Abstract
Background
Effective oral hygiene measures carried out on a regular basis are vital to maintain good oral health. One‐to‐one oral hygiene advice (OHA) within the dental setting is often provided as a means to motivate individuals and to help achieve improved levels of oral health. However, it is unclear if one‐to‐one OHA in a dental setting is effective in improving oral health and what method(s) might be most effective and efficient.
Objectives
To assess the effects of one‐to‐one OHA, provided by a member of the dental team within the dental setting, on patients' oral health, hygiene, behaviour, and attitudes compared to no advice or advice in a different format.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 10 November 2017); the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 10) in the Cochrane Library (searched 10 November 2017); MEDLINE Ovid (1946 to 10 November 2017); and Embase Ovid (1980 to 10 November 2017). The US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were also searched for ongoing trials (10 November 2017). No restrictions were placed on the language or date of publication when searching the electronic databases. Reference lists of relevant articles and previously published systematic reviews were handsearched. The authors of eligible trials were contacted, where feasible, to identify any unpublished work.
Selection criteria
We included randomised controlled trials assessing the effects of one‐to‐one OHA delivered by a dental care professional in a dental care setting with a minimum of 8 weeks follow‐up. We included healthy participants or participants who had a well‐defined medical condition.
Data collection and analysis
At least two review authors carried out selection of studies, data extraction and risk of bias independently and in duplicate. Consensus was achieved by discussion, or involvement of a third review author if required.
Main results
Nineteen studies met the criteria for inclusion in the review with data available for a total of 4232 participants. The included studies reported a wide variety of interventions, study populations, clinical outcomes and outcome measures. There was substantial clinical heterogeneity amongst the studies and it was not deemed appropriate to pool data in a meta‐analysis. We summarised data by categorising similar interventions into comparison groups.
Comparison 1: Any form of one‐to‐one OHA versus no OHA
Four studies compared any form of one‐to‐one OHA versus no OHA.
Two studies reported the outcome of gingivitis. Although one small study had contradictory results at 3 months and 6 months, the other study showed very low‐quality evidence of a benefit for OHA at all time points (very low‐quality evidence).
The same two studies reported the outcome of plaque. There was low‐quality evidence that these interventions showed a benefit for OHA in plaque reduction at all time points.
Two studies reported the outcome of dental caries at 6 months and 12 months respectively. There was very low‐quality evidence of a benefit for OHA at 12 months.
Comparison 2: Personalised one‐to‐one OHA versus routine one‐to‐one OHA
Four studies compared personalised OHA versus routine OHA.
There was little evidence available that any of these interventions demonstrated a difference on the outcomes of gingivitis, plaque or dental caries (very low quality).
Comparison 3: Self‐management versus professional OHA
Five trials compared some form of self‐management with some form of professional OHA.
There was little evidence available that any of these interventions demonstrated a difference on the outcomes of gingivitis or plaque (very low quality). None of the studies measured dental caries.
Comparison 4: Enhanced one‐to‐one OHA versus one‐to‐one OHA
Seven trials compared some form of enhanced OHA with some form of routine OHA.
There was little evidence available that any of these interventions demonstrated a difference on the outcomes of gingivitis, plaque or dental caries (very low quality).
Authors' conclusions
There was insufficient high‐quality evidence to recommend any specific one‐to‐one OHA method as being effective in improving oral health or being more effective than any other method. Further high‐quality randomised controlled trials are required to determine the most effective, efficient method of one‐to‐one OHA for oral health maintenance and improvement. The design of such trials should be cognisant of the limitations of the available evidence presented in this Cochrane Review.
Plain language summary
One‐to‐one oral hygiene advice for oral health
Review question
The aim of this review was to assess the effects of one‐to‐one oral hygiene advice, provided by a member of the dental team within the dental setting, on patients' oral health, hygiene, behaviour, and attitudes compared to no advice or advice in a different format.
Background
Poor oral hygiene habits are known to be associated with high rates of dental decay and gum disease. The dental team routinely assess oral hygiene methods, frequency and effectiveness or otherwise of oral hygiene routines carried out by their patients; one‐to‐one oral hygiene advice is regularly provided by members of the dental team with the aim of motivating individuals and improving their oral health. The most effective method of delivering one‐to‐one advice in the dental setting is unclear. This review's aim is to determine if providing patients with one‐to‐one oral hygiene advice in the dental setting is effective and if so what is the best way to deliver this advice.
Study characteristics
Authors from Cochrane Oral Health carried out this review and the evidence is up to date to 10 November 2017. We included research where individual patients received oral hygiene advice from a dental care professional on a one‐to‐one basis in a dental clinic setting with a minimum of 8 weeks follow‐up.
In total, within the identified 19 studies, oral hygiene advice was provided by a hygienist in eight studies, dentist in four studies, dental nurse in one study, dentist or hygienist in one study, dental nurse and hygienist in one study, and dental nurse oral hygiene advice to the control group with further self‐administration of the intervention in one study. It was unclear in three of the studies which member of the dental team carried out the intervention. Over half of the studies (10 of the 19) were conducted in a hospital setting, with only five studies conducted in a general dental practice setting (where oral hygiene advice is largely delivered).
Key results
Overall we found insufficient evidence to recommend any specific method of one‐ to‐one oral hygiene advice as being more effective than another in maintaining or improving oral health.
The studies we found varied considerably in how the oral hygiene advice was delivered, by whom and what outcomes were looked at. Due to this it was difficult to readily compare these studies and further well designed studies should be conducted to give a more accurate conclusion as to the most effective method of maintaining or improving oral health through one‐to‐one oral hygiene advice delivered by a dental care professional in a dental setting.
Quality of the evidence
We judged the quality of the evidence to be very low due to problems with the design of the studies.
Summary of findings
Background
Description of the condition
Dental caries and periodontal disease are the two most prevalent dental conditions globally; both are largely preventable. The accumulation of dental plaque, a microbial biofilm on the tooth surface is a primary aetiological factor in both diseases (Löe 1965; Löe 1972).
When the microbial biofilm is exposed to carbohydrate sources via the host's diet it can lead to the localised lowering of the pH which results in the chemical dissolution of the tooth surface (Sheiham 2015). If this process goes undisturbed the caries process can lead to a lesion or 'cavity' in the exposed tooth. Although the affected carious tooth often remains asymptomatic in the early stages of the disease, the longer term consequences can be toothache, sepsis or ultimately tooth loss. Estimates of the prevalence of caries varies worldwide but the 2010 global burden of disease study estimated that untreated caries in permanent teeth was the most prevalent condition worldwide affecting an estimated 2.4 billion people (Kassebaum 2015). Self‐care prevention strategies include: reducing free sugars intake; disruption of plaque biofilm (toothbrushing and interdental cleaning aids); using a fluoride dentifrice or fluoride mouthwash or both (Marinho 2003; Marinho 2004a; Marinho 2004b; Marinho 2016). Self‐care strategies can be supplemented by professional interventions including fluoride gels and varnishes (Marinho 2003; Marinho 2004a; Marinho 2004b).
The accumulation of dental plaque also results in the inflammation of the periodontium (supporting structures) of the teeth. The undisturbed plaque biofilm initially causes swelling, loss of texture, characteristic redness of the gingiva and liability to gingival bleeding (gingivitis). At this early stage the disease is reversible by the disruption of the dysbiotic pathogenic biofilm allowing a return to healthy periodontal tissues. Periodontal disease, like dental caries often goes unnoticed by patients at its earliest stages. If left undisturbed in susceptible patients, gingival inflammation can lead to the irreversible loss of supporting structures (periodontitis), which can present as gingival recession, sensitivity of the exposed root, tooth mobility, drifting of the teeth or ultimately tooth loss. Studies suggest that 50% to 90% of adults in the UK and USA have gingivitis. The global burden of disease study estimated that severe periodontitis affects approximately 11% of the global population or 743 million people worldwide (Kassebaum 2014).
Oral self‐care prevention involves disruption of the plaque biofilm (toothbrushing and interdental cleaning aids) (Poklepovic 2013; Yaacob 2014) and control of systemic risk factors (e.g. smoking) (Tonetti 2017). There is evidence to suggest effective dental plaque control sustained over the longer term can achieve reduced experience of periodontal disease, dental caries and ultimately tooth mortality rates (Axelsson 2004).
Description of the intervention
The patient's own oral self‐care is a crucial component of the prevention strategies for dental caries and periodontal disease. Ultimately the aim of oral hygiene advice (OHA) is to enable a patient to improve their own oral self‐care and consequently their oral health. To improve a patient's oral self‐care, OHA can be delivered in a variety of formats by any appropriately trained member of the dental team. The content of OHA can include: toothbrushing and toothpaste advice, interdental cleaning advice, mouthwash advice, denture hygiene advice and/or orthodontic fixed/removable appliance hygiene advice.
How the intervention might work
OHA may work through improving patient knowledge, attitudes, and behaviours. Helping patients to understand their disease process and the preventative actions required may motivate them to change their oral self‐care behaviours thus reducing their risk of oral disease. In addition, OHA may improve patients' confidence in carrying out oral self‐care and/or highlight barriers and enablers to facilitate improved oral self‐care. Adequate oral hygiene regimens help prevent dental caries and periodontal disease.
Why it is important to do this review
Current clinical guidance recommends that OHA should be reinforced regularly and tailored to individual patients' needs to attain sustainable benefits (NICE 2015). Advice or information regarding toothbrushing is currently not uniformly provided to all patients. In England in 2009, 78% of adults reported receiving advice on cleaning their teeth and gums, an increase from 63% in 1998 (Chadwick 2011). Within the child population, oral health advice varies widely in both content and to whom it is provided (Tickle 2003) with younger children being less likely to receive advice than older children (Tsakos 2015).
Although OHA is taught as an obligatory part of the undergraduate dental curriculum (given OHA frequently forms part of routine dental care), there remains uncertainty as to how, where and by whom OHA should be provided to be effective. Previously, the main focus of preventing oral disease has been to increase patient knowledge and awareness regarding oral health, with the hope that this would lead to improved oral health status (Towner 1993). This has been superceded by oral health promotion, which aims not only to increase public knowledge, but also to utilise a multidisciplinary approach aimed at the underlying determinants of oral health (WHO 1986; WHO 2003). A systematic review of oral health promotion concluded that the evidence for long‐term reduction in plaque and gingival bleeding outcomes, based on mainly short‐term interventions, was limited. There were also conflicting conclusions regarding the relative effectiveness of different types/styles of educational interventions employed (Watt 2005). A recent systematic review of psychological approaches to behaviour change for improved plaque control in periodontal management concluded that interventions based on the use of goal setting, self‐monitoring and planning are effective in improving oral health‐related behaviours as assessed by oral health status (Newton 2015), although these conclusions were based upon observational studies as well as randomised controlled trials.
Although there are a variety of healthcare professionals who may be involved in the delivery of OHA, both within the dental surgery and within the wider community setting, there does not appear to be any systematic reviews published previously which assess the effect of the individual providing OHA.
Objectives
To assess the effects of one‐to‐one oral hygiene advice, provided by a member of the dental team within the dental setting, on patients' oral health, hygiene, behaviour, and attitudes compared to no advice or advice in a different format.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) in which participants who were provided with one‐to‐one oral hygiene advice (OHA) by a member of the dental team in the dental setting were compared to participants not provided with OHA. We also included RCTs which compared different formats (e.g. DVD, leaflet, video, etc.) of one‐to‐one OHA provided within the dental setting or OHA provided by different members of the dental team within the dental setting. We only included studies with a minimum follow‐up period of 8 weeks. The unit of randomisation could be individual participants, clusters of individuals, or dental setting.
Types of participants
We included studies that involved children (with or without guardian/parent) or adult participants who were fit and healthy or groups of individuals who had well defined medical conditions. Studies involving participants who wore a removable prosthesis or orthodontic appliance of any kind were excluded.
Types of interventions
We included studies that involved OHA being provided by any member of the dental team within the dental setting environment (i.e. within a general dental practice, community dental setting or dental hospital setting) on a one‐to‐one basis in which the comparator was no advice or advice in an alternative format or advice from a different member of the dental team. Dental team members included dentist, dental nurse, dental hygienist and dental therapist. Studies were included if the OHA took place alongside an intervention aiming to change dietary behaviour or smoking behaviour, although these multi‐intervention studies were planned to be subjected to a subgroup analysis if any such studies had met the inclusion criteria.
We excluded any studies where additional measures were provided to the intervention group but not the control group (e.g. dental prophylaxis, dental scaling, application or provision of fluoride containing products, provision of toothbrushes, etc.).
Types of outcome measures
Outcome measures considered in this review included clinical status, patient‐centred and economic factors. The outcome measure must have been assessed at least 8 weeks following the intervention.
Primary outcomes
Clinical status factors
Periodontal health (e.g. plaque levels, gingivitis, and probing depths).
Caries (e.g. dmft/DMFT or other indices).
Secondary outcomes
Patient‐centred factors
Patient‐reported behaviour changes (e.g. toothbrushing/flossing/mouthwash use).
Patient satisfaction with advice provided.
Patient‐reported changes in knowledge, attitudes, and quality of life.
Economic factors
Cost effectiveness.
Other outcomes
Any adverse events due to OHA reported in the included trials.
Search methods for identification of studies
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 10 November 2017) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 10) in the Cochrane Library (searched 10 November 2017) (Appendix 2);
MEDLINE Ovid (1946 to 10 November 2017) (Appendix 3);
Embase Ovid (1980 to 10 November 2017) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 10 November 2017) (Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 10 November 2017) (Appendix 6).
Bibliographic references of identified RCTs and articles identified as relevant to the review retrieved in the electronic search were also searched (e.g. review articles and systematic reviews). In addition, emails were sent to authors of identified potentially eligible RCTs asking them for other known unpublished or ongoing research. Relevant sections of the reports were translated of non‐English publications identified from title, abstract, or keyword, to be of interest.
Data collection and analysis
Selection of studies
The search was designed to be sensitive and included controlled clinical trials, these were filtered out early in the selection process if they were not randomised.
At least two review authors reviewed and analysed independently all reports identified by the search on the basis of title, keywords, and abstract (where this was available) to see if the study was likely to be relevant. Where it was not possible to classify an article based on title, keywords and abstract, we obtained the full article. The full report was obtained of all potentially eligible studies, and also of studies where the title/abstract provided insufficient information to make a decision on eligibility.
The review authors were not blinded with respect to report authors, journals, date of publication, sources of financial support, or results. The inclusion criteria, as outlined above, were applied and those studies deemed suitable for inclusion by at least two review authors were included. We contacted study authors for missing information or clarity regarding methods, results, etc. where required and feasible. We linked multiple publications of the same study under one single study title. Those studies excluded are cited with reasons for exclusion reported.
Two review authors independently and in duplicate assessed the eligibility of the non‐English language reports. Relevant sections of the reports were translated with the assistance of Cochrane Oral Health and non‐English reports that met the inclusion criteria had data extraction and risk of bias completed.
Data extraction and management
A data extraction form was designed and piloted prior to full use. At least two review authors independently extracted data from each of the potentially eligible studies; any disagreements between the two review authors undertaking data extraction was resolved by discussion and if necessary the involvement of a third review author. If necessary we contacted the study author, where possible, to clarify any unclear or inadequate characteristics before a final decision on inclusion was made. Data recorded included the following:
general study information: authors, title, year research completed, year research published, country, ethics and consent process, financial support and conflicts of interest, country, contact address;
methods: research objective, sample size calculation, allocation procedures, follow‐up period, degree of blindness in outcome assessment;
participants: inclusion/exclusion criteria, age, medical factors, baseline periodontal health/caries status/behaviours/knowledge/attitudes/quality of life;
intervention and comparators: type of OHA, format of OHA, advice duration, advice frequency, personnel providing advice;
outcomes: primary and secondary outcomes and outcome measures.
Assessment of risk of bias in included studies
The risk of bias of included trials for the seven domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources for bias was undertaken independently and in duplicate by at least two review authors as part of the data extraction process and in accordance with the guidelines in theCochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). Where possible, we contacted study authors for missing information or clarification of their study methods. Any disagreements were resolved by discussion between the review authors with Cochrane Oral Health being consulted where there was continuing disagreement.
We allocated the level of bias for each domain as high or low as per the criteria below; for those domains where insufficient information was available or not described in sufficient detail to allow a definitive judgement then the level of bias was recorded as unclear.
Random sequence generation
Low: the investigators describe a random component in the sequence generation process such as referring to a random number table, using a computer random number generator, etc..
High: the investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, (e.g. sequence generated by odd or even date of birth, etc.).
Allocation concealment
Low: participants and investigators enrolling participants could not foresee assignment because an appropriate method was used to conceal allocation (e.g. central allocation, etc.).
High: participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on using an open random allocation schedule, etc..
Blinding of participants and personnel
Low: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
High: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment
Low: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
High: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods.
High: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting
Low: the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
High: not all of this study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Other potential sources for bias
Low: the study appears to be free of other sources of bias.
High: there is as least one important risk of bias, for example a potential source of bias related to the specific study design used.
We summarised the risk of bias as follows.
| Risk of bias | In outcome | In included studies |
| Low risk of bias | Low risk of bias in all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | High risk of bias for one or more key domains | The proportion of information from studies at high risk is sufficient to affect the interpretation of results |
Measures of treatment effect
For continuous outcomes (e.g. plaque/gingivitis scores), where studies used the same scale, we used the mean values and standard deviations reported in the studies in order to express the estimate of effect of the intervention as mean difference (MD) with 95% confidence interval (CI). Where different scales were used, we expressed the treatment effect as standardised mean difference (SMD) and 95% CI.
For dichotomous outcomes (e.g. attachment loss/no attachment loss), we expressed the estimate of effect as a risk ratio (RR) with 95% CI. Had we included any cross‐over studies, we would have extracted appropriate data following the methods outlined by Elbourne 2002, and would have used the generic inverse variance method to enter log RRs or MD/SMD and standard error into Review Manager 5 (Review Manager 2014).
Unit of analysis issues
The participant was the unit of analysis. Had we included any cross‐over studies, these should have analysed data using a paired t‐test, or other appropriate statistical test, to take into account the paired nature of the data. Cluster‐RCTs should have analysed results taking account of the clustering present in the data, otherwise we would have used the methods outlined in Section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions in order to perform an approximately correct analysis (Higgins 2011).
Dealing with missing data
We attempted, where feasible, to contact the author(s) of studies to obtain missing data or for clarification. We did not use any further statistical methods or carry out any further imputation to account for missing data.
Assessment of heterogeneity
Heterogeneity was assessed by examining the types of participants, interventions and outcomes in each study. It was agreed in advance that meta‐analysis would only be attempted if studies of similar comparisons reporting the same outcome measures were included in the review.
If it had been appropriate to perform meta‐analysis, we would have assessed the possible presence of heterogeneity visually by inspecting the point estimates and CIs on the forest plots; if the CIs had poor overlap then heterogeneity would have been considered to be present. We would also have assessed heterogeneity statistically using a Chi2 test, where a P value < 0.1 would have been considered to indicate statistically significant heterogeneity. Furthermore, we would have quantified heterogeneity using the I2 statistic. A guide to interpretation of the I2 statistic given in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions is as follows (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Assessment of reporting bias within studies has already been described in the section Assessment of risk of bias in included studies. Reporting biases can occur when reporting (or not reporting) research findings is related to the results of the research (e.g. a study that did not find a statistically significant difference/result may not be published). Reporting bias can also occur if ongoing studies are missed (but that may be published by the time the systematic review is published), or if multiple reports of the same study are published, or if studies are not included in a systematic review due to not being reported in the language of the review authors. If there had been more than 10 studies included in a meta‐analysis, we would have assessed the possible presence of reporting bias by testing for asymmetry in a funnel plot. If present, we would have carried out statistical analysis using the methods described by Egger 1997 for continuous outcomes and Rücker 2008 for dichotomous outcomes. However, we did attempt to limit reporting bias in the first instance by conducting a detailed, sensitive search, including searching for ongoing studies, and any studies not reported in English were translated by a member of Cochrane Oral Health.
Data synthesis
We would only have carried out a meta‐analysis where studies of similar comparisons reported the same outcomes. We would have combined MDs (we would have used SMD where studies had used different scales) for continuous outcomes, and would have combined RRs for dichotomous outcomes, using a fixed‐effect model if there were only two or three studies, or a random‐effects model if there were four or more studies.
We would have used the generic inverse variance method to include data from cross‐over studies in meta‐analyses as described in Section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Elbourne 2002; Higgins 2011). Where appropriate, we would have combined the results from cross‐over studies with parallel group studies, using the methods described by Elbourne 2002.
Subgroup analysis and investigation of heterogeneity
If it had been required, subgroup analyses would have been undertaken where OHA had taken place alongside an intervention aiming to change dietary behaviour or smoking behaviour, personnel providing advice and frequency of advice.
If there had been a number of similar studies, sensitivity analysis would have been considered to determine whether conclusions reached would be affected by different inclusion criteria. However, given the heterogeneity of studies included this was not required.
Presentation of main results
We produced a 'Summary of findings' table for each comparison. We included gingivitis, plaque and dental caries. We used GRADE methods (GRADE 2004), and the GRADEpro GDT online tool for developing 'Summary of findings' tables (www.guidelinedevelopment.org). We assessed the quality of the body of evidence for each comparison and outcome by considering the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias. We categorised the quality of each body of evidence as high, moderate, low, or very low.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The literature search resulted in 15,188 references following de‐duplication. In addition, we also handsearched the references of seven relevant systematic reviews (Harris 2012; Kay 1998; Kay 2016a; Kay 2016b; Khokhar 2016; Newton 2015; Yevlahova 2009) and two reviews (Pastagia 2006; Watt 2005), identifying two further studies (Hetland 1982; Little 1997). Four review authors screened the titles and abstracts against the inclusion criteria for this review, independently and in duplicate, and 15,076 references were found to be ineligible for this review (including four potentially eligible references but they had abstracts‐only published with no further information regarding the study available). Full‐text copies of the remaining references were obtained and examined independently and in duplicate, excluding 84 studies at this stage. Two studies are awaiting classification and four are ongoing. Nineteen studies reported in 24 papers met the inclusion criteria for this review. Handsearching of the references of included studies and correspondence from authors identified no further studies for inclusion. This process is illustrated in Figure 1 PRISMA flow diagram.
1.
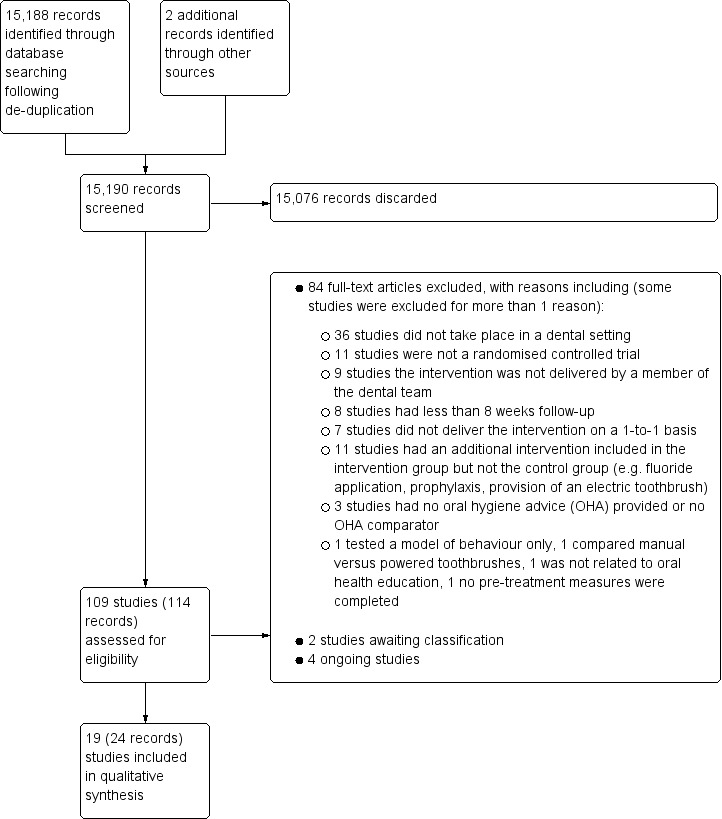
Study flow diagram.
Included studies
Characteristics of trial design and setting
Nineteen studies met the inclusion criteria of the review and were included (Characteristics of included studies). All 19 studies were of parallel‐group design, 10 of which had two trial arms (Aljafari 2017; Baab 1986; Bali 1999; Glavind 1985; Jönsson 2006; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Münster Halvari 2012; Tedesco 1992), four of which had three arms (Hetland 1982; Hoogstraten 1983; Memarpour 2016; Söderholm 1982), two of which had four arms (Hugoson 2007; Weinstein 1996), and two of which had six arms (Schmalz 2018; Van Leeuwen 2017). One study was a cluster‐randomised design with oral hygiene advice (OHA) randomised by cluster (routine or personalised OHA) and scale and polish treatment by parallel randomisation with three arms (Ramsay 2018).
Ten of the included studies were conducted in hospital/graduate clinics (Aljafari 2017; Baab 1986; Bali 1999; Jönsson 2006; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Schmalz 2018; Söderholm 1982; Van Leeuwen 2017), four in a dental practice setting (Glavind 1985; Hoogstraten 1983; Münster Halvari 2012; Ramsay 2018), one in public healthcare settings (Memarpour 2016), one study involved one clinic in a public dental setting and the other a general dental practice (Hugoson 2007), one was in a factory which had two dental units and chairs installed for the purpose of the study (Hetland 1982), and two trials did not specify which dental setting they were conducted in (Tedesco 1992; Weinstein 1996).
Fifteen of the studies were single centre with one study reporting two centres (Hugoson 2007), one study with three centres (Glavind 1985), one study with five centres (Memarpour 2016), and one study with 63 centres (Ramsay 2018).
Four studies were conducted in Sweden (Hugoson 2007; Jönsson 2006; Jönsson 2009; Söderholm 1982), three in the USA (Baab 1986; Lepore 2011; Tedesco 1992), two in the Netherlands (Hoogstraten 1983; Van Leeuwen 2017), two in Norway (Hetland 1982; Münster Halvari 2012), one in Germany (Schmalz 2018), and one in Denmark (Glavind 1985), Spain (López‐Jornet 2014), Italy (Weinstein 1996), England (Aljafari 2017), Austria (Bali 1999), Iran (Memarpour 2016), and one study across the United Kingdom in Scotland and North East England (Ramsay 2018).
Only seven studies mentioned sample size calculations (Aljafari 2017; Jönsson 2009; Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Van Leeuwen 2017). Four of these studies achieved their sample sizes and had the required number of participants at follow‐up (Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018). Jönsson 2009 reported their sample size calculation but noted that they did not recruit or follow up this number of participants; the original examiner could not continue to complete recruitment and a second examiner could not be recruited in time. The authors also noted that the original power analysis was based on an intervention that was less effective than the intervention investigated in their study. Aljafari 2017 reported that only 55% of the recruited sample completed the telephone follow‐up 3 months after the child's dental care under general anaesthetic; the authors recommended the results be interpreted with caution given the sample size calculation deemed a sample of 45 participants in each group would have been required to provide 80% power, at the 5% significance level, to detect effects of 0.6 and above. At 3‐month follow‐up there were 28 patients and 31 patients available from the intervention and control group, respectively (Aljafari 2017). Van Leeuwen 2017 achieved their sample size in five of the six groups at 13 months such that the recruitment target was achieved at the 4‐month review for all groups, but not thereafter.
Eleven studies reported their funding source with six having received some form of public funding (Aljafari 2017; Baab 1986; Hugoson 2007; Münster Halvari 2012; Ramsay 2018; Tedesco 1992), one with dental association research funds (Glavind 1985), one with national health research and development funding (Van Leeuwen 2017 ), one with a university grant (Memarpour 2016), and one study reported joint funding sources between research council, public funding and the Pfizer oral care award (Jönsson 2009). One study acknowledged Philips GmbH (Hamburg, Germany), CP GABA (GmbH, Hamburg, Germany), and GlaxoSmithKline Oral Health Care GmbH (Brühl, Germany) for providing materials for the study (Schmalz 2018).
Characteristics of the participants
A total of 4232 participants were recruited, with numbers included in each trial ranging from 33 to 1877. Three studies investigated children with a mean age of 20 months, 3 years and six years old and age ranges from 1 year to 10 years old (Aljafari 2017; Lepore 2011; Memarpour 2016). In the remaining adult population studies, the age range of the sample was reported in 15 studies with the mean age of adults ranging from 20 years to 58 years old and age range from 15 years to 91 years old (Baab 1986; Bali 1999; Glavind 1985; Hetland 1982; Hoogstraten 1983; Jönsson 2006; Jönsson 2009; López‐Jornet 2014; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996).
Two studies restricted their inclusion criteria to participants who were known to have a specific medical condition: diabetes (Bali 1999) and hyposalivation (López‐Jornet 2014).
The dental condition pre‐treatment required participants to be caries free in three studies (Memarpour 2016; Schmalz 2018; Van Leeuwen 2017) and only those with caries were included in another study (Aljafari 2017). Eight studies reported that the included individuals were only those with plaque present (Glavind 1985) or those who had active or previously treated periodontal disease patients (Baab 1986; Glavind 1985; Jönsson 2006; Jönsson 2009; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996). One study reported including those individuals with healthy periodontal status, gingivitis or moderate periodontal disease (Ramsay 2018). One study specified that there were to be no periodontal pockets ≥4.0 mm, as measured by a pocket probe, and/or serious bone loss visualized by digital X rays during the dental examination (Münster Halvari 2012), and another specified there to be no periodontal attachment loss greater than 1.5 mm, radiographic evidence of bone loss greater than 25% (anterior/posterior bite‐wing radiographs or periapical radiographs), or previous periodontal therapy (Tedesco 1992); another specified there to be no pockets of 4 mm to 5 mm in combination with gingival recession or pockets of ≥ 6 mm, as assessed according to the Dutch Periodontal Screening Index (DPSI) scores 3+ and 4 (Van Leeuwen 2017).
Characteristics of the interventions
Intervention type
A variety of interventions were used in the included studies, none of which were entirely identical.
Two studies used self‐inspection/instruction manuals (Baab 1986; Glavind 1985) with a further incorporating self‐assessment as part of the intervention (Weinstein 1996).
One study reported participants received one‐to‐one advice on toothbrushing technique (Schmalz 2018).
One used a computer game (Aljafari 2017), one included instruction and viewing of an educational film (Hoogstraten 1983), and another included showing patients a phase‐contrast slide of their own subgingival flora on a video monitor (Tedesco 1992).
One study reported the intervention group receiving once‐only professional individual oral hygiene instruction (Van Leeuwen 2017), one with a three‐visit oral hygiene instruction program delivered on a once a week basis (Hetland 1982), one with intensive patient education in oral hygiene with additional control examinations (Bali 1999), another providing oral hygiene instruction in addition to a pamphlet and toothbrush (Memarpour 2016), or similar instruction at a variable number of appointments (Söderholm 1982).
One study reported a motivational–behavioural skills protocol designed following principles of self‐efficacy theory (López‐Jornet 2014).
Six studies provided information tailored to the patients needs (Bali 1999; Hugoson 2007; Jönsson 2009; Lepore 2011; Ramsay 2018; Weinstein 1996), one of which was with the addition of cognitive behavioural therapy (Jönsson 2009), another including Social Cognitive Theory and Implementation Intention Theory (Ramsay 2018) and another with the inclusion of positive social reinforcement (Weinstein 1996).
A negotiation and self‐selected goals method described as Client Self‐Care Commitment Model was reported in one study (Jönsson 2006).
A competency enhancing intervention in addition to standard autonomy‐supportive treatment was described in one study (Münster Halvari 2012).
Member of dental team delivering intervention
It was unclear in three of the studies which member of the dental team carried out the intervention (Bali 1999; López‐Jornet 2014; Weinstein 1996) but after reviewing the text the review authors were confident the intervention was undertaken by a member of the dental team. In the remainder of the studies the interventions were delivered by various members of the dental team.
A hygienist delivered the intervention in eight of the studies (Baab 1986; Hoogstraten 1983; Hugoson 2007; Jönsson 2006; Jönsson 2009; Münster Halvari 2012; Tedesco 1992; Van Leeuwen 2017).
A dentist delivered the intervention in four studies (Glavind 1985; Lepore 2011; Memarpour 2016; Schmalz 2018).
A dentist or hygienist delivered the intervention in one study (Ramsay 2018).
A hygienist and dental nurse delivered the intervention in one study (Söderholm 1982).
A dental nurse delivered the intervention in one study (Hetland 1982) and control OHA in another study (Aljafari 2017).
Further self‐administration of the intervention at home took place in one study (Aljafari 2017).
Frequency of intervention
The frequency of the intervention varied with three studies reporting the intervention to be delivered on a one‐off basis (Lepore 2011; Schmalz 2018; Van Leeuwen 2017).The remaining studies reported various intervention frequencies other than one study being unclear (Hoogstraten 1983).
Two studies reported two intervention visits (Glavind 1985; Memarpour 2016).
Two studies reported three intervention visits (Hetland 1982; Jönsson 2006).
Four studies reported four intervention visits (Bali 1999; López‐Jornet 2014; Münster Halvari 2012; Tedesco 1992).
Two studies reported five intervention visits (Baab 1986; Söderholm 1982).
One study reported three or six intervention visits (Hugoson 2007).
One intervention was initially delivered in the clinic and then used at home as per the patients wishes (Aljafari 2017), one study reported a median of nine intervention visits (Jönsson 2009), and one reported reinforcement of OHA was provided at the discretion of the dentist/hygienist during the trial and recorded (Ramsay 2018). The study by Weinstein 1996 varied in the frequency of intervention from three clinical visits with twice weekly phone call to periodontist to report self‐evaluated plaque score, 16 visits with OHA (twice weekly examinations by periodontist) or 16 visits with OHA (twice weekly examinations by periodontist) and daily completion of an oral hygiene task checklist.
Intensity of intervention
The intensity of the intervention was not reported in nine studies (Baab 1986; Bali 1999; Jönsson 2006; Lepore 2011; Ramsay 2018; Schmalz 2018; Tedesco 1992; Weinstein 1996; Van Leeuwen 2017), two were largely administered at home (Aljafari 2017; Glavind 1985), one remained unclear (López‐Jornet 2014), and one varied between patients (Jönsson 2009).
For those studies who did report the intensity of the intervention, the intensity varied considerably.
Approximately 30 minutes to 40 minutes intervention noted in one study (Hoogstraten 1983).
Two intervention appointments of 30 minutes to 40 minutes each in one study (Memarpour 2016).
Forty‐five minutes for the first intervention visit, 30 minutes for the second intervention visit and 15 minutes for the third intervention visit in one study (Hetland 1982).
Approximately 45 minutes intervention was noted in one study (Münster Halvari 2012).
Between 120 minutes and 150 minutes was reported in two studies (Hugoson 2007; Söderholm 1982).
Prophylaxis as part of intervention
Twelve studies reported some form of prophylactic measures at baseline (Baab 1986; Glavind 1985; Hetland 1982; Hoogstraten 1983; Hugoson 2007; Jönsson 2006; Lepore 2011; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992). One study reported prophylactic measures throughout (Jönsson 2009) and six did not report any prophylactic measures (Aljafari 2017; Bali 1999; López‐Jornet 2014; Memarpour 2016; Van Leeuwen 2017; Weinstein 1996).
Characterictics of the outcomes
Primary outcomes ‐ Clinical status
Periodontal health
Gingivitis
Fifteen studies reported on the presence of gingivitis (Baab 1986; Bali 1999; Glavind 1985; Hetland 1982; Hugoson 2007; Jönsson 2006; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017).
Of these studies:
the gingival index by Löe and Sillness was used by seven studies (Hetland 1982; Hugoson 2007; Jönsson 2006; Jönsson 2009; Münster Halvari 2012; Schmalz 2018; Tedesco 1992);
bleeding on probing was reported in five studies (Baab 1986; Bali 1999; Glavind 1985; Ramsay 2018; Söderholm 1982);
gingival inflammation evaluated using the Papilla Bleeding Index (PBI) (Lange 1977) was reported in one study (Schmalz 2018);
one study (Van Leeuwen 2017) reported that gingival health was assessed at six sites (mesio‐buccal, mid‐buccal, disto‐buccal, mesio‐lingual, mid‐lingual and disto‐lingual) around the selected quadrants by scoring bleeding on marginal probing (BOMP) on a scale of 0 to 2 (Lie 1998; Van der Weijden 1994);
one study reported that gingival health was recorded but did not specify what scoring system was employed (Lepore 2011);
one study reported a bleeding index was recorded but did not specify what scoring system was employed (López‐Jornet 2014).
Probing depth
Four studies reported on probing depths (Bali 1999; Jönsson 2006; Jönsson 2009; Ramsay 2018). One study reported on Community Periodontal Index of Treatment Needs (CPITN) score (López‐Jornet 2014) with an additional study only reporting pocket depths of > 5 mm (Söderholm 1982), and a further study only reporting on pocket depths > 4 mm (Hetland 1982).
Plaque levels
Fifteen studies reported on plaque levels using various plaque indices (Baab 1986; Bali 1999; Glavind 1985; Hetland 1982; Hugoson 2007; Jönsson 2006; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Münster Halvari 2012; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996).
Of these studies:
three studies (Hugoson 2007; Münster Halvari 2012; Tedesco 1992) used the Löe and Sillness plaque index (Löe 1963; Löe 1967) with a further two studies (Jönsson 2006; Jönsson 2009) reporting the accumulation of plaque being recorded using Silness and Löe (Silness 1964);
one study (López‐Jornet 2014) reported a plaque extension index (PEI) following plaque disclosure using a 2% aqueous erythrosine solution. After rinsing once with water, plaque deposits were assessed using the Quigley and Hein index, modified by Turesky et al (Turesky 1970), with scores from 0 to 5 with another study (Van Leeuwen 2017) reporting a similar process by measuring plaque at six sites after disclosing with Mira‐2‐Ton® with scores also based on the modified Quigley and Hein (Quigley 1962) plaque index (QHPI) with a scale of 0 to 5. One study reported a similar method with plaque extension evaluated using the plaque index by Quigley and Hein (QHI) modified by Turesky et al after using a plaque disclosing agent (Mira‐2‐Ton®, Hager score 5 = plaque extending to the coronal third) in addition to recording the Marginal Plaque Index (MPI) by Deinzer et al to differentiate plaque extension at the gingival margin (Deinzer 2014) (Schmalz 2018). A further study also reported using the Turesky hygiene index (Bali 1999);
two studies (Baab 1986; Weinstein 1996) reported on presence or absence of disclosed plaque at the gingival margin (O'Leary 1972);
two studies reported on the presence of disclosed plaque; one did not refer to a specific index (Glavind 1985) and the other reported the percentage of tooth surfaces (mesial, buccal, distal and lingual) with plaque (Söderholm 1982);
two studies reported that a plaque score was recorded but did not specify what scoring system was employed (Hetland 1982; Lepore 2011).
Dental caries
Two studies reported on DMFT (number of decayed, missing or filled permanent teeth) (Bali 1999; Hetland 1982). One study reported on dmft (number of decayed, missing or filled primary teeth) (Memarpour 2016), and a further study reported on dental caries but appeared to use the terms DMFS (number of decayed, missing or filled permanent surfaces) and dmft interchangeably within the text of the primary paper (Lepore 2011).
Secondary outcomes ‐ Patient‐centred factors
Patient‐reported behaviour changes
Five studies reported on patient‐reported behaviour change regarding:
time spent brushing and the number of oral hygiene aids used (Baab 1986);
child's dietary habits, and the child's self‐reported snacking and toothbrushing practices (Aljafari 2017);
oral health behaviour (Ramsay 2018);
oral self‐care habits (Jönsson 2006);
self‐reported brushing and flossing behaviours and self‐efficacy assessed via Oral Health Behavior Expectation Scale (Tedesco 1992).
One of the studies specifically reported on patient‐reported health indices:
the General Oral Health Assessment Index (GOHAI) and self‐rated oral health (Jönsson 2009).
Patient satisfaction with advice provided
One study reported on patient satisfaction with provider of advice and patient satisfaction with advice format:
parent and child satisfaction with their educational intervention (Aljafari 2017).
Patient‐reported changes in knowledge, attitudes and quality of life
Five studies reported on patient‐reported changes in knowledge, attitudes and quality of life following provision of advice:
child's dietary knowledge (Aljafari 2017);
dental knowledge, attitude, behaviour, and fear of dental treatment (Hoogstraten 1983; Jönsson 2009);
patient‐related dental quality of life and confidence in oral hygiene self‐efficacy (Slade 1997) (Ramsay 2018);
parents' knowledge and performance regarding oral health (Memarpour 2016);
oral health‐related quality of life measure (OHQoL‐UK) (Jönsson 2009).
One study used multiple psychological outcome measures including (Münster Halvari 2012):
autonomy orientation (Dental Care Autonomy Orientation Scale) adapted in the present study from the Exercise Causality Orientations Scale (Rose 2001) and the General Causality Orientations Scale (Deci 1985);
perceived autonomy support (6‐item version of the Health Care Climate Questionnaire (Williams 1996));
autonomous motivation for the dental project (Evaluation of Dental Project Scale (Halvari 2006));
autonomous motivation for dental home care (a 3‐item identified subscale of the Self‐Regulation for Dental Home Care Questionnaire (Halvari 2012));
perceived dental competence (Dental Coping Beliefs Scale (Wolfe 1996)) using the five items with the best factor loadings (Halvari 2006) and two added items from a previous study (Halvari 2010);
dental health behaviour assessed by a 4‐item formative composite scale (Halvari 2010).
A further study (Tedesco 1992) reported on the theory of reasoned action variables assessed via Theory of Reasoned Action Oral Health Scale (Fishbein 1980).
Secondary outcome ‐ Economic factors
Only one study reported on economic net benefits of OHA (Ramsay 2018).
Other outcomes
No adverse events due to OHA were reported in the included trials.
Excluded studies
We excluded 84 studies from the review (see Characteristics of excluded studies). Below is a summary of the reasons for excluding these studies (some studies were excluded for more than one reason).
Thirty‐six studies did not take place in a dental setting.
Eleven studies were not a randomised controlled trial.
Nine studies the intervention was not delivered by a member of the dental team.
Eight studies had less than 8 weeks follow‐up.
Seven studies did not deliver the intervention on a one‐to‐one basis.
Eleven studies had an additional intervention included in the intervention group but not the control group (e.g. fluoride application, prophylaxis, provision of an electric toothbrush).
Three studies had no OHA provided or no OHA comparator.
One study tested a model of behaviour only.
One study compared manual versus powered toothbrushes.
One study was not related to oral health education.
In one study no pre‐treatment measures were completed.
Awaiting classification studies
The authors of two published trial protocols were contacted for further information but no reply was received (Gao 2013; IRCT2014062618248N1).
Ongoing studies
The author of one ongoing trial confirmed that data collection was completed but data analysis was not complete at the current time (ACTRN12605000607673).
Risk of bias in included studies
We assessed risk of bias based on the information reported in the included studies in the first instance. We subsequently contacted authors for further information for missing information or clarification. Two authors replied and provided information for two studies (Aljafari 2017; Schmalz 2018).
Allocation
Random sequence generation
We assessed nine studies to be at low risk of bias for this domain (Aljafari 2017; Baab 1986; Hugoson 2007; Jönsson 2006; Jönsson 2009; López‐Jornet 2014; Memarpour 2016; Ramsay 2018; Van Leeuwen 2017). There were 10 studies that had insufficient information to make a judgement and we assessed them as at unclear risk of bias (Bali 1999; Glavind 1985; Hetland 1982; Hoogstraten 1983; Lepore 2011; Münster Halvari 2012; Schmalz 2018; Söderholm 1982; Tedesco 1992; Weinstein 1996).
Allocation concealment
We assessed four studies to be at low risk of bias for this domain (Aljafari 2017; Jönsson 2009; Ramsay 2018; Van Leeuwen 2017). None of the studies assessed were deemed to be at high risk of bias for this domain. There were 15 studies that had insufficient information to make a judgement and we assessed them as at unclear risk of bias (Baab 1986; Bali 1999; Glavind 1985; Hetland 1982; Hoogstraten 1983; Hugoson 2007; Jönsson 2006; Lepore 2011; López‐Jornet 2014; Memarpour 2016; Münster Halvari 2012; Schmalz 2018; Söderholm 1982; Tedesco 1992; Weinstein 1996).
Blinding
Blinding of participants and personnel (performance bias)
We assessed all 19 studies to be at unclear risk of bias for this domain (Aljafari 2017; Baab 1986; Bali 1999; Glavind 1985; Hetland 1982; Hoogstraten 1983; Hugoson 2007; Jönsson 2006; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996). It is not possible to blind participants to this intervention and it is unclear the influence this would have on the risk of bias for this domain.
Blinding of outcome assessment (detection bias)
We assessed 13 studies to be at low risk of bias for this domain (Aljafari 2017; Baab 1986; Hetland 1982; Hoogstraten 1983; Hugoson 2007; Jönsson 2006; Jönsson 2009; López‐Jornet 2014; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Van Leeuwen 2017). There were six studies that had insufficient information to make a judgement and we assessed them as at unclear risk of bias (Bali 1999; Glavind 1985; Lepore 2011; Memarpour 2016; Tedesco 1992; Weinstein 1996).
Incomplete outcome data
We assessed 11 studies to be at low risk of bias for this domain (Glavind 1985; Jönsson 2006; Jönsson 2009; López‐Jornet 2014; Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017). We assessed two studies at high risk of bias for this domain (Aljafari 2017; Bali 1999). There were six studies that had insufficient information to make a judgement and we assessed them as at unclear risk of bias (Baab 1986; Hetland 1982; Hoogstraten 1983; Hugoson 2007; Lepore 2011; Weinstein 1996).
Selective reporting
We assessed 16 studies to be at low risk of bias for this domain (Aljafari 2017; Baab 1986; Glavind 1985; Hetland 1982; Hoogstraten 1983; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996). We assessed one study at high risk of bias for this domain (Hugoson 2007). There were two studies that had insufficient information to make a judgement and we assessed them as at unclear risk of bias (Bali 1999; Jönsson 2006).
Other potential sources of bias
We assessed 16 studies to be at low risk of bias for this domain (Aljafari 2017; Bali 1999; Glavind 1985; Hetland 1982; Hugoson 2007; Jönsson 2006; Jönsson 2009; López‐Jornet 2014; Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996). There were three studies that had insufficient information to make a judgement and we assessed them as at unclear risk of bias (Baab 1986; Hoogstraten 1983; Lepore 2011).
Overall risk of bias
We assessed none of the studies as being at low risk of bias.
We assessed three studies as being at high risk of bias. These studies had at least one domain assessed as high risk of bias (Aljafari 2017; Bali 1999; Hugoson 2007).
We assessed 16 studies as being at unclear risk of bias. These studies had at least one domain judged to be at unclear risk of bias, but no domains judged to be at high risk of bias (Baab 1986; Glavind 1985; Hetland 1982; Hoogstraten 1983; Jönsson 2006; Jönsson 2009; Lepore 2011; López‐Jornet 2014; Memarpour 2016; Münster Halvari 2012; Ramsay 2018; Schmalz 2018; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996).
The results of the risk of bias assessments are presented graphically in Figure 2 and Figure 3.
2.
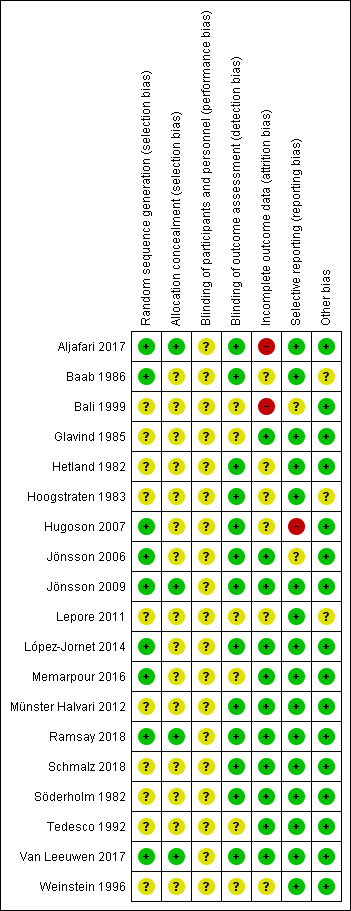
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
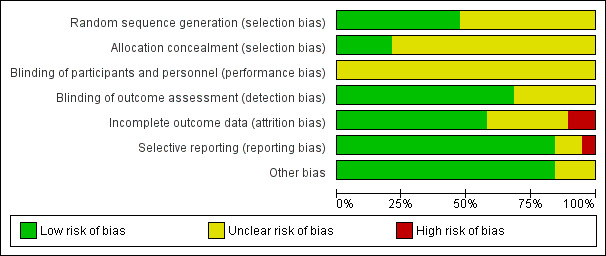
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Summary of findings ‐ Any form of one‐to‐one oral hygiene advice (OHA) versus no OHA.
| Any form of 1‐to‐1 OHA versus no OHA in a dental setting for oral health | |||
|
Patient or population: children or adults Settings: dental surgery/office setting Intervention: any form of 1‐to‐1 OHA Comparison: no OHA | |||
| Outcomes | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Periodontal health: gingivitis |
477 participants (2 studies); follow‐up: 235 participants | ⊕⊝⊝⊝ very low1 | 1 small study in adults had contradictory results at 3 months and 6 months The other study in adults showed evidence of a benefit for OHA at all time points of 12 months, 24 months, and 36 months |
| Periodontal health: plaque levels |
477 participants (2 studies); follow‐up: 235 participants | ⊕⊕⊝⊝ low2 | Both studies at all time points consistently showed a benefit in plaque reduction for OHA |
| Dental caries | 377 participants (2 studies); follow‐ up: 244 participants | ⊕⊝⊝⊝ very low3 | 1 study in adults reported only "small and statistically insignificant changes" but no usable data reported The other study on infants provided very low‐quality evidence of a benefit for OHA at 8 months and 12 months, but not at 4 months |
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||
11 study at high risk and 1 at unclear risk of bias. 1 study small with contradictory results. Inconsistency between studies, unable to pool data. The number of appointments and intensity of the interventions would not be applicable in routine dental practice. Downgraded for risk of bias, inconsistency and indirectness. 21 study at high risk and 1 at unclear risk of bias. The number of appointments and intensity of the interventions would not be applicable in routine dental practice. Downgraded for risk of bias and indirectness. 32 unclear risk of bias studies. Inconsistency between studies, unable to pool data. The number of appointments and intensity of the interventions would not be applicable in routine dental practice. Downgraded for risk of bias and inconsistency and indirectness.
Summary of findings 2. Summary of findings ‐ Personalised one‐to‐one OHA versus routine one‐to‐one OHA.
| Personalised 1‐to‐1 OHA versus routine 1‐to‐1 OHA in a dental setting for oral heath | |||
|
Patient or population: children or adults Settings: dental surgery/office setting Intervention: personalised 1‐to‐1 OHA in a dental setting Comparison: routine 1‐to‐1 OHA in a dental setting | |||
| Outcomes | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Periodontal health: gingivitis |
2209 participants (4 studies); follow‐up: 1635 participants | ⊕⊝⊝⊝ very low1 | 1 large study in adults showed little or no difference between groups at 36 months 1 study in adults showed evidence of a benefit for personalised OHA at 3 months and 12 months 1 study in children reported "statistically significant (P < 0.05) improvement" for personalised OHA but did not report usable data 1 study showed little or no difference between groups at 3 months |
| Periodontal health: plaque levels |
332 participants (3 studies); follow‐up: 308 participants | ⊕⊝⊝⊝ very low2 | 1 study in adults showed evidence of a benefit for personalised OHA at 3 months and 12 months 1 study in children reported "statistically significant (P < 0.05) improvement" for personalised OHA but did not report usable data 1 study showed little or no difference between groups at 3 months |
| Dental caries | 69 participants (1 study); follow‐ up: 69 participants | ⊕⊝⊝⊝ very low3 | 1 study in children between the ages of 1 year and 6 years old reported "statistically significant (P < 0.05) improvement" for personalised OHA but did not report usable data |
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||
14 unclear risk of bias studies. Inconsistency between studies, unable to pool data. Setting of 3 studies is secondary care, and intervention in 1 study (number of appointments and/or intensity required) is not applicable to routine dental practice. Downgraded for risk of bias, inconsistency and indirectness. 23 unclear risk of bias studies. Inconsistency between studies, unable to pool data. Setting of 3 studies is secondary care, and intervention in 1 study (number of appointments and/or intensity required) is not applicable to routine dental practice. Downgraded for risk of bias, inconsistency and indirectness. 31 unclear risk of bias study. No usable data. Setting not applicable to routine dental practice, so downgraded for indirectness.
Summary of findings 3. Summary of findings ‐ Self‐management versus professional OHA.
| Self‐management versus professional OHA in a dental setting for oral heath | |||
|
Patient or population: children or adults Settings: dental surgery/office setting Intervention: self‐management Comparison: professional OHA in a dental setting | |||
| Outcomes | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Periodontal health: gingivitis |
185 participants (4 studies); follow‐up: 181 participants | ⊕⊝⊝⊝ very low1 | 1 study in adults reported "significant differences between groups at each observation were not found" but no usable data reported 1 study in adults reported "no statistically significant differences in gingival bleeding scores were found between the 2 treatment groups at any of the 3 examinations" but no usable data reported 1 study in adults showed little or no difference between groups at 3 months 1 study in adults with hyposalivation provided weak evidence of a benefit in gingivitis for professional OHA at 2 months |
| Periodontal health: plaque levels |
185 participants (4 studies); follow‐up: 181 participants | ⊕⊝⊝⊝ very low1 | 1 study in adults reported "mean plaque scores did not differ significantly between groups" but no usable data reported 1 study in adults reported "no statistically significant differences were found between the 2 groups at any of the examination times" but no usable data reported 1 study in adults provided weak evidence of a benefit in plaque reduction at 3 months for self‐management 1 study in adults with hyposalivation provided very weak evidence of a benefit in plaque reduction for professional OHA at 2 months |
| Dental caries | No studies were found that looked at dental caries | ||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||
14 unclear risk of bias studies. Inconsistency between studies, unable to pool data. 3 studies were in secondary care and 3 of the interventions of 3 trials (number of appointments and/or intensity required) are not applicable to routine dental practice therefore we also downgraded for indirectness.
Summary of findings 4. Summary of findings ‐ Enhanced one‐to‐one OHA versus one‐to‐one OHA.
| Enhanced 1‐to‐1 OHA versus 1‐to‐1 OHA in a dental setting for oral heath | |||
|
Patient or population: children or adults Settings: dental surgery/office setting Intervention: enhanced 1‐to‐1 OHA in a dental setting Comparison: 1‐to‐1 OHA in a dental setting | |||
| Outcomes | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Periodontal health: gingivitis |
782 participants (5 studies); follow‐up: 430 participants | ⊕⊝⊝⊝ very low1 | 2 studies in adults did not report usable data 1 study in adults provided low‐quality evidence of a benefit in gingivitis reduction at 5.5 months for enhanced OHA 3 studies found little or no difference between groups across all time points |
| Periodontal health: plaque levels |
802 participants (6 studies); follow‐up: 440 participants | ⊕⊝⊝⊝ very low2 | 2 studies in adults did not report usable data 1 study in adults provided low‐quality evidence of a benefit in plaque reduction at 5.5 months for enhanced OHA 1 study in adults found little or no difference between groups at 3 months 1 study in adults found little or no difference between groups across all time points 1 study in adults provided weak evidence of a benefit in plaque reduction for 1 of the enhanced OHA at 2 months |
| Dental caries | 121 participants (1 study); follow‐ up: 70 participants | ⊕⊝⊝⊝ very low3 | 1 study in adults did not report usable data |
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||
11 study at high and 4 at unclear risk of bias. Unable to pool data. 2 of the included studies were in secondary care. Interventions of 4 trials (number of appointments and/or intensity) are not applicable in routine dental practice. Downgraded for risk of bias, inconsistency and indirectness. 21 study at high and 5 at unclear risk of bias. Unable to pool data. 2 of the included studies were in secondary care. Interventions of 5 trials (number of appointments and/or intensity) are not applicable in routine dental practice. Downgraded for risk of bias, inconsistency and indirectness. 31 high risk of bias study. Did not report usable data. Setting and intervention not applicable to routine dental care, therefore also downgraded for indirectness.
We have included 19 studies that investigated various forms and delivery methods of one‐to one oral hygiene advice (OHA) in a dental setting.
We have summarised data in additional tables, categorising trials to similar interventions groups where possible (Table 5; Table 6; Table 7; Table 8).
1. Comparison 1 ‐ Any form of one‐to‐one oral hygiene advice (OHA) versus no OHA.
| Hetland 1982 | |||||||||
|
Intervention: OHA provided by dental nurse Frequency: 3 intervention visits with OHA, each visit 1 week apart Intensity: 45 minutes first visit, 30 minutes at second visit, 15 minutes at third visit Control: no OHA (Adults; primary care setting) | |||||||||
| Endpoint | Gingivitis (GI) | Plaque (visible plaque after staining) | Caries (DMFT) | ||||||
| OHA | No OHA | OHA | No OHA | No summary statistics or effect estimates reported. Authors report "Only small and statistically insignificant changes occurred in the 3 groups during the experimental period" | |||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) |
n Mean (SD) |
n Mean (SD) |
MD (95% CI) |
||||
| 3 months | n = 23 1.63 (0.23) |
n = 25 1.38 (0.41) |
0.25 (0.05 to 0.45) |
n = 23 0.21 (0.19) |
n = 25 0.62 (0.28) |
‐0.41 (‐0.54 to ‐0.28) | |||
| 6 months | 23 0.98 (0.39) |
25 1.36 (0.46) |
‐0.38 (‐0.62 to ‐0.14) |
n = 23 0.22 (0.19) |
n = 25 0.66 (0.24) |
‐0.44 (‐0.56 to ‐0.32) | |||
| Hoogstraten 1983 | |||||||||
|
Intervention: OHA provided by dental hygienist with or without film (combined groups). (For info, the 2 relevant arms of this study also appear in Comparison 4 enhanced OHA) Frequency: not fully reported Intensity: not fully reported Control: no OHA (Adults; primary care setting) | |||||||||
| Endpoint | Gingivitis | Plaque | Caries | ||||||
| 12 months | Outcome not measured | Outcome not measured | Outcome not measured | ||||||
| Hugoson 2007 | |||||||||
|
Intervention: preventive care instruction by hygienist Frequency: OHA provided 3 endpoints in 4 visits Intensity: approximately 125 minutes Control: no OHA (Adults; mixed public dental/primary care setting) | |||||||||
| Endpoint |
Gingivitis (Full mouth number of sites with gingivitis) |
Plaque (Plaque index score) |
Caries | ||||||
| OHA | No OHA | OHA | No OHA | Outcome not measured | |||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||
| 12 months | n = 98 22.2 (18.1) |
n = 97 30.2 (16.5) |
‐8.00 (‐12.86 to ‐3.14) |
n = 98 29.9 (25.2) |
n = 97 42.9 (23.9) |
‐13.00 (‐19.89 to ‐6.11) | |||
| 24 months | n = 97 19.0 (17.7) |
n = 96 26.7 (18.2) |
‐7.70 (‐12.77 to ‐2.63) |
n = 97 24.4 (23.1) |
n = 96 39.4 (24.1) |
‐15.00 (‐21.66 to ‐8.34) | |||
| 36 months | 93 19.4 (17.4) |
94 28.5 (17.0) |
‐9.10 (‐14.03 to ‐4.17) |
n = 93 24.5 (23.6) |
n = 94 37.6 (24.3) |
‐13.10 (‐19.97 to ‐6.23) | |||
| Memarpour 2016 | |||||||||
|
Intervention: OHA provided by dentist plus pamphlet Frequency: 2 'intervention' visits and 3 review visits Intensity: 30 minutes to 40 minutes Control: no OHA (Infants; primary care setting) | |||||||||
| Endpoint | Gingivitis | Plaque | Caries (proportion of infants developing caries) | ||||||
| Outcome not measured | Outcome not measured | OHA | No OHA | ||||||
| n events/n participants | n events/n participants | RR (95% CI) | |||||||
| 4 months | 2/97 | 3/96 |
0.66 (0.11 to 3.86) |
||||||
| 8 months | 3/94 | 15/94 |
0.20 (0.06 to 0.67) |
||||||
| 12 months | 4/85 | 29/88 |
0.14 (0.05 to 0.39) |
||||||
CI = confidence interval; DMFT = number of decayed, missing or filled permanent teeth; GI = gingival index (Löe 1963); MD = mean difference; n = number; OHA = oral hygiene advice; RR = risk ratio; SD = standard deviation.
2. Comparison 2 ‐ Personalised one‐to‐one OHA versus routine one‐to‐one OHA.
| Ramsay 2018 | |||||||||||
|
Intervention: personalised 1‐to‐1 OHA provided by the dentist or hygienist Frequency: reinforcement of OHA was provided at the discretion of the dentist/hygienist Intensity: not reported Control: routine 1‐to‐1 OHA provided by dentist or hygienist Frequency: reinforcement of OHA was provided at the discretion of the dentist/hygienist Intensity: not reported (Adults; primary care setting) | |||||||||||
| Endpoint | Gingivitis (GI) | Plaque | Caries | ||||||||
| Personalised | Routine | Outcome not measured | Outcome not measured | ||||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) |
|||||||||
| 36 months | 712 39.2 (23.8) |
615 38.7 (24.9) |
‐2.50 (‐8.30 to 3.30) | ||||||||
| Jönsson 2009 | |||||||||||
|
Intervention: individually tailored oral health education programme based on cognitive behavioural therapy Frequency: median number of visits: 9 Intensity: approximately 60 minutes for each session up to the 3‐month follow‐up, and approximately 45 minutes for the maintenance period. An extra 10 minutes was needed for the first 2 sessions Control: preventive care instruction by hygienist Frequency: median number of intervention visits: 8 Intensity: approximately 60 minutes for each session up to the 3‐month follow‐up, and approximately 45 minutes for the maintenance period (Adults, moderate to advanced periodontitis; secondary care setting) | |||||||||||
| Endpoint | Gingivitis (GI) | Plaque (modified PI) | Caries | ||||||||
| Personalised | Routine | Personalised | Routine | Outcome not measured | |||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) |
n Mean (SD) |
n Mean (SD) |
MD (95% CI) |
||||||
| 3 months | 54 0.27 (0.14) |
56 0.52 (0.20) |
‐0.25 (‐0.31 to ‐0.19) | 54 0.17 (0.11) |
56 0.32 (0.22) |
‐0.15 (‐0.21 to ‐0.09) | |||||
| 12 months | 53 0.21 (0.16) |
55 0.50 (0.17) |
‐0.29 (‐0.35 to ‐0.23) | 53 0.14 (0.13) |
55 0.31 (0.16) |
‐0.17 (‐0.22 to ‐0.12) | |||||
| Lepore 2011 | |||||||||||
|
Intervention: personalised oral health action plan delivered by the dentist Frequency: 1 initial visit Intensity: not reported Control: routine OHA delivered by the dentist Frequency: 1 initial visit Intensity: not reported (Children; secondary care setting) | |||||||||||
| Endpoint | Gingivitis (not specified) | Plaque (index not reported) | Caries (dmft) | ||||||||
| Personalised | Routine | Personalised | Routine | Personalised | Routine | ||||||
| n Mean change (post‐pre) (SD) |
n Mean change (post‐pre) (SD) |
MD (95% CI) |
n Mean change (post‐pre) (SD) |
n Mean change (post‐pre) (SD) |
MD (95% CI) |
n Mean change (post‐pre) (SD) |
n Mean change (post‐pre) (SD) |
MD (95% CI) |
|||
| 2 months | 37 ‐0.45 (Not reported) |
32 0.00 (Not reported) |
Not estimable | 37 ‐0.97 (Not reported) |
32 ‐0.10 (Not reported) |
Not estimable | 37 0.00 (Not reported) |
32 0.00 (Not reported) |
Not estimable | ||
| Schmalz 2018 | |||||||||||
|
Intervention: tailored toothbrush instruction (oscillation rotation, sonic action, manual) provided by the dentist plus OHA Frequency: 1 visit Intensity: not reported Control: OHA and no tailored toothbrush instruction (Adults; secondary care setting) | |||||||||||
| Endpoint | Gingivitis (GI) | Plaque (modified QHI) | Caries | ||||||||
| Personalised | Routine | Personalised | Routine | Outcome not measured | |||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||||
| 3 months | 66 0.97 (0.13) |
65 0.99 (0.09) |
‐0.03 (‐0.06 to 0.01) | 66 1.13 (0.32) |
65 1.17 (0.33) |
‐0.04 (‐0.15 to 0.08) | |||||
CI = confidence interval; dmft = number of decayed, missing or filled primary teeth; GI = gingival index (Löe 1963); MD = mean difference; modified QHI = Turesky Modified Quigley and Hein plaque index; n = number; OHA = oral hygiene advice; PI = plaque index (Silness 1964); SD = standard deviation.
3. Comparison 3 ‐ Self‐management versus professional OHA.
| Aljafari 2017 | |||||||||
|
Intervention: computer game group: the child and parent played the computer game on a touch tablet and received a copy of it on a DVD to play at home Frequency: 1‐off in clinic and thereafter delivered at home Intensity: not reported Control: the child and parent received verbal oral health education from a dental nurse with a health education qualification Frequency: 1‐off intervention Intensity: not reported (High caries risk children; secondary care setting) | |||||||||
| Endpoint | Gingivitis | Plaque | Caries | ||||||
| Outcome not measured | Outcome not measured | Outcome not measured | |||||||
| Baab 1986 | |||||||||
|
Intervention: OHA using a self‐inspection plaque index Frequency: 5 visits with repeated OHA Intensity: 30 minutes Control: traditional instruction using professional monitoring of disclosed plaque provided by the hygienist Frequency: 5 visits with repeated OHA Intensity: 30 minutes (Adults; secondary care setting) | |||||||||
| Endpoint | Gingivitis (BOP) | Plaque (O'Leary) | Caries (DMFT) | ||||||
| 3 months | No numerical data reported | No numerical data reported | Outcome not measured | ||||||
| 6 months | |||||||||
| Glavind 1985 | |||||||||
|
Intervention: self‐examination manual and self‐instruction in OHA manual Frequency: 2 visits, 1 with OHA Intensity: not reported Control: OHA provided by dentist Frequency: 2 visits, 1 with OHA Intensity: 25 minutes to 30 minutes (Adults; primary care setting) | |||||||||
| Endpoint | Gingivitis (mean percentage of presence of gingival bleeding) | Plaque (mean percentage of presence of plaque) | Caries | ||||||
| 3 months | No numerical data reported | No numerical data reported | Outcome not measured | ||||||
| 6 months | |||||||||
| Jönsson 2006 | |||||||||
|
Intervention: Client Self‐Care Commitment Model Frequency: 4 visits in total, 3 visits with OHA Intensity: not reported Control: OHA provided by the hygienist Frequency: 3 visits in total, 2 visits with OHA Intensity: not reported (Adults, receiving periodontal therapy; secondary care setting) | |||||||||
| Endpoint | Gingivitis (GI) | Plaque (PI) | Caries | ||||||
| Self‐care | Professional | Self‐care | Professional | Outcome not measured | |||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||
| 3 months | 19 0.38 (0.20) |
16 0.39 (0.14) |
0.01 (‐0.12 to 0.10) | 19 0.25 (0.11) |
16 0.33 (0.11) |
‐0.08 (‐0.15 to ‐0.01) | |||
| López‐Jornet 2014 | |||||||||
|
Intervention: motivational – behavioural skills protocol provided by the dental care professional Frequency: 4 visits Intensity: "which required a longer time" Control: instruction in conventional oral hygiene procedures Frequency: 4 visits Intensity: 20 minutes (Adults with hyposalivation; secondary care setting) | |||||||||
| Endpoint | Gingivitis (not specified) | Plaque (modified QHI) | Caries | ||||||
| Theory‐based | Conventional | Theory‐based | Conventional | Outcome not measured | |||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||
| 2 months | 30 18.4 (25.8) |
30 6.1 (13.4) |
12.30 (1.90 to 22.70) | 30 0.2 (0.1) |
30 0.3 (0.2) |
‐0.10 (‐0.18 to ‐0.02) | |||
BOP = bleeding on probing; CI = confidence interval; DMFT = number of decayed, missing or filled permanent teeth; GI = gingival index (Löe 1963); MD = mean difference; modified QHI = Turesky Modified Quigley and Hein plaque index; n = number; OHA = oral hygiene advice; O'Leary = disclosed plaque at the gingival margin (O'Leary 1972); PI = plaque index (Löe 1967); SD = standard deviation.
4. Comparison 4 ‐ Enhanced one‐to‐one OHA versus one‐to‐one OHA.
| Bali 1999 | |||||||||||
|
Intervention: professional OHA plus intensive patient education in oral hygiene Frequency: 4 visits with repeated OH instruction Intensity: not reported Control: professional OHA Frequency: 1 visit Intensity: not reported (Adults, type 1 diabetes; secondary care setting) | |||||||||||
| Endpoint | Gingivitis (BOP) | Plaque (Turesky hygiene index) | Caries | ||||||||
| Enhanced OHA | OHA | Enhanced OHA | OHA | Enhanced OHA | OHA | ||||||
| n Median Range |
n Median Range |
MD (95% CI) | n Median Range |
n Median Range |
MD (95% CI) | n Median Range |
n Median Range |
MD (95% CI) | |||
| 6 months | 37 50.0 0, 100 |
37 57.1 0, 100 |
Not estimable | 37 2 0, 3.7 |
37 2.1 0, 3.9 |
Not estimable | 37 18 3, 26 |
37 18 1, 32 |
Not estimable | ||
| 12 months | 36 64.6 0, 100 |
34 65.8 0, 100 |
Not estimable | 36 1.7 0, 3.6 |
34 2 0.2, 3.6 |
Not estimable | 36 18.5 4, 31 |
34 19 1, 32 |
Not estimable | ||
| Hoogstraten 1983 | |||||||||||
|
Intervention: OHA provided by dental hygienist ± film Frequency: not fully reported Intensity: not fully reported Control: OHA provided by dental hygienist Frequency: not fully reported Intensity: not fully reported (Adults; primary care setting) | |||||||||||
| Endpoint | Gingivitis | Plaque | Caries | ||||||||
| 12 months | Outcome not measured | Outcome not measured | Outcome not measured | ||||||||
| Münster Halvari 2012 | |||||||||||
|
Intervention: standard autonomy supportive treatment plus competence enhancing intervention Frequency: 4 visits Intensity: 45 minutes Control: standard autonomy supportive treatment Frequency: 4 visits Intensity: 20 minutes (Adults; primary care setting) | |||||||||||
| Endpoint | Gingivitis (GI) | Plaque (PI) | Caries | ||||||||
| Enhanced OHA | OHA | Enhanced OHA | OHA | Outcome not measured | |||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||||
| 5.5 months | 70 1.17 (0.10) |
71 1.49 (0.16) |
‐0.32 (‐0.36 to ‐0.28) | 70 0.51 (0.19) |
71 0.90 (0.27) |
‐0.39 (‐0.47 to ‐0.31) | |||||
| Söderholm 1982 | |||||||||||
|
Intervention: OHA provided by dental nurse and hygienist Frequency: 5 visits with OHA Intensity: 150 minutes Control: OHA provided by dental nurse and hygienist Frequency: 2 visits with OHA Intensity: 120 minutes (Adults; primary care setting) | |||||||||||
| Endpoint | Gingivitis (percentage bleeding after probing pocket depths) | Plaque (disclosed plaque scores) | Caries | ||||||||
| Enhanced OHA | OHA | OHA | No OHA | Outcome not measured | |||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||||
| 3 months | 19 6.7 (5.67) |
22 6.7 (5.16) |
0.00 (‐3.34 to 3.34) | 19 15.8 (15.26) |
22 18.7 (12.66) |
‐2.90 (‐11.56 to 5.76) | |||||
| Tedesco 1992 | |||||||||||
|
Intervention: routine OHA plus personalised video Frequency: 7 visits in total, 4 visits with OHA Intensity: not reported Control: routine OHA Frequency: 7 visits in total, 4 visits with OHA Intensity: not reported (Adults; setting not reported) | |||||||||||
| Endpoint | Gingivitis (GI) | Plaque (PI) | Caries | ||||||||
| Enhanced OHA | OHA | Enhanced OHA | OHA | Outcome not measured | |||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||||
| 3 months | 80 0.97 (not reported) |
38 1.08 (not reported) |
Not estimable | 80 0.92 (not reported) |
38 1.00 (not reported) |
Not estimable | |||||
| 9 months | 62 1.06 (not reported) |
29 1.05 (not reported) |
Not estimable | 62 0.92 (not reported) |
29 0.92 (not reported) |
Not estimable | |||||
| Van Leeuwen 2017 | |||||||||||
|
Intervention: personal OHA provided by the hygienist plus OHA leaflet Frequency: 3‐week treatment phase, intervention administered once Intensity: not reported Control: OHA leaflet (Adults; secondary care) | |||||||||||
| Endpoint | Gingivitis (GI) | Plaque (modified QHPI) | Caries | ||||||||
| Enhanced OHA | OHA | Enhanced OHA | OHA | Outcome not measured | |||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | n Mean (SD) |
n Mean (SD) |
MD (95% CI) | ||||||
| 4 months | 43 57.8 (13.6) |
44 59.1 (13.6) |
‐1.30 (‐7.02 to 4.42) | 43 1.86 (0.29) |
44 1.86 (0.33) |
0.00 (‐0.13 to 0.13) | |||||
| 7 months | 43 57.8 (14.6) |
44 60.8 (13.3) |
‐3.00 (‐8.87 to 2.87) | 43 1.92 (0.32) |
44 1.95 (0.36) |
‐0.03 (‐0.17 to 0.11) | |||||
| 10 months | 43 54.5 (15.6) |
44 56.1 (14.1) |
‐1.60 (‐7.85 to 4.65) | 43 1.95 (0.42) |
44 2.01 (0.37) |
‐0.06 (‐0.23 to 0.11) | |||||
| 12 months | 43 61.7 (17.3) |
44 62.6 (13.7) |
‐0.90 (‐7.47 to 5.67) | 43 1.85 (0.34) |
44 1.82 (0.37) |
0.03 (‐0.12 to 0.18) | |||||
| Weinstein 1996 | |||||||||||
|
Intervention 1: twice weekly phone call to periodontist to report self‐evaluated plaque score Intervention 2: OHA plus twice weekly examinations by periodontist Intervention 3: OHA plus twice weekly examinations by periodontist plus daily completion of oral hygiene task checklist Frequency 1: 3 visits Frequency 2: 16 visits Frequency 3: 16 visits Intensity: not reported Control: routine OHA Frequency: 3 visits Intensity: not reported (Adults presenting with long‐term periodontic problems; setting not reported) | |||||||||||
| Endpoint | Gingivitis | Plaque | Caries | ||||||||
| Outcome not measured | Enhanced OHA (1) | OHA | Outcome not measured | ||||||||
| n Mean (SD) |
n Mean (SD) |
MD (95% CI) | |||||||||
| 2 months | 5 0.32 (0.08) |
5 0.38 (0.16) |
‐0.06 (‐0.22 to 0.10) | ||||||||
| Enhanced OHA (2) | OHA | ||||||||||
| 2 months | 5 0.23 (0.08) |
5 0.38 (0.16) |
‐0.15 (‐0.31 to 0.01) | ||||||||
| Enhanced OHA (3) | OHA | ||||||||||
| 2 months | 5 0.15 (0.03) |
5 0.38 (0.16) |
‐0.23 (‐0.37 to ‐0.09) | ||||||||
BOP = bleeding on probing; CI = confidence interval; GI = gingival index (Löe 1963); MD = mean difference; modified QHPI = Quigley 1962; n = number; OHA = oral hygiene advice; PI = plaque index (Löe 1967); SD = standard deviation.
Comparison 1: Any form of one‐to‐one OHA versus no OHA
Four studies compared any form of one‐to‐one OHA versus no OHA (Hetland 1982; Hoogstraten 1983; Hugoson 2007; Memarpour 2016). Two of these studies reported outcomes of gingivitis and plaque levels (Hetland 1982; Hugoson 2007). Two studies reported dental caries (Hetland 1982; Memarpour 2016). The remaining study did not include clinical outcomes (Hoogstraten 1983). Outcomes were reported up to 6 months in Hetland 1982, 36 months in Hugoson 2007, and 12 months in Hoogstraten 1983 and Memarpour 2016.
Gingivitis
The Hetland 1982 was a small study in adults that had contradictory results (very weak evidence) at 3 months and 6 months. There was a small difference favouring the control arm of no OHA at 3 months: mean difference (MD) 0.25, 95% confidence interval (CI) 0.05 to 0.45. At 6 months the small difference favoured the OHA intervention: MD ‐0.38, 95% CI ‐0.62 to ‐0.14.
The Hugoson 2007 study in adults showed very low‐quality evidence of a benefit for OHA at 12 months: MD ‐8.0, 95% CI ‐12.86 to ‐3.14; 24 months: MD ‐7.70, 95% CI ‐12.77 to ‐2.63; and 36 months: MD ‐9.10, 95% CI ‐14.03 to ‐4.17.
Plaque levels
Both studies at all time points consistently showed low‐quality evidence of a benefit in plaque reduction for OHA. The Hetland 1982 showed a benefit of: MD ‐0.44, 95% CI ‐0.56 to ‐0.32 at 6 months; at 36 months the Hugoson 2007 study demonstrated a benefit of MD ‐13.10, 95% CI ‐19.97 to ‐6.23.
Dental caries
The Hetland 1982 study did not provide summary statistics or effect estimates, reporting only "small and statistically insignificant changes" between groups. The Memarpour 2016 study provided weak evidence of a benefit for OHA at 8 months: risk ratio (RR) 0.20, 95% CI 0.06 to 0.67; and 12 months: RR 0.14, 95% CI 0.05 to 0.39 (173 participants), but not at 4 months: RR 0.66, 95% CI 0.11 to 3.86. Overall, the evidence for dental caries was graded as very low‐quality evidence.
Comparison 2: Personalised one‐to‐one OHA versus routine one‐to‐one OHA
Four studies compared personalised OHA versus routine OHA (Jönsson 2009; Lepore 2011; Ramsay 2018; Schmalz 2018). All four studies reported gingivitis as an outcome (Jönsson 2009; Lepore 2011; Ramsay 2018; Schmalz 2018). Three of the studies that reported gingivitis also reported plaque levels as an outcome (Jönsson 2009; Lepore 2011; Schmalz 2018). Only one of the studies reported dental caries (Lepore 2011). Outcomes were reported up to 2 months (Lepore 2011), 3 months (Schmalz 2018), 12 months (Jönsson 2009), and 36 months (Ramsay 2018).
Gingivitis
The large Ramsay 2018 study in adults showed little or no difference between groups at 36 months: MD ‐2.50, 95% CI ‐8.30 to 3.30.
The Jönsson 2009 study in adults showed weak evidence of a benefit for personalised OHA at 3 months: MD ‐0.25, 95% CI ‐0.31 to ‐0.19 and 12 months: MD ‐0.29, 95% CI ‐0.35 to ‐0.23.
The Lepore 2011 study in children reported "statistically significant (P < 0.05) improvement" for personalised OHA at 2 months but did not report usable data.
The Schmalz 2018 study showed little or no difference between groups at 3 months: MD ‐0.03, 95% CI ‐0.06 to 0.01.
Overall, the evidence for gingivitis was graded as very low‐quality evidence.
Plaque levels
The Jönsson 2009 study in adults showed weak evidence of a benefit for personalised OHA at 3 months: MD ‐0.15, 95% CI ‐0.21 to ‐0.09 and 12 months: MD ‐0.17, 95% CI ‐0.22 to ‐0.12.
The Lepore 2011 study in children reported "statistically significant (P < 0.05) improvement" for personalised OHA but did not report usable data.
The Schmalz 2018 study showed little or no difference between groups at 3 months: MD ‐0.04, 95% CI ‐0.15 to 0.08.
Overall, the evidence for plaque levels was graded as very low‐quality evidence.
Dental caries
The Lepore 2011 study in children between the ages of 1 year and 6 years old reported "statistically significant (P < 0.05) improvement" for personalised OHA but did not report usable data. Overall, the evidence for dental caries was graded as very low‐quality evidence.
Comparison 3: Self‐management versus professional OHA
Five trials compared some form of self‐management with some form of professional OHA (Aljafari 2017; Baab 1986; Glavind 1985; Jönsson 2006; López‐Jornet 2014). Four studies reported gingivitis and plaque levels (Baab 1986; Glavind 1985; Jönsson 2006; López‐Jornet 2014). None of the studies reported dental caries. Outcomes were reported up to 2 months (López‐Jornet 2014), 3 months (Jönsson 2006), and 6 months (Baab 1986; Glavind 1985).
Gingivitis
The Baab 1986 study in adults reported "significant differences between groups at each observation were not found" but no usable data reported.
The Glavind 1985 study in adults reported "no statistically significant differences in gingival bleeding scores were found between the two treatment groups at any of the three examinations" but no usable data reported.
The Jönsson 2006 study in adults showed little or no difference between groups at 3 months: MD ‐0.01, 95 % CI ‐0.12 to 0.10.
The López‐Jornet 2014 study in adults with hyposalivation showed a benefit in gingivitis for professional OHA at 2 months: MD 12.30 95% CI 1.90 to 22.70.
Overall, the evidence for gingivitis was graded as very low quality.
Plaque levels
The Baab 1986 study in adults reported "mean plaque scores did not differ significantly between groups" but no usable data reported.
The Glavind 1985 study in adults reported "no statistically significant differences were found between the two groups at any of the examination times" but no usable data reported.
The Jönsson 2006 study in adults showed a benefit in plaque reduction at 3 months for self‐management: MD ‐0.08, 95 % CI ‐0.15 to ‐0.01.
The López‐Jornet 2014 study in adults with hyposalivation showed a benefit in gingivitis for professional OHA at 2 months: MD ‐0.10 95% CI ‐0.18 to ‐0.02.
Overall, the evidence for plaque levels was graded as very low quality.
Dental caries
No studies were found that looked at dental caries.
Comparison 4: Enhanced one‐to‐one OHA versus one‐to‐one OHA
Seven trials compared some form of enhanced OHA with some form of routine OHA (Bali 1999; Hoogstraten 1983; Münster Halvari 2012; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996). Five of the studies investigating enhanced OHA versus OHA reported gingivitis (Bali 1999; Münster Halvari 2012; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017). Six of the studies investigating enhanced OHA versus routine OHA reported gingivitis (Bali 1999; Münster Halvari 2012; Söderholm 1982; Tedesco 1992; Van Leeuwen 2017; Weinstein 1996). Only the Bali 1999 study reported dental caries. Outcomes were reported up to 2 months (Weinstein 1996), 3 months (Söderholm 1982), 5.5 months (Münster Halvari 2012), 9 months (Tedesco 1992), and 12 months (Bali 1999; Hoogstraten 1983; Van Leeuwen 2017).
Gingivitis
Two studies in adults did not report usable data (Bali 1999; Tedesco 1992).
Three studies found little or no difference between groups across all time points (Münster Halvari 2012; Söderholm 1982; Van Leeuwen 2017).
The Münster Halvari 2012 study showed low‐quality evidence of a benefit in reduction of gingivitis for enhanced OHA at 5.5 months: MD ‐0.32, 95% CI ‐0.36 to ‐0.28.
The Söderholm 1982 study reported little or no difference between groups at 3 months: MD 0.00, 95% CI ‐3.34 to 3.34.
The Van Leeuwen 2017 study reported little or no difference between groups at 12 months: MD ‐0.90, 95% CI ‐7.47 to 5.67.
Overall, the evidence for gingivitis was graded as very low quality.
Plaque levels
Two studies (Bali 1999; Tedesco 1992) in adults did not report usable data.
The Münster Halvari 2012 study in adults showed low‐quality evidence of a benefit in plaque reduction for enhanced OHA at 5.5 months: MD ‐0.39 95% CI ‐0.47 to ‐0.31.
The Söderholm 1982 study reported little or no difference between groups at 3 months: MD ‐2.90, 95% CI ‐11.56 to 5.76.
The Van Leeuwen 2017 study reported little or no difference between groups at all time points. The mean difference at 12 months was 0.03, 95% CI ‐0.12 to 0.18.
The Weinstein 1996 study included three intervention groups of enhanced OHA compared with the control OHA. Only one of the intervention groups (intervention group 3) showed a small benefit in plaque reduction at 2 months: MD ‐0.23, 95% CI ‐0.37 to ‐0.09.
Overall, the evidence for plaque levels was graded as very low quality.
Dental caries
The Bali 1999 study in adults investigated dental caries but did not report usable data.
Discussion
Summary of main results
We have identified 19 studies suitable for inclusion to this review. A total of 4232 participants were recruited, with numbers included in each trial ranging from 33 to 1877. The member of the dental team delivering the oral hygiene advice (OHA) was a hygienist in eight studies, dentist in four studies, dental nurse in one study, dentist or hygienist in one study, dental nurse and hygienist in one study, and dental nurse OHA to the control group with further self‐administration of the intervention in one study. It was unclear in three of the studies which member of the dental team carried out the intervention.
Due to the heterogeneity of the studies with respect to participants, interventions, settings and outcome measures we were unable to pool the data. We summarised data by categorising similar interventions into comparison groups.
Comparison 1: Any form of one‐to‐one OHA versus no OHA
Four studies compared any form of one‐to‐one OHA versus no OHA and the results are summarised in Table 1.
Two studies reported the outcome of gingivitis. Although one small study in adults had contradictory results at 3 months and 6 months, the other study in adults showed very low‐quality evidence of a benefit for OHA at all time points of 12 months, 24 months and 36 months.
The same two studies reported the outcome of plaque. There was low‐quality evidence that these interventions showed a benefit for OHA in plaque reduction at all time points.
Two studies reported the outcome of dental caries at 6 months and 12 months respectively. There was very low‐quality evidence of a benefit for OHA at 12 months.
Comparison 2: Personalised one‐to‐one OHA versus routine one‐to‐one OHA
Four studies compared personalised OHA versus routine OHA and the results are summarised in Table 2.
There was little evidence available that any of these interventions demonstrated a difference on the outcomes of gingivitis, plaque or dental caries (very low quality).
Comparison 3: Self‐management versus professional OHA
Five trials compared some form of self‐management with some form of professional OHA and the results are summarised in Table 3.
There was little evidence available that any of these interventions demonstrated a difference on the outcomes of gingivitis or plaque (very low quality). None of the studies measured dental caries.
Comparison 4: Enhanced one‐to‐one OHA versus one‐to‐one OHA
Seven trials compared some form of enhanced OHA with some form of routine OHA and the results are presented in Table 4.
There was little evidence available that any of these interventions demonstrated a difference on the outcomes of gingivitis, plaque or dental caries (very low quality).
Overall completeness and applicability of evidence
Our search strategy was wide and retrieved 15,188 studies with a further two studies identified by handsearching. We identified 19 studies for inclusion having also handsearched the references from systematic reviews, reviews and those papers included. As such we are confident that the actions completed have resulted in a robust review of the evidence currently available. Furthermore, the evidence identified studies carried out in a number of dental settings, different countries, different age populations and healthy patients or those with specified medical or oral health conditions. In addition, there was a variety of dental care professionals who delivered a mixture of different interventions over a variety of time frames and frequencies. The sample size and length of follow‐up of included studies were also variable.
Regarding the applicability of the evidence, due to the heterogeneity of study design, study interventions and outcome measures it was not possible to carry out a meta‐analysis to determine if any specific intervention was more effective in maintaining or improving oral health, hygiene, behaviour, and attitudes compared to no advice or advice in a different format. There was also a dearth of high‐quality studies with low risk of bias. The majority of studies were carried out in secondary care, as such it may be difficult to extrapolate the evidence to the wider population as the majority of the general population access dental care in primary care. We also noted that the frequency and intensity of intervention varied significantly between studies with some reporting multiple intervention appointments, as many as median of nine (Jönsson 2009), and up to 150 minutes intervention time. The follow‐up time was limited in most of the included studies. As such the applicability of the evidence is not largely generalisable.
Quality of the evidence
The body of the evidence is very low quality as presented in Table 1; Table 2; Table 3; Table 4.
Potential biases in the review process
The search strategy produced a large number of results with very few additional studies found via handsearching. However, while this process was deemed robust, it is possible that some small studies were not identified though we are confident that all larger studies were found.
We were not blinded to the names of authors, publications, institutions, etc. of studies at the time of reviewing which may have introduced bias to the review with conflict of interests declared in advance of the review process; the primary author had no conflict of interest and those who did declare conflicts of interest were not involved in reviewing any of the related data (i.e. the IQuaD Study (Ramsay 2018)).
Agreements and disagreements with other studies or reviews
In agreement with our review, a number of previous reviews have noted the variety of oral health promotion interventions investigated and the resultant heterogeneity in studies (Kay 2016a; Kay 2016b; Watt 2005; Yevlahova 2009). Furthermore, due to the variable quality of studies and findings, it is not possible to estimate the intervention effect (Watt 2005). As a result, direct comparisons between studies is difficult due to the lack of standardised and validated outcome measurement tools (Kay 1998).
As previously stated, due to the variety of different outcomes and outcome measures used there is difficulty in assessing and summarizing the effect of interventions on plaque levels (Watt 2005). Watt et al also commented that in the short term, i.e. up to 6 months‐post intervention, substantial reductions in plaque reduction can be expected following educational interventions but longer term studies are limited in number, so whether or not such short‐term improvements are maintained is impossible to determine (Watt 2005) which has been echoed by others (Kay 2016b).
The systematic review by Kay et al, which focused on oral health promotion activities that could be delivered in the context of general dental practice, aimed to change individual's knowledge, attitudes or behaviours in order to influence their oral health, also noted that the quality of the studies were very variable. Furthermore, the authors similarly noted that "the outcome measures used to assess knowledge, behaviour and attitudes were ad‐hoc measures and therefore only very rarely allowed direct comparisons between studies and entirely obviated the possibility of meta‐analysing the data. Direct comparison between studies and/or meta‐analysis would have only been possible for studies that measured the same clinical outcomes, and then only if the interventions had been the same. This required level of similarity between studies was not reached" (Kay 2016b). The authors note that this is similar to a previous publication by the same authors (Kay 2016a).
One review concluded that there was evidence to show that motivational interviewing "may be useful in the dental surgery setting and this application should be researched further" (Kay 2016b). The current systematic review only found two studies suitable for inclusion on this topic (López‐Jornet 2014; Münster Halvari 2012) and we did not think the available evidence allowed adequate comparisons.
Regarding previous review findings regarding OHA and dental caries prevention, Kay et al reported that "there is still no evidence that caries can be prevented by oral health promotion although this apparent lack of effect may be due, in part, to the short follow‐up (< 3 years) in the majority of studies" (Kay 2016a).
Authors' conclusions
Implications for practice.
There was insufficient high‐quality evidence to recommend any specific one‐to‐one oral hygiene advice (OHA) method as being effective in improving oral health or being more effective than any other method.
Implications for research.
The quality of study reporting was variable with the use of CONSORT recommended for future studies (www.consort‐statement.org).
Many of the studies did not make the exact nature of the OHA intervention entirely clear; more detailed description of intervention(s) following the 'Criteria for Reporting the Development and Evaluation of Complex Interventions in health care: revised guideline (CReDECI 2)' (Möhler 2015) would be of benefit to facilitate easier comparison.
Researchers conducting future studies should ensure that methods of randomisation are adequate and clearly reported along with ensuring allocation concealment, appropriate blinding and sample size determined at design stage. In addition, appropriate and validated patient‐centred outcome measures should be included in future studies along with the need to determine the cost effectiveness of interventions.
The vast majority of the studies had a short follow‐up; longer follow‐up periods would be beneficial to determine longer term effects of one‐to‐one OHA.
Given the vast majority of dental care is provided in a primary care environment, future studies within this environment that take into account the likely time and financial limitations such an environment are likely to impose (e.g. frequency and intensity of intervention), would be advantageous to allow future research to be applicable to the wider population with such interventions needing to be both clinically and financially effective and efficient.
Acknowledgements
We would like to extend our thanks to Anne Littlewood for completion of searches, and Luisa M Fernandez Mauleffinch for her editorial assistance and copy editing. We would also like to thank Anette Blümle, Dominic Lamont and Falk Schwendicke for their assistance with translation. Also, thanks to Craig Ramsay and Anne Marie Glenny for advice on thematic considerations, and Helen Worthington, Philip Riley, Paul Brocklehurst and Derek Richards for peer review comments.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
From October 2012, searches were undertaken on Cochrane Register of Studies using the following search strategy: #1 ("dental health education" or "health promotion":TI,AB) AND (INREGISTER) #2 (("oral health" AND (instruct* or advice or advise* or educat* or teach* or train* or demonstrat* or supervis*)):TI,AB) AND (INREGISTER) #3 (("oral health" AND (behavior* or behaviour* or "patient compliance" or motivat*) AND (change OR changed OR changing or modify OR modified OR modification)) :TI,AB) AND (INREGISTER) #4 (attitude* AND "oral health") or (attitude AND "oral care") or (attitude AND "dental health") or (attitude AND "mouth hygiene") or (attitude AND "oral hygiene"):TI,AB) AND (INREGISTER) #5 (#1 or #2 or #3 or #4) AND (INREGISTER) Previous to October 2012, searches of the trials register were done using Procite software, using the following search strategy:
("dental health education" or "Health promotion" or ("oral health" AND (instruct* or advice or advise* or educat* or teach* or train* or demonstrat* or supervis*)) or behavior* or behaviour* or "patient compliance" or motivat* or ((behavior* OR behaviour*) AND (change OR changed OR changing or modify OR modified OR modification)) or "feedback device*" or "feed‐back device*" or (attitude* AND "oral health") or (attitude AND "oral care") or (attitude AND "dental health") or (attitude AND "mouth hygiene") or (attitude AND "oral hygiene"))
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 ORAL HEALTH/ #2 Exp STOMATOGNATHIC DISEASES/ #3 Exp HALITOSIS #4 ((dental or tooth or teeth or enamel or root*) AND (decay* or caries or carious or white next spot* or plaque or reminerali* or deminerali*)) #5 (periodont* or gingivitis or (gingiva* next inflamm*) or (gingiva* next bleed*) or (gingival next pocket*) or (periodont* next pocket*) or (periodont* near attachment) or (gingiva* near attachment) #6 stomatitis or (mouth next ulcer*) or (oral next ulcer*) or (oral next candidi*) or (aphthous next ulcer*) or (mouth near aphthae) or (oral near aphthae) or (mucositis near oral) or (mucositis near mouth) or xerostomi* #7 (oral next health) or (dental next health) or orthodontic* #8 ("tooth wear" or ((tooth or dental or teeth or enamel) and (erosion or abrasion))) #9 (halitosis or (mouth next odour*) or (mouth next odor*) or (mouth next malodour*) or (mouth next malodor*) or (oral next malodour) OR (oral next malodour) or (breath near malodour*) or (breath near odour*) or (breath near odor*)) #10 (bottle next caries) or (nursing next caries) or (bottle next decay*) or ((early next childhood) and (caries or decay*)) #11 MOUTH NEOPLASMS/ #12 (oral next cancer*) or ((gingival or mouth or lip* or tongue or (salivary next gland) or palatal or parotid or sublingual or submandibular) AND (cancer* or carcinoma* or neoplasm* or tumour* or tumor*)) #13 leukoplaki* #14 hairy next tongue #15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 #16 Exp ORAL HYGIENE/ #17 Exp MOUTHWASHES/ #18 Exp DENTIFRICES/ #19 (oral next hygiene) or (mouth near care) or (dental near care) or (care near teeth) or (mouth next hygiene) or (plaque near control*) or (plaque near remov*) #20 toothbrush* or tooth‐brush* or toothpaste* or dentifrice* or mouthwash* or mouth‐wash* or mouthrinse* or mouth‐rinse* or fluoride* #21 ((interdental next clean*) or (inter‐dental next clean*) or (tooth near clean*) or (teeth near clean*) or (denture* near hygiene) or (denture* near clean*) or (tongue next scrap*) or (tongue next brush*) or (chewing next stick*) or (chewing next gum*) or (orthodontic next appliance near clean*)) #22 (chewing‐gum or sugar‐free next gum) #23 ((dental or tooth or teeth or interdental* or inter‐dental*) and floss*) #24 ((dental next plaque next index) or (dental next plaque next indices) or (DMF* next index) or (DMF next indices) or (dmf* next index) or (dmf* next indices) or (periodontal next index) or (periodontal next indices) or (oral next hygiene next index) or (oral next hygiene indices) or (gingival next index)) #25 #16 or #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 or #24 #26 Exp HEALTH EDUCATION DENTAL #27 Exp HEALTH PROMOTION #28 instruct* or advice or advise* or educat* or teach* or train* #29 (((health* near promot*)) and (dental or teeth or mouth or periodont* or gingival* or (oral next health))) #30 ((demonstrat* near toothbrush*) or (demonstrat* near "tooth brush*") or (demonstrat* near tooth‐brush*) or (demonstrat* near floss*) or (demonstrat*near "oral hygiene*") or (demonstrat* near "interdental cleaning") or (demonstrat* near wood‐stick*) or (demonstrat* near "wood stick*") or (demonstrat* near "interdental massag*")) #31 ((supervis* near toothbrush*) or (supervis* near floss*) or (supervis* near "oral hygiene") or (supervis* near "interdental cleaning") or (supervis* near wood‐stick*) or (supervis* near "wood stick*") or (supervis* near "interdental massag*")) #32 #26 or #27 or #28 or #29 or #30 or #31 #33 HEALTH BEHAVIOR/ #34 PATIENT COMPLIANCE/ #35 ADOLESCENT BEHAVIOR/ #36 MOTIVATION/ #37 ((behavior* OR behaviour*) AND (change OR changed OR changing or modify OR modified OR modification)) #38 “feed back device*” or “feedback device*” #39 (attitude* near (oral next health)) or (attitude near (oral next care)) or (attitude near (dental next health)) or (attitude near “mouth hygiene”) or (attitude near “oral hygiene”) #40 (((oral next hygiene) near improv*) or (“oral health” near improv*) or ("gingival health" near improv*) or ("periodontal health" near improv*) or ("periodontal condition" near improv*) or (caries near reduc*)) #41 #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 #42 ((#15 OR #25) AND (#32 OR #41))
Appendix 3. MEDLINE Ovid search strategy
1. ORAL HEALTH/ 2. exp Stomatognathic Diseases/ 3. exp HALITOSIS/ 4. ((dental or tooth or teeth or enamel or root$) and (decay$ or caries or carious or white spot$ or plaque or reminerali$ or deminerali$)).mp. 5. (periodont$ or gingivitis or "gingival$ inflamm$" or "gingival$ bleed$" or "gingival pocket$" or "periodont$ pocket$" or (periodont$ adj3 attachment) or (gingival$ adj3 attachment)).mp. 6. (stomatitis or "mouth ulcer$" or "oral ulcer$" or "oral candidi$" or "aphthous ulcer$" or (mouth adj3 aphthae) or (oral adj3 aphthae) or (mucositis adj3 oral) or (mucositis adj3 mouth)).mp. 7. ("oral health" or "dental health" or orthodontic$).mp. 8. ("tooth wear" or ((tooth or dental or teeth or enamel) and (erosion or abrasion))).mp. 9. (halitosis or "mouth odour$" or "mouth odor$" or "mouth malodour$" or "mouth malodor$" or "oral malodour$" or "oral malodour" or (breath adj3 malodour$) or (breath adj3 odour$) or (breath adj3 odor$)).mp. 10. (("bottle caries" or "nursing caries" or "bottle decay$" or "early childhood") and (caries or decay$)).mp. 11. exp Mouth Neoplasms/ 12. (("oral cancer$" or ((gingival or mouth or lip$ or tongue or salivary) adj (gland or palatal or parotid or sublingual or submandibular))) and (cancer$ or carcinoma$ or neoplasm$ or tumour$ or tumor$)).mp. 13. leukoplaki$.mp. 14. hairy tongue.mp. 15. or/1‐14 16. exp ORAL HYGIENE/ 17. exp MOUTHWASHES/ 18. exp DENTIFRICES/ 19. ("oral hygiene" or (mouth adj3 care) or (dental adj3 care) or (care adj3 teeth) or (mouth adj3 hygiene) or (plaque adj4 control$) or (plaque adj4 remov$)).mp. 20. (toothbrush$ or tooth‐brush$ or toothpaste$ or dentifrice$ or mouthwash$ or mouth‐wash$ or mouthrinse$ or mouth‐rinse$ or fluoride$).mp. 21. ("interdental clean$" or "inter‐dental clean$" or (tooth adj4 clean$) or (teeth adj4 clean$) or (denture$ adj4 hygiene) or (denture$ adj4 clean$) or "tongue scrap$" or (tongue adj3 brush$) or "chewing stick$" or "chewing gum$" or ("orthodontic appliance$" adj3 clean$)).mp. 22. (chewing‐gum or "sugar‐free gum").mp. 23. ((dental or tooth or teeth or interdental$ or inter‐dental$) and floss$).mp. 24. ("dental plaque index" or "dental plaque indices" or "DMF? index" or "DMF? indices" or "periodontal index" or "periodontal indices" or "oral hygiene index" or "oral hygiene indices" or "gingival index").mp. 25. or/16‐24 26. Health Education, Dental/ 27. exp Health Promotion/ 28. (instruct$ or advice or advise$ or educat$ or teach$ or train$).mp. 29. ((health$ adj3 promot$) and (dental or teeth or mouth or periodont$ or gingival$ or "oral health")).mp. 30. ((demonstrate$ adj4 toothbrush$) or (demonstrate$ adj4 "tooth brush$") or (demonstrat$ adj3 tooth‐brush$) or (demonstrate$ adj3 floss$) or (demonstrate$ adj3 "oral hygiene$") or (demonstrat$ adj3 "interdental cleaning") or (demonstrate$ adj3 wood‐stick$) or (demonstrate$ adj3 "wood stick$") or (demonstrate$ adj3 "interdental massage$")).mp. 31. ((supervise$ adj3 toothbrush$) or (supervise$ adj3 floss$) or (supervise$ adj3 "oral hygiene") or (supervise$ adj3 "interdental cleaning") or (supervise$ adj3 wood‐stick$) or (supervise$ adj3 "wood stick$") or (supervise$ adj3 "interdental massage$")).mp. 32. or/26‐31 33. HEALTH BEHAVIOR/ 34. PATIENT COMPLIANCE/ 35. ADOLESCENT BEHAVIOR/ 36. MOTIVATION/ 37. ((behavior$ or behaviour$) and (change or changed or changing or modify or modified or modification)).mp. 38. ("feed back device$" or "feedback device$").mp. 39. ((attitude$ adj3 "oral health") or (attitude adj3 "oral care") or (attitude adj3 "dental health") or (attitude adj3 "mouth hygiene") or (attitude adj3 "oral hygiene")).mp. 40. (("oral hygiene" adj3 improv$) or ("oral health" adj3 improv$) or ("gingival health" adj3 improv$) or ("periodontal health" adj3 improv$) or ("periodontal condition" adj3 improv$) or (caries adj3 reduc$)).mp. 41. or/33‐40 42. (15 or 25) and (32 or 41)
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1 “oral health” 2 Exp MOUTH DISEASE/ 3 HALITOSIS/ 4 ((dental or tooth or teeth or enamel or root$) AND (decay$ or caries or carious or “white spot$” or plaque or reminerali$ or deminerali$)) 5 (periodont$ or gingivitis or “gingival$ inflamm$” or “gingival$ bleed$” or “gingival pocket$ or “periodont$ pocket$ or (periodont$ adj3 attachment) or (gingival$ adj3 attachment)) 6 stomatitis or “mouth ulcer$ or “oral ulcer$ or “oral candidi$ or “aphthous ulcer$” or (mouth adj3 aphthae) or (oral adj3 aphthae) or (mucositis adj3 oral) or (mucositis adj3 mouth) or xerostomi$ 7 “oral health” or “dental health” or orthodontic$ 8 ("tooth wear" or ((tooth or dental or teeth or enamel) and (erosion or abrasion))) 9 (halitosis or “mouth odour$” or “mouth odor$” or “mouth malodour$ or “mouth malodor$ or “oral malodour$” OR “oral malodour” or (breath adj3 malodour$) or (breath adj3 odour$) or (breath adj3 odor$)) 10 “bottle caries” or “nursing caries” or “bottle decay$” or (“early childhood”) and (caries or decay$)) 11 MOUTH TUMOR/ 12 (((oral next cancer$) or (gingival or mouth or lip$ or tongue or (salivary next gland) or palatal or parotid or sublingual or submandibular) AND (cancer$ or carcinoma$ or neoplasm$ or tumour$ or tumor$)) 13 leukoplaki$ 14 “hairy tongue” 15 OR/1‐14 16 “Mouth hygiene” 17 mouthwash$ 18 TOOTHPASTE/ 19 “oral hygiene” or (mouth adj3 care) or (dental adj3 care) or (care adj3 teeth) or (mouth adj3 hygiene) or (plaque adj4 control$) or (plaque adj4 remov$) 20 toothbrush$ or tooth‐brush$ or toothpaste$ or dentifrice$ or mouthwash$ or mouth wash$ or mouthrinse$ or mouth‐rinse$ or fluoride$ 21 (“interdental clean$” or “inter‐dental clean$ or (tooth adj4 clean$) or (teeth adj4 clean$) or (denture$ near hygiene) or (denture$ adj4 clean$) or “tongue scrap$” or (tongue adj3 brush$) or “chewing stick$” or “chewing gum$” or (“orthodontic appliance$” adj3 clean$)) 22 (chewing‐gum or sugar‐free next gum) 23 ((dental or tooth or teeth or interdental$ or inter‐dental$) and floss$) 24 (“dental plaque index” or “dental plaque indices” or “DMF$ index” or “DMF indices” or “dmf* index” or “dmf4 indices” or “periodontal index” or “periodontal indices” or “oral hygiene index” or (“oral hygiene indices”) or “gingival index”)) 25 OR/16‐24 26 DENTAL HEALTH EDUCATION/ 27 HEALTH PROMOTION/ 28 instruct$ or advice or advise$ or educat$ or teach$ or train$ 29 (((health$ adj3 promot$)) and (dental or teeth or mouth or periodont$ or gingival$ or “oral health”)) 30 ((demonstrate$ adj4 toothbrush$) or (demonstrate$ "tooth brush$") or (demonstrat$ adj3 tooth‐brush$) or (demonstrate$ adj3 floss$) or (demonstrate$ adj3 "oral hygiene$") or (demonstrat$ adj3 "interdental cleaning") or (demonstrate$ adj3 woodstick$) or (demonstrate$ adj3 "wood stick$") or (demonstrate$ near "interdental massage$")) 31 ((supervise$ adj3 toothbrush$) or (supervise$ adj3 floss$) or (supervise$ adj3 "oral hygiene") or (supervise$ adj3 "interdental cleaning") or (supervise$ adj3 wood‐stick$) or (supervise$ adj3 "wood stick$") or (supervise$ adj3 "interdental massage$")) 32 OR/26‐31 33 HEALTH BEHAVIOR/ 34 PATIENT COMPLIANCE/ 35 (adolescen$ adj3 (behavior or behaviour)) 36 MOTIVATION/ 37 ((behavior$ OR behaviour$) AND (change OR changed OR changing or modify OR modified OR modification)) 38 “feed back device$” or “feedback device$” 39 ((attitude$ adj3 “oral health”) or (attitude adj3 “oral care”) or (attitude adj3 “dental health”) or (attitude adj3 “mouth hygiene”) or (attitude adj3 “oral hygiene”) 40 ((“oral hygiene” adj3 improv$) or (“oral health” adj3 improv$) or ("gingival health" adj3 improv$) or ("periodontal health" adj3 improv$) or ("periodontal condition" adj3 improv$) or (caries adj3 reduc$)) 41 OR/33‐40 42 ((15 OR 25) AND (32 OR 41))
This subject search was linked to an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information).
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
oral hygiene and advice oral hygiene and promotion
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
"oral hygiene" and advice "oral health" and advice "oral hygiene" and promotion "oral health" and promotion
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aljafari 2017.
| Methods |
Trial design: parallel (2 arms) Location: King's College Hospital, London, UK Number of centres: 1 Study Duration: "Recruitment took place between October 2013 and October 2014" with 3‐month follow‐up |
|
| Participants |
Participants: children referred for extraction of decayed teeth under general anaesthesia Inclusion criteria: "included all healthy children scheduled for GA due to dental caries, given that they were 4–10 years of age and were accompanied by a parent/guardian with sufficient English proficiency to enable consent" Exclusion criteria: lacking English proficiency to enable consent, child having learning disability, already recruited to other research projects, legal guardian not present Age at baseline (years): intervention group: 6.76 years (SD = 1.49); control group: 6.15 (SD = 1.57) Gender: intervention group: 25 (45%) female and 30 (55%) male; control group: 23 (43%) female and 31 (57%) male Number randomised: 109; intervention group: 55; control group: 54 Number evaluated: 59 (intervention group: 28; control group: 31) |
|
| Interventions | The education messages were based on the recommendations for high‐caries‐risk children in the second edition of Delivering Better Oral Health (DBOH 2009) Intervention: computer game group: the child and parent played the computer game on a touch tablet and received a copy of it on a DVD to play at home Control: one‐to‐one health educator group: the child and parent received verbal oral health education from a dental nurse with a health education qualification Prophylaxis provided: none reported Member of dental team delivering intervention: self‐administration at home for intervention group, dental nurse for control group Frequency of intervention: intervention group: 1‐off in clinic and thereafter delivered at home; control group: 1‐off intervention Intensity of intervention (length of time): not reported Setting: secondary care Disease level: high caries rate |
|
| Outcomes | (1) Parent and child satisfaction with their educational intervention, scored using a 100‐mm visual analogue scale (VAS), where the highest satisfaction = 100 mm, collected immediately following delivery (2) Child's dietary knowledge, scored using a Pictorial Dietary Quiz (PDQ) (Rice 2008), taken at baseline, immediately following the educational intervention and at a 3‐month follow‐up dental visit (3) Child's dietary habits, scored by the parent using the Children's Dietary Questionnaire (CDQ) (Magarey 2009), at baseline and at a 3‐month telephone call. The CDQ has 4 parameters: fruits and vegetables; full‐fat dairy; sweetened drinks; and non‐core foods (those containing high amounts of saturated fat, added salt, or added sugars) (4) The child's self‐reported snacking and toothbrushing practices recorded in a diary that is given out on the day of the intervention and returned to the researcher at the GA visit In addition to those measures, children in the computer game group verbally reported their views on the content of the game directly to the dental nurse‐educator, who recorded it verbatim, and completed a 'Secret Password Questionnaire' that verified whether or not they had played the game at home |
|
| Notes |
Funding: "The study is funded by King's College London and did not receive any external funding" Sample size calculation: "The primary outcome measure used to calculate the sample size was the participant's satisfaction with the educational intervention. This was assessed on a 100‐mm VAS. Assuming a standard deviation of 25 mm, and aiming to detect a difference of at least 15 mm between the groups, a sample of 45 participants in each group was needed to provide 80% power at the 5% significance level and to detect effects of size 0.6 and above" Adverse effects: not reported Declarations/conflicts of interest: "The authors declare that they have no competing interests" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised using a computer‐generated grid" |
| Allocation concealment (selection bias) | Low risk | Quotes: "dental nurse‐educator allocated the participants according to the randomisation grid and administered the immediate post‐intervention measures. She also delivered the oral health education to the control group." "After the baseline measures are completed, AA will introduce the participants to the dental nurse and leave, to ensure that he remains blinded to group allocation. The nurse will then allocate the participants to either the video‐game group or the control group according to the randomization grid and will apply the intervention accordingly" Comment: allocation method unlikely to have affected outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The lead author (AA) collected the baseline measures. A dental nurse‐educator allocated the participants according to the randomisation grid and administered the immediate post‐intervention measures. She also delivered the oral health education to the control group. All data were coded and anonymised. AA administered the telephone and dental follow‐up measures. He remained blinded to group allocation until after the statistical analysis was completed" Comments: dental nurse‐educators were aware of intervention allocation and provided oral health education to the control group which may have introduced bias as confirmed with author. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher (AA) will remain blinded all through data collection and input. The statistician will also be blinded during the analysis. Only after data collection is complete will one researcher (MTH) break the randomization code to input the group allocation within the pre‐existing data set and enable between‐group analyses. The statistician and lead researcher (AA) will remain blinded" Further information from author: "allocation was completely concealed from AA until after AA had completed the analysis. MTH had code break in safe, in an envelope until after the stats had been completed, checked and discussed with a statistician" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes: "Only 55% of the recruited sample completed the telephone follow‐up 3 months after the child's GA. Hence, the reported dietary improvements need to be interpreted with caution." "A sample of 45 participants in each group will be needed to provide 80 % statistical power" Comment: although this is a high disease risk and hard to reach population, the attrition rate is very high |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the pre‐published protocol were reported |
| Other bias | Low risk | Comments: no apparent other bias |
Baab 1986.
| Methods |
Trial design: parallel (2 arms) Location: University of Washington's Graduate Periodontics Clinic, USA Number of centres: 1 Study Duration: February, March and April, 1982. Participants followed‐up at 6 months |
|
| Participants |
Participants: the subjects had completed active periodontal treatment, including surgery, at least 1 year previously at the University of Washington's Graduate Periodontics Clinic, and were being seen by dental hygienists every 3 months for oral hygiene instruction, scaling and root planing Inclusion criteria: those selected had at least 20 teeth present, including 2 contralateral molars Exclusion criteria: no physical handicaps affecting vision or manual dexterity Age at baseline (years): range 30 to 76 Gender: 18 (55%) women and 15 (45%) men Number randomised: 33 (no group breakdown) Number evaluated: 31 (intervention group 15; control group 16) |
|
| Interventions |
Intervention: OH instruction using a self‐inspection plaque index. Basic treatment including:
‐ baseline questionnaire, plaque and bleeding indices, OH skill assessment, plaque index (after OH), OH instruction and scaling;
‐ OH instruction at 2 weeks;
‐ plaque and bleeding indices, OH skill assessment, plaque index (after OH) and OH instruction at 1.5 months;
‐ plaque and bleeding indices, OH skill assessment, plaque index (after OH), OH instruction and scaling at 3 months;
‐ plaque and bleeding indices, OH skill assessment and plaque index (after OH) at 6 months. Oral hygiene instruction for the 15 patients in the self‐inspection group consisted of the following steps. First, the "Oral self‐inspection manual" (Baab 1983) was used to guide the patients to score the presence or absence of disclosed plaque on 6 index teeth. Teeth 11, 26, and 36 were to be assessed from the lingual, using a lighted dental mirror (Mirolite #711®), and 16, 41, and 46 were to be scored from the facial. If an index tooth was missing, the next tooth distally was used. A line drawing of each index tooth indicated 2 interdental areas and 1 radicular area, near the gingiva, to be scored. The self‐inspection plaque index (SIPI) consisted of the total surfaces out of 18 (3 surfaces per tooth) with disclosed plaque. Patients were guided by the manual to interpret their scores and to correct deficiencies in plaque removal. No particular brushing stroke was advocated because evidence is lacking that any one technique is superior to another (Rugg‐Gunn 1979). After the instructional session, the patients were given 24 erythrosin disclosing wafers (Xpose"*), the lighted dental mirror, the manual and the oral hygiene tools they had selected. Patients were asked to examine the 6 teeth for disclosed plaque once each week following personal oral hygiene. During follow‐ up visits at 2 weeks, 1.5 months and 3 months, the hygienist provided immediate feedback on the patients' SIPI accuracy, and reviewed the patients accuracy and reviewed the patients' self‐scoring charts Control: traditional instruction using professional monitoring of disclosed plaque. Basic treatment including: ‐ baseline questionnaire, plaque and bleeding indices, OH skill assessment, plaque index (after OH), OH instruction and scaling; ‐ OH instruction at 2 weeks; ‐ plaque and bleeding indices, OH skill assessment, plaque index (after OH) and OH instruction at 1.5 months; ‐ plaque and bleeding indices, OH skill assessment, plaque index (after OH), OH instruction and scaling at 3 months; ‐ plaque and bleeding indices, OH skill assessment and plaque index (after OH) at 6 months. The 16 patients in the traditional group were shown where disclosed plaque remained after the oral hygiene skills assessment. The hygienist observed the patients' cleaning and afterward gave feedback regarding how long they spent brushing the facial and lingual surfaces and how effectively they used oral hygiene aids. The dental hygienist was given a relatively free hand regarding the instructional methods; emphasis was placed on plaque removal skills using the various oral hygiene aids, rather than on self‐assessment of disclosed plaque. Information concerning the SIPI was not provided, and disclosing wafers and lighted dental mirrors were not dispensed 30 minutes were spent on oral hygiene instructions at follow‐up visits for both groups Prophylaxis provided: scaling at baseline and 3 months Member of dental team delivering intervention: hygienist Frequency of intervention: intervention: 5 visits with repeated OH instruction; control: 5 visits with repeated OH instruction Intensity of intervention (length of time): intervention: 30 minutes; control: 30 minutes Setting: secondary care Disease level: high caries rate |
|
| Outcomes | Oral hygiene and gingival health were assessed by recording the presence or absence of disclosed plaque at the gingival margin (O'Leary 1972) and bleeding upon measurement probing (Van der Velden 1979). Mean observed time (seconds) spent brushing the facial and lingual surfaces during the oral hygiene skill assessment. Total time (minutes) spent by patients in oral hygiene procedures during the oral hygiene skill assessment at the initial and subsequent examinations (mean + standard deviation). Mean number of oral hygiene aids selected by subjects for the oral hygiene skill assessment at the initial and subsequent examinations | |
| Notes |
Funding: this study was supported by a Biomedical Research Support Grant #RR‐ 05346 from the National Institutes of Health Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned by rolling of dice to 2 groups, self‐inspection and traditional, before the initial examination" |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The dental hygienist was given a relatively free hand regarding the instructional methods" Comments: personnel were aware of intervention allocation. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "2 raters, who were unaware of the group assignments, examined the patients initially, and at 1.5, 3 and 6 months" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 2 dropped out, unsure of allocation |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Unclear risk | Comment: SIPI group and traditional group were significantly different at baseline regarding plaque scores and time spent brushing facial aspects of teeth |
Bali 1999.
| Methods |
Trial design: parallel (2 arms) Location: Wien‐Lainz Hospital, Austria Number of centres: 1 Duration: enrolment took place between beginning of April 1995 until end of March 1996. Follow‐up completed at 6 months and 12 months |
|
| Participants |
Participants: eligible diabetic patients attending the Wien‐Lainz Hospital Inclusion criteria: type I diabetes for at least 3 years, aged between 14 years and 50 years. Minimum of 10 own teeth Exclusion criteria: motor disability, severe visual impairment, acute diabetic foot, myocardial infarction or cerebral insult within the last 6 months Age at baseline (years): control group: mean age 34.4 years, SD 9.5; intervention group: mean age 33.9 years, SD 9.5 Gender: not reported Number randomised: 121 (intervention group: 61; control group: 60) Number evaluated: 70 (intervention group: 36; control group: 34) |
|
| Interventions |
Intervention: professional OHA after enrolment into the study + intensive patient education in oral hygiene (dental cleaning techniques, use of dental floss or interdental brushes or both) with 3 control examinations within 3 weeks to examine (by staining the plaque) the improvement in dental cleaning with training of the dental cleaning technique repeated in cases where plaque, according to a Turesky‐Index, of 2.0 or more was found Control: professional OHA after enrolment into the study Prophylaxis provided: not reported Member of dental team delivering intervention: not explicitly specified, however after reviewing the text the review authors were confident the intervention was undertaken by a member of the dental team Frequency of intervention: intervention: 4 visits with repeated OH instruction; control: 1 visit Intensity of intervention (length of time): not reported Setting: secondary care Disease level: not reported |
|
| Outcomes | Main outcome measure was bleeding on probing. Secondary outcome measures were plaque index (Turesky hygiene index); probing pocket depth at 6 measurement points per tooth; DMFT | |
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated randomly to the intervention or control group" Comment: insufficient information on randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: insufficient information available on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported Comment: insufficient information to determine any bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported Comment: insufficient information to determine any bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts (42%) were not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | The predefined measures "panoramic X‐ray" and "HbA1c" are reported initially but not in the result section. Panoramic X‐ray was completed to document the dental chart and to quantify the caries. HbA1C was measured to determine the quality of the metabolic adjustment Comment: DMFT is reported in the results section with further panoramic X‐ray findings/completion not specifically reported. Repeat HbA1C not reported as planned to be repeated though changes may have had an impact on oral health outcomes |
| Other bias | Low risk | No other bias apparent |
Glavind 1985.
| Methods |
Trial design: parallel (2 arms) Location: general dental practice, Denmark Number of centres: 3 Study Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 6 months |
|
| Participants |
Participants: 55 adults who sought treatment in 3 different general dental practices in Denmark Inclusion criteria: plaque on more than 30% of their tooth surfaces Exclusion criteria: not reported Age at baseline (years): range 20 years to 62 years, mean 34 years; no group breakdown provided Gender: "The distribution of patients in the 2 groups was similar with regard to sex and age" Number randomised: 55 (intervention group: 29; control group: 26) Number evaluated: 55 (intervention group: 29; control group: 26 |
|
| Interventions |
Intervention: a self‐instructing group (S) comprising 29 patients were given a self‐examination manual (Glavind 1979) at their initial appointment and a self‐instruction manual in oral hygiene measures for use at home at their 2‐week appointment Control: a conventional personal instruction group (C) comprising 26 patients who received information about the presence of disease in the mouth and the need for improved oral cleanliness. They were given instructions in proper oral hygiene techniques at the chair‐side by a dentist for approximately 25 minutes to 30 minutes at their 2‐week appointment Prophylaxis provided: scaling of teeth was completed in 2 sessions (weeks 1 and 2) and all patients were provided with oral hygiene aids such as a toothbrush, toothpicks, disclosing tablets and a lighted mouth mirror Treatment provided: treatment of carious lesions was undertaken according to need during the experimental period Member of dental team delivering intervention: dentist Frequency of intervention: intervention: 2 visits, 1 with OHA; control: 2 visits, 1 with OHA Intensity of intervention (length of time): intervention: self‐instruction manual for use at home; control: 25 minutes to 30 minutes Setting: primary care Disease level: plaque levels |
|
| Outcomes | In order to evaluate the effect of the 2 different modes of instruction, the presence or absence of disclosed dento‐gingival plaque (Plak‐lite) and gingival bleeding by gentle probing was scored on all tooth surfaces initially and after 3 months and 6 months. Plaque was also scored 1 week after instruction. The presence or absence of gingival bleeding was assessed for each surface while plaque was recorded for each quadrant as the number of mesial‐, facial‐, lingual‐ and distal tooth surfaces with disclosed plaque. The percentage of tooth surfaces with plaque and gingival bleeding was calculated and presented to the patient in graphical form in order to obtain feedback from the result of the treatment. A questionnaire about the treatment was answered by the patients of the self‐instruction group at the 2‐week appointment | |
| Notes |
Funding: quote: "This study has been supported by a grant from the Research Foundation of the Danish Dental Association" Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These patients were divided randomly into 2 treatment groups" Comment: no further detail on randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "Conventional group..... were given instructions in proper oral hygiene techniques at the chair side by a dentist...", "The scoring was performed by the 3 general dental practitioners (EP, HC, HR) who treated the patients in their private offices. At the time of scoring, the dentist did not know to which group the patient belonged" Comments: blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The scoring was performed by the 3 general dental practitioners (EP, HC, HR) who treated the patients in their private offices. At the time of scoring, the dentist did not know to which group the patient belonged" Comment: insufficient information provided to determine if same dentists provided intervention and follow‐up outcome measure assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "2 patients in each group did not show up for the 3‐month examination", "The questionnaire on self‐examination and self‐instruction in oral hygiene was answered by 26 out of the 29 patients in the self‐instruction group (S)" |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Hetland 1982.
| Methods |
Trial design: parallel (3 arms) Location: dental office at factory, Sandnes, Southwestern Norway Number of centres: 1 Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 24 weeks |
|
| Participants |
Participants: 77 industrial and commercial firm employees Inclusion criteria: participants selected at random by the personnel division of the firm among a total of 1500 employees. The test persons were to be between 25 years and 45 years of age and to have at least 20 remaining teeth. The sample was to include industrial workers as well as office and retail store employees Exclusion criteria: not reported Age at baseline (years): range 25 years to 44 years Gender: follow‐up: control Group A: 21 (84%) males, 4 (16%) females; intervention Group C: 19 (83%) males, 4 (17%) females Number randomised: 77 participants Number evaluated: 71 (control Group A: 25; intervention Group B: 23; intervention Group C: 25) |
|
| Interventions |
Interventions: ‐ Group C: the participants in Group C were subjected to depuration, removal of overhangs or polishing. They were given instructions in oral hygiene procedures utilizing a 3‐visit program with 1 week between each visit. Instructions in oral hygiene were carried out by 4 dental chair‐side assistants who had received an intensive course of 40 hours' duration ‐ Group B: received OHA alone ‐ not included further in review Control: ‐ Group A: the participants in Group A received depuration of the teeth utilizing an ultrasonic apparatus (Cavitron®, Cavitron Ultra‐ sonics Inc, Long Island City, NY, USA, or Odontoson® (Goof, Herlev, Denmark) and an Ivory number 2/3 sealer. Gross overhangs were removed using a diamond point and the teeth were polished with rubber‐cup and pumice (as per Group C). A maximum of 45 minutes were allocated per person for these procedures. In Group A no oral hygiene instructions were given Prophylaxis provided: removal of retentive factors and polishing at initial appointment Member of dental team delivering intervention: dental nurse Frequency of intervention: intervention: 3 intervention visits with OHA; control: no oral hygiene but appointment for prophylaxis lasted 45 minutes Intensity of intervention (length of time): intervention Group C: 45 minutes OHA at first visit, 30 minutes for the second visit and 15 minutes for third visit. Control: no OHA provided Setting: dental surgery attached to factory Disease level: not reported |
|
| Outcomes | At the initial examination which took approximately 30 minutes the following registrations were performed in this sequence: DMF teeth, gingival index (Löe 1967), retention index (Björby 1967), dental surfaces harbouring visible plaque alter staining (Diaplac* tablets, Astra, Sweden) registered both from the vestibular and lingual aspect, registration of pockets of 4 mm depth or more using calibrated 'Hilming' periodontal probes (Brincker, Copenhagen, Denmark). For gingival index scoring, all dental surfaces were examined and attempts to provoke bleeding were performed with a pocket probe by carrying this back and forth within the sulcus. A score of 2 was given even if bleeding was observed only after some time The following parameters were calculated on the basis of clinical examinations: (a) DMF teeth in percentage (DMFT), (b) gingival index as mean for all surfaces (GI), (c) retention index as mean for all surfaces (RI), (d) number of pockets per remaining tooth (P/T), (e) proportion of remaining teeth with at least 1 pocket (TP), and (f) proportion of surfaces harbouring visible plaque (PS) The participants were interviewed with respect to smoking habits (0, 0 to 10, > 10 cigarettes per day) |
|
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On the basis of the findings at the initial examination the participants were divided into three experimental groups. Group A, Group B and Group C. The three groups were matched by stratified randomization with respect to number of participants, sex, age, smoking habits, occupation and dental findings at the start of the experiment. Several alternative assignment algorithms were examined" Comment: unclear regarding randomisation method employed |
| Allocation concealment (selection bias) | Unclear risk | Not provided Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Instructions in oral hygiene were carried out by four dental chair‐side assistants who had received an intensive course of 40 hours' duration" Comment: blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The three groups were re‐examined 4, 12 and 24 weeks later utilizing the same indices and following the same procedures as at the initial examination. The participants were examined by the same examiner during the entire experiment. The examiners were unaware of whether or not oral hygiene instructions had been given to the particular participant. The examiners had been calibrated during a 1‐week period prior to the start of the experiments" Comment: examiners reported to be blind to experimental group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: small dropout rate across the trial (8%). Insufficient information provided to assess whether dropouts were balanced |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Hoogstraten 1983.
| Methods |
Trial design: parallel (3 arms) Location: group practice, Abcoude, the Netherlands Number of centres: 1 Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 12 months |
|
| Participants |
Participants: 108 male and female inhabitants of Abcoude Inclusion criteria: recently registered patients of the group practice, between the age of 15 years and 60 years old Exclusion criteria: no full dentures and no more than 1 person per family admitted to the sample Age at baseline (years): range 15 to 60 Gender: not reported Number randomised: 150 (intervention Group A: 50; intervention Group B: 50; control Group C: 50) Number evaluated: 108 (intervention Group A: 36; intervention Group B: 36; intervention Group C: 36) |
|
| Interventions |
Interventions: ‐ Group A: subjects received standard information and instruction on dental hygiene. The instruction, given by 1 of 4 female hygienists during about 30 minutes, consisted of information concerning the relations between dental health and sugar consumption, oral hygiene, different methods of oral hygiene, the use of fluoride, information about regular control visits to the dentist, etc. ‐ Group B: subjects received the identical standard information as Group A subjects. Before that, however, they were shown a 10‐minute film (a Dutch version of "4 Tons of Teeth") presenting more or less the same issues as the hygienist's standard presentation Control: ‐ Group C: received no instruction at all (control condition); special care was taken to insure that these subjects were excluded from information on dental hygiene Prophylaxis provided: scaling and polishing at initial appointment Member of dental team delivering intervention: hygienist Frequency of intervention: intervention Group A: not clearly reported; appears to be 1 intervention visit with OHA. Intervention Group B: not clearly reported; appears to be 1 visit with OHA and 10‐minute film. Control: no OHA provided Intensity of intervention (length of time): intervention Group A: 30 minutes OHA. Intervention Group B: 30 minutes OHA and 10‐minute video. Control: no OHA provided Setting: primary care Disease level: not reported |
|
| Outcomes | "The questionnaire contained items on dental knowledge, attitude, behaviour, and fear of dental treatment. The subject's knowledge was tapped with 14 multiple choice items. Each item had 5 alternatives, 1 of which was correct. The knowledge scores thus ranged from 0 to 14. The attitude towards dental hygiene was measured with 10 items of the summated‐rating format with scale points ranging from 1 (agree) to 5 (disagree). The higher the score, the more positive the subject's attitude. Fear of dental treatment was assessed with 1 item, with scale points ranging from 1 (very fearful) to 5 (not fearful). 6 aspects of self‐reported dental behaviour were measured: sugar consumption (range 1‐5), brushing frequency (range 1‐5), brushing moment (range 1‐6), use of toothbrush (range 1‐6), use of interdental stimulators (range 1‐5) and oral hygiene (range 1‐5). Again, the higher the score, the more positive the subjects behaviour" "Half of the subjects of each condition received a pre‐test (filling in a questionnaire). The other half did not complete this pre‐test. Through this 2 x 3 design, not only the effect of the educational programs can be assessed, but also the possible effect of the pre‐test on subsequent post‐test scores" |
|
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "They were randomly selected", "Subjects were assigned at random to 1 of 3 conditions" Comment: insufficient information provided on the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comments: blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: patients completed a questionnaire and would have been aware of the intervention provided or otherwise. The outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "As usual in longitudinal studies, however, there was a certain dropout of subjects due to reasons such as moving house and leaving no change of address, emigration or an occasional refusal to cooperate" Comment: insufficient information to assess bias |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Unclear risk | Quote: Group A "Whilst presenting the information to the patient, the hygienist performed regular preventive measures, such as scaling and polishing" Comment: unclear if Group B and Group C received the same regular preventive measures. No response from contact author regarding further information |
Hugoson 2007.
| Methods |
Trial design: parallel (4 arms) Location: 2 clinics: a large Public Dental Service (PDS) clinic and a private 2‐dentist practice in Jönköping, Sweden Number of centres: 2 Study Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 3 years |
|
| Participants |
Participants: 400 subjects aged 20 years to 27 years, 211 males and 189 females, participated in the study Inclusion criteria: young adults regularly seeking dental care where the individual was not planning to move from Jönköping within the next few years Exclusion criteria: not reported Age at baseline (years): 20 to 27 Gender: 189 (47%) females and 211 (53%) males Number randomised: 400 participants Number evaluated: 348 participants. The dropout rates during the study were 2.3% (9 individuals), 4.3% (17 individuals), and 6.5% (26 individuals) after 1 year, 2 years, and 3 years, respectively, or a total of 13% (52 individuals) after 3 years |
|
| Interventions | In all test groups, a soft toothbrush (Swedish brand 'TePe', Malmo, Sweden) and either toothpicks (TePe) or dental floss (Johnson & Johnson dentotape, Sollentuna, Sweden) were used at the oral hygiene instruction sessions. Instructions on the Bass method (Bass 1954a) were given if the subject's own brushing technique proved unsatisfactory, and all subjects were advised to start their brushing lingually in the lower jaw molar region. The patients were informed, according to routine procedures at the clinic, that the best way of establishing the habit of interdental cleaning is to clean the teeth proximally before brushing. In groups 2 and 3, the oral hygiene instruction or re‐instruction was carried out after the participants used a disclosing Diaplack tablet. The proximal aids, dental floss or toothpicks, were chosen depending on anatomical conditions (e.g. crowded teeth), and the degree of gingivitis when toothpicks were recommended in cases with severe proximal inflammation or 'open' proximal spaces. The subjects were asked to demonstrate and practice the cleaning technique in their own mouths Group 1: the control group: no organized prophylactic measures for caries and gingivitis/periodontitis within the framework of the study but had to answer a questionnaire about knowledge of dental diseases and oral hygiene behaviour. The subjects were recalled at 12‐month intervals for follow‐up examinations, identical to the baseline examination, over the next 3 years Group 2: the Karlstad model. All individuals received prophylactic care every second month (6 times/year) according to the Karlstad model for adult individuals. At the first visit, approximately 30 minutes, information on caries and gingivitis/periodontitis was presented and oral hygiene instruction was given based on plaque disclosure. At the next 5 visits, approximately 20 minutes each at 2‐month intervals, the individual's oral status was reviewed and, when necessary, information or oral hygiene instruction was repeated. Half the number of the individuals were also randomly chosen to have no other preventive measures (20). The other individuals were randomly chosen to undergo professional tooth cleaning at each visit. The cleaning was performed crosswise in 2 quadrants, which meant that the teeth in the right maxilla and the left mandible were professionally cleaned in 25 individuals (21) and in the left maxilla and the right mandible in 25 individuals (22). The 1‐year follow‐up comprised the same measures undertaken at the baseline examination. The remedial measures undertaken during the first year were repeated for the next 2 years with yearly follow‐ups, the last 1 being the 3‐year follow‐up NOTE: group (21) and group (22) received prophylactic care every 2 months and are not included further Group 3: individual educational. All individuals underwent an individual basic preventive programme according to the National Swedish Board of Health and Welfare. The programme comprised 3 visits at 2‐week intervals during the first year. At the first visit, approximately 30 minutes, information on caries and gingivitis/periodontitis was presented and oral hygiene instruction was given based on plaque disclosure. The individual's oral status was reviewed at the next 2 visits of approximately 20 minutes and 15 minutes, respectively, and when necessary, information and hygiene instruction were repeated. The 1‐year follow‐up comprised the measures undertaken at the baseline examination. Directly after the follow‐up, the individuals were scheduled for a 30‐minute repetition of indicated information and oral hygiene instruction. The same was done at the 2‐year follow‐up, after which the individuals were called for a 3‐year follow‐up Group 4: group education. All individuals underwent the remedial measures recommended by the National Swedish Board of Health and Welfare for dental health preventive programmes for adults but modified for group‐based information with 3 visits that had essentially the same content as the programme followed by Group 3. The time required was approximately 60 minutes for the first visit, 30 minutes for the second and 15 minutes for the third.The programme was conducted as group activities with 10 individuals in each group NOTE: given the group nature of the intervention this group is not included further Prophylaxis provided: "Caries restorative measures and scaling were undertaken when needed to bring the oral hygiene of all participants up to the same baseline standard" "Each year, all the test subjects were given enough fluoride toothpaste (Acta 0.22% sodium fluoride corresponding to 0.1% fluoride, Astra‐Wallco AB) to last for 1 year (8 tubes)" Member of dental team delivering intervention: hygienist Frequency of intervention: Group 1: 4 visits total, no OHA. Group 20: 6 visits, each with repeated OHA. Group 3: 4 visits, OHA provided 3 times Intensity of intervention (length of time): Group 1: no OHA. Grp 20: approximately 130 minutes. Group 3: approximately 125 minutes Setting: mixed setting: public dental setting and primary care Disease level: young adults regularly seeking dental care |
|
| Outcomes | Presence of plaque and gingivitis, clinical caries, restorative dental care, attachment level, pocket depth, and supra and subgingival calculus Plaque and gingivitis were recorded on the buccal, lingual, mesial, and distal tooth surfaces of all teeth except the third molars. The presence of plaque was recorded after the tooth surfaces were dried with air according to the criteria for the plaque index (PLI). A PLI score of 1, 2, or 3 was considered to be a positive indicator of plaque, and the surface was registered as positive. The presence of gingivitis was recorded according to the criteria for the gingival index (GI). A GI score of 2 or 3 was used as a measure of gingivitis. Thus, bleeding was registered after the pocket probe had been applied to the opening of the gingival pocket and passed along the tooth surface in question (Löe 1967) |
|
| Notes |
Funding: "Financial support to this study has been given by the Jönköping County Council and The Institute for postgraduate dental Education, Jönköping, Sweden" "The cost analysis took into account both the direct and indirect costs where direct costs were the time the personnel sat aside the individual for each program and indirect costs the patients sat aside and the relation between these costs" Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: "The authors declare that they have no conflict of interests" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomly assigned, by help of a randomising table, into 4 groups of 100 individuals each. Responsible for the randomisation was one of the authors" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "The study was performed as a randomized controlled study with the outcome of the result blind"; "The remedial measures were carried out by another dental hygienist. The programmes followed a detailed, written working plan to ensure that all patients received the same information and instructions" Comment: blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The dental hygienist who carried out the baseline examination of the patient also examined the patient annually and was unaware of which group the patient belonged to and which programme of preventive measures the participant was following" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: (Hugoson 2007) "The dropout rates during the study were 2.3% (9 individuals), 4.3% (17 individuals), and 6.5% (26 individuals) after 1, 2, and 3 years, respectively, or a total of 13% (52 individuals) after 3 years. The main reasons for the dropouts (see Hugoson 2003 ). The dropouts were evenly distributed between the groups" Previous paper from research group reported (Hugoson 2003): "The dropout rates during the study's first 3 years were 1% (4 individuals), 1.8% (7 individuals) and 3.8% (15 individuals) after 1, 2 and 3 years, respectively, in total 6.5% (26 individuals) after 3 years" Comment: disparity between publications regarding dropout numbers. No response from contact author regarding further clarification |
| Selective reporting (reporting bias) | High risk | Comment: a number of clinical outcomes not reported upon (e.g. clinical caries, restorative dental care (other than 1 extraction), pocket depths, attachment levels and calculus). No response from contact author regarding further information |
| Other bias | Low risk | No apparent other bias |
Jönsson 2006.
| Methods |
Trial design: parallel (2 arms) Location: Department of Periodontology, the County Council of Uppsala, Sweden Number of centres: 1 Study Duration: 3‐month follow‐up |
|
| Participants |
Participants: patients of the Department of Periodontology who demonstrated progress of disease and insufficient compliance at 1 year to 2 years review following initial periodontal therapy Inclusion criteria: individuals 20 years to 80 years of age with insufficient compliance (i.e. individuals who reported interdental cleaning (tooth picks or interdental brushes) less than 5 times a week combined with a dental plaque score > 0.20 according to Silness and Löe (Silness 1964)). To avoid missing the individuals who over‐reported their interdental cleaning, patients who reported interdental cleaning 5 times or more a week but nevertheless showed a dental plaque score > 0.40, were also included Exclusion criteria: not reported Age at baseline (years): intervention group: 54.8 ± 11.7 (25–74); control group: 58.1 ± 9.9 (41–78) Gender: intervention group: female/male, 9/10; control group: female/male, 8/8 Number randomised: 37 Number evaluated: 35 (intervention group: 19; control group: 16) |
|
| Interventions |
Intervention: Client Self‐Care Commitment Model (CSCCM) ‐ Visit 1. The first visit started with the initiation phase. The patient presented their own explanatory model of self‐care methods and disease processes, experiences of earlier treatments, and their beliefs about the reasons for disease progress. Thereafter, the last periodontal status was demonstrated, discussed, and compared with previous status. In the assessment phase, the dental hygienist (DH) used interview strategies with the help of an interview guide to disclose patient's perceptions of self‐care behaviours, knowledge of biomedical facts, and illness experiences. As an additional component of the commitment process, the DH provided the patient with the explanatory model of periodontitis. Depending on the patient responses, the DH provided further information if necessary. The negotiation phase started with the DH exploring the patients present oral hygiene status by using an Erytrosin‐based colouring disclosure agent (Rondell Red; Nordenta, Enköping, Sweden) after which appropriate dental cleaning aids were introduced. Thereafter, the DH and the patient discussed and negotiated with the purpose to achieve an improvement in oral hygiene. The DH assisted the patient to establish self‐selected goals in the commitment phase. A goal was established from the patient's individual requirements for toothbrush frequencies (i.e. how often and when during the day), interdental cleaning frequencies (i.e. how often and when during the week), and tooth surfaces of particular importance for cleaning. The patient made the decisions with the assisting of the DH. The result of the decision was documented in a written commitment containing: type of cleaning aid, frequencies and special areas. If there was enough time available at the first visit, scaling was performed ‐ Visit 2. At the next visit (after 1 week to 2 weeks) the client reported their compliance with the established self‐care commitment (evaluation phase). The oral hygiene status was checked by using an Erytrosin‐based colouring disclosure agent (Rondell Red; Nordenta, Enköping, Sweden) and new instructions and adjustments of technique were discussed if necessary. If the clients had new requirements for the commitment, adjustments were made. Necessary scaling and polishing were provided ‐ Visit 3. Approximately 4 weeks after baseline and the written commitment. The aim with the visit was to check if the patients had found the self‐selected goals realistic and if any changes were necessary. No other treatment was performed ‐ Visit 4. The final evaluation was performed 12 weeks to 14 weeks after the first visit. The patients were given the second questionnaire.The same dentist performed the same clinical assessments as at baseline. The data from the clinical assessments were analysed and discussed with the patient. The commitment was also evaluated and adjusted if necessary. Finally, the recall intervals were discussed and established Control: ‐ Visit 1. The latest periodontal status was demonstrated, discussed and compared with previous status. New information about the periodontal disease was given if necessary. The oral hygiene instructions was performed, controlled and adjusted if necessary, by using a colouring disclosure agent (diaplaque Oral Pharma). Necessary scaling and polishing were provided ‐ Visit 2. At the next visit (after 1 week to 2 weeks), the oral hygiene status was checked by using a colouring disclosure agent (diaplaque Oral Pharma) and new instructions and adjustments of technique were discussed if necessary. Necessary scaling and polishing were provided ‐ Visit 3. The final evaluation was performed 12 weeks to 14 weeks after the first visit. The patients were given the second questionnaire. The same dentist performed the same clinical assessments as at baseline. The data from the clinical assessments were analysed and discussed with the patient. Finally, the recall intervals were discussed and established Prophylaxis provided: necessary scaling and polishing were provided at the first visit for the control group and where time allowed for the intervention group, and for both groups at the second visit Member of dental team delivering intervention: hygienist Frequency of intervention: intervention group: 4 visits total, 3 visits with OHA; control group: 3 visits, with 2 visits with OHA Intensity of intervention (length of time): intervention group: not reported; control group: not reported Setting: secondary care Disease level: patients of the Department of Periodontology who demonstrated progress of disease and insufficient compliance at 1 year to 2 years review following initial periodontal therapy |
|
| Outcomes |
The clinical examination: consisted of plaque index (PI) according to Löe 1967, gingival index (GI) according to Löe 1967, probing pocket depth (PD) measuring 6 sites (mesio‐, mid‐, disto‐buccal, and mesio‐, mid‐, disto‐lingual) per tooth, and bleeding on probing recorded on 4 sites as absent or present and summarized as a percentage index for all teeth Participant reported: all participants were given a questionnaire at baseline and at the end of the study. The questionnaire covered oral self‐care habits such as frequency of toothbrushing and interdental cleaning, type of interdental cleaning aid, time spent and reasons for cleaning the teeth and finally demographic data. At the end of the study, there were additional questions regarding possible change in their oral self‐care |
|
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was organized by giving the 10 individuals a number. By using a lottery, the first 5 numbers were included to the intervention group and the rest to the control group" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was organized by giving the 10 individuals a number. By using a lottery, the first 5 numbers were included to the intervention group and the rest to the control group" Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "37 individuals, were included in a randomized single‐blind control trial to test an intervention based on the CSCCM" Comment: study reported as single‐blind trial and that the outcome assessor was blinded to allocation. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical assessments were performed at baseline and at the final evaluation by the same examiner, an experienced periodontist (NO) who was blind to the group allocation" Comment: study reported as single‐blind trial and that the outcome assessor was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 of the patients dropped out; 1 became ill, and 1 declined treatment at the clinic for Periodontology" Comment: unclear which group these dropped out from. Given low numbers unlikely to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | Quote: "All participants were given a questionnaire at baseline and at the end of the study. The questionnaire covered oral self‐care habits such as frequency of toothbrushing and interdental cleaning, type of interdental cleaning aid, time spent and reasons for cleaning the teeth and finally demographic data. At the end of the study, there were additional questions regarding possible change in their oral self‐care. For the intervention group, the questionnaire also included questions if the written commitment had any influence on their oral self‐care habits" Comment: not all questionnaire data presented |
| Other bias | Low risk | No apparent other bias |
Jönsson 2009.
| Methods |
Trial design: parallel (2 arms) Location: Department of Periodontology, Sweden Number of centres: 1 Study Duration: recruitment March 2006 to March 2007. Participants followed‐up at 12 months |
|
| Participants |
Participants: participants were recruited among subjects with moderate to advanced periodontitis referred to the clinic and examined during the period from March 2006 to March 2007 Inclusion criteria: participants clinically diagnosed with chronic periodontitis and scheduled to undergo a dental hygiene treatment (i.e. non‐surgical periodontal debridement and intervention influencing oral hygiene), aged between 20 and 65, literate in Swedish, and had a plaque index (PlI) according to Silness 1964 of ≥ 0.3. The criteria for PlI were set for 2 reasons. Firstly, a high standard of oral hygiene and a plaque level between 20% and 40% is suggested as a level compatible with maintenance of periodontal health (Axelsson 2004; Lang 2003). Secondly, as both interventions aimed to improve oral hygiene habits, subjects with low plaque scores would have clinical efforts focused on other aspects besides oral hygiene Exclusion criteria: "...if they knew that they could not be available during any part of the study period, suffered from a serious disease that precluded regular sessions, and if explorative periodontal surgery was necessary before the dental hygiene treatment" Age at baseline (years): intervention group: 52.4 ± 8.4; control group: 50.1 ± 10.3 Gender: intervention group: male: 25 (43.9%), female: 32 (56.1%); control group: male: 28 (50%), female: 28 (50%) Number randomised: 113 (intervention group: 57; control group: 56) Number evaluated: 107 (intervention group: 52; control group: 55) |
|
| Interventions |
Intervention: the individually tailored oral health educational programme was based on the perspective of behavioural medicine (i.e. an integration of cognitive behavioural principles and non‐surgical periodontal treatment). The programme comprised 7 separate components with different tactics for tailoring each individual's personal goals regarding oral health and dental hygiene habits
Control: the control conditions were chosen to be equivalent to the best possible routine oral health preventive programme for patients with periodontal problems. The programme used corresponded to the description by Nyman 1984 and by Rylander 1997. The programme (labelled individual educational programme) has been tested on young adults with satisfactory results (Hugoson 2007)
Prophylaxis provided: in both groups, the participants visited the dental hygienist once a week until the scaling treatment was finished and there was an oral hygiene control performed after 1 month Quote: "Non‐surgical treatment and supportive periodontal care programme: non‐surgical root surface debridement was integrated into both programmes and undertaken during the initial dental hygiene treatment (visits 1 to 5), mainly performed with hand instruments (LMs Gracys curette of 5 various designs and LMs Svärdström 1/3 and 2/4). There was some supplementary scaling after the 3‐month follow‐ up and during the supportive maintenance care. At each session for both groups, the teeth were cleaned with polishing paste AV 170, and with flossing on proximal surfaces. For both groups, supportive maintenance care was scheduled every third month after the initial dental hygiene treatment, i.e. 3 and 6 months after the 3‐month follow‐up. The supportive maintenance care sessions included checking oral hygiene status with disclosure solutions, and if necessary, re‐instruction. For the experimental group these sessions included relapse prevention procedures and, when needed new goals for oral hygiene practise were discussed" Member of dental team delivering intervention: hygienist Frequency of intervention: intervention group: median number of intervention visits: 9; control group: median number of intervention visits: 8 Intensity of intervention (length of time): appointment time was approximately 60 minutes for each session up to the 3‐month follow‐up, and approximately 45 minutes for the maintenance period. In the intervention group, an extra 10 minutes was needed for the first 2 sessions Setting: secondary care Disease level: moderate to advanced periodontitis |
|
| Outcomes | "Probing pocket depth (PPD) measured at 6 surfaces of each tooth, and bleeding on probing (BoP) in connection with the measurement of periodontal pockets. The presence of plaque was recorded according to Silness & Löe (Silness 1964) PlI. In the present study, criteria 2 and 3 were combined, i.e. all visible plaque was judged as the same amount. The presence of gingival inflammation was recorded according to the criteria for the gingival index (GI) of Löe & Silness (Löe 1963). Experience from patients treated for periodontal disease at the clinic indicates that few patients have spontaneous bleeding and ulcerations; therefore, criteria 2 and 3 were considered to be equally severe. Thus, the highest score for both PlI and GI was 2. Both PlI and GI were recorded on the buccal, lingual, mesial, and distal tooth surfaces of all teeth. The mesial and distal surfaces were recorded from the lingual perspective. To minimize the risk of underestimating PlI and GI scores, the assessment was performed from the lingual aspect, as proximal surfaces are more accessible from the lingual aspect for the observer, but are probably more difficult for the individual patient when performing their oral hygiene. The assessments for PlI, GI, PPD, and BoP were performed with a mirror and a periodontal probe (CC Williams Probe 1‐2‐3‐5‐7‐8‐9‐10, Hu‐Friedys, Chicago, IL, USA)" "Oral hygiene behaviour was assessed through questionnaires covering oral self‐care habits such as frequency of toothbrushing and interdental cleaning, type of toothbrush and interdental cleaning aid, and time and place for oral cleaning. The toothbrushing and interdental cleaning were classified into 2 categories: brushing at least twice a day/less often and, cleaning proximal surfaces once a day/less often" "To evaluate participants' opinion about the interventions and satisfaction with the treatment, 6 questions were posed at the 12‐month follow‐up: (1) performance of oral self‐care (much better, better, no difference, and worse) compared with before treatment; (2) satisfaction with oral health (much more satisfied, more satisfied, and no difference, worse) compared with before treatment; (3) compliance with skills obtained during the treatment (daily, several times a week, some times per week, a couple of times during a month, seldom/never); (4) likelihood to maintain new habits (very likely, likely, fairly likely, and not likely); (5) satisfaction with care given by the dental hygienist (very satisfied, satisfied, fairly unsatisfied, and very unsatisfied); and (6) whether the treatment had been worthwhile (time and cost)(yes, absolutely, yes to some extent, neither yes nor no, and no)" |
|
| Notes |
Funding: "This study was supported by the Swedish Research Council, Uppsala County Council, Swedish Patent Revenue Fund for Research in Preventive Odontology, Pfizer Oral Care Award, and the support of the authors institutions" Sample size calculation: "A power calculation, with data from a previous study (Jönsson 2006), based on the detection of a difference in the mean GI interproximally of 20% between treatment groups indicated that 75 participants were required in each group (α = 0.05, β = 0.2)" "The power analyses revealed that about 150 participants were required for the study. Although the desired number of participants was not reached further inclusion was discontinued for 2 reasons. First, the examiner was unable to participate in the whole process of the requirement and there were difficulties in introducing a new examiner to the study with short notice, and second, the original power analysis was based on an intervention judged as being less effective than the present one. Therefore, the effect size was probably underestimated" Adverse effects: not reported Declarations/conflicts of interest: "The authors declare that there are no conflicts of interest in this study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly allocated to an individually tailored oral health educational programme (experimental group) or to a standard treatment programme (control group). The randomization was made in blocks of various sizes by a random computer table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was secured by (i) having a person not involved with the clinic perform the randomization i.e. neither the examiner nor the therapist could influence the allocation of group belongings and (ii) providing the dental hygienists with sealed consecutively numbered envelopes containing only the assignment for an individual subject. The dental hygienist had not met the patient before the assignment. The sample was stratified for smoking and allocated to the 2 dental hygienists who performed the treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A randomized, evaluator‐blinded, controlled trial with 2 different active treatments was performed" Comment: study reported as evaluator‐blinded. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same examiner, who was blind to group membership, performed all clinical measurements throughout the course of the study" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "6 participants (4 women and 2 men) dropped out during the study period. In the experimental group, 1 participant discontinued treatment before the intervention started due to economic reasons and a further 2 were lost at the 3‐month follow‐up; 1 became seriously ill and 1 moved temporarily from the county, but came back into the study at the 12‐month follow‐up. Another 2 participants dropped out between the 3‐ and the 12‐month follow‐up. In the control group, 1 participant discontinued treatment" |
| Selective reporting (reporting bias) | Low risk | Probing pocket depths not reported in primary publication but subsequently reported |
| Other bias | Low risk | No apparent other bias |
Lepore 2011.
| Methods |
Trial design: parallel (2 arms) Location: Columbia University Medical Center, USA Number of centres: 1 Study duration: recruitment and follow‐up dates not reported. Participants followed‐up at 2 months |
|
| Participants |
Participants: 69 paediatric‐child patients between ages 1 and 6 years Inclusion criteria: not reported Exclusion criteria: not reported Age (years): between ages 1 and 6 years, with the majority (90%) being 2 years to 5 years (mean age of 3 years) Gender: not reported Number randomised: 69 (intervention group: 37; control group: 32) Number evaluated: 69 (intervention group: 37; control group: 32) |
|
| Interventions |
Intervention: dental prophylaxis and topical fluoride application, routine oral health instructions (as given to the control group). In addition a personalized oral health action plan which consisted of an assessment of the patient's current caries risk and a list of suggestions on how to improve that status. The parent and dentist together chose 1 particular suggestion they felt was achievable Control: dental prophylaxis and topical fluoride application, routine, verbally dispensed oral hygiene and diet instructions targeting the specific needs of the patient Prophylaxis provided: both groups received a dental prophylaxis and topical fluoride varnish application Member of dental team delivering intervention: dentist Frequency of intervention: intervention group: 1 initial visit with OHA; control group: 1 initial visit with OHA Intensity of intervention (length of time): intervention group: not reported; control group: not reported Setting: secondary care Disease level: caries risk |
|
| Outcomes | Data collected included DMFS, gingival health and plaque scores. During the first visit, the parents were questioned regarding the oral hygiene and diet behaviour of the child in order to fulfil the 6 survey topics (frequency of toothbrushing with a fluoridated dentifrice, parent‐assisted toothbrushing, bottle use, sippy cup use, frequency of juice consumption and frequency of between‐meal snacking). Data for the reported behaviour was categorized as low, moderate or high caries risk and assigned a numerical value of 0, 1 or 2, respectively | |
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "divided randomly into control and intervention groups" Comment: insufficient information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "32 patients in the control group received routine, verbally dispensed oral hygiene and diet instructions targeting the specific needs of the patient. In addition to the routine oral health instructions given to the control group, the 37 patients in the intervention group received a personalized oral health action plan" and "The parent and dentist together chose 1 particular suggestion they felt was achievable" Comment: blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "Finally, since this was a single blind study, upon follow‐up examination, experimenter bias has to be considered and results may be skewed" and "...This discrepancy could probably be accounted for considering the other 3 major limitations of this study: the sample size, observation time and potential examiner bias" Comment: unclear on who was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no dropouts reported, however difference in group allocation (37 versus 32). The authors may not have reported any possible dropouts, only reporting those available at the end of the study |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes in methods section were reported. Unclear what plaque index and gingival health index were used but unlikely to influence outcome |
| Other bias | Unclear risk | Unclear baseline comparability and ITT not completed Method states DMFS to be scored but within abstract and results table dmft is reported |
López‐Jornet 2014.
| Methods |
Trial design: parallel (2 arms) Location: Department of Oral Medicine, Murcia University Dental School, Murcia, Spain Number of centres: 1 Study duration: 8‐week follow‐up of patients attending during 2009 and 2010 |
|
| Participants |
Participants: patients with hyposalivation attending the Department of Oral Medicine Inclusion criteria: "..patients complaining of dry mouth as assessed by a response of 30 mm or greater on at least 1 of 8 Dry Mouth Visual Analogue Scale (VAS) questions; subjects with an unstimulated whole salivary flow rate of less than 1.5 mL/15 minutes; patients who had never received periodontal treatment or been taught the brushing technique; and patients who were considered sufficiently fit in terms of physical capacity to implement hygiene measures" Exclusion criteria: "..patients younger than 18 years; patients with insufficient manual dexterity; edentulous patients; and subjects with an uncontrolled medical condition that might require changes in medication during the course of the study" Age (years): 56.7 ± 15.4 (range, 31 years to 84 years old) Gender: overall: female: 48 (80%), male: 12 (20%); intervention group: 22 (73%) females and 8 (27%) males; control group: 26 (87%) females and 4 (13%) males Number randomised: 60 (intervention group: 30; control group: 30) Number evaluated: 60 (intervention group: 30; control group: 30) |
|
| Interventions | Quote: "In both groups, oral hygiene instruction was given once every 15 days with a total of 4 sessions over the 8‐week period" Intervention: advice on conventional hygiene procedures using a motivational–behavioural skills protocol designed following principles of self‐efficacy theory, which required a longer time. This was a systematic method, consisting of 6 steps designed to help patients make effective lifestyle changes and to identify and resolve problems. It consisted of the following steps: step 1: creating confidence and commitment; step 2: increasing self‐awareness of behaviour; step 3: developing and implementing an action plan; step 4: evaluating the plan; step 5: maintaining change; and step 6: preventing relapse (Kakudate 2009) Control: instruction in conventional hygiene procedures for 20 minutes based on the Bass method of oral health education (Quigley 1962) Prophylaxis provided: not reported Member of dental team delivering intervention: dental care professional Frequency of intervention: intervention group: 4 visits with OHA; intervention group: 4 visits with OHA Intensity of intervention (length of time): intervention group: quote "which required a longer time;" control group: 20 minutes Setting: secondary care Disease level: dry mouth with no history of periodontal treatment |
|
| Outcomes | Quotes: "On the first visit, patient data were registered (age, gender, medical history, disease process, drug history, family history and clinical signs and symptoms), and then plaque extension index, bleeding index and CPITN were measured," "the periodontal health of all patients was assessed on the first visit and at the end of the oral hygiene instruction programme," "all patients were subjected to a periodontal clinical examination performed at 6 sites per tooth (excluding third molars) to determine the plaque extension index (PEI) following plaque disclosure using a 2% aqueous erythrosine solution. A cotton swab was submerged in the solution for 10 seconds and was then applied to the tooth surfaces. After rinsing once with water, plaque deposits were assessed using the Quigley and Hein index, modified by Turesky et al, with scores from 0 to 5. Periodontal condition was assessed by means of the World Health Organization (WHO) Community Periodontal Index of Treatment Needs (CPITN), using the specially designed WHO periodontal probe (23/CP11, Hu‐Friedy, Leimen, Germany) with a sensing force of no more than 20 g. Each patient's mouth was divided into sextants, and each sextant was examined providing that there were ≥ 2 teeth present and that these were not indicated for extraction. The teeth examined were 1.7, 1.6, 1.1, 2.1, 2.6, 2.7, 4.7, 4.6, 4.1, 3.1, 3.6, and 3.7. The highest index found in each sextant was recorded according to the following scores: 0 = good periodontal health; 1 = gingival bleeding; 2 = calculus detected during probing; 3 = pocket 4 mm to 5 mm in depth; and 4 = pocket ≥ 6 mm in depth. The periodontal condition of each patient was reported as the worst sextant CPITN score" | |
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported Additional information from author correspondence: "..intervention was provided in a dental clinic setting....The advice were (made) by dental care professional" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to 2 groups – control group and intervention group – by a single researcher using a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In both groups, oral hygiene instruction was given once every 15 days with a total of 4 sessions over the 8‐week period" Comment: blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same examiner (CRA), blinded to group assignment, performed all clinical measurements throughout the study" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data, all participants followed‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Memarpour 2016.
| Methods |
Trial design: parallel (3 arms) Location: Shiraz, Iran Number of centres: 5 Duration: recruitment began in December 2012 and was completed within 4 months. Follow‐up appointments were scheduled at 4 months, 8 months, and 12 months after the initial dental appointment for each child |
|
| Participants |
Participants: 300 children participants Inclusion criteria: "The main inclusion criterion was age between 12 and 24 months at the beginning of the study. All children had resided since birth in Shiraz, where water fluoridation levels are similar (< 0.07 ppm) in all areas. All participants had at least 4 erupted primary teeth. None of the teeth showed signs of non‐cavitated or cavitated caries (dmft = 0). The parents did not use any oral hygiene methods such as toothbrushing or any fluoride products at home or in other clinics. All parents were interviewed to explain the aim of the study, and all parents provided their informed consent in writing. A schedule of appointments was provided and parents were asked to attend the healthcare center to receive both free health and dental care service during the study period" Exclusion criteria: "The exclusion criteria were medical history of systemic diseases, drug allergies, congenital physical or mental disabilities, oral or dental anomalies or disabilities, and unwillingness to participate due to lack of time" Age at baseline (months): mean age intervention Group A: 20.70 ± 7.04; intervention Group B: 21.04 ± 8.86; control: 19.73 ± 8.24 Gender: intervention Group A: 48 (48%) female and 52 (52%) male; intervention Group B: 47 (47%) female and 53 (53%) male; control group: 42 (42%) female and 58 (58%) male Number randomised: 300 (intervention Group A: 100; intervention Group B: 100; control Group: 100) Number evaluated: ‐ month 4 follow‐up: total 288 (intervention Group A: 97, intervention Group B: 95, control group: 96) ‐ month 8 follow‐up: total 281 (intervention Group A: 94, intervention Group B: 93, control group: 94) ‐ month 12 follow‐up: total 260 (intervention Group A: 85, intervention Group B: 87, control group: 88) |
|
| Interventions |
Intervention Group A: at the first appointment, parents in group 2 completed the questionnaires and received a free gift bag that contained an educational pamphlet and a toothbrush. The pamphlet explained the importance of caring for primary teeth, the factors that influence Severe Early Childhood Caries (SECC), instructions on how to prevent SECC with a non‐cariogenic diet and feeding methods, and instructions for oral hygiene. Parents received face‐to‐face oral health instructions that were included in the pamphlet and were trained in the proper use of a toothbrush (Kagihara 2009). Children in this group received a placebo fluoride varnish as previously described. Subsequent appointments were scheduled until the end of the follow‐up period, and the placebo varnish was applied at baseline and at the 6‐month follow‐up appointment Intervention Group B: the parents received oral health instruction as described for group 2. The dentist cleaned the children's teeth by brushing and isolated them with cotton rolls. Fluoride varnish that contained 5% sodium fluoride (DuraShield, Sultan Healthcare, Hackensack, NJ, USA) was applied with a disposable brush to all tooth surfaces and left for 1 minute. A small amount of varnish was applied to all primary teeth at baseline. The parents were advised not to allow the child to eat rough (abrasive) foods for the remainder of the day (Holve 2008) and not to brush until the following day (Weyant 2013). The fluoride varnish was applied again 6 months later. This group is not further included in this review Control: mothers completed the questionnaires, and the children's teeth were examined at baseline. Control group participants did not receive any oral healthcare intervention. The placebo, a water‐based coloured solution similar to fluoride varnish, was painted over the tooth surfaces. The solution was tasteless and odourless. The baseline procedure for placebo application was repeated at the follow‐up appointment 6 months later Prophylaxis provided: no baseline prophylaxis Member of dental team delivering intervention: dentist Frequency of intervention: each group attended for 2 'intervention' appointments and 3 review appointments. Each child received a new toothbrush every 3 months Intensity of intervention (length of time): all procedures including completion of the questionnaires, oral hygiene instruction and performing the intervention took about 30 minutes to 40 minutes for each child in all groups Setting: public health care centres Disease level: none of the teeth showed signs of non‐cavitated or cavitated caries (dmft = 0) |
|
| Outcomes |
Clinical outcomes: "The primary outcome was the influences of the interventions on the incidence of caries. The children's teeth were examined by 2 trained dentists with the arm‐cradling or knee‐to‐knee positions. First the dentists were given instructions by a senior author (MM) with a role‐playing technique on how to perform the oral examination, interview the mothers, provide oral health instructions, and complete the questionnaire. To ensure consistency, under supervision by the senior author, both examiners assessed 15 children as part of the training for this research (pilot study) during 1 week. The examiners used disposable dental mirrors, a head light and ball‐ended World Health Organization CPI probes according to their criteria for the diagnosis of dental caries (WHO 1997). Initially the teeth were cleaned with a toothbrush, then wiped with a cotton roll and allowed to air dry. We used the dmft index to record the presence of any caries. This index is defined by 'd' indicating a decayed tooth, 'm' indicating a missing tooth due to decay, and 'f' indicating a filled tooth. Both dentists were present at the follow‐up appointments. The Kappa values for caries detection showed a high level of agreement between the 2 examiners at all 3 follow‐up time points (range 0.940–1). In case of disagreement during the evaluations, consensus evaluations were obtained between the examiners. The data were collected and recorded at each follow‐up period" Knowledge and performance outcomes: "Parents' knowledge and performance regarding oral health was assessed as the secondary outcome. The questionnaire addressed (1) demographic information of the child, i.e. age, gender, place of birth, general health status, and dental history; (2) parents' information, including level of education, employment, family income, and mother's age; (3) parents' knowledge, evaluated as their knowledge about factors that contribute to SECC, the role of brushing and fluoride in caries prevention, bottle feeding of milk or sweet liquids, and the importance of caring for primary teeth, and (4) parents' performance on activities to ensure the child's oral health, including brushing the child's teeth, frequency of brushing, frequency of snacking, method of milk feeding, sharing utensils with the child during meals and checking the child's teeth" |
|
| Notes |
Funding: "Vice‐Chancellery of Research of Shiraz University of Medical Sciences, Shiraz, Iran. Grant No 92‐6158" Sample size calculation: "A sample size of 60 persons per group was estimated to be sufficient to detect a clinically significant difference of 20% between groups in caries incidence using a 2‐tailed test of proportions between 2 groups with 80% power and a 5% level of significance. This difference represents a 30% incidence of caries in the control group [Kumarihamy 2011; Mohebbi 2006;Mohebbi 2008] and a 10% incidence of caries in the fluoride varnish group 12 months after the start of the study. However, because of the long study period, a sample size of 300 children (100 in each group) was finally selected at baseline to compensate for the high rate of probable withdrawal (> 50%)" Adverse effects: not reported Declarations/conflicts of interest: quote: "There are no conflicts of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A staff member randomly assigned 100 children from the list of children with dmft = 0 to each group using a block randomization method (total n = 300), with a block length of B = 6. Random allocation sequences were generated by a statistician with a random number table and were concealed from the 2 examiners until the start of the study" Comment: adequate random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "..and were concealed from the 2 examiners until the start of the study" Comment: insufficient information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Parents were blinded to the study groups. They were not aware which group their child had been randomized to regarding the use of fluoride varnish or a placebo" Comment: the dentists were likely to know the patients allocation. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Parents were blinded to the study groups. They were not aware which group their child had been randomized to regarding the use of fluoride varnish or a placebo" Comment: the parents and children (aged 12 months to 14 months) would be unlikely to know what group they were allocated to. The dentists may have known the patients allocation when clinically assessing participants teeth and parent's performance so unclear if this may have affected outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants accounted for, explanation of dropouts provided |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Münster Halvari 2012.
| Methods |
Trial design: parallel (2 arms) Location: Colosseum Dental Clinic in Sandvika, Norway Number of centres: 1 Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 5.5 months |
|
| Participants |
Participants: 158 adults Inclusion criteria: adult participants that showed up at the clinic, understood Norwegian, and gave informed consent Exclusion criteria: periodontal pockets ≥ 4.0 mm, as measured by a pocket probe, and/or serious bone loss visualized by digital X rays during the dental examination; significant additional oral or other diseases; pregnant women Age at baseline (years): range 18 to 32, mean age 23.31 years, SD =3.5 Gender: total: 112 (71%) female and 46 (29%) male Number randomised: 158 (intervention group: 79; control group: 79) Number evaluated: 141 (intervention group: 70; control group: 71) |
|
| Interventions | Quotes: "When participants first arrived at Time 1a (T1a; ........), they completed a survey assessing autonomy orientation, autonomous motivation for dental home care, perceived dental competence, dental behaviours, and demographics. The standard oral examination. The exam lasted about 45 minutes for all participants, during which time T1a oral health variables were assessed. The DH was autonomy supportive when giving the information about the examination. The DH addressed an introduction to the exam (5 minutes); measures of dental plaque and gingivitis on all teeth surfaces (112 for patients with all teeth intact; 20 minutes); clinical and X‐ray exam for caries (5 minutes); and pocket exam (5 minutes). The final dialogue lasted about 10 minutes and included information on how caries looks and how to detect it on patients' own X rays, and the importance of choice and self‐initiation regarding treatment options in order to promote an informed basis for patient choice and decision making (Beauchamp & Childress 2001)" and "Immediately thereafter, a 45‐minute intervention took place for the experimental group, whereas control group participants went directly to a 45‐minute standard teeth cleaning. The experimental group received the cleaning after the intervention. The cleaning in both groups was done in an autonomy‐supportive way" Intervention: "The DH started the intervention by asking participants about their perceived oral health and problems, and listening to and acknowledging their feelings and perspectives, before giving competence‐related information about their perceived oral health and problems. Based on this conversation, the contents of the intervention were (a) education in plaque‐related diseases such as gingivitis, periodontitis, and caries; (b) demonstrating effective brushing and flossing, with participants practicing these tasks and receiving positive feedback and corrections; (c) giving health promotion and disease preventive information, and offering rationales for the dental behaviours by explaining the relations of behaviours to disease prevention and health; (d) giving information about the value of fluorides and regular meals; and (e) offering choice and options concerning their dental home care" Control: "This 45‐minute cleaning was given to the control group after the exam and to the experimental group after the intervention. The DH focused on the importance of removing calculus in order to make participants' dental home care easier to perform. The cleaning made the baseline the same for both groups." "The control group was viewed as having received usual care; however, because of ethical considerations, the emphasis on patient autonomy support in this clinic likely made this usual‐care condition more autonomy supportive than would have been the case in other clinics. If that were so, it would make the test of the intervention more stringent than if usual care had been provided without the autonomy support, as the primary difference between the 2 groups would likely have been just the competence enhancing intervention itself and/or any possible interaction between the intervention and the autonomy support" Prophylaxis provided: scaling at baseline Member of dental team delivering intervention: hygienist Frequency of intervention: intervention group: 1 visit with OHA; control group: 1 visit with OHA Intensity of intervention (length of time): intervention group: 90 minutes; control group: 45 minutes Setting: primary care Disease level: routinely attending adults with no signs of periodontal disease |
|
| Outcomes |
Clinical outcomes: dental plaque (dental plaque index (Löe 1967)), gingivitis (dental gingival index (Löe 1967)). Both plaque and gingivitis were assessed on distal, buccal, mesial, and lingual tooth surfaces on all teeth except third molars. A participant's scores were the sums for plaque and gingivitis, respectively, divided by the total number of surfaces measured. For the purpose of reliability estimation for plaque and gingivitis, the average scores of each of the 4 teeth quadrants as indicators, and used observations of plaque in the 4 teeth quadrants in modelling of latent indicators for plaque in Structural Equation Modeling (SEM) Psychological outcomes (questionnaires): autonomy orientation (Dental Care Autonomy Orientation Scale), perceived autonomy support (6‐item version of the Health Care Climate Questionnaire (Williams 1996)), autonomous motivation for the dental project (Evaluation of Dental Project Scale (Halvari 2006)), autonomous motivation for dental home care (a 3‐item identified subscale of the Self‐Regulation for Dental Home Care Questionnaire (Halvari 2012)); perceived dental competence (Dental Coping Beliefs Scale (Wolfe 1996) using the 5 items with the best factor loadings (Halvari 2006) and 2 added items from a previous study (Halvari 2010)); dental health behaviours (dental health behaviour was assessed by a 4‐item formative composite scale (Halvari 2010)) |
|
| Notes |
Funding: "The Faculty of Odontology, University of Oslo, for funding the 4‐year PhD period; the Norwegian Ministry of Health, who funded the study" Sample size calculation: "A power analysis using data from a previous study (Halvari 2006) indicated that the necessary number of participants in each group should be 14 for dental plaque, 18 for gingivitis, and 56 for perceived competence to detect significant differences (using t tests) between averages for the experimental and control groups with a power of .90 (α = .05). Based on the power estimates and an unknown participant dropout from Time 1 to Time 2, 79 participants were randomly assigned to each condition and were considered to be sufficient" Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the exam, 79 participants were randomly assigned to each condition" Comment: insufficient information on the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Immediately thereafter, a 45‐minute intervention took place for the experimental group, whereas control group participants went directly to a 45‐minute standard teeth cleaning. The experimental group received the cleaning after the intervention. The cleaning in both groups was done in an autonomy‐supportive way" Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This 45‐minute cleaning was given to the control group after the exam and to the experimental group after the intervention" Comment: the dental hygienist providing the intervention also provided the baseline 'cleaning' and would therefore be aware of the allocation. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At T2 (after 5.5 months), participants responded to all the same questionnaires completed before the teeth exam at T1a, except autonomy orientation and demographics, which were not included at T2. Then, the second teeth examinations were conducted by a different DH than the one for T1a. The second DH was blind to experimental conditions" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants accounted for, explanation of dropouts provided |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | Quote: "207 potential participants from the University of Oslo indicated interest in the study on motivation and dental behaviour after seeing a poster or being approached by the researcher... They were informed that they would get a free dental examination, a free dental cleaning, and a chance to win travel worth NOK 10000 (about USD 1700)" Comment: unlikely to influence outcome |
Ramsay 2018.
| Methods |
Trial design: parallel (6 arms) Location: general dental practices in Scotland and the North East of England Number of centres: 63 Study Duration: participants recruited between Febrruary 2012 and March 2013 with a 3‐year follow‐up |
|
| Participants |
Participants: adults attending National Health Service general dental practices in Scotland and the North East of England Inclusion criteria: adult patients (≥ 18 years of age) with periodontal health, gingivitis or moderate periodontitis (Basic Periodontal Examination (BPE) score 0 to 3) who are dentate; have attended for a check‐up at least twice in the previous 2 years; and receive their dental care in part or fully as a National Health Service patient Exclusion criteria: patients with severe periodontal disease with a BPE score of 4 (probing depth > 6 mm and/or furcation involvements or attachment loss of 7 mm or more) in any sextant on the basis more extensive periodontal care is indicated. Patients with an uncontrolled chronic medical condition (e.g. diabetes, immunocompromised) Age at baseline (years): intervention group: mean 47.4 years (SD 16.1); control group: mean 48.3 years (SD 15.3) Gender: intervention group: 387 males (38%), 321 females (62%); control group: 290 males (33%), 576 females (67%) Number randomised: 1877 participants Number evaluated: 1237 participants |
|
| Interventions |
Intervention: personalised OHA: "Personalised OHA intervention based upon Social Cognitive Theory (Bandura 1998) and Implementation Intention Theory (Gollwitzer 1999) was delivered. The content of the advice delivered was personalised according to the dentist's/hygienist's assessment of the needs of the patient. At a minimum the content included advice and instruction in self‐diagnosis (e.g. bleeding gums on brushing indicates the presence of reversible gingival inflammation) and advice and instruction on toothbrushing and flossing (frequency and technique). Upon completion of the advice, the dentist agreed an action plan with the patient. The feasibility and utility of including personalised biofeedback (Chapple 2008) in the personalised OHA intervention was considered by the research team and the Periodontal Advisory Group." "In addition, the Control and Intervention groups were further randomised at patient‐individual randomisation level to no periodontal instrumentation (PI), 6 monthly PI or 12 monthly PI" Control: routine OHA: "Routine OHA indicates current practice. There is no published information describing 'routine' OHA, but anecdotal evidence suggests that this is often the provision of minimal advice (e.g. 'you need to brush your teeth more frequently' or no advice)" Prophylaxis provided: at baseline all patients received OHA according to cluster level randomisation. A full mouth supra‐ and subgingival PI was carried out by the dentist/hygienist on all participants prior to randomisation. No time limit was set on this treatment and dentists/hygienists were instructed to scale the teeth and root surfaces until they were free of all deposits and smooth to probing Member of dental team delivering intervention: dentist or hygienist Frequency of intervention: reinforcement of OHA was provided at the discretion of the dentist/hygienist during the trial and recorded Intensity of intervention (length of time): no information available Setting: primary care setting Disease level: periodontal health, gingivitis or moderate periodontitis (Basic Periodontal Examination (BPE) score 0 to 3) |
|
| Outcomes | The primary clinical outcome was inflammation at the gingival margin measured by running a University of North Carolina probe circumferentially around each tooth just within the gingival sulcus or pocket (gingival index of Löe (Löe 1967)). After 30 seconds, bleeding was recorded as being present or absent on the buccal and lingual surface and reported as the percentage of sites (twice the number of teeth) with bleeding. Oral hygiene self‐efficacy was measured on a 1 to 7 scale with 7 being extremely confident Economic net benefits were calculated as mean willingness to pay for services and outcomes (obtained from a general population discrete choice experiment) minus mean health service perspective costs Secondary outcomes were calculus and clinical probing depth, patient‐reported dental quality of life (OHIP‐14) (Slade 1997), oral health behaviour and intention, additional private scale and polish treatments, referral and having a plan for better self‐care. Providers' beliefs relating to providing OHA and maintenance of periodontal health were collected at 3 years |
|
| Notes |
Funding: "Funded by the Health Technology Assessment Programme of the National Institute for Health Research, Current Controlled Trials number ISRCTN56465715" Sample size calculation: "Oral health advice (OHA): to calculate the sample size required to estimate the main effect of OHA, it is recognised that the data are contained within a cluster RCT. Assuming a conservative estimate of the intracluster correlation (ICC) of 0.0531, a cluster RCT of 50 dentists collecting information from 25 patient participants each (25*25 = 625 patients per arm) will have 90% power to detect a difference of 7.5%. Should the correlation be 0.1, the trial will still have approximately 80% power to detect a difference of 7.5%" Adverse effects: none reported Declarations/conflicts of interest: all authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization system was used and managed by the Centre for Healthcare Randomised Trials (CHaRT), University of Aberdeen, UK. Both dentist and patient participants underwent randomization with even allocation. The minimization algorithms for the practice allocation were, employs dental hygienist (yes/no) and has 3 or more dentists (yes/no), and for patient allocation absence of gingival bleeding on probing (yes/no), highest BPE score 3 (yes/no) and current smoking (yes/no)" |
| Allocation concealment (selection bias) | Low risk | Quotes: "Dentist allocation to OHA group: recruited dentists will be allocated to routine or personalised OHA by minimisation on 2 factors ‐ (i) practice employs dental hygienist (yes/no) and (ii) practice size (2 or less dentists in practice/3 or more dentists)" and "Patient participant allocation to PI group: allocation will take place once the outcome assessor has completed the baseline outcome assessment and will be minimised on (i) absence of gingival bleeding on probing (yes/no), (ii) highest sextant BPE score (BPE less than 3/BPE 3) and (iii) current smoking (yes/no)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "Both dentist and patient participants underwent randomization with even allocation. The minimization algorithms for the practice allocation were, employs dental hygienist (yes/no) and has 3 or more dentists (yes/no), and for patient allocation absence of gingival bleeding on probing (yes/no), highest BPE score 3 (yes/no) and current smoking (yes/no);" "This is a 5‐year multicentre, randomized, open trial with blinded outcome evaluation" and "Patient participant allocation to PI group...... A letter will be sent to patient participants to inform them of their trial group allocation and the practice will be contacted by the TCOD to arrange the first intervention appointment" Comments: study reported as evaluator blinded however personnel delivering intervention would know group allocation. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinical outcomes were recorded by blinded assessors at baseline and 3 years. Outcome assessor training was provided to ensure intra‐ and interassessor alignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Attendance (70%) at the 3‐year clinical examination and return of the final questionnaire (75%) was balanced across groups" Comment: all participants accounted for and reported on |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Schmalz 2018.
| Methods |
Trial design: parallel (6 arms) Location: Department of Cariology, Endodontology and Periodontology, University of Leipzig, Germany Number of centres: 1 Duration: between May 2015 and November 2016. Participants followed‐up at 12 weeks |
|
| Participants |
Participants: a total of 162 participants were screened for eligibility, of which 150 were included into the study Inclusion criteria: healthy oral conditions (i.e. no active carious lesions, which require invasive treatment (D‐T = 0), and no periodontal treatment need (PSR/PSI ≤ 2)); a minimum number of 20 remaining teeth; age between 18 years and 30 years; ability to give informed consent and voluntary participation Exclusion criteria: age < 18 years or > 30 years; inability to participate due to severe general diseases; diseases affecting motor skills; presence of metabolic diseases (diabetes mellitus); infectious diseases (hepatitis A/B/C, HIV); renal insufficiency; seizure or neurological disorders; pregnancy; addiction (alcohol, drugs); required antibiotic prophylaxis due to endocarditis risk or immunosuppression (e.g. due to organ transplantation); dental background (dentist, dental student, or related occupation); allergies against using toothpaste or other materials used within the study; presence of dental (carious lesions with a cavitation of the tooth surface) and/or periodontal treatment need (periodontal probing depth > 3.5 mm) Age at baseline (years): all groups: 23.44 ± 2.89; Group A1 and Group A2: 22.98 ± 2.52; Group B1 and Group B2: 23.66 ± 2.95; Group C1 and Group C2: 23.70 ± 3.13 Gender: all: female 112 (81%), male 27 (19%); Group A1 and Group A2: female 38 (83%), male 8 (17%); Group B1 and Group B2: female 35 (78%), male 10 (22%); Group C1 and Group C2: female 39 (81%), male 9 (19%) Number randomised: all 150 participants. Group A1: 25; Group A2: 25; Group B1: 25; Group B2: 25; Group C1: 25; Group C2: 25 Number evaluated: all 131 participants. Group A1: 21; Group A2: 22; Group B1: 22; Group B2: 22; Group C1: 22; Group C2: 22 |
|
| Interventions |
Group A1: powered toothbrush (Professional Care™ 7000, Procter & Gamble GmbH, Schwalbach am Taunus, Germany) with no instruction
Group A2: powered toothbrush (Professional Care™ 7000, Procter & Gamble GmbH, Schwalbach am Taunus, Germany) received 1 standardized instruction of the toothbrush system according to the manufacturers recommendations Group B1: powered toothbrush (SoniCare™, Philips GmbH, Hamburg, Germany) with no instruction Group B2: powered toothbrush (SoniCare™, Philips GmbH, Hamburg, Germany) received 1 standardized instruction of the toothbrush system according to the manufacturers recommendations Group C1: manual toothbrush (elmex®INTERX, CP GABA GmbH, Hamburg, Germany) with no instruction Group C2: manual toothbrush (elmex®INTERX, CP GABA GmbH, Hamburg, Germany) received instruction on 1 occasion to use the Fones technique All participants used the same toothpaste (Sensodyne® Fluoride; GlaxoSmithKline Oral Health Care GmbH, Brühl, Germany) during the whole study All groups were required to brush twice daily for 2 minutes to 3 minutes and to abandon other oral hygiene aids such as dental floss and/or interdental brushes or mouthrinses Prophylaxis provided: participants received a professional tooth cleaning including the removal of supragingival calculus, biofilm, and extrinsic discolourations as well as polishing of the tooth surfaces Member of dental team delivering intervention: dentist Frequency of intervention: instruction groups: 1 visit with OHA Intensity of intervention (length of time): not reported Setting: secondary care Disease level: healthy individuals with no active carious lesions and not requiring periodontal treatment |
|
| Outcomes | Clinical outcomes: "Gingival inflammation was evaluated using the Papilla Bleeding Index (PBI) (Lange 1977) and the Gingival Index (GI) by Löe and Silness (Löe 1963). To assess PBI, gingival sulcus was spread out using a periodontal probe (PCP 15, Hu‐Friedy, Chicago, IL, USA); the PBI score ranges from score 0 (no bleeding) to score 4 (profuse bleeding). Furthermore, the GI was used to assess changes of the gingiva. A score from 0 (normal gingiva, inflammation‐free, no discolouration, no bleeding) to score 3 (severe inflammation, reddening and swelling, tendency toward spontaneous bleeding or ulceration) was used. After assessment of gingival inflammation, plaque extension was evaluated using the Plaque Index by Quigley and Hein (QHI) modified by Turesky et al after using a plaque disclosing agent (Mira‐2‐Ton®, Hager score 5 = plaque extending to the coronal third). Furthermore, the Marginal Plaque Index (MPI) by Deinzer et al was used to differentiate plaque extension at the gingival margin (Deinzer 2014). The evaluation was performed on 8 measuring points at each tooth (score 0 = no plaque; score 1 = plaque)" | |
| Notes |
Funding: "Philips GmbH (Hamburg, Germany), CP GABA (GmbH, Hamburg, Germany), and GlaxoSmithKline Oral Health Care GmbH (Brühl, Germany) for providing materials for the study" Sample size calculation: "This RCT should detect differences based on PBI and QHI of an effect size δ = 1 with a power of 80%. Under estimation of normal distribution, for a 2‐sided t test at the significance level of 5%, 20 participants in each group were required. To compensate potential dropouts, 20 to 25 participants per group should be included" Adverse effects: not reported Declarations/conflicts of interest: "The authors declare that they have no conflict of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly divided into 3 groups (OR, SA, MTB; n = 50 each group) with 2 subgroups each: participants receiving no instructions (NI) and participants receiving instructions (I) (n = 25 per group). Randomization and attribution to groups were performed by an independent examiner (DZ). The plaque accumulation at screening examination of each patient was classified into 3 categories using the baseline modified Quigley–Hein Plaque Index (mod QHI) as follows: good (2). Based on these categories, as well as smoking habits, gender, and left‐handedness, a matching was performed according to previous studies of this working group (Schmalz 2017b; Schmickler 2014; Schmickler 2016), to ensure comparable groups" Comment: insufficient information available regarding randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned regarding participant blinding Quote: "With respect to their subgroup (I or NI), participants got a brush‐specific instruction (DZ)" Comment: personnel delivering intervention (DZ) would have been aware of intervention group. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All study‐related examinations were performed under standardized conditions by a skilled, calibrated, and blinded dentist (kappa > 0.8, KK) in the Department of Cariology, Endodontology and Periodontology, University of Leipzig, Germany" Comment: outcome assessor blinded to participant group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropouts reported and similar across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No apparent other bias |
Söderholm 1982.
| Methods |
Trial design: parallel (3 arms) Location: Department of Periodontology, School of Dentistry, University of Lund, Malmö, Sweden Number of centres: 1 Study Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 12 weeks |
|
| Participants |
Participants: 69 white‐collar employees at a shipyard in Malmö, Sweden Inclusion criteria: not reported Exclusion criteria: not reported Age at baseline (years): 5‐Visit Group: 35.3 years (SE 0.8); 2‐Visit Group: 36.3 years (SE 0.8); Control Group: 35.6 years (SE 35.6) Gender: 5‐Visit Group: female 3 (13%), male 20 (87%); 2‐Visit Group: female 3 (13%), male 20 (87%); Control Group: female 3 (13%), male 20 (87%) Number randomised: 69 (5‐Visit Group: 23; 2‐Visit Group: 23; Control Group: 23) Number evaluated: 41 (5‐Visit Group: 19; 2‐Visit Group: 22; Control Group: not available) |
|
| Interventions |
Intervention: 5‐Visit Group: plaque control program given during 5 visits. The interval between each of the visits was 2 days to 3 days. Each visit was scheduled as a 30‐ minute appointment 2‐Visit Group: plaque control program given during 2 visits. The 2 visits were spaced 7 days to 10 days apart and were scheduled as 60‐minute appointments. No other treatment except the oral hygiene instruction was provided at the plaque control visits. Oral hygiene instructions were given by 2 plaque control nurses. The intervention program was based on the following educational aids and principles: ‐ a step‐by‐step approach was used in teaching the patients the performance of oral hygiene procedures. New devices or techniques were not recommended or used until the patients had tried and found that those previously used were insufficient ‐ the teaching was as far as possible based upon positive reinforcement. Negative criticism was kept at a minimum ‐ plaque‐disclosing dyes and mouth mirrors were used and given to the patients ‐ repeated charting of plaque was used for instruction and feedback to the patients Control: did not receive OHA, however they were omitted at the 12‐week examination as many of these patients had started restorative care and had been given OHA. This group is not included further in this review Prophylaxis provided: "After the initial examination all participants received a dental prophylaxis. This was given in 1 visit and consisted of scaling and removal of large overhangs to remove interference with plaque control. The treatment was provided by a dental hygienist and completed within 1 hour. 2 months later the experiment started (baseline examination). This delay was used in order to ensure that reduction in bleeding tendency and periodontal pocket depth resulting from the initial prophylaxis would have occurred prior to the start of the experiment" Member of dental team delivering intervention: dental nurse and hygienist Frequency of intervention: intervention groups: 5 visits with OHA or 2 visits with OHA Intensity of intervention (length of time): 5‐Visit Group: 150 minutes; 2‐Visit Group: 120 minutes Setting: secondary care Disease level: not reported |
|
| Outcomes | Plaque score: the percentage of tooth surfaces (mesial, buccal, distal and lingual) with plaque. Plaque was defined as the soft material on the tooth surfaces which stains dark red with an erythrosine‐disclosing solution (Diaplac Rondell, Astra, Sweden) and which could be removed easily with the blunt side of a probe. The mesial and distal of the teeth were evaluated from the vestibular aspect Periodontal pocket score: percentage of tooth surfaces (mesial, buccal, distal and lingual) with periodontal pockets equal to or greater than 5 mm Gingival bleeding score: percentage of gingival areas (mesial, buccal, distal and lingual) showing bleeding after probing for pocket measurement Number of teeth Age |
|
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In an attempt to obtain comparable groups of subjects the individuals were first matched in combinations of 3 with the help of a computer. Different weight was attached to the above 5 recordings. The plaque score was given the greatest weight followed by their periodontal pocket score, gingival bleeding score, number of teeth and age, respectively. The 3 individuals in a matched combination were randomly placed in 1 of the 3 study groups. Males and females were treated separately. Each group originally contained 23 individuals (20 males and 3 females)" Comment: insufficient information on the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "The treatment was provided by a dental hygienist and completed within 1 hour" and "Oral hygiene instructions were given by 2 plaque control nurses. At random, half of the participants in each of the 2 experimental groups was assigned to 1 of the nurses and the remaining half to the other nurse" Comment: patient and personnel would have been aware of allocation (5 visits versus 2 visits). Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The clinical examinations were performed by 2 of the authors (GS and NN). Each of these examiners scored the same subjects throughout the experiment. The 2 examiners scored equal numbers of subjects in each of the 3 study groups, and were not aware to which study group the individual subjects belonged" and "Each of these (2) examiners scored the same subjects throughout the experiment...and were not aware to which study group the individual subjects belonged" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐week follow‐up data reported for intervention groups as per study protocol with additional 12‐, 24‐, 36‐ and 48‐month data also reported 12‐month follow‐up 5‐Visit Group: 23 to 19; 2‐Visit Group: 23 to 22 24‐month follow‐up 5‐Visit Group: 19 to 17; 2‐Visit Group: 22 to 21 36‐month follow‐up 5‐Visit Group: 17 to 19; 2‐Visit Group: 21 to 19 48‐month follow‐up 5‐Visit Group: 19 to 18; 2‐Visit Group: 19 to 20 Comment: all groups data reported as per initial study protocol |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Tedesco 1992.
| Methods |
Trial design: parallel (2 arms) Location: Oral Health Enhancement Clinic, Greater Buffalo metropolitan area, New York, USA Number of centres: 1 Study Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 9 months |
|
| Participants |
Participants: individuals between the ages of 21 and 65 were recruited from the Greater Buffalo metropolitan area Inclusion criteria: participants were required to have mild to moderate gingivitis, defined as at least 55% of oral sites with gingival bleeding on probing (scores of 2 or 3 on the Löe and Silness gingival index (Löe 1963; Silness 1964)) and to have at least at least 10 teeth per dental arch Exclusion criteria: all prospective participants were required to have a screening visit prior to acceptance into the study and were disqualified if they had any area with periodontal attachment loss greater than 1.5 mm, radiographic evidence of bone loss greater than 25% (anterior/posterior bitewing radiographs or periapical radiographs), or previous periodontal therapy. Study participants were required to be in good health (no diabetes or heart disease), not pregnant, and not taking medications, including antibiotics, for any chronic diseases Age at baseline (mean years): intervention group: 32.9; control group: 31.3 Gender: intervention group: female 56 (51%), male 55 (49%); control group: female 24 (43%), male 32 (57%) Number randomised: 167 (intervention group: 111; control group: 56) Number evaluated: 91 (intervention group: 62; control group: 29) |
|
| Interventions | Participants visited the clinic 7 times over a 14‐month period. Visits 1 and 2 provided baseline measures, and visits 2 through 5 were intervention (experimental) or control visits. Visits 6 and 7 were 3‐month and 9‐month follow‐up visits, respectively Intervention: at visit 2, study participants received traditional oral hygiene instruction in the same manner as control group participants. The same standardized delivery and management of the visit were employed. Before the second prophylaxis was performed, however, study participants were shown a phase contrast slide of their own subgingival flora on a video monitor. A videotape record was made simultaneously. During video monitor viewing, the hygienist described and labelled the patient's oral pathogens and explained their association to the development of periodontal disease. The patient was encouraged to ask questions and offer his or her reactions to the video monitor display. The patient received a complete prophylaxis and then a new slide was made, viewed on the video monitor and recorded on videotape to show the subject the relative absence of bacteria. The hygienist emphasized that this was the picture of a healthy mouth. Emphasis was placed on how the patient can control the health of his/her mouth and always "possess" this picture of wellness. Visits 3, 4, and 5, following 1 month after each other, repeated the intervention, with the subject watching the videotape from the previous visit and viewing a fresh slide on the video monitor made at the visit in progress. During these visits emphasis was placed on the patient's accomplishments; i.e. performance accomplishments. The patient's and hygienist's conversations and descriptions of the video monitor display were linked to the plaque index and gingival index scores, problem areas, etc., detected clinically by the hygienist. At visits 6 (3‐months post‐intervention) and 7 (9‐months post‐intervention) assessments were taken as follow‐up procedures, and a final prophylaxis was performed before terminating study participation Control: at visit 2 control group participants received traditional oral hygiene instruction that included brushing and flossing techniques from a hygienist. Study participants were also given feedback on their entry state of oral hygiene, with problem areas for improvement emphasized. The hygienist also explained the range and meaning of plaque index and gingival index scores. Control group participants were encouraged to present problems and ask questions. Before dismissal, a complete oral prophylaxis was performed. Psychological and social assessment instruments were also administered during these visits. At visits, 3, 4, and 5 patients were given feedback on how clean their teeth and gingival surfaces were, and what areas needed most improvement, and assessments (clinical, psychological, and social) were taken. At visits 6 and 7 follow‐up assessments were taken and a final prophylaxis was performed Prophylaxis provided: prophylaxis provided to both groups Member of dental team delivering intervention: hygienist Frequency of intervention: intervention group: 7 visits with 4 visits of OHA; control group: 7 visits with 4 visits of OHA Intensity of intervention (length of time): intervention group: not reported; control group: not reported Setting: not reported Disease level: mild to moderate gingivitis |
|
| Outcomes | Self‐efficacy assessed via Oral Health Behaviour Expectation Scale Theory of reasoned action variables assessed via Theory of Reasoned Action Oral Health Scale Self‐reported brushing and flossing behaviours Plaque accumulation (Löe and Silness plaque index, Löe 1963) Gingival inflammation (Löe and Silness gingival index, Silness 1964) Brushing and flossing skill observation |
|
| Notes |
Funding: "The study was supported by NIH‐NIDR grant DE‐07335" Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to experimental or control groups" Comment: insufficient information on the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned Comment: insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All procedures were trained to a written script and intervention protocol. In addition to periodic recalibration exercises, the hygienist was periodically monitored for appropriate intervention delivery, communication, and patient feedback" Comment: patient and personnel would have been aware of allocation. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "1 hygienist completed all the plaque and gingival assessments following the screening. She received calibration training prior to her work on this project. All procedures were trained to a written script and intervention protocol. In addition to periodic recalibration exercises, the hygienist was periodically monitored for appropriate intervention delivery, communication, and patient feedback" Comment: it is possible that there was only 1 hygienist involved who provided intervention and outcome assessment. No response from contact author regarding further information. Unclear if outcome would have been influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "At the last intervention visit (Visit 5), 18.6% of the sample did not return. This dropout rate increased to 28.7% and 45.5% by Visit 6, (3‐month follow‐up), and Visit 7 (9‐month follow‐up), respectively. Dropout rates were similar for both experimental and control groups; by the end of the study, 52% of the control group and 56% of the experimental group remained. Dropouts at Visits 6 and 7 were disproportionally represented in the lower income and education groups (less than high school education and income under $20,000). In addition, there was a difference for race, with proportionally more non‐Caucasian minorities leaving the study by Visits 6 and 7 than Caucasian subjects (who outnumbered minority groups 4:1 at the beginning of the study). When compared with participants, dropouts at Visits 6 and 7 had higher plaque and gingival index scores at Visits 5 and 6. The same pattern emerged for per cent "0" gingival index scores; that is, dropouts had a lower percentage of non‐bleeding sites (poorer oral health status) when compared with all study participants" |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | Quote: "Prospective subjects were offered 3 free cleanings and free oral health education for their participation in 7 visits at the Oral Health Enhancement Clinic" Comment: unlikely to influence outcome |
Van Leeuwen 2017.
| Methods |
Trial design: parallel (6 groups) Location: Department of Periodontology of the Academic Centre for Dentistry, Amsterdam, the Netherlands Number of centres: 1 Study Duration: recruitment November 2009 to November 2010. Participants followed‐up at 12 months |
|
| Participants |
Participants: "Participants were students recruited from different universities and colleges in and around Amsterdam by e‐mail and flyers" Inclusion criteria: ≥ 18 years of age, have a minimum of 5 evaluable teeth per quadrant, and have moderate to advanced gingivitis (≥ 40% bleeding on marginal probing (BOMP)) (Lie 1998; Van der Weijden 1994) Exclusion criteria: open caries, pockets of 4 mm to 5 mm in combination with gingival recession or pockets of ≥ 6 mm, as assessed according to the Dutch Periodontal Screening Index (DPSI) scores 3+ and 4 (Mantilla Gómez 2001; Van der Velden 2009), orthodontic appliances or removable (partial) dentures, a history of allergic reactions to erythrosine and/or any of the mouthrinse components, pregnancy, systemic disease or any adverse medical history or long‐term medication that might interfere with the response variables. No restriction with respect to smoking status was applied Age at baseline (mean years): Control I: 21.1 (SD 2.66); Control II: 21.4 (SD 2.99); OHI: 21.2 (SD 2.91); PP: 21.6 (3.36); OA + CHX: 21.9 (SD 3.17); COMBI: 20.6 (SD 2.19) Gender: male/female: Control I: 13/27; Control II: 11/33; OHI: 10/33; PP: 7/35; OA + CHX: 6/34; COMBI 6/32 Number randomised: 267 (Control I: 44, Control II: 45, OHI: 44, PP: 45, OA + CHX: 44, COMBI: 45) Number evaluated: 247 (Control I: 40, Control II: 44, OHI: 43, PP: 42, OA + CHX: 40, COMBI: 38) |
|
| Interventions | "All participants performed a similar basic oral hygiene regimen of brushing twice daily for 2 minutes with a fluoride‐containing dentifrice for the full duration of the study. At day 0 participants were instructed to brush according to the details provided in a written oral hygiene instruction leaflet describing the Bass method technique (Bass 1954a; Bass 1954b)" Control I and Control II: the participants in control groups I and II were instructed to adhere to this basic oral hygiene regimen only. To minimize the potential Hawthorne effect (Adair 1984), the clinical parameters of Control I were assessed at day 0, baseline (3 weeks later) and 12 months later. The clinical parameters of all other groups were assessed at day 0, baseline, 4, 7, 10 and 12 months OHI Group: the oral hygiene instruction group (OHI) received the same interventions as those in the control groups. Additionally they received professional individual oral hygiene instruction (at day 0) PP Group: the professional prophylaxis group (PP) received a professional oral prophylaxis from an experienced dental hygienist at day 0. This group is not further included in this review OA + CHX Group: "During the treatment phase from day 0 until baseline, the oxygenating‐agent (OA) and chlorhexidine (CHX) group (OA + CHX) were instructed to rinse with a combination of the OA Bocasan (Oral‐B Laboratories, Boston, MA, USA) and CHX 0.20% (Corsodyl; GlaxoSmithKline, Zeist, the Netherlands) twice daily for 3 weeks in addition to the basic oral hygiene." This group is not included further in this review COMBI Group: "In addition to the basic oral hygiene regimen, participants in the COMBI group received all 3 supplementary preventive interventions: OHI, PP and OA + CHX." This group is not included further in this review Prophylaxis provided: only for those in the PP Group. "Subjects were not allowed to use any other dental products or interdental cleaning aids during the study and/or to undergo a dental prophylaxis during routine dental check‐ups" Member of dental team delivering intervention: hygienist Frequency of intervention: 3‐week treatment phase, intervention administered once Intensity of intervention (length of time): not reported Setting: Department of Periodontology of the Academic Centre for Dentistry, Amsterdam Disease level: not reported ‐ students in Amsterdam |
|
| Outcomes |
Baseline, 4 months, 7 months, 10 months, 12 months: ‐ staining was assessed (NLHH) at 4 sites per tooth, according to the Grundemann Modification of the Stain Index (GMSI), on a scale of 0 to 3 (Gründemann 2000) ‐ gingival health was assessed at 6 sites (mesio‐buccal, mid‐buccal, disto‐buccal, mesio‐lingual, mid‐lingual and disto‐lingual) around the selected quadrants by scoring BOMP on a scale of 0 to 2 (Lie 1998; Van der Weijden 1994) ‐ dental plaque was also assessed at 6 sites after disclosing with Mira‐2‐Ton (Hager & Werken GmbH & Co KG, Duisburg, Germany), and scores were based on the modified Quigley and Hein (Quigley 1962) plaque index (QHPI) with a scale of 0 to 5 12 months only: "Upon completion of the clinical assessments, all participants were asked to complete a questionnaire designed to evaluate their attitudes towards the assigned intervention.The participants answered the questions by placing a vertical mark on a 10‐cm‐long uncalibrated line (the visual analogue scale, VAS); the left of this line represented the 'negative' extreme, whereas the right represented the 'positive' extreme. As such, a mean score of 5 would represent an 'average' score, being neither positive nor negative" |
|
| Notes |
Funding: The Netherlands Organisation for Health Research and Development Sample size calculation: "The sample size of 40 participants per group was calculated a priori (PS: Power and Sample Size program) (Dupont 1990) based on a pooled standard deviation (r) of 0.23 (as taken from the gingivitis scores in a previous 6‐month mouthrinse study by Paraskevas et al (Paraskevas 2005) and a detectable difference (d) of 0.18 (between groups) with an a = 0.05 to obtain 80% power" Adverse effects: not reported Declarations/conflicts of interest: "ACTA Dental Research BV received financial support from The Netherlands Organization for Health Research and Development (ZonMW). The authors designed, performed and analysed the study project without any interference from other parties. Van der Weijden, Rosema and Slot have formerly received either external advisor fees, lecturer fees or research grants from companies that produce mouthwash products. Among these were Colgate, Dentaid, GABA, Johnson & Johnson Lactona, Oral‐B, Philips, Procter & Gamble, Sara Lee, Sunstar, and Unilever. All authors declare that they have no conflicts of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization for group and quadrant selection was performed (by CB) using true random numbers generated by sampling and processing a source of entropy outside the computer. The source was atmospheric noise, which was sampled and fed into a computer without any buffering mechanisms in the operating system (www.random.org). The study co‐ordinator assigned the participants to their randomly chosen group" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed by using sequentially numbered, opaque, sealed envelopes (SNOSE‐method) (Schulz 2002)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The participants were not informed about the group allocation" Comment: personnel delivering oral hygiene instruction intervention would be aware of which group participant was in. Blinding of participants was not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The examiners were blinded to treatment randomization, and the records of earlier examinations were not available at the time of re‐examination" and "The examiners were blinded with respect to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants lost to follow‐up accounted for including reason (e.g. "used another dentifrice," "absent without notice," etc.) |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
Weinstein 1996.
| Methods |
Trial design: parallel (4 arms) Location: Italy Number of centres: 1 Study Duration: recruitment and follow‐up dates not reported. Participants followed‐up at 60 days |
|
| Participants |
Participants: "All 20 subjects presented periodontic problems for which long‐term behavioural maintenance of oral hygiene routines were prescribed by the periodontist" Inclusion criteria: not reported Exclusion criteria: not reported Age at baseline (mean years): not reported by group, age range was 32 to 50 years Gender: group breakdown not provided: female 9 (45%), male 11 (55%) Number randomised: 20 (Intervention Group A: 5; Intervention Group B: 5; Intervention Group C: 5; and Control Group D: 5) Number evaluated: 20 (Intervention Group A: 5; Intervention Group B: 5; Intervention Group C: 5; and Control Group D: 5) |
|
| Interventions | "Core procedures and the duration of visits were kept standard for all groups" "Like all the other groups, these patients had also received the same instructions and suggestions concerning oral hygiene, etc." Intervention group A: "Received standard instructions on Bass' technique....were required to call in the results of the plaque score self‐evaluations twice weekly to their periodontist who provided them with additional verbal feedback about their maintenance status. ..The results of plaque score self‐evaluation using a detecting pill were subjective and as a consequence, the patients' feedback was approximate according to the value of the self‐evaluation. These patients, like any other group, had to visit their periodontist monthly for the control of their full mouth plaque score (FMPS)" Intervention Group B: "...examined directly by their periodontists 2 x weekly who provided them with objective feedback about their status. In addition the periodontists gave them positive social reinforcement contingent upon having maintained the plaque score within acceptable limits" Intervention Group C: this group received the same interventions as those in Intervention Group B. Additionally they carried out a self‐monitoring system by completing an 'oral hygiene task checklist' Control (Group D): "Consisted of instructions and demonstrations of Bass' technique of dental flossing, etc.. The patients were invited to take again medical examination after 1 and 2 months. In that occasion the full mouth plaque was notified" Prophylaxis provided: none reported Member of dental team delivering intervention: not explicitly specified, however after reviewing the text the review authors were confident the intervention was undertaken by a member of the dental team Frequency of intervention: Intervention Group A: 3 visits with twice week phone call to periodontist to report self‐evaluated plaque score; Intervention Group B: approximately 16 visits with OHA (twice weekly examinations by periodontist); Intervention Group C: approximately 16 visits with OHA (twice weekly examinations by periodontist) and daily completion of oral hygiene task checklist; Control Group D: 3 visits Intensity of intervention (length of time): Intervention Group A: not reported; Intervention Group B: not reported; Intervention Group C: not reported; Control Group D: not reported Setting: not reported Disease level: periodontal patients ‐ disease level not reported |
|
| Outcomes | The effects on compliance of the different methods of patient's treatment were measured using the Full Mouth Plaque Score (FMPS) according to the procedure suggested by O'Leary, Drake and Naylor (O'Leary 1972) | |
| Notes |
Funding: not reported Sample size calculation: not reported Adverse effects: not reported Declarations/conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Were assigned to 1 of 4 groups and given regular evaluation appointment times at random" Comment: insufficient information provided on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Were assigned to 1 of 4 groups and given regular evaluation appointment times at random" Comment: insufficient information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided on who provided interventions. The different groups had markedly different number of appointments Comment: insufficient information provided to make judgement but blinding of participants was likely to be not possible; it is unclear the influence this would have on the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported Comment: insufficient information provided to make judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reported dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section were reported |
| Other bias | Low risk | No apparent other bias |
BOMP = bleeding on marginal probing; DH = dental hygienist; DMFS = number of decayed, missing or filled permanent surfaces; dmft = number of decayed, missing or filled primary teeth; DMFT = number of decayed, missing or filled permanent teeth; GA = general anaesthesia; ITT = intention to treat; OH = oral hygiene; OHA = oral hygiene advice; ppm = parts per million; PSR/PSI = periodontal screening; RCT = randomised controlled trial; SD = standard deviation; SE = standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albandar 1994 | Not in a dental setting Intervention not provided on a 1‐to‐1 basis |
| Aleksejūnienė 2018 | School‐based: not in a dental setting |
| Almomani 2006 | "Education room": not in a dental setting |
| Almomani 2009 | Intervention not delivered by member of dental team |
| Arrow 2013 | Not in a dental setting: confirmed with author |
| Arunakul 2012 | Not in a dental setting |
| Arunakul 2015 | Intervention not wholly delivered in a dental setting |
| Axelsson 1981 | Following baseline examination the test group participants received oral prophylaxis in addition to instruction and practice in oral hygiene techniques at regular interval; the control group only received annual review and further treatment as appropriate. No additional information from author available |
| Bahri 2015 | Not in a dental setting Intervention not delivered by member of dental team: confirmed with author |
| Blinkhorn 1981 | Test group had addition of fluoride tablet use |
| Blinkhorn 2003 | Intervention groups not treated equally: fluoride issued to intervention group throughout study |
| Brand 2013 | Intervention not provided by a member of the dental team |
| Brukienė 2012 | Not in a dental setting |
| Choi 2012 | Not in a dental setting |
| Clarkson 2009 | Electric toothbrush provided as part of intervention |
| Crawford 1975 | Fluoride provided to intervention group |
| Daly 2009 | Not a randomised controlled trial |
| Davies 2007 | Not in a dental setting |
| de Andrade Meyer 2010 | Less than 8‐week follow‐up Compared 2 different toothpaste formulations |
| Dermen 2014 | Not in a dental setting |
| Džiaugytė 2017 | Not in a dental setting |
| Esfahanizadeh 2011 | Not in a dental setting |
| Ferrazzano 2008 | Not a randomised controlled trial |
| Franz‐Ritscherle 1983 | Not in a dental setting |
| Freitas‐Fernandes 2002 | Not in a dental setting |
| Gathece 2011 | Not in a dental setting |
| Glavind 1981 | Not a randomised controlled trial |
| Glavind 1983 | Not a randomised controlled trial |
| Godard 2011 | Less than 8‐week follow‐up |
| Graehn 1984 | Not in a dental setting |
| Harnacke 2012 | Not in a dental setting |
| Harrison 2007 | Intervention not delivered by member of dental team |
| Hietasalo 2009 | Experimental group received oral health advice plus fluoride toothpaste + fluoride and xylitol lozenges therefore unable to compare to control group regarding the effect of 1‐to‐1 oral health advice only |
| Ismail 2011 | Intervention not delivered by member of dental team Additional information from author: "The interviews were conducted by trained non‐dental interviewers" |
| Iwata 1981 | Not a randomised controlled trial Compares educational programme versus educational programme + fee reduction |
| Jiang 2014 | Intervention not in a dental setting Additional information from author: "The fluoride varnish was mostly applied by a dentist in a community setting, usually in a community or social services centre" |
| Ju 2017 | Not in a dental setting |
| Jönsson 2012 | Testing a model of behaviour only |
| Kakudate 2009 | Less than 8‐week follow‐up |
| Kasaj 2004 | Less than 8‐week follow‐up |
| Langan 1976 | Less than 8‐week follow‐up |
| Lee 2009 | Intervention group received supragingival scaling on a monthly basis compared with control group who did not |
| Legler 1971 | Part of intervention not delivered on 1‐to‐1 basis: movies presented in the waiting room environment as part of intervention |
| Lim 1996 | Not in a dental setting |
| Lim 1996b | Not in a dental setting |
| Little 1997 | Part of intervention not delivered on 1‐to‐1 basis |
| Mahantesha 2015 | Setting of intervention unclear. No additional information available from authors |
| Makuch 2011 | Not in a dental setting |
| Mayer 2003 | Not in a dental setting |
| Meurman 2009 | Advice not all provided by a dental care professional (provided by public maternity health nurses as well as dental hygienists) Xylitol lozenges provided to children in the intervention group who were positive for Streptococci mutans |
| Moltzer 1986 | No pre‐treatment measurements completed with which to compare effect of intervention |
| Moskovitz 2009 | Not a randomised controlled trial |
| Ohrn 2009 | Not a randomised controlled trial |
| Park 2015 | Less than 8‐week follow‐up |
| Park 2011 | Not in a dental setting |
| Patthoff 1981 | Not a randomised controlled trial |
| Picard 2014 | Less than 8‐week follow‐up |
| Pickrell 2007 | Not related to oral health education |
| Pine 2007 | Not in a dental setting |
| Plutzer 2008 | Not in a dental setting: printed information leaflets provided to pregnant women at antenatal clinics |
| Plutzer 2012 | Not in a dental setting |
| Reinhardt 2010 | Not in a dental setting |
| Roscher 2004 | Manual versus powered toothbrush trial |
| Saengtipbovorn 2017 | Not in a dental setting |
| Sandeep 2014 | Not in a dental setting |
| Sato 2008 | Not in a dental setting |
| Schiffner 2007 | Intervention groups recieved oral aides or fluoride mouthwash or both |
| Schneider 1981 | No oral hygeine advice provided |
| Schneider 1982 | No oral hygiene advice provided |
| Schüz 2009 | Intervention not delivered by a member of dental team |
| Seow 2003 | Not in a dental setting |
| Sniehotta 2007 | Not a 1‐to‐1 intervention |
| Stenman 2012 | Intervention not delivered by a member of the dental team |
| Stenman 2017 | Intervention not provided by a member of the dental team (clinical psychologist) |
| Strippel 2010 | Not in a dental setting |
| Söderholm 1982b | Not a randomised controlled trial |
| Tan 1978 | Intervention groups received a group lecture in addition to individualised instruction as part of the dental healthcare instruction |
| Tan 1979 | Intervention groups received a group lecture in addition to individualised instruction as part of the dental healthcare instruction |
| Telford 1974 | Not a randomised controlled trial |
| Triana 1985 | Less than 8‐week follow‐up |
| Valle 2004 | Not in a dental setting |
| Zanin 2007 | Not a 1‐to‐1 intervention: lectures and group discussion |
| Zee 2006 | No oral hygiene advice comparator |
| Ziebolz 2009 | Not a randomised controlled trial: quasi‐randomised |
Characteristics of studies awaiting assessment [ordered by study ID]
Gao 2013.
| Methods | Randomised controlled trial stratified by parental education and child's caries experience at baseline |
| Participants | 3‐year old children enrolled in kindergarten grade 1 (K1), who have unfavourable oral health behaviour(s) (i.e. a child who needs intervention). Unfavourable oral health behaviours are defined as "brush teeth less often than twice a day" and/or "snack 3 times or more a day." Each child will be required to join this study together with his/her parent (mother or father) who spends the most time with him/her, so that a parent‐child dyad can be recruited. A child will be excluded if (i) he/she has a serious medical condition or (ii) his/her parents are non‐Chinese speaking and cannot understand the oral health materials and counselling, which will be in Chinese |
| Interventions | Conventional oral health education versus conventional oral health education and motivational interviewing versus conventional oral health education and motivational interviewing and interactive risk assessment |
| Outcomes | The primary outcome will be caries increment in children and the proportion of caries‐free children Other outcomes: changes in parental efficacy in protecting children's oral health and changes in children's dental behaviours |
| Notes | HKCTR‐1455 Author contacted for further information: no response so unable to clarify further |
IRCT2014062618248N1.
| Methods | Randomised controlled trial; 2‐stage sampling method (clinical examination and questionnaire‐based) |
| Participants | 2nd grade high school students who voluntarily participated in the study |
| Interventions | Educational intervention was designed based on the data collected from pre‐test and carried out in 5 50‐minute to 60‐minute sessions within 4 weeks |
| Outcomes | Main outcomes: dental plaque and oral health behaviour Other outcomes: toothbrushing |
| Notes | Author contacted for further information: no response so unable to clarify further |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12605000607673.
| Trial name or title | Oral Health Education Logan Programme (OHELP) (The effect of a personalised oral health education programme on clinical and molecular risk factors for oral and general health in an at risk population) |
| Methods | Parallel randomised controlled trial |
| Participants | 18‐ to 60‐year old patients with a minimum of 12 teeth and not requiring antibiotic cover for dental treatment |
| Interventions | The oral health promotion programme being tested will involve oral health therapists/hygienists providing an individualised oral health promotion programme, in addition to standard care, over 1 to 4 visits. This will include motivation enhancement, goal setting, problem solving, assertion skills, followed by monthly reporting on adherence until the 2‐year examination |
| Outcomes | Main outcome: the level of oral disease Other outcomes: clinical and molecular risk factors for oral and general health such as diet, oral care, smoking, alcohol consumption, body mass index (BMI) |
| Starting date | 3 January 2003 |
| Contact information | Dr Mary Cullinan, Oral Care Research Programme, Oral Biology and Pathology, The University of Queensland, Brisbane QLD 4072, Australia m.cullinan@uq.edu.au |
| Notes | Author contacted: data collection completed, data analysis to be completed |
ISRCTN24958829.
| Trial name or title | Dental RECUR trial (Comparison of a new with standard child and family primary care service to reduce the re‐occurrence of childhood dental caries (Dental RECUR Trial)) |
| Methods | Randomised controlled trial |
| Participants | Children aged 5 years to 7 years old who are having 1 or more teeth removed at a secondary care centre |
| Interventions | All participants are given help to find and register with a local family dentist if they wish. Every child is given a free dental checkup 2 years after the extraction. This is done at the child's school by a dentist trained in looking for dental decay in children. All families are invited to attend a review appointment at some point in the first 6 weeks following tooth extraction. The session will be delivered by a dental nurse. For families in the new service group, the session will look at ways to help prevent further decay in the child's remaining first teeth and permanent teeth. The new service group families are also invited for an appointment with their dentist every 3 months for a year. At the end of the year the child will go back to visiting their dentist as normal. For families in the usual follow‐up care group, the session will look at future dental development for their child. At the end of the session families in this group will go back to visiting their dentist as normal |
| Outcomes | Main outcome: dental caries experience after 2 years Other outcomes: 1. parental readiness to change and beliefs about caring for their children's teeth. Measured by the modified Contemplation Ladder 2. parental self‐efficacy in relation to the implementation of dental health‐related behaviours for the study children. Measured by the Child Oral Health Behaviours Questionnaire and Parenting Self‐Efficacy Scale 3. oral cleanliness by plaque assessment of anterior teeth at dental examinations 4. use of dental services; child oral health behaviours including dietary behaviours. Measured by the Child Oral Health Behaviours Questionnaire 5. NHS costs will be measured: from a public sector, multiagency perspective, they will: 5.1. fully cost the community‐based DR‐BNI and prevention in dental practice programme (as compared with costs of usual dental care) 5.2. record study participant dental service use, primary and secondary care health service use, social care and special educational service use (using a CSRI, costed using national unit costs) 5.3. conduct a primary cost‐effectiveness analysis (using dental caries rates as measure of effectiveness) 5.4. conduct a secondary cost‐consequences study relating costs to a range of consequences spanning measures of: dental decay in participating child, regular dental attendance, parent participation in better oral health behaviours (e.g. sugar‐free bedtime routine) and school attendance, and where appropriate, potential child neglect |
| Starting date | 1 November 2013 |
| Contact information | Ms Louise Robinson, Research and Development Directorate, Salford Royal Hospitals NHS Trust, Mayo Building, 3rd Floor, Stott Lane, Salford M6 8HD, United Kingdom louise.robinson@srft.nhs.uk |
| Notes | Author contacted: data collection will be completed November 2018, no interim measures available |
ISRCTN38542397.
| Trial name or title | Is photographic evidence a valuable aide to oral hygiene advice in 9‐ to 16‐year olds? |
| Methods | Randomised controlled trial |
| Participants | Patients with visible plaque (tartar) levels on 3 or more tooth surfaces (i.e. a minimum plaque score of 3); attending Barking and Dagenham Special Needs and Community Dental Service; aged between 9 years to 16 years old; who have the ability to brush their own teeth unsupervised; and have no conditions that would contraindicate measuring the bleeding from the gums |
| Interventions | Group 1: standard intervention (control) including manual toothbrush and standardized oral and written toothbrushing instruction based on the Bass technique Group 2: as with standard intervention with the addition of standardized photographic evidence of the subjects oral health status and instructions how best to use this |
| Outcomes | Modified plaque and bleeding indices Questionnaire (how did they use the information given, what was most helpful) |
| Starting date | 1 December 2006 |
| Contact information | Dr Zahra Syed, Upminster Dental Clinic, 230 St Mary's Lane, Upminster RM14 3DH, United Kingdom +44 01708 796786 |
| Notes | Author contacted for further information: no response so unable to clarify further |
NCT01118143.
| Trial name or title | Oral health literacy tailored communication (Effects of communication tailored to oral health literacy level of adult dental patients: a randomised controlled trial) |
| Methods | Randomised parallel controlled trial |
| Participants | Inclusion criteria: participants > 20 years of age; who master the Norwegian language; who are registered at the university dental clinic (IKO) Exclusion criteria: people who do not master the Norwegian language; strongly visually impaired people that have difficulty in reading; people with a history of heart attack in the past 6 months; immunocompromised people and organ‐transplanted people; people with other conditions that might lead to more disadvantages than advantages by participating |
| Interventions | Behavioural: tailored oral health literacy instruction The intervention includes oral hygiene instruction tailored to the level of oral health literacy, and will include information about the outcome of the clinical examination. Providing relevant information to the participant will be in focus, and there will be given a personalized demonstration as well as guidance on how to conduct proper oral hygiene practices at home. The aim of the intervention is to improve oral hygiene and attitudes towards dental care by taking the literacy level of the participant into consideration. Other Name: lifestyle counselling |
| Outcomes | Main outcome: gingival index (time frame: 6 months after intervention) Other outcome: plaque index (time frame: 6 months after intervention) |
| Starting date | June 2010 |
| Contact information | Professor Jan O Bergdahl, University of Tromsoe, Norway |
| Notes | Author contacted: data collection completed, data analysis to be completed/published |
Differences between protocol and review
We made the following amendments to the review.
Controlled clinical trials were not included as per Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011).
Objective changed from 'To determine the benefits and harms of one‐to‐one oral hygiene ...' to 'To assess the effects of one‐to‐one oral hygiene...'.
Oral infection removed from primary outcome clinical status factors.
Periodontal health primary outcome clinical status factor to include plaque levels.
Secondary outcomes patient‐centred factors compressed to:
patient‐reported behaviour changes (e.g. toothbrushing/flossing/mouthwash use),
patient satisfaction with advice provided,
patient‐reported changes in knowledge, attitudes and quality of life.
Additional databases searched:
ClinicalTrials.gov (to November 2017),
World Health Organization International Clinical Trials Registry Platform (to November 2017).
Quality assessment completed as per Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011).
Data analysis guided by the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011).
Contributions of authors
Janet E Clarkson (JC) conceived the idea for the review. JC, Francesca A Soldani (FS), Linda Young (LY), Kate Jones (KJ), and Tanya Walsh (TW) wrote the protocol. JC, FS, LY, KJ and Thomas Lamont (TL) designed the final review. FS co‐ordinated the review. FS, TL, KJ and LY screened abstracts. FS, TL, Rizwana Lala (RL) and KJ carried out data extraction. TL and FS compiled the characteristics of included studies tables. FS, TL, RL and KJ assessed the risk of bias for the included studies. TW provided statistical data advice. TL, FS and KJ drafted the background section. FS and TL drafted the body of the review. TW, KJ, RL, LY and JC commented on and edited the draft review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
Janet E Clarkson, Linda Young and Thomas Lamont were all authors of Ramsay 2018. They were not involved in the data extraction or risk of bias assessment of this trial. Janet E Clarkson is also Co‐ordinating Editor of Cochrane Oral Health. Francesca A Soldani: none known. Kate Jones: none known. Tanya Walsh: none known. Tanya Walsh is an Editor with Cochrane Oral Health. Rizwana Lala: none known.
New
References
References to studies included in this review
Aljafari 2017 {published and unpublished data}
- Aljafari A, Gallagher JE, Hosey MT. Can oral health education be delivered to high‐caries‐risk children and their parents using a computer game? – A randomised controlled trial. International Journal of Paediatric Dentistry 2017;27(6):476‐85. [DOI: 10.1111/ipd.12286] [DOI] [PubMed] [Google Scholar]
- Aljafari A, Rice C, Gallagher JE, Hosey MT. An oral health education video game for high caries risk children: study protocol for a randomized controlled trial. Trials 2015;16:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baab 1986 {published data only}
- Baab D, Weinstein P. Longitudinal evaluation of a self‐inspection plaque index in periodontal recall patients. Journal of Clinical Periodontology 1986;13(4):313‐8. [DOI] [PubMed] [Google Scholar]
Bali 1999 {published data only}
- Bali C, Gurdet C, Hauer G, Handler I, Fischer R. Evaluation of the effect of professional dental cleaning and education in dental hygiene in type 1 diabetic patients. Acta Medica Austriaca 1999;26(5):159‐62. [PubMed] [Google Scholar]
Glavind 1985 {published data only}
- Glavind L, Christensen H, Pedersen E, Rosendahl H, Attström R. Oral hygiene instruction in general dental practice by means of self‐teaching manuals. Journal of Clinical Periodontology 1985;12(1):27‐34. [DOI] [PubMed] [Google Scholar]
Hetland 1982 {published data only}
- Hetland L, Midtun N, Kristoffersen T. Effect of oral hygiene instructions given by paraprofessional personnel. Community Dentistry and Oral Epidemiology 1982;10(1):8‐14. [DOI] [PubMed] [Google Scholar]
Hoogstraten 1983 {published data only}
- Hoogstraten J, Moltzer G. Effects of dental health care instruction on knowledge, attitude, behavior and fear. Community Dentistry and Oral Epidemiology 1983;11(5):278‐82. [DOI] [PubMed] [Google Scholar]
Hugoson 2007 {published data only}
- Hugoson A, Lundgren D, Asklöw B, Borgklint G. Effect of three different dental health preventive programmes on young adult individuals: a randomized, blinded, parallel group, controlled evaluation of oral hygiene behaviour on plaque and gingivitis. Journal of Clinical Periodontology 2007;34(5):407‐15. [DOI] [PubMed] [Google Scholar]
- Hugoson A, Lundgren D, Asklöw B, Borgklint G. The effect of different dental health programmes on young adult individuals. A longitudinal evaluation of knowledge and behaviour including cost aspects. Swedish Dental Journal 2003;27(3):115‐30. [PubMed] [Google Scholar]
Jönsson 2006 {published data only}
- Jönsson B, Lindberg P, Oscarson N, Ohrn K. Improved compliance and self‐care in patients with periodontitis ‐ a randomized control trial. International Journal of Dental Hygiene 2006;4(2):77‐83. [DOI: 10.1111/j.1601-5037.2006.00175.x] [DOI] [PubMed] [Google Scholar]
Jönsson 2009 {published data only}
- Jönsson B, Ohrn K. Evaluation of the effect of non‐surgical periodontal treatment on oral health‐related quality of life: estimation of minimal important differences 1 year after treatment. Journal of Clinical Periodontology 2014;41(3):275‐82. [DOI] [PubMed] [Google Scholar]
- Jönsson B, Ohrn K, Lindberg P, Oscarson N. Cost‐effectiveness of an individually tailored oral health educational programme based on cognitive behavioural strategies in non‐surgical periodontal treatment. Journal of Clinical Periodontology 2012;39(7):659‐65. [DOI: 10.1111/j.1600-051X.2012.01898.x] [DOI] [PubMed] [Google Scholar]
- Jönsson B, Ohrn K, Lindberg P, Oscarson N. Evaluation of an individually tailored oral health educational programme on periodontal health. Journal of Clinical Periodontology 2010;37(10):912‐9. [DOI: 10.1111/j.1600-051X.2010.01590.x] [DOI] [PubMed] [Google Scholar]
- Jönsson B, Ohrn K, Oscarson N, Lindberg P. The effectiveness of an individually tailored oral health educational programme on oral hygiene behaviour in patients with periodontal disease: a blinded randomized‐controlled clinical trial (one‐year follow‐up). Journal of Clinical Periodontology 2009;36(12):1025‐34. [DOI: 10.1111/j.1600-051X.2009.01453.x] [DOI] [PubMed] [Google Scholar]
Lepore 2011 {published data only}
- Lepore LM, Yoon RK, Chinn CH, Chussid S. Evaluation of behavior change goal‐setting action plan on oral health activity and status. New York State Dental Journal 2011;77(6):43‐7. [PUBMED: 22338818] [PubMed] [Google Scholar]
López‐Jornet 2014 {published data only}
- López‐Jornet P, Fabio CA, Consuelo RA, Paz AM. Effectiveness of a motivational–behavioural skills protocol for oral hygiene among patients with hyposalivation. Gerodontology 2014;31(4):288–95. [DOI: 10.1111/ger.12037] [DOI] [PubMed] [Google Scholar]
Memarpour 2016 {published data only}
- Memarpour M, Dadaein S, Fakhraei E, Vossoughi M. Comparison of oral health education and fluoride varnish to prevent early childhood caries: a randomized clinical trial. Caries Research 2016;50(5):433–42. [DOI: 10.1159/000446877] [DOI] [PubMed] [Google Scholar]
Münster Halvari 2012 {published data only}
- Münster Halvari AE, Halvari H, Bjørnebekk G, Deci EL. Self‐determined motivational predictors of increases in dental behaviors, decreases in dental plaque, and improvement in oral health: a randomized clinical trial. Health Psychology 2012;31(6):777‐88. [DOI: 10.1037/a0027062] [DOI] [PubMed] [Google Scholar]
Ramsay 2018 {published data only}
- Ramsay CR, Clarkson JE, Duncan A, Lamont TJ, Heasman PA, Boyers D, et al. Improving the Quality of Dentistry (IQuaD): a cluster factorial randomised controlled trial comparing the effectiveness and cost‐benefit of oral hygiene advice and/or periodontal instrumentation with routine care for the prevention and management of periodontal disease in dentate adults attending dental primary care. Health Technology Assessment 2018;22(38):1‐144. [DOI: 10.3310/hta22380] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmalz 2018 {published data only}
- Schmalz G, Kiehl K, Schmickler J, Rinke S, Schmidt J, Krause F, et al. No difference between manual and different power toothbrushes with and without specific instructions in young, oral healthy adults ‐ results of a randomized clinical trial. Clinical Oral Investigations 2018;22(3):1147‐55. [DOI: 10.1007/s00784-017-2200-5] [DOI] [PubMed] [Google Scholar]
Söderholm 1982 {published data only}
- Söderholm G, Nobréus N, Attström R, Egelberg J. Teaching plaque control. I. A five‐visit versus a two‐visit program. Journal of Clinical Periodontology 1982;9(3):203‐13. [PUBMED: 6954162] [DOI] [PubMed] [Google Scholar]
Tedesco 1992 {published data only}
- Tedesco LA, Keffer MA, Davis EL, Christersson LA. Effect of a social cognitive intervention on oral health status, behavior reports, and cognitions. Journal of Periodontology 1992;63(7):567‐75. [DOI: 10.1902/jop.1992.63.7.567] [DOI] [PubMed] [Google Scholar]
Van Leeuwen 2017 {published data only}
- Leeuwen M, Rosema N, Versteeg PA, Slot DE, Hennequin‐Hoenderdos NL, Weijden GA. Effectiveness of various interventions on maintenance of gingival health during 1 year – a randomized clinical trial. International Journal of Dental Hygiene 2017;15(4):e16–e27. [DOI] [PubMed] [Google Scholar]
Weinstein 1996 {published data only}
- Weinstein R, Tosolin F, Ghilardi L, Zanardelli E. Psychological intervention in patients with poor compliance. Journal of Clinical Periodontology 1996;23(3 Pt 2):283‐8. [DOI: 10.1111/j.1600-051X.1996.tb02090.x] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albandar 1994 {published data only}
- Albandar JM, Buischi YA, Mayer MP, Axelsson P. Long‐term effect of two preventive programs on the incidence of plaque and gingivitis in adolescents. Journal of Periodontology 1994;65(6):605‐10. [DOI] [PubMed] [Google Scholar]
Aleksejūnienė 2018 {published data only}
- Aleksejūnienė J, Brukienė V. A cluster randomized theory‐guided oral hygiene trial in adolescents ‐ a latent growth model. International Journal of Dental Hygiene 2018;16(2):e23‐e30. [DOI: 10.1111/idh.12286] [DOI] [PubMed] [Google Scholar]
Almomani 2006 {published data only}
- Almomani F, Brown C, Williams KB. The effect of an oral health promotion program for people with psychiatric disabilities. Psychiatric Rehabilitation Journal 2006;29(4):274‐81. [DOI] [PubMed] [Google Scholar]
Almomani 2009 {published data only}
- Almomani F, Williams K, Catley D, Brown C. Effects of an oral health promotion program in people with mental illness. Journal of Dental Research 2009;88(7):648‐52. [DOI] [PubMed] [Google Scholar]
Arrow 2013 {published data only}
- Arrow P, Raheb J, Miller M. Brief oral health promotion intervention among parents of young children to reduce early childhood dental decay. BMC Public Health 2013;13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arunakul 2012 {published data only}
- Arunakul M, Kuphasuk Y, Boonyathanasit R. Effectiveness of oral hygiene instruction media on periodontal health among hearing impaired children. Southeast Asian Journal of Tropical Medicine and Public Health 2012;43(5):1297‐303. [PubMed] [Google Scholar]
Arunakul 2015 {published data only}
- Arunakul M, Asvanund Y, Tantakul A, Mitrakul K, Srisatjaluk R, Vongsavan K. Effectiveness of an oral hygiene education program combined with fluoride mouthrinse among visually impaired students in Bangkok, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2015;46(2):354‐9. [PubMed] [Google Scholar]
Axelsson 1981 {published data only}
- Axelsson P, Lindhe J. Effect of oral hygiene instruction and professional toothcleaning on caries and gingivitis in schoolchildren. Community Dentistry and Oral Epidemiology 1981;9(6):251‐5. [DOI] [PubMed] [Google Scholar]
Bahri 2015 {published data only}
- Bahri N, Tohidinik HR, Bahri N, Iliati HR, Moshki M, Darabi F. Educational intervention to improve oral health beliefs and behaviors during pregnancy: a randomized‐controlled trial. Journal of the Egyptian Public Health Association 2015;90(2):41‐5. [DOI] [PubMed] [Google Scholar]
Blinkhorn 1981 {published data only}
- Blinkhorn AS, Downer MC, Mackie IC, Bleasdale RS. Evaluation of a practice based preventive programme for adolescents. Community Dentistry and Oral Epidemiology 1981;9(6):275‐9. [DOI] [PubMed] [Google Scholar]
Blinkhorn 2003 {published data only}
- Blinkhorn AS, Gratrix D, Holloway PJ, Wainwright‐Stringer YM, Ward SJ, Worthington HV. A cluster randomised, controlled trial of the value of dental health educators in general dental practice. British Dental Journal 2003;195(7):395‐400. [DOI: 10.1038/sj.bdj.4810566] [DOI] [PubMed] [Google Scholar]
Brand 2013 {published data only}
- Brand VS, Bray KK, MacNeill S, Catley D, Williams K. Impact of single‐session motivational interviewing on clinical outcomes following periodontal maintenance therapy. International Journal of Dental Hygiene 2013;11(2):134‐41. [DOI] [PubMed] [Google Scholar]
Brukienė 2012 {published data only}
- Brukienė V, Aleksejūnienė J. Is the authoritative parenting model effective in changing oral hygiene behavior in adolescents?. Health Education Research 2012;27(6):1081‐90. [DOI] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi HS, Ahn HY. Effects of mothers involved in dental health program for their children. Journal of Korean Academy of Nursing 2012;42(7):1050‐61. [DOI] [PubMed] [Google Scholar]
Clarkson 2009 {published data only}
- Clarkson JE, Young L, Ramsay CR, Bonner BC, Bonetti D. How to influence patient oral hygiene behavior effectively. Journal of Dental Research 2009;88(10):933‐7. [DOI: 10.1177/0022034509345627] [DOI] [PubMed] [Google Scholar]
Crawford 1975 {published data only}
- Crawford AN, McAllan LH, Murray JJ, Brook AH. Oral hygiene instruction and motivation in children using manual and electric toothbrushes. Community Dentistry and Oral Epidemiology 1975;3(6):257‐61. [PUBMED: 1059513] [DOI] [PubMed] [Google Scholar]
Daly 2009 {published data only}
- Daly CG. Prescribing good oral hygiene for adults. Australian Prescriber 2009;32(3):72‐5. [Google Scholar]
Davies 2007 {published data only}
- Davies GM, Duxbury JT, Boothman NJ, Davies RM. Challenges associated with the evaluation of a dental health promotion programme in a deprived urban area. Community Dental Health 2007;24(2):117‐21. [PubMed] [Google Scholar]
de Andrade Meyer 2010 {published data only}
- Andrade Meyer AC, Mello Tera T, Rocha JC, Jardini MA. Clinical and microbiological evaluation of the use of toothpaste containing 1% chlorhexidine and the influence of motivation on oral hygiene in patients with motor deficiency. Special Care in Dentistry 2010;30(4):140‐5. [DOI] [PubMed] [Google Scholar]
Dermen 2014 {published data only}
- Dermen KH, Ciancio SG, Fabiano JA. A pilot test of motivational oral health promotion with alcohol‐dependent inpatients. Health Psychology 2014;33(4):392‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Džiaugytė 2017 {published data only}
- Džiaugytė L, Aleksejūnienė J, Brukienė V, Pečiulienė V. Self‐efficacy theory‐based intervention in adolescents: a cluster randomized trial‐focus on oral self‐care practice and oral self‐care skills. International Journal of Paediatric Dentistry 2017;27(1):37‐46. [DOI] [PubMed] [Google Scholar]
Esfahanizadeh 2011 {published data only}
- Esfahanizadeh N. Dental health education programme for 6‐year‐olds: a cluster randomised controlled trial. European Journal of Paediatric Dentistry 2011;12(3):167‐70. [PubMed] [Google Scholar]
Ferrazzano 2008 {published data only}
- Ferrazzano GF, Cantile T, Sangianantoni G, Ingenito A. Effectiveness of a motivation method on the oral hygiene of children. European Journal of Paediatric Dentistry 2008;9(4):183‐7. [PubMed] [Google Scholar]
Franz‐Ritscherle 1983 {published data only}
- Franz‐Ritscherle A, Lange DE. Effectiveness of various audiovisual technics to improve oral hygiene behavior. Deutsche Zahnarztliche Zeitschrift 1983;38(9):811‐5. [PubMed] [Google Scholar]
Freitas‐Fernandes 2002 {published data only}
- Freitas‐Fernandes LB, Novaes AB Jr, Feitosa AC, Novaes AB. Effectiveness of an oral hygiene program for Brazilian orphans. Brazilian Dental Journal 2002;13(1):44‐8. [PubMed] [Google Scholar]
Gathece 2011 {published data only}
- Gathece LW, Wang'ombe JK, Ng'ang'a PM, Wanzala PN. Effect of health education on oral hygiene and gingival status of persons living with HIV attending comprehensive care centres in Nairobi, Kenya. African Journal of AIDS Research 2011;10(4):495‐500. [DOI] [PubMed] [Google Scholar]
Glavind 1981 {published data only}
- Glavind L, Zeuner E, Attström R. Oral hygiene instruction of adults by means of a self‐instructional manual. Journal of Clinical Periodontology 1981;8(3):165‐76. [DOI] [PubMed] [Google Scholar]
Glavind 1983 {published data only}
- Glavind L, Zeuner E, Attstrom R. Evaluation of various feedback mechanisms in relation to compliance by adult patients with oral home care instructions. Journal of Clinical Periodontology 1983;10(1):57‐68. [PUBMED: 6572635] [DOI] [PubMed] [Google Scholar]
Godard 2011 {published data only}
- Godard A, Dufour T, Jeanne S. Application of self‐regulation theory and motivational interview for improving oral hygiene: a randomized controlled trial. Journal of Clinical Periodontology 2011;38(12):1099‐105. [DOI] [PubMed] [Google Scholar]
Graehn 1984 {published data only}
- Graehn G. A health education program for pre‐school children. 1. Results on health knowledge, health behavior, and oral hygiene. Stomatologie der DDR 1984;34(4):226‐35. [PubMed] [Google Scholar]
Harnacke 2012 {published data only}
- Harnacke D, Mitter S, Lehner M, Munzert J, Deinzer R. Improving oral hygiene skills by computer‐based training: a randomized controlled comparison of the modified Bass and the Fones techniques. PLoS One 2012;7(5):e37072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2007 {published data only}
- Harrison R, Benton T, Everson‐Stewart S, Weinstein P. Effect of motivational interviewing on rates of early childhood caries: a randomized trial. Pediatric Dentistry 2007;29(1):16‐22. [PubMed] [Google Scholar]
Hietasalo 2009 {published data only}
- Hietasalo P, Seppa L, Lahti S, Niinimaa A, Kallio J, Aronen P, et al. Cost‐effectiveness of an experimental caries‐control regimen in a 3.4‐yr randomized clinical trial among 11‐12‐yr‐old Finnish schoolchildren. European Journal of Oral Sciences 2009;117(6):728‐33. [DOI] [PubMed] [Google Scholar]
Ismail 2011 {published data only}
- Ismail AI, Ondersma S, Jedele JM, Little RJ, Lepkowski JM. Evaluation of a brief tailored motivational intervention to prevent early childhood caries. Community Dentistry and Oral Epidemiology 2011;39(5):433‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwata 1981 {published data only}
- Iwata BA, Becksfort CM. Behavioral research in preventive dentistry: educational and contingency management approaches to the problem of patient compliance. Journal of Applied Behavior Analysis 1981;14(2):111‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2014 {published data only}
- Jiang EM, Lo EC, Chu CH, Wong MC. Prevention of early childhood caries (ECC) through parental toothbrushing training and fluoride varnish application: a 24‐month randomized controlled trial. Journal of Dentistry 2014;42(12):1543‐50. [DOI] [PubMed] [Google Scholar]
Jönsson 2012 {published data only}
- Jönsson B, Baker SR, Lindberg P, Oscarson N, Ohrn K. Factors influencing oral hygiene behaviour and gingival outcomes 3 and 12 months after initial periodontal treatment: an exploratory test of an extended Theory of Reasoned Action. Journal of Clinical Periodontology 2012;39(2):138‐44. [DOI] [PubMed] [Google Scholar]
Ju 2017 {published data only}
- Ju X, Brennan D, Parker E, Mills H, Kapellas K, Jamieson L. Efficacy of an oral health literacy intervention among Indigenous Australian adults. Community Dentistry and Oral Epidemiology 2017;45(5):413‐26. [DOI] [PubMed] [Google Scholar]
Kakudate 2009 {published data only}
- Kakudate N, Morita M, Sugai M, Kawanami M. Systematic cognitive behavioral approach for oral hygiene instruction: a short‐term study. Patient Education and Counseling 2009;74(2):191‐6. [DOI] [PubMed] [Google Scholar]
Kasaj 2004 {published data only}
- Kasaj A, Sculean A, Willershausen B. Study of the usefulness of the DetecTar System in improving patient compliance and oral hygiene. Parodontologie 2004;15(4):343‐53. [Google Scholar]
Langan 1976 {published data only}
- Langan MJ, Yearick ES. The effects of improved oral hygiene on taste perception and nutrition of the elderly. Journal of Gerontology 1976;31(4):413‐8. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee HK, Choi SH, Won KC, Merchant AT, Song KB, Jeong SH, et al. The effect of intensive oral hygiene care on gingivitis and periodontal destruction in type 2 diabetic patients. Yonsei Medical Journal 2009;50(4):529‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legler 1971 {published data only}
- Legler DW, Gilmore RW, Stuart GC. Dental education of disadvantaged adult patients: effects on dental knowledge and oral health. Journal of Periodontology 1971;42(9):565‐70. [DOI] [PubMed] [Google Scholar]
Lim 1996 {published data only}
- Lim LP, Davies WI. Comparison of various modalities of "simple" periodontal therapy on oral cleanliness and bleeding. Journal of Clinical Periodontololgy 1996;23(6):595‐600. [DOI] [PubMed] [Google Scholar]
Lim 1996b {published data only}
- Lim LP, Davies WI, Yuen KW, Ma MH. Comparison of modes of oral hygiene instruction in improving gingival health. Journal of Clinical Periodontology 1996;23(7):693‐7. [DOI] [PubMed] [Google Scholar]
Little 1997 {published data only}
- Little SJ, Hollis JF, Stevens VJ, Mount K, Mullooly JP, Johnson BD. Effective group behavioral intervention for older periodontal patients. Journal of Periodontal Research 1997;32(3):315‐25. [DOI] [PubMed] [Google Scholar]
Mahantesha 2015 {published data only}
- Mahantesha T, Nara A, Kumari PR, Halemani PK, Buddiga V, Mythri S. A comparative evaluation of oral hygiene using Braille and audio instructions among institutionalized visually impaired children aged between 6 years and 20 years: a 3‐month follow‐up study. Journal of International Society of Preventive and Community Dentistry 2015;5(Suppl 2):S129‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makuch 2011 {published data only}
- Makuch A, Reschke K, Rupf S. Effective teaching of tooth‐brushing to preschool children. Journal of Dentistry for Children 2011;78(1):9‐12. [PubMed] [Google Scholar]
Mayer 2003 {published data only}
- Mayer MP, Paiva Buischi Y, Oliveira LB, Gjermo O. Long‐term effect of an oral hygiene training program on knowledge and reported behavior. Oral Health and Preventive Dentistry 2003;1(1):37‐43. [PubMed] [Google Scholar]
Meurman 2009 {published data only}
- Meurman P, Pienihäkkinen K, Eriksson AL, Alanen P. Oral health programme for preschool children: a prospective, controlled study. International Journal of Paediatric Dentistry 2009;19(4):263‐73. [DOI] [PubMed] [Google Scholar]
Moltzer 1986 {published data only}
- Moltzer G, Hoogstraten J. The effect of three methods of dental health care instruction and dental knowledge, attitude, behaviour, and fear. Community Dental Health 1986;3(1):83‐9. [PubMed] [Google Scholar]
Moskovitz 2009 {published data only}
- Moskovitz M, Abud W, Ram D. The influence of an oral health education program provided in a community dental clinic on the prevalence of caries among 12‐14 year‐old children. Journal of Clinical Pediatric Dentistry 2009;33(3):259‐64. [DOI] [PubMed] [Google Scholar]
Ohrn 2009 {published data only}
- Ohrn K, Sanz M. Prevention and therapeutic approaches to gingival inflammation. Journal of Clinical Periodontology 2009;36(Suppl 10):20‐6. [DOI] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park MS, Choi‐Kwon S. The effects of oral care education on caregivers' knowledge, attitude, & behavior toward oral hygiene for elderly residents in a nursing home. Journal of Korean Academy of Nursing 2011;41(5):684‐93. [DOI] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park DY, Lee BJ, Kim BO, Yu SJ. Effect of the education interval and method on improving patients' plaque control ability. Journal of Korean Academy of Oral Health 2015;39(2):145‐51. [Google Scholar]
Patthoff 1981 {published data only}
- Patthoff DE, Horton AM Jr, Vecchio MB. Behavioral dentistry: a multiple‐baseline study incorporating contracting with patients. Perceptual and Motor Skills 1981;53(2):383‐6. [DOI] [PubMed] [Google Scholar]
Picard 2014 {published data only}
- Picard AJ, Estrella MR, Boynton J, Maxwell A, Inglehart MR. Educating parents of children receiving comprehensive dental care under general anesthesia with visual AIDS. Pediatric Dentistry 2014;36(4):329‐35. [PubMed] [Google Scholar]
Pickrell 2007 {published data only}
- Pickrell JE, Heima M, Weinstein P, Coolidge T, Coldwell SE, Skaret E, et al. Using memory restructuring strategy to enhance dental behaviour. International Journal of Paediatric Dentistry 2007;17(6):439‐48. [DOI] [PubMed] [Google Scholar]
Pine 2007 {published data only}
- Pine CM, Curnow MM, Burnside G, Nicholson JA, Roberts AJ. Caries prevalence four years after the end of a randomised controlled trial. Caries Research 2007;41(6):431‐6. [DOI] [PubMed] [Google Scholar]
Plutzer 2008 {published data only}
- Plutzer K, Spencer AJ. Efficacy of an oral health promotion intervention in the prevention of early childhood caries. Community Dentistry and Oral Epidemiology 2008;36(4):335‐46. [DOI] [PubMed] [Google Scholar]
Plutzer 2012 {published data only}
- Plutzer K, Spencer AJ, Keirse MJ. Reassessment at 6‐7 years of age of a randomized controlled trial initiated before birth to prevent early childhood caries. Community Dentistry and Oral Epidemiology 2012;40(2):116‐24. [DOI] [PubMed] [Google Scholar]
Reinhardt 2010 {published data only}
- Reinhardt CH, Noack MJ, Wassmer G, Hurrelmann K, Klein K. A strategy for encouraging young adults' adoption of a preferred oral hygiene technique. Oral Health and Preventive Dentistry 2010;8(1):3‐8. [PubMed] [Google Scholar]
Roscher 2004 {published data only}
- Roscher T, Rösing CK, Gjermo P, Aass AM. Effect of instruction and motivation in the use of electric and manual toothbrushes in periodontal patients. A comparative study. Brazilian Oral Research 2004;18(4):296‐300. [DOI] [PubMed] [Google Scholar]
Saengtipbovorn 2017 {published data only}
- Saengtipbovorn S. Efficacy of Motivational Interviewing in Conjunction with Caries Risk Assessment (MICRA) programmes in improving the dental health status of preschool children: a randomised controlled trial. Oral Health & Preventive Dentistry 2017;15(2):123‐9. [DOI] [PubMed] [Google Scholar]
Sandeep 2014 {published data only}
- Sandeep V, Vinay C, Madhuri V, Rao VV, Uloopi KS, Sekhar RC. Impact of visual instruction on oral hygiene status of children with hearing impairment. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2014;32(1):39‐43. [DOI] [PubMed] [Google Scholar]
Sato 2008 {published data only}
- Sato T, Abe T, Ichikawa M, Fukushima Y, Nakamoto N, Koshikiya N, et al. A randomized controlled trial assessing the effectiveness of professional oral care by dental hygienists. International Journal of Dental Hygiene 2008;6(1):63‐7. [DOI] [PubMed] [Google Scholar]
Schiffner 2007 {published data only}
- Schiffner U, Bahr M, Effenberger S. Plaque and gingivitis in the elderly: a randomized, single‐blind clinical trial on the outcome of intensified mechanical or antibacterial oral hygiene measures. Journal of Clinical Periodontology 2007;34(12):1068‐73. [DOI: 10.1111/j.1600-051X.2007.01150.x] [DOI] [PubMed] [Google Scholar]
Schneider 1981 {published data only}
- Schneider HG, Friedländer B, Richter C, Unger E, Ziesenitz S, Bendrien S. Experience with plaque‐disclosing agents. Stomatologie der DDR 1981;31(12):904‐9. [PubMed] [Google Scholar]
Schneider 1982 {published data only}
- Schneider HG, Rasch K. The motivation of preschool children toward oral and dental hygiene. Stomatologie der DDR 1982;32(4):261‐5. [PubMed] [Google Scholar]
Schüz 2009 {published data only}
- Schüz B, Wiedemann AU, Mallach N, Scholz U. Effects of a short behavioural intervention for dental flossing: randomized‐controlled trial on planning when, where and how. Journal of Clinical Periodontology 2009;36(6):498‐505. [DOI] [PubMed] [Google Scholar]
Seow 2003 {published data only}
- Seow WK, Cheng E, Wan V. Effects of oral health education and tooth‐brushing on mutans streptococci infection in young children. Pediatric Dentistry 2003;25(3):223‐8. [PubMed] [Google Scholar]
Sniehotta 2007 {published data only}
- Sniehotta FF, Araújo Soares V, Dombrowski SU. Randomized controlled trial of a one‐minute intervention changing oral self‐care behavior. Journal of Dental Research 2007;86(7):641‐5. [DOI] [PubMed] [Google Scholar]
Söderholm 1982b {published data only}
- Söderholm G, Egelberg J. Teaching plaque control. II. 30‐minute versus 15‐minute appointments in a three‐visit program. Journal of Clinical Periodontology 1982;9(3):214‐22. [6954163] [DOI] [PubMed] [Google Scholar]
Stenman 2012 {published data only}
- Stenman J, Lundgren J, Wennström JL, Ericsson JS, Abrahamsson KH. A single session of motivational interviewing as an additive means to improve adherence in periodontal infection control: a randomized controlled trial. Journal of Clinical Periodontology 2012;39(10):947‐54. [DOI] [PubMed] [Google Scholar]
Stenman 2017 {published data only}
- Stenman J, Wennström JL, Abrahamsson KH. A brief motivational interviewing as an adjunct to periodontal therapy ‐ a potential tool to reduce relapse in oral hygiene behaviours. A three‐year study. International Journal of Dental Hygiene 2017 Aug 24 [Epub ahead of print]. [DOI: 10.1111/idh.12308] [DOI] [PubMed]
Strippel 2010 {published data only}
- Strippel H. Effectiveness of structured comprehensive paediatric oral health education for parents of children less than two years of age in Germany. Community Dental Health 2010;27(2):74‐80. [PubMed] [Google Scholar]
Tan 1978 {published data only}
- Tan HH, Saxton CA. Effect of a single dental health care instruction and prophylaxis on gingivitis. Community Dentistry and Oral Epidemiology 1978;6(4):172‐5. [DOI] [PubMed] [Google Scholar]
Tan 1979 {published data only}
- Tan HH. Effect of dental health care instruction and prophylaxis on knowledge, attitude and behavior in Dutch military personnel. Community Dentistry and Oral Epidemiology 1979;7(5):252‐8. [DOI] [PubMed] [Google Scholar]
Telford 1974 {published data only}
- Telford AB, Murray JJ. The effect of systematic chairside oral hygiene instruction on gingivitis and oral cleanliness in children. Community Dentistry and Oral Epidemiology 1974;2(2):50‐7. [PUBMED: 4528776] [PubMed] [Google Scholar]
Triana 1985 {published data only (unpublished sought but not used)}
- Triana Cue M, Nazco Ríos C. Gingival bleeding and motivation [Sangramiento gingival y motivación]. Revista Cubana de Estomatología 1985;22(2):118‐24. [PubMed] [Google Scholar]
Valle 2004 {published data only}
- Valle DD, Carvalho Vianna RB, Quintanilha LE, Abreu FV. Evaluation of an oral health promotion program using different indicators. Journal of Clinical Pediatric Dentistry 2004;29(1):87‐92. [DOI] [PubMed] [Google Scholar]
Zanin 2007 {published data only}
- Zanin L, Meneghim MC, Assaf AV, Cortellazzi KL, Pereira AC. Evaluation of an educational program for children with high risk of caries. Journal of Clinical Pediatric Dentistry 2007;31(4):246‐50. [DOI] [PubMed] [Google Scholar]
Zee 2006 {published data only}
- Zee KY, Lee DH, Corbet EF. Repeated oral hygiene instructions alone, or in combination with metronidazole dental gel with or without subgingival scaling in adult periodontitis patients: a one‐year clinical study. Journal of the International Academy of Periodontology 2006;8(4):125‐35. [PubMed] [Google Scholar]
Ziebolz 2009 {published and unpublished data}
- Ziebolz D, Herz A, Brunner E, Hornecker E, Mausberg RF. Individual versus group oral hygiene instruction for adults. Oral Health & Preventive Dentistry 2009;7(1):93‐9. [PUBMED: 19408821] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gao 2013 {published data only}
- Gao X, Lo EC, McGrath C, Ho SM. Innovative interventions to promote positive dental health behaviors and prevent dental caries in preschool children: study protocol for a randomized controlled trial. Trials 2013;14:118. [DOI: 10.1186/1745-6215-14-118] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2014062618248N1 {unpublished data only}
- IRCT2014062618248N1. The effect of educational intervention based on Pender's Health Promotion Model on oral and dental health behaviors among high school students. apps.who.int/trialsearch/Trial3.aspx?trialid=IRCT2014062618248N1 (first received 25 October 2014).
References to ongoing studies
ACTRN12605000607673 {unpublished data only}
- ACTRN12605000607673. Oral Health Education Logan Programme (OHELP) [The effect of a personalised oral health education programme on clinical and molecular risk factors for oral and general health in an at risk population]. www.anzctr.org.au/ACTRN12605000607673.aspx (first received 12 September 2005).
ISRCTN24958829 {unpublished data only}
- ISRCTN24958829. Dental RECUR Trial [Comparison of a new with standard child and family primary care service to reduce the re‐occurrence of childhood dental caries (Dental RECUR Trial)]. www.isrctn.com/ISRCTN24958829 (first received 27 September 2013).
ISRCTN38542397 {unpublished data only}
- ISRCTN38542397. Is photographic evidence a valuable aide to oral hygiene advice in 9‐16 year olds?. www.isrctn.com/ISRCTN38542397 (first received 28 September 2007).
NCT01118143 {unpublished data only}
- NCT01118143. Oral health literacy tailored communication [Effects of communication tailored to oral health literacy level of adult dental patients: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT01118143 (first received 6 May 2010).
Additional references
Adair 1984
- Adair JG. The Hawthorne effect: a reconsideration of the methodological artifact. Journal of Applied Psychology 1984;69(2):334‐45. [Google Scholar]
Axelsson 2004
- Axelsson P, Nyström B, Lindhe J. The long‐term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. Journal of Clinical Periodontology 2004;31(9):749‐57. [DOI] [PubMed] [Google Scholar]
Baab 1983
- Baab DA, Weinstein P. Oral hygiene instruction using a self inspection plaque index. Community Dentistry and Oral Epidemiology 1983;11(3):174‐9. [DOI] [PubMed] [Google Scholar]
Bandura 1998
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health 1998;13:623‐49. [Google Scholar]
Bass 1954a
- Bass CC. An effective method of personal oral hygiene. Journal of the Louisiana State Medical Society 1954;106(2):57‐73. [PubMed] [Google Scholar]
Bass 1954b
- Bass CC. An effective method of personal oral hygiene; part II. Journal of the Louisiana State Medical Society 1954;106(3):100‐12. [PubMed] [Google Scholar]
Björby 1967
- Björby Å, Löe H. The relative significance of different local factors in the initiation and development of periodontal inflammation. Scandinavian Symposium in Periodontology 1966. Journal of Periodontal Research 1967;2(1):76‐7 (Abs No 20). [Google Scholar]
Chadwick 2011
- Chadwick B, White D, Lader D, Pitts N. 5: Preventive behaviour and risks to oral health ‐ a report from the Adult Dental Health Survey 2009. Leeds: The Health and Social Care Information Centre; 2011 March.
Chapple 2008
- Chapple ILC, Hill K. Getting the message across to periodontitis patients: the role of personalised biofeedback. International Dental Journal 2008;65(5 Supplement 1):294‐306. [Google Scholar]
DBOH 2009
- Department of Health, British Association for the Study of Community Dentistry. Delivering Better Oral Health: an evidence‐based toolkit for prevention. London: Department of Health; 2009 April.
Deci 1985
- Deci EL, Ryan RM. The general causality orientation scale: self‐determination in personality. Journal of Research in Personality 1985;19:109–34. [Google Scholar]
Deinzer 2014
- Deinzer R, Jahns S, Harnacke D. Establishment of a new marginal plaque index with high sensitivity for changes in oral hygiene. Journal of Periodontology 2014;85(12):1730‐8. [DOI] [PubMed] [Google Scholar]
Dupont 1990
- Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Controlled Clinical Trials 1990;11(2):116‐28. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fishbein 1980
- Fishbein M, Azjen I. Understanding Attitudes and Predicting Social Behaviour. Englewood Cliffs: Prentice‐Hall, Inc., 1980. [Google Scholar]
Glavind 1979
- Glavind L, Attström R. Periodontal self‐examination. A motivational tool in periodontics. Journal of Clinical Periodontology 1979;6(4):238‐51. [DOI] [PubMed] [Google Scholar]
Gollwitzer 1999
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. American Psychologist 1999;54:493‐503. [Google Scholar]
Gründemann 2000
- Gründemann LJ, Timmerman MF, Ijzerman Y, Weijden GA, Weijden GA. Stain, plaque and gingivitis reduction by combining chlorhexidine and peroxyborate. Journal of Clinical Periodontology 2000;27(1):9‐15. [DOI] [PubMed] [Google Scholar]
Halvari 2006
- Halvari A, Halvari H. Motivational predictors of change in oral health: an experimental test of self‐determination theory. Motivation and Emotion 2006;30:295‐305. [Google Scholar]
Halvari 2010
- Halvari AEM, Halvari H, Bjørnebekk G, Deci EL. Motivation and anxiety for dental treatment: testing a self‐determination theory model of oral self‐care behavior and dental clinic attendance. Motivation and Emotion 2010;34:15‐33. [Google Scholar]
Halvari 2012
- Halvari AEM, Halvari H, Bjørnebekk G, Deci EL. Motivation for dental home care: testing a self‐determination theory model. Journal of Applied Social Psychology 2012;42:1‐39. [Google Scholar]
Harris 2012
- Harris R, Gamboa A, Dailey Y, Ashcroft A. One‐to‐one dietary interventions undertaken in a dental setting to change dietary behaviour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD006540.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holve 2008
- Holve S. An observational study of the association of fluoride varnish applied during well child visits and the prevention of early childhood caries in American Indian children. Maternal and Child Health Journal 2008;12 Suppl 1:64–7. [DOI] [PubMed] [Google Scholar]
Hugoson 2003
- Hugoson A, Lundgren D, Asklöw B, Borgklint G. The effect of different dental health programmes on young individuals: a longitudinal evaluation of knowledge and behaviour including cost aspects. Swedish Dental Journal 2003;27(3):115‐30. [PubMed] [Google Scholar]
Kagihara 2009
- Kagihara LE, Niederhauser VP, Stark M. Assessment, management, and prevention of early childhood caries. Journal of the American Academy of Nurse Practitioners 2009;21(1):1–10. [DOI] [PubMed] [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990‐2010: a systematic review and meta‐regression. Journal of Dental Research 2014;93(11):1045‐53. [DOI: 10.1177/0022034514552491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassebaum 2015
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. Journal of Dental Research 2015;94(5):650‐8. [DOI: 10.1177/0022034515573272] [DOI] [PubMed] [Google Scholar]
Kay 1998
- Kay EJ, Locker D. A systematic review of the effectiveness of health promotion aimed at improving oral health. Community Dental Health 1998;15:132‐44. [PubMed] [Google Scholar]
Kay 2016a
- Kay E, Vascott D, Hocking A, Nield H, Dorr C, Barrett H. A review of approaches for dental practice teams for promoting oral health. Community Dentistry and Oral Epidemiology 2016;44(4):313‐30. [DOI] [PubMed] [Google Scholar]
Kay 2016b
- Kay EJ, Vascott D, Hocking A, Nield H. Motivational interviewing in general dental practice: a review of the evidence. British Dental Journal 2016;221(12):785‐91. [DOI] [PubMed] [Google Scholar]
Khokhar 2016
- Khokhar MA, Khokhar WA, Clifton AV, Tosh GE. Oral health education (advice and training) for people with serious mental illness. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD008802.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumarihamy 2011
- Kumarihamy SL, Subasinghe LD, Jayasekara P, Kularatna SM, Palipana PD. The prevalence of early childhood caries in 1–2 year olds in a semi‐urban area of Sri Lanka. BMC Research Notes 2011;4:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 2003
- Lang NP, Tonetti MS. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (STP). Oral Health and Preventive Dentistry 2003;1(1):7–16. [PubMed] [Google Scholar]
Lange 1977
- Lange DE, Plagmann H, Eenboom A, Promesberger A. Clinical methods for the objective evaluation of oral hygiene. Deutsche Zahnarztliche Zeitschrift 1977;32(1):44‐7. [PubMed] [Google Scholar]
Lange 1986
- Lange DE. Odontologie in der Täglichen Praxis. Berlin: Quintessenz Verlag, 1986. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lie 1998
- Lie MA, Timmerman MF, Velden U, Weijden GA. Evaluation of 2 methods to assess gingival bleeding in smokers and non‐smokers in natural and experimental gingivitis. Journal of Clinical Periodontology 1998;25(9):695‐700. [DOI] [PubMed] [Google Scholar]
Löe 1963
- Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontologica Scandinavica 1963;21:533–51. [DOI] [PubMed] [Google Scholar]
Löe 1965
- Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. Journal of Periodontology 1965;36:177‐87. [DOI] [PubMed] [Google Scholar]
Löe 1967
- Löe H. The Gingival Index, the Plaque Index and the Retention Index systems. Journal of Periodontology 1967;38(6):Suppl:610‐6. [DOI] [PubMed] [Google Scholar]
Löe 1972
- Löe H, Fehr FR, Schiott CR. Inhibition of experimental caries by plaque prevention. The effect of chlorhexidine mouthrinses. Scandinavian Journal of Dental Research 1972;80(1):1‐9. [DOI] [PubMed] [Google Scholar]
Magarey 2009
- Magarey A, Golley R, Spurrier N, Goodwin E, Ong F. Reliability and validity of the Children's Dietary Questionnaire; a new tool to measure children's dietary patterns. International Journal of Pediatric Obesity 2009;4(4):257‐65. [DOI] [PubMed] [Google Scholar]
Mantilla Gómez 2001
- Mantilla Gómez S, Danser MM, Sipos PM, Rowshani B, Velden U, Weijden GA. Tongue coating and salivary bacterial counts in healthy/gingivitis subjects and periodontitis patients. Journal of Clinical Periodontolology 2001;28(10):970‐8. [DOI] [PubMed] [Google Scholar]
Marinho 2003
- Marinho VCC, Higgins JPT, Logan S, Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD002782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2004a
- Marinho VCC, Higgins JPT, Sheiham A, Logan S. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002781.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2004b
- Marinho VCC, Higgins JPT, Sheiham A, Logan S. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002780.pub2] [DOI] [Google Scholar]
Marinho 2016
- Marinho VCC, Chong LY, Worthington HV, Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD002284.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohebbi 2006
- Mohebbi SZ, Virtanen JI, Vahid‐Golpayegani M, Vehkalahti MM. Early childhood caries and dental plaque among 1‐3‐year‐olds in Tehran, Iran. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2006;24(4):177‐81. [DOI] [PubMed] [Google Scholar]
Mohebbi 2008
- Mohebbi SZ, Virtanen JI, Vahid‐Golpayegani M, Vehkalahti MM. Feeding habits as determinants of early childhood caries in a population where prolonged breastfeeding is the norm. Community Dentistry and Oral Epidemiology 2008;36(4):363‐9. [DOI] [PubMed] [Google Scholar]
Möhler 2015
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16:204. [DOI: 10.1186/s13063-015-0709-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2015
- Newton TJ, Asimakopoulou K. Managing oral hygiene as a risk factor for periodontal disease: a systematic review of psychological approaches to behaviour change for improved plaque control in periodontal management. Journal of Clinical Periodontology 2015;42 (Suppl 16):S36‐S46. [DOI] [PubMed] [Google Scholar]
NICE 2015
- NICE. Oral health promotion: general dental practice. NICE guideline [NG30]. www.nice.org.uk/guidance/ng30 (accessed prior to 25 April 2018).
Nyman 1984
- Nyman S, Bratthall D, Böhlin E. The Swedish dental health program for adults. International Dental Journal 1984;34(2):130–4. [PubMed] [Google Scholar]
O'Leary 1972
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. Journal of Periodontology 1972;43(1):38. [DOI] [PubMed] [Google Scholar]
Paraskevas 2005
- Paraskevas S, Versteeg PA, Timmerman MF, Velden U, Weijden GA. The effect of a dentifrice and mouth rinse combination containing amine fluoride/stannous fluoride on plaque and gingivitis: a 6‐month field study. Journal of Clinical Periodontology 2005;32(7):757‐64. [DOI] [PubMed] [Google Scholar]
Pastagia 2006
- Pastagia J, Nicoara P, Robertson PB. The effect of patient‐centered plaque control and periodontal maintenance therapy on adverse outcomes of periodontitis. Journal of Evidence‐based Dental Practice 2006;6(1):25‐32. [DOI] [PubMed] [Google Scholar]
Poklepovic 2013
- Poklepovic T, Worthington HV, Johnson TM, Sambunjak D, Imai P, Clarkson JE, et al. Interdental brushing for the prevention and control of periodontal diseases and dental caries in adults. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD009857.pub2] [DOI] [PubMed] [Google Scholar]
Quigley 1962
- Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. Journal of the American Dental Assocication 1962;65:26‐9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rice 2008
- Rice C, Hosey MT. Interactive oral health education – a randomised controlled trial. European Archives of Paediatric Dentistry 2008;9(Suppl 2):20‐1 (Abs No O44). [Google Scholar]
Rose 2001
- Rose EA, Markland D, Parfitt G. The development and initial validation of the Exercise Causality Orientations Scale. Journal of Sports Sciences 2001;19(6):445‐62. [DOI] [PubMed] [Google Scholar]
Rugg‐Gunn 1979
- Rugg‐Gunn AJ, Macgregor ID, Edgar WM, Ferguson MW. Toothbrushing behaviour in relation to plaque and gingivitis in adolescent schoolchildren. Journal of Periodontal Research 1979;14(3):231‐8. [DOI] [PubMed] [Google Scholar]
Rylander 1997
- Rylander H, Lindhe J. Chapter 15: Cause‐related periodontal therapy. Periodontology and Oral Implants. 3rd Edition. Copenhagen: Munksgaard, 1997:438‐60. [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [DOI] [PubMed] [Google Scholar]
Schmalz 2017b
- Schmalz G, Müller M, Schmickler J, Rinke S, Haak R, Mausberg RF, et al. Influence of manual and power toothbrushes on clinical and microbiological findings in initial treatment of periodontitis ‐ a randomized clinical study. American Journal of Dentistry 2017;30(1):40‐6. [PubMed] [Google Scholar]
Schmickler 2014
- Schmickler J, Wurbs S, Wurbs S, Lange K, Rinke S, Hornecker E, et al. Influence of the utilization time of different manual toothbrushes on oral hygiene assessed during a 6‐month observation period: a randomized clinical trial. Journal of Periodontology 2014;85(8):1050–8. [DOI] [PubMed] [Google Scholar]
Schmickler 2016
- Schmickler J, Wurbs S, Wurbs S, Kramer K, Rinke S, Hornecker E, et al. The influence of the utilization time of brush heads from different types of power toothbrushes on oral hygiene assessed over a 6‐month observation period: a randomized clinical trial. American Journal of Dentistry 2016;29(6):307‐14. [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359:515‐9. [DOI] [PubMed] [Google Scholar]
Sheiham 2015
- Sheiham A, James WPT. Diet and dental caries: the pivotal role of free sugars reemphasized. Journal of Dental Research 2015;94(10):1341‐7. [DOI] [PubMed] [Google Scholar]
Silness 1964
- Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica 1964;22:121‐35. [DOI] [PubMed] [Google Scholar]
Slade 1997
- Slade GD. Derivation and validation of a short‐form oral health impact profile. Community Dentistry and Oral Epidiemiology 1997;25(4):284‐90. [DOI] [PubMed] [Google Scholar]
Tickle 2003
- Tickle M, Milsom KM, King D, Blinkhorn AS. The influences on preventive care provided to children who frequently attend the UK General Dental Service. British Dental Journal 2003;194(6):329‐32. [DOI] [PubMed] [Google Scholar]
Tonetti 2017
- Tonetti MS, Jepsen S, Jin L, Otomo‐Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. Journal of Clinical Periodontology 2017;44(5):456‐62. [DOI: 10.1111/jcpe.12732] [DOI] [PubMed] [Google Scholar]
Towner 1993
- Towner E. The history of dental health education: a case study of Britain. In: Schou L, Blinkhorn A editor(s). Oral Health Promotion. Oxford: Oxford University Press, 1993. [Google Scholar]
Tsakos 2015
- Tsakos G, Hill K, Chadwick B, Anderson T. Children's Dental Health Survey 2013. Report 1: Attitudes, behaviours and children's dental health England, Wales and Northern Ireland, 2013. Leeds: The Health and Social Care Information Centre; 2015 March.
Turesky 1970
- Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analog of vitamin C. Journal of Periodontology 1970;41:41‐3. [DOI] [PubMed] [Google Scholar]
Van der Velden 1979
- Velden U. Probing force and the relationship of the probe tip to the periodontal tissues. Journal of Clinical Periodontolology 1979;6(2):106‐14. [DOI] [PubMed] [Google Scholar]
Van der Velden 2009
- Velden U. The Dutch periodontal screening index validation and its application in The Netherlands. Journal of Clinical Periodontology 2009;36(12):1018‐24. [DOI] [PubMed] [Google Scholar]
Van der Weijden 1994
- Weijden GA, Timmerman MF, Saxton CA, Russell JI, Huntington E, Velden U. Intra‐/inter‐examiner reproducibility study of gingival bleeding. Journal of Periodontal Research 1994;29:236‐41. [DOI] [PubMed] [Google Scholar]
Watt 2005
- Watt RG, Marinho VC. Does oral health promotion improve oral hygiene and gingival health?. Periodontology 2000 2005;37:35‐47. [DOI] [PubMed] [Google Scholar]
Weyant 2013
- Weyant RJ, Tracy SL, Anselmo TT, Beltrán‐Aguilar ED, Donly KJ, Frese WA, et al. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. Journal of the American Dental Association 2013;144(11):1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1986
- World Health Organization. The Ottawa Charter for Health Promotion. www.who.int/healthpromotion/conferences/previous/ottawa/en/ (accessed prior to 25 April 2018).
WHO 1997
- World Health Organization. Oral health surveys – basic methods, 4th edition. www.who.int/iris/handle/10665/41905 (accessed prior to 25 April 2018).
WHO 2003
- World Health Organization. World Oral Health Report 2003. www.who.int/oral‐health/publications/world‐oral‐health‐report‐2003/en/ (accessed prior to 25 April 2018).
Williams 1996
- Williams GC, Deci ED. Internalization of biopsychosocial values by medical students: a test of self‐determination theory. Journal of Personality and Social Psychology 1996;70:767–79. [DOI] [PubMed] [Google Scholar]
Wilson 1992
- Wilson PH. Principles and Practice of Relapse Prevention. New York: Guildford Press, 1992. [Google Scholar]
Wolfe 1996
- Wolfe GR, Stewart JM, Meader LA, Hartz GW. Use of dental coping beliefs scale to measure cognitive changes following oral hygiene interventions. Community Dentistry and Oral Epidemiology 1996;24(1):37–41. [DOI] [PubMed] [Google Scholar]
Yaacob 2014
- Yaacob M, Worthington HV, Deacon SA, Deery C, Walmsley AD, Robinson PG, et al. Powered versus manual toothbrushing for oral health. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002281.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yevlahova 2009
- Yevlahova D, Satur J. Models for individual oral health promotion and their effectiveness: a systematic review. Australian Dental Journal 2009;54:190‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Soldani 2008
- Soldani FA, Young L, Jones K, Walsh T, Clarkson JE. One‐to‐one oral hygiene advice provided in a dental setting for oral health. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007447] [DOI] [PMC free article] [PubMed] [Google Scholar]


