Abstract
Background
Progesterone, a female sex hormone, is known to induce secretory changes in the lining of the uterus essential for successful implantation of a fertilized egg. It has been suggested that a causative factor in many cases of miscarriage may be inadequate secretion of progesterone. Therefore, clinicians use progestogens (drugs that interact with the progesterone receptors), beginning in the first trimester of pregnancy, in an attempt to prevent spontaneous miscarriage. This is an update of a review, last published in 2013.
Objectives
To assess the efficacy and safety of progestogens as a preventative therapy against recurrent miscarriage.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (6 July 2017) and reference lists from relevant articles, attempting to contact trial authors where necessary, and contacted experts in the field for unpublished works.
Selection criteria
Randomized or quasi‐randomized controlled trials comparing progestogens with placebo or no treatment given in an effort to prevent miscarriage.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. Two reviewers assessed the quality of the evidence using the GRADE approach.
Main results
Twelve trials (1,856 women) met the inclusion criteria. Eight of the included trials compared treatment with placebo and the remaining four trials compared progestogen administration with no treatment. The trials were a mix of multicenter and single‐center trials, conducted in India, Jordan, UK and USA. In five trials women had had three or more consecutive miscarriages and in seven trials women had suffered two or more consecutive miscarriages. Routes, dosage and duration of progestogen treatment varied across the trials. The majority of trials were at low risk of bias for most domains. Ten trials (1684 women) contributed data to the analyses.
The meta‐analysis of all women, suggests that there may be a reduction in the number of miscarriages for women given progestogen supplementation compared to placebo/controls (average risk ratio (RR) 0.73, 95% confidence interval (CI) 0.54 to 1.00, 10 trials, 1684 women, moderate‐quality evidence). A subgroup analysis comparing placebo‐controlled versus non‐placebo‐controlled trials, trials of women with three or more prior miscarriages compared to women with two or more miscarriages and different routes of administration showed no clear differences between subgroups for miscarriage.
None of the trials reported on any secondary maternal outcomes, including severity of morning sickness, thromboembolic events, depression, admission to a special care unit, or subsequent fertility.
There was probably a slight benefit for women receiving progestogen seen in the outcome of live birth rate (RR 1.07, 95% CI 1.00 to 1.13, 6 trials, 1411 women, moderate‐quality evidence). We are uncertain about the effect on the rate of preterm birth because the evidence is very low‐quality (RR 1.13, 95% CI 0.53 to 2.41, 4 trials, 256 women, very low‐quality evidence). No clear differences were seen for women receiving progestogen for the other secondary outcomes including neonatal death, fetal genital abnormalities or stillbirth. There may be little or no difference in the rate of low birthweight and trials did not report on the secondary child outcomes of teratogenic effects or admission to a special care unit.
Authors' conclusions
For women with unexplained recurrent miscarriages, supplementation with progestogen therapy may reduce the rate of miscarriage in subsequent pregnancies.
Keywords: Female; Humans; Pregnancy; Abortion, Habitual; Abortion, Habitual/prevention & control; Abortion, Spontaneous; Abortion, Spontaneous/epidemiology; Abortion, Spontaneous/prevention & control; Live Birth; Live Birth/epidemiology; Placebos; Placebos/administration & dosage; Pregnancy Trimester, Second; Premature Birth; Premature Birth/epidemiology; Progestins; Progestins/therapeutic use; Randomized Controlled Trials as Topic
Progestogen for preventing miscarriage
What is the issue?
Early pregnancy loss, also known as miscarriage, generally occurs in the first trimester. For some women and their partners, miscarriages can happen several times, also known as recurrent miscarriages. While there are sometimes causes for miscarriages that are found, often no clear reasons can be found. The hormone called progesterone prepares the womb (uterus) to receive and support the newly fertilized egg during the early part of pregnancy. It has been suggested that some women who miscarry may not make enough progesterone in the early part of pregnancy. Supplementing these women with medications that act like progesterone (these are called progestogens) has been suggested as a possible way to prevent recurrent miscarriage.
Why is this important?
Having miscarriages can be both physically and emotionally difficult for women and their partners. Finding a therapy to help reduce recurrent miscarriages could help them avoid a miscarriage and have a live baby.
What evidence did we find?
We searched for evidence on 6 July 2017 and identified a total of 13 trials that enrolled a total of 2556 women with a history of recurrent miscarriages. These trials found that giving progestogen medication to women with recurrent miscarriages early in their pregnancy may help lower the rates of miscarriage in that pregnancy from 27.5% to 20.1%. We believe that these findings are based on evidence of only moderate quality, so we cannot be certain about the results. We did not find that giving the progestogen medication by mouth, as a shot (injection), or in the vagina, was any better than any of the other ways. We also found that the trials showed that giving progestogen to women with prior recurrent miscarriages made the chances of having a live baby in the current pregnancy slightly higher. We are uncertain about the effect on the rate of preterm birth because the evidence is very low‐quality. We did not find evidence of improvement in other outcomes such as newborn death, stillbirth, low birthweight, or newborn birth defects for women given progestogens.
What does this mean?
We found evidence from randomized controlled trials that giving progestogen medication may prevent miscarriage for women with recurrent previous miscarriages.
Summary of findings
Summary of findings for the main comparison.
Progestogen compared to placebo/no treatment for preventing miscarriage in women with recurrent miscarriage of unclear etiology
| Progestogen compared to placebo/no treatment for preventing miscarriage in women with recurrent miscarriage of unclear etiology | ||||||
|
Patient or population: women with recurrent miscarriage of unclear etiology Setting: mix of multicenter and single‐center trials based in Egypt, India, Jordan, UK and USA Intervention: progestogen Comparison: placebo/no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with progestogen | |||||
| Miscarriage (all trials) | Study population | average RR 0.73 (0.54 to 1.00) | 1684 (10 RCTs) | ⊕⊕⊕⊝ Moderate1 |
||
| 275 per 1000 | 201 per 1000 (149 to 275) | |||||
| Live birth rate | Study population | RR 1.07 (1.00 to 1.13) | 1411 (6 RCTs) | ⊕⊕⊕⊝ Moderate1 |
||
| 701 per 1000 | 750 per 1000 (701 to 793) | |||||
| Preterm birth | Study population | RR 1.13 (0.53 to 2.41) | 256 (4 RCTs) | ⊕⊝⊝⊝ Very low1,2 | ||
| 75 per 1000 | 84 per 1000 (40 to 180) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious limitations in study design due to some of the studies being at high or unclear risk for selection bias.
2 We downgraded (2) levels for very serious limitations in imprecision due to small number of events, small sample size and wide CI crossing the line of no effect.
Background
Description of the condition
The term miscarriage refers to the loss of a pregnancy prior to the fetus being viable. This can be defined as reaching 20 or 24 weeks' gestation depending on the source of the data. Vaginal bleeding during the first 20 weeks of pregnancy, with or without pain, is known as threatened miscarriage. This can present with anything from spots of blood to potentially fatal shock (McBride 1991). Once dilation of the cervix has begun, miscarriage is inevitable (Lede 2005).
Ten per cent to 15% of all clinically recognized pregnancies end in miscarriage (Regan 1989), with 1% to 2% of couples suffering recurrent early losses (traditionally defined as three or more spontaneous miscarriages; Coulam 1991). It is thought, however, that the true incidence of early spontaneous miscarriage may be much higher (Grudzinskas 1995; Howie 1995; Simpson 1991). Miscarriage is an important cause of morbidity and mortality, especially in low‐income countries (Neilson 2006).
It has been estimated that, in over half of miscarriages, a chromosomal abnormality is present (Burgoyne 1991; Szabo 1996). Other risk factors include maternal age greater than 35 years, multiple pregnancies, uterine malformations, polycystic ovaries, autoimmune factors (such as phospholipid antibodies, lupus anticoagulant and cardiolipin antibodies), genetic disorders, poorly controlled diabetes, and having had two or more miscarriages (Lede 2005). Many of these conditions can lead to recurrent miscarriage, traditionally defined as three or more miscarriages (Royal 2001). For many women and their partners, a cause of recurrent miscarriage may never be found. With the development of ultrasound and improvements in pregnancy testing, pregnancies can be diagnosed earlier, even if destined for early miscarriage. In the past, these may not have been detected. Thus, the number of women reporting early pregnancy loss may increase.
The occurrence of a miscarriage may induce significant emotional distress in both partners. Initial emotional numbness and denial, anxiety, shock, sense of loss, sadness, emptiness, anger, inadequacy, blame and jealousy, depression, sleep disturbance, social withdrawal, anger and marital disturbance have all been described as emotional responses to pregnancy loss (Atkin 1998; Dyregrove 1987; Vance 1991; Woods 1987).
Description of the intervention
Progesterone and other progestogens can be given to women orally, vaginally, intramuscularly, or by other routes. Supplementing a pregnancy with progestin often begins early in the first trimester after a positive pregnancy test is obtained (under 14 weeks' gestation) and can continue throughout the first trimester and beyond. The therapeutic intervention can be with natural progesterone or with any other progestogen that interacts with and stimulates the progesterone receptor.
How the intervention might work
Progesterone, a female sex hormone, is known to induce secretory changes in the lining of the uterus essential for successful implantation of a fertilized egg. It is secreted chiefly by the corpus luteum, a group of cells formed in the ovary after the follicle ruptures during the release of the egg. It has been suggested that a causative factor in many cases of miscarriage may be inadequate secretion of progesterone during the luteal phase of the menstrual cycle and in the early weeks of pregnancy. Therefore, clinicians use progestogens, beginning in the first trimester of pregnancy, in an attempt to prevent spontaneous miscarriage. Their use is particularly common with assisted reproductive technologies. For women with recurrent miscarriages, whose evaluation has not yielded a specific cause, the presumed diagnosis of a deficiency in progesterone as a cause of the miscarriages has led to women begin given supplemental progestins to help 'support' the pregnancy.
A review of pregnancy rates following hormonal treatments for various conditions concluded that the benefits of therapy are uncertain (Karamadian 1992). A 1989 meta‐analysis of six trials concluded that exogenous progesterone supplementation after conception does not improve pregnancy outcomes (Goldstein 1989; Regan 1989). It was concluded that low levels of progesterone in early pregnancy reflected an already failed pregnancy (Royal 2001).
Concerns have been raised that the use of progestogens, with their uterine‐relaxant properties, in women with fertilized defective ova may cause a delay in spontaneous abortion. Reports also suggest a potential association between intrauterine exposure to progesterone containing drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. The risk of hypospadias (deformities of the penis or urethra, or both), 5 to 8 per 1000 male births in the general population, may be increased with exposure to these drugs (Briggs 2011). However, some trials do not report increased risk with exposure to these drugs and progesterone supplementation is commonly utilized in assisted reproduction settings. There are insufficient data to quantify the risk to exposed female fetuses. Due to some of these reports and the fact that the urogenital groove is fused by 16 weeks, some have recommended that progesterone‐containing drugs be avoided in the first 16 weeks of pregnancy (Briggs 2011).
Why it is important to do this review
Several Cochrane Reviews have been initiated to investigate different interventions for the prevention of miscarriage (Alfirevic 2012; Bamigboye 2003; Lede 2005; Morley 2013; Porter 2006; Rumbold 2011). The aim of this updated review is to study all available data to determine the efficacy and safety of administering prophylactic progesterone in an attempt to prevent pregnancy loss in the population of women with recurrent miscarriages. There is a separate Cochrane Review on the use of progestogens for treating women with threatened miscarriage (Wahabi 2018). This is an update of a review last published in 2013 (Haas 2013).
Objectives
To assess the efficacy and safety of progestogens as a preventative therapy against recurrent miscarriage.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized trials comparing progestogens with placebo or no treatment, given for the prevention of miscarriage, were eligible for inclusion. Cluster‐randomized trials were also eligible for inclusion, but cross‐over design trials were not eligible.
Types of participants
Women who were diagnosed with recurrent miscarriages (usually of unknown origin) and who began treatment with progestins in the first trimester of pregnancy. We placed no restriction on the age of participants or past obstetric history otherwise. Where specified, we limited the analysis to singleton pregnancies. We excluded women achieving pregnancy by in‐vitro fertilization.
Types of interventions
Progestogen therapy, either natural or synthetic, given prophylactically to prevent recurrent miscarriage (i.e. loss during the first 20 weeks of pregnancy) versus placebo therapy or no therapy, regardless of dose, mode of administration or treatment duration. We considered trials pertaining to administration of progestogens starting before pregnancy if treatment continued after pregnancy was confirmed.
Types of outcome measures
Primary outcomes
Miscarriage ‐ defined by trial authors but typically as pregnancy loss less than 20 weeks' gestation
Secondary outcomes
Mother
Maternal adverse events (from progestogen)
Severity of 'morning sickness' ‐ intensified headache, nausea, breast tenderness
Reported thromboembolic events
Depression
Admission to special care unit
Subsequent fertility
Child
Live birth rate (defined as number of live births in entire study population)
Preterm birth (< 37 weeks' gestation)
Neonatal death
Fetal genital abnormalities
Stillbirth (fetal loss after 20 weeks' gestation in women with a viable pregnancy after 20 weeks)
Low birthweight less than 2500 g
Teratogenic effects (impairing normal fetal development)
Admission to special care unit
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (6 July 2017)
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (6 July 2017) for unpublished, planned and ongoing trial reports using the terms listed in Appendix 1.
Searching other resources
We searched the citation lists of relevant publications, review articles, abstracts of scientific meetings and included studies for both published and unpublished works but did not find any additional references.
We contacted experts in the field for unpublished works. None were revealed to us.
We obtained all reports that described (or may have described) randomized controlled trials of prophylactic progestogen to prevent pregnancy loss for women with recurrent pregnancy loss. We did not apply any language or date restrictions and attempted to make to contact with trial authors when we required additional information.
Data collection and analysis
For methods used in the previous version of this review, see Haas 2013.
For this update, we used the following methods, based on a standard template used by Cochrane Pregnancy and Childbirth, to assess the reports that we identified as a result of the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential trials that we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Additionally, as this Cochrane Review's focus had shifted specifically to women with recurrent miscarriage, we re‐evaluated the prior included trials and excluded ones that did not report on women with recurrent miscarriages.
Data extraction and management
We designed a form to extract data. For eligible trials, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager 5 software (Review Manager 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE Handbook in order to assess the quality of the body of evidence relating to the outcome of miscarriage for the main comparisons of progestogen versus placebo/no treatment (Schünemann 2013). We assessed the following outcomes using GRADE:
miscarriage;
live birth rate;
preterm birth.
We used the GRADEpro Guideline Development Tool (GRADEpro GDT 2015), to import data from Review Manager 5 (Review Manager 2014), in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for the above outcome using the GRADE approach (Schünemann 2013). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
None of our outcomes have continuous data. If future updates add continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardized mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomized trials
We did not include cluster‐randomized trials in this Cochrane Review. In future updates, if we identify any high‐quality cluster‐randomized trials, we will consider including them in the analyses along with individually randomized trials. We will adjust their sample sizes using the methods described in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Cross‐over trials
Cross‐over trials are not included as they are inappropriate to the question.
Other unit of analysis issues
If we identify and include trials with more than two treatment groups, we will assess the most appropriate way to include the data. This may be by combining groups to create a pair‐wise comparison or to select the most appropriate pair of interventions and exclude the others. We did not identify any such trials.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003), and Chi² statistics (Deeks 2017). We regarded heterogeneity as substantial if an I² statistic was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots, see Figure 1. We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
Figure 1.
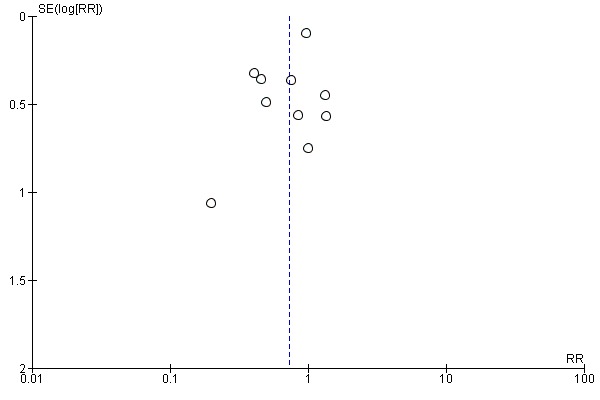
Funnel plot of comparison 1. Progestogen versus placebo/no treatment, outcome 1.1 Miscarriage (all trials)
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. Where we used random‐effects meta‐analysis, we treated the random‐effects summary as the average of range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned not to combine trials. If we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We conducted planned subgroup analyses for the following subgroups:
placebo‐controlled trials only versus trials not having placebo control;
women with at least three previous consecutive miscarriages versus women with at least two prior miscarriages;
route of administration of progestogen: oral, intramuscular or vaginal versus placebo.
We used only the primary outcome in subgroup analysis: miscarriage.
We assessed subgroup differences by interaction tests available within Review Manager 5 (Review Manager 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analysis to explore the effect of risk of bias. This involved analysis of trials based on low risk of bias in order to assess for any substantive difference to the overall results.
Results
Description of studies
Results of the search
See: Figure 2 for full details of the updated search process.
Figure 2.
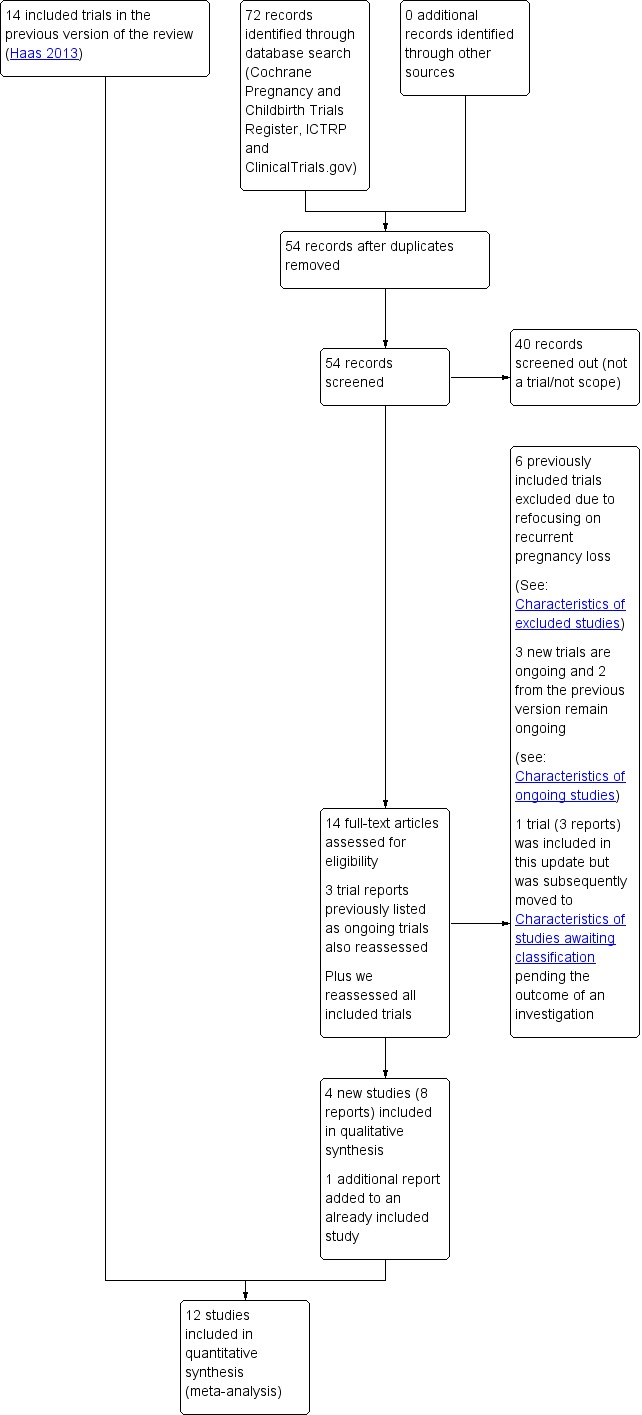
Study flow diagram
The July 2017 search retrieved 14 new trial reports to assess, plus there were three previously ongoing trials to reassess. One of the previously ongoing trials had been published and we included it (Coomarasamy 2015). We included three other, newly identified trials (Agarwal 2016; Ghosh 2014; Kumar 2014). Three of the reports were ongoing trials listed in trials registries (IRCT2013100114853N1; ACTRN12611000401954; NCT02706470). Attempts to contact the authors were not fruitful. One study (Ismail 2017) was previously included in this update but has now been moved to studies awaiting classification pending the outcome of a formal investigation ‐ see .'Studies awaiting classification' below.
In this update the scope changed to include specifically women with recurrent pregnancy loss. Four trials included in previous versions of the review were therefore excluded from this update as the populations dealt with women having threatened miscarriage symptoms (Berle 1980; Gerhard 1987; Moller 1965; Tognoni 1980). One previously included trial was excluded as it only enrolled women getting a genetic amniocentesis (Corrado 2002), and one previously included trial was excluded as it only enrolled women receiving in‐vitro fertilization or other reproductive assistance (Nyboe Anderson 2002).
This resulted in a total of 12 included trials and five Ongoing studies.
Included studies
Trial design characteristics
Interventions
Route of administration
Seven trials administered treatment orally (Agarwal 2016; El‐Zibdeh 2005; Ghosh 2014; Goldzieher 1964; Klopper 1965; Kumar 2014; MacDonald 1972); three administered treatment intramuscularly (Le Vine 1964; Reijnders 1988; Shearman 1963); one used vaginal micronized progesterone (Coomarasamy 2015); and one used progestogen pellets inserted into the gluteal muscle (Swyer 1953).
Dosage and type of progestogen
Of the trials that administered treatment orally, one study used a dose of 10 mg/day medroxyprogesterone (Goldzieher 1964), one study used a twice‐daily dose of cyclopentyl enol ether of progesterone (Enol Luteovis), (Klopper 1965), while three used 10 mg of oral dydrogesterone either twice daily (El‐Zibdeh 2005; Ghosh 2014) or three times daily (MacDonald 1972). One trial gave participants 20 mg of oral dydrogesterone daily (Kumar 2014). One trial used 200 mg oral micronized progesterone twice daily (Agarwal 2016).
In the three trials that administered treatment intramuscularly, two trials used a dose of 500 mg of hydroxyprogesterone caproate (Le Vine 1964; Reijnders 1988), while the third study used a staggered dose, also of hydroxyprogesterone caproate, of between 250 to 500 mg depending on week of gestation (Shearman 1963).
The remaining two trials (Coomarasamy 2015; Swyer 1953) delivered treatment via 400 mg micronized progesterone vaginally, 400 mg progesterone pessaries, and six times 25 mg progesterone pellets inserted within the gluteal muscle, respectively.
Duration of treatment
There was a wide variation in treatment duration between trials. One study continued treatment until the 24th week of pregnancy (Shearman 1963); and one study continued treatment until miscarriage or until 36 weeks' gestation (Le Vine 1964). Several trials continued treatment until the 12th week of gestation (Coomarasamy 2015; El‐Zibdeh 2005; Ghosh 2014; Reijnders 1988). One trial continued therapy to 16 weeks' gestation (Agarwal 2016) and one continued up to 20 weeks' gestation (Kumar 2014). In the remaining four trials treatment duration was either not stated or unclear (Goldzieher 1964; Klopper 1965; MacDonald 1972; Swyer 1953), although Klopper 1965 stated that they hospitalized participants starting at under 10 weeks' gestation until they were 18 weeks' gestation but was unclear how long the treatment lasted.
Placebo/control
Eight of the included trials compared treatment with placebo (Coomarasamy 2015; Goldzieher 1964; Klopper 1965; Kumar 2014; Le Vine 1964; MacDonald 1972; Reijnders 1988; Shearman 1963). The remaining four trials compared progestogen administration with no treatment (Agarwal 2016; El‐Zibdeh 2005; Ghosh 2014; Swyer 1953).
Contribution to meta‐analysis
One study included women with recurrent pregnancy loss among all recruited women but did not report outcomes for this group separately, so did not contribute to the meta‐analysis (Reijnders 1988) and one study compared oral or vaginal progestogen to a control group of women without a history of recurrent pregnancy loss (Ghosh 2014) and thus did not have an adequate control group so did not contribute data to the meta‐analysis. All other included trials contributed data to at least one comparison.
Baseline characteristics of participants
Number of prior miscarriages
Five trials required women to have had three or more consecutive miscarriages (Coomarasamy 2015; El‐Zibdeh 2005; Ghosh 2014; Kumar 2014; Le Vine 1964), and seven trials required women to have suffered two or more consecutive miscarriages (Agarwal 2016; Goldzieher 1964; Klopper 1965; MacDonald 1972; Reijnders 1988; Shearman 1963; Swyer 1953). In addition, one study (MacDonald 1972) required women to have cervical mucus ferning as evidence of "significant hormonal imbalance".
Only one study excluded women who had experienced a live birth (Klopper 1965).
Gestation
Most other trials recruited women in the first trimester of pregnancy with various cut‐offs of gestational age. One trial accepted women to the 20th gestational week (Le Vine 1964).
Studied outcomes for trials contributing to meta‐analysis
Miscarriage: 10 trials included miscarriage as an outcome.
Live birth rate: specifically reported by two trials (Coomarasamy 2015; Swyer 1953). For other trials, extrapolated from data if they presented birth data and reported individual or group outcomes and either reported a number of stillbirths or did not note any stillbirths
Preterm birth: four trials reported preterm delivery (El‐Zibdeh 2005; Goldzieher 1964; Le Vine 1964; Swyer 1953).
Intrauterine fetal death/still birth: two trials reported intrauterine fetal death/still birth as an outcome (Coomarasamy 2015; Swyer 1953).
Fetal genital abnormalities/teratogenic effects, fetal deformities: three trials (Coomarasamy 2015; El‐Zibdeh 2005; Le Vine 1964) reported fetal or genital abnormalities, or both, as an outcome.
Neonatal death: three trials contributed data for neonatal death (Coomarasamy 2015; El‐Zibdeh 2005; Swyer 1953).
Low birthweight less than 2500 g: several trials reported birthweight as an outcome but only one categorized it as less than 2500 g (Kumar 2014).
Severity of morning sickness, intensified headache, nausea, or breast tenderness: no trials reported on these as separate outcomes.
Thromboembolic events: no trials reported thromboembolic rates as an outcome.
Admission to special care unit: no trials reported admission to special care units as an outcome.
Maternal depression: no trials reported maternal depression as an outcome.
Subsequent fertility: no trials reported subsequent fertility as an outcome.
Support/sponsorship
Six trials reported what appeared to be support or sponsorship from pharmaceutical companies (Goldzieher 1964; MacDonald 1972; Shearman 1963; Klopper 1965; Reijnders 1988; Swyer 1953). Two reports were funded by governmental grants from India (Agarwal 2016; Kumar 2014), and one by governmental grants from the UK (Coomarasamy 2015). One study specified that no support was obtained (Ghosh 2014). Two trials did not mention any support (El‐Zibdeh 2005; Le Vine 1964). (See Characteristics of included studies).
All of the trials either declared no competing interests (Coomarasamy 2015; Ghosh 2014; Kumar 2014) or did not state if any interests existed (Agarwal 2016; El‐Zibdeh 2005; Goldzieher 1964; Klopper 1965; Le Vine 1964; MacDonald 1972; Reijnders 1988; Shearman 1963). One study mentioned specifically that one of the authors was grateful to a provider of the study drug but did not clarify the relationship (Swyer 1953).
Number of centers
Some of the trials were multicenter (Coomarasamy 2015; Goldzieher 1964; Klopper 1965; Shearman 1963; Swyer 1953), the rest were either single‐center trials (El‐Zibdeh 2005; Ghosh 2014; Kumar 2014; MacDonald 1972) or the number of centers was unclear (Agarwal 2016; Le Vine 1964; Reijnders 1988).
Excluded studies
On obtaining the full papers, we found that five trials were not randomized controlled trials (Check 1985; Check 1987a; Daya 1988; Rock 1985; Sidelnikova 1990), and it was unclear if there was any randomization in another (Check 1987b). Five trials were not concerned with investigating the effects of progestogen for miscarriage, but were concerned with investigating other effects of progesterone, such as on preterm birth or other outcomes (Brenner 1962; Johnson 1975; Kyrou 2011; Sondergaard 1985; Turner 1966), three used progestogen combination therapy rather than progestogen alone (Check 1995; Prietl 1992; Shu 2002), one did not administer progestogen during pregnancy (Clifford 1996), and one compared two types of progestogen rather than progestogen with no treatment or a placebo group (Smitz 1992). One trial was excluded due to using progestogen therapy after 20 weeks' gestation to prevent preterm labor (Norman 2006). We excluded one additional trial due to being terminated before data collection was complete (Fuchs 1966).
As stated above, for this update we excluded six previously included trials; four for treating threatened miscarriage (Berle 1980; Gerhard 1987; Moller 1965; Tognoni 1980), and two for ineligible patient populations (Corrado 2002; Nyboe Anderson 2002). See Characteristics of excluded studies for more details.
Studies awaiting classification
Since publication of the 2018 update of this review, we have been advised that the Ismail 2017 study is currently the subject of an investigation by the Journal of Maternal‐Fetal & Neonatal Medicine. We have now moved this study from 'included studies' to 'studies awaiting classification' until the outcome of the investigation is known.
Risk of bias in included studies
See Figure 3; Figure 4 for a summary of risk of bias in included trials.
Figure 3.
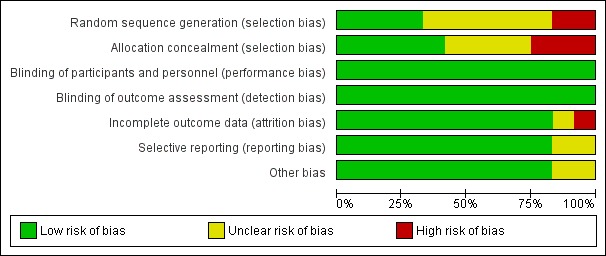
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Figure 4.
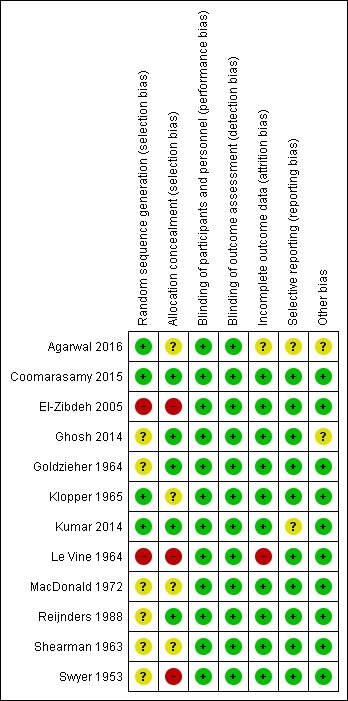
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Of the 12 trials that met the inclusion criteria, four had low risk of bias for random sequence generation. Three of the trials reported computer‐generated or other adequate methods of randomization (Agarwal 2016; Coomarasamy 2015; Kumar 2014), and one used a random table produced by a statistician (Klopper 1965). We found high risk of bias for two trials: one used alternation (Le Vine 1964), and one randomized by day of the week (El‐Zibdeh 2005). The risk of bias for random sequence generation was unclear for five other trials (Ghosh 2014; Goldzieher 1964; MacDonald 1972; Reijnders 1988; Shearman 1963). In one study (Swyer 1953), two centers took part. One center allocated by alternation, while the paper stated that the other used "randomization". However, the method of randomization was not stated and thus risk of bias is unclear.
Regarding allocation concealment, of the 12 trials that met the inclusion criteria, we assessed five as low risk of bias. These trials used sequentially numbered ampules provided by a central source or the pharmaceutical company (Coomarasamy 2015; Kumar 2014; Reijnders 1988); or sequentially numbered, sealed envelopes (Ghosh 2014). One study used sequentially numbered bottles, and the code was not broken until the end of the trial (Goldzieher 1964).
Three trials used clearly inadequate methods of allocation concealment, based on the day of the week or alternating A/B groups (El‐Zibdeh 2005; Le Vine 1964; Swyer 1953); while the allocation in the remaining four trials was unclear (Agarwal 2016; Klopper 1965; MacDonald 1972; Shearman 1963).
Blinding
Some trials blinded participants and providers but the majority of trials did not report on blinding of participants, providers, or outcome assessors. However, because the outcomes of miscarriage, live birth, etc. are unlikely to be influenced by knowledge of assignment group, per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), we assessed these also as low risk of bias, even when blinding was not explicitly stated.
Eight trials used double‐blinding, where both the participant and treating provider do not know the allocation (Coomarasamy 2015; Goldzieher 1964; Klopper 1965; Kumar 2014; Le Vine 1964; MacDonald 1972; Reijnders 1988; Shearman 1963). One study used single‐blinding by keeping study investigators and sonologists unaware of the type of protocol used, but double‐blinding was not possible due to differing drug delivery methods (Ghosh 2014). The remaining three trials did not mention efforts at blinding but as the outcomes were unlikely to be influenced by blinding efforts, we assessed all as low risk of bias (Agarwal 2016; El‐Zibdeh 2005; Swyer 1953).
Few trials stated if outcome assessors specifically were blinded. However, some stated that "study personnel" of some type were blinded (Coomarasamy 2015; Ghosh 2014; Shearman 1963). One stated that the randomization was unknown until the study was complete, thus highly likely that the outcome assessors were blinded (Kumar 2014). The remainder did not clearly state that outcome assessors were blinded but it is unlikely that knowledge of assignment would influence outcomes being assessed and so these were assessed as being at low risk of bias.
Incomplete outcome data
Most trials had low rates of attrition and accounted for participants well.
One study reported a large number of dropouts (Le Vine 1964). Fifty‐six women were randomized but 26 women were excluded from the analysis: 16 were found not to be pregnant after they had been randomized, and 10 failed to return for injections.
Selective reporting
The majority of trials reported on all planned outcomes relevant to this review.
Other potential sources of bias
Agarwal 2016 was only reported as an abstract of results located in a trials registry. As a full report was unavailable, it is unclear if there are other potential sources of bias. Ghosh 2014 reported that a large group of the women were still pregnant and did not have final outcomes so we rated it as unclear risk of other bias, as there could be outcome differences at the time of delivery that were not reported. We assessed all the other trials as low risk of other potential sources of bias.
Publication bias
The funnel plot of study size and effect size did not visually suggest evidence of publication bias (Figure 1).
See Characteristics of included studies tables for individual details.
Effects of interventions
See: Table 1
Twelve trials (1,856 women) met the inclusion criteria. Ten trials (1684 women) contributed data to the analyses.
Progestogen versus placebo/no treatment
Primary outcomes
Miscarriage
The meta‐analysis of the 10 included trials (1684 women) suggests that progestogen supplementation may reduce the miscarriage rate compared to placebo/control women (27.5% versus 20.1%, average risk ratio (average RR) 0.73, 95% confidence interval (CI) 0.54 to 1.00; Tau² = 0.08; Chi² = 14.53, df = 9 (P = 0.10); I² = 38%; moderate‐quality evidence; Analysis 1.1, Table 1). This is based on moderate‐quality evidence with evidence being downgraded for limitations in study design.
Analysis 1.1.
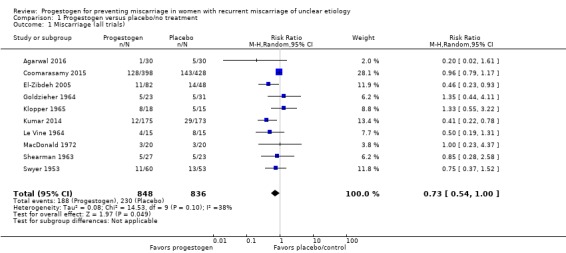
Comparison 1 Progestogen versus placebo/no treatment, Outcome 1 Miscarriage (all trials).
A sensitivity analysis eliminating the four trials at potentially higher risk of bias (having one or more areas assessed as potentially high risk of bias: Agarwal 2016; El‐Zibdeh 2005; Le Vine 1964; Swyer 1953) lessened the certainty of this effect (average RR 0.86, 95% CI 0.60 to 1.24).
Trials with placebo controls
We carried out a subgroup analysis comparing trials that did use a placebo control and those that did not use a placebo (Agarwal 2016; El‐Zibdeh 2005; Swyer 1953). There was no strong evidence of a subgroup difference as indicated by the subgroup interaction test (test for subgroup differences: Chi² = 1.68, df = 1 (P = 0.19), I² = 40.6%). Both of these subgroups demonstrated that progestogen may reduce miscarriage rates (trials with placebo controls: average RR 0.82, 95% CI 0.58 to 1.16, 7 trials, 1381 women; trials without placebo controls: average RR 0.55, 95% CI 0.34 to 0.90, 3 trials, 303 women; Analysis 1.2).
Analysis 1.2.
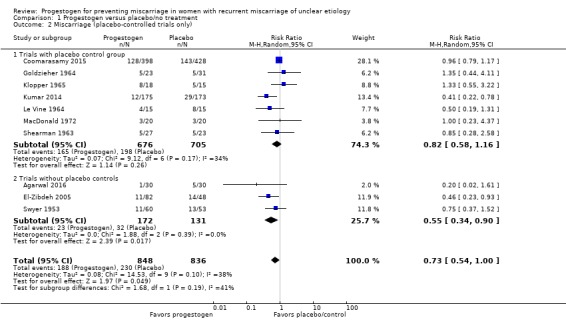
Comparison 1 Progestogen versus placebo/no treatment, Outcome 2 Miscarriage (placebo‐controlled trials only).
Women with a history of three or more consecutive miscarriages
Five trials enrolled only women who had suffered three or more consecutive miscarriages (Coomarasamy 2015; El‐Zibdeh 2005; Ghosh 2014; Kumar 2014; Le Vine 1964). The rest of the trials enrolled women with two or more prior miscarriages or did not specify. The two largest trials (Coomarasamy 2015; Kumar 2014) enrolled women with three or more prior miscarriages. There was no evidence of subgroup differences in these groups of trials in their effects for the two populations (test for subgroup differences: Chi² = 2.08, df = 1 (P = 0.15), I² = 51.9%). The meta‐analysis showed a probable reduction in miscarriage rates for women with a history of three or more miscarriages who were given progestogen supplementation (average RR 0.59, 95% CI 0.34 to 1.01, 4 trials, 1334 women; Analysis 1.3). Because of a clinical trend toward evaluating women with only two prior miscarriages for causes, the subgroup analysis also analyzed women with at least two prior miscarriages. Progestogens probably make little or no difference in miscarriage rates for women with at least two or more miscarriages, although some of these women had more than two miscarriages prior to enrolment (average RR 0.98, 95% CI 0.64 to 1.52, 5 trials, 290 women; Analysis 1.3). Thus it is not possible to discern the impact of progestogen on women who only have two prior miscarriages.
Analysis 1.3.
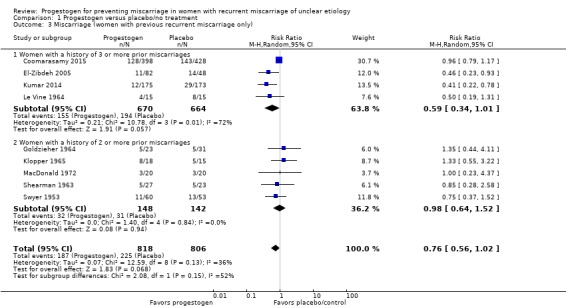
Comparison 1 Progestogen versus placebo/no treatment, Outcome 3 Miscarriage (women with previous recurrent miscarriage only).
Route of administration of progestogen
There was no evidence of a subgroup difference based on the route of administration of the progestogen as indicated by the subgroup interaction test (test for subgroup differences: Chi² = 2.66, df = 2 (P = 0.27), I² = 24.7%). Six trials compared oral progestogen with placebo/no treatment and contributed to the meta‐analysis (Agarwal 2016; El‐Zibdeh 2005; Goldzieher 1964; Klopper 1965; Kumar 2014; MacDonald 1972). The meta‐analysis showed that there was probably little or no difference in miscarriage rates with oral progestogen supplementation compared to women who received a placebo or no treatment, although rates were lower with progestogens (average RR 0.66, 95% CI 0.39 to 1.13, 6 trials, 665 women; Analysis 1.4).
Analysis 1.4.
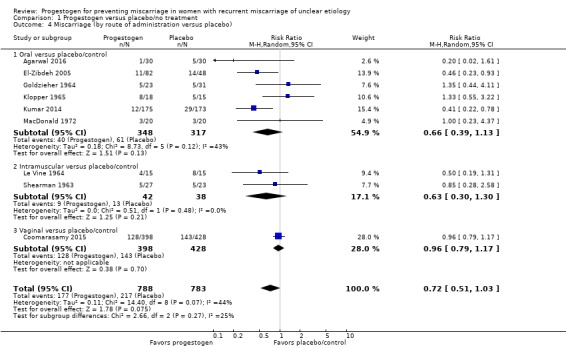
Comparison 1 Progestogen versus placebo/no treatment, Outcome 4 Miscarriage (by route of administration versus placebo).
Two trials compared intramuscular progestogen with placebo/no treatment (Le Vine 1964; Shearman 1963). The meta‐analysis showed no clear difference in miscarriages between the progestogen and placebo group (average RR 0.63, 95% CI 0.30 to 1.30, 2 trials, 80 women; Analysis 1.4).
Only one trial compared vaginally‐administered progestogen with placebo/no treatment (Coomarasamy 2015). There was probably little or no difference between the two groups with respect to the incidence of recurrent miscarriage (average RR 0.96, 95% CI 0.79 to 1.17, 1 trial, 826 women; Analysis 1.4).
Secondary outcomes
Live birth rate
Two trials specifically reported live birth rate (Coomarasamy 2015; Swyer 1953), and we were able to extrapolate the live birth rate from the results given in a further four trials (Goldzieher 1964; Kumar 2014; Le Vine 1964; MacDonald 1972). The evidence suggests that live birth rates may be increased for women receiving progestogen supplementation compared to controls (RR 1.07, 95% CI 1.00 to 1.13, 6 trials, 1411 women; moderate‐quality evidence; Analysis 1.5).
Analysis 1.5.
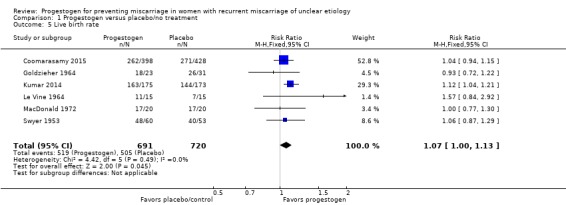
Comparison 1 Progestogen versus placebo/no treatment, Outcome 5 Live birth rate.
Preterm birth
Four trials reported an incidence of premature birth (El‐Zibdeh 2005; Goldzieher 1964; Le Vine 1964; Swyer 1953). We are uncertain about the effect on the rate of preterm birth because the quality of this evidence is very low‐quality (RR 1.13, 95% CI 0.53 to 2.41, 4 trials, 256 women, very low‐quality evidence; Analysis 1.6).
Analysis 1.6.
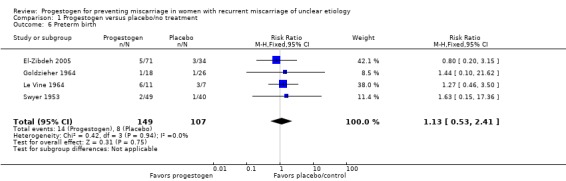
Comparison 1 Progestogen versus placebo/no treatment, Outcome 6 Preterm birth.
Neonatal death
Three trials reported neonatal death as an outcome (Coomarasamy 2015; El‐Zibdeh 2005; Swyer 1953). There were few events in these trials and there probably is little or no difference between women receiving progestogen and controls (RR 1.74, 95% CI 0.36 to 8.46, 3 trials, 724 women; Analysis 1.7).
Analysis 1.7.
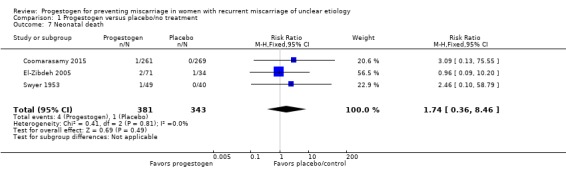
Comparison 1 Progestogen versus placebo/no treatment, Outcome 7 Neonatal death.
Fetal genital abnormalities
Three trials reported fetal genital abnormalities or virilization as an outcome (Coomarasamy 2015; El‐Zibdeh 2005; Le Vine 1964). Two trials reported no incidence of fetal abnormalities (El‐Zibdeh 2005; Le Vine 1964). One study reported a single case of fetal genital abnormality in both groups (Coomarasamy 2015). There were no differences in fetal genital anomalies (RR 1.04, 95% CI 0.07 to 16.50; 3 trials, 665 women; Analysis 1.8). One study reported two anomalies in the progestogen group (neural tube defect and non‐immune hydrops) and one case of multiple anomalies in the control group in a baby with Down's syndrome (El‐Zibdeh 2005). No genital anomalies were noted in that study.
Analysis 1.8.
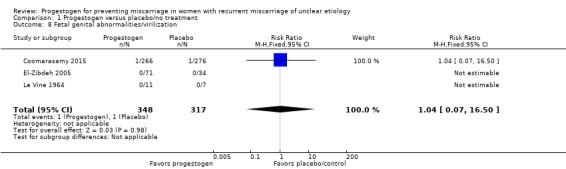
Comparison 1 Progestogen versus placebo/no treatment, Outcome 8 Fetal genital abnormalities/virilization.
Stillbirth
Two trials reported rates of stillbirth (Coomarasamy 2015; Swyer 1953). Swyer 1953 did not have any cases of stillbirth. There appears to be little or no difference in stillbirth rates (RR 0.53, 95% CI 0.05 to 5.79, 2 trials, 644 women; Analysis 1.9).
Analysis 1.9.
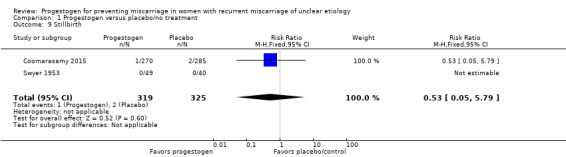
Comparison 1 Progestogen versus placebo/no treatment, Outcome 9 Stillbirth.
Low birthweight (less than 2500 g)
One study reported low birthweight by this definition (Kumar 2014). There may be little or no difference in the rate of low birthweight babies (RR 0.67, 95% CI 0.36 to 1.26, 1 trial, 348 women; Analysis 1.10).
Analysis 1.10.
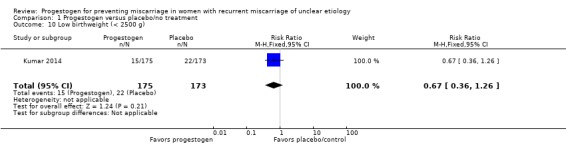
Comparison 1 Progestogen versus placebo/no treatment, Outcome 10 Low birthweight (< 2500 g).
Maternal adverse events
One study listed maternal adverse effects as outcomes (Le Vine 1964) but did not note any events.
Other secondary outcomes
None of the trials reported on our remaining planned secondary mother or child outcomes:
severity of 'morning sickness' ‐ intensified headache, nausea, breast tenderness;
reported thromboembolic events;
depression;
admission to special care unit;
subsequent fertility.
Discussion
Summary of main results
The aim of this review was to assess the effectiveness of progestogens to prevent recurrent miscarriage. There has been much speculation that progestogens may reduce the miscarriage recurrence rate, and the results of this meta‐analysis show that moderate‐quality evidence demonstrates that progestogen supplementation probably reduces the miscarriage rate for women with recurrent miscarriage. A subgroup analysis comparing placebo‐controlled versus non‐placebo‐controlled trials, trials of women with three or more prior miscarriages compared to women with two or more miscarriages and different routes of administration showed no clear differences between subgroups for miscarriage.
The secondary outcome of live birth rate was also probably improved with progesterone therapy (moderate‐quality evidence). This is mainly driven by the primary outcome of reduced miscarriages, as the rates of stillbirth, if reported at all, were typically very low or zero.
We are uncertain about the effect on the rate of preterm birth because the quality of this evidence is very low. It is clear from the literature about preventing preterm birth that progestogen therapy, vaginally or intramuscularly, can reduce preterm birth rates for some women (Dodd 2013; Keirse 1990). Thus, this finding should be interpreted with caution. Perhaps longer duration therapy in women with recurrent pregnancy loss may be warranted for future study.
The meta‐analysis showed there may be little or no difference in stillbirths, neonatal death or the number of fetal abnormalities (including virilization and hypospadias) in babies whose mothers had been given progestogens whilst in utero.
No trials reported any adverse maternal effects. There were many other planned secondary outcomes not reported. We may need to revise the outcome list for this topic and possibly unify it around a core set of outcomes for trials of miscarriage prevention.
Overall completeness and applicability of evidence
This updated review adds two recently published trials, which together strengthen the evidence that progestogen therapy probably reduces miscarriage rates for women with recurrent miscarriages. However, there are five trials that are ongoing or about to begin that may yet impact the evidence base, and so this conclusion may change in further updates of this review.
As most of the included trials had different entry criteria and duration of therapy, determining a uniform protocol for treatment may be difficult.
Quality of the evidence
Overall, we judged most of the trials to be of low risk of bias for most of the categories. The majority of the trials were conducted before robust reporting of methods was instituted. For many of the older trials, generally for trials at the time the trials were performed, the overall quality appears to be reasonable. The more recent publications were generally of better quality with low risks of bias. This difference in reporting standards is commonly seen in reviews that include trials published before uniform reporting guidelines such as CONSORT were developed (Schulz 2010).
Overall, the evidence ranged from very low to moderate the quality based on the GRADE system (Schünemann 2013). We assessed quality of evidence for the primary outcome of miscarriage and the main secondary outcomes of live birth rate and preterm birth. Limitations in study design led to downgrading of the evidence to moderate quality for the outcomes of miscarriage and live birth rate. Limitations in study design and very serious limitations in imprecision led to downgrading of the evidence to very low‐quality for the outcome preterm birth.
Potential biases in the review process
We took steps to minimize bias at every step of the review process: a comprehensive search of the literature was performed in order to identify all relevant studies and two authors independently assessed studies, performed data extraction and GRADE assessments. None of the review authors were involved in any of the included or excluded trials. We are unaware of any potential biases inherent in refocusing this update on women with recurrent pregnancies alone. The addition of a third review author in this update allowed for assessment and consensus around the study assessments. There was no funding provided for this review.
Agreements and disagreements with other studies or reviews
Two other published systematic reviews agree with our findings. One review, Coomarasamy 2011, included only four trials (all included in this updated review) and found benefit to progestogen. A more recent systematic review, Saccone 2017, included all trials included in this review. Thus, this updated Cochrane Review is in agreement with and includes more trials than prior published systematic reviews. A separate Cochrane Review has also been conducted on the use of progestogens for treating women with threatened miscarriage (Wahabi 2018), and this also suggests a benefit in terms of a reduction in miscarriage in those women treated with progestogens.
Authors' conclusions
For women with unexplained recurrent miscarriages, supplementation with progestogen therapy may reduce the rate of miscarriage in subsequent pregnancies.
As noted, the included trials utilized many different treatment protocols including when the progestogen was started and how long into gestation it was continued. Coming to a more standard treatment protocol for study in future trials, along with establishing a uniform core outcome set, would be beneficial for future trials. This would also aid in translation into practice. Including patient‐centered outcomes such as progestogen tolerability would also be appropriate in future trials. More data comparing the different routes or types of progestogens may also be beneficial.
Acknowledgements
The review authors would like to thank Lynn Hampson for her search of the Cochrane Pregnancy and Childbirth Trials Register.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
progesterone AND miscarriage
progesterone AND abortion
progestagen AND miscarriage
progestogen AND miscarriage
progestagens AND abortion
progestogens AND abortion
progesterone AND pregnancy loss
progestagen AND pregnancy loss
progestagens AND pregnancy loss
progestogen AND pregnancy loss
Data and analyses
Comparison 1.
Progestogen versus placebo/no treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage (all trials) | 10 | 1684 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 1.00] |
| 2 Miscarriage (placebo‐controlled trials only) | 10 | 1684 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 1.00] |
| 2.1 Trials with placebo control group | 7 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 2.2 Trials without placebo controls | 3 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.90] |
| 3 Miscarriage (women with previous recurrent miscarriage only) | 9 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.56, 1.02] |
| 3.1 Women with a history of 3 or more prior miscarriages | 4 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.01] |
| 3.2 Women with a history of 2 or more prior miscarriages | 5 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.64, 1.52] |
| 4 Miscarriage (by route of administration versus placebo) | 9 | 1571 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.03] |
| 4.1 Oral versus placebo/control | 6 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.13] |
| 4.2 Intramuscular versus placebo/control | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.30, 1.30] |
| 4.3 Vaginal versus placebo/control | 1 | 826 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.17] |
| 5 Live birth rate | 6 | 1411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.13] |
| 6 Preterm birth | 4 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.53, 2.41] |
| 7 Neonatal death | 3 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.36, 8.46] |
| 8 Fetal genital abnormalities/virilization | 3 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.50] |
| 9 Stillbirth | 2 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.79] |
| 10 Low birthweight (< 2500 g) | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.26] |
What's new
| Date | Event | Description |
|---|---|---|
| 14 November 2019 | Amended | We have made further amendments to this review to clarify the reasons for moving Ismail 2017 from Included to Characteristics of studies awaiting classification. We have edited text in results/studies awaiting classification', characteristics of studies awaiting classification, and published notes, in order to make the following information more visible. Since publication of the 2018 update of this review, we have been advised that the Ismail 2017 study is currently the subject of an investigation by the Journal of Maternal‐Fetal & Neonatal Medicine. We have now moved this study from 'included studies' to 'Characteristics of studies awaiting classification' until the outcome of the investigation is known. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 16 October 2019 | Amended | One study, previously included (Ismail 2017), has been moved to Characteristics of studies awaiting classification, pending clarification about the study data. We have added a Published notes to clarify that this review will no longer be updated in its current form. |
| 6 July 2017 | New search has been performed | Search updated. 'Summary of findings' table incorporated |
| 6 July 2017 | New citation required but conclusions have not changed | Title amended and focus of review changed from treatment of all miscarriage to treatment of recurrent miscarriage. This was done to eliminate treatment for threatened miscarriage as that is covered in a separate Review (Wahabi 2018). Five trials added (Agarwal 2016; Coomarasamy 2015; Ghosh 2014; Ismail 2017a; Kumar 2014); six trials previously included are now excluded (Berle 1980; Corrado 2002; Gerhard 1987; Moller 1965; Nyboe Anderson 2002; Tognoni 1980). They were excluded as they dealt strictly with women with threatened miscarriage or a different population. Co‐author added. |
| 1 August 2013 | New search has been performed | Search updated and four new trials identified. One was included (MacDonald 1972), two excluded (Kyrou 2011; Shu 2002), and one is ongoing (Coomarasamy 2012). Three previously included trials (Moller 1965a; Moller 1965b; Moller 1965c) are in fact three separate reports from one trial (Moller 1965a). |
| 1 August 2013 | New citation required but conclusions have not changed | No changes to conclusions. |
| 12 May 2009 | Amended | Contact details updated |
| 11 February 2008 | Amended | Converted to new review format |
| 31 January 2008 | New citation required but conclusions have not changed | This update has been prepared by a new review team. |
| 31 January 2008 | New search has been performed | Search updated. Four new trials identified: we included El‐Zibdeh 2005 and excluded Norman 2006. Two are ongoing (NCT00193674; Walch 2005). The inclusion of El‐Zibdeh 2005 narrows the confidence intervals (CI) of the outcomes it contained, including narrowing the CI for women with a history of three or more miscarriages; thus leading to the strengthening of the conclusions somewhat. |
Differences between protocol and review
For the 2017 update, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). We also changed the scope of the review to exclude trials reporting women with symptoms of threatened miscarriage. We also included a 'Summary of findings' table and redefined/revised our reported outcomes: live birth rate; preterm birth was changed to "preterm birth (less than 37 weeks' gestation)"; stillbirth changed to "stillbirth (fetal loss after 20 weeks' gestation in women with a viable pregnancy after 20 weeks)".
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Unit of randomization: pregnancy Method of randomization: unclear Timing of randomization: unclear Blinding: no Power calculation: unknown Number of centers: 1 90 women total enrolled; 30 randomized to progesterone, 30 to nothing, and 30 healthy controls; 300 women analyzed Source of funding: All India Institute of Medical Science |
|
| Participants | Women with a history of ≥ 2 consecutive miscarriages of unknown cause. Euthyroid Exclusion: history of repeated miscarriages of known cause Age: 21‐40 Location: India Timing and duration: August 2013‐unknown |
|
| Interventions | 200 mg oral micronized progesterone twice daily up until 16 weeks' gestation Control: no intervention Healthy control group |
|
| Outcomes | Cytokines, "perinatal outcome", occurrence of obstetric complications like ICP, pre‐eclampsia | |
| Notes | Trial registry summary from Clinical Trials Registry ‐ India Dates of study: first enrollment 12 August 2013, completion date not specified Funding sources: All India Institute of Medical Sciences, New Dehli (government medical college) Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only intervention group took drug but unlikely that knowledge of assignment would influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract results from trials registry |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Only abstract results available from trials registry |
| Other bias | Unclear risk | Unclear. Only abstract results available from trials registry |
| Methods | Unit of randomization: pregnancy Method of randomization: computer‐generated Timing of randomization: before conception, reconfirmed after became pregnant Blinding: yes Power calculation: yes, needed 376 women in each group, planned to recruit 790 women to allow for loss to follow‐up Number of centers: multiple ‐ recurrent miscarriage clinics across the UK (36 sites) and Netherlands (9 sites) 836 women randomized, 826 women had results for primary outcome to analyze Source of funding: UK NIHR |
|
| Participants | Women with ≥ 3 unexplained miscarriages trying to actively conceive naturally. Exclusions: unable to conceive after 1 year of being in trial, recognized thrombophilic condition, uterine anomalies, abnormal parental karyotype, or other identifiable cause of recurrent miscarriage. Age: 18‐39 years Location: UK and Netherlands Timing and duration: June 2010‐October 2013 |
|
| Interventions | 400 mg micronized progesterone vaginal suppositories twice daily. Began therapy "soon after receiving a positive" urine pregnancy test (no later than 6 weeks of gestation) through 12 weeks of duration. Control: matched placebo |
|
| Outcomes | Live birth after 24 weeks (primary), clinical pregnancy, ongoing pregnancy with fetal heart activity at 12 weeks, miscarriage (loss < 24 weeks), gestational age at delivery, neonatal survival, congenital anomalies, exploratory outcomes ‐ pre‐eclampsia SGA, PPROM, hemorrhage, neonatal outcomes | |
| Notes | PROMISE trial Dates of study: June 2010‐October 2013 Funding sources: UK NIHR Declarations of interest: disclosure forms on file at NEJM.org, not stated in paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Assignment through secure internet facility |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants, physicians, and trial nurses were unaware of the study‐group assignments throughout the trial" Comment: identical drug and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 total lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No other biases identified |
| Methods | Unit of randomization: pregnancy Method of randomization: day of week attending clinic Timing of randomization: presentation for new pregnancy Blinding: unclear Power calculation: no Number of centers: 1 180 women randomized, 0 exclusions, 180 women analyzed Source of funding: not stated |
|
| Participants | Women (< 35 years old) with ≥ 3 consecutive unexplained abortions with same husband with a new pregnancy Age: treatment 22% age 20‐24 years, 36% age 25‐29 years, 41.5% age 30‐34 years, control: 21% age 20‐24 years, 48% age 25‐29 years, 31% age 30‐34 years Location: Jordan Timing and duration: 1994‐2000 |
|
| Interventions | 10 mg oral dydrogesterone, twice daily. 5000 IU IM hCG every 4 days, or no treatment Control: no treatment Duration: until miscarriage or 12th gestational week |
|
| Outcomes | Miscarriage, preterm delivery, fetal malformations, perinatal death (not analyzed in review: hospitalization for vaginal bleeding) | |
| Notes | Dates of study: 1994‐2000 Funding sources: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by day of the week |
| Allocation concealment (selection bias) | High risk | Allocation by day of the week |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be incomplete data |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Methods | Unit of randomization: pregnancy Method of randomization: unclear Timing of randomization: before the 13th week of gestation Blinding: single Power calculation: no, listed as pilot study Number of centers: 1 ‐ tertiary infertility care unit in India 101 women randomized 2 women excluded 133 women analyzed (added controls without miscarriage history) Source of funding: not stated |
|
| Participants | Women with ≥ 3 miscarriages in the first trimester (up to 12 weeks' gestation) followed by a spontaneous conception confirmed by viable fetus on ultrasound Exclusions: abnormal thyroid, prolactin, or any other medication in the last 3 months. Also excluded if there was an apparent cause of miscarriages by multiple lab and chromosomal testing. Also excluded luteal phase defects diagnosed by midluteal serum progesterone. Age: 23‐40 years Location: India Timing and duration: taken up to 12 weeks |
|
| Interventions | Intervention: 10 mg oral dydrogesterone twice daily (50 women) or 100 mg micronized vaginal progesterone three times daily (51 women), taken up to 12 weeks Control: women without a history of miscarriage (32 women) |
|
| Outcomes | Uterine doppler blood flow parameters, ongoing clinical pregnancy (at least 1 viable fetus at 28 weeks' gestation), viable delivery, miscarriage rates | |
| Notes | Emailed contact author to ask about "ongoing pregnancy" group. if still pregnant at time of analysis, only able to utilize miscarriage data Dates of study: not stated Funding sources: "no financial support received" Declarations of interest: trial authors report "no conflicts of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study co‐ordinator did a "simple randomization" |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes prepared by study co‐ordinator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded due to different routes of administration but study personnel were "unaware of the type of protocol being used" and considered single‐blinded but unlikely that knowledge of assignment would influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel unaware of protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women excluded from analysis for adequate reasons |
| Selective reporting (reporting bias) | Low risk | Reported on outcomes collected |
| Other bias | Unclear risk | "ongoing pregnancy" group still pregnant at analysis so could not determine outcome for them other than that they did not have a miscarriage |
| Methods | Unit of randomization: pregnancy Method of randomization: sequentially numbered bottles. It is not clear who was responsible for the coding. Timing of randomization: unclear Blinding: yes (double) Power calculation: no Number of centers: 2 main centers 54 women randomized, 0 women excluded, 54 women analyzed Source of funding: Upjohn |
|
| Participants | Women who had either:
All women had to have a urinary pregnanediol of < 5 mg/d before 8 weeks' gestation and/or < 7 mg/d by 14 weeks' gestation. Age: not stated Location: USA |
|
| Interventions | 10 mg/d of oral medroxyprogesterone Control: placebo Duration: not stated |
|
| Outcomes | Miscarriage Preterm delivery | |
| Notes | Dates of study: not stated Funding sources: supported by a grant from the Upjohn Company, Kalamazoo, Michigan Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered bottles. Code not broken until end of trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None apparent |
| Methods | Unit of randomization: pregnancy Method of randomization: random schedules generated by a statistician Timing of randomization: before 10 weeks' gestation Power calculation: no Blinding: yes (double) Number of centers: 2 33 women randomized, 33 women analyzed Source of funding: not stated |
|
| Participants | Women who had ≥ 2 miscarriages, no pregnancy beyond 28 weeks' gestation, were < 10 weeks into the current pregnancy and with no other obvious causes of miscarriage | |
| Interventions | 50 mg twice daily of oral cyclopentyl enol ether of progesterone Control: placebo Duration: not stated |
|
| Outcomes | Miscarriage | |
| Notes | Dates of study: not stated Funding sources: somewhat unclear although the authors acknowledge "the firm of Vister who supplied the steroid and allowed their product to be subjected to a much more rigorous test than is usual in this field." Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random schedules generated by statistician |
| Allocation concealment (selection bias) | Unclear risk | Given according to a random schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded design ‐ neither participants nor physician knew which of the 2 they were getting. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but likely blinded, but unlikely that knowledge of assignment would influence outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods | Unit of randomization: pregnancy Method of randomization: computer‐generated Timing of randomization: early pregnancy Blinding: yes Power calculation: yes, needed 163 women in each group, planned to recruit 180 women to allow for loss to follow‐up Number of centers: 2 antenatal clinics from one Center 180 women randomized, 826 women had results for primary outcome to analyze Source of funding: Indian Council of Medical Research |
|
| Participants | Women with history of idiopathic, ≥ 3 first‐trimester pregnancy losses and currently in the first trimester with a live pregnancy (preferably 2‐8 weeks' gestation). Exclusion: known cause of recurrent miscarriage, women who had taken an injection of hCG or hydroxyprogesterone Age: 18‐35 Location: India |
|
| Interventions | 20 mg dydrogesterone (taken as 2 x 10 mg tablets) from time of enrolment to 20 weeks Control: identical placebo Additional control group of women without a history of miscarriage who were age‐matched, healthy, pregnant women with at least 1 live birth |
|
| Outcomes | Cytokine profiles, pregnancy outcomes (from results section) ‐ miscarriage, EGA at delivery, preterm delivery, cesarean LBW, SGA | |
| Notes | Dates of study: May 2010‐April 2013 Funding sources: Indian Council of Medical Research Declarations of interest: all trial authors state no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization sequence generated by computers |
| Allocation concealment (selection bias) | Low risk | Coded numbers on packets each containing 60 tablets of drug or placebo, sequentially distributed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomization unknown until after study completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 lost to follow‐up with appropriate reasons |
| Selective reporting (reporting bias) | Unclear risk | All outcomes recorded not stated in methods |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Unit of randomization: pregnancy Method of randomization: women were alternated between 'Group A' and 'Group B'. It is unclear who decided which group would be treatment and which would be placebo Timing of randomization: within the 16th week of pregnancy Power calculation: no Blinding: yes (double) Number of centers: not stated 56 women randomized, 26 women excluded, 30 women analyzed Source of funding not stated |
|
| Participants | Women who had had 3 consecutive miscarriages, were < 16 weeks' gestation and with no signs of threatened miscarriage in the current pregnancy Age: 20‐42 Location: USA Timing and duration: unknown |
|
| Interventions | 500 mg/week IM of hydroxyprogesterone caproate Duration: until miscarriage or the 36th week of gestation Control: placebo |
|
| Outcomes | Miscarriage Side effects of treatment suffered by the mother Deformities in the baby Preterm birth | |
| Notes | Dates of study: not stated Funding sources: not stated Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating group A and B |
| Allocation concealment (selection bias) | High risk | Alternating group A and B |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 56 women randomized, 26 women excluded, 30 women analyzed |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods | Unit of randomization: pregnancy Method of randomization: unclear Timing of randomization: after they were found to have evidence of hormonal imbalance on cervical mucus smears Power calculation: no Blinding: yes (double) Number of centers: 1 specialized antenatal center 40 women randomized, 0 women excluded, 40 women analyzed Source of funding: grant from N.V. Philips‐Duphar |
|
| Participants | Women with ≥ 2 consecutive proven abortions and then subsequent pregnancy with cervical mucus ferning present | |
| Interventions | 2 x 5 mg tablets of dydrogesterone tablets 3 times daily, increased to 4 tablets 3 times daily if ferning persisted, no duration specified Control: placebo |
|
| Outcomes | Miscarriage | |
| Notes | Dates of study: not stated Funding sources: grant from N.V. Philips‐Duphar Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods | Unit of randomization: pregnancy Method of randomization: sequentially‐coded ampules supplied by the drug company Timing of randomization: 6‐7 weeks' gestation Blinding: yes (double) Number of centers: not stated Power calculation: yes (80% with 40 in each group) 64 women randomized, 0 women excluded, 64 women analyzed Source of funding: Schering |
|
| Participants | Women who fell into ≥ 1 of the following criteria:
Evidence of a viable fetus at 6 weeks of pregnancy was required to be enrolled in the trial Age: not stated Location: Netherlands Timing and duration: not stated |
|
| Interventions | 500 mg/week IM of hydroxyprogesterone caproate Control: placebo Duration: from 7 weeks' gestation to 12 weeks' gestation |
|
| Outcomes | Miscarriage Preterm delivery | |
| Notes | Impossible to tell which were results for the women with recurrent pregnancy loss Dates of study: not stated Funding sources: Schering Nederland BV for providing 17‐OHP‐C and for financial support Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sequentially coded ampules supplied by central location |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specifically stated but likely blinded and unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods | Unit of randomization: pregnancy Method of randomization: unclear. The ampules were said to be coded but who coded these and how women were then allocated to the coded ampules is not stated. Timing of randomization: before the 12th week of gestation Blinding: yes (double) Power calculation: no Number of centers: 2 50 women randomized, 0 women excluded, 50 women analyzed Source of funding: preparations supplied by Schering |
|
| Participants | Women having had ≥ 2 consecutive abortions and who had low or falling pregnanediol levels Exclusions: women with uterine malformations Age: not stated Location: London, UK Duration and timing: not stated |
|
| Interventions | Up to 8 weeks' gestation: 250 mL/week IM hydroxyprogesterone 8‐11 weeks' gestation: 375 mL/week IM of 17‐a‐hydroxyprogesterone 12‐16 weeks' gestation: 500 mL/week IM of 17‐a‐hydroxyprogesterone 17‐20 weeks' gestation: 375 mg/week IM of 17‐a‐hydroxyprogesterone 21‐24 weeks' gestation: 250 mg/week IM of 17‐a‐hydroxyprogesterone Control: placebo |
|
| Outcomes | Miscarriage | |
| Notes | Source of funding: preparations supplied by Schering Dates of study: not stated Funding sources: "the preparations used in this study were made available by Dr. Jurgen Friebel, Schering AG, Berlin" Declarations of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Solution A and B ‐ the "correct identity of these substances is not known to any person taking part in this study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods | Unit of randomization: pregnancy Method of randomization of allocation: there were 2 centers in this study. 1 allocated by alternation. It was stated in the paper that the other center used "randomisation". However, method of randomization was not given. Timing of randomization: before 12 weeks' gestation Blinding: unclear Power calculation: no Number of centers: 2 113 women were enrolled, 0 women were excluded, 113 women were analyzed Source of funding: not stated |
|
| Participants | Women having had ≥ 2 consecutive miscarriages before 12 weeks' gestation Exclusions: women with any other known complicating factor (positive Wassermann reaction, extensive cervical tear, uterine malformation, associated medical disease, etc) Age: not stated Location: London, UK Timing and duration: not stated |
|
| Interventions | 6 x 25 mg progesterone pellets inserted within the gluteal muscle either:
Control: no treatment Duration: unclear |
|
| Outcomes | Miscarriage Preterm delivery Stillbirth Live birth rate |
|
| Notes | Dates of study: not stated Funding sources: stated that an author was grateful to Organon Laboratories, Ltd for "generous supplies of progesterone implants" Declarations of interest: not stated but it did state that "one of us (D.D.) is grateful" to Dr. Tindall of Organon but no details of why only that author was grateful were given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | There were 2 centers in this study. 1 allocated by alternation. It was stated in the paper that the other center used "randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated but unlikely that knowledge of assignment would influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None noted |
hCG: human chorionic gonadotropin;IM: intramuscular; IU: international unit: LBW: low birthweight; NIHR: National Institute for Health Research; PPROM: preterm premature rupture of the membranes; SGA: small‐for‐gestational age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berle 1980 | Included women who "presented with bleeding" of threatened miscarriage, wrong population |
| Brenner 1962 | Outcomes of this study were not applicable to this review. Treatment was not given to women until 38 weeks' gestation. The outcome measured was time from onset of labor to delivery |
| Check 1985 | No method of randomization was used for this trial. |
| Check 1987a | No method of randomization was used for this trial. |
| Check 1987b | It is unclear as to whether there was any randomization. The authors of this review attempted, but failed, to contact the trial authors. |
| Check 1995 | The intervention considered in this trial is progesterone in association with immunotherapy rather than progesterone alone. |
| Clifford 1996 | In this trial, progesterone was not taken during pregnancy. |
| Corrado 2002 | Trial of women undergoing amniocentesis, not with recurrent miscarriage |
| Daya 1988 | This trial was not an RCT. |
| Fuchs 1966 | This trial was terminated before the results were of sufficient size to statistically analyze. Therefore, data are incomplete. |
| Gerhard 1987 | Trial of women with threatened miscarriage. |
| Johnson 1975 | The outcome measure of this trial was preterm delivery rather than miscarriage. |
| Kyrou 2011 | Comparison of stopping progestin at 12 weeks vs continuing. Wrong comparison |
| Moller 1965 | Trial of women with threatened miscarriage. |
| Norman 2006 | This trial was excluded because progesterone was given after 20 weeks' gestation to prevent preterm labor. |
| Nyboe Anderson 2002 | Trial of women after IVF or ICSI, not with recurrent miscarriage |
| Prietl 1992 | The active intervention given in this trial was a combination of progesterone and oestrogen, rather than progesterone alone |
| Rock 1985 | This trial was not an RCT. |
| Shu 2002 | Wrong population and intervention |
| Sidelnikova 1990 | This trial was not an RCT. |
| Smitz 1992 | This trial compared intramuscular progesterone versus intravaginal progesterone in the luteal phase followed by all participants receiving progesterone and oestrogen on the day prior to ovocyte puncture. There was no placebo or 'no treatment' control and the treatment given after commencement of pregnancy was a combination of progesterone and oestrogen rather than progesterone alone. |
| Sondergaard 1985 | This trial was conducted to ascertain the efficacy of progesterone to prevent preterm birth rather than miscarriage. |
| Tognoni 1980 | Trial of women with threatened miscarriage. |
| Turner 1966 | The outcome of this trial is not relevant to the current systematic review. Progesterone was not given to prevent miscarriage and was not given until the 30/40. |
ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilisation; RCT: randomized controlled trial; VS: versus
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unit of randomization: pregnancy Method of randomization: computer‐generated tables Timing of randomization: before trying to conceive Power calculation: yes, needed 350 in each arm Blinding: yes (double) Number of centers: 1 ‐ Recurrent Miscarriage Clinic of Assiut Women's Health Hospital 700 women randomized, 675 women analyzed Source of funding: not stated |
| Participants | All women who presented to the clinic, "regular marital life with the same partner". Not specifically stated the inclusion criteria or definition of recurrent miscarriage but assumed it is similar to others based on other text as at least 3 unexplained miscarriages. Exclusion: known cause of recurrent miscarriage, antiphospholipid antibody syndrome, consanguinity Age: 20‐39 Location: Egypt |
| Interventions | 400 mg progesterone pessary suppositories twice daily Control: matched placebo pessaries manufactured to look identical Treatment began immediately after documentation of ovulation using ultrasound. Continued until 28 weeks of gestation. Stopped if did not become pregnant that cycle ‐ allowed up to 6 cycles |
| Outcomes | Miscarriage rate (primary), viable pregnancy > 20 weeks, live birth rate, vaginal bleeding, preterm delivery, cytokine levels |
| Notes | Dates of study: September 2012‐November 2015 Funding sources: NIHR‐HTA, UK Declarations of interest: the trial authors reported "no conflicts of interest". This study will remain in awaiting classification pending clarification regarding the study data. Since publication of the 2018 update of this review, we have been advised that the Ismail 2017 study is currently the subject of an investigation by the Journal of Maternal‐Fetal & Neonatal Medicine. Consequently, we have now moved this study from 'included studies' to 'studies awaiting classification' until the outcome of the investigation is known. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Does using progesterone reduce the miscarriage rate in high risk pregnancies? Pregnancy Maintenance Trial (PMTrial) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Australia/New Zealand Trial Registry ACTRN12611000401954. Listed as Recruiting. Emailed scientific contact Dr. McLindon‐ no reply |
| Trial name or title | Effect of concomitant administration of vaginal progesterone and vitamin D3 in the treatment of unexplained recurrent abortion in pregnant women. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Iranian trial registry IRCT2013100114853N1 lists as complete. Attempted to contact PI, no response |
| Trial name or title | Habitual abortion study |
| Methods | |
| Participants | Pregnant women with history of 3 idiopathic miscarriages |
| Interventions | Oral dydrogesterone vs placebo |
| Outcomes | Change in IFN/IL‐10 ratio |
| Starting date | 2003 |
| Contact information | Gereon Raddatz, gereon.raddatz@solvay.com |
| Notes |
| Trial name or title | A randomized, controlled trial of cyclosporin a for women with unexplained recurrent miscarriage |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Listed on clinicaltrials.gov registry NCT02706470 as not yet open to enrolment. |
| Trial name or title | The prevention of miscarriage study (PROMIS) |
| Methods | |
| Participants | Pregnant women with history of at least 3 spontaneous miscarriages with same partner and negative standard evaluation |
| Interventions | 20 mg daily oral dydrogesterone vs placebo tablet |
| Outcomes | Change in Interferon‐gamma/Interleukin‐10 ratio from baseline and "pregnancy outcomes" |
| Starting date | |
| Contact information | Dr. Katharina Walch, Vienna, Austria, katharina.walch@meduniwien.ac.at |
| Notes |
Contributions of authors
David Haas: is the guarantor of the review; prepared the 2013 and current update; performed independent data extraction and quality assessment of the included trials, and commented on all drafts of the review.
Taylor Hathaway: performed independent data extraction and quality assessment of included trials for the updates; commented on the drafts for this update.
Patrick Ramsey: performed independent data extraction and quality assessment of included trials for the updates; commented on the drafts for this update.
Sources of support
Internal sources
None (internal Department of OB/GYN funding), USA.
External sources
None, Other.
Declarations of interest
David M Haas: none known
Taylor J Hathaway: none known
Patrick S Ramsey: I serve as an officer in Section 5 of District XI of the American College and Congress of Obstetricians and Gynecologists. I received compensation for this general work with ACOG but have not engaged in any specific work relevant to the topic of this review. I also serve on the Data Safety Monitoring Board for the KV Pharmaceutical RCT designed to evaluate the efficacy of 17‐hydroxyprogesterone caproate to prevent preterm birth. I received compensation for service as the DSMB chair from an independent data management organization and not from the pharmaceutical company.
Notes
This review will no longer be updated in its current form. The review will be superseded by a new review called, 'Progestogens for preventing miscarriage: a systematic review and network meta‐analysis' (protocol in preparation).
Since publication of the 2018 update of this review, we have been advised that the Ismail 2017 study is currently the subject of an investigation by the Journal of Maternal‐Fetal & Neonatal Medicine. We have now moved this study from 'included studies' to 'Characteristics of studies awaiting classification' until the outcome of the investigation is known.
Edited (no change to conclusions)
References
References to studies included in this review
- CTRI/2016/09/007278. Role of inflammatory markers in recurrent pregnancy loss and effect of oral micronized therapy on these cases. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=14740 (first received 16 September 2016).
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141‐8. [DOI] [PubMed] [Google Scholar]; Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. PROMISE: first‐trimester progesterone therapy in women with a history of unexplained recurrent miscarriages ‐ a randomised, double‐blind, placebo‐controlled, international multicentre trial and economic evaluation. Health Technology Assessment 2016;20(41):7‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]; ISRCTN92644181. First trimester progesterone therapy in women with a history of unexplained recurrent miscarriages: a randomised double‐blind placebo‐controlled multi‐centre trial (The PROMISE [PROgesterone in recurrent MIScarriagE] Trial). controlled‐trials.com/ISRCTN92644181 (first received 17 March 2009). [DOI] [PMC free article] [PubMed]
- Zibdeh M. Randomized clinical trial comparing the efficacy of dydrogesterone and human chorionic gonadotropin. Climacteric 2002;5(Suppl 1):136. [Google Scholar]; Zibdeh M. Randomized study comparing the efficacy of reducing spontaneous abortion following treatment with progesterone and human chorionic gonadotropin (hCG). Fertility and Sterility 1998;70(3 Suppl 1):S77‐S78. [Google Scholar]; El‐Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):431‐4. [DOI] [PubMed] [Google Scholar]
- CTRI/2013/02/003406. Assessment of sub‐endometrial blood flow parameters following dydrogesterone and micronized vaginal progesterone administration in women with idiopathic recurrent spontaneous miscarriage. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5847 (first received 18 February 2013). [DOI] [PubMed]; Chakravarty BN, Ganesh A, Chowdhuri K, Shyam T, Ghosh S, Chattopadhyay R. Assessment of endometrial vascularity following dydrogesterone and micronized progesterone administration in idiopathic recurrent miscarriage‐a preliminary study. Human Reproduction 2012;27(Suppl 2):P089. [Google Scholar]; Ghosh S, Chattopadhyay R, Goswami S, Chaudhury K, Chakravarty B, Ganesh A. Assessment of sub‐endometrial blood flow parameters following dydrogesterone and micronized vaginal progesterone administration in women with idiopathic recurrent miscarriage: a pilot study. Journal of Obstetrics & Gynaecology Research 2014;40(7):1871‐6. [DOI] [PubMed] [Google Scholar]
- Goldzieher JW. Double‐blind trial of a progestin in habitual abortion. JAMA 1964;188(7):651‐4. [DOI] [PubMed] [Google Scholar]
- Klopper A, MacNaughton M. Hormones in recurrent abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1022‐8. [Google Scholar]
- Kumar A, Begum N, Prasad S, Aggarwal S, Sharma S. Oral dydrogesterone treatment during early pregnancy to prevent recurrent pregnancy loss and its role in modulation of cytokine production: a double‐blind, randomized, parallel, placebo‐controlled trial. Fertility and Sterility 2014;102(5):1357‐63.e3. [DOI] [PubMed] [Google Scholar]
- Vine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgery 1964;72:30‐6. [PubMed] [Google Scholar]
- MacDonald RR, Goulden R, Oakey RE. Cervical mucus, vaginal cytology and steroid excretion in recurrent abortion. Obstetrics & Gynecology 1972;40(3):394‐402. [PubMed] [Google Scholar]
- Reijnders FJ, Thomas CM, Doesburg WH, Rolland R, Eskes TK. Endocrine effects of 17 alpha‐hydroxyprogesterone caproate during early pregnancy: a double‐blind clinical trial. British Journal of Obstetrics and Gynaecology 1988;95:462‐8. [DOI] [PubMed] [Google Scholar]
- Shearman R, Garrett W. Double‐blind study of effect of 17‐hydroxyprogesterone caproate on abortion rate. British Medical Journal 1963;1:292‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shearman RP. Hormonal treatment of habitual abortion. In: UK editor(s). In: Progress In Infertility. Kistner RW (ed). Shearman 1963. London: J & A Churchill, 1968:767‐77. [Google Scholar]
- Swyer GI, Daley D. Progesterone implantation in habitual abortion. British Medical Journal 1953;1:1073‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Berle P, Budenz M, Michaelis J. Is hormonal therapy still justified in imminent abortion?. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184:353‐8. [PubMed] [Google Scholar]
- Brenner W, Hendricks C. Effect of medroxyprogesterone acetate upon the duration and characteristics of human gestation labour. American Journal of Obstetrics and Gynecology 1962;83(8):1094‐8. [DOI] [PubMed] [Google Scholar]
- Check J, Wu C‐H, Adelson H. Decreased abortion in HMG‐induced pregnancies. International Journal of Fertility 1985;30(3):45‐7. [PubMed] [Google Scholar]
- Check J, Chase J, Nowroozi K, Wu C, Adelson H. Progesterone therapy to decrease first‐trimester spontaneous abortions in previous aborters. International Journal of Fertility 1987;32(3):192‐9. [PubMed] [Google Scholar]
- Check J, Chase J, Wu C, Adelson H, Teichman M, Rankin A. The efficacy of progesterone in achieving successful pregnancy: prophylactic use during luteal phase in anovulatory women. International Journal of Fertility 1987;32(2):135‐8. [PubMed] [Google Scholar]
- Check JH, Tarquini P, Gandy P, Lauer C. A randomized study comparing the efficacy of reducing the spontaneous abortion rate following lymphocyte immunotherapy and progesterone treatment vs progesterone alone in primary habitual aborters. Gynecologic and Obstetric Investigation 1995;39:257‐61. [DOI] [PubMed] [Google Scholar]
- Clifford K, Rai R, Watson H, Franks S, Regan L. Does suppressing luteinising hormone secretion reduce the miscarriage rate? Results of a randomised controlled trial. BMJ 1996;312:1508‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado F, Dugo C, Cannata M, Bartolo M, Scilipoti A, Stella N. A randomised trial of progesterone prophylaxis after midtrimester amniocentesis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;100(2):196‐8. [DOI] [PubMed] [Google Scholar]
- Daya S, Ward S, Burrows E. Progesterone profiles in luteal phase defect cycles and outcome of progesteron treatment in patients with recurrent spontaneous abortion. American Journal of Obstetrics and Gynecology 1988;158(2):225‐32. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Olsen P. An attempted double‐blind controlled trial of progesterone therapy in habitual abortion. Ugeskrift for Laeger 1966;128:1461‐2. [PubMed] [Google Scholar]
- Gerhard I, Gwinner B, Eggert‐Kruse W, Runnebaum B. Double‐blind controlled trial of progesterone substitution in threatened abortion. Biological Research in Pregnancy and Perinatology 1987;8:26‐34. [PubMed] [Google Scholar]
- Johnson J, Austin K, Jones G, Davis G, King T. Efficacy of 17 alpha‐hydroxyprogesterone caproate on the prevention of premature labour. New England Journal of Medicine 1975;298(14):675‐80. [DOI] [PubMed] [Google Scholar]
- Kyrou D, Fatemi HM, Zepiridis L, Riva A, Papanikolaou EG, Tarlatzis BC, et al. Does cessation of progesterone supplementation during early pregnancy in patients treated with recFSH/GnRH antagonist affect ongoing pregnancy rates? A randomized controlled trial. Human Reproduction 2011;26(5):1020‐4. [DOI] [PubMed] [Google Scholar]
- Moller K, Fuchs F. Double blind controlled trial of 6‐methyl‐17‐acetoxyprogesterone in threatened abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1042‐4. [Google Scholar]
- Norman JE. Double blind randomised placebo controlled study of progesterone for the prevention of preterm birth in twins (STOPPIT). National Research Register. www.nrr.nhs.uk (accessed 6 July 2006).
- Nyboe Anderson A, Popovic‐Todorovic B, Schmidt K, Loft A, Lindhard A, Hojgaard A, et al. Progesterone supplement during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Human Reproduction 2002;17(2):357‐61. [DOI] [PubMed] [Google Scholar]
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17alpha‐hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction 1992;7(1):1‐5. [DOI] [PubMed] [Google Scholar]
- Rock J, Colston Wentz A, Cole K, Kimball A, Zacur H, Early S, et al. Fetal malformations following progesterone therapy during pregnancy: a preliminary report. Fertility and Sterility 1985;44(1):17‐9. [DOI] [PubMed] [Google Scholar]
- Shu J, Miao P, Wang RJ. Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive early recurrent spontaneous abortion. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi//Chinese Journal of Integrated Traditional and Western Medicine 2002;22(6):414‐6. [PubMed] [Google Scholar]
- Sidelnikova VM, Demidova EM, Borisova IF, Dondukova TM, Absava GI, Korkhov VV. The use of acetomepegrenol in the therapy of threatened abortion. Akusherstvo i Ginekologiia 1990;66(9):37‐40. [PubMed] [Google Scholar]
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. Randomized prospective trial comparing supplementation of the luteal phase and of early pregnancy by natural progesterone given by intramuscular or vaginal administration. Revue Francaise de Gynecologie et d Obstetrique 1992;87:507‐16. [PubMed] [Google Scholar]
- Sondergaard F, Ottesen B, Detlefsen GU, Schierup L, Pederson SC, Lebech PE. Progesterone treatment of cases of threatened pre‐term delivery in women with a low level of plasma progesterone. Contraception, Fertilite, Sexualite 1985;13(12):1227‐31. [Google Scholar]
- Tognoni G, Ferrario L, Inzalaco M, Crosignani P. Progestogens in threatened abortion. Lancet 1980;2(8206):1242‐3. [DOI] [PubMed] [Google Scholar]
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95:222‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Ismail A, Nasr A, Amin A, Al‐Inany H. Peri‐conceptional progesterone treatment in women with unexplained recurrent miscarriage, a randomized double‐blind controlled trial. Human Reproduction 2015;30:i194, abstract no: P‐167. [Google Scholar]; Ismail AM, Abbas AM, Ali MK, Amin AF. Peri‐conceptional progesterone treatment in women with unexplained recurrent miscarriage: a randomized double‐blind placebo‐controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 23 January 2017 [Epub ahead of print]. [DOI] [PubMed]; NCT01670929. Pr‐conceptional progesterone for unexplained recurrent miscarriage. clinicaltrials.gov/ct2/show/record/NCT01670929 (first received 17 August 2012).
References to ongoing studies
- ACTRN12611000401954. Does using progesterone reduce the miscarriage rate in high risk pregnancies? Pregnancy Maintenance Trial (PMTrial). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000401954 18 April 2011.
- IRCT2013100114853N1. Effect of concomitant administration of vaginal progesterone and vitamin D3 in the treatment of unexplained recurrent abortion in pregnant women. en.search.irct.ir/view/15255 (first received 30 October 2013).
- NCT00193674. Oral dydrogesterone treatment during the first trimester of pregnancy in women with recurrent miscarriage: a double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. clinicaltrials.gov/ct2/show/NCT00193674 (first received 11 Septamber 2005). [DOI] [PubMed]
- NCT02706470. A randomized, controlled trial of cyclosporin a for women with unexplained recurrent miscarriage. clinicaltrials.gov/ct2/show/record/NCT02706470 (first received 12 March 2016).
- Walch K, Hefler L, Nagele F. Oral dydrogesterone treatment during the first trimester of pregnancy: the prevention of miscarriage study (PROMIS). A double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. Journal of Maternal‐Fetal & Neonatal Medicine 2005;18(4):265‐9. [DOI] [PubMed] [Google Scholar]
Additional references
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008991.pub2] [DOI] [PubMed] [Google Scholar]
- Atkin LC, Arcelus M, Fernandez A, Tolbert K. La Psicologia en el Ambito Perinatal. Mexico D.F: INPER, 1988. [Google Scholar]
- Bamigboye AA, Morris J. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004353] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 9th Edition. Philadelphia: Lippincott Williams & Wilkins, 2011. [Google Scholar]
- Burgoyne PS, Holland K, Stephens R. Incidence of numerical chromosome anomalies in human pregnancy estimation from induced and spontaneous abortion data. Human Reproduction 1991;6(4):555‐65. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Truchanowicz EG, Rai R. Does first trimester progesterone prophylaxis increase the live birth rate in women with unexplained recurrent miscarriages?. BMJ 2011;342:d1914. [DOI] [PubMed] [Google Scholar]
- Coulam CB. Epidemiology of recurrent spontaneous abortion. American Journal of Reproductive Immunology 1991;26:23‐7. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
- Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004947.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyregrove A, Mathieson SB. Stillbirth, neonatal death and SIDS: parental reactions. Scandanavian Journal of Psychology 1987;28:104‐14. [DOI] [PubMed] [Google Scholar]
- Goldstein P, Berrier J, Rosen S, Sacks HS, Chalmers TC. A meta‐analysis of randomised control trials of progestational agents in pregnancy. British Journal of Obstetrics and Gynaecology 1989;96:265‐74. [DOI] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Grudzinskas JG. Endocrinocolgical and metabolical assessment of early pregnancy. In: Chamberlain G editor(s). Tumbull's Obstetrics. London: Pearson Professional Ltd, 1995. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available fromwww.training.cochrane.org/handbook.
- Howie PG. Abortion and ectopic pregnancy. In: Whitfield CR editor(s). Dewhurst's Textbook of Obstetrics and Gynaecology for Postgraduates. Oxford: Blackwell Science Ltd, 1995. [Google Scholar]
- Karamadian LM, Grimes DA. Luteal phase deficiency. Effect of treatment on pregnancy rates. American Journal of Obstetrics and Gynecology 1992;167:1391‐8. [DOI] [PubMed] [Google Scholar]
- Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. British Journal of Obstetrics and Gynaecology 1990;97(2):149‐54. [DOI] [PubMed] [Google Scholar]
- Lede R, Duley L. Uterine muscle relaxant drugs for threatened miscarriage. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002857.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WZ. Spontaneous abortion. American Family Physician 1991;43:175‐82. [PubMed] [Google Scholar]
- Morley LC, Simpson N, Tang T. Human chorionic gonadotrophin (hCG) for preventing miscarriage. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD008611.pub2] [DOI] [PubMed] [Google Scholar]
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002253.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter TF, LaCoursiere Y, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000112.pub2] [DOI] [PubMed] [Google Scholar]
- Regan L, Braude PB, Trembath PR. Influences of past reproductive performance on risk of spontaneous abortion. BMJ 1989;299:541‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Royal College of Obstetricians and Gynaecologists. The management of recurrent miscarriage. www.rcog.org.uk/guidelines/recurrent.html(accessed 2001).
- Rumbold A, Middleton P, Pan N, Crowther CA. Vitamin supplementation for preventing miscarriage. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD004073.pub3] [DOI] [PubMed] [Google Scholar]
- Saccone G, Schoen C, Franasiak JM, Scott RT Jr, Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta‐analysis of randomized, controlled trials. Fertility and Sterility 2017;107(2):430‐8. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:Epub 24 March. [DOI] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
- Simpson JL. Fetal wastage. In: Gabe SG, Simpson JL editor(s). Obstetrics. Normal and Problem Pregnancies. New York: Churchill Livingstone Inc, 1991. [Google Scholar]
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
- Szabo I, Szilagyi A. Management of threatened abortion. Early Pregnancy 1996;2(4):233‐40. [PubMed] [Google Scholar]
- Vance JC, Foster WJ, Najman JM, Embelton, Thearle MJ, Hogden FM. Early parental responses to sudden infant death, stillbirth or neonatal death. Medical Journal of Australia 1991;155(5):292‐7. [DOI] [PubMed] [Google Scholar]
- Wahabi HA, Fayed AA, Esmaeil SA, Bahkali KH. Progestogen for treating threatened miscarriage. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD005943.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JR, Esposito JL. Pregnancy Loss: Medical Therapeutics and Practical Considerations. Baltimore: Williams and Wilkins, 1987. [Google Scholar]
References to other published versions of this review
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003511.pub2] [DOI] [PubMed] [Google Scholar]
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD003511.pub3] [DOI] [PubMed] [Google Scholar]
- Oates‐Whitehead RM, Haas DM, Carrier JAK. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003511] [DOI] [PubMed] [Google Scholar]


