Abstract
Background
Dysphagia (swallowing problems), which is common after stroke, is associated with increased risk of death or dependency, occurrence of pneumonia, poor quality of life, and longer hospital stay. Treatments provided to improve dysphagia are aimed at accelerating recovery of swallowing function and reducing these risks. This is an update of the review first published in 1999 and updated in 2012.
Objectives
To assess the effects of swallowing therapy on death or dependency among stroke survivors with dysphagia within six months of stroke onset.
Search methods
We searched the Cochrane Stroke Group Trials Register (26 June 2018), the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library (searched 26 June 2018), MEDLINE (26 June 2018), Embase (26 June 2018), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (26 June 2018), Web of Science Core Collection (26 June 2018), SpeechBITE (28 June 2016), ClinicalTrials.Gov (26 June 2018), and the World Health Organization International Clinical Trials Registry Platform (26 June 2018). We also searched Google Scholar (7 June 2018) and the reference lists of relevant trials and review articles.
Selection criteria
We sought to include randomised controlled trials (RCTs) of interventions for people with dysphagia and recent stroke (within six months).
Data collection and analysis
Two review authors independently applied the inclusion criteria, extracted data, assessed risk of bias, used the GRADE approach to assess the quality of evidence, and resolved disagreements through discussion with the third review author (PB). We used random‐effects models to calculate odds ratios (ORs), mean differences (MDs), and standardised mean differences (SMDs), and provided 95% confidence intervals (CIs) for each.
The primary outcome was functional outcome, defined as death or dependency (or death or disability), at the end of the trial. Secondary outcomes were case fatality at the end of the trial, length of inpatient stay, proportion of participants with dysphagia at the end of the trial, swallowing ability, penetration aspiration score, or pneumonia, pharyngeal transit time, institutionalisation, and nutrition.
Main results
We added 27 new studies (1777 participants) to this update to include a total of 41 trials (2660 participants).
We assessed the efficacy of swallowing therapy overall and in subgroups by type of intervention: acupuncture (11 studies), behavioural interventions (nine studies), drug therapy (three studies), neuromuscular electrical stimulation (NMES; six studies), pharyngeal electrical stimulation (PES; four studies), physical stimulation (three studies), transcranial direct current stimulation (tDCS; two studies), and transcranial magnetic stimulation (TMS; nine studies).
Swallowing therapy had no effect on the primary outcome (death or dependency/disability at the end of the trial) based on data from one trial (two data sets) (OR 1.05, 95% CI 0.63 to 1.75; 306 participants; 2 studies; I² = 0%; P = 0.86; moderate‐quality evidence). Swallowing therapy had no effect on case fatality at the end of the trial (OR 1.00, 95% CI 0.66 to 1.52; 766 participants; 14 studies; I² = 6%; P = 0.99; moderate‐quality evidence). Swallowing therapy probably reduced length of inpatient stay (MD ‐2.9, 95% CI ‐5.65 to ‐0.15; 577 participants; 8 studies; I² = 11%; P = 0.04; moderate‐quality evidence). Researchers found no evidence of a subgroup effect based on testing for subgroup differences (P = 0.54). Swallowing therapy may have reduced the proportion of participants with dysphagia at the end of the trial (OR 0.42, 95% CI 0.32 to 0.55; 1487 participants; 23 studies; I² = 0%; P = 0.00001; low‐quality evidence). Trial results show no evidence of a subgroup effect based on testing for subgroup differences (P = 0.91). Swallowing therapy may improve swallowing ability (SMD ‐0.66, 95% CI ‐1.01 to ‐0.32; 1173 participants; 26 studies; I² = 86%; P = 0.0002; very low‐quality evidence). We found no evidence of a subgroup effect based on testing for subgroup differences (P = 0.09). We noted moderate to substantial heterogeneity between trials for these interventions. Swallowing therapy did not reduce the penetration aspiration score (i.e. it did not reduce radiological aspiration) (SMD ‐0.37, 95% CI ‐0.74 to ‐0.00; 303 participants; 11 studies; I² = 46%; P = 0.05; low‐quality evidence). Swallowing therapy may reduce the incidence of chest infection or pneumonia (OR 0.36, 95% CI 0.16 to 0.78; 618 participants; 9 studies; I² = 59%; P = 0.009; very low‐quality evidence).
Authors' conclusions
Moderate‐ and low‐quality evidence suggests that swallowing therapy did not have a significant effect on the outcomes of death or dependency/disability, case fatality at the end of the trial, or penetration aspiration score. However, swallowing therapy may have reduced length of hospital stay, dysphagia, and chest infections, and may have improved swallowing ability. However, these results are based on evidence of variable quality, involving a variety of interventions. Further high‐quality trials are needed to test whether specific interventions are effective.
Plain language summary
Swallowing therapy for difficulties with swallowing in stroke survivors who have had a recent stroke
Question
We wanted to assess the effectiveness of swallowing therapy for stroke survivors with dysphagia (difficulty in swallowing). We looked at swallowing therapy in survivors up to six months after stroke.
Background
Stroke often results in difficulty swallowing. This can lead to choking, chest infections, poorer quality of life, longer hospital stay, and increased risk of death or discharge to a care home. Therapy to improve swallowing aims to speed up recovery of swallowing function and reduce these risks.
Study characteristics
This is an update of the review originally published in 1999 and previously updated in 2012. We have now included a total of 41 studies (2660 participants), and the evidence is current to June 2018. Swallowing therapy comprises several different treatment types, and we looked at eight of these: acupuncture (11 studies), behavioural interventions (nine studies), drug therapy (three studies), neuromuscular electrical stimulation (NMES; six studies), pharyngeal electrical stimulation (PES; four studies), physical stimulation (three studies), transcranial direct current stimulation (tDCS; two studies), and transcranial magnetic stimulation (TMS; nine studies).
Key results
Swallowing therapy did not result in less death or disability among stroke survivors, nor did it lead to a safer swallow after treatment. However, some individual swallowing therapies seemed to reduce hospital length of stay, lessen the chance of getting a chest infection or pneumonia, or improve swallowing ability and recovery from swallowing problems. Many of the swallowing therapies involved different methods of delivery, so it is still not clear which approach is most effective for each type of therapy.
Quality of the evidence
The quality of the evidence was generally very low, low, or moderate. Additional high‐quality studies are needed.
Summary of findings
Summary of findings for the main comparison. Swallowing therapy compared to placebo for dysphagia in acute and subacute stroke.
| Swallowing therapy compared to placebo for dysphagia in acute and subacute stroke | ||||||
| Patient or population: dysphagia in acute and subacute stroke Setting: in hospital Intervention: swallowing therapy Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with swallowing therapy | |||||
| Death or dependency at end of trial | Study population | OR 1.05 (0.63 to 1.75) | 306 (2 RCTs) | ⊕⊕⊕⊝ Moderate | a | |
| 693 per 1000 | 703 per 1000 (587 to 798) | |||||
| Case fatality at end of trial | Study population | OR 1.00 (0.66 to 1.52) | 766 (14 RCTs) | ⊕⊕⊕⊝ Moderate | b | |
| 197 per 1000 | 197 per 1000 (140 to 272) | |||||
| Length of inpatient stay (days) | Mean length of inpatient stay (days) ranged from 19 to 119 | MD 2.9 lower (5.65 lower to 0.15 lower) | ‐ | 577 (8 RCTs) | ⊕⊕⊕⊝ Moderate | c |
| Proportion of participants with dysphagia at end of trial | Study population | OR 0.42 (0.32 to 0.55) | 1487 (23 RCTs) | ⊕⊕⊝⊝ Low | d | |
| 570 per 1000 | 357 per 1000 (298 to 421) | |||||
| Swallowing ability | Mean swallowing ability was 0 | SMD 0.66 lower (1.01 lower to 0.32 lower) | ‐ | 1173 (26 RCTs) | ⊕⊝⊝⊝ Very low | e |
| Penetration aspiration score | Mean penetration aspiration score was 0 | SMD 0.37 lower (0.74 lower to 0 ) | ‐ | 303 (11 RCTs) | ⊕⊕⊝⊝ Low | f |
| Adverse event: chest infection or pneumonia | Study population | OR 0.34 (0.17 to 0.71) | 676 (10 RCTs) | ⊕⊝⊝⊝ Very low | g | |
| 343 per 1000 | 151 per 100 (82 to 271) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level due to lack of precision (one study split into two trials).
bDowngraded by one level for indirectness of the evidence (i.e. multiple different interventions).
cDowngraded by one level due to indirectness of the evidence (i.e. multiple different interventions). Note also that two studies had unclear blinding.
dDowngraded by two levels due to indirectness of the evidence and blinding ‐ a large number of studies did not clarify blinding status.
eDowngraded by three levels due to indirectness of the evidence (i.e. multiple different interventions), considerable heterogeneity, and fair number of studies did not clarify blinding status.
fDowngraded by two levels due to indirectness of the evidence (i.e. multiple different interventions) and moderate heterogeneity.
gDowngraded by three levels due to indirectness of the evidence (i.e. multiple different interventions), substantial heterogeneity, and fair number of studies did not clarify blinding status.
Background
Description of the condition
Dysphagia after stroke is common, affecting 27% to 64% of stroke survivors (Gordon 1987; Wolfe 1993; Odderson 1995; Smithard 1996; Mann 2000; Singh 2006a; Rofes 2013). Although dysphagia improves spontaneously in many people with stroke (by two weeks in about half), some will die and 15% of stroke survivors will still have swallowing problems at one month (Smithard 1993); many of these individuals require long‐term feeding with significant impairment of function, recovery, and quality of life (Barer 1989; Smithard 1997; Mann 1999; Perry 2004). Complications of dysphagia include aspiration leading to chest infection and pneumonia, malnutrition, inability to rehabilitate, increased risk of infection, prolonged length of stay in hospital, and increased risk of death (Smithard 1993; Odderson 1995; Finestone 1996; Smithard 1996; Sharma 2001; Martino 2005; Arnold 2016). Early identification and management of dysphagia have been shown to reduce pneumonia rates (Odderson 1995; Ramsey 2003; Hinchey 2005; Lakshminarayan 2010). Cohen 2016 recently reviewed this topic.
Description of the intervention
Speech and language therapists (SLTs) often administer interventions for treating dysphagia. These interventions involve behavioural approaches that may be compensatory or rehabilitative in nature. Compensatory approaches include modification of fluid and food consistencies, postural techniques such as adopting a chin tuck position, and swallow strategies such as a supraglottic swallow. Rehabilitative methods include swallowing exercises that focus on muscle strength; resistance or skill training, or both, such as tongue exercises, effortful swallow, and Mendelsohn’s manoeuvre (Mendelsohn 1987); and the Shaker exercise (Shaker 2002). Rehabilitative methods also include peripheral sensory stimulation, such as physical stimulation with tactile, thermal, or sour stimulation (Lazarra 1986; Logemann 1991; Logemann 1993; Rosenbek 1996; U1111‐1188‐0335); carbonation (Krival 2008); electrical stimulation (Power 2006); and air pulses (Theurer 2013). Researchers have also studied chemical and pharmacological agents, including capsaicin, black pepper oil, cabergoline, angiotensin‐converting enzyme (ACE) inhibitors, and nifedipine (Arai 2003; Ebihira 2004; Ebihira 2005).
Practitioners in China routinely use acupuncture techniques to treat dysphagia (Wong 2012).
Several other stimulation methods to promote recovery from dysphagia post stroke have emerged in recent years, in particular peripheral and central stimulation methods. Peripheral methods include pharyngeal electrical stimulation (PES), as reported in Scutt 2015, and neuromuscular surface electrical stimulation (NMES), as described in Chen 2016. Central stimulation methods, also known as non‐invasive brain stimulation, include transcranial magnetic stimulation (TMS) (Momosaki 2016; Pisegna 2016), as well as transcranial direct current stimulation (tDCS) (Momosaki 2016; Pisegna 2016).
How the intervention might work
The swallowing network is asymmetrically represented in both cerebral hemispheres, with one hemisphere showing dominance for swallowing (Hamdy 1998). Following unilateral stroke, TMS studies have demonstrated that recovery from dysphagia is associated with improved function of the non‐lesioned hemisphere (Hamdy 1998). The aim of most of the interventions described in this review is to accelerate this process of plasticity in acute and sub‐acute stroke patients with dysphagia. The exact process by which this is achieved is not fully understood, although it is thought that some interventions specifically aim to improve swallowing by enhancing sensory drive to the brain, causing increased activity in motor swallowing areas.
Why it is important to do this review
Dysphagia post stroke affects quality of life, carries increased risks of mortality and dependency (Smithard 1996; Arnold 2016), prolongs hospital stay (Smithard 1996; Smithard 1997; Arnold 2016), increases healthcare costs, and often leads to discharge from hospital to a care home (Smithard 1996; Arnold 2016). Despite all of this, the previous two versions of this review concluded in 1999 and 2012 that overall, current evidence for interventions was insufficient, and that no definitive treatments for dysphagia were available (Bath 1999; Geeganage 2012).
An updated version of this review is therefore needed to appraise current evidence regarding the effectiveness of interventions for dysphagia post stroke. This information will provide support for clinical practice; will inform stroke survivors, clinicians, and healthcare funders regarding which interventions are most effective; and may help guide policy and funding decisions. This review assesses the effectiveness of swallowing therapy for treatment of dysphagia in stroke survivors with acute or subacute stroke.
Objectives
To assess the effects of swallowing therapy on death or dependency among stroke survivors with dysphagia within six months of stroke onset.
Methods
Criteria for considering studies for this review
Types of studies
We identified randomised controlled trials (RCTs) of swallowing therapy for stroke survivors with acute or subacute stroke and dysphagia.
We excluded trials if they compared two or more active treatments (i.e. treatment was confounded), recruited participants after six months following stroke onset, involved a large proportion of participants with non‐stroke causes of dysphagia, or used a cross‐over design by which we could not just use data from the first treatment phase.
For this third version of the review, we removed most trials examining postural studies and all trials examining modified fluids because they lacked a true control group. We also excluded trials of free water protocols, oral hygiene, cough reflex testing, and swallow screening, as we do not consider these to be interventions for dysphagia per se. We also excluded trials involving the use of antibiotics.
Types of participants
Definitions
Acute or subacute stroke
Participants recruited with a clinical diagnosis of stroke within six months of onset.
Stroke type
Ischaemic or haemorrhagic.
Dysphagia
Diagnosed clinically (water swallow tests, modified diet or fluid assessments, swallowing test scores) by a clinician (typically a nurse or SLT), or by a videofluoroscopy swallow study (VFSS) or fibreoptic endoscopic evaluation of swallowing (FEES).
Types of interventions
Acupuncture versus no acupuncture or routine acupuncture or sham acupuncture
Behavioural interventions such as swallowing exercises, or positioning versus limited, usual, or no treatment
Drug intervention versus none or placebo
Neuromuscular electrical stimulation (NMES) versus none or sham stimulation
Pharyngeal electrical stimulation (PES) versus none or sham stimulation
Physical stimulation such as thermal or tactile versus limited, usual, or no treatment
Transcranial direct current stimulation (tDCS) versus none or sham stimulation
Transcranial magnetic stimulation (TMS) versus none or sham stimulation
We combined different interventions, collectively referred to as 'swallowing therapy', for the purpose of analysing their effects on the main outcomes. Given that the science of intervention development for dysphagia is at an early stage, it is reasonable to ask the question whether any intervention is better than no intervention, and to try to establish where the most positive effects are seen and for what topics more research is needed.
Types of outcome measures
We obtained information on the following outcome measures, as available, for each trial.
Primary outcomes
Functional outcome assessed as death or dependency (modified Rankin Scale: mRS > 2), or death or disability (Barthel Index: BI < 60), at the end of the trial
We chose functional outcome (i.e. death or dependency/disability) as the primary outcome because dysphagia is associated with increased risk of death or dependency in acute and subacute stroke. Whilst swallowing therapy aims to reduce dysphagia, we needed to assess whether evidences shows that people receiving swallowing therapy are less likely to die or remain dependent. We listed other important outcomes relevant to swallowing function as secondary outcomes.
Secondary outcomes
Case fatality at the end of the trial
Length of inpatient stay
Proportion of patients with dysphagia at the end of the trial
Swallowing ability based on assessments of dysphagia impairment using the dysphagia severity rating scale (DSRS), the functional oral intake scale (FOIS), the dysphagia outcome and severity scale (DOSS), or water swallowing tests
Penetration Aspiration score determined by VFSS and FEES and quantified on a scale such as the Penetration Aspiration Scale (PAS)
Chest infection or pneumonia, determined clinically or radiologically
Swallow timings from VFSS measurements (e.g. pharyngeal transit time (PTT))
Nutritional measure based on blood albumin
Institutionalisation with discharge to a residential, care, or nursing home, or to an extended care facility
Neurological impairment within four weeks (e.g. using National Institutes of Health Stroke Scale (NIHSS) or Scandinavian Stroke Scale)
Quality of life (e.g. using Short Form‐36 (SF‐36) or EuroQoL (measure of health‐related quality of life))
Search methods for identification of studies
See the Cochrane Stroke Group search methods. We searched for trials in all languages and arranged translation of relevant articles published in languages other than English. We have listed publications requiring translation in the Characteristics of studies awaiting classification section.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched on 26 June 2018). In addition, we searched:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (Appendix 1) in the Cochrane Library (searched 26 June 2018);
MEDLINE Ovid (1946 to 26 June 2018) (Appendix 2);
Embase (1974 to 26 June 2018) (Appendix 3);
Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO) (1982 to 26 June 2018) (Appendix 4);
Science Citation Index Expanded, Social Sciences Citation Index, Conference Proceedings Citation Index‐ Science (Web of Science Core Collection; 1900 to 26 June 2018) (Appendix 5); and
SpeechBITE (searched 28 June 2018) (Appendix 6).
In an effort to identify further published, unpublished, and ongoing trials, we searched:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 26 June 2018; Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 26 June 2018; Appendix 8); and
Google Scholar (searched 7 June 2018; Appendix 9).
Searching other resources
Additionally, we searched the reference lists of relevant trials and review articles and our own reference lists.
For a previous version of this review (Geeganage 2012), we contacted researchers and the UK Royal College of Speech and Language Therapists Special Interest Group for information on adult‐acquired dysphagia trials.
Data collection and analysis
Selection of studies
For this update, two review authors (HSL, LE) scanned the titles and abstracts of records identified through searches of electronic bibliographic databases and excluded obviously irrelevant articles. We independently reviewed the full text of remaining studies and selected relevant trials according to the listed inclusion criteria; we resolved disagreements through discussion with the third review author (PB).
Data extraction and management
For this update, two review authors (HSL, LE) extracted data using a predefined proforma, and entered the data into RevMan 5 (RevMan 2014); we resolved disagreements through discussion and consultation with the third review author (PB). We assessed information on randomisation, blinding, numbers of participants randomised, timing of treatment from stroke, types of dysphagia therapy, participant withdrawals and losses to follow‐up, and relevant outcomes (Types of outcome measures). We aggregated outcome data from dose escalation or dose comparison trials into one active treatment group.
Assessment of risk of bias in included studies
We assessed potential for bias using the 'Risk of bias' tool as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This assessment includes sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other issues.
Measures of treatment effect
We assessed weighted estimate of the typical treatment effect across trials using odds ratios (ORs) and 95% confidence intervals (CIs) for binary data, mean differences (MDs) and 95% CIs for continuous data, and standardised mean differences (SMDs) and 95% CIs for continuous data based on different scales. We performed analyses using RevMan 5 (RevMan 2014). We calculated OR using the Mantel‐Haenszel method, and MDs using the inverse variance method.
Unit of analysis issues
When outcome measures included different scores, we converted these to grades in the same direction of mild to severe and analysed them using MDs. When studies compared graduations of therapy (high‐medium‐low intensity), we divided the middle‐intensity group in two and analysed study data by comparing high intensity versus medium intensity, and medium intensity versus low intensity or no treatment. Similarly, if a trial compared high‐ versus low‐frequency stimulation or unilateral versus bilateral stimulation, we divided control group participants equally between treatment groups to prevent control participants from being counted more than once, and thereby artificially narrowing the CIs. We entered each set of data as a separate trial.
Dealing with missing data
If a trial publication did not provide relevant data or if data were missing but we felt it appropriate otherwise, we placed studies into Characteristics of studies awaiting classification.
Assessment of heterogeneity
We used the random‐effects model to assess heterogeneity by looking at forest plots to see how CIs overlapped (non‐overlapping studies are exhibiting statistical heterogeneity) along with the I² statistic (Higgins 2011). We defined thresholds for interpreting heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions, whereby 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We assessed selective outcome reporting as reported in the 'Risk of bias' table (Characteristics of included studies).
Data synthesis
We performed meta‐analysis using functionality within RevMan 5 (RevMan 2014): we used random‐effects models (Mantel‐Haenszel method) and presented data as number (%) or mean (standard deviation), with OR, MD, or SMD. We used random‐effects models because we expected that trials would be heterogeneous in design and delivery, including different types of participants and interventions.
Grade and 'Summary of findings' table
We assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for the following main outcomes of analysis.
Death or dependency/disability at the end of the trial.
Case fatality at the end of the trial.
Length of inpatient stay.
Proportion of participants with dysphagia at the end of the trial.
Swallowing ability.
Penetration aspiration score.
Adverse event: chest infection or pneumonia.
We have presented in Table 1 key findings of the review, including a summary of the quantity of data, the magnitude of effect size, and the overall quality of evidence.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on the eight different types of swallowing therapy to provide more specific information pertaining to the different interventions. We assessed for significant subgroup interactions by testing for subgroup differences for each main outcome.
Sensitivity analysis
We did not perform sensitivity analyses due to the small number of studies.
Results
Description of studies
We identified 27 new RCTs involving a total of 1777 acute or subacute stroke survivors with dysphagia.
Results of the search
We have presented the PRISMA study flow diagram in Figure 1. In total, we identified 2902 references, removed 860 duplicates, and screened 2042 records. We excluded 1874 records, leaving a total of 168 records. After full‐text review, we excluded 41 studies. We added these newly excluded studies to the existing list of 39 excluded studies, for a total of 80 (Excluded studies). We added 22 studies into the ongoing studies section (Ongoing studies). We also added 78 new studies to the eight existing studies awaiting classification, yielding a total of 86 (Studies awaiting classification); these studies have been completed and are awaiting publication or are awaiting translation, or we are seeking full‐text articles. External assessment of this review led to a request to further update the searches; an updated search revealed further potentially relevant studies, and we have added these to the Studies awaiting classification section; we will assess these when we prepare the next update of this review. Finally, we added 27 new studies to the existing 14 studies, yielding a total of 41 included studies (47 data sets) (Included studies). This resulted in the addition of 1777 participants to the existing 883, for a total of 2660 participants.
1.

Study Flow Diagram, *86 studies awaiting classification.
Included studies
We included 41 trials in this updated review (mean participant age 67.8 years). These trials looked at various forms of swallowing therapy after stroke.
When outcome measures included different scores, we converted these to grades in the same direction of mild to severe and analysed them using mean differences (MDs). Two studies compared graduations of therapy (high‐medium‐low intensity) (Yuan 2003i; Yuan 2003ii; Carnaby 2006i; Carnaby 2006ii;); here, we divided the middle‐intensity group in two and analysed the study data by comparing high intensity versus medium intensity, and medium intensity versus low intensity or no treatment. Similarly, one trial of TMS compared high‐ versus low‐frequency stimulation or unilateral versus bilateral stimulation (Kim 2012i; Kim 2012ii; Du 2016i; Du 2016ii; Park 2016 (a) i; Park 2016 (a) ii); here, we divided control group participants equally between treatment groups to prevent control participants from being counted more than once and thereby artificially narrowing the confidence intervals (CIs). We entered each set of data as a separate trial; hence, although the total number of included studies was 41, the total number of data sets entered for analysis was 47.
Acupuncture
Eleven studies tested acupuncture in 998 participants (Liu 2000; Han 2004; Liu 2004; Wei 2005; Jia 2006a; Bai 2007i; Bai 2007ii; Huang 2010; Chan 2012; Chen 2016a; Xia 2016a).
Behavioural interventions
Nine studies investigated behavioural interventions in 632 participants (Yuan 2003i; Yuan 2003ii; Song 2004; Carnaby 2006i; Carnaby 2006ii; Kang 2012; Zheng 2014; Heo 2015; Park 2016b). Behavioural interventions consisted of swallowing exercises, environmental modifications such as upright positioning for feeding, safe swallowing advice, dietary modifications, kinesio‐taping, and expiratory muscle strength training.
Drug therapy
Three studies assessed several different drugs in 148 participants (Perez 1997; Lee 2015; Warusevitane 2015). Drug interventions included nifedipine in 17 participants (Perez 1997), lisinopril in 71 participants (Lee 2015), and metoclopramide in 60 participants (Warusevitane 2015).
Neuromuscular electrical stimulation (NMES)
Six studies tested NMES in 312 participants (Lim 2009; Xia 2011; Park 2012; Lee 2014; Li 2014; Terre 2015). Researchers most often compared NMES versus traditional dysphagia therapy. One study combined NMES and effortful swallow (Park 2012).
Pharyngeal electrical stimulation (PES)
Four studies involving 214 participants assessed PES (Jayasekeran 2010a; Jayasekeran 2010b; STEPS 2016; Vasant 2016).
Physical stimulation (thermal, tactile)
Three studies enrolled 155 participants. Types of stimulation included tactile stimulation (Bath 1997), electrical stimulation (Power 2006), and Tongyan spray (Feng 2012).
Transcranial direct current stimulation (tDCS)
Two studies assessed tDCS in 34 participants (Kumar 2011; Shigematsu 2013).
Transcranial magnetic stimulation (TMS)
Nine studies involving 167 participants investigated TMS (Khedr 2009; Khedr 2010; Kim 2012i; Kim 2012ii; Park 2013; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii).
Excluded studies
We excluded 80 studies from this updated review, most commonly because investigators compared two active treatments (confounded) or because the trials were not RCTs. We excluded 10 studies as reported outcomes were not relevant to this review. We excluded 11 studies because of lack of outcome data; some of these might be relevant to this review should outcome data become available (Characteristics of excluded studies).
Risk of bias in included studies
Key sources of bias follow; we have summarised risk of bias in Figure 2.
2.
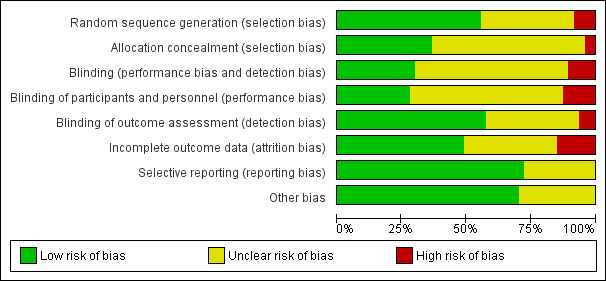
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Random sequence generation
Randomisation by computer occurred in 15 studies (low risk of bias) (Bath 1997; Perez 1997; Carnaby 2006i; Carnaby 2006ii; Jayasekeran 2010a; Jayasekeran 2010b; Park 2012; Park 2013; Lee 2014; Li 2014; Lee 2015; Terre 2015; Chen 2016a; STEPS 2016; Vasant 2016).
Randomisation via random number tables occurred in 10 studies (low risk of bias) (Song 2004; Bai 2007i; Bai 2007ii; Chan 2012; Feng 2012; Shigematsu 2013; Warusevitane 2015; Du 2016i; Du 2016ii; Xia 2016a).
Simple randomisation occurred in four studies (low risk of bias) (Han 2004; Kumar 2011; Heo 2015; Park 2016b).
Method of randomisation was unclear in 16 studies (unclear risk of bias) (Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Wei 2005; Power 2006; Khedr 2009; Huang 2010; Khedr 2010; Xia 2011; Kang 2012; Kim 2012i; Kim 2012ii; Zheng 2014; Park 2016a (i); Park 2016a (ii)).
Two studies used non‐randomised methods (high risk of bias) (Jia 2006a; Lim 2009).
Allocation concealment
Researchers ensured allocation concealment in 17 studies (low risk of bias) (Han 2004; Carnaby 2006i; Carnaby 2006ii; Khedr 2009; Chan 2012; Feng 2012; Park 2012; Park 2013; Shigematsu 2013; Li 2014; Lee 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016b; Vasant 2016).
Allocation concealment was unclear in 28 studies (unclear risk of bias) (Bath 1997; Perez 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Song 2004; Wei 2005; Power 2006; Bai 2007i; Bai 2007ii; Huang 2010; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Kumar 2011; Xia 2011; Kang 2012; Kim 2012i; Kim 2012ii; Lee 2014; Zheng 2014; Heo 2015; Terre 2015; Park 2016a (i); Park 2016a (ii); STEPS 2016; Xia 2016a).
Two studies did not ensure allocation concealment (high risk of bias) (Jia 2006a; Lim 2009).
Baseline prognostic factors matching between intervention and control groups
Baseline factors were similar in 34 studies (low risk of bias) (Perez 1997; Song 2004; Carnaby 2006i; Carnaby 2006ii; Bai 2007i; Bai 2007ii; Khedr 2009; Jayasekeran 2010b; Khedr 2010; Xia 2011; Chan 2012; Feng 2012; Kang 2012; Kim 2012i; Kim 2012ii; Park 2012; Park 2013; Shigematsu 2013; Lee 2014; Li 2014; Zheng 2014; Heo 2015; Lee 2015; Terre 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Park 2016b; STEPS 2016; Vasant 2016; Xia 2016a).
Baseline factor matching was unclear in 13 studies (unclear risk of bias) (Bath 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Han 2004; Liu 2004; Wei 2005; Jia 2006a; Power 2006; Lim 2009; Huang 2010; Jayasekeran 2010a; Kumar 2011).
Blinding
Performance bias
Both participants and investigators were blinded in three studies (low risk of bias) (Perez 1997; Kumar 2011; Warusevitane 2015).
Participants were blinded in nine studies (low risk of bias) (Khedr 2009; Chan 2012; Park 2012; Park 2013; Terre 2015; Du 2016i; Du 2016ii; STEPS 2016; Vasant 2016).
Both participants and investigators were unblinded in five studies (high risk of bias) (Carnaby 2006i; Carnaby 2006ii; Chen 2016a; Park 2016a (i); Park 2016a (ii)).
Blinding of participants and investigators was uncertain in 14 studies (unclear risk of bias) (Bath 1997; Han 2004; Bai 2007i; Bai 2007ii; Lim 2009; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Xia 2011; Shigematsu 2013; Li 2014; Lee 2015; Park 2016b; Xia 2016a).
Detection bias
Outcomes were blinded in 28 studies (low risk of bias) (Perez 1997; Han 2004; Wei 2005; Carnaby 2006i; Carnaby 2006ii; Khedr 2009; Lim 2009; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Xia 2011; Chan 2012; Park 2012; Park 2013; Shigematsu 2013; Li 2014; Lee 2015; Terre 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Park 2016b; STEPS 2016; Vasant 2016; Xia 2016a).
Outcomes were not blinded in three studies (high risk of bias) (Bath 1997; Bai 2007i; Bai 2007ii).
Overall, 16 studies did not report on any blinding procedures (i.e. for participants, investigators, or outcome assessors) (unclear risk of bias) (Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Song 2004; Wei 2005; Jia 2006a; Power 2006; Huang 2010; Feng 2012; Kang 2012; Kim 2012i; Kim 2012ii; Lee 2014; Zheng 2014; Heo 2015).
Incomplete outcome data
Ten studies reported no loss of participants during follow‐up (low risk of bias) (Han 2004; Jayasekeran 2010a; Chan 2012; Kang 2012; Kim 2012i; Kim 2012ii; Park 2013; Shigematsu 2013; Lee 2014; Warusevitane 2015).
Twelve studies reported loss of participants during follow‐up, but we judged them to be at low risk of bias (Perez 1997; Carnaby 2006i; Carnaby 2006ii; Khedr 2009; Khedr 2010; Feng 2012; Park 2012; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Vasant 2016).
We judged seven studies to be at high risk of bias due to incomplete outcome data (Lim 2009; Jayasekeran 2010b; Li 2014; Lee 2015; Chen 2016a; Park 2016b; STEPS 2016).
Loss of participants during follow‐up was unclear in 18 studies (unclear risk of bias) (Bath 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Liu 2004; Song 2004; Wei 2005; Jia 2006a; Power 2006; Bai 2007i; Bai 2007ii; Huang 2010; Kumar 2011; Xia 2011; Zheng 2014; Heo 2015; Terre 2015; Xia 2016a).
Data were not available for quality of life.
Selective reporting
We judged 34 studies to be at low risk of reporting bias (Perez 1997; Carnaby 2006i; Carnaby 2006ii; Power 2006; Khedr 2009; Jayasekeran 2010a; Jayasekeran 2010b; Khedr 2010; Kumar 2011; Xia 2011; Chan 2012; Feng 2012; Kang 2012; Kim 2012i; Kim 2012ii; Park 2012; Park 2013; Shigematsu 2013; Lee 2014; Li 2014; Zheng 2014; Heo 2015; Lee 2015; Terre 2015; Warusevitane 2015; Chen 2016a; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii); Park 2016b; STEPS 2016; Vasant 2016; Xia 2016a).
In the remaining 13 studies, it was unclear if reported data were complete (unclear risk of bias) (Bath 1997; Liu 2000; Yuan 2003i; Yuan 2003ii; Han 2004; Liu 2004; Song 2004; Wei 2005; Jia 2006a; Bai 2007i; Bai 2007ii; Lim 2009; Huang 2010).
Other potential sources of bias
We assessed seven studies based on translations of the original text (Yuan 2003i; Yuan 2003ii; Song 2004; Wei 2005; Bai 2007i; Bai 2007ii; Huang 2010). Native Chinese speakers performed translations from Chinese to English.
We aggregated outcome data from dose escalation or comparison trials to form one active treatment group in one trial (Jayasekeran 2010b).
Effects of interventions
See: Table 1
Summary of findings for main outcomes of swallowing therapy in general
We entered the important outcomes in this review into Table 1, and we reported outcomes for 'swallowing therapy' versus 'no swallowing therapy'. This means that overall, for each outcome (e.g. length of inpatient stay), we combined several different interventions to test for efficacy. In this way, we have provided information on the effectiveness of swallowing therapy as a whole for each outcome. We assessed three additional outcomes (pharyngeal transit time, institutionalisation, and nutrition) but did not include them in Table 1 (a maximum of seven outcomes are allowed); therefore, we did not assess the quality of studies for these outcomes using the GRADE approach, and we have not reported their outcomes in the main findings.
We also undertook subgroup analysis for each different type of intervention.
The number of outcomes reported varied considerably across studies.
Primary outcome of death or dependency/disability at end of trial in one trial (split into two data sets).
Case fatality at end of trial in 14 trials.
Length of inpatient stay in eight trials.
Proportion of patients with dysphagia at end of trial in 23 trials.
Swallowing ability in 26 trials.
Penetration aspiration score (PAS) in 11 trials.
Chest infections or pneumonia in nine trials.
Swallow timing in six trials.
Nutrition in three trials.
Institutionalisation in three trials.
Primary outcome
Functional outcome: death or dependency or death or disability at end of trial
Swallowing therapy had no effect on death or dependency, or death or disability, at end of trial (odds ratio (OR) 1.05, 95% confidence interval (CI) 0.63 to 1.75; 306 participants; 2 studies; I² = 0%; P = 0.86: moderate‐quality evidence; Analysis 1.1). One trial (two data sets) of behavioural interventions reported on this outcome.
1.1. Analysis.
Comparison 1 Swallowing therapy, Outcome 1 Functional outcome ‐ death or dependency, death or disability at end of trial.
Secondary outcomes
Case fatality at end of trial
Swallowing therapy had no effect on case fatality at end of trial (OR 1.00, 95% CI 0.66 to 1.52; 766 participants; 14 studies; I² = 6%; P = 0.99: moderate‐quality evidence; Analysis 1.2). Trials of behavioural interventions, drug therapy, pharyngeal electrical stimulation, physical stimulation, and transcranial magnetic stimulation reported on this outcome.
1.2. Analysis.
Comparison 1 Swallowing therapy, Outcome 2 Case fatality at end of trial.
Length of inpatient stay
Swallowing therapy probably reduced length of inpatient stay (mean difference (MD) ‐2.90, 95% CI ‐5.65 to ‐0.15; 577 participants; 8 studies; I² = 11%; P = 0.04: moderate‐quality evidence; Analysis 1.3). Trials of behavioural interventions and PES reported on this outcome. Subgroup analysis showed that the interventions did not differ (Analysis 1.3).
1.3. Analysis.
Comparison 1 Swallowing therapy, Outcome 3 Length of inpatient stay (days).
Proportion of participants with dysphagia at end of trial
Swallowing therapy probably reduced the proportion of participants with dysphagia at end of trial (OR 0.42, 95% CI 0.32 to 0.55; 1487 participants; 23 studies; I² = 0%; P = 0.00001: low‐quality evidence; Analysis 1.4). Trials of acupuncture, behavioural interventions, drug therapy, NMES, PES, physical stimulation, and tDCS reported on this outcome. Subgroup analysis showed that acupuncture (OR 0.31, 95% CI 0.20 to 0.49; 676 participants; 8 studies; I² = 0%; P < 0.00001) and behavioural interventions (OR 0.45, 95% CI 0.28 to 0.74; 511 participants; 6 studies; I² = 28%; P = 0.001) each reduced dysphagia but did not differ from each other (P = 0.91; Analysis 1.4).
1.4. Analysis.
Comparison 1 Swallowing therapy, Outcome 4 Proportion of participants with dysphagia at end of trial.
Swallowing ability
Swallowing therapy probably improved swallowing ability (standardised mean difference (SMD) ‐0.66, 95% CI ‐1.01 to ‐0.32; 1173 participants; 26 studies; I² = 86%; P = 0.0002: very low‐quality evidence; Analysis 1.5). Trials of acupuncture, behavioural interventions, drug therapy, NMES, PES, physical stimulation, tCDS, and TMS reported on this outcome. Subgroup analysis showed that behavioural interventions (SMD ‐0.56, 95% CI ‐1.07 to ‐0.05; 121 participants; 3 studies; I² = 47%; P = 0.03) and TMS (SMD ‐1.29, 95% CI ‐2.37 to ‐0.21; 141 participants; 8 studies; I² = 85%; P = 0.02) each improved swallowing ability but did not differ from each other (P = 0.09; Analysis 1.5). Review authors noted moderate to substantial heterogeneity between trials (Analysis 1.5).
1.5. Analysis.
Comparison 1 Swallowing therapy, Outcome 5 Swallowing ability.
Penetration aspiration score
Swallowing therapy did not significantly reduce aspiration assessed as penetration aspiration score (SMD ‐0.37, 95% CI ‐0.74 to ‐0.00; 303 participants; 11 studies; I² = 46%; P = 0.05: low‐quality evidence; Analysis 1.6). Trials of behavioural interventions, NMES, PES, and TMS reported on this outcome. However, given that results show no overall benefit, we have not commented on subgroup analysis (Analysis 1.6).
1.6. Analysis.
Comparison 1 Swallowing therapy, Outcome 6 Penetration aspiration score.
Chest infection or pneumonia
Swallowing therapy probably reduced the incidence of chest infection or pneumonia (OR 0.36, 95% CI 0.16 to 0.78; 618 participants; 9 studies; I² = 59%; P = 0.009: very low‐quality evidence; Analysis 1.7). Trials of behavioural interventions, drug therapy, NMES, and PES reported on this outcome. Subgroup analysis showed that drug therapy (OR 0.06, 95% CI 0.01 to 0.21; 60 participants; 1 study; I² not applicable; P < 0.0001) significantly reduced the incidence of chest infection or pneumonia at end of trial ‐ a result that differed significantly from other interventions (P = 0.008; Analysis 1.7).
1.7. Analysis.
Comparison 1 Swallowing therapy, Outcome 7 Chest infection or pneumonia.
Pharyngeal transit time (PTT)
Swallowing therapy may have reduced PTT (MD ‐0.23, 95% CI ‐0.32 to ‐0.15; 187 participants; 6 studies; I² = 29%; P < 0.00001; Analysis 1.8). Trials of drug therapy, NMES, PES, and physical stimulation reported on this outcome. Subgroup analysis showed that NMES (MD ‐0.23, 95% CI ‐0.39 to ‐0.08; 126 participants; 3 studies; I² = 63%; P = 0.003; Analysis 1.8) and physical stimulation in one small study (MD ‐0.19; 95% CI ‐0.34 to ‐0.04; 16 participants; 1 study; I² not applicable; P = 0.01) each reduced PTT but did not differ from each other, i.e. these findings are likely due to chance and not‐significant. (P = 0.98; Analysis 1.8).
1.8. Analysis.
Comparison 1 Swallowing therapy, Outcome 8 Pharyngeal transit time (seconds).
Institutionalisation
Swallowing therapy did not reduce the incidence of institutionalisation (OR 0.75, 95% CI 0.47 to 1.19; 447 participants; 3 studies; I² = 0%; P= 0.22; Analysis 1.9). Trials of behavioural interventions and pharyngeal electrical stimulation reported on this outcome.
1.9. Analysis.
Comparison 1 Swallowing therapy, Outcome 9 Institutionalisation.
Nutrition (albumin)
Swallowing therapy did not reduce nutrition (MD 0.37, 95% CI ‐1.5 to 2.24; 169 participants; 3 studies; I² = 0%; P = 0.70; Analysis 1.10). Trials of behavioural interventions and pharyngeal electrical stimulation reported on this outcome.
1.10. Analysis.
Comparison 1 Swallowing therapy, Outcome 10 Nutritional (albumin).
Detailed subgroup analysis: summary of findings per type of intervention
Not all interventions addressed all outcomes. We have reported available data.
Acupuncture
Acupuncture resulted in significant results (i.e. < 1.0) for reducing the proportion of participants with dysphagia at end of trial. However, these findings may be due to chance, given that testing for subgroup differences did not yield significant results. Acupuncture did not reduce swallowing ability. Data on the effects of acupuncture on other outcomes were not available.
Proportion of participants with dysphagia at end of trial (OR 0.31, 95% CI 0.20 to 0.49; 676 participants; 8 studies; I² = 0%; P < 0.00001; Analysis 1.4).
Swallowing ability (SMD ‐0.55, 95% CI ‐1.20 to 0.11; 496 participants; 6 studies; I² = 91%; P = 0.10). We noted significant heterogeneity (Analysis 1.5).
Behavioural interventions
Behavioural interventions produced significant results (i.e. < 1.0) for improving swallowing ability and reducing the proportion of participants with dysphagia at the end of the trial. However, both of these findings may be due to chance, given that testing for subgroup differences for each outcome did not yield significant results. Although behavioural interventions also reduced penetration aspiration score (i.e. < 1.0), results show no overall benefit for this outcome and this finding is likely due to chance. Behavioural interventions did not reduce length of inpatient stay, chest infection or pneumonia, case fatality at end of trial, functional outcome, institutionalisation, or nutrition. Behavioural interventions addressed more outcomes when compared with most interventions.
Swallowing ability (SMD ‐0.56, 95% CI ‐1.07 to ‐0.05; 121 participants; 3 studies; I² = 47%; P = 0.03; Analysis 1.5).
Proportion of participants with dysphagia at end of trial (OR 0.45, 95% CI 0.28 to 0.74; 511 participants; 6 studies; I² = 28%; P = 0.001; Analysis 1.4).
Penetration aspiration score (SMD ‐0.88, 95% CI ‐1.68 to ‐0.08; 27 participants; 1 study; I² not applicable; P = 0.03; Analysis 1.6).
Length of inpatient stay (MD ‐2.70, 95% CI ‐5.68 to 0.28; 370 participants; 4 studies; I² = 19%; P = 0.08; Analysis 1.3).
Chest infection or pneumonia (OR 0.56, 95% CI 0.31 to 1.00; 473 participants; 6 studies; I² = 21%; P = 0.05; Analysis 1.7).
Case fatality at end of trial (OR 0.83, 95% CI 0.46 to 1.51; 306 participants; 2 studies; I² = 0%; P = 0.54; Analysis 1.2).
Functional outcome (OR 1.05, 95% CI 0.63 to 1.75; 306 participants; 2 studies; I² = 0%; P = 0.86; Analysis 1.1).
Institutionalisation (OR 0.76, 95% CI 0.39 to 1.48; 306 participants; 2 studies; I² = 12%; P = 0.42; Analysis 1.9).
Nutrition (albumin) (MD 0.20, 95% CI ‐4.77 to 5.17; 64 participants; 2 studies; I² = 0%; P = 0.94; Analysis 1.10).
Drug therapy
Drug therapy was probably effective for reducing chest infection or pneumonia in one study ‐ a result that differed from those of other interventions. Drug therapy did not improve swallowing ability, nor did it reduce case fatality, proportion of participants with dysphagia at end of trial, or pharyngeal transit time. Data on effects of drug therapy on other outcomes were not available.
Chest infection or pneumonia (OR 0.06, 95% CI 0.01 to 0.21; 60 participants; 1 study; I² not applicable; P < 0.0001; Analysis 1.7).
Swallowing ability (SMD ‐0.46, 95% CI ‐0.93 to 0.01; 71 participants; 1 study; I² not applicable; P = 0.06; Analysis 1.5).
Case fatality (OR 1.40, 95% CI 0.31 to 6.28; 148 participants; 3 studies; I² = 70%; P = 0.66; Analysis 1.2).
Proportion of participants with dysphagia at end of trial (OR 0.48, 95% CI 0.07 to 3.35; 17 participants; 1 study; I² not applicable; P = 0.46; Analysis 1.4).
Pharyngeal transit time (MD ‐0.21, 95% CI ‐0.91 to 0.49; 17 participants; 1 study; I² not applicable; P = 0.56; Analysis 1.8).
Neuromuscular electrical stimulation (NMES)
NMES was probably effective for reducing pharyngeal transit time (i.e. < 1.0). NMES did not reduce the proportion of participants with dysphagia at end of trial or penetration aspiration score, and did not improve swallowing ability.
Pharyngeal transit time (MD ‐0.23, 95% CI ‐0.39 to ‐0.08; 126 participants; 3 studies; I² = 63%; P = 0.003; Analysis 1.8).
Proportion of participants with dysphagia at end of trial (OR 0.51, 95% CI 0.18 to 1.49; 76 participants; 2 studies; I² = 7%; P = 0.22; Analysis 1.4).
Penetration aspiration score (SMD 0.57, 95% CI ‐0.38 to 1.52; 18 participants; 1 study; I² not applicable; P = 0.24; Analysis 1.6).
Swallowing ability (SMD ‐1.34, 95% CI ‐3.39 to 0.71; 100 participants; 2 studies; I² = 93%; P = 0.20; Analysis 1.5).
Pharyngeal electrical stimulation (PES)
PES studies addressed many outcomes but did not show an effect for case fatality, length of inpatient stay, proportion of participants with dysphagia at end of trial, swallowing ability, penetration aspiration score, chest infection or pneumonia, pharyngeal transit time, institutionalisation, or nutrition.
Case fatality (OR 0.92, 95% CI 0.38 to 2.26; 215 participants; 4 studies; I² = 0%; P = 0.86; Analysis 1.2).
Length of inpatient stay (MD ‐6.05, 95% CI ‐16.40 to 4.31; 207 participants; 4 studies; I² = 27%; P = 0.25; Analysis 1.3).
Proportion of participants with dysphagia at end of trial (OR 0.55, 95% CI 0.15 to 2.11; 66 participants; 3 studies; I² = 0%; P = 0.39; Analysis 1.4).
Swallowing ability (SMD 0.06, 95% CI ‐0.22 to 0.34; 194 participants; 3 studies; I² = 0%; P = 0.69; Analysis 1.5).
Penetration aspiration score (SMD ‐0.17, 95% CI ‐0.53 to 0.19; 177 participants; 4 studies; I² = 12%; P = 0.35; Analysis 1.6).
Chest infection (OR 0.43, 95% CI 0.06 to 3.09; 28 participants; 1 study; I² not applicable; P = 0.40; Analysis 1.7).
Pharyngeal transit time (MD ‐0.15, 95% CI ‐0.67 to 0.37; 28 participants; 1 study; I² not applicable; P = 0.56; Analysis 1.8).
Institutionalisation (OR 0.73, 95% CI 0.36 to 1.48; 141 participants; 1 study; I² not applicable; P = 0.38; Analysis 1.9).
Nutrition (MD 0.40; 95% CI‐1.62 to 2.42; 105 participants; 1 study; I² not applicable; P = 0.70; Analysis 1.10).
Physical stimulation (thermal, tactile)
Physical stimulation reduced pharyngeal transit time in one small study (i.e. < 1.0). However, these findings may be due to chance, given that testing for subgroup differences did not yield significant findings.
Physical stimulation had no effect on case fatality at end of trial nor on proportion of participants with dysphagia at end of trial and did not improve swallowing ability.
Pharyngeal transit time (MD ‐0.19, 95% CI ‐0.34 to ‐0.04; 16 participants; 1 study; I² not applicable; P = 0.01; Analysis 1.8).
Case fatality at end of trial (OR 1.05, 95% CI 0.16 to 6.92; 19 participants; 1 study; I² not applicable; P = 0.96; Analysis 1.2).
Proportion of participants with dysphagia at end of trial (OR 0.65, 95% CI 0.07 to 5.85; 127 participants; 2 studies; I² = 0%; P = 0.70; Analysis 1.4).
Swallowing ability (SMD ‐0.30, 95% CI ‐1.29 to 0.68; 16 participants; 1 study; I² not applicable; P = 0.55; Analysis 1.5).
Transcranial direct current stimulation (tDCS)
tDCS did not alter the proportion of participants with dysphagia at end of trial and did not improve swallowing ability. Data on other outcomes were not available.
Proportion of participants with dysphagia at end of trial (OR 0.29, 95% CI 0.01 to 8.39; 14 participants; 1 study; I² not applicable; P = 0.47; Analysis 1.4).
Swallowing ability (SMD ‐0.33, 95% CI ‐2.22 to 1.56; 34 participants; 2 studies; I² = 85%; P = 0.73; Analysis 1.5).
Transcranial magnetic stimulation (TMS)
TMS improved swallowing ability at end of trial (i.e. < 1.0), although this finding may be due to chance, given that testing for subgroup differences did not yield significant results. We also noted considerable heterogeneity. TMS did not alter case fatality at end of trial nor penetration aspiration score. Data on other outcomes were not available.
Swallowing ability (SMD ‐1.29, 95% CI ‐2.37 to ‐0.21; 141 participants; 8 studies = 8; I² = 85%; P = 0.02; Analysis 1.5).
Case fatality at end of trial (OR 0.28, 95% CI 0.03 to 2.93; 78 participants; 4 studies; I² = 0%; P = 0.29; Analysis 1.2).
Penetration aspiration score (SMD ‐0.53, 95% CI ‐1.22 to 0.16; 81 participants; 5 studies; I² = 51%; P = 0.13; Analysis 1.6).
In summary, acupuncture, behavioural interventions, and TMS appeared to be individually effective for reducing some outcomes. However, as results of testing for subgroup differences were not significant, none of these interventions are convincingly different from the summary result. Drug therapy was the only intervention that was significantly less than 1.0, and findings were significantly different for testing of subgroup differences, although this result was based on very low‐quality evidence.
Discussion
Summary of main results
We included 41 studies in this updated review of swallowing therapy in people with stroke. We identified 22 additional studies that are ongoing (Characteristics of ongoing studies), along with 86 studies that are awaiting classification (Characteristics of studies awaiting classification).
Researchers assessed eight types of stimulatory techniques ‐ acupuncture, behavioural therapy, drug therapy, neuromuscular electrical stimulation (NMES), pharyngeal electrical stimulation (PES), physical stimulation, transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation (TMS). Swallowing therapy had no effect on functional outcomes (death or dependency, or death or disability), although only one trial reported this outcome (two data sets). Swallowing therapy also had no effect on case fatality at end of trial, nor on penetration aspiration score. However, swallowing therapy probably reduced length of inpatient stay, the proportion of participants with dysphagia at end of trial, and the incidence of chest infection or pneumonia (with one study reporting significant effects for drug therapy). Swallowing therapy also probably improved swallowing ability. In the absence of significant effects on the primary outcome, statistically significant findings in secondary and explanatory outcomes are hypothesis‐generating and might reflect chance, for example, due to multiple‐comparison testing. Hence, further trials are needed to test these observations.
Overall completeness and applicability of evidence
Results of this review are incomplete at this time because of the significant number of ongoing studies and those awaiting classification identified by review authors. Nevertheless, the addition of new studies to this version of the review has tightened confidence intervals, although the overall conclusion that dysphagia treatment does not alter functional outcome has not changed.
Quality of the evidence
The quality of evidence ranged from very low and low through moderate to high, as presented in Table 1. The most common reasons for reduced quality of evidence were lack of blinding, moderate to considerable heterogeneity between trials, and lack of precision (i.e. inclusion of multiple different interventions).
Potential biases in the review process
Results of the present analysis are subject to several caveats. First, we combined different interventions together for analysis, to assess whether trial results show any effect of swallowing therapy as a whole as opposed to no intervention or usual care. This means that decisions on which specific types of interventions are effective cannot be made upon analysis of these data. Future reviews will focus on assessing effects of specific interventions on main outcomes. Second, we excluded 80 studies from the analysis. One common reason for exclusion is that studies compared two active treatments without including a control or placebo group. We also excluded trials due to lack of uniformity in usage of outcome measures and lack of data on clinical outcomes, such as dependency, mortality, institutionalisation, and chest infection or pneumonia. Further, included trials used various swallowing assessment techniques, cortical excitability techniques, and videofluoroscopic measurements. So, trialists are encouraged to design future trials that include a control or placebo group, and to incorporate standard outcome measures. Third, a further 86 studies are awaiting assessment, subject to the availability of full‐text articles; such omission of multiple studies will inevitably bias review results. Fourth, with regard to acupuncture, data from three studies may have been confounded due to use of 'routine' acupuncture or a different type of acupuncture as control, variation in delivery of therapy, and risk of language bias, in that some of the acupuncture literature is available in full only in Chinese language journals. Similarly, we included data from an NMES study (Park 2012), which considered sensory stimulation as a control; therefore we cannot be certain that this trial is not confounded. Last, the present analysis included only studies up to six months from stroke onset, and the effects of later treatments for post‐stroke dysphagia remain unclear.
It is important to note that many trials are ongoing and should add substantially to the existing data once complete.
Agreements and disagreements with other studies or reviews
This is the largest, most inclusive, and most up‐to‐date review on this topic. It combines all current interventions for dysphagia in the acute and subacute phases of stroke. A number of separate systematic reviews exploring individual interventions for stroke survivors have been published, including some examining acupuncture in stroke (Xie 2008; Long 2012; Wong 2012), behavioural interventions in neurogenic dysphagia (Ashford 2009), TMS in stroke and acquired brain injury (Yang 2015; Liao 2016; Momosaki 2016; Pisegna 2016), tDCS in stroke and acquired brain injury (Yang 2015; Momosaki 2016; Pisegna 2016), NMES in stroke and neurological impairment (Chen 2016; Ding 2016), and PES in stroke (Scutt 2015). However, these reviews have examined the efficacy of individual interventions, whereas the current review has examined the efficacy of swallowing therapy overall; hence direct comparisons are difficult to make.
Authors' conclusions
Implications for practice.
Information on effects of swallowing therapy on the primary outcome of death or dependency/disability continues to be insufficient. Although some swallowing therapies appear to have a beneficial effect on some outcomes, these results are based on lower‐quality evidence. At present, clinical decisions cannot be based on reliable evidence from clinical trials.
Implications for research.
On the basis of existing studies and the need to exclude many others, future trials should consider the following design issues.
Patients: include only those who have post‐stroke dysphagia, and limit recruitment to a particular temporal phase after stroke. Researchers must specify clearly the time from stroke onset to randomisation when reporting trials. Trialists should aim for larger numbers of participants, ideally from multiple centres.
Comparator: in the absence of any proven treatment, the control group should receive only standard care, with the treatment group receiving standard care plus the intervention being tested.
Outcomes: studies need to ensure that standardised outcome measures are used to allow comparison of trials. Functional outcome (death or dependency) should be included in future trials, as should the number of participants who develop chest infection or pneumonia, or who have signs of aspiration. Trials should include outcomes of relevance to health economics, such as length of inpatient stay and discharge to an institution, as well as quality of life outcomes (e.g. EuroQoL Group Quality of Life Questionnaire based on five dimensions (EuroQoL‐5D), Swallowing Quality of Life Questionnaire (SWAL‐QOL)).
Methods: researchers should endeavour to examine common parameters (i.e. use similar methods), so that results can be compared more readily across different studies.
Quality of research: trialists must report full information on randomisation, allocation concealment, blinding of treatment and outcome assessment, and attrition.
Future research: further research is needed to discover which components of swallowing therapy are beneficial. A number of studies assessing interventions for dysphagia are ongoing (22 studies), and findings of these studies will add further information on this topic (Characteristics of ongoing studies). Several studies of mixed groups of chronic dysphagia have been done or are ongoing: a systematic review of these studies may further inform the management of acute and subacute dysphagia post stroke.
What's new
| Date | Event | Description |
|---|---|---|
| 28 March 2018 | New citation required but conclusions have not changed | More significant outcomes reported as compared to the 2012 review, but largely based on moderate‐ to low‐quality evidence. Changes made to authorship |
| 28 March 2018 | New search has been performed | New studies added. 14 studies (883 participants) included in the 2012 review. 27 studies (1777 participants) added to this updated review. Total number of included studies reported is 41 (2660 participants). Focus of this review is limited to treatment of dysphagia in acute and subacute stroke (nutritional, feeding, and fluid support removed from this review and will become the focus of a separate review) |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 4, 1999
| Date | Event | Description |
|---|---|---|
| 14 March 2012 | New citation required but conclusions have not changed | Changes made to authorship. No changes made to conclusions |
| 14 March 2012 | New search has been performed | Results of 27 new studies involving 6567 participants added to the review. Total of 33 studies involving 6779 participants now included. 15 new ongoing studies also added. Modifications made to analysis method, types of stroke patients included, and outcome measures assessed (Differences between protocol and review) |
| 13 April 2008 | Amended | Review converted to new review format |
Acknowledgements
We thank the following people who were review authors in previous versions of this review.
Version 1 (1999): Jean Kerr, Morwenna Collins, Cameron Sellars, and David Smithard; they variously contributed to searches, data extraction, analysis and interpretation of data, and updating of the review.
Version 2 (2012): Jessica Beavan, Sharon Ellendar, and Chamilla Geeganage; they variously undertook searches, data extraction, and analysis and interpretation of data, and updated the review.
We thank the Cochrane Stroke Group for assistance in identifying trials and conducting searches, and their editors and external assessor for comments on the review. Several trialists and other interested healthcare staff reviewed the draft of the first version and made comments ‐ we thank each of them: CGMI Baeten (Netherlands), MS Dennis (UK), BR Garon (USA), GJ Hankey (Australia), GKT Holmes (UK), PR Mills (UK), B Norton (UK), C Ormiston (USA), J Rosenbek (USA), and G Vanhooren (Belgium). We also thank D Luo and G Lan, who translated five of the papers from Chinese into English. Finally, we are grateful to the funding bodies that supported this research. Naturally any mistakes are our own. We would be very grateful to be informed of any completed or ongoing trials that are not listed in the review, and to know of outcome data from existing trials that have not been included.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor: [Cerebrovascular Disorders] this term only
MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only
MeSH descriptor: [Brain Ischemia] explode all trees
MeSH descriptor: [Carotid Artery Diseases] explode all trees
MeSH descriptor: [Cerebral Small Vessel Diseases] explode all trees
MeSH descriptor: [Intracranial Arterial Diseases] explode all trees
MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
MeSH descriptor: [Intracranial Hemorrhages] explode all trees
MeSH descriptor: [Stroke] explode all trees
MeSH descriptor: [Stroke, Lacunar] this term only
(stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva*):ti,ab,kw (Word variations have been searched)
((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw (Word variations have been searched)
((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher*) near/5 (h?emorrhag* or h?ematoma* or bleed*)):ti,ab,kw (Word variations have been searched)
{or #1‐#13}
MeSH descriptor: [Deglutition] this term only
MeSH descriptor: [Deglutition Disorders] explode all trees
((swallow* or deglutit* or dysphag*) near/3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)):ti,ab,kw (Word variations have been searched)
MeSH descriptor: [Pharynx] this term only
MeSH descriptor: [Pharyngeal Muscles] this term only
((pharyn* or oropharyn*) near/3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)):ti,ab,kw (Word variations have been searched)
{or #15‐#20}
#14 and #21
Appendix 2. MEDLINE search strategy
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or stroke, lacunar/
(stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$).tw.
((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
or/1‐4
Deglutition/
exp Deglutition Disorders/
((swallow$ or deglutit$ or dysphag$) adj5 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
Pharynx/ or pharyngeal muscles/
((pharyn$ or oropharyn$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
or/6‐10
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
random$.ab.
trial.ab.
groups.ab.
or/12‐18
5 and 11 and 19
Previous version of search strategy
stroke.mp.
infarction.mp.
exp cerebral infarction/
exp cerebrovascular disease/
cerebrovascular disease.mp.
hemorrhage.mp.
exp cerebral hemorrhage/
cerebral haemorrhage.mp.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
(dysphagia or deglutition or swallowing or deglutition disorders or swallowing disorders or malnutrition or undernutrition).mp.
(intervention or supplementation or feeding or nutrition or nutritional supplementation or therapy or swallowing therapy or tube feeding or fluid or fluid supplementation or sip feeding or feeding route or timing or diet or hydration).mp.
10 or 11
9 and 12
(randomized controlled trial.pt. or controlled clinical trial.pt.or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) and humans.sh.
13 and 14
Appendix 3. Embase search strategy
cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/
(stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$).tw.
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
or/1‐4
dysphagia/
swallowing/
((swallow$ or deglutit$ or dysphag$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
exp pharynx/
((pharyn$ or oropharyn$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
or/6‐10
Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
Randomization/
Controlled clinical trial/ or "controlled clinical trial (topic)"/
control group/ or controlled study/
clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
Crossover Procedure/
Double Blind Procedure/
Single Blind Procedure/ or triple blind procedure/
placebo/ or placebo effect/
(random$ or RCT or RCTs).tw.
(controlled adj5 (trial$ or stud$)).tw.
(clinical$ adj5 trial$).tw.
((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
(cross‐over or cross over or crossover).tw.
(placebo$ or sham).tw.
trial.ti.
(assign$ or allocat$).tw.
controls.tw.
or/12‐31
5 and 11 and 32
Previous version of search strategy
stroke.mp.
infarction.mp.
exp brain Infarction/
cerebrovascular disease.mp.
exp cerebrovascular disease/
hemorrhage.mp.
exp cerebral hemorrhage/
cerebral haemorrhage.mp.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
(dysphagia or deglutition or swallowing or deglutition disorders or swallowing disorders or malnutrition or undernutrition).mp.
(intervention or supplementation or feeding or nutrition or nutritional supplementation or therapy or swallowing therapy or tube feeding or fluid or fluid supplementation or sip feeding or feeding route or timing or diet or hydration).mp.
10 or 11
09 and 12
((RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/) or ((RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab) and human*.ec,hw,fs.
13 and 14
Appendix 4. CINAHL search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units")
S2 TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex )
S3 TI ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery ) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) OR AB ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery ) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*))
S4 TI (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) OR AB (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ))
S5 S1 OR S2 OR S3 OR S4
S6 (MH "Deglutition") OR (MH "Gagging")
S7 (MH "Deglutition Disorders")
S8 TI ( (swallow* or deglutit* or dysphag*) N3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*) ) OR AB ( (swallow* or deglutit* or dysphag*) N3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*) )
S9 TI ((swallow* or deglutit* or dysphag*) N3 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)) OR AB ((swallow* or deglutit* or dysphag*) N3 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test))
S10 S6 OR S7 OR S8 OR S9
S11 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design
S12 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study")
S13 TI random* or AB random*
S14 AB "latin square" or TI "latin square"
S15 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over)
S16 MH Placebos
S17 TI ( ((singl* or doubl* or trebl* or tripl*) N3 (blind* or mask*)) ) OR AB ( ((singl* or doubl* or trebl* or tripl*) N3 (blind* or mask*)) )
S18 TI Placebo* or AB Placebo* or SU Placebo*
S19 MH Clinical Trials
S20 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial)
S21 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20
S22 S5 AND S10 AND S21
Previous version of review search strategy
S1. stroke
S2. infarction
S3. brain Infarction
S4. cerebrovascular disease
S5. hemorrhage
S6. cerebral hemorrhage
S7. cerebral haemorrhage
S8. S1 or S2 or S3 or S4 or S5 or S6 or S7
S9. dysphagia or deglutition or swallowing or deglutition disorders or swallowing disorders or malnutrition or undernutrition
S10. intervention or supplementation or feeding or nutrition or nutritional supplementation or therapy or swallowing therapy or tube feeding or fluid or fluid supplementation or sip feeding or feeding route or timing or diet or hydration
S11. S9 or S10
S12. S8 and S11
S13. randomised controlled trials or controlled clinical trial or randomized or clinical trials
S14. S12 and S13
Appendix 5. Web of Science search strategy
1. TS=(stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva*)
2. TS=((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery) NEAR/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)) 3. TS=((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher*) NEAR/5 (h?emorrhag* or h?ematoma* or bleed*)) 4. #3 OR #2 OR #1 5. TS=((swallow* or deglutit* or dysphag*) NEAR/3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)) 6. TS=((pharyn* or oropharyn*) NEAR/3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)) 7. #6 OR #5 8. TS=(random* or RCT or RCTs)
9. TS=(controlled NEAR/5 (trial* or stud*)) 10. TS=(clinical* NEAR/5 trial*) 11. TS=((control or treatment or experiment* or intervention) NEAR/5 (group* or subject* or patient*))
12. TS=((control or experiment* or conservative) NEAR/5 (treatment or therapy or procedure or m.anage*))
13. TS=((singl* or doubl* or tripl* or trebl*) NEAR/5 (blind* or mask*))
14. TS=(cross‐over or cross over or crossover) 15. TS=(placebo* or sham) 16. TS=trial
17. #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 18. #17 AND #7 AND #4
Previous version of review search strategy
stroke
infarction
brain infarction
cerebrovascular disease
hemorrhage
cerebral haemorrhage
cerebral hemorrhage
1 or 2 or 3 or 4 or 5 or 6 or 7
dysphagia or deglutition or swallowing or deglutition disorders or swallowing disorders
randomized controlled trial or controlled clinical trial randomized or placebo or clinical trials or trial
8 and 9 and 10
Appendix 6. SpeechBITE search stategy
Speech Pathology Practice Area: Dysphagia
Type of intervention: Swallowing/ feeding
Within this population: Stroke/CVA
Research Design : Randomised Controlled Trial
Age group: Adults
Speech Pathology Practice Area: Dysphagia
Type of intervention: Swallowing/ feeding
Within this population: Stroke/CVA
Research Design: Non Randomised Controlled Trial
Age group: Adults
Appendix 7. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov)
( Dysphagia AND ( Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke ) ) [DISEASE]
Appendix 8. World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch)
stroke AND swallowing OR stroke AND dysphagia
Appendix 9. Google Scholar
Stroke
Dysphagia
Interventions
Randomised Controlled Trials
Data and analyses
Comparison 1. Swallowing therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional outcome ‐ death or dependency, death or disability at end of trial | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.75] |
| 1.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.63, 1.75] |
| 2 Case fatality at end of trial | 14 | 766 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.52] |
| 2.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 2.2 Drug therapy | 3 | 148 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.31, 6.28] |
| 2.3 Pharyngeal electrical stimulation | 4 | 215 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.26] |
| 2.4 Physical stimulation (thermal, tactile) | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.92] |
| 2.5 Transcranial magnetic stimulation | 4 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.93] |
| 3 Length of inpatient stay (days) | 8 | 577 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐5.65, ‐0.15] |
| 3.1 Behavioural interventions | 4 | 370 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.68, 0.28] |
| 3.2 Pharyngeal electrical stimulation | 4 | 207 | Mean Difference (IV, Random, 95% CI) | ‐6.05 [‐16.40, 4.31] |
| 4 Proportion of participants with dysphagia at end of trial | 23 | 1487 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.32, 0.55] |
| 4.1 Acupuncture | 8 | 676 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.49] |
| 4.2 Behavioural interventions | 6 | 511 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.28, 0.74] |
| 4.3 Drug therapy | 1 | 17 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.07, 3.35] |
| 4.4 Neuromuscular electrical stimulation | 2 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.49] |
| 4.5 Pharyngeal electrical stimulation | 3 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 2.11] |
| 4.6 Physical stimulation (thermal, tactile) | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.07, 5.85] |
| 4.7 Transcranial direct current stimulation | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 8.39] |
| 5 Swallowing ability | 26 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.01, ‐0.32] |
| 5.1 Acupuncture | 6 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.20, 0.11] |
| 5.2 Behavioural intervention | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.07, ‐0.05] |
| 5.3 Drug therapy | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.93, 0.01] |
| 5.4 Neuromuscular electrical stimulation | 2 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐3.39, 0.71] |
| 5.5 Pharyngeal electrical stimulation | 3 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.34] |
| 5.6 Physical stimulation (thermal, tactile) | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.29, 0.68] |
| 5.7 Transcranial direct current stimulation | 2 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐2.22, 1.56] |
| 5.8 Transcranial magnetic stimulation | 8 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.37, ‐0.21] |
| 6 Penetration aspiration score | 11 | 303 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.74, ‐0.00] |
| 6.1 Behavioural intervention | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.68, ‐0.08] |
| 6.2 Neuromuscular electrical stimulation | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.38, 1.52] |
| 6.3 Pharyngeal electrical stimulation | 4 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.53, 0.19] |
| 6.4 Transcranial magnetic stimulation | 5 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.22, 0.16] |
| 7 Chest infection or pneumonia | 9 | 618 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.16, 0.78] |
| 7.1 Behavioural interventions | 6 | 473 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.00] |
| 7.2 Drug therapy | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.21] |
| 7.3 Neuromuscular electrical stimulation | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Pharyngeal electrical stimulation | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.09] |
| 8 Pharyngeal transit time (seconds) | 6 | 187 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.32, ‐0.15] |
| 8.1 Drug therapy | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.91, 0.49] |
| 8.2 Neuromuscular electrical stimulation | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.39, ‐0.08] |
| 8.3 Pharyngeal electrical stimulation | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.67, 0.37] |
| 8.4 Physical stimulation (thermal, tactile) | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] |
| 9 Institutionalisation | 3 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.47, 1.19] |
| 9.1 Behavioural interventions | 2 | 306 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.39, 1.48] |
| 9.2 Pharyngeal electrical stimulation | 1 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.48] |
| 10 Nutritional (albumin) | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐1.50, 2.24] |
| 10.1 Behavioural interventions | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.77, 5.17] |
| 10.2 Pharyngeal electrical stimulation | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.62, 2.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bai 2007i.
| Methods | Random numbers table Outcomes not blinded (medium‐intensity vs low‐intensity data set) |
|
| Participants | 1 centre in China
111 participants within 2 weeks of stroke Baseline characteristics similar No cross‐overs or dropouts identified Dysphagia defined by Watian swallow test |
|
| Interventions | A1: shallow needling (control) (n = 35) = low intensity A2: single deep needling (n = 18) = medium intensity B: deep multi‐needling | |
| Outcomes | Watian drinking test grade Return to normal diet | |
| Notes | Exclusions: needle phobia, infection risk, dementia, inability to co‐operate with treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation via a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Translated from Chinese language |
Bai 2007ii.
| Methods | (High vs medium data set) | |
| Participants | As data set 1 | |
| Interventions | A1: shallow needling (control) A2: single deep needling (n = 17) = medium intensity B: deep multi‐needling (n = 40) = high intensity | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation via a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Translated from Chinese |
Bath 1997.
| Methods | Computerised randomisation by minimisation Unblinded outcome assessment Analysis by ITT Cross‐overs: 3 NGT to PEG, 0 PEG to NGT Balancing of baseline prognostic factors between treatment groups unclear | |
| Participants | 1 centre in UK 19 participants: 8 male Mean age 77 (SD 11) years 13 ischaemic stroke, 6 haemorrhagic stroke 100% CT Enrolment within 2 weeks of stroke onset | |
| Interventions | Factorial trial: PEG vs NGT; intensive vs conservative swallowing therapy PEG: NGT: up to 3 NGTs Intensive swallowing therapy: as for conservative, plus voluntary control (tongue‐holding), sensory stimulation (tactile, oromotor exercises, swallow practice) Conservative swallowing therapy: review, advice regarding feeding route, postural/dietary modification, safe swallowing methods | |
| Outcomes | Primary outcomes: resumption of safe feeding at 12 weeks, weight loss < 5% at 6 weeks, discharge by 6 weeks Secondary outcomes: impairment, disability, handicap, quality of life, tube failures, chest infection, oropharyngeal delay time (by videofluoroscopy) at 4 weeks | |
| Notes | Exclusions: oro‐gastrointestinal disease, concurrent severe illness, coagulopathy, premorbid dependency, severe dementia, psychiatric illness Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by minimisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | None identified |
Carnaby 2006i.
| Methods | Computerised randomisation Blinded outcome assessments by SLT ITT (Control vs low‐intensity data set) Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in Australia 306 participants; baseline characteristics similar Enrolment within 2 weeks of stroke onset: mean/median 2 days, range 0 to 12 days Clinical and videofluoroscopic evidence of dysphagia |
|
| Interventions | Rx 1: standardised high‐intensity swallowing therapy (n = 102) Rx 2: standardised low‐intensity swallowing therapy (n = 102); split into (n = 51) for each data set C: usual care (n = 102) Treatment for up to 1 month | |
| Outcomes | Outcomes: time to return to normal diet; aspiration pneumonia; dysphagia (PHAD score < 85) | |
| Notes | Trial completed and published 2006 Exclusions: previous swallowing therapy, head and neck surgery, inability to consent Follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation based on a computer‐generated random numbers list generated via the SPSS statistical package |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule held at the trial office, remote from the study environment; assignment to 1 of 3 treatment options by a telephone call to the trial office made by the study speech pathologist |
| Blinding (performance bias and detection bias) All outcomes | High risk | All people involved in the study unaware of treatment allocation, apart from participants and the study speech pathologist who treated participants Assigned to high‐intensity and low‐intensity groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and speech pathologist aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed by an independent speech pathologist, who was unaware of treatment allocation, every month for 6 months after randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up before 6‐month analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Carnaby 2006ii.
| Methods | (High‐intensity vs low‐intensity data set) | |
| Participants | As data set 1 | |
| Interventions | High intensity (n = 102) Low intensity (n = 51) | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation based on a computer‐generated random numbers list obtained via the SPSS statistical package |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule held at trial office, remote from the study environment; assignment to 1 of 3 treatment options by a telephone call to the trial office made by the study speech pathologist |
| Blinding (performance bias and detection bias) All outcomes | High risk | All people involved in the study unaware of treatment allocation, apart from participants and the study speech pathologist who treated participants Assigned to high‐intensity and low‐intensity groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed by an independent speech pathologist, who was unaware of treatment allocation, every month for 6 months after randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up before 6‐month analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | None identified |
Chan 2012.
| Methods | Randomisation by random sequences on black paper Single‐blind (participants blinded): outcome assessors blinded |
|
| Participants | 1 centre in Hong Kong 87 participants with neurogenic dysphagia with similar baseline characteristics 60 (69%) participants with dysphagia due to cerebral infarct < 6 months; other causes of neurogenic dysphagia include intracranial haemorrhage, vascular dementia, Parkinson's disease Clinical evidence of dysphagia |
|
| Interventions | All groups given routine swallowing therapy Rx 1: true acupuncture (n = 20) Rx 2: sham acupuncture that did not puncture true acupoints lying on a meridian (n = 19) C: routine swallowing therapy only (n = 48) Treatment for up to 4 weeks |
|
| Outcomes | Outcomes: Royal Brisbane Hospital Outcome Measure Scale (RBHOMS), swallow function by consistencies of ingested food and fluid | |
| Notes | Exclusions: structural oral, pharyngeal, or oesophageal disease; severe primary disease of the liver, kidneys, hematopoietic system, or endocrine system; malignant tumour or infectious disease; inability to follow commands Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random sequences |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single (participants) blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single (participants) blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Chen 2016a.
| Methods | Computer‐generated random numbers by independent research staff Assessors blinded |
|
| Participants | Multi‐centre trial in China 250 participants; 148 male 100% stroke within 2 to 7 days Dysphagia identified by bedside swallowing assessment and videofluoroscopic swallowing study Baseline characteristics and prognostic values similar between both groups |
|
| Interventions | Rx: acupuncture and conventional stroke rehabilitation care C: conventional stroke rehabilitation care only Duration: 3 weeks Follow‐up: 7 weeks |
|
| Outcomes | Primary outcome: NIHSS index Secondary outcomes: FMA for motor function, rate of recovery based on BSA, VFSS, MMSE, and MoCA |
|
| Notes | Exclusions: serious heart, liver, and kidney‐related diseases; blood coagulation dysfunction; inability to complete the MMSE test or bedside swallowing assessment; congenital disabilities; posterior circulation infarcts; receiving thrombolytic; participated in other clinical trials within previous 3 months; pregnant or breastfeeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers provided by independent research staff |
| Allocation concealment (selection bias) | Low risk | Random numbers placed into sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and acupuncturist aware of treatment allocations. All allopathic medical staff and rehabilitation therapists blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and acupuncturist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants lost to follow‐up; 4 discontinued intervention. Not all participants given VFSS examination |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Du 2016i.
| Methods | Randomisation by sequentially numbered sealed envelopes Blinded outcome assessments by trained neurologist (Sham vs low‐frequency (1 Hz) data set) Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in China 40 participants; baseline characteristics similar Enrolment within 2 months of stroke onset confirmed by CT or MRI scan Clinical evidence of dysphagia |
|
| Interventions | Rx 1: 1 Hz rTMS to unaffected hemisphere (n = 13) Rx 2: 3 Hz rTMS to affected hemisphere (n = 13) C: sham rTMS (n = 12), split into n = 6 for each data set Treatment for up to 5 days | |
| Outcomes | Outcomes: swallow score using Standardised Swallow Assessment (SSA), BI, mRS, and measures of mylohyoid MEPs | |
| Notes | Exclusions: other concomitant neurological diseases, fever, infection, prior administration of tranquilliser, severe aphasia or cognitive impairment, inability to complete the follow‐up, and other contraindications for rTMS Follow‐up: up to 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by sequentially numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant blinded; outcome assessor blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded ‐ measures evaluated by a trained neurologist who was blinded to participants' group allocation throughout |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Only NIHSS not recorded at the end; all other measures reported on for all 3 time points |
| Other bias | Low risk | None identified |
Du 2016ii.
| Methods | (High‐frequency vs sham data set) | |
| Participants | As data set 1 | |
| Interventions | High = 102 (high intensity) Sham = 51 (low intensity) | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by sequentially numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant blinded; outcome assessor blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded ‐ measures evaluated by a trained neurologist who was blinded to participants' group allocation throughout |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Only NIHSS not recorded at the end; all other measures reported on for all 3 time points |
| Other bias | Low risk | None identified |
Feng 2012.
| Methods | Randomisation by random numbers table Blinding unclear Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in China 122 participants; baseline characteristics similar Enrolment within 2 weeks to 6 months of stroke onset Clinical evidence of dysphagia 2 participants lost to follow‐up |
|
| Interventions | Rx: tongyan spray (n = 60) C: placebo (n = 60) Treatment for up to 28 days | |
| Outcomes | Outcomes: swallow safety and function using the SSA | |
| Notes | Exclusions: consciousness disorder; unstable life sign and accompanied by serious diseases (heart, kidney, etc.), non‐compliance with examination and treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Concealed via sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participant dropouts (1 from each group) |
| Selective reporting (reporting bias) | Low risk | All outcomes listed reported |
| Other bias | Low risk | None identified |
Han 2004.
| Methods | Randomisation by sealed opaque envelope. Assessors blinded | |
| Participants | People with acute stroke, dysphagia, and dysarthria 1 centre in China 66 participants 100% with stroke within 30 days of onset. Degrees of dysphagia not stated |
|
| Interventions | Rx: scalp and neck acupuncture with electroacupuncture with standard Western medical treatment C: standard Western medical treatment only |
|
| Outcomes | Dysphagia at end of trial after 3 treatment sessions | |
| Notes | Exclusions: reduced consciousness, poor compliance, infections at acupoints | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by sealed opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Allocations concealed by opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | None identified |
Heo 2015.
| Methods | Participants were randomly allocated for radiographic inspection and treatment with or without kinesiotaping by drawing lots Blinding unknown |
|
| Participants | 1 centre in Republic of Korea 44 participants 100% with dysphagia and stroke within 3 months of diagnosis Baseline characteristics similar |
|
| Interventions | Rx: kinesiotaping C: no kinesiotaping |
|
| Outcomes | Kinematic analysis of movement of the hyoid bone (movements measured in both horizontal and vertical sections) Angular variation of the epiglottis using human anatomy‐based co‐ordinates Swallow score: FDS |
|
| Notes | Exclusions: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Huang 2010.
| Methods | Method of randomisation unknown Blinding unknown Only data for groups 2 and 3 included |
|
| Participants | 1 centre in China 97 participants with post‐stroke dysphagia |
|
| Interventions | Group 1: electrical stimulation (n = 35) Group 2: rehabilitation training (n = 30) Group 3: acupuncture (n = 32) |
|
| Outcomes | Swallowing function | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unknown |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unknown |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Translated from Chinese language |
Jayasekeran 2010a.
| Methods | Dose comparison protocol (only data from the group that were stimulated once a day over 3 days were included) Computerised randomisation by minimisation Blinded outcome measures Balancing of prognostic baseline factors between treatment groups unclear |
|
| Participants | 1 centre in UK 10 participants with acute anterior circulation cerebral infarct (< 3 weeks) Mean age 73 years |
|
| Interventions | Rx: bedside pharyngeal electrical stimulation C: sham stimulation Duration: once daily for 3 consecutive days |
|
| Outcomes | Airway aspiration at 2 weeks' post intervention | |
| Notes | Exclusion: dementia, pacemaker or implantable cardiac defibrillator, severe receptive aphasia, unstable cardiopulmonary status, distorted oropharyngeal anatomy (e.g. pharyngeal pouch), brainstem stroke, dysphagia resulting from conditions other than hemispheric stroke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by minimisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Jayasekeran 2010b.
| Methods | Parallel‐group design protocol Computerised randomisation by minimisation Blinded outcome measures Prognostic baseline factors between treatment groups similar |
|
| Participants | 2 centres in UK 28 participants with acute anterior circulation cerebral infarct or haemorrhage (< 3 weeks) Mean age 75 years |
|
| Interventions | Rx: bedside pharyngeal electrical stimulation C: sham stimulation Duration: once daily for 3 consecutive days |
|
| Outcomes | Airway aspiration at 2 weeks post intervention | |
| Notes | Exclusion: dementia, pacemaker or implantable cardiac defibrillator, severe receptive aphasia, unstable cardiopulmonary status, distorted oropharyngeal anatomy (e.g. pharyngeal pouch), brainstem stroke, dysphagia resulting from conditions other than hemispheric stroke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by minimisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Jia 2006a.
| Methods | Randomisation: participants randomised in visiting sequence Blinding: unclear ITT: unclear Balancing of all prognostic factors not reported; only for age, gender, and stroke duration |
|
| Participants | 1 centre in China 72 inpatients, stroke confirmed by CT or MRI scan but unclear patient inclusion criteria ‐ 2 out of 5 symptoms as hemiplegia, coma, slurred speech, unilateral sensory disturbance, wry mouth and tongue; difficulty in swallowing Mean age: treatment group = 55.4 years, control = 54.8 years |
|
| Interventions | Group 1: acupuncture + rehabilitation training Group 2: rehabilitation training only |
|
| Outcomes | Primary outcomes: therapeutic assessment of swallowing function using 1 to 10 point scale with categories basic cure; marked improvement; improvement and failure | |
| Notes | Not having above symptoms; cannot co‐operate to do chemical examination and treatment; severe primary disease in the liver, kidneys, hematopoietic system, and endocrine system | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants randomised in visiting sequence |
| Allocation concealment (selection bias) | High risk | Allocation not concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Only 1 outcome chosen and reported ‐ improvement in swallowing at end of trial |
| Other bias | Unclear risk | Unclear |
Kang 2012.
| Methods | Method of randomisation unclear Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in Korea 25 participants; baseline characteristics similar Enrolment within 6 weeks of stroke onset Clinical and videofluoroscopic evidence of dysphagia |
|
| Interventions | Rx: additional exercise programme for dysphagia with thermal‐tactile stimulation C: thermal‐tactile stimulation only Treatment for up to 2 months | |
| Outcomes | Videofluoroscopy, Functional Oral Intake Scale, transition from tube to oral feeding, incidence of aspiration pneumonia | |
| Notes | Exclusions: previous history of other diseases, which may have caused dysphagia; severe cognitive disorder, such as dementia; inability to carry out videofluoroscopy due to incapability of sitting posture; inability to follow study instructions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Blinding unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Unclear |
Khedr 2009.
| Methods | Method of randomisation unclear: participants were assigned randomly to receive real or sham rTMS using closed envelopes Blinded outcome assessment Allocation sequence concealed from participants Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in Egypt 26 participants between 5th and 10th days post stroke (monohemispheric) Mean age 56 years |
|
| Interventions | Rx: repetitive transcranial magnetic stimulation of the affected motor cortex (n = 14) C: sham stimulation (n = 12) |
|
| Outcomes | Primary outcome: score on the dysphagia rating scale Secondary outcomes: motor power of hand grip, BI, measures of oesophageal motor evoked potentials from both hemispheres before and 1 month after sessions |
|
| Notes | Exclusion: head injury or neurological disease other than stroke, unstable cardiac dysrhythmia, fever, infection, hyperglycaemia, prior administration of tranquilliser | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Low risk | Allocation sequence concealed from participants |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and outcome assessors not aware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants informed of which group they had been allocated to at the end of the last assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants apart from 1 in the sham treatment group who died completed the trial and follow‐up periods |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Khedr 2010.
| Methods | Method of randomisation unclear: participants from both the lateral medullary infarction (LMI) group and the other brainstem infarction group were each randomly classified into 2 groups ‐ to receive real or sham repetitive transcranial magnetic stimulation Blinded primary outcome assessment Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in Egypt Total of 22 participants with hemispheric stroke split into having lateral medullary infarction or other brainstem infarction Mean age 58 years |
|
| Interventions | Rx: repetitive transcranial magnetic stimulation of the affected motor cortex (n = 11) C: sham stimulation (n = 11) |
|
| Outcomes | Primary outcome: score on the dysphagia rating scale Secondary outcomes: motor power of hand grip, BI, NIHSS |
|
| Notes | Exclusion: head injury or neurological disease other than stroke, unstable cardiac dysrhythmia, fever, infection, hyperglycaemia, epilepsy, prior administration of tranquilliser | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants apart from 2 in the sham treatment group who died completed the trial and follow‐up periods |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Kim 2012i.
| Methods | Method of randomisation unclear Blinding unclear (High frequency data set vs control) |
|
| Participants | 1 centre in Korea 30 participants with acute brain injury; baseline characteristics similar Clinical and videofluoroscopic evidence of dysphagia |
|
| Interventions | Rx 1: high‐frequency (5 Hz) rTMS (n = 10)
Rx 2: low‐frequency (1 Hz) rTMS (n = 10) (Using high frequency data set) C: sham stimulation. (n = 10); control = 5 Treatment for 2 weeks |
|
| Outcomes | Functional Dysphagia Scale and Penetration Aspiration Scale | |
| Notes | Exclusions: prior diagnosis of another neurological disease, unstable medical condition, severe cognitive impairment, severe aphasia, history of seizure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Kim 2012ii.
| Methods | (Low‐frequency data set vs control) | |
| Participants | As data set 1 | |
| Interventions | Low‐frequency rTMS = 10 Control (sham stimulation) = 5 | |
| Outcomes | As data set 1 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Kumar 2011.
| Methods | Randomisation via simple randomisation Double‐blind Analysis by ITT unclear Balancing of prognostic baseline factors between treatment groups unclear |
|
| Participants | 1 centre in USA 14 participants with subacute (24 to 168 hours) unilateral hemispheric infarction Mean age 75 years |
|
| Interventions | Rx: anodal transcranial direct current stimulation C: sham stimulation For 5 consecutive days |
|
| Outcomes | Swallowing impairment using dysphagia outcome and severity scale | |
| Notes | Exclusions: difficulty following instructions because of obtundation or cognitive impairment, pre‐existing swallowing problems; other contraindications to transcranial direct current stimulation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation via simple randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported and explained |
| Other bias | Low risk | None identified |
Lee 2014.
| Methods | Randomisation via computer‐generated block randomisation Blinding unclear Analysis by ITT unclear Prognostic baseline factors between treatment groups similar |
|
| Participants | 1 centre in Korea 57 participants with dysphagic stroke within 10 days of onset (men 42, women 15) Mean age 65 years |
|
| Interventions | Rx: NMES combined with traditional dysphagia therapy (n = 31) C: traditional dysphagia therapy only (n = 26) 5 days per week for 3 weeks |
|
| Outcomes | Swallowing function, Functional Oral Intake Scale | |
| Notes | Exclusion: presence of dysphagia before stroke, previous history, unstable cardiopulmonary status, serious psychological disorder or epilepsy, tumour or radiotherapy of the head and neck region, swallowing therapy before participation in the present study, unstable medical conditions that may interfere with VFSS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appeared to have been followed up at 12 weeks |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Lee 2015.
| Methods | Randomisation by computer‐generated random sequence Outcome assessors blinded |
|
| Participants | Multi‐centre trial in Hong Kong 93 participants with cerebrovascular disease; onset unclear although study states recent hospitalisation in the previous 3 months Baseline characteristics and prognostic factors similar |
|
| Interventions | Rx: lisinopril 2.5 mg once daily at bedtime C: placebo |
|
| Outcomes | Incidence of pneumonia, mortality, and Royal Brisbane Hospital Outcome Measure Scale score | |
| Notes | Exclusion: life expectancy < 6 months, baseline systolic blood pressure less than 100 mm Hg, known intolerance to ACE inhibitors, current use of ACE inhibitor or angiotensin receptor blockers, symptomatic chronic lung disease or cardiac failure, frequent withdrawal of enteral tube by patients, serum creatinine > 150 mmol/L, serum potassium > 5.1 mmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocations concealed by coding files kept confidential to all parties involved until the end of the trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All parties involved not aware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All parties involved not aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 participants did not complete trial |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Li 2014.
| Methods | Randomisation via minimisation software Single‐blind ‐ assessors blinded No significant differences in baseline comparability tests in all groups of participants |
|
| Participants | Recruitment through newspaper advertisements and flyers in China 118 participants with dysphagia and hemispheric stroke |
|
| Interventions | Rx 1: neuromuscular electrical stimulation (VitalStim) Rx 2: combined NMES and traditional swallowing therapy C: traditional swallowing therapy (Data from Rx 2 vs control used in this review) |
|
| Outcomes | Swallow score, oral transit time, pharyngeal transit time, laryngeal closure duration, PAS | |
| Notes | Exclusion: progressive stroke, other neurological disease, neoplastic disease, previous surgery to swallowing apparatus, nasogastric tube | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation via minimisation software |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and technicians not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17 participant dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Lim 2009.
| Methods | Method of randomisation unclear: participants divided into 2 groups according to order of enrolment Blinding of outcomes unclear Analysis by ITT unclear Balancing of prognostic baseline factors between treatment groups ‐ not reported for dysphagia severity, only for previous treatment of pneumonia |
|
| Participants | 1 centre in Korea 22 participants with CT or MRI confirmed stroke < 6 months from onset Mean age 64 years |
|
| Interventions | Rx: neuromuscular electrical stimulation + thermal‐tactile stimulation (n = 13) C: thermal‐tactile stimulation (n = 9) |
|
| Outcomes | Swallow function scoring system, PAS and PTT | |
| Notes | Exclusions: inability to receive treatment for 1 hour, neurological disease other than stroke, combined behavioural disorder that interfered with administration of therapy, current illness or upper gastrointestinal disease, inability to give informed consent because of cognitive impairment or receptive aphasia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants divided into 2 groups according to order of enrolment |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Doctor blinded to groups performed videofluoroscopic examination; measured PTT as well as swallow function scoring system and Rosenbek penetration aspiration scale. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36 enrolled to the study. Only 28 participants completed the study (16 in the experimental group and 12 in the control group) |
| Selective reporting (reporting bias) | Unclear risk | Swallow scores not fully reported (unclear on the range of median values) |
| Other bias | Low risk | None identified |
Liu 2000.
| Methods | Method of randomisation unclear Blinding of outcomes unclear Analysis by ITT unclear Balancing of prognostic baseline factors between treatment groups unclear |
|
| Participants | 1 centre in China 84 participants with bulbar palsy and CT/MRI‐documented stroke: 54 men, 30 women Age 50 to 78 years Infarct 56, haemorrhage 28 Enrolment within 2 months of stroke onset |
|
| Interventions | Rx: acupuncture ‐ Tiantu (CV 22), Lieque (LU 7), Zhaohai (KI 6) ‐ once daily for 10 days (n = 54) C: (n = 30) |
|
| Outcomes | Outcome: bulbar function (phonation, swallowing, cough reflex) Timing unclear |
|
| Notes | Exclusions: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ no clear aim of study |
| Other bias | Unclear risk | Unclear |
Liu 2004.
| Methods | RCT | |
| Participants | 1 centre in China 82 participants with cerebral infarction or haemorrhage and CT/MRI‐documented stroke: 49 men, 33 women Age 40 to 80 years Infarct 72, haemorrhage 10 Enrolment within 6 months of stroke onset |
|
| Interventions | Rx: scalp acupuncture + sublingual needling (n = 44) C: scalp acupuncture + control needling (n = 38) |
|
| Outcomes | Recovery of function (swallowing food and water, movement of the tongue, disappearance of dyslalia and hoarseness) | |
| Notes | Exclusion: severe arrhythmia, coma, asthma, dilating myocardiopathy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear aim of study ‐ only 1 outcome reported |
| Other bias | Unclear risk | Unclear |
Park 2012.
| Methods | Computer‐generated randomisation sequence Outcomes and participants blinded |
|
| Participants | Study in Korea 20 participants with stroke > 1 month Baseline characteristics similar, except stimulation intensities. Unclear baseline degree of dysphagia between groups Dysphagia defined by videofluoroscopy |
|
| Interventions | Rx: effortful swallow with infrahyoid motor electrical stimulation C: effortful swallow with infrahyoid sensory electrical stimulation (placebo stimulation) |
|
| Outcomes | Vertical laryngeal and hyoid movements, maximum width of UES opening, PAS | |
| Notes | Exclusions: subarachnoid haemorrhage, carotid stenosis, inability to overcome stimulation, which was determined by observation and palpation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Automated assignment system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and outcome assessors blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participant dropouts (1 from each group) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Park 2013.
| Methods | Computer‐generated randomisation sequence Outcomes and participants blinded |
|
| Participants | Study in Korea
18 participants with stroke > 1 month Baseline characteristics similar Dysphagia confirmed by videofluoroscopy |
|
| Interventions | Rx: active high‐frequency rTMS (5 Hz) at the contralesional intact cortex C: sham rTMS |
|
| Outcomes | VDS, PAS | |
| Notes | Exclusions: metal implants or a pacemaker in the body, history of seizures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Automated assignment system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and outcome assessors blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Park 2016a (i).
| Methods | Randomisation unclear Outcome assessor blinded (unilateral stimulation vs sham data set) |
|
| Participants | 1 centre in Korea 35 participants with subacute stroke defined as onset < 3 months Swallowing dysfunction confirmed by videofluoroscopy Baseline characteristics similar 2 participants lost to follow‐up |
|
| Interventions | Rx 1: unilateral stimulation group with (10 Hz) rTMS on ipsilesional cortex and sham on contralesional cortex (n = 11) Rx 2: bilateral stimulation group with (10 Hz) rTMS on ipsilesional and contralesional cortex (n = 11) C: sham rTMS over bilateral hemispheres (n = 11) Control group split into n = 5 for data set 1 and n = 6 for data set 2 Therefore for this data set, unilateral stimulation (n = 11) vs sham stimulation (n = 5) |
|
| Outcomes | Clinical Dysphagia Scale, Dysphagia Outcome and Severity Scale, PAS, VDS | |
| Notes | Exclusion: history of swallowing problems caused by other underlying neurological diseases, such as Parkinson’s disease, dementia, or motor neuron disease; history of intractable seizure; metallic implants in the brain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blinding unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blinded (assessors only) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported only as single‐blinded (assessors only) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Park 2016a (ii).
| Methods | As per Park 2016a (bilateral stimulation vs sham data set) |
|
| Participants | As data set 1 | |
| Interventions | Bilateral stimulation (n = 11) vs sham stimulation (n = 6) | |
| Outcomes | As data set 1 | |
| Notes | As data set 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blinding unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blinded (assessors only) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported only as single‐blinded (assessors only) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Park 2016b.
| Methods | Randomisation by randomly selected envelopes containing a code specifying the group Outcomes partially blinded (for VFSS only but not for sEMG evaluation) |
|
| Participants | 1 centre in Korea 33 participants with dysphagia (inclusion criteria states stroke onset within 6 months) Dysphagia confirmed by videofluoroscopy Baseline demographics and prognostic factors balanced |
|
| Interventions | Rx: EMST with a 70% threshold value of maximal expiratory pressure, using an EMST device C: training with sham device Treatment for 4 weeks |
|
| Outcomes | Swallow function using VFSS, PAS, Functional Oral Intake Scale | |
| Notes | Exclusion: stroke before that resulting in dysphagia; severe oro‐facial pain including trigeminal neuropathy; significant malocclusion or facial asymmetry; unstable breathing and pulse; tracheostomy; severe communication disorder such as severe aphasia; inadequate lip closure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by randomly selected envelopes containing a code specifying the group |
| Allocation concealment (selection bias) | Low risk | Concealed by coded envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes partially blinded (surface EMG evaluation not blinded; however this outcome not relevant in this review) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Perez 1997.
| Methods | Computerised randomisation Triple‐blind trial; outcomes assessed by blinded therapist Analysis by ITT No cross‐overs or losses to follow‐up 1 participant withdrawn with heart failure (nifedipine group) Baseline prognostic factors balanced between treatment groups |
|
| Participants | 1 centre in UK 17 participants; 8 men Mean age 77 (SD 7) years All first ischaemic stroke 100% CT Enrolment 2 weeks after stroke |
|
| Interventions | Rx: nifedipine (30 mg orally daily, Bayer, UK) (n = 8) Pl: matching tablet; treatment for 4 weeks (n = 9) |
|
| Outcomes | Primary outcome: clinical improvement in swallowing Other outcomes: incidence of silent aspiration, pharyngeal transit time and response duration, swallowing delay (all assessed by videofluoroscopy), death |
|
| Notes | Exclusions: inability to sit, high clinical risk of aspiration, receptive dysphasia, cognitive impairment, pre‐stroke dysphagia, existing neurological or psychiatric disease, current treatment with calcium channel blockers or aminophylline Follow‐up: 4 weeks. 1 participant withdrawn with heart failure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blind trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by blinded therapist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant withdrawn with heart failure (nifedipine group) No cross‐overs |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Power 2006.
| Methods | Method of randomisation unclear CT scans analysed by a neuroradiologist who was blinded to patients' clinical presentation and videofluoroscopic swallowing status Baseline data not including dysphagia severity of baseline groups |
|
| Participants | 1 centre in UK 16 participants |
|
| Interventions | Rx: actual electrical stimulation following threshold setting exercise to faucial pillars C: single episode of sham electrical stimulation following threshold setting exercise | |
| Outcomes | Changes on videofluoroscopy 60 minutes post intervention | |
| Notes | Exclusions: prior dysphagia, intercurrent illness, other neurological disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Shigematsu 2013.
| Methods | Participants randomised using code numbers issued by coauthor Outcomes blinded |
|
| Participants | 1 centre in Japan
20 participants with stroke > 4 weeks Baseline characteristics similar Clinical, video endoscopic, and videofluoroscopic evidence of dysphagia |
|
| Interventions | Rx: 1‐mA anodal tDCS C: sham tDCS (n = 10) Treatment for 10 days |
|
| Outcomes | Dysphagia Outcome and Severity Scale, PAS, VFSS, video endoscopic evaluation of dysphagia | |
| Notes | Exclusions: subarachnoid haemorrhage, history of epileptic seizures, severe consciousness disturbance, organic neck disease, history of surgery except for tracheotomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via code numbers issued by coauthor |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by code numbers |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes blinded (rehabilitation doctor and speech‐language hearing therapists did not know participants' group allocation) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Results of the Dysphagia Outcome and Severity Scale reported pre‐, post‐, and at 1‐month follow‐up |
| Other bias | Low risk | None identified |
Song 2004.
| Methods | Method of randomisation: random numbers table Allocation method and concealment unclear |
|
| Participants | 1 centre in China 53 participants; 46 men All dysphagia identified by water swallow test Baseline characteristics reported as similar |
|
| Interventions | Rx: nurse‐led swallowing exercises, oral stimulation and oral care (n = 29) C (n = 24) Follow‐up: 1 month |
|
| Outcomes | Primary and secondary outcomes not defined Resolution of dysphagia by water swallow test and dietary ability, pneumonia rates |
|
| Notes | Exclusions and whether ITT not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Translated from Chinese language |
STEPS 2016.
| Methods | Computerised randomisation Single‐blind; outcome assessor blinded Analysis by ITT Baseline characteristics balanced |
|
| Participants | International, multi‐centre trial 162 participants; 94 men Mean age 74.4 years Dysphagia identified clinically and by videofluoroscopy |
|
| Interventions | Rx: active pharyngeal electrical stimulation C: sham pharyngeal electrical stimulation Follow‐up: up to 12 weeks |
|
| Outcomes | Primary: change in PAS at 2 weeks from baseline Secondary: safety outcomes, clinical dysphagia (Dysphagia Severity Rating Scale, PAS at 12 weeks), dependency (mRS), activities of daily living/disability (BI), impairment (NIHSS), health‐related quality of life (European Quality of Life‐5 Dimensions (EQ‐5D), nutritional measures (weight, mid‐arm circumference, and blood albumin)) |
|
| Notes | Exclusions: history of dysphagia, dysphagia from a condition other than stroke, advanced dementia, implanted pacemaker or cardiac defibrillator in situ, unstable cardiopulmonary status or a condition that compromised cardiac or respiratory status, distorted oropharyngeal anatomy, additional diagnosis of progressive neurological disorder, receiving continuous oxygen treatment, pregnant or nursing mother | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Researcher delivering the intervention not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Assessor and participant blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 181 participants randomised; only 123 participants completed all 3 treatments |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Terre 2015.
| Methods | Computerised randomisation Double‐blinded study Outcome assessors blinded |
|
| Participants | Study completed in Spain 20 participants with neurological oropharyngeal dysphagia (14 stroke participants in the posterior circulation; 6 with traumatic brain injury) Baseline characteristics similar between groups All within 5 months of diagnosis Dysphagia identified by videofluoroscopy and Functional Oral Intake Scale |
|
| Interventions | Rx: active NMES with conventional therapy C: sham NMES with conventional therapy |
|
| Outcomes | Clinical, videofluoroscopic, and oesophageal manometric analyses of swallow; Functional Oral Intake Scale | |
| Notes | Exclusion: previous stroke or traumatic brain injury, previous dysphagia secondary to any other etiology, other metabolic or neurological disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Vasant 2016.
| Methods | Computerised randomisation Single‐blind trial; outcomes assessed by blinded therapist Analysis by ITT |
|
| Participants | 3 centres in UK 36 participants; 22 men All dysphagia identified by bedside screening swallow test and videofluoroscopy Baseline characteristics reported as similar 1 participant withdrawn and lost to follow‐up Baseline prognostic factors similar between groups |
|
| Interventions | Rx: pharyngeal electrical stimulation n = 18 C: sham n = 18 Duration: 3 days Follow‐up: 3 months |
|
| Outcomes | Death, swallow function, dysphagia | |
| Notes | Exclusions: advanced dementia, other neurological conditions that may explain dysphagia, previous history of dysphagia, presence of cardiac pacemaker or implanted cardiac defibrillator, diagnosis other than stroke (e.g. brain tumour), significant structural abnormalities of the mouth or throat and requiring continuous oxygen treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through a concealed computer programme |
| Allocation concealment (selection bias) | Low risk | Concealed via a computerised programme |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Researcher delivering the intervention not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and assessors blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant lost to follow‐up (withdrawn), 2 participants (1 from each group) died before follow‐up at 3 months |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Warusevitane 2015.
| Methods | Randomisation via a random numbers list generated by an independent statistician Double‐blind Analysis by ITT unclear |
|
| Participants | 1 centre in UK 60 participants within 7 days of acute ischaemic or haemorrhagic stroke confirmed by CT scan of the brain who required nasogastric feeds for > 24 hours Mean age: 78 No significant differences between baseline characteristics |
|
| Interventions | Rx: 10 mg metoclopramide (10 mL) C: 10 mL normal saline Treatment duration: 21 days or until NGT no longer needed |
|
| Outcomes | Swallowing impairment using dysphagia outcome and severity scale | |
| Notes | Exclusions: signs and symptoms of pneumonia after stroke onset, history of chronic neurodegenerative disease that could affect swallowing (e.g. Parkinson disease, motor neuron disease), oesophageal disorders, contraindications to metoclopramide. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by numbers list generated by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Allocation sequence concealed from participants |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher and medical team involved in participants' care blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 60 participants analysed at end of trials (none excluded) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Wei 2005.
| Methods | Method of randomisation unclear Outcomes blinded |
|
| Participants | 1 centre in China 68 participants; timing post stroke unclear but suggests acute Dysphagia defined by water swallow test | |
| Interventions | Rx: Shuiti acupoint injection with stellate ganglion block for 40 days of treatment (n = 32) C: standard medical care, which included some acupuncture (n = 33) | |
| Outcomes | Resolution of dysphagia: water swallow test score BI Chinese Neurological Score Fugl‐Meyer Assessment | |
| Notes | Exclusions: needle phobia, organ failure, head and neck tumours Exclusions and dropouts accounted for but not analysed by ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Translated from Chinese language |
Xia 2011.
| Methods | Method of randomisation unclear Outcomes blinded |
|
| Participants | 1 centre in China
120 participants, timing post stroke unclear but suggests acute
Dysphagia defined by water swallow test Baseline characteristics similar |
|
| Interventions | Rx 1: combined VitalStim therapy + conventional swallowing training (n = 40) Rx 2: VitalStim therapy (n = 40) C: conventional swallowing training (n = 40) For the purpose of this review, treatment group Rx 1 used as the treatment arm only |
|
| Outcomes | VFSS, Standardised Swallowing Assessment (SSA), surface EMG, Swallowing Quality of Life (SWAL‐QOL) | |
| Notes | Exclusion criteria not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Unclear |
Xia 2016a.
| Methods | Randomisation by random numbered tables Outcomes blinded |
|
| Participants | 1 centre in China
124 participants, timing post stroke unclear but suggests acute based on mean days from onset of stroke
Dysphagia identified by videofluoroscopy and Dysphagia Outcome Severity Scale No significant differences in baseline characteristics between groups |
|
| Interventions | Rx: combined acupuncture with standard swallowing training (n = 62)
C: standard swallowing training only (n = 62) Treatment for 4 weeks |
|
| Outcomes | Primary: Standardized Swallowing Assessment, Dysphagia Outcome Severity Scale Secondary: Modified BI, Swallowing Quality of Life (SWAL‐QOL) |
|
| Notes | Exclusion: presence of serious diseases of the liver, kidney, hematological system, or endocrine system; psychiatric disorders; severe cognitive impairment; severe aphasia; other diseases that potentially impaired swallowing function, such as head and neck tumours, oesophageal neoplasms, craniocerebral injury, myasthenia gravis, and Guillain‐Barre syndrome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participant dropouts from study in total |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Yuan 2003i.
| Methods | Method of randomisation unclear Blinding unclear (traditional liquid diet with swallowing therapy vs control) |
|
| Participants | 1 centre in China 64 participants; timing unclear All dysphagia as defined by Watian Swallow Test | |
| Interventions | R1: enteral nutrition agent with thickener and swallowing therapy (n = 18) R2: traditional liquid diet and swallowing therapy (n = 22). This data set was split (n= 11)* C: liquid diet only and no swallowing therapy (n = 24) (R1 and R2 had NGTs for an uncertain amount of time) *Compared in data set 1 | |
| Outcomes | Length of stay, pneumonia rates, nutritional measures, resolution of dysphagia (swallow test grade) | |
| Notes | Exclusions: terminal illness, organ failure Unclear if any blinding of interventions or outcomes occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Translated from Chinese language |
Yuan 2003ii.
| Methods | (Enteral nutrition agent with thickener and swallowing therapy vs traditional liquid diet and swallowing therapy data set) | |
| Participants | As data set 1 | |
| Interventions | R1: enteral nutrition agent with thickener and swallowing therapy (n = 18) R2: traditional liquid diet and swallowing therapy (n = 22). This data set was split (n = 11) | |
| Outcomes | As data set 1 | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Zheng 2014.
| Methods | Randomisation unclear Blinding unclear |
|
| Participants | 1 centre in China
88 participants; onset of stroke within 2 weeks
Dysphagia identified by water swallow test Baseline characteristics similar |
|
| Interventions | Rx: individualised multi‐disciplinary rehabilitation programme (n = 44)
C: conventional rehabilitation programme (n = 44) Treatment for 4 weeks |
|
| Outcomes | Swallowing function by the water swallow test | |
| Notes | Exclusion: comprehension difficulty, such as Wernicke aphasia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Unclear |
ACE: angiotensin‐converting enzyme BI: Barthel Index BSA: body surface area C: control group CT: computed tomography EMG: electromyography EMST: expiratory muscle strength training EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on five dimensions FDS: Functional Dysphagia Scale FMA: Fugl‐Meyer Assessment Hz: Hertz ITT: intention‐to‐treat analysis LMI: lateral medullary infarction MD: mean difference MEPs: motor evoked potentials MMSE: Mini Mental State Examination MoCA: Montreal Cognitive Assessment MRI: magnetic resonance imaging mRS: modified Rankin Scale NGT: nasogastric tube NIHSS: National Institutes of Health Stroke Scale NMES: neuromuscular electrical stimulation OR: odds ratio PAS: Penetration Aspiration Scale PEG: percutaneous endoscopic gastrostomy PHAD: Paramatta Hospital's Assessment for Dysphagia score Pl: placebo group PTT: pharyngeal transit time RBHOMS: Royal Brisbane Hospital Outcome Measure Scale rTMS: repetitive transcranial magnetic stimulation Rx: treatment group SD: standard deviation sEMG: surface electromyography SLT: speech and language therapy SPSS: Statistical Package for the Social Sciences SSA: Standardised Swallow Assessment SWAL‐QOL: Swallowing Quality of Life Questionnaire tDCS: transcranial direct current stimulation UES: upper oesophageal sphincter VDS: videofluoroscopic dysphagia scale VFSS: videofluoroscopy swallow study
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akamatsu 2009 | RCT assessing transcutaneous electrical stimulation vs control 12 participants with chronic stroke and episodes of choking while eating or drinking Outcome: latency time in swallowing reflex Excluded: no relevant outcome data |
| Aoki 2016 | Study looking at effect of implementing multi‐disciplinary swallowing team approach in lowering the rate of pneumonia (between‐team organisation vs after‐team organisation) Outcomes: rates of pneumonia Excluded: not a true RCT |
| Arai 2003 | RCT Group 1: cabergoline (n = 13) Group 2: amantadine (n = 14) Group 3 : ACE inhibitor (n = 12) Group 4: control Excluded: (1) > 3 months post stroke; (2) definition of aspiration non‐standard; (3) randomisation unclear; (4) insufficient information |
| Beom 2011 | Study comparing conventional dysphagia management (CDM) vs CDM with repetitive electrical stimulation of the suprahyoid muscles Outcomes: swallow score Excluded: not true RCT ‐ non‐concurrent comparative design |
| Beom 2015 | Randomised trial in dysphagic participants with stroke, traumatic brain injury, or brain tumour NMES on suprahyoid (Stimplus) vs NMES on suprahyoid and infrahyoid (VitalStim) Outcomes: swallow scores Excluded: confounded ‐ comparison between 2 treatment groups |
| Byeon 2016 | Randomised trial comparing neuromuscular electrical stimulation vs thermal‐tactile stimulation in subacute stroke patients with dysphagia Outcomes: swallow scores (Functional Dysphagia Scale using VFSS) Excluded: confounded ‐ comparing 2 active treatments |
| Bülow 2008 | RCT assessing neuromuscular electrical stimulation vs traditional swallowing therapy in 25 stroke patients with dysphagia Outcomes: video radiographic swallowing evaluation, nutritional status, oral motor function test, visual analogue scale for self‐evaluation of complaints Excluded: (1) no available outcome data, (2) confounded, comparing 2 direct treatments |
| Cai 2015 | Randomised trial comparing tongue acupuncture vs conventional (neck and wrist) acupuncture in post‐stroke dysphagia patients Outcomes: dysphagia at end of trial, NIHSS, pneumonia Excluded: (1) confounded ‐ both groups received active treatment |
| Chaudhuri 2006 | RCT assessing effectiveness of electric stimulation vs traditional dysphagia therapy in participants with acute stroke (< 6 weeks) Outcomes: American Speech Language Hearing Association National outcome measurement system swallowing level Excluded: no available outcome data |
| Chen 2002 | RCT assessing tongue acupuncture + ice massage + general medical treatment (n = 50) vs general medical treatment (n = 46) in acute dysphagic stroke patients Outcome: dysphagia recovery assessed by videofluoroscopy Excluded: no available outcome data |
| Chen 2003 | RCT assessing electroacupuncture + rehabilitation (n = 34) vs rehabilitation alone (n = 34) in dysphagia patients with pseudobulbar palsy including stroke Treated for 10 days Outcome: dysphagia recovery after stroke Excluded: no available outcome data |
| ChiCTR‐ONC‐17012326 | RCT examining effects of acupuncture and rTMS for acute patients ‐ duration of stroke and dysphagia between 1 and 6 months. Outcomes: VFSS score Excluded: confounded ‐ comparing acupuncture and rTMS |
| ChiCTR‐TRC‐14005233 | RCT comparing validity and safety of telerehabilitation (exercise rehabilitation and myoelectrical feedback) vs conventional rehabilitation in dysphagic patients with ischaemic cerebral stroke Outcomes: Barthel Index assessment; NIHSS assessment; water drinking test assessment; surface electromyography Excluded: confounded ‐ comparing 2 active treatment groups |
| DePippo 1994 | RCT comparing 3 active interventions in 115 dysphagic stroke patients taught compensatory swallowing techniques Group 1: patient/family choice of diet and food consistency (n = 38) Group 2: therapist‐prescribed diet and food consistency (n = 38) Group 3: therapist‐prescribed diet and food consistency, with daily reinforcement of compensatory swallowing techniques (n = 39) Outcomes: pneumonia, dehydration, caloric‐nitrogen deficit, death Excluded: 3 active treatment groups with no control group (confounded) |
| Dou 2012 | Randomised trial comparing effects of active vs passive balloon dilatation therapy on swallowing function in participants with cricopharyngeal dysfunction due to neurological disorders Outcomes: swallow score, changes in upper oesophageal sphincter opening Excluded: confounded ‐ comparison between 2 active treatments |
| Ebihira 2004 | RCT Group 1: theophylline 200 mg once daily Group 2: placebo N = 85 with 'mild to moderate' dysphagia (definition unclear) Outcome: latency of swallow Excluded: (1) nursing home residents (not acute), proportion of stroke patients not stated; (2) > 3 months post stroke |
| Ebihira 2005 | RCT Group1: capsaicin troche 1.5 mcg (n = 34) Group 2: placebo (blinded) (n = 33) for 4 weeks Excluded: (1) 'predominantly' stroke (% not stated) nursing home‐dependent residents; (2) definition of dysphagia unclear; (3) > 3 months post stroke; (4) outcomes: latency of swallow not relevant to review |
| El‐Tamawy 2015 | RCT evaluating effects of a designed physical therapy programme that consists of therapeutic physical exercises in addition to neuromuscular electrical stimulation on severe swallowing disorders (oropharyngeal dysphagia) in people with acute ischaemic cerebrovascular stroke Outcomes: oral transit time, hyoid/laryngeal elevation, oesophageal sphincter opening, incidence of penetration and aspiration Excluded: no available outcome data |
| Fraser 2002 | RCT including 16 acute stroke (< 4 days from ictus) participants with dysphagia TMS vs none Outcome: pharyngeal electromyographic responses Excluded: no relevant outcome data |
| Freed 1996 | Controlled clinical trial comparing 3 active interventions in 112 participants with aspiration Group 1: electrical stimulation Group 2: thermal stimulation Group 3: both ‐ failed thermal stimulation followed by electrical stimulation Outcome: regain oral intake Excluded: (1) dysphagia of mixed aetiology (stroke ?%); (2) not an RCT; (3) 2 active treatment groups with no control group (confounded) |
| Freed 2001 | Quasi‐RCT (alternate assignment) comparing electrical stimulation vs thermal‐tactile stimulation in 110 dysphagic stroke patients Outcome: swallow score Excluded: (1) 2 active treatment groups with no control group (confounded) |
| Hagg 2015 | Prospective comparative study of 2 groups of post‐stroke 4‐quadrant facial dysfunction and dysphagic patients ‐ palatal plate training (2005‐2008) vs training with oral IQoro® (2009‐2012) Outcome: facial activity, swallow function Excluded: (1) not a true RCT, (2) confounded ‐ comparing 2 active treatment protocols |
| Inui 2017 | Quasi‐experimental study to compare the incidence of pneumonia as a dependent variable between before (control) and after (intervention group) intervention with pyriform sinus suctioning as an independent variable Outcomes: incidence of pneumonia Excluded: (1) not an RCT ‐ not randomised |
| ISRCTN18137204 | RCT comparing electrical pharyngeal stimulation vs sham stimulation in severely dysphagic tracheotomised stroke patients Outcomes: intention to decannulate based on FEES performance; feeding status at discharge (dysphagia severity rating scale, functional oral intake scale); mRS; length of stay (ICU/hospital), time from stimulation to discharge Excluded: outcomes not relevant to the review |
| ISRCTN97286108 | RCT assessing dose response of transcranial direct current stimulation for dysphagia after acute stroke Outcome: swallow safety Excluded: trial terminated due to problems in recruitment (according to study author) |
| Jin 2014a | RCT assessing effects of magnetic‐ball sticking therapy at auricular points against acupuncture in 90 participants with chronic post‐stroke dysphagia Outcomes: swallow score (VFSS), PAS, pneumonia, malnutrition Excluded: (1) confounded ‐ all participants received treatment, (2) duration of stroke unknown |
| KCT0001907 | Study looking at effects of NMES according to electrode placement in stroke patients with dysphagia Outcomes: videofluoroscopic dysphagia scale; PAS; functional oral intake scale Excluded: (1) confounded (comparing electrode placement on suprahyoid vs infrahyoid), (2) time post onset unclear |
| Kikuchi 2014 | Double‐blind RCT on participants > 65 years old with stroke and dysphagia from 2 hospitals and 2 nursing homes in Sendai, Japan Group 1: press needles (Pyonex; Seirin Corporation, Shizuoka, Japan) at 2 points on the legs (ST36 and KI3) Group 2: sham patches on acupuncture points Group 3: press needles on sham points Excluded: no relevant outcomes |
| Kobayashi 1996 | Randomised crossover trial assessing levodopa in 27 participants with basal ganglia infarction and 20 healthy volunteers Outcomes: swallowing latency Excluded: (1) cross‐over trial, (2) outcomes (swallowing latency) not relevant to this review, (3) < 50% stroke |
| Kulnik 2015 | Single‐blind RCT in acute stroke patients Expiratory training vs inspiratory training vs sham training Outcomes: peak expiratory cough flow of maximal voluntary cough, pneumonia Excluded: most participants do not have clinical dysphagia |
| Kushner 2013 | Case‐control study comparing the efficacy of NMES in addition to traditional dysphagia therapy including progressive resistance training vs that of traditional dysphagia therapy/progressive resistance training alone in participants with acute post‐stroke dysphagia Outcomes: swallow score, dysphagia at end of trial Excluded: non‐randomised trial |
| Lan 2013 | Single‐blind clinical intervention trial comparing biomechanical properties of swallowing in brainstem stroke patients with dysphagia following modified balloon dilation therapy vs regular dysphagia therapy Outcomes: Functional Oral Intake Scale, pharyngeal maximum pressures and duration, and upper oesophageal sphincter residual pressure and duration during swallowing were measured using high‐resolution manometry Excluded: non‐randomised trial |
| Logemann 2009 | RCT assessing traditional swallowing therapy or the Shaker exercise in participants with prolonged oropharyngeal dysphagia and aspiration Outcomes: occurrence of aspiration (preswallow, intraswallow, postswallow) at 6‐week follow‐up period; occurrence of residue in the oral cavity, valleculae, or pyriform sinuses; Performance Status Scale for Diet Excluded: (1) head and neck cancer and stroke (< 50%); (2) no relevant outcome data |
| Ma 2014 | Randomised trial comparing acupoint injection, neural electrical stimulation, combination of both and swallowing training Outcomes: swallow function using water swallow test Excluded: confounded ‐ comparing 3 active treatments |
| Ma 2015 | Randomised trial comparing effects of acupuncture and neck‐skin electrical stimulation on dysphagia in participants with cerebral infarction Outcomes: swallow function using water swallow test and food‐intake scale Excluded: confounded ‐ comparing 2 active treatments |
| Maeda 2017 | RCT 43 participants who were prescribed in‐hospital dysphagia rehabilitation (most with history of stroke) Sensory stimulation vs sham stimulation Outcomes: cough latency times, functional oral intake scale scores, oral nutritional intake Excluded: (1) majority of participants without stroke (48.8% stroke participants), (2) timing of stroke unclear |
| Mao 2016 | Non‐randomised interventional study Standard swallowing training vs standard swallowing training with acupuncture All participants with post‐stroke dysphagia Excluded: not an RCT ‐ not randomised |
| McCullough 2012 | Cross‐over study investigating effects of intensive exercise using Mendelsohn manoeuvre on swallowing movement All 18 participants with stroke and dysphagia Outcomes: videofluoroscopic swallow assessment, swallow score Excluded: (1) not a true RCT ‐ cross‐over design, (2) majority of participants chronic |
| McCullough 2013 | Cross‐over study assessing effect of Mendelsohn manoeuvre on hyoid movement All 18 participants with post‐stroke dysphagia Outcomes: assessment of hyoid movements, upper oesophageal sphincter opening Excluded: (1) not a true RCT ‐ cross‐over design, (2) no relevant outcomes |
| Mepani 2009 | RCT comparing traditional swallowing therapy vs Shaker exercise in 6 stroke and 5 cancer patients Outcome: deglutitive thyrohyoid shortening before and after completion of assigned therapy regimen Excluded: (1) no time of onset for stroke patients, (2) no separate results for stroke, (3) no relevant outcome data |
| Messaggi‐Sartor 2015 | RCT comparing effects of short‐term inspiratory and expiratory muscle training on respiratory muscle strength in subacute stroke patients Outcomes: respiratory muscle strength (maximum inspiratory and expiratory pressures) Excluded: (1) outcomes not relevant to review, (2) not all participants had dysphagia |
| Michou 2010 | RCT comparing transcranial magnetic stimulation vs sham stimulation in 12 stoke participants with dysphagia Outcome: pharyngeal electromyographic responses Excluded: no relevant outcome data |
| Michou 2011 | RCT comparing transcranial magnetic stimulation vs pharyngeal electrical stimulation vs paired associative stimulation vs sham stimulation in 14 dysphagic stroke participants Outcome: videofluoroscopic swallowing assessments Excluded: no available outcome data |
| Nakamura 2013 | Cross‐over study assessing the effect of ice massage in triggering the swallow reflex Outcomes: videofluoroscopic assessment of swallowing Excluded: not a true RCT ‐ cross‐over design |
| Nakayama 1998 | RCT comparing 5 mg imidapril or placebo in randomised, double‐blind, cross‐over design. Participants were normotensive patients with at least 1 episode of aspiration and healthy volunteers Outcome: swallowing reflex Excluded: no relevant outcome data |
| Nam 2012 | Randomised trial comparing 2 neuromuscular stimulation techniques (VitalStim vs Stimplus DP 200) Outcomes: swallow function using videofluoroscopic swallowing studies Excluded: confounded ‐ comparison of 2 treatment groups |
| NCT00376506a | Implanted neuroprosthesis (neuro control implantable receiver‐stimulator) to stimulate the laryngeal nerve vs sensory training in dysphagic participants including stroke > 6 months post onset Excluded: (1) no control group, 2 active groups compared, (2) no outcome data |
| NCT00376506b | RCT assessing intramuscular stimulation device implanted in the neck vs vibrotactile stimulation of the throat in 20 participants with dysphagia secondary to stroke or chronic neurological disease Outcome: swallowing safety for 10 mL of thin liquid and 5 mL of pudding with and without stimulation Excluded: comparing 2 active treatments vs no control (confounded) |
| NCT01971320 | Single‐blind RCT comparing active vs fake Urostim I stimulation in hemispheric stroke patients with oropharyngeal dysphagia Outcomes: evaluation of oropharyngeal dysphagia symptoms Excluded: no outcome data as trial terminated due to lack of recruitment |
| Nishiyama 2010 | RCT comparing nicergoline (15 mg tds) vs control in 50 ischaemic stroke patients Outcome: substance P level Excluded: no relevant outcome data |
| Ortega 2016 | RCT comparing 2 x 10‐day treatment groups (transient receptor potential vanilloid 1 agonist vs transcutaneous sensory electrical stimulation) Outcomes: swallow function (videofluoroscopic), dysphagia at end of trial Excluded: (1) < 50% participants with stroke ‐ duration unknown, (2) confounded ‐ comparing 2 active treatments |
| Permsirivanich 2009 | RCT Group 1: NMES (n = 12) Group 2: rehabilitation swallowing therapy (n = 11) All stroke Excluded: confounded, i.e. comparison of 2 active treatments |
| Pownall 2008 | RCT assessing thickened fluids vs postural and/or swallowing strategies in 50 participants with post‐stroke dysphagia: a further group of participants who were not dysphagic for liquids and who were given normal fluids compared with RCT Outcome: development of chest infection and dehydration Excluded: no control group ‐ 2 interventional groups were compared in the RCT |
| Pryor 2011 | RCT comparing NMSE vs vibrotactile stimulation in dysphagic participants Outcomes: swallow function, PAS Excluded: (1) mixed patient population, (2) confounded ‐ comparison of 2 active interventions |
| Reidnauer 2006 | RCT comparing vital stimulation (and electrotherapy intervention) vs traditional treatment in post‐stroke participants with dysphagia Outcomes: swallow scores Excluded: no available outcome data |
| Rofes 2014 | Double‐blind RCT comparing effects of 2 doses of piperine (dual TRPV1/TRPA1 agonist) on the swallow response of dysphagic participants Participants were randomised into 2 groups: 1 group received 150 lM piperine and the other group received 1 mM Outcome: PAS, swallowing analysis with videofluoroscopic images Excluded: dose‐response trial ‐ all groups received treatment (either low or high dose of piperine) |
| Rosenbek 1991 | Randomised cross‐over trial assessing thermal stimulation in 7 male dysphagic participants with multiple previous strokes Outcome: duration of stage transition Excluded: (1) cross‐over trial, (2) most participants recruited > 3 months after stroke onset, (3) randomisation status unclear |
| Rosenbek 1996 | Randomised cross‐over trial assessing thermal stimulation in 23 dysphagic participants with multiple previous strokes Outcome: duration of stage transition, total swallow duration Excluded: (1) cross‐over trial, (2) 14 participants recruited > 3 months after stroke onset |
| Rosenbek 1998 | Dose comparison RCT of thermal stimulation (150, 300, 450, 600 trials per week) in 45 dysphagic stroke participants recruited within 12 weeks Outcome: number of trials delivered, treatment time, duration of stage transition, aspiration (PAS) Excluded: no control group |
| Sdravou 2012 | Interventional study comparing effects of carbonated thin liquids vs non‐carbonated thin liquids on oropharyngeal swallowing in adults with neurogenic dysphagia Outcomes: oral transit time, pharyngeal transit time, PAS Excluded: (1) non‐RCT, (2) many participants with chronic stroke (> 6 months) |
| Seki 2005 | Randomised trial Group 1: acupuncture (n = 18) Group 2: no intervention (n = 14) Excluded: (1) incomplete outcome data, (2) time from stroke unclear |
| Shaker 2002a | RCT comparing head‐raising exercise vs sham exercise in 27 dysphagic participants Outcomes: upper oesophageal sphincter function, functional swallow status Excluded: (1) dysphagia of mixed aetiology (cerebrovascular disease 56%), (2) most participants recruited > 3 months after stroke onset, (3) individual patient data unavailable, so not possible to analyse subgroup of appropriate participants |
| She 2014 | RCT comparing acupuncture in 8 neck‐occiput points vs meridian points Outcomes: speech and swallowing dysfunction at end of trial Excluded: (1) confounded ‐ comparing 2 different treatment groups |
| SQACU01 2001 | RCT comparing acupuncture vs sham acupuncture for 16 sessions in participants with dysphagia due to recent stroke Outcomes: tube feeding, pneumonia, mortality, each at 6 months Excluded: no outcome data |
| Steele 2016 | RCT comparing 2 treatment protocols: tongue pressure profile training or tongue pressure strength‐and‐accuracy training Outcomes: swallow function Excluded: confounded ‐ comparison between 2 treatment protocols |
| Sukthankar 1994 | RCT assessing swallowing therapy (biofeedback) in 9 participants with dysphagia secondary to stroke or head injury
Group 1: regular therapy (n = 4)
Group 2: regular therapy and oral exercises (n = 2)
Group 3: regular therapy and oral exercises with visual and audio biofeedback (n = 3) Excluded: (1) dysphagia of mixed aetiology, (2) outcome measures (tongue and lip motor force) not relevant to this review |
| Suntrup 2015 | RCT comparing electrical pharyngeal stimulation vs sham stimulation (control) in severely dysphagic tracheotomised stroke participants Outcomes: ability to decannulate based on FEES performance; feeding status at discharge (FOIS); mRS; length of stay (ICU/hospital) and time from stimulation to discharge Excluded: outcomes (decannulation) not relevant to review (only data regarding decannulation available before trial unblinded) |
| Suzuki 2012 | Randomised trial investigating the relationship between body position during nasogastric feed and aspiration pneumonia in acute stroke participants Outcomes: aspiration pneumonia rates Excluded: pseudo‐randomised study; assessment of body position |
| Tai 2014 | Quasi‐experimental trial to investigate effectiveness of the chin‐down swallowing technique in improvement of dysphagia in stroke participants Outcomes: Dysphasia Assessment Scale and Swallow Self‐assessment Excluded: not an RCT ‐ not randomised |
| Teramoto 2008 | RCT assessing swallowing function using cilostazol vs placebo in 48 participants with dysphagia secondary to stroke Outcome: swallowing function Excluded: (1) onset of stroke to randomisation, 1 to 6 months, (2) cross‐over study, no access to data on the first phase |
| Terre 2012 | Randomised, alternating, cross‐over study assessing effectiveness of chin‐down posture in preventing aspiration in participants with neurogenic dysphagia secondary to acquired brain injury Outcomes: aspiration prevention Excluded: (1) pseudo‐randomised study, (2) assessment of posture |
| Toyama 2014 | Non‐randomised interventional study comparing NMES and conventional treatment vs conventional treatment only Outcomes: swallow scores (VDS, FOIS), hyoid and laryngeal displacement Excluded: not an RCT ‐ not randomised |
| Ueda 2004 | 21 participants Group 1: functional swallowing training (n = 11) Group 2: oral care (n = 11) in nursing home residents (% stroke unknown) who are tube fed Excluded: (1) < 50% stroke, (2) non‐acute, (3) randomisation unclear |
| Varma 2006 | Group 1: motor control programme (n = 30) Group 2: home exercise programme (n = 30) Randomisation method unclear Excluded: (1) insufficient data, (2) outcome methods unclear |
| Wang 2016 | Randomised interventional trial comparing differences in effects between awn‐like needle at Tiantu (CV 22) and filiform needle for dysphagia after cerebral infarction Outcomes: standard swallowing assessment scale and modified Bathel index Exlcuded: confounded ‐ comparing 2 different treatment groups |
| Xia 2016 | RCT with 130 participants with post‐stroke dysphagia In treatment group, acupuncture based on meridian differentiation was adopted. The main acupoints were Neiguan (PC 6), Shuigou (GV 26), Sanyinjiao (SP 6), Fengchi (GB 20), Lianquan (CV 23), Jialianquan (Extra), Jinjin (EX‐HN 12), Yuye (EX‐HN 13), etc. Control group: points were selected 5 cm lateral to the acupoints used in the observation groups and stimulated with shallow puncture Outcomes: standardised swallowing assessment, VFSS, modified Barthel Index and swallowing‐related quality of life (SWAL‐QOL) Excluded: confounded ‐ comparing 2 treatments |
| Zhang 2011 | RCT comparing different depth of Chonggu (EX‐HN 27) by electroacupuncture in participants with dysphagia after stroke Chonggu (EX‐HN 27) deep insertion group (n = 99) Chonggu (EX‐HN 27) shallow insertion group (n = 94) Traditional acupuncture group (n = 90) Outcomes: Kubota's Water Drinking Test Scale, standard swallowing function scale, and TCM Scale of Dysphagia After Stroke Excluded: no available outcome data |
| Zhang 2018a | RCT comparing effects of electroacupuncture with different frequencies in participants with dysphagia after stroke Low‐frequency (2 Hz) electroacupuncture group vs high‐frequency (100 Hz) electroacupuncture group Outcomes: VFSS, standardised swallowing assessment Excluded: not an RCT ‐ dose‐response study (no control group) |
| Zhang 2018b | Randomised interventional trial to assess clinical improvement of nursing intervention in swallowing dysfunction of elderly stroke participants Conventional nursing service vs nursing interventions (psychological intervention, health education, rehabilitation exercises, diet intervention) Outcomes: dysphagia at end of trial, functional outcomes (GQOL‐74) Excluded: confounded ‐ comparing 2 different treatment groups |
| Zhao 2015 | Randomised trial of participants with stroke and swallowing disorders Group A: normal acupuncture Group B: NMES combined with acupuncture with uniform reinforcing‐reducing manipulation as well as the piercing and blood‐letting method Outcomes: Kubota water test, dysphagia at end of trial Excluded: confounded ‐ comparison between 2 treatment groups |
ACE: angiotensin‐converting enzyme CDM: conventional dysphagia management CXR: chest x‐ray FEES: Fibreoptic Endoscopic Evaluation of Swallowing FIM: Functional Independence Measure FOIS: Functional Oral Intake Scale GQOL‐74: Generic Quality of Life Inventory ICU: intensive care unit IOro®: Orofacial device mRS: modified Rankin Scale NGT: nasogastric tube NIHSS: National Institutes of Health Stroke Scale NMES: neuromuscular electrical stimulation PEG: percutaneous endoscopic gastrostomy RCT: randomised controlled trial rTMS: repetitive transcranial magnetic stimulation SAH: subarachnoid haemorrhage SWAL‐QOL: Swallowing Quality of Life Questionnaire TCM: Traditional Chinese Medicine TMS: transcranial magnetic stimulation VDS: videofluoroscopic dysphagia scale VFSS: videofluoroscopy swallow study
Characteristics of studies awaiting assessment [ordered by study ID]
Azimov 2017.
| Methods | RCT although randomisation method unclear |
| Participants | 34 participants with ischaemic stroke and dysphagia at onset 2 to 7 points of PAS Scale |
| Interventions | Experimental group: amantadine (200 mg/d) and levodopa (125 mg/d) after standard treatment (n = 17) Control group: standard treatment, including citicoline and anticholinesterase (n = 17) |
| Outcomes | PAS divided into group PAS score 2 to 4 and group PAS score 5 to 7; recheck after 2 months |
| Notes | Study completed; awaiting full published data |
Carnaby 2012.
| Methods | RCT |
| Participants | 53 stroke participants from a subacute rehabilitation facility |
| Interventions | Group 1: usual care Group 2: McNeill Dysphagia Therapy plus sham NMES Group 3: McNeill Dysphagia Therapy plus active NMES |
| Outcomes | Increase of 10 or more points on the Mann Assessment of Swallowing and improvement of 2 or more scale points on the Functional Oral Intake Scale, without significant weight loss or complication |
| Notes | In the process of retrieving full‐text article and data |
Chang 2014.
| Methods | RCT |
| Participants | 74 participants with dysphagia after stroke |
| Interventions | Functional electrical stimulation vs a combination of electrical stimulation and acupuncture |
| Outcomes | Swallow score, removal rate of nasogastric tube |
| Notes | In the process of retrieving full‐text article |
Chaudhuri 2008.
| Methods | RCT |
| Participants | People with stroke and dysphagia |
| Interventions | Traditional dysphagia treatment vs combined neuromuscular electrical stimulation and traditional treatment |
| Outcomes | Swallow score (ASHA NOMS) |
| Notes | Awaiting published data (full text) |
Chen 2017.
| Methods | RCT |
| Participants | People with dysphagia due to stroke (onset 2 to 7 days) |
| Interventions | Levetiracetam (Keppra) vs carbidopa/levodopa (Sinemet) vs placebo |
| Outcomes | Qualitative and quantitative swallow function |
| Notes | Study published; in the process of extracting data |
Cheng 2005.
| Methods | RCT |
| Participants | People with Ischaemic stroke with pseudobulbar palsy |
| Interventions | Early throat muscle training vs control |
| Outcomes | Effects on vertebral and basilar artery blood flow |
| Notes | In the process of retrieving full‐text article |
Cheng 2014.
| Methods | RCT |
| Participants | 180 participants with post‐stroke dysphagia |
| Interventions | Group 1 (Acupuncture A): acupuncture at Lianquan (CV 23) Group 2 (Acupuncture B): acupuncture at Hegu (LI 4) and Neiguan (PC 6) Group 3 (Control): rehabilitation group |
| Outcomes | NIHSS scores, VFSS scale, pneumonia, clinical efficacy |
| Notes | In the process of retrieving full‐text article |
ChiCTR‐TRC‐07000010.
| Methods | RCT |
| Participants | People with dysphagia in the convalescence phase of stroke (2 and 6 months) |
| Interventions | Combination of body acupuncture, scalp acupuncture, and electroacupuncture vs routine rehabilitation training |
| Outcomes | Safety and tolerability of acupuncture |
| Notes | Study completed; awaiting published data |
ChiCTR‐TRC‐08000463.
| Methods | RCT |
| Participants | People with stroke 2 to 60 days from onset |
| Interventions | Dysphagia therapeutic apparatus on acupoints vs regular dysphagia rehabilitation vs both |
| Outcomes | Swallowing function and mastication function |
| Notes | Study completed; awaiting published data |
ChiCTR‐TRC‐14004235.
| Methods | RCT |
| Participants | People with dysphagia symptoms appearing within 1 to 6 months after stroke |
| Interventions | Modified Dihuang Yinzi Decoction (herb treatment group) vs control |
| Outcomes | Swallowing rehabilitation improvement diagnosed by videofluoroscopy, adverse events |
| Notes | Study completed; awaiting published data |
ChiCTR‐TRC‐14004955.
| Methods | Randomised parallel controlled trial |
| Participants | 60 people with stroke; onset of stroke at least 2 times but occurrence of stroke at least 1 month before admission |
| Interventions | Manipulation + sham tDCS Manipulation + tDCS |
| Outcomes | Lingual movement; buccofacial apraxia; Modified Assessment of Swallowing Ability; VFSS; EEG non‐linear analysis |
| Notes | Study likely completed; website not updated; awaiting published data |
Choi 2017.
| Methods | RCT |
| Participants | Stroke survivors with dysphagia |
| Interventions | Experimental group: Shaker exercise + conventional therapy (n = 16) Control group: conventional therapy (n = 16) |
| Outcomes | PAS and oral diet level |
| Notes | In the process of retrieving full‐text article |
Chu 2017.
| Methods | RCT |
| Participants | Dysphagia patients with pseudobulbar palsy |
| Interventions | Basic treatment vs GAO neck acupuncture at Fengchi (GB 20), Yiming (EX‐HN 14), Gongxue (Extra), Lianquan (CV 23), Wai Jinjin Yuye (Extra), Tunyan (Extra), Zhiqiang (Extra), Fayin (Extra) with basic treatment |
| Outcomes | Repetitive saliva‐swallowing test, standardised swallowing assessment, swallow quality‐of‐life questionnaire |
| Notes | In the process of retrieving full‐text article |
de Fraga 2017.
| Methods | RCT |
| Participants | 10 participants with ischaemic stroke and speech therapy‐diagnosed oropharyngeal dysphagia |
| Interventions | Rx: myofunctional therapy plus voice therapy C: myofunctional therapy only |
| Outcomes | Swallow function |
| Notes | Study published; in the process of extracting data |
Eom 2017.
| Methods | RCT |
| Participants | Stroke patients with oropharyngeal dysphagia |
| Interventions | Resistance expiratory muscle strength training vs sham expiratory muscle strength training |
| Outcomes | Videofluoroscopic dysphagia scale, PAS |
| Notes | In the process of retrieving full‐text article |
Erfmann 2017.
| Methods | RCT |
| Participants | Subacute stroke patients with oropharyngeal dysphagia |
| Interventions | Expiratory muscle strength training; no further details available |
| Outcomes | No further details available at the time |
| Notes | In the process of retrieving text |
Fan 2007.
| Methods | RCT |
| Participants | 60 post‐stroke patients with dysphagia |
| Interventions | Experimental group: acupuncture plus Western drugs Control group: Western drugs |
| Outcomes | Swallowing test |
| Notes | In the process of retrieving full‐text article |
Feng 2016.
| Methods | RCT |
| Participants | 60 cases of post‐stroke dysphagia |
| Interventions | Rx: deep acupuncture at Lianquan (CV 23) and Yifeng (TE 17) with swallowing training C: swallowing training only |
| Outcomes | VFSS dysphagia evaluation scale and Watian water swallow test |
| Notes | In the process of retrieving full‐text article |
Gao 2016.
| Methods | RCT |
| Participants | 90 patients with dysphagia after cerebral infarction |
| Interventions | Chin tuck resistance vs Shaker exercise vs control |
| Outcomes | VFSS, Self‐Rating Depression Scale, PAS |
| Notes | In the process of retrieving full‐text article |
Guillen‐Sola 2017.
| Methods | RCT |
| Participants | Subacute ischaemic stroke (1 to 3 weeks) and dysphagia confirmed by videofluoroscopic study with a score ≥ 3 on the 8‐point PAS |
| Interventions | Group I: standard swallow therapy Group II: inspiratory and expiratory muscle training + standard swallow therapy Group III: neuromuscular electrical stimulation of suprahyoid muscles, sham inspiratory and expiratory muscle training, and standard swallow therapy |
| Outcomes | Respiratory muscle function (baseline, 3 weeks, and 3 months), severity of dysphagia (PAS) (baseline and 3 months), and occurrence of respiratory complications (chest x‐ray, fever); also volume‐viscosity swallow test (V‐VST), Functional Oral Intake Scale, and Dysphagia Outcome and Severity Scale (baseline, 3 weeks, and 3 months) |
| Notes | Study published; in the process of extracting data |
Hamada 2017.
| Methods | Study design not clear |
| Participants | 56 people with acute stroke and dysphagia |
| Interventions | General dysphagia therapy vs combination of surface electrical stimulation and general dysphagia therapy |
| Outcomes | Pulmonary infection |
| Notes | In the process of retrieving full‐text article |
Hong 2011.
| Methods | RCT |
| Participants | People with cerebral apoplexy and dysphagia |
| Interventions | Strengthened diet nursing vs control |
| Outcomes | Incidence of aspiration, malnutrition, dehydration |
| Notes | In the process of retrieving full‐text article |
Huang 2008.
| Methods | RCT |
| Participants | 66 participants with dysphagia post‐ischaemic stroke |
| Interventions | Group 1: electro‐acupuncture group Group 2: rehabilitation training combined with acupoint percutaneous electrical stimulation Group 3: rehabilitation training combined with acupoint token puncturing |
| Outcomes | Quality of life scale specified for dysphagia (name not stated) |
| Notes | In process of retrieving full‐text article |
Huang 2014.
| Methods | RCT |
| Participants | People with acute stroke and dysphagia |
| Interventions | Traditional swallowing vs oropharyngeal NMES vs combined NMES/traditional swallowing |
| Outcomes | Swallow score, PAS, VFSS |
| Notes | In process of retrieving relevant outcome data |
Huimin 2015.
| Methods | RCT |
| Participants | 76 people with pharyngeal dysphagia after stroke |
| Interventions | Surface electromyographic biofeedback with conventional therapy vs conventional therapy only |
| Outcomes | Degree of openness of upper oesophageal sphincter, pharyngeal transit time, maximum displacement of the hyoid bone |
| Notes | In the process of retrieving full‐text article |
Jefferson 2008.
| Methods | RCT |
| Participants | People with chronic stroke and dysphagia |
| Interventions | Repetitive transcranial magnetic stimulation vs sham stimulation over the unaffected pharyngeal motor cortex |
| Outcomes | Measurements of cortico‐pharyngeal excitability |
| Notes | In the process of retrieving full‐text article |
Ji‐Ye 2017.
| Methods | RCT |
| Participants | Dysphagia patients with ischaemic stroke and pseudobulbar palsy |
| Interventions | Oral aspirin vs acupuncture (XNJ‐AI at Fengchi (GB 20)) with oral aspirin |
| Outcomes | Water‐swallowing test, plasma thromboxane B2 and 6‐keto‐prostaglandin F1a levels |
| Notes | In the process of retrieving full‐text article |
Jia 2006.
| Methods | RCT |
| Participants | 40 cases of post‐apoplectic dysphagia with 2 out of 5 symptoms such as hemiplegia, coma, slurred speech, unilateral sensory disturbance, dry mouth and tongue, difficulty in swallowing |
| Interventions | Treatment group was treated by acupuncturing points Fengchi (GB 20), Tianzhu (BL 10), Tongli (HT 5), and Lianquan (CV 23) plus rehabilitation exercises Control group only by rehabilitation exercise |
| Outcomes | Therapeutic effect assessed by 1 to 10 point scale |
| Notes | Study published; in the process of extracting data |
Jiang 2014.
| Methods | RCT |
| Participants | People with stroke and dysphagia |
| Interventions | Electroacupuncture group vs VitalStim group vs combined group |
| Outcomes | Water swallow test, swallow score |
| Notes | In the process of retrieving full‐text article |
Jing 2016.
| Methods | RCT |
| Participants | 60 people with dysphagia after stroke |
| Interventions | NMES with conventional therapy vs conventional therapy only |
| Outcomes | Curative effects, swallowing function, aspiration, laryngeal elevation, food residue, food intake scores |
| Notes | In the process of retrieving full‐text article |
Kim 2017.
| Methods | RCT |
| Participants | People with post‐stroke oropharyngeal dysphagia confirmed by VFSS |
| Interventions | Tongue‐to‐palate resistance training vs control |
| Outcomes | Swallowing function ‐ videofluoroscopic dysphagia scale and PAS |
| Notes | Study published; in the process of extracting data |
Koch 2015.
| Methods | RCT |
| Participants | People with stroke and dysphagia |
| Interventions | Swallowing training using surface electromyography as biofeedback vs standard treatment |
| Outcomes | Swallow score |
| Notes | In the process of retrieving full‐text article |
Konecny 2018.
| Methods | RCT |
| Participants | 54 people with early‐stage stroke and dysphagia |
| Interventions | Transcutaneous electrical nerve stimulation of suprahyoid muscles vs control |
| Outcomes | Swallow function ‐ videofluoroscopic study, oral transit time, pharyngeal transit time |
| Notes | Study published; in the process of extracting data |
Koyama 2017.
| Methods | RCT |
| Participants | 16 participants with stroke‐related dysphagia |
| Interventions | Modified jaw opening exercise vs control |
| Outcomes | Swallow function ‐ videofluorographic swallowing study, distance between the mental spine and the hyoid bone, hyoid displacement |
| Notes | Study published; in the process of extracting data |
Lee 2015b.
| Methods | RCT |
| Participants | 24 people with dysphagia after ischaemic stroke |
| Interventions | Treatment: 10 Hz rTMS over the brain cortex where motor evoked potential was obtained from the suprahyoid muscle Control: 10 Hz rTMS over the brain cortex where motor evoked potential was obtained from the abductor pollicis brevis muscle |
| Outcomes | Functional Dysphagia Scale, PAS, Dysphagia Outcome and Severity Scale |
| Notes | Study published; in the process of extracting data |
Li 2008.
| Methods | RCT |
| Participants | 60 people with ischaemic stroke and dysphagia |
| Interventions | Group 1: acupuncture group and routine treatment and rehabilitation training Group 2: routine treatment and rehabilitation training |
| Outcomes | Not stated |
| Notes | In the process of retrieving full‐text article |
Li 2009.
| Methods | RCT |
| Participants | 60 people post stroke with dysphagia |
| Interventions | Experimental group: acupuncture plus feeding and swallowing rehabilitation training Control group: swallowing and feeding rehabilitation training |
| Outcomes | Swallowing test |
| Notes | In the process of retrieving full‐text article |
Li 2016.
| Methods | RCT |
| Participants | 60 people with pseudobulbar palsy paralysis dysphagia |
| Interventions | Treatment: 5 needles of the Nape acupuncture Control: routine acupuncture (Lian Quan, Tong Li, Zhao Hai) |
| Outcomes | Curative effect dysphagia (unclear) |
| Notes | In the process of retrieving full‐text article |
Liu 2018.
| Methods | RCT |
| Participants | 100 people with dysphagia caused by pseudobulbar palsy |
| Interventions | Nape acupuncture with rehabilitative swallowing training vs rehabilitative swallowing training only |
| Outcomes | Repetitive saliva‐swallowing test, water swallow test, standardised swallowing assessment, swallow quality‐of‐life questionnaire (SWAL‐QOL) |
| Notes | In the process of retrieving full‐text article |
Ma 2016.
| Methods | RCT |
| Participants | 80 people with dysphagia and pseudobulbar palsy |
| Interventions | Quick needle insertion at Aqiang point vs routine acupuncture at Lianquan (CV 23) |
| Outcomes | Water swallow test, curative rate |
| Notes | In the process of retrieving full‐text article |
Malik 2017.
| Methods | RCT |
| Participants | People with dysphagia (95% of patients with stroke aetiology) |
| Interventions | Thermal stimulation vs swallowing manoeuvres vs combination of both |
| Outcomes | Function Outcome Swallowing Scale |
| Notes | Study published; in the process of extracting data |
Mehndiratta 2017.
| Methods | RCT |
| Participants | 98 people with dysphagia within the first month after ischaemic stroke |
| Interventions | Sensory‐level electrical stimulation to bilateral masseter muscles vs sham stimulation |
| Outcomes | Bedside Dysphagia Score, Neurological Examination Dysphagia Score, Total Dysphagia Score, Mann Assessment of Swallowing Ability test, flexible fibreoptic endoscopic evaluation of swallowing |
| Notes | Study published; in the process of extracting data |
Meng 2015.
| Methods | RCT |
| Participants | 251 people with dysphagia after stroke |
| Interventions | Group 1: deep acupuncture with conventional glossopharyngeum acupuncture Group 2: shallow acupuncture with conventional glossopharyngeum acupuncture Group 3: conventional glossopharyngeum acupuncture only (control) |
| Outcomes | Water swallowing test evaluation scale |
| Notes | In the process of retrieving full‐text article |
Meng 2018.
| Methods | RCT |
| Participants | 30 people with post‐stroke dysphagia |
| Interventions | 2 groups given surface NMES at different sites of patients' neck vs control |
| Outcomes | Water swallow test, repetitive saliva swallowing test, dysphagia outcome and severity scale |
| Notes | In the process of retrieving full‐text article |
Moon 2017.
| Methods | RCT |
| Participants | 18 people with stroke and dysphagia |
| Interventions | Expiratory muscle strength training vs control |
| Outcomes | Functional dysphagia scale, PAS, vallecular residue, pyriform sinuses residue |
| Notes | Study published; in the process of extracting data |
Moon 2018.
| Methods | RCT |
| Participants | 16 people with subacute stroke and dysphagia |
| Interventions | Tongue pressure strength and accuracy training vs control |
| Outcomes | Maximum isometric tongue pressures of the anterior and posterior tongue, Mann Assessment of Swallowing Ability, Swallowing‐Quality of Life |
| Notes | In the process of retrieving full‐text article |
NCT00722111.
| Methods | Randomised, open label |
| Participants | 200 people post stroke |
| Interventions | Group 1: lingual press (high‐intensity, oral, non‐swallowing) Group 2: effortful swallowing (high‐intensity swallowing) Group 3: natural swallowing (high‐frequency, low‐intensity swallowing) Group 4: non‐oral sham (control) exercise |
| Outcomes | Composite score of PAS and Residue Scale with no worsening of either at baseline, week 4, and week 8 |
| Notes | Study completed; awaiting published data |
NCT01081444.
| Methods | RCT |
| Participants | People with dysphagia and first episode of stroke |
| Interventions | Active vs sham rTMS |
| Outcomes | Videofluoroscopy and high‐resolution manometry |
| Notes | Study completed; awaiting published data |
NCT01085903.
| Methods | Randomised, double‐blind (participant, investigator), cross‐over assignment |
| Participants | People with stroke, neglect, dysphagia |
| Interventions | Modafinil 200 mg once daily vs placebo for 3 days |
| Outcomes | Predicting response to modafinil among participants with neglect, dysphagia |
| Notes | Study completed; awaiting published data |
NCT01777672.
| Methods | RCT |
| Participants | 100 people with oropharyngeal dysphagia due to stroke episode within last 3 months |
| Interventions | Control group: recommendations from patient healthcare providers Experimental group 1: oral TRPV1 (natural capsaicin) plus recommendations from patient healthcare providers Experimental group 2: pharyngeal electrical stimulation plus recommendations from patient healthcare providers Experimental group 3: transcutaneous electrical stimulation plus recommendations from patient healthcare providers |
| Outcomes | VFSS‐PAS, oropharyngeal reconfiguration, timing and extent of hyoid motion, bolus propulsion force of tongue Episodes of aspiration pneumonia and lower respiratory tract infection Clinical outcomes of nutritional status, complications and clinical symptoms, mortality rates, cause of death |
| Notes | Study completed; awaiting published data |
NCT02090231.
| Methods | RCT |
| Participants | Post‐stroke dysphagia more than 3 months |
| Interventions | Real 5 Hz rTMS vs sham 5 Hz rTMS |
| Outcomes | Dysphagia severity, swallow function |
| Notes | Study completed; awaiting published data |
NCT02379182.
| Methods | RCT |
| Participants | 90 people with stroke > 3 months |
| Interventions | Control group: standard clinical care Sensory group: transcutaneous electrical stimulation at sensory level Motor group: transcutaneous electrical stimulation at motor level |
| Outcomes | PAS; incidence of all adverse events; change in pharyngeal residue prevalence; change in Eating Assessment Tool‐10 scores; frequency of chest infection; time from randomisation to death |
| Notes | Study completed; awaiting published data |
Nowicki 2003.
| Methods | RCT |
| Participants | People with stroke and dysphagia |
| Interventions | Manual + electro‐acupuncture (6 to 8 treatments 2 to 3 times per week for 3 weeks) vs control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
Oshima 2009.
| Methods | Unclear design (not stated in abstract) |
| Participants | 218 people with stroke complicated by dysphagia |
| Interventions | Group 1: swallowing training with nutritional and high‐risk management Group 2: control (none of the above) |
| Outcomes | Time taken to oral intake, nutritional status, incidence rate of infection, activities of daily living |
| Notes | In the process of retrieving full‐text article |
Pan 2015.
| Methods | RCT |
| Participants | 70 people with post‐stroke dysphagia |
| Interventions | Acupoint massage vs control |
| Outcomes | Improvement rate in swallow function |
| Notes | In the process of retrieving full‐text article |
Park 2017.
| Methods | RCT |
| Participants | 40 participants with dysphagia after stroke 6 months < stroke onset |
| Interventions | Group 1: head lift exercise and conventional dysphagia therapy Group 2: conventional dysphagia therapy |
| Outcomes | Movement of hyolaryngeal complex; PAS |
| Notes | Study completed; in the process of retrieving data |
Park 2018.
| Methods | RCT |
| Participants | People with dysphagia following subacute stroke |
| Interventions | Chin tuck against resistance exercise vs control |
| Outcomes | Functional dysphagia scale, PAS |
| Notes | In the process of retrieving full‐text article |
Shao 2017.
| Methods | RCT |
| Participants | 64 people with post‐stroke upper oesophageal sphincter dystrophy and severe dysphagia |
| Interventions | Drug therapy and conventional swallowing rehabilitation training vs columnar balloon dilatation combined with drug therapy and conventional swallowing rehabilitation training |
| Outcomes | Upper sphincter dynamics and dysphagia scores |
| Notes | In the process of retrieving full‐text article |
Su 2010.
| Methods | RCT |
| Participants | 60 people with dysphagia after stroke |
| Interventions | Group 1: electroacupuncture Group 2: swallowing training |
| Outcomes | VFSS and Kubota water swallowing function test |
| Notes | In the process of retrieving full‐text article |
Sun 2008.
| Methods | RCT |
| Participants | People with dysphagia after stroke |
| Interventions | Acupuncture at Lianquan, Yamen, and Tian Zhu acupoints vs VitalStim therapy |
| Outcomes | Swallowing function |
| Notes | In the process of retrieving full‐text article |
Sun 2018.
| Methods | RCT |
| Participants | People with stroke and dysphagia |
| Interventions | Treatment group treated by intradermal needle‐embedding at Lianquan (CV 23), Jialianquan‐point, Yifeng (TE 17), Ashi‐point, etc. (once every other day for 20 days) on the basis of treatments used in the control group Control group was treated with conventional medicines, NMES of the bilateral midlines of the neck, and swallowing function training |
| Outcomes | Swallowing function (0 to 10 point scaling), surface electromyography |
| Notes | Study published; in the process of extracting data |
Suntrup‐Krueger 2018.
| Methods | RCT |
| Participants | People with dysphagia due to stroke |
| Interventions | Experimental group: transcranial direct current stimulation vs sham group: sham stimulation |
| Outcomes | Fibreoptic Endoscopic Dysphagia Severity Scale, diet at discharge, dysphagia severity rating score, endoscopically assessed swallow function |
| Notes | Study completed; in the process of retrieving data |
Tageldin 2017.
| Methods | RCT |
| Participants | 30 people with dysphagia following brain stem infarction |
| Interventions | rTMS vs sham rTMS on bilateral supratentorial motor area |
| Outcomes | Modified dysphagia outcome and severity scale |
| Notes | Study completed; awaiting full published data |
Umay 2017.
| Methods | RCT |
| Participants | 98 people with dysphagia within the first month after ischaemic stroke |
| Interventions | Sensory‐level electrical stimulation vs sham sensory‐level electrical stimulation to bilateral masseter muscles |
| Outcomes | Bedside Dysphagia Score, Neurological Examination Dysphagia Score, Total Dysphagia Score, and Mann Assessment of Swallowing Ability test, flexible fibreoptic endoscopic evaluation of swallowing |
| Notes | Study published; in the process of extracting data |
Wang 2010.
| Methods | RCT |
| Participants | 84 people with cerebral stroke and dysphagia |
| Interventions | Group 1: routine therapy and acupuncture Group 2: routine therapy |
| Outcomes | Not stated |
| Notes | In the process of retrieving full‐text article |
Wang 2014.
| Methods | RCT |
| Participants | 54 nasal feeding patients with pseudobulbar palsy or bulbar palsy after acute ischaemic stroke |
| Interventions | Integrated swallowing function rehabilitation training vs routine treatment |
| Outcomes | Swallow score, oral intake function |
| Notes | In the process of retrieving full‐text article |
Wang 2015.
| Methods | RCT |
| Participants | 91 people with post‐stroke deglutition disorders |
| Interventions | Acupuncture using the Tong Guan Li Qiao needling method vs control |
| Outcomes | Standard Swallowing Assessment (Modified Barthel Index), Swallowing‐related Quality of Life, Hamilton Depression Scale |
| Notes | In the process of retrieving full‐text article |
Wang 2017.
| Methods | RCT |
| Participants | 96 people with dysphagic stroke |
| Interventions | Observation group to receive Rood intervention; control group to receive routine oral intervention |
| Outcomes | Swallowing function, nutritional status and interventional effect ‐ no further details |
| Notes | Study published; in the process of extracting data |
Wei 2017.
| Methods | RCT |
| Participants | 30 people with upper oesophageal sphincter dysfunction due to unilateral brainstem stroke |
| Interventions | Modified balloon dilatation therapy vs control |
| Outcomes | Amplitude of bilateral submental motor evoked potentials induced by transcranial magnetic stimulations over bilateral motor cortex, diameters of upper oesophageal sphincter opening, maximal displacement of hyoid |
| Notes | Study published; in the process of extracting data |
Wu 2011.
| Methods | RCT |
| Participants | 229 people with dysphagia after stroke |
| Interventions | Group 1: acupuncture Group 2: acupuncture and rehabilitation training Group 3: control group with rehabilitation training |
| Outcomes | Traditional Chinese medicine swallowing assessment, swallowing test, Swallowing Quality of Life Scale ‐ SWAL‐QOL |
| Notes | In the process of retrieving full‐text article |
Wu 2013.
| Methods | RCT |
| Participants | 90 people with dysphagia after stroke |
| Interventions | Group 1: routine acupuncture group + routine treatment and swallowing training Group 2: acupuncture kinesitherapy simultaneously at ezhongxian, lianquan (RN23), jialianquan points + routine treatment, and swallowing training Group 3: routine treatment and swallowing training |
| Outcomes | Water drinking test and brainstem auditory evoked potential |
| Notes | In the process of retrieving full‐text article |
Xia 2010.
| Methods | RCT |
| Participants | 120 people with dysphagia after stroke |
| Interventions | Experimental group: feeding‐swallowing training and acupuncture treatment Control group: feeding‐swallowing training |
| Outcomes | Standardised Swallowing Assessment, VFSS, Modified Barthel Index, Swallowing Quality of LIfe Scale ‐ SWAL‐QOL |
| Notes | In the process of retrieving full‐text article |
Xie 2011.
| Methods | RCT |
| Participants | 148 people with stroke and dysphagia |
| Interventions | Acupuncture group (body acupuncture, electrical acupuncture, and scalp acupuncture) vs rehabilitation group |
| Outcomes | Intention‐to‐treat analysis and on‐treatment/per‐protocol analysis, Watian swallowing ability, pulmonary infection rate, mortality |
| Notes | In the process of retrieving full‐text article |
Xu 2013.
| Methods | RCT |
| Participants | 140 people with stroke |
| Interventions | Experimental group: acupuncture and Western medicine Control group: Western medicine |
| Outcomes | Water drinking test |
| Notes | In the process of retrieving full‐text article |
Xue 2004.
| Methods | RCT |
| Participants | People with post‐stroke dysphagia |
| Interventions | Early rehabilitation + acupuncture vs control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
Yang 2008.
| Methods | RCT |
| Participants | People with post‐stroke dysphagia |
| Interventions | Functional electrical stimulation 40 minutes/d vs functional electrical stimulation 40 minutes twice daily |
| Outcomes | Swallowing function |
| Notes | In the process of retrieving full‐text article |
Yang 2012.
| Methods | RCT |
| Participants | People with post‐stroke dysphagia diagnosed using VFSS |
| Interventions | Anodal tDCS group (1 mA for 20 minutes) vs sham group (1 mA for 30 seconds) |
| Outcomes | Functional dysphagia scale |
| Notes | In the process of retrieving full‐text article |
Zeng 2017.
| Methods | RCT |
| Participants | 112 people with cerebral infarction and dysphagia |
| Interventions | NMES vs control |
| Outcomes | Water‐drinking test, Hamilton Anxiety Scale test, Hamilton Depression Scale |
| Notes | In the process of retrieving full‐text article |
Zhang 2007.
| Methods | RCT |
| Participants | People with stroke, dysphagia, and poor elevation of the larynx |
| Interventions | Comparison of 2 methods of larynx elevation (15 minutes, 5 × day for 4 weeks) |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
Zhang 2015.
| Methods | RCT |
| Participants | 198 people with dysphagia after stroke |
| Interventions | Huoshe Liyan Decoction vs control |
| Outcomes | Efficacy rate, swallow function (unclear) |
| Notes | In the process of retrieving full‐text article |
Zhang 2016.
| Methods | RCT |
| Participants | People with dysphagia with medullary infarction |
| Interventions | Traditional swallowing therapy vs sensory approach combined with traditional swallowing therapy vs motor approach combined with traditional swallowing therapy |
| Outcomes | Swallow function, quality of life, cognition |
| Notes | In the process of retrieving relevant data |
Zhang 2017.
| Methods | RCT |
| Participants | 80 people with stroke and dysphagia |
| Interventions | Vitalstim Electroacupuncture of Fengchi (GB 20), Jinjin (EX‐HN 12) and Yuye (EX‐HN 13) with a Vitalstim Electrostimulator, and manual acupuncture stimulation of Lianquan (CV 23), Tiantu (CV 22) vs control. Both groups received conventional therapy |
| Outcomes | Kubota swallowing ability test, dysphagia subscale (0 to 6 scores) of the neurological deficit degrees, videofluorography assessment, Medical Outcomes Study Item Short Form Health Survey (SF‐36) |
| Notes | In the process of retrieving full‐text article |
Zhen 2014.
| Methods | RCT |
| Participants | 97 people with post‐stroke deglutition dysfunction |
| Interventions | Group A: acupuncture with conventional treatment Group B: VitalStim electric stimulation with conventional treatment Group C: conventional treatment only |
| Outcomes | Swallow function (water‐drinking test, stethocatharsis scoring, and fluoroscopic examination) |
| Notes | In the process of retrieving full‐text article |
Zhong 2003.
| Methods | RCT |
| Participants | People with stroke and dysphagia 15 to 40 days post stroke |
| Interventions | Head acupuncture vs body acupuncture vs control |
| Outcomes | Not available in the study summary |
| Notes | In the process of retrieving full‐text article |
Zhu 2015a.
| Methods | RCT |
| Participants | People with dysphagia after stroke |
| Interventions | Conventional training vs surface electromyographic biofeedback treatment with conventional training |
| Outcomes | Upper oesophageal sphincter opening, pharyngeal transit time |
| Notes | In the process of retrieving full‐text article |
Zhu 2015b.
| Methods | RCT |
| Participants | 68 people with dysphagia after ischaemic stroke |
| Interventions | Combined treatment group (n = 34) receiving swallowing training, feeding strategies, and low‐frequency electrical stimulation Control group (n = 34) receiving swallowing training and feeding strategies |
| Outcomes | VFSS, Standardized Swallowing Assessment |
| Notes | Study published; in the process of extracting data |
ASHA‐NOMS: American Speech‐Language‐Hearing Association National Outcomes Measurement System EEG: electroencephalography Hz: Hertz NIHSS: National Institutes of Health Stroke Scale NMES: neuromuscular electrical stimulation PAS: Penetration Aspiration Scale RCT: randomised controlled trial rTMS: repetitive transcranial magnetic stimulation SWAL‐QOL: Swallowing Quality of Life Questionnaire tDCS: transcranial direct current stimulation TRPV1: transient receptor potential vanilloid 1 VFSS: videofluoroscopic swallow study V‐VST: volume‐viscosity swallow test
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐ICR‐15006004.
| Trial name or title | Clinical observation of YiShen‐TongQiao acupuncture on pharyngeal dysphagia after stroke |
| Methods | RCT |
| Participants | 90 stroke patients with pharyngeal dysphagia |
| Interventions | Observational group: YiShen‐TongQiao acupuncture treatment Control group: rehabilitation training |
| Outcomes | Kubota drinking water test score; Swallow Quality of Life |
| Starting date | 2015 |
| Contact information | Yu Chuan; yuchuan106@126.com |
| Notes | Funding: general planning project of BeiJing Municipal Science and Technology Project of Traditional Chinese Medicine |
ChiCTR‐IOR‐17010505.
| Trial name or title | Fire N needle for patients with dysphagia caused by post‐stroke pseudobulbar palsy: a randomized controlled clinical trial |
| Methods | Randomised, parallel controlled trial |
| Participants | 64 participants with dysphagia after stroke, 30 to 75 years old, onset time < 8 months |
| Interventions | Group A: fire needle Group B: rehabilitation treatment of dysphagia |
| Outcomes | Watian water test evaluation, TengShi swallowing disorder evaluation, swallowing‐related quality of life, dysphagia assessment scale of Traditional Chinese Medicine, pulse oximetry |
| Starting date | 2017, but not yet recruiting |
| Contact information | Xiaolu Qian; qian_xiaolu@163.com |
| Notes | Funding: Shanghai Municipal Commission of Health and Family Planning |
ChiCTR‐IOR‐17011359.
| Trial name or title | The study on the effect of electroacupuncture at Lianquan and Fengfu on one side of brain swallowing function |
| Methods | Randomised parallel controlled trial |
| Participants | 30 participants aged 18 to 65 years; inclusion criteria not clear |
| Interventions | Electroacupuncture group Sham acupuncture group |
| Outcomes | MEP of mylohyoid muscle Resting motion threshold of mylohyoid muscle |
| Starting date | 2017 |
| Contact information | Lin Wang; 373670740@qq.com |
| Notes | Funding: Education Department of Guangdong |
ChiCTR‐IPC‐14005435.
| Trial name or title | Research on mechanism of central regulation of transcranial magnetic stimulation on post‐stroke dysphagia patients |
| Methods | Randomised parallel controlled trial, phase 1 |
| Participants | 20 virtual lesion group; 20 stroke patient group; 20 control |
| Interventions | Virtual lesion group: continuous theta burst stimulation Patient group: transcranial magnetic stimulation Control: conventional treatments |
| Outcomes | MEP; pharyngeal pressure waveform; upper oesophageal sphincter pressure waveform; centre network of swallowing |
| Starting date | 2013 |
| Contact information | Yue Lan; bluemooning@163.com |
| Notes | Funding: National Science Foundation of China |
ChiCTR‐ROC‐17011673.
| Trial name or title | Neuromodulation on post‐stroke patients: a clinical control trial based on mapping swallowing musculature motor cortex |
| Methods | Clinical control (randomisation unclear) |
| Participants | 120 participants with dysphagia post stroke |
| Interventions | Experimental group: TMS Control group: sham TMS |
| Outcomes | Pharyngeal musculature MEP; MEP amplitude; latency of MEP; hotspot |
| Starting date | 2017 |
| Contact information | Wanqi Li; 1170782244@qq.com |
| Notes | Funding: ‐ |
ChiCTR1800014337.
| Trial name or title | High frequency repetitive transcranial magnetic stimulation in the rehabilitation of post‐stroke swallowing disorder |
| Methods | Randomised parallel controlled trial |
| Participants | 40 participants with acute stroke (> 2 weeks post onset) with dysphagia |
| Interventions | High‐frequency rTMS + routine swallow training vs routine swallow training alone |
| Outcomes | Surface EMG; VFSS; Standardised Swallowing Study; VGF (no explanation provided on website); PAS; water drinking test scale for depression |
| Starting date | 2018 |
| Contact information | Zhu Qixiu; szjzqxsx@163.com |
| Notes | Funding: Shandong Province Science and Technology Plan |
ChiCTR1800015837.
| Trial name or title | A randomized controlled clinical study on stroke with dysphagia with treatment of combined of traditional Chinese and west medicine |
| Methods | Randomised parallel controlled trial |
| Participants | 242 stroke patients with dysphagia from 2 weeks to 6 months |
| Interventions | Treatment: acupuncture treatment based on surface electromyography Control: traditional acupuncture treatment |
| Outcomes | Water swallow test rating scale of depression, Standardized Swallowing Assessment, videofluoroscopic swallowing study |
| Starting date | 2016 |
| Contact information | Guoping Zhou; doctorzgp@sina.com |
| Notes | Funding: Construction of High‐level University Scientific Research Funding |
ISRCTN14124645.
| Trial name or title | Metoclopramide and selective oral decontamination for avoiding pneumonia after stroke (MAPS‐2) Trial |
| Methods | 2 × 2 factorial double‐blind randomised controlled trial (treatment) |
| Participants | Acute stroke within 9 hours of clinical onset |
| Interventions | Metoclopramide and placebo paste Metoclopramide and antibiotic paste Placebo metoclopramide and antibiotic paste Placebo metoclopramide and placebo paste |
| Outcomes | Mortality up to the end of the study (90 days), pneumonia within 14 days, number of days of antibiotic treatment for pneumonia within the first 30 days, neurological recovery (NIHSS), disability (mRS), quality of life (EuroQol) |
| Starting date | 1 January 2017 |
| Contact information | Christine Roffe ‐ Institute for Applied Clinical Sciences (IACS), Keele University Guy Hilton Research Centre, Thornburrow Drive, Hartshill ST4 7QB, Stoke‐on‐Trent, United Kingdom |
| Notes | Funding: Health Technology Assessment Programme |
ISRCTN68981054.
| Trial name or title | Treatment of dysphagia after stroke with He's santong needling method: a prospective randomized controlled study |
| Methods | RCT |
| Participants | 60 stroke patients with oral and pharyngeal dysphagia |
| Interventions | Experimental group: He's santong needling method acupuncture combined with swallowing rehabilitation Control group: swallowing rehabilitation |
| Outcomes | Dynamics of swallowing function measured using FEES and Caiteng 7 Rank Swallowing Quality of Life ‐ SWAL‐QOL, Modified MASA, surface EMG |
| Starting date | 2017 |
| Contact information | Bin Li; libin@bjzhongyi.com |
| Notes | Funding: Beijing Traditional Chinese Medicine Administration Administrative Project |
NCT01758991.
| Trial name or title | Therapeutic Impact of tDCS on dysphagia in the acute phase of stroke (improving swallowing after stroke with transcranial direct current stimulation (iSWAT)) |
| Methods | RCT |
| Participants | 100 acute stroke patients with dysphagia |
| Interventions | Experimental group: tDCS Control group: sham tDCS |
| Outcomes | Videofluoroscopy; fiberoptic endoscopic evaluation of swallowing; NIHSS; clinical records; swallowing quality of life ‐ SWAL‐QOL |
| Starting date | 2013 |
| Contact information | Katalin de Fays; katalin.defays@uclouvain.be |
| Notes | Funding: University Hospital of Mont‐Godinne; Université Catholique de Louvain |
NCT01919112.
| Trial name or title | Non‐invasive brain stimulation for swallowing recovery after a dysphagic stroke |
| Methods | RCT |
| Participants | Moderate to severe dysphagic patients with acute stroke documented by imaging |
| Interventions | High dose vs low dose vs sham (control) anodal tDCS |
| Outcomes | Improvement in swallowing |
| Starting date | 2013 |
| Contact information | Sandeep Kumar; Beth Israel Deaconess Medical Center; 617‐632‐8917; skumar@bidmc.harvard.edu |
| Notes | Funding: Beth Israel Deaconess Medical Center |
NCT02322411.
| Trial name or title | Effects of device‐facilitated isometric progressive resistance oropharyngeal (I‐PRO) therapy on dysphagia related outcomes in patients post‐stroke |
| Methods | Randomised controlled pilot study |
| Participants | 30 ischaemic stroke patients within 6 months of acute stroke diagnosis |
| Interventions | Group 1: 12 weeks of Isometric Progressive Resistance Oropharyngeal Therapy plus compensatory treatment Group 2: compensatory treatment only |
| Outcomes | Change in maximum isometric tongue pressures; bolus flow durational measures; swallowing‐related pressures; swallowing quality of life ‐ SWAL‐QOL; functional oral intake scale; pneumonia diagnoses; hospital admissions |
| Starting date | 2014 |
| Contact information | Nicole Pulia; nicolepulia@gmail.com |
| Notes | Sponsors and collaborators: University of Wisconsin, Madison |
NCT02470078.
| Trial name or title | Randomised controlled trial of pharyngeal electrical stimulation for the treatment of post‐extubation dysphagia in acute stroke patients |
| Methods | Randomised parallel assignment trial |
| Participants | 60 stroke patients with severe dysphagia post extubation due to acute stroke |
| Interventions | Pharyngeal electrical stimulation vs sham stimulation |
| Outcomes | Pneumonia rate; reintubation rate; length of stay; PEG tube placement; swallowing function; time until oral nutrition |
| Starting date | 2015 |
| Contact information | Rainer Dziewas; dziewas@uni‐muenster.de |
| Notes | Funding: University Hospital Muenster |
NCT02576470.
| Trial name or title | Motor learning in dysphagia rehabilitation |
| Methods | Randomised, parallel assignment trial |
| Participants | 21 to 100 years with a swallowing problem |
| Interventions | Investigating 3 forms of biofeedback for training swallowing manoeuvres or compensatory techniques and pairing with adjuvant techniques ‐ tDCS, TMS, and financial reward. Group 1: VFSS biofeedback Group 2: submental EMG biofeedback Group 3: mixed VFSS and submental EMG biofeedback Group 4: VFSS biofeedback with anodal tDCS and TMS Group 5: submental EMG biofeedback with anodal tDCS and TMS Group 6: mixed VFSS, submental EMG with anodal tDCS and TMS. Group 7: VFSS with sham tDCS Group 8: submental EMG with sham tDCS Group 9: mixed VFSS and submental EMG with sham tDCS Group 10: VFSS with financial reward Group 11: submental EMG with financial reward Group 12: mixed VFSS and submental EMG with financial reward |
| Outcomes | PAS, targeted dysphagia training biofeedback using VFSS images, submental EMG measures and both VFSS and submental EMG measures; dysphagia manoeuvres, kinematic analysis, financial reward analysis |
| Starting date | |
| Contact information | |
| Notes | Study completed; awaiting full published data |
NCT02960737.
| Trial name or title | Dysphagia evaluation after stroke‐incidence and effect of oral screen intervention on swallowing dysfunction (DESIRE) |
| Methods | Interventional, randomised, parallel assignment. Double‐blind (investigator, outcomes assessor) |
| Participants | Acute stroke patients 6 (± 2) weeks after first‐time transient ischaemic attack and stroke |
| Interventions | Experimental group: intensive training with oral screen and traditional compensatory swallowing training Control group: no intervention; traditional compensatory swallowing training only |
| Outcomes | Swallowing ability, swallowing function, lip force, swallowing quality of life, dysarthria, oral health, activities of daily living, global disability, NIHSS |
| Starting date | 2016 |
| Contact information | Patricia Hägglund, PhD Student; +46907850000; patricia.hagglund@umu.se |
| Notes | Sponsor: Umeå University |
NCT03021252.
| Trial name or title | The RETORNUS‐2 study: impact of respiratory muscle training on swallowing disorders in stroke patients |
| Methods | Interventional, randomised, parallel assignment; single‐blind (outcomes assessor) |
| Participants | Stroke onset 1 month |
| Interventions | Experimental group: high‐intensity inspiratory and expiratory muscle training (IEMT) (IEMT + standard swallow therapy) vs control Sham IEMT Sham IEMT + standard swallow therapy |
| Outcomes | Change in dysphagia severity, change in respiratory muscle strength |
| Starting date | 2017 |
| Contact information | Anna Guillen‐Sola; aguillen@parcdesalutmar.cat |
| Notes | Funding: Parc de Salut Mar |
NCT03247374.
| Trial name or title | Bio‐feedback treatment versus standard treatment for dysphagic post‐stroke patients: a randomized controlled trial |
| Methods | RCT |
| Participants | 40 patients (> 6 weeks onset) with post‐stroke dysphagia |
| Interventions | Experimental group: biofeedback (visual and verbal feedback) Control group: standard SLT (verbal feedback) |
| Outcomes | Functional Oral Intake Scale; change in pooling score during endoscopic evaluation; PAS |
| Starting date | 2017 |
| Contact information | Sara Nordio; sara.nordio@ospedalesancamillo.net |
| Notes | Funding: IRCCS San Camillo, Venezia, Italy |
NCT03274947.
| Trial name or title | The utility of cerebellar transcranial magnetic stimulation in the neurorehabilitation of dysphagia after stroke |
| Methods | RCT |
| Participants | 72 participants with post‐stroke dysphagia within 6 weeks of symptom onset |
| Interventions | Protocol 1 Experimental group: cerebellar TMS Control group: sham TMS Protocol 2 Experimental group: low‐level cerebellar TMS stimulation (once per day for 3 days) plus standard SLT Experimental group: high‐level cerebellar TMS stimulation (twice per day for 5 days) plus standard SLT Control group: sham stimulation (twice per day for 5 days) plus standard SLT |
| Outcomes | Protocol 1: videofluoroscopy before and at 1 hour Protocol 2: videofluoroscopy; functional oral intake scale; dysphagia severity rating scale; feeding status; mRS |
| Starting date | 2017 |
| Contact information | Shaheen Hamdy; shaheen.hamdy@manchester.ac.uk |
| Notes | Funding: University of Manchester, Medical Research Council University of Nottingham |
NCT03358810.
| Trial name or title | Pharyngeal electrical stimulation evaluation for dysphagia after stroke |
| Methods | RCT |
| Participants | 270 acute ischaemic or hemorrhagic cerebral stroke within 7 to 28 days of baseline VFSS |
| Interventions | Experimental group: pharyngeal electrical stimulation Control group: sham pharyngeal electrical stimulation |
| Outcomes | PAS (based on VFSS); time to removal of NG/PEG tube/transition to oral feeding or first diet upgrade; functional oral intake scale |
| Starting date | 2017 |
| Contact information | Phagenesis Ltd. |
| Notes | Funding: Phagenesis Ltd.; Regulatory and Clinical Research Institute; Cytel |
NCT03499574.
| Trial name or title | A randomized controlled feasibility trial of dysphagia therapy using biofeedback in patients with acute stroke |
| Methods | RCT |
| Participants | Participants with new diagnosis of acute stroke and dysphagia |
| Interventions | Experimental: biofeedback using surface EMG with usual care Control: usual care only |
| Outcomes | Dysphagia Severity Rating Scale, Functional Oral Intake Scale, PAS, Dysphagia Handicap Index, modified Rankin Scale, NIHSS, mortality, incidence of pneumonia |
| Starting date | 2018 |
| Contact information | Timothy England; timothy.england@nottingham.ac.uk |
| Notes | Funding: University of Nottingham |
PACTR201710002724163.
| Trial name or title | Effect of transcutaneous electrical nerve stimulation and conventional therapy in post‐stroke dysphagic patients: a randomized controlled trial |
| Methods | RCT |
| Participants | Dysphagic patients following ischaemic stroke less than 1 month (aged 45 to 70 years) |
| Interventions | TENS vs TENS + conventional treatment vs conventional treatment |
| Outcomes | Swallow function |
| Starting date | 2017 |
| Contact information | Rami Maged; ramimaged@hotmail.com |
| Notes | Funding: Taheal Rehabilitation Centre |
U1111‐1188‐0335.
| Trial name or title | Program of rehabilitation with therapeutic efficacy control in oropharyngeal dysphagia after stroke |
| Methods | Randomised, parallel trial |
| Participants | 20 participants with dysphagia after stroke |
| Interventions | Group 1: neuromuscular electrical stimulation associated with sour taste swallowing and cold temperature Group 2: stimulation of swallowing sour taste and cold temperature |
| Outcomes | Decreased episodes of penetration and aspiration (verified by objective examination of swallowing), nasoendoscopy |
| Starting date | 2015 |
| Contact information | Paula Cristina Cola, paccola@hotmail.com |
| Notes | Funding: Faculdade Filosofia e Ciências de Marília |
C: control EMG: electromyography EuroQoL: European Quality of Life Scale FEES: Fibreoptic Endoscopic Evaluation of Swallowing MASA: Mann Assessment of Swallowing Ability MEP: motor evoked potential mRS: modified Rankin Scale NG: nasogastric NIHSS: National Institutes of Health Stroke Scale PAS: Penetration Aspiration Scale PEG: percutaneous endoscopic gastroscopy RCT: randomised controlled trial rTMS: repetitive transcranial magnetic stimulation Rx: treatment SD: standard deviation SLT: speech and language therapy SWAL‐QOL: Swallowing Quality of Life Questionnaire tDCS: transcranial direct current stimulation TMS: transcranial magnetic stimulation VFSS: videofluoroscopy swallow study VGF: no explanation provided on website as to abbreviation
Differences between protocol and review
Separation of dysphagia treatment from nutritional support
For this version of the review, we removed all trials related to nutritional support and feeding to allow focus on swallowing therapy for post‐stroke dysphagia.
Modification of analysis method
We changed the analysis method from fixed‐effect to random‐effects models (odds ratio (OR), mean difference (MD)) because we noted the presence of significant trial and statistical heterogeneity. Two studies included more than one interventional group (Yuan 2003; Carnaby 2006), producing different treatment intensities. In these cases, we divided the low‐intensity (middle) groups and entered data from the study as two data sets (e.g. data set 1: medium (M), low (L), or none; and data set 2: high (H) or medium (M)). Similarly, in the case of repetitive transcranial magnetic stimulation, when a trial compared high‐ versus low‐frequency stimulation or unilateral versus bilateral stimulation (Kim 2012i; Kim 2012ii; Du 2016i; Du 2016ii; Park 2016a (i); Park 2016a (ii)), we divided control group participants equally between treatment groups to prevent counting control participants more than once, thereby artificially narrowing the confidence intervals (CIs).
We combined different interventions, collectively referred to as 'swallowing therapy', for the purposes of analysing their effects on main outcomes to evaluate whether any intervention is better than no intervention, and to try to establish where the most positive effects are seen, and where more research is needed.
Modification of type of stroke patients
We excluded trials in which a majority of participants did not present with stroke, along with trials for which enrolment occurred after six months.
Addition or modification of outcome measures
Modification of search strategies: we have revised and updated the search strategies used for this review to account for newly identified relevant terms keywords and indexing terms. We have included both versions of each search strategy in the review appendices.
We divided swallowing therapy into subcategories: acupuncture, drug therapy, NMES, PES, physical stimulation (thermal, tactile), tDCS, and TMS.
We added additional outcome measures, especially focusing on intermediate outcomes: chest infection or pneumonia rates and penetration aspiration scores. We retained outcomes related to improvement of dysphagia as listed with proportion of participants with dysphagia at end of trial. However, we also included changes in some measurements on videofluoroscopy (pharyngeal transit time) and changes in swallowing ability as determined by change in swallow scores. We included discharge destination within the outcome 'institutionalisation': the number of participants discharged to long‐term care.
Contributions of authors
Philip Bath: conceived and designed the review, undertook searches, analysed and interpreted data, wrote the original review, and updated the review in 2007 (interim update), 2012, and 2018.
Han Sean Lee: undertook searches, extracted data, analysed and interpreted data, and updated the review in 2018.
Lisa Everton: undertook searches and data extraction, analysed and interpreted data, and updated the review in 2018.
Sources of support
Internal sources
King's College Hospital Audit Committee, UK.
Division of Stroke, University of Nottingham, UK.
External sources
South Thames NHS Executive, UK.
Trent NHS Executive, UK.
Wolfson Foundation, UK.
The Stroke Association, UK.
Royal College of Physicians, UK.
Dunhill Medical Trust, UK.
-
National Institutes of Health Research Stroke Research Network, UK.
Support for recruitment of patients into UK‐based trials
National Institutes of Health Research ‐ Cochrane Incentive Scheme, UK.
Declarations of interest
PB was chief investigator of two included trials (Bath 1997, academic; STEPS 2016, commercial ‐ funded by Phagenesis Ltd); he consults for this company and receives honoraria and expenses for this work; he did not contribute to decisions on PES studies including deciding which trials should be included and extracting outcome data. No pharmaceutical or device companies, or other commercial entities, were involved in data analysis, data interpretation, writing of this review, or comments on it.
SL: none known.
LE: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bai 2007i {published data only}
- Bai J, Li B, Wang Z, Gao W, Wang L. The role of different needling manipulation in adjusting swallow period obstacle of dysphagia after stroke. Zhongguo Zhenjiu 2007;27(1):35‐7. [PubMed] [Google Scholar]
Bai 2007ii {published data only}
- Bai J, Li B, Wang Z, Gao W, Wang L. The role of different needling manipulation in adjusting swallow period obstacle of dysphagia after stroke. Zhongguo Zhenjui 2007;27(1):35‐7. [PubMed] [Google Scholar]
Bath 1997 {unpublished data only}
- Bath PMW, Kerr J, Collins M. Factorial trial of swallowing versus conventional therapy, and PEG versus nasogastric tube feeding, in dysphagic patients with recent stroke. Data on file 1997.
Carnaby 2006i {published and unpublished data}
- Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurology 2006;5:31‐7. [DOI] [PubMed] [Google Scholar]
- Mann G, Baxter K, Hankey G, Davis B, Stewart‐Wynne E. Treatment for swallowing disorders following acute stroke: a randomised controlled trial. Stroke Society of Australia Annual Scientific Meeting. 1997.
- Mann G, Hankey G, Davis B, Stewart‐Wynne E. Swallowing therapy after acute stroke study (STAASS): where are we now?. Journal of Clinical Neuroscience 1999;6(3):281. [Google Scholar]
Carnaby 2006ii {published data only}
- Carnaby G, Hankey GJ, Pizzi J. Behavioural interventions for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurology 2006;5:31‐7. [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan S, Or K, Sun W, Ng K, Lo S, Lee Y. Therapeutic effects of acupuncture for neurogenic dysphagia ‐ a randomized controlled trial. Journal of Traditional Chinese Medicine 2012;32(1):25‐30. [DOI] [PubMed] [Google Scholar]
Chen 2016a {published data only}
- * Chen L, Fang J, Ma R, Gu X, Chen L, Li J, et al. Additional effects of acupuncture on early comprehensive rehabilitation in patients with mild to moderate acute ischemic stroke: a multicenter randomized controlled trial. BMC Complementary and Alternative Medicine 2016;16:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Fang J, Ma R, Froym R, Gu X, Li J, et al. Acupuncture for acute stroke: study protocol for a multicenter, randomized, controlled trial. Trials 2014;15:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2016i {published data only}
- Du J, Yang F, Liu L, Hu J, Cai B, Liu W, et al. Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomized, double‐blind clinical trial. Clinical Neurophysiology 2016;127:2907‐13. [DOI] [PubMed] [Google Scholar]
Du 2016ii {published data only}
- Du J, Yang F, Liu L, Hu J, Cai B, Liu W, et al. Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomized, double‐blind clinical trial. Clinical Neurophysiology 2016;127:2907‐13. [DOI] [PubMed] [Google Scholar]
Feng 2012 {published data only}
- Feng X, Hao W, Ding Z, Sui Q, Guo H, Fu J. Clinical study on tongyan spray for post‐stroke dysphagia patients: a randomized controlled trial. Chinese Journal of Integrative Medicine 2012;18(5):345‐9. [DOI] [PubMed] [Google Scholar]
Han 2004 {published data only}
- Han JC. An observation on the therapeutic effect of acupuncture for bulbar palsy after acute stroke. Henan Journal of Practical Nervous Diseases 2004;7(3):81‐2. [Google Scholar]
Heo 2015 {published data only}
- Heo SY, Kim KM. Immediate effects of kinesio taping on the movement of the hyoid bone and epiglottis during swallowing by stroke patients with dysphagia. Journal of Physical Therapy Science 2015;27:3355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2010 {published data only}
- Huang Z, Huang F, Yan HX, Min Y, Gao Y, Tan BD, et al. Dysphagia after stroke treated with acupuncture or electric stimulation: a randomized controlled trial. Zhongguo Zhen Jiu 2010;30(12):969‐73. [PubMed] [Google Scholar]
Jayasekeran 2010a {published data only}
- Jayasekeran V, Singh S, Tyrrell P, Michou E, Jefferson S, Mistry S, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology 2010;138(5):1737‐46. [DOI] [PubMed] [Google Scholar]
Jayasekeran 2010b {published data only}
- Jayasekeran V, Singh S, Tyrrell P, Michou E, Jefferson S, Mistry S, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology 2010;138(5):1737‐46. [DOI] [PubMed] [Google Scholar]
Jia 2006a {published data only}
- Jia H, Zhang Y. Treatment of 40 cases of post‐apoplectic dysphagia by acupuncture plus rehabilitation exercise. Journal of Acupuncture and Tuina Science 2006;4(6):336‐8. [Google Scholar]
Kang 2012 {published data only}
- Kang J, Park R, Lee S, Kim J, Yoon S, Jung K. The effect of bedside exercise program on stroke patients with dysphagia. Annals of Rehabilitation Medicine 2012;26:512‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khedr 2009 {published data only}
- Khedr EM, Abo‐Elfetoh N, Rothwell JC. Treatment of post‐stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurologica Scandinavica 2009;119(3):155‐61. [DOI] [PubMed] [Google Scholar]
Khedr 2010 {published data only}
- Khedr EM, Abo‐Elfetoh N. Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. Journal of Neurology Neurosurgery and Psychiatry 2010;81:495‐9. [DOI] [PubMed] [Google Scholar]
Kim 2012i {published data only}
- Kim L, Chun MH, Kim BR, Lee SJ. Effect of repetitive transcranial magnetic stimulation on patients with brain injury and dysphagia. Annals of Rehabilitation Medicine 2011;35:765‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012ii {published data only}
- Kim L, Chun MH, Kim BR, Lee SJ. Effect of repetitive transcranial magnetic stimulation on patients with brain injury and dysphagia. Annals of Rehabilitation Medicine 2011;35:765‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke‐related dysphagia: a pilot study. Stroke 2011;42(4):1035‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01132066. Transcranial direct current stimulation (TDCS) for facilitating swallowing improvement after an acute unilateral hemispheric stroke. clinicaltrials.gov/show/NCT01132066 (first received 27 May 2010).
Lee 2014 {published data only}
- Lee KW, Kim SB, Lee JH, Lee SJ, Ri JW, Park JG. The effect of early neuromuscular electrical stimulation therapy in acute/subacute ischemic stroke patients with dysphagia. Annals of Rehabilitation Medicine 2014;38(2):153‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee JS, Chui PY, Ma HM, Auyeung TW, Kng C, Law T, et al. Does low dose angiotensin converting enzyme inhibitor prevent pneumonia in older people with neurologic dysphagia ‐ a randomized placebo‐controlled trial. Journal of the American Medical Directors Association 2015;16(8):702‐7. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li L, Shi J, Yin J, Qiao B, Li Y, Huang R. Study of transcutaneous neuromuscular electrical stimulation (VitalStim) therapy for post‐stroke dysphagia. European Journal of Physical and Rehabilitation Medicine 2014;Jul:23. [PubMed] [Google Scholar]
Lim 2009 {published data only}
- Lim KB, Lee HJ, Lim SS, Choi YI. Neuromuscular electrical and thermal‐tactile stimulation for dysphagia caused by stroke: a randomized controlled trial. Journal of Rehabilitation Medicine 2009;41(3):174‐8. [DOI] [PubMed] [Google Scholar]
Liu 2000 {published data only}
- Liu L. Acupuncture treatment of bulbar palsy ‐ a report of 54 cases. Journal of Traditional Chinese Medicine 2000;20(1):30‐2. [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liu Y. Treatment of pseudobulbar paralysis by scalp acupuncture and sublingual needling. Journal of Traditional Chinese Medicine 2004;24(1):26‐7. [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park J, Kim Y, Oh J, Lee H. Effortful swallowing training combined with electrical stimulation in post‐stroke dysphagia: a randomized controlled study. Dysphagia 2012;27:521‐7. [DOI] [PubMed] [Google Scholar]
Park 2013 {published data only}
- Park J, Oh J, Lee J, Yeo J, Ryu KH. The effect of 5Hz high‐frequency rTMS over contralesional pharyngeal motor cortex in post‐stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterology and Motility 2013;25:324–e250. [DOI] [PubMed] [Google Scholar]
Park 2016a (i) {published data only}
- Park E, Kim MS, Chang WH, Oh SM, Kim YK, Lee A, Kim Y. Effects of bilateral repetitive transcranial magnetic stimulation on post‐stroke dysphagia. Brain Stimulation 2017;10:75‐82. [DOI] [PubMed] [Google Scholar]
Park 2016a (ii) {published data only}
- Park E, Kim MS, Chang WH, Oh SM, Kim YK, Lee A, Kim Y. Effects of bilateral repetitive transcranial magnetic stimulation on post‐stroke dysphagia. Brain Stimulation 2017;10:75‐82. [DOI] [PubMed] [Google Scholar]
Park 2016b {published data only}
- Park JS, Oh DH, Chang MY, Kim KM. Effects of expiratory muscle strength training on oropharyngeal dysphagia in subacute stroke patients: a randomised controlled trial. Journal of Oral Rehabilitation 2016;43:364‐72. [DOI] [PubMed] [Google Scholar]
Perez 1997 {published and unpublished data}
- Perez I, Smithard DG, Davies H, Kalra L. Pharmacological treatment of dysphagia in stroke. Dysphagia 1998;13:12‐6. [DOI] [PubMed] [Google Scholar]
- Smithard D, Perez I, Kalra L. Pharmacological treatment of dysphagia in stroke. Age and Ageing 1997;26 Suppl 1:40. [DOI] [PubMed] [Google Scholar]
- Smithard D, Perez I, Kalra L. Pharmacological treatment of dysphagia in stroke. Cerebrovascular Diseases 1997;7 Suppl 4:36. [DOI] [PubMed] [Google Scholar]
Power 2006 {published data only}
- Power ML, Fraser DH, Hobson A, Singh S, Tyrell P, Nicholson DA, et al. Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia 2006;21(1):49‐55. [DOI] [PubMed] [Google Scholar]
Shigematsu 2013 {published data only}
- Shigematsu T, Fujishima I, Ohno K. Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabilitation and Neural Repair 2013;27(4):363‐9. [DOI] [PubMed] [Google Scholar]
Song 2004 {published data only}
- Song QL. Swallowing and ingesting training and nursing in patients with swallowing disorders after stroke. Chinese Journal of Clinical Rehabilitation 2004;8(19):3722‐3. [Google Scholar]
STEPS 2016 {published data only}
- Bath PM, Scutt P, Love J, Clavé P, Cohen D, Dziewas R, et al. Pharyngeal electrical stimulation for treatment of dysphagia in subacute stroke: a randomized controlled trial. Stroke 2016;47:1562‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J, Bath PMW. A multi‐centre, double blind, randomised controlled clinical investigation to validate the EPS1 device as a treatment for stroke‐induced dysphagia: a study of Swallowing Treatment using Electrical Pharyngeal Stimulation (STEPS Study). Clinical Investigational Plan. Data on file 2012.
Terre 2015 {published data only}
- Terre R, Mearin F. A randomized controlled study of neuromuscular electrical stimulation in oropharyngeal dysphagia secondary to acquired brain injury. European Journal of Neurology 2015;22(4):687‐e44. [DOI] [PubMed] [Google Scholar]
Vasant 2016 {published data only}
- Vasant D, Michou E, Tyrrell P, Jayasekeran V, Mistry S, O'Leary N, et al. Pharyngeal electrical stimulation (PES) In dysphagia post‐acute stroke: a double‐blind, randomised trial. Gut 2014;63(1):A31. [Google Scholar]
- Vasant, DH, Michou E, O'Leary N, Vail A, Mistry S, Hamdy S, et al. Pharyngeal electrical stimulation in dysphagia poststroke: a prospective, randomized single‐blinded interventional study. Neurorehabilitation and Neural Repair 2016;30(9):866‐75. [DOI] [PubMed] [Google Scholar]
Warusevitane 2015 {published data only}
- Warusevitane AB, Karunatilake DS, Sim J, Lally F, Roffe C. Safety and effect of metoclopramide to prevent pneumonia in patients with stroke fed via nasogastric tubes trial. Stroke 2015;46:454‐60. [DOI] [PubMed] [Google Scholar]
Wei 2005 {published data only}
- Wei LL. Effect of shuiti acupoint injection with stellate ganglion block on swallow dysfunction after stroke. Chinese Journal of Clinical Rehabilitation 2005;9(9):106‐7. [Google Scholar]
Xia 2011 {published data only}
- Xia W, Zheng C, Lei Q, Tang Z, Hua Q, Zhang Y, et al. Treatment of post‐stroke dysphagia by vitalStim therapy coupled with conventional swallowing training. Journal of Huazhong University of Science and Technology ‐ Medical Sciences 2011;31(1):73‐6. [DOI] [PubMed] [Google Scholar]
Xia 2016a {published data only}
- Xia W, Zheng C, Zhu, Tang Z. Does the addition of specific acupuncture to standard swallowing training improve outcomes in patients with dysphagia after stroke? A randomized controlled trial. Clinical Rehabilitation 2016;30(3):237‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2003i {published data only}
- Yuan ZH, Huang LL, Chen ZL. Coagulant and enteral nutrition agents in the rehabilitation of deglutition disorders for patients with acute stroke. Chinese Journal of Clinical Rehabilitation 2003;7(28):3834‐5. [Google Scholar]
Yuan 2003ii {published data only}
- Yuan MZ, Huang LR, Chen ZL. Coagulant and enteral nutrition agent in the rehabilitation of deglutition disorders for patients with acute stroke. Chinese Journal of Clinical Rehabilitation 2003;7(28):3834‐5. [Google Scholar]
Zheng 2014 {published data only}
- Zheng L, Li Y, Liu Y. The individualized rehabilitation interventions for dysphagia: a multidisciplinary case control study of acute stroke patients. International Journal of Clinical and Experimental Medicine 2014;7(10):3789‐94. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akamatsu 2009 {published data only}
- Akamatsu C, Ebihara T, Ishizuka S, Fujii M, Seki K, Arai H, et al. Improvement of swallowing reflex after electrical stimulation to lower leg acupoints in patients after stroke. Journal of the American Geriatric Society 2009;57(10):1959‐60. [DOI] [PubMed] [Google Scholar]
Aoki 2016 {published data only}
- Aoki S, Hosomi N, Hirayama J, Nakamori M, Yoshikawa M, Nezu T, et al. The multidisciplinary swallowing team approach decreases pneumonia onset in acute stroke patients. PLOS ONE 2016;11(5):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arai 2003 {published data only}
- Arai T, Ekizawa K. Cabergoline and silent aspiration in elderly patients with stroke. Journal of the American Geriatrics Society 2003;51(12):1815. [DOI] [PubMed] [Google Scholar]
Beom 2011 {published data only}
- Beom J, Kim SJ, Han TR. Electrical stimulation of the suprahyoid muscles in brain‐injured patients with dysphagia: a pilot study. Annals of Rehabilitation Medicine 2011;35:322‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beom 2015 {published data only}
- Beom J, Oh B, Choi KH, Kim W, Song YJ, You DS, et al. Effect of electrical stimulation of the suprahyoid muscles in brain‐injured patients with dysphagia. Dysphagia 2015;30:423‐9. [DOI] [PubMed] [Google Scholar]
Bülow 2008 {published data only}
- Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia 2008;23(3):302‐9. [DOI] [PubMed] [Google Scholar]
Byeon 2016 {published data only}
- Byeon H, Koh HW. Comparison of treatment effect of neuromuscular electrical stimulation and thermal‐tactile stimulation on patients with sub‐acute dysphagia caused by stroke. Journal of Physical Therapy Science 2016;28:1809‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2015 {published data only}
- Cai H, Ma B, Gao X, Gao H. Tongue acupuncture in treatment of post‐stroke dysphagia. International Journal of Clinical and Experimental Medicine 2015;8(8):14090‐4. [PMC free article] [PubMed] [Google Scholar]
Chaudhuri 2006 {published data only}
- Chaudhuri G, Brady S, Caldwell R. Electric stimulation for dysphagia flowing stroke: pilot data. Archives of Physical Medicine and Rehabilitation 2006;87(11):e51. [Google Scholar]
Chen 2002 {published data only}
- Chen F, Zhang X. Tongue acupuncture therapy plus ice stimulation for treating 50 cases of dysphagia at the acute stage of sanguineous apoplexy. Henan Traditional Chinese Medicine 2002;22(2):59. [Google Scholar]
Chen 2003 {published data only}
- Chen Y, Li SY, Wang Y. The impression on the deglutition disorders due to pseudobulbar palsy treated with electroacupuncture integrated rehabilitation. Chinese Journal of Clinical Rehabilitation 2003;7(3):430‐1. [Google Scholar]
ChiCTR‐ONC‐17012326 {published data only}
- ChiCTR‐ONC‐17012326. Therapeutic effect of acupuncture and rTMS for dysphagia after unilateral hemispheric stroke of pharyngeal stage: a multi‐center cohort study. www.chictr.org.cn/showproj.aspx?proj=21029 (first received 10 August 2017).
ChiCTR‐TRC‐14005233 {published data only}
- ChiCTR‐TRC‐14005233. The application for telemedicine in post‐stroke rehabilitation. www.chictr.org.cn/showprojen.aspx?proj=4343 (first received 16 September 2014).
DePippo 1994 {published data only}
- DePippo KL, Holas MA, Reding MJ. Dysphagia therapy following stroke: a controlled trial. Neurology 1993;43:A234‐5. [DOI] [PubMed] [Google Scholar]
- DePippo KL, Holas MA, Reding MJ, Lesser ML, Mandel FS. Dysphagia therapy following stroke: a controlled trial. Neurology 1992;42:249. [DOI] [PubMed] [Google Scholar]
- DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology 1994;44:1655‐60. [DOI] [PubMed] [Google Scholar]
Dou 2012 {published data only}
- Dou Z, Zu Y, Wen H, Wan G, Jiang L, Hu Y. The effect of different catheter balloon dilatation modes on cricopharyngeal dysfunction in patients with dysphagia. Dysphagia 2012;27:514‐20. [DOI] [PubMed] [Google Scholar]
Ebihira 2004 {published data only}
- Ebihara T, Takahasi H, Ebihira S, Okazaki T, Sasaki T, Wabanto A, et al. Theophylline improved swallowing reflex in elderly nursing home patients. Jourmal of the American Geriatrics Society 2004;52(10):1787‐8. [DOI] [PubMed] [Google Scholar]
Ebihira 2005 {published data only}
- Ebihara T, Takahashi H, Ebihara S, Okazaki T, Sasaki T, Watando A. Capsaicin trouche for swallowing dysfunction in older people. Journal of American Geriatrics Society 2005;53:824‐8. [DOI] [PubMed] [Google Scholar]
El‐Tamawy 2015 {published data only}
- El‐Tamawy MS, Darwish MH, El‐Azizib HS, Abdelalim AM, Taha SI. The influence of physical therapy on oropharyngeal dysphagia in acute stroke patients. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2015;52(3):201‐5. [Google Scholar]
Fraser 2002 {published data only}
- Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 2002;34(5):831‐40. [DOI] [PubMed] [Google Scholar]
Freed 1996 {published data only}
- Freed M, Christian MO, Beytas EM, Tucker H, Kotton B. Electrical stimulation of the neck: a new effective treatment for dysphagia. Dysphagia 1996;11:159. [Google Scholar]
Freed 2001 {published data only}
- Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respiratory Care 2001;46(5):466‐74. [PubMed] [Google Scholar]
Hagg 2015 {published data only}
- Hagg M, Tibbling L. Effect of oral IQoro® and palatal plate training in post‐stroke, four‐quadrant facial dysfunction and dysphagia: a comparison study. Acta Oto‐Laryngologica 2015;135(9):962‐8. [DOI] [PubMed] [Google Scholar]
Inui 2017 {published data only}
- Inui Y, Kamakuyra Y, Fukada J, Yoneda M, Kataoka E, Usami Y, et al. Development of pyriform sinus suctioning programs for aspiration pneumonia prevention during the acute stroke. Dysphagia 2017;32:767‐76. [DOI] [PubMed] [Google Scholar]
ISRCTN18137204 {published data only}
- ISRCTN18137204. Benefit of PHAryngeal electrical STimulation for early de‐cannulation in TRACheotomised stroke patients with neurogenic dysphagia: a prospective randomized single‐blinded interventional study (PHAST TRAC study). www.isrctn.com/ISRCTN18137204 (first received 23 February 2015).
- Minten J, Tweel I, Dziewas R, Bath PM, Hamdy S. Benefit of PHAryngeal electrical STimulation for early de‐cannulation in TRACheotomised stroke patients with neurogenic dysphagia: a prospective randomised single‐blinded interventional study (PHAST TRAC study). Data on file 2015. [DOI] [PubMed]
ISRCTN97286108 {published data only}
- ISRCTN97286108. Non‐invasive brain stimulation for dysphagia after acute stroke. http://www.isrctn.com/ISRCTN97286108 (first received 2 April 2015).
Jin 2014a {published data only}
- Jin HP, Wu QY, Zhang W, Xie JJ, Chen JC. Post‐stroke dysphagia in chronic stage treated with magnetic‐ball sticking therapy at the auricular points: a randomized controlled trial. Zhongguo Zhen Jiu 2014;34(1):9‐14. [PubMed] [Google Scholar]
KCT0001907 {published data only}
- KCT0001907. Effects of neuromuscular electrical stimulation according to electrode placement in stroke patients with dysphagia. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=6225 (first received 4 August 2015).
Kikuchi 2014 {published data only}
- Kikuchi A, Seki T, Takayama S, Ishizuka S, Yaegashi N. Effect of press needles on swallowing reflex in older adults with cerebrovascular disease: a randomized double‐blind controlled trial. Journal of the American Geriatrics Society 2014;62(12):2430‐40. [DOI] [PubMed] [Google Scholar]
Kobayashi 1996 {published data only}
- Kobayashi H, Nakagawa T, Sekizawa K, Arai H, Sasaki H. Levodopa and swallowing reflex. Lancet 1996;348:1320‐1. [DOI] [PubMed] [Google Scholar]
Kulnik 2015 {published data only}
- Kulnik ST, Birring SS, Moxham J, Rafferty GF, Klara L. Does respiratory muscle training improve cough flow in acute stroke? Pilot randomized controlled trial. Stroke 2015;46:447‐53. [DOI] [PubMed] [Google Scholar]
Kushner 2013 {published data only}
- Kushner DS, Peters K, Eroglu ST, Perless‐Carroll M, Johnson‐Greene D. Neuromuscular electrical stimulation efficacy in acute stroke feeding tube‐dependent dysphagia during inpatient rehabilitation. American Journal of Physical Medicine and Rehabilitation 2013;92(6):486‐95. [DOI] [PubMed] [Google Scholar]
Lan 2013 {published data only}
- Lan Y, Xu G, Dou Z, Wan G, Yu F, Lin T. Biomechanical changes in the pharynx and upper sphincter after modified balloon dilatation in brainstem stroke patients with dysphagia. Neurogastroenterology and Motility 2013;25:821‐9. [DOI] [PubMed] [Google Scholar]
Logemann 2009 {published data only}
- Logemann JA, Rademaker A, Pauloski BR, Kelly A, Stangl‐McBreen C, Antinoja J, et al. A randomized study comparing the Shaker exercise with traditional therapy: a preliminary study. Dysphagia 2009;24(4):403‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2014 {published data only}
- Ma FX, Cao GP, Li WL. Post‐stroke dysphagia treated with acupoint injection combined with neural electrical stimulation. Zhongguo Zhenjiu 2014;34(12):1169‐73. [PubMed] [Google Scholar]
Ma 2015 {published data only}
- Ma JN, Wang ZL, Ning LN, Yang H, Xiong J. Observation on therapeutic effects of acupuncture combined with cutaneous electrical stimulation for dysphagia in patients with cerebral infarction. Chen Tzu Yen Chiu Acupuncture Research 2015;40(3):238‐41. [PubMed] [Google Scholar]
Maeda 2017 {published data only}
- Maeda K, Koga T, Akagi J. Interferential current sensory stimulation, through the neck skin, improves airway defense and oral nutrition intake in patients with dysphagia: a double‐blind randomized controlled trial. Clinical Interventions in Aging 2017;12:1879‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2016 {published data only}
- Mao L, Li L, Mao Z, Han Y, Zhang X, Yao J, Li M. Therapeutic effect of acupuncture combining standard swallowing training for post‐stroke dysphagia: a prospective cohort study. Chinese Journal of Integrative Medicine 2016;22(7):525‐31. [DOI] [PubMed] [Google Scholar]
McCullough 2012 {published data only}
- McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary MA. Effects of Mendelsohn maneuver on measures of swallowing duration post‐stroke. Topics in Stroke Rehabilitation 2012;19(3):234‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCullough 2013 {published data only}
- McCullough GH, Kim Y. Effects of the Mendelsohn maneuver on extent of hyoid movement and UES opening post‐stroke. Dysphagia 2013;28:511‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mepani 2009 {published data only}
- Mepani R, Antonik S, Massey B, Kern M, Logemann J, Pauloski B, et al. Augmentation of deglutitive thyrohyoid muscle shortening by the shaker exercise. Dysphagia 2009;24:26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Messaggi‐Sartor 2015 {published data only}
- Messaggi‐Sartor M, Guillen‐Solà A, Depolo M, Duarte E, Rodríguez DA, Barrera M, et al. Inspiratory and expiratory muscle training in subacute stroke ‐ a randomized clinical trial. American Academy of Neurology 2015;85:564‐72. [DOI] [PubMed] [Google Scholar]
Michou 2010 {published data only}
- Michou E, Mistry S, Jefferson S, Singh S, Rothwell J, Hamdy S. Addressing oropharyngeal dysphagia post stroke with neurostimulation interventions: a pilot study. International Journal of Stroke 2010;5 Suppl 3:61‐2. [Google Scholar]
- Michou E, Mistry S, Jefferson S, Singh S, Hamdy SA. Preliminary study of neurostimulation based interventions in the treatment of chronic dysphagia post stroke. Gut 2010;59(1):A27. [Google Scholar]
Michou 2011 {published data only}
- Michou E, Mistry S, Jefferson S, Singh S, Rothwell J, Tyrrell P, et al. Neurostimulation techniques benefit stroke patients with chronic oropharyngeal dysphagia: preliminary results from a randomised controlled study. Cerebrovascular Diseases 2011;31(Suppl 2):58. [Google Scholar]
Nakamura 2013 {published data only}
- Nakamura T, Fujishima I. Usefulness of ice massage in triggering the swallow reflex. Journal of Stroke and Cerebrovascular Diseases 22;4:378‐82. [DOI] [PubMed] [Google Scholar]
Nakayama 1998 {published data only}
- Nakayama K, Sekizawa K, Sasaki H. ACE inhibitor and swallowing reflex. Chest 1998;113(5):1425. [DOI] [PubMed] [Google Scholar]
Nam 2012 {published data only}
- Nam H, Beom J, Oh BM, Han BR. Kinematic analysis of hyoid bone and vocal cord after laryngeal electrical stimulation therapy in dysphagia. Neurorehabilitation and Neural Repair 2012;26(4):433. [Google Scholar]
NCT00376506a {published data only}
- NCT00376506. A comparison of an implanted neuroprosthesis with sensory training for improving airway protection in chronic dysphagia. https://clinicaltrials.gov/ct2/show/NCT00376506 (first received 15 September 2006).
NCT00376506b {published data only}
- NCT00376506. A comparison of an implanted neuroprosthesis with sensory training for improving airway protection in chronic dysphagia. clinicaltrials.gov/ct2/show/NCT00376506 (first received 15 September 2006).
NCT01971320 {published data only}
- NCT01971320. Evaluation of transcutaneous electrical stimulation in post stroke dysphagia. clinicaltrials.gov/show/NCT01971320 (first received 29 October 2013).
Nishiyama 2010 {published data only}
- Nishiyama Y, Abe A, Ueda M, Katsura K, Katayama Y. Nicergoline increases serum substance P levels in patients with an ischaemic stroke. Cerebrovascular Diseases 2010;29(2):194‐8. [DOI] [PubMed] [Google Scholar]
Ortega 2016 {published data only}
- Ortega O, Rofes L, Martin A, Arreola V, Lo I, Clave P. A comparative study between two sensory stimulation strategies after two weeks treatment on older patients with oropharyngeal dysphagia. Dysphagia 2016;31:706‐16. [DOI] [PubMed] [Google Scholar]
Permsirivanich 2009 {published data only}
- Permsirivanich W, Tipchatyotin S, Wongchai M, Leelamanit V, Setthawatcharawanich S, Sathirapanya P, et al. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. Journal of the Medical Association of Thailand 2009;92(2):259‐65. [PubMed] [Google Scholar]
Pownall 2008 {published data only}
- Pownall S, Enderby P, Hendra T, Marshall M. Are thickened fluids worth the trouble? A pilot RCT of dysphagia management. Proceedings of the 3rd UK Stroke Forum Conference. Harrogate, UK: The Stroke Association, 2008:86‐7.
Pryor 2011 {published data only}
- Pryor J, Leonard R, Belafsky P. A prospective, randomized trial of two dysphagia therapies: neuromuscular electrical stimulation and vibrotactile stimulation. Dysphagia 2011;26(4):466. [Google Scholar]
Reidnauer 2006 {published data only}
- Reidnauer S, Repsher S, Stryker D, Segal M. Vital stimulation may be more effective than traditional treatment in improving swallowing after stroke. Stroke 2006;37(2):737. [Google Scholar]
Rofes 2014 {published data only}
- Rofes L, Arreola V, Martin A, Clave P. Effect of oral piperine on the swallow response of patients with oropharyngeal dysphagia. Journal of Gastroenterology 2014;29:1517‐23. [DOI] [PubMed] [Google Scholar]
Rosenbek 1991 {published data only}
- Rosenbek JC, Robbins J, Fishback B, Levine RL. Effects of thermal application on dysphagia after stroke. Journal Speech and Hearing Research 1991;34:1257‐68. [DOI] [PubMed] [Google Scholar]
Rosenbek 1996 {published data only}
- Rosenbek JC. Effects of thermal stimulation on dysphagia after stroke. Journal of Rehabilitation Research and Development 1990;28(1):151. [Google Scholar]
- Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia 1996;11:225‐33. [DOI] [PubMed] [Google Scholar]
Rosenbek 1998 {published data only}
- Rosenbek JC, Robbins JA, Willford WO, Kirk G, Schiltz A, Sowell TW, et al. Comparing treatment intensities of tactile‐thermal application. Dysphagia 1998;13:1‐9. [DOI] [PubMed] [Google Scholar]
Sdravou 2012 {published data only}
- Sdravou K, Walshe M. Effects of carbonated liquids on oropharyngeal swallowing measures in people with neurogenic dysphagia. Dysphagia 2012;27:240‐50. [DOI] [PubMed] [Google Scholar]
Seki 2005 {published data only}
- Seki T, Iwasaki K, Arai H, Sasaki H, Hayashi H, Yamada S, et al. Acupuncture for dysphagia in post stroke patients: a video fluoroscopic study. Journal of the American Geriatrics Society 2005;53(6):1083‐4. [DOI] [PubMed] [Google Scholar]
Shaker 2002a {published data only}
- Easterling C, Kern M, Nitschke T, Grande B, Kazandijan M, Dikeman K, et al. Restoration of oral feeding in 17 tube fed patients by the Shaker exercise. Dysphagia 2000;15(2):105. [Google Scholar]
- Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, et al. Rehabilitation of swallowing by exercise in tube‐fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 2002;122:1314‐21. [DOI] [PubMed] [Google Scholar]
She 2014 {published data only}
- She RP, Ge CH. Clinical observation on medulla oblongata palsy after brainstem infarction treated with electroacupuncture at eight‐neck‐occiput points. Zhongguo Zhen Jiu 2014;34(6):539‐42. [PubMed] [Google Scholar]
SQACU01 2001 {published data only}
- Heng D. SQACU01 ‐ a randomised trial of acupuncture as adjuvant therapy for dysphagia due to recent stroke. Clinical Trials and Epidemiology Research Unit Annual Report. Singapore: Clinical Trials and Epidemiology Research Unit, 2001:41. [Google Scholar]
Steele 2016 {published data only}
- Steele CM. Tongue pressure profile training for dysphagia post stroke (TPPT): study protocol for an exploratory randomized controlled trial. Trials 2013; Vol. 14:126. [DOI] [PMC free article] [PubMed]
- Steele CM, Bayley MT, Peladeau‐Pigeon M, Nagy A, Namasivayam AM, Stokely S, et al. A randomized trial comparing two tongue‐pressure resistance training protocols for post‐stroke dysphagia. Dysphagia 2016;31:452‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sukthankar 1994 {published data only}
- Sukthankar SM, Reddy NP, Canilang EP, Stephenson L, Thomas R. Design and development of portable biofeedback systems for use in oral dysphagia rehabilitation. Medical Engineering and Physics 1994;16:430‐5. [DOI] [PubMed] [Google Scholar]
Suntrup 2015 {published data only}
- DRKS00005509. A single‐centre, double blind, randomised controlled clinical trial to evaluate the effect of electrical pharyngeal stimulation as a treatment for stroke‐related dysphagia in tracheotomized stroke patients. www.drks.de/DRKS00005509 (first received 15 January 2014).
- Suntrup S, Marian T, Schröder JB, Suttrup I, Muhle P, Oelenberg S, et al. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: a randomized controlled trial. Intensive Care Medicine 2015;41(9):1629‐37. [DOI] [PubMed] [Google Scholar]
Suzuki 2012 {published data only}
- Suzuki H, Takeda S, Nakazaki M, Sone S, Mori T. The appropriate body position during nasal‐gastric tube feeding to prevent the aspiration pneumonia in acute stroke patients. Cerebrovascular Diseases 2012;33(2):464. [Google Scholar]
Tai 2014 {published data only}
- Tai S, Chang Y, Chang L. On the use of the chin‐down posture for dysphagia in stroke patients. Cerebrovascular Diseases 2014;38:105. [Google Scholar]
- Tai S, Huang HM. The effectiveness of the chin‐down posture in the improvement of dysphagia in stroke patients. http://hdl.handle.net/10755/602716 (first received 21 March 2016).
Teramoto 2008 {published data only}
- Teramoto S, Yamamoto H, Yamaguchi Y, Ishii M, Hibi S, Kume H. Antiplatelet cilostazol, an inhibitor of type III phosphodiesterase, improves swallowing function in patients with a history of stroke. Journal of the American Geriatrics Society 2008;56(6):1153‐4. [DOI] [PubMed] [Google Scholar]
Terre 2012 {published data only}
- Terre R, Mearin F. Effectiveness of chin‐down posture to prevent tracheal aspiration in dysphagia secondary to acquired brain injury. A videofluoroscopy study. Neurogastroenterology and Motility 2012;24:414. [DOI] [PubMed] [Google Scholar]
Toyama 2014 {published data only}
- Toyama K, Matsumoto S, Kurasawa M, Setoguchi H, Noma T, Takenaka K, et al. Novel neuromuscular electrical stimulation system for treatment of dysphagia after brain injury. Neurologia Medico‐Chirurgica 2014;54:521‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMIN000015406. Effect of electrical stimulation in post‐stroke patients with dysphagia: a feasibility study. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000017918 (first received 10 October 2014).
Ueda 2004 {published data only}
- Ueda K, Yamada Y, Toyosata A, Nomura S, Saitho E. Effects of functional training of dysphagia to prevent pneumonia for patients on tube feeding. Gerontology 2004;21:108‐11. [DOI] [PubMed] [Google Scholar]
Varma 2006 {published data only}
- Varma AK. The effect of motor control on oro‐facial dysfunctions in stroke patients under Indian conditions; 5th World Stroke Congress; 2004 Jun 23‐26; Vancouver, Canada. 2006;e319.
Wang 2016 {published data only}
- Wang Z, Ma J, Ning L. Clinical observation of dysphagia after cerebral infarction treated with awn‐like needle at Tiantu (CV 22). Chinese Acupuncture and Moxibustion 2016;36(10):1019‐22. [DOI] [PubMed] [Google Scholar]
Xia 2016 {published data only}
- Xia W, Zheng C, Xia J, Zhang Y. Post‐stroke dysphagia treated with acupuncture of meridian differentiation: a randomized controlled trial. Chinese Acupuncture and Moxibustion 2016;36(7):673‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang ZL, Zhao SH, Chen GH, Ji XQ, Xue L, Yang YQ, et al. Randomized controlled study on dysphagia after stroke treated with deep insertion of Chonggu (EX‐HN 27) by electroacupuncture. Zhongguo Zhen Jiu 2011;31(5):385‐90. [PubMed] [Google Scholar]
Zhang 2018a {published data only}
- Zhang L, Xu N, Li R, Wang L. Clinical study of electroacupuncture with different frequencies at Lianquan (CV 23) and Fengfu (GV 16) for stroke dysphagia. Chinese Acupuncture and Moxibustion 2018;38(2):115‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2018b {published data only}
- Zhang R, Ju X. Clinical improvement of nursing intervention in swallowing dysfunction of elderly stroke patients. Biomedical Research 2018;29(6):1099‐102. [Google Scholar]
Zhao 2015 {published data only}
- Zhao K, Wang Z, Cao W, Zhang Y, Song S, Kang W, et al. Therapeutic efficacy of swallowing neuromuscular electrical stimulation combined with acupuncture for post‐stroke dysphagia. World Journal of Acupuncture‐Moxibustion 2015;25(1):19‐23. [Google Scholar]
References to studies awaiting assessment
Azimov 2017 {published data only}
- Azimov A, Sadykov R, Rakhimbaeva G. Dopaminergic medicines can treat dysphagia in ischemic stroke. Journal of the Neurological Sciences 2017;381 Suppl 1:396. [Google Scholar]
Carnaby 2012 {published data only}
- Carnaby G, LaGorio L, Crary M, Miller D. A randomized double blind trial of neuromuscular electrical stimulation + McNeill dysphagia therapy (MDTP) after stroke (ANSRS). Dysphagia 2012;27:569‐620. [Google Scholar]
Chang 2014 {published data only}
- Chang L, He PL, Zhou ZZ, Li YH. Efficacy observation of dysphagia after acute stroke treated with acupuncture and functional electric stimulation. Zhongguo Zhenjiu 2014;34(8):737‐40. [PubMed] [Google Scholar]
Chaudhuri 2008 {published data only}
- Chaudhuri G, Brady S, Caldwell R, Wesling M, Quill A. Neuromuscular electrical stimulation (NMES) for dysphagia treatment following acute ischaemic stroke. Dysphagia 2008;23(4):441. [Google Scholar]
Chen 2017 {published data only}
- Chen D, Xing H Jiang Q, Xiang Y, Guo H. Role of levetiracetam in the rehabilitation of dysphagia due to stroke. International Journal of Pharmacology 2017;13(6):603‐11. [Google Scholar]
Cheng 2005 {published data only}
- Cheng XL, Zhao CS, Wang H, Ma L. Effects of early throat muscle training on vertebral‐basilar artery blood flow in patients with pseudobulbar palsy. Chinese Journal of Clinical Rehabilitation 2005;9(25):17‐9. [Google Scholar]
Cheng 2014 {published data only}
- Cheng FX, Chen T. Efficacy observation of post‐stroke dysphagia treated with acupuncture at Lianquan (CV 23). Zhongguo Zhen Jiu 2014;34(7):627‐30. [PubMed] [Google Scholar]
ChiCTR‐TRC‐07000010 {published data only}
- ChiCTR‐TRC‐07000010. Randomized controlled study on the acupuncture for dysphagia in convalescence phase of apoplexy. http://www.chictr.org.cn/showprojen.aspx?proj=9515 (first received 6 February 2007).
ChiCTR‐TRC‐08000463 {published data only}
- ChiCTR‐TRC‐08000463. Clinical evaluation of dysphagia therapeutic apparatus on cerebrovascular disease. Chinese Clinical Trial Registry (ChiCTR) www.chictr.org/ (first received 3 November 2008).
ChiCTR‐TRC‐14004235 {published data only}
- ChiCTR‐TRC‐14004235. Clinical research of modified Dihuang Yinzi Decoction combined swallowing rehabilitation and videofluoroscopy on post‐stroke dysphagia patients: a pilot trial. www.chictr.org/en/proj/show.aspx?proj=6601 (first received 20 January 2014).
ChiCTR‐TRC‐14004955 {published data only}
- ChiCTR‐TRC‐14004955. Effect of transcranial direct current stimulation on dysphagia after stroke. http://www.chictr.org.cn/showproj.aspx?proj=4618 (first received 16 July 2014).
Choi 2017 {published data only}
- Choi J‐B, Shim S‐H, Yang J‐E, Kim H‐D, Lee D‐H, Park J‐S. Effects of Shaker exercise in stroke survivors with oropharyngeal dysphagia. NeuroRehabilitation 2017;41(4):753‐7. [DOI] [PubMed] [Google Scholar]
Chu 2017 {published data only}
- Chu J, Liu X, Chen F, Hong F, Bao Y. Effects of GAO's neck acupuncture on swallowing function and quality of life in patients with post‐stroke pseudobulbar palsy: a randomized controlled trial. Chinese Acupuncture and Moxibustion 2017;37(7):691‐5. [DOI] [PubMed] [Google Scholar]
de Fraga 2017 {published data only}
- Fraga BFD, Almeida STD, Santana MG, Cassol M. Efficacy of myofunctional therapy associated with voice therapy in the rehabilitation of neurogenic oropharyngeal dysphagia: a pilot study. International Archives of Otorhinolaryngology 2017;DOI:10.1055/s‐0037‐1605597:[Ref 27900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eom 2017 {published data only}
- Eom M, Chang M, Oh D, Kim H, Han N, Park J. Effects of resistance expiratory muscle strength training in elderly patients with dysphagic stroke. Neuro Rehabilitation 2017;41(4):747‐52. [DOI] [PubMed] [Google Scholar]
Erfmann 2017 {published data only}
- Erfmann, K. Effects of expiratory muscle strength training (EMST) on oropharyngeal dysphagia in subacute stroke patients: a randomised controlled trial. Journal of Clinical Practice in Speech‐Language Pathology 2017;19(2):111. [Google Scholar]
Fan 2007 {published data only}
- Fan C, Jiang H, Wu L. Clinical observations on acupuncture treatment of postapoplectic dysphagia. Shanghai Journal of Acupuncture and Moxibustion 2007;26:6‐7. [Google Scholar]
Feng 2016 {published data only}
- Feng S, Cao S, Du S, Yin T, Mai F, Chen X, et al. Acupuncture combined with swallowing training for post‐stroke dysphagia: a randomized controlled trial. Zhongguo Zhen Jiu 2016;36(4):347‐50. [PubMed] [Google Scholar]
Gao 2016 {published data only}
- Gao J, Zhang HJ. Effects of chin tuck against resistance exercise versus Shaker exercise on dysphagia and psychological state after cerebral infarction. European Journal of Physical and Rehabilitation Medicine 2016;53(3):426‐32. [DOI] [PubMed] [Google Scholar]
Guillen‐Sola 2017 {published data only}
- * Guillén‐Solà A, Messagi Sartor M, Bofill‐Soler N, Duarte E, Barrera MC, Marco E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: a randomized controlled trial. Clinical Rehabilitation 2017;31(6):761‐71. [DOI] [PubMed] [Google Scholar]
- Guillen‐Sola A, Messagi‐Sartor M, Barrera De Paz C, Bofill‐Soler N, Rodriguez DA, Duarte E, et al. Effects of neuromuscular electrostimulation and respiratory muscle training in acute/subacute dysphagic stroke patients. Retornus: a randomized control trial. Dysphagia 2015;30(2):236‐7. [Google Scholar]
Hamada 2017 {published data only}
- Hamada S, Yamaguchi H, Hiroyoshi H. Does sensory transcutaneous electrical stimulation prevent pneumonia in the acute stage of stroke? A preliminary study. International Journal of Rehabilitation Research 2017;40(1):94‐6. [DOI] [PubMed] [Google Scholar]
Hong 2011 {published data only}
- Hong Z, Yulin W, Qin Y. Influence of diet nursing care on the prognosis of patients with poststroke dysphagia. Chinese Nursing Research 2011;25(1C):211‐3. [Google Scholar]
Huang 2008 {published data only}
- Huang YL, Liang FR, Chang HS, Hu KM, He J, Li N, et al. Effect of acupuncture on quality of life in post‐ischemic stroke patients with dysphagia. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28:505‐8. [PubMed] [Google Scholar]
Huang 2014 {published data only}
- * Huang K, Liu T, Huang Y, Leong C, Lin W, Pong Y. Functional outcome in acute stroke patients with oropharyngeal dysphagia after swallowing therapy. Journal of Stroke and Cerebrovascular Diseases 2014;23(10):2547‐53. [DOI] [PubMed] [Google Scholar]
- NCT03048916. Dysphagia after different swallowing therapies. https://www.clinicaltrials.gov/ct2/show/record/NCT03048916 (first received 1 August 2010).
Huimin 2015 {published data only}
- Huimin Z, Yongchao Y, Jiang R, Li L, Yao W, Weibo S, Jie Z. Effect of surface electromyographic biofeedback on the pharyngeal phase activities in patients with dysphagia after stroke. Chinese Journal of Cerebrovascular Diseases 2015;12(11):572‐6. [Google Scholar]
Jefferson 2008 {published data only}
- Jefferson S, Hamdy S, Michou E, Mistry S, Singh S. Neurostimulation is able to increase cortical bulbar excitability following dysphagic stroke. Proceedings of the 3rd UK Stroke Forum Conference; 2008 Dec 2‐4. Harrogate: The Stroke Association, 2008.
Jia 2006 {published data only}
- Jia H‐L, Zhang Y‐C. Treatment of 40 cases of post‐apoplectic dysphagia by acupuncture plus rehabilitation exercise. Journal of Acupuncture and Tuina Science 2006;4(6):336‐8. [Google Scholar]
Jiang 2014 {published data only}
- Jiang W, Tan B, Zhou Y, Jia G, Wu X, Jia L, et al. Clinical study on treatment of patients with dysphagia after stroke by improved Vitalstim electroacupuncture. Journal of Shanghai Jiaotong University (Medical Science) 2014;34(9):1361‐4. [Google Scholar]
Jing 2016 {published data only}
- Jing Q, Yang X, Reng Q. Effect of neuromuscular electrical stimulation in patients with post‐stroke dysphagia. Medical Science Technology 2016;57:1‐5. [Google Scholar]
Ji‐Ye 2017 {published data only}
- Ji‐Ye L. Influence of acupoint‐injection on TXB2 and 6‐keto‐PGF1a in patients with pseudobulbar palsy: a randomized controlled trial. Journal of Acupuncture and Tuina Medicine 2017;1:22‐6. [Google Scholar]
Kim 2017 {published data only}
- Kim HD, Choi JB, Yoo SJ, Chang MY, Lee SW, Park JS. Tongue‐to‐palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagia. Journal of Oral Rehabilitation 2017;44:59–64. [DOI] [PubMed] [Google Scholar]
Koch 2015 {published data only}
- Koch I, Meneghello F, Piccione F. Preliminary data of swallowing training using sEMG as biofeedback. Journal of the Neurological Sciences 2015;357:e353. [Google Scholar]
Konecny 2018 {published data only}
- Konecny P, Elfmark M. Electrical stimulation of hyoid muscles in post‐stroke dysphagia. Biomedical Papers of the Medical Faculty of the University Palacky Olomouc Czechoslovakia 2018;162(1):40‐2. [DOI] [PubMed] [Google Scholar]
Koyama 2017 {published data only}
- Koyama Y, Sugimoto A, Hamano T, Kasahara T, Toyokura M, Masakado Y. Proposal for a modified jaw opening exercise for dysphagia: a randomized, controlled trial. Tokai Journal of Experimental and Clinical Medicine 2017;42(2):71‐8. [PubMed] [Google Scholar]
Lee 2015b {published data only}
- Lee JH, Kim SB, Lee KW, Lee SJ, Lee JU. Effect of repetitive transcranial magnetic stimulation according to the stimulation site in stroke patients with dysphagia. Annals of Rehabilitation Medicine 2015;39(3):432‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li J, Li J. Acupuncture used to treat dysphagia induced by ischemic stroke. Journal of Beijing University of Traditional Chinese Medicine 2008;15:17‐9. [Google Scholar]
Li 2009 {published data only}
- Li H, Yue G, Liu D, Zhou H. Clinical observations on acupuncture plus rehabilitation training for improving postapoplectic dysphagia. Shanghai Journal of Acupuncture and Moxibustion 2009;28:388‐9. [Google Scholar]
Li 2016 {published data only}
- Li Y, Ren K, Xing R, Peng J, Zhang Z, Zhao J. Clinical research of the five needles combined with rehabilitation training treatment dysphagia after stroke. Pakistan Journal of Pharmaceutical Sciences 2016;29(5 Suppl):1745‐8. [PubMed] [Google Scholar]
Liu 2018 {published data only}
- Liu XP, Chen FY, Chu JM, Bao YH. Effects of nape acupuncture combined with swallowing rehabilitation on dysphagia in pseudobulbar palsy. Journal of Traditional Chinese Medicine 2018;38(1):117‐24. [PubMed] [Google Scholar]
Ma 2016 {published data only}
- Ma P, Xu S, Tian W, Duan H, Wang C, Shan Y, et al. Efficacy observation of post‐stroke pseudo‐bulbar palsy treated with quick needle insertion therapy at Aqiang point. Chinese Acupuncture and Moxibustion 2016;36(10):1027‐30. [DOI] [PubMed] [Google Scholar]
Malik 2017 {published data only}
- Malik SN, Khan MSG, Ehsaan F, Tul‐Ain Q. Effectiveness of swallow maneuvers, thermal stimulation and combination both in treatment of patients with dysphagia using functional outcome swallowing scale. Biomedical Research (India) 2017;28(4):1479‐82. [Google Scholar]
Mehndiratta 2017 {published data only}
- Mehndiratta MM, Gupta P, Kaur M. The effect of sensory‐level electrical stimulation of the masseter muscle in early stroke patients with dysphagia. Neurology India 2017;65(4):743‐5. [DOI] [PubMed] [Google Scholar]
Meng 2015 {published data only}
- Meng Y, Wang C, Shang S, Ning L, Zhou L, Han K. Effects of different acupuncture depths of Lianquan (CV 23) for dysphagia after stroke: a randomized controlled trial. Zhongguo Zhen Jiu 2015;35(10):990‐4. [PubMed] [Google Scholar]
Meng 2018 {published data only}
- Meng P, Zhang S, Wang Q, Wang P, Han C, Gao J, Yue S. The effect of surface neuromuscular electrical stimulation on patients with post‐stroke dysphagia. Journal of Back & Musculoskeletal Rehabilitation 2018;31(2):363‐70. [DOI] [PubMed] [Google Scholar]
Moon 2017 {published data only}
- Moon JH, Jung J, Won YS, Cho H, Cho K. Effects of expiratory muscle strength training on swallowing function in acute stroke patients with dysphagia. Journal of Physical Therapy Science 2017;29:609‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moon 2018 {published data only}
- Moon JH, Hahm SC, Won YS, Cho HY. The effects of tongue pressure strength and accuracy training on tongue pressure strength, swallowing function, and quality of life in subacute stroke patients with dysphagia: a preliminary randomized clinical trial. International Journal of Rehabilitation Research 2018; Vol. 41, issue 3:204‐10. [DOI: 10.1097/MRR.0000000000000282] [DOI] [PubMed]
NCT00722111 {published data only}
- NCT00722111. Exercise for swallowing problems after stroke. https://clinicaltrials.gov/ct2/show/NCT00722111 (first received 25 July 2008).
NCT01081444 {published data only}
- NCT01081444. Repetitive transcranial stimulation (rTMS) in post stroke dysphagia. clinicaltrials.gov/ct2/show/record/NCT01081444?term=NCT01081444&rank=1 (first received 5 March 2010).
NCT01085903 {published data only}
- NCT01085903. Identifying and treating arousal related deficits in neglect and dysphagia. https://clinicaltrials.gov/ct2/show/NCT01085903 (first received 12 March 2010).
NCT01777672 {published data only}
- NCT01777672. Effect of afferent oropharyngeal pharmacological and electrical stimulation on swallow response and on activation of human cortex in stroke patients with oropharyngeal dysphagia (OD). A randomized controlled trial. clinicaltrials.gov/show/NCT01777672 (first received 29 January 2013).
NCT02090231 {published data only}
- NCT02090231. The effect of repetitive transcranial magnetic stimulation for post‐stroke dysphagia recovery. https://clinicaltrials.gov/ct2/show/NCT02090231 (first received 18 March 2014).
NCT02379182 {published data only}
- NCT02379182. Randomized controlled trial to evaluate the effect of vitalstim in patients with chronic post‐stroke oropharyngeal dysphagia. clinicaltrials.gov/show/NCT02379182 (first received 4 March 2015).
Nowicki 2003 {published data only}
- Nowicki NC, Averill A. Acupuncture for dysphagia following stroke. Medical Acupuncture 2003;14(3):17‐9. [Google Scholar]
Oshima 2009 {published data only}
- Oshima F, Takezawa H, Hamanaka M, Imai K, Makino M, Oda K, et al. Usefulness of nutritional management and swallowing training during the acute phase of cerebral infarction and the incidence rate of infection. Dysphagia 2009;24:453. [Google Scholar]
Pan 2015 {published data only}
- Pan MZ, Chen J, Lin L. Effect of traditional Chinese medicine rehabilitation nursing on functional rehabilitation of dysphagia in stroke patients. Chinese Medicine Modern Distance Education of China 2015;13(23):107‐9. [Google Scholar]
Park 2017 {published data only}
- * Park JS, Hwang NK, Oh DH, Chang MY. Effect of head lift exercise on kinematic motion of the thyolaryngeal complex and aspiration in patients with dysphagic stroke. Journal of Oral Rehabilitation 2017;44:385–91. [DOI] [PubMed] [Google Scholar]
- KCT0001901. Effect of shaker exercise on motion of hyolaryngeal complex and aspiration in stroke patients with oropharyngeal dysphagia. http://cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=6221 (first received 30 October 2015).
Park 2018 {published data only}
- Park J, An D, Oh D, Chang M. Effect of chin tuck against resistance exercise on patients with dysphagia following stroke: a randomized pilot study. NeuroRehabilitation 2018;42(2):191‐7. [DOI] [PubMed] [Google Scholar]
Shao 2017 {published data only}
- Shao W‐B, Wang Y, Jiang W‐W, Tian L, Zhang J. Clinical study of columnar balloon dilatation therapy for severe dysphagia caused by upper esophageal sphincter achalasia after stroke. Chinese Journal of Contemporary Neurology and Neurosurgery 2017;17(3):185‐91. [Google Scholar]
Su 2010 {published data only}
- Su X, Lai X. The clinical study on "tongdutiaoshen" (an acupuncture treatment) for treatment of dysphagia after stroke. Journal of Clinical Acupuncture and Moxibustion 2010;26:3‐6. [Google Scholar]
Sun 2008 {published data only}
- Sun J, Mi Z, Wang H, Xu D, Chen H. Study on therapeutic effect of acupuncture on dysphagia after stroke. Journal of Rehabilitation Medicine 2008;169 Suppl 46:Abstract PP003‐139. [Google Scholar]
Sun 2018 {published data only}
- Sun D, Xu W, Chen N, Li S‐M, Fu T. Clinical effectiveness of intradermal needle‐embedding therapy for swallowing function in stroke patients with dysphagia. Acupuncture Research 2018;43(2):118‐22. [DOI] [PubMed] [Google Scholar]
Suntrup‐Krueger 2018 {published data only}
- NCT01970384. Transcranial direct current stimulation for dysphagia therapy in acute stroke patients. https://clinicaltrials.gov/ct2/show/NCT01970384 (first received 28 October 2013).
- Suntrup‐Krueger S, Ringmaier C, Muhle P, Wollbrink A, Kemmling A, Hanning U, et al. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Annals of Neurology 2018;83(2):328‐40. [DOI] [PubMed] [Google Scholar]
Tageldin 2017 {published data only}
- Tageldin E, Khalil M, Bahnasy W, Fouda B. Evaluation of possible role of repetitive transcranial magnetic stimulation for dysphagic patients with brain stem infarction. Neurology 2017;88(16 Suppl 1):P5.156. [Google Scholar]
Umay 2017 {published data only}
- Umay EK, Yaylaci A, Saylam G, Gundogdu I, Gurcay E, Akcapinar D, et al. The effect of sensory level electrical stimulation of the masseter muscle in early stroke patients with dysphagia: a randomized controlled study. Neurology India 2017;65(4):734‐42. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang Y. Clinical observation on cerebral stroke with dysphagia with treatment of combined traditional Chinese and west medicine. Heilongjiang Medicine Journal 2010;24:625‐6. [Google Scholar]
Wang 2014 {published data only}
- Wang Z, Song W, Qu Y, Huang X, Wang L. Efficacy of integrated swallowing function rehabilitation training in patients with nasal feeding during acute ischemic stroke. Chinese Journal of Cerebrovascular Diseases 2014;11(7):342‐6. [Google Scholar]
Wang 2015 {published data only}
- Wang Q. Clinical study on Tong Guan Li Qiao needling method for post‐stroke deglutition disorders. Shanghai Journal of Acupuncture and Moxibustion 2015;34:721‐3. [Google Scholar]
Wang 2017 {published data only}
- Wang L, Qiu X, Ye LJ. Effects of rood intervention and routine oral intervention on malnutrition in stroke patients with dysphagia. World Chinese Journal of Digestology 2017;25(21):1980‐4. [Google Scholar]
Wei 2017 {published data only}
- Wei X, Yu F, Dai M, Xie C, Wan G, Wang Y, et al. Change in excitability of cortical projection after modified catheter balloon dilatation therapy in brainstem stroke patients with dysphagia: a prospective controlled study. Dysphagia 2017;32:645‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu P, Liang F, Li Y, Yang L, Huang Y, Li A, et al. Clinical observation on acupuncture plus rehabilitation training for dysphagia after stroke ‐ a multi‐centered random‐controlled trial. Journal of Traditional Chinese Medicine 2011;52:45‐8. [Google Scholar]
Wu 2013 {published data only}
- Wu YL, Wang L, Tuo S, Yu X, Wang Q. Clinical study on the effects of acupuncture kinesiotherapy for dysphagia caused by pseudobulbar paralysis after stroke. Chinese Journal of Rehabilitation Medicine 2013;28(8):739‐42, 757. [Google Scholar]
Xia 2010 {published data only}
- Xia W, Zheng C, Zhu S, Tang Z, Wang H, Hua Q, et al. Combination of feeding swallowing training and acupuncture: an effective rehabilitation method for dysphagia post stroke. Acta Med Univ Sci Technol Huazhong Journal of Huazhong University of Science and Technology. Medical Sciences 2010;39:614‐9. [Google Scholar]
Xie 2011 {published data only}
- Xie Y, Liu H, Zhou W. Effect of acupuncture on dysphagia of convalescent stroke patients. Chinese Journal of Integrative Medicine 2011;31:736‐40. [PubMed] [Google Scholar]
Xu 2013 {published data only}
- Xu JY, Zhou ZL, Wu J. Clinical observation on the treatment of post‐stroke dysphagia by Tiaoshen Tongluo Acupuncture combined with Tongue 3‐needle and acupuncturing Double Yifeng Acupoints. Journal of Zhejiang University of Traditional Chinese Medicine 2013;37(9):1117‐8, 1132. [Google Scholar]
Xue 2004 {published data only}
- Xue W. Early rehabilitation combined with acupuncture treatment on patients with allo‐swallowing because of pseudo‐medulla oblongata paralysis after apoplexy. Chinese Journal of Composite Clinical Medicine 2004;6(12):25‐6. [Google Scholar]
Yang 2008 {published data only}
- Yang C, Lee J, Joo M, Shin Y. The effect of double application of functional electrical stimulation in patients with dysphagia after stroke. Journal of Rehabilitation Medicine 2008;169(Suppl 46):169‐70 (Abstract PP003‐142). [Google Scholar]
Yang 2012 {published data only}
- Yang EJ, Baek SR, Shin J, Lim JY, Jang HJ, Kim YK, et al. Effects of transcranial direct current stimulation (tDCS) on post‐stroke dysphagia. Restorative Neurology and Neuroscience. 2012;30(4):303‐11. [DOI] [PubMed] [Google Scholar]
Zeng 2017 {published data only}
- Zeng Y, Yip J, Cui H, Guan L, Zhu H, Zhang W, et al. Efficacy of neuromuscular electrical stimulation in improving the negative psychological state in patients with cerebral infarction and dysphagia. Neurological Research 2018;40(6):473‐9. [DOI: 10.1080/01616412.2018.1451015] [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang J, Zhao C, Jin M, Zhou Y, Wang C, Zhao X, et al. A new effective method for larynx elevation could avoid a special abnormal swallowing mode. Stroke 2007;38(2):571. [Google Scholar]
Zhang 2015 {published data only}
- Zhang C. Analysis of Huoshe Liyan Decoction on treatment of 198 cases of stroke patients with dysphagia. Liaoning Journal of Traditional Chinese Medicine 2015;42:1436‐8. [Google Scholar]
Zhang 2016 {published data only}
- Zhang M, Tao T, Zhang ZB, Zhu X, Fan WG, Pu LJ, et al. Effectiveness of neuromuscular electrical stimulation on patients with dysphagia with medullary infarction. Archives of Physical Medicine and Rehabilitation 2016;97:355‐62. [DOI] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- Zhang SY, Liu SB, Chen YM, Liao KL, Xiang Y, Pan D. Clinical trials for treatment of stroke patients with dysphagia by Vitalstim electroacupuncture combined with swallowing rehabilitation training. Acupuncture Research 2017;42(2):168‐72. [PubMed] [Google Scholar]
Zhen 2014 {published data only}
- Zhen H. Clinical observations of treatments of post‐stroke deglutition dysfunction with acupuncture and electric stimulation. Physical Medicine and Rehabilitation 2014;6(8S2):S115. [Google Scholar]
Zhong 2003 {published data only}
- Zhong C‐M, Rong G, He F‐Z, Jin H‐Y. Comparison of head and body acupuncture in the treatment of deglutition disorders in subacute period of stroke. Chinese Journal of Clinical Rehabilitation 2003;7(19):2706‐7. [Google Scholar]
Zhu 2015a {published data only}
- Zhu H, Yang Y, Rao J, Liu L, Wang Y, Shao W, Zhang J. Effect of surface electromyographic biofeedback on the pharyngeal phase activities in patients with dysphagia after stroke. Chinese Journal of Cerebrovascular Diseases 2015;11:572‐6. [Google Scholar]
Zhu 2015b {published data only}
- Zhu Z Z, Cui LL, Yin MM, Yu Y, Wang HT. Effects of swallowing training combined with low ‐frequency electrical stimulation on dysphagia after ischemic stroke. Chinese Journal of Contemporary Neurology and Neurosurgery 2015;15(4):285‐9. [Google Scholar]
References to ongoing studies
ChiCTR1800014337 {published data only}
- ChiCTR1800014337. High frequency repetitive transcranial magnetic stimulation in the rehabilitation of post‐stroke swallowing disorder. http://www.chictr.org.cn/showprojen.aspx?proj=23332 (first received 6 January 2018).
ChiCTR1800015837 {published data only}
- ChiCTR1800015837. A randomized controlled clinical study on stroke with dysphagia with treatment of combined of traditional Chinese and West medicine. http://www.chictr.org.cn/showprojen.aspx?proj=20656 (first received 24 April 2018).
ChiCTR‐ICR‐15006004 {published data only}
- ChiCTR‐ICR‐15006004. Clinical observation of YiShen‐TongQiao acupuncture on pharyngeal dysphagia after stroke. http://www.chictr.org.cn/showproj.aspx?proj=10470 (first received 25 February 2015).
ChiCTR‐IOR‐17010505 {published data only}
- ChiCTR‐IOR‐17010505. Fire needle for patients with dysphagia caused by post‐stroke pseudobulbar palsy: a randomized controlled clinical trial. http://www.chictr.org.cn/showprojen.aspx?proj=17738 (first received 23 January 2017).
ChiCTR‐IOR‐17011359 {published data only}
- ChiCTR‐IOR‐17011359. The study on the effect of electro‐acupuncture at Lianquan and Fengfu on one side of brain swallowing function. http://www.chictr.org.cn/showproj.aspx?proj=19078 (first received 11 May 2017).
ChiCTR‐IPC‐14005435 {published data only}
- ChiCTR‐IPC‐14005435. Research on mechanism of central regulation of transcranial magnetic stimulation on post‐stroke dysphagia patients. http://www.chictr.org.cn/showproj.aspx?proj=9785 (first received 17 October 2017).
ChiCTR‐ROC‐17011673 {published data only}
- ChiCTR‐ROC‐17011673. Neuromodulation on post‐stroke patients: a clinical control trial based on mapping swallowing musculature motor cortex. www.chictr.org.cn/showproj.aspx?proj=19921 (first received 16 June 2017).
ISRCTN14124645 {published data only}
- ISRCTN14124645. Metoclopramide and selective oral decontamination for avoiding pneumonia after stroke. http://www.isrctn.com/ISRCTN14124645 (first received 10 October 2016).
ISRCTN68981054 {published data only}
- ISRCTN68981054. Treatment of dysphagia after stroke with He's santong needling method: a prospective randomized controlled study. http://www.isrctn.com/ISRCTN68981054 (first received 25 September 2017).
NCT01758991 {published data only}
- NCT01758991. Improving swallowing after stroke with transcranial direct current stimulation (iSWAT). https://clinicaltrials.gov/ct2/show/NCT01758991 (first received 1 January 2013).
NCT01919112 {published data only}
- NCT01919112. Fostering eating after stroke with transcranial direct current stimulation. https://clinicaltrials.gov/ct2/show/record/NCT01919112 (first received 8 August 2013).
NCT02322411 {published data only}
- NCT02322411. Effects of device‐facilitated isometric progressive resistance oropharyngeal (I‐PRO) therapy on dysphagia related outcomes in patients post‐stroke (StrokeStrong). clinicaltrials.gov/show/NCT02322411 (first received 23 December 2014).
NCT02470078 {published data only}
- NCT02470078. Pharyngeal electrical stimulation for the treatment of post‐extubation dysphagia in acute stroke. https://clinicaltrials.gov/ct2/show/NCT02470078 (first posted 12 June 2015).
NCT02576470 {published data only}
- Humbert IA, Vose A. Kinematic visual biofeedback is best when training novel swallowing behaviors in dysphagic patients after stroke. Stroke 2018;49:ATP150. [Google Scholar]
- NCT02576470. Applying motor learning principles to dysphagia rehabilitation. https://clinicaltrials.gov/ct2/show/NCT02576470 (first received 15 October 2015).
NCT02960737 {published data only}
- NCT02960737. Dysphagia evaluation after stroke ‐ incidence and effect of oral screen intervention on swallowing dysfunction. clinicaltrials.gov/show/NCT02960737 (first received 10 November 2016).
NCT03021252 {published data only}
- NCT03021252. Respiratory muscle training in stroke swallowing disorders RETORNUS‐2. https://clinicaltrials.gov/ct2/show/NCT03021252 (first received 13 January 2017).
NCT03247374 {published data only}
- NCT03247374. Bio‐feedback treatment versus standard treatment for dysphagic post‐stroke patients: a randomized controlled trial (bio‐feedback treatment for dysphagic post‐stroke patients (BIO_DYS)). https://clinicaltrials.gov/ct2/show/NCT03247374 (first received 11 August 2017).
NCT03274947 {published data only}
- NCT03274947. The utility of cerebellar transcranial magnetic stimulation in the neurorehabilitation of dysphagia after stroke. https://clinicaltrials.gov/ct2/show/NCT03274947 (first received 7 September 2017).
NCT03358810 {published data only}
- NCT03358810. Pharyngeal electrical stimulation evaluation for dysphagia after stroke (PhEED). https://clinicaltrials.gov/ct2/show/NCT03358810 (first received 2 December 2017).
NCT03499574 {published data only}
- NCT03499574. Feasibility study of biofeedback in dysphagia therapy post stroke. https://www.clinicaltrials.gov/ct2/show/record/NCT03499574?id=NCT03499574&rank=1 (first received 17 April 2018).
PACTR201710002724163 {published data only}
- PACTR201710002724163. Effect of transcutaneous electrical nerve stimulation and conventional therapy in post‐stroke dysphagic patients: a randomized controlled trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201710002724163 (first received 26 October 2017).
U1111‐1188‐0335 {published data only}
- U1111‐1188‐0335. Program of rehabilitation with therapeutic efficacy control in oropharyngeal dysphagia after stroke. www.ensaiosclinicos.gov.br/rg/RBR‐33grwq/ (first received 26 September 2016).
Additional references
Arnold 2016
- Arnold M, Liesirova K, Broeg‐Morvay A, Meisterernst J, Schlager M, Mono M‐L, et al. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLoS ONE 2016;11(2):e0148424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashford 2009
- Ashford J, McCabe D, Wheeler‐Hegland K, Frymark T, Mullen R, Musson N, et al. Evidence‐based systematic review: oropharyngeal dysphagia behavioral treatments. Part III. Impact of dysphagia treatments on populations with neurological disorders. Journal of Rehabilitation, Research and Development 2009;46(2):195‐204. [PubMed] [Google Scholar]
Barer 1989
- Barer D. The natural history and functional consequences of dysphagia after hemisphere stroke. Journal of Neurology, Neurosurgery and Psychology 1989;52:236‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carnaby 2006
- Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurology 2006;5:31‐7. [DOI] [PubMed] [Google Scholar]
Chen 2016
- Chen YW, Chang KH, Chen HC, Liang WM, Wang YH, Lim YN. The effects of surface neuromuscular electrical stimulation on post‐stroke dysphagia: a systemic review and meta‐analysis. Clinical Rehabilitation 2016;30(1):24‐35. [DOI] [PubMed] [Google Scholar]
Cohen 2016
- Cohen DL, Roffe C, Beavan J, Blackett B, Fairfield CA, Hamdy S, et al. Post stroke dysphagia: a review and design considerations for future trials. International Journal Stroke 2016;11(4):399‐411. [DOI] [PubMed] [Google Scholar]
Ding 2016
- Ding R, Ma F. Effectiveness of neuromuscular electrical stimulation on dysphagia treatment in patients with neurological impairments – a systematic review and metaanalysis. Annals of Otolaryngology and Rhinology 2016;3(12):1151. [Google Scholar]
Finestone 1996
- Finestone HM, Greene‐Finestone LS, Wilson ES, Teasell RW. Prolonged length of stay and reduced functional improvement rate in malnourished stroke rehabilitation patients. Archives of Physical Medicine and Rehabilitation 1996;77:340‐5. [DOI] [PubMed] [Google Scholar]
Gordon 1987
- Gordon C, Langton‐Hewer R, Wade D. Dysphagia in acute stroke. BMJ 1987;295:411‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamdy 1998
- Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 1998;115(5):1104‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinchey 2005
- Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S, the Stroke Practice Improvement Network Investigators. Formal dysphagia screening protocols prevent pneumonia. Stroke 2005;36:1972‐6. [DOI] [PubMed] [Google Scholar]
Krival 2008
- Krival K, Pelletier C, Kelchner L. Effects of carbonate vs thin and thickened liquids on swallowing in adults with stroke. Dysphagia 2008;23:428. [Google Scholar]
Lakshminarayan 2010
- Lakshminarayan K, Tsai AW, Tong X, Vazquez G, Peacock JM, George MG, et al. Utility of dysphagia screening results in predicting poststroke pneumonia. Stroke 2010;41(12):2849‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazarra 1986
- Lazarra G, Lazarus C, Logemann J. Impact of thermal stimulation on the triggering of the swallow reflex. Dysphagia 1986;1:73‐7. [Google Scholar]
Liao 2016
- Liao X, Xing G, Guo Z, Jin Y, Tang Q, He B, et al. Repetitive transcranial magnetic stimulation as an alternative therapy for dysphagia after stroke: a systematic review and meta‐analysis. Clinical Rehabilitation 2017;31(3):289‐98. [DOI] [PubMed] [Google Scholar]
Logemann 1991
- Logemann J. Approaches to management of disordered swallowing. Clinical Gastroenterology 1991;5:269‐80. [DOI] [PubMed] [Google Scholar]
Logemann 1993
- Logemann J. Non‐invasive approaches to deglutitive aspiration. Dysphagia 1993;8:331‐3. [DOI] [PubMed] [Google Scholar]
Long 2012
- Long Y‐B, Wu X‐P. A meta‐analysis of the efficacy of acupuncture in treating dysphagia in patients with a stroke. Acupuncture in Medicine 2012;00:1‐7. [DOI] [PubMed] [Google Scholar]
Mann 1999
- Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke 1999;30:744‐8. [DOI] [PubMed] [Google Scholar]
Mann 2000
- Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovascular Diseases 2000;10:380‐6. [DOI] [PubMed] [Google Scholar]
Martino 2005
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36(12):2756‐63. [DOI] [PubMed] [Google Scholar]
Mendelsohn 1987
- Mendelsohn MS, McConnell FM. Function in the pharyngoesophageal segment. Laryngoscope 1987;97(4):483‐9. [DOI] [PubMed] [Google Scholar]
Momosaki 2016
- Momosaki R, Kinoshita S, Kakuda W, Yamada N, Abo M. Noninvasive brain stimulation for dysphagia after acquired brain injury. A systematic review. Journal of Medical Investigation 2016;63(3‐4):153‐8. [DOI] [PubMed] [Google Scholar]
Odderson 1995
- Odderson IR, Keaton JC, McKenna BS. Swallow management in patients on an acute stroke pathway: quality is cost effective. Archives of Physical Medicine and Rehabilitation 1995;76:1130‐3. [DOI] [PubMed] [Google Scholar]
Perry 2004
- Perry L. Eating and dietary intake in communication impaired stroke survivors: a cohort study from acute stage hospital admission to 6 months post stroke. Clinical Nutrition 2004;23:1333‐43. [DOI] [PubMed] [Google Scholar]
Pisegna 2016
- Pisegna JM, Kaneoka A, Pearson Jr WG, Kumar S, Langmore SE. Effects of non‐invasive brain stimulation on post‐stroke dysphagia: a systematic review and meta‐analysis of randomized controlled trials. Clinical Neurophysiology 2016;127(1):956‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsey 2003
- Ramsey DJC, Smithard D, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke 2003;34:1252‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rofes 2013
- Rofes L, Vilardell N, Clavé P. Post‐stroke dysphagia: progress at last. Neurogastroenterology and Motility 2013;25(4):278‐82. [DOI] [PubMed] [Google Scholar]
Scutt 2015
- Scutt P, Lee HS, Hamdy S, Bath PM. Pharyngeal electrical stimulation for treatment of poststroke dysphagia: individual patient data meta‐analysis of randomised controlled trials. Stroke Research and Treatment 2015;2015:1‐8. [DOI: 10.1155/2015/429053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaker 2002
- Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, et al. Rehabilitation of swallowing by exercise in tube‐fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 2002;122(5):1314‐21. [DOI] [PubMed] [Google Scholar]
Sharma 2001
- Sharma JC, Fletcher S, Vassallo M, Ross I. What influences outcome after stroke ‐ pyrexia or dysphagia?. International Journal of Clinical Practice 2001;55(1):17‐20. [PubMed] [Google Scholar]
Singh 2006a
- Singh S, Hamdy S. Dysphagia in stroke patients. Postgraduate Medical Journal 2006;82:383‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smithard 1993
- Smithard D, Kenwick D, Martin D, O'Neill P. Chest infection following acute stroke: does aspiration matter?. Age and Ageing 1993;22 Suppl 3:24‐9. [Google Scholar]
Smithard 1996
- Smithard DG, O'Neill PA, Park C, Morris J, Wyatt R, England R, et al. Complications and outcome after acute stroke. Does dysphagia matter?. Stroke 1996;27:1200‐4. [DOI] [PubMed] [Google Scholar]
Smithard 1997
- Smithard DG, O'Neil PA, England RE, Park, CL, Wyatt, R, Martin DF, et al. The natural history of dysphagia following stroke. Dysphagia 1997;12(4):188‐93. [DOI] [PubMed] [Google Scholar]
Theurer 2013
- Theurer JA, Johnston JL, Fisher J, Darling S, Stevens RC, Taves D, et al. Proof‐of‐principle pilot study of oropharyngeal air‐pulse application in individuals with dysphagia after hemispheric stroke. Archives of Physical Medicine and Rehabilitation 2013;94(6):1088‐94. [DOI] [PubMed] [Google Scholar]
Wolfe 1993
- Wolfe C, Taub N, Woodrow J, Richardson E, Warburton F, Burney P. Patterns of acute stroke care in three districts of southern England. Journal of Epidemiology and Community Health 1993;47:144‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong ISY, Ng KF, Tsang HWH. Acupuncture for dysphagia following stroke: a systematic review. European Journal of Integrative Medicine. 2012;4(2):141‐50. [Google Scholar]
Xie 2008
- Xie Y, Wang L, He J, Wu T. Acupuncture for dysphagia in acute stroke. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2015
- Yang SN, Pyun S‐B, Kim HJ, Ahn HS, Rhyu BJ. Effectiveness of non‐invasive brain stimulation in dysphagia subsequent to stroke: a systematic review and meta‐analysis. Dysphagia 2015;30:383‐91. [DOI] [PubMed] [Google Scholar]
Yuan 2003
- Yuan ZH, Huang LL, Chen ZL. Coagulant and enteral nutrition agents in the rehabilitation of deglutition disorders for patients with acute stroke. Chinese Journal of Clinical Rehabilitation 2003;7(28):3834‐5. [Google Scholar]
References to other published versions of this review
Bath 1999
- Bath PMW, Bath FJ, Smithard DG. Interventions for dysphagia in acute stroke. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000323] [DOI] [PubMed] [Google Scholar]
Geeganage 2012
- Geeganage C, Beavan J, Ellender S, Bath PMW. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000323.pub2] [DOI] [PubMed] [Google Scholar]


