Abstract
Background
Red blood cell (RBC) transfusion is a common treatment for anaemia in many conditions. The safety and efficacy of transfusing RBC units that have been stored for different durations before a transfusion is a current concern. The duration of storage for a RBC unit can be up to 42 days. If evidence from randomised controlled trials (RCT) were to indicate that clinical outcomes are affected by storage duration, the implications for inventory management and clinical practice would be significant.
Objectives
To assess the effects of using red blood cells (RBCs) stored for a shorter versus a longer duration, or versus RBCs stored for standard practice duration, in people requiring a RBC transfusion.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, PubMed (for epublications), LILACS, Transfusion Evidence Library, Web of Science CPCI‐S and four international clinical trial registries on 20 November 2017.
Selection criteria
We included RCTs that compared transfusion of RBCs of shorter versus longer storage duration, or versus standard practice storage duration.
Data collection and analysis
We used standard Cochrane methods.
Main results
We included 22 trials (42,835 participants) in this review.
The GRADE quality of evidence ranged from very low to moderate for our primary outcome of in‐hospital and short‐term mortality reported at different time points.
Transfusion of RBCs of shorter versus longer storage duration
Eleven trials (2249 participants) compared transfusion of RBCs of shorter versus longer storage duration. Two trials enrolled low birth weight neonates, two enrolled children with severe anaemia secondary to malaria or sickle cell disease, and eight enrolled adults across a range of clinical settings (intensive care, cardiac surgery, major elective surgery, hospitalised in‐patients, haematology outpatients). We judged only two trials to be at low risk of bias across all domains; most trials had an unclear risk for multiple domains.
Transfusion of RBCs of shorter versus longer storage duration probably leads to little or no difference in mortality at seven‐day follow‐up (risk ratio (RR) 1.42, 95% confidence interval (CI) 0.66 to 3.06; 1 trial, 3098 participants; moderate quality evidence) or 30‐day follow‐up (RR 0.85, 95%CI 0.50 to 1.45; 2 trials, 1121 participants; moderate quality evidence) in adults undergoing major elective cardiac or non‐cardiac surgery.
For neonates, no studies reported on the primary outcome of in‐hospital or short‐term mortality. At 40 weeks gestational age, the effect of RBCs of shorter versus longer storage duration on the risk of death was uncertain, as the quality of evidence is very low (RR 0.90, 95% CI 0.41 to 1.85; 1 trial, 52 participants).
The effect of RBCs of shorter versus longer storage duration on the risk of death in children with severe anaemia was also uncertain within 24 hours of transfusion (RR 1.50, 95% CI 0.43 to 5.25; 2 trials, 364 participants; very low quality evidence), or at 30‐day follow‐up (RR 1.40, 95% CI 0.45 to 4.31; 1 trial, 290 participants; low quality evidence).
Only one trial, in children with severe anaemia (290 participants), reported adverse transfusion reactions. Only one child in each arm experienced an adverse reaction within 24 hours of transfusion.
Transfusion of RBCs of shorter versus standard practice storage duration
Eleven trials (40,588 participants) compared transfusion of RBCs of shorter versus standard practice storage duration. Three trials enrolled critically ill term neonates; two of these enrolled very low birth weight neonates. There were no trials in children. Eight trials enrolled critically ill and non‐critically ill adults, with most being hospitalised. We judged four trials to be at low risk of bias across all domains with the others having an unclear risk of bias across multiple domains.
Transfusion of RBCs of shorter versus standard practice storage duration probably leads to little or no difference in adult in‐hospital mortality (RR 1.05, 95% CI 0.97 to 1.14; 4 trials, 25,704 participants; moderate quality evidence), ICU mortality (RR 1.06, 95% CI 0.98 to 1.15; 3 trials, 13,066 participants; moderate quality evidence), or 30‐day mortality (RR 1.04, 95% CI 0.96 to 1.13; 4 trials, 7510 participants;moderate quality evidence).
Two of the three trials that enrolled neonates reported that there were no adverse transfusion reactions. One trial reported an isolated case of cytomegalovirus infection in participants assigned to the standard practice storage duration group. Two trials in critically ill adults reported data on transfusion reactions: one observed no difference in acute transfusion reactions between arms (RR 0.67, 95% CI 0.19 to 2.36, 2413 participants), but the other observed more febrile nonhaemolytic reactions in the shorter storage duration arm (RR 1.48, 95% CI 1.13 to 1.95, 4919 participants).
Trial sequential analysis showed that we may now have sufficient evidence to reject a 5% relative risk increase or decrease of death within 30 days when transfusing RBCs of shorter versus longer storage duration across all patient groups.
Authors' conclusions
The effect of storage duration on clinically important outcomes has now been investigated in large, high quality RCTs, predominantly in adults. There appears to be no evidence of an effect on mortality that is related to length of storage of transfused RBCs. However, the quality of evidence in neonates and children is low. The current practice in blood banks of using the oldest available RBCs can be continued safely. Additional RCTs are not required, but research using alternative study designs, should focus on particular subgroups (e.g. those requiring multiple RBC units) and on factors affecting RBC quality.
Plain language summary
Transfusion of red blood cells stored for a shorter duration versus red blood cells stored for a longer duration for all conditions
Review question
In people needing a blood transfusion, is it better to give blood that has been stored for a shorter time compared to a longer time?
Background
After donation, red blood cells (blood) can be stored for up to 42 days before being transfused to a person in need of a blood transfusion. It is not known whether blood stored for this long might be harmful, particularly in vulnerable patient groups, such as those needing intensive care.
Study characteristics
We investigated the consequences of giving transfusions of:
‐ blood stored for a shorter duration versus blood stored for a longer duration, and
‐ blood stored for a shorter duration versus blood stored for a standard length of time ('standard practice storage duration'; this period varies between hospitals)
to anyone needing a blood transfusion.
We searched the medical literature to 20 November 2017. We identified 22 studies, which included a range of 42,835 participants (newborn babies less than 4 weeks old (neonates), children, and adults). Eleven studies (2249 participants) compared transfusion of blood stored for a shorter duration versus transfusion of blood stored for a longer duration, and the other 11 studies (40,588 participants) compared transfusion of blood stored for a shorter duration versus transfusion of standard practice storage duration blood.
Quality of evidence
We assessed the quality of evidence for our results, which ranged from very low to moderate quality. These judgements are based on the number of trials and participants contributing data to each result, how similar the results were between trials, and the reliability of the trial methods used. Future research is highly likely to change the findings of results judged to be of very low quality, but is less likely to change the findings of other results, such as risk of death, for which we rated the evidence as moderate.
Key results Transfusion of blood stored for a shorter duration versus blood stored for a longer duration
Eight studies focused on adults, two on children with severe anaemia (low blood count) and two on low birth weight neonates. In adults undergoing major surgery, transfusion of blood stored for a shorter duration versus blood stored for a longer duration probably leads to little or no difference in risk of death up to 30 days but the quality of evidence is moderate. In children we were uncertain whether transfusion of blood stored for a shorter duration increases or decreases risk of death, because the quality of the evidence is very low.
Transfusion of blood stored for a shorter duration versus standard practice storage duration blood
Eight studies focused on adults, most of whom were in hospital. Three studies enrolled critically ill neonates. Transfusion of blood of shorter storage duration versus blood of standard practice storage duration probably leads to little or no difference in risk of death in adults in the 30 days after transfusion. Whether transfusion of blood of shorter storage duration increases or decreases risk of death in critically ill neonates up to 30 days after transfusion is uncertain because the quality of evidence is very low.
Two studies in critically ill adults provided information on adverse reactions to transfusion, but had conflicting results. While one study found more transfusions associated with fever in the participants who received blood stored for a shorter duration than those who received standard practice storage duration blood, another study found no differences between the two groups.
Conclusion
We observed no clear difference in the risk of death at different time points between transfusion of blood stored for a shorter duration versus blood stored for a longer duration or versus blood stored for the standard practice storage duration.
Summary of findings
Background
Description of the condition
The haemoglobin (Hb) contained within red blood cells (RBCs) is essential for oxygen transportation. Anaemia is defined by the World Health Organization (WHO) as a Hb concentration lower than 13 g/dL in men and lower than 12 g/dL in non‐pregnant women, and describes a clinical state in which oxygen transport is disturbed and tissue hypoxia may occur (Beutler 2006). Anaemia has no single cause; rather it is the consequence of a variety of factors. In high‐income countries, the overall prevalence of anaemia is estimated to be 10% (McLean 2009), however, this figure varies significantly with demographic profiles and patterns of co‐morbid diagnoses (McLean 2009; Tettamanti 2010). Children and older adults are most commonly affected by anaemia, for example, almost 90% of preterm infants with birth weights under 1.0 kg are anaemic (Martin 2010). In later life, rates rise again, largely because of the increasing incidence of co‐morbid diagnoses.
The aetiology of anaemia can be broadly divided into disease processes that impair RBC production and those in which the life span or distribution of RBCs is altered. In the former group, disorders such as acquired and iatrogenic marrow dysfunction, nutritional deficiency, and cytokine‐driven processes such as anaemia due to chronic disease are commonplace. In the latter group, examples include disease processes such as pathological bleeding and immune haemolysis.
When possible, reversing the primary cause of anaemia remains the treatment of choice, however this cannot always be achieved. Furthermore, when severe anaemia results in life‐threatening organ dysfunction, rapid correction is required. In these instances, RBC transfusion is the only viable treatment capable of restoring tissue oxygenation.
Description of the intervention
Red blood cell transfusion
Red blood cell transfusion has been a commonplace treatment for anaemia since the 1990s (Alter 2008), and is very widely practiced. Annually, in the UK, around 1.7 million RBC units are issued for transfusion (NICE 2015). This equates to transfusion of approximately 36 units per 1000 population per year, which is a figure not dissimilar to other high‐income countries (Cobain 2007; NICE 2015). It is surprising, for such a ubiquitous intervention, that a recent systematic overview concluded that rigorous clinical trial data were lacking to support the benefits of many current transfusion practices (Wilkinson 2011). Indeed, evidence obtained from randomised controlled trials (RCTs) indicates little or no benefit from RBC transfusion at higher recipient haemoglobin concentration thresholds (commonly termed 'liberal' policies for RBC transfusion) (Carson 2012; Carson 2016).
Red blood cell transfusions are also associated with some well‐described risks (Stainsby 2006); these are biological products and hazards such as bacterial and viral contamination and allergic reactions. The Serious Hazards of Transfusion (SHOT) scheme estimated that, in 2014, the risk of transfusion‐related morbidity was 63.5 per million blood components issued (SHOT 2015). Key amongst these risks is the potential long‐debated risk posed by RBCs with a prolonged storage duration (see below) (Schrier 1979). Therefore, practice guidelines now promote more restrictive policies for RBC transfusion in many clinical settings (Carson 2013; Carson 2016). Despite this, RBC transfusion remains a very common intervention; for example, while up to 60% of patients admitted to critical care units develop anaemia (Vincent 2002; Corwin 2004), only 10% to 15% have a history of chronic anaemia before admission to the intensive care unit (ICU). Unless modified by RBC transfusion, haemoglobin values typically decrease by about 0.5 g/dL/day during critical illness for reasons that include anaemia of inflammation associated with acute illness, haemodilution, co‐morbidities, bleeding and phlebotomy (Walsh 2010). As a result, 20% to 50% of critically ill patients receive a RBC transfusion, especially those with multiple organ failure. About 8% to 10% of the UK blood supply is transfused to patients in ICUs.
Red blood cell units and their storage
The uncertainty regarding the clinical consequences of transfusing RBC units that have been stored for longer periods before transfusion is a major concern. The debate about potential harm related to transfusion of a product that has been stored for a longer period was re‐ignited by the authors of the Transfusion Requirements In Critical Care (TRICC) trial (Hebert 1999). This landmark RCT compared liberal and restrictive transfusion practices in critically ill patients ‐ not length of product storage ‐ and investigators showed that restricting transfusions to maintain Hb concentration at 7 g/dL to 9 g/dL was safe, and superior to more liberal RBC use in some subgroups of patients. Crucially, the study authors suggested that the common practice of storing RBC units for prolonged periods might contribute to the unexpected adverse effects of liberal transfusion.
This suggestion is biologically plausible in view of the body of evidence that has demonstrated changes in many cellular and physiological properties of RBCs. These in vitro changes, which occur during RBC storage, are commonly known as the 'storage lesion' (D'Alessandro 2010; Glynn 2010). The storage lesion includes biochemical, metabolic and mechanical changes to RBCs, all of which may impair oxygen delivery. The term also encompasses changes that occur in the storage medium, which theoretically could mediate inflammatory or oxidative tissue damage (Sharifi 2000; Kucukakin 2011). The most commonly described biochemical and metabolic components of the storage lesion are impaired nitric oxide metabolism (Stapley 2012), depletion of cellular 2,3‐diphosphoglycerate (Vora 1989), and dysfunction of the membrane sodium‐potassium pump (D'Alessandro 2010). Nitric oxide depletion induces vasoconstriction, which, in turn impairs blood flow and oxygenation (Stapley 2012); depleted 2,3‐diphosphoglycerate reduces the oxygen affinity of haemoglobin (Sohmer 1979); while dysfunction of the membrane sodium‐potassium pump results in harmful potassium leakage from the RBCs into extracellular fluids (Hess 2010). Mechanical changes to the red cell membrane impair fluidity and red cell flow (Hess 2010), may reduce transit of RBCs through the microscopic vasculature of organs such as lungs and kidneys (Roback 2011a), and may impair oxygen uptake and delivery. Changes caused by the storage medium include the generation of inflammatory mediators such as the soluble CD40 ligand, interleukin‐6 (IL‐6) and interleukin‐8 (IL‐8) (Khan 2006; Kucukakin 2011). Potential oxidative damage may also arise from superoxide generation in the storage media (Kucukakin 2011).
Extended RBC storage, as described above, is fundamental to effective management of blood stocks. In the UK, due to stock rotation processes, the average duration of storage of a RBC unit at the time of transfusion is 18 to 21 days (NHSBT 2012). This is very similar to the situation throughout Europe and North America (Bennett‐Guerrero 2009; Heddle 2012). Changes in the storage lesion, as described above, may be well established by this time. Currently, it is biologically plausible that critically ill patients may be being denied the benefits of RBCs that have been stored for a shorter duration, and are being exposed to the additional clinical risks posed by RBC units that have been stored for a longer duration. Limited clinical data support this notion. Cohort studies have described associations between RBC storage duration and a wide range of clinically important adverse outcomes (including infection, organ failure, increased hospital stay and death) (Vamvakas 1999; Mynster 2000; Leal‐Noval 2003; Basran 2006; Koch 2008). However, these effects are not universally described (Vamvakas 2000; van de Watering 2006); although this important message is provided in the literature, many study authors point to the presence of significant confounding factors in the evidence (Steiner 2009). In particular, the strong linkage between the total volume transfused (which itself is strongly associated with the presence of co‐morbidities, severity of illness and worse prognosis) and the average duration of storage of RBC units issued makes inferring causality very difficult (Vamvakas 2010).
How the intervention might work
The goal of RBC transfusion is to improve tissue oxygenation through increasing the red cell mass. It is commonly presumed that lower Hb concentrations represent an accurate measure of diminished oxygen‐carrying capacity, which can be corrected in part by RBC transfusion. The processing methods for RBC collection and storage for transfusion have been studied for many years (Alter 2008). After blood collection, whole blood is centrifuged, plasma is depleted and RBCs are resuspended in an optimal additive solution ‐ a solution of additives that is designed to optimise and maintain the integrity of the RBCs ‐ for storage within specially designed bags. This process, in conjunction with effective refrigeration, has allowed the duration storage of RBC units to be significantly extended (D'Alessandro 2010). Many countries store RBC units routinely for up to 42 days. This period is defined by the arbitrary requirement that, after storage, more than 75% of RBCs should survive in the recipient's circulation at 24 hours (Roback 2011b). An extended shelf‐life facilitates stock management and is fundamental to effective blood banking. Amongst blood providers and blood banks, it is standard practice to issue the oldest stock first in preference to newer stock (Stanger 2012). This ensures that the demand for blood can be met and minimises wastage of a precious and financially costly resource (Stanger 2012).
Why it is important to do this review
If studies were to indicate that clinical outcomes are affected by storage duration, the implications for inventory management and clinical practice would be significant. Clinicians would expect to use RBC units stored for a shorter duration, as they would be safer and more efficacious than RBC units stored for longer periods. Implementation of such a strategy would place considerable strain on blood providers and blood banks. It might also result in increased wastage, higher financial costs and could threaten blood supplies (Glynn 2010). There is an urgent need to reconcile this issue ‐ keenly felt by clinicians, blood services and policy makers alike ‐ because of the potential harms to patients and massive logistical implications.
Although numerous published reviews have addressed this question (including Lelubre 2009; Zimrin 2009; Vamvakas 2010; Vamvakas 2011; Wang 2012; Lelubre 2013; Alexander 2016), some have included observational study data, or have not included recently published and ongoing trials in this area, or have only focused on one outcome (e.g. mortality in Chai‐Adisaksopha 2017). Since the previous iteration of this review (Brunskill 2015), several trials previously noted as ongoing have been completed and published, specifically: ABLE (Lacroix 2015), RECESS (Steiner 2015), TOTAL (Dhabangi 2015), INFORM (Heddle 2016), and TRANSFUSE (Cooper 2017). Incorporation of these within the review will increase the number of participants available for analysis significantly. Therefore, there is a need to update this Cochrane Review so that new guidelines and policies will be based on the most recent evidence pertaining to the effects of duration of storage on RBCs.
Objectives
To assess the effects of using red blood cells (RBCs) stored for a shorter versus a longer duration, or versus RBCs stored for standard practice duration, in people requiring a RBC transfusion.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing transfusion of RBCs of shorter storage duration with transfusion of RBCs of longer storage duration.
Types of participants
People of any age (neonates, children and adults) requiring RBC transfusion for investigator‐diagnosed and ‐defined anaemia of any aetiology.
Types of interventions
No consensus has been reached about what defines shorter storage duration or longer storage duration RBC units. Arbitrarily defining shorter and longer storage durations for the purposes of this review is scientifically unsound, invites legitimate criticism of the clinical validity of the review's results and risks exclusion of a large proportion of available data. Consequently, we included all definitions of shorter and longer storage durations for RBCs. Studies comparing the following interventions were eligible for inclusion.
Transfusion of RBCs of shorter storage duration versus longer storage duration.
Transfusion of RBCs of shorter storage duration versus standard practice storage duration. Here, the duration of storage of the RBCs stored for longer was dictated by standard inventory management practice of each study site.
Types of outcome measures
Primary outcomes
Mortality measured at two time points: in hospital (with time points as defined by the participant group) and short term (up to 30 days).
Secondary outcomes
Long‐term mortality (more than 30 days)
Incidence of hospital‐acquired infection (as defined by the study authors)
Duration of organ support: respiratory (invasive and non‐invasive ventilation), haemodynamic (inotropic) and renal (haemofiltration)
Length of hospital and ICU stay
Adverse transfusion reactions
Economic or blood stock inventory outcomes (as reported by the study authors)
We were interested in data addressing any of the above outcomes, at any reported time points.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
In this 2018 update we searched the following electronic databases and ongoing trials databases on 20 November 2017:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2017, Issue 10);
MEDLINE (OvidSP, 1948 to 20 November 2017);
Embase (OvidSP, 1974 to 20 November 2017);
CINAHL (EBSCOHost, 1982 to 20 November 2017);
PubMed (epublications ahead of print only, 20 November 2017);
LILACS (Latin American Caribbean Health Sciences Literature) (Bireme, 1982 to 20 November 2017);
Transfusion Evidence Library (1980 to 20 November 2017);
Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S, 1990 to 20 November 2017);
ClinicalTrials.gov (20 November 2017);
World Health Organization International Clinical Trials Registry Search Platform (ICTRP) (20 November 2017);
UMN‐CTR Japanese Clinical Trials Registry (20 November 2017);
Hong Kong Clinical Trials Registry (HKUCTR, 20 November 2017).
For the original version of this review we also searched the following three electronic databases up to 29 September 2014. However we did not need to search them for the update, as these databases have now been incorporated in the World Health Organization International Clinical Trials Registry Search Platform (ICTRP):
International Standard Randomised Controlled Trial Number Register (ISRCTN, 29 September 2014);
EU Clinical Trials Register (EU‐CTR, 29 September 2014);
Japan Primary Registries Network (29 September 2014).
All search strategies are reported in Appendix 1. We combined searches in MEDLINE, Embase and CINAHL with adaptations of the Cochrane RCT search filter, as detailed in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Searching other resources
We checked references of all identified trials, relevant review articles and current treatment guidelines for further relevant literature. We limited these searches to 'first‐generation' reference lists.
Data collection and analysis
Selection of studies
One review author (CD) removed the duplicates and did the initial screening of titles and abstracts of references identified through electronic searches, excluding only those references which were clearly irrelevant (for example, when the intervention did not contain RBCs). These excluded references were not validated. For this update, two review authors (SB, AS) independently screened the remaining references and subsequently retrieved full texts for those requiring assessment for inclusion using a study‐specific eligibility form.
Data extraction and management
Two review authors (AS and MD) independently undertook data extraction for the studies identified for inclusion through the 2017 update search using a piloted, study‐specific data extraction form. Disagreements were resolved by consensus between the review authors. One review author (SB) entered the data into Review Manager 5 software (Review Manager 2014).
We undertook data extraction in accordance with guidance detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We anticipated potential issues with protocol non‐adherence. With regard to the intervention, we expected heterogeneity within the definitions of what constituted shorter and longer storage durations for the RBC units. Consequently, we specifically extracted intervention data for:
storage duration of RBC units transfused in the intervention group and in the control group; and
total volume of RBC transfusion in both groups.
In expectation of possible heterogeneity of RBC product specifications, we specifically extracted data on the use of:
irradiated blood;
whole blood; and
red cell leucoreduction ‐ leucoreduction is the filtering process by which white blood cells (leukocytes) are removed from whole blood before transfusion. White blood cells are removed because they confer no benefit to recipients, but can carry pathogens and cause adverse transfusion reactions.
In this update, SB undertook subcategorisation of studies by their definitions of shorter and longer storage durations for RBCs.
Assessment of risk of bias in included studies
Two review authors (AS and MD) assessed risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We resolved disagreements by discussion. For each of the included trials, we assessed risk of bias (as low, high or unclear) for the following domains.
Generation of random sequence (selection bias)
Concealment of treatment allocation (selection bias)
Blinding of participants and personnel (person(s) delivering treatment) to treatment allocation (performance bias)
Blinding of outcome assessors to treatment allocation (detection bias)
Completeness of the outcome data (including checks for possible attrition bias through withdrawals, loss to follow‐up and protocol violations)
Selective reporting of outcomes (reporting bias)
Other sources of bias (other bias). We assessed whether each trial was free of problems, other than those listed above, that could put it at risk of bias
Measures of treatment effect
We carried out separate analyses according to the duration of follow‐up after treatment, this included: in hospital (with time points as defined by the individual studies), short term (up to 30 days), and long term (more than 30 days after receipt of study intervention). We expressed dichotomous data for each arm in individual studies as a proportion, or risk, and the treatment effect as an average risk ratio (RR) (i.e. using a random‐effects model) with 95% confidence intervals (CI), calculated using Mantel‐Haenszel methods. We expressed treatment effects for continuous data outcomes as mean differences (MD) and used a 95% CI in all instances. Where outcomes were measured in the same way across studies, we expressed continuous data for each arm in individual studies as a mean and standard deviation (SD), and the treatment effect as the MD.
With regard to the incidence of hospital‐acquired infection, we were going to estimate the incidence ratio as the ratio of new observed cases divided by expected numbers of cases for patients exposed to RBC transfusion. However, as we did not have sufficient homogenous data to permit meta‐analysis, we did not calculate incidence rates. Instead we reported incidences of, and calculated RRs for, different infections within each study.
We reported, but did not perform a formal analysis, of all reported data on length of hospital stay, length of stay in ICUs and duration of mechanical ventilation. Trials presented data on length of hospital stay and length of ICU stay as median values (with interquartile ranges), which are appropriate and robust ways to report these data, as these outcomes usually are not distributed normally; or as means (with SDs), which is usually an inappropriate way to report these particular outcomes (as length of stay is usually a skewed distribution). We have presented data for duration of mechanical ventilation as means (with SDs) and as number of participants requiring mechanical ventilation. When presented as means (with SDs), the same principles apply as for length of stay (i.e. that this is usually an inappropriate way to report an outcome of duration because usually it is skewed). We tabulated data for these outcomes and have reported them narratively within the text.
In the absence of appropriate skills in our facility to analyse economic and blood stock inventory outcomes fully at this time, we have presented these data in a narrative format. One review author (SB) entered all data into Review Manager 2014, and these were checked by a second review author (MT).
Unit of analysis issues
No unit of analysis issues arose while this review was performed; we identified no eligible cross‐over or cluster‐randomised trials. The unit of randomisation was the patient.
Dealing with missing data
We had no overall concern regarding missing data; but we did email the authors of four trials to seek clarification and request additional data (Dhabangi 2015; Steiner 2015; Cooper 2017; Spadaro 2017).
We are grateful to the authors of these four studies who responded to our emails as follows.
Cooper 2017: although the unadjusted hazard ratio for patient survival to follow‐up was reported as having been measured by the investigators, the data were not published in the trial report. The investigators sent us the hazard ratio data, but we have not used them in this version of the review because other comparable studies did not report this type of data.
Dhabangi 2015: we received clarification about the time points for mortality for the four participants who died after the first 24‐hour observation period.
Spadaro 2017: we received clarification regarding the time points at which inotropic and renal replacement therapy were reported.
Steiner 2015: we requested information about interquartile ranges for length of stay in hospital and in the ICU, and the median number of RBC units transfused per participant up to the seventh postoperative day, along with the number of transfusion‐related serious adverse events. The authors have responded indicating that they will send these data, and we hope to receive them soon.
When denominator data allowed, we converted reported percentages into actual numbers of participants for two studies (Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]), so we could calculate outcome RRs for these studies. We noted levels of attrition for all included trials.
Assessment of heterogeneity
With the addition of new trials in this 2018 update, we undertook assessments of statistical heterogeneity for three outcomes. Primarily, we explored clinical heterogeneity within studies. The clinical heterogeneity that we identified included age of the participants (neonates versus adult participants); underlying clinical diagnosis of participants across trials; duration of storage of the RBCs used for the intervention (we noted a range of storage duration for RBCs within each of the intervention arms, and in many instances, the range of the two intervention groups overlapped, see Table 3); and heterogeneity in the techniques used to measure outcomes (see Table 4, Table 5 and Table 6). We found no clinical heterogeneity due to trial design or risk of bias.
1. Characteristics of the interventions.
| Quantity of RBCs transfused | Duration of storage of RBCs as defined in the Methods | Duration of storage of RBCs received | |||||||
| Trial | Types of RBCs (RBC additive solutions)a | Shorter storage duration | Longer storage duration/standard practice storage duration | Shorter storage duration | Longer storage duration/standard practice storage duration | Shorter storage duration | Longer storage duration/standard practice storage duration | Further information: shorter storage duration | Further information: longer storage duration/standard practice storage duration |
| Transfusion of shorter storage duration RBCs vs transfusion of longer storage duration RBCs | |||||||||
| Neonate participant population | |||||||||
| Fernandes 2005 | Irradiated and leucoreduced (CPDA‐1) | Number of transfusions per infant transfused: mean 4.2 (SD 3.1); range 1 to 13 transfusions Mean RBC volume transfused: 62.9 (SD 45.1) mL/kg |
Number of transfusions per infant transfused: mean 4.4 (SD 4.0); range 1 to 20 transfusions Mean RBC volume transfused: 65.3 (SD 58.3) mL/kg |
RBCs stored for < 3 days | RBCs stored for ≤ 28 days | Mean: 1.6 (SD 0.6) days | Mean: 9.0 (SD 8.9) days | ‐‐ | ‐‐ |
| Liu 1994 | Irradiated (CPDA‐1) | 12 infants received 55 RBC transfusions during the study | 13 infants received 73 RBC transfusions during the study | Quint packs stored for < 5 days When the need for multiple transfusions was anticipated, attempts were made to limit donor transfusions by reserving multiple quint packs from the same donor for a participant. | Assigned a single adult unit of PRBC, which was reserved for that infant up to the recommended storage time of 35 days | Mean: 3.5 (SD 1.2) days | Mean: 12.9 (SD 10.9) days | ‐‐ | ‐‐ |
| Paediatric participant population | |||||||||
| Dhabangi 2013 | Not stated (SAGM) | Volume of RBCs transfused, mean: 12.7 (SD 2.6) mL/kg | Volume of RBCs transfused, mean: 12.7 (SD 2.2) mL/kg | Stored for 1 to 10 days | Stored 21 to 35 days | Mean: 7.8 (SD 1.8) days | Mean: 27.2 (SD 3.9) days | ‐‐ | ‐‐ |
| Dhabangi 2015 | Leukoreduced CP2D‐AS‐3 | Not stated | Not stated | Stored for 1 to 10 days | Stored for 25 to 35 days | Median: 8 (IQR 7 to 9) days | Median: 32 (IQR 30 to 34) days | ‐‐ | ‐‐ |
| Adult participant population | |||||||||
| Bennett‐Guerrero 2009 [1], | Leucoreduced (AS‐1 or AS‐3) |
Number of units transfused per participant, median: 4 (IQR 2.5 to 6.5) | Number of units transfused per participant, median: 5 (IQR 4 to 10) | Mean: 7(SD 4) days | Mean: 21 (SD 4) days Transfusions in this arm were within the target window for only 5 of 10 transfused participants |
Mean: 6 (V2) days |
Mean: 18 (SD 7) days | Shortest stored RBC unit, mean: 5 (SD 1) days. Maximum storage duration, mean: 7 (SD 2) days |
Shortest stored RBC unit, mean: 17 (SD 7) days Maximum storage duration, mean: 20 (SD 7) days |
| Neuman 2013 | Not stated | Not stated | Not stated | Stored for < 10 days | Stored for > 21 days | Mean: 9.1 (SD 3.1) days | Mean 29.8 (SD 5.7) days | ‐‐ | ‐‐ |
| Schulman 2002 | Not stated | Number of transfusions per infant transfused: mean 9.3 (SD 1.9) | Number of transfusions per infant transfused: mean 10.6 (SD 3.35) | Stored for < 7 days | Stored for > 20 days | Not stated | Not stated | ‐‐ | ‐‐ |
| Spadaro 2017 | Non‐leucoreduced (SAGM) |
Number of units transfused per participant, median: 2 (IQR 2 to 3) | Number of units transfused per participants, median: 2 (IQR 2 to 4) | Stored for ≤ 14 days | Stored for > 14 days | Median: 6 (IQR 5 to 10 days) | Median: 15 (IQR 11 to 20 days) | Per protocol analysis, median: 6 (IQR 5 to 10) days | Per protocol analysis, median: 18 (IQR 14 to 22) days |
| Steiner 2015 | Leucoreduced (AS‐1, AS‐3 or AS‐5) | Number of units transfused per participant, median: 4 (IQR 2 to 6) | Number of units transfused per participants, median: 3 (IQR 2 to 6) | Stored for ≤ 10 days | Stored for > 21 days | Mean: 7.8 (SD 4.8) days | Mean: 28.3 (SD 6.7) days | ‐‐ | ‐‐ |
| Walsh 2004 | Leucoreduced (SAGM) | Mean RBC volume transfused: 307 (SD 25) mL | Mean RBC volume transfused: 344 (SD 26) mL | RBC units stored for ≤ 5 days | RBC units stored for ≥ 20 days | Median: 2 (IQR 2 to 2.25) days | Median: 28 (IQR 26.75 to 31) days | Range = 2 to 3 | Range = 22 to 32 |
| Yuruk 2013 | Leucoreduced (SAGM) | Number of RBC bags: median: 3 (IQR 2 to 3) | Number of RBC bags: median: 3 (IQR 3 to 3) | Storage duration of < 1 week | Storage duration of 3 to 4 weeks | Median: 7 (IQR 5 to 7) days | Median: 23 (IQR 22 to 28) days | ‐‐ | ‐‐ |
| Shorter storage duration RBCtransfusion vs standard practice storage duration | |||||||||
| Neonate participant population | |||||||||
| Fergusson 2012 | Irradiated and leucoreduced (not stated) | Mean number of transfusion episodes: 5.01 (SD 4.00). Range 1 to 10 transfusion episodes |
Mean number of transfusion episodes: 4.94 (SD 3.88) Range 1 to 10 transfusion episodes |
Stored for ≤ 7 days | Range 2 to 42 days In this group, units were divided into aliquots to increase usage and reduce waste. Each aliquot was designated for use in a single infant up to its expiry date. |
Mean: 5.10 (SD 2.05) days | Mean: 14.58 (SD 8.26) days | Median: 5.00 (IQR 4.00 to 6.00) days | Median: 13.00 (IQR 8.00 to 19.00) days |
| Strauss 1996 | Irradiated (CPDA‐1 or AS‐1) |
Mean number of transfusions per infant: 3.9 (SD 2.6) | Mean number of transfusions per infant: 4.7 (SD 2.8) | Stored for < 7 days | Stored for < 42 days From 1 dedicated donor who consented to donate a second unit when needed by the infant. |
Stored for < 7 days = 100% of RBC transfusions (58 units transfused) | Stored for < 7 days = 17% of RBC transfusions (n = 11 units) Stored for 7 to 12 days = 36% of RBC transfusions (n = 24 units) |
‐‐ | The following constituted 47% of transfusions given 15 to 21 days = 16 units 22 to 28 days = 7 units 29 to 35 days = 3 units 36 to 42 days = 5 units |
| Strauss 2000 | Irradiated, leucoreduced (CPDA‐1 or AS‐3) |
Mean number of transfusions per infant: 6.7 (SD 3.9) Total number of transfusions given = 40 |
Mean number of transfusions per infant: 3.5 (SD 2.1) Total number of transfusions given = 28 |
Stored for < 7 days | Stored for< 42 days (RBC units from dedicated donors only) | All units < 7 days | 28 units transfused in this arm: stored for < 7 days = 5 units; stored for 7 to 14 days = 9 units; stored for 15 to 42 days = 14 units | ‐‐ | ‐‐ |
| Adult participant population | |||||||||
| Aubron 2012 | Leucoreduced (SAGM) | Number of units transfused per participant, mean: 3.2 (SD 2.6) | Number of units transfused per participant, mean: 3.8 (SD 3.6) | "Freshest" | "Compatible, non expired units with the longest storage duration at the time of transfusion request” | Mean: 12.1 (SD 3.8) days Mean storage duration range of RBCs: 3 to 19 days |
Mean: 23 (SD 8.4) days Mean storage duration range of RBCs: 7 to 41.5 days |
Minimum storage duration,
mean: 9.5 (SD 4.5) days Maximum storage duration, mean: 15 (SD 6.5) days |
Minimum storage duration,
mean: 20.5 (SD 8.9) days Maximum storage duration, mean: 26 (SD 9.2) days |
| Bennett‐Guerrero 2009 [2] | Leucoreduced (AS‐1 or AS‐3) |
Number of units transfused per participants, median: 5 (IQR 2 to 7) | Number of units transfused per participants, median: 4 (IQR 2 to 5) | Less than 21 days | Unit standard of care = RBC unit stored for longest out first | Mean: 11 (4) days | Mean: 16 (SD 5) days | Shortest stored RBC unit, mean:
10 (SD 5) days Maximum storage duration, mean: 12 (SD 4) days |
Shortest stored RBC unit, mean: 13 (SD 4) days Maximum storage duration, mean: 18 (SD 7) days No RBC unit was stored for > 31 days |
| Cooper 2017 | Leucoreduced (SAGM) | Number of units transfused per participant, median: 2 (IQR 1 to 4) | Number of units transfused per participant, median: 2 (IQR 1 to 4) | "Freshest available" | "Oldest available" | Mean: 11.8 (SD 5.3) days | Mean: 22.4 (SD 7.5) days | Median: 10.7 (IQR 8.3 to 14.1) days | Median: 21.4 (IQR 16.7 to 27.4) days |
| Hebert 2005 | Leucoreduced (CPD‐2 and AS‐3) |
Median number of RBC units transfused: 3 (IQR 2 to 5) | Median number of RBC units transfused: 2 (IQR 2 to 4) | Storage duration < 8 days | This group received RBCs issued from the hospital blood bank with the longest storage time in accordance with standard blood bank procedure. To ensure the maximum separation in terms of duration of storage between groups, participants were allocated only on days when average duration of storage of RBCs in the blood bank exceeded 15 days. | Median: 4 days | Median: 19 days | Overall 73% of participants received RBCs with storage times that correspond to treatment allocation more than 90% of the time. Compliance target of 90% was attained by 91% of patients allocated to shorter storage duration arm compared with 59% in the standard duration arm. | Overall 73% of participants received RBCs with storage times that correspond to treatment allocation more than 90% of the time. Compliance target of 90% was attained by 91% of participants allocated to shorter storage duration arm compared with 59% in standard duration arm. |
| Heddle 2012 | Leucoreduced (SAGM) |
Total number of RBC units transfused: 1157 Median: 2 (IQR 2 to 4) Range 1 to 102 |
Total number of RBC units transfused: 2369 Median: 2 (IQR 2 to 5) Range 1 to 38 |
“Freshest available” | “Oldest in the inventory” | Maximum storage duration, mean: 12.0 (SD 6.8) days | Maximum storage duration, mean: 26.6 (SD 7.8) days | Range 2 to 27 days and 33 to 37 days | Range 3 to 42 days |
| Heddle 2016 | Leucoreduced (SAGM) | Total number of RBC units transfused: 25,466 Number of units transfused per participant median: 1 (IQR 1 to 2) Range 1 to 87 |
Total number of RBC units transfused: 50,890 Number of units transfused per participant, median: 1 (IQR 1 to 2) Range 1 to 58 |
"Freshest available" | "Oldest available" | Mean: 13.0 (SD 7.6) days | Mean: 23.6 (SD 8.9) days | Median: 11 (IQR 8 to 16) days | Median: 23 (IQR 16 to 31) days |
| Kor 2012 | Leucoreduced (not stated) | 1 unit | 1 unit | Single unit of RBCs stored for < 5 days Subsequent transfusions (after the first study transfusion) were standard issue |
Single unit of standard issue RBCs (median = 21 days) | Median: 4.0 (IQR 3.0 to 5.0) days | Median: 26.5 (IQR 21.0 to 36.0) days | ‐‐ | 4 participants received an RBC unit that had been stored for ≤ 14 days; 9 participants received an RBC unit that had been stored between 15 and 21 days, and 37 participants received an RBC unit that had been stored for > 21 days. |
| Lacroix 2015 | Leucoreduced (SAGM) |
Total number of RBC units transfused: 5198 | Total number of RBC units transfused: 5210 | "Freshest available" < 8 days |
"Standard issue" | Mean: 6.1 (SD 4.9) days | Mean: 22.0 (SD 8.4) days | Mean number of RBC units per participant who received at least 1 transfusion: 4.3 (SD 5.2) | Mean number of RBC units per participant who received at least 1 transfusion: 4.3 (SD 5.5) |
aType of RBCs relates to details of whether trials report that RBCs were leucoreduced or irradiated, or whether whole blood rather than RBCs were transfused. The nature of the blood that was transfused is recorded here.
Abbreviations
AS‐1: additive solution‐1, a commercial additive solution containing sodium chloride, dextrose, adenine, mannitol AS‐3: additive solution‐3, a commercial additive solution containing sodium chloride, dextrose, adenine, tri‐sodium citrate, citric acid, sodium phosphate AS‐5: additive solutionl‐5, a commercial additive solution containing sodium chloride, mannitol, adenine, dextrose CPDA‐1: citrate, phosphate, dextrose, adenine 1 CPD‐2: citrate, phosphate, dextrose‐2 CPD‐2‐AS‐3: citrate, phosphate, dextrose‐2 with additive solution‐3 IQR: interquartile range PRBC = packed red blood cells RBCs = red blood cells SAGM = saline adenine glucose mannitol SD: standard deviation
2. Duration of organ support.
| Trial | Shorter storage duration | Longer storage duration/standard practice storage duration |
| Transfusion of RBCs of shorter vs longer storage duration | ||
| Respiratory support (invasive and non‐invasive ventilation) | ||
| Adult participant population | ||
| Bennett‐Guerrero 2009 [1] | Duration of mechanical ventilation, mean = 31 (SD 33) hours Percentage (number)a of participants on any vasopressor > 48 hours after surgery = 17% (2) |
Duration of mechanical ventilation, mean = 16 (SD 6) hours Percentage (number)a of participants on any vasopressor > 48 hours after surgery = 25% (3)] |
| Transfusion of RBCs of shorter vs standard practice storage duration | ||
| Respiratory support (invasive and non‐invasive ventilation) | ||
| Adult participant population | ||
| Aubron 2012 | Duration of mechanical ventilation, median 156 (IQR 6.1 to 253) days | Duration of mechanical ventilation, median 9.85 (IQR 0 to 198) days |
| Cooper 2017 | Number of days alive and free of mechanical ventilation, median = 25 (IQR 11 to 28) days | Number of days alive and free of mechanical ventilation, median = 25 (IQR 13 to 28) days |
| Renal support (haemofiltration) | ||
| Adult participant population | ||
|
Hebert 2005 Number of participants on dialysis over the 30 days of the study |
0% (n = 0) | 6% (n = 2) |
| Cooper 2017 | Percentage (number)a of participants requiring: renal replacement therapy = 13.9% (342) Days alive and free of renal replacement therapy, median: 28 (IQR 22 to 28) days |
Percantage (number)a of participants requiring: renal replacement therapy = 14.6% (360) Days alive and free of renal replacement therapy, median: 22 (IQR 22 to 28) days |
| Lacroix 2015 | Duration of extrarenal epuration, mean: 2.5 (SD 10.1) days | Duration of extrarenal epuration, mean: 2.5 (SD 8.3) days |
| Haemodynamic support (vasopressors, inotropes) | ||
| Adult participant population | ||
| Lacroix 2015 | Duration of cardiac or vasoactive drugs, mean: 7.1 (SD 10.2) days | Duration of cardiac or vasoactive drugs, mean: 7.5 (SD 11.2) days |
aAs the number of participants included in the analysis for this outcome was known, the number reported here was calculated for the purposes of this review.
Abbreviations
IQR: interquartile range SD: standard deviation
3. Length of stay: hospital and intensive care unit.
| Trial | Shorter storage duration | Longer storage duration/standard practice storage duration |
| Transfusion of RBCs of shorter vs longer storage duration | ||
| Neonate participant population | ||
|
Fernandes 2005 Length of hospital stay: mean (SD) |
60.8 (SD 37.3) days | 62.6 (SD 48.3) days |
| Paediatric participant population | ||
|
Dhabangi 2015 Length of hospital stay: median (IQR) |
4 (IQR 2, 6) days* | 4 (IQR 3 to 7) days* |
| Adult participant population | ||
|
Bennett‐Guerrero 2009 [1] Length of hospital stay: mean (SD) |
10 (SD 9) days | 8.6 (SD 4) days |
|
Steiner 2015 Length of hospital stay: median |
8 days (IQR not reported) | 8 days (IQR not reported) |
|
Spadaro 2017 Length of hospital stay: median (IQR) |
10 (IQR 6 to 17) days* | 9 (IQR 7 to 17) days* |
|
Bennett‐Guerrero 2009 [1] Length of ICU stay: mean (SD) |
51 (SD 67) hours | 51 (SD 56) hours |
|
Steiner 2015 Length of ICU stay: median |
3 days (IQR not reported) | 3 days (IQR not reported) |
|
Spadaro 2017 Length of ICU stay: median (IQR) |
1 (IQR 1 to 6) days* | 3(IQR 2 to 5) days* |
| Transfusion of RBCs of shorter vs standard practice storage duration | ||
| Neonate participant population | ||
|
Fergusson 2012 Length of ICU stay: median (IQR) |
84 (IQR 50 to 104) days* | 77 (IQR 50 to 104) days* |
|
Strauss 1996 Percentage discharge from hospital before 84 days |
14% | 10% |
| Adult participant population | ||
|
Aubron 2012 Length of hospital stay: median (IQR) |
21 (IQR 12 to 38) days | 17 (IQR 8 to 27) days |
|
Bennett‐Guerrero 2009 [2] Length of hospital stay: mean (SD) |
10 (SD 7) days | 13 (SD 14) days |
|
Cooper 2017 Length of hospital stay: median (IQR) |
14.5 (IQR 7.4 to 27.5) days | 14.7 (IQR 7.4 to 28.3) days |
|
Heddle 2016 Length of hospital stay: median (IQR) |
10 (IQR 5 to 19) days | 10 (IQR 5 to 20) days |
|
Lacroix 2015 Length of hospital stay: mean (SD) |
34.4 (SD 39.5) days | 33.9 (SD 38.8) days |
|
Aubron 2012 Length of ICU stay: median (IQR) |
11 (IQR 5 to 15) days | 7 (IQR 3 to 17) days |
|
Bennett‐Guerrero 2009 [2] Length of ICU stay: mean (SD) |
69 (SD 136) hours | 47 (SD 51) hours |
|
Cooper 2017 Length of ICU stay: median (IQR) |
4.2 (IQR 2.0 to 9.3) days | 4.2 (IQR 1.9 to 9.4) days |
|
Lacroix 2015 Length of ICU stay: mean (SD) |
15.3 (SD 15.4) days | 15.3 (SD 14.8) days |
Abbreviations
IQR: interquartile range SD: standard deviation
4. Assessment of economic and blood stock inventory.
| Trial | Shorter storage duration | Longer storage duration/standard practice storage duration |
| Transfusion of RBCs of shorter vs longer storage duration | ||
| Adult participant population | ||
|
Yuruk 2013 Number of bags of RBCs transfused to adult haematology participants: median (IQR) |
3 (IQR 2 to 3) bags | 3 (IQR 3 to 3) bags |
|
Steiner 2015 Number of RBC units transfused per participant to postoperative day 7: median (need IQR) Number of RBC units transfused per participant "throughout the study period': median (IQR) |
3 units (IQR not reported) 4 (IQR 2 to 6) units |
3 units (IQR not reported) 3 units (IQR 2 to 6) |
|
Spadaro 2017 Number of RBC units transfused per participant during surgery: median (IQR) |
2 (IQR 2 to 3) units | 2 (IQR 2 to 4) units |
| Transfusion of shorter storage duration RBCvs transfusion of standard practice storage duration | ||
| Adult participant population | ||
|
Heddle 2016 Number of RBC units transfused per participant: median (IQR) |
2 (IQR 2 to 4) units | 2 (IQR 2 to 4) units |
|
Cooper 2017 Number of RBC units transfused per participant: median (IQR) |
2 (IQR 1 to 4) units | 2 (IQR 1 to 4) units |
Abbreviations
IQR: interquartile range
Assessment of reporting biases
We did not assess publication bias using funnel plots, as we had fewer than 10 studies in any one outcome analysis.
Data synthesis
The comparisons made within the primary studies were diverse because of the absence of uniform definitions of shorter and longer storage durations for RBCs. After data extraction, we assessed whether the included studies were suitable ‐ in terms of the definitions of shorter and longer storage durations they employed ‐ for inclusion in a single meta‐analysis. Unfortuantely, we found them not to be suitable (we noted a range of durations of storage of RBCs within each of the intervention arms, see Table 3), and there were also differences in clinical outcomes measured between trials (see also Assessment of heterogeneity).
We undertook meta‐analyses using Review Manager 5 on three occasions, employing a random‐effects model due to the anticipated heterogeneity arising from differences in participant characteristics and duration of storage of RBCs (Review Manager 2014).
Within each included trial, we analyzed all participants in the treatment groups to which they had been randomised.
We constructed 'Summary of findings' tables using GRADEpro GDT (GRADEpro GDT). We focused our summary of findings on the primary outcome of mortality up to 30 days. We made an assessment of the quality of the evidence based on study design limitations, inconsistency of results, indirectness of evidence, imprecision, and publication bias as described in the GRADE handbook (Schünemann 2011), with consideration of the optimal information size generated from trial sequential analysis (TSA).
Trial sequential analysis
We provided a sample size estimate showing how many participants would be needed to be included in a meta‐analysis for it to produce reliable results. We used TSA methods to explore treatment effects attained before the required sample size was reached (TSA 2011), by using TSA beta 0.9 software (TSA 2011). We applied TSA for the outcome of short‐term all‐cause mortality (up to 30 days).
We performed TSAs for this outcome on all trials regardless of risk of bias and also on trials with overall low risk of bias. We included all definitions of shorter and longer storage durations (or standard practice storage duration) for RBCs. This provided the required information size (the total number of participants) necessary to detect a statistically significant underlying effect. We estimated a mortality risk of 25% in the control group based on a mean of the observed ICU mortality of the control arms in the three recent large studies (Lacroix 2015; Heddle 2016; Cooper 2017). We calculated the information size necessary for a relative risk reduction (RRR) of 5%.
When we calculated cumulative Z‐curves that crossed trial sequential monitoring boundaries, we determined that statistical significance had been reached and the overall type I error rate had been maintained. We produced futility boundaries such that if the cumulative Z‐curve crossed the futility threshold, the evidence showed that the two treatments did not differ more than the anticipated effect size. We used the O’Brien Fleming alpha‐spending function with an overall type I error rate of 5% and with 80% statistical power to derive two‐sided sequential monitoring and futility boundaries.
Subgroup analysis and investigation of heterogeneity
If the data had been sufficient, we would have undertaken subgroup analyses based on age of participants (e.g. 'neonates', 'children' or 'adults'), and transfusion indications (long‐term transfusion dependence versus support during an acute illness), to look for differences in treatment effects.
Sensitivity analysis
If the data had been sufficient, we would have undertaken sensitivity analyses to explore aspects of methodology. These would have explored the effects of removing trials at high or unclear risk for the following bias domains: selection bias (reflecting lack of confirmation of random sequence generation and allocation concealment); detection bias (reflecting lack of assessor blinding); and attrition bias (reflecting high levels of missing data).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Through the searches we ran to November 2017, we identified 4909 references, 1822 of which we excluded in the first screening because they were duplicates or were clearly irrelevant to the scope of this review. We screened the remaining 3087 references by title and abstract and excluded 3021 of them, because they did not meet the inclusion criteria for participants, or interventions, or both, or were not RCTs.
We obtained the full text of 66 references. We deemed 22 trials (reported in 50 of the 66 references) to be eligible for inclusion (Liu 1994; Strauss 1996; Strauss 2000; Schulman 2002; Walsh 2004; Fernandes 2005; Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Fergusson 2012; Heddle 2012; Kor 2012; Dhabangi 2013; Neuman 2013; Yuruk 2013; Dhabangi 2015; Lacroix 2015; Steiner 2015; Heddle 2016; Cooper 2017; Spadaro 2017); four trials are awaiting assessment (NCT00458783, NCT01534676; NCT02050230; NCT02724605), and there is one ongoing trial (NCT01977547). We excluded 11 studies because they did not meet the eligibility criteria of this review (Wasser 1989; Eshleman 1994; Hod 2011; Seitelbach 2011; Lebiedz 2012; Yamal 2015; Bao 2017; Rapido 2017; Rodrigues 2015; Chantepie 2015; Murphy 2017) (see Characteristics of excluded studies). We have reported full details in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 1).
1.
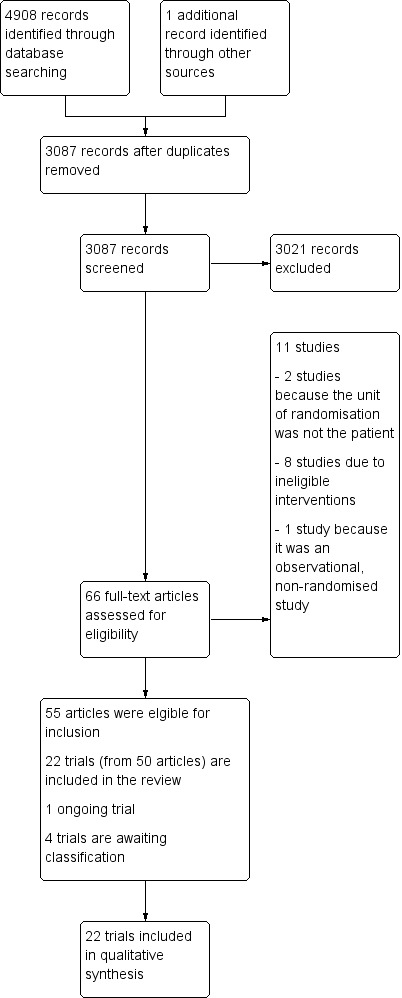
A study flow diagram
Included studies
Participants in 21 trials were in‐patients (i.e. in hospital) (Liu 1994; Strauss 1996; Strauss 2000; Schulman 2002; Walsh 2004; Fernandes 2005; Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Fergusson 2012; Heddle 2012; Kor 2012; Dhabangi 2013; Neuman 2013; Dhabangi 2015; Lacroix 2015; Steiner 2015; Heddle 2016; Cooper 2017; Spadaro 2017). Participants in the remaining trial were outpatients of the haematology department (Yuruk 2013).
One manuscript reported data from two separate trials, which will be reported throughout as Bennett‐Guerrero 2009 [1] and Bennett‐Guerrero 2009 [2]. One trial was included in the content of a letter (Schulman 2002). Two trials were reported as conference abstracts (Schulman 2002; Neuman 2013).
Six of the 22 trials were feasibility trials for larger studies that were planned and have now been completed: Aubron 2012 for the TRANSFUSE trial (Cooper 2017); Dhabangi 2013 for the TOTAL trial (Dhabangi 2015); Hebert 2005 for the ABLE trial (Lacroix 2015); Bennett‐Guerrero 2009 [1] and Bennett‐Guerrero 2009 [2] for the RECESS trial (Steiner 2015); and Heddle 2012) for the INFORM trial (Heddle 2016). See Characteristics of included studies for details of these trials.
Sample sizes
A total of 42,635 participants was randomly assigned in the 22 trials. The number of participants assigned to each trial ranged from 17 in Schulman 2002, to 31,497 in Heddle 2016, with eight trials including more than 100 participants: Heddle 2012 (n = 910), Fergusson 2012 (n = 377), Dhabangi 2015 (n = 290), Lacroix 2015 (n = 2510), Steiner 2015 (n = 1481), Heddle 2016 (n = 31,497), Cooper 2017 (n = 4994), and Spadaro 2017 (n = 199). The total number of participants included per outcome is detailed in the Effects of interventions section and ranged from 23 to 23,281.
Setting
Eight trials were multi‐centred with participants from across Canada (Hebert 2005; Fergusson 2012, Lacroix 2015, Heddle 2016), the USA (Steiner 2015, Heddle 2016), Europe (Lacroix 2015, Cooper 2017), the Middle East (Heddle 2016, Cooper 2017), or Australia (Aubron 2012, Heddle 2016, Cooper 2017). The remaining 14 single‐centre trials were conducted in the USA (Liu 1994; Strauss 1996; Strauss 2000; Schulman 2002; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Kor 2012; Neuman 2013), Brazil (Fernandes 2005), Canada (Heddle 2012), Scotland (Walsh 2004), the Netherlands (Yuruk 2013), Uganda (Dhabangi 2013; Dhabangi 2015), and Italy (Spadaro 2017).
Participants
The eligible trials included neonatal, paediatric or adult participants. Five trials enrolled very low birth weight premature neonates (Liu 1994; Strauss 1996; Strauss 2000; Fernandes 2005; Fergusson 2012), two trials focused on paediatric participants with severe malarial anaemia (Dhabangi 2013; Dhabangi 2015), and 15 trials included adult participants (Schulman 2002; Walsh 2004; Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Heddle 2012; Kor 2012; Neuman 2013; Yuruk 2013; Lacroix 2015; Steiner 2015; Heddle 2016; Cooper 2017; Spadaro 2017). In the trials with adult participants, the reasons for the hospital stay included cardiac surgery (Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Steiner 2015), haematological treatment for anaemia (Yuruk 2013), treatment for critical illness (Walsh 2004; Aubron 2012; Lacroix 2015; Cooper 2017), admission to a level 1 trauma centre (Schulman 2002), need for intubation and mechanical ventilation (Kor 2012), postoperative RBC transfusion after elective noncardiac surgery (Spadaro 2017), and the "requirement for a RBC transfusion" (with no further details given) (Heddle 2012; Neuman 2013; Heddle 2016).
Interventions
The definition (i.e. in the trial protocol or Methods section that defined the duration of storage of the RBCs) and the actual duration of storage of RBCs transfused to participants differed markedly across intervention arms and between trials (see Table 3). This was the case for trials that compared RBCs of shorter versus longer storage duration, or versus RBCs of standard practice storage duration.
Eleven trials compared transfusion of RBCs of shorter versus longer storage duration (Liu 1994; Schulman 2002; Walsh 2004; Fernandes 2005; Bennett‐Guerrero 2009 [1]; Dhabangi 2013; Neuman 2013; Yuruk 2013; Dhabangi 2015; Steiner 2015; Spadaro 2017).
Eleven trials compared transfusion of RBCs of shorter storage duration versus standard practice storage duration (Strauss 1996; Strauss 2000; Hebert 2005; Bennett‐Guerrero 2009 [2]; Aubron 2012; Fergusson 2012; Heddle 2012; Kor 2012; Lacroix 2015; Heddle 2016; Cooper 2017). The durations of storage for the standard practice storage duration RBCs was reported as: "a range of 2 to 42 days" in Fergusson 2012, "oldest in the inventory" in Bennett‐Guerrero 2009 [2], Heddle 2012, Lacroix 2015, Heddle 2016, and Cooper 2017, and less than 42 days old in Strauss 1996 and Strauss 2000.
Across all the trials, the defined durations for shorter storage of RBCs ranged from "freshest available" (Aubron 2012), to "less than 21 days old" (Bennett‐Guerrero 2009 [2]). The actual duration of storage for these RBCs ranged from a mean of 1.6 (SD 0.6) days in Fernandes 2005 to a mean of 13.0 (SD 7.6) days in Heddle 2016.
The additive solutions used to preserve RBCs ahead of transfusion and whether RBCs were leucoreduced and/or irradiated also differed between trials (see Table 3).
One trial reported instances of non‐compliance with allocated treatment (Hebert 2005). The other 21 trials either did not mention compliance with treatment allocation in their reports, or reported that there was no non‐compliance with treatment allocation.
Outcomes
No trial measured all outcomes of interest in this review.
Follow‐up periods for the primary outcomes measured ranged from within four hours of RBC transfusion to 180 days after randomisation. Details of the primary outcomes measured can be found in the Characteristics of included studies table. All studies reported measuring secondary outcomes, which are also detailed in the Characteristics of included studies table.
Excluded studies
For the full list of excluded studies, see Characteristics of excluded studies.
We excluded 11 studies from this review following assessment of their full text. We excluded two because the unit of randomisation was the intervention (Eshleman 1994; Seitelbach 2011), another because it was an observational, non‐randomised study (Lebiedz 2012), and another eight studies that assessed ineligible interventions (Wasser 1989; Hod 2011; Chantepie 2015; Rodrigues 2015; Yamal 2015; Bao 2017; Murphy 2017; Rapido 2017).
Ongoing studies and studies awaiting classification
We identified one ongoing RCT (NCT01977547; see Characteristics of ongoing studies table).
There are four studies awaiting classification (NCT00458783; NCT01534676; NCT02050230; NCT02724605), see Characteristics of studies awaiting classification table. According to ClinicalTrials.gov, NCT00458783 has completed (accessed 9 October 2014); NCT01534676 terminated after recruiting only three participants, and is also identified as completed (accessed 31 October 2018); NCT02050230 was also terminated due to slow recruitment of participants (last updated on ClinicalTrials.gov in October 2016, accessed 15 November 2018); and NCT02724605 completed in January 2018 (accessed 31 October 2018). To date, no further details have been reported for any of these studies.
Risk of bias in included studies
Allocation
Random sequence generation
We assessed 15 studies as being at low risk of bias for random sequence generation because they used either a web‐based system (Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Heddle 2012; Kor 2012; Lacroix 2015; Steiner 2015; Heddle 2016; Cooper 2017), or an interactive voice response system (and thereafter referral to a manual of unique random numbers generated by an independent statistician before study activation to determine trial arm allocation) (Fergusson 2012), or mixing of sealed envelopes for 20 minutes by three people (Dhabangi 2015), or a random numbers table (Liu 1994), or randomly permuted block sizes of four and six (Spadaro 2017), or random length block randomisation by an external, independent research unit (Walsh 2004).
We assessed seven studies as being at unclear risk of bias for random sequence generation, because there was insufficient information on which to base an assessment (Strauss 1996; Strauss 2000; Schulman 2002; Fernandes 2005; Dhabangi 2013; Neuman 2013; Yuruk 2013). We assessed no trials as having a high risk of bias for this domain (see Figure 2; Figure 3).
2.
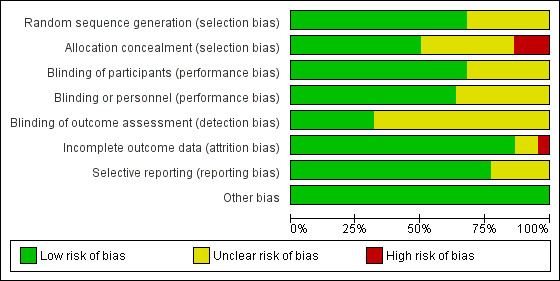
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Twenty‐two studies are included in this review.
3.
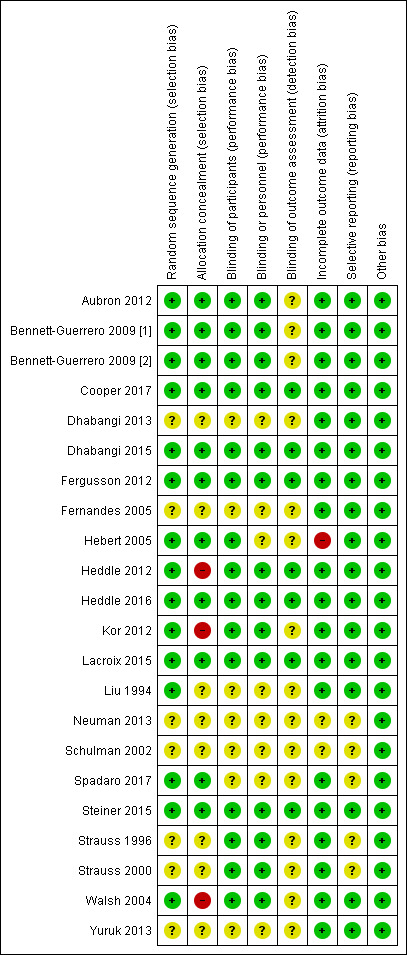
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation concealment
We assessed 11 studies as being at low risk of bias for allocation concealment, because either participants' specific identification numbers were used with only the transfusion service scientist unblinded to treatment allocation (Aubron 2012; Steiner 2015), or a computerised randomisation schedule was maintained by non‐trial personnel (Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]), or only the study statistician at the co‐ordinating centre was aware of the randomisation codes (Lacroix 2015), or generation of random sequence occurred in a way that was outside the control of the treating clinician (Fergusson 2012; Heddle 2016; Cooper 2017); or allocation details were stored in sequentially numbered, sealed opaque envelopes (Hebert 2005; Dhabangi 2015; Spadaro 2017).
We assessed three studies as being at high risk of bias for allocation concealment, because either randomisation was performed using an unconcealed paper‐based sequence held in the blood bank (Heddle 2012), or the envelopes containing details of treatment allocation were not sequentially numbered (Kor 2012), or were not sequentially numbered and opaque (Walsh 2004).
We assessed eight studies as being at unclear risk of bias for allocation concealment, because there was insufficient information on which to base an assessment (Liu 1994; Strauss 1996; Strauss 2000; Schulman 2002; Fernandes 2005; Dhabangi 2013; Neuman 2013; Yuruk 2013).
Blinding
We have reported details regarding blinding to treatment allocation separately for participants, study personnel and outcome assessors.
Blinding of participants
We assessed 15 studies as being at low risk of bias for blinding of participants. In seven of these studies, participants received intensive care treatment (Walsh 2004; Hebert 2005; Aubron 2012; Kor 2012), or were neonates whose parents were blinded to treatment allocation (Strauss 1996; Strauss 2000; Fergusson 2012). For two of these studies, RBC units were provided by the hospital’s transfusion service in a blinded manner from induction of general anaesthesia to postoperative day 7, so participants were blinded to study group assignment (Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]). In Lacroix 2015 and Cooper 2017, opaque labels and/or obscuring stickers were used to cover the expiration and/or collection dates on blood units. In four studies participants were unblinded to treatment allocation, but as no outcomes were measured subjectively, we did not believe knowledge of treatment allocation would constitute a risk of bias in these trials (Heddle 2012; Dhabangi 2015; Steiner 2015; Heddle 2016).
We assessed seven studies as being at unclear risk of bias for this domain, because there was insufficient information on which to make an assessment of risk of bias (Liu 1994; Schulman 2002; Fernandes 2005; Dhabangi 2013; Neuman 2013; Yuruk 2013; Spadaro 2017). We assessed no trials as being at high risk of bias.
Blinding of study personnel
We assessed 14 trials as having low risk of bias for blinding of study personnel to treatment allocation. In 10 of these trials, study personnel were reported to be blinded to treatment allocation and it is unlikely that blinding could have been broken (Strauss 1996; Strauss 2000; Walsh 2004; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Fergusson 2012; Kor 2012; Lacroix 2015; Cooper 2017). In Heddle 2012, Dhabangi 2015, Steiner 2015, and Heddle 2016, study personnel were unblinded to treatment allocation, but as no outcomes were measured subjectively, we did not believe that knowledge of treatment allocation constituted a risk of bias in these trials.
We assessed eight studies as being at unclear risk of bias for blinding of study personnel, because insufficient information was reported to permit assessment of risk of bias (Liu 1994; Schulman 2002; Fernandes 2005; Hebert 2005; Dhabangi 2013; Neuman 2013; Yuruk 2013; Spadaro 2017). We assessed no trials as being at high risk of bias.
Blinding of outcome assessors
We judged seven trials to be at low risk of detection bias because they reported blinding of outcome assessors (Fergusson 2012; Heddle 2012; Dhabangi 2015; Lacroix 2015; Steiner 2015; Heddle 2016; Cooper 2017). In Fergusson 2012, neonatologists blinded to study group allocation adjudicated composite outcomes independently. In Heddle 2012, Steiner 2015, Dhabangi 2015, and Heddle 2016, outcome assessors were not blinded, but as outcomes were objective, we believe that such knowledge would not have had an impact on outcome assessment.
We assessed 15 studies as being at unclear risk of bias for blinding of outcome assessors, because there was insufficient information reported about how outcomes were assessed (Liu 1994; Strauss 1996; Strauss 2000; Schulman 2002; Walsh 2004; Fernandes 2005; Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Kor 2012; Dhabangi 2013; Neuman 2013; Yuruk 2013; Spadaro 2017). We assessed no trials as being at a high risk of bias for this domain.
Incomplete outcome data
We assessed 20 trials as being at low risk of attrition bias, as all participants who were randomly assigned and received a RBC transfusion were included in the analysis of outcome data; there was minimal participant loss to follow‐up and reasons for loss to follow‐up and missing data were balanced in terms of numbers across intervention groups (Liu 1994; Strauss 1996; Strauss 2000; Walsh 2004; Fernandes 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Fergusson 2012; Heddle 2012; Kor 2012; Dhabangi 2013; Neuman 2013; Yuruk 2013; Lacroix 2015; Steiner 2015; Dhabangi 2015; Heddle 2016; Cooper 2017; Spadaro 2017). Information needed to assess attrition bias was insufficient in Schulman 2002.
One trial was at high risk of attrition bias (Hebert 2005). We made this assessment because outcome data for mortality outcomes at 30‐day and 90‐day follow‐up were incomplete.
Selective reporting
In 18 trials, investigators reported all prespecified outcomes in the Results section, and we deemed them to be at low risk of reporting bias (Liu 1994; Walsh 2004; Fernandes 2005; Hebert 2005; Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]; Aubron 2012; Fergusson 2012; Heddle 2012; Kor 2012; Dhabangi 2013; Neuman 2013; Yuruk 2013; Dhabangi 2015; Lacroix 2015; Steiner 2015; Heddle 2016; Cooper 2017).
Three trials did not define the outcomes they were interested in measuring in their Methods; therefore, it is impossible to identify whether reporting bias was present in these trials (Strauss 1996; Strauss 2000; Schulman 2002), and we have rated them as being at unclear risk of bias. We have rated one other trial as being at unclear risk of bias because only the primary outcome was prospectively registered (Spadaro 2017). We assessed no trials as having high risk of bias.
Other potential sources of bias
We have no concerns about other potential sources of bias.
Effects of interventions
Summary of findings for the main comparison. Transfusion of RBCs of shorter vs longer storage duration.
| Transfusion of RBCs of shorter vs longer storage duration for all conditions | ||||||
|
Patient or population: all conditions
Setting: hospital
Intervention: transfusion of RBCs of shorter storage duration Comparison: transfusion of RBCs of longer storage duration | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with transfusion of RBCs of longer storage duration | Risk with transfusion of RBCs of shorter storage duration | |||||
| Mortality: in‐hospital mortality (within 24 hours) | Study population ‐ paediatric participants | RR 1.71 (0.41 to 7.10) | 290 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 21 per 1000 | 35 per 1000 (8 to 147) | |||||
| Mortality: in‐hospital mortality (within 7 days) | Study population ‐ paediatric participants | RR 3.00 (0.13 to 71.34) | 74 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Mortality: in‐hospital mortality (within 7 days) | Study population ‐ adults | RR 1.42 (0.66 to 3.06) | 1098 (1 RCT) | ⊕⊕⊕⊝ MODERATE 4 | ||
| 20 per 1000 | 28 per 1000 (13 to 60) | |||||
| Mortality: time‐point not defined | Study population ‐ adults | RR 2.25 (0.55 to 9.17) | 17 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 6 7 | ||
| 222 per 1000 | 500 per 1000 (122 to 1000) | |||||
| Mortality: short term (up to 30 days) | Study population ‐ paediatric participants | RR 1.44 (0.46 to 4.48) | 290 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 34 per 1000 | 50 per 1000 (16 to 154) | |||||
| Mortality: short term (up to 30 days) | Study population ‐ adults | RR 0.85 (0.50 to 1.45) | 1121 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 8 | ||
| 51 per 1000 | 43 per 1000 (25 to 74) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level for indirectness, as the trial was not designed to assess the impact of transfusion on mortality, rather it was designed to assess if storage duration influenced the efficacy of RBCs in the particular participant population.
2 Downgraded 1 level for imprecision, due to very small number of events.
3 Downgraded 1 level, as no information was reported to permit assessment of biases due to selection, performance and detection. However there were no concerns with regard to attrition or reporting bias.
4 Downgraded 1 level for indirectness, as the trial was not designed to assess the impact of transfusion on mortality, but was designed to assess the effect of storage duration on organ dysfunction in elective cardiac surgery patients.
5 Downgraded 1 level for imprecision, due to small sample size of 17 participants.
6 Downgraded 2 levels, as no information was reported to permit assessment of the risk of selection, performance, detection, attrition and reporting biases. The study was reported as part of a letter.
7 Downgraded 1 level for indirectness, as the trial was not designed to assess the impact of transfusion on mortality, but was designed to assess the feasibility of running a larger trial and whether the blood bank in the investigating hospital could provide an adequate inventory of RBCs.
8 Downgraded 1 level for indirectness, as the trials were not designed to assess the impact of transfusion on mortality, rather Steiner 2015 was designed to assess the effect of storage duration on organ dysfunction in elective cardiac surgery patients and Bennett‐Guerrero 2009 was a feasibility trial designed to assess recruitment and randomisation rates and to see if adequate separation of duration of storage between the two intervention arms was feasible (Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]).
Summary of findings 2. Transfusion of RBCs of shorter vs standard practice storage duration.
| Transfusion of RBCs of shorter vs standard practice storage duration for all conditions | ||||||
| Patient or population: all conditions Setting: hospital Intervention: transfusion of RBCs of shorter storage duration Comparison: transfusion of RBCs of standard practice storage duration | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with transfusion of RBCs of standard practice storage duration | Risk with transfusion of RBCs of shorter storage duration | |||||
| Mortality: in‐hospital mortality (time points varied) | Study population ‐ adults | RR 1.05 (0.97 to 1.14) | 25754 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 88 per 1000 | 92 per 1000 (85 to 100) | |||||
| Mortality: in‐ICU mortality | Study population ‐ adults | RR 1.06 (0.98 to 1.15) | 13066 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | ||
| 145 per 1000 | 153 per 1000 (142 to 167) | |||||
| Mortality: short‐term mortality (up to 30 days) ‐ neonates | Study population ‐ neonates | RR 0.30 (0.01 to 7.02) | 40 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 5 | ||
| 53 per 1000 | 16 per 1000 (1 to 369) | |||||
| Mortality: short‐term mortality (up to 30 days) ‐ adults | Study population ‐ adults | RR 1.04 (0.96 to 1.13) | 7510 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 6 | ||
| 223 per 1000 | 232 per 1000 (214 to 252) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level for high risk of bias in 2 trials; selection bias in Heddle 2012 and attrition bias in Hebert 2005.
2 Downgraded 1 level for high risk of bias in 1 trial: attrition bias in Hebert 2005.
3 Downgraded 1 level for risk of bias, as information was not reported that would permit assessment of selection, detection and reporting biases.
4 Downgraded 1 level for indirectness, as the study was designed to measure safety of the duration of storage of RBCs.
5 Downgraded 1 level for imprecision due to small number of events.
6 Downgraded 1 level for high risk of bias in 2 trials; selection bias in Kor 2012 and attrition bias in Hebert 2005.
We have reported outcome data in this review by comparison of interventions and then by outcome. We subdivided these sections further into trials of neonate, child and adult participants.
When denominator data allowed, we converted reported percentages into actual numbers of participants in two trials to enable us to calculate outcome RRs for these trials (Bennett‐Guerrero 2009 [1]; Bennett‐Guerrero 2009 [2]).
Diversity in the clinical setting; age of participants (see Characteristics of included studies); variability in what constituted shorter, longer and standard practice storage durations of RBCs within each of the trials; (see Table 3), and differences in measurement of outcomes, have meant that we had few opportunities to pool data for any of our primary and secondary outcomes. Therefore throughout this section, data are predominantly reported for each trial.
Transfusion of RBCs of shorter versus longer storage duration
Eleven trials, with 1864 participants, reported data for this comparison. Two trials included neonate participants (Liu 1994; Fernandes 2005), two trials included paediatric participants (Dhabangi 2013; Dhabangi 2015), and seven trials included adult participants (Schulman 2002; Walsh 2004; Bennett‐Guerrero 2009 [1]; Neuman 2013; Yuruk 2013; Steiner 2015; Spadaro 2017).
Primary outcome: Mortality: in hospital (with time points as defined by the participant group) and short term (up to 30 days)
Trials with neonate participants
No trial in this participant population reported data for this outcome.
Trials with paediatric participants
Dhabangi 2013 and Dhabangi 2015 provided data on mortality within 24 hours of the start of transfusion. Overall, there was no evidence of a difference in the risk of death between the shorter and longer storage duration intervention arms (RR 1.50, 95% CI 0.43 to 5.25; 2 trials, 364 participants, very low quality evidence; Analysis 1.1). Dhabangi 2015 also reported data on mortality at 30‐day follow‐up and found no evidence of a difference in the risk of death between the shorter and longer storage duration intervention arms (RR 1.40, 95% CI 0.45 to 4.31; 290 participants; low quality evidence; Analysis 1.1). The overall mortality event rate was low in both trials Analysis 1.1.
1.1. Analysis.
Comparison 1 Transfusion of shorter storage duration RBCs vs longer storage duration RBCs, Outcome 1 Mortality.
Trials with adult participants
Steiner 2015 reported mortality data at postoperative day 7. Investigators reported no difference in the risk of death between the shorter and longer storage duration intervention arms (RR 1.42, 95% CI 0.66 to 3.06; 1098 participants, moderate quality evidence; Analysis 1.1).
Bennett‐Guerrero 2009 [1] and Steiner 2015 reported data on mortality at up to 30 days after cardiac surgery. In these trials, there was no evidence of a difference in mortality between the shorter and longer storage duration intervention arms (RR 0.85, 95% CI 0.50 to 1.45; 1121 participants, moderate quality evidence; Analysis 1.1). This analysis was dominated by the Steiner 2015 study, which provided 96% of the participants in the analysis.
Schulman 2002 reported mortality in trauma patients, but did not report the time points of the deaths. Investigators reported no difference in the risk of death between the shorter and longer storage duration intervention arms (RR 2.25, 95% CI 0.55 to 9.17; 17 participants, very low quality evidence; Analysis 1.1).
Secondary outcome: Long‐term mortality (more than 30 days)
Trials with neonate participants
Fernandes 2005 reported mortality at up to 40 weeks' postconceptual age and observed no difference in the risk of death reported between the shorter and longer storage duration intervention arms (RR 0.90, 95% CI 0.44 to 1.85; 52 participants, very low quality evidence; Analysis 1.1).
Trials with paediatric participants
The trials investigating this participant population did not report data for this outcome (Dhabangi 2013; Dhabangi 2015).
Trials with adult participants
Spadaro 2017 reported all‐cause mortality at 90 days following non‐cardiac surgery. Investigators reported no difference in the risk of death between the shorter and longer storage duration intervention arms (RR 1.58, 95% CI 0.68 to 3.64; 199 participants, moderate quality evidence; Analysis 1.1).
Secondary outcome: Incidence of hospital‐acquired infection
Trials with neonate participants
Fernandes 2005 reported data for this outcome as the number of neonates developing clinical sepsis and necrotising enterocolitis. The trialists observed no differences between the shorter and longer storage duration intervention arms in incidence of clinical sepsis (RR 1.25, 95% CI 1.00 to 1.56; 52 participants; Analysis 1.2), or necrotising enterocolitis (RR 1.50, 95% CI 0.48 to 4.70; 52 participants; Analysis 1.2). The overall number of neonates developing clinical sepsis was high at 96% in the shorter storage arm and 77% in the longer storage arm. The overall number of neonates developing necrotising enterocolitis was considerably lower, at 23% in the shorter storage arm and 15% in the longer storage arm.
1.2. Analysis.
Comparison 1 Transfusion of shorter storage duration RBCs vs longer storage duration RBCs, Outcome 2 Incidence of hospital‐acquired infection in neonates.
Trials with paediatric participants
The trials investigating this participant population did not report data for this outcome (Dhabangi 2013; Dhabangi 2015).
Trials with adult participants
Steiner 2015 reported data for this outcome as the number of participants who had developed "infections and infestations" 28 days after surgery, and reported no difference between the shorter and longer storage duration arms (RR 0.89, 95% CI 0.60 to 1.32; 1098 participants; Analysis 1.3). The percentage of participants who developed an infection by day 28 was 8% in the shorter storage arm and 9% in the longer storage arm (Analysis 1.3).
1.3. Analysis.
Comparison 1 Transfusion of shorter storage duration RBCs vs longer storage duration RBCs, Outcome 3 Incidence of hospital‐acquired infection in an adults.
Spadaro 2017 reported the total number of participants who developed at least one infection, and the number of participants with a specific infection (sepsis, pulmonary, wound, peritonitis or urinary tract) by day 28. The trialists observed no differences between the shorter and longer storage duration arms for the risk of participants developing at least one infection (RR 0.85, 95% CI 0.52 to 1.41; 199 participants), sepsis (RR 0.73, 95% CI 0.26 to 2.02; 199 participants), pulmonary infection (RR 2.26, 95% CI 0.60 to 8.51; 199 participants), peritonitis (RR 1.94, 95% CI 0.36 to 10.35; 199 participants), or urinary tract infection (RR 1.94, 95% CI 0.18 to 21.06; 199 participants). Spadaro 2017 did observe a difference in the risk of participants developing a wound infection by day 28 between the two intervention arms, with fewer wound infections reported in the shorter storage duration arm (RR 0.32, 95% CI 0.12 to 0.86; 199 participants; Analysis 1.3).
Secondary outcome: Duration of respiratory support (invasive and non‐invasive ventilation), haemodynamic support (inotropic) and renal support (haemofiltration)
Respiratory support was reported as duration of mechanical ventilation and as the number of participants requiring mechanical ventilation. In terms of reporting of the duration of mechanical ventilation, this type of reporting requires the same considerations as length of stay, in that it usually has a skewed distribution and hence is better reported as a median value with interquartile ranges (IQR); none of the included trials reported the outcome in this way. For the trials that reported data on the duration of mechanical ventilation as means and SDs, we did not analyse the data, but reported them narratively and via a table (see Table 4).
Trials with neonate participants
In Fernandes 2005 researchers reported no difference between the shorter and longer storage arms in the mean duration (days) that mechanical ventilation was required (MD ‐6.40, 95% CI ‐18.83 to 6.03; 52 participants; Analysis 1.4). No trial reported data on haemodynamic or renal support in the neonate population.
1.4. Analysis.
Comparison 1 Transfusion of shorter storage duration RBCs vs longer storage duration RBCs, Outcome 4 Duration of organ support.
Trials with paediatric participants
The trials investigating this participant population did not report data for this outcome (Dhabangi 2013; Dhabangi 2015).
Trials with adult participants
Bennett‐Guerrero 2009 [1] reported the mean duration of mechanical ventilation and measured the number of participants on any vasopressor for more than 48 hours after surgery. Investigators observed no difference between the shorter and longer storage arms in the number of participants on any vasopressor for more than 48 hours after surgery (RR 0.61, 95% CI 0.12 to 3.00; 23 participants (note: results not shown in analyses)). Adult participants in the longer storage arm required fewer hours of mechanical ventilation than those in the shorter storage arm (see Table 4).
In Spadaro 2017, investigators found no difference between the shorter and longer storage arms in the number of participants requiring inotropic support (RR 1.07; 95% CI 0.47 to 2.40; 199 participants), or renal support (RR 0.65, 95% CI 0.11 to 3.79, 199 participants) up to 28 days post surgery (Analysis 1.5).
1.5. Analysis.
Comparison 1 Transfusion of shorter storage duration RBCs vs longer storage duration RBCs, Outcome 5 Number of participants requiring organ support (adults).
Secondary outcome: Length of hospital and ICU stay
Trials with neonate participants
No trial reported median (IQR) data for this outcome. Fernandes 2005 reported mean (with SD) length of hospital stay. We have not reported these data: the actual data are provided in Table 5.
Trials with paediatric participants
Dhabangi 2015 reported median (IQR) length of hospital stay. We did not analyse these data: the actual data are presented in Table 5.
Trials with adult participants
Bennett‐Guerrero 2009 [1] reported mean (SD) length of hospital stay. Steiner 2015 and Spadaro 2017 reported median (IQR) ICU and hospital length of stay. We did not analyse these data: the actual data are presented in Table 5.
Secondary outcome: Adverse transfusion reactions
Trials with paediatric participants
Only one trial reported adverse transfusion reactions (Dhabangi 2015). One child in each group experienced a reaction that was related to transfusion within the first 24 hours of transfusion (facial oedema in the shorter storage arm and hives in the longer storage arm).
Trials with adult participants
Although Steiner 2015 reported serious adverse events that occurred in both intervention arms by day 28, the investigators did not specify those that were related to transfusion.
Secondary outcome: Assessment of economic or blood stock inventory outcomes
Trials with neonate participants
Fernandes 2005 and Liu 1994 reported mean number of donor transfusions per infant (i.e. mean number of donors to which each infant was exposed). This is an outcome of particular interest in very low birth weight, premature neonates. In practice, premature infants are routinely exposed to longer storage RBCs because of a 'dedicated paed pack' donor policy introduced in the 1980s to minimise the risk of transmission of viruses through transfusions from multiple donors. These packs are aliquots made from one batch of adult blood donation. These aliquots are stored and an aliquot is used each time the same infant requires an RBC transfusion. By design, this leads to higher rates of transfusion of longer storage duration RBCs. This creates a blood stock management activity. Investigators noted a difference in the mean number of donor transfusions per infant that favoured the longer storage arm (i.e. fewer donors exposed to) in both trials (MD 2.80, 95% CI 1.46 to 4.14; 52 participants in Fernandes 2005; and MD 1.70, 95% CI 0.07 to 3.33; 25 participants in Liu 1994: Analysis 1.6).
1.6. Analysis.
Comparison 1 Transfusion of shorter storage duration RBCs vs longer storage duration RBCs, Outcome 6 Economic or blood stock inventory outcomes.
Trials with paediatric participants
The trials investigating this participant population did not report data for this outcome (Dhabangi 2013; Dhabangi 2015).
Trials with adult participants
Spadaro 2017, Steiner 2015, and Yuruk 2013 reported the median (IQR) number of RBC units transfused per participant during surgery (Spadaro 2017); number of RBC units transfused per participant up to postoperative day 7 and 'throughout the study period' (Steiner 2015); and number of bags of RBCs transfused to adult haematology participants (Yuruk 2013).
With the exception of the outcome 'throughout the study period' in Steiner 2015, overall the median number of units transfused in the shorter and longer storage duration arms were the same. The Steiner 2015 'throughout the study period' data showed that participants in the shorter storage arm received more RBC units than those in the longer storage arm (a median of 4 (IQR 2 to 6) units compared to 3 (IQR 2 to 6) units). We did not analyse these data: the actual data are presented in Table 6.
Transfusion of RBCs of shorter versus standard practice storage duration
Eleven studies that randomised 40,588 participants reported data for this comparison. Three studies included neonates (Strauss 1996; Strauss 2000; Fergusson 2012), and eight studies included adults (Hebert 2005; Bennett‐Guerrero 2009 [2]; Aubron 2012; Heddle 2012; Kor 2012; Cooper 2017; Heddle 2016; Lacroix 2015).
We use the term 'standard practice storage' when referring to the current standard practice storage duration intervention arm.
We calculated the number of participants included in the analyses of mortality at 30‐day and 90‐day follow‐ups in both intervention arms in Hebert 2005 from data provided in the study authors' Table 2 (page 1437 in Hebert 2005), as this value was not reported in the text. In this table Hebert 2005 reported the overall number of participants included in these analyses, as well as events and percentage event rates. From these figures, it was possible to calculate the actual number of participants included in these analyses per intervention.
Primary outcome: Mortality: in hospital (with time points as defined by the participant group) and short term (up to 30 days)
Trials with neonate participants
Strauss 1996 reported data on mortality up to 30 days. The investigators observed no differences in the number of reported deaths between the shorter and standard practice storage intervention arms (average RR 0.30, 95% CI 0.01 to 7.02; 40 participants, very low quality evidence; Analysis 2.1).
2.1. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 1 Mortality.
Trials with adult participants
Mortality data were reported by eight trials at a range of time points. Four trials reported in‐hospital mortality (Aubron 2012; Hebert 2005; Heddle 2012; Heddle 2016); three reported mortality in intensive care (Hebert 2005; Heddle 2016; Lacroix 2015); and five reported mortality at 30 days post intervention (Bennett‐Guerrero 2009 [2]; Hebert 2005; Kor 2012; Lacroix 2015; Cooper 2017) (see Characteristics of included studies table). In Bennett‐Guerrero 2009 [2], there were no reported deaths at 30‐day follow‐up.
Investigators reported no evidence of a difference in the number of reported deaths between the shorter and standard practice storage arms at any of the reported time points:
in hospital: four trials reported in‐hospital mortality with a median length of stay ranging from 10 to 21 days. When combined, there was no evidence of a difference in mortality between the two arms (average RR 1.05, 95% CI 0.97 to 1.14; 25,704 participants, moderate quality evidence; Analysis 2.1);
in ICU: three trials reported ICU mortality with a median length of stay ranging from 10 to 15 days. When combined, there was no evidence of a difference in ICU mortality between the two arms (average RR 1.06, 95% CI 0.98 to 1.15; 13,066 participants, moderate quality evidence; Analysis 2.1);
at 30‐day follow‐up across the combined data of five trials (average RR 1.04, 95% CI 0.96 to 1.13; I2 = 0%; 7510 participants, moderate quality evidence; Analysis 2.1) (Bennett‐Guerrero 2009 [2]; Hebert 2005; Kor 2012; Lacroix 2015; Cooper 2017).
Secondary outcome: Long‐term mortality
Trials with neonate participants
Fergusson 2012 reported data on mortality at 90‐day follow‐up with no difference in the risk of death reported between the shorter and standard practice storage arms (average RR 0.97, 95% CI 0.61 to 1.54; 377 participants; moderate quality evidence; Analysis 2.1).
Trials with adult participants
Hebert 2005,Lacroix 2015 and Cooper 2017 reported data on mortality at 90‐day follow‐up and observed no difference in the risk of death reported between the shorter and standard practice storage arms (average RR 1.04, 95% CI 0.97 to 1.12; I2 = 0%; 7398 participants, high quality evidence; Analysis 2.1).
Secondary outcome: Incidence of in‐hospital infection
Trials with neonate participants
Fergusson 2012 observed no differences between shorter and standard practice storage arms for the risk of premature neonates with necrotising enterocolitis (average RR 1.01, 95% CI 0.51 to 2.00; 377 participants; Analysis 2.2), clinically suspected infections (average RR 1.01, 95% CI 0.90 to 1.12; 377 participants; Analysis 2.2), or confirmed infections (average RR 1.06, 95% CI 0.91 to 1.22; 377 participants; Analysis 2.2).
2.2. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 2 Incidence of hospital‐acquired infection in a neonates.
Trials with adult participants
Lacroix 2015 reported data on hospital‐acquired infections (including hospital‐acquired pneumonia, peritonitis, mediastinitis, and bacteraemia) and observed no difference in the risk of acquiring a hospital infection between the shorter and standard practice storage arms (average RR 1.09, 95% CI 0.97 to 1.22; 2413 participants; Analysis 2.3).
2.3. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 3 Incidence of hospital‐acquired infection in adults.
Cooper 2017 reported data on 'new bloodstream infection' in the ICU and observed no difference in the risk of acquiring a new blood stream infection between the shorter and standard practice storage arms (average RR 0.90, 95% CI 0.57 to 1.41; 4919 participants; Analysis 2.3).
Secondary outcome: Duration of respiratory support (invasive and non‐invasive ventilation), haemodynamic support (inotropic) and renal support (haemofiltration)
Duration of respiratory support was reported as duration of mechanical ventilation and as the number of participants requiring mechanical ventilation. As mentioned previously, reporting of duration of mechanical ventilation requires the same considerations as length of stay, in that it is usually a skewed distribution and hence is better reported as a median IQR value. Since none of the included trials reported this outcome in this way, we did not analyse the data, but reported them narratively in a table (see Table 4).
Trials with neonate participants
Fergusson 2012 reported data on respiratory support in neonates, and noted no difference between the shorter and standard practice storage arms for the risk of premature neonates requiring mechanical ventilation (average RR 0.99, 95% CI 0.90 to 1.10; 377 participants; Analysis 2.4), or high‐frequency ventilation (average RR 1.02, 95% CI 0.79 to 1.31; 377 participants; Analysis 2.4). Full study data are reported in Table 4.
2.4. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 4 Number of participants requiring organ support.
No trial reported data on haemodynamic or renal support in neonates.
Trials with adult participants
Five trials reported data on respiratory support in adults (Hebert 2005; Bennett‐Guerrero 2009 [2]; Aubron 2012; Lacroix 2015; Cooper 2017), and three reported data on renal support (Hebert 2005; Lacroix 2015; Cooper 2017). One trial reported data on haemodynamic support (Lacroix 2015). A variety of methods were used to measure this outcome. We did not undertake a meta‐analysis because of clinical diversity (ICU setting in Aubron 2012, and Cooper 2017; cardiac surgery in Hebert 2005), and because of the ways in which the outcomes were measured.
Respiratory support
When pooled, the Aubron 2012, Hebert 2005 and Cooper 2017 trials reported no differences between the shorter and standard practice storage arms for the risk of participants requiring mechanical ventilation (average RR 1.15, 95% CI 0.92 to 1.44; 3 studies, 5027 participants; I2 = 72%; Analysis 2.4). Due to the high heterogeneity, we are uncertain of the true effect.
In Bennett‐Guerrero 2009 [2], investigators noted no differences between the shorter and standard practice storage arms for the risk of participants taking any vasopressor for more than 48 hours after surgery (average RR 3.67, 95% CI 0.46 to 29.49; 20 participants; Analysis 2.4).
In Hebert 2005, researchers reported no differences between the shorter and standard practice storage arms for the risk of participants requiring prolonged invasive mechanical ventilation (more than 48 hours) (average RR 1.67, 95% CI 0.60 to 4.64; 57 participants; Analysis 2.4), or prolonged mechanical ventilation (more than 48 hours) (average RR 1.34, 95% CI 0.60 to 2.98; 57 participants; Analysis 2.4), or vasoactive drugs, an aortic balloon pump or ventricular assist devices for more than 48 hours (average RR 1.49, 95% CI 0.45 to 4.98; 57 participants; Analysis 2.4).
The Lacroix 2015 trial reported no differences between the shorter and standard practice storage arms in the mean number of days for which mechanical ventilation was required (MD 0.3, 95% CI ‐1.01 to 1.61; 2413 participants; Analysis 2.5).
2.5. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 5 Duration of organ support.
In Aubron 2012, researchers reported the median (with IQR) duration of mechanical ventilation and observed that it was shorter in the standard practice storage arm than in the shorter storage arm. Cooper 2017 reported no difference in the median days alive and free of invasive mechanical ventilation between arms (25 days). We did not analyse these median data: actual data are presented in Table 4.
Haemodynamic support
In Lacroix 2015, researchers reported no difference between the shorter and standard practice storage arms for the mean number of days for which cardiac or vasoactive drugs were required (MD ‐0.40, 95% CI ‐1.25 to 0.45; 2413 participants; Analysis 2.5).
Renal support
Hebert 2005 and Cooper 2017 reported the number of participants who required renal dialysis at 30 days and observed no difference in risk between the shorter and standard practice storage arms (average RR 0.95, 95% CI 0.83 to 1.09; I2 = 0%; 4976 participants; Analysis 2.4).
In Lacroix 2015, researchers reported the number of days for which participants required renal support and observed no difference in risk between the shorter and standard practice storage arms (MD 0.20, 95% CI ‐0.54 to 0.94; 2413 participants; Analysis 2.5).
Cooper 2017 reported that participants in the shorter storage arm were alive and free of renal replacement therapy for more days than those in the standard practice storage arm (median (IQR)). We did not analyse these median data: the actual data are presented in Table 4.
Secondary outcome: Length of hospital and ICU stay
Trials with neonate participants
Strauss 1996 reported percentage data for length of hospital stay, and Fergusson 2012 reported median (IQR) data for the duration of intensive care unit stay. These data are available in Table 5.
Strauss 1996 reported the percentage of neonates discharged up to day 84: 67% in the shorter storage arm versus 53% in the standard practice storage arm. These data are available in Table 5.
Fergusson 2012 presented data as medians (IQR). Neonates in the shorter storage arm required a longer stay in the ICU. These data are available in Table 5.
Trials with adult participants
Aubron 2012, Cooper 2017 and Heddle 2016 presented length of stay data as medians (plus IQR). We did not analyse these data, but report them in Table 5. Bennett‐Guerrero 2009 [2] and Lacroix 2015 reported mean (with SD) length of hospital stay. We did not report these data but present them in Table 5.
In Aubron 2012, participants in the shorter storage arm required a longer stay in hospital and a longer stay in the ICU than those in the standard practice storage arm.
In Cooper 2017, participants in the shorter storage arm required a shorter stay in hospital than those in the standard practice storage arm, but there was no difference in the length of ICU stay between the two arms.
In Heddle 2016, there was no difference in the length of hospital stay between the shorter and standard practice storage arms.
Secondary outcome: Adverse transfusion reactions
Trials with neonate participants
Three trials (with 431 neonates) reported data for this outcome (Strauss 1996; Strauss 2000; Fergusson 2012). We did not undertake a meta‐analysis because event data were lacking. No adverse transfusion reactions were reported in Strauss 1996 or Strauss 2000, however, one diagnosis of cytomegalovirus (CMV) infection within a 90‐day ICU stay was reported in a neonate assigned to the standard practice storage arm in Fergusson 2012.
Trials with adult participants
Lacroix 2015 reported the number of participants who experienced an acute transfusion reaction and Cooper 2017 reported the number who experienced a febrile nonhaemolytic transfusion reaction. We did not meta‐analyse these data due to differences in the specifics of the transfusion reactions.
In Lacroix 2015, investigators observed no difference between the shorter and standard practice storage arms for risk of participants experiencing an acute transfusion reaction (average RR 0.67, 95% CI 0.19 to 2.36; 2413 participants; Analysis 2.6).
2.6. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 6 Number of participants experiencing an adverse transfusion reaction.
In Cooper 2017, investigators observed that participants in the standard practice storage arm experienced a lower risk of febrile nonhaemolytic transfusion reactions than those in the shorter storage arm (average RR 1.48, 95%CI 1.13 to 1.95; 4919 participants; Analysis 2.6).
Secondary outcome: Assessment of economic or blood stock inventory outcomes
Trials with neonate participants
Fergusson 2012, Strauss 1996 and Strauss 2000 reported the mean number of donor transfusions per infant (i.e. mean number of donors to which infant each infant was exposed). Differences in the mean numbers of donor transfusions per infant favoured the standard practice storage arm (i.e. exposed to fewer donors) in all trials (MD 1.62, 95% CI 1.17 to 2.07; 377 participants in Fergusson 2012; MD 2.10, 95% CI 0.82 to 3.38; 29 participants in Strauss 1996; MD 4.60, 95% CI 2.28 to 6.92; 21 participants in Strauss 2000; Analysis 2.7).
2.7. Analysis.
Comparison 2 Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs, Outcome 7 Economic or blood stock inventory outcomes.
Trials with adult participants
Cooper 2017, Heddle 2016 and Lacroix 2015 reported the number of RBC units transfused per participant. In Lacroix 2015, investigators observed no difference between the shorter and standard practice storage arms in the mean number of RBC units transfused per participant (MD 0.00, 95% CI ‐0.43 to 0.43; 2413 participants; Analysis 2.7).
Heddle 2016 and Cooper 2017 reported the median (IQR) number of RBC units transfused per participant. In both trials, the median number was identical in both intervention arms. We did not analyse these data, but present them in Table 6.
Trial sequential analysis
The TSA for short‐term all‐cause mortality showed that the cumulative Z‐curve crossed the non‐inferiority boundaries after 80.9% of the required information size in the analysis with trials of low overall risk of bias and with 84.1% of the required information size in the analysis that included all trials. These results suggest that we may now have sufficient evidence to reject a 5% relative risk decrease or increase of death within 30 days when transfusing RBCs of shorter versus longer storage duration.
Discussion
Summary of main results
This 2018 update of our 2015 review, has included six additional randomised controlled trials (RCTs) with an additional 40,771 participants, which represents a marked increase from the 1864 participants included in Brunskill 2015. In total, this 2018 update includes 22 RCTs that randomised 42,635 participants across a wide range of clinical settings.
Two main features were noted across these 22 RCTs:
There was marked clinical heterogeneity, as the trials were conducted in a variety of clinical settings, specifically critically ill neonates, children, hospitalised adults, cardiac surgery and adult intensive care.
There was wide diversity in the size, methodology and reporting of primary outcomes – from the highly pragmatic INFORM trial (Heddle 2016), which recruited over 30,000 participants and reported on in‐hospital mortality to smaller trials such as TOTAL (Dhabangi 2015), which closely evaluated physiology or efficacy end‐points, or both.
We have summarised the main findings for our two pre‐defined comparisons.
Transfusion of RBCs of shorter versus longer storage duration
Eleven trials, with 1864 participants, compared transfusion of RBCs of shorter versus longer storage duration. All trials contributed to mortality endpoints; and there was no evidence of difference in risk of mortality between the arms at any of our predefined time points (Analysis 1.1).
Not all studies provided data for our other predefined secondary clinical outcomes. For those that did, there were no significant differences reported between the arms for incidence of infection, requirements for organ support, or length of stay. Only one trial provided information on adverse reactions (Dhabangi 2015).
Assessment of economic and blood stock inventory outcomes were reported as the mean number of donor transfusions in two neonatal trials (i.e. the mean number of donors to which each infant was exposed).
Transfusion of RBCs of shorter versus standard practice storage duration
Eleven trials, with 40,588 participants, compared transfusion of RBCs of shorter versus standard practice storage duration. The large number of participants was due to one study, Heddle 2016, which provided qualitative data for 24,736 participants. All trials provided data on mortality. There was no evidence of a difference in risk of mortality between the arms at any of our predefined time points (Analysis 2.1). There were no clear differences reported between the arms for secondary clinical outcomes across all studies. We judged the quality of evidence to be high for long‐term mortality.
Only one study of critically ill participants contributed data to the outcome of adverse transfusion reactions (Cooper 2017). The trialists observed fewer febrile non‐haemolytic transfusion reactions in the standard practice storage arm than in the shorter storage arm. Otherwise, for studies that provided data on our secondary clinical outcomes, there were no significant differences reported between both arms for incidence of infection, requirements for organ support, or length of stay. In the three neonatal trials, participants who received RBCs of standard practice storage duration had a significantly fewer number of donor exposures than those who received RBCs of shorter storage duration.
Results from the Trial Sequential Analysis indicate that we may now have enough evidence to reject a 5% relative risk decrease/increase of death within 30 days when transfusing shorter storage blood versus blood of any longer storage duration across a range of clinical settings.
We identified four trials that are awaiting further assessment (NCT00458783; NCT01534676; NCT02050230; NCT02724605), and one ongoing RCT in critically ill paediatric patients that will be incorporated into future updates of this systematic review (NCT01977547). The ongoing RCT will evaluate changes in organ dysfunction as a primary outcome.
Overall completeness and applicability of evidence
This 2018 review update includes 22 studies that randomised a total of 42,635 participants, and now provides the most up‐to‐date evidence to assess the consequences of transfusion of RBCs of shorter versus longer storage duration. The trials were set across a wide range of clinical settings, and included the large INFORM trial that recruited across all hospitalised patients (Heddle 2016), which suggests broad applicability of the findings. However, certain subgroups of participants were less well represented as distinct groups, for example, those suffering from bleeding or trauma, in whom multiple transfusions are often required. The large number of participants, overall, was heavily influenced by one study (Heddle 2016).
In the previous version of this review (Brunskill 2015), we discussed several factors that affected applicability of the evidence. These included different baseline risks between different participant groups, differences in underlying physiology, small sample sizes (mainly due to pilot or feasibility studies), predominance of physiological efficacy outcome measures over clinically important outcomes, the effect of multiple units of transfusion and concerns about effects of extremes of storage duration. Some of these have now been addressed with the results of large RCTs, which were powered to detect differences in clinically important outcomes such as mortality and morbidity.
Twelve trials provided data on physiological markers of oxygen consumption or altered micro‐circulation for assessing the clinical effect of storage duration on the efficacy of storage duration. We were unable to undertake meta‐analysis of any participant population due to widespread differences in the measurements used to assess efficacy (see Table 7). In addition, such physiological markers can be altered by many other interventions in critically ill patients (e.g. fluid resuscitation, vasopressors/intotropes, renal replacement therapy), and therefore trying to disentangle the specific effect of storage duration of blood is likely to be very difficult.
5. Physiological markers of oxygen consumption or alterations in microcirculation.
| Trial | Shorter storage duration | Longer storage duration/standard practice storage duration |
| Transfusion of RBCs of shorter vs longer storage duration | ||
| Neonatal participant population | ||
| Liu 1994 | Mean change (before and after transfusion) in:
|
Mean change (before and after transfusion) in:
|
| Paediatric participant population | ||
| Dhabangi 2013 | Resolution of lactic acidosis within 4 hours of RBC transfusion = 92% (n = 34) | Resolution of lactic acidosis within 4 hours of RBC transfusion = 81% (n = 30) |
| Dhabangi 2015 | Proportion of participants with a lactate of 3 mmol/L or lower at 8 hours after RBC transfusion = 58% (83/143) Cerebral tissue oxygen saturation (%)
|
Proportion of participants with a lactate of 3 mmol/L or lower at 8 hours after RBC transfusion = 61% (87/143) Cerebral tissue oxygen saturation (%)
|
| Adult participant population | ||
| Bennett‐Guerrero 2009 [1] | PaO2:FiO2 ratio, mean at:
Lactic acid, concentration 2 hours post operation: mean = 1.9 (SD 1.6) |
PaO2:FiO2 ratio, mean at:
Lactic acid, concentration 2 hours post operation: mean = 2.5 (SD 1.5) |
| Neuman 2013 | "patients had a [mean (SD)] 0.191 (0.438) and 0.069 (0.242) µM increase in N02 at 1 and 24 hours after transfusion respectively" "Patients receiving fresh pRBC [PRBC] had no significant change in FMD" (i.e. flow‐mediated dilation, a measure of vascular endothelial function) No significant change in FHb (plasma‐free Hb) was noted before or after a RBC transfusion |
"patients had a [mean (SD)] 0.191 (0.438) and 0.069 (0.242) µM increase in N02 at 1 and 24 hours after transfusion respectively" "Patients receiving fresh pRBC [PRBC] had no significant change in FMD" (flow‐mediated dilation, a measure of vascular endothelial function) No significant change in FHb (plasma‐free Hb) was noted before or after a RBC transfusion |
| Walsh 2004 | Median (plus IQR) from baseline to post‐transfusion period Intragastric arterial difference in PCO2 (Pg‐PaCO2 gap) = ‐0.15 (IQR ‐0.31 to ‐0.01) PgCO2,kPa = ‐0.46 (IQR ‐1.36 to ‐0.18) PgCO2 ‐PaCO2,kPa = ‐0.41 (IQR ‐0.82 to 0.10) pHi = 0.02 (IQR ‐0.01 to 0.05) Lactate = 0.10 (IQR ‐0.16 to 0.21) mmol/L Arterial pH = 0.00 (IQR ‐0.001 to 0.001) HCO3 (actual) = 0.85 (IQR ‐1.44 to ‐0.53) mmol/L Arterial base excess = ‐0.70 (IQR ‐1.42 to ‐0.45) |
Median (plus IQR) from baseline to post‐transfusion period Intragastric arterial difference in PCO2 (Pg‐PaCO2 gap) = 0.02 (IQR ‐0.29 to 0.11) PgCO2,kPa = 0.16 (IQR ‐0.36 to 1.19) PgCO2 ‐PaCO2,kPa = 0.37 (IQR ‐0.17 to 1.66) pHi = ‐0.02 (IQR ‐0.06 to 0.01) Lactate = 0.03 (IQR ‐0.10 to 0.23) mmol/L Arterial pH = 0.00 (IQR ‐0.001 to 0.001) HCO3 (actual) = ‐0.29 (IQR ‐0.90 to 0.07) mmol/L Arterial base excess = ‐0.71 (IQR ‐1.5 to ‐0.42) |
| Yuruk 2013 | Mean changes in microcirculatory density (measured before and 30 minutes after transfusion) from 17 (SD 3) mm/mm2 to 19 (SD 3) mm/mm2. | Mean changes in microcirculatory density (measured before and 30 minutes after transfusion) from 17 (SD 3) mm/mm2 to 19 (SD 2) mm/mm2. |
| Transfusion of shorter storage duration RBCs vs transfusion of standard practice storage durationRBCs | ||
| Neonatal participant population | ||
| Fergusson 2012 | Percentage (number) of participants requiring:
|
Percentage (number) of participants requiring:
|
| Strauss 1996 | Mean change from before to after transfusion:
|
Mean change from before to after transfusion:
|
| Strauss 2000 | Mean change from before to after transfusion:
|
Mean change from before to after transfusion:
|
| Adult participant population | ||
| Bennett‐Guerrero 2009 [2] | PaO2:FiO2 ratio, mean at:
Lactic acid, concentration 2 hours post operation: mean = 2.2 (SD 1.1) |
PaO2:FiO2 ratio, mean at
Lactic acid, concentration 2 hours post operation: mean = 2.4 (SD 1.0) |
| Kor 2012 | Mean change from before to after transfusion PaO2:FiO2 = 2.5 (SD 49.3) mmHg |
Mean change from before to after transfusion PaO2:FiO2 = ‐9.0 (SD 69.8) mmHg |
Abbreviations
Hb: haemoglobin IQR: interquartile range Pg‐PaCO2: intragastric minus arterial partial pressure of carbon dioxide PgCO2,kPa; intragastric partial pressure of carbon dioxide pHi: intramucosal gastric pH PRBC: packed red blood cells RBC: red blood cell SD: standard deviation
However, despite the high quality of some of the newer trials, certain factors were still present which limited our ability to perform a complete meta‐analysis. In particular, outcomes such as mortality were not standardised across the trials and were reported at multiple time points ranging from 10 days to 90 days in adult participants. Trial processes, such as separation strategies, were also not similar across all the trials. Therefore, even the addition of these recent large RCTs has not altered, but has confirmed, the overall conclusion of our previous review where we reported that no clear difference in mortality was observed between the different storage durations of RBCs.
Quality of the evidence
We summarised the GRADE quality of evidence in separate 'Summary of findings' tables for different comparisons (Table 1; Table 2). The overall quality of evidence for our mortality outcomes ranged from very low to moderate. Due to the potential underlying physiological differences between neonates, children and adults we subdivided our 'Summary of findings' tables into these groups. We downgraded studies for indirectness when the primary outcome of the trial(s) in that particular population was different to our chosen outcome of in‐hospital and short‐term (up to 30 days) mortality, and for indirectness when there were a very small number of deaths. Across the different subpopulations we found no issues with inconsistency.
The previous version of this review described difficulties in assessing risk of bias mainly due to lack of information (Brunskill 2015), which led to many domains being marked as being at an unclear risk of bias. In this 2018 update, we identified six new trials, four of which were large multicentre RCTs at low risk of bias across all domains. Some trials were unable to blind participants and clinicians due to regulatory restrictions concerning blood labelling, but we felt this was not an important issue as we used objective end‐points, such as mortality. The majority of trials were at low risk for attrition and reporting bias.
Potential biases in the review process
We identified no biases in the review process. The strengths of this review lie in the robust and comprehensive methods employed to find and assess all relevant trials. We have followed standard Cochrane methods for data extraction, analyzed our results with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a; Higgins 2011b), and referred to a statistician when necessary. We have had a clinician and a methodologist working at all stages, independently of each other, to control for any bias that might ensue from knowledge of clinical or systematic review methods. Where necessary, we contacted trial authors to obtain missing data.
A potential weakness of this systematic review was our decision not to predefine the interventions of shorter, longer and standard practice storage duration, but rather to use the terminology used by the primary studies. This has meant that the distribution of RBC storage duration within the intervention groups is not always clear, and there is substantial cross‐over and overlap of storage durations between intervention groups.
Agreements and disagreements with other studies or reviews
This review is an update of a Cochrane Review published in 2015. The original review included 16 studies with 1864 participants. This updated review included six more studies with 40,771 more participants. In the previous review, we chose not to perform a meta‐analysis, but did perform it in this update, a result of the inclusion of large, high‐quality trials targeted towards our predefined clinically important outcomes (albeit at different time points). These trials also included large numbers of particularly vulnerable patient groups e.g. critically ill people.
The findings of this review are similar to those of other very recent systematic reviews. Rygard 2018 specifically focused on critically ill patients and found no evidence of benefit of transfusion of blood of shorter storage duration. The findings of their trial sequential analysis also agree with ours. They reported reaching the required information size to reject a 10% relative risk increase/decrease of death; we have enough evidence to reject an even smaller (5%) relative risk increase/decrease of death within 30 days, although we acknowledge that most of the data come from adult participants. Chai‐Adisaksopha 2017 performed a systematic review and meta‐analysis, which included 14 trials and 26,374 hospitalised patients of all ages, and also found no evidence of an effect of storage duration of RBCs on overall in‐hospital mortality.
Authors' conclusions
Implications for practice.
Findings from 22 randomised controlled trials (RCTs) conducted worldwide across a range of clinical settings show no evidence of an effect of transfusing red blood cells (RBCs) that have been stored for a shorter duration versus RBCs that have been stored for a longer duration, or RBCs that have been stored for the duration that is standard practice in particular institutions, on risk of mortality. In adults requiring a blood transfusion, current practice of using the oldest available RBCs does not need to change at present. The quality of evidence in neonates and children was low and current practice may be altered by the findings of an ongoing RCT in critically ill children.
Implications for research.
This review has more implications for research than for current practice, but we are not advocating that more RCTs should be conducted. We remain unclear about the impact of RBC storage duration on certain groups such as critically ill trauma patients or bleeding patients who require multiple units of transfusion. The quality of evidence for neonates and children in this review was low, but the results of a multi‐centre RCT in critically ill children are awaited. The confidence intervals in our meta‐analysis, and other meta‐analyses, do not exclude the possibility of increased mortality with RBCs of shorter storage duration across all patient groups. Further RCTs are unlikely to answer these questions and alternative methodologies, such as individual patient data analysis should be explored as a means of providing greater understanding of the impact of RBC storage duration on clinical outcomes. This could be complemented with mechanistic studies to investigate further the impact of duration of storage, particularly at the extremes of storage.
Further research should also focus on factors affecting RBC quality, which was the focus of many of the earlier published trials. Donor age and sex have been associated with RBC fragility and haemolysis and variability in collection and processing has also been reported (Spinella 2015).
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2018 | New citation required and conclusions have changed | The review has been updated with the inclusion of six new studies which involved over 40,000 participants. |
| 27 November 2018 | New search has been performed | The search has been updated to 20 November 2017. The authors of the review have changed. |
Acknowledgements
We would like to thank the authors of the previous review (Brunskill 2015); Susan Brunskill, Kirstin Wilkison, Carolyn Dorée, Marialena Trivella and Simon Stanworth.
We would also like to thank Richard Gregg for his work in leading preparation of the protocol of the previous review, screening the first set of references (with Susan Brunskill) and developing and piloting data extraction forms.
We would like to thank the peer reviewers: Fiona Clay, Walter Dzik, Leslie Levine, Christopher Breen and Katriina Heikkila.
This project was supported by the UK National Institute for Health Research (NIHR), through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Erythrocyte Transfusion] explode all trees #2 (red cell* or red blood cell* or erythrocyte* or RBC* or blood) near/1 (transfus* or infus* or hypertransfus* or retransfus*) #3 MeSH descriptor: [Blood Transfusion] this term only #4 MeSH descriptor: [Blood Component Transfusion] this term only #5 #3 or #4 #6 MeSH descriptor: [Erythrocytes] this term only #7 red cell* or red blood cell* or erythrocyte* or RBC* or whole blood #8 #6 or #7 #9 #5 and #8 #10 (red cell* or red blood cell* or erythrocyte* or RBC* or whole blood):ti #11 #1 or #2 or #9 or #10 #12 MeSH descriptor: [Blood Preservation] explode all trees #13 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*):ti #14 #12 or #13 #15 #11 and #14 #16 ((red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) near/5 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*)):ti #17 #15 or #16
MEDLINE (Ovid SP)
1. ERYTHROCYTE TRANSFUSION/ 2. ((blood or erythrocyte* or red cell* or red blood cell* or RBC*) adj1 (transfus* or infus* or retransfus*)).ti,ab. 3. BLOOD TRANSFUSION/ or BLOOD COMPONENT TRANSFUSION/ 4. ERYTHROCYTES/ 5. (red cell* or red blood cell* or erythrocyte* or RBC* or whole blood).tw. 6. 4 or 5 7. 3 and 6 8. (RBC* or red cell* or red blood cell* or erythrocyte* or whole blood).ti. 9. 1 or 2 or 7 or 8 10. exp Blood Preservation/ 11. *Time Factors/ 12. (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*).ti. 13. 10 or 11 or 12 14. 9 and 13 15. ((red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) adj5 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*)).ti. 16. ((red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) adj3 (store* or storage or storing or preserv* or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest)).ab. 17. 14 or 15 or 16
EMBASE (Ovid SP)
1. ERYTHROCYTE TRANSFUSION/ 2. ((blood or erythrocyte* or red cell* or red blood cell* or RBC*) adj1 (transfus* or infus* or retransfus*)).ti,ab. 3. BLOOD TRANSFUSION/ or BLOOD COMPONENT THERAPY/ 4. ERYTHROCYTE/ or ERYTHROCYTE CONCENTRATE/ 5. (red cell* or red blood cell* or erythrocyte* or RBC* or whole blood).tw. 6. 4 or 5 7. 3 and 6 8. (RBC* or red cell* or red blood cell* or erythrocyte* or whole blood).ti. 9. 1 or 2 or 7 or 8 10. BLOOD STORAGE/ 11. ERYTHROCYTE PRESERVATION/ 12. STORAGE TIME/ 13. *TIME/ 14. (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*).ti. 15. 10 or 11 or 12 or 13 or 14 16. 9 and 15 17. ((red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) adj5 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*)).ti. 18. ((red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) adj3 (store* or storage or storing or preserv* or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest)).ab. 19. 16 or 17 or 18
CINAHL (EBSCOhost)
1. (MH "Erythrocyte Transfusion") 2. TI ((red cell* or red blood cell* or erythrocyte* or RBC* or blood) N1 (transfus* or infus* or retransfus*)) OR AB ((red cell* or red blood cell* or erythrocyte* or RBC* or blood) N1 (transfus* or infus* or retransfus*)) 3. (MH "Blood Transfusion") OR (MH "Blood Component Transfusion") 4. (MH "Erythrocytes") 5. TI (red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) OR AB (red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) 6. S4 OR S5 7. S3 AND S6 8. TI (red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) 9. S1 OR S2 OR S7 OR S8 10. (MH "Blood Preservation") 11. (MM "Time Factors") 12. TI (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*) 13. S10 OR S11 OR S12 14. S9 AND S13 15. TI ((red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) N5 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*)) 16. AB ((red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) N3 (store* or storage or storing or preserv* or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest)) 17. S14 OR S15 OR S16
PubMed (e‐publications only)
(red cell*[TI] OR blood[TI] OR erythrocyte*[TI] OR transfus*[TI] or RBC*[TI]) AND (random* or trial* OR controlled OR control group OR blind* OR systematic*) AND (age[TI] or aged[TI] or aging[TI] or fresh*[TI] or old[TI] or older[TI] or oldest[TI] or new[TI] or newer[TI] or newest[TI] or young[TI] or younger[TI] or youngest[TI] or store*[TI] or storage[TI] or storing[TI] or preserv*[TI]) AND (publisher[sb] NOT pubstatusnihms)
Transfusion Evidence Library (www.transfusionevidencelibrary.com)
(red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) [in Record Title] AND (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*) [in Record Title]
LILACS
db:("LILACS") AND type_of_study:("clinical_trials" OR "systematic_reviews" OR "guideline" OR "evidence_synthesis") AND (tw:("red cells" OR "red blood cells" OR "age of blood" OR "blood transfusion" OR transfus*) OR ti:(blood)) AND limit:("humans")
Web of Science (CPCI‐S)
Title: (red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) AND Title: (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*) AND RCT Filter/Topic: (random* or blind* or trial* or control*)
ISRCTN and EU Clinical Trials Register
(red cells OR red blood cells OR erythrocytes OR RBCs OR blood OR transfusion) AND (age OR aged OR aging OR fresh OR fresher OR freshest OR old OR older OR oldest OR new OR newer OR newest OR young OR younger OR youngest OR stored OR storage OR storing OR preserved)
ClinicalTrials.gov
Search Terms: (age OR aging OR fresh OR fresher OR freshest OR old OR older OR oldest OR new OR newer OR newest OR young OR younger OR youngest OR stored OR storage) AND transfusion AND red blood cells
WHO ICTRP
Title: (age OR aged OR aging OR fresh OR fresher OR freshest OR old OR older OR oldest OR new OR newer OR newest OR young OR younger OR youngest OR stored OR storage OR storing) Intervention: transfusion
UMN‐CTR Japanese Clinical Trials Registry and the Hong Kong Clinical Trials Registry
Title: (age OR aged OR aging OR fresh OR fresher OR freshest OR old OR older OR oldest OR new OR newer OR newest OR young OR younger OR youngest OR stored OR storage OR storing) Intervention: transfusion
Data and analyses
Comparison 1. Transfusion of shorter storage duration RBCs vs longer storage duration RBCs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 In‐hospital mortality: within 24 hours (children) | 2 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.43, 5.25] |
| 1.2 In‐hospital: within 7 days (children) | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.34] |
| 1.3 In‐hospital mortality: within 7 days (adults) | 1 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.66, 3.06] |
| 1.4 Mortality: time‐point not defined (adults) | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.55, 9.17] |
| 1.5 Short‐term mortality: up to 30 days (children) | 1 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.45, 4.31] |
| 1.6 Short‐term mortality: up to 30 days (adults) | 2 | 1121 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.50, 1.45] |
| 1.7 Long‐term mortality: > 30 days (neonates) | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.44, 1.85] |
| 1.8 Long‐term mortality: > 30 days (adults) | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.68, 3.64] |
| 2 Incidence of hospital‐acquired infection in neonates | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Number of clinical sepsis events | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.00, 1.56] |
| 2.2 Number of necrotising enterocolitis events | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.48, 4.70] |
| 3 Incidence of hospital‐acquired infection in an adults | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Number of participants developing infections and infestations (as classified under serious advere events) to day 28 | 1 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.60, 1.32] |
| 3.2 Number of participants developing at least 1 postoperative infection by day 28 | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.41] |
| 3.3 Number of participants developing sepsis by day 28 | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.02] |
| 3.4 Number of participants developing a pulmonary infection by day 28 | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.60, 8.51] |
| 3.5 Number of participants developing a wound infection by day 28 | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.86] |
| 3.6 Number of participants developing peritonitis by day 28 | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.36, 10.35] |
| 3.7 Number of participants develoiping a urinary tract infection by day 28 | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.18, 21.06] |
| 4 Duration of organ support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Duration of mechanical ventialation (neonates) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants requiring organ support (adults) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Number of adult participants requiring renal support up to day 28 post surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Number of patients requiring inotropic drug support up to day 28 post surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Economic or blood stock inventory outcomes | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Mean donor exposure (neonate participant population) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Transfusion of shorter storage duration RBCs vs standard practice storage duration RBCs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 In‐hospital mortality: time‐points varied (adults) | 4 | 25754 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.14] |
| 1.2 In‐ICU mortality (adults) | 3 | 13066 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.3 Short‐term mortality: up to 30 days (neonates) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.02] |
| 1.4 Short‐term mortality: up to 30 days (adults) | 5 | 7530 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.13] |
| 1.5 Long‐term mortality: up to 90 days (neonates) | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.61, 1.54] |
| 1.6 Long‐term mortality: at 90 days (adults) | 3 | 7398 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| 2 Incidence of hospital‐acquired infection in a neonates | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Necrotising enterocolitis (Bell criteria ≥ 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinically suspected infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Confirmed infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Incidence of hospital‐acquired infection in adults | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Hospital‐acquired infections (adults) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 New blood stream infection whilst in ICU (adults) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants requiring organ support | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Number of neonates requiring mechanical ventilation | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.10] |
| 4.2 Number of neonates requiring high‐frequency ventilation | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.31] |
| 4.3 Number of adults requiring mechanical ventilation | 3 | 5027 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.92, 1.44] |
| 4.4 Number of adults requiring mechanical ventilation for > 48 hours | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.60, 2.98] |
| 4.5 Number of adults requiring prolonged invasive ventilation for > 48 hours | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.60, 4.64] |
| 4.6 Number of adults requiring vasoreactive drugs or an aortic balloon pump or a ventricular assist device for > 48 hours | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.45, 4.98] |
| 4.7 Number of adults on any vasopressor > 48 hours after surgery | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [0.46, 29.49] |
| 4.8 Number of adults requiring renal support | 2 | 4976 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| 5 Duration of organ support | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Duration (days) of mechanical ventilation (adults) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Duration (days) of cardiac or vasoreactive drugs (adults) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Duration (days) of renal support (adults) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of participants experiencing an adverse transfusion reaction | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Adults | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Economic or blood stock inventory outcomes | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Mean donor exposure (neonates) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Mean number of RBC units transfused per participant (adults) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aubron 2012.
| Methods | Parallel‐group, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: none reported Duration of follow‐up: for the duration of ICU stay |
|
| Participants |
Inclusion criteria: aged > 18 years; hospitalised in ICU and prescribed at least 1 RBC unit Exclusion criteria: receipt of palliative care or history of organ transplantation or of haematological disease Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: 'freshest available', i.e. shortest storage time Comparator: “compatible, non expired [RBC] units with the longest storage duration at the time of transfusion request”, as per November 2010 Australian transfusion practice Number of participants enrolled: 52 Number of participants randomly assigned to the interventions: 25:26 Number completing assessment: 25:26 Compliance with assigned treatments: nothing stated in the text about compliance with assigned interventions |
|
| Outcomes |
Primary: feasibility trial
Secondary
|
|
| Notes |
Population nationality: Australian Sources of funding: Australian Red Cross Blood Service and ANZIC Research Centre, Monash University, Australia Declarations of interest (among primary researchers): "None of the authors have any conflict of interest" (page 1201) Dates the study was conducted: September 2010 to January 2011 This is a feasibility study for the 'TRANSFUSE (STandaRd Issue TrANsfusion versuS Fresher RBC Use in intenSive carE – a randomized controlled trial)' trial. The TRANSFUSE trial is listed in this review under Cooper 2017 (Kaukonen 2013 report). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned 1:1 by ICU staff using a web‐based system. System generated a participant‐specific identification number, which maintained blinding of clinical and ICU research staff. |
| Allocation concealment (selection bias) | Low risk | Participant‐specific identification number was sent (in writing and by telephone) to the transfusion service with a notification of transfusion request. The unique number when applied to a printed randomisation schedule of participant study numbers held only by the transfusion laboratory allowed the unblinded transfusion service scientist to issue trial‐specific RBC units. The transfusion service computer system ensured that all subsequent units were similarly allocated while the participant was in the ICU. |
| Blinding of participants (performance bias) | Low risk | All participants were treated in an ICU whilst involved in the trial. |
| Blinding or personnel (performance bias) | Low risk | Manuscript stated that all ICU staff involved in the care of participants (research co‐ordinator, bedside nurse, treating intensivist, study investigators) were blinded to study arm assignment. However 2 ICU nurses were unblinded to treatment assignment, in order to perform the necessary checking of blood products ahead of a transfusion. Trialists took steps to measure whether study arm assignment was revealed by these nurses. Investigators in this study measured no subjective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial report did not state who undertook the outcome assessment, so information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One enrolled participant declined to consent. All randomly assigned participants were included in the outcome analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Bennett‐Guerrero 2009 [1].
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: start of the surgical process Sample size calculation: none reported Duration of follow‐up: to postoperative day 30 |
|
| Participants |
Inclusion criteria: aged > 18 years; high morbidity and mortality risk defined as a preoperative Parsonnet risk score > 15 and scheduled for non‐emergency CABG and/or cardiac valve repair or replacement surgery using CPB Exclusion criteria: patient refusal of RBC products; haemodialysis or peritoneal dialysis within 7 days before surgery; planned significant concomitant procedure; planned avoidance of CPB or a minimally invasive surgical approach; planned use of an alternative to heparinisation; (scheduled) participation in an experimental drug or device trial within 30 days of study entry; history of significant abnormality or RBC disorders; positive antibody screen; known allergic reactivity to blood products requiring washed RBCs; significant immunocompromise and patients with blood type O (due to insufficient quantities of middle‐aged or old O negative units) Transfusion indication: support during acute illness |
|
| Interventions |
Comparisons: transfusion of RBCs of shorter vs transfusion of longer storage duration Intervention: mean storage (SD) 7 (4) days Comparator: mean storage (SD) 21 (4) days Number of participants enrolled: 845 (of whom 771 were excluded from the trial because, 255 had a Parsonnet score of < 15;183 were planned off‐pump surgery or minimally invasive surgery; 155 were allocated to other clinical studies; 97 had a variety of clinical reasons detailed within the trial that precluded eligibility; 46 for local centre administration reasons; 24 for patient specific reasons and 11 for unknown reasons) Number of participants randomly assigned to the interventions: 12:11 Number completing assessment: 12:11 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions. All participants in this trial underwent surgery, and all were included in the feasibility analysis. 20 (87%) participants received at least 1 unit of RBCs; 20 (87%) participants were excluded from the organ injury analysis |
|
| Outcomes |
Primary: feasibility trial
Secondary
|
|
| Notes |
Population nationality: USA Sources of funding: Duke University internal funds Declarations of interest (among primary researchers): manuscript reported "None" Dates the study was conducted: conducted over a 15‐month period, but no start and end dates reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computerised randomisation schedule that was stratified by the attending surgeon and maintained by the hospital’s transfusion service” (page 1376) |
| Allocation concealment (selection bias) | Low risk | Quote: “Computerised randomisation schedule that was stratified by the attending surgeon and maintained by the hospital’s transfusion service” (page 1376) |
| Blinding of participants (performance bias) | Low risk | Quote: “Red blood cells units provided by the hospital’s transfusion service in a blinded manner from induction of general anaesthesia to post‐operative day 7such that patients, care providers, study personnel and investigators were blinded to the study group assignment” (page 1376) Quote: “To maintain blinding, the transfusion service obscured the expiration date of red blood cell units with a removable sticker that contained the institutional review board number and study contact information in case a clinician had any questions” (page 1376) Investigators in this study measured no subjective outcomes. |
| Blinding or personnel (performance bias) | Low risk | Quote: “Red blood cell units provided by the hospital’s transfusion service in a blinded manner from induction of general anaesthesia to post‐operative day 7 such that patients, care providers, study personnel and investigators were blinded to the study group assignment” (page 1376) Quote: “To maintain blinding, the transfusion service obscured the expiration date of red blood cell units with a removable sticker that contained the institutional review board number and study contact information in case a clinician had any questions” (page 1376) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not state who undertook the outcome assessment, so information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants were included in all of the outcome analyses for organ injury analysis. Three participants (no details were reported as to which intervention arm) were excluded from the organ injury analysis. |
| Selective reporting (reporting bias) | Low risk | Outcome data of interest to this review were reported in supplemental tables. All assessments detailed in the Methods section were reported in the Results section and in supplemental tables. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Bennett‐Guerrero 2009 [2].
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: start of the surgical process Sample size calculation: none reported Duration of follow‐up: to postoperative day 30 |
|
| Participants |
Inclusion criteria: aged > 18 years; high morbidity and mortality risk defined as a preoperative Parsonnet risk score > 15 and scheduled for non‐emergent CABG and/or cardiac valve repair or replacement surgery using CPB Exclusion criteria: patient refusal of RBC products; haemodialysis or peritoneal dialysis within 7 days before surgery; planned significant concomitant procedure; planned avoidance of CPB or a minimally invasive surgical approach; planned use of an alternative to heparinisation; (scheduled) participation in an experimental drug or device trial within 30 days of study entry; history of significant abnormality or RBC disorders; positive antibody screen; known allergic reactivity to blood products requiring washed RBCs; significant immunocompromise and patients with blood type O (due to insufficient quantities of middle‐aged or old O negative units) Transfusion indication: support during acute illness |
|
| Interventions |
Comparisons: transfusion of RBCs of shorter vs standard practice storage duration Intervention: stored for < 21 days Comparator: unit standard of care = RBC units stored for longest out first Number of participants enrolled: 845 (of whom 771 were excluded from the trial because, 255 had a Parsonnet score of < 15;183 were planned off‐pump surgery or minimally invasive surgery; 155 were allocated to other clinical studies; 97 had a variety of clinical reasons detailed within the trial that precluded eligibility; 46 for local centre administration reasons; 24 for patient specific reasons and 11 for unknown reasons) Number of participants randomly assigned to the interventions: not stated; 22 were randomly assigned but surgery was cancelled in 2 participants Number completing assessment: 9:11 Compliance with assigned treatments: Nothing is stated in the text about compliance with assigned interventions. All participants in this trial underwent surgery, and all were included in the feasibility and organ injury analysis. 18 (90%) participants received at least 1 unit of RBCs |
|
| Outcomes |
Primary: feasibility trial
Secondary
|
|
| Notes |
Population nationality: USA Sources of funding: Duke University (North Carolina) internal funds Declarations of interest (among primary researchers): manuscript reported "None" Dates the study was conducted: conducted over a 15‐month period, but no start and end dates reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computerised randomisation schedule that was stratified by the attending surgeon and maintained by the hospital’s transfusion service” (page 1376) |
| Allocation concealment (selection bias) | Low risk | Quote: “Computerised randomisation schedule that was stratified by the attending surgeon and maintained by the hospital’s transfusion service” (page 1376) |
| Blinding of participants (performance bias) | Low risk | Quote: “Red blood cell units provided by the hospital’s transfusion service in a blinded manner from induction of general anaesthesia to post‐operative day 7 such that patients, care providers, study personnel and investigators were blinded to the study group assignment” (page 1376) “To maintain blinding, the transfusion service obscured the expiration date of red blood cell units with a removable sticker that contained the institutional review board number and study contact information in case a clinician had any questions” (page 1376) Investigators in this study measured no subjective outcomes. |
| Blinding or personnel (performance bias) | Low risk | Quote: “Red blood cell units provided by the hospital’s transfusion service in a blinded manner from induction of general anaesthesia to post‐operative day 7 such that patients, care providers, study personnel and investigators were blinded to the study group assignment” (page 1376 )Quote: “To maintain blinding, the transfusion service obscured the expiration date of red blood cells units with a removable sticker that contained the institutional review board number and study contact information in case a clinician had any questions” (page 1376) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial report did not state who undertook the outcome assessment, so information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 participants were randomly assigned into the study. Surgery was cancelled for 2 participants (it is unclear to which intervention arm they had been randomly assigned). Only 20 participants received a RBC transfusion and were included in the outcome analyses. |
| Selective reporting (reporting bias) | Low risk | Outcome data of interest to this review were reported in supplemental tables. All assessments detailed in the Methods section were reported in the Results section and in supplemental tables. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Cooper 2017.
| Methods | Parallel‐group, double‐blind, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: 90‐day follow up for the primary outcome (all‐cause mortality), 180 day follow‐up for death and quality of life |
|
| Participants |
Inclusion criteria: > 18 years; admitted to ICU; anticipated stay of ≥ 24 hours; requirement for RBC transfusions Exclusion criteria: previous transfusion; cardiac surgery; haematologic cancer; organ transplantation; pregnancy; expected death in < 24 hours; objection to receiving human blood products; co‐enrolment; lack of clinician equipoise Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shortest storage available vs standard practice storage duration Intervention: shortest available storage Comparator: longest storage in inventory Number of participants enrolled: 6353 Number of participants randomly assigned to the interventions: 2490:2504 Number completing assessment:2457:2462 Compliance with assigned treatments: 2454:2491 received assigned treatment |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: Australia, New Zealand, Ireland, Finland, Saudi Arabia Sources of funding: Australian National Health and Medical Research Council, Australian Red Cross Blood Service, Health Research Council of New Zealand, Irish Health Research Board Declarations of interest (among primary researchers): Manuscript reported "Dr. Cooper reports receiving consulting fees, paid to Monash University, from Eustralis Pharmaceuticals. No other potential conflict of interest relevant to this article was reported" Dates the study was conducted: November 2012 to December 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation with computer‐generated schedule with variable‐block randomisation |
| Allocation concealment (selection bias) | Low risk | Identification number provided to hospital transfusion services for treatment allocation without involvement of clinicians |
| Blinding of participants (performance bias) | Low risk | Only blood bank staff unblinded |
| Blinding or personnel (performance bias) | Low risk | Only blood bank staff unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only blood bank staff unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High rates of follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. Prospectively registered |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Dhabangi 2013.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: none reported Duration of follow‐up: 24 hours |
|
| Participants |
Inclusion criteria: positive blood smear for malaria; severe anaemia (Hb < 5 g/dL); lactic acidosis (blood lactate > 5 mmol/L); written informed consent from parent or guardian Exclusion criteria: known or concurrent cardiac disease; undergoing transfusion of blood products other than packed RBCs Transfusion indication: support during acute illness Age range: shorter storage group = mean 27.6 (SD 16.6) months; longer storage group = mean 23.1 (SD 15.2) months |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: stored for 1 to 10 days Comparator: stored for 21 to 35 days Number of participants enrolled: 250 (a total of 176 participants were not eligible because of a blood lactate concentration of < 5 mM (n = 89), Hb > 5 g/dL(n = 62), age < 6 or > 60 months (n = 12) and blood screen negative for malaria parasite (n = 9) or excluded because there were no study bloods (n = 3) or consent was declined (n = 1)). Number of participants randomly assigned to the interventions: 37:37 Number completing assessment: 37:37 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions |
|
| Outcomes |
Primary: feasibility trial
Secondary
|
|
| Notes |
Population nationality: Ugandan Source of funding: Canadian Institute for Health Resaerch (MCT 75527) Declarations of interest (among primary researchers): "The authors declare that they have no competing interests" Dates the study was conducted: December 2010 to August 2011 This was a feasibility study for 'TOTAL' (RBC transfusion in severe anemia with lactic acidosis). The TOTAL trial is an ongoing trial listed in this review under NCT01586923a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit assessment of risk of selection bias. The study reported that "eligible children were randomized using sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit assessment of risk of selection bias. Details regarding allocation were concealed within a sealed envelope. No further details were reported regarding whether the envelopes were sequentially numbered or opaque, or both. |
| Blinding of participants (performance bias) | Unclear risk | Information was insufficient to permit assessment of risk of performance bias. |
| Blinding or personnel (performance bias) | Unclear risk | Information was insufficient to permit assessment of risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Dhabangi 2015.
| Methods | Parallel‐group, non‐inferiority, randomised controlled trial, single‐centre study Randomly assigned at: point of severe anaemia (Hb < 5 g/dL) Sample size calculation: yes Duration of follow‐up: 8 hours for primary outcome, 30 days for proportion of participants returning to good health |
|
| Participants |
Inclusion criteria: children aged 6 to 60 months; severe anaemia (Hb < 5 g/dL); lactic acidosis (lactate > 5) Exclusion criteria: children undergoing transfusion of blood products other than packed RBCs; suspected or known concurrent cardiac disease; severe acute malnutrition Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: stored for 1 to 10 days (shorter storage) Comparator: stored for 25 to 35 days (longer storage) Number of participants enrolled: 349 Number of participants randomly assigned to the interventions: 145:145 Number completing assessment: 140:142 Compliance with assigned treatments: 145:145 received assigned treatment |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: Ugandan Source of funding: National Institutes of Health grant (1R21HL109518‐01A1). Nonin Corporation supplied the equipment used for noninvasive cerebral oxygen measurements. Declarations of interest (among primary researchers): "Dr Stowell reports serving on the scientific review committee for Haemonetics Corporation. No other disclosures were reported." Dates the study was conducted: February 2013 to May 2015 (follow‐up completed June 2015) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes were thoroughly mixed for 20 minutes by 3 individuals. |
| Allocation concealment (selection bias) | Low risk | Identical, opaque sealed envelopes |
| Blinding of participants (performance bias) | Low risk | Unblinded, but outcomes were objective and measurable and unlikely to be affected by blinding. |
| Blinding or personnel (performance bias) | Low risk | Unblinded, but outcomes were objective and measurable and unlikely to be affected by blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High level of data completeness |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. Prospectively registered. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Fergusson 2012.
| Methods | Parallel‐group, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: 90 days |
|
| Participants |
Inclusion criteria: neonates admitted to neonatal ICU; consent form signed by parents; birth weight < 1250 g; required 1 or more RBC transfusions for anaemia Exclusion criteria: premature infants who had already received RBCs and were scheduled to receive an exchange transfusion or a directed donation; had a rare blood type; were moribund on admission to neonatal ICU; were not expected to survive because of a severe congenital abnormality; whose attending clinician team specifically requested 'fresh' RBCs; whose families were experiencing mitigating circumstances Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: storage duration ≤ 7 days Comparator: range of storage duration 2 to 42 days. In this group, units were divided into aliquots to increase usage and reduce waste. Each aliquot was designated for use in a single infant up to its expiry date Number of participants enrolled: 1752 Number of participants randomly assigned to the interventions: 188:189 Number completing assessment: 188:189 Compliance with assigned treatments: manuscript reported a blood bank non‐compliance rate < 4%, identified upon data safety monitoring board first interim analysis (page 1446/7) |
|
| Outcomes |
Primary
These complications had to occur from receipt of the initial transfusion to up to 90 days' neonatal ICU stay in order to be recorded as a primary outcome. Data were presented for each individual outcome as well as for composite outcomes. For this review, we reported and used the data for individual outcomes only. Secondary
These complications had to occur after receipt of the initial transfusion to up to 90 days' neonatal ICU stay in order to be measured as a secondary outcome. Data were presented for each individual outcome and for composite outcomes. For this review, we reported and used the data for individual outcomes only. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Interactive voice response system whereby following eligibility screening by the research co‐ordinator, the system generated a unique number, the research co‐ordinator then telephoned the hospital blood bank staff and reported the number. In turn the blood bank staff referred to a manual of unique numbers generated by an independent statistician prior to study activation to determine the study intervention allocated to the randomized patient" (page 1445) Schedule stratified by site in variable blocks of 6 and 8. |
| Allocation concealment (selection bias) | Low risk | This was undertaken and managed outside the control of the treating clinician. Allocation occurred only after an order to transfuse was received, and only if a supply of RBCs stored for ≤ 7 days was available at the time of allocation |
| Blinding of participants (performance bias) | Low risk | Participants were neonates, and the manuscript reported that “Infants’ families were blinded to treatment allocation” (page 1445). |
| Blinding or personnel (performance bias) | Low risk | Manuscript reported that “study investigators, research co‐ordinators (and) attending care teams … were blinded to treatment allocation” (page 1445). Study protocol stated that each blood bag was issued with a label that could not be altered, but did bear details of the expiry date of the unit. Therefore it is possible that the clinician checking and transfusing the units would be unblinded to treatment allocation, but once units were transfused, the paperwork and notes did not bear details of expiry dates. The study authors believed that this method limited information bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Manuscript reported that “individual elements of the composite outcome were adjudicated independently by 2 neonatologists blinded to the study group allocation" (page 1445). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing. |
| Selective reporting (reporting bias) | Low risk | Study protocol was available, and all of the study's prespecified outcomes that were of interest in this systematic review were reported in the prespecified way. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Fernandes 2005.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: not stated |
|
| Participants |
Inclusion criteria: gestational age < 37 weeks; birth weight < 1500 g; received at least 1 RBC transfusion during hospital stay and parents signed consent form Exclusion criteria: major congenital malformations; haemolytic anaemia of any aetiology; total or partial exchange transfusion during hospitalisation; requiring rapid infusion of packed RBCs for haemorrhage and requiring more than 1 RBC transfusion within 24 hours Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: stored for < 3 days Comparator: stored for ≤ 28 days Number of participants enrolled: 108 Number of participants randomly assigned to the interventions: 26:26 Number completing assessment: 26:26 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions |
|
| Outcomes |
Primary: number of donor transfusions Secondary: none specifically stated, but the following outcomes were reported
|
|
| Notes |
Population nationality: Brazilian Sources of funding: Fundo de Auxilio aos Docentes e Alunos da Universidade Federal de Sao Paulo, Brazil Declarations of interest (among primary researchers): none reported Dates the study was conducted: 15 May 2002 to 15 December 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit assessment of risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit assessment of risk of selection bias. |
| Blinding of participants (performance bias) | Unclear risk | Information was insufficient to permit assessment of risk of performance bias. |
| Blinding or personnel (performance bias) | Unclear risk | Information was insufficient to permit assessment of risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing. |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Hebert 2005.
| Methods | Parallel‐group, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: duration of hospital stay (up to 30 days) |
|
| Participants |
Inclusion criteria: required at least 1 unit of allogeneic RBCs; accepted invitation to participate in the study and to undergo elective or urgent cardiac surgical procedures or admitted to an ICU Exclusion criteria: aged < 16 years; enrolment in another interventional study; previous enrolment in this study; had a terminal illness with a life expectancy < 3 months; not expected to survive > 12 hours post ICU admission; not provided with all required therapeutic interventions to sustain life; unable to receive blood products because of difficulties with cross‐matching or a history of unexplained severe transfusion reaction; receipt of a blood transfusion in the 6 months before enrolment; had undergone a bone marrow transplant; had a malignant haematological disease within a year of study enrolment; or was recipient of a heart transplant or artificial heart Transfusion indication: not stated |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: stored for < 8 days Comparator: RBCs issued from the hospital blood bank with the longest storage time in accordance with standard blood bank procedure. To ensure maximum separation of durations of storage between groups, participants were allocated only on days when the average duration of storage of RBCs in the blood bank exceeded 15 days. Number of participants enrolled: not stated Number of participants randomly assigned to the interventions: 33:33 Number completing assessment: 26:31 Compliance with assigned treatments: some non‐compliance: 2 participants in the shorter storage intervention arm were non‐compliant with randomisation as they did not receive RBCs stored for < 8 days. These participants were excluded from outcome analyses. Another 7 participants (5 in the shorter storage arm and 2 in the standard practice storage arm) did not receive any transfusions. Trialists also reported compliance with allocated RBC storage times. Compliance target was 90%. This was attained in 91% of participants in the shorter storage arm and 59% of participants in the standard practice storage duration intervention arm. |
|
| Outcomes |
Primary: feasibility trial
Secondary
Investigators measured outcomes over the duration of the hospital stay. |
|
| Notes |
Population nationality: Canadian Sources of funding: not stated Declarations of interest (among primary researchers): none reported Dates the study was conducted: 28 October 1999 to 31 May 2001 This was a feasibility study for the 'Age of Blood Evaluation' (ABLE) trial. The ABLE trial is listed in this review under Lacroix 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation process was started by a telephone request from the attending team for a RBC transfusion. The randomisation schedule consisted of a computer‐generated random listing of the 2 treatment allocations blocked into groups of 4 and 6 and stratified by centre and categories of cardiac surgery or critical care. |
| Allocation concealment (selection bias) | Low risk | As above, on receipt of the phone call, a blood bank technologist who was not involved in clinical care opened a sequentially numbered sealed opaque envelope. |
| Blinding of participants (performance bias) | Low risk | Few details were given, but as participants were adults and the trial did not measure subjective outcomes, we have judged blinding of participants to be at low risk of bias. |
| Blinding or personnel (performance bias) | Unclear risk | No further details were given regarding blinding than were reported for participants (above); therefore risk of performance bias was unclear because information was insufficient for assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further details were given regarding blinding than were reported for participants (above); therefore risk of detection bias was unclear because information was insufficient for assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were incomplete for participants in the analysis of 30‐day and 90‐day outcomes. All participants who received RBCs of correct storage duration based on the intervention group they were randomly assigned to were included in the analysis of mortality rates in the ICU and in hospital (shorter storage, n = 26; standard practice storage, n = 31). At 30‐day follow‐up assessment, the numbers included in the intervention arms were: shorter storage, n = 24; standard practice storage, n = 29; at 90‐day follow‐up: shorter storage, n = 20; standard practice storage, n = 29). No details were reported regarding what had happened to the missing participants at 30‐day and 90‐day follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes defined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Heddle 2012.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: no Duration of follow‐up: “until discharge or 31 January 2011 at which time they were censored” |
|
| Participants |
Inclusion criteria: requirement for a blood transfusion Exclusion criteria: medical indication for blood with a short storage duration (e.g. sickle cell disease); scheduled to receive directed or autologous donations; expected to receive massive blood transfusion (defined as > 10 units of blood transfused within 24 hours); received a transfusion as an outpatient Transfusion indication: support during an acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: “freshest available”, i.e. those stored for shortest duration Comparator: “oldest in the inventory", i.e. those stored for longest duration Number of participants enrolled: 1129 Number of participants randomly assigned to the interventions: 309:601 Number completing assessment: 309:601 Compliance with assigned treatments: 5% (n = 46) of participants did not receive RBC transfusion quite as intended: 19 received a massive transfusion; 19 received transfusions before the study was implemented and 8 were transfused in a day‐surgery clinic with same‐day discharge. All were included in the outcome analyses |
|
| Outcomes |
Primary: feasibility trial
Secondary
|
|
| Notes |
Population nationality: Canadian Sources of funding: Canadian Institutes for Health Research, Canadian Blood Services and Health Canada Declarations of interest (among primary researchers): "The authors have no conflicts of interest to disclose" Dates the study was conducted: 14 June 2010 to 19 December 2010 This was the feasibility study for the 'Informing Fresh versus Old Red cell Management' (INFORM)' trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer generated random allocation schedule was used to assign [participants] 1:2 to freshest available or standard issue blood" (page 1204) (The unbalanced randomisation was to maintain a contrast of at least 10 days in the mean maximum storage duration of transfused RBCs between the two treatment groups and to avoid outdating of RBCs). |
| Allocation concealment (selection bias) | High risk | Manuscript reported that for the first 24 weeks of the study, "the randomisation was performed using an unconcealed paper‐based sequence held in the blood bank" (page 1204). Manuscript further reported that in the (additional) last 3 weeks of the trial, allocation was concealed through a secure website (thus low risk of selection bias). However as allocation was unconcealed over most of the trial, this dimension has been judged to be at high risk of bias overall. |
| Blinding of participants (performance bias) | Low risk | Participants were not blinded, but as outcomes were objective, we believe that such knowledge would not have had an effect on outcome assessment, hence our judgement of low risk of bias. |
| Blinding or personnel (performance bias) | Low risk | Study personnel were not blinded, but as outcomes were objective, we believe that such knowledge would not have had an effect on outcome assessment, hence our judgement of low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not blinded, but as outcomes were objective, we believe that such knowledge would not have had an effect on outcome assessment, hence our judgement of low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Heddle 2016.
| Methods | Parallel‐group, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: 30 days |
|
| Participants |
Inclusion criteria: hospitalised adults (>18 years); requirement for RBC transfusion Exclusion criteria: specific requirement for 'fresh' blood, i.e. shorter storage duration (e.g. sickle disease, transfusion dependent thalassaemia); 'fresh' RBCs ordered by care provider; preplanned directed or autologous donation; request for uncross‐matched blood; anticipated massive transfusion as communicated from the clinical area Transfusion indication: support during an acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: “freshest available”, i.e. shortest storage duration Comparator: “oldest in the inventory", i.e. longest storage duration Number of participants enrolled: 24,736 Number of participants randomly assigned to the interventions: 8215:16,521 Number completing assessment: primary assessment (6936:13,922), secondary assessment (8215:16521) Compliance with assigned treatment: manuscript stated "No crossovers occurred during the study" |
|
| Outcomes |
Primary
Secondary
Primary analyses restricted to participants with types A or O blood. Secondary analyses included participants with any blood type. |
|
| Notes |
Population nationality: Australian, Canadian, Israeli, USA Sources of funding: Grants from the Canadian Institutes of Health Research (NSP‐258611 and MOP‐119584) and by funding to the McMaster Centre for Transfusion Research from the Canadian Blood Services and Health Canada Declarations of interest (among primary researchers): Dr Arnold reported grant support from the Canadian Institutes for Health Research, Health Canada, and Canadian Blood Services outside the submitted work. Dr Devereaux reported grant support from Abbott Diagnostics, Boehringer Ingelheim, Covidien, Octapharma, Roche Diagnostics, and Stryker outside the submitted work. Dr Eikelboom reported grant support and personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, Daiichi‐Sankyo, Eli Lilly, GlaxoSmithKline, Janssen, and Sanofi‐Aventis outside the submitted work. Ms Heddle reported grant support from the Canadian Institutes of Health Research, Canadian Blood Services, and Health Canada during the conduct of the study. Dr Kurz reported grant support from the Canadian Institutes of Health Research during the conduct of the study. Dates the study was conducted: April 2012 to October 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule stratified according to study centre and participant blood type |
| Allocation concealment (selection bias) | Low risk | Within each stratum, treatment assignments were made with the use of random block sizes (3 and 6). |
| Blinding of participants (performance bias) | Low risk | Unblinded, as regulatory agencies require blood suppliers to label all RBC products with the date of blood collection or expiration. However, primary outcome (mortality) was objective and measurable and unlikely to be affected by lack of blinding. |
| Blinding or personnel (performance bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 20% of participants excluded post‐randomisation but in a prespecified manner. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. Prospectively registered |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Kor 2012.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: outcome‐dependent over a 48‐hour interval post RBC transfusion or for mortality at hospital discharge |
|
| Participants |
Inclusion criteria: endotracheally intubated; mechanically ventilated ICU participants; arterial access in situ; Hb concentration < 9.5 g/dL Exclusion criteria: concurrent transfusion of another blood product; emergency transfusion and/or haemodynamic instability as defined by upward titration of vasoactive medications in the 2‐hour interval between RBC transfusions Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: single unit of RBCs stored for < 5 days. Subsequent transfusions (after first study transfusion) were standard issue Comparator: single unit of standard issue RBCs (median = 21 days) Number of participants enrolled: 665 Number of participants randomly assigned to the interventions: 50:50 Number completing assessment: 49:50 Compliance with assigned treatments: 1 participant randomly assigned to the study was excluded before administration of a RBC transfusion because an appropriately cross‐matched 'fresh' unit of RBCs could not be found |
|
| Outcomes |
Primary
Secondary
aChanges measured between baseline (measurements were obtained in the 2‐hour interval before transfusion) and post transfusion (on completion of the intervention of RBC transfusion and within 2 hours of initiation of the intervention of RBC transfusion) |
|
| Notes |
Population nationality: USA Sources of funding: National Institutes of Health grant and Department of Critical Care Medicine, Mayo Clinic College of Medicine Declarations of interest (among primary researchers): all authors declared receiving NIH grant award monies Dates the study was conducted: June 2008 to May 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple, computer‐generated list" (page 844) |
| Allocation concealment (selection bias) | High risk | Manuscript reported that treatment allocation was determined by personnel within the blood bank at the time of RBC issue by opening 'sealed, opaque envelopes' (page 844). No details were given regarding whether the envelopes were sequentially numbered, hence our assessment of high risk of bias for allocation concealment. |
| Blinding of participants (performance bias) | Low risk | Manuscript reported that participants were blinded to treatment allocation. Moreover participants were mechanically ventilated when transfused whilst in the trial, so knowledge of treatment allocation does not seem important, hence judgement of low risk of bias. |
| Blinding or personnel (performance bias) | Low risk | Trial stated that all investigators were blinded to treatment allocation status for the duration of the study procedures, as the clinical service responsible for ordering the blood product was not involved in administration of the RBC transfusion. The manuscript further stated that the product expiration date was not concealed during the transfusion process, so there would have been the potential to work out a participant's allocation using the expiration date. However we believe that working out a participant's allocation by RBC unit expiration date was unlikely, and that as all outcomes are objective and most were measured while participants were ventilated, the risk of performance bias was low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial did not state who undertook the outcome assessment, so information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who received the study intervention were included in the outcome analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Lacroix 2015.
| Methods | Parallel‐group, blinded, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: 90 days |
|
| Participants |
Inclusion criteria: request for a first RBC unit transfusion in the ICU with an anticipated length of invasive and/or non‐invasive (CPAP or BIPAP) mechanical ventilation of at least 48 hours Exclusion criteria: age < 16 years; previously enrolled into ABLE; already received RBC transfusion; life expectancy < 3 months; cardiac surgery; decision to withdraw care; requirement for > 1 unit of uncross‐matched blood; rare blood types; objection to blood products Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: stored for < 8 days Comparator: longest storage duration available Number of participants enrolled: 2510 Number of participants randomly assigned to the interventions: 1253:1257 Number completing assessment: 1211:1219 Compliance with assigned treatments: adherence to transfused protocol was 95.4% for all RBCs transfused with 100% of participants in the standard‐blood group receiving only RBCs stored for < 8 days) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: Canadian, British, French, Dutch, Belgian Sources of funding: peer‐reviewed grants from Canadian Institutes of Health Research (177453), Fonds de Recherche du Quebec‐Sante (24460), National Institute for Health Research Evaluation, Trials and Studies Coordinating Centre Health Technology Assessment Programme, French Ministry of Health Programme Hospitalier de Recherche Clinique (12 July 2011) Declarations of interest (among primary researchers): Dr Bardiaux reported grant support from the Canadian Institutes of Health Research, Fonds de la Recherche du Québec, NETSCC Health Technology Assessment, and Programme Hospitalier de Recherche Clinique, Ministère de la Santé, France, during the conduct of the study; and personal fees from Etablissement Français du Sang (French transfusion public service), outside the submitted work. Dr Campbell reported grant support from the National Institute for Health Research Health Technology Assessment Clinical Evaluation and Trials during the conduct of the study. Ms. Clayton reported grant support from the Canadian Institutes of Health Research during the conduct of the study. Dr Fergusson reported grant support from the Canadian Institutes of Health Research during the conduct of the study. Dr Marshall reported personal fees from AKPA Pharma and Roche Diagnostics outside the submitted work. Dr Tiberghien reported grant support from the Canadian Institutes of Health Research, Fonds de la Recherche du Québec, NETSCC Health Technology Assessment, and Programme Hospitalier de Recherche Clinique, Ministère de la Santé, France, during the conduct of the study; and personal fees from Etablissement Français du Sang (French transfusion public service), outside the submitted work. Dr Tinmouth reported grant support from the Canadian Institutes of Health Research and grant support and personal fees from Canadian Blood Services during the conduct of the study; grant support from the Canadian Institutes of Health Research and Novartis, grant support and personal fees from Canadian Blood Services, and personal fees from Amgen and GlaxoSmithKline outside the submitted work. Dr van de Watering reported personal fees from Sanquin Blood Supply, Netherlands, outside the submitted work. Dates the study was conducted: March 2009 to May 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated assignment sequence with stratification according to study site |
| Allocation concealment (selection bias) | Low risk | Use of permuted blocks of varying sizes of 6, 8, or 10 |
| Blinding of participants (performance bias) | Low risk | Opaque sticker was affixed over the expiration and collection dates on the blood units, or the labels, were changed, so that medical team would be unaware of the treatment‐group assignments. |
| Blinding or personnel (performance bias) | Low risk | Blood transfusion technologists refrained from releasing information on storage duration to all clinical and research personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High level of completeness |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. Prospectively registered. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Liu 1994.
| Methods | Parallel‐group, randomised controlled trial, multi‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: 24 ± 6 hours post transfusion |
|
| Participants |
Inclusion criteria: infants weighing < 1.5 kg at the time of first or second blood transfusion Exclusion criteria: an exchange transfusion anticipated > 5 days after the first transfusion; active bleeding or coagulopathy; renal failure; very premature sick neonates not expected to survive > 48 hours; not expected to need > 1 transfusion Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: quint packs stored for < 5 days. When the need for multiple transfusions was anticipated, attempts were made to limit donor transfusions by reserving multiple quint packs from the same donor for a participant. Comparator: assigned a single adult unit of packed RBCs, which was reserved for that infant up to the recommended storage time of 35 days. All transfusions were delivered by syringe pump during a 4‐hour period. Number of participants enrolled: not stated Number of participants randomly assigned to the interventions: 12:13 Number completing assessment: 12:13 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: USA Sources of funding: none stated Declarations of interest (among primary researchers): none reported Dates the study was conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random numbers table” |
| Allocation concealment (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit assessment of risk of selection bias. |
| Blinding of participants (performance bias) | Unclear risk | Information was insufficient to permit assessment of risk of performance bias. |
| Blinding or personnel (performance bias) | Unclear risk | Information was insufficient to permit assessment of risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Neuman 2013.
| Methods | Parallel‐group, randomised controlled trial Randomly assigned at: point of transfusion Sample size calculation: none reported Duration of follow‐up: 24 hours |
|
| Participants |
Inclusion criteria: hospital in‐patients receiving cross‐matched packed RBCs for clinical indications Exclusion criteria: not stated Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion RBCs of shorter vs longer storage duration Intervention: storage duration < 10 days Comparator: storage duration > 21 days Number of participants enrolled: 44 Number of participants randomly assigned to the interventions: 20:24 Number completing assessment: 20:24 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: USA Source of funding: not stated Declarations of interest (among primary researchers): "The authors declare that they have nothing to disclose" Dates the study was conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. |
| Blinding of participants (performance bias) | Unclear risk | Information was insufficient to assess whether an important risk of performance bias existed. |
| Blinding or personnel (performance bias) | Unclear risk | Information was insufficient to assess whether an important risk of performance bias existed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to assess whether an important risk of detection bias existed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No outcome data were missing. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes defined in the Methods section were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Schulman 2002.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion, but only if there were at least 15 units of shorter and standard practice storage duration RBCs available in the blood bank. Sample size calculation: no Duration of follow‐up: not stated |
|
| Participants |
Inclusion criteria: admission to level 1 trauma centre; patients with blood type A only Exclusion criteria: not stated Transfusion indication: support during acute illness Participants were considered for analysis only if 2 or more units of type‐specific blood were transfused |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: storage duration < 11 days Comparator: storage duration > 20 days Number of participants enrolled: not stated Number of participants randomly assigned to the interventions: 8:9 Number completing assessment: 8:9 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions |
|
| Outcomes | Mortality (time point not defined) | |
| Notes | This was noted as a pilot trial and was published in a letter only: no full publication for the trial could be found Population nationality: USA Sources of funding: none stated Declarations of interest (among primary researchers): none reported Dates the study was conducted: August 2000 to July 2001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. |
| Blinding of participants (performance bias) | Unclear risk | Information was insufficient to assess whether an important risk of performance bias existed. |
| Blinding or personnel (performance bias) | Unclear risk | Information was insufficient to assess whether an important risk of performance bias existed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to assess whether an important risk of detection bias existed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information was insufficient to assess whether an important risk of attrition bias existed. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to assess whether an important risk of reporting bias existed. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Spadaro 2017.
| Methods | Parallel‐group, double‐blind randomised controlled trial, single‐centre study Randomly assigned at: preoperative cross‐matching Sample size calculation: yes Duration of follow‐up: 28 days for primary outcome, 90 days for all‐cause mortality |
|
| Participants |
Inclusion criteria: adults (> 18 years) undergoing scheduled noncardiac surgery Exclusion criteria: received RBC transfusion in the 30 days before surgery; suspected or documented infection 30 days prior to surgery; on chronic corticosteroid therapy or other immunosuppressive therapy; active malignant haematologic illness; any other congenital or acquired immunodeficiency Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: "fresh blood" versus "old blood", i.e. shorter vs longer storage duration Intervention: storage duration < 14 days Comparator: storage duration > 24 days Number of participants enrolled: 199 Number of participants randomly assigned to the interventions: 101:98 Number completing assessment: 101:98 Compliance with assigned treatments: 182 out of 199 received assigned treatment ‐ 101 (100%) in the intervention group and 81 (82%) in the comparator group. 17 participants did not receive the comparator treatment because units stored for > 14 days were not available. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: Italian Sources of funding: University of Ferrera, Italy Declarations of interest (among primary researchers): none reported Dates the study was conducted: August 2013 to July 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using sealed and numbered envelopes with randomly permuted block sizes of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered envelopes |
| Blinding of participants (performance bias) | Unclear risk | Trial protocol said trial was double‐blinded, but no information about how this was done. |
| Blinding or personnel (performance bias) | Unclear risk | Trial protocol said trial was double‐blinded, but no information about how this was done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Microbiologist assessing infection was blinded, but unclear if those assessing other outcomes were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Only the primary outcome was prospectively registered. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Steiner 2015.
| Methods | Parallel‐group, randomised controlled trial, multi‐centre study Randomly assigned at: no earlier than 1 day before scheduled surgery and randomisation only performed if transfusion service had enough RBCs stored to meet the cross‐match request Sample size calculation: yes Duration of follow‐up: postoperative day 7, hospital discharge or death, whichever occurred first |
|
| Participants |
Inclusion criteria: ≥ 12 years old; ≥ 40 kg; scheduled complex cardiac surgery; for those aged ≥ 18 a TRUST (Transfusion Risk Understanding Scoring Tool) score of ≥ 4 was required Exclusion criteria: planned use of autologous or directed donations; washed or volume‐reduced blood components or blood components from which additive solution had been removed; severe renal dysfunction; use of an intra‐aortic balloon pump for treatment of cardiogenic shock; planned deep hypothermic circulatory arrest; previous RBC transfusion during current admission; concurrent or recent enrolment in another clinical study Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: stored for < 10 days Comparator: stored for > 21 days Number of participants enrolled: 1709 Number of participants randomly assigned to the interventions: 742:739 Number completing assessment: 538:569 Compliance with assigned treatments: 478 (89%) in the shorter storage and 488 (87%) in the longer storage group |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: USA Sources of funding: supported by the National Heart, Lung and Blood Institute Declarations of interest (among primary researchers): Dr Assmann reported grant support from the NHLBI during the conduct of the study. Dr Bennett‐Guerrero reported grant support from the NIH/NHLBI during the conduct of the study, and grant support and personal fees from Haemonetics Inc. outside the submitted work. Dr Carson reported grant support and personal fees from the National Heart, Lung, and Blood Institute and personal fees from St. Michaels Hospital and the Canadian Institutes of Health Research outside the submitted work. In addition, Dr Carson reported he will apply to the NIH for a grant that will support trial evaluating transfusion thresholds in patients with acute coronary syndrome. Dr Cushing reported grant support from the National Heart, Lung and Blood Institute during the conduct of the study. Ms D'Andrea reported grant support from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Delaney reported personal fees and non‐financial support from Novartis/Progenika/Grifols and Immucor/Bioarray, and personal fees from Williams Kastner outside the submitted work. Ms Granger reported grant support from the NHLBI during the conduct of the study. Dr Hunsaker reported grants from the National Heart Lung and Blood Institute during the conduct of the study. Dr Levy reported grant support and personal fees from Boehringer Ingelheim, CSL Behring, Grifols, and Janssen outside the submitted work. Dr McCullough reported personal fees from Fenwal, Division of Fresenius Kabe outside the submitted work. Dr McFarland reported grant support from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Ness reported grant support from the NHLBI during the conduct of the study and personal fees from Terumo BCT and New Health Sciences outside the submitted work. Dr Ortel reported grant support from the NHLBI during the conduct of the study; grant support from Eisai and the Centers for Disease Control and Prevention, grant support, personal fees and other support from Instrumentation Laboratory, and personal fees from Daiichi‐Sankyo and Bayer outside the submitted work. Dr Puca reported grant support from the NHLBI during the conduct of the study. Dr Raife reported grant support from the National Institutes of Health during the conduct of the study. Dr Roberts reported grant support from the National Institutes of Health during the conduct of the study. Dr Sloan reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute during the conduct of the study. Dr Stowell reported grant support from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Triulzi reported grant support from the NHLBI during the conduct of the study; personal fees from Fresenius‐Fenwal and Carmell, and non‐financial support from the American Red Cross and Canadian Blood Services outside the submitted work. Dates the study was conducted: January 2010 to January 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme used permuted blocks within strata and institutional balancing |
| Allocation concealment (selection bias) | Low risk | Only blood bank staff with appropriate security level were allowed to access treatment arm assignment. Expiration date was not obscured on RBC units due to legal requirements, however, storage duration was not included on the unit labels. |
| Blinding of participants (performance bias) | Low risk | Unblinded, but outcome measures were objective and measurable and unlikely to be affected by lack of blinding. |
| Blinding or personnel (performance bias) | Low risk | Unblinded, but outcome measures were objective and measurable and unlikely to be affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High level of data completeness |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. Prospectively registered trial |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Strauss 1996.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: up to 84 days from birth or until discharge or death if either occurred before 84 days |
|
| Participants |
Inclusion criteria: very low birth weight infants (0.6 kg to 1.3 kg); admission to neonatal ICU Exclusion criteria: infant's survival or need for prolonged support seemed unlikely Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: storage duration < 7 days Comparator: storage duration < 42 days. From 1 dedicated donor who consented to donate a second unit when needed by the infant Number of participants enrolled: not stated Number of participants randomly assigned to the interventions: 21:19 Number completing assessment: for mortality, 21:19; for physiological markers of oxygen consumption or alterations in microcirculation and assessment of economic or blood stock inventory outcomes, 15:14 Compliance with assigned treatments: 29% (n = 6) of participants in the shorter storage duration intervention arm and 26% (n = 5) of participants in the standard practice storage duration arm did not receive any transfusions All participants randomly assigned to the shorter storage duration arm received the allocated intervention: RBCs stored for < 7 days |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Population nationality: USA Sources of funding: Program Project Grant, a Clinical Research Centre Grant, National Institutes of Health Declarations of interest (among primary researchers): none reported Dates the study was conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. Manuscript reported that "envelopes arranged according to a table of random numbers” but provided no further details. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. Manuscript reported that "envelopes arranged according to a table of random numbers” but provided no further details, such as if the envelopes were sequentially numbered and opaque. |
| Blinding of participants (performance bias) | Low risk | Participants were neonates and their parents were not informed of the result of randomisation. |
| Blinding or personnel (performance bias) | Low risk | Manuscript reported that the neonatal ICU staff were not informed of the results of randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to assess whether an important risk of detection bias existed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who received a RBC transfusion were included in the outcome analysis for all outcomes. |
| Selective reporting (reporting bias) | Unclear risk | It was unclear what outcomes trialists wanted to measure; therefore information was insufficient to permit assessment of risk of selection bias. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Strauss 2000.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: not stated Sample size calculation: yes Duration of follow‐up: up to 84 days from birth or until discharge or death if either occurred before 84 days |
|
| Participants |
Inclusion criteria: premature infants weighing between 0.6 kg and 1.3 kg Exclusion criteria: not stated Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs standard practice storage duration Intervention: storage duration < 7 days Comparator: storage duration < 42 days (RBC units from dedicated donors only) Number of participants enrolled: not stated Number of participants randomly assigned to the interventions: 10:11 Number completing assessment: 6:8 Compliance with assigned treatments: all participants randomly assigned to the shorter storage duration arm received the allocated intervention, RBCs stored for < 7 days |
|
| Outcomes |
Primary: whether AS‐3 RBCs could safely satisfy all RBC needs of an individual very low birth weight infant Secondary: assessment of potential risks, both clinical reactions and laboratory abnormalities (glucose, sodium, potassium and calcium) of transfusing stored AS‐1 RBCs (measured as change from pre to post transfusion (not further defined)) |
|
| Notes |
Population nationality: USA Sources of funding: Program Project Grant, a Clinical Research Centre Grant and a National Institutes of Health and Research Grant Declarations of interest (among primary researchers): none reported Dates the study was conducted: 1992 to 1994 Other: the trial was stopped before completion because of the Liu 1994 trial, which demonstrated the feasibility and safety of transfusing RBCs stored for longer rather than RBCs stored for a shorter duration to limit donor transfusion of preterm infants who received transfusions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. Manuscript reported that "envelopes arranged according to a table of random numbers” but provided no further details. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. Manuscript reported that "envelopes arranged according to a table of random numbers” but provided no further details, such as if the envelopes were sequentially numbered and opaque. |
| Blinding of participants (performance bias) | Low risk | Participants were neonates, and their parents were not informed of the results of randomisation. |
| Blinding or personnel (performance bias) | Low risk | Manuscript reported that the neonatal intensive care unit staff were not informed of the results of random arm assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to assess whether an important risk of detection bias existed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who received a RBC transfusion were included in each outcome analysis. Of note, the trial was stopped early "before completion because of a report (Liu 1994) that demonstrated the feasibility and safety of transfusing stored rather than fresh RBCs to limit donor transfusion of preterm infants who received transfusion" (page 216) Therefore the calculated sample size of 40 infants (20 per arm) was never reached. Instead the trial randomly assigned 22 participants (10 in the shorter storage duration intervention arm and 11 in the standard practice storage duration arm), 14 participants (6 participants from the shorter storage duration arm and 8 from the standard practice storage duration arm) who received a RBC transfusion and were included in the analysis of outcomes. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear what outcomes trialists wanted to measure; therefore information was insufficient to permit assessment of risk of selection bias |
| Other bias | Low risk | We have no concerns about other potential sources of bias |
Walsh 2004.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: yes Duration of follow‐up: a total of 10.5 hours (baseline = 2.5 hours; transfusion period = 3 hours; post‐transfusion period = 5 hours) |
|
| Participants |
Inclusion criteria: ICU physician had decided to transfuse the participant with 2 units of RBCs to increase haemoglobin concentration in the absence of clinical bleeding; transfusion could be deferred 12 to 18 hours to enable relatives' assent to be sought when necessary; haemoglobin concentration at the time of screening was < 9 g/dL; participants must not have received a RBC transfusion for at least 48 hours before baseline measurements were to start Exclusion criteria: presence of clinically apparent bleeding; contradiction to placement of a nasogastric tube; required frequent changes in respiratory or cardiovascular support because of physiological instability; not expected to survive > 48 hours; previous gastric surgery; postoperative liver transplant patient; age < 16 years; pregnancy Transfusion indication: support during acute illness |
|
| Interventions |
Comparison: transfusion of RBCs stored for shorter vs longer storage duration Intervention: stored for ≤ 5 days Comparator: stored for ≥ 20 days Number of participants enrolled: 50 Number of participants randomly assigned to the interventions: 10:12 Number completing assessment: 10:12 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions. |
|
| Outcomes |
Primary
Secondary Change in indexes of tissue hypoxia and organ perfusion between pre and post transfusion (pre transfusion measures taken in the 2.5 hours before transfusion, and post‐transfusion measures taken in the 5 hours post RBC transfusion. All the following indexes were measured.
|
|
| Notes |
Population nationality: Scottish Sources of funding: Mason Medical Research Foundation, Effective Use of Blood Group of the Scottish National Blood Transfusion Service and the Royal Infirmary of Edinburgh Intensive Care Unit Research Fund Declarations of interest (among primary researchers): none reported Dates the study was conducted: November 1999 to December 2000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned only if RBCs of both storage durations were available. Random length block randomisation was carried out by the Health Services Research Unit, University of Aberdeen. |
| Allocation concealment (selection bias) | High risk | Information was insufficient to assess whether an important risk of bias existed. Manuscript reported "Nonresealable envelopes were opened for individual patients by blood bank staff only after they had established that compatible blood of each age range was available" but provided no further details, such as if the envelopes were sequentially numbered and opaque. |
| Blinding of participants (performance bias) | Low risk | All participants were treated in an ICU while involved in the trial. |
| Blinding or personnel (performance bias) | Low risk | Quote: “To ensure that all individuals in the intensive care unit were blinded to the age of the transfused units, special blood pack labels and forms were printed for the study. These obscured the collection and expiry dates but stated a time within which the blood must be transfused, which allowed full checking before administration” (page 365). Additionally, trial outcomes were objective, and consisted of physiological markers of oxygenation and mortality. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial did not state who undertook the outcome assessment, so information was insufficient to permit assessment of risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who received treatment were included in the data analysis. It was not clear how many were assigned randomly to each arm, but the number not included at each stage from enrolment to receipt of study treatment was reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section of the trial were reported in the Results section. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Yuruk 2013.
| Methods | Parallel‐group, randomised controlled trial, single‐centre study Randomly assigned at: point of transfusion Sample size calculation: no Duration of follow‐up: measurements were performed before and 30 minutes after transfusion of the last RBC unit |
|
| Participants |
Inclusion criteria: anaemic haematological outpatients requiring monthly RBC transfusions Exclusion criteria: age < 18 years; chemotherapy < 4 months previously; use of vasopressor‐inotropic, sedative or analgesic and anticoagulant pharmacological drugs; indication to receive irradiated and/or washed blood products Transfusion indication: Hb level < 9.6 g/dL. Transfusion was stopped after Hb rose above this threshold or after infusion of 4 RBC units |
|
| Interventions |
Comparison: transfusion of RBCs of shorter vs longer storage duration Intervention: stored for < 1 week Comparator: stored for 3 to 4 weeks Number of participants enrolled: 20 Number of participants randomly assigned to the interventions: 10:10 Number completing assessment: 10:10 Compliance with assigned treatments: nothing was stated in the text about compliance with assigned interventions. |
|
| Outcomes |
Primary: not stated Secondary
|
|
| Notes |
Population nationality: Dutch Sources of funding: Landsteiner Foundation for Blood Research (Grant 20056‐0621) Declarations of interest (among primary researchers): none reported Dates the study was conducted: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to assess whether an important risk of selection bias existed. |
| Blinding of participants (performance bias) | Unclear risk | Information was insufficient to assess whether an important risk of performance bias existed. |
| Blinding or personnel (performance bias) | Unclear risk | Information was insufficient to assess whether an important risk of performance bias existed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to assess whether an important risk of detection bias existed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who received a RBC transfusion were included in the outcome analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the Methods section were reported in the Results. |
| Other bias | Low risk | We have no concerns about other potential sources of bias. |
Abbreviations
ABLE: Age of Blood Evaluation trial ANZICS: Australian and New Zealand Intensive Care Society AS‐1: additive solution‐1, a commercial additive solution containing sodium chloride, dextrose, adenine, mannitol AS‐3: additive solution‐3, a commercial additive solution containing sodium chloride, dextrose, adenine, tri‐sodium citrate, citric acid, sodium phosphate BIPAP: bilevel positive airway pressure BUN: blood urea nitrogen test CABG: coronary artery bypass graft CPAP: continuous positive airway pressure CPB: cardiopulmonary bypass Hb: haemoglobin ICU: intensive care unit MODS: Multiple Organ Dysfunction score NHLBI: National Heart, Lung, and Blood Institute NIH: National Institutes of Health PaO2:FiO2 = ratio of partial pressure arterial oxygen and fraction of inspired oxygen; comparison between the oxygen level in the blood and the oxygen concentration that is breathed. PCO2: partial pressure of carbon dioxide pHi: gastric intramuscosal pH RBC: red blood cell SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bao 2017 | Ineligible intervention: this study compared a no transfusion versus transfusion strategy. The length of storage of blood in the transfusion group was non‐randomised. |
| Chantepie 2015 | Ineligible intervention: this study compared liberal and restrictive transfusion thresholds, instead of length of storage of blood transfused, in patients with haematological malignancies. |
| Eshleman 1994 | Unit of randomisation: in this study (reported as a conference abstract), the intervention was the unit of randomisation, not the participant. Minimal intervention, participant and outcome data were reported, making interpretation of findings difficult |
| Hod 2011 | This was a non‐randomised study in healthy volunteers which used an ineligible intervention (autologous blood). |
| Lebiedz 2012 | This was an observational, non‐randomised study (but is mentioned in some reviews as an RCT). |
| Murphy 2017 | Ineligible intervention: this trial compared the addition of a rejuvenating solution to red cells compared to standard issue red cells. |
| Rapido 2017 | This was a randomised study in healthy volunteers that used an ineligible intervention (autologous blood) and only measured biochemical parameters (ineligible outcomes). |
| Rodrigues 2015 | Ineligible intervention: this study compared two different strategies of blood product resuscitation (a 1:1:1 ratio versus point‐of‐care guided therapy) in patients with trauma requiring massive transfusion. |
| Seitelbach 2011 | Unit of randomisation: participants in this abstract report were not randomly assigned between intervention arms, rather they received both interventions. No details on study methodology were given, and no outcome data were reported |
| Wasser 1989 | Ineligible intervention: this trial compared 2 units of 'fresh' whole blood, i.e. transfused within 12 hours of collection with stored blood (i.e. donated 2 to 5 days before transfusion and stored at 4°C). This comparison does not fit into any of the comparison arms of interest for this review. |
| Yamal 2015 | Ineligible intervention: secondary analysis of a 2 x 2 factorial trial comparing the effects of erythropoietin and 2 haemoglobin transfusion thresholds on neurological recovery after traumatic brain injury. The storage duration of RBC units was not randomised. |
Abbreviation
RBC: red blood cell
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00458783.
| Methods | Parallel‐arm, randomised, controlled trial |
| Participants |
Inclusion criteria: all primary and reoperative adult (≥ 18 years) cardiac surgical patients undergoing CPB for CABG, CABG with a valve procedure, isolated valve procedures, ascending aortic aneurysm or dissection repair alone or combined with CABG and valve procedures Exclusion criteria: descending thoracic aortic aneurysm repair; left or right ventricular assist devices; those unable to receive blood for religious reasons |
| Interventions | Participants undergoing cardiac surgery will be randomly assigned into 1 of 2 groups:
|
| Outcomes |
Primary outcome: to determine whether length of storage of RBCs is related to postoperative morbid outcomes up to 30 days post surgery Secondary outcome: none reported |
| Notes | ClinicalTrials.gov identifier: NCT00458783 Study start date: April 2007 Study completion date: February 2014 Principal Investigator: Collen Koch, The Cleveland Clinic, Ohio, USA Target number of participants: not stated |
NCT01534676.
| Methods | Parallel‐arm, randomised, controlled trial |
| Participants |
Inclusion criteria (recipient)
Exclusion criteria (recipient)
Inclusion criteria (donor)
Exclusion criteria (donor)
|
| Interventions | Eligible participants to be randomised between:
|
| Outcomes |
Primary outcome: non‐transferrin‐bound iron level 2 hours after transfusion Secondary outcome: none reported |
| Notes | ClinicalTrials.gov identifier: NCT01534676 Study start date: February 2012 Study completion date: 3 participants were recruited and the study was terminated in March 2013. Principal Investigator: Steven L. Spitalnik, Professor of Pathology and Cell Biology, Columbia University, USA Target number of participants: not stated |
NCT02050230.
| Methods | Parallel‐arm, randomised, controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria:
|
| Interventions | Adult participants in Intensive care will be randomised to:
|
| Outcomes |
Primary outcome: pulmonary arterial pressure and pulmonary vascular resistance Secondary outcome: systemic pressure and systemic vascular resistance |
| Notes | ClinicalTrials.gov identifier: NCT02050230 Study start date: January 2014 Study completion date:October 2016 Principal Investigator: David M Baron, Medical University of Vienna, Austria Target number of participants: not stated |
NCT02724605.
| Methods | Parallel‐arm, randomised, controlled trial |
| Participants |
Inclusion criteria:.ASA I‐II physical status patients aged between 2 and 10 years with an RBC transfusion volume within 10 mL/kg to 20 mL/kg; non‐crush, trauma paediatric patients needing intraoperative RBC transfusion Exclusion criteria: patients with renal failure (serum creatinine 1.5 mg/dL), hepatic insufficiency, furosemide diuresis, succinylcholine for rapid sequence induction or electrolyte disorders |
| Interventions | Paediatric trauma patients will be randomly assigned into 1 of 2 groups:
|
| Outcomes |
Primary outcome: serum potassium level at 1 hour after RBC transfusion Secondary outcomes
|
| Notes | ClinicalTrials.gov identifier: NCT02724605 Study start date: Januray 2016 Study completion date: August 2017 Principal Investigator: Abdelrady S Ibrahim, Assiut University, Asyut, Egypt Target number of participants: 60, 30 per treatment arm. |
Abbreviations
ASA I‐II: American Society of Anesthesiologists physical status classification bpm: beats per minute BUN: blood urea nitrogen test CABG: coronary artery bypass graft CPB: cardiopulmonary bypass Hb: haemoglobin RBC: red blood cell
Characteristics of ongoing studies [ordered by study ID]
NCT01977547.
| Trial name or title | Age of blood in children in pediatric intensive care units (ABC PICU) |
| Methods | Randomised controlled trial |
| Participants | Patients are considered eligible to participate in the trial if 1 of the following occurs:
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: short storage: RBC storage duration ≤ 7 days Comparator: standard issue: RBC storage duration of 2 to 42 days with expected average length of storage of about 17 to 21 days |
| Outcomes |
Primary outcome
NPMODS is defined as the proportion of participants who die during the 28 days after randomisation, or who develop NPMODS. For participants with no organ dysfunction at randomisation, new MODS is the development of ≥ 2 concurrent organ dysfunctions during the 28 days after randomisation. For participants with 1 organ dysfunction at randomisation, new MODS is the development of at least 1 other concurrent organ dysfunction after randomisation. Participants with MODS (i.e. concurrent dysfunction of ≥ 2 organ systems) at randomisation can develop progressive MODS, defined as development of at least 1 additional concurrent organ dysfunction at or during the 28 days after randomisation. All deaths will be considered to be due to progressive MODS. NPMODS will be monitored up to 28 days or until ICU discharge because it is almost never observed beyond this time in children. Secondary outcomes
|
| Starting date | January 2014 |
| Contact information | Rachel Jacobs; jacobs_r@kids.wustl.edu Lucy Clayton; lucy.clayton@recherche‐ste‐justine.qc.ca |
| Notes | Estimated primary completion date: June 2018 Estimated enrolment: 1538 ClinicalTrials.gov identifier: NCT01977547 Sponsor: National Heart, Lung, and Blood Institute (NHLBI) |
Abbreviations
ICU: intensive care unit IgA: immunoglobulin A RBC: red blood cell
Differences between protocol and review
In this review we have separated our reporting of data on 'Effects of Interventions' between trials of neonates and trials of adult participants (a predefined subgrouping for this review). The decision to present the data in this way was due to innate clinical differences between these two groups of participants. The physiology of the neonate is fundamentally different from that of the adult, and this particularly applies to extremely premature neonates.
In this first update, we removed one of the outcomes that had been included in the original version of the review: clinically accepted measures of multiple organ dysfunction (e.g. Multiple Organ Dysfunction Score (MODS), Sequential Organ Failure Assessment (SOFA) score) and numbers of dysfunctional organs reported per participant. We removed this outcome because the area is saturated in terms of trials and we do not expect new trials to present data that help to progress this outcome. We have chosen to keep length of stay as an outcome measure although we do recognise its limitations such as skewness of data and external factors (e.g. organisational pressures, clinician preferences) that can influence it. However, we believe that it is an outcome that is important to patients. We have not attempted to analyse it by performing a meta‐analysis and have presented the results descriptively.
We chose to perform a meta‐analysis due to the availability of clinically relevant outcome data from large, high quality RCTs.
We undertook a trial sequential analysis (TSA) for the primary outcome of short term mortality. Such an analysis was not a requirement of the original version of this review and therefore we were not able to predetermine the information size required to produce a reliable result from a meta‐analysis. Instead we determined the value ranges for the TSA using the findings of the recent larger multicentre trials (Steiner 2015; Lacroix 2015; Heddle 2016; Cooper 2017).
Contributions of authors
All review authors contributed to the review update and preparation of the final review manuscript. Akshay Shah and Susan Brunskill prepared the first draft of the manuscript and thereafter incorporated comments from all the other review authors.
Akshay Shah: data extraction, risk of bias assessments, screening of references, data analysis, critical care content expert
Susan Brunskill: screening of references, data analysis, Review Manager 5 data entry, methodological expert
Michael Desborough: data extraction, risk of bias assessments, transfusion medicine content expert
Carolyn Dorée: creator of the updated search strategy
Marialena Trivella: statistical expert
Simon Stanwroth: transfusion medicine content expert
Sources of support
Internal sources
NHS Blood and Transplant, Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
Akshay Shah: This report is independent research supported by the National Institute for Health Research (NIHR Doctoral Research Fellowship, Dr Akshay Shah, DRF‐2017‐10‐094). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Susan Brunskill: none known
Michael Desborough: none known
Carolyn Dorée: none known
Marialena Trivella: I was a Training Co‐ordinator at the UK Cochrane Centre and am a statistical editor or peer reviewer for five Cochrane groups (Anaesthesia, Wounds, Injuries, Breast Cancer and Sexually Transmitted Infections). My work with the Cochrane groups is independent of my involvement in this review. My involvement in this review did not involve a financial relationship.
Simon Stanworth: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aubron 2012 {published data only}
- Aubron C, Syres G, Nichol A, Bailey M, Board J, Magrin G, et al. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion 2012;52:1196‐202. [DOI] [PubMed] [Google Scholar]
Bennett‐Guerrero 2009 [1] {published data only}
- Bennett‐Guerrero E, Stafford‐Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, et al. A prospective, double blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion 2009;49:1375‐83. [DOI] [PubMed] [Google Scholar]
Bennett‐Guerrero 2009 [2] {published data only}
- Bennett‐Guerrero E, Stafford‐Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, et al. A prospective, double blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion 2009;49:1375‐83. [DOI] [PubMed] [Google Scholar]
Cooper 2017 {published data only}
- Cooper DJ, McQuilten ZK, Nichol A, Ady B, Aubron C, Bailey M, et al. Age of red cells for transfusion and outcomes in critically ill adults. New England Journal of Medicine 2017;377:1858‐67. [DOI] [PubMed] [Google Scholar]
- Kaukonen KM, Cooper DJ. Age of blood and mortality in critically ill patients: the TRANSFUSE trial. Intensive Care Medicine 2013;39:S386. [Google Scholar]
Dhabangi 2013 {published data only}
- Dhabangi A, Mworozi E, Lubega IR, Cserti‐Gazdewich CM, Maganda A, Dzik WH. The effect of blood storage age on treatment of lactic acidosis by transfusion in children with severe malarial anaemia: a pilot, randomized, controlled trial. Malaria Journal 2013;12(55):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhabangi 2015 {published and unpublished data}
- Dhabangi A, Ainomugisha B, Cserti‐Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia. The TOTAL randomized clinical trial. JAMA 2015;314(23):2514‐23. [DOI] [PubMed] [Google Scholar]
- NCT01586923. RBC Transfusion in Severe Anemia With Lactic Acidosis (TOTAL). www.clinicaltrials.gov.
- Shah A. Clarification of the time‐points of the additional deaths beyond the study's initial 24‐ hour observation period. Email to: W Dzik 10 January 2018.
Fergusson 2012 {published data only}
- Fergusson D, Hutton B, Hogan DL, LeBel L, Blajchman MA, Ford JC, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfusion Medicine Reviews 2009;23(1):55‐61. [DOI] [PubMed] [Google Scholar]
- Fergusson DA. Assessing the clinical effect of RBCs in neonates: the age of red blood cells in premature infants (ARIPI) trial. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology 2012;40:203‐38. [Google Scholar]
- Fergusson DA. The age of red blood cells in neonates ‐ The ARIPI trial. Transfusion Alternatives in Transfusion Medicine 2012;12(2):5. [Google Scholar]
- Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez‐Bouali N, Smyth JA, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low‐birth‐weight infants: the ARIPI randomized trial. JAMA 2012;308(14):1443‐51. [DOI] [PubMed] [Google Scholar]
- Fergusson DA, Hebert PC, LeBel L, Rouvinez‐Bouali NG, Smyth JA, Sankaran K, et al. Age of red blood cells in premature infants (ARIPI). Transfusion 2012;52:11A‐12A. [DOI] [PubMed] [Google Scholar]
Fernandes 2005 {published data only}
- Cunha DH, Kopelman BI, Santos AM, Guinsburg R, Terzian CN, Kuwano ST, et al. Can red blood cells stored up to 30 days be safely transfused to very low birthweight infants?. Pediatric Research 2004;55(68):2622. [Google Scholar]
- Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, Areco KN, Guinsburg R, Araujo Peres C, et al. Transfusions of CPDA‐1 red blood cells stored for up to 28 days decrease donor exposures in very low‐birth‐weight premature infants. Transfusion Medicine 2005; Vol. 15:467‐73. [DOI] [PubMed]
Hebert 2005 {published data only}
- Hebert PC, Chin‐Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesthesia and Analgesia 2005;100:1433‐8. [DOI] [PubMed] [Google Scholar]
Heddle 2012 {published data only}
- Heddle NM, Cook RJ, Arnold DM, Crowther MA, Warkentin TE, Webert KE, et al. The effect of blood storage duration on in‐hospital mortality: a randomized controlled pilot feasibility trial. Transfusion 2012;52:1203‐12. [DOI] [PubMed] [Google Scholar]
- Heddle NM, Eikelboom JW, Cook RJ, Liu Y, Barty RL, Warkentin TE, et al. Can we do a 25,000 patient pragmatic study to address the age of blood and mortality controversy? A pilot to determine feasibility. Transfusion 2011;51:193A. [Google Scholar]
Heddle 2016 {published data only}
- Cook RJ, Heddle NM, Lee KA, Arnold DM, Crowther MA, Devereaux PJ, et al. Red blood cell storage and in‐hospital mortality: a secondary analysis pf the INFORM randomised controlled trial. Lancet Haematology 2017;4(11):e544‐e552. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Cook RJ, Barty R, Liu Y, Arnold DM, Crowther MA, et al. Rationale and design of the Informing Fresh versus Old Red Cell Management (INFORM) trial: an international pragmatic randomized trial. Transfusion Medicine Reviews 2016;3(1):25‐9. [DOI] [PubMed] [Google Scholar]
- Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of short‐term vs. long‐term blood storage of mortality after transfusion. New England Journal of Medicine 2017;375(20):1937‐45. [DOI] [PubMed] [Google Scholar]
- Heddle NM, Cook RJ, Barty R, Liu Y, Arnold DM, Crowther MA, et al. Informing Fresh Versus Old red cell Management (INFORM) trial: a large international pragmatic randomized trial. Transfusion. 2016; Vol. 56:5a. [DOI] [PubMed]
Kor 2012 {published data only}
- Kor DJ, Kashyap R, Weiskopf R, Wilson G, Buskirk CM, Winters JL, et al. The impact of red blood cell storage duration on short‐term pulmonary function and immunologic status in mechanically ventilated patients. American Journal of Respiratory and Critical Care Medicine 2011;183:1. [Google Scholar]
- Kor DJ, Kashyap R, Weiskopf RB, Wilson GA, Buskirk CM, Winters JL, et al. Fresh red blood cell transfusion and short‐term pulmonary, immunologic, and coagulation status: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2012;185(8):842‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00751322. Transfusion‐induced alterations of pulmonary and immune function in mechanically ventilated patients (TRALI2). CliicalTrials.gov record number NCT00751322.
Lacroix 2015 {published data only}
- Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfusion Medicine Reviews 2011;25(3):197‐205. [DOI] [PubMed] [Google Scholar]
- Lacroix J, Hebert PC, Fergusson DA, Tinmoith A, Cook DJ, Marshall JC, et al. Length of red blood cell storage and clinical outcomes in transfused critical ill adults ‐ the Age of Blood Evaluation (ABLE) randomised controlled trial. Transfusion Medicine. 2015; Vol. 25 (Suppl 1):6.
- Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. New England Journal of Medicine 2015;372(15):1410‐8. [DOI] [PubMed] [Google Scholar]
- Lehr A, Fergusson D, Sabri E, Hebert P, Tinmouth A, Lacroix J. Age of blood evaluation: a subgroup analysis or perioperative critically ill adults. Critical Care Medicine 2016;44(12 Suppl 1):460. [DOI] [PubMed] [Google Scholar]
- Mack JP, Kahn SR, Tinmouth A, Fergusson D, Hebert PC, Lacroix J. Dose dependent effect of stored red blood: results of a sub‐group analysis of the age of blood evaluation (ABLE) trial. Blood 2016;128:22. [Google Scholar]
- Stanworth SJ. Discussions about the ABLE trial. Telephone conference: principal investigators of the ABLE trial 5 August 2014.
- Walsh T. The Age of Blood Evaluation Study (ABLE). www.hta.ac.uk/2535. NIHR HTA programme, (accessed 05 April 2013).
Liu 1994 {published data only}
- Liu EA, Mannino FL, Lane TA. Prospective, randomized trial of the safety and efficacy of a limited donor exposure transfusion program for premature neonates. The Journal of Pediatrics 1994;125(1):92‐6. [DOI] [PubMed] [Google Scholar]
Neuman 2013 {published data only}
- Neuman R, Karatela SK, Menon V, Newman JL, Polhemus D, Sher S, et al. Hospitalised patients transfused with storage aged (but not fresh) red blood cells (RBCS) demonstrate impaired endothelial function and significant reductions in circulating nitrite levels. Transfusion 2013;53(Suppl):135A. [Google Scholar]
- Neuman R, Menon V, Karatela S, Newman J, Sher S, Ashraf K, et al. Endothelial dysfunction precipitated by transfusion of storage‐aged but not fresh red blood cells. Vascular Medicine 2013;61:E2066. [Google Scholar]
- Neuman RB, Polhemus D, Karatela S. Transfusion of storage‐aged, but not fresh red blood cells in hospitalised patients results in endothelial dysfunction and decreased circulating nitrite levels. Nitric Oxide: Biology and Chemistry 2013;21:s20. [Google Scholar]
Schulman 2002 {published data only}
- Schulman CI, Nathe K, Brown M, Cohn S. Impact of age of transfused blood in the trauma patient. Journal of Trauma Injury 2002;52(6):1224‐5. [DOI] [PubMed] [Google Scholar]
Spadaro 2017 {published data only}
- NCT01976234. Stored RBC Transfusion and Immonomodulation. www.clinicaltrials.gov.
- Spadaro S, Taccone FS, Fogagnolo A, Fontana V, Ragazzi R, Verri M, et al. The effects of storage of red blood cells on the development of postoperative infections after noncardiac surgery. Transfusion 2017;57:2727‐37. [DOI] [PubMed] [Google Scholar]
Steiner 2015 {published data only}
- Steiner ME. Discussions about the RECESS trial. Email to M Murphy 30 September 2014.
- Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the red cell storage duration study (RECESS). Artificial Cells, Blood Substitutes and Biotechnology 2012;40(3):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the red cell storage duration study (RECESS) (Section 7). Transfusion and Apheresis Science 2010;43:107‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan DR, Delaney M, et al. Effects of red‐cell storage duration on patients undergoing cardiac surgery. New England Journal of Medicine 2015;372(15):1419‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strauss 1996 {published data only}
- Strauss RG, Burmeister L, James T, Miller J, Johnson K, Bell E. A randomized trial of fresh versus stored RBCS for neonatal transfusions. Transfusion 1994;34;10(S):S269. [DOI] [PubMed] [Google Scholar]
- Strauss RG, Burmeister LF, Johnson K, James T, Miller J, Cordle DG, et al. AS‐1 red cells for neonatal transfusions: a randomized trial assessing donor exposure and safety. Transfusion 1996;36(10):873‐8. [DOI] [PubMed] [Google Scholar]
Strauss 2000 {published data only}
- Strauss RG, Burmeister L, James T, Miller J, Johnson K, Bell E. A randomized trial of fresh versus stored RBCS for neonatal transfusions. Transfusion 1994;34;10(S):S269. [DOI] [PubMed] [Google Scholar]
- Strauss RG, Burmeister LF, Cordle DG. Feasibility and safety of AS‐3 red blood cells stored up to 42 days for neonatal transfusions. Transfusion 1999;39(10S):S507‐0400. [DOI] [PubMed] [Google Scholar]
- Strauss RG, Burmeister LF, Johnson K, Cress G, Cordle D. Feasibility and safety of AS‐3 red blood cells for neonatal transfusions. Journal of Pediatrics 2000;136(2):215‐9. [DOI] [PubMed] [Google Scholar]
Walsh 2004 {published data only}
- Maginnis M, Maciver C, Cairns A, McArdle F, Scott N, Walsh TS, et al. Behind the scenes of the age of stored red cells randomized trial ‐ crucial role played by blood bank staff. Transfusion Medicine 2001;37(Suppl 1):P40XIX. [Google Scholar]
- Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients?. Critical Care Medicine 2004;32(2):364‐71. [DOI] [PubMed] [Google Scholar]
Yuruk 2013 {published data only}
- Yuruk K, Milstein MJ, Bezemer R, Barteks SA, Biemond BJ, Ince C. Transfusion of banked red blood cells and the effects on hemorrheology and microvascular hemodynamics in anemic hematology outpatients. Transfusion 2013;53:1346‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bao 2017 {published data only}
- Bao HX, Tong P, Li C, Du J, Chen BY, Huang Z, et al. Efficacy of fresh packed red blood transfusion in organophosphate poisoning. Medicine (Baltimore) 2017;96(11):e6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chantepie 2015 {published data only}
- Chantepie 2015. Transfusion strategy in hematological intensive care unit. www.clinicaltrials.gov, NCT024661264. [DOI] [PMC free article] [PubMed]
Eshleman 1994 {published data only}
- Eshleman JR, Akinbi H, Pleasure J, Asakura T, Magee D, Smith L, et al. Prospective double‐blind study of small volume neonatal transfusion with RBCs up to 35 days old. Transfusion 1994; Vol. 34, issue 10S:S126.
Hod 2011 {published data only}
- Hod EA, Brittenham GM, Billote GB, Francis RO, Ginsburg YZ, Hendrickson JE, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non‐transferrin‐bound iron. Blood 2011;118(25):6675‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lebiedz 2012 {published data only}
- Lebiedz P, Glasmeyer S, Hilker E, Yilmaz‐Neuhaus A, Karaboutas T, Reinecke H, et al. Influence of red blood cell storage time non clinical course and cytokine profile in septic shock patients. Transfusion Medicine and Hemotherapy 2012;39:271‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2017 {published data only}
- Murphy 2017. Red cell rejuvenation for the attenuation of transfusion associated organ injury in cardiac surgery. www.clinicaltrials.gov, NCT03167788.
Rapido 2017 {published data only}
- Rapido F, Brittenham GM, Bandyopadhyay S, Carpia F, L'Acqua C, McMahon DJ, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. Journal of Clinical Investigation 2017;127(1):375‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodrigues 2015 {published data only}
- Rodrigues 2015. Strategy of transfusion in trauma patients ‐ STATA trial. www.clinicaltrials.gov, NCT02416817.
Seitelbach 2011 {published data only}
- Chia J, Seitelbach M, Chin‐Yee IH, Hsia CC. Quality of life outcomes of the age of blood in highly transfusion dependent patients with myelodysplasia: prospective randomized controlled N‐of‐1 studies. Blood 2010;116(21):4411. [Google Scholar]
- Seitelbach M, Chin‐Yee IH, Kinney J, Chia J, Ormond K, Mahon J, et al. Age of blood does not affect quality of life and hemoglobin: N‐of‐1 trials in transfusion‐dependent patients. Blood 2011;118:3369. [Google Scholar]
Wasser 1989 {published data only}
- Wasser MN, Houbiers JG, D'Amaro J, Hermans J, Huysmans HA, Konijnenburg GC, et al. The effect of fresh versus stored blood on post‐operative bleeding after coronary bypass surgery: a prospective randomized study. British Journal of Haematology 1989;72:81‐4. [DOI] [PubMed] [Google Scholar]
Yamal 2015 {published data only}
- Yamal JM, Benoit JS, Doshi P, Rubin ML, Tilley BC, Hannay HJ, et al. Association of transfusion red blood cell storage age and blood oxygenation, long‐term neurologic outcome, and mortality in traumatic brain injury. Journal of Trauma and Acute Care Surgery 2015;79(5):843‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00458783 {published data only}
- NCT00458783. Red Cell Storage Duration and Outcomes in Cardiac Surgery (The Clevelend Trial). clinicaltrials.gov/show/NCT00458783.
NCT01534676 {published data only}
- NCT01534676. Effects of Transfusion or Older Stored Red Cells. www.clinicaltrials.gov.
NCT02050230 {published data only}
- NCT02050230. Hemodynamic effects of stored blood transfusion in intensive care patients. www.clinicaltrials.gov.
NCT02724605 {published data only}
- NCT02724605. The Effect of Red Blood Cells Storage Duration on Biochemical Changes in Pediatric Patients. www.clinicaltrials.gov.
References to ongoing studies
NCT01977547 {published data only}
- NCT01977547. Age of Blood in Children in Pediatric Intensive Care Units (ABC PICU). clinicaltrials.gov/show/NCT01977547.
Additional references
Alexander 2016
- Alexander PE, Barty R, Fei Y, Vandvik PO, Pai M, Siemieniuk RA, et al. Transfusion of fresher versus older red blood cells in hospitalized patients: a systematic review and meta‐analysis. Blood 2016;127(4):400‐10. [DOI] [PubMed] [Google Scholar]
Alter 2008
- Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood 2008;112:2617‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basran 2006
- Basran S, Frumento RJ, Cohen A, Lee S, Du Y, Nishanian E, et al. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesthesia Analgesia 2006;103:15‐20. [DOI] [PubMed] [Google Scholar]
Bennett‐Guerrero 2009
- Bennett‐Guerrero E, Stafford‐Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, et al. A prospective, double‐blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion 2009;49:1375‐83. [DOI] [PubMed] [Google Scholar]
Beutler 2006
- Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration?. Blood 2006;107:1747‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002042.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2013
- Carson JL, Carless PA, Hébert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013;309:83‐4. [DOI] [PubMed] [Google Scholar]
Carson 2016
- Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD002042.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chai‐Adisaksopha 2017
- Chai‐Adisaksopha C, Alexander PE, Guyatt G, Crowther MA, Heddle NM, Devereaux PJ, et al. Mortality outcomes in patients transfused with fresher versus older red blood cells: a meta‐analysis. Vox Sanguinis 2017;112(3):268‐78. [DOI] [PubMed] [Google Scholar]
Cobain 2007
- Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Medicine 2007;17:1‐15. [DOI] [PubMed] [Google Scholar]
Corwin 2004
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: Anemia and blood transfusion in the critically ill ‐ current clinical practice in the United States. Critical Care Medicine 2004;32:39‐52. [DOI] [PubMed] [Google Scholar]
D'Alessandro 2010
- D'Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfusion 2010;8:82‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glynn 2010
- Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion 2010;50:1164‐9. [DOI] [PubMed] [Google Scholar]
Hebert 1993
- Hebert PC, Drummond AJ, Singer J, Bernard GR, Russell JA. A simple, multiple system organ failure scoring system predicts mortality of patient who have severe sepsis syndrome. Chest 1993;104:230‐5. [DOI] [PubMed] [Google Scholar]
Hebert 1999
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New England Journal of Medicine 1999;340:409‐17. [DOI] [PubMed] [Google Scholar]
Hess 2010
- Hess JR. Red cell changes during storage. Transfusion and Apheresis Science 2010;43:51‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Khan 2006
- Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion‐related acute lung injury. Blood 2006;108:2455‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koch 2008
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red‐cell storage and complications after cardiac surgery. New England Journal of Medicine 2008;358:1229‐39. [DOI] [PubMed] [Google Scholar]
Kucukakin 2011
- Kucukakin B, Kocak V, Lykkesfeldt J, Nielsen HJ, Magnussen K, Rosenberg J, et al. Storage‐induced increase in biomarkers of oxidative stress and inflammation in red blood cell components. Scandinavian Journal of Clinical Laboratory Investigation 2011;71:299‐303. [DOI] [PubMed] [Google Scholar]
Leal‐Noval 2003
- Leal‐Noval SR, Jara‐Lopez I, Garcia‐Garmendia JL, Marin‐Niebla A, Herruzo‐Aviles A, Camacho‐Larana P, et al. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology 2003;98:815‐22. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Lelubre 2009
- Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality?. Transfusion 2009;49:1384‐94. [DOI] [PubMed] [Google Scholar]
Lelubre 2013
- Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Critical Care 2013;17(2):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2010
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: final data for 2008. National Vital Statistics Report 2010;59:1‐71. [PubMed] [Google Scholar]
McLean 2009
- McLean E, Cogswell M, Egli I, Wojdyla D, Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993‐2005. Public Health Nutrition 2009;12:444‐54. [DOI] [PubMed] [Google Scholar]
Mynster 2000
- Mynster T, Nielsen HJ. The impact of storage time of transfused blood on postoperative infectious complications in rectal cancer surgery. Scandinavian Journal of Gastroenterology 2000;35:212‐7. [DOI] [PubMed] [Google Scholar]
NHSBT 2012
- Hospital Services, NHS Blood and Transplant, Fiton, North Bristol. Hospital Services (Issues) department data [personal communication]. Email to: Richard Gregg 15 March 2012.
NICE 2015
- National Institute for Health and Care Excellence. Blood Transfusion. NICE guideline14 2015. [www.nice.org.uk/guidance/NG24.] [PubMed]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roback 2011a
- Roback JD. Vascular effects of the red blood cell storage lesion. American Society of Hematology Education Book 2011;2011:475‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roback 2011b
- Roback JD. American Association of Blood Banks. 17th Edition. Bethesda: American Association of Blood Banks, 2011. [Google Scholar]
Rygard 2018
- Rygard SL, Jonsson AB, Madsen MB, Perner A, Holst LB, Johansson PI, et al. Effects of shorter versus longer storage time of transfused red blood cells in adult ICU patients: a systematic review with meta‐analysis and trial sequential analysis. Intensive Care Medicine 2018;44(2):204‐17. [DOI] [PubMed] [Google Scholar]
Schrier 1979
- Schrier SL, Hardy B, Bensch K, Junga I, Krueger J. Red blood cell membrane storage lesion. Transfusion 1979;19:158‐65. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org 2011.
Sharifi 2000
- Sharifi S, Dzik WH, Sadrzadeh SM. Human plasma and tirilazad mesylate protect stored human erythrocytes against the oxidative damage of gamma‐irradiation. Transfusion Medicine 2000;10:125‐30. [DOI] [PubMed] [Google Scholar]
SHOT 2015
- Bolton‐Maggs PH (editor), Poles D, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2014 Annual SHOT Report. www.shotuk.org/wp‐content/uploads/myimages/report‐2014.pdf (accessed 19 December 2018).
Sohmer 1979
- Sohmer PR, Dawson RB. The significance of 2,3‐DPG in red blood cell transfusions. CRC Critical Reviews in Clinical Laboratory Sciences 1979;11:107‐74. [DOI] [PubMed] [Google Scholar]
Spinella 2015
- Spinella PC, Acker J. Storage duration and other measures of quality of red blood cells for transfusion. JAMA 2015;314(23):2509‐10. [DOI] [PubMed] [Google Scholar]
Stainsby 2006
- Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfusion Medicine Reviews 2006;20:273‐82. [DOI] [PubMed] [Google Scholar]
Stanger 2012
- Stanger SH, Yates N, Wilding R, Cotton S. Blood inventory management: hospital best practice. Transfusion Medicine Reviews 2012;26:153‐63. [DOI] [PubMed] [Google Scholar]
Stapley 2012
- Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, et al. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion‐related toxicity. Biochemistry Journal 2012;446:499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2009
- Steiner ME, Stowell C. Does red blood cell storage affect clinical outcome? When in doubt, do the experiment. Transfusion 2009;49:1286‐90. [DOI] [PubMed] [Google Scholar]
Tettamanti 2010
- Tettamanti M, Lucca U, Gandini F, Recchia A, Mosconi P, Apolone G, et al. Prevalence, incidence and types of mild anemia in the elderly: the "Health and Anemia" population‐based study. Haematologica 2010;95:1849‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. Trial Sequential Analysis. Version beta 0.9. Copenhagen, Denmark: Centre for Clinical Intervention Research, Rigshospitalet, 2012.
Vamvakas 1999
- Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion 1999;39:701‐10. [DOI] [PubMed] [Google Scholar]
Vamvakas 2000
- Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion 2000;40:101‐9. [DOI] [PubMed] [Google Scholar]
Vamvakas 2010
- Vamvakas EC. Meta‐analysis of clinical studies of the purported deleterious effects of "old" (versus "fresh") red blood cells: are we at equipoise?. Transfusion 2010;50:600‐10. [DOI] [PubMed] [Google Scholar]
Vamvakas 2011
- Vamvakas EC. Purported deleterious effects of "old" versus "fresh" red blood cells: an updated meta‐analysis. Transfusion 2011;51(5):1122‐3. [DOI] [PubMed] [Google Scholar]
van de Watering 2006
- Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion 2006;46:1712‐8. [DOI] [PubMed] [Google Scholar]
Vincent 2002
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA 2002;288:1499‐507. [DOI] [PubMed] [Google Scholar]
Vora 1989
- Vora S, West C, Beutler E. The effect of additives on red cell 2,3 diphosphoglycerate levels in CPDA preservatives. Transfusion 1989;29:226‐9. [DOI] [PubMed] [Google Scholar]
Walsh 2010
- Walsh TS, Wyncoll DLA, Stanworth SJ. Managing anaemia in critically ill adults. BMJ 2010;341:c4408. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta‐analysis. Transfusion 2012;52(6):1184‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2011
- Wilkinson KL, Brunskill SJ, Doree C, Hopewell S, Stanworth S, Murphy MF, et al. The clinical effects of red blood cell transfusions: an overview of the randomized controlled trials evidence base. Transfusion Medicine Reviews 2011;25:145‐55 e2. [DOI] [PubMed] [Google Scholar]
Zimrin 2009
- Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sanguinis 2009;96:93‐103. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brunskill 2015
- Brunskill SJ, Wilkinson KL, Doree C, Trivella M, Stanworth S. Transfusion of fresher versus older red blood cells for all conditions. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD010801.pub2] [DOI] [PubMed] [Google Scholar]


