Abstract
Tens of millions of contrast enhanced magnetic resonance imaging (MRI) exams are performed annually around the world. The contrast agents, which improve diagnostic accuracy, are almost exclusively small, hydrophilic gadolinium(III) based chelates. In recent years concerns have arisen surrounding the long term safety of these compounds, and this has spurred research into alternatives. There has also been a push to develop new molecularly targeted contrast agents or agents that can sense pathological changes in the local environment. This comprehensive review describes the state of the art of clinically approved contrast agents, their mechanism of action, and factors influencing their safety. From there we describe different mechanisms of generating MR image contrast such as relaxation, chemical exchange saturation transfer, and direct detection and the types of molecules that are effective for these purposes. Next we describe efforts to make safer contrast agents either by increasing relaxivity, increasing resistance to metal ion release, or by moving to gadolinium(III)-free alternatives. Finally we survey approaches to make contrast agents more specific for pathology either by direct biochemical targeting or by the design of responsive or activatable contrast agents.
Graphical Abstract
1. Introduction
Gadolinium(III) based contrast agents (GBCAs), as a class, are one of the most successful examples of inorganic drugs, along with the platinum anticancer compounds and the technetium-99m radiopharmaceuticals used for cardiac and bone imaging. This success comes from the diagnostic and prognostic information that these agents provide, as well as their very favorable safety profile when compared to other imaging agents or drugs. GBCAs are used in about 40% of all magnetic resonance imaging (MRI) exams and in about 60% of neuro MRI exams. This represents about 40 million administrations of GBCAs worldwide.1
The growth of MRI and contrast-enhanced MRI has been remarkable. When one of us reviewed the field in 1999 we noted that since the approval of Gd-DTPA in 1988, about 30 metric tons of gadolinium metal ion had been cumulatively administered to patients worldwide over that 11 year period.2 Now almost 50 tons of gadolinium are administered annually. The market for GBCAs is over one billion dollars per year. What has driven this growth?
GBCAs have been so successful because they provide essential diagnostic information that often cannot be obtained with other noninvasive techniques. A prime example is using GBCAs to detect blood brain barrier (BBB) disruption. GBCAs do not cross the BBB, so contrast enhancement of the brain can arise from pathologies such as multiple sclerosis, primary or metastatic cancer, or stroke. The absence of contrast enhancement can also enable a differential diagnosis when other symptoms are considered. GBCAs also detect increased vascular permeability associated with lesions outside the brain as well, for example in breast cancer detection and staging. GBCAs are also widely used to image the blood vessels to detect blockages or aneurysms, or to guide surgical interventions. One can also exploit the kinetics of contrast enhancement after injection, for example to measure regional perfusion of the heart.3
Another factor in the success of GBCAs is that their effect is immediate. Unlike in nuclear medicine where the patient receives the radiopharmaceutical and then waits a period before imaging, in MRI the contrast agent is administered while the patient is in the scanner and diagnostic images appear within minutes. This practical aspect allows more patient throughput and facilitates workflow in radiology practice. Unlike radioactive tracers used in nuclear medicine, GBCAs are shelf-stable and do not need to be synthesized on demand. Moreover there is no ionizing radiation associated with GBCAs or MRI in general. A further factor is that GBCAs shorten the T1 of protons and this allows for faster imaging (higher throughput) and for imaging that is less sensitive to artifacts from motion (better quality images).
Gadolinium is not the only element that can be used to generate MR image contrast. Iron oxide nanoparticles and a manganese(II) complex have been approved for imaging the liver, although these were not commercially successful. In our 1999 review we focused solely on gadolinium(III) complexes as contrast agents. At that time it was clear that the Gd(III) complexes would dominate the field. In this current review we focus more broadly. In the intervening time a number of changes have occurred to cause us to cast a wider net.
For 18 years following GBCA approval for human use, these compounds were considered to be among the safest pharmaceuticals. Indeed the rate of immediate adverse events is very low (<1 per thousand injections) and usually mild with severe adverse events occurring at about once per 40,000 injections.4 However in 2006 GBCAs were associated with a devastating and potentially fatal condition called nephrogenic systemic fibrosis (NSF).5–6 NSF occurs in patients with poor kidney function and its onset can occur months after the last GBCA administration. More recently it has been recognized that some fraction of the injected gadolinium can remain in the body for long periods, although the chemical form of Gd(III) and its whole body distribution is still not well understood. Repeat usage of GBCAs can result in a buildup of residual Gd(III) to the point where it can be detectable by MRI or other methods.7 Together, these findings have led to renewed interest in finding alternatives to using gadolinium(III) for MR contrast.
New techniques for contrast agent detection have also emerged or been improved. In a seminal 2000 paper Ward and Balaban proposed generating image contrast by saturating exchangeable hydrogen resonances (e.g. N-H protons) which then chemically transfer this saturation to bulk water resulting in signal loss.8 There have been a number of clever approaches to exploit this mechanism involving small molecules, metal chelates, and nanoparticles. Direct detection of nuclei such as C-13 or F-19 had been limited because of very low sensitivity when compared with imaging water. However gains in hyperpolarization technology have enabled imaging of hyperpolarized C-13 labeled molecules in humans.9 Pyruvate can be hyperpolarized, injected into the body and then quantified, along with its metabolic transformation into alanine and lactate. Imaging pyruvate metabolism may be useful in staging cancers. Fluorine-19 in the form of perfluorocarbon emulsions can be used to label cells and 19F MRI can be used to image the location and concentration of these cell therapies once they are injected into the body.10
The ability of GBCAs to delineate pathology is based mainly on non-specific distribution. Most GBCAs have an extracellular distribution and are eliminated via the kidneys. Pathology is detected because of altered anatomy (e.g. a narrowed blood vessel) or physiology (e.g. leaky blood brain barrier). GBCAs that distribute to the liver can detect liver lesions that don’t take up the contrast agent. However there is a great potential to move beyond passive distribution and create contrast agents that are biochemically targeted and potentially more specific to disease.
Contrast agents are detected indirectly by their effect on bulk water molecules. The magnitude of this effect is strongly influenced by the chemical properties of the contrast agent and can be potentially be modulated to produce a stronger signal in certain environments. Activatable contrast agents, whose signal is altered by pH, enzymatic activity, temperature change, or ion flux, represent an exciting and growing area of research.
The goals of this review are to provide an overview of the state of art of approved MRI contrast agents, their applications, and the risks associated with them; to provide an understanding of the molecular mechanisms that give rise to MR contrast from relaxation and exchange; to review the coordination chemistry of these agents and the factors responsible for designing an agent safe for human use. We then move from this foundation to review new gadolinium(III)- and non-gadolinium(III) based contrast agents, molecularly targeted agents, and activatable agents. The review should provide the reader with an understanding of the state of the art of MRI contrast agents, the current challenges in the field, and the strategies employed to overcome these challenges.
We start with a description of contrast agents that have been approved for human use. Since these are so well studied, they also the form the basis of descriptions of mechanism of action as well as stability and safety. The field of MRI contrast agents has grown tremendously in the last decades. It has not been possible to cover all of the outstanding chemistry reported. For reasons of scope, we focus primarily on discrete molecular entities (small molecules, metal chelates). While there is some discussion of nanoparticle based agents, this is limited to formulations that have been studied in humans and some exemplary representatives of targeting and/or high relaxivity. We also focus on contrast agents, molecules that generate image contrast in MRI, and provide only a brief overview of agents that are directly detected like hyperpolarized nuclei or perfluorocarbon emulsions.
2. Standard of care and new frontiers
2.1. Applications
MR contrast agents are an absolutely integral component of modern radiology. The first MR contrast agent was made available in 1988 for imaging blood-brain barrier abnormalities and since then contrast enhanced MRI has played an increasingly important role is diagnostic medicine.11 Today, there have been a dozen FDA approved MR contrast agents and 8 of these agents are still commercially available in the United States. Contrast enhanced MRI is now routinely used for imaging lesions in the central nervous system, breast, and abdomen, for angiographic imaging, cardiac imaging, and articular imaging, amongst numerous additional applications. Tens of millions of contrast enhanced examinations are ordered annually worldwide.
All 8 of the MR contrast agents commercially available in the United Stated are small molecule Gd(III) complexes. Seven of the commercially available agents received their primary indication for imaging lesions of the central nervous system (CNS), but one agent (Gd-EOB-DTPA) received FDA approval for liver imaging applications. There are four MR contrast agents that have FDA approval but are no longer currently marketed. The Gd(III)-complex MS-325 was approved for angiographic (blood vessel) imaging. A Mn(II) complex (Mn-DPDP) and a superparamagnetic iron-oxide nanoparticle (SPION) formulation (ferumoxide) were approved for liver imaging, and an oral SPION formulation was approved for gastrointestinal imaging (ferumoxsil).
The seven commercially available Gd(III)-based MRI contrast agents with indications for CNS lesions are administered as an intravenous bolus. These contrast agents are classified as extracellular fluid (ECF) contrast agents. They rapidly extravasate from the bloodstream into the tissue interstitial, extracellular spaces and are rapidly eliminated from the systemic circulation via filtration through the kidneys. Two of these agents are partially excreted through the liver and into the bile and feces.
The contrast enhancement observed after injection reflects the distribution of the exogenously administered agent, and extracellular agents can be ideal for identifying pathologic tissue. For example, tumors are typically characterized by hyper-vascularity, compromised endothelium, and/or an underdeveloped lymphatic drainage system and thus temporarily retain greater concentrations of the contrast agent than healthy tissue and for longer periods of time. The contrast agent thus provides conspicuous contrast enhancement of the tumor in the minutes after injection. Important information about the tumor vascularity and perfusion can be learned by acquiring scans during the contrast agents first pass through the arteries or by dynamically monitoring accumulation and clearance of the contrast agent from the tumor. In the seconds immediately following bolus injection, extracellular MRI contrast agents can also provide high-resolution imaging of the blood vessels to detect vascular pathologies such as stenoses, occlusions, and aneurisms. Contrast agents that are partially eliminated via the hepatobiliary system provide strong contrast enhancement of liver parenchyma as the agent transits the liver in the minutes after the blood signal has largely returned to baseline. This contrast is useful for differential diagnosis of liver malignancies which are devoid of normal hepatocytes.
2.2. New Directions
Contrast agents with biochemical specificity
The clinically available ECF agents are specifically designed to passively move through patients with little or no interactions with proteins or cells.12 The signal observed is strictly reflective of the agent’s distribution. ECF agents provide superb contrast to differentiate anatomical structures or to characterize pathophysiology, but do so in a nonspecific manner. However, new contrast agents with the capability to detect or quantify pathologic biomarkers could revolutionize the specificity with which MRI can be used to detect, characterize, and quantify pathologic tissue.
There are two general approaches to increased biochemical specificity. The first is to couple the contrast agent to a targeting vector to localize the agent to a specific protein or cell type. However the concentration of a monomeric contrast agent needed for detection is at least in the micromolar range. Thus abundant targets need to be selected, large assemblies of the imaging reporter must be used, or some other mechanism of target accumulation must be exploited. The second approach is to modulate the contrast generating signal in response to some stimulus. For example the hydration state of the contrast agent may change with pH resulting in increased or decreased signal. Or the contrast agent may be chemically transformed by the action of a specific enzyme, resulting in a change in signal. Sections 5 and 6 will highlight innovate approaches towards biochemically specific targeted and activatable MRI contrast agents, respectively.
3. Background and Theory
3.1. Classification of contrast agents: Biodistribution and applications
MR contrast agents can be administered either (1) intravenously; (2) orally or (3) by inhalation. Intravenous MR contrast agents may be categorized in 3 types: (a) extracellular fluid (ECF); (b) blood pool; and (c) target/organ-specific agents (Figure 1).
Figure 1:
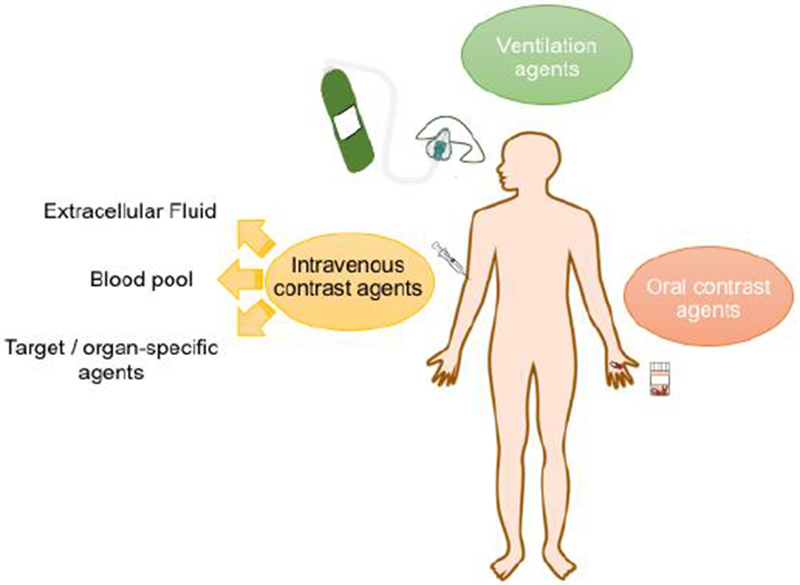
Route of administration of MRI contrast agents.
3.1.1. Intravenous MRI contrast agents
a) Extracellular fluid (ECF) agents are distributed between the intravascular and cellular space. ECF agents are low molecular weight chelates and after intravenous injection, they travel to the heart and then out to the systemic arteries, leaking into the extravascular, extracellular space. They are rapidly eliminated from the body through the renal pathway (see Figure 2). ECF agents are the most commonly used contrast agents and represent >98% of all contrast agents sold in the United States.13 They are generally employed to image arterial abnormalities and to detect altered tissue endothelium, e.g. disrupted blood brain barrier. ECF agents approved for clinical use all consist of a nine-coordinate Gd(III) ion chelated by an octadentate polyaminocarboxylate ligand with a water co-ligand (Figure 3). The ECF agents approved for human use are listed in Table 1. Like all drugs, contrast agents have a commercial name like “Dotarem®” and a generic name, in this case gadoterate meglumine. There is also a chemical abbreviation, and in this case Gd-DOTA or [Gd(DOTA)(H2O)]−. In the literature the reference to the commercial name can be confusing. In the past, the ECF agents were dominantly sold by a single supplier such that gadoterate meglumine = Dotarem. But now there are increasing numbers of generic products. For example GE Healthcare now sells its own version of gadoterate meglumine which is marketed as “Clariscan®” which has the same Gd-DOTA chelate that is used in Dotarem®. Clariscan was also the name given to an iron oxide nanoparticle formulation NC100150 that underwent clinical trials in the 1990s, but was never approved for commercial use. In general it is best to avoid the use of commercial names to avoid confusion over the compound involved and to avoid product promotion for a particular commercial entity.
Figure 2:
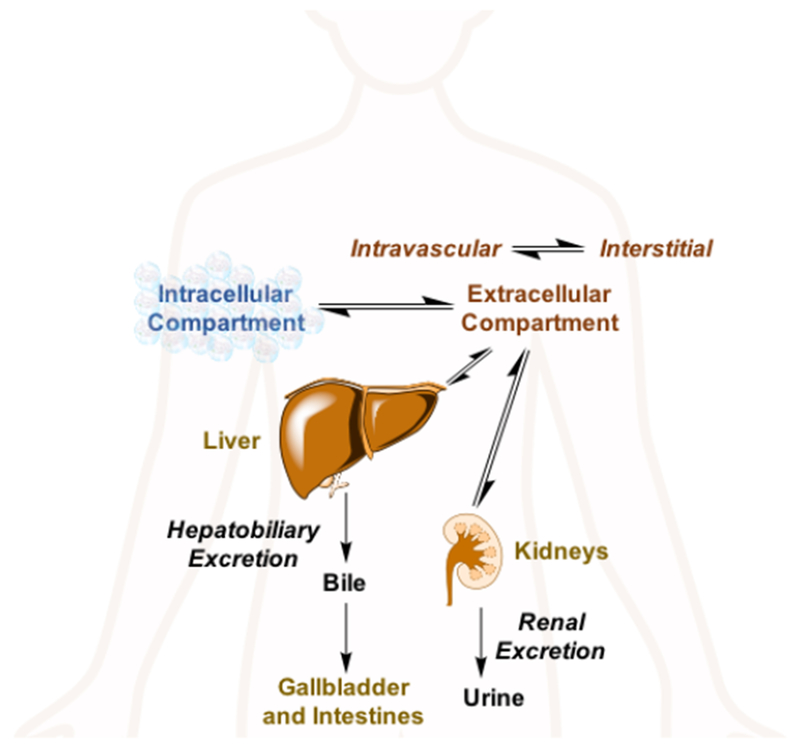
Main distribution sites and excretion pathways for intravenously administered soluble metal complexes.15
Figure 3:
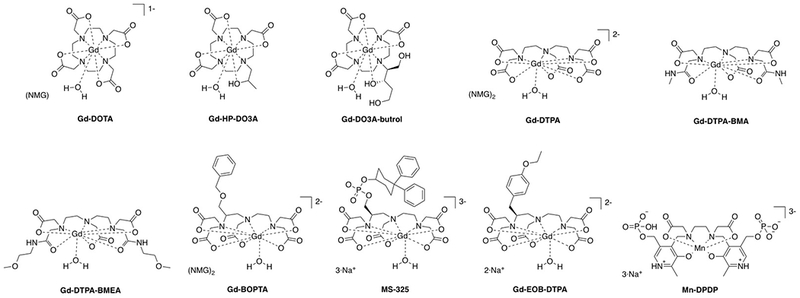
Commercially approved T1 contrast agents (NMG = meglumine).
Table 1:
ECF MRI contrast agents that have been used in the clinic.
| ECF agent (Trade name) | ECF agent (Chemical code) | ECF agent (Generic name) | Approval date |
|---|---|---|---|
| Dotarem, Clariscan | Gd-DOTA | gadoterate meglumine | 1989 (Europe) 2013 (United States) |
| ProHance | Gd-HPDO3A | gadoteridol | 1992 |
| Gadovist (Europe) Gadavist (United States) |
Gd-DO3A-butrol | gadobutrol | 1998 (Europe) 2011 (United States) |
| Magnevista | Gd-DTPA | gadopentate dimeglumine | 1988 |
| Omniscana | Gd-DTPA-BMA | gadodiamide | 1993 |
| Optimarka | Gd-DTPA-BMEA | gadoversetamide | 1999 |
| Multihanceb, c | Gd-BOPTA | gadobenate dimeglumine | 2004 |
agents suspended by the European Medicines Agency in 2017.
agent available for limited, liver-specific indications in the EU.
multipurpose agent that is also suitable for liver imaging.14
b) Blood pool agents are compounds that are, after injection, restricted to the intravascular space and which provide high contrast for imaging the arteries and veins. In the 1990s there were many blood pool agents under development. There were three main strategies employed, all of which resulted in compounds that underwent human clinical trials: (1) low molecular weight compounds that bind noncovalently to human serum albumin upon intravenous injection which restricts distribution to the intravascular space. An example is MS-325 (gadofosveset trisodium which had commercial names of Angiomark, Vasovist, and Ablavar) (Figure 3). MS-325 was 80-90% bound to HSA but the unbound fraction could be eliminated through the kidneys.16–21 MS-325 was approved for commercial use but is no longer commercially available. The compound B22956 (Figure 4) was another albumin-binding Gd-DTPA based agent that progressed to Phase 2 clinical trials.22–26 (2) Large discrete Gd(III)-based compounds that were too large to rapidly extravasate across the endothelial barrier, but still small enough to be filtered by the kidney. The dendrimer Gadomer (aka Gadomer-17) (Figure 4) was a 17 kDa dendrimer with 24 macrocyclic Gd(III)-chelates that advanced to Phase 2 clinical trials.27–30 Gadomer combined increased size with a high Gd(III) payload for high relaxivity. The compound P792 (Figure 4) was a single Gd-DOTA derivative the chelate at the barycenter and 4 large hydrophilic branches to increase the hydrodynamic radius.31–34 This compound was also used in Phase 2 clinical trials. (3) Small iron oxide nanoparticles that had a high T1 relaxivity. These compounds are truly intravascular and have long blood circulation times. The nanoparticles are not cleared from the body, rather they eventually accumulate in the liver and spleen and the iron is incorporated into the body’s pool of iron. Iron oxide particles that underwent Phase 2 and/or Phase 3 angiography clinical trials were NC100150,35–36 VSOP-C184,37–38 and AMI-227.39–43 None of these was ultimately approved for human use. The SPION ferumoxytol was approved for use as an intravenous iron replacement therapy in anemic patients. Ferumoxytol has excellent T1 relaxivity and has been used off-label at some institutions as a blood pool agent in humans. Off-label means that the compound is not being used for its approved packaging label (in this case to treat anemia).
Figure 4:
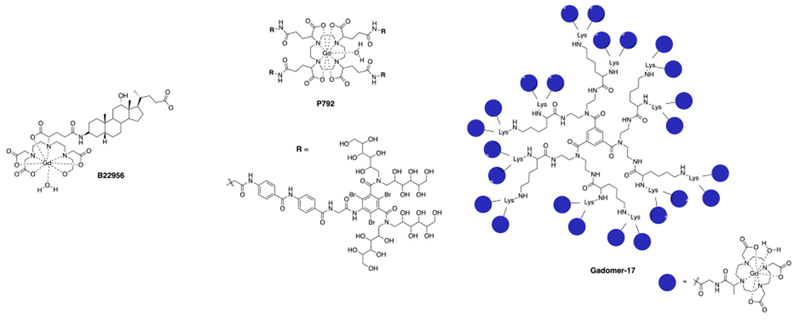
Chemical structures of the Gd(III)-based blood pool contrast agents (charges and counter ions are omitted for simplicity).
The lack of approved and commercially available blood pool agents is driven by market demand. In the 1990s when most of these agents were being developed, the MR scanner performance was insufficient to support dynamic contrast enhanced MR angiography (MRA). Dynamic MRA involves very rapid injection of a contrast agent and rapid imaging to get an image of the contrast agent as it traversed the systemic arteries after injection but before it arrived in the venous circulation. However power injectors (for reproducible rapid injection) and higher performing scanners with faster gradients (for faster imaging) enabled contrast enhanced MRA to be routinely performed with ECF agents, and there is now much lower demand for dedicated blood pool agents.
c) Organ-specific agents are capable of targeting specific organs or tissues, for example the liver, spleen, or lymph nodes. ECF agents are eliminated via the kidneys. Altering the elimination pathway to include the liver can enable liver-specific imaging. Table 3 lists examples of contrast agents that are approved for commercial use or were studied in human clinical trials. For instance, the approved MRI contrast agent gadoxetic acid is rapidly taken up by hepatocytes and can therefore be used to image the liver and biliary system.44 Gadobenate can also be used for liver imaging but its hepatocyte uptake is much lower than that of gadoxetic acid.45 A Mn(II)-based example is the agent mangafodipir (Mn-DPDP)46, that dissociates after injection to the free Mn(II) ion. The Mn(II) is then rapidly taken up by hepatocytes, cardiomyocytes, and pancreatic cells, and can be employed to image the liver, myocardium, or pancreas. Organ-specific agents that are based on iron oxide particles exhibit a different distribution. SPIONs are imported into the cells of the reticuloendothelial system through phagocytosis, which enables selective access to the liver, spleen, lymph nodes, tumor associated macrophages, and bone marrow.47 Depending on the SPION size and coating, different tissues can be targeted. For instance ferumoxide48 and ferucarbotran49 were both rapidly taken up by the Kuppfer cells in the liver and enabled liver-specific imaging. Ferumoxtran-10 has a longer circulating time and can be used for blood pool imaging, but with time also significantly accumulates in lymph nodes which enables cancer staging applications.50 None of the approved SPIONs, nor mangafodipir are commercially available anymore, and to the best of our knowledge clinical development has been discontinued.
Table 3:
Organ-specific MRI contrast agents that have been used clinically or received approval for clinical trials.
| Organ-specific agent (Trade name) | Organ-specific agent (Short name) | Organ-specific agent (Generic name) | Approval date | Applications |
|---|---|---|---|---|
|
Approved and commercially available: | ||||
| Primovist (Europe) Eovist (United States) |
Gd-EOB-DTPA | disodium gadoxetic acid | 2005 (Europe) 2008 (United States) |
liver |
| Multihancea | Gd-BOPTA | gadobenate dimeglumine | 1998 (Europe) 2004 (United States) |
liver |
|
Approved for use as an iron replacement therapy. Not approved, but used off-label for MRA | ||||
| Feraheme | ferumoxytol (USPIO) | 2009 (United States) 2013 (Europe) |
brain lesions, abdominal organs, lymph nodes, vascular walls | |
|
Agent withdrawn from one or all major markets: | ||||
| Teslascan | Mn-DPDP | mangafodipir trisodium | 1997 | liver, myocardium |
| Feridex (United States) Endorem (Europe) |
AMI-25 | ferumoxides (SPIO) | 1996 (United States) | liver |
| Resovist | SH U 555 A | ferucarbotran (SPIO) | 2001 (Europe) | liver |
|
Used in clinical trials but clinical development of the agents has been discontinued: | ||||
| Dy-DTPA-BMA | sprodiamde injection | - | myocardium, brain perfusions | |
| Sinerem/Combidex | AMI-227 | ferumoxtran-10 (USPIO) | - | metastatic lymph nodes, macrophage imaging |
Gd-BOPTA is predominantly used as an ECF agent but does provide some hepatobiliary enhancement and can be used for liver imaging
3.1.2. Oral contrast agents
Oral contrast agents are orally administered and are suitable for imaging the gastrointestinal tract (GI) imaging. Gd(III)- and Mn(II)-based agents, SPIOs, barium sulfate suspensions as well as fruit juice rich in manganese (e.g. blueberry pineapple juice) have been investigated as oral contrast agents.51–56 The following Table 4 summarizes the OCAs that are currently available for clinical application. These are not widely used.
Table 4:
OCAs that are approved for clinical application.
| OC agent (Trade name) | OC agent (Short name) | OC agent (Generic name) | Approval date |
|---|---|---|---|
| Lumirem/GastroMARK | AMI-121 | ferumoxsil (MPIO) | 1996 |
| Ferriseltz | - | ferric ammonium citrate | 1992 |
| LumenHance | - | manganese chloride | 1997 |
3.1.3. Ventilation agents
Ventilation agents are contrast agents that are inhaled to improve the diagnostic value of MRI for the lungs. Gadolinium(III)-based aerosols and oxygen gas are paramagnetic and after inhalation they can be used to estimate ventilation in the lungs by its effect on lung water T1. Direct detection is also used with hyperpolarized gases (3He, 129Xe) and with inert perfluorinated gases such as SF6.57
3.2. Classification of contrast agents: Biophysical mechanism of action
Contrast agents can also be classified in terms of their contrast generating mechanism of action. We have grouped contrast agents into four major classes (1) paramagnetic; (2) superparamagnetic; (3) chemical exchange saturation transfer (CEST); and (4) direct detection.
3.2.1. Paramagnetic contrast agents
Paramagnetic contrast agents are by far the most prominent MRI contrast agents. They are also called positive contrast agents because they increase MR signal in regions where they distribute.
Paramagnetic contrast agents comprise a metal ion that has unpaired electrons. Gd(III) with its half-filled f7 shell and high spin Mn(II)- and Fe(III)-complexes with their half-filled d5 shells all exhibit a large magnetic susceptibility compared to other metal ions. The symmetric S electronic ground state also endows these compounds with a relatively slow electronic relaxation rate which is necessary to promote strong nuclear relaxation. Of all the potential metal complexes that could be imagined, discrete Gd(III) chelates have been the most successful paramagnetic contrast agents so far and clearly dominate the contrast agents used in the clinic. We will start with a description of approved contrast agents as this will inform discussions of newer agents.
Clinically used Gd(III)-based contrast agents (GBCAs) all utilize an octadentate polyaminopolycarboxylato-based ligand and have a ninth coordination site available for water ligation. The coordinated water ligand can be rapidly exchanged with bulk water molecules. GBCAs that have been approved for clinical use are illustrated in Figure 3. As outlined above, some of these are no longer available for commercial.
To date, only two manganese-based contrast agents have ever been approved: Mn-DPDP (Figure 3) and an orally administered contrast agent that consists of liposomal encapsulated manganese chloride (LumenHance).58
Contrast agents containing Gd(III) shorten the observed longitudinal (T1) and the transverse (T2) relaxation time of water protons in their vicinity. The rate constants corresponding to the T1 or T2 relaxation times are defined as 1/T1 and 1/T2, respectively, which are generally referred to as relaxation rates. In this review, we will use the general term relaxation rate when referring to the rate constant of spin relaxation. On T1-weighted scans, tissue with short T1 appears bright and thus T1-shortening contrast agents generally make signal bright. On the contrary, T2-weighted scans show tissue with long T2 as being bright, so T2-shortening agents reduce signal. The extent to which a contrast agent can change the T1 or T2 of solvent water is termed relaxivity r1 or r2, respectively; this is sometimes referred to as longitudinal (r1) and transverse (r2) relaxivity. Relaxivity is defined by equation 1, where (1/Ti0) is the inherent relaxation rate of the tissue, (1/Ti) is the relaxation rate in the presence of contrast agent (ri) and [CA] is the concentration of the contrast agent.
| (1) |
Low molecular weight Gd(III) complexes have similar r1 and r2 values in water that have very little variation with field at magnetic fields generally used for MRI (Figure 5).
Figure 5:

Longitudinal (left) and transverse (right) relaxivities (mM−1s−1) of some commercial gadolinium(III)-based contrast agents at different magnetic fields (green = 0.47 T, red = 1.5 T, blue = 3 T) in human plasma at 37 ºC.59
The relaxation times of water hydrogen nuclei in pure water are very long. But in different tissues the relaxation times can be much shorter due to the interaction of water with macromolecules and also because of the presence of endogenous paramagnetic species such as ferritin.60 In the body, water T2 is generally 5 – 20 times shorter than T1. As a result, the effect of a Gd(III) contrast agent will be much more pronounced on T1. This is illustrated in Figure 6 where the endogenous relaxation rates of grey matter at 1.5 T are plotted (solid). Addition of 1 mM Gd-DOTA (r1 = 3.6 mM−1s−1, r2 = 4.3 mM−1s−1) will increase the 1/T1 and 1/T2 by 3.6 and 4.3 s−1, respectively. Because of the low baseline T1 relaxation rate, the effect of the contrast agent on 1/T1 is much larger (+ 428 %) than on 1/T2 (+ 41 %). Because of this effect, Gd(III) based agents are often referred to as T1-contrast agents because on a percentage basis they have a much larger effect on T1.
Figure 6:
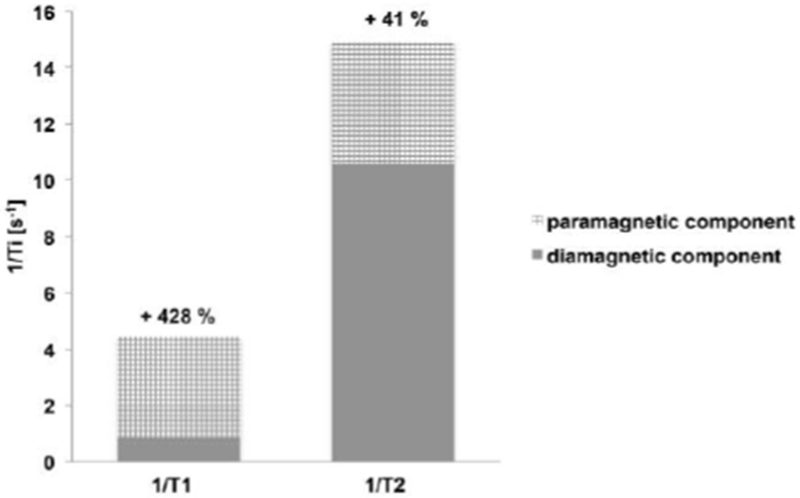
Paramagnetic contribution of 1 mM of T1 contrast agent Gd-DOTA on 1/T1 and 1/T2 in cortical grey matter at 1.5 T.
Figure 7 is an exemplary T1 weighted image of the brain of a patient with glioblastoma and shows the effect of the T1 contrast agent gadoteridol at 3 T. Twenty minutes after injection of gadoteridol, the tumor becomes strongly and positively enhanced (image on right) in comparison to the T1 weighted image acquired before injection (left).61
Figure 7:
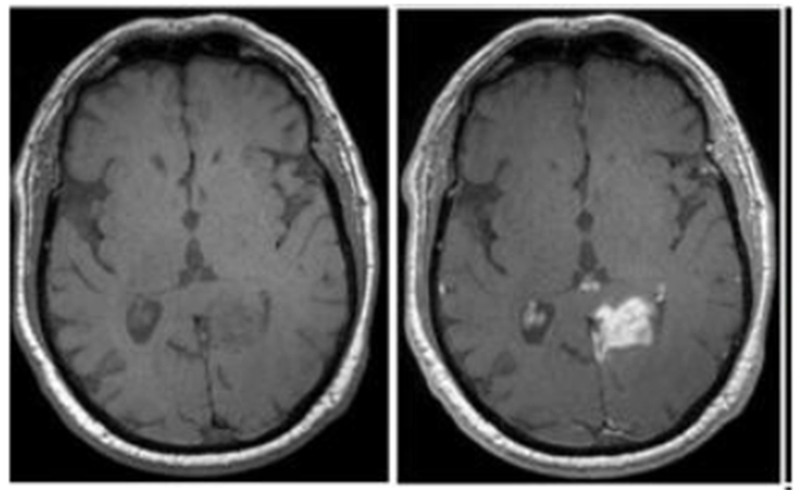
Axial T1-weighted MR images obtained at 3 T before (left) and 20 minutes after intravenous gadoteridol administration (right) of patient with glioblastoma. Reproduced with permission of Ref.61 (URL: https://pubs.rsna.org/doi/abs/10.1148/radiol.12111472). Copyright 2013 Radiological Society of North America (RSNA®).
3.2.2. Superparamagnetic contrast agents
Superparamagnetic contrast agents are colloidal materials made up of particles (~ 5-200 nm in diameter) in suspension. These particles are composed of small crystallites (1-10 nm) that contain thousands of magnetic ions, usually iron, that are randomly oriented. In the presence of an external magnetic field, the crystallites align with the field, leading to a superspin which renders the material magnetic. The total spin of the particle is much larger than the sum of the individual metal ion spins which can result in a very high relaxivity. In the absence of the applied field, the material is no longer magnetic. The crystallites are made of a core of nonstoichiometric metal (usually iron) oxides covered by a coating, such as dextran, citrate, oleate, or other nonimmunogenic polymers in order to avoid aggregation and reduce their toxicity.62–66 The first superparamagnetic contrast agents had very high r2/r1 ratios and predominantly affected T2. As a result they were referred to as T2-agents or negative contrast agents because they provide darkened MR images. However there are many examples of superparamagnetic iron oxide particles that also have a large r1 and can serve as effective T1 or T2 contrast agents.
Superparamagnetic iron oxides can be classified in three groups according to the size of the particulate: (1) Ultra-Small Superparamagnetic Iron Oxide (USPIO) particles with a diameter of d < 50 nm; (2) Small Superparamagnetic Iron Oxide (SPIO) particles with a diameter of 1 μm > d > 50 nm; and (3) Micron-sized Particles of Iron Oxide (MPIO) with a diameter in excess of a micron.67 Iron oxides that have been approved for clinical use or that have undergone human clinical trials are listed in Table 2 and 3 above. These agents are also referred to generically as Superparamagnetic Iron Oxide Nanoparticles (SPION).
Table 2:
Examples of blood pool contrast agents that have been used in human clinical trials.
| Blood pool agent (Trade name) | Blood pool agent (Chemical code) | Blood pool agent (Generic name) | Approval date |
|---|---|---|---|
|
Approved for use as an iron replacement therapy. Not approved, but used off-label for MRA | |||
| Feraheme | ferumoxytol (USPIO) | 2009 (United States) 2013 (Europe) |
|
| Approved for MRA but no longer commercially available: | |||
| Ablavar (formerly: Vasovist, Angiomark) | MS-325 | gadofosveset trisodium | 2005 (Europe) 2008 (United States) |
|
Used in clinical trials but clinical development of the agents has been discontinued: | |||
| B22956 | Gadocoletic acid | - | |
| Gadomer | SH L 643A | Gadomer-17 | - |
| Vistarem | P792 | Gadomelitol | - |
| Clariscan | NC-100150 | PEG-feron (USPIO) | - |
| VSOP-C184 | - | ||
| Sinerem/Combidex | AMI-227 | ferumoxtran-10 (USPIO) | - |
Figure 8 shows the effect of a T2 agent on tissue relaxation times. Although the contrast agent shortens T1, the effect on T2 is very large. The large magnetic moment of superparamagnetic relaxation agents also generates a local magnetic inhomogeneity and shortens the T2* of tissue which creates signal loss on gradient echo T2* weighted images.
Figure 8:
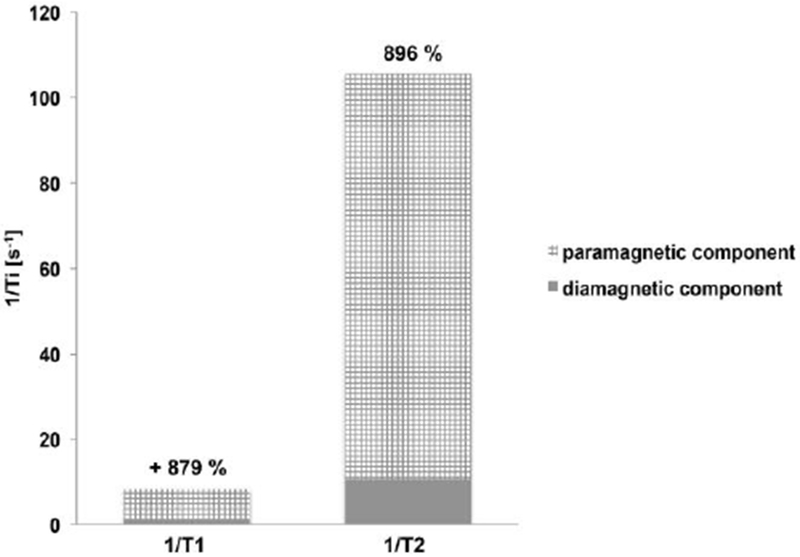
Paramagnetic contribution of 1 mM of T2 contrast agent ferucarbotran on 1/T1 and 1/T2 in cortical grey matter at 1.5 T.
Exemplary images from a patient with primary central nervous system (CNS) lymphoma are shown in Figure 9, before and 24 hours after intravenous injection of ferumoxytol.68 T2-weighted images obtained before ferumoxytol show regions of hyperintensity due to tumor associated edema. After 24 hours, ferumoxytol has accumulated in the tumors and several areas of deep white matter lesions are enhanced through strong signal loss due to ferumoxytol uptake in these regions.
Figure 9:
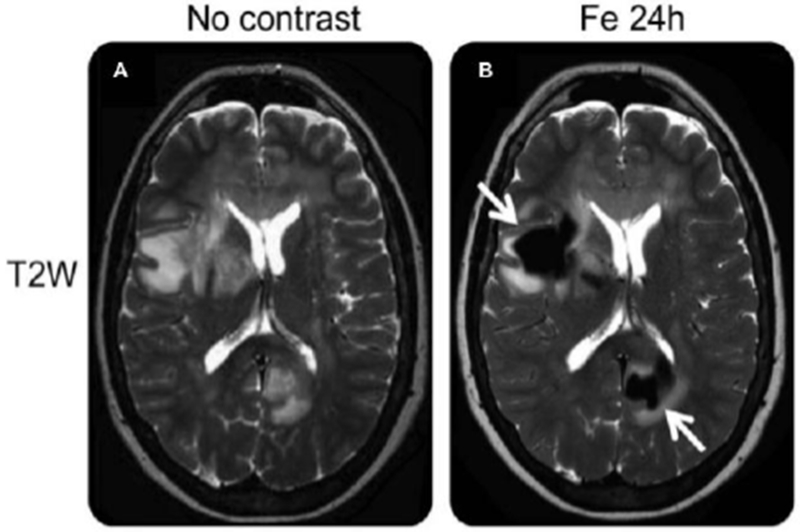
T2-weighted images obtained before and 24 hours after intravenous ferumoxytol administration show deep white matter lesions, demonstrated through several areas of confluent, focal, strong signal loss due to ferumoxytol uptake (arrows). Reproduced with permission of Ref.68 (URL: http://n.neurology.org/content/neurology/81/3/256). Copyright 2013 Wolters Kluwer Health, Inc.
3.2.3. Chemical Exchange Saturation Transfer (CEST) Agents
CEST agents are molecules that possess exchangeable protons, such as NH, OH, or exchangeable water molecules, that resonate at a chemical shift that is different from the bulk water signal. In a CEST experiment, the exchangeable protons of the CEST agent are irradiated with an off-resonance saturation pulse. Their magnetization (i.e. spin polarization) is transferred to the protons of the bulk water pool by exchange between both pools (saturated proton pool and bulk water proton pool), which leads to a reduced intensity of the water signal. This in turn generates MRI contrast.
For a CEST agent to be effective there needs to be good frequency separation between the exchangeable protons and the bulk water resonances, especially since the bulk water resonance is broadened in vivo because of local magnetic field inhomogeneities.69 To overcome this problem, the chemical shift difference needs to be maximized. This can be achieved by either increasing the magnetic field strength (higher field strengths for the MR experiment) or by using paramagnetic CEST (ParaCEST) agents. Typically, ParaCEST agents contain paramagnetic ions to shift the signal of the labile proton tens to hundreds of ppm away from the bulk water resonance.
Some CEST contrast agents are known molecules that are already approved for intravenous administration. Examples include glucose (exchangeable OH) and the X-ray contrast agent Iopamidol (exchangeable NH) (Figure 10), that are currently being used in human clinical trials.70–72
Figure 10:
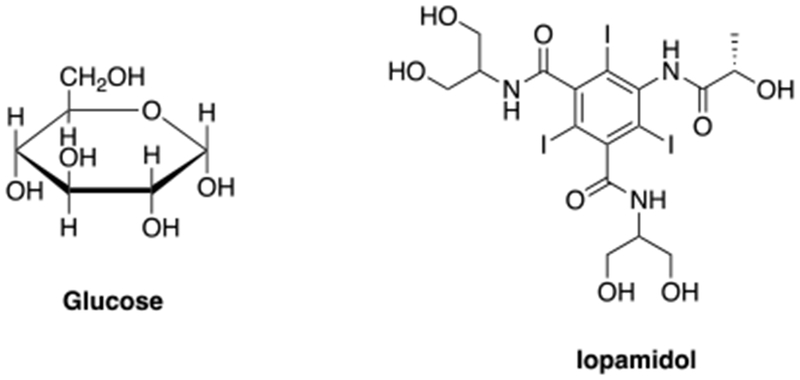
CEST agents that are approved for use in clinical trials: glucose and iopamidol.70–72
3.2.4. Direct detection agents
MRI is an insensitive technique. To overcome this low sensitivity clinical MRI detects hydrogen, the most sensitive NMR nucleus, and primarily detects water because of its very high (10s of molar) concentration in tissue. Other proton containing molecules or heteronuclei can be detected, such as 13C, 23Na, 31P, and 19F, but very high concentrations are usually required. Nonetheless there are numerous examples of direct detection agents. The T1-, T2-, and CEST agents described above are contrast agents that change the local MR water signal and create image contrast. These agents are not directly detected, but are seen via their effect on the bulk water signal. Direct detection agents do not generate contrast and in general have little to no background signal, but do suffer from poor sensitivity.
To overcome the sensitivity problem nanoparticle containing perfluorocarbon (PFCs) emulsions, providing huge payloads of 19F atoms, have been used for direct detection in 19F MRI.73–74 These perfluorocarbons are the most biologically inert organic xenobiotics and usually, even at high doses, their toxicity is very low.75 Selected examples of PCFs are illustrated in Figure 11.
Figure 11:
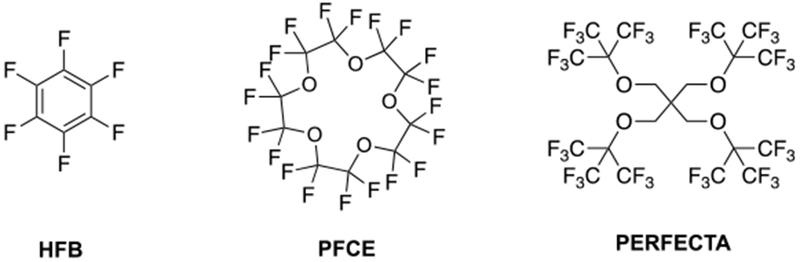
Chemical structures of some PFCs: left, hexafluorobenzene (HFB); center, perfluoro-15-crown-5-ether (PFCE); right, tetra(perfluorotertbutyl)pentaerythritol (PERFECTA).
To overcome the low water solubility of traditional PFCs, a new generation of hydrophilic fluorinated molecules has emerged. Annapragada and coworkers developed stable liposomal formulations of hydrophilic fluorinated molecules that allow simultaneous imaging of multiple targets (Figure 12).76
Figure 12:
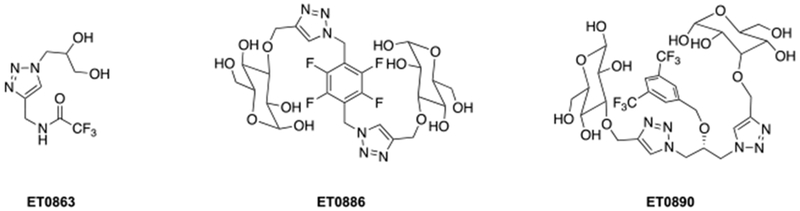
Examples of hydrophilic fluorinated molecules studied by Annapragada and coworkers.76
The natural abundance of 19F (100%), its spin (1/2), its high gyromagnetic ratio (40.08 MHz/T, 1H: 42.58 MHz/T), and sensitivity (83% of 1H) makes 19F a promising candidate for direct detection imaging. In addition, the chemical shifts of 19F containing compounds vary over a broad range (>350 ppm) and the in vivo background signal is very low due to the natural abundance of the element in the human body. In addition, the concentration of agent can be measured directly from the intensity of the signal. While not widely available, it is possible to modify MRI hardware to directly detect both 1H and 19F, and in this way the visualization of the 19F agent can be localized to the anatomic 1H (water) image.
It is also possible to directly detect the 1H resonance of a direct detect agent, but because of the narrow chemical shift range of 1H, there is usually a significant background signal from metabolites. A further limitation of direct detection agents is the long T1 of the nucleus being detected. After excitation one has to wait until the T1 magnetization is sufficiently recovered before applying a second excitation pulse. The acquisition time window in vivo is limited because the agent may be washing out of tissue with time, and there is a practical limit of keeping the subject in the scanner. If the T1 was very short, then excitation pulses could be acquired continuously allowing for signal averaging. Aime et al. showed over 20 years ago that a paramagnetic complex exhibiting a large chemical shift could overcome these problems to some extent.77 Yb-DOTMA has 4 equivalent CH3 groups (12 H per molecule) that are well shifted outside the diamagnetic window. Moreover the Yb(III) ion causes efficient T1 relaxation of the CH3 protons but does not result in severe linebroadening. The chemical shift of the CH3 group is very sensitive to temperature and several lanthanide complexes of DOTMA have been used in MR thermometry applications.78–79 Yang et al. took a different approach and encapsulated sodium 3-(trimethylsilyl)-1-propanesulfonate (DSS) at a high concentration inside liposomes.80 DSS has a zero ppm chemical shift, outside the range of metabolites. A small amount of Gd-HPDO3A was included to shorten the T1 of the DSS protons. They showed that liposomes could be directly detected at concentrations as low as 50 pM.
Parker and coworkers used a rational, systematic approach to design what they termed ParaSHIFT agents (Figure 13). They first designed compounds with a large number of equivalent fluorine atoms and incorporated a lanthanide ion at an optimal distance to the fluorine to optimize T1 shortening. In this way, they showed that the sensitivity could be increased by a factor of 25 compared to the diamagnetic compound. More recently they have extended this approach to lanthanide complexes with a shifted proton signal (e.g. from a t-butyl group with 9 equivalent protons).81–82
Figure 13:
Examples of fluorinated compounds studied by Parker and coworkers in order to boost the 19F MRI – SNR.83–84
The insensitivity of NMR arises from the low energy difference between the ground and excited state which results in a large population of spins in the excited state at thermal equilibrium. If the nuclear spins could be polarized far beyond thermal equilibrium conditions, then the sensitivity could be increased by several orders of magnitude. The polarization of spins is enhanced by a non-equilibrium distribution of nuclear spins called the hyperpolarized state.85 This technique requires nuclei with long T1 values such that relaxation does not occur in the time the molecule is hyperpolarized to when the image is acquired, and includes nuclei such as 3He, 129Xe, 13C, 15N, 6Li.86
Initially hyperpolarization was applied to gases like 3He and 129Xe which have very long T1 values. These gases are used clinically at a number of sites to image the lung architecture after inhalation of the gas.87–90 In the last decade 13C hyperpolarization has been widely employed and there is now a commercial apparatus available to hyperpolarized 13C for human applications. For example, hyperpolarized [1-13C] pyruvate was used by Nelson et al. in patients with prostate cancer.9 Pyruvate is metabolized to lactate, alanine, and bicarbonate in a matter of seconds, and each of these can be visualized and quantified because of the chemical shift differences between these metabolites. The ratio of pyruvate metabolites can be used to grade tumor aggressiveness and monitor treatment response.
The most widely used method for hyperpolarization is dynamic nuclear polarization (DNP).91 In this method, a stable organic radical, usually trityl, is frozen along with the compound to be hyperpolarized. This sample is placed inside a DNP polarizer, comprising a superconducting magnet (B0 = 3 to 5 T) and a liquid helium cooled sample space (maintained cold at 1 to 5 K). Under these conditions the radical’s unpaired electrons are aligned with the external magnetic field, which leads to a hyperpolarized state, and this electron hyperpolarization is transferred to the 13C nucleus using microwave radiation close to the MR frequency of the electron spin. Before the hyperpolarized sample can be used in vivo, it needs to be rapidly dissolved in an appropriate solvent, heated to physiological temperature, and separated from the radical. Generally a superheated solvent at 180 ºC and 10 bar is used.92
Another frequently used method for polarization is the parahydrogen-induced polarization (PHIP)93 that induces hyperpolarization through the reaction of parahydrogen (its two proton spins are aligned antiparallel) with an unsaturated substrate containing double or triple bonds.91 Typically, parahydrogen is introduced through catalytic hydrogenation to the unsaturated compound. Subsequently, the nuclear polarization of a vicinal 13C nucleus is achieved by converting the nonequilibrium spin polarization of parahydrogen by diabatic-adiabatic magnetic field cycling or by radiofrequency pulses.94
A third and related approach is signal amplification by reversible exchange (SABRE). SABRE involves the transient coordination of a substrate molecule (usually containing a coordinating atom like a pyridyl N) to an organometallic catalyst. Parahydrogen (H2) also coordinates the catalyst and then transfers its polarization to the substrate in a catalytic manner. Unlike in PHIP, the substrate molecule is unchanged.95–96
3.3. Biophysics of magnetic resonance imaging
There are many ways to achieve contrast in a MR image without giving an exogenous contrast agent.3, 97–99 Chemical shift difference can provide contrast between mobile lipids and water. The density of water protons in tissue also provides contrast (low in lung and bone, higher in soft tissue and fluids). There are a number of contrast mechanisms that exploit differences in the properties of water in different tissues. For instance the relaxation times T1, T2, T1ρ (T1 in the rotating frame) can be very different in adjacent tissues and images can be acquired using pulse sequences that are weighted to provide more (or less) signal intensity for a short relaxation time. Contrast can also be generated from physicochemical properties of water such as diffusion and flow. Magnetization transfer from the solid-like macromolecules to bulk water is another mechanism, as is saturation transfer (discussed in CEST contrast). Exogenous contrast agents can further exploit these mechanisms by amplifying the effect. The majority of contrast agents serve to shorten the relaxation times of water, while CEST agents exploit a saturation transfer mechanism. In this section we describe the mechanisms by which contrast agents affect relaxation times or saturation transfer.
3.3.1. Relaxation agents
Relaxation agents are primarily described by their “relaxivity” (r1 or r2), that is defined as the extent to which the contrast agent can modify the relaxation rate of tissue water (1/T1 or 1/T2) The conventional units for relaxivity are mM−1s−1 (per millimolar per second, and sometimes L·mol−1s−1). Sometimes, the relaxation rate enhancement of the contrast agent is referred to as 1/Tip (i = 1,2) as the paramagnetic contribution to the relaxation rate.
Figure 14 shows the effect of a typical ECF agent on the relaxation rate (a) and on the relaxation time (b) of three different tissues: heart (T10 = 1200 ms), liver (T10 = 590 ms), and subcutaneous fat (T10 = 340 ms).100 Panel (a) shows that increasing the contrast agent concentration increases the tissue relaxation rate in the same linear fashion for all three tissues, although the intercept differs among the tissue types. The slope of the lines in panel (a) is the relaxivity, r1. Panel (b) shows the same data expressed as relaxation times. At lower concentrations (left part of the graph), the amount of contrast agent has a higher effect on tissue with longer T1, whereas at higher concentrations of contrast agent (right part of the graph), all tissues approach a similar relaxation time. Thus the detection sensitivity of the contrast agent will be greater in tissues with long relaxation times.
Figure 14:
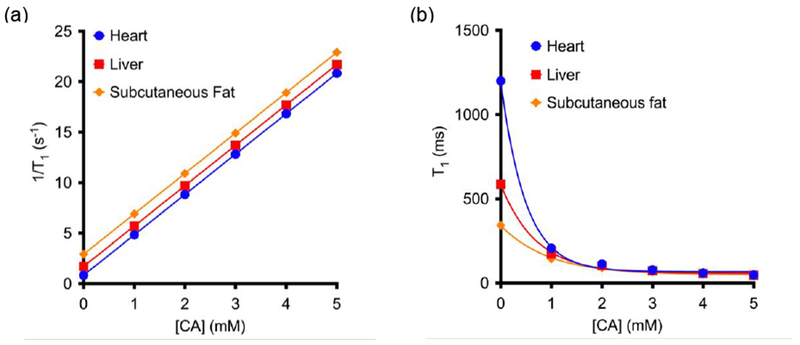
Change in the longitudinal relaxation time (a) and in the relaxivity (b) as a function of the concentration of contrast agent in three different tissues: heart (T10 = 1200 ms), liver (T10 = 590 ms) and subcutaneous fat (T10 = 340 ms); supposing in every case r1 = 4 mM−1s−1.
The relaxation rate of the tissue (1/T10) varies with tissue type, usually in the range 0.5 – 2 s−1 for most tissues and tumors, and increases with magnetic field strength.60, 101 In designing experiments and new contrast agents it is important to consider both the relaxivity of the agent and the inherent relaxation rate of the tissue, since both are involved in determining the signal change observed. Figure 15 illustrates this point using relaxivity data for Gd-DOTA59 and MS-32515 measured in human plasma and compared to brain grey matter (GM) and white matter (WM) T1 values measured at 1.5 and 4.7 T.60 The relaxation times of GM and WM increase by about 50% with increasing field while Gd-DOTA relaxivity only decreases by about 10%. As a result, the percent change in brain tissue relaxation rate that one would observe for a given Gd-DOTA concentration is actually higher at 4.7 T, despite its lower relaxivity at this field strength. Even for MS-325 where the relaxivity at 1.5 T is more than double that at 4.7 T, the change in brain tissue relaxation rate for a 0.3 mM MS-325 concentration is only about 40% higher at 1.5 T.
Figure 15:
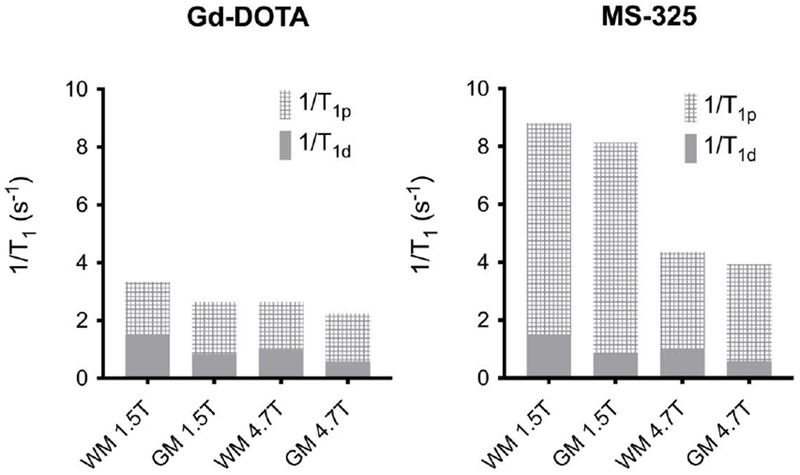
Effect of field strength on relaxivity and contrast in the case of Gd-DOTA (left) and MS-325 (right) in white matter (WM) and grey matter (GM).
In the case of transverse relaxation, the T2 of tissues is generally very short and usually decreases with increasing field strength. Thus much higher concentrations of contrast agent and/or much higher relaxivities are required to measurably affect T2.102
T1-contrast agents
Brownian motion of a paramagnetic complex or particle generates a fluctuating magnetic field that induces relaxation in nearby water molecules. In order to transmit the relaxation effect to the bulk, the exchange between the water interacting with the metal ion and the bulk needs to be fast (>106 s−1). Paramagnetic induced T1 relaxation directly depends on the spin quantum number (as a function of S(S+1)) and inversely on the distance (rMH) between the metal ion and the proton of the water as a function of 1/rMH6. Gadolinium(III) with S = 7/2 and high spin manganese(II) or iron(III) with S = 5/2 have been the most widely used as contrast agents. The shortest rMH distance can be achieved through a direct bond between the metal ion and the water molecule, thus occupying a coordination position in the inner-coordination sphere of the metal ion. The exchange rate of this water molecule must be fast (> 106 s−1) in order to maximize the transfer of the relaxation effect to the bulk water. The absence of ligand field stabilization energy of Gd(III) and Mn(II) makes their complexes quite labile, thus favoring the fast chemical exchange of the bound water molecule. These ions also have a symmetric S electronic ground state which results in electronic relaxation typically in the nanosecond timescale, and this allows an efficient nuclear T1 relaxation.
From a chemical standpoint, three contributions to relaxivity can be considered:103
Inner-sphere relaxation where a water ligand directly bound to the metal is relaxed and transmits the relaxation effect to the bulk water through exchange with another water molecule.
Second-sphere relaxation where hydrogen bonded water molecules in the second coordination sphere, or exchangeable hydrogen atoms (such as O-H, N-H) undergo relaxation and exchange.
Outer-sphere relaxation, where water molecules that diffuse close to the paramagnetic compound can also be relaxed.
Because of the difficulty to experimentally distinguish water molecules in the second-sphere from outer-sphere water, these two groups are often referred to as outer-sphere water molecules. Relaxivity can be factored into these two components of inner-sphere r1IS and outer-sphere (r1OS relaxivity (equation 2):
| (2) |
It should be noted that r1OS can represent a major component of the observed relaxivity and is about 40% of the relaxivity of approved ECF agents.
Inner-sphere relaxivity
For water ligands in the inner-sphere, relaxivity depends on the extrinsic parameters applied magnetic field strength and temperature, and on intrinsic molecular factors including: the number of water molecules in the inner-coordination sphere, q; the kinetics of water exchange kex = 1/τm where τm is the mean residency time of the water ligand; the rotational dynamics of the molecule, described by a rotational correlation time τR; the electron spin S of the complex; and the electronic relaxation times T1e and T2e, which are sometimes referred to as τS. Figure 16 summarizes these molecular factors along with outer-sphere relaxation that is described by the translational diffusion correlation time τD and a distance of closest approach a (Figure 16).
Figure 16:
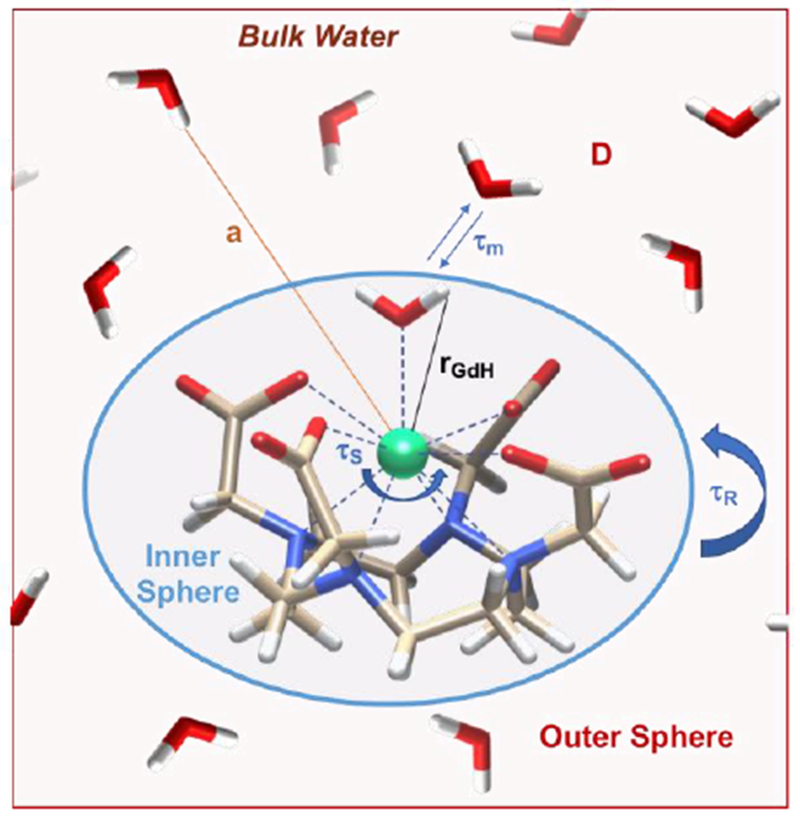
Pictorial description of the parameters that influence the relaxivity of a MRI contrast agent.
Combining the definition of relaxivity with the Bloch equations for a system in fast two site exchange, where one pool (bulk water) is present at much higher concentration than the other pool (metal bound water), one arrives at equations 3 and 4 which describe inner-sphere relaxivity. Here Tim is the Ti (i = 1 or 2) of the coordinated water molecule and is described by the Solomon-Bloembergen-Morgan equations of paramagnetic relaxation theory,104–108 equations 5 and 6. Here rMH is the metal ion to water hydrogen distance, γH is the proton magnetogyric ratio, g is the electronic g factor, μB is the Bohr magneton, µ0 is the vacuum permeability, and ωS and ωH are the Larmor frequencies of the electron and proton, respectively. There is also a correlation time for the magnetic fluctuation (τc) that is the shorter of the rotational correlation time (τR), the electronic relaxation time (T1e or T2e), or the water residency time (τm) defined by equation 7. The T1 effect is dipolar and 1/T1m depends on the electronic spin state of the complex, indicating that larger S values give greater relaxation. It depends on both the nuclear and electron magnetogyric ratios indicating that relaxation is greater for H than for other nuclei with lower γ values.109 The T2 effect is contributed by both dipolar (through space) and scalar (contact, through bonds) interactions. The scalar contribution depends on the hyperfine coupling constant between the metal ion and the water proton and a correlation time τsc for that interaction, equation 8. The scalar contribution to proton relaxation is small for Gd(III) complexes but can be appreciable for Mn(II) and Fe(III).
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
Because the electron Larmor frequency is much higher than the proton frequency, the term ωsτc2 becomes very large by about 0.1 T and thus the “7” term in equation 5 becomes negligible at applied fields above 0.1 T and reduces to equation 9 where C represents the physical constants in equation 5. For simple metal complexes, T1m is always large (several microseconds) compared with the mean residency time of the metal-bound water molecule (submicrosecond at 37 °C for most Gd(III) and Mn(II) complexes, so T1m >>τm). For such complexes, the dominant correlation time at most imaging fields (B0 > 0.2T) will be the rotational correlation time. In these cases, inner-sphere relaxivity can be approximated to equation 10 and depends only q and the rotational correlation time.
In these complexes, the relaxivity is almost always limited by the fast rotation of the complex (short τR, ~ 50 – 200 ps at 37 ºC).110–111 The relaxivity could be increased by slowing down its rotation (longer τR) by enhancing the molecular weight of the paramagnetic compound. For instance, this can be accomplished by immobilizing the complex, covalently or non-covalently, on a macromolecule.
The fluctuating field can also be caused by the water molecules that are in contact with the metal complex, but this is only observed when the rotational correlation time is very long and water exchange is extremely fast.112
Electronic relaxation
At very low fields (< 0.1 T), electronic relaxation is the dominant correlation time for Gd(III) and Mn(II) complexes. The influence of the electronic relaxation on the relaxivity of paramagnetic complexes depends mainly on the decay of the electron spin magnetization in the direction parallel to the external magnetic field (T1e). This decay is too fast in order to be directly measured, but the transverse electronic relaxation (T2e) can be estimated by EPR. For high spin ions like Gd(III) and Mn(II), the main relaxation mechanism is a transient zero-field splitting (ZFS). ZFS describes interelectronic interactions in paramagnetic compounds with two or more unpaired electrons. The modulation of the zero-field splitting depends on the perturbation of the ligand field through rotation, vibration and other motions. This splitting is the result of inter-electronic repulsion, spin-orbit coupling and the action of the ligand field on the unpaired electrons of the paramagnetic metal center. The spin Hamiltonian formalism can be written as follows (equation 11):113
| (11) |
where D and E describe the magnitude of the operator parallel and perpendicular to the z axis. This perturbation lifts the 2S+1 degeneracy at zero field, giving S + 1/2 (for half-integer S) doubly degenerated zero-field spin states or S + 1 (for integer S) zero-field spin states where one of them is non-degenerated (since mS = 0), that can be written as linear combinations of the Zeeman mS states. If an external magnetic field (B0) is applied, the doubly degenerated levels will be lifted. In the case of high spin ions in a symmetrical environment, such as the aqua ions of Cr(III), Fe(III), Mn(II), Gd(III) or Eu(II), the zero-field splitting is averaged out. An extension of this reasoning can be applied for Gd(III) and Eu(II) complexes with polyaminocarboxylates, where f electrons are mostly shielded from the ligand field and result in a small, but non-zero ZFS.
Although Gd(III) and Mn(II) complexes have non-zero ZFS, the effect of this static ZFS can usually be ignored at most field strengths where the Zeeman energy is much larger than the ZFS energy. However NMRD relaxometers can operate at very low proton Larmor frequencies, e.g. 10 kHz, and there the Zeeman energy may be lower than the ZFS. In such instances the widely used SBM relaxation theory does not apply.114–117 As alternatives to Gd(III) are increasingly proposed it is important to consider the magnitude of the ZFS of those complexes. If the ZFS is much larger than the Zeeman energy, then SBM theory may no longer apply.
In addition to this static ZFS, solvent collisions can induce distortions in the molecule resulting in a transient ZFS, and this transient ZFS can serve as an efficient relaxation mechanism. The transient ZFS broadens the line in EPR spectra.118 The electronic relaxation rates are given by equations 12 and 13,119 where τv is a correlation time that describes the modulation of the transient ZFS, and Δ2 is the square of the trace of the ZFS tensor.
| (12) |
| (13) |
Thus 1/T1e decreases with the square of increasing applied field. As the field is increased, eventually 1/T1e < 1/τR and rotation dominates the overall correlation time. For instance, at 0.02 MHz, the relaxivity of Gd-DOTA is r10.02MHz = 11.3 mM−1s−1 compared to 7.7 mM−1s−1 for Gd-DTPA at 25 °C, owing to the much longer T1e of Gd-DOTA. But, at 20 MHz, the relaxivity of these two complexes is almost identical: r120MHz = 4.7 mM−1s−1 for Gd-DOTA and 4.8 mM−1s−1 for Gd-DTPA.120 Complexes with higher symmetry will have smaller ZFS energies and thus, longer electronic relaxation times. For example, the axial ZFS parameter D is 24 mT for Gd-DOTA but 56 mT for Gd-DTPA.107. Intramolecular electronic relaxation between several Gd(III) ions can shorten T1e if the metals are very close ( << 10 Å).121–122 However this effect is only manifest on relaxivity at low fields. In the design of novel optimized MRI contrast agents, electronic relaxation is usually not taken into account, since its contribution to relaxivity is negligible at the magnetic field strengths that are typically used in clinic (≥1.5 T).
The metal-water hydrogen distance (rMH)
Because this distance enters as the inverse sixth power to the relaxation rate, small changes in rMH could have a profound effect on relaxivity, especially if this distance could be tuned. Caravan, Raitsimring and coworkers showed that the Gd-H(water) distance could be determined on frozen solutions using high field proton electron nuclear double resonance (1H ENDOR) spectroscopy.121, 123–128 They investigated a range of q=1 and q=2 complexes with different water exchange kinetics and found that rGdH was consistently 3.05 Å distributed within a range of ±0.07 Å. That is, there is a normal distribution of Gd-H distances ranging from 2.98 – 3.12 Å with the average distance being 3.05 Å (note that the initial paper gave a distance of 3.1Å obtained at a lower field; this was refined by measurements at very high field).121, 127 They compared the DTPA derivatives Gd-DTPA, Gd-BOPTA, and MS-325 and found that the ENDOR spectra were superimposable indicating a common rGdH, although earlier reports using indirect measures of rGdH had proposed differences in rGdH among these complexes.129–130 They measured Gd-DOTA, Gd-DOTMA, Gd-HPDO3A, and two Gd-DOTAla derivatives and again found rGdH = 3.05 Å and no difference in the ENDOR spectra. They also measured 3 different q=2 complexes, and there too observed the same rGdH value, see Figure 17.
Figure 17:
Gadolinium(III) complexes studied by 1H ENDOR where the Gd-H(water) distance was determined.
Number of water molecules (q)
Inner-sphere relaxivity is directly proportional to the number of water ligands (see equations 3 and 4). Increasing the hydration number (q) should increase the inner-sphere relaxivity, but an increase in q is often accomplished at the cost of decreased thermodynamic stability and/or kinetic inertness.131 A second consideration is that two cis coordinated water ligands can often be displaced by a coordinating anion in vivo. Thus it is important to know the hydration state of the complex in solution. Unlike certain other metal ions, the coordination number of Gd(III) and high spin Mn(II) varies and may not be easily predicted. Gd(III) complexes in aqueous solution are often CN9 but many CN8 and even CN7 and CN10 examples exist.132–133 Mn(II) complexes in aqueous solution tend to be CN6 and CN7.
There are several physical methods that allow estimation of the number of water ligands coordinated to Gd(III). There are surrogate approaches where Gd(III) is replaced by another Ln(III) similar in ionic radius. For instance the induced paramagnetic shift of H217O is proportional to q under fast exchange conditions. Geraldes and Peters and coworkers showed that the H217O shift induced by the Dy(III) complex could be measured and compared to [Dy(H2O)8]3+ to estimate q for the new complex.134 However, this method requires large amounts of sample (>10 mM).
Another method is based on luminescence lifetime measurements of the corresponding Eu(III) or Tb(III) analogs measured in both H2O and D2O. Coordinated H2O quenches the emission of Eu(III) or Tb(III) more efficiently than D2O. The reason for this lies in the different energy levels of the vibrational overtones of the O-H and O-D oscillators. The vibrational overtones of O-D are lower in energy in comparison to an O-H oscillator, which makes the energy transfer from Eu(III) or Tb(III) excited states into the O-D oscillators higher harmonics less likely. Horrocks and Sudnick showed that a simple empirical equation could be used to relate these decay rate constants to q,135 and some refinements on this approach have been reported.136–137 A benefit of this approach is that low µM concentrations can be used and the method is also generally useful for estimating q in complex matrices such as biological samples.
Another more direct method is electron-nuclear double resonance (ENDOR) spectroscopy of the Gd(III) complex.126 Raitsimring et al. used high field pulsed 17O ENDOR and showed that the presence of coordinated water ligands was associated with two narrow lines with a splitting of about 1.33 MHz originating from the −1/2 ⇔ +1/2 transitions of the 17O nuclei.125 By comparison of the amplitude of these transitions to the Gd(III) aqua ion (q = 8), the number of coordinated waters in the unknown complex could be determined.
As noted above, the Gd-H(water) distance determined by 1H ENDOR was invariable, and about 3.05 Å. As a result the position of the ENDOR transition associated with this coordinated water was constant. Raitsimring et al. exploited this effect to estimate q by 1H ENDOR. The difference in the intensity of the 1H ENDOR spectra measured in H2O and in D2O is proportional to the number of exchangeable protons. This technique allows the direct utilization of the gadolinium(III) compound and also protein bound Gd(III)-complexes can be directly measured.124, 126
Theoretical calculations can also provide an idea of the hydration number of the Gd(III), however it should be cautioned that the energy difference between CN9 and CN8 Gd(III) complexes is small and can also be strongly influenced by the presence of solvent water. X-Ray crystallography provides evidence of the hydration number in the solid state, but again, this may not reflect the hydration number in solution.109 Furthermore weakly coordinating ions such as nitrate or acetate may crystallize coordinated to a Gd(III) complex, but these will typically be displaced by water ligands in aqueous solution.
Gale et al. developed a simple NMR method to measure the hydration number of Mn(II) complexes.138 They demonstrated that the maximum in a plot of transverse O-17 relaxivity (r2O) versus temperature was directly proportional to the number of coordinated water ligands. This technique could be performed with micromolar concentrations of chelate, allowing for its use in interrogating the hydration number of protein-bound chelates. From the same data set, one can also determine the water exchange kinetics.
The tumbling of the molecule (τR)
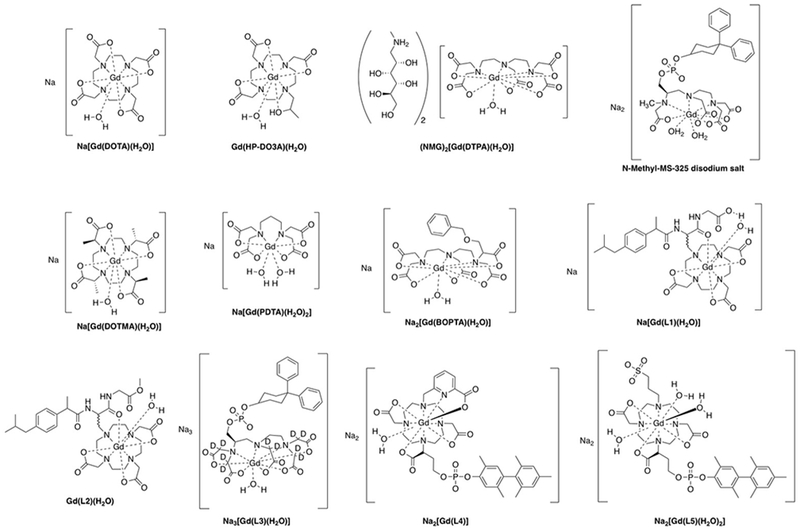
As discussed above, at the most common field strengths used for imaging, the dominant correlation time is almost always rotational diffusion. For instance, for small monomeric gadolinium(III) complexes, τR is about 0.1 ns and at low magnetic field strength and 37 ºC, slowing down the rotation will result in an increase of the relaxivity. Reducing the rate of molecular tumbling (that is, making τR longer) will enhance the relaxivity of a T1 contrast agent at low field strength. However at higher fields, relaxivity will increase with increasing τR up to a point, after which relaxivity will decrease with further increases in τR. This is because of the 3τc/(1+ωH2τc2) term in eq 5; as τc increases, relaxivity will increase until the product ωH2τc2 > 1 which will occur at high fields and/or high values of τc. This is illustrated in Figure 18 where field-dependent relaxivity profiles, so called nuclear magnetic relaxation dispersion (NMRD), are shown for Gd(III) complexes with increasing rotational correlation times. Figure 18A shows the NMRD profile for MS-325 in phosphate buffered saline where τR is on the order of 0.1 ns. In the presence of human serum albumin (Figure 18C), much of the MS-325 is protein bound with an effective τR of about 10 ns.17–18, 139–141 Slow rotation results in a multifold increase in relaxivity at low fields, but at 7 tesla the relaxivity has dispersed to the point that the unbound form of MS-325 has slightly higher relaxivity. Figure 18B shows the per Gd(III) NMRD profile of EP-1084 which is a contrast agent comprising 4 Gd-DTPA chelates and two fibrin binding peptides.142 This compound has a relatively high molecular weight resulting in an intermediate τR. While the relaxivity of EP-1084 is lower than MS-325/HSA at low fields, by 1.5 T the relaxivity is almost the same and at 3 T and 7T the relaxivity of EP-1084 is higher than MS-325/HSA. All three examples involved a Gd-DTPA derivative where the differences in relaxivity arose solely because of differences in rotational correlation time. Strategies to optimize the rotational correlation time are further discussed in section: 4.2 High relaxivity Gd(III)-contrast agents: 4.2.2. Optimizing the rotational correlation time (τR) of Gd(III)-based contrast agents.
Figure 18:
Experimental 1H NMRD profiles showing relaxivity (per Gd(III)) versus field strength for Gd-DTPA derivatives with short (A, MS-325), intermediate (B, EP-1084), and long (C, MS-325 in HSA solution) rotational correlation times.
Rotational diffusion can be estimated through several physical methods, such as NMR relaxation, fluorescence lifetime measurements, or can be calculated from the Stokes-Einstein equation.2
The exchange rate of the water molecule
The water exchange rate kex (1/τm where τm is the residency time of the coordinated water) is another key and tunable molecular parameter. Relaxivity has a 1/(T1m + τm) dependence. For small Gd(III) and Mn(II) complexes, T1m >> τm, and so these complexes tend not to be limited by slow water exchange. However if rotation is slowed and T1m is decreased, then water exchange may limit relaxivity. For other metal ions, water exchange may be slow relative to T1m.143
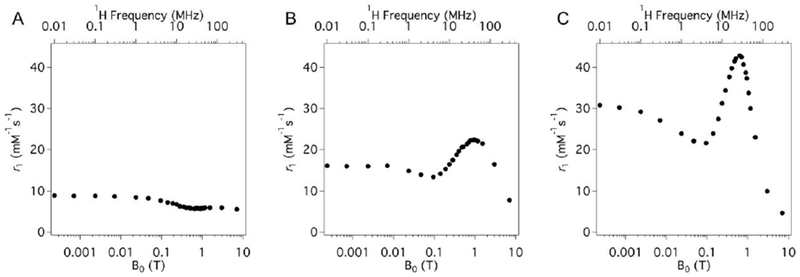
Figure 19 shows a simulation of the effect of the water exchange on gadolinium(III) complexes at three different magnetic fields: 0.47, 3.0, and 9.4 T. At each of these field strengths, the rotational correlation time (τR) is near the optimum value for that specific field strength, that is 20, 1.5 and 0.5 ns, respectively.144 Note that the y-axis range differs because the theoretical maximum relaxivity decreases with increasing field strength. At 0.47 T, very high relaxivities can be attained, but only for a narrow range of water exchange rates. At the higher fields a broader range of water exchange rates can yield near optimal relaxivity.
Figure 19:
Effect of water residency time (τm, ns) for an inner-sphere water on r1 (—) and r2 (---) under optimal rotational conditions (τR, ns) at 0.47 T (A, 20 ns), 3 T (B, 0.5 ns), and 9.4 T (C, 0.5 ns). The optimal τm range increases as field increases. Reproduced with permission from Ref.144 (URL: http://dx.doi.org/10.1002/cmmi.267). Copyright 2009 John Wiley and Sons.
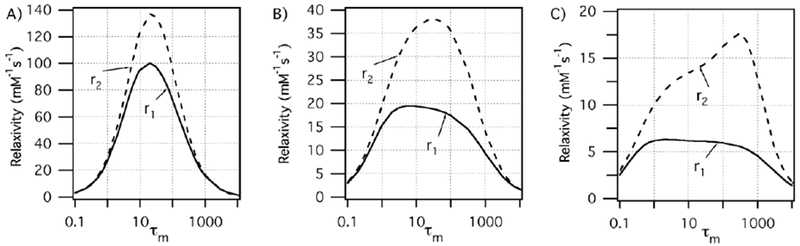
Relaxivity is limited by both very slow (long τm) and very fast exchange (Figure 19). In the latter case, if exchange is too fast then water is not present long enough for there to be a high probability of relaxation. Modifications of τm are usually related to the stabilization or destabilization of intermediates that participate in the water exchange mechanism and/or with changes in the population of the isomers present in solution. For instance, for DOTA-type ligands, it was found that water exchange is around 50 times faster in the twisted-square antiprismatic (TSAP) isomer compared to the square antiprismatic (SAP) isomer. The main explanation for this is the steric crowding at the water binding site, which favors the release of the metal-bound water in a dissociative mechanism.145 Figure 20 shows a simplified vision of the SAP and TSAP conformations in a DOTA-type complex. From a geometric point of view, these two isomers can be distinguished by the twist angle between the plane defined by the chelating oxygen atoms and the chelating nitrogen atoms: ~ 25 °for the TSAP isomer and ~40 ° in the case of the SAP form.
Figure 20:
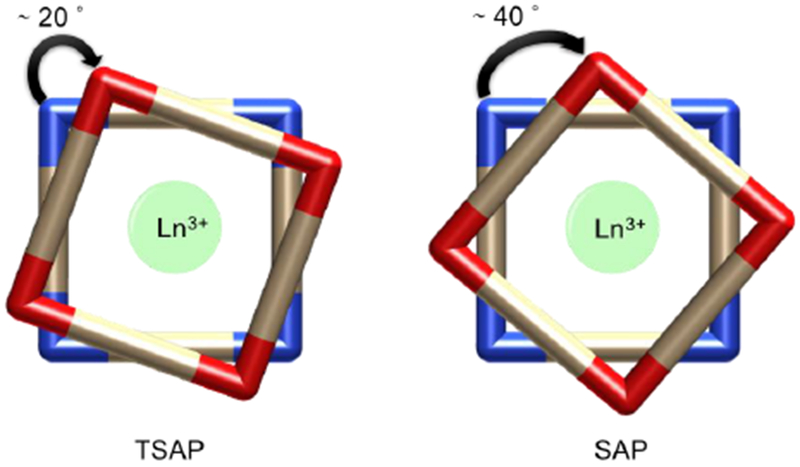
TSAP and SAP isomers of DOTA-type ligands of macrocyclic lanthanide chelates.
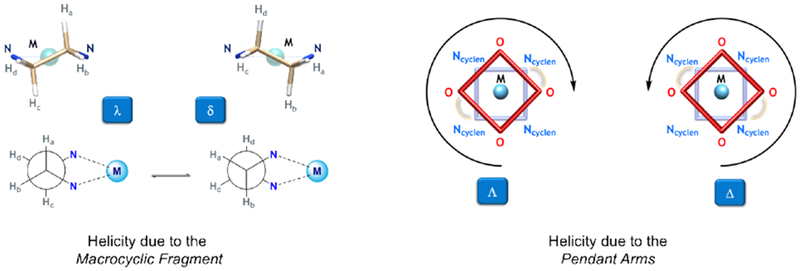
For DOTA-like structures, two sources of chirality should be considered: the orientation of the chelates (δδδδ or λλλλ) and the helicity of the pendant arms [Δ (clockwise arrangement) or Λ (counter clockwise arrangement)] (Figure 21).146–148
Figure 21:
Chirality sources within DOTA-like macrocyclic structures.
The TSAP isomer has the same stereochemistry at both positions [Δ(δδδδ) or Λ(λλλλ)], whereas the SAP isomer has the opposite stereochemistry at the two positions [Δ(λλλλ) or Λ(δδδδ)]. Interconversion between the two isomers is possible by ring inversion (δδδδ ↔ λλλλ) or by arm rotation (Δ ↔ Λ). Both, arm rotation and ring inversion performed in a successive manner or concerted way lead to an enantiomerization process. Because of steric repulsion between the nitrogen and oxygen donors, the distance between the N4 and O4 plane is increased in the TSAP isomer,149 thereby making the metal center less accessible for the coordination of a water molecule.145 On the other hand, within this isomer more bulky pendant arms can be accommodated. Substitution on the α position of the pendant arms slows or completely eliminates the arm rotation process,150–153 while substitution on the cyclen backbone restricts the ring inversion motion.154–157 Substitution at both positions effectively locks the macrocycle into a single conformation.158–161
The water exchange rate can be measured from the temperature dependence of the 17O NMR transverse relaxation rate of solvent water in the presence and absence of the metal complex at high fields.143
Outer-sphere relaxivity
In addition to the inner-sphere contribution to relaxivity, the second-sphere and in general, the outer-sphere contribution also needs to be taken into account. The relaxivity derived from the water molecules diffusing close to the gadolinium(III) complex can be predicted from the hard-sphere model of Hwang and Freed,162 where the relaxation depends primarily on the diffusion coefficient of water and the distance of closest approach to the metallic center.
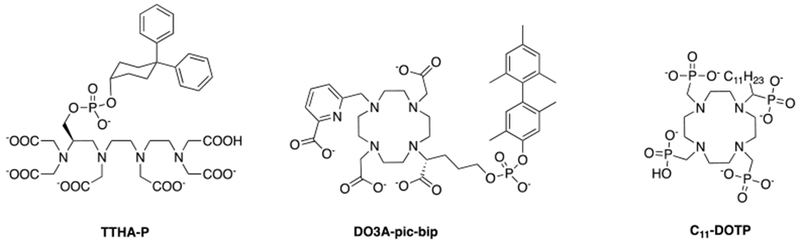
Second-sphere relaxivity is an operational definition that refers to complexes that have water molecules or exchangeable protons in the second coordination sphere that have a residency time longer than the diffusion lifetime. Second-sphere relaxivity is described by the same equations as inner-sphere relaxivity but here the dominant correlation time is the residency time of the second-sphere protons. In general, this effect is seen when independently determined molecular parameters cannot account for the observed relaxivity.109 Figure 22 shows some selected examples of q = 0 complexes with an important second sphere effect.
Figure 22:
Ligands forming HSA-binding Gd(III) complexes without any water molecules in the inner-sphere (q = 0).17
For instance, the relaxivities of [Gd(TTHA-P)]3– in buffer and in the presence of HSA are 2.1 mM−1s−1 and 8.0 mM−1s−1, respectively and the relaxivities of [Gd(DO3A-pic-bip)]2– in buffer and in the presence of HSA are 3.1 mM−1s−1 and 7.0 mM−1s−1, respectively. Although these values are lower than that of q = 1 compounds, their relaxivity is non-negligible. When bound to human serum albumin, the relaxivity is increased by 3 to 4-fold due to the long lived proton(s) near the Gd(III)-center.17 [Gd(C11-DOTP)]5− is also a q = 0 complex but its relaxivity is much higher than that of [Gd(TTHA-P)]3−, that is a molecule of similar size. The reason for this is the negatively charged surface due to the phosphonate moieties, which also furnish a favorable arrangement of the water molecules in the second coordination sphere.
T2-contrast agents
In the case of T2-contrast agents, as T2 is shortened, the water linewidth increases, so the signal decreases, leading to a negative image contrast. At the molecular level, the rotation of the complex in solution creates a fluctuating magnetic field that gives rise to T2 and can be described according to the Solomon-Bloembergen-Morgan (SBM) theory, equation 6.2 Pure T2 relaxation requires access of the water molecule to the contrast agent. For discrete complexes SBM theory can also describe T2 relaxation. However for 1/T2m, there is a field independent term for both dipolar and scalar relaxation such that r2 does not decrease at high fields the way that r1 does. In addition there is an additional relaxation mechanism called Curie spin relaxation that results in increased r2 at high fields, especially for ions with large magnetic moments.163
T2* takes into account the inhomogeneity of the local magnetic field (B0) and is related to T2 by equation 14:
| (14) |
The magnetic susceptibility of the paramagnetic agent causes local changes in B0 and induces this T2* effect. This magnetic susceptibility depends on the concentration of the contrast agent and its molar susceptibility. Since Tb(III) and Dy(III) have larger moments than Gd(III), their complexes could be used as contrast agents with increased susceptibility.164 These effects can be pronounced in vivo where compartmentalization of the contrast agent naturally occurs. For instance as the contrast agent passes through the blood vessels in the brain there is a large susceptibility gradient between the blood, where the contrast agent resides, and the brain tissue where the contrast agent is absent. This creates a large T2* relaxation effect and is used clinically to measure brain perfusion.165
Iron oxide nanoparticles have much larger magnetic susceptibilities than discrete coordination complexes. SPIONs are all T2-contrast agents, that de-phase the spins of the nearby protons of the water molecules, leading to a decrease in the signal. In the case of superparamagnetic particles, outer-sphere theory for T2 relaxivity predicts that their effectiveness is highly dependent on both the saturation magnetization (MS) value and the effective radius (r) (equation 15):
| (15) |
where D is the diffusivity of water molecules, l is the thickness of an impermeable surface coating, and κ = V*/C where V* is the volume fraction and C is the total iron concentration.
Recently, many novel platforms, such as carbon nanotubes (iron oxide-doped),166 zeolites (Dy(III)-doped),65 and metal-organic frameworks (MOFS) (Dy(III) and Gd(III)-doped)167 have been studied as potential T2-based contrast agents.
Another type of T2 contrast is derived from chemical exchange and is denoted T2ex.168–169 The effect arises from chemical exchange of protons that have a different frequency than bulk water. Here the ideal chemical exchange rate (104 – 107 Hz) of protons is slower than what is required for good T1 relaxation agents.170 Large pseudocontact shift of the protons that are close to a paramagnetic MRI contrast agent can produce a very large MR frequency for the proton, that depends on the external magnetic field applied and that can generate a large T2ex relaxation effect.92 However this mechanism produces relaxivities that are much lower than dipolar relaxation from Gd(III) or Mn(II) complexes.
3.3.2. Chemical Exchange Saturation Transfer
Comparison of pre- and post-injection images allows the detection of enhanced regions using an exogenous relaxation agent. Ideally these pre- and post-injection images should be acquired during the same scanning session, otherwise there may be problems of co-registering the pre- and post-injection data sets because the subject moved. However for targeted imaging and imaging with nanoparticles it often takes hours for the agent to accumulate at its target and/or for the background signal to clear. Another potential limitation of relaxation agents is that it can be difficult to quantify the agent in vivo, especially if the relaxivity changes.171–172 Theoretically, all of these shortcomings can be solved by using chemical exchange saturation transfer (CEST) agents.45
CEST agents contain a pool of labile protons that can be exchanged with the protons of the bulk water in an intermediate to slow rate on the NMR time scale (kex ≤ Δω, where Δω is the chemical shift frequency difference between the exchangeable pool frequency and the bulk water frequency.170, 173 A slow water exchange rate is optimal for the production of a CEST signal at a low radiofrequency pulse for in vivo studies.174 The application of a presaturation pulse at the frequency of the exchangeable protons leads to the transfer of some saturated spins into the water pool, which attenuates the signal of the bulk water.175–177 One of the major advantages of this technique is that despite the small concentration of the solute molecules (µM to mM range), the signal can be amplified several orders of magnitude171 through the transmission of the magnetization to the bulk water.178
When a radiofrequency (RF) pulse is applied at the resonance frequency of the labile protons of the CEST agent then saturation will occur. Because of chemical exchange, the magnetic saturation will spontaneously be transferred to the bulk water over time (Figure 23A). This saturation transfer will lead to a decrease in the water signal, through the continuous transfer of excited protons to the pool water (Figure 23B, C).179
Figure 23:
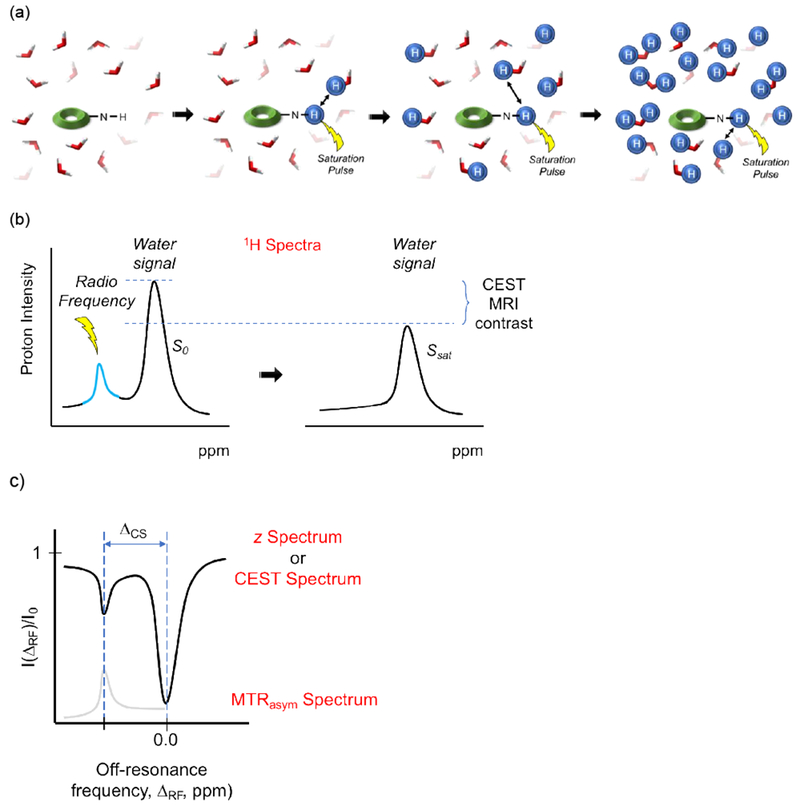
(a) Illustration of a CEST process: exchangable amine protons of a CEST agent are selectively saturated using radio frequency (RF) irradiation, leading to a reduction of the amine 1H signal intensity. Because of chemical exchange, the saturated protons (shown as blue spheres) are transferred to the bulk water pool. Continous chemical exchange during the saturation experiment leads to the amplification of the water signal reduction. (b) Continuous application of RF pulses leads to the saturation of more bulk water protons, thereby decreasing the 1H-NMR signal. (c) Z-spectrum (top): normalized water intensity (I/I0) vs off-resonance frequency (ΔRF), taking as 0.0 ppm the water resonance and the magnetization transfer ratio asymmetry (MTRasym) (ΔCS = separation in chemical shift between the two proton pools) spectrum (bottom): which shows the Z-spectrum asymmetry vs off-resonance frequency (ΔRF).180–181
The CEST effect is sensitive to environmental parameters, such as temperature, pH, membrane fluidity and cation concentration, as well as the concentration of the CEST agent.171 The exchange will continue until a steady state is reached or until the RF pulse is turned off. Using an exchange model consisting of two distinguishable pools (a small solute pool and large bulk water pool, with no back exchange of saturated protons), the proton transfer ratio (PTR) in the steady state can be estimated under the assumption that the radiofrequency irradiation of the solute pool does not affect the bulk water pool (Equations 16 and 17):176
| (16) |
where
| (17) |
According to these equations, the CEST effect increases with the fractional concentration xs, saturation efficiency α, the exchange rate ksw, and the longitudinal relaxation time of bulk water T1w.182 The CEST effect depends on the applied field. High fields allow a better frequency separation in the slow-exchange condition and reduces interference of direct water saturation. Assuming that transverse relaxation is negligible, the saturation efficiency at a given power (B1) can be estimated as follows (Equation 18):
| (18) |
For analyzing the CEST effect, the most common metric used is the “zero-order-spillover correction”, called Magnetization Transfer Ratio asymmetry (MTRasym) (Equation 19):
| (19) |
where I(ΔCS) and I(-ΔCS) are signal intensities acquired with RF irradiation applied on-resonance with the exchanging pool (I(ΔCS)) and at the frequency symmetric around water (I(-ΔCS)), and I0 is the reference signal intensity acquired without RF pre-saturation. In the z-spectrum, the normalized water signal intensity is monitored vs the frequency of the off-resonance saturation.175, 183 The CEST signal is commonly represented as a percent decrease in total bulk water intensity. Assuming that the saturation of the bound water signal is complete, the net magnetization of water protons at steady-state (Mz/M0) can be estimated as follows (Equation 20):
| (20) |
where c is the concentration of the CEST agent, q is the number of exchanging protons, 55.5 represents the molar concentration of bulk water, T1 is the relaxation time of bulk water, and τM is the exchange lifetime of the exchanging proton (1/τM = kex).184
Common CEST agents include amino acids, sugars, nucleotides or heterocyclic rings in their structure.185 The paramagnetic metal ion present in ParaCEST agents can be a lanthanide (such as Eu(III), Tm(III), Yb(III)) or a transition metal ion (Fe(II), Co(II) or Ni(II)), with a short electronic relaxation time. In this case, the election of the macrocyclic backbone as well as the pendant arms are key to control the oxidation state and the spin state of the metal, and hence, the stability of the complex.174, 186
There are four categories of exogenous CEST contrast agents: diamagnetic (DiaCEST), paramagnetic (ParaCEST), liposomal (lipoCEST), and hyperpolarized gas (hyperCEST – see in section on hyperpolarized molecules).176, 187–188 Even if all rely on exchanging protons, the nature of the exchanging protons is different. For example, in the case of diamagnetic agents (DiaCEST agents), the exchangeable protons usually belong to –NH and –OH groups, and the chemical shift difference (Δω) with the bulk water protons is less than 6 ppm (Figure 24). This relatively small chemical shift difference requires slow exchange rates in order to be detected, and this slow exchange usually limits the magnitude of the CEST effect. In the case of paramagnetic (ParaCEST) agents (chelates that contain paramagnetic ions), the chemical shift difference (Δω) of the labile protons with the bulk water protons can be much larger, up to a few hundred ppm, depending on the metal structure. This larger shift difference means that there can be much faster proton exchange, but that still obeys the slow exchange condition.171
Figure 24:
Exogenous agents (blue) and endogenous biomolecules (red) that can be detected when applying a saturation pulse at the magnetic resonance frequencies listed in the chart. (A) Aryl acid agents, (B) aryl amide agents, (C) amides, (D) glutamate, (E) amines, (F) glucose, and (G) lactic acid.189 Paramagnetic compounds have much wider chemical shift ranges.
LipoCEST agents are composed of liposomes that encapsulate a chemical shift agent in the inner cavity, thus shifting the resonating frequency of intraliposomal water protons by 1 to 5 ppm from that of extraliposomal water protons (Figure 25).190 The separation in chemical shift (Δω) between the two proton pools in slow exchange (Δω > kex, kex = exchange rate) is described in equation 21:
| (21) |
Figure 25:
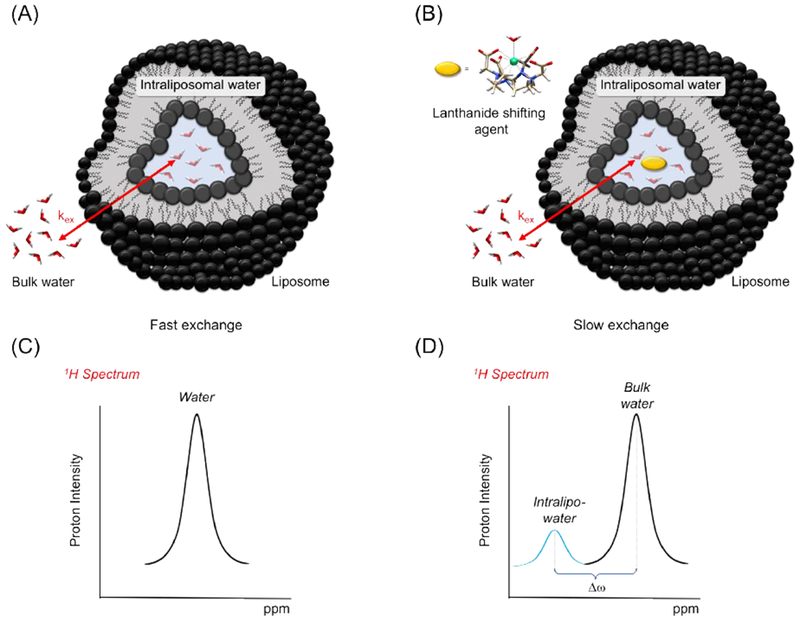
Simplified scheme of a liposome (A) and a lipoCEST agent (B). 1H NMR spectra focused on the region of the water signal, in a regular liposome (C) and in a lipoCEST agent (D).
Liposomes present large amounts of equivalent water molecules with exchangeable protons (106 - 108) in their inner cavities, that are in exchange with the bulk water protons.190 By introducing a shift agent inside the liposome, the resonance of the intraliposomal water is shifted relative to the water outside the liposome. The shift agent in LipoCEST agents is usually a lanthanide complexes in which the ninth coordination site of the metal ion is occupied by a water molecule in fast exchange with the intraliposomal water molecules. The CEST effect is generated by applying a radiofrequency pulse at the resonance frequency of the intraliposomal water.
HyperCEST is a technique that combines CEST with hyperpolarized MRI, and has been typically been performed with 129Xe. 129Xe is very soluble in organic solvents and aqueous solutions (including blood plasma), and possesses a highly polarizable electron cloud (being highly sensitive to the molecular environment and thus allowing the display of well-resolved chemical shifts of solvents, small molecules or proteins it associates with) and can be easily hyperpolarized, usually using spin-exchange optical pumping (SEOP) where the polarization is transferred from electronically polarized rubidium atoms in the vapor state to 129Xe nuclei.191 Because the natural abundance of xenon in the air is at the trace level, no background signal is observed. 129Xe can be encapsulated in molecular cages that have been functionalized to bind to a desired target, however direct detection of the hyperpolarized encapsulated Xe is limited because the Xe can diffuse out of the cage, with only about 1% of the cage being occupied at a given time.192 Schröder et al. showed that the caged 129Xe resonance could be saturated and the saturation transferred to the larger unbound 129Xe resonance in a manner that increased the detection sensitivity over 3300 fold relative to direct detection of the bound species.193 This had led to various targeted, caged 129Xe compounds.187, 193–197 A further potential benefit of 129Xe is that it can be inhaled and transferred to the blood stream in the lungs. Thus the hyperpolarized signal can be replenished over time by having the subject breath 129Xe enriched air.
CEST benefits from imaging at high field (7 T and above), since high magnetic fields increase the chemical shift in Hz, and can improve the specificity of the technique when several CEST agents are present. Also, at higher fields the T1 relaxation time of water is increased, making possible a longer accumulation of the saturation of the bulk water, thus increasing the detectable CEST effect.179
While CEST has several advantages compared to relaxation agents, it also has some inherent limitations. A primary limitation is the sensitivity which is about two orders of magnitude lower than relaxation agents.170 In addition, in the body there is an endogenous magnetization transfer effect that can interfere with the CEST signal. Finally, there are limitations to the saturation power that can be applied otherwise dangerous heating of the subject will occur. Thus while ParaCEST agents with very fast exchanging protons may exhibit theoretically large CEST effects compared to DiaCEST agents, this gain may not necessarily be realized in vivo because of the inability to apply enough radiofrequency power to achieve full saturation.
In order to overcome these shortcomings, Sherry and coworkers prepared europium complexes with highly shifted exchange sites (large Δω) and very slow water molecule exchange rates, which allows its activation through relatively low-power RF pulses. 170, 198–201 In general, there is a correlation between the polarity of the ligand pendant arms and the Eu(III)-bound water lifetime (τM = 1/kex): phosphonates ≥ carboxylates >> alkyl groups ≥ simple amides.202 For ParaCEST agents, the optimal residence time of the bound water molecule (τm) is 10−4 – 10−2 s, depending the magnetic field applied during the presaturation period. 54
3.4. Thermodynamic stability/kinetic inertness of Gd(III)-based MRI contrast agents
High stability is essential for all metal-based MRI contrast agents used in medicine due to the well known toxicities of the dissociated metal ions. For example, Gd(III) ions can irreversibly bind to skeletal tissue and are also known to block Ca(II) binding sites.2, 203
The prediction of in vivo stability of newly developed Gd(III)-based contrast agents is based on fundamental physical properties, like solution thermodynamics, selectivity and dissociation kinetics. The thermodynamic stability constant KGdL (equation 22) provides the affinity of Gd(III) for a given ligand and gives the amount of dissociated Gd(III) that is released in the environment if the system reaches equilibrium.
| (22) |
The thermodynamic stability constants for the commercially approved GBCAs (Figure 3) are all large (Table 5), so that the equilibrium lies heavily on the side of the intact complex GdL. Table 5 shows as well that more basic ligands form thermodynamically more stable complexes with Gd(III), which is expected given that the Gd(III) ion is a hard acid and therefore prefers hard donor atoms. The linear correlation between the sum of the ligand protonation constants (Σ log K1-n) and thermodynamic stability (log KGdL) was pointed out many years ago.204 For instance, the DOTA ligand is much more basic than the DTPA-BMA ligand (sum of ligand protonation constants: Σ log K1-4 = 30.94 vs. Σ log K1-4 = 19.3, respectively)205 and forms a thermodynamically much more stable complex with Gd(III) (log KGdL = 24.7 vs. log KGdL = 16.85, respectively). The stability constant does not factor in competition with other ions or with acid. Very basic ligands will also show strong competition with proton. The conditional stability constant, Kcond, is a useful number that gives the equilibrium constant at a given pH, e.g. pH 7.4, and is defined by equation 23,
| (23) |
where K1, K2, K3, …, Kn are the stepwise protonation constants of the ligand.
Table 5:
Thermodynamic and conditional (pH 7.4) stability constants for commercially approved Gd(III)-based MRI contrast agents.206
| Charge | Log KGdL | Log Kcond | |
|---|---|---|---|
| [Gd(DTPA)(H2O)]2− | −2 | 22.46207 | 18.4 |
| [Gd(DTPA-BMA)(H2O)] | 0 | 16.85203 | 14.8 |
| [Gd(DTPA-BMEA)(H2O)] | 0 | 16.6208 | 15.0 |
| [Gd(BOPTA)(H2O)]2− | −2 | 22.6208 | 18.4 |
| [Gd(EOB-DTPA)(H2O)]2− | −2 | 23.46209 | 18.7 |
| MS-325 | −3 | 22.1210 | 18.9 |
| [Gd(DOTA)(H2O)]− | −1 | 24.7211 | 17.2 |
| [Gd(HP-DO3A)(H2O)] | 0 | 23.8208 | 17.1 |
| [Gd(DO3A-butrol)(H2O)] | 0 | 21.8212 | 14.7 |
Note that [Gd(DOTA)(H2O)]− with the highest basicity among these compounds exhibits the highest thermodynamic stability constant (24.7211). In contrast, [Gd(DOTA)(H2O)]− exhibits one of the lowest conditional stability constants (17.2), which stems from its high basicity and therefore high competition for protons. Note, that the thermodynamic stability constant and the conditional stability constant for a complex with a less basic ligand like [Gd(DTPA-BMA)(H2O)] are much closer together (16.9203 vs. 14.8) since there is much less competition for protons at pH 7.4.206 An alternate value sometimes reported is pM (−log[M]) which refers to the unchelated metal ion under a given set of conditions ([M]total,[L]total, pH), where the higher the pM value, the more stable the complex.
In the body, there is of course competition from other metal ions and from coordinating anions. There are software programs that can model these systems of equilibria provided the individual stability constants are all known. In a highly cited paper, Cacheris et al. introduced a selectivity factor (Ksel), that also considers the stability constants for the corresponding Zn(II), Cu(II), and Ca(II)-complexes as well as pH (equation 24).79
| (24) |
The authors found selectivity factors for [Gd(DTPA-BMA)(H2O)] and [Gd(DTPA)(H2O)]2− of log Ksel = 9.04 and log Ksel = 7.04, respectively, which reversed the stability trend usually seen for this two compounds ([Gd(DTPA-BMA)(H2O)]: log KGdL = 16.85 and log Kcond = 14.90 (pH 7.4) vs. ([Gd(DTPA)(H2O)]2−: log KGdL = 22.46 and log Kcond = 17.70 (pH 7.4)). The authors argued that this higher selectivity value for [Gd(DTPA-BMA)(H2O)] explained acute toxicity results chelates: LD50 = 14.8 vs 5.6 mmol kg−1 for [Gd(DTPA-BMA)(H2O)] vs. [Gd(DTPA)(H2O)]2−; LD50 is the dose at which half of the animals die. The authors claimed that the lower acute toxicity of [Gd(DTPA-BMA)(H2O)] was caused by an decreased amount of Gd(III) release in comparison to [Gd(DTPA)(H2O)]2−.80 However, in several studies it was found that actually more Gd(III) is released from administration of [Gd(DTPA-BMA)(H2O)] than from [Gd(DTPA)(H2O)]2−.213–216 This demonstrates that care has to be taken when predicting the in vivo stability or toxicity of Gd(III)-based contrast agents using only thermodynamic stability data.
In addition to thermodynamics, the rate at which Gd(III) can dissociate from the complex or be exchanged with an endogenous cation is an equally important factor. In this regard, the approved macrocyclic contrast agents are much more kinetically inert than the approved acyclic agents.
Unlike the thermodynamic stability constant, there is no uniform measure of inertness. This is because different complexes will undergo decomplexation or transmetallation by different mechanistic pathways. For example, for transmetallation of approved agents with Zn(II), the rate is first order in [Zn] for acyclic chelates, but is zeroth order for the macrocyclic chelates. Note, that exchange rate constants are only comparable if the exchange mechanism per metal complex is the same. Kinetic inertness is reported in various ways. A common approach is to measure the dissociation rate constant at a low pH. Another is to measure the rate of Gd(III) release in the presence of another metal ion or by a competing ligand. Laurent et al. used a semi-quantitative method where they incubated the chelate with a mixture of Zn(II) and phosphate.110, 217 Frenzel et al. studied the rate of Gd(III) release from approved agent when incubated in human plasma.218 Table 6 shows different kinetic measures for Gd(III) dissociation from commercially approved contrast agents under acidic conditions, in the presence of Zn(II) and after incubation in human plasma.
Table 6:
Kinetic measures for dissociation of Gd(III) from commercially approved MRI contrast agents under different conditions.
| Acid-catalyzed dissociation rate constants219 | 1 eq. Zn, phosphate buffer pH 7.0, 37 °C110, 217 | 10 mM phosphate, human plasma, 37 °C206, 220 | |
|---|---|---|---|
| k1 /M−1 s−1 | t0.8/mina | t2%/hb | |
| [Gd(DTPA)(H2O)]2− | 0.58 | 260-280 | 8.73 |
| [Gd(DTPA-BMA)(H2O)] | 12.7 | 50-60 | 0.51 |
| [Gd(DTPA-BMEA)(H2O)] | 8.6 | 0.89 | |
| [Gd(BOPTA)(H2O)]2− | 0.41 | 600 | 9.08 |
| [Gd(EOB-DTPA)(H2O)]2− | 0.16 | 1500 | 51.3 |
| MS-325 | 2.9 × 10−2 | 3800 | 34.4 |
| [Gd(DOTA)(H2O)]− | (8.4 × 10−6)221 (1.8 × 10−6)222 |
>5000 | > 1000 |
| [Gd(HP-DO3A)(H2O)] | (6.4 × 10−4)204 (2.6 × 10−4)223 |
>5000 | > 1000 |
| [Gd(DO3A-butrol)(H2O)] | 2.8 × 10−5 | >5000 | > 1000 |
competition against Zn(II) - 2.5 mM Gd(III)-based contrast agent, 2.5 mM ZnCl2, pH = 7.0, 50 mM phosphate buffer (t0.8 = time for R1 to reach 80% of its initial relaxivity).
t2% = time when 2% of the Gd(III)-ions are released from the complex.
Under all applied conditions, the macrocyclic Gd(III) complexes are the most kinetically inert which is in line with what has been observed in in vivo studies where Gd(III) release was examined in animal models.224 The rigidity of the macrocyclic Gd(III) complexes leads to considerably slower dissociation rates in comparison to the linear Gd(III) chelates. In general, it is believed that kinetic inertness is the most critical factor with respect to Gd(III) release. Among the acyclic chelates, kinetic inertness also appears to predict Gd(III) release in vivo.213–214, 216, 224–226
It is obvious that novel gadolinium(III)-based MR contrast agents have to meet or exceed the stability/inertness properties of the approved contrast agents. This is especially important for targeted and/or macromolecular MRI contrast agents that are expected to have longer in vivo retention times than simple hydrophilic complexes. Kinetic inertness combined with thermodynamic stability is the best predictor of in vivo toxicity so far, but the ultimate standard is how the compounds behave in vivo.
There is also a strong selection bias in our understanding of contrast agent safety that comes from the data available for the approved contrast agents. Toxicity may arise from different causes. While much can be learned from the approved agents, it should be obvious that for new complexes, increased stability/inertness may not necessarily correlate with safety/toxicity. Ultimately these compounds would need to be tested in vivo.
4. Contrast agents of improved safety
4.1. Safety concerns
4.1.1. Nephrogenic systemic fibrosis
There is a low incidence of adverse drug reactions associated with the approved contrast agents and for years these products were considered very safe. Prince et al. reviewed data for 160,000 GBCA enhanced MRI studies and found that acute adverse events occurred at a rate of 5.9 per 10,000 exams, and that severe adverse events occurred about 1 in 40,000 injections.4 They also mined the FDA adverse events reporting system database found reports on 40 GBCA associated deaths between 2004-2009 that were unrelated to nephrogenic systemic fibrosis (NSF) which gave with an incidence per million doses of 0.15, 0.19, 0.97, 2.7, and 0.7 for gadodiamide, gadoversetimide, gadopentetate dimeglumine, gadobenate dimeglumine, and gadoteridol, respectively. In general the use of GBCAs is considered to be very safe.
Throughout the 1990s and early 2000s it was commonplace to administer repeat doses of GBCA within a short time frame and to administer double and triple dose injections for angiography or perfusion weighted scans.165, 227 However, in 2006 a strong link between Gd(III)-based contrast agents and a devastating disease termed nephrogenic systemic fibrosis (NSF) was identified in renally impaired patients.5–6 The precise mechanism of NSF onset remains undetermined but it appears to be strongly connected to exposure to gadolinium.
The risk of NSF increases with diminishing renal function. GBCAs are eliminated almost exclusively via renal filtration and thus the agent dwells for longer in renally impaired subject, prolonging the time window for Gd(III) exposure.228–230 Renal function is often assessed by creatinine glomerular filtration rate (GFR) and the GBCA elimination time increases with decreasing GFR. For example, GBCAs typically exhibit blood half-lives on the order of 90 min in patients with normal renal function (GFR > 90 mL/min/1.73).230–236 In subjects experiencing moderate renal insufficiency GFR = 30 – 60 mL/min/1.73) the half-life is increased to 4 – 8 h. In cases of severe renal impairment (GFR < 30 mL/min/1.73 m2) half-lives ranging from 18 to 34 h have been reported. Gd(III) recovery in the urine of patients with normal renal fucntion is typically >90% within 12 h of GBCA injection but recovery decreases with decreasing renal function. For example, Gd(III) recovery as low as 76% after 5 days was reported for patients with GFR under 10 mL/min/1.73 m2,230. NSF incidence among the 8 FDA approved contrast agents appears to reflect the relative kinetic inertness of the Gd(III) complex as determined by in vitro assays.237–238
Following the linkage between NSF and gadolinium exposure, regulatory agencies like FDA placed restrictions on the use of GBCAs. In 2010 FDA contraindicated the use of Gd-DTPA, Gd-DTPA-BMA, and Gd-DTPA-BMEA in patients with GFR < 30 mL/min/1.73 m2, and recommended to test for GFR and to avoid use of all GBCAs in patients with impaired renal function.239 The American College of Radiology (ACR) 2015 manual recommends GFR screening of any patients with known or suspected renal impairment.240 The ACR also recommends extreme caution in administering any Gd(III) contrast agent to patients of GFR < 40 mL/min/1.73 m2, suffering acute injury, or in need of dialysis. These guidelines are necessary and have essentially eliminated new incidences of NSF.
4.1.2. Brain and body retention of Gd(III)
Mounting evidence since 2013 indicates that Gd(III) from contrast agents is irreversibly retained in the central nervous system (CNS). Concerns were first raised after reports of prolonged and non-diminishing signal enhancement in the dentate nucleus and globus pallidus of patients receiving Gd(III) contrast agents.241 The relative degree of CNS enhancement correlated to the number of contrast enhanced examinations received.242 A 2015 analysis of autopsy specimens confirmed that the CNS enhancement was indeed due to Gd(III) deposition.7 As with NSF risk, the risks of CNS Gd(III) accumulation associated with each agent appear to reflect kinetic inertness – a greater degree of CNS accumulation is observed following exposure to the less kinetically inert linear agents.243–244 However, significantly elevated signal in the dentate nucleus has also been observed in Multiple Sclerosis patients exclusively receiving multiple doses of the macrocyclic agents Gd-DO3A-butrol or Gd-DOTA.245–246 Here too, dentate nucleus signal enhancement reflects cumulative dose. A conflicting study did not detect any significantly elevated signal in the dentate nucleus or globus pallidus of a brain tumor patients receiving multiple doses of Gd-DO3A-butrol exclusively.247 The long term effects of Gd(III) deposition in the CNS remain unknown and no toxic effect has yet been identfied. However, these new findings have triggered a high degree of anxiety about the safety of Gd(III)-based agents and stimulated a new wave of regulatory activity and debate.
Gadolinium deposition has also been identified in the body of patients with normal renal function after receiving GBCAs. Gd(III) retention has been identified in femoral heads extracted from patients receiving hip replacement surgery who had previously received Gd-DTPA-BMA or Gd-HPDO3A.248 Bone retention was 2.5 greater in the patients who received Gd-DTPA-BMA. A 2016 autopsy study demonstrating elevated bone levels of gadolinium by mass spectrometry in adult patients after receiving linear or macrocyclic GBCAs.249 A 2018 study quantified gadolinium retention in tibeas of healthy normal volunteers 5 years after a single dose of Gd-DO3A-butrol using X-ray fluorescence.250 The study identified long term bone accumulation that increased with cumulative dose. An average accumulation of 2.5 nmol Gd(III) per g bone mineral for every mmol/kg GBCA administered was observed. Another study identified a positive correlation between liver gadolinium and iron concentrations in pediatric patients with iron overload after receiving Gd-DOTA.251
A few studies have attempted to discern the speciation of accumulated Gd(III). Phosphate bound Gd(III) was identified by extended X-ray absorbance fine structure (EXAFS) analysis in the skin of NSF patients, which is consistent with dechelated Gd(III).252 In another study, water soluble Gd(III) was extracted from the biopsied skin of NSF patients and analyzed by HPLC-ICP-MS.253 The water soluble Gd(III)-containing species was identified as Gd-HPDO3A. After extraction of the water soluble Gd(III)-containing species the skin sample was analyzed by laser ablation ICP-MS which showed that the distribution of insoluble Gd(III) was strongly correlated with phosphorous distribution.253 A study of the speciation of accumulated gadolinium in the brains of rats after repeat exposure to high doses of commercially available GBCAs revealed that Gd(III) accumulated after treatment with linear agents existed in 3 forms: intact chelate, soluble macromolecule associated Gd(III), and insoluble Gd(III) deposits, whereas Gd(III) accumulated after treatment with macrocylic agents existed solely as intact chelate.254 Another study comparing speciation of Gd(III) retained in the rat brain after repeat administration of Gd-DOTA and Gd-DTPA-BMA demonstrated that Gd(III) from Gd-DOTA exists as the intact chelator where Gd(III) from Gd-DTPA-BMA is predominatly recovered as insoluble Gd(III).255
Chelation therapy has been considered as a method to remediate Gd(III) accumulation. Levels of Gd(III) accumulated in pediatric patients suffering iron overload after Gd-DOTA treatment was shown to decrease after iron chelation therapy.251 An oral chelator formulation is currently under development as a Gd(III) remediation treatment.256
There have also been reports of NSF-like and neurologic symptoms in patients with normal renal function who have received GBCAs.257–259 However these cases have primarily been identified through patient self-reporting and the linkage to gadolinium remains controversial.
4.1.3. Current regulatory status
In 2010, the FDA forbade the use of the 3 least kinetically inert agents (Gd-DTPA, Gd-DTPA-BMA, Gd-DTPA-BMEA) in patients with GFR ≤30 mL/min/1.73 m2. 238–239 The FDA also labelled all contrast agents with a boxed warning that advises against exceeding the recommended dose and against repeat dosing.238–239 Following the reports of brain deposition, the European Medicines Agency announced in 2017 that it would suspend the marketing authorization for 3 of the 8 available intravenous formulations (Gd-DTPA, Gd-DTPA-BMA, Gd-DTPA-BMEA), while limiting the use of Gd-DTPA-EOB and Gd-BOPTA to liver imaging applications.260 FDA did not recommend any product withdrawals but did add new warning labels advising caution and prudence when ordering an MRI scan.261–262 Japan’s Pharmaceuticals and Medical Devices similarly updated their GBCA package inserts.
Limiting the use of GBCAs in patients with impaired renal function eliminates NSF risk but creates challenges in patient management. For instance an estimated 16% of US adults suffer chronic kidney disease stage 3 (GFR ≤60 mL/min/1.73 m2) or greater.263 Besides their kidney disease, these patients often have other co-morbidities like diabetes or cardiovascular disease.264–265 Physicians managing these patients must now carefully weigh the risk versus benefit of contrast enhanced examinations that would otherwise be routine for patients with normal renal function. There are often no good clinical alternatives available to replace GBCA enhanced scans. Intravenous X-ray contrast agents, which can be used for blood vessel imaging also poses a potentially severe nephrotoxicity risk to renally impaired patients.266
The current concerns over the safety of MRI contrast agents is driving chemistry innovations towards the design of imaging products of enhanced safety. Four main approaches have been taken. One approach is to focus on developing higher relaxivity gadolinium(III) based contrast agents which can be administered at a lower dose than current GBCAs. A second approach is to identify Gd(III) complexes that are even more inert to Gd(III) dissociation/transmetallation. A third approach is to use molecular targeting to improve specificity and lower the dose. Another approach is to develop a next generation of MRI contrast agents that moves away from Gd(III) altogether. We will review progress towards both these goals below.
It should be kept in mind though that GBCAs have a very good safety profile over all, and so far it is better than the more limited experience with iron oxide based nanoparticles. For instance with the iron oxide ferumoxytol, the FDA found 79 anaphylactic reactions in its Adverse Event Reporting System between June 2009 and June 30, 2014, involving patients aged 19 to 96 years.267 Half of the reactions occurred with the first dose of ferumoxytol, and three-quarters began during infusion or within 5 minutes of completion. Eighteen of those 79 patients died despite immediate medical intervention. The incidence rates of anaphylaxis or serious hypersensitivity reaction varied between 0.2% and 0.9%, depending on the patient population in clinical trials of ferumoxytol.268 This is in the range of 2 – 10 serious events per 1000 injections. On the other hand GBCAs have a more favorable safety profile, with a rate of 0.04 serious events per 1000 injections (40 per million). Over a 5 year reporting period the total number of deaths associated with GBCAs was 41 deaths out of 51 million GBCA administrations (0.9 deaths per million administrations). New compounds will have to meet a high safety standard.
4.2. High relaxivity Gd(III)-based contrast agents
There are currently 7 Gd(III)-based ECF agents that are FDA approved and these have r1 values in blood plasma in the range of 3.6 to 6.3 mM−1s−1 at 1.5 and 3T. For clinical use, the approved dose range from 0.1 to 0.3 mmol kg−1. For those agents, it is estimated that the local concentrations of Gd(III) must be around 125 µM to robustly observe contrast differences in tissue (this corresponds to a relaxation rate change of about 0.5 s−1). Regarding absolute sensitivity, the detection limit for the MRI contrast agent Gd-HPDO3A (Figure 3) in mouse skeletal muscle was reported to be 30 µM.269 Hence, for targeted MR contrast agents with similar T1 relaxivity values as the commercial contrast agents, the biological target should be present in excess of 100 µM.
Research has focused on improving sensitivity and expanding the scope of clinical MR contrast agents for higher efficiency, without sacrificing safety. Relaxivity is a measure of the sensitivity of the contrast agent. The typical T1 relaxivity values of commercially available gadolinium(III) based contrast agents are relatively small compared to what is theoretically possible.270 Contrast agents with higher relaxivity could provide greater tissue enhancement for better detection of smaller lesions, or could produce equivalent contrast at a lower dose compared to existing approved compounds, which may lower the risk of Gd(III)-induced toxicity. For targeted or activatable contrast agents, using high relaxivity compounds would also allow the detection of lower concentration targets.
Several molecular parameters that govern the T1 relaxivity of MR contrast agents need to be tuned to achieve higher relaxivity.271 Such parameters are the hydration number (q), the rotational correlation time (τR) and the water residency time (τm). An optimum relaxivity can be reached if the hydration number q is increased (q = 1 in approved contrast agents), if the rotational correlation time (τR) relative to commercial CAs is increased,270 and if the coordinated water residency time τm is decreased to 1-30 ns (τm = 150-1000 ns for approved contrast agents).
4.2.1. Increasing the hydration number q of Gd(III)-based contrast agents
Inner-sphere relaxivity is directly proportional to hydration number. There has been considerable effort to generate coordination cages capable of forming highly stable and kinetically inert Gd(III) complexes (inert with respect to Gd(III) dissociation) with an extended inner-sphere hydration number. The hydration number can be increased by lowering the denticity of the multidentate co-ligand, e.g. using heptadentate instead of octadentate chelators. However, this often reduces the thermodynamic stability and/or kinetic inertness of the resulting Gd(III) complex, and increases the risk of Gd(III) release into the body. Increasing the hydration sphere also raises the risk of anion binding and displacement of coordinated water ligands. For instance, the stable q = 2 complex Gd-DO3A can form ternary complexes with endogenous coordinating anions such as phosphate or bicarbonate.272 Anion coordination results in the displacement of the coordinating water ligands, and as a result the T1 relaxivity is reduced.
Figure 26 shows 5 ligand systems that form stable gadolinium(III) complexes with increased hydration number: the heptadentate chelators PCTA, AAZTA, CyPic3A, and aDO3A and the hexadentate chelator tacn(1-Me-3,2-hopo)3. Gd-PCTA is thermodynamically less stable than Gd-DOTA (log Kcond = 16.2 vs. log Kcond = 17.2),206 but it is kinetically quite inert. In acid-assisted gadolinium decomplexation experiments Gd-PCTA reacts only an order of magnitude faster than Gd-DOTA, which makes it superior to the approved macrocyclic contrast agents Gd-HPDO3A and all commercially approved acyclic contrast agents. Due to its two metal-bound water molecules, the T1 relaxivity of Gd-PCTA (r1 = 6.9 mM−1s−1 at 20 MHz, 25 °C) is higher than that of the approved ECF agents.273–276
Figure 26:
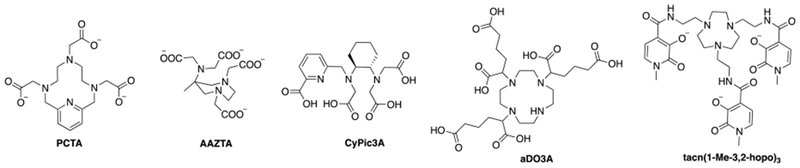
Ligand systems: PCTA, AAZTA, CyPic3A, aDO3A and tacn(1-Me-3,2-hopo)3 that form thermodynamically stable Gd(III)-complexes with extended hydration sphere.
Gd-AAZTA (log Kcond = 16.4)206 is also thermodynamically less stable than Gd-DOTA, but its kinetic inertness may be sufficient for in vivo applications. Although it was shown that Gd-AAZTA reacts faster in acid-catalyzed decomplexation experiments than Gd-DTPA, it undergoes transmetallation reactions with Cu(II) or Eu(III) more slowly than Gd-DTPA.277 Its hydration sphere features two water molecules leading to a high T1 relaxivity of 7.1 mM−1s−1 (20 MHz, 25 °C).278–280
Gd-CyPic3A affords two metal-coordinated water molecules (q = 2) and an r1 value of 5.70 mM−1s−1 (60 MHz, 37 °C), which is 1.7 fold higher than that of Gd-DTPA measured under the same conditions (r1 = 3.26 mM−1s−1). Its thermodynamic stability is comparable to that of the approved contrast agent Gd-HPDO3A and its kinetic inertness is in the range of acyclic clinically used Gd(III) complexes. It was also shown that the complex is resistant to anion coordination.281
Parker and coworkers synthesized Gd-aDO3A, a bishydrated Gd-DO3A derivative with remarkably high relaxivity values (r1 = 12.3 mM−1s−1 at 20 MHz, 25 °C). Unlike Gd-DO3A, Gd-aDO3A is not prone to inner-sphere water displacement by endogenous anions, presumably because of repulsion by the negatively charged pendant carboxylate groups. The kinetic inertness of this complex was reported to be 10 times higher than that of Gd-DTPA.282
Raymond and coworkers developed a series of thermodynamic stable Gd(III) complexes that utilized three hydroxypyridinone (HOPO) bidentate chelators. These complexes exhibited hydration numbers of up to q = 3 and hence, they were able to obtain exceptionally high T1 relaxivity values (e.g. 13.1 mM−1s−1 at 20 MHz, 25 °C for Gd-tacn(1-Me-3,2-hopo)3). Moreover, those complexes are resistant to anion coordination.283–287
Recently, Fries and coworkers reported a comparative study of the experimental high relaxivity agent P03277 (Figure 27, left) with the approved contrast agent gadobutrol (Figure 3).288 P03277 is a Gd-PCTA derivative with a low molecular weight (0.97 kDa) and a measured value of q = 1.7. Its T1 relaxivity in human blood plasma at 37 °C is approximately 2.5 times higher than that of gadobutrol at clinical field strength (12.8 vs. 5.2 mM−1s−1 (1.5 T) and 11.6 vs. 5.0 mM−1s−1 (3.0 T), respectively). Increases in relaxivity due to increased hydration should be maintained at high fields, and P03277 has 2-fold higher relaxivity than gadobutrol (9.3 vs. 4.8 mM−1s−1 at 7.0 T, 8.6 vs. 4.7 mM−1s−1 at 9.4 T, respectively in human plasma, 37°C). An in vivo comparison of both agents in a rat model of hepatic colorectal cancer metastases at 9.4 T revealed that P03277 has similar pharmacokinetics as gadobutrol but demonstrates significantly better tumor enhancement properties as depicted in Figure 27, right. P03277 is currently undergoing human clinical trials (NCT02633501, www.clinicaltrials.gov).
Figure 27:
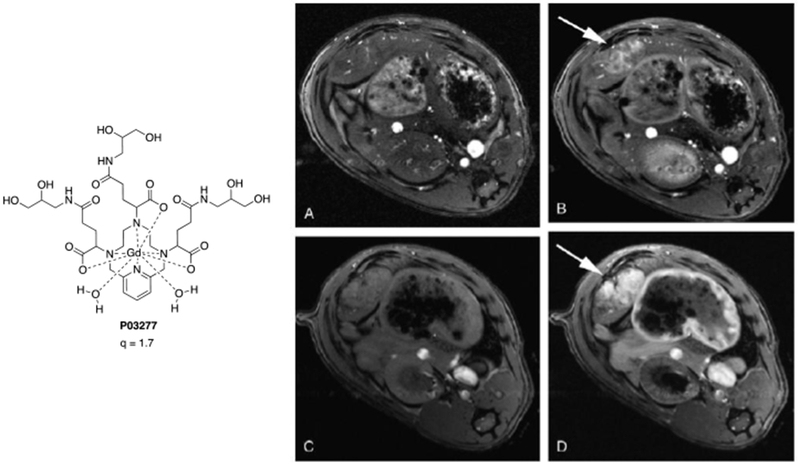
Left: Structure of P03277; Right: Axial T1-weigthed images of the hepatic metastasis A) before and B) after intravenous injection of 0.1 mmol kg−1 gadobutrol,; C) before and D) after intravenous injection of 0.1 mmol kg−1 P03277. Arrow shows tumor. Reproduced with permission from Ref.288 (URL: https://journals.lww.com/10.1097/RLI.0000000000000192). Copyright 2015 Wolters Kluwer Health, Inc.
4.2.2. Optimizing the rotational correlation time (τR) of Gd(III)-based contrast agents
As discussed above, r1 for simple chelates is limited by fast rotation at all accessible field strengths above 0.1 T. Slowing down the rotational motion of the complex is an effective way to increase relaxivity. This can be achieved by increasing the molecular weight of the contrast agent. This in turn can be achieved by making the complex larger, or by the covalent or non-covalent binding of the contrast agent to a macromolecule (e.g. a protein, a polypeptide, a nanosphere, or micellar nanoparticles). The linear dependence of the molecular weight of monohydrated Gd-DOTA derivatives and their T1 relaxivities (0.5 T, 25 °C) is illustrated in Figure 28, left.289 Similarly, the rotational correlation time exhibits the same linear correlation with the molecular weight of those Gd(III) complexes Figure 28, right.
Figure 28:
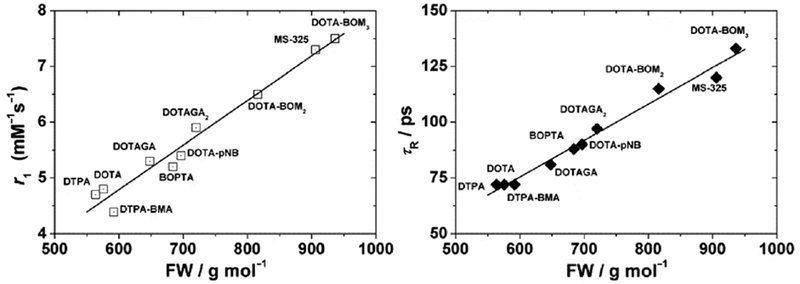
Left: Plot of r1 (0.5 T and 25 °C) for monohydrated DOTA-based Gd(III) complexes vs. molecular weight (R = 0.984). Right: Plot of τR evaluated from the NMRD profiles, vs. molecular weight (R = 0.991). Reproduced with permission from Ref.289 (URL http://dx.doi.org/10.1039/9781788010146-00121). Copyright 2018 Royal Society of Chemistry.
In order to attach paramagnetic agents to macromolecules, bifunctional chelates have been developed.290 Typically, they are based on the chelators DOTA or DTPA and are bearing an electrophilic group, such as isothiocyanates, N-hydroxysuccinimide ester, maleimides etc., that is available for conjugation to nucleophilic groups on macromolecules. Excellent reviews are available, in which those agents are covered.291–293
In an early example, Gd-DTPA chelates were covalently attached to HSA yielding the macromolecular contrast agent albumin-(Gd-DTPA)19 with a molecular weight of 92 kDa. The T1 relaxivity per Gd(III) ion was found to be 3 times higher than that of the monomeric chelate (20 MHz, 37 °C), which is attributed to the larger molecular weight of the contrast agent and hence, its higher rotational correlation time.294–296 Gd-DTPA has been also conjugated to poly-L-lysine.297 The resulting macromolecular compound (average molecular weight: 49 kDa) possesses a r1 value that is also 3 times higher than that of the monomeric chelate.298
Aside from tethering a Gd(III) complex to a macromolecular structure through a linker, peptides or proteins were designed with embedded Gd(III) binding sides. Franklin and coworkers prepared the peptide chimera GdP3W, a 4 kDa metallopeptide with an EF-hand Gd(III) binding site and DNA binding domain.299 The rigid structure and extended hydration sphere (q = 2) of GdP3W furnishes a r1 value that is 6 times higher than that of Gd-DTPA (60 MHz, 37 °C). Moreover, a 100 % increase in its T1 relaxivity was observed upon binding to DNA.
Another example are the protein-based Gd(III) MRI contrast agents (ProCAs).300 Yang et al. showed that engineered proteins chelated with Gd(III) (average molecular weight: 12 kDa) have relaxivities that are 20-times higher than that of Gd-DTPA, and are inert to Gd(III) release.301 Protein engineering with tumor targeting modalities allowed imaging of e.g. prostate or liver cancer in mouse models.302–303
Another strategy to increase the molecular weight of the MR contrast agent is to link multiple Gd(III) complexes together, which in turn also enhances the number of Gd(III) ions per molecule and therefore the overall contrast. In this approach, the coupling of multiple Gd(III) compounds to spherically shaped carriers, e.g. dendrimers, is more effective than linking Gd(III) complexes in a linear fashion. Although there is internal motion in both instances (see below), dendrimers rotate in a more isotropic fashion whereas linear oligomers rotate anisotropically. Linear oligomers will rotate more rapidly about their long axis and this faster rotation will limit relaxivity. A Gd-DO3A derivative assembled to a spherical dendrimer59 has 40% higher relaxivity per gadolinium ion than the same Gd-DO3A derivative assembled to a linear dextran polymer,304 although the molecular weight of the linear polymer (52 kDa) is higher than that of the dendrimer (17 kDa). The different types of motion are depicted in Figure 30.109
Figure 30:
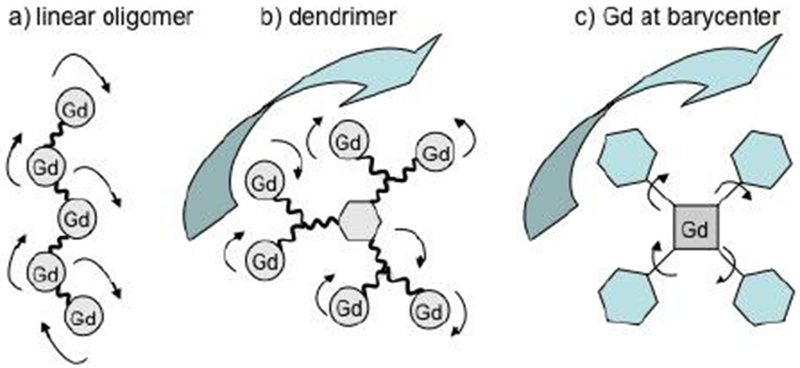
Different strategies to increase relaxivity by modulating rotational motion: A) linear polymer of Gd(III)-complexes that rotates anisotropic (low relaxivity); B) Gd(III) chelates assembled to a dendrimer that rotates isotropically, but internal motion is still possible (higher relaxivity); C) Gd(III)-complex at the barycenter of the molecule, so that it can only rotate at the rate of the entire molecule (highest relaxivity).109
Internal Motion
The nuclear relaxation induced by the chelate is dependent on the rotation about the M-H(water) vector. To this point we have considered that the rotational motion of this vector is isotropic and mirrors that of the complex. This is a good approximation in many cases. But there are often internal rotations that are occurring faster than the overall reorientation of the entire molecule. The simplest of these is rotation about the M-O bond. Over 40 years ago, Dwek pointed out that for water coordinated to a metal ion, fast rotation about the metal-oxygen bond will decrease the efficiency of the paramagnetic relaxation effect.305 For MRI contrast agents, this fast intramolecular motion will reduce the relaxivity of the complex.
Direct experimental assessment of water rotation is challenging. Using the Ln-DOTAM system where the water exchange rate is very slow, Dunand et al. were able to measure the 17O and 1H relaxation times of the coordinated water for the Eu(III) complex and determine the effect of this fast rotation.306–307 However water exchange in most lanthanide complexes is too fast for direct observation of the coordinated water ligand. Nonetheless, this fast intramolecular rotation is assumed to occur in many Gd(III) complexes, including those used as commercial MRI contrast agents.144, 293 The effect of this fast rotation is to reduce inner-sphere relaxivity by about 30%.
Recently Boros et al. showed that this fast water rotation could be reduced by the formation of an intramolecular hydrogen bond between the coordinated water hydrogen and a suitable H-bond acceptor (Figure 31), and that the resultant complex would have a higher relaxivity than similar compounds lacking the H-bond acceptor.123 In that study the authors used Gd-DOTAla derivatives with pendant H-bond acceptors as well as control compounds that were structurally very similar but lacking this H-bond acceptor. There are several reports of gadolinium(III) complexes having anomalously high relaxivity compared to related complexes of similar size, shape, and charge. For instance, work of Lowe et al. describes Gd(III) complexes with a pH-dependent, intramolecular ligation of a β-arylsulfonamide nitrogen which results in changes in the hydration state at the lanthanide center.308 The relaxivity observed for the q = 2 complexes was found to be anomalously high, and possibly indicative of interaction with the bound water by pendant, non-coordinating carboxylates. Similarly, Dumas et al. described Gd(III) complexes with unexpectedly enhanced relaxivity that possess pendant non-coordinating carboxylate donor arms.309–310 Previously, these cases of high relaxivity have usually been ascribed to a second-sphere effect wherein water molecules in the second hydration sphere have extended residency times because of interactions with H-bond acceptor(s) on the chelating ligand. However in many of those complexes it is likely that the H-bond acceptor could also be interacting with the inner-sphere coordinated water and hindering the water rotation.
Figure 31:

Restricting the water ligand mobility (specifically rotation about the Gd-O bond), in Gd(III) complexes through interaction with an intramolecular H-bond acceptor. Reproduced with permission from Ref.123 (URL: http://dx.doi.org/10.1002/anie.201702274). Copyright 2017 John Wiley and Sons.
For more complicated molecules such as multimeric agents or chelates (non)covalently attached to a macromolecule, there can be additional internal motions that reduce relaxivity. For example if the chelate is covalently attached to a macromolecule by a linker group, then the chelate can rotate about this linker so that the rotation of the chelate is decoupled from the macromolecule and the relaxivity is not as high as predicted based on the overall motion of the macromolecule.311–314 For these complex systems where there are a number of internal motions, the data are usually analyzed by a model free approach described originally by Lipari and Szabo.315 They showed that the usual spectral density function for isotropic motion could be replaced by two terms, one that reflected the overall rotation (or global motion (τg)) of the molecule and another that reflected internal (or local) motions (τl) (equation 25),
| (25) |
| (26) |
| (27) |
with C as a constant (as in equation 9) and F as a measure for the degree of isotropic motion.
The relative contributions to the global and local motions is given by an order parameter S2 (sometimes denoted F to avoid confusion with the spin quantum number). At one extreme, the rotational motion of the chelate is completely decoupled from the macromolecule (F = 0). On the other extreme (F = 1), the chelate is immobilized on the macromolecule such that internal motion is prevented, and the spectral density function is the same as for isotropic rotation. In Figure 32 the effect of internal motion on relaxivity is simulated for a system with a very long rotational correlation time (10 ns) associated with a macromolecule and a local rotational correlation time of 0.1 ns at proton Larmor frequencies ranging from 1-100 MHz. Figure 32 shows that internal motion can dramatically reduce relaxivity. However the effect of internal motion on relaxivity is more pronounced at lower fields (<1 T).109
Figure 32:
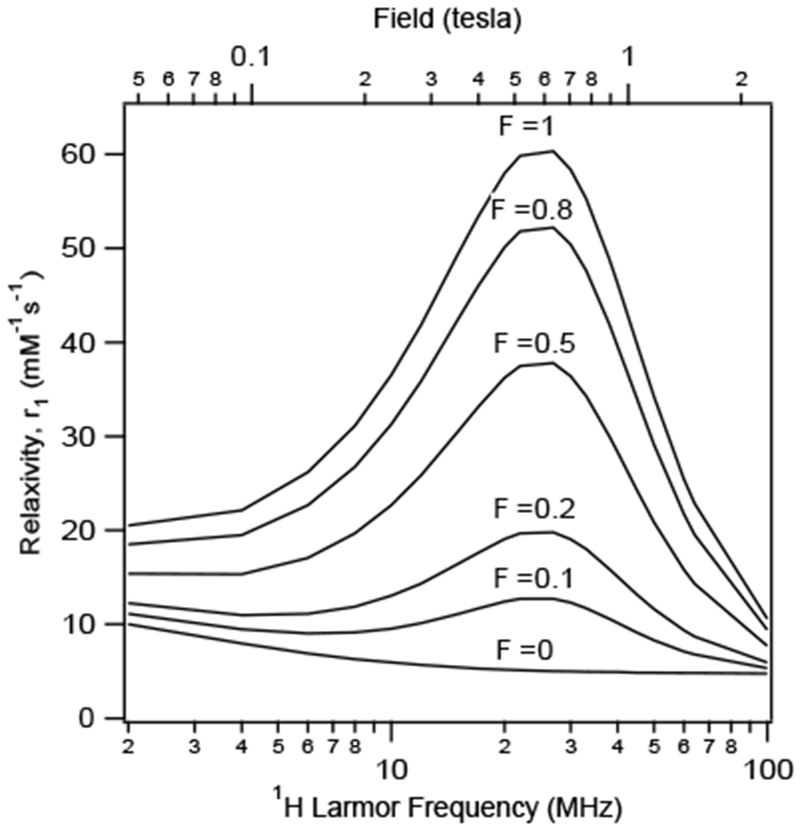
Simulation illustrating the effect of internal motion on the field dependent T1 relaxivity (hypothetical Gd(III)-compound with a global correlation time of 10 ns and a local correlation time with 0.1 ns. F gives the degree of internal motion.109
Strategies to minimize internal motion
One effective strategy to minimize internal motion is to force the metal-water proton vector to rotate isotropically with the entire molecule. Parker and coworkers showed that this can be done by placing metal at the molecular barycenter (Figure 30) to create contrast agents with low molecular weight (3 – 7 kDa) but still remarkably high relaxivity in the region of 20 – 40 mM−1s−1.31, 316–317 In these studies they used 4 large hydrophilic pendant arms to increase the molecular size of Gd-DOTA derivatives. The blood pool agent P792 utilized the same strategy.32
Another approach is to limit internal motion by connecting the chelate to the macromolecular structure by two points of attachment. Early examples were the self-assembled metallostar compounds, where the rigid and multimeric structure of the MR contrast agent is formed in the presence of Fe(II) ions.318–320
In another example, Zhang et al. combined a multimeric approach with protein binding to boost relaxivity.321 They synthesized a tetrameric Gd-DTPA oligomer with two HSA binding groups (Figure 33). In the absence of protein, the relaxivity is relatively low because of the flexible multimeric structure. But upon binding to HSA the contrast agent is rigidified and rotational motion slowed, dramatically increasing relaxivity. This is shown schematically in Figure 33. For small molecules like MS-325, relaxivity is low (Figure 33, A) but is increased several fold upon protein binding (Figure 33, B). However for multimeric agents, the gain in relaxivity upon protein binding is often offset by the internal motion (Figure 33, C). But using a dual anchor strategy overcomes this relaxivity loss (Figure 33, D)). Indeed, the authors obtained an enhanced r1 of 39.1 mM−1s−1 (20 MHz, 37 °C, pH 7.4) for their dual binding Gd(III) tetramer in the presence of HSA (Figure 33, E)). This was 50 % higher than the relaxivity obtained for the same tetramer with only one HSA binding unit (schematic illustration in Figure 33, C)) where r1 = 26.2 mM−1s−1 measured under the same conditions. On a molecular basis, the relaxivity is 4 times higher.
Figure 33:
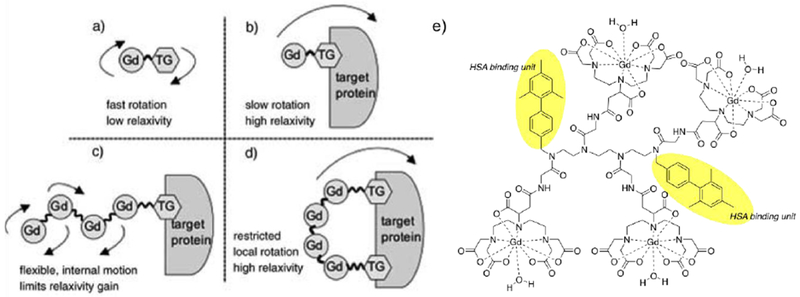
Strategies for increasing relaxivity: A) Gd(III)-complex with targeting moiety that tumbles fast (low relaxivity); B) Binding of the Gd(III)-complex to the targeting protein slows tumbling and the relaxivity increases; C) Gd(III)-based multimer bound to the target protein, relaxivity might be limited through internal motion; D) Gd(III)-based multimer bound to the target protein via two points of attachment, limited internal motion increases relaxivity; E) Structure of the Gd-DTPA-based multimer. Reproduced with permission from Ref.321 (URL: http://dx.doi.org/10.1002/anie.200502245). Copyright 2005 John Wiley and Sons.
Another example of a dual anchor strategy applied to liposome based contrast agents was reported by Botta, Tei, and coworkers.322–323 Amphiphilic Gd(III)-chelates can be incorporated into liposomal bilayers resulting in increased relaxivity. However if the chelate has one alkyl chain for incorporation, significant internal motion can occur. The authors used Gd-DOTA(GA)2 derivatives that had near optimal water exchange kinetics and two points of attachment to the lipid bilayer. As a result relaxivity was doubled compared to the analogous compound with only a single point of attachment.
Contrast agents with very long rotational correlation times (on the order of 10 ns) can have near optimal relaxivity at low fields (0.5 – 1 T), but for these types of compounds the relaxivity falls off dramatically at higher field strength. For higher field strengths, one requires an intermediate rotational correlation time (on the order of 1 ns) which will result in very good relaxivity at 1.5 T and close to optimal relaxivity at 3 T and 7 T.
As an example, Figure 34 shows the influence of the magnetic field strength on the T1 relaxivity of three compounds with different rotational correlation times: Gd-HPDO3A (short τR, see Figure 3), Gd3L3 (intermediate τR, see Figure 35), and MS-325 bound to HSA (long τR, see Figure 3).324 Gd3L3 employs the Gd-DOTAla chelate which allows it to be incorporated into a peptide structure via two points of attachment, thereby limiting internal motion. Figure 34, A presents r1 per molecule and Figure 34, B presents r1 per Gd(III). At 0.47 T, the compounds with intermediate or long τR exhibit similar molecular T1 relaxivities, but with increasing field strength (here, to 11.7 T), the difference between those agents becomes larger. Gd3L3 with its intermediate correlation time is superior to HSA-bound MS-325, 50 % higher at 1.5 T and 350 – 450 % higher at 4.7 – 11.7 T. On a per Gd(III) basis, Gd3L3 has 50 – 220 % higher r1 than HSA-bound MS-325 at high fields. Note that the T1 relaxivity of Gd-HPDO3A (short τR) stays roughly the same at all field strengths.
Figure 34:
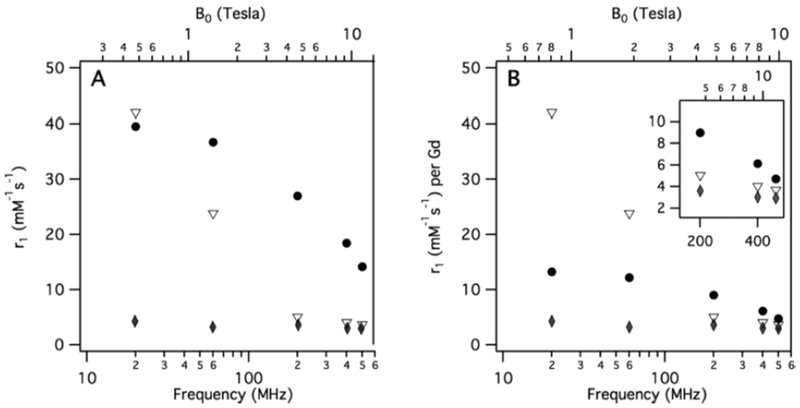
T1 relaxivities of Gd3L3 (sphere), MS-325 with excess HSA (triangle) and Gd-HPDO3A (diamond) as a function of magnetic field strength at 37 °C. A) T1 relaxivity plotted per molecule, at 60 MHz and higher frequency: molecules with intermediate correlation time (Gd3L3) are much more potent relaxation agents than slow (MS-325 with excess HSA) or fast Gd-HPDO3A tumbling compounds; B) T1 relaxivity plotted per Gd(III)-ion shows the same trend. Reproduced from Ref.324 (URL: http://dx.doi.org/10.1021/ja309187m). Copyright 2012 American Chemical Society.
Figure 35:
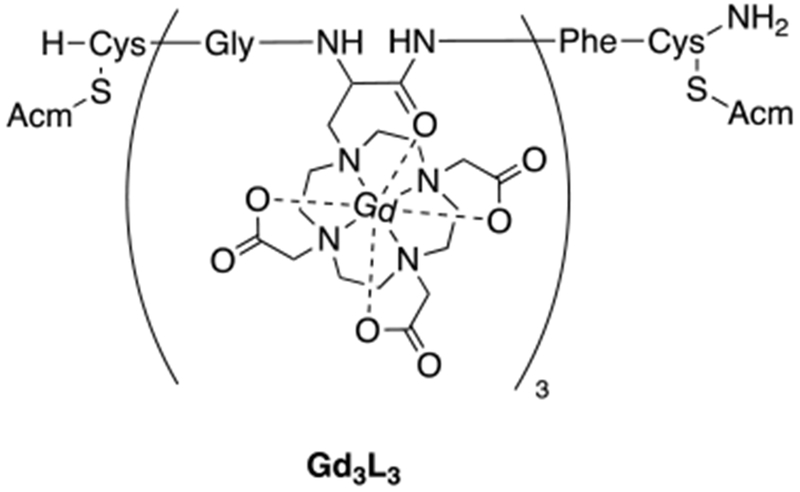
Chemical structure of the Gd3L3.
Since clinical imaging is increasingly moving to higher field strengths, it is important to develop contrast agents that are applicable at high field. For 3 – 7 T, a rotational correlation time in the range of 0.5 – 2 ns is desirable. Still, it should rotate isotropically with limited internal motion. Recently, several examples for high field Gd(III)-based MR contrast agents were reported.320, 324–330
It should be noted that the same strategies apply to Mn(II), Fe(III), and Mn(III) chelates. Slowing down rotation by protein binding results in large increases in relaxivity at lower field strengths.331–333 Similarly, rigid multimers with multiple chelates can provide intermediate rotational correlation times that provide enhanced high field relaxivity.334
4.2.3. Tuning the water residency time (τm) of Gd(III)-based CAs
Another parameter that can be tuned to increase r1 is the water residency time (τm). The coordinated water must be in rapid exchange with bulk solvent in order to transmit the relaxation effect to the solvent. Relaxivity can be limited if τm is too slow or too fast. It is important to note that for fast tumbling complexes T1m >> τm, and so changing τm has little to no effect on relaxivity. However once the rotational correlation time begins to be optimized, then τm can play a major role.
As an example, optimal values for contrast agents with a water residency time similar to Gd-DTPA or Gd-DOTA (τm ~100 ns at 37 °C) are around 10 ns (Figure 36, left). However, incorporation of the gadolinium(III) compound into a macromolecule might affect the other parameters that influence the relaxivity. MS-325 is a Gd-DTPA derivative that can non-covalently bind to the plasma protein human serum albumin (HSA) (Figure 36, right). MS-325 (unbound) has a τR of 115 ps. Once bound to HSA, MS-325 (bound) has a τR of 10.1 ns, which causes a dramatic increase in T1 relaxivity. However, the observed T1 relaxivity is not as high as it theoretically should be, because another parameter that governs relaxivity, the water residency time (τR) is influenced by this modification. Indeed, MS-325 (unbound) has a τR of 69 (± 20) ns and MS-325 (bound) has a τR of 170 (± 40) ns. The water residency time becomes crucial when the rotational motion is slowed, which limits the observed T1 relaxivity.17 This shows that the lability of the water molecule at the metal side can be influenced when a supramolecular adduct is formed.335
Figure 36:
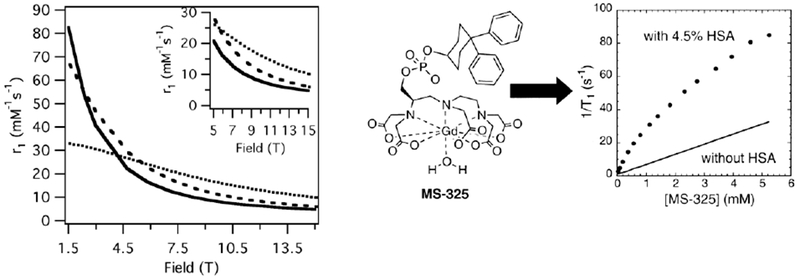
Left: Effect of rotational correlation time on T1 relaxivity as a function of field for a Gd(III) complex with a water residency time 100 ns. τR = 0.1 ns (…), 1.0 ns (---), 10 ns (—). Reproduced with permission from Ref.144 (URL: http://dx.doi.org/10.1002/cmmi.267). Copyright 2009 John Wiley and Sons. Right: Effect of HSA binding on T1 relaxation rate for MS-325. Reproduced from Ref.17 (URL: http://dx.doi.org/10.1021/ja017168k). Copyright 2002 American Chemical Society.
The effect of water exchange of slow tumbling systems is also seen when comparing MS-325 and its bis(N-methyl)amide derivative: MS-325-BMA (Figure 37). Gd(III) complexes with amide oxygen donor atoms are known to have slower water exchange rates compared to the analogous carboxylate derivatives.111 The water exchange rate of MS-325-BMA is 10 times slower than the water exchange rate of MS-325. Since both MS-325 and MS-325-BMA have a similar size, their rotational correlation times and T1m values should be similar in phosphate buffered saline (PBS) or when bound to HSA. In PBS the relaxivity of these complexes is alike indicating that T1m >> τm. However, once bound to HSA the relaxivity of MS-325 is 3 times higher than that of MS-325-BMA. When bound to HSA, T1m is shortened to the point that the long τm of MS-325-BMA limits its relaxivity.109
Figure 37:
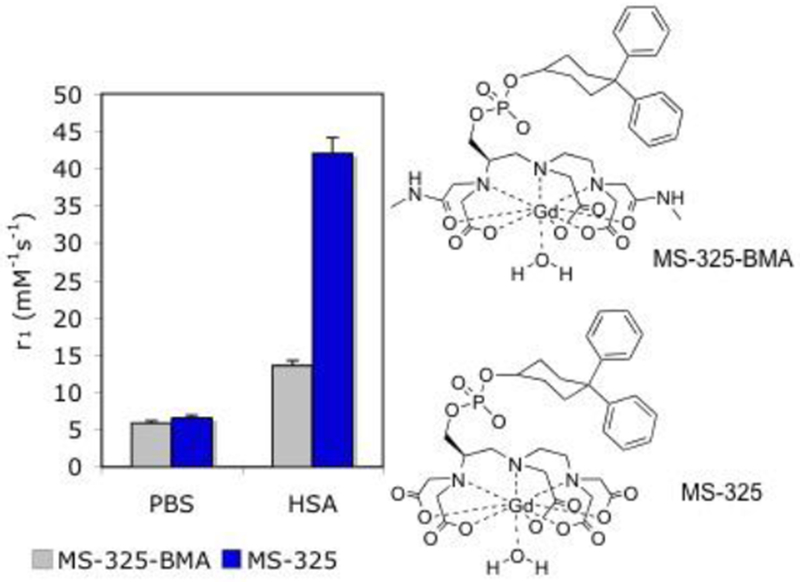
Relaxivity of 0.1 mM MS-325 and MS-325-BMA in pH 7.4 phosphate buffered saline or 0.67 mM HSA solution at 37 °C and 0.47 T. MS-325 is 88 % bound to HSA under these conditions and MS-325-BMA is 83 % bound.109
The water exchange rate can be accelerated by increasing the steric compression around the water binding site. This was originally demonstrated by Merbach and co-workers in octadentate chelates, such as Gd-DTPA (Figure 3) and Gd-EPTPA (Figure 39).111, 143, 336–338 However, for these Gd-DTPA derivatives, the rate enhancement is achieved at the cost of lower thermodynamic stability.
Figure 39:
Chemical structures of Gd-EPTPA, Gd-DO3A-pyNox, Gd-DO3APABn, and Gd-ebpatcn.
The steric crowding in Gd-DOTA derivatives can also be enhanced by introducing bulky groups. Dumas et al. found that exchanging one acetate arm by a different donor group led to an increase in τm in the following order: phosphonates ~ phenolates < alpha-substituted acetates < acetate < hydroxamates ~ sulphonamides < acetamide ~ pyridyl ~ imidazole, which is also in line with the general trend that neutral donors slow down water exchange kinetics in comparison to negatively charged donor groups.112 In these complexes, the coordinated water is released through a dissociative exchange process that is favored by steric crowding.270
In total, Dumas et al. reported 38 Gd-DOTA derivatives with HSA binding groups, and the water residency time varied over 3 orders of magnitude. In the absence of HSA, r1 was similar for all compounds with a given q values and correlated with molecular weight. As expected the relaxivity did not correlate with τm. The influence of τm on relaxivity becomes apparent when the relaxivity was measured in the presence of HSA. Slow tumbling shortens T1m and now τm can limit relaxivity. Figure 38 shows the relaxivity for this series of chelates bound to HSA as a function of their water residency time at two different field strengths (0.47 T and 1.4 T). As predicted by theory there is an optimal water residency time at low field for slow tumbling compounds, which peaks between 10 ns and 30 ns (0.47 T). When increasing the field strength to 1.4 T, the range of τm for optimal relaxivity becomes broader.
Figure 38:
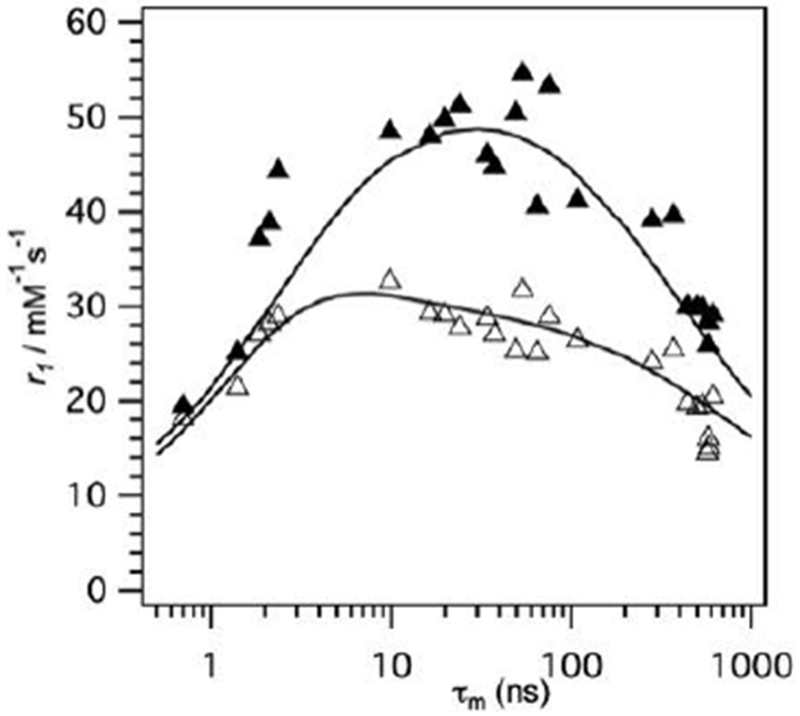
Relaxivity of slow tumbling HSA-bound Gd-DOTA derivatives plotted vs. measured water residency time at 37 °C at 20 MHz (filled triangles) or 60 MHz (open triangles), respectively. τm limits relaxivity at a given field strength if it is either too short or too long. The range of τm for optimal relaxivity becomes broader at higher field. Reproduced with permission from Ref.112 (URL: https://journals.lww.com/10.1097/RLI.0b013e3181ee5a9e). Copyright 2010 Wolters Kluwer Health, Inc.
With phosphonate groups in particular, the steric crowding combined with the favorable arrangement of the water molecules in the second coordination sphere leads to a faster water exchange rate.340 For instance, Gd-DO3A-pyNox (Figure 39), a Gd-DO3A derivative bearing a sterically demanding group has a water residency time of 39.0 ns (25 °C) while Gd-DOTA has a water residency time of 244 ns (25 °C).341 The water residency time in a monophosphinic acid derivative Gd-DO3APABn (Figure 39) was found to be even faster with τm = 16.2 ns (25 °C).340 Boros et al. recently showed that for slow tumbling systems with exceptionally fast water exchange that the water residency time becomes the correlation time influencing relaxvity and results in a useful strategy to achieve high field, high relaxivity.342
4.2.4. High relaxivity Gd(III)-nanomaterials
Gd(III)-nanomaterials have been the subject of intensive research for the past two decades since they have unique potential to overcome the sensitivity disadvantage of contrast agents. Their ability to carry high Gd(III) payloads combined with their long rotational correlation times makes them excellent candidates in the design of high relaxivity MRI contrast agents. Additionally, these materials offer great flexibility in terms of surface modifications for e.g. multifunction or bioconjugation for therapeutic or diagnostic applications, respectively.
Nanoparticles engineered as MRI contrast agents have been based on carbon nanotubes and fullerenes, polymers and dendrimers, gold, silicon, viral nanoparticles, and liposomes and micelles. A comprehensive overview of the recent progress on Gd(III)-nanomaterials as MRI contrast agents is beyond the scope of this review. Excellent reviews are available that cover those agents.343–347
An impressive example for a Gd(III)-nanosystem with exceptional high relaxivity was reported by Wilson and coworkers. They developed gadonanotubes (Gd3+n@US tubes), that consist of aquated Gd(III)-ion clusters confined within single-walled carbon nanotube capsules.348 At clinical field strength, the r1 values were found to be 40 times higher than that of the Gd(III)-based ECF contrast agents (r1 = ~170 mM−1s−1 per Gd(III)-ion, 60 MHz, 40 °C).
Meade and coworkers took a different approach and conjugated Gd-DO3A derivatives to the surface of nanodiamonds.349 The resulting Gd(III)-nanodiamond conjugate affords a T1 relaxivity that is 10 times higher than that of the monomeric Gd(III)-complex (r1 = 58.8 mM−1s−1 per Gd(III) ion, 60 MHz, 37 °C).
In a recent example, it was shown that water permeable silica nanoparticles with confined monohydrated Gd-ebpatcn chelates (Figure 39) feature a nanosized contrast agent with an extraordinary high r1 value of 84 mM−1s−1 at 35 MHz (25 °C).350
In another example, Chuburu and coworkers incorporated Gd-DOTA chelates into a polysaccharide-based hydrogel (Gd-DOTA-NPs).351 As illustrated in Figure 40, the T1 relaxivity of the Gd(III)-loaded nanoparticle was found to be 24 times higher than that of the monomeric Gd-DOTA complex (r1 = 72.3 mM−1s−1 per Gd(III)-ion, 60 MHz, 37 °C).
Figure 40:

NMRD relaxivity profiles of Gd-DOTA-NPs at different concentrations (T = 37 °C) showing the characteristic shape of a compound with slow rotational correlation times. These results indicate that the rotational motion of Gd-DOTA inside the hydrogel is restricted. Dotted line: NMRD relaxivity profile of Gd-DOTA (T = 37 °C). Reproduced with permission from Ref.351 (URL: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201203190). Copyright 2012 John Wiley and Sons.
4.3. Increasing resistance to Gd(III) release
Trivalent gadolinium is an oxyphilic hard cation that favors coordination numbers of 8 and 9 with negatively charged oxygen atom donors preferred to neutral oxygen donor atoms. This becomes apparent by comparing the stability constants of [Gd(DOTA)(H2O)]− and [Gd(HP-DO3A)(H2O)], in which a negatively charged acetato donor was replaced by an uncharged hydroxyl donor (Figure 3; log KGdL = 24.7 vs. log KGdL = 23.8, respectively).
The formation of 5-membered chelate rings is generally preferred, whereas the chelate ring size is one of the dominating factors that govern stability. It is well known that changes in chelate ring size greatly influence stability. As an example introducing one or two additional methylene units in the macrocyclic backbone of Gd-DOTA gives Gd-TRITA and Gd-TETA (Figure 41), respectively and causes a drop in thermodynamic stability in 5 orders of magnitude each.206 Such a drop in stability can also be observed by changing the pendant acetato moiety against a pendant propionato moiety, which also increases the chelate ring size from a 5-membered to a 6-membered ring.352
Figure 41:
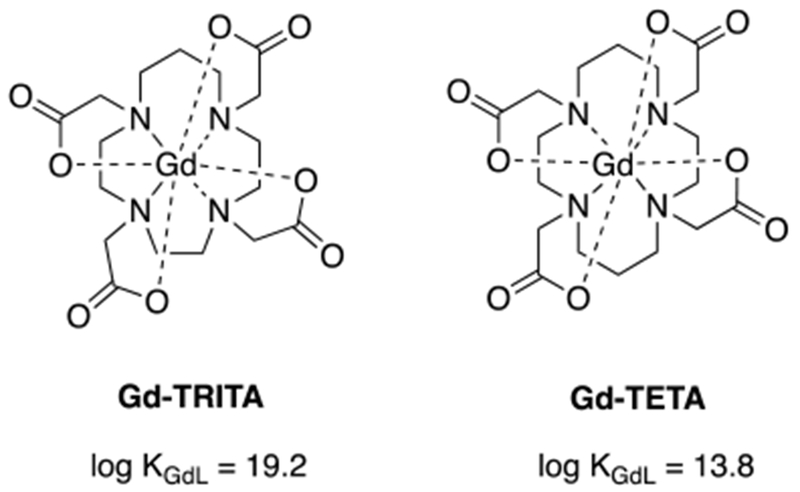
Thermodynamic stability of Gd-TRITA and Gd-TETA.353
Expanding the macrocyclic backbone by additional methylene units does not only decrease the thermodynamic stability, but also increases the kinetic lability. This can be explained by a higher degree of flexibility that is introduced within the macrocyclic backbone with every additional methylene unit. This in turn accelerates the dissociation rates. Hence, Gd-DOTA is kinetically 3 orders of magnitude more inert than Gd-TRITA and 6 orders of magnitude more inert than Gd-TETA.354 Moreover, this example nicely illustrates that macrocyclic chelators do not automatically guarantee superior kinetic inertness. Other macrocyclic systems that show rather fast dissociation kinetics were reported recently.131, 355 Increasing the chelate ring size of the pendent carboxylate by replacing an acetato group through a propionato group also lowers the kinetic inertness of the system.352
Another factor that has been identified to increase the kinetic inertness of the Gd(III)-based agent is to reduce the basicity of the ligand. In those systems, protonation of the chelator is less favored. As a result proton-catalyzed dissociation is further hampered, which decelerates the dissociation rate. Indeed, Gd-DOTA-tetraamide based complexes (Figure 42) that are less basic than the parent DOTA compound were found to be considerably more inert but at the same time they are 10-11 orders of magnitude thermodynamically less stable than Gd-DOTA.221
Figure 42:
Chemical structure of selected ligand systems.
Recently, a highly rigid open-chain derivative Gd-cddadpa was reported with dissociation rates that become comparable to inert macrocyclic complexes and sufficient thermodynamic stability (log KGdL = 20.68, Figure 42).356 Its kinetic inertness was characterized by studying the rate of the metal exchange reaction occurring with Cu(II) (c = 1 µM, 25 °C) at physiological pH. Under those conditions, the dissociation half-life of Gd-cddadpa is 3 orders of magnitude higher than that of Gd-DTPA (t1/2 = 1.49 × 105 h vs. t1/2 = 202 h, respectively) and in the range of that of Gd-DO3A (t1/2 = 2.10 × 105 h).
Ln(III) complexes of a cross-bridged cyclam derivative containing two picolinate pendant arms show exceptional kinetic inertness, which even overcome that of Ln-DOTA complexes by far (Figure 42).357 Under very harsh conditions, such as 2 M HCl, no sign of dissociation has been observed for those complexes for at least 5 month. In contrast, Ln-DOTA has a half-live of <12 hours when subjected to the same conditions. Unfortunately, the corresponding Eu(III) cross-bridged cyclam derivative only exhibits a hydration state of q = 0.25 – 0.35 limiting the relaxivity of this class of contrast agent. However, the correlation between the rigidity of a molecular framework and the kinetic properties of the resulting complex is once more highlighted with this example.
Recently, Law and coworkers reported a series of chiral, backbone substituted Gd-DOTA derivatives, that are kinetically more inert than the parent Gd-DOTA complex.358 For instance, Gd-L2A (SAP isomer) (Figure 42) showed almost no sign of Gd(III) release in 1 M HCl after 8 days while t1/2 for Gd-DOTA is <25 hours when subjected to the same conditions. Notably, these compounds afford modestly higher r1 values than Gd-DOTA (e.g. GdL2A: r1 = 4.7 mM−1s−1, 60 MHz, human blood plasma, 37 °C) and possess very fast water exchange rates that are in an optimal range for high field imaging (e.g. Gd-L2A: τm = 6.6 ns at 37 °C).
A special kind of MR contrast agent are gadofullerenes that are based on carbon nanomaterials. Fullerenes are closed up, hollow and spheroidal structures that are composed of carbon atoms. Gd(III) can be entrapped within those carbon cages to yield paramagnetic particles, such as Gd@C60, Gd@C82 or Gd3N@C80. The high chemical stability of fullerenes can resist any potential metabolic cage-opening process and hence, completely prevents the release of Gd(III) ions.359–361
4.4. Gadolinium(III)-free alternatives
4.4.1. Gd(III)-free small molecule relaxation agents
The Gd(III) ion is a potent relaxation agent due to its 7 unpaired electrons and isotropic electronic ground state but there are a few other metal ions – namely high spin Mn(II), high spin Mn(III), high spin Fe(III), and Eu(II) that can serve as effective relaxation agents.102
The d5 Mn(II) ion is a very effective relaxation agent due to its preference for a high-spin (S=5/2) electronic configureuration and characteristically long T1e.102 Aqueous Mn(II) complexes typically have coordination numbers (CN) of 6 or 7.362 Whereas Gd(III) complexes can typically accommodate both an octadentate chelator and a water co-ligand, Mn(II) chelators cannot exceed hexadenticity and simultaneously accommodate an exchangeable water ligand. In general the stability constants of Mn complexes are lower compared to Gd(III) complexes utilizing comparable donor groups, and the Mn complexes tend to be more labile. For example, log KML for [Gd(DTPA)(H2O)]2– and [Mn(EDTA)(H2O)]− are 22.5 and 13.9, respectively.2, 362 The dissociation half-lives of [Gd(DTPA)(H2O)]2− and [Mn(EDTA)(H2O)]− in the presence of 10 µM challenging Zn(II) at pH 7.4 are 330 h and 0.07 h, respectively.362–363 The lower stability/inertness of Mn(II) complexes is due to its lower charge-to-radius ratio and its absence of ligand field stabilization energy. The poor stability and kinetic inertness of Mn(II) complexes have been the main barriers towards the development of Mn based contrast agents that are capable of replacing Gd(III) complexes.
A number of Mn complexes supported by various linear and macrocyclic ligands have been evaluated as potential MRI contrast agents. (Figure 43, Table 8).362 Linear ligands like EDTA form 7-coordinate ternary Mn complexes with a water co-ligand that are also amongst the most thermodynamically stable Mn complexes. A number of high-relaxivity Mn-EDTA derivatives have been evaluated as MRI contrast agents. The benzyloxymethyl (BOM) functionalized complexes Mn-EDTA-BOM and Mn-EDTA-BOM2 were amongst the first reported Mn complexes of very high relaxivity. The peripheral BOM functional group promotes high affinity interactions with HSA and Mn-EDTA-BOM and Mn-EDTA-BOM2 exhibit r1 values of 55.3 mM−1s−1 and 48.0 mM−1s−1, respectively upon HSA binding at 20 MHz and 25 °C. Mn-L1 is a high-relaxivity, serum albumin-binding Mn-EDTA derivative analogous to MS-325. Mn-L1 was used to conspicuously detect narrowing of the carotid artery and jugular vein in a rabbit model of arterial and venous stenosis. A hexameric Mn-EDTA derived agent was also designed for high-relaxivity at higher (>3.0 T) field strengths. At 4.7 T and 37 °C, a relaxivity of was 6.6 mM−1s−1 per Mn was measured for the hexamer, whereas the monomeric EDTA-derived chelator had a relaxivity of 3.0 mM−1s−1.
Figure 43:
Mn(II) complexes previously considered in the context of MRI contrast.
Table 8:
Comparison of r1, r2, hydration state (q), water exchange rate (kex), enthalpy of activation of water exchange (ΔH≠), and Mn-H(water) hyperfine coupling constant for Mn(II) complexes that have been considered as MRI contrast agents.
| r1298 (mM−1s−1) | r1310 (mM−1s−1) | r2310 (mM−1s−1) | q | kex310 (× 107 s−1) | ΔH≠ | Ao/ħ (× 107 rad/s) | |
|---|---|---|---|---|---|---|---|
| PyC3A | 2.8a, 3.3b | 2.1a, 2.5b | 4.9a | 1 | 11 | 37.2 | 2.87 |
| EDTA332 | 3.0c | 2.2a | 3.7a | 1 | 59 | 36.7 | 3.79 |
| EDTA-BOM332 | 3.6b | -- | -- | 1 | 19 | 43.1 | 3.79 |
| EDTA-BOM2332 | 4.3b | -- | -- | 1 | 25 | 38.4 | 3.79 |
| EDTA-Tyr iso334 | -- | 3.2a, 3.6b | 1 | 30 | 35.6 | 2.92 | |
| Mn-453138, 331 | 5.8b | -- | -- | 0.8 | 30 | 21.1 | 3.33 |
| CDTA138 | -- | 2.1a,d | 3.5a,d | 1 | 27 | 35.8 | 3.14 |
| AAZTA368 | 1.6b368 | -- | -- | 0 | -- | -- | -- |
| AAZ3A368 | 2.5b | -- | -- | 0<q<1 | 7.0 | 22.8 | -- |
| MeAAZ3A368 | 2.0b | -- | -- | 0<q<1 | 20 | 26.5 | -- |
| AAZ3MA368 | 1.9b | -- | -- | 0<q<1 | 18 | 16.7 | -- |
| HBET371 | -- | 2.8a | 9.4a | 1 | 370 | 33.8 | 3.54 |
| HBET-OMe371 | -- | 3.1a | 11.1a | 1 | 360 | 40.7 | 4.15 |
| HBET-NO2371 | -- | 2.3a | 4.8a | 0.5 | 48 | 41.2 | 3.48 |
| CyHBET371 | -- | 3.3a | 6.0a | 1 | 770 | 41.2 | 3.36 |
| CyHBET-OMe371 | -- | 3.3a | 5.8a | 1 | 300 | 20.7 | 4.02 |
| CyHBET-NO2371 | -- | 2.3a | 3.7a | 1 | 190 | 31.3 | 3.97 |
| ENOTA376 | 3.4b | 2.7b | -- | 1 | 7.9 | 20.5 | 3.27 |
| 9-aneN2O-2A365 | 2.8b | 2.3b | -- | 1 | 2.3 | 38.8 | 3.33 |
| 9-aneN2O-2P365 | 5.1b | 4.3b | -- | 1 | 140 | 11.7 | 3.33 |
| 1,4-DO2A364 | 2.1b | -- | -- | 0<q<1 | 110 | 29.4 | 4.10 |
| 1,7-DO2A364 | 1.5b | -- | -- | 0 | -- | -- | -- |
| DO1A364 | 2.4b | -- | -- | 1 | 500 | 17.6 | 3.94 |
| 12-pyN4A366 | 2.4b | 1.9b | -- | 1 | 330 | 13.0 | 3.66 |
| 12-pyN4P366 | 2.8b | 2.3b | -- | 1 | 250 | 14.0 | 3.99 |
| 15-pyN5367 | 4.5b | 3.6b | -- | 2 | 13 | 37.7 | 3.86 |
| 15-pyN3O2367 | 3.6b | 3.1b | -- | 2 | 0.69 | 35.3 | 3.86 |
| 15-pyN5367 | 4.5b | 3.6b | -- | 2 | 13 | 37.7 | 3.86 |
1.4 T;
0.47 T;
0.56 T.
EDTA type ligands form Mn complexes that are very rapidly dissociated but replacement of the ethylendiamine backbone with a trans-1,2-cyclohexylenediamine (trans-1,2,-cyclohexylenediaminetetraacetic acid, CyDTA) linker increase kinetic inertness by nearly 3 orders of magnitude (Table 9).363 CyDTA also supports Mn complexes of q = 1. Recent work evaluating the kinetic inertness of phenylenediaminetetraacetic acid (PhDTA) shows that this ligand too provides a very kinetically inert Mn complex.356
Table 9:
Dissociation half-lives of Mn(II) complexes in the presence of competing divalent metal ions. Conditions: 10 µM Mn(II) complex, 10 µM Cu(II) or Zn(II), pH 7.4.
| ligand | t1/2 (h) |
|---|---|
| EDTAa | 0.08 |
| CyDTAa | 12 |
| PhDTA | 19.1 |
| NOTAb | 74 |
| PyN5b | 11.4 |
| 12-pyN4Ab | 2.4 |
| nompab | 0.02 |
| dompab | 0.16 |
| pcmab | 2.4 |
| NJC-L1a | 0.02 |
| NJC-L2a | 2.8 |
| NJC-L3a | 55 |
Cu(II),
Zn(II).
Although macrocyclic ligands often form complexes that are more inert that linear ligands, Mn complexes with hexadentate macrocylic ligands like NOTA form 6-coordinate Mn complexes that are q = 0 and thus low-relaxivity.362 The macrocyclic chelator 1,4-DO2A has been shown to support a q = 1 Mn(II) complex but we are unaware of any studies to evaluate the kinetic inertness of this complex.364 However, a number of pentadentate ligands have been reported that form q = 1 or 2 Mn(II) complexes that are less thermodynamically stable than the complexes formed by linear and macrocyclic hexadentate chelators but may be sufficiently inert to merit evaluation as MRI contrast agents.365–367
The chelate Mn-PyC3A was rationally designed as a potential alternative to Gd(III)-based extracellular agents.377 The rigidifying trans-1,2-cyclohexylenediamine backbone provides high thermodynamic stability and a high degree of kinetic inertness but leaves a vacant coordination site for coordinately of a rapidly exchanging water co-ligand. Upon challenge with 25 mol equiv. Zn(II) at pH 6.0, Mn-PyC3A was 20 times more inert than Gd-DTPA. Mn-PyC3A has a very modest affinity for blood plasma proteins and this provides a r1 of 3.8 mM−1s−1 in blood plasma at 1.4T, 37 °C, comparable to the r1 of Gd-DTPA and Gd-DOTA measured under similar conditions (4.1 and 3.6 mM−1-s−1, respectively).59 Mn-PyC3A also provides comparable contrast to Gd(III) contrast agents in vivo. Side-by-side comparison of Mn-PyC3A vs. Gd-DTPA in MR angiography performed in a non-human primate model revealed no statistically significant difference in vessel vs. adjacent muscle contrast-to-noise ratios.378 The cyclohexylene backbone and N-pyridyl donor also provide a degree of lipophilicity which promotes mixed renal and hepatobiliary clearance.377 Partial hepatobiliary clearance is a favorable attribute in the context of renal impairment, as reduced kidney function should less profoundly influence elimination of an agent with an alternate route of elimination. Mn-PyC3A was also demonstrated to be rapidly eliminated and highly robust against metabolic transformation or degradation.378
High spin Mn(III) complexes such as Mn-porphyrin and Mn complexes of 1,2-phenylene diamine derived bis-amidate ligands (Mn-PDA) have also been demonstrated as effective MRI contrast agents.379–380 Both porphyrins and PDA type ligands form quaternary Mn(III) complexes with two water co-ligands. The complexes are octahedral with the tetradentate porphyrin and PDA ligands comprising the equatorial plane and the water co-ligands occupying the apical positions. The water exchange rates of Mn-TPPS and Mn-TPMPyP were shown be 1.4 × 107 s−1 and 1.0 × 107 s−1 at 298 K.381
The relaxivity of Mn(III)-porphyrins has been described as “anomalously” high considering the S=2 spin state.379 The mechanisms that dictate the relaxivity of Mn(III) complexes are largely underexplored relative to those of Gd(III) and Mn(II), but it is believed that the high relaxivity of Mn(III) porphyrin is at least partially due to elongation of the singly occupied dz2 orbital which lies across the Mn(III)-OH2 bond axis. This favorable asymmetry effectively reduces the distance between the Mn(III) spin density and the water 1H nuclei and it has been proposed that the r6 term in equation 5 may not adequetly describe the efficiency with which the Mn(III) ion induces 1H relaxation.379 NMRD data recorded on Mn(III) porphyrin complexes indicate that T1e is rapid compared to Gd(III) and Mn(II) complexes and provides significant contributions to the correlation time up to 100 MHz.102, 379, 382 Fast electronic relaxation will limit the relaxivity gains from slow rotation. For example, cyclodextrin encapsulation of the Mn(III) complex Mn(III)-tpps results in only a 2-fold increase in r1 at 0.47 T, while the corresponding Mn(II) sister complex, Mn(II)-tpps, showed a 6-fold increase.382
Anionic Mn(III)-porphyrin complexes have been considered as Gd(III)-free contrast agents. For example, the tetracarboxylate functionalized complex Mn(III)-TCP was shown exhibited comparable T1 enhancement and biodistribution to Gd-DTPA in a mouse model.383 Dimerization of a tri-sulfonated Mn(III)-porphyrin resulted in a complex that exhibited a per Mn relaxivity that is 3-fold greater than Gd-DTPA at 3 T and 25 °C.384 This porphyrin dimer was shown to be an effective blood pool imaging agent in a rat model.385 Mn(III)-mesoporphyrin has been considered for use as a hepatobiliary contrast agent and was shown to be effective for delineation of liver tumors and liver abcesses.386–387 A trisulfonated Mn(III)-porphyrin conjugated to a 30,000 kDa dextran-derived polymer was used to visualize lesions in a mouse model of hepatoma.388 The Mn-PDA complexes were shown to be cell permeable and have been further elaborated for detection of intracellular enzymes.380
High spin Fe(III) is also an effective relaxation agent. Fe(III) complexes are typically much more stable even than Gd(III) complexes. For example, log KML for Fe-DTPA is 27.3 whereas log KML for Gd-DTPA is 22.5. The complexes Fe-DTPA (q = 0) and Fe-CDTA (q = 1) have been evaluated as extracellular contrast agents in a breast cancer xenograft mouse model.389 The relaxivity of Fe-DTPA and Fe-CyDTA are 0.9 mm−1s−1 and 2.2 mM−1s−1, respectively, in blood serum at 9.4 T, room temperature, compared to a value of 4.1 mM−1s−1 for Gd-DTPA under the same conditions. Up to five-fold larger doses of the Fe(III) complexes were required to visualize the tumors with comparable conspicuity to that observed with Gd-DTPA. Coordinatively saturated (q = 0) complexes such as Fe-HBED and Fe-EHPG have been evaluated as potential hepatobiliary MRI contrast agents.390–392
High spin Fe(II) has also been considered as a contrast agent. The q = 1 Fe(II) complexes Fe-DPTACN (DPTACN = 1,4-dipicolyl-1,4,7-triazacyclononane) and Fe-DTTACN (DTTACN = 1,4-ditetrazoylmethyl-1,4,7-triazacyclononane) have been explored as MRI contrast agents.393–394 A relaxivity of 0.6 mM−1s−1 was recorded for both Fe(II) complexes at 7 T. Direct of Fe-DTTACN was shown to provide prolonged contrast enhancement in a mouse model after direct intramuscular injection into a mouse model.393
The Eu(II) ion is isoelectronic with Gd(III) and complexes of Eu(II) have been demonstrated to be potent relaxation agents. The Eu(II) (aq) ion has one of the fastest water exchange rates measured.395–396 However, the Eu(II) oxidation state is disfavored relative to the diamagnetic Eu(III) ion in the polyaminocarboxylato ligands typically utilized for Gd(III)-based contrast agents.397–398 Eu(II)-based MRI contrast agents can be stabilized by 2.2.2 cryptands comprised of neutral donor groups, Figure 47.399–400 The 2.2.2 cryptand cavity is ideally suited to Ln(II) binding and the soft nature of the neutral donors accommodate the Eu(II) oxidation state. Eu(II)-2.2.2 cryptands form ten coordinate quaternary complexes with 2 rapidly exchanging water co-ligands and thus afford high relaxivity contrast agents. The relaxivity values and associated molecular parameters for previously reported Eu(II) cryptand complexes are summarized in Table 10. Like Gd(III) the symmetric S electronic ground state of Eu(II) is characterized by a T1e on the order of nanoseconds and high relaxivity Eu(II) complexes can be achieved by increasing τR. For example, the relaxivity of the biphenyl appended complex Eu(II)-2.2.2-BiPh increased from 4.2 mM−1s−1 to 8.7 mM−1s−1 and 12.5 mM−1s−1 upon forming inclusion complexes with to β-cyclodextrin and poly-β-cyclodextrin, respectively, and to 16.6 mM−1s−1 upon binding to HSA. Intriguingly, the relaxivity values of Eu(II) complexes at 7 T are typically ~30-35 % greater than the values recorded at 3 T and in this regard Eu(II) agents have received consideration for imaging applications at field strength >3 T.401–403
Figure 47:
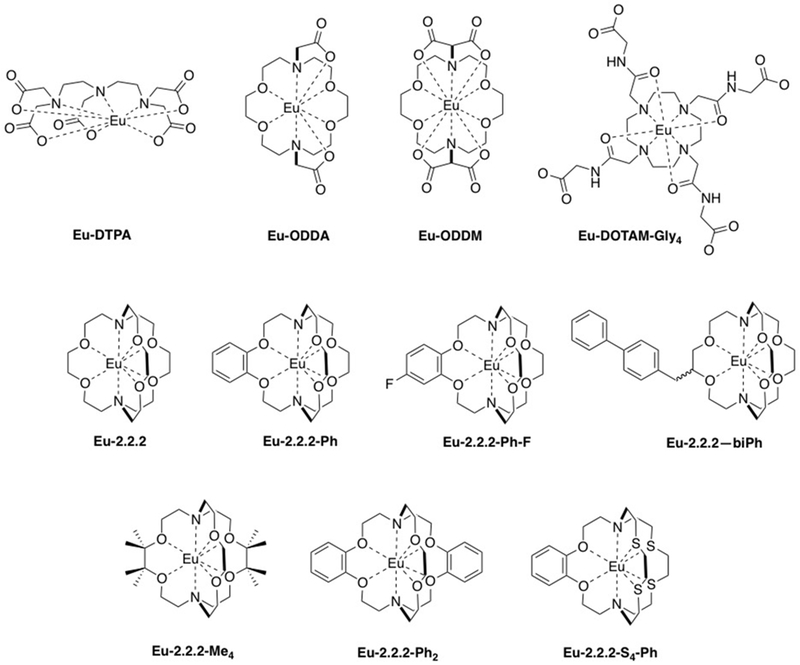
Eu(II) complexes considered as potential MRI contrast agents.
Table 10:
Summary of relaxivity, water exchange kinetics, electronic relaxation, thermodynamic stability (in 0.15M NMe4Cl electrolyte solution), and oxidation potentials of selected Eu(II) complexes.
| r1 (mM−1s−1) | q | τm310 (ns) | ΔH‡ | T1e310 (ns) | Log KML | E1/2 (V) | |
|---|---|---|---|---|---|---|---|
| Eu-2.2.2 | 2.1a, 2.7b | 2 | 2 | 33.4, 30.6 | 1.1,c 3.0d | 13.0f | −0.21g, −0.336h |
| Eu-2.2.2-Ph | 3.7a, 3.3b | 2 | 10 | 39.9 | 1.2,c 3.8d | -- | −.208h |
| Eu-2.2.2-BiPh | 4.2a, 4.8b | 2 | 12 | 31.1 | 1.3,c 4.2d | -- | -- |
| Eu-2.2.2-BiPh / b-CD | 8.7a | 2 | 12 | -- | 39,c 155d | -- | -- |
| Eu-2.2.2-BiPh / poly-bCD | 16.6a | 2 | 12 | -- | 56,c 215d | -- | -- |
| Eu-2.2.2-BiPh / HSA | 12.5a | 2 | 12 | -- | 191,c 763d | -- | -- |
| Eu-2.2.2-Ph-F | -- | -- | -- | -- | -- | -- | −0.079h |
| Eu-2.2.2-Me4 | -- | -- | -- | -- | -- | -- | −0.169h |
| Eu-2.2.2-Ph2 | -- | -- | -- | -- | -- | -- | −0.211h |
| Eu-2.2.2-S4-Ph | -- | -- | -- | -- | -- | -- | −0.035h |
| Eu-ODDM | -- | -- | -- | -- | -- | 13.1e | −0.92g |
| Eu-ODDA | -- | 1 | -- | 22.5 | 4.0,c 15d | 9.9e | −0.82g |
| Eu-DTPA | -- | 1 | -- | 26.3 | 2.3,c 8.7d | 10.1e | −1.35g |
| Eu-DOTAM-Gly4 | -- | -- | -- | -- | -- | -- | −.701g |
1.4 T, 37 °C;
7 T;
1.5 T,
3.0 T.
Determined indirectly via cyclic voltammetry methods
vs. Ag/AgCl,
vs. Fc/Fc+.
Thermodynamic stability and kinetic inertness have been evaluated for a few Eu(II)-based agents. Potentiometric titration of the Eu(II) complexes of ODDM, ODDA, and DTPA yielded log K values ranging from 9.9-13.1.398 Similarly, log KML = 13 for Eu-2.2.2 was determined indirectly through electrochemical measurements.399 These Eu(II) complexes are roughly 10 orders of magnitude less stable the Gd(III)-based contrast agents but they are remarkably inert. Evaluation of the kinetic inertness of complexes Eu-2.2.2 and Eu-2.2.2-Ph was evaluated under a standard set of conditions that has been applied to all of the clinical agents (2.5 mM contrast agent, 2.5 mM ZnCl2 as challenging ion, pH 7.0 phosphate)217, 404 indicates the Eu(II) complexes are comparably inert to the macrocyclic agents such as Gd-DOTA and Gd-HPDO3A.401
Although capable of supporting the Eu(II) oxidation state, the 2.2.2 cryptand complexes reported to date still undergo oxidization to the corresponding Eu(III) complex under aerobic conditions.405–407 Eu-2.2.2. is rapidly oxidized in the bloodstream, providing no positive contrast enhancement 3 min after intravenous injection in mice.408 However, prolonged contrast enhancement on the order of minutes following intratumoral injection of Eu-2.2.2 and for hours following intratumoral injection of Eu-2.2.2-F.406, 408 The oxidation kinetics of EuCl2, Eu-2.2.2-F, Eu-DOTAM-Gly4 have been evaluated in the presence of the bromate anion and the biologically relevant oxidant glutathione disulfide.409 The data suggests that the kinetics of Eu(III) formation reflect the overpotential between Eu(II) and chemical oxidant. It is thus feasible that stable intravenous Eu(II) formulations could be developed by ligand modifications to shift the oxidation potential to more positive values.
Nitroxide radicals, Figure 48, have also been considered as MRI contrast agents but are much less effective relaxation agents than metal ion based systems.411–415 Nitroxide r1 values typically range between 0.1 – 0.5 mM−1s−1. The low relaxivity compared to paramagnetic metal ions is due to the low spin quantum number (S = ½) as well as the fact the nitroxide radicals do not benefit from inner sphere interactions to place the 1H into close proximity with the paramagnet. Indeed, nitroxide radicals require stabilization from adjacent sterically encumbering and hydrophobic functional groups.
Figure 48:
The nitroxide radicals 3-CP and chex have been evaluated as MRI contrast agents.
Nitroxide radicals are rapidly reduced to diamagnetic hydroxylamines and typically exhibit in vivo half-lives on the order of minutes.416–420 Nitroxide reduction kinetics can be influenced by their local chemical environment. For example, it was shown that replacing the nitroxide adjacent geminal dimethyl groups of 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxy (3-CP) with spirocylohexyl (chex) groups results in a 2-fold decrease in the rate of reduction by excess ascorbate.421 Upon incorporation of chex into a polyethyleneglycol decorated polypropylenimine dendrimers, a fraction of dendrimeric nitroxides become 20-fold more resistant to ascorbate reduction than the corresponding monomer.422 These nitroxide functionalized dendrimers provide strong and persistent intravascular contrast enhancement that persists out to at least 1 h in a mouse model. A recently developed nitroxide and PEG functionalized bottlebrush arm star polymer was used to visualize subcutaneous tumors in a mouse model 20 h following intravenous injection.423
4.4.2. Iron-oxide nanoparticles
SPIONs are generally too large to extravasate into extracellular spaces and are not excreted. The ultimate fate of SPIONs is macrophage capture and subsequent metabolism to labile iron.424 Particle distribution is largely a function of size.425 SPIONs of > 80 nm diameter are very rapidly captured by macrophages. However, smaller particles can evade macrophage capture and exhibit circulatory lifetimes that span hours to days.426 Particles > 80 nm diameter are well suited for imaging of the reticuloendothelial system (RES). For example, the SPION formulations ferumoxide and ferucarbotran, which are comprised of 120 – 180 nm dextran coated SPIONs and 45 – 60 nm carboxymethyldextran coated SPIONs, respectively, were developed for the detection of liver lesions.49, 424, 427 Liver tissue is populated with phagocytic Kupffer cells but malignant hepatocellular lesions are typically devoid of Kupffer cells. The strong SPION T2* effect thus renders liver parenchyma hypointense relative to lesions containing comparatively low populations of phagocytic cells. Similarly, the SPION T2* effect has been used to differentiate normal from malignant, macrophage deficient lymph nodes.50, 428 SPION opsonization also offers an effective mechanism to detect and visualize the dynamics of inflammation.429–431 SPION enhanced MRI has been used to visualize atherosclerotic plaque,432–434 the macrophage infiltration following stoke and myocardial infarction,435–437 as well the detection and monitoring of numerous other disease states characterized by an acute inflammatory response.
The smaller SPIONs are more slowly captured by the RES and are eliminated from the bloodstream with half-lives on the order of hours to days. These smaller particles typically have good T1 relaxivity as well and are thus ideal for contrast enhanced MR angiography. Ferumoxytol is a SPION formulation with an FDA indication for iron replacement therapy that occasionally receives off label use as an angiographic contrast agent.438 For example, ferumoxytol has been used for aortic imaging,439 renal artery imaging,440 detection of arteriovenous fistula,441 detection of deep vein thrombosis,442 and to characterize the vasculature of brain tumors.443
Recently, a new class of iron-oxide nanoparticles small enough to clear via glomerular filtration were introduced.444 These particles, termed exceedingly small SPIONs (ES-SPIONs), are derived from a maghemite core which does not affect T2 relaxation as strongly as magnetite. The maghemite core and < 5.5 nm particle diameter provide an r2/r1 of 2.1, which is lower than that of any other SPION. The favorable signal generating and excretion profile of ES-SPIONs implicate these particles as potential candidates for Gd(III) free angiography agents.
Oral SPION formulations comprised have also been developed for contrast enhanced imaging of gastrointestinal structures. Ferumoxsil is a formulation comprised of poly-N-(2-aminoethyl)-3-aminopropyl siloxane magnetite particles ~300 nm in diameter.54 An oral formulation named ferristene comprised of particles ~3500 nm in diameter has also been developed for contrast enhanced MRI of the bowels.47
SPIONs typically have a higher incidence of serious adverse drug reactions than Gd(III)-based agents.445–446 SPION immunogenicity is influenced by the nature of the surface coating and it may be possible to develop formulations of improved safety via careful tuning of chemistry at the particle surface.447 Because SPIONs are metabolized to labile iron rather than excreted, there are also toxicity concerns related to iron overload.448 On the other hand, some SPIONs are highly resistant to metabolism and can persist for prolonged periods in the liver. This can also poses a toxicity concern. For example, development of the USPION formulation feruglose, which was designed for MR angiography, was discontinued due to concerns over long term liver retention.47
A very large number of SPION contrast agents have been proposed and several are currently approved for imaging indications in the US and Europe. However, none are currently marketed.
4.4.3. CEST agents
Although a very large number of CEST agents have been reported over the last decade very few have been pursued with the goal of potentially replacing relaxation agents. CEST agents are detected with poor sensitivity relative to relaxation agents and thus larger doses are required to generate conspicuous MRI contrast. For this reason, the vast majority of CEST agent development has focused on utilizing the versatility of the CEST effect for specialized molecular imaging applications and are discussed in the sections below.
Iodinated X-ray contrast agents have been evaluated as alternatives to Gd(III)-based relaxation agents, Figure 49.449 The X-ray agents contain multiple CEST active exchangeable amide and alcohol protons, exhibit comparable pharmacokinetics to commercial Gd(III) agents, and are well tolerated at the high doses required for CEST detection. Side-by-side comparisons of contrast enhancement generated using 10 mmol/kg of iopamidol, iohexol, and iodixanol compared to the clinically indicated 0.1 mmol/kg dose of Gd-HPDO3A were performed in a murine tumor model.450 CEST enhancement of the tumor expressed as (% saturation transfer) generated using the iodinated agents correlated with Gd-HPDO3A contrast enhancement (expressed as % signal intensity increase). There was also a strong correlation between the extravasation fractions calculated using the iodinated contrast agents and the Gd-HPDO3A enhanced data.
Figure 49:
Iodinated X-ray contrast agents have been evaluated as DiaCEST contrast agents.
A number of paramagnetic metal-ligand complexes have also been evaluated as CEST agents. Paramagnetic ions can shift labile proton resonances by up to 500 ppm from the bulk water resonance and thus permits utilization of a number of proton exchanging functional groups that would otherwise violate the Δω > kex constraint in an analogous diamagnetic system. For example, saturation of the exchangeable Eu(III) coordinated water of Eu-DOTA tetramide complexes has been demonstrated to provide a strong CEST effect at 7 T despite the fact that water exchange occurs on the order of 104 - 105 s−1, which far exceeds the slow kex required to observe the CEST effect with a diamagnetic agent, typically <103 s−1, at the same field strength.451 CEST observation is enabled by the Eu induced >14,000 Hz shift. For a paramagnetic CEST agent, the benefits of a large paramagnetically shifted exchangeable proton resonances must be carefully balanced against the effects of paramagnetic relaxation. For example, the amide protons of Dy-DOTAM (Figure 50) provide a greater per exchangeable CEST effect compared to the Dy(III) bound water because the amide protons are less effectively relaxed by the Dy(III) paramagnet.452 Thus, the majority of newly developed paramagnetic CEST agents utilize second sphere exchangeable protons as the spectroscopically saturable handle. Amides, alcohols, amines, N-hydroxylamines, diazoles, and benzimidazoles have all been considered as second sphere proton exchangers. There are elegant examples of paramagnetic CEST agents prepared with lanthanide ions including Nd(III), Eu(III), Tb(III), Tm(III), Dy(III), Yb(III) and transition metal ions such as Fe(II), Co(II), Cu(II) and Ni(II).199, 453–461
Figure 50:
The exchangeable amide protons of Dy-DOTAM provide a greater CEST effect than the protons of the exchangeable water co-ligand because the amide protons are further from the Dy(III) ion and thus experience a weaker paramagnetic relaxation effect.
5. Targeted contrast agents
Biochemically targeted agents are designed to adhere with high specificity to a molecular target, and provide prolonged local contrast enhancement after clearance of the unbound agent. There are myriad biochemical targets that if visible by MRI could profoundly impact the detection, staging, prognosis, and treatment monitoring of disease, as well as elucidating complex biology. However, developing biochemically targeted MR contrast agents is extremely challenging. A number of considerations must be accounted for in developing a suitable biochemically targeted agent; appropriate affinity for the target, high-specificity for the target, rapid clearance of the unbound agent relative to washout of the target bound agent, and high relaxivity in the target bound form.
5.1. Case study: EP-2104R, a fibrin-targeted contrast agent
We introduce this section with a detailed case study of a fibrin-targeted contrast agent to highlight the challenges and strategies available to develop a biochemically targeted agent. To the best of our knowledge, the fibrin-targeted agent EP-2104R is the only biochemically-targeted MRI contrast agent that has been used to directly detect a pathologic biomarker in humans.462–463 Fibrin is the most highly abundant protein constituent of thrombus (clotted blood) at up to 100s of µM, but it is not present in circulating blood or any healthy tissue. Fibrin is formed by the actions of the enzymes thrombin and Factor XIII on fibrinogen. Fibrinogen circulates in blood at about 7 µM and is polymerized and crosslinked into the insoluble protein fibrin during the clotting cascade. An immediate challenge is to identify a targeting vector that recognizes fibrin specifically over circulating fibrinogen to which it shares great homology. Kolodziej et al. reported three families of short, cyclic peptides that were identified by phage display to selectively bind fibrin.464 The specificity was engineered by first screening the phage libraries against fibrinogen, removing fibrinogen binders, and then screening the depleted library against fibrin.
EP-2104R is comprised of 4 Gd-DOTA chelators appended to a 6 amino acid, disulfide bridged cyclic peptide, Figure 51A. The peptide contains some unnatural amino acids found to improve fibrin affinity.142, 464–466 The 4 Gd-DOTA chelators are included to increase the relaxivity of the compound. It was found that conjugation of the chelates at both the C- and N-termini resulted in compounds that were more resistant to in vivo metabolism, but at the same time did not substantially decrease fibrin affinity.467 The per Gd(III) relaxivity of EP-2104R was 11.1 mM−1s−1 in buffer and increased to 24.9 mM−1s−1 when bound to fibrin (0.47 T, 37 °C). EP-2014R binds with little to no affinity to fibrinogen, albumin or other blood proteins and this evidenced by the observation that relaxivity in blood plasma is virtually unchanged from that recorded in buffer. The high fibrin-binding affinity (Kd = 1.6 for human fibrin) is reflected in the >2-fold relaxivity increase in the presence of fibrin.466
Figure 51:
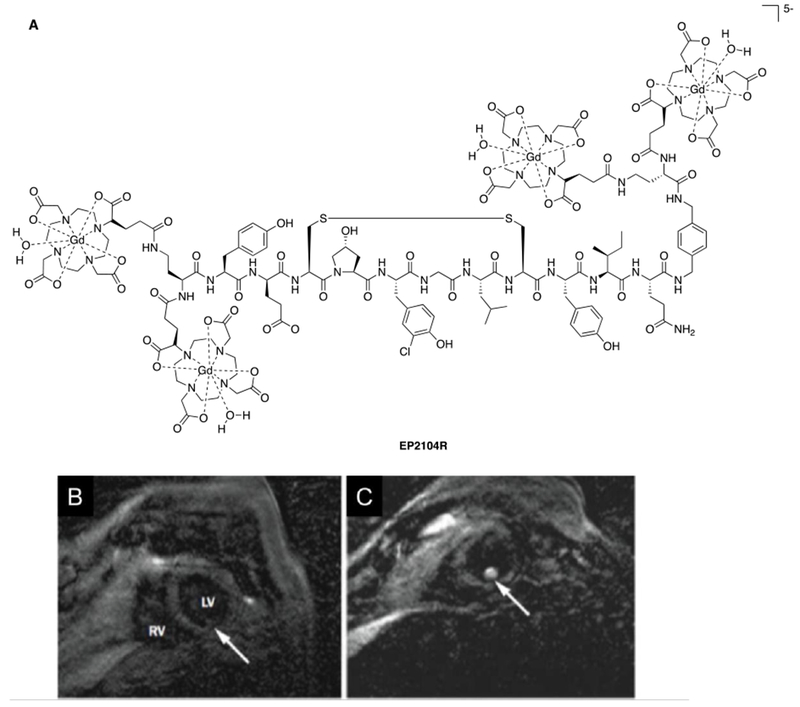
A) Fibrin-targeting contrast agent EP-2104R is the only biochemically targeted agent to be used to detect a pathologic biomarker in humans. B) Short axis view of the heart before EP-2104R injection. The arrow indicates the location of a left ventricular thrombus that is difficult to discern in the baseline scan. C) The thrombus is highly conspicuous for several hours after EP-2104R injection. Reproduced with permission from Ref.463 (URL: https://link.springer.com/article/10.1007%2Fs00330-008-0965-2). Copyright 2008 Springer Nature.
In humans, EP2104R exhibits comparable distribution to an extracellular contrast agent and is rapidly eliminated via renal filtration with the blood signal returning to near baseline values within 6 hours after injection.462 EP-2104R showed efficacy in detecting thrombus in the heart, aorta, carotid arteries, deep veins, and pulmonary emoboli.462
5.2. Challenges and New Frontiers
MR imaging of fibrin provides but one illustrative example of the potential impact of a biochemically specific MRI contrast agent. Successful translation of EP-2104R was the culmination of years of dedicated research and development effort and provides several valuable lessons for the design of a biochemically targeted contrast agent. In this section we will review prior accounts describing the design and application of MRI contrast agents targeting various biomarkers in order to illustrate the challenges and innovative solutions toward this new frontier in contrast enhanced MRI. To extend this work to other targets, a number of technical challenges must be addressed. Identifying targeting vectors to bind to a specific molecular target with high affinity and high specificity is itself very difficult. Appending a contrast generating moiety to the targeting vector in a manner that does not compromise target affinity adds another layer of complication.
A third challenge is that micromolar concentrations of metal ion are required to generate detectable contrast with T1- or T2-weighted imaging sequences. Few biochemical targets are present at high enough concentration to be imaged with a targeted agent containing only a single metal ion. However, very innovative strategies have been explored to amplify contrast upon encountering its biochemical target. These strategies can be broadly divided into 4 general approaches, each with their own strengths and weaknesses: (1) increase the contrasting payload by conjugating multiple relaxation agents to the targeting vector. This strategy was successfully pursued in the EP-2104R example with 4 chelates per peptide. Such agents are chemically well defined molecules that can be reproducibly synthesized. They are relatively small molecules which allows them to rapidly distribute in the body, bind to the target, and see the unbound agent be quickly eliminated from the body. The small size also typically results in ultimately complete elimination from the body. Drawbacks include the increasing complexity of the molecule and its synthesis, as well as the dearth of relevant molecular targets at high enough concentration. (2) Take advantage of an endogenous mechanism such as receptor mediated internalization to accumulate the targeted agent at MR visible concentrations. This is in principle a very attractive technique however the internalization process must be very efficient to accumulate the micromolar concentrations required for detection. (3) Utilize nanoparticles that bring hundreds or thousands of metal ions to increase the detection sensitivity into the low nanomolar range. (4) Use nanoparticle approaches to deliver a very high payload of non-endogenous (i.e. background free) magnetically resonant nuclei such a F-19. Both nanoparticle approaches are attractive solutions to overcoming sensitivity but bring challenges in terms of reproducibility, pharmacokinetics, and elimination. For clinical translation, the agent must be well defined and there are obvious challenges in controlling the distribution of nanoparticles both with respect to their size and their surface derivatization, although these can be overcome. Nanoparticles typically distribute in the blood and are captured by macrophages in the liver, spleen, and lymph nodes. Tumor accumulation can occur either via capture in tumor associated macrophages or via the enhanced permeability of many tumors, but these process take several hours. In general nanoparticles are not completely eliminated from the body and this can also create concerns for clinical translation.
5.3. Fibrin
As discussed above, fibrin is the main protein constituent of clotted blood and also present in the tumor microenvironment making it a useful protein target in the molecular imaging of thrombosis,462, 468 cancer,469 and other disease states that possess a coagulative component. In addition to the human EP-2104R imaging described above a few additional fibrin targeting contrast agents merit note. A Mn-based fibrin seeking contrast agent, Mn-FBP, was also reported, Figure 52.377 This agent uses the same peptide as EP-2104R but the 4 Gd-DOTA chelators are replaced with 4 Mn-PyC3A moieties. Mn-FBP demonstrated equivalent fibrin affinity to EP-2104R in vitro and provided equivalent contrast enhancement of arterial thrombi in rats. The high-payload strategy was extended even further in a liposomal fibrin-targeted agents, where fibrin antibody was conjuguated to liposome comprised of self-assembled amphiphilic Gd(III) complexes.470–472 The direct nuclear detection strategy has been pursued by conjugating 19F perfluorocarbon nanoemulsions to anti-plasmin peptide fragments which become chemically crosslinked to fibrin.473
Figure 52:
The Mn-based fibrin-seeking contrast agent Mn-FBP is comprised of 4 Mn-PyC3A chelators conjugated to a fibrin-binding peptide.
5.4. Serum albumin
Albumin is a serum protein that is present at ~660 µM and thus represents an abundant target for intravascular contrast agents. Small molecule agents with high affinity for albumin will confine largely to the blood pool (the 66 kDa protein does not extravasate) and provide prolonged and strong intravascular contrast. For example, MS-325 is an FDA approved albumin targeting agent that non-covalently binds to the albumin drug binding site II with Kd = 85 µM.17, 139, 474–475 MS-325 binding is promoted by a 4,4-diphenylcyclohexyl moiety connected to the Gd-DPTA chelate via a phosphodiester linkage.16, 476 The µM binding of MS-325 affinity ensures that a small fraction (10-20%) of MS-325 remains unbound at all times, and this unbound fraction can be cleared via glomerular filtration. Le Chatelier’s principle ensures that this unbound fraction is constantly reestablished. Albumin is also present in the lymphatic system, and MS-325 has been used to detect metastatic invasion of lymph nodes.477 In certain pathologies albumin can leak out of the blood vessels into the interstitial space. Montesi et al. showed that patients with idiopathic pulmonary fibrosis had extensive vascular leak in their lungs, readily detected by MS-325 enhanced MRI.478 A Mn-EDTA analogue of MS-325 has also been reported.331
A number of additional albumin targeting functionalities also have been appended to relaxation agents for intravascular imaging. For example, the Gd-DTPA based agents B22956/1, which has been extensively evaluated as an angiographic contrast agent, utilizes 3-deoxycholic acid as an albumin targeting group.23–26, 479–482 A biological function of serum albumin is to transport lipopilic anions. The high concentration of serum albumin and its ability to promiscuously binding many classes of compounds makes it possible to generate a large number of albumin-targeted agents.483–485 Additional examples of albumin binding MRI contrast agents have been reported,333, 476, 486–489 but there are too many to acknowledge even for this extensive review.
5.5. Type I Collagen
Type I collagen is the most abundant protein constituent of connective tissue. In fibrosis, or organ scarring, collagen is overexpressed and is present at 10s of µM concentration. Fibrosis occurs in a number of major diseases such as myocardial infarction, heart failure, atrial fibrillation, hepatitis and cirrhosis, diabetic nephropathy, pulmonary fibrosis, inflammatory bowel disease, and a number of cancers. The type I collagen-targeting contrast agents EP-3533,490 EP-3600,491 and CM-101492 were developed by conjugating Gd-DTPA or Gd-DOTA chelators to peptides that were identified by high-throughput screening methods to bind to type I collagen with high specificity, Figure 53. These collagen-targeting agents all capitalize on the increased payload strategy, with 3 Gd(III) chelates per peptide in each compound. EP-3533 has been used to stage liver fibrosis in mice and rats and also to monitor response to drug therapy.493–494 It was also shown to be effective to detect and quantify the extent of myocardial infarction,495 to stage tumor associated fibrosis,496 and to stage pulmonary fibrosis in mouse models.497 EP-3600 was used to image perfusion defects in a porcine model,491 and CM-101 was reported for imaging liver fibrosis in rat and mouse models.492
Figure 53:
A) Collagen-targeting MRI contrast agents are derived from a collagen-binding peptide identified via high-throughput screen to possess high specificity and affinity for typeI collagen. B,C) The collagen-targeted agents EP-3533 and CM-101 are comprised of the collagen-binding peptide and 3 Gd-DTPA or Gd-DOTA chelators connected to the peptide N-terminus and lysine ε-side chains via thiourea or amide linkages, respectively. C) EP-3600 is comprised of the collagen-binding peptide and a trimeric Gd-DOTA containing scaffold via the peptide N-terminus.
Figure 54 compares liver imaging data acquired in healthy rats and rats experiencing liver fibrosis resulting from bile duct ligation (BDL). Figure 54A compares the time course of liver-to-muscle contrast to noise ratio (CNR) observed in control (sham surgery) and BDL rats following injection of equal doses CM-101. CM-101 is rapidly distributed and eliminated and liver contrast in control rats is accordingly diminished to near baseline levels within 15 min of injection. In the fibrotic liver however, CM-101 adheres to collagen and provides high liver CNR at delayed time points after washout of the unbound agent. Figures 54B-C further illustrate this concept by comparing liver CNR integrated over 0-30 min for the sham surgery and BDL rats and liver CNR between sham surgery and BDL rats 10 min after CM-101 injection. Figure 54D compares T1-weighted MR images of the sham and BDL (fibrotic) rats prior to and 15 min after CM-101 injection.
Figure 54:
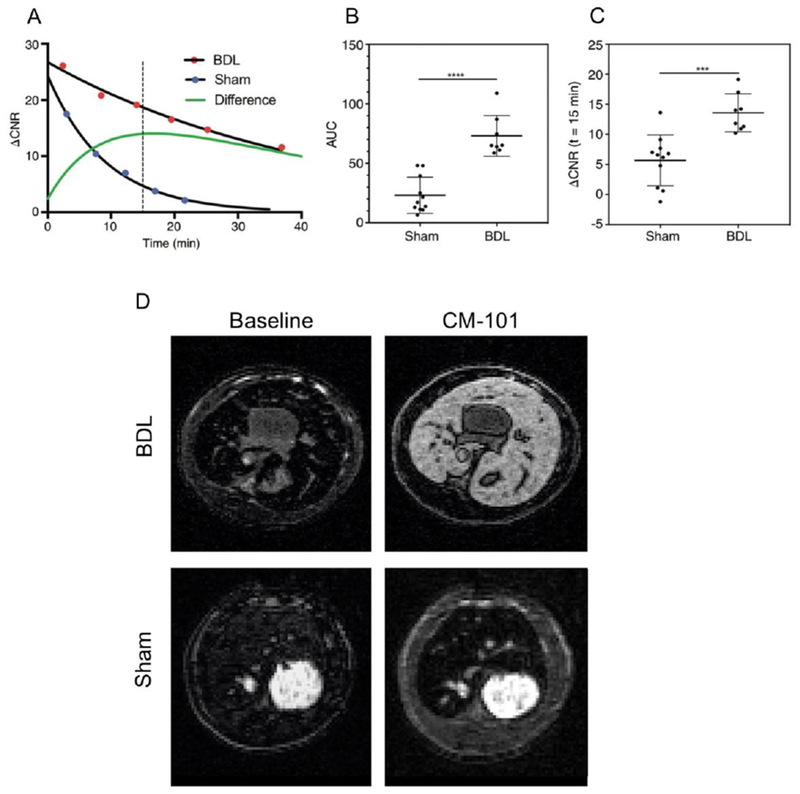
Change in contrast-to-noise ratio (CNR) following CM-101 injection in bile duct ligated (BDL, liver fibrosis) and sham operated (healthy non-fibrotic liver) rats. A) Representative dynamic time courses of the change in CNR between liver and muscle tissue for BDL (red dots) and sham (blue dots) treated rats following injection of CM-101. The maximal difference in ΔCNR (green line) between sham operated and BDL rats was observed at approximately 15-20 minutes following CM-101 injection. B) A statistically significant difference in the area under the ΔCNR curve (AUC) between sham operated and BDL rats was observed. C) A statistically significant difference in ΔCNR between sham operated and BDL rats was also observed at 15 minutes post CM-101 injection. D) Representative T1-weighted images acquired pre CM-101 (baseline) and 15 minutes post CM-101 injection. Notice that the fibrotic liver is markedly enhanced with CM-101 but healthy liver is not. Reproduced with permission from Ref.492 (URL: https://pubs.rsna.org/doi/10.1148/radiol.2017170595). Copyright 2017 Radiological Society of North America (RSNA®).
5.6. Fibrogenesis
Fibrogenesis, or the active deposition of scar tissue, is characterized by high levels of reactive aldehydes which are generated as part of a lysyl oxidase (LOX) mediated collagen crosslinking process. LOX catalyzes oxidative deamination of lysine side chains to form allysine, which goes on to participate in condensation reactions with nearby lysine and allysine residues, resulting in crosslinking between collagen strands. Although allysine is reactive, the transiently generated aldehyde is present in sufficiently abundant concentrations for MR detection using an aldehyde-targeting imaging probe.498 Allysine has been targeted using Gd(III)-based agents functionalized with hydrazide and oxyamine targeting moieties, which attach to allysine via reversible formation of hydrazone and oxime bonds. The hydrazide containing, aldehyde targeting agent Gd-Hyd, Figure 55A, was used to measure the natural history of bleomycin induced fibrogenesis in the lungs of mice, as well as the response to treatment with a LOX inhibitor.499 Gd-Hyd was also capable of monitoring liver fibrogenesis in a CCl4 mouse model.
Figure 55:
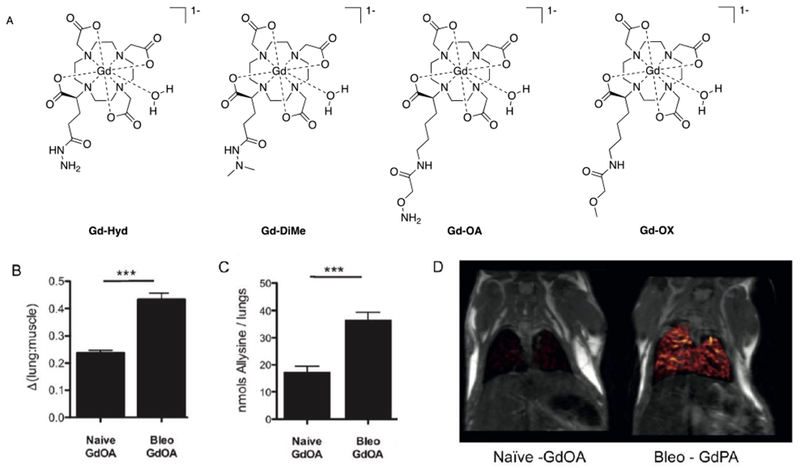
A) Allysine-targeted agents Gd-Hyd and Gd-OA, and their corresponding non-targeted control agents Gd-DiMe and Gd-OX. B-D) Imaging and ex vivo tissue analyses for Gd-OA (allysine-targeting) an in naive (no fibrogenesis) and bleomycin (Bleo) injured (active fibrogenesis) mice. B) Quantification of the change in lung-to-muscle signal intensity ratio following injection of Gd-OA to naïve and Bleo injured mice. C) Quantification of allysine in naïve and Bleo injured mice. D) Coronal MR images of naïve and bleomycin injured mice where the false color overlay is the difference image of the post Gd-OA image and the baseline image. Reproduced with permission from Ref.500 (URL: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201704773). Copyright 2017 John Wiley and Sons.
Similarly, Gd-OA, Figure 55A, with a pendant oxyamine moiety was used to detect bleomycin-induced pulmonary fibrogenesis in mice.500 Figures 55B-D compare lung imaging data and lung allysine content in normal, naïve mice vs. mice experiencing pulmonary fibrogenesis as a result of bleomycin injury. Figure 55B compares the change in lung-to-adjacent muscle signal intensity ratio following contrast agent injection, and demonstrates strong lung enhancement in the bleomycin treated mice. The MRI measurement reflects ex vivo quantification of tissue allysine content, Figure 55C, which is present at high micromolar concentrations in the injury lung. Contrast enhancement is clearly evident in the lungs of the bleomycin injured mice as compared to naïve mice, Figure 55D.
The aldehyde-targeting mechanism of both agents was confirmed using the control agents Gd-DiMe and Gd-OX, Figure 55A, which exhibit comparable pharmacokinetics and relaxivity but are incapable of forming hydrazone and oxime bonds, respectively. These control agents provide no contrast enhancement in tissues experiencing active fibrosis.
5.7. Fibronectin
Aggressively metastatic tumors are typically characterized by interstitial coagulation and thus proteins such as fibrin and fibronectin represent promising prognostic molecular imaging targets. In addition to the fibrin-binding peptides discussed above, other high-throughput screens have yielded low-molecular weight peptides that bind to an epitope formed by the fibrin-fibronectin complex with good affinity.501 Two small fibrin-fibronectin targeting peptides CGLIIQKNEC (CLT1) and CREKA have been utilized for molecular imaging of cancer, Figure 56A. CLT1 conjugated to Gd-DTPA via an amide linkage at the peptide N-terminus was shown to provide strong delayed enhancement of human colon carcinoma xenografts in mice long after probe washout.502–503 High payload fibronectin-targeting contrast agents have also been developed by appending a tetrameric Gd(III)-complex to the N-terminus, Figure 56B, or a trimeric Gd(III)-complex to the cysteine-S, Figure 56C, of the CREKA peptide. These higher relaxivity agents have been used to effectively detect orthotopic prostate and breast tumors in mouse models as well as micrometastases <0.5 mm in diameter, which are difficult to detect using conventional extracellular contrast agents.504–506 The fibronectin selectivity of the CREKA-based agent was further evidenced by comparing localization of a fluorescent peptide with a fibronectin specific immunohistochemical stain using fluorescence microscopy ex vivo. Imaging performed with an otherwise identical agent built from the scrambled CERAK peptide (non-binding control) does not provide delayed tumor enhancement.
Figure 56:
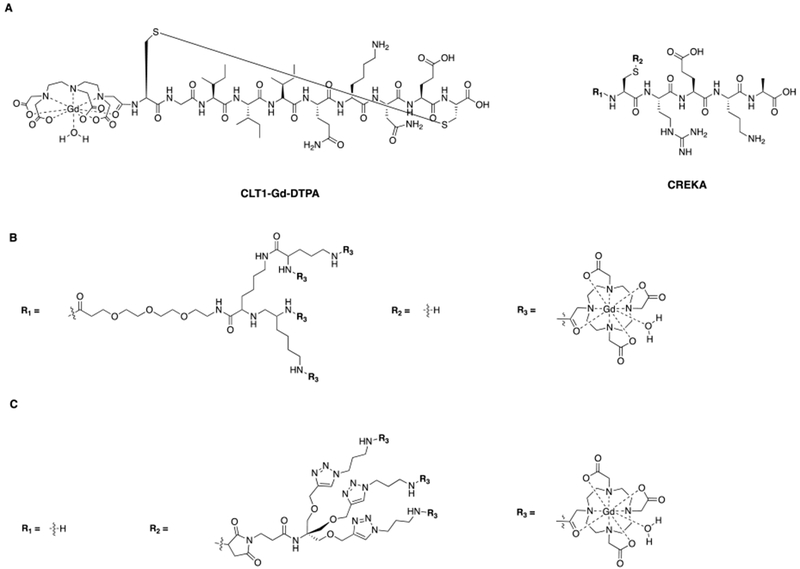
The CLT1 and CREKA peptides identified via high-throughput screen to bind to the fibrin-fibronectic complex with high affinity. A) A fibronectin-targeting agents comprised of Gd-DTPA conjugated to CLT1 via the peptide N-terminus and high payload fibronectin-targeting agents comprising multimeric Gd(III)-complexes (R1 and R2) conjugated to the to the CREKA peptide. B) One high-payload fibronectin-targeting agent was assembled by conjugating a tetrameric Gd-DOTA scaffold to the CREKA N-terminus, C) another was assembled via conjugation of a trimeric Gd-DOTA scaffold to the cysteine side chain S.
Fibronectin-targeted “platelet-mimetic” SPIONs have been developed that promote further particle accumulation around particles bound to vessel wall.503 These fibronectin-targeting SPIONs accumulate in the vessel walls of proliferating tumors and were used for fluorescence-based imaging of human breast cancer xenografts in mice. The fibronectin-targeting SPIONs did not localize to the same tumor phenotype in fibrinogen knockout mice which are incapable of expressing the fibrin-fibronectin complex, further confirming the mechanism of tumor localization. These particle have not yet been applied to MR imaging studies, however, but one could expect the “platelet-mimetic” mechanism of SPION accumulation to provide very strong contrast enhancement in similar models.
5.8. Elastin
Elastin is an abundant protein component of load bearing tissues and levels of this protein are largely increased as part of the compensatory response following injury. Molecular imaging of elastin was performed with the elastin-seeking contrast agent, Gd-ESMA, Figure 57A, which comprises an elastin-binding small molecule conjugated to Gd-DTPA via an amide linkage involving one of the DTPA carboxylates. Gd-ESMA was used to monitor changes in elastin composition in the vessel wall of arterial aneurysm,507 vascular remodeling following atherosclerotic plaque disruption,508 and changes in the extracellular matrix of cardiac tissue following myocardial infarction.509–510
Figure 57:
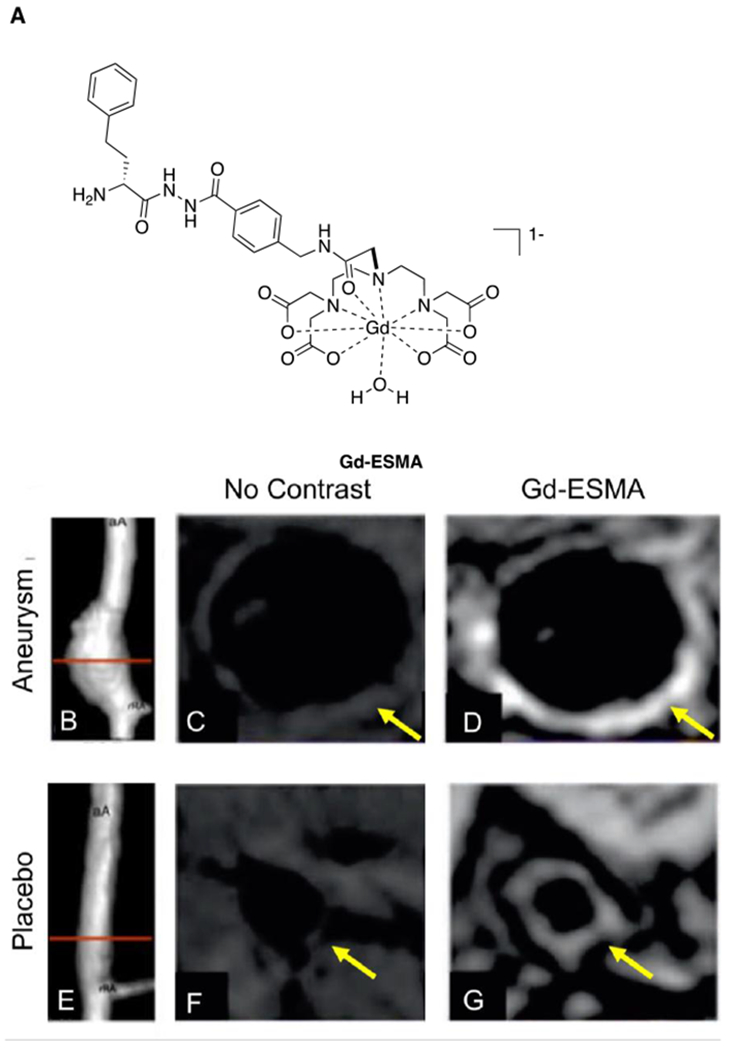
(A) Elastin-targeting MRI contrast agent Gd-ESMA. (B-G) show MR imaging of the suprarenal abdominal aorta in placebo treated mice (B-D) and mice bearing a pharmacologically-induced abdominal aortic aneurysm (E-G). Time of flight MR angiograms are shown in (B) and (E), the red lines mark the axial cross sections of the vessels depicted in images (B-D) and (F-G). Images (C-D) and (F-G) were acquired using the same T1-weighted protocol. (C) and (F) were acquired without contrast enhancement, whereas (D) and (G) were contrast-enhanced using Gd-ESMA. The aneurysm bearing vessel is strongly enhanced compared to the vessel in the negative control animal because an increase in elastin content accompanies the compensatory remodeling of vessel wall at the site of the dilation. Reproduced with permission from Ref.507 (URL: https://www.ahajournals.org/doi/full/10.1161/CIRCIMAGING.113.001131). Copyright 2014 Wolters Kluwer Health, Inc.
The images in Figure 57B-G show the abdominal aortas of a mouse bearing a pharmacologically-induced abdominal aortic aneurysm (B-D) and a placebo treated mouse (E-G). The red lines in the angiograms shown in Figures 57B and 57E mark the axial cross sections of the vessels depicted in images Figure 57C-D and Figure 57F-G, respectively. The cross sectional images were collected with no contrast enhancement and several minutes after Gd-ESMA injection. Elastin is present in all aortas and the vessel wall in the placebo treated mouse is slightly enhanced but the compensatory remodeling of the vessel wall at the site of the aneurysm results in increased elastin content in the vessel wall, which is very strongly contrast enhanced.
5.9. Myelin
A number of neurodegenerative disorders are characterized by disruption to the myelin sheath surrounding the axons of white matter nerve cells. A myelin-seeking contrast agent was developed by appending a Gd(III) complex to a 3-(4-aminophenyl)-2H-chromen-2-one, termed Case Myelin Compound, or CMC, Figure 58.511–512 CMC was identified from a screen of myelin adhering coumarins as the molecule with greatest affinity to myelin. Myelin localization of CMC was confirmed by MR imaging of contrast agent incubated slices of excised mouse brain in vitro, and comparing the patterns of coumarin fluorescence to myelin specific immunohistochemical staining.512 CMC was also shown to bind myelin sheaths in vivo following intravenous injection.513 Intracranially administered CMC enabled MR visualization of pharmacologically induced demyelination in a rat model in vivo.511
Figure 58:
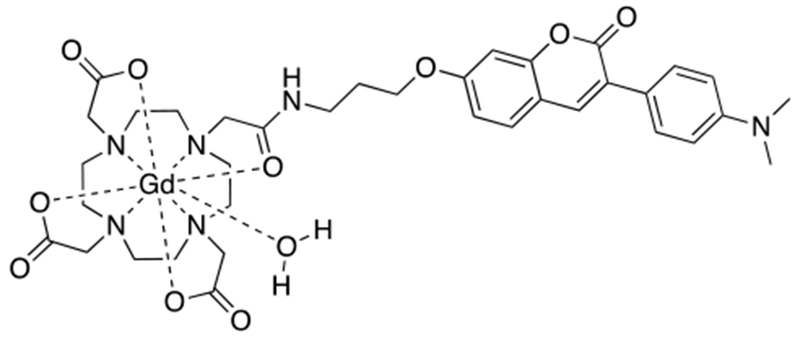
Myelin targeting MRI contrast agent, Case Myelin Compound.
5.10. Amyloid-β peptides
Insoluble amyloid-β (Aβ) peptides are an important biomarker for detection of Alzheimer’s Disease. Aβ is a particularly challenging target because most contrast agents are incapable of penetrating the intact blood-brain barrier. However, innovative chemistry has yielded a handful of contrast agents that can cross the blood-brain barrier and provide enhancement at the site of Aβ deposits. For example, an Aβ fragment containing amino acids 1-40 (Aβ1-40) conjugated to Gd-DTPA and putrescine at the N- and C-termini, respectively, was shown to penetrate the blood brain barrier and adhere to Aβ depositions.514 Incorporation of the polyamine putrescine confers blood brain barrier permeability. This effect had been previously demonstrated with putrescine conjugated proteins.515 The Aβ1-40 aggregates with the insoluble Aβ. In vivo imaging was not performed with this agent but ex vivo brain slices from an Alzheimer’s Disease mouse model, excised after injection of the agent, were imaged with MRI. The T1 enhancement patterns of the brain slices localized well with Thioflavin S staining for insoluble Aβ. A similar strategy has been taken to access Aβ-deposits with USPIONs. The USPIONs were decorated with a Aβ1-42 as the targeting vector and polyethylene glyocol in order to increase membrane permeability.516 The Aβ-seeking nanoparticles provided strongly enhanced T2* contrast 4h after injection in the brains of Alzheimer’s bearing mice compared to wild type mice. In another study, an Aβ-targeted agent comprising Gd-DTPA coupled to N-terminus of a K6Aβ1-30 peptide was co-injected with mannitol in order to relax the blood-brain barrier in a mouse model.517 A series of Gd-DOTA complexes conjugated to Pittsburgh Compound B, a well-established PET tracer for imaging Aβ deposits, were shown to bind to Aβ1-40 in vitro.518–519
Alzheimer’s disease can also be accompanied by neuro-inflammation that can significantly compromise endothelial integrity. Aβ-targeted agents comprising SPIONs coupled to Aβ protein precursor antibodies (Anti-AβPP) have been shown to access Aβ depositions via this endogenous mechanism of blood-brain barrier breakdown. Imaging was performed ex vivo on brain slices and correlated to Aβ histologic staining after administration of the Anti-AβPP nanoparticles to Alzheimer’s bearing mice.520
5.11. Organic Anion Transporting Peptides
Organic anion transporting peptides (OATPs) are highly expressed on the surface of hepatocytes. OATP mediated internalization has been utilized to develop liver targeting agents such as the commercially available Gd-BOPTA and Gd-EOB-DTPA.521–523 Although these clinical agents are not typically thought of as biochemically-targeted contrast agents, the strong liver contrast observed following injection is the result of receptor mediated pooling of the agent in hepatocytes. OATPs are highly abundant on the surface of normal hepatocytes but are under-expressed on the surface of malignant cells and thus OATP-targeted agents are used to render malignant hepatocellular lesions conspicuously hypointense relative to healthy liver parenchyma. Hepatocellular phase images are typically acquired 20 – 40 min after injection in humans. OATP agents are also used in the differential diagnosis of hepatocellular lesions from benign, dense clusters of normal hepatocytes termed focal nodular hyperplasia (FNH). FNH typically exhibit avid arterial phase enhancement similar to many malignant lesions but unlike malignant lesions FNH are strongly enhanced by OATP-targeting agents during the delayed hepatocellular phase.524 Recently, a Mn based hepatocyte seeking agent was developed by conjugating Mn-EDTA to a benzothiazole aniline (BTA) moiety.525 Hepatocyte uptake presumably occurs via OATP mediated transport, but the mechanism has not yet been formally interrogated. A 0.05 mmol/kg dose of Mn-EDTA-BTA was used to visualize liver tumors in a mouse model with high contrast. The liver tumors were hypointense relative to the strongly enhanced liver parenchyma, consistent with previously developed OATP targeting agents.
Patrick et al. utilized the tremendous accumulating power of OATP as a gene reporter. They expressed the OATP receptor into cancer cells and implanted them into mice. Using Gd-EOB-DTPA for detection, they showed that cells expressing the reporter showed readily reversible, intense, and positive contrast (up to 7.8-fold signal enhancement) in T1-weighted magnetic resonance images acquired in vivo.526
5.12. Proliferating cells
Increased glucose metabolism is a hallmark feature of proliferating tumors. Malignant cells have high glycolytic activity compared to non-cancerous cells and typically overexpress insulin independent glucose transporters. Indeed, this is the physiologic basis of clinical [18F]fluorodeoxyglucose (FDG) PET scans to detect malignancies. Innovative studies have demonstrated that analogous imaging can be performed using glucose and glucose derivatives as DiaCEST MRI contrast agents, making use of the chemical exchange of the hydroxyl protons. This targeted imaging is another example of receptor mediated concentration of contrast agent. The efficacy of this approach has been demonstrated in both animal models and human patients.527–530 It was recently shown that glucose can be used to generate strong contrast enhancement in newly diagnosed, untreated glioblastomas using chemical exchange spin locking scans – which are analogous to CEST scans but more clinically robust and sensitive to hydroxyl proton exchange.531 Figure 59B shows glucose enhanced visualization of the proliferative microenvironment in a human glioblastoma patient.
Figure 59:

Comparison of (A) Gd-DOTA enhanced and (B) glucose enhanced imaging in a 45 year old woman with glioblastoma, the Gd-DOTA and glucose enhanced images are fused in (C) Glucose accumulates in proliferating cells and provides strong contrast enhancement in T1ρ-weighted images. The glucose enhanced images provide strong signal intensity in the dorsomedial regions of the tumor that overlap with T1-enhancement, but also strong enhancement beyond the blood-brain barrier disruption. Reproduced with permission from Ref.531 (URL: https://pubs.rsna.org/doi/10.1148/radiol.2017162351). Copyright 2017 Radiological Society of North America (RSNA®).
The hallmark leaky vasculature of solid tumors enables targeting cancer cells and extracellular proteins with macromolecular agents, although the pharmacokinetics of these agents are markedly slower and imaging must be performed hours later than would typically be done using biochemically-targeted small molecule agents. The high metabolic demands of proliferating cells lead to increased consumption of nutrients such as iron and this increased nutrient demand has been exploited for targeted molecular imaging (i.e. receptor mediated accumulation). For example, transferrin-SPION conjugates have been used to image mammary carcinoma in a rat model.532
The protein nucleolin is highly overexpressed on the surface of continuously proliferating cells and plays a critical role in promoting anti-apoptotic activity. A proliferation-seeking contrast agent was developed by derivatizing SPIONs with AS1411 aptamers, which bind surface expressed nucleolin with high affinity. This agent was shown to selectively adhere to cells with surface expressed nucleolin and was successfully used to delineate proliferating tumors via T2-weighted scans in a mouse model.533
Angiogenesis is another hallmark feature of the proliferative microenvironment as the formation of new blood vessels enables continued nourishment of the proliferating cells and an avenue for metastasis. In this regard, contrast agents have been developed to target the ανβ3 integrin receptors highly expressed on endothelial cells participating in angiogenesis. An ανβ3-seeking contrast agent was developed by linking a biotinylated ανβ3 –specific monoclonal antibody, DM101, to a biotin-coated Gd(III)-labelled perfluorocarbon nanoparticle. Avidin, which binds biotin with sub-pM affinity was used to link the biotinylated particle and antibody.534 The antibody based contrast agent provided 25% signal enhancement at the locus of corneal angiogenesis in a rabbit model 4h following intravenous injection, whereas no contrast enhancement was observed at the same time point using the paramagnetic nanoparticle alone, the corresponding non-binding isotype control, or administration of the ανβ3-seeking agent following pre-injection receptor blocking using a competing substrate. Numerous ανβ3-seeking agents have been developed using the cyclic tripeptide RGD as the integrin-binding moiety. For example, RGD functionalized SPIONs have been used to detect ανβ positive vasculature.535–536 One study demonstrated that relative SPION induced change in intratumoral T2 was shown to correlate with immunohistochemically determined ανβ3 per unit area coverage.536 Polymeric and liposomal Gd(III)-based agents and CEST reporters have also been developed as high-payload integrin-targeting agents.537–538
High-affinity folate receptors (HFR) are overexpressed on several tumor phenotypes originating from endothelial cells. A HFR targeting contrast agent was developed by conjugating DOTA to folate via bis(aminoethyl)glycol linker which formed amide bonds with both DOTA and folate carboxylates, Figure 60. This small molecule agent was successfully used to provide delayed enhancement of a HFR-positive human ovarian cancer xenograft in a mouse model for hours after injection of Gd-DOTA-folate, whereas no delayed enhancement was observed in control mice bearing HFR-negative xenografts.539 A high relaxivity, folate-targeting Gd(III)-based agent of undisclosed structure has also been developed. This agent, termed P866, was used to provide strong contrast enhancement of HFR positive tumors in mice for hours after injection.540–541 Contrast enhancement due to HFR binding was confirmed via imaging with a non-HFR binding control agent and by imaging using P866 under conditions where HFR was blocked by injection of free folate. Folate functionalized SPIONs have also been used for visualization of HRF-positive tumors.541 HFR has also been targeted with high-payload agent comprising a 4th generation PAMAM dendrimer functionalized with both folate and Gd-DTPA. A typical dendrimer was functionalized with 3-5 folate molecules but dozens of Gd-DTPA complexes. These folate targeting agent were shown to provide strong contrast enhancement of HFR-positive ovarian cancer xenografts in mice 24h after injection.542 Although no imaging was performed using a non-targeted control dendrimer, enhancement due to a folate-targeting mechanism was retrospectively confirmed by comparing Gd(III) retention in tumors using targeted and non-targeted dendrimers labelled with 153Gd(III) by gamma counting methods.543 A number of groups have also developed HFR-targeted agents using polymers and liposomes to deliver a high-payload of Gd(III)-based contrast agent.544–547
Figure 60:
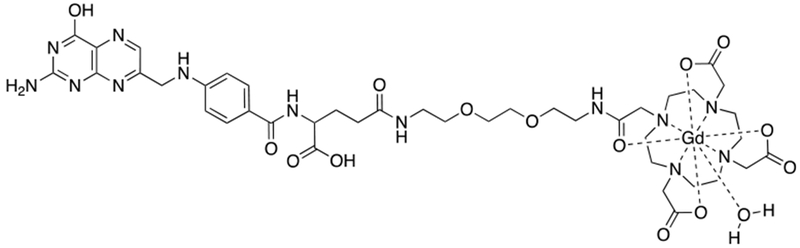
High-affinity folate receptor-targeting MRI contrast agent comprised of folate conjugated to Gd-DOTA via a bis(aminoethyl)glyocol linker.
5.13. Inflammation
Inflammation has been imaged using agents that target the cell adhesion molecules that play a critical role in the early stages of inflammation by recruiting circulating leukocytes to the site of injury. A “leukocyte-mimetic” SPION agent was developed by coating 1 µm diameter SPIONs with antibodies against the cell adhesion molecules P-selectin and vascular cell adhesion molecule-1 (VCAM-1).548 These particles were used to image endothelial activation during the onset of atherosclerosis in the aorta of mice. MR contrast correlated with lesion macrophage content. Flow cytometry experiments confirmed that these “leukocyte-mimetic” agents adhered to activated endothelial cells and were not internalized by phagocytic macrophages. A SPION functionalized with P-selectin antibodies was also used to monitor the dynamics of spinal cord endothelial activation in mouse models of chronic and relapsing multiple sclerosis.549 The cell adhesion molecule E-selectin has been targeted using a dextran-coated SPION functionalized with a small molecule mimic of the E-selectin targeting polysaccharide sialyl Lewisx (sLex) was used to image liver endothelial cell activation in a mouse model of hepatitis.550 This small molecule sLex mimic was also conjugated to Gd-DTPA type contrast agents.551 The resultant agent, Gd-DTPA-B(SLex)A Figure 61, was shown to exhibit a ~67% increase in blood-half life in a rat model of fulminant hepatitis (30 min vs 49 min in healthy vs. hepatitis rats, respectively) which was attributed to interactions with endothelial cell adhesion molecules in the diseased rats.552 In mice, Gd-DTPA-B(SLex)A, was shown to provide strongly enhanced intravascular contrast out to 1 h after injection, whereas blood signal returned to near baseline within minutes after injection of Gd-DTPA.552
Figure 61:

Gd-DTPA-B(SLex)A is an E-selectin-targeting agent. This agent has been used to image endothelial cell activation in a mouse model of fulminant hepatitis.
The inflammatory microenvironment is rich with phagocytic macrophages. Phagocytosis has been pursued by a number of groups as an endogenous mechanism to concentrate macromolecular contrast agents such as SPIONs,430, 553 Gd(III)-loaded liposomes,554 and 19F perfluorocarbon nano-emulsions555–557 for targeted imaging of inflammation.
5.14. Apoptosis
Apoptosis is programmed cell death in response to cell stress or cell signals but dysregulated apoptosis is often encountered in the proliferative microenvironment of solid tumors. Apoptotic cells are characterized by highly negatively charged phosphatidylserine rich cell membrane and this unique cell surface has been used as a targeting mechanism. A high payload, protein-based apoptosis-targeting agent was developed by conjugated Gd-DTPA to the surface exposed lysines of glutathione-S-transferase-C2A fusion protein, which binds to the phosphatidylserine rich surface of apoptotic cells with high affinity.558 This protein based agent was shown to bind selectively to apoptotic and necrotic cells in vitro and was used to image apoptotic cells in a mouse model of lymphoma. Another way to target apoptosis with high payload is to use Gd(III)-chelate loaded liposomes conjugated to Anexin 5, which also has high affinity for apoptotic cells.559 These liposomal agents have been used for targeted imaging of apoptosis in atherosclerotic plaque in mice.560 Anexin 5 functionalized SPIONs have also been used to image cardiomyocyte apoptosis in a mouse model of myocardial ischemia.561
5.15. Necrosis
There are high concentrations of extracellular DNA in acutely necrotic tissue, as macromolecular detritus requires hours to days to clear from the newly necrotic region. In this regard, an extracellular DNA-binding agent was developed for molecular imaging of necrosis.562 The agent DNA-binding agent, termed Gd-TO, Figure 62, is comprised of Gd-DOTA coupled to the DNA intercalator thiazole orange. Gd-TO was used for molecular imaging of acute necrosis following myocardial infarction in a mouse model. The dynamics of extracellular DNA clearance were monitored by Gd-TO enhanced imaging at various time points between 0 to 72h after myocardial infarction.562
Figure 62:
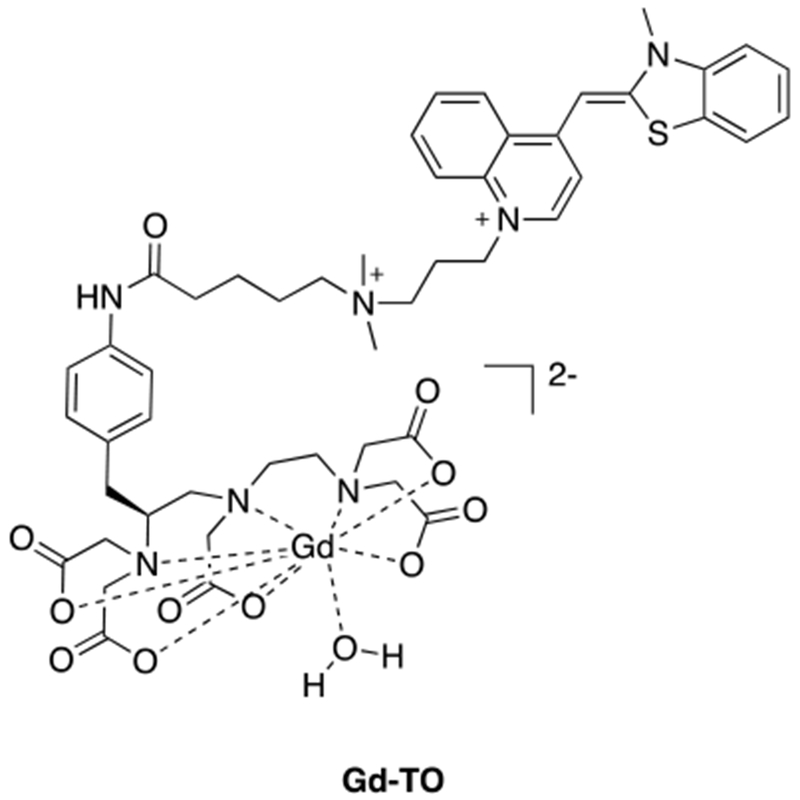
Gd-TO binds to extracellular DNA via DNA intercalation of the appended thiazole orange dye. This agent has been used to image necrosis in a mouse model of myocardial infarction and to monitor the dynamics of extracellular DNA clearance after an acute necrotic event.
6. Activatable contrast agents
Besides direct targeting, molecular MRI can also exploit changes in relaxivity or chemical exchange to detect specific biological processes or pathologies. Ion flux, pH, enzymatic activity, chemical potential (redox), and temperature are all features of the microenvironment that can be altered in disease processes. Activatable contrast agents, sometimes called responsive or ‘smart’ contrast agents, exhibit altered relaxivity or altered CEST effect in response to a stimulus. Unlike targeted agents which accumulate at a specific site because of protein binding or cellular internalization, activatable agents produce signal change because they themselves are chemically altered. Activatable contrast agents that are capable of revealing biochemical or physiologic abnormalities may offer unique insights into the human body and enable the earlier and more precise diagnosis of specific diseases, as well as monitoring the treatment of these disorders.
This section provides an overview of the chemistry, properties and applications of activatable para- and diamagnetic contrast agents. The probes discussed here can be viewed as MR sensors of physiological events whose relaxivity or CEST effect depends on the absence/presence of the biological stimulus of interest.
As outlined in section 3.3, the T1 relaxivity of a paramagnetic metal complex is primarily determined by three factors: q, the number of water molecules in the inner coordination sphere, τm, the residence lifetime of these inner-sphere water molecules, and τR, the rotational correlation time of the molecule. Thus, for the development of a biochemically responsive contrast agent, one or more of these molecular factors must be changed by a biochemical stimulus. In the ideal case, the agent features an “off” state associated with low relaxivity in the absence of the biochemical trigger and an “on” state associated with high relaxivity in the presence of the trigger. Similarly, superparamagnetic nanoparticles can exhibit activatable T2* change based on aggregation of the particles. CEST agents can be made responsive by altering the chemical exchange rate or the chemical shift of the exchangeable hydrogen in response to a stimulus, which causes the CEST effect to appear, disappear or change in magnitude.
6.1. Case study: A Gd(III)-based β-galactosidase sensor
Pioneering work in the development of activatable Gd(III)-based contrast agents was led by Meade and coworkers with their studies on agents responsive to the enzyme β-galactosidase.563 β-galactosidase catalyzes the hydrolysis of β-galactosides and is commonly used in molecular biology as a reporter marker to monitor gene expression.564–565 The Meade group sought to make a MR probe that could report on β-galactosidase function and gene expression. The initial sensor (Figure 63A) consisted of a Gd-DO3A chelate coupled to a galactopyranose moiety that acts as a lid for the access of water to the inner sphere coordination cage. Enzymatic cleavage of the glucosidic linkage irreversibly frees a coordination site to yield a Gd-HPDO3A-like complex with an increased number of inner-sphere water molecules resulting in higher relaxivity. Using lifetime luminescence measurements on the Tb analog, the authors estimated that the hydration state of the complex changes from 0.7 to 1.2 upon cleavage of the sugar group. The relaxivity change was modest but could be improved by modifying the complex to introduce an additional α-methyl group within the molecular scaffold as depicted in Figure 63B-C.566 This modification appears to reduce the relaxivity of the uncleaved complex with the galactopyranose moiety intact, presumably by limiting water access to the gadolinium(III) center. Using this second generation probe, β-galactosidase activity could be detected in living Xenopus laevis embryos (Figure 63D). Two embryos were injected with the activatable contrast agent, while one embryo was additionally injected with β-galactosidase mRNA. The MR signal intensity was 45 – 65 % greater within the embryo that was treated with β-galactosidase mRNA, thereby demonstrating the detection of β-galactosidase activity.
Figure 63:
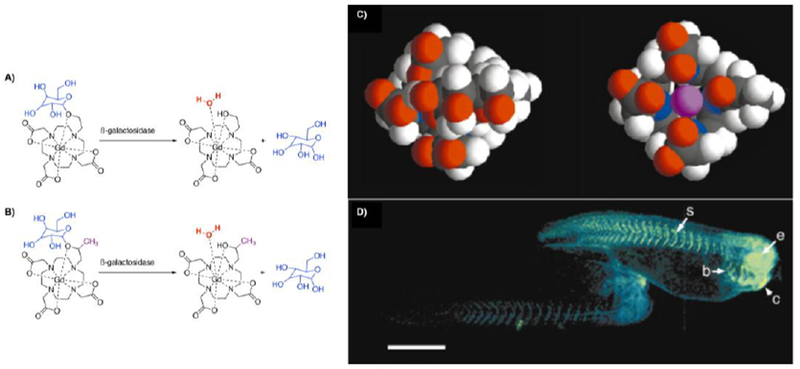
Detecting enzyme activity with a Gd(III)-based MR contrast agent through changes in T1 relaxation caused by modulation of inner-sphere hydration state. Chemical structures of the first (A) and second (B) generation β-galactosidase sensors. C) Enzymatic cleavage of β-galactose frees one coordination side that becomes accessible by water molecules. D) Two living Xenopus laevis embryos were injected with the second generation of the β-galactosidase sensor. The embryo shown on the right was also injected with β-galactosidase mRNA. The pseudocolor rendering of MR images shows that the signal strength is 45 – 65 % greater in the embryo treated with ß-galactosidase mRNA, demonstrating the detection of β-galactosidase activity. Labeled anatomy: (e) eye, (c) cement gland, (s) somite, (b) brachial arches. Reproduced with permission from Ref.566 (URL: http://dx.doi.org/10.1038/73780). Copyright 2000 Springer Nature.
This study demonstrates the power of activatable agents to detect biochemical events, notably enzymatic activity. In this case, 55 M water signal was imaged and this signal was augmented by 0.5 mM contrast agent which in turn was augmented by a 4 µM enzyme concentration. Thus activatable agents offer an avenue to detect low concentration molecular events, despite the sensitivity limitations of MRI. The study also points to some requisites for activatable agents. The difference in relaxivity (or CEST effect) between the “off” and “on” state should be large to detect meaningful change. Additionally, the reaction to activate the agent must be very fast; for enzyme sensing, the kinetics must be fast enough to turnover micromolar concentrations of agents in a short period of time.
6.2. Challenges and New Frontiers
Activatable agents have the same primary challenge as all the other MR contrast agents described in this review: detection sensitivity. For relaxation agents, micromolar concentrations of metal ion are required and millimolar concentrations are needed for CEST. Direct detection typically requires even higher concentrations. Regardless of the mechanism of activation and the magnitude of change in signal generating property (relaxivity, chemical exchange rate), the activatable agent can only be effective if it is present at concentrations above its detection threshold.567
Another consideration is whether the activation is reversible or irreversible. Reversible responsive MRI contrast agents return to their original state when they no longer interact with their specific biomarker. This makes them especially suitable for monitoring rapidly changing physiological conditions, e.g. ion flux. But in this case the MR detection sensitivity is target-limited, which means that the biomarker of interest has to be present in relatively high concentrations to be detectable with a reversible activatable probe. Irreversible responsive agents do not return to their original state in the absence of their specific biomarker. With irreversible responsive agents, signal change is one way and oscillations in biomarker concentration cannot be detected. On the other hand, the complete conversion to the activated state can greatly amplify detection sensitivity.
There are two main approaches to enhance dynamic range of both reversible and irreversible responsive agents. One strategy is to maximize the signal change between the inactivate and the activated agent. This can be done by optimizing the relaxivity (or CEST effect) of the activated agent or by suppressing the relaxivity of the inactive form, or a combination of both. Again, one must keep in mind that the overall detection threshold must be reached, so for relaxation agents the relaxivity of the activated agent must be high enough to render the agent detectable at physiologically achievable concentrations. In another strategy the responsive agent is not only “turned-on” in the presence of a particular biological stimulus but additionally retained and thereby accumulated at the target. This approach can be seen as a combination of targeted and activatable probes and will be further discussed in section 7.
Another question to address is whether one wants to simply detect the presence of a biomarker, e.g. the presence of a specific enzyme, or whether one wants to quantify a biomarker, e.g. measure pH. Quantification presents a particular challenge because the MR signal depends not only on the response to the biochemical stimulus (relaxivity change) but also on the concentration of the imaging probe. Even for qualitative detection, it is necessary to insure that the signal change observed is a result of activation and not just pooling of the inactive agent. Three general methods have been employed to address this problem: (1) the utilization of ratiometric methods where the measured effect is independent of concentration; (2) successive injections of responsive and non-responsive agents with similar structure and pharmacokinetics where the signal change of the non-responsive agent is used to estimate the concentration; or (3) utilization of a bimodal agent and simultaneous bimodal imaging, where one image modality (PET or SPECT) is used to quantify the agent and this is used to deconvolute the MR signal and quantify the degree of activation. These strategies will be discussed on the basis of exemplary examples.
As seen below, a large number of activatable agents have been reported. However unlike with targeted agents, there have been relatively few successful examples of activatable agents being deployed in vivo. On the other hand, the ability to image and quantify enzymatic activity, ion flux, redox status, pH, etc deep within tissue with submillimeter resolution would have a tremendous impact on our understanding of biology and pathobiology. The development of truly functional activatable agents remains a worthy goal.
Excellent reviews of activatable MR contrast agents have been published in recent years.185, 567–572 In this section, our primary goal is to provide an overview of the different strategies and creative ideas that have been employed in the design of activatable MR contrast agents. Due to the large number of examples in the literature, this section is not meant to be comprehensive. We regret the omission of interesting studies for the sake of conciseness.
6.3. pH-responsive molecular imaging agents
Decreased extracellular pH values are associated with cancer and various ischemic diseases. pH quantification could be a useful biomarker for early identification of disease or to monitor treatment efficacy.573 Hence, the development of smart MR contrast agents, which show pH-dependent relaxivity profiles or CEST effects has been the subject of intensive research for the past two decades.
6.3.1. pH-responsive Gd(III) based contrast agents
An early and effective example of a pH-responsive agent was Gd-DOTA-4AmP (Figure 64A) reported by Sherry and coworkers.574–575 This complex is a DOTA derivative where the carboxylate groups have been derivatized as amides with pendant phosphonate groups. Inner-sphere water exchange is extremely slow for this complex, but exchange of protons of the bound water molecule with bulk water is fast and is catalyzed by pendant phosphonates. Protonation of the phosphonate groups, which have pKa values in the physiological range, alters this prototropic exchange rate and also alters second-sphere relaxivity. The relaxivity is 51 % higher at pH 6 compared to pH of 9.5. The magnitude of the pH dependent relaxivity effect can be increased by slowing the overall tumbling rate of the molecule. Coupling of this agent to the macromolecule G5-PAMAM dendrimer yielded a product that contained on average 96 molecules of Gd-DOTA-4AmP and exhibits an average hydrodynamic volume consistent with a molecular weight of ~ 140 kDa. The T1 relaxivity of this macromolecular sensor increases by 122 % (r1 = 10.8 to 24.0 mM−1s−1) over the pH range of 9.5 to 6.576
Figure 64:
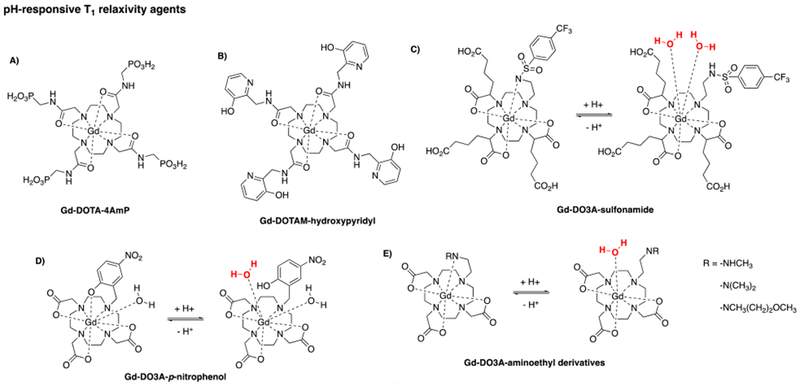
Selected pH-responsive T1 relaxivity agents.574,308, 577, 580–582
The Sherry group reported another example of modulating prototropic exchange and water distribution in the second coordination sphere. The Gd-DOTA tetraamide with pendant hydroxypyridyl moieties was used to image changes in pH values (Figure 64B).577 Deprotonation of the amides in those moieties result in intramolecular acid-base pair interaction with the phenolic protons, leading to a highly organized second hydration sphere. As a result, the relaxivity of this agent is enhanced at higher pH (pH 8.5) and decreases by 84 % at lower pH (pH 6).
The pH-dependent relaxivity changes of most gadolinium(III) based pH sensors are realized through modulation of the inner-sphere hydration state, q. An impressive example was reported by Parker and coworkers who designed a Gd-DO3A derivative with a pendant sulfonamide (Figure 64C).308 The hydration state changes from q = 0 to q = 2, associated with the on/off ligation of the sulfonamide nitrogen donor which occurs in a region of great interest for extracellular pH change. Decreasing the pH from 7.4 to 6.8 resulted in a r1 enhancement of 35 % when measured in a simulated extracellular anion background and 48 % when measured in human serum. Bis-hydrated Gd-DO3A is known to coordinate endogenous anions like bicarbonate and phosphate.308, 578–579 The Parker group employed pendant anionic carboxylate groups (Figure 64C) which served to block anion coordination and inner-sphere water displacement. The pendant carboxylate groups also resulted in unexpectedly higher relaxivity than expected based on molecular weight, which is presumably due to a second sphere effect. In addition, this complex had modest affinity to HSA while maintaining its pH dependent T1 relaxivity profile.487 Increasing the molecular weight and thereby the rotational correlation time of the Gd(III) compound through binding to HSA should dramatically increase the initial r1 enhancement (Gd(III)-complex without HSA: r1 = 2.4 to 9.0 mM−1s−1 from pH 5 to 8.5, 37 °C, 0.47 T). In fact, binding of the Gd(III)-complex to HSA resulted in an unexpectedly low r1 enhancement of 42 % (pH 5) and 260 % (pH 8.5), respectively, which was attributed to displacement of inner-sphere water ligands when the complex is bound to HSA. This finding was confirmed using a derivative with increased HSA affinity.487
Another donor group that can be protonated around neutral pH is phenolate. Sherry and coworkers showed that a Gd-DO3A derivative with a pendant p-nitrophenolate arm underwent a switch in hydration state from q = 1 to q = 2 (Figure 64D) as pH was lowered.580 The protonation and dissociation of the phenolate oxygen donor atom resulted in a 71 % enhancement in r1 from pH 9 to 5. Moreover, at low and at high pH, the compound is resistant to water ligand displacement by anion coordination.
In a recent study, Giovenzana and coworkers exploited the suitability of Gd-aminoethyl-DO3A derivatives as a pH-sensitive platform (Figure 64E).581–582 On average, their compounds showed a percentage enhancement around 100 % in the pH region of 9 to 5, also provoked by a switch in hydration state.
A major obstacle in applying these systems to image tissue pH is the unknown concentration of the contrast agents in tissue. In order to measure pH, one must know the relaxivity, and relaxivity depends on both concentration and T1 change. MRI can measure T1 change but the challenge of measuring concentration must be addressed.
Gillies, Sherry and coworkers applied a dual injection strategy to determine concentration. They administered the pH-independent agent Gd-DOTP and measured the time dependent signal change. They then administered pH-responsive Gd-DOTA-4AmP (Figure 64A) and measured the signal change with time.583–584 With the assumption that both agents had similar tissue biodistribution and pharmacokinetics, the differences in the signal-versus-time curves for Gd-DOTA-4AmP and Gd-DOTP were attributed to differences in relaxivity. As a result, the extracellular tissue pH could be successfully mapped in the kidneys in a renal acidosis model and in a tumor in a brain tumor model.583–584
The same group used a different strategy to map pH in a a rat brain glioma model.585 Instead of a sequential injection of two agents, this time they mixed Gd-DOTA-4AmP with Dy-DOTP. Dy-DOTP has extremely low r1 but does exhibit a large R2* effect. By coinjecting an appropriate mixture, Gd-DOTA-4AmP dominates the ΔR1 effect, while Dy-DOTP dominates the ΔR2* effect. Concentration of Dy-DOTP was estimated from ΔR2*, and since the ratio of Gd:Dy was known, the Gd-DOTA-4AmP concentration could be estimated and pH determined from the ΔR1 measurement. This again assumed that both contrast agents had the same distribution and pharmacokinetics. Simply replacing Gd(III) with Dy(III) would likely give identical pharmacokinetics.
Rather than injecting two different agents, Aime and coworkers proposed a ΔR2/ΔR1 ratiometric method.586 As described above (sections 3.3 & 4.2.b), slow tumbling agents show increased r1 at low fields but this decreases rapidly with increasing field strengths. On the other hand r2 is high at low fields and remains constant or increases with increasing field strength. Thus the r2/r1 ratio increases with increasing field strength but also increases at a given field strength when τR is increasing. This ratio is independent of concentration. This group designed a Gd-DOTA functionalized polypeptide (poly-L-ornithine, Figure 65A) that exhibits a pH-dependent conformation. At low pH it assembles randomly and exhibits a shorter τR while at higher pH an ordered α-helix is formed resulting in an increase in τR. This resulted in a r2/r1 ratio that increased with pH. By measuring ΔR2/ΔR1 (the difference in relaxation rate in the presence and absence of the agent), the authors could detect pH changes that were independent of contrast agent concentration. A limitation of this type of approach is that the r2 relaxivity must be very high to measurably change T2 in tissue where T2 values are generally very short. That is, although ΔR2/ΔR1 is independent of concentration, if the measured ΔR2 and ΔR1 values are small then the error associated with the measurement will be very large.
Figure 65:
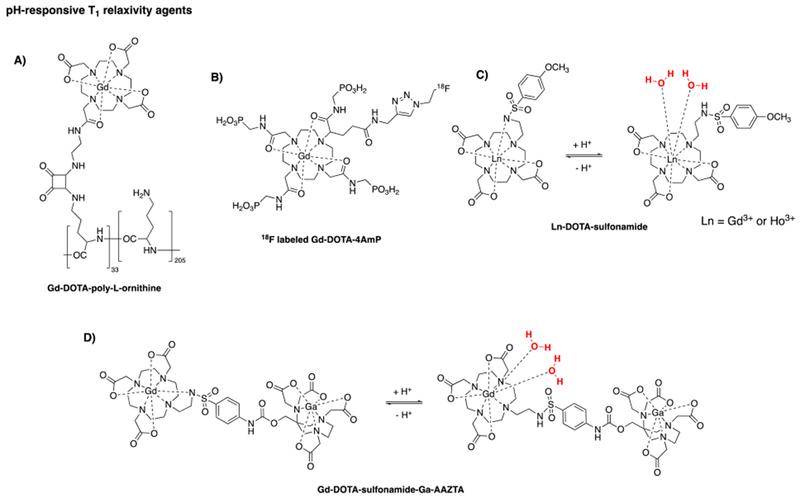
A third strategy to determine concentration independently was reported by Frullano et al. They incorporated the radionuclide 18F into the framework of the already established pH-responsive contrast agent Gd-DOTA-4AmP (Figure 65B). By mixing the 18F compound with the stable 19F analog, they achieved 18F and Gd(III) concentrations suitable for detection by both PET and MRI. Using a simultaneous MR-PET scanner, they could achieve absolute quantification with PET while contemporaneously measuring T1 with MRI.587 Combining the PET (concentration) and MRI (ΔR1) measurements, they could compute relaxivity and hence obtain pH maps.
Other bimodal activatable pH agents have since been reported. Aime and coworkers proposed a dual MR-SPECT agent by adding a small amount of the gamma-emitting 166Ho(III) analog to a previously described pH-responsive Gd-DO3A derivative with a pendant sulfonamide308 (Figure 65C).588 This system is actually two separate compounds but it is assumed that the Ho(III) complex would have the same biodistribution and pharmacokinetics as the Gd(III) analog, which is a reasonable assumption. A limitation of this approach is that there are no simultaneous MR-SPECT scanners, while MR-PET scanners are commercially available. For in vivo measurements, the MRI and PET (or SPECT) measurement must be simultaneous otherwise the concentration measured would not be reflective of the concentration present when T1 was measured. Another elegant bimodal MR-PET pH sensor was invented by Tei and coworkers.589 They prepared a heteroditopic complex that contains Gd(III) as a MRI reporter and 68Ga(III) as the PET reporter (Figure 65D). The dimeric ligand consists of two different chelating cages: a DO3A-sulfonamide derivative for the encapsulation of Gd(III) (Figure 65C) and one chelator based on AAZTA for the complexation of 68Ga(III). A sulfonamide moiety acts as a linker between the two chelating cages and provides pH-sensitivity at the same time since the on/off ligation of the sulfonamide nitrogen donor to the Gd(III) center is pH dependent. Hence, a switch in hydration state (low pH: q = 2/high pH: q = 0) leads to a percentage enhancement in r1 of 150 % over the pH range of 8.5 to 5.
6.3.2. pH-responsive CEST contrast agents
CEST agents can also be used as pH-responsive agents since their mechanism of action is based on the selective saturation of exchangeable protons, followed by the transfer of the saturated protons to the bulk water leading to an alteration in water signal intensity. Typically, for a chemical species with labile protons the exchange rate kex of those protons with bulk water is pH-modulated due to base catalysis of proton exchange.
pH-responsive DiaCEST agents
In their seminal 2000 paper describing CEST imaging, Ward and Balaban also demonstrated that proton chemical exchange agents could be used to image pH.8 They showed that dihydrouracil (Figure 66A) with its two labile amide protons can be employed as a pH sensitive imaging agent. They further showed how a ratiometric method could be used to measure pH in a way that was not dependent on the concentration of the agent. In general, the magnitude of the CEST effect for one type of exchangeable proton is different and has a different pH dependence than another exchangeable proton. For example in dihydrouracil selective irradiation of each of the amide N-H protons results in a different CEST effect. The ratio of these two CEST effects is pH dependent and independent of concentration. Similar methods could be applied using two different CEST contrast agents where the signal of one is ratioed against the other. However in this case, the two agents should have similar pharmacokinetic properties. Longo, Sun, et al. showed that one does not need two separate exchangeable proton pools for quantitative pH CEST imaging. They recognized that the efficiency of the saturation power in creating saturation transfer also has a pH dependence. They showed that by ratioing the CEST effect at different saturation powers that they could quantify pH using the compound Iobitridol.590
Figure 66:
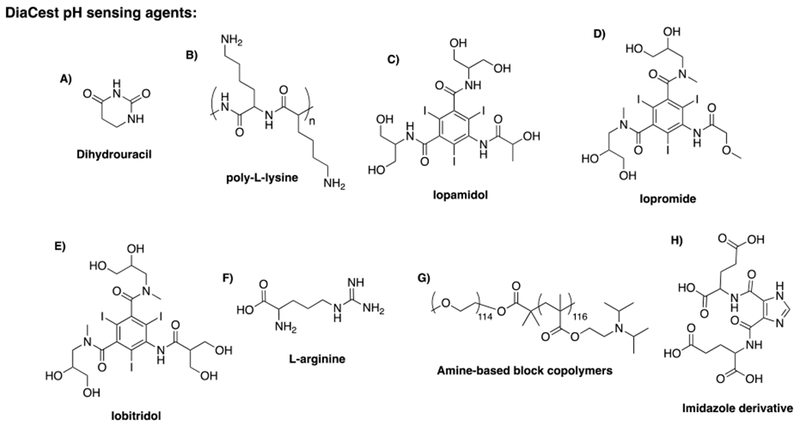
A limitation of diamagnetic CEST agents is their poor sensitivity requiring the administration of high concentrations to obtain measurable MR contrast. Two strategies can be adopted to improve their sensitivity with a concentration threshold in the micromolar range: 1) the chemical shift between the exchangeable proton resonance and the bulk water resonance is increased by using strong de-shielding groups like e.g. aromatic rings, halogen substituents or paramagnetic shift reagents or 2) the number of mobile protons per molecule is increased by using macromolecular systems like dendrimers and peptides.
Applying the latter strategy, van Zijl and coworkers used a poly-L-lysine scaffold to measure the pH dependence of its amide proton exchange rates in the physiologic range (Figure 66B).591 The pH dependent NH proton exchange and its relationship to CEST imaging is illustrated in Figure 65. As shown in the high-resolution 1H-NMR spectrum of poly-L-lysine (Figure 67, left), at lower pH (pH = 6), the NH proton exchange is rather slow, resulting in a sharp NH proton resonance. In contrast, at the same pH, the corresponding CEST spectrum (Figure 67, right) shows only a rather small CEST effect. Increasing the pH to 7.9 accelerates the NH proton exchange, which dramatically broadens the corresponding NH proton signal in the 1H-NMR spectrum and in turn amplifies the CEST signal.
Figure 67:
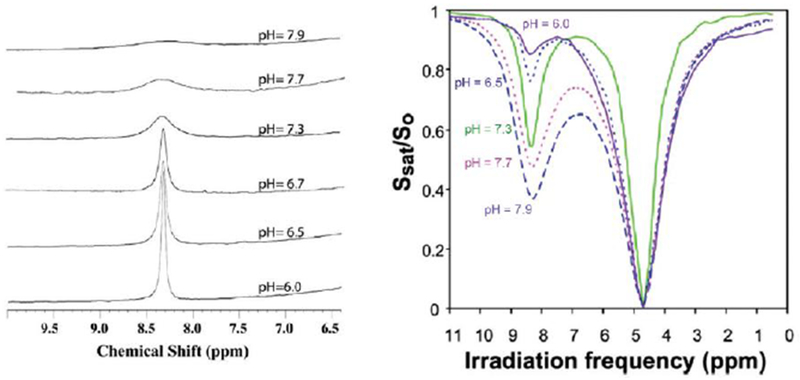
pH dependency of proton exchange and its relationship to CEST. Left: 1H-NMR spectra of poly-L-lysine at different pH. Right: CEST spectra of poly-L-lysine at different pH. The proton exchange accelerates with increasing pH, which broadens the corresponding 1H-NMR signal but amplifies the CEST signal. Reproduced with permission from Ref.591 (URL: http://dx.doi.org/10.1002/mrm.20818). Copyright 2006 John Wiley and Sons.
X-ray contrast agents are given at very high doses, literally 10’s of grams of these highly soluble iodinated aromatic compounds. Aime and coworkers recognized that X-ray agents like Iopamidol, also contain two different amide proton pools with downfield chemical shifts (Figure 66C), and thus could be used as a pH sensor in MR imaging applications.449 Iopamidol was successfully employed to acquire pH maps of kidney cortex, medulla, and renal pelvis in healthy mice and to image the pH evolution of an acute kidney injury mouse model.72, 596 Additionally, in a breast cancer mouse model, tumor pH mapping was performed using Iopamidol in a combined PET and MR-CEST imaging study.597 Other approved CT contrast agents with similar structure: Iopromide592 (Figure 66D) and Iobitridol590 (Figure 66E) were also utilized in vivo for accessing changes in tumor acidosis.
In another approach, liposomes were filled with L-arginine, a molecule with multiple exchangeable NH protons (Figure 66F) and subsequently assembled to microcapsules (LipoCEST). With those pH-nanosensors, transplanted cell viability could be monitored in vivo.593
Gao and coworkers reported ionizable, tertiary amine-based block copolymers (Figure 66G) as pH sensors for MR-CEST imaging. Near physiological pH, the polymers form micelles turning the CEST signal “off” whereas in an acidic environment the CEST signal is turned “on” through dissociation of those micelles.594
Recently, McMahon and coworkers showed that imidazoles (Figure 66H) can also be used as DiaCEST MRI pH sensors as demonstrated by in vivo mapping of kidney pH in mice.595
pH-responsive ParaCEST agents
One limitation of DiaCEST agents is their small chemical shift difference from bulk water which can have interference from endogenous biomolecules that generate CEST in a similar range. A second limitation is sensitivity. For CEST the chemical shift difference (in Hz) must be greater than the proton exchange rate. Faster exchange would produce a larger CEST effect but if exchange is faster than the chemical shift difference then the chemical shifts merge and CEST is impossible. One can increase the shift difference by moving to higher fields or by turning to ParaCEST agents. These exhibit a much larger chemical shift difference between the exchangeable proton resonance and the bulk water resonance, allowing faster exchange rates to be sampled with concomitant increased sensitivity.
As outlined in section 3.3, the water exchange rate between the inner sphere of a paramagnetic center and bulk water is an important parameter that governs CEST sensitivity. The water exchange rate strongly depends on geometric or steric factors that are created by macrocyclic ligands encapsulating the paramagnetic ion. Recently, Udugamasooriya and coworkers reported design principles for Eu(III)-DOTAM-based ParaCEST MR contrast agents to optimize the water exchange rate and thereby, enhance the CEST sensitivity.598–599
Aime and coworkers investigated the ability of Yb-DOTAM-Gly as a pH-responsive ParaCEST agent (Figure 68A).600 The compound contains four chemically equivalent amide protons in close proximity to a paramagnetic ytterbium center. Applying an irradiating radiofrequency pulse at the amide N-H resonances at different pH values gave a change in the net saturation transfer effect from 70 % to 10 % in the pH region of 6 to 8.8. Moreover, the authors discovered that the concentration of the ParaCEST agent and the saturation transfer effect are not linearly related. They applied a ratiometric approach by using two agents differing only in the coordinated lanthanide center: Yb-DOTAM-Gly and Eu-DOTAM-Gly. In the latter agent, the amide protons are only slightly shifted in a region where they cannot longer be distinguished from bulk water signals. The CEST spectrum obtained for a mixture containing both complexes gave three peaks, one for the bulk water, one for the amide protons associated with Yb-DOTAM-Gly and for the metal coordinated water protons associated with Eu-DOTAM-Gly. Under the assumption that the saturation transfer effect of the two different sets of protons (amide protons vs. metal coordinated water protons) have a different pH dependency, the observed CEST effect depends only on the relative concentration ratio of both lanthanide complexes. An improvement of this ratiometric concept was demonstrated by irradiating two kinds of mobile protons (amide protons and metal coordinated water protons), that are part of the same paramagnetic DOTAM-Gly complex. The single-molecule CEST procedure was realized through employment of lighter Ln(III) ions, like e.g. Pr(III), Nd(III) and Eu(III) where Pr-DOTAM-Gly (Figure 68A) showed the highest accuracy and sensitivity in its pH dependent behavior in the pH region 6.0 – 8.0.601–602 However, detecting the CEST effect from metal-bound water molecule can require high saturation power that can hamper the safe in vivo application of those agents because of local heating effects.603
Figure 68:
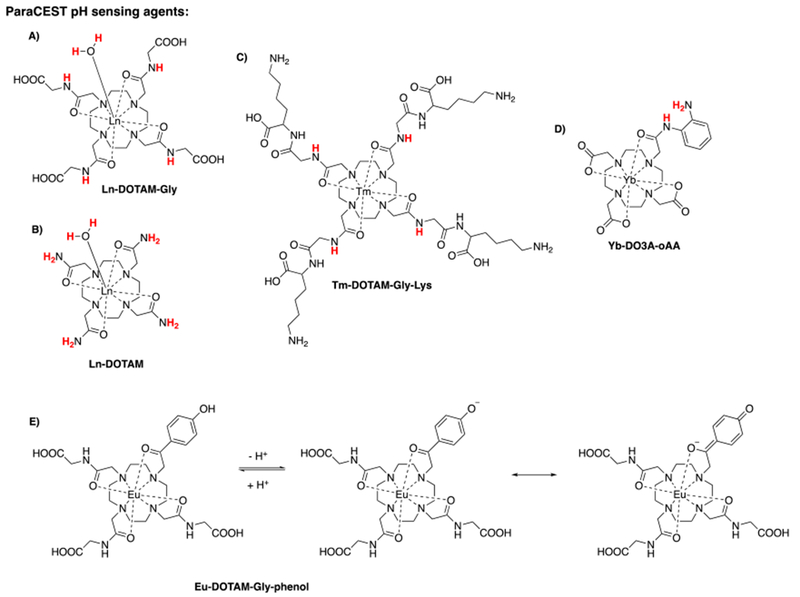
Selected pH-responsive ParaCEST agents (protons employed for CEST imaging are shown in red).600–606
Pikkemmat and coworkers functionalized different generations of poly(propylene imine) dendrimers with Yb-DOTAM (Figure 68B) to obtain macromolecular ParaCEST agents, thereby combining the two strategies (increasing the chemical shift and the number of exchangeable protons) to improve the sensitivity of the MR contrast agent (vide supra).604 In this way they could reduce the detection sensitivity by a factor of 15 and observe a 5 % CEST effect at 20 µM dendrimer solution.
Another paramagnetic DOTAM-Gly derivative: Tm-DOTAM-Gly-Lys (Figure 68C) was used as pH responsive ParaCEST agent.605 Interestingly, in this study the pH was determined from the linewidth of the asymmetric magnetization transfer ratio curve, which has an exponential relation to pH. As a result, pH mapping was successfully realized independent from agent concentration and temperature for a given saturation pulse. This method was also successfully demonstrated in vivo in healthy mice.
Yb-DO3A-oAA (Figure 68D) has two different exchangeable proton pools which also enables pH imaging via an intramolecular ratiometric approach. This compound was used to assess pH in a mouse model of MDA-MB-231 mammary carcinoma.603, 607–608
A direct readout of pH by MR imaging is provided by a Eu-DOTAM-Gly derivative (Figure 68E).606 In this elegant example, one of the amide side chains of the DOTAM-Gly ligand is replaced by a ketone oxygen donor, which in turn is directly conjugated to an ionizable phenolic moiety. Deprotonation of the phenolic proton leads to conjugation of the resulting quinone-like structure with the acetyl oxygen donor coordinated to the europium center. Since the metal-bound water exchange rate is extremely sensitive to alterations in the electronic structure of the ligand pendant arms, the CEST signal can be manipulated through this effect. With a pKa value of 6.7 for the phenolic entity, the agent has ideal properties for sensing pH in biological systems and shows a large change in exchange frequency over the pH range of 6.0 to 7.6. Using ratiometric CEST imaging, the pH could be determined independent of Eu-DOTAM-Gly derivative concentration. Utilization of this frequency-dependent ParaCEST agent allowed imaging of the pH gradient in kidneys of healthy mice.609
Aime and coworkers used the Yb(III) analog of the approved contrast agent Gd-HPDO3A as a pH responsive ParaCEST agent (Figure 3).610 The labile hydroxylic proton shows a pH-dependent CEST effect in the pH region of 5.0 to 7.0 (37 °C). Moreover, the chemical shift of the hydroxylic proton is temperature dependent making Yb-HPDO3A a multi-responsive ParaCEST agent that can report on pH and temperature simultaneously. Yb-HPDO3A exists as two major isomeric forms in solution with different chemical shifts for the hydroxylic protons, and this enables a ratiometric approach in a single-molecule CEST procedure. This agent was successfully employed for in vivo pH mapping of the tumor region in a melanoma murine model, as depicted in Figure 69.611
Figure 69:
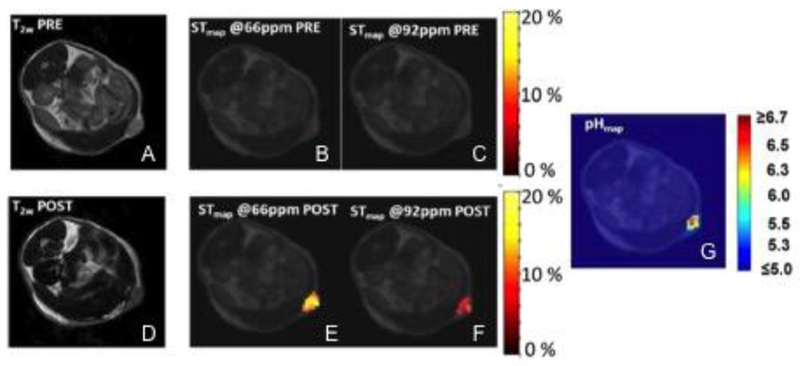
T2W images (7 T) acquired pre (A) and 2 min post (D) i.v. injection of Yb-HPDO3A (dose: 1.2 mmol kg−1); STmaps calculated at 66 ppm pre (B) and 2 min post (E) i.v. injection of Yb-HPDO3A; STmaps calculated at 92 ppm pre (C) and 2 min post (F) i.v. injection of Yb-HPDO3A; G) pH map of a region of a tumor isolated in slice one calculated from the corresponding ST maps. Reproduced with permission from Ref.611 (URL: http://dx.doi.org/10.1002/mrm.24664). Copyright 2014 John Wiley and Sons.
Recently, a pH responsive transition-metal based ParaCEST probe was reported.612 The high-spin Fe(II) complex of a 2-amino-6-picolyl-appended cyclen ligand shows a pH dependent frequency shift at pH 7.7 to 4.8 (37 °C). This shift in the CEST peak correlates with the protonation of the unbound pendant 2-amino-6-picolyl units as illustrated in Figure 70. Noteworthy, in this example the CEST peak changes in frequency with changing pH and not in intensity. In contrast to intensity-responsive contrast agents, frequency-responsive probes do not require a correction for tissue concentration of the probe.
Figure 70:

First frequency-responsive transition-metal based ParaCEST agent that reports on pH.612
6.3.3. pH-responsive ParaSHIFT contrast agents
Recently, the Parker group developed a ParaSHIFT agent for accurate pH and temperature mapping using MR chemical shift imaging (Figure 71).613 Unlike indirect detection methods, e.g. CEST methodology where the interaction between the contrast agent and bulk water is detected, in this approach the MR signal is enhanced by employing paramagnetically shifted resonances and increased relaxivity from Ln(III) complexes itself. Their Ln-DOTP-like ParaSHIFT agent contains a pendant pyridine moiety, which in turn is labeled with a homotopic tbutyl group, whose protons undergo fast relaxation in the field range 1 to 7 T. In direct proximity to the tbutyl group, the pyridine ligand also contains a phosphonate group, whose pKa lies in the physiologically relevant pH region. As a result, the chemical shift of the adjacent tbutyl group varies as a function of pH. Using the corresponding Dy(III) and Tm(III) complexes in a ratiometric CEST experiment, the pH and temperature in the liver, kidney and bladder in healthy mice could be mapped. In comparison to the ParaCEST MR contrast agents discussed in this section, the sensitivity of this agent is substantially enhanced. Consequently, the in vivo experiment could be performed using a dose comparable to clinical MR contrast agents (0.05 mmol kg−1 of a 1:1 mixture of the Dy(III)/Tm(III) complexes).
Figure 71:
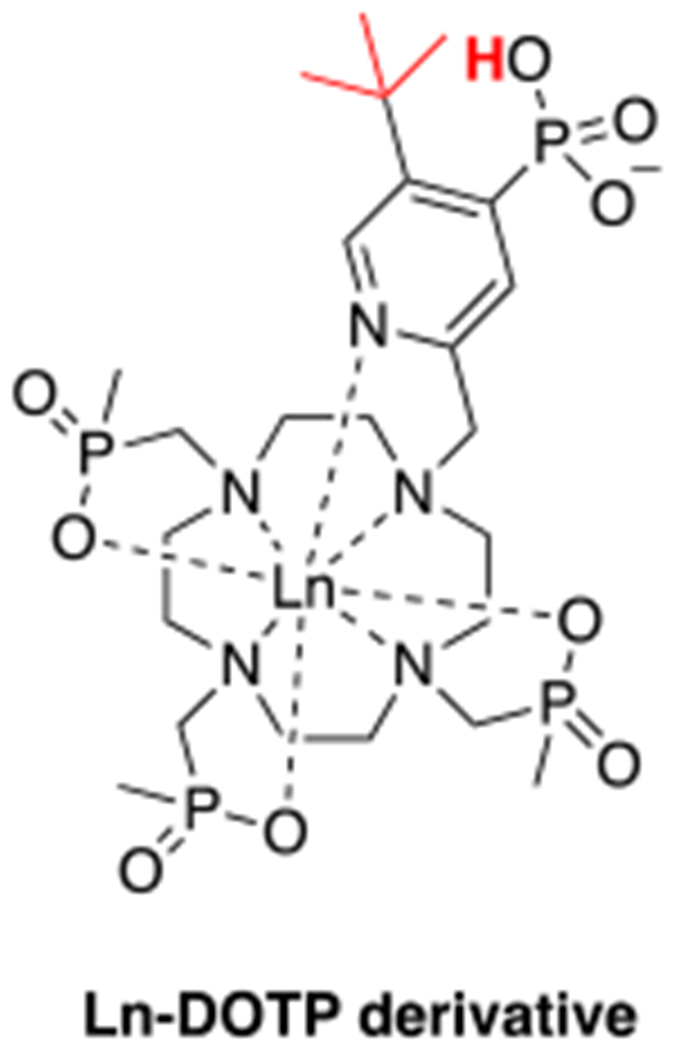
pH-responsive ParaSHIFT agent.613
6.3.4. pH-switchable contrast agents
Macromolecular and nanoparticle based agents have been reported that undergo a pH dependent transition resulting in signal change and/or accumulation. These agents cannot quantify pH but they may be able to detect regions of tissue with low pH. For example, Gd(III) containing linear614 and spherical615 copolymers have been developed, that undergo pH dependent conformational changes. At low pH, the polymers show a higher degree of cross-linking, i.e. the polymers shrink, which alters the rotational correlation time and hence, enhances r1.
pH sensitive polymeric micelles encapsulating Gd(III) complexes can be disassembled under acidic conditions.616 The release of the Gd(III) chelates into solution decreases the T1 relaxation time constant of the system. Similarly, acidification of a pH sensitive liposome provokes the release of Gd(III) chelates into the environment.617
pH responsive manganese oxide nanoparticles have also been reported.618–619 In this approach the poor stability of MnO under weak acidic conditions is exploited. The more acidic tumor microenvironment dissolves MnO, which in turn causes a relaxivity enhancement by releasing Mn(II) ions into the environment.
In another impressive example, Mn(II) ions were confined in pH sensitive calcium phosphate nanoparticles comprising a poly(ethylene glycol) shell.620 Upon activation at lower pH in the solid tumor microenvironment, calcium phosphate disintegrates and Mn(II)-ions are released into the environment. In a C26 tumor-bearing BALB/C nude mice model, it was shown that the released Mn(II)-ions bind to surrounding proteins, which rapidly brightens solid tumors and also detects micrometastases in the liver.
Recently, it was shown that SPIOs can be released from hydrogels621 or polymeric micelles622 under acidic condition, which changes their superparamagnetism and therefore their T2*-relaxivity.
6.4. Enzyme-responsive molecular imaging agents
The central role of enzymes is to act as biological catalysts, thereby being responsible for most of the functions in cellular and molecular biology. In many disease processes specific enzymes are upregulated or become expressed on cell surfaces or in the extracellular space. Noninvasive sensing of enzymatic activity is, in principle, a powerful tool to detect disease activity and to monitor the effects of therapeutic interventions. As outlined above, the design of a contrast agent that is responsive to enzymatic activity benefits from the fact that: 1) the catalytic rate of an enzyme can be high enough to make it feasible to modulate the contrast generating properties of an activatable agent present at micro- to milliomolar concentrations, and 2) the high specificity of enzymatic reactions so that a change in MR signal can be attributed to a specific molecular event.
6.4.1. Enzyme-responsive T1/T2*-based contrast agents
Modulation of the hydration state
An established strategy to modulate MR response to enzymatic activity is to utilize a contrast agent that undergoes a change in hydration state upon activation.
Meade and coworkers used a Gd(III) chelate to detect oncologically significant β-glucuronidase.623 Their agent consists of a Gd-DO3A moiety bearing a pendant β-glucuronic acid moiety connected by a self-immolative linker to the macrocycle (Figure 72A). The glucuronic acid moiety acts as a mask that can be enzymatically cleaved, which in turn triggers the “self-destruction” of the linkage into two byproducts, as illustrated in Figure 72A, resulting in the release of a Gd-DO3A derivative with an altered hydration state. As a result, the relaxivity in human blood serum decreases by 27 % upon enzyme activation. Interestingly, performing the same experiment in a buffer mimicking in vivo anion concentrations leads to an increase in T1 relaxivity by 17 %, which nicely illustrates the influence of buffer compositions upon the efficacy of an MRI contrast agent.
Figure 72:
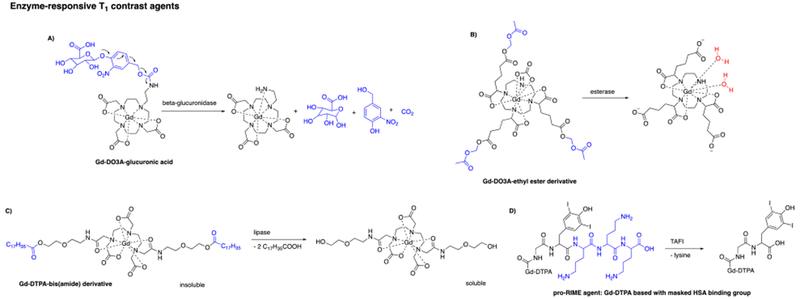
Selected enzyme-responsive T1 MRI contrast agents (enzyme cleavable entities are shown in blue).623–625, 630
Lowe and coworkers exploited the known tendency of neutral Gd-DO3A complexes to avidly bind bicarbonate anions in vivo and form ternary q = 0 complexes.624 They synthesized a neutral Gd-DO3A derivative with three pendant ethyl ester groups (Figure 72B). This complex forms a stable, q = 0 complex with bicarbonate but in the presence of an esterase the ethyl esters are hydrolyzed to liberate three pendant carboxylates. The resultant anionic complex repels the coordinating anions, which in turn allows water molecules to bind to the paramagnetic center. The change in hydration state from q = 0 to q = 2 leads to an increase in T1 relaxivity by 84 % (5.7 to 10.5 mM−1s−1).
Modulation by change in solubility
Insoluble T1 agents have low relaxivity because of poor access of exchangeable water to the complex. Hoehn and coworkers invented an insoluble Gd-DTPA-bis(amide) based, inactive contrast agent (Figure 72C) that can be internalized into dendritic cells.625 Lipase triggered cleavage of the stearic acid ester groups renders the contrast agent soluble with access to bulk water. As a result an increase in MR signal is observed.
In a similar approach, but in the opposite sense, Lepage and coworkers designed a Gd-DOTA based agent linked to a PEG-peptide sequence that exhibits excellent water solubility through the PEG linkage.626–628 A solubility switch is catalyzed through MMP enzymes, which cleave the PEG peptide, thereby making the chelate insoluble in water. As a consequence, bulk water access to the chelate is reduced resulting in a decrease in MR signal. This strategy was applied in MMP-2-rich tumor bearing mice and they showed that the contrast agent can additionally be accumulated in tumor cells overexpressing the targeted enzyme.
Figureueiredo et al. encapsulated Gd-HPDO3A into anionic liposomes. In the presence of the cationic protein protamine, these Gd(III)-containing liposomes form poorly soluble aggregates with a relaxivity of only 0.2 mM−1s−1. In the presence of the enzyme trypsin, the protamine is degraded releasing the liposomes and causing a 9-fold relaxivity increase to 1.8 mM−1s−1.629
Modulation of the rotational correlation time
Another strategy in the design of enzyme responsive T1 contrast agents is to cause the rotational correlation time to change upon activation. The general phenomenon of having contrast agent relaxivity increase upon binding to a receptor was termed RIME (receptor-induced magnetization enhancement) mechanism. Nivorozhkin et al. synthesized a low relaxivity, pro-RIME agent (Figure 72D), which consists of Gd-DTPA and a HSA binding moiety that is masked by a HSA shielding group. Activation by a human carboxypeptidase B, thrombin-activatable fibrinolysis inhibitor (TAFI) releases the shielding group resulting in HSA binding, thereby increasing the relaxivity. Activation in the presence of HSA by micromolar enzyme concentration led to an increase in r1 of over 150 % at 37 °C.630
In a similar approach, Wang and coworkers developed the enzymatic contrast agent Gd-DOTA-FPG, which is based on the Gd-DOTA scaffold equipped with a pendant galactopyranose moiety. This agent can be activated with β-galactosidase. In the presence of β-galactosidase and HSA, an increase in T1 relaxivity by 60 % was observed.631
Another Gd-DTPA based RIME agent that shows affinity to HSA after activation with the enzyme ß-galactosidase was invented by Nagano and coworkers. The authors report an r1 enhancement of 60 %.632
Recently, Wang and coworkers published a Gd(III)-based chelate with a peptide ligand that is cleaved by legumain, which causes the agent to bind to HSA, boosting the T1 relaxivity of the agent by 170 %.633
A different strategy to decelerate τR upon activation exploits the enzyme-catalyzed oligomerization of monomeric contrast agents. Bogdanov et al. synthesized Gd-D-DOTA, a Gd-DOTA derivative that contains a pendant hydroxyphenol moiety (Figure 73A).635 In the presence of peroxidase and hydrogen peroxide, the hydroxyphenol moiety serves as electron donor during enzymatic hydrogen peroxide reduction, which in turn generates hydroxyphenol radicals. The converted Gd(III)-monomers then undergo rapid condensation into paramagnetic dimers or oligomers. As a result, r1 increases by 200 % (3.75 to 11.50 mM−1s−1, 0.47 T).
Figure 73:
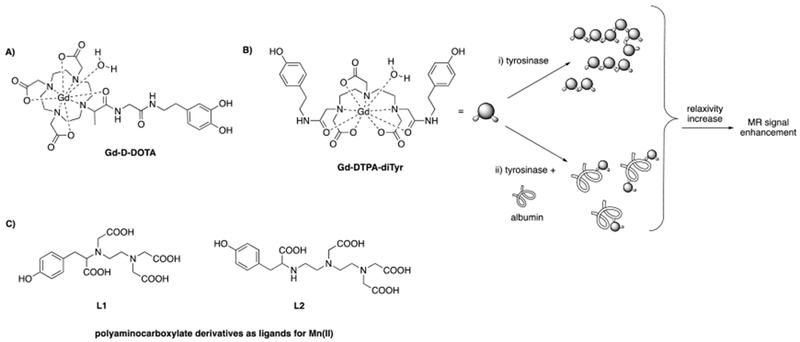
A) Chemical structure of D-DOTA(Gd); B) Interaction of Gd-DTPA-diTyr with activated tyrosinase can result in: a) oligomers and b) contrast agent-albumin conjugates. Both products yield an increase in relaxivity. Reproduced with permission from Ref.637 (URL: http://dx.doi.org/10.1002/cbic.200700157). Copyright 2007 John Wiley and Sons. C) Tyrosinase-responsive Mn(II) ligands.634
A second generation of this probe employs Gd-DTPA units bearing either two hydroxytryptamide or hydroxyphenetylamide moieties, respectively.636 The latter compound (Gd-DTPA-diTyr; Figure 73B) was used to sense tyrosinase activity.637 In the presence of activated tyrosinase, either oligomeric structures were formed (Figure 73B(i)) or in the presence of tyrosinase and HSA, also paramagnetic complex-protein conjugates can be formed (Figure 73B(ii)). Both products exhibit an increased rotational correlation time upon activation. Hence, both products yield an increase in relaxivity.
Botta and coworkers exploited tyrosine modified Mn(II)-based polyaminocarboxylates as enzyme-responsive MRI contrast agents (Figure 73C (L1 and L2)).634 In the presence of the enzyme tyrosinase, r1 increased by 50 % for L1 and by 350 % for L2, respectively. For Mn-L1, tyrosinase destabilizes the Mn(II) complex which leads to the release of Mn(II) ions and consequently, to an increase in MR signal. For Mn-L2, it is believed that the enzyme tyrosinase is catalyzing the formation of oligomeric species, which increases the rotational correlation time of the agent and hence r1.
The enzyme-catalyzed degradation of a polymer containing MRI contrast agent can be employed to observe changes in MRI contrast. Degradation of the polymer accelerates the rotational tumbling time of the MRI agent leading to a decrease in its T1 relaxivity upon enzyme activation. For example, hyaluronan-Gd-DTPA-beads were attached to aggarose-avidin beads.638–639 Degradation of the hyaluronan by the enzyme hyaluronidase to lower molecular weight fragments led to altered relaxation rates.
Daldrup-Link and coworkers took a different approach in the design of an enzyme-responsive Gd(III)-based MR contrast agent.640 They designed a caspase-3-sensitive nanoaggregation MRI probe (C-SNAM) that employs a Gd-DOTA moiety coupled to a DEVD peptide sequence through an amino luciferin-based linkage that contains a terminal disulfide entity (Figure 74 left). Enzymatic cleavage of the DEVD peptide and GSH mediated reduction of the disulfide entity triggers an intramolecular cyclization reaction, thereby yielding a rigid and hydrophobic product (Figure 74 left), that will subsequently self-assemble into Gd(III) containing nanoparticles. The prolonged rotational correlation time of the resulting nanoparticles amplifies the T1 relaxivity by 90 % (r1 = 10.2 mM−1s−1 to 19.0 mM−1s−1 (1 T). Caspase-3 is a common cell apoptosis biomarker.641 To investigate if this agent can be applied for in vivo monitoring of transplanted stem cell viability, the authors transplanted viable FLuc-eGFP-transduced rASCs into an osteochondral defect of the left distal femur and MMC-treated apoptotic FLuc-eGFP-transduced rASCs into an osteochondral defect of the right distal femur of athymic female Harlan rats. Intra-articular injection of C-SNAM provoked significant MR signal enhancement in apoptotic matrix associated stem cell implants (MASI) compared to viable MASI. The MR signal enhancement can be attributed to the increased relaxivity of the probe upon activation, but also to prolonged tissue retention in apoptotic MASI through its nanoaggregation properties (Figure 74 right).
Figure 74:
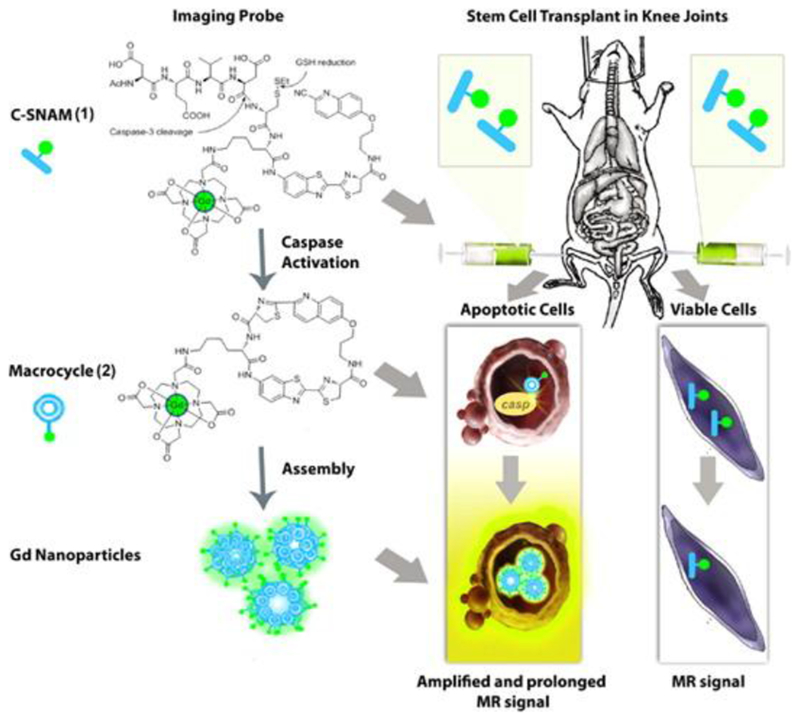
Design and mechanism of action of the caspase-3-sensitive nanoaggregation MRI probe (C-SNAM). (left) Chemical structure of C-SNAM (1). Caspase-3 catalyzed DEVD peptide cleavage and GSH mediated disulfide reduction triggers a biocompatible intramolecular cyclization reaction that yields the rigid and hydrophobic macrocycle (2). Subsequent self-assembly to Gd(III) containing nanoparticles increases r1 by 90 %. (right) Mechanism of action in vivo: (top) Intra-articular injection of C-SNAM into rat knee joints with implants of apoptotic and viable stem cells. (bottom) Caspase-3 mediated activation leads to an enhanced MRI signal through increased T1 relaxivity and retention in apoptotic stem cell transplants. Reproduced from Ref.640 (URL: https://doi.org/10.1021/nn504494c). Copyright 2015 American Chemical Society.
Based on the same approach, the Rao group employed caspase-3-activatable C-SNAMs to image chemotherapy-induced tumor apoptosis in mice642 and to accurately monitor the response of tumors to either metronomic chemotherapy or radiation therapy.643
Enzymes that catalyze the aggregation of SPIOs can increase the superparamagnetism of the nanoparticles and hence, their T2* relaxivity. For instance, the hydrophilic coating of SPIONs can be cleaved by MMP-2 or MMP-9 enzymes, which leads to aggregation of the nanoparticles.644–646 Aggregation can be also induced by polymerization of phenolic SPIONs through the enzyme peroxidase.647 In contrast, also the disaggregation of nanoparticles can be catalyzed by enzymes, which in turn reduces the superparamagnetism of the nanoparticles and therefore decrease their T2* relaxivity.648–650
6.4.2. Enzyme-responsive paramagnetic 19F-based contrast agents
Kikuchi and coworkers proposed a paramagnetic relaxation-based 19F MRI probe to detect enzyme activity. Here a Gd(III) complex is placed in proximity to 19F nuclei such that the T2 is shortened dramatically resulting in attenuation of the 19F MRI signal. If the Gd(III) complex is released from the vicinity of the 19F nuclei, it will result in an increase in the 19F T2 and hence enhance the 19F MRI signal. They coupled Gd-DOTA to trifluoromethoxybenzene via a DEVD peptide linker (Gd-DOTA-DEVD-Tfb) (Figure 75).651 The 19F signal was broadened into the baseline because of the presence of the nearby Gd(III) ion. In the presence of the enzyme caspase-3, the DEVD peptide sequence was cleaved releasing the 19F reporter from the agent and resulted in a measurable 19F MR signal. This probe was further modified to a dual-function agent capable of detecting caspase-3 activity via fluorescence measurements and 19F MRI.652
Figure 75:
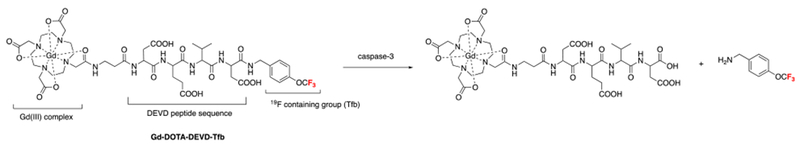
Chemical structure of Gd-DOTA-DEVD-Tfb.651
Tethering a 19F reporter molecule to Gd-DOTA via a different enzyme-cleavable linkage enabled the detection of β-lactamase653 and β-galactosidase654 activity, respectively.
6.4.3. Enzyme-responsive CEST contrast agents
Enzyme-responsive ParaCEST agents
ParaCEST agents can also be utilized for the detection of enzyme activity. The chemical exchange rate of the labile proton is changed by enzyme-catalyzed alteration of the covalent bond structure of the ParaCEST agent. As a result the CEST effect appears, disappears or changes in magnitude.
The first enzyme sensing ParaCEST agent was developed by Pagel and coworkers.655 A Tm-DOTA derivative was coupled to a DEVD peptide sequence (Figure 76A1), which can be selectively hydrolyzed by caspase-3, thereby converting the DOTA-amide into a DOTA-amine. Under physiological conditions, the disappearance of the CEST effect was observed upon caspase-3 activation. A limitation of a “turn-off” effect is in quantifying the absence of signal. In a second study the same group successfully exploited the simultaneous application of the enzyme-responsive DEVD-Tm-DOTA agent (Figure 76A1) and an unresponsive ParaCEST agent, Yb-DOTAM-Gly (Figure 68A), to quantify the enzymatic transformation in a ratiometric approach.656 In this strategy, the unresponsive probe is “always on” and accounts for all factors that could alter the CEST effect except for the enzyme to be measured. This comparative measurement to improve the detection of enzyme activity is referred to as “catalyCEST MRI”.
Figure 76:
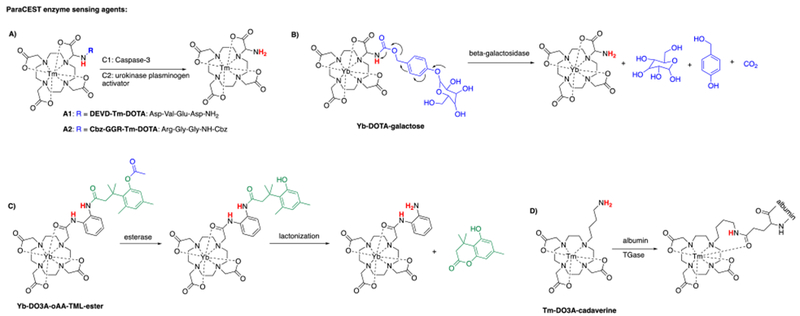
Selected enzyme-responsive ParaCEST MRI contrast agents (enzyme cleavable entities are shown in blue).655, 657, 659–660, 662
The Pagel group extended this method for the in vivo detection of urokinase plasminogen activator (uPa) in a Capan-2 pancreatic tumor mice model where Cbz-GGR-Tm-DOTA (Figure 76A2) served as a uPa responsive probe and Eu-DOTAM-Gly as control probe.657 This proof-of-concept study represents the first report of in vivo catalyCEST MRI. Replacement of the peptide sequence of a Tm-DOTA derivative enabled the detection of cathepsin-D activity.658
Additionally, modularly designed Yb-DOTA derivatives have been used as enzyme sensing ParaCEST agents. For instance the Yb-DOTA complex in Figure 76B is coupled to a β-galactose ligand via a self-immolative linker. Selective cleavage of the sugar by β-galactosidase results in elimination of the linker group and the amide bond is hydrolyzed to an amine thereby generating a CEST effect.659
Yb-DO3A-oAA-TML-ester (Figure 76C) is capable of sensing esterases, which cleave the ester of the chelator (shown in blue, Figure 76C).660 The tri-methyl lock moiety can then undergo lactonization (shown in green, Figure 76C) also resulting in the conversion from an amide into an amine in the Yb-DO3A-oAA contrast agent. A similar probe Yb-DO3A-oAA-TML-Q is activated by DT-diaphorase.661
It was also shown that for enzyme activated ParaCEST agents, the CEST effect can be generated through the opposite reaction, i.e. formation of an amide bond from an amine group. Conjugation of Tm-DO3A-cadaverine (Figure 76D) to albumin by transglutaminase produced a “turn-on” CEST effect.662 Concurrently, a reduced CEST signal from albumin could be observed.
Enzyme-responsive DiaCEST agents
DiaCEST agents have also been developed to detect enzyme activity. Gilad and coworkers reported that the enzyme cytosine deaminase (CDase) catalyzed deamination of cytosine to uracil or F5C to 5FU, respectively (Figure 77A).663 As a result, the previously observed CEST effect is “switched off”.
Figure 77:

Selected enzyme-responsive DiaCEST MRI contrast agents (enzyme cleavable entities are shown in blue).663, 665–669
Another example is the DiaCEST peptide (LRRASLG)8 which shows high CEST contrast due to its basic, positively charged lysine and arginine residues. Phosphorylation of this peptide through the enzyme protein kinase A creates a strong negatively charged group within the molecule, which slows down the water exchange between the exchangeable protons and the bulk water protons. Consequently, the authors observed a reduced CEST effect in in vitro experiments.664
Recently, (Phe-Arg)-4-amino-2-hydroxybenzoic acid (Figure 77B) was proposed as a catalyCEST MRI agent that contains an enzyme-responsive entity and also a non-responsive reference. After cleavage of the dipeptidyl ligand through the enzyme cathepsin B (shown in blue, Figure 77B), the aryl-amide proton is converted into an aryl-amine proton, i.e. the CEST signal observed for the aryl amide proton disappears. The salicylic acid moiety is largely unresponsive to cathepsin B activity and acts as non-responsive reference.665
The modular design of salicylic acid derivatives (Figure 77C) facilitated several “turn-on” catalyCEST MRI agents capable of sensing sulfatase666, alkaline phosphatase667, and the presence of the two enzymes esterase and sulfatase at the same time.668 Additionally, a glutamyl derivative of salicylic acid (Figure 77D) was successfully employed in mouse models of human ovarian cancers to selectively detect γ-glutamyl transferase.669
6.5. Redox potential-responsive molecular imaging agents
The intra- and extracellular redox environment is tightly regulated in order to maintain normal physiological processes. In disease states the redox environment can become disrupted, so that altered redox is generally associated with wide range of pathophysiological conditions. The redox environment is regulated by metabolites, including NAD/NADH, peroxides, thiol/disulfides (e.g. glutathione), nitric oxide, and oxidase enzymes. Activatable MR contrast agents can be designed to be altered by redox active metabolites. The design of redox sensitive probes can involve a change in oxidation state of the ligand or the metal center to provide the desired change in MR contrast with altered redox environment.
6.5.1. Redox potential-responsive T1 contrast agents
One strategy to sense the redox changes is the modulation of the hydration state of the contrast agent upon activation. Louie and coworkers employed this strategy by introducing a merocyanine moiety into a Gd-DO3A complex in a way that the oxygen donor of merocyanine could coordinate to the Gd(III) center (Figure 78A).670–671 After treatment with NADH, the merocyanine group was converted into its spirooxazine isomer, incapable of coordinating the metal center and hence, leaving space for a water molecule to coordinate to the Gd(III) ion. As a result the hydration state of the complex changed from q = 1 to q = 2, thereby increasing r1 by 55 %.
Figure 78:
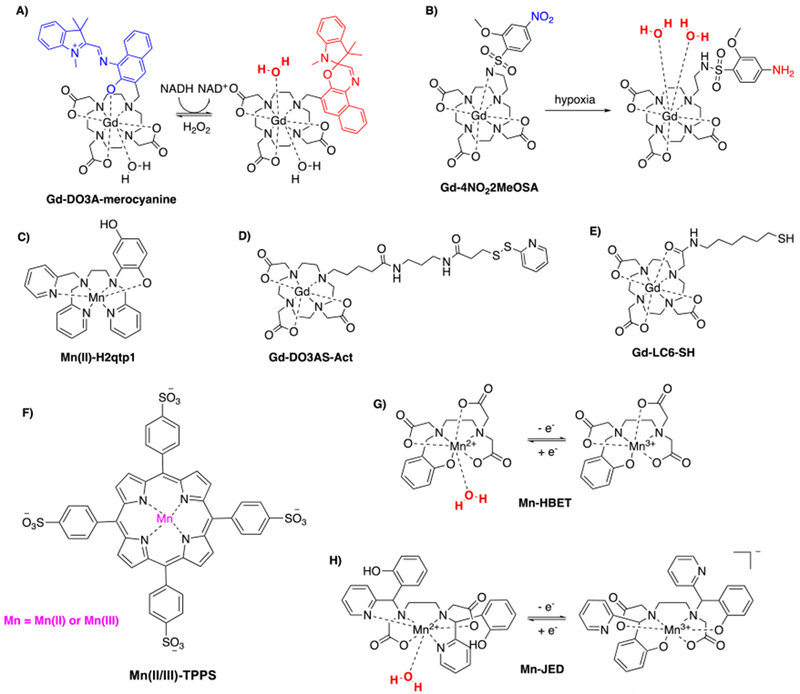
Selected redox potential-responsive T1 contrast agents.382, 657, 670–673, 675–679, 686, 689
Iwaki et al. showed that Gd-4NO22MeOSA (Figure 78B) could undergo reduction to the corresponding aminobenzenesulfonamide in the presence of rat liver microsomes under hypoxic conditions, but not under normoxic conditions.672 The agent consists of a Gd-DO3A derivative with a pendant nitrobenzenesulfonamide where the sulfonamide nitrogen is deprotonated and coordinated to the Gd(III) center at neutral pH. Reduction to the aminobenzenesulfonamide raises the pka value of the sulfonamide nitrogen resulting in protonation and dissociation from the Gd(III) ion. As a result, the hydration state of the complex switches from q = 0 to q = 2 and r1 increases by 70 %.
Goldsmith and coworkers developed Mn(II)-H2qtp1 (Figure 78C), a Mn(II) based complex with a redox-active ligand.673–675 The hexadentate ligand contains a dihydroxybenzyl group that can be oxidized to the weaker coordinating benzoquinone using hydrogen peroxide. As a consequence, r1 increases from 4.73 to 5.3 mM−1s−1, presumably because of greater aquation of the Mn(II) ion. By adding a second dihydroxybenzyl group into the ligand scaffold, r1 could be further increased from 5.46 to 7.17 mM−1s−1.
Another example for a redox potential-responsive agent is Gd-DO3AS-Act (Figure 78D).676 The Gd-DO3A based agent bears a 2-pyridyldithio functionality readily available for disulfide exchange reactions. An exchange reaction with glutathione introduces an additional pendant carboxylic acid moiety within the molecular scaffold, which can coordinate the Gd(III) center. This in turn leads to the displacement of the inner-sphere water molecule and the hydration state of the complex switches from q = 1 to q = 0, thereby decreasing r1 by 50 %.
Another thiol-bearing Gd-DOTA-monoamide derivative (Gd-LC6-SH) (Figure 78E) was attached to HSA via a disulfide bridge.677–679 When bound to albumin the T1 relaxivity of the gadolinium(III) complex is higher than in the unbound form due to the slower tumbling rate of the Gd(III)-HSA adduct. In a reducing environment, the gadolinium(III) complex is cleaved from HSA by reduction of the disulfide bond, which decreases r1 by 55 %. In a proof-of principle study, Gd-LC6-SH was applied as a MR reporter of tumor redox status in in vivo experiments with mice bearing Mia-PaCa-2 or NCI-N87 tumor xenografts.680
Conjugation of thiol-bearing Gd-DO3A derivatives to the surface of liposomes via disulfide linkage led to paramagnetic, supramolecular adducts with slow rotational correlation times. The disulfide linkage makes them sensitive to reducing environments, which was shown in in vitro experiments. Free thiol mediated cleavage of the Gd(III) complexes from the surface of the liposomes accelerated τR and decreased the r1 from 13.6 to 6.5 mM−1s−1.681
Aime and coworkers first proposed the utilization of the Mn(II)/Mn(III) couple as an activation mechanism for the development of redox sensitive T1 contrast agents.382 They used the well known Mn(III)-TPPS (Figure 78F) complex which had been used as a contrast agent for tumor imaging.682–685 They also prepared the Mn(II) complex by reducing with dithionite. The relaxation mechanism is different for the two complexes at 0.47 T: the dominant correlation time for the Mn(III) complex is the electronic relaxation time, while for Mn(II) it is the rotational correlation time. At 0.47 T, Mn(III)-TPPS has a higher relaxivity than Mn(II)-TPPS. However when poly-β-cyclodextrin is added, host-guest complexes form resulting in a longer rotational correlation time. This has a dramatic effect on the relaxivity of Mn(II)-TPPS (r1 = 40.8 mM−1s−1) but much less so with Mn(III)-TPPS (15.2 mM−1s−1). In the presence of 40 torr pO2 (pO2 of venous blood vessels is approx. 40 torr) the Mn(II)-TPPS:CD adduct was completely oxidized.
Loving et al. took a different approach to the Mn(II)/Mn(III) couple.686 They showed that the HBET ligand could stabilize both oxidation states, but the Mn(II)-HBET complex (Figure 78G) is seven coordinate, q = 1, while Mn(III)-HBET is six coordinate q = 0. In addition to the change in hydration state, the difference in spin state will also result in a lower relaxivity for the Mn(III) form. At pH 7.4, 37 °C, 1.4 T, r1 was 2.8 mM−1s−1 in the divalent state and 1.1 mM−1s−1 in the trivalent state. Loving et al. further showed that this system could be reversibly oxidized with hydrogen peroxide and concomitant signal loss or reduced with glutathione and concomitant signal increase. This work was further extended by synthesizing a series of derivatives where the electronic substituent on the aromatic ring was varied from electron withdrawing to electron donating.371 They also examined the effect of changing from an ethylenediamine to a cyclohexamine diamine backbone on relaxivity, thermodynamic stability, pH-dependent complex speciation, hydration state, water exchange kinetics of the Mn(II) complexes, and pseudo-first order reduction kinetics. They found that they could improve the relaxivity turn-on to a factor of 7.5 (r1 = 0.5 mM−1s−1 for Mn(III)-CyHBET compared to 3.3 mM−1s−1 for Mn(II)-CyHBET).
Mn-HBET and Mn-CyHBET provided strong proof of concept for Mn complexes as redox-activated MRI contrast agents but the Mn(II) oxidation state is favored exclusively in blood plasma. Mn(II) and Mn(III) favor disparate and distinct coordination environments and developing a ligand capable of supporting complexes where both oxidation states can persist in blood plasma is challenging. Mn(II) is best stabilized by poly-amino/carboxylate/pyridyl ligands like EDTA or N,N’-bis(pyridyl)-ethylenediaminediacetic acid (BPED), whereas Mn(III) favors more electron releasing ligands phenolate containing ligands like HBED, Figure 43. For example, Mn(III)-EDTA will decompose via disproportionation to Mn(II) and Mn(IV),687 whereas the Mn(II) complex of HBED will spontaneously oxidize to Mn(III) upon O2 exposure.688 Mn-HBET was designed as an initial compromise between the Mn(II) and Mn(III) ligand preferences but the redox potential of Mn-HBET is >500 mV greater than cysteine/cysteine disulfide redox couple. Attempts to depress the Mn(II)/Mn(IIII) redox potential via incorporating electron releasing substituents onto the phenolato-O donor resulted in ligand-metal auto-redox upon oxidation to Mn(III). One solution was to develop a chelator that was capable of isomerizing between binding modes that favor Mn(III) (BPED) or Mn(II) (HBED) exclusively. The chelator JED (Janus HBED/BPED, Figure 78H) was demonstrated to support stable and high-relaxivity complexes of both Mn(III) and Mn(II) in blood plasma. Switching between the Mn(III) and Mn(II) oxidation states resulted in a 9-fold increase in blood plasma r1 at 1.4T, 37 °C. Importantly, a large Mn(II) vs. Mn(III) r1 differential was maintained up to 11.7T.689 Interconversion between the oxidation states can be triggered in the presence of peroxidase enzymes or addition of excess L-cysteine.
A novel procedure for the MR based assessment of hypoxia was reported by Aime and coworkers.690 In this innovative example, the combined utilization of Gd-DOTP- and Gd-HPDO3A-labeled red blood cells made it feasible to map tumor hypoxia in a transplanted breast tumor mouse model. Gd-DOTP-labeled red blood cells act as a vascular oxygenation-responsive agent. Gd-DOTP shows 5 times higher affinity for deoxy-hemoglobin in comparison to oxy-hemoglobin. As a result, Gd-DOTP-labeled red blood cells show far higher T1 relaxivity in hypoxic regions than in normoxic areas. Gd-HPDO3A-labeled red blood cells were used to furnish the local concentration of red blood cells. In the corresponding in vivo study, firstly Gd-HPDO3A-labeled red blood cells were administered and MRI vascular volume maps were acquired. Afterwards, Gd-DOTP-labeled red blood cells were injected into the same animal and T1-weighted images were acquired. The ratio between those two images gave a relative MRI deoxygenation map that reports on O2 content independently from vascular volume (Figure 79).
Figure 79:
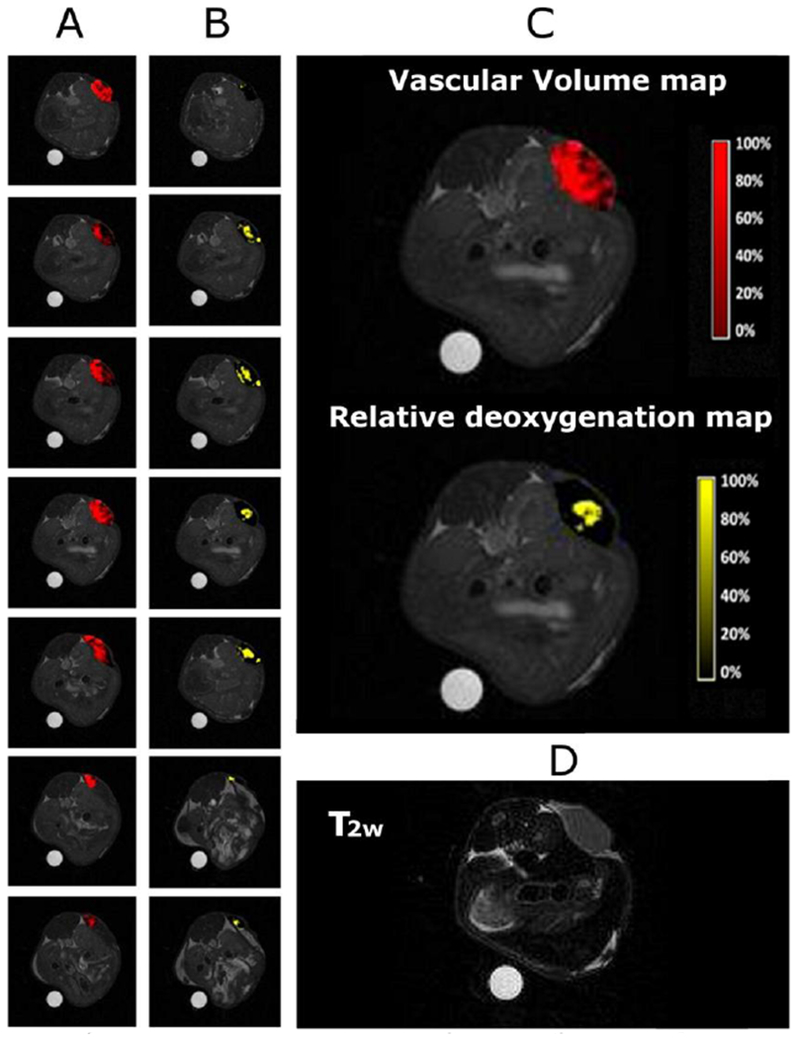
Vascular volume and relative deoxygenation maps of a representative tumor. (A) Vascular volume maps of 7 slices covering the tumor; (B) relative deoxygenation maps of 7 slices covering the tumor; (C) magnification of vascular volume (top) and relative deoxygenation (bottom) maps of the central slice of tumor; (D) T2w image of the central slice of tumor. Reproduced from Ref.690 (URL: https://doi.org/10.1021/acsnano.5b02604). Copyright 2015 American Chemical Society.
6.5.2. Redox potential-responsive ParaCEST contrast agents
Pagel and coworkers proposed Yb-DO3A-oAA (Figure 80) as a responsive ParaCEST agent capable of detecting NO in the presence of O2.691 This compound exhibits two CEST effects, caused by the exchangeable protons from the amine and the amide hydrogens, respectively. In the presence of NO and O2, the compound undergoes an irreversible covalent conversion to a dinuclear species bridged through a triazene (Figure 80A), thereby losing both CEST effects.
Figure 80:
Selected redox potential responsive ParaCEST agents.184, 201, 691–693
Ln-DOTA-tetraamide derivatives exhibit sufficiently slow water exchange kinetics that the shifted, coordinated water ligand can act as a CEST reporter. Water exchange kinetics can be tuned by altering the substituents on the amide groups either with respect to charge, lipophilicity, or electronic structure.451 Sherry and coworkers developed a series of Eu(III)-based ParaCEST agents where oxidation or reduction altered the water exchange rate and, in turn, the CEST signal. These authors reported an activatable agent with two pendant N-methylquinolinium moieties,692 designed to mimic the NADH/NAD+ couple. In its oxidized form (Figure 80B (blue)), the agent is nearly CEST MRI silent but was “turned-on” after irreversible reduction with NADH to the corresponding dihydroquinoline derivative (Figure 80B (red)). The larger CEST signal upon reduction is attributed to the slower water exchange rate of the reduced species.
Another agent utilized a pendant 9-anthryl group (Figure 80C (blue)) that acts as a specific reactive center for singlet oxygen (1O2), a highly unstable reactive oxygen species (ROS) that is associated with cancer and cardiovascular diseases.201 Oxidation of the 9-anthryl moiety with 1O2 irreversibly forms the stable endoperoxide derivative (Figure 80C (red)). Like in the case of Yb-DO3A-oAA (vide supra), the non-equilibrium probe design was chosen to form a sufficient amount of oxidized species to allow CEST detection considering the short-live and low concentration of 1O2. Binding of singlet oxygen successfully shifted the position of the metal-bound water CEST peak. Moreover, ratiometric CEST imaging in in vitro systems revealed the potential of this agent to sense 1O2 with high chemical specificity, rapid reaction kinetics as well as high kinetic and thermodynamic stability.
A different mechanistic approach by the Sherry group was to employ two redox-sensitive nitroxide free radicals, as amide substituents (Figure 80D). The paramagnetic nitroxide radicals serve to shorten the T1 relaxation time of bulk water protons, and this short T1 has the effect of nullifying the CEST effect because water relaxes too quickly upon saturation.184 Nitroxides can be rapidly oxidized or reduced to their diamagnetic derivatives, e.g. under hypoxic conditions reduction of nitroxide radicals has been demonstrated. Formation of the diamagnetic nitroxide (Figure 80D, (red)) through either a biological or chemical means would lengthen the T1 of bulk water protons and restore the CEST signal. Injection of the probe in healthy mice showed that the probe was still intact after excretion in the bladder, i.e. the nitroxide radicals were not reduced by biological processes. Subsequent administration of the reducing agent L-ascorbic acid activated the agent as evidenced by the strong CEST signal in the bladder.
LipoCEST agents contain a fast exchanging, paramagnetic Ln(III) shift reagent encapsulated inside a liposome. If the rate of water exchange across the liposomal membrane is slow enough, then there will be two water resonances observed in the NMR spectrum: the bulk water outside the liposome and the shifted water inside the liposome. Selective saturation of the intraliposomal resonance generates CEST with high sensitivity due to the large number of water molecules inside the liposome.293, 694 Terreno and coworkers developed a disulfide-based, redox sensitive lipoCEST agent.695 They attached a Gd-DO3A derivative to the outer surface of the liposome via a disulfide linker (Figure 81). The Gd(III)-modified liposome is CEST silent because of the T1-shortening effect of the Gd(III), but redox-mediated disulfide reduction results in release of the Gd(III) complexes and restoration of the lipoCEST signal (Figure 81). It is worth noting that the linkage between the Gd(III) complex and the lipoCEST agent is easily exchangeable, so that these agents could be made sensitive to a variety of other stimuli through sophisticated choice of the biodegradable linker.
Figure 81:
A) Schematic representation of the dual T1-CEST liposomal agent. The CEST contrast is activated after cleavage of the Gd(III)-complexes by a biological trigger, e.g. reducing environment. B) MR images of a phantom at 7 T and 37 °C of 1) LipoCEST agent 2) LipoCEST agent modified with free thiol groups on the surface 3) LipoCEST agent modified witch Gd-DO3A derivative via disulfide linkage (LipoCEST-SS-DO3A) 4) Treatment of LipoCEST-SS-DO3A with the reducing agent TCEP. Left: T1 weighted images; right: CEST map upon radiation at 3.5 ppm overlaid on a T2-weighted image of the phantom. Reproduced with permission from Ref.695 (URL: http://dx.doi.org/10.1039/C1CC10172B). Copyright 2011 Royal Society of Chemistry.
Allen and coworkers demonstrated that the Eu(II)/Eu(III) redox switch can be exploited for molecular imaging.405 The authors encapsulated a Eu(II)-cryptate (Eu(2.2.2)2+) in liposomes to form a dual mode contrast agent, which shows T1 enhancement in the reduced form and also exhibits a CEST effect. Oxidation of divalent Eu(2.2.2)2+ to Eu(2.2.2)3+ silenced the T1 enhancement but maintained the CEST effect with similar intensity. Interestingly, the CEST effect was independent from the europium concentration within the liposome, suggesting that the CEST effect is due to the liposome membrane itself rather than the europium compounds within. Detection of both the T1 enhancement and CEST effect implies that the agent has not responded to an oxidative trigger, but loss of T1 enhancement indicates irreversible activation by oxidation. The liposomes were kinetically stable for both oxidation states of europium.
Morrow and coworkers reported a triazamacrocyclic cobalt complex with exchangeable protons located on pendant pyrazole groups (Figure 80E).693 The paramagnetic Co(II) center causes a large shift of these exchangeable protons relative to the water resonance, whereas the in the oxidized form with diamagnetic Co(III), these resonances are much closer in frequency to bulk water. The system exhibits a reversible Co(II)/Co(III) redox couple that is tunable over the biologically relevant range of −80 to −280 mV (versus normal hydrogen electrode) by varying the ligand substituents.
6.6. Metal ion-responsive molecular imaging agents
Metal ions such as Ca(II), Cu(II), and Zn(II) play an important role in cell signaling. The ability to noninvasively image ion flux would enable studies to elucidate organ function and also to identify pathological states characterized by altered metal ion homeostasis. There are a number of challenges in developing metal ion-responsive contrast agents. In addition to the challenges of sensitivity and appropriate dynamic range that affect all responsive agents, for detecting metal ion flux one requires specificity for the ion of interest and, perhaps more importantly, sufficiently fast reversible binding kinetics to sense metal ion concentration change in real time. For T1 agents, the strategies of coupling metal binding to modulation of the rotational correlation time or to modulation of the hydration number have been employed. There have also been ion-responsive ParaCEST agents and SPIONs reported in recent years.
6.6.1. Zn(II)- responsive MR contrast agents
The first Zn(II) responsive contrast agents were published by Nagano and coworkers.696–697 They synthesized a series of Gd-DTPA-bisamide complexes sensitive to Zn(II) ions and their best candidate is depicted in Figure 82A. In the presence of Zn(II) ions, the authors posit that the chelator undergoes a geometrical reconfiguration to coordinate Zn(II), which in turn displaces the inner-sphere water molecule. As a consequence, r1 decreases from 4.8 to 3.4 mM−1s−1 after addition of 1 equivalent of Zn(II). The binding affinity for Zn(II) was not determined in this study, but notably, this sensor selectively responds to Zn(II) ions over Na(I), K(I), Ca(II) and Mg(II).
Figure 82:
Meade and coworkers reported a Gd-DO3A derivative with a pendant iminodiacetate group for zinc binding (Figure 82B).698–699 Coordination of Zn(II) changes the hydration number for Gd(III) from q = 0 to q = 1, i.e. the sensor “turns on” in the presence of zinc with a fairly high r1 change of 121 %. This sensor was selective for Zn(II) over Na(I), K(I), Ca(II) and Mg(II), but also showed some response for Cu(II). In general, it is believed that a simultaneous Cu(II) response does not interfere in in vivo experiments since the Cu(II) concentration is typically much lower in tissue than the Zn(II) concentration. The Kd for Zn(II) is 240 µM and in vitro MR images showed that zinc concentrations as low as 100 µM could be detected with this agent, which is within the physiologically relevant region. The Zn(II) concentration in vesicles of certain types of glutamatergic neuronal, prostate, and pancreatic cells can reach 300 µM.571 However, the detection of lower zinc levels in vivo is still desirable.
Esqueda et al. reported the Gd-DOTA-bisamide agent Gd-CP027 that binds up to two equivalents of Zn(II) using pendant N,N-bis-(2-pyridyl-methyl) ethylene diamine (BPEN) groups as amide substituents (Figure 83A).700 In the presence of Zn(II) in a buffer system, Gd-CP027 displays only a modest 20% increase in r1. However, in the presence of Zn(II) and HSA, a large 165 % r1 change is observed (r1 = 6.6 to 17.4 mM−1s−1). Coordination of Zn(II) to Gd-CP027 enables its binding to a subdomain of HSA, which increases the rotational correlation time of the agent and hence its relaxivity (Figure 83B). In the absence of Zn(II), Gd-CP027 shows no affinity for HSA. This sensor exhibits sensitivity for Zn(II) ions over Ca(II) and Mg(II), but does respond to Cu(II). The authors demonstrated in in vitro experiments, that in the presence of HSA, zinc concentrations as low as 30 µM could be detected and a remarkably high binding affinity for zinc (Kd = 33.6 nM) was reported. Ultimately, Gd-CP027 was applied in vivo. The authors were able to image the glucose stimulated Zn(II) secretion in the mouse pancreas or prostate gland.701–702 The zinc content in prostate cancer tissue is approximately sixfold decreased in comparison to healthy prostate tissue. Using Gd-CP027, it was feasible to distinguish between healthy and malignant mouse prostate by imaging the differential secretion of Zn(II). As illustrated in Figure 83C, treatment of healthy mice with glucose and Gd-CP027 led to clear contrast enhancement in the healthy mice prostate tissue (bottom). On the other hand, stimulation of prostate tumor-bearing mice with glucose revealed that different stages of tumor development could be discriminated from each other (middle & top) since the zinc concentration decreases with progressing disease.
Figure 83:
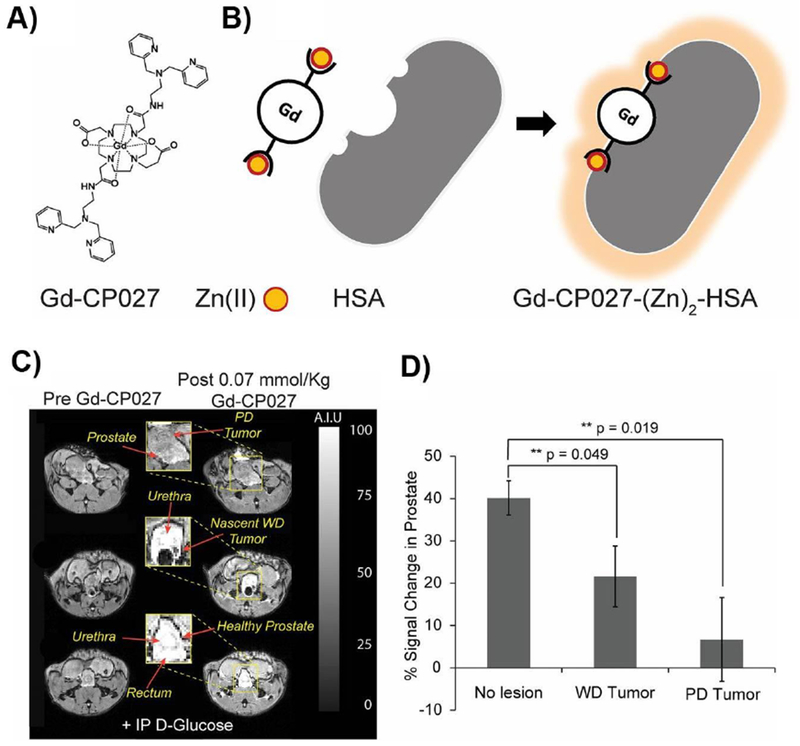
A) Structure of Gd-CP027; B) Gd-CP027 binds to Zn(II) and forms a complex with HSA; C) Glucose sensitive contrast enhanced (GSCE) T1 weighted MR images at 9.4 T of the prostate of mice during various stages of tumor development: (Bottom) image of a normal healthy prostate (Middle) image of malignant prostate with WD tumor shows clear hypointensity due to the presumable lack of intracellular Zn(II) (top) image of malignant prostate with PD shows no GSCE D) Average GSCE measured over the entire prostate of mice (WD tumor = well differentiated tumors, PD tumor = poorly differentiated tumor). Reproduced from Ref.702 (URL: http://www.pnas.org/content/113/37/E5464). Copyright 2016 National Academy of Science.
ParaCEST agents can also be used as Zn(II)-sensitive MR contrast agents. For instance, a Eu-DOTAM derivative equipped with two BPEN units for Zn(II) binding was reported by Sherry and coworkers (Figure 84A).703 In the absence of Zn(II) ions, the agent shows an intermediate to slow water exchange between the Eu-OH2 and bulk water molecules. Coordination of Zn(II) ions through the four pendant pyridine units leads to a mononuclear complex close to the europium center. Unexpectedly, the water exchange at the europium center accelerated resulting in disappearance of the CEST signal. The effect was even more dramatic at higher pH (pH 8) suggesting that there is a water ligand also coordinated to Zn(II) that can be partially deprotonated at pH 8 to give Zn-OH. The close proximity of the Zn-OH species to the Eu(III)-bound water molecule is believed to catalyze the prototropic exchange between the Eu(III)-bound water molecule and bulk water (Figure 84B). CEST imaging experiments revealed that the agent is selective for Zn(II) over Ca(II) and Mg(II) ions and the effective sensitivity range is estimated to be 5 to 120 nM.
Figure 84:
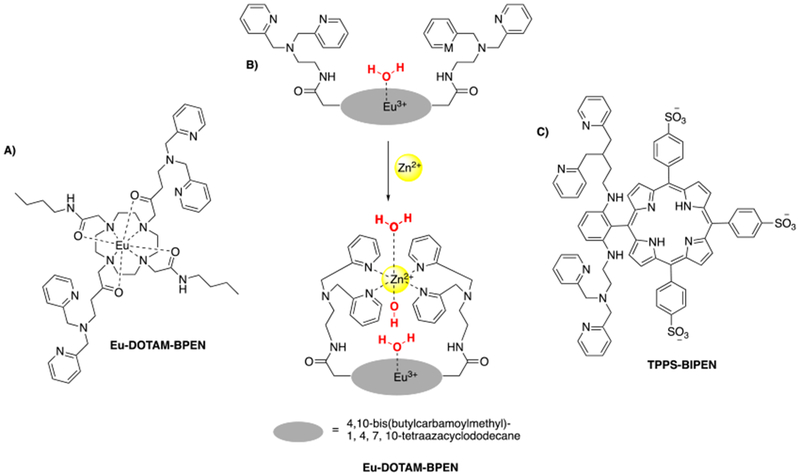
Water-soluble Mn(III)-porphyrins were also employed as MRI based zinc sensors (Figure 84C).704 Notably, the porphyrin TPPS-BIPEN, Figure 84C is a fluorescent sensor for Zn(II). When Mn(III) is coordinated by the porphyrin ligand and the complex binds Zn(II), the contrast agent is able to permeate cell membranes making it the first cell permeable MRI sensor for metal ions reported. Due to an unknown mechanism, the T1 relaxivity decreases from 8.70 to 6.65 mM−1s−1 (24 %) upon addition of 1 equivalent of Zn(II). Interestingly, this trend reverses when the Mn(III)-based agent is treated with Zn(II) ions in a cellular context. Incubation of HEK-293 cells with Mn(III)-porphyrin led to an increase in the T1 relaxivity in the presence of Zn(II) ions. Additionally, T2-weighted images of these cells showed also an increase in relaxivity associated with zinc addition. In a follow-up study, the authors reported Zn(II) sensitivity of their contrast agent when it was directly injected into the brain of rats.705
6.6.2. Ca(II)- responsive MR contrast agents
Meade and coworkers reported the first Ca(II) ion sensitive contrast agent Gd-DOPTA (Figure 85A).706–707 Gd-DOPTA consists of two Gd-DO3A units bridged by a modified 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (BAPTA) unit, which is known to have specificity for Ca(II) over other metals. In the absence of Ca(II), the anionic carboxylate oxygen donor atoms of the BAPTA unit bind to the gadolinium(III) centers yielding a q = 0 complex with r1 = 3.26 mM−1s−1. In the presence of Ca(II), the BAPTA carboxylate groups preferentially coordinate Ca(II), thereby allowing water molecules to coordinate the gadolinium(III) centers, which changes the hydration state per Gd(III) ion from q = 0 to q = 1. As a result r1 increases by 80 % to 5.76 mM−1s−1. Gd-DOPTA shows selectivity for Ca(II) over Mg(II) and a binding affinity for calcium of Kd = 0.96 µM. In the human body, the intracellular Ca(II) concentration is in the micromolar range while the extracellular Ca(II) concentration is in the millimolar range. Thus, Gd-DOPTA has a MRI response in the intracellular concentration range and a possible application of such an agent is to sense Ca(II) fluctuations in biological settings. This probe was further developed by masking the BAPTA units with ethyl esters to firstly increase cell labeling and prevent extracellular Ca(II) binding (Figure 85B).708 Once inside the cell, the ethyl ester groups can be cleaved by the enzyme esterase, thereby unmasking four carboxylates that coordinate to the Gd(III) centers and block access to coordinated water ligands. Subsequent intracellular Ca(II) binding results in an increase in hydration number and facilitates an increase in relaxivity by 66 %. This probe design allows for discrimination between extra- and intracellular Ca(II) ions.
Figure 85:
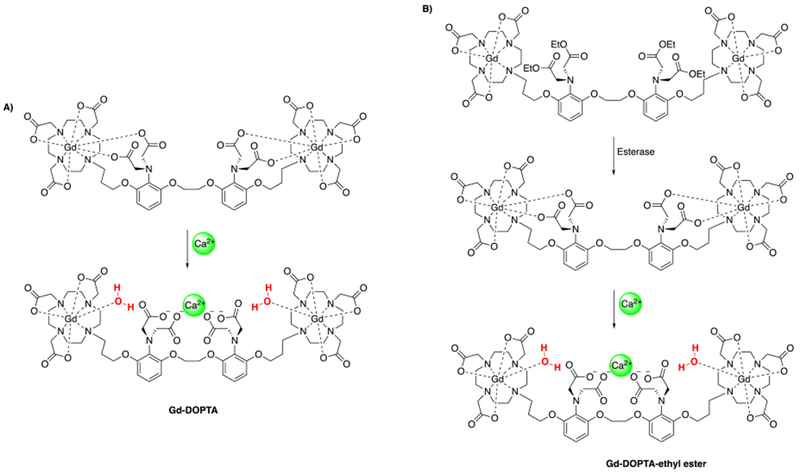
A) Ca(II)-responsive MRI contrast agent706–707 B) Ca(II)-responsive MRI contrast agent that can discriminate between extra- and intracellular Ca(II) ions.708
Gd-DOPTRA, a Gd(III)-based Ca(II) sensor with the highest “turn-on” response based on a switch in hydration state was reported by Dhingra et al. (Figure 86A).709 The agent consists of a Gd-DO3A unit equipped with a pendant calcium-responsive o-aminophenol-N,N,O-triacetate (APTRA) unit. In the absence of Ca(II)-ions, the APTRA unit can coordinate to the Gd(III) center yielding a q = 0 complex. In the presence of Ca(II), the APTRA unit preferably coordinates Ca(II) ions yielding a q = 1 complex resulting in a doubling of r1 (3.5 to 6.9 mM−1s−1). Gd-DOPTRA shows selectivity for Ca(II) over Mg(II) and Zn(II) and exhibits a binding affinity for calcium in the micromolar range (Kd = 11 µM). However in biological media at 37 °C, r1 only increased by 25 % in the presence of Ca(II). Presumably, this attenuated response is due to anion binding to the Gd(III) center in the presence of Ca(II) that also blocks water access and dampens r1.
Figure 86:
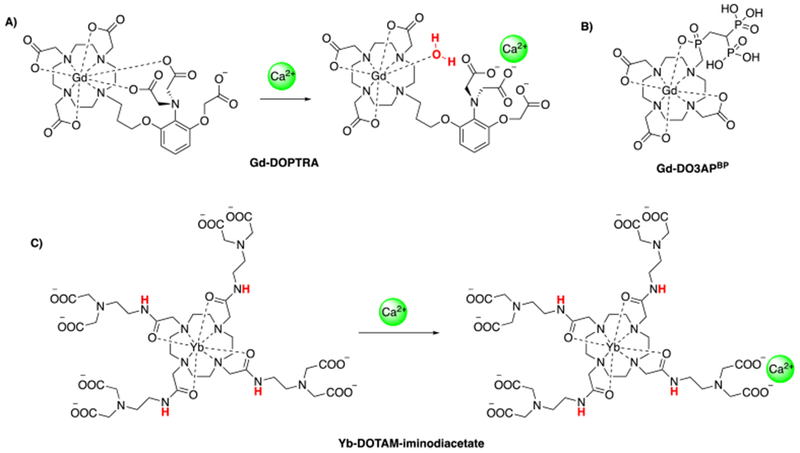
Selected Ca(II) binding MRI contrast agents.706–707, 709, 711
Kubíček et al. described Gd-DO3APBP which is a Gd-DO3A derivative with a pendant biphosphate ligand as a Ca(II) sensor (Figure 86B).710 Ca(II) binding to the bisphosphonate creates a coordination oligomer that has a slower tumbling time than the corresponding monomer and hence, a higher T1 relaxivity. However, Gd-DO3APBP is not selective for Ca(II) over Mg(II) or Zn(II). In the presence of ≥3 equivalents of either of these ions, r1 increases by 200 to 500 %.
An exemplary ParaCEST agent able to sense Ca(II) is based on a Yb-DOTA-tetraamide chelator with four iminodiacetate arms (Figure 86C).711 In the absence of Ca(II), a CEST effect is observed for this compound originating from the slow exchange of the amide protons as well as the Yb-OH2 water ligand. Upon coordination of Ca(II) ions via the pendant iminodiacetate groups, the exchange of the amide protons is slowed down resulting in a marked reduction in the CEST effect. A similar effect is observed in the presence of Mg(II).
Another strategy for sensing Ca(II) ions was proposed by Jasanoff and coworkers.712–713 These authors developed calmodulin modified SPIONs for calcium detection using changes in T2 relaxation. When the calmodulin groups bind Ca(II) this results in an aggregation of the nanoparticles. Particle aggregation is a well known phenomenon that results in increased r2. Their best candidate exhibits a r2 change from 200 to 34 mM−1s−1 which is in a convenient range for intracellular calcium detection (EC50 = 1.4 µM). However, the cell membrane impermeability of this agent greatly limits its in vivo application.
Angelovski and coworkers designed a system that combined a change in hydration number with a change in rotational correlation time to amplify Ca(II) sensing.714 They incorporated an amphiphilic calcium sensor into a liposome bilayer. The sensor consists of a Gd-DO3A chelate coupled to a Ca(II) chelator, which is in turn coupled to a lipophilic alkyl chain (Figure 87). The paramagnetic liposome system exhibits r1 = 7.3 mM−1s−1 (15 °C, 20 MHz) in the absence of Ca(II) ions at physiological pH, but after saturation with Ca(II) ions, r1 increases by a remarkable 400 % (r1 = 38.1 mM−1s−1). This can be explained through additive effects that occur upon Ca(II) binding. Coordination of Ca(II) through the incorporated calcium chelator causes a conformational change within the amphiphilic ligand that on the one hand changes the hydration state of the Gd-DO3A unit from q = 0 to q = 1. At the same time, the fast intramolecular rotation is slowed down, which further enhances r1 (Figure 87). To the best of our knowledge, this has been the highest change in T1 relaxivity for a Ca(II)-responsive Gd(III)-based contrast agent at physiological pH reported so far.
Figure 87:
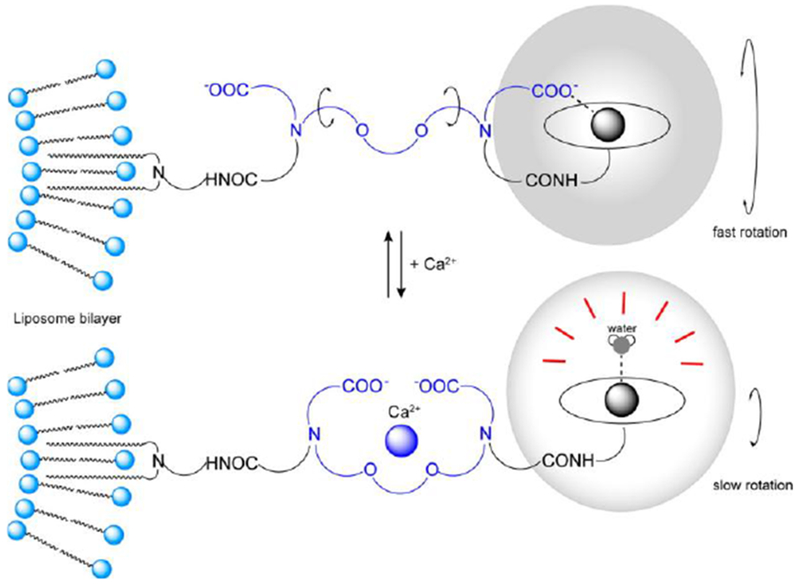
Schematic mechanism that causes the change in T1 relaxivity in the presence of Ca(II)-ions of a liposomal Ca(II)-responsive Gd(III)-based contrast agent. Reproduced from Ref.714 (URL: https://doi.org/10.1021/acs.biomac.5b01668). Copyright 2016 American Chemical Society.
6.6.3. Cu(I)/Cu(II)- responsive MR contrast agents
Chang and coworkers developed a variety of MRI-based copper(I) sensors.715–716 The authors utilized Gd-DO3A derivatives coupled to acetate or thioether-rich receptor ligands that are capable of coordinating copper ions. In this molecular framework, the Gd(III) center is shielded from coordinating water molecules in the absence of copper ions. Here a picolyl group coordinates Gd(III) in the absence of Cu(I), but is displaced by copper binding resulting in coordination of a water ligand to Gd(III), i.e. the hydration state of the complex changes from q = 0 to q = 1 or 2 (Figure 88A). For the thioether-tethered Gd-DO3A chelate, that is depicted in Figure 88B, the highest enhancement in r1 exploiting a hydration state mechanism has been observed after treatment with 1 equivalent of Cu(I). The T1 relaxivity increases by 360 % from 1.5 to 6.9 mM−1s−1. Additionally, this sensor shows impressive selectivity for Cu(I) over Na(I), K(I), Ca(II), Mg(II), Fe(II), Fe(III), Cu(II), and Zn(II) and remarkably high affinity for Cu(I) (Kd = 0.26 pM). However, the presence of coordinating anions can compete with the inner-sphere water molecule and affect the relaxivity response to Cu(I) binding. The authors overcame this problem by installation of additional carboxylate groups on the periphery of the molecular scaffold (Figure 88C), a known strategy to reduce the sensitivity to biologically abundant coordinating anions.717 By maintaining the selectivity and the sensitivity for Cu(I), the relaxivity response to Cu(I) ions was only slightly affected by this modification. In the absence of Cu(I) ions, the contrast agent exhibits a relatively low T1 relaxivity (2.6 mM−1s−1), whereas addition of Cu(I) triggers a 340 % enhancement in r1 to 11.4 mM−1s−1. As a second modification, the authors conjugated their Cu(I) sensor to an octaarginine peptide (Figure 88D) to enable cellular uptake of the contrast agent.718 The agent shows a Cu(I) induced r1 enhancement of 220 % (3.9 to 12.5 mM−1s−1). In vitro experiments demonstrated that this Cu(I) sensor can report on labile copper pools in a disease model where differences in copper accumulation between cells bearing a mutant copper transporter and wildtype cells could be detected.
Figure 88:
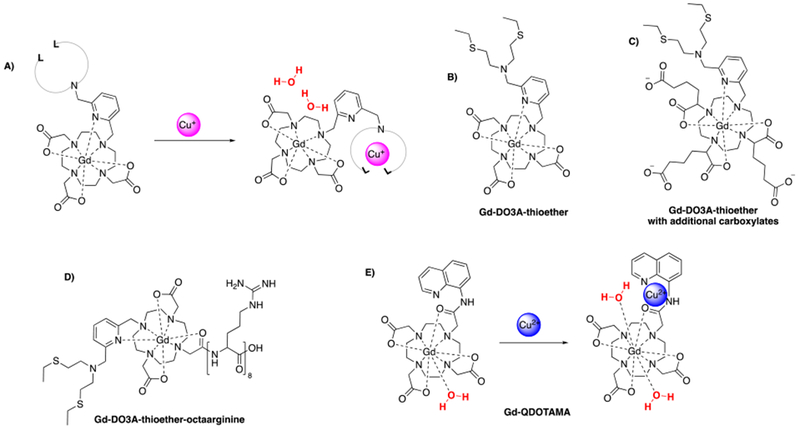
A) Schematic illustration of a copper binding Gd(III)-based probe that changes its hydration state in the presence of copper ions. B) – E) Selected Cu(I)/Cu(II) binding MRI contrast agents.716–719
Chen and coworkers developed Gd-QDOTAMA as a Cu(II) responsive agent that consists of a Gd-DO3A derivative with a pendant quinoline based ligand, chosen to selectively coordinate Cu(II) ions (Figure 88E).719 Cu(II) coordination provokes a switch in the hydration state of the agent from q = 1 to q = 2. Upon addition of 1 equiv. of Cu(II) ions, r1 increases from 4.27 to 7.29 mM−1s−1 (71 %). This agent shows high selectivity for Cu(II) over Na(I), K(I), Ca(II), Mg(II), Fe(II), Fe(III), and Zn(II). The authors estimate the Cu(II) dissociation constant to be Kd = 0.16 nM.
6.7. Other responsive molecular imaging agents
6.7.1. Neurotransmitter-responsive MRI contrast agents
Neuroscience, the study of the brain and nervous system, is inspired by the desire to understand the complexity of the brain and behavior. To facilitate its various functions, the brain contains billions of neurons which interact to form circuits and giving rise of a complex network. The major mode for communication between these nerve cells is mediated through chemical neurotransmission.720–721 Consequently, neuroimaging techniques can provide indispensable insights into the mechanisms of neural functions both in health and disease.722–724 Such studies may enhance the understanding of physiology and pathophysiology of the brain and as a consequence improve the diagnosis and treatment of brain disorders.
Recently, Jasanoff and co-workers developed the first neurotransmitter sensitive MRI contrast agents which are based on advanced molecular engineering techniques (directed evolution725) to provide paramagnetic metalloprotein-based molecular MRI probes for neuroimaging.726–727 In this approach, sophisticated protein engineering gave a paramagnetic heme iron-containing probe that is selective for the neurotransmitter dopamine. Specific binding of dopamine to a side that is close to the heme iron altered T1-weighted MRI signals. Using this neurotransmitter sensing technique, the authors were able to quantitatively map neurotransmitter release patterns deep within the living brain. The chief limitation of the technology as it now stands is its relative insensitivity - only neurotransmitter concentrations in excess of 2 µM could be detected.
A crown ether appended Gd(III)-complex capable of sensing amino acid neurotransmitters was reported by Toth, Angelovski, and coworkers (Figure 89).728 The complex offers ditopic binding for zwitterionic neurotransmitters via interactions (a) between the positively charged and coordinatively unsaturated Gd(III) ion and the carboxylate function and (b) between a pendant triazacrown ether and the amine function of the neurotransmitter. Relaxometric titrations with different neurotransmitters, like γ-aminobutyric acid or glutamate remarkably decreased the T1 relaxivity of their agent. The T1 relaxivity change can be attributed to a switch in hydration state from q = 1.2 to q = 0.4. This complex was successfully employed to monitor neural activity in acute mouse brain slices by MRI.
Figure 89:
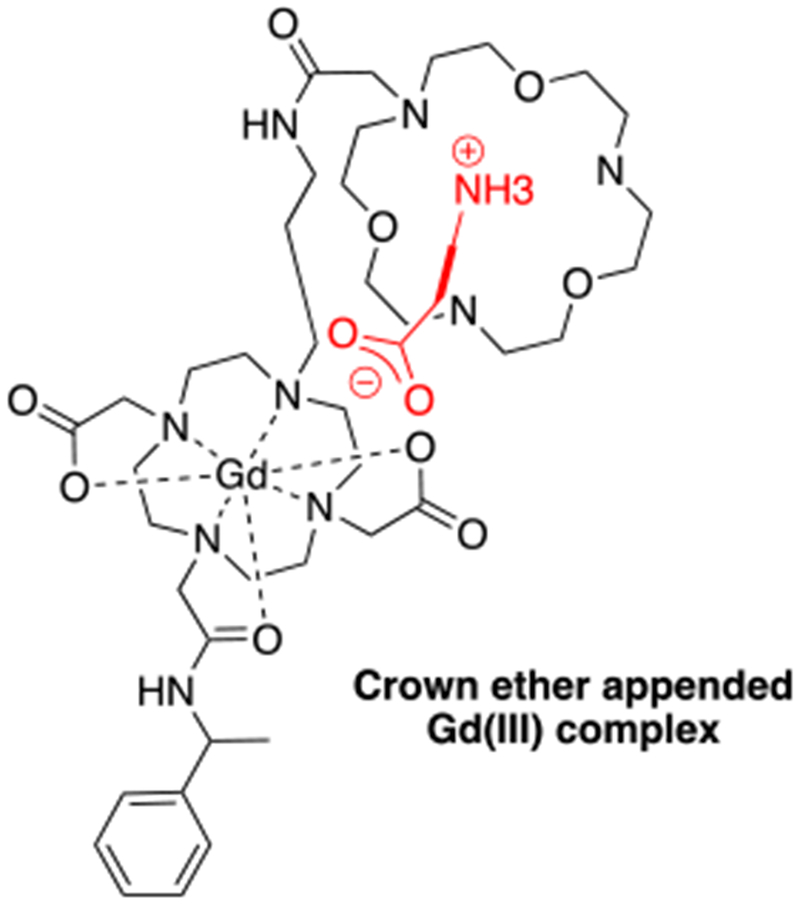
A crown ether appended Gd(III)-complex capable of sensing zwitterionic amino acid neurotransmitters. 728
6.7.2. Temperature-responsive MRI contrast agents
Recently, noninvasive temperature monitoring with MRI has attracted attention since thermotherapy is rapidly emerging. To this end, Zheng and coworkers developed a thermosensitive microgel based on a manganese porphyrin core incorporated in a cross-linked poly(N-isopropylacrylamide) material (Figure 90).729 Subtle temperature changes rapidly swell or shrink those microgels, i.e. the volume of the microgel is changed with its lower critical solution temperature (LCST, 29 – 33 °C). As the properties of the microgel change, the embedded paramagnetic complexes experience changes in their rotational motion, which in turn influences r1. Consequently, within a few degrees of temperature variation, an increase in r1 of 73 % could be observed.
Figure 90:
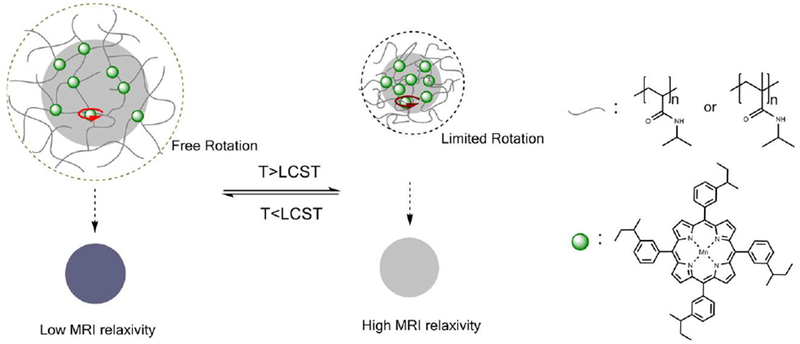
Schematic concept of thermosensitive microgels based on a manganese porphyrin core incorporated in a cross-linked poly(N-isopropylacrylamide) material. LCST: lower critical solution temperature 29 – 33 °C. Reproduced from Ref.729 (URL: https://doi.org/10.1021/acsmacrolett.5b00058). Copyright 2015 American Chemical Society.
Another concept utilizes thermosensitive liposomes that release a Gd-HPDO3A when heated up.730 As a result the water-solubility of the Gd(III)-agent is increased, which in turn decreases its T1 relaxivity.
Since chemical exchange rates are dependent on the temperature, ParaCEST agents have also been used to generate temperature-dependent CEST effects.605, 731–733
It was also demonstrated that Fe(II) or Co(II)-based ParaSHIFT agents might be promising candidates for MR thermometry applications.734–735
6.7.3. Light-responsive MRI contrast agents
The most prominent example for a light-responsive MRI contrast agent couple is merocyanine-Gd-DO3A / spirooxazine-Gd-DO3A, which is also sensitive to the redox environment and was discussed above (Figure 78A). Following the same mechanism as discussed above, r1 is decreased after irradiation with visible light at 563 nm and can be increased again by irradiation with UV light, respectively.670–671
This concept was further applied for the development of a light-responsive T2* contrast agent.736 Louie and coworkers coupled a spyropyran derivative to dextran sulfate coated iron nanoparticles. Light-induced conformational changes of spyropyran between its hydrophopic and hydrophilic isomer led to aggregation or dispersion of the nanoparticles, respectively (Figure 91). Visible light induced aggregation of the particles increased their superparamagnetism and hence, their T2* relaxivity.
Figure 91:
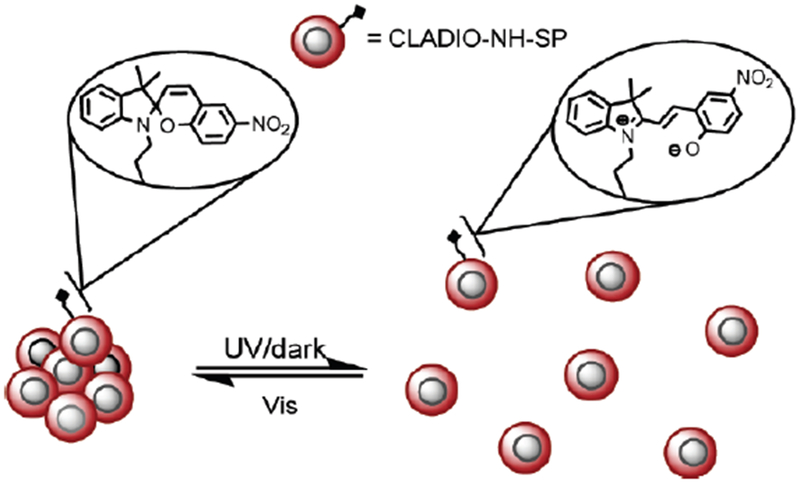
Proposed mechanism of light-induced reversible aggregation of iron oxide nanoparticles coupled to a spyropyran derivative. Reproduced from Ref.736 (URL: https://doi.org/10.1021/ja100254m). Copyright 2010 American Chemical Society.
6.7.4. DNA-responsive MRI contrast agents
Detecting specific nucleic acid sequences within the in vitro and in vivo context is extremely challenging using MRI. This is due to the extremely low concentrations of DNA, which is generally far below 1 µM. MRI contrast agents have been designed that are nonspecific to a DNA sequence to be able to track many types of gene delivery systems which can carry a high payload of DNA above the MRI detection threshold. It was shown, that the CEST effect of a polymeric Eu(III)-based ParaCEST agent could be influenced by interaction of the compound with DNA at 1 mM monomer concentration.737
In a different approach, magnetite spheres with DNA intercalators were bound to DNA duplexes (0.5 mM base pair concentration) for nucleic acid detection with a T2* MRI contrast agent.738 However, the extreme aggregation of the iron oxide nanoparticles reduced the water accessibility and therefore the superparamagnetism of the system, so that the expected decrease of T2* was reversed in this example.
Summaries of the T1- and CEST activatable probes are listed in Tables 11 and 12, respectively.
Table 11:
Summary of T1-based activatable MRI contrast agents.
| Responsive MRI probe | Stimulus | Mechanistic change | r1 switch (mM−1s−1) | % change | Ref. |
|---|---|---|---|---|---|
| Gd-DOTA-4AmP | pH | Second sphere effect, τm | 3.5 to 5.3 (pH 9.5 to 6) at 20 MHz | 51% increase | 574–575 |
| G5-PAMAM dendrimer | pH | Second sphere effect, τm | 10.8 to 24.0 (pH 9.5 to 6) at 20 MHz | 122% increase | 576 |
| Gd-DOTA-tetramide-hydroxypyridyl | pH | Second sphere effect | ~5.6 to ~3.1 (pH 8.5 to 6) at 20 MHz | 85% decrease | 577 |
| Gd-DO3A-sulfonamide | pH | q | ~5.4 to 8.0 (pH 7.4 to 6.8) at 65.6 MHz | 48% increase | 308 |
| Gd-DO3A-p-nitrophenolic arm | pH | q | 4.1 to 7.0 (pH 9 to 5) at 20 MHz | 71% increase | 580 |
| Gd-aminoethyl-DO3A derivatives | pH | q | (pH 9 to 5) at 20 MHz | average: ~100% increase | 581–582 |
| Gd-DO3A-sulfonamide-Ga-AAZTA | pH | q | 3.7 to 10.1 (pH 8.5 to 5) at 20 MHz | 150% increase | 589 |
| Gd-DO3A-glucuronic acid | β-glucuronidase | q | 4.99 to 3.63 (human serum) at 60 MHz | 27% decrease | 623 |
| Gd-DO3A-ethyl ester | esterase | q | 5.7 to 10.5 at 20 MHz | 84% increase | 624 |
| Gd-DTPA fatty acid complex | lipase | solubilty switch | inactive form: insoluble active form: 4.7 at 20 MHz | - | 625 |
| Gd-DOTA-PEG-peptide | MMP | solubility switch | - | - | 626–628 |
| Gd-loaded, protamine-linked liposomes | trypsin | solubilty switch | 0.2 to 1.8 | 800 % increase | 629 |
| Gd-DTPA with a lysine-masked HSA binding group | TAFI | τR | 9.8 to 26.5 (37 °C) at 20 MHz | 150% increase | 630 |
| Gd-DTPA-FPG | β-galactosidase | τR | 7.6 to 15.5 (37 °C) at 20 MHz | 103% increase | 631 |
| Gd-DTPA with masked HSA binding group | β-galactosidase | τR | 3.5 to 5.5 (37 °C) at 20 MHz | 57% increase | 632 |
| Gd-DTPA-derivative linked to a peptide | legumain | τR | 27.1 to 73.5 (in cell lysate) at 20 MHz | 170 % increase | 633 |
| Gd-D-DOTA | peroxidase | τR | 3.75 to 11.5 at 20 MHz | 200 % increase | 635 |
| C-SNAM | caspase-3 | τR | 10.2 to 19.0 at 42.6 MHz | 90 % increase | 640 |
| Mn-polyaminocarboxylates | tyrosinase | L1: release of Mn(II) L3: τR | - | - | 634 |
| Gd-DO3A-merocyanine | NADH | q | 5.6 to 8.6 at 60 MHz | 55 % increase | 670 |
| Gd-4NO22MeOSA | hypoxia | q | 2.1 to 3.6 at 20 MHz | 70 % increase | 672 |
| Mn(II)-H2qtp1 | hydrogen peroxide | q | 4.73 to 5.3 at 128 MHz | 10 % increase | 657 |
| Mn(II)-H4qtp2 | hydrogen peroxide | q | 5.46 to 7.17 at 128 MHz | 30 % increase | 675 |
| Gd-DO3AS-Act | free thiols | q | 8.1 to 4.1 at 20 MHz | 50 % decrease | 676 |
| Gd-LC6-SH | free thiols | τR | 5.3 to 2.33 at 200 MHz | 55 % decrease | 677–679 |
| Gd-SS-liposomes | free thiols/free radicals | τR | 13.6 to 6.5 s−1 mM−1 at 20 MHz | 50 % decrease | 681 |
| Supramolecular Mn-porphyrine adducts | p(O2) | oxidation | 40.8 to 15.2 at 20 MHz | 60 % decrease | 382 |
| Mn-HBET | glutathione/hydrogen peroxide | reduction/oxidation | Mn(II): 2.76 Mn(III): 1.05 at 60 MHz | 160 % increase/60 % decrease | 686 |
| Mn-JED | L-cysteine/hydrogen peroxide | reduction/oxidation | Mn(II): 3.3 Mn(III): 0.5 at 60 MHz | 560 % increase/80 % decrease | 689 |
| Gd-DTPA-bisamide derivative | Zn(II) | q | 4.8 to 3.4 at 300 MHz | 30 % decrease | 696–697 |
| Gd-DO3A-iminodiacetate | Zn(II) | q | 2.3 – 5.1 at 60 MHz | 120 % increase | 698–699 |
| Gd-CP027 | Zn(II) | τR | 6.6 – 17.4 at 23 MHz | 165 % increase | 700 |
| Mn-porphyrin-BPEN | Zn(II) | unknown | 8.7 to 6.7 at 200 MHz | 24% decrease | 704 |
| Gd-DOPTA | Ca(II) | q | 3.26 – 5.76 at 500 MHz | 80 % increase | 706–707 |
| Gd-BAPTA-ethyl esters | esterase/Ca(II) | q | 7.6 to 12.6 at 60 MHz | 66% increase | 708 |
| Gd-DOPTRA | Ca(II) | q | 3.5 to 6.9 at 400 MHz | 97% increase | 709 |
| Gd-DO3APBP | Zn(II), Ca(II), Mg(II) | τR | - | 200 – 500 % increase | 710 |
| Gd(III)-liposomes | Ca(II) | q, τR | 7.3 to 38.1 at 20 MHz | 400% increase | 714 |
| Gd-DO3A-thioether | Cu(I) | q | 1.5 to 6.9 at 60 MHz | 360% increase | 715–716 |
| Gd-DO3A-thioether-carboxylate | Cu(I) | q | 2.6 to 11.4 at 60 MHz | 340% increase | 717 |
| Gd-DO3A-thioether-octaarginine | Cu(I) | q | 3.9 to 12.5 at 60 MHz | 220% increase | 718 |
| Gd-QDOTAMA | Cu(II) | q | 4.3 to 7.3 at 400 MHz | 71% increase | 719 |
| Gd-DO2A-triazacrown ether | zwitterionic neurotransmitter | q | at 300 MHz | average: 70% decrease | 728 |
| Mn-porphyrin microgel | temperature | τR | 8.4 to 14.5 at 128 MHz | 73 % increase | 729 |
Table 12:
Summary of activatable CEST agents.
| Responsive MRI probe | Stimulus | Ref. |
|---|---|---|
| Dihydrouracil | pH | 8 |
| Poly-L-lysine | pH | 591 |
| Iopamidol | pH | 449 |
| Iopromide | pH | 592 |
| Iobitridol | pH | 590 |
| L-arginine filled liposomes | pH | 593 |
| Amine-based block copolymers | pH | 594 |
| Imidazoles | pH | 595 |
| Yb-DOTAM-Gly | pH | 600 |
| Yb-DOTAM-poly(propylene imine) dendrimers | pH | 604 |
| Tm-DOTAM-Gly-Lys | pH | 605 |
| Eu-DOTAM-Gly-phenol | pH | 606, 609 |
| Yb-DO3A-oAA | pH | 603 |
| Yb-HPDO3A | pH | 610 |
| Pyridine-Ln-DOTP-tButyl (ParaSHIFT) | pH | 613 |
| Fe(II)-2-amino-6-picolyl-cyclen | pH | 612 |
| DEVD-Tm-DOTA | caspase-3 | 655 |
| Cbz-GGR-Tm-DOTA | urokinase plasminogen activator | 657 |
| Yb-DOTA-β-galactose | β-galactosidase | 659 |
| Yb-DO3A-oAA-TML-ester | esterase | 661 |
| Yb-DO3A-oAA-TML-Q | DT-diaphorase | 661 |
| TM-DO3A-cadaverine | transglutaminase | 662 |
| Cytosine | cytosine deaminase | 663 |
| F5C | cytosine deaminase | 663 |
| (LRRASLG)8 | Protein Kinase A | 664 |
| (Phe-Arg)-4-amino-2-hydroxybenzoic acid | Cathepsin B | 665 |
| Salicylic acid derivatives | sulfatase or alkaline phosphatase | 666–667 |
| Salicylic acid derivatives | Simultaneous detection: esterase and sulfatase | 666–668 |
| Yb-DO3A-oAA | NO | 691 |
| Eu-DOTAM-N-methylqunolinium | NADH | 692 |
| Eu-DOTAM-anthryl | 1O2 | 201 |
| Eu-DOTAM-nitroxide | hypoxia | 184 |
| Gd(III) modified liposomes | reducing environment | 695 |
| Eu(2.2.2)-liposomes | oxidation | 405 |
| Triazamacrocyclic cobalt complex | oxidation/reduction | 693 |
| Eu-DOTAM-BPEN | Zn(II) | 703 |
| Yb-DOTA-tetraamide-iminodiacetate | Ca(II)/Mg(II) | 711 |
| polymeric Eu-DOTAM derivative | DNA | 737 |
7. Activation and Retention
An effective strategy to overcome major challenges to achieving biochemically targeted or activated MRI contrast is to combine the two approaches. There are multiple advantages to combining activation and retention. For example, off target contrast enhancement can be avoided by developing a non-targeted “pro-agent” that is enzymatically activated to accumulate at a tissue or cell target. Another advantage is that the relatively weak relaxivity changes achieved with most activatable agents can be overcome if the activated agent is accumulated in the aberrant microenvironment after washout of the unactivated agent.
The activation and retention strategy has been applied to image acute inflammation using the myeloperoxidase sensing agent Gd-MPO, comprised of Gd-DTPA with two appended 5-hydroxytryptamines.739–740 Myeloperoxidase is secreted by activated neutrophils and serves to catalytically amplify the reactivity of reactive oxygen species released during neutrophil burst by converting hydrogen peroxide into electrophilic forms of oxygen such as ferryl heme and hypochlorouos acid. Myeloperoxidase mediated oxidation of Gd-MPO results in 5-hydroxytryptamine-based radicals that oligomerize to multimeric MPO and form covalent bonds to surface exposed tyrosines in nearby proteins. Self-oligomerization of Gd-MPO does lead to relaxivity increase at field strenghts < 3.0 T but the strong, delayed enhancement Gd-MPO provides at the inflammatory microenvironment is also due to retention of the oxidized agent. Gd-MPO has been used to image the acute cardiovascular inflammation in mouse models of myocardial infarction, stroke, and vasculitis, and in a rabbit model of aneurism.741–744 Gd-MPO has also been used to image neuroinflammation in a mouse model of multiple sclerosis.745
Figure 92 shows Gd-MPO enhanced imaging of myeloperoxidase activity in a mouse model of myocardial infarction. The images in Figures 92A-C demonstrate the capability of Gd-MPO to detect differences in myeloperoxidase activity in the infarct bearing hearts of wild type, heterozygous, and homozygous myeloperoxidase knockout mice, respectively. The infarct zone (solid yellow arrows) vs remote myocardium (open yellow block arrows) CNR is correlated tightly with MPO activity, Figure 92D.
Figure 92:
A-C) T1-weighted images showing short axis views of the infarct bearing hearts of wild type, heterozygous, and homozygous myeloperoxidase knockout mice, respectively. The myocardial infarction is donoted by the solid yellow arrows and the remote myocardium is denoted by the open yellow block arrows. D) Infract zone vs. remote myocardium CNR is tightly correlated to myeloperoxidase activity within the infarction. Reproduced with permission from Ref.742 (URL: https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.107.756510). Copyright 2008 Wolters Kluwer Health, Inc.
The activation and retention strategy was also applied to a EP-2104R derived agent designed to differentiate active thrombosis from stable clot.746 The cysteine-disulfide bridge of EP-2104R is key to fibrin affinity, and binding affinity is obviated upon breaking this bridge to form open chain, mixed disulfides. An open chain, mixed disulfide form of EP-2104R was converted back to EP-2104R via action of protein disulfide isomerase (PDI) enzymes which are highly expressed and active on the surface of activated platelets. The mixed disfulfide pro-agent was shown provide strong enhancement of clotted fibrin in the presence of PDI using in vitro phantoms. No clot enhancement was observed in control samples that did not contain PDI.746
Neutrally charged and cell membrane permeant Mn-porphyrin and Mn-PDA agents have been functionalized with acetoxymethyl esters that are cleaved by cytosolic estarases to yield anionic, membrane impermeable carboxylate functionalized complexes.380, 747 These intracellularly activated agents have been considered in cell labelling applications. For example, the tetra-acetoxymethyl functionalized complex Mn-AMP was used to label mouse embryonic cells. The Mn-AMP labelled cells remained strongly T1-enhanced as the cells differentiated to cardiomyoctes in vitro.748 this strategy can ostensibly be extended to image cell populations expressing high levels of myriad other forms of intracellular enzymatic activities.
The activation and retention approach has been applied to develop contrast agents that accumulate in the microenvironments with high activities of matrix metalloprotease (MMP) enzymes.626–628 These water soluble agents comprise Gd-DOTA conjugated to PEGylated peptides that are proteolytically cleaved to yield hydrophobic peptides and thus a contrast agent of poor solubility. This approach has been used to image MMP expression in various mouse xenograft models of cancer. For example, an MMP-7 reactive agent was shown to provide strong contrast enhancement of human colon cancer xenografts but strongly attenuated contrast in mice treated with MMP inhibitors.626 In another study, an MMP-2 reactive agent provided strong tumor enhancement in a mouse model of breast cancer.627 The MMP-2 reactive agent was also capable of differentiating levels of MMP-2 expression between two different mouse models of cancer.628
8. Cell Tracking
A potentially highly impactful application of MRI contrast agents is in cell tracking. Labelling exogenously implanted or endogenously occurring cells with an MRI contrast agent enables non-invasive longitudinal tracking of cell mobility, survival, and differentiation in vivo.
One very promising area for MR cell tracking technology is tracking the fate of implanted cells during transplant or regenerative therapy. For example, ferucarbotran-labelled cells were used to confirm successful transplantation and survival of β-cell containing pancreatic islets in a rat model of Type I diabetes.749 The islet cells were isolated from healthy rats and were labelled by incubation in ferucarbotran containing cell culture. The pancreatic islets remained conspicuously hypointense for beyond 4 weeks after transplantation. Normal blood sugar levels were obtained in the diabetic rats within 1 week of islet implantation. This technology was extended to clinical trials performed on islet transplantation patients.750–751 Injection of the contrast agent labelled cells was safe in all patients. Figures 93 shows the liver of a Type I diabetes patient receiving ferucarbotran labelled pancreatic islet transplant via portal vein injection. Figures 93A-E were acquired prior to, 1 day, 1 week, 4 weeks, and 28 weeks after transplantation, respectively. The dark spots of signal loss are due to ferucarbotran loaded islets (white arrows). MR cell tracking demonstrates that 60% of the implanted islets do not survive 1 week past engraftment injection, but most of the surviving islets persist out to 24 weeks. The patients in this trial exhibited an increase in C-peptide levels, a biomarker of endogenous insulin production, and 50-80% less insulin was required to achieve near normal blood sugar levels.751
Figure 93:
Coronal view of the liver of a Type I diabetes patient receiving implantation of ferucarbotran labelled pancreatic islets via portal vein injection. A-E) Images were acquired prior to, 1 day, 1 week, 4 weeks, and 28 weeks after transplantation, respectively. The images show that roughly 60% of the engrafted islet do not survive past 1 week after engraftment, but most of the surviving islets persist out past 24 weeks. Reproduced with permission from Ref.751 (URL: https://doi.org/10.1097%2FTP.0b013e3181ffba5e). Copyright 2010 Wolters Kluwer Health, Inc.
Islet transplantation has also been tracked by labelling with perfluorocarbon nanoparticles and F-19 MRI.752 This direct F-19 detection strategy offers the advantage of quantitative islet tracking with zero background interference.
MR cell tracking is also used to image the homing of immune cells in immunotherapy. In a clinical trial of autologous dendritic cell therapy, immature dendritic cells isolated from the blood were labelled with ferumoxides.753 Immature dendritic cells are phagocytic and readily internalized the SPIONs. The ferumoxide-labelled dendritic cells were injected into a lymph node and migration to and accumulation in other lymph nodes was tracked by MRI. MR tracking of the dendritic cells enabled retrospective identification of false negatives in the clinical trial due to a high rate of mis-injection during intranodular implantation.753 Immunotherapy cell tracking has also been performed used F-19 MRI. In a clinical trial of stage 4 colorectal cancer immunotherapy, dendritic cells isolated from the patient were combined with lysate of surgically resected piece of the tumor in order to induce presentation of tumor specific antigens, and incubated with perfuorocabon nanoparticles. The F-19 labelled cells were administered intradermally and diminishment of the F-19 signal, due either to cell migration or to cell death and dispersion of the F-19 nanoparticles, was monitored over the course of 24 h.10
Immune cells can also be labelled in vivo via a technique called magneto-transfection. Here, vaccine-contrast agent conjugates are injected which enables visualization of adaptive immune system activation. The process by which antigen-presenting dendritic cells localize in lymph nodes and initiate immune response has been monitored using MRI in a tumor vaccine mouse model following injection of a SPION labelled vaccine.754
There are a number of examples where MR cell tracking has been used to monitor the fate of stem cells during cell therapy. In a clinical trial of nerve regeneration in patients suffering traumatic brain injury, autologous neural stem cells isolated from surgically excised tissue where labelled with ferumoxides and reinjected adjacent to the injury and cell migration to the injured tissue was monitored over the course of 3 weeks.755 In another clinical study, autologous CD34+ marrow stem cells were labelled with magnetic beads. Labelling was achieved by conjugating the magnetic beads to antibodies specific to CD34 antigen. The labelled stem cells were injected intrathecally and cell migration and accumulation within the lesion was observed over the course of 24 weeks.756 Cells have also been tracked via labelling with CEST contrast agents. Immortalized mouse skeletal myoblasts were labelled with Eu-HP-DO3A via hypertonic swelling of the cells and used for imaging in a mouse model of cardiac cell therapy.757 The Eu-HP-DO3A labelled cells implanted in the wall of the left ventricle of the heart provided over 30-fold greater MTRasym than surrounding tissue or unlabeled cells immediately after injection. Cell survival and cell rejection were modelled by implanting cells in in both syngeneic and allogeneic mouse models. In the syngeneic mice the cells provided strong CEST contrast out to 20 days past injection, whereas the cells were rejected and no contrast was observed within 20 days of implantation in the allogenic mice.
Cell tracking has also been used to monitor the dynamics of acute inflammation following tissue injury. The time course of monocyte infiltration into the site of myocardial infarction was visualized using T1-weighted MRI following injection of Gd-DTPA loaded liposomes.554 Here, monocyte labelling was performed in vivo by exploiting the phagocytic nature of monocytes. The time course of R1 enhancement in the infarcted myocardium correlated strongly with ex vivo histologic measures of monocyte infiltration. A similar approach was taken to image monocyte infiltration into tumors in a mouse model using F-19 perfluorocarbon nanoparticles.758 Here too, the particles were administered intravenously and accumulated in monocytes by endogenous mechanisms. Tumor F-19 signal intensity correlated with histologic counting of monocytes. In another study, analogously labelled F-19 monocytes were also used to image inflammation in a rat model of multiple sclerosis.759 The F-19 signal was used to quantitatively differentiate relative macrophage burden on the spinal cord of MS rats, MS rats receiving daily prophylactic cyclophosphamide treatment (an immunosuppressant), and healthy control rats.
Cells can also be engineered to express reporter genes that provide endogenous MRI contrast. In one proof of concept study, mice were treated intranasally with adenoviral vector to deliver a reporter gene that results in expression of a ferritin protein engineered to localize in the cytoplasm.760 Intranasal administration of the reporter gene carrying viral vector resulted in strong T1-contrast of epithelial olfactory neurons. No contrast enhancement was observed upon treatment with the same vector carrying only a green fluorescent protein reporter gene. In another study the oncovirus G47Δ, which is currently being evaluated in humans for treatment of progressive glioblastoma, was engineered to carry a lysine rich protein (LRP) CEST reporter and oncovirus transduction was imaged in a rat model of glioma.761 Figures 94A-D show CEST MTRassym maps overlaid with T2-weighted images of the glioma bearing brain prior to and 8h after intratumoral injection G47Δ carrying the LRP reporter and LRP empty control virus. A >1% increase in magnetization MTRassym was observed within 8 hours whereas no change in CEST contrast was observed after injection of LRP-empty control, Figures 94E-F. LRP expression did not demonstrate any measureable effect on the efficacy of the oncolytic viral therapy.
Figure 94:
CEST MTRassym maps overlaid with T2-weighted images of glioma bearing rat brain prior to and 8h after injection G47Δ carrying the LRP reporter and LRP empty control virus. (A,B) Images recorded prior to and 8h after direct intratumoral injection of G47Δ carrying the LRP reporter. (C,D) Images recorded prior to and 8h after direct intratumoral injection of LRP empty G47Δ. (E) Comparison of MTRassym prior to and 8h after treatment with virus carrying the LRP reporter gene and LRP empty control. (F) Comparison of ΔMTRassym observed 8h after treatment with virus carrying the LRP reporter gene and LRP empty control. Reproduced with permission from Ref.761 (URL: https://pubs.rsna.org/doi/10.1148/radiol.14140251). Copyright 2015 Radiological Society of North America (RSNA®).
There are also reporter genes that do not provide contrast but can lead to expression of receptors that can concentrate exogenously administered contrast agents within the cell, or amplify the signal by reaction with activatable MRI contrast agents. Some of these strategies have been described in previous sections. For example, the OATP seeking agent Gd-EOB-DTPA has been used to track the fate of OATP expressing cells in vivo.526 Transduction of the lacZ operon was confirmed using the β-galactosidase activated contrast agent.566 The divalent metal ion transporter (DMT1), which accumulates Mn(II) with high affinity has been used as an MR gene reporter.762 The DMT1 DNA construct was cloned into a viral vector for transduction into murine glioma cells and intracranially implanted into mice. The resultant DMT1 positive tumors were strongly enhanced in a T1-weighted scan 24h after MnCl2 injection, whereas DMT negative control tumors were not T1 enhanced.
9. Conclusions
The progress in MRI contrast agents has been mixed. When one of us co-wrote a review on the topic in 1999 there had been several contrast agents approved for use in the preceding decade and there were a number of new agents in clinical development. However in the intervening time very few new agents were approved and many products, like the iron oxide nanoparticles, have been discontinued because of low utilization rates. The lack of a commercially successful new contrast agent has had a chilling effect on the clinical development of new agents. Private investors are reluctant to spend tens of millions of dollars to fund the clinical development of new imaging agents unless they believe that future sales of the agent will be a multiple of that investment. A successful new contrast agent must address a large market size and deliver useful diagnostic information that justifies the cost of the contrast agent and the imaging procedure. A further barrier to commercialization is the difficulty in obtain proof of concept data in humans. Unlike diagnostic radiopharmaceuticals which are given at microscopic doses, contrast agents are administered in gram quantities necessitating kilo manufacturing and rigorous preclinical safety studies before clinical trials can commence. Together this represents a few million dollars of investment before human studies can begin.
On the other hand the need for specific molecular information is growing. We are entering into a new era of precision medicine where treatments are tailored to the individual. New powerful, but expensive, therapies are being developed, but many of these therapies are only effective in a subset of patients. Increasingly, noninvasive methods like imaging are being sought to stratify patients to therapies that will benefit them and avoid ineffective and/or costly treatments. In 1999 we reported the first examples of activatable and targeted probes, but now there are numerous examples some of which are well validated in animal models. Some of the targeted or activatable probes described here may find utility in the context of precision medicine.
Fifteen years ago the safety of the commercial gadolinium(III) chelates was almost unquestioned. But the advent of NSF and brain deposition has led to increased regulatory scrutiny. We believe that there is a strong need for improved contrast agents that are less retained, provide higher signal, and/or are more specific. It will be interesting to watch this field continue to evolve as chemists, biologists, physicists, engineers, and physicians continue to innovate.
Figure 29:
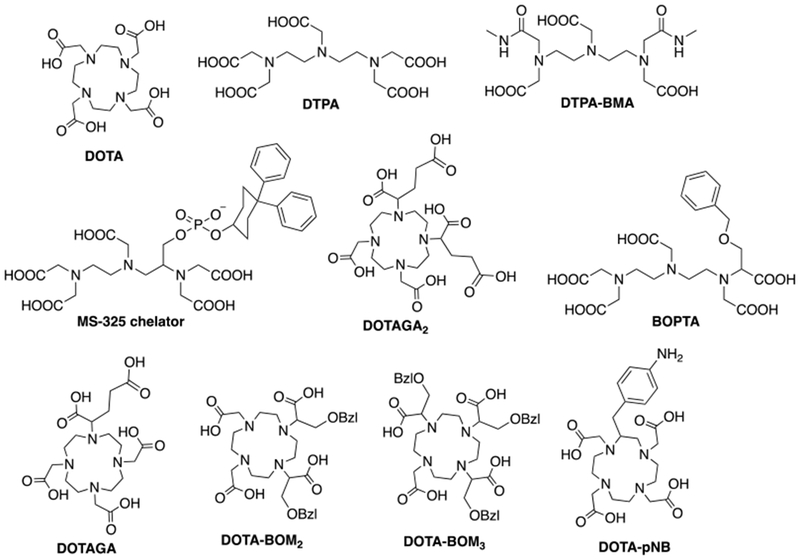
Chemical structure of the chelators mentioned in Figure 28.289
Figure 44:
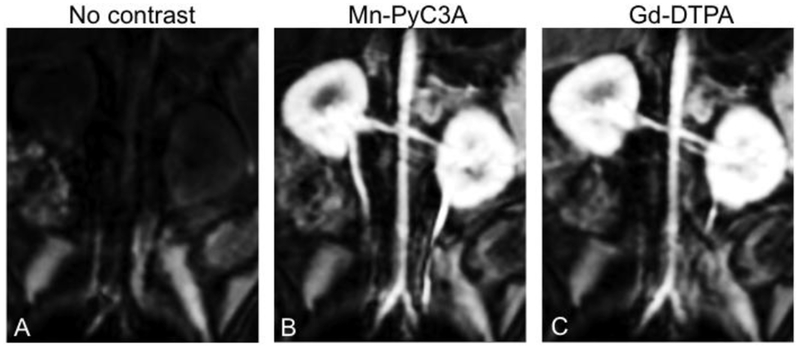
Multiplanar reformatted coronal images from three-dimensional T1-weighted gradient echo (volume-interpolated breathhold examination) sequence acquired at 3.0 T show abdominal aorta and renal arteries, A, prior to injection of contrast agent, B, 9 seconds after injection of 0.1 mmol/kg Mn-PyC3A, and, C, 9 seconds after injection of 0.1 mmol/kg Gd-DTPA. Reproduced with permission from Ref.378 (URL: https://pubs.rsna.org/doi/abs/10.1148/radiol.2017170977). Copyright 2017 Radiological Society of North America (RSNA®).
Figure 45:
Mn(III) complexes explored as high-relaxivity MRI contrast agents.
Figure 46:
(A) The Fe(III) complexes Fe-HBED and Fe-EHPG and (B) have been considered as hepatobiliary specific contrast agents. (B) The Fe(II) complexes Fe-DPTACN and Fe-DTTACN have been considered as contrast agents.
Table 7:
Comparison of formation constants of previously reported Mn chelates. In each group, chelates are ordered by conditional log KML at pH 7.4.
| log KML | log KML pH 7.4 | pMna | |
|---|---|---|---|
| Acyclic Chelates, q < 1 | |||
| AAZTA368 | 14.19b | 11.31b | 8.15b |
| DTPA363 | 14.54b | 10.93b | 7.77b |
| EGTA363 | 11.60b | 8.82b | 6.91b |
| AAZ3A368 | 11.00b | 8.15b | 6.58b |
| AAZ3MA368 | 10.67b | 7.77b | 6.39b |
| BIMP363 | 9.74b | 7.57b | 6.30b |
| MeAAZ3A368 | 11.43b | 7.03b | 6.47b |
| PMDPA369 | 11.37f | 6.82f | 5.94f |
| TMDTA363 | 9.70b | 6.54b | 5.81b |
| DPDP370 | 15.10d | 6.33d | 5.72d |
| HBED370 | 14.78d | 6.33d | 5.72d |
| PLED370 | 12.56d | 6.09d | 5.61d |
| Acyclic Chelates, q ≥ 1 | |||
| CyDTA363, 371 | 14.32b, 14.69d | 12.34b, 12.64d | 8.67b, 8.82d |
| PyC3A | 13.86b | 11.34b, 11.40c | 8.06b |
| EDTA-BOM2332 | 13.90e | 11.02e | 8.12e |
| EDTA-BOM332 | 13.50e | 10.62e | 7.82e |
| EDTA332, 363, 371 | 12.46b, 12.61d, 13.88e | 10.49b, 10.67d, 11.02e | 7.82b, 7.83d, 8.01e |
| CyHBET-NO2371 | 13.66d | 10.10d | 7.55d |
| HBET-NO2371 | 11.29d | 8.73d | 7.01d |
| HBET371 | 13.07d | 7.97d | 6.62d |
| HBET-OMe371 | 13.32d | 7.91d | 6.48d |
| CyHBET371 | 14.16d | 7.76d | 6.68d |
| CyHBET-OMe371 | 14.61d | 6.95d | 6.24d |
| Macrocyclic Chelates, q < 1 | |||
| DOTA372 | 19.89g | 13.27g | 8.94g |
| DO3A372 | 19.49g | 12.25g | 8.62g |
| HPDO3A372 | 17.89g | 12.18g | 8.59g |
| NOTA373-374 | 14.90g, 16.3g | 10.88g, 10.52g | 7.94g, 7.76g |
| 1,4-DO2Ai372 | 16.13g | 10.10g | 7.55g |
| 1,7-DO2A372 | 14.54g | 8.21g | 6.61g |
| Macrocylic Chelators, q ≥ 1 | |||
| 15-pyN5367 | 10.89g | 7.72g | 6.37g |
| 12-pyN4P366 | 14.06g | 7.35g | 6.19g |
| 12-pyN4A366 | 11.54g | 7.14g | 6.09g |
| NO2A365 | 11.56h | 7.11h | 5.96h |
| NODAHA375 | 10.15f | 6.43f | 5.76f |
| NODAHep 375 | 10.98f | 6.37f | 5.73f |
| NODABA375 | 9.90f | 6.10f | 5.61f |
| 15-pyN3O2367 | 7.18g | 5.20g | 5.27g |
| 9-aneN2O-2P365 | 10.61g | 5.07g | 5.23g |
| 9-aneN2O-2A365 | 7.43g | 4.36g | 5.06g |
| 9-aneN2O-2PH 365 | 4.30g | 3.34g | 5.01g |
| 9-aneN2O-2PPh 365 | 4.82g | 2.98g | 5.00g |
pMn at 10 µM MnL, pH 7.4;
I = 0.15 M NaCl, 25 °C;
determined from Kcomp with CDTA challenge, pH 7.4 Tris 50 mM, RT;
I = 0.10 M NaCl, 25 °C;
I = 1.0 M KCl, 25 °C;
I = 0.1 M KCl, 25 °C;
I = 0.1 M Me4NCl, 25 °C;
I = 0.1 M NaNO3, 25 °C.
Mn-DO2A is 0 < q < 1.
10. Acknowledgments
J.W. thanks the German Research Foundation (DFG, research fellowship 320225462) for support. E.M.G acknowledges grant support from the National Heart Lung and Blood Institute (K25HL128899, U54HL119145) and the National Institute for Biomedical Imaging and Bioengineering (NIBIB, R21EB022804). P.C. is grateful for funding from NIBIB (R01EB009062, xR21EB009738), the National Cancer Institute (R01CA161221), the National Institute for Diabetes and Digestive and Kidney Diseases (U01104302), the National Instititute for Neurological Diseases and Stroke (R01NS091552) as well as to Pfizer, Sanofi, and Siemens for supporting our work on MRI contrast agents over the last decade. Julianne Johnson is gratefully acknowledged for her editorial and administrative contributions. Dr. Pauline Désogère is warmly acknowledged for many helpful discussions. Patrick Sheedy is thanked for his help with table 11 and table 12.
Biographies
12. Author information
Jessica Wahsner is a post-doctoral fellow at the A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School. She received both her M.Sc. (2011) and Ph.D. (2015) in chemistry at the Ruhr-University Bochum, Germany, under the mentorship of Prof. Michael Seitz. Receiving a fellowship by the German Research Foundation (DFG), she started her current position as part of Prof. Peter Caravan’s group in 2016. Her research interests primarily focus on the development of small molecule PET probes for the molecular imaging of fibrotic disease and of bimodal PET-MR agents for quantitative pH imaging.
Eric M. Gale is an Assistant Professor in Radiology at the A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School. He received his B.A. in Chemistry from Rutgers University in 2006 and his Ph.D. in chemistry from the University of Georgia under the mentorship of Todd Harrop, where he synthesized and studied the reactivity of Ni complexes that model the nickel superoxide dismutase active site. He received his postdoctoral training under the mentorship of Peter Caravan between 2012-2015, where he primarily focused on studying the chemistry of Mn complexes in the context of MRI. He joined the faculty in 2015. His current research interests center around developing Gd-free alternatives for commercially available contrast agents and on developing redox active transition metal complexes for molecular MR imaging. He is a co-founder of Reveal Pharmaceuticals.
Aurora Rodriguez-Rodriguez was a postdoctoral researcher for two years (2016-2017) at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital and Harvard Medical School, working in the group of Prof. Peter Caravan. She received her Bachelor´s Degree in Chemistry (2008), her Master´s Degree in Environmental and Fundamental Chemistry (2009) and her Ph.D. in Chemistry (2014) from the University of A Coruña (Spain), under the supervision of Prof. María Teresa Rodríguez Blas and Dr. Carlos Platas Iglesias. She was awarded with a 12 months postdoctoral fellowship from the Conseil Général du Finistère 29 (France) in 2014 to work in the group ChASaM at the University of Brittany (France) under the mentorship of Prof. Raphaël Tripier and Dr. Véronique Patinec. Her research is focus on the design, synthesis, characterization and application of new contrast agents for molecular imaging.
Peter Caravan is a native of Bay Roberts, Newfoundland, Canada. He received a B.Sc.(Hons) from Acadia University in Nova Scotia and a Ph.D. in Inorganic Chemistry at the University of British Columbia, where he was a National Sciences and Engineering Research Council (NSERC) post-graduate scholar, under the mentorship of Professor Chris Orvig. He was a NSERC post-doctoral fellow at the Université de Lausanne, Switzerland where we worked with Professor André Merbach. He then joined Epix Pharmaceuticals in Cambridge, MA where he worked on the development of targeted MRI contrast agents including the FDA-approved blood pool agent gadofosveset (MS-325). In 2007 he was recruited to establish a translational molecular imaging lab at the A. A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School. In 2014 he was appointed Co-Director of the Institute for Innovation in Imaging (I3) which focuses on the clinical translation and commercialization of new radiological technologies including contrast agents. He is a National Institutes of Health funded researcher with a focus of development, application, and clinical translation of novel molecular MRI and PET probes.
11. References
- (1).Runge VM Critical Questions Regarding Gadolinium Deposition in the Brain and Body after Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA’s Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents. Invest. Radiol 2017, 52, 317–323. [DOI] [PubMed] [Google Scholar]
- (2).Caravan P; Ellison JJ; McMurry TJ; Lauffer RB Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev 1999, 99, 2293–2352. [DOI] [PubMed] [Google Scholar]
- (3).Edelman RR; Hesselink JR; Zlatkin MB; Crues JV III Clinical Magnetic Resonance Imaging - Volume 3 Elsevier Health: St. Louis, 2006. [Google Scholar]
- (4).Prince MR; Zhang HL; Zou ZT; Staron RB; Brill PW Incidence of Immediate Gadolinium Contrast Media Reactions. Am. J. Roentgenol 2011, 196, W138–W143. [DOI] [PubMed] [Google Scholar]
- (5).Grobner T Gadolinium – A Specific Trigger for the Development of Nephrogenic Fibrosing Dermopathy and Nephrogenic Systemic Fibrosis? Nephrol. Dial. Transplant 2006, 21, 1104–1108. [DOI] [PubMed] [Google Scholar]
- (6).Marckmann P; Skov L; Rossen K; Dupont A; Damholt MB; Heaf JG; Thomsen HS Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging. J. Am. Soc. Nephrol 2006, 17, 2359–2362. [DOI] [PubMed] [Google Scholar]
- (7).Kanda T; Fukusato T; Matsuda M; Toyoda K; Oba H; Kotoku J; Haruyama T; Kitajima K; Furui S Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects Without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [DOI] [PubMed] [Google Scholar]
- (8).Ward KM; Balaban RS Determination of pH Using Water Protons and Chemical Exchange Dependent Saturation Transfer (CEST). Magn. Reson. Med 2000, 44, 799–802. [DOI] [PubMed] [Google Scholar]
- (9).Nelson SJ; Kurhanewicz J; Vigneron DB; Larson PEZ; Harzstark AL; Ferrone M; van Criekinge M; Chang JW; Bok R; Park I, et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [13C]pyruvate. Sci. Transl. Med 2013, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ahrens ET; Helfer BM; O’Hanlon CF; Schirda C Clinical Cell Therapy Imaging Using a Perfluorocarbon Tracer and Fluorine-19 MRI. Magn. Reson. Med 2014, 72, 1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lohrke J; Frenzel T; Endrikat J; Alves FC; Grist TM; Law M; Lee JM; Leiner T; Li KC; Nikolaou K, et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther 2016, 33, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang Y; Spiller M; Caravan P Evidence for Weak Protein Binding of Commercial Extracellular Gadolinium Contrast Agents. Magn. Reson. Med 2010, 63, 609–616. [DOI] [PubMed] [Google Scholar]
- (13).FDA (Food and Drug administration). Medical Imaging Drugs Advisory Commitee: Gadolinium Retention after Gadolinium Based Contrast Magnetic Resonance Imaging in Patients with Normal Renal Function (September 2017). Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf. Accessed on April 6, 2018.
- (14).Runge VM A Comparison of Two MR Hepatobiliary Gadolinium Chelates: Gd-BOPTA and Gd-EOB-DTPA. J. Comput. Assist. Tomo 1998, 22, 643–650. [DOI] [PubMed] [Google Scholar]
- (15).Aime S; Caravan P Biodistribution of Gadolinium-Based Contrast Agents, Including Gadolinium Deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lauffer RB; Parmelee DJ; Dunham SU; Ouellet HS; Dolan RP; Witte S; McMurry TJ; Walovitch RC MS-325: Albumin-Targeted Contrast Agent for MR Angiography. Radiology 1998, 207, 529–538. [DOI] [PubMed] [Google Scholar]
- (17).Caravan P; Cloutier NJ; Greenfield MT; McDermid SA; Dunham SU; Bulte JWM; Amedio JC; Looby RJ; Supkowski RM; Horrocks WD, et al. The Interaction of MS-325 with Human Serum Albumin and Its Effect on Proton Relaxation Rates. J. Am. Chem. Soc 2002, 124, 3152–3162. [DOI] [PubMed] [Google Scholar]
- (18).Eldredge HB; Spiller M; Chasse JM; Greenfield MT; Caravan P Species Dependence on Plasma Protein Binding and Relaxivity of the Gadolinium-Based MRI Contrast Agent MS-325. Invest. Radiol 2006, 41, 229–243. [DOI] [PubMed] [Google Scholar]
- (19).Oliveira IS; Hedgire SS; Li W; Ganguli S; Prabhakar AM Blood Pool Contrast Agents for Venous Magnetic Resonance Imaging. Cardiovasc. Diagn. Ther 2016, 6, 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Goyen M Gadofosveset-Enhanced Magnetic Resonance Angiography. Vasc. Health Risk Manag 2008, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Goyen M; Edelman M; Perreault P; O’Riordan E; Bertoni H; Taylor J; Siragusa D; Sharafuddin M; Emile R Mohler I; Breger R, et al. MR Angiography of Aortoiliac Occlusive Disease: A Phase III Study of the Safety and Effectiveness of the Blood-Pool Contrast Agent MS-325. Radiology 2005, 236, 825–833. [DOI] [PubMed] [Google Scholar]
- (22).Paetsch I; Huber ME; Bornstedt A; Schnackenburg B; Boesiger P; Stuber M; Fleck E; Cavagna F; Nagel E Improved Three‐Dimensional Free‐Breathing Coronary Magnetic Resonance Angiography Using Gadocoletic Acid (B‐22956) for Intravascular Contrast Enhancement. J. Magn. Reson. Imaging 2004, 20, 288–293. [DOI] [PubMed] [Google Scholar]
- (23).Cavagna FM; Lorusso V; Anelli PL; Maggioni F; de Haen C Preclinical Profile and Clinical Potential of Gadocoletic Acid Trisodium Salt (B22956/1), A New Intravascular Contrast Medium for MRI. Acad. Radiol 2002, 9, 491–494. [DOI] [PubMed] [Google Scholar]
- (24).La Noce A; Stoelben S; Scheffler K; Hennig J; Lenz HM; La Ferla R; Lorusso V; Maggioni F; Cavagna F B22956/1, A New Intravascular Contrast Agent for MRI: First Administration to Humans - Preliminary Results. Acad. Radiol 2002, 9, 404–406. [DOI] [PubMed] [Google Scholar]
- (25).Preda A; Novikov V; Moglich M; Turetschek K; Shames DM; Brasch RC; Cavagna FM; Roberts TPL MRI Monitoring of Avastin (TM) Antiangiogenesis Therapy Using B22956/1, A New Blood Pool Contrast Agent, in an Experimental Model of Human Cancer. J. Magn. Reson. Imaging 2004, 20, 865–873. [DOI] [PubMed] [Google Scholar]
- (26).de Haen C; Anelli PL; Lorusso V; Morisetti A; Maggioni F; Zheng J; Uggeri F; Cavagna FM Gadocoletic Acid Trisodium Salt (B22956/1) - A New Blood Pool Magnetic Resonance Contrast Agent with Application in Coronary Angiography. Invest. Radiol 2006, 41, 279–291. [DOI] [PubMed] [Google Scholar]
- (27).Jaspers K; Versluis B; Leiner T; Dijkstra P; Oostendorp M; van Golde JM; Post MJ; Backes WH MR Angiography of Collateral Arteries in a Hind Limb Ischemia Model: Comparison between Blood Pool Agent Gadomer and Small Contrast Agent Gd-DTPA. PLOS ONE 2011, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Stiriba SE; Frey H; Haag R Dendritic Polymers in Biomedical Applications: From Potential to Clinical Use in Diagnostics and Therapy. Angew. Chem. Int. Ed 2002, 41, 1329–1334. [DOI] [PubMed] [Google Scholar]
- (29).Herborn CU; Barkhausen J; Paetsch I; Hunold P; Mahler M; Shamsi K; Nagel E Coronary Arteries: Contrast-Enhanced MR Imaging with SH L 643A—Experience in 12 Volunteers. Radiology 2003, 229, 217–223. [DOI] [PubMed] [Google Scholar]
- (30).Herborn CU; Schmidt M; Bruder O; Nagel E; Shamsi K; Barkhausen J MR Coronary Angiography with SH L 643 A: Initial Experience in Patients with Coronary Artery Disease. Radiology 2004, 233, 567–573. [DOI] [PubMed] [Google Scholar]
- (31).Port M; Corot C; Rousseaux O; Raynal I; Devoldere L; Idée J-M; Dencausse A; Greneur SL; Simonot C; Meyer D P792: A Rapid Clearance Blood Pool Agent for Magnetic Resonance Imaging: Preliminary Results. Magn. Reson. Mater. Phy 2001, 12, 121–127. [DOI] [PubMed] [Google Scholar]
- (32).Port M; Corot C; Raynal I; Idee J-M; Dencausse A; Lancelot E; Meyer D; Bonnemain B; Lautrou J Physicochemical and Biological Evaluation of P792, a Rapid-Clearance Blood-Pool Agent for Magnetic Resonance Imaging. Invest. Radiol 2001, 36, 445–454. [DOI] [PubMed] [Google Scholar]
- (33).Elst LV; Raynal I; Port M; Tisnès P; Muller RN In vitro Relaxometric and Luminescence Characterization of P792 (Gadomelitol, Vistarem®), an Efficient and Rapid Clearance Blood Pool MRI Contrast Agent. Eur. J. Inorg. Chem 2005, 2005, 1142–1148. [Google Scholar]
- (34).Gaillard S; Kubiak C; Stolz C; Bonnemain B; Chassard D Safety and Pharmacokinetics of P792, a New Blood-Pool Agent: Results of Clinical Testing in Nonpatient Volunteers. Invest. Radiol 2002, 37, 161–166. [DOI] [PubMed] [Google Scholar]
- (35).Klein C; Nagel E; Schnackenburg B; Bornstedt A; Schalla S; Hoffmann V; Lehning A; Fleck E The Intravascular Contrast Agent Clariscan™ (NC 100150 injection) for 3D MR Coronary Angiography in Patients with Coronary Artery Disease. Magn. Reson. Mater. Phy 2000, 11, 65–67. [DOI] [PubMed] [Google Scholar]
- (36).Kellar KE; Fujii DK; Gunther WHH; Briley‐Sæbø K; Bjørnerud A; Spiller M; Koenig SH NC100150 Injection, A Preparation of Optimized Iron Oxide Nanoparticles for Positive-Contrast MR Angiography. J. Magn. Reson. Imaging 2000, 11, 488–494. [DOI] [PubMed] [Google Scholar]
- (37).Wagner S; Schnorr J; Pilgrimm H; Hamm B; Taupitz M Monomer-Coated Very Small Superparamagnetic Iron Oxide Particles as Contrast Medium for Magnetic Resonance Imaging: Preclinical In Vivo Characterization. Invest. Radiol 2002, 37, 167–177. [DOI] [PubMed] [Google Scholar]
- (38).Wagner M; Wagner S; Schnorr J; Schellenberger E; Kivelitz D; Krug L; Dewey M; Laule M; Hamm B; Taupitz M Coronary MR Angiography Using Citrate‐Coated Very Small Superparamagnetic Iron Oxide Particles as Blood‐Pool Contrast Agent: Initial Experience in Humans. J. Magn. Reson. Imaging 2011, 34, 816–823. [DOI] [PubMed] [Google Scholar]
- (39).Jung CW; Jacobs P Physical and Chemical Properties of Superparamagnetic Iron Oxide MR Contrast Agents: Ferumoxides, Ferumoxtran, Ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [DOI] [PubMed] [Google Scholar]
- (40).Weissleder R; Elizondo G; Wittenberg J; Rabito CA; Bengele HH; Josephson L Ultrasmall Superparamagnetic Iron Oxide: Characterization of a New Class of Contrast Agents for MR Imaging. Radiology 1990, 175, 489–493. [DOI] [PubMed] [Google Scholar]
- (41).Mayo-Smith WW; Saini S; Slater G; Kaufman JA; Sharma P; Hahn PF MR Contrast Material for Vascular Enhancement: Value of Superparamagnetic Iron Oxide. Am. J. Roentgenol 1996, 166, 73–77. [DOI] [PubMed] [Google Scholar]
- (42).Anzai Y; Blackwell KE; Hirschowitz SL; Rogers JW; Sato Y; Yuh WT; Runge VM; Morris MR; McLachlan SJ; Lufkin RB Initial Clinical Experience with Dextran-Coated Superparamagnetic Iron Oxide for Detection of Lymph Node Metastases in Patients with Head and Neck Cancer. Radiology 1994, 192, 709–715. [DOI] [PubMed] [Google Scholar]
- (43).Anzai Y; Prince MR Iron Oxide‐Enhanced MR Lymphography: The Evaluation of Cervical Lymph Node Metastases in Head and Neck Cancer. J. Magn. Reson. Imaging 1997, 7, 75–81. [DOI] [PubMed] [Google Scholar]
- (44).Ahn SS; Kim M-J; Lim JS; Hong H-S; Chung YE; Choi J-Y Added Value of Gadoxetic Acid–Enhanced Hepatobiliary Phase MR Imaging in the Diagnosis of Hepatocellular Carcinoma. Radiology 2010, 255, 459–466. [DOI] [PubMed] [Google Scholar]
- (45).An SK; Lee JM; Suh K-S; Lee NJ; Kim SH; Kim YJ; Han JK; Choi BI Gadobenate Dimeglumine-Enhanced Liver MRI as the Sole Preoperative Imaging Technique: A Prospective Study of Living Liver Donors. Am. J. Roentgenol 2006, 187, 1223–1233. [DOI] [PubMed] [Google Scholar]
- (46).Toft KG; Hustvedt SO; Grant D; Martinsen I; Gordon PB; Friisk GA; Korsmo ÅJ; Skotland T Metabolism and Pharmacokinetics of MnDPDP in Man. Acta Radiol. 1997, 38, 677–689. [DOI] [PubMed] [Google Scholar]
- (47).Wang Y-XJ Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application. Quant. Imaging Med. Surg 2011, 1, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Clement O; Siauve N; Cuénod C-A; Frija G Liver Imaging With Ferumoxides (Feridex®): Fundamentals, Controversies, and Practical Aspects. Top. Magn. Reson. Imag 1998, 9, 167–182. [PubMed] [Google Scholar]
- (49).Reimer P; Balzer T Ferucarbotran (Resovist): A New Clinically Approved RES-Specific Contrast Agent for Contrast-Enhanced MRI of the Liver: Properties, Clinical Development, and Applications. Eur. Radiol 2003, 13, 1266–1276. [DOI] [PubMed] [Google Scholar]
- (50).Harisinghani MG; Barentsz J; Hahn PF; Deserno WM; Tabatabaei S; van de Kaa CH; de la Rosette J; Weissleder R Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. New Engl. J. Med 2003, 348, 2491–2499. [DOI] [PubMed] [Google Scholar]
- (51).Jacobs KE; Behera D; Rosenberg J; Gold G; Moseley M; Yeomans D; Biswal S Oral Manganese as an MRI Contrast Agent for the Detection of Nociceptive Activity. NMR Biomed. 2012, 25, 563–569. [DOI] [PubMed] [Google Scholar]
- (52).Arthurs OJ; Graves MJ; Edwards AD; Joubert I; Set PA; Lomas DJ Interactive Neonatal Gastrointestinal Magnetic Resonance Imaging Using Fruit Juice as an Oral Contrast Media. BMC Med. Imaging 2014, 14, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Li KCP; Tart RP; Fitzsimmons JR; Storm BL; Mao J; Rolfes RJ Barium Sulfate Suspension as a Negative Oral MRI Contrast Agent: In Vitro and Human Optimization Studies. Magn. Reson. Imaging 1991, 9, 141–150. [DOI] [PubMed] [Google Scholar]
- (54).Hahn PF; Stark DD; Lewis JM; Saini S; Elizondo G; Weissleder R; Fretz CJ; Ferrucci JT First Clinical Trial of a New Superparamagnetic Iron Oxide for Use as an Oral Gastrointestinal Contrast Agent in MR Imaging. Radiology 1990, 175, 695–700. [DOI] [PubMed] [Google Scholar]
- (55).Kaminsky S; Laniado M; Gogoll M; Kornmesser W; Clauss W; Langer M; Claussen C; Felix R Gadopentetate Dimeglumine as a Bowel Contrast Agent: Safety and Efficacy. Radiology 1991, 178, 503–508. [DOI] [PubMed] [Google Scholar]
- (56).Giovagnoni A; Fabbri A; Maccioni F Oral Contrast Agents in MRI of the Gastrointestinal Tract. Abdom. Imaging 2002, 27, 367–375. [DOI] [PubMed] [Google Scholar]
- (57).Mosbah K; Ruiz-Cabello J; Berthezène Y; Crémillieux Y Aerosols And Gaseous Contrast Agents for Magnetic Resonance Imaging of the Lung. Contrast Media. Mol. Imag 2008, 3, 173–190. [DOI] [PubMed] [Google Scholar]
- (58).Pan DPJ; Schmieder AH; Wickline SA; Lanza GM Manganese-Based MRI Contrast Agents: Past, Present, and Future. Tetrahedron 2011, 67, 8431–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Rohrer M; Bauer H; Mintorovitch J; Requardt M; Weinmann HJ Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Invest. Radiol 2005, 40, 715–724. [DOI] [PubMed] [Google Scholar]
- (60).Rooney WD; Johnson G; Li X; Cohen ER; Kim S.-g.; Ugurbil K; Springer CS Magnetic Field and Tissue Dependencies of Human Brain Longitudinal 1H2O Relaxation in Vivo. Magn. Reson. Med 2007, 57, 308–318. [DOI] [PubMed] [Google Scholar]
- (61).Gahramanov S; Muldoon LL; Varallyay CG; Li X; Kraemer DF; Fu R; Hamilton BE; Rooney WD; Neuwelt EA Pseudoprogression of Glioblastoma after Chemo- and Radiation Therapy: Diagnosis by Using Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging with Ferumoxytol versus Gadoteridol and Correlation with Survival. Radiology 2013, 266, 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).De Leõn-Rodríguez LM; Martins AF; Pinho MC; Rofsky NM; Sherry AD Basic MR Relaxation Mechanisms and Contrast Agent Design. J. Magn. Reson. Imaging 2015, 42, 545–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Schellenberger EA; Bogdanov A; Ho D; Tait J; Weissleder R; Josephson L Annexin V – CLIO : A Nanoparticle for Detecting Apoptosis by MRI. Mol. Imaging 2002, 1, 102–107. [DOI] [PubMed] [Google Scholar]
- (64).Yeh T.-c.; Zhang W; Ildstad ST; Ho C In Vivo Dynamic MRI Tracking of Rat T-Cells Labeled with Superparamagnetic Iron-Oxide Particles. Magn. Reson. Med 1995, 33, 200–206. [DOI] [PubMed] [Google Scholar]
- (65).Hyon B; Na B; Song IC; Hyeon T Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater 2009, 744, 2133–2148. [Google Scholar]
- (66).Bannas P; Graumann O; Balcerak P; Peldschus K; Kaul MG; Hohenberg H; Haag F; Adam G; Ittrich H; Koch-Nolte F Quantitative Magnetic Resonance Imaging of Enzyme Activity on the Cell Surface : In Vitro and In Vivo Monitoring of ADP-Ribosyltransferase 2 on T Cells. Mol. Imaging 2010, 9, 211–222. [PubMed] [Google Scholar]
- (67).Vogl TJ; Hammerstingl R; Schwarz W; Mack MG; Müller PK; Pegios W; Keck H; Eibl-Eibesfeldt A; Hoelzl J; Woessmer B, et al. Superparamagnetic Iron Oxide-Enhanced versus Gadolinium-Enhanced MR Imaging for Differential Diagnosis of Focal Liver Lesions. Radiology 1996, 198, 881–887. [DOI] [PubMed] [Google Scholar]
- (68).Farrell BT; Hamilton BE; Dósa E; Rimely E; Nasseri M; Gahramanov S; Lacy CA; Frenkel EP; Doolittle ND; Jacobs PM, et al. Using Iron Oxide Nanoparticles to Diagnose CNS Inflammatory Diseases and PCNSL. Neurology 2013, 81, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Guivel-Scharen V; Sinnwell T; Wolff SD; Balaban RS Detection of Proton Chemical Exchange between Metabolites and Water in Biological Tissues. J. Magn. Reson 1998, 133, 36–45. [DOI] [PubMed] [Google Scholar]
- (70).Chan KWY; McMahon MT; Kato Y; Liu G; Bulte JWM; Bhujwalla ZM; Artemov D; Van Zijl PCM Natural D-Glucose as a Biodegradable MRI Contrast Agent for Detecting Cancer. Magn. Reson. Med 2012, 68, 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Walker-Samuel S; Ramasawmy R; Torrealdea F; Rega M; Rajkumar V; Johnson SP; Richardson S; Goncalves M; Parkes HG; rstad E, et al. In Vivo Imaging of Glucose Uptake and Metabolism in Tumors. Nat. Med 2013, 19, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Longo DL; Dastrù W; Digilio G; Keupp J; Langereis S; Lanzardo S; Prestigio S; Steinbach O; Terreno E; Uggeri F, et al. Iopamidol as a Responsive MRI-Chemical Exchange Saturation Transfer Contrast Agent for PH Mapping of Kidneys: In Vivo Studies in Mice at 7 T. Magn. Reson. Med 2011, 65, 202–211. [DOI] [PubMed] [Google Scholar]
- (73).Ruiz-Cabello J; Barnett BP; Bottomley PA; Bulte JWM Fluorine (19F) MRS and MRI in Biomedicine. NMR Biomed. 2011, 24, 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Díaz-López R; Tsapis N; Fattal E Liquid Perfluorocarbons as Contrast Agents for Ultrasonography and 19F-MRI. Pharm. Res 2010, 27, 1–16. [DOI] [PubMed] [Google Scholar]
- (75).Tirotta I; Dichiarante V; Pigliacelli C; Cavallo G; Terraneo G; Bombelli FB; Metrangolo P; Resnati G 19 F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem. Rev 2015, 115, 1106–1129. [DOI] [PubMed] [Google Scholar]
- (76).Tanifum EA; Patel C; Liaw ME; Pautler RG; Annapragada AV Hydrophilic Fluorinated Molecules for Spectral 19F MRI. Sci. Rep 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Aime S; Botta M; Fasano M; Terreno E; Kinchesh P; Calabi L; Paleari L A New Ytterbium Chelate as Contrast Agent in Chemical Shift Imaging and Temperature Sensitive Probe for MR Spectroscopy. Magn. Reson. Med 1996, 35, 648–651. [DOI] [PubMed] [Google Scholar]
- (78).Coman D; Trubel HK; Hyder F Brain Temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): Comparison between TmDOTP(5−) and TmDOTMA(−). NMR Biomed. 2010, 23, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Hekmatyar SK; Hopewell P; Pakin SK; Babsky A; Bansal N Noninvasive MR Thermometry Using Paramagnetic Lanthanide Complexes of 1,4,7,10‐Tetraazacyclodoecane‐α,α′,α″,α‴‐Tetramethyl‐1,4,7,10‐Tetraacetic Acid (DOTMA4–). Magn. Reson. Med 2005, 53, 294–303. [DOI] [PubMed] [Google Scholar]
- (80).Yang Y; Schühle DT; Dai G; Alford J; Caravan P 1H Chemical Shift Magnetic Resonance Imaging Probes with High Sensitivity for Multiplex Imaging. Contrast Media. Mol. Imag 2012, 7, 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Mason K; Rogers NJ; Suturina EA; Kuprov I; Aguilar JA; Batsanov AS; Yufit DS; Parker D PARASHIFT Probes: Solution NMR and X-ray Structural Studies of Macrocyclic Ytterbium and Yttrium Complexes. Inorg. Chem 2017, 56, 4028–4038. [DOI] [PubMed] [Google Scholar]
- (82).Senanayake PK; Rogers NJ; Finney KLNA; Harvey P; Funk AM; Wilson JI; O’Hogain D; Maxwell R; Parker D; Blamire AM A New Paramagnetically Shifted Imaging Probe for MRI. Magn. Reson. Med 2017, 77, 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Chalmers KH; De Luca E; Hogg NHM; Kenwright AM; Kuprov I; Parker D; Botta M; Wilson JI; Blamire AM Design Principles and Theory of Paramagnetic Fluorine-Labelled Lanthanide Complexes as Probes for 19F Magnetic Resonance: A Proof-of-Concept Study. Chem. Eur. J 2010, 16, 134–148. [DOI] [PubMed] [Google Scholar]
- (84).Chalmers KH; Kenwright AM; Parker D; Blamire AM 19F-lanthanide Complexes with Increased Sensitivity for 19F-MRI: Optimization of the MR Acquisition. Magn. Reson. Med 2011, 66, 931–936. [DOI] [PubMed] [Google Scholar]
- (85).Månsson S; Johansson E; Magnusson P; Chai C-M; Hansson G; Petersson JS; Ståhlberg F; Golman K 13C Imaging—A New Diagnostic Platform. Eur. Radiol 2005, 16, 57–67. [DOI] [PubMed] [Google Scholar]
- (86).Golman K; Ardenkjaer-Larsen JH; Petersson JS; Mansson S; Leunbach I Molecular Imaging with Endogenous Substances. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 10435–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Mugler JP; Altes TA Hyperpolarized 129Xe MRI of the Human Lung. J. Magn. Reson. Imaging 2013, 37, 313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Beek E. J. R. v.; Wild JM; Kauczor HU; Schreiber W; Mugler JP; Lange E. E. d. Functional MRI of the Lung Using Hyperpolarized 3‐Helium Gas. J. Magn. Reson. Imaging 2004, 20, 540–554. [DOI] [PubMed] [Google Scholar]
- (89).Roos JE; McAdams HP; Kaushik SS; Driehuys B Hyperpolarized Gas MRI: Technique and Applications. Magn. Reson. Imaging C 2015, 23, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Fain S; Schiebler ML; McCormack DG; Parraga G Imaging of Lung Function Using Hyperpolarized Helium-3 Magnetic Resonance Imaging: Review of Current and Emerging Translational Methods and Applications. J. Magn. Reson. Imaging 2010, 32, 1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Ardenkjaer-Larsen JH; Fridlund B; Gram A; Hansson G; Hansson L; Lerche MH; Servin R; Thaning M; Golman K Increase in Signal-to-Noise Ratio of > 10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Sinharay S; Pagel MD Advances in Magnetic Resonance Imaging Contrast Agents for Biomarker Detection. Annu. Rev. Anal. Chem 2016, 9, 95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Annis DS; Mosher DF; Roberts DD Towards Hyperpolarized 13 C-Succinate Imaging of Brain Cancer. J. Magn. Reson 2007, 186, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Geraldes C; Laurent S Classification and Basic Properties of Contrast Agents for Magnetic Resonance Imaging. Contrast Media. Mol. Imag 2009, 4, 1–23. [DOI] [PubMed] [Google Scholar]
- (95).Pravdivtsev AN; Yurkovskaya AV; Vieth H-M; Ivanov KL RF-SABRE: A Way to Continuous Spin Hyperpolarization at High Magnetic Fields. J. Phys. Chem. B 2015, 119, 13619–13629. [DOI] [PubMed] [Google Scholar]
- (96).Halse ME Perspectives for Hyperpolarisation in Compact NMR. TRAC-Tend Anal. Chem 2016, 83, 76–83. [Google Scholar]
- (97).Miyazaki M; Akahane M Non-Contrast Enhanced MR Angiography: Established Techniques. J. Magn. Reson. Imaging 2012, 35, 1–19. [DOI] [PubMed] [Google Scholar]
- (98).Wheaton AJ; Miyazaki M Non-Contrast Enhanced MR Angiography: Physical Principles. J. Magn. Reson. Imaging 2012, 36, 286–304. [DOI] [PubMed] [Google Scholar]
- (99).Chavhan GB; Babyn PS; John P; Yoo SJ; RIgsby CK Pediatric Body MR Angiography: Principles, Techniques, and Current Status in Body Imaging. Am. J. Roentgenol 2015, 205, 173–184. [DOI] [PubMed] [Google Scholar]
- (100).Bazelaire CMJD; Duhamel GD; Rofsky NM; Alsop DC Radiology of Abdominal and Pelvic Tissues Measured in Vivo at 3 . 0 T : Preliminary Results 1. Radiology 2004, 230, 652–659. [DOI] [PubMed] [Google Scholar]
- (101).Bottomley PA; Hardy CJ; Argersinger RE; Allen-Moore G A Review of 1h Nuclear Magnetic Resonance Relaxation in Pathology: Are T1 and T2 Diagnostic? Med. Phys 1987, 14, 1–37. [DOI] [PubMed] [Google Scholar]
- (102).Lauffer RB Paramagnetic Metal Complexes as Water Proton Relaxation Agents for NMR Imaging: Theory and Design. Chem. Rev 1987, 87, 901–927. [Google Scholar]
- (103).Edelman RR; Micha JH, Clinical Magnetic Resonance Imaging In Clinical Magnetic Resonance Imaging, 3rd ed.; Saunders: Philadelphia, 2005. [Google Scholar]
- (104).Bloembergen N Spin Relaxation Processes in a Two-Proton System. Phys. Rev 1956, 104, 1542–1547. [Google Scholar]
- (105).Solomon I; Bloembergen N Nuclear Magnetic Interactions in the HF Molecule. J. Chem. Phys 1956, 25, 261–266. [Google Scholar]
- (106).Bloembergen N Proton Relaxation Times in Paramagnetic Solutions. J. Chem. Phys 1957, 27, 572–573. [Google Scholar]
- (107).Bloembergen N; Morgan LO Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation. J. Chem. Phys 1961, 34, 842–850. [Google Scholar]
- (108).Solomon I Relaxation Processes in a System of Two Spins. Phys. Rev 1955, 99, 559–565. [Google Scholar]
- (109).Caravan P Strategies for Increasing the Sensitivity of Gadolinium Based MRI Contrast Agents. Chem. Soc. Rev 2006, 35, 512–523. [DOI] [PubMed] [Google Scholar]
- (110).Laurent S; Elst LV; Muller RN Comparative Study of the Physicochemical Properties of Six Clinical Low Molecular Weight Gadolinium Contrast Agents. Contrast Media. Mol. Imag 2006, 1, 128–137. [DOI] [PubMed] [Google Scholar]
- (111).Powell DH; Dhubhghaill OMN; Pubanz D; Helm L; Lebedev YS; Schlaepfer W; Merbach AE Structural and Dynamic Parameters Obtained from 17O NMR, EPR, and NMRD Studies of Monomeric and Dimeric Gd3+ Complexes of Interest in Magnetic Resonance Imaging: An Integrated and Theoretically Self-Consistent Approach. J. Am. Chem. Soc 1996, 118, 9333–9346. [Google Scholar]
- (112).Dumas S; Jacques V; Sun W-C; Troughton JS; Welch JT; Chasse JM; Schmitt-Willich H; Caravan P High Relaxivity Magnetic Resonance Imaging Contrast Agents Part 1: Impact of Single Donor Atom Substitution on Relaxivity of Serum Albumin-Bound Gadolinium Complexes. Invest. Radiol 2010, 45, 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Atherton NM Principles of Electron Spin Resonance. Ellis Horwood Ltd: New York, 1993. [Google Scholar]
- (114).Bertini I; Kowalewski J; Luchinat C; Nilsson T; Parigi G Nuclear Spin Relaxation in Paramagnetic Complexes of S=1: Electron Spin Relaxation Effects. J. Chem. Phys 1999, 111, 5795–5807. [Google Scholar]
- (115).Bertini I; Luchinat C; Parigi G Solution NMR of Paramagnetic Molecules. Elsevier: Amsterdam, 2001. [Google Scholar]
- (116).Kowalewski J; Luchinat C; Nilsson T; Parigi G Nuclear Spin Relaxation in Paramagnetic Systems: Electron Spin Relaxation Effects under Near-Redfield Limit Conditions and Beyond. J. Phys. Chem. A 2002, 106, 7376–7382. [Google Scholar]
- (117).Kruk D; Nilsson T; Kowalewski J Nuclear Spin Relaxation in Paramagnetic Systems with Zero-Field Splitting and Arbitrary Electron Spin. Phys. Chem. Chem. Phys 2001, 3, 4907–4917. [Google Scholar]
- (118).Rubinstein MB,A; Luz Z Electronic and Nuclear Relaxation in Solutions of Transition Metal Ions with Spin S=3/2 and 5/2. Mol. Phys 1971, 20, 67–80. [Google Scholar]
- (119).Aime SB,M; Fasano M; Terreno E Lanthanide(III) Chelates for NMR Biomedical Applications. Chem. Soc. Rev 1998, 27, 19–29. [Google Scholar]
- (120).Fries PH; Belorizky E Electronic Relaxation of Paramagnetic Metal Ions and NMR Relaxivity in Solution: Critical Analysis of Various Approaches and Application to a Gd(Iii)-Based Contrast Agent. J. Chem. Phys 2005, 123. [DOI] [PubMed] [Google Scholar]
- (121).Raitsimring AM; Astashkin AV; Poluektov OG; Caravan P High Field Pulsed EPR and ENDOR of Gd3+ Complexes in Glassy Solutions. Appl. Magn. Reson 2005, 28, 281–295. [Google Scholar]
- (122).Borel A; Helm L; Merbach ÂE Molecular Dynamics Simulations of MRI-Relevant Gd III Chelates : Direct Access to Outer-Sphere Relaxivity. Chem. Eur. J 2001, 7, 600–610. [DOI] [PubMed] [Google Scholar]
- (123).Boros E; Srinivas R; Kim H-K; Raitsimring AM; Astashkin AV; Poluektov OG; Niklas J; Horning AD; Tidor B; Caravan P Intramolecular Hydrogen Bonding Restricts Gd–Aqua-Ligand Dynamics. Angew. Chem. Int. Ed 2017, 56, 5603–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Caravan P; Amedio JC Jr.; Dunham SU; Greenfield MT; Cloutier NJ; McDermid SA; Spiller M; Zech SG; Looby RJ; Raitsimring AM, et al. When are Two Waters Worse Than One? Doubling the Hydration Number of a Gd–DTPA Derivative Decreases Relaxivity. Chem. Eur. J 2005, 11, 5866–5874. [DOI] [PubMed] [Google Scholar]
- (125).Raitsimring AM; Astashkin AV; Baute D; Goldfarb D; Poluektov OG; Lowe MP; Zech SG; Caravan P Determination of the Hydration Number of Gadolinium(III) Complexes by High-Field Pulsed O-17 ENDOR Spectroscopy. ChemPhysChem 2006, 7, 1590–1597. [DOI] [PubMed] [Google Scholar]
- (126).Zech SG; Sun WC; Jacques V; Caravan P; Astashkin AV; Raitsimring AM Probing the Water Coordination gf Protein-Targeted MRI Contrast Agents by Pulsed ENDOR Spectroscopy. ChemPhysChem 2005, 6, 2570–2577. [DOI] [PubMed] [Google Scholar]
- (127).Caravan P; Astashkin AV; Raitsimring AM The Gadolinium(III)-Water Hydrogen Distance in MRI Contrast Agents. Inorg. Chem 2003, 42, 3972–3974. [DOI] [PubMed] [Google Scholar]
- (128).Astashkin AV; Raitsimring AM; Caravan P Pulsed ENDOR Study Of Water Coordination to Gd3+ Complexes in Orientationally Disordered Systems. J. Phys. Chem. A 2004, 108, 1990–2001. [Google Scholar]
- (129).Vander Elst L; Maton F; Laurent S; Seghi F; Chapelle F; Muller RN A Multinuclear MR Study of Gd-EOB-DTPA: Comprehensive Preclinical Characterization of an Organ Specific MRI Contrast Agent. Magn. Reson. Med 1997, 38, 604–614. [DOI] [PubMed] [Google Scholar]
- (130).Muller RN; Raduchel B; Laurent S; Platzek J; Pierart C; Mareski P; Vander Elst L Physicochemical Characterization of MS-325, a New Gadolinium Complex, by Multinuclear Relaxometry. Eur. J. Inorg. Chem 1999, 1999, 1949–1955. [Google Scholar]
- (131).Polasek M; Caravan P Is Macrocycle a Synonym for Kinetic Inertness in Gd(III) Complexes? Effect of Coordinating and Noncoordinating Substituents on Inertness and Relaxivity of Gd(III) Chelates with DO3A-like Ligands. Inorg. Chem 2013, 52, 4084–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Benson MC,T; Saunders L; Sommerer S Synthesis, Structure, Computational Studies and Magnetic Properties of a Ten-Coordinate Gadolinium(III) Complex. Inorg. Chim. Acta 1997, 248, 127–130. [Google Scholar]
- (133).Caravan P; Hedlund T; Liu S; Sjoberg S; Orvig C Potentiometric, Calorimetric, and Solution NMR Studies of a Tridentate Ligand Which Has a Marked Preference for the Formation of Bis- versus Mono(Ligand) Lanthanide Complexes, and which Exhibits High Selectivity for Heavier Lanthanides. J. Am. Chem. Soc 1995, 117, 11230–11238. [Google Scholar]
- (134).Alpoim MC; Urbano AM; Geraldes CFGC; Peters JA Determination of the Number of Inner-Sphere Water Molecules in Lanthanide(III) Polyaminocarboxylate Complexes. J. Chem. Soc., Dalton Trans 1992, 0, 463–467. [Google Scholar]
- (135).Horrocks WD Jr.; Sudnick DR Lanthanide Ion Probes of Structure in Biology. Laser-Induced Luminescence Decay Constants Provide a Direct Measure of the Number of Metal-Coordinated Water Molecules. J. Am. Chem. Soc 1979, 101, 334–340. [Google Scholar]
- (136).Beeby A; M. Clarkson I; S. Dickins R; Faulkner S; Parker D; Royle L; de Sousa A; A. Gareth Williams J; Woods M Non-Radiative Deactivation of the Excited States of Europium, Terbium and Ytterbium Complexes by Proximate Energy-Matched OH, NH and CH Oscillators: An Improved Luminescence Method for Establishing Solution Hydration States. J. Chem. Soc., Perkin Trans. 2 1999, 0, 493–504. [Google Scholar]
- (137).Supkowski RM; Horrocks WD On the Determination of the Number of Water Molecules, q, Coordinated To Europium(III) Ions In Solution From Luminescence Decay Lifetimes. Inorg. Chim. Acta 2002, 340, 44–48. [Google Scholar]
- (138).Gale EM; Zhu J; Caravan P Direct Measurement of the Mn(II) Hydration State in Metal Complexes and Metalloproteinst through 17O NMR Line Widths. J. Am. Chem. Soc 2013, 135, 18600–18608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Caravan P; Parigi G; Chasse JM; Cloutier NJ; Ellison JJ; Lauffer RB; Luchinat C; McDermid SA; Spiller M; McMurry TJ Albumin Binding, Relaxivity, and Water Exchange Kinetics of the Diastereoisomers of MS-325, a Gadolinium(III)-Based Magnetic Resonance Angiography Contrast Agent. Inorg. Chem 2007, 46, 6632–6639. [DOI] [PubMed] [Google Scholar]
- (140).Zech SG; Eldredge HB; Lowe MP; Caravan P Protein Binding to Lanthanide(III) Complexes Can Reduce the Water Exchange Rate at the Lanthanide. Inorg. Chem 2007, 46, 3576–3584. [DOI] [PubMed] [Google Scholar]
- (141).Zhou X; Caravan P; Clarkson RB; Westlund PO On the Philosophy of Optimizing Contrast Agents. An Analysis of 1H NMRD Profiles and ESR Lineshapes of the Gd(III) Complex MS-325+HSA. J. Magn. Reson 2004, 167, 147–160. [DOI] [PubMed] [Google Scholar]
- (142).Nair SA; Kolodziej AF; Bhole G; Greenfield MT; McMurry TJ; Caravan P Monovalent and Bivalent Fibrin-Specific MRI Contrast Agents for Detection of Thrombus. Angew. Chem. Int. Ed 2008, 47, 4918–4921. [DOI] [PubMed] [Google Scholar]
- (143).Helm L; Merbach AE Inorganic and Bioinorganic Solvent Exchange Mechanisms. Chem. Rev 2005, 105, 1923–1960. [DOI] [PubMed] [Google Scholar]
- (144).Caravan P; Farrar CT; Frullano L; Uppal R Influence of Molecular Parameters and Increasing Magnetic Field Strength on Relaxivity of Gadolinium- and Manganese-Based T1 Contrast Agents. Contrast Media. Mol. Imag 2009, 4, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Aime S; Botta M; Garda Z; Kucera BE; Tircso G; Young VG; Woods M Properties, Solution State Behavior, and Crystal Structures of Chelates of DOTMA. Inorg. Chem 2011, 50, 7955–7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Miller KJ; Saherwala AA; Webber BC; Wu Y; Sherry AD; Woods M The Population of SAP and TSAP Isomers in Cyclen-Based Lanthanide (III) Chelates Is Substantially Affected by Solvent. Inorg. Chem 2010, 49, 8662–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Tircso G; Webber BC; Kucera BE; Young VG; Woods M Analysis of the Conformational Behavior and Stability of the SAP and TSAP Isomers of Lanthanide (III) NB-DOTA-Type Chelates. Inorg. Chem 2011, 50, 7966–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Mayer F; Platas-Iglesias C; Helm L; Peters JA; Djanashvili K 17O NMR and Density Functional Theory Study of the Dynamics of the Carboxylate Groups in DOTA Complexes of Lanthanides in Aqueous Solution. Inorg. Chem 2011, 51, 170–178. [DOI] [PubMed] [Google Scholar]
- (149).Hermann P; Kotek J; Kubicek V; Lukes I Gadolinium (III) Complexes as MRI Contrast Agents : Ligand Design And Properties of the Complexes. Dalton Trans 2008, 23, 3027–3047. [DOI] [PubMed] [Google Scholar]
- (150).Howard JAK; Kenwright AM; Moloney JM; Parker D; Port M; Navet M; Rousseau O; Woods M Structure and Dynamics of all of the Stereoisomers of Europium Complexes of Tetra (Carboxyethyl) Derivatives of Dota: Ring Inversion is Decoupled from Cooperative Arm Rotation in the RRRR and RRRS Isomers. Chem. Commun 1998, 0, 1381–1382. [Google Scholar]
- (151).Dunand FA; Dickins RS; Parker D; Merbach AE Towards Rational Design of Fast Water-Exchanging Gd (dota-Like) Contrast Agents? Importance of the M/m Ratio. Chem. Eur. J 2001, 7, 5160–5167. [DOI] [PubMed] [Google Scholar]
- (152).Bari LD; Pintacuda G; Salvadori P Solution Equilibria in YbDOTMA , a Chiral Analogue of One of the Most Successful Contrast Agents for MRI, GdDOTA. Eur. J. Inorg. Chem 2000, 2000, 75–82. [Google Scholar]
- (153).Aime S; Botta M; Ermondi G; Terreno E; Anelli PL; Fedeli F; Uggeri F Relaxometric , Structural , and Dynamic NMR Studies of DOTA-like Ln (III) Complexes (Ln = La, Gd, Ho, Yb) Containing a p-Nitrophenyl Substituent. Inorg. Chem 1996, 35, 2726–2736. [Google Scholar]
- (154).Woods M; Kovacs Z; Zhang S; Sherry AD Towards the Rational Design of Magnetic Resonance Imaging Contrast Agents: Isolation of the Two Coordination Isomers of Lanthanide DOTA-Type Complexes. Angew. Chem. Int. Ed 2003, 42, 5889–5892. [DOI] [PubMed] [Google Scholar]
- (155).Woods M; Kovacs Z; Kiraly R; Brucher E; Zhang S; Sherry AD Solution Dynamics and Stability of Lanthanide (III) (S)-2-(p-Nitrobenzyl)DOTA Complexes. Inorg. Chem 2004, 43, 2845–2851. [DOI] [PubMed] [Google Scholar]
- (156).Ranganathan RS; Pillai RK; Raju N; Fan H; Nguyen H; Tweedle MF; Desreux JF; Jacques V Polymethylated DOTA Ligands. 1. Synthesis of Rigidified Ligands and Studies on the Effects of Alkyl Substitution on Acid – Base Properties and Conformational Mobility. Inorg. Chem 2002, 41, 6846–6855. [DOI] [PubMed] [Google Scholar]
- (157).Ranganathan RS; Raju N; Fan H; Zhang X; Tweedle MF; Desreux JF; Jacques V Polymethylated DOTA Ligands. 2. Synthesis of Rigidified Lanthanide Chelates and Studies on the Effect of Alkyl Substitution on Conformational Mobility and Relaxivity. Inorg. Chem 2002, 41, 6856–6866. [DOI] [PubMed] [Google Scholar]
- (158).Avecilla F; Peters JA; Geraldes CFGC X-ray Crystal Structure of a Sodium Salt of [Gd(DOTP)]5-: Implications for Its Second-Sphere Relaxivity and the 23Na NMR Hyperfine Shift Effects of [Tm(DOTP)]5-. Eur. J. Inorg. Chem 2003, 2003, 4179–4186. [Google Scholar]
- (159).Rudovsky J; Cigler P; Kotek J; Hermann P; Vojtisek P; Lukes I; Peters JA; Vander Elst L; Muller RN Lanthanide(III) Complexes of a Mono (methylphosphonate) Analogue of H(4)dota: The Influence of Protonation of the Phosphonate Moiety on the TSAP/SAP Isomer Ratio and the Water Exchange Rate. Chem. Eur. J 2005, 11, 2373–2384. [DOI] [PubMed] [Google Scholar]
- (160).Vojtisek P; Cigler P; Kotek J; Rudovsky J; Hermann P; Lukes I Crystal Structures of Lanthanide (III) Complexes with Cyclen Derivative Bearing Three Acetate and One Methylphosphonate Pendants. Inorg. Chem 2005, 44, 5591–5599. [DOI] [PubMed] [Google Scholar]
- (161).Heffern MC; Matosziuk LM; Meade TJ Lanthanide Probes for Bioresponsive Imaging. Chem. Rev 2014, 114, 4496–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Freed JH Dynamic Effects of Pair Correlation Functions on Spin Relaxation by Translational Diffusion in Liquids. II. Finite Jumps and Independent T1 Processes. J. Chem. Phys 1978, 68, 4034–4037. [Google Scholar]
- (163).Gueron M Nuclear Relaxation in Macromolecules by Paramagnetic Ions: A Novel Mechanism. J. Magn. Reson 1975, 19, 58–66. [Google Scholar]
- (164).Caravan P; Greenfield MT; Bulte JW Molecular Factors That Determine Curie Spin Relaxation in Dysprosium Complexes. Magn. Reson. Med 2001, 46, 917–922. [DOI] [PubMed] [Google Scholar]
- (165).Tombach B; Benner T; Reimer P; Schuierer G; Fallenberg EM; Geens V; Wels T; Sorenson AG Do Highly Concentrated Gadolinium Chelates Improve MR Brain Perfusion Imaging? Intraindividually Controlled Randomized Crossover Concentration Comparison Study of 0.5 versus 1.0 mol/l Gadobutrol. Radiology 2003, 226, 880–888. [DOI] [PubMed] [Google Scholar]
- (166).Choi JH; Nguyen FT; Barone PW; Heller DA; Moll AE; Patel D; Boppart SA; Strano MS Multimodal Biomedical Imaging with Asymmetric Single-Walled Carbon Nanotube / Iron Oxide Nanoparticle Complexes. Nano Lett 2007, 7, 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Pereira GA; Peters JA; Paz FAA; Geraldes CFGC Evaluation of [ Ln(H2cmp)(H2O)] Metal Organic Framework Materials for Potential Application as Magnetic Resonance Imaging Contrast Agents. Inorg. Chem 2010, 49, 2969–2974. [DOI] [PubMed] [Google Scholar]
- (168).Vold RL; Daniel ES; Chan SO Magnetic Resonance Measurements of Proton Exchange in Aqueous Urea. J. Am. Chem. Soc 1970, 92, 6771–6776. [Google Scholar]
- (169).Granot J; Fiat D Effect of Chemical Exchange on the Transverse Relaxation Rate of Nuclei in Solution Containing Paramagnetic Ions. J. Magn. Reson 1974, 15, 540–548. [Google Scholar]
- (170).Sherry AD; Wu Y The Importance of Water Exchange Rates in the Design of Responsive Agents for MRI. Curr. Opin. Chem. Biol 2013, 17, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Hancu I; Dixon WT; Woods M; Vinogradov E; Sherry AD; Lenkinski RE CEST and PARACEST MR Contrast Agents. Acta Radiol. 2010, 51, 910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Huinink HP; Sanders HMHF; Erich SJF; Nicolay K; Strijkers GJ; Merkx M; Adan OCG High-Resolution NMR Imaging of Paramagnetic Liposomes Targeted to a Functionalized Surface. Magn. Reson. Med 2008, 59, 1282–1286. [DOI] [PubMed] [Google Scholar]
- (173).Soesbe TC; Wu Y; Dean Sherry A Advantages of Paramagnetic Chemical Exchange Saturation Transfer (CEST) Complexes Having Slow to Intermediate Water Exchange Properties as Responsive MRI Agents. NMR Biomed. 2013, 26, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Morrow JR; Tóth É Magnetic Resonance Imaging Contrast Agents. Inorg. Chem 2017, 56, 6029–6034. [DOI] [PubMed] [Google Scholar]
- (175).Vinogradov E; Sherry AD; Lenkinski RE CEST: From Basic Principles to Applications, Challenges and Opportunities. J. Magn. Reson 2013, 229, 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Zijl P. v.; Yadav N Chemical Exchange Saturation Transfer (CEST): What is in a Name and What isn’t? Magn. Reson. Med 2011, 65, 927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Yang X; Yadav NN; Song X; Ray Banerjee S; Edelman H; Minn I; van Zijl PCM; Pomper MG; McMahon MT Tuning Phenols with Intra-Molecular Bond Shifted HYdrogens (IM-SHY) as diaCEST MRI Contrast Agents. Chemistry 2014, 20, 15824–15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Zhou J; Zijl P. C. M. v. Chemical Exchange Saturation Transfer Imaging and Spectroscopy. Prog. Nucl. Mag. Res. Sp 2006, 48, 109–136. [Google Scholar]
- (179).Wu B; Warnock G; Zaiss M; Lin C; Chen M; Zhou Z; Mu L; Nanz D; Tuura R; Delso G An Overview of CEST MRI for Non-MR Physicists. EJNMMI Phys. 2016, 3, 19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Liu G; Song X; Chan KWY; Mcmahon MT Nuts and Bolts of Chemical Exchange Saturation Transfer MRI. NMR Biomed. 2013, 26, 810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Li Y; Chen H; Xu J; Yadav NN; Chan KWY; Luo L; McMahon MT; Vogelstein B; van Zijl PCM; Zhou S, et al. CEST Theranostics: Label-Free MR Imaging of Anticancer Drugs. Oncotarget 2016, 7, 6369–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Dula AN; Smith SA; Gore JC Application of Chemical Exchange Saturation Transfer (CEST) MRI for Endogenous Contrast at 7 Tesla. J. Neuroimaging 2013, 23, 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Bryant LH Jr.; Hodges MW; Bryant RG Test of Electron Delocalization Effects on Water-Proton Spin-Lattice Relaxation by Bromination of [Tetrakis(4-sulfonatopheny)porphine]manganese. Inorg. Chem 1999, 38, 1002–1005. [DOI] [PubMed] [Google Scholar]
- (184).Ratnakar SJ; Soesbe TC; Lumata LL; Do QN; Viswanathan S; Lin C-Y; Sherry AD; Kovacs Z Modulation of CEST Images in Vivo by T1 Relaxation: A New Approach in the Design of Responsive PARACEST Agents. J. Am. Chem. Soc 2013, 135, 14904–14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Hingorani DV; Yoo B; Bernstein AS; Pagel MD Detecting Enzyme Activities with Exogenous MRI Contrast Agents. Chem. Eur. J 2014, 20, 9840–9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Dorazio SJ; Olatunde AO; Tsitovich PB; Morrow JR Comparison of Divalent Transition Metal Ion ParaCEST MRI Contrast Agents. J. Biol. Inorg. Chem 2014, 19, 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Stevens TK; Palaniappan KK; Ramirez RM; Francis MB; Wemmer DE; Pines A HyperCEST Detection of a 129Xe-Based Contrast Agent Composed of Cryptophane-A Molecular Cages on a Bacteriophage Scaffold. Magn. Reson. Med 2013, 69, 1245–1252. [DOI] [PubMed] [Google Scholar]
- (188).Zaiss M; Schnurr M; Bachert P Analytical Solution for the Depolarization of Hyperpolarized Nuclei by Chemical Exchange Saturation Transfer Between Free and Encapsulated Xenon (HyperCEST). J. Chem. Phys 2012, 136. [DOI] [PubMed] [Google Scholar]
- (189).Jones KM; Pollard AC; Pagel MD Clinical Applications of Chemical Exchange Saturation Transfer (CEST) MRI. J. Magn. Reson. Imaging 2018, 47, 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Castelli DDT,E; Longo D; Aime S Nanoparticle‐Based Chemical Exchange Saturation Transfer (CEST) Agents. NMR Biomed. 2013, 26, 839–849. [DOI] [PubMed] [Google Scholar]
- (191).Walker TGH,W Spin-Exchange Optical Pumping of Noble-Gas Nuclei. Rev. Mod. Phys 1997, 69, 629–642. [Google Scholar]
- (192).Wei Q; Seward GK; Hill PA; Patton B; Dimitrov IE; Kuzma NN; Dmochowski IJ Designing 129Xe NMR Biosensors for Matrix Metalloproteinase Detection. J. Am. Chem. Soc 2006, 128, 13274–13283. [DOI] [PubMed] [Google Scholar]
- (193).Schroder L; Lowery TJ; Hilty C; Wemmer DE; Pines A Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science 2006, 314, 446–449. [DOI] [PubMed] [Google Scholar]
- (194).Chambers JM; Hill PA; Aaron JA; Han Z; Christianson DW; Kuzma NN; Dmochowski IJ Cryptophane Xenon-129 Nuclear Magnetic Resonance Biosensors Targeting Human Carbonic Anhydrase. J. Am. Chem. Soc 2009, 131, 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Rose HM; Witte C; Rossella F; Klippel S; Freund C; Schroder L Development of an Antibody-Based, Modular Biosensor for 129Xe NMR Molecular Imaging of Cells at Nanomolar Concentrations. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 11697–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Schilling F; Schroder L; Palaniappan KK; Zapf S; Wemmer DE; Pines A MRI Thermometry Based on Encapsulated Hyperpolarized Xenon. ChemPhysChem 2010, 11, 3529–3533. [DOI] [PubMed] [Google Scholar]
- (197).Wang Y; Dmochowski IJ An Expanded Palette of Xenon-129 NMR Biosensors. Acc. Chem. Res 2016, 49, 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Fernando WS; Martins AF; Zhao P; Wu Y; Kiefer GE; Platas-Iglesias C; Sherry AD Breaking the Barrier to Slow Water Exchange Rates for Optimal Magnetic Resonance Detection of ParaCEST Agents. Inorg. Chem 2016, 55, 3007–3014. [DOI] [PubMed] [Google Scholar]
- (199).Zhang S; Michaudet L; Burgess S; Sherry AD The Amide Protons of an Ytterbium(III) Dota Tetraamide Complex Act as Efficient Antennae for Transfer of Magnetization to Bulk Water. Angew. Chem. Int. Ed 2002, 41, 1919–1921. [PubMed] [Google Scholar]
- (200).Woods M; Woessner DE; Sherry AD Paramagnetic lanthanide Complexes as PARACEST Agents for Medical Imaging. Chem. Soc. Rev 2006, 35, 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Song B; Wu Y; Yu M; Zhao P; Zhou C; Kiefer GE; Sherry AD A Europium(III)-Based PARACEST Agent for Sensing Singlet Oxygen by MRI. Dalton Trans 2013, 42, 8066–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Zhang SR; Merritt M; Woessner DE; Lenkinski RE; Sherry AD PARACEST Agents: Modulating MRI Contrast via Water Proton Exchange. Acc. Chem. Res 2003, 36, 783–790. [DOI] [PubMed] [Google Scholar]
- (203).Cacheris WP; Quay SC; Rocklage SM The Relationship Between Thermodynamics and the Toxicity of Gadolinium Complexes. Magn. Reson. Imaging 1990, 8, 467–481. [DOI] [PubMed] [Google Scholar]
- (204).Kumar K; Chang CA; Tweedle MF Equilibrium and Kinetic Studies of Lanthanide Complexes of Macrocyclic Polyamino Carboxylates. Inorg. Chem 1993, 32, 587–593. [Google Scholar]
- (205).Sherry AD; Caravan P; Lenkinski RE Primer on Gadolinium Chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Schühle DT; Caravan P Metal-Based MRI Contrast Agents. In Comprehensive Inorganic Chemistry II (Second Edition), Poeppelmeier K, Ed. Elsevier: Amsterdam, 2013. [Google Scholar]
- (207).Smith RM; Martell AE Critical Stability Constants, Enthalpies and Entropies for the Formation of Metal Complexes of Aminopolycarboxylic Acids and Carboxylic Acids. Sci. Total Environ 1987, 64, 125–147. [Google Scholar]
- (208).Port M; Idée J-M; Medina C; Robic C; Sabatou M; Corot C Efficiency, Thermodynamic and Kinetic Stability of Marketed Gadolinium Chelates and Their Possible Clinical Consequences: A Critical Review. BioMetals 2008, 21, 469–490. [DOI] [PubMed] [Google Scholar]
- (209).Schmitt-Willich H; Brehm M; Ewers CLJ; Michl G; Müller-Fahrnow A; Petrov O; Platzek J; Radüchel B; Sülzle D Synthesis and Physicochemical Characterization of a New Gadolinium Chelate: The Liver-Specific Magnetic Resonance Imaging Contrast Agent Gd-EOB-DTPA. Inorg. Chem 1999, 38, 1134–1144. [DOI] [PubMed] [Google Scholar]
- (210).Caravan P; Comuzzi C; Crooks W; McMurry TJ; Choppin GR; Woulfe SR Thermodynamic Stability and Kinetic Inertness of MS-325, a New Blood Pool Agent for Magnetic Resonance Imaging. Inorg. Chem 2001, 40, 2170–2176. [DOI] [PubMed] [Google Scholar]
- (211).Cacheris WP; Nickle SK; Sherry AD Thermodynamic Study Of Lanthanide Complexes of 1,4,7-Triazacyclononane-N,N’,N”-Triacetic Acid (NOTA) and 1,4,7,10-Tetraazacyclododecane-N,N’,N”,N’“-Tetraacetic Acid (DOTA). Inorg. Chem 1987, 26, 958–960. [Google Scholar]
- (212).Bellin MF; Vasile M; Morel-Precetti S Currently Used Non-Specific Extracellular MR Contrast Media. Eur. Radiol 2003, 13, 2688–2698. [DOI] [PubMed] [Google Scholar]
- (213).Sieber MA; Pietsch H; Walter J; Haider W; Frenzel T; Weinmann H-J A Preclinical Study to Investigate the Development of Nephrogenic Systemic Fibrosis: A Possible Role for Gadolinium-Based Contrast Media. Invest. Radiol 2008, 43, 65–75. [DOI] [PubMed] [Google Scholar]
- (214).Sieber MA; Lengsfeld P; Frenzel T; Golfier S; Schmitt-Willich H; Siegmund F; Walter J; Weinmann H-J; Pietsch H Preclinical Investigation to Compare Different Gadolinium-Based Contrast Agents Regarding Their Propensity to Release Gadolinium In Vivo and to Trigger Nephrogenic Systemic Fibrosis-Like Lesions. Eur. Radiol 2008, 18, 2164–2173. [DOI] [PubMed] [Google Scholar]
- (215).Wedeking P; Kumar K; Tweedle MF Dissociation of Gadolinium Chelates in Mice: Relationship to Chemical Characteristics. Magn. Reson. Imaging 1992, 10, 641–648. [DOI] [PubMed] [Google Scholar]
- (216).Tweedle MF; Wedeking P; Kumar K Biodistribution of Radiolabeled, Formulated Gadopentetate, Gadoteridol, Gadoterate, and Gadodiamide in Mice and Rats. Invest. Radiol 1995, 30, 372–380. [DOI] [PubMed] [Google Scholar]
- (217).Laurent S; Elst LV; Copoix F; Muller RN Stability of MRI Paramagnetic Contrast Media: A Proton Relaxometric Protocol for Transmetallation Assessment. Invest. Radiol 2001, 36, 115–122. [DOI] [PubMed] [Google Scholar]
- (218).Frenzel T; Lengsfeld P; Schirmer H; Hutter J; Weinmann HJ Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37 Degrees C. Invest. Radiol 2008, 43, 817–828. [DOI] [PubMed] [Google Scholar]
- (219).Do QN; Ratnakar JS; Kovacs Z; Tircso G; Krisztian Kalman F; Baranyai Z; Brucher E; Toth I Chapter 1 General Synthetic and Physical Methods In Contrast Agents for MRI: Experimental Methods, The Royal Society of Chemistry: 2018. [Google Scholar]
- (220).Palinkas Z; Baranyai Z; Brucher E; Rozsa B Kinetics of the Exchange Reactions between Gd(DTPA)(2-), Gd(BOPTA)(2-), and Gd(DTPA-BMA) Complexes, Used As MRI Contrast Agents, and the Triethylenetetraamine-Hexaacetate Ligand. Inorg. Chem 2011, 50, 3471–3478. [DOI] [PubMed] [Google Scholar]
- (221).Pasha A; Tircsó G; Benyó ET; Brücher E; Sherry AD Synthesis and Characterization of DOTA-(amide)4 Derivatives: Equilibrium and Kinetic Behavior of Their Lanthanide(III) Complexes. Eur. J. Inorg. Chem 2007, 2007, 4340–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Wang X; Jin T; Comblin V; Lopez-Mut A; Merciny E; Desreux JF A Kinetic Investigation of the Lanthanide DOTA Chelates. Stability and Rates of Formation and of Dissociation of a Macrocyclic Gadolinium(III) Polyaza Polycarboxylic MRI Contrast Agent. Inorg. Chem 1992, 31, 1095–1099. [Google Scholar]
- (223).Tóth É; Király R; Platzek J; Radüchel B; Brücher E Equilibrium and Kinetic Studies on Complexes of 10-[2,3-dihydroxy-(1-hydroxymethyl)-propyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate. Inorg. Chim. Acta 1996, 249, 191–199. [Google Scholar]
- (224).Sieber MA; Steger-Hartmann T; Lengsfeld P; Pietsch H Gadolinium-Based Contrast Agents and NSF: Evidence From Animal Experience. J. Magn. Reson. Imaging 2009, 30, 1268–1276. [DOI] [PubMed] [Google Scholar]
- (225).Idée JM; Port M; Robic C; Medina C; Sabatou M; Corot C Role of Thermodynamic and Kinetic Parameters in Gadolinium Chelate Stability. J. Magn. Reson. Imaging 2009, 30, 1249–1258. [DOI] [PubMed] [Google Scholar]
- (226).Fretellier N; Idée J-M; Dencausse A; Karroum O; Guerret S; Poveda N; Jestin G; Factor C; Raynal I; Zamia P, et al. Comparative In Vivo Dissociation of Gadolinium Chelates in Renally Impaired Rats: A Relaxometry Study. Invest. Radiol 2011, 46, 292–300. [DOI] [PubMed] [Google Scholar]
- (227).Filippi M; Rovaris M; Capra R; Gasperini C; Yousry TA; Sormani MP; Prandini F; Horsfield MA; Martinelli V; Bastianello S, et al. A Multi-Centre Longitudinal Study Comparing the Sensitivity of Monthly MRI after Standard and Triple Dose Gadolinium-DTPA for Monitoring Disease Activity in Multiple Sclerosis - Implications for Phase II Clinical Trials. Brain 1998, 121, 2011–2020. [DOI] [PubMed] [Google Scholar]
- (228).Schuhmann-Giampieri G; Krestin G Pharmacokinetics of Gd-DTPA in Patients with Chronic Renal Failure. Invest. Radiol 1991, 26, 975–979. [DOI] [PubMed] [Google Scholar]
- (229).Joffe P; Thomsen HS; Meusel M Pharmacokinetics of Gadodiamide Injection in Patients with Severe Renal Insufficiency and Patients Undergoing Hemodialysis or Continuous Ambulatory Peritoneal Dialysis. Acad. Radiol 1998, 5, 491–502. [DOI] [PubMed] [Google Scholar]
- (230).Tombach B; Bremer C; Reimer P; Schaefer RM; Ebert W; Geens V; Heindel W Pharmacokinetics of 1M Gadobutrol in Patients with Chronic Renal Failure. Invest. Radiol 2000, 35, 35–40. [DOI] [PubMed] [Google Scholar]
- (231).Weinmann HJ; Laniado M; Mutzel W Pharmacokinetics of GdDTPA/dimeglumine after Intravenous Injection into Healthy Volunteers. Physiol. Chem. Phys. Med. NMR 1984, 16, 167–172. [PubMed] [Google Scholar]
- (232).VanWagoner M; O’Toole M; Worah D; Leese PT; Quay SC A Phase I Clinical Trial with Gadodiamide Injection, a Nonionic Magnetic Resonance Imaging Enhancement Agent. Invest. Radiol 1991, 26, 980–986. [DOI] [PubMed] [Google Scholar]
- (233).McLachlan SJ; Eaton S; De Simone DN Pharmacokinetic Behavior of Gadoteridol Injection. Invest. Radiol 1992, 27 Suppl 1, 12–15. [PubMed] [Google Scholar]
- (234).Van Wagoner M; Worah D Gadodiamide injection. First Human Experience with the Nonionic Magnetic Resonance Imaging Enhancement Agent. Invest. Radiol 1993, 28 Suppl 1, 44–48. [PubMed] [Google Scholar]
- (235).Swan SK; Baker JF; Free R; Tucker RM; Barron B; Barr R; Seltzer S; Gazelle GS; Maravilla KR; Barr W, et al. Pharmacokinetics, Safety, and Tolerability of Gadoversetamide Injection (OptiMARK) in Subjects with Central Nervous System or Liver Pathology and Varying Degrees of Renal Function. J. Magn. Reson. Imaging 1999, 9, 317–321. [DOI] [PubMed] [Google Scholar]
- (236).Baker JF; Kratz LC; Stevens GR; Wible JH Jr. Pharmacokinetics and Safety of the MRI Contrast Agent Gadoversetamide Injection (OptiMARK) in Healthy Pediatric Subjects. Invest. Radiol 2004, 39, 334–339. [DOI] [PubMed] [Google Scholar]
- (237).Assessment Report for Gadolinium-Containing Contrast Agents, Proc.No. EMEA/H/A-31/1097. European Medicines Agency: 2010. [Google Scholar]
- (238).Gadolinium-Based Contrast Agents and Nephrogenic Systemic Fibrosis FDA Briefing Document. Advisory Committee: 2009. [Google Scholar]
- (239).Yang L; Krefting I; Gorovets A; Marzella L; Kaiser J; Boucher R; Rieves D Nephrogenic Systemic Fibrosis and Class Labeling of Gadolinium-Based Contrast Agents by the Food and Drug Administration. Radiology 2012, 265, 248–253. [DOI] [PubMed] [Google Scholar]
- (240).ACR Manual on Contrast Media v10.1. American College of Radiology: U.S.A., 2015. [Google Scholar]
- (241).Kanda T; Kawaguchi H Hyperintense Dentate Nucleas and Globus Pallidus on Unenhanced T1-Weighted MR Images are Associated with Gadolinium-Based Contrast Media. Neuroradiology 2013, 55, 1268–1269. [Google Scholar]
- (242).Kanda T; ishii K; Kawaguchi H; Kitajima K; Takenaka D High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-Based Contrast Material. Radiology 2014, 270, 834–841. [DOI] [PubMed] [Google Scholar]
- (243).Kanda T; Osawa M; Oba H; Toyoda K; Kotoku J; Haruyama T High Signal Intensity in Dentate Nucleus on Unenhanced T1-Weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 2015, 275, 803–809. [DOI] [PubMed] [Google Scholar]
- (244).Zhang Y; Cao Y; Shih GL; Hecht EM; Prince MR Extent of Signal Hyperintensity on Unenhanced T1-Weighted Brain MR Images after More than 35 Administrations of Linear Gadolinium-Based Contrast Agents. Radiology 2017, 282, 516–525. [DOI] [PubMed] [Google Scholar]
- (245).Stojanov DA; Aracki-Trenkic A; Vojinovic S; Benedeto-Stojanov D; Ljubisavljevic S Increasing Signal Intensity within the Dentate Nucleus and Globus Pallidus on Unenhanced T1W Magnetic Resonance Images in Patients with Relapsing-Remitting Multiple Sclerosis: Correlation with Cumulative Dose of a Macrocyclic Gadolinium-Based Contrast Agent, Gadobutrol. Eur. Radiol 2016, 26, 807–815. [DOI] [PubMed] [Google Scholar]
- (246).Splendiani A; Perri M; Marsecano C; Vellucci V; Michelini G; Barile A; Di Cesare E Effects of Serial Macrocyclic-Based Contrast Materials Gadoterate Meglumine and Gadobutrol Administrations on Gadolinium-Related Dentate Nuclei Signal Increases in Unenhanced T1-Weighted Brain: A Retrospective Study in 158 Multiple Sclerosis (MS) Patients. Radiol. Med 2018, 123, 125–134. [DOI] [PubMed] [Google Scholar]
- (247).Radbruch A; Weberling LD; Kieslich PJ; Hepp J; Kickingereder P; Wick W; Schlemmer HP; Bendszus M High-Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted Images Evaluation of the Macrocyclic Gadolinium-Based Contrast Agent Gadobutrol. Invest. Radiol 2015, 50, 805–810. [DOI] [PubMed] [Google Scholar]
- (248).Gibby WA; Gibby KA; Gibby WA Comparison of Gd DTPA-BMA (Omniscan) versus GdHP-DO3A (ProHance) Retention in Human Bone Tissue by Inductively Coupled Plasma Atomic Emission Spectroscopy. Invest. Radiol 2004, 39, 138–142. [DOI] [PubMed] [Google Scholar]
- (249).Murata N; Gonzalez-Cuyar LF; Murata K; Fligner C; Dills R; Hippe D; Maravilla KR Macrocyclic and Other Non–Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue Preliminary Results From 9 Patients With Normal Renal Function. Invest. Radiol 2016, 51, 447–453. [DOI] [PubMed] [Google Scholar]
- (250).Lord ML; Chettle DR; Grafe JL; Noseworthy MD; McNeill FE Observed Deposition of Gadolinium in Bone Using a New Noninvasive in Vivo Biomedical Device: Results of a Small Pilot Feasibility Study. Radiology 2018, 287, 96–103. [DOI] [PubMed] [Google Scholar]
- (251).Maximova N; Gregori M; Zennaro F; Sonzogni A; Simeone R; Zanon D Hepatic Gadolinium Deposition and Reversibility after Contrast Agent-Enhanced MR Imaging of Pediatric Hematopoietic Stem Cell Transplant Recipients. Radiology 2016, 281, 418–426. [DOI] [PubMed] [Google Scholar]
- (252).George SJ; Webb SM; Abraham JL; Cramer SP Synchrotron X-Ray Analyses Demonstrate Phosphate-Bound Gadolinium in Skin in Nephrogenic Systemic Fibrosis. Brit. J. Dermatol 2010, 163, 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Birka M; Wentker KS; Lusmoller E; Arheilger B; Wehe CA; Sperling M; Stadler R; Karst U Diagnosis of Nephrogenic Systemic Fibrosis by means of Elemental Bioimaging and Speciation Analysis. Anal. Chem 2015, 87, 3321–3328. [DOI] [PubMed] [Google Scholar]
- (254).Frenzel T; Apte C; Jost G; Schockel L; Lohrke J; Pietsch H Quantification and Assessment of the Chemical Form of Residual Gadolinium in the Brain After Repeated Administration of Gadolinium-Based Contrast Agents Comparative Study in Rats. Invest. Radiol 2017, 52, 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (255).Gianolio E; Bardini P; Arena F; Stefania R; Di Gregorio E; Iani R; Aime S Gadolinium Retention in the Rat Brain: Assessment of the Amounts of Insoluble Gadolinium-containing Species and Intact Gadolinium Complexes after Repeated Administration of Gadolinium-Based Contrast Agents. Radiology 2017, 285, 839–849. [DOI] [PubMed] [Google Scholar]
- (256).Rees JA; Deblonde GJP; An DD; Ansoborlo C; Gauny SS; Abergel RJ Evaluating the Potential of Chelation Therapy to Prevent and Treat Gadolinium Deposition from MRI Contrast Agents. Sci. Rep 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Burke LMB; Ramalho M; AlObaidy M; Chang E; Jay M; Semelka RC Self-Reported Gadolinium Toxicity: A Survey of Patients with Chronic Symptoms. Magn. Reson. Imaging 2016, 34, 1078–1080. [DOI] [PubMed] [Google Scholar]
- (258).Semelka RC; Commander C; Jay M; Burke LMB; Ramalho M Presumed Gadolinium Toxicity in Subjects With Normal Renal Function: A Report of 4 Cases. Invest. Radiol 2016, 51, 661–665. [DOI] [PubMed] [Google Scholar]
- (259).Semelka RC; Ramalho J; Vakharia A; AlObaidy M; Burke LM; Jay M; Ramalho M Gadolinium Deposition Disease: Initial Description of a Disease That Has Been Around for a While. Magn. Reson. Imaging 2016, 34, 1383–1390. [DOI] [PubMed] [Google Scholar]
- (260).Dos Santos Ferreira D; Jesus de Oliveira Pinto BL; Kumar V; Cardoso VN; Fernandes SO; Souza CM; Cassali GD; Moore A; Sosnovik DE; Farrar CT, et al. Evaluation of Antitumor Activity and Cardiac Toxicity of a Bone-Targeted PH-Sensitive Liposomal Formulation in a Bone Metastasis Tumor Model in Mice. Nanomedicine 2017, 13, 1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Gadolinium-Based Contrast Agents & Nephrogenic Systemic Fibrosis FDA Briefing Document. FDA Advisory Committee: U.S.A, 2015. [Google Scholar]
- (262).Desogere P; Tapias LF; Hariri LP; Rotile NJ; Rietz TA; Probst CK; Blasi F; Day H; Mino-Kenudson M; Weinreb P, et al. Type I Collagen-Targeted PET Probe for Pulmonary Fibrosis Detection and Staging in Preclinical Models. Sci. Transl. Med 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).United States Renal Data System, 2016 USRDS Annual Data Report Volume 1: Chronic Kidney Disease in the United States; National Institutes of Health, National Institue of Diabetes Digestive and Kidney Diseases: Bethesda, MD, 2016. [Google Scholar]
- (264).United States Renal Data System, 2013 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in The United States; National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases: Bethesda, MD, 2013. [Google Scholar]
- (265).Daly C Is Early Chronic Kidney Disease an Important Risk Factor for Cardiovascular Disease? A Background Paper Prepared for the UK Consensus Conference on Early Chronic Kidney Disease. Nephrol. Dial. Transplant 2007, 22, ix19–ix25. [DOI] [PubMed] [Google Scholar]
- (266).Solomon RJ; Natarajan MK; Doucet S; Sharma SK; Staniloae CS; Katholi RE; Gelormini JL; Labinaz M; Moreyra AE Cardiac Angiography in Renally Impaired Patients (CARE) Study: A Randomized Double-Blind Trial of Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease. Circulation 2007, 115, 3189–3196. [DOI] [PubMed] [Google Scholar]
- (267).FDA Orders Stricter Warnings for Ferumoxytol (Feraheme). Medscape Medical News: 2015. [Google Scholar]
- (268).Wang CL; Graham DJ; Kane RC; Xie DQ; Wernecke M; Levenson M; MaCurdy TE; Houstoun M; Ryan Q; Wong S, et al. Comparative Risk of Anaphylactic Reactions Associated With Intravenous Iron Products. JAMA-J. Am. Med. Assoc 2015, 314, 2062–2068. [DOI] [PubMed] [Google Scholar]
- (269).Wedeking P; Sotak CH; Telser J; Kumar K; Chang CA; Tweedle MF Quantitative Dependence of MR Signal Intensity on Tissue Concentration of Gd(HP-DO3A) in the Nephrectomized Rat. Magn. Reson. Imaging 1992, 10, 97–108. [DOI] [PubMed] [Google Scholar]
- (270).Werner EJ; Datta A; Jocher CJ; Raymond KN High-Relaxivity MRI Contrast Agents: Where Coordination Chemistry Meets Medical Imaging. Angew. Chem. Int. Ed 2008, 47, 8568–8580. [DOI] [PubMed] [Google Scholar]
- (271).Siriwardena-Mahanama BN; Allen MJ Strategies for Optimizing Water-Exchange Rates of Lanthanide-Based Contrast Agents for Magnetic Resonance Imaging. Molecules 2013, 18, 9352–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Supkowski RM; Horrocks WD Displacement of Inner-Sphere Water Molecules from Eu3+ Analogues of Gd3+ MRI Contrast Agents by Carbonate and Phosphate Anions: Dissociation Constants from Luminescence Data in the Rapid-Exchange Limit. Inorg. Chem 1999, 38, 5616–5619. [DOI] [PubMed] [Google Scholar]
- (273).Kim WD; Kiefer GE; Maton F; McMillan K; Muller RN; Sherry AD Relaxometry, Luminescence Measurement, Electrophoresis, and Animal Biodistribution of Lanthanide(III) Complexes of Some Polyaza Macrocyclic Acetates Containing Pyridine. Inorg. Chem 1995, 34, 2233–2243. [Google Scholar]
- (274).Aime S; Botta M; Geninatti Crich S; Giovenzana GB; Jommi G; Pagliarin R; Sisti M Synthesis and NMR Studies of Three Pyridine-Containing Triaza Macrocyclic Triacetate Ligands and Their Complexes with Lanthanide Ions. Inorg. Chem 1997, 36, 2992–3000. [DOI] [PubMed] [Google Scholar]
- (275).Port M; Raynal I; Vander Elst L; Muller RN; Dioury F; Ferroud C; Guy A Impact of Rigidification on Relaxometric Properties of a Tricyclic Tetraazatriacetic Gadolinium Chelate. Contrast Media. Mol. Imag 2006, 1, 121–127. [DOI] [PubMed] [Google Scholar]
- (276).Tircsó G; Benyó ET; Suh EH; Jurek P; Kiefer GE; Sherry AD; Kovács Z (S)-5-(p-Nitrobenzyl)-PCTA, a Promising Bifunctional Ligand with Advantageous Metal Ion Complexation Kinetics. Bioconjugate Chem. 2009, 20, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Brücher E; Tircsó G; Baranyai Z; Kovács Z; Sherry AD Stability and Toxicity of Contrast Agents In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, Merbach A; Helm L; Tóth É, Eds. John Wiley & Sons, Ltd: 2013. [Google Scholar]
- (278).Aime S; Calabi L; Cavallotti C; Gianolio E; Giovenzana GB; Losi P; Maiocchi A; Palmisano G; Sisti M [Gd-AAZTA]-: A New Structural Entry for an Improved Generation of MRI Contrast Agents. Inorg. Chem 2004, 43, 7588–7590. [DOI] [PubMed] [Google Scholar]
- (279).Elemento EM; Parker D; Aime S; Gianolio E; Lattuada L Variation of Water Exchange Dynamics with Ligand Structure and Stereochemistry in Lanthanide Complexes Based on 1,4-Diazepine Derivatives. Org. Biomol. Chem 2009, 7, 1120–1131. [DOI] [PubMed] [Google Scholar]
- (280).Gugliotta G; Botta M; Giovenzana GB; Tei L Fast and Easy Access to Efficient Bifunctional Chelators for MRI Applications. Bioorg. Med. Chem. Lett 2009, 19, 3442–3444. [DOI] [PubMed] [Google Scholar]
- (281).Gale EM; Kenton N; Caravan P [Gd(CyPic3A)(H2O)2]-: a Stable, Bis(Aquated) and High-Relaxivity Gd(III) Complex. Chem. Commun 2013, 49, 8060–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Messeri D; Lowe MP; Parker D; Botta M A Stable, High Relaxivity, Diaqua Gadolinium Complex that Suppresses Anion and Protein Binding. Chem. Commun 2001, 0, 2742–2743. [Google Scholar]
- (283).Datta A; Raymond KN Gd–Hydroxypyridinone (HOPO)-Based High-Relaxivity Magnetic Resonance Imaging (MRI) Contrast Agents. Acc. Chem. Res 2009, 42, 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Thompson MK; Botta M; Nicolle G; Helm L; Aime S; Merbach AE; Raymond KN A Highly Stable Gadolinium Complex with a Fast, Associative Mechanism of Water Exchange. J. Am. Chem. Soc 2003, 125, 14274–14275. [DOI] [PubMed] [Google Scholar]
- (285).Thompson MK; Misselwitz B; Tso LS; Doble DMJ; Schmitt-Willich H; Raymond KN In Vivo Evaluation of Gadolinium Hydroxypyridonate Chelates: Initial Experience as Contrast Media in Magnetic Resonance Imaging. J. Med. Chem 2005, 48, 3874–3877. [DOI] [PubMed] [Google Scholar]
- (286).Werner EJ; Avedano S; Botta M; Hay BP; Moore EG; Aime S; Raymond KN Highly Soluble Tris-hydroxypyridonate Gd(III) Complexes with Increased Hydration Number, Fast Water Exchange, Slow Electronic Relaxation, and High Relaxivity. J. Am. Chem. Soc 2007, 129, 1870–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (287).Hajela S; Botta M; Giraudo S; Xu J; Raymond KN; Aime S A Tris-hydroxymethyl-Substituted Derivative of Gd-TREN-Me-3,2-HOPO: An MRI Relaxation Agent with Improved Efficiency. J. Am. Chem. Soc 2000, 122, 11228–11229. [Google Scholar]
- (288).Fries P; Müller A; Seidel R; Robert P; Denda G; Menger MD; Schneider G; Buecker A P03277—A New Approach to Achieve High-Contrast Enhancement: Initial Results of an Experimental Extracellular Gadolinium-Based Magnetic Resonance Contrast Agent. Invest. Radiol 2015, 50, 835–842. [DOI] [PubMed] [Google Scholar]
- (289).Helm L; Morrow JR; Bond CJ; Carniato F; Botta M; Braun M; Baranyai Z; Pujales-Paradela R; Regueiro-Figueroa M; Esteban-Gomez D, et al. Chapter 2: Gadolinium-Based Contrast Agents In Contrast Agents for MRI: Experimental Methods, The Royal Society of Chemistry: 2018. [Google Scholar]
- (290).Frullano L; Caravan P Strategies for the Preparation of Bifunctional Gadolinium(III) Chelators. Curr. Org. Synth 2011, 8, 535–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (291).Villaraza AJL; Bumb A; Brechbiel MW Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev 2010, 110, 2921–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Venditto VJ; Regino CAS; Brechbiel MW PAMAM Dendrimer Based Macromolecules as Improved Contrast Agents. Mol. Pharm 2005, 2, 302–311. [DOI] [PubMed] [Google Scholar]
- (293).Aime S; Castelli DD; Crich SG; Gianolio E; Terreno E Pushing the Sensitivity Envelope of Lanthanide-Based Magnetic Resonance Imaging (MRI) Contrast Agents for Molecular Imaging Applications. Acc. Chem. Res 2009, 42, 822–831. [DOI] [PubMed] [Google Scholar]
- (294).Lauffer RB; Brady TJ Preparation and Water Relaxation Properties of Proteins Labeled with Paramagnetic Metal Chelates. Magn. Reson. Imaging 1985, 3, 11–16. [DOI] [PubMed] [Google Scholar]
- (295).Schmiedl U; Ogan M; Paajanen H; Marotti M; Crooks LE; Brito AC; Brasch RC Albumin Labeled with Gd-DTPA as an Intravascular, Blood Pool-Enhancing Agent for MR Imaging: Biodistribution and Imaging Studies. Radiology 1987, 162, 205–210. [DOI] [PubMed] [Google Scholar]
- (296).Ogan MD; Schmiedl U; Moseley ME; Grodd W; Paajanen H; Brasch RC Albumin Labeled with Gd-DTPA: An Intravascular Contrast-Enhancing Agent for Magnetic Resonance Blood Pool Imaging: Preparation and Characterization. Invest. Radiol 1987, 22, 665–671. [PubMed] [Google Scholar]
- (297).Spanoghe M; Lanens D; Dommisse R; Van der Linden A; Alderweireldt F Proton Relaxation Enhancement by means of Serum Albumin and Poly-L-lysine Labeled with DTPA-Gd3+: Relaxivities as a Function of Molecular Weight and Conjugation Efficiency. Magn. Reson. Imaging 1992, 10, 913–917. [DOI] [PubMed] [Google Scholar]
- (298).Schuhmann-Giampieri G; Schmitt-Willich H; Frenzel T; Press W-R; Weinmann H-J In Vivo and In Vitro Evaluation of Gd-DTPA-Polylysine as a Macromolecular Contrast Agent for Magnetic Resonance Imaging. Invest. Radiol 1991, 26, 969–974. [DOI] [PubMed] [Google Scholar]
- (299).Caravan P; Greenwood JM; Welch JT; Franklin SJ Gadolinium-Binding Helix-Turn-Helix Peptides: DNA-Dependent MRI Contrast Agents. Chem. Commun 2003, 0, 2574–2575. [DOI] [PubMed] [Google Scholar]
- (300).Xue S; Qiao J; Jiang J; Hubbard K; White N; Wei L; Li S; Liu ZR; Yang JJ Design of ProCAs (Protein‐Based Gd3+ MRI Contrast Agents) with High Dose Efficiency and Capability for Molecular Imaging of Cancer Biomarkers. Med. Res. Rev 2014, 34, 1070–1099. [DOI] [PubMed] [Google Scholar]
- (301).Yang JJ; Yang J; Wei L; Zurkiya O; Yang W; Li S; Zou J; Zhou Y; Maniccia ALW; Mao H, et al. Rational Design of Protein-Based MRI Contrast Agents. J. Am. Chem. Soc 2008, 130, 9260–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Wei L; Li S; Yang J; Ye Y; Zou J; Wang L; Long R; Zurkiya O; Zhao T; Johnson J, et al. Protein-Based MRI Contrast Agents for Molecular Imaging of Prostate Cancer. Mol. Imaging Biol 2011, 13, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Xue S; Yang H; Qiao J; Pu F; Jiang J; Hubbard K; Hekmatyar K; Langley J; Salarian M; Long RC, et al. Protein MRI Contrast Agent with Unprecedented Metal Selectivity and Sensitivity for Liver Cancer Imaging. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 6607–6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (304).Casali C; Janier M; Canet E; Obadia JF; Benderbous S; Corot C; Revel D Evaluation of Gd-DOTA-Labeled Dextran Polymer as an Intravascular MR Contrast Agent for Myocardial Perfusion. Acad. Radiol 1998, 5, 214–218. [DOI] [PubMed] [Google Scholar]
- (305).Dwek R Nuclear Magnetic Resonance in Biochemistry: Applications To Enzyme Systems. Clarendon Press: Oxford University, 1973. [Google Scholar]
- (306).Dunand FA; Aime S; Merbach AE First 17O NMR Observation of Coordinated Water on Both Isomers of [Eu (DOTAM)(H2O)] 3+: A Direct Access to Water Exchange and its Role in the Isomerization. J. Am. Chem. Soc 2000, 122, 1506–1512. [Google Scholar]
- (307).Dunand FA; Borel A; Merbach AE How Does Internal Motion Influence the Relaxation of the Water Protons in LnIIIDOTA-like Complexes? J. Am. Chem. Soc 2002, 124, 710–716. [DOI] [PubMed] [Google Scholar]
- (308).Lowe MP; Parker D; Reany O; Aime S; Botta M; Castellano G; Gianolio E; Pagliarin R pH-Dependent Modulation of Relaxivity and Luminescence in Macrocyclic Gadolinium and Europium Complexes Based on Reversible Intramolecular Sulfonamide Ligation. J. Am. Chem. Soc 2001, 123, 7601–7609. [DOI] [PubMed] [Google Scholar]
- (309).Dumas S; Jacques V; Sun W-C; Troughton JS; Welch JT; Chasse JM; Schmitt-Willich H; Caravan P High Relaxivity MRI Contrast Agents Part 1: Impact of Single Donor Atom Substitution on Relaxivity of Serum Albumin-Bound Gadolinium Complexes. Invest. Radiol 2010, 45, 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Jacques V; Dumas S; Sun W-C; Troughton JS; Greenfield MT; Caravan P High Relaxivity MRI Contrast Agents Part 2: Optimization of Inner- and Second-Sphere Relaxivity. Invest. Radiol 2010, 45, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Rudovský J; Botta M; Hermann P; Hardcastle KI; Lukeš I; Aime S PAMAM Dendrimeric Conjugates with a Gd–DOTA Phosphinate Derivative and Their Adducts with Polyaminoacids: The Interplay of Global Motion, Internal Rotation, and Fast Water Exchange. Bioconjugate Chem. 2006, 17, 975–987. [DOI] [PubMed] [Google Scholar]
- (312).Nicolle GM; Tóth É; Eisenwiener K-P; Mäcke HR; Merbach AE From Monomers to Micelles: Investigation of the Parameters Influencing Proton Relaxivity. J. Biol. Inorg. Chem 2002, 7, 757–769. [DOI] [PubMed] [Google Scholar]
- (313).Carniato F; Tei L; Dastru W; Marchese L; Botta M Relaxivity Modulation in Gd-Functionalised Mesoporous Silicas. Chem. Commun 2009, 0, 1246–1248. [DOI] [PubMed] [Google Scholar]
- (314).Yerly F; Borel A; Helm L; Merbach AE MD Simulations of Acyclic and Macrocyclic Gd3+-Based MRI Contrast Agents: Influence of the Internal Mobility on Water Proton Relaxivity. Chem. Eur. J 2003, 9, 5468–5480. [DOI] [PubMed] [Google Scholar]
- (315).Lipari G; Szabo A Model-Free Approach to the Interpretation of Nuclear Magnetic Resonance Relaxation in Macromolecules. 1. Theory and Range of Validity. J. Am. Chem. Soc 1982, 104, 4546–4559. [Google Scholar]
- (316).Port M; Meyer D; Bonnemain B; Corot C; Schaefer M; Rousseaux O; Simonot C; Bourrinet P; Benderbous S; Dencausse A, et al. P760 and P775: MRI Contrast Agents Characterized by New Pharmacokinetic Properties. Magn. Reson. Mater. Phy 1999, 8, 172–176. [DOI] [PubMed] [Google Scholar]
- (317).Fulton DA; O’Halloran M; Parker D; Senanayake K; Botta M; Aime S Efficient Relaxivity Enhancement in Dendritic Gadolinium Complexes: Effective Motional Coupling in Medium Molecular Weight Conjugates. Chem. Commun 2005, 0, 474–476. [DOI] [PubMed] [Google Scholar]
- (318).Jacques V; Desreux JF New Classes of MRI Contrast Agents In Contrast Agents I: Magnetic Resonance Imaging, Krause W, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 2002. [Google Scholar]
- (319).Livramento JB; Tóth É; Sour A; Borel A; Merbach AE; Ruloff R High Relaxivity Confined to a Small Molecular Space: A Metallostar-Based, Potential MRI Contrast Agent. Angew. Chem. Int. Ed 2005, 44, 1480–1484. [DOI] [PubMed] [Google Scholar]
- (320).Livramento JB; Weidensteiner C; Prata MIM; Allegrini PR; Geraldes CFGC; Helm L; Kneuer R; Merbach AE; Santos AC; Schmidt P, et al. First In Vivo MRI Assessment of a Self-Assembled Metallostar Compound Endowed with a Remarkable High Field Relaxivity. Contrast Media. Mol. Imag 2006, 1, 30–39. [DOI] [PubMed] [Google Scholar]
- (321).Zhang Z; Greenfield MT; Spiller M; McMurry TJ; Lauffer RB; Caravan P Multilocus Binding Increases the Relaxivity of Protein-Bound MRI Contrast Agents. Angew. Chem. Int. Ed 2005, 44, 6766–6769. [DOI] [PubMed] [Google Scholar]
- (322).Kielar F; Tei L; Terreno E; Botta M Large Relaxivity Enhancement of Paramagnetic Lipid Nanoparticles by Restricting the Local Motions of the Gd(III) Chelates. J. Am. Chem. Soc 2010, 132, 7836–7837. [DOI] [PubMed] [Google Scholar]
- (323).Filippi M; Remotti D; Botta M; Terreno E; Tei L GdDOTAGA(C18)2: An Efficient Amphiphilic Gd(III) Chelate for the Preparation of Self-Assembled High Relaxivity MRI Nanoprobes. Chem. Commun 2015, 51, 17455–17458. [DOI] [PubMed] [Google Scholar]
- (324).Boros E; Polasek M; Zhang Z; Caravan P Gd(DOTAla): A Single Amino Acid Gd-complex as a Modular Tool for High Relaxivity MR Contrast Agent Development. J. Am. Chem. Soc 2012, 134, 19858–19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Paris J; Gameiro C; Humblet V; Mohapatra PK; Jacques V; Desreux JF Auto-Assembling of Ditopic Macrocyclic Lanthanide Chelates with Transition-Metal Ions. Rigid Multimetallic High Relaxivity Contrast Agents for Magnetic Resonance Imaging. Inorg. Chem 2006, 45, 5092–5102. [DOI] [PubMed] [Google Scholar]
- (326).Song Y; Kohlmeir EK; Meade TJ Synthesis of Multimeric MR Contrast Agents for Cellular Imaging. J. Am. Chem. Soc 2008, 130, 6662–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Pierre VC; Botta M; Aime S; Raymond KN Fe(III)-Templated Gd(III) Self-AssembliesA New Route toward Macromolecular MRI Contrast Agents. J. Am. Chem. Soc 2006, 128, 9272–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (328).Mastarone DJ; Harrison VSR; Eckermann AL; Parigi G; Luchinat C; Meade TJ A Modular System for the Synthesis of Multiplexed Magnetic Resonance Probes. J. Am. Chem. Soc 2011, 133, 5329–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Livramento JB; Sour A; Borel A; Merbach AE; Tóth É A Starburst-Shaped Heterometallic Compound Incorporating Six Densely Packed Gd3+ Ions. Chem. Eur. J 2006, 12, 989–1003. [DOI] [PubMed] [Google Scholar]
- (330).Fulton DA; Elemento EM; Aime S; Chaabane L; Botta M; Parker D Glycoconjugates of Gadolinium Complexes for MRI Applications. Chem. Commun 2006, 0, 1064–1066. [DOI] [PubMed] [Google Scholar]
- (331).Troughton JS; Greenfield MT; Greenwood JM; Dumas S; Wiethoff AJ; Wang J; Spiller M; McMurry TJ; Caravan P Synthesis and Evaluation of a High Relaxivity Manganese(II)-Based MRI Contrast Agent. Inorg. Chem 2004, 43, 6313–6323. [DOI] [PubMed] [Google Scholar]
- (332).Aime S; Anelli PL; Botta M; Brocchetta M; Canton S; Fedeli F; Gianolio E; Terreno E Relaxometric Evaluation of Novel Manganese(II) Complexes for Application as Contrast Agents in Magnetic Resonance Imaging. J. Biol. Inorg. Chem 2002, 7, 58–67. [DOI] [PubMed] [Google Scholar]
- (333).Cheng W; Ganesh T; Martinez F; Lam J; Yoon H; Macgregor RB; Scholl TJ; Cheng H-LM; Zhang X.-a. Binding of a Dimeric Manganese Porphyrin to Serum Albumin: Towards a Gadolinium-Free Blood-Pool T 1 MRI Contrast Agent. J. Biol. Inorg. Chem 2014, 19, 229–235. [DOI] [PubMed] [Google Scholar]
- (334).Zhu J; Gale EM; Atanasova I; Rietz TA; Caravan P Hexameric MnII Dendrimer as MRI Contrast Agent. Chem. Eur. J 2014, 20, 14507–14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Tóth É; Pubanz D; Vauthey S; Helm L; Merbach AE The Role of Water Exchange in Attaining Maximum Relaxivities for Dendrimeric MRI Contrast Agents. Chem. Eur. J 1996, 2, 1607–1615. [Google Scholar]
- (336).Toth E; Burai L; Brucher E; Merbach E, Tuning A Water-Exchange Rates on (carboxymethyl)iminobis(ethylenenitrilo)tetraacetate (dtpa)-type Gadolinium(III) Complexes. J. Chem. Soc., Dalton Trans 1997, 1587–1594. [Google Scholar]
- (337).Laus S; Ruloff R; Tóth É; Merbach AE GdIII Complexes with Fast Water Exchange and High Thermodynamic Stability: Potential Building Blocks for High-Relaxivity MRI Contrast Agents. Chem. Eur. J 2003, 9, 3555–3566. [DOI] [PubMed] [Google Scholar]
- (338).Jaszberenyi Z; Sour A; Toth E; Benmelouka M; Merbach AE Fine-Tuning Water Exchange on GdIII Poly(Amino Carboxylates) by Modulation of Steric Crowding. Dalton Trans. 2005, 0, 2713–2719. [DOI] [PubMed] [Google Scholar]
- (339).Boros E; Gale EM; Caravan P MR Imaging Probes: Design and Applications. Dalton Trans. 2015, 44, 4804–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Rudovsky J; Kotek J; Hermann P; Lukes I; Mainero V; Aime S Synthesis of a Bifunctional Monophosphinic Acid DOTA Analogue Ligand and its Lanthanide(III) Complexes. A Gadolinium(III) Complex Endowed with an Optimal Water Exchange Rate for MRI Applications. Org. Biomol. Chem 2005, 3, 112–117. [DOI] [PubMed] [Google Scholar]
- (341).Polasek M; Rudovsky J; Hermann P; Lukes I; Elst LV; Muller RN Lanthanide(III) Complexes of a Pyridine N-Cxide Analogue of DOTA: Exclusive M Isomer Formation Induced by a Six-Membered Chelate Ring. Chem. Commun 2004, 0, 2602–2603. [DOI] [PubMed] [Google Scholar]
- (342).Boros E; Karimi S; Kenton N; Helm L; Caravan P Gd(DOTAlaP): Exploring the Boundaries of Fast Water Exchange in Gadolinium-Based Magnetic Resonance Imaging Contrast Agents. Inorg. Chem 2014, 53, 6985–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (343).Michael AB; Xin Y; Nicole FS Engineering Gd-Loaded Nanoparticles to Enhance MRI Sensitivity via T 1 Shortening. Nanotechnology 2013, 24, 462001–462020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (344).Ching-Hui H; Andrew T Gd-Based Macromolecules and Nanoparticles as Magnetic Resonance Contrast Agents for Molecular Imaging. Curr. Top. Med. Chem 2013, 13, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (345).Yang C-T; Padmanabhan P; Gulyas BZ Gadolinium(iii) Based Nanoparticles for T1-Weighted Magnetic Resonance Imaging Probes. RSC Advances 2016, 6, 60945–60966. [Google Scholar]
- (346).Nicolay K; Strijkers G; Grüll H Gd‐Containing Nanoparticles as MRI Contrast Agents In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, Merbach A; Helm L; Tóth É, Eds. John Wiley & Sons, Ltd: 2013. [Google Scholar]
- (347).Sitharaman B; Wilson LJ Gadofullerenes and Gadonanotubes: A New Paradigm for High-Performance Magnetic Resonance Imaging Contrast Agent Probes. J. Biomed. Nanotechnol 2007, 3, 342–352. [Google Scholar]
- (348).Sitharaman B; Kissell KR; Hartman KB; Tran LA; Baikalov A; Rusakova I; Sun Y; Khant HA; Ludtke SJ; Chiu W, et al. Superparamagnetic Gadonanotubes are High-Performance MRI Contrast Agents. Chem. Commun 2005, 0, 3915–3917. [DOI] [PubMed] [Google Scholar]
- (349).Manus LM; Mastarone DJ; Waters EA; Zhang X-Q; Schultz-Sikma EA; MacRenaris KW; Ho D; Meade TJ Gd(III)-Nanodiamond Conjugates for MRI Contrast Enhancement. Nano Lett. 2010, 10, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Wartenberg N; Fries P; Raccurt O; Guillermo A; Imbert D; Mazzanti M A Gadolinium Complex Confined in Silica Nanoparticles as a Highly Efficient T1/T2 MRI Contrast Agent. Chem. Eur. J 2013, 19, 6980–6983. [DOI] [PubMed] [Google Scholar]
- (351).Courant T; Roullin VG; Cadiou C; Callewaert M; Andry MC; Portefaix C; Hoeffel C; Goltstein M. C. d.; Port M; Laurent S, et al. Hydrogels Incorporating GdDOTA: Towards Highly Efficient Dual T1/T2 MRI Contrast Agents. Angew. Chem. Int. Ed 2012, 51, 9119–9122. [DOI] [PubMed] [Google Scholar]
- (352).Balogh E; Tripier R; Fouskova P; Reviriego F; Handel H; Toth E Monopropionate Analogues of DOTA4- and DTPA5-: Kinetics of Formation and Dissociation of their Lanthanide(III) Complexes. Dalton Trans. 2007, 3572–3581. [DOI] [PubMed] [Google Scholar]
- (353).Clarke ET; Martell AE Stabilities of Trivalent Metal Ion Complexes of the Tetraacetate Derivatives of 12-, 13- and 14-Membered Tetraazamacrocycles. Inorg. Chim. Acta 1991, 190, 37–46. [Google Scholar]
- (354).Balogh E; Tripier R; Ruloff R; Toth E Kinetics of Formation and Dissociation of Lanthanide(III) Complexes with the 13-Membered Macrocyclic Ligand TRITA4. Dalton Trans. 2005, 1058–1065. [DOI] [PubMed] [Google Scholar]
- (355).Pálinkás Z; Roca-Sabio A; Mato-Iglesias M; Esteban-Gómez D; Platas-Iglesias C; de Blas A; Rodríguez-Blas T; Tóth É Stability, Water Exchange, and Anion Binding Studies on Lanthanide(III) Complexes with a Macrocyclic Ligand Based on 1,7-Diaza-12-crown-4: Extremely Fast Water Exchange on the Gd3+ Complex. Inorg. Chem 2009, 48, 8878–8889. [DOI] [PubMed] [Google Scholar]
- (356).Tircsó G; Regueiro-Figueroa M; Nagy V; Garda Z; Garai T; Kálmán FK; Esteban-Gómez D; Tóth É; Platas-Iglesias C Approaching the Kinetic Inertness of Macrocyclic Gadolinium(III)-Based MRI Contrast Agents with Highly Rigid Open-Chain Derivatives. Chem. Eur. J 2016, 22, 896–901. [DOI] [PubMed] [Google Scholar]
- (357).Rodríguez-Rodríguez A; Esteban-Gómez D; Tripier R; Tircsó G; Garda Z; Tóth I; de Blas A; Rodríguez-Blas T; Platas-Iglesias C Lanthanide(III) Complexes with a Reinforced Cyclam Ligand Show Unprecedented Kinetic Inertness. J. Am. Chem. Soc 2014, 136, 17954–17957. [DOI] [PubMed] [Google Scholar]
- (358).Dai L; Jones CM; Chan WTK; Pham TA; Ling X; Gale EM; Rotile NJ; Tai WC-S; Anderson CJ; Caravan P, et al. Chiral DOTA Chelators as an Improved Platform for Biomedical Imaging and Therapy Applications. Nat. Commun 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (359).Bolskar RD Gadofullerene MRI Contrast Agents. Nanomedicine 2008, 3, 201–213. [DOI] [PubMed] [Google Scholar]
- (360).Chen Z; Ma L; Liu Y; Chen C Applications of Functionalized Fullerenes in Tumor Theranostics. Theranostics 2012, 2, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Aihara J -i. Kinetic Stability of Metallofullerenes as Predicted by the Bond Resonance Energy Model. Phys. Chem. Chem. Phys 2001, 3, 1427–1431. [Google Scholar]
- (362).Drahoš B; Lukeš I; Tóth E Mn(II) Complexes as Potential Contrast Agents for MRI. Eur. J. Inorg. Chem 2012, 2012, 1975–1986. [Google Scholar]
- (363).Kálmán FK; Tircsó G Kinetic Inertness of the Mn Complexes Formed with AAZTA and Some Open-Chain EDTA Derivatives. Inorg. Chem 2012, 51, 10065–10067. [DOI] [PubMed] [Google Scholar]
- (364).Rolla GA; Platas-Iglesias C; Botta M; Tei L; Helm L 1H and 17O NMR Relaxometric and Computational Study on Macrocyclic Mn(II) Complexes. Inorg. Chem 2013, 52, 3268–3279. [DOI] [PubMed] [Google Scholar]
- (365).Drahoš B; Pniok M; Havlíčková J; Kotek J; Císařová I; Hermann P; Lukeš I; Tóth E Mn2+ Complexes of 1-oxa-4,7-Diazacyclononane Based Ligands with Acetic, Phosphonic, and Phosphinic Acid Pendant Arms: Stability and Relaxation Studies. Dalton Trans. 2011, 30, 10131–10146. [DOI] [PubMed] [Google Scholar]
- (366).Drahoš B; Kotek J; Císařová I; Hermann P; Helm L; Lukeš I; Tóth E Mn2+ Complexes with 12-member Pyridine Based Macrocycles Bearing Carboxylate or Phosphonate Pendant Arm: Crystallographic, Thermodynamic, Kinetic, Redox and 1H/17O Relaxation Studies. Inorg. Chem 2011, 50, 12785–12801. [DOI] [PubMed] [Google Scholar]
- (367).Drahoš B; Kotek J; Hermann P; Lukeš I; Tóth E Mn2+ Complexes with Pyridine Containing 15-Membered Macrocycles: Thermodynamic, Kinetic, Crystallographic and 1H/17O Relaxation Studies. Inorg. Chem 2010, 49, 3224–3238. [DOI] [PubMed] [Google Scholar]
- (368).Tei L; Gugliotta G; Fekete M; Kálmán FK; Botta M Mn(II) Complexes of Novel Hexadentate AAZTA-like Chelators: A Solution Thermodynamics and Relaxometric Study. Dalton Trans. 2011, 40, 2025–2032. [DOI] [PubMed] [Google Scholar]
- (369).Su H; Wu C; Zhu J; Miao T; Wang D; Xia C; Zhao X; Gong Q; Song B; Ai H Rigid Mn(II) Chelate as Efficient MRI Contrast Agent for Vascular Imaging. Dalton Trans. 2012, 41, 14480–14483. [DOI] [PubMed] [Google Scholar]
- (370).Rocklage SM; Cacheris WP; Quay SC; Hahn FE; Raymond KN Manganese(II) N,N’-dipyrodoxylethelynediamine-N,N’-diacetate 5,5’-bis(phosphate). Synthesis and Characterization of a Paramagnetic Chelate for Magnetic Resonance Imaging Enhancement. Inorg. Chem 1989, 28, 477–485. [Google Scholar]
- (371).Gale EM; Mukherjee S; Liu C; Loving GS; Caravan P Structure-Redox-Relaxivity Relationships for Redox Responsive Manganese-Based Magnetic Resonance Imaging Probes. Inorg. Chem 2014, 53, 10748–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Bianchi A; Calabi L; Giorgi C; Losi P; Mariani P; Palano D; Paoli P; Rossi P; Valtancoli B Thermodynamic and Structural Aspects of Manganese(II) Complexes with Polyaminopolycarboxylic Ligands Based upon 1,4,7,10- tetraazacyclododecane (cyclen). Crystal Structure of Dimeric [MnL]2–2CH3OH Containing the New Ligand 1,4,7,10-Tetraaza- Cyclododecane-1,4-Diacetate. J. Chem. Soc., Dalton Trans 2001, 0, 917–922. [Google Scholar]
- (373).Cortes S; Brücher E; Geraldes CFGC; Sherry AD Potentiometry and NMR Studies of 1,5,9-Triazacyclododecane-N,N’,N’’-Triacetic Acid and its Metal Ion Complexes. Inorg. Chem 1990, 29, 5–9. [Google Scholar]
- (374).Drahoš B; Kubíček V; Bonnet CS; Hermann P; Lukeš I; Tóth E Dissociation kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951. [DOI] [PubMed] [Google Scholar]
- (375).de Sá A; Bonnet CS; Geraldes CFGC; Tóth É; Ferreira PMT; André JP Thermodynamic Stability and Relaxation Studies of Small, Triaza-Macrocyclic Mn(II) Chelates. Dalton Trans. 2013, 42, 4522–4532. [DOI] [PubMed] [Google Scholar]
- (376).Balogh E; He Z; Hsieh W; Liu S; Tóth E Dinuclear Complexes Formed with the Triazacyclononane Derivative ENOTA4-: High-Pressure 17O NMR Evidence of an Associative Water Exchange on [Mn(II)2(ENOTA)(H2O)2]. Inorg. Chem 2007, 46, 238–250. [DOI] [PubMed] [Google Scholar]
- (377).Gale EM; Atanasova I; Blasi F; Ay I; Caravan P A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc 2015, 137, 15548–15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (378).Gale EM; Wey HY; Ramsay I; Yen YF; Sosnovik D; Caravan P A Manganese-Based Alternative to Gadolinium: Contrast Enhanced MR Angiography, Pharmacokinetics, and Metabolism. Radiology 2018, 286, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Koenig SH; Brown RD III; Spiller M The Anamolous Relaxivity of Mn3+(TPPS4). Magn. Reson. Med 1987, 4, 252–260. [DOI] [PubMed] [Google Scholar]
- (380).Barandov A; Bartelle BB; Gonzalez BA; White WL; Lippard SJ; Jasanoff A Membrane-Permeable Mn(III) Complexes for Molecular Magnetic Resonance Imaging of Intracellular Targets. J. Am. Chem. Soc 2016, 138, 5483–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (381).Dees A; Zahl A; Puchta R; van Eikema Hommes NJR; Heinemann FW; Ivanović-Burmazović Water Exchange on Seven-Coordinate Mn(II) Complexes with Macrocyclic Pentadentate Ligands: Insight in the Mechanism of Mn(II) SOD Mimetics. Inorg. Chem 2007, 46, 2459–2470. [DOI] [PubMed] [Google Scholar]
- (382).Aime S; Botta M; Gianolio E; Terreno E A p(O2)-Responsive MRI Contrast Agent Based on the Redox Switch of Manganese(II / III) – Porphyrin Complexes. Angew. Chem. Int. Ed 2000, 39, 747–750. [DOI] [PubMed] [Google Scholar]
- (383).Cheng HLM; Haedicke IE; Cheng WR; Nofiele JT; Zhang XA Gadolinium-Free T-1 Contrast Agents for MRI: Tunable Pharmacokinetics of a New Class of Manganese Porphyrins. J. Magn. Reson. Imaging 2014, 40, 1474–1480. [DOI] [PubMed] [Google Scholar]
- (384).Cheng W; Haedicke IE; Nofiele J; Martinez F; Beera K; Scholl TJ; Cheng H-LM; Zhang X.-a. Complementary Strategies for Developing Gd-Free High-Field T1 MRI Contrast Agents Based on Mn(III) Porphyrins. J. Med. Chem 2014, 57, 516–520. [DOI] [PubMed] [Google Scholar]
- (385).Cheng W; Ganesh T; Martinez F; Lam J; Yoon H; Macgregor RB; Scholl TJ; Cheng H-LM; Zhang X.-a. Binding of a Dimeric Manganese Porphyrin to Serum Albumin: Towards a Gadolinium-Free Blood-Pool T-1 MRI Contrast Agent. J. Biol. Inorg. Chem 2014, 19, 229–235. [DOI] [PubMed] [Google Scholar]
- (386).Schmiedl UP; Nelson JA; Starr FL; Schmidt R Hepatic Contrast-Enhancing Properties of Manganese-Mesoporphyrin and Manganese-TPPS4. A Comparative Magnetic Resonance Imaging Study in Rats. Invest. Radiol 1992, 27, 536–542. [DOI] [PubMed] [Google Scholar]
- (387).Schmiedl UP; Nelson JA; Robinson DH; Michalson A; Starr F; Frenzel T; Ebert W; Schumanngiampieri G Pharmaceutical Properties, Biodistribution, and Imaging Characteristics of Manganese-Mesoporphyrin. A Potential Hepatobiliary Contrast Agent for Magnetic Resonance Imaging. Invest. Radiol 1993, 28, 925–932. [DOI] [PubMed] [Google Scholar]
- (388).Zhang Z; He R; Yan K; Guo QN; Lu YG; Wang XX; Lei H; Li ZY Synthesis and In Vitro and In Vivo Evaluation of Manganese(III) Porphyrin-Dextran as a Novel MRI Contrast Agent. Bioorg. Med. Chem. Lett 2009, 19, 6675–6678. [DOI] [PubMed] [Google Scholar]
- (389).Boehm-Sturm P; Haeckel A; Hauptmann R; Mueller S; Kuhl CK; Schellenberger EA Low-Molecular-Weight Iron Chelates May Be an Alternative to Gadolinium-Based Contrast Agents for T1-Weighted Contrast-Enhanced MR Imaging. Radiology 2018, 286, 537–546. [DOI] [PubMed] [Google Scholar]
- (390).Lauffer RB; Greif WL; Stark DD; Vincent AC; Saini S; Wedeen VJ; Brady TJ Iron-EHPG as an Hepatobilliary MR Contrast Agent: Initial Imaging and Biodistribution Studies. J. Comput. Assist. Tomogr 1985, 9, 431–438. [DOI] [PubMed] [Google Scholar]
- (391).Larsen SK; Jenkins BG; Memon NG; Lauffer RB Structure-Affinity Relationships in the Binding of Unsubstituted Iron Phenolate Complexes to Human Serum Albumin. Molecular Structure of Iron(III) N, N’-bis(2-hydroxybenzyl)ethylenediamine-N,N’-diacetate. Inorg. Chem 1990, 29, 1147–1152. [Google Scholar]
- (392).Jenkins BG; Armstrong E; Lauffer RB Site-Specific Water Proton Relaxation Enhancement of Iron(III) Chelates Noncovalently Bound to Human Serum Albumin. Magn. Reson. Med 1991, 17, 164–178. [DOI] [PubMed] [Google Scholar]
- (393).Touti F; Singh AK; Maurin P; Canaple L; Beuf O; Samarut J; Hasserodt J An Electroneutral Macrocyclic Iron(II) Complex That Enhances MRI Contrast in Vivo. J. Med. Chem 2011, 54, 4274–4278. [DOI] [PubMed] [Google Scholar]
- (394).Wang J; Gondrand C; Touti F; Hasserodt J A Pair of Highly Biotolerated Diamagnetic and Paramagnetic Iron(II) Complexes Displaying Electroneutrality. Dalton Trans. 2015, 44, 15391–15395. [DOI] [PubMed] [Google Scholar]
- (395).Caravan P; Tóth E; Rockenbauer A; Merbach AE Nuclear and Electronic Relaxation of Eu2+(aq): An Extremely Labile Aqua Ion. J. Am. Chem. Soc 1999, 121, 10403–10409. [Google Scholar]
- (396).Caravan P; Merbach AE An Extreme Water Exchange Rate: The Europium(II) Aqua Ion. Chem. Commun 1997, 0, 2147–2148. [Google Scholar]
- (397).Seibig S; Toth E; Merbach AE Unexpected Differences in the Dynamics and in the Nuclear and Electronic Relaxation Properties of the Isoelectronic Eu-II(DTPA)(H2O) (3-) and Gd-III(DTPA)(H2O) (2-) complexes (DTPA = diethylenetriamine pentaacetate). J. Am. Chem. Soc 2000, 122, 5822–5830. [Google Scholar]
- (398).Burai L; Toth E; Seibig S; Scopelliti R; Merbach AE High-Pressure NMR Kinetics, Part 95 - Solution and Solid-State Characterization of Eu-II Chelates: A Possible Route Towards Redox Responsive MRI Contrast Agents. Chem. Eur. J 2000, 6, 3761–3770. [DOI] [PubMed] [Google Scholar]
- (399).Burai L; Scopelliti R; Toth E Eu-II-Cryptate with Optimal Water Exchange and Electronic Relaxation: A Synthon for Potential pO(2) Responsive Macromolecular MRI Contrast Agents. Chem. Commun 2002, 0, 2366–2367. [DOI] [PubMed] [Google Scholar]
- (400).Gamage NDH; Mei YJ; Garcia J; Allen MJ Oxidatively Stable, Aqueous Europium(II) Complexes through Steric and Electronic Manipulation of Cryptand Coordination Chemistry. Angew. Chem. Int. Ed 2010, 49, 8923–8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Garcia J; Kuda-Wedagedara ANW; Allen MJ Physical Properties of Eu2+-Containing Cryptates as Contrast Agents for Ultrahigh-Field Magnetic Resonance Imaging. Eur. J. Inorg. Chem 2012, 2012, 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (402).Kuda-Wedagedara ANW; Allen MJ Enhancing Magnetic Resonance Imaging with Contrast Agents for Ultra-High Field Strengths. Analyst 2014, 139, 4401–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (403).Garcia J; Neelavalli J; Haacke EM; Allen MJ Eu-II-Containing Cryptates as Contrast Agents for Ultra-High Field Strength Magnetic Resonance Imaging. Chem. Commun 2011, 47, 12858–12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Laurent S; Vander Elst L; Henoumon C; Muller RN How to Measure the Transmetallation of a Gadolinium Complex. Contrast Media. Mol. Imag 2010, 5, 305–308. [DOI] [PubMed] [Google Scholar]
- (405).Ekanger LA; Ali MM; Allen MJ Oxidation-Responsive Eu2+/3+-Liposomal Contrast Agent for Dual-Mode Magnetic Resonance Imaging. Abstr. Pap. Am. Chem. Soc 2014, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (406).Ekanger LA; Polin LA; Shen Y; Haacke EM; Martin PD; Allen MJ A EuII-Containing Cryptate as aRedoxSensor in Magnetic ResonanceImaging of Living Tissue. Angew. Chem. Int. Ed 2015, 54, 14398–14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Basal LA; Yan Y; Shen YM; Haacke EM; Mehrmohammadi M; Allen MJ Oxidation-Responsive, Eu-II/III-Based, Multimodal Contrast Agent for Magnetic Resonance and Photoacoustic Imaging. ACS Omega 2017, 2, 800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (408).Ekanger LA; Polin LA; Shen YM; Haacke EM; Allen MJ Evaluation of Eu-II-Based Positive Contrast Enhancement after Intravenous, Intraperitoneal, and Subcutaneous Injections. Contrast Media. Mol. Imag 2016, 11, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (409).Ekanger LA; Basal LA; Allen MJ The Role of Coordination Environment and pH in Tuning the Oxidation Rate of Europium(II). Chem. Eur. J 2017, 23, 1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Lenora CU; Carniato F; Shen YM; Latif Z; Haacke EM; Martin PD; Botta M; Allen MJ Structural Features of Europium(II)-Containing Cryptates That Influence Relaxivity. Chem. Eur. J 2017, 23, 15404–15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (411).Lovin J; Wesbey G; Engelstad B; Brasch R; Sosnovsky G; Moseley M; McNamara M; Ehman R Structure-Relaxivity Relationships for Piperidine Nitroxide Spin Labels - MRI Contrast Agents. Invest. Radiol 1984, 19, 24–24. [Google Scholar]
- (412).Vallet P; Vanhaverbeke Y; Bonnet PA; Subra G; Chapat JP; Muller RN Relaxivity Enhancement of Low Molecular Weight Nitroxide Stable Free Radicals: Importance of Structure and Medium. Magn. Reson. Med 1994, 32, 11–15. [DOI] [PubMed] [Google Scholar]
- (413).Winalski CS; Shortkroff S; Mulkern RV; Schneider E; Rosen GM Magnetic Resonance Relaxivity of Dendrimer-Linked Nitroxides. Magn. Reson. Med 2002, 48, 965–972. [DOI] [PubMed] [Google Scholar]
- (414).Emoto MC; Yamada K; Yamato M; Fujii HG Novel Ascorbic Acid-Resistive Nitroxide in a Lipid Emulsion: An Efficient Brain Imaging Contrast Agent for MRI of small rodents. Neurosci. Lett 2013, 546, 11–15. [DOI] [PubMed] [Google Scholar]
- (415).Bye N; Hutt OE; Hinton TM; Acharya DP; Waddington LJ; Moffat BA; Wright DK; Wang HX; Mulet X; Muir BW Nitroxide-Loaded Hexosomes Provide MRI Contrast in Vivo. Langmuir 2014, 30, 8898–8906. [DOI] [PubMed] [Google Scholar]
- (416).Hyodo F; Matsumoto K; Matsumoto A; Mitchell JB; Krishna MC Probing The Intracellular Redox Status of Tumors with Magnetic Resonance Imaging and Redox-Sensitive Contrast Agents. Cancer Res. 2006, 66, 9921–9928. [DOI] [PubMed] [Google Scholar]
- (417).Davis RM; Matsumoto S; Bernardo M; Sowers A; Matsumoto KI; Krishna MC; Mitchell JB Magnetic Resonance Imaging of Organic Contrast Agents in Mice: Capturing the Whole-Body Redox Landscape. Free Radic. Biol. Med 2011, 50, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (418).Bakalova R; Zhelev Z; Aoki I; Saga T Tissue Redox Activity as a Hallmark of Carcinogenesis: From Early to Terminal Stages of Cancer. Clin. Cancer Res 2013, 19, 2503–2517. [DOI] [PubMed] [Google Scholar]
- (419).Zhelev Z; Aoki I; Gadjeva V; Nikolova B; Bakalova R; Saga T Tissue Redox Activity as a Sensing Platform for Imaging of Cancer Based on Nitroxide Redox Cycle. Eur. J. Cancer 2013, 49, 1467–1578. [DOI] [PubMed] [Google Scholar]
- (420).Zhelev Z; Bakalova R; Aoki I; Lazarova D; Saga T Imaging of Superoxide Generation in the Dopaminergic Area of the Brain in Parkinson’s Disease, Using Mito-TEMPO. ACS Chem. Neurosci 2013, 4, 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Vianello F; Momo F; Scarpa M; Rigo A Kinetics of Nitroxide Spin Label Removal in Biological Systems: An In Vitro And In Vivo ESR Study. Magn. Reson. Imaging 1995, 13, 219–226. [DOI] [PubMed] [Google Scholar]
- (422).Rajca A; Wang Y; Boska M; Paletta JT; Olankitwanit A; Swanson MA; Mitchell DG; Eaton SS; Eaton GR; Rajca S Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc 2012, 134, 15724–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Nguyen HVT; Chen QX; Paletta JT; Harvey P; Jiang Y; Zhang H; Boska MD; Ottaviani MF; Jasanoff A; Rajca A, et al. Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Central Sci. 2017, 3, 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (424).Weissleder R; Stark DD; Engelstad BL; Bacon BR; Compton CC; White DL; Jacobs P; Lewis J Superparamagnetic Iron Oxide: Pharmacokinetics and Toxicity. Am. J. Roentgenol 1989, 152, 167–173. [DOI] [PubMed] [Google Scholar]
- (425).Chouly C; Pouliquen D; Lucet I; Jeune JJ; Jallet P Development of Superparamagnetic Nanoparticles for MRI: Effect of Particle Size, Charge and Surface Nature on Biodistribution. J. Microencapsul 1996, 13, 245–255. [DOI] [PubMed] [Google Scholar]
- (426).Gale EM; Caravan P; Rao AG; McDonald RJ; Winfield M; Fleck RJ; Gee MS Gadolinium-Based Contrast Agents in Pediatric Magnetic Resonance Imaging. Pediatr. Radiol 2017, 47, 507–521. [DOI] [PubMed] [Google Scholar]
- (427).Reimer P; Muller M; Marx C; Wiedermann D; Muller R; Rummeny EJ; Ebert W; Shamsi K; Peters PE T1 Effects of a Bolus-Injectable Superparamagnetic Iron Oxide, SH U 555 A: Dependence on Field Strength and Plasma Concentration Preliminary Clinical Experience with Dynamic T1-Weighted MR Imaging. Radiology 1998, 209, 831–836. [DOI] [PubMed] [Google Scholar]
- (428).Heesakkers RAM; Jager GJ; Hövels AM; de Hoop B; van den Bosch HCM; Raat F; Witjes JA; Mulders PFA; van der Kaa CH; Barentsz JO Prostate Cancer: Detection of Lymph Node Metastases Outside the Routine Surgical Area with Ferumoxtran-10 - Enhanced MR Imaging. Radiology 2009, 251, 408–414. [DOI] [PubMed] [Google Scholar]
- (429).Weissleder R; Nahrendorf M; Pittet MJ Imaging Macrophages with Nanoparticles. Nat. Mater 2014, 13, 125–138. [DOI] [PubMed] [Google Scholar]
- (430).Corot C; Petry KG; Trivedi R; Saleh A; Jonkmanns C; Le Bas JF; Blezer E; Rausch M; Brochet B; Foster-Gareau P, et al. Macrophage Imaging in Central Nervous System and in Carotid Atherosclerotic Plaque Using Ultrasmall Superparamagnetic Iron Oxide in Magnetic Resonance Imaging. Invest. Radiol 2004, 39, 619–625. [DOI] [PubMed] [Google Scholar]
- (431).Neuwelt A; Sidhu N; Hu CAA; Mlady G; Eberhardt SC; Sillerud LO Iron-Based Superparamagnetic Nanoparticle Contrast Agents for MRI of Infection and Inflammation. Am. J. Roentgenol 2015, 204, W302–W313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (432).Kooi ME; Cappendijk VC; Cleutjens KB; Kessels AG; Kitslaar PJ; Borgers M; Frederik PM; Daemen MJ; van Engelshoven JM Accumulation of Ultrasmall Superparamagnetic Particles of Iron Oxide in Human Atherosclerotic Plaques can be Detected by In Vivo Magnetic Resonance Imaging. Circulation 2003, 107, 2453–2458. [DOI] [PubMed] [Google Scholar]
- (433).Taupitz M; Schmitz SA; Beyersdorff D; Wagner S; Hamm Iii BK Magnetic Resonance Imaging of Atherosclerotic Plaques Using Superparamagnetic Iron Oxide Particles. Radiology 2000, 217, 286–286. [DOI] [PubMed] [Google Scholar]
- (434).Schmitz SA; Taupitz M; Wagner S; Wolf KJ; Beyersdorff D; Hamm B Magnetic Resonance Imaging of Atherosclerotic Plaques Using Superparamagnetic Iron Oxide Particles. J. Magn. Reson. Imaging 2001, 14, 355–361. [DOI] [PubMed] [Google Scholar]
- (435).Saleh A; Schroeter M; Ringelstein A; Hartung HP; Siebler M; Modder U; Jander S Iron Oxide Particle-Enhanced MRI Suggests Variability of Brain Inflammation at Early Stages after Ischemic Stroke. Stroke 2007, 38, 2733–2737. [DOI] [PubMed] [Google Scholar]
- (436).Nighoghossian N; Wiart M; Cakmak S; Berthezene Y; Derex L; Cho TH; Nemoz C; Chapuis F; Tisserand GL; Pialat JB, et al. Inflammatory Response after Ischemic Stroke - A USPIO-Enhanced MRI Study in Patients. Stroke 2007, 38, 303–307. [DOI] [PubMed] [Google Scholar]
- (437).Alam SR; Shah ASV; Richards J; Lang NNN; Barnes G; Joshi N; MacGillivray T; McKillop G; Mirsadraee S; Payne J, et al. Ultrasmall Superparamagnetic Particles of Iron Oxide in Patients With Acute Myocardial Infarction Early Clinical Experience. Circ. Cardiovasc. Imaging 2012, 5, 559–565. [DOI] [PubMed] [Google Scholar]
- (438).Bashir MR; Bhatti L; Marin D; Nelson RC Emerging Applications for Ferumoxytol as a Contrast Agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [DOI] [PubMed] [Google Scholar]
- (439).Stabi KL; Bendz LM Ferumoxytol Use as an Intravenous Contrast Agent for Magnetic Resonance Angiography. Ann. Pharmacother 2011, 45, 1571–1575. [DOI] [PubMed] [Google Scholar]
- (440).Bashir MR; Jaffe TA; Brennan TV; Patel UD; Ellis MJ Renal Transplant Imaging Using Magnetic Resonance Angiography With a Nonnephrotoxic Contrast Agent. Transplantation 2013, 96, 91–96. [DOI] [PubMed] [Google Scholar]
- (441).Sigovan M; Gasper W; Alley HF; Owens CD; Saloner D USPIO-Enhanced MR Angiography of Arteriovenous Fistulas in Patients with Renal Failure. Radiology 2012, 265, 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (442).Hansch A; Betge S; Poehlmann G; Neumann S; Baltzer P; Pfeil A; Waginger M; Boettcher J; Kaiser WA; Wolf G, et al. Combined Magnetic Resonance Imaging of Deep Venous Thrombosis and Pulmonary Arteries after a Single Injection of a Blood Pool Contrast Agent. Eur. Radiol 2011, 21, 318–325. [DOI] [PubMed] [Google Scholar]
- (443).Thompson EM; Guillaume DJ; Dósa E; Li X; Nazemi KJ; Gahramanov S; Hamilton BE; Neuwelt EA Dual Contrast Perfusion MRI in a Single Imaging Session for Assessment of Pediatric Brain Tumors. J. Neurooncol 2012, 109, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (444).Wei H; Bruns OT; Kaul MG; Hansen EC; Barch M; Wisniowska A; Chen O; Chen Y; Li N; Okada S, et al. Exceedingly Small Iron Oxide Nanoparticles as Positive MRI Contrast Agents. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (445).Bernd H; De Kerviler E; Gaillard S; Bonnemain B Safety and Tolerability of Ultrasmall Superparamagnetic Iron Oxide Contrast Agent Comprehensive Analysis of a Clinical Development Program. Invest. Radiol 2009, 44, 336–342. [DOI] [PubMed] [Google Scholar]
- (446).Schiller B; Bhat P; Sharma A Safety and Effectiveness of Ferumoxytol in Hemodialysis Patients at 3 Dialysis Chains in the United States Over a 12-Month Period. Clin. Ther 2014, 36, 70–83. [DOI] [PubMed] [Google Scholar]
- (447).Unterweger H; Janko C; Schwarz M; Dezsi L; Urbanics R; Matuszak J; Orfi E; Fulop T; Bauerle T; Szebeni J, et al. Non-Immunogenic Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles: A Biocompatible, Size-Tunable Contrast Agent for Magnetic Resonance Imaging. Int. J. Nanomed 2017, 12, 5223–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (448).Singh N; Jenkins GJS; Asadi R; Doak SH Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev. 2010, 1, 5358–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (449).Aime S; Calabi L; Biondi L; De Miranda M; Ghelli S; Paleari L; Rebaudengo C; Terreno E Iopamidol: Exploring the Potential Use of a Well-Established X-ray Contrast Agent for MRI. Magn. Reson. Med 2005, 53, 830–834. [DOI] [PubMed] [Google Scholar]
- (450).Anemone A; Consolino L; Longo DL MRI-CEST Assessment of Tumour Perfusion Using X-ray Iodinated Agents: Comparison with a Conventional Gd-Based Agent. Eur. Radiol 2017, 27, 2170–2179. [DOI] [PubMed] [Google Scholar]
- (451).Ratnakar SJ; Woods M; Lubag AJM; Kovács Z; Sherry AD Modulation of Water Exchange in Europium(III) DOTA-Tetraamide Complexes Via Electronic Substituent Effects. J. Am. Chem. Soc 2008, 130, 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (452).Woods M; Pasha A; Zhao PY; Tircso G; Chowdhury S; Kiefer G; Woessner DE; Sherry AD Investigations into Whole Water, Prototropic and Amide Proton Exchange in Lanthanide(III) DOTA-Tetraamide Chelates. Dalton Trans. 2011, 40, 6759–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (453).Sherry AD; Woods M Chemical Exchange Saturation Transfer Contrast Agents for Magnetic Resonance Imaging. Annu. Rev. Biomed. Eng 2008, 10, 391–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).Dorazio SJ; Olatunde AO; Spernyak JA; Morrow JR CoCEST: Cobalt(II) Amide-Appended ParaCEST MRI Contrast Agents. Chem. Commun 2013, 49, 10025–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Dorazio SJ; Tsitovich PB; Siters KE; Spernyak JA; Morrow JR Iron(II) PARACEST MRI Contrast Agents. J. Am. Chem. Soc 2011, 133, 14154–14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (456).Nwe K; Andolina CM; Hang CH; Morrow JR PARACEST Properties of a Dinuclear Neodymium(III) Complex Bound to DNA or Carbonate. Bioconjugate Chem. 2009, 20, 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (457).Olatunde AO; Bond CJ; Dorazio SJ; Cox JM; Benedict JB; Daddario MD; Spernyak JA; Morrow JR Six, Seven or Eight Coordinate Fe-II, Co-II or Ni-II Complexes of Amide-Appended Tetraazamacrocycles for ParaCEST Thermometry. Chem. Eur. J 2015, 21, 18290–18300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (458).Olatunde AO; Dorazio SJ; Spernyak JA; Morrow JR The NiCEST Approach: Nickel(II) ParaCEST MRI Contrast Agents. J. Am. Chem. Soc 2012, 134, 18503–18505. [DOI] [PubMed] [Google Scholar]
- (459).Du K; Harris TD A Cu-2(II) Paramagnetic Chemical Exchange Saturation Transfer Contrast Agent Enabled by Magnetic Exchange Coupling. J. Am. Chem. Soc 2016, 138, 7804–7807. [DOI] [PubMed] [Google Scholar]
- (460).Ali MM; Woods M; Suh EH; Kovacs Z; Tircso G; Zhao PY; Kodibagkar VD; Sherry AD Albumin-Binding PARACEST Agents. J. Biol. Inorg. Chem 2007, 12, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (461).Pasha A; Lin M; Tircso G; Rostollan CL; Woods M; Kiefer GE; Sherry AD; Sun X Synthesis and Evaluation of Lanthanide Ion DOTA-Tetraamide Complexes Bearing Peripheral Hydroxyl Groups. J. Biol. Inorg. Chem 2009, 14, 421–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (462).Vymazal J; Spuentrup E; Cardenas-Molina G; Wiethoff AJ; Hartmann MG; Caravan P; Parsons EC Jr. Thrombus Imaging with Fibrin-Specific Gadolinium-Based MR Contrast Agent EP-2104R. Invest. Radiol 2009, 44, 697–704. [DOI] [PubMed] [Google Scholar]
- (463).Spuentrup E; Botnar RM; Wiethoff AJ; Ibrahim T; Kelle S; Katoh M; Ozgun M; Nagel E; Vymazal J; Graham PB, et al. MR Imaging of Thrombi Using EP-2104R, a Fibrin-Specific Contrast Agent: Initial Results in Patients. Eur. Radiol 2008, 18, 1995–2005. [DOI] [PubMed] [Google Scholar]
- (464).Kolodziej AF; Nair SA; Graham P; McMurry TJ; Ladner RC; Wescott C; Sexton DJ; Caravan P Fibrin Specific Peptides Derived by Phage Display: Characterization of Peptides and Conjugates for Imaging. Bioconjugate Chem. 2012, 23, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (465).Kolodziej AF; Zhang Z; Overoye-Chan K; Jacques V; Caravan P Peptide Optimization and Conjugation Strategies in the Development of Molecularly Targeted Magnetic Resonance Imaging Contrast Agents. Methods Mol. Biol 2014, 1088, 185–211. [DOI] [PubMed] [Google Scholar]
- (466).Overoye-Chan K; Koerner S; Looby RJ; Kolodziej AF; Zech SG; Deng Q; Chasse JM; McMurry TJ; Caravan P EP-2104R: a Fibrin-Specific Gadolinium-Based MRI Contrast Agent for Detection of Thrombus. J. Am. Chem. Soc 2008, 130, 6025–6039. [DOI] [PubMed] [Google Scholar]
- (467).Zhang Z; Kolodziej AF; Qi J; Nair SA; Wang X; Case AW; Greenfield MT; Graham PB; McMurry TJ; Caravan P Effect of Peptide-Chelate Architecture on Metabolic Stability of Peptide-Based MRI Contrast Agents. New J. Chem 2010, 10, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (468).Uppal R; Ay I; Dai G; Kim YR; Sorenson AG; Caravan P Molecular MRI of Intracranial Thrombus in a Rat Ischemic Stroke Model. Stroke 2010, 41, 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (469).Uppal R; Medarova Z; Farrar CT; Dai G; Moore A; Caravan P Molecular Imaging of Fibrin in a Breast Cancer Xenograft Mouse Model. Invest. Radiol 2012, 47, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (470).Winter PM; Caruthers SD; Yu X; Song SK; Chen J; Miller B; Bulte JW; Robertson JD; Gaffney PJ; Wickline SA, et al. Improved Molecular Imaging Contrast Agent for Detection of Human Thrombus. Magn. Reson. Med 2003, 50, 411–416. [DOI] [PubMed] [Google Scholar]
- (471).Flacke S; Fischer S; Scott MJ; Fuhrhop RJ; Allen JS; McLean M; Winter P; Sicard GA; Gaffney PJ; Wickline SA, et al. Novel MRI Contrast Agent for Molecular Imaging of Fibrin Implications for Detecting Vulnerable Plaques. Circulation 2001, 104, 1280–1285. [DOI] [PubMed] [Google Scholar]
- (472).Yu X; Song SK; Chen J; Scott MJ; Fuhrhop RJ; Hall CS; Gaffney PJ; Wickline SA; Lanza GM High-Resolution MRI Characterization of Human Thrombus Using a Novel Fibrin-Targeted Paramagnetic Nanoparticle Contrast Agent. Magn. Reson. Med 2000, 44, 867–872. [DOI] [PubMed] [Google Scholar]
- (473).Temme S; Grapentin C; Quast C; Jacoby C; Grandoch M; Ding Z; Owenier C; Mayenfels F; Fischer JW; Schubert R, et al. Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging With Targeted Perfluorocarbon Nanoemulsions. Circulation 2015, 131, 1405–1414. [DOI] [PubMed] [Google Scholar]
- (474).Caravan P Protein-Targeted Gadolinium-Based Magnetic Resonance Imaging (MRI) Contrast Agents: Design and Mechanism of Action. Acc. Chem. Res 2009, 42, 851–862. [DOI] [PubMed] [Google Scholar]
- (475).Caravan P; Comuzzi C; Crooks W; McMurry TJ; Choppin GR; Woulfe SR Thermodynamic Stability and Kinetic Inertness of the MS-325, a new Blood Pool Agents for Magnetic Resonance Imaging. Inorg. Chem 2001, 40, 2170–2176. [DOI] [PubMed] [Google Scholar]
- (476).McMurry TJ; Parmelee DJ; Sajiki H; Scott DM; Oullet HS; Walovitch RC; Tyeklár Z; Dumas S; Bernard P; Nadler S, et al. The Effect of a Phosphodiester Linking Group on Albumin Binding, Blood Half-Life, and Relaxivity of Intravascular Diethylenetriaminepentaacetato Aquo Gadolinium(III) MRI Contrast Agents. J. Med. Chem 2002, 45, 3465–3474. [DOI] [PubMed] [Google Scholar]
- (477).Herborn CU; Lauenstein TC; Vogt FM; Lauffer RB; Debatin JF; Ruehm SG Interstitial MR Lymphography with MS-325: Characterization of Normal and Tumor-Invaded Lymph Nodes in a Rabbit Model. Am. J. Roentgenol 2002, 179, 1567–1572. [DOI] [PubMed] [Google Scholar]
- (478).Montesi S; Rao R; Liang L; Caravan P; Sharma A; Digumarthy S; Seethamraju R; Tager AM Use Of Gadofosveset-Enhanced Lung Mri To Assess Ongoing Lung Injury In Fibrotic Interstitial Lung Disease. Am. J. Resp. Crit. Care 2017, 195. [Google Scholar]
- (479).Lorusso V; Pascolo L; Fernetti C; Visigalli M; Anelli P; Tiribelli C In Vitro and In Vivo Hepatic Transport of the Magnetic Resonance Imaging Contrast Agent B22956/1: Role of MRP Proteins. Biochem. Biophys. Res. Commun 2002, 293, 100–105. [DOI] [PubMed] [Google Scholar]
- (480).Boschi F; Marzola P; Sandri M; Nicolato E; Galie M; Fiorini S; Merigo F; Lorusso V; Chaabane L; Sbarbati A Tumor Microvasculature Observed Using Different Contrast Agents: A Comparison Between Gd-DTPA-Albumin and B-22956/1 in an Experimental Model of Mammary Carcinoma. Magn. Reson. Mat. Phys. Biol. Med 2008, 21, 169–176. [DOI] [PubMed] [Google Scholar]
- (481).Ramponi S; Rebaudengo C; Cabella C; Grotti A; Vultaggio S; Aime S; Morisetti A; Lorusso V Contrast-Enhanced MRI of Murine Sponge Model for Progressive Angiogensis Assessed with Gadoteridol(ProHance) and Gadocoletic Acid Trisodium salt(B22956/1). J. Magn. Reson. Imaging 2008, 27, 872–880. [DOI] [PubMed] [Google Scholar]
- (482).Gianolio E; Cabella C; Serra SC; Valbusa G; Arena F; Maiocchi A; Miragoli L; Tedoldi F; Uggeri F; Visigalli M, et al. B25716/1: A Novel Albumin-Binding Gd-AAZTA MRI Contrast Agent with Improved Properties in Tumor Imaging. J. Biol. Inorg. Chem 2014, 19, 715–726. [DOI] [PubMed] [Google Scholar]
- (483).Boros E; Caravan P Structure-Relaxivity Relationships of Serum Albumin Targeted MRI Probes Based on a Single Amino Acid Gd Complex. J. Med. Chem 2013, 56, 1782–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (484).Caravan P; Zhang Z Structure - relaxivity relationships among Targeted MR contrast agents. Eur. J. Inorg. Chem 2012, 2012, 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Iki N; Boros E; Nakamura M; Baba R; Caravan P Gd3TCAS2: An Aquated Gd(3+)-Thiacalix[4]arene Sandwich Cluster with Extremely Slow Ligand Substitution Kinetics. Inorg. Chem 2016, 55, 4000–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (486).Boros E; Caravan P Probing the Structure-Relaxivity Relationship of Bis-Hydrated Gd(DOTAla) Derivatives. Inorg. Chem 2015, 54, 2403–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).Moriggi L; Yaseen MA; Helm L; Caravan P Serum Albumin Targeted, pH-Dependent Magnetic Resonance Relaxation Agents. Chem. Eur. J 2012, 18, 3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (488).Jung KH; Kim HK; Park JA; Nam KS; Lee GH; Chang Y; Kim TJ Gd Complexes of DO3A-(Biphenyl-2,2 ‘-bisamides) Conjugates as MRI Blood-Pool Contrast Agents. ACS Med. Chem. Lett 2012, 3, 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (489).Jung KH; Kim HK; Lee GH; Kang DS; Park JA; Kim KM; Chang YM; Kim TJ Gd Complexes of Macrocyclic Diethylenetriaminepentaacetic Acid (DTPA) Biphenyl-2,2’-bisamides as Strong Blood-Pool Magnetic Resonance Imaging Contrast Agents. J. Med. Chem 2011, 54, 5385–5394. [DOI] [PubMed] [Google Scholar]
- (490).Caravan P; Das B; Dumas S; Epstein FH; Helm PA; Jacques V; Koerner S; Kolodziej A; Shen L; Sun WC, et al. Collagen-Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angew. Chem. Int. Ed 2007, 46, 8171–8173. [DOI] [PubMed] [Google Scholar]
- (491).Spuentrup E; Ruhl KM; Botnar RM; Wiethoff AJ; Buhl A; Jacques V; Greenfield MT; Krombach GA; Günther RW; Vangel MG, et al. Molecular Magnetic Resonance Imaging of Myocardial Perfusion with EP-3600, a Collagen-Specific Contrast Agent. Circulation 2009, 119, 1768–1775. [DOI] [PubMed] [Google Scholar]
- (492).Farrar CT; Gale EM; Kennan R; Ramsay I; Masia R; Arora G; Looby K; Wei L; Kalpathy-Cramer J; Bunzel MM, et al. CM-101: Type I Collagen-Targeted MR Imaging Probe for Detection of Liver Fibrosis. Radiology 2018, 287, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (493).Zhu B; Wei L; Rotile N; Day H; Rietz T; Farrar CT; Lauwers GY; Tanabe KK; Rosen B; Fuchs BC, et al. Combined Magnetic Resonance Elastography and Collagen Molecular Magnetic Resonance Imaging Accurately Stage Liver Fibrosis in a Rat Model. Hepatology 2017, 65, 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (494).Farrar CT; DePeralta DK; Day H; Rietz TA; Wei L; Lauwers GY; Keil B; Subramaniam A; Sinskey AJ; Tanabe KK, et al. 3D Molecular MR imaging of Liver Fibrosis and Response to Rapamycin Therapy in a Bile Duct Ligation Rat Model. J. Hepatol 2015, 63, 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (495).Helm PA; Caravan P; French BA; Jacques V; Shen L; Xu Y; Beyers RJ; Roy RJ; Kramer CM; Epstein FH Postinfarction Myocardial Scarring in Mice: Molecular MR Imaging with Use of a Collagen-targeting Contrast Agent. Radiology 2008, 3, 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (496).Polasek M; Yang Y; Schuhle DT; Yaseen MA; Kim YR; Sung YS; Guimaraes AR; Caravan P Molecular MR Imaging of Fibrosis in a Mouse Model of Pancreatic Cancer. Sci. Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (497).Caravan P; Yang Y; Zachariah R; Schmitt A; Mino-Kenudson M; Chen HH; Sosnovik DE; Dai G; Fuchs BC; Lanuti M Molecular Magnetic Resonance Imaging of Pulmonary Fibrosis in Mice. Am. J. Respir. Cell Mol. Biol 2013, 49, 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (498).Waghorn PA; Oliveira BL; Jones CM; Tager AM; Caravan P High Sensitivity HPLC Method for Determination of the Allysine Concentration in Tissue by Use of a Naphthol Derivative. J. Chromatogr. B 2017, 1064, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (499).Chen HH; Waghorn PA; Wei L; Tapias LF; Schuhle DT; Rotile NJ; Jones CM; Looby RJ; Zhao G; Elliott JM, et al. Molecular Imaging of Oxidized Collagen Quantifies Pulmonary and Hepatic Fibrogenesis. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (500).Waghorn PA; Jones CM; Rotile NJ; Koerner SK; Ferreira DS; Chen HH; Probst CK; Tager AM; Caravan P Molecular Magnetic Resonance Imaging of Lung Fibrogenesis with an Oxyamine-Based Probe. Angew. Chem. Int. Ed 2017, 56, 9825–9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (501).Pilch J; Brown DM; Komatsu M; Jarvinen TAH; Yang M; Peters D; Hoffman RM; Ruoslahti E Peptides Selected for Binding to Clotted Plasma Accumulate in Tumor Stroma and Wounds. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 2800–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (502).Ye FR; Jeong EK; Jia ZJ; Yang TX; Parker D; Lu ZR A Peptide Targeted Contrast Agent Specific to Fibrin-Fibronectin Complexes for Cancer Molecular Imaging with MRI. Bioconjugate Chem. 2008, 19, 2300–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (503).Simberg D; Duza T; Park JH; Essler M; Pilch J; Zhang LL; Derfus AM; Yang M; Hoffman RM; Bhatia S, et al. Biomimetic Amplification of Nanoparticle Homing to Tumors. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (504).Zhou ZX; Wu XM; Kresak A; Griswold M; Lu ZR Peptide Targeted Tripod Macrocyclic Gd(III) Chelates For Cancer Molecular MRI. Biomaterials 2013, 34, 7683–7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (505).Zhou ZX; Qutaish M; Han Z; Schur RM; Liu YQ; Wilson DL; Lu ZR MRI Detection of Breast Cancer Micrometastases with a Fibronectin-Targeting Contrast Agent. Nat. Commun 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (506).Tan MQ; Burden-Gulley SM; Li W; Wu XM; Lindner D; Brady-Kalnay SM; Gulani V; Lu ZR MR Molecular Imaging of Prostate Cancer with a Peptide-Targeted Contrast Agent in a Mouse Orthotopic Prostate Cancer Model. Pharm. Res 2012, 29, 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (507).Botnar RM; Wiethoff AJ; Ebersberger U; Lacerda S; Blume U; Warley A; Jansen CHP; Onthank DC; Cesati RR; Rezavi R, et al. In Vivo Assessment of Aortic Aneurysm Wall Integrity Using Elastin-Specific Molecular Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2015, 7, 679–689. [DOI] [PubMed] [Google Scholar]
- (508).Phinikaridou A; Andia ME; Indermuehle A; Onthank DC; Cesati RR; Smith A; Robinson SP; Saha P; Botnar RM Vascular Remodeling and Plaque Vulnerability in a Rabbit Model of Atherosclerosis: Comparison of Delayed Enhancement MR Imaging with an Elastin-Specific Contrast Agent and Unenhanced Black-Blood MR Imaging. Radiology 2014, 271, 390–399. [DOI] [PubMed] [Google Scholar]
- (509).Wildgruber M; Bielicki I; Aichler M; Kosanke K; Feuchtinger A; Settles M; Onthank DC; Cesati RR; Robinson SP; Huber AM, et al. Assessment of Myocardial Infarction and Postinfarction Scar Remodeling With an Elastin-Specific Magnetic Resonance Agent. Circ. Cardiovasc. Imaging 2014, 7, 321–329. [DOI] [PubMed] [Google Scholar]
- (510).Protti A; Lavin B; Dong X; Lorrio S; Robinson S; Onthank DC; Shah AM; Botnar RM Assessment of Myocardial Remodeling Using an Elastin/Tropoelastin Specific Agent with High Field Magnetic Resonance Imaging (MRI). J. Am. Heart Assoc 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (511).Frullano L; Zhu JQ; Wang CN; Wu CY; Miller RH; Wang YM Myelin Imaging Compound (MIC) Enhanced Magnetic Resonance Imaging of Myelination. J. Med. Chem 2012, 55, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (512).Frullano L; Wang CN; Miller RH; Wang YM A Myelin-Specific Contrast Agent for Magnetic Resonance Imaging of Myelination. J. Am. Chem. Soc 2011, 133, 1611–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (513).Wang CN; Popescu DC; Wu CY; Zhu JQ; Macklin W; Wang YM In Situ Fluorescence Imaging of Myelination. J. Histochem. Cytochem 2010, 58, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (514).Poduslo JF; Wengenack TM; Curran GL; Wisniewski T; Sigurdsson EM; Macura SI; Borowski BJ; Jack CR Molecular Targeting Of Alzheimer’s Amyloid Plaques for Contrast-Enhanced Magnetic Resonance Imaging. Neurobiol. Dis 2002, 11, 315–329. [DOI] [PubMed] [Google Scholar]
- (515).Poduslo JF; Curran GL Polyamine Modification Increases the Permeability of Proteins at the Blood-Nerve and Blood-Brain Barriers. J. Neurochem 1996, 66, 1599–1609. [DOI] [PubMed] [Google Scholar]
- (516).Wadghiri YZ; Li JL; Wang JH; Hoang DM; Sun YJ; Xu H; Tsui W; Li YS; Boutajangout A; Wang A, et al. Detection of Amyloid Plaques Targeted by Bifunctional USPIO in Alzheimer’s Disease Transgenic Mice Using Magnetic Resonance Microimaging. PLOS ONE 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (517).Sigurdsson EM; Wadghiri YZ; Mosconi L; Blind JA; Knudsen E; Asuni A; Scholtzova H; Tsui WH; Li Y; Sadowski M, et al. A Non-Toxic Ligand for Voxel-Based MRI Analysis of Plaques in AD Transgenic Mice. Neurobiol. Aging 2008, 29, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (518).Martins AF; Dias DM; Morfin J-F; Lacerda S; Laurents DV; Toth E; Geraldes CFGC Interaction of PiB-Derivative Metal Complexes with Beta-Amyloid Peptides: Selective Recognition of the Aggregated Forms. Chem. Eur. J 2015, 21, 5413–5422. [DOI] [PubMed] [Google Scholar]
- (519).Martins AF; Morfin JF; Geraldes C; Toth E Gd3+ Complexes Conjugated to Pittsburgh Compound B: Potential MRI Markers of Beta-Amyloid Plaques. J. Biol. Inorg. Chem 2014, 19, 281–295. [DOI] [PubMed] [Google Scholar]
- (520).Sillerud LO; Solberg NO; Chamberlain R; Orlando RA; Heidrich JE; Brown DC; Brady CI; Vander Jagt TA; Garwood M; Vander Jagt DL SPION-Enhanced Magnetic Resonance Imaging of Alzheimer’s Disease Plaques in A beta PP/PS-1 Transgenic Mouse Brain. J. Alzheimers Dis 2013, 34, 349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (521).Van Montfoort JE; Stieger B; Meijer DKF; Weinmann HJ; Meier PJ; Fattinger KE Hepatic Uptake of the Magnetic Resonance Imaging Contrast Agent Gadoxetate by the Organic Anion Transporting Polypeptide Oatp1. J. Pharmacol. Exp. Ther 1999, 290, 153–157. [PubMed] [Google Scholar]
- (522).Junqiang L; Yinzhong W; Li Z; Shunlin G; Xiaohui W; Yanan Z; Kehu Y Gadoxetic Acid Disodium (Gd-EOBDTPA)-Enhanced Magnetic Resonance Imaging for the Detection of Hepatocellular Carcinoma: a Meta-Analysis. J. Magn. Reson. Imaging 2014, 39, 1079–1087. [DOI] [PubMed] [Google Scholar]
- (523).de Haen C; La Ferla R; Maggioni F Gadobenate Dimeglumine 0.5 M Solution for Injection (MultiHance) as Contrast Agent for Magnetic Resonance Imaging of the Liver: Mechanistic Studies in Animals. J. Comput. Assist. Tomo 1999, 23, 169–179. [DOI] [PubMed] [Google Scholar]
- (524).Purysko AS; Remer EM; Coppa CP; Obuchowski NA; Schneider E; Veniero JC Characteristics and Distinguishing Features of Hepatocellular Adenoma and Focal Nodular Hyperplasia on Gadoxetate Disodium-Enhanced MRI. Am. J. Roentgenol 2012, 198, 115–123. [DOI] [PubMed] [Google Scholar]
- (525).Islam MK; Kim S; Kim HK; Park S; Lee GH; Kang HJ; Jung JC; Park JS; Kim TJ; Chan Y Manganese Complex of Ethylenediaminetetraacetic Acid (EDTA)-Benzothiazole Aniline (BTA) Conjugate as a Potential Liver-Targeting MRI Contrast Agent. J. Med. Chem 2017, 60, 2993–3001. [DOI] [PubMed] [Google Scholar]
- (526).Patrick PS; Hammersley J; Loizou L; Kettunen MI; Rodrigues TB; Hu DE; Tee SS; Hesketh R; Lyons SK; Soloviev D, et al. Dual-Modality Gene Reporter for In Vivo Imaging. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (527).Xu X; Yadav NN; Knutsson L; Hua J; Kalyani R; Hall E; Laterra J; Blakeley J; Strowd R; Pomper M, et al. Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography 2015, 1, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (528).Rivlin M; Tsarfaty I; Navon G Functional Molecular Imaging of Tumors by Chemical Exchange Saturation Transfer MRI of 3-O-Methyl-D-Glucose. Magn. Reson. Med 2014, 72, 1375–1380. [DOI] [PubMed] [Google Scholar]
- (529).Rivlin M; Navon G Glucosamine and N-Acetyl Glucosamine as New CEST MRI Agents for Molecular Imaging of Tumors. Sci. Rep 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (530).Rivlin M; Horev J; Tsarfaty I; Navon G Molecular Imaging of Tumors and Metastases Using Chemical Exchange Saturation Transfer (CEST) MRI. Sci. Rep 2013, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (531).Paech D; Schuenke P; Koehler C; Windschuh J; Mundiyanapurath S; Bickelhaupt S; Bonekamp D; Baumer P; Bachert P; Ladd ME, et al. T1 rho- Weighted Dynamic Glucose-Enhanced MR Imaging in the Human Brain. Radiology 2017, 285, 914–922. [DOI] [PubMed] [Google Scholar]
- (532).Kresse M; Wagner S; Pfefferer D; Lawaczeck R; Elste V; Semmler W Targeting of Ultrasmall Superparamagnetic Iron Oxide (USPIO) Particles to Tumor Cells In Vivo by Using Transferrin Receptor Pathways. Magn. Reson. Med 1998, 40, 236–242. [DOI] [PubMed] [Google Scholar]
- (533).Hwang DW; Ko HY; Lee JH; Kang H; Ryu SH; Song IC; Lee DS; Kim S A Nucleolin-Targeted Multimodal Nanoparticle Imaging Probe for Tracking Cancer Cells Using an Aptamer. J. Nucl. Med 2010, 51, 98–105. [DOI] [PubMed] [Google Scholar]
- (534).Anderson SA; Rader RK; Westlin WF; Null C; Jackson D; Lanza CM; Wickline SA; Kotyk JJ Magnetic Resonance Contrast Enhancement of Neovasculature with alpha(v)beta(3)-Targeted Nanoparticles. Magn. Reson. Med 2000, 44, 433–439. [DOI] [PubMed] [Google Scholar]
- (535).Chen K; Xie J; Xu HY; Behera D; Michalski MH; Biswal S; Wang A; Chen XY Triblock Copolymer Coated Iron Oxide Nanoparticle Conjugate for Tumor Integrin Targeting. Biomaterials 2009, 30, 6912–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (536).Zhang CF; Jugold M; Woenne EC; Lammers T; Morgenstern B; Mueller MM; Zentgraf H; Bock M; Eisenhut M; Semmler W, et al. Specific Targeting of Tumor Angiogenesis by RGD-conjugated Ultrasmall Sperparamagnetic Iron Oxide Particles Using a Clinical 1.5-T Magnetic Resonance Scanner. Cancer Res. 2007, 67, 1555–1562. [DOI] [PubMed] [Google Scholar]
- (537).Liu YJ; Yang Y; Zhang CF A Concise Review of Magnetic Resonance Molecular Imaging of Tumor Angiogenesis by Targeting Integrin Alpha v Beta 3 with Magnetic Probes. Int. J. Nanomed 2013, 8, 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (538).Crich SG; Terreno E; Aime S Nano-sized and Other Improved Reporters for Magnetic Resonance Imaging Of Angiogenesis. Adv. Drug Deliv. Rev 2017, 119, 61–72. [DOI] [PubMed] [Google Scholar]
- (539).Kalber TL; Kamaly N; So PW; Pugh JA; Bunch J; McLeod CW; Jorgensen MR; Miller AD; Bell JD A Low Molecular Weight Folate Receptor Targeted Contrast Agent for Magnetic Resonance Tumor Imaging. Mol. Imaging Biol 2011, 13, 653–662. [DOI] [PubMed] [Google Scholar]
- (540).Corot C; Robert P; Lancelot E; Prigent P; Ballet S; Guilbert I; Raynaud JS; Raynal I; Port M Tumor Imaging Using P866, a High-Relaxivity Gadolinium Chelate Designed for Folate Receptor Targeting. Magn. Reson. Med 2008, 60, 1337–1346. [DOI] [PubMed] [Google Scholar]
- (541).Wang ZJ; Boddington S; Wendland M; Meier R; Corot C; Daldrup-Link H MR Imaging of Ovarian Tumors Using Folate-Receptor-Targeted Contrast Agents. Pediatr. Radiol 2008, 38, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Konda SD; Aref M; Brechbiel M; Wiener EC Development of a Tumor-targeting MR Contrast Agent Using the High-Affinity Folate Receptor - Work in Progress. Invest. Radiol 2000, 35, 50–57. [DOI] [PubMed] [Google Scholar]
- (543).Konda SD; Wang S; Brechbiel M; Wiener EC Biodistribution of a Gd-153-Folate Dendrimer, Generation=4, in Mice with Folate-Receptor Positive and Negative Ovarian Tumor Xenografts. Invest. Radiol 2002, 37, 199–204. [DOI] [PubMed] [Google Scholar]
- (544).Ding N; Lu Y; Lee RJ; Yang C; Huang L; Liu J; Xiang GY Folate Receptor-Targeted Fluorescent Paramagnetic Bimodal Liposomes for Tumor Imaging. Int. J. Nanomed 2011, 6, 2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (545).Kamaly N; Kalber T; Thanou M; Bell JD; Miller AD Folate Receptor Targeted Bimodal Liposomes for Tumor Magnetic Resonance Imaging. Bioconjugate Chem. 2009, 20, 648–655. [DOI] [PubMed] [Google Scholar]
- (546).Nakamura T; Kawano K; Shiraishi K; Yokoyama M; Maitani Y Folate-Targeted Gadolinium-Lipid-Based Nanoparticles as a Bimodal Contrast Agent for Tumor Fluorescent and Magnetic Resonance Imaging. Biol. Pharm. Bull 2014, 37, 521–527. [DOI] [PubMed] [Google Scholar]
- (547).Sideratou Z; Tsiourvas D; Theodossiou T; Fardis M; Paleos CM Synthesis and Characterization of Multifunctional Hyperbranched Polyesters as Prospective Contrast Agents for Targeted MRI. Bioorg. Med. Chem. Lett 2010, 20, 4177–4181. [DOI] [PubMed] [Google Scholar]
- (548).McAteer MA; Mankia K; Ruparelia N; Jefferson A; Nugent HB; Stork LA; Channon KM; Schneider JE; Choudhury RP A Leukocyte-Mimetic Magnetic Resonance Imaging Contrast Agent Homes Rapidly to Activated Endothelium and Tracks With Atherosclerotic Lesion Macrophage Content. Arterioscler. Thromb. Vasc. Biol 2012, 32, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (549).Fournier AP; Quenault A; de Lizarrondo SM; Gauberti M; Defer G; Vivien D; Docagne F; Macrez R Prediction of Disease Activity in Models of Multiple Sclerosis by Molecular Magnetic Resonance Imaging of P-selectin. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 6116–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (550).Boutry S; Laurent S; Vander Elst L; Muller RN Specific E-selectin Targeting with a Superparamagnetic MRI Contrast Agent. Contrast Media. Mol. Imag 2006, 1, 15–22. [DOI] [PubMed] [Google Scholar]
- (551).Laurent S; Vander Elst L; Fu YJ; Muller RN Synthesis and Physicochemical Characterization of Gd-DTPA-B(sLe(x))A, a New MRI Contrast Agent Targeted to Inflammation. Bioconjugate Chem. 2004, 15, 99–103. [DOI] [PubMed] [Google Scholar]
- (552).Boutry S; Burtea C; Laurent S; Toubeau G; Vander Elst L; Muller RN Magnetic Resonance Imaging of Inflammation with a Specific Selectin-Targeted Contrast Agent. Magn. Reson. Med 2005, 53, 800–807. [DOI] [PubMed] [Google Scholar]
- (553).Yancy AD; Olzinski AR; Hu TC; Lenhard SC; Aravindhan K; Gruver SM; Jacobs PM; Willette RN; Jucker BM Differential Uptake of ferumoxtran-10 and ferumoxytol, Ultrasmall Superparamagnetic Iron Oxide Contrast Agents in Rabbit: Critical Determinants of Atherosclerotic Plaque Labeling. J. Magn. Reson. Imaging 2005, 21, 432–442. [DOI] [PubMed] [Google Scholar]
- (554).Naresh NK; Xu Y; Klibanov AL; Vandsburger MH; Meyer CH; Leor J; Kramer CM; French BA; Epstein FH Monocyte and/or Macrophage Infiltration of Heart after Myocardial Infarction: MR Imaging by Using T1-shortening Liposomes1. Radiology 2012, 2, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (555).Jacoby C; Borg N; Heusch P; Sauter M; Bönner F; Kandolf R; Klingel K; Schrader J; Flögel U Visualization of Immune Cell Infiltration in Experimental Viral Myocarditis by 19F MRI In Vivo. Magn. Reson. Mater. Phy 2014, 27, 101–106. [DOI] [PubMed] [Google Scholar]
- (556).Bönner F; Merx MW; Klingel K; Begovatz P; Flögel U; Sager M; Temme S; Jacoby C; Ravesh MS; Grapentin C, et al. Monocyte Imaging Aftermyocardial Infarction with 19FMRI at 3 T: A Pilot Study in Explanted Porcine Hearts. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 612–620. [DOI] [PubMed] [Google Scholar]
- (557).Bönner F; Jacoby C; Temme S; Borg N; Ding Z; Schrader J; Flögel U Multifunctional MR Monitoring of the Healing Process after Myocardial Infarction. Basic Res. Cardiol 2014, 109, 430–445. [DOI] [PubMed] [Google Scholar]
- (558).Krishnan AS; Neves AA; de Backer MM; Hu DE; Davletov B; Kettunen MI; Brindle KM Detection of Cell Death in Tumors by Using MR Imaging and a Gadolinium-Based Targeted Contrast Agent. Radiology 2008, 246, 854–862. [DOI] [PubMed] [Google Scholar]
- (559).van Tilborg GAF; Mulder WJM; Deckers N; Storm G; Reutelingsperger CPM; Strijkers GJ; Nicolay K Annexin A5-Functionalized Bimodal Lipid-Based Contrast Agents for the Detection of Apoptosis. Bioconjugate Chem 2006, 17, 741–749. [DOI] [PubMed] [Google Scholar]
- (560).van Tilborg GAF; Vucic E; Strijkers GJ; Cormode DP; Mani V; Skajaa T; Reutelingsperger CPM; Fayad ZA; Mulder WJM; Nicolay K Annexin A5-Functionalized Bimodal Nanoparticles for MRI and Fluorescence Imaging of Atherosclerotic Plaques. Bioconjugate Chem. 2010, 21, 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (561).Sosnovik DE; Schellenberger EA; Nahrendorf M; Novikov MS; Matsui T; Dai G; Reynolds F; Grazette L; Rosenzweig A; Weissleder R, et al. Magnetic Resonance Imaging of Cardiomyocyte Apoptosis with a Novel Magneto-Optical Nanoparticle. Magn. Reson. Med 2005, 54, 718–724. [DOI] [PubMed] [Google Scholar]
- (562).Huang S; Chen HH; Yuan H; Dai G; Schühle DT; Mekkaoui C; Ngoy S; Liao R; Caravan P; Josephson L, et al. Molecular MRI of Actue Necrosis with a Novel DNA-Binding Gadolinium Chelate: Kinetics of Cell Death and Clearance in Infarcted Myocardium. Circ. Cardiovasc. Imaging 2011, 4, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (563).Moats RA; Fraser SE; Meade TJA “Smart” Magnetic Resonance Imaging Agent That Reports on Specific Enzymatic Activity. Angew. Chem. Int. Ed 1997, 36, 726–728. [Google Scholar]
- (564).Serebriiskii IG; Golemis EA Uses of lacZ to Study Gene Function: Evaluation of β-Galactosidase Assays Employed in the Yeast Two-Hybrid System. Anal. Biochem 2000, 285, 1–15. [DOI] [PubMed] [Google Scholar]
- (565).Nolan GP; Fiering S; Nicolas JF; Herzenberg LA Fluorescence-activated Cell Analysis and Sorting of Viable Mammalian Cells Based on Beta-d-galactosidase Activity after Transduction of Escherichia coli lacZ. Proc. Natl. Acad. Sci. U. S. A 1988, 85, 2603–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (566).Louie AY; Hüber MM; Ahrens ET; Rothbächer U; Moats R; Jacobs RE; Fraser SE; Meade TJ In Vivo Visualization of Gene Expression Using Magentic Resonance Imaging. Nat. Biotechnol 2000, 18, 321–325. [DOI] [PubMed] [Google Scholar]
- (567).Hingorani DV; Bernstein AS; Pagel MD A Review of Responsive MRI Contrast Agents: 2005–2014. Contrast Media. Mol. Imag 2015, 10, 245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Tu C; Osborne EA; Louie AY Activatable T 1 and T 2 Magnetic Resonance Imaging Contrast Agents. Ann. Biomed. Eng 2011, 39, 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (569).Louie A MRI Biosensors: A Short Primer. J. Magn. Reson. Imaging 2013, 38, 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (570).Do QN; Ratnakar JS; Kovács Z; Sherry AD Redox- and Hypoxia-Responsive MRI Contrast Agents. ChemMedChem 2014, 9, 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (571).Que EL; Chang CJ Responsive Magnetic Resonance Imaging Contrast Agents as Chemical Sensors for Metals in Biology and Medicine. Chem. Soc. Rev 2010, 39, 51–60. [DOI] [PubMed] [Google Scholar]
- (572).Davies G-L; Kramberger I; Davis JJ Environmentally Responsive MRI Contrast Agents. Chem. Commun 2013, 49, 9704–9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Pérez-Mayoral E; Negri V; Soler-Padrós J; Cerdán S; Ballesteros P Chemistry of Paramagnetic and Diamagnetic Contrast Agents for Magnetic Resonance Imaging and Spectroscopy. Eur. J. Radiol 2008, 67, 453–458. [DOI] [PubMed] [Google Scholar]
- (574).Zhang S; Wu K; Sherry AD A Novel pH-Sensitive MRI Contrast Agent. Angew. Chem. Int. Ed 1999, 38, 3192–3194. [PubMed] [Google Scholar]
- (575).Kálmán FK; Woods M; Caravan P; Jurek P; Spiller M; Tircsó G; Király R; Brücher E; Sherry AD Potentiometric and Relaxometric Properties of a Gadolinium-Based MRI Contrast Agent for Sensing Tissue pH. Inorg. Chem 2007, 46, 5260–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (576).Ali MM; Woods M; Caravan P; Opina ACL; Spiller M; Fettinger JC; Sherry AD Synthesis and Relaxometric Studies of a Dendrimer-Based pH-Responsive MRI Contrast Agent. Chem. Eur. J 2008, 14, 7250–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (577).Woods M; Zhang S; Ebron VH; Sherry AD pH-Sensitive Modulation of the Second Hydration Sphere in Lanthanide(III) Tetraamide–DOTA Complexes: A Novel Approach to Smart MR Contrast Media. Chem. Eur. J 2003, 9, 4634–4640. [DOI] [PubMed] [Google Scholar]
- (578).Bruce JI; Dickins RS; Govenlock LJ; Gunnlaugsson T; Lopinski S; Lowe MP; Parker D; Peacock RD; Perry JJB; Aime S, et al. The Selectivity of Reversible Oxy-Anion Binding in Aqueous Solution at a Chiral Europium and Terbium Center: Signaling of Carbonate Chelation by Changes in the Form and Circular Polarization of Luminescence Emission. J. Am. Chem. Soc 2000, 122, 9674–9684. [Google Scholar]
- (579).Aime S; Gianolio E; Terreno E; Giovenzana GB; Pagliarin R; Sisti M; Palmisano G; Botta M; Lowe MP; Parker D Ternary Gd(III)L-HSA Adducts: Evidence for the Replacement of Inner-Sphere Water Molecules by Coordinating Groups of the Protein. Implications for the Design of Contrast Agents for MRI. J. Biol. Inorg. Chem 2000, 5, 488–497. [DOI] [PubMed] [Google Scholar]
- (580).Woods M; Kiefer GE; Bott S; Castillo-Muzquiz A; Eshelbrenner C; Michaudet L; McMillan K; Mudigunda SDK; Ogrin D; Tircsó G, et al. Synthesis, Relaxometric and Photophysical Properties of a New pH-Responsive MRI Contrast Agent: The Effect of Other Ligating Groups on Dissociation of a p-Nitrophenolic Pendant Arm. J. Am. Chem. Soc 2004, 126, 9248–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (581).Giovenzana GB; Negri R; Rolla GA; Tei L Gd-Aminoethyl-DO3A Complexes: A Novel Class of pH-Sensitive MRI Contrast Agents. Eur. J. Inorg. Chem 2012, 2012, 2035–2039. [Google Scholar]
- (582).Baranyai Z; Rolla GA; Negri R; Forgács A; Giovenzana GB; Tei L Comprehensive Evaluation of the Physicochemical Properties of LnIII Complexes of Aminoethyl-DO3A as pH-Responsive T1-MRI Contrast Agents. Chem. Eur. J 2014, 20, 2933–2944. [DOI] [PubMed] [Google Scholar]
- (583).Raghunand N; Howison C; Sherry AD; Zhang S; Gillies RJ Renal and Systemic pH Imaging by Contrast-Enhanced MRI. Magn. Reson. Med 2003, 49, 249–257. [DOI] [PubMed] [Google Scholar]
- (584).Garcia-Martin ML; Martinez GV; Raghunand N; Sherry AD; Zhang S; Gillies RJ High resolution pHe Imaging of Rat Glioma using pH-dependent Relaxivity. Magn. Reson. Med 2006, 55, 309–315. [DOI] [PubMed] [Google Scholar]
- (585).Martinez GV; Zhang X; García-Martín ML; Morse DL; Woods M; Sherry AD; Gillies RJ Imaging the Extracellular pH of Tumors by MRI after Injection of a Single Cocktail of T1 and T2 Contrast Agents. NMR Biomed. 2011, 24, 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (586).Aime S; Fedeli F; Sanino A; Terreno E A R2/R1 Ratiometric Procedure for a Concentration-Independent, pH-Responsive, Gd(III)-Based MRI Agent. J. Am. Chem. Soc 2006, 128, 11326–11327. [DOI] [PubMed] [Google Scholar]
- (587).Frullano L; Catana C; Benner T; Sherry AD; Caravan P Bimodal MR–PET Agent for Quantitative pH Imaging. Angew. Chem. Int. Ed 2010, 49, 2382–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (588).Gianolio E; Maciocco L; Imperio D; Giovenzana GB; Simonelli F; Abbas K; Bisi G; Aime S Dual MRI-SPECT Agent for pH-Mapping. Chem. Commun 2011, 47, 1539–1541. [DOI] [PubMed] [Google Scholar]
- (589).Vologdin N; Rolla GA; Botta M; Tei L Orthogonal Synthesis of a Heterodimeric Ligand for the Development of the GdIII-GaIII ditopic Complex as a Potential pH-sensitive MRI/PET Probe. Org. Biomol. Chem 2013, 11, 1683–1690. [DOI] [PubMed] [Google Scholar]
- (590).Longo DL; Sun PZ; Consolino L; Michelotti FC; Uggeri F; Aime S A General MRI-CEST Ratiometric Approach for pH Imaging: Demonstration of in Vivo pH Mapping with Iobitridol. J. Am. Chem. Soc 2014, 136, 14333–14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (591).McMahon MT; Gilad AA; Zhou J; Sun PZ; Bulte JWM; van Zijl PCM Quantifying Exchange Rates in Chemical Exchange Saturation Transfer Agents Using the Saturation Time and Saturation Power Dependencies of the Magnetization Transfer Effect on the Magnetic Resonance Imaging Signal (QUEST and QUESP): Ph Calibration for Poly-L-lysine and a Starburst Dendrimer. Magn. Reson. Med 2006, 55, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (592).Chen LQ; Howison CM; Jeffery JJ; Robey IF; Kuo PH; Pagel MD Evaluations of Extracellular pH within In Vivo Tumors using AcidoCEST MRI. Magn. Reson. Med 2014, 72, 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (593).Chan KWY; Liu G; Song X; Kim H; Yu T; Arifin DR; Gilad AA; Hanes J; Walczak P; van Zijl PCM, et al. MRI-detectable pH Nanosensors Incorporated into Hydrogels for In Vivo Sensing of Transplanted-Cell Viability. Nat. Mater 2013, 12, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (594).Zhang S; Zhou K; Huang G; Takahashi M; Dean Sherry A; Gao J A Novel Class of Polymeric pH-responsive MRI CEST Agents. Chem. Commun 2013, 49, 6418–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (595).Yang X; Song X; Ray Banerjee S; Li Y; Byun Y; Liu G; Bhujwalla ZM; Pomper MG; McMahon MT Developing Imidazoles as CEST MRI pH Sensors. Contrast Media. Mol. Imag 2016, 11, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (596).Longo DL; Busato A; Lanzardo S; Antico F; Aime S Imaging the pH Evolution of an Acute Kidney Injury Model by Means of Iopamidol, a MRI-CEST pH-responsive Contrast Agent. Magn. Reson. Med 2013, 70, 859–864. [DOI] [PubMed] [Google Scholar]
- (597).Longo DL; Bartoli A; Consolino L; Bardini P; Arena F; Schwaiger M; Aime S In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016, 76, 6463–6470. [DOI] [PubMed] [Google Scholar]
- (598).Napolitano R; Soesbe TC; De León-Rodríguez LM; Sherry AD; Udugamasooriya DG On-Bead Combinatorial Synthesis and Imaging of Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Agents To Identify Factors That Influence Water Exchange. J. Am. Chem. Soc 2011, 133, 13023–13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (599).Singh J; Rustagi V; Zhang S; Sherry AD; Udugamasooriya DG On-Bead Combinatorial Synthesis and Imaging of Europium(III)-Based ParaCEST Agents Aids in Identification of Chemical Features that Enhance CEST Sensitivity. Magn. Reson. Chem 2017, 55, 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (600).Aime S; Barge A; Delli Castelli D; Fedeli F; Mortillaro A; Nielsen FU; Terreno E Paramagnetic Lanthanide(III) Complexes as pH-sensitive Chemical Exchange Saturation Transfer (CEST) Contrast Agents for MRI Applications. Magn. Reson. Med 2002, 47, 639–648. [DOI] [PubMed] [Google Scholar]
- (601).Terreno EP; Castelli DDP; Cravotto GP; Milone LP; Aime SP Ln(III)-DOTAMGly Complexes: A Versatile Series to Assess the Determinants of the Efficacy of Paramagnetic Chemical Exchange Saturation Transfer Agents for Magnetic Resonance Imaging Applications. Invest. Radiol 2004, 39, 235–243. [DOI] [PubMed] [Google Scholar]
- (602).Aime S; Delli Castelli D; Terreno E Novel pH-Reporter MRI Contrast Agents. Angew. Chem. Int. Ed 2002, 41, 4334–4336. [DOI] [PubMed] [Google Scholar]
- (603).Liu G; Li Y; Sheth VR; Pagel MD Imaging in Vivo Extracellular pH with a Single Paramagnetic Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Contrast Agent. Mol. Imaging 2012, 11, 47–57. [PMC free article] [PubMed] [Google Scholar]
- (604).Pikkemaat JA; Wegh RT; Lamerichs R; van de Molengraaf RA; Langereis S; Burdinski D; Raymond AYF; Janssen HM; de Waal BFM; Willard NP, et al. Dendritic PARACEST Contrast Agents for Magnetic Resonance Imaging. Contrast Media. Mol. Imag 2007, 2, 229–239. [DOI] [PubMed] [Google Scholar]
- (605).McVicar N; Li AX; Suchý M; Hudson RHE; Menon RS; Bartha R Simultaneous In Vivo pH and Temperature Mapping Using a PARACEST-MRI Contrast Agent. Magn. Reson. Med 2013, 70, 1016–1025. [DOI] [PubMed] [Google Scholar]
- (606).Wu Y; Soesbe TC; Kiefer GE; Zhao P; Sherry AD A Responsive Europium(III) Chelate That Provides a Direct Readout of pH by MRI. J. Am. Chem. Soc 2010, 132, 14002–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (607).Sheth VR; Liu G; Li Y; Pagel MD Improved pH Measurements with a Single PARACEST MRI Contrast Agent. Contrast Media. Mol. Imag 2012, 7, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (608).Sheth VR; Li Y; Chen LQ; Howison CM; Flask CA; Pagel MD Measuring In Vivo Tumor pHe with CEST-FISP MRI. Magn. Reson. Med 2012, 67, 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (609).Wu Y; Zhang S; Soesbe TC; Yu J; Vinogradov E; Lenkinski RE; Sherry AD pH Imaging of Mouse Kidneys In Vivo Using a Frequency-Dependent ParaCEST Agent. Magn. Reson. Med 2016, 75, 2432–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (610).Delli Castelli D; Terreno E; Aime S YbIII-HPDO3A: A Dual pH- and Temperature-Responsive CEST Agent. Angew. Chem. Int. Ed 2011, 50, 1798–1800. [DOI] [PubMed] [Google Scholar]
- (611).Delli Castelli D; Ferrauto G; Cutrin JC; Terreno E; Aime S In Vivo Maps of Extracellular pH in Murine Melanoma by CEST–MRI. Magn. Reson. Med 2014, 71, 326–332. [DOI] [PubMed] [Google Scholar]
- (612).Tsitovich PB; Cox JM; Spernyak JA; Morrow JR Gear Up for a pH Shift: A Responsive Iron(II) 2-Amino-6-picolyl-Appended Macrocyclic paraCEST Agent That Protonates at a Pendent Group. Inorg. Chem 2016, 55, 12001–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (613).Finney K-LNA; Harnden AC; Rogers NJ; Senanayake PK; Blamire AM; O’Hogain D; Parker D Simultaneous Triple Imaging with Two PARASHIFT Probes: Encoding Anatomical, pH and Temperature Information using Magnetic Resonance Shift Imaging. Chem. Eur. J 2017, 23, 7976–7989. [DOI] [PubMed] [Google Scholar]
- (614).Okada S; Mizukami S; Kikuchi K Application of a Stimuli‐Responsive Polymer to the Development of Novel MRI Probes. ChemBioChem 2010, 11, 785–787. [DOI] [PubMed] [Google Scholar]
- (615).Okada S; Mizukami S; Kikuchi K Switchable MRI Contrast Agents Based on Morphological Changes of pH-responsive Polymers. Bioorg. Med. Chem 2012, 20, 769–774. [DOI] [PubMed] [Google Scholar]
- (616).Kim KS; Park W; Hu J; Bae YH; Na K A Cancer-Recognizable MRI Contrast Agents Using pH-responsive Polymeric Micelle. Biomaterials 2014, 35, 337–343. [DOI] [PubMed] [Google Scholar]
- (617).Torres E; Mainini F; Napolitano R; Fedeli F; Cavalli R; Aime S; Terreno E Improved Paramagnetic Liposomes for MRI Visualization of pH Triggered Release. J. Control. Release 2011, 154, 196–202. [DOI] [PubMed] [Google Scholar]
- (618).Kim T; Cho EJ; Chae Y; Kim M; Oh A; Jin J; Lee ES; Baik H; Haam S; Suh JS, et al. Urchin‐Shaped Manganese Oxide Nanoparticles as pH‐Responsive Activatable T1 Contrast Agents for Magnetic Resonance Imaging. Angew. Chem. Int. Ed 2011, 50, 10589–10593. [DOI] [PubMed] [Google Scholar]
- (619).Chen Y; Yin Q; Ji X; Zhang S; Chen H; Zheng Y; Sun Y; Qu H; Wang Z; Li Y, et al. Manganese Oxide-Based Multifunctionalized Mesoporous Silica Nanoparticles for pH-responsive MRI, Ultrasonography and Circumvention of MDR in Cancer Cells. Biomaterials 2012, 33, 7126–7137. [DOI] [PubMed] [Google Scholar]
- (620).Mi P; Kokuryo D; Cabral H; Wu H; Terada Y; Saga T; Aoki I; Nishiyama N; Kataoka K A pH-Activatable Nanoparticle with Signal-Amplification Capabilities for Non-Invasive Imaging of Tumour Malignancy. Nat. Nanotechnol 2016, 11, 724–730. [DOI] [PubMed] [Google Scholar]
- (621).Lin C-W; Tseng SJ; Kempson IM; Yang S-C; Hong T-M; Yang P-C Extracellular Delivery of Modified Oligonucleotide and Superparamagnetic Iron Oxide Nanoparticles from a Degradable Hydrogel Triggered by Tumor Acidosis. Biomaterials 2013, 34, 4387–4393. [DOI] [PubMed] [Google Scholar]
- (622).Gao GH; Im GH; Kim MS; Lee JW; Yang J; Jeon H; Lee JH; Lee DS Magnetite-Nanoparticle-Encapsulated pH-Responsive Polymeric Micelle as an MRI Probe for Detecting Acidic Pathologic Areas. Small 2010, 6, 1201–1204. [DOI] [PubMed] [Google Scholar]
- (623).Duimstra JA; Femia FJ; Meade TJ A Gadolinium Chelate for Detection of β-Glucuronidase: A Self-Immolative Approach. J. Am. Chem. Soc 2005, 127, 12847–12855. [DOI] [PubMed] [Google Scholar]
- (624).Giardiello M; Lowe MP; Botta M An Esterase-Activated Magnetic Resonance Contrast Agent. Chem. Commun 2007, 0, 4044–4046. [DOI] [PubMed] [Google Scholar]
- (625).Himmelreich U; Aime S; Hieronymus T; Justicia C; Uggeri F; Zenke M; Hoehn M A Responsive MRI Contrast Agent to Monitor Functional Cell Status. NeuroImage 2006, 32, 1142–1149. [DOI] [PubMed] [Google Scholar]
- (626).Lepage M; Dow WC; Melchior M; You Y; Fingleton B; Quarles CC; Pepin C; Gore JC; Matrisian LM; McIntyre JO Noninvasive Detection of Matrix Metalloproteinase Activity In Vivo using a Novel Magnetic Resonance Imaging Contrast Agent with a Solubility Switch. Mol. Imaging 2007, 6, 393–403. [PubMed] [Google Scholar]
- (627).Lebel R; Jastrzębska B; Therriault H; Cournoyer M-M; McIntyre JO; Escher E; Neugebauer W; Paquette B; Lepage M Novel Solubility-Switchable MRI Agent Allows the Noninvasive Detection of Matrix Metalloproteinase-2 Activity In Vivo in a Mouse Model. Magn. Reson. Med 2008, 60, 1056–1065. [DOI] [PubMed] [Google Scholar]
- (628).Jastrzȩbska B; Lebel R; Therriault H; McIntyre JO; Escher E; Guérin B; Paquette B; Neugebauer WA; Lepage M New Enzyme-Activated Solubility-Switchable Contrast Agent for Magnetic Resonance Imaging: From Synthesis to in Vivo Imaging. J. Med. Chem 2009, 52, 1576–1581. [DOI] [PubMed] [Google Scholar]
- (629).Figueiredo S; Moreira JN; Geraldes CFGC; Aime S; Terreno E Supramolecular Protamine/Gd-loaded Liposomes Adducts as Relaxometric Protease Responsive Probes. Bioorg. Med. Chem 2011, 19, 1131–1135. [DOI] [PubMed] [Google Scholar]
- (630).Nivorozhkin AL; Kolodziej AF; Caravan P; Greenfield MT; Lauffer RB; McMurry TJ Enzyme-Activated Gd3+ Magnetic Resonance Imaging Contrast Agents with a Prominent Receptor-Induced Magnetization Enhancement. Angew. Chem. Int. Ed 2001, 40, 2903–2906. [DOI] [PubMed] [Google Scholar]
- (631).Chang Y-T; Cheng C-M; Su Y-Z; Lee W-T; Hsu J-S; Liu G-C; Cheng T-L; Wang Y-M Synthesis and Characterization of a New Bioactivated Paramagnetic Gadolinium(III) Complex [Gd(DOTA-FPG)(H2O)] for Tracing Gene Expression. Bioconjugate Chem. 2007, 18, 1716–1727. [DOI] [PubMed] [Google Scholar]
- (632).Hanaoka K; Kikuchi K; Terai T; Komatsu T; Nagano T A Gd3+-Based Magnetic Resonance Imaging Contrast Agent Sensitive to β-Galactosidase Activity Utilizing a Receptor-Induced Magnetization Enhancement (RIME) Phenomenon. Chem. Eur. J 2008, 14, 987–995. [DOI] [PubMed] [Google Scholar]
- (633).Chen Y-J; Wu S-C; Chen C-Y; Tzou S-C; Cheng T-L; Huang Y-F; Yuan S-S; Wang Y-M Peptide-Based MRI Contrast Agent and Near-Infrared Fluorescent Probe for Intratumoral Legumain Detection. Biomaterials 2014, 35, 304–315. [DOI] [PubMed] [Google Scholar]
- (634).Rolla GA; Tei L; Fekete M; Arena F; Gianolio E; Botta M Responsive Mn(II) Complexes for Potential Applications in Diagnostic Magnetic Resonance Imaging. Bioorg. Med. Chem 2011, 19, 1115–1122. [DOI] [PubMed] [Google Scholar]
- (635).Alexei Bogdanov J; Matuszewski L; Bremer C; Petrovsky A; Weissleder R Oligomerization of Paramagnetic Substrates Result in Signal Amplification and Can be Used for MR Imaging of Molecular Targets. Mol. Imaging 2002, 1, 16–32. [DOI] [PubMed] [Google Scholar]
- (636).Querol M; Chen JW; Weissleder R; Bogdanov A DTPA-bisamide-Based MR Sensor Agents for Peroxidase Imaging. Org. Lett 2005, 7, 1719–1722. [DOI] [PubMed] [Google Scholar]
- (637).Querol M; Bennett DG; Sotak C; Kang HW; Bogdanov A A Paramagnetic Contrast Agent for Detecting Tyrosinase Activity. ChemBioChem 2007, 8, 1637–1641. [DOI] [PubMed] [Google Scholar]
- (638).Shiftan L; Israely T; Cohen M; Frydman V; Dafni H; Stern R; Neeman M Magnetic Resonance Imaging Visualization of Hyaluronidase in Ovarian Carcinoma. Cancer Res. 2005, 65, 10316–10323. [DOI] [PubMed] [Google Scholar]
- (639).Shiftan L; Neeman M Kinetic Analysis of Hyaluronidase Activity Using a Bioactive MRI Contrast Agent. Contrast Media. Mol. Imag 2006, 1, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (640).Nejadnik H; Ye D; Lenkov OD; Donig JS; Martin JE; Castillo R; Derugin N; Sennino B; Rao J; Daldrup-Link H Magnetic Resonance Imaging of Stem Cell Apoptosis in Arthritic Joints with a Caspase Activatable Contrast Agent. ACS Nano 2015, 9, 1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (641).Abu-Qare AW; Abou-Donia MB Biomarkers of Apoptosis: Release of Cytochrome c, Activation of Caspase-3, Induction of 8-hydroxy-2’-deoxyguanosine, Increased 3-nitrotyrosine, and Alteration of p53 Gene. J. Toxicol. Environ. Health B Crit. Rev 2001, 4, 313–332. [DOI] [PubMed] [Google Scholar]
- (642).Ye D; Shuhendler AJ; Pandit P; Brewer KD; Tee SS; Cui L; Tikhomirov G; Rutt B; Rao J Caspase-responsive Smart gadolinium-Based Contrast Agent for Magnetic Resonance Imaging of Drug-Induced Apoptosis. Chem. Sci 2014, 5, 3845–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (643).Shuhendler AJ; Ye D; Brewer KD; Bazalova-Carter M; Lee K-H; Kempen P; Dane Wittrup K; Graves EE; Rutt B; Rao J Molecular Magnetic Resonance Imaging of Tumor Response to Therapy. Sci. Rep 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (644).Granot D; Shapiro EM Release Activation of Iron Oxide Nanoparticles: (REACTION) A Novel Environmentally Sensitive MRI Paradigm. Magn. Reson. Med 2011, 65, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (645).Matsumura S; Aoki I; Saga T; Shiba K A Tumor-Environment-Responsive Nanocarrier That Evolves Its Surface Properties upon Sensing Matrix Metalloproteinase-2 and Initiates Agglomeration to Enhance T2 Relaxivity for Magnetic Resonance Imaging. Mol. Pharm 2011, 8, 1970–1974. [DOI] [PubMed] [Google Scholar]
- (646).Schellenberger E; Rudloff F; Warmuth C; Taupitz M; Hamm B; Schnorr J Protease-Specific Nanosensors for Magnetic Resonance Imaging. Bioconjugate Chem. 2008, 19, 2440–2445. [DOI] [PubMed] [Google Scholar]
- (647).Perez JM; Simeone FJ; Tsourkas A; Josephson L; Weissleder R Peroxidase Substrate Nanosensors for MR Imaging. Nano Lett. 2004, 4, 119–122. [Google Scholar]
- (648).Perez JM; Josephson L; O’Loughlin T; Högemann D; Weissleder R Magnetic Relaxation Switches Capable of Sensing Molecular Interactions. Nat. Biotechnol 2002, 20, 816–820. [DOI] [PubMed] [Google Scholar]
- (649).Zhao M; Josephson L; Tang Y; Weissleder R Magnetic Sensors for Protease Assays. Angew. Chem 2003, 115, 1413–1416. [DOI] [PubMed] [Google Scholar]
- (650).Yu SS; Scherer RL; Ortega RA; Bell CS; O’Neil CP; Hubbell JA; Giorgio TD Enzymatic- and Temperature-Sensitive Controlled Release of Ultrasmall Superparamagnetic Iron Oxides (USPIOs). J. Nanobiotechnol 2011, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (651).Mizukami S; Takikawa R; Sugihara F; Hori Y; Tochio H; Wälchli M; Shirakawa M; Kikuchi K Paramagnetic Relaxation-Based 19F MRI Probe To Detect Protease Activity. J. Am. Chem. Soc 2008, 130, 794–795. [DOI] [PubMed] [Google Scholar]
- (652).Mizukami S; Takikawa R; Sugihara F; Shirakawa M; Kikuchi K Dual‐Function Probe to Detect Protease Activity for Fluorescence Measurement and 19F MRI. Angew. Chem. Int. Ed 2009, 48, 3641–3643. [DOI] [PubMed] [Google Scholar]
- (653).Matsushita H; Mizukami S; Mori Y; Sugihara F; Shirakawa M; Yoshioka Y; Kikuchi K 19F MRI Monitoring of Gene Expression in Living Cells through Cell‐Surface β‐Lactamase Activity. ChemBioChem 2012, 13, 1579–1583. [DOI] [PubMed] [Google Scholar]
- (654).Mizukami S; Matsushita H; Takikawa R; Sugihara F; Shirakawa M; Kikuchi K 19F MRI Detection of [small beta]-galactosidase Activity for Imaging of Gene Expression. Chem. Sci 2011, 2, 1151–1155. [Google Scholar]
- (655).Yoo B; Pagel MD A PARACEST MRI Contrast Agent To Detect Enzyme Activity. J. Am. Chem. Soc 2006, 128, 14032–14033. [DOI] [PubMed] [Google Scholar]
- (656).Yoo B; Raam MS; Rosenblum RM; Pagel MD Enzyme-responsive PARACEST MRI Contrast Agents: A New Biomedical Imaging Approach for Studies of the Proteasome. Contrast Media. Mol. Imag 2007, 2, 189–198. [DOI] [PubMed] [Google Scholar]
- (657).Yoo B; Sheth VR; Howison CM; Douglas MJK; Pineda CT; Maine EA; Baker AF; Pagel MD Detection of In Vivo Enzyme Activity with CatalyCEST MRI. Magn. Reson. Med 2014, 71, 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (658).Suchy M; Ta R; Li AX; Wojciechowski F; Pasternak SH; Bartha R; Hudson RHE A Paramagnetic Chemical Exchange-Based MRI Probe Metabolized by Cathepsin D: Design, Synthesis and Cellular Uptake Studies. Org. Biomol. Chem 2010, 8, 2560–2566. [DOI] [PubMed] [Google Scholar]
- (659).Chauvin T; Durand P; Bernier M; Meudal H; Doan B-T; Noury F; Badet B; Beloeil J-C; Tóth É Detection of Enzymatic Activity by PARACEST MRI: A General Approach to Target a Large Variety of Enzymes. Angew. Chem. Int. Ed 2008, 47, 4370–4372. [DOI] [PubMed] [Google Scholar]
- (660).Li Y; Sheth VR; Liu G; Pagel MD A Self-Calibrating PARACEST MRI Contrast Agent That Detects Esterase Enzyme Activity. Contrast Media. Mol. Imag 2011, 6, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (661).Daryaei I; Jones KM; Pagel MD Detection of DT-diaphorase Enzyme with a ParaCEST MRI Contrast Agent. Chem. Eur. J 2017, 23, 6514–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (662).Hingorani DV; Randtke EA; Pagel MD A CatalyCEST MRI Contrast Agent That Detects the Enzyme-Catalyzed Creation of a Covalent Bond. J. Am. Chem. Soc 2013, 135, 6396–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (663).Liu G; Liang Y; Bar-Shir A; Chan KWY; Galpoththawela CS; Bernard SM; Tse T; Yadav NN; Walczak P; McMahon MT, et al. Monitoring Enzyme Activity Using a Diamagnetic Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc 2011, 133, 16326–16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (664).Airan RD; Bar-Shir A; Liu G; Pelled G; McMahon MT; van Zijl PCM; Bulte JWM; Gilad AA MRI Biosensor for Protein Kinase A Encoded By a Single Synthetic Gene. Magn. Reson. Med 2012, 68, 1919–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (665).Hingorani DV; Montano LA; Randtke EA; Lee YS; Cárdenas-Rodríguez J; Pagel MD A Single Diamagnetic CatalyCEST MRI Contrast Agent That Detects Cathepsin B Enzyme Activity by Using a Ratio of Two CEST Signals. Contrast Media. Mol. Imag 2016, 11, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (666).Sinharay S; Fernández-Cuervo G; Acfalle JP; Pagel MD Detection of Sulfatase Enzyme Activity with a CatalyCEST MRI Contrast Agent. Chem. Eur. J 2016, 22, 6491–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (667).Daryaei I; Mohammadebrahim Ghaffari M; Jones KM; Pagel MD Detection of Alkaline Phosphatase Enzyme Activity with a CatalyCEST MRI Biosensor. ACS Sens 2016, 1, 857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (668).Fernández-Cuervo G; Sinharay S; Pagel MD A CatalyCEST MRI Contrast Agent that Can Simultaneously Detect Two Enzyme Activities. ChemBioChem 2016, 17, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (669).Sinharay S; Randtke EA; Jones KM; Howison CM; Chambers SK; Kobayashi H; Pagel MD Noninvasive Detection of Enzyme Activity in Tumor Models of Human Ovarian Cancer Using CatalyCEST MRI. Magn. Reson. Med 2017, 77, 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (670).Tu C; Osborne EA; Louie AY Synthesis and Characterization of a Redox- and Light-sensitive MRI Contrast Agent. Tetrahedron 2009, 65, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (671).Tu C; Nagao R; Louie AY Multimodal Magnetic-Resonance/Optical-Imaging Contrast Agent Sensitive to NADH. Angew. Chem. Int. Ed 2009, 48, 6547–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (672).Iwaki S; Hanaoka K; Piao W; Komatsu T; Ueno T; Terai T; Nagano T Development of Hypoxia-sensitive Gd3+-Based MRI Contrast Agents. Bioorg. Med. Chem. Lett 2012, 22, 2798–2802. [DOI] [PubMed] [Google Scholar]
- (673).Yu M; Beyers RJ; Gorden JD; Cross JN; Goldsmith CR A Magnetic Resonance Imaging Contrast Agent Capable of Detecting Hydrogen Peroxide. Inorg. Chem 2012, 51, 9153–9155. [DOI] [PubMed] [Google Scholar]
- (674).Yu M; Ambrose SL; Whaley ZL; Fan S; Gorden JD; Beyers RJ; Schwartz DD; Goldsmith CR A Mononuclear Manganese(II) Complex Demonstrates a Strategy To Simultaneously Image and Treat Oxidative Stress. J. Am. Chem. Soc 2014, 136, 12836–12839. [DOI] [PubMed] [Google Scholar]
- (675).Yu M; Ward MB; Franke A; Ambrose SL; Whaley ZL; Bradford TM; Gorden JD; Beyers RJ; Cattley RC; Ivanović-Burmazović I, et al. Adding a Second Quinol to a Redox-Responsive MRI Contrast Agent Improves Its Relaxivity Response to H2O2. Inorg. Chem 2017, 56, 2812–2826. [DOI] [PubMed] [Google Scholar]
- (676).Carrera C; Digilio G; Baroni S; Burgio D; Consol S; Fedeli F; Longo D; Mortillaro A; Aime S Synthesis and Characterization of a Gd(III) Based Contrast Agent Responsive to Thiol Containing Compounds. Dalton Trans. 2007, 0, 4980–4987. [DOI] [PubMed] [Google Scholar]
- (677).Raghunand N; Jagadish B; Trouard TP; Galons J-P; Gillies RJ; Mash EA Redox-sensitive Contrast Agents for MRI Based on Reversible Binding of Thiols to Serum Albumin. Magn. Reson. Med 2006, 55, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (678).Jagadish B; Guntle GP; Zhao D; Gokhale V; Ozumerzifon TJ; Ahad AM; Mash EA; Raghunand N Redox-active Magnetic Resonance Imaging Contrast Agents: Studies with Thiol-bearing 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetracetic Acid Derivatives. J. Med. Chem 2012, 55, 10378–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (679).Raghunand N; Guntle GP; Gokhale V; Nichol GS; Mash EA; Jagadish B Design, Synthesis, and Evaluation of 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic Acid Derived, Redox-Sensitive Contrast Agents for Magnetic Resonance Imaging. J. Med. Chem 2010, 53, 6747–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (680).Guntle GP; Jagadish B; Mash EA; Powis G; Dorr RT; Raghunand N Tumor Xenograft Response to Redox-Active Therapies Assessed by Magnetic Resonance Imaging Using a Thiol-Bearing DOTA Complex of Gadolinium. Transl. Oncol 2012, 5, 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (681).Gløgård C; Stensrud G; Aime S Novel Radical-Responsive MRI Contrast Agent Based on Paramagnetic Liposomes. Magn. Reson. Chem 2003, 41, 585–588. [Google Scholar]
- (682).Furmanski P; Longley C Metalloporphyrin Enhancement of Magnetic Resonance Imaging of Human Tumor Xenografts in Nude Mice. Cancer Res 1988, 48, 4604–4610. [PubMed] [Google Scholar]
- (683).Fiel RJ; Musser DA; Mark EH; Mazurchuk R; Alletto JJ A Comparative Study of Manganese Meso-Sulfonatophenyl Porphyrins: Contrast-Enhancing Agents for Tumors. Magn. Reson. Imaging 1990, 8, 255–259. [DOI] [PubMed] [Google Scholar]
- (684).Ogan M; Revel D; Brasch R Metalloporphyrin Contrast Enhancement of Tumors in Magnetic Resonance Imaging A Study of Human Carcinoma, Lymphoma, and Fibrosarcoma in Mice. Invest. Radiol 1987, 22, 822–828. [DOI] [PubMed] [Google Scholar]
- (685).Fiel RJ; Button TM; Gilani S; Mark EH; Musser DA; Henkelman RM; Bronskill MJ; van Heteren JG Proton Relaxation Enhancement by Manganese(III)TPPS4 in a Model Tumor System. Magn. Reson. Imaging 1987, 5, 149–156. [DOI] [PubMed] [Google Scholar]
- (686).Loving GS; Mukherjee S; Caravan P Redox-Activated Manganese-Based MR Contrast Agent. J. Am. Chem. Soc 2013, 135, 4620–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (687).Hamm RE; Suwyn MA Preparation and Characterization of Some Aminopolycarboxylate Complexes of Manganese(III). Inorg. Chem 1967, 6, 139–142. [Google Scholar]
- (688).Frost AE; Freedman HH; Westerback SJ; Martell AE Chelating Tendencies of N,N’-Ethylenebis- [2-(0-hydroxyphenyl) ]-glycine. J. Am. Chem. Soc 1958, 80, 530–536. [Google Scholar]
- (689).Gale EM; Jones CM; Ramsay I; Farrar CT; Caravan P A Janus Chelator Enables Biochemically Responsive MRI Contrast with Exceptional Dynamic Range. J. Am. Chem. Soc 2016, 138, 15861–15864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (690).Di Gregorio E; Ferrauto G; Gianolio E; Lanzardo S; Carrera C; Fedeli F; Aime S An MRI Method To Map Tumor Hypoxia Using Red Blood Cells Loaded with a pO2-Responsive Gd-Agent. ACS Nano 2015, 9, 8239–8248. [DOI] [PubMed] [Google Scholar]
- (691).Liu G; Li Y; Pagel MD Design and Characterization of a New Irreversible Responsive PARACEST MRI Contrast Agent That Detects Nitric Oxide. Magn. Reson. Med 2007, 58, 1249–1256. [DOI] [PubMed] [Google Scholar]
- (692).Ratnakar SJ; Viswanathan S; Kovacs Z; Jindal AK; Green KN; Sherry AD Europium(III) DOTA-tetraamide Complexes as Redox-Active MRI Sensors. J. Am. Chem. Soc 2012, 134, 5798–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (693).Tsitovich PB; Spernyak JA; Morrow JR A Redox-Activated MRI Contrast Agent that Switches Between Paramagnetic and Diamagnetic States. Angew. Chem. Int. Ed 2013, 52, 13997–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (694).Aime S; Delli Castelli D; Terreno E Highly Sensitive MRI Chemical Exchange Saturation Transfer Agents Using Liposomes. Angew. Chem. Int. Ed 2005, 44, 5513–5515. [DOI] [PubMed] [Google Scholar]
- (695).Terreno E; Boffa C; Menchise V; Fedeli F; Carrera C; Castelli DD; Digilio G; Aime S Gadolinium-doped LipoCEST Agents: A Potential Novel Class of Dual 1H-MRI Probes. Chem. Commun 2011, 47, 4667–4669. [DOI] [PubMed] [Google Scholar]
- (696).Hanaoka K; Kikuchi K; Urano Y; Nagano T Selective Sensing of Zinc Ions with a Novel Magnetic Resonance Imaging Contrast Agent. J. Chem. Soc., Perkin Trans 2 2001, 1840–1843. [Google Scholar]
- (697).Hanaoka K; Kikuchi K; Urano Y; Narazaki M; Yokawa T; Sakamoto S; Yamaguchi K; Nagano T Design and Synthesis of a Novel Magnetic Resonance Imaging Contrast Agent for Selective Sensing of Zinc Ion. Chem. Biol 2002, 9, 1027–1032. [DOI] [PubMed] [Google Scholar]
- (698).Major JL; Parigi G; Luchinat C; Meade TJ The Synthesis and In Vitro Testing of a Zinc-activated MRI Contrast Agent. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 13881–13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (699).Major JL; Boiteau RM; Meade TJ Mechanisms of ZnII-Activated Magnetic Resonance Imaging Agents. Inorg. Chem 2008, 47, 10788–10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (700).Esqueda AC; López JA; Andreu-de-Riquer G; Alvarado-Monzón JC; Ratnakar J; Lubag AJM; Sherry AD; De León-Rodríguez LM A New Gadolinium-Based MRI Zinc Sensor. J. Am. Chem. Soc 2009, 131, 11387–11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (701).Lubag AJM; De Leon-Rodriguez LM; Burgess SC; Sherry AD Noninvasive MRI of β-cell Function Using a Zn2+-responsive Contrast Agent. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 18400–18405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (702).Clavijo Jordan MV; Lo S-T; Chen S; Preihs C; Chirayil S; Zhang S; Kapur P; Li W-H; De Leon-Rodriguez LM; Lubag AJM, et al. Zinc-sensitive MRI Contrast Agent Detects Differential Release of Zn(II) Ions from the Healthy vs. Malignant Mouse Prostate. Proc. Natl. Acad. Sci. U. S. A 2016, 113, E5464–E5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (703).Trokowski R; Ren J; Kálmán FK; Sherry AD Selective Sensing of Zinc Ions with a PARACEST Contrast Agent. Angew. Chem. Int. Ed 2005, 44, 6920–6923. [DOI] [PubMed] [Google Scholar]
- (704).Zhang X.-a.; Lovejoy KS; Jasanoff A; Lippard SJ Water-Soluble Porphyrins as a Dual-Function Molecular Imaging Platform for MRI and Fluorescence Zinc Sensing. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 10780–10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (705).Lee T; Zhang X.-a.; Dhar S; Faas H; Lippard SJ; Jasanoff A In Vivo Imaging with a Cell-Permeable Porphyrin-Based MRI Contrast Agent. Chem. Biol 2010, 17, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (706).Li W.-h.; Fraser SE; Meade TJ A Calcium-Sensitive Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc 1999, 121, 1413–1414. [Google Scholar]
- (707).Li W.-h.; Parigi G; Fragai M; Luchinat C; Meade TJ Mechanistic Studies of a Calcium-Dependent MRI Contrast Agent. Inorg. Chem 2002, 41, 4018–4024. [DOI] [PubMed] [Google Scholar]
- (708).MacRenaris KW; Ma Z; Krueger RL; Carney CE; Meade TJ Cell-Permeable Esterase-Activated Ca(II)-Sensitive MRI Contrast Agent. Bioconjugate Chem 2016, 27, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (709).Dhingra K; Maier ME; Beyerlein M; Angelovski G; Logothetis NK Synthesis and Characterization of a Smart Contrast Agent Sensitive to Calcium. Chem. Commun 2008, 0, 3444–3446. [DOI] [PubMed] [Google Scholar]
- (710).Kubíček V; Vitha T; Kotek J; Hermann P; Vander Elst L; Muller RN; Lukeš I; Peters JA Towards MRI Contrast Agents Responsive to Ca(II) and Mg(II) Ions: Metal-Induced Oligomerization of Dota–Bisphosphonate Conjugates. Contrast Media. Mol. Imag 2010, 5, 294–296. [DOI] [PubMed] [Google Scholar]
- (711).Angelovski G; Chauvin T; Pohmann R; Logothetis NK; Tóth É Calcium-responsive Paramagnetic CEST Agents. Bioorg. Med. Chem 2011, 19, 1097–1105. [DOI] [PubMed] [Google Scholar]
- (712).Atanasijevic T; Shusteff M; Fam P; Jasanoff A Calcium-sensitive MRI Contrast Agents Based on Superparamagnetic Iron Oxide Nanoparticles and Calmodulin. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 14707–14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (713).Atanasijevic T; Jasanoff A Preparation of Iron Oxide-Based Calcium Sensors for MRI. Nat. Protoc 2007, 2, 2582–2589. [DOI] [PubMed] [Google Scholar]
- (714).Garello F; Vibhute S; Gündüz S; Logothetis NK; Terreno E; Angelovski G Innovative Design of Ca-Sensitive Paramagnetic Liposomes Results in an Unprecedented Increase in Longitudinal Relaxivity. Biomacromolecules 2016, 17, 1303–1311. [DOI] [PubMed] [Google Scholar]
- (715).Que EL; Chang CJ A Smart Magnetic Resonance Contrast Agent for Selective Copper Sensing. J. Am. Chem. Soc 2006, 128, 15942–15943. [DOI] [PubMed] [Google Scholar]
- (716).Que EL; Gianolio E; Baker SL; Wong AP; Aime S; Chang CJ Copper-Responsive Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc 2009, 131, 8527–8536. [DOI] [PubMed] [Google Scholar]
- (717).Que EL; Gianolio E; Baker SL; Aime S; Chang CJ A Copper-activated Magnetic Resonance Imaging Contrast Agent with Improved Turn-On Relaxivity Response and Anion Compatibility. Dalton Trans 2010, 39, 469–476. [DOI] [PubMed] [Google Scholar]
- (718).Que EL; New EJ; Chang CJ A Cell-Permeable Gadolinium Contrast Agent for Magnetic Resonance Imaging of Copper in a Menkes Disease Model. Chem. Sci 2012, 3, 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (719).Li W-S; Luo J; Chen Z-N A Gadolinium(III) Complex with 8-Amidequinoline Based Ligand as Copper(II) Ion Responsive Contrast Agent. Dalton Trans 2011, 40, 484–488. [DOI] [PubMed] [Google Scholar]
- (720).De-Miguel FF; Fuxe K Extrasynaptic Neurotransmission as a Way of Modulating Neuronal Functions. Front. Physiol 2012, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (721).Hyman SE Neurotransmitters. Curr. Biol 2005, 15, R154–R158. [DOI] [PubMed] [Google Scholar]
- (722).Devor A; Bandettini Peter A.; Boas David A.; Bower James M.; Buxton Richard B.; Cohen Lawrence B.; Dale Anders M.; Einevoll Gaute T.; Fox Peter T.; Franceschini Maria A., et al. The Challenge of Connecting the Dots in the B.R.A.I.N. Neuron 2013, 80, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (723).Klohs J; Rudin M Unveiling Molecular Events in the Brain by Noninvasive Imaging. Neuroscientist 2011, 17, 539–559. [DOI] [PubMed] [Google Scholar]
- (724).Rajendra DB Neurotransmitter Imaging: Basic Concepts and Future Perspectives. Curr. Med. Imaging Rev 2011, 7, 98–103. [Google Scholar]
- (725).Bloom JD; Meyer MM; Meinhold P; Otey CR; MacMillan D; Arnold FH Evolving Strategies for Enzyme Engineering. Curr. Opin. Struct. Biol 2005, 15, 447–452. [DOI] [PubMed] [Google Scholar]
- (726).Lee T; Cai LX; Lelyveld VS; Hai A; Jasanoff A Molecular-Level Functional Magnetic Resonance Imaging of Dopaminergic Signaling. Science 2014, 344, 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (727).Shapiro MG; Westmeyer GG; Romero PA; Szablowski JO; Küster B; Shah A; Otey CR; Langer R; Arnold FH; Jasanoff A Directed Evolution of a Magnetic Resonance Imaging Contrast Agent for Noninvasive Imaging of Dopamine. Nat. Biotechnol 2010, 28, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (728).Oukhatar F; Même S; Même W; Szeremeta F; Logothetis NK; Angelovski G; Tóth É MRI Sensing of Neurotransmitters with a Crown Ether Appended Gd3+ Complex. ACS Chem. Neurosci 2015, 6, 219–225. [DOI] [PubMed] [Google Scholar]
- (729).Zheng X; Qian J; Tang F; Wang Z; Cao C; Zhong K Microgel-Based Thermosensitive MRI Contrast Agent. ACS Macro Lett 2015, 4, 431–435. [DOI] [PubMed] [Google Scholar]
- (730).de Smet M; Langereis S; den Bosch S. v.; Grüll H Temperature-sensitive Liposomes for Doxorubicin Delivery under MRI Guidance. J. Control. Release 2010, 143, 120–127. [DOI] [PubMed] [Google Scholar]
- (731).Zhang S; Malloy CR; Sherry AD MRI Thermometry Based on PARACEST Agents. J. Am. Chem. Soc 2005, 127, 17572–17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (732).Li AX; Wojciechowski F; Suchy M; Jones CK; Hudson RHE; Menon RS; Bartha R A Sensitive PARACEST Contrast Agent for Temperature MRI: Eu3+-DOTAM-glycine (Gly)-phenylalanine (Phe). Magn. Reson. Med 2008, 59, 374–381. [DOI] [PubMed] [Google Scholar]
- (733).Stevens TK; Milne M; Elmehriki AAH; Suchý M; Bartha R; Hudson RHE A DOTAM-Based ParaCEST Agent Favoring TSAP Geometry for Enhanced Amide Proton Chemical Shift Dispersion and Temperature Sensitivity. Contrast Media. Mol. Imag 2013, 8, 289–292. [DOI] [PubMed] [Google Scholar]
- (734).Tsitovich PB; Cox JM; Benedict JB; Morrow JR Six-coordinate Iron(II) and Cobalt(II) ParaSHIFT Agents for Measuring Temperature by Magnetic Resonance Spectroscopy. Inorg. Chem 2016, 55, 700–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (735).Tsitovich PB; Tittiris TY; Cox JM; Benedict JB; Morrow JR Fe(II) and Co(II) N-methylated CYCLEN Complexes as ParaSHIFT Agents with Large Temperature Dependent Shifts. Dalton Trans. 2018, 47, 916–924. [DOI] [PubMed] [Google Scholar]
- (736).Osborne EA; Jarrett BR; Tu C; Louie AY Modulation of T2 Relaxation Time by Light-Induced, Reversible Aggregation of Magnetic Nanoparticles. J. Am. Chem. Soc 2010, 132, 5934–5935. [DOI] [PubMed] [Google Scholar]
- (737).Wu Y; Carney CE; Denton M; Hart E; Zhao P; Streblow DN; Sherry AD; Woods M Polymeric PARACEST MRI Contrast Agents as Potential Reporters for Gene Therapy. Org. Biomol. Chem 2010, 8, 5333–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (738).Smolensky ED; Peterson KL; Weitz EA; Lewandowski C; Pierre VC Magnetoluminescent Light Switches – Dual Modality in DNA Detection. J. Am. Chem. Soc 2013, 135, 8966–8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (739).Rodríguez E; Nilges M; Weissleder R; Chen JW Activatable Magnetic Resonance Imaging Agents for Myeloperoxidase Sensing: Mechanism of Activation, Stability, and Toxicity. J. Am. Chem. Soc 2010, 132, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (740).Shazeeb MS; Xie Y; Gupta S; Bogdanov AA Jr. A Novel Paramagnetic Substrate for Detecting Myeloperoxidase Activity In Vivo. Mol. Imaging 2012, 11, 433–443. [PMC free article] [PubMed] [Google Scholar]
- (741).Su HS; Nahrendorf M; Panizzi P; Breckwoldt MO; Rodriguez E; Iwamoto Y; Aikawa E; Weissleder R; Chen JW Vasculitis: Molecular Imaging by Targeting the Inflammatory Enzyme Myeloperoxidase. Radiology 2012, 262, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (742).Nahrendorf M; Sosnovik D; Chen JW; Panizzi P; Figueiredo J-L; Aikawa E; Libby P; Swirski FK; Weissleder R Activatable Magnetic Resonance Imaging Agent Reports Myeloperoxidase Activity in Healing Infarcts and Noninvasively Detects the Antiinflammatory Effects of Atorvastatin on Ischemia-Reperfusion Injury. Circulation 2008, 117, 1153–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (743).Breckwoldt MO; Chen JW; Stangenberg L; Aikawa E; Rodriguez E; Qiu S; Moskowitz MA; Weissleder R Tracking the Inflammatory Response in Stroke In Vivo by Sensing the Enzyme Myeloperoxidase. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 18584–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (744).DeLeo MJ III; Gounis MJ; Hong B; Ford JC; Wakhloo AK; Bogdanov AA Jr. Carotid Artery Brain Aneurysm Model: In Vivo Molecular Enzyme-Specific MR Imaging of Active Inflammation in a Pilot Study. Radiology 2009, 252, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (745).Forghani R; Wojtkiewicz GR; Zhang YN; Seeburg D; Bautz BRM; Pulli B; Milewski AR; Atkinson WL; Iwamoto Y; Zhang ER, et al. Demyelinating Diseases: Myeloperoxidase as an Imaging Biomarker and Therapeutic Target. Radiology 2012, 263, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (746).Loving GS; Caravan P Activation and Retention: A Magnetic Resonance Probe for the Detection of Acute Thrombosis. Angew. Chem. Int. Ed 2014, 53, 1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (747).Haedicke IE; Li T; Zhu YLK; Martinez F; Hamilton AM; Murrell DH; Nofiele JT; Cheng HLM; Scholl TJ; Foster PJ, et al. An Enzyme-Activatable and Cell-Permeable Mn-III Porphyrin as a Highly Efficient T-1 MRI Contrast Agent for Cell Labeling. Chem. Sci 2016, 7, 4308–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (748).Loai S; Haedicke I; Mirzaei Z; Simmons CA; Zhang X; Cheng HL Positive-Contrast Cellular MRI of Embryonic Stem Cells for Tissue Regeneration Using a Highly Efficient T-1 MRI Contrast Agent. J. Magn. Reson. Imaging 2016, 44, 1456–1463. [DOI] [PubMed] [Google Scholar]
- (749).Jirak D; Kriz J; Herynek V; Andersson B; Girman P; Burian M; Saudek F; Hajek M MRI of Transplanted Pancreatic Islets. Magn. Reson. Med 2004, 52, 1228–1233. [DOI] [PubMed] [Google Scholar]
- (750).Toso C; Valle JP; Morel P; Ris F; Demuylder-Mischler S; Lepetit-Coiffe M; Marangon N; Saudek F; Shapiro AMJ; Bosco D, et al. Clinical Magnetic Resonance Imaging of Pancreatic Islet Grafts after Iron Nanoparticle Labeling. Am. J. Transplant 2008, 8, 701–706. [DOI] [PubMed] [Google Scholar]
- (751).Saudek F; Jirak D; Girman P; Herynek V; Dezortova M; Kriz J; Peregrin J; Berkova Z; Zacharovona K; Hajek M Magnetic Resonance Imaging of Pancreatic Islets Transplanted Into the Liver in Humans. Transplantation 2010, 90, 1602–1606. [DOI] [PubMed] [Google Scholar]
- (752).Barnett BP; Ruiz-Cabello J; Hota P; Ouwerkerk R; Shamblott MJ; Lauzon C; Walczak P; Gilson WD; Chacko VP; Kraitchman DL, et al. Use of Perfluorocarbon Nanoparticles for Non-Invasive Multimodal Cell Tracking of Human Pancreatic Islets. Contrast Media. Mol. Imag 2011, 6, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (753).de Vries IJM; Lesterhuis WJ; Barentsz JO; Verdijk P; van Krieken JH; Boerman OC; Oyen WJG; Bonenkamp JJ; Boezeman JB; Adema GJ, et al. Magnetic Resonance Tracking of Dendritic Cells in Melanoma Patients for Monitoring of Cellular Therapy. Nat. Biotechnol 2005, 23, 1407–1413. [DOI] [PubMed] [Google Scholar]
- (754).Long CM; van Laarhoven HWM; Bulte JWM; Levitsky HI Magnetovaccination as a Novel Method to Assess and Quantify Dendritic Cell Tumor Antigen Capture and Delivery to Lymph Nodes. Cancer Res 2009, 69, 3180–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (755).Zhu JH; Zhou LF; XingWu FG Tracking Neural Stem Cells in Patients with Brain Trauma. New Engl. J. Med 2006, 355, 2376–2378. [DOI] [PubMed] [Google Scholar]
- (756).Callera F; Melo C Magnetic Resonance Tracking of Magnetically Labeled Autologous Bone Marrow CD34(+) Cells Transplanted into the Spinal Cord via Lumbar Puncture Technique in Patients with Chronic Spinal Cord Injury: CD34(+) Cells’ Migration into the Injured Site. Stem Cells Dev 2007, 16, 461–466. [DOI] [PubMed] [Google Scholar]
- (757).Pumphrey AL; Ye SJ; Yang ZS; Simkin J; Gensel JC; Abdel-Latif A; Vandsburger MH Cardiac Chemical Exchange Saturation Transfer MR Imaging Tracking of Cell Survival or Rejection in Mouse Models of Cell Therapy. Radiology 2017, 282, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (758).Khurana A; Chapelin F; Xu HY; Acevedo JR; Molinolo A; Nguyen Q; Ahrens ET Visualization of Macrophage Recruitment in Head and Neck Carcinoma Model Using Fluorine-19 Magnetic Resonance Imaging. Magn. Reson. Med 2018, 79, 1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (759).Zhong J; Narsinh K; Morel PA; Xu HY; Ahrens ET In Vivo Quantification of Inflammation in Experimental Autoimmune Encephalomyelitis Rats Using Fluorine-19 Magnetic Resonance Imaging Reveals Immune Cell Recruitment outside the Nervous System. PLOS ONE 2015, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (760).Iordanova B; Hitchens TK; Robison CS; Ahrens ET Engineered Mitochondrial Ferritin as a Magnetic Resonance Imaging Reporter in Mouse Olfactory Epithelium. PLOS ONE 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (761).Farrar CT; Buhrman JS; Liu G; Kleijn A; Lamfers ML; McMahon MT; Gilad AA; Fulci G Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy. Radiology 2015, 275, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (762).Bartelle BB; Szulc KU; Suero-Abreu GA; Rodriguez JJ; Turnbull DH Divalent Metal Transporter, DMT1: A Novel MRI Reporter Protein. Magn. Reson. Med 2013, 70, 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]


