Abstract
Background
Automated systems use closed‐loop control to enable ventilators to perform basic and advanced functions while supporting respiration. SmartCare™ is a unique automated weaning system that measures selected respiratory variables, adapts ventilator output to individual patient needs by operationalizing predetermined algorithms and automatically conducts spontaneous breathing trials (SBTs) when predetermined thresholds are met.
Objectives
The primary objective of this review was to compare weaning time (time from randomization to extubation as defined by study authors) between invasively ventilated critically ill adults weaned by automated weaning and SBT systems versus non‐automated weaning strategies.
As secondary objectives, we ascertained differences between effects of alternative weaning strategies on clinical outcomes (time to successful extubation, time to first SBT and first successful SBT, mortality, ventilator‐associated pneumonia, total duration of ventilation, lengths of intensive care unit (ICU) and hospital stay, use of non‐invasive ventilation (NIV), adverse events and clinician acceptance).
The third objective of our review was to use subgroup analyses to explore variations in weaning time, length of ICU stay, mortality, ventilator‐associated pneumonia, use of NIV and reintubation according to (1) the type of clinician primarily involved in implementing the automated weaning and SBT strategy, (2) the ICU (as a reflection of the population involved) and (3) the non‐automated (control) weaning strategy utilized.
We conducted a sensitivity analysis to evaluate variations in weaning time based on (4) the methodological quality (low or unclear versus high risk of bias) of the included studies.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 5; MEDLINE (1966 to 31 May 2013); EMBASE (1988 to 31 May 2013); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 31 May 2013), Evidence‐Based Medicine Reviews and Ovid HealthSTAR (1999 to 31 May 2013), as well as conference proceedings and trial registration websites; we also contacted study authors and content experts to identify potentially eligible trials.
Selection criteria
Randomized and quasi‐randomized trials comparing automated weaning and SBT systems versus non‐automated weaning strategies in intubated adults.
Data collection and analysis
Two review authors independently assessed trial quality and abstracted data according to prespecified criteria. Sensitivity and subgroup analyses were planned to assess the impact on selected outcomes of the following: (1) the type of clinician primarily involved in implementing automated weaning and SBT systems, (2) the ICU (as a reflection of the population involved) and (3) the non‐automated (control) weaning strategy utilized.
Main results
We pooled summary estimates from 10 trials evaluating SmartCare™ involving 654 participants. Overall, eight trials were judged to be at low or unclear risk of bias, and two trials were judged to be at high risk of bias. Compared with non‐automated strategies, SmartCare™ decreased weaning time (mean difference (MD) ‐2.68 days, 95% confidence interval (CI) ‐3.99 to ‐1.37; P value < 0.0001, seven trials, 495 participants, moderate‐quality evidence), time to successful extubation (MD ‐0.99 days, 95% CI ‐1.89 to ‐0.09; P value 0.03, seven trials, 516 participants, low‐quality evidence), length of ICU stay (MD ‐5.70 days, 95% CI ‐10.54 to ‐0.85; P value 0.02, six trials, 499 participants, moderate‐quality evidence) and proportions of participants receiving ventilation for longer than seven and 21 days (risk ratio (RR) 0.44, 95% CI 0.23 to 0.85; P value 0.01 and RR 0.39, 95% CI 0.18 to 0.86; P value 0.02). SmartCare™ reduced the total duration of ventilation (MD ‐1.68 days, 95% CI ‐3.33 to ‐0.03; P value 0.05, seven trials, 521 participants, low‐quality evidence) and the number of participants receiving ventilation for longer than 14 days (RR 0.61, 95% CI 0.37 to 1.00; P value 0.05); however the estimated effects were imprecise. SmartCare™ had no effect on time to first successful SBT, mortality or adverse events, specifically reintubation. Subgroup analysis suggested that trials with protocolized (versus non‐protocolized) control weaning strategies reported significantly shorter ICU stays. Sensitivity analysis excluded two trials with high risk of bias and supported a trend toward significant reductions in weaning time favouring SmartCare™.
Authors' conclusions
Compared with non‐automated weaning strategies, weaning with SmartCare™ significantly decreased weaning time, time to successful extubation, ICU stay and proportions of patients receiving ventilation for longer than seven days and 21 days. It also showed a favourable trend toward fewer patients receiving ventilation for longer than 14 days; however the estimated effect was imprecise. Summary estimates from our review suggest that these benefits may be achieved without increasing the risk of adverse events, especially reintubation; however, the quality of the evidence ranged from low to moderate, and evidence was derived from 10 small randomized controlled trials.
Plain language summary
SmartCare™ versus non‐automated weaning strategies for weaning time in invasively ventilated critically ill adults
The process of discontinuing mechanical ventilation is known as weaning. During weaning, the work of breathing is transferred from the ventilator to the patient. Weaning is typically achieved by clinicians reducing ventilator support and/or conducting tests to determine whether a patient can breathe on his/her own. SmartCare™ is a unique system that automates this process by measuring selected respiratory variables, adapting ventilator output to meet individual patient needs and automatically conducting tests of spontaneous breathing to determine the earliest time when patients can breathe on their own.
We identified 10 trials of moderate quality involving 654 participants and comparing SmartCare™ versus non‐automated weaning strategies. Compared with non‐automated strategies, SmartCare™ significantly decreased weaning time, time to successful removal from breathing machines and time spent in the ICU, with fewer patients receiving breathing machine support for longer than seven days and 21 days, and no increase in adverse events. SmartCare™ also showed a favourable trend toward fewer patients receiving ventilation for longer than 14 days, with no increase in adverse events. Subgroup analyses suggested more beneficial effects on weaning time in trials comparing SmartCare™ to a protocolized weaning strategy versus a non‐protocolized control strategy. Sensitivity analyses, which excluded two trials with high risk of bias, supported significant reductions in weaning time with SmartCare™.
Summary of findings
Background
Invasive ventilation has enabled clinicians to support respiration until the factors precipitating respiratory compromise can be identified and addressed. However, invasive mechanical ventilation is associated with the development of important complications, including ventilator‐associated pneumonia (VAP), sinusitis, upper airway pathology, respiratory muscle weakness, prolonged lengths of intensive care unit (ICU) and hospital stay and mortality (Cook 1998; Dries 1997; Heyland 1999; Mancebo 1996; Niederman 1984; Papazian 1996; Pingleton 1988; Vincent 1995). Consequently, identifying the earliest time for liberation from mechanical ventilation and thereby limiting the duration of invasive ventilation are important goals in providing care to critically ill patients.
Description of the condition
The process of discontinuing mechanical ventilation is known as weaning. Weaning accounts for approximately 40% of the time spent on mechanical ventilation (Esteban 1994; Esteban 2002). Transferring the work of breathing from ventilator to patient may occur abruptly in some patients and gradually in others (Lessard 1996), with approximately 75% of patients resuming the work of breathing without difficulty (Brochard 1994; Esteban 1995). For other patients, however, liberation from invasive ventilation is challenging.
Identifying when patients are ready to be weaned is often arbitrary, with the clinician relying on subjective assessments (Sahn 1973) and objective measurements of various respiratory variables in an effort to identify the optimal time to discontinue mechanical ventilation. Clinicians often underestimate the chance of a patient successfully discontinuing mechanical ventilation (Afessa 1999; Stroetz 1995). Recent literature supports the use of strategies to facilitate timely discontinuation of mechanical support, including early identification of weaning candidates (Ely 1996; Kollef 1997; Marelich 2000), conduct of spontaneous breathing trials (SBTs) (Esteban 1997; Esteban 1999; Perren 2002) and use of specific modes to reduce support in patients who fail an SBT (Brochard 1994; Esen 1992; Esteban 1995). Despite large‐scale implementation, many barriers to implementing weaning protocols in clinical practice are known, including the requirement for broad, educational interventions and multi‐disciplinary compliance with them (Ely 1999; Vitacca 2000).
Description of the intervention
Several modes of mechanical ventilation are available. Selection of one of these as an initial mode of support or as a way to transition patients to extubation depends upon the patient's ability to breathe spontaneously, underlying co‐morbidities and clinical circumstances. With volume‐controlled ventilation (VCV), clinicians may set several parameters depending on the ventilator used: tidal volume, respiratory rate, peak flow rate, flow pattern (or inspiratory flow time), inspiratory‐to‐expiratory ratio (I:E), fractional concentration of oxygen (FiO2) and positive end‐expiratory pressure (PEEP) delivered; inspiration terminates after delivery of the preset tidal volume. Synchronized intermittent mechanical ventilation (SIMV) and assist control (AC) are two commonly used modes of volume‐limited ventilation. With SIMV and AC, clinicians set the respiratory rate and the tidal volume. Patients can increase their minute ventilation by initiating spontaneous breaths with variable tidal volume (SIMV) or by triggering additional breaths delivered at a preset tidal volume in AC.
With pressure‐controlled ventilation (PCV), clinicians may set various parameters including I:E ratio, inspiratory time, inspiratory pressure level, respiratory rate, FiO2 and PEEP. Inspiration ends after a set inspiratory pressure is delivered with pressure‐controlled ventilation for a set inspiratory time. With PCV, tidal volumes vary according to airway resistance, compliance, endotracheal tube resistance, inspiratory pressure and end‐expiratory alveolar pressure. Compared with VCV, PCV limits airway pressure during inspiration.
With pressure support (PS), patients trigger breaths that are supported up to a predetermined inspiratory pressure level. Unlike PCV, the ventilator cycles into expiration after inspiratory flow has decreased to a predetermined level. PS is thus a spontaneous mode of ventilation whereby all breaths are initiated by the patient and are supported by a preset pressure. This preset pressure can be titrated up or down by the clinician according to the respiratory status of the patient. Finally, PS can be used in combination with SIMV (SIMV + PS) such that triggered breaths during the spontaneous period are supported by a preselected PS level (Banner 1997). With SIMV + PS, the end of the inspiratory period may occur after a set time for an SIMV breath, or following a predetermined decrease in flow after a PS breath. SIMV can provide a range of ventilatory support. With SIMV, patients can trigger a mandatory volume breath (during the SIMV period) or a spontaneous breath (if triggering occurs earlier in a spontaneous period) before taking the next mandatory breath.
Weaning can be accomplished by several methods. Patients under controlled ventilation (VCV or PCV) can be taken abruptly off the ventilator to test whether they can breathe unassisted for a single testing period (spontaneous breathing trial (SBT) or T‐piece trial) or for periods of increasing duration (progressive T‐piece trials). With SIMV, the mandatory breath rate is reduced in a stepwise manner. Consequently, spontaneous breaths must increase if minute ventilation is to be maintained to the point where a patient can support his/her ventilation without assistance. In PS, the level of pressuresupporting breaths can be progressively decreased to the point where every inspiration is unassisted.
Early attempts were made to enable interaction between patients and ventilator‐adapted SIMV and PS (Strickland 1991; Strickland 1993). More recently, investigators have conducted pilot trials (Bouadma 2005) and retrospective studies (Kataoka 2007) of automated systems that adapt PS alone. Automated systems use closed‐loop control to perform basic and advanced functions while supporting respiration. Closed‐loop systems adapt ventilator output by comparing measured and targeted values of selected respiratory variables and either minimizing or equilibrating (negative feedback) or amplifying (positive feedback) the differences between these values (Burns 2008). Automated modes of mechanical ventilation use more sophisticated closed‐loop systems to enable interaction between patient and ventilator.
How the intervention might work
Several closed‐loop, automated systems are currently marketed. Mandatory minute ventilation (MMV) (Evita 4, Draeger Medical Inc., Luebeck, Germany) combines features of controlled ventilation with mandatory and spontaneous breaths as VCV + PS or SIMV + PS. Clinicians can set tidal volume (VT), mandatory breath rate, level of PS provided during spontaneous breaths and a target minute ventilation (VE). Based on the patient's spontaneous respiratory rate, MMV adapts the mandatory respiratory rate to achieve the target VE. Adaptive support ventilation (ASV) (Galileo, Raphael and Hamilton—G5, Hamilton Medical AG, Rhaezuens, Switzerland) is an automated system that adapts inspiratory pressure in PCV or PS mode to achieve a target VT . ASV targets a desired VE, set as a percentage of normal ventilation, and seeks the optimal VT and respiratory rate (least energy expenditure) to achieve this VE using Otis' equation. Neither MMV nor ASV automates the conduct of SBTs. Conversely, SmartCare™ (Draeger Medical Inc.) measures selected respiratory variables, adapts ventilator output by operationalizing predetermined algorithms and automates the conduct of SBTs (Burns 2008). To initiate SmartCare™, end‐users enter the patient's weight, the presence or absence of chronic obstructive pulmonary disease (COPD) or a central neurological disorder, the type of airway prosthesis used (tracheostomy or oro/nasal endotracheal tube) and the type of humidification applied (heated humidification or heat and moisture exchanger). The first three variables establish limits for respiratory rate, VT and partial pressure of end‐tidal carbon dioxide (PETCO2), and the latter two items determine the threshold to cycle into an SBT (ranging from 5 to 12 cm H2O). SmartCare™ categorizes patients into one of eight diagnostic categories based on average measurements of these variables that are made every two to five minutes. With SmartCare™, patients may breathe with a respiratory rate ranging from 15 to 30 breaths/min (RR min), alternatively 34 breaths/min for patients with neurological disease (RR max), a VT above a minimum threshold (VT min = 250 mL if weight < 55 kg, or VT min = 300 mL if weight > 55 kg) and a PETCO2 below a maximum threshold (max PETCO2 = 55 mmHg, or max PETCO2 = 65 mmHg for patients with COPD). SmartCare™ ascribes a state of normal ventilation when a patient's ventilatory measurements fall within these constraints. If the patient's measured values fall outside of these constraints, an alternate diagnosis is made, and the system adjusts the level of PS provided up or down to achieve these targets.
SmartCare™ automatically initiates an SBT (or 'observation period') when predetermined PS thresholds are reached, provided the patient is in a state of normal ventilation and PEEP is < 5 cm H2O. SBTs are of 30 minutes' to two hours' duration. Upon successful completion of an SBT, the ventilator issues a directive, stating that the patient is 'ready for separation from ventilator.' Clinicians must ensure that patients meet specific criteria before proceeding with the extubation. With the SmartCare™ system, clinicians control titration of FiO2 and PEEP. Consequently, if PEEP is not titrated to ≤ 5 cm H2O, an SBT will not be conducted. Clinicians can specify whether the automated algorithms are applied during the day only or continuously.
Why it is important to do this review
Regardless of the mode of ventilation used for weaning, limiting the duration of invasive ventilation and development of intubation‐related complications is an important goal in providing care for critically ill patients. Systems that automate weaning and SBT conduct obviate the need for clinicians to recognize and manually adjust ventilator settings to wean and conduct SBTs. Consequently, with automated systems, ventilator weaning is unencumbered by limited clinician availability in the busy ICU setting. In this review, we will identify, critically appraise and synthesize the best current evidence comparing automated weaning and SBT systems versus non‐automated weaning strategies in liberating critically ill adult patients from invasive ventilation.
Objectives
The primary objective of this review was to compare weaning time (time from randomization to extubation as defined by study authors) between invasively ventilated critically ill adults weaned by automated weaning and SBT systems versus non‐automated weaning strategies.
As secondary objectives, we ascertained differences between effects of alternative weaning strategies on clinical outcomes (time to successful extubation, time to first SBT and first successful SBT, mortality, ventilator‐associated pneumonia, total duration of ventilation, length of intensive care unit (ICU) and hospital stay, use of non‐invasive ventilation (NIV), adverse events and clinician acceptance).
The third objective of our review was to use subgroup analyses to explore variations in weaning time, length of ICU stay, mortality, ventilator‐associated pneumonia, use of NIV and reintubation according to (1) the type of clinician primarily involved in implementing the automated weaning and SBT strategy, (2) the ICU (as a reflection of the population involved) and (3) the non‐automated weaning strategy utilized.
We conducted a sensitivity analysis to evaluate variations in weaning time based on (4) the methodological quality (low or unclear versus high risk of bias) of the included studies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized trials comparing automated weaning and SBT systems versus non‐automated weaning strategies. Whereas an RCT was defined as a study that generates an unpredictable sequence for allocating participants to study groups (e.g. a random number table, computer‐generated random numbers, shuffling of envelopes, throwing of dice) (Higgins 2011), quasi‐randomized trials were defined as trials in which participants were allocated to treatment arms by alternate or predictable assignment.
Types of participants
We included trials investigating predominantly critically ill adults requiring invasive mechanical ventilation. We used authors' definitions of adult, as criteria for admission to adult ICUs may vary internationally. We did not restrict studies to specific population characteristics, including sex, age, race or the presence of selected risk factors. We excluded trials that evaluated participants requiring planned short‐term ventilation (i.e. postoperative patients) or exclusively tracheostomized participants.
Types of interventions
We included RCTs and quasi‐randomized trials that compared automated weaning and SBT systems versus non‐automated weaning strategies. Non‐automated strategies included usual care, standard care, protocolized care and other strategies (as defined by the study authors) but did not involve use of a nearly fully automated system. Recognizing that AC, intermittent mechanical ventilation (IMV), SIMV and pressure support (PS) ventilation are the most frequently used modes of weaning, we excluded modes that were not usually used for weaning (e.g. AutoFlow, Draeger Medical Inc.) and pressure‐regulated volume control (Maquet‐Dynamed, Tyco, Canada); nearly fully automated systems (e.g. Adaptive Support Ventilation (ASV), Hamilton Medical AG, Bonaduz, Switzerland); modes that switch from pressure control (PC) to PS (i.e. Automode, Siemens Medical Solutions, Erlangen, Germany); and strategies in which modifications of PS were linked to inspiratory flow (automatic tube compensation). We excluded studies that (1) compared alternative weaning strategies in the postoperative setting (i.e. planned short‐term ventilation for most participants, for example, cardiac surgical patients); (2) explored the use of NIV in this regard (i.e. extubation to NIV); (3) evaluated exclusively tracheostomized participants; or (4) explored the use of a nearly fully automated closed‐loop system (invasively or non‐invasively applied) in the control arm. If ambiguity existed as to what constituted a simple mode (set point control) without full automation, we referenced the classification system proposed by Chatburn et al (Chatburn 2004).
Types of outcome measures
Primary outcomes
The primary outcome was weaning time (time from randomization to extubation) as defined by the study authors.
Secondary outcomes
Secondary outcomes included:
time to successful extubation (time from randomization to successful extubation as defined by study authors);
time to first SBT and first successful SBT (time from randomization to first SBT and first successful SBT as defined by study authors);
mortality (the most protracted duration at time points reported by study authors);
VAP as defined by study authors;
total duration of ventilation (time from initiation of invasive ventilation to discontinuation or extubation) as defined by study authors;
Length of ICU stay;
use of NIV following extubation;
adverse events (including but not limited to reintubation, self‐extubation, requirement for tracheostomy and prolonged ventilation as defined by study authors);
clinician acceptance of alternative weaning strategies; and
length of hospital stay.
To be included, studies had to report at least one of the aforementioned primary or secondary outcomes.
Search methods for identification of studies
Electronic searches
We used database‐specific search strategies to search the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 5; (Appendix 1); MEDLINE (1966 to 31 May 2013) (Appendix 2); EMBASE (1988 to 31 May 2013) (Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 31 May 2013) (Appendix 4), Evidence‐Based Medicine Reviews (Appendix 5) and Ovid HealthSTAR (1999 to 31 May 2013) (Appendix 6) to identify potentially eligible trials. We based our search strategies on the optimally sensitive search strategies of The Cochrane Collaboration to identify RCTs in MEDLINE and EMBASE (Dickersin 1994; Lefebvre 2001; Robinson 2002). We combined our subject search terms in MEDLINE with the Cochrane highly sensitive search strategy for identifying RCTs, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted our MEDLINE search strategy to other databases. We did not limit our search by language or publication status.
Searching other resources
We contacted the first authors of all included studies and content experts to obtain additional information on unpublished trials or trials in progress. We searched the bibliographies of all retrieved trials and review papers for potentially relevant trials. Additionally, we handsearched conference proceedings from five scientific meetings (Annual Congress of the European Society of Intensive Care Medicine (2001‐2012), College of Chest Physicians (2003‐2012), American Thoracic Society (2004‐2013), International Symposia of Intensive Care and Emergency Medicine and Critical Care Medicine (2004‐2013) and Critical Care Medicine (2004‐2012)) to identify abstracts of RCTs that met our inclusion criteria. Finally, we searched for ongoing trials on the following websites: www.controlled‐trials.com and http://clinicaltrials.gov.
Data collection and analysis
We utilized the methods of the Cochrane Anaesthesia Review Group. Two review authors (KB, FL) independently screened titles and abstracts identified by electronic and manual searches, and one review author each screened conference proceedings (JF) and trial registration websites (KB).
Selection of studies
Two review authors (KB, JF) retrieved and evaluated the full‐text versions of potentially relevant trials. Two review authors (KB, JF) independently selected trials that met the study inclusion criteria by using a checklist developed for this purpose (Appendix 7). We resolved disagreements through discussion and, if agreement could not be reached, in consultation with a third review author (ML). We recorded reasons for study exclusion in the Characteristics of excluded studies table. One review author (JF) handsearched conference proceedings.
Data extraction and management
The same two review authors (KB, JF) independently extracted data using a standardized data collection form (Appendix 7) that included information regarding name of first author, year of publication, study design, study population and study setting. In addition to information pertaining to participant characteristics, study inclusion and exclusion criteria, details of compared interventions, clinicians involved in implementing weaning strategies and study outcomes, we extracted information regarding study methodology. This included method of randomization, allocation concealment, frequency and handling of withdrawals and adherence to the intention‐to‐treat principle. Most trials used median and interquartile ranges as summary statistics for continuous outcomes, suggesting that data were skewed. When mean and standard deviation were not provided, we approximated the mean from the median and estimated the standard deviation as the interquartile range divided by 1.33 (Higgins 2011) to pool outcomes. We attempted to contact the first authors of all included trials to obtain missing data or to clarify study design features, when necessary. We resolved disagreements through discussion and in consultation with a third review author (ML) as required. We did not blind review authors to the names of study authors, investigators or institutions, nor were they blinded to study results.
Assessment of risk of bias in included studies
The quality of all included trials was assessed by two review authors (KB, JF), independently and in duplicate. We judged study quality on the basis of the following (Higgins 2011).
1. Was sequence generation truly random?
Adequate sequence generation included reference to a random number table, use of a computer random number generator, coin tossing, shuffling of cards or envelopes, throwing of dice, drawing of lots or minimization.
2. Was allocation adequately concealed? Adequate allocation concealment included central randomization (e.g. allocation by a central office unaware of participant characteristics unless based on stratification), such as an on‐site computer system combined with allocation kept in a locked unreadable computer file that could be accessed only after the characteristics of an enrolled participant had been entered; sequentially numbered, sealed, opaque envelopes; or another, similar approach, which ensured that the person generating the allocation sequence did not administer it.
3. Was knowledge of the allocated interventions adequately prevented during the study? Blinding of study participants and personnel from study intervention allocation after inclusion of participants is not feasible; however, we judged whether outcome assessors were separate from the individuals administering or supervising assigned interventions.
4. Were withdrawals described, and did they occur with similar frequency between intervention and control groups?
5. Were participants analysed according to the intervention to which they were allocated, whether or not they received it? Within studies, we described what was reported for each domain and contacted study authors for further information.
6. Were reports of the study free of the suggestion of selective outcome reporting?
7. Did the trial stop early for benefit? What was the impact of early stopping of the trial, if applicable?
Following evaluation, we assigned a judgement related to the risk of bias for each domain as follows. a) Low risk of bias: all criteria met. b) Unclear risk of bias: one or more criteria unclear. c) High risk of bias: one or more criteria not applied or met.
A judgement of 'Yes' indicated low risk of bias, 'No' indicated high risk of bias and 'Unclear' indicated an unknown or unclear risk of bias.
For example, low risk of bias was assigned when allocation concealment was adequate (including central randomization, such as allocation by a central office unaware of participant characteristics unless based on stratification; an on‐site computer system combined with allocation kept in a locked unreadable computer file that could be accessed only after the characteristics of an enrolled participant had been entered; sequentially numbered, sealed, opaque envelopes; or other, similar approaches that ensured that the person who generated the allocation sequence did not administer it). We assigned unclear risk of bias when allocation concealment was unclear or when study authors did not clearly report their approach, and high risk of bias when allocation concealment was not applied. We evaluated the impact of methodological quality (low or unclear versus high risk of bias) on weaning time. We constructed a 'Risk of bias' (RoB) table to depict the results.
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) to assess the quality of the body of evidence in our review associated with specific outcomes (weaning time, time to successful extubation, time to first SBT and first successful SBT, mortality, total duration of mechanical ventilation, length of ICU stay and reintubation) and constructed a 'Summary of findings' (SoF) table using GRADE software. The GRADE approach is used to appraise the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considered within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Two review authors (KB, JF) entered data into Review Manager (RevMan 5.1) for statistical analysis.
Measures of treatment effect
We summarized treatment effects using risk ratio (RR) and mean difference (MD) for binary and continuous outcomes, respectively.
Unit of analysis issues
We used proportions for binary outcomes and preferentially used mean and standard deviation, when reported or available through correspondence with study authors, in pooled analyses. Summary estimates constitute the unit of analysis in this review.
Dealing with missing data
For published reports with insufficient or ambiguous information, we contacted investigators to inquire about study methods and missing data.
Assessment of heterogeneity
We assessed clinical heterogeneity by judging, qualitatively, differences between studies with regard to participant populations enrolled, weaning strategies implemented and study outcomes reported. We conducted statistical tests of heterogeneity and assessed the impact of heterogeneity for each outcome using the I2 statistic. This statistic describes the percentage of total variance across studies that is attributable to heterogeneity rather than chance (Higgins 2003). We considered an I2 statistical threshold of 0% to 40%, 30% to 60%, 50% to 90% and > 75% to represent between study heterogeneity that might not be important, moderate, substantial or considerable, respectively (Higgins 2011). To limit overlap and to operationalize these thresholds, we considered the mutually exclusive I2 intervals of 0% to 30%, 31% to 50%, 51% to 74% and > 75% to represent unimportant, moderate, substantial and considerable heterogeneity, respectively. For outcomes that were qualitatively similar, and in the absence of important heterogeneity, we performed meta‐analysis using random‐effects (RE) models and reported summary estimates along with their associated 95% confidence intervals (CIs).
Assessment of reporting biases
Publication bias occurs when published trials are not fully representative of all completed trials, as positive trials (large and small) tend to be published more often than negative trials, especially small negative trials. We examined funnel plots (a graphical display) for asymmetry and size of the treatment effect for the primary outcome against trial precision (one/standard error) to assess for publication bias, if sufficient (at least 10) studies were identified (Egger 1997).
Data synthesis
We used RE models to pool data quantitatively using Review Manager 5.1 software (RevMan 5.1) when studies were clinically similar overall. We summarized the evidence in the SoF table.
Among the included studies, interventions were continuously applied and outcomes were reported at multiple time points. We recognized that performance of multiple analyses increases the chance of spurious positive findings. Although many statistical approaches have been developed to adjust for multiple testing, no consensus has been reached regarding when multiplicity should be taken into consideration. Further, adjustments for multiple testing are not routinely conducted in systematic reviews. We highlighted the primary outcome and the six secondary outcomes in this protocol as key outcomes featured in the SoF table. We emphasized estimation of intervention effects rather than testing to determine them and considered planned subgroup analyses as exploratory in nature.
Subgroup analysis and investigation of heterogeneity
A priori, we planned to perform subgroup analyses to assess the impact of the following study design features on weaning time, length of ICU stay, mortality, VAP, use of NIV and reintubation.
Type of clinician principally involved in implementing the automated weaning strategy (i.e. registered respiratory therapist (RRT) versus other. including mixed clinicians), as defined by the study authors.
Type of ICU (i.e. medical‐surgical and purely surgical versus purely medical, including coronary care units), as defined by the study authors.
Type of non‐automated weaning strategy (predominantly protocolized versus predominantly non‐protocolized care or other), as defined by the study authors.
A priori, we anticipated that subgroup analyses would be underpowered. We viewed subgroup analyses as exploratory, given their tendency to generate misleading conclusions (Oxman 1992; Yusuf 1991). For these outcomes, we tested the differences in RR between subcategories using a Chi2 test (Borenstein 2008). We considered P value < 0.05 to be statistically significant.
Sensitivity analysis
A priori, we planned a sensitivity analysis to assess the impact on weaning time of excluding studies with high risk of bias.
Results
Description of studies
Results of the search
We screened 2636 unique citations to identify 20 articles potentially meeting our inclusion criteria (Figure 1). Among these, we identified 13 randomized trials (Beale 2007; Bifulco 2008; Burns 2013a; Jiang 2006; Lellouche 2006; Lim 2012; Liu 2013; Ma 2010; Papirov 2007; Reardon 2011; Rose 2008; Stahl 2009; Wong 2008) potentially meeting our study inclusion criteria, including one quasi‐randomized trial (Jiang 2006). Through correspondence, one study author confirmed that the trial never started (Beale 2007), another acknowledged that the trial was stopped because of slow recruitment after enrolment of three participants (Wong 2008) and a final study author confirmed that the trial included exclusively tracheostomized participants and stopped prematurely because of the need to return the study ventilators (Papirov 2007). Five trials (Beale 2007; Papirov 2007; Reardon 2011; Stahl 2009; Wong 2008) were identified on trial registration websites. We identified no weaning and SBT systems used for weaning, as opposed to short‐term ventilation (e.g. postoperative patients), other than SmartCare™.
1.
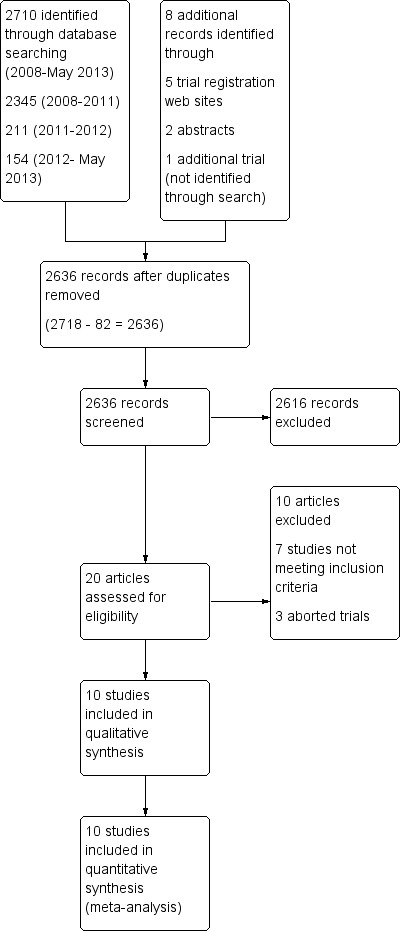
Study flow diagram.
Included studies
Ten trials (Bifulco 2008; Burns 2013a; Jiang 2006; Lellouche 2006; Lim 2012; Liu 2013; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) provided summary estimates and were included in this review. Of these, two trials were published in abstract form (Bifulco 2008; Lim 2012) and two were published in Chinese (Jiang 2006; Ma 2010). Two included trials were identified on trial registration websites (Reardon 2011; Stahl 2009), of which one provided partial study results (Reardon 2011). One trial was published in full and was available as a dissertation (Stahl 2009). Full details of participants, interventions and outcomes for each trial are provided in the Characteristics of included studies table.
Excluded studies
We excluded seven studies (Chen 2008; Donglemans 2007; Jolliet 2006; Jouvet 2007; Kataoka 2007; Schadler 2012; Taniguchi 2009) (see Characteristics of excluded studies), in addition to three aborted trials (Beale 2007; Papirov 2007; Wong 2008). The two review authors (KB, JF) achieved complete agreement on study selection. All study authors (Bifulco 2008; Burns 2013a; Jiang 2006; Lellouche 2006; Lim 2012; Liu 2013; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) provided additional information regarding study methods or results.
Of the included trials, eight were single‐centre studies (Bifulco 2008; Jiang 2006; Lim 2012; Liu 2013; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) and two were multi‐centre trials (Burns 2013a; Lellouche 2006). Trials were conducted in Australia (Rose 2008), Canada (Burns 2013a), China (Jiang 2006; Liu 2013; Ma 2010), Europe (Bifulco 2008; Lellouche 2006; Stahl 2009), Singapore (Lim 2012) and the United States (Reardon 2011). Study populations included medical or critical care unit (CCU) (Jiang 2006; Lim 2012; Reardon 2011), surgical (Stahl 2009), medical‐surgical (Bifulco 2008; Lellouche 2006; Liu 2013; Ma 2010), medical‐surgical trauma (Rose 2008) and multi‐disciplinary (Burns 2013a) participant populations. One trial (Jiang 2006) was conducted at a military hospital and included exclusively male participants.
Weaning candidates were identified daily during multi‐disciplinary rounds (Reardon 2011) after at least 24 hours (Bifulco 2008; Burns 2013a; Lellouche 2006; Lim 2012; Rose 2008; Stahl 2009) or more than 48 hours of mechanical ventilation (Ma 2010; Reardon 2011), or when the illness causing respiratory failure had been controlled (Jiang 2006). One trial (Jiang 2006) included 23 participants nasotracheally intubated and 15 who had a tracheostomy. Ten trials were screened daily or daily when feasible to identify weaning (Bifulco 2008; Burns 2013a; Jiang 2006; Lellouche 2006; Lim 2012; Liu 2013; Reardon 2011; Rose 2008; Stahl 2009) and SBT (Burns 2013a; Ma 2010) candidates. Whereas five trials included tolerance of PS or a formal PS trial (Bifulco 2008; Burns 2013a; Lellouche 2006; Lim 2012; Rose 2008) among their inclusion criteria, three trials included participants who had failed a prerandomization SBT (Burns 2013a; Liu 2013; Ma 2010). Four trials specified PS thresholds of 15 cm H2O (Burns 2013a; Lellouche 2006; Lim 2012) or higher (Bifulco 2008; Lim 2012) with IPV ≤ 30 cm H2O (Lim 2012), and another trial specified maximum pressure support of 20 cm H2O to achieve VT > 200 mL (Rose 2008). Prerandomization SBTs were conducted using T‐piece (Burns 2013a; Ma 2010), PS (Burns 2013a; Liu 2013) or continuous positive airway pressure (CPAP) (Burns 2013a; Liu 2013) and were of 30 to 120 minutes' duration (Burns 2013a; Liu 2013; Ma 2010). One trial used a staged process and included participants who tolerated a PS trial and were too early to undergo an SBT or had failed an SBT (Burns 2013a). Other trials specified inclusion of participants capable of initiating breaths (Reardon 2011) or of performing spontaneous breathing (Jiang 2006; Liu 2013; Ma 2010; Stahl 2009). Inclusion criteria also specified threshold PEEP levels ≤ 5 cm H2O (Bifulco 2008; Jiang 2006; Liu 2013; Ma 2010), ≤ 8 cm H2O (Lellouche 2006; Rose 2008), < 8 cm H2O (Reardon 2011) or ≤ 10 cm H2O (Burns 2013a; Lim 2012; Stahl 2009), as well as FiO2 levels (Burns 2013a; Jiang 2006; Lellouche 2006; Lim 2012; Liu 2013; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) or partial pressure of oxygen in arterial blood (PaO2)/FiO2 ratios (Bifulco 2008; Jiang 2006;Lim 2012; Liu 2013; Ma 2010; Rose 2008). Two trials (Lim 2012; Rose 2008) specified a plateau pressure ≤ 30 cm H2O, and another trial (Lellouche 2006) specified among its inclusion criteria use of inspiratory pressures not greater than 30 cm H2O. One trial specified inclusion of a volume‐ or pressure‐targeted mandatory mode for > 24 hours (Rose 2008), and others specified use of assisted modes of ventilation (Lellouche 2006; Lim 2012).
Control ventilation strategies of included studies
Control group ventilation strategies varied amongst the included trials. Trials specified comparing SmartCare™ versus an evidence‐based standard of care (Reardon 2011), a paper‐based weaning protocol (Burns 2013a; Ma 2010), a written weaning guideline (Liu 2013) and a conventional weaning protocol typically based on usual or local practice (Bifulco 2008; Jiang 2006; Lellouche 2006; Lim 2012; Rose 2008; Stahl 2009). One trial (Lellouche 2006) affirmed the presence of paper‐based weaning and SBT guidelines at four of five participating centres. Two trials specified use of SIMV with PS (Jiang 2006; Ma 2010), and five trials used PS (Burns 2013a; Liu 2013; Reardon 2011; Rose 2008; Stahl 2009) in the control arm if tolerated. Other trials used a combination of modes, including PS (predominant mode), ACV, SIMV and SBTs (T‐piece, PS or CPAP trials) (Lellouche 2006), initial ACV (rarely PCV) transitioned to SIMV with/without PS or PS alone with SBTs conducted at the discretion of physicians/RRTs (Lim 2012) and PS with T‐piece trials (Reardon 2011).
Support was gradually reduced in some trials (Bifulco 2008; Jiang 2006; Ma 2010; Stahl 2009). One trial titrated to a respiratory zone of comfort with no constraints as to the size or frequency of PS adjustments (Rose 2008), and another specified gradual reduction of PS with single steps of not more than 10 cm H2O (Stahl 2009). In another trial (Burns 2013a), the level of PS was reevaluated at least every four to six hours and was titrated to avoid respiratory distress or need for assistance. One trial (Bifulco 2008) reduced PS by 2 cm H2O based on clinical response, with frequency of reductions determined by clinicians. Selected trials reduced support to an SIMV rate of 4 breaths/min and PS 7 to 8 cm H2O for two hours (alternatively, PS 5 cm H2O in tracheostomized participants) (Ma 2010), PS of 10 cm H2O with ≤ 5 cm H2O PEEP for 30 minutes to two hours (Reardon 2011) or PS 7 cm H2O (intubated patients) or 5 cm H2O (tracheostomized participants) (Rose 2008). One trial (Reardon 2011) adjusted PS to maintain VT of 6 to 8 cc/kg ideal body weight. Another trial (Jiang 2006) conducted SBTs while endeavouring to reduce time on mechanical ventilation until participants were ventilator free and returned participants to mechanical ventilation when respiratory rate > 32 breaths/min, heart rate > 100 beats/min or pulse oximetry (SpO2) < 90%. Participants on PS were screened at least daily for SBTs in one trial (Burns 2013a). Four centres in another study (Lellouche 2006) used a combination of PS and SBTs for weaning, with one centre using PS to wean participants who could not tolerate an initial SBT and conducting SBTs in participants who were not weaned in PS mode.
Post‐randomization SBTs in the control arm weaning strategy were conducted using a T‐piece for five minutes following two hours of observation on SIMV with PS (Ma 2010) and either a two‐hour T‐piece trial or periods of ventilator disconnection with spontaneous breathing (Jiang 2006). Although SBTs were conducted on minimal PS (7 cm H2O) for 60 minutes in one trial (Rose 2008), they were performed using a T‐piece (or trach mask) or CPAP (≤ 5 cm H2O) or PS 5 to 7 cm H2O with PEEP ≤ 5 cm H2O (with heated humidification (HH)) or 10 to 12 cm H2O with PEEP ≤ 5 cm H2O (with heat and moisture exchangers (HMEs)) for 30 to 120 minutes in another trial (Burns 2013a). A final trial used PS < 7 cm H2O or T‐piece trials in intubated participants and trach mask trials in participants with a tracheostomy (Liu 2013). Of four centres in one study (Lellouche 2006) with a weaning protocol, one conducted 20‐minute T‐piece trials up to two to three times per day following an initial SBT failure, while others conducted two‐hour SBTs on T‐piece or PS 7 cm H2O daily for participants not in PS mode, or preferentially performed 30‐minute SBTs using PS 10 cm H2O (alternatively, T‐piece or CPAP 5 cm H2O) following at least twice‐daily screening. The final centre conducted SBTs using PS 7 cm H2O (without HME) and 12 cm H2O (with HME) or T‐piece for 30 minutes to two hours with daily screening (Lellouche 2006). In another trial (Reardon 2011), control participants were weaned with SBTs using T‐piece or PS ≤ 10 cm H2O with PEEP ≤ 5 cm H2O for 30 minutes to two hours. A final trial (Liu 2013) specified daily screening with conduct of 30‐minute SBTs with CPAP 5 cm H2O alone or with added PS 5 to 8 cm H2O.
Five trials permitted return to controlled or assist‐control ventilation upon meeting selected criteria (Burns 2013a; Lellouche 2006; Liu 2013; Reardon 2011; Stahl 2009), with one trial specifying that a single return to controlled ventilation and two weaning trials were permitted for each participant (Stahl 2009). Other trials specified use of volume‐controlled ventilation (Reardon 2011) in participants who no longer met weaning criteria or returned participants to SIMV with PS in the event of SBT intolerance (Ma 2010).
Physicians titrated ventilator support in six trials (Bifulco 2008; Jiang 2006; Lellouche 2006; Liu 2013; Ma 2010; Stahl 2009), with one trial (Jiang 2006) specifying that attending clinicians were responsible for implementing SmartCare™, while physicians not involved with the study implemented the control strategy. One trial (Bifulco 2008) specified that SmartCare™ was managed by physicians, including residents in training, who did not participate in the care or weaning of participants in the conventional arm. Another trial (Ma 2010) specified that the main research physician managed participants in the control arm and selected SmartCare™ settings. Two trials (Lim 2012; Liu 2013) specified that RRTs implemented the SmartCare™ strategy, and both RRTs and physicians (Lim 2012) or physicians (Liu 2013) implemented the control weaning strategy. Different physicians provided care to participants assigned to alternative study groups in one trial (Bifulco 2008). In another trial (Rose 2008), ventilator titration was performed primarily by nurses, with physicians directing participant care during twice‐daily structured rounds. In two trials conducted in North America (Burns 2013a; Reardon 2011), weaning was conducted primarily by RRTs with physician support.
SmartCare™ strategies
Few studies provided additional details pertaining to modifiable settings on the SmartCare™ weaning system. One study reported setting trigger sensitivity at 2 L/min and FiO2 between 30% and 45% (Jiang 2006). Three trials did not permit night rest (Burns 2013a; Lellouche 2006; Stahl 2009), and two trials (Bifulco 2008; Ma 2010) activated the night rest option. One trial (Ma 2010) set PS at 5 to 15 cm H2O, FiO2 at 40% and PEEP at 3 cm H2O, while another trial (Burns 2013a) set maximum inspiratory pressure at 35 cm H2O, maximum respiratory rate at 40 breaths/min and level of end tidal carbon dioxide (ETCO2) limits of 15 mmHg and 70 mmHg. This trial used a PEEP/FiO2 chart in both study groups and clustered humidification strategies (HH and HME) within participating ICUs (Burns 2013a). Two trials (Bifulco 2008; Lellouche 2006) used passive humidification (HME) to warm inspired air, and one trial (Stahl 2009) used active humidification (HH).
Both weaning strategies
Sedation was administered at the discretion of the attending physician in one trial (Lellouche 2006), managed according to written sedation protocols titrated by critical care nurses to Richmond Agitation‐Sedation Scale (RASS) or the Riker Sedation‐Agitation Scale (SAS) scores in another trial (Burns 2013a) and managed by a sedation protocol with daily awakening in another trial (Liu 2013); sedation was not reported in the remaining trials (Bifulco 2008; Jiang 2006; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009).
Risk of bias in included studies
See Figure 2 and Characteristics of included studies.
2.
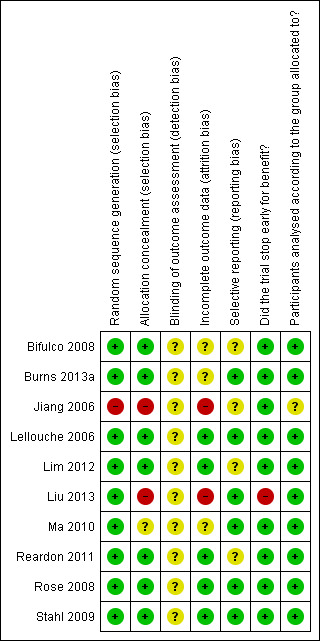
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation (selection bias)
In all trials, allocation to treatment group was done by random assignment, with one trial assigning weaning strategies based on odd or even numbers distributed at hospital admission (Jiang 2006). One trial each specified use of the minimum balance index for randomization (based on the sequence of ICU admission) (Ma 2010) and a random digit table (Liu 2013). Seven trials reported using computer‐generated randomization sequence burns (Bifulco 2008; Burns 2013a; Lellouche 2006; Lim 2012; Rose 2008; Reardon 2011; Stahl 2009); of these, two trials (Reardon 2011; Rose 2008) used on‐line random number generator systems (www.randomization.com and www. random.org). One trial (Liu 2013) reported using a random number table.
Allocation
Two trials each reported use of sequentially numbered, sealed, opaque envelopes (Rose 2008; Stahl 2009); one trial used sequentially numbered, sealed envelopes held by the trial co‐ordinator/RRT (Lim 2012), and one trial used opaque envelopes (Reardon 2011). Group allocation was communicated by telephone in one trial (Bifulco 2008) and by electronic mail messages from a central site in two trials (Burns 2013a; Lellouche 2006). In one trial, allocation was not concealed (Jiang 2006). In another trial (Ma 2010), the minimum balance index based on gender, age and Acute Physiology and Chronic Health Evaluation (APACHE) score, with points assigned for each category, was used for allocation by assigning participants to the strategy with the lowest number of cumulative points. In two trials, allocation was the responsibility of both the researcher implementing the study (Ma 2010) and an RRT (Liu 2013) who held the randomization list. Once participants had been randomly assigned, one investigator (Ma 2010) confirmed that the assigned treatment was initiated, and another (Liu 2013) confirmed that physicians did not know the assigned treatment until the ventilator was brought to the bedside.
Blinding
Blinding was not possible given the nature of the interventions being investigated, and blinded outcome assessment was not reported in any trial. Individuals assessing outcomes were not separate from individuals supervising or administering the study interventions in all 10 trials (Bifulco 2008; Burns 2013a; Jiang 2006; Lellouche 2006; Lim 2012; Liu 2013; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009).
Incomplete outcome data
One investigator affirmed that participants dropped out of the study as the result of infection (e.g. VAP) or self‐extubation, and the distribution of withdrawals between treatment groups was unknown (Jiang 2006). This trial (Jiang 2006) reported on 13 participants in the smartcare (SC) arm and 25 in the SBT (control) arm, suggesting the potential for an imbalance between groups in terms of randomization or withdrawals. In another trial reporting on similar numbers of participants in each study arm, study authors (Ma 2010) affirmed participant attrition due to consent withdrawals and VAP, which occurred equally between treatment groups. Two additional trials reported study withdrawals (Bifulco 2008; Burns 2013a) and specified the number of withdrawals by group assignment. The largest trial (Lellouche 2006) reported that post randomization, two participants were withdrawn because extubation preceded electronic assignment, and one participant was excluded after consent was withdrawn, but treatment assignment was not specified. Meanwhile, one thesis (Stahl 2009) reported that the first 10 participants who failed an initial attempt at weaning were discontinued from the study. The protocol was subsequently modified to permit a second weaning attempt. Additionally, postrandomization withdrawals occurred with similar frequency between treatment groups (four per group). Through correspondence, we clarified that these 10 participants were included in the final analysis, and outcomes were included in the analyses when possible. Two trials (Reardon 2011; Rose 2008) reported no study withdrawals or dropouts. Five participants in the SmartCare™ group (20.8%) and four participants in the physician‐controlled local protocol group (16.7%) who died were not included in the analyses in one trial (Liu 2013). Similarly, although no participants were withdrawn in one trial (Lim 2012), one participant died and did not contribute data to selected outcomes.
Selective reporting
Outcome reporting was complete in five trials (Burns 2013a; Lellouche 2006; Liu 2013; Rose 2008; Stahl 2009), and summary data were provided through correspondence for a fifth trial (Ma 2010). The authors of two trials (Bifulco 2008; Jiang 2006) affirmed that they intended to collect additional outcomes, but fewer data were collected because of early stopping and limited personnel availability (Bifulco 2008) and as a result of transfer of the principal investigator to another hospital (Jiang 2006). We anticipated that selected ICU outcomes (duration of mechanical ventilation, ICU mortality and length of ICU stay) could have been reported in at least two trials (Jiang 2006; Ma 2010). One trial (Reardon 2011) reported partial trial results on a trial registration website, and another in an abstract publication (Lim 2012).
Other potential sources of bias
Stopping early for benefit
Three trials reached full recruitment (Burns 2013a; Jiang 2006; Lellouche 2006). One trial stopped early for benefit following an interim statistical analysis, which suggested that 40 to 50 participants would be sufficient (Liu 2013). Six trials stopped early for futility (Bifulco 2008; Lim 2012; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) due to funding and/or personnel constraints (Bifulco 2008), time constraints and the need to fulfil graduate degree requirements (Ma 2010; Rose 2008; Stahl 2009), slow or delayed recruitment (Reardon 2011; Stahl 2009) and sample size recalculation (Rose 2008; Stahl 2009).
Analysis according to allocated weaning strategy
Nine trials reported or affirmed analysis of participants by treatment group assignment (Bifulco 2008; Burns 2013a; Lellouche 2006; Lim 2012; Liu 2013; Ma 2010; Reardon 2011; Rose 2008; Stahl 2009) while adhering to the intention‐to‐treat principle or a modified intention‐to‐treat principle as the result of withdrawals or deaths. Analysis by intention‐to‐treat was uncertain in one trial (Jiang 2006), with important imbalances reported between the numbers of participants in the treatment arms.
Effects of interventions
Summary of findings for the main comparison. Continuous outcomes automated versus non‐automated weaning.
| SmartCare™ versus non‐automated weaning for weaning time in invasively ventilated critically ill adults | ||||||
| Patient or population: patients with weaning time in invasively ventilated critically ill adults Settings: Intervention: SmartCare™ versus non‐automated weaning | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect MD (95% CI) |
No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Average duration | Estimated duration | |||||
| Control | SmartCare™ versus non‐automated weaning | |||||
| Weaning time (from randomization to extubation) based on ICU type: purely medical | Mean weaning time (from randomization to extubation) based on ICU type—purely medical—in the control groups was 13 days | Mean weaning time (from randomization to extubation) based on ICU type—purely medical—in the intervention groups was 4.78 lower (6.2 to 3.36 lower) | 38 (1 study) | ⊕⊕⊕⊝ moderatea | ||
| Weaning time (from randomization to extubation) based on ICU type: medical‐surgical or surgical only | Mean weaning time (from randomization to extubation) based on ICU type—medical‐surgical or surgical only—in the control groups was 3 to 11 days | Mean weaning time (from randomization to extubation) based on ICU type—medical‐surgical or surgical only—in the intervention groups was 1.85 lower (2.67 to 1.04 lower) | 457 (6 studies) | ⊕⊕⊝⊝ lowa,b | ||
| Time to successful extubation | Mean time to successful extubation in the control groups was 1 to 10 days | Mean time to successful extubation in the intervention groups was 0.99 lower (1.89 to 0.09 lower) | 516 (7 studies) | ⊕⊕⊝⊝ lowa,c | ||
| Time to first successful spontaneous breathing trial | Mean time to first successful spontaneous breathing trial in the control groups was 0 to 6 days | Mean time to first successful spontaneous breathing trial in the intervention groups was 1.72 lower (6.23 lower to 2.78 higher) | 175 (2 studies) | ⊕⊕⊕⊝ moderated | ||
| Total duration of mechanical ventilation | Mean total duration of mechanical ventilation in the control groups was 3 to 17 days | Mean total duration of mechanical ventilation in the intervention groups was 1.68 lower (3.33 to 0.03 lower) | 521 (7 studies) | ⊕⊕⊝⊝ lowa,c | ||
| Intensive care unit length of stay (based on type of control arm): predominantly protocolized control strategy | Mean intensive care unit length of stay based on type of control arm—predominantly protocolized control strategy—in the control groups was 23 to 37 days | Mean length of intensive care unit stay based on type of control arm—predominantly protocolized control strategy—in the intervention groups was 9.84 lower (17.02 to 2.66 lower) | 337 (4 studies) | ⊕⊕⊝⊝ lowa,c | ||
| Intensive care unit length of stay (based on type of control arm): predominantly non‐protocolized control strategy | Mean intensive care unit length of stay based on type of control arm—predominantly non‐protocolized control strategy—in the control groups was 10 to 20 days | Mean intensive care unit length of stay based on type of control arm—predominantly non‐protocolized control strategy—in the intervention groups was 1.26 lower (4.1 lower to 1.59 higher) | 162 (2 studies) | ⊕⊕⊕⊝ moderatec | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOne trial with high risk of bias. bLower CI crosses effect size of 0.5 (st mean difference). cConfidence Interval crosses effect size 0.5 (st mean diff). dNumber of participants is less than 400.
Summary of findings 2. Binary outcomes: automated versus non‐automated weaning.
| SmartCare™ versus non‐automated weaning strategies for weaning time in invasively ventilated critically ill adults | ||||||
| Patient or population: patients with weaning time in invasively ventilated critically ill adults Settings: Intervention: SmartCare™ versus non‐automated weaning | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SmartCare™ versus non‐automated weaning | |||||
| Most protracted measure of mortality | Study population | RR 1.15 (0.74 to 1.79) | 470 (6 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 203 per 1000 | 233 per 1000 (150 to 363) | |||||
| Moderate | ||||||
| 228 per 1000 | 262 per 1000 (169 to 408) | |||||
| Adverse event: reintubation | Study population | RR 0.88 (0.64 to 1.22) | 491 (6 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 244 per 1000 | 215 per 1000 (156 to 297) | |||||
| Moderate | ||||||
| 243 per 1000 | 214 per 1000 (156 to 296) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOne trial has high risk of bias. bFewer than 300 events.
1.1, 2.1 and 3.1 Weaning time (randomization to extubation)
Weaning time (time from randomization to first extubation) was reported in seven trials (Bifulco 2008; Burns 2013a; Jiang 2006; Lellouche 2006; Liu 2013; Ma 2010; Rose 2008) involving 495 participants. Pooled results showed a significant reduction in weaning time (MD ‐2.68 days, 95% CI ‐3.99 to ‐1.37; P value < 0.0001) favouring SmartCare™ in the presence of substantial heterogeneity (I² = 68%; P value 0.005) (Analysis 1.1; Analysis 2.1; Analysis 3.1) (Table 1). Weaning time was not reported separately in survivors and non‐survivors in any trial, and nine participants were excluded from one trial (Liu 2013) reporting this outcome.
1.1. Analysis.
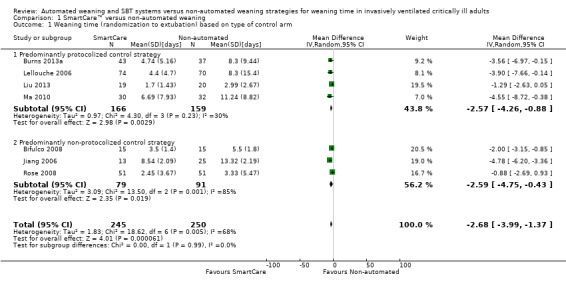
Comparison 1 SmartCare™ versus non‐automated weaning, Outcome 1 Weaning time (randomization to extubation) based on type of control arm.
2.1. Analysis.
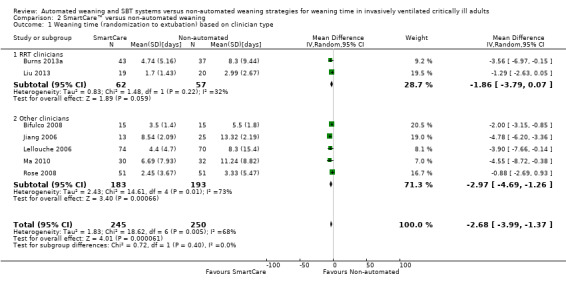
Comparison 2 SmartCare™ versus non‐automated weaning, Outcome 1 Weaning time (randomization to extubation) based on clinician type.
3.1. Analysis.
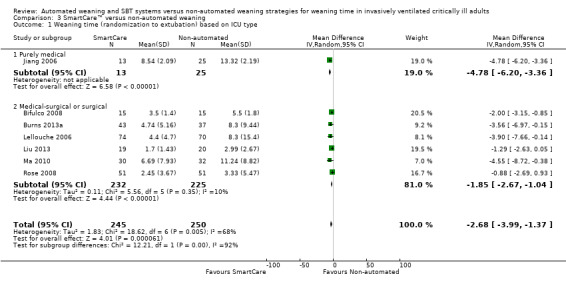
Comparison 3 SmartCare™ versus non‐automated weaning, Outcome 1 Weaning time (randomization to extubation) based on ICU type.
4.1 Time to successful extubation
Time to successful extubation was reported in seven trials (Burns 2013a; Lellouche 2006; Lim 2012; Liu 2013; Reardon 2011; Rose 2008; Stahl 2009) involving 516 participants. Pooled results demonstrated a trend toward reduced time to successful extubation (MD ‐0.99 days, 95% CI ‐1.89 to ‐0.09; P value 0.03) using SmartCare™ with unimportant heterogeneity (I² = 29%; P value 0.20) (Analysis 4.1) (Table 2).
4.1. Analysis.
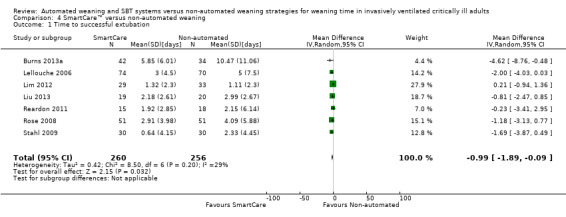
Comparison 4 SmartCare™ versus non‐automated weaning, Outcome 1 Time to successful extubation.
5.1 and 6.1 Time to first spontaneous breathing trial and first successful spontaneous breathing trial
Only one trial reported time to first spontaneous breathing trial (Burns 2013a). Time to first successful SBT was reported in two trials (Burns 2013a; Rose 2008) involving 175 participants. Pooled results showed a non‐significant reduction in time to first successful SBT (MD ‐1.72 days, 95% CI ‐6.23 to 2.78; P value 0.45) with considerable heterogeneity (I² = 96%; P value < 0.00001) (Analysis 5.1; Analysis 6.1) (Table 2).
5.1. Analysis.
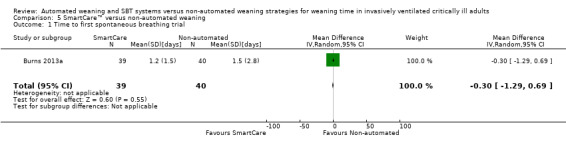
Comparison 5 SmartCare™ versus non‐automated weaning, Outcome 1 Time to first spontaneous breathing trial.
6.1. Analysis.
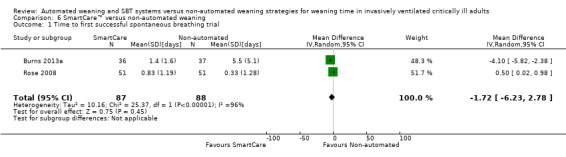
Comparison 6 SmartCare™ versus non‐automated weaning, Outcome 1 Time to first successful spontaneous breathing trial.
7.1, 8.1 and 9.1 Most protracted measure of mortality
We pooled the most protracted measure of mortality reported in six trials (Burns 2013a; Lellouche 2006; Liu 2013; Reardon 2011; Rose 2008; Stahl 2009) involving 470 participants. Aggregated data demonstrated no effect of SmartCare™ on mortality (RR 1.15, 95% CI 0.74 to 1.79; P value 0.53) with unimportant heterogeneity (I² = 21%; P value 0.27) (Analysis 8.1; Analysis 9.1; Analysis 7.1).
8.1. Analysis.
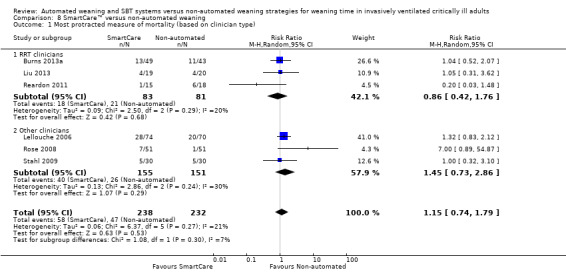
Comparison 8 SmartCare™ versus non‐automated weaning, Outcome 1 Most protracted measure of mortality (based on clinician type).
9.1. Analysis.
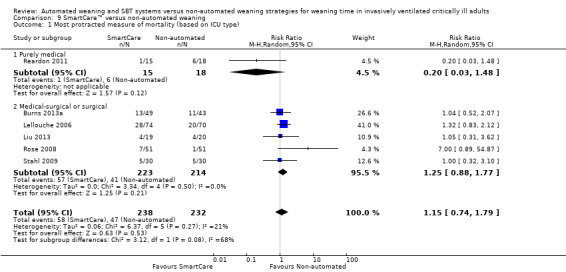
Comparison 9 SmartCare™ versus non‐automated weaning, Outcome 1 Most protracted measure of mortality (based on ICU type).
7.1. Analysis.
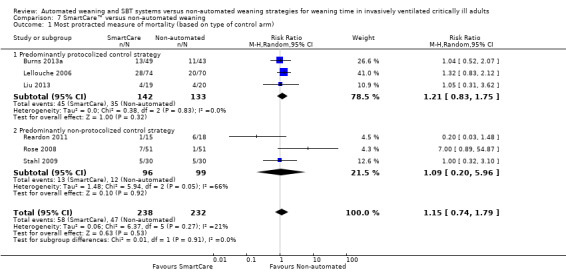
Comparison 7 SmartCare™ versus non‐automated weaning, Outcome 1 Most protracted measure of mortality (based on type of control arm).
10.1 ICU mortality
Pooled data from four trials (Burns 2013a; Lellouche 2006; Liu 2013; Stahl 2009) involving 335 participants showed no effect of automated weaning with SmartCare™, compared with non‐automated weaning, on ICU mortality (RR 0.97, 95% CI 0.62 to 1.50; P value 0.88) in the absence of heterogeneity (I² = 0%; P value 0.95) (Analysis 10.1).
10.1. Analysis.
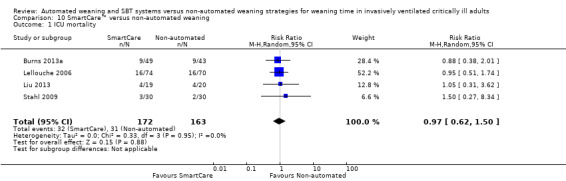
Comparison 10 SmartCare™ versus non‐automated weaning, Outcome 1 ICU mortality.
11.1 Hospital mortality
Hospital mortality was reported in four trials (Burns 2013a; Lellouche 2006; Reardon 2011; Stahl 2009) involving 329 participants. Pooled data showed no effect of SmartCare™, compared with non‐automated weaning, on hospital mortality (RR 1.09, 95% CI 0.71 to 1.67; P value 0.68) with unimportant heterogeneity (I² = 15%; P value 0.32) (Analysis 11.1).
11.1. Analysis.
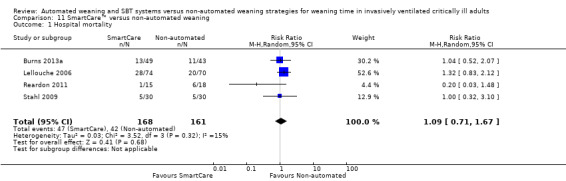
Comparison 11 SmartCare™ versus non‐automated weaning, Outcome 1 Hospital mortality.
12.1 Ventilator‐associated pneumonia
We found no effect of SmartCare™ on the proportion of participants developing ventilator‐associated pneumonia (RR 0.88, 95% CI 0.64 to 1.21; P value 0.42) in four trials (Burns 2013a; Lellouche 2006; Liu 2013; Ma 2010) including 337 participants with no heterogeneity (I² = 0%; P value 0.72) (Analysis 12.1).
12.1. Analysis.
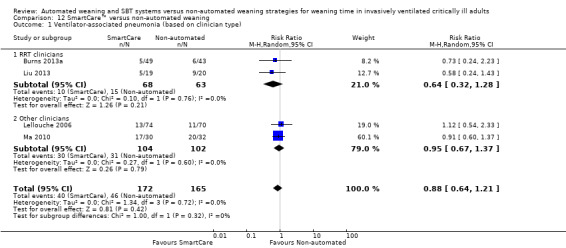
Comparison 12 SmartCare™ versus non‐automated weaning, Outcome 1 Ventilator‐associated pneumonia (based on clinician type).
13.1 Total duration of mechanical ventilation
The total duration of mechanical ventilation was reported in seven trials (Bifulco 2008; Burns 2013a; Lellouche 2006; Lim 2012; Liu 2013; Rose 2008; Stahl 2009) involving 520 participants. Pooled data showed a significant reduction in total duration of mechanical ventilation of 1.8 days favouring SmartCare™ (MD ‐1.68 days, 95% CI ‐3.33 to ‐0.03; P value 0.05) with substantial heterogeneity (I² = 53%; P value 0.05) (Analysis 13.1).
14.1 and 15.1 Length of intensive care unit stay
Length of ICU stay was reported by six trials involving 499 participants (Burns 2013a; Lellouche 2006; Liu 2013; Ma 2010; Rose 2008; Stahl 2009). Pooled data showed a significantly reduced length of ICU stay with SmartCare™ weaning (MD ‐5.70 days, 95% CI ‐10.54 to ‐0.85; P value 0.02) amidst substantial heterogeneity (I² = 66%; P value 0.01) (Analysis 14.1; Analysis 15.1).
14.1. Analysis.
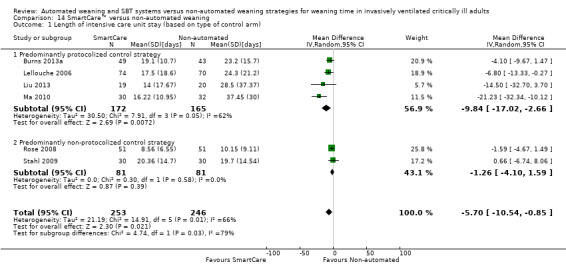
Comparison 14 SmartCare™ versus non‐automated weaning, Outcome 1 Length of intensive care unit stay (based on type of control arm).
15.1. Analysis.
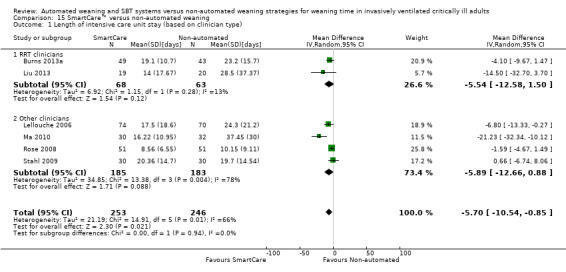
Comparison 15 SmartCare™ versus non‐automated weaning, Outcome 1 Length of intensive care unit stay (based on clinician type).
16.1 and 17.1 Use of non‐invasive ventilation following extubation
Four trials (Burns 2013a; Lellouche 2006; Liu 2013; Rose 2008) involving 377 participants reported use of NIV following extubation. Pooled data showed no effect of SmartCare™ on postextubation NIV use (RR 0.68, 95% CI 0.44 to 1.06; P value 0.09) with unimportant heterogeneity (I² = 2%; P value 0.38) (Analysis 16.1; Analysis 17.1).
16.1. Analysis.
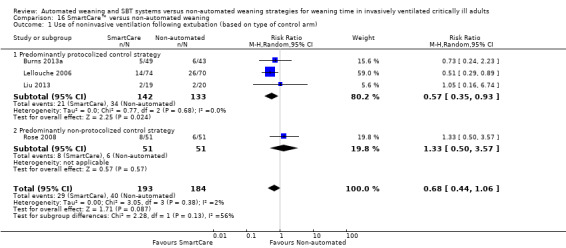
Comparison 16 SmartCare™ versus non‐automated weaning, Outcome 1 Use of noninvasive ventilation following extubation (based on type of control arm).
17.1. Analysis.
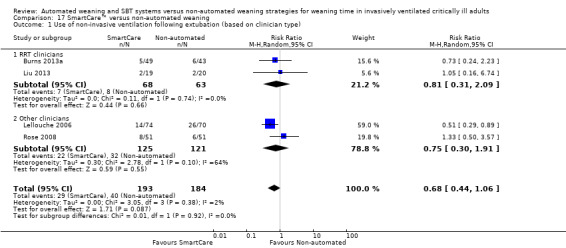
Comparison 17 SmartCare™ versus non‐automated weaning, Outcome 1 Use of non‐invasive ventilation following extubation (based on clinician type).
18.1 and 19.1 Adverse event: reintubation
Reintubation was reported in six trials (Burns 2013a; Lellouche 2006; Liu 2013; Ma 2010; Rose 2008; Stahl 2009) involving 491 participants, and no effect of SmartCare™ compared with non‐automated weaning was observed (RR 0.88, 95% CI 0.64 to 1.22; P value 0.44) with no heterogeneity (I² = 0%; P value 0.71) (Analysis 18.1; Analysis 19.1).
18.1. Analysis.
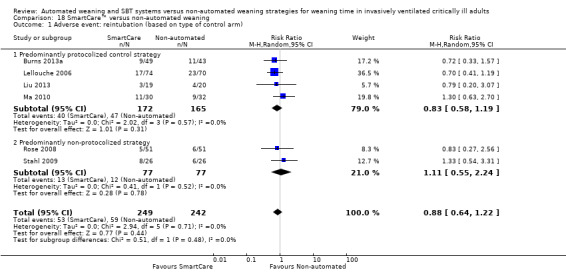
Comparison 18 SmartCare™ versus non‐automated weaning, Outcome 1 Adverse event: reintubation (based on type of control arm).
19.1. Analysis.
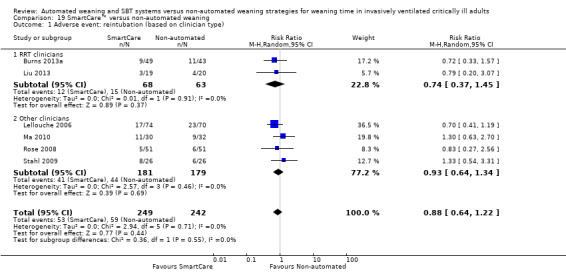
Comparison 19 SmartCare™ versus non‐automated weaning, Outcome 1 Adverse event: reintubation (based on clinician type).
20.1 Adverse event: self‐extubation
Self‐extubation was reported in only three trials (Burns 2013a; Lellouche 2006; Liu 2013) involving 263 participants. SmartCare™ had no effect on rate of self‐extubation (RR 0.86, 95% CI 0.36 to 2.03; P value 0.72) with no heterogeneity (I² = 0%; P value 0.62) (Analysis 20.1).
20.1. Analysis.
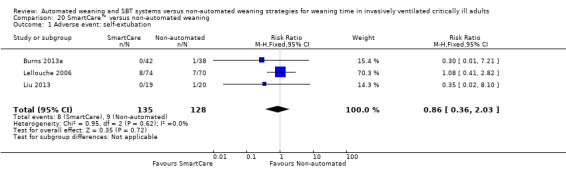
Comparison 20 SmartCare™ versus non‐automated weaning, Outcome 1 Adverse event: self‐extubation.
21.1 Adverse event: tracheostomy
Five trials (Burns 2013a; Lellouche 2006; Liu 2013; Ma 2010; Rose 2008) involving 439 participants reported tracheostomy rates. Pooled data did not support a reduced tracheostomy rate with SmartCare™ (RR 0.86, 95% CI 0.56 to 1.31; P value 0.48) amidst moderate heterogeneity (I² = 40%; P value 0.15) (Analysis 21.1).
21.1. Analysis.
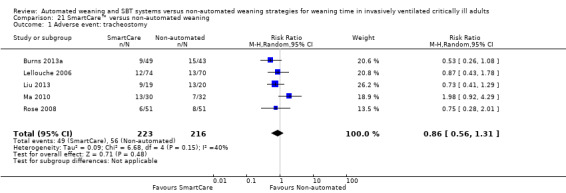
Comparison 21 SmartCare™ versus non‐automated weaning, Outcome 1 Adverse event: tracheostomy.
22.1 Adverse event: pneumothorax
We found no effect of SmartCare™ on pneumothorax (RR 0.55, 95% CI 0.17 to 1.73; P value 0.30) in three trials (Burns 2013a; Lellouche 2006; Ma 2010) involving 298 participants with no heterogeneity (I² = 0%; P value 0.70) (Analysis 22.1).
22.1. Analysis.
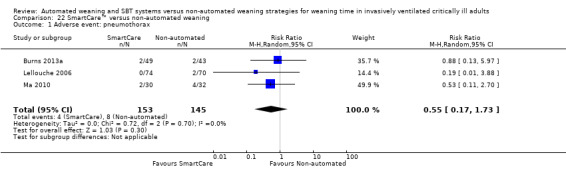
Comparison 22 SmartCare™ versus non‐automated weaning, Outcome 1 Adverse event: pneumothorax.
23.1 and 24.1 Prolonged mechanical ventilation (> seven days and > 14 days)
Two trials reported the proportions of participants requiring prolonged mechanical ventilation for > seven days among 77 participants (Jiang 2006; Liu 2013) and noted a significant reduction favouring SmartCare™ (RR 0.44, 95% CI 0.23 to 0.85; P value 0.01) with no heterogeneity (I² = 0%; P value 0.75) (Analysis 23.1). Three trials (Jiang 2006; Lellouche 2006; Rose 2008) reported on 284 participants requiring more than 14 days of mechanical ventilation. The pooled data analysis revealed a nearly significant decrease in the proportions of participants requiring mechanical ventilation for longer than 14 days with SmartCare™ (RR 0.61, 95% CI 0.37 to 1.00; P value 0.05) with no heterogeneity (I² = 0%; P value 0.93); however, the upper bound of the CI for the summary estimate did not exclude cases of no effect (Analysis 23.1; Analysis 24.1).
23.1. Analysis.
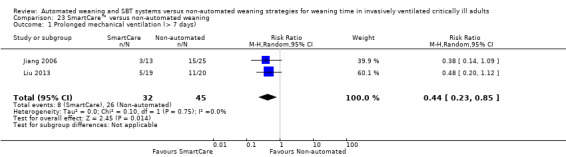
Comparison 23 SmartCare™ versus non‐automated weaning, Outcome 1 Prolonged mechanical ventilation (> 7 days).
24.1. Analysis.
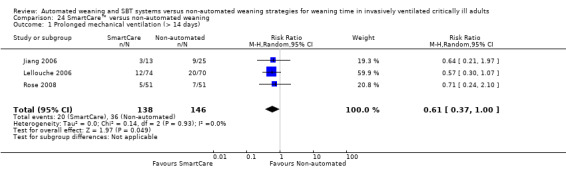
Comparison 24 SmartCare™ versus non‐automated weaning, Outcome 1 Prolonged mechanical ventilation (> 14 days).
25.1 Prolonged mechanical ventilation (> 21 days)
Pooled results of three trials (Burns 2013a; Lellouche 2006; Liu 2013) involving 258 participants showed a significant decrease in the proportions of participants requiring mechanical ventilation for longer than 21 days (RR 0.39, 95% CI 0.18 to 0.86; P value 0.02) favouring SmartCare™ with no heterogeneity (I² = 0%; P value 0.86) (Analysis 25.1).
25.1. Analysis.
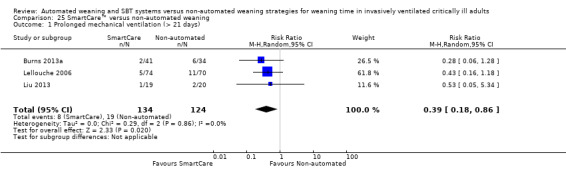
Comparison 25 SmartCare™ versus non‐automated weaning, Outcome 1 Prolonged mechanical ventilation (> 21 days).
26.1 Length of hospital stay
Upon pooling effect estimates from three trials (Burns 2013a; Lellouche 2006; Rose 2008) involving 338 participants, we found a non‐significant reduction in length of hospital stay (MD ‐2.14 days, 95% CI ‐7.18 to 2.89; P value 0.40) favouring SmartCare™ with no heterogeneity (I² = 0%; P value 0.75) (Analysis 26.1).
26.1. Analysis.
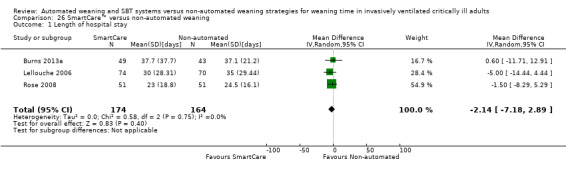
Comparison 26 SmartCare™ versus non‐automated weaning, Outcome 1 Length of hospital stay.
Additional reported outcomes
No trial reported on clinician comfort with alternative weaning protocols or quality of life. Single trials reported time from meeting discontinuation criteria to extubation (Rose 2008), acceptance of alternative weaning strategies by physicians and RRTs (Burns 2013a) and of sedation protocols by critical care nurses (Burns 2013a) and weaning success rates (Stahl 2009).
Four trials (Burns 2013a; Jiang 2006; Lellouche 2006; Stahl 2009) involving 335 participants reported time from initiation of mechanical ventilation to randomization. Time to reintubation was reported by two trials involving 34 participants (Burns 2013a; Stahl 2009), and three trials (Burns 2013a; Lellouche 2006; Rose 2008) involving 338 participants reported death on mechanical ventilation. Although the number of ventilator adjustments was reported in two trials (Ma 2010; Stahl 2009) involving 114 participants, one trial reported the numbers of changes to PS, PEEP and FiO2 per hour (Stahl 2009) separately, and the other trial reported the number of ventilator adjustments per participant (Ma 2010). Consequently, we considered these outcomes to be qualitatively too dissimilar for pooling.
Subgroup analyses
The type of clinician (RRT vs other) involved in implementing SmartCare™ had no effect on weaning time, overall mortality, VAP, length of ICU stay, NIV use or reintubation.
Subgroup analysis of the type of ICU demonstrated a significant subgroup effect on weaning time (Chi2 = 12.21; P value 0.0005) for purely medical participants (one trial; MD ‐4.78, 95%CI ‐6.20 to ‐3.36) versus medical‐surgical or surgical participants (six trials; MD ‐1.85, 95% CI ‐2.67 to ‐1.04). In addition, SmartCare™ demonstrated a trend towards benefit (Chi2 = 3.12; P value 0.08) for overall mortality when one trial with purely medical participants (RR 0.20, 95% CI 0.03 to 1.48) was compared with five trials with mixed medical‐surgical and surgical populations (RR 1.25, 95% CI 0.88 to 1.77) with wide confidence intervals. Subgroup analyses were inestimable for VAP, ICU stay, NIV use and reintubation.
Subgroup analysis of the type of non‐automated control strategy used for ICU stay significantly (Chi2= 4.74; P value 0.03) favoured predominantly protocolized control strategies (four trials; MD ‐9.84, 95% CI ‐17.02 to ‐2.66) versus predominantly non‐protocolized control weaning strategies (two trials; MD ‐1.26 days, 95% CI ‐4.10 to 1.59). Similarly, we found a trend (Chi2 = 2.28; P value 0.13) towards less frequent use of NIV following extubation in three trials comparing SmartCare™ versus a protocolized control weaning strategy (RR 0.57, 95% CI 0.35 to 0.93) versus one trial using a non‐protocolized control weaning strategy (RR 1.33, 95% CI 0.50 to 3.57). Subgroup analysis of the impact of the alternative control weaning strategies on VAP was inestimable and was not significantly different for weaning time, overall mortality and reintubation.
Sensitivity analysis
Exclusion of two trials with high risk of bias (Jiang 2006; Liu 2013) supported a trend towards benefit of SmartCare™ for weaning time (RR ‐2.14 days, 95% CI ‐3.20 to ‐1.07; P value < 0.0001; I2 = 14%; P value 0.32) (Analysis 27.1) with non‐significant between‐subgroup differences (P value 0.06).
27.1. Analysis.
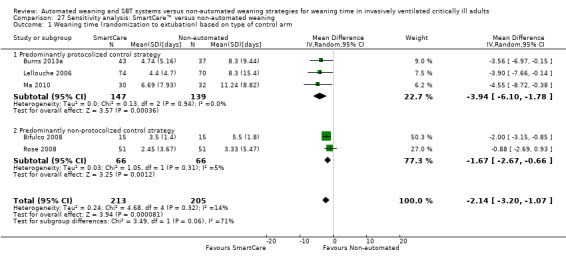
Comparison 27 Sensitivity analysis: SmartCare™ versus non‐automated weaning, Outcome 1 Weaning time (randomization to extubation) based on type of control arm.
Publication bias
We did not assess for publication bias, as only seven trials reported on the primary outcome.
Discussion
Summary of main results
We identified 10 trials of predominantly moderate quality comparing SmartCare™ versus non‐automated weaning strategies involving 654 participants. Compared with non‐automated strategies, SmartCare™ significantly decreased weaning time (Table 1), time to successful extubation (Table 1), length of ICU stay (Table 1), total duration of mechanical ventilation (Table 1) and proportions of participants who received mechanical ventilation for longer than seven days and 21 days. Although not achieving statistical significance, SmartCare™ demonstrated a trend toward fewer participants receiving ventilation (for longer than 14 days) without increased adverse events, compared with non‐automated weaning strategies. SmartCare™ demonstrated no effect on time to first successful SBT (Table 1), mortality (Table 2), length of hospital stay and rates of VAP, reintubation (Table 2), self‐extubation and tracheostomy in a small number of trials reporting these outcomes. Subgroup analysis suggested that trials with protocolized (versus non‐protocolized) control weaning strategies had significantly shorter ICU stays, and sensitivity analysis supported a trend toward reduced weaning time after two trials with high risk of bias were excluded.
Overall completeness and applicability of evidence
We found important differences in the summary estimate of effect favouring SmartCare™ in seven trials reporting weaning time and in seven trials reporting time to successful extubation. Time to successful extubation, although less frequently reported, is the more important outcome, as it limits the potential bias of shortening weaning time by extubating participants early only to subsequently fail and require reintubation. Through correspondence with investigators, we were able to clarify the outcomes reported and to pool them separately in our review. In addition, we identified a positive effect of SmartCare™ in reducing length of ICU stay (six trials) and total duration of mechanical ventilation (seven trials). However, we did not find evidence of a beneficial effect of SmartCare™ compared with non‐automated weaning strategies in four or fewer trials reporting time to first SBT, time to first successful SBT, length of hospital stay and rates of NIV use following extubation and self‐extubation. Despite reducing time to successful extubation and total duration of mechanical ventilation, SmartCare™ had no effect on the incidence of ICU and hospital mortality, and of VAP. However, unlike the non‐invasive approach to weaning, which has been shown to significantly reduce mortality and VAP compared with continued invasive weaning in a meta‐analysis (Burns 2013b), SmartCare™ requires an indwelling airway. In this circumstance and in the absence of large reductions in weaning time, SmartCare™ would not be expected to favourably impact rates of mortality and intubation and mechanical ventilation–related complications. Notwithstanding, reductions in weaning time, time to successful extubation, ICU stay and total duration of mechanical ventilation may lead to important reductions in length of hospital stay and may favourably impact resource utilization. These benefits may be even greater outside of the academic or closed ICU setting. It is important to note that the reintubation rate (six trials) was similar between SmartCare™ and non‐automated weaning strategies, suggesting that SmartCare™ did not lead to erroneous or premature extubation. Although SmartCare™ demonstrated favourable trends toward fewer participants requiring prolonged ventilation at greater than 14 days (three trials), it had no effect on tracheostomy rate (five trials).
This review was strengthened by an extensive search for relevant trials. We conducted duplicate, independent citation screening and data abstraction and corresponded with all lead investigators to clarify study methods. Pooling of results in a meta‐analysis implicitly assumes that the studies are sufficiently similar with respect to the populations studied, study interventions, outcomes and methodological quality that one could reasonably expect a comparable underlying treatment effect. We exclusively used random‐effects models for pooling data, which take into consideration between‐study and within‐study variation. We planned separate reviews to evaluate the impact of SmartCare™ in the postoperative setting and in those requiring invasive ventilation for a longer time, acknowledging the differences between patients who require disconnection and those who require more formal weaning and discontinuation strategies. A priori, we planned to perform sensitivity and subgroup analyses to explain anticipated differences among study results. We expected heterogeneity across studies in pooling continuous outcomes commonly reported in weaning trials.
Quality of the evidence
In summary estimates, we found that SmartCare™ significantly reduced weaning time, length of ICU stay and total duration of mechanical ventilation—all with substantial heterogeneity and time to successful extubation with heterogeneity. To this end, the impact of heterogeneity was unimportant for only three outcomes demonstrating benefit with SmartCare™ (time to successful extubation and proportions requiring mechanical ventilation for longer than seven days and 21 days). SmartCare™ showed a trend toward fewer patients requiring ventilation for > 14 days amidst no heterogeneity (Higgins 2011). Most trials used median and interquartile ranges as summary statistics for continuous outcomes, suggesting that data were skewed.
To limit selection bias, nine trials used random sequence generation, and seven trials reported strategies to conceal allocation concealment. Five trials each had completed outcome reporting, and six trials were judged to be free of selective outcome reporting. One trial stopped early for perceived benefit, and nine trials adhered to intention‐to‐treat in reporting results. Overall, eight trials were judged to be at moderate risk of bias, and two trials were judged to be at high risk of bias. All trials were downgraded because of lack of blinding of outcome assessors. Variable reporting of key outcomes and summary statistics for continuous outcomes among the included trials, heterogeneity in reporting the primary outcome and the absence of a single, large, adequately powered RCT comparing SmartCare™ versus a non‐automated weaning strategy limit the strength of inferences that can be made from this review. Other GRADE considerations of consistency of effect, indirectness and publication bias were not thought to be important, necessitating downgrading.
Potential biases in the review process
When mean and standard deviation were not available, we approximated the mean from the median and estimated the standard deviation as the interquartile range divided by 1.33 (Higgins 2011) for pooling of outcomes. Although this approach is widely used to pool continuous outcomes, the accuracy of these estimations for aggregate outcomes is unknown. In addition to variability in outcome reporting, trials included in our meta‐analysis varied in how they identified weaning candidates and titrated and discontinued mechanical support. Multi‐disciplinary protocols to identify weaning candidates and to conduct SBTs have been shown to reduce the duration of mechanical ventilation (Kollef 1997; Marelich 2000; Perren 2002). For patients failing an SBT, PS or intermittent or once‐daily SBTs are favoured over SIMV to facilitate discontinuation of support (Brochard 1994; Butler 1999; Esen 1992; Esteban 1995; Jounieaux 1994; Tomlinson 1989).
Daily screening and criteria to identify candidates for an SBT or weaning readiness were applied in all 10 trials. Among their inclusion criteria, five trials included a PS trial to ensure that weaning candidates could be supported by using SmartCare™, and only three trials demonstrated that weaning candidates were not ready for extubation by including patients who failed a prerandomization SBT. Methods for identifying and including weaning candidates may impact study estimates of the duration of ventilation but are unlikely to result in between‐group performance bias. To this end, four trials (Burns 2013a; Jiang 2006; Lellouche 2006; Stahl 2009) provided estimates of the duration of ventilation from initiation to randomization, which were similar between groups with unimportant between‐group heterogeneity. Conversely, unequal or inconsistent use of weaning protocols and the frequency with which SBTs were permitted in the non‐automated weaning strategy represent important postrandomization study features that could bias estimates of the duration of ventilation in unblinded weaning trials. Trials specified comparing SmartCare™ versus an evidence‐based standard of care (Reardon 2011), a paper‐based weaning protocol or written guidelines (Burns 2013a; Liu 2013; Ma 2010) and a conventional weaning protocol, typically based on usual or local practice (Bifulco 2008; Jiang 2006; Lellouche 2006; Lim 2012; Rose 2008; Stahl 2009), with one trial (Lellouche 2006) affirming weaning guidelines at four of five participating centres. Two trials specified use of SIMV with PS (Jiang 2006; Ma 2010), and five trials specified use of PS (Burns 2013a; Liu 2013; Reardon 2011; Rose 2008; Stahl 2009) in the control arm. Three trials used a combination of modes (Lellouche 2006; Lim 2012) or PS with T‐piece trials (Reardon 2011).
Seven trials (Burns 2013a; Jiang 2006; Lellouche 2006; Liu 2013; Ma 2010; Reardon 2011; Rose 2008) utilized postrandomization SBTs as part of their control weaning strategy. Although patients who survive to wean may be at lower risk for death compared with those in studies evaluating initial mechanical ventilation strategies, only four trials (Burns 2013a; Lellouche 2006; Liu 2013; Rose 2008) specifically reported deaths on mechanical ventilation, and no trial conducted a competing risk analysis or reported weaning time separately among survivors and non‐survivors. Another important factor that may impact duration of ventilation is sedation administration (Brook 1999). To this end, two trials used a sedation guide (Burns 2013a) or a protocol with daily interruption (Liu 2013) in both treatment groups to limit performance bias during weaning, and two trials (Burns 2013a; Lellouche 2006) reported non‐significant differences between treatment groups in the sedation administered. Finally, our search for RCTs comparing alternative weaning strategies has not been updated since May 2013; additional trials may have been published since that time.
Clinicians endeavour to optimize the time to liberation from invasive ventilation while minimizing the risks associated with failed attempts at extubation and the complications associated with prolonged invasive ventilation (Epstein 1997). SmartCare™, by automating and titrating the level of PS provided and the conduct of SBTs in the busy ICU setting, reduces the time spent weaning and in the ICU without increasing the risk of complications. Notwithstanding, a large trial is required to substantiate our findings and to determine whether SmartCare™ reduces weaning‐related complications. Moreover, the optimal timing for transitioning patients to SmartCare™ and the effects of this weaning strategy in non‐academic settings remain to be determined.
Agreements and disagreements with other studies or reviews
This is the first systematic review and meta‐analysis comparing the effects of automated weaning and SBT systems (limited to trials evaluating SmartCare™) versus non‐automated weaning strategies on important clinical outcomes. Unlike other weaning systems, SmartCare™ integrates several strategies (use of a weaning protocol and use of PS mode) demonstrated to be of benefit in previous weaning trials, and automates the conduct of SBTs. We excluded one trial in which most participants required discontinuation (following short‐term ventilation), in an effort to evaluate the effect of SmartCare™ in a homogeneous population that required weaning. In this trial (Schadler 2012), 26% of those given automated weaning and 31% of control participants were ventilated for longer than four days, with approximately 10% of the study population ventilated for longer than 14 days. Because only three trials included SBT failure among their inclusion criteria, a treatment effect may have been diluted in both treatment groups by inclusion of patients who may not truly have required weaning.
Authors' conclusions
Implications for practice.
Compared with non‐automated weaning strategies, weaning with SmartCare™ decreased weaning time, time to successful extubation, length of ICU stay and proportions of participants requiring prolonged ventilation for longer than seven days and 21 days. Summary estimates from our review suggest that these benefits may be achieved without increasing the risk of adverse events. However, the quality of evidence was low to moderate in most included RCTs. In the absence of a single large RCT, summary estimates from 10 small trials included in this meta‐analysis suggest that SmartCare™ significantly reduces weaning time, time to successful extubation, ICU discharge and proportions of patients receiving prolonged ventilation without increasing adverse events.
Implications for research.
A well‐designed, adequately powered RCT with explicitly defined end points to compare alternative approaches to weaning is justified.
Several unanswered questions remain regarding the role of SmartCare™ in weaning in the ICU. These include the following.
Does SmartCare™ reduce intubation and weaning–related complications?
Does SmartCare™ reduce time to successful extubation and proportions of patients requiring protracted ventilation?
Does the cause of respiratory failure (COPD versus other) influence the effectiveness of SmartCare™ weaning?
Does illness severity at the time of randomization, or duration of mechanical ventilation before randomization, influence the effectiveness of SmartCare™ weaning?
Is there a role for SmartCare™ in non‐academic ICUs?
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
In future iterations of the review, we will consider including other strategies that investigate nearly fully automated systems, which automate alterations in the level of support provided and in the conduct of SBTs.
We did not include an SoF table for the outcome of time to first SBT, as only one trial reported this outcome.
Acknowledgements
We would like to thank David Lightfoot for help in preparing the search strategies, Karen Hovhannisyan for conducting the revised searches and Jane Cracknell for editorial advice provided during preparation of this review.
We would also like to thank Harald Herkner (content editor: review), Mathew Zacharias (content editor: protocol), Nathan Pace (statistical editor) and peer reviewers (Janet Wale and Drs. Rodrigo Cavallazzi, Ross Freebairn and Thordis Thomsen).
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor Ventilators, Mechanical explode all trees #2 MeSH descriptor Ventilator Weaning explode all trees #3 MeSH descriptor Ventilators, Negative‐Pressure explode all trees #4 (ventilat* or wean*):ti,ab #5 invasive near ventil* #6 artificial near respirat* #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Therapy, Computer‐Assisted explode all trees #9 automat* near system* #10 smartcare or (smart near care) #11 computer near assist* #12 (#8 OR #9 OR #10 OR #11) #13 (#7 AND #12) #14 (#10 OR #13)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp ventilators, mechanical/or exp ventilator weaning/ or exp ventilators, negative‐pressure/ or ventilat$.mp. or (invasive adj3 ventil*).mp. or wean*.mp. or (artificial adj3 respirat*).mp.
2.exp "Therapy, Computer‐Assisted"/ or (automat* adj3 system*).mp. or (smartcare or (smart adj3 care)).mp. or (computer adj3 assist*).mp.
3. 1 and 2
4. (randomized controlled trial.pt. or controlled clinical trial.pt.or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals.sh not (humans.sh and animals.sh))
5. 3 and 4
6. smartcare.mp.
7. 6 or 5
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp Ventilator/ or (ventilat$ or wean$).mp
2. (artificial adj3 respirat*).mp. or exp Artificial Ventilation/
3. ((mechanical or invasive) adj3 ventil*).mp.
4. 1 or 2 or 3
5. exp Computer System/ or (computer adj3 assist*).mp. or (automat* adj3 system*).mp.
6. SmartCare.mp. or (Smart adj3 care).mp
7. 4 and (or/5‐6)
8. ((((singl* or doubl* or tripl*) adj3 blind) or crossover).ti,ab. or multicenter.ab. or placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh.
9. 8 and 7
10. SmartCare.mp.
11. 9 or 10
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
Appendix 4. CINAHL (EBSCOhost) search strategy
S1. TX ventilator or (MM "Ventilators, Mechanical") or (MM "Pressure Support Ventilation") or (MH "Ventilation, High Frequency+")
S2. TX computer assisted or (MH "Decision Making, Computer Assisted+") or (MH "Computers and Computerization+")
S3. S1 and S2
S4. TX smartcare or TX smart care
S5. (MM "Random Assignment" or MH "Clinical Trials+" or MM "Placebos" or ( (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies") ) or MM "Multi center Studies" ) or ( MM "Crossover Design" or TI ( random* or placebo* or multi?center or crossover ) or AB ( random* or placebo* or multi?center or crossover ) or TI trial* or AB ( controlled and study ))
S6. S5 and (S4 or S3)
Appendix 5. All Evidence‐Based Medicine reviews
We will use the same strategy as per the Cochrane Database of Systematic Reviews to search other Evidence‐Based Medicine Reviews, including ACP Journal Club, DARE, CCTR, CMR, HTA and NHSEED.
1. ventilator$.mp. or ventilation.mp.
2. Artificial respirat$.mp. [mp=ti, ot, ab, tx, kw, ct, sh, hw]
3. 1 or 2
4. computer assisted.mp.
5. SmartCare.mp.
6. 4 or 5
7. 3 and 6
Appendix 6. Ovid HealthSTAR search strategy
(1999 to date)
1. exp ventilator, mechanical/ or exp ventilator weaning/ or exp ventilators, negative‐pressure/ or ventilat$.mp.
2.*"Therapy, Computer‐Assisted"/ and ventilat$.mp.
3. (smartcare or (smart adj1 care)).mp.
4. 1 and (2 or 3)
Appendix 7. Data extraction form
Data abstraction form—SC weaning systematic review and meta‐analysis
Name of data abstractor (first, last) ______________ __________________
1. Study ID
First author surname, year of publication _______________ ___________________
Is this a duplicate publication?
□ No
□ Yes, please provide details _____________________________________________
2. Study eligibility
a. Study design
Is the study clearly randomized? □ Yes □ Unclear □ No
Is the study pseudorandomized? □ Yes □ Unclear □ No
b. Study participants
Are the participants adults? □ Yes □ Unclear □ No
Are the participants invasively ventilated? □ Yes □ Unclear □ No
c. Study Interventions
Was one group weaned using SmartCare™? □ Yes □ Unclear □ No
Was another group weaned using a non‐ □ Yes □ Unclear □ No
automated weaning strategy (i.e. not involving
a closed‐loop system)?
d. Study outcomes
Did the study report any of the following outcomes?
Time from randomization to extubation □ Yes □ Unclear □ No
Time to successful extubation □ Yes □ Unclear □ No
Time to first spontaneous breathing trial □ Yes □ Unclear □ No
Time to first successful SBT □ Yes □ Unclear □ No
Mortality, specify time point(s)_________ □ Yes □ Unclear □ No
Mortality, specify time point(s)_________ □ Yes □ Unclear □ No
Ventilator‐associated pneumonia □ Yes □ Unclear □ No
Total duration of mechanical ventilation □ Yes □ Unclear □ No
Length of intensive care unit stay □ Yes □ Unclear □ No
Length of hospital stay □ Yes □ Unclear □ No
Use of NIV following extubation □ Yes □ Unclear □ No
Adverse events (including but not limited to □ Yes □ Unclear □ No
reintubation, self‐extubation, tracheostomy,
prolonged ventilation or other adverse event)
Clinician acceptance of weaning strategies □ Yes □ Unclear □ No
e. Exclusion criteria
Did the author report on a study in which:
Most participants required planned short‐term ventilation □ Yes □ Unclear □ No
Study explored use of NIV in discontinuation/weaning □ Yes □ Unclear □ No
Study evaluated exclusively tracheostomized □ Yes □ Unclear □ No
participants
f. Does the study meet all of the above criteria and meet none of the exclusion criteria? □ Yes □ No
If yes, please proceed to page 2.
Decision □ Include □ Exclude, reason__________________________________
□ Additional information is required before a decision can be made
3. Information source
How was the article/abstract identified?
Search of electronic databases? □ Yes □ No
Search of trials registries? □ Yes □ No
Manual searches of conference proceedings? □ Yes □ No
Unpublished data? □ Yes □ No
4. Potential sources of bias
Adequate/Yes (criteria appropriately applied and described in the report or acknowledged from the primary author of the study) Unclear (criteria not described or impossible to acquire from the study author) Inadequate/No (criteria inappropriately applied)
Selection bias
Method of randomization?
Describe the method used to generate the allocation sequence. Specify______________________________________
Check grade. □ Adequate □ Unclear □ Inadequate
Time of randomization
(e.g. admission, upon meeting criteria) Specify_______________________________________
Allocation concealment
Describe the method used to conceal the random allocation Specify_______________________________________
sequence. Check grade. □ Adequate □ Unclear □ Inadequate □ Not used
Detection bias
Outcome assessor blinding?
Were outcomes assessors separate from individuals
administering or supervising assigned interventions? Specify_______________________________________
Check ONE. □ Yes □ Unclear □ No
Attrition bias
Dropouts/withdrawals?
Were any withdrawals/dropouts described? (Check ONE) □ Yes □ Unclear □ No
Did they occur with similar frequency between study groups? (Check ONE) □ Yes □ No
Intention‐to‐treat analysis?
Were all participants analysed according to the group to which they were
initially assigned, whether they received it or not?
Check ONE.
□ All participants entered into trial (indicate 1 of 2 below)
□ 15% or fewer excluded
□ more than 15% excluded
□ Unclear
□ Not analysed as intention‐to‐treat
Overall quality classification
Overall summary (assign ONE category) □ All criteria met □ One or more criteria unclear □ One or more criteria not applied
5. Setting
Country/countries ____________________________________________________________
Number of participating ICUs ______________________________________________________________
Types of ICU(s) □ Medical □ Surgical □ Medical‐surgical □ Cardiac‐surgical
(check all that apply) □ Coronary care unit □ Other, specify___________________________
6. Participants
| Criterion |
SmartCare™ group (n= ) |
Control group 1 (n= ) |
Control group 2 (n= ) |
|
No. randomly assigned |
|||
|
No. analysed |
|||
|
Reasons for differences (if any) |
|
||
|
Inclusion criteria |
______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ |
||
|
Exclusion criteria |
______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ |
||
7. Study interventions
|
Did the study include readiness to wean criteria? (If yes, please list) Did the study screen daily for these criteria? |
□ Yes □ Unclear □ No _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ □ Yes □ Unclear □ No |
|
Did the study include an SBT? If yes, what technique was used for the SBT? (e.g. PS, T‐tube, CPAP, other, not specified) If yes, what was the duration of SBT? If yes, criteria for SBT failure provided? |
□ Yes □ Unclear □ No _________________________________________________________ _________________________________________________________ _________________________________________________________ □ Yes □ Unclear □ No If yes, please list criteria: _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ |
|
Control arm weaning strategy Control strategy described? If yes, how was weaning guided in the control arm? If yes, what mode or technique was used in the control arm? Type of clinician responsible for implementing the control strategy? (check ALL that apply) |
□ Yes □ Unclear □ No □ Protocol □ Usual practice (clinician discretion) □ Other, please specify______________________________________ _________________________________________________________ _________________________________________________________ □ SIMV □ PS □ Daily T‐piece □ Intermittent (multiple daily) T‐piece □ Combination of the above, please specify ________________________________________________________ □ Other, please specify ________________________________________________________ □ Physician □ Nurse □ Respiratory therapist □ Kinesiotherapist □ Other, specify___________________________________________ □ Mixed, specify___________________________________________ |
|
SmartCare™ weaning arm Was SmartCare™ used in the intervention arm? Type of clinician responsible for implementing SmartCare™ strategy? (check ALL that apply) |
□ Yes □ Unclear □ No □ Physician □ Nurse □ Respiratory therapist □ Kinesiotherapist □ Other, specify___________________________________________ □ Mixed, specify___________________________________________ |
8. Study outcomes
| Weaning time (time from randomization to extubation) | □ Yes □ Unclear □ No |
| Time to successful extubation | □ Yes □ Unclear □ No |
| Time to first SBT | □ Yes □ Unclear □ No |
| Time to first successful SBT | □ Yes □ Unclear □ No |
| Mortality time point #1 __________ time point #2__________ time point #3__________ |
□ Yes □ Unclear □ No □ Yes □ Unclear □ No |
| Ventilator‐associated pneumonia | □ Yes □ Unclear □ No |
| Total duration of mechanical ventilation (from initiation to extubation) | □ Yes □ Unclear □ No |
| Length of ICU stay | □ Yes □ Unclear □ No |
| Length of hospital stay | □ Yes □ Unclear □ No |
| Use of non‐invasive ventilation following extubation | □ Yes □ Unclear □ No |
| Adverse events: (please check) reintubation self‐extubation requirement for tracheostomy prolonged mechanical ventilation _________days other (specify) ____________________________ |
□ Yes □ Unclear □ No □ Yes □ Unclear □ No □ Yes □ Unclear □ No □ Yes □ Unclear □ No □ Yes □ Unclear □ No |
| Clinician acceptance of weaning strategies |
□ Yes □ Unclear □ No |
Continuous outcomes
| Outcomes | Unit of measurement | Intervention group | Control group | 95% CI or additional information | |||||
| n |
Mean (SD) |
Median (IQR) | n | Mean (SD) | Median (IQR) | P value | |||
| Weaning time (time from randomization to extubation) | |||||||||
| Time to successful extubation | |||||||||
| Time to first SBT | |||||||||
| Time to first successful SBT | |
||||||||
| Total duration of mechanical ventilation (from initiation to extubation) | |||||||||
| Length of ICU stay | |||||||||
| Length of hospital stay | |||||||||
| Clinician acceptance of weaning strategies | |||||||||
| Other, please specify _____________________________ |
|||||||||
Dichotomous outcomes
| Outcomes |
Intervention group (n = ) |
Control group (n = ) |
P value | Additional information |
| Mortality time point #1 | ||||
| Mortality time point #2 | ||||
| Mortality time point #3 | ||||
| Ventilator‐associated pneumonia | ||||
| Use of non‐invasive ventilation following extubation |
||||
| Adverse events: Reintubation Self‐extubation Requirement for tracheostomy Prolonged mechanical ventilation _________days Other (specify) _________________________ |
||||
| Other outcome, please specify |
Please specify the numerator and the denominator for each outcome.
Other information that you believe is relevant to the results:
| Please provide data obtained from the primary author, additional results extrapolated from graphs, figures etc., in the space provided below |
| Additional concerns/points to be clarified? |
Data and analyses
Comparison 1. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning time (randomization to extubation) based on type of control arm | 7 | 495 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐3.99, ‐1.37] |
| 1.1 Predominantly protocolized control strategy | 4 | 325 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐4.26, ‐0.88] |
| 1.2 Predominantly non‐protocolized control strategy | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐4.75, ‐0.43] |
Comparison 2. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning time (randomization to extubation) based on clinician type | 7 | 495 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐3.99, ‐1.37] |
| 1.1 RRT clinicians | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐3.79, 0.07] |
| 1.2 Other clinicians | 5 | 376 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐4.69, ‐1.26] |
Comparison 3. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning time (randomization to extubation) based on ICU type | 7 | 495 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐3.99, ‐1.37] |
| 1.1 Purely medical | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐4.78 [‐6.20, ‐3.36] |
| 1.2 Medical‐surgical or surgical | 6 | 457 | Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐2.67, ‐1.04] |
Comparison 4. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to successful extubation | 7 | 516 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.89, ‐0.09] |
Comparison 5. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first spontaneous breathing trial | 1 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.29, 0.69] |
Comparison 6. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first successful spontaneous breathing trial | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐6.23, 2.78] |
Comparison 7. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Most protracted measure of mortality (based on type of control arm) | 6 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.74, 1.79] |
| 1.1 Predominantly protocolized control strategy | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.75] |
| 1.2 Predominantly non‐protocolized control strategy | 3 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.20, 5.96] |
Comparison 8. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Most protracted measure of mortality (based on clinician type) | 6 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.74, 1.79] |
| 1.1 RRT clinicians | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.76] |
| 1.2 Other clinicians | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.73, 2.86] |
Comparison 9. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Most protracted measure of mortality (based on ICU type) | 6 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.74, 1.79] |
| 1.1 Purely medical | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.48] |
| 1.2 Medical‐surgical or surgical | 5 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.88, 1.77] |
Comparison 10. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ICU mortality | 4 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.62, 1.50] |
Comparison 11. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital mortality | 4 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.71, 1.67] |
Comparison 12. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ventilator‐associated pneumonia (based on clinician type) | 4 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.21] |
| 1.1 RRT clinicians | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.28] |
| 1.2 Other clinicians | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.37] |
Comparison 14. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of intensive care unit stay (based on type of control arm) | 6 | 499 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐10.54, ‐0.85] |
| 1.1 Predominantly protocolized control strategy | 4 | 337 | Mean Difference (IV, Random, 95% CI) | ‐9.84 [‐17.02, ‐2.66] |
| 1.2 Predominantly non‐protocolized control strategy | 2 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐4.10, 1.59] |
| 2 Total duration of mechanical ventilation | 7 | 521 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐3.33, ‐0.03] |
14.2. Analysis.
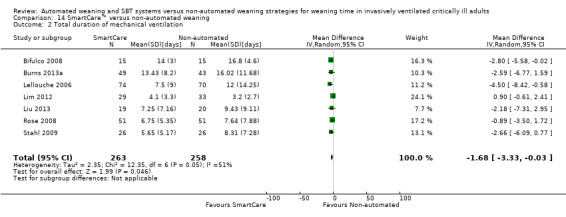
Comparison 14 SmartCare™ versus non‐automated weaning, Outcome 2 Total duration of mechanical ventilation.
Comparison 15. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of intensive care unit stay (based on clinician type) | 6 | 499 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐10.54, ‐0.85] |
| 1.1 RRT clinicians | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐5.54 [‐12.58, 1.50] |
| 1.2 Other clinicians | 4 | 368 | Mean Difference (IV, Random, 95% CI) | ‐5.89 [‐12.66, 0.88] |
Comparison 16. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of noninvasive ventilation following extubation (based on type of control arm) | 4 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.44, 1.06] |
| 1.1 Predominantly protocolized control strategy | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.35, 0.93] |
| 1.2 Predominantly non‐protocolized control strategy | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.50, 3.57] |
Comparison 17. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of non‐invasive ventilation following extubation (based on clinician type) | 4 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.44, 1.06] |
| 1.1 RRT clinicians | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.31, 2.09] |
| 1.2 Other clinicians | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.30, 1.91] |
Comparison 18. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: reintubation (based on type of control arm) | 6 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.22] |
| 1.1 Predominantly protocolized control strategy | 4 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.19] |
| 1.2 Predominantly non‐protocolized strategy | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
Comparison 19. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: reintubation (based on clinician type) | 6 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.22] |
| 1.1 RRT clinicians | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.37, 1.45] |
| 1.2 Other clinicians | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.64, 1.34] |
Comparison 20. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: self‐extubation | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.36, 2.03] |
Comparison 21. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: tracheostomy | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.56, 1.31] |
Comparison 22. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: pneumothorax | 3 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.17, 1.73] |
Comparison 23. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged mechanical ventilation (> 7 days) | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.85] |
Comparison 24. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged mechanical ventilation (> 14 days) | 3 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.37, 1.00] |
Comparison 25. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged mechanical ventilation (> 21 days) | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.86] |
Comparison 26. SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 3 | 338 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐7.18, 2.89] |
Comparison 27. Sensitivity analysis: SmartCare™ versus non‐automated weaning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weaning time (randomization to extubation) based on type of control arm | 5 | 418 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐3.20, ‐1.07] |
| 1.1 Predominantly protocolized control strategy | 3 | 286 | Mean Difference (IV, Random, 95% CI) | ‐3.94 [‐6.10, ‐1.78] |
| 1.2 Predominantly non‐protocolized control strategy | 2 | 132 | Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐2.67, ‐0.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bifulco 2008.
| Methods | ||
| Participants | Ventilated for at least 24 hours Eligible for discontinuation of mechanical ventilation using usual criteria for weaning readiness Successful preinclusion test with PS > 15 cm H2O |
|
| Interventions | SmartCare™ versus conventional weaning protocol (used in ICU) | |
| Outcomes | Weaning time (time from randomization to extubation) Total duration of mechanical ventilation (from initiation to extubation) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based central randomization (statistics department) |
| Allocation concealment (selection bias) | Low risk | Study arm communicated by telephone |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Individuals assessing outcomes were not separate from individuals supervising or administering the study interventions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two withdrawals from the SmartCare™ arm were reported, and one withdrawal from the control arm, representing 3 of 30 (10%) participants |
| Selective reporting (reporting bias) | Unclear risk | Minimal outcomes collected because of early stopping and limited availability of personnel. Study authors confirmed that they intended to collect data on VAP rate, duration of mechanical ventilation and mortality |
| Did the trial stop early for benefit? | Low risk | Stopped early because of limited funding and personnel |
| Participants analysed according to the group allocated to? | Low risk | All participants with data were included in the analysis on the basis of treatment assignment. A modified intention‐to‐treat analysis was conducted because of study withdrawals |
Burns 2013a.
| Methods | ||
| Participants | Invasive mechanical ventilation > 24 hours At least partial reversal of condition precipitating invasive ventilation Stabilization of other organ system failures SpO2 ≥ 90% with FiO2 ≤ 0.7 with PEEP ≤ 12 cm H2O Weight > 35 kg Successful completion of pressure support trial after 60 to 120 minutes and either too early for an SBT or for successful completion of an SBT Excluded:
|
|
| Interventions | SmartCare™ versus paper‐based weaning protocol | |
| Outcomes | Weaning time (time from randomization to extubation) Time to successful extubation Time to first SBT Time to first successful SBT Time from initiation to randomization Time to reintubation Length of ICU stay Length of hospital stay ICU mortality Hospital mortality Death on mechanical ventilation Ventilator‐associated pneumonia (nosocomial pneumonia) Use of non‐invasive ventilation following extubation Adverse event: reintubation Adverse event: self‐extubation Adverse event: tracheostomy Prolonged mechanical ventilation > 21 days Clinician acceptance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, central randomization. Stratified by centre, COPD and central neurological disease. If both COPD and central neurological disease, the latter was prioritized |
| Allocation concealment (selection bias) | Low risk | Electronic mail system |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research co‐ordinators and respiratory therapists assessed and recorded study outcomes; were not separate from individuals supervising or administering the study interventions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two protocolized weaning and 3 automated weaning participants were withdrawn immediately after randomization. In addition, 2 automated weaning participants were withdrawn while on protocol. Outcomes for the latter participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Study authors reported all primary and secondary outcomes |
| Did the trial stop early for benefit? | Low risk | Target sample size was 90 |
| Participants analysed according to the group allocated to? | Low risk | Participants were maintained in the group to which they were assigned for analysis. All participants with data were analysed according to treatment assignment. A modified intention‐to‐treat analysis was conducted because of study withdrawals |
Jiang 2006.
| Methods | ||
| Participants | Underlying disease that caused respiratory failure had been controlled P/F ratio > 200 mmHg, PEEP ≤ 5 cm H2O, FiO2 ≤ 0.40 Stable haemodynamics with no acute pulmonary oedema or hypotension and no vasoconstrictive medications Capable of spontaneous breathing |
|
| Interventions | SmartCare™ versus spontaneous breathing trials/periods of spontaneous breathing | |
| Outcomes | Weaning time (time from randomization to extubation) Time from initiation to randomization Clinician workload (blood gas sampling) Prolonged mechanical ventilation > 7 days Prolonged mechanical ventilation > 14 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudorandomized |
| Allocation concealment (selection bias) | High risk | Based on number (odd/even) assigned at hospital admission |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Attending physicians were responsible for implementing SmartCare™ while physicians not involved with the study implemented the control strategy; study personnel were not blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals occurred as the result of ventilator‐associated infection and self‐extubation. We are uncertain as to numbers and distribution of withdrawals between the 2 treatment groups. This trial reported on 13 participants in the SC arm and 25 in the SBT (control) arm, suggesting the potential for an imbalance between groups in randomization or withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Did not report key outcomes including total duration of mechanical ventilation, ICU mortality, length of ICU stay and adverse events. Study author affirmed that he left the hospital; however, data on outcomes pertaining to ICU stay could have been reported |
| Did the trial stop early for benefit? | Low risk | Study authors intended to enrol in the trial 10 to 20 participants, presumably per arm |
| Participants analysed according to the group allocated to? | Unclear risk | Unsure whether participants were analysed according to treatment assignment, given discrepancy in number of participants included in each study arm. It is likely that not all randomly assigned participants were included, but we are uncertain as to whether participants were analysed by the group to which they were assigned |
Lellouche 2006.
| Methods | ||
| Participants | Mechanical ventilation for at least 24 hours and ventilated using an assisted mode 18 to 85 years old could be enrolled at an early stage when plateau pressure < 30 cm H2O with tidal volume ≤ 8 cc/kg on assist‐control ventilation, PEEP ≤ 8 cm H2O and P/F ratio > 150 or SaO2 > 90% with FiO2 ≤ 0.5 Epinephrine or norepinephrine ≤ 1000 mcg/h Body temperature > 36°C and < 39°C Stable neurological status with Glasgow Coma Scale > 4 on little or no sedation Excluded:
|
|
| Interventions | SmartCare™ versus usual care | |
| Outcomes | Weaning time (time from randomization to extubation) Time to successful extubation Total duration of mechanical ventilation (from initiation to extubation) Time from initiation to randomization Length of ICU stay Length of hospital stay ICU mortality Hospital mortality Death on mechanical ventilation Ventilator‐associated pneumonia Use of non‐invasive ventilation following extubation Adverse event: reintubation Adverse event: self‐extubation Adverse event: tracheostomy Adverse event: pneumothorax Prolonged mechanical ventilation > 14 days Prolonged mechanical ventilation > 21 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization system |
| Allocation concealment (selection bias) | Low risk | Electronic mail from central site |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Site investigators assessed and recorded study outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were withdrawn because extubation preceded electronic assignment, and 1 participant was excluded after consent was withdrawn. Assigned treatment groups for these 3 participants were not provided |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Did the trial stop early for benefit? | Low risk | Intended to enrol 75 participants per group |
| Participants analysed according to the group allocated to? | Low risk | Analysed according to assigned strategy; however, 1 study withdrawal and 1 participant extubated before electronic assignment were reported |
Lim 2012.
| Methods | ||
| Participants | Single‐centre study involving a coronary care unit and including participants between 21 and 85 years of age, with stable neurological status, on an assisted mode of mechanical ventilation for > 24 hours Excluded:
|
|
| Interventions | Participants were randomly assigned 1:1 to knowledge‐based weaning (SmartCare™) or usual care APACHE II score was used to stratify illness severity |
|
| Outcomes | Primary outcome: total weaning time (from inclusion to extubation without reintubation for 72 hours) Adjusted for APACHE II score Total duration of mechanical support |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes held by trial co‐ordinator/RRT or designate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study co‐ordinators collected outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant withdrew from the study. Only one death prevented computation of time to extubation. Total of 5 deaths occurred during the study |
| Selective reporting (reporting bias) | Unclear risk | Yes, reported outcomes were limited in this abstract publication. Study author provided additional data for the 62 participants ultimately included in this trial (originally 54 participants) and reported time to successful extubation and total duration of mechanical ventilation |
| Did the trial stop early for benefit? | Low risk | Stopped early for futility. Investigators sought to recruit 75 participants per study arm |
| Participants analysed according to the group allocated to? | Low risk | Yes. No participant was withdrawn following randomization, and no cross‐overs occurred; however, 1 participant who died was excluded from the outcome analyses |
Liu 2013.
| Methods | ||
| Participants | 48 participants who failed an initial SBT (identified using daily screening) were randomly assigned to computer‐driven weaning with SmartCare™ or physician‐controlled local practice Excluded:
|
|
| Interventions | Participants were randomly assigned to SmartCare™ weaning or physician‐controlled local practice guided by a local, written weaning guideline | |
| Outcomes | Weaning time (randomization to first extubation) with and without NIV Total duration of mechanical ventilation Length of ICU stay ICU mortality Ventilator‐associated pneumonia Use of non‐invasive ventilation following extubation Adverse event: self‐extubation Adverse event: reintubation (within 48 hours) Adverse event: tracheostomy Prolonged mechanical ventilation > 7 days Prolonged mechanical ventilation > 21 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random digit table developed by investigative team |
| Allocation concealment (selection bias) | High risk | Randomization list was held by RRT. Physicians involved in caring for participants did not know the enrolled group until they saw the ventilator at the bedside; however, the implementing RRT held the list of random digits |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | RRTs, not blinded to treatment assignment, assessed outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on 9 participants (5 intervention group; 4 control group) who died before extubation were not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting |
| Did the trial stop early for benefit? | High risk | Stopped early for benefit. Study authors intended to enrol 100 participants but stopped after an interim analysis, which suggested that 40 to 50 participants would be sufficient |
| Participants analysed according to the group allocated to? | Low risk | Yes. All participants with data were analysed according to treatment assignment. A modified intention‐to‐treat analysis was conducted because of study withdrawals |
Ma 2010.
| Methods | ||
| Participants | Age ≥ 18 years Not ventilated at time of ICU admission Ventilation time > 48 hours Improvement in condition after treatment Stable vital signs Participants meeting following criteria for weaning:
Did not include participants who passed an SBT |
|
| Interventions | SmartCare™ (SC) versus synchronized intermittent mandatory ventilation, pressure ventilation (SP) group with T‐piece trials | |
| Outcomes | Weaning time (time from randomization to extubation) Length of ICU stay Clinician workload (ventilator adjustments per participant) Ventilator‐associated pneumonia Adverse event: reintubation Adverse event: tracheostomy Adverse event: pneumothorax Adverse event: other—subcutaneous emphysema |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimum balance index based on ICU admission sequence |
| Allocation concealment (selection bias) | Unclear risk | Strategy for allocation was based on gender, age, APACHE score, with points assigned to each category (gender: M vs F, age 18 to 44, 45 to 64, ≥ 65 and APACHE < 10, 11 to 15, > 15). Theoretical permutations were run (if assigned to SC or SP weaning), and permutation with lowest cumulative number of points determined treatment assignment for the next participant. Allocation was the responsibility of the researcher implementing the study. Participant assignment was not changed or reconsidered following randomization (i.e. cross‐overs) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | One investigator assessed and recorded study outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study withdrawals were due to consent withdrawal and VAP. We are uncertain of the numbers, but study authors confirmed that they were equally distributed between treatment groups. This study reported on 30 participants in the SC arm and 32 in the SP (control) arm |
| Selective reporting (reporting bias) | Low risk | Study author did not report summary continuous outcomes but provided subgroup data to enable computation of weaning time and length of ICU stay. This trial followed participants in the ICU but did not report ICU mortality |
| Did the trial stop early for benefit? | Low risk | Stopped early for futility. Study authors intended to enrol 100 participants. However, because of time constraints and graduate degree requirements, the trial was stopped early |
| Participants analysed according to the group allocated to? | Low risk | Study author confirmed that participants were analysed according to treatment assignment at admission |
Reardon 2011.
| Methods | ||
| Participants | Age > 18 years Initiated on mechanical ventilation via endotracheal tube Admitted to medical intensive care unit and medical intensive care unit team Required mechanical ventilation for longer than 48 hours Meets specified weaning criteria Excluded:
|
|
| Interventions | SmartCare™ versus evidence‐based standard of care for mechanical ventilation discontinuation (weaned with T‐piece SBTs or with PS) | |
| Outcomes | Time to successful extubation Mortality: 28 day Composite of death during weaning, ventilator‐associated pneumonia during weaning, self‐extubation and reintubation (not reported separately) Adverse event: other—serious adverse events Adverse event: other—other adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used an on‐line random number generator (www.random.org) with permuted blocks of 4, stratified by cause of respiratory failure (neurological, obstructive lung disease or other) |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Site investigators and study co‐ordinators ascertained and recorded outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals nor dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Results of this trial are not fully published. Study authors intended to collect data on (1) primary outcome: weaning duration and (2) secondary outcomes: ICU stay, total duration of mechanical ventilation, hospital stay, inpatient mortality, sedation requirements, number of SBTs before extubation and complications (including death during weaning, VAP, self‐extubation and reintubation as a composite outcome) Limited results reported on trial registration website (www.clinicaltrials.gov) include weaning duration, hospital deaths, complications (composite outcome) and serious adverse event rate and other adverse events |
| Did the trial stop early for benefit? | Low risk | Trial was stopped because of slow study recruitment (i.e. for futility) |
| Participants analysed according to the group allocated to? | Low risk | Study authors reported using an intention‐to‐treat analysis on www.clinicaltrials.gov |
Rose 2008.
| Methods | ||
| Participants | Mechanical ventilation with volume‐ or pressure‐targeted mandatory modes for > 24 hours Drager Evita XL ventilator with SmartCare™/PS (v 1.1) software available for use immediately before randomization PEEP ≤ 8 cm H2O P/F ratio > 150 mmHg or SaO2 ≥ 90% with FiO2 ≤ 0.50 Plateau pressure ≤ 30 cm H2O Haemodynamic stability (epinephrine or norepinephrine ≤ 16.5 mcg/min or dopamine ≤ 500 mcg/min) Body temperature 36°C to 39°C Stable neurological status with Glasgow Coma Scale > 4 No anticipated requirement for transport or surgery within 2 hours Successful completion of SBT using PS (max 20 cm H2O) to achieve VT > 200 mL |
|
| Interventions | SmartCare™ versus usual care | |
| Outcomes | Time to successful extubation Time to first successful SBT Total duration of mechanical ventilation (from initiation to extubation) Time from meeting criteria for discontinuation to actual extubation Length of ICU stay Length of hospital stay Mortality before separation potential Mortality before successful extubation Use of non‐invasive ventilation following extubation Adverse event: reintubation Adverse event: tracheostomy Prolonged mechanical ventilation > 14 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Website‐based (www.randomization.com) computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Principal study investigator assessed and recorded study outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from all randomly assigned participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Did the trial stop early for benefit? | Low risk | Target sample was 222, of which 102 participants were enrolled. Trial was stopped early for futility based on graduate degree requirements and sample size recalculation |
| Participants analysed according to the group allocated to? | Low risk | Analyses were performed on an intention‐to‐treat basis |
Stahl 2009.
| Methods | ||
| Participants | Age 18 to 80 years Body weight between 35 kg and 200 kg Invasively mechanical ventilated via endotracheal tube or tracheostomy for ≥ 24 hours Ramsey Score ≤ 3 Spontaneous breathing mode with PEEP ≤ 10 Sufficient arterial oxygenation with PaO2 > 55 mmHg/75 cm H2O or SaO2 > 90% on FiO2 ≤ 0.50. Haemodynamically stable (dopamine < 5 mcg/kg/min) Rectal temperature ≤ 39°C Haemoglobin ≥ 70 g/L pH > 7.20 |
|
| Interventions | ||
| Outcomes | Time to successful extubation Total duration of mechanical ventilation (from initiation to extubation) Time from initiation to randomization Time to reintubation Length of ICU stay Clinician workload (physician changes in ventilator settings, FiO2, PEEP and nurses' cleaning of cuvette) ICU mortality Hospital mortality Adverse event: reintubation Proportion successfully extubated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors used a computer‐generated randomization system (Rita software (version 1.13a)) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Individuals assessing outcomes were not separate from individuals supervising or administering study interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | First 10 participants who failed an initial attempt at weaning were discontinued from the study. The protocol was subsequently modified to permit a second weaning attempt. Dropouts occurred with similar frequency between study groups (4 per group) post randomization (8/60 (13.3%)), and their outcomes were included in the analyses when possible. These 8 participants could not be extubated. The first 10 participants who failed an attempt at weaning were included in the final analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Did the trial stop early for benefit? | Low risk | Stopped early for futility after 60 of 108 planned participants were enrolled |
| Participants analysed according to the group allocated to? | Low risk | Yes, study authors adhered to the intention‐to‐treat principle |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beale 2007 | This 500‐participant trial, identified on a trial registration website, proposed to compare the intensive care unit (ICU) standard ventilator weaning protocol versus the SmartCare™ automated weaning system in patients likely to need mechanical ventilation for a period of 48 hours, but it was never launched |
| Chen 2008 | This non‐randomized trial compared 109 participants who were treated with adaptive support ventilation versus 110 participants whose condition was managed by a respiratory therapist–driven protocol |
| Donglemans 2007 | This trial compared adaptive support ventilation versus pressure control/pressure support in 122 fast‐track coronary artery bypass surgery participants. The trial did not evaluate SmartCare™ |
| Jolliet 2006 | This feasibility study was non‐randomized and reported on the use of SmartCare™ during non‐invasive ventilation in participants with acute respiratory failure |
| Jouvet 2007 | This randomized single‐centre trial evaluated SmartCare™ in a paediatric population |
| Kataoka 2007 | This retrospective study reported on the experience of a single centre in using SmartCare™ after off‐pump coronary artery bypass surgery for early extubation |
| Papirov 2007 | This 60‐participant pilot randomized controlled trial (RCT) was designed to compare computer‐driven weaning with SmartCare™ versus physician‐directed weaning in elderly patients at a geriatric rehabilitation hospital and regional weaning centre (non‐ICU setting). To be included, patients had to have stabilization of the acute health problems that prompted admission to the referral hospital. The study was terminated after an undisclosed number of participants had been enrolled because of a request to return the study ventilators. The trial was excluded, as it included exclusively tracheostomized participants (confirmed by study author) |
| Schadler 2012 | This study evaluated SmartCare™ in a postoperative population |
| Taniguchi 2009 | This trial compared manual versus automatic reduction in pressure support in a randomized trial of 106 postoperative participants. The automated system used mandatory rate ventilation with a Taema‐Horus Ventialtor (Air Liquid, France) |
| Wong 2008 | This randomized trial, identified on a trial registration website, was stopped for futility after 3 participants were enrolled over 18 months despite attempts to modify study inclusion criteria |
Differences between protocol and review
In addition to evaluating the method of allocation concealment, incomplete outcome reporting, selective reporting, whether trials stopped early for benefit and adherence to the intention‐to‐treat principle, we evaluated random sequence generation.
All trials included in this review were unblinded because of the nature of the interventions applied. We contacted all study authors to determine whether outcome assessors were separate from the individuals administering or supervising assigned interventions.
We considered I2 statistical thresholds of 0% to 40%, 30% to 60%, 50% to 90% and > 75% to represent between‐study heterogeneity that might not be important, moderate, substantial or considerable, respectively (Higgins 2011).
In the final phases of protocol development, we were asked to limit the outcomes of interest to at most seven outcomes. At this time, we arbitrarily highlighted the primary outcome and the first six secondary outcomes listed, believing that the first six outcomes likely represented the most important outcomes to be reported in a weaning systematic review and meta‐analysis. This decision was likely influenced by prior experience in conducting a systematic review comparing invasive and non‐invasive weaning strategies. Since the time of publication of the protocol, we (JF and KB) adjudicated which outcomes would be most important to patients and clinicians, and decided that reintubation (a potential adverse event) should be included among the most important outcomes reported in the SoF. Unlike the non‐invasive weaning review, we found no compelling reason to think that VAP rates would be different between weaning strategies, as the alternative weaning strategies compared in this review were invasively applied and titrated. Consequently, we decided to prioritize reintubation and to remove VAP from the outcomes reported in the SoF.
In the protocol, we listed time to first and time to first successful SBT in the protocol as a single outcome. However, in our review, only one trial reported time to first SBT. and pooling was not possible for this outcome. However, two trials reported on time to first successful SBT; we included this outcome in the SoF table. Consequently, we were able to include only time to first successful SBT in our SoF table.
The protocol was intended to focus on comparison of SmartCare™ versus other non‐automated weaning strategies, as SmartCare™ is a unique weaning system that automates not only weaning but also the conduct of SBTs. However during the review process, we searched for RCTs that tested all automated weaning and SBT systems. We did not identify RCTs that tested any other automated weaning and SBT systems in patients meeting our inclusion criteria. Consequently, we replaced the proprietary name in the title with ‘automated weaning and SBT systems’ to reflect this.
Contributions of authors
Karen EA Burns (KB) , Francois Lellouche (FL), Rosane Nisenbaum (RN), Martin R Lessard (ML), Jan O Friedrich (JF)
Conceiving of the review: KB.
Co‐ordinating the review: KB.
Undertaking manual searches: JF.
Screening search results: KB, FL.
Organizing retrieval of papers: KB.
Screening retrieved papers against inclusion criteria: KB, FL.
Adjudicating disagreements regarding trials for inclusion: ML.
Appraising quality of papers: KB, JF.
Abstracting data from papers: KB, JF.
Adjudicating disagreements regarding study quality and methods: ML.
Writing to authors of papers for additional information: KB, JF.
Providing additional data about papers: KB.
Obtaining and screening data on unpublished studies: KB, FL.
Managing data for the review: KB.
Entering data into Review Manager (RevMan 5.1): KB.
Analysing RevMan statistical data: KB, RN.
Performing other statistical analysis not using RevMan: RN.
Performing double entry of data: KB, JF.
Interpreting data: KB.
Making statistical inferences: KB, RN.
Writing the review: KB, FL, JF, ML.
Securing funding for the review: not applicable.
Performing previous work that served as the foundation of the present study: not applicable.
Serving as guarantor for the review (one review author): KB.
Taking responsibility for reading and checking the review before submission: KB, FL, ML, RN, JF.
Sources of support
Internal sources
New source of support, Other.
External sources
-
CIHR Clinician Scientist Award, Canada.
Drs Burns and Friedrich hold CIHR Clinician Scientist Phase 2 Awards
Declarations of interest
Drs Burns and Lellouche hold a $5000 CDN travel bursary from Draeger Medical Inc. (Canada) for the purpose of conducting site visits to participating centres in the WEAN Study. The WEAN Study is an investigator‐initiated trial comparing SmartCare™ and protocolized weaning, for which the co‐principal investigators (Drs Burns and Lellouche) obtained funding from peer‐review, non‐industry sources for implementation. Draeger Medical Inc. provided ventilators and ventilator upgrades for the WEAN study and a central randomization system using electronic mail correspondence (Draeger Medical, Germany). Draeger Medical was not involved in any aspects of study design and oversight, data management or data analysis.
Drs Burns, Lellouche and Lessard have self‐identified as investigators of trials that apply the interventions in question. However, the methods used in conducting this review do not permit bias from these authors in selection, data extraction or risk of bias assessment of any included studies.
Drs Friedrich and Nisenbaum have no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Bifulco 2008 {published and unpublished data}
- Bifulco F, Donato L, Giuricin F, Narni Mancinelli V, Vicchio C, Servillo G. Weaning with SmartCare: our experience. 21st ESICM Annual Congress 2008:S79. [Google Scholar]
Burns 2013a {published and unpublished data}
Jiang 2006 {published and unpublished data}
- Jiang H, Yu S, Wang L. Comparison of SmartCare and spontaneous breathing trials for weaning old patients with chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases 2006;29:545‐8. [PUBMED: 17074269 ] [PubMed] [Google Scholar]
Lellouche 2006 {published and unpublished data}
- Lellouche F, Mancebo J, Jolliet P, Roeselar J, Schortgen F, Dojat M, et al. A multicentre randomzied trial of computer‐driven protocolized weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 2006;174:894‐900. [PUBMED: 16840741] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2012 {published and unpublished data}
- Lim ETS, Hamid N, Beh NC, Phua GC, Tan J. Comparison of knowledge‐based weaning (KBW) and physician‐driven weaning of mechanically ventilated patients in the coronary care unit. European Heart Journal 2012;33:714:P4188. [Google Scholar]
Liu 2013 {published and unpublished data}
- Liu L, Xu X, Yang Y, Huang Y, Liu S, Qiu H. Computer‐driven automated weaning reduces weaning duration in difficult‐to‐wean patients. Chinese Medical Journal 2013;126(10):1814‐8. [PubMed] [Google Scholar]
Ma 2010 {published and unpublished data}
- Ma YJ, Yang XJ, Cao XY, Ma XG. Comparison of computer‐driven weaning and physician‐directed weaning from mechanical ventilation: a randomized prospective study. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:174‐8. [PUBMED: 20450634] [PubMed] [Google Scholar]
Reardon 2011 {published and unpublished data}
- Reardon C. Clinical Trial of a Computer‐driven Weaning System for Patients Requiring Mechanical Ventilation. Clinical Trials.gov 2011. [Other: NCT00606554]
Rose 2008 {published and unpublished data}
- Rose L, Presneill JJ, Johnston L, Cade JF. A randomized, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare/PS. Intensive Care Medicine 2008;34:1788‐95. [PUBMED: 18575843 ] [DOI] [PubMed] [Google Scholar]
Stahl 2009 {published and unpublished data}
- Stahl C, Dahmen G, Ziegler A, Muhl E. Comparison of automated protocol‐based versus non‐protocol‐based physician‐directed weaning from mechanical ventilation: a controlled clinical trial. Intensivmed 2009;46:441‐6. [Google Scholar]
References to studies excluded from this review
Beale 2007 {unpublished data only}
- Beale R. Comparison of an automated weaning programme and a standard clinical weaning protocol for weaning critically ill patients. www.controlled‐trials.com 2007. [ISRCTN82559457] [Google Scholar]
Chen 2008 {published data only}
- Chen CW, Wu CP, Dai YL, Perng WC, Huang YC. Adaptive support ventilation (ASV) facilitates ventilator weaning in a medical ICU with limited respiratory therapist support. American Journal of Respiratory and Critical Care Medicine. 2008; Vol. 177:A379.
Donglemans 2007 {published data only}
- Dongelmans DA, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Pressure controlled/pressure support (PC/PS) versus adaptive support ventilation (ASV) in post‐cardiac surgery patients—a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine. 2007; Vol. 175:A433.
Jolliet 2006 {published data only}
- Jolliet P, Battisti A, Roeseler J, Tasseaux D. Automatic adjustment of pressure support (PS) with a computer‐driven knowledge‐based system (SmartCare) during noninvasive ventilation (NIV) in acute respiratory failure. Proceedings of the American Thoracic Society. 2006; Vol. 3:A472.
Jouvet 2007 {published data only}
- Jouvet P, Farges C, Hatzakis G, Monir A, Lesage F, Dupic L, Brochard L, et al. Weaning children from mechanical ventilation with a computer‐driven system (closed‐loop protocol): a pilot study. Pediatric Critical Care Medicine 2007;8:425‐32. [PUBMED: 17693913 ] [DOI] [PubMed] [Google Scholar]
Kataoka 2007 {published data only}
- Kataoka G, Murai N, Kodera K, Sasaki A, Asano R, Ikeda M, et al. Clinical experience with SmartCare after off‐pump coronary artery bypass for early extubation. Journal of Artificial Organs 2007;10:218‐22. [PUBMED: 18071851] [DOI] [PubMed] [Google Scholar]
Papirov 2007 {unpublished data only}
- Papirov G. Computer driven management of weaning following prolonged mechanical ventilation: a pilot study. http://clinicaltrials.gov/show/NCT00502489. [DOI] [PubMed]
Schadler 2012 {published data only}
- Schadler D, Engel C, Gunnar E, Pulletz S, Haake N, Frerichs I, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. American Journal of Respiratory and Critical Care Medicine 2012;185:637‐44. [PUBMED: 22268137] [DOI] [PubMed] [Google Scholar]
Taniguchi 2009 {published data only}
- Taniguchi C, Eid RC, Saghabi C, Souza R, Silva E, Knobel E, et al. Automatic versus manual pressure support reduction in the weaning of post‐operative patients: a randomized controlled trial. Critical Care 2009;13:R6. [DOI: 10.1186/cc7695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2008 {unpublished data only}
- Wong J. Automated Ventilator Controlled Weaning vs Daily Spontaneous Breathing Trial in Difficult to Wean ICU Patients. www.clinicaltrials.gov. December 2008. [NCT00813839] [Google Scholar]
Additional references
Afessa 1999
- Afessa B, Hogans L, Murphy R. Predicting 3‐day and 7‐day outcomes of weaning from mechanical ventilation. Chest 1999;116:456‐61. [PUBMED: 10453876 ] [DOI] [PubMed] [Google Scholar]
Banner 1997
- Banner MJ, Lampotang S, Blanch PB. Mechanical ventilation. In: Civetta JM editor(s). Critical Care. Philadelphia, PA: Lippincott‐Raven, 1997. [Google Scholar]
Borenstein 2008
- Borenstein N, Hedge LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Bouadma 2005
- Bouadma L, Lellouche F, Cabello B, Taille S, Mancebo J, Dojat M, et al. Computer‐driven management of prolonged mechanical ventilation and weaning: a pilot study. Intensive Care Medicine 2005;10:1446‐50. [PUBMED: 16132889] [DOI] [PubMed] [Google Scholar]
Brochard 1994
- Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1994;150:896‐903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brook 1999
- Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Channon W, et al. Effect of a nursing‐implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine 1999;27(12):2609‐15. [PUBMED: 10628598] [DOI] [PubMed] [Google Scholar]
Burns 2008
- Burns KE, Lellouch F, Lessard MR. Automating the weaning process with advanced closed‐loop systems. Intensive Care Medicine 2008;34(10):1757‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burns 2013b
- Burns KEA, Meade MO, Premji A, Adhikari NKJ. Noninvasive positive‐pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD004127.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 1999
- Butler R, Keenan SP, Inman KJ, Sibbald WJ, Block G. Is there a preferred technique for weaning the difficult‐to‐wean patient? A systematic review of the literature. Critical Care Medicine 1999;27(11):2331‐6. [PUBMED: 10579244] [DOI] [PubMed] [Google Scholar]
Chatburn 2004
- Chatburn RL. Computer control of mechanical ventilation. Respiratory Care 2004;49(5):507‐17. [MEDLINE: ] [PubMed] [Google Scholar]
Cook 1998
- Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator‐associated pneumonia in critically ill patients. Annals of Internal Medicine 1998;129(6):433‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dries 1997
- Dries DJ. Weaning from mechanical ventilation. Journal of Trauma, Injury, Infection and Critical Care 1997;43(2):372‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 1996
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect of the duration of mechanical ventilation on identifying patients capable of breathing spontaneously. New England Journal of Medicine 1996;335:1864‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ely 1999
- Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist‐driven protocol for ventilator weaning. American Journal of Respiratory and Critical Care Medicine 1999;159(2):439‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Epstein 1997
- Epstein SK, Ciabotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997;112:186‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esen 1992
- Esen F, Denkel T, Telci L, Kesecioglu J, Tutuncu AS, Akpir K, et al. Comparison of pressure support ventilation and intermittent mandatory ventilation during weaning in patients with acute respiratory failure. Advances in Experimental Medicine and Biology 1992;317:371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1994
- Esteban A, Alia I, Ibanez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest 1994;106:1188‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1995
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. New England Journal of Medicine 1995;332:388‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1997
- Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, et al. Extubation outcome after spontaneous breathing trials with t‐tube or pressure support ventilation. American Journal of Respiratory and Critical Care Medicine 1997;156:459‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 1999
- Esteban A, Alia I, Tobin MJ. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1999;159:512‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 2002
- Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐d international study. JAMA 2002;287:345‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heyland 1999
- Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun‐Buisson C. The attributable morbidity and mortality of ventilator associated pneumonia in the critically ill patient. The Canadian Critical Care Trials Group. American Journal of Respiratory and Critical Care Medicine 1999;159:1249‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Section 7.7.3.5 Medians and interquartile ranges. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jounieaux 1994
- Jounieaux V, Duran A, Levi‐Valensi P. Synchronized intermittent mandatory ventilation with and without pressure support ventilation in weaning patient with COPD from mechanical ventilation. Chest 1994;105:1204‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kollef 1997
- Kollef MH, Shapiro SD, Silver P, John RE, Prentice D, Sauer S, et al. A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation. Critical Care Medicine 1997;25:567‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2001
- Lefebvre C, Clarke M. Identifying randomized trials. Egger M, Davey Smith G, Altman D editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001. [NLM ID 101093083] [Google Scholar]
Lessard 1996
- Lessard MR, Brochard LJ. Weaning from mechanical support. Clinics in Chest Medicine 1996;17(3):475‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mancebo 1996
- Mancebo J. Weaning from mechanical ventilation. European Respiratory Journal 1996;9(9):1923‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marelich 2000
- Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses. Effect on weaning time and incidence of ventilator associated pneumonia. Chest 2000;118:459‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niederman 1984
- Niederman MS, Ferranti RD, Ziegler A, Merrill W, Reynolds HY. Respiratory infection complicating long‐term tracheostomy: the implication of persistent gram‐negative tracheobronchial colonization. Chest 1984;85:39‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman A, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Papazian 1996
- Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis JP, et al. Effect of ventilator‐associated pneumonia on mortality and morbidity. American Journal of Respiratory and Critical Care Medicine 1996;154(1):91‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perren 2002
- Perren A, Domenighetti G, Mauri S, Genini F, Vizzardi N. Protocol‐directed weaning from mechanical ventilation; clinical outcome in patients randomized for a 30‐minute and 120‐minute trial with pressure support. Intensive Care Medicine 2002;28:1058‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pingleton 1988
- Pingleton SK. Complications of acute respiratory failure. American Review of Respiratory Diseases 1988;137:1463‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.6. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sahn 1973
- Sahn SA, Lakshminarayan S. Bedside criteria for discontinuation of mechanical ventilation. Chest 1973;63(6):1002‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strickland 1991
- Strickland JH, Hasson JH. A computer‐controlled ventilator weaning system. Chest 1991;100:1096‐9. [PUBMED: 1914564] [DOI] [PubMed] [Google Scholar]
Strickland 1993
- Strickland JH Jr, Hasson JH. A computer‐controlled ventilator weaning system: a clinical trial. Chest 1993;103:1220‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stroetz 1995
- Stroetz RW, Hubmayer RD. Tidal volume maintenance during weaning with pressure support. American Journal of Respiratory and Critical Care Medicine 1995;152:1034‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tomlinson 1989
- Tomlinson JR, Miller KS, Lorch DF, Smith L, Reines HD, Sahn SA. A prospective comparison of IMV and T‐piece weaning from mechanical ventilation. Chest 1989;96:348‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vincent 1995
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas‐Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe (EPIC). JAMA 1995;274:639‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Vitacca 2000
- Vitacca M, Clini E, Porta R, Ambrosino N. Preliminary results on nursing workload in a dedicated weaning center. Intensive Care Medicine 2000;26(6):796‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yusuf 1991
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. Journal of the American Medical Association 1991;266(1):93‐8. [MEDLINE: ] [PubMed] [Google Scholar]


