Abstract
Mitral regurgitation is the second most common valvular disorder requiring surgical intervention worldwide. This review summarizes the current understanding of primary, degenerative mitral regurgitation with respect to etiology, comprehensive assessment, natural history and management. The new concept of staging of the valvular disorders, newer predictors of adverse and controversy of “watchful waiting” versus “early surgical intervention” for severe, asymptomatic, primary mitral regurgitation are addressed.
Introduction
The retrograde flow of blood from the left ventricle through the mitral valve into the left atrium defines mitral regurgitation. Mitral regurgitation is the most common valvular disorder in the United States, affecting more than 2 million individuals, with a striking increase in prevalence with advanced age.1,2 From a population-based study, the prevalence of mitral regurgitation is greater than 10% in adults older than 75 years, with no significant difference in age-adjusted rates between men and women.2 In the Euro Heart Survey, moderate or severe mitral regurgitation requiring surgical intervention was the second most common form of valvular abnormality, behind only aortic stenosis (mitral regurgitation 31.5% versus aortic stenosis 43.1%).3
This review will highlight the etiology, pathophysiology and natural history of primary mitral valve regurgitation. Predictors of adverse outcomes will be described followed by discussion of treatment and timing of surgery, the latter delving into the controversy between “watchful waiting” versus “early surgical intervention” for severe, asymptomatic primary mitral regurgitation.
Mechanisms and Causes of Mitral Regurgitation
Mitral regurgitation can occur due to disease of the mitral valve leaflets and/or abnormalities of the mitral valve apparatus or secondary to left ventricular dysfunction. Functionally the mitral valve apparatus consists of several components;4,5
-
-
The mitral annulus
-
-
The anterior and posterior mitral valve leaflets
-
-
The chordae
-
-
The anterolateral and posteromedial papillary muscles
-
-
The left ventricular myocardium underlying the papillary muscles
Dysfunction or altered anatomy of any of these components can lead to mitral regurgitation. The mechanism of mitral regurgitation may be described as primary or secondary. Primary mitral regurgitation, sometime called degenerative or organic, is due to an intrinsic lesion of the mitral valve apparatus. Secondary mitral regurgitation, sometimes called functional or ischemic, is a disease of the left ventricle; the left ventricular remodeling in dilated cardiomyopathy or the segmental wall motion abnormalities in ischemic cardiomyopathy, can displace the papillary muscles apically and laterally, causing tethering and malcoaptation of the mitral valve leaflets, which leads to secondary mitral regurgitation.6
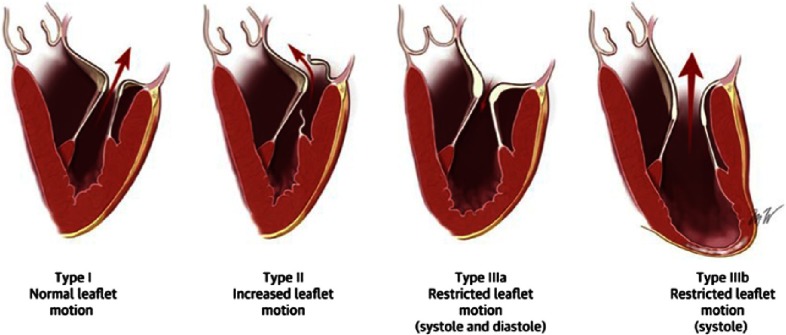
For surgical intervention, the Carpentier Classification is used to group the causes of mitral regurgitation into 3 types, based on the mobility of the mitral valve leaflets [Figure 1]7:
Figure 1. Mitral valve anatomy and Carpentier Classification of mitral regurgitation (from ref # [32]).
-
-
Carpentier type I mitral regurgitation is characterized by normal leaflet mobility. The mitral regurgitation is usually due to a dilated mitral annulus and less often due to a perforated leaflet from endocarditis.8 In normal adults, the mitral annulus is soft and flexible and its contraction contributes significantly to the mitral valve closure. Mitral regurgitation due to annular dilation can occur in any form of heart disease associated with dilation of the left ventricle and especially dilated cardiomyopathy. The annular dilation occurs primarily along the posterior annular section and the associated mitral regurgitation jet is most often centrally directed.8
-
-
Carpentier type II mitral regurgitation is characterized by increased leaflet mobility and it is usually due to leaflet or chordae pathology. The most common cause of primary mitral regurgitation is degenerative mitral valve disease,9,10 predominately mitral leaflet prolapse and/or flail.11,12 (Figure 2)
-
-
There are two major phenotypes of the degenerative mitral valve disease/prolapse: a) fibroelastic deficiency and b) Barlow’s disease.13,14 Fibroelastic deficiency is usually seen in individuals older than 60 years. It is often characterized by single chordal rupture and prolapse of an isolated scallop, most commonly the P2. The associated mitral regurgitation jet is usually eccentric and directed opposite to the prolapsing scallop17 (Movie 1) Barlow’s disease is typically seen in younger patients, 40–60 years old, who present with a chronic murmur. It is characterized by excess leaflet tissue throughout. The leaflets and the chordae appear thickened, redundant and elongated. Multiple scallops of both anterior and posterior leaflets prolapse or may flail into the left atrium during systole15,16 (Movie 2). Of note, these 2 forms of mitral valve prolapse represent the two ends of a spectrum. In clinical practice, most of the patients fall between these two extremes.
-
-
Carpentier type III mitral regurgitation is the result of reduced leaflet mobility and it is further classified to types IIIa and IIIb. In type IIIa mitral regurgitation the leaflet mobility is reduced in both systole and diastole. This is usually seen in rheumatic valve disease or as a result of radiation therapy. Rheumatic mitral regurgitation is characterized by some degree of commissural fusion, but chordal fusion and shortening and leaflet retraction are more prominent findings.18 Similar pathology is noted in post- radiation mitral regurgitation. 19 In type IIIb, the mitral regurgitation is due to myocardial ischemia and/or ventricular remodeling, while the leaflets appear anatomically normal, with reduced mobility during systole.20,21 The cause is papillary muscle displacement with apical tethering and loss of coaptation of the leaflets.
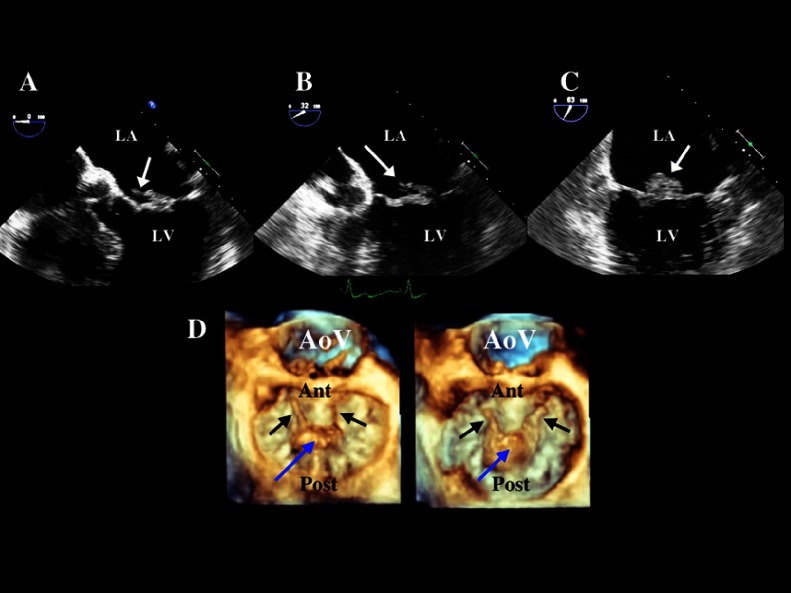
Figure 2. The TEE images demonstrates fibroelastic deficiency with prolapse and flail of P2 Scallop with torn chordae (white arrows) in 2D imaging in three different mid-esophageal TEE views (panel A, B, C) and in 3D imaging (blue arrow = P2 scallop prolapse) (black arrows = torn chordae).
Movie 1. The TEE demonstrates fibroelastic deficiency with prolapse and flail of the P2 scallop shown in 2D imaging (upper left), with color Doppler of the eccentric mitral regurgitation (upper right) and 3D TEE imaging en face from left atrium (lower right) and tilted laterally (lower left).
(“Movie files are available at https://globalcardiologyscienceandpractice.com ”)
Movie 2. The TEE demonstrates bi-leaflet mitral valve prolapse (Barlow’s disease) shown in 2D imaging in three different mid-esophageal views (upper right, upper left, lower right panels) and 3D en face view from the left atrium (lower left panel).
(“Movie files are available at https://globalcardiologyscienceandpractice.com ”)
Pathophysiology of Chronic Mitral Regurgitation
Mitral regurgitation results in left ventricular volume overload, due to an increase in the total stroke volume, as blood is ejected both forward into the aorta and backward into the left atrium. The compensatory response is hypertrophy of the myocardium, progressive dilation and increase in the left ventricular end- diastolic volume, with initial normalization of the wall stress. Long- standing mitral regurgitation causes progressive left ventricular dilation and decline in the left ventricular contractility and ejection fraction. These structural and functional changes, may be clinically silent and precede functional limitations and symptoms. The left atrium also gradually dilates and its compliance increases, in an attempt to maintain normal left atrial pressure. Eventually an increase in the left atrial and left ventricular diastolic pressures and an increase in pulmonary vascular resistance cause the symptoms of heart failure and clinical decompensation.22–24
Presentation
Most patients with severe, chronic mitral regurgitation remain asymptomatic for many years, due to the compensatory pathophysiologic mechanisms described above. Symptoms of dyspnea and heart failure eventually develop as the compensatory mechanisms begin to fail and left ventricular dilation and systolic dysfunction occur.25 Symptoms may occur in patients with preserved left ventricular function who have elevated pulmonary venous pressures or develop atrial fibrillation. By the time the symptoms due to reduced cardiac output and/ or pulmonary congestion become apparent, serious and sometimes irreversible left ventricular dysfunction has occurred.26
Diagnosis and evaluation of mitral regurgitation
Echocardiography plays a pivotal role in the diagnosis of mitral valve regurgitation, the determination mechanism/ cause, the quantification of its severity, and its effect/ consequences on the left ventricle.27 Once defined, further imaging data is used to determine prognosis, timing of surgical intervention and feasibility of successful surgical repair.
The underlying cause of the mitral regurgitation, such as mitral valve prolapse, chordal rupture, can be often determined by transthoracic echocardiography but detailed analysis requires transesophageal.5,28 Transthoracic echocardiography can differentiate primary from secondary regurgitation and provide anatomic information that support repair over replacement of the valve.29,30
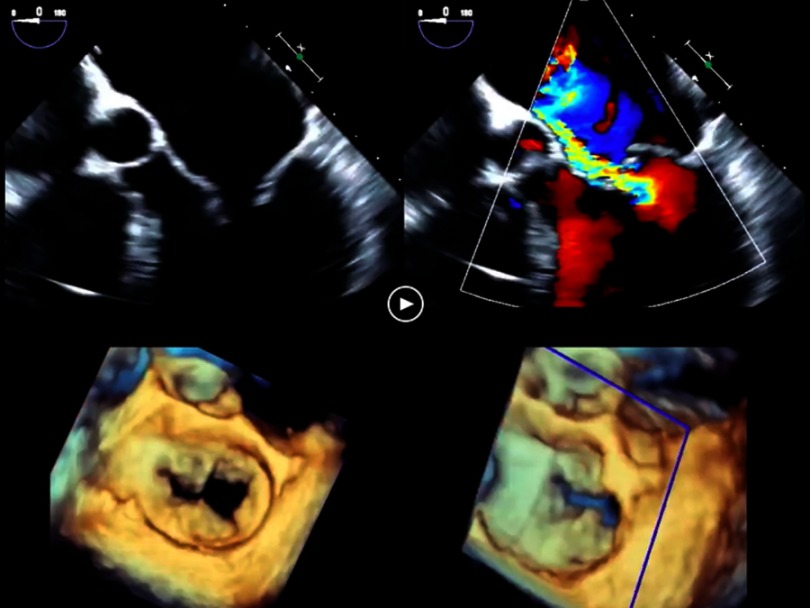
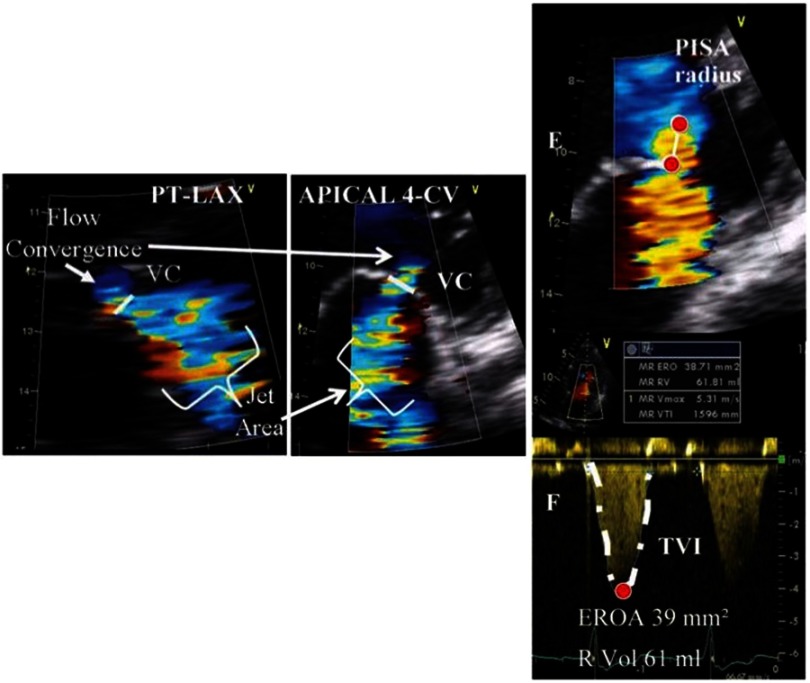
Doppler echocardiography provides significant information on the severity of the mitral regurgitation. Qualitative assessment of the regurgitant jet area using color flow Doppler is influenced by the cause of the regurgitation and the jet eccentricity and it should not be used alone for the grading of the lesion severity.31 Quantitative methods, which measure the regurgitant volume, the regurgitant fraction and the effective regurgitant orifice area (EROA) appear to have greater accuracy and are currently recommended by the ASE guidelines and the European Association of Echocardiography.27,32 Quantification of the EROA can be performed by using the Proximal Isovelocity Surface Area (PISA) (Figure 3A)33,34 or by calculation of the aortic and mitral stroke volumes.35 Severe primary mitral regurgitation is diagnosed with an EROA of 40 mm2, while a smaller EROA ≥20 mm2 is consistent with severe mitral regurgitation in patients with ischemic disease.27 The vena contracta, defined as the narrowest portion of the regurgitant jet, also predicts the severity of mitral regurgitation (Figure 3B).36 Flow reversal in the pulmonary veins and high peak mitral inflow velocity support the diagnosis of severe mitral regurgitation (Table 1).37
Figure 3. (A) (left panel): Assessment of mitral regurgitation using the vena contracta width (from ref [32]).
(B) (right panel): Quantitative assessment of mitral regurgitation using the Proximal Isovelosity Surface Area method (PISA) (from [32]).
Table 1. Qualitative and quantitative parameters useful in grading mitral regurgitation severity (from [27]).
| Mild | Moderate | Severe | ||
|---|---|---|---|---|
| Structural parameters | ||||
| LA size | Normal* | Normal or dilated | Usually dilated** | |
| LV size | Normal* | Normal or dilated | Usually dilated** | |
| Mitral leaflets or support apparatus | Normal or abnormal | Normal or abnormal | Abnormal/ Flail leaflet/ Ruptured papillary muscle | |
| Doppler parameters | ||||
| Color flow jet areaζ | Small, central jet (usually <4 cm2 or <20% of LA area) | Variable | Large central jet (usually >10 cm2 or >40% of LA area) or variable size wall- impinging jet swirling in LA | |
| Mitral inflow–PW | A wave dominantϕ | Variable | E wave dominantϕ (E usually 1.2 m/s) | |
| Jet density–CW | Incomplete or faint | Dense | Dense | |
| Jet contour –CW | Parabolic | Usually parabolic | Early peaking–triangular | |
| Pulmonary vein flow | Systolic dominance§ | Systolic blunting§ | Systolic flow reversal† | |
| Quantitative parametersψ | ||||
| VC width (cm) | <0.3 | 0.3–0.69 | ≥0.7 | |
| R Vol (ml/beat) | <30 | 30–44 | 45–59 | ≥60 |
| RF (%) | <30 | 30–39 | 40–49 | ≥50 |
| EROA (cm2) | <0.20 | 0.20–0.29 | 0.30–0.39 | ≥0.40 |
Notes.
- CW
- Continuous wave
- LA
- left atrium
- EROA
- effective regurgitant orifice area
- LV
- left ventricle
- PW
- pulsed wave
- RF
- regurgitant fraction
- R Vol
- regurgitant volume
- VC
- vena contracta
Unless there are other reasons for LA or LV dilation. Normal 2D measurements: LV minor axis ≤2.8 cm/m2, LV end-diastolic volume ≤82 ml/m2, maximal LA antero-posterior diameter ≤2 cm/m2, maximal LA volume ≤36 ml/m2 (2,33,35).
Exception: acute mitral regurgitation.
At a Nyquist limit of 50–60 cm/s.
Pulmonary venous systolic flow reversal is specific but not sensitive for severe MR.
Usually above 50 years of age or in conditions of impaired relaxation, in the absence of mitral stenosis or other causes of elevated LA pressure.
Unless other reasons for systolic blunting (eg. atrial fibrillation, elevated left atrial pressure).
Quantitative parameters can help sub-classify the moderate regurgitation group into mild-to-moderate and moderate-to-severe.
Transthoracic echocardiography is also very helpful in the follow-up of patients with mitral regurgitation. Based on the stage of the disease, an echocardiogram is repeated every 3–5 years for Stage A, every 1–2 years for Stage B, and every 6–12 months for stage C mitral regurgitation.6
Exercise echocardiography has its role in the evaluation of mitral regurgitation, by providing information on the severity of the regurgitation and the hemodynamic abnormalities (e.g., pulmonary hypertension) during exercise.38 It is a useful tool to evaluate symptoms in patients that appear to have only mild regurgitation, to determine the functional capacity39 and the changes in hemodynamics in patients who appear stable or asymptomatic.40
Cardiac MRI is the most accurate non- invasive technique for measurement of end- diastolic and end-systolic volumes and left ventricular mass. Although visualization of mitral valve structure is more reliable by echocardiography, CMR may provide a more accurate assessment of the severity of regurgitation.41 In patients in whom discrepancy exists between mitral regurgitation severity by clinical findings and echo results, further evaluation with CMR may be helpful.42
Left heart catheterization, with coronary angiography and ventriculography is indicated in the following circumstances in the evaluation of mitral regurgitation: a) discrepancy between clinical findings and echocardiographic data, b) detection and severity assessment of associated valvular lesions and c) presence and extent of coronary artery disease, especially in preparation for surgical intervention.6
Stages of Mitral Regurgitation
The 2014 ACC/ AHA valve guidelines introduced stages for each valve lesion,6 which are similar to the stages proposed in the Heart Failure guidelines. Each stage describes the progression of the valvular disorder taking into account the presence or absence of symptoms, the severity of the valve disorder, the response of the left ventricle to the effect of the disorder, the effect on pulmonary circulation and heart rhythm. The staging system provides the physician a better way to monitor the progression of the valvular disease, defines the clinical and echocardiographic follow-up and helps make management decisions based on the stage of the disorder in order to treat complications and effects of the disorder in a more timely fashion. Table 2 provides a summary of the stages proposed for primary mitral regurgitation.
Table 2. Stages of primary mitral regurgitation (reproduced from [6]).
| Grade | Definition | Valve Anatomy | Valve Hemodynamics* | Hemodynamic Consequences | Symptoms |
|---|---|---|---|---|---|
| A | At risk of MR | • Mild mitral valve prolapse with normal coaptation • Mild valve thickening and leaflet restriction |
• No MR jet or small central jet area <20% LA on Doppler • Small vena contracta <0.3 cm |
• None | • None |
| B | Progressive MR | • Severe mitral valve prolapse with normal coaptation • Rheumatic valve changes with leaflet restriction and loss of central coaptation • Prior IE |
• Central jet MR 20%-40% LA or late systolic eccentric jet MR • Vena contracta <0.7 cm • Regurgitant volume <60 mL • Regurgitant fraction <50% • ERO <0.40 cm2 • Angiographic grade 1–2+ |
• Mild LA enlargement • No LV enlargement • Normal pulmonary pressure |
• None |
| C | Asymptomatic severe MR | • Severe mitral valve prolapse with loss of coaptation or flail leaflet • Rheumatic valve changes with leaflet restriction and loss of central coaptation • Prior IE • Thickening of leaflets with radiation heart disease |
• Central jet MR >40% LA or holosystolic eccentric jet MR • Vena contracta ≥0.7 cm • Regurgitant volume ≥60 mL • Regurgitant fraction ≥50% • ERO 0.40 cm2 • Angiographic grade 3–4+ |
• Moderate or severe LA enlargement • LV enlargement • Pulmonary hypertension may be present at rest or with exercise • C1: LVEF >60% and LVESD <40 mm • C2: LVEF ≤60% and LVESD ≥40 mm |
• None |
| D | Symptomatic severe MR | • Severe mitral valve prolapse with loss of coaptation or flail leaflet • Rheumatic valve changes with leaflet restriction and loss of central coaptation • Prior IE • Thickening of leaflets with radiation heart disease |
• Central jet MR >40% LA or holosystolic eccentric jet MR • Vena contracta ≥0.7 cm • Regurgitant volume ≥60 mL • Regurgitant fraction ≥50% • ERO 0.40 cm2 • Angiographic grade 3–4+ |
• Moderate or severe LA enlargement • LV enlargement • Pulmonary hypertension present |
• Decreased exercise tolerance • Exertional dyspnea |
Notes.
Several valve hemodynamic criteria are provided for assessment of MR severity, but not all criteria for each category will be present in each patient. Categorization of MR severity as mild, moderate, or severe depends on data quality and integration of these parameters in conjunction with other clinical evidence.
- ERO
- effective regurgitant orifice
- IE
- infective endocarditis
- LA
- left atrium/atrial
- LV
- left ventricular
- LVEF
- left ventricular ejection fraction
- LVESD
- left ventricular end-systolic dimension
- MR
- mitral regurgitation
Natural History, Progression and Predictors of Outcomes in Mitral Regurgitation
The natural history of organic mitral regurgitation is highly variable and depends on a combination of parameters that include the regurgitant volume, the cause of the regurgitation and its effect on the left ventricle. Asymptomatic patients with mild primary mitral regurgitation usually remain stable for many years. Severe mitral regurgitation develops only is a small percentage of these patients, due to intervening infective endocarditis or chordal rupture.
Different series examined the natural history of patients with severe mitral regurgitation. Early series reported widely variable mortality rates, ranging from 27% to 97% at 5-year follow-up.43,44 That variation may be explained by poorly defined severity of mitral regurgitation, various selection biases and small study populations.43–48 A series from Ling et al. examined 229 patients with mitral regurgitation due to flail leaflet, many of who were symptomatic, had atrial fibrillation or evidence of left ventricular dysfunction.49 Patients who were treated medically had a mortality rate significantly higher than the expected (6.3% yearly mortality, p = 0.016, when compared with the expected rate in the US population according to the 1990 census). Death or need for surgery was almost unavoidable within 10 years of diagnosis and surgical correction improved long- term survival.49,50
Two recent series involved patients with mitral regurgitation who were initially asymptomatic and had a normal left ventricular function.51,52 Enriquez-Sarano et al. examined prospectively 456 patients with asymptomatic organic mitral regurgitation and showed that the 5-year mortality from any cause was 22% and the cardiac mortality was 14% in patients managed medically. Cardiac surgery was ultimately performed in 232 patients and was associated with improved survival.51 Rosenhek et al. followed a series of 132 asymptomatic patients with severe degenerative mitral regurgitation. Survival without the need of surgery was 92% at 2 years, 78% at 4 years and 65% at 6 years. A total of 38 patients developed indications for surgery and those with a flail leaflet tended to develop criteria for surgery slightly, but not significantly earlier.52
Despite the lack of randomized trials, all the prospective, observational data showed that in asymptomatic patients with initially preserved ejection fraction, severe mitral regurgitation has a high likelihood of requiring surgery over the next 6–10 years, because of heart failure symptoms or left ventricular dysfunction.53,54
Many predictors of different clinical outcomes and especially mortality have been identified in patients with primary mitral regurgitation. Ling et al. showed that in patients with mitral regurgitation due to flail leaflets, older age, presence of symptoms and lower ejection fraction are independent predictors of mortality.50
Enriquez-Sarano et al. demonstrated that the Effective Regurgitant Orifice Area (EROA) is a powerful predictor of outcomes in patients with asymptomatic, organic mitral regurgitation.51 When compared to patients with EROA < 20 mm2, those with an orifice of at least 40 mm2 have an increased risk of death from any cause, death from cardiac causes and cardiac events (defined as death from cardiac causes, heart failure and new atrial fibrillation).
Tourneau et al. examined the impact of left atrial volume on clinical outcomes in 492 patients with organic mitral regurgitation and showed that the left atrial index is a predictor of long-term outcomes.55 Patients with a left atrial index ≥60 ml/m2 have lower 5-year survival and more cardiac events than those with mild or no left atrial enlargement. In this cohort, mitral surgery is associated with decreased mortality and cardiac events.55
The MIDA registry included patients with mitral regurgitation due to flail leaflets.56 Pulmonary artery systolic pressure was measured by echocardiography is 437 patients and pulmonary hypertension was observed in 102 patients. Pulmonary hypertension is an independent predictor of all- cause death (adjusted HR: 1.70, p = 0.002), cardiovascular death (adjusted HR: 2.21, p = 0.003) and heart failure (adjusted HR: 1.70, p = 0.002).56 In that registry, mitral valve surgery was beneficial, but it didn’t abolish completely the effects of pulmonary hypertension once it was established.
Atrial fibrillation is a common arrhythmia in patients with chronic mitral regurgitation and its onset is a marker of disease progression.57 Patients with atrial fibrillation have an adverse outcome compared to those who remain in sinus rhythm58 and the development of atrial fibrillation is considered an indication (IIa) for surgical intervention.6
Over the past few years new prognostic markers have emerged. Those include the b- natriuretic peptide (BNP), the use of left ventricular strain59 and the exercise capacity.60
BNP activation in organic mitral regurgitation is primarily due to ventricular and atrial consequences, rather than the degree of mitral regurgitation.61 Higher BNP levels are associated with lower survival and higher combined adverse events (death and heart failure).62
Alashi et al. examined 448 asymptomatic patients with severe primary mitral regurgitation and preserved ejection fraction and demonstrated that abnormal longitudinal strain and higher BNP levels are associated with higher long-term mortality and the combination of two appeared to be a synergistic outcome predictor.63
The importance of exercise capacity in predicting outcomes in patients with severe primary regurgitation was studied by Naji et al.64,65 In 576 patients with primary mitral regurgitation who underwent exercise echocardiography prior to mitral valve surgery, lower achieved METs were associated with worse long-term outcomes. The authors concluded that achieving >100% of age and gender- predicted METs can safely delay mitral valve surgery for at least one year, without an effect on outcomes.65
Kusunose et al. studied 196 patients with moderate to severe, primary asymptomatic mitral regurgitation and showed that resting left ventricular strain, exercise TAPSE and exercise systolic pulmonary arterial pressure are independent predictors of time to surgery.66 Exercise- induced right ventricular dysfunction is an independent predictor of worse outcomes in in this patient cohort. Table 3 summarizes the clinical, biologic and echocardiographic predictors of poor outcome in patients with primary mitral regurgitation.
Table 3. Predictors of poor outcome in primary mitral regurgitation.
| Clinical Characteristics | Biologic Markers | Echo Findings |
|---|---|---|
| Advance age | Elevated BNP | Low ejection fraction (<60%) |
| Symptoms of CHF | EROA (>40 mm2) | |
| Atrial fibrillation | Left atrial volume | |
| Poor exercise capacity | Pulmonary hypertension | |
| Abnormal LV strain |
Management of Mitral Regurgitation
A. Medical management of primary mitral regurgitation
No medical therapy alters the natural history of severe primary mitral regurgitation. Medical therapy for systolic dysfunction, which includes beta-blockers, ACE-I and possibly aldosterone antagonists, is reasonable (Class IIa recommendation) in symptomatic patients with primary mitral regurgitation, who have a left ventricular ejection fraction<60% and in whom surgery is not planned or it will be delayed.6 Diuretics may relieve the symptoms of heart failure, but improvement in symptoms should not delay referral for surgical intervention. If the left ventricular systolic function is normal vasodilator therapy is not indicated for asymptomatic, normotensive patients with chronic primary regurgitation.6 A limited number of studies addressed the use of ACE-I therapy for 1–6 months in chronic asymptomatic mitral regurgitation with preserved systolic function. These studies failed to provide evidence of clinical or hemodynamic benefit.67–71 Hypertension needs to be treated, because the increased left ventricular systolic pressure increases the trans-mitral gradients and worsens the severity of mitral regurgitation.6
B. Indications for surgical intervention in primary mitral regurgitation
Surgical intervention with either mitral valve repair or replacement is indicated in patients with severe mitral regurgitation who develop symptoms or left ventricular dysfunction.6,72 The left ventricular dysfunction is defined as an ejection fraction <60% and/or an end- systolic dimension >40 mm (Class I recommendations). Concomitant mitral valve repair or replacement is also indicated in patients with chronic severe primary mitral regurgitation undergoing cardiac surgery for another indication (Class I recommendation).6,72
Mitral valve repair is reasonable in asymptomatic patients with chronic severe primary mitral regurgitation (Stage C1) and preserved systolic function in whom the likelihood of successful repair is >95% with an expected mortality <1% (Class IIa recommendation).6 Another reasonable indication for mitral valve repair is in asymptomatic patients with chronic severe primary mitral regurgitation (Sage C1) with new onset atrial fibrillation or resting pulmonary hypertension (Class IIa recommendation).6,72
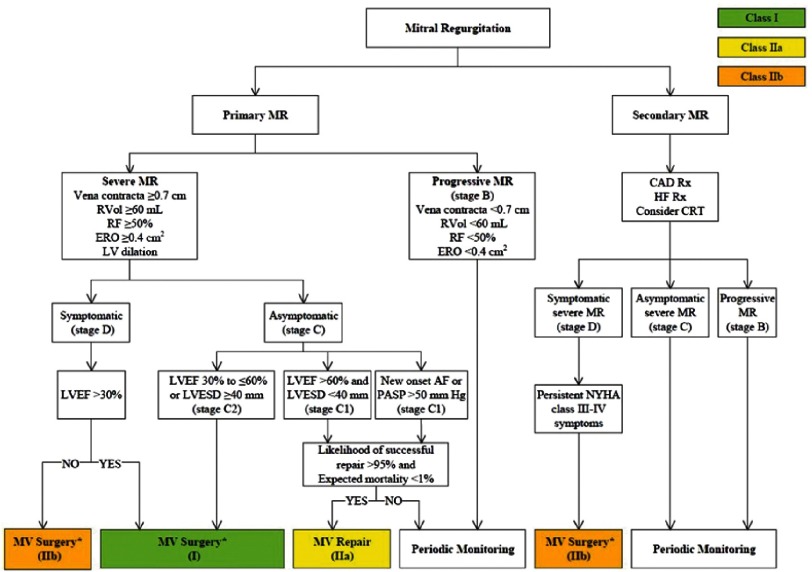
A summary of the recommendations for surgical intervention in primary and functional mitral regurgitation as per the 2014 ACC/AHA valve guidelines is shown below (Figure 4). These recommendations are basically similar to the European valve guidelines, with a couple of caveats: When there is a high likelihood of durable repair at a low surgical risk, Vahanian et al. recommend valve repair in patients with a flail leaflet and LVESD ≥40 mm (Class IIa), while surgery may be considered (Class IIb) if one of the following risk factors is present: left atrial volume ≥60 ml/m3 BSA and sinus rhythm or pulmonary hypertension with exercise (SPAP ≥60 mmHg).72
Figure 4. Summary of the indications for surgical intervention in mitral regurgitation as per the 2014 AHA/ACC valve guidelines (from [6]).
C. Mitral valve repair versus replacement
Mitral valve repair is the preferred treatment for patients with primary mitral regurgitation as it is associated with better outcomes than mitral valve replacement.73–76 Surgical repair of the valve tends to be successful in the following cases: degenerative mitral valve disease, annular dilation, chordal rupture, leaflet perforation due to endocarditis and papillary muscle dysfunction due to ischemia. It is less likely to be successful in older patients with calcified deformed valves, rheumatic heart disease or severe sub-valvular thickening. These cases are usually treated with mitral valve replacement.77–79
There are certain disadvantages associated with the mitral valve replacement that have made it a less favorable strategy. The left ventricular function and the ejection fraction tend to deteriorate after mitral valve replacement, contributing to early and late morbidity and mortality.80,81 That appears to be associated with the loss of support of the mitral valve apparatus, as chordae and papillary muscles are not preserved with valve replacement.82
Another disadvantage of the valve replacement is the prosthesis itself. Mechanical prostheses required life-long anticoagulation and they are associated with an increased risk of thrombosis, bleeding and thromboembolism. Bioprostheses are associated with late structural deterioration and need for repeat intervention. Both types of prostheses have an increased risk of infective endocarditis.6,72
For all these reasons efforts are being made to repair a mitral valve whenever possible. Mitral valve repair is technically a more demanding procedure than replacement and there is a tendency to refer patients to centers of excellence in performing mitral valve repair.83,84 Success of durable repair should be greater than 95%. The most important factor of durable success for mitral repair is the experience of the surgeon.85,86
-
-
Type I mitral regurgitation management: If it is due to annular dilation it can be repaired with an annuloplasty ring, with the goal to reduce annular dilation and increase the coaptation zone.87,88 Type I mitral regurgitation due to endocarditis may also be repaired depending on the amount of leaflet destruction. A small perforation can be patched, while larger lesions may require resection or plication.89
-
-
Type II mitral regurgitation management: Mitral valve repair is the preferred treatment for primary degenerative mitral regurgitation. The feasibility of repair depends on the presence of repairable pathology, which is most likely with excess length and mobility.90 Mitral valve repair for degenerative disease consists of reconstruction of the valve, which is usually accompanied by an annuloplasty ring.91,92 Prolapsed valves are usually treated with resection of the prolapsing segment(s) and plication of the annulus. For the most common form of posterior leaflet prolapse, different repair techniques can be used alone or in combination. These include resection of the prolapsing segment (s),93 plication,94 folding-plasty,95 sliding leaflet-plasty96 or an Alfieri repair,97 which is a leaflet edge-to-edge suturing.
-
-
Type III mitral regurgitation management: Patients with rheumatic disease are usually managed with valve replacement. Management of Type IIIb regurgitation involves the use of an isolated annuloplasty, which accomplishes the immediate goal of repair, but without long- term durability, as the ventricular dysfunction and remodeling are not addressed with the annuloplasty.98,99 Studies show a more reliable reduction in mitral regurgitation with valve replacement,100 with no difference in major outcomes between repair and replacement.99,101
D. Watchful waiting versus early surgical intervention in asymptomatic patients with severe mitral regurgitation
High volume centers of excellence are moving toward a more aggressive surgical approach and they recommend mitral valve repair in asymptomatic patients with severe mitral regurgitation in the absence of left ventricular dysfunction, atrial fibrillation and pulmonary hypertension. That is a Class IIa indication in the 2014 ACC/ AHA guidelines6 and a Class IIb in the 2012 European guidelines.72 The only required condition for that recommendation is a likelihood of successful repair >95% with low associated mortality <1%.84–86
There has been continuous debate in regards to the optimal timing of surgery for asymptomatic patients with severe primary regurgitation who have normal left ventricular systolic function and dimensions, normal pulmonary artery pressure and no episodes of atrial fibrillation. The supporters of the watchful waiting, or “wait and see”, approach argue that surgery should be delayed until Class I or Class IIa indications occur, while the early surgical intervention side supports mitral valve repair before symptoms or any complication of mitral regurgitation happen.
Rosenhek et al. reported excellent results with the watchful waiting strategy.52 The group examined 132 asymptomatic patients with severe degenerative mitral regurgitation due to flail leaflets or valve prolapse. The patients had close clinical and echocardiographic follow-up, at least every 12 months and in some case every 3-6 months and were referred for surgery when a Class I or a IIa trigger was reached. The overall survival at 8 years was 91 ± 3%, which was not different from the expected survival.52
In contrast to the above findings, three groups compared the watchful waiting strategy to the early surgical intervention and showed an advantage of early intervention in terms of long term and operative mortality, as well as repair rates.
Kang et al. studied 447 asymptomatic patients with severe degenerative mitral regurgitation and preserved left ventricular function.102 161 patients were referred for early surgery and 286 were managed with the “wait and see” strategy. They showed that the group treated with early surgery had an improved 7- year event free survival (99% ± 1% versus 85% ± 4% for the watchful waiting group, p = 0.001) and less hospitalization for heart failure.102
Montant et al. showed similar results following prospectively 192 asymptomatic patients with severe degenerative mitral regurgitation.103 67 patients were managed with the conservative approach, while 125 patients underwent early surgical intervention. The 10- year overall survival of the group treated with the conservative strategy was significantly lower (50 ± 7%) compared to the group that received early surgery (86 ± 4%, p < 0.0001). Subgroup analysis of patients with atrial fibrillation and pulmonary hypertension demonstrated similar findings.103
Suri et al. studied 1021 patients with mitral regurgitation due to flail leaflet that were asymptomatic and had normal LVEF and dimensions.104 575 patients were managed conservatively and 446 were referred to surgery within 3 months of diagnosis. Early mitral valve intervention was associated with significant long -term survival benefit and reduced heart failure risk.104
A recent meta- analysis supported an early surgical intervention in asymptomatic severe mitral regurgitation, as it improves survival and increases the likelihood of successful mitral valve repair compared to the watchful waiting strategy.105
In summary, current data favors early mitral valve repair in high volume centers able to achieve high success rate, with low procedural mortality. This is more applicable in the isolated posterior mitral valve prolapse (fibroelastic deficiency) and less likely successful in the Barlow’s disease.
Conclusions
Mitral regurgitation is the most common valvular disorder in the USA and the second most common in Europe. Myxomatous degeneration of the valve is responsible for two thirds of primary mitral regurgitation, which is an intrinsic valve problem in contrast to the secondary regurgitation, which is a disease of the ventricle. Mitral regurgitation is a pure volume overload to the left ventricle, to which the ventricle responds with progressive dilation and eventually with decreased ejection fraction and symptoms of heart failure. Quantitative echocardiography is the main way to evaluate the severity of mitral regurgitation. Cardiac MRI and left/right heart catheterization have an adjunctive role when there is discrepancy between clinical findings and echocardiographic data. Clinical (age, symptoms, poor exercise capacity, atrial fibrillation) and echocardiographic (left ventricular dysfunction, EROA, high SPAP and left atrial volume and abnormal LV strain) parameters predict worse outcomes in mitral regurgitation. Patients with severe primary regurgitation have a high morbidity and mortality rate and in 10 year follow-up 90% will have died or undergone surgery due to development of symptoms. Surgical intervention, preferably valve repair, is indicated in patients with severe primary mitral regurgitation and symptoms or evidence of left ventricular dysfunction, defined as ejection fraction <60% and end-systolic dimension >40 mm. Patients with severe asymptomatic mitral regurgitation can be managed with meticulous follow-up for symptoms and left ventricular dysfunction, but studies appear to favor early valve repair in centers of excellence able to achieve high repair rates (>95%) and low mortality (<1%) especially for patients with localized prolapse. Transcatheter mitral valve repair techniques have emerged in recent years and appear to be safe, especially in elderly people with extensive comorbidities who are frequently denied surgery.106 The future of percutaneous options may change the threshold for intervention and requires careful assessment.
Conflict of interests
The authors have no competing interests to declare.
Authors contributions
EA: contributed to the design of the review, drafted the original manuscript and gave final approval.
ADM: contributed to the concept, created the movies, critically revised the content of the manuscript and gave final approval.
AP: contributed to concept and design of the review, critically revised the content of the manuscript and gave final approval.
The authors read and approved the final manuscript.
References
- 1.Enriquez-Sarano M, Akins CW, et al. Mitral regurgitation. Lancet. 2009;373:1382–94. doi: 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, et al. Burden of valvular heart diseases: A population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Baron G, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 4.Otto CM. Textbook of clinical Echocardiography. 4th edition Saunders; 2009. pp. 311–312. [Google Scholar]
- 5.Enriquez-Sarano M, Freeman W, et al. Functional anatomy of mitral regurgitation: Echocardiographic assessment and implications on outcome. J Am Coll Cardiol. 1999;34:1129–1136. doi: 10.1016/s0735-1097(99)00314-9. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, et al. 2014 AHA/ ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/ American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63(22):2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 7.Carpentier A. Cardiac valve surgery- the “French Correction”. J Thor Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 8.Maslow A. Mitral valve repair: An echocardiographic review: Part 1. J Cardiothorac Vasc Anesth. 2015;29(1):156–177. doi: 10.1053/j.jvca.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Olson LJ, Subramanian R, et al. Surgical pathology of the mitral valve: A study of 712 cases spanning 21 years. Mayo Clin Proc. 1987;62:22–34. doi: 10.1016/s0025-6196(12)61522-5. [DOI] [PubMed] [Google Scholar]
- 10.Griffin BP. Myxomatous mitral valve disease: Valvular Heart Disease- A companion to Braunwald’s Heart Disease. Saunders/ Elsevier; Philadelphia: 2009. pp. 243–249. [Google Scholar]
- 11.Hayek E, Gring CN, et al. Mitral valve prolapse. Lancet. 2005;365:507–518. doi: 10.1016/S0140-6736(05)17869-6. [DOI] [PubMed] [Google Scholar]
- 12.Abramowitz Y, Jilaihawi H, et al. Mitral Annulus Calcification. J Am Coll Cardiol. 2015;66(17):1934–1941. doi: 10.1016/j.jacc.2015.08.872. [DOI] [PubMed] [Google Scholar]
- 13.Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg. 2007;19:90–96. doi: 10.1053/j.semtcvs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Fornes P, Heudes D, et al. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: A histomorphometric study of 130 excised segments. Cardiovasc Pathol. 1999;8:81–92. doi: 10.1016/s1054-8807(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 15.Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve: An auscultatory-electrocardiographic syndrome. Am Heart J. 1966;71(2):166–178. doi: 10.1016/0002-8703(66)90179-7. [DOI] [PubMed] [Google Scholar]
- 16.Barlow JB, Bosman CK, et al. Late systolic murmurs and non- ejection (mid- late) systolic clicks: An analysis of 90 patients. Br Heart J. 1968;30:203–218. doi: 10.1136/hrt.30.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra S, Salgo IS, et al. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three- dimensional echocardiographic images: Objective insight into complexity and planning of mitral valve repair. Circ Cardiovasc Imaging. 2011;4(1):24–32. doi: 10.1161/CIRCIMAGING.109.924332. [DOI] [PubMed] [Google Scholar]
- 18.Remenyi B, ElGuindy A, et al. Valvular heart disease 3: Valvular aspects of rheumatic heart disease. Lancet. 2016;387:1335–1346. doi: 10.1016/S0140-6736(16)00547-X. [DOI] [PubMed] [Google Scholar]
- 19.Brand MD, Abadi CA, et al. Radiation- associated valvular heart disease in Hodgkin’s disease is associated with characteristic thickening and fibrosis of the aortic- mitral curtain. J Heart Valve Dis. 2001;10(5):681–685. [PubMed] [Google Scholar]
- 20.Bursi F, Enriquez-Sarano M, et al. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation. 2005;111(3):295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 21.Birsi F, Enriquez-Sarano M, et al. Mitral regurgitation after myocardial infarction: A review. Am J Med. 2006;119:103–112. doi: 10.1016/j.amjmed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Carabello BA. Mitral regurgitation: Basic pathophysiologic principles. Mod Concepts Cardiovasc Dis. 1988;57:53–58. [Google Scholar]
- 23.Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation: Implications for management. Circulation. 2008;118(22):2298–2303. doi: 10.1161/CIRCULATIONAHA.107.755942. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura R, Vahanian A, et al. Mitral valve disease- current management and future challenges. Lancet. 2016;387:1324–1334. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- 25.Gaasch WH, John RM, et al. Managing asymptomatic patients with chronic mitral regurgitation. Chest. 1995;108:842–847. doi: 10.1378/chest.108.3.842. [DOI] [PubMed] [Google Scholar]
- 26.Wisenbaugh T. Does normal pump function belies muscle dysfunction in patients with chronic severe mitral regurgitation? Circulation. 1988;77(3):515–525. doi: 10.1161/01.cir.77.3.515. [DOI] [PubMed] [Google Scholar]
- 27.Zoghbi WA, Enriquez-Sarano M, et al. Evaluation of the severity of native valvular regurgitation with two- dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 28.Monin JL, Dehant P, et al. Functional assessment of mitral regurgitation by transthoracic echocardiography using standardized imaging planes diagnostic accuracy and outcome implications. J Am Coll Cardiol. 2005;46(2):302–309. doi: 10.1016/j.jacc.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 29.Stewart WJ, Currie PJ, et al. Intraoperative Doppler color flow mapping for decision making in valve repair for mitral regurgitation: Technique and results in 100 patients. Circulation. 1990;81(2):556–566. doi: 10.1161/01.cir.81.2.556. [DOI] [PubMed] [Google Scholar]
- 30.Stewart WJ, Salcedo EE, et al. The value of echocardiography in mitral valve repair. Clev Clin J Med. 1991;58:177–183. doi: 10.3949/ccjm.58.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Enriquez-Sarano M, Tahik A, et al. Color flow imaging compared with quantitative Doppler assessment of severity of mitral regurgitation: Influence of eccentricity of jet and mechanism of regurgitation. J Am Coll Cardiol. 1993;21:1211–1219. doi: 10.1016/0735-1097(93)90248-y. [DOI] [PubMed] [Google Scholar]
- 32.Lancelloti P, Moura L, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11(4):307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 33.Enriquez-Sarano M, Miller FA, et al. Effective mitral regurgitant orifice area: Clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol. 1995;25(3):703–709. doi: 10.1016/0735-1097(94)00434-R. [DOI] [PubMed] [Google Scholar]
- 34.Pu M, Prior DL, et al. Calculation of mitral regurgitant orifice area with the use of a simplified proximal convergence method: Initial clinical application. J Am Soc Echocardiogr. 2001;14:180–185. doi: 10.1067/mje.2001.110139. [DOI] [PubMed] [Google Scholar]
- 35.Enriquez-Sarano M, bailey K, et al. Quantitative Doppler assessment of valvular regurgitation. Circulation. 1993;87(30):841–848. doi: 10.1161/01.cir.87.3.841. [DOI] [PubMed] [Google Scholar]
- 36.Heinle SK, Hall SA, et al. Comparison of vena contracta width by multiplane transesophageal echocardiography with quantitative Doppler assessment of mitral regurgitation. Am J Cardiol. 1998;81(2):175–179. doi: 10.1016/s0002-9149(97)00878-3. [DOI] [PubMed] [Google Scholar]
- 37.Thomas L, Foster E, et al. Peak mitral inflow velocity predicts mitral regurgitation severity. J Am Coll Cardiol. 1998;31(1):174–179. doi: 10.1016/s0735-1097(97)00454-3. [DOI] [PubMed] [Google Scholar]
- 38.Picano E, Pibarot P, et al. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol. 2009;54(24):2251–2260. doi: 10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 39.Massika-Zeitoun D, Johnson BP, et al. Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation: Physiologic and outcome implications. J Am Coll Cardiol. 2006;47:2521–2527. doi: 10.1016/j.jacc.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 40.Leung D, Griffin B, et al. Left ventricular function after valve repair for chronic mitral regurgitation: Predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J Am Coll Cardiol. 1996;28(5):1198–1205. doi: 10.1016/S0735-1097(96)00281-1. [DOI] [PubMed] [Google Scholar]
- 41.Cawley PJ, Maki JH, et al. Cardiovascular magnetic resonance imaging for valvular heart disease: Technique and validation. Circulation. 2009;119(3):468–478. doi: 10.1161/CIRCULATIONAHA.107.742486. [DOI] [PubMed] [Google Scholar]
- 42.Gelfand EV, Hughes S, et al. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance imaging: Optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson. 2006;8(3):503–507. doi: 10.1080/10976640600604856. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MG, Lim WN. The natural history of rheumatic heart disease in the third, fourth and fifth decades of life: Prognosis with special reference to survivorship. Circulation. 1957;16:700–712. doi: 10.1161/01.cir.16.5.700. [DOI] [PubMed] [Google Scholar]
- 44.Horstkotte D, Loogen F, et al. The influence of heart valve replacement on the natural history of isolated mitral, aortic and multivalvular disease. Z Cardiol. 1983;72:494–503. [PubMed] [Google Scholar]
- 45.Munoz S, Gallardo J, et al. Influence of surgery on the natural history of rheumatic mitral and aortic valve disease. Am J Coll Cardiol. 1975;35:234–242. doi: 10.1016/0002-9149(75)90007-7. [DOI] [PubMed] [Google Scholar]
- 46.Rosen SE, Borer JS, et al. Natural history of the asymptomatic/minimally symptomatic patient with severe mitral regurgitation secondary to mitral valve prolapse and normal right and left ventricular performance. Am J Cardiol. 1994;74:374–380. doi: 10.1016/0002-9149(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 47.De Pace NL, Mintz GS, et al. Natural history of the flail mitral leaflet syndrome: A serial 2-dimensional echocardiographic study. Am J Cardiol. 1983;52:789–795. doi: 10.1016/0002-9149(83)90416-2. [DOI] [PubMed] [Google Scholar]
- 48.Hammermeister KE, Fisher L, et al. Prediction of late survival in patients with mitral valve disease from clinical, hemodynamic and quantitative angiographic variables. Circulation. 1978;57:341–349. doi: 10.1161/01.cir.57.2.341. [DOI] [PubMed] [Google Scholar]
- 49.Ling L, Enriquez-Sarano M, et al. Clinical outcome of mitral regurgitation due to flail leaflets. N Eng J Med. 1996;335:1417–1423. doi: 10.1056/NEJM199611073351902. [DOI] [PubMed] [Google Scholar]
- 50.Ling L, Enriquez-Sarano M, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: A long- term outcome study. Circulation. 1997;96:1819–1825. doi: 10.1161/01.cir.96.6.1819. [DOI] [PubMed] [Google Scholar]
- 51.Enriquez-Sarano M, Avierinos JF, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Eng J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 52.Rosenhek R, Rader F, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–2244. doi: 10.1161/CIRCULATIONAHA.105.599175. [DOI] [PubMed] [Google Scholar]
- 53.Avierinos JF, Gersh BJ, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355–1361. doi: 10.1161/01.cir.0000028933.34260.09. [DOI] [PubMed] [Google Scholar]
- 54.Tribouilloy C, Grigioni F, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflet: A long-term follow-up multicenter study. J Am Coll Cardiol. 2009;54:1961–1968. doi: 10.1016/j.jacc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 55.Tourneau T, Messika-Zeitiun D, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010;56:570–578. doi: 10.1016/j.jacc.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 56.Barbieri A, Bursi F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: A multicenter long- term international study. Eur Heart J. 2011;32(6):751–759. doi: 10.1093/eurheartj/ehq294. [DOI] [PubMed] [Google Scholar]
- 57.Eguchi K, Ohtaki E, et al. Pre- operative atrial fibrillation as the key determinant of outcome of mitral valve repair for degenerative mitral regurgitation. Eur Heart J. 2005;26(18):1866–1872. doi: 10.1093/eurheartj/ehi272. [DOI] [PubMed] [Google Scholar]
- 58.Bando K, Kasegawa H, et al. Impact of preoperative and postoperative atrial fibrillation on outcome after mitral valvuloplasty for non- ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2005;129:1032. doi: 10.1016/j.jtcvs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 59.Magne J, Mahjoub H, et al. Prognostic importance of brain natriuretic peptide and left ventricular longitudinal strain in asymptomatic degenerative mitral regurgitation. Heart. 2012;98:584–591. doi: 10.1136/heartjnl-2011-301128. [DOI] [PubMed] [Google Scholar]
- 60.Magne J, Lancelotti P, et al. Exercise- induced changes in degenerative mitral regurgitation. J Am Coll Cardiol. 2010;56:300–309. doi: 10.1016/j.jacc.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 61.Button TM, Stewart RA, et al. Plasma natriuretic peptide level increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003;41:2280–2287. doi: 10.1016/s0735-1097(03)00486-8. [DOI] [PubMed] [Google Scholar]
- 62.Detaint D, Messika-Zeitoun D, et al. B- type natriuretic peptide in organic mitral regurgitation: Determinants and impact on outcome. Circulation. 2005;111(18):2391–2397. doi: 10.1161/01.CIR.0000164269.80908.9D. [DOI] [PubMed] [Google Scholar]
- 63.Alashi A, Mentias A, et al. Synergistic utility of brain natriuretic peptide and left ventricular global longitudinal strain in asymptomatic patients with significant primary mitral regurgitation and preserved systolic function undergoing mitral valve surgery. Circ Cardiovasc Imaging. 2016;9:e004451. doi: 10.1161/CIRCIMAGING.115.004451. [DOI] [PubMed] [Google Scholar]
- 64.Naji P, Griffin B, et al. Predictors of long- term outcomes in patients with significant myxomatous mitral regurgitation undergoing exercise echocardiography. Circulation. 2014a;129(12):1310–1319. doi: 10.1161/CIRCULATIONAHA.113.005287. [DOI] [PubMed] [Google Scholar]
- 65.Naji P, Griffin B, et al. Importance of exercise capacity in predicting outcomes and determining optimal timing of surgery in significant primary mitral regurgitation. J Am Heart Assoc. 2014b;3(5):e001010. doi: 10.1161/JAHA.114.001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kusunose K, Popovic Z, et al. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:167–176. doi: 10.1161/CIRCIMAGING.112.000162. [DOI] [PubMed] [Google Scholar]
- 67.Tishler M, Rowan M, et al. Effect of Enalapril on left ventricular mass and volumes in asymptomatic chronic severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 1998;82:242–245. doi: 10.1016/s0002-9149(98)00325-7. [DOI] [PubMed] [Google Scholar]
- 68.Levine H, Gaasch W. Vasoactive drugs in chronic regurgitant lesions of mitral and aortic valves. J Am Coll Cardiol. 1996;28:1083–1091. doi: 10.1016/S0735-1097(96)00288-4. [DOI] [PubMed] [Google Scholar]
- 69.Host U, Kelbaek H, et al. Effect of Ramipril on mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 1997;80:655–658. doi: 10.1016/s0002-9149(97)00445-1. [DOI] [PubMed] [Google Scholar]
- 70.Schon H. Hemodynamic and morphologic changes after long- term angiotensin converting enzyme inhibition in patients with chronic valvular regurgitation. J Hypertens. 1994;12:S95–104. [PubMed] [Google Scholar]
- 71.Wisenbaugh T, Sinovich V, et al. Six-month pilot study of captopril for mildly symptomatic severe isolated mitral regurgitation and isolated aortic regurgitation. J Heart Valve Dis. 1994;3:197–204. [PubMed] [Google Scholar]
- 72.Vahanian A, Alfieri O, et al. the Joint Task Force on the management of valvular heart disease of the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery Guidelines on the management of valvular heart disease. Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 73.Enriquez-Sarano M, Schaff HV, et al. Valve repair improves the outcome of surgery for mitral regurgitation: A multivariate analysis. Circulation. 1995;91(4):1022–1028. doi: 10.1161/01.cir.91.4.1022. [DOI] [PubMed] [Google Scholar]
- 74.Jokinen JJ, Hippelainen MJ, et al. Mitral valve replacement versus repair: Propensity adjusted survival and quality of life analysis. Ann Thorac Surg. 2007;84(2):451–458. doi: 10.1016/j.athoracsur.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 75.Moss RR, Humphries KH, et al. Outcome of mitral valve repair or replacement: A comparison by propensity score analysis. Circulation. 2003;108(Suppl II):90–97. doi: 10.1161/01.cir.0000089182.44963.bb. [DOI] [PubMed] [Google Scholar]
- 76.Shuhaiber J, Anderson RJ. Meta-analysis of clinical outcomes following mitral valve repair or replacement. Eur J Cardiothorac Surg. 2007;31(2):267–275. doi: 10.1016/j.ejcts.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Fedak PW, McCarthy PM, et al. Evolving concepts and technologies in mitral valve repair. Circulation. 2008;117(7):963–974. doi: 10.1161/CIRCULATIONAHA.107.702035. [DOI] [PubMed] [Google Scholar]
- 78.Adams DH, Anyanwu AC. Seeking a higher standard for degenerative mitral valve repair. Begin with etiology. J Thorac Cardiovasc Surg. 2008;136(3):551–556. doi: 10.1016/j.jtcvs.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 79.Verma S, Mesana TG. Mitral valve repair for mitral valve prolapse. N Engl J Med. 2009;361(23):2261–2269. doi: 10.1056/NEJMct0806111. [DOI] [PubMed] [Google Scholar]
- 80.Crawford MH, Souchek J, et al. Determinants of survival and left ventricular performance after mitral valve replacement. Circulation. 1990;81(4):1173–1181. doi: 10.1161/01.cir.81.4.1173. [DOI] [PubMed] [Google Scholar]
- 81.Enriquez-Sarano M, Tajik A, et al. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: Results and clinical implications. J Am Coll Cardiol. 1994;24(6):1536–1543. doi: 10.1016/0735-1097(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 82.Carabello BA. The current therapy of mitral regurgitation. J Am Coll Cardiol. 2008;52(5):319–326. doi: 10.1016/j.jacc.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 83.Bridgewater B, Hooper T, et al. Mitral repair best practice: Proposed standards. Heart. 2006;92(7):939–944. doi: 10.1136/hrt.2005.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castillo JG, Anyanwu AC, et al. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: Implications for future guidelines. J Thorac Cardiovasc Surg. 2012;144:308–312. doi: 10.1016/j.jtcvs.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 85.Gammie JS, O’Brien SM, et al. Influence of hospital procedural volume on care process ad mortality for patients undergoing elective surgery for mitral regurgitation. Circulation. 2007;115:881–887. doi: 10.1161/CIRCULATIONAHA.106.634436. [DOI] [PubMed] [Google Scholar]
- 86.Vassileva CM, Boley T, et al. Impact of hospital annual mitral procedural volume on mitral valve repair rates and mortality. J Heart Valve Dis. 2012;21(1):41–47. [PubMed] [Google Scholar]
- 87.Deloche A, Jebara V, et al. Valve repair with Carpentier techniques: the second decade. J Thorac Cardiovasc Surg. 1990;99(6):990–1001. [PubMed] [Google Scholar]
- 88.Wan S, Lee AP, et al. Mitral valve repair using a semirigid ring: Patient selection and early outcomes. Asian Cardiovasc Thorac Ann. 2016;24(7):647–652. doi: 10.1177/0218492316659970. [DOI] [PubMed] [Google Scholar]
- 89.Maslow A. Mitral valve repair: An echocardiographic review: Part 2. J Cardiothorac Vasc Anesth. 2015;29(2):439–471. doi: 10.1053/j.jvca.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Kongsaerepong V, Shiota M, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol. 2006;98(4):504–508. doi: 10.1016/j.amjcard.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 91.Cosgrove DM, Chavez AM, et al. Results of mitral valve reconstruction. Circulation. 1986;74(3):I82–87. [PubMed] [Google Scholar]
- 92.Carpentier A, Deloche A, et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg. 1971;61(1):1–13. [PubMed] [Google Scholar]
- 93.Filsoufi F, Carpentier A. Principles of reconstructive surgery in degenerative mitral valve disease. Semin Thorac Cardiovasc Surg. 2007;19(2):103–110. doi: 10.1053/j.semtcvs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Calafiore AM, Di Mauro M, et al. Longitudinal plication of posterior leaflet in myxomatous disease of the mitral valve. Ann Thorac Surg. 2006;81(5):1909–1910. doi: 10.1016/j.athoracsur.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz CF, Grossi EA, et al. Ten- year results of folding plasty in mitral valve repair. Ann Thorac Surg. 2010;89(2):485–488. doi: 10.1016/j.athoracsur.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 96.Perier P, Clausnizer B, et al. Carpentier “sliding leaflet” technique for repair of the mitral valve: Early results. Ann Thorac Surg. 1994;57(2):383–386. doi: 10.1016/0003-4975(94)91001-4. [DOI] [PubMed] [Google Scholar]
- 97.Bhuda SK, McCarthy PM, et al. Edge-to edge (Alfieri) mitral repair: Results in diverse clinical settings. Ann Thorac Surg. 2004;77(5):1598–1606. doi: 10.1016/j.athoracsur.2003.09.090. [DOI] [PubMed] [Google Scholar]
- 98.Magne J, Girerd N, et al. Mitral repair versus replacement for ischemic mitral regurgitation: Comparison of short- term and long- term survival. Circulation. 2009;120:S104–111. doi: 10.1161/CIRCULATIONAHA.108.843995. [DOI] [PubMed] [Google Scholar]
- 99.Acker MA, Parides MK, et al. Mitral valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370(1):23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kron IL, Hung J, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149(3):752–761. doi: 10.1016/j.jtcvs.2014.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calafiore AM, Di Mauro M, et al. Mitral valve surgery for chronic ischemic mitral regurgitation. Ann Thorac Surg. 2004;77:1989–1997. doi: 10.1016/j.athoracsur.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Kang DH, Kim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119(6):797–804. doi: 10.1161/CIRCULATIONAHA.108.802314. [DOI] [PubMed] [Google Scholar]
- 103.Montant P, Chenot F, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: A propensity score- based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg. 2009;138:1339–1348. doi: 10.1016/j.jtcvs.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 104.Suri RM, Vanoverschelde JL, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310(6):609–616. doi: 10.1001/jama.2013.8643. [DOI] [PubMed] [Google Scholar]
- 105.Goldstone AB, Patrick WL, et al. Early surgical intervention or watchful waiting for the management of asymptomatic mitral regurgitation: A systematic review and meta-analysis. Ann Cardiothorac Surg. 2015;4(3):220–229. doi: 10.3978/j.issn.2225-319X.2015.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feldman T, Kar S, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation. 5- year results of EVEREST II. J Am Coll Cardiol. 2015;66(25):2844–2854. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]