Abstract
Background
Central venous access (CVA) is widely used. However, its thrombotic, stenotic and infectious complications can be life‐threatening and involve high‐cost therapy. Research revealed that the risk of catheter‐related complications varied according to the site of CVA. It would be helpful to find the preferred site of insertion to minimize the risk of catheter‐related complications. This review was originally published in 2007 and was updated in 2011.
Objectives
1. Our primary objective was to establish whether the jugular, subclavian or femoral CVA routes resulted in a lower incidence of venous thrombosis, venous stenosis or infections related to CVA devices in adult patients.
2. Our secondary objective was to assess whether the jugular, subclavian or femoral CVA routes influenced the incidence of catheter‐related mechanical complications in adult patients; and the reasons why patients left the studies early.
Search methods
We searched CENTRAL (The Cochrane Library 2011, Issue 9), MEDLINE, CINAHL, EMBASE (from inception to September 2011), four Chinese databases (CBM, WANFANG DATA, CAJD, VIP Database) (from inception to November 2011), Google Scholar and bibliographies of published reviews. The original search was performed in December 2006. We also contacted researchers in the field. There were no language restrictions.
Selection criteria
We included randomized controlled trials comparing central venous catheter insertion routes.
Data collection and analysis
Three authors assessed potentially relevant studies independently. We resolved disagreements by discussion. Dichotomous data on catheter‐related complications were analysed. We calculated relative risks (RR) and their 95% confidence intervals (CI) based on a random‐effects model.
Main results
We identified 5854 citations from the initial search strategy; 28 references were then identified as potentially relevant. Of these, we Included four studies with data from 1513 participants. We undertook a priori subgroup analysis according to the duration of catheterization, short‐term (< one month) and long‐term (> one month) defined according to the Food and Drug Administration (FDA).
No randomized controlled trial (RCT) was found comparing all three CVA routes and reporting the complications of venous stenosis.
Regarding internal jugular versus subclavian CVA routes, the evidence was moderate and applicable for long‐term catheterization in cancer patients. Subclavian and internal jugular CVA routes had similar risks for catheter‐related complications. Regarding femoral versus subclavian CVA routes, the evidence was high and applicable for short‐term catheterization in critically ill patients. Subclavian CVA routes were preferable to femoral CVA routes in short‐term catheterization because femoral CVA routes were associated with higher risks of catheter colonization (14.18% or 19/134 versus 2.21% or 3/136) (n = 270, one RCT, RR 6.43, 95% CI 1.95 to 21.21) and thrombotic complications (21.55% or 25/116 versus 1.87% or 2/107) (n = 223, one RCT, RR 11.53, 95% CI 2.80 to 47.52) than with subclavian CVA routes. Regarding femoral versus internal jugular routes, the evidence was moderate and applicable for short‐term haemodialysis catheterization in critically ill patients. No significant differences were found between femoral and internal jugular CVA routes in catheter colonization, catheter‐related bloodstream infection (CRBSI) and thrombotic complications, but fewer mechanical complications occurred in femoral CVA routes (4.86% or 18/370 versus 9.56% or 35/366) (n = 736, one RCT, RR 0.51, 95% CI 0.29 to 0.88).
Authors' conclusions
Subclavian and internal jugular CVA routes have similar risks for catheter‐related complications in long‐term catheterization in cancer patients. Subclavian CVA is preferable to femoral CVA in short‐term catheterization because of lower risks of catheter colonization and thrombotic complications. In short‐term haemodialysis catheterization, femoral and internal jugular CVA routes have similar risks for catheter‐related complications except internal jugular CVA routes are associated with higher risks of mechanical complications.
Plain language summary
Central venous access sites to prevent venous blood clots, blood vessel narrowing, and infection
Central venous access (CVA) involves a large bore catheter inserted in a vein in the neck, upper chest or groin (femoral) area to give drugs that cannot be given by mouth or via a conventional needle (cannula or tube in the arm). CVA is widely used. However, its thrombotic (causing a blood clot) and infectious complications can be life‐threatening and involve high‐cost therapy. Research has revealed that the risk of catheter‐related complications varies according to the sites of central venous catheter (CVC) insertion. It would be helpful to find the preferred site of insertion to minimize the risk of catheter‐related complications. This review examined whether there was any evidence to show that CVA through any one site (neck, upper chest, or femoral area) is better than the other. Four studies were identified comparing data from 1513 participants. For the purpose of this review, three comparisons were evaluated: 1) internal jugular versus subclavian CVA routes; 2) femoral versus subclavian CVA routes; and 3) femoral versus internal jugular CVA routes. We compared short‐term and long‐term catheter insertion. We defined long‐term as for more than one month and short‐term as for less than one month, according to the Food and Drug Administration (FDA). No randomized controlled trial was found comparing all three CVA routes and reporting the complications of venous stenosis.
Subclavian and internal jugular CVA routes had similar risks for catheter‐related complications in long‐term catheter insertion in cancer patients. Subclavian CVA was preferable to femoral CVA in short‐term catheter insertion because of lower risks of catheter colonization and thrombotic complications. In catheter insertion for short‐term haemodialysis, femoral and internal jugular CVA routes had similar risks for catheter‐related complications except internal jugular CVA routes were associated with higher risks of mechanical complications. Further trials comparing subclavian, femoral and jugular CVA routes are needed.
Summary of findings
Background
Description of the condition
Central venous access (CVA), in which a large bore catheter or venous access device (VAD) is placed in a vein in the neck, groin or upper chest, is needed to give drugs that cannot be given by mouth or via a conventional cannulae in the arm. Drugs administered via these catheters include antibiotics, chemotherapy, intravenous feeding, drugs such as those acting on the heart and blood vessels, blood products and other agents. CVA may also be required in intensive care settings to assess venous and cardiac function or it is used for haemodialysis in patients requiring renal replacement therapy.
Concerns have arisen over the number of catheters where the vein that the catheter is in becomes blocked by a blood clot (thrombosis) or by narrowing of the vessel (stenosis) around the catheter. One third of all thromboses of the upper extremity are related to intravenous catheters (Yellin 1996). The clinical picture of central venous thrombosis is recognized by the development of pain and swelling of the neck and arm and a positive venogram, which is performed by administering a radio‐opaque dye into the affected vein, or a positive doppler ultrasound (Alhimyary 1996). The prognosis for patients with catheter‐related venous thrombosis is good in uncomplicated cases (Hye 1996). However, certain clinical groups, for example patients with malignant disease, appear to be at a greater risk of developing venous thrombosis. Most central venous catheter (CVC) related thrombosis is asymptomatic. A systematic review reported that in cancer patients, 29% (range 5% to 62% ) of patients had asymptomatic CVC‐related thrombosis and 12% (range 5% to 54%) had symptomatic thrombosis. These CVC‐related thromboses can cause postphlebitic syndrome in 15% to 30% of cases and pulmonary embolism in 11% of cases (Kuter 2004). Severe cases of venous thrombosis, involving virtually total occlusion of the superior vena cava, can be life‐threatening, increase hospital stay and require lifelong anticoagulant therapy, which incurs considerable healthcare costs and inconvenience to patients.
Catheter‐related infection is also a major health and economic issue. In the United States, approximately 16,000 catheter‐related bloodstream infections (CRBSI) occur in intensive care units (ICUs) each year, and 500 to 4000 patients die annually of CRBSI (Mermel 2000). Furthermore, these infections lead to an increase in hospital stay and costs. In a cohort study, it was reported that among the patients who developed an episode of CRBSI, the hospital stay was increased by 19.6 days. This represented an added cost of EUR 3124 per episode of CRBSI (Rello 2000). Another controlled clinical study showed that CRBSI was associated with an increase of USD 56,167 (in 1998 USD) in total hospital costs, an increase of USD 71,443 in ICU costs, a 22‐day increase in hospital length of stay, and a 20‐day increase in ICU length of stay (Dimick 2001).
Description of the intervention
To gain CVA, a silicone or polyurethane tube is passed into the venous system, often via the subclavian vein in the shoulder or the internal jugular vein in the neck and less commonly via the femoral vein in the groin. The distal tip of the CVC is ideally positioned in the lower third of the superior vena cava, or the inferior vena cava if a femoral approach is used.
Why it is important to do this review
A number of studies revealed that the risk of catheter‐related complications varied according to the site of CVC insertion. However, the conclusions were controversial. Some studies supported the theory that a higher incidence of catheter‐related infections and thrombosis was associated with femoral access than with other central venous sites and concluded that the subclavian was the preferred site of insertion (Goetz 1998; Lorente 2005; Merrer 2001; Trottier 1995); some reported no increased risk of catheter‐related complications for the different insertion sites (Biffi 2009; Deshpande 2005; Mer 2009); some have even reported a higher risk of complications related to subclavian catheterization (Araujo 2008; Schillinger 1991). These discrepancies in study results may be partly caused by different types of patients and CVC characteristics, diagnostic modalities (venography, ultrasound), the criteria used and, importantly, most of the trials were prospective cohort studies. This meant that one could not rule out selection bias by a preference given to one approach over the others as a result of habits or the experience of the operator and may result in overestimation of the benefits of the more commonly used approach (Timsit 2003). One guideline published in 2007 concluded that no randomized controlled trials had satisfactorily compared infection or thrombosis rates for catheters placed via the jugular, subclavian and femoral sites (Bishop 2007). The recommendations about the preferred site for catheterization were not supported by strong evidence from randomized studies. Hence, a need for well‐designed clinical trials was identified.
There are already two systematic review of this literature. A meta‐analysis, published up to the year 2000, reported that there were significantly more arterial punctures with jugular access compared with subclavian access, but fewer malpositions with jugular access. There was no evidence of any difference in the incidence of haemato‐ or pneumothorax and vessel occlusion in this review (Reusch 2002). Another meta‐analysis, published up to the year 2007, reported that rates of catheter‐related infection and arterial puncture were higher with jugular access as compared to subclavian access (Zhang 2008). Both systematic reviews included non‐randomized studies and concluded that selection bias could not be ruled out. Furthermore, Zhang 2008 and Reusch 2002 only compared internal jugular with subclavian catheterization; there were no data on femoral versus subclavian or femoral versus internal jugular catheterization.
This is an update of a Cochrane review (Hamilton 2007). The aim of that review was to assess the evidence from available randomized studies on whether the incidence of venous thrombosis, venous stenosis or infection related to CVA devices varied with the site of catheterization. In this update we have not limited the comparison to internal jugular versus subclavian catheterization but compared the catheter‐related complications among all three CVA routes.
Objectives
Our primary objective was to establish whether the jugular, subclavian or femoral CVA routes resulted in a lower incidence of venous thrombosis, venous stenosis or infections related to CVA devices in adult patients.
Our secondary objective was to assess whether the jugular, subclavian or femoral CVA routes influenced the incidence of catheter‐related mechanical complications in adult patients; and the reasons why patients left the studies early.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomized controlled trials (RCTs). We excluded quasi‐randomized trials, such as those in which allocation was undertaken based on alphabetical order.
Types of participants
We included adults over 16 years of age with any disease process requiring proposed intravenous therapy via the central venous route, irrespective of sex or severity of illness.
We excluded studies in children (aged less than 16 years) because CVC‐related complications in paediatric patients have been closely linked to age, body size and age‐related immune status, and the optimal site of insertion also depended on factors such as the paediatric patient's age and the need for sedation and analgesia during the insertion procedure (De Jonge 2005).
Types of interventions
Any CVC facilitating the administration of intravenous therapy via the central venous route, irrespective of catheter material, size, and number of lumens.
Only CVCs inserted via the subclavian, jugular or femoral veins, on either the left or right side, were included in this review. We only included studies comparing the complications among those three CVA routes.
We excluded studies of peripherally inserted central catheters (PICC) for the reason that it has been recommended in guidelines that PICC should be avoided for inpatient therapy because of limited catheter longevity and increased incidence of thrombosis, and that they should be more suited to ambulatory or outpatient‐based therapy (Bishop 2007).
Types of outcome measures
Primary outcomes
1. Catheter‐related infectious complications
These complications were defined according to an updated guideline by the Infectious Diseases Society of America (Mermel 2009) and included catheter colonization, phlebitis, exit site infection, tunnel infection, pocket infection, and catheter‐related bloodstream infection.
2. Catheter‐related thrombotic complications
According to the 2008 Standards, Options and Recommendations (SOR) guidelines, catheter‐related thrombotic complications were defined as a mural thrombus extending from the catheter into the lumen of a vessel and leading to partial or total catheter occlusion with or without clinical symptoms (Debourdeau 2009).
3. Venous stenosis
Venous stenosis was considered when there was evidence of unequivocal strictures (more than 30% narrowing of the vessel lumen diameter) with or without collateral circulation (Cimochowski 1990; Hernandez 1998).
Secondary outcomes
1. Mechanical complications
1.1 Immediate mechanical complications: defined as mechanical complications that were detected perioperatively and were related to a procedure. They included arterial puncture, minor bleeding, minor haematoma, major haematomas, pneumothorax, haemothorax or mediastinal haematoma, misplacement of the catheter tip, and air embolism. Complications requiring a specific therapeutic procedure (e.g. pneumothorax necessitating chest tube insertion or haemorrhage requiring blood transfusion or a surgical procedure) were defined as major mechanical complications.
1.2 Late mechanical complications: those reported at least 24 hours after the procedure, such as catheter malfunction, tip dislocation, and CVC fracture.
2. Leaving the studies early
Patients that dropped out of the studies for any reasons.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for randomized controlled trials addressing CVA via the subclavian, internal jugular and femoral CVA routes:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 9); MEDLINE (Ovid SP) (1950 to September 2011); EMBASE (Ovid SP) (1966 to September 2011); and CINAHL (EBSCO host) (1950 to September 2011). In addition, we searched four Chinese databases all of which are public and commonly used Chinese reference databases with conference proceedings and published scientific papers: Chinese Bio‐medicine Database (CBM) (1978 to November 2011); WANFANG DATA (1982 to November 2011); Chongqing VIP Database (1989 to November 2011); and China Academic Journal Network Publishing Database (CAJD) (1978 to November 2011).
For specific information regarding our search strategies please see Appendix 1 (CENTRAL); Appendix 2 (Ovid MEDLINE); Appendix 3 (EMBASE), Appendix 4 (CINAHL) and Appendix 5 (Chinese databases search strategy, details of search showing Chinese characters).
Searching other resources
We searched Google Scholar, bibliographies of published trials and conference proceedings.
We contacted prominent authors in the field for knowledge of unpublished trials.
We inspected the references of included studies and systematic reviews for additional trials.
We did not impose any language or date restrictions.
Data collection and analysis
Selection of studies
Three authors (XG, SP and FW) independently inspected all citations identified from the search. We identified potentially relevant reports and ordered full papers for assessment. We resolved disagreements by consensus. If it was impossible to resolve disagreements, then the studies were added to an awaiting assessment list and we contacted the authors of the papers for clarification.
Data extraction and management
We (XG ,SP and FW) independently extracted data from included studies using a piloted data extraction form to record the following data: study allocation, methods, participant details, type of intervention and outcomes. We resolved any disagreement by consensus or by appealing to a fourth author (CL). We documented our decisions and, if necessary, contacted the study authors for clarification.
Assessment of risk of bias in included studies
Again working independently, XG and SP assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool encouraged consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome assessment, the completeness of outcome data, selective reporting and other biases. We decided not to include studies where the sequence generation was at high risk of bias. If disputes arose as to which category a trial was to be allocated to, then again resolution was sought by discussion and by working with the third review author (CL).
Measures of treatment effect
Where binary outcomes were used, we calculated relative risks (RR) and their 95% confidence intervals (CI) based on a random‐effects model.
Unit of analysis issues
1.Studies with multiple treatment groups
Where a study involved more than two treatment arms, if they were relevant the additional treatment arms were presented in comparisons. Where the additional treatment arms were not relevant, these data were not reproduced.
Dealing with missing data
1. Overall loss of credibility
Data must lose credibility at some level of losses to follow up. We were forced to make a judgment for the very short‐term trials likely to be included in this review. Should more than 30% of data be unaccounted for by 24 hours, we did not reproduce these data or use them within our analyses (Xia 2009).
2. Binary
Where attrition for a binary outcome was between 0% and 40% and outcomes of these patients were described, we included these data as reported. Where these data were not clearly described, we assumed the worst primary outcome and that rates of adverse effects were similar to those patients who did continue to have their data recorded.
All data that were extracted reflected the original allocated group, to allow an intention‐to‐treat analysis. Drop‐outs were identified where this information was given.
Assessment of heterogeneity
1. Clinical heterogeneity
In order to judge clinical heterogeneity, we initially considered all included studies without seeing the comparison data.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I2 statistic
This provided an estimate of the percentage of inconsistency thought to be due to chance alone. An I2 estimate greater than or equal to 50% was interpreted as evidence of a high level of heterogeneity (Higgins 2003).
Assessment of reporting biases
Reporting biases arose when the dissemination of research findings was influenced by the nature and direction of results. These were described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We were aware that funnel plots might be useful in investigating reporting biases but were of limited power to detect small‐study effects (Egger 1997). We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We employed a random‐effects model for analyses because: the random‐effects model was more likely to fit the actual sampling distribution; did not impose the restriction of a common effect size; yielded identical results as the fixed‐effects model in the absence of heterogeneity; allowed the conclusions to be generalized to a wider array of situations (Borenstein 2010).
Subgroup analysis and investigation of heterogeneity
1. Subgroup analysis
If data were available, we considered subgroup analysis on the basis of following.
1.1 Different duration of catheter placement: short term (< one month), long term (> one month) defined according to the FDA (FDA 1995).
1.2 Different usage of catheter (to administer medications or fluids, obtain blood tests, obtain cardiovascular measurements, or haemodialysis).
1.3 Influence of disease process, influence of vessels on either the right or left side.
1.4 Patients receiving anticoagulation agents (warfarin, low molecular weight heparin or conventional heparin).
2. Investigation of heterogeneity
If data were clearly heterogeneous, we checked that data were correctly extracted and entered and that we had made no unit of analysis errors. If the high level of heterogeneity remained, we did not undertake a meta‐analysis at this point. If there was considerable variation in results, and particularly if there was inconsistency in the direction of effect, it might be misleading to quote an average value for the intervention effect. We would have wanted to explore heterogeneity. We intended to assess heterogeneity with the I2 statistic. Where this exceeded 50% we would seek to explain the presence of statistical heterogeneity by considering variations in the characteristics of the studies in our analyses.
Sensitivity analysis
We analysed the effect of our assumptions regarding those participants lost to follow up in a sensitivity analysis for the primary outcome but reported our data, assumptions included, in the final analysis. In addition, we included trials in a sensitivity analysis if they were described as 'double‐blind' but only implied randomization. If we found no substantive differences for the primary outcome, these 'implied randomization' studies were added to the overall results.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified 5854 citations from the initial search strategy; we then identified 28 references as potentially relevant and these were retrieved for further assessment. Of these, four references were eligible for inclusion in this review (see Characteristics of included studies) (Biffi 2009; Merrer 2001; Parienti 2008; Wang 2006). All identified trials were published in full, three in English and one in Chinese (Figure 1).
1.
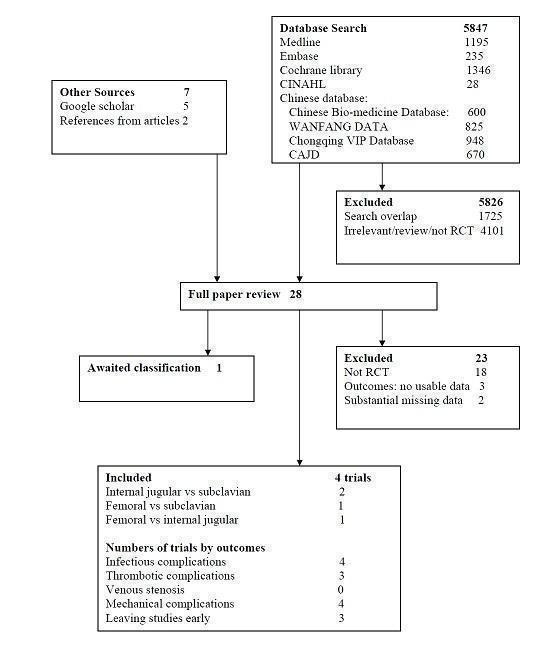
Search results
Included studies
We were able to include four studies with data from 1513 participants (range 200 to 750 participants per trial) (Biffi 2009; Merrer 2001; Parienti 2008; Wang 2006).
1. Duration of catheter placement
In two studies the mean duration of catheter placement was under one month (Merrer 2001; Parienti 2008), which we defined as short‐term catheterization. In the other two studies the duration was beyond one month (Biffi 2009; Wang 2006), which we defined as long‐term catheterization.
2. Study design
All included studies were randomized controlled trials.
3. Participants
All included studies were published between 2001 and 2009. All studies involved participants requiring intravenous therapy via the central venous routes. Ages ranged from 16 to 82 years; most participants were aged between 40 and 60 years. All the studies included both men and women. The participants were critically ill patients (Merrer 2001; Parienti 2008) and cancer patients (Biffi 2009; Wang 2006). Two studies enrolled patients requiring a central venous catheter for the administration of antibiotics, chemotherapy, blood products and parenteral nutrition (Merrer 2001; Wang 2006); one study enrolled participants requiring haemodialysis catheters (Parienti 2008); and one study enrolled cancer patients requiring long‐term central venous totally implantable access ports (TIAP) for repeated administration of chemotherapy (Biffi 2009).
4. Settings
All studies were performed in the in‐hospital setting.
5. Interventions
No randomized controlled trial was found comparing all three insertion sites. We classified interventions into three groups according to the route of catheterization. The interventions in two studies were the internal jugular versus subclavian CVA routes (Biffi 2009; Wang 2006), in one study the interventions were the femoral versus subclavian CVA routes (Merrer 2001), and in one study the interventions were the femoral versus Internal jugular CVA routes (Parienti 2008).
6. Outcomes
6.1 Primary outcomes
6.1.1 Catheter‐related infectious complications
All included studies reported catheter‐related infectious complications. Merrer 2001 and Parienti 2008 reported the complications of catheter colonization and CRBSI; Wang 2006 reported the complications of exit site infection; and Biffi 2009 reported the complications of port‐related bacteraemia or pocket infections, or both.Tunnel infection was not reported in any included study.
6.1.2 Catheter‐related thrombotic complications
Three studies reported catheter‐related thrombotic complications (Biffi 2009; Merrer 2001; Parienti 2008). Wang 2006 did not report the complications of thrombosis.
6.1.3 Venous stenosis
Venous stenosis was not reported in the included studies.
6.2 Secondary outcomes
6.2.1 Mechanical complications
All studies reported data on immediate mechanical complications. Two studies reported late mechanical complications (Biffi 2009;Wang 2006).
6.2.2 Leaving study early
Three studies reported the reasons for leaving the study early (Biffi 2009; Merrer 2001; Parienti 2008).
Excluded studies
We excluded 23 studies. Fourteen studies were not randomized controlled trials but prospective observational studies (Araujo 2008; Chen 2008; Deshpande 2005; Dhawan 2001; Goetz 1998; Gowardman 2008; Lorente 2005; Mer 2009; Moretti 2005; Pang 2009; Richet 1990; Schillinger 1991; Torgay 2005; Young 2005). Trottier 1995 was a randomized controlled trial but it compared the complications of upper access sites (internal jugular and subclavian vein) with lower access sites (femoral vein); it was unclear how many participants were originally randomized to the internal jugular or subclavian vein groups. We identified from another report of the same study that Cui 2008 was a retrospective study. Huang 2009 randomized participants to the femoral, internal jugular and subclavian sites in the first catheterization, however, there were 13 participants whose catheters were changed in the original site through a guidewire, the second catheterization was not randomized, and the data of outcomes for the first catheterization were not available. Moini 2009 compared the complications of a central venous group (internal jugular and subclavian vein) with an external jugular group but the participants were not randomized to internal jugular and subclavian vein respectively. Kaiser 1981 and Senagore 1987 were excluded for the reason that they had substantial missing data (no description about participants' age and sex, duration of catheterization and setting). Miao 2010 was excluded because it allocated participants by their own intention; Wang 2011 was excluded because it allocated participants by the sequence; and Fan 2011 was excluded because the intervention was selected by the patient's condition. For further information please see Characteristics of excluded studies.
2. Studies awaiting assessment
One study is awaiting assessment. In the study of Xie 2003 the number of CRBSI was higher than the number of catheter colonizations, which is unexpected and strange. We are contacting the authors for further details.
3.Ongoing studies
There were no studies classified as ongoing.
Risk of bias in included studies
Judgement of risks are presented in Figure 2 and Figure 3. For detailed information please see Characteristics of included studies.
2.
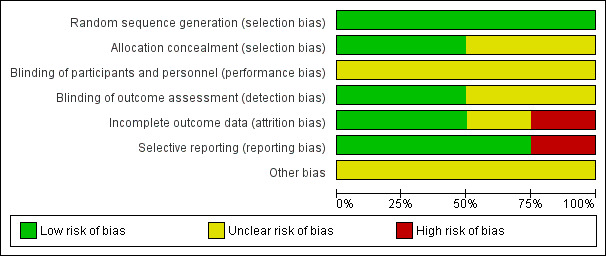
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
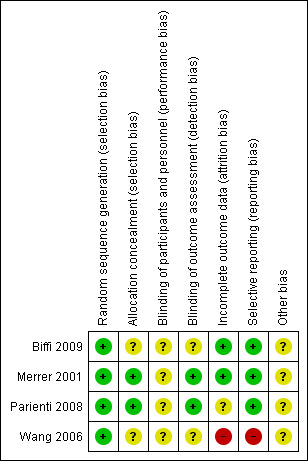
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies were stated to be randomized. All studies described adequate generation of randomization sequences. Two studies reported concealment in detail (Merrer 2001; Parienti 2008); the remaining two studies (Biffi 2009; Wang 2006) did not explain the allocation process and were classified as of 'unclear risk' with a moderate risk of selection bias and overestimation of positive effect.
Blinding
None of the studies described blinding of participants and personnel and they were classified as of 'unclear risk' with a moderate risk of performance bias. Only two studies stated that they used blinding of outcome assessment (Merrer 2001; Parienti 2008). No study tested the success of blinding for participants or evaluators. This may increase the risk of observer bias and also gather further potential for overestimation of positive effects and underestimation of negative ones (Li 2009).
Incomplete outcome data
Three studies reported reasons for leaving the studies early (Biffi 2009; Merrer 2001; Parienti 2008). Wang 2006 only reported on patients who completed the trial, with those leaving the study early not included in the analyses. In the study of Parienti 2008, the number of participants with catheter colonization was inconsistent with the number reported in the subgroup analysis for the effect of body mass index (BMI) on catheter colonization. We contacted the author regarding this issue. The author explained that these apparent inconsistencies were the consequence of data that were missing at random for BMI, making it impossible for them to stratify 20 patients in the femoral group and 18 patients in the internal jugular group according to BMI. We assessed the risk of attrition bias as 'unclear' for this study.
Selective reporting
We checked the methods section of published articles for details of the outcomes that were assessed. Three studies appeared to have reported all outcome measures (Biffi 2009; Merrer 2001; Parienti 2008). Wang 2006 did not report the results of haematoma, air embolism and phlebitis, which were stated in the method section; we classified the risk of selective reporting as 'high' for this study.
Other potential sources of bias
There was no description of other potential sources of bias in all four studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Internal jugular versus subclavian insertion: long‐term catheterization for the prevention of venous thrombosis, stenosis and infection.
| Internal jugular versus subclavian insertion: long‐term catheterization for the prevention of venous thrombosis, stenosis and infection | ||||||
| Patient or population: cancer patients Settings: inpatient Intervention: Internal jugular versus subclavian insertion: long‐term catheterization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | versus subclavian insertion: long‐term catheterization | |||||
| Catheter‐related infectious complications ‐ Exit site infection | Study population | RR 7 (0.88 to 55.86) | 200 (1 study) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 10 per 1000 | 70 per 1000 (9 to 559) | |||||
| Moderate | ||||||
| 10 per 1000 | 70 per 1000 (9 to 559) | |||||
| Catheter‐related infectious complications ‐ Port‐related bacteraemia and/or pocket infections | Study population | RR 0.35 (0.04 to 3.32) | 240 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ||
| 24 per 1000 | 9 per 1000 (1 to 81) | |||||
| Moderate | ||||||
| 24 per 1000 | 8 per 1000 (1 to 80) | |||||
| Catheter‐related thrombotic complications | Study population | RR 1.97 (0.87 to 4.48) | 240 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ||
| 65 per 1000 | 128 per 1000 (57 to 291) | |||||
| Moderate | ||||||
| 65 per 1000 | 128 per 1000 (57 to 291) | |||||
| Immediate mechanical complications ‐ Total mechanical complications | Study population | RR 1 (0.21 to 4.84) | 468 (2 studies) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 13 per 1000 | 13 per 1000 (3 to 62) | |||||
| Moderate | ||||||
| 15 per 1000 | 15 per 1000 (3 to 73) | |||||
| Immediate mechanical complications ‐ Major mechanical complications | Study population | RR 0.33 (0.01 to 8.09) | 468 (2 studies) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 4 per 1000 | 1 per 1000 (0 to 34) | |||||
| Moderate | ||||||
| 5 per 1000 | 2 per 1000 (0 to 40) | |||||
| Late mechanical complications | Study population | RR 1.67 (0.41 to 6.79) | 468 (2 studies) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 13 per 1000 | 21 per 1000 (5 to 86) | |||||
| Moderate | ||||||
| 15 per 1000 | 25 per 1000 (6 to 102) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Blinding not well described. 2 Concealment not well described. 3 Incomplete outcome data addressed. 4 Selective reporting addressed.
Summary of findings 2. Femoral versus subclavian insertion: short‐term catheterization for the prevention of venous thrombosis, stenosis and infection.
| Femoral versus subclavian insertion: short‐term catheterization for the prevention of venous thrombosis, stenosis and infection | ||||||
| Patient or population: critically ill patients Intervention: Femoral versus subclavian insertion: short‐term catheterization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Femoral versus subclavian insertion: short‐term catheterization | |||||
| Catheter‐related infectious complications ‐ Catheter colonization | Study population | RR 6.43 (1.95 to 21.21) | 270 (1 study) | ⊕⊕⊕⊕ high | ||
| 22 per 1000 | 142 per 1000 (43 to 468) | |||||
| Moderate | ||||||
| 22 per 1000 | 141 per 1000 (43 to 467) | |||||
| Catheter‐related infectious complications ‐ Catheter‐related bloodstream infection | Study population | RR 2.03 (0.19 to 22.12) | 270 (1 study) | ⊕⊕⊕⊕ high | ||
| 7 per 1000 | 15 per 1000 (1 to 163) | |||||
| Moderate | ||||||
| 7 per 1000 | 14 per 1000 (1 to 155) | |||||
| Catheter‐related thrombotic complications | Study population | RR 11.53 (2.8 to 47.52) | 223 (1 study) | ⊕⊕⊕⊕ high | ||
| 19 per 1000 | 216 per 1000 (52 to 888) | |||||
| Moderate | ||||||
| 19 per 1000 | 219 per 1000 (53 to 903) | |||||
| Immediate mechanical complications ‐ Total mechanical complications | Study population | RR 0.92 (0.56 to 1.51) | 289 (1 study) | ⊕⊕⊕⊕ high | ||
| 188 per 1000 | 172 per 1000 (105 to 283) | |||||
| Moderate | ||||||
| 188 per 1000 | 173 per 1000 (105 to 284) | |||||
| Immediate mechanical complications ‐ Major mechanical complications | Study population | RR 0.5 (0.09 to 2.67) | 289 (1 study) | ⊕⊕⊕⊕ high | ||
| 28 per 1000 | 14 per 1000 (3 to 74) | |||||
| Moderate | ||||||
| 28 per 1000 | 14 per 1000 (3 to 75) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. Femoral versus internal jugular insertion: short‐term catheterization for the prevention of venous thrombosis, stenosis and infection.
| Femoral versus internal jugular insertion: short‐term catheterization for the prevention of venous thrombosis, stenosis and infection | ||||||
| Patient or population: critically ill patients who were expected to require support with renal replacement therapy Settings: inpatient Intervention: Femoral versus internal jugular insertion: short‐term haemodialysis catheterization | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Femoral versus internal jugular insertion: short‐term catheterization | |||||
| Catheter‐related infectious complications ‐ Catheter colonization | Study population | RR 1.04 (0.8 to 1.36) | 637 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 249 per 1000 | 259 per 1000 (199 to 339) | |||||
| Moderate | ||||||
| 249 per 1000 | 259 per 1000 (199 to 339) | |||||
| Catheter‐related infectious complications ‐ Catheter related bloodstream infection | Study population | RR 0.58 (0.14 to 2.4) | 637 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 16 per 1000 | 9 per 1000 (2 to 38) | |||||
| Moderate | ||||||
| 16 per 1000 | 9 per 1000 (2 to 38) | |||||
| Catheter‐related infectious complication ‐ Subgroup Analysis for the effect of BMI on catheter colonization ‐ Highest BMI tercile (>28.4) | Study population | RR 1.69 (1.08 to 2.65) | 202 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 220 per 1000 | 372 per 1000 (238 to 583) | |||||
| Moderate | ||||||
| 220 per 1000 | 372 per 1000 (238 to 583) | |||||
| Catheter‐related thrombotic complications ‐ Symptomatic deep venous thrombosis | Study population | RR 0.99 (0.14 to 6.98) | 736 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 5 per 1000 | 5 per 1000 (1 to 38) | |||||
| Moderate | ||||||
| 6 per 1000 | 6 per 1000 (1 to 42) | |||||
| Catheter‐related thrombotic complications ‐ Catheter related thrombosis | Study population | RR 0.46 (0.21 to 1.01) | 151 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 227 per 1000 | 104 per 1000 (48 to 229) | |||||
| Moderate | ||||||
| 227 per 1000 | 104 per 1000 (48 to 229) | |||||
| Immediate mechanical complications ‐ Total mechanical complications | Study population | RR 0.51 (0.29 to 0.88) | 736 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 96 per 1000 | 49 per 1000 (28 to 84) | |||||
| Moderate | ||||||
| 96 per 1000 | 49 per 1000 (28 to 84) | |||||
| Immediate mechanical complications ‐ Major mechanical complications | Study population | RR 0.33 (0.03 to 3.16) | 736 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 8 per 1000 | 3 per 1000 (0 to 26) | |||||
| Moderate | ||||||
| 8 per 1000 | 3 per 1000 (0 to 25) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear risk of incomplete outcome data.
See Table 1; Table 2; Table 3.
1. Comparison 1. Internal jugular versus subclavian CVA routes
This comparison contained data from two studies (Biffi 2009; Wang 2006). Both evaluated long‐term catheterization and the participants were 470 cancer patients.
1.1 Primary outcomes
1.1.1 Catheter‐related infectious complications
Both studies reported these outcomes. Based on the data provided in Wang 2006, there was no statistically significant difference in exit site infection in the internal jugular group (7.00%, 7/100) compared with the subclavian group (1.00%, 1/100) (n = 200, one RCT, RR 7.00, 95% CI 0.88 to 55.86). No significant differences were found in Biffi 2009 in port‐related bacteraemia and pocket infections in the internal jugular group (0.85%, 1/117) compared with the subclavian group (2.43%, 3/123) (n = 240, one RCT, RR 0.35, 95% CI 0.04 to 3.32; Analysis 1.1)
1.1. Analysis.
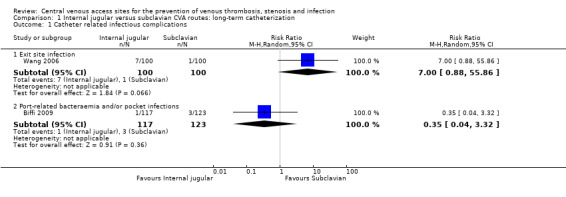
Comparison 1 Internal jugular versus subclavian CVA routes: long‐term catheterization, Outcome 1 Catheter related infectious complications.
1.1.2 Catheter related thrombotic complications
One study reported catheter‐related thrombotic complications (Biffi 2009). There was no significant difference in the catheter‐related thrombotic complications in the internal jugular group (12.82%, 15/117) compared with the subclavian group (6.50%, 8/123) (n = 240, one RCT, RR 1.97, 95% CI 0.87 to 4.48; Analysis 1.2).
1.2. Analysis.
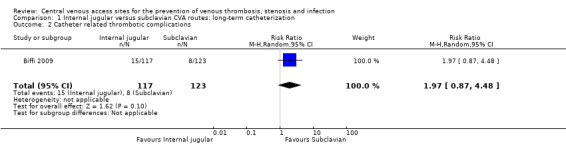
Comparison 1 Internal jugular versus subclavian CVA routes: long‐term catheterization, Outcome 2 Catheter related thrombotic complications.
1.2 Secondary outcomes
1.2.1 Mechanical complications
Both studies reported immediate and late mechanical complications. There were no significant differences in immediate total mechanical complications (1.29%, 3/232 versus 1.27%, 3/236) (n = 468, two RCTs, RR 1.00, 95% CI 0.21 to 4.84; Analysis 1.3) and major mechanical complications(0%, 0/232 versus 0.42%, 1/236) (n = 468, two RCTs, RR 0.33, 95% CI 0.01 to 8.09; Analysis 1.3) in the internal jugular group compared with the subclavian group. Nor was there any difference in late mechanical complications between internal jugular and subclavian CVA sites (2.16%, 5/232 versus 1.27%, 3/236) (n = 468, two RCTs, RR1.67, 95% CI 0.41 to 6.79; Analysis 1.4 ).
1.3. Analysis.
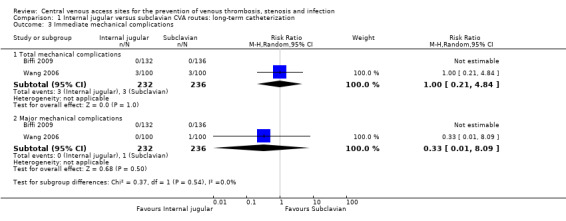
Comparison 1 Internal jugular versus subclavian CVA routes: long‐term catheterization, Outcome 3 Immediate mechanical complications.
1.4. Analysis.
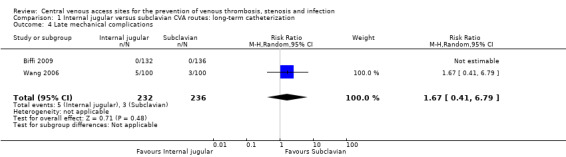
Comparison 1 Internal jugular versus subclavian CVA routes: long‐term catheterization, Outcome 4 Late mechanical complications.
1.2.2 Leaving the studies early
One study reported the reasons for leaving the studies early (Biffi 2009). There were no statistically significant differences in leaving the studies early between the internal jugular group and the subclavian group: because of cancelled operations (1.49%, 2/134 versus 0%, 0/136) (n = 270, one RCT, RR 5.07, 95% CI 0.25 to 104.71), withdrawal of informed consent (1.49%, 2/134 versus 0.74%, 1/136) (n = 270, one RCT, RR 2.03, 95% CI 0.19 to 22.12), or unavailability of data (9.70%, 13/134 versus 8.82%, 12/136) (n = 270, one RCT, RR 1.10, 95% CI 0.52 to 2.32; Analysis 1.5).
1.5. Analysis.
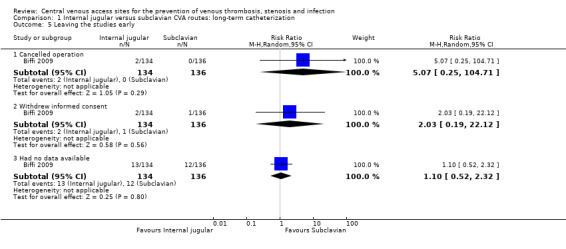
Comparison 1 Internal jugular versus subclavian CVA routes: long‐term catheterization, Outcome 5 Leaving the studies early.
2. Comparison 2. Femoral versus subclavian CVA routes
This comparison only included one study (Merrer 2001), which evaluated short‐term catheterization. Participants were 293 critically ill patients. See Table 4.
1. Femoral versus subclavian CVA routes: short‐term catheterization.
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 1.1 Catheter‐related infectious complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Catheter colonization | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 6.43 [1.95, 21.21] |
| 1.1.2 Catheter‐related bloodstream infection | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.19, 22.12] |
| 1.2 Catheter‐related thrombotic complications | 1 | 223 | Risk Ratio (M‐H,Random, 95% CI) | 11.53 [2.80,47.52] |
| 1.3 Immediate mechanical complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Total mechanical complications | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.56, 1.51] |
| 1.3.2 Major mechanical complications | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.09, 2.67] |
| 1.4 Leaving the studies early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Catheterization was not performed | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.19] |
| 1.4.2 Unsuccessful catheter insertion | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.31, 5.89] |
| 1.4.3 Had grossly contaminated catheter | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.05] |
| 1.4.4 Catheter removed without notification | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.41, 6.89] |
| 1.4.5 Died before catheter removal or before ultrasonographic examination | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.54] |
| 1.4.6 Discharged before ultrasonographic examination | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.33] |
| 1.4.7 Refused ultrasonographic examination | 1 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.07, 1.64] |
2.1 Primary outcomes
2.1.1 Catheter‐related infectious complications
Merrer 2001 reported a significantly higher risk of catheter colonization with the femoral routes (14.18%, 19/134) compared with subclavian routes (2.21%, 3/136) (n = 270, one RCT, RR 6.43, 95% CI 1.95 to 21.21) but no significant difference in the risk of CRBSI between the femoral and subclavian groups (1.49%, 2/134 versus 0.74%, 1/136) (n = 270, one RCT, RR 2.03, 95% CI 0.19 to 22.12).
2.1.2 Catheter‐related thrombotic complications
Merrer 2001 reported that femoral CVA routes (21.55%, 25/116) demonstrated a significant increase in the catheter‐related thrombotic complications compared with subclavian CVA routes (1.87%, 2/107) (n = 223, one RCT, RR 11.53, 95% CI 2.80 to 47.52).
2.2 Secondary outcomes
2.2.1 Mechanical complications
Merrer 2001 assessed immediate mechanical complications. Late mechanical complications were not reported. No significant differences were found in total immediate mechanical complications (17.24%, 25/145 versus 18.75%, 27/144) (n = 289, one RCT, RR 0.92, 95% CI 0.56 to 1.51) and major mechanical complications (1.38%, 2/145 versus 2.78%, 4/144) (n = 289, one RCT, RR 0.50, 95% CI 0.09 to 2.67) between the femoral and subclavian groups.
2.2.2 Leaving the studies early
Various reasons were reported for leaving the studies early. There were no differences between the femoral and subclavian groups for leaving the studies early because of: non‐performance of catheterization (0.68%, 1/146 versus 2.0%, 3/147) (n = 293, one RCT, RR 0.34, 95% CI 0.04 to 3.19); unsuccessful catheter insertion (2.7%, 4/146 versus 2.0%, 3/147) (n = 293, one RCT, RR 1.34, 95% CI 0.31 to 5.89); presence of a grossly contaminated catheter (1.37%, 2/146 versus 1.36%, 2/147) (n = 293, one RCT, RR 1.01, 95% CI 0.14 to 7.05); catheter removal without notification (3.42%, 5/146 versus 2.0%, 3/147) (n = 293, one RCT, RR 1.68, 95% CI 0.41 to 6.89); death before catheter removal or before ultrasonographic examination (13.01%,19/146 versus 14.97%, 22/147) (n = 293, one RCT, RR 0.87, 95% CI 0.49 to 1.54); discharge before ultrasonographic examination (2.7%, 4/146 versus 4.08%, 6/147) (n = 293, one RCT, RR 0.67, 95% CI 0.19 to 2.33); or refusal of ultrasonographic examination (1.37%, 2/146 versus 4.08%, 6/147) (n = 293, one RCT, RR 0.34, 95% CI 0.07 to 1.64).
3. Comparison 3. Femoral versus internal jugular CVA routes
This comparison included only one study (Parienti 2008), which evaluated short‐term catheterization for haemodialysis. Participants were 750 critically ill patients who were expected to require support with renal replacement therapy. See Table 5.
2. Femoral versus internal jugular CVA routes: short‐term catheterization .
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 2.1 Catheter‐related infectious complication | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Catheter colonization | 1 | 637 | Risk Ratio (M‐H,Random, 95% CI) | 1.04 [0.80, 1.36] |
| 2.1.2 Catheter‐related bloodstream infection | 1 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.14, 2.40] |
| 2.2 Catheter‐related infectious complication: Subgroup analysis for the effect of BMI on catheter colonization | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Lowest BMI tercile (<24.2) | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.39, 1.15] |
| 2.2.2 Middle BMI tercile (24.2‐28.4) | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 2.2.3 Highest BMI tercile (>28.4) | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.08, 2.65] |
| 2.3 Catheter‐related thrombosis complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Symptomatic deep venous thrombosis | 1 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.98] |
| 2.3.2 Catheter‐related thrombosis | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.21, 1.01] |
| 2.4 Immediate Mechanical complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Total mechanical complications | 1 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.88] |
| 2.4.2 Major mechanical complications | 1 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.16] |
| 2.5 Leaving the studies early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Withdrew consent | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 5.49] |
| 2.5.2 Did not require catheterization or died before catheterization | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.14, 7.06] |
| 2.5.3 Met exclusion criteria | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.05] |
| 2.5.4 Did not have a catheter culture | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.25] |
3.1 Primary outcomes
3.1.1 Catheter‐related infectious complications
Based on the data provided in Parienti 2008, there were no significant differences between femoral and internal jugular groups in the outcomes of catheter colonization (25.93%, 84/324 versus 24.92%, 78/313) (n = 637, one RCT, RR 1.04, 95% CI 0.80 to 1.36) and CRBSI (0.93%, 3/324 versus 1.60%, 5/313) (n = 637, one RCT, RR 0.58, 95% CI 0.14 to 2.40), but an a priori subgroup analysis for the effect of BMI on catheter colonization demonstrated a significant difference. No differences were found in catheter colonization in the lowest terciles (BMI < 24.2) (n = 195, one RCT, RR 0.67, 95% CI 0.39 to 1.15) and the middle BMI tercile (24.2 to 28.4) (n = 202, one RCT, RR 0.89, 95% CI 0.56 to 1.42) but in the highest BMI tercile (>28.4) the femoral routes were associated with a significant increase in catheter colonization compared with the jugular routes (n = 202, one RCT, RR 1.69, 95% CI 1.08 to 2.65).
3.1.2 Catheter‐related thrombotic complications
Only two participating centres in the study of Parienti 2008 performed ultrasonography to assess the presence of thrombotic complications of the vein, and no significant difference was found in these complications between the femoral and jugular groups (10.53%, 8/76 versus 22.67%, 17/75) (n = 151, one RCT, RR 0.46, 95% CI 0.21 to 1.01). The occurrence of overall symptomatic deep venous thrombosis was similar in both groups (0.54%, 2/370 versus 0.55%, 2/366) (n = 736, one RCT, RR 0.99, 95% CI 0.14 to 6.98).
3.2 Secondary outcomes
3.2.1 Mechanical complications
In Parienti 2008, late mechanical complications were not reported. The risk of total immediate mechanical complications was lower in the femoral (4.86%, 18/370) than the internal jugular routes (9.56%, 35/366) (n = 736, one RCT, RR 0.51, 95% CI 0.29 to 0.88) but there was no significant difference in major mechanical complications between the two groups (0.27%, 1/370 versus 0.82%, 3/366) (n = 736, one RCT, RR 0.33, 95% CI 0.03 to 3.16).
3.2.2 Leaving the studies early
Various reasons were reported for participants leaving the study early. Between the femoral and internal jugular CVA routes they included withdrawal of consent (0.27%, 1/375 versus 0.53%, 2/375), non‐requirement for catheterization or death before catheterization (0.53%, 2/375 versus 0.53%, 2/375), presence of exclusion criteria (0.53%, 2/375 versus 1.33%, 5/375), and lack of a catheter culture (12.27%, 46/375 versus 14.13%, 53/375). No significant differences in these outcomes were found between the two groups.
Discussion
Summary of main results
We were able to include four studies with data from 1513 participants in this review. Two studies compared internal jugular with subclavian CVA routes; one study compared femoral with subclavian CVA routes; and one study compared femoral with internal jugular CVA routes. No randomized controlled trial was found comparing all three sites and reporting the complications of venous stenosis. We undertook an a priori subgroup analysis according to the duration of catheterization, both short‐term (< one month) and long‐term (> one month).
1. Comparison 1. Internal jugular versus subclavian CVA routes
Only data on long‐term catheterization were available for this comparison. Two studies (Biffi 2009; Wang 2006) were included and the participants were 470 cancer patients. Neither study described blinding and concealment. Wang 2006 only reported on patients who completed the trial and had a high risk of selective reporting. Thus, the quality of the evidence was moderate.
Regarding primary outcomes, no significant differences were found between internal jugular and subclavian access in exit site infection, port‐related bacteraemia and pocket infections, and thrombotic complications. Outcomes of catheter colonization and catheter‐related bloodstream infections (CRBSI) were not reported. Regarding secondary outcomes, there were no significant differences in the mechanical complications and reasons for leaving the studies early between the internal jugular and subclavian routes.
2. Comparison 2. Femoral versus subclavian CVA routes
Only data on short‐term catheterization were available and this comparison included one study (Merrer 2001). Participants were 293 critically ill patients. The quality of the evidence was high because the report described randomization, allocation concealment and blinding in detail.
Femoral routes were associated with more catheter colonization and thrombosis than subclavian routes. No significant difference was found in the outcome of CRBSI between the two groups. Late mechanical complications were not reported. There were no significant differences in mechanical complications and reasons for leaving the studies early between the two groups.
3. Comparison 3. Femoral versus Internal jugular CVA routes
This comparison only included one study, which evaluated short‐term catheterization for haemodialysis. The participants were 750 critically ill patients who were expected to require support with renal replacement therapy. The quality of the evidence was assessed as moderate because of an unclear risk of attrition bias in the included study (the number of participants with catheter colonization was inconsistent with the number reported in subgroup analysis for the effect of BMI).
No significant differences were found in the complications of catheter colonization, CRBSI and thrombosis between the femoral and internal jugular routes. An a priori subgroup analysis for the effect of BMI on catheter colonization demonstrated that in patients with the highest BMI tercile (> 28.4), the femoral routes should not be favoured over internal jugular routes. Fewer total mechanical complications occurred in the femoral routes than in the jugular routes but there was no significant difference in major mechanical complications or reasons for leaving the studies early between the two groups.
Overall completeness and applicability of evidence
1. Completeness
There were four randomized studies included in this updated review. No randomized controlled trial was found comparing all three central venous sites and reporting the complications of venous stenosis. Regarding internal jugular versus subclavian insertion sites, only data on long‐term catheterization were available. There is a need for studies of short‐term catheterization comparing internal jugular with subclavian CVA routes. Regarding femoral versus subclavian routes, and femoral versus internal jugular routes, there were no available data on long‐term catheterization.
2. Applicability
Regarding internal jugular versus subclavian routes, the evidence was applicable for long‐term catheterization in cancer patients. There were no significant differences in catheter‐related complications between the two insertion sites (Biffi 2009; Wang 2006). Since cancer patients appear to be at a greater risk of developing venous thrombosis, the evidence from this group may not be applicable to other types of patients.
Regarding femoral versus subclavian routes, the evidence was from data on short‐term catheterization. Subclavian routes were associated with less catheter colonization and fewer thrombotic complications compared with femoral routes. Risks for mechanical complications were similar between the two sites (Merrer 2001). The explanation for these conclusions may be that the subclavian site was easier to sterilize during the insertion procedure and there was less movement of the catheter after placement at this site (Collignon 1988). Femoral routes showed a higher incidence of infectious colonization and thrombotic complications, probably because of the higher bacterial density of local skin flora in the groin area (Lorente 2005).
Regarding femoral versus internal jugular routes, the evidence was also from the data on short‐term catheterization. No significant differences were found between femoral and internal jugular CVA routes in catheter‐related complications except more total mechanical complications occurred in internal jugular CVA routes. However, the evidence was from the data on catheters for haemodialysis where the participants often received routine anticoagulation, which might have contributed to lower rates of thrombosis. These conclusions might differ if studies are performed with catheters used for administrating drugs. Furthermore, guidelines have recommended the use of ultrasound guidance to reduce complications and improve success in central venous catheterization, especially for an internal jugular line (NICE 2002). There were few patients who received internal jugular catheterization with ultrasound guidance in the included study, which further limits the applicability of the conclusion. In regards to mechanical complications, the conclusion may not be fully applicable to hospitals where ultrasound is routinely used.
Quality of the evidence
In this systematic review, most included studies were not of very high reporting quality (see Table 1; Table 2; Table 3; Included studies). Though all included studies described adequate sequence generation, two studies did not describe allocation concealment and blinding (Biffi 2009; Wang 2006), one had an unclear risk of incomplete outcome data (Parienti 2008), one only reported completed trial patients and had a high risk of selective reporting bias (Wang 2006). There was only one study with high reporting quality; this study described randomization, allocation concealment and blinding in detail (Merrer 2001). The overall quality of evidence was moderate.
In conclusion, there appeared to be a considerable risk of bias in the included studies and we have to say that there was still a lack of systematic and strong evidence on optimal insertion sites for clinical practice.
Potential biases in the review process
It has been reported that obtaining and including data from unpublished trials is one obvious way of avoiding publication bias (Higgins 2011). In this review, all included studies were published reports and we could not find any unpublished data. Thus, publication bias may exist. In some included studies the outcomes were not precisely defined and we contacted the study investigators to collect missing information. However, contacting authors of trial reports may lead to overly positive answers (Higgins 2011). Furthermore, there are other possible sources of bias (for example the operator experience, the sterile technique and catheter care) that may significantly influence the outcomes when comparing the preferred site of catheterization.
Agreements and disagreements with other studies or reviews
Regarding internal jugular versus subclavian CVA routes, we found one prospective, non‐randomized observational study which also included a comparison of long‐term catheterization in cancer patients (Araujo 2008). Araujo et al reported no significant difference in the rates of local infection (0.4% versus 1.5%, P = 0.110 ) or totally implantable venous access devices (TIVAD) related sepsis (1.8% versus 2%, P = 0.775) between internal jugular and subclavian sites. These results were similar to the infectious complications of our included studies (Biffi 2009; Wang 2006). The results on thrombosis and mechanical complications remain controversial. Araujo et al reported that thrombosis occurred in 2.0% of participants with subclavian sites and 0.6% for internal jugular sites (P = 0.044). In our included study (Biffi 2009), thrombosis occurred in 6.5% for subclavian sites and 12.8% for internal jugular sites, higher than the data from Araujo 2008 probably because the definitions used for thrombosis were different. The definition of thrombosis in the study of Araujo 2008 was thrombosis in symptomatic cases while Biffi 2009 included thrombosis with and without clinical symptoms. In Araujo 2008, the immediate and late mechanical complications occurred in 5.0% and 10.5%, respectively, for subclavian sites and in 1.6% and 4.9% for internal jugular sites (P < 0.001), which were higher than the data of Biffi 2009. These discrepancies may be partly caused by the use of ultrasound guided access to the subclavian sites in Biffi 2009, while Araujo 2008 did not use ultrasound guidance, and bias due to a higher number of emergency catheterizations in observational studies.
Regarding femoral versus subclavian routes, in the included study for this comparison (Merrer 2001) the rates of catheter colonization and CRBSI were similar to those available in the literature (Goetz 1998; Gowardman 2008; Lorente 2005). The rate of femoral thrombosis (21%) was similar to the 25% rate reported in Trottier 1995.
Regarding femoral versus internal jugular routes, in the included study for this comparison (Parienti 2008) the incidence of colonization (25.43%) was higher than the 2.53% reported in Harb 2005 and 6.2% reported in Souweine 2006. Parienti et al explained that this was because of the different BMIs of participants and different durations of insertion with dialysis catheters. The rate of mechanical complications in internal jugular sites (9.56%) was similar to the 6.3% to 11.8% reported in the literature (McGee 2003). The rate of mechanical complications in the femoral group (4.86%) was lower than the 12.8 to 19.4% reported in McGee 2003, which is probably because of the experienced operators in Parienti 2008 and the randomization process, which avoided the selection of a route according to the operator's preference (Parienti 2008).
We were able to identify two systematic reviews which compared complications of central venous access sites in addition to the earlier version of this Cochrane systematic review (Hamilton 2007). One was in English (Reusch 2002) and the other was in Chinese (Zhang 2008).
Reusch 2002 reported that there were significantly more arterial punctures with jugular access compared with subclavian access, but fewer malpositions with jugular access. There was no evidence of any difference in the incidence of haemato‐ or pneumothorax and vessel occlusion in this review.
Zhang 2008 reported that the rate of catheter‐related infection and arterial puncture were higher with the internal jugular routes than the subclavian routes.
Reusch 2002 and Zhang 2008 collected data from non‐randomized studies and only compared subclavian with internal jugular catheterization; both concluded that selection bias could not be ruled out. To our knowledge, the earlier version of this Cochrane systematic review (Hamilton 2007) was the first to assess the evidence from randomized controlled trials on whether the incidence of venous thrombosis, venous stenosis or infection related to CVA devices varied with the site of catheterization. It identified only one randomized controlled trial (Merrer 2001) that employed one comparison, femoral versus subclavian catheterization, and concluded that subclavian CVA was preferable to femoral CVA. This update includes three new studies (Biffi 2009; Parienti 2008; Wang 2006) and performed an a priori subgroup analysis according to the duration of catheterization. There were three comparisons in our review: subclavian versus internal jugular catheterization; femoral versus subclavian catheterization; femoral versus internal jugular catheterization. Regarding the comparison of subclavian versus internal jugular catheterization, we concluded that there were no significant differences in catheter‐related complications. This was different from the results of Reusch 2002 and Zhang 2008, probably because we only included randomized controlled trials and we included different types of patients and different durations of catheterization.
Authors' conclusions
Implications for practice.
Subclavian and internal jugular CVA routes have similar risks for catheter‐related complications with long‐term catheterization in cancer patients.
Subclavian CVA is preferable to femoral CVA in short‐term catheterization because of lower risks of catheter colonization and thrombotic complications.
In short‐term haemodialysis catheterization, femoral and internal jugular CVA routes have similar risks for catheter‐related complications, except internal jugular CVA routes are associated with higher risks of mechanical complications.
Implications for research.
Overall, no randomized controlled trial was found comparing all three central venous sites and reporting the complications of venous stenosis. Regarding internal jugular versus subclavian insertion sites, there is a need for studies of short‐term catheterization comparing these two sites. Regarding femoral versus subclavian insertion sites and femoral versus internal jugular insertion sites, there is a need for studies of long‐term catheterization and studies with ultrasound guidance catheterization. Further research is required to address these issues.
What's new
| Date | Event | Description |
|---|---|---|
| 12 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 20 January 2012 | New search has been performed | The original search was performed in December 2006. We searched the databases until September 2011. We added search results from four Chinese databases. We included three new studies in the updated review (Biffi 2009; Parienti 2008; Wang 2006). The addition of the new studies provided new evidence that subclavian and internal jugular central venous access (CVA) routes had similar risks for catheter‐related complications in long‐term catheterization of cancer patients. Subclavian CVA routes were preferable to femoral CVA routes in short‐term catheterization because of lower risks of catheter colonization and thrombotic complications. In short‐term haemodialysis catheterization, femoral and internal jugular CVA routes had similar risks for catheter‐related complications, except internal jugular CVA routes were associated with higher risks of mechanical complications. |
| 20 January 2012 | New citation required and conclusions have changed | This review is an update of the previous Cochrane systematic review (Hamilton 2007), which included one randomized controlled trial (Merrer 2001). The previous authors Hamilton HC, Foxcroft D decided not to update the review; new authors (Ge X, Cavallazzi R, Li C, Pan SM, Wang YW, Wang F‐L) have updated this version. We updated the review into RevMan 5.1, which resulted in a slight change in the Methods section, and we included risk of bias and summary of findings tables. |
| 23 January 2008 | New search has been performed | Converted to new review format. |
| 17 April 2007 | Amended | Substantive amendment |
Acknowledgements
In the Methods section we have used the standard text for use in the Methods sections produced by The Cochrane Schizophrenia Group Editorial Base and adapted it as required.
We would like to acknowledge the help of Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia revIew Group (CARG)) and Xiaochun Qiu in finding relevant trials. We would like to thank Lina Wang for her help. Our great thanks to Mrs Jane Cracknell (Managing Editor, CARG) for her enthusiastic help during the preparation of this review.
We would like to thank Professor Andrew Smith (content editor), Cathal Walsh (statistical editor), Teresa Williams, Tajender S Vasu and Jijun Wang (peer reviewers) for their help and editorial advice during the preparation of this review.
Appendices
Appendix 1. CENTRAL
| Search terms |
| #1 (thromb*) or (fibrin*) or (occlu*) or (block*) or (stenos*) or (infect*) #2 (central near venous) or (CVA*) or (jugular* near subclavian*) or (jugular* near femoral*) or (subclavian* near femoral*) #3 #1 and #2 |
Appendix 2. Ovid MEDLINE
| Search terms |
| #1 exp Catheterization, Central Venous/ or (central adj3 venous*).ti,ab. or ((central venous or CVA or jugular or subclav* or femoral or catheter* or cannula) adj5 (access* or rout* or site* or area)).mp. or CVA*.ti,ab. or (CVA adj5 (neck or chest or femor*)).mp. or ((access or CVA*) adj3 devic*).mp #2 exp catheter‐related infections/ or Venous Thrombosis/ or (thromb* or fibrin* or occlu* or block* or stenos* or infect*).ti,ab. or (blood adj5 (clot* or vessel* narrow*)).mp #3 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. #4 1 and 2 and 3 |
Appendix 3. EMBASE (Ovid SP)
| Search terms |
| 1. random$:ab,ti 2. placebo:ab,ti 3. (singl* OR doubl* OR trebl* OR tripl*) AND (blind* OR mask*) 4. ' cross over$' OR crossover$ 5. 'randomized controlled trial'/exp OR 'randomized controlled trial' 6. 'phase 2 clinical trial'/exp OR 'phase 2 clinical trial' 7. 'phase 3 clinical trial'/exp OR 'phase 3 clinical trial' 8. 'double blind procedure'/exp OR 'double blind procedure' 9. 'single blind procedure'/exp OR 'single blind procedure' 10. 'crossover procedure'/exp OR 'crossover procedure' 11. 'latin square design'/de OR 'latin square design' 12. 'placebo'/exp 13. 'multicenter study'/exp OR 'multicenter study' 14. or/1‐13 15. 14# AND [humans]/lim 16. thromb$ 17. fibrin$ 18. occlu$ 19. block$ 20. infect$ 21. stenos$ 22. or/16‐21 23. 'central venous' 24. cva$:ab,ti 25. jugular$:ab,ti AND subclavian$:ab,ti 26. jugular$:ab,ti AND femoral$:ab,ti 27. subclavian$:ab,ti AND femoral$:ab,ti 28. or/23‐27 29. 15# AND 22# AND 28# |
Appendix 4. CINAHL (EBSCO host)
| Search terms |
| S1 (random* or clin* trial* or allocate*). ti, ab. or (clinical trials or double blind studies or triple blind studies or random assignment or meta analysis).MW S2 thromb* or fibrin* or occlu* or block* or stenos* or infect* S3 (central N5 subclavian) or (jugular* N3 subclavian*) or (jugular* N3 femoral*) or (subclavian* N3 femoral*) or CVA* S4 S2 and S3 S5 S1 and S4 |
Appendix 5. Chinese databases search strategy
| Search terms |
|
Chinese Bio‐medicine Database (CBM) (1978 to November 2011) 1.(随机 OR 对照 OR 对比 OR 比较) in title ,abstract 2.(置管 OR 穿刺 OR 留置) in title ,abstract 3.(中心静脉 OR 股静脉 OR 锁骨下静脉 OR 颈内静脉) in title ,abstract 4.(感染 OR 堵塞 OR 堵管 OR 栓塞 OR 血栓 OR 狭窄) in title ,abstract 5. #1 and 2# and 3# and 4# WANFANG DATA (1982 to November 2011) 1.(title:随机 or 对照 or 对比 or 比较) or (abstract:随机 or 对照 or 对比 or 比较) 2.(title:置管 or 穿刺 or 留置)or(abstract:置管 or 穿刺 or 留置) 3.(title:中心静脉 or 股静脉 or 锁骨下静脉 or 颈内静脉)or(abstract: 中心静脉 or 股静脉 or 锁骨下静脉 or 颈内静脉) 4.(title:感染 or 堵管 or 堵塞 or 血栓 or 栓塞 or 狭窄)or(abstract: 感染 or 堵管 or 堵塞 or 血栓 or 栓塞 or 狭窄) 5. 1 and 2 and 3 and 4 Chongqing VIP Database (1989 to November 2011) 1.(T=随机+ T=对照+ T=对比+ T=比较)+(R=随机+ R =对照+ R =对比+ R =比较) 2.(T=中心静脉+ T=股静脉+ T=锁骨下静脉+ T=颈内静脉)+(R=中心静脉+ R=股静脉+R=锁骨下静脉+ R=颈内静脉) 3.(T=感染+ T=堵塞+ T=堵管+ T=栓塞+ T=血栓+ T=狭窄)+(R=感染+ R =堵塞+ R =堵管+ R =栓塞+ R =血栓+ R =狭窄) 4. 1* 2 * 3 China Academic Journal Network Publishing Database(CAJD)(1978 to November 2011) ((TI=随机+对照+对比+比较)or(AB=随机+对照+对比+比较))and((TI=置管+穿刺+留置)or(AB=置管+穿刺+留置))and((TI=中心 静脉+股静脉+锁骨下静脉+颈内静脉)or(AB=中心静脉+股静脉+锁骨下静脉+颈内静脉))and((TI=感染+堵塞+堵管+栓塞+血栓+ 狭窄)or(AB=感染+堵塞+堵管+栓塞+血栓+狭窄)) |
Data and analyses
Comparison 1. Internal jugular versus subclavian CVA routes: long‐term catheterization.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter related infectious complications | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Exit site infection | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.88, 55.86] |
| 1.2 Port‐related bacteraemia and/or pocket infections | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.32] |
| 2 Catheter related thrombotic complications | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.87, 4.48] |
| 3 Immediate mechanical complications | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Total mechanical complications | 2 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.21, 4.84] |
| 3.2 Major mechanical complications | 2 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 4 Late mechanical complications | 2 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.41, 6.79] |
| 5 Leaving the studies early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Cancelled operation | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 5.07 [0.25, 104.71] |
| 5.2 Withdrew informed consent | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.19, 22.12] |
| 5.3 Had no data available | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.52, 2.32] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Merrer 2001.
| Methods | Allocation: Randomized, randomization was performed in blocks of 6, a computer generated random‐numbers table. Blinding: All caregivers and other research personnel were blinded to the randomization. Duration (mean (SD), d): Femoral group: 9.3 (6.2), Subclavian Group:11.0 (6.3). |
|
| Participants | Diagnosis: Patients admitted to ICU, older than 18 years, undergo first central venous catheterization. N=289. Age (mean (SD), y): Femoral group 60.1 (17.3), Subclavian 61.9 (17). Sex: F 97, M 192. Setting: 8 intensive care units (ICUs) in France. |
|
| Interventions | Femoral site insertion (n=145). Subclavian site insertion (n=144). |
|
| Outcomes | Catheter‐related infectious complications: colonization with or without sepsis, Major infectious complication. Catheter‐related thrombotic complications. Mechanical complications. Leaving the study early. |
|
| Notes | Only data of catheter colonization and CRBSI used in the outcome of catheter‐related infectious complications in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed in blocks of 6, by means of a computer generated random‐numbers table. |
| Allocation concealment (selection bias) | Low risk | The trial was concealed in that the site of insertion was given by telephone to the investigators from the central randomization centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All caregivers and other research personnel were blinded to the randomization schedule and the block size. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Description of reasons why terminated. |
| Selective reporting (reporting bias) | Low risk | Reported all data. |
| Other bias | Unclear risk | No description. |
Wang 2006.
| Methods | Allocation: Randomized, drawing of lots. Blinding: No further details. Duration (mean (SD), d): Subclavian group 41.1 (19.8), Internal jugular 36.9 (17.8). |
|
| Participants | Diagnosis: Cancer patients who needed central venous catheterization. N=200. Age (mean (SD), y): Subclavian group 58.3 (17.6), Internal jugular 54.5 (19.0). Sex: F111, M89. Setting: The People's Hospital of Xingtai, Hebei, China. |
|
| Interventions | Subclavian site insertion (n=100). Internal jugular site insertion (n=100). |
|
| Outcomes | Catheter‐related infectious complications: Exit site infection. Mechanical complications. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Those leaving the study early were not included in analyses, only reported completed trial patients. |
| Selective reporting (reporting bias) | High risk | No results for haematoma, air embolism, and phlebitis, which were described in the method section. |
| Other bias | Unclear risk | No description. |
Parienti 2008.
| Methods | Allocation: Randomized, computed generated algorithm. Blinding: Evaluator blinded. Duration (mean (SD), d): Femoral group 6.2(5.5); Internal jugular group 6.9 (7.5). |
|
| Participants | Diagnosis: Critically ill patients requiring catheter insertion for renal replacement therapy, with first venous catheterization and no contraindications for either femoral or jugular access. N=736. Age (mean (SD), y): Femoral group 64.5 (14.9); internal jugular group 65.3 (14.8). Sex: F 242, M 494. Setting: 9 tertiary care university medical centres and 3 general hospitals in France. |
|
| Interventions | Femoral vein insertion (n=370). Internal jugular vein insertion (n=366). |
|
| Outcomes | Catheter‐related infectious complications: Colonization, CRBSI, subgroup analysis for the effect of BMI on catheter colonization. Catheter‐related thrombotic complications. Mechanical complications. Leaving the study early. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computed generated algorithm. |
| Allocation concealment (selection bias) | Low risk | Concealment was obtained by a centralized 24‐hour Internet or telephone service , involving a dynamic semideterminist computed generated algorithm. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluator‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants with catheter colonization was inconsistent with the number reported in subgroup analysis for the effect of BMI. We contacted the author in regards to this issue. The author explained that these apparent inconsistencies were the consequence of missing data at random for body mass index making it impossible for them to stratify 20 patients in the femoral group and 18 patients in the internal jugular group according to BMI. |
| Selective reporting (reporting bias) | Low risk | Reported all data. |
| Other bias | Unclear risk | No description. |
Biffi 2009.
| Methods | Allocation: randomized, randomization was intraoperatively carried out by the data manager of the trial using a computer‐assisted procedure and communicated to the operators. Blinding : No description. Duration (mean (range),d): 596 (0‐1087). |
|
| Participants | Diagnosis: Hospitalized adults (aged 18‐75) with an Eastern Cooperative Oncology Group performance status of zero to two, bearing solid tumours and candidate for iv chemotherapy. Age (mean (SD), y ): 52.0 (11.9). Sex: F 313, M 90. Setting: The European Institute of Oncology in Milan, Italy. |
|
| Interventions | 1. Percutaneous landmark access to the internal jugular site (n=134). 2. Ultrasound (US) guided access to the subclavian site (n=136). 3. Surgical cut‐down access through cephalic vein site (n=133). |
|
| Outcomes | Catheter‐related infectious complications: Port‐related bacteraemia and/or pocket infection. Catheter‐related thrombotic complications. Mechanical complications. Leaving study early. |
|
| Notes | Only interventions 1, 2 included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was intraoperatively carried out by the data manager of the trial using a computer‐assisted procedure and communicated to the operators. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Description of reasons why terminated. |
| Selective reporting (reporting bias) | Low risk | Reported all data. |
| Other bias | Unclear risk | No description. |
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Kaiser 1981 | Allocation: Randomized, table of random numbers. Participants: Patients requiring selective central venous cannulation. Interventions: Internal jugular site insertion n=49, Subclavian site insertion n=51. Substantial missing data: No description about participants' age and sex, duration of catheterization and setting. |
| Senagore 1987 | Allocation: Randomized, no further details. Participants: Patients admitted to ICU and have pulmonary artery catheters placed. Interventions: Internal jugular site insertion n=26, Subclavian site insertion n=40. Outcomes: No usable data‐ no group detail about infectious complications. Substantial missing data: No description about participants' age, sex. |
| Richet 1990 | Allocation: Prospective, observational study. |
| Schillinger 1991 | Allocation: Prospective, observational study. |
| Trottier 1995 | Allocation: Randomized. Participants: Patients from a medical‐surgical ICU requiring no emergent central venous catheterization. Interventions: Upper access site (internal jugular and subclavian vein) n=21 Lower access site (femoral vein) n=24 Outcomes: No usable data‐unclear how many participants originally randomized to internal jugular and subclavian vein group respectively. |
| Goetz 1998 | Allocation: Prospective, observational study. |
| Dhawan 2001 | Allocation:Prospective, observational study. |
| Lorente 2005 | Allocation: Prospective observational study. |
| Deshpande 2005 | Allocation: Prospective observational study. |
| Torgay 2005 | Allocation: Prospective, observational study. |
| Moretti 2005 | Allocation: Prospective observational study. |
| Young 2005 | Allocation: Prospective, observational study. |
| Araujo 2008 | Allocation: Prospective, non‐randomized, observational, study. |
| Chen 2008 | Allocation: Prospective observational study. |
| Cui 2008 | Allocation: Retrospective study from another report of the same study. |
| Gowardman 2008 | Allocation: Prospective, observational Study. |
| Moini 2009 | Allocation: Randomized. Participants: Patients requiring short‐term central venous catheterization. Interventions: Central venous group (internal jugular and subclavian vein) n=34. External jugular group n=34. Outcomes: No usable data, participants not randomized to internal jugular and subclavian vein group respectively. |
| Mer 2009 | Allocation: Prospective, observational study. |
| Huang 2009 | Allocation: Randomized, no further detail. Participants: Patients with various kinds of chronically renal disease. Interventions: Femoral (n=40), internal jugular (n=52), subclavian (n=53). Outcomes: No usable data‐ There were 13 participants changed their catheter in the original site through a guidewire, Huang et al added the data of second catheterization into the first catheterization, but the second catheterization was not randomized and the data for the first catheterization were not available. |
| Pang 2009 | Allocation: Prospective observational study. |
| Miao 2010 | Allocation: quasi‐randomized trials. Allocation by participants' own intention. |
| Fan 2011 | Allocation: not randomized trial. The intervention was selected by the patient's condition. |
| Wang 2011 | Allocation: quasi‐randomized trials. It allocated by the sequence. |
Characteristics of studies awaiting assessment [ordered by study ID]
Xie 2003.
| Methods | Allocation: Randomized, random number table. Blinding: No further details. Duration (mean ( SD), d): Subclavian group 13.2 (3.4), Femoral group 5.5 (2.1). |
| Participants | Diagnosis: Adult patients registered in ICU. N= 171. Age (mean , y ): 47.8. Sex: F 49, M 122. Setting: ICU of Southwest Hospital, Chongqin, China. |
| Interventions | Subclavian insertion (n=57). Femoral insertion (n=57). Peripherally inserted central catheter (n=57). |
| Outcomes | Catheter‐related infectious complications: Exit site infection, Catheter colonization, CRBSI. Mechanical complications. |
| Notes | The number of CRBSI was higher than the number of catheter colonization, which was unexpected and strange, and we are contacting authors for further details. |
Differences between protocol and review
We updated the review into RevMan 5.1 resulting in slight reformatting in the Methods section.
We included risk of bias and summary of findings tables.
We added the outcome 'Leaving the studies early' and included the search results from four Chinese databases.
We revised the criteria for included studies as follows.
In the earlier review (Hamilton 2007), the criteria for including participants were patients requiring long‐term intravenous therapy (the definition for 'long‐term' was intended to be in situ for at least one month). This review included only one study (Merrer 2001). However, the duration of the catheterization in this study was under two weeks. We performed an a priori subgroup analysis according to the duration of catheterization, long‐term (> 1month) and short‐term (< 1 month) in this new updated version.
The earlier review (Hamilton 2007) reported that they included randomized controlled trials or controlled clinical trials, but the results showed that the real selection criterion was only randomized controlled trials, not controlled clinical trials. In this updated version we included only randomized controlled trials to avoid selection bias.
The earlier review (Hamilton 2007) stated the objective "To determine whether the circumference of a long‐term central venous access device influences the incidence of venous thrombosis, venous stenosis or infection related to CVA devices", but there was no description or search terms about the circumference of a central venous access device in the background part and in the search strategies. We deleted this objective in the updated version.
Contributions of authors
Conceiving the review: Xiaoli Ge (XG), Chunbo Li (CL)
Co‐ordinating the review: XG, CL
Undertaking manual searches: none.
Screening search results: XG, Shu Ming Pan (SMP), Fei‐Long Wang (FLW)
Organizing retrieval of papers: XG, SMP
Screening retrieved papers against inclusion criteria: XG, SMP, FLW
Appraising quality of papers: XG, CL
Abstracting data from papers: XG, SMP
Writing to authors of papers for additional information: XG, Rodrigo Cavallazzi (RC)
Providing additional data about papers: XG
Obtaining and screening data on unpublished studies: none
Data management for the review: XG
Entering data into Review Manager (RevMan 5.1): XG, FLW
RevMan statistical data: XG,CL
Other statistical analysis not using RevMan: none
Interpretation of data: XG
Statistical inferences: CLi, XG
Writing the review: XG, RC, Ying Wei Wang (YWW)
Securing funding for the review: XG
Performing previous work that was the foundation of the present study: XG, CL, RCi, YWW
Guarantor for the review (one author): XG
Person responsible for reading and checking review before submission: XG
Sources of support
Internal sources
-
Division of Pulmonary and Critical Care Medicine,University of Louisville, USA.
to Rodrigo Cavallazzi
-
Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, China.
to Chunbo Li
-
Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, China.
to Xiaoli Ge, Shuming Pan, Fei‐long Wang, Yingwei Wang
External sources
-
Program of Xin Hua Hospital Funds,Shanghai Jiao Tong University School of Medicine, China.
to Xiaoli Ge
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Biffi 2009 {published data only}
- Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, et al. Best choice of central venous insertion site for the prevention of catheter‐related complications in adult patients who need cancer therapy: a randomized trial. Annals of Oncology 2009;20(5):935‐40. [PUBMED: 19179550] [DOI] [PubMed] [Google Scholar]
Merrer 2001 {published data only}
- Merrer J, Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286(6):700‐7. [PUBMED: 11495620] [DOI] [PubMed] [Google Scholar]
Parienti 2008 {published data only}
- Parienti JJ, Thirion M, Megarbane B, Souweine B, Ouchikhe A, Polito A, et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA 2008;299(20):2413‐22. [PUBMED: 18505951] [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang S‐X. complications of two different central venous insertion sites. Chinese Journal of Practical Nursing 2006;22(11):46‐7. [Google Scholar]
References to studies excluded from this review
Araujo 2008 {published data only}
- Araujo C, Silva JP, Antunes P, Fernandes JM, Dias C, Pereira H, et al. A comparative study between two central veins for the introduction of totally implantable venous access devices in 1201 cancer patients. European Journal of Surgical Oncology 2008;34(2):222‐6. [PUBMED: 17566692] [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen L‐Z. Comparison of internal jugular vein and femoral vein detention double‐lumen catheter in hemodialysis. Journal of Anhui Health Vocational & Technical College 2008;7(3):68‐9. [Google Scholar]
Cui 2008 {published data only}
- Cui L, Tang M, Shi D‐Y, Ma Q‐L, Wang X‐D. Central venous catheter related infection and dangerous factors after cardiovascular surgery. Chinese Journal of Postgraduates of Medicine 2008;31(20):41‐2. [Google Scholar]
Deshpande 2005 {published data only}
- Deshpande KS, Hatem C, Ulrich HL, Currie BP, Aldrich TK, Bryan‐Brown CW, et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular, and femoral sites in an intensive care unit population. Critical Care Medicine 2005;33(1):13‐20; discussion 234‐5. [PUBMED: 15644643] [DOI] [PubMed] [Google Scholar]
Dhawan 2001 {published data only}
- Dhawan B, Chaudhry R, Hote M, Bhan A, Venugopal P. Infective complications of central venous catheters in cardiac surgical patients. Indian Journal of Pathology & Microbiology 2001;44(2):125‐9. [PUBMED: 11883126] [PubMed] [Google Scholar]
Fan 2011 {published data only}
- Fan G‐J, Xu M‐Z. Application and nursing of different central venous access routs in hemodialysis. Gangxi Medical Journal 2011;33(5):622‐4. [Google Scholar]
Goetz 1998 {published data only}
- Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infection Control and Hospital Epidemiology 1998;19(11):842‐5. [PUBMED: 9831940] [DOI] [PubMed] [Google Scholar]
Gowardman 2008 {published data only}
- Gowardman JR, Robertson IK, Parkes S, Rickard CM. Influence of insertion site on central venous catheter colonization and bloodstream infection rates. Intensive Care Medicine 2008;34(6):1038‐45. [PUBMED: 18317732] [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang G‐L. Prospective comparison study of the safety and efficiency about temporary central venous catheterization on different location in haemodialysis patients. Jinan university Master's degree paper 2009.
Kaiser 1981 {published data only}
- Kaiser CW, Koornick AR, Smith N, Soroff HS. Choice of route for central venous cannulation: subclavian or internal jugular vein? A prospective randomized study. Journal of Surgical Oncology 1981;17(4):345‐54. [PUBMED: 7265974] [DOI] [PubMed] [Google Scholar]
Lorente 2005 {published data only}
- Lorente L, Henry C, Martin MM, Jimenez A, Mora ML. Central venous catheter‐related infection in a prospective and observational study of 2,595 catheters. Critical Care (London, England) 2005;9(6):R631‐5. [PUBMED: 16280064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mer 2009 {published data only}
- Mer M, Duse AG, Galpin JS, Richards GA. Central venous catheterization: a prospective, randomized, double‐blind study. Clinical and Applied Thrombosis/Hemostasis 2009;15(1):19‐26. [PUBMED: 18593746] [DOI] [PubMed] [Google Scholar]
Miao 2010 {published data only}
- Miao L‐Y, Liu J‐F, Li X‐R, Ren Y. Application of various ways of central vein catheterization in hemodialysis. Journal of Qiqihar Medical College 2010;31(18):2862‐4. [Google Scholar]
Moini 2009 {published data only}
- Moini M, Rasouli MR, Kenari MM, Mahmoodi HR. Non‐cuffed dual lumen catheters in the external jugular veins versus other central veins for hemodialysis patients. Saudi Journal of Kidney Diseases and Transplantation 2009;20(1):44‐8. [PUBMED: 19112218] [PubMed] [Google Scholar]
Moretti 2005 {published data only}
- Moretti EW, Ofstead CL, Kristy RM, Wetzler HP. Impact of central venous catheter type and methods on catheter‐related colonization and bacteraemia. The Journal of Hospital Infection 2005;61(2):139‐45. [PUBMED: 16026898] [DOI] [PubMed] [Google Scholar]
Pang 2009 {published data only}
- Pang D‐C, Liao Z‐N, Fan H‐O. Comparison of three central venous insertion sites in patients after neurosurgical operation. Journal of Minimally Invasive Medicine 2009;4(3):241‐3. [Google Scholar]
Richet 1990 {published data only}
- Richet H, Hubert B, Nitemberg G, Andremont A, Buu‐Hoi A, Ourbak P, et al. Prospective multicenter study of vascular‐catheter‐related complications and risk factors for positive central‐catheter cultures in intensive care unit patients. Journal of Clinical Microbiology 1990;28(11):2520‐5. [PUBMED: 2254429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schillinger 1991 {published data only}
- Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrology, Dialysis, Transplantation 1991;6(10):722‐4. [PUBMED: 1754109] [DOI] [PubMed] [Google Scholar]
Senagore 1987 {published data only}
- Senagore A, Waller JD, Bonnell BW, Bursch LR, Scholten DJ. Pulmonary artery catheterization: a prospective study of internal jugular and subclavian approaches. Critical Care Medicine 1987;15(1):35‐7. [PUBMED: 3539524] [PubMed] [Google Scholar]
Torgay 2005 {published data only}
- Torgay A, Pirat A, Candan S, Zeyneloglu P, Arslan G, Haberal M. Internal jugular versus subclavian vein catheterization for central venous catheterization in orthotopic liver transplantation. Transplantation Proceedings 2005;37(7):3171‐3. [PUBMED: 16213340] [DOI] [PubMed] [Google Scholar]
Trottier 1995 {published data only}
- Trottier SJ, Veremakis C, O'Brien J, Auer AI. Femoral deep vein thrombosis associated with central venous catheterization: results from a prospective, randomized trial. Critical Care Medicine 1995;23(1):52‐9. [PUBMED: 8001386] [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang Y‐J, Fu J‐Y, Li X‐J. Comparison of three kinds of central venous access routes in stroke patients. Chinese Rural Health Service Administration 2011;31(2):215‐7. [Google Scholar]
Young 2005 {published data only}
- Young EJ, Contreras G, Robert NE, Vogt NJ, Courtney TM. Incidence and influencing factors associated with exit site infections in temporary catheters for hemodialysis and apheresis. Nephrology Nursing Journal 2005;32(1):41‐50. [PUBMED: 15787083] [PubMed] [Google Scholar]
References to studies awaiting assessment
Xie 2003 {published data only}
- Xie X‐H, Liu M‐Y, Peng J, Wen J. A comparative study on CVC‐related infection via different accesses in ICU. Nursing Journal of Chinese People's Liberation Army 2003;20(7):10‐1. [Google Scholar]
Additional references
Alhimyary 1996
- Alhimyary A, Fernandez C, Picard M, Tierno K, Pignatone N, Chan HS, et al. Safety and efficacy of total parenteral nutrition delivered via a peripherally inserted central venous catheter. Nutrition and Clinical Practice 1996;11(5):199‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bishop 2007
- Bishop L, Dougherty L, Bodenham A, Mansi J, Crowe P, Kibbler C, et al. Guidelines on the insertion and management of central venous access devices in adults. International Journal of Laboratory Hematology 2007;29(4):261‐78. [PUBMED: 17617077] [DOI] [PubMed] [Google Scholar]
Borenstein 2010
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods 2010;1(2):97‐111. [DOI] [PubMed] [Google Scholar]
Cimochowski 1990
- Cimochowski GE, Worley E, Rutherford WE, Sartain J, Blondin J, Harter H. Superiority of the internal jugular over the subclavian access for temporary dialysis. Nephron 1990;54(2):154‐61. [PUBMED: 2314526] [DOI] [PubMed] [Google Scholar]
Collignon 1988
- Collignon P, Soni N, Pearson I, Sorrell T, Woods P. Sepsis associated with central vein catheters in critically ill patients. Intensive Care Medicine 1988;14(3):227‐31. [PUBMED: 3379183] [DOI] [PubMed] [Google Scholar]
De Jonge 2005
- Jonge RC, Polderman KH, Gemke RJ. Central venous catheter use in the pediatric patient: mechanical and infectious complications. Pediatric Critical Care Medicine 2005;6(3):329‐39. [PUBMED: 15857534] [DOI] [PubMed] [Google Scholar]
Debourdeau 2009
- Debourdeau P, Kassab Chahmi D, Gal G, Kriegel I, Desruennes E, Douard MC, et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Annals of Oncology 2009;20(9):1459‐71. [PUBMED: 19525362] [DOI] [PubMed] [Google Scholar]
Dimick 2001
- Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA. Increased resource use associated with catheter‐related bloodstream infection in the surgical intensive care unit. Archives of Surgery 2001;136(2):229‐34. [PUBMED: 11177147] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [PUBMED: 9432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 1995
- U.S. Food, Drug Administration (FDA). Guidance on Premarket Notification [510(k)] Submission for Short‐Term Long‐Tern Intravascular Catheters. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM080766.pdf (accessed 24th March 2011).
Harb 2005
- Harb A, Estphan G, Nitenberg G, Chachaty E, Raynard B, Blot F. Indwelling time and risk of infection of dialysis catheters in critically ill cancer patients. Intensive Care Medicine 2005;31(6):812‐7. [PUBMED: 15834707] [DOI] [PubMed] [Google Scholar]
Hernandez 1998
- Hernandez D, Diaz F, Rufino M, Lorenzo V, Perez T, Rodriguez A, et al. Subclavian vascular access stenosis in dialysis patients: natural history and risk factors. Journal of the American Society of Nephrology 1998;9(8):1507‐10. [PUBMED: 9697674] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hye 1996
- Hye RJ, Stabile BE. Complications of percutaneous vascular access procedures and their management. In: Wilson SE editor(s). Vascular Access: Principles and Practice. 3rd Edition. St Louis: Mosby, 1996:92‐103. [Google Scholar]
Kuter 2004
- Kuter DJ. Thrombotic complications of central venous catheters in cancer patients. The Oncologist 2004;9(2):207‐16. [PUBMED: 15047925] [DOI] [PubMed] [Google Scholar]
Li 2009
- Li C, Xia J, Wang J. Risperidone dose for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007474.pub2] [DOI] [PubMed] [Google Scholar]
McGee 2003
- McGee DC, Gould MK. Preventing complications of central venous catheterization. The New England Journal of Medicine 2003;348(12):1123‐33. [PUBMED: 12646670] [DOI] [PubMed] [Google Scholar]
Mermel 2000
- Mermel LA. Prevention of intravascular catheter‐related infections. Annals of Internal Medicine 2000;132(5):391‐402. [PUBMED: 10691590] [DOI] [PubMed] [Google Scholar]
Mermel 2009
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;49(1):1‐45. [PUBMED: 19489710] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2002
- National Institute for Clinical Excellence (NICE) Guidance on the use of ultrasound locating devices for placing central venous catheters. Technology Appraisal Guidance Number 49. September 2002. http://www.nice.org.uk/nicemedia/pdf/Ultrasound_49_GUIDANCE.pdf(accessed ninth August 2011).
Rello 2000
- Rello J, Ochagavia A, Sabanes E, Roque M, Mariscal D, Reynaga E, et al. Evaluation of outcome of intravenous catheter‐related infections in critically ill patients. American Journal of Respiratory and Critical Care Medicine 2000;162(3 Pt 1):1027‐30. [PUBMED: 10988125] [DOI] [PubMed] [Google Scholar]
Reusch 2002
- Reusch S, Walder B, Tramer MR. Complications of central venous catheters: internal jugular versus subclavian access ‐ a systematic review. Critical Care Medicine 2002;30(2):454‐60. [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration,. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration,, 2011.
Souweine 2006
- Souweine B, Liotier J, Heng AE, Isnard M, Ackoundou‐N'Guessan C, Deteix P, et al. Catheter colonization in acute renal failure patients: comparison of central venous and dialysis catheters. American Journal of Kidney Diseases 2006;47(5):879‐87. [PUBMED: 16632028] [DOI] [PubMed] [Google Scholar]
Timsit 2003
- Timsit JF. What is the best site for central venous catheter insertion in critically ill patients?. Critical Care (London, England) 2003;7(6):397‐9. [PUBMED: 14624670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams C, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Losing participants before the end of the trial erodes credibility of findings. Psychiatric Bulletin 2009;33:254‐7. [Google Scholar]
Yellin 1996
- Yellin AE, Hood DB, Weaver FA. Axillo‐subclavian vein thrombosis. In: Wilson SE editor(s). Vascular Access: Principles and Practice. 3rd Edition. St Louis: Mosby, 1996:104‐9. [Google Scholar]
Zhang 2008
- Zhang LF, Zhao XP, Cai YM, He LC. Meta analysis of two approaches for central venous catheterization trough deep vein puncture. Chinese Journal of Practical Nursing 2008;24(6):57‐60. [Google Scholar]
References to other published versions of this review
Hamilton 2007
- Hamilton HC, Foxcroft D. Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long‐term intravenous therapy. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004084.pub2] [DOI] [PubMed] [Google Scholar]


