Abstract
Background
Ménière's disease or syndrome is a chronic inner ear disorder that results in sporadic attacks of vertigo, sensorineural hearing loss, aural fullness and tinnitus.
There is no definitive treatment for Ménière's disease and treatment options range from dietary modification through medication to surgery.
Modification of diet, including restriction of salt, caffeine and alcohol intake, is a management option that is widely recommended to patients with Ménière's as a first‐line treatment. There has not previously been a systematic review of this intervention.
Objectives
To assess the effects of dietary restriction of salt, caffeine and alcohol intake in patients with Ménière's disease or syndrome.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Central Register of Controlled Trials (CENTRAL); PubMed; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 28 March 2018.
Selection criteria
Randomised controlled trials of dietary modification, specifically salt, caffeine and alcohol restriction or substitution (or both), compared to no modification of these agents or a placebo intervention, in adult patients with Ménière's disease or syndrome.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were control of vertigo or decrease in vertigo attacks, and adverse effects. Secondary outcomes included hearing (change in hearing loss or its progression), tinnitus (severity), perception of aural fullness, well‐being and quality of life (overall changes), and other adverse effects. We planned to use GRADE to assess the quality of the evidence for each outcome.
Main results
We did not identify any studies that met the inclusion criteria for the review.
Authors' conclusions
There is no evidence from randomised controlled trials to support or refute the restriction of salt, caffeine or alcohol intake in patients with Ménière's disease or syndrome.
High‐quality research in this field is warranted. The best evidence may come from a randomised controlled trial comparing dietary interventions (e.g. low salt versus general healthy diet advice), using rigorous methodology for patient selection, randomisation and blinding, and strictly adhering to the Bárány Society diagnostic criteria. However, this research question might be more pragmatically addressed by using information from carefully constructed patient registries that include information on dietary intake of substances of interest such as salt, caffeine and alcohol. It will be important to address the question of any possible harms or unwanted effects of dietary modification.
Plain language summary
Dietary changes in the treatment of Ménière's disease or syndrome
Review question
What is the effect of changing diet by restricting salt, caffeine and alcohol, alone or together with each other, on the symptoms of people with Ménière's disease?
Background
Approximately 200 per 100,000 people suffer from Ménière's. The condition is called Ménière's disease if no cause can be identified and Ménière's syndrome if there is a known reason for its development. Patients who suffer from it experience vertigo (dizzy episodes), hearing loss, a sensation of pressure in their ears and tinnitus (ringing or buzzing in the ears).
At present there is no standard treatment for Ménière's disease and options can range from dietary changes, to medicines and in some cases surgery. Ménière's disease is thought to be caused by disturbance of the volume or composition of the fluid in the inner ear (called endolymph). Dietary intake of salt can affect the concentrations of electrolytes (salts and minerals that can conduct electrical impulses in the body) in the blood, which in turn may affect the composition of the endolymph. Salt intake may therefore contribute to attacks and so restriction in the diet could be used to control both the volume and composition of the endolymph. Caffeine and alcohol intake can result in constriction of blood vessels (vasoconstriction) and could result in a reduction in the blood supply to the inner ear, which may make patients' symptoms worse. Many doctors advise dietary changes as a first‐line treatment as it is thought to be a relatively simple and inexpensive option. We wanted to find out whether dietary changes are effective to ensure that patients are receiving the correct advice about treatment options, and to ensure that potentially more appropriate treatment is not delayed by spending time on ineffective interventions, resulting in unpleasant symptoms and disease progression.
Study characteristics
We searched for high‐quality studies (randomised controlled trials) of dietary changes (salt, caffeine and alcohol restriction or substitution, or both) compared to no restriction in adult patients with Ménière's disease or syndrome. Our search is up to date to March 2018.
Key results
We did not identify any randomised controlled trials that met the inclusion criteria for the review.
Quality of evidence and conclusions
There is no evidence from randomised controlled trials about the restriction of salt, caffeine or alcohol intake in patients with Ménière's disease or syndrome. High‐quality research in this field is needed if this question is to be answered, in the form of a study that uses rigorous methods (for example, randomisation and blinding, or careful use of patient registries) and carefully recruits only patients that meet accepted Ménière's disease diagnostic criteria. It will be important to address the question of any possible harms or unwanted effects of dietary changes.
Summary of findings
for the main comparison.
| Dietary modifications compared with no modification/placebo for Ménière's disease or syndrome | ||||||
|
Patient or population: adults with Ménière's disease or syndrome Settings: hospital and community Intervention: dietary modification Comparison: no modification/placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No modification/placebo | Dietary modification | |||||
| Proportion of patients with control of vertigo or decrease in vertigo attacks | No data | No data | No data | No data | — | — |
| Proportion of patients with adverse effects (e.g. hyponatraemia, mood disturbance and headaches) | No data | No data | No data | No data | — | — |
| Proportion of patients with loss or gain of hearing/reduction in progression of hearing loss | No data | No data | No data | No data | — | — |
| Proportion of patients with a reduction in the severity of tinnitus | No data | No data | No data | No data | — | — |
| Proportion of patients with a reduction in the perception of aural fullness | No data | No data | No data | No data | — | — |
| Overall changes in well‐being and quality of life | No data | No data | No data | No data | — | — |
| Any other adverse effects | No data | No data | No data | No data | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Ménière's disease or syndrome is a chronic inner ear disorder that results in sporadic attacks of vertigo, sensorineural hearing loss, aural fullness and tinnitus. The term 'Ménière's disease' refers to an idiopathic disorder, but a clinically identical presentation can occur secondary to other conditions, such as infections, genetic disorders or trauma, and this is referred to as 'Ménière's syndrome'.
In a large US study the prevalence of Ménière's disease was estimated at 200 per 100,000 people (Alexander 2010). It most commonly affects people between the ages of 40 and 60 years (Harcourt 2014), with a slight female preponderance (da Costa 2002). Acute attacks usually occur in clusters, with those affected often being symptom‐free for months in between. There is a higher frequency of attacks in the initial period after presentation with an eventual reduction but sustained deterioration in hearing (Moffat 1997). The vertiginous episodes cease eventually (Silverstein 1989). However, there can be considerable persistent disability and the economic costs of the condition are estimated to be significant (Salvador‐Carulla 2010; Tyrrell 2016).
At present no 'gold standard' diagnostic test for Ménière's disease exists and the diagnosis is frequently based on the practitioner's assessment of the patient's history and neurotologic evaluation. The American Academy of Otolaryngology – Head and Neck Surgery (AAO‐HNS) has produced diagnostic guidelines (Alford 1972), which have since been revised twice (Ménière's Guide 1995; Pearson 1985). The guidelines state that a diagnosis can be made if the following criteria are met:
at least two spontaneous episodes of rotational vertigo lasting at least 20 minutes;
audiometric confirmation of a sensorineural hearing loss;
tinnitus and/or a perception of aural fullness.
When patients meet the AAO‐HNS criteria and the symptoms are attributed to a specific cause they are classified as having Ménière's syndrome.
Although the pathophysiology of Ménière's disease is unknown, there is thought to be an association with endolymphatic hydrops, which is distortion of the membranous labyrinth due to the over accumulation of endolymph (Hallpike 1938).
At present there is no definitive treatment for Ménière's disease and treatment options range from dietary modification through medication (for example, betahistine (James 2001) or diuretics (Burgess 2006)) to intratympanic injections (Pullens 2011; Phillips 2011) or surgery (Pullens 2013).
Description of the intervention
Modification of diet can include the restriction of salt, caffeine and alcohol intake.
Dietary salt restriction is a potentially simple and relatively inexpensive option that is widely recommended to patients with Ménière's as a first‐line treatment. The level and duration of salt intake restriction that is advised can vary.
Other additional dietary modifications include limiting the intake of caffeine (Luxford 2013) and alcohol (Ménière's Society). Caffeine is a commonly ingested substance found in liquid form in beverages such as tea and coffee, and it is also found in food such as chocolate. Alcohol is widely consumed, with reported figures of 7.8 litres per person per year on average throughout the adult population in 2015 in the UK (Office of National Statistics). As with dietary salt restriction the level and duration of caffeine and or alcohol restriction advised may vary.
How the intervention might work
Disturbance of the volume and/or electrolyte composition of the endolymph is considered to be the cause of the symptoms experienced by patients with Ménière's disease. High dietary intake of salt can affect the concentrations of electrolytes in the blood, which in turn affects the composition of the endolymph. It has been reported that high salt intake can contribute to attacks and it therefore follows that dietary modification can be used to control both the volume and composition of the endolymph (Stahle 1984). Fluctuation in the composition and volume of the endolymph is considered to contribute to the fluctuating nature of the symptoms experienced by sufferers of Ménière's. The observation that water retention can exacerbate the symptoms of Ménière's disease was first documented in 1929 (Dederding 1929). Subsequent uncontrolled studies suggested that the manipulation of salt intake influences the symptoms experienced by those suffering with Ménière's (Furstenberg 1934; Furstenberg 1941). More recent studies of restricted salt intake, usually together with other treatment modalities, such as the use of diuretics, have also suggested better symptom control in patients with Ménière's disease (Klockhoff 1974; Santos 1993). Excessive reduction in salt intake may, however, in extreme and rare cases result in hyponatraemia, although this is more commonly due to specific diseases. Hyponatraemia is associated with conditions ranging from mood disturbance to cerebral oedema and possible death in extreme cases (Thompson 2010).
Alcohol and caffeine, in high concentrations, can both result in vasoconstriction and a reduction in the blood supply to the inner ear, which can exacerbate the symptoms of sufferers. Dietary restriction of these substances may therefore be beneficial in Ménière's patients. Caffeine, however, is a recognised ergogenic aid even at physiological levels and enhances concentration and alertness whilst reducing fatigue. A reduction in the intake of caffeine may therefore lead to withdrawal effects in individuals who are accustomed to its effects, which can result in symptoms ranging from mood disturbance to headaches (Pesta 2013). It is well recognised to have clinical effects on balance and known to produce symptoms of vertigo and vomiting when taken in sufficient doses, reminiscent of an episode of Ménière's. Alcohol also causes vasoconstriction and therefore acts in a similar fashion to caffeine. However, to date no studies have been published that specifically address the effect of reducing/omitting alcohol in the treatment of Ménière's disease, as is routinely recommended. Although no recognised adverse effects of reducing alcohol consumption in individuals with consumption within recommended limits have been documented, a reduction in people who have a dependency on alcohol can result in withdrawal symptoms such as psychotic episodes and delirium tremens (Stern 2010).
Why it is important to do this review
Dietary modification, including the restriction of salt, caffeine and alcohol, is widely recommended to those suffering with Ménière's and generally considered the first‐line treatment. There has been no previous systematic review of the evidence for this advice, which is routinely given to patients. This may delay the use of more appropriate and effective treatments. Restricting the intake of salt, caffeine and alcohol, substances which people may find palatable and that potentially provide a degree of pleasure to their daily lives, may also have an effect on mood or quality of life, with no potential benefit. Dietary modification could also have potentially negative implications for social, work and family life. It is therefore important to conduct a systematic review of randomised controlled trials of these dietary modifications in patients with Ménière's disease or syndrome.
Objectives
To assess the effects of dietary restriction of salt, caffeine and alcohol intake in patients with Ménière's disease or syndrome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials. We planned to include cluster‐randomised and cross‐over trials if identified.
Types of participants
Adult patients, aged 18 and over, with a diagnosis of Ménière's disease or syndrome.
We planned to classify studies according to the diagnostic criteria used to diagnose Ménière's disease or syndrome, grading those using the AAO‐HNS criteria, or equivalent, to define probable, definite or certain Ménière's as grade 'I' studies and the remaining studies as grade 'II'.
Settings included both the community and hospital.
Types of interventions
The intervention of interest was dietary modification: specifically, salt, caffeine and/or alcohol restriction or substitution (or both). The control intervention was no modification.
The main comparison pairs were:
dietary restriction of salt versus no restriction;
dietary restriction of caffeine versus no restriction;
dietary restriction of alcohol versus no restriction.
Other possible comparison pairs included:
dietary restriction of salt versus dietary restriction of caffeine;
dietary restriction of salt versus dietary restriction of alcohol;
dietary restriction of alcohol versus dietary restriction of caffeine;
dietary restriction of salt + caffeine versus no restriction;
dietary restriction of salt + alcohol versus no restriction;
dietary restriction of alcohol + caffeine versus no restriction.
We planned to include studies where multiple dietary restrictions were used in conjunction but to note this in the analysis. We expected that there would be variability in the level and duration of dietary modification, but we planned to deal with this in subgroup analysis.
We excluded studies where dietary modification plus another treatment modality (e.g. a pharmacological agent) was used due to the potential for interactive effects.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Control of vertigo or decrease in the intensity, frequency and duration of vertigo attacks, as suggested by the AAO‐HNS* (Ménière's Guide 1995), using the results of the various questionnaire‐based assessment tools including the Vertigo Symptom Scale, the Vertigo Dizziness Imbalance Questionnaire and the Dizziness Handicap Inventory amongst others.
Adverse effects; any reported adverse effects such as hyponatraemia, mood disturbance and headaches would be noted.
Secondary outcomes
Hearing: change in hearing loss or its progression (or both) as measured by a pure‐tone audiogram. The proportion of patients with progression of hearing loss (more than 15 dB), based on the four‐tone average of thresholds at 0.5 kHz, 1 kHz, 2 kHz and 3 kHz, as measured by a pure‐tone audiogram.
Tinnitus: the proportion of patients with a reduction in tinnitus as measured by using patient‐reported questionnaire scores (the Tinnitus Handicap Index (THI), the Tinnitus Functional Index, the Tinnitus Handicap Questionnaire, the Tinnitus Questionnaire, the Tinnitus Reaction Questionnaire and the Tinnitus Severity Scale).
Perception of aural fullness: the proportion of patients with reduction of aural fullness as measured by using patient‐reported questionnaire scores such as the Vertigo Dizziness Imbalance Questionnaire or a visual analogue scale.
Well‐being and quality of life: overall changes, as reported in the Dizziness Handicap Inventory questionnaire and any other appropriate scale.
Any other adverse effects; these would be noted based on patient‐reported symptoms of potential unpleasant effects, as well as the results of specific questionnaires including the Vertigo Symptom Scale, the Vertigo Dizziness Imbalance Questionnaire and the Dizziness Handicap Inventory amongst others.
*The AAO‐HNS Committee on Hearing and Equilibrium proposed the "control of vertigo" as a main objective outcome measure when assessing therapy in Ménière's disease. The number of attacks six months prior to treatment is compared to the number in the period between 18 and 24 months following treatment. The resulting number indicates the extent of "control of vertigo". The AAO‐HNS further divides the control of vertigo into classes, where Class A (CoV = 0) is complete control and class B (CoV 1 to 40) is substantial control. They recommend a period of at least two years of follow‐up in order to assess fully the effect of the intervention. We also considered studies with shorter periods of follow‐up for this review.
We also anticipated various questionnaire‐based assessment tools being used in the different studies, including the Vertigo Symptom Scale (VSS), the Vertigo Dizziness Imbalance Questionnaire and the Dizziness Handicap Inventory amongst others. We included all forms of questionnaire that address the patient's perception of their symptoms, if used consistently. These questionnaires enable us to assess the impact on the patients' quality of life, functional impairment and disability.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 28 March 2018.
Electronic searches
Published, unpublished and ongoing studies were identified by searching the following databases from their inception:
Cochrane ENT Trials Register (searched via the Cochrane Register of Studies 28 March 2018);
Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 4);
PubMed (1946 to 29 March 2018);
Ovid EMBASE (1974 to 29 March 2018);
EBSCO CINAHL (1982 to 29 March 2018);
Ovid AMED (1985 to 29 March 2018);
Ovid CAB abstracts (1910 to 29 March 2018);
LILACS (searched 29 March 2018);
KoreaMed (searched via Google Scholar 29 March 2018);
IndMed (searched 29 March 2018);
PakMediNet (searched 29 March 2018);
Web of Knowledge, Web of Science (1945 to 29 March 2018);
CNKI (searched via Google Scholar 29 March 2018);
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 29 March 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 29 March 2018);
ISRCTN, www.isrctn.com (searched 29 March 2018).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
Two authors (KH and LM) independently scanned the initial search results to identify studies that appeared to meet the inclusion criteria. We used abstract review to eliminate any studies that were clearly ineligible. If either author identified a paper as potentially suitable, we reviewed the full text of the article. We resolved disagreements by discussion or, failing that, with the input of the third author (AS).
Data extraction and management
Two authors (KH and LM) extracted data independently. We used standardised data extraction forms. There was no blinding of journal, author names or affiliations.
For each study, we planned to extract the following information:
study design;
duration of study;
randomisation;
allocation concealment;
number of participants;
setting of study;
diagnostic criteria;
exclusion criteria;
age and sex distribution of participants;
country of recruitment;
co‐morbidity;
date of study;
number of intervention groups;
type of dietary modification;
outcomes measured and definition of outcomes;
missing data and final sample size.
Assessment of risk of bias in included studies
KH and LM planned to undertake assessment of the risk of bias of the included studies independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We planned to use the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the study and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
We also intended to judge two extra domains: the certainty of the diagnosis of Ménière's (see also Types of participants) and the quality of outcome assessment (see Types of outcome measures). However, we would have reported and addressed these domains in the 'Characteristics of included studies' table rather than consider them as a risk of bias domain.
Measures of treatment effect
We planned to use appropriate statistical tests based on the data. For dichotomous data we planned to calculate an odds ratio (OR), risk ratio (RR) and risk difference (RD, also called absolute risk reduction), as well as the number of participants needed to treat to avoid a case of the disease (number needed to treat to benefit ‐ NNTB) from the pooled results, based on the median risk in the control groups.
For the intervention effect measures of continuous data we planned to calculate the difference in means (mean difference, MD) between the groups, provided that different studies were using the same scale of measurement. We planned to calculate the standardised mean difference (SMD) if different scales were used.
For ordinal data we planned to check to see whether the scale used had been validated. Depending on the number points in these scales (and how the data were reported), we planned either to dichotomise these or analyse them as continuous outcomes.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials allocate groups instead of individuals. The participants in each group may be related in some way, therefore this needs to be taken into account in the analysis otherwise there is a unit of analysis error, which would produce an artificially smaller P value and a risk of false positive results. For this purpose we planned to use a special statistical method, as detailed in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), with the appropriate statistical advice.
Cross‐over trials
Cross‐over trials may have a carry‐over effect. For this reason we planned to use data from cross‐over trials only if data from before the cross‐over could be obtained.
Multi‐arm studies
If we had found studies with more two groups (e.g. two or more active treatments being tested against placebo), we would have established which of the comparisons were relevant to the systematic review and relevant to each of the meta‐analyses that we may implement. If the study design used independent groups, we would have treated the study as having independent comparisons. However, if we had encountered participants that had been included in several groups, there would be a risk of unit of analysis error and we planned to ensure that participants were only included once per meta‐analysis as per chapter 16.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Repeated observations on participants
In longer studies, results may be recorded at more than one time interval. In order to avoid unit of analysis error when combining these results in a single meta‐analysis (and therefore counting the same participants in more than one comparison), we aimed to retrieve individual patient data and perform a time‐to‐event analysis using the whole follow‐up for each participant.
We also aimed to establish short‐term (three months), medium‐term (12 months) and long‐term (over 24 months) effects.
Dealing with missing data
When the required data were not available in published accounts, we planned to contact the principal investigator to request the data. If no useful response could be obtained, we planned to treat missing data differently if they were judged to be 'missing at random', in which case the effect may not be important, or 'not missing at random', where missing data may affect the overall result. In the first case, the data can be ignored. If large numbers of dropouts were found, we planned to conduct sensitivity analysis with different assumptions.
We were alert to potential mislabelling or non‐identification of standard errors and standard deviations. Unless missing standard deviations could be derived from confidence intervals we would not have assumed values for analysis purposes.
Assessment of heterogeneity
We planned to assess studies for clinical, statistical and methodological heterogeneity. If sufficient non‐heterogeneous studies were found, we planned to subject the data to a meta‐analysis with a fixed‐effect model where applicable. If there was statistical heterogeneity, we planned to use random‐effects modelling. If the level of heterogeneity and the appropriate model that should be used was unclear, we planned to take statistical advice.
Assessment of reporting biases
If an individual meta‐analysis contained at least 10 studies, we would have assessed publication bias using funnel plots and Egger's test.
Data synthesis
Analysis was to be on an intention‐to‐treat basis. If the data were compatible we planned to combine data to give summary measures of effect. If data were missing we planned to use available case analysis – using all data (as reported) for all randomised patients available at the end of the trial/time point of interest, regardless of actual treatment received.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analysis based on:
patients meeting versus not meeting the AAO‐HNS criteria for the diagnosis of Ménière's disease or syndrome;
-
the treatment protocol; the subgroups would be based on the specific duration:
complete versus partial restriction of the dietary modification of interest versus modification of multiple simultaneous dietary modifications. This latter group may also be further subdivided based on the level of restriction.
Sensitivity analysis
We planned to conduct a sensitivity analysis by comparing the effect of the inclusion and exclusion of studies with different risk of bias. If we deemed studies to have a high risk of bias, we would have excluded them from the analysis.
GRADE and 'Summary of findings' table
We planned to use the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we planned to apply this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We planned to include a 'Summary of findings' table, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). We would have included the following outcomes in our 'Summary of findings' table(s):
proportion of patients with control of vertigo or decrease in vertigo attacks (as suggested by the AAO‐HNS);
proportion of patients with adverse effects (e.g. hyponatraemia, mood disturbance and headaches);
proportion of patients with loss or gain of hearing/reduction in progression of hearing loss;
proportion of patients with a reduction in the severity of tinnitus;
proportion of patients with a reduction in the perception of aural fullness;
overall changes in well‐being and quality of life;
any other adverse effects.
Results
Description of studies
Results of the search
The searches retrieved 363 records including papers, reviews, conference abstracts and registered clinical trials, of which 124 remained after removing duplicates. Review of the titles and abstracts identified two potential studies for full‐text review. Subsequently, we formally excluded these two studies for the reasons given in the Characteristics of excluded studies table. We identified no ongoing studies and there are no studies awaiting assessment. No studies met the inclusion criteria for the review.
Full details of the search and study selection process are shown in a PRISMA flowchart (Figure 1).
1.
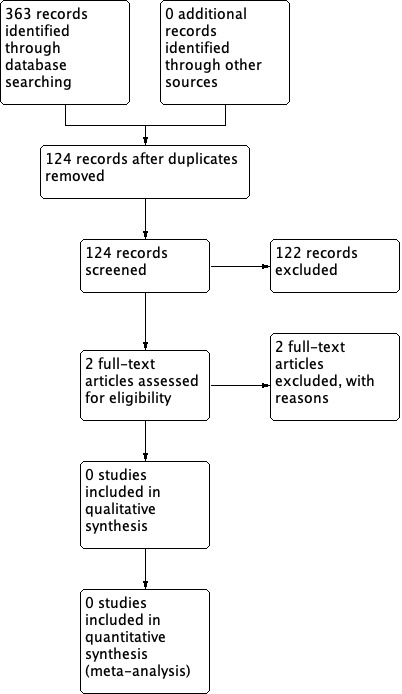
Process for sifting search results and selecting studies for inclusion.
Included studies
We did not identify any studies that met the inclusion criteria for the review.
Excluded studies
We excluded two studies after a full‐text review (see Characteristics of excluded studies).
Acharya 2017 was a double‐blind randomised controlled trial in the outpatient department of an ENT unit in a teaching hospital in Nepal. Patients who were diagnosed with Ménière's disease were randomised into three groups: dietary salt restriction, diuretics or a vasodilator. Outcome measures included the number and severity of vertigo attacks, tinnitus and hearing loss. We excluded this study because the control groups were both active interventions. The study did not have a no treatment (no dietary restriction) or placebo control group.
Bittar 2016 was a randomised controlled trial evaluating the effect of a fractionated diet without glucose as the treatment for labyrinthine disorders associated with the glucose insulin index. We excluded this study because it excluded patients with Ménière's disease and did not assess the restriction of salt, caffeine or alcohol.
Risk of bias in included studies
We did not identify any studies that met the inclusion criteria.
Effects of interventions
See: Table 1
There were no studies meeting our inclusion criteria. See Table 1.
Discussion
Summary of main results
We were unable to identify any randomised controlled trials or quasi‐randomised controlled trials investigating the role of dietary modification (restriction of salt, caffeine and/or alcohol) in the treatment of Ménière's disease or syndrome.
Ménière's disease is a debilitating and progressive condition that can result in the affected person being unable to carry out acts of daily living and can lead to the development of irreversible hearing loss. Modification of diet, including restriction of salt, caffeine and alcohol intake, is an option that is widely recommended to patients with Ménière's as a first‐line treatment, and is in fact featured on both the UK National Health Service (NHS) and Ménière's Society websites (Ménière's Society; NHS 2017). However, it is evident that there is no high‐level evidence on which to base these recommendations. The question was prioritised by a James Lind Alliance patient and public involvement (PPI) initiative in 2011 (JLA 2011), but still cannot be answered.
Overall completeness and applicability of evidence
There are no studies to include in this review.
Quality of the evidence
There is no evidence meeting our inclusion criteria to assess.
Potential biases in the review process
We conducted this review according to a published protocol (Hussain 2016). The search is comprehensive and up to date as of March 2018. We have not identified any specific biases in our review process.
Agreements and disagreements with other studies or reviews
This is the first systematic review that sought to identify randomised controlled trials investigating the role of dietary modification of salt, caffeine and alcohol in the treatment of Ménière's disease. However, other studies have been conducted in an attempt to investigate the effect on the disease. Most recently, Acharya 2017 addressed the role of diet in the control of symptoms in Ménière's disease. Patients were required to meet the American Academy of Otolaryngology – Head and Neck Surgery (AAO‐HNS) diagnostic criteria and they were randomly allocated into the trial. The treatment arms were dietary salt restriction (plus vitamin B complex daily as a placebo) versus 5 mg of amiloride and 40 mg of furosemide, versus 24 mg of betahistine. There was no advice about intake of salt for participants in these latter two arms. The study also did not include a no treatment (no dietary restriction) or placebo control group. Bittar 2016 addressed the impact on vestibular disorders of a fractionated diet with glucose restriction versus no restriction at all. This study, however, excluded patients with Ménière's disease/syndrome and did not look specifically at the impact of restricting salt, caffeine or alcohol intake.
We found one review article that looked at the role of caffeine in the causation or aggravation of various otolaryngology conditions, including Ménière's. The authors were unable to identify any evidence to support or refute this association (Trinidade 2014).
Authors' conclusions
Implications for practice.
There is no evidence from randomised controlled trials (RCTs) to support or refute the restriction of salt intake or other dietary modifications for patients with Ménière's disease or syndrome. There are therefore no implications for the alteration of current practice.
Implications for research.
High‐quality research into the restriction of salt and other dietary modifications for the treatment of Ménière's disease or syndrome is warranted. This intervention is widely recommended to patients without any proven benefit or clear understanding of any potential harms. This may delay the use of more effective treatment options resulting in disease progression and patient suffering or adverse effects. If shown to be effective, however, this is a cost‐neutral treatment option for the medical treatment provider. There is also undoubtedly interest from patients in the potential effectiveness of dietary modifications. The James Lind Alliance patient‐professional priority‐setting group identified the efficacy of dietary intervention for Ménière's disease as one of the top 10 priorities for research into balance disorders (JLA 2011).
It must be acknowledged there has been a move away from the American Academy of Otolaryngology – Head and Neck Surgery (AAO‐HNS) diagnostic criteria within the Ménière's disease community since the start of this review process. The Bárány Society represents an international collaboration of basic scientists, otolaryngologists, oto‐neurologists, physical therapists and other allied health professionals considered experts in the field. A classification committee for an International Classification for Vestibular Disorders (ICVD) was established by the society and developed international consensus diagnostic criteria for Ménière's disease. They worked with the Equilibrium Committee of the AAO‐HNS as part of their process and defined two sets of diagnostic criteria: definite and probable Ménière's disease (Lopez‐Escamez 2016). In view of the fact that the Bárány Society criteria are not dissimilar to the AAO‐HNS definition, we do not believe that our search results will be materially affected and therefore we opted not to repeat the search or change the scope of this review. However, future research should adhere to the newer Bárány Society diagnostic criteria. Any future trial should also be conducted and reported according to the CONSORT statement.
There are methodological challenges with conducting research into dietary interventions in Ménière's disease. Appropriate placebo groups can be difficult to construct for dietary restrictions. Adherence to diets should be assessed objectively where possible, for example by measurement of urinary sodium levels in the case of low salt diets. Comparison of high and low levels of salt intake has been successfully accomplished, for example when looking at salt restriction for the management of hypertension (Graudal 2017). Unlike hypertension, however, Ménière's disease is relatively uncommon (Murdin 2015), and it has a relapsing and remitting natural history. Conducting an adequately powered study of a dietary intervention over a suitable time period is therefore challenging. The best evidence may come from a RCT comparing dietary interventions (e.g. low salt versus general healthy diet advice). However, this research question might be more pragmatically addressed by using information from carefully constructed patient registries that include information on dietary intake of substances of interest such as salt, caffeine and alcohol. It would be important to address the question of any possible harms or unwanted effects of dietary modification.
It must, however, also be acknowledged that a Cochrane‐style review may not be appropriate for addressing this question (Truswell 2005). Adherence to dietary modification is challenging to implement and assess. However, this does not take away from the need for an appropriate RCT to be conducted.
Acknowledgements
We are grateful to Mr Malcolm Hilton for peer reviewing the manuscript for this review and to Joan Blakley for her input as the consumer referee.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | Embase | CINAHL |
| #1 MeSH descriptor: [Endolymphatic Hydrops] explode all trees #2 (meniere or menieres or meniere's):ti,ab,kw #3 ((endolymphatic or cochlea*) and hydrops):ti,ab,kw #4 ((aural or labyrinth*) and (hydrops or syndrome or vertigo)):ti,ab,kw #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Diet, Sodium‐Restricted] explode all trees #7 MeSH descriptor: [Feeding Behavior] this term only #8 MeSH descriptor: [Diet Therapy] this term only #9 MeSH descriptor: [Sodium, Dietary] explode all trees #10 MeSH descriptor: [Caffeine] explode all trees #11 MeSH descriptor: [Drinking Behavior] explode all trees #12 (salt* or sodium*):ti,ab,kw #13 (Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol*):ti,ab,kw #14 ((diet* or food) and (restrict* or modif* or habit* or free*)):ti,ab,kw #15 nutrition*:ti,ab,kw #16 Any MeSH descriptor with qualifier(s): [Diet therapy ‐ DH] #17 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #5 and #17 #19 MeSH descriptor: [Endolymphatic Hydrops] explode all trees and with qualifier(s): [Diet therapy ‐ DH] #20 #18 or #19 |
#1 Search "Endolymphatic Hydrops"[Mesh] #2 Search meniere*[Title/Abstract] #3 Search ((endolymphatic[Title/Abstract] OR cochlea*[Title/Abstract])) AND hydrops[Title/Abstract] #4 Search ((aural[Title/Abstract] OR labyrinth*[Title/Abstract])) AND (hydrops[Title/Abstract] OR syndrome[Title/Abstract] OR vertigo[Title/Abstract]) #5 (#1 OR #2 OR #3 O R #4) #6 Search "Diet, Sodium‐Restricted"[Mesh] #7 Search "Feeding Behavior"[Mesh:NoExp] #8 Search "Diet Therapy"[Mesh:NoExp] #9 Search "Sodium, Dietary"[Mesh] #10 Search "Caffeine"[Mesh] #11 Search "Drinking Behavior"[Mesh] #12 Search (salt*[Title/Abstract] OR sodium*[Title/Abstract]) #13 Search (Caffein*[Title/Abstract] OR decaffein*[Title/Abstract] OR "de caffein*"[Title/Abstract] OR de‐caffein*[Title/Abstract] OR coffee[Title/Abstract] OR tea[Title/Abstract] OR alcohol*[Title/Abstract]) #14 Search ((diet*[Title/Abstract] OR food[Title/Abstract])) AND (restrict*[Title/Abstract] OR modif*[Title/Abstract] OR habit*[Title/Abstract] OR free*[Title/Abstract]) #15 Search nutrition*[Title/Abstract] #16 Search Diet therapy[sh] #17 Search (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #18 (#5 and #17) #19 Search "Endolymphatic Hydrops/diet therapy"[Mesh] #20 (#18 AND #19) |
1 exp Meniere disease/ 2 "meniere*".ti,ab. 3 ((endolymphatic or cochlea*) and hydrops).ti,ab. 4 ((aural or labyrinth*) and (hydrops or syndrome or vertigo)).ti,ab. 5 exp sodium restriction/ 6 exp feeding behavior/ 7 diet therapy/ 8 exp sodium intake/ 9 exp caffeine/ 10 exp drinking behavior/ 11 (salt* or sodium*).ti,ab. 12 (Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol*).ti,ab. 13 ((diet* or food) and (restrict* or modif* or habit* or free*)).ti,ab. 14 "nutrition*".ti,ab. 15 1 or 2 or 3 or 4 16 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 17 15 and 16 |
S40 S38 OR S39 S39 (MH "Endolymphatic Hydrops+/DH") S38 S25 AND S37 S37 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 S36 MW "DH" S35 TX nutrition* S34 TX (diet* or food) and (restrict* or modif* or habit* or free*) S33 TX Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol* S32 TX salt* or sodium* S31 (MH "Drinking Behavior+") S30 (MH "Caffeine") S29 (MH "Sodium, Dietary+") S28 (MH "Diet Therapy") S27 (MH "Food Habits") S26 (MH "Diet, Sodium‐Restricted") S25 S21 OR S22 OR S23 OR S24 S24 TX ((aural or labyrinth*) and (hydrops or syndrome or vertigo)) S23 TX ((endolymphatic or cochlea*) and hydrops) S22 TX meniere* S21 (MH "Endolymphatic Hydrops+") S20 S18 OR S19 S19 (MH "Endolymphatic Hydrops+/DH") S18 S5 AND S17 S17 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 MW "DH" S15 TX nutrition* S14 TX (diet* or food) and (restrict* or modif* or habit* or free*) S13 TX Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol* S12 TX salt* or sodium* S11 (MH "Drinking Behavior+") S10 (MH "Caffeine") S9 (MH "Sodium, Dietary+") S8 (MH "Diet Therapy") S7 (MH "Eating Behavior") S6 (MH "Diet, Sodium‐Restricted") S5 S1 OR S2 OR S3 OR S4 S4 TX ((aural or labyrinth*) and (hydrops or syndrome or vertigo)) S3 TX ((endolymphatic or cochlea*) and hydrops) S2 TX meniere* S1 (MH "Endolymphatic Hydrops+") |
| Web of Science | ICTRP | ClinicalTrials.gov | LILACS |
| #1 TOPIC: (meniere*) #2 TOPIC: ((endolymphatic or cochlea*) and hydrops) #3 TOPIC: ((aural or labyrinth*) and (hydrops or syndrome or vertigo)) #4 #3 OR #2 OR #1 #5 TOPIC: (salt* or sodium* or Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol*) #6 TOPIC: ((diet* or food) and (restrict* or modif* or habit* or free*)) #7 TOPIC: (nutrition*) #8 #7 OR #6 OR #5 #9 #8 AND #4 |
meniere* AND salt* OR meniere* AND sodium* OR meniere* AND caffein* OR meniere* AND alcohol |
Via the Cochrane Register of Studies 1 (meniere or menieres or meniere's) AND INSEGMENT 2 (endolymphatic or cochlea*) and hydrops AND INSEGMENT 3 (aural or labyrinth*) and (hydrops or syndrome or vertigo) AND INSEGMENT 4 #1 OR #2 OR #3 AND INSEGMENT 5 salt* or sodium* AND INSEGMENT 6 Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol* AND INSEGMENT 7 (diet* or food) and (restrict* or modif* or habit* or free*) AND INSEGMENT 8 nutrition* AND INSEGMENT 9 #5 OR #6 OR #7 OR #8 AND INSEGMENT 10 #4 AND #9 AND INSEGMENT 11 (nct*):AU AND INSEGMENT 12 #10 AND #11 Via Clinicaltrials.org (menieres OR meniere's) AND (salt OR sodium OR caffeine OR alcohol) |
(TW:Endolymphatic AND TW:Hydops) OR (TW:Hidropesía AND TW:Endolinfática) OR TW:meniere* AND Controlled Clinical Trials filter |
Search strategies for CENTRAL, PubMed and CINAHL for searches in July 2016 and July 2017
See explanation in Differences between protocol and review.
| CENTRAL | PubMed | CINAHL |
| #1 MeSH descriptor: [Endolymphatic Hydrops] explode all trees #2 (meniere or menieres or meniere's):ti,ab,kw #3 ((endolymphatic or cochlea*) and hydrops):ti,ab,kw #4 ((aural or labyrinth*) and (hydrops or syndrome or vertigo)):ti,ab,kw #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Diet, Sodium‐Restricted] explode all trees #7 MeSH descriptor: [Food Habits] explode all trees #8 MeSH descriptor: [Diet Therapy] this term only #9 MeSH descriptor: [Sodium, Dietary] explode all trees #10 MeSH descriptor: [Caffeine] explode all trees #11 MeSH descriptor: [Drinking Behavior] explode all trees #12 (salt* or sodium*):ti,ab,kw #13 (Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol*):ti,ab,kw #14 ((diet* or food) and (restrict* or modif* or habit* or free*)):ti,ab,kw #15 nutrition*:ti,ab,kw #16 Any MeSH descriptor with qualifier(s): [Diet therapy ‐ DH] #17 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #5 and #17 #19 MeSH descriptor: [Endolymphatic Hydrops] explode all trees and with qualifier(s): [Diet therapy ‐ DH] #20 #18 or #19 |
#1 Search "Endolymphatic Hydrops"[Mesh] #2 Search meniere*[Title/Abstract] #3 Search ((endolymphatic[Title/Abstract] OR cochlea*[Title/Abstract])) AND hydrops[Title/Abstract] #4 Search ((aural[Title/Abstract] OR labyrinth*[Title/Abstract])) AND (hydrops[Title/Abstract] OR syndrome[Title/Abstract] OR vertigo[Title/Abstract]) #5 (#1 OR #2 OR #3 O R #4) #6 Search "Diet, Sodium‐Restricted"[Mesh] #7 Search "Food Habits"[Mesh] #8 Search "Diet Therapy"[Mesh:NoExp] #9 Search "Sodium, Dietary"[Mesh] #10 Search "Caffeine"[Mesh] #11 Search "Drinking Behavior"[Mesh] #12 Search (salt*[Title/Abstract] OR sodium*[Title/Abstract]) #13 Search (Caffein*[Title/Abstract] OR decaffein*[Title/Abstract] OR "de caffein*"[Title/Abstract] OR de‐caffein*[Title/Abstract] OR coffee[Title/Abstract] OR tea[Title/Abstract] OR alcohol*[Title/Abstract]) #14 Search ((diet*[Title/Abstract] OR food[Title/Abstract])) AND (restrict*[Title/Abstract] OR modif*[Title/Abstract] OR habit*[Title/Abstract] OR free*[Title/Abstract]) #15 Search nutrition*[Title/Abstract] #16 Search Diet therapy[sh] #17 Search (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #18 (#5 and #17) #19 Search "Endolymphatic Hydrops/diet therapy"[Mesh] #20 (#18 AND #19) |
S40 S38 OR S39 S39 (MH "Endolymphatic Hydrops+/DH") S38 S25 AND S37 S37 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 S36 MW "DH" S35 TX nutrition* S34 TX (diet* or food) and (restrict* or modif* or habit* or free*) S33 TX Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol* S32 TX salt* or sodium* S31 (MH "Drinking Behavior+") S30 (MH "Caffeine") S29 (MH "Sodium, Dietary+") S28 (MH "Diet Therapy") S27 (MH "Food Habits") S26 (MH "Diet, Sodium‐Restricted") S25 S21 OR S22 OR S23 OR S24 S24 TX ((aural or labyrinth*) and (hydrops or syndrome or vertigo)) S23 TX ((endolymphatic or cochlea*) and hydrops) S22 TX meniere* S21 (MH "Endolymphatic Hydrops+") S20 S18 OR S19 S19 (MH "Endolymphatic Hydrops+/DH") S18 S5 AND S17 S17 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 MW "DH" S15 TX nutrition* S14 TX (diet* or food) and (restrict* or modif* or habit* or free*) S13 TX Caffein* or decaffein* or "de caffein*" or de‐caffein* or coffee or tea or alcohol* S12 TX salt* or sodium* S11 (MH "Drinking Behavior+") S10 (MH "Caffeine") S9 (MH "Sodium, Dietary+") S8 (MH "Diet Therapy") S7 (MH "Food Habits") S6 (MH "Diet, Sodium‐Restricted") S5 S1 OR S2 OR S3 OR S4 S4 TX ((aural or labyrinth*) and (hydrops or syndrome or vertigo)) S3 TX ((endolymphatic or cochlea*) and hydrops) S2 TX meniere* S1 (MH "Endolymphatic Hydrops+") |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acharya 2017 | ALLOCATION: Randomised controlled trial PARTICIPANTS: Patients with Ménière's disease according to AAO‐HNS 1995 guidelines INTERVENTION: Dietary salt restriction (plus vitamin B complex daily as a placebo) versus amiloride 5 mg and furosemide 40 mg (no advice about intake of salt) versus betahistine 24 mg (no advice about intake of salt): study did not include a no treatment (no dietary restriction) or placebo control group |
| Bittar 2016 | ALLOCATION: Randomised controlled trial PARTICIPANTS: Patients with vestibular disorders: patients with Ménière's disease were excluded INTERVENTION: Fractionated diet with glucose restriction versus no restriction: not salt, caffeine or alcohol restriction |
AAO‐HNS: American Academy of Otolaryngology – Head and Neck Surgery
Differences between protocol and review
In March 2018, changes to the searches of CENTRAL, PubMed and CINAHL were required because the National Library of Medicine (NLM) had removed 'Food Habits' from MeSH. The Information Specialist replaced this term with a broader one 'Feeding Behaviour' in CENTRAL and PubMed and 'Eating Behaviour' in CINAHL. The original term was used in previous searches in July 2016 and July 2017. In March 2018, we re‐ran the searches over all years to look for duplicates within the previous two searches and removed them. See Appendix 1 for further details.
Contributions of authors
Kiran Hussain: screened and selected studies, obtained full texts, extracted data and assessed risk of bias, entered data into RevMan and would have carried out and interpreted the analysis, drafted the final review and will have responsibility for updating and maintaining the review.
Louisa Murdin: screened and selected studies, extracted data and assessed risk of bias, drafted the final review.
Anne GM Schilder: provided advice as needed throughout the review, drafted the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Kiran Hussain: Kiran Hussain is a recipient of a small grant from the Ménière's Society (charity).
Louisa Murdin: none known.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported in part by the National Institute of Health Research University College London Hospitals Biomedical Research Centre. Their research is funded by the NIHR and EU Horizon2020. She is the national chair of the NIHR Clinical Research Network ENT Specialty. She is the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. She is co‐investigator on the NIHR PGfAR grant 'Defining best Management for Adults with Chronic RhinOsinusitis: the MACRO Programme'. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she acts as an advisor on clinical trial design and delivery to a range of biotech companies.
New
References
References to studies excluded from this review
Acharya 2017 {published data only}
- Acharya A, Singh MM, Shrestha A. First line treatment of Meniere's disease: a randomized controlled trial. Journal of Lumbini Medical College 2016;4(2):68‐71. [Google Scholar]
Bittar 2016 {published data only}
- Bittar RS, Santos MD, Mezzalira R. Glucose metabolism disorders and vestibular manifestations: evaluation through computerized dynamic posturography. Brazilian Journal of Otorhinolaryngology 2016;82(4):372‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alexander 2010
- Alexander TH, Harris JP. Current epidemiology of Meniere's syndrome. Otolaryngologic Clinics of North America 2010;43(5):965‐70. [DOI] [PubMed] [Google Scholar]
Alford 1972
- Alford BR. Disease: criteria for diagnosis and evaluation of therapy for reporting. Report of subcommittee on equilibrium and its measurement. Transactions of the American Academy of Ophthalmology and Otolaryngology 1972;76:1462‐4. [PubMed] [Google Scholar]
Burgess 2006
- Burgess S, Kundu S. Diuretics for Ménière's disease or syndrome. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003599.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
da Costa 2002
- Costa SS, Sousa LC, Piza MR. Meniere's disease: overview, epidemiology and natural history. Otolaryngologic Clinics of North America 2002;35(3):455‐95. [DOI] [PubMed] [Google Scholar]
Dederding 1929
- Dederding D. Clinical and experimental examination in patients suffering from morbus Meniere including study of problems of bone conduction. Acta Oto‐Laryngologica 1929;10:1‐156. [Google Scholar]
Furstenberg 1934
- Furstenberg AC, Lashmet FH, Lathrop F. Meniere's symptom complex: medical treatment. Annals of Otology, Rhinology and Laryngology 1934;43:1035‐47. [DOI] [PubMed] [Google Scholar]
Furstenberg 1941
- Furstenberg AC, Richardson G, Lathrop FD. Meniere's disease: addenda to medical therapy. Archives of Otolaryngology 1941;34:1038‐92. [Google Scholar]
Graudal 2017
- Graudal N, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD004022.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallpike 1938
- Hallpike C, Cairns H. Observations on the pathology of Menière's syndrome. Journal of Laryngology and Otology 1938;53:625‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Harcourt 2014
- Harcourt J, Barraclough K, Bronstein AM. Meniere's disease. BMJ 2014;12:g6544. [DOI] [PubMed] [Google Scholar]
James 2001
- James A, Burton M. Betahistine for Ménière's disease or syndrome. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001873] [DOI] [PMC free article] [PubMed] [Google Scholar]
JLA 2011
- James Lind Alliance Priority Setting Partnerships. Ear, Nose and Throat (Aspects of Balance) Top 10. http://www.jla.nihr.ac.uk/priority‐setting‐partnerships/ear‐nose‐and‐throat‐aspects‐of‐balance/top‐10‐priorities/ 2011.
Klockhoff 1974
- Klockhoff I, Lindbloom U, Stahle J. Diuretic treatment of Meniere's disease: long‐term results with chlorothiazide. Archives of Otolaryngology 1974;100:262‐5. [DOI] [PubMed] [Google Scholar]
Lopez‐Escamez 2016
- Lopez‐Escamez JA, John C, Won‐Ho C, Joel AG, Måns M, Marco M, et al. Diagnostic criteria for Menière's disease. Consensus document of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology (EAONO), the American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS) and the Korean Balance Society. Acta Otorrinolaringologica Espanoloa 2016;67(1):1‐7. [DOI] [PubMed] [Google Scholar]
Luxford 2013
- Luxford E, Berliner KI, Lee J, Luxford WM. Dietary modification as adjunct treatment in Ménière's disease: patient willingness and ability to comply. Otology & Neurotology 2013;34(8):1438‐43. [DOI] [PubMed] [Google Scholar]
Moffat 1997
- Moffat DA, Ballagh RH. Menière's disease. In: Kerr AG, Booth JB editor(s). Scott‐Brown's Otolaryngology. 3rd Edition. Vol. 3, Oxford: Butterworth‐Heinemann, 1997:1‐50. [Google Scholar]
Murdin 2015
- Murdin L, Schilder AS. Epidemiology of balance system disorders in the community: a systematic review. Otology Neuro‐otology 2015;36(3):387‐92. [DOI] [PubMed] [Google Scholar]
Ménière's Guide 1995
- No authors listed. Committee on Hearing and Equilibrium. Guidelines for the diagnosis and evaluation of therapy in Menière's disease. Otolaryngology ‐ Head and Neck Surgery 1995;113:181‐5. [DOI] [PubMed] [Google Scholar]
Ménière's Society
- Ménière's Society. Other non‐surgical treatments. https://www.menieres.org.uk/ (accessed prior to 8 August 2018).
NHS 2017
- NHS. Ménière's disease. https://www.nhs.uk/conditions/menieres‐disease/ (accessed 22 October 2018).
Office of National Statistics
- Office of National Statistics. Adult drinking habits in England. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/datasets/adultdrinkinghabitsinengland (accessed prior to 8 August 2018).
Pearson 1985
- Pearson BW, Brackmann DE. Committee on hearing and equilibrium guidelines for reporting treatment results in Menière's disease. Otolaryngology ‐ Head and Neck Surgery 1985;93:578‐81. [DOI] [PubMed] [Google Scholar]
Pesta 2013
- Pesta DH, Angadi SS, Burtscher M, Roberts CK. The effects of caffeine, nicotine, ethanol, and tetrahydrocannabinol on exercise performance. Nutrition and Metabolism 2013;10(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2011
- Phillips JS, Westerberg B. Intratympanic steroids for Ménière's disease or syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008514.pub2] [DOI] [PubMed] [Google Scholar]
Pullens 2011
- Pullens B, Benthem PP. Intratympanic gentamicin for Ménière's disease or syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD008234.pub2] [DOI] [PubMed] [Google Scholar]
Pullens 2013
- Pullens B, Verschuur HP, Benthem PP. Surgery for Ménière's disease. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD005395.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salvador‐Carulla 2010
- Salvador‐Carulla L, Gasca VI. Defining disability functioning autonomy and dependency in person‐centered medicine and integrated care. International Journal of Integrated Care 2010;10 Suppl:e025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 1993
- Santos PM, Hall RA, Snyder JM, Hughes LF, Dobie RA. Diuretic and diet effect on Menière's disease evaluated by the 1985 Committee on Hearing and Equilibrium guidelines. Otolaryngology ‐ Head and Neck Surgery 1993;109(4):680‐9. [DOI] [PubMed] [Google Scholar]
Silverstein 1989
- Silverstein H, Smouha E, Jones R. Natural history versus surgery for Menière's disease. Otolaryngology ‐ Head and Neck Surgery 1989;100:6‐16. [DOI] [PubMed] [Google Scholar]
Stahle 1984
- Stahle J. Medical treatment of fluctuant hearing loss in Meniere's disease. American Journal of Otology 1984;5(6):529‐33. [PubMed] [Google Scholar]
Stern 2010
- Stern TA, Gross AF, Stern TW, Nejad SH, Maldonado JR. Current approaches to the recognition and treatment of alcohol withdrawal and delirium tremens: "old wine in new bottles" or "new wine in old bottles". Primary Care Companion to the Journal of Clinical Psychiatry 2010;12(3):PCC.10r00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2010
- Thompson CJ. Hyponatraemia: new associations and new treatments. European Journal of Endocrinology 2010;162:S1‐S3. [DOI] [PubMed] [Google Scholar]
Trinidade 2014
- Trinidade A, Robinson T, Phillips JS. The role of caffeine in otorhinolaryngology: guilty as charged?. European Archives of Oto‐Rhino‐Laryngology 2014;271(8):2097‐102. [DOI] [PubMed] [Google Scholar]
Truswell 2005
- Truswell AS. Some problems with Cochrane reviews of diet and chronic disease. European Journal of Clinical Nutrition 2005;59 Suppl 1:S150‐4; discussion S195‐6. [DOI] [PubMed] [Google Scholar]
Tyrrell 2016
- Tyrrell J, Whinney DJ, Taylor T. The cost of Meniere's disease: a novel multisource approach. Ear and Hearing 2016;37(3):202‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hussain 2016
- Hussain K, Murdin L, Schilder AGM. Restriction of salt intake and other dietary modifications for the treatment of Ménière's disease or syndrome. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012173] [DOI] [PMC free article] [PubMed] [Google Scholar]


