Abstract
Background
Initial goal‐directed resuscitation for hypotensive shock usually includes administration of intravenous fluids, followed by initiation of vasopressors. Despite obvious immediate effects of vasopressors on haemodynamics, their effect on patient‐relevant outcomes remains controversial. This review was published originally in 2004 and was updated in 2011 and again in 2016.
Objectives
Our objective was to compare the effect of one vasopressor regimen (vasopressor alone, or in combination) versus another vasopressor regimen on mortality in critically ill participants with shock. We further aimed to investigate effects on other patient‐relevant outcomes and to assess the influence of bias on the robustness of our effect estimates.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015 Issue 6), MEDLINE, EMBASE, PASCAL BioMed, CINAHL, BIOSIS and PsycINFO (from inception to June 2015). We performed the original search in November 2003. We also asked experts in the field and searched meta‐registries to identify ongoing trials.
Selection criteria
Randomized controlled trials (RCTs) comparing various vasopressor regimens for hypotensive shock.
Data collection and analysis
Two review authors abstracted data independently. They discussed disagreements between them and resolved differences by consulting with a third review author. We used a random‐effects model to combine quantitative data.
Main results
We identified 28 RCTs (3497 participants) with 1773 mortality outcomes. Six different vasopressors, given alone or in combination, were studied in 12 different comparisons.
All 28 studies reported mortality outcomes; 12 studies reported length of stay. Investigators reported other morbidity outcomes in a variable and heterogeneous way. No data were available on quality of life nor on anxiety and depression outcomes. We classified 11 studies as having low risk of bias for the primary outcome of mortality; only four studies fulfilled all trial quality criteria.
In summary, researchers reported no differences in total mortality in any comparisons of different vasopressors or combinations in any of the pre‐defined analyses (evidence quality ranging from high to very low). More arrhythmias were observed in participants treated with dopamine than in those treated with norepinephrine (high‐quality evidence). These findings were consistent among the few large studies and among studies with different levels of within‐study bias risk.
Authors' conclusions
We found no evidence of substantial differences in total mortality between several vasopressors. Dopamine increases the risk of arrhythmia compared with norepinephrine and might increase mortality. Otherwise, evidence of any other differences between any of the six vasopressors examined is insufficient. We identified low risk of bias and high‐quality evidence for the comparison of norepinephrine versus dopamine and moderate to very low‐quality evidence for all other comparisons, mainly because single comparisons occasionally were based on only a few participants. Increasing evidence indicates that the treatment goals most often employed are of limited clinical value. Our findings suggest that major changes in clinical practice are not needed, but that selection of vasopressors could be better individualised and could be based on clinical variables reflecting hypoperfusion.
Plain language summary
Vasopressors for hypotensive shock
Review question
This review seeks unbiased evidence about the effects of different drugs that enhance blood pressure on risk of dying in critically ill patients with impaired blood circulation.
Background
● Circulatory shock is broadly defined as a life‐threatening condition of impaired blood flow resulting in inability of the body to maintain blood delivery to body tissue and to meet oxygen demands.
● Typical signs of shock include low blood pressure, rapid heartbeat and poor organ perfusion indicated by low urine output, confusion or loss of consciousness.
● Death in the intensive care unit ranges from 16% to 60%, depending on the underlying condition: treatment includes fluid replacement followed by use of vasopressor agents, if necessary.
● A vasopressor agent is a drug that causes a rise in blood pressure. Six vasopressor drugs are available and are used successfully to increase blood pressure to reverse circulatory failure in critical care. Differences in their effects on survival are discussed with controversy and must be investigated.
● This review aims to discover whether any of the drugs given alone or in combination were better or worse than the others.
Search date
Evidence is current to June 2015.
Study characteristics
Review authors identified 28 randomized controlled trials involving 3497 critically ill patients with circulatory failure, among whom 1773 died. Patients were followed up to one year.
The following drugs, given alone or in combination, were studied in 12 different comparisons: dopamine, norepinephrine, epinephrine, phenylephrine, vasopressin, and terlipressin.
Key results
In summary, researchers found no significant differences in risk of dying in any comparisons of different drugs given alone or in combination when latest reported death was considered.
Disturbances in the rhythm of the heart were observed more frequently in people treated with dopamine than in those treated with norepinephrine.
Quality of the evidence
The quality of the evidence was high for the comparison of norepinephrine and dopamine, and was very low to moderate for the other comparisons.
Findings were consistent among the few large studies and studies of different quality.
Summary of findings
Background
Description of the condition
Shock is a state of severe systemic deterioration in tissue perfusion, characterized by decreased cellular oxygen delivery and utilization, as well as decreased removal of waste byproducts of metabolism. Hypotension, although common in shock, is not synonymous with shock. Individuals can have hypotension and normal perfusion, whereas patients who have a history of hypertension can have shock without hypotension in the early phase of cardiogenic shock. Shock is the final pre‐terminal event in many diseases. Progressive tissue hypoxia results in loss of cellular membrane integrity, reversion to a catabolic state of anaerobic metabolism and loss of energy‐dependent ion pumps and chemical and electrical gradients. Mitochondrial energy production begins to fail. Multiple organ dysfunction follows localized cellular death, and this is followed by death of the organism (Young 2008). A widely used classification for mechanisms of shock consists of hypovolaemic, cardiogenic, obstructive and distributive (Hinshaw 1972). Septic shock, a form of distributive shock, is the most common form of shock among patients admitted to the intensive care unit, followed by cardiogenic and hypovolaemic shock; obstructive shock is rare. As an example, in a trial of 1600 patients with undifferentiated shock, septic shock occurred in 62%, cardiogenic shock in 16%, hypovolaemic shock in 16%, other types of distributive shock in 4% (e.g. neurogenic shock, anaphylaxis) and obstructive shock in 2% (De Backer 2010).
Currently, the definition of septic shock is more pragmatic because hypotension instead of hypoperfusion is the main clinical criterion. The current standard definition for septic shock (Dellinger 2008) in adults refers to a state of acute circulatory failure characterized by persistent arterial hypotension that is not explained by other causes. Hypotension is defined by systolic blood pressure < 90 mm Hg, mean arterial pressure < 60 mm Hg or a reduction in systolic blood pressure > 40 mm Hg despite adequate volume resuscitation in the absence of other causes for hypotension (Levy 2003). A large study defined shock even more pragmatically, as haemodynamic compromise necessitating administration of vasopressor catecholamines (Sakr 2006).
Estimates of the incidence of shock in the general population vary considerably. From an observational study, 31 cases of septic shock per 100,000 population/y (Esteban 2007) were reported. Many patients develop shock from severe sepsis, which has an incidence of 25 to 300 cases per 100,000 population/y (Angus 2001; Blanco 2008; Sundararajan 2005); among those, 30% are expected to develop septic shock (Esteban 2007).
The frequency of shock at healthcare facilities is somewhat better described. In the large observational study of sepsis occurrence in acutely Ill patients (SOAP), among 3147 critically ill participants from 198 intensive care units (ICUs), 34% had shock; of those, 15% had septic shock (Sakr 2006). Recently a large French observational study published similar numbers indicating that among 10,941 patients admitted to participating ICUs between October 2009 and September 2011, 1495 (13.7%) presented with inclusion criteria for septic shock (Quenot 2013). In another large European ICU cohort study, 32% were found to have septic shock. In a prospective observational study of 293,633 participants with ST‐elevation myocardial infarction from 775 US hospitals, 9% developed cardiogenic shock (Babaev 2005). From an observational study on 2445 participants admitted to a trauma level I centre, 22% were reported to already have shock on admission to the emergency department (ED) (Cannon 2009).
Hospital mortality is high, at around 38% (Sakr 2006), among patients with shock but seems to depend much on shock type. For patients with septic shock, mortality ranges from 46% (Esteban 2007; Sakr 2006) to 61% (Alberti 2005). Mortality in patients with traumatic shock was somewhat lower, at 16% (Cannon 2009). Whereas the incidence of cardiogenic shock was almost constant between 1995 and 2004, mortality has decreased from 60% in 1995 to 48% over the years (Babaev 2005).
Description of the intervention
Vasopressors are a heterogeneous class of drugs with powerful and immediate haemodynamic effects. Vasopressors can be classified according to their adrenergic and non‐adrenergic actions.
Catecholamines are sympathomimetics that act directly or indirectly on adrenergic receptors. Their haemodynamic effects depend on their varying pharmacological properties. They may increase the contractility of myocardial muscle fibres and heart rate (via beta‐adrenergic receptors), but they may also, and sometimes exclusively, increase vascular resistance (via alpha‐adrenergic receptors). Many good textbooks have outlined the detailed mechanisms of action (e.g. see Hoffman 1992; Zaritsky 1994).
The haemodynamic properties of vasopressin, a neurohypophysial peptide hormone, were first reported in 1926 (Geiling 1926). Vasopressin and analogues like terlipressin display their vasopressor effects via vasopressin receptors and serve as newer treatments for patients with shock (Levy 2010).
Utilisation of different vasopressors was described recently in a large European multi‐centre cohort study conducted in 198 ICUs (Sakr 2006). The most frequently used vasopressor was norepinephrine (80%), followed by dopamine (35%) and epinephrine (23%), given alone or in combination. Single‐agent use was reported for norepinephrine (32%), dopamine (9%) and epinephrine (5%). A combination of norepinephrine, dopamine and epinephrine was used in only 2% of patients with shock. Vasopressin and terlipressin were not described in this report. Currently the choice of vasopressors seems to be based mainly on physicians' preferences (Leone 2004). Additionally, clinical guidelines suggest norepinephrine as the first‐line vasopressor in shock states, such as septic shock (Dellinger 2013).
How the intervention might work
Initial goal‐directed resuscitation to support vital functions is essential in the management of shock. First‐line treatment for the manifestation of circulatory failure usually consists of administration of intravenous fluids. If fluid treatment does not restore circulatory function, vasopressors such as norepinephrine, dopamine, epinephrine and vasopressin are recommended.
Why it is important to do this review
Effects of vasopressors on the cardiovascular system are largely undisputed. However, it remains unclear whether a vasopressor of choice is known for the treatment of patients with particular forms of shock or for the treatment of patients with shock in general. We are conducting this systematic review to explore uncertainty arising from conflicting results reported by several studies in this area.
Objectives
Our objective was to compare the effect of one vasopressor regimen (vasopressor alone, or in combination) versus another vasopressor regimen on mortality in critically ill participants with shock. We further aimed to investigate effects on other patient‐relevant outcomes and to assess the influence of bias on the robustness of our effect estimates.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) undertaken to investigate the effects of vasopressors in the treatment of patients with any kind of circulatory failure. For simplicity, we refer to circulatory failure as 'shock' (see also search terms for shock). We were exclusively interested in patient‐relevant outcomes (see below). Such endpoints, particularly death, can be assessed only through parallel‐group trials. Therefore, we excluded cross‐over trials.
Types of participants
We included trials with acutely and critically ill adult and paediatric participants. We excluded trials looking at pre‐term infants with hypotension, as this patient group is covered in another Cochrane review (Subhedar 2003). We excluded animal experiments. The definition of 'hypotensive shock' used was that given by study authors.
Types of interventions
The intervention consisted of administration of different vasopressors, vasopressors versus intravenous fluids and vasopressors versus placebo with or without non‐protocol vasoactive drugs (NPVDs).
Types of outcome measures
Primary outcomes
We looked at total mortality (in the ICU, in hospital and at one year) as the main endpoint. If mortality was assessed at several time points in a study, we used data derived from the latest follow‐up time.
Secondary outcomes
Other pre‐defined outcomes included the following.
-
Morbidity, given as:
ICU length of stay (LOS);
hospital LOS;
duration of vasopressor treatment;
duration of mechanical ventilation;
renal failure (as defined by study authors, such as oliguria or need for renal replacement therapy); and
other.
Measures of health‐related quality of life at any given time, and measures of anxiety and depression (together or separately) at any given time.
Search methods for identification of studies
Electronic searches
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 6) (see Appendix 1, Search filter for CENTRAL); MEDLINE (1966 to June 2015) (see Appendix 2); EMBASE (1989 to June 2015) (see Appendix 3, Search filter for EMBASE); PASCAL BioMed (1996 to June 2015); and BIOSIS (1990 to June 2015) (see Appendix 4 and Appendix 5, Search filter for PASCAL BioMed, CINAHL and BIOSIS); PsycINFO (1978 to June 2015) (see Appendix 6, Search filter for PsycINFO) using the Ovid platform. We searched CINAHL (1984 to June 2015) via EBCSO.
We searched for key words describing the condition or describing the intervention and combined the results by using a methodological filter (RCT filter).
We used a validated RCT filter for MEDLINE and EMBASE (Higgins 2011).
We applied no language restrictions.
Searching other resources
We searched ongoing clinical trials and unpublished studies via the Internet (date of latest search, 24 June 2015) on www.controlled‐trials.com by using the multiple database search option metaRegister of Controlled Trials. This register includes International Standard Randomised Controlled Trial Number (ISRCTN) Register, Action Medical Research, Leukaemia Research Fund, Medical Research Council (UK), NHS Research and Development HTA Programme, ClinicalTrials.gov, Wellcome Trust and UK Clinical Trials Gateway.
Further, we searched textbooks and references of papers selected during the electronic search to look for relevant references. Finally, we contacted experts in the field to identify additional trials (see Acknowledgements).
Data collection and analysis
Selection of studies
We entered all search results into bibliographic software (Endnote X7, The Thomson Corp, USA); we then removed duplicates. At least two review authors (GG, CH, JA, HL, HH) independently screened the studies by title and abstract for exclusion using a template that included inclusion and exclusion criteria. We recorded reasons for exclusion. For the remaining studies, we retrieved full papers. Two review authors independently recorded the inclusion and exclusion criteria in the first section of the data extraction form. We resolved all disagreements through arbitration by a third review author (GG, CH, JA, HL, HH).
Data extraction and management
At least two review authors (GG, CH, JA, HL, HH) abstracted data independently onto a pre‐defined data extraction form and entered the data into RevMan 5.3. We compared results and resolved disagreements by discussion amongst at least three review authors (GG, CH, JA, HL, HH).
Besides data on intervention and outcome, we recorded study and participant characteristics such as age; gender; severity of illness, as given (e.g. acute physiology and chronic health evaluation (APACHE), multiple organ failure (MOF) score, simplified acute physiology score (SAPS)); underlying diagnosis and particular type of shock, given definition of shock; duration of ICU stay before enrolment into study; duration of mechanical ventilation before enrolment; and study setting.
Assessment of risk of bias in included studies
Two review authors independently abstracted data onto a pre‐defined data extraction form. We abstracted whether adequate methods were used to generate a random sequence, whether allocation to treatment was concealed, whether inclusion and exclusion criteria were explicit, if data had been analysed by intention‐to‐treat, whether participant descriptions were adequate, whether care provided during the study period was identical in both groups, whether the outcome description was adequate, whether involved clinical staff were blinded to the intervention and whether the assessor of the outcome was blinded to the intervention. Noteworthy, for some interventions, is that performance bias is inevitable. We compared results and resolved disagreements by discussion amongst at least three review authors. We then entered data into RevMan. We produced a risk of bias graph and a risk of bias table.
Measures of treatment effect
For binary outcomes, we used risk ratio as the standard effect measure. For continuous outcomes, we used the difference in means as the standard effect measure.
Unit of analysis issues
We did not include cluster‐randomized or cross‐over trials in any of the analyses; in the case of multiple treatment groups, we refrained from combining groups to create a single pair‐wise summary comparison; and we declared studies as multi‐arm comparisons to allow for adequate network meta‐analyses.
Dealing with missing data
We did not replace missing data by using any algorithm, but we contacted study authors if we considered missing data essential.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by performing an informal inspection of study characteristics and clinical judgement. We measured statistical heterogeneity with the I2 statistic and heterogeneity with Cochrane Q tests. We did not use a specific threshold of I2 to judge heterogeneity, but as a general rule, we considered an I2 statistic greater than 50% as showing substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to assess reporting bias and small‐study effects graphically by using funnel plots of standard errors versus effect estimates for the primary outcome. We also planned to formally test funnel plot asymmetry by using the arcsine test (Rücker 2008) if 10 or more studies per comparison were available for the primary outcome.
Data synthesis
We combined data quantitatively only if clinical heterogeneity was assumed to be negligible. For standard meta‐analyses, we used RevMan 5.3. We used a random‐effects model to combine risk ratios by default because we expected several different comparisons to show at least some heterogeneity. In two trials (Dünser 2003; Martin 1993), some participants crossed over to the other treatment; these participants were analysed according to the intention‐to‐treat principle, that is, according to the group to which they were initially assigned.
In this update, we decided to add a network meta‐analysis to demonstrate direct and indirect effects simultaneously (Salanti 2014). For the analysis, we used the R package netmeta version 0.7 (R Core Team 2014). Network meta‐analysis is a generalization of pair‐wise meta‐analysis, whereby all pairs of treatments are compared on the basis of the graph‐theoretical method originally developed in electrical network theory. This method is considered equivalent to the frequentist approach to network meta‐analysis (Rücker 2012). For network meta‐analyses, we estimated both fixed‐effect and random‐effects models.
Conceptually, we identified two networks: one comparing different vasopressors or combinations of vasopressors (Figure 1, left‐hand side), and the other comparing vasopressors in combination with otherwise vasoactive catecholamines (Figure 1, right‐hand side). In both networks, the reference group was norepinephrine. We produced network plots indicating available direct comparisons, calculated effects relative to a baseline intervention and present network forest plots.
1.
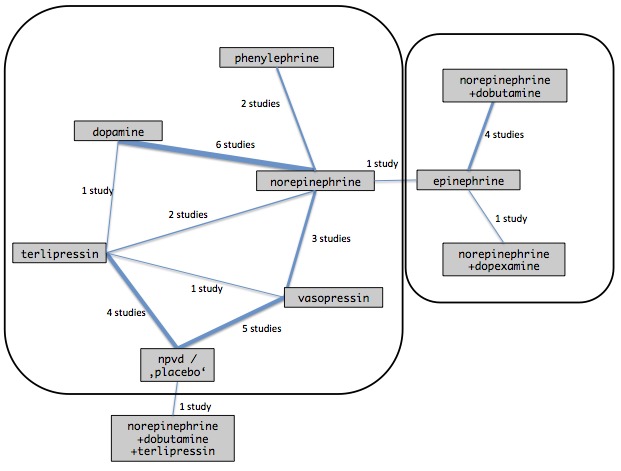
Comparisons including vasopressors identified from the systematic review. The 10 interventions with 31 direct comparisons were derived from 28 studies. Line thickness is proportional to the number of included participants. Boxes indicate the two networks that we formally assessed in our network meta‐analysis. npvd/'placebo' denotes non‐protocol vasoactive drugs or placebo.
We calculated metrics for consistency/homogeneity. Using Rücker's frequentist approach, we derived this content from decomposition of the Q statistic and from the net heat plot (Krahn 2013). We also present analyses on the strength of evidence obtained from matrices derived by the netmeasures function in R (König 2012).
Subgroup analysis and investigation of heterogeneity
We planned no a priori subgroup analyses. We performed a post hoc subgroup analysis of participants with septic shock. We included studies performed in participants with septic shock and studies for which estimates were available for subgroups with septic shock. For this subgroup, we performed a network meta‐analysis comparing different vasopressors or combinations of vasopressors. We used norepinephrine as the reference group because it is currently considered the vasopressor of first choice (Dellinger 2013). We did not perform a subgroup analysis for the network including otherwise vasoactive catecholamines because of the limited number of available studies.
Sensitivity analysis
We planned to perform a sensitivity analysis to assess the influence of risk of bias on the main effects of interventions, and thereby on the robustness of our estimates. We classified studies as 'low risk of bias' and 'no low risk of bias'. We classified studies as having low risk of bias if they had adequate allocation concealment, and if the other bias items in the summary were not believed to have a major influence on the robustness of the single study effect. Unclear or inadequate allocation concealment in any case resulted in classification as a 'study with no low risk of bias'. Our primary outcome was mortality, which was generally considered robust against outcome assessor knowledge of treatment allocation. Lack of blinding of outcome assessors therefore had less influence on assessment of risk of bias for this outcome. On the contrary, this risk of bias item had a strong effect on outcomes for which assessment included individual judgement, as for measures of quality of life. In the sensitivity analysis, we grouped studies according to our classification of 'low risk of bias' and 'no low risk of bias' in a forest plot.
We also performed a post hoc sensitivity analysis to investigate the influence of different time points on mortality outcome assessment.
'Summary of findings'
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (Guyatt 2008) to grade the quality of the body of evidence assessed in our review and constructed a 'Summary of findings' (SOF) table using GRADE software. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
We constructed ‘Summary of findings’ tables that included information about (1) populations (including specification of medium‐risk populations), interventions and comparisons for the standard meta‐analysis of norepinephrine versus dopamine on mortality and for results of the network meta‐analysis on mortality; (2) the source of external information used in the ‘Assumed risk’ column; (3) the GRADE approach to assess the quality of the body of evidence as briefly described above; and (4) any departures from standard methods. We included information on the primary outcome (total mortality) and on arrhythmia within 28 days.
Results
Description of studies
Results of the search
Search result
The electronic search resulted in 1776 hits after removal of duplicates with bibliographic software and one reference from other sources (Figure 2). We identified and retrieved 168 potentially relevant articles (this number included 12 articles identified by reading the references of potentially relevant articles and writing to 14 specialists in the field, five of whom replied; see Acknowledgements). Two trials were not retrievable (Hai Bo 2002; Singh 1966; see Characteristics of studies awaiting classification). Of these 168 articles, 140 did not meet our inclusion criteria after closer inspection for the following reasons.
2.
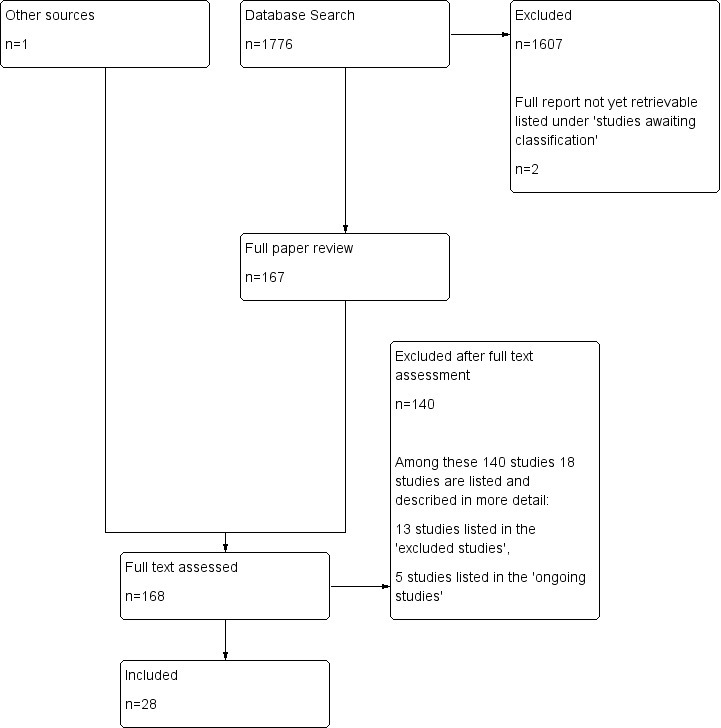
Search flow diagram.
54 trials involved other interventions.
49 were not randomized.
24 were cross‐over trials.
Three were animal studies.
Two trials were duplicates (abstract was presented at a scientific meeting and the report was subsequently published (Martin 1993)).
-
Four other publications (Russell 2008) did not meet criteria.
Two were systematic reviews.
Two eligible RCTs provided none of the pre‐defined outcomes (Morelli 2011; Morelli 2011a).
Of these 140 excluded reports, we identified 13 potentially relevant studies (see Characteristics of excluded studies). Finally, we included 28 studies in our review (Characteristics of included studies).
Included studies
In our original review (Müllner 2004), we included eight studies. In the updated review (Havel 2011), we included 15 new studies. This current update adds five new studies. In total, we have included 28 studies investigating several comparisons among 3497 participants (Figure 1). Six different vasopressors, alone or in combination, were studied in 12 different comparisons. Details are presented in the table Characteristics of included studies. Among these studies, eight were multi‐centre studies (Annane 2007; Choong 2009; De Backer 2010; Han 2012; Lauzier 2006; Malay 1999; Myburgh 2008; Russell 2008) and all but five (Annane 2007; Han 2012; Malay 1999; Myburgh 2008; Svoboda 2012) were performed at university hospitals only.
Eighteen studies were performed in participants with septic shock (Albanese 2005; Annane 2007; Han 2012; Jain 2010; Lauzier 2006; Malay 1999; Marik 1994; Martin 1993; Morelli 2008a; Morelli 2008b; Morelli 2009; Patel 2010; Ruokonen 1993; Russell 2008; Seguin 2002; Seguin 2006; Yildizdas 2008; Svoboda 2012). Three studies included participants with peri‐operative shock (Boccara 2003; Dünser 2003; Luckner 2006). Two studies were performed in paediatric participants (Choong 2009; Yildizdas 2008).
Seventeen studies provided norepinephrine as an intervention (Albanese 2005; Boccara 2003; De Backer 2010; Dünser 2003; Han 2012; Jain 2010; Lauzier 2006; Luckner 2006; Marik 1994; Martin 1993; Mathur 2007; Morelli 2008a; Morelli 2008b; Myburgh 2008; Patel 2010; Ruokonen 1993; Russell 2008); another four studies examined the combination of norepinephrine + dobutamine (Annane 2007; Levy 1997; Levy 2011; Seguin 2002); and one study used the combination of norepinephrine + dopexamine (Seguin 2006).
Nine studies used dopamine (De Backer 2010; Han 2012; Hua 2013; Jain 2010; Marik 1994; Martin 1993; Mathur 2007; Patel 2010; Ruokonen 1993), and six studies used epinephrine (Annane 2007; Levy 1997; Levy 2011; Myburgh 2008; Seguin 2002; Seguin 2006). Eight studies used vasopressin (Choong 2009; Dünser 2003; Han 2012; Lauzier 2006; Luckner 2006; Malay 1999; Morelli 2009; Russell 2008), and another seven studies used terlipressin (Albanese 2005; Boccara 2003; Hua 2013; Morelli 2008a; Morelli 2009; Svoboda 2012; Yildizdas 2008). Two studies used phenylephrine (Jain 2010; Morelli 2008b), and five studies compared vasopressors versus placebo or non‐protocolized vasopressors as add‐on therapy (Choong 2009; Malay 1999; Morelli 2008a; Svoboda 2012; Yildizdas 2008).
Excluded studies
In total, we excluded 140 studies after full‐text assessment, among which 13 studies are listed by reference. We have presented some details of the 13 excluded studies in the table Characteristics of excluded studies. We excluded 10 studies because they reported not on any of our pre‐defined endpoints but on haemodynamic variables and other surrogate endpoints instead (Argenziano 1997; Hentschel 1995; Kinstner 2002; Levy 1999; Majerus 1984; Morelli 2011; Morelli 2011a; Patel 2002; Totaro 1997; Zhou 2002). We excluded one trial in which investigators looked at pre‐term infants with hypotension (Rozé 1993), as this topic is covered in another Cochrane review (Subhedar 2003). One study was a non‐randomized multi‐centre prospective cohort study and therefore was excluded (Sperry 2008). Another study compared low‐dose dopamine versus dopexamine and versus placebo added to norepinephrine with the intention of improving renal and splanchnic blood flow. Low‐dose dopamine at 3 µg/kg/min is not considered to have relevant vasopressor properties; therefore we also excluded this study (Schmoelz 2006).
Studies waiting to be assessed
We have not yet been able to retrieve two particular studies. One, which was published in 1966 (Singh 1966), is a 'comparative study of angiotensin and norepinephrine in hypotensive states', according to the title. As no abstract is available, we do not know how many participants were enrolled. The second study, published in the journal Critical Care Shock in 2002 (Hai Bo 2002), also could not be retrieved. This paper is on the 'renal effect of dopamine, norepinephrine, epinephrine, or norepinephrine‐dobutamine in septic shock'. We do not know whether this study contained original data from human experiments, whether it was randomized and, if so, whether researchers reported relevant outcomes.
Ongoing studies
Our search resulted in 52 potentially relevant ongoing studies. We considered five ongoing studies (Choudhary 2013; Cohn 2007a; Fernandez 2006; Gordon 2014; Lienhart 2007) as relevant (Characteristics of ongoing studies).
Risk of bias in included studies
Methodological quality of included studies
We have presented risk of bias in Figure 3 and Figure 4.
3.
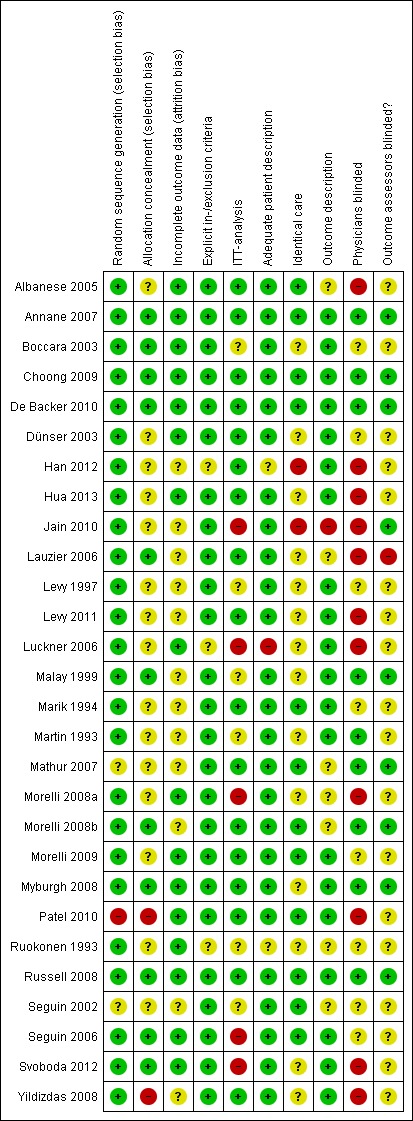
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
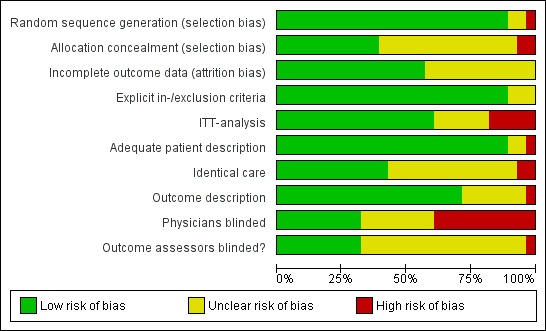
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
GRADE evidence quality varied considerably between comparisons and ranged from high to very low.
Generally, risk of bias in the included studies was moderate. For the comparison of norepinephrine versus dopamine, risk of bias was low, but for the other comparisons, risk of bias was moderate to high. We classified 11 studies as having low risk of bias for the primary outcome of mortality (Annane 2007; Boccara 2003; Choong 2009; De Backer 2010; Lauzier 2006; Malay 1999; Morelli 2008b; Myburgh 2008; Russell 2008; Seguin 2006; Svoboda 2012); only four studies fulfilled all trial quality items (Annane 2007; Choong 2009; De Backer 2010; Russell 2008).
Allocation
Random sequence generation was reported in all but four studies (Levy 2011; Mathur 2007; Patel 2010; Seguin 2002). Allocation concealment was appropriate in 11 studies (Annane 2007; Boccara 2003; Choong 2009; De Backer 2010; Lauzier 2006; Malay 1999; Morelli 2008b; Myburgh 2008; Russell 2008; Seguin 2006; Svoboda 2012) and was not appropriate in three studies (Han 2012; Patel 2010; Yildizdas 2008).
All but eight studies presented intention‐to‐treat analyses; for six studies this item was unclear (Boccara 2003; Levy 1997; Malay 1999; Martin 1993; Ruokonen 1993; Seguin 2002), and for four studies this was not fulfilled (Jain 2010; Luckner 2006; Morelli 2008a; Svoboda 2012).
Blinding
From the available information, identical care for the intervention group and the control group could be assumed for 12 studies (Albanese 2005; Annane 2007; Choong 2009; De Backer 2010; Marik 1994; Mathur 2007; Morelli 2008b; Morelli 2009; Patel 2010; Russell 2008; Seguin 2002; Seguin 2006). An appropriate outcome description was present in 20 studies; for the remaining eight studies, this was unclear (Albanese 2005; Jain 2010; Lauzier 2006; Mathur 2007; Morelli 2008a; Morelli 2008b; Ruokonen 1993; Seguin 2002). Treating personnel were blinded in nine studies (Annane 2007; Choong 2009; De Backer 2010; Malay 1999; Martin 1993; Mathur 2007; Morelli 2008b; Myburgh 2008; Russell 2008). In the same nine studies, outcome assessors were blinded too. In one study, only outcome assessors were blinded (Jain 2010).
Incomplete outcome data
Generally, studies included critically ill participants, for whom follow‐up usually is not of major concern.
Selective reporting
Given the large number of comparisons, each with a few studies only, proper assessment of between‐study reporting bias was not possible. However, when looking at the funnel plot in Figure 5, we could not spot major asymmetry. Within‐study reporting bias: Many of the included studies were performed several years ago, when it was not standard to publish trial protocols. Accordingly, we could not systematically assess selective outcome data reporting because protocols were not available. However, for the primary outcome of 'mortality', we assumed that selective omission of this outcome was unlikely in the ICU setting because this is one of the standard clinical outcomes. Choice of outcome usually is determined by the design (short‐term haemodynamic studies vs longer‐term studies with clinical outcomes).
5.
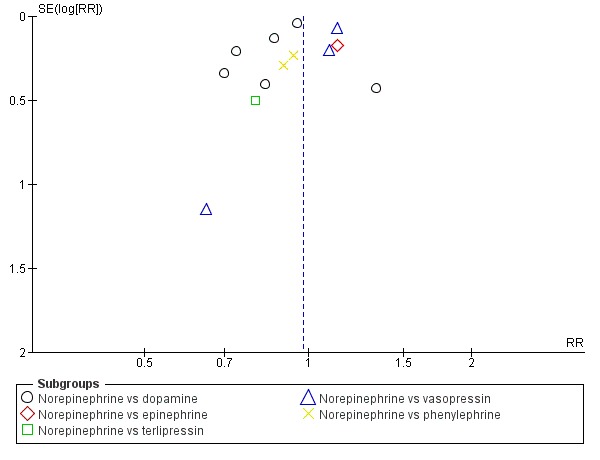
Funnel plot of comparison: 1 norepinephrine, outcome: 1.1 mortality.
Other potential sources of bias
All but two studies explicitly described inclusion and exclusion criteria (Boccara 2003; Ruokonen 1993). Participants were adequately described in all but three studies (Han 2012; Luckner 2006; Ruokonen 1993).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Dopamine compared with norepinephrine for hypotensive shock.
| Dopamine compared with norepinephrine for hypotensive shock | ||||||
| Patient or population: hypotensive shock Settings: critical care units Intervention: dopamine Comparison: norepinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Norepinephrine | Dopamine | |||||
| Total mortalitya | Moderateb | RR 1.07 (0.99 to 1.16)c | 1400 (6 RCTs) | ⊕⊕⊕⊕ HIGHd,e | ||
| 380 per 1000 | 407 per 1000 (376 to 441) | |||||
| Arrhythmia < BR/> follow‐up: range 28 days | Moderatef,g | RR 2.33 (1.45 to 3.85) | 1931 (2 RCTs) | ⊕⊕⊕⊕ HIGHh | ||
| 76 per 1000 | 177 per 1000 (110 to 293) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe largest study reported 12‐month mortality, one study reported 28‐day mortality and one hospital mortality. For the remaining 3 studies, the time point of mortality assessment was undetermined. A sensitivity analysis indicates no influence on effects by differences in mortality definition bSakr 2006
cEstimate from the network meta‐analysis integrating direct and indirect comparisons dFour smaller studies included up to 50 participants, each of whom did not fulfil some of the quality criteria and one high risk of bias study that contributed 252 participants. However, the summary result is mainly made up by the largest study of more than 1000 participants that fulfils all low risk of bias criteria eThe main outcomes of the four smaller studies are haemodynamics and metabolic measures. Mortality is reported only at the end of the results and often is unclear timepoint‐wise. However, the study by De Backer 2010 (which contributes mainly to the summary result) clearly defines mortality endpoints fReinelt 2001 gAnnane 2008 hInformation was obtained from 992 participants, 86% of whom were studied in a low risk of bias study (De Backer 2010); remaining participants were included in a high risk of bias study (Patel 2010). Effects show into the same direction
Summary of findings 2. Terlipressin compared with norepinephrine for hypotensive shock.
| Terlipressin compared with norepinephrine for hypotensive shock | ||||||
| Patient or population: hypotensive shock Settings: critical care units Intervention: terlipressin Comparison: norepinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Norepinephrine | Terlipressin | |||||
| Total mortalitya | Moderateb | RR 0.93 (0.69 to 1.26)c | 231 (7 RCTs) | ⊕⊝⊝⊝ VERY LOWd | ||
| 380 per 1000 | 353 per 1000 (262 to 479) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDirect comparisons based on in‐hospital mortality and mortality at an undetermined point in time. A sensitivity analysis indicates no influence on effects by differences in mortality definition. bSakr 2006
cEstimate from the network meta‐analysis integrating direct and indirect comparisons
dRisk of bias was serious with adequate allocation concealment in one small study that contributed to the estimates only; imprecision of the estimates was very serious, as direct comparisons are based on two small studies only, whereas only one contributed to the estimates
Summary of findings 3. Vasopressin compared with norepinephrine for hypotensive shock.
| Vasopressin compared with norepinephrine for hypotensive shock | ||||||
| Patient or population: hypotensive shock Settings: critical care units Intervention: vasopressin Comparison: norepinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Norepinephrine | Vasopressin | |||||
| Total mortalitya | Moderateb | RR 0.90 (0.78 to 1.03)c | 1108 (8 RCTs) | ⊕⊕⊕⊝ MODERATEd | ||
| 380 per 1000 | 342 per 1000 (296 to 391) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aFor the direct comparison, the largest study reported 90‐day mortality, and 2 studies contributed ICU mortality. A sensitivity analysis indicates no influence on effects by differences in mortality definition bSakr 2006
cEstimate from the network meta‐analysis integrating direct and indirect comparisons
dThe effect is based mainly on 1 moderately large RCT, and direct evidence comes from 3 RCTS including 812 individuals overall
Summary of findings 4. Phenylephrine compared with norepinephrine for hypotensive shock.
| Phenylephrine compared with norepinephrine for hypotensive shock | ||||||
| Patient or population: hypotensive shock Settings: critical care units Intervention: phenylephrine Comparison: norepinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Norepinephrine | Phenylephrine | |||||
| Total mortalitya | Moderateb | RR 1.08 (0.76 to 1.55)c | 86 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWd,e | ||
| 380 per 1000 | 410 per 1000 (289 to 589) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aFor the direct comparison, one study measured ICU mortality; for the other study, the time point of mortality assessment was undetermined bSakr 2006
cEstimate from the network meta‐analysis integrating direct and indirect comparisons
dTwo small studies, with 1 having unclear allocation concealment and an open intervention
eTwo small studies with 86 individuals overall for the direct comparison
Summary of findings 5. Epinephrine compared with norepinephrine for hypotensive shock.
| Epinephrine compared with norepinephrine for hypotensive shock | ||||||
| Patient or population: hypotensive shock Settings: critical care units Intervention: epinephrine Comparison: norepinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Norepinephrine | Epinephrine | |||||
| Total mortalitya | Moderateb | RR 0.88 (0.63 to 1.25) | 269 (1 RCT) | ⊕⊕⊝⊝ LOWc | ||
| 380 per 1000 | 334 per 1000 (239 to 475) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
a90‐Day mortality bSakr 2006
cEffect from only 1 moderately large single RCT
In total, six vasopressors were compared in several combinations and directions (Figure 2). Therefore, we have organized our comparisons to present each vasopressor against all comparators in a separate analysis per outcome. Vasopressors that were used in both study arms were considered as constant between groups and generally were not explicitly described in analyses. For studies with more than two study arms, we used each comparison separately. We refrained from including overall summary effects within analyses to address considerable clinical heterogeneity due to major differences in comparators and,when applicable, to avoid a unit of analysis error.
Main analyses
Primary outcomes
Total mortality
Total mortality was assessed in all included studies. If mortality was assessed at several time points in a study we used data from the latest follow‐up time. Mortality was assessed at an undetermined time point in seven studies (Boccara 2003,; Jain 2010; Levy 1997,; Marik 1994,; Mathur 2007,; Seguin 2002,; Ruokonen 1993).
Norepinephrine was compared with dopamine, epinephrine, terlipressin, vasopressin, phenylephrine and norepinephrine + terlipressin + dobutamine (14 studies; 2607 participants) (Figure 6). In addition, Morelli 2009 compared norepinephrine versus norepinephrine + vasopressin and norepinephrine versus norepinephrine + terlipressin and found no differences in both comparisons (risk ratio (RR) 1.25, 95% confidence interval (CI) 0.69 to 2.26; and RR 1.43, 95% CI 0.75 to 2.70 ‐ data from the single study not presented in analyses). Studies were performed in participants with septic shock (Albanese 2005; Jain 2010; Lauzier 2006; Levy 1997; Marik 1994; Martin 1993; Mathur 2007; Morelli 2008a; Morelli 2008b; Morelli 2009; Patel 2010; Ruokonen 1993; Russell 2008; Seguin 2002; Seguin 2006), in critically ill participants (De Backer 2010; Myburgh 2008), in participants with refractory hypotension after anaesthesia (Boccara 2003) and in adult post‐operative participants (Luckner 2006). None of the comparisons revealed significant differences. Morelli 2008a compared NPVD (including norepinephrine) versus norepinephrine + terlipressin + dobutamine and found no differences in mortality (14/20 vs 14/20; RR 1.00, 95% CI 0.67 to 1.50). The funnel plot, which is presented in Figure 5 shows no indication of relevant asymmetry.
6.
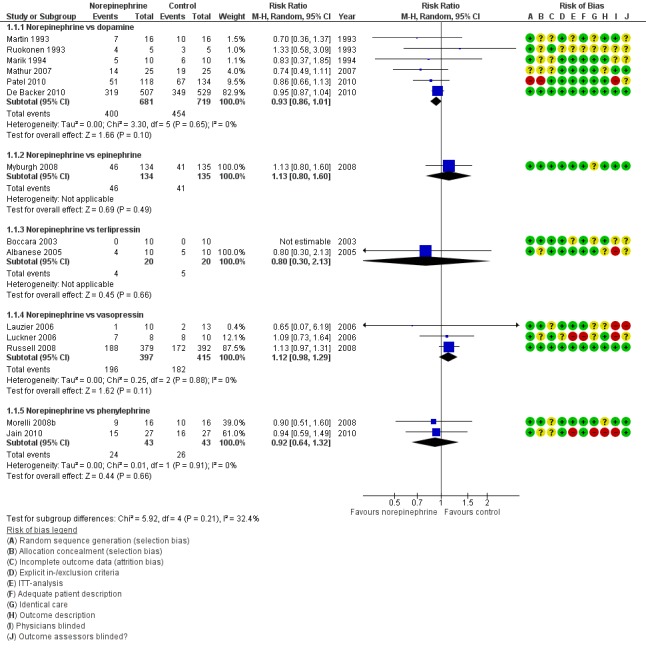
Forest plot of comparison: 1 Norepinephrine, outcome: 1.1 Total mortality.
Epinephrine was compared with norepinephrine, norepinephrine + dobutamine and norepinephrine + dopexamine (six studies; 703 participants) (Analysis 2.1). Overall 298 deaths were observed among the 703 participants. Studies were performed in participants with septic shock (Annane 2007; Levy 1997; Seguin 2002; Seguin 2006), participants with cardiogenic shock (Levy 2011) and critically ill participants (Myburgh 2008). In no comparisons was a significant difference found.
2.1. Analysis.
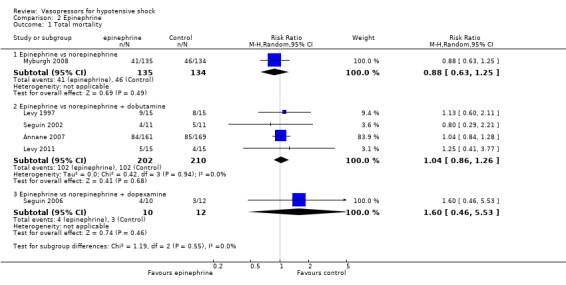
Comparison 2 Epinephrine, Outcome 1 Total mortality.
Vasopressin was compared with placebo (non‐protocol vasoactive drugs), terlipressin and norepinephrine/dopamine (eight studies; 1138 participants) (Analysis 3.1). In the comparisons 'versus placebo/non‐protocol vasoactive drugs', two studies actually used a placebo (Choong 2009; Malay 1999), two studies (Dünser 2003; Morelli 2009) compared fixed‐dose vasopressin + variable‐dose norepinephrine versus variable‐dose norepinephrine and one study compared vasopressin versus norepinephrine or dopamine (Han 2012). Overall 503 deaths were observed among 1138 participants. Studies were performed in participants with septic shock (Dünser 2003; Han 2012; Lauzier 2006; Malay 1999; Morelli 2009; Russell 2008), in adult post‐operative participants (Luckner 2006) and in paediatric participants with vasodilatory shock (Choong 2009). In no comparisons was a significant difference found.
3.1. Analysis.
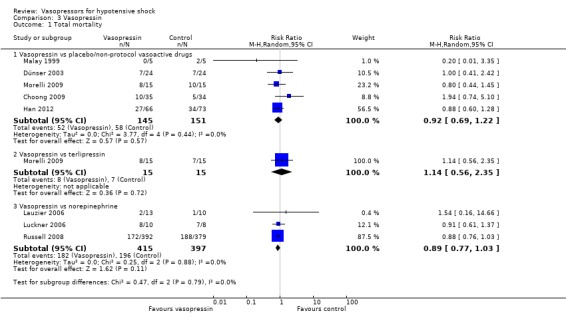
Comparison 3 Vasopressin, Outcome 1 Total mortality.
Terlipressin was compared with placebo (non‐protocol vasoactive drugs), norepinephrine and vasopressin (seven studies; 259 participants) (Analysis 4.1). In the comparisons 'versus placebo/non‐protocol vasoactive drugs', one study actually used a placebo (Yildizdas 2008), and three studies (Morelli 2008a; Morelli 2009; Svoboda 2012) compared fixed‐dose terlipressin + variable‐dose norepinephrine versus variable‐dose norepinephrine. In the same study (Morelli 2008a), investigators compared norepinephrine + terlipressin + dobutamine versus non‐protocol vasoactive drugs and found no difference (14/20 vs 14/20; RR 1.00, 95% CI 0.67 to 1.50). In another study, terlipressin was compared with dopamine (Hua 2013). Overall 150 deaths were observed among 259 participants. Studies were performed in participants with septic shock (Albanese 2005; Han 2012; Morelli 2008a; Morelli 2009; Svoboda 2012), in children with catecholamine‐resistant shock (Yildizdas 2008) and in participants with refractory hypotension after anaesthesia (Boccara 2003). No comparisons revealed a significant difference.
4.1. Analysis.
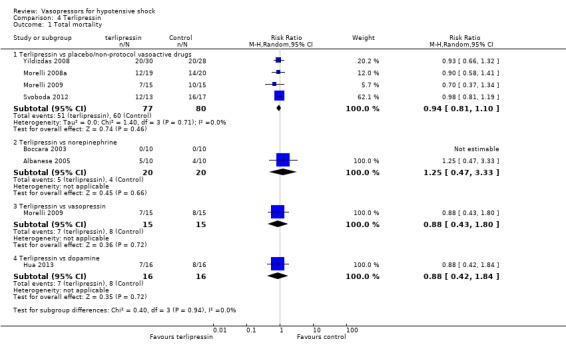
Comparison 4 Terlipressin, Outcome 1 Total mortality.
Dopamine was compared with norepinephrine and with terlipressin (seven studies; 1432 participants) (Analysis 5.1). Overall 869 deaths were observed among 1432 participants. Studies were performed in participants with septic shock (Hua 2013; Marik 1994; Martin 1993; Mathur 2007; Patel 2010; Ruokonen 1993) and in participants with several causes of shock (De Backer 2010). In no comparisons was a significant difference found.
5.1. Analysis.
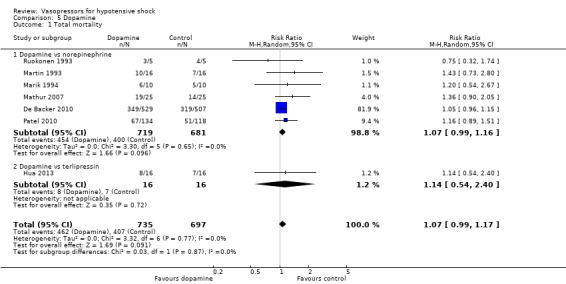
Comparison 5 Dopamine, Outcome 1 Total mortality.
Phenylephrine was compared with norepinephrine in participants with septic shock (two studies; 86 participants) (Jain 2010; Morelli 2008b) (Analysis 1.1). Overall 50 deaths were observed among 86 participants (RR 0.92, 95% CI 0.64 to 1.32).
1.1. Analysis.
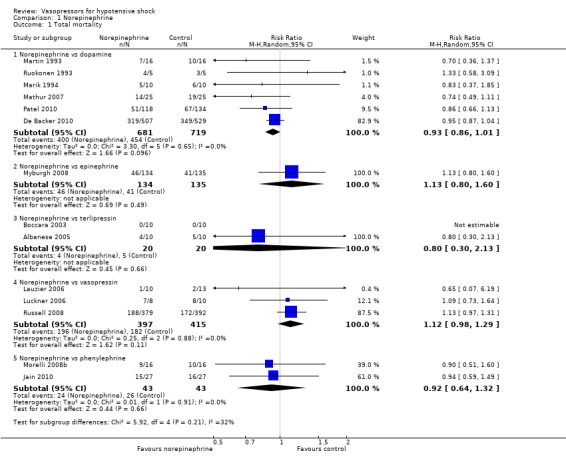
Comparison 1 Norepinephrine, Outcome 1 Total mortality.
In a network that included vasopressors only (Figure 7), we found no differences in effects of any vasopressor versus non‐protocol vasoactive drugs on mortality, as shown in the network forest plot comparing seven vasopressor regimens versus norepinephrine (Figure 8) from 22 studies with 24 pair‐wise comparisons. Heterogeneity/inconsistency: tau2 < 0.0001; I2 statistic = 0%. Test of heterogeneity/inconsistency: Q = 8.39 (d.f. 17), P value = 0.96. Likewise, no hotspots are evident in the heatplot indicating the absence of specific sources of important inconsistency in the network (see www.meduniwien.ac.at/user/harald.herkner/AppendixCARG029_2015.pdf). For the full network assessment, including measures for characterizing a network meta‐analysis (direct evidence proportion, mean path length, minimal parallelism of mixed treatment comparisons and evidence flow per design) (see www.meduniwien.ac.at/user/harald.herkner/AppendixCARG029_2015.pdf).
7.
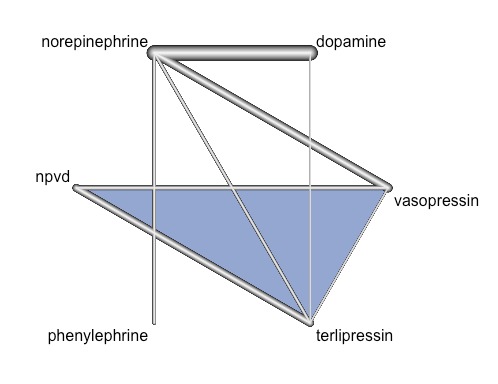
Graphical representation of the evidence network including vasopressors showing available direct comparisons. The lines represent direct comparisons, and line thickness is proportional to precision of the estimates (1/SE).
8.
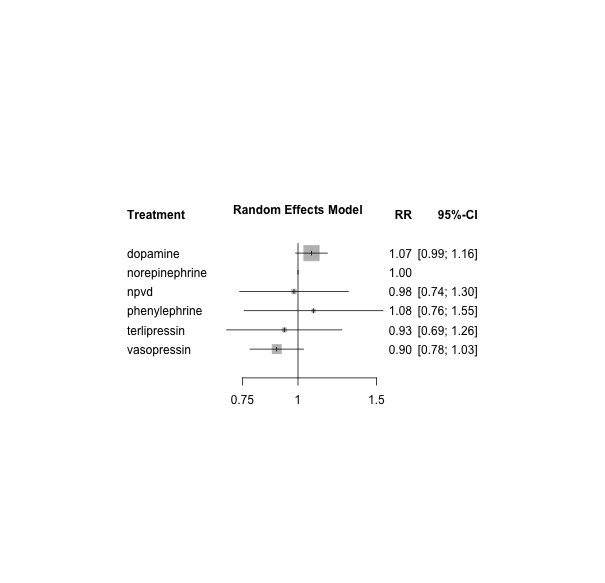
Network forest plot comparing seven vasopressor regimens vs norepinephrine (reference) from 22 studies with 24 pair‐wise comparisons. Heterogeneity/inconsistency: tau2 < 0.0001; I2 statistic = 0%. Test of heterogeneity/inconsistency: Q = 8.48 (d.f. 18), P value = 0.97; 'NPVD' denotes non‐protocol vasoactive drugs with or without placebo. RR denotes risk ratio, as calculated by a fixed‐effect model. RR > 1 indicates increased mortality risk; RR < 1 indicates reduced mortality risk vs norepinephrine (reference).
In a network that included vasopressors and combinations with beta‐agonist action (Figure 9), we found no differences in the effects of any vasopressor versus norepinephrine on mortality, as shown in the network forest plot comparing four vasopressor regimens with a beta‐agonist component versus norepinephrine (Figure 10) from six studies with six pair‐wise comparisons. Heterogeneity/inconsistency: tau2 < 0.0001; I2 statistic = 0%. Test of heterogeneity/inconsistency: Q = 0.42 (d.f. 3), P value = 0.94 (Figure 10). No indication of severe heterogeneity/inconsistency was found, although several analyses were not possible with available data. For the full network assessment, see the Appendix (www.meduniwien.ac.at/user/harald.herkner/AppendixCARG029_2015.pdf).
9.
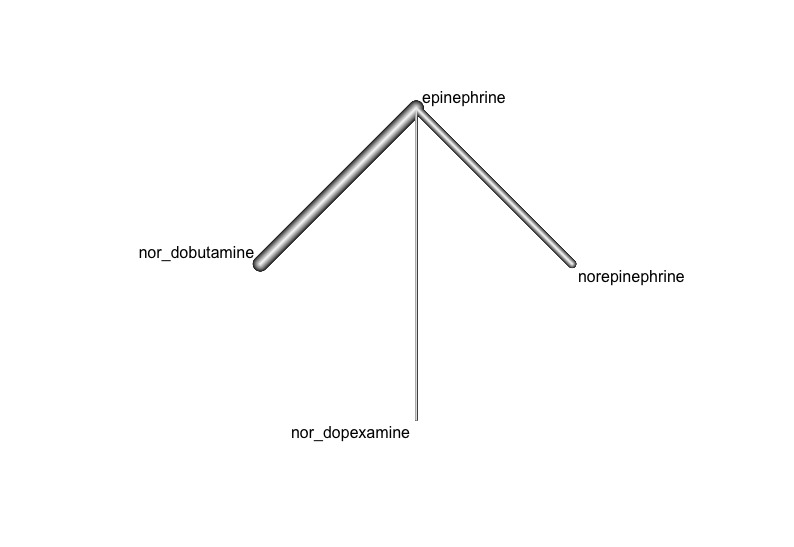
Graphical representation of the evidence network including vasopressors and combinations with beta‐agonist action showing available direct comparisons. The lines represent direct comparisons, and line thickness is proportional to precision of the estimates (1/SE).
10.
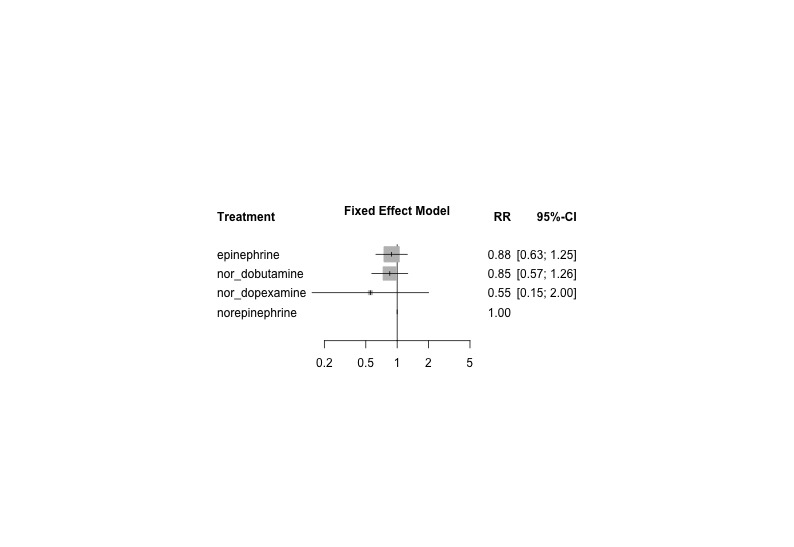
Network forest plot comparing four vasopressor regimens with a beta‐agonist component vs norepinephrine (reference) from 6 studies with 6 pair‐wise comparisons. Heterogeneity/inconsistency: tau2 < 0.0001; I2 statistic = 0%. Test of heterogeneity/inconsistency: Q = 0.42(d.f. 3), P value = 0.94; 'nor_' denotes norepinephrine added to the other drugs as described. RR denotes risk ratio, as calculated by a fixed‐effect model. RR > 1 indicates increased mortality risk; RR < 1 indicates reduced mortality risk vs norepinephrine (reference).
Secondary outcomes
Morbidity
Morbidity was assessed as (1a) ICU LOS; (1b) hospital LOS; (1c) duration of vasopressor treatment; (1d) duration of mechanical ventilation; (1e) renal failure (as defined by study authors as oliguria or renal replacement therapy) and (1f) other. Renal outcomes are presented separately in Table 6.
1. Morbidity outcomes ‐ measures of renal function comparing several vasopressor regimens (each single vasopressor is compared with other available vasopressor regimens).
| Vasopressor | Comparator (Reference) | Outcome | Effect* |
| Norepinephrine |
Vasopressin (Lauzier 2006) Vasopressin (Russell 2008) |
Creatinine clearance Days alive free of renal replacement therapy |
54 mL min‐11.73 m‐2 ± 38 vs 122 mL min‐11.73m‐2 ± 66 , P value < 0.001 (23 (IQR 5 to 28) vs 25 (IQR 6 to 28), P value = 0.64) |
| Norepinephrine + terlipressin + dobutamine (Morelli 2008a) |
Urine output 4 hours after study start |
96 mL/h ± 48 vs 130 mL/h ± 76 (P value < 0.05) |
|
| Norepinephrine + terlipressin (Morelli 2008a) |
Urine output 4 hours after study start | 96 mL/h ± 48 vs 147 mL/h ± 119 (P value = 0.08) |
|
| Dopamine (Mathur 2007) Dopamine (De Backer 2010) |
Post‐treatment urine output Days free of renal support within 28 days |
1.17 mL/kg/h ± 0.47 vs 0.81 mL/kg/h ± 0.75, P value < 0.05 14.0 ± 12.3 days vs 12.8 ± 12.4 days, P value = 0.07 |
|
| Phenylephrine (Morelli 2008b) |
Creatinine clearance Urine output |
No difference (P value = 0.61) No difference (P value = 0.17) |
|
| Terlipressin (Boccara 2003) | Renal failure post‐operatively |
(0/10 vs 0/10) |
|
| Terlipressin + norepinephrine and vasopressin + norepinephrine (Morelli 2009) |
Need for renal replacement therapy |
(8/15 vs 4/15 vs 5/15, P value = 0.29) |
|
| Epinephrine | Norepinephrine + dobutamine (Levy 1997) |
Oliguria reversal |
9/12 vs 10/11 RR 0.36 (95% CI 0.04 to 3.00) |
| Vasopressin |
Norepinephrine (Lauzier 2006) Norepinephrine (Russell 2008) Norepinephrine vs terlipressin (Morelli 2009) |
Creatinine clearance Days alive free of renal replacement therapy Need for renal replacement therapy |
122 mL min‐11.73m‐2 ± 66 vs 54 mL min‐11.73m‐2 ± 38, P value < 0.001 25 (IQR 6 to 28) vs 23 (IQR 5 to 28), P value = 0.64 8/15 vs 4/15 vs 5/15, P value = 0.29 |
| Placebo/non‐protocol vasoactive drugs (Choong 2009) Placebo/non‐protocol vasoactive drugs (Malay 1999) |
Urine output Creatinine |
1.7 mL/kg/h (IQR 0.7 to 3.5) vs 1.5 mL/kg/h (IQR 0.7 to 3.7), P value = 0.65 N/A |
|
| Terlipressin |
Norepinephrine vs vasopressin (Morelli 2009) |
Need for renal replacement therapy |
8/15 vs 4/15 vs 5/15, P value = 0.29 |
| Placebo/non‐protocol vasoactive drugs in patients taking norepinephrine (Morelli 2008a) | Urine output 4 hours after study start |
147 mL/h ± 119 vs 96 mL/h ± 48 (P value = 0.08) |
|
| Dopamine | Norepinephrine (Mathur 2007) Norepinephrine (De Backer 2010) |
Post‐treatment urine output Days free of renal support within 28 days |
0.81 mL/kg/h ± 0.75 vs 1.17 mL/kg/h ± 0.47, P value < 0.05 12.8 ± 12.4 days vs 14.0 ± 12.3 days, P value = 0.07 |
*All effects are presented as outcomes in the vasopressor group (left hand column) versus comparator (second column) with risk ratio or P value for differences between groups
CI = confidence interval
IQR = 25% to 75% interquartile range
N/A = not applicable
RR = risk ratio
Norepinephrine was compared with dopamine, vasopressin, phenylephrine and norepinephrine + terlipressin + dobutamine in terms of (1a) ICU LOS (five studies; 2781 participants) (Analysis 1.2). All studies included participants with septic shock. Investigators reported no differences in (1a) ICU LOS and (1b) hospital LOS (Analysis 1.2; Analysis 1.3). Additionally, Russell 2008 compared norepinephrine versus vasopressin and found no significant differences in (1b) hospital LOS (difference 1.00 day, 95% CI ‐3.01 to 5.01). Further, they reported no significant differences in (1c) vasopressor use (17, interquartile range (IQR) 0 to 24 vs 19, IQR 0 to 24; P value = 0.61) and (1d) days alive free of mechanical ventilation (six, IQR 0 to 20 vs 9, IQR 0 to 20; P value = 0.24). Myburgh 2008 compared norepinephrine with epinephrine and found no differences in the number of (1c) vasopressor‐free days (25 days, IQR 14 to 27 vs 26 days, IQR 19 to 27; P value = 0.31). De Backer 2010 compared norepinephrine with dopamine and found no differences in days free of mechanical ventilation within 28 days (9.5 ± 11.4 days vs 8.5 ± 11.2 days; P value = 0.13). They noted a small difference in (1c) days free of any vasopressor therapy within 28 days (14.2 ± 12.3 days vs 12.6 ± 12.5 days; P value = 0.007). The largest study by De Backer 2010 and a smaller study by Patel 2010 compared dopamine versus norepinephrine and found significant differences in (1f) arrhythmia (Analysis 1.4), including mostly sinus tachycardia (Patel 2010): 25% versus 6%; atrial fibrillation: 21% versus 11% (De Backer 2010), 13% versus 3% (Patel 2010); ventricular tachycardia (De Backer 2010): 2.4% versus 1.0%; and ventricular fibrillation (De Backer 2010): 1.2% versus 0.5%. Boccara 2003 compared noradrenaline with terlipressin and found no differences in (1b) hospital LOS (5 days, IQR 4 to 7 vs 5 days, IQR 4 to 7).
1.2. Analysis.
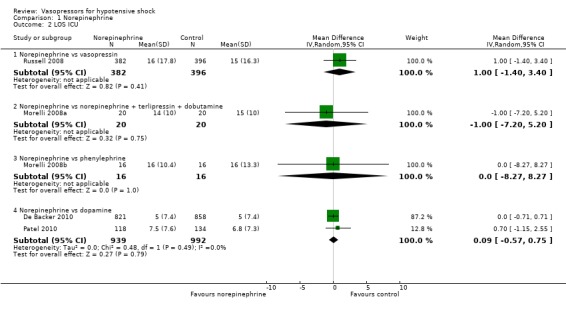
Comparison 1 Norepinephrine, Outcome 2 LOS ICU.
1.3. Analysis.
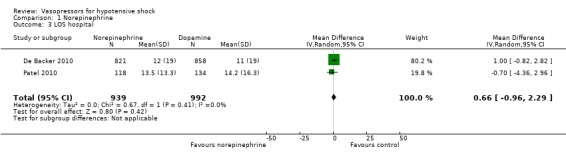
Comparison 1 Norepinephrine, Outcome 3 LOS hospital.
1.4. Analysis.
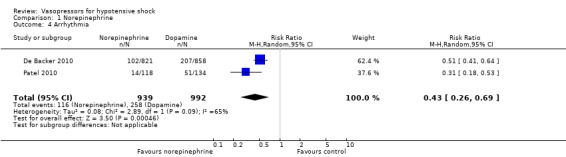
Comparison 1 Norepinephrine, Outcome 4 Arrhythmia.
Epinephrine was compared with norepinephrine + dobutamine in terms of (1a) ICU LOS in participants with septic shock (one study; 330 participants) (Annane 2007). Researchers reported no significant differences in ICU LOS (difference 1.00 day, 95% CI ‐3.01 to 5.01). In the same study (Annane 2007), the number of (1c) vasopressor‐free days until day 90 was reported as a median 53 days (IQR 0 to 86) in the epinephrine group and 66 days (IQR 6 to 86) in the norepinephrine + dobutamine group (P value = 0.18). In the same study, duration of (1c) vasopressor support was presented as a Kaplan‐Meier plot (log‐rank test P value = 0.09). Myburgh 2008 compared epinephrine with norepinephrine and found no differences in the number of (1c) vasopressor‐free days (26 days, IQR 19 to 27 vs 25 days, IQR 14 to 27; P value = 0.31).
Vasopressin was compared with placebo (non‐protocol vasoactive drugs), terlipressin and norepinephrine in terms of (1a) ICU LOS (four studies; 1046 participants) (Analysis 3.2). Studies were performed in participants with septic shock (Morelli 2009; Russell 2008) and paediatric vasodilatory shock (Choong 2009). In no comparison was a significant difference found. Vasopressin was compared with norepinephrine in terms of (1b) hospital LOS in one study (Russell 2008), and no significant difference was found (difference 1.00 day, 95% CI ‐3.01 to 5.01). Further, researchers noted no significant differences in (1c) vasopressor use (19, IQR 0 to 24 vs 17, IQR 0 to 24; P value = 0.61) and (1d) days alive free of mechanical ventilation (9, IQR 0 to 20 vs 6, IQR 0 to 20; P value = 0.24). Choong 2009 compared vasopressin with placebo/non‐protocol vasoactive drugs in paediatric participants and found no differences in (1c) time to vasopressor discontinuation (50 hours, IQR 30 to 219 vs 47, IQR 26 to 87; P value = 0.85) and (1d) mechanical ventilation‐free days until day 30 (17 days, IQR 0 to 24 vs 23 days, IQR 13 to 26; P value = 0.15). Han 2012 compared vasopressin with non‐protocol vasoactive drugs and found no differences in (1a) LOS (five days (IQR 3 to 8) vs five days (IQR 3 to 8) and (1d) duration of mechanical ventilation (4 days (IQR 3 to 6) vs 4 days (IQR 2 to 5); P value > 0.05),
3.2. Analysis.
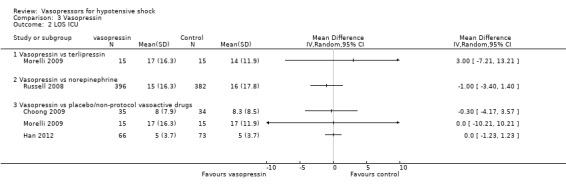
Comparison 3 Vasopressin, Outcome 2 LOS ICU.
Terlipressin was compared with placebo (non‐protocol vasoactive drugs) (six studies; 185 participants) (Morelli 2008a; Morelli 2009; Svoboda 2012; Yildizdas 2008), dopamine (Hua 2013), norepinephrine (Boccara 2003) and vasopressin (Morelli 2009) in terms of (1a) ICU LOS (Analysis 4.2), (1b) hospital LOS (Analysis 4.3), (1c) pressor‐free days until day 28 (Analysis 4.5), (1d) duration of mechanical ventilation (Analysis 4.4) and (1f) serious adverse events (Analysis 4.6). In comparisons summarized as 'versus placebo/non‐protocol vasoactive drugs', one study actually used placebo (Yildizdas 2008); in two studies (Morelli 2008a; Morelli 2009), fixed‐dose terlipressin + variable dose norepinephrine was compared with variable‐dose norepinephrine. Studies were performed in participants with septic shock (Morelli 2008a; Morelli 2009; Svoboda 2012) and in children with catecholamine‐resistant shock (Yildizdas 2008), peri‐operative refractory hypotension (Boccara 2003) and acute respiratory distress syndrome (Hua 2013). In no comparison was a significant difference found.
4.2. Analysis.
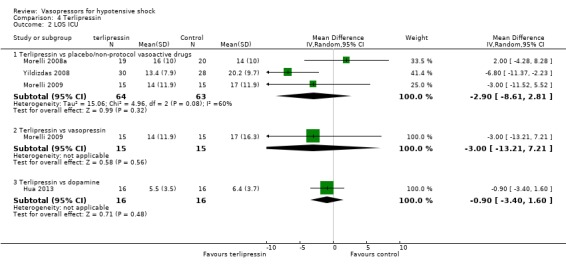
Comparison 4 Terlipressin, Outcome 2 LOS ICU.
4.3. Analysis.
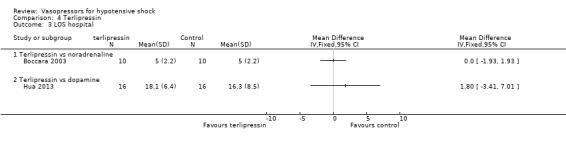
Comparison 4 Terlipressin, Outcome 3 LOS hospital.
4.5. Analysis.
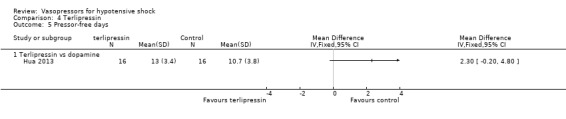
Comparison 4 Terlipressin, Outcome 5 Pressor‐free days.
4.4. Analysis.
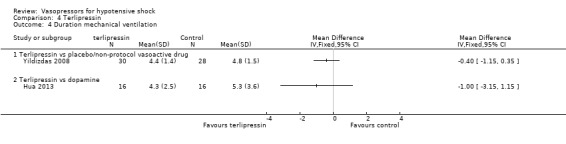
Comparison 4 Terlipressin, Outcome 4 Duration mechanical ventilation.
4.6. Analysis.
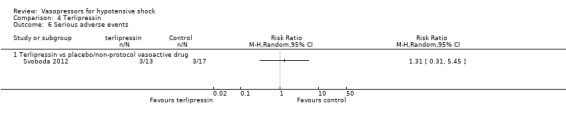
Comparison 4 Terlipressin, Outcome 6 Serious adverse events.
Dopamine was compared with norepinephrine (two studies; 1931 participants; De Backer 2010; Patel 2010) and terlipressin (one study; 32 participants) Hua 2013) in terms of (1a) ICU LOS (Analysis 5.2), (1b) hospital LOS (Analysis 5.3), (1c) pressor‐free days until day 28 (Analysis 5.4) and (1f) arrhythmia (Analysis 5.5). De Backer 2010 and Patel 2010 compared dopamine with norepinephrine and found no differences in (1a) ICU LOS and (1b) hospital LOS (Analysis 5.2; Analysis 5.3). Further, De Backer 2010 assessed (1d) days free of mechanical ventilation within 28 days (8.5 ± 11.2 days vs 9.5 ± 11.4 days; P value = 0.13). Hua 2013 compared dopamine with noradrenaline for duration of mechanical ventilation (5.3 ± 3.6 days vs 4.3 ± 2.5 days; difference 1.00, 95% CI ‐1.15 to 3.15).
5.2. Analysis.
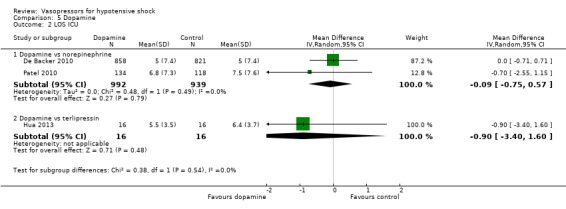
Comparison 5 Dopamine, Outcome 2 LOS ICU.
5.3. Analysis.
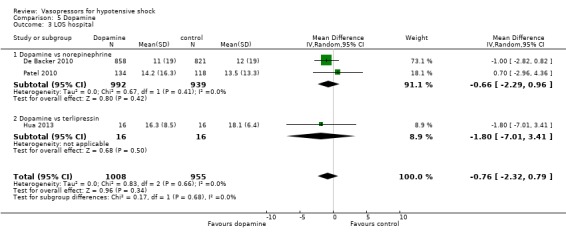
Comparison 5 Dopamine, Outcome 3 LOS hospital.
5.4. Analysis.
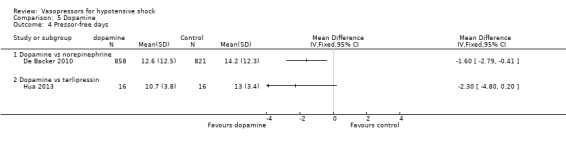
Comparison 5 Dopamine, Outcome 4 Pressor‐free days.
5.5. Analysis.
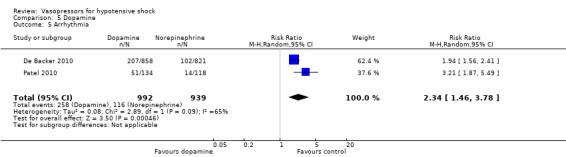
Comparison 5 Dopamine, Outcome 5 Arrhythmia.
De Backer 2010 reported a small difference in (1c) days free of any vasopressor therapy within 28 days (12.6 ± 12.5 days vs 14.2 ± 12.3 days; P value = 0.007; Analysis 5.4). The largest study by De Backer 2010 and a smaller study by Patel 2010 compared dopamine versus norepinephrine and found significant differences in (1f) arrhythmias (Analysis 5.5), including mostly sinus tachycardia (Patel 2010): 25% versus 6%; atrial fibrillation: 21% versus 11% (De Backer 2010), 13% versus 3% (Patel 2010); ventricular tachycardia (De Backer 2010): 2.4% versus 1.0%; and ventricular fibrillation (De Backer 2010): 1.2% versus 0.5%.
Phenylephrine was compared with norepinephrine in participants with septic shock (one study; 32 participants) (Morelli 2008b). Mean (1a) ICU LOS was 16 ± 13 versus 16 ± 10 days (difference 0.00 days, 95% CI ‐8.27 to 8.27).
Measures of health‐related quality of life and measures of anxiety and depression
In no studies were measures of health‐related quality of life assessed. In no studies were measures of anxiety and depression assessed.
Subgroup analysis
Among the network of vasopressors versus combinations of vasopressors, 18 studies provided data on participants with septic shock. In 17 studies, septic shock was an eligibility criterion; in one study, data on a subgroup of participants with septic shock were available for day 28 (De Backer 2010). Overall 20 study arms included 2706 participants with 1370 mortality outcomes. The network forest plot is presented as Figure 11. In this subgroup analysis, dopamine was inferior to norepinephrine (dopamine vs norepinephrine RR 1.11, 95% CI 1.005 to 1.23).
11.
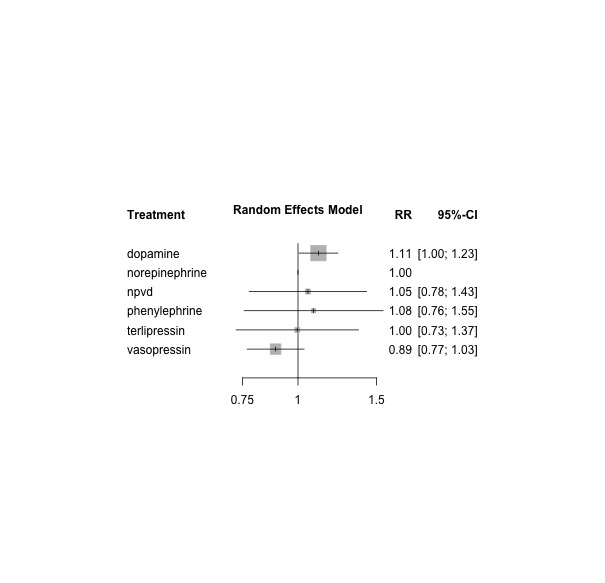
Subgroup analysis in patients with septic shock: network forest plot comparing 7 vasopressor regimens vs norepinephrine (reference) from 18 studies with 20 pair‐wise comparisons. Heterogeneity/inconsistency: tau2 < 0.0001; I2 statistic = 0%. Test of heterogeneity/inconsistency: Q = 5.21, d.f. = 14, P value = 0.98; 'NPVD' denotes non‐protocol vasoactive drugs with or without placebo. RR denotes risk ratio, as calculated by a fixed‐effect model. RR > 1 indicates increased mortality risk; RR < 1 indicates reduced mortality risk vs norepinephrine (reference).
Sensitivity analysis
We classified 11 studies (1148 participants) as having low risk of bias for the primary outcome of mortality (Annane 2007; Boccara 2003; Choong 2009; De Backer 2010; Lauzier 2006; Malay 1999; Morelli 2008b; Myburgh 2008; Russell 2008; Seguin 2006; Svoboda 2012); for the remaining studies, at least some risk of bias could not be excluded because information was lacking, or because high risk of bias was indicated by the study design.
In no comparisons did within‐study risk of bias seem to affect overall estimates (Analysis 6.1; Analysis 7.1; Analysis 8.1; Analysis 9.1; Analysis 10.1).
6.1. Analysis.
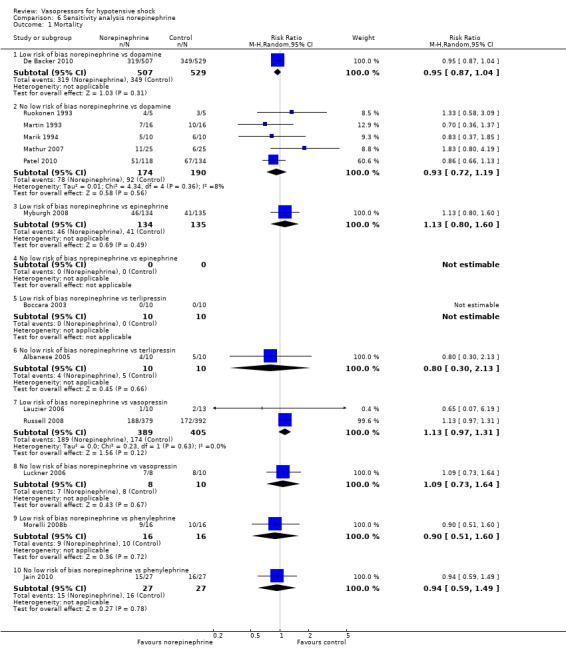
Comparison 6 Sensitivity analysis norepinephrine, Outcome 1 Mortality.
7.1. Analysis.
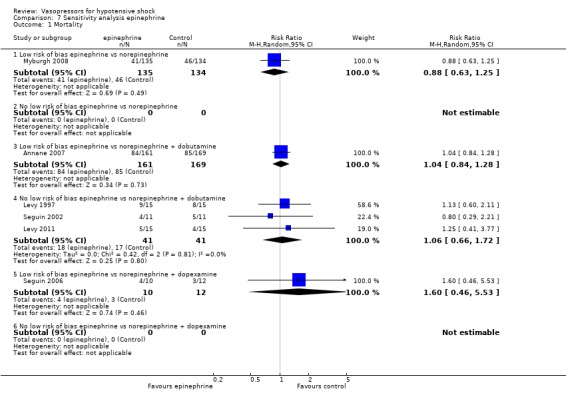
Comparison 7 Sensitivity analysis epinephrine, Outcome 1 Mortality.
8.1. Analysis.
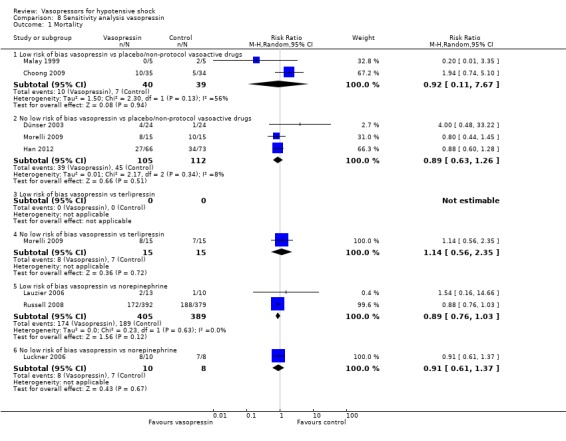
Comparison 8 Sensitivity analysis vasopressin, Outcome 1 Mortality.
9.1. Analysis.
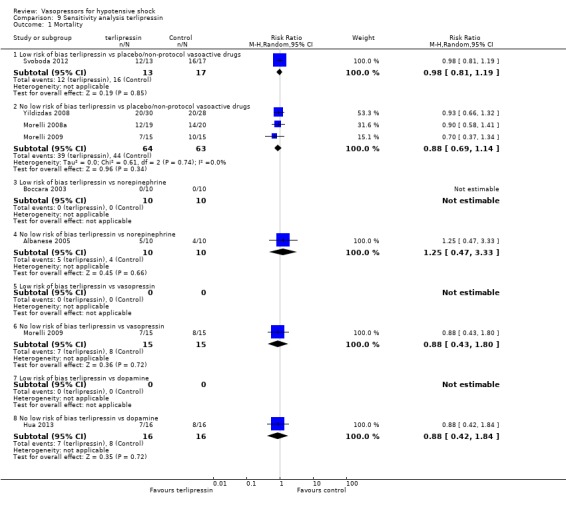
Comparison 9 Sensitivity analysis terlipressin, Outcome 1 Mortality.
10.1. Analysis.
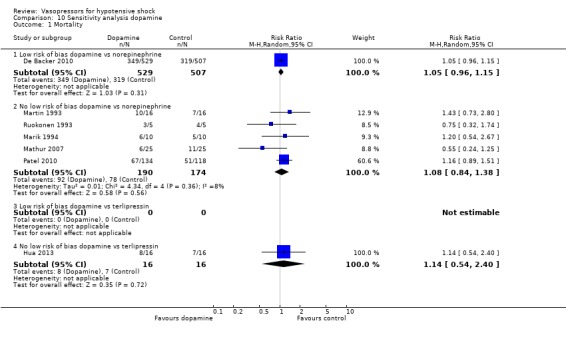
Comparison 10 Sensitivity analysis dopamine, Outcome 1 Mortality.
In all comparisons, heterogeneous mortality outcomes were included (Analysis 1.1; Analysis 2.1; Analysis 3.1; Analysis 4.1; Analysis 5.1).
For the comparison of norepinephrine versus dopamine (six studies; 1400 participants) (Analysis 1.1), effects of using the latest mortality outcome yielded an RR of 0.93 (95% CI 0.86 to 1.01) as compared with an RR of 0.92 (95% CI 0.85 to 1.01; analysis not shown) if 28‐day mortality, hospital mortality and undetermined periods were acknowledged.
For the comparison of epinephrine versus norepinephrine + dobutamine (four studies; 412 participants) (Analysis 2.1), effects of using the latest mortality outcome included an RR of 1.04 (95% CI 0.86 to 1.26) as compared with an RR of 1.19 (95% CI 0.92 to 1.54; analysis not shown) if 28‐day mortality and undetermined periods were acknowledged.
For the comparison of vasopressin versus non‐protocol vasoactive drugs (five studies; 296 participants) (Analysis 3.1), effects of using the latest mortality outcome included an RR of 0.92 (95% CI 0.69 to 1.22) as compared with an RR of 0.90 (95% CI 0.06 to 12.64; analysis not shown) if restricted to studies reporting 24‐hour mortality (Dünser 2003; Malay 1999), and an RR of 1.05 (95% CI 0.63 to 1.75; analysis not shown) if restricted to studies reporting 30‐day or ICU mortality (Choong 2009; Dünser 2003; Morelli 2009).
For the comparison of norepinephrine versus vasopressin (three studies; 812 participants) (Analysis 1.1), effects of using the latest mortality outcome included an RR of 1.12 (95% CI 0.98 to 1.29) as compared with an RR of 1.10 (95% CI 0.94 to 1.30; analysis not shown) if 28‐day mortality and ICU mortality were acknowledged. In the comparison of terlipressin and non‐protocol vasoactive drugs, ICU mortality and 90‐day mortality were combined. Choosing time points other than 90 days had no significant impact on the estimates (Analysis 4.1).
In summary, estimates remained virtually unchanged if definitions of mortality were changed, or if studies with different mortality definitions were compared.
Assessment of reporting bias
Funnel plots of the primary outcome of all comparisons did not suggest major asymmetry. We present the funnel plot for comparison 1.1 (see Figure 5). We identified too few studies per comparison to sensibly perform a formal test for funnel plot asymmetry. Overall, however, reporting bias does not seem to be a major problem in this review and in particular does not explain the results.
Discussion
Summary of main results
We found 28 studies that fulfilled our inclusion criteria. Overall 3497 participants with 1773 mortality outcomes were analysed. Information was derived mainly from six studies (Annane 2007; De Backer 2010; Han 2012; Myburgh 2008; Patel 2010; Russell 2008) that reported on 2797 participants (80% of total) and 1463 mortality outcomes (83% of total mortality outcomes) across all comparisons. Six different vasopressors, given alone or in combination with dobutamine or dopexamine, were compared in 12 different combinations.
All 28 studies reported mortality outcomes. Length of stay (LOS) was reported in 12 studies (Annane 2007; Boccara 2003; Choong 2009; De Backer 2010; Han 2012; Hua 2013; Morelli 2008a; Morelli 2008b; Morelli 2009; Patel 2010; Russell 2008; Yildizdas 2008). Other morbidity outcomes were reported in a variable and heterogeneous way. No data were available on quality of life nor on anxiety and depression outcomes.
In summary, investigators reported no differences in mortality outcomes in any of the studies comparing different vasopressors or combinations. In particular, for the comparison between dopamine and norepinephrine, which included most participants, researchers observed no differences in mortality (Table 1). The same results were obtained by our network meta‐analysis (Figure 9; Figure 10).
De Backer 2010 and Patel 2010 when comparing dopamine versus norepinephrine found higher risk of arrhythmia in the dopamine group (Analysis 1.4). In total, 347 arrhythmia episodes were documented in 1891 participants (Table 1). Other adverse events such as new infectious episodes, skin ischaemias and arterial occlusion did not differ between intervention groups.
We found no differences in other relevant morbidity outcomes within any comparisons. This finding was consistent among the few large studies identified, as well as in studies with different levels of within‐study bias risk. Overall the quality of the evidence ranged from high to very low across comparisons.
Our review included no pre‐defined subgroup analyses; therefore, we cannot make strong inferences about whether effects of vasopressors differ across populations with different causes of shock. However, in one of the large trials comparing norepinephrine versus dopamine (De Backer 2010), a pre‐defined subgroup analysis according to shock type revealed a beneficial effect on 28‐day mortality among participants with cardiogenic shock treated with norepinephrine. However, although subgroups were pre‐defined, randomization was not stratified; moreover the test for subgroup differences (P value = 0.87) suggests that this subgroup effect can be explained by chance alone.
No evidence suggests that any of the investigated vasopressors are clearly superior over others.
Nine studies can be regarded as placebo/non‐protocol vasoactive drug controlled add‐on studies. Morelli 2008a and Morelli 2009 compared norepinephrine versus norepinephrine + terlipressin and dobutamine, which might be seen as add‐on therapy of terlipressin + dobutamine versus no extra vasopressor in participants receiving norepinephrine. Investigators reported no differences in mortality or in LOS. Likewise Morelli 2009 compared a vasopressin + norepinephrine arm versus a norepinephrine alone arm. This add‐on vasopressin therapy did not have an effect. Yildizdas 2008 compared terlipressin versus placebo in paediatric patients with septic shock who did not respond to fluid resuscitation and high‐dose catecholamines, and found no differences in mortality but significant reduction of LOS. This effect was no longer found when data were combined with those from Morelli 2008a. Malay 1999 and Han 2012 studied vasopressin versus non‐protocol vasoactive drugs in participants with septic shock who were already taking catecholamines; Dünser 2003 and Svoboda 2012 compared norepinephrine versus norepinephrine + vasopressin; Choong 2009 compared vasopressin versus placebo in paediatric patients with vasodilatory shock after volume resuscitation under catecholamines. In none of these comparisons could a significant effect on mortality or morbidity be found. This result must not be interpreted as showing no effects of vasopressors versus placebo at all. Moreover, these results indicate that in participants requiring massive vasoactive support, additional vasopressors have no major effect. It is noteworthy that this evidence on placebo/non‐protocol vasoactive drug comparisons comes from a few small studies only and therefore must be interpreted with additional caution.
Overall completeness and applicability of evidence
Even though 28 studies met our inclusion criteria, numerous comparisons were necessary. Accordingly, the actual number of studies per comparison, as well as the number of participants in most studies, was small. Therefore, some comparisons resulted in under‐powered effects. Also only limited subgroup analyses could be performed to investigate potential sources of heterogeneity.
Quality of the evidence
Overall quality of the evidence ranged from high to very low. Only four studies (Annane 2007; Choong 2009; De Backer 2010; Russell 2008) fulfilled all criteria for the risk of bias assessment (Figure 3). However, when only bias items that were assumed to strongly influence the effects were considered, 11 studies were classified as having low risk of bias. Small‐study bias usually tends to overestimate a true effect, but on the other hand, in the case of a null effect, limited power to exclude the absence of an effect may matter more. Many comparisons must be interpreted with caution. In summary, however, within‐study bias does not seem to explain our findings.
Studies were too few for review authors to examine reporting bias in detail. However, as no obvious asymmetry in funnel plots was considered, and given that the comprehensive search strategy used several electronic databases without restrictions, as well as trial registers and experts in the field, reporting bias may not be a major source of distortion.
For the network meta‐analysis, we considered at least two possible clinical elements of concern, including type of shock (septic shock vs other shock) and variability of the set point (mean arterial pressure) for titration of vasopressors. Allowing placebo to be real placebo or an active drug in a network meta‐analysis adds another source of inconsistency, although we considered all such trials as add‐on vasopressor trials. We acknowledge the clinical heterogeneity included in our analyses, but we assume that heterogeneity comes mainly from within the single studies, and that the extra heterogeneity introduced by the network meta‐analysis is less important than the between‐single study heterogeneity.
Potential biases in the review process
Data show no relevant departures from protocol (Müllner 2002) as potential sources of bias and no relevant decisions about analyses. The decision to include a network meta‐analysis was made at the time of publication of the last update (Havel 2011 ).
Agreements and disagreements with other studies or reviews
Several cohort studies have presented different conclusions about the effects of different vasopressors.
In a university hospital‐based cohort study, Martin 2000 studied 97 participants with septic shock. Participants were treated with a mix of catecholamines, mainly to compare high‐dose dopamine versus norepinephrine in a non‐randomized design. Norepinephrine in comparison with other vasopressors was significantly associated with better outcomes. This effect was adjusted for many potential confounders, but treatment allocation may have been poorly controlled.
In a multi‐centre cohort study in 198 European ICUs (Sakr 2006) (occurrence of sepsis in acutely ill patients (SOAP study)), the effects of norepinephrine, dopamine, dobutamine and epinephrine were assessed in 1058 participants with shock. Epinephrine and in particular dopamine were found to worsen outcomes. In a smaller subset of participants with septic shock, epinephrine was associated with poor outcomes and dopamine showed a trend towards poor outcomes. These data were derived from a very heterogeneous sample and, despite extensive multi‐variable adjustments, residual confounding may explain the effects.
Povoa 2009 reported a multi‐centre cohort study from 17 Portuguese ICUs, in which 897 participants with community‐acquired sepsis were studied. In this population, norepinephrine and dobutamine were associated with worse outcomes, whereas dopamine was a predictor for better outcomes. In particular, when participants who received dopamine only were compared with participants who received norepinephrine only, the latter showed significantly worse outcomes. This effect was adjusted for age, sex, admission diagnosis, SAPS II, sequential organ failure assessment score (SOFA score) and inotropic support, but residual confounding cannot reasonably be excluded. Specifically, concern was expressed that the choice of vasopressors was driven by disease severity ‐ simply that sicker participants were more likely to receive norepinephrine than dopamine.
In contrast to the observational evidence, recent reviews (Beale 2004; Holmes 2009; Leone 2008) have been conservative in stating differences between several vasopressors, and have indicated that norepinephrine and dopamine are most often considered to be the vasopressors of choice for participants with shock.
In the meta‐analysis comparing norepinephrine versus dopamine in the setting of septic shock, DeBacker reported significantly increased mortality among participants undergoing therapy with dopamine. This finding is true when only 28‐day mortality is considered, but the effect is attenuated when the latest reported mortality is taken into account (DeBacker 2012). Recently, two additional concordant systematic reviews on vasopressors in septic shock were published; both included mixed treatment comparisons (Avni 2015; Zhou 2015).
Authors' conclusions
Implications for practice.
Vasopressor therapy is an important part of haemodynamic support for patients with shock (Dellinger 2013). Several different vasopressors are available, and for six vasopressors, the effect was assessed in randomized controlled trials. The quality of evidence differs greatly between several comparisons, but, in summary, evidence is insufficient to prove that any of the vasopressors at assessed doses are superior over others in terms of mortality. Dopamine increases the risk for arrhythmia and might confer a mortality disadvantage versus norepinephrine. Most available data involve norepinephrine. The choice of the specific vasopressor may therefore be individualized and left to the discretion of the treating physician. Factors such as experience, physiological effects (e.g. heart rate, intrinsic inotropic effects, splanchnic perfusion), drug interaction with other therapeutics (especially vasopressin and concomitant use of corticosteroids) (Russell 2009), availability and cost should be considered.
Implications for research.
A large number of randomized trials have been conducted, but the sample population for specific comparisons is small. We hope that our review encourages the scientific community to design future studies in a way that outcomes that matter to patients, such as survival, but also long‐term health‐related quality of life, can be evaluated. Such studies ideally would be large, multi‐centre trials based on simple and pragmatic study protocols. Such studies are also needed to evaluate whether surrogate endpoints, such as filling pressures, are of clinical use and, if so, how they should be used. In this context, special concern arises about use of target blood pressure as an endpoint of resuscitation, as achievement of specific blood pressure values may not reflect reversal of the underlying condition, as is shown in the group of participants with persisting "cryptogenic" shock despite normalization of blood pressure (Rivers 2001). A single universally valid blood pressure goal appropriate for all patients with shock probably does not exist at all (Asfar 2014). Furthermore, it is known that blood pressure targets often are not reached in controlled clinical trials (Takala 2010). Moreover, increasing evidence does not support superiority of strict goal‐directed therapy protocols (ARISE 2014; ProCESS 2014).
In the light of currently available evidence, additional well‐designed studies with individualized goals of resuscitation including clinical parameters of end‐organ perfusion and appropriate patient‐relevant outcomes endpoints are urgently needed.
As with all Cochrane reviews, this review will be updated regularly. It is hoped that answers to the questions under study will be found over the next few years.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 5 February 2016 | New citation required and conclusions have changed | We reran the search to June 2015. We included five new RCTs in this updated review (Han 2012; Hua 2013; Jain 2010; Levy 2011; Svoboda 2012). We updated all existing analyses, tables and figures for the newly included studies and for the new features of RevMan 5.3. Shortly before submission of the current update, we identified other studies that might be suitable for inclusion. These studies are listed under Studies awaiting classification |
| 5 February 2016 | New search has been performed | We included a network meta‐analysis A new author, Nathan Pace, joined the review team. Gunnar Gamper took the lead for this update |
| 5 April 2011 | New search has been performed | In this updated systematic review we reorganized all analyses and present new analyses for all comparisons and sensitivity analyses, 'Risk of bias' tables, and a 'Summary of findings' table. |
| 5 April 2011 | New citation required and conclusions have changed | This review is an update of the previous Cochrane systematic review (Müllner 2004) that included eight RCTs and excluded nine studies. In the previous version we searched the databases until November 2003. In this updated version we reran the searches to March 2010. This updated version contains 15 new RCTs (Albanese 2005: Annane 2007; Choong 2009; De Backer 2010; Lauzier 2006; Luckner 2006; Mathur 2007; Morelli 2008a; Morelli 2008b; Morelli 2009; Myburgh 2008; Patel 2010; Russell 2008; Seguin 2006; Yildizdas 2008;) and two new excluded studies (Sperry 2008; Schmoelz 2006) and three new ongoing studies (Cohn 2007a; Fernandez 2006; Lienhart 2007). These new studies changed the conclusion of our review, in particular for the comparison of norepinephrine versus dopamine. Change in authors: Bernhard Urbanek (co‐author Müllner 2004) has left the review team. A new author (Jasmin Arrich) has joined the review team of this updated version. |
| 7 August 2008 | Amended | Minor edit to text |
| 1 August 2008 | Amended | Converted to new review format. |
Notes
In keeping with the decision made after the last update (Havel 2011), we now include a network meta‐analysis to simultaneously model effects from direct and indirect comparisons, given the 10 treatment nodes currently available.
Acknowledgements
For the update review, we would like to thank Andrea Morelli for providing information on two potentially eligible studies, and we'd like to extend special thanks to Dr Jinglan Mu for providing us with a translation of a newly included paper. We would like to thank Anna Lee (Content Editor), Cathal Walsh (Statistical Editor), Djillali Annane and Anthony Gordon (Peer Reviewers) and Roy Buffery (Consumer Referee) for help and editorial advice provided during preparation of this systematic review, and of course, Jane Cracknell (Managing Editor) for help and editorial advice provided during preparation of the review at all stages.
We would like to thank people who contributed to earlier versions of the review, namely, Jane Ballantyne, Anna Lee, Nathan Pace, Mike Grocott, Lance Richard, Ann Møller, Karen Hovhannisyan, Janet Wale, Nete Villebro and Kathie Godfrey. We are also grateful to experts in the field for sharing their knowledge with us: Daniel DeBacker, Djillali Annane, Claude Martin and Jean Louis Vincent. We'd like to extend particular thanks to Djillali Annane for providing a list of potentially relevant articles on vasopressors and inotropic drugs for septic shock.
We would also like to acknowledge Bernhard Urbanek, an author of the original version of this review (Müllner 2004), who did not participate as a review author for updated reviews.
Appendices
Appendix 1. Search filter for the Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor Shock explode all trees #2 MeSH descriptor Systemic Inflammatory Response Syndrome explode all trees #3 MeSH descriptor Shock, Cardiogenic explode all trees #4 MeSH descriptor Shock, Hemorrhagic explode all trees #5 MeSH descriptor Shock, Septic explode all trees #6 MeSH descriptor Shock, Surgical explode all trees #7 MeSH descriptor Shock, Traumatic explode all trees #8 MeSH descriptor Hypotension explode all trees #9 MeSH descriptor Intensive Care, Neonatal explode all trees #10 MeSH descriptor Intensive Care explode all trees #11 circulatory near failure #12 shock or Sepsis Syndrome or Cardiogenic Shock or Hemorrhagic Shock or Haemorrhagic Shock or Septic Shock or Surgical Shock or Traumatic Shock or Anaphylactic Shock or Allergic Shock or Burn Shock #13 hypotension and ((critical near care) or (intensive near care)) #14 neonatal near intensive near care #15 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16 MeSH descriptor Vasoconstrictor Agents explode all trees #17 MeSH descriptor Epinephrine explode all trees #18 MeSH descriptor Norepinephrine explode all trees #19 MeSH descriptor Catecholamines explode all trees #20 MeSH descriptor Orciprenaline explode all trees #21 MeSH descriptor Dobutamine explode all trees #22 MeSH descriptor Dopamine explode all trees #23 MeSH descriptor Vasopressins explode all trees #24 MeSH descriptor Arginine Vasopressin explode all trees #25 MeSH descriptor Deamino Arginine Vasopressin explode all trees #26 MeSH descriptor Lysine Vasopressin explode all trees #27 MeSH descriptor Felypressin explode all trees #28 MeSH descriptor Ornipressin explode all trees #29 Vasoconstrictor near Agents #30 Epinephrine or Norepinephrine or Catecholamines or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin #31 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #32 (#15 AND #31)
Appendix 2. Search terms for MEDLINE (Ovid)
1. exp Vasoconstrictor Agents/ or exp Epinephrine/ or exp Norepinephrine/ or exp Catecholamines/ or exp Orciprenaline/ or exp dobutamine/ or exp Vasopressins/ or exp Argipressin/ or exp Deamino Arginine Vasopressin/ or exp Lypressin/ or exp Felypressin/ or exp Ornipressin/ 2. (Epinephrine or Norepinephrine or Catecholamines or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin or (Vasoconstrictor* adj3 Agent*)).mp. 3. 2 or 1 4. exp Shock, Cardiogenic/ or exp Shock, Hemorrhagic/ or exp shock/ or exp Sepsis Syndrome/ or exp Shock, Septic/ or exp Shock, Surgical/ or exp Shock, Traumatic/ or exp hypotension/ or exp Intensive Care, Neonatal/ or exp Intensive Care/ 5. (shock or Sepsis Syndrome or Cardiogenic Shock or Hemorrhagic Shock or Haemorrhagic Shock or Septic Shock or Surgical Shock or Traumatic Shock or Anaphylactic Shock or Allergic Shock or Burn Shock).mp. 6. ((circulatory adj6 failure) or ((hypotension or neonatal) and (care adj5 (critical or intensive)))).mp. 7. 4 or 5 or 6 8. 3 and 7 9. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) and humans.sh. 10. 8 and 9
Appendix 3. Search filter for EMBASE (Ovid SP)
1. Vasoconstrictor Agent/ or Adrenalin/ or Noradrenalin/ or Catecholamine/ or Orciprenaline/ or Dobutamine/ or Vasopressin Derivative/ or Argipressin/ or "Argipressin[1 Deamino]"/ or Lypressin/ or Felypressin/ or Ornipressin/ 2. (Epinephrine or Norepinephrine or Catecholamines or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin or (Vasoconstrictor* adj3 Agent*)).ti,ab. 3. 1 or 2 4. Cardiogenic Shock/ or Hemorrhagic Shock/ or Septic Shock/ or Shock/ or Sepsis/ or Traumatic Shock/ or Hypotension/ or Newborn Intensive Care/ or Intensive Care/ (141799) 5. (shock or Sepsis Syndrome or Cardiogenic Shock or Hemorrhagic Shock or Haemorrhagic Shock or Septic Shock or Surgical Shock or Traumatic Shock or Anaphylactic Shock or Allergic Shock or Burn Shock or ((circulatory adj6 failure) or ((hypotension or neonatal) and (care adj5 (critical or intensive))))).ti,ab. 6. 4 or 5 7. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) and human*.ec,hw,fs. 8. 6 and 3 and 7
Appendix 4. Search filter for CINAHL (EBSCO Host)
S1. TX circulatory failure S2. MW (shock or Sepsis Syndrome or Carcinogenic Shock or Hemorrhagic Shock or Hemorrhagic Shock or Septic Shock or Surgical Shock Traumatic Shock or Anaphylactic Shock or Allergic Shock or Burn Shock) S3. TX (hypotension and ((critical care) or (intensive care) or (neonatal intensive care))) S4. S3 or S2 or S1 S5. TX Vasopressor or Vasoconstrictor or Epinephrine or Norepinephrine or Catecholamines or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin S6. S5 and S4 S7. TX (PLACEBO* or random* or trial* or control* or compar* or blind*) S8. S6 and S7
Appendix 5. Search filter for BIOSIS Previews and ISI Web of Science
#1 TS=(circulatory failure or shock or Sepsis Syndrome or ((Cardiogenic or Hemorrhagic or Haemorrhagic or Septic or Surgical or Traumatic or Anaphylactic or Allergic or Burn) SAME Shock) or (hypotension SAME (critical care or intensive care or neonatal intensive care))) #2 TS=(Vasopressor* or Vasoconstrictor* or Epinephrine or Norepinephrine or Catecholamine* or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin) #3 TS=(PLACEBO* or random* or ((clinical or controlled) same trial*)) #4 #1 and #2 and #3
Appendix 6. Search filter for PsycINFO (Ovid SP)
1. (circulatory failure or shock or Sepsis Syndrome or Cardiogenic Shock or Hemorrhagic Shock or Haemorrhagic Shock or Septic Shock or Surgical Shock or Traumatic Shock or Anaphylactic Shock or Allergic Shock or Burn Shock or (hypotension and (critical care or intensive care or neonatal intensive care))).af. 2. (Vasopressor or Vasoconstrictor or Epinephrine or Norepinephrine or Catecholamines or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin).ti,ab. 3. (PLACEBO* or random* or trial*).af. 4. 1 and 2 and 3
Appendix 7. Search filter for Pascal BioMed
S1. TX circulatory failure S2. MW (shock or Sepsis Syndrome or Carcinogenic Shock or Hemorrhagic Shock or Hemorrhagic Shock or Septic Shock or Surgical Shock Traumatic Shock or Anaphylactic Shock or Allergic Shock or Burn Shock) S3. TX (hypotension and ((critical care) or (intensive care) or (neonatal intensive care))) S4. S3 or S2 or S1 S5. TX Vasopressor or Vasoconstrictor or Epinephrine or Norepinephrine or Catecholamines or Orciprenaline or dobutamine or dopamine or adrenaline or noradrenaline or Vasopressins or Argipressin or Desmopressin or Lypressin or Felypressin or Ornipressin or Terlipressin or Glypressin S6. S5 and S4 S7. TX (PLACEBO* or random* or trial* or control* or compar* or blind*) S8. S6 and S7
Data and analyses
Comparison 1. Norepinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Norepinephrine vs dopamine | 6 | 1400 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 1.2 Norepinephrine vs epinephrine | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.60] |
| 1.3 Norepinephrine vs terlipressin | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.30, 2.13] |
| 1.4 Norepinephrine vs vasopressin | 3 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.98, 1.29] |
| 1.5 Norepinephrine vs phenylephrine | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.64, 1.32] |
| 2 LOS ICU | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Norepinephrine vs vasopressin | 1 | 778 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.40, 3.40] |
| 2.2 Norepinephrine vs norepinephrine + terlipressin + dobutamine | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐7.20, 5.20] |
| 2.3 Norepinephrine vs phenylephrine | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐8.27, 8.27] |
| 2.4 Norepinephrine vs dopamine | 2 | 1931 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.57, 0.75] |
| 3 LOS hospital | 2 | 1931 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.96, 2.29] |
| 4 Arrhythmia | 2 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.69] |
Comparison 2. Epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Epinephrine vs norepinephrine | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.25] |
| 1.2 Epinephrine vs norepinephrine + dobutamine | 4 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.26] |
| 1.3 Epinephrine vs norepinephrine + dopexamine | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [0.46, 5.53] |
Comparison 3. Vasopressin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Vasopressin vs placebo/non‐protocol vasoactive drugs | 5 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.22] |
| 1.2 Vasopressin vs terlipressin | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.35] |
| 1.3 Vasopressin vs norepinephrine | 3 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
| 2 LOS ICU | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Vasopressin vs terlipressin | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Vasopressin vs norepinephrine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Vasopressin vs placebo/non‐protocol vasoactive drugs | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Terlipressin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Terlipressin vs placebo/non‐protocol vasoactive drugs | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.10] |
| 1.2 Terlipressin vs norepinephrine | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.47, 3.33] |
| 1.3 Terlipressin vs vasopressin | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.43, 1.80] |
| 1.4 Terlipressin vs dopamine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.84] |
| 2 LOS ICU | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Terlipressin vs placebo/non‐protocol vasoactive drugs | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐8.61, 2.81] |
| 2.2 Terlipressin vs vasopressin | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐13.21, 7.21] |
| 2.3 Terlipressin vs dopamine | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.40, 1.60] |
| 3 LOS hospital | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Terlipressin vs noradrenaline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Terlipressin vs dopamine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Duration mechanical ventilation | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Terlipressin vs placebo/non‐protocol vasoactive drug | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Terlipressin vs dopamine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pressor‐free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Terlipressin vs dopamine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Terlipressin vs placebo/non‐protocol vasoactive drug | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Dopamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 7 | 1432 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.99, 1.17] |
| 1.1 Dopamine vs norepinephrine | 6 | 1400 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.99, 1.16] |
| 1.2 Dopamine vs terlipressin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.40] |
| 2 LOS ICU | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Dopamine vs norepinephrine | 2 | 1931 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.75, 0.57] |
| 2.2 Dopamine vs terlipressin | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.40, 1.60] |
| 3 LOS hospital | 3 | 1963 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.32, 0.79] |
| 3.1 Dopamine vs norepinephrine | 2 | 1931 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.29, 0.96] |
| 3.2 Dopamine vs terlipressin | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐7.01, 3.41] |
| 4 Pressor‐free days | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Dopamine vs norepinephrine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Dopamine vs terlipressin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Arrhythmia | 2 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [1.46, 3.78] |
Comparison 6. Sensitivity analysis norepinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias norepinephrine vs dopamine | 1 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 1.2 No low risk of bias norepinephrine vs dopamine | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.19] |
| 1.3 Low risk of bias norepinephrine vs epinephrine | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.60] |
| 1.4 No low risk of bias norepinephrine vs epinephrine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Low risk of bias norepinephrine vs terlipressin | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 No low risk of bias norepinephrine vs terlipressin | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.30, 2.13] |
| 1.7 Low risk of bias norepinephrine vs vasopressin | 2 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.97, 1.31] |
| 1.8 No low risk of bias norepinephrine vs vasopressin | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.73, 1.64] |
| 1.9 Low risk of bias norepinephrine vs phenylephrine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.51, 1.60] |
| 1.10 No low risk of bias norepinephrine vs phenylephrine | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.59, 1.49] |
Comparison 7. Sensitivity analysis epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias epinephrine vs norepinephrine | 1 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.25] |
| 1.2 No low risk of bias epinephrine vs norepinephrine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Low risk of bias epinephrine vs norepinephrine + dobutamine | 1 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.28] |
| 1.4 No low risk of bias epinephrine vs norepinephrine + dobutamine | 3 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.66, 1.72] |
| 1.5 Low risk of bias epinephrine vs norepinephrine + dopexamine | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [0.46, 5.53] |
| 1.6 No low risk of bias epinephrine vs norepinephrine + dopexamine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Sensitivity analysis vasopressin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias vasopressin vs placebo/non‐protocol vasoactive drugs | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.11, 7.67] |
| 1.2 No low risk of bias vasopressin vs placebo/non‐protocol vasoactive drugs | 3 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.26] |
| 1.3 Low risk of bias vasopressin vs terlipressin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 No low risk of bias vasopressin vs terlipressin | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.35] |
| 1.5 Low risk of bias vasopressin vs norepinephrine | 2 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.03] |
| 1.6 No low risk of bias vasopressin vs norepinephrine | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.61, 1.37] |
Comparison 9. Sensitivity analysis terlipressin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias terlipressin vs placebo/non‐protocol vasoactive drugs | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.19] |
| 1.2 No low risk of bias terlipressin vs placebo/non‐protocol vasoactive drugs | 3 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.14] |
| 1.3 Low risk of bias terlipressin vs norepinephrine | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 No low risk of bias terlipressin vs norepinephrine | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.47, 3.33] |
| 1.5 Low risk of bias terlipressin vs vasopressin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 No low risk of bias terlipressin vs vasopressin | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.43, 1.80] |
| 1.7 Low risk of bias terlipressin vs dopamine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 No low risk of bias terlipressin vs dopamine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.84] |
Comparison 10. Sensitivity analysis dopamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias dopamine vs norepinephrine | 1 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 1.2 No low risk of bias dopamine vs norepinephrine | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.38] |
| 1.3 Low risk of bias dopamine vs terlipressin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 No low risk of bias dopamine vs terlipressin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albanese 2005.
| Methods | Single‐centre open‐label randomized controlled study at a tertiary care University hospital; France | |
| Participants | Adult participants with hyperdynamic septic shock after fluid resuscitation Mean age = 66 years, 35% female APACHE II score = 28.5 (N = 20) |
|
| Interventions | Norepinephrine started with 0.3 mcg/kg and increased by 0.3 mcg/kg every 4 minutes until MAP 65 to 75 mm Hg vs Terlipressin 1 mg bolus, followed by second bolus of 1 mg if MAP < 65 mm Hg |
|
| Outcomes | In‐hospital mortality, renal function (urine flow, creatinine clearance up to 8 hours ‐ presented on a graph only, no numbers provided), haemodynamic parameters, blood gas, lactate at 6 hours For the mortality analysis, we used data on in‐hospital mortality |
|
| Notes | No explicit primary outcome defined No funding source Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether allocation was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Appropriately reported |
| Adequate patient description | Low risk | Appropriately reported |
| Identical care | Low risk | For only 6 hours |
| Outcome description | Unclear risk | Implicitly described |
| Physicians blinded | High risk | Open‐label |
| Outcome assessors blinded? | Unclear risk | Not reported |
Annane 2007.
| Methods | Multi‐centre double‐blind randomized controlled trial in 19 ICUs (CATS study); France | |
| Participants | Adult participants with septic shock (study authors' definition) Mean age = 63 years, 39% female SAPS II score = 53, SOFA score = 11 (N = 330) |
|
| Interventions | Epinephrine infusion 0.2 mcg/kg/min (N = 161) vs Norepinephrine infusion 0.2 mcg/kg/min and dobutamine 5 mcg/kg/min (N = 169) Both adjusted according to MAP, pulmonary arterial wedge pressure, cardiac index and response to fluid challenge |
|
| Outcomes | 28‐Day mortality (primary); 7‐, 14‐, 90‐day ICU; hospital mortality; duration of vasopressor therapy; time to haemodynamic success; adverse events For the mortality analysis, we used data on 90‐day mortality |
|
| Notes | The French Ministry of Health provided financial support (1997 Clinical Research Hospital Programme PHRC 1997, AOM 97123) Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization sequence, stratified by centre, 1:1, blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequate |
| Identical care | Low risk | Reported |
| Outcome description | Low risk | Reported |
| Physicians blinded | Low risk | Adequately reported |
| Outcome assessors blinded? | Low risk | Reported |
Boccara 2003.
| Methods | Single‐centre randomized controlled trial, University hospital; France | |
| Participants | Adult participants scheduled for carotid endarterectomy and refractory peri‐operative hypotension (N = 20) | |
| Interventions | Goal‐directed (terlipressin infused in 1 mg intravenous boluses up to 3 mg vs norepinephrine (50 mcg/mL) at initial rate of 10 mL/h, incrementally by 2 mL/h) | |
| Outcomes | Death, stroke, myocardial ischaemias; renal failure; hospital stay For the mortality analysis, we used data on undetermined mortality |
|
| Notes | It is unclear when participants died (peri‐operative or in‐hospital) Conflict of interest not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Implicitly described |
| Allocation concealment (selection bias) | Low risk | Envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Unclear risk | Not reported |
| Adequate patient description | Low risk | Reported |
| Identical care | Unclear risk | Not reported |
| Outcome description | Low risk | Reported |
| Physicians blinded | Unclear risk | Not reported |
| Outcome assessors blinded? | Unclear risk | Not reported |
Choong 2009.
| Methods | Multi‐centre double‐blind randomized controlled trial, University hospital PCCUs (Paediatric Critical Care Unit); Canada | |
| Participants | Paediatric participants with vasodilatory shock (study authors' definition) Mean age = 10 years, 48% female PRISM III score = 13, MODS score = 3; PELOD score = 10.5 (N = 69) |
|
| Interventions | Vasopressin infusion 0.0005 U/kg/min, increased every 5 minutes up to 0.002 U/kg/min (max dose 0.05 U/min) to maintain target MAP for age vs Saline placebo |
|
| Outcomes | Time to vasopressor discontinuation (primary), 30‐day mortality, organ dysfunction, urine output, haemodynamics, vasopressor dose, vasopressin serum levels, vasopressor‐free days until day 30, organ failure‐free days until day 30, mechanical ventilation‐free days until day 30, PCCU LOS, adverse events For the mortality analysis, we used data on 30‐day mortality |
|
| Notes | All other vasopressors were open‐label and were used at the treating physician's discretion Supported by grants from the Canadian Institutes of Health Research (200511MCT‐154713), Heart and Stroke Foundation (NA 5937), Physician Services Incorporated (02–65), Hospital for Sick Children Foundation (XG 02– 071R), Canadian Intensive Care Foundation, Laerdal Foundation and Ferring Inc Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization sequence, 1:1, stratified by centre, permuted blocks within strata |
| Allocation concealment (selection bias) | Low risk | Central telephone‐based randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Appropriate |
| Identical care | Low risk | Appropriate |
| Outcome description | Low risk | Appropriate |
| Physicians blinded | Low risk | Identical syringes |
| Outcome assessors blinded? | Low risk | Appropriate |
De Backer 2010.
| Methods | Multi‐centre randomized controlled trial, 8 University hospitals (SOAP II study); Belgium, Austria, Spain | |
| Participants | Adult participants with shock (study authors' definition) Mean age = 68 years, 57%female APACHE II score = 20, SOFA score = 9 (N = 1679) |
|
| Interventions | Dopamine incrementally increased dose by 2 mcg/kg/min (max 20 mcg) vs Norepinephrine incrementally increased dose by 0.02 mcg/kg/min (max 0.19 mcg) |
|
| Outcomes | 28‐Day mortality (primary); 6‐month‐12‐month‐, ICU‐ and hospital mortality; ICU LOS, hospital LOS, number of days without need for organ support (vasopressors, ventilators, renal replacement therapy), time to reach MAP > 65 mm Hg, adverse events (arrhythmias, myocardial necrosis, skin necrosis, ischaemias in limbs or distal extremities, secondary infections) For the mortality analysis, we used data on 12‐month mortality (main analysis) For the subgroup analysis in septic shock patients, we used 28‐day data available in the meta‐analysis by DeBacker (DeBacker 2012) |
|
| Notes | If patients hypotensive after max dosage, open‐label norepinephrine Supported in part by the European Society of Intensive Care through support from the European Critical Care Research Network Dr Aldecoa reports receiving consulting fees from Covidien. No other potential conflict of interest relevant to this article was reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted blocks of 6 to 10, stratified to centres |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed to day 28; data on outcomes during stay in hospital were available for 1656 participants (98.6%), along with data on 6‐month outcomes for 1443 participants (85.9%) and data on 12‐month outcomes for 1036 participants (61.7%) |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Appropriate |
| Identical care | Low risk | Appropriate |
| Outcome description | Low risk | Reported |
| Physicians blinded | Low risk | Identical syringes |
| Outcome assessors blinded? | Low risk | Appropriate |
Dünser 2003.
| Methods | Single‐centre randomized controlled trial, University hospital; Austria | |
| Participants | Adult surgical and medical participants with vasodilatory shock (N = 48) | |
| Interventions | Fixed dose of vasopressin (4 U/h) + goal‐directed norepinephrine (MAP ≥ 70 mm Hg) vs goal‐directed norepinephrine (MAP ≥ 70 mm Hg) | |
| Outcomes | 24‐Hour mortality; 48‐hour mortality; ICU mortality; ICU LOS For the mortality analysis, we used data on ICU mortality |
|
| Notes | Participants were allowed to receive vasopressin in the norepinephrine group if "failed" This study was supported in part by the Lorenz Böhler Fund Conflict of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'patients were randomly assigned into an AVP group and an NE group' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up during study period |
| Explicit in‐/exclusion criteria | Low risk | Implicitly described |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Appropriate |
| Identical care | Unclear risk | Not reported |
| Outcome description | Low risk | Implicitly described |
| Physicians blinded | Unclear risk | Not reported |
| Outcome assessors blinded? | Unclear risk | Not reported |
Han 2012.
| Methods | Multi‐centre RCT in community hospitals; China | |
| Participants | Patients with septic shock who were older than 16 years with dopamine requirements exceeding 5 µg/kg/min; 29% female, mean 72 years old, average APACHE I score 27.4, SOFA score 9.3 (N = 139) | |
| Interventions | Pituitrin (vasopressin 0.017 to 0.042 U/min) vs standard vasopressors (dopamine or norepinephrine 2 to 20 µg/kg/min) norepinephrine added to both groups to keep haemodynamics stable |
|
| Outcomes | 28‐Day survival, LOS hospital, duration mechanical ventilation; MAP, heart rate, serum creatinine, lactate, norepinephrine dose, heparin, glucocorticoids | |
| Notes | Published in Chinese; funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Explicit in‐/exclusion criteria | Unclear risk | Only high‐level information given |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Unclear risk | Only high‐level information on aetiology given |
| Identical care | High risk | Open‐label intervention; no treatment protocol presented |
| Outcome description | Low risk | Reported |
| Physicians blinded | High risk | Not reported, but from indirect information high risk was assumed |
| Outcome assessors blinded? | Unclear risk | Low risk for mortality outcomes, high risk for other outcomes |
Hua 2013.
| Methods | Single‐centre randomized controlled trial, University hospital; China | |
| Participants | Adult participants with ARDS and septic shock. Mean age 54 years, 44% female, APACHE II score 18.6, lung injury score 2.9. SAPS 45.5 (N = 32) | |
| Interventions | Terlipressin (1.3 μg/kg/h) vs dopamine (up to 20 μg/kg/min) to reach MAP 70 ± 5 mm Hg | |
| Outcomes | 28‐Day mortality, LOS ICU, LOS hospital, pressor‐free days, duration of mechanical ventilation; VEGF, TNFa, haemodynamics, oxygenation | |
| Notes | Funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adaequately described |
| Allocation concealment (selection bias) | Unclear risk | No sufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Reported |
| Adequate patient description | Low risk | Adequately reported |
| Identical care | Unclear risk | No sufficient information |
| Outcome description | Low risk | Reported |
| Physicians blinded | High risk | Not reported |
| Outcome assessors blinded? | Unclear risk | No sufficient information |
Jain 2010.
| Methods | Single‐centre randomized controlled trial, University hospital; India | |
| Participants | Adult participants with septic shock. Mean age 44 years, 48% female, APACHE II score 18.4 Dopamine infusion rate 25 µg/kg/min (N = 32) |
|
| Interventions | Norepinephrine (0.5 to 3.5 µg/kg/min with increments of 0.5 µg/kg/min every 30 minutes) vs phenylephrine (0.5 to 8.5 µg/kg/min with increments of 1 µg/kg/min every 30 minutes) | |
| Outcomes | Survival (time point not reported), main outcomes were haemodynamic parameters within 6 hours | |
| Notes | Source of support: nil Conflict of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three participants in each group excluded |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | High risk | Post‐randomization exclusions in 6 participants |
| Adequate patient description | Low risk | Reported |
| Identical care | High risk | Open‐label intervention, no treatment protocol presented |
| Outcome description | High risk | Time to survival not reported, several post‐randomization exclusions |
| Physicians blinded | High risk | Not reported |
| Outcome assessors blinded? | Low risk | Outcomes were assessed by another physician blinded to the study |
Lauzier 2006.
| Methods | Multi‐centre open‐label randomized controlled trial at University hospitals; Canada, France | |
| Participants | Adult participants with septic shock (ACCP/SCCM definition ACCP/SCCM 1992) mean age = 55 years, 39% female Mean APACHE II score = 23.15, moderate SOFA score = 8.9 (N = 23) |
|
| Interventions | Arginine‐vasopression (AVP) infusion 0.04 to 0.2 U/min (N = 13), at doses > 0.2 U/min rescue therapy with norepinephrine or additional AVP allowed vs Norepinephrine 0.1 to 2.8 mcg/kg/min (N = 10), at doses > 2.8 mcg/kg/min rescue therapy with norepinephrine or additional AVP allowed, both for 48 hours to achieve MAP > 70 mm Hg |
|
| Outcomes | Haemodynamic parameters, organ dysfunction, creatinine clearance based on 2‐hour collection 24 hours after randomization, ICU mortality For the mortality analysis, we used data on ICU mortality |
|
| Notes | Dobutamine allowed if cardiac index < 3 L/min/m2 Funding: not reported Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization list, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three participants died during study period |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Unclear risk | Groups differ in steroid therapy and baseline vasopressor support |
| Outcome description | Unclear risk | Only implicitly described, no primary outcome |
| Physicians blinded | High risk | Open‐label |
| Outcome assessors blinded? | High risk | Not reported |
Levy 1997.
| Methods | Single‐centre randomized controlled trial, University hospital; France | |
| Participants | Adult surgical and medical participants with septic shock (N = 30) | |
| Interventions | Goal‐directed epinephrine vs norepinephrine + fixed dobutamine (5 mcg/kg/min) | |
| Outcomes | Survival (not further clarified); haemodynamics, tonometry For the mortality analysis, we used data on undetermined mortality |
|
| Notes | It is unclear when participants died | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'According to a randomization code' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Explicit in‐/exclusion criteria | Low risk | Explicitly described |
| ITT‐analysis | Unclear risk | Not reported |
| Adequate patient description | Low risk | Reported |
| Identical care | Unclear risk | Not reported |
| Outcome description | Low risk | Implicit |
| Physicians blinded | Unclear risk | Not reported |
| Outcome assessors blinded? | Unclear risk | Not reported |
Levy 2011.
| Methods | Single‐centre randomized controlled trial, University hospital; France | |
| Participants | Adult participants with cardiogenic shock. Mean age 65 years, 30% female, SAP II score 51, SOFA score 8.5 acute or chronic heart failure with EF < 30% or CI < 2.2 L/min/m2 and systolic blood pressure: < 90 mm Hg; MAP < 60 mm Hg or drop in MAP > 30 mm Hg despite dobutamine up to 10 µg/kg/min and dopamine up to 20 µg/kg/min (N = 30) |
|
| Interventions | Epinephrine (mean dose 0.15 µg/kg/min at 24 hours for 5 days) vs norepinephrine (mean dose 0.13 µg/kg/min at 24 hours) + dobutamine (8 ± 2 µg/kg/min for 5 days) Target MAP between 65 and 70 mm Hg and stable CI |
|
| Outcomes | Mortality 28 days. Vasopressor titration, haemodynamic, metabolic, splanchnic and renal parameters at baseline, 1, 6, 12 and 24 hours | |
| Notes | Before study: Start with dobutamine up to 10 µg/kg/min, then dopamine up to 2 to 20 µg/kg/min, then start study drug and concomitantly stop dopamine. Dobutamine stopped in the epinephrine group Funding: not reported Conflits of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code |
| Allocation concealment (selection bias) | Unclear risk | Consecutive patients, …according to the randomization code |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up for study period |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Unclear risk | Open‐label intervention, no treatment protocol presented |
| Outcome description | Low risk | Clear |
| Physicians blinded | High risk | Not reported/not performed |
| Outcome assessors blinded? | Unclear risk | Low risk for mortality, unclear risk for other outcomes |
Luckner 2006.
| Methods | Single‐centre randomized controlled trial, University hospital; Austria | |
| Participants | Adult post‐operative participants (major surgery or cardiac surgery) with MAP < 65 mm Hg and norepinephrine support > 0.5 mcg/kg/min Mean age = 69 years, 39% female Modified Goris score = 12.3 (N = 18) |
|
| Interventions | Arginine‐vasopressin infusion 4 U/h and norepinephrine infusion to achieve and maintain MAP > 65 mm Hg vs Norepinephrine infusion to achieve and maintain MAP > 65 mm Hg |
|
| Outcomes | Cutaneous vascular reactivity and flow motion at 1 hour (primary), haemodynamics, metabolic variables, ICU mortality For the mortality analysis, we used data on ICU mortality |
|
| Notes | One study author has received a grant from Aguettant Laboratories, Lyon, France ‐ a company that has applied for registration of vasopressin with European authorities No personal conflict of interest Funding: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number‐generating computer programme |
| Allocation concealment (selection bias) | Unclear risk | No sufficient detail reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Explicit in‐/exclusion criteria | Unclear risk | No sufficient detail reported |
| ITT‐analysis | High risk | Analysis was performed 'as treated' |
| Adequate patient description | High risk | No sufficient detail reported |
| Identical care | Unclear risk | No sufficient detail reported, in particular on catecholamine therapy |
| Outcome description | Low risk | Reported |
| Physicians blinded | High risk | Unclear |
| Outcome assessors blinded? | Unclear risk | Not reported |
Malay 1999.
| Methods | Single‐centre randomized controlled trial, general hospital; USA | |
| Participants | Adult surgical and trauma participants with septic shock (N = 10) | |
| Interventions | Fixed dose of vasopressin (0.04 U/min) vs placebo | |
| Outcomes | 24‐Hour mortality For the mortality analysis, we used data on 24‐hour mortality |
|
| Notes | Funding: not reported Conflict of interest. not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Unclear risk | Not reported |
| Adequate patient description | Low risk | Adequately reported |
| Identical care | Unclear risk | Not reported |
| Outcome description | Low risk | Clear |
| Physicians blinded | Low risk | Reported |
| Outcome assessors blinded? | Low risk | Reported |
Marik 1994.
| Methods | Single‐centre randomized controlled trial, University hospital; USA | |
| Participants | Adult participants with septic shock; unclear whether medical or surgical (N = 20) | |
| Interventions | Goal‐directed norepinephrine (to achieve MAP > 75 mm Hg) vs dopamine ("... and in case of dopamine … to keep pulse rate < 150/min") | |
| Outcomes | Death For the mortality analysis, we used data on undetermined mortality |
|
| Notes | It is unclear when participants died Funding: not reported Confict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Low risk | Described |
| Outcome description | Low risk | Implicit |
| Physicians blinded | Unclear risk | Not reported |
| Outcome assessors blinded? | Unclear risk | Not reported |
Martin 1993.
| Methods | Single‐centre randomized controlled trial, University hospital; France | |
| Participants | Adult participants with septic shock; unclear whether medical or surgical (N = 32) | |
| Interventions | Goal‐directed dopamine (max 25 mcg/kg/min; if goal not reached, addition of norepinephrine) vs norepinephrine (max 5 mcg/kg/min; if goal not reached, addition of dopamine) | |
| Outcomes | Hospital mortality For the mortality analysis, we used data on hospital mortality. |
|
| Notes | Funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'according to the randomization code' |
| Allocation concealment (selection bias) | Unclear risk | Not reported in sufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete outcome data One participant in each group did not respond to therapy and died during the study period |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Unclear risk | Not reported |
| Adequate patient description | Low risk | Described adequately |
| Identical care | Unclear risk | Not reported |
| Outcome description | Low risk | Clear |
| Physicians blinded | Low risk | Reported |
| Outcome assessors blinded? | Unclear risk | Not reported |
Mathur 2007.
| Methods | Single‐centre randomized controlled trial, University hospital; India | |
| Participants | Adult participants with septic shock (study authors' definition) Mean age = 54 years, 36% female APACHE II score = 25 (N = 50) |
|
| Interventions | Dopamine infusion 10 mcg/kg/min, increased by 2.5 mcg/kg/min every 15 minutes (up to 25 mcg/kg/min) to achieve goal vs Norepinephrine infusion 0.5 mcg/kg/min, increased by 0.25 mcg/kg/min every 15 minutes (up to 2.5 mcg/kg/min) to achieve goal Goal (to be achieved and maintained for 6 hours): SBP > 90 mm Hg and SVRI > 1100 dynes*s/cm5*m2 and CI > 4 L/min/m2 and IDO2 > 550 mL/min/m2 and IVO2 > 150 mL/min/m2 |
|
| Outcomes | Haemodynamic parameters, haemodynamic response (goal achieved), urine output, mortality For the mortality analysis, we used data on undetermined mortality |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'were randomly allocated into two groups' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete data not reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequate |
| Identical care | Low risk | Reported |
| Outcome description | Unclear risk | Implicitly described |
| Physicians blinded | Low risk | Reported |
| Outcome assessors blinded? | Low risk | Performed |
Morelli 2008a.
| Methods | Single‐centre open‐label randomized controlled pilot study, University hospital; Italy | |
| Participants | Adult participants with septic shock (ACCP/SCCM 1992ACCP/SCCM 1992) and norepinephrine support > 0.9 mcg/kg/min Mean age = 66 years, 27% female SAPS score = 60 (N = 59) |
|
| Interventions |
vs
vs
We used comparisons 1 vs 2 and 1 vs 3, but not 2 vs 3, because this is virtually a comparison of dobutamine vs control. Dobutamine is not considered a vasopressor |
|
| Outcomes | Organ dysfunction, urine output 2 and 4 hours after study start, haemodynamics, vasopressor requirements, ICU LOS, ICU mortality For the mortality analysis, we used data on ICU mortality |
|
| Notes | This study was funded by an independent research grant from the Department of Anesthesiology and Intensive Care of the University of Rome, ‘La Sapienza’ Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based procedure reported |
| Allocation concealment (selection bias) | Unclear risk | No means to conceal the allocation reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | High risk | One participant with inappropriate noradrenaline dosing excluded from the analysis |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Unclear risk | Not reported in sufficient detail |
| Outcome description | Unclear risk | Not reported in sufficient detail |
| Physicians blinded | High risk | Not performed |
| Outcome assessors blinded? | Unclear risk | Not reported |
Morelli 2008b.
| Methods | Single‐centre double‐blind randomized controlled study, University hospital; Italy | |
| Participants | Adult participants with septic shock (Surviving Sepsis Campaign 2008 criteria; Dellinger 2008) Mean age = 70 years, 34% female Simplifies APACHE II score = 56 (N = 32) |
|
| Interventions | Norepinephrine infusion to achieve MAP 65 to 75 mm Hg vs Phenylephrine infusion to achieve MAP 65 to 75 mm Hg For both arms, explicit dosing schemes are not reported |
|
| Outcomes | Plasma disappearance rate of indocyanine green (PDR) and blood clearance of indocyanine green (CBI) (primary), haemodynamics, organ function, ICU LOS, ICU mortality Creatinine clearance and urine output presented only graphically, P values reported For the mortality analysis, we used data on ICU mortality |
|
| Notes | Noradrenaline dose at baseline was 0.8 ± 0.7 mcg/kg/min Dobutamine was added to achieve mixed venous oxygen saturation > 64% if necessary Funding: The present study was funded by an independent research grant from the Department of Anesthesiology and Intensive Care of the University of Rome 'La Sapienza' Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based procedure |
| Allocation concealment (selection bias) | Low risk | Double‐blind study drug |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completely reported, open‐label therapy after 12h |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Low risk | Reported |
| Outcome description | Unclear risk | Only primary outcome described in the Methods |
| Physicians blinded | Low risk | Performed |
| Outcome assessors blinded? | Low risk | Performed |
Morelli 2009.
| Methods | Single‐centre randomized controlled trial, University hospital; Italy | |
| Participants | Adult participants with septic shock (Surviving Sepsis Campaign criteria 2008; Dellinger 2008) Mean age 66 years, 27% female SAPS II score = 60 (N = 45) |
|
| Interventions | Terlipressin infusion 1.3 mcg/kg/h vs Arginine‐vasopressin infusion 0.03 U/min vs Norepinephrine infusion 15 mcg/min For all groups, open‐label norepinephrine was added to achieve MAP 65 to 75 mm Hg |
|
| Outcomes | Norepinephrine requirements (primary), haemodynamics, metabolic parameters, blood gas, cytokine levels, ICU mortality, ICU LOS, adverse events, renal replacement therapy, creatinine clearance and urine output at 48 hours (data presented in a graph only, with intragroup comparisons) For the mortality analysis, we used data on ICU mortality |
|
| Notes | Funding: This study was funded by an independent research grant from the Department of Anesthesiology and Intensive Care of the University of Rome 'La Sapienza' Conflict of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based procedure |
| Allocation concealment (selection bias) | Unclear risk | No concealment procedure reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Described adequately |
| Identical care | Low risk | Described |
| Outcome description | Low risk | Clear |
| Physicians blinded | Unclear risk | Not explicitly reported |
| Outcome assessors blinded? | Unclear risk | Not explicitly reported |
Myburgh 2008.
| Methods | Multi‐centre double‐blind randomized controlled trial, 4 multi‐disciplinary university hospital ICUs; Australia | |
| Participants | Adult ICU participants requiring vasopressors for any reason Mean age = 60 years, 39% female APACHE II score = 22 (N = 280) |
|
| Interventions | Switch from the vasopressor at inclusion to
or
To achieve MAP > 70 mm Hg (or individualized by treating physicians), no restriction on other vasopressors except study drugs |
|
| Outcomes | Time to achieve MAP goal, drug‐free days from randomization (primary); mortality at days 28, 90 For the mortality analysis, we used data on 90‐day mortality |
|
| Notes | Subgroup analysis: septic shock, circulatory failure 22 patients withdrawn by treating physicians for adverse events Funding for statistical analysis of this study from the Australian and New Zealand College of Anaesthetists (Project grant: 06/024). Financial contribution from participating institutions that provided substantial support from internal funds Conflict of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator (StatMate, GraphPad), stratified by centre in invariable blocks |
| Allocation concealment (selection bias) | Low risk | Independently prepared double‐blind study drugs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported explicitly |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Described |
| Identical care | Unclear risk | Sufficient detail not described |
| Outcome description | Low risk | Clear |
| Physicians blinded | Low risk | Reported |
| Outcome assessors blinded? | Low risk | Reported |
Patel 2010.
| Methods | Single‐centre prospective quasi‐randomized open‐label clinical trial in a medical intensive care unit, University hospital; USA | |
| Participants | Adult fluid‐resuscitated participants with septic shock Age not stated, 54% female APACHE II score = 28 SOFA score = 12, (N = 252) |
|
| Interventions | Dopamine 5 to 20 mcg/kg/min to pre‐determined max of 20 mcg/kg/min vs Norepinephrine 5 to 20 mcg/min, to pre‐determined max of 20 mcg/kg/min as the initial vasopressor If haemodynamic goal was not achieved (MAP > 60 mm Hg and/or SBP > 90 mm Hg): add (1) vasopressin 0.04 U/min and (2) phenylephrine (25 to 200 mcg/min) If ScvO2 < 70%: add dobutamine |
|
| Outcomes | Primary: all‐cause 28 day mortality. Secondary: organ dysfunction, hospital and ICU LOS and safety (primarily occurrence of arrhythmias) For the mortality analysis, we used data on 28‐day mortality |
|
| Notes | SOFA score as secondary outcome not explicitly presented Funding: The dopamine vs norepinephrine trial was not funded Conflict of interest: None of the authors has any financial involvement nor commercial association that might pose a real or perceived conflict of interest in connection with this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Even and odd calender day enrolment |
| Allocation concealment (selection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completely reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Reported |
| Adequate patient description | Low risk | But age not stated |
| Identical care | Low risk | Reported |
| Outcome description | Low risk | Clear |
| Physicians blinded | High risk | Not blinded |
| Outcome assessors blinded? | Unclear risk | But not explicitly reported |
Ruokonen 1993.
| Methods | Single‐centre randomised controlled trial, University hospital; Finland | |
| Participants | Adult, medical participants with septic shock (N = 10) | |
| Interventions | Goal‐directed norepinephrine vs dopamine Participants in the norepinephrine group received additional low‐dose dopamine (not considered a vasopressor) |
|
| Outcomes | Death For the mortality analysis, we used data on undetermined mortality |
|
| Notes | It is unclear when participants died; duration of the intervention is unclear Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'patients received in random order ...' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Explicit in‐/exclusion criteria | Unclear risk | Not reported |
| ITT‐analysis | Unclear risk | Not reported |
| Adequate patient description | Unclear risk | Not reported |
| Identical care | Unclear risk | Not reported |
| Outcome description | Unclear risk | Not reported |
| Physicians blinded | Unclear risk | Not reported |
| Outcome assessors blinded? | Unclear risk | Not reported |
Russell 2008.
| Methods | Multi‐centre double‐blind randomized controlled trial, University hospitals (VASST study); Canada, Australia | |
| Participants | Adult participants with septic shock defined by ACCP/SCCM 1992 criteria (ACCP/SCCM 1992) Mean age 61 years, 39% female APACHE score = 27 (N = 778) |
|
| Interventions | Vasopressine infusion 0.01 U/min, increased by 0.005 U/min every 10 minutes (max 0.03 U/min) until MAP 65 to 75 mm Hg vs Norepinephrine infusion 5 mcg/min, increased by 2.5 mcg/min every 10 minutes (max 15 mcg/min) until MAP 65 to 75 mm Hg Additional open‐label vasopressors allowed if MAP not reached at maximum doses of study drugs |
|
| Outcomes | 28‐Day mortality (primary), 90‐day mortality, days alive and organ dysfunction free (renal replacement therapy, mechanical ventilation) until day 28, days alive and free of SIRS until day 28, days alive and free of corticosteroids until day 28, ICU LOS and hospital, serious adverse events For the mortality analysis, we used data on 90‐day mortality |
|
| Notes | Funding: supported by a grant (MCT 44152) from the Canadian Institutes of Health Research Drs Russell, Walley and Gordon report serving as officers and holding stock in Sirius Genomics, which has submitted a patent, owned by the University of British Columbia and licenced to Sirius Genomics that is related to the genetics of vasopressin. The University of British Columbia has also submitted a patent related to use of vasopressin in septic shock. Drs Russell, Walley and Gordon report that they are inventors on this patent. Drs Russell and Walley report that they received consulting fees from Ferring, which manufactures vasopressin. Dr Russell reported that he received grant support from Sirius Genomics, Novartis and Eli Lilly; and Dr Wally, from Sirius Genomics. No other potential conflict of interest relevant to this article was reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated procedure, stratified by centre and severity of shock, variable permutated blocks (size 2 to 6) |
| Allocation concealment (selection bias) | Low risk | Central telephone‐based allocation system |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Midway through the trial, the executive committee, unaware of all data and in conference with the data and safety monitoring committee, determined that participants who had undergone randomization but had never received an infusion would not be included in the primary analysis, as their omission would be equally distributed between groups, would be unrelated to treatment assignment and would not bias outcome ascertainment. We increased the total number of participants enrolled to maintain target sample size after removal of such participants from the analysis 3 participants withdrew consent after receiving study drug |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Adequate |
| Identical care | Low risk | Described |
| Outcome description | Low risk | Adequately described |
| Physicians blinded | Low risk | Reported |
| Outcome assessors blinded? | Low risk | Performed |
Seguin 2002.
| Methods | Single‐centre randomized controlled trial, University hospital; France | |
| Participants | Adult participants with septic shock; unclear whether medical or surgical (N = 22) | |
| Interventions | Goal‐directed epinephrine vs norepinephrine + fixed dobutamine (5 mcg/kg/min) | |
| Outcomes | Death For the mortality analysis, we used data on undetermined mortality |
|
| Notes | It is unclear when participants died | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No sufficient information |
| Allocation concealment (selection bias) | Unclear risk | No sufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Unclear risk | No sufficient information |
| Adequate patient description | Low risk | Reported |
| Identical care | Low risk | Described adequately |
| Outcome description | Unclear risk | No sufficient information |
| Physicians blinded | Unclear risk | No sufficient information |
| Outcome assessors blinded? | Unclear risk | No sufficient information |
Seguin 2006.
| Methods | Single‐centre randomized controlled trial, University hospital; France | |
| Participants | Adult participants with septic shock (study authors' definition) Mean age = 66 years, 23% female SAPS II score = 54, SOFA score = 10 (N = 22) |
|
| Interventions | Dopexamine (DX) infusion 0.5 mcg/kg/min and norepinephrine (NE) infusion 0.2 mcg/kg/min If cardiac index > 3 L/kg/min, NE increased by 0.2 mcg/kg/min every 3 minutes until MAP 70 to 80 mm Hg If cardiac index < 3 L/kg/min, DX increased by 0.5 mcg/kg/min every 3 minutes until MAP 70 to 80 mm Hg vs Epinephrine infusion 0.2 mcg/kg/min. Increased by 0.2 mcg/kg/min every 3 minutes until MAP 70 to 80 mm Hg |
|
| Outcomes | Gastromucosal blood flow (primary), haemodynamics, 28‐day mortality, 90‐day mortality For the mortality analysis, we used data on 90‐day mortality |
|
| Notes | Funding: This study was supported by Grant from Rennes University Hospital and Rennes 1 University, 2001 Clinical Research Program, Rennes, France Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Appropriately reported |
| Allocation concealment (selection bias) | Low risk | Appropriately reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appropriately reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | High risk | Not performed |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Low risk | Described |
| Outcome description | Low risk | Adequately described |
| Physicians blinded | Unclear risk | No sufficient information |
| Outcome assessors blinded? | Unclear risk | No sufficient information |
Svoboda 2012.
| Methods | Single‐centre randomized controlled trial, community hospital; Czech Republic | |
| Participants | Adult patients with late advanced refractory septic shock, with blood pressure < 90 mm Hg systolic or < 70 mm Hg mean, who need norepinephrine > 0,6 µg/kg/min for longer than 24 hours after adequate volume resuscitation unless CVP > 12 mm Hg. Mean age = 73 years, 40% female. SOFA score 18, MODS 14 (N = 30) | |
| Interventions | Terlipressin (4 mg/24 h) vs 'no terlipressin' | |
| Outcomes | Mortality at day 3, day 7, day 14, day 28, day 90; haemodynamic response, norepinephrine requirement, changes in MODS and SOFA score, differences in laboratory variables and safety of treatment (serious adverse events)
|
|
| Notes | Both groups received “conventional open label norepinephrine” Grant of IGA MZ CR NR 9284‐3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random treatment list |
| Allocation concealment (selection bias) | Low risk | Opaque sealed numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data complete |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | High risk | Excluded from analysis were participants who did not survive 3 hours after start of study drug infusion (also for mortality estimates) |
| Adequate patient description | Low risk | Adequately described |
| Identical care | Unclear risk | Open‐label intervention. Open‐label norepinephrine to maintain goal MAP. Target blood pressure determined by the doctor in charge for each individual participant, usually 70 ± 5 mm Hg |
| Outcome description | Low risk | Described |
| Physicians blinded | High risk | Not blinded |
| Outcome assessors blinded? | Unclear risk | Low risk for mortality, high risk for adverse outcomes |
Yildizdas 2008.
| Methods | Single‐centre randomized controlled trial, University hospital PICU (Paediatric Intensive Care Unit); Turkey | |
| Participants | Paediatric participants with septic shock (Hayden 1994; ACCP/SCCM 1992) and non‐response to fluid resuscitation and high‐dose catecholamines Mean age = 28 months, 47% female PRISM score = 29 (N = 58) |
|
| Interventions | Terlipressin bolus 20 mcg/kg every 6 hours up to 96 hours (if MAP < 2 SD for age and at discretion of treating physicians) vs Placebo |
|
| Outcomes | ICU mortality, ICU LOS, biochemical markers, mechanical ventilation, haemodynamics, adverse events (digital ischaemias). Urine output narratively described as unchanged within the intervention group, but no numbers or between‐group comparisons presented) For the mortality analysis, we used data on ICU mortality |
|
| Notes | Placebo administration not described in sufficient detail Funding: not reported Conflict of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Open random table |
| Allocation concealment (selection bias) | High risk | Open random table |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Implicitly reported |
| Explicit in‐/exclusion criteria | Low risk | Described explicitly |
| ITT‐analysis | Low risk | Performed |
| Adequate patient description | Low risk | Described |
| Identical care | Unclear risk | Catecholamine therapy not described in sufficient detail |
| Outcome description | Low risk | Clear |
| Physicians blinded | High risk | Not blinded |
| Outcome assessors blinded? | Unclear risk | Not reported |
ACCP/SCCM = American College of Chest Physicians/Society of Critical Care Medicine; APACHE´= Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; AVP = arginine‐vasopressin; CBI = blood clearance of indocyanine green; CI = cardiac index; CV = cardiovascular; EF = ejection fraction; h = hour; ICU = intensive care unit; IDO2 = index of oxygen delivery; IVO2 = oxygen consumption, indexed; LOS = length of stay; MAP = mean arterial pressure; MODS = multiple organ dysfunction score; NE = norepinephrine; PCCU = Paediatric Critical Care Unit; PELOD = paediatric logistic organ dysfunction score; PICU = paediatric intensive care unit; PRISM = Paediatric Risk of Mortality score; RCT = randomized controlled trial; SAPS = simplified acute physiology score; SBP = systolic blood pressure; ScvO2 = central venous oxygen saturation; SIRS = systemic Inflammatory response syndrome; SOFA = sequential organ failure assessment; SOAP = sepsis occurrence in acutely ill patients; SVRI = systemic vascular resistance index; TNFa = tumour necrosis factor alpha; U = units; VASST (study) = Vasopressin And Septic Shock Trial; VEGF = vascular endothelial growth factor; 2SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Argenziano 1997 | No relevant endpoints (study population: vasodilatory shock after left ventricular assist device placement) Intervention: vasopressin or saline placebo |
| Hentschel 1995 | No relevant endpoints and other population (study population: hypotensive pre‐term infants) Intervention: dobutamine or dopamine |
| Kinstner 2002 | No relevant endpoints (study population: adult patients with septic shock) Intervention: arginine‐vasopressin or placebo Study population: adults with septic shock NB: published only as abstract |
| Levy 1999 | No relevant endpoints (study population: adult patients with septic shock) Intervention: dobutamine or dopexamine |
| Majerus 1984 | No relevant endpoints (study population: adult patients after abdominal surgery with septic shock) Intervention: dopamine or dobutamine |
| Morelli 2011 | No relevant endpoints |
| Morelli 2011a | No relevant endpoints |
| Patel 2002 | No relevant endpoints (study population: adult patients with severe septic shock) Intervention: norepinephrine or vasopressin |
| Rozé 1993 | Other population (study population: pre‐term infants with refractory shock) Intervention: dopamine vs dobutamine |
| Schmoelz 2006 | Participants with septic shock treated with norepinephrine received dopexamine (2 mcg/kg/min), dopamine (3 mcg/kg/min) or saline placebo Comparison between dopamine and saline placebo as add‐on therapy was initially considered relevant. However, the dopamine dose (3 mcg/kg/min) is not considered to have vasopressor properties and accordingly does not fulfil our inclusion criteria for vasopressor vs placebo |
| Sperry 2008 | Data from a multi‐centre prospective cohort study in patients with blunt injury and haemorrhagic shock, not a randomized study |
| Totaro 1997 | No relevant endpoints (study population: adult patients with hypotension after cardiopulmonary bypass) Intervention: epinephrine or norepinephrine |
| Zhou 2002 | No relevant endpoints (study population: adults with septic shock) Intervention: norepinephrine, epinephrine and norepinephrine‐dobutamine in a cross‐over fashion |
Characteristics of studies awaiting assessment [ordered by study ID]
Agrawal 2011.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Chawla 2014.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Chen 2012.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Cohn 2007.
| Methods | Randomized placebo‐controlled parallel‐group efficacy study |
| Participants | Age 18+, systolic blood pressure < 90 mm Hg, clinical evidence of acute traumatic injury, infusion of study drug within 1 hour after shock onset |
| Interventions | Vasopressin bolus 4 U, followed by continuous infusion 2.4 U/h for 5 hours vs Placebo |
| Outcomes | Organ dysfunction, 28‐day mortality |
| Notes | Estimated enrolment: 333 participants NCT00420407 |
Hai Bo 2002.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Not available |
Hajjar 2013.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
High 2008.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Hussain 2014.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Liu 2010.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Oliveira 2014.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Plotkin 2007.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Singh 1966.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Not available |
Ventura 2014.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Wu 2010.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Zambolim 2014.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Zhuangyu 2011.
| Methods | Study report under retrieval |
| Participants | Study report under retrieval |
| Interventions | Study report under retrieval |
| Outcomes | Study report under retrieval |
| Notes | Study report under retrieval |
Characteristics of ongoing studies [ordered by study ID]
Choudhary 2013.
| Trial name or title | A Prospective Open Label Randomized Non Inferiority Trial to Compare the Efficacy and Safety of Monotherapy With Noradrenaline and Terlipressin in Patients of Cirrhosis With Septic Shock Admitted to Intensive Care Unit. |
| Methods | Prospective open‐label randomized non‐inferiority trial |
| Participants | Patients of cirrhosis with septic shock admitted to intensive care unit |
| Interventions | Terlipressin versus noradrenaline |
| Outcomes | Mortality, organ failure, LOS, adverse events |
| Starting date | April 2013 |
| Contact information | drashokdm@hotmail.com |
| Notes | NCT01836224 |
Fernandez 2006.
| Trial name or title | Terlipressin in Septic Shock in Cirrhosis; Effects on Survival of Terlipressin Administration in Cirrhotic Patients With Severe Sepsis or Septic Shock. A Randomized, Open Labelled Controlled Trial |
| Methods | Treatment, randomized, open‐label, uncontrolled, single group assignment, safety/efficacy study |
| Participants | Adults with liver cirrhosis and septic shock |
| Interventions | Terlipressin 1, 1.5 and 2 mg/4 h intravenously in patients with body weight < 50 kg, between 50 and 70 kg and > 70 kg, respectively, until 24 hours after shock resolution + dopamine (1 to 20 µg/kg/min) and/or norepinephrine (0.05 to 4 µg/kg/min) until shock resolution vs Dopamine (1 to 20 µg/kg/min) and/or norepinephrine (0.05 to 4 µg/kg/min) until shock resolution |
| Outcomes | Hospital survival (primary), refractory shock, variceal bleeding, hepatorenal syndrome |
| Starting date | October 2006 |
| Contact information | Javier Fernandez, MD; Jfdez@clinic.ub.es; Hospital Clinic Barcelona, Catalonia, Spain |
| Notes | NCT00628160 |
Gordon 2014.
| Trial name or title | VAsopressin vs Noradrenaline as Initial therapy in Septic sHock |
| Methods | Double‐blind factorial (2 × 2) randomized controlled trial |
| Participants | Adult patients with septic shock, being treated in 19 intensive care units (ICUs) in the UK |
| Interventions | Vasopressin or noradrenaline (Study Drug 1) by continuous infusion to stabilize blood pressure. If maximum limit of Study Drug 1 is reached, then Study Drug 2 (hydrocortisone or placebo) will be administered |
| Outcomes | Renal failure‐free days at day 28 post randomization, renal outcomes, survival, other organ failure, biomarkers |
| Starting date | 01/10/2012 |
| Contact information | http://www.vanishtrial.co.uk |
| Notes | ISRCTN20769191; EudraCT number 2011‐005363‐24 |
Lienhart 2007.
| Trial name or title | Vasopressin for the Therapy of Persistent Traumatic Hemorrhagic Shock. The VITRIS.at Study |
| Methods | European multi‐centre randomized controlled study in the pre‐hospital emergency medical helicopter setting |
| Participants | Adult pre‐hospital traumatic haemorrhagic shock despite standard treatment within 60 minutes |
| Interventions | Vasopressin (10IU IV) vs saline placebo up to 3 injections at least 5 minutes apart |
| Outcomes | Hospital admission rate (primary), haemodynamics, fluid requirements, hospital discharge rate |
| Starting date | January 2009 |
| Contact information | www.vitris.at |
| Notes |
NCT 00379522 EudraCT‐number 2006‐004252‐20 |
NOVEL 2015.
| Trial name or title | NOrepinephrine and VasoprEssin Versus Norepinephrine aLone in Critically Ill Patients With Septic Shock (NOVEL) |
| Methods | Open‐label, non‐randomized |
| Participants | Individuals with septic shock |
| Interventions | Norepinephrine (0.05 to 0.5 mcg/kg/min) and vasopressin (0.04 units/min) will be given by continuous infusion to achieve and maintain target mean arterial pressure (65 to 75 mm Hg) |
| Outcomes | Time to goal MAP |
| Starting date | November 2015 |
| Contact information | Drayton Hammond, PharmD |
| Notes |
Vasoactive Drugs in Intensive Care Unit 2015.
| Trial name or title | Vasoactive Drugs in Intensive Care Unit |
| Methods | Randomized parallel‐group double‐blind controlled trial |
| Participants | ICU patients with shock |
| Interventions | Phenylephrine and vasopressin vs norepinephrine and epinephrine |
| Outcomes | Hospital mortality (primary), haemodynamics, length of stay, ICU complications, functional status |
| Starting date | May 2014 |
| Contact information | John P. Kress, MD, University of Chicago |
| Notes |
ICU = intensive care unit
Contributions of authors
GG: selecting, reading, and comparing titles, abstracts and papers; drafting a data extraction sheet; extracting data from studies; drafting the update review.
CH: revising the protocol for content and clarity; building a database for data extraction; selecting, reading and comparing titles, abstracts and papers; extracting data from studies; drafting a data extraction sheet; reading and correcting the full review.
HL: selecting, reading and comparing titles, abstracts and papers; reading and correcting the full review.
JA: performing the literature search; selecting, reading and comparing titles, abstracts and papers; drafting a data extraction sheet; extracting data from studies; preparing the SoF table; reading and correcting the full review.
NLP: planning and performing network meta‐analyses; reading and correcting the full review.
MM: conceiving of the initial review; drafting the protocol; performing the literature search; selecting, reading and comparing titles, abstracts and papers; drafting a data extraction sheet; extracting data from studies; drafting the first review; reading and correcting the full review.
HH: conceiving of the update review; overseeing the search process; serving as arbiter for trial selection in case of discrepancies and arbiter for data extraction in case of discrepancies; performing statistical analyses; drafting the update review.
Sources of support
Internal sources
Medical University Vienna, where most of the review authors are employed, Austria.
External sources
No sources of support supplied
Declarations of interest
Gunnar Gamper: no conflict of interest.
Christof Havel: no conflict of interest.
Jasmin Arrich: no conflict of interest.
Heidrun Losert: no conflict of interest.
Nathan Leon Pace: no conflict of interest.
Marcus Müllner: no conflict of interest.
Harald Herkner: no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Albanese 2005 {published data only}
- Albanese J, Leone M, Delmas A, Martin C. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Critical Care Medicine 2005;33(9):1897‐902. [PUBMED: 16148457] [DOI] [PubMed] [Google Scholar]
Annane 2007 {published data only}
- Annane D, Vignon P, Renault A, Bollaert PE, Charpentier C, Martin C, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007;370(9588):676‐84. [PUBMED: 17720019] [DOI] [PubMed] [Google Scholar]
Boccara 2003 {published data only}
- Boccara G, Ouattara A, Godet G, Dufresne E, Bertrand M, Riou B, et al. Terlipressin versus norepinephrine to correct refractory arterial hypotension after general anesthesia in patients chronically treated with renin‐angiotensin system inhibitors. Anesthesiology 2003;98:1338‐44. [PUBMED: 12766641] [DOI] [PubMed] [Google Scholar]
Choong 2009 {published data only}
- Choong K, Bohn D, Fraser DD, Gaboury I, Hutchison JS, Joffe AR, et al. Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2009;180(7):632‐9. [PUBMED: 19608718] [DOI] [PubMed] [Google Scholar]
De Backer 2010 {published data only}
- Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. The New England Journal of Medicine 2010;362(9):779‐89. [PUBMED: 20200382] [DOI] [PubMed] [Google Scholar]
Dünser 2003 {published data only}
- Dünser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, et al. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation 2003;107:2313‐9. [PUBMED: 12732600] [DOI] [PubMed] [Google Scholar]
Han 2012 {published data only}
- Han X‐D, Sun H, Huang X‐Y, Zhang S‐Y, Wang Y‐D, Ren K, et al. [A clinical study of pituitrin versus norepinephrine in the treatment of patients with septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue/Chinese Critical Care Medicine/Zhongguo Weizhongbing Jijiuyixue 2012;24(1):33‐7. [PubMed] [Google Scholar]
Hua 2013 {published data only}
- Hua F, Wang X, Zhu L. Terlipressin decreases vascular endothelial growth factor expression and improves oxygenation in patients with acute respiratory distress syndrome and shock. Journal of Emergency Medicine 2013;44(2):434‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2010 {published data only}
- Jain G, Singh DK. Comparison of phenylephrine and norepinephrine in the management of dopamine‐resistant septic shock. Indian Journal of Critical Care Medicine 2010;14(1):29‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauzier 2006 {published data only}
- Lauzier F, Levy B, Lamarre P, Lesur O. Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial. Intensive Care Medicine 2006;32(11):1782‐9. [PUBMED: 17019548] [DOI] [PubMed] [Google Scholar]
Levy 1997 {published data only}
- Levy B, Bollaert PE, Charpentier C, Nace L, Audibert G, Bauer P, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Medicine 1997;23:282‐7. [PUBMED: 9083230] [DOI] [PubMed] [Google Scholar]
Levy 2011 {published data only}
- Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine‐dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Critical Care Medicine 2011;39(3):450‐5. [PUBMED: 21037469] [DOI] [PubMed] [Google Scholar]
Luckner 2006 {published data only}
- Luckner G, Dunser MW, Stadlbauer KH, Mayr VD, Jochberger S, Wenzel V, et al. Cutaneous vascular reactivity and flow motion response to vasopressin in advanced vasodilatory shock and severe postoperative multiple organ dysfunction syndrome. Critical Care 2006;10(2):R40. [PUBMED: 16542484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malay 1999 {published data only}
- Malay MB, Ashton RC Jr, Landry DW, Townsend RN. Low‐dose vasopressin in the treatment of vasodilatory septic shock. Journal of Trauma 1999;47:699‐703. [PUBMED: 10528604] [DOI] [PubMed] [Google Scholar]
Marik 1994 {published data only}
- Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA 1994;272:1354‐7. [PUBMED: 7933396] [PubMed] [Google Scholar]
Martin 1993 {published data only}
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock?. Chest 1993;103:1826‐31. [PUBMED: 8404107] [DOI] [PubMed] [Google Scholar]
Mathur 2007 {published data only}
- Mathur S, Dhunna R, Chakraborty A. Comparison of norepinephrine and dopamine in the management of septic shock using impedance cardiography. Indian Journal of Critical Care Medicine 2007;11(4):186‐91. [EMBASE: 2008016347] [Google Scholar]
Morelli 2008a {published data only}
- Morelli A, Ertmer C, Lange M, Duenser M, Rehberg S, Aken H, et al. Effects of short‐term simultaneous infusion of dobutamine and terlipressin in patients with septic shock: the DOBUPRESS study. British Journal of Anaesthesia 2008;100(4):494‐503. [PUBMED: 18308741] [DOI] [PubMed] [Google Scholar]
Morelli 2008b {published data only}
- Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Laderchi A, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Critical Care 2008;12(6)(R143):1‐11. [PUBMED: 19017409 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morelli 2009 {published data only}
- Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Cecchini V, et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Critical Care 2009;13(4):R130. [PUBMED: 19664253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myburgh 2008 {published data only}
- Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J, et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Medicine 2008;34(12):2226‐34. [PUBMED: 18654759] [DOI] [PubMed] [Google Scholar]
Patel 2010 {published data only}
- Patel GP, Grahe JS, Sperry M, Singla S, Elpern E, Lateef O, et al. Efficacy and safety of dopamine versus norepinephrine in the management of septic shock. Shock 2010;33(4):375‐80. [PUBMED: 19851126] [DOI] [PubMed] [Google Scholar]
Ruokonen 1993 {published data only}
- Ruokonen E, Takala J, Kari A, Saxen H, Mertsola J, Hansen EJ. Regional blood flow and oxygen transport in septic shock. Critical Care Medicine 1993;21:1296‐303. [PUBMED: 8370292] [DOI] [PubMed] [Google Scholar]
Russell 2008 {published data only}
- Gordon AC, Russell JA, Walley KR, Singer J, Ayers D, Storms MM, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Medicine 2010;36(1):83‐91. [DOI] [PubMed] [Google Scholar]
- Gordon AC, Wang N, Walley KR, Ashby D, Russell JA. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest 2012;142(3):593‐605. [DOI] [PubMed] [Google Scholar]
- Russell JA, Fjell C, Hsu JL, Lee T, Boyd J, Thair S, et al. Vasopressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock. American Journal of Respiratory and Critical Care Medicine 2013;188:356‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Walley KR, Gordon AC, Cooper DJ, Hébert PC, Singer J, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Critical Care Medicine 2009;37(3):811‐8. [PUBMED: 19237882] [DOI] [PubMed] [Google Scholar]
- Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. New England Journal of Medicine 2008;358(9):877‐87. [PUBMED: 18305265] [DOI] [PubMed] [Google Scholar]
Seguin 2002 {published data only}
- Seguin P, Bellissant E, Le‐Tulzo Y, Laviolle B, Lessard Y, Thomas R, et al. Effects of epinephrine compared with the combination of dobutamine and norepinephrine on gastric perfusion in septic shock. Clinical Pharmacology and Therapeutics 2002;71:381‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Seguin 2006 {published data only}
- Seguin P, Laviolle B, Guinet P, Morel I, Malledant Y, Bellissant E. Dopexamine and norepinephrine versus epinephrine on gastric perfusion in patients with septic shock: a randomized study [NCT00134212]. Critical Care 2006;10(1)(R32):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svoboda 2012 {published data only}
- Svoboda P, Scheer P, Kantorova I, Doubek J, Dudra J, Radvan M, et al. Terlipressin in the treatment of late phase catecholamine‐resistant septic shock. Hepato‐Gastroenterology 2012;59(116):1043‐7. [DOI] [PubMed] [Google Scholar]
Yildizdas 2008 {published data only}
- Yildizdas D, Yapicioglu H, Celik U, Sertdemir Y, Alhan E. Terlipressin as a rescue therapy for catecholamine‐resistant septic shock in children. Intensive Care Medicine 2008;34(3):511‐7. [PUBMED: 18092150] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Argenziano 1997 {published data only}
- Argenziano M, Choudhri AF, Oz MC, Rose EA, Smith CR, Landry DW. A prospective randomized trial of arginine vasopressin in the treatment of vasodilatory shock after left ventricular assist device placement. Circulation 1997;96 Suppl II:286‐90. [PUBMED: 9386112] [PubMed] [Google Scholar]
Hentschel 1995 {published data only}
- Hentschel R, Hensel D, Brune T, Rabe H, Jorch G. Impact on blood pressure and intestinal perfusion of dobutamine or dopamine in hypotensive preterm infants. Biology of the Neonate 1995;68:318‐24. [PUBMED: 8835086] [DOI] [PubMed] [Google Scholar]
Kinstner 2002 {published data only}
- Kinstner C, Germann P, Ullrich R, Landry D, Sladen R. Infusion of arginine‐vasopressin (AVP) enhances blood pressure and renal function while preserving cerebral and splanchnic perfusion in patients in septic shock. Anesthesiology Abstracts of Scientific Papers, Annual Meeting. 2002:Abstract No A‐439.
Levy 1999 {published data only}
- Levy B, Nace L, Bollaert PE, Dousset B, Mallie JP, Larcan A. Comparison of systemic and regional effects of dobutamine and dopexamine in norepinephrine‐treated septic shock. Intensive Care Medicine 1999;25:942‐8. [PUBMED: 10501749] [DOI] [PubMed] [Google Scholar]
Majerus 1984 {published data only}
- Majerus TC, Chodoff P, Borel CO. Dopamine and dobutamine in septic shock. A comparison. Archives Internationales de Physiologie et de Biochimie 1984;92:S65‐7. [PUBMED: 6085242] [DOI] [PubMed] [Google Scholar]
Morelli 2011 {published data only}
- Morelli A, Donati A, Ertmer C, Rehberg S, Kampmeier T, Orecchioni A. Effect of vasopressinergic receptor agonists on sublingual microcirculation in norepinephrine‐dependent septic shock. Critical Care 2011;15:R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morelli 2011a {published data only}
- Morelli A. Short term effects of terlipressin bolus infusion on sublingual microcirculatory blood flow during septic shock. Intensive Care Medicine 2011;37:963‐9. [DOI] [PubMed] [Google Scholar]
Patel 2002 {published data only}
- Patel BM, Chittock DR, Russell JA, Walley KR. Beneficial effects of short‐term vasopressin infusion during severe septic shock. Anesthesiology 2002;96:576‐82. [PUBMED: 11873030] [DOI] [PubMed] [Google Scholar]
Rozé 1993 {published data only}
- Roze JC, Tohier C, Maingueneau C, Lefevre M, Mouzard A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Archives of Disease in Childhood 1993;69:59‐63. [PUBMED: 8346957] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmoelz 2006 {published data only}
- Schmoelz M, Schelling G, Dunker M, Irlbeck M. Comparison of systemic and renal effects of dopexamine and dopamine in norepinephrine‐treated septic shock. Journal of Cardiothoracic and Vascular Anesthesia 2006;20(2):173‐8. [PUBMED: 16616656] [DOI] [PubMed] [Google Scholar]
Sperry 2008 {published data only}
- Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht B G, Moore EE, et al. Early use of vasopressors after injury: caution before constriction. Journal of Trauma 2008;64(1):9‐14. [PUBMED: 18188092] [DOI] [PubMed] [Google Scholar]
Totaro 1997 {published data only}
- Totaro RJ, Raper RF. Epinephrine‐induced lactic acidosis following cardiopulmonary bypass. Critical Care Medicine 1997;25:1693‐9. [PUBMED: 9377884] [DOI] [PubMed] [Google Scholar]
Zhou 2002 {published data only}
- Zhou SX, Qiu HB, Huang YZ, Yang Y, Zheng RQ. Effects of norepinephrine, epinephrine, and norepinephrine‐dobutamine on systemic and gastric mucosal oxygenation in septic shock. Acta Pharmacologica Sinica 2002;23:654‐8. [PUBMED: 12100762 ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Agrawal 2011 {published data only}
- Agrawal A, Gupta A, Consul S, Shastri P. Comparative study of dopamine and norepinephrine in the management of septic shock. Saudi Journal of Anaesthesia 2011;5:162‐6. [PUBMED: 21804796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawla 2014 {published data only}
- Chawla L. Busse L, Brasha‐Mitchell E, Davison D, Honiq J, Alotaibi Z, et al. Intravenous angiotensin II for the treatment of high output shock (ATHOS trial): a pilot study. Critical Care 2014;18(5):534‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen H, Zeng Z. Comparison of the effect and complications between dopamine and norepinephrine on treatment of septic shock. Jiangxi Medical Journal 2012;47:565‐7. [Google Scholar]
Cohn 2007 {published data only}
- Cohn SM, McCarthy J, Stewart RM, Jonas RB, Dent DL, Michalek JE. Impact of low‐dose vasopressin on trauma outcome: prospective randomized study. World Journal of Surgery 2011;35(2):430‐9. [DOI: 10.1007/s00268-010-0875-8] [DOI] [PubMed] [Google Scholar]
Hai Bo 2002 {published data only}
- Hai Bo Q, Yi Y, Shao Xia Z, Ying Zi H, Rui Qiang Z. Renal effect of dopamine, norepinephrine, epinephrine, or norepinephrine‐dobutamine in septic shock. Critical Care and Shock 2002;5:9‐14. [Google Scholar]
Hajjar 2013 {published data only}
- Hajjar L, Vincent JL, Rhodes A, Annane D, Galas F, Almeida J, et al. Vasopressin versus norepinephrine for the management of shock. Critical Care 2013;17(Suppl 2):S83‐P222. [Google Scholar]
High 2008 {published data only}
- High K. Impact of dopamine and norepinephrine on renal perfusion in patients with septic shock. Journal of Herbal Medicine 2008;30:1188. [Google Scholar]
Hussain 2014 {published data only}
- Hussain T, Zahir J, Akbar A, Qureshi QA, Rehman HR. Efficacy of phenylephrine versus noradrenaline in management of patients presenting with septic shock in the intensive care unit. Rawal Medical Journal 2014;2:136‐40. [Google Scholar]
Liu 2010 {published data only}
- Liu P, Chen T, Zhang Y. Comparison evaluation of resuscitation effect of norepinephrine and dopamine on the treatment of septic shock. Clinical Education of General Practice 2010;8:265‐7. [Google Scholar]
Oliveira 2014 {published data only}
- Oliveira S, Dessa F, Rocha C, Oliveira F. Early vasopressin application in shock study. Critical Care 2014;Suppl:S56. [Google Scholar]
Plotkin 2007 {published data only}
- Plotkin LL. Use of vasopressin to correct hemodynamic disorders in patients with abdominal sepsis. Anesteziologiia i Reanimatologiia 2007;2:47‐9. [PubMed] [Google Scholar]
Singh 1966 {published data only}
- Singh S, Malhotra RP. Comparative study of angiotensin and nor‐adrenaline in hypotensive states (shock). The Journal of the Association of Physicians of India 1966;14:639‐45. [PUBMED: 4292372] [PubMed] [Google Scholar]
Ventura 2014 {published data only}
- Ventura AMc, Goes PF, Fernandes I de COF, Hsin SH, Souza de C, Gaiga L, et al. Randomized double‐blind trial of dopamine or epinephrine as first‐line vasoactive drugs in fluid refractory pediatric septic shock. Pediatric Critical Care Medicine 2014;4(1):5. [Google Scholar]
Wu 2010 {published data only}
- Wu J, Chen J, Ou Y, Yang C, Chen M, Hwuang SW, et al. Effect of dopamine and norepinephrine on hemodynamics and oxygen metabolism of tissue in patients with septic shock. Chinese Archives of General Surgery 2010;4:117‐21. [Google Scholar]
Zambolim 2014 {published data only}
- Zambolim C, Nagaoka D, Fukushima J, Park C, Carneiro J, Osawa E, et al. Vasopressin versus norepinephrine for the management of septic shock in cancer patients. Critical Care 2014;Suppl:S57. [Google Scholar]
Zhuangyu 2011 {published data only}
- Zhuangyu Y. Effect of norepinephrine and dopamine on infectious tissue oxygen metabolismand hemodynamics in patients with shock. Shandong Medicine Journal 2011;51:93‐4. [Google Scholar]
References to ongoing studies
Choudhary 2013 {published data only}
- Choudhary A. Efficacy and safety of monotherapy with noradrenaline and terlipressin in patients of cirrhosis with septic shock admitted to intensive care unit. NCT01836224.
Fernandez 2006 {published data only}
- Terlipressin in Septic Shock in Cirrhosis; Effects on Survival of Terlipressin Administration in Cirrhotic Patients With Severe Sepsis or Septic Shock. A Randomized, Open Labelled Controlled Trial. Ongoing study October 2006.
Gordon 2014 {published data only}
- Gordon AC, Mason AJ, Perkins GD, Ashby D, Brett SJ. Protocol for a randomised controlled trial of VAsopressin versus Noradrenaline as Initial therapy in Septic sHock (VANISH). BMJ open 2014;4(7):e005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lienhart 2007 {published data only}
- Lienhart H G, Wenzel V, Braun J, Dorges V, Dunser M, Gries A, et al. Vasopressin for therapy of persistent traumatic hemorrhagic shock. The VITRIS.at study. [German]. Anaesthetist 2007;56(2):145‐50. [DOI] [PubMed] [Google Scholar]
NOVEL 2015 {published data only}
- NOrepinephrine and VasoprEssin Versus Norepinephrine aLone in Critically Ill Patients With Septic Shock (NOVEL). clinicaltrials.gov. [NCT02454348]
Vasoactive Drugs in Intensive Care Unit 2015 {published data only}
- Vasoactive Drugs in Intensive Care Unit. clinicaltrials.gov. [NCT02118467]
Additional references
ACCP/SCCM 1992
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine 1992;20:864‐74. [PUBMED: 1597042] [PubMed] [Google Scholar]
Alberti 2005
- Alberti C, Brun‐Buisson C, Chevret S, Antonelli M, Goodman SV, Martin C, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. American Journal of Respiratory and Critical Care Medicine 2005;171(5):461‐8. [PUBMED: 15531752] [DOI] [PubMed] [Google Scholar]
Angus 2001
- Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 2001;29(7):1303‐10. [PUBMED: 11445675] [DOI] [PubMed] [Google Scholar]
Annane 2008
- Annane D, Sébille V, Duboc D, Heuzey JY, Sadoul N, Bouvier E, et al. Incidence and prognosis of sustained arrhythmias in critically ill patients. American Journal of Respiratory and Critical Care Medicine 2008;178:20‐5. [PUBMED: 18388358] [DOI] [PubMed] [Google Scholar]
ARISE 2014
- The ARISE Investigators and the ANZICS Clinical Trials Group. Goal‐directed resuscitation for patients with early septic shock. New England Journal of Medicine 2014;371:1496‐506. [DOI] [PubMed] [Google Scholar]
Asfar 2014
- Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood‐pressure target in patients with septic shock. New England Journal of Medicine 2014;370(17):1583‐93. [DOI] [PubMed] [Google Scholar]
Avni 2015
- Avni T, Lador A, Lev S, Leibovici L, Paul M, Grossman A. Vasopressors for the treatment of septic shock: systematic review and meta‐analysis. PLoS One 2015;10(8):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babaev 2005
- Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294(4):448‐54. [PUBMED: 16046651] [DOI] [PubMed] [Google Scholar]
Beale 2004
- Beale RJ, Hollenberg SM, Vincent JL, Parrillo JE. Vasopressor and inotropic support in septic shock: an evidence‐based review. Critical Care Medicine 2004;32 Suppl(11):455‐65. [PUBMED: 15542956] [DOI] [PubMed] [Google Scholar]
Blanco 2008
- Blanco J, Muriel‐Bombin A, Sagredo V, Taboada F, Gandia F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Critical Care (London, England) 2008;12(6):R158. [PUBMED: 19091069] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cannon 2009
- Cannon CM, Braxton CC, Kling‐Smith M, Mahnken JD, Carlton E, Moncure M. Utility of the shock index in predicting mortality in traumatically injured patients. The Journal of Trauma 2009;67(6):1426‐30. [PUBMED: 20009697] [DOI] [PubMed] [Google Scholar]
DeBacker 2012
- DeBacker D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta‐analysis. Critical Care Medicine 2012;40(3):725‐30. [DOI] [PubMed] [Google Scholar]
Dellinger 2008
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine 2008;36(1):296‐327. [PUBMED: 18158437] [DOI] [PubMed] [Google Scholar]
Dellinger 2013
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Critical Care Medicine 2013;41(2):580‐637. [DOI] [PubMed] [Google Scholar]
Esteban 2007
- Esteban A, Frutos‐Vivar F, Ferguson ND, Penuelas O, Lorente JA, Gordo F, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Critical Care Medicine 2007;35(5):1284‐9. [PUBMED: 17414725] [DOI] [PubMed] [Google Scholar]
Geiling 1926
- Geiling EMK, Campbell DJ. Variations in blood pressure induced by repeated injections of extracts of the posterior lobe of the pituitary gland. Journal of Pharmacological and Experimental Therapeutics 1926;29:449–60. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 1994
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. www.cochrane‐handbook.org.
Hinshaw 1972
- Hinshaw LB, Cox BG. The Fundamental Mechanisms of Shock. New York: Plenum Press, 1972. [Google Scholar]
Hoffman 1992
- Hoffman B, Lefkowitz RJ. Catecholamines and sympathomimetic drugs. In: A Goodman Gilman editor(s). The Pharmacological Basis of Therapeutics. 8th Edition. Singapore: McGraw‐Hill, 1992:187‐220. [Google Scholar]
Holmes 2009
- Holmes CL, Walley KR. Vasoactive drugs for vasodilatory shock in ICU. Current Opinion in Critical Care 2009;15(5):398‐402. [PUBMED: 19542884] [DOI] [PubMed] [Google Scholar]
Krahn 2013
- Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta‐analyses. BMC Medical Research Methodology 2013;13(35):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
König 2012
- König J, Krahn U, Binder H. Visualizing the flow of evidence in network meta‐analysis and characterizing mixed treatment comparisons. Statistics in Medicine 2012;32:5414‐29. [DOI] [PubMed] [Google Scholar]
Leone 2004
- Leone M, Vallet B, Teboul JL, Mateo J, Bastien O, Martin C. Survey of the use of catecholamines by French physicians. Intensive Care Medicine 2004;30(5):984‐8. [PUBMED: 14997293] [DOI] [PubMed] [Google Scholar]
Leone 2008
- Leone M, Martin C. Vasopressor use in septic shock: an update. Current Opinion in Anaesthesiology 2008;21(2):141‐7. [PUBMED: 18443479] [DOI] [PubMed] [Google Scholar]
Levy 2003
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine 2003;31(4):1250‐6. [PUBMED: 12682500] [DOI] [PubMed] [Google Scholar]
Levy 2010
- Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, et al. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Medicine 2010;36(12):2019‐29. [PUBMED: 20862451] [DOI] [PubMed] [Google Scholar]
Martin 2000
- Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Critical Care Medicine 2000;28(8):2758‐65. [PUBMED: 10966247] [DOI] [PubMed] [Google Scholar]
Povoa 2009
- Povoa PR, Carneiro AH, Ribeiro OS, Pereira AC. Influence of vasopressor agent in septic shock mortality. Results from the Portuguese Community‐Acquired Sepsis Study (SACiUCI study). Critical Care Medicine 2009;37(2):410‐6. [PUBMED: 19114885] [DOI] [PubMed] [Google Scholar]
ProCESS 2014
- The ProCESS Investigators. A randomized trial of protocol‐based care for early septic shock. New England Journal of Medicine 2014;370:1683‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quenot 2013
- Quenot J‐P, Binquet C, Kara F, Martinet O, Ganster F, Navellou J‐C, et al. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Critical Care 2013;17(R65):1‐10. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
R Core Team 2014 [Computer program]
- R Core Team. R: A language and environment for statistical computing.. Vienna, Austria: R Foundation for Statistical Computing, 2014.
Reinelt 2001
- Reinelt P Karth DG, Geppert A, Heinz G. Incidence and type of cardiac arrhythmias in critically ill patients: a single center experience in a medical‐cardiological ICU. Intensive Care Medicine 2001;27:1466‐73. [PUBMED: 11685339] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivers 2001
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. The New England Journal of Medicine 2001;345:1368‐77. [PUBMED: 11794169] [DOI] [PubMed] [Google Scholar]
Russell 2009
- Russell JA, Walley KR, Gordon AC, Cooper DJ, Hébert PC, Singer J, et al. Interaction of vasopressin infusion, corticosteroid treatment, and the mortality of septic shock. Critical Care Medicine 2009;37(3):811‐8. [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rucker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Rücker 2012
- Rücker G. Network meta‐analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3:312‐24. [DOI] [PubMed] [Google Scholar]
Sakr 2006
- Sakr Y, Reinhart K, Vincent JL, Sprung CL, Moreno R, Ranieri VM, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Critical Care Medicine 2006;34(3):589‐97. [PUBMED: 16505643] [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta‐analysis. PLOS ONE 2014;9(7):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subhedar 2003
- Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001242; CD001242] [DOI] [PubMed] [Google Scholar]
Sundararajan 2005
- Sundararajan V, Macisaac CM, Presneill JJ, Cade JF, Visvanathan K. Epidemiology of sepsis in Victoria, Australia. Critical Care Medicine 2005;33(1):71‐80. [PUBMED: 15644651] [DOI] [PubMed] [Google Scholar]
Takala 2010
- Takala J. Should we target blood pressures in Sepsis?. Critical Care Medicine 2010;38:S609‐13. [DOI] [PubMed] [Google Scholar]
Young 2008
- Young WF. SHOCK. In: Stone CK, Humphries R editor(s). CURRENT Diagnosis and Treatment Emergency Medicine. 6th Edition. New York: LANGE CURRENT Series/McGraw‐Hill, 2008:160. [Google Scholar]
Zaritsky 1994
- Zaritsky AL. Catecholamines, inotropic medications, and vasopressor agents. In: Chernow B editor(s). Essentials of Critical Care Pharmacology. 2nd Edition. Baltimore, MD: Williams & Wilkins, 1994:255‐72. [Google Scholar]
Zhou 2015
- Zhou F, Mao Z, Zeng X, Kang H, Liu H, Pan L, et al. Vasopressors in septic shock: a systematic review and network meta‐analysis. Therapeutics and Clinical Risk Management 2015;11:1047‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Havel 2011
- Havel C, Arrich J, Losert H, Gamper G, Müllner M, Herkner H. Vasopressors for hypotensive shock. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD003709.pub3] [DOI] [PubMed] [Google Scholar]
Müllner 2002
- Müllner M, Urbanek B, Havel C, Losert H, Gamper G. Vasopressors for shock. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003709] [DOI] [PubMed] [Google Scholar]
Müllner 2004
- Müllner M, Urbanek B, Havel C, Losert H, Gamper G, Herkner H. Vasopressors for shock. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003709] [DOI] [PubMed] [Google Scholar]


