Abstract
Background
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are syndromes of severe respiratory failure that are associated with substantial mortality and morbidity. Artifical ventilatory support is commonly required and may exacerbate lung injury. Partial liquid ventilation (PLV) has been proposed as a less injurious form of ventilatory support for these patients. Although PLV has been shown to improve gas exchange and to reduce inflammation in experimental models of ALI, a previous systematic review did not find any evidence to support or refute its use in humans with ALI and ARDS.
Objectives
The primary objective of this review was to assess whether PLV reduced mortality (at 28 d, at discharge from the intensive care unit (ICU), at discharge from hospital and at one, two and five years) in adults with ALI or ARDS when compared with conventional ventilatory support.
Secondary objectives were to determine how PLV compared with conventional ventilation with regard to duration of invasive mechanical ventilation, duration of respiratory support, duration of oxygen therapy, length of ICU stay, length of hospital stay, incidence of infection, long‐term cognitive impairment, long‐term health related quality of life, long‐ term lung function, long‐term morbidity costs and adverse events. The following adverse events were considered: hypoxia (arterial PO2 <80 mm Hg), pneumothorax (any air leak into the pleural space requiring therapeutic intervention), hypotension (systolic blood pressure < 90 mm Hg sustained for longer than two minutes or requiring treatment with fluids or vasoactive drugs), bradycardia (heart rate < 50 beats per minute sustained for longer than one minute or requiring therapeutic intervention) and cardiac arrest (absence of effective cardiac output).
Search methods
In this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL Issue 10, 2012, in The Cochrane Library; MEDLINE (Ovid SP, 1966 to November 2012); EMBASE (Ovid SP, 1980 to November 2012) and CINAHL (EBSCOhost,1982 to November 2012) for published studies. In our original review, we searched until May 2004.
Grey literature was identified by searching conference proceedings and trial registries and by contacting experts in the field.
Selection criteria
As in the original review, review authors selected randomized controlled trials that compared PLV with other forms of ventilation in adults (16 y of age or older) with ALI or ARDS, reporting one or more of the following: mortality; duration of mechanical ventilation, respiratory support, oxygen therapy, stay in the intensive care unit or stay in hospital; infection; long‐term cognitive impairment or health‐related quality of life; long‐term lung function or cost.
Data collection and analysis
Two review authors independently evaluated the quality of the relevant studies and extracted the data from included studies.
Main results
In this updated review, one new eligible study was identified and included, yielding a total of two eligible studies (including a combined total of 401 participants). Of those 401 participants, 170 received 'high'‐dose partial liquid ventilation (i.e. a mean dose of at least 20 mL/kg), 99 received 'low‐dose' partial liquid ventilation (i.e. a dose of 10 mL/kg) and 132 received conventional mechanical ventilation (CMV). Pooled estimates of effect were calculated for all those who received 'high'‐dose PLV versus conventional ventilation. No evidence indicated that 'high'‐dose PLV either reduced mortality at 28 d (risk ratio (RR) 1.21, 95% confidence interval (CI) 0.79 to 1.85, P = 0.37) or increased the number of days free of CMV at 28 d (mean difference (MD) ‐2.24, 95% CI ‐4.71 to 0.23, P = 0.08). The pooled estimate of effect for bradycardia in those who received PLV was significantly greater than in those who received CMV (RR 2.51, 95% CI 1.31 to 4.81, P = 0.005). Pooled estimates of effect for the following adverse events-hypoxia, pneumothorax, hypotension and cardiac arrest-all showed a nonsignificant trend towards a higher occurrence of these events in those treated with PLV. Because neither eligible study addressed morbidity or mortality beyond 28 d, it was not possible to determine the effect of PLV on these outcomes.
Authors' conclusions
No evidence supports the use of PLV in ALI or ARDS; some evidence suggests an increased risk of adverse events associated with its use.
Plain language summary
No evidence of benefit of partial liquid ventilation in adults with acute lung injury and some evidence of increased risk associated with its use
Seriously ill adults can get a severe lung disease called acute lung injury or acute respiratory distress syndrome, which stops enough oxygen from getting into the blood. About half of these patients die, and for those who survive, it can take several years to get back to near normal.
At the height of their illness, many of these patients are unable to breathe properly and need the assistance of a breathing machine called a ventilator, which pushes gas into the lungs under pressure through a process called artifical ventilation. Artifical ventilation can cause further damage to the lungs. The need to support breathing for these patients, while avoiding further lung damage, has led to a search for gentler types of ventilation.
One such gentler type of ventilation is called partial liquid ventilation. It uses a special liquid called perfluorocarbon instead of the gas used by traditional ventilators.
The purpose of this systematic review was to determine whether patients with acute lung injury who received partial liquid ventilation were less likely to die or were more likely to recover completely than those who received traditional gas ventilation.
To provide the best possible answer to this question, this review was conducted in a special preplanned way with the intention of putting together the results of all selected studies to produce an overall measure of the value of partial liquid ventilation. Two eligible studies (including a total of 401 participants) were found, and a comparison was made between those who received similar doses of perfluorocarbon and those who received traditional ventilation. No evidence indicated that partial liquid ventilation reduced the risk of death or the duration of artifical ventilation, and some evidence suggested that it may increase the risk of complications, including low blood oxygen levels, low heart rate, low blood pressure, air leakage from the lungs and cardiac collapse.
Summary of findings
for the main comparison.
| Partial liquid ventilation compared with conventional mechanical ventilation for acute lung injury and acute respiratory distress syndrome | ||||||
|
Patient or population: mechanically ventilated participants with acute lung injury and acute respiratory distress syndrome Settings: Intensive care in Europe and North America Intervention: Partial liquid ventilation Comparison: Conventilation mechanical ventilation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional mechanical ventilation | Partial liquid ventilation | |||||
| 28 d mortality | 1.8 per 1000 | 2.18 per 1000 | 1.21 (0.79 to 1.85) |
302 (2) |
⊕⊕⊝⊝ low1 | |
| Number of days free of mechanical ventilation in a 28 d period | The number of days free of mechanical ventilation in the control groups during a 28 d period ranged from 3.7 to 22.3 d | The mean number of days free of mechanical ventilation in a 28 d period was 2.24 (4.71 to 0.23) d less in the PLV group than in the CMV group | 302 (2) |
⊕⊕⊝⊝ low1 | ||
| Adverse events | ||||||
| Hypoxia | 2.4 per 1000 | 4.2 per 1000 | 1.77 (0.97 to 3.24) |
302 (2) |
⊕⊕⊝⊝ low1 | |
| Pneumothorax | 1.0 per 1000 | 2.0 per 1000 | 2.06 (0.71 to 5.95) |
302 (2) |
⊕⊕⊝⊝ low1 | |
| Hypotension | 2.2 per 1000 | 3.0 per 1000 | 1.38 (0.87 to 2.19) |
302 (2) |
⊕⊕⊝⊝ low1 | |
| Bradycardia | 0.8 per 1000 | 2.0 per 1000 | 2.51 (1.31 to 4.81) |
302 (2) |
⊕⊕⊝⊝ low1 | |
| Cardiac arrest | 0.5 per 1000 | 0.7 per 1000 | 1.31 (0.56 to 3.04) |
302 (2) |
⊕⊕⊝⊝ low1 | |
| *The basis for the assumed risk (e.g. the mean control group risk across included studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1.The 'low' quality grade was assigned on the basis that only two studies were eligible for inclusion in this meta‐analysis, limiting the quantity of data available for analysis, and based on the fact that both studies excluded those with severe nonpulmonary organ dysfunction, limiting the generalizability of these results.
Background
Description of the condition
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are characterized by the development of noncardiogenic pulmonary oedema and hypoxaemia (Bernard 1994). By definition, it is a secondary illness, occurring in response to an initial primary illness or insult, including severe pneumonia, pancreatitis, sepsis, trauma, shock and massive blood transfusion (Bernard 1994; Raneiri 2012). Since it was first described more than four decades ago, ALI remains a major cause of acute respiratory failure, accounts for in excess of 200,000 intensive care unit (ICU) admissions annually and requires more than 3.5 million d of inpatient care per year. It carries a mortality rate of 35% to 44% and substantial survivor morbidity (Ashbaugh 1967; Herridge 2011; Rubenfeld 2005; Rubenfeld 2007).
Pathophysiological changes seen in ALI include endothelial inflammation, increased microvascular permeability, alveolar and interstitial oedema and de‐activation of surfactant, leading to atelectasis, ventilation/perfusion mismatch and reduced lung compliance (Matthay 2011; Ware 2000). The resultant hypoxaemia and increased work of breathing, superimposed on the initial causative acute illness, mean that many patients are unable to maintain effective spontaneous breathing and require artificial ventilatory support (Tobin 2001).
Mechanical ventilation can exacerbate lung injury by causing ventilator‐induced lung injury-a constellation of structural damage caused by volume and pressure changes and biotrauma due to activation of inflammatory cascades (de Prost 2011). This has led to a search for less traumatic ways of providing respiratory support for these patients. Lung protective ventilation strategies, limiting tidal volumes and pressures, have been shown to improve outcomes (ARDS Network 2000). Other techniques that have been explored include the use of nitric oxide, prone positioning, high‐frequency oscillatory ventilation and extracorporal membrane oxygenation (ECMO) (Adhikari 2007; Derdak 2002; Taccone 2009; Combes 2012). To date, none of these interventions have been shown to confer a substantial survival benefit in unselected participants with ALI (Adhikari 2007; Derdak 2002; Taccone 2009; Combes 2012).
Description of the intervention
Partial liquid ventilation has been proposed as a possibly beneficial form of ventilatory support in ALI (Wiedemann 2000). Using liquids to facilitate gas exchange was first proposed in the 1960s, when Kylstra and colleagues showed that mammals could breathe in a liquid medium (Kylstra 1962). Initial experiments using saline required hyperbaric conditions to dissolve enough oxygen in solution (Kylstra 1962). This led to a search for fluids that could carry large amounts of oxygen and carbon dioxide at atmospheric pressure. Only silicone oils and perfluorocarbons were found to have these properties, and as silicone oils proved toxic, only perfluorocarbons were investigated further (Clark 1966). These synthetic fluorinated hydrocarbons are nontoxic, have an oxygen‐carrying capacity three times that of blood and low surface tension and are chemically and metabolically inert, being eliminated by evaporation (Kaisers 2003).
Initial studies of liquid ventilation involved filling the lungs completely with liquid and administering a liquid tidal volume. This technique, which is called total liquid ventilation, necessitates the use of a specially designed ventilator (Kaisers 2003). It remains largely experimental.
In 1991, Fuhrman and colleagues described an animal experiment in which they filled the lungs to functional residual capacity with perfluorocarbon (PFC) and provided a gaseous tidal volume (Fuhrman 1991). This technique, in which the lungs are partially filled with liquid, is called partial liquid ventilation (PLV) (Kaisers 2003). It can be used with commercially available ventilators and has been the focus of much study in both animals and humans (Hernan 1996; Hirschl 1995 Effects of interventions; Hirschl 1996; Wiedemann 2000).
How the intervention might work
The rationale for the use of PLV in ALI centres on the physical properties of perfluorocarbons, which make them particularly useful in the context of increased surface tension at the air‐gas interface, which occurs in this condition (Kaisers 2003; Matthay 2011).
The very low surface tension of PFCs gives them surfactant‐like properties, thereby improving lung compliance. Twice as dense as water, they gravitate to dependent regions of the lung, reopening collapsed alveoli and acting as liquid positive end‐expiratory pressure (PEEP) (Davies 1999; Kaisers 2003). Perfluorocarbons are thought to improve ventilation/perfusion matching by compressing blood vessels (through PFC‐filled alveoli) in dependent regions of the lung, with consequent diversion of blood flow to the gas‐filled alveoli in nondependent regions (Davies 1999; Kaisers 2003). The kinetic effects of liquid ventilation may assist with mobilization of debris and secretions (Tawfic 2010). Aside from their physical properties, animal studies have shown that PFCs have anti‐inflammatory effects that may reduce lung injury and inflammation (Pakulla 2004: Zhu 2010).
Why it is important to do this review
It has been eight years since the original review of this topic was conducted. Since then, important advances have been made in our approach to ventilatory support of patients with ALI (ARDS Network 2000), and awareness has increased regarding the importance of quality in the reporting of randomized controlled trials (RCTs) (Dechartres 2011; Falagas 2009).
Objectives
The primary objective of this review was to assess whether PLV reduced mortality (at 28 d, at discharge from the intensive care unit (ICU), at discharge from hospital and at one, two and five years) in adults with ALI or ARDS when compared with conventional ventilatory support.
Secondary objectives were to determine how PLV compared with conventional ventilation with regard to duration of invasive mechanical ventilation, duration of respiratory support, duration of oxygen therapy, length of ICU stay, length of hospital stay, incidence of infection,long‐term cognitive impairment, long‐ term health related quality of life, long‐term lung function, long‐term morbidity costs and adverse events. The following adverse events were considered: hypoxia (arterial PO2 < 80 mm Hg), pneumothorax (any air leak into the pleural space requiring therapeutic intervention), hypotension (systolic blood pressure < 90 mm Hg sustained for longer than two minutes or requiring treatment with fluids or vasoactive drugs), bradycardia (heart rate < 50 beats per minute sustained for longer than one minute or requiring therapeutic intervention) and cardiac arrest (absence of effective cardiac output).
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs.
Types of participants
We included mechanically ventilated adults with ALI ± ARDS.
We defined adults as persons aged 16 y or older.
ALI and ARDS were defined according to American European Consensus Conference (AECC) criteria (i.e. the definition in use until 2012).
Definition of ALI (Bernard 1994):
Acute onset of respiratory failure;
Bilateral opacities on chest x‐ray (CXR) consistent with pulmonary oedema;
Pulmonary artery wedge pressure < 18 mm Hg or no clinical evidence of raised left atrial pressure; and
Partial pressure of oxygen in the blood (PaO2)/fraction of inspired oxygen (FiO2) ratio ≤300 mm Hg.
Definition of ARDS (Bernard 1994):
Acute onset of respiratory failure;
Bilateral opacities on CXR consistent with pulmonary oedema;
Pulmonary artery wedge pressure < 18 mm Hg or no clinical evidence of raised left atrial pressure; and
PaO2/FiO2 ≤ 200 mm Hg.
Types of interventions
PLV compared with other forms of ventilatory management without the use of PFC.
Types of outcome measures
One or more of the following outcomes must be reported.
Primary outcomes
Mortality (at 28 d, at discharge from ICU, at discharge from hospital and at one, two and five years).
Secondary outcomes
Duration of invasive mechanical ventilation (i.e. duration of ventilatory support delivered via endotracheal tube or tracheostomy).
Duration of respiratory support (i.e. duration of any form of artifical support with ventilation, including both invasive ventilation as defined above and noninvasive ventilation delivered via face mask, nasal mask or hood.
Duration of oxygen therapy.
Length of stay in the ICU.
Length of stay in hospital.
Infection (sepsis, pneumonia).
Long‐term cognitive impairment.
Long‐term health‐related quality of life.
Long‐term lung function.
Long‐term morbidity costs.
Adverse events.
Search methods for identification of studies
Electronic searches
In this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 10, 2012, in The Cochrane Library;Appendix 1); MEDLINE (Ovid SP, 1966 to November 2012; Appendix 2); EMBASE (Ovid SP, 1980 to November 2012; Appendix 3) and CINAHL (EBSCOhost, 1982 to November 2012; Appendix 4). In the previous version (Davies 2004), the databases were searched to May 2004.
We identified RCTs of PLV in ALI or ARDS from MEDLINE using the Medical Subject Heading (MeSH) 'RESPIRATORY DISTRESS SYNDROME, ADULT' or the text words 'ARDS', 'ALI' or 'acute lung injury' and the MeSH heading 'FLUOROCARBONS' or the text word 'partial liquid ventilation'.
Searching other resources
We searched the proceedings of major annual Critical Care conferences (i.e. American Thoracic Society, International Symposium on Intensive Care and Emergency Medicine, Society of Critical Care Medicine, European Society of Intensive Care Medicine, American College of Chest Physicans, Intensive Care Society UK and Canadian Critical Forum).
We searched the metaRegister of Controlled Trials for relevant ongoing trials using the search terms 'liquid ventilation', 'partial liquid ventilation', 'liquid ventilation', 'acute respiratory distress syndrome', 'acute lung injury', 'ALI', 'ARDS' and 'perfluorocarbon'.
Finally we contacted experts in the field of PLV research to identify unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (IMG and AS) independently selected studies according to the following process. We assessed each title and abstract retrieved by the search strategy to determine whether they met the eligibility criteria for inclusion. We excluded studies that were clearly ineligible (animal studies and those addressing interventions other than liquid ventilation) at this stage. For all other studies, we examined full‐text versions to determine eligibility. We assessed methodological quality using the criteria detailed above, and the study was excluded or accepted for inclusion. We resolved any discrepancies by discussion between these two review authors. One review author (IMG) was responsible for contacting primary study authors when additional details were required for determination of study eligibility and quality.
Data extraction and management
A comprehensive data extraction form was designed by one review author (IMG), using all items in the checklist recommended in Section 7 of the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011). Two review authors (IMG and AS) independently extracted data. We entered data initially onto the data extraction form and then into the appropriate fields in RevMan. RevMan entries were checked against the primary study report by two review authors (IMG and AS).
Assessment of risk of bias in included studies
The risk of bias in included studies was assessed independently by two review authors (IMG, AS), using the Cochrane Collaboration tool for assessing risk of bias, as described in Section 8 of the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011). We resolved any discrepancies by discussion between the assessing review authors. For each included primary study, bias was assessed in the domains detailed below. For each domain, we determined the risk of bias as 'high', 'low' or 'unclear', and each judgement was supported by direct quotes from the primary reports, where applicable.
Random sequence generation
Probably done/low risk: where the study report described a random method of sequence generation.
Probably not done/high risk: where the study report did not describe a random method of sequence generation.
Uncertain/unclear risk: where the study report did not provide enough information to reveal whether or not a random method was used.
Allocation concealment
Probably done/low risk: where the study report described a centralized method of intervention allocation or a method by which investigators could not feasibly foresee the treatment assignments of individual participants.
Probably not done/high risk: where the study report did not describe a centralized method of intervention allocation or a method by which investigators could not feasibly foresee the treatment assignments of individual participants.
Uncertain/unclear risk: where the study report did not provide enough information to reveal whether or not the method used was likely to ensure allocation concealment.
Performance bias
Blinding of participants and personnel.
Probably done/low risk: where the study report described blinding of participants and key study personnel.
Probably not done/low risk: where the study report did not describe a blinded method, or blinding was incomplete but measures were taken to ensure and determine equality of ancillary treatment between groups.
Probably not done/ high risk: where the study report did not describe a blinded method, or blinding was incomplete and measures were not taken to ensure and determine equality of ancillary treatment between groups.
Probably not done/ unclear risk: where the study report did not describe a blinded method, or blinding was incomplete and the authors did not describe measures taken to ensure and determine equality of ancillary treatment between groups.
Detection bias
Blinding of outcome assessment.
Probably done/low risk: where the study report described blinding of participants and key study personnel.
Probably not done/low risk: where the study report did not describe a blinded method, or blinding was incomplete but outcome measures addressed were objective.
Probably not done/ high risk: where the study report did not describe a blinded method, or blinding was incomplete and outcome measures addressed were subjective.
Probably not done/ unclear risk: where the study report does not provide enough information to reveal the extent or adequacy of blinding or its likely impact on outcome assessment.
Attrition bias
Incomplete outcomes data.
Low risk: no missing outcome data for prespecified outcomes, or missing data equally balanced between groups, or reasons for missing data unlikely to be related to the intervention or to the outcome or unlikely to have a significant influence on the effect estimate or missing data dealt with by using appropriate imputation methods.
High risk: missing data for prespecified outcomes and any of the following missing data unequally balanced between groups, or reasons for missing data likely to be related to the intervention or to the outcome or likely to have a significant influence on the effect estimate or missing data not dealt with by using appropriate imputation methods.
Unclear risk: the study report does not provide enough information to reveal the extent of missing data or the influence of missing data on study results.
Reporting bias
Outcome reporting bias.
Low risk: all prespecified outcomes reported completely.
High risk: one or more prespecified outcomes not reported or reported incompletely.
Unclear risk: not enough information to reveal the adequacy of outcome reporting.
Other bias
Low risk: no other potential source of bias identified.
High risk: other potential sources of bias identified.
Unclear risk: report not complete enough to allow accurate determination of the presence or absence of other sources of bias.
Measures of treatment effect
We performed the meta‐analyses using Review Manager software (RevMan 5.1). We included two eligible studies and compared all participants who received similar dosages of perflurocarbon with those who received conventional mechanical ventilation. We used a random‐effects model and calculated categorical outcomes as risk ratios (RRs) and continuous outcomes as mean differences (MDs), with 95% confidence intervals (CIs) for both.
Unit of analysis issues
None identified.
Dealing with missing data
For all missing data, we planned to determine whether data were 'missing completely at random', missing at random' or 'missing not at random'. Data were judged to be 'missing completely at random' and 'missing at random' if the reasons the data were missing were 'highly unlikely' and 'unlikely', respectively, to be related to the intervention or the outcome being addressed. Data were judged to be 'missing not at random' if the reason the data were missing was 'likely' related to the intervention or to the outcomes being addressed. For data judged to be 'missing not at random', we planned to determine the degree of influence this was likely to have on the effect estimates from the study and on the adequacy of statistical measures used to take account of the missing data.
Where appropriate alternative analyses were used to take account of missing data, we planned to use effect estimates from those analyses to contribute to our pooled estimates of effect.
We planned to discuss all available details regarding missing data and the likely impact of this on the findings of this review, and, if appropriate, to conduct a sensitivity analysis to evaluate the potential impact of incomplete outcome data on the pooled estimate of effect.
Assessment of heterogeneity
Included studies were assessed for clinical heterogeneity by comparing the following factors: study participants, setting, interventions and ancillary treatments. Methodological heterogeneity was assessed by determining and comparing the risk of bias in included studies.
Where included studies were adequately homogenous in terms of clinical and methodological aspects, statistical heterogeneity was assessed by performing a visual inspection of forest plots and the Chi2 test (assessing the P value) and by calculating the I2 statistic. A P value less than 0.10 and I2 in excess of 50% were taken as indicative of significant heterogeneity.
Assessment of reporting biases
To determine the presence or absence of reporting bias, we planned to examine funnel plots for each meta‐analysis that included ten or more studies to determine if they were symmetrical. Where plots were visually asymmetrical, the meta‐analysis was judged to be potentially biased because of small study effects or reporting bias.
Data synthesis
It was planned that a meta‐analysis would be performed if all of the following conditions were met:
No significant clinical or statistical heterogeneity of the included studies;
Inclusion of at least two eligible studies deemed to have low individual risks of bias; and
No substantial reporting bias.
Subgroup analysis and investigation of heterogeneity
If adequate numbers of eligible studies were identified (at least three), we planned to perform subgroup analyses to determine whether the results differ by:
Population:
Age;
Severity of overall illness (e.g. Acute Physiology and Chronic Health Evaluation (APACHE) score or Simplified Acute Physiology Score (SAPS)) or severity of ALI or ARDS; and
Aetiology of ALI or ARDS (e.g. septicaemia, pneumonia, trauma, burns).
Mortality and other outcomes have been shown to vary by the age of the patient, the initial severity of ALI or ARDS or of the patient's condition (e.g. by APACHE score) and the underlying cause of ALI or ARDS (Monchi 1998; Suntharalingam 2001; Ware 2000).
Intervention:
Initial amount or dose of perflurocarbon (PFC);
Whether continuous PLV or intermittent doses of PFC are used; and
Type of PFC (e.g. perflubron, Rimar).
The optimal dose of PFC that should be used when PLV is initiated is unknown. Variations in the technique of PLV may also include giving an initial dose of PFC with or without further top‐up doses to maintain partial filling of the lungs. Various types of PFC with different physical and chemical properties may be used (Davies 1999).
Co‐interventions used in addition to PLV:
Inhaled nitric oxide;
Surfactant;
The prone position; and
High‐frequency ventilation.
Whilst the mainstay of treatment for ALI or ARDS is mechanical ventilation, additional therapies have been considered, and some of these have been subjected to RCTs. Adjuncts to mechanical ventilation have included inhaled nitric oxide, endogenous surfactant, prone positioning and high‐frequency ventilation (Conner 2000); all can be used in conjunction with PLV.
Sensitivity analysis
If adequate numbers of eligible studies of good methodological quality were identified (three or more), we planned to conduct the following sensitivity analyses to test how robust our findings are under the following conditions:
Analysis excluding all studies considered to be at high or unclear risk of bias; and
Analysis excluding studies with missing data considered to be 'missing not at random'.
Results
Description of studies
Results of the search
In combining our original searches from the period 1966 to May 2004 with updated searches covering the period from May 2004 to November 2012, we retrieved a total of 749 citations. We reviewed the titles and abstracts and excluded 739 citations at this stage, as it was clear from the abstract that they would not be eligible for inclusion. These included in vitro and animal studies, studies of interventions other than liquid ventilation, studies not performed in adults, review articles, commentaries and duplicate reports .
Of the remaining 10 articles, we excluded seven, as they were not randomized controlled trials; one was excluded because it had not yet been completed, and the remaining two were found to be eligible for inclusion (Figure 1).
1.
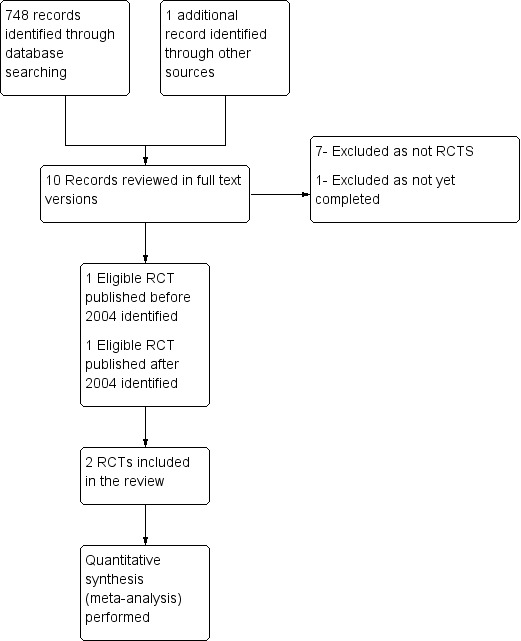
Study flow diagram.
Included studies
Two studies were eligible for inclusion in this updated review. The first study (Hirschl 2002) was the only study included in the original review because although the second study (Kacmarek 2006) had been completed at the time the original review was conducted, the results were not published until 2006.
The study included in the original review (Hirschl 2002) was a multi‐centred RCT conducted between July 1995 and August 1996 at 18 centres in North America. This was a pilot study that sought to evaluate the safety and efficacy of PLV in adults with ARDS. The mean dose of PFC used was 22 mL/kg. As detailed below (Characteristics of included studies), this study raised some concerns related to changes in oxygenation criteria for recruitment and the exclusion of patients with multiple organ failure, both of which may influence the generalizabilty of results.
The additional study included in this review (Kacmarek 2006) was a multi‐centred RCT that was conducted at 56 centres in Europe and North America between December 1998 and December 2000. It enrolled 311 participants, achieving its target sample size, although the sample size was changed on two occasions in response to protocol amendments and interim analyses. Investigators evaluated two doses of PFC-'low dose' of 10 mL/kg and 'high dose' of 20 mL/kg-using a control group that received CMV alone. All three groups received standardized ventilatory support, target gas exchange criteria for weaning were defined clearly a priori and the numbers of weaning attempts per day were equal between groups when these criteria were met and were not met. The study was generally well conducted and was clearly reported. The long period between the end of the study and publication of results, as highlighted by the authors, emphasizes the problems associated with publication of negative trials and the importance of negative results to medical knowledge.
For both studies, participant follow‐up appeared complete, and no missing data were identified.
Neither of the included studies (Hirschl 2002 ;Kacmarek 2006) addressed mortality beyond 28 d, duration of stay in intensive care or of stay in hospital, morbidity, quality of life or cost‐effectiveness.
Excluded studies
We excluded a total of seven completed studies from the review. Details of these are provided in the Characteristics of excluded studies table. Apart from one new included study (Kacmarek 2006), no new completed human adult studies in ARDS/ALI participants of any design were identified in the new search (May 2004 to November 2012). Most reports on PLV were animal studies, review articles, letters or commentaries.
Risk of bias in included studies
We assessed risk of bias using the methods detailed above. We have detailed the results in the Characteristics of included studies section and have summarized them in Figure 2 and in Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Other bias-bias resulting from imbalance in baseline prognostic variables and competing interests.
3.
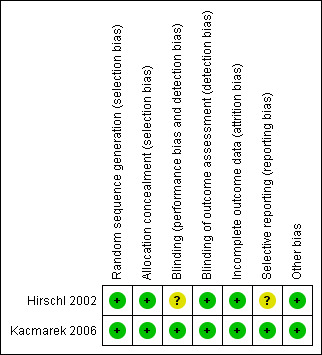
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Other bias-bias resulting from imbalance in baseline prognostic variables and competing interests.
Allocation
In the study by Hirschl and colleagues (Hirschl 2002), the allocation sequence was generated randomly, and a centralized intervention assignment was used. These factors reduced the risk of selection bias. However, participants randomly assigned to PLV were randomly assigned on average 25 hours after other participants were randomly assigned to conventional ventilation. Those in the PLV group therefore had a longer duration of mechanical ventilation before randomization and may have been at a different stage in their disease process compared with those in the conventional ventilation group.The trial by Kacmarek and colleagues (Kacmarek 2006) used a randomized computer method to generate the allocation sequence. Methods used to ensure allocation concealment are not detailed in the trial report. The corresponding author was contacted for clarification, but no further information was obtained. However, given that a computer generated method of allocation was used, the overall risk of selection bias due to these elements was judged to be low.
Blinding
Neither of the two included studies (Hirschl 2002; Kacmarek 2006) used blinding of participants or of key study personnel. However, given that the outcome measures of both studies were unambiguous and objective, the lack of blinding alone is unlikely to have had a significant impact on outcome ascertainment. In the study by Hirschl (Hirschl 2002), investigators did have access to the interim data and were aware of the preliminary results. It was on this basis that they decided to change the inclusion criteria. Also although ventilation management guidelines were provided, no measures were taken to ensure or to measure adherence to these guidelines, and no guidelines were provided regarding the discontinuation of PLV. It is therefore possible that participants may not have been treated equally with respect to ventilation management, weaning decisions and discontinuation of PLV.
Incomplete outcome data
The outcome data were assumed to be complete for both included studies based on the fact that all enrolled participants were followed up in both studies. The study by Kacmarek (Kacmarek 2006) provides a clear statement of follow‐up: “311 patients who were enrolled were followed up for the 28 day study period". The trial by Hirschl (Hirschl 2002) does not provide such a statement but does provide enough information, based on adverse event data to allow the reasonable assumption that all enrolled participants were followed up for outcome assessment.
Selective reporting
No selective reporting bias was judged to be present in either of the two included studies (Hirschl 2002; Kacmarek 2006).
Other potential sources of bias
The risk of bias from other sources was judged to be low in both studies (Hirschl 2002; Kacmarek 2006).
Effects of interventions
See: Table 1
The two identified eligible studies addressed similar populations, interventions and outcomes and were therefore reasonably clinically homogenous.The trial by Kacmarek (Kacmarek 2006) employed two doses of PLV-a higher dose of 20 mL/kg and a lower dose of 10 mL/kg. Only the higher dose was used to calculate pooled estimates of effect for the outcomes below, as the lower dose was substantially less than the mean dose of 22 mL/kg used in the trial by Hirschl (Hirschl 2002). Assessment of statistical heterogeneity is limited by the small number of included studies, but the CIs for all comparisons overlap, and I2 values were 0 and 20% for 28 mortality‐free and ventilator‐free days, respectively.
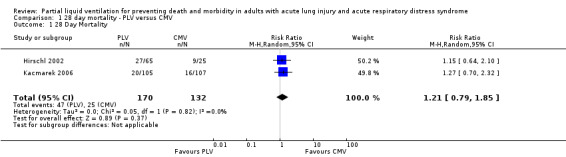
Mortality
The overall pooled estimate for the RR of mortality at 28 d of PLV was 1.21 (95% CI 0.79 to 1.85, P = 0.37) (Figure 4) .
4.

Forest plot of comparison 1. 28 day mortality, PLV versus CMV Outcome:28 Day Mortality.
Both included studies suggested a trend towards higher mortality when PLV was used, but neither study showed a significant difference. Hirschl 2002 reported a 28 day mortality of 36% in the CMV group and of 42% in the PLV group (RR of death with use of PLV 1.15, P = 0.63). Kacmarek 2006 showed a 28 day mortality of 15% in the CMV group compared with 26.3% in the low‐dose PLV group (RR 1.75, P = 0.06) and 19% in the high‐dose PLV group (RR 1.27, P = 0.39).
Duration of ventilation
'Days free of mechanical ventilation' was used in both eligible trials as a measure of duration of ventilation. The overall pooled estimate of the effect of PLV on days free of mechanical ventilation at 28 d was an MD of ‐2.24 (95% CI ‐4.71 to 0.23, P = 0.08) (Figure 5) .
5.
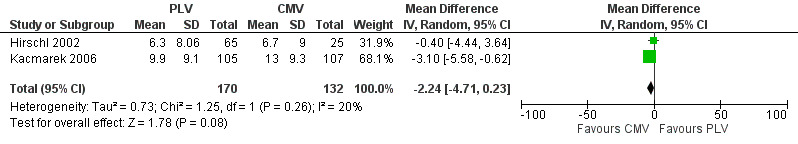
Forest plot of comparison 2.1. Ventilator‐Free Days ‐ PLV versus CMV ‐ Outcome: Days Free of Mechanical Ventilation at Day 28.
Hirschl 2002 did not show a significant difference in the number of days free from the ventilator at 28 d between those who received PLV and those who received CMV (CMV = 6.7 d (standard deviation (SD) 9), PLV = 6.3 d (SD 8.06), MD ‐0.40, P = 0.85). Kacmarek 2006 showed significantly more days free from the ventilator at 28 d in the CMV group than in the low‐dose PLV group (CMV = 13 d (SD 9.3), low dose PLV = 7.4 d (SD 8.5), MD 5.6 d, P < 0.001) and in the high‐dose PLV group (high‐dose PLV = 9.9 d (SD 9.1), MD 3.10, P = 0.043).Time to unassisted ventilation was significantly shorter in the CMV group than in both the low‐dose PLV group (12.5 vs 18.9 days, MD 6.2 d, P < 0.001) and the high‐dose PLV group (12.5 vs 13.9 d, MD 1.4 days, P = 0.017). Also the percentage of participants alive and off ventilation at 28 d was significantly greater in the CMV group than in the low‐dose PLV group (76% vs 53%, P < 0.001) or the high‐dose PLV group (76% vs 61%, P = 0.027).
Adverse events
Both studies reported data on the following adverse events: hypoxia, pneumothoraces, hypotension, bradycardia and cardiac arrest. Pooled estimates of effect were calculated for each of these outcomes. The RR of bradycardia in those who received PLV was 2.51 (95% CI 1.31 to 4.81, P = 0.005) (Figure 6). Pooled estimates for other adverse events in those who received PLV were as follows: hypoxia RR 1.77 (95% CI 0.97 to3.24, P = 0.06), pneumothorax RR 2.06 (95% CI 0.71 to 5.95, P = 0.18), hypotension RR 1.38 (95% CI 0.87 to 2.18, P = 0.22), cardiac arrest RR 1.31 (95% CI 0.56 to 3.04, P = 0.54).
6.

Forest plot of comparison 3.4. Adverse Events ‐ Outcome: Bradycardia.
Both included studies reported high numbers of PLV‐related adverse events. Kacmarek reported statistically significantly more adverse events in those receiving PLV (Kacmarek 2006) and noted that most episodes of hypoxia and hypotension occurred during the first five days of drug delivery; investigators attributed these events to the need to interrupt ventilatory support to administer the PFC. A similar observation was made by Hirsch, with most adverse events occurring at the time of PFC dosing (Hirschl 2002).
Other outcomes
Duration of respiratory support, duration of oxygen therapy, length of stay in the ICU, length of stay in the hospital, infection (sepsis, pneumonia), long‐term cognitive impairment, long‐term health related quality of life, long‐term lung function and costs were not addressed by either trial.
Discussion
Summary of main results
Mortality
This review suggests a trend toward higher 28 day mortality with the use of PLV versus CMV; however, the difference did not reach statistical significance (RR 1.21, 95% CI 0.79 to 1.83, P = 0.37).
Duration of ventilation
Similarly, this review suggests a trend toward fewer ventilator‐free days when PLV versus CMV is used (MD 2.24 d, 95% CI 4.71 to ‐ 0.23, P = 0.08).
Adverse events
Participants who received PLV were significantly more likely to suffer bradycardia (RR 2.51, CI 1.31 to 4.81, P = 0.005), and the pooled estimates of effect for other adverse events uniformly suggested trends toward higher risk for those receiving PLV, but these findings were not statistically significant.
Overall completeness and applicability of evidence
The findings of this review are subject to the following important limitations.
First, there is a lack of evidence. Only two studies were eligible for inclusion in this review (Hirschl 2002; Kacmarek 2006).
Second, both studies used ventilator‐free days as the primary endpoint. Ventilator‐free days is a rather ambiguous concept that when used as a primary outcome measure carries an unacceptably high risk of showing that a treatment that increases mortality is actually superior (Schoenfeld 2002); it is an outcome of dubious clinical relevance. Although 28 day mortality serves as a slightly more informative outcome measure, it provides vital outcome information only up to this limited time point. Neither study measured morbidity, which is known to be substantial and persistent in survivors of ALI/ARDS (Herridge 2011). Unfortunately, the current ongoing study of PLV in adults with ALI is assessing the oxygenation index and pulmonary mechanics as primary outcome measures, with survival again reported as a secondary outcome measure (Chen 2011).
Third, this review used the AECC definition (Bernard 1994) of ARDS to identify those used in the individual studies and in the original review (Davies 2004). Both included studies excluded patients with serious nonpulmonary organ dysfunction and severe shock. Multiple‐organ failure is the most common cause of death in those who die with ALI; therefore, the findings of the included studies may not be representative of patients with ARDS in general (Stapleton 2005).
Finally, both studies preceded the era of lung protective ventilation and used tidal volumes well in excess of 6 mL/kg (Hirschl 2002 used mean tidal volumes of 9 mL/kg actual body weight, and Kacmarek 2006 used tidal volumes of 9 mL/kg predicted body weight). Both the intervention groups and the control groups received similar tidal volumes; therefore, it is unlikely that tidal volume had a significant influence on the results of either study. However, we cannot be sure what role if any PLV has when used in accordance with ARDS Network guidelines (ARDS Network 2000).
Given the limitations detailed above, our findings should be treated with caution. It is possible that additional research that uses more participant‐relevant outcomes, current definitions of ARDS (The ARDS Definition Task Force 2012) and lung protective ventilation strategies may produce different results.
Quality of the evidence
The quality of both studies was reasonable in terms of risk of bias; however, both studies were subject to the limitations detailed above. Futhermore, both studies excluded patients with severe nonpulmonary organ dysfunction, limiting the generalizabilty of their results and hence of the results of this review .
Potential biases in the review process
Every effort was made to minimize selection and reporting bias by comprehensively searching both mainstream and grey literature to identify all relevant studies, with two review authors (IMG and AS) separately applying a priori defined selection criteria to select appropriate studies for inclusion.
The small number of eligible studies may contribute to content bias. The findings of this review and meta‐analysis need to be interpreted in the light of these limitations.
Agreements and disagreements with other studies or reviews
The findings of this review are consistent with those of existing studies in humans, namely, that PLV has not been shown to offer any survival benefit over CMV in participants with ALI and ARDS. On the basis of results of the most recent human studies, PLV in fact may be inferior to CMV, and it may be associated with increased risk of adverse events (Kacmarek 2006;Hirschl 2002). Several narrative reviews include sections on PLV, all of which acknowledge that in the light of current evidence, this strategy cannot be recommended in ALI/ARDS (Anzueto 2006; Lynch 2006).
The results of animal studies differ from those of human studies and of this review. Several animal models of ALI have shown promising results in terms of improvements noted as reduced inflammation and improved oxygenation and short‐term survival (Pakulla 2004; Zhu 2010). However, as is often the case, promising animal data do not necessarily translate into useful treatments for humans.
Authors' conclusions
Implications for practice.
No evidence supports the use of PLV in adults with ALI or ARDS, and some evidence suggests that PLV may be associated with increased risk of adverse events.
Implications for research.
Although no current evidence supports the use of PLV in practice, available evidence is limited. Further clinical research may therefore be appropriate but would require very careful monitoring for adverse events and would be most informative if it employed the current definition of ARDS and lung protective ventilation strategies, and if it addressed morbidity as well as mortality beyond 28 d.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 9 August 2013 | Amended | Acknowledgement section updated. |
| 10 July 2013 | New search has been performed | This review is an update of a previous Cochrane systematic review of the same title (Davies 2004) that included only one study. The literature search for this current version was extended from May 2004 to November 2012, with preservation of the original criteria for study inclusion and review objectives. The abstract, background, plain language summary, methods, results and discussion sections were rewritten to take account of relevant new information on acute lung injury and partial liquid ventilation and current guidance for the completion of Cochrane reviews (Higgins 2011). Risk of bias assessment with tables and figures was included. One new eligible study was identified and included (Kacmarek 2006), yielding a total of two eligible studies. Pooled estimates of effect of partial liquid ventilation on mortality, days free of mechanical ventilation at 28 days and adverse events were calculated. |
| 10 July 2013 | New citation required and conclusions have changed | This update was conducted by four new team members (IMG, AS, NDF, RP) working together with one of the original review authors (MWD). The findings of this updated review differed slightly from those of the original review (Davies 2004) in that previously no evidence was found to support or refute the role of partial liquid ventilation in adults with ALI/ARDS; new evidence suggests that it is not superior to conventional mechanical ventilation and may be associated with a higher incidence of adverse events. |
| 2 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Mike Bennett (content editor), Nathan Pace (Statistical editor), Rodrigo Cavallazzi, Neill Adhikari (peer reviewers) and Janet Wale (consumer editor) for their help and editorial advice during the preparation of this updated systematic review.
We would like to thank the following individuals who reviewed and commented on Davies 2004 before its initial publication: Dr Mike Bennett, Prof Nathan Pace, Dr Antonio Anzueto, Dr John Carlisle, Dr Ann Møller, Janet Wale and Nete Villebro.
We also would like to thank JF Fraser, who was a review author of the first version of this review (Davies 2004).
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Respiratory Distress Syndrome, Adult explode all trees #2 MeSH descriptor Acute Lung Injury explode all trees #3 ALI or ARDS or acute lung injury #4 (#1 OR #2 OR #3) #5 MeSH descriptor Liquid Ventilation explode all trees #6 MeSH descriptor Fluorocarbons explode all trees #7 ventilation #8 (#5 OR #6 OR #7) #9 (#4 AND #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp RESPIRATORY DISTRESS SYNDROME, ADULT/ or exp Acute Lung Injury/ or (ALI or ARDS or acute lung injury).mp. 2. exp FLUOROCARBONS/ or exp Liquid Ventilation/ or fluorocarbon*.af. or (liquid adj3 ventilation).mp. 3. 1 and 2
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp respiratory‐distress‐syndrome/ or exp respiratory‐distress/ or exp acute‐lung‐injury/ or (ARDS or ALI or (acute adj3 lung injur*)).mp. 2. exp fluorocarbon‐/ or exp liquid‐ventilation/ or fluorocarbon*.af. or (liquid adj3 ventilation).mp. 3. 1 and 2
Appendix 4. Search strategy for CINAHL (EBSCO host)
S1 ((MM "Respiratory Distress Syndrome, Acute") OR (MM "Respiratory Distress Syndrome+") OR (MM "Acute Lung Injury+") ) OR TX ( ALI or ARDS or acute lung injury ) S2 ( (MM "Fluorocarbons") OR (MM "Ventilation, Liquid") ) OR TX fluorocarbon* OR TX liquid ventilation S3 S1 and S2
Appendix 5. Acknowlegements
We would like to thank Mike Bennett (content editor), Nathan Pace (statistical editor), Rodrigo Cavallazzi and Neill Adhikari (peer reviewers) and Janet Wale (consumer editor) for their help and editorial advice during the preparation of this protocol for the systematic review.
Data and analyses
Comparison 1. 28 day mortality ‐ PLV versus CMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 28 Day Mortality | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.79, 1.85] |
1.1. Analysis.
Comparison 1 28 day mortality ‐ PLV versus CMV, Outcome 1 28 Day Mortality.
Comparison 2. Ventilator Free Days ‐ PLV versus CMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days Free of Mechanical Ventilation at Day 28 | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐4.71, 0.23] |
2.1. Analysis.

Comparison 2 Ventilator Free Days ‐ PLV versus CMV, Outcome 1 Days Free of Mechanical Ventilation at Day 28.
Comparison 3. Adverse Events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypoxia | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.97, 3.24] |
| 2 Pneumothorax | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.71, 5.95] |
| 3 Hypotension | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.87, 2.19] |
| 4 Bradycardia | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.31, 4.81] |
| 5 Cardiac Arrest | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.56, 3.04] |
3.1. Analysis.

Comparison 3 Adverse Events, Outcome 1 Hypoxia.
3.2. Analysis.
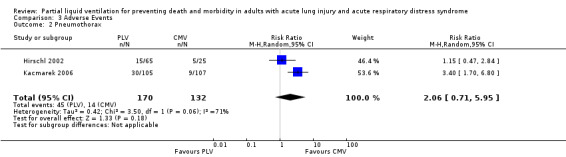
Comparison 3 Adverse Events, Outcome 2 Pneumothorax.
3.3. Analysis.
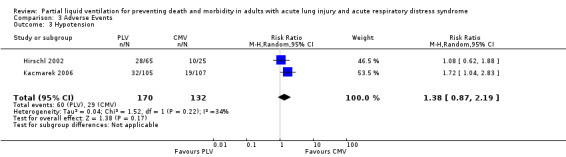
Comparison 3 Adverse Events, Outcome 3 Hypotension.
3.4. Analysis.
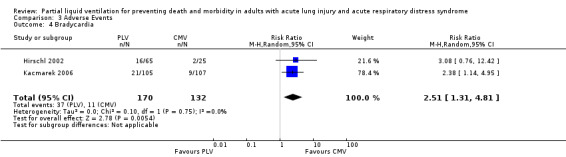
Comparison 3 Adverse Events, Outcome 4 Bradycardia.
3.5. Analysis.
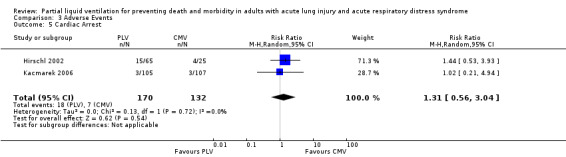
Comparison 3 Adverse Events, Outcome 5 Cardiac Arrest.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hirschl 2002.
| Methods | Multi‐centre, randomized controlled trial Done between July 1995 and August 1996 | |
| Participants | 90 participants with ALI/ARDS from 18 centres in the USA Inclusion criteria:
The first 45 participants were stratified according to Murray lung Injury score ≤ 2.5 or > 2.5. They had their PaO2/FiO2 ratios determined at an FiO2 of 1 The second 45 participants also had to have an APACHE 2 score of < 30. They had their PaO2/FiO2 ratios determined at an FiO2 of ≥ 0.5 Exclusion criteria:
|
|
| Interventions | Randomly assigned to receive PLV or CMV for a maximum of four days for the first 45 participants and a maximum of five days for subsequent participants. Groups were allocated at a PLV‐to‐CMV ratio of 2:1. Perflurocarbon was administered at a dosage of 5 mL/kg increments based on ideal body weight to a maximum of 30 mL/kg. Each participant was assessed every four hours for the presence of a meniscus visible within the endotracheal tube during transient ventilator disconnect. If none was present, an additional 1 to 5 mL/kg aliquot of perflubron was administered. PLV was discontinued at the discretion of the investigator; no guidelines or rules regarding discontinuation of PLV were provided The mean duration of perflubron administration was 80 ± 3 h, with a range of 17 to 120 h | |
| Outcomes |
Primary outcome:
Secondary outcomes included:
Results: This study showed no difference in:
Adverse events: The authors do not provide P values or confidence intervals for all adverse event outcomes but report higher incidence of adverse events in those receiving PLV (99%) than in those receiving CMV (96%). Hypoxia, hypotension, pneumothoraces, bradycardia, respiratory acidosis and cardiac arrest were all more common in the partial liquid ventilation group Other relevant outcomes that were not reported: The following outcomes, which we consider clinically relevant, were not reported:
|
|
| Notes | There are some concerns with this study regarding the following: Methodological rigour and external validity: Concerns regarding methodological rigour and generalizability of results due to changes in selection criteria and primary endpoints during the course of the study and exclusion of patients with refractory shock and renal, hepatic and haematological dysfunction Adverse event reporting: The authors conclude that “PLV may be performed reasonably safely in adult patients with respiratory failure with few adverse events, which appear to be transient, self limited and with appropriate vigilance, manageable”. This statement does not accurately represent the findings of the study, which showed a higher incidence of hypoxia, hypotension, pneumothoraces, bradycardia and cardiac arrest in the partial liquid ventilation group, most of which occurred at times of perfluorocarbon dosing, and at least four episodes of cardiac arrest were attributable to treatment in this group Miscellaneous: A large number of post hoc analyses are reported. Post hoc analyses showed more rapid discontinuation of ventilation in the PLV arm (P = 0.045), although participants who were randomly assigned to PLV had a longer length of CMV before randomization (P = 0.12). The time lag involved may explain the difference in rapidity of discontinuation, as those randomly assigned later may already be in the recovery phase of their illness 18 centres were involved, although only 4 centres enrolled more than 5 participants of the total 90 participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed according to a 2 or 6 block design ...“ Comment: Probably done, as although the authors do not tell us the exact method of randomization used, they do tell us that randomization was performed |
| Allocation concealment (selection bias) | Low risk | Quote: “After granting of informed consent, a central office at Alliance Pharmaceutical was contacted for group assignment” Comment: Probably done, as a centralized randomization process is described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quotes:"This was a prospective, non blinded, randomized study…” "After entry of 45 patients evaluation of the data suggested a trend ...." "No monitoring committee was used" "There was no follow up to ensure that investigators adhered to these (ventilation) guidelines" Comment: Not done, as the authors clearly state from the outset that no blinding was employed. The outcome measures addressed-'ventilator free days', '28 day mortality' and 'physiological indices'-were objective and therefore were unlikely to be influenced by the lack of blinding. However, the fact that at least some of the investigators had access to unblinded interim data, together with the lack of any method of ensuring or measuring adherence to ventilation guidelines, raise concerns over possible performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding of outcome measures, but the outcomes addressed were unambiguous and therefore were unlikely to be influenced significantly by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors enrolled 90 participants but do not provide a statement of completeness of follow‐up or any details of losses to follow‐up. Results for primary and secondary outcomes are not presented in a way that allows the reader to determine whether all participants were followed up for the duration of the study. However, adverse event data are presented in terms of absolute numbers, and for each event, these numbers add up to 90, so it is likely that all included participants were followed up for outcome |
| Selective reporting (reporting bias) | Unclear risk | Quotes: “No significant difference in the number of days free from ventilation at 28 days, the incidence of mortality or any pulmonary related parameter was noted” “PLV may be performed safely in adult patients with respiratory failure with few adverse events, which appear to be transient, self limited and, with appropriate vigilance, manageable“ Comments: The authors provide unbiased reports for the primary and secondary outcome measures of ventilator‐free days and 28 day mortality. Both of these are clearly defined in the methods section of the report, and both are reported in an unbiased way However, the severity, seriousness and significance of adverse events are under‐appreciated and under‐reported |
| Other bias | Low risk | Participants randomly assigned to the PLV group were randomly assigned an average of 25 h later than those who were randomly assigned to the CMV group However, this baseline imbalance in pre‐randomization of duration of ventilation is unlikely to have resulted in significant bias |
Kacmarek 2006.
| Methods | Prospective, multi‐centre, randomized controlled trial Done between December 1998 and December 2000 | |
| Participants | 311 participants with ARDS from 56 centres in North America and Europe Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Randomly assigned to receive:
Five participants did not receive the intended intervention (two randomly assigned to low‐dose PLV and three randomly assigned to high‐dose PLV never received PLV). However, analysis was performed on the basis of 'intention to treat'. |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Results: This study reported:
Adverse events: Statistically significantly (P < 0.05) more episodes of pneumothoraces, hypoxia and hypotension in PLV groups than in the CMV group. The authors state that most of the hypoxic and hypotensive events in the PLV group occurred during the first 5 d of drug delivery and were associated with initial and subsequent filling of the lungs with perfluorocarbon. They attributed this to the need to interrupt ventilator support to administer the perfluorocarbon. Other relevant outcomes that were not reported: The following outcomes, which we considered clinically relevant, were not reported:
|
|
| Notes | Although the study was generally well conducted and well reported, there are some concerns regarding the following: Internal validity: Investigators changed the target sample size on two occasions in response to protocol amendments and interim analyses, and the rationale for these changes is not well explained or justified. Initially a total sample size of 480 was estimated for a power of ≥ 90% for a two‐sided t test, to detect a 3 VFD difference between groups with an overall type 1 error of 5%. After protocol amendments, this was decreased to 260, and the number of VFD considered to represent a significant difference was increased from three to four. After interim analysis, the sample size was subsequently increased to 309 with a power of 80% for a two‐sided t test to detect a 4 VFD difference between groups with an overall type 1 error of 5%. External validity: This study excluded patients with shock and severe nonpulmonary organ dysfunction, and the strict oxygenation criteria for inclusion meant that only those with severe lung injury were included. This limits its generalizability to a subset of critically ill patients with severe lung injury without multiple organ dysfunction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned to one of 4 groups...Group assignment was performed using a computerized randomization system” Comment: Probably done, as the investigators describe a random method. |
| Allocation concealment (selection bias) | Low risk | Quote:"Group assignment was performed using a computerized randomization system." Comment: Probably done, as the investigators describe a random method. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding of participants or personnel is described, but evidence for equal treatment of the two groups with respect to ancillary treatment comes from the fact that both groups received standardized ventilatory support, target gas exchange criteria for weaning were defined a priori and the number of weaning attempts per day was equal between the groups when these criteria were met and were not met. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment is described, but the objective nature of the outcome measures used (i.e. ‘ventilator free days’, ‘time to unassisted ventilation’, ‘time to resolution of ARDS’, ‘percentage of patients alive and off ventilation at 28 days’ and ‘28 day mortality’) means that this is unlikely to result in significant bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “311 patients who were enrolled were followed up for the 28 day study period“ Comment: Probably done, as all enrolled participants were followed up for the study period, and outcome data were reported for all enrolled participants. |
| Selective reporting (reporting bias) | Low risk | Quote “The primary outcome was ventilator free days during the 28 days following randomization. The secondary outcomes were mortality, time to unassisted ventilation …." Comment: Study protocol is not available, but the primary and secondary outcomes as reported are clearly stated in the methods section of the report. |
| Other bias | Low risk | |
- A‐a = alveolar‐arterial.
- ALI = acute lung injury.
- APACHE = acute physiology and chronic health evaluation system.
- ARDS = acute respiratory distress syndrome.
- CMV = conventional mechanical ventilation.
- CXR = chest x‐ray.
- d = days.
- ETT = endotracheal tube.
- FiO2 = fraction of inspired oxygen.
- ICU = intensive care unit.
- PaO2 = arterial oxygen tension.
- PEEP = positive end‐expiratory pressure.
- PFC = perfluorocarbon liquid.
- PLV = partial liquid ventilation.
- Ppc,we = pulmonary capillary wedge pressure.
- s = seconds.
- VFD = ventilator‐free days.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hirschl 1995 | Case series Not randomized No control group |
| Hirschl 1996 | Not randomized No control group |
| Hirschl 1998 | Not randomized No control group |
| Kazerooni 1996 | Not randomized No control group |
| Meaney 1997 | Case series Not randomized No control group |
| Reickert 2001 | Not randomized No control group |
| Schuster 2001 | This study compared chest radiograph filling patterns in participants with acute lung injury who had received low‐dose (10 mL/kg) or high‐dose perflubron (20ml/kg). There was no control group that did not receive perflubon. |
Characteristics of ongoing studies [ordered by study ID]
Chen 2011.
| Trial name or title | Perfluorocarbon (PFC) Inhalation Treatment of Acute Lung Injury/Acute Respiratory Distress Syndrome |
| Methods | Randomized controlled single‐blind trial with cross‐over assignment |
| Participants | Mechanically ventilated adults with acute lung injury |
| Interventions | Experimental: Perfluorocarbon Placebo Comparator: Sterile Water for Injection |
| Outcomes | Primary outcome measures: oxygenation index, respiratory mechanics Secondary outcome measures: 3‐y survival, ventilator‐free days, 28‐d mortality |
| Starting date | August 2011 |
| Contact information | Contact: Zhixin Liang, MD |
| Notes |
Differences between protocol and review
We rewrote the abstract, background, plain language summary, methods, results and discussion sections to take account of relevant new information on acute lung injury and partial liquid ventilation and current guidance for the completion of Cochrane reviews (Higgins 2011). We included risk of bias assessment with tables and figures.
This updated version was conducted by four new team members (IMG, AS, NDF, RP) working together with one of the original authors (MWD).
Contributions of authors
Imelda M Galvin (IMG), Andrew Steel (AS), Ruxandra Pinto (RP), Niall D Ferguson (NDF), Mark W Davies (MWD).
MWD conceived the original review, of which this is an updated version.
IMG coordinated the updated version.
IMG and AS undertook the manual search, screened the search results, retrieved relevant papers, extracted data and assessed quality and risk of bias for included studies.
IMG wrote this updated version of the review.
RP provided statistical expertise.
NDF and MWD provided content expertise on the subjects of acute lung injury and partial liquid ventilation.
All five review authors contributed to the content of the review and reviewed and agreed on the final version.
Sources of support
Internal sources
Grantley Stable Neonatal Unit, Royal Women's Hospital, Brisbane, Australia.
Dept of Paediatrics and Child Health, University of Queensland, Brisbane, Australia.
Royal Children's Hospital, Brisbane, Australia.
Royal Children's Hospital Foundation, Royal Children's Hospital, Brisbane, Queensland, Australia.
Dept of Intensive Care Medicine, The Prince Charles Hospital, Brisbane, Australia.
Interdepartmental Division of Critical Care, University of Toronto, Canada, Canada.
Department of Critical Care Medicine, Sunnybrooke Hospital, Toronto, Canada.
External sources
No sources of support supplied
Declarations of interest
Imelda M Galvin: none known.
Andrew Steel: Dr Steel has received honoraria for lecturing and unrestricted educational grants for the Critical Care Education Program at the University Health Network from Hospira Pharmaceuticals, Canada. No conflict of interest with his current academic activity is known.
Ruxandra Pinto: none known.
Niall D Ferguson: A randomized trial in which NDF is co‐principal investigator has received in‐kind support in the form of loaned equipment from CareFusion Inc. CareFusion Inc. provided in‐kind support in the form of loaned equipment (ventilators) used in the conduct of an international peer review funded randomized controlled trial of high‐frequency oscillation versus conventional ventilation in participants with severe ARDS and provides in‐kind support in the form of loaned equipment (ventilators) used in the conduct of an international peer review funded randomized controlled trial of high‐frequency oscillation versus conventional ventilation in participants with severe ARDS.
Mark W Davies: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Hirschl 2002 {published data only}
- Bartlett R, Croce M, Hirschl R, Gore D, Wiedemann H, Davis K, et al. A phase II randomized, controlled trial of partial liquid ventilation (PLV) in adult patients with acute hypoxemic respiratory failure (AHRF). Critical Care Medicine. 1997; Vol. 25:A35.
- Croce MA, Fabian TC, Patton JH Jr, Melton SM, Moore M, Trenthem LL. Partial liquid ventilation decreases the inflammatory response in the alveolar environment of trauma patients. The Journal of Trauma 1998;45(2):273‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hirschl RB, Croce M, Gore D, Wiedemann H, Davis K, Zwischenberger J, et al. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2002;165:781‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kacmarek 2006 {published data only}
- Kacmarek RM, Wiedemann HP, Lavin PT, Wedel MK, Tutuncu AS, Slutsky AS. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. American Journal of Respiratory & Critical Care Medicine 2006;173(8):882‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hirschl 1995 {published data only}
- Hirschl RB, Pranikoff T, Gauger P, Schreiner RJ, Dechert R, Bartlett RH. Liquid ventilation in adults, children, and full‐term neonates. Lancet 1995;346:1201‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hirschl 1996 {published data only}
- Hirschl RB, Pranikoff T, Wise C, Overbeck MC, Gauger P, Schreiner RJ, et al. Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA 1996;275(5):383‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hirschl 1998 {published data only}
- Hirschl RB, Conrad S, Kaiser R, Zwischenberger JB, Bartlett RH, Booth F, et al. Partial liquid ventilation in adult patients with ARDS: a multicenter phase I‐II trial. Adult PLV Study Group. Annals of Surgery 1998;228(5):692‐700. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazerooni 1996 {published data only}
- Kazerooni EA, Pranikoff T, Cascade PN, Hirschl RB. Partial liquid ventilation with perflubron during extracorporeal life support in adults: radiographic appearance. Radiology 1996;198(1):137‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meaney 1997 {published data only}
- Meaney JF, Kazerooni EA, Garver KA, Hirschl RB. Acute respiratory distress syndrome: CT findings during partial liquid ventilation. Radiology 1997;202:570‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reickert 2001 {published data only}
- Reickert CA, Pranikoff T, Overbeck MC, Kazerooni EA, Massey KD, Bartlett RH, et al. The pulmonary and systemic distribution and elimination of perflubron from adult patients treated with partial liquid ventilation. Chest 2001;119:515‐22. [DOI] [PubMed] [Google Scholar]
Schuster 2001 {published data only}
- Schuster DP, Lange NR, Tutuncu A, Wedel M, LiquiVent Study Group. Clinical correlation with changing radiographic appearance during partial liquid ventilation. Chest 2001;119(5):1503‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Chen 2011 {published data only}
- Perfluorocarbon (PFC) Inhalation Treatment of Acute Lung Injury/Acute Respiratory Distress Syndrome. Ongoing study August 2011.
Additional references
Adhikari 2007
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ 2007;334:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anzueto 2006
- Anzueto A, Guntapalli K. Adjunctive therapy to mechanical ventilation: surfactant therapy, liquid ventilation, and prone position. Clinics in Chest Medicine 2006;27:637‐54. [DOI] [PubMed] [Google Scholar]
ARDS Network 2000
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England Journal of Medicine 2000;342(18):1301‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ashbaugh 1967
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2(7511):319‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernard 1994
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American‐European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 1994;149(3):818‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clark 1966
- Clark LC, Gollan F. Survival of mammals breathing organic liquids equilibrated with oxygen at atmospheric pressure. Science 1966;152:1755‐66. [PUBMED: 5938414] [DOI] [PubMed] [Google Scholar]
Combes 2012
- Combes A, Bacchetta M, Brodie D, Müller T, Pellegrino V. Extracorporeal membrane oxygenation for respiratory failure in adults. Current Opinion in Critical Care 2012;18(1):99‐104. [DOI] [PubMed] [Google Scholar]
Conner 2000
- Conner BD, Bernard GR. Acute respiratory distress syndrome: potential pharmacological interventions. Clinics in Chest Medicine 2000;21(3):563‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davies 1999
- Davies MW. Liquid ventilation. Journal of Paediatrics and Child Health 1999;35(5):434‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Prost 2011
- Prost N, Ricard JD, Saumon G, Dreyfuss D. Ventilator‐induced lung injury: historical perspectives and clinical implications. Annals of Intensive Care. 2011;1:28. [DOI: 10.1186/2110-5820-1-28] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2011
- Dechartres A, Charles P, Hopewell S, Philippe R, Altman DG. Reviews assessing the quality or the reporting of randomized controlled trials are increasing over time but raised questions about how quality is assessed . Journal of Clinical Epidemiology 2011;64:136‐44. [DOI] [PubMed] [Google Scholar]
Derdak 2002
- Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, et al. High‐frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. American Journal of Respiratory Critical Care Medicine 2002;166:801‐8. [DOI] [PubMed] [Google Scholar]
Falagas 2009
- Falagas ME, Grigori T, Ioannidou E. A systematic review of trends in the methodological quality of randomized controlled trials in various research fields. Journal of Clinical Epidemiology 2009;62:227‐31. [DOI] [PubMed] [Google Scholar]
Fuhrman 1991
- Fuhrman BP, Paczan PR, Francisis M. Perfluorocarbon‐associated gas exchange. Critical Care Medicine 1991;19(5):712‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hernan 1996
- Hernan LJ, Fuhrman BP, Kasier RE, Penfil S, Foley C, Papo MC, et al. Perflurocarbon associated gas exchange in normal and acid injured large sheep. Critical Care Medicine 1996;24:475‐81. [DOI] [PubMed] [Google Scholar]
Herridge 2011
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz‐Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine 2011;364:1293‐304. [PUBMED: 21470008] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kaisers 2003
- Kaisers U, Kelly KP, Busch T. Liquid ventilaton. British Journal of Anaesthesia 2003;91:143‐51. [PUBMED: 12821573] [DOI] [PubMed] [Google Scholar]
Kylstra 1962
- Kylstra JA, Tissing MO, Maen A. Survival in air after breathing fluid. Lancet 1962;2:1170. [13927778] [DOI] [PubMed] [Google Scholar]
Lynch 2006
- Lynch JE, Cheek JM, Zwischenberger JB. Adjuncts to mechanical ventilation in ARDS. Seminars in Thoracic Cardiovascular Surgery 2006;18:20‐7. [16766249] [DOI] [PubMed] [Google Scholar]
Matthay 2011
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annual Review of Pathology 2011;6:147‐63. [PUBMED: 20936936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monchi 1998
- Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, Laurent I, et al. Early predictive factors of survival in the acute respiratory distress syndrome: a multivariate analysis. American Journal of Respiratory and Critical Care Medicine 1998;158(4):1076‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pakulla 2004
- Pakulla MA, Seidel D, Obal D, Loer SA. Hydrochloric acid‐induced lung injury: effects of early partial liquid ventilation on survival rate, gas exchange, and pulmonary neutrophil accumulation. Intensive Care Medicine 2004;30:2110‐9. [PUBMED: 15448887] [DOI] [PubMed] [Google Scholar]
Raneiri 2012
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307(23):2526‐33. [PMID: 22797452] [DOI] [PubMed] [Google Scholar]
Rubenfeld 2005
- Rubenfeld GD, Galdwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. New England Journal of Medicine 2005;353:1685‐93. [PUBMED: 16236739] [DOI] [PubMed] [Google Scholar]
Rubenfeld 2007
- Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest 2007;131:554‐62. [PUBMED: 17296661] [DOI] [PubMed] [Google Scholar]
Schoenfeld 2002
- Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Critical Care Medicine 2002;30:1772‐7. [DOI] [PubMed] [Google Scholar]
Stapleton 2005
- Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 2005;128:525‐32. [DOI] [PubMed] [Google Scholar]
Suntharalingam 2001
- Suntharalingam G, Regan K, Keogh BF, Morgan CJ, Evans TW. Influence of direct and indirect etiology on acute outcome and 6‐month functional recovery in acute respiratory distress syndrome. Critical Care Medicine 2001;29(3):562‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taccone 2009
- Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009;302:1977‐84. [DOI] [PubMed] [Google Scholar]
Tawfic 2010
- Tawfic QA, Kausalya R. Liquid ventilation. Oman Medical Journal 2010;26:4‐9. [PUBMED: 22043370] [DOI] [PMC free article] [PubMed] [Google Scholar]
The ARDS Definition Task Force 2012
- The ARDS Definition Task Force. Acute respiratory distress syndrome-the Berlin definition. JAMA 2012;307(23):2526‐33. [DOI] [PubMed] [Google Scholar]
Tobin 2001
- Tobin MJ. Advances in mechanical ventilation. The New England Journal of Medicine 2001;344(26):1986‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ware 2000
- Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England Journal of Medicine 2000;342(18):1334‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wiedemann 2000
- Wiedemann HP. Partial liquid ventilation for acute respiratory distress syndrome. Clinics in Chest Medicine 2000;21(3):543‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhu 2010
- .Zhu YB, Wang Q, Liu YL, Li XF, Li JA, Lü XD, et al. Effect of partial liquid ventilation on oleic acid‐induced inflammatory responses in piglets. Chinese Medical Journal 2010;123:2088‐93. [PUBMED: 20819547] [PubMed] [Google Scholar]
References to other published versions of this review
Davies 2004
- Davies MW, Fraser JF. Partial liquid ventilation for preventing death and morbidity in adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003707.pub2] [DOI] [PubMed] [Google Scholar]


