Abstract
Background
Early accurate detection of all skin cancer types is important to guide appropriate management, to reduce morbidity and to improve survival. Basal cell carcinoma (BCC) is almost always a localised skin cancer with potential to infiltrate and damage surrounding tissue, whereas a minority of cutaneous squamous cell carcinomas (cSCCs) and invasive melanomas are higher‐risk skin cancers with the potential to metastasise and cause death. Dermoscopy has become an important tool to assist specialist clinicians in the diagnosis of melanoma, and is increasingly used in primary‐care settings. Dermoscopy is a precision‐built handheld illuminated magnifier that allows more detailed examination of the skin down to the level of the superficial dermis. Establishing the value of dermoscopy over and above visual inspection for the diagnosis of BCC or cSCC in primary‐ and secondary‐care settings is critical to understanding its potential contribution to appropriate skin cancer triage, including referral of higher‐risk cancers to secondary care, the identification of low‐risk skin cancers that might be treated in primary care and to provide reassurance to those with benign skin lesions who can be safely discharged.
Objectives
To determine the diagnostic accuracy of visual inspection and dermoscopy, alone or in combination, for the detection of (a) BCC and (b) cSCC, in adults. We separated studies according to whether the diagnosis was recorded face‐to‐face (in person) or based on remote (image‐based) assessment.
Search methods
We undertook a comprehensive search of the following databases from inception up to August 2016: Cochrane Central Register of Controlled Trials; MEDLINE; Embase; CINAHL; CPCI; Zetoc; Science Citation Index; US National Institutes of Health Ongoing Trials Register; NIHR Clinical Research Network Portfolio Database; and the World Health Organization International Clinical Trials Registry Platform. We studied reference lists and published systematic review articles.
Selection criteria
Studies of any design that evaluated visual inspection or dermoscopy or both in adults with lesions suspicious for skin cancer, compared with a reference standard of either histological confirmation or clinical follow‐up.
Data collection and analysis
Two review authors independently extracted all data using a standardised data extraction and quality assessment form (based on QUADAS‐2). We contacted authors of included studies where information related to the target condition or diagnostic thresholds were missing. We estimated accuracy using hierarchical summary ROC methods. We undertook analysis of studies allowing direct comparison between tests. To facilitate interpretation of results, we computed values of sensitivity at the point on the SROC curve with 80% fixed specificity and values of specificity with 80% fixed sensitivity. We investigated the impact of in‐person test interpretation; use of a purposely‐developed algorithm to assist diagnosis; and observer expertise.
Main results
We included 24 publications reporting on 24 study cohorts, providing 27 visual inspection datasets (8805 lesions; 2579 malignancies) and 33 dermoscopy datasets (6855 lesions; 1444 malignancies). The risk of bias was mainly low for the index test (for dermoscopy evaluations) and reference standard domains, particularly for in‐person evaluations, and high or unclear for participant selection, application of the index test for visual inspection and for participant flow and timing. We scored concerns about the applicability of study findings as of ‘high’ or 'unclear' concern for almost all studies across all domains assessed. Selective participant recruitment, lack of reproducibility of diagnostic thresholds and lack of detail on observer expertise were particularly problematic.
The detection of BCC was reported in 28 datasets; 15 on an in‐person basis and 13 image‐based. Analysis of studies by prior testing of participants and according to observer expertise was not possible due to lack of data. Studies were primarily conducted in participants referred for specialist assessment of lesions with available histological classification. We found no clear differences in accuracy between dermoscopy studies undertaken in person and those which evaluated images. The lack of effect observed may be due to other sources of heterogeneity, including variations in the types of skin lesion studied, in dermatoscopes used, or in the use of algorithms and varying thresholds for deciding on a positive test result.
Meta‐analysis found in‐person evaluations of dermoscopy (7 evaluations; 4683 lesions and 363 BCCs) to be more accurate than visual inspection alone for the detection of BCC (8 evaluations; 7017 lesions and 1586 BCCs), with a relative diagnostic odds ratio (RDOR) of 8.2 (95% confidence interval (CI) 3.5 to 19.3; P < 0.001). This corresponds to predicted differences in sensitivity of 14% (93% versus 79%) at a fixed specificity of 80% and predicted differences in specificity of 22% (99% versus 77%) at a fixed sensitivity of 80%. We observed very similar results for the image‐based evaluations.
When applied to a hypothetical population of 1000 lesions, of which 170 are BCC (based on median BCC prevalence across studies), an increased sensitivity of 14% from dermoscopy would lead to 24 fewer BCCs missed, assuming 166 false positive results from both tests. A 22% increase in specificity from dermoscopy with sensitivity fixed at 80% would result in 183 fewer unnecessary excisions, assuming 34 BCCs missed for both tests. There was not enough evidence to assess the use of algorithms or structured checklists for either visual inspection or dermoscopy.
Insufficient data were available to draw conclusions on the accuracy of either test for the detection of cSCCs.
Authors' conclusions
Dermoscopy may be a valuable tool for the diagnosis of BCC as an adjunct to visual inspection of a suspicious skin lesion following a thorough history‐taking including assessment of risk factors for keratinocyte cancer. The evidence primarily comes from secondary‐care (referred) populations and populations with pigmented lesions or mixed lesion types. There is no clear evidence supporting the use of currently‐available formal algorithms to assist dermoscopy diagnosis.
Plain language summary
Does dermoscopy improve the accuracy of diagnosing basal cell or squamous cell skin cancer (BCC or cSCC) compared to using the naked eye alone?
What is the aim of the review?
We wanted to find out whether using a handheld illuminated microscope (dermatoscope or ‘dermoscopy’) is any better at diagnosing basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC) compared to just looking at the skin with the naked eye. We included 24 studies to answer this question.
Why is improving diagnosis of BCC or cSCC important?
There are a number of different types of skin cancer. BCC and cSCC are less serious than melanoma skin cancer, because they usually grow more slowly and BCC does not spread to other organs in the body. Making the correct diagnosis of BCC or cSCC is still important, because their treatment may differ. A missed BCC (known as a false negative result) can result in disfigurement and the need for more major surgery. A missed cSCC can spread to other parts of the body. Diagnosing BCC or cSCC when they are not actually present (a false positive result) may mean unnecessary treatment, e.g. surgical removal which may result in a disfiguring scar, and worry to patients if the lesion (a mole or area of skin with an unusual appearance in comparison with the surrounding skin) is benign (not a cancer), or may result in wrong treatment, e.g. a non‐surgical therapy, being used if the lesion is misdiagnosed.
What was studied in the review?
A dermatoscope is a handheld magnifier that includes a light source. Dermoscopy is often used by skin specialists to help diagnose skin cancer. It is also being used more by community doctors.
As well as seeing whether dermoscopy added anything to visual inspection alone overall, we also wanted to find out whether dermoscopy accuracy was different when used in a face‐to‐face consultation or when used on images of skin lesions sent to specialists. We also tried to find out whether the accuracy of dermoscopy was improved by use of a checklist, or if it was better when used by a skin specialist compared to a non‐specialist.
What are the main results of the review?
The review included 24 studies reporting information for people with lesions suspected of skin cancer.
Diagnosis of BCC with the patient present
We found 11 relevant studies. Eight studies (including 7017 suspicious skin lesions) investigated the accuracy of visual inspection on its own and seven studies (with 4683 suspicious skin lesions) investigated the accuracy of dermoscopy added to visual inspection (four of which reported data for both visual inspection on its own and for dermoscopy added to visual inspection). The results suggest that dermoscopy is more accurate than visual inspection on its own, both for identifying BCC correctly and for excluding things that are not BCCs.
The results can be illustrated using a group of 1000 lesions, of which 170 (17%) are BCC. In order to see how much better dermoscopy is in identifying BCC correctly when compared to just looking at the skin, we have to assume that both lead to the same number of lesions being falsely diagnosed as BCC (we assumed that 166 of the 830 lesions without BCC would have an incorrect diagnosis of BCC). In this fixed situation, adding dermoscopy to visual inspection would correctly identify an extra 24 BCCs (158 compared with 134) that would have been missed by just looking at the skin alone. In other words, more BCC cancers would be correctly identified.
In order to see how much better dermoscopy is in deciding if a skin lesion is not a BCC when compared to just looking at the skin, we have to assume that both lead to the same number of BCCs being correctly diagnosed (in this case we assumed that 136 out of the 170 BCCs would be correctly diagnosed). In this situation, adding in dermoscopy to visual inspection would reduce the number of lesions being wrongly diagnosed as being BCC by 183 (a reduction from 191 in the visual inspection group to eight people in the dermoscopy group). In other words, more lesions that were not BCC would be correctly identified, and fewer people would end up being sent for surgery.
Image‐based diagnosis of BCC
Eleven studies concerning BCC diagnosis using either clinical photographs or magnified images from a dermatoscope were included. Four studies, (including 853 suspicious skin lesions) used visual inspection of photographs and nine studies (including 2271 suspicious lesions) used dermoscopic images (two studies reported data for diagnosis using both photographs and using dermoscopic images). Results were very similar to the in‐person studies.
Value of checklists and observer expertise
There was no evidence that use of a checklist to help visual inspection or dermoscopy interpretation improved diagnostic accuracy. There was not enough evidence to examine the effect of clinical expertise and training.
Diagnosis of cSCC
There was not enough evidence to reliably comment on the accuracy of either test for the detection of cSCCs.
How reliable are the results of the studies of this review?
Most of our studies made a reliable final diagnosis by lesion biopsy and by following people up over time to make sure the skin lesion remained negative for skin cancer. Some studies used expert diagnosis to confirm the absence of skin cancer, which is less reliable*. Poor reporting of what was done in the studies made it difficult for us to judge how reliable they were. Some studies excluded certain types of skin lesion and some did not describe how a positive test result to trigger referral to a specialist or treatment was defined.
Who do the results of this review apply to?
Eleven studies were done in Europe (46%), and the rest in North America (n = 3), Asia (n = 5), Oceania (n = 2), or multiple countries (n = 3). People included in the studies were on average between 30 and 74 years old. The percentage of people with BCC ranged between 1% and 61% for in‐person studies and between 2% and 63% in studies using images. Almost all studies were done with people referred from primary care to specialist skin clinics. Over half of studies considered the ability of dermoscopy and visual inspection to diagnose any skin cancer, including melanoma and BCC, while 10 (42%) focused on just BCC. Variation in the expertise of doctors doing the examinations and differences in the definitions used to decide when a test was positive make it unclear how dermoscopy should be carried out and what level of training is needed in order to achieve the accuracy observed in studies.
What are the implications of this review?
When used by specialists, dermoscopy may be a useful tool to help diagnose BCC correctly when compared with visual inspection alone. It is not clear whether dermoscopy should be used by general practitioners to correctly identify people with suspicious lesions who need to be seen by a specialist. Checklists to help interpret dermoscopy do not seem to help improve accuracy for BCC. Further research is needed, to see if dermoscopy is useful in primary care.
How up‐to‐date is this review?
The review authors searched for and used studies published up to August 2016.
*In these studies biopsy, clinical follow‐up or specialist clinician diagnosis were the reference standards (means of establishing the final diagnosis).
Summary of findings
Summary of findings'. 'Summary of findings table.
| Question: | What is the diagnostic accuracy of dermoscopy, in comparison to visual inspection, for the detection of keratinocyte skin cancer in adults? | |||||||
| Population: | Adults with skin lesions: suspicious for keratinocyte skin cancers, basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC) (e.g. non‐pigmented lesions); suspicious for any skin cancer, including melanoma (e.g. those with pigmented lesions only or mixed populations of pigmented and non‐pigmented lesions); or those at high risk of developing keratinocyte skin cancer | |||||||
| Index test: | Dermoscopy with or without the use of any established algorithms or checklist to aid diagnosis, including: in‐person evaluations (face‐to‐face diagnosis), and image‐based evaluations (diagnosis based on assessment of a dermoscopic image) | |||||||
| Comparator test | Visual inspection including: in‐person evaluations, and image‐based evaluations (diagnosis based on assessment of a clinical image) | |||||||
| Primary Target condition: | BCC or cSCC | |||||||
| Reference standard: | Histology with or without long‐term follow‐up | |||||||
| Action: | If accurate, negative results will stop patients having unnecessary excision or biopsy of skin lesions; positive results could inform the use of nonsurgical management options | |||||||
| Number of studies | Total lesions | Total malignancies | ||||||
| Quantity of evidence | 24 | Visual Inspection: 8805 Dermoscopy: 6855 |
Visual Inspection: 2579 Dermoscopy: 1444 |
|||||
| Limitations | ||||||||
| Risk of bias: (in‐person (14); image‐based (12)) | Potential risk of bias for participant selection from use of case‐control type design (3 image‐based), inappropriate exclusion criteria (3; 2) or lack of detail (8; 4). All visual inspection and dermoscopy interpretation considered blinded to reference standard diagnosis. Visual Inspection risk of bias not clear due to thresholds not clearly prespecified (8; 4). Threshold prespecification better reported for dermoscopy (6; 6). Low risk for reference standard (13; 11); high risk from use of expert diagnosis or > 20% of benign lesions with no histology (1; 1). High risk for participant flow due to differential verification (1; 1), and exclusions following recruitment (5; 6); timing of tests was not mentioned in (7; 7) | |||||||
| Applicability of evidence to question: (in‐person (14); image‐based (12)) | High concern for participants (14; 12) due to restriction to those with histopathology results (13; 11) and including multiple lesions per participant (9; 2). High concern for Visual Inspection (7; 4) from lack of description of diagnostic thresholds. High concern for dermoscopy (3; 9) from no description of diagnostic thresholds (2; 4) or reporting of average or consensus diagnoses (2; 7). Dermoscopic image interpretation blinded to clinical images (10 image‐based). Unclear applicability of reference standard due to insufficient information concerning the expertise of the histopathologist (13; 11) | |||||||
| FINDINGS: | ||||||||
| We included 24 studies. 14 studies reported data for in‐person visual inspection (n = 11) or in‐person dermoscopy (n = 8); 12 studies reported data for image‐based visual inspection (n = 4) or image‐based dermoscopy (n = 10). Two studies report both in‐person and image‐based data. The findings presented are based on results for the 21 studies reporting data for BCC alone or for cSCC alone. Due to the observed heterogeneity between studies, the results presented are points estimated from summary ROC curves rather than average sensitivity and specificity operating points. These are presented for illustrative purposes and should not be quoted as the actual performance of visual inspection or dermoscopy. We did not undertake analyses of studies by degree of prior testing due to a lack of relevant information provided in the study publications, most studies apparently being conducted in referred populations, and small study subgroups. There was not enough evidence to assess the use of algorithms or structured checklists for dermoscopy (or visual inspection) | ||||||||
| Test (for BCC): | In‐person visual inspection alone versus visual inspection plus dermoscopy for the detection of BCC – any algorithm or threshold | |||||||
| Data analysed | Visual inspection | 8 datasets ‐ 7017 lesions; 1586 cases | ||||||
| Dermoscopy | 7 datasets ‐ 4683 lesions; 363 cases | |||||||
| Resultsa | Sensitivity | Fixed specificity | Fixed sensitivity | Specificity | ||||
| Visual inspection | 79% | 80% | 80% | 77% | ||||
| Dermoscopy | 93% | 99% | ||||||
| Numbers applied to a hypothetical cohort of 1000 lesionsb | ||||||||
| TP | FN | FP | TN | TP | FN | FP | TN | |
| At a prevalence of 10% | VI: 79 D: 93 ↑ 14 |
VI: 21 D: 7 ↓ 14 |
180 | 720 | 80 | 20 | VI: 207 D: 9 ↓198 |
VI: 693 D: 891 ↑198 |
| At a prevalence of 17% | VI: 134 D: 158 ↑24 |
VI: 36 D: 12 ↓ 24 |
166 | 664 | 136 | 34 | VI: 191 D: 8 ↓183 |
VI: 639 D: 822 ↑183 |
| At a prevalence of 53% | VI: 419 D: 493 ↑ 74 |
VI: 111 D: 37 ↓ 74 |
94 | 376 | 424 | 106 | VI: 108 D: 5 ↓103 |
VI: 362 D: 465 ↑103 |
| Consistency: | Wide range in prevalence of BCC; includes pigmented and non‐pigmented lesion populations and participants suspected of BCC or suspected of any malignancy, including melanoma. Sensitivities highly heterogeneous, particularly for visual‐inspection evaluations. Specificity for BCC lower in studies of non‐pigmented lesions | |||||||
| Test (for BCC): | Image‐based visual inspection alone versus visual inspection plus dermoscopy for the detection of BCC – any algorithm or threshold | |||||||
| Data analysed | Visual inspection | 4 datasets ‐ 853 lesions; 156 cases | ||||||
| Dermoscopy | 9 datasets ‐ 2271 lesions; 737 cases | |||||||
| Results | Sensitivity | Fixed specificity | Fixed sensitivity | Specificity | ||||
| Visual inspection | 85% | 80% | 80% | 87% | ||||
| Dermoscopy | 93% | 96% | ||||||
| Numbers applied to a hypothetical cohort of 1000 lesionsc | ||||||||
| TP | FN | FP | TN | TP | FN | FP | TN | |
| At a prevalence of 11% | VI: 94 D: 102 ↑ 8 |
VI: 16 D: 8 ↓ 8 |
178 | 712 | 88 | 22 | VI: 116 D: 36 ↓80 |
VI: 774 D: 854 ↑80 |
| At a prevalence of 16% | VI: 136 D: 149 ↑13 |
VI: 24 D: 11 ↓ 13 |
168 | 672 | 128 | 32 | VI: 109 D: 34 ↓75 |
VI: 731 D: 806 ↑75 |
| At a prevalence of 47% | VI: 400 D: 437 ↑ 37 |
VI: 70 D: 33 ↓ 37 |
106 | 424 | 376 | 94 | VI: 69 D: 21 ↓48 |
VI: 461 D: 509 ↑48 |
| Consistency: | Wide range in prevalence of BCC; includes mixed populations, as for in‐person evaluations. Sensitivities highly heterogeneous for visual inspection evaluations. | |||||||
| Test (for cSCC): | Visual inspection or dermoscopy for the detection of cSCC | |||||||
| Datasets | Lesions | Cases | Sensitivity | (95%CIs) | Specificity | (95%CI) | ||
| Visual inspection (in‐person) | 2 | 2684 | 538 | 57% | (53%, 61%) | 79% | (77%, 81%) | |
| Dermoscopy (image‐based) | 2 | 717 | 119 | 55% | (29%, 79%) | 84% | (32%, 98%) | |
aNumbers for a hypothetical cohort of 1000 lesions are presented for two illustrative examples of points on the SROC curves: firstly for the sensitivities of tests at fixed specificities of 80%; and secondly for the specificities of tests at fixed sensitivities of 80%. bNumbers estimated at 25th, 50th (median) and 75% percentiles of BCC prevalence observed across 11 studies reporting in‐person evaluations of visual inspection (reported in eight studies) or visual inspection plus dermoscopy (reported in seven studies). cNumbers estimated at 25th, 50th (median) and 75% percentiles of BCC prevalence observed across 11 studies reporting image‐based diagnosis using clinical photographs (reported in four studies) or dermoscopic images (reported in nine studies)
Background
This review is one of a series of Cochrane Diagnostic Test Accuracy (DTA) Reviews on the diagnosis and staging of melanoma and keratinocyte skin cancers as part of the National Institute for Health Research (NIHR) Cochrane Systematic Reviews Programme. Appendix 1 shows the content and structure of the programme.
Target condition being diagnosed
The commonest skin cancers in white populations are those arising from keratinocyte cells: basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) (Gordon 2013; Madan 2010). BCC is the more common of the two keratinocyte carcinomas, and approximately one‐third of people with a BCC will subsequently develop a second (Flohill 2013). In 2003, the World Health Organization (WHO) estimated that between two and three million ‘non‐melanoma’ skin cancers (of which BCC and cSCC are estimated to account for around 80% and 16% of cases respectively) and 132,000 melanoma skin cancers occur globally each year (WHO 2003).
Rather than defining BCC and cSCC by what they are not (i.e. non‐melanoma skin cancer), we collectively refer to these conditions using the preferred and more accurate term of 'keratinocyte carcinoma' in this DTA review (Karimkhani 2015). We define (a) BCC and (b) cSCC as the primary target conditions for this review. We also examine accuracy for the target condition of (c) any skin cancer, including keratinocyte skin cancer, melanoma or intra‐epidermal melanocytic variants and any other skin cancer. We have examined the accuracy of visual inspection for the diagnosis of melanoma in a previous review (Dinnes 2018a) and in a further review, we examine the potential benefit of dermoscopy added to visual inspection for the diagnosis of melanoma (Dinnes 2018b). Appendix 2 provides a glossary of terms used.
Basal cell carcinoma
BCC can arise from multiple stem cell populations, including from the follicular bulge and interfollicular epidermis (Grachtchouk 2011). Growth is usually localised, but it can infiltrate and damage surrounding tissue, which if left untreated can cause considerable destruction and disfigurement, particularly when located on the face (Figure 1). The four main types of BCC are superficial, nodular, morphoeic (infiltrative), and pigmented. Lesions typically present as slow‐growing asymptomatic papules, plaques, or nodules, which may bleed or form ulcers that do not heal (Firnhaber 2012). People with a BCC often present themselves to healthcare professionals with a non‐healing lesion rather than specific symptoms such as pain. Many lesions are diagnosed incidentally (Gordon 2013).
1.

Sample photograph of superficial spreading melanoma(left), BCC (centre) and SCC (right). Copyright © 2012 Dr Rubeta Matin: reproduced with permission.
BCC most commonly occurs on sun‐exposed areas of the head and neck (McCormack 1997), and are more common in men and in people over the age of 40. A rising incidence of BCC in younger people has been attributed to increased recreational sun exposure (Bath‐Hextall 2007a; Gordon 2013; Musah 2013). Other risk factors include Fitzpatrick skin types I and II (Fitzpatrick 1975; Lear 1997; Maia 1995); previous skin cancer history; immunosuppression; arsenic exposure; and genetic predisposition, such as in basal cell naevus (Gorlin) syndrome (Gorlin 2004; Zak‐Prelich 2004). Annual incidence is increasing worldwide; Europe has experienced an average increase of 5.5% per year since the 1970s, the USA 2% per year, while estimates for the UK show incidence appears to be increasing more steeply at a rate of an additional 6/100,000 persons a year (Lomas 2012). The rising incidence has been attributed to an ageing population, changes in the distribution of known risk factors, particularly ultraviolet radiation, and improved detection due to the increased awareness amongst both practitioners and the general population (Verkouteren 2017). Hoorens 2016 points to evidence for a gradual increase in the size of BCCs over time, with delays in diagnosis ranging from 19 to 25 months.
According to National Institute for Health and Care Excellence (NICE) guidance (NICE 2010), low‐risk BCCs are nodular lesions occurring in people older than 24 years who are not immunosuppressed and do not have Gorlin syndrome. Furthermore, lesions should be located below the clavicle; should be small (less than 1 cm) with clinically well‐defined margins; not recurrent following incomplete excision or other treatment; and not in awkward or highly‐visible locations (NICE 2010). Superficial BCCs are also typically low risk and may be amenable to medical treatments such as cryotherapy, photodynamic therapy or topical immunomodulatory therapy, e.g. 5% Imiquimod cream (Kelleners‐Smeets 2017). Assigning BCCs as low or high risk influences the management options (Batra 2002; Randle 1996).
Advanced locally‐destructive BCC can be found on the H‐area of the face (Lear 2014), can arise from long‐standing untreated lesions, or from a recurrence of aggressive basal cell carcinoma after primary treatment (Lear 2012). Very rarely, BCC may metastasise to regional and distant sites resulting in death; this is particularly true for large neglected lesions in those who are immunosuppressed, or those with Gorlin syndrome (McCusker 2014). Rates of metastasis are reported at 0.0028% to 0.55% with very poor survival rates (Lo 1991). It is recognised that basosquamous carcinoma (more like a high‐risk SCC in behaviour and not considered a true BCC) is likely to have accounted for many cases of apparent metastases of BCC, hence, the spuriously high reported incidence in some studies of up to 0.55%, which is not seen in clinical practice (Garcia 2009).
Squamous cell carcinoma of the skin
Primary cSCC arises from the keratinising cells of the epidermis or its appendages. cSCC typically presents with an ulcer or firm (indurated) papule, plaque, or nodule (Griffin 2016), often with an adherent crust (Madan 2010) (Figure 1). cSCC can arise in the absence of a precursor lesion, or may develop from pre‐existing actinic keratosis or Bowen's disease (considered by some clinicians to be cSCC in situ); the estimated annual risk of progression is less than 1% to 20% for newly‐arising lesions (Alam 2001) and 5% for pre‐existing lesions (Kao 1986). It remains locally invasive for a variable length of time, but has the potential to spread to the regional lymph nodes or via the bloodstream to distant sites, especially in immunosuppressed individuals (Lansbury 2010). High‐risk lesions are those arising on the lip or ear; recurrent cSCC; lesions arising on non‐exposed sites; within scars or chronic ulcers; tumours more than 20 mm in diameter and those with a histological depth of invasion exceeding 4 mm; and poor differentiation status on pathological examination (Motley 2009). Perineural nerve invasion (PNI) of at least 0.1 mm in diameter is a further documented risk factor for high‐risk cSCC (Carter 2013).
Chronic ultraviolet light exposure through recreation or occupation is strongly linked to cSCC occurrence (Alam 2001). It is particularly common in people with fair skin and in less common genetic disorders of pigmentation, such as albinism, xeroderma pigmentosum, and recessive dystrophic epidermolysis bullosa (RDEB) (Alam 2001). Other recognised risk factors include immunosuppression; chronic wounds; arsenic or radiation exposure; certain drug treatments, such as voriconazole and BRAF mutation inhibitors; and previous skin cancer history (Baldursson 1993; Chowdri 1996; Dabski 1986; Fasching 1989; Lister 1997; Maloney 1996; O'Gorman 2014). In solid organ transplant recipients, cSCC is the most common form of skin cancer; the risk of developing cSCC has been estimated at 65 to 253 times that of the general population (Hartevelt 1990; Jensen 1999; Lansbury 2010). Overall, local and metastatic recurrence of cSCC at five years is estimated at 8% and 5% respectively. The five‐year survival rate of metastatic cSCC of the head and neck is around 60% (Moeckelmann 2018).
Treatment
Treatment options for BCC and cSCC include surgery, other destructive techniques such as cryotherapy or electrodesiccation and topical chemotherapy. A Cochrane Review of 27 randomised controlled trials (RCTs) of interventions for BCC found very little good‐quality evidence for any of the interventions used (Bath‐Hextall 2007b). Complete surgical excision of primary BCC has a reported five‐year recurrence rate of less than 2% (Griffiths 2005; Walker 2006), leading to significantly fewer recurrences than treatment with radiotherapy (Bath‐Hextall 2007b). After apparent clear histopathological margins (serial vertical sections) after standard excision biopsy with 4 mm surgical peripheral margins taken, there is a five‐year reported recurrence rate of around 4% (Drucker 2017). Mohs micrographic surgery, whereby horizontal sections of the excised specimen are microscopically examined perioperatively, and re‐excision is undertaken until the margins are tumour‐free, can be considered for high‐risk lesions where standard wider excision margins might lead to incomplete excision or considerable functional and/or cosmetic impairment (Bath‐Hextall 2007b; Motley 2009; Lansbury 2010; Stratigos 2015). Bath‐Hextall 2007b found a single trial comparing Mohs micrographic surgery with a 3 mm surgical margin excision in BCC (Smeets 2004), showing non‐significantly lower recurrence at 10 years with Mohs micrographic surgery (4.4% compared to 12.2% after surgical excision, P = 0.10) (Van Loo 2014).
The main treatments for high‐risk BCC are wide local excision, Mohs micrographic surgery and radiotherapy. For low‐risk or superficial subtypes of BCC, or for small and/or multiple BCCs at low‐risk sites (Marsden 2010), destructive techniques other than excisional surgery may be used (e.g. electrodesiccation and curettage or cryotherapy (Alam 2001; Bath‐Hextall 2007b)). Alternatively, non‐surgical (or non‐destructive) treatments may be considered (Bath‐Hextall 2007b; Drew 2017; Kim 2014), including topical chemotherapy such as imiquimod (Williams 2017), 5‐fluorouracil (5‐FU) (Arits 2013), ingenol mebutate (Nart 2015) and photodynamic therapy (PDT) (Roozeboom 2016). Non‐surgical treatments are most frequently used for superficial forms of BCC, with one head‐to‐head trial suggesting topical imiquimod is superior to PDT and 5‐FU (Jansen 2018). Although non‐surgical techniques are increasingly used, they do not allow histological confirmation of tumour clearance, and their efficacy is dependent on accurate characterisation of the histological subtype and depth of tumour, and so a baseline diagnostic biopsy can be helpful. The 2007 systematic review of BCC interventions found limited evidence from very small RCTs for these approaches (Bath‐Hextall 2007b), which have only partially been filled by subsequent studies (Bath‐Hextall 2014; Kim 2014; Roozeboom 2012). Most BCC trials have compared interventions within the same treatment class, and few have compared medical versus surgical treatments (Kim 2014).
Vismodegib, a first‐in‐class Hedgehog signalling pathway inhibitor, is now available for the treatment of metastatic or locally‐advanced BCC based on the pivotal study ERIVANCE BCC (Sekulic 2012). It is licensed for use in people with BCC where surgery or radiotherapy is inappropriate, e.g. for treating locally‐advanced periocular and orbital BCCs with orbital salvage of patients who otherwise would have required exenteration (Wong 2017). However, NICE has recently recommended against the use of vismodegib based on cost effectiveness and uncertainty of evidence (NICE 2017).
A systematic review of interventions for primary cSCC found only one RCT eligible for inclusion (Lansbury 2010). Current practice therefore relies on evidence from observational studies, as reviewed in Lansbury 2013, for example. Surgical excision with predetermined margins is usually the first‐line treatment (Motley 2009; Stratigos 2015). Estimates of recurrence after Mohs micrographic surgery, surgical excision, or radiotherapy, which are likely to have been evaluated in higher‐risk populations, have shown pooled recurrence rates of 3%, 5.4% and 6.4%, respectively, with overlapping confidence intervals; the review authors advise caution when comparing results across treatments (Lansbury 2013).
Index test(s)
For the purposes of our series of reviews, each component of the diagnostic process, including visual inspection during clinical examination, is considered a diagnostic or index ‘test', the accuracy of which can be established in comparison with a reference standard of diagnosis, either alone or in combination with other available technologies that may assist the diagnostic process. In this review, two index tests are under consideration: visual inspection and dermoscopy, both of which can be undertaken in person (in a face‐to‐face consultation) or image‐based (remote diagnosis using images). As dermoscopy is effectively added to visual inspection of a skin lesion when it is undertaken in person, we effectively have three index tests: visual inspection alone (in person or using images), visual inspection plus dermoscopy (in‐person dermoscopy), and dermoscopy alone (image‐based dermoscopy).
Visual inspection
Clinical history‐taking and visual inspection (and palpation) of the lesion, surrounding skin and comparison with other lesions identified on complete examination of the body, is fundamental to the diagnosis of skin cancer. In the UK, clinical examination is typically done at two decision points: first in primary care where a decision is made to refer, treat (if low‐risk BCC is suspected), or reassure, and then a second time by a dermatologist or other secondary‐care clinician where a treatment decision is made if appropriate.
Visual inspection of a lesion involves clinical reasoning based on both non‐analytical and analytical pattern recognition strategies (Elstein 2002; Norman 1989; Norman 2009). Non‐analytical pattern recognition uses subconscious intuitive processes, while analytical pattern recognition uses more explicit rules based on hypothetico‐deductive reasoning (Norman 2009). The balance between non‐analytical and analytical reasoning varies between clinicians, according to factors such as constitutional reasoning style preference, experience and familiarity with the diagnostic question.
Unlike for melanoma, where a number of diagnostic algorithms or checklists have been developed to help recognise melanomas (Friedman 1985; MacKie 1985; MacKie 1990; Nachbar 1994; Pehamberger 1993; Sober 1979; Steiner 1987; Stolz 1994), visual inspection for keratinocyte skin cancers relies primarily on pattern recognition. Accuracy has been shown to vary according to the expertise of the clinician. Primary‐care physicians have been reported to miss over half of BCCs (Offidani 2002) and to inappropriately diagnose one‐third of BCCs (Gerbert 2000). In contrast, an Australian study found that skin‐cancer specialists were able to detect 89% of BCCs compared to 79% for general practitioners (GPs), with corresponding specificities of 79% (specialists) and 83% (GPs) (Youl 2007b).
Visual inspection of a digital photograph or ‘macroscopic’ image of a suspicious skin lesion can also be undertaken as part of a teledermatology consultation, whereby clinical photographs, dermoscopic images, or both, are taken by non‐specialist clinicians and forwarded to a dermatologist, to obtain a specialist opinion (Chuchu 2018a). Images can also be encompassed in a store‐and‐forward smartphone application whereby a photograph of a concerning lesion is taken by the smartphone user and forwarded for an assessment of skin‐cancer risk by a specialist clinician (Chuchu 2018b). Images are often accompanied by a summary of the medical history and demographic information as part of a consultation package (Ndegwa 2010). According to UK guidelines, both clinical and dermoscopic images must be sent for ‘full dermatology’, i.e. as a replacement for a face‐to‐face consultation, whereas for ‘triage teledermatology’ dermoscopic images should be sent where facilities permit (BAD 2013).
Dermoscopy
Dermoscopy (also referred to as dermatoscopy or epiluminescence microscopy (ELM)) has become a widely‐used tool for the specialist clinician and is also increasingly being used in primary‐care settings. It uses a hand‐held microscope and incident light (with or without oil immersion) to reveal subsurface images of the skin at increased magnification of x10 to x100 (Kittler 2011) (Figure 2). It is particularly useful for the identification of melanoma when used by specialists (Dinnes 2018b), but its role in the diagnosis of keratinocyte skin cancers is less clearly established.
2.

Dermatoscope. Copyright © 2018 HEINE Optotechnik: reproduced with permission.
The visual nature of dermoscopic interpretation means that when used on an in‐person basis, dermoscopy is essentially added to visual inspection of a skin lesion and similar non‐analytical and analytical pattern recognition strategies are employed to reach a dermoscopic diagnosis. Dermoscopic histological correlations have been established for the diagnosis of melanoma, allowing a number of diagnostic algorithms to be developed based on lesion colour, aspect, pigmentation pattern, and skin vessels (Dinnes 2018b). However, the diagnosis of keratinocyte skin cancers using dermoscopy again relies predominantly on subjective pattern recognition. Features of BCC on dermoscopy include arborising (branching of) blood vessels, superficial fine telangiectasia (abnormally tortuous and dilated blood vessels), grey‐blue ovoid nests and globules, in‐focus dots, spoke wheels and maple‐leaf‐like areas, concentric structures, ulceration, multiple small erosions, shiny white‐red structureless areas, and short white streaks (Tzellos 2014). Features favouring cSCC on dermoscopy include the presence of keratin, white circles, radial telangiectasia and blood spots (Rosendahl 2012a; Zalaudek 2012).
In modern practice, dermoscopic images are frequently obtained for skin lesions that are recommended for excision and are also obtained for lesions that have not yet met the diagnostic threshold for excision but are to be monitored over time in case of any further suspicious changes. Dermoscopic images are also a key component of teledermatology consultations, usually accompanied by digital photographs and other pertinent information (Chuchu 2018a), as discussed above.
Clinical pathway
The diagnosis of skin lesions occurs in primary‐, secondary‐, and tertiary‐care settings by both generalist and specialist healthcare providers. In the UK, people with concerns about a new or changing lesion will present to their general practitioner rather than directly to a specialist in secondary care. If the general practitioner has concerns, then a referral is usually made to a specialist in secondary care – usually a dermatologist, but sometimes to a surgical specialist such as a plastic surgeon or an ophthalmic surgeon. Suspicious skin lesions may also be identified in a referral setting, for example by a general surgeon, and referred for a consultation with a skin cancer specialist (Figure 3). Skin cancers identified by other specialist surgeons (such as an ear, nose, and throat (ENT) specialist or maxillofacial surgeon) will usually be diagnosed and treated without further referral.
3.

Current clinical pathway for people with skin lesions.
Current UK guidelines recommend that all suspicious pigmented lesions presenting in primary care should be assessed by taking a clinical history and visual inspection using the seven‐point checklist (MacKie 1990); lesions suspected to be melanoma or cSCC should be referred for appropriate specialist assessment within two weeks (Chao 2013; Marsden 2010; NICE 2015). Evidence is emerging, however, to suggest that excision of melanoma by GPs is not associated with increased risk compared with outcomes in secondary care (Murchie 2017). In the UK, low‐risk BCCs are usually recommended for routine referral, with urgent referral for those in whom a delay could have a significant impact on outcomes, for example due to large lesion size or critical site (NICE 2015). Appropriately‐qualified generalist care providers increasingly undertake management of low‐risk BCCs in the UK, such as by excision of low‐risk lesions (NICE 2010). Similar guidance is in place in Australia (CCAAC Network 2008).
For referred lesions, the specialist clinician will use history‐taking, visual inspection of the lesion (in conjunction with other skin lesions), palpation of the lesion and associated regional nodal basins in conjunction with dermoscopic examination to inform a clinical decision. If melanoma is suspected, then urgent 2 mm excision biopsy is recommended (Lederman 1985; Lees 1991); for cSCC predetermined surgical margin excision or a diagnostic biopsy may be considered. BCCs and pre‐malignant lesions potentially eligible for nonsurgical treatment may undergo a diagnostic biopsy before initiation of therapy if there is diagnostic uncertainty. Equivocal melanocytic lesions for which a definitive clinical diagnosis cannot be reached may undergo surveillance to identify any lesion changes that would indicate excision biopsy or reassurance and discharge for those lesions that remain stable over a period of time.
Theoretically, teledermatology consultations may aid appropriate triage of lesions into urgent referral; non‐urgent secondary‐care referral (e.g. for suspected basal cell carcinoma); or where available, referral to an intermediate care setting, e.g. clinics run by GPs with a special interest in dermatology. The distinction between setting and examiner qualifications and experience is important, as specialist clinicians might work in primary‐care settings (for example, in the UK, GPs with a special interest in dermatology and skin surgery who have undergone appropriate training), and generalists might practice in secondary‐care settings (for example, plastic surgeons who do not specialise in skin cancer). The level of skill and experience in skin cancer diagnosis will vary for both generalist and specialist care providers and will also impact on test accuracy.
Prior test(s)
Although smartphone applications and community‐based teledermatology services can increasingly be directly accessed by people who have concerns about a skin lesion (Chuchu 2018b), visual inspection of a suspicious lesion by a clinician is usually the first in a series of tests to diagnose skin cancer. In the UK this usually takes place in primary care, but in many countries people with suspicious lesions can present directly to a specialist setting. Although dermoscopy is frequently combined with visual inspection of a lesion in secondary‐care settings, it is also increasingly used in primary care, particularly in countries such as Australia (Youl 2007a).
Consideration of the degree of prior testing that study participants have undergone is key to interpretation of test accuracy indices, as these are known to vary according to the disease spectrum (or case‐mix) of included participants (Lachs 1992; Leeflang 2013; Moons 1997; Usher‐Smith 2016). Spectrum effects are often observed when tests that are developed further down the referral pathway have lower sensitivity and higher specificity when applied in settings with participants with limited prior testing (Usher‐Smith 2016). Studies of individuals with suspicious lesions at the initial clinical presentation stage ('test‐naïve') are likely to have a wider range of differential diagnoses and include a higher proportion of people with benign diagnoses compared with studies of participants who have been referred for a specialist opinion on the basis of visual inspection (with or without dermoscopy) by a generalist practitioner. Furthermore, studies in more specialist settings may focus on equivocal or difficult‐to‐diagnose lesions rather than lesions with a more general level of clinical suspicion. However this direction of effect is not consistent across tests and diseases, the mechanisms in action often being more complex than prevalence alone, and can be difficult to identify (Leeflang 2013). A simple categorisation of studies according to primary, secondary or specialist setting may therefore not always adequately reflect these key differences in disease spectrum that can affect test performance.
Role of index test(s)
When diagnosing potentially life‐threatening conditions, the consequences of falsely reassuring a person that they do not have skin cancer can be serious and potentially fatal, as the resulting delay to diagnosis means that the window for successful early treatment may be missed. To minimise these false‐negative diagnoses, a good diagnostic test will demonstrate high sensitivity and a high negative predictive value (NPV), i.e. so that very few of those with a negative test result will actually have a malignant lesion. Giving falsely‐positive test results (meaning the test has poor specificity and a high false‐positive rate) resulting in the removal of lesions that turn out to be benign is arguably less of an error than missing a potentially fatal lesion, but is not cost‐free. False‐positive diagnoses not only cause unnecessary scarring from the biopsy or excision procedure, but also increase anxiety (particularly during the time that people wait for results) and increase healthcare costs as the number of lesions that need to be removed to yield one malignant diagnosis increases.
Delay in diagnosis of a BCC as a result of a false‐negative test is not as serious as for melanoma, because BCCs are usually slow‐growing and very unlikely to metastasise (Betti 2017). However, delayed diagnosis can result in a larger and more complex excision with consequent greater morbidity. Very sensitive diagnostic tests for BCC, however, may compromise on lower specificity leading to a higher false‐positive rate, and an enormous burden of skin surgery, such that a balance between sensitivity and specificity is needed. The situation for cSCC is more similar to melanoma in that the consequences of falsely reassuring a person that they do not have skin cancer can be serious and potentially fatal, given that removal of an early cSCC is usually curative. Thus, a good diagnostic test for cSCC should demonstrate high sensitivity and a corresponding high negative predictive value. A test that can also reduce false positive clinical diagnoses without missing true cases of cSCC has patient and resource benefits.
Alternative test(s)
A number of other tests have been reviewed as part of our series of Cochrane DTA Reviews on the diagnosis of keratinocyte skin cancers, including reflectance confocal microscopy (RCM) (Dinnes 2018c), computer‐assisted diagnosis (CAD) or artificial intelligence‐based techniques using dermoscopic or spectroscopic images (Ferrante di Ruffano 2018a), optical coherence tomography (OCT) (Ferrante di Ruffano 2018b), high‐frequency ultrasonography (Dinnes 2018d) and exfoliative cytology (Ferrante di Ruffano 2018c). Evidence permitting, we will compare the accuracy of available tests in an overview review, exploiting within‐study comparisons of tests and allowing the analysis and comparison of commonly‐used diagnostic strategies where tests may be used singly or in combination.
We also considered and excluded a number of tests from this review, such as tests used for monitoring people (e.g. total body photography of those with large numbers of pigmented lesions). We also did not assess histopathological confirmation following lesion excision, because it is the established reference standard for skin cancer diagnosis and will be one of the standards against which the index tests are evaluated in these reviews.
Rationale
This series of reviews of diagnostic tests used to assist the clinical diagnosis of BCC and cSCC in clinical practice or research settings, aims to identify the most accurate approaches to diagnosis, and to provide clinical and policy decision‐makers with the highest possible standard of evidence on which to base diagnostic and treatment decisions. With the increasing availability of a wider range of tests, there is a need to differentiate and appropriately triage keratinocyte skin cancers to avoid sending too many people with benign or low‐risk lesions for a specialist opinion whilst not missing those people who have lesions that require treatment.
There is a lack of systematic reviews in the field. A 2007 review of a range of tests for diagnosis of BCC did not report the use of systematic methods for study inclusion or extraction and did not appear to apply any quality assessment (Mogensen 2007). Critical questions of comparative test accuracy and the impact of examiner, prior testing, and underlying risk status remain unanswered for the NHS. With the increasing availability of digital imaging systems and computerised instruments, there is a further need for an up‐to‐date analysis of their accuracy in comparison with visual inspection or dermoscopy.
This review follows a generic protocol which covers the full series of Cochrane DTA Reviews for the diagnosis of keratinocyte skin cancer (Dinnes 2015a). The Background and Methods sections of this review therefore use some text that was originally published in the protocol (Dinnes 2015a) and text that overlaps some of our other reviews (Dinnes 2018a; Dinnes 2018b).
Objectives
To determine the diagnostic accuracy of visual inspection and dermoscopy, alone or in combination, for the detection of BCC in adults.
To determine the diagnostic accuracy of visual inspection and dermoscopy, alone or in combination, for the detection of cSCC in adults.
For both visual inspection and dermoscopy, we estimated accuracy separately according to whether the diagnosis was based on a face‐to‐face (in person) encounter or based on remote (image‐based) assessment. We therefore aimed to compare tests in the following way:
To estimate incremental accuracy for the diagnosis of BCC in adults, (a) from dermoscopy added to in‐person visual inspection of a skin lesion, or (b) from dermoscopic image‐based assessment in comparison to visual inspection of a clinical photograph.
To estimate incremental accuracy for the diagnosis of cSCC in adults, (a) from dermoscopy added to in‐person visual inspection of a skin lesion, or (b) from dermoscopic image‐based assessment in comparison to visual inspection of a clinical photograph.
We also proposed to analyse data according to the prior testing undergone by study participants (comparing those with limited prior testing with those referred for further evaluation of a suspicious skin lesion). However, this was not possible due to limited data.
Secondary objectives
For the identification of BCC or cSCC:
To compare the accuracy of dermoscopy added to in‐person visual inspection versus visual inspection alone, where both tests have been evaluated in the same studies (direct test comparisons);
To compare the accuracy of image‐based dermoscopy versus visual inspection of digital photographs, where both tests have been evaluated in the same studies (direct test comparisons);
To determine the diagnostic accuracy of individual algorithms used to assist visual inspection;
To determine the diagnostic accuracy of individual algorithms used to assist dermoscopy;
To determine the effect of observer experience on diagnostic accuracy.
To assess an alternative target condition:
To determine the diagnostic accuracy of visual inspection or dermoscopy, alone or in combination, for the detection of any skin cancer, and to compare the accuracy of dermoscopy with that of visual inspection alone.
Investigation of sources of heterogeneity
We set out to address a range of potential sources of heterogeneity for investigation across our series of reviews, as outlined in our generic protocol (Dinnes 2015a) and as described in Appendix 3; however, our ability to investigate these was necessarily limited by the available data on each individual test reviewed.
The sources of heterogeneity that we investigated for this review were:
In‐person versus image‐based evaluations
Use of a diagnostic algorithm: no algorithm reported versus any named algorithm used
Disease prevalence: 0% to 25%; > 25%
Observer expertise.
Methods
Criteria for considering studies for this review
Types of studies
We included test‐accuracy studies that allow comparison of the result of the index test with that of a reference standard, including the following:
studies where all participants receive a single index test and a reference standard;
studies where all participants receive more than one index test(s) and reference standard;
studies where participants are allocated (by any method) to receive different index tests or combinations of index tests and all receive a reference standard (between‐person comparative studies (BPC));
studies that recruit series of participants unselected by true disease status (referred to as case series for the purposes of this review);
diagnostic case‐control studies that separately recruit diseased and non‐diseased groups (see Rutjes 2005); however, we did not include studies that compared results for malignant lesions to those for healthy skin (i.e. with no lesion present);
both prospective and retrospective studies;
studies where previously‐acquired clinical or dermoscopic images were retrieved and prospectively interpreted for study purposes.
We excluded studies from which we could not extract 2 x 2 contingency data or if they included fewer than five cases of basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC), or fewer than five benign lesions. The size threshold of five is arbitrary. However, such small studies are unlikely to add precision to estimates of accuracy.
Studies available only as conference abstracts were excluded; however, attempts were made to identify full papers for potentially relevant conference abstracts (Searching other resources).
Participants
We included studies in adults with lesions suspicious for skin cancer. These could include participants:
with lesion characteristics suspicious for keratinocyte skin cancers, including BCC or cSCC
with lesion characteristics suspicious for any skin cancer, including melanoma (e.g. restricted to those with pigmented lesions only, or including both pigmented and non‐pigmented lesion types);
those at high risk of developing BCC or cSCC
We excluded studies that recruited only participants with malignant or benign final diagnoses.
We excluded studies conducted in children or which clearly reported inclusion of more than 50% of participants aged 16 and under.
Index tests
Studies reporting accuracy data for visual inspection or dermoscopy, or both, with diagnosis made either in person (face‐to‐face diagnosis) or image‐based (diagnosis based on photographs or dermoscopic images, remotely from the study participant) were eligible for inclusion. We included all established algorithms or checklists to assist diagnosis.
Studies developing new algorithms or methods of diagnosis (i.e. derivation studies) wereincluded if they:
used a separate independent 'test set' of participants or images to evaluate the new approach; or
investigated lesion characteristics that had previously been suggested as associated with BCC or cSCC, and the study reported accuracy based on the presence or absence of specific combinations of characteristics.
Studies were excluded if they:
used a statistical model to produce a data‐driven equation, or algorithm based on multiple diagnostic features, with no separate test set
used cross‐validation approaches such as 'leave‐one‐out' cross‐validation (Efron 1983)
evaluated the accuracy of the presence or absence of individual lesion characteristics or morphological features, with no overall diagnosis of malignancy
reported accuracy data for ‘clinical diagnosis’ with no clear description of whether the reported data related to visual inspection alone or included dermoscopy in all study participants
were based on the experience of a skin cancer‐specific clinic, where dermoscopy may or may not have been used on an individual basis.
Although primary‐care clinicians can have a specialist interest in skin cancer, for the purposes of this review we considered primary‐care physicians as generalist practitioners and dermatologists as specialists. Within each group, we extracted any reporting of special interest or accreditation in skin cancer.
Target conditions
The primary target conditions were the detection of:
BCC, including all subtypes;
Invasive cSCC (we did not consider cutaneous SCC in situ, such as Bowen’s disease, as disease‐positive)
We considered an additional target condition in secondary analyses, namely the detection of:
any skin cancer, including BCC, cSCC, melanoma or any rare skin cancer (e.g. Merkel cell cancer), as long as skin cancers other than melanoma made up more than 50% of the disease‐positive group. Data from studies in which melanoma accounted for more than 50% of skin cancers were included in our reviews of visual inspection and of dermoscopy compared to visual inspection for the diagnosis of melanoma (Dinnes 2018a; Dinnes 2018b).
Reference standards
The ideal reference standard was histopathological diagnosis in all eligible lesions. A qualified pathologist or dermatopathologist should perform histopathology. Ideally, reporting should be standardised, detailing a minimum dataset to include the type of skin cancer (BCC, cSCC) and subtype of BCC, and may also refer to the tumour, node, and metastasis (TNM) classification of staging for cSCC (Royal College of Pathologists 2014). We did not apply the reporting standard as a necessary inclusion criterion, but extracted any pertinent information.
Partial verification (applying the reference test only to a subset of those undergoing the index test) was of concern, given that lesion excision or biopsy are unlikely to be carried out for all clinically‐benign skin lesions within a representative population sample. We therefore accepted clinical follow‐up of benign lesions as an eligible reference standard, whilst recognising the risk of differential verification bias (as misclassification rates of histopathology and follow‐up will differ).
Additional eligible reference standards included cancer registry follow‐up and 'expert opinion' with no histology or clinical follow‐up. Cancer registry follow‐up is considered less desirable than active clinical follow‐up, as follow‐up is not carried out within the control of the study investigators. Furthermore, if participant‐based analyses are presented as opposed to lesion‐based analyses, it may be difficult to determine whether the detection of a malignant lesion during follow‐up is the same lesion that originally tested negative on the index test.
All of the above are eligible reference standards, with the following caveats:
all study participants with a final diagnosis of the target disorder must have a histological diagnosis, either subsequent to the application of the index test or after a period of clinical follow‐up, and
at least 50% of all participants with benign lesions must have either a histological diagnosis or clinical follow‐up to confirm benignity.
Search methods for identification of studies
Electronic searches
The Information Specialist (SB) carried out a comprehensive search for published and unpublished studies. A single large literature search was conducted to cover all topics in the programme grant (see Appendix 1 for a summary of reviews included in the programme grant). This allowed for the screening of search results for potentially relevant papers for all reviews at the same time. A search combining disease related terms with terms related to the test names, using both text words and subject headings was formulated. The search strategy was designed to capture studies evaluating tests for the diagnosis or staging of skin cancer. As the majority of records were related to the searches for tests for staging of disease, a filter using terms related to cancer staging and to accuracy indices was applied to the staging test search, to try to eliminate irrelevant studies, for example, those using imaging tests to assess treatment effectiveness. A sample of 300 records that would be missed by applying this filter was screened and the filter adjusted to include potentially relevant studies. When piloted on MEDLINE, inclusion of the filter for the staging tests reduced the overall numbers by around 6000. The final search strategy, incorporating the filter, was subsequently applied to all bibliographic databases as listed below (Appendix 4). The final search result was cross‐checked against the list of studies included in five systematic reviews; our search identified all but one of the studies, and this study was not indexed on MEDLINE. The Information Specialist devised the search strategy, with input from the Information Specialist from Cochrane Skin. No additional limits were used.
We searched the following bibliographic databases to 29 August 2016 for relevant published studies:
MEDLINE via OVID (from 1946);
MEDLINE In‐Process & Other Non‐Indexed Citations via OVID; and
Embase via OVID (from 1980).
We searched the following bibliographic databases to 30 August 2016 for relevant published studies:
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 7, 2016, in the Cochrane Library;
Cochrane Database of Systematic Reviews (CDSR) Issue 8, 2016 in the Cochrane Library;
Cochrane Database of Abstracts of Reviews of Effects (DARE) Issue 2, 2015;
CRD Health Technology Assessment (HTA) database Issue 3, 2016; and
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCO from 1960).
We searched the following databases for relevant unpublished studies using a strategy based on the MEDLINE search:
CPCI (Conference Proceedings Citation Index), via Web of Science™ (from 1990; searched 28 August 2016); and
SCI Science Citation Index Expanded™ via Web of Science™ (from 1900, using the 'Proceedings and Meetings Abstracts' Limit function; searched 29 August 2016).
We searched the following trials registers using the search terms 'melanoma', 'squamous cell', 'basal cell' and 'skin cancer' combined with 'diagnosis':
Zetoc (from 1993; searched 28 August 2016).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov); searched 29 August 2016.
NIHR Clinical Research Network Portfolio Database (www.nihr.ac.uk/research‐and‐impact/nihr‐clinical‐research‐network‐portfolio/); searched 29 August 2016.
The World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/); searched 29 August 2016.
We aimed to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or in progress). We applied no date limits.
Searching other resources
We have screened any relevant systematic reviews identified by the searches for their included primary studies, and have included any missed by our searches. We have checked the reference lists of all included papers, and subject experts within the author team have reviewed the final list of included studies. We have conducted no electronic citation searching.
Data collection and analysis
Selection of studies
At least one review author (JDi or NC), screened all titles and abstracts, with any queries discussed and resolved by consensus. A pilot screen of 539 MEDLINE references showed good agreement (89% with a kappa of 0.77) between screeners. We included primary test accuracy studies and test accuracy reviews (for scanning of reference lists) of any test used to investigate suspected melanoma, BCC, or cSCC at initial screening. Inclusion criteria (Appendix 5) were applied independently by both a clinical reviewer (from one of a team of 12 clinician reviewers) and a methodologist reviewer (JDi or NC) to all full‐text articles, with disagreements resolved by consensus or by a third party (JDe, CD, HW, or RM). We contacted authors of eligible studies when insufficient data were presented, to allow for the construction of 2 x 2 contingency tables.
Data extraction and management
One clinical (as detailed above) and one methodologist reviewer (JDi, NC or LFR) independently extracted data for details of the study design, participants, index test(s) or test combinations and criteria for index test positivity, reference standards, and data required to populate a 2 x 2 diagnostic contingency table for each index test, using a piloted data extraction form. We extracted data at all available index test thresholds, resolving disagreements by consensus or by a third party (JDe, CD, HW, and RM).
We contacted authors of included studies where information relating to the diagnostic threshold was missing. We contacted authors of conference abstracts published from 2013 to 2015 to ask whether full data were available. If we could not identify a full paper, we marked conference abstracts as 'pending' and will revisit them in a future review update.
Dealing with multiple publications and companion papers
Where we found multiple reports of a primary study, we maximised yield of information by collating all available data. Where there were inconsistencies in reporting or overlapping study populations, we contacted study authors for clarification in the first instance. If this contact with authors was unsuccessful, we used the most complete and up‐to‐date data source where possible.
Assessment of methodological quality
We assessed risks of bias and applicability of included studies using the QUADAS‐2 checklist (Whiting 2011), tailored to the topic of skin cancer (see Appendix 6). We piloted the modified QUADAS‐2 tool on a small number of full‐text articles included across the full series of diagnostic test accuracy reviews. One clinical and one methodologist reviewer (JDi, NC or LFR) independently assessed quality for the remaining studies, resolving any disagreement by consensus or by a third party where necessary (JDe, CD, HW, and RM).
Statistical analysis and data synthesis
We planned separate analyses according to the point that study participants have reached in the clinical pathway, the clarity with which the pathway could be determined, and the evaluation of in‐person versus image‐based diagnosis.
Our unit of analysis was the lesion rather than the person. This is because (i) in skin cancer initial treatment is directed to the lesion rather than systemically (thus it is important to be able to correctly identify cancerous lesions for each person), and (ii) it is the most common way in which the primary studies reported data. Although there is a theoretical possibility of correlations of test errors when the same people contribute data for multiple lesions, most studies include very few people with multiple lesions and any potential impact on findings is likely to be very small, particularly in comparison with other concerns regarding risk of bias and applicability. For each analysis, we included only one dataset per study, to avoid multiple counting of lesions. We retrieved few studies comparing algorithms, but where we assessed multiple algorithms in an individual study, we selected datasets on the following preferential basis:
‘no algorithm’ reported; data presented for clinician’s overall diagnosis or management decision
pattern analysis or pattern recognition
ABCD algorithm (or derivatives of) or other established algorithm such as seven‐point checklist, Menzies algorithm or three‐point checklist
New algorithm developed by study authors
For the diagnosis of BCC (or cSCC), we considered any melanomas or cSCCs (BCCs) that were positively identified in the ‘disease‐negative’ group (i.e. that were mistaken for BCCs) false‐positive results. The clinical management of a lesion considered to be a BCC might be quite different from that for a melanoma or cSCC, and could potentially lead to a negative outcome for the participants concerned; for example, if a treatment other than excision was initiated.
For each index test, algorithm or checklist under consideration, we plotted estimates of sensitivity and specificity on coupled forest plots and in receiver operating characteristic (ROC) space. For tests where commonly‐used thresholds were reported we estimated summary operating points (summary sensitivities and specificities) with 95% confidence and prediction regions using the bivariate hierarchical model (Chu 2006; Reitsma 2005). Where inadequate data were available for the model to converge, we simplified the model, first by assuming no correlation between estimates of sensitivity and specificity and secondly by setting estimates of near‐zero variance terms to zero (Takwoingi 2017). Where all studies reported 100% sensitivity (or 100% specificity) we summed the number with disease (or no disease) across studies and used them to compute a binomial exact 95% confidence interval.
We drew comparisons between visual inspection and dermoscopy results with:
a. all visual inspection and all dermoscopy data from all studies, and then
b. only using data from studies that reported both visual inspection data and dermoscopy data for the same lesions, to enable a robust direct comparison (Takwoingi 2013).
We made comparisons between tests by comparing summary ROC curves using the hierarchical summary receiver‐operator curves (HSROC) model (Rutter 2001) rather than by estimating average operating points, as this approach allows incorporation of data at different thresholds as could arise with different algorithms or checklists. We used an HSROC model that assumed a constant SROC shape between tests and subgroups, but allowed for differences in threshold and accuracy by the addition of covariates. We assessed the significance of the differences between tests by the likelihood ratio test (LR test) assessing differences in both accuracy and threshold, and by a Wald test on the parameter estimate testing for differences in accuracy alone. We provide the P values from both tests in the Tables with the results from the LR test cited in the text, on the basis that differences in threshold between tests is likely. We fitted simpler models when convergence was not achieved due to small numbers of studies, first assuming symmetric SROC curves (setting the shape term to zero), and then setting random‐effects variance estimates to zero.
We present estimates of accuracy from HSROC models as diagnostic odds ratios (DORs) (estimated where the SROC curve crosses the sensitivity = specificity line) with 95% confidence intervals. We present differences between tests and subgroups from HSROC analyses as relative diagnostic odds ratios (RDORs) with 95% confidence intervals. To facilitate interpretation in terms of rates of false‐positive and false‐negative diagnoses, we have computed values of sensitivity at the point on the SROC curve with 80% specificity and of specificity at the point on the SROC curve with 80% sensitivity. We chose these 80% values as they lie within the estimates for most of the analyses. These results should only be considered as illustrative examples of possible sensitivities (and specificities) and differences in sensitivities (and specificities) that could be expected.
Where data were insufficient to estimate HSROC curves (e.g. for the analysis of cSCC),we estimated summary operating points (summary sensitivities and specificities) with 95% confidence and prediction regions using the bivariate hierarchical model (Chu 2006; Reitsma 2005).
For computation of likely numbers of true‐positive, false‐positive, false‐negative and true‐negative findings in the 'Summary of findings' table, we applied these indicative values to the lower quartile, median and upper quartiles of the prevalence observed in the study groups.
We fitted bivariate models using the xtmelogit command in STATA 15, and HSROC models using the NLMIXED procedure in the SAS statistical software package (SAS 2012) and the metadas macro (Takwoingi 2010).
Investigations of heterogeneity
We investigated heterogeneity, comparisons between algorithms and according to observer experience by comparing summary ROC curves using the HSROC model (Rutter 2001), with additional covariates for differences in threshold and accuracy as used for comparing tests.
Sensitivity analyses
We did not conduct any sensitivity analyses.
Assessment of reporting bias
Because of uncertainty about the determinants of publication bias for diagnostic accuracy studies and the inadequacy of tests for detecting funnel plot asymmetry (Deeks 2005), we did not perform tests to detect publication bias.
Results
Results of the search
We identified and screened 34,517 unique references for inclusion. Of these, we reviewed 1051 full‐text papers for eligibility for any one of the suite of reviews of tests to assist in the diagnosis of melanoma or keratinocyte skin cancer. Of the 1051 full‐text papers assessed, we eliminated 848 from all reviews in our series (see Figure 4 PRISMA flow diagram of search and eligibility results).
4.
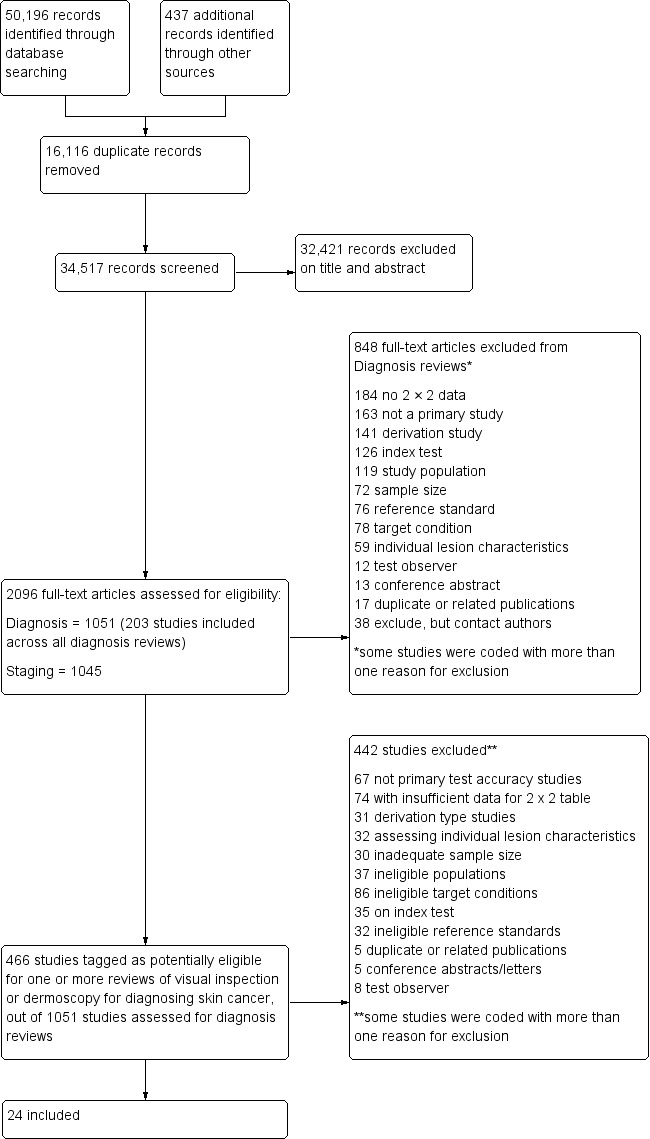
PRISMA flow diagram.
Of the 466 studies tagged as potentially eligible for any of our reviews of visual inspection or dermoscopy, we include 24 publications in this review. Exclusions were mainly due to the inability to construct a 2 x 2 contingency table based on the data presented (n = 74); the use of ineligible index tests (n = 35; for example: reporting of data for ‘clinical diagnosis’ or for serial use of the index test in a follow‐up context); assessment of individual lesion characteristics (n = 32); or derivation‐type studies developing new algorithms or checklists without a separate training and test set of lesions (n = 31). Other reasons for exclusion included not meeting our requirements for an eligible reference standard (n = 32), ineligible study populations (n = 37) (for example, recruiting only malignant or only benign lesions), inadequate sample size (n = 30), ineligible definition of the target condition (n = 86; including those eligible only for reviews of the detection of melanoma) or with test interpretation by medical students or laypersons (n = 8). A list of the 442 publications excluded from this review with reasons for exclusion is available in Characteristics of excluded studies, with a list of all studies excluded from the full series of reviews available as a separate pdf (please contact skin.cochrane.org for a copy of the pdf).
We contacted the authors of 17 publications concerned with the evaluation of visual inspection or dermoscopy for further data to allow study inclusion; we received responses from four authors with regard to seven publications. Two authors provided additional data but these were insufficient to allow inclusion of the studies (Cabrijan 2008; Warshaw 2009a; Warshaw 2009b; Warshaw 2010a), one replied indicating that dermoscopy was not necessarily used in all study participants (Youl 2007a; Youl 2007b) and one replied but was unable to access the data needed (Fabbrocini 2008). We contacted the authors of a further seven included studies for further details of study methods, and received a responses for four studies; three provided further information about the diagnostic thresholds used (Amirnia 2016; Durdu 2011; Stanganelli 2000) and one provided full anonymised study data (Rosendahl 2011).
The 24 included study publications report on a total of 24 cohorts of lesions and provide 27 visual inspection datasets (8805 lesions; 2579 malignancies) and 33 dermoscopy datasets (6855 lesions; 1444 malignancies). We provide a summary of the tests and target conditions evaluated in each study in Appendix 7. Six studies contributed data for in‐person visual inspection alone (Chang 2013; Cooper 2002; Ek 2005; Hacioglu 2013; Schwartzberg 2005; Steiner 1987); three for dermoscopy added to visual inspection (Amirnia 2016; Durdu 2011; Gokdemir 2011); and five for both in‐person visual inspection alone and combined with dermoscopy (Argenziano 2006; Carli 2002a; Markowitz 2015; Stanganelli 2000; Ulrich 2015). Two studies contributed data for image‐based visual inspection of clinical photographs alone (Lorentzen 1999; Nori 2004); eight for image‐based dermoscopy (Altamura 2010; Carli 2002a; Hacioglu 2013 ; Lorentzen 2008; Menzies 2000; Navarrete Dechent 2016; Witkowski 2016; Zalaudek 2006); and two for both image‐based visual inspection and image‐based dermoscopy (Carli 2002b; Rosendahl 2011). Five studies compared the accuracy of visual inspection with or without dermoscopy to other tests, including: exfoliative cytology (Durdu 2011); computer‐assisted diagnosis (CAD) (Hacioglu 2013); optical coherence tomography (OCT) (Markowitz 2015; Ulrich 2015); and radiographic contrast medium (RCM) (Witkowski 2016). Thirteen studies also contributed data to our reviews of visual inspection (n = 9) and/or dermoscopy (n = 9) for the detection of melanoma (Dinnes 2018a; Dinnes 2018b).
Methodological quality of included studies
We summarise the overall methodological quality of all included studies according to in‐person or image‐based approaches to dermoscopy or to visual inspection. We present 14 studies reporting data for in‐person visual inspection (n = 11) and/or in‐person dermoscopy (added to visual inspection) (n = 8) in Figure 5, with results by study presented in Figure 6. Twelve studies reporting data for image‐based visual inspection (n = 4) and/or image‐based dermoscopy (n = 10) are presented in Figure 7, with results by study presented in Figure 8. Two studies appear in both sets of figures: Carli 2002a evaluated the accuracy of image‐based dermoscopy as well as in‐person visual inspection and dermoscopy, while Hacioglu 2013 reported data for in‐person visual inspection and image‐based dermoscopy.
5.

Risk of bias and applicability concerns graph for in‐person studies: review authors' judgements about each domain presented as percentages across included studies
6.

Risk of bias and applicability concerns summary for in‐person evaluations: review authors' judgements about each domain for each included study
7.

Risk of bias and applicability concerns graph for image‐based evaluations: review authors' judgements about each domain presented as percentages across included studies
8.

Risk of bias and applicability concerns summary for image‐based evaluations: review authors' judgements about each domain for each included study
In‐person evaluations
We judged the risk of bias to be low for most of the studies in only two of five quality domains assessed (dermoscopy index test, reference standard); we judged risk of bias to be high or unclear for most of the studies for participant selection, visual inspection index test, and flow and timing (Figure 5). We rated applicability of study findings as of high or unclear concern in all four domains (participant selection, dermoscopy index tests, visual inspection index tests, reference standards) assessed for all studies apart from one.
For participant selection: we rated three of the 14 studies (21%) at low risk of bias, and three (21%) at high risk (Figure 5) due to exclusion of lesions by size (Hacioglu 2013), or because of missing (Ulrich 2015) or equivocal pathology (Ek 2005). Five studies (36%) did not report the method of participant selection and eight (57%) did not clearly describe exclusions from the study. We rated all studies at high concern for applicability of participants, primarily due to inclusion of lesions selected for biopsy or excision based on the clinical or dermoscopic diagnosis. We judged only one to have included a representative population (Stanganelli 2000). Nine cohorts (64%) also included multiple lesions per participant (Chang 2013; Cooper 2002; Durdu 2011; Ek 2005; Gokdemir 2011; Markowitz 2015; Schwartzberg 2005; Stanganelli 2000; Ulrich 2015) and three did not clearly report the number of included participants (Argenziano 2006; Carli 2002a; Steiner 1987).
For the index test domain: there are eight evaluations of in‐person dermoscopy and 11 evaluations of in‐person visual inspection (Figure 5). For dermoscopy, we rated six evaluations (75%) at low risk of bias, and two did not provide sufficient information to allow us to fully judge the risk of bias. We rated all studies to have made the diagnosis blinded to the reference standard result, given that this is always undertaken prior to histology; six (75%) also clearly reported prespecification of the diagnostic threshold (all using named algorithms or pattern). We judged that all 11 visual‐inspection evaluations had made the diagnosis blinded to the reference standard result. Only three clearly reported prespecification of the threshold used, with two reporting use of formal algorithms (Argenziano 2006; Stanganelli 2000) and one describing the process by which the diagnosis was reached (Ulrich 2015).
We recorded high concern for the applicability of the index tests for three in‐person evaluations of dermoscopy (37%) and for seven evaluations of visual inspection (64%) (Figure 5). For the dermoscopy evaluations this was due to the presentation of average (Argenziano 2006) or consensus diagnoses (Carli 2002a), as opposed to the diagnosis of a single observer, and a lack of description of the diagnostic threshold used (Gokdemir 2011). Only two studies provided sufficient information on which to judge the level of observer expertise in dermoscopy (Carli 2002a; Gokdemir 2011). For visual inspection, we noted high concerns due to the presentation of average (Argenziano 2006) or consensus (Carli 2002a; Steiner 1987) diagnoses, or lack of detail about the threshold for diagnosis (Carli 2002a; Chang 2013; Cooper 2002; Ek 2005; Hacioglu 2013; Steiner 1987). Most studies (7/11) did not provide sufficient information on which to judge the level of observer expertise in lesion diagnosis.
For the reference standard: We judged all studies except Stanganelli 2000 at low risk of bias due to the use of an acceptable reference standard (73%) (Figure 5). In Stanganelli 2000 only 8% of included lesions underwent excision, with the remaining 3110 ‘benign’ diagnosed assumed to be benign based on cancer registry follow‐up. Blinding of the reference standard to the index test was recorded but did not contribute to the overall risk of bias for this domain. Blinding of the reference standard was reported in only one study (Amirnia 2016). The applicability of the reference standard was of low concern in one evaluation reporting pathology review by an expert histopathologist (Argenziano 2006), and we rated the remaining 13 (93%) as unclear.
For participant flow and timing: We rated five studies at low risk of bias (36%), three as unclear (21%), and six at high risk of bias (43%) (Figure 5). Of those at high risk, one did not use the same reference standard for all participants (Stanganelli 2000), and five did not include all participants in the analysis. Seven studies were unclear on the interval between the application of the index test and excision for histology.
Image‐based evaluations
Across the 12 studies providing image‐based data, we rated risk of bias to be high or unclear for at least half of the studies in all domains, apart from the reference standard domain (Figure 7). We also scored applicability of study findings as of high concern in almost all studies, apart from for the reference standard domain.
For participant selection: We judged six of the 12 evaluations (50%) at high risk of bias, four did not provide sufficient information to judge this domain, and two were at low risk of bias (Figure 7). Three studies (25%) used a case‐control design with separate sampling of malignant and benign lesions (Altamura 2010; Menzies 2000; Nori 2004), and two (17%) excluded lesions on the basis of size (Hacioglu 2013) or type of lesion (Navarrete Dechent 2016, excluding seborrhoeic keratosis). Five evaluations (42%) did not report the method of participant selection and six (50%) did not clearly describe exclusions from the study. We rated all evaluation cohorts at high concern for applicability of participants, primarily due to the restricted inclusion of lesions selected for excision or biopsy. Two studies also reported including multiple lesions per participant (Navarrete Dechent 2016; Rosendahl 2011).
For the index test domain: There are 10 evaluations of image‐based dermoscopy and four evaluations of visual inspection of clinical images (Figure 7). Insufficient information was provided on which to judge the risk of bias for visual inspection, due to unclear prespecification of the threshold for diagnosis of skin cancer. For dermoscopy, we rated five evaluations (50%) at low risk of bias, four as unclear (36%) and one at high risk. The high‐risk study developed a new algorithm for dermoscopy using characteristics previously suggested to be associated with BCC, but did not use a separate training set to develop the algorithm (Navarrete Dechent 2016). Four studies did not clearly report prespecification of the diagnostic threshold used (Altamura 2010; Carli 2002b; Hacioglu 2013; Witkowski 2016).
We had high concern for the applicability of the index tests for all four visual‐inspection and nine of 10 dermoscopy evaluations, due to the use of image‐based interpretations. None of the visual‐inspection evaluations provided further information on the participants concerned, and two presented average (Lorentzen 1999) or consensus (Carli 2002b) diagnoses. None of the four provided sufficient detail about the diagnostic threshold used. For dermoscopy, nine studies reported blinded interpretation of dermoscopic images and six reported average (Lorentzen 2008; Zalaudek 2006) or consensus (Carli 2002a; Carli 2002b; Navarrete Dechent 2016) diagnoses, or were not clear on the data provided (Menzies 2000). One study reported presentation of the clinical photograph of the lesion alongside the dermoscopic image (Rosendahl 2011), and also presented data for a single observer. Four studies provide insufficient information on the diagnostic threshold (Carli 2002b; Hacioglu 2013; Lorentzen 2008; Witkowski 2016) and four did not provide details of the observer expertise (Hacioglu 2013; Menzies 2000; Witkowski 2016; Zalaudek 2006).
For the reference standard: We judged 11 (92%) of the 12 included image‐based studies at low risk of bias (Figure 7). We considered Nori 2004 to be at high risk, as it did not meet our criteria for an adequate reference standard (histology or clinical follow‐up in at least 80% of benign lesions). Blinding of the reference standard to the original clinical diagnosis was not reported in any study. We judged the applicability of the reference standard to be of unclear concern in 11 studies, due to a lack of detail about the expertise of the histopathologist or by a dermatopathologist. Nori 2004 was of high concern, due to the use of expert opinion for classifying the final diagnosis of some lesions.
For participant flow and timing: Six studies were at high risk of bias (50%), four at low risk (33%) and two (17%) did not provide enough information on which to judge this domain (Figure 7). Of those at high risk, one evaluations did not use the same reference standard for all participants (differential verification) (Nori 2004), and none of the six included all participants in the analysis. Seven studies (58%) were unclear on the interval between the application of the index test and lesion excision, with only five (42%) considered to report consecutive diagnosis and excision or biopsy (Carli 2002b; Hacioglu 2013; Lorentzen 1999; Menzies 2000; Witkowski 2016).
Findings
1. Target condition: BCC
Twenty‐one studies reported accuracy data for the detection of BCC. Twelve studies provided data for visual inspection alone; eight evaluations were conducted in person and four were image‐based. Fifteen studies reported accuracy data for the detection of BCC by using dermoscopy; seven evaluations were in person and nine were image‐based. One study reported dermoscopy data for both in‐person and image based dermoscopy (Carli 2002a).
We provide summary details of the in‐person and image‐based studies in Appendix 8. We present results for the primary analyses in Table 2, with heterogeneity investigations presented in Table 3 and Table 4. Forest plots of study data for each analysis are shown in Figure 9 and Figure 10; summary estimates for in‐person comparisons are depicted in Figure 11 and Figure 12, and for image‐based comparisons in Figure 13 and Figure 14.
1. Comparison of visual inspection and dermoscopy for detection of BCC.
| Test | Datasets | Lesions (BCCs) | DOR (95% CI) | Specificity at 80% sensitivity | Sensitivity at 80% specificity | Relative DOR (95% CI) | P value (LR)a | P value (Wald)b | |
| In‐person evaluations | |||||||||
| Visual inspection | 8 | 7017 (1586) |
19.9 (7.8 to 51.2) |
77% | 79% | 8.2 (3.5 to 19.3) |
< 0.001 | < 0.001 | |
| Visual inspection + Dermoscopy |
7 | 4683 (363) |
164 (56.8 to 475) |
99% | 93% | ||||
| In‐person evaluations (direct studies) | |||||||||
| Visual inspection | 4 | 3974 (257) |
12.8 (3.3 to 48.8) |
36% | 71% | 7.5 (2.7 to 21.3) |
< 0.001 | < 0.001 | |
| Visual inspection + Dermoscopy |
4 | 3974 (258) |
96.2 (21.1 to 439) |
97% | 87% | ||||
| Image‐based evaluations | |||||||||
| Visual inspection (clinical images) | 4 | 853 (156) |
26.8 (11.9, 60.4) |
87% | 85% | 3.9 (1.2, 5.0) |
0.006 | 0.025 | |
| Dermoscopic images | 9 | 2271 (737) |
75.7 (21.3, 269) |
96% | 93% | ||||
| Image‐based evaluations (direct studies) | |||||||||
| Visual inspection (clinical images) | 2 | 516 (82) |
81.1 (39.1, 168) |
95%c | 95%c | Not estimable | Not estimable | Not estimable | |
| Dermoscopic images | 2 | 516 (79) |
275.5 (112, 678) |
99%c | 99%c | ||||
BCC ‐ basal cell carcinoma; DOR ‐ diagnostic odds ratio; RDOR ‐ relative diagnostic odds ratio; CI ‐ confidence interval; LR ‐ likelihood ratio.
aTests whether there is a difference in test performance between defined groups in terms of either DOR or threshold. bTests the significance of the difference in DOR between defined groups at a particular SROC curve intercept value. cComputed assuming symmetric SROC curve.
2. Investigations of sources of heterogeneity for studies of visual inspection for detection of BCC.
| Test | Datasets | Lesions (BCCs) |
DOR (95% CI) |
Specificity at 80% sensitivity | Sensitivity at 80% specificity | Relative DOR (95% CI) | P value (LR)a | P value (Wald)b |
| Difference in‐person and image based | ||||||||
| In‐person | 8 | 7017 (1586) |
11.9 (4.4 to 32.2) |
64% | 74% | 0.45 (0.26 to 9.2) |
0.88 | 0.62 |
| Image | 4 | 853 (156) |
18.5 (4.3 to 80.6) |
78% | 79% | |||
| Prevalence | ||||||||
| 0% ‐ 25% | 6 | 4643 (168) |
50.5 (17.1 to 149) |
94% | 91% | 9.7 (2.3 to 40.8) |
0.002 | 0.002 |
| > 25% | 6 | 3227 (1574) |
5.2 (2.3 to 11.7) |
50% | 60% | |||
BCC ‐ basal cell carcinoma; DOR ‐ diagnostic odds ratio; RDOR ‐ relative diagnostic odds ratio; CI ‐ confidence interval; LR ‐ likelihood ratio
aTests whether there is a difference in test performance between defined groups in terms of either DOR or threshold. bTests the significance of the difference in DOR between defined groups at a particular SROC curve intercept value.
3. Investigations of sources of heterogeneity for studies of dermoscopy for detection of BCC.
| Test | Datasets | Lesions (cases) | DOR (95% CI) | Specificity at 80% sensitivity | Sensitivity at 80% specificity | Relative DOR (95% CI) | P value (LR)a | P value (Wald)b |
| Difference in person and image based | ||||||||
| In person | 7 | 4683 (363) |
388 (68.6 to 2194) |
100% | 96% | 4.0 (0.46 to 33.8) |
0.39 | 0.21 |
| Image | 9 | 2271 (737) |
98.2 (21.6 to 446) |
98% | 91% | |||
| Use of an algorithm | ||||||||
| No algorithm | 9 | 5427 (338) |
371 (86.9 to 1587) |
100% | 98% | 7.8 (0.90 to 68.2) |
0.004 | 0.06 |
| Any algorithm | 7 | 1527 (762) |
47.4 (10.2 to 219) |
94% | 90% | |||
| Prevalence (in‐person studies) | ||||||||
| 0% ‐ 25% | 9 | 5524 (349) | 309 (69.2 to 1380) |
100% | 97% | 4.5 (0.49 to 41.8) |
0.04 | 0.18 |
| > 25% | 7 | 1430 (751) |
68.4 (13.2 to 356) |
96% | 91% | |||
BCC ‐ basal cell carcinoma; DOR ‐ diagnostic odds ratio; RDOR ‐ relative diagnostic odds ratio; CI ‐ confidence interval; LR ‐ likelihood ratio
aTests whether there is a difference in test performance between defined groups in terms of either DOR or threshold. bTests the significance of the difference in DOR between defined groups at a particular SROC curve intercept value.
9.
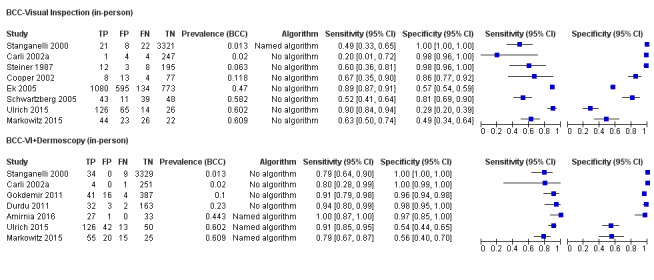
In‐person evaluations of the accuracy of visual inspection and visual inspection plus dermoscopy (VI+Dermoscopy) according to BCC prevalence and use of a formal algorithm
10.

Image‐based evaluations of the accuracy of visual inspection and dermoscopy alone according to BCC prevalence and use of a formal algorithm
11.

Comparison of the accuracy of visual inspection with visual inspection plus dermoscopy (VI+Dermoscopy) for detection of BCC from in‐person studies
12.
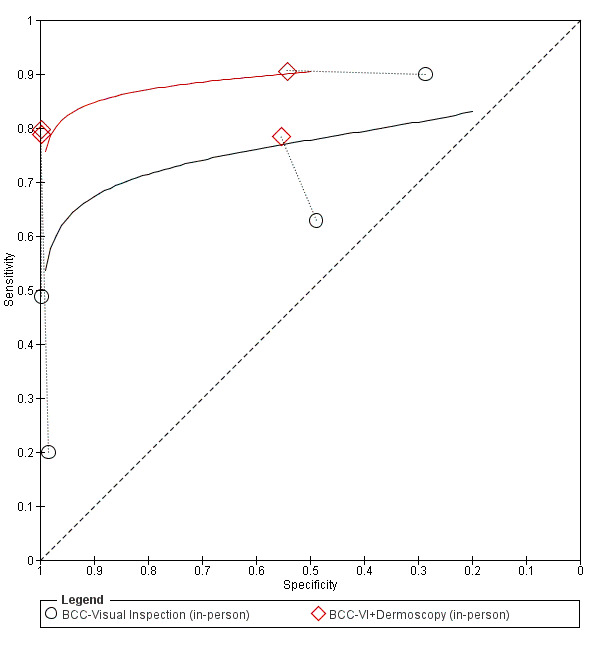
Paired comparisons of the accuracy of visual inspection with visual inspection plus dermoscopy for detection of BCC from in‐person studies
13.
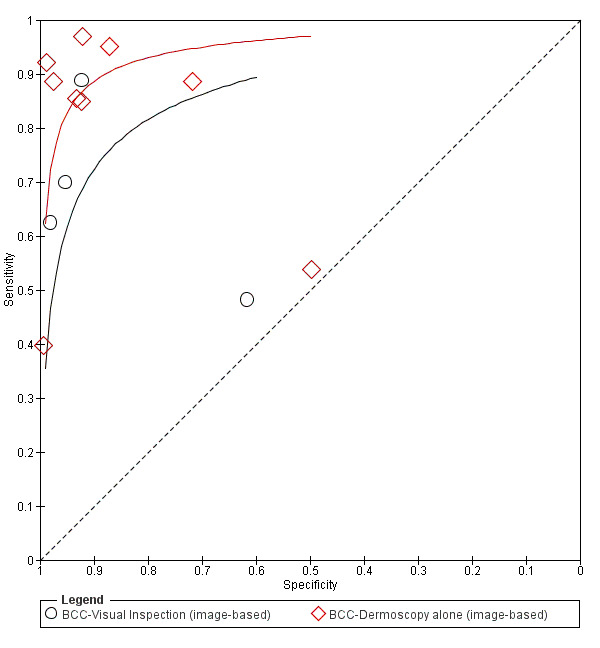
Comparison of the accuracy of image‐based visual inspection with image‐based dermoscopy for detection of BCC
14.

Paired comparisons of the accuracy of visual inspection with visual inspection plus dermoscopy for detection of BCC from image‐based studies
Analyses by clinical pathway and in‐person versus image‐based design
Attempts to classify studies according to where on the clinical pathway they had been conducted were hindered by lack of information. We considered that only eight studies had provided a clear description of the prior testing of included participants and only three were conducted in a limited prior testing population, as opposed to studies in participants referred for specialist assessment (Appendix 8). We were therefore unable to analyse data by pathway for either visual inspection or for dermoscopy.
We found no clear differences in accuracy between studies undertaken in person and those which evaluated images (Table 3 and Table 4). The accuracy of visual inspection was non‐significantly lower for in‐person studies of visual inspection compared to image‐based (relative diagnostic odds ratio (RDOR) 0.45, 95% confidence interval (CI) 0.26 to 9.2, LR test P = 0.88) (Table 3; Figure 15), while the accuracy of in‐person dermoscopy was non‐significantly higher compared to diagnosis based on dermoscopic images (RDOR 4.0, 95% CI 0.46 to 33.8; LR test P = 0.39) (Table 4; Figure 16). The lack of effect observed is probably due to other sources of heterogeneity, particularly given the much bigger and highly‐significant effect observed for this analysis for the detection of melanoma (Dinnes 2018a). We elected to undertake our primary analyses separately for in‐person and image‐based analyses, to be consistent with the approach used in the melanoma review.
15.

Comparison of the accuracy of visual inspection for detection of BCC between in‐person and image‐based
16.
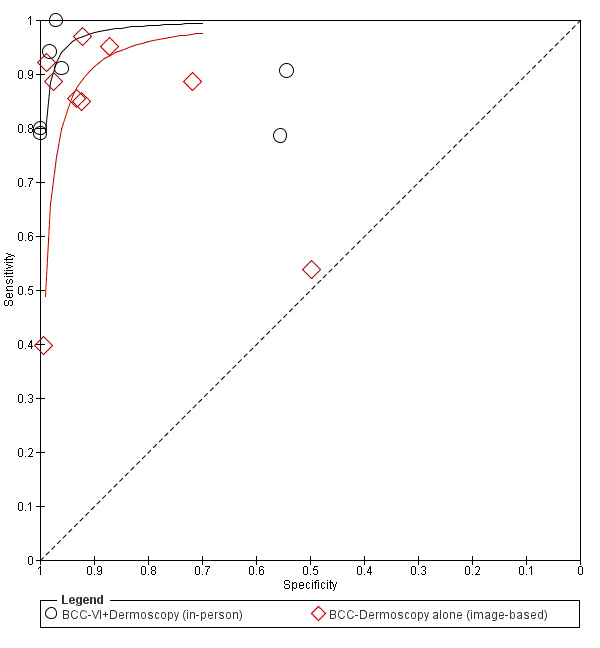
Comparison of the accuracy of dermoscopy for detection of BCC between in‐person (VI+Dermoscopy) and image‐based (Dermoscopy alone)
In‐person evaluations
The 11 studies reporting in‐person evaluations of visual inspection alone (n = 4; Cooper 2002; Ek 2005; Schwartzberg 2005; Steiner 1987), for visual inspection plus dermoscopy (n = 3; Amirnia 2016; Durdu 2011; Gokdemir 2011) or for both (n=4; Carli 2002a; Markowitz 2015; Stanganelli 2000; Ulrich 2015) were all conducted in referred populations undergoing biopsy or excision (Appendix 9). Three were considered to have been conducted in participants with equivocal lesions (Markowitz 2015; Steiner 1987; Ulrich 2015) and one in participants at high risk for developing skin cancer following renal transplantation (Cooper 2002). Seven evaluations were prospective case series, one was retrospective (Stanganelli 2000), and three did not clearly report the direction of the design (Amirnia 2016; Carli 2002a; Gokdemir 2011).
Five of the 11 studies primarily aimed to examine accuracy for the detection of BCC (Amirnia 2016; Markowitz 2015; Schwartzberg 2005; Ulrich 2015) or ‘non‐melanoma’ skin cancer (Cooper 2002), while the remaining six also provided data for our reviews of visual inspection or dermoscopy or both for the diagnosis of melanoma (Dinnes 2018a; Dinnes 2018b). Two evaluations included any lesion considered suspicious for skin cancer (Ek 2005; Cooper 2002); two included lesions suspicious for BCC (Amirnia 2016; Schwartzberg 2005), one of these restricted to lesions on the face (Amirnia 2016); five included only pigmented lesions (Carli 2002a; Durdu 2011; Gokdemir 2011; Stanganelli 2000; Steiner 1987) and two to non‐pigmented ‘pink’ lesions (Markowitz 2015; Ulrich 2015), one of these restricted to head and neck lesions only (Markowitz 2015). The prevalence of BCC ranged from 1% (Stanganelli 2000) to 61% (Markowitz 2015); median 17% (interquartile range (IQR) 10, 53%). The lowest prevalence was generally observed in the studies in pigmented lesions (1% to 10% in four studies) and the highest in non‐pigmented or lesions suspicious for BCC (58% to 61% in three studies). Six studies reported including invasive melanoma or melanoma in situ (Carli 2002a; Durdu 2011; Ek 2005; Gokdemir 2011; Stanganelli 2000; Steiner 1987) and two included cSCC (Cooper 2002; Ek 2005) in the disease‐negative group.
Diagnosis was recorded by dermatologists or clinicians presumed to be dermatologists (based on author’s institutions) in most of the studies (9/11; 82%), a mixed group of dermatology residents (trainees) and consultants (Cooper 2002) or plastic surgery residents, consultants and a clinical assistant (Ek 2005). Where reported (n = 7), the number of observers ranged from 1 to 17 (median 2).
Test accuracy was reported for a single observer in just over half of the evaluations (n = 6), for a consensus of two or three observers in two (Carli 2002a; Steiner 1987), and this information was not reported by the remaining three evaluations (Ek 2005; Gokdemir 2011; Markowitz 2015).
Visual inspection (in‐person)
Across the eight evaluations of visual inspection, no formal algorithm to assist diagnosis was reported in 87% (n = 7) and one reported using the ABCD approach (Stanganelli 2000). Sensitivity ranged from 20% to 90% and specificity from 29% to 100% (Figure 9). Examinations in six studies were undertaken by dermatologists, (or were assumed to be dermatologists, based on study institution) and in two studies by consultant or registrar dermatologists (Cooper 2002) or plastic surgeons (Ek 2005). The lowest sensitivities were reported in studies restricted to pigmented lesions, particularly Carli 2002a and Stanganelli 2000. We pooled results across algorithms and thresholds as a summary ROC curve (7017 lesions; 1586 BCCs; Figure 11). Estimates of accuracy obtained from the curve suggest that the specificity of visual inspection would be 77% at a fixed threshold of 80% sensitivity, and sensitivity would be 79% at a fixed threshold of 80% specificity (Table 2). We chose these 80% fixed values as they lie within the estimates for most of the analyses and should only be considered as illustrative examples of the values that might be achieved based on the observed data (Statistical analysis and data synthesis). Of the three datasets which included melanomas in the disease‐negative group (Carli 2002a; Stanganelli 2000; Steiner 1987), five of the 15 false positive results were melanoma mistaken for BCCs (Carli 2002a; Steiner 1987).
Dermoscopy added to visual inspection
For the seven evaluations of dermoscopy added to visual inspection, two did not report using any algorithm to assist diagnosis (Durdu 2011; Gokdemir 2011), two used pattern analysis (Carli 2002a; Stanganelli 2000), and three used formal algorithms to assist diagnosis, including the three‐point checklist for BCC (Amirnia 2016) and the Marghoob and colleagues (Marghoob 2010) two‐step approach for classifying skin lesions (Markowitz 2015; Ulrich 2015). Sensitivity ranged from 79% to 100% and specificity from 54% to 100% (Figure 9). The low specificities of 54% (Ulrich 2015) and 56% (Markowitz 2015) appeared as outliers (with non‐overlapping confidence intervals), all other studies having specificities of 96% or above. Both studies included particularly high percentages of BCC (60% to 61%) and included non‐pigmented lesions with a high clinical suspicion of being BCC.
We pooled results across algorithms and thresholds as a summary ROC curve (4683 lesions; 363 BCCs; Figure 11). Estimates of accuracy obtained from the curve suggest that the specificity of dermoscopy would be 99% at a fixed threshold of 80% sensitivity, and sensitivity would be 93% at a fixed threshold of 80% specificity (Table 2). Of the four datasets which included melanomas in the disease‐negative group (Carli 2002a; Durdu 2011; Gokdemir 2011; Stanganelli 2000), three of the 19 false‐positive results were melanoma mistaken for BCCs (Durdu 2011; Gokdemir 2011).
Comparison of in‐person dermoscopy added to visual inspection versus visual inspection alone
The accuracy of visual inspection was compared with the accuracy of dermoscopy estimated from (a) all eight in‐person visual inspection and all seven dermoscopy studies (Figure 11) and (b) estimated from direct comparisons in the subset of four studies that evaluated both visual inspection and dermoscopy on an in‐person basis (3974 lesions; 258 BCCs; Figure 12). In both comparisons the accuracy of dermoscopy in addition to visual inspection exceeded that of visual inspection alone (Table 2). In (a) the diagnostic odds ratio (DOR) for dermoscopy was 8.2 (95% CI 3.5 to 19.3; LR test P < 0.001) times that of visual inspection alone; in (b) it was 7.5 (95% CI 2.7 to 21.3; LR test P < 0.001) times that of visual inspection alone. These effects correspond to predicted differences in specificity of (a) 22% (99% versus 77%) and (b) 61% (97% versus 36%) at a fixed sensitivity of 80% (Table 2) and predicted differences in sensitivity of (a) 14% (93% versus 79%) and (b) 16% (87% versus 71%) at a fixed specificity of 80% (Table 2).
Image‐based evaluations
The 11 studies reporting image‐based diagnosis using clinical photographs (n = 2; Lorentzen 1999; Nori 2004), dermoscopic images (n = 7; Altamura 2010; Carli 2002a; Lorentzen 2008; Menzies 2000; Navarrete Dechent 2016; Witkowski 2016; Zalaudek 2006) or both (n = 2; Carli 2002b; Rosendahl 2011) were primarily conducted in referred populations undergoing biopsy or excision (Appendix 9). Two studies were conducted in a limited prior testing setting, recruiting participants from primary care (Rosendahl 2011) or from a private dermatology practice (Navarrete Dechent 2016). Of the remaining nine, one was conducted in participants with equivocal lesions (Witkowski 2016). Two evaluations used a case‐control design, separately recruiting diseased and non‐diseased participants (Altamura 2010; Menzies 2000), one was a prospective case series (Lorentzen 1999), five retrospectively selected series of images for prospective interpretation within the context of the study (Navarrete Dechent 2016; Nori 2004; Rosendahl 2011; Witkowski 2016; Zalaudek 2006), and three did not clearly report the direction of the design (Carli 2002a; Carli 2002b; Lorentzen 2008).
Five of the 11 studies primarily aimed to examine accuracy for the detection of BCC (Altamura 2010; Menzies 2000; Navarrete Dechent 2016; Nori 2004; Witkowski 2016), while the remaining six also provided data for our reviews of visual inspection or dermoscopy or both for the diagnosis of melanoma (Dinnes 2018a; Dinnes 2018b). Four evaluations included any lesion, pigmented or non‐pigmented (Altamura 2010; Lorentzen 1999; Lorentzen 2008; Zalaudek 2006); four included only pigmented lesions (Carli 2002a; Carli 2002b; Menzies 2000; Rosendahl 2011); two included non‐pigmented lesions only (Navarrete Dechent 2016; Witkowski 2016), and one included biopsy‐confirmed BCCs and lesions with a range of common diagnoses (Nori 2004). The prevalence of BCC ranged from 2% (Carli 2002a) to 63% (Navarrete Dechent 2016); median 16% (IQR 11, 47%). The highest prevalence was generally observed in the studies in non‐pigmented lesions or lesions suspicious for BCC (44% to 63% in four studies, one of which used a case‐control design; Altamura 2010). All studies apart from Nori 2004 reported including invasive melanoma or melanoma in situ, and five also included cSCC in the disease‐negative group (Altamura 2010; Navarrete Dechent 2016; Nori 2004; Rosendahl 2011; Witkowski 2016).
Diagnosis was recorded by dermatologists or clinicians presumed to be dermatologists (based on author’s institutions) in most of the studies (9/11; 73%), or by a mixed group of clinicians in two (Lorentzen 1999; Zalaudek 2006). Where reported (n = 9), the number of observers ranged from two (reported for five studies) to 150 (median 2).
Test accuracy was reported for a single observer in four studies, for a consensus of two observers in three (Carli 2002a; Carli 2002b; Navarrete Dechent 2016), the average across observers in three (Lorentzen 1999; Lorentzen 2008; Zalaudek 2006), and this information was not reported by one (Menzies 2000).
Visual inspection of clinical photographs
The four evaluations of image‐based visual inspection reported no formal algorithm to have been used to assist diagnosis. Sensitivity ranged from 48% to 89%, and specificity from 62% to 98% (Figure 10). We pooled results as a summary ROC curve (853 lesions; 156 BCCs; Figure 13). Estimates of accuracy obtained from the curve suggest that the specificity of image‐based visual inspection would be 87% at a fixed threshold of 80% sensitivity, and sensitivity would be 85% at a fixed threshold of 80% specificity (Table 2). Of the three datasets which included melanoma in the disease‐negative group (Carli 2002b; Lorentzen 1999; Rosendahl 2011), three of 39 false‐positive results were melanoma mistaken for BCCs (Rosendahl 2011).
Dermoscopic image‐based diagnosis
Of the nine evaluations of image‐based dermoscopy, two did not report using any algorithm to assist diagnosis (Carli 2002b; Witkowski 2016), three used pattern analysis (Carli 2002a; Lorentzen 2008; Rosendahl 2011), and four used formal algorithms to assist diagnosis, including the three‐point checklist (Zalaudek 2006), the Menzies algorithm for BCC (Menzies 2000) or a modification thereof (Altamura 2010), or a new algorithm ‘shiny white blotches and strands' (Navarrete Dechent 2016). Only one study provided the clinical photograph alongside the dermoscopic image (Rosendahl 2011), with the rest reporting blinded dermoscopy interpretations. Sensitivity ranged from 40% to 97% and specificity from 50% to 100% (Figure 10). We observed particularly low sensitivities in Carli 2002a and Navarrete Dechent 2016 (which respectively had the lowest (2%) and highest (63%) prevalence of BCC), the latter also reporting the lowest specificity (50%). All other studies reported sensitivities of 85% or above and specificities of 72% or more.
We pooled results across algorithms and thresholds as a summary ROC curve (2271 lesions; 737 BCCs; Figure 13). Estimates of accuracy obtained from the curve suggest that the specificity of dermoscopy would be 96% at a fixed threshold of 80% sensitivity, and sensitivity would be 93% at a fixed threshold of 80% specificity (Table 2). All nine evaluations included melanomas in the disease‐negative group; 23 of the 178 false‐positive results were melanomas mistaken for BCCs in five studies (Menzies 2000; Navarrete Dechent 2016; Rosendahl 2011; Witkowski 2016; Zalaudek 2006) and 45 were cSCCs mistaken for BCCs (Navarrete Dechent 2016; Witkowski 2016). Navarrete Dechent 2016 alone was responsible for 53 false positives (44 cSCC and nine melanomas).
Comparison of diagnosis based on dermoscopic images versus visual inspection of images
We compared the accuracy of image‐based visual inspection with the accuracy of dermoscopy estimated from (a) all four image‐based visual inspection and all nine dermoscopy studies (Figure 13), and (b) estimated from direct comparisons in the subset of two studies that evaluated both clinical photographs and dermoscopic images (516 lesions; 79 BCCs; Figure 14). In both comparisons the accuracy of dermoscopy in addition to visual inspection exceeded that of visual inspection alone (Table 2). In (a) the DOR for dermoscopy was 3.9 (95% CI 1.2 to 5.0, LR test P = 0.006) times that of visual inspection alone, and in (b) the RDOR was not estimable but the DOR of 275.5 (95% CI 112 to 678) for dermoscopy exceeded visual inspection alone (DOR 81.1, 95% CI 39.1 to 168). These effects correspond to predicted differences in specificity of (a) 9% (96% versus 87%) and (b) 4% (99% versus 95%) at a fixed sensitivity of 80% (Table 2), and predicted differences in sensitivity of (a) 8% (93% versus 85%) and (b) 4% (99% versus 95%) at a fixed specificity of 80% (Table 2).
Secondary analyses for the detection of BCC
Covariate investigations
Table 3 and Table 4 report the results of the heterogeneity investigations for visual inspection and for dermoscopy respectively. As discussed above, we found no clear differences in accuracy between studies undertaken in person and those which evaluated images for either test. Although our primary analyses are presented separately for in‐person and image‐based approaches, due to a paucity of data we have based all subsequent covariate investigations on the complete datasets for each test.
Visual inspection: Due to a lack of data, we could not investigate the use of a formal algorithm versus no formal algorithm for visual inspection. Observed accuracy was significantly higher, however, where disease prevalence of BCC was 25% or less (RDOR 9.7, 95% CI 2.3 to 40.8; LR test P = 0.002), compared to those where disease prevalence was greater than 25% (Table 3). This result appears to be driven by lower specificities with non‐overlapping confidence intervals in the studies in the higher‐prevalence group, most of which were conducted in populations with lesions suspicious for BCC (Schwartzberg 2005; Ulrich 2015; Markowitz 2015; Nori 2004). Sensitivities reported in these studies were largely within the range of those reported by studies in the lower prevalence group (Appendix 10).
Dermoscopy: Observed accuracy was somewhat higher in studies using no formal algorithm to assist diagnosis, as opposed to those reporting use of an algorithm (RDOR 7.8, 95% CI 0.90 to 68.2; LR test P = 0.004) Table 4. Accuracy was also non‐significantly higher where disease prevalence of BCC was 25% or less (RDOR 4.5, 95% CI 0.49 to 41.8; LR test P = 0.04), compared to those with disease prevalence greater than 25% (Table 4). There is considerable overlap in the studies included in the ‘named algorithm’ and higher‐prevalence groups (with six of the seven same studies appearing in each group: Altamura 2010; Amirnia 2016; Markowitz 2015; Menzies 2000; Navarrete Dechent 2016; Ulrich 2015). It seems likely that both factors play a role in the observed differences in accuracy (Appendix 10).
Analyses by algorithms used to assist diagnosis
We provide details of the algorithms used to assist diagnosis in Appendix 9. We report results by algorithm used (or not used) in Table 5 for each of the target conditions under consideration in this review.
4. Algorithm and threshold analysis for each definition of the target condition.
| Target condition Test | No Datasets | Lesions (Cases) | Pooled Sensitivity (95% CI) | Pooled Specificity (95% CI) | No studies | Lesions (Cases) | Pooled Sensitivity (95% CI) | Pooled Specificity (95% CI) |
| a. BCC – Visual inspection | IN‐PERSON | IMAGE‐BASED | ||||||
| No algorithm at any threshold | 7 | 3645 (1543) | 0.68 (0.48 to 0.83) | 0.82 (0.55 to 0.95) | 4 | 853 (156) | 0.71 (0.51 to 0.86) | 0.92 (0.76 to 0.98) |
| No algorithm at BCC possible | 1 | 141 (82) | 0.89 (0.80 to 0.95) | 0.37 (0.25 to 0.51) | 1 | 105 (58) | 0.78 (0.65 to 0.87) | 0.38 (0.25 to 0.54) |
| ABCD threshold not reported | 1 | 3372 (43) | 0.49 (0.33 to 0.65) | 1.00 (1.00 to 1.00) | ‐ | ‐ | ‐ | ‐ |
| Schwartzberg algorithm | 1 | 141 (82) | 0.89 (0.80 to 0.95) | 0.37 (0.25 to 0.51) | ‐ | ‐ | ‐ | ‐ |
| b. BCC – Dermoscopy | IN‐PERSON | IMAGE‐BASED | ||||||
| Algorithm threshold not reported | 2 | 648 (79) | 0.92 (0.84 to 0.97) | 0.97 (0.95 to 0.98) | 2 | 313 (121) | 0.85 (0.78 to 0.90) | 0.93 (0.88 to 0.96) |
| Pattern analysis | 2 | 3628 (48) | 0.79 (0.65 to 0.88) | 1.00 (1.00 to 1.00) | 2 | 582 (85) | 0.89 (0.81 to 0.94) | 0.98 (0.96 to 0.99) |
| 3 point at ≥ 2 | 1 | 61 (27) | 1.00 (0.87 to 1.00) | 0.97 (0.85 to 1.00) | 1 | 150 (18) | 0.89 (0.65 to 0.99) | 0.72 (0.63 to 0.79) |
| 2‐step algorithm | 2 | 346 (209) | 0.86 (0.76 to 0.92) | 0.55 (0.46 to 0.63) | ‐ | ‐ | ‐ | ‐ |
| Menzies for BCC (new) | ‐ | ‐ | ‐ | ‐ | 1 | 213 (71) | 0.97 (0.90 to 1.00) | 0.92 (0.87 to 0.96) |
| Menzies for BCC (revised) | ‐ | ‐ | ‐ | ‐ | 1 | 300 (150) | 0.95 (0.91 to 0.98) | 0.87 (0.81 to 0.92) |
| New SWS at ≥ 1 | ‐ | ‐ | ‐ | ‐ | 1 | 457 (287) | 0.54 (0.48 to 0.60) | 0.50 (0.42 to 0.58) |
| Chaos/clues | ‐ | ‐ | ‐ | ‐ | 1 | 463 (72) | 0.99 (0.93 to 1.00) | 0.55 (0.50 to 0.60) |
| c. cSCC – Visual inspection | IN‐PERSON | IMAGE‐BASED | ||||||
| No algorithm at threshold NR | 2 | 2684 (538) | 0.59 (0.42 to 0.82) | 0.79 (0.77 to 0.81) | ‐ | ‐ | ‐ | ‐ |
| d. cSCC – Dermoscopy | IN‐PERSON | IMAGE‐BASED | ||||||
| No algorithm at threshold NR | ‐ | ‐ | ‐ | ‐ | 1 | 260 (13) | 0.77 (0.46 to 0.95) | 0.97 (0.94 to 0.99) |
| SWS at > 1 char | ‐ | ‐ | ‐ | ‐ | 1 | 457 (106) | 0.42 (0.32 to 0.51) | 0.49 (0.43 to 0.54) |
| e. Any – Visual inspection | IN‐PERSON | IMAGE‐BASED | ||||||
| No algorithm at threshold NR | 4 | 3533 (1968) | 0.91 (0.79 to 0.96) | 0.61 (0.25 to 0.87) | 2 | 517 (124) | 0.77 (0.68 to 0.83) | 0.84 (0.80 to 0.87) |
| ABCD at threshold NR | 1 | 85 (53) | 0.57 (0.42 to 0.70) | 0.50 (0.32 to 0.68) | ‐ | ‐ | ‐ | ‐ |
| f. Any – Dermoscopy | IN‐PERSON | IMAGE‐BASED | ||||||
| No algorithm at threshold NR | 1 | 200 (46) | 0.98 (0.88 to 1.00) | 0.98 (0.94 to 1.00) | 3 | 393 (187) | 0.89 (0.84 to 0.93) | 0.79 (0.73 to 0.84) |
| No algorithm at excise | ‐ | ‐ | ‐ | ‐ | 1 | 260 (140) | 0.95 (0.90 to 0.98) | 0.53 (0.44 to 0.62) |
| Pattern analysis | ‐ | ‐ | ‐ | ‐ | 1 | 463 (104) | 0.79 (0.70 to 0.86) | 0.88 (0.85 to 0.91) |
| 3 point at ≥ 2 | 1 | 77 (39) | 0.85 (0.69 to 0.94) | 0.26 (0.13 to 0.43) | ‐ | ‐ | ‐ | ‐ |
| Menzies for BCC (revised) | ‐ | ‐ | ‐ | ‐ | 1 | 213 (142) | 0.95 (0.90 to 0.98) | 0.92 (0.83 to 0.97) |
| SWS | ‐ | ‐ | ‐ | ‐ | 1 | 457 (414) | 0.50 (0.45 to 0.55) | 0.63 (0.47 to 0.77) |
| Chaos/Clues | ‐ | ‐ | ‐ | ‐ | 1 | 463 (104) | 0.92 (0.85 to 0.97) | 0.58 (0.53 to 0.63) |
BCC ‐ basal cell carcinoma; CI ‐ confidence interval; SWS ‐ shiny white streaks; NR ‐ not reported
For the diagnosis of BCC, Table 5 highlights the lack of available data for formal algorithms to diagnose BCC, particularly for visual inspection. Although a number of dermoscopic algorithms have been evaluated for the diagnosis of BCC, only the Menzies algorithm appears to show promise in terms of increasing sensitivity without sacrificing the specificity which can be achieved by observer diagnosis alone (with no algorithm). The data, however, come from the same study which developed the algorithm using dermoscopic images, and it remains to be seen whether results can be replicated on an in‐person basis (Menzies 2000).
Analyses by observer experience
Observer experience was generally poorly described in the study reports (Appendix 8), but we attempted broad classifications by reported expertise in visual inspection or dermoscopy, regardless of an in‐person or image‐based approach to diagnosis. The resulting study subgroups were small, and results highly heterogeneous, so we could undertake no further analyses by observer expertise. None of the included studies provided direct comparisons of observer accuracy according to expertise or qualifications.
2. Target condition: cSCC
Four studies reported accuracy data for the detection of cSCC. Two studies provided data for in‐person visual inspection (Cooper 2002; Ek 2005) and two for image‐based dermoscopy (Navarrete Dechent 2016; Witkowski 2016) (Appendix 8). We present results for the primary analyses in Table 6. Forest plots of study data are given in Figure 17.
5. Comparison of visual inspection and dermoscopy for the detection of cSCC.
| Test | Datasets | Lesions (cSCC) | DOR (95% CI) | Summary sensitivity | Summary specificity |
| In‐person evaluations | |||||
| Visual inspection | 2 | 2684 (538) |
5.0 (4.1 to 6.1) |
0.57 (0.53 to 0.61) |
0.79 (0.77 to 0.81) |
| Visual inspection + Dermoscopy |
0 | ‐ | ‐ | ‐ | ‐ |
| Image‐based evaluations | |||||
| Visual inspection (clinical images) | 0 | ‐ | ‐ | ‐ | ‐ |
| Dermoscopic images | 2 | 717 (119) |
6.5 (0.45 to 93.2) |
0.55 (0.29 to 0.79) |
0.84 (0.32 to 0.98) |
cSCC ‐ cutaneous squamous cell carcinoma; DOR ‐ diagnostic odds ratio; CI ‐ confidence interval
17.

Evaluations of the accuracy of visual inspection or dermoscopy for detecting invasive melanoma cSCC
Visual inspection (in‐person)
Both studies of visual inspection were conducted in secondary clinic specialist clinics, one of which was provided for renal transplant recipients (Cooper 2002). Both studies included participants with a range of different lesion types that might be observed in clinical practice. The prevalence of cSCC was 21% (Cooper 2002) and 20% (Ek 2005). Both studies reported data for observers’ correct diagnosis of cSCC using no formal algorithm.
Pooled sensitivity and specificity (2684 lesions; 538 cSCCs) were 57% (95% CI 53% to 61%) and 79% (95% CI 77% to 81%) respectively. In Cooper 2002 none of the 12 BCCs was mistaken for a cSCC, but in Ek 2005, 119 of 1214 included BCCs were diagnosed as cSCCs (accounting for 28% of the false positives in this study).
Dermoscopic image‐based diagnosis
The two studies evaluating dermoscopic images were both conducted in participants with non‐pigmented lesions: Navarrete Dechent 2016, using their own new algorithm for detection of BCC based on the presence of shiny white streaks and blotches (but also reporting accuracy data for detection of cSCC using the algorithm), and Witkowski 2016, using no algorithm. Navarrete Dechent 2016 primarily recruited participants with malignant lesions (90% of lesions), whereas Witkowski 2016 included participants with a wider range of different lesion types that might be observed in clinical practice. The prevalence of cSCC was 23% (Navarrete Dechent 2016) and 5% (Witkowski 2016).
Pooled sensitivity and specificity (717 lesions; 119 cSCCs) were 55% (95% CI 29% to 79%) and 84% (95% CI 32% to 98%) respectively. Both sensitivity and specificity were considerably higher in Witkowski 2016 compared to Navarrete Dechent 2016, and the resulting confidence intervals were therefore extremely wide.
Comparison of dermoscopy versus visual inspection
No formal comparison of visual inspection and dermoscopy is possible for the detection of cSCC, as visual inspection data are from in‐person studies and dermoscopy from image‐based studies.
3. Target condition: Any skin cancer
In this section we present the results for studies of visual inspection for the identification of any skin cancer, according to the approach taken for diagnosis: in‐person or image‐based evaluations. We present summary characteristics of studies in Appendix 8, forest plots of study data in Figure 18 and Figure 19, and results of meta‐analyses in Table 7, Figure 20 and Figure 21.
18.

Forest plot of tests: 27 Any ‐Visual inspection (in‐person), 29 Any ‐VI+Dermoscopy (in‐person).
19.
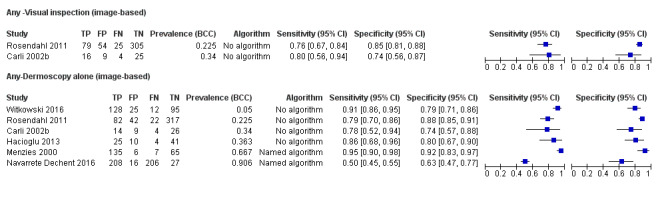
Forest plot of tests: 28 Any ‐Visual inspection (image‐based), 30 Any‐Dermoscopy alone (image‐based).
6. Comparison of visual inspection and dermoscopy for the detection of any skin cancer.
| Test | Datasets | Lesions (cases) | DOR (95% CI) | Specificity at 80% sensitivity | Sensitivity at 80% specificity | Relative DOR (95% CI) | P value (LR)a | P value (Wald)b |
| In‐person evaluations | ||||||||
| Visual inspection | 5 | 3618 (2021) |
28.7 (5.0 to 166) |
88% | 84% | NE | NE | NE |
| Visual inspection + Dermoscopy |
2 | 277 (85) |
126 (9.1 to 1751) |
NE | NE | |||
| Image‐based evaluations | ||||||||
| Visual inspection (clinical images) | 2 | 517 (124) |
16.3 (4.4 to 59.9) |
79% | 78% | 1.5 (0.76 to 3.0) |
0.50 | 0.24 |
| Dermoscopic images | 6 | 1526 (847) |
24.5 (7.6 to 79.3) |
84% | 86% | |||
DOR ‐ diagnostic odds ratio; RDOR ‐ relative diagnostic odds ratio; CI ‐ confidence interval; LR ‐ likelihood ratio; NE – not estimated; data not estimated due to extreme differences in results between the two studies of dermoscopy added to visual inspection
aTests whether there is a difference in test performance between defined groups in terms of either DOR or threshold. bTests the significance of the difference in DOR between defined groups at a particular SROC curve intercept value.
20.
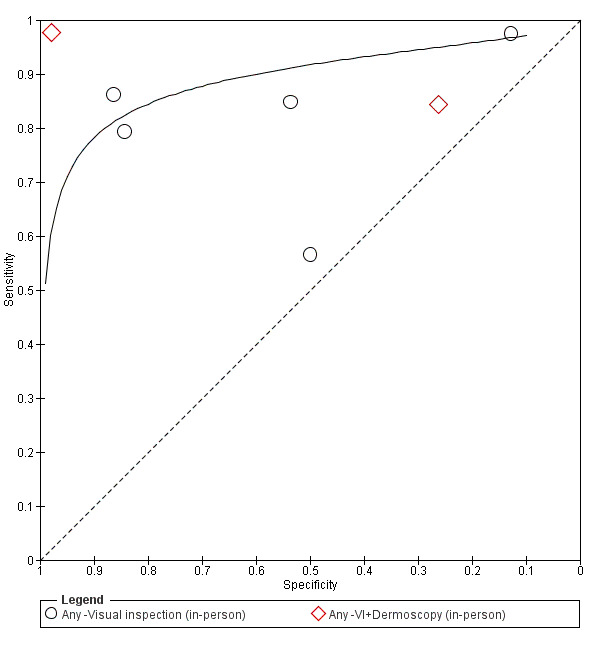
Comparison of the accuracy of visual inspection with visual inspection plus dermoscopy (VI+Dermoscopy) for detection of any skin cancer (Any). SROC curve estimated only for in‐person visual inspection.
21.

Comparison of the accuracy of image‐based visual inspection with image‐based dermoscopy (Dermoscopy alone) for detection of any skin cancer (Any)
In‐person evaluations
Five studies evaluated the accuracy of in‐person visual inspection for the detection of any skin cancer (Argenziano 2006; Chang 2013; Cooper 2002; Ek 2005; Hacioglu 2013) and two evaluated in‐person dermoscopy (Argenziano 2006; Durdu 2011). Three of these also reported accuracy data separately for BCC alone (Cooper 2002; Durdu 2011; Ek 2005) or for cSCC (Cooper 2002; Ek 2005).
All studies were based in secondary care or specialist referral clinics, apart from Argenziano 2006 which recruited participants from primary care (although only lesions selected for excision by an expert could be included). The prevalence of skin cancer ranged from 20% (Chang 2013) to 68% (Ek 2005). Studies included any lesion type, apart from Durdu 2011 which restricted inclusion to pigmented lesions only. Diagnoses were recorded by GPs (Argenziano 2006), dermatologists or assumed to be dermatologists based on study institution (Chang 2013; Durdu 2011; Hacioglu 2013) or by a clinician with mixed experience (Cooper 2002; Ek 2005). All studies used a histological reference standard.
Visual inspection
Studies either used no algorithm to aid diagnosis, or reported using the ABCD approach to diagnosis (Argenziano 2006). Sensitivities ranged from 57% to 98%; specificities ranged from 13% to 86% (Figure 18). In meta‐analysis the DOR was 28.7 (95% CI 5.0 to 166) (3618 lesions; 2021 skin cancer cases). Estimates of accuracy obtained from the curve suggest that the specificity of visual inspection would be 88% at a fixed threshold of 80% sensitivity, and sensitivity would be 84% at a fixed threshold of 80% specificity (Table 7).
Dermoscopy added to visual inspection
The two studies of in‐person dermoscopy reported data using the three‐point checklist (Argenziano 2006) and the ABCD approach (Durdu 2011) (Figure 18). In Argenziano 2006, GPs' diagnosis had a sensitivity of 85% (95% CI 69% to 94%) and specificity of 26% (95% CI 13% to 43%) for the subgroup of lesions selected for excision by an expert clinician. Of the six malignancies missed by GPs, four were BCCs, one cSCC and one melanoma. Durdu 2011 reported a sensitivity of 98% (95% CI 88% to 100%) and specificity 98% (95% CI 94% to 100%) for their sample of pigmented lesions which could not be diagnosed by a dermatologist with visual inspection alone.
In meta‐analysis the DOR was 126 (95% CI 9.1 to 1751) (277 lesions; 85 skin cancer cases) (Table 7). We could not obtain estimates of accuracy from the SROC curve due to extreme differences in results between the two studies (evidenced by the very wide range in confidence intervals around the DOR).
Comparison of in‐person dermoscopy versus visual inspection alone
No formal comparison of visual inspection and dermoscopy added to visual inspection was possible, due to the observed heterogeneity in results for the two dermoscopy studies (Figure 20).
Image‐based evaluations
Six studies reported data for image‐based diagnosis for the detection of any skin cancer. Two evaluated the accuracy of image‐based visual inspection (Carli 2002b; Rosendahl 2011) and all six evaluated diagnosis using dermoscopic images (Carli 2002b; Hacioglu 2013; Menzies 2000; Navarrete Dechent 2016; Rosendahl 2011; Witkowski 2016). Five of these also reported accuracy data separately for BCC alone (Carli 2002b; Menzies 2000; Navarrete Dechent 2016; Rosendahl 2011; Witkowski 2016) or for cSCC (Navarrete Dechent 2016; Witkowski 2016).
Two studies were conducted in a limited prior testing setting, recruiting participants from primary care (Rosendahl 2011) or from a private dermatology practice (Navarrete Dechent 2016). Of the remaining four, one was considered to have been conducted in participants with equivocal lesions (Witkowski 2016). Four of the six studies primarily aimed to examine accuracy for the detection of BCC (Menzies 2000; Navarrete Dechent 2016; Witkowski 2016) or ‘non‐melanoma’ skin cancer (Hacioglu 2013), with the remaining two also providing data for the diagnosis of melanoma (Carli 2002b; Rosendahl 2011). Three studies included only pigmented lesions (Carli 2002b; Menzies 2000; Rosendahl 2011); two included only non‐pigmented lesions (Navarrete Dechent 2016; Witkowski 2016) and one described lesions as ‘suspicious for malignancy’ (Hacioglu 2013). All studies apart from Hacioglu 2013 reported including invasive melanoma or melanoma in situ as disease‐negative and four also included cSCC (all apart from Carli 2002b and Menzies 2000) in the disease‐negative group. Diagnosis was recorded by dermatologists or by dermatology trainees (Navarrete Dechent 2016). All studies used a histological reference standard.
Visual inspection of images
The two included studies used no algorithm to aid diagnosis and both included pigmented lesions only (Carli 2002b; Rosendahl 2011). Sensitivities were 80% (95% CI 56% to 94%) and 76% (95% CI 67% to 84%) and specificities 74% (95% CI 56% to 87%) and 85% (95% CI 81% to 88%) in Carli 2002b and Rosendahl 2011, respectively (Figure 19).
In meta‐analysis the DOR was 16.3 (95%CI 4.4 to 59.9) (517 lesions; 124 skin cancer cases). Estimates of accuracy obtained from the curve suggest that the specificity of visual inspection would be 79% at a fixed threshold of 80% sensitivity, and sensitivity would be 78% at a fixed threshold of 80% specificity (Table 7).
Dermoscopic image‐based diagnosis
The six studies used no algorithm to assist diagnosis in three (Carli 2002b; Hacioglu 2013; Witkowski 2016), pattern analysis in one (Rosendahl 2011), and new algorithms for detection of BCC in two (Menzies 2000; Navarrete Dechent 2016).
Sensitivity ranged from 50% to 95% and specificity from 63% to 92% (Figure 19). We pooled results across algorithms and thresholds as a summary ROC curve (1526 lesions; 847 BCCs; Figure 21). Estimates of accuracy obtained from the curve suggest that the specificity of dermoscopy would be 84% at a fixed threshold of 80% sensitivity, and sensitivity would be 86% at a fixed threshold of 80% specificity (Table 7).
Comparison of diagnosis using dermoscopic images versus visual inspection of images
We compared accuracy using data from both visual inspection studies and all dermoscopy studies (Figure 21). The accuracy of diagnosis using dermoscopic images was non‐significantly higher than that based on clinical photographs (Table 7), with an RDOR of 1.5 (95% CI 0.76 to 3.0, LR test P = 0.50). Differences were marginal in sensitivity and specificity between tests in the two studies providing paired data.
Discussion
Summary of main results
We have evaluated visual inspection and the addition of dermoscopy for the detection of keratinocyte skin cancers in a range of study populations, on both an in‐person basis and using clinical photographs or dermoscopic images. Although a small number of published algorithms to assist diagnosis are available, most of the data relate to diagnosis without the use of an algorithm and relate to the detection of BCC rather than cSCC. Studies either did not recruit sufficient numbers of participants with cSCC to meet our inclusion criteria (i.e. five or more confirmed cSCCs) or did not present accuracy data for cSCC. For the detection of BCC, sensitivities and specificities were highly heterogeneous, especially for visual inspection. There was some suggestion that this heterogeneity was related to the case‐mix of included lesions, with studies in non‐pigmented lesions or those with a high index of suspicion of BCC having lower and more variable specificity, in comparison to those including pigmented lesions or lesions suspicious for any skin cancer. Studies were generally at high or unclear risk of bias across most domains assessed, particularly for image‐based interpretations, and of high or unclear concern about the applicability of the evidence, limiting the strength of conclusions that we can draw.
Table 1 presents key results for the primary target conditions of BCC and cSCC, and translates summary estimates to a hypothetical cohort of 1000 lesions. Due to the observed heterogeneity between studies, the results presented are points estimated from summary ROC curves rather than average sensitivity and specificity operating points. We present these for illustrative purposes, and they should not be quoted as the actual performance of visual inspection or dermoscopy. Due to the high risk of bias, concerns about applicability, the high level of unexplained heterogeneity and the necessity of the SROC curve analytical approach, we cannot confidently estimate the actual false‐negative and false‐positive rates for either test. Nevertheless, on average, the addition of dermoscopy to in‐person visual inspection of a lesion increases sensitivity and specificity for the diagnosis of BCC.
Sensitivity: At a fixed specificity of 80%, the use of dermoscopy increased the sensitivity of in‐person visual inspection by 14%, from 79% to 93%. Assuming BCC prevalence of 10%, 17% and 53% in a cohort of 1000 lesions, a test sensitivity of 93% would reduce the number of BCCs missed in comparison to using visual inspection alone by 14, 24 and 74 (resulting in 7, 12 and 37 BCCs missed). A test specificity of 80% (for both visual inspection and visual inspection plus dermoscopy) would result in 180, 166 and 94 false‐positive test results, i.e. lesions considered to be BCC which might then undergo unnecessary biopsy or treatment, in this case of benign lesions mistaken for BCCs, or inappropriate management, in the case of melanomas or cSCCs mistaken for BCCs.
Specificity: At a fixed sensitivity of 80%, the use of dermoscopy increased the specificity of in‐person visual inspection by 22%, from 77% to 99%. Applying these results to a cohort of 1000 lesions at the same three prevalences of disease, both tests would miss 20, 34 or 106 BCCs with the addition of dermoscopy reducing false positives by 198, 183 and 103 per 1000 from 207, 191 and 108 lesions mistaken as BCCs using visual inspection alone.
We found a similar pattern for image‐based comparisons of visual inspection and dermoscopy, although the differences in sensitivity and specificity were smaller (Table 1). It is notable that for the in‐person evaluations, up to a third of observed false‐positive results were melanomas mistaken for BCCs (33% (5/15) of false positives for visual inspection and 16% (3/19) for dermoscopy). This is of particular concern if non‐surgical treatment without biopsy is under consideration for lesions clinically presumed to be BCCs. In contrast to our review of dermoscopy versus visual inspection alone for the diagnosis of melanoma (Dinnes 2018b), there were no statistically significant differences between in‐person and image‐based evaluations for the diagnosis of BCC. Insufficient data were available to consider the effect of where in the clinical pathway the study was positioned, the use of formally‐developed algorithms to assist diagnosis of BCC, or the effect of observer experience on accuracy. In Dinnes 2018b, however, we were able to demonstrate that observer expertise and training in dermoscopy does improve accuracy for the diagnosis of melanoma.
Data for the detection of cSCC were limited, but suggest pooled sensitivity of 57% (95% CI 53% to 61%) and specificity of 79% (95% CI 77% to 81%) for visual inspection (in‐person), and sensitivity of 55% (95% CI 29% to 79%) and specificity of 84% (95% CI 32% to 98%) for dermoscopy (image‐based).
Strengths and weaknesses of the review
The strengths of this review include an in‐depth and comprehensive electronic literature search, systematic review methods including double extraction of papers by both clinicians and methodologists, and contact with authors to allow study inclusion or clarify data. We adopted a clear analysis structure focusing on estimating incremental gains in accuracy. We undertook a detailed and replicable analysis of methodologic quality.
The main concerns for the review are a result of relatively small numbers of studies, variation in the spectrum of included lesions and poor reporting of primary studies, hindering the assessment of study quality and limiting the conclusions that we can draw from the data. Our review of visual inspection for the diagnosis of melanoma identified a general trade‐off between sensitivity and specificity along the clinical pathway, with higher sensitivity and lower specificity in limited prior testing studies compared to those in referred populations (Dinnes 2018a). The lack of data from limited prior testing populations in this review and the lack of detailed information on the prior testing of participants included in referred populations meant that we could detect no clear patterns in sensitivity or specificity. We found some evidence of more variable accuracy, especially in terms of specificity, in studies with a higher prevalence of BCC or those conducted in populations of non‐pigmented lesions, or both. Many of these studies, however, also used new algorithms for detection of BCC rather than relying on the clinician’s diagnosis. The quality of dermatoscope and the resultant images may vary greatly, and there are further variations such as whether they are used with oil immersion or other light sources. None of our included studies provided enough detail to evaluate such effects on test performance. All of these factors together make it difficult to fully determine the cause of the observed heterogeneity.
Given these limitations, our results should be considered as exploratory rather than conclusive. We have, however, identified a clear suggestion of benefit from dermoscopy for the diagnosis of BCC, which requires further investigation. This is the first systematic review, to our knowledge, to have examined this critical question of dermoscopy use for the diagnosis of BCC, particularly given the increasing availability of newer imaging tests such as optical coherence tomography (OCT) or radiocontrast medium (RCM) which purport to assist in the diagnosis of BCC (Dinnes 2018c; Ferrante di Ruffano 2018b).
Applicability of findings to the review question
Our findings are particularly relevant to the use of visual inspection and dermoscopy for the diagnosis of BCC in referral settings. Limited data were available to consider accuracy in primary care or according to observer experience. We cannot be clear as to the likely error rates of visual inspection or dermoscopy in any particular lesion population, due to varying definitions and lack of clarity about the clinical pathway and any prior testing undergone.
Authors' conclusions
Implications for practice.
Dermoscopy may be a valuable tool to support visual inspection of a suspicious skin lesion for the diagnosis of BCC. The evidence primarily comes from secondary‐care (referred) populations and populations with pigmented lesions or mixed lesion types. There is no clear evidence supporting the use of formal algorithms to assist diagnosis.
Implications for research.
Surveys and qualitative research documenting dermoscopy use in a primary‐care setting in different countries and healthcare systems would help to better understand the purpose for which dermoscopy is being used. It may be that it is mainly used for triaging suspected melanoma (or high‐risk keratinocyte skin cancer) for urgent secondary referral; alternatively, dermoscopy may be used to differentiate between types of skin cancer (melanoma, BCC or cSCC) with a view to initial treatment of some lesions in primary care and referral of others to a secondary‐care setting. Prospective studies evaluating the use of dermoscopy in primary care for all forms of suspected skin cancer could better define where the gains might reside in terms of triage, and help to quantify diagnostic test accuracy. The need not to miss potentially lethal cancers such as melanomas must be balanced against the avoidance of unnecessary referral and biopsy resulting in raised morbidity and cost.
Further prospective evaluation of dermoscopy added to visual inspection in populations with a high clinical suspicion of BCC in both a primary‐care and secondary‐care setting by users with defined expertise is also likely to be warranted. Such evaluations should be conducted on an in‐person basis with prospective recruitment of consecutive series of participants and with systematic follow‐up of non‐excised lesions to avoid over‐reliance on a histological reference standard that can only provide information on excised cases. A clear identification of the level of training and experience required to achieve good results is required. It is unclear whether further research is warranted on the potential additional value of dermoscopy to visual inspection for lesions that are suspected to be cSCC in a primary‐ and secondary‐care setting, unless they are conducted in specific populations such as people with immunosuppression or who have received organ transplants in whom cSCC is a common problem.
Given the mixed results to date, it is unclear whether further research is warranted into the added value of dermoscopy algorithms to assist diagnosis above pattern recognition of characteristic morphological features. Any future research study needs to be clear about the diagnostic pathway followed by study participants prior to study enrolment, and should conform to the updated Standards for Reporting of Diagnostic Accuracy (STARD) guideline (Bossuyt 2015).
What's new
| Date | Event | Description |
|---|---|---|
| 19 December 2018 | Amended | Affiliations, Disclaimer and Sources of support updated |
Acknowledgements
Members of the Cochrane Skin Cancer Diagnostic Test Accuracy Group include:
the full project team (Susan Bayliss, Naomi Chuchu, Clare Davenport, Jonathan Deeks, Jacqueline Dinnes, Lavinia Ferrante di Ruffano, Kathie Godfrey, Rubeta Matin, Colette O'Sullivan, Yemisi Takwoingi, Hywel Williams)
our 12 clinical reviewers (Rachel Abbott, Ben Aldridge, Oliver Bassett, Sue Ann Chan, Alana Durack, Monica Fawzy, Abha Gulati, Jacqui Moreau, Lopa Patel, Daniel Saleh, David Thompson, Kai Yuen Wong) and two methodologists (Lavinia Ferrante di Ruffano and Louise Johnston), who assisted with full‐text screening, data extraction and quality assessment across the entire suite of reviews of diagnosis and staging and skin cancer;
our expert advisor and co‐author on the review, Hamid Tehrani, and on the protocol for keratinocyte skin cancer, Fiona Bath‐Hextall; and
all members of our Advisory Group (Jonathan Bowling, Seau Tak Cheung, Colin Fleming, Matthew Gardiner, Abhilash Jain, Susan O’Connell, Pat Lawton, John Lear, Mariska Leeflang, Richard Motley, Paul Nathan, Julia Newton‐Bishop, Miranda Payne, Rachael Robinson, Simon Rodwell, Julia Schofield, Neil Shroff, Hamid Tehrani, Zoe Traill, Fiona Walter, Angela Webster).
Cochrane Skin editorial base wishes to thank Robert Dellavalle, who was the Dermatology Editor for this review; and the clinical referee, Andrew Affleck. We also wish to thank the Cochrane DTA editorial base and colleagues, as well as Kate Cahill, who copy‐edited this review.
Appendices
Appendix 1. Current content and structure of the Programme Grant
| LIST OF REVIEWS | Number of studies | |
| Diagnosis of melanoma | ||
| 1 | Visual inspection | 49 |
| 2 | Dermoscopy +/‐ visual inspection | 104 |
| 3 | Teledermatology | 22 |
| 4 | Smartphone applications | 2 |
| 5a | Computer‐assisted diagnosis – dermoscopy‐based techniques | 42 |
| 5b | Computer‐assisted diagnosis – spectroscopy‐based techniques | Review amalgamated into 5a |
| 6 | Reflectance confocal microscopy | 18 |
| 7 | High‐frequency ultrasound | 5 |
| Diagnosis of keratinocyte skin cancer (BCC and cSCC) | ||
| 8 | Visual inspection +/‐ Dermoscopy | 24 |
| 5c | Computer‐assisted diagnosis – dermoscopy‐based techniques | Review amalgamated into 5a |
| 5d | Computer‐assisted diagnosis – spectroscopy‐based techniques | Review amalgamated into 5a |
| 9 | Optical coherence tomography | 5 |
| 10 | Reflectance confocal microscopy | 10 |
| 11 | Exfoliative cytology | 9 |
| Staging of melanoma | ||
| 12 | Imaging tests (ultrasound, CT, MRI, PET‐CT) | 38 |
| 13 | Sentinel lymph node biopsy | 160 |
| Staging of cSCC | ||
| Imaging tests review | Review dropped; only one study identified | |
| 13 | Sentinel lymph node biopsy | Review amalgamated into 13 above (n = 15 studies) |
Appendix 2. Glossary of terms
| Term | Definition |
| Atypical intraepidermal melanocytic variant | Unusual area of darker pigmentation contained within the epidermis that may progress to an invasive melanoma; includes melanoma in situ and lentigo maligna |
| Atypical naevi | Unusual looking but noncancerous mole or area of darker pigmentation of the skin |
| BRAF V600 mutation | BRAF is a human gene that makes a protein called B‐Raf which is involved in the control of cell growth. BRAF mutations (damaged DNA) occur in around 40% of melanomas, which can then be treated with particular drugs. |
| BRAF inhibitors | Therapeutic agents which inhibit the serine‐threonine protein kinase BRAF mutated metastatic melanoma. |
| Breslow thickness | A scale for measuring the thickness of melanomas by the pathologist using a microscope, measured in mm from the top layer of skin to the bottom of the tumour. |
| Congenital naevi | A type of mole found on infants at birth |
| Dermoscopy | Whereby a handheld microscope is used to allow more detailed, magnified, examination of the skin compared to examination by the naked eye alone |
| False negative | An individual who is truly positive for a disease, but whom a diagnostic test classifies them as disease‐free. |
| False positive | An individual who is truly disease‐free, but whom a diagnostic test classifies them as having the disease. |
| Histopathology/Histology | The study of tissue, usually obtained by biopsy or excision, for example under a microscope. |
| Incidence | The number of new cases of a disease in a given time period. |
| Index test | A diagnostic test under evaluation in a primary study |
| Lentigo maligna | Unusual area of darker pigmentation contained within the epidermis which includes malignant cells but with no invasive growth. May progress to an invasive melanoma |
| Lymph node | Lymph nodes filter the lymphatic fluid (clear fluid containing white blood cells) that travels around the body to help fight disease; they are located throughout the body often in clusters (nodal basins). |
| Melanocytic naevus | An area of skin with darker pigmentation (or melanocytes) also referred to as ‘moles’ |
| Meta‐analysis | A form of statistical analysis used to synthesise results from a collection of individual studies. |
| Metastases/metastatic disease | Spread of cancer away from the primary site to somewhere else through the bloodstream or the lymphatic system. |
| Micrometastases | Micrometastases are metastases so small that they can only be seen under a microscope. |
| Mitotic rate | Microscopic evaluation of number of cells actively dividing in a tumour. |
| Morbidity | Detrimental effects on health. |
| Mortality | Either (1) the condition of being subject to death; or (2) the death rate, which reflects the number of deaths per unit of population in relation to any specific region, age group, disease, treatment or other classification, usually expressed as deaths per 100, 1000, 10,000 or 100,000 people. |
| Multidisciplinary team | A team with members from different healthcare professions and specialties (e.g. urology, oncology, pathology, radiology, and nursing). Cancer care in the National Health Service (NHS) uses this system to ensure that all relevant health professionals are engaged to discuss the best possible care for that patient. |
| Prevalence | The proportion of a population found to have a condition. |
| Prognostic factors/indicators | Specific characteristics of a cancer or the person who has it which might affect the patient’s prognosis. |
| Receiver operating characteristic (ROC) plot | A plot of the sensitivity and 1 minus the specificity of a test at the different possible thresholds for test positivity; represents the diagnostic capability of a test with a range of binary test results |
| Receiver operating characteristic (ROC) analysis | The analysis of a ROC plot of a test to select an optimal threshold for test positivity |
| Recurrence | Recurrence is when new cancer cells are detected following treatment. This can occur either at the site of the original tumour or at other sites in the body. |
| Reference Standard | A test or combination of tests used to establish the final or ‘true’ diagnosis of a patient in an evaluation of a diagnostic test |
| Reflectance confocal microscopy (RCM) | A microscopic technique using infrared light (either in a handheld device or a static unit) that can create images of the deeper layers of the skin |
| Sensitivity | In this context the term is used to mean the proportion of individuals with a disease who have that disease correctly identified by the study test |
| Specificity | The proportion of individuals without the disease of interest (in this case with benign skin lesions) who have that absence of disease correctly identified by the study test |
| Staging | Clinical description of the size and spread of a patient’s tumour, fitting into internationally agreed categories. |
| Subclinical (disease) | Disease that is usually asymptomatic and not easily observable, e.g. by clinical or physical examination. |
| Systemic treatment | Treatment, usually given by mouth or by injection, that reaches and affects cancer cells throughout the body rather than targeting one specific area. |
Appendix 3. Proposed sources of heterogeneity
i. Population characteristics
general versus higher‐risk populations
patient population: Primary/secondary/specialist unit
lesion suspicion: general suspicion/atypical/equivocal/NR
lesion type: any pigmented; melanocytic
inclusion of multiple lesions per participant
ethnicity
ii. Index test characteristics
the nature of and definition of criteria for test positivity
observer experience with the index test
approaches to lesion preparation (e.g. the use of oil or antiseptic gel for dermoscopy)
iii. Reference standard characteristics
reference standard used
whether histology‐reporting meets pathology‐reporting guidelines
use of excisional versus diagnostic biopsy
whether two independent dermatopathologists reviewed histological diagnosis
iv. Study quality
consecutive or random sample of participants recruited
index test interpreted blinded to the reference standard result
index test interpreted blinded to the result of any other index test
presence of partial or differential verification bias (whereby only a sample of those subject to the index test are verified by the reference test or by the same reference test with selection dependent on the index test result)
use of an adequate reference standard
overall risk of bias
Appendix 4. Final search strategies
Melanoma search strategies to August 2016
Database: Ovid MEDLINE(R) 1946 to August week 3 2016
Search strategy:
1 exp melanoma/
2 exp skin cancer/
3 exp basal cell carcinoma/
4 basalioma$1.ti,ab.
5 ((basal cell or skin) adj2 (cancer$1 or carcinoma$1 or mass or masses or tumour$1 or tumor$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or lesion$1 or malignan$ or nodule$1)).ti,ab.
6 (pigmented adj2 (lesion$1 or mole$ or nevus or nevi or naevus or naevi or skin)).ti,ab.
7 (melanom$1 or nonmelanoma$1 or non‐melanoma$1 or melanocyt$ or non‐melanocyt$ or nonmelanocyt$ or keratinocyt$).ti,ab.
8 nmsc.ti,ab.
9 (squamous cell adj2 (cancer$1 or carcinoma$1 or mass or masses or tumor$1 or tumour$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or epithelial or lesion$1 or malignan$ or nodule$1) adj2 (skin or epiderm$ or cutaneous)).ti,ab.
10 (BCC or CSCC or NMSC).ti,ab.
11 keratinocy$.ti,ab.
12 Keratinocytes/
13 or/1‐12
14 dermoscop$.ti,ab.
15 dermatoscop$.ti,ab.
16 photomicrograph$.ti,ab.
17 exp epiluminescence microscopy/
18 (epiluminescence adj2 microscop$).ti,ab.
19 (confocal adj2 microscop$).ti,ab.
20 (incident light adj2 microscop$).ti,ab.
21 (surface adj2 microscop$).ti,ab.
22 (visual adj (inspect$ or examin$)).ti,ab.
23 ((clinical or physical) adj examin$).ti,ab.
24 3 point.ti,ab.
25 three point.ti,ab.
26 pattern analys$.ti,ab.
27 ABCD$.ti,ab.
28 menzies.ti,ab.
29 7 point.ti,ab.
30 seven point.ti,ab.
31 (digital adj2 (dermoscop$ or dermatoscop$)).ti,ab.
32 artificial intelligence.ti,ab.
33 AI.ti,ab.
34 computer assisted.ti,ab.
35 computer aided.ti,ab.
36 neural network$.ti,ab.
37 exp diagnosis, computer‐assisted/
38 MoleMax.ti,ab.
39 image process$.ti,ab.
40 automatic classif$.ti,ab.
41 image analysis.ti,ab.
42 SIAscop$.ti,ab.
43 Aura.ti,ab.
44 (optical adj2 scan$).ti,ab.
45 MelaFind.ti,ab.
46 SIMSYS.ti,ab.
47 MoleMate.ti,ab.
48 SolarScan.ti,ab.
49 VivaScope.ti,ab.
50 (high adj3 ultraso$).ti,ab.
51 (canine adj2 detect$).ti,ab.
52 ((mobile or cell or cellular or smart) adj ((phone$1 adj2 app$1) or application$1)).ti,ab.
53 smartphone$.ti,ab.
54 (DermoScan or SkinVision or DermLink or SpotCheck).ti,ab.
55 Mole Detective.ti,ab.
56 Spot Check.ti,ab.
57 (mole$1 adj2 map$).ti,ab.
58 (total adj2 body).ti,ab.
59 exfoliative cytolog$.ti,ab.
60 digital analys$.ti,ab.
61 (image$1 adj3 software).ti,ab.
62 (teledermatolog$ or tele‐dermatolog$ or telederm or tele‐derm or teledermoscop$ or tele‐dermoscop$ or teledermatoscop$ or tele‐dermatoscop$).ti,ab.
63 (optical coherence adj (technolog$ or tomog$)).ti,ab.
64 (computer adj2 diagnos$).ti,ab.
65 exp sentinel lymph node biopsy/
66 (sentinel adj2 node).ti,ab.
67 nevisense.mp. or HFUS.ti,ab.
68 electrical impedance spectroscopy.ti,ab.
69 history taking.ti,ab.
70 patient history.ti,ab.
71 (naked eye adj (exam$ or assess$)).ti,ab.
72 (skin adj exam$).ti,ab.
73 physical examination/
74 ugly duckling.mp. or UD.ti,ab.
75 ((physician$ or clinical or physical) adj (exam$ or triage or recog$)).ti,ab.
76 ABCDE.mp. or VOC.ti,ab.
77 clinical accuracy.ti,ab.
78 Family Practice/ or Physicians, Family/ or clinical competence/
79 (confocal adj2 microscop$).ti,ab.
80 diagnostic algorithm$1.ti,ab.
81 checklist$.ti,ab.
82 virtual imag$1.ti,ab.
83 volatile organic compound$1.ti,ab.
84 dog$1.ti,ab.
85 gene expression analy$.ti,ab.
86 reflex transmission imag$.ti,ab.
87 thermal imaging.ti,ab.
88 elastography.ti,ab.
89 or/14‐88
90 (CT or PET).ti,ab.
91 PET‐CT.ti,ab.
92 (FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical$).ti,ab.
93 exp Deoxyglucose/
94 deoxy‐glucose.ti,ab.
95 deoxyglucose.ti,ab.
96 CATSCAN.ti,ab.
97 exp Tomography, Emission‐Computed/
98 exp Tomography, X‐ray computed/
99 positron emission tomograph$.ti,ab.
100 exp magnetic resonance imaging/
101 (MRI or fMRI or NMRI or scintigraph$).ti,ab.
102 exp echography/
103 Doppler echography.ti,ab.
104 sonograph$.ti,ab.
105 ultraso$.ti,ab.
106 doppler.ti,ab.
107 magnetic resonance imag$.ti,ab.
108 or/90‐107
109 (stage$ or staging or metasta$ or recurrence or sensitivity or specificity or false negative$ or thickness$).ti,ab.
110 "Sensitivity and Specificity"/
111 exp cancer staging/
112 or/109‐111
113 108 and 112
114 89 or 113
115 13 and 114
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations 29 August 2016
Search strategy:
1 basalioma$1.ti,ab.
2 ((basal cell or skin) adj2 (cancer$1 or carcinoma$1 or mass or masses or tumour$1 or tumor$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or lesion$1 or malignan$ or nodule$1)).ti,ab.
3 (pigmented adj2 (lesion$1 or mole$ or nevus or nevi or naevus or naevi or skin)).ti,ab.
4 (melanom$1 or nonmelanoma$1 or non‐melanoma$1 or melanocyt$ or non‐melanocyt$ or nonmelanocyt$ or keratinocyt$).ti,ab.
5 nmsc.ti,ab.
6 (squamous cell adj2 (cancer$1 or carcinoma$1 or mass or masses or tumor$1 or tumour$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or epithelial or lesion$1 or malignan$ or nodule$1) adj2 (skin or epiderm$ or cutaneous)).ti,ab.
7 (BCC or CSCC or NMSC).ti,ab.
8 keratinocy$.ti,ab.
9 or/1‐8
10 dermoscop$.ti,ab.
11 dermatoscop$.ti,ab.
12 photomicrograph$.ti,ab.
13 (epiluminescence adj2 microscop$).ti,ab.
14 (confocal adj2 microscop$).ti,ab.
15 (incident light adj2 microscop$).ti,ab.
16 (surface adj2 microscop$).ti,ab.
17 (visual adj (inspect$ or examin$)).ti,ab.
18 ((clinical or physical) adj examin$).ti,ab.
19 3 point.ti,ab.
20 three point.ti,ab.
21 pattern analys$.ti,ab.
22 ABCD$.ti,ab.
23 menzies.ti,ab.
24 7 point.ti,ab.
25 seven point.ti,ab.
26 (digital adj2 (dermoscop$ or dermatoscop$)).ti,ab.
27 artificial intelligence.ti,ab.
28 AI.ti,ab.
29 computer assisted.ti,ab.
30 computer aided.ti,ab.
31 neural network$.ti,ab.
32 MoleMax.ti,ab.
33 image process$.ti,ab.
34 automatic classif$.ti,ab.
35 image analysis.ti,ab.
36 SIAscop$.ti,ab.
37 Aura.ti,ab.
38 (optical adj2 scan$).ti,ab.
39 MelaFind.ti,ab.
40 SIMSYS.ti,ab.
41 MoleMate.ti,ab.
42 SolarScan.ti,ab.
43 VivaScope.ti,ab.
44 (high adj3 ultraso$).ti,ab.
45 (canine adj2 detect$).ti,ab.
46 ((mobile or cell or cellular or smart) adj ((phone$1 adj2 app$1) or application$1)).ti,ab.
47 smartphone$.ti,ab.
48 (DermoScan or SkinVision or DermLink or SpotCheck).ti,ab.
49 Mole Detective.ti,ab.
50 Spot Check.ti,ab.
51 (mole$1 adj2 map$).ti,ab.
52 (total adj2 body).ti,ab.
53 exfoliative cytolog$.ti,ab.
54 digital analys$.ti,ab.
55 (image$1 adj3 software).ti,ab.
56 (teledermatolog$ or tele‐dermatolog$ or telederm or tele‐derm or teledermoscop$ or tele‐dermoscop$ or teledermatoscop$ or tele‐dermatoscop$).ti,ab.
57 (optical coherence adj (technolog$ or tomog$)).ti,ab.
58 (computer adj2 diagnos$).ti,ab.
59 (sentinel adj2 node).ti,ab.
60 nevisense.mp. or HFUS.ti,ab.
61 electrical impedance spectroscopy.ti,ab.
62 history taking.ti,ab.
63 patient history.ti,ab.
64 (naked eye adj (exam$ or assess$)).ti,ab.
65 (skin adj exam$).ti,ab.
66 ugly duckling.mp. or UD.ti,ab.
67 ((physician$ or clinical or physical) adj (exam$ or triage or recog$)).ti,ab.
68 ABCDE.mp. or VOC.ti,ab.
69 clinical accuracy.ti,ab.
70 (Family adj (Practice or Physicians)).ti,ab.
71 (confocal adj2 microscop$).ti,ab.
72 clinical competence.ti,ab.
73 diagnostic algorithm$1.ti,ab.
74 checklist$.ti,ab.
75 virtual imag$1.ti,ab.
76 volatile organic compound$1.ti,ab.
77 dog$1.ti,ab.
78 gene expression analy$.ti,ab.
79 reflex transmission imag$.ti,ab.
80 thermal imaging.ti,ab.
81 elastography.ti,ab.
82 or/10‐81
83 (CT or PET).ti,ab.
84 PET‐CT.ti,ab.
85 (FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical$).ti,ab.
86 deoxy‐glucose.ti,ab.
87 deoxyglucose.ti,ab.
88 CATSCAN.ti,ab.
89 positron emission tomograph$.ti,ab.
90 (MRI or fMRI or NMRI or scintigraph$).ti,ab.
91 Doppler echography.ti,ab.
92 sonograph$.ti,ab.
93 ultraso$.ti,ab.
94 doppler.ti,ab.
95 magnetic resonance imag$.ti,ab.
96 or/83‐95
97 (stage$ or staging or metasta$ or recurrence or sensitivity or specificity or false negative$ or thickness$).ti,ab.
98 96 and 97
99 82 or 98
100 9 and 99
Database: Embase 1974 to 29 August 2016
Search strategy:
1 *melanoma/
2 *skin cancer/
3 *basal cell carcinoma/
4 basalioma$.ti,ab.
5 ((basal cell or skin) adj2 (cancer$1 or carcinoma$1 or mass or masses or tumour$1 or tumor$1 or neoplasm$ or adenoma$ or epithelioma$ or lesion$ or malignan$ or nodule$)).ti,ab.
6 (pigmented adj2 (lesion$1 or mole$ or nevus or nevi or naevus or naevi or skin)).ti,ab.
7 (melanom$1 or nonmelanoma$1 or non‐melanoma$1 or melanocyt$ or non‐melanocyt$ or nonmelanocyt$ or keratinocyt$).ti,ab.
8 nmsc.ti,ab.
9 (squamous cell adj2 (cancer$1 or carcinoma$1 or mass or tumor$1 or tumour$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or epithelial or lesion$1 or malignan$ or nodule$1) adj2 (skin or epiderm$ or cutaneous)).ti,ab.
10 (BCC or cscc).mp. or NMSC.ti,ab.
11 keratinocyte.ti,ab.
12 keratinocy$.ti,ab.
13 or/1‐12
14 dermoscop$.ti,ab.
15 dermatoscop$.ti,ab.
16 photomicrograph$.ti,ab.
17 *epiluminescence microscopy/
18 (epiluminescence adj2 microscop$).ti,ab.
19 (confocal adj2 microscop$).ti,ab.
20 (incident light adj2 microscop$).ti,ab.
21 (surface adj2 microscop$).ti,ab.
22 (visual adj (inspect$ or examin$)).ti,ab.
23 ((clinical or physical) adj examin$).ti,ab.
24 3 point.ti,ab.
25 three point.ti,ab.
26 pattern analys$.ti,ab.
27 ABCD$.ti,ab.
28 menzies.ti,ab.
29 7 point.ti,ab.
30 seven point.ti,ab.
31 (digital adj2 (dermoscop$ or dermatoscop$)).ti,ab.
32 artificial intelligence.ti,ab.
33 AI.ti,ab.
34 computer assisted.ti,ab.
35 computer aided.ti,ab.
36 neural network$.ti,ab.
37 MoleMax.ti,ab.
38 exp diagnosis, computer‐assisted/
39 image process$.ti,ab.
40 automatic classif$.ti,ab.
41 image analysis.ti,ab.
42 SIAscop$.ti,ab.
43 (optical adj2 scan$).ti,ab.
44 Aura.ti,ab.
45 MelaFind.ti,ab.
46 SIMSYS.ti,ab.
47 MoleMate.ti,ab.
48 SolarScan.ti,ab.
49 VivaScope.ti,ab.
50 confocal microscop$.ti,ab.
51 (high adj3 ultraso$).ti,ab.
52 (canine adj2 detect$).ti,ab.
53 ((mobile or cell$ or cellular or smart) adj ((phone$1 adj2 app$1) or application$1)).ti,ab.
54 smartphone$.ti,ab.
55 (DermoScan or SkinVision or DermLink or SpotCheck).ti,ab.
56 Spot Check.ti,ab.
57 Mole Detective.ti,ab.
58 (mole$1 adj2 map$).ti,ab.
59 (total adj2 body).ti,ab.
60 exfoliative cytolog$.ti,ab.
61 digital analys$.ti,ab.
62 (image$1 adj3 software).ti,ab.
63 (optical coherence adj (technolog$ or tomog$)).ti,ab.
64 (teledermatolog$ or tele‐dermatolog$ or telederm or tele‐derm or teledermoscop$ or tele‐dermoscop$ or teledermatoscop$).mp. or tele‐dermatoscop$.ti,ab.
65 (computer adj2 diagnos$).ti,ab.
66 *sentinel lymph node biopsy/
67 (sentinel adj2 node).ti,ab.
68 nevisense.ti,ab.
69 HFUS.ti,ab.
70 electrical impedance spectroscopy.ti,ab.
71 history taking.ti,ab.
72 patient history.ti,ab.
73 (naked eye adj (exam$ or assess$)).ti,ab.
74 (skin adj exam$).ti,ab.
75 *physical examination/
76 ugly duckling.ti,ab.
77 UD sign$.ti,ab.
78 ((physician$ or clinical or physical) adj (exam$ or recog$ or triage)).ti,ab.
79 ABCDE.ti,ab.
80 clinical accuracy.ti,ab.
81 *general practice/
82 (confocal adj2 microscop$).ti,ab.
83 clinical competence/
84 diagnostic algorithm$.ti,ab.
85 checklist$1.ti,ab.
86 virtual image$1.ti,ab.
87 volatile organic compound$1.ti,ab.
88 VOC.ti,ab.
89 dog$1.ti,ab.
90 gene expression analys$.ti,ab.
91 reflex transmission imaging.ti,ab.
92 thermal imaging.ti,ab.
93 elastography.ti,ab.
94 dog$1.ti,ab.
95 gene expression analys$.ti,ab.
96 reflex transmission imaging.ti,ab.
97 thermal imaging.ti,ab.
98 elastography.ti,ab.
99 or/14‐93
100 PET‐CT.ti,ab.
101 (CT or PET).ti,ab.
102 (FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical$).ti,ab.
103 exp Deoxyglucose/
104 CATSCAN.ti,ab.
105 deoxyglucose.ti,ab.
106 deoxy‐glucose.ti,ab.
107 *positron emission tomography/
108 *computer assisted tomography/
109 positron emission tomograph$.ti,ab.
110 *nuclear magnetic resonance imaging/
111 (MRI or fMRI or NMRI or scintigraph$).ti,ab.
112 *echography/
113 Doppler.ti,ab.
114 sonograph$.ti,ab.
115 ultraso$.ti,ab.
116 magnetic resonance imag$.ti,ab.
117 or/100‐116
118 (stage$ or staging or metasta$ or recurrence or sensitivity or specificity or false negative$ or thickness$).ti,ab.
119 "Sensitivity and Specificity"/
120 *cancer staging/
121 or/118‐120
122 117 and 121
123 99 or 122
124 13 and 123
Database: Cochrane Library (Wiley) 2016 searched 30 August 2016 CDSR Issue 8 of 12 2016 CENTRAL Issue 7 of 12 2016 HTA Issue 3 of 4 July 2016 DARE Issue 3 of 4 2015
Search strategy:
#1 melanoma* or nonmelanoma* or non‐melanoma* or melanocyt* or non‐melanocyt* or nonmelanocyt* or keratinocyte*
#2 MeSH descriptor: [Melanoma] explode all trees
#3 "skin cancer*"
#4 MeSH descriptor: [Skin Neoplasms] explode all trees
#5 skin near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*)
#6 nmsc
#7 "squamous cell" near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*) near/2 (skin or epiderm* or cutaneous)
#8 "basal cell" near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*)
#9 pigmented near/2 (lesion* or nevus or mole* or naevi or naevus or nevi or skin)
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 dermoscop*
#12 dermatoscop*
#13 Photomicrograph*
#14 MeSH descriptor: [Dermoscopy] explode all trees
#15 confocal near/2 microscop*
#16 epiluminescence near/2 microscop*
#17 incident next light near/2 microscop*
#18 surface near/2 microscop*
#19 "visual inspect*"
#20 "visual exam*"
#21 (clinical or physical) next (exam*)
#22 "3 point"
#23 "three point"
#24 "pattern analys*"
#25 ABDC
#26 menzies
#27 "7 point"
#28 "seven point"
#29 digital near/2 (dermoscop* or dermatoscop*)
#30 "artificial intelligence"
#31 "AI"
#32 "computer assisted"
#33 "computer aided"
#34 AI
#35 "neural network*"
#36 MoleMax
#37 "computer diagnosis"
#38 "image process*"
#39 "automatic classif*"
#40 SIAscope
#41 "image analysis"
#42 "optical near/2 scan*"
#43 Aura
#44 MelaFind
#45 SIMSYS
#46 MoleMate
#47 SolarScan
#48 Vivascope
#49 "confocal microscopy"
#50 high near/3 ultraso*
#51 canine near/2 detect*
#52 Mole* near/2 map*
#53 total near/2 body
#54 mobile* or smart near/2 phone*
#55 cell next phone*
#56 smartphone*
#57 "mitotic index"
#58 DermoScan or SkinVision or DermLink or SpotCheck
#59 "Mole Detective"
#60 "Spot Check"
#61 mole* near/2 map*
#62 total near/2 body
#63 "exfoliative cytolog*"
#64 "digital analys*"
#65 image near/3 software
#66 teledermatolog* or tele‐dermatolog* or telederm or tele‐derm or teledermoscop* or tele‐dermoscop* or teledermatoscop* or tele‐dermatolog*
#67 "optical coherence" next (technolog* or tomog*)
#68 computer near/2 diagnos*
#69 sentinel near/2 node*
#70 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69
#71 ultraso*
#72 sonograph*
#73 MeSH descriptor: [Ultrasonography] explode all trees
#74 Doppler
#75 CT or PET or PET‐CT
#76 "CAT SCAN" or "CATSCAN"
#77 MeSH descriptor: [Positron‐Emission Tomography] explode all trees
#78 MeSH descriptor: [Tomography, X‐Ray Computed] explode all trees
#79 MRI
#80 MeSH descriptor: [Magnetic Resonance Imaging] explode all trees
#81 MRI or fMRI or NMRI or scintigraph*
#82 "magnetic resonance imag*"
#83 MeSH descriptor: [Deoxyglucose] explode all trees
#84 deoxyglucose or deoxy‐glucose
#85 "positron emission tomograph*"
#86 #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85
#87 stage* or staging or metasta* or recurrence or sensitivity or specificity or "false negative*" or thickness*
#88 MeSH descriptor: [Neoplasm Staging] explode all trees
#89 #87 or #88
#90 #89 and #86
#91 #70 or #90
#92 #10 and #91
#93 BCC or CSCC or NMCS
#94 keratinocy*
#95 #93 or #94
#96 #10 or #95
#97 nevisense
#98 HFUS
#99 "electrical impedance spectroscopy"
#100 "history taking"
#101 "patient history"
#102 naked next eye near/1 (exam* or assess*)
#103 skin next exam*
#104 "ugly duckling" or (UD sign*)
#105 MeSH descriptor: [Physical Examination] explode all trees
#106 (physician* or clinical or physical) near/1 (exam* or recog* or triage*)
#107 ABCDE
#108 "clinical accuracy"
#109 MeSH descriptor: [General Practice] explode all trees
#110 confocal near microscop*
#111 "diagnostic algorithm*"
#112 MeSH descriptor: [Clinical Competence] explode all trees
#113 checklist*
#114 "virtual image*"
#115 "volatile organic compound*"
#116 dog or dogs
#117 VOC
#118 "gene expression analys*"
#119 "reflex transmission imaging"
#120 "thermal imaging"
#121 elastography
#122 #97 or #98 or #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108 or #109 or #110 or #111 or #112 or #113 or #114 or #115 or #116 or #117 or #118 or #119 or #120 or #121
#123 #70 or #122
#124 #96 and #123
#125 #96 and #90
#126 #125 or #124
#127 #10 and #126
Database: CINAHL Plus (EBSCO) 1937 to 30 August 2016
Search strategy:
S1 (MH "Melanoma") OR (MH "Nevi and Melanomas+")
S2 (MH "Skin Neoplasms+")
S3 (MH "Carcinoma, Basal Cell+")
S4 basalioma*
S5 (basal cell) N2 (cancer* or carcinoma* or mass or masses or tumor* or tumour* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*)
S6 (pigmented) N2 (lesion* or mole* or nevus or nevi or naevus or naevi or skin)
S7 melanom* or nonmelanoma* or non‐melanoma* or melanocyt* or non‐melanocyt* or nonmelanocyt*
S8 nmsc
S9 TX BCC or cscc or NMSC
S10 (MH "Keratinocytes")
S11 keratinocyt*
S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
S13 dermoscop* or dermatoscop* or photomicrograph* or (3 point) or (three point) or ABCD* or menzies or (7 point) or (seven point) or AI or Molemax or SIASCOP* or Aura or MelaFind or SIMSYS or MoleMate or SolarScan or smartphone* or DermoScan or SkinVision or DermLink or SpotCheck
S14 (epiluminescence or confocal or incident or surface) N2 (microscop*)
S15 visual N1 (inspect* or examin*)
S16 (clinical or physical) N1 (examin*)
S17 pattern analys*
S18 (digital) N2 (dermoscop* or dermatoscop*)
S19 (artificial intelligence)
S20 (computer) N2 (assisted or aided)
S21 (neural network*)
S22 (MH "Diagnosis, Computer Assisted+")
S23 (image process*)
S24 (automatic classif*)
S25 (image analysis)
S26 SIAScop*
S27 (optical) N2 (scan*)
S28 (high) N3 (ultraso*)
S29 elastography
S30 (mobile or cell or cellular or smart) N2 (phone*) N2 (app or application*)
S31 (mole*) N2 (map*)
S32 total N2 body
S33 exfoliative cytolog*
S34 digital analys*
S35 image N3 software
S36 teledermatolog* or tele‐dermatolog* or telederm or tele‐derm or teledermoscop* or tele‐dermoscop* or teledermatoscop* or tele‐dermatoscop* teledermatolog* or tele‐dermatolog* or telederm or tele‐derm or teledermoscop*
S37 (optical coherence) N1 (technolog* or tomog*)
S38 computer N2 diagnos*
S39 sentinel N2 node
S40 (MH "Sentinel Lymph Node Biopsy")
S41 nevisense or HFUS or checklist* or VOC or dog*
S42 electrical impedance spectroscopy
S43 history taking
S44 "Patient history"
S45 naked eye
S46 skin exam*
S47 physical exam*
S48 ugly duckling
S49 UD sign*
S50 (physician* or clinical or physical) N1 (exam*)
S51 clinical accuracy
S52 general practice
S53 (physician* or clinical or physical) N1 (recog* or triage)
S54 confocal microscop*
S55 clinical competence
S56 diagnostic algorithm*
S57 checklist*
S58 virtual image*
S59 volatile organic compound*
S60 gene expression analys*
S61 reflex transmission imag*
S62 thermal imaging
S63 S13 or S14 or S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62
S64 CT or PET
S65 PET‐CT
S66 FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical*
S67 (MH "Deoxyglucose+")
S68 deoxy‐glucose or deoxyglucose
S69 CATSCAN
S70 CAT‐SCAN
S71 (MH "Deoxyglucose+")
S72 (MH "Tomography, Emission‐Computed+")
S73 (MH "Tomography, X‐Ray Computed")
S74 positron emission tomograph*
S75 (MH "Magnetic Resonance Imaging+")
S76 MRI or fMRI or NMRI or scintigraph*
S77 echography
S78 doppler
S79 sonograph*
S80 ultraso*
S81 magnetic resonance imag*
S82 S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81
S83 stage* or staging or metasta* or recurrence or sensitivity or specificity or (false negative*) or thickness
S84 (MH "Neoplasm Staging")
S85 S83 OR S84
S86 S82 AND S85
S87 S63 OR S86
S88 S12 AND S87
Database: Science Citation Index SCI Expanded (Web of Science) 1900 to 30 August 2016
Conference Proceedings Citation Index (Web of Science) 1900 to 1 September 2016
Search strategy:
#1 (melanom* or nonmelanom* or non‐melanoma* or melanocyt* or non‐melanocyt* or nonmelanocyt* or keratinocyt*)
#2 (basalioma*)
#3 ((skin) near/2 (cancer* or carcinoma or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*))
#4 ((basal) near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*))
#5 ((pigmented) near/2 (lesion* or mole* or nevus or nevi or naevus or naevi or skin))
#6 (nmsc or BCC or NMSC or keratinocy*)
#7 ((squamous cell (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*))
#8 (skin or epiderm* or cutaneous)
#9 #8 AND #7
#10 #9 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#11 ((dermoscop* or dermatoscop* or photomicrograph* or epiluminescence or confocal or "incident light" or "surface microscop*" or "visual inspect*" or "physical exam*" or 3 point or three point or pattern analy* or ABCDE or menzies or 7 point or seven point or dermoscop* or dermatoscop* or AI or artificial or computer aided or computer assisted or neural network* or Molemax or image process* or automatic classif* or image analysis or siascope or optical scan* or Aura or melafind or simsys or molemate or solarscan or vivascope or confocal microscop* or high ultraso* or canine detect* or cellphone* or mobile* or phone* or smartphone or dermoscan or skinvision or dermlink or spotcheck or spot check or mole detective or mole map* or total body or exfoliative psychology or digital or image software or optical coherence or teledermatology or telederm* or teledermoscop* or teledermatoscop* or computer diagnos* or sentinel))
#12 ((nevisense or HFUS or impedance spectroscopy or history taking or patient history or naked eye or skin exam* or physical exam* or ugly duckling or UD sign* or physician* exam* or physical exam* or ABCDE or clinical accuracy or general practice or confocal microscop* or clinical competence or diagnostic algorithm* or checklist* or virtual image* or volatile organic or VOC or dog* or gene expression or reflex transmission or thermal imag* or elastography))
#13 #11 or #12
#14 ((PET or CT or FDG or deoxyglucose or deoxy‐glucose or fluorodeoxy* or radiopharma* or CATSCAN or positron emission or computer assisted or nuclear magnetic or MRI or FMRI or NMRI or scintigraph* or echograph* or Doppler or sonograph* or ultraso* or magnetic reson*))
#15 ((stage* or staging or metast* or recurrence or sensitivity or specificity or false negative* or thickness*))
#16 #14 AND #15
#17 #16 OR #13
#18 #10 AND #17
Refined by: DOCUMENT TYPES: (MEETING ABSTRACT OR PROCEEDINGS PAPER)
Appendix 5. Full‐text inclusion criteria
The title and abstract screening will lead to the retrieval of a large number of full text journal papers and conference abstracts from which to populate the four sets of test accuracy reviews and the intervention review. The systematic reviews will largely be carried out sequentially, beginning with the reviews of tests for melanoma diagnosis; however, the full‐text papers need to be screened at the beginning of the Programme Grant and papers meeting the inclusion criteria tagged accordingly by review.
The table below summarises the inclusion criteria to be applied; these will be transferred to an Excel spreadsheet or Google Forms so that pertinent information can be recorded about each eligible study and reasons for exclusion recorded about each ineligible study.
| Criterion | Inclusion | Exclusion |
| Study design |
For diagnostic and staging reviews
|
|
| Target condition |
|
|
| Population |
For diagnostic reviews
For staging reviews
|
|
| Index tests |
For diagnosis
For staging
Any test combination and in any order Any test positivity threshold Any variation in testing procedure (e.g. radioisotope used) |
|
| Reference standard |
For diagnostic studies
For studies of imaging tests for staging
For studies of SLNB accuracy for staging
|
For diagnostic studies
|
| BCC: basal cell carcinoma; cSCC: cutaneous squamous cell carcinoma; CT: computed tomography; FNAC: fine needle aspiration cytology; LND: lymph node dissection; MRI: magnetic resonance imaging; PET: positron emission tomography; PET‐CT: positron emission tomography computed tomography; RCT: randomised controlled trial; SCC: squamous cell carcinoma; SLN+: positive sentinel lymph node; SLn: negative sentinel lymph node; SLNB: sentinel lymph node biopsy. | ||
Appendix 6. Quality assessment (based on QUADAS‐2)
The following tables use text that was originally published in the QUADAS‐2 tool by Whiting and colleagues (Whiting 2011).
| Item | Response (delete as required) |
| PARTICIPANT SELECTION (1) ‐ RISK OF BIAS | |
| 1) Was a consecutive or random sample of participants or images enrolled? |
Yes – if paper states consecutive or random No – if paper describes other method of sampling Unclear – if participant sampling not described |
| 2) Was a case‐control design avoided? |
Yes – if consecutive or random or case‐control design clearly not used No – if study described as case‐control or describes sampling specific numbers of participants with particular diagnoses Unclear – if not described |
3) Did the study avoid inappropriate exclusions, e.g.,
|
Yes – if inappropriate exclusions were avoided No – if lesions were excluded that might affect test accuracy, e.g., 'difficult to diagnose' lesions, or where disagreement between evaluators was observed Unclear – if not clearly reported but there is suspicion that difficult to diagnose lesions may have been excluded |
4) For between‐person comparative studies only (i.e., allocating different tests to different study participants):
|
For A)
For B)
For C)
|
| Could the selection of participants have introduced bias? For non‐comparative and within‐person comparative studies
For between‐person comparative studies
|
For non‐comparative and within‐person comparative studies
For between‐person comparative studies
|
| PARTICIPANT SELECTION (1) ‐ CONCERNS REGARDING APPLICABILITY | |
1) Are the included participants and chosen study setting appropriate to answer the review question, i.e., are the study results generalisable?
|
A) For studies that will contribute to the analysis of participants with a primary presentation of a skin lesion (i.e., test naive) Yes – if participants included in the study appear to be generally representative of those who might present in a usual practice setting No – if study participants appear to be unrepresentative of usual practice, e.g., in terms of severity of disease, demographic features, presence of differential diagnosis or co‐morbidity, setting of the study, and previous testing protocols Unclear – if insufficient details are provided to determine the generalisability of study participants B) For studies that will contribute to the analysis of referred participants (i.e., who have already undergone some form of testing) Yes – if study participants appear to be representative of those who might be referred for further investigation. If the study focuses only on those with equivocal lesions, for example, we would suggest that this is not representative of the wider referred population No – if study participants appear to be unrepresentative of usual practice, e.g., if a particularly high proportion of participants have been self‐referred or referred for cosmetic reasons. Other factors to consider include severity of disease, demographic features, presence of differential diagnosis or co‐morbidity, setting of the study, and previous testing protocols Unclear – if insufficient details are provided to determine the generalisability of study participants |
| 2) Did the study avoid including participants with multiple lesions? |
Yes – if the difference between the number of included lesions and number of included participants is less than 5% No – if the difference between the number of included lesions and number of included participants is greater than 5% Unclear – if it is not possible to assess |
Is there concern that the included participants do not match the review question?
|
|
| INDEX TEST (2) ‐ RISK OF BIAS (to be completed per test evaluated) | |
| 1) Was the index test or testing strategy result interpreted without knowledge of the results of the reference standard? |
Yes – if index test described as interpreted without knowledge of reference standard result or, for prospective studies, if index test is always conducted and interpreted prior to the reference standard No – if index test described as interpreted in knowledge of reference standard result Unclear – if index test blinding is not described |
| 2) Was the diagnostic threshold at which the test was considered positive (i.e., BCC or cSCC present) prespecified? |
Yes – if threshold was prespecified (i.e., prior to analysing study results) No – if threshold was not prespecified Unclear – if not possible to tell whether or not diagnostic threshold was prespecified |
| 3) For within‐person comparisons of index tests or testing strategies (i.e., > 1 index test applied per participant): was each index test result interpreted without knowledge of the results of other index tests or testing strategies? |
Yes – if all index tests were described as interpreted without knowledge of the results of the others No – if the index tests were described as interpreted in the knowledge of the results of the others Unclear – if it is not possible to tell whether knowledge of other index tests could have influenced test interpretation N/A – if only 1 index test was evaluated |
| Could the conduct or interpretation of the index test have introduced bias? For non‐comparative and between‐person comparison studies
For within‐person comparative studies
|
For non‐comparative and between‐person comparison studies
For within‐person comparative studies
|
| INDEX TEST (2) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Was the diagnostic threshold to determine presence or absence of disease established in a previously published study? E.g., previously evaluated/established
|
Yes – if a previously evaluated/established tool to aid diagnosis of BCC or cSCC was used or if the diagnostic threshold used was established in a previously published study No – if an unfamiliar/new tool to aid diagnosis of BCC or cSCC was used, if no particular algorithm was used, or if the objective threshold reported was chosen based on results in the current study Unclear – if insufficient information was reported |
| 2) Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? Study results can only be reproduced if the diagnostic threshold is described in sufficient detail. This item applies equally to studies using pattern recognition and those using checklists or algorithms to aid test interpretation |
Yes – if the criteria for diagnosis of BCC or cSCC were reported in sufficient detail to allow replication No – if the criteria for diagnosis of BCC or cSCC were not reported in sufficient detail to allow replication Unclear – if some but not sufficient information on criteria for diagnosis to allow replication were provided |
| 3) Was the test interpretation carried out by an experienced examiner? |
Yes – if the test was interpreted by 1 or more speciality‐accredited dermatologists, or by examiners of any clinical background with special interest in dermatology and with any formal training in the use of the test No – if the test was not interpreted by an experienced examiner (see above) Unclear – if the experience of the examiner(s) was not reported in sufficient detail to judge or if examiners described as 'Expert' with no further detail given N/A – if system‐based diagnosis, i.e., no observer interpretation |
Is there concern that the index test, its conduct, or interpretation differ from the review question?
|
|
| REFERENCE STANDARD (3) ‐ RISK OF BIAS | |
| 1) Is the reference standard likely to correctly classify the target condition? A) Disease‐positive ‐ 1 or more of the following:
B) Disease‐negative ‐ 1 or more of the following:
|
A) Disease‐positive Yes – if all participants with a final diagnosis of BCC or cSCC underwent 1 of the listed reference standards No – if a final diagnosis of BCC or cSCC for any participant was reached without histopathology Unclear – if the method of final diagnosis was not reported for any participant with a final diagnosis of BCC or cSCC or if the length of clinical follow‐up used was not clear or if a clinical follow‐up reference standard was reported in combination with a participant‐based analysis and it was not possible to determine whether the detection of a malignant lesion during follow‐up is the same lesion that originally tested negative on the index test B) Disease‐negative Yes – if at least 80% of benign diagnoses were reached by histology and up to 20% were reached by clinical follow‐up for a minimum of 6 (or 3) months following the index test No – if more than 20% of benign diagnoses were reached by clinical follow‐up for a minimum of 6 (or 3) months following the index test or if clinical follow‐up period was less than 6 (or 3) months Unclear – if the method of final diagnosis was not reported for any participant with benign diagnosis |
| 2) Were the reference standard results interpreted without knowledge of the results of the index test? Please score this item for all studies even though histopathology interpretation is usually conducted with knowledge of the clinical diagnosis (from visual inspection or dermoscopy or both). We will deal with this by not including the response to this item in the 'Risk of bias' assessment for these tests. For reviews of all other tests, this item will be retained |
Yes – if the reference standard diagnosis was reached blinded to the index test result No – if the reference standard diagnosis was reached with knowledge of the index test result Unclear – if blinded reference test interpretation was not clearly reported |
| Could the reference standard, its conduct, or its interpretation have introduced bias? For visual inspection/dermoscopy evaluations
For all other tests
|
For visual inspection/dermoscopy evaluations
For all other tests
|
| REFERENCE STANDARD (3) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Are index test results presented separately for each component of the target condition (i.e., separate results presented for those with invasive melanoma, melanoma in situ, lentigo maligna, severe dysplasia, BCC, and cSCC)? |
Yes – if index test results for each component of the target condition can be disaggregated No – if index test results for the different components of the target condition cannot be disaggregated Unclear – if not clearly reported |
| 2) Expert opinion (with no histological confirmation) was not used as a reference standard 'Expert opinion' means diagnosis based on the standard clinical examination, with no histology or lesion follow‐up ***do not complete this item for teledermatology studies |
Yes – if expert opinion was not used as a reference standard for any participant No – if expert opinion was used as a reference standard for any participant Unclear – if not clearly reported |
| 3) Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? |
Yes – if histology interpretation was reported to be carried out by an experienced histopathologist or dermatopathologist No – if histology interpretation was reported to be carried out by a less experienced histopathologist Unclear – if the experience/qualifications of the pathologist were not reported |
Is there concern that the target condition as defined by the reference standard does not match the review question?
***For teledermatology studies only
|
***For teledermatology studies only
|
| FLOW AND TIMING (4): RISK OF BIAS | |
| 1) Was there an appropriate interval between index test and reference standard? A) For histopathological reference standard, was the interval between index test and reference standard ≤ 1 month? B) If the reference standard includes clinical follow‐up of borderline/benign‐appearing lesions, was there at least 6 (or 3) months' follow‐up following application of index test(s) for studies of BCC (or cSCC)? |
A) Yes – if study reports ≤ 1 month between index and reference standard No – if study reports > 1 month between index and reference standard Unclear – if study does not report interval between index and reference standard B) Yes – if study reports ≥ 6 (or 3 for cSCC) months' follow‐up No – if study reports < 6 (or 3 for cSCC) months' follow‐up Unclear – if study does not report length of clinical follow‐up |
| 2) Did all participants receive the same reference standard? |
Yes – if all participants underwent the same reference standard No – if more than 1 reference standard was used Unclear – if not clearly reported |
| 3) Were all participants included in the analysis? |
Yes – if all participants were included in the analysis No – if some participants were excluded from the analysis Unclear– if not clearly reported |
| 4) For within‐person comparisons of index tests Was the interval between application of index tests ≤ 1 month? |
Yes – if study reports ≤ 1 month between index tests No – if study reports > 1 month between index tests Unclear – if study does not report interval between index tests |
| Could the participant flow have introduced bias? For non‐comparative and between‐person comparison studies
For within‐person comparative studies
|
For non‐comparative and between‐person comparison studies
For within‐person comparative studies
|
| BCC = basal cell carcinoma; cSCC = cutaneous squamous cell carcinoma | |
Appendix 7. Summary of tests and target conditions evaluated per study
| In‐person | Image‐based | Other tests evaluated in study | Target conditions reported | Appears in melanoma review | |||||
| Visual inspection | Dermoscopy added to VI | Visual inspection | Dermoscopic images | BCC | SCC | KER | |||
| Altamura 2010 | ‐ | ‐ | ‐ | X | ‐ | X | ‐ | ‐ | ‐ |
| Amirnia 2016 | ‐ | X | ‐ | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Argenziano 2006 | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| Carli 2002a | X | X | ‐ | X | ‐ | X | ‐ | ‐ | X |
| Carli 2002b | ‐ | ‐ | X | X | ‐ | X | ‐ | X | X |
| Chang 2013 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| Cooper 2002 | X | ‐ | ‐ | ‐ | ‐ | X | X | X | |
| Durdu 2011 | ‐ | X | ‐ | ‐ | Exfoliative cytology | X | ‐ | X | X |
| Ek 2005 | X | ‐ | ‐ | ‐ | ‐ | X | X | X | X |
| Gokdemir 2011 | ‐ | X | ‐ | ‐ | ‐ | X | ‐ | ‐ | X |
| Hacioglu 2013 | X | ‐ | ‐ | X | CAD | ‐ | ‐ | X | ‐ |
| Lorentzen 1999 | ‐ | ‐ | X | ‐ | ‐ | X | ‐ | ‐ | X |
| Lorentzen 2008 | ‐ | ‐ | ‐ | X | ‐ | X | ‐ | ‐ | X |
| Markowitz 2015 | X | X | ‐ | ‐ | OCT | X | ‐ | ‐ | ‐ |
| Menzies 2000 | ‐ | ‐ | ‐ | X | ‐ | X | ‐ | X | ‐ |
| Navarrete Dechent 2016 | ‐ | ‐ | ‐ | X | ‐ | X | X | X | ‐ |
| Nori 2004 | ‐ | ‐ | X | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Rosendahl 2011 | ‐ | ‐ | X | X | ‐ | X | ‐ | X | X |
| Schwartzberg 2005 | X | ‐ | ‐ | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Stanganelli 2000 | X | X | ‐ | ‐ | ‐ | X | ‐ | ‐ | X |
| Steiner 1987 | X | ‐ | ‐ | ‐ | ‐ | X | ‐ | ‐ | X |
| Ulrich 2015 | X | X | ‐ | ‐ | OCT | X | ‐ | ‐ | ‐ |
| Witkowski 2016 | ‐ | ‐ | ‐ | X | RCM | X | X | X | ‐ |
| Zalaudek 2006 | ‐ | ‐ | ‐ | X | ‐ | X | ‐ | ‐ | X |
| Footnotes: BCC – basal cell carcinoma; CAD – computer‐assisted diagnosis; cSCC – cutaneous squamous cell carcinoma; KER ‐ any skin cancer; OCT ‐ optical coherence tomography; RCM – reflectance confocal microscopy; VI ‐ visual inspection | |||||||||
Appendix 8. Summary study details
|
Study author
Outcomes reported Pathway |
Study type Country Setting |
Inclusion criteria |
Index tests (algorithm) Diagnostic approach |
Threshold |
Observer qualifcation (number) Experience |
Reference standard Final diagnoses Prevalence (Any) Exclusions (if reported) |
|
| In‐person evaluations | |||||||
|
Amirnia 2016 BCC Referred (selected on reference) (c) |
NC NR‐CS Iran Secondary 61 / 61 |
Patients suspected of BCC or melanocytic naevi of the face who were referred to dermatology clinic | Dermoscopy (3‐point checklist plus dermatoscopic criteria of melanocytic naevi and BCC) In person |
≥ 2 characteristics present; diagnosis of BCC | Dermatologist (assumed) (n = NR; experience NR) Single observer |
Histology BCC 27 Benign 28 27/61; 44% |
|
|
Argenziano 2006 Any Limited prior testing; selected on refererence standard (c) |
BPC RCT Italy, Spain Primary NR / 85 (Full sample 1203 lesions*) |
Patients asking for screening or exhibiting 1 or more skin tumours as seen during routine physical examination (patient‐finding screening). Participating PCPs randomised to either visual inspection alone or visual inspection plus dermoscopy; only excised lesions can be included for each arm. |
VI (ABCD) Dermoscopy (3‐point checklist) In person |
Subjective impression; dx of malignancy | GPs (n = 37) All trained in ABCD rule Single observer |
Histology MEL 6 BCC 37; SCC 10 Benign 32 53/85; 62% NB: Only those patients who were considered to have lesions suggestive of skin cancer had histology and could be included; rest had expert diagnosis (making full dataset ineligible for this review) |
|
|
Carli 2002a BCC (MEL) Referred (selected on reference) (u) |
WPC NR‐CS Italy Secondary NR/256 |
Clinically equivocal or suspicious PSL subjected to excisional biopsy at the Institute of Dermatology | 1. VI (no algorithm)
2. Dermoscopy (pattern) In‐person (Dermoscopy – image‐based) |
Subjective impression | Dermatologist (n = 2; High experience – “extensive experience in both clinical and dermoscopic diagnosis”) Consensus of 2 |
Histology MM 40; MiS 14 BCC 5 BN 177; SN 16; SK 4 BCC: 5/256; 2% No exclusions reported NB: BCC (VI): 2 MMS were FP; BCC (Derm – pattern): all MM TN |
|
|
Chang 2013 Any Referred (selected on reference) (u) |
NC R‐CS Taiwan Secondary 676/769 |
Potentially malignant biopsied or excised skin lesions (nontumour specimens excluded) | VI (no algorithm) In person |
Subjective impression; definitely malignant | Dermatologists; n = 25 Board‐certified Single observer |
Histology MM 4; MiS 4 BCC: 110; cSCC: 20 'Benign' diagnoses: 595 Skin cancer: 152/769; 20% Exclusions: Poor‐quality index test image; mis‐registered or poor‐quality images (unfocused or containing a motion artifact) |
|
|
Cooper 2002 BCC cSCC Any Follow‐up (c) |
NC P‐CS UK Spec. clinic NR/102 |
Patients attending the open‐access dermatology renal transplant clinic with suspicious lesions | VI (No algorithm) In person |
NR; correct diagnosis of malignancy | Mixed (n = 2; experience NR) Single observer |
Histology BCC 12; cSCC 21 KA 2; BD 19; Solar 16; viral warts 7; other 25 BCC: 12/102; 12% SCC: 21/102; 21% Exclusions: BCC: 3 SCCs were FP |
|
|
Durdu 2011 BCC Any (MEL) Referred (selected on reference) (u) |
WPC P‐CS Secondary Turkey 176/200 |
PSL that could not be diagnosed with only dermatologic physical examination; 2 x 2 included for melanocytic subset | Dermoscopy (No algorithm (ABCD for diagnosis of melanoma only) Also evaluated exfoliative cytology In person |
NR | Dermatologist (n = 1; experience NR) Single observer |
Histology MEL 10; BCC: 34; Other malignant 2 SK 24; BN 100; DF 12; Warts 16; Dirt 1; Other 1 BCC: 34/200; 17% ‐ |
|
|
Ek 2005 BCC cSCC Any (MEL) Referred (selected on reference) (c) |
NC P‐CS Aus. Specialist clinic 1223/2582 |
Lesions excised for which malignancy could not be excluded | VI (no algorithm) In person |
Subjective impression | Plastic surgeon (n = 4 or 5; mixed experience; 3 consultants, 1 plastic surgery trainee (usually 1st year, on 6‐month rotation) and a clinical assistant) Unclear |
Histology MEL 23 BCC 1214; SCC 517; BD 188; SK 63; 577 other benign (incl 330 solar keratosis) BCC: 1214/2582; 47% SCC: 517/2582; 20% Exclusions: Incomplete or incorrectly entered proformas were excluded – 79 patients with 96 lesions NB for BCC: 202 SCC and 6 MM were counted as FPs |
|
|
Gokdemir 2011 BCC [MEL] Referred (selected on reference) (u) |
NC NR‐CS Secondary Turkey 362/449 |
Patients with melanocytic and non‐melanocytic skin lesions with dermoscopic and histologic diagnoses | Dermoscopy (no algorithm) Unclear if in‐person or image‐based |
Subjective assessment (dx of MM) | Dermatologist (n = NR; experience High “at least 2 years’ experience with Molemax II”) Unclear obs interp |
Histology MEL 13; BCC: 45 Benign: 390 BCC: 45/448; 10% NB for BCC: 1 MM was counted as FP |
|
|
Hacioglu 2013 Any Referred (selected on reference) (u) |
WPC NR‐CS Turkey Secondary 76 / 80 |
Patients with skin lesions <12 mm diameter suspicious for malignancy; lesions that had a crusted or rough surface were excluded. NB aim is diagnose non melanoma skin cancers |
VI (no algorithm) In‐person [Also evaluates image‐based dermoscopy and CAD] |
Subjective impression; diagnosis of BCC/cSCC | Dermatologist (assumed) (n = 1; experience NR) Single observer |
Histology MM 3; BCC 24; cSCC 3; basosquamous 2 SK 19; AK 8; intradermal nevus 4; DF 3; KA 2; Other 12 Skin cacner: 29/80; 36% Study reports 0 excluded from analysis after histopathology results NB: 3 MM considered disease negative by authors; cannot be disaggregated |
|
|
Markowitz 2015 BCC Equivocal lesions (selected on reference) (u) |
WPC P‐CS US Secondary 100 / 115 |
Adults with ≤ 3 suspicious lesions, if they had ≥ 1 clinically challenging pink lesions, on the head or neck, that was suspicious for BCC, and to be biopsied to rule BCC in or out, and if they were eligible for Mohs surgery | VI (no algorithm) Dermoscopy (2‐step algorithm Marghoob 2010) In‐person (Also evaluates OCT) |
Possible BCC | Dermatologist (assumed) (n = NR; experience NR) Unclear |
Histology BCC 70 Benign 45 BCC: 70/115; 61% No exclusions reported |
|
|
Schwartzberg 2005 BCC Referred (selected on reference) (u) |
WPC‐algs P‐CS US Secondary 141/141 |
Patients with suspected BCC undergoing biopsy | VI (no algorithm; own new algorithm) In‐person |
BCC certain or likely (Confidence level 1 or 2) | Dermatologist (assumed) (n = 17; experience NR) Single |
Histology BCC 82 Benign 59 BCC: 82/141; 58% ‐ |
|
|
Stanganelli 2000 BCC Any (MEL) Referred (unselected on reference) (u) |
WPC R‐CS Italy Specialist clinic NR/3372 |
PSL referred by dermatologists and general practitioners either for pre‐surgical assessment or consultation | 1. VI (ABCD) 2. Dermoscopy (pattern analysis) In person |
NR Subjective impression | NR (assumed dermatologist ‐ described as one of the co‐authors; n = 1) Single observer |
Histology / Registry FU MEL 55 BCC 43; Benign 3274 43/3372; 1% No exclusions reported NB for BCC: all MMs were TN for VI and for dermoscopy |
|
|
Steiner 1987 BCC Any (MEL) Equivocal (selected on reference) (u) |
WPC P‐CS Austria Spec. clinic NR / 318 |
Small (< 10 mm) diagnostically equivocal PSL; no absolute agreement on clinical diagnosis among investigating clinicians at a pigmented lesion clinic | 1. VI (no algorithm) In person (also evaluated dermoscopy) |
Subjective impression | Dermatologists (n = 3; High experience ‐ "experienced dermatologists") Consensus of 3 observers |
Histology MM 49; MiS 24 BCC 20 BN 143; SK 20; lentigo simplex and naevoid lentigo 19; Other 15 BCC: 20/318; 9% No exclusions reported NB: Dermoscopy data excluded as no breakdown of incorrect diagnoses For BCC (VI): 3 MMs were counted as FP |
|
|
Ulrich 2015 BCC Equivocal (selected on reference) (u) |
WPC P‐CS Germany Secondary 155/231 |
Patients with non‐pigmented pink lesions with clinical suspicion of BCC requiring biopsy for diagnostic confirmation. Pink lesions defined as clinically unclear erythematous papule or plaque; either reddish macules, patches or small papules with or without scale |
VI (no algorithm) Dermoscopy (2‐step algorithm Marghoob 2012) In person (Also evaluates OCT) |
Clinical characteristics of BCC | Dermatologist (assumed) (n = NR; experience NR) Single observer |
Histology *BCC 141 Benign 94 BCC:141/235; 60% Exclusions: Histology was missing for 21 lesions, and 1 case was found to have a combination of both BCC and SK or AK, leaving 235 lesions for analysis NB: 231 diagnoses available for VI (140 BCC) and 231 for dermoscopy (139 BCCs) |
|
| Image‐based evaluations | |||||||
|
Altamura 2010 BCC Referred (selected on reference) (c) |
NC RP‐CCS Secondary Italy; Aus; Austria NR/300 |
Skin lesions randomly selected from digital databases at dermatology departments and tertiary referral centre; all excised | Dermoscopy (Menzies for BCC (rev)) Image‐based (none) |
Diagnosis of BCC | Dermatologist (assumed) (n = 3; experience High) observers experienced in dermatoscopic evaluation Single observer |
Histology MM 40; MiS 10; BCC 150; cSCC 2 BN 50; SK 20; AK 12; DF 10; Other 6 BCC: 150/300; 50% NB: MM and cSCC results not disaggregated from Disease negative group |
|
|
Carli 2002a BCC (MEL) Referred (selected on reference) (u) |
WPC R‐CS Italy Secondary NR/256 |
Clinically equivocal or suspicious PSL subjected to excisional biopsy at the Institute of Dermatology | (Dermoscopy – image‐based) In person (Also evaluates in‐person VI and dermoscopy (see above)) |
Subjective impression | Dermatologist (n = 2; High experience – “extensive experience in both clinical and dermoscopic diagnosis”) Consensus of 2 |
Histology MM 40; MiS 14 BCC 5 BN 177; SN 16; SK 4 BCC: 5/256; 2% No exclusionsne reported NB for BCC: all MEL were test negative |
|
|
Carli 2002b BCC Any (MEL) Referred (selected on reference) (u) |
WPC R‐CS Italy Secondary NR / 57 |
Clinically suspicious or equivocal PSL undergoing excision for diagnostic purposes; all ≤ 14mm diameter | 1. VI (NR)
2. Dermoscopy (NR) Image‐based (blinded) |
NR | Dermatologists (n = 2) High experience ('with experience in the field of '); consensus of 2 |
Histology MM 6, MiS 5 BCC 10 BN 31, SK 1; Other 4 BCC; 10/57; 18% Exclusions: 4 ‘not evaluables’ excluded (NB these differ between clinical images and dermoscopic images (1 MM excluded from VI analysis) |
|
|
Hacioglu 2013 Any Referred (selected on reference) (u) |
WPC NR‐CS Turkey Secondary 76/80 |
Patients with skin lesions < 12 mm diameter suspicious for malignancy; lesions that had a crusted or rough surface were excluded. NB aim is diagnose non‐melanoma skin cancers |
Dermoscopy (no algorithm) Image‐based (blinded) (Also evaluates in‐person VI and CAD) |
Subjective impression; diagnosis of BCC/cSCC | Dermatologist (assumed) (n = 1; experience NR) Single observer |
Histology MM 3; BCC 24; cSCC 3; basosquamous 2 SK 19; AK 8; intradermal naevus 4; DF 3; KA 2; Other 12 Skin cancer: 29/80; 36% Exclusions: Study reports 0 excluded from analysis after histopathology results B: 3 MM considered disease‐negative by study authors; cannot be disaggregated |
|
|
Lorentzen 1999 BCC (MM) Referred (selected on reference) (c) |
WPC P‐CS Specialist clinic Denmark 232/232 |
Patients with lesions suspicious for CMM referred to outpatients clinic | 1. VI (no algorithm) 2. Dermoscopy (no algorithm) Image based (clinical image) |
Subjective impression; correct dx of M | Mixed: Dermatologist (n = 4; experience High (4‐5 years daily experience) & 'non‐expert dermatology residents' (n = 5; 1 ‐ 2 years interest and formal training in dermatoscopy) Average |
Histology MM 49; BCC 16 SK 12; BN 137 Other: 18 (SN, BD plus others) BCC: 16/232; 7% Exclusions Poor‐quality index test image 10 cases excluded NB for BCC: MM results not disaggregated |
|
|
Lorentzen 2008 BCC MM Any Referred (selected on reference) (c) |
WPC NR‐CS Specialist clinic Denmark 119/119 |
Patients referred to the specialist naevus clinic; compared classic dermoscopy to acrylic globe magnifer | Dermoscopy (Kenet risk stratification) Image‐based (blinded) |
NR | Dermatologist (n = NR) Average |
Histology MM 24; BCC 13 BN 69; Mild/moderate dysplasia 2; SK 9; Other 2 BCC: 13/119; 11% Exclusions: 1 dermatofibroma |
|
|
Menzies 2000 BCC Any (MM‐excl) Referred (selected on reference) (u) |
NC RP‐CCS Spec. clinic Aus; US Test set: NR/213 (Full sample 426) |
PSL with dermoscopic images and histological diagnoses | Dermoscopy (Menzies for BCC (new)) Image‐based (none) |
Absence of pigment network and ≥ 1 other char present; Dx | Dermatologist (assumed) (n = 2; experience NR) NR | Histology MM 71; BCC 71 BN 59; SK 5; Solar 3; DF 1; Other 3 BCC: 71/213; 33% NB: Included 142 BCCs, 142 invasive melanomas and 142 randomly‐sampled benign For BCC: 5 MM classed as FP |
|
|
Navarrete Dechent 2016 BCC cSCC Any (MEL excl) Referred (selected on reference) (u) |
NC RP‐CS Spec clinic US NR/457 |
Consecutively excised nonpigmented lesions; no discernible pigment on clinical or dermoscopic images. | Dermoscopy (Shiny white blotches and strands (new)) Image‐based (blinded) |
≥ 1 char present | Dermatologist (assumed) and medical student (n = 2; experience NR) Consensus of 2 |
Histology MEL 21; BCC 287; cSCC 106 lichen planus–like keratosis 39; Naevus 4 BCC: 287/457; 63% cSCC: 106/457; 23% NB for BCC: 9 MM and 44 cSCC were counted as FP |
|
|
Nori 2004 BCC Referred (selected on reference) (u) |
WPC RP‐NR Secondary US; Spain 105 (VI) Full sample: 145/152 |
Biopsy confirmed BCC and convenience sample of non‐BCC with 'range of common diagnoses'; lesions with superior clinical image quality selected for VI | VI (no algorithm) Image based (blinded) (Also evaluates RCM) |
Subjective impression: High/Med probability of BCC | Dermatologist (n = 2; experience NR) Single observer |
Histology and Expert opinion* BCC 58 Benign 47 (Full sample includes 83 BCC; 4 SCC; 65 benign) BCC: 58/105; 55% NB: 15 lesions not biopsied because the clinical diagnosis was considered diagnostic (e.g.SK) cSCC results not disaggregated |
|
|
Rosendahl 2011 BCC Any (MEL) Limited prior test (selected on reference) (u) |
WPC‐algs R‐CS Aus. Primary 389/463 |
PSL submitted for histology from the primary‐care skin cancer practice of 1 author | 1. VI (no algorithm) 2. Dermoscopy (pattern; chaos and clues) |
1. Subjective impression 2. NR; both characteristics present |
Dermatologist (n = 1) High experience (confirmed by author); Single observer |
Histology MM 9; MiS 20 BCC 72; SCC 5 BN 217; BD 18; AK 14*; BNM 140 AK were considered malignant by study authors but not by review team BCC: 72/463; 16% Exclusions: 3 poor‐quality images excluded NB for BCC (VI): 3 MM were counted as FP; for BCC (Derm chaos/clues) 23 MM/MiS were counted as FPs; and for BCC (Pattern) 1 MM was counted as FP |
|
|
Witkowski 2016 BCC cSCC Any (MEL excl) Equivocal (selected on reference) (u) |
WPC RP‐CS Secondary Italy NR/260 |
Consecutive clinically equivocal ‘pink’ cutaneous lesions with absent pigmentation or containing < 10% pigment and absence of pigment network. All lesions were excised at first visit or follow‐up video dermoscopy control visit |
Dermoscopy (No algorithm) Image based (blinded) (Also evaluates RCM) |
NR | Dermatologist (assumed) (n = NR; experience NR) Single | Histology MEL 12; BCC 114; cSCC 13; Other malig 1 BN 47; SN 6; SL/SK/LPLK/AK 25; DF 18 Other 24 BCC: 114/260; 44% cSCC: 13/260; 5% NB for BCC: 1 MM and 1 cSCC were counted as FP |
|
|
Zalaudek 2006 BCC Any (MEL) Referred (selected on reference) (u) |
NC R‐CS Specialist clinic Italy NR/165 |
Random sample of excised, equivocal and nonequivocal, PSL and and non‐PSLs with melanin or haemoglobin pigmentation in all or part of the lesion. | Dermoscopy (3PCL) Image‐based (age, site, gender) |
≥ 2 characteristics present | Mixed (n = 150; experience NR) Average result |
Histology Full sample: MM 18; MiS 11 BCC: 18 79 BN; 26 SK; 8 vascular; 3 DF BCC: 18/150; 12% Exclusions: 15 used for training purposes NB for BCC: 7 MM were counted as FP |
|
| Footnotes: 3PCL ‐ three‐ point checklist; 7PCL ‐ seven‐point checklist; AK – actinic keratosis; BCC – basal cell carcinoma; BD – Bowen’s disease; BN – benign naevi; BPC – between person comparison (of tests); c ‐ clearly positioned on clinical pathway; CAD – computer‐assisted diagnosis; CCS – case control study; CS – case series; cSCC – cutaneous squamous cell carcinoma; DF – dermatofibroma; dx ‐ diagnosis; FP ‐ false positive; FU – follow‐up; KA ‐ keratoacanthoma; LPLK ‐ lichen planus‐like keratosis; LS – lentigo simplex; MEL: invasive melanoma or atypical intraepidermal melanocytic lesions; MiS – melanoma in situ (or lentigo maligna); MM – malignant (invasive) melanoma; NC – non comparative; NR – not reported; OCT ‐ optical coherence tomography; P – prospective; PLC – pigmented lesion clinic; PSL – pigmented skin lesion; R – retrospective; RCM – reflectance confocal microscopy; SK – seborrheic keratosis; SL ‐ solar lentigo; SN – Spitz naevi; TN ‐ true negative; u – unclear position on clinical pathway; WPC – within person comparison (of tests). |
|||||||
Appendix 9. Content of algorithms for BCC
|
Menzies algorithm for pigmented BCC Menzies 2000 |
Menzies (revised; pigmented and non‐pigmented BCC) Altamura 2010 |
Two‐step algorithm (Marghoob 2010); non‐pigmented BCC Markowitz 2015 |
3‐point checklist plus dermoscopic criteria (pigmented BCC) Amirnia 2016 |
Shiny White Structures (SWSs); non‐pigmented BCC Navarrete Dechent 2016 |
| No pigment network (Negative feature absent) > 1 positive feature present 1. Spoke wheel areas (well‐circumscribed radial projections) 2. Large grey‐blue ovoid nests (well circumscribed, confluent or near confluent pigmented ovoid or elongated areas, larger than globules, not intimately connected to a pigmented tumor body 3. Arborizing telangiectasia (telangiectasia with distinct treelike branching) 4. Multiple grey‐blue globules (as opposed to multiple grey‐blue dots) 5. Maple leaflike areas (brown to grey‐blue discrete bulbous extensions forming leaflike pattern 6. Ulceration (absence of epidermis often associated with congealed blood; not due to recent trauma). |
'Classic' BCC patterns for pigmented BCC (Menzies 2000) 1. ulceration, 2. multiple blue/grey globules, 3. leaflike areas, 4. large blue/grey ovoid nests, 5. spoke‐wheel areas, 6. arborizing telangiectasia Plus 'Non‐classic' patterns
|
Dermoscopic features consistent with BCC:
|
1. Asymmetry in colour or structure in one or two orthogonal axis asymmetric 2. Pigment network with irregular holes and thick lines atypical network 3. Any kind of blue or white colour Blue ‐ white structures Dermoscopic criteria of BCC
|
SWSs were classified as 1. blotches (clods; discrete, small or large structure‐less areas); 2. strands (long thick or thin lines, randomly distributed or parallel, not orthogonally oriented); 3. rosettes (cluster of 4 white dots in a 4‐leaf clover–like arrangement); and 4. short white lines (crystalline structures and chrysalis; fine lines that intersect or are oriented orthogonally to each other) 5. non‐specified. All lesions also evaluated for Menzies 2000 criteria; ‘featureless’ lesions further evaluated for:
|
| BCC ‐ basal cell carcinoma | ||||
Appendix 10. Forest plots for covariate investigations by prevalence and use of an algorithm
22.

Forest plot of tests: 1 BCC‐Visual Inspection (in‐person), 2 BCC‐Visual Inspection (image‐based).
23.
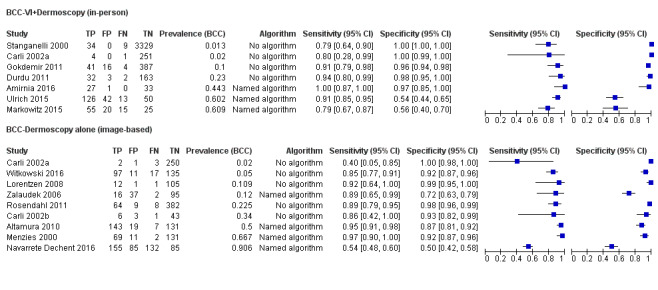
Forest plot of tests: 3 BCC‐VI+Dermoscopy (in‐person), 4 BCC‐Dermoscopy alone (image‐based).
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
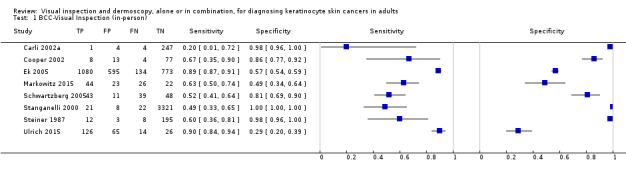
BCC‐Visual Inspection (in‐person).
2. Test.

BCC‐Visual Inspection (image‐based).
3. Test.
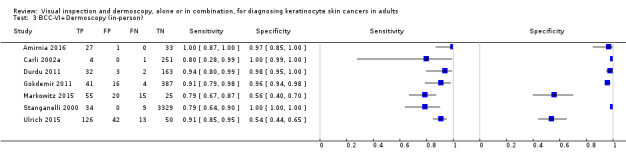
BCC‐VI+Dermoscopy (in‐person).
4. Test.
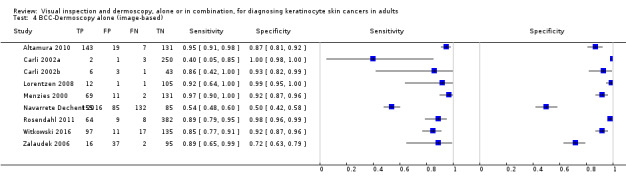
BCC‐Dermoscopy alone (image‐based).
5. Test.
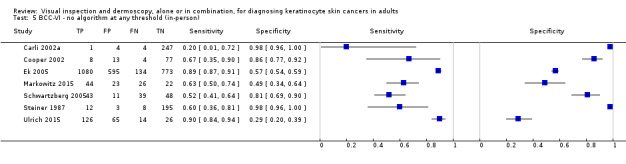
BCC‐VI ‐ no algorithm at any threshold (in‐person).
6. Test.

BCC‐VI ‐ no algorithm at BCC possible (in‐person).
7. Test.

BCC‐VI ‐ ABCD at threshold NR (in‐person).
8. Test.

BCC‐VI ‐ Schwartzberg algorithm (in‐person).
9. Test.

BCC‐VI ‐ no algorithm at any threshold (image‐based).
10. Test.

BCC‐VI ‐ no algorithm at BCC possible (image‐based).
11. Test.

BCC‐ VI+Dermoscopy no algorithm at NR (in‐person).
12. Test.

BCC‐VI+Dermoscopy pattern analysis_obs_dx (in‐person).
13. Test.

BCC‐ VI+Dermoscopy 3 point at >= (in‐person).
14. Test.

BCC‐VI+Dermoscopy Two step_obs_dx (in‐person).
15. Test.

BCC‐Dermoscopy ‐ no algorithm at any threshold (image‐based).
16. Test.

BCC‐Dermoscopy ‐ pattern analysis at NR (image‐based).
17. Test.

BCC‐Dermoscopy ‐ Menzies for BCC(rev)_obsdx (image‐based).
18. Test.

BCC‐Dermoscopy ‐ Menzies for BCC(new) ‐ 1 char absent&>=1 other +ve (image‐based).
19. Test.

BCC‐Dermoscopy ‐ 3 point checklist at >= 2 (image‐based).
20. Test.

BCC‐Dermoscopy ‐ new SWS at >=1 (image‐based).
21. Test.

BCC‐Dermoscopy ‐ Chaos/clues (image‐based).
22. Test.

cSCC‐Visual inspection (in‐person).
23. Test.

cSCC‐Dermoscopy alone (image‐based).
24. Test.

cSCC‐VI ‐ no algorithm at NR (in‐person).
25. Test.

cSCC‐Dermoscopy ‐ no algorithm at NR (image‐based).
26. Test.

cSCC‐Dermoscopy ‐ SWS at >1 char (image‐based).
27. Test.
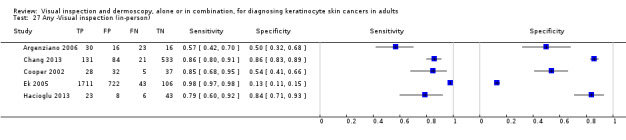
Any ‐Visual inspection (in‐person).
28. Test.
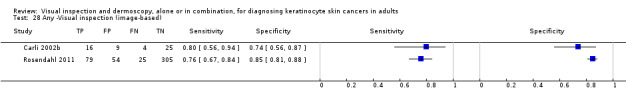
Any ‐Visual inspection (image‐based).
29. Test.

Any ‐VI+Dermoscopy (in‐person).
30. Test.

Any‐Dermoscopy alone (image‐based).
31. Test.
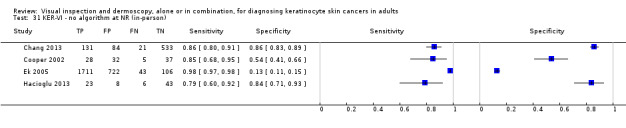
KER‐VI ‐ no algorithm at NR (in‐person).
32. Test.

KER‐VI ‐ ABCD at NR (in‐person).
33. Test.

KER‐VI ‐ no algorithm at NR (image‐based).
34. Test.

KER‐ VI+Dermoscopy no algorithm at NR (in‐person).
35. Test.

KER‐VI+Dermoscopy ‐ 3 point at >=2 (in‐person).
36. Test.

KER‐Dermoscopy ‐ no algorithm at any threshold (image‐based).
37. Test.

KER‐Dermoscopy ‐ no algorithm at excise (image‐based).
38. Test.

KER‐ Dermoscopy ‐ pattern at NR (image‐based).
39. Test.

KER‐Dermoscopy‐ SWS (image‐based).
40. Test.

KER‐Dermoscopy ‐ Chaos/Clues (image‐based).
41. Test.

KER‐Dermoscopy ‐ Menzies for BCC(rev)_obsdx (image‐based).
42. Test.
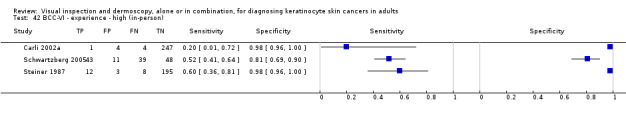
BCC‐VI ‐ experience ‐ high (in‐person).
43. Test.

BCC‐VI ‐ experience ‐ mixed (in‐person).
44. Test.

BCC‐VI ‐ experience ‐ NR (in‐person).
45. Test.

BCC‐VI ‐ experience ‐ high (image‐based).
46. Test.

BCC‐VI ‐ experience ‐ mixed (image‐based).
47. Test.

BCC‐VI ‐ experience ‐ NR (image‐based).
48. Test.

BCC‐VI+Dermoscopy ‐ experience ‐ high (in‐person).
49. Test.
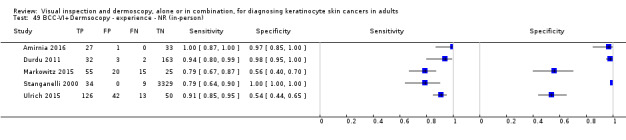
BCC‐VI+Dermsocopy ‐ experience ‐ NR (in‐person).
50. Test.
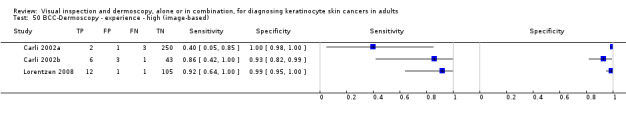
BCC‐Dermoscopy ‐ experience ‐ high (image‐based).
51. Test.

BCC‐Dermoscopy ‐ experience ‐ mixed (image‐based).
52. Test.

BCC‐Dermoscopy ‐ experience ‐ trained (image‐based).
53. Test.
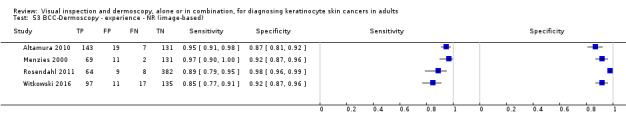
BCC‐Dermoscopy ‐ experience ‐ NR (image‐based).
54. Test.
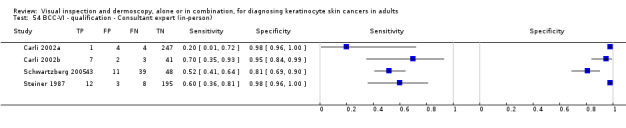
BCC‐VI ‐ qualification ‐ Consultant expert (in‐person).
55. Test.
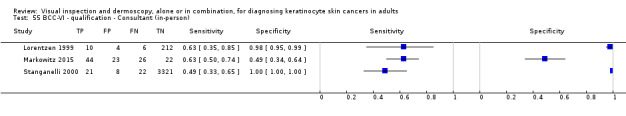
BCC‐VI ‐ qualification ‐ Consultant (in‐person).
56. Test.

BCC‐VI ‐ qualification ‐ Mixed (Secondary care) (in‐person).
57. Test.

BCC‐VI ‐ qualification ‐ Consultant expert (image‐based).
58. Test.

BCC‐VI ‐ qualification ‐ Consultant (image‐based).
59. Test.

BCC‐VI+Dermoscopy ‐ qualification ‐ Consultant expert (in‐person).
60. Test.

BCC‐VI+Dermoscopy ‐ qualification ‐ Consultant (in‐person).
61. Test.

BCC‐Dermoscopy ‐ qualification ‐ Consultant expert (image‐based).
62. Test.

BCC‐Dermoscopy ‐ qualification ‐ Consultant (image‐based).
63. Test.

BCC‐Dermoscopy ‐ qualification ‐ Resident (image‐based).
64. Test.

BCC‐Dermoscopy ‐ qualification ‐ Mixed (dermoscopy trained) (image‐based).
65. Test.

cSCC‐VI ‐ experience ‐ mixed (in‐person).
66. Test.

cSCC‐VI ‐ experience ‐ NR (in‐person).
67. Test.

cSCC‐Dermoscopy ‐ experience ‐ trained (image‐based).
68. Test.

cSCC‐Dermoscopy ‐ experience ‐ NR (image‐based).
73. Test.

KER‐VI ‐ experience ‐ high (in‐person).
74. Test.

KER‐VI ‐ experience ‐ mixed (in‐person).
75. Test.

KER‐VI ‐ experience ‐ NR (in‐person).
76. Test.

KER‐VI ‐ experience ‐ high (image‐based).
77. Test.

KER‐VI ‐ experience ‐ NR (image‐based).
78. Test.

KER‐VI+Dermoscopy ‐ experience ‐ trained (in‐person).
80. Test.

KER‐VI+Dermoscopy ‐ experience ‐ NR (in‐person).
81. Test.

KER‐Dermoscopy ‐ experience ‐ high (image‐based).
82. Test.

KER‐Dermoscopy ‐ experience ‐ trained (image‐based).
83. Test.

KER‐Dermoscopy ‐ experience ‐ NR (image‐based).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altamura 2010.
| Study characteristics | |||
| Patient sampling |
Study design: Case control Data collection: Retrospective Period of data collection January 1991 ‐ May 2007 Country Italy, Australia and Austria Test set derived. BCC characteristics assessed on a random sample of BCC lesions; observer accuracy for diagnosis of BCC assessed on a separately‐derived random sample of 4 lesion types |
||
| Patient characteristics and setting |
Inclusion criteria: Skin lesions randomly selected from digital image databases of all lesions excised; separately sampled BCCs, melanomas, 50 melanocytic naevi, and nonmelanocytic skin lesions Setting: Secondary; Departments of Dermatology of the University of L'Aquila. Specialist unit; tertiary referral centre of the Sydney Melanoma Diagnostic Center (Sydney, Australia) Prior testing: Unclear; all selected for excision Setting for prior testing: Unspecified Exclusion criteria: Poor‐quality images excluded (considered under Flow and Timing) Sample size (patients): Not reported Sample size (lesions): No. included: 300 Participant characteristics: Not reported for test set of images Lesion characteristics: Not reported in full for test set of images. BCC included 38 pigmented, 38 heavily pigmented, 37 nonpigmented, and 37 lightly pigmented); median Breslow thickness for melanomas 0.4 mm; range 0 ‐ 2.7 mm. Non‐BCC lesions reportedly had "a similar degree and distribution of pigmentation" |
||
| Index tests |
Dermoscopy Modified version of Menzies algorithm for BCC (Menzies 2000) Method of diagnosis: Dermoscopic images Prior test data: No further information used; images were scored "without knowledge of any clinical data of the patients and lesions" Diagnostic threshold: Observer diagnosis of BCC. On diagnosis of a BCC, observer was asked to report the presence or absence of 'classic' and 'nonclassic' BCC dermatoscopic patterns as identified in the first phase of the study (assessment of 609 confirmed BCCs for global and local dermatoscopic features as described in Menzies 2000 and Menzies 1996a; 'classic' BCC patterns were defined as those associated with pigmented BCC (i.e. ulceration, multiple blue/grey globules, leaflike areas, large blue/grey ovoid nests, spoke‐wheel areas, and arborising telangiectasia), 'nonclassic' patterns were dermoscopic features "representing a possible variation on the theme of the (classic) patterns ... (i.e. short fine superficial telangiectasia, multiple small erosions, concentric structures, multiple in‐focus blue/gray dots)". Diagnosis based on: Single observer (n = 3) Observer qualifications: Likely dermatologists; described as "3 observers experienced in dermatoscopic evaluation". It is unclear whether the same observer participated in the first phase of the study Experience in practice: Assumed high "experienced in dermatoscopic evaluation" Experience with index test: Assumed high |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: None provided; states "blinded to the histopathologic diagnosis" Target condition (Final diagnoses): BCC: 150; melanoma (invasive): 40; melanoma (in situ): 10; cSCC: 2 Melanocytic naevi 50 (including 28 atypical, 9 Spitz/ Reed, 5 blue, 5 dermal, 3 compound); Nonmelanocytic naevi 50 (20 seborrhoeic keratosis, 12 AKs, 10 Dermatofibromas, 4 haemangiomas, 1 eccrine poroma, 1 viral wart) |
||
| Flow and timing |
Participant exclusions: Poor‐quality index test image "large lesions present on the database but not completely comprised within the field of view were not included in the study" Index test to reference standard interval: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | Unclear | ||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Amirnia 2016.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Unclear Period of data collection February 2012 ‐ February 2014 Country Iran |
||
| Patient characteristics and setting |
Inclusion criteria: Randomly‐selected patients suspected of BCC or melanocytic naevi of the face, referred to dermatology clinic for excision or examination; all included lesions were excised Setting: Secondary (general dermatology) Prior testing: Selected for excision (no further detail) Setting for prior testing: NR Exclusion criteria: NR Sample size (patients): N eligible: 67; N included: 61 Sample size (lesions): N eligible: NR; N included: 61 Participant characteristics: Mean age: 49.5 (± 18.9; 24 ‐ 81). Male: 25 (41%) Lesion characteristics: Face (100%). mean lesion duration 6 years and 10 months (1 month to 20 years). |
||
| Index tests |
Dermoscopy; 3‐point checklist Method of Diagnosis: In‐person diagnosis Prior test Clinical examination Diagnostic threshold: Presence of 2 or more criteria. Asymmetry in colour or structure in 1 or 2 orthogonal axis asymmetric; pigment network with irregular holes and thick lines atypical network; any kind of blue or white colour Diagnosis based on: Single observer (N NR) Observer qualifications: NR; assume dermatologist Experience in practice: NR Experience with index test: NR |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone (biopsy) Target condition (Final diagnoses): BCC: 27; melanocytic naevi: 28; sebhorrheic keratosis:1; 1 reaction to foreign substance, 1 folliculitis associated with calcification, 1 abscess; 2 reported as "in situ carcinoma" but not further described |
||
| Flow and timing |
Participant exclusions: NR Index test to reference standard interval: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Unclear | |||
Argenziano 2006.
| Study characteristics | |||
| Patient sampling |
Study design: Randomised controlled trial allocating primary‐care physicians to use either visual inspection alone or visual inspection plus dermoscopy (only excised lesions can be included for each arm) Data collection: Prospective Period of data collection May 2003 ‐ Sept 2004 Country Italy and Spain |
||
| Patient characteristics and setting |
Inclusion criteria: Patients asking for screening or exhibiting 1 or more skin tumours as seen during routine physical examination (patient‐finding screening) were considered for inclusion; those undergoing excision were included in this review (i.e. those deemed sufficiently suspicious by the Expert evaluation). PCPs were invited to participate in the trial; only those who attended the training sessions and who then screened patients and referred them to the Pigmented Lesion Clinics were randomised Setting: Primary Prior testing: No prior testing Setting for prior testing: N/A Exclusion criteria: NR Sample size (patients): N eligible: 3271 patients screened; 1325 participants allocated to Naked Eye observation (VI) and 1197 participants allocated to dermoscopy observation; N included: 162 received histology after Expert evaluation at the PLC Sample size (lesions): 85 in VI arm and 77 in Dermoscopy arm underwent excision Participant characteristics: Based on full sample: mean age 40, range 2 ‐ 90 (VI group)/41, range 3 ‐ 94 (dermoscopy group). Male 498 (38%): VI group/451 (38%) dermoscopy Lesion characteristics NR |
||
| Index tests |
Visual inspection (VI) ABCD (control arm of RCT comparing naked‐eye examination to naked eye plus dermoscopy) Method of diagnosis: In‐person diagnosis Prior test data: N/A in‐person diagnosis Diagnostic threshold: Qualitative NR; Described in Intro as: simple morphologic features summarised by the asymmetry, border irregularity, colour variegation, and diameter 5 mm (ABCD) Diagnosis based on: Average (N = 37) Observer qualifications: Primary care physicians Experience in practice: Not described Experience with index test: Not described Other detail: Pre‐randomisation all participating PCPs underwent training in ABCD rule for clinical diagnosis and 3‐point checklist for dermoscopy Dermoscopy 3‐point rule (intervention arm of RCT) Method of diagnosis: In person diagnosis Prior test data: N/A in‐person diagnosis Diagnostic threshold: ≥ 2 characteristics present (algorithm is based on the recognition of only 3 individual features: dermoscopic asymmetry (in colour or structure or both, not in shape), atypical network (pigmented network with thick lines and irregular distribution), and blue‐white structures (presence of any blue or white colour within the lesion). Each PCP in both groups examined the individual lesions and scored the patient outcome, as banal or suggestive of skin cancer Diagnosis based on: Average (N = 36) Observer qualifications: Primary care physicians Experience in practice: Not described Experience with dermoscopy: Not described Dermoscopy training: All PCPs received training (2‐hour session) on the clinical ABCD rule for diagnosis of melanoma, basic recognition of nonmelanoma skin cancers including BCC and SCC plus a 2‐hour session describing the dermoscopy 3‐point checklist |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone All lesions considered suggestive of skin cancer at the PLC were excised and subsequently diagnosed histopathologically. Equivocal lesions by histopathologic examination were reviewed by a second independent pathologist and a final diagnosis made Target condition (Final diagnoses): Melanoma (in situ and invasive, or not reported): 12; BCC: 66; cSCC: 14 sebhorrheic keratosis: 13; melanocytic naevi 51; other: 6 |
||
| Flow and timing |
Excluded participants: Data can only be extracted for those with histology (i.e. patients considered to have lesions suggestive of skin cancer); remainder had expert diagnosis (not included in the final 2 x 2 data extracted) Time interval to reference test: NR Time interval between index test(s): N/A (RCT) |
||
| Comparative | RCT examining effect of making dermoscopy available to primary care practitioners | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | High | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Yes | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | |||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Carli 2002a.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Unclear. Visual inspection and in‐vivo dermoscopy diagnoses recorded at time of patient consultation; Ex vivo (image‐based) dermoscopy interpretation undertaken retrospectively Period of data collection June 1997 ‐ December 1998 Country Italy |
||
| Patient characteristics and setting |
Inclusion criteria: Clinically equivocal or suspicious pigmented skin lesions subjected to excisional biopsy at the Institute of Dermatology Setting: Secondary (not further specified) Prior testing: Clinical or dermatoscopic suspicion, or both Setting for prior testing: Secondary Exclusion criteria: NR Sample size (patients): NR Sample size (lesions): 256 Participant characteristics: NR Lesion characteristics Of the cutaneous melanomas, 14 (25.9%) were in situ melanoma (Clark level I); 18 (33.3%) were invasive with < 0.75 mm thickness; 19 (35.3%) were of intermediate thickness (0.76 – 1.50 mm); and 3 (5.5%) were > 1.5 mm. The median thickness of invasive melanomas was 0.94 mm ± 0.5 (SD) (range 0.2 – 6) |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: In‐person diagnosis Prior test data: Unclear Other test data: Clinical examination and in vivo dermoscopy were performed before excision by 2 trained dermatologists and diagnosis reached Diagnostic threshold: NR Diagnosis based on: Consensus (2 observers); final clinical diagnosis was based on agreement between the 2 observers. In case of disagreement, the opinion of a third observer (BG) was considered to be the judge for the diagnosis Observer qualifications: Dermatologist Experience in practice: High experience or ‘Expert’; described as “dermatologists with extensive experience in both clinical and dermoscopic diagnosis of pigmented skin lesions” Dermoscopy Pattern analysis Method of diagnosis: In‐person diagnosis and image‐based diagnosis. Clinical examination and in vivo dermoscopy were performed before excision by 2 trained dermatologists and diagnosis reached. Dermoscopic images were re‐analysed by the same 2 observers at the end of the inclusion period (December 1998), blind to the previous clinical and histological diagnoses Prior test data: N/A for in person; For image‐based: slides of dermoscopic images were evaluated using a viewer that made it impossible to analyse the clinical features of the lesion; both observers had access to clinical information, including the age of the participant, the site of the lesion, the history of change over time as reported by the participant at the time of in vivo examination Diagnostic threshold: Dermoscopic diagnosis was based on the ELM pattern analysis criteria, using the same diagnostic categories used for clinical diagnosis; characteristics investigated included pigment network, pigmentation, hypopigmentation, brown globules, black dots, pseudopods, radial streaming, grey‐blue veil, atypical vascular pattern Test observers as described for Visual Inspection (above) |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Target condition (final diagnoses): Melanoma (invasive): 40; Melanoma (in situ): 14; BCC: 5; Sebhorrheic keratosis: 4; Common melanocytic naevi: 90; Melanocytic naevi: 78; Blue naevi: 9; Spitz reed naevi: 16 |
||
| Flow and timing | Excluded participants: NR Time interval to reference test: NR | ||
| Comparative | In person clinical examination and dermoscopy Time interval between index test(s): the interval between the time in‐vivo dermoscopy and re‐evaluation of dermoscopic images was reported as 1 year |
||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Unclear | |||
Carli 2002b.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: NR Period of data collection NR Country Italy |
||
| Patient characteristics and setting |
Inclusion criteria: Clinically‐suspicious or equivocal pigmented skin lesions undergoing excision for diagnostic purposes; only lesions with a diameter of 14 mm or less were included Setting: Secondary (general dermatology) Prior testing: Clinical suspicion of malignancy without dermatoscopic suspicion Setting for prior testing: Secondary (general dermatology) Exclusion criteria: NR Sample size (patients): N included: NR Sample size (lesions): N included: 57 Participant characteristics: NR Lesion characteristics: Thickness ≤ 1mm: 11 cases (5 in situ, 6 invasive); All ≤ 14 mm diameter |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: Clinical photographs; Fixed‐focus distance of 10 cm; images observed using a viewer in 2 separate diagnostic sessions Prior test data: No further information used; contact (dermoscopic) images viewed first and then distant images (clinical), without knowing the classification of the contact image of the individual lesions Diagnostic threshold: NR Diagnosis based on: Consensus (2 observers); N = 2 Observer qualifications: Dermatologist Experience in practice: High experience or ‘Expert’; states "with experience in the field of PSL" Experience with dermoscopy: High experience/‘Expert’ users; "experienced in the field of PSLs" Other detail: Used an AF micro Nikkor 60 lens objective mounted on a Nikon f50 camera, with a fixed‐focus distance of 10 cm Dermoscopy No algorithm Method of diagnosis: Dermoscopic images Prior test data: No further information used; contact (dermoscopic) images viewed first and then distant images (clinical), without knowing the classification of the contact image of the individual lesions Diagnostic threshold: NR Test observers As described for Visual Inspection (above) Any other detail Dermaphot device placed directly on the lesion without previous application of oil; only lesions with a diameter of 14 mm or less were included in the study. The image has an automatic, original magnification of x 10 |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone (not further described) Target condition (Final diagnoses): Melanoma (invasive): 6; melanoma (in situ): 5; BCC: 10 'Benign' diagnoses: 36 |
||
| Flow and timing |
Excluded participants: No exclusions reported Time interval to reference test: Photographic procedures performed consecutively prior to surgery |
||
| Comparative | Photographic procedures performed consecutively prior to surgery | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual inspection (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Chang 2013.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Retrospective Period of data collection: Jan 2006 ‐ Jul 2009 Country: Taiwan |
||
| Patient characteristics and setting |
Inclusion criteria: Potentially malignant biopsied or excised skin lesions (non‐tumour specimens excluded) Setting: Secondary (general dermatology) Prior testing: Selected for excision (no further detail) Setting for prior testing: Secondary (general dermatology) Exclusion criteria: Prior surgery; image mis‐registered or poor‐quality images (unfocused or containing a motion artefact) (considered under Flow and Timing) Sample size (patients): N eligible: 3964; N included: 676 Sample size (lesions): N eligible: 4192; N included: 769 Participant characteristics: Mean age: 47.6 (SD 21.0); Male: 296; 43.8% Lesion characteristics: NR |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: In‐person diagnosis Prior test data: N/A in‐person diagnosis Diagnostic threshold: NR; clinicians’ impressions prior to biopsy were classified as ‘‘benign’’, ‘‘malignant’’, or ‘‘indeterminate’’. When the clinicians were not confident enough to make a definite benign or malignant diagnosis, the clinical impression was considered as ‘‘indeterminate’’ data extracted for malignant vs rest and malignant/indeterminate vs rest Diagnosis based on: Single observer; board‐certified staff dermatologists from institute; N = 25 Observer qualifications: Dermatologist Experience in practice: Board certified Experience with index test: High |
||
| Target condition and reference standard(s) |
Reference standard: Histology (not further described) Target condition (Final diagnoses): Melanoma (invasive): 4; melanoma (in situ): 4; BCC: 110; cSCC: 20 'Benign' diagnoses: 595 |
||
| Flow and timing |
Excluded participants: Mis‐registered or poor‐quality images (unfocused or containing a motion artefact) as a study inclusion criterion Time interval to reference test: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Low | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | |||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Cooper 2002.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection May 2000 ‐ September 2000 Country UK |
||
| Patient characteristics and setting |
Inclusion criteria: Patients attending the open‐access dermatology renal transplant clinic with lesions suspicious for malignancy or premalignancy and booked for biopsy Setting: Specialist unit; dermatology renal transplant clinic Prior testing: Clinical suspicion Setting for prior testing: Specialist unit Exclusion criteria: NR Sample size (patients): N eligible: 70; N included: NR Sample size (lesions): N eligible: 125; N included: 102 Participant characteristics: Mean age: 60; Male: 75% Lesion characteristics Head/neck: 43; 34.4%; Limbs: 21; 16.8%; 3 genitals; 2.4% |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: In‐person diagnosis Prior test data: N/A in‐person diagnosis Diagnostic threshold: Observer provisional diagnosis Diagnosis based on: Single observer (N = 2) Observer qualifications: Consultant dermatologist and a registrar Experience in practice: Not described Experience with index test: Not described |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone (biopsy, no further details) Target condition (Final diagnoses): BCC: 12; cSCC: 23 (incl 2 keratoacanthoma); Bowen's disease 19; viral warts 7; solar keratoses 16; other 25 |
||
| Flow and timing |
Participant exclusions: 23 lesions did not undergo biopsy; 11 resolved prior to biopsy, 6 patients died (10 lesions) and 2 patients failed to attend (2 lesions). No diagnosis was made in a further 3 samples Index test to reference standard interval: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | Unclear | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | |||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Durdu 2011.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection Jan 2006 ‐ January 2009 Country Turkey |
||
| Patient characteristics and setting |
Inclusion criteria: Pigmented skin lesions that could not be diagnosed with only dermatologic physical examination Setting: Secondary (general dermatology) Prior testing: Clinical examination and dermoscopy Setting for prior testing: Secondary (general dermatology) Exclusion criteria: None reported Sample size (patients): N included: 176 Sample size (lesions): N included: 200 Participant characteristics: Mean age: 48 (4 ‐ 85). Male: 64; 36.4% Lesion characteristics: 9% nodulo‐ulcerative, 56% papular, 17% macular, 10% nodular, 8% plaque |
||
| Index tests |
Dermoscopy: No algorithm Method of diagnosis: In‐person diagnosis Prior test data: Clinical examination Diagnostic threshold: 2‐step process: step 1 melanocytic and non‐melanocytic were differentiated (Braun 2005; Zalaudek 2008); step 2 ABCD applied to melanocytic lesions for diagnosis of melanoma only (threshold > 5.45). Previously reviewed dermoscopic characteristics used to diagnose non‐melanocytic lesions Diagnosis based on: Single observer; N = 2; 1 for dermoscopy diagnosis and 1 for Tzanck smear Observer qualifications: Dermatologist Experience in practice: Not described Experience with dermoscopy: Not described |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone (excisional biopsies (N = 166) or punch biopsy (N = 34) Details: "Biopsy specimens were stained with hematoxylin and eosin. Immunohistochemical (anti‐S‐100 and human melanoma black [HMB]‐45) and histochemical (Fontana‐Masson) stains were also applied, if necessary"; interpretation by a 'pathologist' Target condition (Final diagnoses): Melanoma (in situ and invasive, or not reported): 10; BCC: 34; 1 pigmented mammary Paget disease; 1 pigmented metastatic mammary carcinoma Sebhorrheic keratosis: 24; Benign melanocytic naevus: 100; Dermatofibroma 12; Warts 16; Dirt 1; hereditary hemorrhagic telangiectasia 1 |
||
| Flow and timing |
Participant exclusions: NR Time interval to reference test: Appears consecutive. Following dermoscopic examination and cytology "either a punch or an excisional biopsy specimen was taken from the lesions and was examined histopathologically" |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | Yes | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Ek 2005.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection January 2001 ‐ December 2002 Country Australia |
||
| Patient characteristics and setting |
Inclusion criteria: Lesions excised at tertiary referral centre for the management of cancers; only those lesions in which malignancy could not be excluded were included Setting: Specialist unit (skin cancer/pigmented lesions clinic) Prior testing: Selected for excision (no further detail) Setting for prior testing: Specialist unit (skin cancer/pigmented lesions clinic) Exclusion criteria: Punch, shave or incisional biopsies and palliative excisions. Equivocal pathology report (N = 56). Sample size (patients): N eligible: 1302; N included: 1223 Sample size (lesions): N eligible: 2678; N included: 2582 Participant characteristics: Mean age: 73.6 (16 – 102). Male: 784 (64.1%); History of melanoma/skin cancer (%) 224; 8.7% recurrent lesions Lesion characteristics: Head/neck: 61%; Trunk: 14.4%; Limbs: 24.6% |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: In‐person diagnosis Prior test data: N/A in person diagnosis Diagnostic threshold: NR pre‐operative diagnosis Diagnosis based on: Unclear; likely single (N = 5) Observer qualifications: 3 consultants, a plastic surgery trainee and a clinical assistant Experience in practice: Mixed (low and high experience combined); Plastic surgery trainee usually 1st year, on 6‐month rotation; clinical assistant described as having “many years of experience” Other detail: Some results are presented for consultant, senior registrar and registrar but underlying participant numbers are not provided per observer to allow separate 2 x 2 estimation. The Discussion does describe the “six MM misdiagnosed as benign … as .. assessed by non‐consultants” |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Target condition (Final diagnoses): Melanoma (in situ and invasive, or not reported): 23; BCC: 1214; cSCC: 517 'Benign' diagnoses: 188 (7.3%) SCC in situ (Bowen’s disease), 330 (12.8%) solar keratoses, 63 (2.4%) seborrhoeic keratoses, 247 (9.6%) were other benign lesions |
||
| Flow and timing |
Excluded participants: Lesions with incomplete or incorrectly entered pro formas were excluded (N = 40) Index to reference interval: Consecutive; used pre‐operative clinical diagnosis of lesions undergoing biopsy |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Are the included patients and chosen study setting appropriate? | Unclear | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | |||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Gokdemir 2011.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: NR Period of data collection: 2005 ‐ 2009 Country: Turkey |
||
| Patient characteristics and setting |
Inclusion criteria: Patients with melanocytic and non‐melanocytic skin lesions excised due to dermoscopic suspicion of malignancy or dysplasia Setting: Secondary (general dermatology) Prior testing: NR Setting for prior testing: Unspecified Exclusion criteria: NR Sample size (patients): N eligible: 1264; N included: 362 Sample size (lesions): N included: 449 Participant characteristics: Mean age 40.3 (± 1.08), range 1 ‐ 89; Male: 160; 44.2% Lesion characteristics: NR |
||
| Index tests |
Dermoscopy No algorithm Method of diagnosis: Unclear; appears to be in‐person diagnosis Prior test data: Clinical examination Diagnostic threshold: Not reported; diagnosis of melanoma Diagnosis based on: Unclear (N NR) Observer qualifications: Dermatologist Experience in practice: Not described Experience with dermoscopy: High experience ‐ at least 2 years experience with Molemax II |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone; not further described Target condition (Final diagnoses): Melanoma (in situ and invasive, or not reported): 13; BCC: 45 Benign: Not described |
||
| Flow and timing |
Participant exclusions: None reported Index test to reference standard interval: Not reported |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Unclear | |||
Hacioglu 2013.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Unclear; diagnoses recorded at initial consultation but unclear whether the study was prospective in design. Also report prospective interpretation of previously‐acquired images (SIAscopy and dermoscopy) Period of data collection January 2009 ‐ January 2010 Country Turkey |
||
| Patient characteristics and setting |
Inclusion criteria: Patients with skin lesions < 12 mm in diameter, suspicious for malignancy; only excised lesions included Setting: Secondary (general dermatology) Prior testing: Selected for excision Setting for prior testing: Unspecified Exclusion criteria: lesion size > 12 mm; lesions with a crusted or rough surface Sample size (patients): N included: 76 Sample size (lesions): N included: 80 Participant characteristics: Mean age: 57.6 (SD 15.48: range 23 ‐ 84). Male: 45 (52%) Lesion characteristics: NR |
||
| Index tests |
Visual inspection (VI): No algorithm Method of diagnosis: In person; "clinical diagnosis based on the patient's history and dermatological findings." NB: unclear whether dermoscopy was used to inform initial diagnosis; dermoscopy use not described but dermoscopic images later evaluated Prior test data: N/A in‐person diagnosis Diagnostic threshold: Observer diagnosis Diagnosis based on: Single observer (N = 3) Observer qualifications: NR; likely dermatologist Experience in practice: Not described; 3 investigators ‐ 1 made preliminary clinic diagnosis and evaluated Siascope images 8 months later; second investigator evaluated all Siascope images; a third investigator evaluated dermoscopic images Experience with index test: Not described Dermoscopy: No algorithm Method of diagnosis: Dermoscopic images Prior test data: No further information used; "a third investigator (EBB), also blinded to the previous diagnoses, evaluated all the lesions using dermatoscopic images only." Diagnostic threshold: Observer diagnosis Observers: As described above. |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: Skin biopsies (3 or 4 mm in size) Target condition (Final diagnoses): BCC: 24; melanoma (in situ and invasive, or not reported): cSCC 3; Basosquamous cancer 2; sebhorrhoeic keratosis: 19; actinic keratosis 8; intradermal naevus 4; dermatofibroma 3; keratoacanthoma 2; Other 12 ‐ including: epidermal proliferation, pseudoepithelial hyperplasia, solar degeneration, lichen simplex chronicus, compound naevus, dysplastic naevus, prurigo nodularis, chronic inflammatory granulation, dysplastic junctional naevus |
||
| Flow and timing |
Participant exclusions: NR Index test to reference standard interval: Appears consecutive; "Images ... were obtained ... and skin biopsies ... were taken" |
||
| Comparative | 3. Time interval between index test(s): 8 months between visual and SIAscopetime between visual/SIAscope and dermatoscopy not reported | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Lorentzen 1999.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection 1994 ‐ 1997 Country Denmark |
||
| Patient characteristics and setting |
Inclusion criteria: Patients with lesions suspicious for CMM referred to outpatients clinic; only excised included Setting: NR Prior testing: Clinical suspicion of malignancy without dermatoscopic suspicion Setting for prior testing: NR Exclusion criteria: Poor‐quality index test image (considered under flow/timing) Sample size (patients): N eligible: 242; N included: 232 Sample size (lesions): N eligible: 242; N included: 232 NB: Not all cases were assessed by all observers; 2 x 2 are based on presented sensitivity and specificity estimates for full dataset of lesions; "the dermatoscopy experts assessed almost all cases (98 ± 100%), whereas the non‐expert group completed fewer assessments, from 76 to 98%". Participant characteristics: NR Lesion characteristics: NR |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: Clinical photographs Prior test data: No further information used; no option to change clinical diagnosis after viewing dermoscopic image Other test data: Dermoscopic images presented to observer subsequent to diagnosis using clinical images alone; clinical images presented before dermoscopic images Diagnostic threshold: NR; clinical diagnosis Diagnosis based on: Average; N = 9 Observer qualifications: Dermatologist Experience in practice: High; moderate; mixed (average reported); 4 'experienced dermatologists' (4 ‐ 5 years daily experience) & 5 'non‐expert dermatology residents' (1 ‐ 2 years interest and formal training in dermatoscopy Experience with index test: High; moderate; mixed |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: a co‐author from Dept of Pathology "re‐evaluated all cases to confirm the pathology diagnosis, which was used as the gold standard in this study" Target condition (Final diagnoses): Melanoma (invasive): 49 'malignant melanoma'; BCC: 16; sebhorrheic keratosis: 12; benign naevus: 137 (pigmented naevi = 116; blue naevi = 16; atypical naevi = 5); Other: 18 (Spitz naevi, Bowen's disease, sarcoid, naevus spilus, hemangioma, and others) |
||
| Flow and timing |
Excluded participants: 10 cases were "considered unfit for evaluation" due to poor‐quality image Reference interval: "biopsy specimens...were obtained after the clinical and dermatoscopic photographs had been performed" |
||
| Comparative | tbc | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual inspection (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Lorentzen 2008.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: NR Period of data collection: NR Country: Denmark |
||
| Patient characteristics and setting |
Inclusion criteria: Patients referred to the specialist naevus clinic for lesion excision Setting: Specialist unit (skin cancer/pigmented lesions clinic) Prior testing: NR Setting for prior testing: NR Exclusion criteria: Not specified Sample size (patients): N eligible: 120; N included: 119 Sample size (lesions): N included: 119 Participant characteristics: NR Lesion characteristics: NR |
||
| Index tests |
Dermoscopy: Mixed/no algorithm; describes using "the risk stratification and pattern analysis procedure as described by Kenet 2001 and Lorentzen 2000". Method of diagnosis: Dermoscopic images; compared accuracy using standard dermoscopy images (Dermaphot) and images obtained using a globe magnifier. Slides were randomised and evaluated on 2 different occasions with 3‐week intervals Prior test data: No further information used Diagnostic threshold: Observer correct diagnosis of each lesion type Diagnosis based on: Unclear (assumed average) (N NR) Observer qualifications: Dermatologist Experience in practice: High; "dermatologists who have performed dermatoscopy for 5–10 years, published scientific papers on dermatoscopy and carried out pre‐ and post specialist training in dermatoscopy" Experience with dermoscopy: High |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: used haematoxylin‐eosin staining as well as histochemistry performed using S‐100 and HMB‐45 on suspect melanoma lesions Target condition (Final diagnoses): Melanoma (invasive): 24; BCC: 13; mild/moderate dysplasia: 2; sebhorrheic keratosis: 9; haemangioma: 2; naevus pigmentosus: 69 |
||
| Flow and timing |
Excluded participants: 1 dermatofibroma excluded Time interval to reference test: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Markowitz 2015.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection: NR Country: USA |
||
| Patient characteristics and setting |
Inclusion criteria: Consecutive patients with at least 1 clinically‐challenging pink lesion on the head or neck that was suspicious for BCC and was therefore to be biopsied to rule BCC in or out; all eligible for Mohs surgery. 'Clinically‐challenging' defined as lesions that did not have the usual characteristics of BCC, such as ulceration, bleeding, crusting, isolated pink scaly patches, or pearly papules Setting: Secondary (general dermatology) Prior testing: Clinical suspicion of malignancy without dermatoscopic suspicion Setting for prior testing: Secondary (general dermatology) Exclusion criteria: Previous history of skin cancer/prior treatment at site; > 3 lesions per participant Sample size (patients): N included: 100 Sample size (lesions): N included: 115 Participant characteristics: NR Lesion characteristics: NR |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: In‐person diagnosis Prior test data: N/A in‐person diagnosis Diagnostic threshold: Observer diagnosis of possible BCC; "lesions were diagnosed based on the patient’s clinical history of a nonhealing area of concern or the clinician’s inability to rule out BCC" Diagnosis based on: Unclear; appears that diagnoses made in clinic after acquisition of each type of image Number of examiners Not specified Observer qualifications: Not described; likely dermatologist Experience in practice: Not described Experience with index test: Not described Dermoscopy: 2‐step algorithm Method of diagnosis: In‐person diagnosis; images also taken but diagnosis made in person Prior test data: Clinical examination; diagnoses made after each step in the clinical process Diagnostic threshold: Observer diagnosis of possible BCC; 2‐step algorithm described as similar to Marghoob 2010 and Malvehy 2002. Lesions inspected for dermoscopic features consistent with BCC ... "including arborized vessels, pink white shiny background, blue/grey ovoid nests, ash leaf pattern, dot‐globular‐like pattern, spoke wheel, and crystalline‐like structures" Test observers: As described for Visual Inspection (above) |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: A biopsy was taken and the final diagnosis and lesion depth based on histopathology Target condition (Final diagnoses): BCC: 70; 'Benign' diagnoses: 45 |
||
| Flow and timing |
Participant exclusions: NR Index test to reference standard interval: Consecutive; After "the patient was returned for standard‐of‐care treatment. A biopsy was taken" |
||
| Comparative | Time interval between index test(s): consecutive | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Low | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Menzies 2000.
| Study characteristics | |||
| Patient sampling |
Study design Case control Data collection Retrospective image selection/Prospective interpretation Period of data collection: NR Country: Australia and USA Test set derived: Sample randomly divided into training and test sets |
||
| Patient characteristics and setting |
Inclusion criteria: Pigmented skin lesions with dermoscopic images and histological diagnoses; BCCs, invasive melanomas and clinically atypical 'nonmelanoma' lesions separately sampled Study setting: Specialist unit; Sydney Melanoma Unit and Florida Skin and Cancer Unit databases Prior testing: Selected for excision (no further detail) Exclusion criteria: NR Sample size (patients): NR Sample size (lesions) N included: 213 Participants Characteristics: NR Lesion characteristics: Median Breslow thickness for invasive melanoma (71/213) was 0.67 mm for the test set |
||
| Index tests |
Dermoscopy: Own new algorithm (Menzies) for diagnosis of pigmented BCC Method of diagnosis: Dermoscopic images; images studies on a viewer Prior test: No further information used Diagnostic threshold: Pigment network absent with at least 1 positive feature present: ulceration, large blue‐grey ovoid nests, multiple blue‐grey globules, maple leaflike areas, spoke wheel areas, arborising (treelike) telangiectasia (all defined in detail) Diagnosis based on: Unclear; training set images assessed by 2 observers; unclear if consensus or average and whether same observers also assessed the test set images; N = 2 Observer qualification: NR: likely dermatologists Observer experience in practice: NR Observer experience with index test: NR Derivation aspect: Training set was assessed for the presence/absence of 45 dermoscopic features and a simple model constructed using negative features with low sensitivity and high specificity for invasive melanoma and benign nonmelanoma lesions. The optimal model was then evaluated on the test set of images |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone (not further described) Target condition (Final diagnoses): Test set: BCC: 71; melanoma (invasive): 71; sebhorrheic keratosis: 5; ephelis 1; solar lentigo 3; common naevus 19; dysplastic naevus 38; blue naevus 2; dermatofibroma 1; haemangioma 1; Other 1 |
||
| Flow and timing |
Participant exclusions: NR Index test to reference standard interval: PSLs photographed prior to excision |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Navarrete Dechent 2016.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Retrospective image selection/Prospective interpretation Period of data collection: 2009 ‐ 2012 Country: USA |
||
| Patient characteristics and setting |
Inclusion criteria: Consecutively‐excised nonpigmented lesions with no discernible pigment on clinical or dermoscopic images Setting: Specialist unit; Memorial Sloane Kettering Cancer Centre Prior testing: Selected for excision (no further detail) Setting for prior testing: Specialist unit Exclusion criteria: Collision tumours, dermatofibromas and seborrhoeic keratoses were excluded Sample size (patients): N eligible: 2375; N included: NR Sample size (lesions): N eligible: 2891; N included: 457 Participant characteristics: Mean age: 64.3 (SD 14.1); Male: 282; 61.7% Lesion characteristics: Head/neck: 134; 29.3%; trunk: 124; 27.1%; upper extremity 84; 18.4%; lower extremity 113; 24.7%; genitalia 1; 0.2%; missing 1; 0.2% |
||
| Index tests |
Dermoscopy: Own new algorithm (shiny white streaks (SWSs)) Method of diagnosis: Dermoscopic images; Each individual lesion’s close‐up clinical (cropped images without patient identifiers) and dermoscopic images were reviewed for inclusion by a single author Prior test data: No further information used Diagnostic threshold: Presence of any SWSs; these were classified as (1) blotches (also known as clods; discrete, small or large structureless areas); (2) strands (long thick or thin lines, randomly distributed or parallel, and not orthogonally oriented); (3) rosettes (cluster of 4 white dots in a 4‐leaf clover–like arrangement); and (4) short white lines (also known as crystalline structures and chrysalis; fine lines that intersect or are oriented orthogonally to each other) (Liebman 2012; Liebman 2011). Shiny white structures that could not be classified into one of these specific morphologies were categorised as nonspecified. (All lesions were also evaluated for Menzies criteria (Menzies 2000); those without Menzies criteria were considered featureless and were further evaluated for presence of: SFT; multiple in‐focus, blue‐grey dots; multiple small erosions;and concentric structures). Diagnosis based on: Consensus (2 observers); N = 2 Observer qualifications: 1 observer appears to be a dermatologist and the other was a medical student (based on authors' institutions); both trained by a third observer (expert dermoscopist) who also acted as arbitrator in case of any disagreement Experience in practice: Not described Experience with index test: Trained; Described as "trained in dermoscopic analysis by an expert dermoscopist" Any other detail: Images were captured with a Nikon 1 camera (Nikon USA, Inc) using Dermlite DL2 pro HR for polarized images and Dermlitefluid for nonpolarised images at 10‐fold magnification(3Gen, LLC) |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Target condition (Final diagnoses): BCC: 287; cSCC: 106; melanoma (in situ and invasive, or not reported): 21; lichen planus–like keratosis 39; naevus 4 |
||
| Flow and timing |
Participant exclusions: NR Index test to reference standard interval: Appears consecutive; "Standard procedures in this practice included capturing clinical and dermoscopic images of all lesions selected for biopsy" |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Unclear | |||
Nori 2004.
| Study characteristics | |||
| Patient sampling |
Study design: Case control Data collection: Retrospective image selection/Prospective interpretation Period of data collection 2 years ‐ date range not specified Country USA and Spain |
||
| Patient characteristics and setting |
Inclusion criteria: Biopsy‐confirmed BCC and convenience sample of non‐BCC with "'range of common diagnoses"; of these images with superior clinical quality were selected for clinical assessment Setting: Secondary (general dermatology); Private care Prior testing: Most underwent biopsy but no detail of selection process Setting for prior testing: Unspecified Exclusion criteria: NR Sample size (patients): N included: 145 Sample size (lesions): N included: 152; 105 in VI analysis Participant characteristics: Male: 98; 64% Lesion characteristics: Face/ears: 35%; trunk: 13%; limbs: extremities 45%; back 7%; only 7 of 69 non‐BCC lesions "had BCC on the list of possible differential diagnoses" |
||
| Index tests |
Visual inspection (VI): No algorithm Method of diagnosis: Clinical photographs; "set of randomised clinical images was ... analysed in a blinded fashion by two dermatologists" Prior test data: No further information used Diagnostic threshold: High and high/medium probability of BCC. Lesions assigned to: high probability (BCC until proven otherwise), medium probability (would biopsy to rule out BCC), and low probability (no biopsy needed) Diagnosis based on: Single observer (N = 2) Observer qualifications: Dermatologist Experience in practice: Not described Experience with index test: Not described |
||
| Target condition and reference standard(s) |
Reference standard Histological diagnosis plus other. Histology not further described Expert opinion: 15 lesions were not biopsied (e.g. lesions like seborrhoeic keratosis) because the clinical diagnosis was considered diagnostic Target condition (Final diagnoses): BCC: 83; 58 in VI analysis; cSCC: 4 'Benign' diagnoses: 65 |
||
| Flow and timing |
Participant exclusions: 47 lesions were not included because of poor clinical image quality Index test to reference standard interval: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test Visual inspection (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | No | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Rosendahl 2011.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Retrospective image selection/Prospective interpretation Period of data collection 30‐month period; dates NR Country Australia |
||
| Patient characteristics and setting |
Inclusion criteria: Consecutive series of pigmented lesions submitted for histology from the primary‐care skin cancer practice of 1 author Setting: Primary‐care skin cancer practice Prior testing: Selected for excision (no further detail) Setting for prior testing: Primary Exclusion criteria: Poor image quality (considered under Flow and Timing) Sample size (patients): N included: 389 Sample size (lesions): N eligible: 466 pigmented lesions out of 1959 lesions excised or biopsied; N included: 463 Participant characteristics: Mean age: 57 (SD 17). Male: 67.4% Lesion characteristics: (53.1%) melanocytic. Lesion site: 17.7% head or face; trunk: 52.1%; 27.6% extremities; 2.2% palms or soles. melanoma thickness: ≤ 1 mm: 1/29 melanoma (3.4%) |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: Clinical photographs overview and close‐up image presented Prior test data: No further information used Other test data: Dermoscopic images presented to observer subsequent to diagnosis using clinical images alone Diagnostic threshold: Clinical diagnosis/subjective impression. Observers gave a diagnosis with level of confidence (from 0 for definitely benign to 100 for definitely malignant) after viewing the clinical images. (NB used authors' threshold for detection of any skin cancer which includes lesions clinically considered to be MM, BCC pigmented epithelial carcinoma including SCC, keratoacanthoma, actinic keratosis and Bowen's disease as test positive; review only considered histologically‐confirmed MM, BCC or invasive SCC to be disease‐positive) Diagnosis based on: Single observer (N NR) Observer qualifications: Expert dermatologist (based on author communication). Experience in practice: Expert Experience with dermoscopy: Expert Dermoscopy Pattern analysis; new algorithm ‐ Chaos and clues Method of diagnosis: Clinical photographs (1 overview and 1 close‐up), followed by 1 dermoscopic image presented to a blinded observer on a computer screen Prior test data: Clinical image only; Diagnosis made based on clinical image before presentation of dermoscopic image Diagnostic threshold: Observers gave a diagnosis with level of confidence (from 0 for definitely benign to 100 for definitely malignant) Chaos and clues short algorithm ‐ each assessed for evidence of ‘‘chaos’’ (asymmetry of colour or structure); if present then ‘‘clues’’ searched for. Chaos ‐ asymmetry of structure and colour defined according to the basic principles of pattern analysis as revised by Kittler 2007. Clues included: eccentric structure‐less zone (any colour except skin colour), grey or blue structures, peripheral black dots or clods, segmental radial lines or pseudopods, polymorphous vessels, white lines, thick reticular or branched lines, and parallel lines on ridges (acral lesions) Observers as for visual inspection |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: Excise or biopsy Target condition (Final diagnoses): Melanoma (invasive): 9; melanoma (in situ): 20; BCC: 72; cSCC: 5 (including 2 keratoacanthoma); 'Benign' diagnoses: 18 Bowen's disease and 14 actinic keratosis, 217 benign melanocytic plus additional 140 benign non‐melanocytic *authors considered Bowen's disease, actinic keratosis and keratoacanthoma as malignant"; all considered benign for review analysis |
||
| Flow and timing |
Excluded participants: Lesions were excluded due to poor image quality (N = 3) Time interval to reference test: Unclear; lesions 'routinely photographed' if scheduled for excision or biopsy but not further described |
||
| Comparative | Time interval between index test(s): consecutive | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Are the included patients and chosen study setting appropriate? | Yes | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Visual inspection (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | No | ||
| Was the test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Schwartzberg 2005.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection October 2002 ‐ December 2003 Country USA |
||
| Patient characteristics and setting |
Inclusion criteria: Patients with suspected BCC undergoing biopsy; dermatology faculty performing biopsies on patients in whom BCC was a consideration were asked to complete a study questionnaire Setting: Secondary; refers to 'Dermatology faculty' Prior testing: Clinical suspicion Setting for prior testing: Unspecified Exclusion criteria: NR Sample size (patients): N eligible: 161; N included: 141. If multiple biopsies were performed on the same participant, only the first biopsy performed was included in the study Sample size (lesions): N eligible: 161; N included: 141 Participant characteristics: Mean age: 64 (28 ‐ 92); Male: 65%; Immunosuppresion (%) 5.7% Lesion characteristics: Pigmented: 19%; non‐pigmented: 81%; ulcerated (%): 25%; erythematous 49%, telangiectasis 60%, pearly border 75%, crusty 33%, scaly 41%. Head/neck: 61%; mean lesion area was 31 mm2 (range 1 mm2 – 1.8 cm2) |
||
| Index tests |
Visual inspection (VI) No algorithm Method of diagnosis: In‐person diagnosis Prior test data: No further information used Diagnostic threshold: Clinical diagnosis (certainty of diagnosis of BCC); plus combinations of characteristics predictive of BCC Diagnosis based on: Single observer Number of examiners 17 (11 full‐time faculty members and 6 part‐time faculty) Observer qualifications: Likely all dermatologists; (1 full‐time faculty member and 1 part‐time faculty member perform Mohs surgery and the others perform dermatologic surgery within the context of their general dermatology practice) Experience in practice: Assumed high Experience with index test: Not described Other detail: Information about the lesions being biopsied was collected, including: length of time the lesion was present, the location, and the presence of telangiectasias, ulceration, crusting, surrounding erythema, scale, pigmentation, or a pearly border, or both. Multivariate logistic regression analysis using backward selection used to id best predictors of BCC diagnosis |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: Dermatology faculty performed biopsies. No further detail Target condition (Final diagnoses): BCC: 82; Other diagnoses not reported apart from FPs for those with clinical certainty level 1 (6 were actinic keratoses, 2 were dermal naevi, and 1 each were scar, dermal elastosis, and trichoepithelioma) |
||
| Flow and timing |
Participant exclusions: NR Index test to reference standard interval: Consecutive; diagnoses recorded prior to dermatology faculty performing biopsies |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Stanganelli 2000.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Retrospective Period of data collection 1994 ‐ 1996 Country Italy |
||
| Patient characteristics and setting |
Inclusion criteria: Patients with pigmented skin lesions referred by dermatologists and general practitioners either for pre‐surgical assessment or consultation Setting: Specialist unit (skin cancer/pigmented lesions clinic) Prior testing: Patients referred for pre‐surgical assessment or consultation indicating they have had prior tests Setting for prior testing: Primary: some patients referred for consultation only; dermoscopy findings are reported back and management decision remains with referring clinician; Secondary (general dermatology) Exclusion criteria: NR Sample size (patients): N eligible: 1556 Sample size (lesions): N eligible: 3372; N included: 3372 Participant characteristics: Median age 30 years, range 10 to 94; Male: 522 (34%) Lesion characteristics: NR |
||
| Index tests |
Visual inspection (VI) ABCD Method of diagnosis: In‐person diagnosis Prior test data: N/A in‐person diagnosis Other test data: Dermoscopic and clinical images subsequently presented separately to observer subsequent to diagnosis using clinical images alone Diagnostic threshold: NR Diagnosis based on: Single observer; N = 1 Observer qualifications: NR; described as 1 of the co‐authors and study based in skin cancer clinic ‐ likely dermatologist Experience in practice: Not described Experience with dermoscopy: Not described Other detail: A crude clinical image (magn x 6 and x 10) was recorded in the digital database Dermoscopy: Pattern analysis Method of diagnosis: Unclear; participants seen in person but dermoscopic diagnosis made based on digital ELM image (by same clinician as in‐person clinical dx) Prior test data: Combined clinical/dermoscopy diagnosis Diagnostic threshold: Diagnosis described as based on an integrated synopsis of the patterns most commonly described in the literature (Steiner 1993) and generally associated with known histologic counterparts. Features were assessed described in detail with multiple references, including: presence of pigment network, sharp margins, abrupt edge of pigment network, branched streaks, pseudopods, radial streaming, brown globules, pigment dots, whitish or whitish‐blue veil, grey‐blue areas, white or depigmented areas, maple leaf areas, milia‐cysts, horny plugs and vascular patterns. Test observers: As described for Visual Inspection (above) Experience with dermoscopy: Any other detail. The equipment consisted of a Leica Wild M‐650 stereomicroscope (Leica AG, Heerbrugg, Switzerland), a Sony 3ccd DXC‐930P colour video camera, an AT‐Vista videographics adapter, and IBM personal computer, a Sony Trinitron Analog PVM‐2043MD monitor, and the DBDERMO MIPS software |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis plus follow‐up; histology report of known surgical excisions (n = 262) plus a cancer registry‐based follow‐up of benign cases (N = 3110) Target condition (Final diagnoses): Melanoma (in situ and invasive, or not reported): 55; BCC: 43; 'Benign' diagnoses: 3274 |
||
| Flow and timing |
Excluded participants: None reported Time interval to reference test: NR |
||
| Comparative | Time interval between index test(s): not clearly reported just indicated that D‐ELM was performed soon after clinical examination | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Are the included patients and chosen study setting appropriate? | Yes | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| Low | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| High | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | Yes | ||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Steiner 1987.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection: Not specified Country: Austria |
||
| Patient characteristics and setting |
Inclusion criteria: Small (< 10 mm) pigmented skin lesions considered diagnostically equivocal in that there was no absolute agreement on the clinical diagnosis among investigating clinicians at a pigmented lesions clinic Setting: Specialist unit (skin cancer/pigmented lesions clinic) Prior testing: Clinical suspicion of malignancy without dermatoscopic suspicion Setting for prior testing: Specialist unit (skin cancer/pigmented lesions clinic) Exclusion criteria: > 10 mm diameter Sample size (patients): NR Sample size (lesions): 318 Participant characteristics: NR Lesion characteristics: NR |
||
| Index tests |
Visual inspection (VI): No algorithm Method of diagnosis: In‐person diagnosis Prior test data: N/A Other test data: Dermoscopy undertaken by same clinician(s) subsequent to clinical evaluation Diagnostic threshold: NR Diagnosis based on: Consensus (3 observers) "All lesions were independently seen and diagnosed by the three investigators, and the diagnosis that appeared most probable to at least two of the three investigators was recorded as the clinical"; N = 3 Observer qualifications: Dermatologist Experience in practice: High experience or ‘Expert’; "experienced dermatologists" Experience with dermoscopy: Unclear; not explicitly described. Discussion describes ELM as standard procedure in clinic Study reported data for dermoscopy, but a breakdown of incorrect diagnoses by final diagnosis was not provided to allow a 2 x 2 to be estimated |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Target condition (Final diagnoses): Melanoma (invasive): 49; melanoma (in situ): 15; BCC: 20; lentigo maligna 9 (also includes lentigo maligna melanoma); Sebhorrheic keratosis: 20; junctional naevi 39; blue naevus 29; dysplastic naevus 75; lentigo simplex and naevoid lentigo 19; angioma/ angiokeratoma 15 |
||
| Flow and timing |
Excluded participants: None reported Time interval to reference test: Assumed consecutive; following diagnosis, lesions subsequently excised |
||
| Comparative | Time interval between index test(s): consecutive | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Ulrich 2015.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Prospective Period of data collection: April 2013 ‐ March 2014 Country: Germany |
||
| Patient characteristics and setting |
Inclusion criteria: Patients with non‐pigmented pink lesions with clinical suspicion of BCC requiring biopsy for diagnostic confirmation. Pink lesions defined as clinically‐unclear erythematous papule or plaque; either reddish macules, patches or small papules with or without scale Setting: Multicentre study; authors' institutions included Dermatology departments (N = 4) and private dermatology offices (N = 3) Prior testing: Clinical suspicion of malignancy Setting for prior testing: Unspecified Exclusion criteria: Lesions with the typical clinical appearance of BCC on clinical examination (such as the presence of a pearly border, central ulceration and obvious telangiectasias), as well as pigmented lesions, were excluded from the protocol. Patients with unstable or uncontrolled clinically‐significant medical conditions were excluded. Lesions with missing histology also excluded (N = 21) Sample size (patients): N eligible: 164; N included: 155 Sample size (lesions): N eligible: 256; N included: 235 (different sets of 231 lesions were available for each test) Participant characteristics: Median age: 70 (33 ‐ 90) Lesion characteristics Head/neck: 41%; upper body 48.8% |
||
| Index tests |
Visual inspection (VI): No algorithm Method of diagnosis: In‐person diagnosis; "All assessments were documented before the histological results were available" Prior test data: N/A in‐person diagnosis Diagnostic threshold: Clinical diagnosis of BCC; describes diagnostic criteria as "pink or red lesions that could be either macules, patches or small papules with or without scale", but these also form part of inclusion criteria Diagnosis based on: Single observer; in‐clinic diagnosis (N NR) Observer qualifications: Not described; probably dermatologists, given authors' institutions Experience in practice: Not described Experience with index test: Not described Dermoscopy; No algorithm (referenced Marghoob 2012) Method of diagnosis: In‐person diagnosis Prior test data: Clinical examination Diagnostic threshold: Observer diagnosis of BCC: scattered vascular global pattern with loose haphazard distribution; shiny white to red structures with or without chrysalis‐like structures; small fine telangiectasias appearing as fine, kinked vessels of small calibre, with length < 1 mm in superficial BCC and larger arborising vessels in more invasive BCC (nodular/infiltrative) Observers: As above Any other detail After clinical examination dermoscopy was carried out using a Dermlite ProHr (3Gen Inc., San Juan Capistrano, CA, USA), attached to a Sony Cybershot DSC‐W710 camera (Sony, Tokyo, Japan) (supplied by MDL). As polarised light was used, no preparation of the area under examination was necessary |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Details: a biopsy or excision of the lesion was taken and sent for histological analysis Target condition (Final diagnoses): BCC: 141 (as different sets of 231 lesions were available for each test, the number diseased per 2 x 2 varies); 'Benign' diagnoses: 94 |
||
| Flow and timing |
Participant exclusions: Histology was missing for 21 lesions, and 1 case was found to have a combination of both BCC and SK or AK, leaving 235 lesions for analysis in the ITT group Index test to reference standard interval: Consecutively done after index test "All diagnostic steps had to be completed before histological confirmation was made" |
||
| Comparative | Time interval between index test(s): consecutive | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Visual Inspection (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Dermoscopy (in‐person) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
Witkowski 2016.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Retrospective image selection/Prospective interpretation Period of data collection: January 2009 – 2011 Country: Italy |
||
| Patient characteristics and setting |
Inclusion criteria: Consecutive clinically‐equivocal ‘pink’ cutaneous lesions with absent pigmentation or containing < 10% pigment and absence of pigment network. All lesions were excised at first visit or follow‐up video dermoscopy control visit and had available digital dermoscopy images and a complete standard set of RCM images, with histopathology reports Setting: Secondary (general dermatology) Prior testing: Clinical suspicion of malignancy without dermatoscopic suspicion Setting for prior testing: Secondary (general dermatology) Exclusion criteria: Benign diagnosis made with high confidence; lack of histological report as a result of the lesion not being excised Sample size (patients): NR Sample size (lesions): N eligible: 3869 consecutive cases were reviewed; N included: 260 Participant characteristics: NR Lesion characteristics: NR |
||
| Index tests |
Dermoscopy No algorithm Method of diagnosis: Dermoscopic images Prior test data: No further information used Diagnostic threshold: Correct diagnosis (of BCC, MM and SCC) and correct management decision (excise or not) Diagnosis based on: Single observer (N = 2; 1 reader evaluated only dermoscopic images while the second reader evaluated RCM images) Observer qualifications: Not clear; only given initials of the reader, likely dermatologist Experience in practice: Not described Experience with index test: Not described Any other detail: Digital dermoscopy images were obtained with DermLite FOTO System (DermLite Photo 3Gen, San Juan Capistrano, CA, USA) |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone Target condition (Final diagnoses): BCC: 114; cSCC: 13; melanoma (in situ and invasive, or not reported): 12; Other malignant: 1 syringoid eccrine carcinoma; sebhorrheic keratosis: 25 grouped solar lentigo/seborrhoeic keratosis/lichen planus‐like keratosis/actinic keratosis (SL/SK/LPLK/AK); benign naevus: 47 naevi; 6 Spitz naevi; 18 dermatofibromas (DF), 4 vascular lesions, and 20 other type benign lesions. Other types of benign lesions included 1 clear cell acanthoma, 1 discoid lupus, 10 inflammatory lesions, 1 perivascular hyperplasia, 4 granulomatous hyperacanathosis reactions, 1 papulous fibrosis, 1 eccrine poroma, and 1 eczematous lesion |
||
| Flow and timing | Excluded participants: Around 357 cases were excluded due to the lack of a histopathology report, as a result of the lesion not being excised, or a benign diagnosis was made with high confidence Time interval to reference test: lesions excised at first visit or follow‐up video dermoscopy control visit | ||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Low | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| Low | |||
Zalaudek 2006.
| Study characteristics | |||
| Patient sampling |
Study design: Case series Data collection: Retrospective image selection/Prospective interpretation Period of data collection February 2003 ‐ January 2004 Country Naples, Italy |
||
| Patient characteristics and setting |
Inclusion criteria: Excised, equivocal and nonequivocal, pigmented and nonpigmented skin lesions with good image quality and melanin or haemoglobin pigmentation in all or part of the lesion Setting: Specialist unit; specialized Pigmented Lesion Clinic database Prior testing: Selected for excision (no further detail) Setting for prior testing: Specialist unit Exclusion criteria: NR Sample size (patients): NR Sample size (lesions): Eligible: 2621; Included: 150 (plus 15 lesions used for training purposes) Participant characteristics: NR Lesion characteristics 37/165 (26%) considered equivocal on clinical and dermoscopic grounds Thickness/depth: Mean Breslow 0.9 mm |
||
| Index tests |
Dermoscopy: 3‐point checklist Method of diagnosis: Dermoscopic images, "optimized for colour, brightness and contrast by using Adobe photoshop standards" Prior test data: Age, site, and gender provided Diagnostic threshold: 1+ criteria present indicates malignancy (asymmetry ‐ in colour and/or structure, not in shape; atypical network ‐ pigment network with thick lines and irregular holes; and blue‐white structures ‐ presence of any blue and/or white colour within the lesion) Diagnosis based on: Average (N = 150 out of 170 participating observers, who finished all 15 training cases and performed at least 1 evaluation of the main set of images (test set). Participation was open to all individuals regardless of professional profile and experience in dermoscopy; study was advertised through personal communication, e‐mail correspondences, adverts during congresses and courses, as well as via the website (www.dermoscopy.org)) Observer qualifications: For full sample of 170: dermatologists (N = 125); GPs (N = 15); other professionals in the field of skin lesions (N = 12); medical students (N = 7); other medical specialty (N = 11) Experience in practice: Not described Experience with dermoscopy: Mixed; 146/170 (86%) reported some experience with dermoscopy; 24 with no dermoscopy experience, 45 (26%) with > 5 years experience Dermoscopy training: A web‐based tutorial was provided to describe the concept of the 3‐point checklist of dermoscopy including complete definitions of criteria and example images. Following web‐based tutorial, observers initially scored a random sample of 15 images, receiving real‐time feedback for that case as judged by an expert observer Training format: Online |
||
| Target condition and reference standard(s) |
Reference standard: Histological diagnosis alone (no further details) Target condition (Final diagnoses): Melanoma (invasive): 18; melanoma (in situ): 11; BCC: 18; 79 melanocytic naevi; 26 seborrhoeic keratoses; 8 vascular tumours and 3 dermatofibromas |
||
| Flow and timing |
Participant exclusions: Poor‐quality index test image as exclusion criterion Index test to reference standard interval: Not described |
||
| Comparative | |||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Are the included patients and chosen study setting appropriate? | No | ||
| Did the study avoid including participants with multiple lesions? | Unclear | ||
| Low | High | ||
| DOMAIN 2: Index Test Dermoscopy (image based) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| For studies reporting the accuracy of multiple diagnostic thresholds, was each threshold or algorithm interpreted without knowledge of the results of the others? | |||
| Was the test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpretation carried out by an experienced examiner? | Unclear | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Expert opinion (with no histological confirmation) was not used as a reference standard | Yes | ||
| Was histology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the referral diagnosis? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| If the reference standard includes clinical follow‐up of borderline/benign appearing lesions, was there a minimum follow‐up following application of index test(s) of at least: 3 months for melanoma or cSCC or 6 months for BCC? | |||
| If more than one algorithm was evaluated for the same test, was the interval between application of the different algorithms 1 month or less? | |||
| High | |||
AK – actinic keratosis; BCC – basal cell carcinoma; BD – Bowen’s disease; BN – benign naevi; BPC – between‐person comparison (of tests); CAD – computer‐assisted diagnosis; CCS – case control study; CS – case series; cSCC – cutaneous squamous cell carcinoma; DF – dermatofibroma; ELM ‐ epiluminescence microscopy (dermoscopy); FU – follow‐up; LS – lentigo simplex; MiS – melanoma in situ (or lentigo maligna); MM – malignant melanoma; N ‐ number; N/A ‐ not applicable; NC – non‐comparative; NR – not reported; P – prospective; PCP ‐ primary‐care physician; PLC – pigmented lesion clinic; PSL – pigmented skin lesion; R – retrospective; RCM – reflectance confocal microscopy; SK – seborrhoeic keratosis; SN – Spitz naevi; WPC – within‐person comparison (of tests).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbasi 2004 | Not a primary study Systematic review |
| Ahnlide 2013 | Ineligible index test 'clinical diagnosis' study |
| Ahnlide 2016 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Akasu 1996 | Insufficient data for 2 x 2 table No 2 x 2 data only describing the dermoscopic features present in the lesions |
| Al Jalbout 2013 | Inadequate sample size Case study |
| Alarcon 2014 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Aldridge 2011a | Ineligible test observer Medical students and lay persons |
| Aldridge 2011b | Ineligible test observer |
| Aldridge 2013 | Insufficient data for 2 x 2 table Not test accuracy study |
| Alendar 2009 | Ineligible reference standard Only 7 reported verified histologically |
| Altamura 2006 | Assesses individual lesion characteristics only Insufficient data for 2 x 2 table Looking for characteristics associated with acral melanoma; does not give 2 x 2 for overall diagnosis |
| Annessi 2007 | Ineligible target condition; does not report data for BCC or cSCC |
| Antonio 2013 | Ineligible target condition Atypical naevi does not fall within our definition of D+ |
| Antoszewski 2015 | Inadequate sample size All excised lesions were benign. Insufficient data for 2 x 2 table |
| Aoyagi 2010 | Inadequate sample size |
| Arevalo 2008 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Argenziano 1997 | Wrong study population Only melanoma included |
| Argenziano 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Argenziano 1999 | Wrong study population Only includes melanoma |
| Argenziano 2002 | Not a primary study |
| Argenziano 2003 | Insufficient data for 2 x 2 table Table V gives se/sp data for 108 lesions but cannot derive the number of melanoma for this subset of the original 128 Contact authors; contacted 10 May 2016 and 24 June 2016 |
| Argenziano 2004a | Assesses individual lesion characteristics only Only lesions with vascular structures included; presence of 10 different characteristics assessed. 2 x 2 would be possible |
| Argenziano 2004b | Not a primary study Letter |
| Argenziano 2008 | Ineligible index test Surveillance/monitoring study |
| Argenziano 2010 | Ineligible index test Test used for follow‐up looking at dermoscopic features of melanomas diagnosed 1 yr after follow‐up Insufficient data for 2 x 2 table |
| Argenziano 2011 | Ineligible target condition Inadequate sample size Only 2 melanomas |
| Argenziano 2011a | Ineligible target condition 5 melanoma metastases included as D+ |
| Argenziano 2011b | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Argenziano 2012 | Ineligible reference standard no follow‐up of test negatives |
| Argenziano 2014 | Insufficient data for 2 x 2 table |
| Armstrong 2011 | Ineligible reference standard No reference standard results presented for the screened lesions; just compares naked eye judgements with dermoscopy |
| Ascierto 1998 | Insufficient data for 2 x 2 table The data presented do not contribute to the review Duplicate or related publication. Data included in Ascierto 2003 |
| Ascierto 2000 | Insufficient data for 2 x 2 table Contact authors For excised lesions, study cross‐tabulates ELM high/very high‐risk classification against some histological classification (Table 2). Number D+ = 580 (2 x 2: 504, 79, 76, 2072); 580 not mentioned anywhere else in paper (contacted 10 May 2016 and 24 June 2016) |
| Ascierto 2003 | Not a primary study |
| Ascierto 2010 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Badertscher 2015 | Insufficient data for 2 x 2 table |
| Bafounta 2001 | Not a primary study Systematic review |
| Bajaj 2016 | Ineligible reference standard Unclear ref standard for benign diagnoses |
| Banky 2005 | Ineligible target condition Ineligible index test |
| Barzegari 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Basarab 1996 | Wrong study population Not all suspected of skin cancer Insufficient data for 2 x 2 table |
| Bauer 2000 | Ineligible index test Does not provide 2 x 2 data for visual inspection alone |
| Bauer 2005 | Ineligible index test Follow‐up/monitoring study |
| Bauer 2006 | Ineligible index test Dermoscopy used to improve histopathology diagnosis |
| Becker 1954 | Not a primary study |
| Benati 2015 | Assesses individual lesion characteristics only |
| Benelli 1999 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Benelli 2000a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Benelli 2000b | Insufficient data for 2 x 2 table Only inter‐rater reliability data given (n = 25); authors have published much larger evaluations of 7FFM and ABCD |
| Benelli 2001 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Benvenuto‐Andrade 2006 | Insufficient data for 2 x 2 table Diagnostic confidence rather than accuracy |
| Benvenuto‐Andrade 2007 | Insufficient data for 2 x 2 table Agreement on lesion characterisation; not test accuracy |
| Binder 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Binder 1995 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Binder 1997 | Insufficient data for 2 x 2 table Training study; only ROC curves/AUC presented pre‐ and post‐training Contact authors (contacted 10 May 2016 and 24 June 2016) |
| Binder 1999 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Blum 2003a | Not a primary study |
| Blum 2003b | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Blum 2003c | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Blum 2004a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Blum 2004b | Not a primary study Comment paper |
| Blum 2004c | Not a primary study Letter Letter only; limited data presented ‐ evaluates '3‐colour' rule as developed By MacKie 2002 (excluded as assessment of individual lesion features only) |
| Blum 2004d | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Blum 2004e | Not a primary study Letter |
| Blum 2006 | Ineligible target condition Differentiates melanocytic from non‐melanocytic lesions only |
| Blum 2011 | Wrong study population Mucosal lesions only |
| Blum 2014 | Inadequate sample size case studies |
| Boespflug 2015 | Wrong study population Study aim is estimate the efficacy of an online spaced educational training for dermoscopy |
| Bolognia 1990 | Ineligible reference standard No ref standard diagnosis for index test negatives |
| Bono 1996 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Bono 2001 | Insufficient data for 2 x 2 table Aim of the study is to determine what features are present in amelanotic cutaneous melanoma |
| Bono 2002a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Bono 2002b | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Bono 2006 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Borsari 2010 | Assesses individual lesion characteristics only Contact authors Paper focuses on diagnostic prediction of dermoscopic island for early melanoma, however the Methods describe the calculation of the total dermoscopy score and the 7‐point checklist score; mean scores on each checklist per lesion type are then presented (no reply from authors) |
| Borsari 2015 | Assesses individual lesion characteristics only |
| Borve 2012 | Wrong study population Includes participants without skin lesions Inadequate sample size < 5 BCC |
| Bourne 2012 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Bowns 2006 | Ineligible index test; teledermatology study |
| Braun 2000 | Derivation study This is a pilot study on the new "wobble sign" in ELM no training/test sets used |
| Braun 2007 | Assesses individual lesion characteristics only |
| Braun‐Falco 1990 | Insufficient data for 2 x 2 table Not a test accuracy study |
| Broganelli 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Brown 2000 | Not a primary study Systematic review |
| Brown 2009 | Ineligible test observer lay persons |
| Buhl 2012 | Ineligible index test Follow‐up/monitoring Duplicate or related publication. Same participants as Haenssle 2010a #191 |
| Burki 2015 | Not a primary study |
| Burr 2015 | Not a primary study |
| Burton 1998 | Ineligible reference standard Can only get 2 x 2 data for referral accuracy Insufficient data for 2 x 2 table |
| Bystryn 2003 | Not a primary study Letter |
| Cabrijan 2008 | Insufficient data for 2 x 2 table Cannot get 2 x 2; reports % correct diagnoses for each different lesion classification and not % misdiagnosed as melanoma or melanomas missed Contact authors Study states "Dermatoscopic diagnosis were conformable with pathohistological diagnosis in 75 cases (72.82%) out of 103. The highest conformation was in diagnosing melanoma, in 5 out of 6 cases (83.3%)." which would give us sensitivity; do you have data on numbers mis classified as melanoma, i.e false positives? (author replied 5 July 2016 with some data but not sufficient to allow 2 x 2) |
| Canpolat 2011 | Derivation study Looks at dermoscopic characteristics of acral lesions; only 4 suspicious lesions excised |
| Cardenas 2009 | Wrong study population Includes participants with palpable lesions; not all suspected of having skin cancer |
| Carli 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Carli 1998 | Inadequate sample size se/sp data are based on sample with only 4 MM |
| Carli 2000 | Ineligible target condition Only lesions histologically classified as common naevi or naevi with architectural disorder with/without cytological atypia were considered for the study |
| Carli 2003a | Ineligible reference standard Only 39/1042 with ref test |
| Carli 2003b | Inadequate sample size |
| Carli 2003c | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Carli 2003d | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Carli 2004a | Inadequate sample size < 5 MM per arm Insufficient data for 2 x 2 table |
| Carli 2004b | Ineligible index test; can only estimate 2 x 2 for the full time period 1997 to 2001 across all observers, but dermoscopy was only introduced routinely in 1998, so some diagnoses prior to that will have been with visual inspection alone, and observers were classed as dermoscopy ’users’ (those working in pigmented lesion clinics) and nonusers (general dermatology). Contact authors Author passed away; unable to make contact with co‐authors |
| Carli 2004c | Ineligible index test 'Clinical diagnosis' ‐ Dataset covers 1997‐2001, but dermoscopy routinely introduced 1998; authors contacted but no response |
| Carli 2005 | Insufficient data for 2 x 2 table Contact authors Study presents % MM correctly classified by naked eye ± dermoscopy but does not give any detail on FPs, is this available anywhere and/or are these lesions included in any subsequent publications? Author passed away; unable to make contact with co‐authors |
| Carlos‐Ortega 2007 | Insufficient data for 2 x 2 table Gives se/sp for visual inspection and dermoscopy in the English abstract. 68 participants/70 lesions were included but only 36 seem to have had visual inspection results and all underwent dermoscopy. Two observers performed each test blinded to each other. Table I gives 22 with BCC and 11 with melanoma overall (N D+ not reported for those with VI results), but using either or both of these numbers with the se/sp provided does not give the same PPV and NPV as given by the authors Contact authors Data not clearly presented for 2 x 2; translator suggested alternative but still does not work out to what is in paper; tried contacting authors twice, no reply as of 28 July 2016 |
| Carrera 2016 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Carroll 1998 | Derivation study Derivation study; proposes new dermoscopic criteria for dx of BCC Insufficient data for 2 x 2 table |
| Chen 2001 | Not a primary study Systematic review comparing PCP accuracy with dermatologist accuracy. |
| Chen 2006 | Insufficient data for 2 x 2 table Only given AUC |
| Chen 2013 | Ineligible test observer |
| Chiaravalloti 2014 | Wrong study population Includes melanoma only |
| Ciudad‐Blanco 2014 | Wrong study population Includes melanoma only Assesses individual lesion characteristics only Insufficient data for 2 x 2 table |
| Collas 1999 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Coras 2003 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Cornell 2015 | Ineligible test observer |
| Cox 2008 | Ineligible reference standard Se and sp estimates for diagnosis of melanoma for both the seven‐point checklist and the revised (10‐point) checklist; reference standard not reported for any of the 381 TWR referrals for melanoma Contact authors Author contacted 10 May 2016; co‐author contacted 24 June 2016 |
| Cristofolini 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Cristofolini 1997 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Dal Pozzo 1999 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| De Giorgi 2006 | Inadequate sample size < 5 cases of participants with a final melanoma diagnosis |
| De Giorgi 2011 | Duplicate or related publication. Assesses same lesions as in Carli 2003c but different observers |
| De Giorgi 2012 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| De Troya‐Martin 2008 | Wrong study population Only MM included |
| DeCoste 1993 | Insufficient data for 2 x 2 table Not given the total number of D+/D‐ or total number of lesions included. Just given the sens/spec values |
| Delfino 1997 | Assesses individual lesion characteristics only Derivation study Insufficient data for 2 x 2 table Only reports association of each characteristics with D+/D‐, not 2 x 2 |
| Di Carlo 2014 | Ineligible index test. Videothermography not relevant for the review and there are no 2x2 data for dermoscopy Derivation study. Only includes AK and BCC; no 2x2 for dermoscopy |
| Di Chiacchio 2010 | Ineligible target condition Excluding nail bed melanoma Insufficient data for 2 x 2 table There are insufficient data to extract for a 2 x 2 table |
| Di Meo 2016 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Di Stefani 2007 | Inadequate sample size < 5 malignant |
| Dolianitis 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Dreiseitl 2009 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Duff 2001 | Ineligible index test Does not evaluate visual inspection alone |
| Dummer 1993 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Dummer 1995 | Assesses individual lesion characteristics only |
| Edmondson 1999 | Ineligible reference standard It seems that the reference standard here is expert diagnosis. This is not a teledermatology paper |
| Elwan 2016 | Inadequate sample size Derivation study Insufficient data for 2 x 2 table |
| Emmons 2011 | Insufficient data for 2 x 2 table Not test accuracy study; promoting primary prevention |
| Engelberg 1999 | Inadequate sample size Only 1 confirmed melanoma and 3 BCC |
| English 2003 | Insufficient data for 2 x 2 table No accuracy data given |
| English 2004 | Insufficient data for 2 x 2 table No accuracy data |
| Fabbrocini 2008 | Insufficient data for 2 x 2 table There is insufficient data provided for each index test to populate 2 x 2 table Contact authors As we can only include DTA studies ‐ Do you have a cross tabulation of each clinician's diagnosis (e.g. at threshold of 3 or more on 7‐point checklist) against the histological diagnosis and/or a cross‐tabulation of the remote diagnosis against the face‐to‐face diagnoses? (author reply; 30 June 2016 cannot access data needed) |
| Feci 2015 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Federman 1995 | Insufficient data for 2 x 2 table Not test accuracy |
| Feldmann 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Ferrara 2002 | Ineligible index test This study looks at histopathological and dermoscopic disagreements not necessarily looking at how well dermoscopy differentiates between benign and malignant diagnosis |
| Ferrari 2015 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Ferris 2015 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Fidalgo 2003 | Insufficient data for 2 x 2 table Duplicate or related publication. Appears to be superseded by Serrao 2006 Contact authors Paper provides % of MM and of DN with DNAOS scores of >=5.5 and >7, is it possible for you to provide the same information for the remaining 127 lesions in the study? Also can you advise as to whether any of the 247 lesions included in this study, overlap with the 652 reported in Serrao 2006 (#1144)? (author contacted 10 May 2016; 24 June 2016) |
| Fikrle 2013 | Ineligible reference standard Follow‐up study < 50% of study participants have their final diagnosis reached by histopathology |
| Freeman 1963 | Insufficient data for 2 x 2 table Only gives % correct for each lesion type Contact authors Tables 2 and 3 appear to give % correct diagnoses per lesion type, but do not give data on numbers misclassified as melanoma, or other malignancy, i.e. FPs. Author responded; paper too old, cannot provide data |
| Friedman 1985 | Not a primary study |
| Friedman 2008 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Fruhauf 2012 | Ineligible reference standard 35/219 underwent histology; 13 followed up; 171 expert clinical Dx |
| Fueyo‐Casado 2009 | Ineligible reference standard < 50% of the study population received histology as a test. No information given on those who were followed up |
| Funt 1963 | Ineligible index test Insufficient data for 2 x 2 table No 2 x 2 data |
| Gachon 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Gerbert 1996 | Ineligible target condition No breakdown of final diagnoses for included lesions Insufficient data for 2 x 2 table Only gives % correct for each lesion type; not sens/spec |
| Gerbert 1998 | Insufficient data for 2 x 2 table |
| Gereli 2010 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Giacomel 2005 | Wrong study population Only BCC included |
| Giacomel 2014 | Inadequate sample size |
| Giannotti 2004 | Not a primary study A review |
| Gill 2015 | Inadequate sample size Derivation study |
| Gilmore 2009 | Derivation study Principle of lacunarity has been looked at before but not this particular application/approach to it Ineligible reference standard It is possible to get 2 x 2 for 'standard dermoscopy criteria' but dermoscopy‐negative were not excised and assumed benign; 201/312 underwent excision so theoretically eligible |
| Gilmore 2010 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Glud 2009 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Grana 2003 | Ineligible index test Assesses individual lesion characteristics only Only looking at lesion border |
| Green 1991 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Green 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Grichnik 2003 | Inadequate sample size |
| Grichnik 2004 | Not a primary study Editorial |
| Grimaldi 2009 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Grob 1998 | Not a primary study |
| Guibert 2000 | Ineligible reference standard Not designed as an accuracy study, only observational. Cannot get 2 x 2 data > 50% of study participants did not receive histology as reference standard |
| Guillod 1996 | Derivation study |
| Gunduz 2003 | Inadequate sample size Case study |
| Gutierrez 2013 | Ineligible index test Test to improve histopathology diagnosis |
| Haenssle 2006 | Ineligible index test Surveillance study estimating accuracy of different approaches to follow‐up |
| Haenssle 2010a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Haenssle 2010b | Insufficient data for 2 x 2 table Does not report specificity Duplicate or related publication. Same participants as Haenssle 2010a #191 |
| Hallock 1998 | Ineligible index test 'clinical diagnosis'; dermoscopy used for 3 of 4 years |
| Haniffa 2007 | Ineligible reference standard Looks like approximately 20% of participants received a final diagnosis by histology. 179 biopsies were performed. Total sample was 881 lesions |
| Har‐Shai 2001 | Ineligible index test 'clinical diagnosis' |
| Haspeslagh 2016 | Assesses individual lesion characteristics only Insufficient data for 2 x 2 table |
| Hauschild 2014 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Heal 2008 | Insufficient data for 2 x 2 table Sensitivities and PPVs are given so theoretically a 2 x 2 could be worked out but the numbers do not appear to work out Author response; the 2 x 2 table the Cochrane researchers want to create is not possible for our results, because sensitivity and PPV are based on different sample sizes |
| Healsmith 1994 | Ineligible reference standard Benign lesions described as 'clinically diagnosed' rather than histology/follow‐up |
| Henning 2007 | Derivation study First application of CASH algorithm |
| Henning 2008 | Exclude as a derivation study |
| Herschorn 2012 | Not a primary study Systematic review |
| Higgins 1992 | Wrong study population Includes only benign lesions Inadequate sample size No melanomas Insufficient data for 2 x 2 table No malignant cases |
| Hirata 2011 | Ineligible target condition Ineligible index test |
| Hoffmann 2003 | Derivation study Uses 'leave one out' cross validation procedure Insufficient data for 2 x 2 table Only giving ROC values not able to extract a 2 x 2 table |
| Hoorens 2016 | Ineligible index test Ineligible reference standard No info on numbers undergoing histology; and no follow‐up reported for benign appearing lesions Insufficient data for 2 x 2 table |
| Huang 1996 | Assesses individual lesion characteristics only Border irregularity not overall dx Insufficient data for 2 x 2 table |
| Hubener 1956 | Insufficient data for 2 x 2 table |
| Ishioka 2009 | Ineligible index test ‐ include for teledermatology only |
| Iyatomi 2006 | Derivation study Uses 'leave one out' procedure and same lesions and tumour extraction method as Iyatomi 2008 Insufficient data for 2 x 2 table |
| Iyatomi 2008 | Derivation study The performance was evaluated by averaging both combinations (training and test sets) they did not present the data separately; uses 'leave one out' procedure Insufficient data for 2 x 2 table Not test accuracy; compares automated with manual extraction of tumour area |
| Jamora 2003 | Ineligible reference standard No referene standard for index test negatives |
| Janda 2014 | Inadequate sample size Only 1 case of melanoma, 1 case of BCC and 1 of SCC |
| Jensen 2015 | Not a primary study Comment paper |
| Johr 2002 | Not a primary study |
| Jolliffe 2001 | Ineligible index test Provides data for clinical diagnosis (including dermoscopy for some cases) |
| Jonna 1998 | Insufficient data for 2 x 2 table Only included index test positives to get PPV, not worth author contact on this one |
| Kaddu 1997 | Inadequate sample size Sample size < 5; not test accuracy |
| Kawabata 1998 | Derivation study Aim of the study is to correlate findings between dermoscopy and histology findings of acral melanoma Insufficient data for 2 x 2 table Not test accuracy |
| Kawabata 2001 | Wrong study population MM of the nail bed |
| Keefe 1990 | Ineligible reference standard Only 28% (60/214) of non‐melanoma group had excision |
| Kefel 2012 | Derivation study No test set, first use of polarised light dermoscopy, various neural networks tested Insufficient data for 2 x 2 table |
| Kelly 1986 | Ineligible target condition Cannot disaggregate the severely dysplastic/in situ MM Inadequate sample size Unclear whether > 5 in situ melanoma |
| Kenet 1994 | Not a primary study Insufficient data for 2 x 2 table Not an accuracy study |
| Kittler 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Kittler 1999 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Kittler 2001 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Kittler 2002 | Not a primary study Systematic review |
| Kittler 2006 | Conference abstract |
| Koga 2011 | Ineligible reference standard ˜ 23% of participants have their final diagnosis reached by histopathology 43/191 |
| Koh 1990 | Ineligible reference standard Screening study; no adequate reference standard |
| Kopf 1975 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Korotkov 2012 | Not a primary study Narrative review |
| Krahn 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Kreusch 1992 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Kroemer 2011 | Ineligible index test Provides data for clinical diagnosis (including dermoscopy for some cases) |
| Krol 1991 | Ineligible reference standard No follow‐up reported for those who were test‐negative |
| Kurvers 2015 | Ineligible index test Collective intelligence ‐ majority rule and quorum rule applied to large number of test interpreter decisions Duplicate or related publication. Re‐analyses data from 2 previously published studies to determine whether collective intelligence (i.e majority rules or quorum rules across a large number of observers) imporves test accuracy. We have excluded 1 of these studies as the number of melanomas is not provided (Argenziano 2003) and included the other in dermoscopy review (Zalaudek 2006) |
| Kvedar 1997 | Wrong study population Not all suspected of skin cancer |
| Lallas 2015 | Derivation study Develops new algorithm and does not use separate training/test sets of lesions |
| Langley 2001 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Langley 2007 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Lechner 2015 | Not a primary study Erratum |
| Lewis 1999 | Insufficient data for 2 x 2 table Study appears to meet all eligibility criteria but disease prevalence not given alongside se/sp Contact authors Authors contacted 10 May 2016; email returned |
| Liebman 2011 | Not a primary study Comment |
| Liebman 2012 | Not a primary study Comment |
| Lindelöf 1994 | Wrong study population Only malignant melanoma Insufficient data for 2 x 2 table Not enough information given to derive a 2 x 2 table. Only given for a sample of 50 participants who had a strong suspicion of melanoma clinically. Do not know what happened to those with no suspicion clinically |
| Lipoff 2008 | Ineligible target condition Study does not differentiate MM from benign/other but looks to identify lesion characteristics that might help id those at risk for MM |
| Liu 2012 | Derivation study Asymmetry detection; 10‐fold cross‐validation Insufficient data for 2 x 2 table |
| Lorentzen 2000 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Luttrell 2012 | Ineligible test observer Accuracy data only given for lay persons; this population of test observers is not eligible |
| Machet 2005 | Wrong study population This is a staging study |
| MacKenzie‐Wood 1998 | Wrong study population Only malignant diagnosis |
| MacKie 1971 | Insufficient data for 2 x 2 table Only gives % with correct diagnosis rather than numbers misclassified as malignant |
| MacKie 1990 | Not a primary study |
| MacKie 1991 | Not a primary study Letter |
| MacKie 2002 | Assesses individual lesion characteristics only Presence of 3 or more colours on dermoscopy |
| Mahendran 2005 | Ineligible index test Face‐to‐face is 'clinical diagnosis', i.e. visual inspection ± use of dermoscopy |
| Mahon 1997 | Not a primary study A summary of a comparison of two screening checklists |
| Malvehy 2014 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Marghoob 1995 | Not a primary study Letter |
| Marghoob 2007 | Not a primary study |
| Marghoob 2010 | Not a primary study |
| Massi 2001 | Assesses individual lesion characteristics only |
| Mayer 1997 | Not a primary study Systematic review |
| McCarthy 1995 | Not a primary study Leaflet |
| McGovern 1992 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Menzies 1996a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Menzies 1996b | Assesses individual lesion characteristics only Only given the SE/SP of individual characteristics; lesions make up the training set for Menzies 1996a (#1971) |
| Menzies 1999 | Not a primary study |
| Menzies 2001 | Ineligible index test Monitoring purposes |
| Menzies 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Menzies 2008 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Menzies 2009 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Menzies 2011 | Ineligible index test Surveillance study; data used to id factors predictive of lesion changes |
| Menzies 2013 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Moffatt 2006 | Ineligible index test 'clinical diagnosis' |
| Mohammad 2015 | Wrong study population Only includes BCC |
| Morales Callaghan 2008 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Morrison 2001 | Insufficient data for 2 x 2 table Study gives % correct diagnosis within each histology group and then gives the % ‘correct’ diagnosis of skin cancer as 22% for FP and 87% for dermatologist. But these statistics appear to have been reached by taking the mean of the % correct diagnoses across the malignant groups and do not equate to sensitivity, i.e. If you take the mean of the FP correct (%) for the 4 malignant groups you get: (40 + 22 + 25 + 0)/4 = 21.75% and then the same for the 'dermatologist correct' (%) column: (95 + 77 + 75 + 100)/4 = 86.75% |
| Morton 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Mun 2016 | Ineligible reference standard Only 37% of benign group underwent adequate reference standard |
| Nachbar 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Nathansohn 2007 | Insufficient data for 2 x 2 table Not test accuracy; follow‐up study |
| Nilles 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Osborne 1998 | Ineligible reference standard Not clear what the ref standard is Insufficient data for 2 x 2 table |
| Osborne 1999 | Wrong study population Only participants with melanoma included |
| Pagnanelli 2003 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Pan 2008 | Derivation study Looking to id characteristics assoc with superficial BCC; 2 x 2 could be extracted for combination of 3 selected characteristics. Dermoscopic features selected based on prior studies but only participants with 3 diagnoses included: BCC, intra‐ep carcinoma and psoriasis |
| Panasiti 2009 | Assesses individual lesion characteristics only Ineligible reference standard Of the 1543 lesions analysed on 321 received histopathology diagnosis. The accuracy data is based on this (only 20%); unclear what happened to the 80% of participants as no mention of follow‐up |
| Parslew 1997 | Wrong study population Not all suspected of skin cancer |
| Pazzini 1996 | Insufficient data for 2 x 2 table |
| Pehamberger 1987 | Insufficient data for 2 x 2 table Not test accuracy. This is a descriptive paper defining dermoscopic criteria. It is not a study testing accuracy of dermoscopy. From the authors final sign‐off it looks like part 2 of this paper may have details on accuracy(Steiner 1987). |
| Pellacani 2002 | Not a primary study |
| Pellacani 2006 | Derivation study Looks at detection of asymmetry between clinicians and computer Insufficient data for 2 x 2 table 2 x 2 could be derived for overall asymmetry or border cut‐off but not overall diagnosis |
| Pellacani 2007 | Assesses individual lesion characteristics only Derivation study Looking at blue hue |
| Pellacani 2009 | Ineligible target condition Focus is on identifying Spitz naevi from melanoma and ‘clark’ naevi and is looking to derive useful RCM characteristics. Although some data are given in the text for an RCM score > 3 it is difficult to work out which are FP and which FN |
| Perednia 1992 | Insufficient data for 2 x 2 table Not test accuracy |
| Peris 2002 | Wrong study population Only participants with BCC diagnosis included |
| Perrinaud 2007 | Ineligible index test Does not provide data for visual inspection alone |
| Phan 2010 | Insufficient data for 2 x 2 table Not test accuracy investigating dermoscopic features of acral melanoma including of the nail apparatus; no accuracy data given |
| Piccolo 2000 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Piccolo 2002 | Not a primary study Insufficient data for 2 x 2 table Not enough data to populate 2 x 2 table. No breakdown of index test results and ref standard |
| Piccolo 2002a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Piccolo 2004 | Ineligible index test; include for teledermatology anyway |
| Piccolo 2006 | Inadequate sample size 3 MMs, but also 1 lentigo and 14 dysplastic nevus; data not presented to allow se/sp estimation Assesses individual lesion characteristics only Derivation study Derivation for hypoluminescence microscopy |
| Piccolo 2014 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Pizzichetta 2001a | Wrong study population Population in study only those with malignant disease |
| Pizzichetta 2001b | Insufficient data for 2 x 2 table Observer agreement only |
| Pizzichetta 2002 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Pizzichetta 2004 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Pizzichetta 2007 | Wrong study population Only participants with melanoma included |
| Pizzichetta 2010 | Inadequate sample size Case study |
| Pizzichetta 2013 | Assesses individual lesion characteristics only Presence of negative pigmented network |
| Pralong 2012 | Wrong study population Only melanoma participants included |
| Provost 1998 | Insufficient data for 2 x 2 table Not test accuracy; only reports concordance |
| Pupelli 2013 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Quéreux 2011 | Ineligible index test Self‐administered questions to patients attending a GP surgery before their appointment to determine whether they are at high risk of melanoma, which is meant to highlight to the GP which patient to examine during their consultation |
| Rader 2014 | Assesses individual lesion characteristics only Insufficient data for 2 x 2 table |
| Rajpara 2009 | Not a primary study Systematic review |
| Rallan 2006 | Ineligible index test No data can be extracted for visual inspection alone |
| Rampen 1988 | Wrong study population Only melanoma included |
| Rao 1997 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Reeck 1999 | Wrong study population Only includes index test negatives, i.e. those considered benign by referring clinician Ineligible target condition |
| Reggiani 2015 | Not a primary study Systematic review of kerationcyte skin cancer |
| Riddell 1961 | Wrong study population All malignant |
| Rigel 1993 | Not a primary study |
| Rigel 1997 | Not a primary study |
| Rigel 2012 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Robati 2014 | Ineligible reference standard No follow‐up of patients not referred to dermatology clinics, who did not receive histopathology |
| Robinson 2010 | Ineligible index test Self‐examination |
| Ronger 2002 | Assesses individual lesion characteristics only |
| Rosado 2003 | Not a primary study Systematic review |
| Rosendahl 2012a | Assesses individual lesion characteristics only |
| Rosendahl 2012b | Not a primary study |
| Rossi 2000 | Ineligible reference standard Unclear reference standard in disease‐negative |
| Roush 1986 | Ineligible target condition Only dysplastic naevus |
| Rubegni 2002 | Not a primary study |
| Rubegni 2005 | Not a primary study Editorial |
| Rubegni 2010 | Derivation study Uses 'leave one out' procedure Insufficient data for 2 x 2 table |
| Rubegni 2012 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Rubegni 2016 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Sahin 2004 | Assesses individual lesion characteristics only Insufficient data for 2 x 2 table No accuracy data given, study looking at dermoscopic features of LM |
| Saida 2002 | Assesses individual lesion characteristics only Descriptive study looking at presence (%) of certain features. Not looking at accuracy. Has paragraph on diagnostic value of this specific feature quoting sens & spec but this is based upon unpublished observations and the data are not given in this paper |
| Saida 2004 | Assesses individual lesion characteristics only |
| Sakakibara 2010 | Assesses individual lesion characteristics only Only looking at different vascular structures |
| Salerni 2011 | Inadequate sample size < 5 cases |
| Salerni 2012 | Ineligible index test Surveillance study Insufficient data for 2 x 2 table |
| Salerni 2013 | Not a primary study Systematic review of surveillance with digital dermoscopy |
| Salvio 2011 | Not a primary study Inadequate sample size |
| Sanchez‐Martin 2012 | Wrong study population Only BCC cases |
| Savk 2004 | Not a primary study Letter |
| Sawada 2013 | Not a primary study |
| Sboner 2003 | Derivation study Describes 10‐fold cross‐validation process for training/testing classifier |
| Sboner 2004 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Schindewolf 1994 | Ineligible index test Evaluates CAD not VI |
| Schmoeckel 1987 | Not a primary study |
| Schulz 2001 | Ineligible target condition Melanoma metastases |
| Scope 2008 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Scope 2015 | Not a primary study |
| Segura 2009 | Ineligible index test; RCM evaluation |
| Seidenari 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Seidenari 2004 | Insufficient data for 2 x 2 table No data to populate 2 x 2 table, just ROC curve values given Contact authors TABLE 5 provides AUC values for each diagnosis for both formats and observers; we are particularly interested in accuracy for the diagnosis of melanoma, are you able to provide data in 2 x 2 format, e.g. for melanoma 'certain' against final diagnosis and for melanoma 'certain or fairly certain' against final diagnosis? (no reply from authors) |
| Seidenari 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Seidenari 2006a | Wrong study population Assessing best means of follow‐up in patients with previous melanoma ‐ total body exam versus only lesions > 2 cm. No melanoma identified |
| Seidenari 2006b | Assesses individual lesion characteristics only Looks like this study is only looking at asymmetry judgement |
| Seidenari 2007 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Seidenari 2012 | Assesses individual lesion characteristics only Looks at individual lesion characteristics to distinguish melanoma in situ, also gives mean ABCD and 7‐point scores Insufficient data for 2 x 2 table Contact authors Table 3 provides mean ABCD and 7‐point checklist scores, are you able to provide us with a cross‐tabulation of results with each checklist at 'standard' thresholds against final diagnosis? e.g. ABCD > 4.75 and > 5.45 for MIS and benign groups 7‐point checklist: presence of 2or more characteristics and 3 or more characteristics? (no reply) |
| Seidenari 2013 | Ineligible index test |
| Serrao 2006 | Ineligible index test; include for CAD review only |
| Sgouros 2014 | Ineligible index test; include for CAD review only |
| Shakya 2012 | Ineligible target condition SCC in situ is not included in target condition |
| Shariff 2010 | Ineligible reference standard |
| Shitara 2014 | Assesses individual lesion characteristics only |
| Shitara 2015 | Wrong study population Includes only melanoma |
| Skvara 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Sondak 2015 | Not a primary study Comment paper |
| Soyer 1987 | Insufficient data for 2 x 2 table Not test accuracy |
| Soyer 1995 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Soyer 2001 | Not a primary study Editorial |
| Soyer 2004 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Stanganelli 1998a | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Stanganelli 1998b | Insufficient data for 2 x 2 table Cannot derive specificity; only gives 'exact diagnoses' for MM and 2 benign categories and not number benign misdiagnosed as MM |
| Stanganelli 1999 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Stanganelli 2005 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Stanganelli 2015 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Stanley 2003 | Assesses individual lesion characteristics only Fuzzy histogram is based on the lesion's colour, which is an individual lesion characteristic |
| Stathopoulos 2015 | Insufficient data for 2 x 2 table Only includes index test‐positive participants, i.e. no FN or TN results |
| Steiner 1993 | Assesses individual lesion characteristics only Derivation study |
| Stephens 2013 | Inadequate sample size |
| Stoecker 2009 | Derivation study Translucency Insufficient data for 2 x 2 table Data presented only as ROC curve and AUC |
| Stoecker 2011 | Assesses individual lesion characteristics only Derivation study Uses 'leave one out' procedure Insufficient data for 2 x 2 table Data presented only as ROC curve and AUC |
| Stolz 1994 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Stolz 2002 | Not a primary study |
| Stratigos 2007 | Ineligible reference standard Insufficient data for 2 x 2 table |
| Stricklin 2011 | Assesses individual lesion characteristics only |
| Strumia 2003 | Conference abstract; letter only |
| Tan 2009 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Tandjung 2015 | Ineligible target condition 'Malignant' includes: AK, Bowen's, dysplastic naevus, lentigo maligna, SCC, BCC, MM, keratoacanthoma Ineligible index test GPs sent images for telederm opinion; then free to send for biopsy or not; results shown are only for those that wer biopsied, according to TD advice |
| Tasli 2012 | Not a primary study Systematic review looking at frequency of publications ion dermoscopy |
| Teban 2003 | Wrong study population Classification of Clark naevi into 12 types Insufficient data for 2 x 2 table No 2 x 2 data; classification of Clark naevi into 12 types |
| Tenenhaus 2010 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Terrill 2009 | Ineligible index test Whole‐body skin examination after participants referred on for further assessment by a specialist Insufficient data for 2 x 2 table |
| Terstappen 2007 | Wrong study population Includes only BCC ‐ looking for BCC characteristics on Siascope Derivation study Derivation study; first application of Siascope to pigmented BCC; 21/25 lesions were BCCs |
| Terushkin 2010a | Inadequate sample size Only 2 invasive SCCs Insufficient data for 2 x 2 table |
| Terushkin 2010b | Insufficient data for 2 x 2 table Not test accuracy ‐ reports final diagnoses of those excised over a number of time periods and benign‐malignant ratio |
| Thomas 1998 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Thomson 2005 | Not a primary study Letter |
| Torrey 1941 | Ineligible target condition Includes non‐cutaneous lesions |
| Tromme 2012 | Ineligible reference standard Inadequate reference test for disease‐negatives; expert dx only |
| Troyanova 2003 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Tschandl 2012 | Ineligible index test Differentiating melanocytic from non‐melanocytic lesions |
| Tschandl 2015 | Ineligible test observer Medical students |
| Unlu 2014 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Van der Leest 2011 | Ineligible reference standard Inadequate reference test for test‐negatives; expert dx only |
| Van der Rhee 2010 | Ineligible reference standard < 50% of disease‐negative have an adequate reference standard |
| Van der Rhee 2011 | Inadequate sample size < 5 cases |
| Vasili 2010 | Conference abstract |
| Verduzco‐Martinez 2013 | Wrong study population Only BCC |
| Vestergaard 2008 | Not a primary study Systematic review; check reference list |
| Viglizzo 2004 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Wagner 1985 | Insufficient data for 2 x 2 table |
| Walter 2010 | Not a primary study Clinical trial protocol |
| Walter 2012 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Walter 2013 | Ineligible reference standard Final diagnosis reached by histology or expert opinion; no follow‐up of non‐excised lesions reported in this paper. Walter 2012 does report follow‐up for enough benign lesions for control arm (weighted 7PCL) data to be included. Authors contacted and confirmed calculations (02 March 2016) |
| Wang 2008 | Insufficient data for 2 x 2 table Not test accuracy; no details of misdiagnoses of benign lesions as malignant |
| Warshaw 2009a | Insufficient data for 2 x 2 table Duplicate or related publication. Subgroup of participants from Warshaw 2010a Contact authors Study presents diagnostic accuracy of teledermatology and clinic diagnosis in comparison to histopathology; we need the underlying 2 x 2 contingency tables (see Warshaw 2010a for author response) |
| Warshaw 2009b | Insufficient data for 2 x 2 table Duplicate or related publication. Subgroup of participants from Warshaw 2010a Contact authors Study presents diagnostic accuracy of teledermatology and clinic diagnosis in comparison to histopathology; we need the underlying 2 x 2 contingency tables (see Warshaw 2010afor author response)] |
| Warshaw 2010a | Insufficient data for 2 x 2 table Contact authors Study presents diagnostic accuracy of teledermatology and clinic diagnosis in comparison to histopathology. Author only able to provide numbers test‐positive and ‐negative for melanoma and not for the final 2 cells of the 2 x 2; data provided showed higher sensitivity for melanoma as the primary diagnosis rather than as the ‘aggregate’ diagnosis and the 2 x 2 using the authors' data and the accuracy figures from the paper showed more T+ from the primary diagnosis as opposed to the aggregate |
| Warshaw 2010b | Insufficient data for 2 x 2 table As per Warshaw 2009a; this 2010 paper presents combined data for pigmented and nonpigmented lesions |
| Weismann 2002 | Not a primary study |
| Wells 2012 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Westbrook 2006 | Insufficient data for 2 x 2 table |
| Westerhoff 2000 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Whitaker‐Worth 1998 | Wrong study population Ineligible test observer Mixed medical student/clinicians Insufficient data for 2 x 2 table Not test accuracy study |
| Whited 1998 | Inadequate sample size |
| Wilkes 2010 | Not a primary study |
| Williams 1991 | Insufficient data for 2 x 2 table |
| Winkelmann 2015a | Duplicate or related publication. |
| Winkelmann 2015b | Duplicate or related publication. |
| Winkelmann 2016 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Wolf 1998 | Ineligible index test Clinical diagnosis study; test clearly described ‐ "concerning the clinical diagnosis, we were not able to ascertain from the clinical data sheet whether the referring physicians used additional diagnostics techniques such as dermoscopy" |
| Yadav 1993 | Insufficient data for 2 x 2 table Not test accuracy |
| Yamaura 2005 | Derivation study Gene amplification in acral lesions |
| Yelamos 2016 | Not a primary study. Commentary on Guitera 2016 |
| Yoo 2015 | Conference abstract |
| Youl 2007a | Ineligible index test; evaluates 'clinical diagnosis' Contact authors; author replied ‐ dermoscopy used in some but not all lesions |
| Youl 2007b | Ineligible index test; evaluates 'clinical diagnosis' Contact authors; author replied ‐ dermoscopy used in some but not all lesions |
| Zaballos 2013 | Wrong study population They do not have enough benign cases to include as full report |
| Zalaudek 2010 | Not a primary study Editorial |
| Zaumseil 1983 | Ineligible target condition; does not present data for detection of BCC or cSCC |
| Zell 2008 | Inadequate sample size Case study |
| Zortea 2014 | Derivation study Although data are divided into training and test sets, the test set data are used more than once over 20 realisations of each model, especially the melanomas, for which the same 10 are used in each realisation |
| Zou 2001 | Not a primary study Study uses results from Stolz 1994 Insufficient data for 2 x 2 table Just showing ROC curves |
7PCL ‐ 7‐point checklist; AK ‐ actinic keratosis; BCC ‐ basal cell carcinoma; CAD ‐ computer‐assisted diagnosis; D+ ‐ disease positive; Dx ‐ diagnosis; FN ‐ false negative; FP ‐ false positive; LM ‐ lentigo meligna; MM ‐ malignant melanoma; NPV ‐ negative predictive value; PCP ‐ primary‐care physician; PPV ‐ positive predictive value; ROC: receiver operating characteristic; se ‐ sensitivity; SCC ‐ squamous cell carcinoma; sp ‐ specificity; VI ‐ visual inspection.
Differences between protocol and review
The proposed primary objective to analyse studies according to the prior testing undergone by study participants (comparing those with limited prior testing with those referred for further evaluation of a suspicious skin lesion) was not possible due to limited data.
The primary objectives were also amended to conduct separate analyses by in‐person/image‐based diagnosis rather than to investigate the effect on accuracy as a secondary objective, as originally proposed in the generic protocol. We took this decision very early in the review process and based it on the fact that a diagnosis based on a dermoscopic image or clinical photograph cannot approximate the context of a face‐to‐face patient/clinician consultation, and was not based on observed results.
We expanded the secondary objectives for the detection of BCC or cSCC to include: test comparisons restricted to studies where both tests were evaluated in the same studies (direct test comparisons); and investigations of the accuracy of individual algorithms used to assist visual inspection or dermoscopy, and any effect from observer experience on diagnostic accuracy.
The secondary objective has been changed from "for the detection of any skin cancer" to "for the detection of any skin cancer in adults, where keratinocyte skin cancers make up at least 50% of included skin cancers" in order to keep the focus on keratinocyte skin cancers for this review and in order not to replicate analyses conducted for the review of RCM for melanoma. These changes also affect the definition of the secondary target condition in the Methods section.
Sources of heterogeneity that could be investigated were restricted due to lack of data.
We amended the text to clarify that studies available only as conference abstracts would be excluded from the review unless full papers could be identified; studies available only as conference abstracts do not allow a comprehensive assessment of study methods or methodological quality.
We clarified the participant inclusion criteria to make it clear that studies of only malignant or benign lesions would be excluded.
To improve clarity of methods, this text from the protocol “We will include studies developing new algorithms or methods of diagnosis (i.e. derivation studies) if they use a separate independent ’test set’ of participants or images to evaluate the new approach.We will also include studies using other forms of cross validation, such as ’leave‐one‐out’ cross‐validation (Efron 1983). We will note for future reference (but not extract) any data on the accuracy of lesion characteristics individually, e.g. the presence or absence of a pigment network or detection of asymmetry.”
has been replaced with “Studies developing new algorithms or methods of diagnosis (i.e. derivation studies) were included if they:
used a separate independent 'test set' of participants or images to evaluate the new approach, or
investigated lesion characteristics that had previously been suggested as associated with melanoma and the study reported accuracy based on the presence or absence of particular combinations of characteristics.
Studies were excluded if they:
used a statistical model to produce a data driven equation, or algorithm based on multiple diagnostic features, with no separate test set
used cross‐validation approaches such as 'leave‐one‐out' cross‐validation (Efron 1983)
evaluated the accuracy of the presence or absence of individual lesion characteristics or morphological features, with no overall diagnosis of malignancy
reported accuracy data for ‘clinical diagnosis’ with no clear description as to whether the reported data related to visual inspection alone or included dermoscopy in all study participants
were based on the experience of a skin cancer‐specific clinic, where dermoscopy may or may not have been used on an individual patient basis."
We proposed to supplement the database searches by searching the annual meetings of appropriate organisations (e.g. British Association of Dermatologists Annual Meeting, American Academy of Dermatology Annual Meeting, European Academy of Dermatology and Venereology Meeting, Society for Melanoma Research Congress, World Congress of Dermatology, European Association of Dermato Oncology), but due to the volume of evidence retrieved from database searches and time restrictions we were unable to do this.
As per the change to secondary objectives, this text from the protocol "For our secondary objective, the target condition will include any skin lesion requiring excision. We will include studies reporting data for keratinocyte skin cancer combined, and not differentiated according to BCC or cSCC, in this analysis, along with any melanoma or rare skin cancer (e.g. Merkel or amelanotic melanoma) that may be detected. We will not consider in situ cancers or actinic keratosis as disease‐positive" has been changed to:
"An additional definition of the target condition was considered in secondary analysis, the detection of:
any skin cancer, including BCC, cSCC, melanoma, or any rare skin cancer (e.g. Merkel cell cancer), as long as skin cancers other than melanoma made up more than 50% of the disease positive group. Data from studies in which melanoma accounted for more than 50% of skin cancers were included in the reviews of visual inspection and dermoscopy with and without visual inspection for the diagnosis of melanoma (Dinnes 2018a; Dinnes 2018b)."
For quality assessment, we further tailored the QUADAS‐2 tool according to the review topic.
In terms of analysis, we did not restrict analysis of per‐patient data, due to lack of data. We did not perform heterogeneity investigations or sensitivity analyses as planned, due to lack of data.
Contributions of authors
JD was the contact person with the editorial base. JD co‐ordinated contributions from the co‐authors and wrote the final draft of the review. SB conducted the literature searches. JD, NC, LJ, KYW, RBA, AD, AG and SAC screened papers against eligibility criteria. JD and NC obtained data on ongoing and unpublished studies. JD, NC, LJ, KYW, RBA, AD, AG and SC appraised the quality of papers. JD, NC, LJ, KYW, RBA, AD, AG and SC extracted data for the review and sought additional information about papers. JD and NC entered data into RevMan. JD, JLB and JJD analysed and interpreted data. JD, JJD, NC, JJB, YT and CD worked on the methods sections. JD, AJ, FW, LJ, KYW, RBA, AD, AG, SC, RNM, HT, and HCW drafted the clinical sections of the background and responded to the clinical comments of the referees. JD, JJD, CD, and YT responded to the methodology and statistics comments of the referees. CO was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. JD is the guarantor of the update.
Disclaimer
This project presents independent research supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group and Cochrane Programme Grant funding, and the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group
-
NIHR Systematic Review Programme, UK.
This project was funded by an NIHR Cochrane Systematic Reviews Programme Grant (13/89/15)
NIHR clinical fellowship, UK.
-
NIHR Birmingham Biomedical Research Centre, UK.
JD, JJD and YT receive support from the NIHR Birmingham Biomedical Research Centre
Declarations of interest
Jacqueline Dinnes: nothing to declare. Jonathan J Deeks: nothing to declare. Naomi Chuchu: nothing to declare. Rubeta N Matin: "my institution received a grant for a Barco NV commercially sponsored study to evaluate digital dermoscopy in the skin cancer clinic. My institution also received Oxfordshire Health Services Research Charitable Funds for carrying out a study of feasibility of using the Skin Cancer Quality of Life Impact Tool (SCQOLIT) in non melanoma skin cancer. I have received royalties for the Oxford Handbook of Medical Dermatology (Oxford University Press). I have received payment from Public Health England for the "Be Clear on Cancer" skin cancer report. I have no conflicts of interest to declare that directly relate to the publication of this work." Kai Yuen Wong: nothing to declare. Roger Benjamin Aldridge: nothing to declare. Alana Durack: nothing to declare. Abha Gulati: nothing to declare. Sue Ann Chan: nothing to declare. Louise Johnston: nothing to declare. Susan E Bayliss: nothing to declare. Jo Leonardi‐Bee: nothing to declare. Yemisi Takwoingi: nothing to declare. Clare Davenport: nothing to declare. Colette O'Sullivan: nothing to declare. Hamid Tehrani: nothing to declare. Hywel C Williams: I am director of the NIHR HTA Programme. HTA is part of the NIHR which also supports the NIHR systematic reviews programme from which this work is funded.
Edited (no change to conclusions)
References
References to studies included in this review
Altamura 2010 {published data only}
- Altamura D, Menzies SW, Argenziano G, Zalaudek I, Soyer HP, Sera F, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. Journal of the American Academy of Dermatology 2010;62(1):67‐75. [ER4:15465845; PUBMED: 19828209] [DOI] [PubMed] [Google Scholar]
Amirnia 2016 {published data only}
- Amirnia M, Ranjkesh MR, Azimpouran M, Karkon‐Shayan F, Alikhah H, Jafari‐Asl M, et al. Comparative study of dermatoscopic and histopathologic results in facial basal cell carcinoma and melanocytic nevi. Asian Pacific Journal of Cancer Prevention 2016;17(1):425‐9. [PUBMED: 26838250] [DOI] [PubMed] [Google Scholar]
Argenziano 2006 {published data only}
- Argenziano G, Puig S, Zalaudek I, Sera F, Corona R, Alsina M, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. Journal of Clinical Oncology 2006;24(12):1877‐82. [ER4:17940973; PUBMED: 16622262] [DOI] [PubMed] [Google Scholar]
Carli 2002a {published data only}
- Carli P, Giorgi V, Argenziano G, Palli D, Giannotti B. Pre‐operative diagnosis of pigmented skin lesions: in vivo dermoscopy performs better than dermoscopy on photographic images. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(4):339‐46. [ER4:15465882; PUBMED: 12224689] [DOI] [PubMed] [Google Scholar]
Carli 2002b {published data only}
- Carli P, Giorgi V, Salvini C, Mannone F, Chiarugi A. The gold standard for photographing pigmented skin lesions for diagnostic purposes: contact versus distant imaging. Skin Research and Technology 2002;8(4):255‐9. [PUBMED: 12423545] [DOI] [PubMed] [Google Scholar]
Chang 2013 {published data only}
- Chang WY, Huang A, Yang CY, Lee CH, Chen YC, Wu TY, et al. Computer‐aided diagnosis of skin lesions using conventional digital photography: a reliability and feasibility study. PloS One 2013;8(11):e76212. [ER4:15465893; PUBMED: 24223698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2002 {published data only}
- Cooper SM, Wojnarowska F. The accuracy of clinical diagnosis of suspected premalignant and malignant skin lesions in renal transplant recipients. Clinical and Experimental Dermatology 2002;27(6):436‐8. [ER4:20569444; PUBMED: 12372077] [DOI] [PubMed] [Google Scholar]
Durdu 2011 {published data only}
- Durdu M, Baba M, Seckin D. Dermatoscopy versus Tzanck smear test: a comparison of the value of two tests in the diagnosis of pigmented skin lesions. Journal of the American Academy of Dermatology 2011;65(5):972‐82. [ER4:15465910; PUBMED: 21565420] [DOI] [PubMed] [Google Scholar]
Ek 2005 {published data only}
- Ek EW, Giorlando F, Su SY, Dieu T. Clinical diagnosis of skin tumours: how good are we?. ANZ Journal of Surgery 2005;75(6):415‐20. [DOI: 10.1111/j.1445-2197.2005.03394.x; ER4:20569451; PUBMED: 15943729] [DOI] [PubMed] [Google Scholar]
Gokdemir 2011 {published data only}
- Gokdemir A, Guler Ozden M, Bek Y, Aydin F, Senturk N, Canturk T, et al. Dermoscopic and histopathological correlation in melanocytic and non‐melanocytic lesions [Melanositik ve non‐melanositik lezyonlarda dermoskopik ve histopatolojik tani korelasyonu]. Turkiye Klinikleri Dermatoloji 2011;21(1):7‐16. [Google Scholar]
Hacioglu 2013 {published data only}
- Hacioglu S, Saricaoglu H, Baskan EB, Uner SI, Aydogan K, Tunali S. The value of spectrophotometric intracutaneous analysis in the noninvasive diagnosis of nonmelanoma skin cancers. Clinical and Experimental Dermatology 2013;38(5):464‐9. [ER4:15465947; PUBMED: 23777487] [DOI] [PubMed] [Google Scholar]
Lorentzen 1999 {published data only}
- Lorentzen H, Weismann K, Petersen CS, Larsen FG, Secher L, Skodt V. Clinical and dermatoscopic diagnosis of malignant melanoma. Assessed by expert and non‐expert groups. Acta Dermato‐Venereologica 1999;79(4):301‐4. [ER4:17941062; PUBMED: 10429989] [DOI] [PubMed] [Google Scholar]
Lorentzen 2008 {published data only}
- Lorentzen HF, Eefsen RL, Weismann K. Comparison of classical dermatoscopy and acrylic globe magnifier dermatoscopy. Acta Dermato‐Venereologica 2008;88(2):139‐42. [ER4:15465993; PUBMED: 18311441] [DOI] [PubMed] [Google Scholar]
Markowitz 2015 {published data only}
- Markowitz O, Schwartz M, Feldman E, Bienenfeld A, Bieber AK, Ellis J, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. Journal of Clinical and Aesthetic Dermatology 2015;8(10):14‐20. [ER4:25012306; PUBMED: 26557214] [PMC free article] [PubMed] [Google Scholar]
Menzies 2000 {published data only}
- Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Archives of Dermatology 2000;136(8):1012‐6. [PUBMED: 10926737] [DOI] [PubMed] [Google Scholar]
Navarrete Dechent 2016 {published data only}
- Navarrete‐Dechent C, Bajaj S, Marchetti MA, Rabinovitz H, Dusza SW, Marghoob AA. Association of shiny white blotches and strands with nonpigmented basal cell carcinoma: evaluation of an additional dermoscopic diagnostic criterion. JAMA Dermatology 2016;152(5):546‐52. [ER4:25233592; PUBMED: 26792406] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nori 2004 {published data only}
- Nori S, Rius‐Diaz F, Cuevas J, Goldgeier M, Jaen P, Torres A, et al. Sensitivity and specificity of reflectance‐mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. Journal of the American Academy of Dermatology 2004;51(6):923‐30. [ER4:15466027; PUBMED: 15583584] [DOI] [PubMed] [Google Scholar]
Rosendahl 2011 {published data only}
- Rosendahl C, Tschandl P, Cameron A, Kittler H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. Journal of the American Academy of Dermatology 2011;64(6):1068‐73. [ER4:15466083; PUBMED: 21440329] [DOI] [PubMed] [Google Scholar]
Schwartzberg 2005 {published data only}
- Schwartzberg JB, Elgart GW, Romanelli P, Ma F, Federman DG, Kirsner RS. Accuracy and predictors of basal cell carcinoma diagnosis. Dermatologic Surgery 2005;31(5):534‐7. [ER4:20569493; PUBMED: 15962736] [DOI] [PubMed] [Google Scholar]
Stanganelli 2000 {published data only}
- Stanganelli I, Serafini M, Bucch L. A cancer‐registry‐assisted evaluation of the accuracy of digital epiluminescence microscopy associated with clinical examination of pigmented skin lesions. Dermatology 2000;200(1):11‐6. [ER4:15466129; PUBMED: 10681607] [DOI] [PubMed] [Google Scholar]
Steiner 1987 {published data only}
- Steiner A, Pehamberger H, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. II. Diagnosis of small pigmented skin lesions and early detection of malignant melanoma. Journal of the American Academy of Dermatology 1987;17(4):584‐91. [ER4:17940992; PUBMED: 3668003] [DOI] [PubMed] [Google Scholar]
Ulrich 2015 {published data only}
- Ulrich M, Braunmuehl T, Kurzen H, Dirschka T, Kellner C, Sattler E, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. British Journal of Dermatology 2015;173(2):428‐35. [ER4:25012284; PUBMED: 25904111] [DOI] [PubMed] [Google Scholar]
Witkowski 2016 {published data only}
- Witkowski AM, Ludzik J, DeCarvalho N, Ciardo S, Longo C, DiNardo A, et al. Non‐invasive diagnosis of pink basal cell carcinoma: how much can we rely on dermoscopy and reflectance confocal microscopy?. Skin Research and Technology 2016;22(2):230‐7. [ER4:25012281; PUBMED: 26338448] [DOI] [PubMed] [Google Scholar]
Zalaudek 2006 {published data only}
- Zalaudek I, Argenziano G, Soyer HP, Corona R, Sera F, Blum A, et al. Three‐point checklist of dermoscopy: an open internet study. British Journal of Dermatology 2006;154(3):431‐7. [ER4:15466171; PUBMED: 16445771] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbasi 2004 {published data only}
- Abbasi NR, Shaw HM, Rigel DS, Friedman RJ, McCarthy WH, Osman I, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004;292(22):2771‐6. [DOI] [PubMed] [Google Scholar]
Ahnlide 2013 {published data only}
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Dermato‐Venereologica 2013;93(3):305‐8. [DOI] [PubMed] [Google Scholar]
Ahnlide 2016 {published data only}
- Ahnlide I, Bjellerup M, Nilsson F, Nielsen K. Validity of ABCD rule of dermoscopy in clinical practice. Acta Dermato‐Venereologica 2016;96(3):367‐72. [ER4:25012370; PUBMED: 26351008] [DOI] [PubMed] [Google Scholar]
Akasu 1996 {published data only}
- Akasu R, Sugiyama H, Araki M, Ohtake N, Furue M, Tamaki K. Dermatoscopic and videomicroscopic features of melanocytic plantar nevi. American Journal of Dermatopathology 1996;18(1):10‐8. [DOI] [PubMed] [Google Scholar]
Alarcon 2014 {published data only}
- Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. British Journal of Dermatology 2014;170(4):802‐8. [ER4:17941078; PUBMED: 24124911] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aldridge 2011a {published data only}
- Aldridge RB, Glodzik D, Ballerini L, Fisher RB, Rees JL. Utility of non‐rule‐based visual matching as a strategy to allow novices to achieve skin lesion diagnosis. Acta Dermato‐Venereologica 2011;91(3):279‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aldridge 2011b {published data only}
- Aldridge RB, Zanotto M, Ballerini L, Fisher RB, Rees JL. Novice identification of melanoma: not quite as straightforward as the ABCDs. Acta Dermato‐Venereologica 2011;91(2):125‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aldridge 2013 {published data only}
- Aldridge RB, Naysmith L, Ooi ET, Murray CS, Rees JL. The importance of a full clinical examination: assessment of index lesions referred to a skin cancer clinic without a total body skin examination would miss one in three melanomas. Acta Dermato‐Venereologica 2013;93(6):689‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alendar 2009 {published data only}
- Alendar F, Drljevic I, Drljevic K, Alendar T. Early detection of melanoma skin cancer. Bosnian Journal of Basic Medical Sciences 2009;9(1):77‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Jalbout 2013 {published data only}
- Al Jalbout S, Moscarella E, Longo C, Argenziano G, Piana S, Zalaudek I. Dermoscopy should always be performed... even in clear‐cut cases!. Journal of the American Academy of Dermatology 2013;69(4):e159‐60. [DOI] [PubMed] [Google Scholar]
Altamura 2006 {published data only}
- Altamura D, Altobelli E, Micantonio T, Piccolo D, Fargnoli MC, Peris K. Dermoscopic patterns of acral melanocytic nevi and melanomas in a white population in central Italy. Archives of Dermatology 2006;142(9):1123‐8. [DOI] [PubMed] [Google Scholar]
Annessi 2007 {published data only}
- Annessi G, Bono R, Sampogna F, Faraggiana T, Abeni D. Sensitivity, specificity, and diagnostic accuracy of three dermoscopic algorithmic methods in the diagnosis of doubtful melanocytic lesions: the importance of light brown structureless areas in differentiating atypical melanocytic nevi from thin melanomas. Journal of the American Academy of Dermatology 2007;56(5):759‐67. [ER4:15465846; PUBMED: 17316894] [DOI] [PubMed] [Google Scholar]
Antonio 2013 {published data only}
- Antonio JR, Soubhia RM, D'Avila SC, Caldas AC, Tridico LA, Alves FT. Correlation between dermoscopic and histopathological diagnoses of atypical nevi in a dermatology outpatient clinic of the Medical School of Sao Jose do Rio Preto, SP, Brazil. Anais Brasileiros de Dermatologia 2013;88(2):199‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antoszewski 2015 {published data only}
- Antoszewski B, Fijalkowska M, Stabryla P, Kasielska‐Trojan A. Dermatoscopy as a helpful tool in plastic surgeon's practice ‐ a preliminary study. Polski Przeglad Chirurgiczny 2015;87(12):609‐13. [DOI] [PubMed] [Google Scholar]
Aoyagi 2010 {published data only}
- Aoyagi S, Hata H, Izumi K, Iitani MM, Shimizu H. Diagnostic pitfalls of using dermoscopic features to differentiate between malignant melanoma and pigmented seborrhoeic keratosis. Acta Dermato‐Venereologica 2010;90(4):440‐1. [DOI] [PubMed] [Google Scholar]
Arevalo 2008 {published data only}
- Arevalo A, Altamura D, Avramidis M, Blum A, Menzies S. The significance of eccentric and central hyperpigmentation, multifocal hyper/hypopigmentation, and the multicomponent pattern in melanocytic lesions lacking specific dermoscopic features of melanoma. Archives of Dermatology 2008;144(11):1440‐4. [ER4:19728335; PUBMED: 19015418] [DOI] [PubMed] [Google Scholar]
Argenziano 1997 {published data only}
- Argenziano G, Fabbrocini G, Carli P, Giorgi V, Delfino M. Epiluminescence microscopy: criteria of cutaneous melanoma progression. Journal of the American Academy of Dermatology 1997;37(1):68‐74. [DOI] [PubMed] [Google Scholar]
Argenziano 1998 {published data only}
- Argenziano G, Fabbrocini G, Carli P, Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7‐point checklist based on pattern analysis. Archives of Dermatology 1998;134(12):1563‐70. [ER4:15465850; PUBMED: 9875194] [DOI] [PubMed] [Google Scholar]
Argenziano 1999 {published data only}
- Argenziano G, Fabbrocini G, Carli P, Giorgi V, Delfino M. Clinical and dermatoscopic criteria for the preoperative evaluation of cutaneous melanoma thickness. Journal of the American Academy of Dermatology 1999;40(1):61‐8. [DOI] [PubMed] [Google Scholar]
Argenziano 2002 {published data only}
- Argenziano G, Soyer HP, Chimenti S, Argenziano G, Ruocco V. Impact of dermoscopy on the clinical management of pigmented skin lesions. Clinics in Dermatology 2002;20(3):200‐2. [DOI] [PubMed] [Google Scholar]
Argenziano 2003 {published data only}
- Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. Journal of the American Academy of Dermatology 2003;48(5):679‐93. [DOI] [PubMed] [Google Scholar]
Argenziano 2004a {published data only}
- Argenziano G, Zalaudek I, Corona R, Sera F, Cicale L, Petrillo G, et al. Vascular structures in skin tumors: a dermoscopy study. Archives of Dermatology 2004;140(12):1485‐9. [DOI] [PubMed] [Google Scholar]
Argenziano 2004b {published data only}
- Argenziano G, Zalaudek I, Soyer HP. Which is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology?. British Journal of Dermatology 2004;151(2):512‐3. [DOI] [PubMed] [Google Scholar]
Argenziano 2008 {published data only}
- Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow‐up protocols. British Journal of Dermatology 2008;159(2):331‐6. [DOI] [PubMed] [Google Scholar]
Argenziano 2010 {published data only}
- Argenziano G, Kittler H, Ferrara G, Rubegni P, Malvehy J, Puig S, et al. Slow‐growing melanoma: a dermoscopy follow‐up study. British Journal of Dermatology 2010;162(2):267‐73. [DOI] [PubMed] [Google Scholar]
Argenziano 2011 {published data only}
- Argenziano G, Catricala C, Ardigo M, Buccini P, Simone P, Eibenschutz L, et al. Dermoscopy of patients with multiple nevi: Improved management recommendations using a comparative diagnostic approach. Archives of Dermatology 2011;147(1):46‐9. [DOI] [PubMed] [Google Scholar]
Argenziano 2011a {published data only}
- Argenziano G, Longo C, Cameron A, Cavicchini S, Gourhant J Y, Lallas A, et al. Blue‐black rule: a simple dermoscopic clue to recognize pigmented nodular melanoma. British Journal of Dermatology 2011;165(6):1251‐5. [DOI] [PubMed] [Google Scholar]
Argenziano 2011b {published data only}
- Argenziano G, Catricala C, Ardigo M, Buccini P, Simone P, Eibenschutz L, et al. Seven‐point checklist of dermoscopy revisited. British Journal of Dermatology 2011;164(4):785‐90. [ER4:15465848; PUBMED: 21175563] [DOI] [PubMed] [Google Scholar]
Argenziano 2012 {published data only}
- Argenziano G, Zalaudek I, Hofmann‐Wellenhof R, Bakos RM, Bergman W, Blum A, et al. Total body skin examination for skin cancer screening in patients with focused symptoms. Journal of the American Academy of Dermatology 2012;66(2):212‐9. [DOI] [PubMed] [Google Scholar]
Argenziano 2014 {published data only}
- Argenziano G, Moscarella E, Annetta A, Battarra VC, Brunetti B, Buligan C, et al. Melanoma detection in Italian pigmented lesion clinics. Giornale Italiano di Dermatologia e Venereologia 2014;149(2):161‐6. [PubMed] [Google Scholar]
Armstrong 2011 {published data only}
- Armstrong A. Dermoscopy: An Evidence‐Based Approach for the Early Detection of Melanoma. UNF Theses and Dissertations (Available at digitalcommons.unf.edu/etd/302/) 2011.
Ascierto 1998 {published data only}
- Ascierto PA, Satriano RA, Palmieri G, Parasole R, Bosco L, Castello G. Epiluminescence microscopy as a useful approach in the early diagnosis of cutaneous malignant melanoma. Melanoma Research 1998;8(6):529‐37. [DOI] [PubMed] [Google Scholar]
Ascierto 2000 {published data only}
- Ascierto PA, Palmieri G, Celentano E, Parasole R, Caraco C, Daponte A, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions. The Melanoma Cooperative Study. British Journal of Dermatology 2000;142(5):893‐8. [DOI] [PubMed] [Google Scholar]
Ascierto 2003 {published data only}
- Ascierto PA, Palmieri G, Botti G, Satriano RA, Stanganelli I, Bono R, et al. Early diagnosis of malignant melanoma: Proposal of a working formulation for the management of cutaneous pigmented lesions from the Melanoma Cooperative Group. International Journal of Oncology 2003;22(6):1209‐15. [PubMed] [Google Scholar]
Ascierto 2010 {published data only}
- Ascierto PA, Palla M, Ayala F, Michele I, Caraco C, Daponte A, et al. The role of spectrophotometry in the diagnosis of melanoma. BMC Dermatology 2010;10:5. [ER4:19728329; PUBMED: 20707921] [DOI] [PMC free article] [PubMed] [Google Scholar]
Badertscher 2015 {published data only}
- Badertscher N, Tandjung R, Senn O, Kofmehl R, Held U, Rosemann T, et al. A multifaceted intervention: no increase in general practitioners' competence to diagnose skin cancer (minSKIN) ‐ randomized controlled trial. Journal of the European Academy of Dermatology & Venereology 2015;29(8):1493‐9. [DOI] [PubMed] [Google Scholar]
Bafounta 2001 {published data only}
- Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta‐analysis using techniques adapted to the evaluation of diagnostic tests. Archives of Dermatology 2001;137(10):1343‐50. [DOI] [PubMed] [Google Scholar]
Bajaj 2016 {published data only}
- Bajaj S, Marchetti MA, Navarrete‐Dechent C, Dusza SW, Kose K, Marghoob AA. The role of color and morphologic characteristics in dermoscopic diagnosis. JAMA Dermatology 2016;152(6):676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banky 2005 {published data only}
- Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Archives of Dermatology 2005;141(8):998‐1006. [DOI] [PubMed] [Google Scholar]
Barzegari 2005 {published data only}
- Barzegari M, Ghaninezhad H, Mansoori P, Taheri A, Naraghi ZS, Asgari M. Computer‐aided dermoscopy for diagnosis of melanoma. BMC Dermatology 2005;5:8. [ER4:15465860; PUBMED: 16000171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Basarab 1996 {published data only}
- Basarab T, Munn SE, Jones RR. Diagnostic accuracy and appropriateness of general practitioner referrals to a dermatology out‐patient clinic. British Journal of Dermatology 1996;135(1):70‐3. [PubMed] [Google Scholar]
Bauer 2000 {published data only}
- Bauer P, Cristofolini P, Boi S, Burroni M, Dell'Eva G, Micciolo R, et al. Digital epiluminescence microscopy: usefulness in the differential diagnosis of cutaneous pigmentary lesions. A statistical comparison between visual and computer inspection. Melanoma Research 2000;10(4):345‐9. [DOI] [PubMed] [Google Scholar]
Bauer 2005 {published data only}
- Bauer J, Blum A, Strohhacker U, Garbe C. Surveillance of patients at high risk for cutaneous malignant melanoma using digital dermoscopy. British Journal of Dermatology 2005;152(1):87‐92. [DOI] [PubMed] [Google Scholar]
Bauer 2006 {published data only}
- Bauer J, Leinweber B, Metzler G, Blum A, Hofmann‐Wellenhof R, Leitz N, et al. Correlation with digital dermoscopic images can help dermatopathologists to diagnose equivocal skin tumours. British Journal of Dermatology 2006;155(3):546‐51. [DOI] [PubMed] [Google Scholar]
Becker 1954 {published data only}
- Becker S. PItfalls in the diagnosis and treatment of melanoma. A.M.A. Archives of Dermatology and Syphilology 1954;69(1):11‐30. [DOI] [PubMed] [Google Scholar]
Benati 2015 {published data only}
- Benati E, Argenziano G, Kyrgidis A, Moscarella E, Ciardo S, Bassoli S, et al. Melanoma and naevi with a globular pattern: confocal microscopy as an aid for diagnostic differentiation. British Journal of Dermatology 2015;173(5):1232‐8. [DOI] [PubMed] [Google Scholar]
Benelli 1999 {published data only}
- Benelli C, Roscetti E, Pozzo VD, Gasparini G, Cavicchini S. The dermoscopic versus the clinical diagnosis of melanoma. European Journal of Dermatology 1999;9(6):470‐6. [ER4:18375029; PUBMED: 10491506] [PubMed] [Google Scholar]
Benelli 2000a {published data only}
- Benelli C, Roscetti E, Dal Pozzo V. The dermoscopic (7FFM) versus the clinical (ABCDE) diagnosis of small diameter melanoma. European Journal of Dermatology 2000;10(4):282‐7. [PubMed] [Google Scholar]
Benelli 2000b {published data only}
- Benelli C, Roscetti E, Dal Pozzo V. Reproducibility of a dermoscopic method (7FFM) for the diagnosis of malignant melanoma. European Journal of Dermatology 2000;10(2):110‐4. [PubMed] [Google Scholar]
Benelli 2001 {published data only}
- Benelli C, Roscetti E, Dal Pozzo V. Reproducibility of the clinical criteria (ABCDE rule) and dermatoscopic features (7FFM) for the diagnosis of malignant melanoma. European Journal of Dermatology 2001;11(3):234‐9. [ER4:18375028; PUBMED: 11358731] [PubMed] [Google Scholar]
Benvenuto‐Andrade 2006 {published data only}
- Benvenuto‐Andrade C, Dusza SW, Hay JL, Agero AL, Halpern AC, Kopf AW, et al. Level of confidence in diagnosis: clinical examination versus dermoscopy examination. Dermatologic Surgery 2006;32(5):738‐44. [DOI] [PubMed] [Google Scholar]
Benvenuto‐Andrade 2007 {published data only}
- Benvenuto‐Andrade C, Dusza SW, Agero AL, Scope A, Rajadhyaksha M, Halpern AC, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Archives of Dermatology 2007;143(3):329‐38. [DOI] [PubMed] [Google Scholar]
Binder 1994 {published data only}
- Binder M, Steiner A, Schwarz M, Knollmayer S, Wolff K, Pehamberger H. Application of an artificial neural network in epiluminescence microscopy pattern analysis of pigmented skin lesions: a pilot study. British Journal of Dermatology 1994;130(4):460‐5. [ER4:18375032; PUBMED: 8186110] [DOI] [PubMed] [Google Scholar]
Binder 1995 {published data only}
- Binder M, Schwarz M, Winkler A, Steiner A, Kaider A, Wolff K, et al. Epiluminescence microscopy. A useful tool for the diagnosis of pigmented skin lesions for formally trained dermatologists. Archives of Dermatology 1995;131(3):286‐91. [ER4:18375031; PUBMED: 7887657] [DOI] [PubMed] [Google Scholar]
Binder 1997 {published data only}
- Binder M, Puespoeck‐Schwarz M, Steiner A, Kittler H, Muellner M, Wolff K, et al. Epiluminescence microscopy of small pigmented skin lesions: short‐term formal training improves the diagnostic performance of dermatologists. Journal of the American Academy of Dermatology 1997;36(2 Pt 1):197‐202. [DOI] [PubMed] [Google Scholar]
Binder 1999 {published data only}
- Binder M, Kittler H, Steiner A, Dawid M, Pehamberger H, Wolff K. Reevaluation of the ABCD rule for epiluminescence microscopy. Journal of the American Academy of Dermatology 1999;40(2 Pt 1):171‐6. [ER4:15465864; PUBMED: 10025741] [DOI] [PubMed] [Google Scholar]
Blum 2003a {published data only}
- Blum A. Amelanotic/hypomelanotic melanoma‐‐is dermatoscopy useful for diagnosis?. Journal der Deutschen Dermatologischen Gesellschaft 2003;1(8):666‐7. [PubMed] [Google Scholar]
Blum 2003b {published data only}
- Blum A, Rassner G, Garbe C. Modified ABC‐point list of dermoscopy: A simplified and highly accurate dermoscopic algorithm for the diagnosis of cutaneous melanocytic lesions. Journal of the American Academy of Dermatology 2003;48(5):672‐8. [ER4:15465867; PUBMED: 12734495] [DOI] [PubMed] [Google Scholar]
Blum 2003c {published data only}
- Blum A, Soyer HP, Garbe C, Kerl H, Rassner G, Hofmann‐Wellenhof R. The dermoscopic classification of atypical melanocytic naevi (Clark naevi) is useful to discriminate benign from malignant melanocytic lesions. British Journal of Dermatology 2003;149(6):1159‐64. [ER4:15465868; PUBMED: 14674892] [DOI] [PubMed] [Google Scholar]
Blum 2004a {published data only}
- Blum A, Hofmann‐Wellenhof R, Luedtke H, Ellwanger U, Steins A, Roehm S, et al. Value of the clinical history for different users of dermoscopy compared with results of digital image analysis. Journal of the European Academy of Dermatology and Venereology : JEADV 2004;18(6):665‐9. [ER4:15465865; PUBMED: 15482291] [DOI] [PubMed] [Google Scholar]
Blum 2004b {published data only}
- Blum A. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. British Journal of Dermatology 2004;151(2):511‐2. [DOI] [PubMed] [Google Scholar]
Blum 2004c {published data only}
- Blum A, Clemens J, Argenziano G. Three‐colour test in dermoscopy: a re‐evaluation. British Journal of Dermatology 2004;150(5):1040. [DOI] [PubMed] [Google Scholar]
Blum 2004d {published data only}
- Blum A, Luedtke H, Ellwanger U, Schwabe R, Rassner G, Garbe C. Digital image analysis for diagnosis of cutaneous melanoma. Development of a highly effective computer algorithm based on analysis of 837 melanocytic lesions. British Journal of Dermatology 2004;151(5):1029‐38. [ER4:15465866; PUBMED: 15541081] [DOI] [PubMed] [Google Scholar]
Blum 2004e {published data only}
- Blum A, Hofmann‐Wellenhof R. Simplified dermoscopic diagnosis of acral melanocytic lesions: mountains and valleys. Australasian Journal of Dermatology 2004;45(4):235‐6. [DOI] [PubMed] [Google Scholar]
Blum 2006 {published data only}
- Blum A, Clemens J, Argenziano G. Modified dermoscopic algorithm for the differentiation between melanocytic and nonmelanocytic skin tumors. Journal of Cutaneous Medicine and Surgery 2006;10(2):73‐8. [DOI] [PubMed] [Google Scholar]
Blum 2011 {published data only}
- Blum A, Simionescu O, Argenziano G, Braun R, Cabo H, Eichhorn A, et al. Dermoscopy of pigmented lesions of the mucosa and the mucocutaneous junction: results of a multicenter study by the International Dermoscopy Society (IDS). Archives of Dermatology 2011;147(10):1181‐7. [DOI] [PubMed] [Google Scholar]
Blum 2014 {published data only}
- Blum A, Ellwanger U, Luedtke H. Features Amplifying Dermoscopy (FAD) for better evaluation in difficult pigmented and non‐pigmented melanocytic skin tumors. Journal der Deutschen Dermatologischen Gesellschaft 2014;12(1):77‐9. [DOI] [PubMed] [Google Scholar]
Boespflug 2015 {published data only}
- Boespflug A, Guerra J, Dalle S, Thomas L. Enhancement of customary dermoscopy education with spaced education e‐learning: a prospective controlled trial. JAMA Dermatology 2015;151(8):847‐53. [DOI] [PubMed] [Google Scholar]
Bolognia 1990 {published data only}
- Bolognia JL, Berwick M, Fine JA. Complete follow‐up and evaluation of a skin cancer screening in Connecticut. Journal of the American Academy of Dermatology 1990;23(6 Pt 1):1098‐106. [DOI] [PubMed] [Google Scholar]
Bono 1996 {published data only}
- Bono A, Tomatis S, Bartoli C, Cascinelli N, Clemente C, Cupeta C, et al. The invisible colours of melanoma. A telespectrophotometric diagnostic approach on pigmented skin lesions. European Journal of Cancer 1996;32(4):727‐9. [DOI: ; ER4:20569437] [DOI] [PubMed] [Google Scholar]
Bono 2001 {published data only}
- Bono A, Maurichi A, Moglia D, Camerini T, Tragni G, Lualdi M, et al. Clinical and dermatoscopic diagnosis of early amelanotic melanoma. Melanoma Research 2001;11(5):491‐4. [DOI] [PubMed] [Google Scholar]
Bono 2002a {published data only}
- Bono A, Bartoli C, Cascinelli N, Lualdi M, Maurichi A, Moglia D, et al. Melanoma detection. A prospective study comparing diagnosis with the naked eye, dermatoscopy and telespectrophotometry. Dermatology 2002;205(4):362‐6. [ER4:15465870; PUBMED: 12444332] [DOI] [PubMed] [Google Scholar]
Bono 2002b {published data only}
- Bono A, Bartoli C, Baldi M, Tomatis S, Bifulco C, Santinami M. Clinical and dermatoscopic diagnosis of small pigmented skin lesions. European Journal of Dermatology 2002;12(6):573‐6. [ER4:18375034; PUBMED: 12459531] [PubMed] [Google Scholar]
Bono 2006 {published data only}
- Bono A, Tolomio E, Trincone S, Bartoli C, Tomatis S, Carbone A, et al. Micro‐melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. British Journal of Dermatology 2006;155(3):570‐3. [ER4:15465872; PUBMED: 16911283] [DOI] [PubMed] [Google Scholar]
Borsari 2010 {published data only}
- Borsari S, Longo C, Ferrari C, Benati E, Bassoli S, Schianchi S, et al. Dermoscopic island: a new descriptor for thin melanoma. Archives of Dermatology 2010;146(11):1257‐62. [DOI] [PubMed] [Google Scholar]
Borsari 2015 {published data only}
- Borsari S, Longo C, Piana S, Moscarella E, Lallas A, Alfano R, et al. When the 'Ugly Duckling' Loses Brothers, It Becomes the 'Only Son of a Widowed Mother'. Dermatology 2015;231(3):222‐3. [DOI] [PubMed] [Google Scholar]
Borve 2012 {published data only}
- Borve A, Holst A, Gente‐Lidholm A, Molina‐Martinez R, Paoli J. Use of the mobile phone multimedia messaging service for teledermatology. Journal of Telemedicine and Telecare 2012;18(5):292‐6. [DOI] [PubMed] [Google Scholar]
Bourne 2012 {published data only}
- Bourne P, Rosendahl C, Keir J, Cameron A. BLINCK‐A diagnostic algorithm for skin cancer diagnosis combining clinical features with dermatoscopy findings. Dermatology Practical & Conceptual 2012;2(2):202a12. [ER4:17941081; PUBMED: 23785600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowns 2006 {published data only}
- Bowns IR, Collins K, Walters SJ, McDonagh AJ. Telemedicine in dermatology: a randomised controlled trial. Health Technology Assessment (Winchester, England) 2006;10(43):iii‐iv, ix‐xi, 1‐39. [DOI] [PubMed] [Google Scholar]
Braun 2000 {published data only}
- Braun RP, Krischer J, Saurat JH. The "wobble sign" in epiluminescence microscopy as a novel clue to the differential diagnosis of pigmented skin lesions. Archives of Dermatology 2000;136(7):940‐2. [DOI] [PubMed] [Google Scholar]
Braun 2007 {published data only}
- Braun RP, Gaide O, Oliviero M, Kopf AW, French LE, Saurat JH, et al. The significance of multiple blue‐grey dots (granularity) for the dermoscopic diagnosis of melanoma. British Journal of Dermatology 2007;157(5):907‐13. [DOI] [PubMed] [Google Scholar]
Braun‐Falco 1990 {published data only}
- Braun‐Falco O, Stolz W, Bilek P, Merkle T, Landthaler M. The dermatoscope. A simplification of epiluminescent microscopy of pigmented skin changes. Hautarzt 1990;41(3):131‐6. [PubMed] [Google Scholar]
Broganelli 2005 {published data only}
- Broganelli P, Chiaretta A, Sacerdote C, Pippione M. The epiluminescence microscopy in the ambulatory clinical practice: Diagnostic accuracy and usefulness of videodermatoscopic monitoring [L'epiluminescenza nella pratica clinica ambulatoriale: Accuratezza diagnostica ed utilita del monitoraggio videodermatoscopico]. Giornale Italiano di Dermatologia e Venereologia 2005;140(1):15‐25. [ER4:18375073] [Google Scholar]
Brown 2000 {published data only}
- Brown N. Exploration of diagnostic techniques for malignant melanoma: an integrative review. Clinical Excellence for Nurse Practitioners 2000;4(5):263‐71. [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown NH, Robertson KM, Bisset YC, Rees JL. Using a structured image database, how well can novices assign skin lesion images to the correct diagnostic grouping?. Journal of Investigative Dermatology 2009;129(10):2509‐12. [DOI] [PubMed] [Google Scholar]
Buhl 2012 {published data only}
- Buhl T, Hansen‐Hagge C, Korpas B, Kaune KM, Haas E, Rosenberger A, et al. Integrating static and dynamic features of melanoma: the DynaMel algorithm. Journal of the American Academy of Dermatology 2012;66(1):27‐36. [DOI] [PubMed] [Google Scholar]
Burki 2015 {published data only}
- Burki TK. Total body exam or lesion detection screening for skin cancer?. Lancet Oncology 2015;16(16):e590. [DOI] [PubMed] [Google Scholar]
Burr 2015 {published data only}
- Burr S. The assessment, history taking and differential diagnosis of pigmented skin lesions. Dermatological Nursing 2015;14(4):(5p). [Google Scholar]
Burton 1998 {published data only}
- Burton RC, Howe C, Adamson L, Reid AL, Hersey P, Watson A, et al. General practitioner screening for melanoma: sensitivity, specificity, and effect of training. Journal of Medical Screening 1998;5(3):156‐61. [DOI] [PubMed] [Google Scholar]
Bystryn 2003 {published data only}
- Bystryn JC. Dermoscopic and histopathologic diagnosis of equivocal melanocytic skin lesions: an interdisciplinary study on 107 cases. Cancer 2003;97(7):1817; author reply 1817‐8. [DOI] [PubMed] [Google Scholar]
Cabrijan 2008 {published data only}
- Cabrijan L, Lipozencic J, Batinac T, Lenkovic M, Gruber F, Stanic ZZ. Correlation between clinical‐dermatoscopic and histopathologic diagnosis of skin tumors in our patients. Collegium Antropologicum 2008;32(Suppl 2):195‐7. [PubMed] [Google Scholar]
Canpolat 2011 {published data only}
- Canpolat F, Akış HK, Akay BN, Erdem C. Dermoscopic features of acral melanocytic nevi [Akral melanositik nevüslerin dermoskopik özellikleri]. Archives of the Turkish Dermatology and Venerology / Turkderm 2011;45(4):193‐7. [DOI: 10.4274/turkderm.04568] [DOI] [Google Scholar]
Cardenas 2009 {published data only}
- Cardenas E, Sosa A, Bezaury P, Madrid JV, Reyes E, Topete RO. Usefulness of high resolution ultrasound of 17 Mhz in palpable skin lesions. An analysis of 27 patients [Utilidad del ultrasonido de alta resolucion de 17 MHz en lesiones cutaneas palpables. Analisis de 27 pacientes]. Dermatologia Revista Mexicana 2009;53(3):119‐24. [Google Scholar]
Carli 1994 {published data only}
- Carli P, Giorgi V, Donati E, Pestelli E, Giannotti B. Epiluminescence microscopy reduces the risk of removing clinically atypical, but histologically common, melanocytic lesions [La microscopia a epiluminescenza (Elm) riduce il rischio di asportare lesioni melanocitarie clinicamente sospette ma istologicamente comuni]. Giornale Italiano di Dermatologia e Venereologia 1994;129(12):599‐605. [EMBASE: 25118646; ER4:18375075] [Google Scholar]
Carli 1998 {published data only}
- Carli P, Giorgi V, Naldi L, Dosi G. Reliability and inter‐observer agreement of dermoscopic diagnosis of melanoma and melanocytic naevi. Dermoscopy Panel. European Journal of Cancer Prevention 1998;7(5):397‐402. [DOI] [PubMed] [Google Scholar]
Carli 2000 {published data only}
- Carli P, Giorgi V, Massi D, Giannotti B. The role of pattern analysis and the ABCD rule of dermoscopy in the detection of histological atypia in melanocytic naevi. British Journal of Dermatology 2000;143(2):290‐7. [DOI] [PubMed] [Google Scholar]
Carli 2003a {published data only}
- Carli P, Giorgi V, Giannotti B, Seidenari S, Pellacani G, Peris K, et al. Skin cancer day in Italy: method of referral to open access clinics and tumor prevalence in the examined population. European Journal of Dermatology 2003;13(1):76‐9. [PubMed] [Google Scholar]
Carli 2003b {published data only}
- Carli P, Mannone F, Giorgi V, Nardini P, Chiarugi A, Giannotti B. The problem of false‐positive diagnosis in melanoma screening: the impact of dermoscopy. Melanoma Research 2003;13(2):179‐82. [DOI] [PubMed] [Google Scholar]
Carli 2003c {published data only}
- Carli P, Quercioli E, Sestini S, Stante M, Ricci L, Brunasso G, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. British Journal of Dermatology 2003;148(5):981‐4. [ER4:15465890; PUBMED: 12786829] [DOI] [PubMed] [Google Scholar]
Carli 2003d {published data only}
- Carli P, Giorgi V, Chiarugi A, Nardini P, Mannone F, Stante M, et al. Effect of lesion size on the diagnostic performance of dermoscopy in melanoma detection. Dermatology 2003;206(4):292‐6. [ER4:15465883; PUBMED: 12771468] [DOI] [PubMed] [Google Scholar]
Carli 2004a {published data only}
- Carli P, Giorgi V, Chiarugi A, Nardini P, Weinstock MA, Crocetti E, et al. Addition of dermoscopy to conventional naked‐eye examination in melanoma screening: a randomized study. Journal of the American Academy of Dermatology 2004;50(5):683‐9. [DOI] [PubMed] [Google Scholar]
Carli 2004b {published data only}
- Carli P, Giorgi V, Crocetti E, Mannone F, Massi D, Chiarugi A, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the 'dermoscopy era': a retrospective study 1997‐2001. British Journal of Dermatology 2004;150(4):687‐92. [DOI] [PubMed] [Google Scholar]
Carli 2004c {published data only}
- Carli P, Nardini P, Crocetti E, Giorgi V, Giannotti B. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry‐based study. Melanoma Research 2004;14(5):403‐7. [DOI] [PubMed] [Google Scholar]
Carli 2005 {published data only}
- Carli P, Chiarugi A, Giorgi V. Examination of lesions (including dermoscopy) without contact with the patient is associated with improper management in about 30% of equivocal melanomas. Dermatologic Surgery 2005;31(2):169‐72. [DOI] [PubMed] [Google Scholar]
Carlos‐Ortega 2007 {published data only}
- Carlos‐Ortega B, Sanchez‐Alva ME, Ysita‐Morales A, Angeles‐Garay U. Correlation among simple observation and dermoscopy in the study of pigmented lesions of the skin. Revista Medica del Instituto Mexicano del Seguro Social 2007;45(6):541‐8. [PubMed] [Google Scholar]
Carrera 2016 {published data only}
- Carrera C, Marchetti MA, Dusza SW, Argenziano G, Braun RP, Halpern AC, et al. Validity and reliability of dermoscopic criteria used to differentiate nevi from melanoma aweb‐based international dermoscopy society study. JAMA Dermatology 2016;152(7):798‐806. [ER4:25233595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 1998 {published data only}
- Carroll DM, Billingsley EM, Helm KF. Diagnosing basal cell carcinoma by dermatoscopy. Journal of Cutaneous Medicine and Surgery 1998;3(2):62‐7. [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen SC, Bravata DM, Weil E, Olkin I. A comparison of dermatologists' and primary care physicians' accuracy in diagnosing melanoma (Structured abstract). Archives of Dermatology 2001;137(12):1627‐34. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen SC, Pennie ML, Kolm P, Warshaw EM, Weisberg EL, Brown KM, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. Journal of General Internal Medicine 2006;21(7):678‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen LL, Liebman TN, Soriano RP, Dusza SW, Halpern AC, Marghoob AA. One‐year follow‐up of dermoscopy education on the ability of medical students to detect skin cancer. Dermatology 2013;226(3):267‐73. [DOI] [PubMed] [Google Scholar]
Chiaravalloti 2014 {published data only}
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. Journal of Clinical and Aesthetic Dermatology 2014;7(8):18‐22. [PMC free article] [PubMed] [Google Scholar]
Ciudad‐Blanco 2014 {published data only}
- Ciudad‐Blanco C, Aviles‐Izquierdo JA, Lazaro‐Ochaita P, Suarez‐Fernandez R. Dermoscopic findings for the early detection of melanoma: an analysis of 200 cases. Actas Dermo‐Sifiliograficas 2014;105(7):683‐93. [DOI] [PubMed] [Google Scholar]
Collas 1999 {published data only}
- Collas H, Delbarre M, Preville PA, Courville P, Neveu C, Dompmartin A, et al. Evaluation of the diagnosis of pigmented tumors of the skin and factors leading to a decision to excise [Evaluation du diagnostic des tumeurs pigmentées de la peau et des éléments conduisant à une décision d'exérèse]. Annales de Dermatologie et de Vénéréologie 1999;126(6‐7):494‐500. [ER4:21450600] [PubMed] [Google Scholar]
Coras 2003 {published data only}
- Coras B, Glaessl A, Kinateder J, Klovekorn W, Braun R, Lepski U, et al. Teledermatoscopy in daily routine‐‐results of the first 100 cases. Current Problems in Dermatology 2003;32:207‐12. [DOI] [PubMed] [Google Scholar]
Cornell 2015 {published data only}
- Cornell E, Robertson K, McIntosh RD, Rees JL. Viewing exemplars of melanomas and benign mimics of melanoma modestly improves diagnostic skills in comparison with the ABCD method and other image‐based methods for lay identification of melanoma. Acta Dermato‐Venereologica 2015;95(6):681‐5. [DOI] [PubMed] [Google Scholar]
Cox 2008 {published data only}
- Cox NH, Madan V, Sanders T. The U.K. skin cancer 'two‐week rule' proforma: assessment of potential modifications to improve referral accuracy. British Journal of Dermatology 2008;158(6):1293‐8. [DOI] [PubMed] [Google Scholar]
Cristofolini 1994 {published data only}
- Cristofolini M, Zumiani G, Bauer P, Cristofolini P, Boi S, Micciolo R. Dermatoscopy: usefulness in the differential diagnosis of cutaneous pigmentary lesions. Melanoma Research 1994;4(6):391‐4. [ER4:15465898; PUBMED: 7703719] [DOI] [PubMed] [Google Scholar]
Cristofolini 1997 {published data only}
- Cristofolini M, Bauer P, Boi S, Cristofolini P, Micciolo R, Sicher MC. Diagnosis of cutaneous melanoma: Accuracy of a computerized image analysis system (Skin View). Skin Research and Technology 1997;3(1):23‐7. [ER4:17941039; PUBMED: 27333169] [DOI] [PubMed] [Google Scholar]
Dal Pozzo 1999 {published data only}
- Dal Pozzo V, Benelli C, Roscetti E. The seven features for melanoma: a new dermoscopic algorithm for the diagnosis of malignant melanoma. European Journal of Dermatology 1999;9(4):303‐8. [ER4:18375041; PUBMED: 10356410] [PubMed] [Google Scholar]
DeCoste 1993 {published data only}
- DeCoste SD, Stern RS. Diagnosis and treatment of nevomelanocytic lesions of the skin: A community‐based study. Archives of Dermatology 1993;129(1):57‐62. [PubMed] [Google Scholar]
De Giorgi 2006 {published data only}
- Giorgi V, Trez E, Salvini C, Duquia R, Villa D, Sestini S, et al. Dermoscopy in black people. British Journal of Dermatology 2006;155(4):695‐9. [DOI] [PubMed] [Google Scholar]
De Giorgi 2011 {published data only}
- Giorgi V, Grazzini M, Rossari S, Gori A, Alfaioli B, Papi F, et al. Adding dermatoscopy to naked eye examination of equivocal melanocytic skin lesions: effect on intention to excise by general dermatologists. Clinical and Experimental Dermatology 2011;36(3):255‐9. [ER4:15465901; PUBMED: 21091756] [DOI] [PubMed] [Google Scholar]
De Giorgi 2012 {published data only}
- Giorgi V, Savarese I, Rossari S, Gori A, Grazzini M, Crocetti E, et al. Features of small melanocytic lesions: does small mean benign? A clinical‐dermoscopic study. Melanoma Research 2012;22(3):252‐6. [ER4:18375042; PUBMED: 22430838] [DOI] [PubMed] [Google Scholar]
Delfino 1997 {published data only}
- Delfino M, Fabbrocini G, Argenziano G, Magliocchetti N, Nofroni I. A statistical analysis of the characteristics of pigmented skin lesions using epiluminescence microscopy. Journal of the European Academy of Dermatology and Venereology 1997;9(3):243‐8. [Google Scholar]
De Troya‐Martin 2008 {published data only}
- Troya‐Martin M, Blazquez‐Sanchez N, Fernandez‐Canedo I, Frieyro‐Elicegui M, Funez‐Liebana R, Rivas‐Ruiz F. Dermoscopic study of cutaneous malignant melanoma: descriptive analysis of 45 cases. Actas Dermo‐Sifiliograficas 2008;99(1):44‐53. [PubMed] [Google Scholar]
Di Carlo 2014 {published data only}
- Carlo A, Elia F, Desiderio F, Catricala C, Solivetti FM, Laino L. Can video thermography improve differential diagnosis and therapy between basal cell carcinoma and actinic keratosis?. Dermatologic Therapy 2014;27(5):290‐7. [DOI] [PubMed] [Google Scholar]
Di Chiacchio 2010 {published data only}
- Chiacchio N, Hirata SH, Enokihara MY, Michalany NS, Fabbrocini G, Tosti A. Dermatologists' accuracy in early diagnosis of melanoma of the nail matrix. Archives of Dermatology 2010;146(4):382‐7. [DOI] [PubMed] [Google Scholar]
Di Meo 2016 {published data only}
- Meo N, Stinco G, Bonin S, Gatti A, Trevisini S, Damiani G, et al. CASH algorithm versus 3‐point checklist and its modified version in evaluation of melanocytic pigmented skin lesions: The 4‐point checklist. Journal of Dermatology 2016;43(6):682‐5. [ER4:25012343; PUBMED: 26589251] [DOI] [PubMed] [Google Scholar]
Di Stefani 2007 {published data only}
- Stefani A, Zalaudek I, Argenziano G, Chimenti S, Soyer HP. Feasibility of a two‐step teledermatologic approach for the management of patients with multiple pigmented skin lesions. Dermatologic Surgery 2007;33(6):686‐92. [DOI] [PubMed] [Google Scholar]
Dolianitis 2005 {published data only}
- Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Archives of Dermatology 2005;141(8):1008‐14. [ER4:15465906; PUBMED: 16103330] [DOI] [PubMed] [Google Scholar]
Dreiseitl 2009 {published data only}
- Dreiseitl S, Binder M, Hable K, Kittler H. Computer versus human diagnosis of melanoma: evaluation of the feasibility of an automated diagnostic system in a prospective clinical trial. Melanoma Research 2009;19(3):180‐4. [DOI] [PubMed] [Google Scholar]
Duff 2001 {published data only}
- Duff CG, Melsom D, Rigby HS, Kenealy JM, Townsend PL. A 6 year prospective analysis of the diagnosis of malignant melanoma in a pigmented‐lesion clinic: even the experts miss malignant melanomas, but not often. British Journal of Plastic Surgery 2001;54(4):317‐21. [DOI] [PubMed] [Google Scholar]
Dummer 1993 {published data only}
- Dummer W, Doehnel KA, Remy W. Videomicroscopy in differential diagnosis of skin tumors and secondary prevention of malignant melanoma. Hautarzt 1993;44(12):772‐6. [ER4:18375044; PUBMED: 8113040] [PubMed] [Google Scholar]
Dummer 1995 {published data only}
- Dummer W, Blaheta HJ, Bastian BC, Schenk T, Brocker EV, Remy W. Preoperative characterization of pigmented skin lesions by epiluminescence microscopy and high‐frequency ultrasound. Archives of Dermatology 1995;131(3):279‐85. [PubMed] [Google Scholar]
Edmondson 1999 {published data only}
- Edmondson PC, Curley RK, Marsden RA, Robinson D, Allaway SL, Willson CD. Screening for malignant melanoma using instant photography. Journal of Medical Screening 1999;6(1):42‐6. [DOI] [PubMed] [Google Scholar]
Elwan 2016 {published data only}
- Elwan NM, Eltatawy RA, Elfar NN, Elsakka OM. Dermoscopic features of acral pigmented lesions in Egyptian patients: a descriptive study. International Journal of Dermatology 2016;55(2):187‐92. [PUBMED: 26341359] [DOI] [PubMed] [Google Scholar]
Emmons 2011 {published data only}
- Emmons KM, Geller AC, Puleo E, Savadatti SS, Hu SW, Gorham S, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. Journal of the American Academy of Dermatology 2011;64(2):282‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engelberg 1999 {published data only}
- Engelberg D, Gallagher RP, Rivers JK. Follow‐up and evaluation of skin cancer screening in British Columbia. Journal of the American Academy of Dermatology 1999;41(1):37‐42. [DOI] [PubMed] [Google Scholar]
English 2003 {published data only}
- English DR, Burton RC, Mar CB, Donovan RJ, Ireland PD, Emery G. Evaluation of aid to diagnosis of pigmented skin lesions in general practice: controlled trial randomised by practice. BMJ 2003;327(7411):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
English 2004 {published data only}
- English DR, Mar C, Burton RC. Factors influencing the number needed to excise: excision rates of pigmented lesions by general practitioners. Medical Journal of Australia 2004;180(1):16‐9. [DOI] [PubMed] [Google Scholar]
Fabbrocini 2008 {published data only}
- Fabbrocini G, Balato A, Rescigno O, Mariano M, Scalvenzi M, Brunetti B. Telediagnosis and face‐to‐face diagnosis reliability for melanocytic and non‐melanocytic 'pink' lesions. Journal of the European Academy of Dermatology and Venereology 2008;22(2):229‐34. [DOI] [PubMed] [Google Scholar]
Feci 2015 {published data only}
- Feci L, Cevenini G, Nami N, Fagiolini A, Perotti R, Miracco C, et al. Influence of ambient stressors and time constraints on diagnostic accuracy of borderline pigmented skin lesions. Dermatology 2015;231(3):269‐73. [ER4:25012339] [DOI] [PubMed] [Google Scholar]
Federman 1995 {published data only}
- Federman D, Hogan D, Taylor JR, Caralis P, Kirsner RS. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. Journal of the American Academy of Dermatology 1995;32(5, Part 1):726‐9. [DOI] [PubMed] [Google Scholar]
Feldmann 1998 {published data only}
- Feldmann R, Fellenz C, Gschnait F. The ABCD rule in dermatoscopy: analysis of 500 melanocytic lesions. Hautarzt 1998;49(6):473‐6. [ER4:15465916; PUBMED: 9675574] [DOI] [PubMed] [Google Scholar]
Ferrara 2002 {published data only}
- Ferrara G, Argenziano G, Soyer HP, Corona R, Sera F, Brunetti B, et al. Dermoscopic and histopathologic diagnosis of equivocal melanocytic skin lesions: an interdisciplinary study on 107 cases. Cancer 2002;95(5):1094‐100. [DOI] [PubMed] [Google Scholar]
Ferrari 2015 {published data only}
- Ferrari B, Pupelli G, Farnetani F, Carvalho NT, Longo C, Reggiani C, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. Journal of the European Academy of Dermatology and Venereology 2015;29(6):1135‐40. [DOI: 10.1111/jdv.12769; ER4:20569458] [DOI] [PubMed] [Google Scholar]
Ferris 2015 {published data only}
- Ferris LK, Harkes JA, Gilbert B, Winger DG, Golubets K, Akilov O, et al. Computer‐aided classification of melanocytic lesions using dermoscopic images. Journal of the American Academy of Dermatology 2015;73(5):769‐76. [ER4:25012337; PUBMED: 26386631] [DOI] [PubMed] [Google Scholar]
Fidalgo 2003 {published data only}
- Fidalgo A, Caldas Lopes L, Macedo Ferreira A. Digital dermatoscopy: One‐year experience with the DANAOS system. Skin Cancer 2003;18(4):211‐8. [Google Scholar]
Fikrle 2013 {published data only}
- Fikrle T, Pizinger K, Szakos H, Panznerova P, Divisova B, Pavel S. Digital dermatoscopic follow‐up of 1027 melanocytic lesions in 121 patients at risk of malignant melanoma. Journal of the European Academy of Dermatology and Venereology 2013;27(2):180‐6. [DOI] [PubMed] [Google Scholar]
Freeman 1963 {published data only}
- Freeman RG, Knox JM. Clinical accuracy in diagnosis of skin tumors. Geriatrics 1963;18:546‐51. [PUBMED: 13959467] [PubMed] [Google Scholar]
Friedman 1985 {published data only}
- Friedman RJ, Rigel DS. The clinical features of malignant melanoma. Dermatologic Clinics 1985;3(2):271‐83. [PubMed] [Google Scholar]
Friedman 2008 {published data only}
- Friedman RJ, Gutkowicz‐Krusin D, Farber MJ, Warycha M, Schneider‐Kels L, Papastathis N, et al. The diagnostic performance of expert dermoscopists vs a computer‐vision system on small‐diameter melanomas. Archives of Dermatology 2008;144(4):476‐82. [ER4:15465921; PUBMED: 18427041] [DOI] [PubMed] [Google Scholar]
Fruhauf 2012 {published data only}
- Fruhauf J, Leinweber B, Fink‐Puches R, Ahlgrimm‐Siess V, Richtig E, Wolf IH, et al. Patient acceptance and diagnostic utility of automated digital image analysis of pigmented skin lesions. Journal of the European Academy of Dermatology and Venereology 2012;26(3):368‐72. [DOI] [PubMed] [Google Scholar]
Fueyo‐Casado 2009 {published data only}
- Fueyo‐Casado A, Vazquez‐Lopez F, Sanchez‐Martin J, Garcia‐Garcia B, Perez‐Oliva N. Evaluation of a program for the automatic dermoscopic diagnosis of melanoma in a general dermatology setting. Dermatologic Surgery 2009;35(2):257‐9; discussion 260‐2. [DOI] [PubMed] [Google Scholar]
Funt 1963 {published data only}
- Funt TR. Early recognition of cutaneous malignant melanoma in adults. Journal of the Florida Medical Association 1963;50:280‐2. [PubMed] [Google Scholar]
Gachon 2005 {published data only}
- Gachon J, Beaulieu P, Sei JF, Gouvernet J, Claudel JP, Lemaitre M, et al. First prospective study of the recognition process of melanoma in dermatological practice. Archives of Dermatology 2005;141(4):434‐8. [ER4:15465924; PUBMED: 15837860] [DOI] [PubMed] [Google Scholar]
Gerbert 1996 {published data only}
- Gerbert B, Maurer T, Berger T, Pantilat S, McPhee SJ, Wolff M, et al. Primary care physicians as gatekeepers in managed care: Primary care physicians' and dermatologists' skills at secondary prevention of skin cancer. Archives of Dermatology 1996;132(9):1030‐8. [PubMed] [Google Scholar]
Gerbert 1998 {published data only}
- Gerbert B, Bronstone A, Wolff M, Maurer T, Berger T, Pantilat S, et al. Improving primary care residents’ proficiency in the diagnosis of skin cancer. Journal of General Internal Medicine 1998;13(2):91‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gereli 2010 {published data only}
- Gereli MC, Onsun N, Atilganoglu U, Demirkesen C. Comparison of two dermoscopic techniques in the diagnosis of clinically atypical pigmented skin lesions and melanoma: seven‐point and three‐point checklists. International Journal of Dermatology 2010;49(1):33‐8. [ER4:15465929; PUBMED: 20465608] [DOI] [PubMed] [Google Scholar]
Giacomel 2005 {published data only}
- Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatologic Surgery 2005;31(12):1710‐3. [DOI] [PubMed] [Google Scholar]
Giacomel 2014 {published data only}
- Giacomel J, Lallas A, Zalaudek I, Argenziano G. Dermoscopic "signature" pattern of pigmented and nonpigmented lentigo maligna. Journal of the American Academy of Dermatology 2014;70(2):e33‐5. [DOI] [PubMed] [Google Scholar]
Giannotti 2004 {published data only}
- Giannotti B, Carli P. Improvement of early diagnosis of melanoma in a mediterranean population: The experience of the Florence melanoma clinic [Novita in tema di diagnosi precoce del melanoma cutaneo: L'esperienza del gruppo Fiorentino]. Giornale Italiano di Dermatologia e Venereologia 2004;139(2):89‐96. [Google Scholar]
Gill 2015 {published data only}
- Gill L, Wang S, Mancebo SE, Lim HW, Kohen LL. Dermoscopic features of acral melanocytic nevi in patients with skin types V and VI: A cross‐sectional study. Journal of the American Academy of Dermatology 2015;73(6):1059‐61. [DOI] [PubMed] [Google Scholar]
Gilmore 2009 {published data only}
- Gilmore S, Hofmann‐Wellenhof R, Muir J, Soyer HP. Lacunarity analysis: a promising method for the automated assessment of melanocytic naevi and melanoma. PLoS One 2009;4(10):e7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilmore 2010 {published data only}
- Gilmore S, Hofmann‐Wellenhof R, Soyer HP. A support vector machine for decision support in melanoma recognition. Experimental Dermatology 2010;19(9):830‐5. [ER4:15465935; PUBMED: 20629732] [DOI] [PubMed] [Google Scholar]
Glud 2009 {published data only}
- Glud M, Gniadecki R, Drzewiecki KT. Spectrophotometric intracutaneous analysis versus dermoscopy for the diagnosis of pigmented skin lesions: prospective, double‐blind study in a secondary reference centre. Melanoma Research 2009;19(3):176‐9. [ER4:18375045; PUBMED: 19319002] [DOI] [PubMed] [Google Scholar]
Grana 2003 {published data only}
- Grana C, Pellacani G, Cucchiara R, Seidenari S. A new algorithm for border description of polarized light surface microscopic images of pigmented skin lesions. IEEE Transactions on Medical Imaging 2003;22(8):959‐64. [DOI] [PubMed] [Google Scholar]
Green 1991 {published data only}
- Green A, Martin N, McKenzie G, Pfitzner J, Quintarelli F, Thomas BW, et al. Computer image analysis of pigmented skin lesions. Melanoma Research 1991;1(4):231‐6. [ER4:17941055; PUBMED: 1823631] [DOI] [PubMed] [Google Scholar]
Green 1994 {published data only}
- Green A, Martin N, Pfitzner J, O'Rourke M, Knight N. Computer image analysis in the diagnosis of melanoma. Journal of the American Academy of Dermatology 1994;31(6):958‐64. [ER4:15465938; PUBMED: 7962777] [DOI] [PubMed] [Google Scholar]
Grichnik 2003 {published data only}
- Grichnik JM. Dermoscopy of melanocytic neoplasms: subpatterns of dysplastic/atypical nevi. Archives of Dermatology 2003;139(12):1696. [DOI] [PubMed] [Google Scholar]
Grichnik 2004 {published data only}
- Grichnik JM. Dermoscopy of melanocytic neoplasms: familial patterns. Archives of Dermatology 2004;140(5):642. [DOI] [PubMed] [Google Scholar]
Grimaldi 2009 {published data only}
- Grimaldi L, Silvestri A, Brandi C, Nisi G, Brafa A, Calabro M, et al. Digital epiluminescence dermoscopy for pigmented cutaneous lesions, primary care physicians, and telediagnosis: a useful tool?. Journal of Plastic, Reconstructive & Aesthetic Surgery 2009;62(8):1054‐8. [ER4:15465940; PUBMED: 18547883] [DOI] [PubMed] [Google Scholar]
Grob 1998 {published data only}
- Grob JJ, Bonerandi JJ. The 'ugly duckling' sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Archives of Dermatology 1998;134(1):103‐4. [DOI] [PubMed] [Google Scholar]
Guibert 2000 {published data only}
- Guibert P, Mollat F, Ligen M, Dreno B. Melanoma screening: report of a survey in occupational medicine. Archives of Dermatology 2000;136(2):199‐202. [DOI] [PubMed] [Google Scholar]
Guillod 1996 {published data only}
- Guillod JF, Schmid P, Fischer S, Salomon D, Saurat JH. Detection and classification of pigmented skin lesions by dermatoscopic digital image processing. Dermatology 1996;193(2):169. [Google Scholar]
Gunduz 2003 {published data only}
- Gunduz K, Koltan S, Sahin MT, E Filiz E. Analysis of melanocytic naevi by dermoscopy during pregnancy. Journal of the European Academy of Dermatology and Venereology 2003;17(3):349‐51. [DOI] [PubMed] [Google Scholar]
Gutierrez 2013 {published data only}
- Gutierrez R, Rueda A, Romero E. Learning semantic histopathological representation for basal cell carcinoma classification. SPIE Proceedings: Medical Imaging 2013: Digital Pathology. 29 March 2013; Vol. 8676. [DOI: 10.1117/12.2007117] [DOI]
Haenssle 2006 {published data only}
- Haenssle HA, Krueger U, Vente C, Thoms KM, Bertsch HP, Zutt M, et al. Results from an observational trial: digital epiluminescence microscopy follow‐up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. Journal of Investigative Dermatology 2006;126(5):980‐5. [DOI] [PubMed] [Google Scholar]
Haenssle 2010a {published data only}
- Haenssle HA, Korpas B, Hansen‐Hagge C, Buhl T, Kaune KM, Rosenberger A, et al. Seven‐point checklist for dermatoscopy: performance during 10 years of prospective surveillance of patients at increased melanoma risk. Journal of the American Academy of Dermatology 2010;62(5):785‐93. [DOI] [PubMed] [Google Scholar]
Haenssle 2010b {published data only}
- Haenssle HA, Korpas B, Hansen‐Hagge C, Buhl T, Kaune KM, Johnsen S, et al. Selection of patients for long‐term surveillance with digital dermoscopy by assessment of melanoma risk factors. Archives of Dermatology 2010;146(3):257‐64. [DOI] [PubMed] [Google Scholar]
Hallock 1998 {published data only}
- Hallock GG, Lutz DA. Prospective study of the accuracy of the surgeon's diagnosis in 2000 excised skin tumors. Plastic and Reconstructive Surgery 1998;101(5):1255‐61. [DOI] [PubMed] [Google Scholar]
Haniffa 2007 {published data only}
- Haniffa MA, Lloyd JJ, Lawrence CM. The use of a spectrophotometric intracutaneous analysis device in the real‐time diagnosis of melanoma in the setting of a melanoma screening clinic. British Journal of Dermatology 2007;156(6):1350‐2. [DOI] [PubMed] [Google Scholar]
Har‐Shai 2001 {published data only}
- Har‐Shai Y, Hai N, Taran A, Mayblum S, Barak A, Tzur E, et al. Sensitivity and positive predictive values of presurgical clinical diagnosis of excised benign and malignant skin tumors: a prospective study of 835 lesions in 778 patients. Plastic and Reconstructive Surgery 2001;108(7):1982‐9. [DOI] [PubMed] [Google Scholar]
Haspeslagh 2016 {published data only}
- Haspeslagh M, Vossaert K, Lanssens S, Noe M, Hoorens I, Chevolet I, et al. Comparison of ex vivo and in vivo dermoscopy in dermatopathologic evaluation of skin tumors. JAMA Dermatology 2016;152(3):312‐7. [DOI] [PubMed] [Google Scholar]
Hauschild 2014 {published data only}
- Hauschild A, Chen SC, Weichenthal M, Blum A, King HC, Goldsmith J, et al. To excise or not: impact of MelaFind on German dermatologists' decisions to biopsy atypical lesions. Journal der Deutschen Dermatologischen Gesellschaft 2014;12(7):606‐14. [ER4:17941085; PUBMED: 24944011] [DOI] [PubMed] [Google Scholar]
Heal 2008 {published data only}
- Heal CF, Raasch BA, Buettner PG, Weedon D. Accuracy of clinical diagnosis of skin lesions. British Journal of Dermatology 2008;159(3):661‐8. [DOI] [PubMed] [Google Scholar]
Healsmith 1994 {published data only}
- Healsmith MF, Bourke JF, Osborne JE, Graham‐Brown RA. An evaluation of the revised seven‐point checklist for the early diagnosis of cutaneous malignant melanoma. British Journal of Dermatology 1994;130(1):48‐50. [DOI] [PubMed] [Google Scholar]
Henning 2007 {published data only}
- Henning JS, Dusza SW, Wang SQ, Marghoob AA, Rabinovitz HS, Polsky D, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. Journal of the American Academy of Dermatology 2007;56(1):45‐52. [DOI] [PubMed] [Google Scholar]
Henning 2008 {published data only}
- Henning JS, Stein JA, Yeung J, Dusza SW, Marghoob AA, Rabinovitz HS, et al. CASH algorithm for dermoscopy revisited. Archives of Dermatology 2008;144(4):554‐5. [PUBMED: 18427058] [DOI] [PubMed] [Google Scholar]
Herschorn 2012 {published data only}
- Herschorn A. Dermoscopy for melanoma detection in family practice. Canadian Family Physician 2012;58(7):740‐5, e372‐8. [PMC free article] [PubMed] [Google Scholar]
Higgins 1992 {published data only}
- Higgins EM, Hall P, Todd P, Murthi R, Du Vivier AW. The application of the seven‐point check‐list in the assessment of benign pigmented lesions. Clinical and Experimental Dermatology 1992;17(5):313‐5. [DOI] [PubMed] [Google Scholar]
Hirata 2011 {published data only}
- Hirata SH, Yamada S, Enokihara MY, Chiacchio N, Almeida FA, Enokihara MM, et al. Patterns of nail matrix and bed of longitudinal melanonychia by intraoperative dermatoscopy. Journal of the American Academy of Dermatology 2011;65(2):297‐303. [PUBMED: 21531039] [DOI] [PubMed] [Google Scholar]
Hoffmann 2003 {published data only}
- Hoffmann K, Gambichler T, Rick A, Kreutz M, Anschuetz M, Grunendick T, et al. Diagnostic and neural analysis of skin cancer (DANAOS). A multicentre study for collection and computer‐aided analysis of data from pigmented skin lesions using digital dermoscopy. British Journal of Dermatology 2003;149(4):801‐9. [DOI] [PubMed] [Google Scholar]
Hoorens 2016 {published data only}
- Hoorens I, Vossaert K, Pil L, Boone B, Schepper S, Ongenae K, et al. Total‐body examination vs lesion‐directed skin cancer screening. JAMA Dermatology 2016;152(1):27‐34. [DOI] [PubMed] [Google Scholar]
Huang 1996 {published data only}
- Huang CL, Wasti Q, Marghoob AA, Kopf AW, David M, Rao BK, et al. Border irregularity: Atypical moles versus melanoma. European Journal of Dermatology 1996;6(4):270‐3. [Google Scholar]
Hubener 1956 {published data only}
- Hubener LF, McMullan FH. Malignant melanoma: A statistical review of clinical and histological diagnoses. A.M.A. Archives of Dermatology 1956;74(6):618‐9. [PUBMED: 13371918] [PubMed] [Google Scholar]
Ishioka 2009 {published data only}
- Ishioka P, Tenorio JM, Lopes PR, Yamada S, Michalany NS, Amaral MB, et al. A comparative study of teledermatoscopy and face‐to‐face examination of pigmented skin lesions. Journal of Telemedicine and Telecare 2009;15(5):221‐5. [DOI] [PubMed] [Google Scholar]
Iyatomi 2006 {published data only}
- Iyatomi H, Oka H, Saito M, Miyake A, Kimoto M, Yamagami J, et al. Quantitative assessment of tumour extraction from dermoscopy images and evaluation of computer‐based extraction methods for an automatic melanoma diagnostic system. Melanoma Research 2006;16(2):183‐90. [DOI] [PubMed] [Google Scholar]
Iyatomi 2008 {published data only}
- Iyatomi H, Oka H, Celebi ME, Ogawa K, Argenziano G, Soyer HP, et al. Computer‐based classification of dermoscopy images of melanocytic lesions on acral volar skin. Journal of Investigative Dermatology 2008;128(8):2049‐54. [DOI] [PubMed] [Google Scholar]
Jamora 2003 {published data only}
- Jamora MJ, Wainwright BD, Meehan SA, Bystryn JC. Improved identification of potentially dangerous pigmented skin lesions by computerized image analysis. Archives of Dermatology 2003;139(2):195‐8. [DOI] [PubMed] [Google Scholar]
Janda 2014 {published data only}
- Janda M, Loescher LJ, Banan P, Horsham C, Soyer HP. Lesion selection by melanoma high‐risk consumers during skin self‐examination using mobile teledermoscopy. JAMA Dermatology 2014;150(6):656‐8. [DOI] [PubMed] [Google Scholar]
Jensen 2015 {published data only}
- Jensen JD, Elewski BE. The ABCDEF rule: Combining the 'ABCDE rule' and the "ugly duckling sign" in an effort to improve patient self‐screening examinations. Journal of Clinical and Aesthetic Dermatology 2015;8(2):15. [PMC free article] [PubMed] [Google Scholar]
Johr 2002 {published data only}
- Johr RH. Dermoscopy: alternative melanocytic algorithms‐the ABCD rule of dermatoscopy, Menzies scoring method, and 7‐point checklist. Clinics in Dermatology 2002;20(3):240‐7. [DOI] [PubMed] [Google Scholar]
Jolliffe 2001 {published data only}
- Jolliffe VM, Harris DW, Whittaker SJ. Can we safely diagnose pigmented lesions from stored video images? A diagnostic comparison between clinical examination and stored video images of pigmented lesions removed for histology. Clinical and Experimental Dermatology 2001;26(1):84‐7. [DOI] [PubMed] [Google Scholar]
Jonna 1998 {published data only}
- Jonna BP, Delfino RJ, Newman WG, Tope WD. Positive predictive value for presumptive diagnoses of skin cancer and compliance with follow‐up among patients attending a community screening program. Preventive Medicine 1998;27(4):611‐6. [DOI] [PubMed] [Google Scholar]
Kaddu 1997 {published data only}
- Kaddu S, Soyer HP, Wolf IH, Rieger E, Kerl H. Reticular lentigo. Hautarzt 1997;48(3):181‐5. [DOI] [PubMed] [Google Scholar]
Kawabata 1998 {published data only}
- Kawabata Y, Tamaki K. Distinctive dermatoscopic features of acral lentiginous melanoma in situ from plantar melanocytic nevi and their histopathologic correlation. Journal of Cutaneous Medicine and Surgery 1998;2(4):199‐204. [DOI] [PubMed] [Google Scholar]
Kawabata 2001 {published data only}
- Kawabata Y, Ohara K, Hino H, Tamaki K. Two kinds of Hutchinson's sign, benign and malignant. Journal of the American Academy of Dermatology 2001;44(2, Part 1):305‐7. [DOI] [PubMed] [Google Scholar]
Keefe 1990 {published data only}
- Keefe M, Dick DC, Wakeel RA. A study of the value of the seven‐point checklist in distinguishing benign pigmented lesions from melanoma. Clinical and Experimental Dermatology 1990;15(3):167‐71. [DOI] [PubMed] [Google Scholar]
Kefel 2012 {published data only}
- Kefel S, Guvenc P, LeAnder R, Stricklin SM, Stoecker WV. Discrimination of basal cell carcinoma from benign lesions based on extraction of ulcer features in polarized‐light dermoscopy images. Skin Research and Technology 2012;18(4):471‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 1986 {published data only}
- Kelly JW, Crutcher WA, Sagebiel RW. Clinical diagnosis of dysplastic melanocytic nevi. A clinicopathologic correlation. Journal of the American Academy of Dermatology 1986;14(6):1044‐52. [DOI] [PubMed] [Google Scholar]
Kenet 1994 {published data only}
- Kenet RO, Fitzpatrick TB. Reducing mortality and morbidity of cutaneous melanoma: a six year plan. B). Identifying high and low risk pigmented lesions using epiluminescence microscopy. Journal of Dermatology 1994;21(11):881‐4. [DOI] [PubMed] [Google Scholar]
Kittler 1998 {published data only}
- Kittler H, Seltenheim M, Pehamberger H, Wolff K, Binder M. Diagnostic informativeness of compressed digital epiluminescence microscopy images of pigmented skin lesions compared with photographs. Melanoma Research 1998;8(3):255‐60. [ER4:17941060; PUBMED: 9664147] [DOI] [PubMed] [Google Scholar]
Kittler 1999 {published data only}
- Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Morphologic changes of pigmented skin lesions: a useful extension of the ABCD rule for dermatoscopy. Journal of the American Academy of Dermatology 1999;40(4):558‐62. [ER4:15465976; PUBMED: 10188673] [DOI] [PubMed] [Google Scholar]
Kittler 2001 {published data only}
- Kittler H, Binder M. Risks and benefits of sequential imaging of melanocytic skin lesions in patients with multiple atypical nevi. Archives of Dermatology 2001;137(12):1590‐5. [ER4:20569472; PUBMED: 11735709] [DOI] [PubMed] [Google Scholar]
Kittler 2002 {published data only}
- Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncology 2002;3(3):159‐65. [DOI] [PubMed] [Google Scholar]
Kittler 2006 {published data only}
- Kittler H. Value of follow‐up of pigmented skin lesions by digital dermatoscopy. Journal of Investigative Dermatology 2006;126(Suppl 2):S20. [Google Scholar]
Koga 2011 {published data only}
- Koga H, Saida T. Revised 3‐step dermoscopic algorithm for the management of acral melanocytic lesions. Archives of Dermatology 2011;147(6):741‐3. [DOI] [PubMed] [Google Scholar]
Koh 1990 {published data only}
- Koh HK, Caruso A, Gage I, Geller AC, Prout MN, White H, et al. Evaluation of melanoma/skin cancer screening in Massachusetts. Preliminary results. Cancer 1990;65(2):375‐9. [DOI] [PubMed] [Google Scholar]
Kopf 1975 {published data only}
- Kopf AW, Mintzis M, Bart RS. DIagnostic accuracy in malignant melanoma. Archives of Dermatology 1975;111(10):1291‐2. [DOI: 10.1001/archderm.1975.01630220055001; ER4:21450617] [DOI] [PubMed] [Google Scholar]
Korotkov 2012 {published data only}
- Korotkov K, Garcia R. Computerized analysis of pigmented skin lesions: a review. Artificial Intelligence in Medicine 2012;56(2):69‐90. [DOI] [PubMed] [Google Scholar]
Krahn 1998 {published data only}
- Krahn G, Gottlober P, Sander C, Peter RU. Dermatoscopy and high frequency sonography: two useful non‐invasive methods to increase preoperative diagnostic accuracy in pigmented skin lesions. Pigment Cell Research 1998;11(3):151‐4. [ER4:15465981; PUBMED: 9730322] [DOI] [PubMed] [Google Scholar]
Kreusch 1992 {published data only}
- Kreusch J, Rassner G, Trahn C, Pietsch‐Breitfeld B, Henke D, Selbmann HK. Epiluminescent microscopy: a score of morphological features to identify malignant melanoma. Pigment Cell Research 1992;Suppl 2:295‐8. [PUBMED: 1409432] [DOI] [PubMed] [Google Scholar]
Kroemer 2011 {published data only}
- Kroemer S, Fruhauf J, Campbell TM, Massone C, Schwantzer G, Soyer HP, et al. Mobile teledermatology for skin tumour screening: diagnostic accuracy of clinical and dermoscopic image tele‐evaluation using cellular phones. British Journal of Dermatology 2011;164(5):973‐9. [DOI] [PubMed] [Google Scholar]
Krol 1991 {published data only}
- Krol S, Keijser LM, Rhee HJ, Welvaart K. Screening for skin cancer in The Netherlands. Acta Dermato‐Venereologica 1991;71(4):317‐21. [PubMed] [Google Scholar]
Kurvers 2015 {published data only}
- Kurvers RH, Krause J, Argenziano G, Zalaudek I, Wolf M. Detection accuracy of collective intelligence assessments for skin cancer diagnosis. JAMA Dermatology 2015;151(12):1346‐53. [DOI] [PubMed] [Google Scholar]
Kvedar 1997 {published data only}
- Kvedar JC, Edwards RA, Menn ER, Mofid M, Gonzalez E, Dover J, et al. The substitution of digital images for dermatologic physical examination. Archives of Dermatology 1997;133(2):161‐7. [PUBMED: 9041828] [PubMed] [Google Scholar]
Lallas 2015 {published data only}
- Lallas A, Kyrgidis A, Koga H, Moscarella E, Tschandl P, Apalla Z, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. British Journal of Dermatology 2015;173(4):1041‐9. [DOI] [PubMed] [Google Scholar]
Langley 2001 {published data only}
- Langley RG, Rajadhyaksha M, Dwyer PJ, Sober AJ, Flotte TJ, Anderson RR. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. Journal of the American Academy of Dermatology 2001;45(3):365‐76. [DOI: ; ER4:20569473; PUBMED: 11511832] [DOI] [PubMed] [Google Scholar]
Langley 2007 {published data only}
- Langley RG, Walsh N, Sutherland AE, Propperova I, Delaney L, Morris SF, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology 2007;215(4):365‐72. [ER4:15465985; PUBMED: 17912001] [DOI] [PubMed] [Google Scholar]
Lechner 2015 {published data only}
- Lechner SC, Pereira LC, Reategui E, Gordon C, Byrne M, Hooper MW, et al. Erratum to : Acceptability of a rinse screening test for diagnosing head and neck squamous cell carcinoma among black Americans. Journal of Racial and Ethnic Health Disparities 2015;2(Numb 1):68. [DOI] [PubMed] [Google Scholar]
Lewis 1999 {published data only}
- Lewis K, Gilmour E, Harrison PV, Patefield S, Dickinson Y, Manning D, et al. Digital teledermatology for skin tumours: a preliminary assessment using a receiver operating characteristics (ROC) analysis. Journal of Telemedicine and Telecare 1999;5(Suppl 1):S57‐8. [DOI] [PubMed] [Google Scholar]
Liebman 2011 {published data only}
- Liebman TN, Scope A, Rabinovitz H, Braun RP, Marghoob AA. Rosettes may be observed in a range of conditions. Archives of Dermatology 2011;147(12):1468. [DOI] [PubMed] [Google Scholar]
Liebman 2012 {published data only}
- Liebman TN, Rabinovitz HS, Balagula Y, Jaimes‐Lopez N, Marghoob AA. White shiny structures in melanoma and BCC. Archives of Dermatology 2012;148(1):146. [DOI] [PubMed] [Google Scholar]
Lindelöf 1994 {published data only}
- Lindelöf B, Hedblad MA. Accuracy in the clinical diagnosis and pattern of malignant melanoma at a dermatological clinic. Journal of Dermatology 1994;21(7):461‐4. [DOI] [PubMed] [Google Scholar]
Lipoff 2008 {published data only}
- Lipoff JB, Scope A, Dusza SW, Marghoob AA, Oliveria SA, Halpern AC. Complex dermoscopic pattern: a potential risk marker for melanoma. British Journal of Dermatology 2008;158(4):821‐4. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu Z, Sun J, Smith L, Smith M, Warr R. Distribution quantification on dermoscopy images for computer‐assisted diagnosis of cutaneous melanomas. Medical & Biological Engineering & Computing 2012;50(5):503‐13. [DOI] [PubMed] [Google Scholar]
Lorentzen 2000 {published data only}
- Lorentzen H, Weismann K, Kenet RO, Secher L, Larsen FG. Comparison of dermatoscopic ABCD rule and risk stratification in the diagnosis of malignant melanoma. Acta Dermato‐Venereologica 2000;80(2):122‐6. [PubMed] [Google Scholar]
Luttrell 2012 {published data only}
- Luttrell MJ, McClenahan P, Hofmann‐Wellenhof R, Fink‐Puches R, Soyer HP. Laypersons' sensitivity for melanoma identification is higher with dermoscopy images than clinical photographs. British Journal of Dermatology 2012;167(5):1037‐41. [DOI] [PubMed] [Google Scholar]
Machet 2005 {published data only}
- Machet L, Nemeth‐Normand F, Giraudeau B, Perrinaud A, Tiguemounine J, Ayoub J, et al. Is ultrasound lymph node examination superior to clinical examination in melanoma follow‐up? A monocentre cohort study of 373 patients. British Journal of Dermatology 2005;152(1):66‐70. [DOI] [PubMed] [Google Scholar]
MacKenzie‐Wood 1998 {published data only}
- MacKenzie‐Wood AR, Milton GW, Launey JW. Melanoma: accuracy of clinical diagnosis. Australasian Journal of Dermatology 1998;39(1):31‐3. [DOI] [PubMed] [Google Scholar]
MacKie 1971 {published data only}
- MacKie RM. An aid to the preoperative assessment of pigmented lesions of the skin. British Journal of Dermatology 1971;85(3):232‐8. [PUBMED: 5111687] [DOI] [PubMed] [Google Scholar]
MacKie 1990 {published data only}
- MacKie RM. Clinical recognition of early invasive malignant melanoma. BMJ 1990;301(6759):1005‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKie 1991 {published data only}
- MacKie RM, Doherty VR. Seven‐point checklist for melanoma. Clinical and Experimental Dermatology 1991;16(2):151‐3. [DOI] [PubMed] [Google Scholar]
MacKie 2002 {published data only}
- MacKie RM, Fleming C, McMahon AD, Jarrett P. The use of the dermatoscope to identify early melanoma using the three‐colour test. British Journal of Dermatology 2002;146(3):481‐4. [DOI] [PubMed] [Google Scholar]
Mahendran 2005 {published data only}
- Mahendran R, Goodfield MJ, Sheehan‐Dare RA. An evaluation of the role of a store‐and‐forward teledermatology system in skin cancer diagnosis and management. Clinical and Experimental Dermatology 2005;30(3):209‐14. [DOI] [PubMed] [Google Scholar]
Mahon 1997 {published data only}
- Mahon SM. A comparison of findings from two checklists for the early detection of skin cancer. Missouri Nurse 1997;66(2):12. [PubMed] [Google Scholar]
Malvehy 2014 {published data only}
- Malvehy J, Hauschild A, Curiel‐Lewandrowski C, Mohr P, Hofmann‐Wellenhof R, Motley R, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: An international, multicentre, prospective and blinded clinical trial on efficacy and safety. British Journal of Dermatology 2014;171(5):1099‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marghoob 1995 {published data only}
- Marghoob AA, Slade J, Kopf AW, Rigel DS, Friedman RJ, Perelman RO. The ABCDs of melanoma: why change?. Journal of the American Academy of Dermatology 1995;32(4):682‐4. [DOI] [PubMed] [Google Scholar]
Marghoob 2007 {published data only}
- Marghoob AA, Korzenko AJ, Changchien L, Scope A, Braun RP, Rabinovitz H. The beauty and the beast sign in dermoscopy. Dermatologic Surgery 2007;33(11):1388‐91. [DOI] [PubMed] [Google Scholar]
Marghoob 2010 {published data only}
- Marghoob AA, Braun R. Proposal for a revised 2‐step algorithm for the classification of lesions of the skin using dermoscopy. Archives of Dermatology 2010;146(4):426‐8. [PUBMED: 20404234] [DOI] [PubMed] [Google Scholar]
Massi 2001 {published data only}
- Massi D, Giorgi V, Carli P, Santucci M. Diagnostic significance of the blue hue in dermoscopy of melanocytic lesions: a dermoscopic‐pathologic study. American Journal of Dermatopathology 2001;23(5):463‐9. [DOI] [PubMed] [Google Scholar]
Mayer 1997 {published data only}
- Mayer J. Systematic review of the diagnostic accuracy of dermatoscopy in detecting malignant melanoma. Medical Journal of Australia 1997;167(4):206‐10. [DOI] [PubMed] [Google Scholar]
McCarthy 1995 {published data only}
- McCarthy JT. ABCDs of melanoma. Cutis 1995;56(6):313. [PubMed] [Google Scholar]
McGovern 1992 {published data only}
- McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions. A comparison of the Glasgow seven‐point checklist and the American Cancer Society's ABCDs of pigmented lesions. Journal of Dermatologic Surgery and Oncology 1992;18(1):22‐6. [ER4:18375119; PUBMED: 1740563] [DOI] [PubMed] [Google Scholar]
Menzies 1996a {published data only}
- Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Archives of Dermatology 1996;132(10):1178‐82. [ER4:21450627; PUBMED: 8859028] [PubMed] [Google Scholar]
Menzies 1996b {published data only}
- Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Research 1996;6(1):55‐62. [DOI] [PubMed] [Google Scholar]
Menzies 1999 {published data only}
- Menzies SW. Automated epiluminescence microscopy: human vs machine in the diagnosis of melanoma. Archives of Dermatology 1999;135(12):1538‐40. [DOI] [PubMed] [Google Scholar]
Menzies 2001 {published data only}
- Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short‐term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Archives of Dermatology 2001;137(12):1583‐9. [DOI] [PubMed] [Google Scholar]
Menzies 2005 {published data only}
- Menzies SW, Bischof L, Talbot H, Gutenev A, Avramidis M, Wong L, et al. The performance of SolarScan: an automated dermoscopy image analysis instrument for the diagnosis of primary melanoma.[Erratum appears in Arch Dermatol. 2006 May;142(5):558 Note: Virol, Alexandra [corrected to Varol, Alexandra]]. Archives of Dermatology 2005;141(11):1388‐96. [ER4:20569478; PUBMED: 16301386] [DOI] [PubMed] [Google Scholar]
Menzies 2008 {published data only}
- Menzies SW, Kreusch J, Byth K, Pizzichetta MA, Marghoob A, Braun R, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Archives of Dermatology 2008;144(9):1120‐7. [DOI] [PubMed] [Google Scholar]
Menzies 2009 {published data only}
- Menzies SW, Emery J, Staples M, Davies S, McAvoy B, Fletcher J, et al. Impact of dermoscopy and short‐term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. British Journal of Dermatology 2009;161(6):1270‐7. [DOI: 10.1111/j.1365-2133.2009.09374.x; ER4:15466005; PUBMED: 19747359] [DOI] [PubMed] [Google Scholar]
Menzies 2011 {published data only}
- Menzies SW, Stevenson ML, Altamura D, Byth K. Variables predicting change in benign melanocytic nevi undergoing short‐term dermoscopic imaging. Archives of Dermatology 2011;147(6):655‐9. [DOI] [PubMed] [Google Scholar]
Menzies 2013 {published data only}
- Menzies SW, Moloney FJ, Byth K, Avramidis M, Argenziano G, Zalaudek I, et al. Dermoscopic evaluation of nodular melanoma. JAMA Dermatology 2013;149(6):699‐709. [DOI] [PubMed] [Google Scholar]
Moffatt 2006 {published data only}
- Moffatt CR, Green AC, Whiteman DC. Diagnostic accuracy in skin cancer clinics: the Australian experience. International Journal of Dermatology 2006;45(6):656‐60. [DOI] [PubMed] [Google Scholar]
Mohammad 2015 {published data only}
- Mohammad EA, Mansour M, Parichehr K, Farideh D, Amirhossein R, Ahmad SA. Assessment of clinical diagnostic accuracy compared with pathological diagnosis of basal cell carcinoma. Indian Dermatology Online Journal 2015;6(4):258‐62. [PUBMED: 26225330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morales Callaghan 2008 {published data only}
- Morales‐Callaghan AM, Castrodeza‐Sanz J, Martinez‐Garcia G, Peral‐Martinez I, Miranda‐Romero A. Correlation between clinical, dermatoscopic, and histopathologic variables in atypical melanocytic nevi. Actas Dermo‐Sifiliograficas 2008;99(5):380‐9. [ER4:17941068; PUBMED: 18501170] [PubMed] [Google Scholar]
Morrison 2001 {published data only}
- Morrison A, O'Loughlin S, Powell FC. Suspected skin malignancy: a comparison of diagnoses of family practitioners and dermatologists in 493 patients. International Journal of Dermatology 2001;40(2):104‐7. [DOI] [PubMed] [Google Scholar]
Morton 1998 {published data only}
- Morton CA, MacKie RM. Clinical accuracy of the diagnosis of cutaneous malignant melanoma. British Journal of Dermatology 1998;138(2):283‐7. [ER4:20569481; PUBMED: 9602875] [DOI] [PubMed] [Google Scholar]
Mun 2016 {published data only}
- Mun JH, Ohn J, Kim WI, Park SM, Kim MB. Dermoscopy of melanomas on the trunk and extremities in Asians.(Erratum appears in PLoS One. 2016;11(8):e0161419). PLoS One 2016;11(7):e0158374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nachbar 1994 {published data only}
- Nachbar F, Stolz W, Merkle T, Cognetta AB, Vogt T, Landthaler M, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. Journal of the American Academy of Dermatology 1994;30(4):551‐9. [ER4:15466022; PUBMED: 8157780] [DOI] [PubMed] [Google Scholar]
Nathansohn 2007 {published data only}
- Nathansohn N, Orenstein A, Trau H, Liran A, Schachter J. Pigmented lesions clinic for early detection of melanoma: preliminary results. Israel Medical Association Journal 2007;9(10):708‐12. [PubMed] [Google Scholar]
Nilles 1994 {published data only}
- Nilles M, Boedeker RH, Schill WB. Surface microscopy of naevi and melanomas‐‐clues to melanoma. British Journal of Dermatology 1994;130(3):349‐55. [DOI] [PubMed] [Google Scholar]
Osborne 1998 {published data only}
- Osborne JE, Bourke JF, Holder J, Colloby P, Graham‐Brown RA. The effect of the introduction of a pigmented lesion clinic on the interval between referral by family practitioner and attendance at hospital. British Journal of Dermatology 1998;138(3):418‐21. [DOI] [PubMed] [Google Scholar]
Osborne 1999 {published data only}
- Osborne JE, Bourke JF, Graham‐Brown RA, Hutchinson PE. False negative clinical diagnoses of malignant melanoma. British Journal of Dermatology 1999;140(5):902‐8. [DOI] [PubMed] [Google Scholar]
Pagnanelli 2003 {published data only}
- Pagnanelli G, Soyer HP, Argenziano G, Talamini R, Barbati R, Bianchi L, et al. Diagnosis of pigmented skin lesions by dermoscopy: web‐based training improves diagnostic performance of non‐experts. British Journal of Dermatology 2003;148(4):698‐702. [ER4:15466036; PUBMED: 12752126] [DOI] [PubMed] [Google Scholar]
Pan 2008 {published data only}
- Pan Y, Chamberlain AJ, Bailey M, Chong AH, Haskett M, Kelly JW. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque‐features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. Journal of the American Academy of Dermatology 2008;59(2):268‐74. [DOI] [PubMed] [Google Scholar]
Panasiti 2009 {published data only}
- Panasiti V, Devirgiliis V, Curzio M, Roberti V, Gobbi S, Masciangelo R, et al. The reticular point of view in dermatoscopy. Journal of the American Academy of Dermatology 2009;61(4):605‐10. [DOI] [PubMed] [Google Scholar]
Parslew 1997 {published data only}
- Parslew RA, Rhodes LE. Accuracy of diagnosis of benign skin lesions in hospital practice: a comparison of clinical and histological findings. Journal of the European Academy of Dermatology and Venereology 1997;9(2):137‐41. [Google Scholar]
Pazzini 1996 {published data only}
- Pazzini C, Pozzi M, Betti R, Vergani R, Crosti C. Improvement of diagnostic accuracy in the clinical diagnosis of pigmented skin lesions by epiluminescence microscopy. Skin Cancer 1996;11(2):159‐61. [Google Scholar]
Pehamberger 1987 {published data only}
- Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. Journal of the American Academy of Dermatology 1987;17(4):571‐83. [DOI] [PubMed] [Google Scholar]
Pellacani 2002 {published data only}
- Pellacani G, Seidenari S. Comparison between morphological parameters in pigmented skin lesion images acquired by means of epiluminescence surface microscopy and polarized‐light videomicroscopy. Clinics in Dermatology 2002;20(3):222‐7. [DOI] [PubMed] [Google Scholar]
Pellacani 2006 {published data only}
- Pellacani G, Grana C, Seidenari S. Algorithmic reproduction of asymmetry and border cut‐off parameters according to the ABCD rule for dermoscopy. Journal of the European Academy of Dermatology & Venereology 2006;20(10):1214‐9. [DOI] [PubMed] [Google Scholar]
Pellacani 2007 {published data only}
- Pellacani G, Bassoli S, Longo C, Cesinaro AM, Seidenari S. Diving into the blue: in vivo microscopic characterization of the dermoscopic blue hue. Journal of the American Academy of Dermatology 2007;57(1):96‐104. [DOI] [PubMed] [Google Scholar]
Pellacani 2009 {published data only}
- Pellacani G, Longo C, Ferrara G, Cesinaro AM, Bassoli S, Guitera P, et al. Spitz nevi: In vivo confocal microscopic features, dermatoscopic aspects, histopathologic correlates, and diagnostic significance. Journal of the American Academy of Dermatology 2009;60(2):236‐47. [DOI] [PubMed] [Google Scholar]
Perednia 1992 {published data only}
- Perednia DA, Gaines JA, Rossum AC. Variability in physician assessment of lesions in cutaneous images and its implications for skin screening and computer‐assisted diagnosis. Archives of Dermatology 1992;128(3):357‐64. [PubMed] [Google Scholar]
Peris 2002 {published data only}
- Peris K, Altobelli E, Ferrari A, Fargnoli MC, Piccolo D, Esposito M, et al. Interobserver agreement on dermoscopic features of pigmented basal cell carcinoma. Dermatologic Surgery 2002;28(7):643‐5. [DOI] [PubMed] [Google Scholar]
Perrinaud 2007 {published data only}
- Perrinaud A, Gaide O, French LE, Saurat JH, Marghoob AA, Braun RP. Can automated dermoscopy image analysis instruments provide added benefit for the dermatologist? A study comparing the results of three systems. British Journal of Dermatology 2007;157(5):926‐33. [DOI] [PubMed] [Google Scholar]
Phan 2010 {published data only}
- Phan A, Dalle S, Touzet S, Ronger‐Savle S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. British Journal of Dermatology 2010;162(4):765‐71. [DOI] [PubMed] [Google Scholar]
Piccolo 2000 {published data only}
- Piccolo D, Smolle J, Argenziano G, Wolf IH, Braun R, Cerroni L, et al. Teledermoscopy‐‐results of a multicentre study on 43 pigmented skin lesions. Journal of Telemedicine and Telecare 2000;6(3):132‐7. [DOI] [PubMed] [Google Scholar]
Piccolo 2002 {published data only}
- Piccolo D, Peris K, Chimenti S, Argenziano G, Soyer HP. Jumping into the future using teledermoscopy. SKINmed 2002;1(1):20‐4. [DOI] [PubMed] [Google Scholar]
Piccolo 2002a {published data only}
- Piccolo D, Ferrari A, Peris K, Diadone R, Ruggeri B, Chimenti S. Dermoscopic diagnosis by a trained clinician vs. a clinician with minimal dermoscopy training vs. computer‐aided diagnosis of 341 pigmented skin lesions: a comparative study. British Journal of Dermatology 2002;147(3):481‐6. [ER4:15466057; PUBMED: 12207587] [DOI] [PubMed] [Google Scholar]
Piccolo 2004 {published data only}
- Piccolo D, Soyer HP, Chimenti S, Argenziano G, Bartenjev I, Hofmann‐Wellenhof R, et al. Diagnosis and categorization of acral melanocytic lesions using teledermoscopy. Journal of Telemedicine & Telecare 2004;10(6):346‐50. [DOI] [PubMed] [Google Scholar]
Piccolo 2006 {published data only}
- Piccolo D, Fargnoli MC, Ferrara G, Lozzi GP, Altamura D, Ventura T, et al. Hypoepiluminescence microscopy of pigmented skin lesions: new approach to improve recognition of dermoscopic structures. Dermatologic Surgery 2006;32(11):1391‐7. [DOI] [PubMed] [Google Scholar]
Piccolo 2014 {published data only}
- Piccolo D, Crisman G, Schoinas S, Altamura D, Peris K. Computer‐automated ABCD versus dermatologists with different degrees of experience in dermoscopy. European Journal of Dermatology 2014;24(4):477‐81. [ER4:17941089; PUBMED: 24721784] [DOI] [PubMed] [Google Scholar]
Pizzichetta 2001a {published data only}
- Pizzichetta MA, Argenziano G, Talamini R, Piccolo D, Gatti A, Trevisan G, et al. Dermoscopic criteria for melanoma in situ are similar to those for early invasive melanoma. Cancer 2001;91(5):992‐7. [PubMed] [Google Scholar]
Pizzichetta 2001b {published data only}
- Pizzichetta MA, Talamini R, Piccolo D, Argenziano G, Pagnanelli G, Burgdorf T, et al. The ABCD rule of dermatoscopy does not apply to small melanocytic skin lesions. Archives of Dermatology 2001;137(10):1376‐8. [PubMed] [Google Scholar]
Pizzichetta 2002 {published data only}
- Pizzichetta MA, Talamini R, Piccolo D, Trevisan G, Veronesi A, Carbone A, et al. Interobserver agreement of the dermoscopic diagnosis of 129 small melanocytic skin lesions. Tumori 2002;88(3):234‐8. [ER4:18375049; PUBMED: 12195762] [DOI] [PubMed] [Google Scholar]
Pizzichetta 2004 {published data only}
- Pizzichetta MA, Talamini R, Stanganelli I, Puddu P, Bono R, Argenziano G, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. British Journal of Dermatology 2004;150(6):1117‐24. [ER4:15466066; PUBMED: 15214897] [DOI] [PubMed] [Google Scholar]
Pizzichetta 2007 {published data only}
- Pizzichetta MA, Stanganelli I, Bono R, Soyer HP, Magi S, Canzonieri V, et al. Dermoscopic features of difficult melanoma. Dermatologic Surgery 2007;33(1):91‐9. [DOI] [PubMed] [Google Scholar]
Pizzichetta 2010 {published data only}
- Pizzichetta MA, Canzonieri V, Massarut S, Baresic T, Borsatti E, Menzies SW. Pitfalls in the dermoscopic diagnosis of amelanotic melanoma. Journal of the American Academy of Dermatology 2010;62(5):893‐4. [DOI] [PubMed] [Google Scholar]
Pizzichetta 2013 {published data only}
- Pizzichetta MA, Talamini R, Marghoob AA, Soyer HP, Argenziano G, Bono R, et al. Negative pigment network: an additional dermoscopic feature for the diagnosis of melanoma. Journal of the American Academy of Dermatology 2013;68(4):552‐9. [DOI] [PubMed] [Google Scholar]
Pralong 2012 {published data only}
- Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. British Journal of Dermatology 2012;167(2):280‐7. [DOI] [PubMed] [Google Scholar]
Provost 1998 {published data only}
- Provost N, Kopf AW, Rabinovitz HS, Stolz W, DeDavid M, Wasti Q, et al. Comparison of conventional photographs and telephonically transmitted compressed digitized images of melanomas and dysplastic nevi. Dermatology 1998;196(3):299‐304. [DOI] [PubMed] [Google Scholar]
Pupelli 2013 {published data only}
- Pupelli G, Longo C, Veneziano L, Cesinaro AM, Ferrara G, Piana S, et al. Small‐diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. British Journal of Dermatology 2013;168(5):1027‐33. [ER4:15466070; PUBMED: 23301553] [DOI] [PubMed] [Google Scholar]
Quéreux 2011 {published data only}
- Quéreux G, Lequeux Y, Cary M, Jumbou O, Nguyen JM, Dreno B. Feasibility and effectiveness of a melanoma targeted screening strategy. Melanoma Research 2011;21:e1‐2. [Google Scholar]
Rader 2014 {published data only}
- Rader RK, Payne KS, Guntupalli U, Rabinovitz HS, Oliviero MC, Drugge RJ, et al. The pink rim sign: location of pink as an indicator of melanoma in dermoscopic images. Journal of Skin Cancer 2014;2014:719740. [PUBMED: 24639898] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajpara 2009 {published data only}
- Rajpara SM, Botello AP, Townend J, Ormerod AD. Systematic review of dermoscopy and digital dermoscopy/ artificial intelligence for the diagnosis of melanoma. British Journal of Dermatology 2009;161(3):591‐604. [DOI] [PubMed] [Google Scholar]
Rallan 2006 {published data only}
- Rallan D, Dickson M, Bush NL, Harland CC, Mortimer P, Bamber JC. High‐resolution ultrasound reflex transmission imaging and digital photography: potential tools for the quantitative assessment of pigmented lesions. Skin Research and Technology 2006;12(1):50‐9. [DOI] [PubMed] [Google Scholar]
Rampen 1988 {published data only}
- Rampen FH, Rumke P. Referral pattern and accuracy of clinical diagnosis of cutaneous melanoma. Acta Dermato‐venereologica 1988;68(1):61‐4. [PubMed] [Google Scholar]
Rao 1997 {published data only}
- Rao BK, Marghoob AA, Stolz W, Kopf AW, Slade J, Wasti Q, et al. Can early malignant melanoma be differentiated from atypical melanocytic nevi by in vivo techniques? Part I. Clinical and dermoscopic characteristics. Skin Research and Technology 1997;3(1):8‐14. [EMBASE: 27145858; ER4:17941048] [DOI] [PubMed] [Google Scholar]
Reeck 1999 {published data only}
- Reeck MC, Chuang TY, Eads TJ, Faust HB, Farmer ER, Hood AF. The diagnostic yield in submitting nevi for histologic examination. Journal of the American Academy of Dermatology 1999;40(4):567‐71. [DOI] [PubMed] [Google Scholar]
Reggiani 2015 {published data only}
- Reggiani C, Manfredini M, Mandel VD, Farnetani F, Ciardo S, Bassoli S, et al. Update on non‐invasive imaging techniques in early diagnosis of non‐melanoma skin cancer. Giornale Italiano di Dermatologia e Venereologia 2015;150(4):393‐405. [PubMed] [Google Scholar]
Riddell 1961 {published data only}
- Riddell JM Jr. A report of 300 patients with skin cancer. Texas State Journal of Medicine 1961;57:588‐92. [PUBMED: 13741469] [PubMed] [Google Scholar]
Rigel 1993 {published data only}
- Rigel DS, Friedman RJ. The rationale of the ABCDs of early melanoma. Journal of the American Academy of Dermatology 1993;29(6):1060‐1. [DOI] [PubMed] [Google Scholar]
Rigel 1997 {published data only}
- Rigel DS. Epiluminescence microscopy in clinical diagnosis of pigmented skin lesions?. Lancet 1997;349(9065):1566‐7. [DOI] [PubMed] [Google Scholar]
Rigel 2012 {published data only}
- Rigel DS, Roy M, Yoo J, Cockerell CJ, Robinson JK, White R. Impact of guidance from a computer‐aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Archives of Dermatology 2012;148(4):541‐3. [ER4:15466080; PUBMED: 22351788] [DOI] [PubMed] [Google Scholar]
Robati 2014 {published data only}
- Robati RM, Toossi P, Karimi M, Ayatollahi A, Esmaeli M. Screening for skin cancer: a pilot study in Tehran, Iran. Indian Journal of Dermatology 2014;59(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2010 {published data only}
- Robinson JK, Turrisi R, Mallett K, Stapleton J, Pion M. Comparing the efficacy of an in‐person intervention with a skin self‐examination workbook. Archives of Dermatology 2010;146(1):91‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ronger 2002 {published data only}
- Ronger S, Touzet S, Ligeron C, Balme B, Viallard AM, Barrut D, et al. Dermoscopic examination of nail pigmentation. Archives of Dermatology 2002;138(10):1327‐33. [DOI] [PubMed] [Google Scholar]
Rosado 2003 {published data only}
- Rosado B, Menzies S, Harbauer A, Pehamberger H, Wolff K, Binder M, et al. Accuracy of computer diagnosis of melanoma: a quantitative meta‐analysis. Archives of Dermatology 2003;139(3):361‐7; discussion 366. [DOI] [PubMed] [Google Scholar]
Rosendahl 2012a {published data only}
- Rosendahl C, Cameron A, Argenziano G, Zalaudek I, Tschandl P, Kittler H. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Archives of Dermatology 2012;148(12):1386‐92. [DOI] [PubMed] [Google Scholar]
Rosendahl 2012b {published data only}
- Rosendahl C, Cameron A, McColl I, Wilkinson D. Dermatoscopy in routine practice: 'Chaos and Clues'. Australian Family Physician 2012;41(7):482‐7. [PubMed] [Google Scholar]
Rossi 2000 {published data only}
- Rossi CR, Vecchiato A, Bezze G, Mastrangelo G, Montesco MC, Mocellin S, et al. Early detection of melanoma: an educational campaign in Padova, Italy. Melanoma Research 2000;10(2):181‐7. [PubMed] [Google Scholar]
Roush 1986 {published data only}
- Roush GC, Kirkwood JM, Ernstoff M, Somma SJ, Duray PH, Klaus SN, et al. Reproducibility and validity in the clinical diagnosis of the nonfamilial dysplastic nevus: work in progress. Recent Results in Cancer Research 1986;102:154‐8. [DOI] [PubMed] [Google Scholar]
Rubegni 2002 {published data only}
- Rubegni P, Burroni M, Dell'eva G, Andreassi L. Digital dermoscopy analysis for automated diagnosis of pigmented skin lesions. Clinics in Dermatology 2002;20(3):309‐12. [DOI] [PubMed] [Google Scholar]
Rubegni 2005 {published data only}
- Rubegni P, Burroni M, Andreassi A, Fimiani M. The role of dermoscopy and digital dermoscopy analysis in the diagnosis of pigmented skin lesions. Archives of Dermatology 2005;141(11):1444‐6. [DOI] [PubMed] [Google Scholar]
Rubegni 2010 {published data only}
- Rubegni P, Cevenini G, Burroni M, Bono R, Sbano P, Biagioli M, et al. Objective follow‐up of atypical melanocytic skin lesions: a retrospective study. Archives of Dermatological Research 2010;302(7):551‐60. [DOI] [PubMed] [Google Scholar]
Rubegni 2012 {published data only}
- Rubegni P, Cevenini G, Nami N, Argenziano G, Saida T, Burroni M, et al. Dermoscopy and digital dermoscopy analysis of palmoplantar 'equivocal' pigmented skin lesions in Caucasians. Dermatology 2012;225(3):248‐55. [ER4:15466088; PUBMED: 23182753] [DOI] [PubMed] [Google Scholar]
Rubegni 2016 {published data only}
- Rubegni P, Tognetti L, Argenziano G, Nami N, Brancaccio G, Cinotti E, et al. A risk scoring system for the differentiation between melanoma with regression and regressing nevi. Journal of Dermatological Science 2016;83(2):138‐44. [ER4:25012293; PUBMED: 27157925] [DOI] [PubMed] [Google Scholar]
Sahin 2004 {published data only}
- Sahin MT, Oztürkcan S, Ermertcan AT, Güneş AT. A comparison of dermoscopic features among lentigo senilis/initial seborrheic keratosis, seborrheic keratosis, lentigo maligna and lentigo maligna melanoma on the face. Journal of Dermatology 2004;31(11):884‐9. [PUBMED: 15729860] [DOI] [PubMed] [Google Scholar]
Saida 2002 {published data only}
- Saida T, Oguchi S, Miyazaki A. Dermoscopy for acral pigmented skin lesions. Clinics in Dermatology 2002;20(3):279‐85. [DOI] [PubMed] [Google Scholar]
Saida 2004 {published data only}
- Saida T, Miyazaki A, Oguchi S, Ishihara Y, Yamazaki Y, Murase S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Archives of Dermatology 2004;140(10):1233‐8. [DOI] [PubMed] [Google Scholar]
Sakakibara 2010 {published data only}
- Sakakibara A, Kamijima M, Shibata S, Yasue S, Kono M, Tomita Y. Dermoscopic evaluation of vascular structures of various skin tumors in Japanese patients. Journal of Dermatology 2010;37(4):316‐22. [DOI] [PubMed] [Google Scholar]
Salerni 2011 {published data only}
- Salerni G, Lovatto L, Carrera C, Palou J, Alos L, Puig‐Butille JA, et al. Correlation among dermoscopy, confocal reflectance microscopy, and histologic features of melanoma and basal cell carcinoma collision tumor. Dermatologic Surgery 2011;37(2):275‐9. [DOI] [PubMed] [Google Scholar]
Salerni 2012 {published data only}
- Salerni G, Carrera C, Lovatto L, Puig‐Butille JA, Badenas C, Plana E, et al. Benefits of total body photography and digital dermatoscopy ("two‐step method of digital follow‐up") in the early diagnosis of melanoma in patients at high risk for melanoma. Journal of the American Academy of Dermatology 2012;67(1):e17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salerni 2013 {published data only}
- Salerni G, Teran T, Puig S, Malvehy J, Zalaudek I, Argenziano G, et al. Meta‐analysis of digital dermoscopy follow‐up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. Journal of the European Academy of Dermatology and Venereology 2013;27(7):805‐14. [DOI] [PubMed] [Google Scholar]
Salvio 2011 {published data only}
- Salvio AG, Assumpcao Junior A, Segalla JG, Panfilo BL, Nicolini HR, Didone R. One year experience of a model for melanoma continuous prevention in the city of Jau (Sao Paulo), Brazil. Anais Brasileiros de Dermatologia 2011;86(4):669‐74. [DOI] [PubMed] [Google Scholar]
Sanchez‐Martin 2012 {published data only}
- Sanchez‐Martin J, Vazquez‐Lopez F, Perez‐Oliva N, Argenziano G. Dermoscopy of small basal cell carcinoma: study of 100 lesions 5 mm or less in diameter. Dermatologic Surgery 2012;38(6):947‐50. [DOI] [PubMed] [Google Scholar]
Savk 2004 {published data only}
- Savk E, Sahinkaras E, Okyay P, Karaman G, Erkek M, Sendur N. Interobserver agreement in the use of the ABCD rule for dermoscopy. Journal of Dermatology 2004;31(12):1041‐3. [DOI] [PubMed] [Google Scholar]
Sawada 2013 {published data only}
- Sawada M, Tanaka M. Self‐assembly of a simple low‐cost dermoscope for examination of skin lesions. Dermatology Practical and Conceptual 2013;3(4):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sboner 2003 {published data only}
- Sboner A, Eccher C, Blanzieri E, Bauer P, Cristofolini M, Zumiani G, et al. A multiple classifier system for early melanoma diagnosis. Artificial Intelligence in Medicine 2003;27(1):29‐44. [DOI] [PubMed] [Google Scholar]
Sboner 2004 {published data only}
- Sboner A, Bauer P, Zumiani G, Eccher C, Blanzieri E, Forti S, et al. Clinical validation of an automated system for supporting the early diagnosis of melanoma. Skin Research and Technology 2004;10(3):184‐92. [ER4:15466104; PUBMED: 15225269] [DOI] [PubMed] [Google Scholar]
Schindewolf 1994 {published data only}
- Schindewolf T, Schiffner R, Stolz W, Albert R, Abmayr W, Harms H. Evaluation of different image acquisition techniques for a computer vision system in the diagnosis of malignant melanoma. Journal of the American Academy of Dermatology 1994;31(1):33‐41. [DOI] [PubMed] [Google Scholar]
Schmoeckel 1987 {published data only}
- Schmoeckel C, Braun‐Falco O. Diagnosis of early malignant melanoma: sensitivity and specificity of clinical and histological criteria. In: Elder DE editor(s). Pathobiology of Malignant Melanoma. Pigment Cell. Vol. 8, Philadelphia, PA: Karger, 1987:96‐106. [ISBN: 978‐3‐8055‐4348‐4] [Google Scholar]
Schulz 2001 {published data only}
- Schulz H. Epiluminescent microscopy aspects of initial cutaneous melanoma metastases. Hautarzt 2001;52(1):21‐5. [DOI] [PubMed] [Google Scholar]
Scope 2008 {published data only}
- Scope A, Dusza SW, Halpern AC, Rabinovitz H, Braun RP, Zalaudek I, et al. The "ugly duckling" sign: agreement between observers. Archives of Dermatology 2008;144(1):58‐64. [ER4:15465911; PUBMED: 18209169] [DOI] [PubMed] [Google Scholar]
Scope 2015 {published data only}
- Scope A, Braun RP. The recognition process in dermoscopy: analytic approach vs heuristic approach. JAMA Dermatology 2015;151(7):704‐6. [DOI] [PubMed] [Google Scholar]
Segura 2009 {published data only}
- Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two‐step method for the diagnosis of melanoma by reflectance confocal microscopy. Journal of the American Academy of Dermatology 2009;61(2):216‐29. [DOI] [PubMed] [Google Scholar]
Seidenari 1998 {published data only}
- Seidenari S, Pellacani G, Pepe P. Digital videomicroscopy improves diagnostic accuracy for melanoma. Journal of the American Academy of Dermatology 1998;39(2 Pt 1):175‐81. [ER4:15466116; PUBMED: 9704824] [DOI] [PubMed] [Google Scholar]
Seidenari 2004 {published data only}
- Seidenari S, Pellacani G, Righi E, Nardo A. Is JPEG compression of videomicroscopic images compatible with telediagnosis? Comparison between diagnostic performance and pattern recognition on uncompressed TIFF images and JPEG compressed ones. Telemedicine Journal and E‐health 2004;10(3):294‐303. [DOI] [PubMed] [Google Scholar]
Seidenari 2005 {published data only}
- Seidenari S, Pellacani G, Martella A. Acquired melanocytic lesions and the decision to excise: role of color variegation and distribution as assessed by dermoscopy. Dermatologic Surgery 2005;31(2):184‐9. [ER4:15466115; PUBMED: 15762212] [DOI] [PubMed] [Google Scholar]
Seidenari 2006a {published data only}
- Seidenari S, Longo C, Giusti F, Pellacani G. Clinical selection of melanocytic lesions for dermoscopy decreases the identification of suspicious lesions in comparison with dermoscopy without clinical preselection. British Journal of Dermatology 2006;154(5):873‐9. [DOI] [PubMed] [Google Scholar]
Seidenari 2006b {published data only}
- Seidenari S, Pellacani G, Grana C. Asymmetry in dermoscopic melanocytic lesion images: a computer description based on colour distribution. Acta Dermato‐Venereologica 2006;86(2):123‐8. [DOI] [PubMed] [Google Scholar]
Seidenari 2007 {published data only}
- Seidenari S, Grana C, Pellacani G. Colour clusters for computer diagnosis of melanocytic lesions. Dermatology 2007;214(2):137‐43. [ER4:15466111; PUBMED: 17341863] [DOI] [PubMed] [Google Scholar]
Seidenari 2012 {published data only}
- Seidenari S, Ferrari C, Borsari S, Bassoli S, Cesinaro AM, Giusti F, et al. The dermoscopic variability of pigment network in melanoma in situ. Melanoma Research 2012;22(2):151‐7. [DOI] [PubMed] [Google Scholar]
Seidenari 2013 {published data only}
- Seidenari S, Arginelli F, Dunsby C, French PM, Konig K, Magnoni C, et al. Multiphoton laser tomography and fluorescence lifetime imaging of melanoma: morphologic features and quantitative data for sensitive and specific non‐invasive diagnostics. PLoS One 2013;8(7):e70682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serrao 2006 {published data only}
- Serrao VV, Baptista J, Paris F, Lopes LC, Fidalgo A, Ferreira A. Digital dermoscopy. Review of 652 lesions analysed by the DANAOS system. Skin Cancer 2006;21(4):185‐98. [Google Scholar]
Sgouros 2014 {published data only}
- Sgouros D, Lallas A, Julian Y, Rigopoulos D, Zalaudek I, Longo C, et al. Assessment of SIAscopy in the triage of suspicious skin tumours. Skin Research and Technology 2014;20(4):440‐4. [DOI] [PubMed] [Google Scholar]
Shakya 2012 {published data only}
- Shakya NM, LeAnder RW, Hinton KA, Stricklin SM, Rader RK, Hagerty J, et al. Discrimination of squamous cell carcinoma in situ from seborrheic keratosis by color analysis techniques requires information from scale, scale‐crust and surrounding areas in dermoscopy images. Computers in Biology and Medicine 2012;42(12):1165‐9. [DOI] [PubMed] [Google Scholar]
Shariff 2010 {published data only}
- Shariff Z, Roshan A, Williams AM, Platt AJ. 2‐Week wait referrals in suspected skin cancer: does an instructional module for general practitioners improve diagnostic accuracy?. Surgeon 2010;8(5):247‐51. [DOI] [PubMed] [Google Scholar]
Shitara 2014 {published data only}
- Shitara D, Ishioka P, Alonso‐Pinedo Y, Palacios‐Bejarano L, Carrera C, Malvehy J, et al. Shiny white streaks: a sign of malignancy at dermoscopy of pigmented skin lesions. Acta Dermato‐Venereologica 2014;94(2):132‐7. [DOI] [PubMed] [Google Scholar]
Shitara 2015 {published data only}
- Shitara D, Nascimento M, Ishioka P, Carrera C, Alos L, Malvehy J, et al. Dermoscopy of naevus‐associated melanomas. Acta Dermato‐Venereologica 2015;95(6):671‐5. [DOI] [PubMed] [Google Scholar]
Skvara 2005 {published data only}
- Skvara H, Teban L, Fiebiger M, Binder M, Kittler H. Limitations of dermoscopy in the recognition of melanoma. Archives of Dermatology 2005;141(2):155‐60. [ER4:20569495; PUBMED: 15724011] [DOI] [PubMed] [Google Scholar]
Sondak 2015 {published data only}
- Sondak VK, Glass LF, Geller AC. Risk‐stratified screening for detection of melanoma. JAMA 2015;313(6):616‐7. [DOI] [PubMed] [Google Scholar]
Soyer 1987 {published data only}
- Soyer HP, Smolle J, Kerl H, Stettner H. Early diagnosis of malignant melanoma by surface microscopy. Lancet 1987;2(8562):803. [DOI] [PubMed] [Google Scholar]
Soyer 1995 {published data only}
- Soyer HP, Smolle J, Leitinger G, Rieger E, Kerl H. Diagnostic reliability of dermoscopic criteria for detecting malignant melanoma. Dermatology 1995;190(1):25‐30. [ER4:18375054; PUBMED: 7894091] [DOI] [PubMed] [Google Scholar]
Soyer 2001 {published data only}
- Soyer HP, Argenziano G, Talamini R, Chimenti S. Is dermoscopy useful for the diagnosis of melanoma?. Archives of Dermatology 2001;137(10):1361‐3. [DOI] [PubMed] [Google Scholar]
Soyer 2004 {published data only}
- Soyer HP, Argenziano G, Zalaudek I, Corona R, Sera F, Talamini R, et al. Three‐point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology 2004;208(1):27‐31. [DOI] [PubMed] [Google Scholar]
Stanganelli 1998a {published data only}
- Stanganelli I, Serafini M, Cainelli T, Cristofolini M, Baldassari L, Staffa M, et al. Accuracy of epiluminescence microscopy among practical dermatologists: a study from the Emilia‐Romagna region of Italy. Tumori 1998;84(6):701‐5. [ER4:18375055; PUBMED: 10080681] [DOI] [PubMed] [Google Scholar]
Stanganelli 1998b {published data only}
- Stanganelli I, Bucchi L. Epiluminescence microscopy versus clinical evaluation of pigmented skin lesions: effects of Operator's training on reproducibility and accuracy. Dermatology and Venereology Society of the Canton of Ticino. Dermatology 1998;196(2):199‐203. [DOI] [PubMed] [Google Scholar]
Stanganelli 1999 {published data only}
- Stanganelli I, Seidenari S, Serafini M, Pellacani G, Bucchi L. Diagnosis of pigmented skin lesions by epiluminescence microscopy: determinants of accuracy improvement in a nationwide training programme for practical dermatologists. Public Health 1999;113(5):237‐42. [ER4:15466128; PUBMED: 10557118] [DOI] [PubMed] [Google Scholar]
Stanganelli 2005 {published data only}
- Stanganelli I, Brucale A, Calori L, Gori R, Lovato A, Magi S, et al. Computer‐aided diagnosis of melanocytic lesions. Anticancer Research 2005;25(6C):4577‐82. [ER4:15466126; PUBMED: 16334145] [PubMed] [Google Scholar]
Stanganelli 2015 {published data only}
- Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow‐up improves melanoma detection accuracy. British Journal of Dermatology 2015;172(2):365‐71. [ER4:20569496] [DOI] [PubMed] [Google Scholar]
Stanley 2003 {published data only}
- Stanley RJ, Moss RH, Stoecker W, Aggarwal C. A fuzzy‐based histogram analysis technique for skin lesion discrimination in dermatology clinical images. Computerized Medical Imaging and Graphics 2003;27(5):387‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stathopoulos 2015 {published data only}
- Stathopoulos P, Ghaly G, Sisodia B, Harrop C. Positive predictive value of clinical diagnosis of head and neck non‐melanoma skin malignancies. How accurate are we?. Oral and Maxillofacial Surgery 2015;19(Numb 4):387‐90. [DOI] [PubMed] [Google Scholar]
Steiner 1993 {published data only}
- Steiner A, Binder M, Schemper M, Wolff K, Pehamberger H. Statistical evaluation of epiluminescence microscopy criteria for melanocytic pigmented skin lesions. Journal of the American Academy of Dermatology 1993;29(4):581‐8. [DOI] [PubMed] [Google Scholar]
Stephens 2013 {published data only}
- Stephens A, Fraga‐Braghiroli N, Oliviero M, Rabinovitz H, Scope A. Spoke wheel‐like structures in superficial basal cell carcinoma: a correlation between dermoscopy, histopathology, and reflective confocal microscopy. Journal of the American Academy of Dermatology 2013;69(5):e219‐21. [DOI] [PubMed] [Google Scholar]
Stoecker 2009 {published data only}
- Stoecker WV, Gupta K, Shrestha B, Wronkiewiecz M, Chowdhury R, Stanley RJ, et al. Detection of basal cell carcinoma using color and histogram measures of semitranslucent areas. Skin Research and Technology 2009;15(3):283‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoecker 2011 {published data only}
- Stoecker WV, Wronkiewiecz M, Chowdhury R, Stanley RJ, Xu J, Bangert A, et al. Detection of granularity in dermoscopy images of malignant melanoma using color and texture features. Computerized Medical Imaging and Graphics 2011;35(2):144‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolz 1994 {published data only}
- Stolz W, Riemann A, Cognetta AB, Pillet L, Abmayer W, Holzel D, et al. ABCD rule of dermatoscopy: A new practical method for early recognition of malignant melanoma. European Journal of Dermatology 1994;4(7):521‐7. [EMBASE: 24349113; ER4:18375098] [Google Scholar]
Stolz 2002 {published data only}
- Stolz W, Schiffner R, Burgdorf WH. Dermatoscopy for facial pigmented skin lesions. Clinics in Dermatology 2002;20(3):276‐8. [DOI] [PubMed] [Google Scholar]
Stratigos 2007 {published data only}
- Stratigos A, Nikolaou V, Kedicoglou S, Antoniou C, Stefanaki I, Haidemenos G, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. Journal of the European Academy of Dermatology and Venereology 2007;21(1):56‐62. [DOI] [PubMed] [Google Scholar]
Stricklin 2011 {published data only}
- Stricklin SM, Stoecker WV, Oliviero MC, Rabinovitz HS, Mahajan SK. Cloudy and starry milia‐like cysts: how well do they distinguish seborrheic keratoses from malignant melanomas?. Journal of the European Academy of Dermatology and Venereology 2011;25(10):1222‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strumia 2003 {published data only}
- Strumia R, Montanari A. Low positive predictive value of ABCD‐E rule for dermatoscopy of small melanocytic naevi. Melanoma Research 2003;13(6):631‐2. [PUBMED: 14646628] [DOI] [PubMed] [Google Scholar]
Tan 2009 {published data only}
- Tan E, Levell NJ. Regular clinical dermatoscope use with training improves melanoma diagnosis by dermatologists. Clinical and Experimental Dermatology 2009;34(8):e876‐8. [ER4:17941000; PUBMED: 20055853] [DOI] [PubMed] [Google Scholar]
Tandjung 2015 {published data only}
- Tandjung R, Badertscher N, Kleiner N, Wensing M, Rosemann T, Braun RP, et al. Feasibility and diagnostic accuracy of teledermatology in Swiss primary care: process analysis of a randomized controlled trial. Journal of Evaluation in Clinical Practice 2015;21(2):326‐31. [DOI] [PubMed] [Google Scholar]
Tasli 2012 {published data only}
- Tasli L, Kacar N, Argenziano G. A scientometric analysis of dermoscopy literature over the past 25 years. Journal of the European Academy of Dermatology and Venereology 2012;26(9):1142‐8. [DOI] [PubMed] [Google Scholar]
Teban 2003 {published data only}
- Teban L, Pehamberger H, Wolff K, Binder M, Kittler H. Clinical value of a dermatoscopic classification of Clark nevi. Journal der Deutschen Dermatologischen Gesellschaft 2003;1(4):292‐6. [DOI] [PubMed] [Google Scholar]
Tenenhaus 2010 {published data only}
- Tenenhaus A, Nkengne A, Horn JF, Serruys C, Giron A, Fertil B. Detection of melanoma from dermoscopic images of naevi acquired under uncontrolled conditions. Skin Research and Technology 2010;16(1):85‐97. [ER4:17941001] [DOI] [PubMed] [Google Scholar]
Terrill 2009 {published data only}
- Terrill PJ, Fairbanks S, Bailey M. Is there just one lesion? The need for whole body skin examination in patients presenting with non‐melanocytic skin cancer. ANZ Journal of Surgery 2009;79(10):707‐12. [DOI] [PubMed] [Google Scholar]
Terstappen 2007 {published data only}
- Terstappen K, Larko O, Wennberg AM. Pigmented basal cell carcinoma‐‐comparing the diagnostic methods of SIAscopy and dermoscopy. Acta Dermato‐Venereologica 2007;87(3):238‐42. [DOI] [PubMed] [Google Scholar]
Terushkin 2010a {published data only}
- Terushkin V, Braga JC, Dusza SW, Scope A, Busam K, Marghoob AA, et al. Agreement on the clinical diagnosis and management of cutaneous squamous neoplasms. Dermatologic Surgery 2010;36(10):1514‐20. [DOI] [PubMed] [Google Scholar]
Terushkin 2010b {published data only}
- Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Archives of Dermatology 2010;146(3):343‐4. [DOI] [PubMed] [Google Scholar]
Thomas 1998 {published data only}
- Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology 1998;197(1):11‐7. [ER4:15466141; PUBMED: 9693179] [DOI] [PubMed] [Google Scholar]
Thomson 2005 {published data only}
- Thomson MA, Loffeld A, Marsden JR. More skin cancer detected from nonurgent referrals. British Journal of Dermatology 2005;153(2):453‐4. [DOI] [PubMed] [Google Scholar]
Torrey 1941 {published data only}
- Torrey FA, Levin EA. Comparison of the clinical and the pathologic diagnoses of malignant conditions of the skin. Archives of Dermatology 1941;43(3):532. [ER4:21450650] [Google Scholar]
Tromme 2012 {published data only}
- Tromme I, Sacre L, Hammouch F, Legrand C, Marot L, Vereecken P, et al. Availability of digital dermoscopy in daily practice dramatically reduces the number of excised melanocytic lesions: results from an observational study. British Journal of Dermatology 2012;167(4):778‐86. [DOI] [PubMed] [Google Scholar]
Troyanova 2003 {published data only}
- Troyanova P. A beneficial effect of a short‐term formal training course in epiluminescence microscopy on the diagnostic performance of dermatologists about cutaneous malignant melanoma. Skin Research and Technology 2003;9(3):269‐73. [ER4:17941004; PUBMED: 12877690] [DOI] [PubMed] [Google Scholar]
Tschandl 2012 {published data only}
- Tschandl P, Rosendahl C, Kittler H. Accuracy of the first step of the dermatoscopic 2‐step algorithm for pigmented skin lesions. Dermatology Practical and Conceptual 2012;2(3):203a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tschandl 2015 {published data only}
- Tschandl P, Kittler H, Schmid K, Zalaudek I, Argenziano G. Teaching dermatoscopy of pigmented skin tumours to novices: comparison of analytic vs. heuristic approach. Journal of the European Academy of Dermatology and Venereology 2015;29(6):1198‐204. [DOI] [PubMed] [Google Scholar]
Unlu 2014 {published data only}
- Unlu E, Akay BN, Erdem C. Comparison of dermatoscopic diagnostic algorithms based on calculation: The ABCD rule of dermatoscopy, the seven‐point checklist, the three‐point checklist and the CASH algorithm in dermatoscopic evaluation of melanocytic lesions. Journal of Dermatology 2014;41(7):598‐603. [ER4:15466145; PUBMED: 24807635] [DOI] [PubMed] [Google Scholar]
Van der Leest 2011 {published data only}
- Leest RJ, Vries E, Bulliard JL, Paoli J, Peris K, Stratigos AJ, et al. The Euromelanoma skin cancer prevention campaign in Europe: characteristics and results of 2009 and 2010. Journal of the European Academy of Dermatology and Venereology 2011;25(12):1455‐65. [DOI] [PubMed] [Google Scholar]
Van der Rhee 2010 {published data only}
- Rhee JI, Bergman W, Kukutsch NA. The impact of dermoscopy on the management of pigmented lesions in everyday clinical practice of general dermatologists: a prospective study. British Journal of Dermatology 2010;162(3):563‐7. [DOI] [PubMed] [Google Scholar]
Van der Rhee 2011 {published data only}
- Rhee JI, Bergman W, Kukutsch NA. Impact of dermoscopy on the management of high‐risk patients from melanoma families: a prospective study. Acta Dermato‐Venereologica 2011;91(4):428‐31. [DOI] [PubMed] [Google Scholar]
Vasili 2010 {published data only}
- Vasili E, Shkodrani E, Harja D, Labinoti L, Zoto A. Retrospective study of 70 patients with NMSC. Melanoma Research 2010;20:e63. [Google Scholar]
Verduzco‐Martinez 2013 {published data only}
- Verduzco‐Martinez AP, Quinones‐Venegas R, Guevara‐Gutierrez E, Tlacuilo‐Parra A. Correlation of dermoscopic findings with histopathologic variants of basal cell carcinoma. International Journal of Dermatology 2013;52(6):718‐21. [DOI] [PubMed] [Google Scholar]
Vestergaard 2008 {published data only}
- Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta‐analysis of studies performed in a clinical setting. British Journal of Dermatology 2008;159(3):669‐76. [DOI] [PubMed] [Google Scholar]
Viglizzo 2004 {published data only}
- Viglizzo G, Rongioletti F. Clinical, dermoscopic and pathologic correlation of pigmentary lesions observed in a dermoscopy service in the year 2003 [Correlazione clinico‐dermoscopico‐patologica delle lesioni cutanee pigmentate osservate in un servizio di dermoscopia nell'anno 2003]. Giornale Italiano di Dermatologia e Venereologia 2004;139(4):339‐44. [EMBASE: 39456561; ER4:18375099] [Google Scholar]
Wagner 1985 {published data only}
- Wagner RF, Wagner D, Tomich JM, Wagner KD, Grande DJ. Residents’corner: diagnoses of skin disease: dermatologists vs. nondermatologists. Journal of Dermatologic Surgery and Oncology 1985;11(5):476‐9. [DOI] [PubMed] [Google Scholar]
Walter 2010 {published data only}
- Walter FM, Morris HC, Humphrys E, Hall PN, Kinmonth AL, Prevost AT, et al. Protocol for the MoleMate UK Trial: a randomised controlled trial of the MoleMate system in the management of pigmented skin lesions in primary care [ISRCTN 79932379]. BMC Family Practice 2010;11:36. [PUBMED: 20459846] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walter 2012 {published data only}
- Walter FM, Morris HC, Humphrys E, Hall PN, Prevost AT, Burrows N, et al. Effect of adding a diagnostic aid to best practice to manage suspicious pigmented lesions in primary care: randomised controlled trial. BMJ 2012;345:e4110. [ER4:15466154; PUBMED: 22763392] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walter 2013 {published data only}
- Walter FM, Prevost AT, Vasconcelos J, Hall PN, Burrows NP, Morris HC, et al. Using the 7‐point checklist as a diagnostic aid for pigmented skin lesions in general practice: a diagnostic validation study. British Journal of General Practice 2013;63(610):e345‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang SQ, Dusza SW, Scope A, Braun RP, Kopf AW, Marghoob AA. Differences in dermoscopic images from nonpolarized dermoscope and polarized dermoscope influence the diagnostic accuracy and confidence level: a pilot study. Dermatologic Surgery 2008;34(10):1389‐95. [DOI] [PubMed] [Google Scholar]
Warshaw 2009a {published data only}
- Warshaw EM, Lederle FA, Grill JP, Gravely AA, Bangerter AK, Fortier LA, et al. Accuracy of teledermatology for pigmented neoplasms.[Erratum appears in J Am Acad Dermatol. 2010 Feb;62(2):319]. Journal of the American Academy of Dermatology 2009;61(5):753‐65. [DOI] [PubMed] [Google Scholar]
Warshaw 2009b {published data only}
- Warshaw EM, Lederle FA, Grill JP, Gravely AA, Bangerter AK, Fortier LA, et al. Accuracy of teledermatology for nonpigmented neoplasms. Journal of the American Academy of Dermatology 2009;60(4):579‐88. [DOI] [PubMed] [Google Scholar]
Warshaw 2010a {published data only}
- Warshaw EM, Gravely AA, Bohjanen KA, Chen K, Lee PK, Rabinovitz HS, et al. Interobserver accuracy of store and forward teledermatology for skin neoplasms. Journal of the American Academy of Dermatology 2010;62(3):513‐6. [DOI] [PubMed] [Google Scholar]
Warshaw 2010b {published data only}
- Warshaw, EM, Gravely AA, Nelson DB. Accuracy of teledermatology/teledermoscopy and clinic‐based dermatology for specific categories of skin neoplasms. Journal of the American Academy of Dermatology 2010;63(255):348‐52. [DOI] [PubMed] [Google Scholar]
Weismann 2002 {published data only}
- Weismann K, Lorentzen HF, Larsen FG. Diagnostic pearl: bright field globe magnifier diascopy for large pigmented skin lesions: a practical approach to epiluminescence microscopy. Journal of the American Academy of Dermatology 2002;47(2):304‐6. [DOI] [PubMed] [Google Scholar]
Wells 2012 {published data only}
- Wells R, Gutkowicz‐Krusin D, Veledar E, Toledano A, Chen SC. Comparison of diagnostic and management sensitivity to melanoma between dermatologists and MelaFind: a pilot study. Archives of Dermatology 2012;148(9):1083‐4. [ER4:15466163] [DOI] [PubMed] [Google Scholar]
Westbrook 2006 {published data only}
- Westbrook RH, Goyal N, Gawkrodger DJ. Diagnostic accuracy for skin cancer: comparison of general practitioner with dermatologist and dermatopathologist. Journal of Dermatological Treatment 2006;17(1):57‐8. [DOI] [PubMed] [Google Scholar]
Westerhoff 2000 {published data only}
- Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. British Journal of Dermatology 2000;143(5):1016‐20. [ER4:15466164] [DOI] [PubMed] [Google Scholar]
Whitaker‐Worth 1998 {published data only}
- Whitaker‐Worth DL, Susser WS, Grant‐Kels JM. Clinical dermatologic education and the diagnostic acumen of medical students and primary care residents. International Journal of Dermatology 1998;37(11):855‐9. [DOI] [PubMed] [Google Scholar]
Whited 1998 {published data only}
- Whited JD, Mills BJ, Hall RP, Drugge RJ, Grichnik JM, Simel DL. A pilot trial of digital imaging in skin cancer. Journal of Telemedicine and Telecare 1998;4(2):108‐12. [DOI] [PubMed] [Google Scholar]
Wilkes 2010 {published data only}
- Wilkes D. The use of dermoscopy in medical photography for the early detection of skin cancer. Journal of Visual Communication in Medicine 2010;33(4):169‐73. [DOI] [PubMed] [Google Scholar]
Williams 1991 {published data only}
- Williams HC, Smith D, Du Vivier A. Melanoma: differences observed by general surgeons and dermatologists. International Journal of Dermatology 1991;30(4):257‐61. [DOI] [PubMed] [Google Scholar]
Winkelmann 2015a {published data only}
- Winkelmann RR, Hauschild A, Tucker N, White R, Rigel DS. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. Journal of Clinical and Aesthetic Dermatology 2015;8(10):27‐9. [PMC free article] [PubMed] [Google Scholar]
Winkelmann 2015b {published data only}
- Winkelmann RR, Yoo J, Tucker N, White R, Rigel DS. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. Journal of Clinical and Aesthetic Dermatology 2015;8(9):21‐4. [PMC free article] [PubMed] [Google Scholar]
Winkelmann 2016 {published data only}
- Winkelmann RR, Farberg AS, Tucker N, White R, Rigel DS. Enhancement of international dermatologists' pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis. Journal of Clinical and Aesthetic Dermatology 2016;9(7):53‐55. [ER4:25701735] [PMC free article] [PubMed] [Google Scholar]
Wolf 1998 {published data only}
- Wolf IH, Smolle J, Soyer HP, Kerl H. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Research 1998;8(5):425‐9. [DOI] [PubMed] [Google Scholar]
Yadav 1993 {published data only}
- Yadav S, Vossaert KA, Kopf AW, Silverman M, Grin‐Jorgensen C. Histopathologic correlates of structures seen on dermoscopy (epiluminescence microscopy). American Journal of Dermatopathology 1993;15(4):297‐305. [DOI] [PubMed] [Google Scholar]
Yamaura 2005 {published data only}
- Yamaura M, Takata M, Miyazaki A, Saida T. Specific dermoscopy patterns and amplifications of the cyclin D1 gene to define histopathologically unrecognizable early lesions of acral melanoma in situ. Archives of Dermatology 2005;141(11):1413‐8. [DOI] [PubMed] [Google Scholar]
Yelamos 2016 {published data only}
- Yelamos O, Nehal KS. Integrating clinical information, dermoscopy and reflectance confocal microscopy to improve the diagnostic accuracy and confidence of amelanotic and lightly pigmented melanomas. British Journal of Dermatology 2016;175(6):1147‐8. [DOI] [PubMed] [Google Scholar]
Yoo 2015 {published data only}
- Yoo J, Tucker N, White R, Rigel D. The impact of probability of melanoma information provided by a multispectral digital skin lesion analysis device (MSDSLA) on resident dermatologists' decisions to biopsy clinical atypical lesions. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB177. [EMBASE: 71895455] [Google Scholar]
Youl 2007a {published data only}
- Youl PH, Raasch BA, Janda M, Aitken JF. The effect of an educational programme to improve the skills of general practitioners in diagnosing melanocytic/pigmented lesions. Clinical and Experimental Dermatology 2007;32(4):365‐70. [PUBMED: 17433042] [DOI] [PubMed] [Google Scholar]
Youl 2007b {published data only}
- Youl PH, Baade PD, Janda M, Mar CB, Whiteman DC, Aitken JF. Diagnosing skin cancer in primary care: how do mainstream general practitioners compare with primary care skin cancer clinic doctors?. Medical Journal of Australia 2007;187(4):215‐20. [DOI] [PubMed] [Google Scholar]
Zaballos 2013 {published data only}
- Zaballos P, Banuls J, Cabo H, Llambrich A, Salsench E, Puig S, et al. The usefulness of dermoscopy for the recognition of basal cell carcinoma‐‐seborrhoeic keratosis compound tumours. Australasian Journal of Dermatology 2013;54(3):208‐12. [DOI] [PubMed] [Google Scholar]
Zalaudek 2010 {published data only}
- Zalaudek I, Argenziano G, Marghoob AA, Pellacani G, Soyer HP. Dermoscopy and skin cancer. Dermatology Research and Practice 2010;2010:867059. [PUBMED: 21789037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaumseil 1983 {published data only}
- Zaumseil RP, Fiedler H, Gstöttner R. Clinical diagnostic accuracy of the malignant melanoma of the skin [Klinisch‐diagnostische treffsicherheit beim malignen melanom der haut]. Dermatologische Monatsschrift 1983;169(2):101‐5. [ER4:21450660; PUBMED: 6840366] [PubMed] [Google Scholar]
Zell 2008 {published data only}
- Zell D, Kim N, Olivero M, Elgart G, Rabinovitz H. Early diagnosis of multiple primary amelanotic/hypomelanotic melanoma using dermoscopy. Dermatologic Surgery 2008;34(9):1254‐7. [DOI] [PubMed] [Google Scholar]
Zortea 2014 {published data only}
- Zortea M, Schopf TR, Thon K, Geilhufe M, Hindberg K, Kirchesch H, et al. Performance of a dermoscopy‐based computer vision system for the diagnosis of pigmented skin lesions compared with visual evaluation by experienced dermatologists. Artificial Intelligence in Medicine 2014;60(1):13‐26. [DOI] [PubMed] [Google Scholar]
Zou 2001 {published data only}
- Zou KH. Comparison of correlated receiver operating characteristic curves derived from repeated diagnostic test data. Academic Radiology 2001;8(3):225‐33. [DOI] [PubMed] [Google Scholar]
Additional references
Alam 2001
- Alam M, Ratner D. Cutaneous squamous‐cell carcinoma [Review]. New England Journal of Medicine 2001;344(13):975‐83. [PUBMED: 11274625] [DOI] [PubMed] [Google Scholar]
Arits 2013
- Arits AH, Mosterd K, Essers BA, Spoorenberg E, Sommer A, Rooij MJ, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal‐cell carcinoma: a single blind, non‐inferiority, randomised controlled trial. Lancet Oncology 2013;14(7):647‐54. [DOI: 10.1016/S1470-2045(13)70143-8; PUBMED: 23683751] [DOI] [PubMed] [Google Scholar]
BAD 2013
- British Association of Dermatology. Quality standards for Teledermatology using ‘store and forward’ images. www.bad.org.uk/shared/get‐file.ashx?itemtype=document&id=794. London: British Association of Dermatology, (accessed 30 July 2018).
Baldursson 1993
- Baldursson B, Sigurgeirsson B, Lindelof B. Leg ulcers and squamous cell carcinoma. An epidemiological study and a review of the literature. Acta Dermato‐Venereologica 1993;73(3):171‐4. [PUBMED: 8105611] [DOI] [PubMed] [Google Scholar]
Bath‐Hextall 2007a
- Bath‐Hextall F, Leonardi‐Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. International Journal of Cancer 2007;121(9):2105‐8. [PUBMED: 17640064] [DOI] [PubMed] [Google Scholar]
Bath‐Hextall 2007b
- Bath‐Hextall FJ, Perkins W, Bong J, Williams HC. Interventions for basal cell carcinoma of the skin. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003412.pub2] [DOI] [PubMed] [Google Scholar]
Bath‐Hextall 2014
- Bath‐Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W, Miller PS, et al. Surgical excision versus imiquimod 5% cream for nodular and superficial basal‐cell carcinoma (SINS): a multicentre, non‐inferiority, randomised controlled trial. Lancet Oncology 2014;15(1):96‐105. [PUBMED: 24332516] [DOI] [PubMed] [Google Scholar]
Batra 2002
- Batra RS, Kelley LC. A risk scale for predicting extensive subclinical spread of nonmelanoma skin cancer. Dermatologic Surgery 2002;28(2):107‐12; discussion 112. [PUBMED: 11860418] [DOI] [PubMed] [Google Scholar]
Betti 2017
- Betti R, Moneghini L, Mapelli ET, Bulfamante G, Cerri A. Growth rate of different basal cell carcinoma subtypes. European Journal of Dermatology 2017;27(5):544‐5. [PUBMED: 29084641] [DOI] [PubMed] [Google Scholar]
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI: 10.1136/bmj.h5527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2005
- Braun RP, Rabinovitz HS, Oliviero M, Kopf AW, Saurat JH. Dermoscopy of pigmented skin lesions. Journal of the American Academy of Dermatology 2005;52(1):109‐21. [PUBMED: 15627088] [DOI] [PubMed] [Google Scholar]
Carter 2013
- Carter JB, Johnson MM, Chua TL, Karia PS, Schmults CD. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11‐year cohort study. JAMA Dermatology 2013;149(1):35‐41. [DOI: 10.1001/jamadermatol.2013.746; PUBMED: 23324754] [DOI] [PubMed] [Google Scholar]
CCAAC Network 2008
- Cancer Council Australia & Australian Cancer Network. Basal Cell Carcinoma, Squamous Cell Carcinoma (and related lesions) ‐ a guide to clinical management in Australia. www.cancer.org.au/content/pdf/HealthProfessionals/ClinicalGuidelines/Basal_cell_carcinoma_Squamous_cell_carcinoma_Guide_Nov_2008‐Final_with_Corrigendums.pdf. Sydney: Cancer Council Australia & Australian Cancer Network, (accessed 30 July 2018).
Chao 2013
- Chao D, London Cancer North and East. London Cancer, Guidelines for Cutaneous Malignant Melanoma Management August 2014. www.londoncancer.org/media/76373/london‐cancer‐melanoma‐guidelines‐2013‐v0.pdf. London: London Cancer North and East Alliance, (accessed 30 July 2018).
Chowdri 1996
- Chowdri NA, Darzi MA. Postburn scar carcinomas in Kashmiris. Burns 1996;22(6):477‐82. [PUBMED: 8884010] [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole S. Bivariate meta‐analysis for sensitivity and specificity with sparse data: a generalized linear mixed model approach (letter to the Editor). Journal of Clinical Epidemiology 2006;59(12):1331‐3. [PUBMED: 17098577] [DOI] [PubMed] [Google Scholar]
Chuchu 2018a
- Chuchu N, Dinnes J, Takwoingi Y, Matin RN, Bayliss SE, Davenport C, et al. Teledermatology for diagnosing skin cancer in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuchu 2018b
- Chuchu N, Takwoingi Y, Dinnes J, Matin RN, Bassett O, Moreau JF, et al. Smartphone applications for triaging adults with skin lesions that are suspicious for melanoma. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013192] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabski 1986
- Dabski K, Stoll HL Jr, Milgrom H. Squamous cell carcinoma complicating late chronic discoid lupus erythematosus. Journal of Surgical Oncology 1986;32(4):233‐7. [PUBMED: 3736067] [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [PUBMED: 16085191] [DOI] [PubMed] [Google Scholar]
Dinnes 2018a
- Dinnes J, Deeks JJ, Grainge MJ, Chuchu N, Ferrante di Ruffano L, Matin RN, et al. Visual inspection for diagnosing cutaneous melanoma in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinnes 2018b
- Dinnes J, Deeks JJ, Chuchu N, Ferrante di Ruffano L, Matin RN, Thomson DR, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD011902.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinnes 2018c
- Dinnes J, Deeks JJ, Chuchu N, Saleh D, Bayliss SE, Takwoingi Y, et al. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinnes 2018d
- Dinnes J, Bamber J, Chuchu N, Bayliss SE, Takwoingi Y, Davenport C, et al. High‐frequency ultrasound for diagnosing skin cancer in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drew 2017
- Drew BA, Karia PS, Mora AN, Liang CA, Schmults CD. Treatment patterns, outcomes, and patient satisfaction of primary epidermally limited nonmelanoma skin cancer. Dermatologic Surgery 2017;43(12):1423‐30. [DOI: 10.1097/DSS.0000000000001225; PUBMED: 28661992] [DOI] [PubMed] [Google Scholar]
Drucker 2017
- Drucker A, Adam GP, Langberg V, Gazula A, Smith B, Moustafa F, et al. Treatments for Basal Cell and Squamous Cell Carcinoma of the Skin. Comparative Effectiveness Reviews, No. 199. Rockville (MD): Agency for Healthcare Research and Quality (US), 2017. [PubMed] [Google Scholar]
Efron 1983
- Efron B. Estimating the error rate of a prediction rule: improvement on cross‐validation. Journal of the American Statistical Association 1983;78(382):316‐31. [Google Scholar]
Elstein 2002
- Elstein AS, Schwartz A. Clinical problem solving and diagnostic decision making: selective review of the cognitive literature. BMJ (Clinical Research Ed.) 2002;324(7339):729‐32. [PUBMED: 11909793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fasching 1989
- Fasching MC, Meland NB, Woods JE, Wolff BG. Recurrent squamous‐cell carcinoma arising in pilonidal sinus tract‐‐multiple flap reconstructions. Report of a case. Diseases of the Colon and Rectum 1989;32(2):153‐8. [PUBMED: 2914529] [DOI] [PubMed] [Google Scholar]
Ferrante di Ruffano 2018a
- Ferrante di Ruffano L, Takwoingi Y, Dinnes J, Chuchu N, Bayliss SE, Davenport C, et al. Computer‐assisted diagnosis techniques (dermoscopy and spectroscopy‐based) for diagnosing skin cancer in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrante di Ruffano 2018b
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, Chuchu N, Bayliss SE, Davenport C, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013189] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrante di Ruffano 2018c
- Ferrante di Ruffano L, Dinnes J, Chuchu N, Bayliss SE, Takwoingi Y, Davenport C, et al. Exfoliative cytology for diagnosing basal cell carcinoma and other skin cancers in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD013187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Firnhaber 2012
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. American Family Physician 2012;86(2):161‐8. [PUBMED: 22962928] [PubMed] [Google Scholar]
Fitzpatrick 1975
- Fitzpatrick TB. Soleil et peau. Journal de Médecine Esthétique 1975;2:33‐4. [Google Scholar]
Flohill 2013
- Flohil SC, Leest RJ, Arends LR, Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta‐analysis. European Journal of Cancer 2013;49(10):2365‐75. [PUBMED: 23608733] [DOI] [PubMed] [Google Scholar]
Garcia 2009
- Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. Journal of the American Acadademy of Dermatology 2009;60(1):137‐43. [PUBMED: 19103364] [DOI] [PubMed] [Google Scholar]
Gerbert 2000
- Gerbert B, Bronstone A, Maurer T, Hofmann R, Berger T. Decision support software to help primary care physicians triage skin cancer: a pilot study. Archives of Dermatology 2000;136(2):187‐92. [PUBMED: 10677094] [DOI] [PubMed] [Google Scholar]
Gordon 2013
- Gordon R. Skin cancer: an overview of epidemiology and risk factors. Seminars in Oncology Nursing 2013;29(3):160‐9. [PUBMED: 23958214] [DOI] [PubMed] [Google Scholar]
Gorlin 2004
- Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genetics in Medicine 2004;6(6):530‐9. [PUBMED: 15545751] [DOI] [PubMed] [Google Scholar]
Grachtchouk 2011
- Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, et al. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. Journal of Clinical Investigation 2011;121(5):1768‐81. [PUBMED: 21519145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2016
- Griffin LL, Ali FR, Lear JT. Non‐melanoma skin cancer. Clinical Medicine 2016;16(1):62‐5. [PUBMED: 26833519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2005
- Griffiths RW, Suvarna SK, Stone J. Do basal cell carcinomas recur after complete conventional surgical excision?. British Journal of Plastic Surgery 2005;58(6):795‐805. [PUBMED: 16086990] [DOI] [PubMed] [Google Scholar]
Guitera 2016
- Guitera, P, Menzies, S. W, Argenziano, G, Longo, A, Drummond, M, Scolyer, R. A, Pellacani, G. Dermoscopy and in vivo confocal microscopy are complimentary techniques for the diagnosis of difficult amelanotic and light colored skin lesions. Br J Dermatol 2016;175(6):1311‐19. [DOI: 10.1111/bjd.14749] [DOI] [PubMed] [Google Scholar]
Hartevelt 1990
- Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation 1990;49(3):506‐9. [PUBMED: 2316011] [DOI] [PubMed] [Google Scholar]
Jansen 2018
- Jansen MH, Mosterd K, Arits AH, Roozeboom MH, Sommer A, Essers BA, et al. Five‐year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5‐fluorouracil in patients with superficial basal cell carcinoma. Journal of Investigative Dermatology 2018;138(3):527‐33. [DOI: 10.1016/j.jid.2017.09.033; PUBMED: 29045820] [DOI] [PubMed] [Google Scholar]
Jensen 1999
- Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long‐term immunosuppressive therapy regimens. Journal of the American Academy of Dermatology 1999;40(2 Pt 1):177‐86. [PUBMED: 10025742] [DOI] [PubMed] [Google Scholar]
Kao 1986
- Kao GF. Carcinoma arising in Bowen's disease. Archives of Dermatology 1986;122(10):1124‐6. [PUBMED: 3767398] [PubMed] [Google Scholar]
Karimkhani 2015
- Karimkhani C, Boyers LN, Dellavalle RP, Weinstock MA. It's time for "keratinocyte carcinoma" to replace the term "nonmelanoma skin cancer". Journal of the American Academy of Dermatology 2015;72(1):186‐7. [PUBMED: 25497921] [DOI] [PubMed] [Google Scholar]
Kelleners‐Smeets 2017
- Kelleners‐Smeets NW, Mosterd K, Nelemans PJ. Treatment of low‐risk basal cell carcinoma. Journal of Investigative Dermatology 2017;137(3):539‐40. [PUBMED: 28235442] [DOI] [PubMed] [Google Scholar]
Kenet 2001
- Kenet RO, Kenet BJ. Risk stratification. A practical approach to using epiluminescence microscopy/dermoscopy in melanoma screening. Dermatologic Clinics 2001;19(2):327‐35. [PUBMED: 11556241] [DOI] [PubMed] [Google Scholar]
Kim 2014
- Kim DD, Tang JY, Ioannidis JP. Network geometry shows evidence sequestration for medical vs. surgical practices: treatments for basal cell carcinoma. Journal of Clinical Epidemiology 2014;67(4):391‐400. [PUBMED: 24491794] [DOI] [PubMed] [Google Scholar]
Kittler 2007
- Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical and Conceptual 2007;13(1):‐. [Google Scholar]
Kittler 2011
- Kittler H, Rosendahl C, Cameron A, Tschandl P. Dermatoscopy. An Algorithmic Method Based on Pattern Analysis. Austria: Facultas.WUV, 2011. [ISBN‐10: 3708907175] [Google Scholar]
Lachs 1992
- Lachs MS, Nachamkin I, Edelstein PH, Goldman J, Feinstein AR, Schwartz JS. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Annals of Internal Medicine 1992;117(2):135‐40. [PUBMED: 1605428] [DOI] [PubMed] [Google Scholar]
Lansbury 2010
- Lansbury L, Leonardi‐Bee J, Perkins W, Goodacre T, Tweed JA, Bath‐Hextall FJ. Interventions for non‐metastatic squamous cell carcinoma of the skin. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007869.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lansbury 2013
- Lansbury L, Bath‐Hextall F, Perkins W, Stanton W, Leonardi‐Bee J. Interventions for non‐metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ 2013;347:f6153. [PUBMED: 24191270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lear 1997
- Lear JT, Tan BB, Smith AG, Bowers W, Jones PW, Heagerty AH, et al. Risk factors for basal cell carcinoma in the UK: case‐control study in 806 patients. Journal of the Royal Society of Medicine 1997;90(7):371‐4. [PUBMED: 9290417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lear 2012
- Lear JT. Oral hedgehog‐pathway inhibitors for basal‐cell carcinoma. New England Journal of Medicine 2012;366(23):2225‐6. [PUBMED: 22670909] [DOI] [PubMed] [Google Scholar]
Lear 2014
- Lear JT, Corner C, Dziewulski P, Fife K, Ross GL, Varma S, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. British Journal of Cancer 2014;111(8):1476‐81. [DOI: 10.1038/bjc.2014.270; PUBMED: 25211660] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lederman 1985
- Lederman JS, Sober AJ. Does biopsy type influence survival in clinical stage I cutaneous melanoma?. Journal of the American Academy of Dermatology 1985;13(6):983‐7. [PUBMED: 4078105] [DOI] [PubMed] [Google Scholar]
Leeflang 2013
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ 2013;185(11):E537‐44. [PUBMED: 23798453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 1991
- Lees VC, Briggs JC. Effect of initial biopsy procedure on prognosis in stage I invasive cutaneous malignant melanoma: review of 1086 patients. British Journal of Surgery 1991;78(9):1108‐10. [PUBMED: 1933198] [DOI] [PubMed] [Google Scholar]
Lister 1997
- Lister RK, Black MM, Calonje E, Burnand KG. Squamous cell carcinoma arising in chronic lymphoedema. British Journal of Dermatology 1997;136(3):384‐7. [PUBMED: 9115922] [PubMed] [Google Scholar]
Lo 1991
- Lo JS, Snow SN, Reizner GT, Mohs FE, Larson PO, Hruza GJ. Metastatic basal cell carcinoma: report of twelve cases with a review of the literature. Journal of the American Academy of Dermatology 1991;24(5 Pt 1):715‐9. [PUBMED: 1869642] [DOI] [PubMed] [Google Scholar]
Lomas 2012
- Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. British Journal of Dermatology 2012;166(5):1069‐80. [PUBMED: 22251204] [DOI] [PubMed] [Google Scholar]
MacKie 1985
- MacKie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. British Journal of Dermatology 1985;113(2):167‐74. [PUBMED: 4027184] [DOI] [PubMed] [Google Scholar]
Madan 2010
- Madan V, Lear JT, Szeimies RM. Non‐melanoma skin cancer. Lancet 2010;375(9715):673‐85. [PUBMED: 20171403] [DOI] [PubMed] [Google Scholar]
Maia 1995
- Maia M, Proenca NG, Moraes JC. Risk factors for basal cell carcinoma: a case‐control study. Revista de Saude Publica 1995;29(1):27‐37. [PUBMED: 8525311] [DOI] [PubMed] [Google Scholar]
Maloney 1996
- Maloney ME. Arsenic in dermatology. Dermatologic Surgery 1996;22(3):301‐4. [PUBMED: 8599743] [DOI] [PubMed] [Google Scholar]
Malvehy 2002
- Malvehy J, Puig S. Follow‐up of melanocytic skin lesions wtih digital total‐body photography and digital dermoscopy: a two step method. Clinics in Dermatology 2002;20(3):297‐304. [PUBMED: 12074871] [DOI] [PubMed] [Google Scholar]
Marghoob 2012
- Marghoob AA, Malvehy J, Braun R, editor(s). An Atlas of Dermoscopy. Second Edition. London: Informa Healthcare, 2012. [ISBN 9780415458955] [Google Scholar]
Marsden 2010
- Marsden JR, Newton‐Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. BAD Guidelines: Revised UK guidelines for the management of cutaneous melanoma 2010. British Journal of Dermatology 2010;163(2):238‐56. [PUBMED: 20608932] [DOI] [PubMed] [Google Scholar]
McCormack 1997
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of the histological subtypes of basal cell carcinoma. A possible indicator of differing causes. Archives of Dermatology 1997;133(5):593‐6. [PUBMED: 9158412] [PubMed] [Google Scholar]
McCusker 2014
- McCusker M, Basset‐Seguin N, Dummer R, Lewis K, Schadendorf D, Sekulic A, et al. Metastatic basal cell carcinoma: Prognosis dependent on anatomic site and spread of disease. European Journal of Cancer 2014;50(4):774‐83. [PUBMED: 24412051] [DOI] [PubMed] [Google Scholar]
Moeckelmann 2018
- Moeckelmann N, Ebrahimi A, Dirven R, Liu J, Low TH, Gupta R, et al. Analysis and comparison of the 8th edition American Joint Committee on Cancer (AJCC) nodal staging system in cutaneous and oral squamous cell cancer of the head and neck. Annals of Surgical Oncology 2018;25(6):1730‐6. [DOI: 10.1245/s10434-018-6340-x; PUBMED: 29352431] [DOI] [PubMed] [Google Scholar]
Mogensen 2007
- Mogensen M, Jemec GB. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: a review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatologic Surgery 2007;33(10):1158‐74. [PUBMED: 17903149] [DOI] [PubMed] [Google Scholar]
Moons 1997
- Moons KG, Es GA, Deckers JW, Habbema JD, Grobbee DE. Limitations of sensitivity, specificity, likelihood ratio, and bayes' theorem in assessing diagnostic probabilities: a clinical example [Review]. Epidemiology 1997;8(1):12‐7. [PUBMED: 9116087] [DOI] [PubMed] [Google Scholar]
Motley 2009
- Motley RJ, Preston PW, Lawrence CM. Multi‐professional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. www.bad.org.uk/library‐media%5Cdocuments%5CSCC_2009.pdf (accessed 30 July 2018).
Murchie 2017
- Murchie P, Amalraj Raja E, Brewster DH, Iversen L, Lee AJ. Is initial excision of cutaneous melanoma by General Practitioners (GPs) dangerous? Comparing patient outcomes following excision of melanoma by GPs or in hospital using national datasets and meta‐analysis. European Journal of Cancer 2017;86:373‐84. [PUBMED: 29100192] [DOI] [PubMed] [Google Scholar]
Musah 2013
- Musah A, Gibson JE, Leonardi‐Bee J, Cave MR, Ander EL, Bath‐Hextall F. Regional variations of basal cell carcinoma incidence in the U.K. using The Health Improvement Network database (2004‐10). British Journal of Dermatology 2013;169(5):1093‐9. [PUBMED: 23701520] [DOI] [PubMed] [Google Scholar]
Nart 2015
- Nart IF, Armayones SG, Medina FV, Orti MB, Orpinell XB. Basal cell carcinoma treated with ingenol mebutate. Journal of the American Academy of Dermatology 2015;5(Suppl 1):AB180. [Google Scholar]
Ndegwa 2010
- Ndegwa S, Prichett‐Pejic W, McGill S, Murphy G, Severn M. Teledermatology services: rapid review of diagnostic, clinical management, and economic outcomes. www.cadth.ca/media/pdf/H0502_Teledermatology_Report_e.pdf. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH), (accessed 30 July 2018).
NICE 2010
- National Institute of Health and Care Excellence. NICE Guidance on Cancer Services. Improving outcomes for people with skin tumours including melanoma (update). www.nice.org.uk/guidance/csgstim. NICE, (accessed 30 July 2018).
NICE 2015
- National Institute for Health and Care Excellence. Suspected cancer: recognition and referral [NG12]. www.nice.org.uk/guidance/ng12. London: National Institute for Health and Clinical Excellence, (accessed 30 July 2018).
NICE 2017
- National Institute for Health and Care Excellence. Vismodegib for treating basal cell carcinoma. www.nice.org.uk/guidance/ta489. London: NICE, (accessed 30 July 2018).
Norman 1989
- Norman GR, Rosenthal D, Brooks LR, Allen SW, Muzzin LJ. The development of expertise in dermatology. Archives of Dermatology 1989;125(8):1063‐8. [PUBMED: 2757402] [PubMed] [Google Scholar]
Norman 2009
- Norman G, Barraclough K, Dolovich L, Price D. Iterative diagnosis. BMJ 2009;339:b3490. [DOI: 10.1136/bmj.b3490] [DOI] [PubMed] [Google Scholar]
O'Gorman 2014
- O'Gorman SM, Murphy GM. Photosensitizing medications and photocarcinogenesis. Photodermatology, Photoimmunology & Photomedicine 2014;30(1):8‐14. [PUBMED: 24393207] [DOI] [PubMed] [Google Scholar]
Offidani 2002
- Offidani A, Simonetti O, Bernardini ML, Alpagut A, Cellini A, Bossi G. General practitioners' accuracy in diagnosing skin cancers. Dermatology 2002;205(2):127‐30. [PUBMED: 12218226] [DOI] [PubMed] [Google Scholar]
Pehamberger 1993
- Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. Journal of the American Academy of Dermatology 1987;17(4):571‐83. [PUBMED: 3668002] [DOI] [PubMed] [Google Scholar]
Randle 1996
- Randle HW. Basal cell carcinoma. Identification and treatment of the high‐risk patient. Dermatologic Surgery 1996;22(3):255‐61. [PUBMED: 8599737] [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
Roozeboom 2012
- Roozeboom MH, Arits AH, Nelemans PJ, Kelleners‐Smeets NW. Overall treatment success after treatment of primary superficial basal cell carcinoma: a systematic review and meta‐analysis of randomized and nonrandomized trials. British Journal of Dermatology 2012;167(4):733‐56. [PUBMED: 22612571] [DOI] [PubMed] [Google Scholar]
Roozeboom 2016
- Roozeboom MH, Arits AH, Mosterd K, Sommer A, Essers BA, Rooij MJ, et al. Three‐year follow‐up results of photodynamic therapy vs. imiquimod vs. fluorouracil for treatment of superficial basal cell carcinoma: a single‐blind, noninferiority, randomized controlled trial. Journal of Investigative Dermatology 2016;136(8):1568‐74. [PUBMED: 27113429] [DOI] [PubMed] [Google Scholar]
Royal College of Pathologists 2014
- Royal College of Pathologists. Standards and datasets for reporting cancers. Dataset for the histological reporting of primary invasive cutaneous squamous cell carcinoma and regional lymph nodes. www.rcpath.org/Resources/RCPath/Migrated%20Resources/Documents/G/G124_DatasetSquamous_May14.pdf. London: Royal College of Pathologists, 2014 (accessed 30 July 2018).
Rutjes 2005
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case‐control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51(8):1335‐41. [PUBMED: 15961549] [DOI] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [PUBMED: 11568945] [DOI] [PubMed] [Google Scholar]
SAS 2012 [Computer program]
- SAS Institute Inc.. SAS 2012. Version 9.3. Cary, NC, USA: SAS Institute Inc., 2012.
Sekulic 2012
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal‐cell carcinoma. New England Journal of Medicine 2012;366(23):2171‐9. [PUBMED: 22670903] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeets 2004
- Smeets NW, Krekels GA, Ostertag JU, Essers BA, Dirksen CD, Nieman FH, et al. Surgical excision vs Mohs' micrographic surgery for basal‐cell carcinoma of the face: randomised controlled trial. Lancet 2004;364(9447):1766‐72. [PUBMED: 15541449] [DOI] [PubMed] [Google Scholar]
Sober 1979
- Sober AJ, Fitzpatrick TB, Mihm MC, Wise TG, Pearson BJ, Clark WH, et al. Early recognition of cutaneous melanoma. JAMA 1979;242(25):2795‐9. [PubMed] [Google Scholar]
STATA 15 [Computer program]
- StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC, 2017.
Stratigos 2015
- Stratigos A, Garbe C, Lebbe C, Malvehy J, Marmol V, Pehamberger H, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus‐based interdisciplinary guideline. European Journal of Cancer 2015;51(14):1989‐2007. [DOI] [PubMed] [Google Scholar]
Takwoingi 2010
- Takwoingi Y, Deeks J. MetaDAS: a SAS macro for meta‐analysis of diagnostic accuracy studies. User Guide Version 1.3. 2010. www.methods.cochrane.org/sites/methods.cochrane.org.sdt/files/public/uploads/MetaDAS%20Readme%20v1.3%20May%202012.pdf (accessed 30 July 2018).
Takwoingi 2013
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544‐54. [PUBMED: 23546566] [DOI] [PubMed] [Google Scholar]
Takwoingi 2017
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2017;26(4):1896‐1911. [DOI: 10.1177/0962280215592269; PUBMED: 26116616] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzellos 2014
- Tzellos T, Kyrgidis A, Mocellin S, Chan AW, Pilati P, Apalla Z. Interventions for melanoma in situ, including lentigo maligna. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD010308] [DOI] [PMC free article] [PubMed] [Google Scholar]
Usher‐Smith 2016
- Usher‐Smith JA, Sharp SJ, Griffin SJ. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ 2016;353:i3139. [DOI: 10.1136/bmj.i3139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Loo 2014
- Loo E, Mosterd K, Krekels GA, Roozeboom MH, Ostertag JU, Dirksen CD, et al. Surgical excision versus Mohs' micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow‐up. European Journal of Cancer 2014;50(17):3011‐20. [DOI] [PubMed] [Google Scholar]
Verkouteren 2017
- Verkouteren JA, Ramdas KH, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. British Journal of Dermatology 2017;177(2):359‐72. [DOI: 10.1111/bjd.15321.] [DOI] [PubMed] [Google Scholar]
Walker 2006
- Walker P, Hill D. Surgical treatment of basal cell carcinomas using standard postoperative histological assessment. Australasian Journal of Dermatology 2006;47(1):1‐12. [PUBMED: 16405477] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
WHO 2003
- WHO. Skin cancer FAQ. www.who.int/uv/faq/skincancer/en/index2.html (accessed 3rd February 2018).
Williams 2017
- Williams HC, Bath‐Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W, et al. Surgery versus 5% imiquimod for nodular and superficial basal cell carcinoma: 5‐year results of the SINS randomized controlled trial. Journal of Investigative Dermatology 2017;137(3):614‐9. [DOI: 10.1016/j.jid.2016.10.019; PUBMED: 27932240] [DOI] [PubMed] [Google Scholar]
Wong 2017
- Wong KY, Fife K, Lear JT, Price RD, Durrani AJ. Vismodegib for locally advanced periocular and orbital basal cell carcinoma: A review of 15 consecutive cases. Plastic and Reconstructive Surgery Global Open 2017;5(7):e1424. [PUBMED: 28831360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zak‐Prelich 2004
- Zak‐Prelich M, Narbutt J, Sysa‐Jedrzejowska A. Environmental risk factors predisposing to the development of basal cell carcinoma. Dermatologic Surgery 2004;30(2 Pt 2):248–252. [PUBMED: 14871217] [DOI] [PubMed] [Google Scholar]
Zalaudek 2008
- Zalaudek I, Giacomel J, Cabo H, Stefani A, Ferrara G, Hofmann‐Wellenhof R, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology 2008;216(1):14‐23. [PUBMED: 18032894] [DOI] [PubMed] [Google Scholar]
Zalaudek 2012
- Zalaudek I, Giacomel J, Schmid K, Bondino S, Rosendahl C, Cavicchini S, et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: a progression model. Journal of the American Academy of Dermatology 2012;66(4):589‐97. [PUBMED: 21839538] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dinnes 2015a
- Dinnes J, Wong KY, Gulati A, Chuchu N, Leonardi‐Bee J, Bayliss SE, et al. Tests to assist in the diagnosis of keratinocyte skin cancers in adults: a generic protocol. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011901] [DOI] [Google Scholar]
Dinnes 2015b
- Dinnes J, Matin RN, Moreau JF, Patel L, Chan SA, Wong KY, et al. Tests to assist in the diagnosis of cutaneous melanoma in adults: a generic protocol. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011902] [DOI] [Google Scholar]


