Abstract
Background
Nonsteroidal anti‐inflammatory drugs (NSAIDs) can play a major role in the management of acute pain in the peri‐operative period. However, there are conflicting views on whether NSAIDs are associated with adverse renal effects.
Objectives
The primary objective of this review was to determine the effects of NSAIDs on postoperative renal function in adults with normal preoperative renal function.
Search methods
Electronic searches for relevant randomised and quasi‐randomised controlled trials in Cochrane Central Register of Controlled Trials, MEDLINE and EMBASE were performed. Attempts were also made to identify trials from citation lists of relevant trials, review articles and clinical practice guidelines. Handsearching of conference abstracts published in major anaesthetic journals was also performed.
Selection criteria
The inclusion criteria were randomised or quasi‐randomised comparisons of individual NSAIDs with either each other or placebo for treatment of postoperative pain, with relevant postoperative renal outcome measures, in adult surgical patients with normal renal function.
Data collection and analysis
The data were extracted independently by two authors. The primary outcome measure was creatinine clearance within the first two days after surgery. Secondary outcome measures included serum creatinine, urine volume, urinary sodium level, urinary potassium level, fractional excretion of sodium, fractional excretion of potassium and need for dialysis. Mean differences (MD) for continuous outcomes and risk ratio (RR) and risk difference (RD) for dichotomous outcomes were estimated with 95% confidence intervals (CI).
Main results
Twenty‐three trials (1459 patients) fulfilled the selection criteria for this review. NSAIDs reduced creatinine clearance by 16 mL/min (95% CI 5 to 28) and potassium output by 38 mmol/day (95% CI 19 to 56) on the first day after surgery compared to placebo. There was no significant difference in serum creatinine on the first day (0 μmol/L, 95% CI ‐3 to 4) compared to placebo. No significant reduction in urine volume during the early postoperative period was found. There was no significant difference in serum creatinine in the early postoperative period between patients receiving diclofenac, ketorolac, indomethacin, ketoprofen or etodolac. No cases of postoperative renal failure requiring dialysis were described. The trials were not heterogeneous for the primary outcome.
Authors' conclusions
NSAIDs caused a clinically unimportant transient reduction in renal function in the early postoperative period in patients with normal preoperative renal function. NSAIDs should not be withheld from adults with normal preoperative renal function because of concerns about postoperative renal impairment.
Plain language summary
NSAIDs used for pain relief after surgery may have only small, temporary negative effects on kidney function in adults with normal renal function
Nonsteroidal anti‐inflammatory drugs (NSAIDs) can be used to try and relieve pain after surgery. However, there have been concerns about the possible harmful effects of these drugs on the kidneys. The review of trials found that NSAIDs can cause small, temporary negative effects on the kidneys in adults, but no one in the trials experienced renal failure or serious kidney problems. These results may not apply to children or adults with decreased kidney function
Background
Optimal postoperative pain management can include the selective use of nonsteroidal anti‐inflammatory drugs (NSAIDs) with or without supplemental opioids. The Royal College of Anaesthetists published Guidelines for the use of nonsteroidal anti‐inflammatory drugs in the peri‐operative period, an overview of the benefits and risks of using NSAIDs (RCA 1998). It concluded that in patients who had undergone major surgery, NSAIDs were not sufficiently effective as the sole agent and that renal function should be monitored regularly in these patients and in at‐risk patients (RCA 1998).
The peri‐operative use of NSAIDs may be limited because of concern with side effects relating to the gastrointestinal, coagulation and renal systems (Feldman 1997; Romsing 1997; Strom 1996). More recently, there has been intense interest in the cardiovascular effects of the selective inhibitors of cyclooxygenase 2. Recent evidence from a meta‐analysis of observational studies of selective and nonselective inhibitors of cyclooxygenase 2 (McGettigan 2006) suggest that there is an increase cardiovascular risk associated with rofecoxib and diclofenac, but not with celecoxib, naproxen, piroxicam or ibuprofen. For anaesthesiologists, particular attention has been given to the possibility of renal toxicity caused by the use of NSAIDs during the peri‐operative period (Myles 1998). NSAIDs may produce either acute, reversible or permanent renal toxicity and a variety of effects on electrolyte and water homeostasis (Murray 1993). The most important renal complication after surgery is acute renal failure. Acute renal failure is characterised by a deterioration of renal function over a period of hours to days, resulting in the failure of the kidney to excrete nitrogenous waste products and to maintain fluid and electrolyte homeostasis (Thadhani 1996). Morbidity and mortality are highly associated with postoperative acute renal failure (Novis 1994). While the definition of acute renal failure and renal insufficiency varies among studies, one study showed that the overall incidence of postoperative renal insufficiency was 18% after major surgery, with a subsequent hospital mortality rate of 13% (Hou 1983).
Although there have been case reports describing adverse renal effects of NSAIDs (Sivarajan 1997; Smith 1993), the evidence from randomised controlled trials (RCTs) is inconclusive. Several such studies (Aitken 1992; Irwin 1995; Perttunen 1992; Power 1992) showed that NSAIDs caused changes in electrolyte balance and urine output. In contrast, others have failed to show any significant effect of NSAIDS on renal function (Brinkmann 1998; Jones 2000, Laisalmi 2001a; Perttunen 1999; Turner 1994; Varrassi 1994). Most of these RCTs were limited to the effects of NSAIDs on the renal system within the first 48 hours in adults with normal renal function, and did not address the longer term effects on renal function or the safety of NSAIDs in patients with impaired renal function. In some of these trials, the results may have been imprecise because the sample size was insufficient to detect important differences. More recently, in a meta‐analysis of trials examining the use of adjunctive use of NSAIDs with narcotic analgesia in cardiothoracic surgery (Bainbridge 2006) showed no significant risk of renal dysfunction (odds ratio 0.95, 95% CI 0.37 to 2.46). Therefore, it is unclear whether there is a clinically significant effect of NSAIDs on renal function in the early postoperative period.
Objectives
To assess the effects of NSAIDs on postoperative renal function in adults with normal preoperative renal function.
We wished to test the following hypotheses:
Treatment with NSAIDs is more harmful on the renal system than placebo in the early postoperative period (first 48 hours after surgery)
Individual NSAIDs have similar harmful effects on the renal system in the early postoperative period (first 48 hours after surgery)
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐randomised (allocation based on alternation, date of birth, hospital medical record number) controlled trials of NSAID treatment versus placebo for treatment of postoperative pain.
All RCTs and quasi‐randomised controlled trials that compared two or more NSAID for treatment of postoperative pain.
Types of participants
All adult surgical patients with normal preoperative renal function.
Types of interventions
NSAID treatments (ketorolac, ibuprofen, diclofenac, indomethacin, tenoxicam, ketoprofen, etodolac, parecoxib) versus placebo. Variable doses and all routes of administration of NSAID treatment during the peri‐operative period were considered.
Types of outcome measures
Each of the following outcomes (within the first 48 hours after surgery) were recorded where available
Primary outcome
Change in creatinine clearance for timed urine measurement. We chose creatinine clearance as the primary outcome as it is a better measure of the glomerular filtration rate (renal function) than serum creatinine.
Secondary outcomes
Calculated creatinine clearance (based on the Cockcroft‐Gault formula from serum creatinine)
Serum creatinine
Urine volume
Urinary sodium level
Urinary potassium level
Fractional excretion of sodium
Fractional excretion of potassium
Need for dialysis
As there is no benefit of frusemide (Bennett‐Jones 2006) and dopamine (Sear 2005) for the treatment of acute renal failure, these were no longer considered as secondary outcomes. The long term harmful effects of NSAIDs are not considered. The need for dialysis is included as it is an important for the consumer.
Search methods for identification of studies
Relevant trials were obtained from the following sources:
The Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library, Issue 2, 2006)
Electronic databases: MEDLINE 1966‐May 2006, EMBASE 1980‐May 2006
Reference lists of relevant articles, reviews, trials and clinical practice guidelines (ANZCA 2005; RCA 1998)
Pharmaceutical industry representatives
Handsearching conference abstracts (1990 to May 1999) published in Acta Anaesthesiologica Scandinavica, Anaesthesia, Anaesthesia and Intensive Care, Anesthesia and Analgesia, Anesthesiology, British Journal of Anaesthesia and Canadian Journal of Anaesthesia.
There were no language restrictions. The first author was contacted to clarify issues related to data extraction.
All publications which described RCTs, clinical trials, and controlled trials were obtained using the optimal sensitive search strategy method (Chalmers 1995). In addition, the following MESH and text words were included in the MEDLINE electronic search strategy: NSAIDs, nonsteroidal, kidney failure, postoperative renal failure, postoperative. The MEDLINE search was modified to search for relevant trials in EMBASE.
Data collection and analysis
The selection of trials for inclusion in the review was performed independently by the authors (AL and MC). Trials were examined for duplicate data. Data was abstracted independently, by AL and MC, using a standardised data collection form. Discrepancies were resolved by discussion, or, if no consensus was reached, advice was sought from a third party (JC). The quality of eligible trials were assessed independently, under open conditions. The quality of allocation concealment was graded as A‐adequate, B‐unclear, or C‐inadequate, as previously described (Schulz 1995). Blinding, losses to follow‐up, method of randomisation, intention‐to‐treat analysis and power calculations were recorded.
The duration of treatment, type, and dose of NSAIDs, patient population, type of surgery, and anaesthetic details were collected. The primary outcome was change in creatinine clearance on Day 1 (0 to 24th hours) and Day 2 (24th to 48th hour) after surgery. A creatinine clearance reduction of 50% was chosen a priori as the threshold for a clinically important change. If the article reported measurements taken at multiple time points, the values at or near 24 or 48 hours after surgery were selected for analyses, because the 24 and 48 hour time points were most often reported in these studies. In cases where results were presented in graphs and no actual data were given, the data were extracted from the graphs or the primary author was contacted for clarification.
The DerSimonian and Laird random‐effects model was used to combine data for both continuous and dichotomous outcomes, because we expected that the treatments and conditions in these studies would be heterogeneous. This model incorporates both between‐study (different treatment effects) and within‐study (sampling error) variability (Mosteller 1996). The pooled risk ratio (RR) and risk difference (RD) and 95% confidence interval (95% CI) were calculated for dichotomous data (need for dialysis). Number needed to harm estimates (1/RD) were calculated to compare the harmful effects of NSAIDs.
For continuous outcomes, the mean and standard deviation for each treatment group, before and after the operation, were collected. The mean change from baseline to follow‐up, between treatment groups, was not given in trials. Therefore, as the correlation coefficient between preoperative and postoperative measures was unknown, we assumed a correlation of 0.50 (Follmann 1992). A sensitivity analysis was carried out assuming zero correlation. The standard deviation between preoperative and postoperative measures for each treatment group was estimated using a method outlined in the Cochrane Collaboration Handbook. When the median and interquartile range were reported, we assumed that the mean was equivalent to the median and estimated the standard deviation to be interquartile range/1.35 (O'Rourke 2002).
For each continuous outcome, the mean difference in each study was defined:
mean difference = [NSAIDs (post ‐ pre)] ‐ [Placebo (post ‐ pre)]
where "post" represented a postoperative measure and "pre" represented a preoperative measure. A postoperative measure was either at Day 1 or Day 2 after surgery.
A mean difference (MD) method was used to pool continuous data for each of the following outcomes: creatinine clearance, serum creatinine, urine volume, sodium output, potassium output, fractional excretion of sodium and fractional excretion of potassium. These were analysed separately for Day 1 and Day 2. These results are reported as MD and 95% CI.
Heterogeneity was analysed using the Q‐statistic with a threshold for the P value < 0.10 and the I² test (Higgins 2003). Subgroup analyses were done to estimate the robustness of results according to the type and dose (single versus multiple dose regimen, or comparison of two or more dosage regimen) of NSAID given. Subgroup analyses on trial quality, type of surgery (cardiac versus noncardiac) and cyclooxygenase inhibitor (selective versus nonselective) were not performed as there were insufficient number of trials for a meaningful interpretation to be made. Publication bias was to be assessed using a funnel plot, however there were insufficient studies to do so.
Results
Description of studies
Forty‐two RCTs of NSAIDs for postoperative pain with relevant renal outcome measures were identified.
Trials excluded from this review
Nineteen trials were excluded from the review. One of these (Fredman 1999) examined the effect of diclofenac on intra operative renal blood flow and glomerular filtration rate, and did not collect postoperative renal outcome measures. The other study (Horneffer 1990) was excluded because NSAIDs (ibuprofen or indomethacin) were administered two days after cardiac surgery to treat post‐pericardiotomy syndrome. Acute renal failure up to 30 days after surgery was the outcome used in several RCTs (Forrest 2002; Nussmeier 2005; Nussmeier 2006; Ott 2003), but there was a small percentage of patients with a history of renal insufficiency included in these trials. No additional relevant renal outcome measures were reported in the second paper by Laisalmi (Laisalmi 2001b)
Trials included in this review
Twenty‐three RCTs (1459 patients) met the criteria for inclusion in the review. The trials were conducted between 1992 and 2006. The participants were all adults with normal preoperative renal function. Patients underwent various types of surgery, ranging from minor orthopaedic surgery (Irwin 1995) to major abdominal surgery (Castiglione 1997; Rao 2000). Five studies described patients undergoing cardiac surgery (Hynninen 2000; Immer 2003; Khalil 2006; Kulik 2004; Rapanos 1999). Where specific details were given, surgery was on an elective basis. All patients underwent general anaesthesia, with additional regional anaesthesia in three studies (Brinkmann 1998; Jones 2000; Perttunen 1992). Adequate preoperative and postoperative hydration treatment was described in only one study (Slaven 1998). We did not collect data on fluid or blood losses during surgery. There was insufficient data for meta‐analysis in nine studies (Castiglione 1997; Chow 2001; Kostamovaara 1996; Nuutinen 1991; Parker 1994; Rao 2000; Ready 1994; Turner 1994; Varrassi 1994). Reasons for insufficient data for pooling are outlined in the notes section of each trial (see Table of Included Studies), and included lack of data for "pre" and "post" surgery and insufficient details to met the outcome definition above.
NSAIDs examined included diclofenac, ketorolac, indomethacin, ketoprofen, tenoxicam, ibuprofen, naproxen, etodolac and parecoxib. Etodolac (Immer 2003) and parecoxib (Khalil 2006) are selective cyclooxygenase (COX)‐2 inhibitors. The route of NSAID administration varied (intravenous bolus, intravenous infusions, suppositories, intramuscular, orally or combinations of these). A single dose NSAID regimen was used in four studies (Brinkmann 1998; Jones 2000; Khalil 2006; Slaven 1998). Creatinine clearance was collected in nine studies (Nuutinen 1991; Aitken 1992; Brinkmann 1998; Immer 2003; Irwin 1995; Jones 2000; Khalil 2006; Nuutinen 1991; Slaven 1998) but sufficient data for meta‐analysis was available in seven studies (Aitken 1992; Brinkmann 1998; Jones 2000; Khalil 2006; Power 1992; Slaven 1998). The intermittent ketorolac arm (10 mg every four hours intramuscular) was chosen randomly over the continuous ketorolac arm (intramuscular infusion) in the Aitken 1992 trial for the purposes of this review. Data from the Perttunen 1999 trial using the diclofenac arm, not ketorolac arm, was pooled for comparisons between NSAIDs and placebo. Diclofenac, not ketoprofen or indomethacin, was pooled for comparisons between NSAIDs and placebo in another trial (Hynninen 2000).
Risk of bias in included studies
Ten trials (Hynninen 2000; Jones 2000; Laisalmi 2001a; Kulik 2004; Khalil 2006; Perttunen 1999; Rao 2000; Rapanos 1999; Ready 1994; Turner 1994) had adequate allocation of concealment (A). The remaining trials received an allocation score of B (unclear). Double‐blinding was used in 20 trials and single blinding was used in one trial (Slaven 1998). The majority of trials did not specifically state that they had used an intention‐to‐treat analysis. Power calculations were done in six trials (Hynninen 2000; Jones 2000; Khalil 2006; Kulik 2004; Rao 2000; Rapanos 1999). Withdrawals were less than 10% in all trials, except Chow 2001(15%), Kulik 2004 (16%) and Ready 1994 (31%).
Effects of interventions
NSAIDs versus placebo
All studies pooled for analysis had mild to moderate amounts of heterogeneity, except serum creatinine on Day 2. There were no reported cases of postoperative renal failure requiring dialysis. None of the studies estimated creatinine clearance based on the Cockcroft‐Gault formula. When creatinine clearance was pooled from all trials, NSAIDs significantly reduced creatinine clearance by 16 mL/min (95% CI 5 to 28) on Day 1 (Analysis 1.1.1). This was equivalent to 18% (95% CI 6% to 31%) reduction from the preoperative level. A sensitivity analysis (assuming zero correlation between measurements over time) showed that NSAIDs reduced creatinine clearance by 1 to 36% on Day 1. On Day 2, there was no significant reduction (Analysis 1.1.2). A subgroup analysis based on dosing regimen showed that multiple NSAID dosing was associated with a significant reduction in creatinine clearance on Day 1 (‐25 mL/min, 95% CI ‐7 to ‐42). In comparison, single NSAID dose administration in three studies (Brinkmann 1998; Jones 2000; Slaven 1998) was not significantly associated with a reduction in creatinine clearance (‐10 mL/min, 95% CI ‐26 to +5). However, overall comparisons of the subgroups showed no significant difference (P = 0.23). The overall comparison of the subgroups (multiple versus single dosing) on Day 2 showed no significant difference (P = 0.71).
1.1. Analysis.
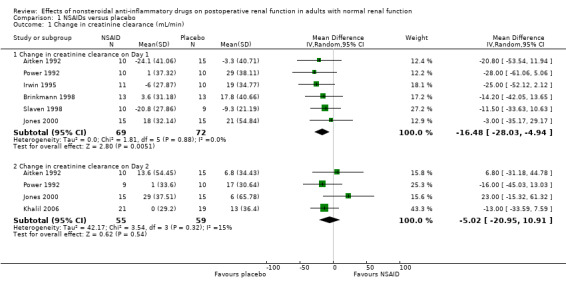
Comparison 1 NSAIDs versus placebo, Outcome 1 Change in creatinine clearance (mL/min).
There was no significant difference in serum creatinine between NSAIDs and placebo on Day 1 or Day 2 (Analysis 1.2). Despite an inadequate definition of oliguria, the proportion of patients given ketorolac or placebo who became oliguric was similar (4% versus 3% respectively; P = 0.72) (Ready 1994). There was no significant reduction in urine volume on Day 1 (‐15 mL/min, 95% CI ‐32 to +1) or on Day 2 (‐3 mL/min, 95% CI ‐19 to +14) after surgery (Analysis 1.3). There was no significant reduction in urinary sodium levels on Day 1 or Day 2 (Analysis 1.4). However, there was significant reduction in urinary potassium levels on Day 1 (‐38 mmol/L, 95% CI ‐56 to ‐19; Analysis 1.5.1), but not on Day 2 (‐15 mmol/L, 95% CI ‐39 to +9; Analysis 1.5.2). The reductions in fractional sodium and potassium excretion were not significant on Day 1 or on Day 2 (Analysis 1.6).
1.2. Analysis.
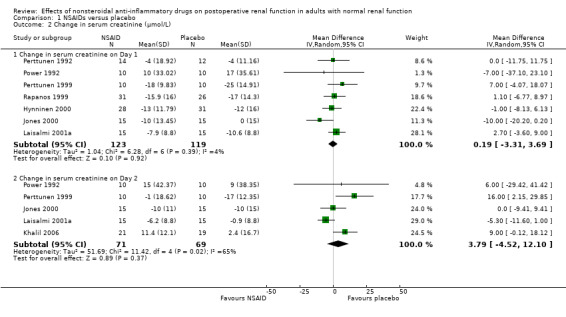
Comparison 1 NSAIDs versus placebo, Outcome 2 Change in serum creatinine (μmol/L).
1.3. Analysis.
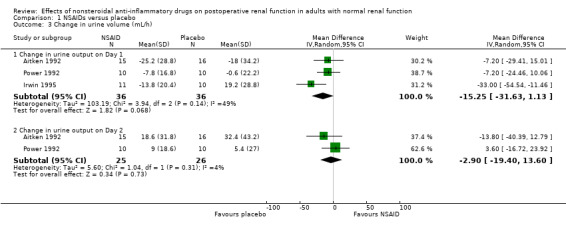
Comparison 1 NSAIDs versus placebo, Outcome 3 Change in urine volume (mL/h).
1.4. Analysis.
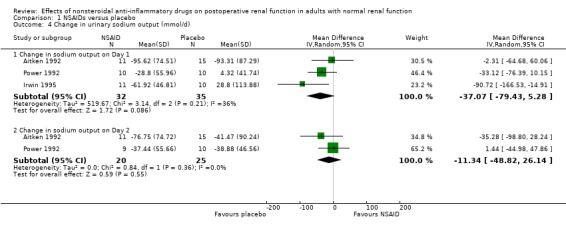
Comparison 1 NSAIDs versus placebo, Outcome 4 Change in urinary sodium output (mmol/d).
1.5. Analysis.
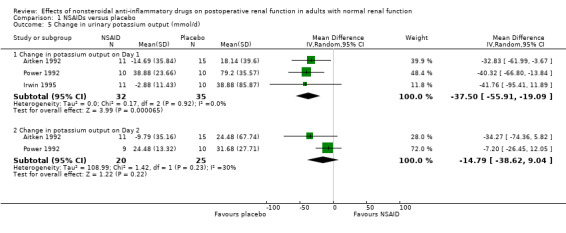
Comparison 1 NSAIDs versus placebo, Outcome 5 Change in urinary potassium output (mmol/d).
1.6. Analysis.
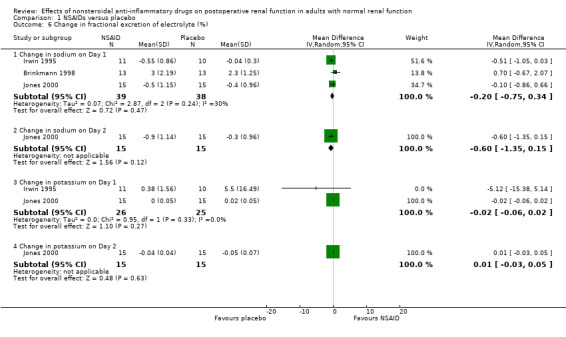
Comparison 1 NSAIDs versus placebo, Outcome 6 Change in fractional excretion of electrolyte (%).
NSAID versus NSAID
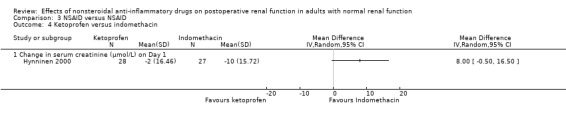
There were three trials (Hynninen 2000; Immer 2003; Perttunen 1999) that directly compared different types of NSAIDs. There was a significant reduction in serum creatinine associated with diclofenac compared to ketoprofen on Day 1(Hynninen 2000) (Analysis 3.2.1). On Day 2, there was no significant reduction in serum creatinine associated with diclofenac compared to ketorolac (Perttunen 1999; Analysis 3.1.2) or etodolac (Immer 2003; Analysis 3.5.1).
3.2. Analysis.
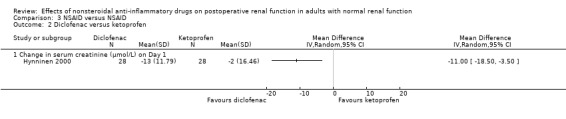
Comparison 3 NSAID versus NSAID, Outcome 2 Diclofenac versus ketoprofen.
3.1. Analysis.
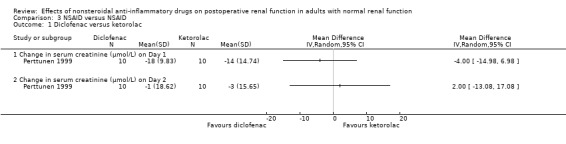
Comparison 3 NSAID versus NSAID, Outcome 1 Diclofenac versus ketorolac.
3.5. Analysis.
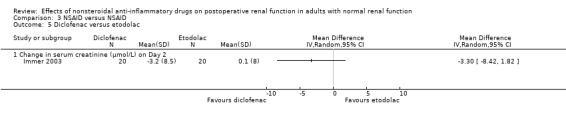
Comparison 3 NSAID versus NSAID, Outcome 5 Diclofenac versus etodolac.
There was one trial (Castiglione 1997) that assessed two ketorolac dose regimens (270 mg versus 240 mg over 48 hours). They found no significant differences in serum creatinine levels on Day 2 between the two regimens.
Discussion
This systematic review has shown that NSAIDs caused a clinically unimportant reduction in renal function on the first day after surgery in patients with normal preoperative renal function. The reduction in creatinine clearance on the first day by NSAIDs was up to 31% (up to 36% using sensitivity analysis of zero correlation of measurements over time), which is less than the clinically important reduction threshold set a priori. The fact that this reduction did not affect urine volume or that no patients required dialysis confirms that it is clinically unimportant. There was no evidence of a reduction in creatinine clearance by NSAIDs on the second day after surgery.
Overall, transient postoperative creatinine clearance and electrolyte homeostasis disturbances attributed to the use of NSAIDs were found. The mechanism by which NSAIDs affect the renal system is complex. Inhibition of prostaglandin synthesis by NSAIDs can decrease distal tubular flow rate and sodium delivery, by reducing the glomerular filtration rate and increasing tubular reabsorption of sodium (Bugge 1995). Inhibition of prostaglandins leads to a moderate decline in aldosterone, which may contribute to potassium retention (Bugge 1995). The mode of action at the cellular level of NSAIDs in producing renal impairment is reviewed elsewhere (Murray 1993).
There was no strong evidence that NSAIDs caused postoperative renal failure in adults with normal preoperative renal function. None of the adults required dialysis for acute renal failure. A retrospective cohort (Feldman 1997) of inpatients receiving parenteral ketorolac and opioids for two days showed that the ketorolac group were at no greater risk of acute renal failure compared to the opioid group (adjusted RR 0.86, 95% CI 0.63 to 1.17). However, it is plausible that NSAIDs may cause postoperative renal failure in patients with pre‐existing impaired renal blood flow, such as the elderly, those with heart failure or shock, or patients exposed to other nephrotoxic agents (Thadhani 1996). The information about risk factors for postoperative renal impairment has mainly been derived from a qualitative systematic review (Novis 1994) of observational studies and case reports (Reynolds 2003; Sivarajan 1997; Smith 1993).
A limitation of this review was the use of several surrogate measures of renal function for postoperative renal failure. These renal function tests have varying sensitivity and specificity for predicting the onset of peri‐operative renal dysfunction (Kellen 1994). Serial determination of creatinine clearance is one of the most sensitive tests for predicting the onset of peri‐operative renal dysfunction (Kellen 1994). Creatinine clearance is a better alternative than serum creatinine in measuring renal function (Wijeysundera 2006) as the glomerular filtration rate may be reduced by 75% before serum creatinine becomes abnormal (Kellen 1994). This explains why we found a significant transient reduction in creatinine clearance but no significant increase in serum creatinine. Creatinine clearance involving urine collections over 24 hours overestimates the glomerular filtration rate by 13% (Waller 1991). This confirms our view that the reduction in creatinine clearance was clinically unimportant. As testing creatinine clearance by urine collection is time‐consuming and labour intensive (Kellen 1994), few studies included in this systematic review collected creatinine clearance for more than a day. Aitken 1992, Jones 2000, Khalil 2006 and Power 1992 suggest that there was a trend towards normal renal function on the second day after surgery after the use of NSAIDs. Although none of the NSAIDs versus NSAIDs trials examined creatinine clearance within 48 hours after surgery in this systematic review, the reduction in creatinine clearance from baseline was similar in diclofenac (10%) and etodolac (11%) groups on Day 4 (Immer 2003).
Another consideration is that the different NSAIDs were combined to assess the overall adverse renal effects caused by this class of drugs and there was little evidence of statistical heterogeneity between the studies. All trials used the recommended maximum doses of NSAIDs in adults with normal baseline renal function. While the cyclooxygenase‐1 (COX‐1) and cyclooxygenase‐2 (COX‐2) inhibition ratios would be different for various NSAIDs (Cryer 1998), there is no direct and current evidence to suggest that this makes a difference to the risk of renal impairment. Direct comparisons between diclofenac and ketorolac (Perttunen 1999), diclofenac and indomethacin (Hynninen 2000) and diclofenac and etodolac (Immer 2003) showed similar minor effects on serum creatinine. However, there was some evidence that ketoprofen may be associated with a 20% increase in serum creatinine compared with diclofenac (Hynninen 2000).
Authors' conclusions
Implications for practice.
While the use of NSAIDs as sole analgesics has not been justified, the efficacy of NSAIDs as components of multimodal analgesia has been confirmed (ANZCA 2005). In considering the adverse renal effects of NSAIDs, this review has shown that there was a clinically unimportant transient reduction in renal function in the early postoperative period in a wide variety of surgical settings in patients with normal preoperative renal function. It should be noted that the findings may not be transferable to paediatric patients (in whom the renal effects of postoperative NSAIDs have not been adequately studied) or to those patients with pre‐existing abnormal renal function. NSAIDs should not be withheld in adults with normal preoperative renal function because of concerns about postoperative renal impairment.
Implications for research.
Recent work suggests that different types of NSAIDs inhibit cyclooxygenase‐1 (COX‐1) and cyclooxygenase‐2 (COX‐2) to a varying extent (Cryer 1998). COX‐1 is responsible for the production of prostaglandins in all tissues, while COX‐2 is expressed only after trauma or inflammation (Vane 1997). NSAIDs that have a high COX‐2:COX‐1 ratio may have more potent anti‐inflammatory activity with fewer side‐effects than drugs with lower COX‐2:COX‐1 ratio (Vane 1997). COX‐2 selective inhibitors have not been available in many countries or been used widely by anaesthetists for postoperative pain management because of cardiovascular complications (myocardial infarction, atrial fibrillation, stroke). More trials comparing COX‐2 selective inhibitors with older types of NSAIDs are needed in surgical patients to assess the cost‐incremental benefit of using COX‐2 selective inhibitors and risk reduction of adverse renal effects.
What's new
| Date | Event | Description |
|---|---|---|
| 5 December 2018 | Review declared as stable | This review is no longer being updated. Please refer to: Bell S, Rennie T, Marwick CA, Davey P. Effects of peri‐operative nonsteroidal anti‐inflammatory drugs on post‐operative kidney function for adults with normal kidney function. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No.: CD011274. DOI: 10.1002/14651858.CD011274.pub2. |
| 5 December 2018 | Amended | This review has been replaced by Bell 2018 using the new KDIQO definition for AKI |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Contact details updated. |
| 29 September 2008 | Amended | Converted to new review format. |
| 21 December 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
2018: This review has been replaced by "Effects of peri‐operative nonsteroidal anti‐inflammatory drugs on post‐operative kidney function for adults with normal kidney function" (Bell 2018). THe KDIGO definition for AKI has been used in the new review.
The original work was done at the Centre for Kidney Research, The Children's Hospital at Westmead, Westmead, NSW, Australia. Subsequent updates were done at the Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong.
Acknowledgements
We thank Dr Brinkmann, Dr Walker and Dr Marks for clarification of their data.
Data and analyses
Comparison 1. NSAIDs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in creatinine clearance (mL/min) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Change in creatinine clearance on Day 1 | 6 | 141 | Mean Difference (IV, Random, 95% CI) | ‐16.48 [‐28.03, ‐4.94] |
| 1.2 Change in creatinine clearance on Day 2 | 4 | 114 | Mean Difference (IV, Random, 95% CI) | ‐5.02 [‐20.95, 10.91] |
| 2 Change in serum creatinine (μmol/L) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Change in serum creatinine on Day 1 | 7 | 242 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐3.31, 3.69] |
| 2.2 Change in serum creatinine on Day 2 | 5 | 140 | Mean Difference (IV, Random, 95% CI) | 3.79 [‐4.52, 12.10] |
| 3 Change in urine volume (mL/h) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Change in urine output on Day 1 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐15.25 [‐31.63, 1.13] |
| 3.2 Change in urine output on Day 2 | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐19.40, 13.60] |
| 4 Change in urinary sodium output (mmol/d) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Change in sodium output on Day 1 | 3 | 67 | Mean Difference (IV, Random, 95% CI) | ‐37.07 [‐79.43, 5.28] |
| 4.2 Change in sodium output on Day 2 | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐11.34 [‐48.82, 26.14] |
| 5 Change in urinary potassium output (mmol/d) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Change in potassium output on Day 1 | 3 | 67 | Mean Difference (IV, Random, 95% CI) | ‐37.50 [‐55.91, ‐19.09] |
| 5.2 Change in potassium output on Day 2 | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐14.79 [‐38.62, 9.04] |
| 6 Change in fractional excretion of electrolyte (%) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Change in sodium on Day 1 | 3 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.75, 0.34] |
| 6.2 Change in sodium on Day 2 | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.6 [‐1.35, 0.15] |
| 6.3 Change in potassium on Day 1 | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 6.4 Change in potassium on Day 2 | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
Comparison 2. Multiple versus single NSAID dose regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in creatinine clearance (mL/min) on Day 1 | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Multiple NSAID doses versus placebo | 3 | 66 | Mean Difference (IV, Random, 95% CI) | ‐24.63 [‐42.29, ‐6.98] |
| 1.2 Single NSAID dose versus placebo | 3 | 75 | Mean Difference (IV, Random, 95% CI) | ‐10.40 [‐25.65, 4.86] |
| 2 Change in creatinine clearance (mL/min) on Day 2 | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Multiple NSAID doses versus placebo | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐7.59 [‐30.66, 15.47] |
| 2.2 Single NSAID dose versus placebo | 2 | 70 | Mean Difference (IV, Random, 95% CI) | 1.22 [‐33.27, 35.72] |
2.1. Analysis.
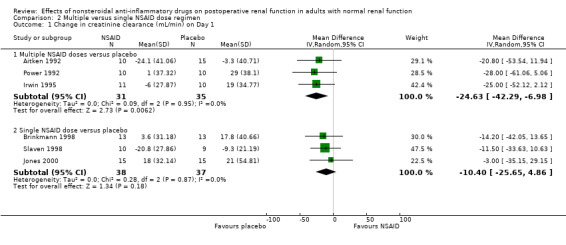
Comparison 2 Multiple versus single NSAID dose regimen, Outcome 1 Change in creatinine clearance (mL/min) on Day 1.
2.2. Analysis.
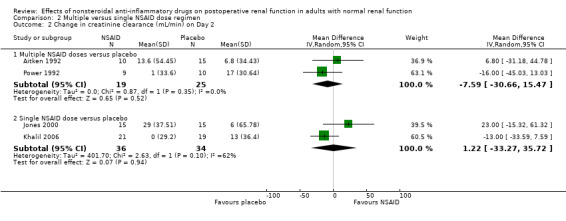
Comparison 2 Multiple versus single NSAID dose regimen, Outcome 2 Change in creatinine clearance (mL/min) on Day 2.
Comparison 3. NSAID versus NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diclofenac versus ketorolac | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Change in serum creatinine (μmol/L) on Day 1 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Change in serum creatinine (μmol/L) on Day 2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Diclofenac versus ketoprofen | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Change in serum creatinine (μmol/L) on Day 1 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Diclofenac versus indomethacin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Change in serum creatinine (μmol/L) on Day 1 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Ketoprofen versus indomethacin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Change in serum creatinine (μmol/L) on Day 1 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Diclofenac versus etodolac | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Change in serum creatinine (μmol/L) on Day 2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.3. Analysis.
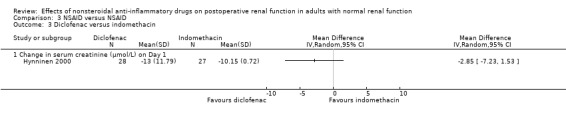
Comparison 3 NSAID versus NSAID, Outcome 3 Diclofenac versus indomethacin.
3.4. Analysis.
Comparison 3 NSAID versus NSAID, Outcome 4 Ketoprofen versus indomethacin.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aitken 1992.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. Four patients withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 67 patients undergoing elective upper abdominal surgery. Exclusions: respiratory insufficiency, hepatic or renal impairment and abuse of alcohol or drugs. | |
| Interventions | Rx 1: ketorolac 12.5 mg/h IM infusion for 30 minutes during surgery then 2.5 mg/h for 47.5 hours, with normal saline injections every 4 hours Rx 2: ketorolac 10 mg every 4 hours IM for 48 hours, first dose during surgery. Pl: Intermittent and continuous infusions of saline to match other groups | |
| Outcomes | Pre‐operative and post‐operative creatinine clearance, urine output, sodium output, potassium output | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Brinkmann 1998.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. No patients withdrew from study. No power calculation. | |
| Participants | 26 (22 males, 4 females) patients undergoing infrarenal aortic surgery. Exclusions: NSAIDs at least 7 days prior to surgery, history of renal disease, evidence for renal artery stenosis on preoperative aortography, drugs likely to alter renal function. | |
| Interventions | Rx: ibuprofen 400 mg IV before skin incision Pl: Placebo aliquot IV before skin incision | |
| Outcomes | Pre‐operative and post‐operative creatinine clearance, fractional excretion of sodium, number of patients given diuretic or dopamine to treat post‐operative renal insufficiency. | |
| Notes | All patients were given post‐operative dopamine. Frusemide 10 mg IV was given when urine output was less than 0.5 mL/kg/h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Castiglione 1997.
| Methods | Blinding not stated. Randomised controlled trial. Randomisation method: not stated. No patients withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 40 patients (18 to 70 years) undergoing major elective abdominal surgery. Exclusions: renal disease, hepatic disease, coagulopathy, history of allergy to NSAIDs or peptic ulcer. | |
| Interventions | Rx 1: 30 mg ketorolac IV at induction, 30 mg IV at skin closure then 30 mg IV every 6 hours for 48 hours. Rx 2: 30 mg ketorolac IV at skin closure then 30 mg IV every 6 hours for 48 hours. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine. | |
| Notes | Article in Italian. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chow 2001.
| Methods | Double‐blind randomised placebo controlled trial. 10 patients withdrew (intraoperative haemostasis concerns, conversion to open surgery, need for an extraction incision, nursing failure to administer the drug, voluntary withdrawal). Sample size not calculated. Per‐protocol analysis. | |
| Participants | 55 (26 males, 29 females) patients undergoing laparoscopic urologic surgery. Exclusions: history of peptic ulcer/gastrointestinal bleeding, pregnancy, history of NSAID allergy/intolerance, or history of renal insufficiency (serum creatinine > 140 μmol/L). | |
| Interventions | Rx1: Ketorolac 15 to 30 mg IV every 6 hours up to 48 hours after surgery. First dose given at end of surgery. Pl: No details. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine. | |
| Notes | Time at which post‐operative serum creatinine was done within the first 48 hours was not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hynninen 2000.
| Methods | Double‐blind randomised, placebo controlled trial. Randomisation and preparation of study drugs into identically shaped suppositories was done by the hospital pharmacy. 6 patients withdrew. Sample size calculated. Per‐protocol analysis done. | |
| Participants | 114 adults undergoing coronary artery bypass grafting. Exclusion criteria: ejection fraction< 20%, previous cardiac surgery, insulin dependent diabetes mellitus, weight > 100 kg or < 60 kg, renal insufficiency (creatinine > 130 μmol/L), allergy to propofol, morphine or NSAID, active peptic ulcer disease, history of gastrointestinal bleeding, age > 75 years, warfarin, dipyridamole or heparin therapy preoperatively. | |
| Interventions | Rx:1: Diclofenac 75 mg suppository twice a day after surgery. Rx2: Ketoprofen 100 mg suppository twice a day after surgery Rx: Indomethacin 100 mg suppository twice a day after surgery Pl: Placebo suppository twice a day after surgery | |
| Outcomes | Pre‐operative and post‐operative serum creatinine. | |
| Notes | 1 patient was withdrawn after one dose of indomethacin because of serum creatinine increase > 20% postoperatively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Immer 2003.
| Methods | Randomisation method: not stated. 60 patients randomly allocated to diclofenac, etodolac or tramadol. No details about blinding. No loss of follow‐up. No power calculation. | |
| Participants | Patients undergoing coronary artery bypass operation. Exclusion: aged more than 70 years, left ventricular ejection fraction less than 30%, previous history of peptic ulcer disease or gastrointestinal bleeding, hepatic or renal insufficiency, known allergy to tramadol or NSAIDs, and preoperative analgesic treatment. Postoperative period exclusion criteria were delayed transfer to the general ward, serum creatinine more than 150 μmol/L, and altered mental status. | |
| Interventions | Rx1: diclofenac 50 mg every 8 hours orally on postoperative days 2 and 3. Rx2: etodolac 300 mg every 8 hours orally on postoperative days 2 and 3 | |
| Outcomes | Pre‐operative and post‐operative serum creatinine. | |
| Notes | Tramadol group (weak opioid) not included in analysis. Postoperative day 1 serum creatinine data not included as study drugs were not given. Creatinine clearance measured on postoperative day 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Irwin 1995.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method not stated. One patient withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 22 males undergoing minor orthopaedic surgery. Exclusions: patients with respiratory, cardiac, hepatic or renal insufficiency, a history of peptic ulcer disease or allergy to aspirin, diclofenac or other prostaglandin inhibiting compounds. | |
| Interventions | Rx: Diclofenac 100 mg suppository before surgery then 100 mg on Day 1 Pl: Placebo suppository before surgery and on Day 1 | |
| Outcomes | Pre‐operative and post‐operative creatinine clearance, urine output, sodium output, potassium output, fractional excretion of sodium, fractional excretion of potassium. | |
| Notes | Day 2 measures were not used as no diclofenac was administered on Day 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Jones 2000.
| Methods | Double‐blind, randomised placebo controlled trial. No patient withdrawal. Sample size calculated. Intention‐to‐treat analysis done. | |
| Participants | 30 women (50 to 70 years) undergoing major gynaecological surgery. Exclusions: renal or hepatic impairment, bleeding diathesis, hypersensitivity to NSAIDs, asthma, medications known to interfere with tenoxicam disposition. | |
| Interventions | Rx: Tenoxicam 20 mg IV given 2 hours before surgery. Pl: Normal saline IV given 2 hours before surgery. | |
| Outcomes | Pre‐operative and post‐operative creatinine clearance, serum creatinine, fractional excretion of sodium and potassium. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Khalil 2006.
| Methods | Double‐blind randomised, placebo controlled trial. Randomisation method: achieved using a random number generator, and the results placed into consecutively numbered sealed envelopes by a third party not involved in the study. The envelopes were opened at close of surgery also by a third party not involved in the study. Intended sample size was 60 patients but study ended early due to manufacturer's global announcement of parecoxib contraindications. | |
| Participants | Adult less than 70 years, scheduled for elective coronary artery bypass grafting. Exclusion: diabetics, patients on anticoagulants, and those with previous cerebrovascular disease. | |
| Interventions | Rx: parecoxib 40 mg IV at end of surgery. Pl: normal saline IV at end of surgery. | |
| Outcomes | Creatinine clearance, serum creatinine, oliguria (urine output < 0.5 mL/kg/h for more than 1 hour that persisted after correction of hypovolaemia and/or hypotension) treated with 40 mg IV bolus frusemide. | |
| Notes | Contacted author to verify creatinine clearance data for each group as there was error in the text on page 175. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kostamovaara 1996.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. Three patients withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 76 (26 males, 50 females) undergoing total hip (n = 62) or knee (n = 14) replacement. Exclusions: hepatic, renal or cardiac failure, bleeding or coagulation disorders, peptic ulcer, asthma, hypersensitivity to aspirin or other NSAIDs, or who were on cytostatic treatment | |
| Interventions | Rx 1: 50 mg ketoprofen IV loading dose for 30 minutes, followed 50 mg ketoprofen infusion over following 11.5 hours. Rx 2: 100 mg ketoprofen IV loading dose for 30 minutes, followed 100 mg ketoprofen infusion over following 11.5 hours. Rx 3: 150 mg ketoprofen IV loading dose for 30 minutes, followed 150 mg ketoprofen infusion over following 11.5 hours. Pl: Isotonic saline infusion for 30 minutes, followed by saline over following 11.5 hours (n = 19). | |
| Outcomes | Pre‐operative and Day 2 serum creatinine. | |
| Notes | Not pooled because serum creatinine was measured after drug had been eliminated (more than 5 half‐life). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kulik 2004.
| Methods | Double‐blind randomised, placebo‐controlled trial. Computer‐generated randomisation schedule. Medications prepared by hospital pharmacy and appeared identical. Sample size calculated. Intention‐to‐treat analysis. | |
| Participants | 98 patients undergoing elective coronary artery bypass graft. Exclusions: left ventricle ejection fraction < 20%, serum creatinine > 130 μmol/L, preoperative use of H2 antagonists, proton pump inhibitors, steroids, NSAIDs (with exception of aspirin), narcotics or illicit drugs, a history of peptic ulcer, liver disease or NSAID allergy. | |
| Interventions | Rx: naproxen 500 mg rectal suppository within 1 hour after arrival in the recovery room, then every 12 hours for a total of 5 doses; followed by naproxen 250 mg orally three times a day for 2 days. Pl: placebo suppositories and placebo tablets administered in a similar way as the treatment group. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine, inotropic use for renal dysfunction. | |
| Notes | Unpublished Day 1 and Day 2 serum creatinine and inotropic use requested from authors. 7 did not receive naproxen because of prolonged cardiopulmonary bypass time, perioperative stroke, anorexia and protocol violations. 9 did not receive placebo because of cardiac arrest, perioperative myocardial infarction, elevated baseline creatinine, excessive chest tube output and protocol violations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Laisalmi 2001a.
| Methods | Double‐blind randomised placebo controlled trial. Randomisation: by sealed envelopes. No patient withdrew. Intention‐to‐treat analysis. No power calculation done. | |
| Participants | 30 women undergoing breast surgery. Exclusions: abnormal renal or hepatic function. | |
| Interventions | Rx: Ketorolac 30 mg IM with premedication, at end of anaesthesia, and 6 hours after anaesthesia. Pl: Normal saline IM with premedication, at end of anaesthesia, and 6 hours after anaesthesia. | |
| Outcomes | Pre‐operative and postoperative serum creatinine. | |
| Notes | No pre‐operative urine output measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Nuutinen 1991.
| Methods | Randomisation method: not stated. Patients randomly allocated to diclofenac or placebo. No details about blinding. No loss to follow‐up. No details about intention to treat analysis or power calculation. | |
| Participants | Patients undergoing total hip replacement. Exclusions: no details. | |
| Interventions | Rx: Diclofenac infusion for 20 hours post‐operatively, a bolus of 75 mg over 30 minutes, followed by infusion of 4 mg/h, then 50 mg three times a day for 10 days in ward. Pl: Normal saline infusion for 20 hours post‐operatively, followed by dextropropoxyfen 65 mg orally. | |
| Outcomes | Creatinine clearance, serum creatinine, urinary sodium level, urinary potassium level, urine volume. | |
| Notes | Data insufficient for pooling. Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Parker 1994.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. Twelve patients withdrew from study. No intention to treat analysis but power calculation done | |
| Participants | 210 women undergoing abdominal hysterectomy. Exclusions: major organ dysfunction, history of allergic reactions to opioid analgesics or NSAIDs, bronchial asthma, gastrointestinal ulceration, bleeding disorders, or concurrent anticoagulant therapy. | |
| Interventions | Rx: Ketorolac 60 mg IV bolus before end of surgery then 30 mg over 30 minutes every 6 hours for 72 hours Pl: 2 mL normal saline IV before end of surgery then 20 mL normal saline IV infusion over 30 minutes every 6 hours for 72 hours | |
| Outcomes | Pre‐operative and hospital discharge serum creatinine. | |
| Notes | Median serum creatinine levels given but time of hospital discharge was variable among women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Perttunen 1992.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. No patients withdrew from study. No power calculation. | |
| Participants | 30 (24 males, 16 females) patients undergoing thoracotomy. Exclusions: aged more than 75 years; clinically manifest cardiac, renal or hepatic failure; history of gastrointestinal bleeding or peptic ulceration, haemorrhagic diathesis and asthma or allergy to aspirin or diclofenac; confusion, estimated preoperative FEV1<1 L/s. | |
| Interventions | Rx: diclofenac 25 mg IV bolus on arrival into recovery room then 2 mg/kg IV infusion for 48 hours Pl: saline infusion started with bolus dose of 25 mL in 15 minutes and continued with a constant rate of 2 mL/kg/d for 48 hours. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine, proportion of patients with urine output less then 100 mL during Day 1. | |
| Notes | No pre‐operative urine output measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Perttunen 1999.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation: by sealed envelopes. No patients withdrew from study. No power calculation. | |
| Participants | 30 (14 males, 16 females) patients undergoing thoracoscopy. Exclusions: patients aged more than 75 years; with cardiac, renal or hepatic failure; history of gastrointestinal bleeding or peptic ulceration, haemorrhagic diathesis and asthma, or allergy to aspirin, NSAIDs or morphine; confusion, preoperative FEV1 < 60% of reference value, sleep apnoea. | |
| Interventions | Rx 1: diclofenac 17 mg IV bolus one hour before anaesthesia then 2 mg/kg/d IV infusion for 48 hours Rx 2: ketorolac 10 mg IV bolus one hour before anaesthesia then 1.2 mg/kg/d IV infusion for 48 hours Pl: saline bolus dose 17 mL in 30 minutes and continued with 2 ml/kg/d for 48 hours. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine. | |
| Notes | No pre‐operative urine output measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Power 1992.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. One patient withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 20 (17 males, 3 females) patients undergoing oesophagogastrectomy. Exclusions: history of peptic ulceration, asthma, previous reactions to NSAID, allergies, evidence of renal insufficiency, diuretic therapy and recent NSAID ingestion. | |
| Interventions | Rx: diclofenac 75 IM at induction then 4 doses (75 mg each) every 12 hours Pl: placebo with same diclofenac regimen. | |
| Outcomes | Pre‐operative and post‐operative creatinine clearance, serum creatinine, urine output, sodium output, potassium output, number of patients on diuretic or dopamine to treat post‐operative renal insufficiency. | |
| Notes | One patient in diclofenac group withdrawn due to low urine output and was later found to have had a reduced preoperative creatinine clearance (45 mL/min). This patient recovered after IV dopamine and frusemide administration. In this study, frusemide 10 mg IV was given if urine flow rate was less than 30 mL/h for two consecutive periods of one hour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rao 2000.
| Methods | Double‐blind, randomised placebo controlled trial. One patient withdrew. Sample size calculated. | |
| Participants | 39 (22 males, 17 women) patients undergoing abdominal surgery. Exclusion: history of previous allergy to ketoprofen, aspirin and other NSAIDs, peptic ulcer disease, significant respiratory, renal or liver disease, history of depression, dementia or substance abuse, pregnant or lactating patients and patients with coagulopathies. | |
| Interventions | Rx: ketoprofen 100 mg IV at end of surgery and 12 hours after surgery Pl: Normal saline IV at end of surgery and 12 hours after surgery. | |
| Outcomes | Urine output | |
| Notes | Oliguria not defined. One patient in ketoprofen group developed transient oliguric renal failure due to hypovolaemia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Rapanos 1999.
| Methods | Double‐blind randomised, placebo controlled trial. Randomisation carried out by the pharmacy department by sequential selection of previously randomised envelopes containing study drugs. Sample size calculated. No patients withdrew after drug allocation. | |
| Participants | 57 adults undergoing elective aortocoronary bypass surgery. Exclusions: previous history of peptic ulcer or gastrointestinal bleeding, hepatic or renal insufficiency, insulin dependent diabetes mellitus, known allergy to aspirin or NSAIDs, use of aspirin in the 5 days prior to surgery, gastro‐epiploic artery conduit, weight < 60 kg, inability to operate patient controlled analgesia device. | |
| Interventions | Rx: Indomethacin 100 mg suppository 2‐3 hours after surgery and again 12 hours later. Pl: Placebo suppository 2‐3 hours after surgery and again 12 hours later. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine. | |
| Notes | This study was not identified in the previous published review. This study was identified from other research work done concurrently by the principal author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ready 1994.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation: by computer, stratified by type of surgery. Sixty‐five patients withdrew from study. Reasons included adverse reactions (premature withdrawal from study due to nausea, vomiting, hypotension, decrease urine output, skin complaints, nervous system events), study administration problems, inadequate analgesia and intercurrent illness. Intention to treat analysis done but no power calculation. | |
| Participants | 207 patients undergoing major orthopaedic, gynaecological or general surgery. Exclusions: know allergy, sensitivity or contraindications to any opioids, aspirin, or NSAIDs, history of active peptic ulcer within preceding 6 months, a history of bleeding problems or anticoagulant use within preceding 4 weeks, pregnancy or breast feeding, history of known or suspected alcohol or drug abuse, or a medical or psychiatric condition that would compromise ability to give informed consent. | |
| Interventions | Rx 1: 30 mg ketorolac IV bolus then 5 mg/h IV for 24 hours Rx 2: 30 mg IV bolus then 15 mg IV every 3 hours for 24 hours Pl: Placebo initial IV infusion bolus, IV infusion and IV bolus every 3 hours | |
| Outcomes | Urine output | |
| Notes | Oliguria was not defined. No significant difference in the incidence of oliguria between the three groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Slaven 1998.
| Methods | Single‐blind, randomised, placebo controlled trial. Randomisation method: not stated. Number of patients withdrew from study unclear. No details about intention to treat analysis or power calculation. | |
| Participants | 20 (16 males, 4 females) patients undergoing elective laminectomies. Exclusions: not stated | |
| Interventions | Rx: tenoxicam 40 mg IV bolus before induction Pl: normal saline 5 mL IV bolus before induction | |
| Outcomes | Pre‐operative and post‐operative creatinine clearance. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Turner 1994.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation: sequential selection of previously precoded envelopes. Two patients withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 50 patients undergoing elective open cholecystectomy. Exclusions: History of peptic ulceration, bleeding disorder, renal impairment or haemorrhoids. | |
| Interventions | Rx: Indomethacin suppositories 200 mg at end of surgery then 100 mg twice daily for 3 days. Pl: Placebo suppositories according to same treatment regimen. | |
| Outcomes | Pre‐operative and post‐operative serum creatinine | |
| Notes | No pre‐operative and post‐operative serum creatinine measures given, rather the mean change was given for each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Varrassi 1994.
| Methods | Double‐blind, randomised, placebo controlled trial. Randomisation method: not stated. Five patients withdrew from study. No details about intention to treat analysis or power calculation. | |
| Participants | 100 patients undergoing elective cholecystectomy. Exclusions: pregnancy, history of peptic ulceration, coagulopathies, impaired renal function, allergy or intolerance to NSAIDs, alcohol or opioid abuse, children, aged more than 65 years. | |
| Interventions | Rx: ketorolac 30 mg IM before surgery then 2 mg/h IV infusion for 24 hours. Pl: normal saline 1 mL IM then 2 mL/h IV infusion for 24 hours. | |
| Outcomes | Post‐operative serum creatinine | |
| Notes | No pre‐operative serum creatinine data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rx = Treatment Pl = Placebo
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bosek 1996 | Did not collect postoperative renal outcome measures |
| Capuzzo 1999 | Patients did not undergo surgery. Study was conducted in an intensive care unit. |
| Dahl 2004 | Groups consisted of ibuprofen, acetaminophen, and combination of ibuprofen and acetaminophen. No placebo group. Ibuprofen groups similar for dose and administration. |
| Desjardins 2002 | Renal outcome (not specifically defined) measured up to 9 days following oral surgery, or 2 weeks after bunionectomy. |
| Forrest 2002 | Included 42 patients with a history of renal insufficiency in the trial. Acute renal failure was defined as 100% increase in serum creatinine and/or oliguria, and/or dialysis, with evidence of increased blood urea and potassium, IV pyelogram, renal biopsy, x‐rays and/or ultrasound at any time during the 30 days after surgery. No data given for the first 2 days after surgery. |
| Forse 1996 | Did not collect postoperative renal outcome measures |
| Fredman 1999 | Did not collect postoperative renal outcome measures |
| Horneffer 1990 | NSAIDs (ibuprofen or indomethacin) were administered two days after cardiac surgery to treat post‐pericardiotomy syndrome |
| Jones 2000a | Did not collect postoperative renal outcome measures |
| Laisalmi 2001b | No relevant postoperative renal outcome measures |
| Murrell 1996 | Did not collect postoperative renal outcome measures |
| Nussmeier 2005 | Included 33 (2%) of patients with renal insufficiency in the trial. Renal failure defined as the need for haemodialysis or peritoneal dialysis after surgery. Severe renal dysfunction defined as a postoperative serum creatinine level of at least 2.0 mg/dL, with an increase of at least 0.7 mg/dL after randomisation. These outcomes were at any time during the 30 days after surgery. No data given for the first 2 days after surgery. |
| Nussmeier 2006 | Included 6 (1%) of patients with renal insufficiency in the trial. Renal failure defined as the need for hemodialysis or peritoneal dialysis after surgery. Severe renal dysfunction defined as a postoperative serum creatinine level of at least 2.0 mg/dL, with an increase of at least 0.7 mg/dL after randomisation. These outcomes were at any time during the 30 days after surgery. No data given for the first 2 days after surgery. |
| O'Hanlon 1996 | No relevant postoperative renal outcome measures |
| O'Hanlon 1996b | Duplicate study of that reported in the European Journal of Anaesthesiology by the same group of authors. |
| Ott 2003 | Abnormal renal function or increase creatinine level (serum creatinine > 2.0 mg/dL and an increase of > 0.7 mg/dL from baseline) at any time during the 30 days after surgery. No data given for the first 2 days after surgery. |
| Reynolds 2003 | Included adults with abnormal renal function in the trial. |
| Rhodes 1992 | No placebo group for comparison. No treatment group was the control group. |
| Rockemann 1996 | No placebo group for comparison. No treatment group was used. |
Contributions of authors
AL initiated and designed the study, extracted the data, conducted statistical analyses, wrote first draft of the review, collated comments from the other authors, and incorporated the comments of the Anaesthesia and Intensive Care and Cochrane peer reviewers into the final version. MGC provided input to the data extraction forms and extracted the data, and commented on all drafts of the review. JCC, JPK and JFK provided input to the design of the study and commented on all drafts of the review.
Sources of support
Internal sources
Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong.
External sources
No sources of support supplied
Declarations of interest
Dr John Knight now works for Johnson & Johnson Pharmaceutical Research & Development. His contribution to this review was while he was Head, Centre for Kidney Research, The Children's Hospital at Westmead.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aitken 1992 {published data only}
- Aitken HA, Burns JW, McArdle CS, Kenny GN. Effects of ketorolac trometamol on renal function. British Journal of Anaesthesia 1992;68(5):481‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brinkmann 1998 {published data only}
- Brinkmann A, Seeling W, Wolf CF, Kneitinger E, Vogt N, Steinbach G, et al. Ibuprofen does not impair renal function in patients undergoing infrarenal aortic surgery with epidural anaesthesia. Intensive Care Medicine 1998;24(4):322‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castiglione 1997 {published data only}
- Castiglione G, Hauf ME, Panascia E, Scuderi C, Crimi G. Does the preoperative administration of ketorolac improve postoperative analgesia? [La somministrazione preoperatoria di ketorolac migliora l'analgesia postoperatoria?]. Minerva Anestesiologica 1997;63(7‐8):237‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Chow 2001 {published data only}
- Chow GK, Fabrizio MD, Steer T, Potter SR, Jarrett TW, Gelman S, et al. Prospective double‐blind study of effect of ketorolac administration after laparoscopic urologic surgery. Journal of Endourology 2001;15(2):171‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hynninen 2000 {published data only}
- Hynninen MS, Cheng DC, Hossain I, Carroll J, Aumbhagavan SS, Yue R, et al. Non‐steroidal anti‐inflammatory drugs in treatment of postoperative pain after cardiac surgery. Canadian Journal of Anaesthesia 2000;47(12):1182‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Immer 2003 {published data only}
- Immer FF, Immer‐Bansi AS, Trachsel N, Berdat PA, Eigenmann V, Curatolo M, Carrel TP. Pain treatment with a COX‐2 inhibitor after coronary artery bypass operation: a randomized trial. Annals of Thoracic Surgery 2003;75(2):490‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Irwin 1995 {published data only}
- Irwin MG, Roulson CJ, Jones RD, Cheng IK, Visram AR, Chan YM. Peri‐operative administration of rectal diclofenac sodium. The effect on renal function in patients undergoing minor orthopaedic surgery. European Journal of Anaesthesiology 1995;12(4):403‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Jones 2000 {published data only}
- Jones RD, Endre Z, Miles W, Prankerd R, Chilvers M, Willgoss D. Tenoxicam i.v. for major gynaecological surgery‐‐effects on renal function. Anaesthesia & Intensive Care 2000;28(5):501‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khalil 2006 {published data only}
- Khalil MW, Chaterjee A, MacBryde G, Sarkar PK, Marks RR. Single dose parecoxib significantly improves ventilatory function in early extubation coronary artery bypass surgery: a prospective randomized double blind placebo controlled trial. British Journal of Anaesthesia 2006;96(2):171‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kostamovaara 1996 {published data only}
- Kostamovaara PA, Laitinen JO, Nuutinen LS, Koivuranta MK. Intravenous ketoprofen for pain relief after total hip or knee replacement. Acta Anaesthesiologica Scandinavica 1996;40(6):697‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulik 2004 {published and unpublished data}
- Kulik A, Ruel M, Bourke ME, Sawyer L, Penning J, Nathan HJ, et al. Postoperative naproxen after coronary artery bypass surgery: a double‐blind randomized controlled trial. European Journal of Cardio‐Thoracic Surgery 2004;26(4):694‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laisalmi 2001a {published data only}
- Laisalmi M, Eriksson H, Koivusalo AM, Pere P, Rosenberg P, Lindgren L. Ketorolac is not nephrotoxic in connection with sevoflurane anesthesia in patients undergoing breast surgery. Anesthesia & Analgesia 2001;92(4):1058‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nuutinen 1991 {published data only}
- Nuutinen L, Laitinen J. The effect of a nonsteroidal antiinflammatory drug, diclofenac, on renal function in patients undergoing total hip replacement operation [abstract]. Acta Anaesthesiologica Scandinavica 1991;35 Suppl(96):31. [Google Scholar]
Parker 1994 {published data only}
- Parker RK, Holtmann B, Smith I, White PF. Use of ketorolac after lower abdominal surgery. Effect on analgesic requirement and surgical outcome. Anesthesiology 1994;80(1):6‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perttunen 1992 {published data only}
- Perttunen K, Kalson E, Heinonen J, Salo J. IV diclofenac in post‐thoracotomy pain. British Journal of Anaesthesia 1992;68(5):474‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perttunen 1999 {published data only}
- Perttunen K, Nilsson E, Kalso E. I.V. diclofenac and ketorolac for pain after thoracoscopic surgery. British Journal of Anaesthesia 1999;82(2):221‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Power 1992 {published data only}
- Power I, Cumming AD, Pugh GC. Effect of diclofenac on renal function and prostacyclin generation after surgery. British Journal of Anaesthesia 1992;69(5):451‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rao 2000 {published data only}
- Rao AS, Cardosa M, Inbasegaran K. Morphine‐sparing effect of ketoprofen after abdominal surgery. Anaesthesia & Intensive Care 2000;28(1):22‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rapanos 1999 {published data only}
- Rapanos T, Murphy P, Szalai JP, Burlacoff L, Lam‐McCulloch J, Kay J. Rectal indomethacin reduces postoperative pain and morphine use after cardiac surgery. Canadian Journal of Anesthesia 1999;46(8):725‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ready 1994 {published data only}
- Ready LB, Brown CR, Stahlgren LH, Egan KJ, Ross B, Wild L, et al. Evaluation of intravenous ketorolac administered by bolus or infusion for treatment of postoperative pain. Anesthesiology 1994;80(6):1277‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Slaven 1998 {published data only}
- Slaven GM, Walker RJ, Zacharias M, Fawcett JP, Hodgson BF. Tenoxicam does not alter renal function during anaesthesia in normal individuals. Australian & New Zealand Journal of Medicine 1998;28(6):772‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turner 1994 {published data only}
- Turner GA, Gorringe J. Indomethacin as adjunct analgesia following open cholecystectomy. Anaesthesia & Intensive Care 1994;22(1):25‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Varrassi 1994 {published data only}
- Varrassi G, Panella L, Piroli A, Marinangeli F, Varrassi S, Wolman I, et al. The effects of perioperative ketorolac infusion on postoperative pain and endocrine‐metabolic response. Anesthesia & Analgesia 1994;78(3):514‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bosek 1996 {published data only}
- Bosek V, Cox CE. Comparison of analgesic effect of locally and systemically administered ketorolac in mastectomy patients. Annals of Surgical Oncology 1996;3(1):62‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Capuzzo 1999 {published data only}
- Capuzzo M, Carrer S, Sgarbi A, Verri M, Alvisi R, Gritti G. The effect of single‐dose non‐steroidal anti‐inflammatory drugs on urinary output. Clinical Intensive Care 1999;10(2):55‐60. [EMBASE: 1999163652] [Google Scholar]
Dahl 2004 {published data only}
- Dahl V, Dybvik T, Steen T, Aune AK, Rosenlund EK, Raeder JC. Ibuprofen vs. acetaminophen vs. ibuprofen and acetaminophen after arthroscopically assisted anterior cruciate ligament reconstruction. European Journal of Anaesthesiology 2004;21(6):471‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Desjardins 2002 {published data only}
- Desjardins PJ, Shu VS, Recker DP, Verburg KM, Woolf CJ. A single preoperative oral dose of valdecoxib, a new cyclooxygenase‐2 specific inhibitor, relieves post‐oral surgery or bunionectomy pain. Anesthesiology 2002;97(3):565‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forrest 2002 {published data only}
- Forrest JB, Camu F, Greer IA, Kehlet H, Abdalla M, Bonnet F, et al. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. British Journal of Anaesthesia 2002;88(2):227‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forse 1996 {published data only}
- Forse A, El‐Beheiry H, Butler PO, Pace RF. Indomethacin and ketorolac given preoperatively are equally effective in reducing early postoperative pain after laparoscopic cholecystectomy. Canadian Journal of Surgery 1996;39(1):26‐30. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Fredman 1999 {published data only}
- Fredman B, Zohar E, Golan E, Tillinger M, Bernheim J, Jedeikin R. Diclofenac does not decrease renal blood flow or glomerular filtration in elderly patients undergoing orthopedic surgery. Anesthesia & Analgesia 1999;88(1):149‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Horneffer 1990 {published data only}
- Horneffer PJ, Miller RH, Pearson TA, Rykiel MR, Reitz BA, Gardner TJ. The effective treatment of postpericardiotomy syndrome after cardiac operations. A randomized placebo‐controlled trial. Journal of Thoracic & Cardiovascular Surgery 1990;100(2):292‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Jones 2000a {published data only}
- Jones RD, Miles W, Prankerd R, Lang C, Chilvers M, Lo SK. Tenoxicam i.v. in major gynaecological surgery‐‐pharmacokinetic, pain relief and haematological effects. Anaesthesia & Intensive Care 2000;28(5):491‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laisalmi 2001b {published data only}
- Laisalmi M, Teppo AM, Koivusalo AM, Honkanen E, Valta P, Lindgren L. The effect of ketorolac and sevoflurane anesthesia on renal glomerular and tubular function. Anesthesia & Analgesia 2001;93(5):1210‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murrell 1996 {published data only}
- Murrell GC, Leake T, Hughes PJ. A comparison of the efficacy of ketorolac and indomethacin for postoperative analgesia following laparoscopic surgery in day patients. Anaesthesia & Intensive Care 1996;24(2):237‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nussmeier 2005 {published data only}
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. New England Journal of Medicine 2005;352(11):1081‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nussmeier 2006 {published data only}
- Nussmeier NA, Whelton AA, Brown MT, Joshi GP, Langford RM, Singla NK, et al. Safety and efficacy of the cyclooxygenase‐2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology 2006;104(3):518‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Hanlon 1996 {published data only}
- O'Hanlon JJ, Beers H, Huss BK, Milligan KR. A comparison of the effect of intramuscular diclofenac, ketorolac or piroxicam on post‐operative pain following laparoscopy. European Journal of Anaesthesiology 1996;13(4):404‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Hanlon 1996b {published data only}
- O'Hanlon JJ, Beers H, Huss BK, Milligan KR. A comparison of the effect of intramuscular diclofenac, ketorolac or piroxicam on postoperative pain following laparoscopy. Ulster Medical Journal 1996;65(1):51‐4. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Ott 2003 {published data only}
- Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. Journal of Thoracic & Cardiovascular Surgery 2003;125(6):1481‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reynolds 2003 {published data only}
- Reynolds LW, Hoo RK, Brill RJ, North J, Recker DP, Verburg KM. The cox‐2 specific inhibitor, valdecoxib, is an effective, opioid‐sparing analgesic in patients undergoing total knee arthroplasty. Journal of Pain & Symptom Management 2003;25(2):133‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rhodes 1992 {published data only}
- Rhodes M, Conacher I, Morritt G, Hilton C. Nonsteroidal antiinflammatory drugs for postthoracotomy pain: a prospective controlled trial after lateral thoracotomy. Journal of Thoracic and Cardiovascular Surgery 1992;103(1):17‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Rockemann 1996 {published data only}
- Rockemann MG, Seeling W, Bischof C, Börstinghaus D, Steffen P, Georgieff M. Prophylactic use of epidural mepivacaine/morphine, systemic diclofenac, and metamizole reduces postoperative morphine consumption after major abdominal surgery. Anesthesiology 1996;84(5):1027‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
ANZCA 2005
- Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute Pain Management: scientific evidence. 2nd Edition. Melbourne: Australian and New Zealand College of Anaesthetists, 2005. [Google Scholar]
Bainbridge 2006
- Bainbridge D, Cheng DC, Martin JE, Novick R, The Evidence‐based perioperative clinical outcomes research (EPiCOR) group. NSAID‐analgesia, pain control and morbidity in cardiothoracic surgery. Canadian Journal of Anesthesia 2006;53(1):46‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennett‐Jones 2006
- Bennett‐Jones DN. Early intervention in acute renal failure. British Medical Journal 2006;333:406‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bugge 1995
- Bugge JF. Renal effects and complications of NSAIDs for routine post‐operative pain relief: Increased awareness of a real problem is needed. Baillieres Clinical Anaesthesiology 1995;9(3):483‐92. [EMBASE: 1995330996] [Google Scholar]
Chalmers 1995
- Chalmers I, Altman DG. Systematic reviews. London: British Medical Journal Publishing Group, 1995. [Google Scholar]
Cryer 1998
- Cryer B, Feldman M. Cyclooxygenase‐1 and cyclooxygenase‐2 selectivity of widely used nonsteroidal anti‐inflammatory drugs. American Journal of Medicine 1998;104(5):413‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feldman 1997
- Feldman HI, Kinman JL, Berlin JA, Hennessy S, Kimmel SE, Farrar J, et al. Parenteral ketorolac: the risk for acute renal failure. Annals of Internal Medicine 1997;126(3):193‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hou 1983
- Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital‐acquired renal insufficiency: a prospective study. American Journal of Medicine 1983;74(2):243‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kellen 1994
- Kellen M, Aronson S, Roizen MF, Barnard J, Thisted RA. Predictive and diagnostic tests of renal failure: a review. Anesthesia & Analgesia 1994;78(1):134‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McGettigan 2006
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. Journal of the American Medical Association 2006;296:1633‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mosteller 1996
- Mosteller F, Colditz GA. Understanding research synthesis (meta‐analysis). Annual Review of Public Health 1996;17:1‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murray 1993
- Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti‐inflammatory drugs. Annual Review of Pharmacology & Toxicology 1993;33:435‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Myles 1998
- Myles PS, Power I. Does ketorolac cause postoperative renal failure: how do we assess the evidence?. British Journal of Anaesthesia 1998;80(4):420‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Novis 1994
- Novis BK, Roizen MF, Aronson S, Thisted RA. Association of preoperative risk factors with postoperative acute renal failure. Anesthesia & Analgesia 1994;78(1):143‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Rourke 2002
- O'Rourke K. Mixed means and medians: a unified approach to deal with disparate outcome summaries. Symposium on systematic reviews: pushing the boundaries. Oxford, 2002:49.
RCA 1998
- Royal College of Anaesthetists. Guidelines for the use of nonsteroidal antiinflammatory drugs in the perioperative period. London: Royal College of Anaesthetists, 1998. [Google Scholar]
Romsing 1997
- Romsing J, Walther‐Larsen S. Peri‐operative use of nonsteroidal anti‐inflammatory drugs in children: analgesic efficacy and bleeding. Anaesthesia 1997;52(7):673‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sear 2005
- Sear JW. Kidney dysfunction in the postoperative period. British Journal of Anaesthesia 2005;95:20‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sivarajan 1997
- Sivarajan M, Wasse L. Perioperative acute renal failure associated with preoperative intake of ibuprofen. Anesthesiology 1997;86(6):1390‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1993
- Smith K, Halliwell RM, Lawrence S, Klineberg PL, O'Connell P. Acute renal failure associated with intramuscular ketorolac. Anaesthesia & Intensive Care 1993;21(5):700‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strom 1996
- Strom BL, Berlin JA, Kinman JL, Spitz PW, Hennessy S, Feldman H, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding. A postmarketing surveillance study. JAMA 1996;275(5):376‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Thadhani 1996
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. New England Journal of Medicine 1996;334(22):1448‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vane 1997
- Vane JR, Botting RM. Mechanism of action of anti‐inflammatory drugs. Advances in Experimental Medicine & Biology 1997;433:131‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Waller 1991
- Waller DG, Fleming JS, Ramsey B, Gray J. The accuracy of creatinine clearance with and without urine collection as a measure of glomerular filtration rate. Postgraduate Medical Journal 1991;67(783):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijeysundera 2006
- Wijeysundera DN, Kartouti K, Beattie WS, Rao V, Ivanov J. Improving the identification of patients at risk of postoperative renal failure after cardiac surgery. Anesthesiology 2006;104:65‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bell 2018
- Bell S, Rennie T, Marwick CA, Davey P. Effects of peri‐operative nonsteroidal anti‐inflammatory drugs on post‐operative kidney function for adults with normal kidney function. Cochrane Database of Systematic Reviews 11, Issue 2018. [DOI: 10.1002/14651858.CD011274.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 1999
- Lee A, Cooper MG, Craig JC, Knight JF, Keneally JP. The effects of nonsteroidal anti‐inflammatory drugs (NSAIDs) on postoperative renal function: a meta‐analysis. Anaesthesia & Intensive Care 1999;27(6):574‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2000
- Lee A, Cooper MC, Craig JC, Knight JF, Keneally JP. Effects of nonsteroidal anti‐inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002765.pub2] [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee A, Cooper MC, Craig JC, Knight JF, Keneally JP. Effects of nonsteroidal anti‐inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD002765.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


