Abstract
Background
Treatment with vitamin K antagonists is associated with increased morbidity and mortality. Reversal therapy with prothrombin complex concentrate (PCC) is used increasingly and is recommended in the treatment of patients with bleeding complications undertaking surgical interventions, as well as patients at high risk of bleeding. Evidence is lacking regarding indication, dosing, efficacy and safety.
Objectives
We assessed the benefits and harms of PCC compared with fresh frozen plasma in the acute medical and surgical setting involving vitamin K antagonist‐treated bleeding and non‐bleeding patients. We investigated various outcomes and predefined subgroups and performed sensitivity analysis. We examined risks of bias and applied trial sequential analyses (TSA) to examine the level of evidence, and we prepared a 'Risk of bias’ table to test the quality of the evidence.
Search methods
We searched the following databases from inception to 1 May 2013: Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (Ovid SP); EMBASE (Ovid SP); International Web of Science; Latin American and Caribbean Health Sciences Literature (LILACS) (via BIREME); the Chinese Biomedical Literature Database; advanced Google and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). We applied a systematic and sensitive search strategy to identify relevant randomized clinical trials and imposed no language or date restrictions. We adapted our MEDLINE search strategy for searches in all other databases. We reran the search in October 2014 and found one potential new study of interest. We added this study to a list of ‘Studies awaiting classification', and we will incorporate this study into the formal review findings at the time of the review update.
Selection criteria
We included randomized controlled trials (RCTs), irrespective of publication status, date of publication, blinding status, outcomes published or language. We contacted investigators and study authors to request relevant data.
Data collection and analysis
Three review authors independently abstracted data and resolved disagreements by discussion. Our primary outcome measures were 'overall mortality longest follow‐up' and 'overall 28‐day mortality'. We performed subgroup analyses to assess the effects of PCC in adults in terms of various clinical and physiological outcomes. We presented pooled estimates of the effects of interventions on dichotomous outcomes as risk ratios (RRs), and on continuous outcomes as mean differences (MDs), with 95% confidence intervals (CIs). We assessed risk of bias by assessing trial methodological components and risk of random error through TSA.
Main results
We included four RCTs with a total of 453 participants and determined that none of these trials had overall low risk of bias. We found six ongoing trials from which we were unable to retrieve further data. Three trials provided data on mortality. Meta‐analysis showed no statistical effect on overall mortality (RR 0.93, 95% CI 0.37 to 2.33; very low quality of evidence). We were unable to associate use of PCC with the number of complications probably related to the intervention (RR 0.92, 95% CI 0.78 to 1.09; very low quality of evidence). Lack of transfusion data and apparent differences in study design prevented review authors from finding a beneficial effect of PCC in reducing the volume of fresh frozen plasma (FFP) transfused to reverse the effect of vitamin K antagonist treatment. The number of new occurrences of transfusion of red blood cells (RBCs) did not seem to be associated with the use of PCC (RR 1.08, 95% CI 0.82 to 1.43; very low quality of evidence). Still, the included studies demonstrate the possibility of equally reversing vitamin K‐induced coagulopathy using PCC without the need for transfusion of FFP. No effect on other predefined outcomes was observed.
Authors' conclusions
In the four included RCTs, use of prothrombin complex concentrate does not appear to reduce mortality or transfusion requirements but demonstrates the possibility of reversing vitamin K‐induced coagulopathy without the need for transfusion of fresh frozen plasma. All included trials have high risk of bias and are underpowered to detect mortality, benefit or harm. Clinical and statistical heterogeneity is high, and definitions of clinically important outcomes such as adverse events are highly dissimilar between trials. Only weak observational evidence currently supports the use of PCC in vitamin K antagonist‐treated bleeding and non‐bleeding patients, and the current systematic review of RCTs does not support the routine use of PCC over FFP. Additional high‐quality research is urgently needed.
Plain language summary
Prothrombin complex concentrate for reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients
Prothrombin complex concentrate (PCC) is a drug that contains a source of proteins involved in the human blood clotting process. Patients medicated with vitamin K antagonists (blood thinning drug) have low blood levels of these important blood clotting proteins. Therefore these patients will be at increased risk of spontaneous and traumatic bleeding events. Also, when these patients experience a bleeding event, this will lead to progressive loss of these important blood clotting proteins. This process causes a vicious circle, thereby increasing risks of illness and death.
In the present Cochrane systematic review, we assessed the benefits and harms of prothrombin complex concentrate in vitamin K antagonist‐treated bleeding and non‐bleeding patients who are undertaking acute surgical intervention. We searched the databases until 1 May 2013. We identified four randomized trials (453 participants) involving neurological and cardiac surgical settings, as well as medical reversal of vitamin K‐intoxication among participants. We found six ongoing trials but were unable to retrieve these data. We reran the search in October 2014 and found one new study of potential interest. We added this study to a list of ‘Studies awaiting classification', and we will incorporate this study into the formal review findings at the time of the review update.
We could not identify any beneficial effects of prothrombin complex concentrate on death. In our predefined outcomes, we identified a decreased volume of fresh frozen plasma transfused for reversal of vitamin K antagonist treatment. We could not identify statistical differences in reduced blood loss, harm or numbers of adverse events in the group treated with PCC. However, all trials were of low quality and small, and all were characterized by a high level of heterogeneity. Therefore, evidence in support of PCC in vitamin K antagonist‐treated bleeding and non‐bleeding patients receiving acute surgical intervention remains weak.
Summary of findings
Summary of findings for the main comparison. Prothrombin complex concentrate versus any comparator for reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients.
| Prothrombin complex concentrate versus any comparator for reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients | ||||||
| Patient or population: patients with vitamin K antagonist‐induced bleeding requiring acute intervention Setting: acute medical and operative setting Intervention: prothrombin complex concentrate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prothrombin complex concentrate | |||||
| Overall mortality ‐ longest follow‐up | Study population | RR 0.93 (0.37 to 2.33) | 421 (3 studies) | ⊕⊝⊝⊝ Very lowa,b,c,d,e | Effect of the use of PCC on mortality was uncertain. None of the included studies were powered to detect differences in mortality. Large confidence intervals. Few participants and few events | |
| 84 per 1000 | 78 per 1000 (31 to 195) | |||||
| Moderate | ||||||
| 91 per 1000 | 85 per 1000 (34 to 212) | |||||
| Number of new occurrences of RBC transfusion | Study population | RR 1.08 (0.82 to 1.43) | 376 (2 studies) | ⊕⊝⊝⊝ Very lowa,c,d,e | Number of new occurrences of blood transfusion did not seem to be associated with use of PCC. Large confidence intervals. Few participants and few events | |
| 319 per 1000 | 344 per 1000 (262 to 456) | |||||
| Moderate | ||||||
| 300 per 1000 | 324 per 1000 (246 to 429) | |||||
| Number of complications probably related to the intervention | Study population | RR 0.92 (0.78 to 1.09) | 442 (4 studies) | ⊕⊝⊝⊝ Very lowa,b,c,d,e,f | Assessment of safety was not uniform, which raises concerns of underreporting. Large confidence intervals. Few participants and few events | |
| 573 per 1000 | 527 per 1000 (447 to 625) | |||||
| Moderate | ||||||
| 563 per 1000 | 518 per 1000 (439 to 614) | |||||
| Transfusion of RBCs | Mean transfusion of RBCs in the intervention groups was 4.52 lower (80.59 lower to 71.55 higher) | 370 (2 studies) | ⊕⊝⊝⊝ Very lowa,c,d,e | Trial sequential analysis of PCC vs FFP on quantity of RBCs transfused from 2 trials led to rejection of an intervention effect of 125 mL based on sparse data and repetitive analyses. Large confidence intervals. Few participants and few events | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. The quality of the evidence is downgraded 3 levels based on risk of bias in the included studies and the relatively small overall number of participants. | ||||||
aOnly poster and abstract published, open‐label study. Industry sponsored. bNon‐blinded study, terminated early and small population. No sample size stated. Lacks a clear definition of exclusion criteria ("clinical evidence of brainstem herniation"), which might lead to investigator‐dependent conclusions. cOpen‐label, non‐blinded study. Industry funded.
Industry funded, small sample size. Describes several breaches of the study protocol.
dImprecision of results (large confidence intervals).
Indirectnes of evidence (few participants and few events).
CI = confidence interval.
FFP = fresh frozen plasma.
PCC = prothrombin complex concentrate.
RBC = red blood cell.
RR = risk ratio.
Background
Description of the condition
Severe bleeding can cause haemorrhagic shock, which is a lethal state of tissue hypoxia ultimately leading to cellular ischaemia and hence organ dysfunction, failure and death (Bruce 2008). An increasing number of patients receive oral anticoagulation therapy with a vitamin K antagonist such as warfarin or phenprocoumon to treat or prevent new occurrences of venous thromboembolism. However this approach requires special consideration with regard to co‐morbidity, interacting medications and potentially lethal haemorrhagic complications. The primary medical indications are atrial fibrillation, venous thromboembolism and mechanical heart valves (Leissinger 2008; Levy 2008; Vang 2011). Large‐scale epidemiological studies have reported new occurrences of major bleeding complications from vitamin K antagonist treatment ranging from 1.1% to 1.5%, with gastrointestinal, urinary and intracranial sites most frequently involved (Bruce 2008; Franchini 2010; Leissinger 2008; Palareti 1996). This potentially lethal condition requires rapid reversal of oral anticoagulant therapy, which traditionally has been executed by large‐volume transfusions of fresh frozen plasma. However, this strategy presents important safety concerns such as volume overload, dilutional coagulopathy, blood group specificity, lack of viral inactivation and risk of transfusion‐related acute lung injury (Kor 2010; Leissinger 2008; Ozgonenel 2007).
Description of the intervention
Inhibition of the biosynthesis of vitamin K‐dependent coagulation factors can be reversed by several treatments. Withholding oral anticoagulation therapy while administering vitamin K induces hepatic de novo synthesis of vitamin K‐dependent coagulation factors. In this context, normalization of haemostasis lasts hours to days. Fresh frozen plasma (FFP) generally has been considered the 'standard of care' for reversal of vitamin K antagonist therapy (Leissinger 2008). It provides partial reversal of the coagulopathy through replacement of exogenous factors II, VII, IX and X but presents important safety concerns and challenges involving 'dosage/international normalized ratio (INR) correction' and time needed to prepare the infusion (Demeyere 2010; Holland 2006). Prothrombin complex concentrate (PCC), originally developed for the treatment of patients with haemophilia B (Ostermann 2007), is derived from the cryoprecipitate supernatant of large plasma pools after removal of antithrombin and factor XI. Prothrombin complex concentrate represents a source of vitamin K‐dependent coagulation factors II, VII, IX and X and the naturally occurring anticoagulants activated proteins C and S. It has a final overall clotting factor concentration approximately 25 times higher than that of normal plasma (Franchini 2010). Increasing availability of purified and recombinant factor IX has led to expanded use of PCC for other indications (e.g. reversal of vitamin K‐induced bleeding disorders) (Franchini 2010).
Prothrombin complex concentrate, which has come into focus increasingly with regard to acute reversal of vitamin K antagonist bleeding disorders, constitutes treatment for two complex clinical subgroups with a similar need for acute intervention but with different therapeutic indications (Levi 2009; Pindur 1999; Schick 2009; Vigué 2009).
Vitamin K antagonist‐treated patients with a bleeding complication requiring acute surgical intervention (e.g. intracranial bleeding).
Vitamin K antagonist‐treated patients with supranormal INR levels and no ongoing bleeding complications who have the need for acute surgical intervention. .
How the intervention might work
Acute administration of PCC to reverse vitamin K‐induced coagulopathy over the past few years has reduced the need for blood transfusions, thereby reducing risks of volume overload, haemodilution coagulopathy and transfusion‐related acute lung injury, as well as possible transmission of viral infection (Bruce 2008; Franchini 2010; Riess 2007; Schick 2009).
Prothrombin complex concentrate theoretically offers several advantages over FFP: decreased time required for initiation of goal‐directed therapeutic interventions (e.g. surgical intervention), as thawing of plasma is bypassed (Taberner 1976; Van Aart 2006); reduced infusion volume, as PCC treatment of 1 to 2 mL/kg corresponds to a volume of approximately 15 mL/kg of FFP, thereby diminishing the risk of a compromised vulnerable cardiovascular system (Franchini 2010); lack of blood group specificity, reducing the risk of transfusion‐related complications; and finally, viral inactivation, which may minimize the risk of pathogen transmission.
Prothrombin complex concentrate seems to have a faster INR correction time than FFP, and several studies have suggested a role for PCC in the setting of acute neurosurgery in reducing clinical progression of intracerebral haemorrhage (Boulis 1999; Franchini 2010; Steiner 2011).
Why it is important to do this review
Use of FFP constitutes a well‐established procedure to reverse vitamin K antagonist‐induced bleeding disorders (Ansell 2008). However, avoiding blood transfusions may result in a beneficial effect in terms of possibly reduced mortality and morbidity (Koch 2006a; Koch 2006b; Theusinger 2009). Infusion of coagulation factor concentrates in low volumes might offer a potential treatment for patients experiencing vitamin K antagonist‐induced bleeding complications and people with supranormal INR levels requiring acute surgical intervention. Prothrombin complex concentrate is already included in American and European guidelines for reversal of acute life‐threatening bleeding related to elevated INR (Ansell 2008; Baglin 2006). However, it is important to stress that rapid reversal of an elevated INR is not necessarily associated with better clinical outcomes. Differentiating between therapeutic indications and clinical risk assessment is vital for balancing possible benefits (e.g. reduced blood transfusions and avoidance of volume overload, dilutional coagulopathy, pathogen transmission, transfusion‐related acute lung injury and the time‐consuming process of thawing blood group‐specific FFP) and drawbacks (e.g. inducing life‐threatening thrombotic events such as acute myocardial infarction, pulmonary embolism or cerebral venous thromboembolism) associated with treating an acute bleeding complication or preventing development of the bleeding complication by using exogenous coagulation factors (Warren 2009). More compelling evidence is needed in this area, and increasing use of PCC should be accompanied by an ongoing systematic assessment of its potential benefits and adverse events. The aim of this review is to assess the evidence suggesting that PCC is beneficial or harmful for vitamin K antagonist‐treated patients undergoing acute surgery compared with placebo, FFP or other haemostatic agents.
Objectives
We assessed the benefits and harms of PCC compared with fresh frozen plasma in the acute medical and surgical setting involving vitamin K antagonist‐treated bleeding and non‐bleeding patients. We investigated various outcomes and predefined subgroups and performed sensitivity analysis. We examined risks of bias and applied trial sequential analyses (TSA) (Wetterslev 2008) to examine the level of evidence, and we prepared a 'Risk of bias’ (ROB) table to test the quality of the evidence (Guyatt 2008).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), irrespective of publication status, date of publication, blinding status, outcomes published or language. We contacted investigators and study authors to request relevant data. We included unpublished trials only if trial data and methodological descriptions were provided in written form or could be retrieved from study authors. We included cluster‐randomized trials because the number of trials eligible for inclusion was expected to be low, but we excluded cross‐over trials and observational studies. We included in this review trials that applied interventional invasive procedures such as endoscopy, interventional radiology and vascular procedures (i.e. embolization, stenting of aneurisms) and less extensive procedures (i.e. nasal balloon tamponade) to extend the general applicability of the evidence.
Types of participants
We included participants who were treated with vitamin K antagonists (VKAs) and were undergoing acute surgery or surgical intervention related to bleeding complications arising from VKA treatment. We also included VKA‐treated participants with supranormal INR values undergoing acute surgery or surgical intervention as the result of co‐morbidity. We excluded trials that examined neonates and participants with hereditary bleeding disorders and/or significant liver dysfunction.
Types of interventions
We included trials on PCC versus FFP. We included trials using any dose of PCC over any duration of administration and/or co‐interventions. Furthermore, we included trials that compared PCC with other haemostatic agents (e.g. vitamin K, recombinant factor VIIa, other plasma derivatives). We undertook separate subgroup analyses of trials in which PCC was compared with other active interventions or was combined with co‐interventions.
PCC versus any comparator.
PCC versus placebo or no treatment.
PCC versus FFP.
PCC versus other haemostatic agents (vitamin K, recombinant factor VIIa, other plasma derivatives, factor‐substitution products).
PCC in combination with other haemostatic agents versus placebo, no treatment or usual treatment with or without haemostatic agents.
Types of outcome measures
Primary outcomes
Overall mortality: We used longest follow‐up data from each trial regardless of the period of follow‐up.
Overall 28‐day mortality: We included data provided as 30‐day mortality in the same analysis.
Secondary outcomes
The number of new occurrences of blood transfusion (e.g. avoidance of transfusion) and quantity of blood products transfused.
Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, disseminated intravascular coagulation), heparin‐induced thrombocytopenia, major immunological and allergic reactions (TRALI), cardiopulmonary overload (TACO), infection, sepsis (e.g. transmission of viral infections)).
Complications during the inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure).
Number of days in hospital.
Mean length of stay in intensive care unit (ICU).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 5; see Appendix 1); MEDLINE (Ovid SP, 1950 to 1 May 2013; see Appendix 2); EMBASE (Ovid SP, 1980 to 1 May 2013; see Appendix 3); International Web of Science (1964 to 1 May 2013; see Appendix 4); Latin American Caribbean Health Sciences Literature (LILACS via BIREME, 1982 to 1 May 2013; see Appendix 5); the Chinese Biomedical Literature Database; advanced Google; and the Cumulative Index to Nursing & Allied Health Literature (CINAHL, 1980 to 1 May 2013; see Appendix 6).
We applied a systematic and sensitive search strategy to identify relevant RCTs with no language or date restrictions. We adapted our MEDLINE search strategy for searching all other databases.
We reran the search in October 2014. We will add new studies of potential interest to a list of Studies awaiting classification, and we will incorporate them into formal review findings at the time of the review update.
Searching other resources
We handsearched the reference lists of reviews, randomized and non‐randomized studies and editorials to look for additional studies. We contacted the main authors of studies to ask about missed, unreported or ongoing studies. We contacted pharmaceutical companies for information on unpublished trials. We searched for ongoing clinical trials and unpublished studies on the following Internet sites (search date to 21 October 2014).
Data collection and analysis
Selection of studies
We assessed reports identified through the described searches and excluded obviously irrelevant reports. Two review authors independently examined these studies for eligibility (MJ, JL). We performed this process without blinding of study authors, institution, journal of publication or results. We resolved disagreements by discussion, and when no agreement was reached, we consulted a third person (AA). We provide a detailed description of the search and assessment.
Data extraction and management
Using a data extraction sheet (Appendix 7), we evaluated each study, entered the data into Review Manager 5 (RevMan 5.3) and checked them for accuracy. If data in the identified reports were unclear, we contacted study authors to invite them to provide further details. Two review authors (MJ, JL) independently extracted data in accordance with Appendix 7. We resolved disagreements by discussion, and if no agreement was reached, we consulted a third person (AW).
Assessment of risk of bias in included studies
Two review authors (MJ, JL) independently assessed risk of bias using the criteria outlined in Higgins 2011. We resolved disagreements by discussion, and if no agreement was reached, we consulted a third person (AW). We addressed each question of validity systematically as described here.
1. Random sequence generation
Assessment of randomization: sufficiency of the method in producing two comparable groups before intervention.
Grade: ’low risk’: a truly random process (e.g. random computer number generator, coin tossing, throwing dice); ’high risk’: any non‐random process (e.g. date of birth, date of admission by hospital or clinic record number or by availability of the intervention); or ’unclear risk’: insufficient information.
2. Allocation concealment
Allocation method prevented investigators or participants from foreseeing assignment.
Grade: ’low risk’: central allocation or sealed opaque envelopes; ’high risk’: using open allocation schedule or other unconcealed procedure; or ’unclear risk’: insufficient information.
3. Blinding
Assessment of appropriate blinding of the team of investigators and participants: person responsible for participant care, participants and outcome assessors.
Grade: ’low risk’: blinding considered adequate if participants and personnel were kept unaware of intervention allocations after inclusion of participants into the study, and if the method of blinding involved a placebo indistinguishable from the intervention, as mortality is an objective outcome; ’high risk’: not double‐blinded, categorized as an open‐label study, or without use of a placebo indistinguishable from the intervention; ’unclear risk’: blinding not described.
4. Incomplete outcome data
Completeness of outcome data, including attritions and exclusions.
Grade: ’low risk’: numbers and reasons for drop‐outs and withdrawals in the intervention groups described, or no drop‐outs or withdrawals was specified; ’high risk’: no description of drop‐outs and withdrawals provided; ’unclear risk’: report gave the impression of no drop‐outs or withdrawals, but this was not specifically stated.
5. Selective reporting
The possibility of selective outcome reporting.
Grade: ’low risk’: reported outcomes prespecified in an available study protocol, or, if this is not available, published report includes all expected outcomes; ’high risk’: not all prespecified outcomes reported, reported using non‐prespecified subscales, reported incompletely or report fails to include a key outcome that would have been expected for such a study; ’unclear risk’: insufficient information.
6. Other bias
Assessment of any possible sources of bias not addressed in domains 1 to 5.
Grade: ’low risk’: report appears to be free of such biases; ’high risk’: at least one important bias is present that is related to study design, early stopping because of some data‐dependent process, extreme baseline imbalance, academic bias, claimed fraudulence or other problems; or ’unclear risk’: insufficient information, or evidence that an identified problem will introduce bias.
With reference to domains 1 to 6 above, we planned to assess the likely magnitude and direction of the bias, and whether it was likely to impact the findings. Impact was explored through sensitivity analyses. Please see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (binary outcomes). These included the following.
Primary outcomes
Overall mortality: We used longest follow‐up data from each trial regardless of the period of follow‐up.
Secondary outcomes
Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, disseminated intravascular coagulation), major immunological and allergic reactions (TRALI), cardiopulmonary overload (TACO), infection, sepsis (e.g. transmission of viral infection)).
Complications during inpatient stay not specific to trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure).
Continuous data
We used mean differences (MDs) if data were continuous and were measured in the same way between trials. We used standardized mean differences (SMDs) to combine trials that measured the same outcome using different scales. These included the following.
Number of new occurrences of blood transfusions (e.g. avoidance of transfusion) and quantity of blood products transfused.
Number of days in hospital.
Mean length of stay in ICU.
Unit of analysis issues
Cross‐over trials
We excluded cross‐over trials from our meta‐analyses because of the potential risk for 'carry‐over' of treatment effect in the context of bleeding.
Studies with multiple intervention groups
In studies designed with multiple intervention groups, we combined groups to create a single pair‐wise comparison (Higgins 2011). In trials with two or more PCC groups receiving different doses, we combined data, when possible, for primary and secondary outcomes.
Dealing with missing data
We contacted the authors of trials with missing data to retrieve the relevant information. For all included studies, we noted levels of attrition and any exclusions. We conducted a sensitivity analysis to explore the impact of included studies with high levels of missing data. In cases of missing data, we chose ’complete‐case analysis’ for our primary outcome, which excludes from the analysis all participants with missing outcomes. Selective outcome reporting occurs when non‐significant results are selectively withheld from publication (Chan 2004); it is defined as selection, on the basis of results, of a subset of the original variables recorded for inclusion in publication of trials (Hutton 2000). The most important types of selective outcome reporting are selective omission of outcomes from reports; selective choice of data for an outcome; selective reporting of different analyses using the same data; selective reporting of subsets of the data; and selective under‐reporting of data (Higgins 2011). Statistical methods devised to detect within‐study selective reporting are still in their infancy. We tried to explore for selective outcome reporting by comparing publications with their protocols, if the latter were available.
Assessment of heterogeneity
We explored heterogeneity by using the I² statistic and the Chi² test. An I² statistic above 50% represents substantial heterogeneity (Higgins 2003). In cases of I² > 0 (mortality outcome), we tried to determine the cause of heterogeneity by performing relevant subgroup analyses. We used the Chi² test to provide an indication of heterogeneity between studies, with P value ≤ 0.1 considered significant.
Assessment of reporting biases
Publication bias arises when dissemination of research findings is influenced by the nature and direction of results (Higgins 2011). We planned to explore the level of publication bias related to the trials included in this review by generating a funnel plot. As only a small number of studies were eligible for inclusion in this review, this step was not performed as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Funding bias is related to possible publication delay or discouragement of undesired results in trials sponsored by the industry (Higgins 2011). To explore the role of funding, we planned to conduct a sensitivity analysis based on our primary endpoint. However, because of lack of data, this approach was not applied.
Data synthesis
We used Review Manager 5 software (RevMan 5.3) to perform meta‐analyses on pre‐stated outcomes from the included trials. If we performed the meta‐analyses and found I² = 0, we reported only results from the fixed‐effect model; in cases of I² > 0, we reported only results from the random‐effects model unless one or two trials contributed more than 60% of the total evidence provided, in which case the random‐effects model may have been biased. We believed that little value was derived by using a fixed‐effect model in cases of substantial heterogeneity, which we expected would be due to the various factors leading to massive bleeding. We pooled studies only in cases of low clinical heterogeneity. When meta‐analysis is used to combine results from several studies with binary outcomes (i.e. event or no event), adverse side effects may be rare but serious, and hence important (Sutton 2002). Most meta‐analytical software does not include trials with ’zero events’ in both arms (intervention vs control) when calculating a risk ratio (RR). Exempting these trials from calculation of an RR and 95% confidence interval (CI) may lead to overestimation of a treatment effect. The Cochrane Collaboration recommends application of the Peto odds ratio (OR), which is the best method of estimating odds ratios when many trials are found with no events in one or both arms (Higgins 2011). However, the Peto method generally is less useful when trials are small, or when treatment effects are large. We planned to conduct a sensitivity analysis by applying the Peto OR if this appeared to be a valid option. However, the trials included did not fulfil criteria for the Peto OR (Effects of interventions).
Trial sequential analysis
Meta‐analyses may result in type 1 errors caused by sparse data and repeated significance testing following updates with new trials (Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). Systematic errors from trials with high risk of bias, outcome reporting bias, publication bias, early stopping for benefit and small trial bias may result in spurious P values. In a single trial, interim analysis increases the risk of type 1 errors. To avoid type 1 errors, group sequential monitoring boundaries (Lan 1983) are applied to decide whether a trial could be terminated early because of a sufficiently small P value, that is, the cumulative Z curve crosses the monitoring boundary. Sequential monitoring boundaries can be applied to meta‐analyses as well and are called 'trial sequential monitoring boundaries'. In 'trial sequential analysis' (TSA), the addition of each trial in a cumulative meta‐analysis is regarded as an interim meta‐analysis, which helps to show whether additional trials are needed (Wetterslev 2008). It seems appropriate to adjust new meta‐analyses for multiple testing on accumulating data to control the overall type 1 error risk in cumulative meta‐analysis (Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2008). The idea in TSA is that if the cumulative Z curve crosses the boundary, a sufficient level of evidence is reached, and no further trials may be needed. However, evidence is insufficient to allow a conclusion if the Z curve does not cross the boundary or does not surpass the required information size. To construct the trial sequential monitoring boundaries (TSMB), the required information size is needed and will be calculated as the least number of participants needed in a well‐powered single trial (Brok 2008; Pogue 1998; Wetterslev 2008). We adjusted the required information size for heterogeneity with the diversity adjustment factor (Wetterslev 2009). We applied TSA, as it prevents an increase in the risk of type 1 errors (< 5%) due to potential multiple updating and testing on accumulating data, whenever new trial results are included in a cumulative meta‐analysis (Pogue 1997; Pogue 1998). This provided important information for estimation of the level of evidence on the experimental intervention (Pogue 1997; Pogue 1998; Thorlund 2009) and the need for additional trials and their required sample size (Wetterslev 2008; Wetterslev 2009). We applied TSMB according to an information size suggested by Wetterslev 2008 and Wetterslev 2009 and an a priori 20% relative risk reduction (RRR) of serious adverse events using a control event proportion suggested by the pooled estimate of the event proportion in included trial control groups. We conducted TSA at least on primary outcomes (Brok 2009; Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2008), and on secondary outcomes if the accrued information size was an acceptable fraction of the estimated required information size to allow meaningful analyses (> 20%). If the actual accrued information size was too small, we provided the required information size given the actual diversity (Wetterslev 2009)
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Benefits and harms of prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) without other haemostatic agents.
Benefits and harms of PCC versus FFP and cryoprecipitate.
Benefits and harms of PCC versus other haemostatic agents (rVIIa, antifibrinolytics, desmopressin, plasma derivatives or other factor‐substitution products).
Benefits and harms of PCC versus FFP in combination with other haemostatic agents.
Benefits and harms of PCC in combination with other haemostatic agents versus 'standard treatment'.
Benefits and harms of PCC in trials investigating the emergency surgery population (defined as those in whom surgery was performed within 24 hours after meeting the indication for surgery) requiring acute surgical intervention due to bleeding complications versus trials investigating the emergency surgery population with no bleeding complications requiring acute surgical intervention due to co‐morbidity.
Benefits and harms of PCC in trials investigating the trauma population versus trials investigating the non‐trauma population.
Benefits and harms of PCC in trials investigating the neurosurgical population versus trials investigating the non‐neurosurgical population.
Benefits and harms of PCC in trials investigating the cardiac surgery population versus trials investigating the non‐cardiac surgery population.
Benefits and harms of PCC in trials investigating the paediatric population (age younger than 18 years, neonates not included) versus trials investigating the adult population.
Benefits and harms of PCC when the pooled intervention effect in trials with a dose regimen higher than the median dose of administered PCC was compared with that in trials with a dose regimen equal to or smaller than the median dose. This comparison was performed to detect possible dependency of the estimate of intervention effect on dose regimen. In cases of considerable between‐trial heterogeneity, we applied meta‐regression.
Efficacy and safety of three‐factor PCC versus four‐factor PCC.
If analyses of various subgroups with binary data were significant, we planned to perform a test of interaction by applying the fixed inverse variance method incorporated in RevMan 5.3. Alternatively, we planned to apply meta‐regression if a fixed‐effect model was not considered sensible because between‐study variability was considerable. We considered P value < 0.05 as indicating significant interaction between the PCC effect on mortality and subgroup category (Higgins 2011, Chapters 9.6.1 and 9.7). We also considered applying Q‐partitioning for interaction and subgroup differences if appropriate (RevMan 5.3).
We did not perform subgroups analysis because data were insufficient. See Differences between protocol and review.
Sensitivity analysis
We planned to conduct the following sensitivity analyses.
Comparison of estimates of the pooled intervention effect in trials with low risk of bias versus estimates from trials with high risk of bias (i.e. trials having at least one inadequate risk of bias component).
Comparison of estimates of the pooled intervention effect in trials based on different components of risk of bias (random sequence generation, allocation concealment, blinding, completeness of outcome data, selective reporting and 'other bias').
Comparison of estimates of the pooled intervention effect in trials with high levels of missing data. In cases of missing data, we applied ’complete‐case analysis’ for primary and secondary outcomes, thereby excluding from the analysis all participants with missing outcome data.
Examination of the role of funding bias by excluding trials that are sponsored exclusively by pharmaceutical and medical devices companies.
Comparison of estimates of pooled intervention effects performed by excluding data from studies published only as abstracts.
Examination of the importance of thromboembolic events when participants with high prior risk of thrombotic events were compared with others.
We calculated RRs with 95% CIs and decided to apply 'complete‐case analysis', if possible, for sensitivity and subgroup analyses based on our primary outcome measure (mortality).
We did not perform sensitivity analysis because data were insufficient. See Differences between protocol and review.
Summary of findings tables
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008; GradePro) to assess the quality of the body of evidence associated with specific outcomes (overall mortality; number of new occurrences of allogenic blood transfusion; numbers of adverse events and complications probably related to the intervention) (Table 1).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
A systematic search of electronic databases combined with a handsearch of references resulted in identification of 1250 publications (Figure 1). Animal studies, reviews, duplicates and trials with irrelevant endpoints or populations resulted in exclusion of 1240 publications. On the basis of title and abstract, 10 publications were included for a full paper review. We identified six ongoing studies but were unable to retrieve any data from the investigators (Ahonen 2013; Frenzel 2008; Innerhofer 2012; Ranucci 2011; Roy 2013; Steiner 2009). We included four RCTs with a total of 453 participants (Boulis 1999; Demeyere 2010; Majeed 2013; Sarode 2013).
1.
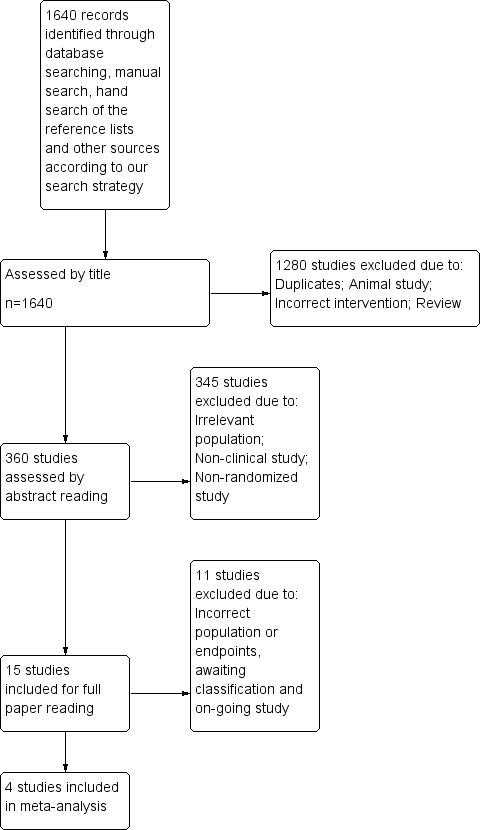
PRISMA. We reran the search in October 2014. We found 1 study of interest (Kerebel 2013). This study was added to a list of ‘Studies awaiting classification' and will be incorporated into formal review findings at the time of the review update .
We reran the search in October 2014 and identified an additional 390 studies and found one study of interest (Kerebel 2013). We will add potential new studies of interest to a list of Studies awaiting classification, and we will incorporate these studies into formal review findings at the time of the review update.
Included studies
See Characteristics of included studies; Appendix 8.
We included four RCTs published from 1999 to 2013 to investigate the potential role of PCC in the VKA‐treated participant with concomitant bleeding and/or need for acute surgical intervention. Sample sizes varied from 21 to 216 adult participants, and trials were categorized as surgical or non‐surgical. All trials assessed the use of weight‐based and INR‐based PCC administration with infusion of FFP. Three trials (Boulis 1999; Majeed 2013; Sarode 2013) investigated the potential of replacing FFP with PCC in the acutely bleeding participant. The fourth study investigated the preemptive replacement of FFP with PCC in the setting of semi urgent cardiac surgery (Demeyere 2010). In all trials but one (Demeyere 2010), investigators co‐administered vitamin K to both intervention and control groups.
Boulis 1999 assessed the effect of PCC administration in decreasing INR in 21 participants with intracranial bleeding potentially requiring acute surgical intervention.
Demeyere 2010 assessed the effect of preemptive PCC administration on numbers of postoperative bleedings, re‐operations and blood transfusions and on plasma concentrations of vitamin K‐dependent coagulation factors as additional outcomes in 40 participants.
Majeed 2013 assessed the effect of preemptive PCC administration in a mixed surgical population of 176 VKA‐treated participants requiring an acute surgical/invasive procedure with haemostatic efficacy during surgery and decreased INR, plasma factor levels and transfusion of red blood cells (RBCs) or whole blood.
Finally, Sarode 2013 assessed the effect of PCC administration on 216 VKA‐treated non‐surgical participants with elevated INR experiencing major bleeding. Additional outcomes included mortality, cause of death, length of hospital stay, length of stay in ICU, number and volume of blood transfusions, type of bleeding, haemostatic efficacy and plasma concentrations of vitamin K‐dependent coagulation factors at various time points (Sarode 2013).
Formulation of PCC (Table 2), dose of PCC and time of PCC administration differed between trials. The mean volume of FFP transfused in the trials by Boulis 1999, Demeyere 2010 and Sarode 2013 varied from 813.5 mL to 2712 mL in the FFP group and from 0 mL to 399 mL in the PCC group.
1. Prothrombin complex concentrates.
| PCC | Factor II | Factor VII | Factor IX | Factor X | Protein C | Protein S | Antitrombin | Heparin | Trial |
| Konyne | 38 IU/mL | 4 IU/mL | 25 IU/mL | 38 IU/mL | Not stated | Not stated | Not stated | Not stated | Boulis 1999 |
| Cofact | 14‐35 IU/mL | 7‐20 IU/mL | 25 IU/mL | 14‐35 IU/mL | 11‐39 IU/mL | 1‐8 IU/mL | ≤ 0.6 IU/mL | Not stated | Demeyere 2010 |
| Beriplex (Kcentra) | 19‐40 IU/mL | 10‐25 IU/mL | 20‐31 IU/mL | 25‐51 IU/mL | 21‐41 IU/mL | 12‐34 IU/mL | 0.2‐1.5 IU/mL | 0.4‐2 IU/mL | Majeed 2013; Sarode 2013 |
Product information sought (contact with study authors and Internet search) and applied when not listed in references. When "not stated", the information was not retrievable.
PCC = prothrombin complex concentrate.
Excluded studies
We excluded four studies (Aart 2006; Eerenberg 2011; Marlu 2012; Taberner 1976).
Aart 2006 is a dose response study that did not include other interventions for comparison. Taberner 1976 did not report on characteristics of participants included in the study (baseline characteristics, morbidity, reasons for anticoagulation), process of randomization, allocation, concealment or bias. Also, they did not report on any of our predefined outcomes. We were not able to ensure the inclusion of relevant participants, hence the study was excluded. Eerenberg 2011 and Marlu 2012 were ex vivo studies that included healthy individuals without bleeding or pending surgery. Researchers reported no relevant outcome measures.
(See Characteristics of excluded studies.)
Ongoing studies
We identified six ongoing trials (see Characteristics of ongoing studies).
Ahonen 2013: 40 participants randomly assigned to PCC + fibrinogen concentrate or fresh frozen plasma (FFP) in the treatment of postpartum haemorrhage. Primary outcome was blood loss.
Frenzel 2008: 200 participants under VKA therapy with the need for urgent surgery or invasive procedures randomly assigned to octaplex or FFP. Primary efficacy endpoint was correction of INR to < 1.5.
Innerhofer 2012: 200 participants experiencing trauma‐induced coagulopathy randomly assigned to coagulation factor concentrates or FFP. Primary outcome measure was multiple organ failure.
Ranucci 2011: 120 participants undergoing cardiac surgery randomly assigned to placebo (saline), fibrinogen concentrate or PCC. Primary outcome was avoidance of allogeneic blood product transfusion.
Roy 2013: 400 VKA‐treated participants with minor craniocerebral trauma admitted to hospital randomly assigned to receive "standard therapy" or preventive reversal of coagulopathy with PCC. Primary outcome measure was percentage of intracranial bleeding diagnosed on computed tomography (CT) scan.
Steiner 2009: 74 VKA‐treated participants with CT‐confirmed intracerebral bleeding randomly assigned to receive PCC or FFP. Primary outcome was INR ≤ 1.2 within three hours after the start of drug infusion.
Risk of bias in included studies
We could classify no trials as having overall ’low risk of bias’. We evaluated the overall quality of trials on the basis of major sources of bias (domains) as previously described. We present the various bias domains in the ’Risk of bias’ graph and in the ’Risk of bias’ summary (Figure 2; Figure 3).
2.
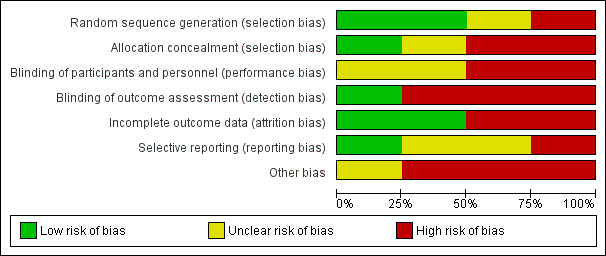
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
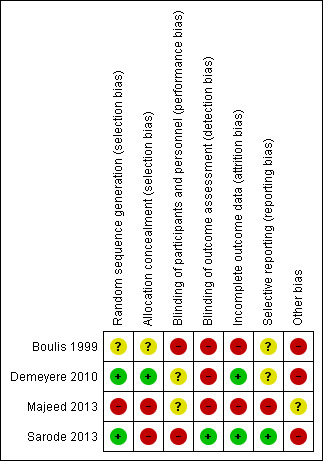
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials provided sufficient information regarding generation of allocation sequence (Demeyere 2010; Sarode 2013). One trial provided allocation concealment (Demeyere 2010). Two trials were designed as open‐label (Majeed 2013; Sarode 2013) and provided no allocation concealment.
Blinding
No included trials reported blinding of participants. Two trials did not blind personnel involved (Boulis 1999; Sarode 2013). One trial did not describe blinding of personnel (Majeed 2013). Finally, one trial (Demeyere 2010) blinded the surgeons involved but did not describe blinding of anaesthesiologists who were in charge of study drug administration. Two trials did not blind outcome assessors (Boulis 1999; Demeyere 2010). One trial did not report on blinding of outcome assessors. Finally, one study provided relevant blinding of outcome assessors (Sarode 2013). Attempts to contact study authors to request further information were not successful.
Incomplete outcome data
All four trials excluded participants after randomization. Two trials excluded participants on the basis of parameters that were not in accordance with exclusion criteria (Boulis 1999; Demeyere 2010). Boulis 1999 excluded 38% of the initial population because of withdrawal of consent and omission in data collection. Demeyere 2010 excluded two participants because vitamin K was erroneously administered. One trial did not describe reasons for exclusion of participants (Majeed 2013), and another trial adequately described the reasons for exclusion (Sarode 2013).
Selective reporting
We were able to compare one trial with its trial registration at www.clinicaltrials.gov (Sarode 2013). The time frame of the co‐primary outcome was changed from one hour and four hours post infusion at trial registration to 24 hours post infusion as reported in the publication. Despite contacting the study authors, we were unable to obtain protocol information for the three remaining trials (Boulis 1999; Demeyere 2010; Majeed 2013). Demeyere 2010 concluded that use of PCC caused less bleeding, even when this was a non‐significant finding. With the exception of the latter, none of the included studies were suspected of selective reporting.
Other potential sources of bias
Funding bias
One study stated that investigators had no financial interest and no apparent link to the medical industry (Boulis 1999). The three remaining trials were supported in varying degrees by the manufacturer of the investigated drug (Demeyere 2010; Majeed 2013; Sarode 2013). Support primarily consisted of financial backing, employment of investigators by the manufacturer, access to free drugs or assistance in preparing and processing the scientific data.
Study design and early stopping
One trial reported a sample size calculation and provided clinically relevant outcomes (Sarode 2013). The three remaining trials provided no sample size calculation (Boulis 1999; Majeed 2013) or based the calculation on pragmatic considerations (Demeyere 2010). Primary outcomes for the three latter trials were based on surrogate parameters. One study (Boulis 1999) reported early stopping as the result of shortage of study drugs.
Baseline imbalance
Demeyere 2010 provided baseline parameters (weight, age and surgical data) with exploratory statistical analyses and reported no baseline differences. Sarode 2013 and Majeed 2013 also provided baseline parameters but without statistical explanatory support. Boulis 1999 concluded that no differences in demographic data, including participant weight, age and indications for warfarin anticoagulation, were present.
Effects of interventions
See: Table 1
Primary outcome 1. Overall mortality longest follow‐up
We will use the longest follow‐up data from each trial regardless of the period of follow‐up. Three trials reported data on "Overall mortality ‐ longest follow up" (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.37 to 2.33; participants = 421; trials = 4; I2 = 43%; very low quality of evidence). All‐cause mortality did not seem to be associated with the use of PCC (Figure 4; Boulis 1999; Majeed 2013; Sarode 2013). The small number of studies eligible for inclusion precluded subgroup and sensitivity analyses referring to the primary outcome.
4.
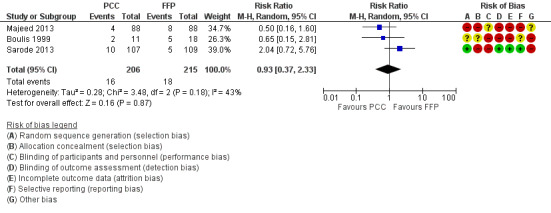
Forest plot of comparison: 1 Mortality, outcome: 1.1 Overall mortality ‐ longest follow‐up.
We conducted trial sequential analysis of effects of PCC versus FFP on all‐cause mortality as reported by three trials (Boulis 1999; Majeed 2013; Sarode 2013) with a control event proportion of 8.4%, diversity of 44% and an anticipated intervention effect of 50% relative risk reduction. Despite the anticipated large intervention effect of 50% relative risk reduction, in this analysis, the cumulative Z‐curve does not break through any boundaries for benefit, harm or futility. The analysis is inconclusive because information was sparse.
Primary outcome 2. Overall 28‐day mortality
We intended to provide data as 30‐day mortality. None of the included studies provided data as 28‐day mortality, hence we were not able to further conduct subgroup or sensitivity analyses.
Secondary outcome 1. Number of new occurrences of blood transfusions (e.g. avoidance of transfusion) and quantity of blood products transfused
The number of new occurrences of blood transfusions (RBCs) was reported by two trials (Majeed 2013; Sarode 2013). The use of PCC did not appear to be associated with fewer new occurrences of RBC transfusions (RR 1.08, 95% CI 0.82 to 1.43; participants = 370; trials = 2; I2 = 0%; very low quality of evidence) (Analysis 2.1). We were unable to further investigate the true distribution of RBC units transfused per participant because data were insufficient (Demeyere 2010; Sarode 2013 ‐ we contacted the study authors but received no relevant reply; Table 3). We were able to obtain data regarding the volume of RBC transfused in two trials (Majeed 2013; Sarode 2013). The volume of RBCs transfused did not appear to be associated with the use of PCC (mean difference (MD) ‐1.85, 95% CI ‐85.89 to 82.20; I2 = 13%; very low quality of evidence) (Figure 5).
2.1. Analysis.
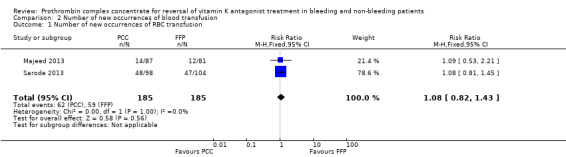
Comparison 2 Number of new occurrences of blood transfusion, Outcome 1 Number of new occurrences of RBC transfusion.
2. Additional findings.
| Analysis | Study | Participants (PCC/FFP) | Notes | Findings | |
| Number of new occurrencesof RBC transfusion | Demeyere 2010 | 20/20 | 19 participants allocated to FFP group received 50 units of packed RBCs 16 participants in PCC group received 33 units of packed RBCs |
True distribution of packed RBCs per participant unknown because of lack of data (study authors contacted but no reply; Included studies). Hence, trial data were not amenable for meta‐analysis | |
| Transfusion of FFP | Boulis 1999 | 6/11 | 6 participants allocated to PCC group received a mean of 399 mL (SD = 271 mL) FFP 11 participants allocated to FFP group received a mean of 2712 mL (SD = 346 mL) FFP |
MD ‐2313 mL; 95% CI ‐2611.04 mL to ‐2014.96 mL; participants = 17; studies = 1 | |
| Demeyere 2010 | 20/20 | None of the participants allocated to PCC group received FFP 20 participants allocated to FFP group received a mean of 1372 mL (SD = 320 mL) FFP |
Secondary to study design and based on lack of events in PCC group, this study was not included in further analysis of FFP transfusion (Data synthesis; Results) | ||
| Sarode 2013 | 103/108 | None of the participants allocated to PCC group received FFP 108 participants allocated to FFP group received a mean of 813.5 mL (SD = 187.5 mL) FFP |
Secondary to study design and based on lack of events in PCC group, this study was not included in further analysis of FFP transfusion | ||
| Length of stay in hospital (days) | Sarode 2013 | 98/104 | 98 participants in PCC group had mean length of stay in hospital of 4.5 days (SD = 0.55) 104 participants in FFP group had mean length of stay in hospital of 4.2 days (SD = 0.55) |
MD = 0.30 days; 95% CI 0.15 days to 0.45 days | |
| Length of stay in ICU (hours) | Sarode 2013 | 98/104 | 98 participants in PCC group had mean stay of 0 hours in ICU (IQR 0‐44.7) 104 participants in FFP group had mean stay of 0 hours in the ICU (IQR 0‐40.0) |
MD = 0.00 hours; 95% CI ‐0.08 hours to 0.08 hours | |
| Number of complications during inpatient stay not specific to trial intervention | Sarode 2013 | 103/109 | 103 participants in PCC group experienced 31 events 109 participants in FFP group experienced 24 events |
RR = 1.37; 95% CI 0.86 to 2.16 | |
| Amount of bleeding | Demeyere 2010 | 20/20 | No statistical differences in 1 hour, 4 hours and 24 hours postoperative chest tube drainage | 1 hour (FFP): mean, mL (SD) 97.0 (± 54.7) 1 hour (PCC): mean, mL (SD) 67.6 (± 46.8) 4 hours (FFP): mean, mL (SD) 163.5 (± 89.8) 4 hours (PCC): mean, mL (SD) 133.5 (± 73.4) 24 hours (FFP): mean, mL (SD) 471.7 (± 294.4) 24 hours (PCC): mean, mL (SD) 439.3 (± 247.3) |
CI = confidence interval.
FFP = fresh frozen plasma.
ICU = intensive care unit.
IQR = interquartile range.
MD = mean difference.
PCC = prothrombin complex concentrate.
RBC = red blood cell.
SD = standard deviation.
5.
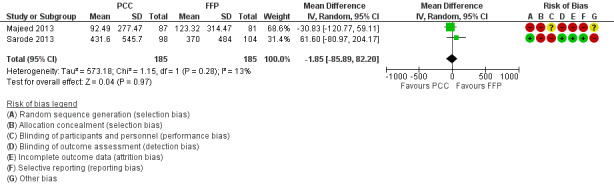
Forest plot of comparison: 3 Quantity of blood products transfused, outcome: 3.1 Transfusion of RBCs.
We conducted trial sequential analysis (TSA) of PCC versus FFP on quantity of RBCs transfused from two trials (Majeed 2013; Sarode 2013) with diversity of 18% and an anticipated mean difference in intervention effect of 125 mL (Figure 6). The cumulative Z‐curve breaks through the boundary for futility (non‐superiority). Therefore the analysis is able to reject an intervention effect of 125 mL in the light of sparse data and repetitive analyses. The TSA‐adjusted confidence interval for the intervention effect estimated in a random‐effects model of ‐1.83 mL is ‐123 mL to 120 mL.
6.
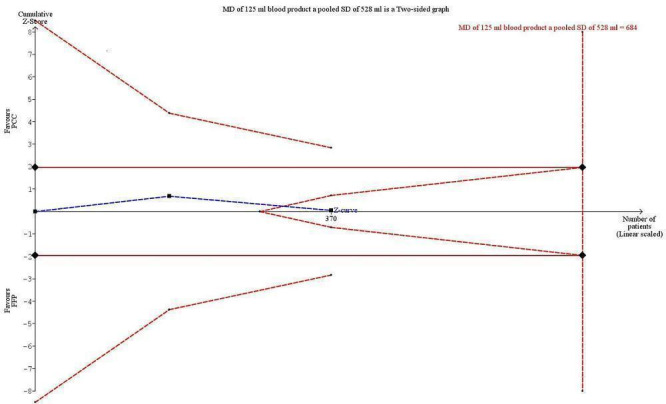
Trial sequential analysis (TSA) of PCC vs FFP on quantity of RBCs transfused from 2 trials with diversity of 18% and an anticipated mean difference in intervention effect of 125 mL. The cumulative Z‐curve breaks through the boundary for futility (non‐superiority). The analysis therefore led to rejection of an intervention effect of 125 mL based on sparse data and repetitive analyses. The TSA adjusted confidence interval on the intervention effect estimated in a random‐effects model of ‐1.83 mL is ‐123 mL to 120 mL.
Three trials reported the number of new occurrences of FFP transfusion (Boulis 1999; Demeyere 2010; Sarode 2013). In the studies of Demeyere 2010 and Sarode 2013, transfusion of FFP was not protocolized in the intervention group. In the study by Boulis 1999, transfusion of FFP was administered to control and intervention groups. As a result of the apparent study design (FFP vs PCC), an obvious difference in the number of new occurrences of FFP transfusion was reported. Analysing data by applying the fixed‐effect model (Data synthesis) showed an apparent effect of PCC on the number of new occurrences of FFP transfusion (RR 0.06, 95% CI 0.03 to 0.12; participants = 264; trials = 3; I2 = 99%; Analysis 2.2). However, applying the random‐effects model showed no association between administration of PCC and the number of new occurrences of FFP transfusion (RR 0.05, 95% CI 0.00 to 270914.97; participants = 264; trials = 3; I2 = 99%). The high degree of heterogeneity and diversity in these results poses important questions regarding internal validity and indicates that data most likely are not appropriate for meta‐analysis. We planned to conduct a corrected analysis by applying the Peto OR if this was seen as a valid option (Data synthesis). However, only two trials (Demeyere 2010; Sarode 2013) included ’zero event’ in one group (PCC/intervention arm), and Demeyere 2010 was a small study with 20 participants in each group. The Peto method generally is less useful when trials are small, or when treatment effects are large. Hence we chose not to proceed with these exploratory analyses.
2.2. Analysis.
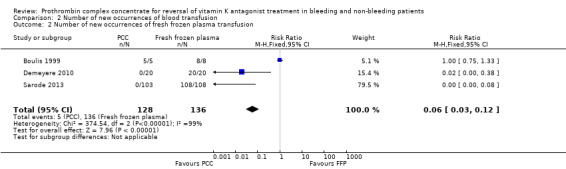
Comparison 2 Number of new occurrences of blood transfusion, Outcome 2 Number of new occurrences of fresh frozen plasma transfusion.
The volume of FFP transfused concomitantly among control and intervention groups differed in three trials (Boulis 1999, FFP arm: mean = 2712 mL; standard deviation (SD) = 346 mL; PCC arm: mean = 399 mL; SD = 271 mL; Demeyere 2010, FFP arm: mean = 1372 mL; SD = 320 mL; PCC arm: no transfusion of FFP; Sarode 2013, FFP arm: mean = 813.5 mL; SD = 187.5 mL; PCC arm: no transfusion of FFP. Majeed 2013 did not report on FFP transfusion requirements. We contacted the study authors, but they did not reply. As a result of the occurrence of two 'zero event' trials (Demeyere 2010; Sarode 2013), we were unable to perform meta‐analysis.
One trial reported on amount of bleeding. Demeyere 2010 reported chest tube drainage at one hour, four hours and 24 hours postoperatively, but researchers were not able to make an association between the use of PCC and the amount of bleeding (Table 3).
Secondary outcome 2. Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, disseminated intravascular coagulation), heparin‐induced thrombocytopenia, major immunological and allergic reactions (TRALI), cardiopulmonary overload (TACO), infection and sepsis (e.g. transmission of viral infection))
All trials reported on complications probably related to trial intervention. We could not show an association between use of PCC and complications (adverse events) probably related to trial intervention (RR 0.92, 95% CI 0.78 to 1.09; I2 = 0%; very low quality of evidence) (Figure 7). One trial specifically reported data on complications not related to trial intervention (Sarode 2013). These primarily involved cardiac ischaemic events, respiratory failure and intracranial haemorrhage. No other trials stated information regarding complications not specific to trial intervention. Demeyere 2010 reported adverse events in 7/20 participants allocated to the PCC group and in 9/20 in the FFP group. Types of adverse events and potential correlation of adverse events to trial intervention were not further investigated by study authors (these authors were contacted but failed to reply). Majeed 2013 reported overall numbers of adverse events (PCC = 41/88; FFP = 44/88) and serious adverse events (PCC = 22/88; FFP = 23/88) but did not classify these events as related or not related to trial intervention.
7.
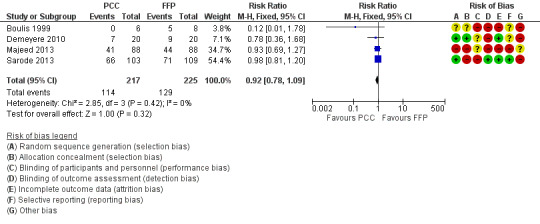
Forest plot of comparison: 4 Adverse events, outcome: 4.1 Number of complications probably related to the intervention.
We conducted trial sequential analysis of PCC versus FFP on adverse events possibly related to the intervention from four trials with a control event proportion of 57%, diversity of 0% and an anticipated intervention effect of 20% relative risk reduction (RRR) (Figure 8). The cumulative Z‐curve breaks through the boundary for futility (non‐superiority). The analysis therefore is able to reject an intervention effect of 20% RRR, in the light of sparse data and repetitive analyses, but an intervention effect less than 20% RRR may be present.
8.
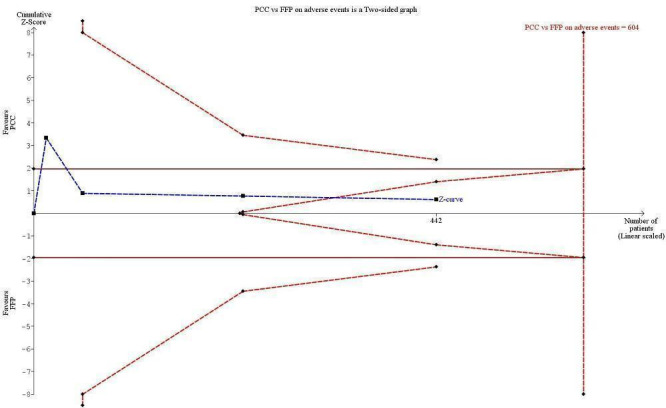
Trial sequential analysis (TSA) of PCC vs FFP on adverse events from 4 trials with a control event proportion of 57%, diversity of 0% and an anticipated intervention effect of 20% relative risk reduction (RRR). The cumulative Z‐curve breaks through the boundary for futility (non‐superiority). The analysis therefore led to rejection of an intervention effect of 20% RRR based on sparse data and repetitive analyses, but the intervention effect may be less than 20% RRR.
As studies eligible for inclusion were few and provided a limited quantity of data along with considerable heterogeneity, we were not able to further assess this subgroup analysis.
Secondary outcome 3. Complications during inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure)
Only one study (Sarode 2013) reported on complications during inpatient stay not specific to the trial intervention. As studies eligible for inclusion were few and provided a limited quantity of data, we were not able to further assess this subgroup analysis.
Secondary outcome 4. Number of days in hospital
Only one study (Sarode 2013) reported on "number of days in hospital". We assessed duration of stay in hospital (Table 3) and found no statistically significant effect. As studies eligible for inclusion were few and provided a limited quantity of data, we were not able to further assess this subgroup analysis.
Secondary outcome 5. Mean length of stay in the ICU
Only one study (Sarode 2013) reported on "number of days in hospital". We assessed duration of ICU stay (Table 3 ) and found no statistically significant effect. As studies eligible for inclusion were few and provided a limited quantity of data, we were not able to further assess this subgroup analysis. No trials reported data on "days free from ventilator".
Finally, none of the trials provided data on quality of life assessment or cost‐benefit analyses.
Discussion
This systematic review included four randomized controlled trials comprising a total of 453 medical and surgical vitamin K antagonist (VKA)‐treated participants undergoing reversal therapy with prothrombin complex concentrate (PCC), fresh frozen plasma (FFP) or both. Mortality may be contested by many as the choice of primary outcome, but it summarizes ultimate harms and benefits simultaneously. Three studies provided data on mortality (Boulis 1999; Majeed 2013; Sarode 2013). None of these studies were powered to detect a difference in mortality. Meta‐analysis showed no statistical benefit of PCC treatment versus FFP (RR 0.93, 95% CI 0.37 to 2.33). We identified use of a medical treatment (PCC) that has been known for many years and that lately has found its way to European and American guidelines for acute reversal of acute life‐threatening bleeding related to elevated international normalized ratio (INR) (Ansell 2008; Baglin 2006). Still, it must be kept in mind that a high level of heterogeneity, bias, differences in study design (87% of the participants included in this review originate from two RCTs designed as non‐inferiority studies) and use of surrogate outcome measures (e.g. INR values and time to INR reversal) contribute to overall low clinical evidence and hence applicability.
However, it is important to stress the fact that use of PCC seems to enable reversal of vitamin K‐induced coagulopathy without the need for transfusion of FFP. Demeyere 2010 showed that the comparator FFP arm required considerably more therapeutic measures to reach the target INR value (20/20 participants) compared with 6/20 participants in the PCC arm. Specifically, the FFP arm required a total of 19.4 L FFP additional to the 8 L intended in the protocol (400 mL × 20 participants). Furthermore, intermittent use of PCC was needed to achieve the target INR value in the FFP arm. In addition, Boulis 1999 showed that introducing the use of PCC reduced the mean volume of FFP transfused from 2712 mL (SD = 346 mL) to 399 mL (SD = 271 mL), and Sarode 2013 showed that total avoidance of FFP was possible for urgent reversal of VKA therapy in major bleeding events, as demonstrated by clinical assessments of bleeding and laboratory measurements of INR and factor levels.
Assessment of safety is not uniform and raises important questions regarding underreporting. Both Demeyere 2010 and Majeed 2013 stated the number of adverse events and classified them as serious or non‐serious. No further elaboration of type of adverse event or possible correlation with medical treatment was investigated. We were unable to associate use of PCC with the number of complications probably related to the intervention (RR 0.92, 95% CI 0.78 to 1.09). Both Boulis 1999 and Sarode 2013 stated the occurrence of "fluid overload" as the most frequent adverse event in the FFP‐treated group. Still, lack of definition and possible correlation with underlying patient co‐morbidity remain to be further investigated. The number of new occurrences of blood transfusions (RBCs) did not seem to be associated with use of PCC (RR 1.08, 95% CI 0.82 to 1.43)
Thus, this review summarizes an area in which the evidence differs widely from clinical practice, and the information provided here should indeed ultimately guide the direction of future research. The quality of evidence for our primary and secondary outcomes was very low. Two of four included studies had high or unclear risk of selection bias and high risk of attrition bias; 50% of included studies had high risk of performance bias and 75% had high risk of detection bias. Most of the included studies had unclear risk of reporting bias. More randomized clinical trials with clinically relevant endpoints and a systematic definition of adverse events are urgently needed.
Summary of main results
Our systematic review comprised a total of 453 participants included in four "high risk of bias" trials performed in a widespread clinical array with a high degree of both clinical and statistical heterogeneity. No statistically significant effect on mortality or number of serious adverse events (e.g. life‐threatening thrombotic events such as acute myocardial infarction, pulmonary embolism or cerebral venous thromboembolism) was observed. Prothrombin complex concentrate appears to enable reversal of vitamin K‐induced coagulopathy without the need for transfusion of fresh frozen plasma and without generating a higher rate of adverse events.
Overall completeness and applicability of evidence
It must be kept in mind that all of the four included trials were small (imprecision of results) and aimed primarily to investigate surrogate outcomes (e.g. time to reverse international normalized ratio (INR), indirectness). Additionally, all trials were characterized by a high degree of heterogeneity in study design and clinical setup. Currently, evidence supporting the use of PCC in bleeding patients is weak. Discussions related to prothrombin complex concentrate treatment of the vitamin K antagonist‐treated patient experiencing acute life‐threatening bleeding or with a need for urgent surgery are often complicated by lack of evidence. A high level of heterogeneity, use of a non‐standardized product (three‐factor PCC vs four‐factor PCC; contents of naturally occurring anticoagulants such as proteins C and S), lack of definition of relevant clinical outcomes (e.g. adverse events) and conflicts of interest often complicate interpretation of results.
Quality of the evidence
Randomized controlled trials (RCTs) are considered the gold standard for evaluating clinical effects and new treatments. Prothrombin complex concentrate has been used in different forms (e.g. three‐factor and four‐factor concentrates) since 1976, and still no well‐designed and powered RCT supports the general applicability of its use. The body of RCTs currently available provide only evidence of low quality. Reasons for downgrading the evidence include risk of bias in included studies, wide confidence intervals and relatively few participants overall.
Potential biases in the review process
The strength of this review lies in the specification that all included studies must have a comparator group. Still, this excludes most of the studies found by a well‐constructed search strategy that minimizes the chance of missing randomized controlled trials not fulfilling inclusion criteria. Authors of this review have no conflicts of interest. We ensured that language would not introduce bias by imposing no limitations on such. With regards to safety outcomes, reporting was not uniform and raises concerns of underreporting. We reran the search strategy in October 2014 and found one study of interest. This study was added to a list of ‘Studies awaiting classification' and will be incorporated into formal review findings at the time of the review update.
Agreements and disagreements with other studies or reviews
The overall conclusions of this review do not contradict the statements and findings of previous reviews and meta‐analysis (Dentali 2011; Lin 2013; Sørensen 2011, Vang 2011). A recent systematic review (Lin 2013) on the use of coagulation factors such as fibrinogen and prothrombin complex concentrate (PCC) concludes that too often surrogate primary endpoints (laboratory parameters) are used instead of preferred patient‐related clinical outcomes (need for allogenic blood transfusion and postoperative chest tube drainage). Also, the authors of this review found the existing body of literature to be characterized by a high degree of bias, underpowered and unable to evaluate safety outcomes, as reporting of adverse events is not uniform and raises concerns of underreporting. Dentali 2011 performed a meta‐analysis of 27 studies evaluating the safety of PCCs for rapid reversal of vitamin K antagonist treatment. Investigators concluded that a low but quantifiable risk of thromboembolism in VKA‐treated participants receiving PCCs for anticoagulation reversal exists, and that these findings should be confirmed in randomized controlled trials. In accordance with these findings, Sørensen 2011 and Vang 2011 (non‐systematic reviews) emphasize the importance of patient risk stratification in relation to possible ongoing bleeding, indications for VKA therapy, risk of thromboembolic events and type of surgery.
Authors' conclusions
Implications for practice.
The current body of evidence based on RCT analysis does not suggest superiority of either FFP or PCC and suggests that both treatment options may induce the occurrence serious adverse events. Fresh frozen plasma has long been considered international “gold standard therapy” for reversal of vitamin K antagonist‐induced coagulopathy, and use of PCC has raised concerns about patient safety due to lack of uniform reporting of adverse events and possible underreporting of serious adverse events. Several non‐randomized studies (Cartmill 2000; Holland 2006; Huttner 2006; Makris 1997; Schick 2009) have indicated that prothrombin complex concentrate is more effective in reversing INR when compared with fresh frozen plasma. PCC can reduce the need for transfusion of FPP, thereby reducing transfusion‐related complications in vitamin K antagonist‐treated patients. Still, no randomized clinical trials have so far been sufficiently designed or powered to detect harms or benefits. Consequently, we were unable to reach conclusions on possible benefits and harms associated with the use of prothrombin complex concentrate. At present, the general and routine use of PCC in clinical practice can be based only on expert opinion and non‐randomized studies. Before choosing this intervention, practitioners should consider awaiting sufficient evidence on patient important outcomes (benefits and harms) to be derived from large randomized trials with low risk of bias.
Implications for research.
Further trials are urgently needed, and great emphasis must be placed on attempts to reduce bias and increase power to show differences in patient‐relevant clinical outcomes (i.e. mortality).
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We thank Dr Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia Review Group (CARG)) for assistance in providing our different search strategies. We thank Jane Cracknell (Managing Editor, CARG) for valuable assistance given during the entire process.
We would like to thank Andy Smith (Content Editor), Marialena Trivella (Statistical Editor)and Laura Green, Michael Makris and Giancarlo M Liumbruno (Peer Reviewers) for help and editorial advice given during preparation of this protocol.
We would like to thank Andrew Smith (Content Editor), Vibeke E Horstmann (Statistical Editor) and Giancarlo M Liumbruno, Laura Green and Michael Makris (Peer Reviewers) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. CENTRAL
((prothrombin near complex) or Beriplex or Octaplex) or ((anticoagulat* therap* or perioperative) near revers*)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. ((prothrombin adj3 complex) or Beriplex or Octaplex).af. 2. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 3. 1 and 2 4. ((anticoagulat* therap* or perioperative) adj5 revers*).af. 5. 3 or 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. ((prothrombin adj3 complex) or Beriplex or Octaplex).ti,ab. 2. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 3. 1 and 2 4. ((anticoagulat* therap* or perioperative) adj5 revers*).af. 5. 3 or 4
Appendix 4. ISI Web of Science
#1 TI=((prothrombin SAME complex) or Beriplex or Octaplex) or TS=((anticoagulat* therap* or perioperative) SAME revers*) #2 TS=(random* or ((controlled or clinical) SAME trial*) or multicenter or placebo* or prospective) or TS=((blind* or mask*) SAME (single or double or triple or treble)) #3 #1 and #2
Appendix 5. LILACS (iAH) search strategy
((prothrombin and complex) or Beriplex or Octaplex) or ((anticoagulat$ therap$ or perioperative) and revers$) [Palavras] and (random$ or ((controlled or clinical) and trial$) or multicenter or placebo$ or prospective or ((blind$ or mask$) and (single or double or triple or treble))) [Palavras]
Appendix 6. CINAHL (EBSCO host) search strategy
S1 TX ((prothrombin N3 complex) or Beriplex or Octaplex) OR TX ((anticoagulat* therap* or perioperative) N5 revers*)
Appendix 7. Data extraction form
Study selection, quality assessment and data extraction form
| First author | Journal/Conference proceedings, etc | Year |
| |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/No*/Unclear |
* Issue relates to selective reporting when authors may have taken measurements for particular outcomes but did not report these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after 3 attempts, study should be excluded.
| Do not proceed if any of the above answers is ‘No’. If study is to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If further references to this trial are identified, link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference proceedings, etc | Year |
| A | The paper listed above | ||
| B | Further papers |
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers/%, etc) | |
| Disease status/type, etc (if applicable) | |
| Patients treated with coagulants other than VKA before intervention | |
| Clinical setting: (mark with an X) | Cardiac Non‐cardiac Neurosurgery Emergency (surgery that should be performed within 24 hours after meeting the indication for surgery) Trauma Obstetrics Paediatrics (age younger than 18 years, neonates not included) Neonates (born preterm) Critically ill (sepsis, septic shock, DIC) Other |
| Other | |
Trial characteristics
SeeAppendix 7, usually completed by just 1 reviewer.
Methodological quality
We recommend that you refer to and use the method described by Juni (Juni 2001).
| Allocation of intervention (random sequence generation?) | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Adequate (random) (low risk of bias) |
| Inadequate (e.g. alternate) (high risk of bias) | |
| Unclear (unclear risk of bias) | |
|
Concealment of allocation (allocation concealment?) Process used to prevent foreknowledge of group assignment in an RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate (low risk of bias) | |
| Inadequate (high risk of bias) | |
| Unclear (unclear risk of bias) | |
| Blinding (blinding of participants and personnel and blinding of outcome assessment?) | |
| Person responsible for participant care | Low risk/High risk |
| Participant | Low risk/High risk |
| Outcome assessor | Low risk/High risk |
| Other (please specify) | Low risk/High risk |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether or not they received it. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes ? No ? Not clear ?
| Incomplete outcome data? | |
| Completeness of outcome data including attrition and exclusions | Grade (circle) |
| Adequate (low risk) | |
| Inadequate (high risk) | |
| Unclear (unclear risk) | |
| Selective reporting? | |
| Possibility of selective outcome reporting | Grade (circle) |
| Adequate (low risk) | |
| Inadequate (high risk) | |
| Unclear (unclear risk) | |
|
Other bias? (bias not addressed in the other domains) | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate (low risk) | |
| Inadequate (high risk) | |
| Unclear (unclear risk) | |
Data extraction
|
Outcomes relevant to your review Copy and paste from ‘Types of outcome measures’ | |
| Reported in paper (circle) | |
| Overall mortality | Yes/No |
| Overall 28‐day mortality (30‐day mortality included) | Yes/No |
| Number of new occurrences of blood transfusion (e.g. avoidance of transfusion) | Yes/No |
| Number of bleeding events | Yes/No |
| Quantity of blood products transfused | Yes/No |
| Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, disseminated intravascular coagulation), major immunological and allergic reactions (TRALI), cardiopulmonary overload (TACO), infection and sepsis (e.g. transmission of viral infection)) | Yes/No |
| Complications during inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure) | Yes/No |
| Yes/No | |
| Yes/No | |
| Number of days in hospital. | Yes/No |
| Mean length of stay in intensive care unit (ICU). | Yes/No |
| For continuous data | |||||||
| Code of paper |
Outcomes (rename) |
Unit of measurement |
Intervention group | Control group | Details if outcome described only in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A, etc | Bleeding events and amount of blood transfused | ||||||
| Number of days in hospital | |||||||
| Mean length of stay in intensive care unit (ICU) | |||||||
| For dichotomous data | |||
| Code of paper | Outcomes (rename) | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| A | Overall mortality | ||
| Overall mortality (28 days) | |||
| Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, DIC), major immunological and allergic reactions, infection and sepsis) | |||
| Complications during inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure) | |||
|
Other information that you feel is relevant to the results Indicate if any data were obtained from the primary study author; or if results were estimated from graphs, etc or were calculated by you using a formula (this should be stated and the formula given). In general, if results not reported in paper(s) are obtained, this should be made clear here to be cited in the review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, list contact names and details | ||
| | ||
| Trial characteristics | |
| Further details | |
| Single‐centre/Multi‐centre | |
| Country/Countries | |
| How was participant eligibility defined? |
|
| How many people were randomly assigned? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose/Frequency of administration | |
| Co‐interventions (including if prothrombin complex concentrate was given as part of a predefined transfusion algorithm) |
|
| Duration of treatment (state weeks/months, etc; if cross‐over trial, give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years, or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Appendix 8. Details of included studies
| RCT | Number of included patients | Setting | Blinding/ Randomization method |
Intervention (PCC) |
Control group (FFP) |
Significant outcomes (intervention vs placebo) |
Bias |
| Boulis 1999 | 21 | Surgical: intracranial bleeding; possible need for neurosurgical intervention |
Not blinded/ Not stated |
1. 10 mg of vitamin K subcutaneous 2. Infusion of FFP at maximum tolerated rate 3. IU (PCC) = kilograms of weight × (target factor level ‐ current factor level)* *Target factor level was set at 50%. Current factor levels of participants were estimated on the basis of the INR at the time of randomization. INRs 2 to 3 were equated with factor concentrations of 10% INRs 3 to 4 were equated with factor concentrations of 5% INRs > 4 were equated with factor concentrations < 1% |
1. 10 mg of vitamin K subcutaneous 2. Infusion of FFP at maximum tolerated rate |
Less FFP‐transfusion 399 ± 271 mL vs 2712 ± 346 mL |
High risk of bias |
| Time to INR‐correction 2.95 ± 0.46 hours vs 8.9 ± 1.51 hours | |||||||
| Rate of INR‐correction 0.63 ± 0.18 [DELTA]INR/h vs 0.18 ± 0.03 [DELTA]INR/h | |||||||
| Demeyere 2010 | 40 | Surgical: semi urgent cardiac surgery |
Surgeons blinded/ Computer programme |
1. After sternotomy, 50% dose of PCC administered* 2. After cardiopulmonary bypass, remaining 50% dose of PCC administered* 3. Additional study product transfusion *Dosage based on participant's body weight and initial and target INRs |
1. 2 units FFP after sternotomy and before cardiopulmonary bypass 2. 2 units FFP administered following cardiopulmonary bypass 3. Additional study product transfusion |
Number of participants reaching INR target after 15 minutes: 7/20 vs 0/20 | High risk of bias |
| Need for additional study product (FFP and/or PCC): 6/20 vs 20/20 participants | |||||||
| Significantly higher level of coagulation factor II and X over time (intervention group) | |||||||
| Significantly higher haematocrit level over time, indicating less dilution (intervention group) | |||||||
| Sarode 2013 | 216 | Non‐surgical: major bleeding |
Open label/ Computer |
1. 5 to 10 mg vitamin K infusion 2. Single‐dose infusion PCC* * Dosage based on INR and body weight. Maximum infusion rate of 3 IU/kg/min. Maximum PCC dose 5000 IU |
1. 5 to 10 mg vitamin K infusion 2. FFP infusion 6 mL/min* *Maximum FFP dose 1500 mL |
INR ≤ 1.3 at 0.5 hour after end of infusion 62.2% vs 9.6% |
High risk of bias |
| Median INR significantly lower until 12 hours after start of infusion | |||||||
| Mean factor levels were significantly higher in the intervention group than in the plasma group at 0.5, 1, 3 and 6 hours (P value < 0.05) apart from factor VII at 6 hours | |||||||
| Majeed 2013 | 176 | Surgical: VKA reversal before urgent surgical procedure |
Open label/ Not stated |
1. 2 to 10 mg vitamin K 2. Single‐dose infusion PCC * Dosage based on INR and body weight. Maximum infusion rate of 3 IU/kg/min |
1. 2 to 10 mg vitamin K 2. Infusion FFP* * Plasma infusion rate at treating physician's discretion |
Effective haemostasis in 89.7% vs 75.3% of participants 14.3% (95% CI 2.8 to 25.8) |
High risk of bias |
| Rapid INR reduction in 55.2% vs 9.9% of participants 45.3% (95% CI 31.9 to 56.4) Fewer fluid overload events in PCC group –9.1% (–18.6 to –0.1) |
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality ‐ longest follow‐up | 3 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.37, 2.33] |
1.1. Analysis.
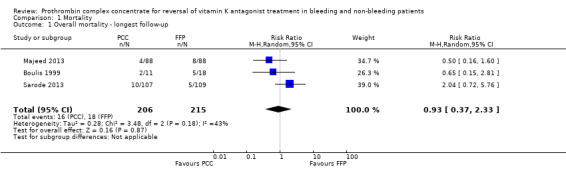
Comparison 1 Mortality, Outcome 1 Overall mortality ‐ longest follow‐up.
Comparison 2. Number of new occurrences of blood transfusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of new occurrences of RBC transfusion | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 2 Number of new occurrences of fresh frozen plasma transfusion | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.03, 0.12] |
Comparison 3. Quantity of blood products transfused.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Transfusion of RBCs | 2 | 370 | Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐85.89, 82.20] |
3.1. Analysis.
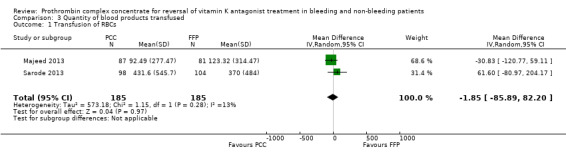
Comparison 3 Quantity of blood products transfused, Outcome 1 Transfusion of RBCs.
Comparison 4. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of complications probably related to the intervention | 4 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
4.1. Analysis.
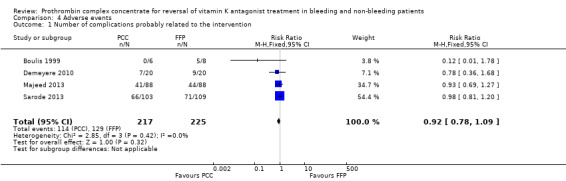
Comparison 4 Adverse events, Outcome 1 Number of complications probably related to the intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boulis 1999.
| Methods | Two‐group, parallel RCT, single centre Overall study quality: high risk of bias Sample size calculation: none reported. Study terminated preterm because of drug shortage Funding: no declarations of interest stated |
|
| Participants | 21 participants randomly assigned to 2 groups: fresh frozen plasma group ((FFP) + vitamin K + furosemide) vs PCC group (FFP + vitamin K + FIXCC + furosemide). Complete follow‐up on 5 participants in PCC group and 8 participants in FFP group Inclusion criteria: CT scan confirmation of subarachnoid, subdural, epidural or intracerebral haemorrhage and documented history of current warfarin usage with prothrombin time > 17 seconds at inclusion Exclusion criteria: clinical evidence of brainstem herniation |
|
| Interventions | After randomization, all participants received 10 mg of vitamin K subcutaneous and infusion of fresh frozen plasma was started at maximal rate tolerated without causing fluid overload. Additionally, furosemide was administered to counteract the latter Intervention: Participants randomly assigned to the FIXCC group were treated with 10 mg vitamin K subcutaneous and FIXCC (Konyne; Bayer). The dose of PCC was calculated as follows: "International Units FIXCC requested = weight (kg) x (target factor level ‐ current factor level)". FIXCC infusion rate was 100 IU/min Control: Participants randomly assigned to the FFP group were treated with 10 mg vitamin K subcutaneous and infusion of FFP at maximal rate tolerated by individual participants |
|
| Outcomes | Rate of INR correction, time to INR correction and total INR correction. Glasgow Coma Scale scores at the time of the initial evaluation and at discharge. Complications of anticoagulation correction. Dose of FIXCC and volume of FFP required to achieve an INR of 1.3 and need for surgery following intervention | |
| Notes |
Objective: to compare the speed of warfarin anticoagulation correction and resulting complications for patients treated with FIXCC compared with standard treatment Country: Michigan, USA PCC: Konyne, Bayer Period of investigation: 1996‐1997 Authors' conclusion: "Significant differences were found in time to INR correction, rate of INR correction and volume of FFP required for correction between groups. Neurological outcomes were unchanged using the Glasgow Coma Scale." Author contacted 25/11‐2013 + 27/11‐2013 (reply: 25/11‐2013 + 27/11‐2013) Bayer Healthcare contacted 28/11‐2013 (reply: 09/12‐2013) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 dropouts (4 due to withdrawal of consent from relatives and 4 result of missed blood drawings). Intention‐to‐treat seems however to have been performed on mortality data |
| Selective reporting (reporting bias) | Unclear risk | Protocol or trial registration not available. Protocol not registered on www.clinicaltrials.gov |
| Other bias | High risk | Study terminated before time for very small sample size. No sample size calculation stated. Lacks a clear definition of exclusion criteria ("clinical evidence of brainstem herniation"), which might lead to investigator‐dependent conclusions. No description of the number of eligible patients and reasons for non‐inclusion. Only body weight and age were described as baseline variables, thus no reasons for anticoagulant treatment or co‐morbidities were accounted for. Authors compared RCT results with historical data from the same centre |
Demeyere 2010.
| Methods | 40 participants, single‐centre, 1:1 parallel RCT Overall study quality: high risk of bias Sample size calculation: none reported (calculation based on pragmatic consideration) Funding: Investigation was supported through a grant from CAF‐DCF – SANQUIN for laboratory analyses. Dr. Paul Strengers is employed by CAF‐DCF and Sanquin |
|
| Participants | 40 participants assigned to urgent or semi urgent cardiac surgery were randomly assigned to receive PCC or FFP before and after cardiopulmonary bypass procedure. Two participants were replaced because surgery was postponed, and 2 other participants were excluded for inadvertently receiving vitamin K. All participants were analysed as intention‐to‐treat (ITT) Inclusion criteria: urgent or semi urgent cardiac surgery under oral anticoagulation therapy with acenocoumarol, phenprocoumon or warfarin; INR ≥ 2.1 and < 7.8; weight < 100 kg Exclusion criteria: renal or hepatic insufficiency; allergic reaction to blood products; disseminated intravascular coagulation; active thrombosis or pulmonary embolism; intracardiac thrombus; treatment with platelet inhibitors (except for aspirin); pregnant or breastfeeding women |
|
| Interventions |
Intervention: Post sternotomy and before cardiopulmonary bypass, participants received half of the calculated
dose of PCC based on their body weight and initial and target INR. The additional half was given following cardiopulmonary bypass and after administration of protamine sulfate. Further doses of PCC were allowed to reach target INR ≤ 1.5 Control: Post sternotomy and before cardiopulmonary bypass, participants received 2 units of FFP. Another 2 units were administered following cardiopulmonary bypass and protamine sulfate. Additional doses of FFP and/or PCC to reach INR target of ≤ 1.5 postoperatively were allowed |
|
| Outcomes |
Primary: number of participants reaching target INR (≤ 1.5) and time needed to reach target INR after completion of cardiopulmonary bypass Secondary: Quantity and number of postoperative bleedings, re‐operations or blood transfusions, plasma concentration of vitamin K‐dependent coagulation factors II, VII, IX and X and haematocrit |
|
| Notes |
Objective: To compare the efficacy of intraoperative administration of PPC versus fresh frozen plasma in patients on oral anticoagulant treatment undergoing heart surgery with cardiopulmonary bypass Country: Belgium PCC: Cofact, Sanquin Period of investigation: 2002‐2004 Authors' conclusion:"PCC reverses anticoagulation safely, faster and with less bleeding than fresh frozen plasma" Author contacted 27/11‐2013 + 29/1‐2014: no relevant reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was generated with the Dynarand Automated Randomization Computer programme |
| Allocation concealment (selection bias) | Low risk | Sealed envelope was opened sequentially by the investigator |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Surgeons were blinded. Participants, anaesthetic personnel and persons in charge of administration of study medication were not accounted for |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants from the PCC group inadvertently received vitamin K and were excluded from the per protocol population. Study authors did not describe at which point in the trial these 2 participants received vitamin K. All participants were treated as ITT |
| Selective reporting (reporting bias) | Unclear risk | Study authors concluded that PCC provides less bleeding based on a non‐significant result |
| Other bias | High risk |
Funding: The investigation was supported through a grant from CAF‐DCF – SANQUIN. Dr. Paul Strengers was employed by CAF‐DCF and Sanquin. Monitoring the study, drafting manuscript and performing statistical analyses were completed by personnel from the medical industry Statistiques: Sample size was small and was based on pragmatic considerations. Between‐groups baseline imbalance is possible because of co‐morbidity and small sample size (12 participants were considered at high risk of thrombosis because of coronary artery bypass grafting) Breach of protocol: FFP group: 4/20 participants before cardiopulmonary bypass were treated with higher doses of FFP (600 mL vs 400 mL), while 5/20 participants were treated with higher doses of FFP post cardiopulmonary bypass. This breach of protocol could inflict fluid overload, increase numbers of adverse advents and affect primary and secondary outcomes PCC group: Several participants received a higher than recommended dose of PCC. This breach of protocol could induce the same effect in primary and secondary outcomes, as mentioned above apart from fluid overload |
Majeed 2013.
| Methods | 176 participants, prospective, phase IIIb, open‐label, non‐inferiority trial Overall study quality: high risk of bias Sample size calculation: none reported Funding: The study was funded by CSL‐Behring |
|
| Participants | 176 VKA‐treated participants were randomly assigned 1:1 to receive a single dose of PCC or FFP before an urgent surgical or invasive procedure. All participants received vitamin K as early as possible Inclusion criteria: Patients (≥ 18 years) currently receiving VKA therapy requiring urgent surgical or invasive procedure within 24 hours of medical product administration. INR greater than or equal to 2 within 3 hours before the start of investigational medicinal product. Informed consent obtained Exclusion criteria: Participants requiring urgent surgical procedures for which according to the surgeon's clinical judgement, an accurate estimate of blood loss was not possible (e.g. ruptured aneurysm). Participants for whom administration of intravenous vitamin K and vitamin K antagonist withdrawal alone could adequately correct the participant's coagulopathy before initiation of the urgent surgical procedure. Administration of intravenous vitamin K longer than 3 hours or administration of oral vitamin K longer than 6 hours before infusion of investigational medicinal product. Participants in whom lowering INR within normal range could present an unacceptable risk for a thromboembolic complication when the INR goal was to lower but not normalize the INR because of risk of a procedure‐associated stroke. Participants who, despite medical management that included close monitoring and diuretics, might not, by investigator assessment, tolerate the total volume of investigational medicinal product required by the protocol Expected need for additional non‐study blood products before infusion of investigational medicinal product. Administration of packed red blood cells was not an exclusion criterion. Expected need for platelet transfusions or desmopressin before day 10. Acute trauma for which reversal of vitamin K antagonist treatment alone would not be expected to control or resolve an acute bleeding complication and/or control the acute bleeding event. Unfractionated or low molecular weight heparin use within 24 hours before randomization or potential need before completion of the procedure. History of thromboembolic event, myocardial infarction, unstable angina pectoris, critical aortic stenosis, cerebral vascular accident, transient ischaemic attack, severe peripheral vascular disease, disseminated intravascular coagulation within 3 months of enrolment; known history of antiphospholipid antibody syndrome or lupus anticoagulant antibodies. Suspected or confirmed serious viral or bacterial infection (e.g. meningitis, sepsis) at time of enrolment. Administration of whole blood, plasma, plasma fractions or platelets within 2 weeks before inclusion into the study. Pre‐existing progressive fatal disease with life expectancy less than 2 months. Known inhibitors to coagulation factors II, VII, IX or X; or hereditary protein C or protein S deficiency; or heparin‐induced, type II thrombocytopenia. Treatment with any other investigational medicinal product within 30 days before inclusion into the study. Presence or history of hypersensitivity to components of the study medication. Pregnant or breast‐feeding women. Prior inclusion in this study or any other CSL Behring‐sponsored Beriplex study. For participants with intracranial haemorrhage with Glasgow Coma Score < 10 (see Appendix 8), Modified Rankin Score > 3 before ICH (see Appendix 9), intracerebral haemorrhage, epidural haematoma, infratentorial haemorrhage, subarachnoid haemorrhage (SAH); participants with a Hunt and Hess Scale > 2, subdural haematoma judged to be an acute subdural haematoma (based on neurosurgeon review); participants with concurrent SAH or parenchymal contusion |
|
| Interventions |
Intervention: administration of vitamin K and 4F‐PCC before urgent surgical/invasive procedure Control: administration of vitamin K and FFP before urgent surgical/invasive procedure Dosage of 4F‐PCC or FFP was based on baseline INR and weight |
|
| Outcomes |
Primary outcome measures:
1. Percentage of participants achieving haemostatic efficacy during surgery. Haemostatic efficacy was rated as excellent, good or poor/none on the basis of prespecified definitions 2. Percentage of participants who had a rapid decrease in INR (time frame: 30 minutes after the end of infusion) Rapid decrease in INR was defined as INR ≤ 1.3 at 30 minutes after completion of infusion Secondary outcome measures: 1. Plasma levels of factors II, VII, IX and X, protein C and protein S 2. Transfusion of packed red blood cells (PRBCs) or whole blood 3. Percentage of participants with INR correction at various times after the start of infusion 4. Percentage of participants who received red blood cells 5. Overall treatment‐emergent adverse events (TEAEs) |
|
| Notes |
Objective: to evaluate efficacy, safety and tolerance of Beriplex P/N (Kcentra) compared with plasma with regard to rapid reversal of coagulopathy induced by vitamin K antagonists in participants who require immediate correction of international normalized ratio (INR) because of emergency surgery Country: United States, Belarus, Bulgaria, Lebanon, Romania and Russian Federation PCC: Beriplex (KCentra) Period of investigation: 2009‐2012 Authors' conclusion:"The study met both co‐primary endpoints and found that 4FPCC was superior to plasma for hemostatic efficacy and for rapid INR reduction. Overall, 4F‐PCC had a favourable safety profile and was well tolerated in this population. This study demonstrates that 4F‐PCC is an effective alternative to plasma for rapid VKA reversal in patients undergoing urgent surgical procedures" Author contacted 01/05‐2014 (no reply) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Poster and abstract published. Results found at www.clinicaltrials.gov (NCT00803101). Study authors contacted 01‐05‐2014, no reply. Reasons for exclusion of participants were death/serious adverse events (PCC = 4/88; fresh frozen plasma = 6/88), withdrawal of consent (PCC = 6/88; fresh frozen plasma = 4/88), loss to follow‐up (PCC = 2/88; fresh frozen plasma = 2/88), protocol violation (PCC = 1/88; fresh frozen plasma = 7/88), surgery finished outside protocol window (PCC = 1/88; FFP = 1/88), participant died beyond the serious adverse event report period (PCC = 1/88; FFP = 0/88), participant did not attend scheduled visit (PCC = 2/88; FFP = 1/88) and no surgery (PCC = 1/88; FFP = 1/88) |
| Selective reporting (reporting bias) | High risk | Only poster and abstract published |
| Other bias | Unclear risk | Trial initiated and funded by CSL Behring |
Sarode 2013.
| Methods | 216 participants, multi‐centre, 1:1 parallel, open‐label, non‐inferiority RCT with stratification for site and type of bleeding Overall study quality: high risk of bias Sample size calculation: yes, based on haemostasis in 85% of plasma group and increased to 90% in PCC group, including 10% dropouts with power of 84% to show non‐inferiority of PCC vs FFP Funding: sponsored by CSL Behring |
|
| Participants | 216 participants with major bleeding randomly assigned, 103 received PCC and 109 received FFP. Four were excluded before treatment because of withdrawal of consent Inclusion criteria: ≥ 18 years of age; receiving VKA therapy with an elevated INR (≥ 2.0 within 3 hours before study treatment) and experiencing an acute major bleeding event were eligible. Acute major bleeding was defined as 1 of the following: life‐threatening or potentially life‐threatening (according to the treating physician); acute bleeding associated with a fall in haemoglobin ≥ 2 g/dL; and bleeding requiring blood product transfusion Exclusion criteria: 1. Expected survival of < 3 days or expected surgery in < 1 day 2. Acute trauma for which reversal of vitamin K antagonist treatment alone would not be expected to control or resolve the acute bleeding event 3. Use of unfractionated or low‐molecular‐weight heparin < 24 hours before enrolment or expected need < 24 hours after start of infusion 4. History of thrombotic event, myocardial infarction, disseminated intravascular coagulation, cerebral vascular accident, transient Ischaemic attack, unstable angina pectoris, severe peripheral vascular disease at ≤ 3 months of enrolment 5. Known history of antiphospholipid antibody syndrome 6. Suspected/confirmed sepsis at enrolment 7. Administration of FFP, plasma fractions or platelets ≤ 2 weeks before study (administration of packed red blood cells permitted) 8. Large blood vessel rupture (e.g. aortic dissection, ruptured aortic aneurysm) 9. Preexisting progressive fatal disease with life expectancy < 2 months 10. Known inhibitors to factors II, VII, IX or X; or hereditary protein C or S deficiency; or heparin‐induced, type II thrombocytopenia 11. Treatment with any other investigational medicinal product ≤ 30 days before study 12. Presence or history of hypersensitivity to components of the study medication 13. Intracranial haemorrhage 14. Glasgow Coma Scale score < 7 15. Intracerebral haematoma volume > 30 cm3 (assessed by ABC/2 formula) 16. For subdural haematoma, maximum thickness ≥ 10 mm, midline shift ≥ 5 mm 17. For subarachnoid haemorrhage, any evidence of hydrocephalus 18. Infratentorial intracranial haemorrhage location 19. Epidural haematomas 20. Intraventricular extension of haemorrhage 21. Modified Rankin Scale score > 3 before intracranial haemorrhage |
|
| Interventions | When randomly assigned, all participants were to receive 5 to 10 mg vitamin K (according to 2008 American College of Chest Physicians guidelines (Ansell 2008) or local clinical practice if different) by slow intravenous infusion Intervention: PCC was administered as a single intravenous dose based on INR and body weight, with a maximum infusion rate of 3 IU/kg/min. Maximum PCC dose 5000 IU Control: Plasma was infused intravenously at a study protocol–recommended infusion rate (median of 6 mL/min). Maximum FFP dose of 1500 mL |
|
| Outcomes |
Primary endpoints: 1. Haemostatic efficacy assessed over a 24‐hour period from start of infusion (haemostatic efficacy rated as excellent, good or poor/none by a blinded, independent endpoint adjudication board) 2. INR correction (≤ 1.3) at 0.5 hour after end of infusion Secondary endpoints: plasma levels of vitamin K‐dependent coagulation factors and natural anticoagulant proteins (proteins C and S). Time to INR correction. Volume of RBC and FFP transfused. Mortality and numbers of adverse events |
|
| Notes |
Objective: to compare INR correction and haemostatic efficacy of PCC vs FFP in the non‐surgical setting of major bleeding Country: 36 sites across the United States and Europe PCC: Kcentra, Beriplex (described as 4F‐PCC in article and Kcentra in www.clinicaltrials.gov) Period of investigation: not stated Authors' conclusion:"PCC is an effective alternative to plasma for urgent reversal of vitamin K antagonist therapy in major bleeding events, as demonstrated by clinical assessments of bleeding and laboratory measurements of international normalized ratio and factor levels" Authors contacted 21/4‐2014 (reply 25/4‐2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned by a centrally managed biased‐coin minimization method |
| Allocation concealment (selection bias) | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Haemostatic efficacy was assessed by a blinded, independent endpoint adjudication board (EAB). An independent data safety monitoring board reviewed unblinded data to assess participant safety. Serious adverse events (AEs) of interest to the data safety monitoring board (thromboembolic events, deaths, late bleeding events) were reviewed by a blinded, independent safety adjudication board (SAB). The sponsor participated in selection of board members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of participants is well described |
| Selective reporting (reporting bias) | Low risk | Compared with clinical registration NCT00708435: Time frame of the co‐primary outcome is changed from 1 hour and 4 hours post infusion in protocol to 24 hours post infusion. Following exclusion criteria are omitted in the final text: pregnant or breast‐feeding women; prior inclusion in this study or in any other CSL Behring‐sponsored Beriplex study |
| Other bias | High risk | Funding: Sponsor participated in selection of board members. Sponsor was responsible for data processing, management, analysis of data according to a predefined statistical analysis plan and reporting of results. Editorial assistance was funded by the sponsor. Dr. Sarode received consulting fees and honoraria from CSL Behring GmbH. Dr. Mangione and B. Durn were employees of CSL Behring LLC. Dr. Schneider was an employee of CSL Behring GmbH. Dr. Goldstein received consulting fees, honoraria and a research grant from CSL Behring GmbH. Dr. Milling received consulting fees from CSL Behring |
AEs = adverse events.
CSL = CSL Behring.
CT = computerized tomography.
EAB = endpoint adjudication board.
FFP = fresh frozen plasma group.
FIXCC = factor IX concentrate.
ICH = intracerebral haemorrhage.
INR = international normalized ratio.
ITT = intention‐to‐treat.
PCC = prothrombin complex concentrate.
PRBCs = packed red blood cells (PRBCs).
RBC = red blood cells.
RCT = randomized controlled trial.
SAB = safety adjudication board.
SAH = subarachnoid haemorrhage.
TEAEs = treatment‐emergent adverse events.
VKA = vitamin K antagonists.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aart 2006 |
Randomized controlled trial: INR reversal due to bleeding/need for surgical intervention 93 participants; single‐centre, open‐label, 1:1 parallel RCT comparing 2 dose regimens of PCC administration. Control group received fixed dose of PCC, while intervention group received PCC at weight‐adjusted dose. No FFP was administered Findings: "Of the patients treated according to the individualized dosing regimen, significantly more patients attained target‐INR 15 minutes after PCC administration than patients treated with standard dose" Reason for exclusion: dose response study; single comparative measure with no transfusion requirements; no relevant outcome measures |
| Eerenberg 2011 |
Randomized cross‐over study: reversal of Dabigatran and Rivaroxaban effect as anticoagulants in healthy individuals Two‐group, parallel, prospective RCT with healthy individuals randomly assigned to 2 groups receiving Rivaroxaban or Dabigatran in total 2.5 days Following this, both groups received a single bolus of 50 IU/kg of PCC or saline. Trial was repeated, with both groups switching intervention treatment Findings:"Prothrombin complex concentrate immediately and completely reverses the anticoagulant effect of rivaroxaban in healthy subjects but has no influence on the anticoagulant action of dabigatran at the PCC dose used in this study" Reason for exclusion: Healthy individuals without bleeding or pending surgery were included. No relevant outcome measures |
| Marlu 2012 |
Randomized cross‐over study: In vitro reversal of Dabigatran and Rivaroxaban effect as anticoagulants in healthy participants Two‐group, parallel, prospective study with healthy individuals randomly assigned to 1 single bolus of 20 mg Rivaroxaban or 150 mg Dabigatran. Blood samples were drawn close to intervention and 2 hours after. After a 15‐day washout period, participants received the other drug. Drawn blood samples were then treated with factor VII, PCC or FEIBA Findings:"PCC strongly corrected ETP‐AUC, whereas rFVIIa only modified the kinetic parameters. FEIBA corrected all parameters.... In conclusion, some non‐specific reversal agents appear to be able to reverse the anticoagulant activity of rivaroxaban or dabigatran" Reason for exclusion: ex vivo study including healthy individuals without bleeding or pending surgery; no relevant outcome measures |
| Taberner 1976 |
Randomized controlled trial: INR reversal of vitamin K antagonist treatment. Unknown setting Two‐group, parallel, prospective RCT, single centre. Eighteen instances of overdose with nicoumalone (Sinthrome) and two with warfarin were studied: 9 participants were randomly assigned to each group. The remaining 2 are not accounted for Findings: "The prothrombin complex concentrate provides a quicker, more controlled but less sustained method of reversing the coumarin defect than vitamin K1" Reason for exclusion: no relevant outcome measures |
ETP‐AUC = endogenous thrombin potential ‐ area under the curve.
FEIBA = activated prothrombin complex concentrate.
FFP = fresh frozen plasma group.
INR = international normalized ratio.
PCC = prothrombin complex concentrate.
RCT = randomized controlled trial.
rFVIIa = recombinant factor VIIa.
Characteristics of studies awaiting assessment [ordered by study ID]
Kerebel 2013.
| Methods | Phase III, prospective, randomized, open‐label study including individuals with objectively diagnosed VKA‐associated intracranial haemorrhage |
| Participants | 59 patients objectively diagnosed with VKA‐associated intracranial haemorrhage between November 2008 and April 2011 at 22 centres in France |
| Interventions | A total of 59 participants were randomly assigned to receive 25 IU/kg PCC (29 participants) or 40 IU/kg PCC (30 participants) |
| Outcomes |
Primary endpoint: international normalized ratio 10 minutes after the end of 4‐factor PCC infusion Secondary endpoints: changes in coagulation factors, global clinical outcomes and numbers of new occurrences of adverse events |
| Notes |
PCC = prothrombin complex concentrate.
VKA = vitamin K antagonist.
Characteristics of ongoing studies [ordered by study ID]
Ahonen 2013.
| Trial name or title | Use of Prothrombin Complex Concentrate and Fibrinogen Compared With Fresh Frozen Plasma in the Treatment of Postpartum Haemorrhage |
| Methods | Randomized, parallel, open‐label efficacy study |
| Participants | 40 participants randomly assigned to PCC + Fibrinogen concentrate or FFP Inclusion criteria: Women with vaginal delivery or by caesarean section with PPH of 2000 mL (the amount of blood loss: in addition to the suctioned blood, sponges, wraps, swabs, etc., are carefully weighed) Exclusion criteria: Women with a history of bleeding tendency or hepatic or renal insufficiency, or PPH exceeding 3000 mL |
| Interventions | Twenty participants in the PCC group will receive 2000 IU of PCC concentrate and 2 grams of fibrinogen concentrate. Twenty participants in the FFP group will receive 4 units of FFP |
| Outcomes |
Primary outcome measures:
Maximum clot firmness (time frame: at the time when blood loss reaches 2000 mL and 45 minutes later): FIBTEM/ROTEM
Endogenous thrombin potential (time frame: at the time when blood loss reaches 2000 mL and 45 minutes later)
Fibrinogen level (time frame: at the time when blood loss reaches 2000 mL and 45 minutes later): Clauss method
Platelet function (time frame: at the time when blood loss reaches 2000 mL and 45 minutes later): PFA‐100 Secondary outcome measures: Blood loss (time frame: within 24 hours after delivery). Suctioned blood, sponges, wraps, swabs, etc., are carefully weighed. The amount of blood loss will be compared between groups |
| Starting date | July 2013 |
| Contact information | Jouni V. Ahonen, Ph.D., M.D.; +358504271852; jouni.ahonen@fimnet.fi |
| Notes | Estimated completion date: December 2014 |
Frenzel 2008.
| Trial name or title | A Randomized, Open‐Label, Efficacy and Safety Study of OCTAPLEX and Fresh Frozen Plasma in Patients Under Vitamin K Antagonist Therapy With the Need for Urgent Surgery or Invasive Procedures |
| Methods | Randomized, parallel, open‐label study |
| Participants | 200 participants will be randomly assigned to determine whether Octaplex works at reversing the effects of anticoagulants when compared with standard treatment of receiving FFP Inclusion criteria: Male or female patients at least 18 years of age Receiving oral anticoagulation with coumadin or warfarin derived agents Need for urgent surgery or an invasive procedure up to 8 hours after admission, or currently hospitalized, when oral or parenteral vitamin K therapy is deemed too slow in its action for reversal of coumadin or warfarin anticoagulant effects International normalized ratio (INR) of 2.0 or above Have given written informed consent or written informed consent has been obtained from legal representative on patient's behalf Able and willing to comply with the procedures laid out in the study protocol. In the case of unconscious and/or incapacitated patients, willingness of legal representative to undergo the procedures laid out in the study protocol Exclusion criteria: Life expectancy of less than 48 hours (e.g. GCS (Glasgow Coma Scale) equal to 3 or head AIS (abbreviated injury score) of 6, requiring continuous inotropic or pressor support, status post cardiac arrest) History within past 6 months of disseminated intravascular coagulation (DIC) or hyperfibrinolysis Known congenital coagulation disorder Known antiphospholipid antibody syndrome or known lupus anticoagulant antibodies Present or past specific factor inhibitor activity Thrombocytopenia < 80,000 or history of heparin‐induced thrombocytopenia (HIT) Received heparin of any type or any non‐coumadin or warfarin anticoagulant immediately before and/or intended to be given within the first hour post infusion Have received vitamin K more than 3 hours before infusion of study drug History of hypersensitivity to plasma‐derived products Pregnant or nursing women Participating in another clinical treatment study currently or during the past 1 month before study inclusion Previously enrolled in this study |
| Interventions |
Experimental group: OCTAPLEX dose will depend on weight and baseline INR of the participant and will be calculated by the responsible treating investigator on the basis of dosing formula and predefined INR to a value < 1.5 Control: FFP given as a continuous intravenous infusion. Dosage should be 10 mL/kg for a participant with an initial INR of < 3 and 15 mL/kg for a participant with an initial INR > 3. Infusion will be given over 90 minutes or as fast as clinically indicated or tolerated by the participant at the discretion of the investigator |
| Outcomes |
Primary outcome measure: Efficacy endpoint is correction of INR to < 1.5 Secondary outcome measure: Number of intraoperative red blood cell units transfused |
| Starting date | April 2008 |
| Contact information | Contact Octapharma for facility details
Philadelphia, Pennsylvania, United States, 19141 Wolfgang Frenzel, M.D. |
| Notes | Study completion date: August 2012. No data available November 6, 2013 Each unit of FFP contains roughly 200 mL |
Innerhofer 2012.
| Trial name or title | RETIC trial: Reversal of Trauma Induced Coagulopathy Using Coagulation Factor Concentrates or Fresh Frozen Plasma |
| Methods | Single‐centre, parallel, open‐label, randomized trial |
| Participants | Severely traumatized patients (ISS > 15) admitted to emergency department with obvious bleeding and/or at risk for significant haemorrhage will be screened by rotational thromboelastometry (ROTEM) assays during ED treatment and subsequent surgical/radiological interventions for coagulopathy Inclusion criteria: Male and female patients ≥ 18 years and ≤ 80 years of age Major trauma (ISS > 15) Clinical signs of ongoing bleeding or at risk for significant haemorrhage as assessed and judged by the ED team in charge of the patient Presence of coagulopathy defined by ROTEM assays as follows: concomitant decreased fibrinogen polymerization (ROTEM/FIBTEM A10 < 7 mm after 10 minutes) Concomitant decreased coagulation factor levels (ROTEM® EXTEM CT > 90 seconds) Exclusion criteria: Lethal injury CPR on the scene Isolated brain injury, burn injury Avalanche injury Administration of fresh frozen plasma or coagulation factor concentrates before ED admission Delayed (> 6 hours after trauma) admittance to ED Known use of oral anticoagulants or platelet aggregation inhibitors within 5 days before injury Known history of severe allergic reaction to plasma products Known history of congenital haemostasis disturbance, IgA or protein C deficiency History of thromboembolic events or heparin‐induced thrombocytopenia (HIT) type 2 within the past year Body weight < 45 kg and > 150 kg Known to be pregnant Jehova's Witness Known participation in another clinical trial Known refusal of participation in this clinical trial |
| Interventions |
Intervention group: receives fibrinogen concentrate and/or PCC and/or FXIII concentrate If FIBTEM A10 < 7 mm: fibrinogen concentrate (RiaSTAP®/CLS Behring) dose at 50 mg/kg body weight intravenously as single dose or repeated, each single vial (1 g) over 5 minutes If EXTEM CT > 90 seconds and FIBTEM A10 > 7 mm: PCC dose at 20 IE/kg body weight PCC intravenously as single dose or repeated, each single dose over 10 minutes If FXIII decreases below 60% as detected by laboratory measurements: FXIII concentrate dose at 20 IU/kg body weight Fibrogammin® P administered with second dose of fibrinogen concentrate (100 mg/kg) intravenously as single dose or repeated, each single dose over 10 minutes Control group: fresh frozen plasma blood type 0, A, B and AB If FIBTEM A10 < 7 mm and/or EXTEM CT > 90 seconds: FFP dose of 15 mL/kg body weight intravenously as single dose or repeated, each single U (200 mL) over 5 minutes Additional treatment/rescue treatment: Treatment failure will be registered if bleeding persists and ROTEM parameters do not improve after 2 times dosages of study drug. In these cases, haemostatic rescue therapy will be administered. CFC (fibrinogen concentrate and/or PCC, and/or FXIII concentrate) will be administered to participants randomly assigned to receive FFP, and FFP will be administered to participants in the CFC group. In cases unresponsive to comprehensive treatment or normal ROTEM combined with diffuse bleeding, other haemostatic medications can be administered (e.g. rFVIIa, DDAVP, VWF/FVIII concentrate) as judged by anaesthetist in charge. Need for and type of any rescue therapy will be documented, and ROTEM will be performed thereafter |
| Outcomes | Multiple organ failure (MOF) until 24 hours on ICU Differences between treatment groups in MOF as assessed by Sequential Organ Failure Assessment score (SOFA) |
| Starting date | March 2012 |
| Contact information | Petra Innerhofer, MD; petra.innerhofer@uki.at |
| Notes | Estimated enrolment: 200 participants; study currently recruiting participants Participating hospital: University Hospital Innsbruck Contacted 24 April 2013; reply 25 April 2013 Relation to the industry: independent |
Ranucci 2011.
| Trial name or title | The ZEro PLASma Trial (ZEPLAST): Avoidance of Fresh Frozen Plasma in Cardiac Surgery |
| Methods | Prospective, randomized, double‐blind, comparative, parallel‐assigned, single‐centre clinical trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
Withdrawal/additional exclusion criteria (exclusion following randomization):
Participants randomly assigned and not withdrawn will be given investigational/place drugs in accordance with allocation All participants randomly assigned and not withdrawn will be tested 20 minutes before removal of aortic cross‐clamping with a thromboelastometric fibrinogen test ROTEM®/FIBTEM |
| Interventions |
Intervention group: fibrinogen concentrate (Haempcpmplettan P®/CSL Behring) and prothrombin complex concentrate (PCC) (Confidex®/CSL Behring) Dose fibrinogen: according to the formula based on ROTEM®/FIBTEM test: (22 [mm] − MCF [mm]) * body weight [kg]/140 [m] = whole g fibrinogen Co‐intervention: After 15 minutes from study drug administration and in the presence of ongoing microvascular bleeding: ROTEM®/EXTEM ‐ prolonged CT time (> 80 seconds): PCC at a weight‐based dose of 7 U/kg body weight Control group: (placebo) saline Study drugs or placebo has to be administered after protamine |
| Outcomes | Primary outcome measures: avoidance of allogeneic blood product transfusion within 30 days (packed red cells, FFP, platelet concentrates, cryoprecipitates) Secondary outcome measures: reduction in allogeneic blood product transfusions within 30 days |
| Starting date | November 2011 |
| Contact information | Marco Ranucci, MD; cardioanestesia@virgilio.it, |
| Notes | Estimated enrolment: 120 participants Participating hospitals: (Italy) IRCCS Policlinico San Donato Funding/relation to the industry: collaborates with CSL Behring Contacted 4 April 2012; reply 4 April 2012 Contacted 24 April 2013; reply 29 April 2013 |
Roy 2013.
| Trial name or title | PREVACT: Preventive REversal of Vitamine K Antagonist in Minor Craniocerebral Trauma |
| Methods | Open‐label, randomized, multi‐centre clinical trial |
| Participants | 400 vitamin K antagonist‐treated patients with minor craniocerebral trauma Inclusion criteria: Admission through emergency department for recent and isolated minor head trauma with at least 1 of the following characteristics: period of alteration in the level of consciousness, period of loss of consciousness (< 30 minutes), post‐traumatic amnesia < 24 hours or any other neurological sign such as convulsion or localized neurological sign Participant receiving anticoagulant treatment with anti‐vitamin K for treatment of atrial fibrillation (AF) Initial ED Glasgow Coma Scale (GCS) score ≥ 13 Achievable follow‐up Informed consent form signed by participant or if he/she is not able, emergency inclusion Exclusion criteria: Delay between minor head trauma and possible preventive administration of PCC > 6 hours Receiving anticoagulant treatment other than anti‐vitamin K (heparin, fondaparinux, dabigatran, rivaroxaban, apixaban) Receiving anticoagulant treatment for reason other than AF Receiving antiplatelet treatment (except low dose of aspirin (≤ 100 mg/d)) Delocalized biology INR in capillary blood < 1.5 if available (only in department in which this analysis is usual practice) Haemorrhage or suspected haemorrhage other than intracranial that could lead to reversion of the anticoagulation Head trauma associated with one or further potential haemorrhagic traumatic lesions Rejected use of products derived from human blood Pregnant Any condition that, as judged by the investigator, would place the participant at increased risk of harm if he/she participated in the study Unable to provide written informed consent or not eligible for an emergency inclusion Without social security registered |
| Interventions | 400 anticoagulated patients with minor craniocerebral trauma are randomly assigned to receive PCC before CT scan of the cerebrum vs PCC administered when CT documented bleeding is present |
| Outcomes |
Primary outcome measures:
Percentage of intracranial bleeding diagnosed on CT scan (time frame: H20 +/‐ 4 hours) Will be analysed in a centralized place by 2 neuroradiologists blind to the randomly assigned group Secondary outcome measures: Volumetric measure of intracranial haemorrhage (time frame: CT scan performed 20 hours +/‐ 4 after inclusion) Will be analysed in a centralized place by 2 neuroradiologists blind to randomly assigned group Percentage of participants with decreased autonomy (time frame: 3 months) Loss of at least 1 point on the Glasgow Outcome Score Extended Percentage of participants with systemic or neurological ischaemic attacks (time frame: 1 month) |
| Starting date | October 2013 |
| Contact information | ROY Pierre‐Marie, Professor; +33(2)‐41‐35‐79‐47; prevact@chu‐angers.fr TAZAROURTE Karim, Physician; +33(2)‐41‐35‐79‐47; prevact@chu‐angers.fr |
| Notes | Study is not yet open for participant recruitment |
Steiner 2009.
| Trial name or title | Multi‐centre, Prospective, Randomized Trial on the Use of Prothrombin Complex and Fresh Frozen Plasma in Patients With Intracerebral Hemorrhage Related to Vitamin K Antagonists |
| Methods | Interventional, randomized, parallel, open‐label pharmacokinetics/dynamics study |
| Participants | 74 vitamin K antagonist‐treated patients with CT‐confirmed intracerebral bleeding randomly assigned to receive PCC or FFP Inclusion criteria: Spontaneous ICH (intraparenchymal), subdural haematoma (SDH) diagnosed by CT scanning ≤ 12 hours after onset of symptoms. In cases of unknown time of symptom onset: time between last seen in healthy condition and first CCT ≤ 12 hours. Receiving vitamin K antagonists (VKA) INR ≥ 2. Exclusion criteria: ICH not related to vitamin K antagonist therapy Secondary ICH related to infarction, haemophilia or other coagulopathy, tumour, haemorrhagic infarction, cerebrovenous thrombosis, aneurysm, arteriovenous malformations (AVM) or severe trauma Deep coma (GCS ≤ 5) at the time of admission or before intubation if intubated outside the hospital Known previous disability (mRS > 2 before stroke occurred) Acute myocardial ischaemia, acute septicaemia, acute crush injury, any history of acute haemorrhagic disseminated intravascular coagulation, acute thrombotic stroke Known history of intermittent claudication Known recent thrombotic event < 30 days Acute or known congestive heart failure (NYHA III‐IV) Pulmonary oedema Known liver failure (Child‐Pugh score C) Known alcohol or other drug abuse Known active malignant disease Known thrombocytopenia (platelets < 50,000/µL), haemorrhagic diathesis (primary defects of coagulation, fibrinolysis, platelets) History of hypersensitivity to investigational products or to any drug with similar chemical structure or to any excipient present in the pharmaceutical form of the investigational product Known allergy to heparin or history of heparin‐induced thrombocytopenia Pregnancy and lactation Concomitant use of antithrombotic (with PTT > 1.5 of normal PTT), thrombolytic treatment Use of aspirin, clopidogrel or dipyridamole or combinations thereof (e.g. Aggrenox®) is not an exclusion criterion. These drugs should be discontinued and not restarted earlier than 24 hours after normalization of INR if indicated Previous participation in this trial |
| Interventions | Group 1: PCC, 30 IU/kg Group 2: FFP, 20 mL/kg |
| Outcomes | Primary outcome measures: INR ≤ 1.2 within 3 hours after start of drug infusion (time frame: 3 hours) (Designated as safety issue: yes) Secondary outcome measures: Safety: number of thromboembolic events (time frame: 90 days) (Designated as safety issue: yes) Efficacy: percentage of volume increase (time frame: 24 hours) (Designated as safety issue: yes) Clinical outcome (time frame: day 90) (Designated as safety issue: no) |
| Starting date | July 2009 |
| Contact information | Contact: Sven Poli, Dr.; +49 6221 56 38746; sven_poli@med.uni‐heidelberg.de |
| Notes | Estimated date of completion: June 2013 Last updated: November 2011 Status: recruiting |
AF = atrial fibrillation.
AIS = abbreviated injury score.
AVM = arteriovenous malformation.
CFC = coagulation factor concentrate.
CPB = cardiopulmonary bypass.
CPR = cardiopulmonary resuscitation.
CT = clotting time.
DIC = disseminated intravascular coagulation.
DDAVP = desmopressin.
ED = emergency department.
FFP = fresh frozen plasma group.
FIBTEM = trade name.
FVIII = factor VII.
GCS = Glasgow Coma Scale.
HCT = haematocrit.
HIT = heparin‐induced thrombocytopenia.
ICH = intracerebral haemorrhage.
INR = international normalized ratio.
ISS = injury severity score.
IU = international units.
MOF = multiple organ failure.
NYHA = New York Heart Association.
PCC = prothrombin complex concentrate.
Differences between protocol and review
We made the following changes to the published protocol (Johansen 2013).
1. Background/Description of the intervention: section updated.
2. Objectives: section amended. Previously the protocol stated, "...compared to fresh frozen plasma, other haemostatic agents and placebo in the perioperative setting of acute surgical intervention in bleeding and non‐bleeding patients". The review now states, "...compared with fresh frozen plasma in the acute medical and surgical setting involving bleeding and non‐bleeding patients".
3. Change to types of studies: We wrote, “We will attempt to adjust the sample size of cluster‐randomized trials using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook) by using an estimate of intercluster correlation co‐efficient (ICC) derived from the trial or from a study of a similar population”. No cluster‐randomized trials were found. Hence, we amended the above mentioned section in the final review.
4. Outcomes: We wrote, “Depending on the time scales of the included studies, we plan to run separate analyses for short‐, medium‐ and long‐term mortality. The ranges of the different time points will be defined in accordance with the definitions of the included studies and any related clinical considerations.” Section amended from final review for insufficient data.
5. Measures of treatment effect/Dichiotomous data/Primary outcomes: We wrote, "2. Overall 28‐day mortality. We will include data provided as 30‐day mortality in the same analysis". Section amended for lack of data.
6. Unit of analysis issues/Cross‐over trials: We wrote, “However, we will include them in the review and discuss their shortcomings and their findings". No cross‐over trials were found. Hence, amended for final review.
7. Trial sequential analysis: We wrote, “We will apply trial sequential monitoring boundaries according to the required diversity‐adjusted information size (Wetterslev 2009), based on an a priori 20% risk ratio reduction (RRR), an intervention effect estimated from the trials with a low risk of bias, and a diversity‐adjusted required information size estimated from the intervention effect suggested by all the trials, employing α = 0.05 and ß = 0.20 and the diversity found among the included trials. We will use the control event proportion suggested by all trials, and in case of the actual diversity being zero, we will do a TSA challenging a situation wherein the diversity would be 25%”. No trials were found to be at "low risk of bias". Hence, section amended from final review.
8. Subgroup analyses and investigations of heterogeneity: We wrote, "We plan the following subgroup analyses:
Benefits and harms of prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP) without other haemostatic agents. Benefits and harms of PCC versus FFP and cryoprecipitate. Benefits and harms of PCC versus other haemostatic agents (rVIIa, antifibrinolytics, desmopressin, plasma derivatives or other factor‐substitution products). Benefits and harms of PCC versus FFP in combination with other haemostatic agents. Benefits and harms of PCC in combination with other haemostatic agents versus 'standard treatment'. Benefits and harms of PCC in trials investigating the emergency surgery population (defined as surgery that should be performed within 24 hours after meeting the indication for surgery) requiring acute surgical intervention because of bleeding complications versus trials investigating the emergency surgery population with no bleeding complication requiring acute surgical intervention because of co‐morbidity. Benefits and harms of PCC in trials investigating the trauma population versus trials investigating the non‐trauma population. Benefits and harms of PCC in trials investigating the neurosurgical population versus trials investigating the non‐neurosurgical population. Benefits and harms of PCC in trials investigating the cardiac surgery population versus trials investigating the non‐cardiac surgery population. Benefits and harms of PCC in trials investigating the paediatric population (age below 18 years, neonates not included) versus trials investigating the adult population.Benefits and harms of PCC by comparing the pooled intervention effect in trials with a dose regimen that was higher than the median dose of administered PCC with trials having a dose regimen equal to or smaller than the median dose. This is done to detect possible dependency of the estimate of intervention effect on the dose regimen. In case of considerable between‐trial heterogeneity, we will apply meta‐regression; efficacy and safety of three‐factor PCC versus four‐factor PCC.
If analyses of various subgroups with binary data are significant, we plan to perform a test of interaction by applying the fixed inverse variance method incorporated in RevMan 5.2. Alternatively, we will apply meta‐regression if a fixed‐effect model is not considered sensible because of considerable between‐study variability. We consider P value < 0.05 as indicating significant interaction between the PCC effect on mortality and the subgroup category (Cochrane Handbook, Chapters 9.6.1 and 9.7). We will also consider applying Q‐partitioning for interaction and subgroup differences if appropriate (RevMan 5.2)". As data were insufficient, we were unable to perform subgroup analyses but will perform subgroup analysis in the updated review if additional data become available.
Subgroup analyses 8 and 9: Benefits and harms of PCC in trials investigating the neurosurgical population versus trials investigating the non‐neurosurgical population; benefits and harms of PCC in trials investigating the cardiac surgery population versus trials investigating the non‐cardiac surgery population.
As studies eligible for inclusion were few, we did not consider it valuable to compare one neurosurgical trial (Boulis 1999) versus one cardiac surgical trial (Demeyere 2010). Comparison of these two studies was very difficult because heterogeneity was pronounced. Thus, because of differences in study design and participant populations in conjunction with lack of data, we found it pointless to further elaborate on this issue. The trial by Majeed 2013 included a mixed surgical population. We were not able to retrieve further relevant predefined data.
9. Sensitivity analyses. We wrote, "We plan the following sensitivity analyses: Comparison of estimates of the pooled intervention effect in trials with low risk of bias versus estimates from trials with high risk of bias (i.e. trials having at least one inadequate risk of bias component). Comparison of estimates of the pooled intervention effect in trials based on different components of risk of bias (random sequence generation, allocation concealment, blinding, completeness of outcome data, selective reporting and 'other bias'). Comparison of estimates of the pooled intervention effect in trials with high levels of missing data. In case of missing data, we will apply ’complete‐case analysis’ for primary and secondary outcomes, thereby excluding from the analysis all participants with missing outcome data. Examination of the role of funding bias by excluding trials that are exclusively sponsored by pharmaceutical and medical devices companies. Comparison of estimates of pooled intervention effects by excluding data from studies published only as abstracts. Examination of the importance of thromboembolic events when participants with high prior risk of thrombotic events were compared with others. We will calculate RRs with 95% CIs and will decide to apply 'complete‐case analysis', if possible, for our sensitivity and subgroup analyses based on our primary outcome measure (mortality)". We were unable to do this in the review because data were insufficient, but we will perform sensitivity analysis in the updated review if sufficient data are available.
10. We changed the title of the review from "Prothrombin complex concentrate for perioperative reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients" to "Prothrombin complex concentrate for reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients".
11. We removed the word "perioperative" from the text for lack of correlation with included studies.
12. We changed secondary outcome 1 from "Incidence of perioperative blood transfusions (e.g. avoidance of transfusion) and amount of blood products transfused" to "Number of new occurrences of blood transfusions (e.g. avoidance of transfusion) and quantity of blood products transfused".
13. We planned to use the principles of the GRADE system and added a 'Summary of findings table' to the ‘Methods’ section.
Contributions of authors
Mathias Johansen1, Anne Wikkelsø2, Jens Lunde3, Jørn Wetterslev4, Arash Afshari5
Conceiving of the review: MJ (Mathias Johansen), AW (Anne Wikkelsø), AA (Arash Afshari), JL (Jens Lunde). Designing the review: MJ, AW, AA. Co‐ordinating the review: MJ. Undertaking manual searches: MJ, JL. Screening search results: MJ, JL, AA. Organizing retrieval of papers: MJ, JL. Screening retrieved papers against inclusion criteria: MJ, JL. Appraising quality of papers: MJ, JL, AA, AW. Abstracting data from papers: MJ, JL. Writing to authors of papers for additional information: MJ. Providing additional data about papers: MJ, JL. Obtaining and screening data on unpublished studies: MJ, JL. Managing data for the review: MJ, JL. Entering data into Review Manager 5 (RevMan 5.3): MJ, JL. Analysing RevMan statistical data: MJ, AA, JW. Performing other statistical analysis not using RevMan: AA, JW. Performing double entry of data (data entered by person one: MJ; data entered by person two: JL). Interpreting data: MJ, AW, AA, JL, JW. Making statistical inferences: AA, JW. Writing the review: MJ. Providing guidance on the review: AA, AW. Securing funding for the review: MJ, AA. Performing previous work that was the foundation of the present study: AW, AA. Serving as guarantor for the review (one review author): MJ. Taking responsibility for reading and checking the review before submission: MJ.
Sources of support
Internal sources
Cochrane Anaesthesia Review Group (CARG), Denmark, Other.
-
Karen Hovhannisyan (CARG), Denmark, Denmark.
Technical support and search strategy design
External sources
No sources of support supplied
Declarations of interest
Mathias Johansen: no conflicts of interest.
Anne Wikkelsø: no conflicts of interest.
Jens Lunde: no conflicts of interest.
Jørn Wetterslev: I am a member of the Copenhagen Trial Unit Task Force, which develops trial sequential analysis theory and software.
Arash Afshari: no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Boulis 1999 {published data only}
- Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin‐related intracranial hemorrhage. Neurosurgery 1999;45(5):1113‐8; (discussion 1118‐9). [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Demeyere 2010 {published data only}
- Demeyere R, Gillardin S, Arnout J, Strengers PF. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sanguinis 2010;99(3):251‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Majeed 2013 {published and unpublished data}
- Majed AR, Goldstein JN, Milling TJ, Lewis B, Goldberg‐Alberts R, Hug BA, et al. Randomized phase IIIb study of rapid vitamin K antagonist reversal in patients requiring an urgent surgical procedure: four‐factor prothrombin complex concentrate is superior to plasma. Blood 2013;122(21):3588. [Google Scholar]
Sarode 2013 {published data only}
- Sarode R, Milling TJ Jr, Refaai MA, Mangione A, Schneider A, Durn BL, et al. Efficacy and safety of a 4‐factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma‐controlled, phase IIIb study. Circulation 2013;128(11):1234‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aart 2006 {published data only}
- Aart LV, Eijkhout HW, Kamphuis JS, Dam M, Schattenkerk ME, Schouten JT, et al. Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thrombosis Research 2006;118:313‐20. [DOI] [PubMed] [Google Scholar]
Eerenberg 2011 {published data only}
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate. Circulation 2011;124:1573‐9. [DOI] [PubMed] [Google Scholar]
Marlu 2012 {published data only}
- Raphael M, Enkelejda H, Adeline P, Albaladejo P, Crackowski JL, Pernod G. Effect of non‐specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. Thrombosis and Haemostasis 2012;108:217‐24. [DOI] [PubMed] [Google Scholar]
Taberner 1976 {published data only}
- Taberner DA, Thomson JM, Poller L. Comparison of prothrombin complex concentrate and vitamin K In oral anticoagulant reversal. BMJ 1976;2(6027):83‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kerebel 2013 {published data only}
- Kerebel D, Joly LM, Honnart D. A French multicenter randomised trial comparing two dose‐regimens of prothrombin complex concentrates in urgent anticoagulation reversal. Critical Care 2013;17(R4):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Ahonen 2013 {published and unpublished data}
- Use of Prothrombin Complex Concentrate and Fibrinogen Compared With Fresh Frozen Plasma in the Treatment of Postpartum Haemorrhage. Ongoing study July 2013.
Frenzel 2008 {published and unpublished data}
- A Randomized, Open‐Label, Efficacy and Safety Study of OCTAPLEX and Fresh Frozen Plasma in Patients Under Vitamin K Antagonist Therapy With the Need for Urgent Surgery or Invasive Procedures. Ongoing study April 2008.
Innerhofer 2012 {published and unpublished data}
Ranucci 2011 {published and unpublished data}
- The ZEro PLASma Trial (ZEPLAST): Avoidance of Fresh Frozen Plasma in Cardiac Surgery. Ongoing study November 2011.
Roy 2013 {published and unpublished data}
- PREVACT: Preventive REversal of Vitamine K Antagonist in Minor Craniocerebral Trauma. Ongoing study October 2013.
Steiner 2009 {published and unpublished data}
- Multi‐centre, Prospective, Randomized Trial on the Use of Prothrombin Complex and Fresh Frozen Plasma in Patients With Intracerebral Hemorrhage Related to Vitamin K Antagonists. Ongoing study July 2009.
Additional references
Ansell 2008
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G, American College of Chest Physicians. Pharmacology and Management of the Vitamin K Antagonists: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):160S‐98S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baglin 2006
- Baglin TP, Keeling DM, Watson HG. Guidelines on Oral Anticoagulation (Warfarin): Third Edition‐‐2005 Update. British Journal of Haematology 2006;132(3):277‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive—Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruce 2008
- Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Critical Care 2008;12(4):R105. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cartmill 2000
- Cartmill M, Dolan G, Byrne JL, Byrne PO. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. British Journal of Neurosurgery 2000;5:458‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dentali 2011
- Dentali F1, Marchesi C, Pierfranceschi MG, Crowther M, Garcia D, Hylek E, et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta‐analysis. Thrombosis and Haemostasis 2011;106(3):429‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Franchini 2010
- Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfusion 2010;8(3):149‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GradePro [Computer program]
- GradePro Working Team. GRADEPro. 3.2 for Windows. http://ims.cochrane.org/revman/other–resources/gradepro/download, 2008.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holland 2006
- Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: the effect of plasma transfusion on coagulation test results. American Society of Clinical Pathologists 2006;126(1):133‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huttner 2006
- Huttner HB, Schellinger PD, Hartmann M, Köhrmann M, Juettler E, Wikner J, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006;6:1465‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series C 2000;49:359‐70. [MEDLINE: ] [Google Scholar]
Koch 2006a
- Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Critical Care Medicine 2006;34:1608‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koch 2006b
- Koch CG, Li L, Duncan AI, Mihaljevic T, Loop FD, Starr NJ, et al. Transfusion in coronary artery bypass grafting is associated with reduced long‐term survival. The Annals of Thoracic Surgery 2006;81(5):1650‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kor 2010
- Kor DJ, Stubbs JR, Gajic O. Perioperative coagulation management—fresh frozen plasma. Best Practice & Research. Clinical Anaesthesiology 2010;24(1):51‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63. [Google Scholar]
Leissinger 2008
- Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. American Journal of Hematology 2008;83:137‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levi 2009
- Levi M. Emergency reversal of antithrombotic treatment. Internal and Emergency Medicine 2009;4(2):137‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levy 2008
- Levy JH, Tanaka KA, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology 2008;109(5):918‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin DM, Murphy LS, Tran M. Use of prothrombin complex concentrates and fibrinogen concentrates in the perioperative setting: a systematic review. Transfusion Medicine Reviews 2013;27:91‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Makris 1997
- Makris M, Greaves M, Phillips WS, Kitchen S, Rosendaal FR, Preston EF. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thrombosis Haemostasis 1997;3:477‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Ostermann 2007
- Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of beriplex P/N prothrombin complex concentrate in healthy volunteers. Thrombosis and Haemostasis 2007;98(4):790‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Ozgonenel 2007
- Ozgonenel B, O'Malley B, Krishen P, Eisenbrey AB. Warfarin reversal emerging as the major indication for fresh frozen plasma use at a tertiary care hospital. American Journal of Hematology 2007;82(12):1091‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palareti 1996
- Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception‐cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Antcoagulant Therapy. Lancet 1996;348(9025):423‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pindur 1999
- Pindur G, Mörsdorf S. The use of prothrombin complex concentrates in the treatment of hemorrhages induced by oral anticoagulation. Thrombosis Research 1999;95(4 Suppl 1):S57‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue JM, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration 2014. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014, 2013.
Riess 2007
- Riess HB, Meier‐Hellmann A, Motsch J, Elias M, Kursten FW, Dempfle CE. Prothrombin complex concentrate (Octaplex) in patients requiring immediate reversal of oral anticoagulation. Thrombosis Research 2007;121(2):9‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schick 2009
- Schick KS, Fertmann JM, Jauch KW, Hoffmann JN. Prothrombin complex concentrate in surgical patients: retrospective evaluation of vitamin K antagonist reversal and treatment of severe bleeding. Critical Care 2009;13(6):R191 (1‐15). [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2011
- Steiner T, Freiberger A, Griebe M, Hüsing J, Ivandic B, Kollmar R, et al. International normalised ratio normalisation in patients with coumarin‐related intracranial haemorrhages—the INCH trial: a randomised controlled multicentre trial to compare safety and preliminary efficacy of fresh frozen plasma and prothrombin complex—study design and protocol. International Journal of Stroke 2011;6(3):271‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sutton 2002
- Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta‐analysis of rare and adverse event data. Expert Review of Pharmacoeconomics & Outcomes Research 2002;2(4):367‐79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sørensen 2011
- Sørensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: Prothrombin complex concentrates ‐ evaluation of safety and thrombogenicity. Critical Care 2011;15(1):201. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theusinger 2009
- Theusinger OM, Spahn DR, Ganter MT. Transfusion in trauma: why and how should we change our current practice?. Current Opinion Anaesthesiology 2009;22(2):305‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Aart 2006
- Aart L, Eijkhout HW, Kamphuis JS, Dam M, Schattenkerk ME, Schouten TJ, et al. Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thrombosis Research 2006;118(3):313‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vang 2011
- Vang ML, Hvas AM, Ravn HB. Urgent reversal of vitamin K antagonist therapy. Acta Anaesthesiologica Scandinavica 2011;55(5):507‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vigué 2009
- Vigué B. Bench‐to‐bedside review: Optimising emergency reversal of vitamin K antagonists in severe haemorrhage ‐ from theory to practice. Critical Care 2009;13(2):209. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 2009
- Warren O, Simon B. Massive, fatal, intracardiac thrombosis associated with prothrombin complex concentrate. Annals of Emergency Medicine 2009;53(6):758‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology [electronic resource] 2009;30(9):86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Johansen 2013
- Johansen M, Wikkelsø A, Lunde J, Wetterslev J, Afshari A. Prothrombin complex concentrate for perioperative reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients requiring acute surgical intervention. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010555] [DOI] [PMC free article] [PubMed] [Google Scholar]


