Abstract
Background
Nutritional support is an essential component of critical care. Malnutrition has been associated with poor outcomes among patients in intensive care units (ICUs). Evidence suggests that in patients with a functional gut, nutrition should be administered through the enteral route. One of the main concerns regarding use of the enteral route is the reduction in gastric motility that is often responsible for limited caloric intake. This increases the risk of aspiration pneumonia as well. Post‐pyloric feeding, in which the feed is delivered directly into the duodenum or the jejunum, could solve these issues and provide additional benefits over routine gastric administration of the feed.
Objectives
To evaluate the effectiveness and safety of post‐pyloric feeding versus gastric feeding for critically ill adults who require enteral tube feeding.
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL;2013 Issue 10), MEDLINE (Ovid) (1950 to October 2013), EMBASE (Ovid) (1980 to October 2013) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO host (1982 to October 2013). We reran the search on 4 February 2015 and will deal with the one study of interest when we update the review.
Selection criteria
Randomized or quasi‐randomized controlled trials comparing post‐pyloric versus gastric tube feeding in critically ill adults.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Anaesthesia, Critical and Emergency Care Group and separately evaluated trial quality and data extraction as performed by each review author. We contacted trials authors to request missing data.
Main results
We pooled data from 14 trials of 1109 participants in a meta‐analysis. Moderate quality evidence suggests that post‐pyloric feeding is associated with low rates of pneumonia compared with gastric tube feeding (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.51 to 0.84). Low‐quality evidence shows an increase in the percentage of total nutrient delivered to the patient by post‐pyloric feeding (mean difference (MD) 7.8%, 95% CI 1.43 to 14.18).
Evidence of moderate quality revealed no differences in duration of mechanical ventilation or in mortality. Intensive care unit (ICU) length of stay was similar between the two groups. The effect on the time required to achieve the full nutrition target was uncertain (MD ‐1.99 hours 95% CI ‐10.97 to 6.99) (very low‐quality evidence). We found no evidence suggesting an increase in the rate of complications during insertion or maintenance of the tube in the post‐pyloric group (RR 0.51, 95% CI 0.19 to 1.364; RR1.63, 95% CI 0.93 to 2.86, respectively); evidence was assessed as being of low quality for both.
Risk of bias was generally low in most studies, and review authors expressed concern regarding lack of blinding of the caregiver in most trials.
Authors' conclusions
We found moderate‐quality evidence of a 30% lower rate of pneumonia associated with post‐pyloric feeding and low‐quality evidence suggesting an increase in the amount of nutrition delivered to these participants. We do not have sufficient evidence to show that other clinically important outcomes such as duration of mechanical ventilation, mortality and length of stay were affected by the site of tube feeding.
Low‐quality evidence suggests that insertion of a post‐pyloric feeding tube appears to be safe and was not associated with increased complications when compared with gastric tube insertion. Placement of the post‐pyloric tube can present challenges; the procedure is technically difficult, requiring expertise and sophisticated radiological or endoscopic assistance.
We recommend that use of a post‐pyloric feeding tube may be preferred for ICU patients for whom placement of the post‐pyloric feeding tube is feasible. Findings of this review preclude recommendations regarding the best method for placing the post‐pyloric feeding tube. The clinician is left with this decision, which should be based on the policies of institutional facilities and should be made on a case‐by‐case basis. Protocols and training for bedside placement by physicians or nurses should be evaluated.
Plain language summary
Post‐pyloric versus gastric tube feeding for critically ill adult patients
Review question.
We reviewed the evidence on benefits and complications of passing a feeding tube into the small bowel instead of placing it in the stomach to feed critically ill adults admitted to the intensive care unit (ICU).
Background
Providing early nutritional support for participants in the ICU is very important. Nutrition is supplied in a special liquid form, which is delivered through a tube placed in the mouth or nose of the person and extended into the stomach (gastric), or the tube may be advanced more distally to reach the small bowel (duodenum or jejunum), in which case it is called a post‐pyloric feeding tube. We wanted to learn about the safety and potential benefits associated with post‐pyloric feeding, as well as potential complications.
Study characteristics
We searched the databases until October 2013 and identified 14 studies (randomized controlled trials) with a total of 1109 participants. We reran the search on 4 February 2015 and will deal with the one study of interest when we update the review. We investigated the benefits of post‐pyloric tube feeding for reducing the rate of pneumonia, decreasing the number of days that a person needs to be dependent on a breathing machine, increasing the percentage of nutrients that can be provided to the participant and reducing the number of deaths. We also investigated potential complications that may occur during insertion of the tube, such as bleeding from the gastrointestinal tract, and complications arising during maintenance of the tube, such as the need to replace the tube.
Key results
We found that post‐pyloric feeding appeared to reduce the rate of pneumonia and increase the amount of nutrition delivered to the patient. Its use did not result in fewer days that a person needed to be dependent on a breathing machine nor in fewer deaths. The target amount of feeding for a person fed with a post‐pyloric tube was reached without delay. Insertion of a post‐pyloric feeding tube appears safe and did not increase the likelihood of complications.
Quality of the evidence
We found evidence of moderate quality for the outcomes of rate of pneumonia, duration of dependency on a breathing machine and rate of death, mainly because identified studies were poorly conducted. With regard to the total quantity of nutrients that can be delivered to patients and complications related to insertion and maintenance of the tube, the quality of evidence was assessed as low. Evidence for the time required to reach the target amount of feeding was very low in that results were not similar across studies and study design issues hindered assessment.
We recommend that a post‐pyloric feeding tube should be used routinely for all ICU patients, when this approach is feasible.
Summary of findings
Summary of findings for the main comparison. Post‐pyloric tube feeding compared with gastric tube feeding for critically ill adult patients.
| Post‐pyloric tube feeding compared with gastric tube feeding for critically ill adult patients | ||||||
| Patient or population: critically ill adult patients Settings: critical care Intervention: post‐pyloric tube feeding Comparison: gastric tube feeding | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Gastric tube feeding | Post‐pyloric tube feeding | |||||
|
Pneumonia (follow‐up from time of enrolment in the study until discontinued enteral nutritional support and commenced concurrent oral nutrition, participant death or discharge from ICU) |
Study population | RR 0.65 (0.51 to 0.84) | 819 (9 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 285 per 1000 | 185 per 1000 (145 to 239) | |||||
| Moderate | ||||||
| 204 per 1000 | 133 per 1000 (104 to 171) | |||||
|
Mortality (follow‐up from time of enrolment in the study until participant death) |
Study population | RR 1.03 (0.83 to 1.29) | 977 (11 studies) | ⊕⊕⊕⊝ Moderatea | ||
| 218 per 1000 | 225 per 1000 (181 to 281) | |||||
| Moderate | ||||||
| 261 per 1000 | 269 per 1000 (217 to 337) | |||||
|
Percentage of total nutrition delivered to participant (follow‐up from time of insertion of the tube until discontinued enteral nutritional support and commenced concurrent oral nutrition) |
Mean percentage of nutritional targets delivered to participants in the intervention groups was 7.8 higher (1.43 to 14.18 higher) | 692 (7 studies) | ⊕⊕⊝⊝ Lowa,b | |||
|
Time required to achieve full nutritional target (in hours) (follow‐up from time of insertion of the tube until discontinued enteral nutrition support and commenced concurrent oral nutrition) |
Mean time required to achieve full nutritional target (in hours) in control groups was 226 hours | Mean time required to achieve full nutritional target (in hours) in intervention groups was 1.99 lower (10.97 lower to 6.99 higher) | 432 (5 studies) | ⊕⊝⊝⊝ Very lowa,c,d | ||
|
Duration of mechanical ventilation in days (follow‐up from the day of start of mechanical ventilation until discontinued mechanical ventilation) |
Mean duration of mechanical ventilation in days in control groups was 279 days | Mean duration of mechanical ventilation in days in intervention groups was 0.92 lower (2.11 lower to 0.28 higher) | 549 (5 studies) | ⊕⊕⊕⊝ Moderatea | ||
|
Complications related to tube insertion (follow‐up from time of tube insertion until removal of the tube) |
Study population | RR 0.51 (0.19 to 1.36) | 324 (4 studies) | ⊕⊕⊝⊝ Lowa,e | ||
| 61 per 1000 | 31 per 1000 (12 to 83) | |||||
| Moderate | ||||||
| 26 per 1000 | 13 per 1000 (5 to 35) | |||||
|
Complications related to tube maintenance (follow‐up from time of tube insertion until removal of the tube) |
Study population | RR 1.63 (0.93 to 2.86) | 638 (7 studies) | ⊕⊕⊝⊝ Lowa,f | ||
| 158 per 1000 | 258 per 1000 (147 to 453) | |||||
| Low | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| High | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe quality of evidence was downgraded one level because of serious risk of bias (no blinding of caregiver or outcome assessor for most of the study). bThe quality of evidence was downgraded one level because of serious inconsistency (I² = 89%). cThe quality of evidence was downgraded one level because of serious inconsistency (I² = 85%). dThe quality of evidence was downgraded one level because of serious imprecision (95% CI ranged from ‐10.97 to 6.99). eThe quality of evidence was downgraded one level because of serious imprecision, total number of events was very small (only one event) and confidence interval was very wide. fThe quality of evidence was downgraded one level because of serious inconsistency (I² =58%).
Background
Description of the condition
Providing nutritional support for critically ill patients is a complex and important task. A paradigm shift has occurred regarding the true value of nutritional support in the intensive care unit (ICU) setting. In the past, the goals of nutritional support were to provide adjunctive therapy to support the stress response, to deliver exogenous nutrients to reduce depletion of lean body mass and to prevent the consequences of protein caloric malnutrition. Today, early feeding provided to a critically ill patient is seen as a therapeutic tool or strategy that can attenuate disease severity, modulate the immune response, reduce complications and favourably impact patient outcomes (ASPEN 2009; Kudsk 2007).
For patients who require nutritional support, early feeding (within 24 to 48 hours of admission to ICU) may be provided by the enteral or parenteral route. According to European, Canadian and American guidelines for enteral and parenteral nutrition, enteral feeding is the preferred method for patients who have a functioning gastrointestinal (GI) tract but who cannot maintain adequate oral intake (ASPEN 2009; Canadian Guidelines 2013; ESPEN 2006). Advantages of enteral nutrition include prevention of gastrointestinal mucosal atrophy; maintenance of intestinal integrity; and prevention of bacterial adherence to the gut wall by stimulated release of secretory immunoglobulin (Ig)A immunoglobulin, which results in prevention of bacterial translocation from the gastrointestinal lumen to the rest of the body (Groos 1996; Jabbar 2003; Kudsk 2001; Kudsk 2002). In addition, enteral nutrition has been reported to reduce infectious complications and is more cost‐effective than parenteral nutrition (Heyland 2003).
Description of the intervention
For short‐term enteral nutrition (for a period less than 30 days), the feeding tube is passed via the nose to the stomach (gastric), or it may be advanced more distally into the duodenum or jejunum (post‐pyloric). Debate continues regarding the best route of enteral nutrition and the true risks and benefits derived by the use of post‐pyloric tube feeding over gastric feeding.
How the intervention might work
Successful enteral feeding of critically ill patients involves the challenges of intolerance and potential adverse effects, which are caused mainly by the gastrointestinal tract dysfunction commonly seen in these patients. Predominant motility abnormalities seen in these patients include antral hypomotility and delayed gastric emptying (Heyland 1996; Ritz 2001; Ukleja 2010). Antral hypomotility and loss of peristaltic activity in the stomach result from disturbed motor function of the proximal and distal stomach (Chapman 2005; Nguyen 2006). Loss of peristaltic activity is not as great in the duodenum as it is in the stomach (Dive 1994). A prospective case series study of critically ill patients showed that motor activity persisted in the duodenum in many circumstances when gastric motility was profoundly suppressed (Bosscha 1998), but the real prevalence of abnormal motility in the small bowel is not known. Mechanisms of abnormal gastrointestinal motility in critically ill patients are not fully understood but could be related to impaired enteric nerve and smooth muscle function; inflammation mediated by cytokines and nitric oxide; surgery leading to gut injury and hypoperfusion; medications, including opioids, dopamine, catecholamines and pressor agents; and hyperglycaemia, electrolyte disturbances, sepsis and increased intracranial pressure (Ukleja 2010l).
The first challenge associated with enteral feeding is increased risk of aspiration pneumonia. A nasoenteric tube can interfere with function of the upper and lower oesophageal sphincters and may predispose a patient to gastroesophageal reflux (GER) and aspiration (Uklaja 2007). Another factor contributing to higher risk of GER is the high volume of gastric contents and gastric distention that results from decreased gastric emptying, which leads to relaxation of the lower oesophageal sphincter and more frequent episodes of GER. It has been found that aspiration of gastric contents is common in critically ill tube‐fed patients and is a major risk factor for pneumonia (Metheny 2006). Advancement of the feeding tube beyond the pylorus should, theoretically, overcome the risk of GER because the pylorus acts as a protective barrier against reflux of nutrient contents back into the stomach. Heyland found that feeding beyond the pylorus was associated with a significant reduction in gastroesophageal regurgitation, suggesting that this location may provide additional antireflux protection (Heyland 2001).
The second important challenge of providing enteral feeding is the inability to ensure that critically ill patients will have the energy that they need, because gastric intolerance and high gastric residual volumes result in frequent interruption of feeding and cessation of delivery of food. Studies have shown that only 50% to 64% of estimated daily energy requirements are actually delivered through enteral feeding (De Jonghe 2001; Elpern 2004; O'Leary 2005; O'Meara 2008; Woodcock 2001). Delivering feedings in a timely manner is an important goal because failure to deliver adequate nutrition, especially to malnourished patients, has been shown to correlate with significantly longer ICU stay, additional days on mechanical ventilation and more frequent infectious complications (Rubinson 2004; Villet 2005). The larger percentage of caloric requirements provided by post‐pyloric feeding may be related to significantly reduced gastric residual volume with the perception of improved tolerance and thus fewer interruptions in delivery of formula than occur with intragastric feeds. Post‐pyloric feeding has been used successfully to maintain enteral nutrition in patients who otherwise would have required parenteral nutrition (Boulton 2004).
Why it is important to do this review
The primary goal of nutritional support for critically ill patients is to provide adequate nutrients while avoiding complications related to the technique of providing nutritional support. Unfortunately, the best way of feeding the critically ill patient is still a topic of debate, including the best route for providing enteral nutrition and the true risks and benefits derived when post‐pyloric tube feeding is selected over gastric feeding.
At least seven previously published meta‐analyses have compared gastric versus post‐pyloric feeding in the critical care setting (Alhazzani 2013; Deane 2013; Heyland 2002; Ho 2006; Jiyong 2013; Marik 2003; Zhang 2013). These meta‐analyses have reached different conclusions regarding the benefits and complications that may result from use of a post‐pyloric feeding tube; this conflict affects both clinical practice and published guidelines. The Canadian Clinical Practice Guidelines recommend routine use of small bowel feeding in clinical units in which small bowel access is feasible (Canadian Guidelines 2013), but American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines state that gastric or small bowel feeding of the critically ill patient is acceptable and recommend small bowel feeding only for patients with high risk of aspiration or with intolerance of gastric feeding (ASPEN 2009).
In the light of recently published studies regarding this issue, a systematic review with meta‐analysis conducted to re‐evaluate potential benefits and adverse effects of early post‐pyloric feeding for critically ill adult patients is warranted
Objectives
To evaluate the effectiveness and safety of post‐pyloric feeding versus gastric feeding for critically ill adults who require enteral tube feeding.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized and quasi‐randomized controlled trials (RCTs). We define quasi‐randomized controlled trials as trials using inadequate randomization methods such as date of birth; day of the week or month of the year; a person's medical record number; or allocation of alternate participants.
We excluded prospective cohort studies.
We considered inclusion of trials that report at least one of our primary or secondary outcomes of interest; however, primary outcomes will be the focus of the review.
Types of participants
We included adult participants 18 years of age and older who received treatment in a critical care setting (including participants with burns, head injury, trauma, brain haemorrhage and cerebrovascular accident) and who were anticipated to require enteral feeding for at least 48 hours after admission to the critical care unit.
Types of interventions
We included trials comparing post‐pyloric versus gastric tube feeding with catheters passed via the nose or mouth. We excluded the following.
Gastrostomy, duodenostomy or jejunostomy feeding.
Aspiration per se as the outcome (without clinical evidence of pneumonia).
Abdominal surgery.
Gastrointestinal bleeding.
Intestinal obstruction.
Types of outcome measures
Primary outcomes
Pneumonia.
Mortality.
Percentage of total nutrition delivered to the participant, which is calculated by comparing the mean estimated caloric need versus the mean actual calories delivered to the participant.
Time required to achieve the full nutritional target: time from participant enrolment in the study to time the nutritional goal rate was reached and continued successfully for four hours.
Secondary outcomes
Intensive care unit (ICU) length of stay.
Duration of mechanical ventilation.
Gastrointestinal complications: vomiting, diarrhoea, high gastric residual volume.
Complications related to tube insertion: epistaxis, pneumothoraces, gastrointestinal bleeding.
Complications related to tube maintenance: need for tube replacement, tube occlusion.
Time required to start feeding: from participant enrolment in the study to start of the feeding.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2013 Issue 10); MEDLINE (Ovid) (1950 to October 2013); EMBASE (Ovid) databases (1980 to October 2013); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO host (1982 to October 2013). We reran the search on 4 February 2015 and will deal with studies of interest when we update the review. We employed the search strategy stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and developed a specific strategy for each database. Our detailed search strategies for CENTRAL, MEDLINE, EMBASE and CINAHL are displayed in Appendix 1, Appendix 2, Appendix 3 and Appendix 4, respectively.
We applied no language or publication restrictions.
We searched the following major databases for ongoing trials.
Searching other resources
We identified further potential studies by examining the references cited in previous relevant Cochrane reviews, other relevant studies, review articles and standard textbooks. We sought relevant information from expert informants on additional published and unpublished studies. We tried to contact the authors of all relevant identified studies to enquire about additional studies potentially suitable for inclusion.
Data collection and analysis
Selection of studies
Three review authors (SA, CM, RJB) independently assessed the titles and abstracts (when available) of all reports identified by the searches. We retrieved full‐text versions and evaluated potentially relevant studies chosen by at least one review author. We (SA, RJB, CM) selected trials that met the inclusion criteria (see Appendix 5 for a copy of the Study Selection Form). Each review author documented the reasons for exclusion of trials. We resolved disagreements by discussion between review author groups. In cases of insufficient published information, to make a decision about inclusion, we contacted the first author of the relevant trial (SA). We compiled a list of eligible trials, along with unique identifiers, on a Form for Eligible Trials (see Appendix 6 for a copy of this form).
Data extraction and management
The first review author (SA) extracted and collected data on all studies and reviewed this information against data extracted independently by two other review authors (CM, RJB). A copy of this paper form is provided in Appendix 7. The data extraction form had been piloted before use; we resolved discrepancies in the data extracted by discussion within the group. When additional information was needed, one review author (SA) contacted the first author of the relevant trial. Excluded trials and reasons for exclusion are listed in the Characteristics of excluded studies table. One review author (SA) entered all data into Review Manager (RevMan 5.3), and the fourth author (FGS) checked all entries.
Quality assessment
We generated a 'Summary of findings' table consisting of information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data from all studies included in the comparison of all important outcomes. We evaluated the following outcomes.
Risk of pneumonia.
Percentage of nutrition delivered to the participant.
Time required to achieve the full nutritional target.
Duration of mechanical ventilation.
Mortality.
Complications related to tube insertion: epistaxis, pneumothoraces, gastrointestinal bleeding.
Complications related to tube maintenance: need for tube replacement, tube occlusion.
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach to interpret findings and the GRADE profiler (GRADEpro 3.6) to import data from RevMan 5.3.
Assessment of risk of bias in included studies
Three review authors (SA, RB, CM) independently, and in duplicate, assessed risk of bias of selected trials on the basis of information provided in the articles. We resolved disagreements by discussion among the group and performed the assessment as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Jüni 2001). A copy of the form that was used to carry out this assessment is provided in Appendix 8.
We considered a trial as having low risk of bias if all of the criteria listed below were assessed as adequate. We considered a trial as having high risk of bias if one or more of the criteria listed below were not assessed as adequate.
Generation of the allocation sequence of interventions
We considered allocation adequate if it was generated by a computer or a random number table algorithm. We judged other processes, such as tossing of a coin, adequate if the whole sequence was generated before the start of the trial, and if the process was performed by a person not otherwise involved in participant recruitment.
We considered allocation inadequate if a non‐random system, such as dates, names or identification numbers, was used.
Concealment of allocation
We considered concealment adequate if the process used prevented participant recruiters, investigators and participants from knowing the intervention allocation for the next participant to be enrolled in the study.
Acceptable systems include a central allocation system, sealed opaque envelopes or an on‐site locked computer.
We considered concealment inadequate if the allocation method allowed participant recruiters, investigators or participants to know the treatment allocation for the next participant to be enrolled in the study, for example, alternate medical record numbers, reference to case record numbers or date of birth, an open allocation sequence or unsealed envelopes.
Blinding
Blinding of the participant is not important because it cannot affect the outcome, but blinding of the caregiver is important for all outcomes because different ways of managing groups can affect the results. Blinding of the outcome assessor is important in trials in which pneumonia is one of the measured outcomes, as the diagnosis of pneumonia is more subjective and depends on interpretation of the chest X‐ray.
We considered blinding adequate if caregivers and outcome assessors for pneumonia were blinded to the intervention. We considered blinding inadequate if caregivers and outcome assessors for pneumonia were not blinded to the intervention.
Incomplete outcome data
We considered incomplete outcome data to be adequately addressed if no outcome data were missing; reasons for missing outcome data were unlikely to be related to a true outcome; or missing outcome data were balanced in quantity across intervention groups, with similar reasons for missing data across groups.
We considered incomplete outcome data to be inadequately addressed if the reason for missing outcome data was likely to be related to true outcome; an imbalance in numbers or reasons for missing data was noted across intervention groups; or ‘as‐treated’ analysis was done with substantial departure of the intervention received from that assigned at randomization.
Selective outcome reporting
We considered the study free of selective reporting bias if the study protocol was available and all of the study’s prespecified (primary and secondary) outcomes of interest for the review were reported in the pre‐specified way; or the study protocol was not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified.
We considered a study as having risk of selective reporting bias if any of the following applied.
Not all of the study’s pre‐specified primary outcomes were reported.
One or more primary outcomes were reported as measurements or analysis methods that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified.
One or more outcomes of interest for the review were reported incompletely, so data cannot be entered into a meta‐analysis.
The study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Other potential threats to validity
We considered the study to be free of other bias if the study was apparently free of other problems that could put it at risk of bias. We considered the study as having risk of other bias if extreme baseline imbalance was evident.
Measures of treatment effect
For continuous outcomes, we used mean differences and standard deviations to summarize the data for each group. For dichotomous outcomes, we estimated effects of the intervention as risk ratios with 95% confidence intervals (CIs).
Dealing with missing data
We attempted to contact the first author or the contact person for all trials with missing data before we made a decision about trial eligibility. If this was unsuccessful, we planned to perform sensitivity analysis to compare the effects of complete case analysis and worst case scenario and last observation carried forward options on the results of individual studies and meta‐analyses. We tolerated a maximum loss of 5% of data (Gamble 2005).
Assessment of heterogeneity
We assessed the clinical heterogeneity of included studies by exploring clinical and methodological characteristics of these studies (e.g. differences in study quality, participants, intervention or outcome assessment). We pooled data in a meta‐analysis, as clinical heterogeneity among selected studies was negligible. We assessed statistical heterogeneity with the I² statistic, thereby estimating the percentage of total variance across studies due to heterogeneity rather than to chance (Higgins 2002), with a value greater than 50% indicating substantial statistical heterogeneity. We assessed possible sources of heterogeneity by performing subgroup and sensitivity analyses when studies and data were sufficient.
Assessment of reporting biases
Publication bias occurs when published studies are not representative of all studies that have been done, usually because positive results tend to be submitted and published more often than negative results. Because detecting publication bias is difficult, we tried to minimize the bias by performing a comprehensive literature search and by using study registries (Glasziou 2001). When we had identified an adequate number of trials for inclusion, we constructed funnel plots and examined them visually to assess the presence of publication bias (Egger 1997), which is associated with asymmetry (Light 1984).
Data synthesis
We generated a quantitative summary measure and performed the analysis using Review Manager software (RevMan 5.3). As the population was varied, we used the random‐effects model for meta‐analysis. For outcomes with a smaller value of the I² statistic, both random‐effects and fixed‐effect methods were carried out, but no difference between random‐effects and fixed‐effect estimates were reported for these outcome variables. We performed all analyses according to the intention‐to‐treat (ITT) principle. We reported a confidence interval at a level of 95%.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses of participants, interventions and outcomes for each of the following.
Subgroups of participants
Participants on mechanical ventilation.
Subgroups of the intervention
Participants for whom the tube was inserted into the jejunum versus the duodenum.
Participants with regular monitoring of the position of the tube versus no monitoring.
Participants for whom other preventive measures for pneumonia, such as head elevation or chlorhexidene mouth wash, were used.
Subgroups of the outcome
A clear and acceptable definition of pneumonia as a new or progressive radiographic infiltrate on chest radiograph associated with clinical features of pneumonia versus no clear or inadequate definition.
Sensitivity analysis
We performed sensitivity analyses to determine the impact of methodological quality on the overall effect estimate. We excluded two trials (Montecalvo 1992; White 2009) because no standardized protocol was provided for management of tube feeding in both groups (Montecalvo 1992), and because high cross‐over and baseline imbalance were noted between groups (White 2009). In this latter trial, 4/45 (8.9%) from the gastric group were crossed over to post‐pyloric feeding because of high gastric residual, and 10/50 (20%) participants from the post‐pyloric group did not receive the intended treatment because it was not possible to insert the feeding tube blindly. Also, baseline characteristics demonstrate that the Acute Physiology and Chronic Health Evaluation (APACHE) II score was higher in the post‐pyloric group.
We will not use results obtained from subgroup and sensitivity analyses to form conclusions. They are provided for hypothesis generation and for testing in future adequately designed studies.
No difference between random‐effects and fixed‐effect estimates was provided for each outcome variable. In cases of missing data, we tolerated 5% maximum loss of data (Gamble 2005).
Results
Description of studies
Full study details are provided in Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
We reran the search on 4 February 2015. The search yielded 4195 studies; after removal of duplicates, we identified 2920 studies.
We performed initial screening by reading the abstract; when it was not clear from the abstract whether a study should be included, or when no abstract was available, we obtained the full paper. We took a total of 53 papers forward for detailed assessment; one paper (Huang 2012) was a secondary publication of Hsu 2009. We excluded a total of 15 papers and included 14 studies in the meta‐analysis, one study is awaiting classification.(see Figure 1).
1.
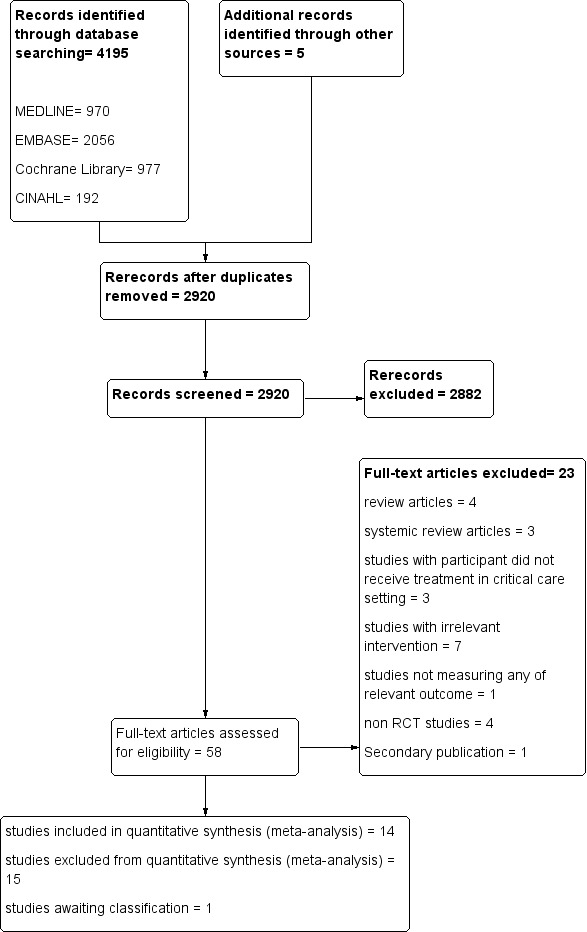
Study flow diagram.
We reran the search on 4 Febuary 2015. We found one study of interest and will deal with this study (Couto 2014) when we update the review.
Included studies
See Characteristics of included studies.
We included 14 studies (Acosta‐Escribano 2010; Boivin 2001; Davies 2002; Davies 2012; Day 2001; Esparza 2001; Hsu 2009; Kearns 2000; Kortbeek 1999; Montecalvo 1992; Montejo 2002; Neumann 2002; White 2009; Zeng 2010). Theses are described in detail in the Characteristics of included studies. The oldest study was undertaken in 1992, and the most recent one in 2012
Excluded studies
See Characteristics of excluded studies
After inspecting the full report, we excluded 15 studies (Bao 2006; Eatock 2005; Graham 1989; Heyland 2001; Kumar 2006; Lin 2006; Minard 2000; Nayak 2008; Olah 1996; Singh 2012; Spain 1995; Strong 1992; Taylor 1998; Taylor 1999; Treux 1995).
Awaiting classification
See Characteristics of studies awaiting classification.
One study (Couto 2014) is awaiting classification. We will deal with this study when we update the review.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We provided full details in Figure 2, in Figure 3 and in the risk of bias tables (found below Characteristics of included studies).
2.
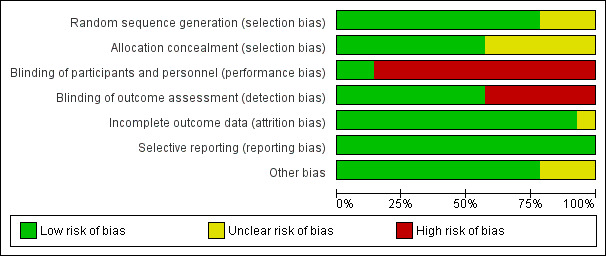
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
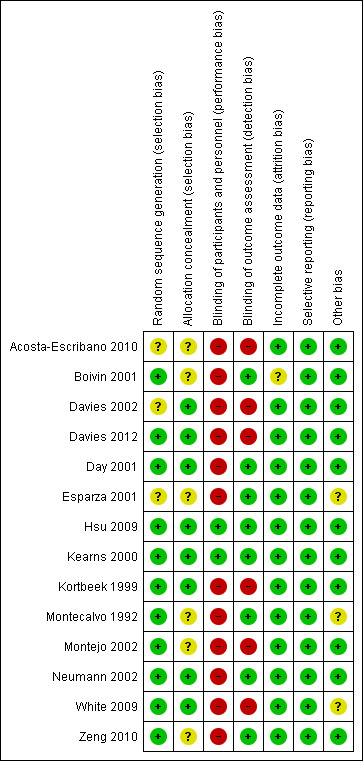
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most trials had low risk of selection bias. Random sequence generation was adequate in all but three trials (Acosta‐Escribano 2010; Davies 2002; Esparza 2001), in which assessment of risk of bias was not possible. Allocation concealment was adequate in most trials, with the exception of six trials (Acosta‐Escribano 2010; Boivin 2001; Esparza 2001; Montecalvo 1992; Montejo 2002; Zeng 2010) in which it was not possible to judge whether it was adequate.
Blinding
Concern has been expressed regarding blinding in most trials. No blinding of participants occurred in any of the studies, but this is unlikely to affect study results. Caregivers were not blinded in all trials, with the exception of two trials (Hsu 2009; Kearns 2000). This was considered to introduce high risk of bias because differences in care provided for the two groups may affect outcomes.
Outcome assessors were not blinded in most studies. We consider this to present high risk of bias only in trials in which pneumonia is one of the outcomes, as the other outcomes are more objective and are less likely to be affected by blinding of the outcome assessor.
Incomplete outcome data
Complete follow‐up was reported for all outcomes. No participant was lost to follow‐up. All trials performed statistical analyses in accordance with ITT, with the exception of three trials (Boivin 2001; Montecalvo 1992; Zeng 2010) in which it was uncertain whether they had used ITT in their analysis or not . In two trials (Boivin 2001; Esparza 2001), no standard deviations were reported for the percentage of goal rates achieved. In Boivin 2001, time from enrolment to insertion of a feeding tube and time from enrolment to initiation of feeding were not available. In two trials (Day 2001; Kortbeek 1999), duration of mechanical ventilation and length of stay were calculated as median and interquartile range (IQR) ‐ not as mean and standard deviation. In Singh 2012, no standard deviations were reported for total length of hospital stay. We failed to get these results even after we contacted the first study author; accordingly these data were not entered into the analysis.
Selective reporting
We detected no reporting bias, and all prespecified outcomes were reported in all studies. The funnel plot for main outcomes showed no publication bias (Figure 4; Figure 5; Figure 6).
4.
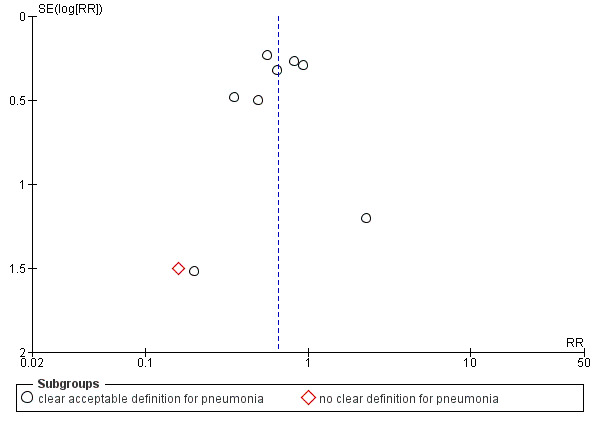
Funnel plot of comparison: 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, outcome: 1.1 Pneumonia.
5.
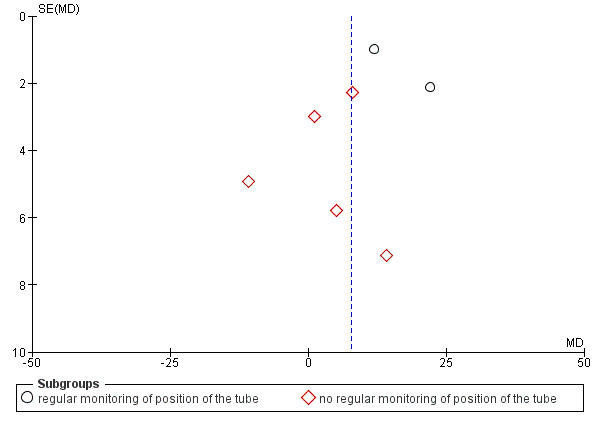
Funnel plot of comparison: 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, outcome: 1.5 Percentage of nutritional targets delivered to participants.
6.
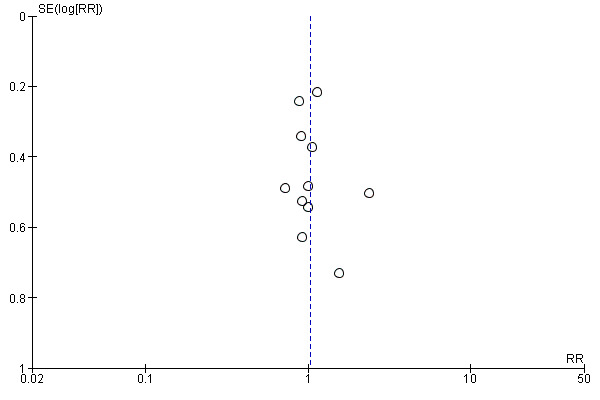
Funnel plot of comparison: 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, outcome: 1.3 Mortality.
Other potential sources of bias
Three trials described methodological issues, indicating possible risk of bias (Esparza 2001; Montecalvo 1992; White 2009). For one trial (Esparza 2001), inclusion and exclusion criteria were not available. For Montecalvo 1992, no clear protocol was provided for management of the feeding tube with regard to when the feeding should be interrupted; this resulted in significant differences in management in both groups and could have a significant effect on outcomes. For White 2009, 4/45 (8.9%) from the gastric group were crossed over to post‐pyloric feeding because of high gastric residual, and 10/50 (20%) from the post‐pyloric group did not receive the intended treatment because the feeding tube could not be inserted blindly. This was described in the protocol and involved ITT analysis, but this cross‐over might affect the outcome.
Effects of interventions
See: Table 1
Primary outcomes
Pneumonia
Nine of the 14 included trials, with a total of 819 participants, reported this outcome (Acosta‐Escribano 2010; Davies 2002; Davies 2012; Day 2001; Hsu 2009; Kortbeek 1999; Montecalvo 1992; Montejo 2002; White 2009). Two studies (Acosta‐Escribano 2010; Hsu 2009) found that post‐pyloric feeding was associated with a statistically significantly lower rate of pneumonia. The remaining seven trials found no statistically significant differences in the incidence of pneumonia. When data from all trials were combined in a meta‐analysis, a statistically significantly lower rate of pneumonia was noted in participants who were fed via the post‐pyloric route (RR 0.65, 95% CI 0.51 to 0.84; I² = 0%) (Analysis 1.1). The quality of evidence was moderate.
1.1. Analysis.
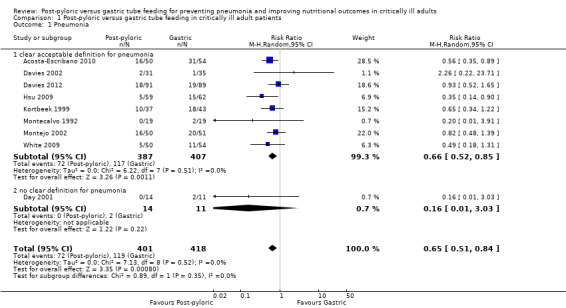
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 1 Pneumonia.
Subgroup analysis
Participants were mechanically ventilated in six trials (Acosta‐Escribano 2010; Davies 2002; Davies 2012; Hsu 2009; Kortbeek 1999; White 2009). Authors for the remaining three trials (Day 2001; Montecalvo 1992; Montejo 2002) did not mention whether participants were mechanically ventilated; this prevented us from performing a subgroup analysis for participants who were not on mechanical ventilation.
Only one study provided no clear definition for pneumonia (Day 2001). Results of this subgroup analysis were not modified much by removal of this study (RR 0.66, 95% CI 0.52 to 0.85; I² = 0%).
Sensitivity analysis
Two of the nine trials (Montecalvo 1992; White 2009) were excluded from the sensitivity analysis. Montecalvo 1992 was excluded because no standardized protocol was available for the management of tube feeding in both groups. White 2009 was excluded because 10/50 participants in the post‐pyloric group and 4/54 participants in the gastric tube group did not receive the intended treatment and were crossed over to the second group. In addition, baseline characteristics show that the APACHE II score was higher in the post‐pyloric group. The result was modified very little by removal of these two studies from the analysis (RR 0.67, 95% CI 0.52 to 0.87; I² = 2%) (Analysis 1.2).
1.2. Analysis.
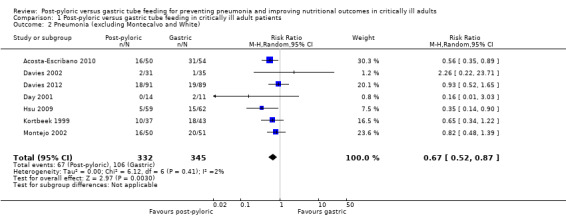
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 2 Pneumonia (excluding Montecalvo and White).
Mortality
Eleven of the included trials, with a total of 977 participants, reported this outcome (Acosta‐Escribano 2010; Boivin 2001; Davies 2002; Davies 2012; Esparza 2001; Hsu 2009; Kearns 2000; Kortbeek 1999; Montecalvo 1992; Montejo 2002; White 2009). Most studies did not specify clearly whether they calculated ICU or hospital mortality, with the exception of one trial (Davies 2012), for which investigators specified mortality as hospital mortality. None of the individual trials and no meta‐analysis of studies revealed any statistically significant differences in mortality (RR 1.03, 95% CI 0.83 to 1.29; I² = 0%) (Analysis 1.3). The quality of evidence was moderate
1.3. Analysis.
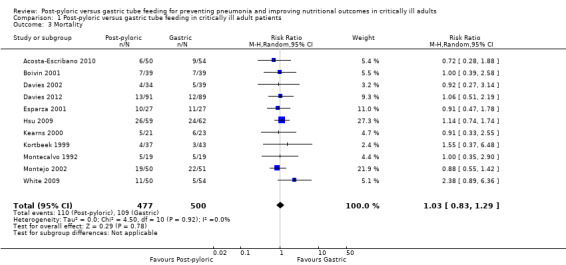
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 3 Mortality.
Sensitivity analysis
In a sensitivity analysis that excluded Montecalvo 1992 and White 2009, the result was not modified (RR .99, 95% CI 0.78 to 1.25; I² = 0%) (Analysis 1.4).
1.4. Analysis.
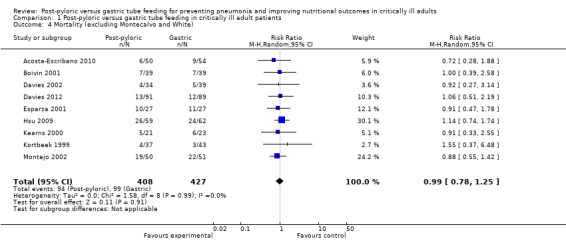
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 4 Mortality (excluding Montecalvo and White).
Percentage of nutritional targets delivered to participants
The percentage of total nutritional targets delivered was reported in 10 of the included trials (Acosta‐Escribano 2010; Boivin 2001; Davies 2012; Day 2001; Esparza 2001; Hsu 2009; Kearns 2000; Montecalvo 1992; Montejo 2002; White 2009).
Slight variation was evident in the description of nutritional intake. It was specified as percentage of the target energy requirement received during the whole study period in Davies 2012; Kearns 2000; and Montejo 2002); as percentage of the daily target energy received during the whole study period in Boivin 2001; Esparza 2001; Day 2001; Hsu 2009; Montecalvo 1992; and White 2009; and as "percentage of mean effective volume of diet" in Acosta‐Escribano 2010.
The standard deviation was not available for three trials (Boivin 2001; Day 2001; Esparza 2001); therefore these studies were not included in the meta‐analysis.
Four trials (Acosta‐Escribano 2010; Hsu 2009; Kearns 2000; Montecalvo 1992) showed a significantly higher percentage of average intake in the post‐pyloric group, and White 2009 reported significantly lower average intake in the post‐pyloric group. On the other hand, results from two studies (Davies 2012; Montejo 2002) revealed no significant differences between the two groups.
When data from the seven trials were combined in a meta‐analysis, with a total of 692 participants, a significantly greater percentage of nutritional intake could be seen in the post‐pyloric group (MD 7.80, 95% CI 1.43 to 14.18; I² = 90%) (Analysis 1.5). The quality of evidence was low.
1.5. Analysis.
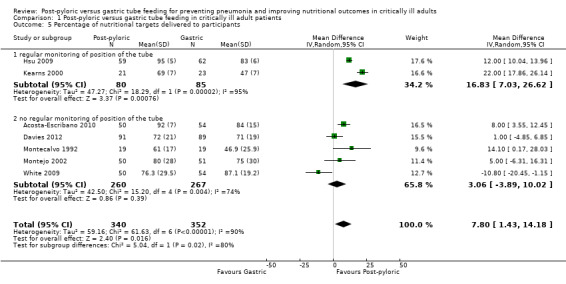
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 5 Percentage of nutritional targets delivered to participants .
Subgroup analysis
In trials with no regular monitoring of the position of the feeding tube (Acosta‐Escribano 2010; Davies 2012; Montecalvo 1992; Montejo 2002; White 2009), no significant differences between groups in average daily intake were reported (MD 3.06, 95% CI ‐3.89 to 10.02; I² = 74%).
The target location of the feeding tube was the duodenum in three trials (Day 2001; Hsu 2009; Kearns 2000) and the jejunum in six trials (Acosta‐Escribano 2010; Davies 2002, Davies 2012; Esparza 2001; Montecalvo 1992; Montejo 2002) the exact position was not clear in five trials (Boivin 2001; Kortbeek 1999; Neumann 2002; White 2009; Zeng 2010). Accordingly, we were not able to conduct a subgroup analysis regarding the exact position of the feeding tube.
Sensitivity analysis
In a sensitivity analysis that excluded two studies (Montecalvo 1992; White 2009), significantly higher average daily intake was noted in the post‐pyloric group (MD 10.19, 95% CI 3.90 to 16.47; I² = 92%) (Analysis 1.6).
1.6. Analysis.
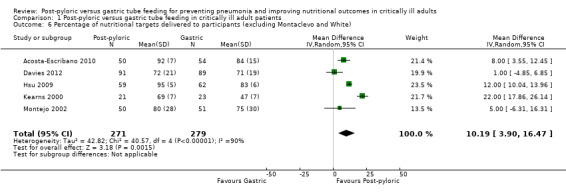
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 6 Percentage of nutritional targets delivered to participants (excluding Montaclevo and White) .
Time required to achieve the full nutritional target (in hours)
Time from the first attempt to insert the feeding tube until attainment of the full nutritional target was reported in six of the 14 included trials (Boivin 2001; Davies 2002; Hsu 2009; Kortbeek 1999; Neumann 2002; White 2009). The standard deviation was not available for Boivin 2001; therefore, this trial was not included in the meta‐analysis. A total of 432 participants were included in the six included trials. Two trials (Hsu 2009; Kortbeek 1999) showed faster time to reach target feed in the post‐pyloric group, two trials (Neumann 2002; White 2009) reported the opposite result and one study (Davies 2002) described no differences between the two groups. When data from all trials were combined in a meta‐analysis, no significant differences were noted in time required to achieve the full nutritional target in both groups (MD ‐1.99 hour, 95% CI ‐10.97 to 6.99; I² = 85%) (Analysis 1.7). The quality of evidence was very low.
1.7. Analysis.
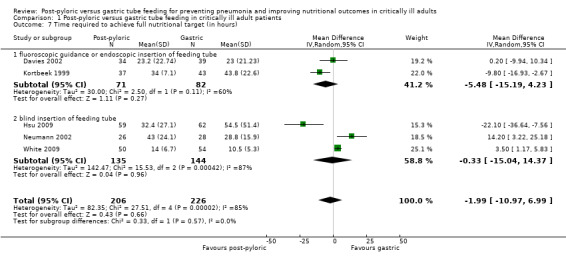
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 7 Time required to achieve full nutritional target (in hours) .
Subgroup analysis
No significant differences in time required to achieve the full nutritional target were observed in trials in which the feeding tube was inserted under fluoroscopic guidance/endoscopy (Davies 2002; Kortbeek 1999) (MD ‐5.5 hour, 95% CI ‐15.2 to 4.2; I² = 60%) and in trials in which insertion was done blindly at the bedside (Hsu 2009; Neumann 2002; White 2009) (MD ‐0.3 hour, 95% CI 15.0 to 24.3; I² = 87%). These results signify the importance of regular monitoring of the position of the feeding tube, because the tube might migrate from the small intestine back to the stomach during the patient's stay in the ICU.
Sensitivity analysis
In a sensitivity analysis that excluded White 2009, the result was not modified (MD ‐3.994, 95% CI ‐17.16 to 9.19; I² = 85%) (Analysis 1.8).
1.8. Analysis.
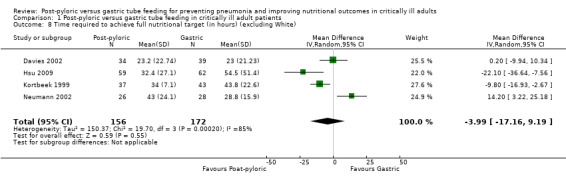
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 8 Time required to achieve full nutritional target (in hours) (excluding White).
Secondary outcomes
ICU length of stay (in days)
Nine of the 14 included trials reported this outcome (Acosta‐Escribano 2010; Davies 2002; Davies 2012; Hsu 2009; Kearns 2000; Kortbeek 1999; Montecalvo 1992; Montejo 2002; White 2009). Two studies (Davies 2012; Kortbeek 1999) reported this outcome as a median value; therefore it was not possible to include them in the analysis. None of the individual trials found any statistically significant difference in ICU length of stay, nor did the meta‐analysis of the seven included studies with a total of 585 participants (MD ‐0.70 day, 95% CI ‐2.31 to 0.91; I² =40%) (Analysis 1.9).
1.9. Analysis.

Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 9 ICU length of stay (in days).
Duration of mechanical ventilation (in days)
Six of the included trials reported this outcome (Acosta‐Escribano 2010; Davies 2012; Hsu 2009; Kortbeek 1999; Montecalvo 1992; White 2009). For Kortbeek 1999, this was reported as a median value; therefore it was not possible to include this information in the analysis. None of the individual trials found any statistically significant differences in duration of mechanical ventilation, nor did the meta‐analysis of the five included studies with a total of 549 participants (MD ‐0.92 day, 95% CI ‐2.11 to 0.28; I² = 10%) (Analysis 1.10). The quality of evidence was moderate.
1.10. Analysis.
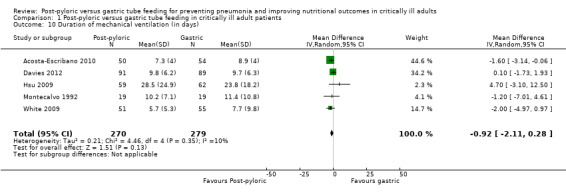
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 10 Duration of mechanical ventilation (in days).
Gastrointestinal complications
Vomiting
Six of the included trials reported this outcome, with a total of 543 participants (Boivin 2001; Davies 2012; Day 2001; Hsu 2009; Montecalvo 1992; Montejo 2002). None of the individual trials found statistically significant differences in vomiting between the two groups, nor did the meta‐analysis of studies report this finding (RR 1.01, 95% CI 0.54 to 1.89; I² = 21%) (Analysis 1.11).
1.11. Analysis.
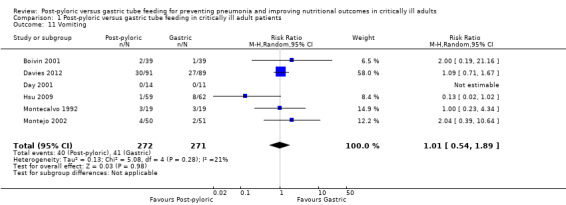
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 11 Vomiting.
Diarrhoea
Eight of the included trials reported this outcome, with a total of 675 participants (Acosta‐Escribano 2010; Davies 2002; Davies 2012; Day 2001; Hsu 2009; Montecalvo 1992; Montejo 2002; Zeng 2010). None of the individual trials found any statistically significant differences in diarrhoea between the two groups, nor did the meta‐analysis of studies (RR 0.96, 95% CI 0.74 0 to 1.25; I² = 0%) (Analysis 1.12).
1.12. Analysis.
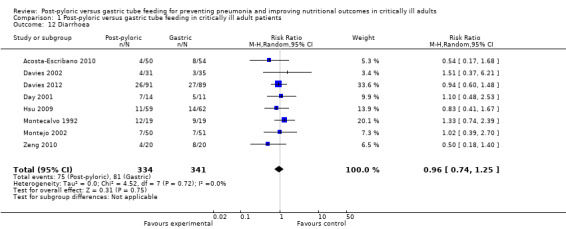
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 12 Diarrhoea.
High gastric residual
We could not include this outcome in our analysis because different studies reported this outcome in different ways. Some studies reported the actual amount of gastric residual, other studies determined how many times the participant had high gastric residual and yet other studies reported the number of participants with high gastric residual.
Complications related to tube insertion (epistaxis, pneumothoraces, gastrointestinal bleeding)
Four of the included trials, with a total of 324 participants, reported this outcome (Davies 2002; Davies 2012; Montecalvo 1992; Zeng 2010). The complication most commonly reported was gastrointestinal bleeding. Montecalvo 1992 reported one case of pneumothorax in the gastric group. None of the other studies reported epistaxis as a complication. Analysis of the data showed no significant differences in complications between the two groups (RR 0.51, 95% CI 0.19 to 1.36; I² = 0%) (Analysis 1.13). The quality of evidence was low.
1.13. Analysis.
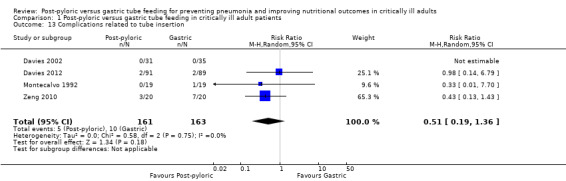
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 13 Complications related to tube insertion.
Complications related to tube maintenance (need for tube replacement, tube occlusion)
This outcome includes complications such as the need for tube replacement, repositioning and blockage. Seven of the included trials, with a total of 638 participants, reported this outcome (Boivin 2001; Davies 2002; Davies 2012; Esparza 2001; Hsu 2009; Montecalvo 1992; Montejo 2002). Only one study (Montejo 2002) showed a significantly higher complication rate in the post‐pyloric group. When data from all trials were combined in a meta‐analysis, no significant difference in complication rates was observed between groups (RR 1.63, 95% CI 0.93 to 2.86; I² = 58%) (Analysis 1.14). The quality of evidence was low.
1.14. Analysis.
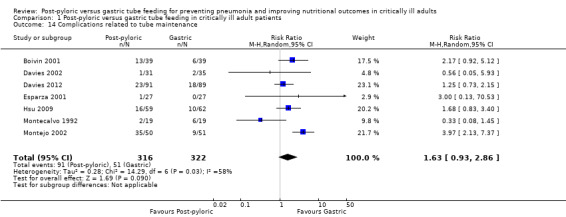
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 14 Complications related to tube maintenance.
Time required to start feeding (in hours)
Time from the first attempt to insert the feeding tube until the start of feeding was reported in six trials (Boivin 2001; Davies 2002; Montecalvo 1992; Montejo 2002; Neumann 2002; White 2009). The standard deviation was not available for Boivin 2001; therefore the result was not included in the meta‐analysis. The feeding tube was inserted blindly by the bedside nurse in two trials (Neumann 2002; White 2009); blindly by the physician in Montecalvo 1992; by endoscopy in Davies 2002; and by endoscopy, under fluoroscopic guidance, blindly or by echo in Montejo 2002. Three trials showed a significant delay in time to start feeding in the post‐pyloric group (Montejo 2002; Neumann 2002; White 2009). When data from five trials were combined in a meta‐analysis with a total participant count of 374, a significant delay in time to start feeding was reported in the post‐pyloric group (MD 11.05 hour, 95% CI 3.05 to 19.05; I² = 90%) (Analysis 1.15).
1.15. Analysis.
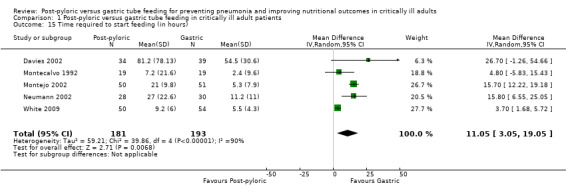
Comparison 1 Post‐pyloric versus gastric tube feeding in critically ill adult patients, Outcome 15 Time required to start feeding (in hours).
Discussion
Summary of main results
Evidence from 14 studies with 1109 participants showed some benefit for post‐pyloric feeding compared with feeding by the gastric route for critically ill adult patients. We found evidence of moderate quality for a lower rate of pneumonia and evidence of low quality for an increase in the percentage of nutrients delivered to the participant. However, these outcomes were not reflected in other important clinical outcomes such as duration of mechanical ventilation, mortality and ICU length of stay, which did not differ between the two groups.
We found no evidence that participants fed by post‐pyloric tube reach their full nutritional target earlier than those fed by gastric tube, regardless of whether the tube was inserted under fluoroscopic guidance/endoscopy or blindly at the bedside. Our results revealed no significant differences in adverse effects between the two groups. No evidence suggested that post‐pyloric feeding was associated with an increase in gastrointestinal complications such as vomiting or diarrhoea, and no evidence was found of an increase in the rate of complications related to tube insertion, such as upper gastrointestinal bleeding, or of complications related to tube maintenance, such as the need for tube replacement, repositioning or blockage.
A significant delay in time to start feeding was reported in the post‐pyloric group; this was expected and was related to the time needed for insertion of the tube. In spite of this delay, no increase in the time required to achieve the full nutritional target was reported. This could be explained by the fact that participants fed through the small bowel may receive a greater percentage of nutritional intake and can “catch up” with the participant fed through the stomach.
Overall completeness and applicability of evidence
In most of the included trials, the studied population was mixed and included surgical and medical participants; this makes the result applicable for most intensive care patients. Generalization of our results is limited because most studies were conducted at single centres and the total number of participants was small. Most studies did not report all expected outcomes; this is why we analysed a limited number of studies for most of our reported outcomes. In addition, the standard deviation was not available for certain important outcomes, some of which were reported as a median value and an IQR instead of a mean and a standard deviation. Accordingly, all of these results were not entered into the meta‐analysis; thus, the results should be interpreted with great caution.
Quality of the evidence
The quality of evidence is summarized in Table 1. Included studies were relatively small, ranging from 25 to 181 participants, with a total of 1109. The small sample size might have created an inflated overall estimate of treatment effect. Although all studies were RCTs, blinding was not properly done, and this is reflected in the moderate quality of evidence for pneumonia, mortality and duration of mechanical ventilation. The increase in the percentage of daily nutrients delivered to the patient with post‐pyloric feeding was judged to be of low quality because of inconsistency and substantial statistical heterogeneity, indicating that this observation should be interpreted with caution. The heterogeneity may be explained in part by the fact that slight variation was noted in the description of nutrient intake among trials. Although time to reach full nutritional target was equal in the two groups, this finding again should be interpreted with caution, as the quality of evidence for this outcome was judged to be very low because of inconsistency and imprecision, which may reflect that insertion of a post‐pyloric tube can be technically difficult and time consuming. The evidence indicating that post‐pyloric tube insertion is not associated with more complications during insertion or maintenance when compared with insertion of a gastric tube was judged to be of low quality because of imprecision and inconsistency, respectively.
Potential biases in the review process
To the best of our knowledge, no potential biases arose from the review process. We have identified all published studies that report post‐pyloric tube feeding.
Agreements and disagreements with other studies or reviews
Over the past decade, seven systematic reviews have compared gastric versus post‐pyloric feeding (Alhazzani 2013; Deane 2013; Heyland 2002; Ho 2006; Jiyong 2013; Marik 2003; Zhang 2013). Of the older meta‐analyses, two (Ho 2006; Marik 2003) failed to demonstrate any clinical benefit derived from post‐pyloric tube feeding, and Heyland 2002 suggested that post‐pyloric tube feeding reduced the risk of pneumonia.
The more recently published analyses concluded that post‐pyloric feeding was able to deliver a greater proportion of the estimated energy requirement (Deane 2013; Zhang 2013), and it reduced the risk of pneumonia (Alhazzani 2013; Deane 2013; Jiyong 2013) without affecting other clinically important outcomes. One of the latest analyses (Jiyong 2013) has some limitations in that studies of both adult and paediatric populations were included, along with a study in which participants received care outside the critical care environment. Consistent with previous meta‐analyses, our analysis confirmed that post‐pyloric feeding may improve nutritional intake and reduce the incidence of pneumonia.
Our analysis incorporated all recent trials that met our inclusion criterion for clinical evidence of pneumonia and excluded studies that considered aspiration per se as their outcome without clinical evidence of pneumonia.
Authors' conclusions
Implications for practice.
We found evidence of moderate quality suggesting that post‐pyloric feeding reduces the rate of pneumonia by 30% and low‐quality evidence suggesting that post‐pyloric feeding may lead to an increase in the amount of nutrition delivered to these participants when compared with gastric tube feeding. We do not have sufficient evidence to show that other clinically important outcomes such as duration of mechanical ventilation, mortality and length of stay were affected by the site of tube feeding. Low‐quality evidence shows that Insertion of a post‐pyloric feeding tube appears to be safe and was not associated with complications such as epistaxis, pneumothoraces and gastrointestinal bleeding when compared with gastric tube insertion. Placement of the post‐pyloric tube can be challenging and technically difficult, requiring expertise and sophisticated radiological or endoscopic assistance.
Given the findings of this review, the best method for placement of the post‐pyloric feeding tube remains unclear. The clinician is left with this decision, which should be based on the policies of institutional facilities and should be made on a case‐by‐case basis. Protocols and training for bedside placement by physicians or nurses should be evaluated.
Implications for research.
In view of conflicting data on the benefits of post‐pyloric feeding, further adequately powered multi‐centre trials are needed. Measurement of clinically important outcomes such as pneumonia and nutritional administration should be well defined and standardised among future studies. Regular monitoring of tube position must be documented, as the tube might migrate during routine patient care. Further studies should target selected critically ill patients with intolerance to gastric feeding.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
We would like to thank Mathew Zacharias (Content Editor); Nathan Pace (Statistical Editor); Paul Marik, Bronagh Blackwood and Alison Avenell (Peer Reviewers), Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia Review Group (CARG)) and Ann E Fonfa (member of the Cochrane Consumer Network) for help and editorial advice provided during preparation of the protocol (Alkhawaja 2010) for this systematic review.
Acknowledgements
We would like to thank Rodrigo Cavallazz (Content Editor); Nathan Pace (Statistical Editor); Danielle Bear, Alison Avenell and Paul Marik (Peer Reviewers); and Patricia Tong (Consumer Referee) for help and editorial advice provided during preparation of this systematic review. Our great thanks to Jane Cracknell (Managing Editor, CARG) for continuous help and support, and Sandra McKeown at the University of Western Ontario for help with the literature search. We would also like to thank Waleed Alhazzani for kindly providing us with the Zeng 2010 article, and Kunming Tao for helping with translation of this article.
Appendices
Appendix 1. CENTRAL search
#1 MeSH descriptor Enteral Nutrition explode all trees #2 MeSH descriptor Gastrostomy explode all trees #3 MeSH descriptor Duodenostomy explode all trees #4 MeSH descriptor Jejunostomy explode all trees #5 MeSH descriptor Intubation, Gastrointestinal explode all trees #6 (gastrostom* or duodenostom* or jejunostom* or PEG or g‐tube* or ng‐tube* or j‐tube* or nj‐tube* or PEJ):ab,ti #7 ((nutrition* or fed or feed* or tube* or intub*) near (gastr* or nasogastr* or stomach or duoden* or nasoduoden* or jejun* or nasojejun* or bowel* or intestine* or post?pylor* or trans?pylor* or nasoenter* or orogastric or gavage)):ab,ti #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Pneumonia explode all trees #10 MeSH descriptor Pneumonia, Aspiration explode all trees #11 MeSH descriptor Intensive Care Units explode all trees #12 MeSH descriptor Burn Units explode all trees #13 MeSH descriptor Respiratory Care Units explode all trees #14 MeSH descriptor Critical Care explode all trees #15 MeSH descriptor Intensive Care explode all trees #16 MeSH descriptor Critical Illness explode all trees #17 MeSH descriptor Craniocerebral Trauma explode all trees #18 MeSH descriptor Burns explode all trees #19 MeSH descriptor Wounds and Injuries explode all trees #20 MeSH descriptor Pancreatitis explode all trees #21 (pneumonia* or critical* ill* or critical* care or intensive care or ICU or burn* or trauma* or head injur* or pancreatitis):ti,ab #22 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 (#8 AND #22) #24 (infant* or child* or adolescent*) #25 (adult* or aged) #26 (#24 AND NOT ( #25 AND #24 )) #27 (#23 AND NOT #26)
Appendix 2. MEDLINE (Ovid SP) search
1. enteral nutrition/ or gastrostomy/ or duodenostomy/ or jejunostomy/ or intubation, gastrointestinal/ or (gastrostom* or duodenostom* or jejunostom* or peg or g‐tube* or ng‐tube* or j‐tube* or nj‐tube* or pej).ab,ti. or ((nutrition* or fed or feed* or tube* or intub*) adj5 (gastr* or nasogastr* or stomach or duoden* or nasoduoden* or jejun* or nasojejun* or bowel* or intestine* or post?pylor* or trans?pylor* or nasoenter* or orogastric or gavage)).ab,ti. 2. pneumonia/ or pneumonia, aspiration/ or pneumonia, lipid/ or intensive care units/ or burn units/ or respiratory care units/ or critical care/ or intensive care/ or critical illness/ or craniocerebral trauma/ or burns/ or "wounds and injuries"/ or pancreatitis/ or (pneumonia* or critical* ill* or critical* care or intensive care or ICU or burn* or trauma* or head injur* or pancreatitis).ab,ti. 3. 1 and 2 4. (infant* or child* or adolescent*).af. 5. (adult* or aged).af. 6. 3 not (4 not (5 and 4)) 7. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 8. 6 and 7
Appendix 3. EMBASE (Ovid SP) search
1. enteric feeding/ or artificial feeding/ or nose feeding/ or nasogastric tube/ or stomach tube/ or stomach intubation/ or gastrostomy/ or percutaneous endoscopic gastrostomy/ or duodenum intubation/ or duodenostomy/ or jejunostomy/ or (g‐tube* or ng‐tube* or gastrostom* or PEG or duodenostom* or jejunostom* or PEJ or j‐tube* or nj‐tube*).ti,ab. or ((nutrition* or fed or feed* or tube* or intub*) adj5 (gastr* or nasogastr* or stomach or duoden* or nasoduoden* or jejun* or nasojejun* or bowel* or intestine* or post?pylor* or trans?pylor* or nasoenter* or orogastric or gavage)).ab,ti. 2. exp pancreatitis/ or injury/ or burn/ or "head and neck injury"/ or multiple trauma/ or critical illness/ or intensive care/ or intensive care unit/ or pneumonia/ or aspiration pneumonia/ or (pneumonia* or critical* ill* or critical* care or intensive care or ICU or burn* or trauma* or head injur* or pancreatitis).ab,ti. 3. 1 and 2 4. (infant* or child* or adolescent*).af. 5. (adult* or aged).af. 6. 3 not (4 not (5 and 4)) 7. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 8. 6 and 7
Appendix 4. CINAHL (EBSCO host) search
S1 ( (MH "Enteral Nutrition") OR (MH "Feeding Tubes") OR (MH "Nasoenteral Tubes") OR (MH "Gastrostomy") OR (MH "Intubation, Gastrointestinal") OR (MH "Jejunostomy Tubes") OR (MH "Jejunostomy") OR (MH "Gastrostomy Tubes") ) OR ( gastrostom* or duodenostom* or jejunostom* or PEG or g‐tube* or ng‐tube* or j‐tube* or nj‐tube* or PEJ ) OR ( ((nutrition* or fed or feed* or tube* or intub*) AND (gastr* or nasogastr* or stomach or duoden* or nasoduoden* or jejun* or nasojejun* or bowel* or intestine* or post?pylor* or trans?pylor* or nasoenter* or orogastric or gavage)) ) S2 ( (MH "Critical Care") OR (MH "Intensive Care Units") OR (MH "Critical Illness") OR (MH "Critically Ill Patients") OR (MH "Trauma") OR (MH "Head Injuries") OR (MH "Burn Patients") OR (MH "Pancreatitis") OR (MH "Pneumonia") OR (MH "Pneumonia, Aspiration") ) OR ( pneumonia* or critical* ill* or critical* care or intensive care or ICU or burn* or trauma* or head injur* or pancreatitis ) S3 S1 and S2 S4 infant* or child* or adolescent* S5 adult* or aged S6 S3 NOT (S4 NOT (S4 AND S5)) S7 ( (MH "Clinical Trials") OR (MH "Randomized Controlled Trials") OR (MH "Random Assignment") OR (MH "Placebos") ) OR PT clinical trial OR AB ( random or placebo or groups or trial* ) S8 S6 and S7
Appendix 5. Study selection form
Study Selection Form
| First author | Journal/Conference proceedings, etc | Year |
Study eligibility
| Type of study | Relevant participants | Relevant intervention | Relevant outcome |
| RCT or quasi‐randomized trial |
|
Transpyloric vs gastric tube feeding with catheters passed via nose or mouth |
|
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
| Do not proceed if any of the above answers is ‘No’. If study is to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into the ‘Table of excluded studies’.. |
Key
GI = Gastrointestinal.
ICU = Intensive care unit.
Appendix 6. Eligible trials form
Eligible Trials Form
| Code each paper | Author(s) | Journal/Conference proceedings, etc | Year |
| A | |||
| B | |||
| C |
Appendix 7. Data extraction form
Data Extraction Form
1‐Participant characteristics
| Further details | |
| Type of patient | Medical/Surgical/Mixed |
| Age (mean, median, range, etc) |
|
| Sex of participants (numbers/%, etc) |
2‐Trial characteristics
| Further details | |
| Single‐centre/Multi‐centre | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomly assigned? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Duration of treatment (state weeks/months, etc; if cross‐over trial, give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years, or if not stated) |
3‐Intervention details
| Nasogastric tube | Post‐pyloric tube | ||
| Feeding tube | size and length | ||
| Initial method of insertion | blind/endoscopy/fluoroscopy | ||
| Number of attempts | |||
| Person inserting tube | doctor/nurse | ||
| Target location | duodenum/jejunum | ||
| Confirmation of placement | yes/no | ||
| Monitoring position of the tube | X‐ray/residual volume not done |
||
| Routine use of H2antagonist or proton pump inhibitor (PPI) | yes/no | ||
| Routine use of prokinetic (not for tube placement) | yes/no | ||
| Application of other intervention to prevent pneumonia such as head elevation, chlorhexidene mouthwash, etc (please specify) |
yes/no |
4‐Outcome
| Information available in paper | |
| Outcome 1 Pneumonia |
Yes/No |
| Outcome 2 Mortality |
Yes/No |
| Outcome 3 Achievement of target nutritional requirement |
Yes/No |
| Outcome 4 Time required to achieve full nutritional target |
Yes/No |
| Outcome 5 ICU length of stay |
|
| Outcome 6 Duration of mechanical ventilation |
Yes/No |
| Outcome 7 Gastrointestinal complications: vomiting, diarrhoea, high gastric residual |
Yes/No |
| Outcome 8 Complications related to tube insertion: epistaxis, pneumothoraces, gastrointestinal bleeding |
Yes/No |
| Outcome 9 Complications related to tube maintenance: need for tube replacement, tube occlusion |
Yes/No |
| Outcome 10 Time required to start feeding: time from patient enrolment in the study to time the feeding was started |
Yes/No |
| For continuous data (with a separate copy for each relevant subgroup) | |||||||
| Code of paper | Outcomes/Unit of measurement | Unit of measurement | Post‐pyloric group | Nasogastric group | Details (if outcome described only in text) | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Outcome 3 Percentage of daily goal calorie fed |
|||||||
| Outcome 4 Time required to achieve full nutritional target |
|||||||
| Outcome 5 ICU length of stay |
|||||||
| Outcome 6 Duration of assisted ventilation |
|||||||
| Outcome 10 Time required to start feeding |
|||||||
| For dichotomous data (with a separate copy for each relevant subgroup) | |||
| Code of paper | Outcomes | Post‐pyloric group (n) n = number of participants, not number of events | Nasogastric group (n) n = number of participants, not number of events |
| Outcome 1 Pneumonia |
|||
| Outcome 2 Mortality |
|||
| Outcome 7 Gastrointestinal complications: vomiting, diarrhoea, high gastric residual |
|||
| Outcome 8 Complications related to tube insertion: epistaxis, pneumothoraces, gastrointestinal bleeding |
|||
| Outcome 9 Complications related to tube maintenance: need for tube replacement, tube occlusion |
|||
| Other information that you believe is relevant to the results Indicate if any data were obtained from the primary study author; if results were estimated from graphs, etc; or if they were calculated by you using a formula (this should be stated and the formula given). In general, if results were not reported in paper(s) but were obtained, this should be made clear here to be cited in the review Freehand |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First study author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give contact name and details | ||
Appendix 8. Quality assessment of eligible trials form
Methodological quality
Trial:
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Comment on allocation by review authors or included study Quote concerning allocation: | Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
| Concealment of allocation Process used to prevent foreknowledge of group assignment in an RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Comment on allocation concealment by review authors or include study quote concerning allocation: | Adequate |
| Inadequate | |
| Unclear | |
| Blinding | |
| Participants | Yes/No/Unclear |
| Caregiver | Yes/No/Unclear |
| Outcome assessor | Yes/No/Unclear |
| Other (please specify) | Yes/No/Unclear |
| Comment on blinding by review authors or include study quote concerning allocation: | |
| Incomplete outcome data |
Yes/No/Unclear |
| Discuss if appropriate | |
| Selective outcome reporting | Yes/No/Unclear |
| Discuss if appropriate | |
| Other potential sources of bias | Yes/No/Unclear |
| Discuss if appropriate | |
Data and analyses
Comparison 1. Post‐pyloric versus gastric tube feeding in critically ill adult patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pneumonia | 9 | 819 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.51, 0.84] |
| 1.1 clear acceptable definition for pneumonia | 8 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.52, 0.85] |
| 1.2 no clear definition for pneumonia | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.03] |
| 2 Pneumonia (excluding Montecalvo and White) | 7 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.52, 0.87] |
| 3 Mortality | 11 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.83, 1.29] |
| 4 Mortality (excluding Montecalvo and White) | 9 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.25] |
| 5 Percentage of nutritional targets delivered to participants | 7 | 692 | Mean Difference (IV, Random, 95% CI) | 7.80 [1.43, 14.18] |
| 5.1 regular monitoring of position of the tube | 2 | 165 | Mean Difference (IV, Random, 95% CI) | 16.83 [7.03, 26.62] |
| 5.2 no regular monitoring of position of the tube | 5 | 527 | Mean Difference (IV, Random, 95% CI) | 3.06 [‐3.89, 10.02] |
| 6 Percentage of nutritional targets delivered to participants (excluding Montaclevo and White) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 10.19 [3.90, 16.47] |
| 7 Time required to achieve full nutritional target (in hours) | 5 | 432 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐10.97, 6.99] |
| 7.1 fluoroscopic guidance or endoscopic insertion of feeding tube | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐5.48 [‐15.19, 4.23] |
| 7.2 blind insertion of feeding tube | 3 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐15.04, 14.37] |
| 8 Time required to achieve full nutritional target (in hours) (excluding White) | 4 | 328 | Mean Difference (IV, Random, 95% CI) | ‐3.99 [‐17.16, 9.19] |
| 9 ICU length of stay (in days) | 7 | 585 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.31, 0.91] |
| 10 Duration of mechanical ventilation (in days) | 5 | 549 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐2.11, 0.28] |
| 11 Vomiting | 6 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.89] |
| 12 Diarrhoea | 8 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.25] |
| 13 Complications related to tube insertion | 4 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.19, 1.36] |
| 14 Complications related to tube maintenance | 7 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.93, 2.86] |
| 15 Time required to start feeding (in hours) | 5 | 374 | Mean Difference (IV, Random, 95% CI) | 11.05 [3.05, 19.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acosta‐Escribano 2010.
| Methods | Randomized controlled trial with 2 parallel groups Single centre Country: Spain |
|
| Participants | Severe traumatic brain injury patient Mechanically ventilated Mean age: 38 years Male: 86% Median APACHE II score: 17 Inclusion criteria 1‐Age between 18 and 80 years 2‐Glasgow coma scale score < 9 3‐Apache II score between 15 and 30 4‐Sequential organ failure assessment (SOFA) index < 6 (excluding the neurological variable) 5‐Mechanical ventilation required on admission 6‐Artificial nutrition required for at least 5 days Exclusion criteria 1‐Chronic renal failure (plasma creatinine > 2 mg/dL) 2‐Hepatic failure (bilirubin > 3 mg/dL) 3‐Contraindication for enteral nutrition: intestinal inflammatory disease, past history of gastric resection, abdominal surgery within 2 months before inclusion, facial trauma 4‐Treatment with steroid or immunosuppressive drugs 5‐Traumatic spinal cord injury 6‐Pregnancy 7‐Body mass index (BMI) > 35 kg/m2 8‐Severe malnutrition with BMI < 18 kg/m2 9‐Life expectancy less than 5 days 10‐Encephalic death on admission 11‐Refusal to participate 12‐Participation in another clinical study Number of participants randomly assigned: 104 Number of participants who received intended treatment (50 post‐pyloric; 54 gastric) Number of participants analysed (50 post‐pyloric; 54 gastric) |
|
| Interventions | Post‐pyloric group: double‐lumen tube (Compat Stay‐Put 9/18) was placed in the jejunum through spontaneous placement or in the radiological suite Position was confirmed by plain abdominal film. No regular monitoring for position of the tube Same feeding protocol was used in both groups Preventive measures were used to reduce risk of pneumonia: head elevation Duration of follow‐up was not mentioned |
|
| Outcomes |
Primary outcome 1‐Incidence of nosocomial pneumonia (score > 6 using Clinical Pulmonary Infection Score criteria) Secondary outcomes 1‐Enteral feeding, gastrointestinal complications: abdominal distension, increased gastric residual volume, diarrhoea, aspiration 2‐Achievement of nutritional goal measured in mean efficacious volume (mean volume of diet administered daily) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patient[s] were randomized in 1:1 ratio to one of two treatment group[s]" Method of randomization was not mentioned Information was insufficient to permit judgement of ’high risk’ or ’low risk’ |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned by study authors Information was insufficient to permit judgement of ’high risk’ or ’low risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Boivin 2001.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: New Mexico, USA |
|
| Participants | Medical, surgical and neuroscience intensive care unit 99% mechanically ventilated Age, years: 49 post‐pyloric; 48 gastric APACHE II score: 17 post‐pyloric; 16 gastric Inclusion criteria 1‐Patients 18 years of age or older needing enteral feed as decided by treating team Exclusion criteria 1‐Patients who were already being fed 2‐Pancreatitis 3‐Burns 4‐Severe head injury (Glasgow Coma Score < 6) 5‐Pregnant 6‐Prisoners 7‐Patients with previous gastric surgery 8‐Ongoing gastrointestinal bleeding 9‐Expected need for tube feeding was < 72 hours Number of participants randomly assigned: 80 Number of participants who received intended treatment: 39 post‐pyloric; 39 gastric Number of participants analysed: 39 post‐pyloric; 39 gastric |
|
| Interventions | Post‐pyloric group: 10‐Fr weighted feeding tube (Flexiflo, Ross Product Div., Abbott Laboratories) was placed blindly by investigator. Target location was not mentioned, and placement was confirmed and monitored by chest and abdominal radiographs Gastric group: nasogastric or orogastric tube placed into the stomach, position confirmed by abdominal radiograph, patients receiving erythromycin (200 mg) every 8 hours to increase gastric motility Same feeding protocol was used in both groups Partiicpants were studied until they were able to tolerate oral intake or until 96 hours had elapsed |
|
| Outcomes |
Primary outcome 1‐Percentage of recommended calories received by participant Secondary outcomes 1‐Time required to achieve full nutritional target 2‐Daily percentage of goal rate 3‐Change in albumin, pre‐albumin 4‐PaO2/FIO2 index (partial pressure of arterial oxygen tension/fraction of inspired oxygen) 5‐Mortality |
|
| Notes | No standard deviations were reported for percentage of goal rate achieved; time from enrolment to insertion of feeding tube; and time from enrolment to initiation of feeding. E‐mail was sent to study author, who replied but did not provide further data until November 2013 Duration of mechanical ventilation was not reported; study authors reported ventilator‐free days; therefore this outcome was not included in our analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number table in blocks of ten" |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of ’high risk’ or ’low risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded, but outcome was not likely to be influenced Personnel: blinding not mentioned by study authors Comments: personnel probably were not blinded; otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned by study authors Comments: outcome not likely to be influenced, as incidence of pneumonia was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data Uncertain whether analysis was done by intention‐to‐treat Standard deviation was not available for some outcomes |
| Selective reporting (reporting bias) | Low risk | Study protocol available, and all primary and secondary outcomes reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Davies 2002.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: Australia |
|
| Participants | Medical and surgical intensive care unit Age, years: 55 post‐pyloric; 53 gastric 90% mechanically ventilated APACHE II score: 17.6 post‐pyloric; 18.6 gastric Inclusion criteria 1‐Patient expected to require nutritional and critical care support for at least 3 days Exclusion criteria 1‐Patients unsuitable for passage of a nasoenteral tube (e.g. facial fractures, coagulopathy) 2‐Patient already receiving nutritional support 3‐Patient expected to die within 48 hours 4‐Recent abdominal surgery was not an exclusion criterion Number of participants randomly assigned: 73 Number of participants who received intended treatment: 34 post‐pyloric; 39 gastric Number of participants analysed: 31 post‐pyloric; 35 gastric |
|
| Interventions | Post‐pyloric group: 126 cm long, 2.1 mm diameter tube (ENTRAL 204–09, Mallinckrodt, St Louis, MO) was inserted into jejunum by endoscopy over a guidewire. Placement was confirmed and monitored by chest and abdominal radiograph every third day Gastric group: nasogastric or orogastric tube placed into the stomach, with position confirmed by abdominal radiograph Same feeding protocol used in both groups Preventive measure to reduce risk of pneumonia: not mentioned Study ceased when participant discontinued enteral nutritional support, commenced concurrent oral nutrition or was discharged from ICU |
|
| Outcomes |
Primary outcome 1‐Tolerance of enteral nutrition, which was measured by gastric residual volume Secondary outcomes 1‐New onset of pneumonia: according to consensus conference definition (clinical criteria, chest X‐ray, changes and microbiological data) 2‐New onset of systemic inflammatory response 3‐New onset of severe sepsis 4‐New onset of septic shock 5‐New onset of acute renal failure 6‐Gastrointestinal bleeding 7‐Diarrhoea 8‐Mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was by sealed envelope" Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was by sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, but outcomes not likely to be influenced Personnel: not mentioned by study authors Comments: personnel probably not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned by study authors Comments: probably outcome assessments not blinded; otherwise this would be mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Davies 2012.
| Methods | Randomized controlled trial with 2 parallel groups Multi‐centre Country: Australia |
|
| Participants | Multidisciplinary, medical‐surgical ICU patient Mechanically ventilated patients on narcotic infusion Mean age: 52 years Male: 74% Median APACHE II score: 20 Inclusion criteria 1‐Age ≥ 18 years 2‐ICU stay < 48 hours 3‐Receiving mechanical ventilation with ≥ 48 hours anticipated duration 4‐Receiving narcotic infusion (morphine ≥ 2 mg/h, fentanyl ≥ 20 mcg/h or meperidine ≥ 20 mg/h) 5‐Elevated GRV (single measurement > 150 mL or 12 hour cumulative volume > 500 mL) Exclusion criteria 1‐Previous anatomy‐altering upper gastrointestinal surgery 2‐Gastric malignancy 3‐Esophageal varices 4‐Current peptic ulceration 5‐Mechanical bowel obstruction 6‐Presence of gastrostomy or jejunostomy 7‐Nutrition therapy before ICU admission 8‐Severe coagulopathy 9‐Pregnancy 10‐Suspected brain death 11‐Death expected within 24 hours 12‐Suspected hypoxic‐ischaemic encephalopathy Number of participants randomly assigned: 181 Number of participants who received intended treatment (91 post‐pyloric; 89 gastric) Number of participants analysed (91 post‐pyloric; 89 gastric) |
|
| Interventions | Post‐pyloric group: spontaneously migrating frictional nasojejunal tube (Tiger Tube, Cook Critical Care, Bloomington, IN) inserted using a standardized method Position confirmed radiologically No regular monitoring of position Same feeding protocol used in both groups Preventive measure to reduce risk of pneumonia: head elevation Study nutrition period continued until 28 days after enrolment, death or ICU discharge, or until participant began to eat, whichever came first |
|
| Outcomes |
Primary outcome 1‐Energy delivery from enteric feeding,calculated as proportion of estimated energy requirements from enrolment to end of ICU stay (up to 28 days) Secondary outcomes 1‐Incidence of ventilator‐associated pneumonia; consensus panel of 3 clinicians, pneumonia diagnosed by at least 2 members on the basis of temperature, white blood cell count, sputum, PO2/FIO2 ratio and chest X‐ray 2‐Duration of mechanical ventilation 3‐Duration of hospitalizations 4‐In‐hospital mortality rate |
|
| Notes | Duration of assisted ventilation and hospital and ICU length of stay were calculated as median and IQR; therefore this was not added to the result. E‐mail was sent to study author to request further information, but no reply until November 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomizations schedule with variable block" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment assignments were concealed before randomizations using a centralized voice‐recognition phone system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "There was no blinding after randomizations" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned by study authors Comments: probably outcome assessment, not blinded; otherwise this would be mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Day 2001.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: USA |
|
| Participants | Neurological intensive care unit Age, years: 53 post‐pyloric; 60 gastric Not stated whether participants were mechanically ventilated APACHE III score: 44.5 post‐pyloric; 51.7 gastric Inclusion criteria 1‐Patient with primary neurological diagnosis 2‐Expected to require enteral feeding for at least 72 hours 3‐Approved for inclusion by their primary physician Exclusion criteria 1‐Patient with documented gastroparesis 2‐Pre‐existing gastrointestinal bleeding 3‐Contraindication to head elevation of at least 30 degrees 4‐Absence of gag reflex Number of participants randomly assigned: 25 Number of participants who received intended treatment: 14 post‐pyloric; 11 gastric Number of participants analysed: 14 post‐pyloric; 11 gastric |
|
| Interventions | All participants had a 10 French, 3 gram weighted tip Corflow Ultra NG enteral feeding tube (Corpak Med Syatem) placed in stomach or duodenum. Tube was inserted blindly and position was confirmed by abdominal X‐ray Same feeding protocol was used in both groups Study nutrition period was continued until 10 days after enrolment or participant death |
|
| Outcomes |
Primary outcome 1‐Percentage of recommended calories and protein received by participant Secondary outcomes 1‐Physiological effect of feeding 2‐Reasons for delay in feeding 3‐Volume of feeding residual 4‐Number of feeding tubes replaced 5‐Cost of feeding 6‐Numbers and types of complications 7‐Aspiration pneumonia: no definition provided |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment was performed using a software‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "the number generated were placed into sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, but outcome not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned by study authors Comments: outcome not likely to be influenced, as incidence of pneumonia was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other source of bias |
Esparza 2001.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: New Mexico, USA |
|
| Participants | Medical intensive care unit Age, years: 45 post‐pyloric; 50 gastric Mechanically ventilated: 96.3% post‐pyloric; 92.6% gastric APACHE II score: 15.8 post‐pyloric; 17.1 gastric Inclusion criteria Not mentioned Exclusion criteria Not mentioned Number of participants randomly assigned: 54 Number of participants who received intended treatment: 24 post‐pyloric; 27 gastric Number of participants analysed: 27 post‐pyloric; 27 gastric |
|
| Interventions | 10 French feeding tube was placed in upper jejunum with the aid of an electromyographic (EMG) electrode 4 cm from the tip (Modified Flexiflo, Ross Product Division, Abbott Laboratories, Columbus, OH, USA), placement was confirmed by a typical gastric or duodenal EMG pattern. Abdominal X‐ray confirmed position of the tube before that start of the feeding. EMG was recorded continuously to monitor correct position Same feeding protocol was used in both groups Participants were studied for up to 8 days, until enteral feeding was no longer required or until isotopic aspiration occurred |
|
| Outcomes |
Primary outcome 1‐Difference in aspiration rate between gastric and post‐pyloric fed participants Secondary outcomes 1‐Average daily percentage of goal fed 2‐Mortality |
|
| Notes | No standard deviations were reported for average daily percentage of recommended calories received by participant. E‐mail was sent to study author in May, but no reply was received until July 13, 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "feeding tube was placed in the randomly assigned position" Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
| Allocation concealment (selection bias) | Unclear risk | Quote: "feeding tube was placed in the randomly assigned position" Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, but outcome not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned by study authors Comments: probably not blinded, but outcome is not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Unclear risk | Exclusion and Inclusion criteria not mentioned |
Hsu 2009.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: Taiwan |
|
| Participants | Medical intensive care unit Age, years: 70 post‐pyloric; 67.9 gastric All participants were mechanically ventilated APACHE II score: 20.5 post‐pyloric; 20.3 gastric Inclusion criteria 1‐Patient without intractable vomiting, severe diarrhoea and paralytic ileus 2‐Anticipated to require enteral feeding for at least 3 days Exclusion criteria 1‐Abdominal surgery 2‐Acute pancreatitis 3‐Gastrointestinal bleeding 4‐Intestinal obstruction 5‐Short bowel syndrome 6‐Chronic renal disease with creatinine > 2.5 mg/dL or liver disease with hepatic encephalopathy requiring protein intake restriction Number of participants randomly assigned: 121 Number of participants who received intended treatment: 59 post‐pyloric; 62 gastric Number of participants analysed: 59 post‐pyloric; 62 gastric |
|
| Interventions | Both groups received a 114 cm long, 12 French enteral feeding tube (Flexiflo, Abbott, Chicago, IL), which was placed by resident doctors in stomach (gastric group) or duodenum (post‐pyloric group) Position confirmed by radiograph and by checking pH of aspirated juice, tube position was monitored by chest radiograph or residual volume Same feeding protocol was used in both groups Study observations continued from placement of nasoenteric tube until 1 of the following events occurred: enteral tube was removed, participant commenced concurrent oral nutrition, participant was discharged from ICU or he or she expired |
|
| Outcomes |
Primary outcomes 1‐Mean daily caloric and protein intake and mean percentage of daily goal caloric fed 2‐Time to achieve nutritional goal rate Secondary outcomes 1‐Duration of ICU and hospital stays 2‐Duration of mechanical ventilation 3‐Blood glucose level 4‐Vomiting 5‐Diarrhoea 6‐Gastrointestinal bleeding 7‐Tube replaced 8‐ Tube clogged 9‐Episodes of residual volume > 100 mL 10‐Fever 11‐Bacteraemia 12‐Ventilator‐associated pneumonia: requires agreement of 2 pulmonologists without knowledge of clinical details. Criteria include:new CXR changes and 1 of the following: A‐Temperature > 38 ° C or < 36 ° C with no other recognized causes B‐White blood cell count > 12,000/cm3 or < 4,000/cm3 C‐For adults > 70 years and altered mental status, need at least 2 of the following: change in respiratory secretions, new or worsening cough or dyspnoea, worsening gas exchange, bronchial breath sound 13‐Mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "group assignment was performed using a software‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "all care givers were blinded to the randomization sequence" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants not blinded, but outcome not likely to be influenced Personnel, quote: "resident doctor insert all feeding tubes to avoid awareness of tube position by nurses" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all radiographs were reviewed by pulmonologist without the knowledge of clinical details" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Kearns 2000.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: California, USA |
|
| Participants | Medical intensive care unit Age, years: 54 post‐pyloric; 49 gastric All participants were mechanically ventilated APACHE II score: 22 postpyloric; 20 gastric Inclusion criteria 1‐People who tolerate enteral feeding 2‐Anticipated to need enteral feeding for 3 days Exclusion criteria 1‐Hypotension 2‐Abdominal surgery 3‐Pancreatitis 4‐Gastrointestinal bleeding 5‐Ileus Number of participants randomly assigned: 44 Number of participants who received intended treatment: 21 post‐pyloric; 23 gastric Number of participants analysed: 21 post‐pyloric; 23 gastric |
|
| Interventions | Post‐pyloric group: 1 of the study authors passed a 104 cm, 12 French polyurethane, styletted feeding tube in the duodenum by manipulating tube using tactile cues until feeling the tip was in position. Radiograph was used to confirm tip location, and positions were monitored by radiograph and by length of exposed feeding tube Gastric group: same feeding tube but placed entirely within the stomach Same feeding protocol was used in both groups Preventive measure to reduce risk of pneumonia: head elevation Study observations continued from placement of nasoenteric tube until the earlier of 2 events: Endotracheal tube was removed or participant was discharged from ICU Duration of follow‐up was not mentioned |
|
| Outcomes |
Primary outcomes 1‐Achievement of caloric goal: percentage of energy expenditures 2‐Incidence of ventilator‐associated pneumonia; defined as presence of new infiltrate on CXR in the presence of 2 of the following: A‐White blood cell count > 10,000/cm3 B‐Temperature > 38.5° C C‐Positive glucose test or blue discolorations in endotracheal secretion Secondary outcomes 1‐Mortality 2‐Duration of tube feeding 3‐Duration of ICU and hospital stays 4‐Number of feeding tubes placed 5‐Incidence of gastrointestinal bleeding 6‐Feeding intolerance 7‐Number of positive blood cultures 8‐Days with diarrhoea |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random number" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants not blinded, but outcomes not likely to be influenced Personnel, quote: "nurses were unaware of the location of the tube's tip" Comments: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all radiographs were reviewed by pulmonologist without the knowledge of clinical details" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Kortbeek 1999.
| Methods | Randomized controlled trial with 2 parallel groups 2 centres Country: Calgary, Canada |
|
| Participants | Trauma intensive care unit Age, years: 33.6 post‐pyloric; 34.7 gastric All participants were mechanically ventilated APACHE III score: 18 post‐pyloric; 18 gastric Inclusion criteria 1‐Major trauma with injury severity score ≥ 16 2‐Requires mechanical ventilator for at least 48 hours Exclusion criteria 1‐Disruption of gastrointestinal tract 2‐Traumatic pancreatitis 3‐Severe physiological instability precluding transportation for fluoroscopy 4‐Prognosis considered to be hopeless 5‐Enrolment in another trial 6‐Prior initiation of nutritional support 7‐Failure of attending physician to enrol participant within 72 hours of admission Number of participants randomly assigned: 80 Number of participants who received intended treatment: 37 post‐pyloric; 40 gastric Number of participants analysed: 37 post‐pyloric; 40 gastric |
|
| Interventions | Nasal or oral transpyloric feeding tube was placed in radiology by using fluoroscopy. Target position of the tube was not mentioned. No regular monitoring for position of the tube Same feeding protocol was used in both groups Preventive measure to reduce risk of pneumonia: not mentioned |
|
| Outcomes |
Primary outcomes 1‐Time from initiation of tube feed to tolerance of full caloric feeding 2‐Ventilator‐associated pneumonia:diagnosed by new chest X‐ray changes and 2 of the following: A‐Temperature > 38.5 ° B‐White blood cell count > 10,000/cm3 or < 3000 /cm3 C‐Purulent sputum D‐Bacterial culture from bronchoalveolar lavage Secondary outcomes 1‐ICU length of stay 2‐Duration of mechanical ventilation 3‐Hospital length of stay 4‐Mortality |
|
| Notes | ICU length of stay and duration of mechanical ventilation were reported as median values; therefore the result could not be included in the meta‐analysis E‐mail was sent to study author, but no reply was received until June 13, 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "software generated randomizations schedule" |
| Allocation concealment (selection bias) | Low risk | Quote:"All caregivers were unaware of the randomization sequence" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, but outcome not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel were not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned by study authors Comments: probably outcome assessment not blinded, otherwise this would be mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Montecalvo 1992.
| Methods | Randomized controlled trial with 2 parallel groups Multi‐centre Country: Boston, USA |
|
| Participants | Surgical and medical intensive care unit Age, years: 50.5 post‐pyloric; 44.8 gastric Not clear what percentage of participants were mechanically ventilated APACHE II score: 17.4 post‐pyloric; 16.4 gastric Inclusion criteria 1‐Patient requires tube feeding for at least 3 days 2‐No contraindication to tube feeding or elective endoscopy 3‐No evidence of gastrointestinal bleeding Exclusion criteria Not mentioned Number of participants randomly assigned: 38 Number of participants who received intended treatment: 19 post‐pyloric; 19 gastric Number of participants analysed: 19 post‐pyloric; 19 gastric |
|
| Interventions | Post‐pyloric group: 60 inch, 12 French feeding tube (Biosearch Medical Products, Somerville) was placed in jejunum endoscopically over guide wire, tube placement was confirmed by X‐ray Gastric group: 43 inch,12 French, radiopaque polyurethane feeding tube was inserted into the stomach Baseline nutritional assessments were similar in both groups, but no standardized protocol was available for management of tube feeding No regular monitoring of tube position Preventive measure to reduce risk of pneumonia: not mentioned Participants were followed for 72 hours after completing tube feeding or for a maximum of 6 weeks of hospitalization |
|
| Outcomes | 1‐Duration of ICU stay 2‐Duration of mechanical ventilation 3‐Days of antibiotic therapy 4‐Days of fever 5‐Percentage of daily goal caloric intake 6‐Increase in serum pre‐albumin concentration 7‐Days of diarrhoea 8‐Number of participants with vomiting 9‐Number of participants with pneumonia: Pneumonia was diagnosed by presence of infiltrate on CXR plus 3 of the following: A‐Purulent sputum > 25 leucocytes, < 10 epithelial and numerous bacteria B‐Purulent sputum > 25 leucocytes < 10 epithelial and nosocomial pathogens C‐Fever > 38.6 ° C D‐Periphral leucocytosis > 10.000 cells/mm3 10‐Number of times tube was clogged 11‐Number of participants with tube replaced 12‐Gastrointestinal bleeding 13‐Tube insertion complication |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patient randomly assigned according to computer generated randomizations code" |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned by study author Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, but outcomes not likely to be influenced Personnel: not mentioned by study authors Comments: Probably personnel were not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all culture were reviewed ina blinded fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Uncertain whether analysis was done according to intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Unclear risk | Baseline nutritional assessments were similar in both groups, but no standardized protocol was available for management of tube feeding Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
Montejo 2002.
| Methods | Randomized controlled trial with 2 parallel groups 14 centres Country: Spain |
|
| Participants | Type of intensive care unit not mentioned Age, years: 57 post‐pyloric; 59 gastric Not clear whether participants were mechanically ventilated APACHE II score: post‐pyloric:18; gastric 19 Inclusion criteria 1‐Over 18 years old 2‐Need enteral feeding for longer than 5 days 3‐No contraindication for enteral feed Exclusion criteria 1‐Anatomical disruption of gastrointestinal tract 2‐Previous gastrointestinal surgery 3‐Contraindication to enteral feeding 4‐Contraindication to gastric endoscopy Number of participants randomly assigned: 101 Number of participants who received intended treatment: 50 post‐pyloric; 51 gastric Number of participants analysed: 50 post‐pyloric; 51 gastric |
|
| Interventions | Post‐pyloric group: Feeding tube (Stay‐Put, Novartis) was placed in jejunum by endoscopy, fluoroscopy guidance, blind technique or echography. Tube placement was confirmed by abdominal X‐ray Gastric group: Standard nasogastric tube was inserted on admission No regular monitoring of tube position Same feeding protocol was used in both groups Preventive measure to reduce risk of pneumonia: not mentioned Participants were followed up prospectively until ICU discharge or after 28 days follow‐up |
|
| Outcomes | 1‐Gastrointestinal complications: abdominal distension, vomiting, diarrhoea, constipation, high gastric residual 2‐Efficacy of diet administration: expressed as ratio between administered and prescribed volumes 3‐Incidence of nosocomial pneumonia: diagnosis according to criteria described by the Centers for Disease Control and Prevention 4‐ICU length of stay 5‐Mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random numbers were generated by computer for group assignment" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned by study author Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded, but outcomes were not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned by study authors Comments: probably outcome assessment not blinded, otherwise this would be mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Neumann 2002.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: California, USA |
|
| Participants | Medical intensive care unit Age, years: (59.6 Post‐pyloric; 58.1Gastric) Not clear if patient were mechanically ventilated or not Inclusion Criteria Candidate for enteral feeding Exclusion criteria 1‐Gastrointestinal obstruction 2‐Ileus 3‐Pancreatitis 4‐Documented gastroparesis 5‐Inability to obtain informed consent Number of participants who were randomized: 60 Number of participants who received intended treatment: (30 Post‐pyloric; 30 Gatric) Number of participants who were analysed: (30 Post‐pyloric; 30 Gastric) |
|
| Interventions | Standard 12‐French feeding tube (Dobhoff, Biosearch Medical Products, Somerville, NJ) was placed gastric or post‐pyloric by nursing staff Target position in post‐pyloric group was not clear. Placement was confirmed by X‐ray No regular monitoring of feeding tube position Same feeding protocol was used in both groups Participants were followed‐up for duration of enteral feeding, until leaving ICU or for a maximum of 14 days |
|
| Outcomes | 1‐Time of initial tube insertion 2‐Onset of feeding 3‐Achievement of goal rate:calculated as mL/h 4‐Adverse outcomes: aspiration, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using a computer generated, individual number" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded, but outcome is not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned by study authors Comments: outcome not likely to be influenced, as incidence of pneumonia was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing date Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
White 2009.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: Australia |
|
| Participants | Medical intensive care unit Age, years: 50 post‐pyloric; 54 gastric All patients were mechanically ventilated APACHE II score: post‐pyloric: 30; gastric: 24.5 Inclusion criteria 1‐18 years old 2‐Expected to require mechanical ventilation for longer than 24 hours Exclusion criteria 1‐Ischaemic bowel 2‐Bowel obstruction 3‐Exacerbation of inflammatory bowel disease 4‐Acute variceal bleeding 5‐Patients at high risk of anastomotic leak Number of participants randomly assigned: 108 Number of participants who received intended treatment: 50 post‐pyloric; 54 gastric Number of participants analysed: 50 post‐pyloric; 54 gastric |
|
| Interventions | Post‐pyloric group received un weighted feeding tube 10 French (Corflo‐Ultra Lite), 110 cm for participants < 80 kg, 140 cm for patients greater than 80 kg. Tube was inserted in duodenum or jejunum blindly by ICU nursing staff, and position was confirmed by abdominal X‐ray Nasogastric tube size of 12, 14 or 16 was inserted for all participants, regardless of the allocation No regular monitoring of tube position Same feeding protocol was used in both groups Preventive measure to reduce risk of pneumonia: head elevation Participants remained in the study until enteral feeding was ceased, or they were discharged from ICU |
|
| Outcomes |
Primary outcomes 1‐Success rate of nurse‐initiated insertion of post‐pyloric tubes 2‐Time taken to insert tube 3‐Time to reach goal feeds and total nutrition received over ICU stay as a proportion of calculated ideal Secondary outcomes 1‐Incidence of ventilator‐associated pneumonia, diagnosis based on new onset of fever, leucocytosis, new pulmonary infiltrate on CXR, increased pulmonary secretions and clinical pulmonary infection score > 6 2‐Gastric residual volume 3‐Mortality |
|
| Notes | Results were reported in median and IQR, e‐mail was sent to study author and he provided us with all results in means and standard deviations Percentage of recommended calories received by participant was not reported in published paper but was provided to us by study author himself 10/50 participants in post‐pyloric group did not receive post‐pyloric tube because of difficulty with insertion; 4/54 participants in gastric tube group did receive post‐pyloric tube because of high gastric residual volume. Participants were analysed according to intention‐to‐treat Baseline characteristics demonstrate that APACHE II score was higher in post‐pyloric group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using a computer generated, random number sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded, but outcomes were not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned by study authors Comments: probably outcome assessment not blinded, otherwise this would be mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all primary and secondary outcomes have been reported |
| Other bias | Unclear risk | 10/50 participants in post‐pyloric group did not receive post‐pyloric tube because of difficulty with insertion; 4/54 participants in gastric tube group did receive post‐pyloric tube because of high gastric residual volume. Participants were analysed according to intention‐to‐treat Baseline characteristics demonstrate that APACHE II score was higher in post‐pyloric group Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
Zeng 2010.
| Methods | Randomized controlled trial with 2 parallel groups 1 centre Country: China |
|
| Participants | Patients with severe craniocerebral injury Mean age: 41 years Male: 63% Median APACHE II score: not mentioned Not mentioned whether participants were on mechanical ventilation Inclusion criteria 1‐Length of hospital stay > 14 days 2‐Glasgow coma scale (GCS) score ≤ 8 3‐No history of liver, kidney or other vital organ disease, without diabetes and other metabolic disease Exclusion criteria Not mentioned Number of participants randomly assigned: 40 Number of participants who received intended treatment: 20 post‐pyloric; 20 gastric Number of participants analysed: 20 post‐pyloric; 20 gastric |
|
| Interventions | Nose jejunal tube or nasogastric tube was placed immediately after patients were admitted to ICU No details available about size of tube, method of insertion, target location, confirmation of placement or monitoring of position of the tube Same feeding protocol was used in both groups Duration of follow‐up was not mentioned |
|
| Outcomes | 1‐Serum albumin at 7 and 14 days after nutritional support 2‐Blood glucose at 7 and 14 days after nutritional support 3‐Lymphocyte count at 7 and 14 days after nutritional support 4‐Complications: gastrointestinal bleeding, reflux, diarrhoea, bloating |
|
| Notes | Article was published in Chinese and was translated with the help of another Chinese author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly divided by random table method" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned Comment: insufficient information to permit judgement of ’high risk’ or ’low risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded, but outcome was not likely to be influenced Personnel: not mentioned by study authors Comments: probably personnel not blinded, otherwise this would be mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned by study authors Comments: outcome not likely to be influenced, as incidence of pneumonia was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data Uncertain whether analysis was done according to intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all outcomes have been reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
APACHE II = Acute Physiology and Chronic Health Evaluation II.
BMI = body mass index.
CXR = chest X‐ray.
EMG = electromyographic.
GCS = Glasgow coma scale.
GRV = gastric residual volume.
ICU = intensive care unit.
IQR = interquartile range.
SOFA = Sequential Organ Failure Assessment.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bao 2006 | Interventions out of area of interest: comparing enteral nutrition (EN) group and total parenteral nutrition (TPN) group in severe acute pancreatitis |
| Eatock 2005 | Participants were not receiving treatment in critical care setting |
| Graham 1989 | Participants were not randomly assigned |
| Heyland 2001 | Not measuring any relevant outcomes |
| Kumar 2006 | Participants were not receiving treatment in critical care setting |
| Lin 2006 | Retrospective review |
| Minard 2000 | Interventions out of area of interest: Study examined the effects of early vs delayed enteral feeding on outcomes in participants with severe head injury |
| Nayak 2008 | Interventions out of area of interest: Study was comparing time taken for insertion of nasoduodenal tube with metoclopramide or cisapride |
| Olah 1996 | Interventions out of area of interest: Study was comparing jejunal vs parenteral feeding Feeding in treatment of acute pancreatitis |
| Singh 2012 | Irrelevant outcomes; only outcome relevant to our review was total length of hospital stay, but no standard deviations were reported for this outcome Pneumonia was not an outcome; investigators reported positive tracheal aspirate without correlating it with radiological findings or the clinical picture |
| Spain 1995 | Retrospective study |
| Strong 1992 | Participants were not receiving treatment in a critical care setting |
| Taylor 1998 | Observational study |
| Taylor 1999 | Interventions out of area of interest: Study was comparing aggressive enteral feeding vs standard enteral feeding, and as part of feeding protocol, participants in intervention group could require a post‐pyloric tube to achieve feeding target |
| Treux 1995 | Interventions out of area of interest: Study was comparing continued enteral feeding vs intermittent bolus delivery |
EN = enteral nutrition.
TPN = total parenteral nutrition.
Characteristics of studies awaiting assessment [ordered by study ID]
Couto 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | Not known |
Differences between protocol and review
We removed the word 'patients' from the title.
In our protocol (Alkhawaja 2010), the third primary outcome was "Achievement of target nutritional requirement: We will calculate the mean percentage of the caloric goal achieved by comparing the mean estimated caloric need versus the mean actual calories delivered to the patient". During our review, we found that the mean percentage of the caloric goal was already mentioned in the studies, and we did not need to calculate it by ourselves. Accordingly, we decided to rephrase the whole sentence in a more clear way: "Percentage of total nutrition delivered to the patient, which is calculated by comparing the mean estimated caloric need versus the mean actual calories delivered to the patient".
In the Background section, we made slight changes. We removed the last paragraph from "Description of the intervention" section and modified and kept this section under a new subtitle ("How the intervention might work").
In the section "Why it is important to do the review", we added a brief description of four new meta‐analyses.
In the Methods section, under the "Data extraction and management" subheading, we added duration of mechanical ventilation to the other important outcomes to be included in the quality assessment in the 'Summary of findings' table.
In the Methods section, under "Assessment of risk of bias in included studies", we modified the blinding criteria from the way they appeared in the protocol. We reconsidered the importance of blinding the participant, and we agreed that blinding of participants is not important in this kind of intervention because it cannot affect the outcomes and accordingly cannot result in bias. Regarding blinding of the outcome assessor, we found that the only subjective outcome that needed to be assessed for the diagnosis was pneumonia; accordingly we considered blinding adequate if caregivers and outcome assessors for pneumonia were blinded to the intervention, and blinding inadequate if caregivers and outcome assessors for pneumonia were not blinded to the intervention.
In the Methods section, under "Measurement of treatment effect", we removed the last paragraph, as we did not calculate the number needed to treat for an additional beneficial outcome (NNTB), the number needed to treat for an additional harmful outcome (NNTH) and the effect size (EFS) for each group on the basis of the standardized mean difference, because of the small number of participants.
In the Methods section, under "Data synthesis", we removed the last sentence as we did not need to use STATA for the analysis.
Contributions of authors
Sana Alkhawaja (SA), Claudio Martin (CM), Ronald J Butler (RJB), Femida Gwadry‐Sridhar (FGS).
Conceiving of the review: SA.
Designing the review: SA.
Co‐ordinating the review: SA.
Undertaking manual searches: SA.
Screening search results:SA, CM, RJB.
Organizing retrieval of papers: SA.
Screening retrieved papers against inclusion criteria: SA, CM, RJB.
Appraising quality of papers: SA, CM, RJB.
Abstracting data from papers: SA, CM, RJB.
Writing to authors of papers for additional information: SA.
Providing additional data on papers: SA.
Obtaining and screening data on unpublished studies: SA.
Managing data for the review: SA.
Entering data into Review Manager (RevMan 5.3): SA.
Analysing RevMan statistical data: SA, FGS.
Performing other statistical analyses not using RevMan: SA, FGS.
Performing double entry of data: SA, FGS.
Interpreting data: SA, CM, RJB, FGS.
Making statistical inferences: FGS.
Writing the review: SA.
Providing guidance on the review: CM, RJB.
Securing funding for the review: N/A.
Performing previous work that served as the foundation of the present study: SA.
Serving as guarantor for the review (one review author): SA.
Taking responsibility for reading and checking the review before submission: SA, CM, RJB.
Sources of support
Internal sources
University of Western Ontario, Canada.
External sources
No sources of support supplied
Declarations of interest
Sana Alkhawaja: none known.
Claudio Martin: none known.
Ronald J Butler: none known.
Femida Gwadry‐Sridhar: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Acosta‐Escribano 2010 {published and unpublished data}
- Acosta‐Escribano J, Fernández‐Vivas M, Grau Carmona T, Caturla‐Such J, Garcia‐Martinez M, Menendez‐Mainer A, et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intensive Care Medicine 2010;36(9):1532‐9. [PUBMED: 20495781] [DOI] [PubMed] [Google Scholar]
Boivin 2001 {published and unpublished data}
- Boivin MA, Levy H. Gastric feeding with erythromycin is equivalent to transpyloric feeding in the critically ill. Critical Care Medicine 2001;29(10):1916‐9. [PUBMED: 11588451] [DOI] [PubMed] [Google Scholar]
Davies 2002 {published and unpublished data}
- Davies AR, Froomes PR, French CJ, Bellomo R, Gutteridge GA, Nyulasi I, et al. Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Critical Care Medicine 2002;30(3):586‐90. [PUBMED: 11990920] [DOI] [PubMed] [Google Scholar]
Davies 2012 {published and unpublished data}
- Davies AR, Morrison SS, Bailey MJ, Bellomo R, Cooper DJ, Doig GS, et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Critical Care Medicine 2012;40(8):2342‐8. [PUBMED: 22809907] [DOI] [PubMed] [Google Scholar]
Day 2001 {published and unpublished data}
- Day L, Stotts NA, Frankfurt A, Stralovich‐Romani A, Volz M, Muwaswes M, et al. Gastric versus duodenal feeding in patients with neurological disease. Journal of Neuroscience Nursing 2001;33(3):148‐9, 155‐9. [PUBMED: 11413660] [DOI] [PubMed] [Google Scholar]
Esparza 2001 {published and unpublished data}
- Esparza J, Boivin MA, Hartshorne MF, Levy H. Equal aspiration rates in gastrically and transpylorically fed critically ill patients. Intensive Care Medicine 2001;27(4):660‐4. [PUBMED: 11398691] [DOI] [PubMed] [Google Scholar]
Hsu 2009 {published and unpublished data}
- Hsu CW, Sun SF, Lin SL, Kang SP, Chu KA, Lin CH, et al. Duodenal versus gastric feeding in medical intensive care unit patients. Critical Care Medicine 2009;37(6):1866‐72. [PUBMED: 19384225] [DOI] [PubMed] [Google Scholar]
- Huang HH, Chang SJ, Hsu CW, Chang TM, Kang SP, Liu MY. Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. Journal of the Academy of Nutrition and Dietetics 2012;112(8):1138‐46. [PUBMED: 22682883] [DOI] [PubMed] [Google Scholar]
Kearns 2000 {published and unpublished data}
- Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM. The incidence of ventilator‐associated pneumonia and success in nutrient delivery with gastric versus small intestinal feeding. Critical Care Medicine 2000;28(6):1742‐6. [PUBMED: 10890612] [DOI] [PubMed] [Google Scholar]
Kortbeek 1999 {published and unpublished data}
- Kortbeek JB, Haigh PI, Doig C. Duodenal versus gastric feeding in ventilated blunt trauma patients. Journal of Trauma 1999;46(6):992‐8. [PUBMED: 10372614] [DOI] [PubMed] [Google Scholar]
Montecalvo 1992 {published and unpublished data}
- Montecalvo MA, Steger KA, Farber HW, Smith BF, Dennis RC, Fitzpatrick GF, et al. Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. Critical Care Medicine 1992;20(10):1377‐87. [PUBMED: 1395657] [DOI] [PubMed] [Google Scholar]
Montejo 2002 {published and unpublished data}
- Montejo JC, Grau T, Acosta J, Ruiz‐Santana S, Planas M, García‐De‐Lorenzo A, et al. Multicenter, prospective, randomized, single‐blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Critical Care Medicine 2002;30(4):796‐800. [PUBMED: 11940748] [DOI] [PubMed] [Google Scholar]
Neumann 2002 {published and unpublished data}
- Neumann DA, DeLegge MH. Gastric versus small‐bowel tube feeding in the intensive care unit: a prospective comparison of efficacy. Critical Care Medicine 2002;30(7):1436‐8. [PUBMED: 12130958 ] [DOI] [PubMed] [Google Scholar]
White 2009 {published and unpublished data}
- White H, Sosnowski K, Tran K, Reeves A, Jones M. A randomised controlled comparison of early post‐pyloric versus early gastric feeding to meet nutritional targets in ventilated intensive care patients. Critical Care Medicine 2009;13(6):1‐8. [PUBMED: 19930728 [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2010 {published and unpublished data}
- Zeng R‐C, Jiang F‐G, Xie Q. Comparison of nose jejunal tube and nasogastric tube in providing early enteral nutrition for patients with severe craniocerebral injury [鼻空肠管和鼻胃管在重型颅脑损伤患者早期肠内营养中应用效果的比较]. Chinese Journal of Clinical Nutrition 2010;18(3):355‐7. [Google Scholar]
References to studies excluded from this review
Bao 2006 {published and unpublished data}
- Bao J, Jing D, Wang P, Wang X. Effect of early jejunum nutrition on the comprehensive therapy for severe acute pancreatitis. Chinese Journal of Gastroenterology 2006;11(5):259‐62. [EMBASE: AN/2006513768 ] [Google Scholar]
Eatock 2005 {published and unpublished data}
- Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, et al. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. American Journal of Gastroenterology 2005;11(2):432‐9. [PUBMED: 15667504] [DOI] [PubMed] [Google Scholar]
Graham 1989 {published and unpublished data}
- Grahm TW, Zadrozny DB, Harrington T. The benefits of early jejunal hyperalimentation in the head‐injured patient. Neurosurgery 1989;25(5):729‐35. [PUBMED: 2511499] [DOI] [PubMed] [Google Scholar]
Heyland 2001 {published and unpublished data}
- Heyland DK, Drover JW, MacDonald S, Novak F, Lam M. Effects of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration. Critical Care Medicine 2001;29(8):1495‐501. [PUBMED: 11505114 ] [DOI] [PubMed] [Google Scholar]
Kumar 2006 {published and unpublished data}
- Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis. Journal of Clinical Gastroenterology 2006;40(5):431‐4. [PUBMED: 16721226 ] [DOI] [PubMed] [Google Scholar]
Lin 2006 {published and unpublished data}
- Lin HH, Hsieh TY, Chu HC, Wu DM, Chao YC, Chang WK. Transpyloric tube feeding for 506 consecutive patients with delayed gastric emptying. Journal of Internal Medicine of Taiwan 2006;17(2):52‐60. [EMBASE: AN/2006304628] [Google Scholar]
Minard 2000 {published and unpublished data}
- Minard G, Kudsk KA, Melton S, Patton JH, Tolley EA. Early versus delayed feeding with an immune‐enhancing diet in patients with severe head injuries. Journal of Parenter and Enteral Nutrtion 2000;24(3):145‐9. [PUBMED: 10850938] [DOI] [PubMed] [Google Scholar]
Nayak 2008 {published and unpublished data}
- Nayak SK, Sherchan M, Dutta Poudel S, Chamling Rai J, Shrestha R, Shretha BC, et al. Assessing placement of nasoduodenal tube and its usefulness in maintaining nutrition in critically ill patients. Nepal Medical College Journal 2008;10(4):249‐53. [PUBMED: 19558064] [PubMed] [Google Scholar]
Olah 1996 {published and unpublished data}
- Oláh A, Pardavi G, Belágyi T, Nagy A, Issekutz A, Mohamed GE. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition 2002;18(3):259‐62. [PUBMED: 11882400] [DOI] [PubMed] [Google Scholar]
Singh 2012 {published and unpublished data}
- Singh N, Sharma B, Sharma M, Sachdev V, Bhardwaj P, Mani K, et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: a noninferiority randomized controlled trial.. Pancreas 2012;41(1):153‐9. [PUBMED: 21775915] [DOI] [PubMed] [Google Scholar]
Spain 1995 {published and unpublished data}
- Spain DA, DeWeese RC, Reynolds MA, Richardson JD. Transpyloric passage of feeding tubes in patients with head injuries does not decrease complications. Journal of Trauma 1995;39(6):1100‐2. [PUBMED: 7500401 ] [DOI] [PubMed] [Google Scholar]
Strong 1992 {published and unpublished data}
- Strong RM, Condon SC, Solinger MR, Namihas BN, Ito‐Wong LA, Leuty JE. Equal aspiration rates from postpylorus and intragastric‐placed small‐bore nasoenteric feeding tubes: a randomized, prospective study. Journal of Parenter and Enteral Nutrition 1992;16(1):59‐63. [PUBMED: 1738222 ] [DOI] [PubMed] [Google Scholar]
Taylor 1998 {published and unpublished data}
- Taylor SJ, Fettes SB. Enhanced enteral nutrition in head injury:effect on the efficacy of nutritional delivery, nitrogen balance, gastric residuals and risk of pneumonia. Journal of Human Nutrition and Dietetics 1998;11:391‐401. [CENTRAL: CN‐00386647] [Google Scholar]
Taylor 1999 {published and unpublished data}
- Taylor SJ, Fettes SB, Jewkes C, Nelson R. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Critical Care Medicine 1999;27(11):2525‐31. [CENTRAL: 10579275] [DOI] [PubMed] [Google Scholar]
Treux 1995 {published and unpublished data}
- Tueux O, Bildstein S, Leger A, Cochard JF, Pinaquy C, Erny P. Influence of delivery mode of enteral nutrition upon gastrointestinal tolerance in posttraumatic intensive care [abstract]. Clinical Nutrition 1995;14(Suppl 2):34. [CENTRAL: CN‐00285188] [Google Scholar]
References to studies awaiting assessment
Couto 2014 {published and unpublished data}
- Couto C, Beck M, Friedman G. A randomized controlled trial comparing early jejunal with gastric nutrition in critical illness. 27th Annual Congress of the European Society of Intensive Care Medicine. Barcelona, Spain: ESICM, 2014:40, S17.
Additional references
Alhazzani 2013
- Alhazzani W, Almasoud A, Jaeschke R, Lo BW, Sindi A, Altayyar S, et al. Small bowel feeding and risk of pneumonia in adult critically ill patients: a systematic review and meta‐analysis of randomized trials. Critical Care 2013;17(4):R127. [PUBMED: 23820047] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASPEN 2009
- McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. Journal of Parenteral and Enteral Nutrition 2009;33(3):277‐316. [PUBMED: 19398613] [DOI] [PubMed] [Google Scholar]
Bosscha 1998
- Bosscha K, Nieuwenhuijs VB, Vos A, Samsom M, Roelofs JM, Karman LM. Gastrointestinal motility and gastric tube feeding in mechanically ventilated patients. Critical Care Medicine 1998;26(9):1510‐7. [PUBMED: 9751586] [DOI] [PubMed] [Google Scholar]
Boulton 2004
- Boulton‐Jones JR, Lewis J, Jobling JC, Teahon K. Experience of post‐pyloric feeding in seriously ill patients in clinical practice. Clinical Nutrition 2004;23(1):35‐41. [PUBMED: 14757391 ] [DOI] [PubMed] [Google Scholar]
Canadian Guidelines 2013
- No authors listed. Critical Care Nutrition. Canadian Clinical Practice Guidelines for nutrition support in mechanically ventilated, critically ill adult patients, updates 2013. www.criticalcarenutrition.com (accessed 30 June 2015).
Chapman 2005
- Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, et al. Antro‐pyloro‐duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut 2005;54(10):1384‐90. [PUBMED: 15923669 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jonghe 2001
- Jonghe B, Appere‐De‐Vechi C, Fournier M. A prospective survey of nutritional support practices in intensive care unit patients: What is prescribed? What is delivered?. Critical Care Medicine 2001;29:8‐12. [PUBMED: 11176150] [DOI] [PubMed] [Google Scholar]
Deane 2013
- Deane AM, Dhaliwal R, Day AG, Ridley EJ, Davies AR, Heyland DK. Comparisons between intragastric and small intestinal delivery of enteral nutrition in the critically ill: a systematic review and meta‐analysis. Critical Care 2013;17(3):R125. [PUBMED: 23799928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dive 1994
- Dive A, Moulart M, Jonard P, Jamart J, Mahieu P. Gastroduodenal motility in mechanically ventilated critically ill patients: a manometrics study. Critical Care Medicine 1994;22:441‐7. [PUBMED: 8124995] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis:principles and procedures. BMJ 1997;315(7121):1533‐7. [PUBMED: 9432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elpern 2004
- Elpern EH, Stutz L, Peterson S, Gurka DP, Skipper A. Outcomes associated with enteral tube feedings in a medical intensive care unit. American Journal of Critical Care 2004;13:221‐7. [PUBMED: 15149056] [PubMed] [Google Scholar]
ESPEN 2006
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN Guidelines on Enteral Nutrition: intensive care. Clinical Nutrition 2006;25(2):210‐23. [PUBMED: 16697087 ] [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [PUBMED: 15878471] [DOI] [PubMed] [Google Scholar]
Glasziou 2001
- Glasziou P, Irwig L, Bain C, Colditz G. Systematic Reviews in HealthCare: A Practical Guide. 1st Edition. Cambridge, UK: Cambridge University Press, 2001. [Google Scholar]
GRADEpro 3.6 [Computer program]
- http://ims.cochrane.org/revman/gradepro. GRADEpro. http://ims.cochrane.org/revman/gradepro, 2010.
Groos 1996
- Groos S, Hunefeld G, Luciano L. Parenteral versus enteral nutrition: morphological changes in human adult intestinal mucosa. Journal of Submicroscopic Cytology and Pathology 1996;28(1):61‐74. [PUBMED: 8929627 ] [PubMed] [Google Scholar]
Heyland 1996
- Heyland DK, Tougas G, King D, Cook DJ. Impaired gastric emptying in mechanically ventilated, critically ill patients. Intensive Care Medicine 1996;22(12):1399‐444. [PUBMED: 8986483] [DOI] [PubMed] [Google Scholar]
Heyland 2002
- Heyland DK, Drover JW, Dhaliwal R, Greenwood J. Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: role of small bowel feeding. Journa Parenteral and Enteral Nutrition 2002;26((6 Suppl)):S51‐5; discussion S56‐7. [PUBMED: 12405623] [DOI] [PubMed] [Google Scholar]
Heyland 2003
- Heyland DK, Dhaliwal R, Drover JW. Canadian clinical practice guidelines for nutritional support in mechanically ventilated, critically ill adult patients. Journal of Parenteral and Enteral Nutrition 2003;27:355‐73. [PUBMED: 12971736] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins J, Thomson S. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine. 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2008. www.cochrane‐handbook.org.
Ho 2006
- Ho KM, Dobb GJ, Webb SA. A comparison of early gastric and post‐pyloric feeding in critically ill patients: a meta‐analysis. Intensive Care Medicine 2006;32:636‐49. [PUBMED: 16570149] [DOI] [PubMed] [Google Scholar]
Jabbar 2003
- Jabbar A, Chang WK, Dryden GW, McClave SA. Gut immunology and the differential response to feeding and starvation. Nutrition in Clinical Practice 2003;18:461‐82. [PUBMED: 16215082] [DOI] [PubMed] [Google Scholar]
Jiyong 2013
- Jiyong J, Tiancha H, Huiqin W, Jingfen J. Effect of gastric versus post‐pyloric feeding on the incidence of pneumonia in critically ill patients: observations from traditional and Bayesian random‐effects meta‐analysis. Clinical Nutrition 2013 Feb;32(1):8‐15. [PUBMED: 22853861] [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman D, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [PUBMED: 11440947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kudsk 2001
- Kudsk KA. Importance of enteral feeding in maintaining gut integrity. Techniques in Gastrointestinal Endoscopy 2001;3:2‐8. [Google Scholar]
Kudsk 2002
- Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. American Journal of Surgery 2002;183(4):390‐8. [PUBMED: 11975926] [DOI] [PubMed] [Google Scholar]
Kudsk 2007
- Kudsk KA. Beneficial effect of enteral feeding. Gastrointestinal Endoscopy Clinic of North America 2007;17(4):647‐62. [PUBMED: 17967372] [DOI] [PMC free article] [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing Up: The Science of Reviewing Research. 1st Edition. Cambridge, Massachusetts: Harvard University Press, 1984. [Google Scholar]
Marik 2003
- Marik PE, Zaloga GP. Gastric versus post‐pyloric feeding: a systematic review. Journal of Critical Care 2003;7:R46‐51. [PUBMED: 12793890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Metheny 2006
- Metheny NA, Clouse RE, Chang YH. Tracheobronchial aspiration of gastric contents in critically ill tube‐fed patients: frequency, outcomes, and risk factors. Critical Care Medicine 2006;34:1007‐15. [PUBMED: 16484901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2006
- Nguyen NQ, Fraser RJ, Chapman M, Bryant LK, Holloway RH, Vozzo R, et al. Proximal gastric response to small intestinal nutrients is abnormal in mechanically ventilated critically ill patients. World Journal of Gastroenterology 2006;21(27):4383‐8. [PUBMED: 16865782 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Leary 2005
- O'Leary‐Kelley CM, Puntillo KA, Barr J, Stotts N, Douglas MK. Nutritional adequacy in patients receiving mechanical ventilation who are fed enterally. American Journal of Critical Care 2005;14:222‐31. [PUBMED: 15840896 ] [PubMed] [Google Scholar]
O'Meara 2008
- O'Meara D, Mireles‐Cabodevila E, Frame F. Evaluation of delivery of enteral nutrition in critically ill patients receiving mechanical ventilation. American Journal of Critical Care 2008;17:53‐61. [PUBMED: 18158390 ] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ritz 2001
- Ritz MA, Fraser R, Edwards N, Matteo AC, Chapman M, et al. Delayed gastric emptying in ventilated critically ill patients: measurement by 13C‐octanoic acid breath test. Critical Care Medicine 2001;29(9):1744‐9. [PUBMED: 11546976 ] [DOI] [PubMed] [Google Scholar]
Rubinson 2004
- Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Critical Care Medicine 2004;32:350‐7. [PUBMED: 14758147 ] [DOI] [PubMed] [Google Scholar]
Uklaja 2007
- Ukleja A, Sanchez‐Fermin M. Gastric versus post‐pyloric feeding: relationship to tolerance, pneumonia risk, and successful delivery of enteral nutrition. Current Gastroenterology Reports 2007;9:309‐16. [PUBMED: 17883980 ] [DOI] [PubMed] [Google Scholar]
Ukleja 2010
- Ukleja A. Altered GI motility in critically ill patients: current understanding of pathophysiology, clinical impact and diagnostic approach. Nutrition in Clinical Practice 2010;25:16‐25. [PUBMED: 20130154 ] [DOI] [PubMed] [Google Scholar]
Villet 2005
- Villet S, Chiolero RL, Bollmann MD. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Cilnical Nutrition 2005;24:502‐9. [PUBMED: 15899538] [DOI] [PubMed] [Google Scholar]
Woodcock 2001
- Woodcock NP, Zeigler D, Palmer MD. Enteral versus parenteral nutrition: a pragmatic study. Journal of Nutrition 2001;17:1‐12. [PUBMED: 11165880] [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang Z, Xu X, Ding J, Ni H. Comparison of postpyloric tube feeding and gastric tube feeding in intensive care unit patients: a meta‐analysis. Nutrition in Clinical Practice 2013;28(3):371‐80. [PUBMED: 23614960] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alkhawaja 2010
- Alkhawaja S, Martin C, Butler RJ, Gwadry‐Sridhar F. Post‐pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adult patients. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008875] [DOI] [PMC free article] [PubMed] [Google Scholar]


