Abstract
Background
Noninvasive positive‐pressure ventilation (NPPV) provides ventilatory support without the need for an invasive airway. Interest has emerged in using NPPV to facilitate earlier removal of an endotracheal tube and to decrease complications associated with prolonged intubation.
Objectives
We evaluated studies in which invasively ventilated adults with respiratory failure of any cause (chronic obstructive pulmonary disease (COPD), non‐COPD, postoperative, nonoperative) were weaned by means of early extubation followed by immediate application of NPPV or continued IPPV weaning. The primary objective was to determine whether the noninvasive positive‐pressure ventilation (NPPV) strategy reduced all‐cause mortality compared with invasive positive‐pressure ventilation (IPPV) weaning. Secondary objectives were to ascertain differences between strategies in proportions of weaning failure and ventilator‐associated pneumonia (VAP), intensive care unit (ICU) and hospital length of stay (LOS), total duration of mechanical ventilation, duration of mechanical support related to weaning, duration of endotracheal mechanical ventilation (ETMV), frequency of adverse events (related to weaning) and overall quality of life. We planned sensitivity and subgroup analyses to assess (1) the influence on mortality and VAP of excluding quasi‐randomized trials, and (2) effects on mortality and weaning failure associated with different causes of respiratory failure (COPD vs. mixed populations).
Search methods
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 5, 2013), MEDLINE (January 1966 to May 2013), EMBASE (January 1980 to May 2013), proceedings from four conferences, trial registration websites and personal files; we contacted authors to identify trials comparing NPPV versus conventional IPPV weaning.
Selection criteria
Randomized and quasi‐randomized trials comparing early extubation with immediate application of NPPV versus IPPV weaning in intubated adults with respiratory failure.
Data collection and analysis
Two review authors independently assessed trial quality and abstracted data according to prespecified criteria. Sensitivity and subgroup analyses assessed (1) the impact of excluding quasi‐randomized trials, and (2) the effects on selected outcomes noted with different causes of respiratory failure.
Main results
We identified 16 trials, predominantly of moderate to good quality, involving 994 participants, most with chronic obstructive pulmonary disease (COPD). Compared with IPPV weaning, NPPV weaning significantly decreased mortality. The benefits for mortality were significantly greater in trials enrolling exclusively participants with COPD (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.24 to 0.56) versus mixed populations (RR 0.81, 95% CI 0.47 to 1.40). NPPV significantly reduced weaning failure (RR 0.63, 95% CI 0.42 to 0.96) and ventilator‐associated pneumonia (RR 0.25, 95% CI 0.15 to 0.43); shortened length of stay in an intensive care unit (mean difference (MD) ‐5.59 days, 95% CI ‐7.90 to ‐3.28) and in hospital (MD ‐6.04 days, 95% CI ‐9.22 to ‐2.87); and decreased the total duration of ventilation (MD ‐5.64 days, 95% CI ‐9.50 to ‐1.77) and the duration of endotracheal mechanical ventilation (MD ‐ 7.44 days, 95% CI ‐10.34 to ‐4.55) amidst significant heterogeneity. Noninvasive weaning also significantly reduced tracheostomy (RR 0.19, 95% CI 0.08 to 0.47) and reintubation (RR 0.65, 95% CI 0.44 to 0.97) rates. Noninvasive weaning had no effect on the duration of ventilation related to weaning. Exclusion of a single quasi‐randomized trial did not alter these results. Subgroup analyses suggest that the benefits for mortality were significantly greater in trials enrolling exclusively participants with COPD versus mixed populations.
Authors' conclusions
Summary estimates from 16 trials of moderate to good quality that included predominantly participants with COPD suggest that a weaning strategy that includes NPPV may reduce rates of mortality and ventilator‐associated pneumonia without increasing the risk of weaning failure or reintubation.
Plain language summary
Use of noninvasive ventilation (a mask ventilator) holds promise as a method to make it easier to remove adults from conventional ventilators.
Patients with acute respiratory failure frequently require endotracheal intubation and mechanical ventilation (invasive positive‐pressure ventilation) to sustain life. Complications of mechanical ventilation include respiratory muscle weakness, upper airway injury, ventilator‐associated pneumonia, sinusitis and associated death. For these reasons, it is important to minimize the duration of mechanical ventilation. Noninvasive positive‐pressure ventilation is achieved with an oronasal, nasal or total face mask connected to a positive‐pressure ventilator and does not require an indwelling artificial airway.
Results from 16 randomized controlled trials, predominantly of moderate to good quality, involving 994 selected participants, approximately two thirds with chronic obstructive pulmonary disease who had respiratory failure and were starting to breathe spontaneously, demonstrate that support with noninvasive ventilation can decrease death, weaning failure, pneumonia and length of stay in the intensive care unit and hospital. Noninvasive weaning also decreased the total duration of ventilation and the time spent on invasive ventilation, as well as the number of participants who received a tracheostomy. Although noninvasive weaning had no effect on the duration of mechanical ventilation related to weaning, it did not increase the reintubation rate. Insufficient data were available to assess its impact on quality of life. Noninvasive weaning significantly reduced mortality in chronic obstructive pulmonary disease studies versus mixed population studies.
Summary of findings
Background
Description of the condition
Patients with acute respiratory failure (ARF) frequently require endotracheal intubation (ETI) and mechanical ventilation to sustain life. Although invasive ventilation is effective, it has been associated with the development of complications, including respiratory muscle weakness, upper airway pathology, ventilator‐associated pneumonia (VAP) (Pingleton 1988) and sinusitis (Niederman 1984). VAP in turn is associated with increased morbidity and a trend toward increased mortality (Heyland 1999). For these reasons, minimizing the duration of invasive mechanical support is an important goal of critical care (MacIntyre 2001).
Description of the intervention
Noninvasive positive‐pressure ventilation (NPPV) may provide a means of avoiding the need for or reducing the duration of invasive mechanical support for intubated patients with ARF. Unlike conventional invasive ventilation, NPPV is achieved with an oronasal, nasal or total face mask or a helmet connected to a ventilator and does not require an artificial airway. Through NPPV, one can (1) administer oxygen, (2) augment tidal volume and (3) apply extrinsic positive end‐expiratory pressure (ePEEP) to counteract intrinsic positive end‐expiratory pressure (iPEEP) (Appendini 1994). NPPV may provide partial ventilatory support to patients recovering from respiratory failure and who require ventilator support but have regained the ability to breathe spontaneously and can be extubated. NPPV has been shown to augment tidal volume, reduce breathing frequency, rest the muscles of respiration and improve gas exchange (Nava 1993). A small, prospective physiologic study of participants with chronic obstructive pulmonary disease (COPD) with hypercapneic respiratory failure who were not capable of fully autonomous breathing demonstrated that although physiologic and clinical responses to the delivery of noninvasive and invasive pressure support (PS) were similar (Vitacca 2001), significantly higher tidal volumes and lower dyspnoea scores were achieved with noninvasive PS (Vitacca 2001).
With acute exacerbation of COPD, the effectiveness of NPPV in decreasing mortality and ETI rates has been demonstrated in randomized trials and meta‐analyses (Keenan 2003; Peter 2002). Data to support the use of NPPV in non‐COPD participants with hypoxaemic respiratory failure are inconclusive at present (Keenan 2004). Many patients with severe respiratory failure, impaired sensorium, haemodynamic instability or difficulty clearing secretions, however, undergo direct intubation or intubation after a failed attempt at noninvasive ventilation (Keenan 2011).
How the intervention might work
To mitigate the effects of complications associated with protracted invasive ventilation, investigators have explored the role of NPPV in weaning, that is, replacing invasive support with noninvasive support in patients who are ready to be weaned but are not ready to be immediately extubated. Because no tracheal prosthesis is used with the NPPV approach and the cough reflex is preserved, the risk for development of VAP is reduced (Antonelli 1998; Nourdine 1999). Additionally, weaning with NPPV may reduce the requirement for sedation (Rathgeber 1997), decrease psychological distress (Criner 1994) and preserve important functions, including speech and oral intake (Mehta 2001). NPPV has been identified by professional organizations, including the American College of Chest Physicians, American Association for Respiratory Care and American College of Critical Care Medicine, as a weaning modality that may decrease the duration of intubation and improve patient outcomes (Meade 2001). Potential limitations of the NPPV approach include forfeiture of a protected airway, desiccation of oral secretions and the ability of NPPV to provide only partial ventilatory support.
Why it is important to do this review
The first report to describe the successful use of NPPV in liberating participants with weaning failure from invasive positive‐pressure ventilation (IPPV) was published in 1992 (Udwadia 1992). Thereafter, four uncontrolled, prospective studies were reported, in which participants with tracheostomies (Goodenberger 1992), participants with tracheostomies and translaryngeal airways (Restrick 1993) and those not meeting conventional discontinuation criteria (Gregoretti 1998; Kilger 1999) were weaned using NPPV. More recently, randomized controlled trials (RCTs) comparing the alternative weaning strategies have been published. The purpose of this review was to critically appraise, summarize and update information on the effects of NPPV weaning compared with IPPV weaning on important clinical outcomes, in light of new evidence derived from RCTs.
Objectives
We evaluated studies in which invasively ventilated adults with respiratory failure of any cause (COPD, non‐COPD, postoperative, nonoperative) were weaned by means of early extubation followed by immediate application of NPPV or continued IPPV weaning.
The primary objective was to determine whether the noninvasive positive‐pressure ventilation (NPPV) strategy reduced all‐cause mortality compared with invasive positive‐pressure ventilation (IPPV) weaning.
Secondary objectives were to ascertain differences between strategies in proportions of weaning failure and ventilator‐associated pneumonia (VAP), intensive care unit (ICU) and hospital length of stay (LOS), total duration of mechanical ventilation, duration of mechanical support related to weaning, duration of endotracheal mechanical ventilation (ETMV), frequency of adverse events (related to weaning) and overall quality of life.
We planned sensitivity and subgroup analyses to assess (1) the influence on mortality and VAP of excluding quasi‐randomized trials, and (2) effects on mortality and weaning failure associated with different causes of respiratory failure (COPD vs mixed populations).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized trials. We excluded trials that did not assess the role of NPPV and IPPV as weaning strategies and studies that compared NPPV and IPPV in the immediate postoperative setting (requiring discontinuation) or after unplanned extubation. Further, we excluded studies that compared the application of NPPV with supplemental oxygen versus unassisted oxygen to prevent respiratory failure and reintubation after elective or unplanned extubation. We also excluded studies that evaluated exclusively tracheostomized participants. We permitted studies conducted outside the ICU setting.
Types of participants
Adults receiving IPPV for ARF or acute‐on‐chronic respiratory failure of any cause (COPD, non‐COPD, postoperative, nonoperative) who were intubated for at least 24 hours were eligible for inclusion. We used authors' definitions of respiratory failure.
Types of interventions
A strategy of sequential extubation and weaning with NPPV was compared with a strategy of weaning using IPPV.
Types of outcome measures
Primary outcomes
The primary outcome was all‐cause mortality, as reported at specific time points by study authors.
Secondary outcomes
Secondary outcomes included the following.
Weaning failure (the reinitiation of mechanical support after discontinuation, or the requirement for protracted mechanical support).
VAP (according to study authors' definitions of VAP).
ICU LOS.
Hospital LOS.
Total duration of mechanical ventilation (defined as the total number of days the participant required mechanical support—invasive or noninvasive).
Duration of ventilation related to weaning (defined as the time from randomization to discontinuation of support, death or study withdrawal or the time until a decision was made to institute home ventilation).
Duration of ETMV (defined as the time wherein mechanical ventilation was delivered through an artificial airway).
Adverse events related to weaning (including reintubation, tracheostomy, cutaneous irritation, nasal abrasions, gastric distension, general medical and specific complications such as sinusitis, arrhythmias, sepsis, pneumonia and barotrauma).
Quality of life (as assessed by study authors).
Search methods for identification of studies
Electronic searches
We used the standard strategy of the Cochrane Anaesthesia Review Group. We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2013, Issue 5; see Appendix 1); MEDLINE via Ovid SP (January 1966 to May 2013; see Appendix 2); and EMBASE via Ovid SP (January 1980 to May 2013; see Appendix 3) to look for RCTs comparing NPPV and IPPV weaning strategies. No language restrictions were applied. To identify RCTs, we combined the MEDLINE search strategy with the Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE, as delineated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Searching other resources
We reviewed the bibliographies of retrieved articles and conference proceedings from the four international meetings published in theAmerican Journal of Respiratory and Critical Care Medicine, Intensive Care Medicine, Critical Care Medicine and Chest (January 1995 to May 2013) to identify potentially relevant trials. Finally, we searched for ongoing trials on the websites www.controlled‐trials.com and http://clinicaltrials.gov.
Data collection and analysis
Two review authors (KEAB, NKJA) independently screened citations, evaluated methodologic quality and abstracted data.
Selection of studies
We assessed trials on the basis of title and abstract. We retrieved potentially eligible trials in full text. We resolved disagreements regarding study selection and data abstraction by consensus or by arbitration with a third review author (MOM).
Data extraction and management
Data on the types of participants, interventions and outcomes included in each trial were extracted using a standardized data extraction form.
Assessment of risk of bias in included studies
The quality of all included trials was assessed by two review authors (KEAB, NKJK), both independently and in duplicate. For each study, we recorded the use of true randomization and the use of concealed allocation to minimize selection bias. Additionally, we evaluated reports of randomized trials for completeness of outcome data and selective outcomes reporting to assess for attrition and reporting biases, respectively. The two review authors evaluated each quality assessment independently and resolved disagreements through discussion and electronic email.
In detail, we judged study quality on the basis of the following (Higgins 2011).
1. Was sequence generation truly random?
Adequate sequence generation included reference to a random number table, use of a computer random number generator, coin tossing, shuffling of cards or envelopes, throwing of dice, drawing of lots or minimization.
2. Was knowledge of the allocated interventions adequately prevented during the study?
Adequate allocation concealment included central randomization (e.g. allocation by a central office unaware of participant characteristics unless based on stratification), such as an on‐site computer system combined with allocation sequence kept in a locked unreadable computer file accessed only after the characteristics of an enrolled participant were entered; sequentially numbered, sealed, opaque envelopes; or another similar approach to ensure that the person generating the allocation sequence did not administer it.
3. Were withdrawals described, and did they occur with similar frequency in intervention and control groups?
4. Were reports of the study free of the suggestion of selective outcome reporting?
We assigned a judgement related to the risk of bias for each domain as follows.
'yes' (criteria appropriately applied and described in the report or acknowledged from the primary author of the study).
'unclear' (criteria not described or impossible to acquire from the author).
'no' (criteria inappropriately applied).
A judgement of 'Yes' indicated a low risk of bias, 'No' indicated a high risk of bias and 'Unclear' indicated an unknown or unclear risk of bias.
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence in our review associated with specific outcomes (weaning time, time to successful extubation, time to first SBT and first successful SBT, mortality, total duration of mechanical ventilation, ICU length of stay and reintubation) and constructed Table 1; and Summary of findings table 2 (SoF tables) using the GRADE software (Higgins 2011). The GRADE approach appraised the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considered within‐study risk of bias (methodologic quality), directness of the evidence, heterogeneity of the data, precision of the effect estimates and risk of publication bias.
Summary of findings for the main comparison. Noninvasive versus invasive weaning for intubated adults with respiratory failure.
| Noninvasive versus invasive weaning for intubated adults with respiratory failure | ||||||
| Patient or population: intubated adults with respiratory failure Settings: Intervention: noninvasive versus invasive weaning | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Noninvasive versus invasive weaning | |||||
| Mortality—COPD | Study population | RR 0.36 (0.24 to 0.56) | 632 (9 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 225 per 1000 | 81 per 1000 (54 to 126) | |||||
| Moderate | ||||||
| 200 per 1000 | 72 per 1000 (48 to 112) | |||||
| Mortality—mixed | Study population | RR 0.81 (0.47 to 1.4) | 362 (7 studies) | ⊕⊕⊝⊝ low1 | ||
| 239 per 1000 | 194 per 1000 (112 to 335) | |||||
| Moderate | ||||||
| 270 per 1000 | 219 per 1000 (127 to 378) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Fewer than 300 events.
Test for subgroup differences (P = 0.02).
Measures of treatment effect
In pooled analyses, we used proportions for binary outcomes and preferentially used mean and standard deviation, when reported or available through correspondence with authors. Continuous outcomes are reported in days. We pooled categorical and continuous data using risk ratio (RR) and mean difference (MD) as respective summary estimates of effect.
Unit of analysis issues
Summary estimates of individual participants randomly assigned to the same intervention in the included trials constitute the unit of analysis in this review. For one trial with three arms, we included the results of two arms relevant to our research question (Girault 2011).
Dealing with missing data
For published reports with insufficient or ambiguous information, we contacted the first study author, when feasible, to clarify study methods.
Assessment of heterogeneity
We evaluated heterogeneity with the Cochran Q statistic (Cochran 1954) using a threshold P value of less than 0.10 (Fleiss 1986). We assessed the impact of heterogeneity on outcomes using the I2 measure (Higgins 2002). We considered an I2 statistic threshold of 0% to 40%, 30% to 60%, 50% to 90% or > 75% to represent between‐study heterogeneity that might not be important, moderate, substantial or considerable, respectively (Higgins 2011).
Assessment of reporting biases
We assessed effects of publication bias on the mortality outcome by constructing and visually inspecting a funnel plot that compared the study estimate of effect (RR) with the standard error of the log RR, while recognizing that the absence of small, negative trials may overinflate the overall summary estimate of effect (Higgins 2011).
Data synthesis
We used random‐effects (RE) models to pool data quantitatively, using Review Manager 5.1 software (RevMan 5.1), when studies were overall clinically similar. If a single outcome was reported at two different time points, we included the more protracted measurement in the pooled analyses.
Subgroup analysis and investigation of heterogeneity
We assessed the impact of the causes of respiratory failure (COPD vs mixed populations) and of studies enrolling at least 50% COPD participants versus less than 50% COPD participants on mortality and weaning failure. Based on identification of important differences in mortality between COPD and mixed populations and on the advice of the editorial team, we assessed the impact of the cause of respiratory failure on all outcomes post hoc. For these outcomes, we tested the difference in RR between subcategories using a Chi2 test (Borenstein 2008). We considered P < 0.05 to be statistically significant.
Sensitivity analysis
A priori, we planned to assess the effects of excluding quasi‐randomized studies on mortality and VAP outcomes.
Results
Description of studies
We identified a total of 16 trials, including 15 randomized trials (Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Tawfeek 2012; Trevisan 2008; Vaschetto 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) and one quasi‐randomized trial (Chen 2001) that met our inclusion criteria (Table 3) (Characteristics of included studies).
1. Populations and interventions in studies of noninvasive ventilation in critically ill adults.
| Study | No of participants | Inclusion criteria (participants) | Inclusion criteria (weaning eligibility) |
Experimental strategy | Control strategy |
| Nava 1998 |
50 | Exacerbation of COPD. Intubated for at least 36 to 48 hours | Simple weaning criteria, 1‐hour SBT failure | Noninvasive pressure support on conventional ventilator delivered with face mask | Invasive PS |
| Girault 1999 | 33 | Acute‐on‐chronic respiratory failure (COPD, restrictive, or mixed populations). Intubated for at least 48 hours | Simple weaning criteria, 2‐hour SBT failure | Flow or pressure mode with nasal or face mask | Flow or pressure mode (PS) |
| Hill 2000 |
21 | Acute respiratory failure | 30‐minute SBT failure | NPPV using VPAP in ST‐A mode | Invasive PS |
| Chen 2001 |
24 |
Exacerbation of COPD. Intubated for at least 48 to 60 hours. Saturation > 88% on FiO2 of 40% |
Day 3+ weaning criteria |
Bilevel NPPV (pressure mode) |
Invasive PS |
| Ferrer 2003 | 43 | Acute respiratory failure and persistent weaning failure. Intubated for at least 72 hours | Two‐hour SBT failure on 3 consecutive days | Bilevel NPPV in ST mode delivered with face or nasal mask | AC or invasive PS |
| Rabie Agmy 2004 |
37 | Exacerbation of COPD | Two‐hour SBT failure | NPPV (proportional assist in timed mode) delivered by face or nasal mask | Invasive PS |
| Wang 2004 | 28 | COPD. Bronchopulmonary infection | PIC window | NPPV (pressure mode) delivered by mask (unspecified) |
SIMV + PS |
| Zheng 2005 |
33 | COPD. Severe pulmonary infection | PIC window | Bilevel NPPV (pressure mode) delivered by face or nasal mask | Invasive PS |
| Zou 2006 |
76 | COPD with severe respiratory failure. Pulmonary infection | PIC window | Bilevel NPPV (pressure, ST mode) delivered by nasal or oronasal mask | SIMV + PS |
| Wang 2005 |
90 | COPD with severe hypercapneic respiratory failure. Pneumonia or purulent bronchitis. Age < 85. Capable of self care in past year | PIC window | Bilevel NPPV (pressure mode) | SIMV + PS |
| Trevisan 2008 | 65 | Invasively ventilated > 48 hours | 30‐minute SBT failure | Bilevel NPPV (pressure mode) delivered by face mask | Invasive mechanical ventilation |
| Prasad 2009 | 30 | COPD. Hypercapneic respiratory failure | Two‐hour SBT failure | Bilevel NPPV (pressure mode) delivered by full face mask | Invasive PS |
| Girault 2011 | 138 | Chronic hypercapneic respiratory failure invasively ventilated for at least 48 hours | Two‐hour SBT failure | Noninvasive PS ± PEEP or bilevel NIV with face mask (initial choice) | Invasive PS with once‐daily SBT with T‐piece or PS ± PEEP |
| Rabie Agmy 2012 |
264 | Acute‐on‐chronic exacerbation of COPD |
Two‐hour SBT failure |
NPPV (pressure, ST mode) | Invasive PS |
| Tawfeek 2012 |
42 | Invasively ventilated for > 48 hours |
Two‐hour SBT failure |
Noninvasive PAV ventilation delivered by face mask | SIMV |
| Vaschetto 2012 | 20 | Hypoxemic respiratory failure invasively ventilated for at least 48 hours | PS with PEEP + inspiratory support, < 25 cm H2O PEEP 8 to 13 cm H2O PaO2/FiO2 200 to 300 mm Hg with FiO2< 0.6 |
Helmet NPPV | Invasive PS with SBT when P/F ratio > 250 mm Hg |
COPD = chronic obstructive pulmonary disease; NPPV = noninvasive positive‐pressure ventilation; PS = pressure support; PEEP = positive end‐expiratory pressure; PIC = pulmonary infection control window; ST = spontaneous timed; AC = assist control; SIMV = synchronized intermittent mandatory ventilation; P/F ratio = ratio of arterial concentration of oxygen to fractional concentration of oxygen administered; SBT = spontaneous breathing trial; PAV = proportional assist ventilation.
Results of the search
We identified 1,506 records through an updated search (Figure 1). Of the 961 unique records, we assessed 14 new articles for eligibility.
1.
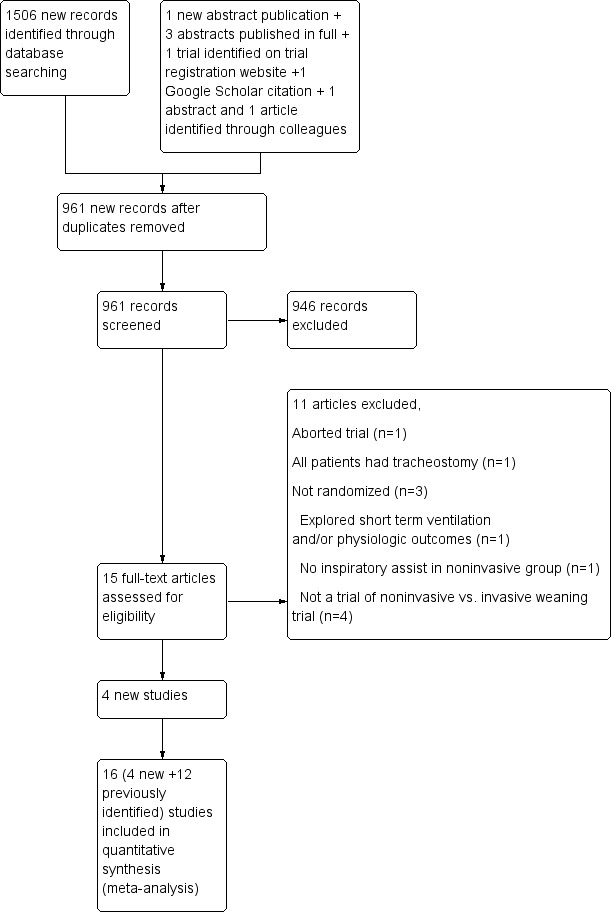
Study flow diagram.
Included studies
Although we identified five additional trials from the updated search (Gao Smith 2006; Girault 2011; Rabie Agmy 2012; Tawfeek 2012; Vaschetto 2012), one author (Gao Smith 2006) confirmed that his trial was aborted after approximately eight participants were enrolled because of the need to fulfil a clinical requirement at another hospital. Consequently, we included in our updated review four newly identified trials (Girault 2011; Rabie Agmy 2012; Tawfeek 2012; Vaschetto 2012), in addition to the 12 previously identified trials. Full details of participants, interventions and outcomes for each trial are provided in the Characteristics of included studies table. Of the 16 included studies, two trials were published only in abstract form (Hill 2000; Rabie 2004), four trials were published in Chinese (Chen 2001; Wang 2004; Zheng 2005; Zou 2006), one trial was a doctoral dissertation, subsequently published in full (Prasad 2009), and another trial was labelled as a pilot RCT (Vaschetto 2012).
Excluded studies
In total, we excluded 20 trials (Celebi 2008; Du 2009; Duan 2012; Gao Smith 2006; Ishikawa 1997; Jiang 1999; Kilic 2008; Kruger 1998; Luo 2001; Matic 2007; Nava 2011; Radojevic 1997; Rong 2012; Rosinha 2002; Vargas 2012; Venkatram 2010; Wang 2000; Wang 2003; Yang 2009; Zheng 2011), including eight newly identified full publications, one aborted trial (Gao Smith 2006), one abstract publication (Vargas 2012) and one trial identified through a Google Scholar search (Rong 2012; see Characteristics of excluded studies). The two review authors achieved complete agreement on study selection.
Study participants were restricted to adults. Of the 16 RCTs identified, nine included exclusively participants with COPD (Chen 2001; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) and seven trials included mixed or non‐COPD populations (Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Tawfeek 2012; Trevisan 2008; Vaschetto 2012). Of the latter trials, COPD was diagnosed in approximately 75% of participants in three trials (Ferrer 2003; Girault 1999; Girault 2011), in approximately one third of participants in two trials (Hill 2000; Trevisan 2008) and in more than 20% of participants in another trial (Tawfeek 2012), and COPD served as an exclusion criterion in the final trial (Vaschetto 2012). Participants were considered difficult to wean in two trials (Girault 1999; Girault 2011) and as persistent weaning failures in another trial (Ferrer 2003). Four trials (Wang 2004; Wang 2005; Zheng 2005; Zou 2006) included participants with COPD with respiratory failure due to pulmonary infection.
Initial prerandomization ventilation strategies integrated predominantly volume‐cycled ventilation strategies (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Nava 1998a; Prasad 2009; Rabie 2004; Wang 2004; Wang 2005; Zou 2006) with or without concurrent or subsequent pressure support (PS). Screening for weaning eligibility was reported to occur daily in three trials (Ferrer 2003; Hill 2000; Rabie 2004) and daily after 48 hours of invasive ventilation in two trials (Girault 1999; Vaschetto 2012). Weaning candidates were identified after at least 24 hours (Prasad 2009); at 36 to 48 hours, including six to eight hours of paralysis (Nava 1998a); after at least 48 hours (Girault 1999; Girault 2011; Tawfeek 2012; Vaschetto 2012); at 48 to 60 hours (Chen 2001); at 72 hours, including six to eight hours of paralysis (Rabie 2004); or after three days (Ferrer 2003) of invasive ventilation. The four trials (Wang 2004; Wang 2005; Zheng 2005; Zou 2006) evaluating participants with COPD with pulmonary infection enrolled participants upon achievement of 'pulmonary infection control' (PIC) window criteria (Wang 2005; Zheng 2005; Zou 2006) or after infection control was achieved (Wang 2004). These criteria included an improved radiograph, temperature and white blood cell count (or percentage of neutrophils) and reduced secretion volume and tenacity (Wang 2004; Wang 2005; Zheng 2005; Zou 2006). Two trials also specified improved haemodynamics, expectoration and level of consciousness (Wang 2004; Zou 2006), and another (Wang 2005) specified minimum ventilator settings (synchronized intermittent mandatory ventilation (SIMV) rate of 10 to 12 breaths/min, PS of 10 to 12 cm H2O). Eligibility for study inclusion and randomization required that participants meet predefined permissive weaning criteria (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Tawfeek 2012; Trevisan 2008; Vaschetto 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) and that they fail a single 30‐minute (Hill 2000; Trevisan 2008), one‐hour (Nava 1998a) or two‐hour (Girault 1999; Girault 2011; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Tawfeek 2012) SBT, or a two‐hour T‐piece trial on three consecutive days (Ferrer 2003).
Weaning strategies
Invasive positive‐pressure group
Participants in the control group were weaned using PS (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Vaschetto 2012; Zheng 2005), assist control (AC) (Ferrer 2003), SIMV with PS (Wang 2004; Wang 2005; Zou 2006) or SIMV alone (Tawfeek 2012). Initial support, after failure of an SBT, was titrated to achieve the prior PaCO2 (Chen 2001; Nava 1998a; Prasad 2009; Rabie 2004; Tawfeek 2012), pH (Chen 2001; Nava 1998a; Prasad 2009; Rabie 2004; Tawfeek 2012) or respiratory rate (Chen 2001; Girault 1999; Hill 2000; Nava 1998a; Prasad 2009; Rabie 2004; Tawfeek 2012). In some trials, initial settings were adjusted to achieve specific flow rates (Girault 1999) or tidal volume (VT) (Hill 2000). The level of PS was gradually reduced in three trials (Ferrer 2003; Girault 2011; Nava 1998a). One study each titrated PS by 2 cm H2O every four hours to clinical tolerance, saturation and respiratory rate (Prasad 2009) or by 2 to 4 cm H2O per day (Rabie 2004). Another trial decreased PS and positive end‐expiratory pressure (PEEP) by 2 cm H2O every two hours until a minimum of 8 and 10 cm H2O, respectively, were attained and titrated support to PaO2/FiO2, PaCO2 and pH (Vaschetto 2012).
Trials of spontaneous breathing (SB), using T‐piece or continuous positive airway pressure (CPAP) < 5 cm H2O or PS, were performed twice daily (Nava 1998a), daily (Ferrer 2003; Tawfeek 2012; Trevisan 2008) or at least once daily (Girault 2011). One study included at least two observation periods per day during PS weaning with optional SBTs (Girault 1999). Participants were considered weaned when (1) they remained stable for at least four hours on an SIMV rate of five breaths per minute with PS of 5 to 7 cm H2O (Wang 2005); (2) blood gases were normalized and participants could spontaneously breathe for longer than three hours with low oxygen requirements (FiO2 ≤ 0.40), acceptable oxygen saturation (SpO2 ≥ 90%) and a normal pH (≥ 7.35) (Wang 2004); or (3) when PS was titrated to < 7 cm H2O (Girault 2011), ≤ 8 cm H2O (Zheng 2005) or ≤ 10 cm H2O (Prasad 2009; Zou 2006) with PEEP of 5 cm H2O and satisfactory blood gases (Prasad 2009; Vaschetto 2012), saturations (Zheng 2005; Zou 2006) and respiratory rate (Prasad 2009; Vaschetto 2012; Zheng 2005; Zou 2006); a tidal volume of approximately 8 mL/kg (Zheng 2005; Zou 2006); and partial pressure of carbon dioxide (PaCO2) between 45 and 60 mm Hg; or at baseline on low FiO2 (Prasad 2009; Zheng 2005; Zou 2006) for longer than four hours (Zheng 2005; Zou 2006). A final trial considered participants to be weaned from invasive PS with arterial saturation ≥ 90% on an FiO2 ≤ 40% with pH ≥ 7.35, RR < 35 breaths/min, haemodynamic stability and the absence of severe dyspnoea or depressed neurological status (Rabie 2004). To discontinue invasive ventilation, participants successfully completed a 30‐minute SBT (Vaschetto 2012), a two‐hour SBT (Ferrer 2003; Hill 2000) or a three‐hour SBT (Chen 2001; Nava 1998a; Wang 2004), or two periods of observation with optional SBTs (Girault 1999). Three trials did not specify SBT duration (Girault 2011; Tawfeek 2012; Trevisan 2008).
Noninvasive ventilation group
Similar to invasive weaning, trials applied different noninvasive weaning protocols. After extubation, NPPV was administered in pressure mode in 13 trials (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Nava 1998a; Prasad 2009; Trevisan 2008; Vaschetto 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006), of which six trials specified use of a spontaneous timed mode (Ferrer 2003; Girault 1999; Hill 2000; Prasad 2009; Rabie Agmy 2012; Zou 2006) or a flow mode (Girault 1999). Two trials (Girault 2011; Vaschetto 2012) did not specify the mode. Two studies used proportional assist ventilation (Rabie 2004; Tawfeek 2012). NPPV was preferentially delivered by face mask (Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Nava 1998a; Prasad 2009; Rabie 2004; Tawfeek 2012, Trevisan 2008; Wang 2005; Zheng 2005; Zou 2006) or nasal mask (Ferrer 2003; Girault 1999; Hill 2000; Rabie 2004; Zheng 2005; Zou 2006). One trial (Vaschetto 2012) used a helmet but also permitted use of full face and oronasal masks in rotation to improve tolerance to NPPV. Initial support was delivered continuously in seven studies (Chen 2001; Ferrer 2003; Hill 2000; Nava 1998a; Prasad 2009; Tawfeek 2012; Vaschetto 2012) and continuously initially and subsequently intermittently in one study (Girault 2011). Alternatively, NPPV was delivered intermittently in one study (Girault 1999) or for at least two (Zou 2006) or six (Girault 2011) hours during the initial application, and in one study until tolerated for 20 to 22 hours per day, spaced by periods of spontaneous ventilation with oxygen for meals and expectoration (Rabie 2004).
The level of support was gradually decreased (Tawfeek 2012; Zheng 2005; Zou 2006), and noninvasive ventilation time was gradually reduced (Zheng 2005; Zou 2006). Some trials permitted fixed or gradually increasing periods of spontaneous breathing (Ferrer 2003; Girault 1999; Hill 2000; Nava 1998a; Rabie 2004), with at least two trials (Nava 1998a; Rabie 2004) specifying two periods of spontaneous breathing per day. Other trials enabled spontaneous breathing when selected criteria were met (Vaschetto 2012) or intermittently between NPPV periods (Girault 2011). In some trials, clinicians titrated PS by 2 cm H2O every two (Vaschetto 2012) or four (Prasad 2009) hours until PS and PEEP targets were achieved (Vaschetto 2012) or by 2 to 4 cm H2O each day (Rabie 2004), according to participant tolerance. In one trial (Vaschetto 2012), the goal of the weaning protocol was specified as maintaining PaO2/FiO2> 225, PaCO2< 50 mm Hg and pH > 7.35. In some trials, clinicians decreased the levels of inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP) to 8 and 4 cm H2O, respectively (Prasad 2009). In other trials, IPAP was reduced to < 10 cm H2O (with NPPV applied for less than two hours per day) (Zheng 2005; Zou 2006), or until the difference between IPAP and EPAP was ≤ 5 cm H2O (Wang 2005). Still other trials considered participants to be weaned from noninvasive support when arterial saturations were ≥ 90% on an FiO2 ≤ 40% with pH ≥ 7.35, RR < 35 breaths/min, haemodynamic stability and absence of severe dyspnoea or depressed neurological status (Rabie 2004), or according to blood gases, clinical status or mechanical ventilation parameters (Girault 2011; Vaschetto 2012; Wang 2004). One trial (Girault 2011) specified the need for daily NPPV for less than six hours or respiratory stability with standard oxygen therapy for at least 12 hours with arterial blood gases (ABGs): PaO2> 64 mm Hg with pH > 7.35 and PaCO2< 60 mm Hg. Criteria for discontinuing noninvasive support included successful completion of a three‐hour (Chen 2001; Nava 1998a), a two‐hour (Hill 2000) or a 30‐minute (Vaschetto 2012) period of spontaneous breathing, a period of observation of undetermined duration (Girault 2011) or at least two periods of spontaneous breathing observed by an attending physician (Girault 1999). One trial (Tawfeek 2012) did not conduct postrandomization periods of spontaneous breathing.
Risk of bias in included studies
2.
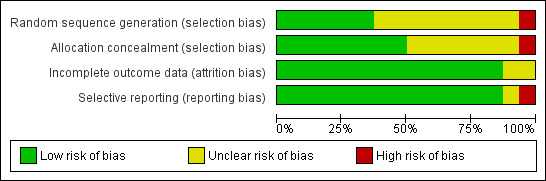
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
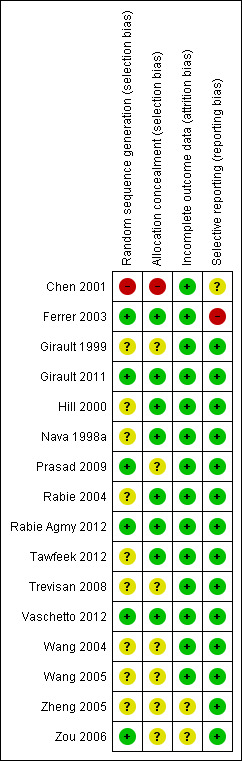
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
In most trials included in this review (Characteristics of included studies), allocation to treatment group was by random assignment, with one quasi‐randomized trial allocating participants according to hospital admission order (Chen 2001). To generate randomization sequences, trials reported use of computer‐generated random tables at each centre (Ferrer 2003), a computer‐generated randomization table using variable blocks of four (Girault 2011), computer‐generated randomization (Rabie Agmy 2012), Kendall and Babington tables (Prasad 2009), a table of random numbers held by an investigator not involved with study enrolment (Vaschetto 2012) and a digital table (Zou 2006). The remaining trials (Girault 1999; Hill 2000; Nava 1998a; Rabie 2004; Tawfeek 2012; Trevisan 2008; Wang 2004; Wang 2005; Zheng 2005) did not provide specific information regarding sequence generation.
To conceal allocation, trials reported using sealed envelopes (Trevisan 2008), opaque envelopes (Girault 1999) or sealed, opaque envelopes (Girault 2011; Hill 2000). Three trials reported using sealed, opaque, sequentially numbered envelopes (Nava 1998a; Tawfeek 2012Vaschetto 2012). One study used a computer‐generated randomization list held by investigators not involved in clinical decisions (Ferrer 2003). The method of allocation concealment was not specified in five trials (Prasad 2009; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) and was confirmed to be concealed through correspondence with one study author for two other trials (Rabie 2004; Rabie Agmy 2012).
Blinding
Because of the nature of the interventions, blinding of caregivers and participants was not possible; however, one trial (Hill 2000) blinded individuals participating in data collection and analysis.
Incomplete outcome data
We assessed for completeness of outcome data in publications by inspecting denominators, when provided. In two trials (Zheng 2005; Zou 2006), denominators were not provided in binary outcomes to ensure complete outcomes reporting.
Selective reporting
Selective outcomes reporting was judged to be unclear in one trial (Chen 2001), which reported clinically important outcomes but did not specify primary and secondary outcomes. Another trial (Ferrer 2003) did not report the proportions of weaning successes and failures in a full trial publication but reported this outcome in a smaller number of participants in an earlier abstract publication, with the authors affirming that they did not continue to collect data on this outcome.
Other potential sources of bias
We did not evaluate other potential sources of bias.
Effects of interventions
Summary of findings 2. Noninvasive versus invasive weaning for intubated adults with respiratory failure.
| Noninvasive versus invasive weaning for intubated adults with respiratory failure | ||||||
| Patient or population: patients with intubated adults with respiratory failure Settings: Intervention: Noninvasive versus invasive weaning | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Noninvasive versus invasive weaning | |||||
| Weaning failure | Study population | RR 0.63 (0.42 to 0.96) | 605 (8 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 362 per 1000 | 228 per 1000 (152 to 348) | |||||
| Moderate | ||||||
| 327 per 1000 | 206 per 1000 (137 to 314) | |||||
| Nosocomial pneumonia | Study population | RR 0.25 (0.15 to 0.43) | 953 (14 studies) | ⊕⊕⊝⊝ low2 | ||
| 296 per 1000 | 74 per 1000 (44 to 127) | |||||
| Moderate | ||||||
| 307 per 1000 | 77 per 1000 (46 to 132) | |||||
| Average duration of ventilation related to weaning | The mean average duration of ventilation related to weaning in the intervention groups was 0.25 lower (2.06 lower to 1.56 higher) | 645 (9 studies) | ⊕⊕⊝⊝ low3,4,5 | |||
| Reintubation | Study population | RR 0.65 (0.44 to 0.97) | 789 (10 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 310 per 1000 | 202 per 1000 (137 to 301) | |||||
| Moderate | ||||||
| 286 per 1000 | 186 per 1000 (126 to 277) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Less than 300 events 2 RR 0.25 (95% CI 0.15 to 0.43) 3 Impact of heterogeneity was considerable (I2 =90%) 4 95% CI spans from a clinically important and significant increase or decrease 5 Uncertain if estimates include non‐survivors due to differential between group mortality (higher in control arm)
Eleven study authors (Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Trevisan 2008; Vaschetto 2012; Wang 2005; Zou 2006) confirmed and supplemented information related to study methods. Overall, the included trials were of moderate to good quality (Figure 2; Figure 3).
Mortality
Sixteen trials involving 994 participants provided mortality data. Mortality was reported at 30 days (Prasad 2009; Tawfeek 2012), at 60 days (Nava 1998a), at 90 days (Ferrer 2003; Girault 1999), at ICU (Girault 2011; Vaschetto 2012) and hospital discharge (Girault 1999; Rabie 2004; Rabie Agmy 2012; Trevisan 2008; Vaschetto 2012; Wang 2005; Zheng 2005; Zou 2006) and at an undefined time point (Chen 2001; Hill 2000; Wang 2004). Strong evidence indicated that NPPV weaning reduced mortality (RR 0.53, 95% confidence interval (CI) 0.36 to 0.80, P = 0.002) with moderate heterogeneity (I2 = 37%, P = 0.07). We noted a significant beneficial effect (Chi2 = 5.12, P = 0.02) of noninvasive weaning on mortality in participants with COPD (nine trials) compared with mixed populations (seven trials) (RR 0.36, 95% CI 0.24 to 0.56, P < 0.00001; RR 0.81, 95% CI 0.47 to 1.40, P = 0.45, respectively; see Analysis 1.1; Table 1).
1.1. Analysis.
Comparison 1 Noninvasive versus invasive weaning, Outcome 1 Mortality.
Proportion of weaning failures
Eight trials, involving 605 participants, reported the proportions of participants successfully weaned (Girault 1999; Girault 2011; Hill 2000; Nava 1998a; Rabie 2004; Rabie Agmy 2012; Tawfeek 2012; Vaschetto 2012). Successful weaning was not defined in two studies (Girault 2011; Rabie 2004) and was defined in two studies as not requiring initiation of NPPV or reintubation within 72 hours (Nava 1998a; Tawfeek 2012) or as not requiring reintubation within 48 hours (Hill 2000). Another trial (Girault 1999) defined weaning failure as the need for reintubation by day five after extubation or, when extubation was not possible, within five days of initiation of weaning efforts in the IPPV group. In this trial (Girault 1999), all participants with weaning failure were reintubated within five days. Successful weaning was defined as the absence of reintubation within three days after extubation (Tawfeek 2012) or, if reintubation or noninvasive ventilation was not required, within 72 hours of suspension of ventilation (Rabie Agmy 2012). Similarly, Vaschetto et al (Vaschetto 2012) defined extubation failure as the inability to sustain spontaneous unassisted breathing for 48 consecutive hours without development of respiratory failure requiring ventilatory support (invasive or noninvasive). With moderate heterogeneity (I2 = 39%, P = 0.12), the pooled data demonstrated a significant reduction in the proportion of weaning failures with noninvasive weaning (RR 0.63, 95% CI 0.42 to 0.96, P = 0.03). Although summary estimates of effect on weaning failure suggested greater benefit in three trials evaluating participants with COPD (RR 0.52, 95% CI 0.36 to 0.74, P = 0.0002) versus five trials involving mixed populations (RR 0.73, 95% CI 0.35 to 1.51, P = 0.40), between‐group differences were not significant (Chi2 = 0.71, P = 0.40; see Analysis 2.1; Summary of findings table 2).
2.1. Analysis.
Comparison 2 Noninvasive versus invasive weaning, Outcome 1 Weaning failure.
Ventilator‐associated pneumonia
Although the proportions of participants developing VAP were reported in 14 trials (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Tawfeek 2012; Trevisan 2008; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) involving 953 participants, criteria for the diagnosis of VAP during weaning were provided in ten trials (Chen 2001; Ferrer 2003; Nava 1998a; Prasad 2009; Tawfeek 2012; Trevisan 2008; Wang 2004; Wang 2005; Zheng 2005; Zou 2006). The pooled estimate demonstrated a beneficial effect of noninvasive weaning in reducing VAP (RR 0.25, 95% CI 0.15 to 0.43, P < 0.00001), with moderate heterogeneity (I2 = 38%, P = 0.07; see Analysis 3.1; Summary of findings table 2). The effect on VAP was not different in participants with COPD compared with mixed populations (P value (subgroup differences) = 0.31).
3.1. Analysis.
Comparison 3 Noninvasive versus invasive weaning, Outcome 1 Nosocomial pneumonia.
Intensive care unit length of stay
Thirteen trials (Ferrer 2003; Girault 1999; Girault 2011; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Trevisan 2008; Vaschetto 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) involving 907 participants evaluated ICU LOS. The aggregate data revealed a significant reduction in ICU LOS of five days favouring noninvasive weaning (MD ‐5.59 days, 95% CI ‐7.90 to ‐3.28, P < 0.00001) amidst considerable heterogeneity (I2 = 77%, P < 0.00001; see Analysis 4.1). The effect on ICU stay was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.14).
4.1. Analysis.
Comparison 4 Noninvasive versus invasive weaning, Outcome 1 LOS ICU.
Hospital length of stay
Ten trials including 803 participants reported hospital LOS (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Rabie 2004; Rabie Agmy 2012; Trevisan 2008; Wang 2005; Zheng 2005; Zou 2006). These trials noted a significant reduction in hospital LOS of six days (MD ‐6.04 days, 95% CI ‐9.22 to ‐2.87, P = 0.0002) amidst considerable heterogeneity (I2 = 78%, P < 0.00001; see Analysis 5.1). The effect on hospital stay was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.39).
5.1. Analysis.
Comparison 5 Noninvasive versus invasive weaning, Outcome 1 LOS hospital.
Mean total duration of mechanical ventilation
We found significant reductions in the total duration of mechanical ventilation in seven trials that included 385 participants (Ferrer 2003; Nava 1998a; Trevisan 2008; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) (MD ‐5.64 days, 95% CI ‐9.50 to ‐1.77, P = 0.004), with considerable heterogeneity (I2 = 86%, P < 0.00001; see Analysis 6.1). The effect on total duration of mechanical ventilation was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.89).
6.1. Analysis.
Comparison 6 Noninvasive versus invasive weaning, Outcome 1 Average total duration of mechanical ventilatory support.
Mean duration of ventilation related to weaning
We found no effect of noninvasive weaning on the duration of mechanical ventilation related to weaning in nine trials involving 645 participants (Chen 2001; Ferrer 2003; Girault 1999; Girault 2011; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Trevisan 2008; Vaschetto 2012) (MD ‐0.25 days, 95% CI ‐2.06 to 1.56, P = 0.79), with considerable heterogeneity (I2 = 90%, P < 0.00001; see Analysis 7.1; Summary of findings table 2). The effect on duration of ventilation related to weaning was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.48).
7.1. Analysis.
Comparison 7 Noninvasive versus invasive weaning, Outcome 1 Average duration of ventilation related to weaning.
Mean duration of endotracheal mechanical ventilation
Twelve trials (Ferrer 2003; Girault 1999; Hill 2000; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Tawfeek 2012; Vaschetto 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) that included 717 participants reported the duration of ETMV. In the presence of considerable heterogeneity (I2 = 87%, P < 0.00001), the summary estimate demonstrated a significant decrease in the duration of ETMV with noninvasive weaning (MD ‐7.44 days, 95% CI ‐10.34 to ‐4.55, P < 0.00001; see Analysis 8.1). The effect on duration of ETMV was not different in participants with COPD versus mixed populations (P‐value (subgroup differences) = 0.81).
8.1. Analysis.
Comparison 8 Noninvasive versus invasive weaning, Outcome 1 Duration of endotracheal mechanical ventilation.
Adverse events
Variability in selection and reporting of adverse events in individual trials precluded pooling of most data.
Reintubation
The rate of reintubation was reported separately from the proportion of weaning failures in ten trials (Ferrer 2003; Girault 1999; Girault 2011; Hill 2000; Rabie Agmy 2012; Tawfeek 2012; Trevisan 2008; Vaschetto 2012; Wang 2005; Zou 2006) involving 789 participants. The pooled estimate supported a significant reduction in reintubation rate with noninvasive weaning (RR 0.65, 95% CI 0.44 to 0.97, P = 0.03), with moderate heterogeneity (I2 = 41%, P = 0.08; see Analysis 9.1; Summary of findings table 2). The effect on reintubation rates was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.13).
9.1. Analysis.
Comparison 9 Noninvasive versus invasive weaning, Outcome 1 Reintubation.
Arrhythmia
The pooled results of three trials (Girault 1999; Girault 2011; Prasad 2009), including 201 participants, demonstrated no effect of noninvasive weaning on development of arrhythmias (RR 0.89, 95% CI 0.34, 2.34, P = 0.81), in the absence of heterogeneity (I2 = 0%, P = 0.63; see Analysis 10.1). The effect on arrhythmia rates was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.44).
10.1. Analysis.
Comparison 10 Noninvasive versus invasive weaning, Outcome 1 Arrhythmia.
Tracheostomy
Seven trials involving 572 participants (Ferrer 2003; Girault 1999; Girault 2011; Rabie Agmy 2012; Tawfeek 2012; Trevisan 2008; Vaschetto 2012) reported the requirement for tracheostomy. The pooled estimated demonstrated a significant reduction in the rate of tracheostomy (RR 0.19, 95% CI 0.08 to 0.47, P = 0.0004), in the presence of unimportant heterogeneity (I2 = 10%, P = 0.35; see Analysis 11.1). The effect on rate of tracheostomy was not different in participants with COPD versus mixed populations (P value (subgroup differences) = 0.22).
11.1. Analysis.
Comparison 11 Noninvasive versus invasive weaning, Outcome 1 Tracheostomy.
Quality of life
Quality of life was not reported.
Sensitivity analysis
Exclusion of a quasi‐randomized trial (Chen 2001) supported significant reductions in pooled mortality (RR 0.60, 95% CI 0.40 to 0.90, P = 0.01) and VAP (RR 0.27, 95% CI 0.16 to 0.45, P < 0.00001) favouring the noninvasive approach to weaning (see Analysis 12.1; Analysis 12.2).
12.1. Analysis.
Comparison 12 Sensitivity analysis: noninvasive versus invasive weaning, Outcome 1 Mortality excluding quasi‐randomized trial.
12.2. Analysis.
Comparison 12 Sensitivity analysis: noninvasive versus invasive weaning, Outcome 2 Nosocomial pneumonia excluding quasi‐randomized trial.
Subgroup analyses
Similarly, subgroup analysis comparing trials that enrolled at least 50% COPD participants (12 trials) versus those enrolling less than 50% COPD participants (four trials) supported a trend towards noninvasive weaning (Chi2 = 2.39, P = 0.12) leading to reduced mortality in trials that enrolled predominantly COPD participants (RR 0.47, 95% CI 0.29 to 0.76, P = 0.002; RR 0.86, 95% CI 0.47 to 1.58, P = 0.63; see Analysis 13.1).
13.1. Analysis.
Comparison 13 Noninvasive versus invasive weaning, Outcome 1 Mortality greater than or equal to 50% COPD versus less than 50% COPD.
We found a nonsignificant effect (Chi2 = 0.15, P = 0.70) of noninvasive weaning on between‐group differences in weaning failure in five trials enrolling at least 50% COPD participants compared with mixed population (three trials) subcategories (RR 0.68, 95% CI 0.46 to 1.01, P = 0.06; RR 0.51, 95% CI 0.12 to 2.18, P = 0.36, respectively; see Analysis 14.1).
14.1. Analysis.
Comparison 14 Noninvasive versus invasive weaning, Outcome 1 Weaning failure greater than or equal to 50% COPD.
Publication bias
Visual inspection of a funnel plot comparing study estimate of effect (RR) with standard error of the log RR for mortality did not reveal important asymmetry.
Discussion
Summary of main results
We identified 16 trials comparing NPPV and IPPV weaning strategies among 994 participants, most with COPD. Compared with IPPV, NPPV significantly decreased mortality (Table 1), weaning failure and VAP (Summary of findings table 2). Amidst significant heterogeneity, NPPV weaning also significantly reduced ICU and hospital LOS, total duration of mechanical ventilation and duration of invasive ventilation. Although noninvasive weaning significantly reduced tracheostomy and reintubation rates, it had no effect on the duration of mechanical ventilation related to weaning (Summary of findings table 2). Exclusion of a single quasi‐randomized trial supported statistically significant reductions in mortality and VAP favouring NPPV. Subgroup analyses suggested that benefits of the noninvasive approach to weaning in terms of mortality were significantly greater in trials enrolling exclusively COPD participants compared with those enrolling mixed populations. Summary estimates from 16 trials of moderate to good quality, including predominantly participants with COPD, demonstrated a positive effect of noninvasive weaning on mortality and VAP without increased risk of weaning failure or reintubation.
Overall completeness and applicability of evidence
Most studies in our review included participants exclusively (Chen 2001; Nava 1998a; Prasad 2009; Rabie 2004; Rabie Agmy 2012; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) or predominantly (Ferrer 2003; Girault 1999) diagnosed with COPD. Patients with chronic airflow limitation may be ideally suited to NPPV given its ability to offset respiratory muscle fatigue and tachypnoea, augment tidal volume and reduce iPEEP. Subgroup analyses suggested greater benefit with noninvasive weaning in COPD participants, with statistical tests of subgroup effects achieving statistical significance. Notwithstanding, inferences from subgroup analyses may be limited by inclusion of COPD participants in mixed population studies and by the small number of trials comparing alternative weaning strategies in participants with respiratory failure of other causes. Whether other causes of respiratory failure are as amenable as COPD to noninvasive weaning remains to be determined in a single, adequately powered RCT.
This review was strengthened by an extensive search for relevant trials. We screened citations and abstracted data independently and in duplicate, and we corresponded with investigators to clarify study methods and outcomes reporting, when needed. Pooling of results in a meta‐analysis implicitly assumes that the included studies are sufficiently similar with respect to populations, study interventions, outcomes and methodologic quality that one could reasonably expect a comparable underlying treatment effect. To this end, we exclusively used random‐effects models for pooling data, which take into consideration both between‐study and within‐study variation. A priori, we planned to perform sensitivity and subgroup analyses to explain anticipated differences among study results.
Quality of the evidence
Studies included in this meta‐analysis varied in the methods used to identify weaning candidates and to titrate and discontinue mechanical support. Multidisciplinary protocols used to identify weaning candidates and to perform daily SBTs reduce the duration of mechanical ventilation (Ely 1996; Ely 2001; Esteban 1997; Esteban 1999; Kollef 1997; Marelich 2000; Perren 2002). For patients failing an SBT, PS or intermittent or once‐daily SBTs are favoured over SIMV to facilitate discontinuation of support (Brochard 1994; Butler 1999; Esen 1992; Esteban 1995; Jounieaux 1994; Tomlinson 1989). Although criteria used to identify candidates for an SBT or for weaning were used in 11 trials, only three trials screened daily for SBT readiness. In addition, four trials each conducted prerandomization SBTs and assessed for resolution of pulmonary infection to identify weaning readiness. The latter strategy represents a novel approach to identifying weaning candidates in selected populations and prioritizes identifying the cause of respiratory failure (bronchopulmonary infection) over meeting conventional weaning criteria. Methods for identifying weaning candidates may have an impact on study estimates of the duration of ventilation; however, these prerandomization study design considerations are less likely to result in important performance bias. Conversely, unequal or inconsistent use of weaning protocols and the frequency with which periods of spontaneous breathing (noninvasive strategy) or SBTs (invasive strategy) were permitted were variably reported among the included trials and represent important postrandomization study design considerations that could bias estimates of the duration of ventilation in unblinded weaning trials. Trials also varied in their selection and reporting of outcomes. An additional important study design consideration for weaning trials that could have an impact on the duration of ventilation is sedation administration (Brook 1999). Only one trial (Hill 2000) in our review used a sedation protocol. Overall, most trials in this review were of moderate quality; three trials were evaluated to be at low risk of bias and two trials were considered to be at high risk of bias.
Potential biases in the review process
In summary estimates, we found that noninvasive weaning significantly reduced mortality, ICU and hospital LOS and total duration of mechanical ventilation; these findings are consistent with and may be due to reduced VAP. However, we cannot ignore the fact that having direct access to respiratory secretions in invasively weaned participants may have resulted in detection bias by enhancing VAP detection in this group compared with the noninvasively weaned group. Inspection of control group rates of VAP in our review shows that they varied widely, ranging from 6.3% (Girault 1999) to 59.1% (Ferrer 2003). Similarly, control group mortality rates in our review ranged from 11.1% (Hill 2000; Rabie 2004) to 60.0% (Prasad 2009). Across trials, 173 total mortality events and 174 total VAP events were reported. Disparate control group mortality and VAP event rates, potential for detection bias in assessment of VAP, total of less than several hundred mortality VAP events (Devereaux 2004; Thorlund 2011) and selection and reporting of continuous outcomes limit the strength of inferences that can be made from this review. In addition, although estimates of the impact of heterogeneity associated with pooled estimates of mortality, VAP and reintubation were considered moderate, estimates associated with most continuous outcomes (ICU and hospital LOS, total duration of mechanical ventilation and duration of endotracheal mechanical intubation) were considerable. To this end, we considered estimates of the impact of heterogeneity to be unimportant (Higgins 2011) for only two outcomes (arrhythmia and tracheostomy rates), which significantly favoured noninvasive weaning. Finally, we noted effect modification with NPPV weaning in subgroup analysis of mortality but not for other outcomes. This finding may reflect differences in the populations studied, physiological benefits of NPPV in COPD participants, ecological bias (findings at the trial level driven by some other differences between trials, such as quality, that may not be confirmed by a within‐trial subgroup analysis), lack of robustness of the mortality subgroup analysis or lack of power in the other subgroup analyses conducted.
Agreements and disagreements with other studies or reviews
In their efforts to optimize the time of liberation from invasive ventilation, clinicians are challenged by a trade‐off between the risks associated with failed extubation and the complications associated with prolonged invasive ventilation (Epstein 1997). Noninvasive weaning, by providing ventilatory support without an artificial airway, offers a potential solution to this trade‐off. Summary estimates from 16 trials of moderate to good quality, most with COPD participants, demonstrated a positive effect of NPPV weaning on mortality and VAP without increased risk of weaning failure or reintubation.
Notwithstanding these data, clinicians may be reluctant to incorporate noninvasive weaning into clinical practice because of the need to surrender a protected airway, concerns regarding the ventilatory support that can be provided with NPPV and increased risk for developing VAP if reintubation is required (Pawar 2003). Promising findings associated with noninvasive weaning require confirmation in a single, large, adequately powered RCT. The optimal timing for transitioning patients to NPPV for weaning remains to be determined. Additionally, whether other causes of respiratory failure are as amenable as COPD to noninvasive weaning remains to be elucidated.
Authors' conclusions
Implications for practice.
Current trials of noninvasive weaning, mainly small trials most with COPD, show near‐consistent positive effects on mortality and VAP without increased risk of weaning failure and reintubation. Although use of NPPV to wean participants with COPD is associated with highly encouraging net clinical benefit (number needed to treat for an additional beneficial outcome (to prevent death) of 8.9 in all participants and 6.5 in the COPD subgroup), most evidence has been obtained from small randomized trials. Given the potential for small event rates to be misleading when results from multiple small RCTs are pooled, additional evidence is required from a large, adequately powered RCT before we can recommend the routine use of NPPV as an adjunct for weaning patients from invasive mechanical ventilation. If consideration is given to adopting this approach to wean patients, we suggest that it be preferentially used in patients with COPD and in highly monitored environments at expert centres.
Implications for research.
A well‐designed, adequately powered RCT with explicitly defined end points that can be used to compare the alternative approaches to weaning is justified.
Several unanswered questions remain regarding the role of noninvasive weaning in the ICU. These include the following.
Does the NPPV strategy decrease the duration of ventilation related to weaning?
Does the cause of respiratory failure (COPD vs other) influence the effectiveness of noninvasive weaning?
Does illness severity at the time of randomization or duration of mechanical ventilation before randomization influence the effectiveness of noninvasive weaning?
What are the consequences of reintubation? Do important trade‐offs exist between weaning failure and consequences of reintubation (VAP, mortality and ICU LOS)?
Can the same potential benefits be realized in diverse populations and at other centres without local expertise in noninvasive weaning?
What is the effect of noninvasive weaning on quality of life?
To address these questions, future trials should consider incorporation of:
stratification based on cause of respiratory failure (COPD, non‐COPD);
daily screening for participant identification;
incorporation of weaning guidelines or protocols;
explicit criteria for discontinuation of mechanical support and reintubation;
identification and control of important co‐interventions, including, but not limited to, sedation and general medical care;
reporting of clinically relevant outcomes, including duration of mechanical support related to weaning, adverse events and quality of life; and
consequences of reintubation for LOS, VAP and mortality.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 4 December 2013 | New search has been performed | Included trials: We included four new trials (Girault 2011; Rabie Agmy 2012; Tawfeek 2012; Vaschetto 2012) in this updated review. For one trial with three arms, we included two arms relevant to our research question (Girault 2011). Quality assessment: In this update, we evaluated and recorded the presence of true randomization and the use of concealed allocation to minimize selection bias. Additionally, we evaluated reports of randomized trials for completeness of outcome data and selective outcomes reported to assess for attrition and reporting biases, respectively. Unlike in the previous review (Burns 2010), we did not include in our quality assessment the use of daily screening to identify participants capable of unassisted breathing; predefined, permissive weaning criteria to identify weaning candidates (including but not limited to minute ventilation, tidal volume (VT), vital capacity, respiratory rate, rapid shallow breathing index, Glasgow Coma Scale, presence of spontaneous ventilatory efforts and a cough reflex, requirement for positive end‐expiratory pressure (PEEP) and ability to maintain arterial oxygen saturation above 90% on a fractional concentration of inspired oxygen (FiO2) of less than 0.50) and performace of spontaneous breathing trials (SBTs). We did not include assessment of the use of weaning protocols or guidelines (in both groups) and criteria for failure of a prerandomization SBT, discontinuation of mechanical ventilation (in both groups) and extubation, reintubation due to poor reporting of these aspects of trial design and implementation and concerns over the reliability of efforts to acquire these details amidst language issues. We contacted authors to ask them to describe specific features of their trials including use of daily screening and a prerandomization SBT; however, we did not include these items in the quality assessment in this update. Exclusion criteria: We updated our exclusion criteria to exclude studies evaluating exclusively tracheostomized participants as (1) tracheostomy was an outcome of this review, (2) these studies typically include a high proportion of participants undergoing prolonged mechanical ventilation and (3) application of the interventions could be different in the setting of a tracheostomy (e.g. participants randomly assigned to noninvasive weaning may meet criteria to return to invasive ventilation per tracheostomy and subsequently be returned to noninvasive ventilation. Similarly, participants randomly assigned to invasive weaning may undergo a series of spontaneous breathing trials (SBTs) before extubation). Summary of findings: We included in this update SoF tables for the outcomes of mortality, weaning failure, ventilator‐associated pneumonia (VAP) and reintubation . |
| 4 December 2013 | New citation required but conclusions have not changed | A new author, Azra Premji, joined the review team in June 2012. |
| 6 July 2010 | New citation required but conclusions have not changed | We invited one new review author (Sean Keenan) to participate in the update to the previously published meta‐analysis (Burns 2003).
|
| 6 July 2010 | New search has been performed | We reran our searches from July 2003 to April 2008. We included seven new RCTs (Prasad 2008; Rabie 2004; Trevisan 2008; Wang 2004; Wang 2005; Zheng 2005; Zou 2006) in this version. Our previous version (Burns 2003) included five RCTS. This new updated version includes 12 RCTs, involving 530 patients in total. Of those 12 RCTs, eight trials included patients with chronic obstructive pulmonary disease and four trials included mixed populations. We identified and excluded three new trials (Matic 2007; Wang 2000; Wang 2003) in this version. One study (Girault 2002) is now completed and awaiting publication. |
| 17 February 2009 | Amended | Converted to new review format |
| 8 July 2004 | New search has been performed | We revised the variance for the duration of ETMV reported in the abstract publication from standard error of the mean to standard deviation. The summary estimate of effect changed slightly from a WMD of ‐6.60 days (95% CI, ‐11.70 to ‐1.87) to a WMD of ‐6.32 days (95% CI, ‐12.12 to ‐0.52). The test for overall effect and the test for heterogeneity remained statistically significant. We revised the variance for the duration of ETMV reported in the abstract publication from standard error of the mean to standard deviation. The summary estimate of effect changed slightly from a WMD of ‐6.60 days (95% CI, ‐11.70 to ‐1.87) to a WMD of ‐6.32 days (95% CI, ‐12.12 to ‐0.52). The test for overall effect and the test for heterogeneity remained statistically significant. In addition, we tested the difference in relative risk (RR) between sub‐categories (COPD versus mixed populations) using a z‐test in the subgroup analyses section of the 'Results'. We considered a P‐value less than or equal to 0.05 to be statistically significant. We revised the number of patients in the computation of the WMD for the duration of mechanical ventilation related to weaning in the study by Girault et al (Girault 1999) to reflect that this outcome was reported in successful patients. The summary estimate of effect changed minimally from a WMD ‐2.71 (95% CI, ‐15.71 to 10.29, P = 0.68) to a WMD of ‐2.72 days (95% CI, ‐15.58 to 10.14, P = 0.68). |
Notes
In the previously published protocol, as part of an a priori sensitivity analysis, we stated that we would assess the impact of the cause of respiratory failure (COPD vs non‐COPD) on (1) the proportion of weaning failures and (2) mortality. In the last version of this review, we identified two studies restricted to participants with COPD and three studies with mixed participant populations. In the absence of individual participant data, we compared studies restricted to COPD participants versus those with mixed participant populations. To explore for potential differences in response to NPPV, we compared studies enrolling at least 50% COPD participants versus those enrolling less than 50% COPD participants, in terms of mortality.
To search EMBASE, we used the following Emtree terms: respiratory failure (explode), positive end‐expiratory pressure (explode) and weaning (explode). In addition, we used the Emtag: artificial ventilation.
In the protocol, we stated that the MEDLINE search strategy would be limited to include the following publication types: clinical trials, controlled clinical trials, randomized controlled trials, multicenter studies and meta‐analyses. In the review, we did not limit the most recent literature search by publication type.
October 2013
Quality assessment
In this update, we evaluated and recorded the presence of true randomization and use of concealed allocation to minimize selection bias. Additionally, we evaluated reports of randomized trials for completeness of outcome data and selective outcomes reporting to assess for attrition and reporting biases, respectively.
Unlike in the previous review (Burns 2010), we did not include in our quality assessment the use of daily screening to identify participants capable of unassisted breathing; inclusion of predefined, permissive weaning criteria to identify weaning candidates (including but not limited to minute ventilation, tidal volume (VT), vital capacity, respiratory rate, rapid shallow breathing index, Glasgow Coma Scale, presence of spontaneous ventilatory efforts and a cough reflex, requirement for PEEP and ability to maintain arterial oxygen saturation above 90% on a fractional concentration of inspired oxygen (FiO2) of less than 0.50) and performance of spontaneous breathing trials (SBTs). We did not include assessment of the use of weaning protocols or guidelines (in both groups) and criteria for failure of prerandomization SBT, discontinuation of mechanical ventilation (in both groups) and extubation, reintubation due to poor reporting of these aspects of trial design and implementation and concerns over the reliability of efforts to acquire these details amidst language issues. We contacted study authors to ask them to describe specific features of their trials, including use of daily screening and a prerandomization SBT; however, we did not include them in the quality assessment in this update.
Summary of findings
We included in this update SoF tables for the outcomes of mortality, weaning failure, VAP and reintubation.
Exclusion criteria
We updated our exclusion criteria to exclude studies evaluating exclusively tracheostomized participants, as (1) tracheostomy was an outcome of this review, (2) these studies typically include a high proportion of participants undergoing prolonged mechanical ventilation and (3) application of the interventions could be different in the setting of a tracheostomy (e.g. participants randomly assigned to noninvasive weaning may meet criteria to return to invasive ventilation per tracheostomy and subsequently may be returned to noninvasive ventilation. Similarly, participants randomly assigned to invasive weaning may undergo a series of SBTs before extubation).
Acknowledgements
We would like to thank the the Cochrane editorial team and our peer reviewers (Dr Harald Herkner, Professor Nathan Pace, Dr Felix Ram, Dr Andrew Jones, Dr Bronagh Blackwood, Dr Eddy Fan and Dr Andrew MacDuff) for their contributions to the review. We would also like to thank the authors of the primary research, who provided additional information pertinent to the design and outcomes of their respective clinical trials, and Dr Sean Keenan, who, although not able to participate in the updated review, provided advice regarding study selection. We thank Haibo Zhang, Haibo Qiu, Wei Ehr Cheng, Zhuxian Feng and Hanpo Yu for their assistance with translating and contacting authors to clarify publication and study design in earlier issues of this review.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Positive‐Pressure Respiration explode all trees #2 post‐extubation or postextubation #3 MeSH descriptor Ventilator Weaning explode all trees #4 #1 OR #2 OR #3 #5 MeSH descriptor Respiratory Insufficiency explode all trees #6 MeSH descriptor Postoperative Complications, this term only #7 #5 OR #6 #8 #4 and #7
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp POSITIVE‐PRESSURE RESPIRATION/ or exp VENTILATOR WEANING/ or post?extubation.mp. 2. POSTOPERATIVE CARE/ or exp RESPIRATORY INSUFFICIENCY/ or POSTOPERATIVE COMPLICATIONS 3. (randomised controlled trial.pt. or controlled clinical trial.pt.or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals.sh not (humans.sh and animals.sh)) 4. 1 and 2 and 3
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp positive‐end‐expiratory‐pressure/ or exp artificial‐ventilation/ or post?extubation*.mp. 2. postoperative‐care/ or exp respiratory‐failure/ 3. ((RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/) or ((RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab) not (animals.sh not (humans.sh and animals.sh)) 4. 1 and 2 and 3
Data and analyses
Comparison 1. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 9 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.56] |
| 1.2 Mixed | 7 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.47, 1.40] |
Comparison 2. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning failure | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.74] |
| 1.2 Mixed | 5 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.51] |
Comparison 3. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nosocomial pneumonia | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 9 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.13, 0.37] |
| 1.2 Mixed | 5 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.93] |
Comparison 4. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LOS ICU | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 8 | 608 | Mean Difference (IV, Random, 95% CI) | ‐6.66 [‐9.41, ‐3.92] |
| 1.2 Mixed | 5 | 299 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐6.78, 0.15] |
Comparison 5. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LOS hospital | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 6 | 524 | Mean Difference (IV, Random, 95% CI) | ‐6.91 [‐10.83, ‐1.00] |
| 1.2 Mixed | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐4.02 [‐9.41, 1.36] |
Comparison 6. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average total duration of mechanical ventilatory support | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 5 | 277 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐10.64, ‐0.91] |
| 1.2 Mixed | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐5.20 [‐11.34, 0.93] |
Comparison 7. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average duration of ventilation related to weaning | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 4 | 355 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐3.12, 0.26] |
| 1.2 Mixed | 5 | 290 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐4.01, 4.35] |
Comparison 8. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of endotracheal mechanical ventilation | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 7 | 558 | Mean Difference (IV, Random, 95% CI) | ‐7.53 [‐11.47, ‐3.60] |
| 1.2 Mixed | 5 | 159 | Mean Difference (IV, Random, 95% CI) | ‐6.85 [‐10.75, ‐2.95] |
Comparison 9. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reintubation | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.35, 0.70] |
| 1.2 Mixed | 7 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.47, 1.43] |
Comparison 10. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Arrhythmia | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 19.78] |
| 1.2 Mixed | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.17] |
Comparison 11. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tracheostomy | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 1 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.60] |
| 1.2 Mixed | 6 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.57] |
Comparison 12. Sensitivity analysis: noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality excluding quasi‐randomized trial | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Nosocomial pneumonia excluding quasi‐randomized trial | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 13. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality greater than or equal to 50% COPD versus less than 50% COPD | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Greater than or equal to 50% COPD | 12 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.76] |
| 1.2 Less than 50% COPD | 4 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.58] |
Comparison 14. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning failure greater than or equal to 50% COPD | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Greater than or equal to 50% COPD | 5 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.01] |
| 1.2 Less than 50% COPD | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.12, 2.18] |
Comparison 15. Noninvasive versus invasive weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning failure | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Nosocomial pneumonia | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Average duration of ventilation related to weaning | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Reintubation | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
15.1. Analysis.
Comparison 15 Noninvasive versus invasive weaning, Outcome 1 Weaning failure.
15.2. Analysis.
Comparison 15 Noninvasive versus invasive weaning, Outcome 2 Nosocomial pneumonia.
15.3. Analysis.
Comparison 15 Noninvasive versus invasive weaning, Outcome 3 Average duration of ventilation related to weaning.
15.4. Analysis.
Comparison 15 Noninvasive versus invasive weaning, Outcome 4 Reintubation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2001.
| Methods | Pseudo‐randomized (n = 24) | |
| Participants | Participants were admitted with an acute exacerbation of COPD. Participants were invasively ventilated through a nasotracheal tube for 48 to 60 hours Inclusion criteria: pH less than 7.35 PaO2 less than 45 mm Hg and RR greater than 30 breaths/min | |
| Interventions | Participants were randomly assigned by alternating day of the month to receive noninvasive ventilation in PS mode or continued weaning with invasive PS. PS and PEEP were gradually decreased to facilitate liberation from mechanical support. Ventilation was discontinued after a three‐hour SBT was completed and discontinuation criteria were met | |
| Outcomes | 1. Mortality 2. VAP 3. Duration of MV related to weaning 4. Hospital LOS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants randomly assigned to group A or B on the basis of order of hospital admission |
| Allocation concealment (selection bias) | High risk | Used order of hospital admission |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None missing |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes not specified. Clinically important outcomes reported |
Ferrer 2003.
| Methods | RCT
(n = 43) Two centres Computer‐generated list held by investigator not involved in participant care |
|
| Participants | Participants with ARF and persistent weaning failure requiring MV for at least 72 hours and failing a two‐hour T‐piece trial on three consecutive days. Participants were identified by daily screening prerandomization | |
| Interventions | Participants were randomly assigned to bilevel positive airway pressure in ST mode or invasive weaning with AC or PS. Daily T‐piece trials were conducted until extubation in the IPPV group. Periods of SB of increasing duration were used to wean NPPV. IPPV was discontinued after successful completion of a two‐hour SBT | |
| Outcomes | 1. ICU mortality 2. 90‐day mortality 3. VAP 4. Duration of MV related to weaning 5. Duration of ETMV 6. Total duration of MV 7. ICU LOS 8. Tracheostomy 9. Reintubation 10. Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned with the use of a computer‐generated table for each centre |
| Allocation concealment (selection bias) | Low risk | Computer‐generated table held by an investigator not involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None missing |
| Selective reporting (reporting bias) | High risk | Proportions of weaning successes and failures not reported in publication of full trial. This outcome was previously reported in a smaller number of participants in an earlier abstract publication (2000). Study authors did not continue to collect data on this outcome |
Girault 1999.
| Methods | RCT
(n = 33) Opaque envelopes |
|
| Participants | Participants with acute‐on‐chronic respiratory failure (COPD, restrictive, mixed) failing a two‐hour T‐piece trial after invasive mechanical ventilation for at least 48 hours. Participants were identified through daily screening | |
| Interventions | Participants were randomly assigned to receive invasive pressure support or NPPV delivered in flow or pressure mode. NPPV was delivered intermittently following extubation, separated by periods of SB of increasing duration. Invasive PS was titrated by 3 to 5 cm H2O according to tolerance. Discontinuation of support followed successful completion of two periods of observation during SB (NPPV) or during PS weaning with optional SBTs (IPPV). Extubation was performed when PS was less than 8 cm H2O in the IPPV group | |
| Outcomes | 1. 90‐day mortality 2. Hospital mortality 3. Successful weaning 4. VAP 5. Duration of MV related to weaning 6. Duration of ETMV 7. Mean daily period of support 8. ICU LOS 9. Hospital LOS 10. Adverse events 11. Reintubation 12. Tracheostomy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. Randomization and introduction of the weaning procedure IPSV or NPPV were done during the 24 hours after the two‐hour SBT |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None missing |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Published manuscript reports on prespecified outcomes |
Girault 2011.
| Methods | RCT (n = 208) three arms, of which two arms (n = 138) were included in the pooled results 13 ICUs/centres Computer‐generated randomization table using variable blocks of four Sealed, opaque envelopes |
|
| Participants | Participants with chronic hypercapneic respiratory failure based on history, chest radiograph, arterial blood gases in steady state and/or bicarbonate level and pulmonary function tests (if available) who were intubated for at least 48 hours, regardless of cause of the disorder. Participants were clinically stable for at least 24 hours and underwent an SBT after meeting weaning criteria as determined by a daily screening evaluation. Participants who failed an SBT were assigned to one of the three treatment groups. Two groups (invasive weaning and NPPV weaning) were included in the pooled analysis | |
| Interventions | Participants were randomly assigned to conventional invasive weaning (n = 69), oxygen‐therapy (n = 70) or noninvasive ventilation (n = 69). Conventional invasive weaning was performed using one or more daily SBTs with the use of a T‐piece or PSV (with or without PEEP) in 20% of participants. In the oxygen‐therapy and NPPV groups, respectively, SBTs were followed by a re‐ventilation period of at least 30 minutes duration and extubation (same day as randomization) or standard oxygen therapy to maintain SaO2> 90% or immediate NPPV with a face mask. NPPV was performed for > six hours and was administered continuously initially and intermittently subsequently with spontaneous breathing periods using supplemental oxygen | |
| Outcomes | 1. Mortality (before eighth day after randomization) 2. Mortality (before 29th day after randomization) 3. ICU mortality (before 29th day after randomization) 4. Hospital mortality (before 29th day after randomization) 5. Total duration of ventilation 6. Duraion of ventilation related to weaning 7. Ventilator‐free days 8. Complications (auto extubation, postextubation stridor, tube obstruction, respiratory encephalopathy, bronchial hypersecretion, nosocomial pneumonia, sinusitis, atelectasis, cardiac arrhythmia, haemodynamic collapse, ACPE, paralytic ileus, gastric distension, mask intolerance) 9. Respiratory support at discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table using variable blocks of four |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes according to centre stratification |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All specific outcomes reported |
Hill 2000.
| Methods | RCT
(n = 21) (abstract) Sealed, opaque envelopes |
|
| Participants | Participants with acute respiratory failure admitted to a medical intensive care unit and failing a 30‐minute T‐piece trial were eligible. Participants were identified through daily screening | |
| Interventions | Participants were randomly assigned to receive VPAP using PS, delivered in ST mode, or invasive PS. In both arms, mechanical support was titrated to RR and tidal volume. Whereas two‐hour T‐piece trials were permitted to discontinue IPPV support in the IPPV group, NPPV was discontinued by gradually increasing periods between NPPV trials until participants were able to breathe spontaneously between NPPV sessions for two hours without increasing RR or dyspnoea | |
| Outcomes | 1. Mortality 2. Successful weaning 3. Duration of ETMV 4. Reintubation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clarified to be randomized. Uncertain sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes (unclear whether sequentially numbered) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None missing |
| Selective reporting (reporting bias) | Low risk | Abstract publication only. Published abstract reports the duration of invasive ventilation |
Nava 1998a.
| Methods | RCT
(n = 50) Three centres Opaque, sealed envelopes |
|
| Participants | Participants admitted with an acute exacerbation of COPD requiring intubation and MV for at least 36 to 48 hours. Relapse was defined as pH less than 7.33, PaO2 less than 45 mm Hg, severe dyspnoea in the absence of pneumonia or one of 11 nonoperative diagnoses. Participants who met permissive criteria and failed a one‐hour T‐piece trial were eligible for inclusion | |
| Interventions | Participants were intubated, sedated and paralysed for the first six to eight hours. Those failing a one‐hour T‐piece trial were randomly assigned to weaning with NPPV or IPPV. NPPV was delivered continuously with at least two periods of SB per day of increasing duration. PS was decreased by 2 to 4 cm H2O per day in the NPPV group. In the IPPV group, PS was titrated to an RR of less than 25 breaths/min, and twice‐daily SBTs were permitted. Discontinuation occurred after successful completion of a three‐hour period of SB (NPPV) or SBT (IPPV) and when discontinuation criteria were met | |
| Outcomes | 1. 60‐day mortality 2. VAP 3. Successful weaning at 60 days 4. Total duration of MV 5. ICU LOS 6. Adverse events 7. Tracheostomy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomly assigned" |
| Allocation concealment (selection bias) | Low risk | Using sealed, opaque, numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Authors report on important outcomes, including weaning outcomes (success or failure), mortality, VAP, total duration of ventilation and ICU length of stay |
Prasad 2009.
| Methods | ||
| Participants | ||
| Interventions | Participants were initially ventilated in AC mode and were treated with muscle relaxants and sedation to achieve standard ventilator settings. After at least 24 hours of MV and meeting permissive criteria, a T‐piece trial was conducted. Participants failing the T‐piece trial were randomly assigned to NPPV or IPPV weaning NPPV and IPPV were initiated in pressure mode (with a full face mask) and with the use of invasive PS, respectively. NPPV was applied continuously (except for meals, expectoration). IPAP and EPAP levels were adjusted to achieve satisfactory blood gases and RR less than 25 breaths/min. Thereafter, noninvasive or invasive PS was decreased by 2 cm H2O every four hours, titrated to good tolerance (monitoring for changes in saturations and RRs). Both noninvasive and invasive PS (above PEEP) were titrated to participant tolerance, blood gases and RR. Once NPPV was decreased to IPAP and EPAP of 8 and 4 cm H2O, respectively, and invasive PS and PEEP were titrated to 10 and 5 cm H2O, respectively, with pH greater than or equal to 7.35, SaO2 greater than or equal to 90%, RR < 30 breaths/min and FiO2 less than or equal to 40%, participants were allowed to breathe spontaneously on a Venturi mask or were extubated to a Venturi mask |
|
| Outcomes | 1. 30‐day mortality 2. ICU mortality 3. Duration of MV related to weaning 4. ICU LOS 5. VAP 6. Duration of ETMV 7. Deaths due to VAP 8. Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported using a Kendall and Babington table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
Rabie 2004.
| Methods | RCT
(n = 37) One centre (abstract) Allocation concealment not described |
|
| Participants | Intubated participants with an acute‐on‐chronic exacerbation of COPD, who failed a two‐hour SBT despite meeting simple weaning criteria | |
| Interventions | After intubation, participants were ventilated in controlled mode, received sedation and paralysis for the first six to eight hours and were treated with PS for an additional 60 hours. A T‐piece trial was carried out once participants achieved a satisfactory neurological status and normal temperature and were haemodynamically stable. Participants failing the T‐piece trial were randomly assigned to NPPV (initiated by face mask or nasal mask using BiPAP in PAV/T mode) or continued invasive PS. IPPV was titrated by 2 to 4 cm H2O per day. NPPV was delivered until well tolerated (20 to 22 hours per day), spaced by periods of spontaneous inhalation of oxygen only during meals and for expectoration. The level of PS was decreased by 2 to 4 cm H2O per day in participants with good tolerance. At least two trials of spontaneous breathing of gradually increasing duration were attempted each day. Criteria for weaning from invasive PS or NPPV were SaO2 of 90% or greater with an FiO2 of 40% or less, pH of 7.35 or more, RR less than 35 breaths/min, haemodynamic stability, absence of severe dyspnoea and depressed neurological status. The absence of any of these criteria was considered failure to wean. Participants were screened daily for weaning criteria | |
| Outcomes | 1. Weaning failure 2. Weaning duration 3. ICU LOS 4. Hospital LOS 5. Reintubation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment assignment "was randomized". Author confirms that he used a central computer and that group allocation was communicated by a computer |
| Allocation concealment (selection bias) | Low risk | The author reported that investigators did not know in advance to which arm the participant would be allocated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Personal communication: "Follow‐up was complete" |
| Selective reporting (reporting bias) | Low risk | The authors reported clinically important outcomes. Note is made that they did not report the duration of ventilation related to weaning, although this was not a prespecified outcome in this study |
Rabie Agmy 2012.
| Methods | RCT
(n = 264) (abstract) Allocation concealment not described |
|
| Participants | Intubated participants with acute‐on‐chronic respiratory failure due to COPD who failed a two‐hour SBT, although they met simple criteria for weaning | |
| Interventions | Conventional invasive PSV (n = 130) was compared with NPPV immediately followed by extubation (n = 134) | |
| Outcomes | 1. Gas exchange 2. Duration of ETMV 3. Weaning failure 4. Nosocomial pneumonia 5. ICU LOS 6. Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was determined using a central computer, and group allocation was communicated by the computer |
| Allocation concealment (selection bias) | Low risk | The author reported that investigators did not know in advance to which arm the participant would be allocated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants appear to be accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | The authors reported on gas exchange, duration of ETMV, weaning failure rates, nosocomial pneumonia rates and ICU and hospital LOS in their full publication |
Tawfeek 2012.
| Methods | RCT (n = 42) One centre Opaque, sealed, numbered envelopes |
|
| Participants | Participants invasively ventilated for longer than 48 hours who failed a two‐hour SBT, despite meeting simple weaning criteria | |
| Interventions | Participants who failed an SBT were randomly allocated to SIMV or noninvasive PAV ventilation In the control SIMV group, ventilatory parameters were adjusted until previous PaCO2 and pH values were reached within the first 60 minutes, and the respiratory rate was < 30 breaths/min. In the PAV group, flow and volume assist PAV were adjusted separately |
|
| Outcomes | 1. Gas exchange 2. Duration of ventilatory support 3. Survival at 30 days 4. Complications: septic shock, pneumothorax and VAP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Random assignment was performed using opaque, sealed and numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors randomly assigned 42 participants (21 per group), and the denominators for reported outcomes reflect 21 participants per group |
| Selective reporting (reporting bias) | Low risk | All specified outcomes were reported |
Trevisan 2008.
| Methods | RCT (n = 65) One centre Sealed envelopes |
|
| Participants | Participants receiving mechanical ventilation for longer than 48 hours who failed a 30‐minute spontaneous breathing trial. Participants considered apt to undergo the weaning procedure were submitted to an SBT. Participants had already been randomly assigned to one of the ventilator modes (NPPV or IPPV) that would be used in the event that they failed an SBT | |
| Interventions | After failing a T‐piece trial, participants were randomly divided into two groups: Participants were extubated and placed on NPPV or were returned to invasive mechanical ventilation. For participants randomly assigned to NPPV, IPAP was delivered according to participant tolerance (varied from 10 to 30 cm H2O), and EPAP was set to maintain gas exchange and FiO2 was set to maintain SpO2 greater than 90%. A face mask was used. Weaning from NPPV was performed on a daily basis by gradually reducing pressure levels until adequate VT and minute ventilation levels could be reached and proper alveolar ventilator established. In the IPPV group, a daily SBT was conducted to evaluate the possibility of extubation | |
| Outcomes | 1. ICU length of stay 2. Hospital length of stay 3. Total length of stay in hospital 4. ICU mortality 5. Hospital mortality 6. Duration of ventilation after randomization 7. Total duration of mechanical ventilation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe the trial as an "experimental randomized clinical trial" and state that a "randomized clinical trial was conducted" but do not provide details regarding sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available. The published manuscript includes prespecified outcomes |
Vaschetto 2012.
| Methods | RCT (n = 20) Sealed opaque, sequentially numbered envelopes |
|
| Participants | Included participants were mechanically ventilated for longer than 48 hours, had a PaO2/FiO2 ratio between 200 and 300 with FiO2< 0.60 in PS mode and total applied pressure (PEEP + inspired pressure) < 25 cm H2O, PaCO2< 50, pH > 7.35, RR < 30 breaths/min, core temperature < 38.5ºC, cough on suctioning, need for tracheobronchial suctioning < two per hour and GCS = 11 | |
| Interventions | Participants with hypoxaemic ARF were randomly assigned to early extubation followed by NPPV via helmet (helmet group) or conventional weaning through endotracheal tube (tube group) | |
| Outcomes | 1. Days of mechanical ventilation and adherence to study protocol (primary outcomes) 2. Weaning failure 2. Hospital mortality 4. ICU mortality 5. Tracheotomy 6. Continuous sedation 7. Weaning time 8. Septic complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors used a table of random numbers, held by investigator not involved in study enrolment |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned with the use of sealed, sequentially numbered, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
Wang 2004.
| Methods | RCT (n = 28) One respiratory intensive care unit Allocation concealment not described |
|
| Participants | 28 invasively ventilated participants with COPD and bronchopulmonary infection. Participants were placed on volume‐controlled AC after intubation and then were switched to SIMV + PS + PEEP. When their infection had been brought under control (decreased sputum, sputum less tenacious and purulent, body temperature less than 37.5°C, WBC less than 10 × 109/L, chest x‐ray improved but not resolved), participants were treated differently | |
| Interventions | Participants in the IPPV group were ventilated until blood gases approached normal values and they had fulfilled the weaning criteria (spontaneous breathing for longer than three hours, FiO2 less than or equal to 40%, SpO2 greater than or equal to 90%, pH greater than or equal to 7.35 and RR less than or equal to 35 breaths/min, with stable haemodynamics and clear consciousness), at which time they were extubated. Participants in the NPPV group were extubated and were switched to mask NPPV with PS + PEEP. In both groups, investigators closely monitored the time infection was brought under control, blood gases and mechanical ventilation parameters | |
| Outcomes | 1. Time to control of lung infection 2. Length of ICU stay 3. Duration of mechanical ventilation 4. Mortality 5. VAP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the abstract that participants were "randomly assigned" and that "28 patients were randomized equally in 2 groups". No mention is made of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The authors reported clinically important outcomes in the context of wanting to explore the effects of and optimal timing for noninvasive weaning |
Wang 2005.
| Methods | RCT (n = 90) 11 teaching hospitals RICUs, MICUs Allocation concealment not described |
|
| Participants | Intubated COPD participants, 85 years of age or younger, with severe hypercapneic respiratory failure due to bronchial pulmonary infection, who were capable of self care in the past year | |
| Interventions | Participants were ventilated with AC (4 to 12 hours) and subsequently with SIMV/PS. Ventiltor rate was gradually decreased to 10 to 12 breaths/min with 10 to 12 cm H2O PS. When the PIC window appeared, participants were randomly assigned to NPPV (BiPAP) or IPPV (continued SIMV/PS). Nonivasive PS (with PEEP of 4 to 6 cm H2O) was adjusted to RR < 28 breaths/min, PaO2 65 to 90 mm Hg and PaCO2 between 45 and 60 mm Hg or at level before extubation. NPPV was considered weaned when PS was decreased until the difference between IPAP and EPAP was less than or equal to 5 cm H2O and the participant was stable. In the IPPV group, participants were weaned using SIMV + PS. Participants were weaned when the SIMV rate had been decreased to 5 breaths/min, the level of PS was 5 to 7 cm H2O and the participant had remained stable for four hours | |
| Outcomes | 1. Hospital mortality 2. Total duration of MV 3. ICU LOS 4. Hospital LOS 5. VAP 6. Duration of ETMV 7. Reintubation 8. Costs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A prospective randomized controlled trial was conducted in 11 teaching hospitals." Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data apparent |
| Selective reporting (reporting bias) | Low risk | The authors wanted to examine the feasibility and efficacy of early extubation with sequential NPPV and reported clinically important outcomes |
Zheng 2005.
| Methods | RCT
(n = 33) Allocation concealment not described |
|
| Participants | Intubated participants with COPD with severe respiratory failure due to pulmonary infection, who were capable of self care in the past year | |
| Interventions | Once pulmonary infection had been significantly controlled (PIC window appeared), participants were randomly assigned to NPPV versus invasive PS. NPPV was administered in BiPAP mode with a face mask. The criteria for the PIC window were (1) chest x‐ray showed improvement in infectious infiltrates, (2) WBC count was less than 10 × 109/L, (3) sputum was decreased and was less purulent or white, with sputum tenacity decreased (lower than grade II). In the IPPV group, participants were weaned with PS. Inspiratory pressure was decreased gradually to less than or equal to 8 cm H2O to keep RR less than 28 breaths/min, VT approximately 8 mL/kg, SpO2 greater than 90% and PaCO2 between 45 and 60 mm Hg or at baseline levels. If participants were stable for four hours with a spontaneous cough, they were extubated. In the NPPV group, participants were ventilated with a nasal or oral mask using BiPAP. Inspiratory pressure and FiO2 were adjusted to keep RR lower than 28 breaths /min, VT approximately 8 mL/kg, SpO2 greater than 90% and PaCO2 between 45 and 60 mm Hg or at pre‐extubation levels. All participants received 4 to 6 cm H2O PEEP to reduce the work of breathing from intrinsic PEEP. The duration of NPPV was longer than two hours initially, and investigators gradually decreased the duration of NPPV and inspiratory pressure daily until NPPV was required for less than two hours per day and inspiratory pressure was less than 10 cm H2O | |
| Outcomes | 1. Hospital mortality 2. Total duration of MV 3. ICU LOS 4. Hospital LOS 5. VAP 6. Time to PIC window 7. Duration of ETMV | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective, randomized, controlled clinical study. No mention of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No denominators reported in binary outcomes |
| Selective reporting (reporting bias) | Low risk | Clinically important outcomes reported |
Zou 2006.
| Methods | RCT
(n = 76) RICU (one hospital) Allocation concealment not described |
|
| Participants | COPD participants (nasotracheally intubated) with respiratory failure due to pulmonary infection | |
| Interventions | All participants were initially treated with AC + SIMV + PS with 3 to 5 cm H2O PEEP. After the PIC window (1) full consciousness, effective expectoration, stable haemodynamics, (2) more noticeable absorption of patchy infectious infiltrates compared to before, with no merging shadows and (3) two or more of the following: (a) temperature lower than 38.0°C, (b) peripheral WBC lower than 10.0 × 109/L or percent neutrophils lower than 78.0% and (c) noticeable decrease in the amount of phlegm, the colour of which had turned white or had become lighter and thickness decreased to below grade II) had been reached, participants were randomly assigned to NPPV or IPPV Whereas NPPV was applied with a face or nasal mask in pressure (ST mode), SIMV with PS was used in the IPPV group. IPAP and EPAP were titrated to respiratory condition, arterial gases, RR < 25 to 28 breaths/min, SpO2 > 90 and PaCO2 between 45 and 60 or at baseline. All participants were kept on noninvasive mechanical ventilation for longer than two hours during the initial application. Noninvasive time was gradually decreased and IPAP was gradually decreased until NPPV was stopped when BiPAP time was less than two hours each day and IPAP level was less than 10 cm H2O. Invasive PS was gradually reduced to less than or equal to 10 cm H2O with FiO2 less than or equal to 50% titrated to the same parameters and to a tidal volume of 8 mL/kg. IPPV was stopped when conditions were stable for longer than four hours and participants were able to swallow and expectorate spontaneously |
|
| Outcomes | 1. Inpatient mortality 2. Overall MV time 3. ICU LOS 4. Hospital LOS 5. VAP 6. Duration of ETMV 7. Reintubation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Digital table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No denominators reported in binary outcomes |
| Selective reporting (reporting bias) | Low risk | Clinically important outcomes reported |
RCT: randomized controlled trial; COPD: chronic obstructive pulmonary disease; b/min: breaths per minute; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; RR: respiratory rate; ARF: acute respiratory failure; MV: mechanical ventilation; AC: assist control; PS: pressure support; PAV: proportional assist ventilation; SIMV: synchronized intermittent mandatory ventilation; PEEP: positive end‐expiratory pressure; NPPV: noninvasive positive‐pressure ventilation; IPPV: invasive positive‐pressure ventilation; IPAP: inspiratory positive airway pressure; EPAP: expiratory positive airway pressure; VPAP: ventilator (delivered) positive airway pressure; SB: spontaneous breathing; SBT: spontaneous breathing trial; ST: spontaneous timed; T: timed mode; LOS: length of stay; VAP: ventilator‐associated pneumonia; ETMV: endotracheal mechanical ventilation; ICU: intensive care unit; PIC: pulmonary infection control window; RICU: respiratory intensive care unit; MICU: medical intensive care unit; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; SpO2: pulse oximetry oxygen saturation; FiO2: fractional concentration of inspired oxygen.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Celebi 2008 | This study randomly assigned postoperatively 100 participants who had undergone coronary artery bypass surgery to the following four groups: (1) recruitment maneuver (RM) with sustained inflation (n = 25), (2) RM combined with NPPV applied for 30‐minute periods every six hours on the first postoperative day after tracheal extubation (n = 25), (3) NPPV after tracheal extubation (n = 25) and (4) a control group that received neither RM or NPPV (n = 25). The authors reported outcomes that included pulmonary function tests, oxygenation index and atelectasis on chest radiograph. This study was excluded because all participants were extubated within six hours of intervention, and because it did not report clinically important outcomes |
| Du 2009 | This trial of 32 elderly participants with ARDS randomly assigned participants to oronasal CPAP or SIMV + PS when the "ARDS control window" appeared. We excluded this trial because it compared extubation versus CPAP with no inspiratory assist. We did not consider CPAP to be a weaning modality |
| Duan 2012 | This randomized trial compared noninvasive weaning (using a face mask) versus continued invasive weaning in 32 exclusively tracheostomized participants. We excluded this trial because (1) tracheostomy was an outcome of our review, (2) this study included a high proportion of participants undergoing prolonged mechanical ventilation and (3) interventions were applied in a different manner in the setting of a tracheostomy. For example, participants randomly assigned to noninvasive weaning could have met criteria to return to invasive ventilation per tracheostomy and subsequently could have been returned to noninvasive ventilation. Similarly, participants randomly assigned to invasive weaning could have undergone a series of SBTs before extubation |
| Gao Smith 2006 | This trial randomly assigned participants who failed a spontaneous breathing trial to standard treatment (placed back on supported breaths) and compared these participants with those who were extubated and placed on noninvasive ventilation. NPPV was initially set at the same support settings as the ventilator and was reduced accordingly by nursing staff. Conventionally ventilated participants underwent daily spontaneous breathing trials. The trial was aborted after approximately eight participants had been enrolled |
| Ishikawa 1997 | This nonrandomized study assessed the role of BIPAP in the management of respiratory failure after cardiovascular surgery. Twenty participants who required respiratory support for longer than 72 hours were studied. BiPAP (n = 8) was compared with unassisted oxygen treatment (n = 12) in the control group. Outcomes reported included respiratory index, alveolar arterial oxygen difference and shunt fraction. This study was excluded as it was not an RCT. In addition, NPPV was not used to facilitate weaning, and physiological end points alone were reported |
| Jiang 1999 | This RCT evaluated the effects of early application of NPPV on extubation outcome in 93 participants after elective (n = 56) or unplanned (n = 37) extubation. After extubation, participants were randomly assigned to receive NPPV or oxygen therapy. This study did not assess the role of NPPV as a weaning modality |
| Kilic 2008 | In this study, 60 participants, after cardiac surgery, were randomly assigned to PS‐CPAP or bilevel positive airway pressure, both administered invasively, with bilevel positive airway pressure continued after extubation. Outcomes included blood gases and haemodynamics on arrival to the ICU and one, two, four, six, eight and 12 hours later. This study was excluded, as both treatment groups were predominantly weaned invasively, participants were ventilated for less than 24 hours and the outcomes reported were physiological |
| Kruger 1998 | This RCT evaluated 572 participants who underwent median sternotomy and hypothermic cardiac arrest for cardiopulmonary bypass. Participants were randomly assigned to receive BiPAP (n = 280) or SIMV with PS (n = 292). Outcomes reported included duration of intubation (reported in hours), proportion of participants extubated within six hours, requirement for postoperative analgesia and reintubation rate. This study did not assess the role of NPPV as a weaning strategy in postoperative participants with respiratory failure |
| Luo 2001 | This RCT evaluated 32 participants with COPD requiring intubation for hypercapneic respiratory failure. Participants were randomly assigned to receive BiPAP (n = 19) or conventional therapy (n = 13). Reported outcomes included gas exchange at 45 minutes and 12 hours after extubation and rates of reintubation. This study assessed the role of NPPV not as a weaning strategy, but rather as an aid in transitioning participants to spontaneous breathing. Moreover, in this study, the comparator group was not mechanically ventilated |
| Matic 2007 | This RCT compared NPPV and IPPV as early treatment strategies in COPD participants requiring more than 24 hours of MV. The goals of the study were to compare (1) the influence of physiological parameters on the choice of mechanical ventilation strategy in the treatment of COPD and (2) outcomes using the alternative mechanical ventilation strategies. This study evaluated NPPV and IPPV as initial approaches to mechanical ventilation. |
| Nava 2011 | In this RCT, 82 participants were randomly assigned to receive NPPV plus standard medical therapy or standard medical therapy (SMT). Outcomes included rate of meeting endotracheal intubation (ETI) criteria, mortality rate, respiratory rate, dyspnoea score and arterial blood gases. This study evaluated the role of NPPV in reducing the rate of ETI criteria, not as a weaning strategy |
| Radojevic 1997 | In this prospective, randomized study, participants received BIPAP or PS in the early postoperative period after undergoing aortocoronary bypass surgery (seven hours plus or minus one hour). Criteria for eligibility included an awake participant with neuromuscular activity. The population studied represents a cohort of participants in the post–acute care setting that did not require formal weaning |
| Rong 2012 | Participants were not consistently randomly assigned |
| Rosinha 2002 | This prospective RCT allocated participants requiring MV for longer than 72 hours to receive NPPV or supplemental oxygen, by mask, after achieving criteria for extubation. Proportion of successful extubations, length of ICU stay and hospital mortality were reported. This study did not assess the role of NPPV as a weaning strategy, as the comparative group received unassisted oxygen alone |
| Vargas 2012 | This multicentre trial, involving 144 participants, randomly assigned participants to receive NPPV for 48 hours after planned extubation or conventional oxygen treatment. This trial did not assess the role of NPPV in weaning participants from mechanical ventilation, and the comparator group received supplemental oxygen alone |
| Venkatram 2010 | This study was a retrospective study that compared participants managed with NPPV (n = 110) and those managed through invasive mechanical ventilation (n = 156). Duration of ventilatory support, hospital and ICU mortality and NPPV failure rate were reported. The study was not randomized |
| Wang 2000 | This study compared early extubation and sequential NPPV application versus continued invasive ventilation in 11 participants with exacerbations of COPD due to pulmonary infection. The intervention group was compared with a cohort of 11 participants who continuously received invasive MV after control of pulmonary infection had been achieved. This was not an RCT |
| Wang 2003 | This study represents a duplicate publication of Wang 2000 |
| Yang 2009 | This study compared standard treatment versus standard treatment with NPPV and did not include invasively ventilated participants |
| Zheng 2011 | This study was a prospective cohort study that included 20 invasively ventilated COPD participants with respiratory failure. Reported outcomes included ventilation and oxygenation index, duration of ETMV, total duration of mechanical ventilation, hospital LOS and rates of reintubation and VAP. This study was excluded, as it was not randomized |
BiPAP: bilevel positive airway pressure; RCT: randomized controlled trial; NPPV: noninvasive positive‐pressure ventilation; SIMV: synchronized intermittent mandatory ventilation; PS: pressure support; ARF: acute respiratory failure; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; MV: mechanical ventilation.
Characteristics of ongoing studies [ordered by study ID]
Perkins 2013.
| Trial name or title | Protocolized trial of invasive and noninvasive weaning off ventilation: the BREATHE trial |
| Methods | RCT (n = 920) http://www.warwick.ac.uk/breathe Allocation concealment not described |
| Participants | Participants with respiratory failure who received invasive ventilation for longer than 48 hours (from the time of intubation) and who failed a spontaneous breathing test (SBT) Inclusion criteria: 1. Male and female participants, age > 16 years 2. Participants with respiratory failure who had received invasive ventilation for longer than 48 hours (from intubation) 3. Failure of an SBT 4. Provision of written informed consent Exclusion criteria: 1. Presence of a tracheostomy 2. Profound neurological deficits 3. Any absolute contraindication to NIV 4. Home ventilation before ICU admission 5. Decision not to reintubate or withdrawal of care 6. Further surgery/procedure requiring sedation planned in the next 48 hours 7. Previous participation in the trial |
| Interventions | Invasive versus noninvasive weaning Protocolized invasive weaning arm: The participant will be restarted on PS ventilation at the previous settings. The level of PS will be titrated to achieve comfort and RR < 30 breaths/min. Causes for distress/fatigue/weaning failure will be sought and corrective treatments initiated as appropriate. The participant will be reassessed every two hours. If no signs of distress are noted, the level of PS will be reduced by 2 cm H2O. If at any stage the participant develops distress/fatigue, the PS will be increased by 2 cm H2O. FiO2 will be titrated to maintain SaO2 > 90%. A further SBT will take place each morning. This cycle will continue until the participant has been extubated (passing an SBT or tolerating PS 5 cm H2O) or a tracheostomy has been performed Protocolized noninvasive weaning arm: Participants will be extubated and immediately provided with NIV with an equivalent level of PS and PEEP to the ventilator settings before extubation. After two hours, if no signs of distress/fatigue are observed, the NIV interface will be removed and the participant will undergo a self‐ventilation trial with supplemental oxygen (equivalent to the previous FiO2) via a standard oxygen mask. If no signs of distress or fatigue develop during the self ventilation trial, the participant will continue to receive unsupported ventilation, with inhaled oxygen provided as required. If the participant subsequently develops signs of distress or fatigue, NIV will be restarted (as below). Otherwise, the participant will continue with unsupported self ventilation. FiO2 will be titrated to maintain SaO2 > 90%. If signs of distress or fatigue develop, NIV will be reinstated at the previous settings. The level of PS will be titrated to achieve participant comfort and a RR < 30 breaths/min. Causes for distress/fatigue/weaning failure will be sought and corrective treatments initiated as appropriate. The participant will be reassessed every two hours. If no signs of distress/fatigue are noted, a further trial of self ventilation will be commenced as described above. NIV will be withdrawn when the participant tolerates 12 hours of unsupported spontaneous ventilation In both groups, the active weaning protocol will occur between 8 am and 10 pm. Unless participants develop signs of fatigue or distress, ventilator settings will not be adjusted overnight |
| Outcomes | Primary: time from randomization to liberation from ventilation Secondary Efficacy: 1. Mortality at 30, 90 and 180 days 2. Duration of invasive mechanical ventilation and total ventilator‐free days (invasive and noninvasive ventilation) 3. Time to meeting ICU discharge criteria (defined as no further requirement for level 2/3 care) 4. Proportion of participants receiving antibiotics for presumed respiratory infection and total antibiotic days 5. Reintubation rates (protocolized end point and actual events) 6. Tracheostomy Safety 1. Adverse events 2. Serious adverse events Patient‐focused outcomes Health‐related quality of life, EuroQol, EQ‐5D and SF12 at baseline (estimated) and at three and six months |
| Starting date | 1 January 2013 |
| Contact information | Mrs Beverley Hoddell, Clinical Trials Unit, Warwick Medical School, Gibbet Hill Road, Coventry, UK b.hoddell@warwick.ac.uk |
| Notes |
Smith 2013.
| Trial name or title | NEXT: Comparison of noninvasive positive pressure ventilation for extubated patients who fail a single spontaneous breathing trial versus conventional weaning |
| Methods | RCT (n = 8) Stopped early because of the need to fulfil clinical requirements at another hospital |
| Participants | Inclusion criteria: 1. Participants will have to meet the criteria for reducing breathing support—will not be weaned until physiologically ready 2. Participants will have to be on a breathing machine attached to a tube in the mouth for at least 48 hours (participants who are on a breathing machine for < 48 hours are not seen as difficult to wean from a ventilator) 3. Age > 18 years—participant should be able to make own legal judgements regarding treatment 4. Written informed consent obtained 5. Failed an attempt to try breathing without help Exclusion criteria: 1. Participants are generally not suitable for NIV (grade 3/4 intubation) 2. Gastric/oesophageal surgery on this admission 3. Participants who would not be ready for reintubation once extubated (by investigator decision????) |
| Interventions | Noninvasive positive‐pressure ventilation versus conventional weaning |
| Outcomes | Primary outcome: duration of time with breathing support tube in the mouth in days Secondary outcomes: length of stay in the intensive care unit and hospital stay in days |
| Starting date | 13 March 2006 |
| Contact information | Dr Fang Gao Department of Anaesthetics, Birmingham Heartlands Hospital, Heart of England NHS Foundation Trust, Bordelsey Green East, Birmingham, UK |
| Notes |
MV: mechanical ventilation; GCS: Glasgow Coma Scale; PaO2/FiO2: ratio of arterial partial pressure of oxygen to fractional concentration of inspired oxygen; PEEP: positive end‐expiratory pressure; SBT: spontaneous breathing trial; PS: pressure support; NPPV: noninvasive positive‐pressure ventilation; VAP: ventilator‐associated pneumonia; ICU: intensive care unit; LOS: length of stay.
Contributions of authors
Karen Burns (KB): proposed the research question and title; designed the protocol; reviewed and retrieved relevant articles; assessed methodologic quality of retrieved articles; abstracted data as the primary review author; entered data; conducted the statistical analysis and prepared the final review.
Neill Adhikari (NA): reviewed and retrieved relevant articles; assessed methodologic quality of retrieved articles; abstracted data as the second review author and revised the final review for important intellectual content.
Azra Premji (AP): assisted in retrieving articles and updating the text of the review. Also revised the final review for methodologic quality and scientific integrity.
Maureen Meade (MM): adjudicated disagreements; supervised the methodologic integrity of the review; reviewed the manuscript for methodologic and scientific integrity.
Sources of support
Internal sources
New source of support, Other.
External sources
Dr Burns is the recipient of a Canadian Institutes of Health Research Clinician Scientist Phase 2 Award, Canada.
Declarations of interest
Karen EA Burns: none known.
Maureen O Meade: Dr Meade has received in‐kind support from industry in the form of equipment loans for use in the context of a multicentre clinical trial.
Azra Premji: none known.
Neill KJ Adhikari: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Chen 2001 {published data only}
- Chen J, Qiu D, Tao D. Time for extubation and sequential noninvasive mechanical ventilation in COPD patients with acute exacerbated respiratory failure who received invasive ventilation. Zhongua Jie He He Hu Xi Za Zhi 2001;24:99‐100. [MEDLINE: ] [PubMed] [Google Scholar]
Ferrer 2003 {published data only}
- Ferrer M, Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, et al. Noninvasive ventilation during persistent weaning failure. American Journal of Respiratory and Critical Care Medicine 2003;168:70‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girault 1999 {published data only}
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute‐on‐chronic respiratory failure: a prospective, randomized controlled study. American Journal of Respiratory and Critical Care Medicine 1999;160:86‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girault 2011 {published and unpublished data}
- Girault C, Bubenheim M, Abroug F, Diehl JL, Elatrous S, Beuret P, et al. Noninvasive ventilation and weaning in patients with chronic hypercapnic respiratory failure. American Journal of Respiratory and Critical Care Medicine 2011;184:672‐9. [www.clinicaltrials.gov (NCT 00213499)] [DOI] [PubMed] [Google Scholar]
Hill 2000 {published data only}
- Hill NS, Lin D, Levy M, O'Brien A, Klinger J, Houtchens J, et al. Noninvasive positive pressure ventilation (NPPV) to facilitate extubation after acute respiratory failure: a feasibility study. American Journal of Respiratory and Critical Care Medicine 2000;161:B18. [Google Scholar]
Nava 1998a {published data only}
- Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease: a randomized, controlled trial. Annals of Internal Medicine 1998;128:721‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasad 2009 {published and unpublished data}
- Prasad S, Chaudhry D, Khanna R. Role of NIV in weaning from mechanical ventilation in patients of chronic obstructive pulmonary disease—An Indian experience. Indian Journal of Critical Care Medicine 2009;13(4):207‐12. [DOI: 10.4103/0972-5229.60173] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabie 2004 {published data only}
- Rabie GM, Mohamed AZ, Mohamed RN. Noninvasive ventilation in the weaning of patients with acute‐on‐chronic respiratory failure due to COPD. Chest 2004; Vol. 126(Suppl 4):755.
Rabie Agmy 2012 {published data only}
- Rabie Agmy GM, Metwally MM. Noninvasive ventilation in the weaning of patients with acute‐on‐chronic respiratory failure due to COPD. Egyptian Journal of Chest Diseases and Tuberculosis 2012;61(1):84‐91. [Google Scholar]
Tawfeek 2012 {published data only}
- Tawfeek MM, Ali‐Elnabtity AM. Noninvasive proportional assist ventilation may be useful in weaning patients who failed a spontaneous breathing trial. Egyptian Journal of Anaesthesia 2012;28:89‐94. [Google Scholar]
Trevisan 2008 {published data only}
- Trevisan CE, Viera SR, and the Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Critical Care 2008;12:136. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaschetto 2012 {published data only}
- Vaschetto R, Turucz E, Dellapiazza F, Guido S, Colombo D, Cammarota G, et al. Noninvasive ventilation after early extubation in patients recovering from hypoxemic acute respiratory failure: a single‐centre feasibility study. Intensive Care Medicine 2012;38(10):1599‐606. [PUBMED: 22825283 ] [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang X, Du X, Zhang W. Observation of the results and discussion on the timing of transition form invasive mechanical ventilation to noninvasive ventilation in COPD patients with concomitant acute respiratory failure. Shandong Medicine 2004;44(7):4‐6. [Google Scholar]
Wang 2005 {published data only}
- Wang C, Zhang Q, Cao Z, Wei L, Cheng Z, Liu S, et al. Collaborating Research Group for Noninvasive Mechanical Ventilation of the Chinese Respiratory Society. Pulmonary infection control window in the treatment of severe respiratory failure of chronic obstructive pulmonary diseases: a prospective, randomized controlled, multi‐centre study. Chinese Medical Journal 2005;118(19):1589‐94. [PubMed] [Google Scholar]
Zheng 2005 {published data only}
- Zheng R, Liu L, Yang Y. Prospective randomized controlled clinical study of sequential non‐invasive following invasive mechanical ventilation in patients with acute respiratory failure induced COPD. Chinese Journal of Emergency Medicine 2005;14(1):21‐5. [Google Scholar]
Zou 2006 {published data only}
- Zou S, Zhou R, Chen P, Luo H, Xiang X, Lu Y, Zhu L. Application of sequential noninvasive following invasive mechanical ventilation in COPD patients with severe respiratory failure by investigating the appearance of pulmonary‐infection‐control‐window. Journal of Central Southern University 2006;31(1):120‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Celebi 2008 {published data only}
- Celebi S, Koner O, Menda F, Omay O, Gunay I, Suzer K, Cakar N. Pulmonary effects of noninvasive ventilation combined with the recruitment maneuver after cardiac surgery. International Anesthesia Research Society 2008;107(2):614‐9. [DOI] [PubMed] [Google Scholar]
Du 2009 {published data only}
- Du LL, Han H, Zhang XJ, Wei L. Randomized control study of sequential non‐invasive following short‐term invasive ventilation in the treatment of acute respiratory distress syndrome as a result of existing pulmonary disease in elderly patients. Chinese Critical Care Medicine 2009;21(7):394‐6. [PUBMED: 19615128 ] [PubMed] [Google Scholar]
Duan 2012 {published data only}
- Duan J, Guo S, Han X, Tang X, Xu L, Xu X, et al. Dual‐mode weaning strategy for difficult‐weaningtracheotomy patients: a feasibility study. Anesthesia and Analagesia 2012;115(3):597‐604. [PUBMED: 22696608] [DOI] [PubMed] [Google Scholar]
Gao Smith 2006 {unpublished data only}
- Gao Smith F, Sutton P. Comparison of noninvasive positive pressure ventilation for extubated patients who fail a single spontaneous breathing trial vs conventional weaning. www.controlled‐trials.com (accessed 28 October 2013). [DOI: 10.1186/ISRCTN32810409; ISRCTN32810409 ] [DOI]
Ishikawa 1997 {published data only}
- Ishikawa S, Ohtaki A, Takahashi T, Koyano T, Hasegawa Y, Ohki S, et al. Noninvasive nasal mask BiPAP management for prolonged respiratory failure following cardiovascular surgery. Journal of Cardiac Surgery 1997;12:176‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jiang 1999 {published data only}
- Jiang JS, Kao SJ, Wang SN. Effect of early application of biphasic positive airway pressure on the outcome of extubation in ventilator weaning. Respirology 1999;4:161‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kilic 2008 {published data only}
- Kilic A, Yapici N, Bicer Y, Coruh T, Aykac Z. Early extubation and weaning with bilevel positive airway pressure ventilation after cardiac surgery (weaning with BIPAP ventilation after cardiac surgery). Southern African Journal of Anesthesia and Analgesia 2008;14(5):25‐31. [Google Scholar]
Kruger 1998 {published data only}
- Kruger M, Walther T, Falk V, Rauch T, Schmitt DV, Autschbach R, et al. Prospective randomization of pressure‐controlled (BiPAP) and volume‐controlled (SIMV) ventilation after cardiac surgery. Intensive Care Medicine 1998;24(Suppl):5. [Google Scholar]
Luo 2001 {published data only}
- Luo H, Cheng P, Zhou R. Sequential BiPAP following invasive mechanical ventilation in COPD patients with hypercapneic respiratory failure. Bulletin of Hunan Medical University 2001;26:563‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Matic 2007 {published data only}
- Matic I, Sakic‐Zdravcevic K, Jurjevic M. Comparison of invasive and noninvasive mechanical ventilation for patients with chronic obstructive pulmonary disease: randomized prospective study. Periodicum Biologorum 2007;109(2):137‐45. [Google Scholar]
Nava 2011 {published data only}
- Nava S, Grassi M, Fanfulla F, Domenighetti G, Carlucci A, Perren A, et al. Non‐invasive ventilation in elderly patients with acute hypercapnic respiratory failure: a randomized controlled trial. Age and Ageing 2011;40:444‐50. [DOI] [PubMed] [Google Scholar]
Radojevic 1997 {published data only}
- Radojevic D, Vuk LJ, Radomir B, Jovic M, Bojic M. PIPAP and PSV in the weaning of cardiosurgical patients from mechanical ventilation. British Journal of Anaesthesia 1997;78(Suppl 1):A161. [Google Scholar]
Rong 2012 {published data only (unpublished sought but not used)}
- Rong F. Application of treating chronic obstructive pulmonary disease patients with respiratory failure with the sequential noninvasive and invasive ventilation. Journal of Bengbu Medical College 2012;37(4):442‐4. [Google Scholar]
Rosinha 2002 {published data only}
- Rosinha SRPOR, Lobo SMA, Sanches HS, Deraldini M, Vidal AMA, Tofoli LT, et al. The use of noninvasive ventilation after weaning of acute respiratory failure prevents reintubation and decreases hospital mortality. American Journal of Respiratory and Critical Care Medicine 2002;165(8):A686. [Google Scholar]
Vargas 2012 {published data only}
- Vargas F, Clavel M, Sanchez P, Garnier S, Boyer A, Bui NH, et al. Sequential and early use of noninvasive ventilation after extubation in patients with chronic respiratory disorders. Americal Journal of Respiratory and Critical Care Medicine 2012;185:A6487. [Google Scholar]
Venkatram 2010 {published data only}
- Venkatram S, Rachmale S, Kanna B, Soni, A. Non‐invasive positive pressure ventilation compared to invasive mechanical ventilation among patients with COPD exacerbations in an inner city MICU‐ Predictors of NPPV use. The Internet Journal of Pulmonary Medicine 2010;12(1). [DOI: 10.5580/284f; ISSN: 1531‐2984] [DOI] [Google Scholar]
Wang 2000 {published data only}
- Wang C, Shang M, Huang K, et al. Sequential non‐invasive following short‐term invasive mechanical ventilation in COPD induced hypercapneic respiratory failure. Chinese Journal of Tuberculosis and Respiratory Diseases 2000;23(4):212‐6. [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang C, Shang M, Huang K, Tong Z, Kong W, Jiang C, et al. Sequential non‐invasive mechanical ventilation following short‐term invasive mechanical ventilation in COPD induced hypercapneic respiratory failure. Chinese Medical Journal 2003;116(1):39‐43. [PubMed] [Google Scholar]
Yang 2009 {published data only}
- Yang SY, Shen JL, Feng EZ. Study on the indication and time of the use of non‐invasive positive pressure ventilation in the patients with acute exacerbation of chronic cor pulmonale combined with type II respiratory failure at high altitude area. Chinese Critical Care Medicine 2009;21(7):440‐1. [ISSN: CN‐00742932] [PubMed] [Google Scholar]
Zheng 2011 {published data only}
- Zheng D, Wang C, Liu R, Gao F, Deng S, Zhou P, et al. The application of improved Glasgow coma scale score of 15 as switching point for invasive‐noninvasive mechanical ventilation in treatment of severe respiratory failure in chronic obstructive pulmonary disease. Chinese Critical Care Medicine 2011;23:224‐7. [PubMed] [Google Scholar]
References to ongoing studies
Perkins 2013 {unpublished data only}
- Protocolized trial of invasive and noninvasive weaning off ventilation: the BREATHE trial. Ongoing study 1 January 2013.
Smith 2013 {unpublished data only}
- NEXT: Comparison of noninvasive positive pressure ventilation for extubated patients who fail a single spontaneous breathing trial versus conventional weaning. Ongoing study 13 March 2006.
Additional references
Antonelli 1998
- Antonelli M, Conti G, Rocco M, Bufi M, DeBlasi RA, Vivino G, Gasparetto A, et al. A comparison of noninvasive positive‐pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. New England Journal of Medicine 1998;339:429‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Appendini 1994
- Appendini L, Patessio A, Zanaboni S, Carone M, Gukov B, Donner CF, et al. Physiological effects of positive end expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1994;149:1069‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borenstein 2008
- Borenstein N, Hedge LV, Higgins JPT, Rothstein HR. Introduction to Meta‐analysis. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Brochard 1994
- Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1994;150:896‐903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brook 1999
- Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, et al. Effect of a nursing‐implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine 1999;27:2609‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Butler 1999
- Butler R, Keenan SP, Inman KJ, Sibbald WJ, Block G. Is there a preferred technique for weaning the difficult‐to‐wean patient? A systematic review of the literature. Critical Care Medicine 1999;27:2331‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cochran 1954
- Cochran W. The combination of estimates from different experiments. Biometrics 1954;10:101‐29. [Google Scholar]
Criner 1994
- Criner GJ, Tzouanakis A, Kreimer DT. Overview of improving tolerance of long‐term mechanical ventilation. Critical Care Clinics 1994;10:845‐66. [MEDLINE: ] [PubMed] [Google Scholar]
Devereaux 2004
- Devereaux PJ, Yusuf S, Yang H, Choi P, Guyatt G. Are the recommendations to use perioperative beta‐blocker therapy in patients undergoing noncardiac surgery based on reliable evidence?. CMAJ 2004;171(3):245‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 1996
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. New England Journal of Medicine 1996;335:1864‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ely 2001
- Ely EW, Meade MO, Haponik EF, Kollef MH, Cook DJ, Guyatt GH, et al. Mechanical ventilator weaning protocols driven by nonphysician health‐care professionals. Evidence‐based clinical practice guidelines. Chest 2001;120 Suppl:454‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Epstein 1997
- Epstein SK, Ciabotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997;112(1):186‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esen 1992
- Esen F, Denkel T, Telci L, Kesecioglu J, Tutunca AS, Akpir K, et al. Comparison of PSV and IMV during weaning in patients with acute respiratory failure. Advances in Experimental Medicine and Biology 1992;317:371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1995
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Vallverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. New England Journal of Medicine 1995;332:345‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1997
- Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, et al. Extubation outcome after spontaneous breathing trials with T‐tube or pressure support ventilation. American Journal of Respiratory and Critical Care Medicine 1997;156:459‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1999
- Esteban A, Alia I, Tobin MJ, Gil A, Gordo F, Vallverdu I, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1999;159:512‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fleiss 1986
- Fleiss JL. Analysis of data from multicentric studies. Controlled Clinical Trials 1986;7:267‐75. [DOI] [PubMed] [Google Scholar]
Goodenberger 1992
- Goodenberger DM, Couser JI, May JJ. Successful discontinuation of ventilation via tracheostomy by substitution of nasal positive pressure ventilation. Chest 1992;102:1277‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gregoretti 1998
- Gregoretti C, Beltrame F, Lucangelo U, Burbi L, Conti G, Turello M, et al. Physiologic evaluation of noninvasive pressure support ventilation in trauma patients with acute respiratory failure. Intensive Care Medicine 1998;24:785‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heyland 1999
- Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun‐Buisson C. The attributable morbidity and mortality of ventilator associated pneumonia in the critically ill patient. The Canadian Critical Care Trials Group. American Journal of Respiratory and Critical Care Medicine 1999;159:1249‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Jounieaux 1994
- Jounieaux V, Duran A, Levi‐Valensi P. Synchronized intermittent mandatory ventilation with and without pressure support ventilation in weaning patients with COPD from mechanical ventilation. Chest 1994;105:1204‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keenan 2003
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbations of COPD benefit from noninvasive positive‐pressure ventilation? A systematic review. Annals of Internal Medicine 2003;138:861‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keenan 2004
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxaemic respiratory failure? A systematic review. Critical Care Medicine 2004;Dec;32(12):2516‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keenan 2011
- Keenan SP, Sinuff T, Burns KEA, Muscedere J, Kutsogiannis J, Mehta S, et al. as the Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group. Clinical practice guidelines for the use of noninvasive positive‐pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. Canadian Medical Association Journal 2011;183(3):E195‐214. [PUBMED: 21324867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilger 1999
- Kilger E, Briegel J, Haller M, Frey L, Schelling G, Stoll C, et al. Effects of noninvasive positive pressure ventilatory support in non‐COPD patients with acute respiratory insufficiency after early extubation. Intensive Care Medicine 1999;25:1374‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kollef 1997
- Kollef MH, Shapiro SD, Silver P, St. John RE, Prentice D, Sauer S, et al. A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation. Critical Care Medicine 1997;25:567‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacIntyre 2001
- MacIntyre NR, Cook DJ, Ely EW, Epstein SK, Fink JB, Heffner JE, et al. Evidence‐based guidelines for weaning and discontinuing ventilatory support. A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001;6 Suppl:375‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marelich 2000
- Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses. Effect on weaning time and incidence of pneumonia. Chest 2000;118:459‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meade 2001
- Meade M, Guyatt G, Sinuff T, Griffith L, Hand L, Toprani G, et al. Trials comparing alternative weaning modes and discontinuation assessments. Chest 2001;120 Suppl:425‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 2001
- Mehta S, Hill NS. Noninvasive ventilation. American Journal of Respiratory and Critical Care Medicine 2001;163:540‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nava 1993
- Nava S, Ambrosino N, Rubini F, Fracchia C, Rampulla C, Torri G, et al. Effect of nasal pressure support ventilation and external positive end expiratory pressure on diaphragmatic function in patients with severe stable COPD. Chest 1993;103:143‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niederman 1984
- Niederman MS, Ferranti RD, Ziegler A, Merrill W, Reynolds HY. Respiratory infection complicating long‐term tracheostomy: the implication of persistent gram‐negative tracheobronchial colonization. Chest 1984;85:39‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nourdine 1999
- Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela C, Ducreux JC. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Medicine 1999;25:567‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pawar 2003
- Pawar M, Mehta Y, Khurana P, Chaudhary A, Kulkami V, Trehan N. Ventilator associated pneumonia: incidence, risk factors, outcome and microbiology. Journal of Cardiothoracic Anaesthesia 2003;17(1):22‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perren 2002
- Perren A, Domenighetti G, Mauri S, Genini F, Vizzardi N. Protocol‐directed weaning from mechanical ventilation; clinical outcome in patients randomized for a 30‐minute or 120‐minute trial with pressure support ventilation. Intensive Care Medicine 2002;28:1058‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peter 2002
- Peter JV, Moran JL, Phillips‐Hughes J, Warn D. Noninvasive ventilation in acute respiratory failure: a meta‐analysis update. Critical Care Medicine 2002;30:555‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pingleton 1988
- Pingleton SK. Complications of acute respiratory failure. American Review of Respiratory Diseases 1988;137:1463‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rathgeber 1997
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mechanical ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis of 596 patients following adult cardiac surgery. European Journal of Anaesthesiology 1997;14:576‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Restrick 1993
- Restrick LJ, Scott AD, Ward EM, Fenech RO, Cornwell WE, Wedjicha JA. Nasal intermittent positive‐pressure ventilation in weaning intubated patients with chronic respiratory disease from assisted intermittent positive pressure ventilation. Respiratory Medicine 1993;87:199‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan) [[computer program] Version 5.1.6]. The Nordic Cochrane Centre, 2011.
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis‐‐a simulation study. PLoS One 2011;6(10):e25491. [PUBMED: 22028777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomlinson 1989
- Tomlinson JR, Miller KS, Lorch DG, Smith L, Reines HD, Sahn SA. A prospective comparison of IMV and T‐piece weaning from mechanical ventilation. Chest 1989;96:348‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Udwadia 1992
- Udwadia ZF, Santis GK, Steven MH, Simonds AK. Nasal ventilation to facilitate weaning in patients with chronic respiratory insufficiency. Thorax 1992;47:715‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitacca 2001
- Vitacca M, Ambrosino N, Clini E, Porta R, Rampulla C, Lanini B, et al. Physiological response to pressure support ventilation delivered before and after extubation in patients not capable of totally spontaneous autonomous breathing. American Journal of Respiratory and Critical Care Medicine 2001;164:638‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Burns 2003
- Burns KEA, Adhikari NKJ, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004127] [DOI] [PubMed] [Google Scholar]
Burns 2009
- Burns KEA, Adhikari NKJ, Keenan SP, Meade MO. Use of non‐invasive ventilation to wean critically ill adults off invasive ventilation: meta‐analysis and systematic review. BMJ 2009;338:b1574. [DOI: 10.1136/bmj.b1574.; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2010
- Burns KEA, Adhikari NKJ, Keenan SP, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD004127.pub2] [DOI] [PubMed] [Google Scholar]


