Abstract
Background
Decompression illness (DCI) is due to bubble formation in the blood or tissues following the breathing of compressed gas. Clinically, DCI may range from a trivial illness to loss of consciousness, death or paralysis. Recompression is the universally accepted standard treatment of DCI. When recompression is delayed, a number of strategies have been suggested in order to improve the outcome.
Objectives
To examine the effectiveness and safety of both recompression and adjunctive therapies in the treatment of DCI.
Search methods
In our previous update we searched until October 2009. In this version we searched CENTRAL (The Cochrane Library, October 2011); MEDLINE (1966 to October 2011); CINAHL (1982 to October 2011); EMBASE (1980 to October 2011); the Database of Randomised Controlled Trials in Hyperbaric Medicine (October 2011); and handsearched journals and texts.
Selection criteria
We included randomized controlled trials that compared the effect of any recompression schedule or adjunctive therapy with a standard recompression schedule. We did not apply language restrictions.
Data collection and analysis
Three authors extracted the data independently. We assessed each trial for internal validity and resolved differences by discussion. Data were entered into RevMan 5.1.
Main results
Two randomized controlled trials enrolling a total of 268 patients satisfied the inclusion criteria. The risk of bias for Drewry 1994 was unclear as this study was presented as an abstract, while Bennett 2003 was rated as at low risk. Pooling of data was not possible. In one study there was no evidence of improved effectiveness with the addition of a non‐steroidal anti‐inflammatory drug (tenoxicam) to routine recompression therapy (at six weeks: relative risk (RR) 1.04, 95% confidence interval (CI) 0.90 to 1.20, P = 0.58) but there was a reduction in the number of compressions required when tenoxicam was added from three to two (P = 0.01, 95% CI 0 to 1). In the other study, the odds of multiple recompressions were lower with a helium and oxygen (heliox) table compared to an oxygen treatment table (RR 0.56, 95% CI 0.31 to 1.00, P = 0.05).
Authors' conclusions
Recompression therapy is standard for the treatment of DCI, but there is no randomized controlled trial evidence for its use. Both the addition of a non‐steroidal anti‐inflammatory drug (NSAID) and the use of heliox may reduce the number of recompressions required, but neither improve the odds of recovery. The application of either of these strategies may be justified. The modest number of patients studied demands a cautious interpretation. Benefits may be largely economic and an economic analysis should be undertaken. There is a case for large randomized trials of high methodological rigour in order to define any benefit from the use of different breathing gases and pressure profiles during recompression therapy.
Plain language summary
Recompression therapy and adjunctive drug therapy for decompression illness (the bends)
Decompression illness (DCI) is due to the presence of bubbles in the tissues or blood vessels following the reduction of surrounding pressure (decompression). It is most commonly associated with breathing compressed gas while diving underwater. The effects of DCI may vary from the trivial to life‐threatening and treatment is usually administered urgently. Recompression is applied while breathing 100% oxygen or a mixture of oxygen and helium (heliox), based on the reduction in bubble size with pressure and more rapid elimination of nitrogen from the bubbles when breathing nitrogen poor mixtures. Recovery without recompression can be slow and incomplete and DCI is responsible for significant health problems in geographical areas where recompression is unavailable. Recompression with 100% oxygen has become universally accepted as the appropriate therapy despite the lack of high quality clinical evidence of effectiveness. This review found only two randomized trials enrolling a total of 268 patients. One trial compared standard oxygen recompression to helium and oxygen recompression, while the other compared oxygen recompression alone to recompression and an adjunctive non‐steroidal anti‐inflammatory drug (NSAID). Both trials suggested that these additional interventions may shorten the course of recompression required. For example, the use of an NSAID reduced the median number of recompression sessions required from three to two. We conclude that there is little evidence for using one recompression strategy over another in the treatment of decompression illness and that the addition of an anti‐inflammatory drug may shorten the course of recompression required. More research is needed.
Summary of findings
Summary of findings for the main comparison. Recompression therapy for decompression illness.
| Recompression therapy for decompression illness | ||||||
| Patient or population: patients with decompression illness Settings: patients referred to a hyperbaric facility for recompression Intervention: tenoxicam (adjunctive therapy) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | tenoxicam | |||||
| Need for second recompression Simple count Follow‐up: mean 6 weeks1 | Study population | RR 0.64 (0.46 to 0.86) | 180 (1 study) | ⊕⊕⊕⊕ high | ||
| 611 per 1000 | 391 per 1000 (287 to 501) | |||||
| Low risk population | ||||||
| 400 per 1000 | 256 per 1000 (188 to 328) | |||||
| High risk population | ||||||
| 800 per 1000 | 512 per 1000 (376 to 656) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All patients reviewed at discharge
2 Assumed risks estimated by authors from the literature review and clinical experience
Background
Description of the condition
Decompression illness (DCI) is the term given to the clinical manifestations of bubble formation in the blood or tissues following a reduction in ambient pressure (Brubakk 1999). Decompression illness most commonly occurs in relation to compressed air or mixed gas diving, but it may also arise in aviators following rapid ascent to altitude or cabin decompression and in astronauts participating in 'space walks' (Moon 2003). The term covers two different problems, arterial gas embolism (AGE) caused by the presence of bubbles in the arterial blood vessels; and decompression sickness (DCS) caused by bubbles in the veins and tissues. Arterial gas embolism may arise with entry of bubbles into the pulmonary veins through damage to lung tissue from the air trapped in the distal airways during ascent (pulmonary barotrauma); or via an abnormal communication between the right and left sides of the heart, where blood can pass from the venous circulation to the arterial circulation without going through the lungs. Direct venous to arterial passage of bubbles in this way avoids the lung capillaries, which act as a very effective filter for bubbles and allow the safe evolution of gas into the expired breath. Decompression sickness may develop when venous and tissue bubbles form from dissolved inert gas that accumulated during the period of time under pressure. Bubbles may cause harm through mechanical distortion of tissues, vascular obstruction or stimulation of immune mechanisms that lead to tissue oedema, haemoconcentration and hypoxia.
Arterial blood vessels are a particular target for damage by intravascular bubbles, where they disrupt the luminal surfactant layers, damage the endothelium and stimulate intraluminal blood elements (particularly white blood cells and platelets) to clump together and obstruct the flow within the vessel. Secondary interactions between these elements result in leaking vessels and further reductions in flow (Helps 1991; Hills 1991; Nossum 1999). This mechanism does not seem to be important with regard to venous bubbles, possibly due to the low pressure nature of this system.
The two pathological entities (AGE and DCS) are difficult to distinguish clinically and are treated with similar strategies (Francis 1988; Smith 1992). It is, therefore, accepted practice to make the clinical diagnosis of 'DCI' in the understanding that one or both of the two pathologies may be operating. We will use the generic term of DCI in this review except when we refer to the specific pathological mechanisms that cause AGE and DCS.
Clinically, DCI has many possible manifestations, from mild, vague constitutional symptoms to sudden loss of consciousness, death or paralysis (Francis 2003). The most important target tissues are the central nervous system and the musculoskeletal system, with musculoskeletal pain being the most common symptom in the early stages. More recently it has been suggested that constitutional symptoms similar to those experienced during viral illness may be a manifestation of DCI (Francis 2003; Rudge 1991). Without an objective method of determining whether symptoms are due to bubble formation these mild symptoms will sometimes result in misdiagnosis. Severe illness is now uncommon in the developed world, but severe DCI leading to permanent disability or death remains a significant problem for poorly trained indigenous commercial divers around the developing world (Francis 2003; Moon 2003). While the overall incidence of DCI in that setting has not been determined, a number of studies have reported both the incidence and prevalence of DCI and its long‐term effects in individual diving populations. In one prospective study the proportion of divers who reported ever having DCI was 94.4%, and 10% had residual signs of spinal injury. Mortality was estimated at 4% of indigenous divers per year in another group (Bourke 1998; Cross 1998). In contrast, the incidence of DCI among recreational divers in Canada was estimated at 0.01% of dives over 14 months (Ladd 2002).
Description of the intervention
Recompression usually involves placing the patient in an airtight vessel, increasing the pressure within that vessel and administering 100% oxygen for respiration. Typically, treatments involve pressurization to between two and six atmospheres absolute (ATA) for periods between two hours and several days. The optimal treatment strategy for differing clinical presentations is not apparent however by far the most commonly used regimen is the United States Navy Treatment Table 6 (USN TT6), a 2.8 ATA maximum pressure, 100% oxygen breathing schedule which lasts a total of four hours and 45 minutes (DAN 2001).
The historical development of recompression treatment tables was well described by Moon and Gorman (Moon 2003). Pol and Wattelle first proposed recompression (while breathing air) as a treatment for DCI in 1854, but it was not used systematically in practice until 1896 during the construction of the Hudson river tunnel. This project involved many workers spending long shifts in a pressurized working chamber known as a caisson (Moir 1896). Mortality of 25% of cases recorded prior to institution of recompression was dramatically reduced with recompression. In a subsequent tunnel project in New York, Keays demonstrated a recurrence rate of symptoms of 13.7% in workers with DCI who were treated with analgesics and 'stimulants' compared to 0.5% when treated with recompression (Keays 1909). Recompression on air became the standard therapy for DCI until the introduction of 100% oxygen breathing during recompression in 1944, following the work of Yarbrough and Behnke (Yarbrough 1939).
How the intervention might work
With the application of pressure and oxygen, or helium and oxygen breathing, there is a reduction in bubble size according to Boyle's Law (the product of pressure and volume is a constant, so if the pressure is doubled then the volume of any bubble is halved), as well as a greatly enhanced movement of nitrogen out of any bubbles down a steep diffusion gradient (Moon 2003). At the same time there is a greatly increased partial pressure of oxygen supplied to the tissues and this may have profound effects on the development of inflammatory changes, ischaemia‐reperfusion injury and endothelial function in the tissues (Thom 2009). Any or all of these mechanisms may contribute to the observed improvement in clinical symptoms and signs of DCI. Whatever the mechanism, a review of the effectiveness of the United States Navy oxygen treatment tables suggests complete relief of symptoms in 50% to 98% of individuals, apparently depending on the severity of illness and period of time elapsed between development of DCI and recompression (Thalmann 1996).
Why it is important to do this review
Many variations of recompression on oxygen, air, and helium and oxygen mixtures have been proposed and used. Although recompression in some form remains the mainstay of treatment for DCI, it is not clear whether there is one particular recompression table to be preferred. In addition, a number of 'first aid' and adjunctive therapies have been applied in the hope of improving rates of complete resolution. Strategies suggested include the maintenance of a horizontal position (to prevent movement of intravascular bubbles into the cerebral circulation); 100% oxygen administration at one atmosphere; and the administration of intravenous or oral fluids, corticosteroids, anticoagulants, non‐steroidal anti‐inflammatory drugs, lignocaine and diazepam. These strategies (and others) have been recently summarized by Moon (Moon 2003). It is important to consider that any one of these strategies, when combined with recompression, might modify the outcome of DCS and AGE in opposite directions.
Objectives
The objective of this review was to examine the effectiveness and safety of both recompression and adjunctive therapies in the treatment of decompression illness. We assessed effectiveness by using a number of clinically important outcomes, including mortality, residual functional disability and severity scoring systems.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized and quasi‐randomized controlled trials that examined the effectiveness and safety of therapy for DCI.
Types of participants
We included patients of any age or sex with DCI. We defined DCI as any symptom or sign, or both, arising after compressed gas breathing (including brief exposures such as during submarine escape training) and assessed clinically as likely to represent bubble injury. We excluded participants suffering from other causes of AGE (for example iatrogenic).
Types of interventions
We included trials comparing interventions that included recompression or an adjunctive therapy, listed in this section, compared with another form of recompression or other therapy. Adjunctive therapies of interest were the administration of intravenous or oral fluids, or both, corticosteroids, anticoagulants, non‐steroidal anti‐inflammatory drugs, local anaesthetic agents such as lignocaine or benzodiazepines such as diazepam.
The comparator group was most likely to include recompression in some form. We accepted any treatment regimen designed to promote recovery after an episode of DCI, including intensive combined therapies. Where regimens differed significantly between studies this was clearly stated and the implications discussed.
Types of outcome measures
We considered studies as eligible for inclusion if they reported any of the following outcome measures.
Primary outcomes
Mortality rate
Severe functional disability rate or death. We took care to ensure death was included as a bad outcome when extracting data
Complete recovery rate
Secondary outcomes
Functional recovery scale (e.g. Royal New Zealand Navy (RNZN) Recovery Score (Mitchell 1998), Dick and Massey Score (Dick 1985), functional outcome scale (Bennett 1995))
Number of recompression sessions required (in studies looking at adjunctive therapies only)
Time to complete recovery
Time to return to diving
Activities of daily living (ADL) (e.g. the Barthel Index)
Quality of life
Adverse events following therapy (e.g. for recompression: barotrauma (aural, sinus, pulmonary in the short and long‐term), and oxygen toxicity (short‐term)). Any other recorded adverse effects were reported and discussed
Search methods for identification of studies
Electronic searches
In our previous update we searched until October 2009. In this version we searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2011, Issue 3) (see Appendix 1); Ovid MEDLINE (1951 to week 1, October 2011) (see Appendix 2); Ovid EMBASE (1980 to week 1, October 2011) (see Appendix 3); CINAHL via EBSCO host (1982 to week 1, October 2011) (see Appendix 4) and an additional database developed in our hyperbaric facility (the Database of Randomised Trials in Hyperbaric Medicine) (Bennett 2004). The search strategy for MEDLINE was adapted for searching in the other databases. We did not apply language restrictions.
Searching other resources
We also handsearched the following relevant publications.
Hyperbaric textbooks (Brubakk 2003; Jain 2009; Kindwall 2005; Mathieu 2006).
Journals (Undersea and Hyperbaric Medicine 1992 to 2011; Hyperbaric Medicine Review 1986 to 1992; South Pacific Underwater Medicine Society (SPUMS) Journal 1973 to 2008; Diving and Hyperbaric Medicine 2008 to 2011; European Journal of Hyperbaric Medicine 1998 to 2008, and Aviation, Space and Environmental Medicine Journal 1980 to 2011).
Conference proceedings (Undersea and Hyperbaric Medical Society, SPUMS, European Undersea and Baromedical Society, International Congress of Hyperbaric Medicine) published from 1980 to October 2011.
We checked the reference lists of the trials and reviews. We also contacted current researchers in the field for information on unpublished data and ongoing trials.
Data collection and analysis
Selection of studies
Records retrieved by the initial search were scanned by MB, JL and JW to exclude obviously irrelevant studies; two authors (MB and JW) then identified trials that may have met the inclusion criteria. Full‐text articles were retrieved and reviewed by three authors (MB, JL and SM) for the purpose of applying inclusion criteria independently. In all instances we resolved differences of opinion by discussion.
Data extraction and management
We contacted the authors of primary studies for them to provide information when missing or incomplete data were encountered. Two of us (MB and JW) assessed the extracted data from each trial and entered the data into RevMan 5.1. We resolved any disagreement by discussion.
Assessment of risk of bias in included studies
We appraised each included study to assess the risk of bias as outlined in Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We appraised each included study according to the eight criteria outlined in the Handbook and included risk of bias tables. All studies were rated as at 'low risk', unclear risk' or 'high risk' of bias for each criterion (see Characteristics of included studies).
Measures of treatment effect
Although pooling of data was not appropriate in this review, the following is our plan for dealing with any such data in the future. For proportions (dichotomous outcomes), we would calculate relative risk (RR) with 95% confidence intervals (CI). All analyses will be made on an intention‐to‐treat (ITT) basis, where possible; where not possible, this will be clearly stated. Where the 95% CI for the absolute risk difference does not cross zero, we will calculate the number needed to treat (NNT) from the standard recompression event rate and the experimental group rate. The 95% CI would be calculated from the 95% CI of the risk difference between the groups.
Dichotomous data
For this analysis of single trials, we examined any differences between treatment groups using a Chi2 analysis. For the pooling of dichotomous outcomes we planned to calculate the summary estimate as a risk ratio (RR) with 95% confidence intervals (CI). We would estimate the RR using the intention‐to‐treat (ITT) data of the treatment group (hyperbaric oxygen therapy (HBOT)) compared with the ITT of the control group. The dichotomous outcomes included the following.
Mortality rate.
Severe functional disability rate, or death.
Complete recovery rate.
Adverse events.
For these outcomes, we plan to analyse the number of reported events in each arm against the number of participants originally randomised to that arm at trial enrolment (ITT). We would then undertake sensitivity analyses to include people (events) potentially lost to follow‐up (see Dealing with missing data).
Continuous data
For any continuous outcomes measured in the same way across trials, we planned to report a mean difference (MD) with 95% CI. We planned to use the standardised mean difference (SMD) where trials measured the same outcome using different methods. The continuous outcomes included the following.
Functional recovery scale (e.g. Royal New Zealand Navy (RNZN) Recovery Score (Mitchell 1998), Dick and Massey Score (Dick 1985), functional outcome scale (Bennett 1995)).
Number of recompression sessions required (in studies looking at adjunctive therapies only).
Time to complete recovery.
Time to return to diving.
Activities of daily living (ADL) (e.g. the Barthel Index).
Quality of life.
Dealing with missing data
For any trials indicating missing data on allocated participants, we used a ‘best‐case’ and ‘worst‐case’ scenario method as cited in Section 16.2 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess any effect of allocation of that missing data. The ‘best‐case’ scenario is that all participants with missing outcomes in the experimental intervention group had good outcomes, and all those with missing outcomes in the control intervention group had poor outcomes. The ‘worst‐case’ scenario is the converse.
Assessment of heterogeneity
We planned to use the I2 statistic to measure statistical heterogeneity among the trials in each analysis. If we identify substantial heterogeneity we will explore it by pre‐specified subgroup analysis. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error. We will consider there to be significant statistical heterogeneity if I2 > 50% (Higgins 2011).
If pre‐specified subgroup analyses do not explain the statistical heterogeneity, we plan to perform a sensitivity analysis by exclusion of poor quality studies.
Assessment of reporting biases
If there were sufficient included trials for any outcome (> 10 randomized controlled trials (RCTs)), we planned to assess whether the review was subject to publication bias by using a funnel plot to graphically illustrate variability between trials. If asymmetry was detected, we would explore causes other than publication bias.
Data synthesis
We planned to undertake statistical pooling using Cochrane RevMan software (version 5.1) (RevMan 2011). We planned to apply a fixed‐effect model where trials examined the same interventions, the populations and methods described were sufficiently similar, and low levels of between‐trial heterogeneity were evident (I2 ≤ 30%) (Higgins 2011). If statistical heterogeneity was detected, we planned to use a random‐effects model to produce an overall summary estimate. As an estimate of the clinical relevance of any difference between the experimental intervention and control intervention we planned to calculate the number needed to treat (NNT) with 95% CI, as appropriate. We undertook and presented a narrative synthesis of all studies.
Subgroup analysis and investigation of heterogeneity
If appropriate data existed, we would plan to consider subgroup analysis based on:
subtype of DCI (DCS (Type I, Type II), AGE;
severity grade;
gas burden;
time elapsed between completion of last dive and treatment. We intended to arbitrarily divide participants in to those being compressed within one hour, one to 12 hours and more than 12 hours since the time of last dive;
time elapsed from appearance of first symptom to treatment (as above for categories);
dose of oxygen received (pressure less than 3.0 ATA versus 3.0 ATA or more and length of treatment course, one session versus multiple sessions);
recompression with oxygen versus helium and oxygen mixtures.
Sensitivity analysis
We had planned a sensitivity analysis by study quality however this was not appropriate.
Results
Description of studies
Results of the search
Following our updated search in October 2011, we had identified a total of 243 publications apparently dealing with the use of HBOT for the treatment of late radiation tissue injury (LRTI). On the basis of screening the titles and abstracts, we excluded 228 records. The remaining 15 reports were retrieved in full text. Examination of the full articles confirmed six were investigations concerning divers but for problems other than DCI, two were reviews without new data, one was a treatment guideline, one was a non‐randomised trial with retrospective controls, one was a trial involving pre‐treatment with a range of adjunctive agents intended to prevent or modify any subsequent illness, one was a letter to the editor with no new data (Moon 2009), and two were reports of planned trials that have since been abandoned before enrolment (Francis 2002 and Hink 2001). These reports were excluded (see 'Characteristics of excluded studies' table) leaving two publications of randomized comparative trials. After appraisal, we included both these trials (Bennett 2003; Drewry 1994). Details are given in the 'Characteristics of included studies ' table.
The results of all three searches were combined and are summarized in Figure 1. In total we have included three reports of two trials (Bennett 2003; Drewry 1994).
1.
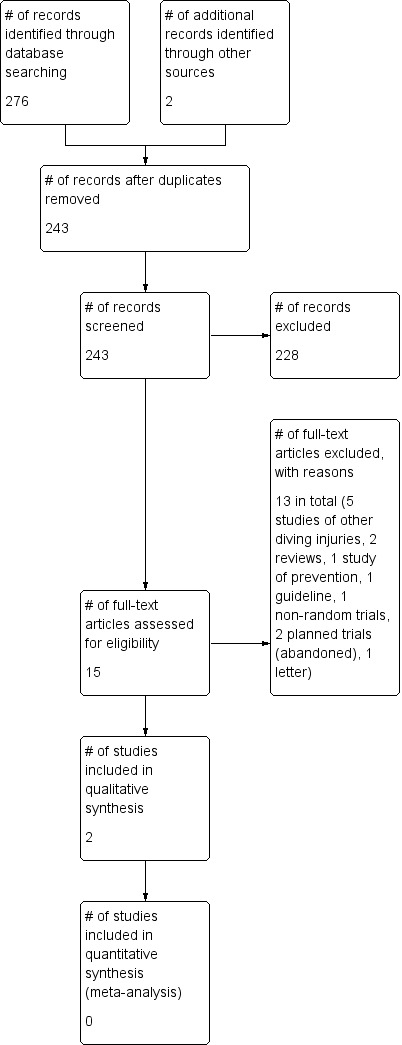
Study flow diagram.
Included studies
In Bennett 2003, 180 participants presenting for management of DCI, with exclusion of those with a clinical diagnosis of AGE, were randomized to either routine recompression therapy or routine recompression therapy with the addition of a non‐steroidal anti‐inflammatory drug (tenoxicam). Those with a firm clinical diagnosis of AGE were excluded on the basis of a lack of expectation of therapeutic benefit and some criticism that the administration of oral medication was not appropriate in this group (personal knowledge). The randomization schedule stratified those enrolled into five groups by disease severity using a clinical scoring system. The recompression schedule was not specified in the protocol but was prescribed at the discretion of the treating physician. In the active therapy arm, tenoxicam 20 mg was administered at the first air break during recompression and daily for seven days, while in the control arm a placebo medication was administered on the same schedule. Results were given for 164 of the 180 patients enrolled (91%). The primary outcome variable in this trial was complete recovery of symptoms and signs measured at completion of recompression therapy and at six weeks. Any mortality was also reported, as was the number of recompression sessions administered.
In Drewry 1994, 88 patients with a clinical diagnosis of DCI were randomized to an initial recompression schedule of either 100% oxygen breathing at 2.8 ATA (equivalent to 18 metres of seawater) pressure, with subsequent higher pressure options on oxygen and nitrogen mixtures if the response was less than an 80% improvement, or a schedule involving breathing 50% oxygen with 50% helium at 2.8 ATA with similar higher pressure options breathing oxygen and helium mixtures in the event of less than 80% improvement. No details were given as to how an 80% improvement was calculated. To date, this trial has been reported as interim results in an abstract only. Eighteen of the 88 participants (20.5%) were withdrawn from analysis due to failure to meet the entry criteria (retrospectively) or because of protocol violations, and a further 14 had not reached final follow‐up. Therefore only 56 participants (64% of those enrolled) had outcomes reported in the abstract. This trial reported the proportion of participants who required multiple compressions prior to discharge.
Excluded studies
These studies are listed in the table 'Characteristics of excluded studies', along with the reasons for exclusion.
Risk of bias in included studies
Details of the identified risks of bias in the included studies is included in 'Characteristics of included studies' and displayed graphically in Figure 2 and Figure 3.
2.
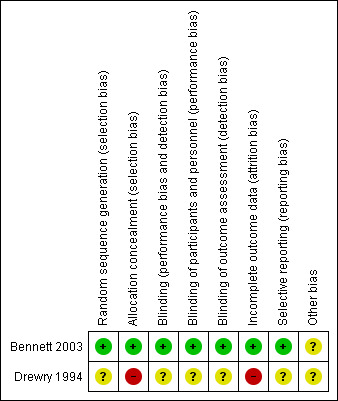
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
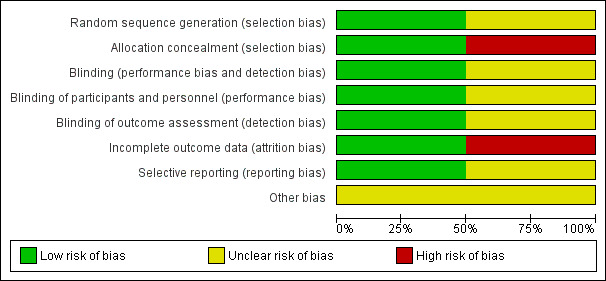
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomization procedures were described in both studies: a computer‐generated number sequence for Bennett 2003 and numbered envelopes for Drewry 1994 (personal communication). Allocation concealment was adequate for Bennett 2003 (central allocation by pharmacy staff) but unclear for Drewry 1994. In the latter trial, it was not clear that the operational staff could not manipulate the group assignment by examination of the allocation envelope prior to recompression. Drewry 1994 stratified the randomization for those presenting up to or later than 48 hours after the onset of symptoms. Bennett 2003 reported the number of participants with severity scores from 1 to 5 in each intervention group (see Table 2).
1. Outcome scores used by Bennett 2003.
| Outcome score |
| 1. Well, no symptoms or signs |
| 2. Minor symptoms or signs not effecting daily life (examples: intermittent tingling in an extremity or minor discomfort not requiring analgesia) |
| 3. Moderate symptoms or signs resulting in some effect on daily life (examples: continued pain requiring analgesia, weakness, hypoaesthesia) |
| 4. Major symptoms or signs significantly effecting life (examples: paraparesis, cognitive dysfunction requiring employment change) |
| 5. Dead |
Blinding
Bennett 2003 described good blinding with the use of a placebo medication, manufactured by the drug company, that was presented in numbered containers. Only the pharmacist who performed the randomization coding held the key. Participants and attending medical officers were blinded in Drewry 1994; however, it may have been possible to discover allocation because of different voice timbre changes when breathing the different compressed gases in the two groups.
Incomplete outcome data
Bennett 2003 lost a total of 16 of 180 participants (9%) at final follow‐up, while Drewry 1994 did not report on 32 of the original 88 participants enrolled (36%). It was not clear to which arm these participants had been allocated.
Selective reporting
Bennett 2003 reported all indicated outcomes. For Drewry 1994, the trial was not completed and a number of planned outcomes have not been reported.
Other potential sources of bias
Bennett 2003 specifically stated the use of an intention‐to‐treat analysis while Drewry 1994 reported multiple violations of the protocol and could not have analysed the data by intention to treat.
Effects of interventions
See: Table 1
Data from the two included studies could not be pooled and were described individually. The data from both Bennett 2003 and Drewry 1994 are reproduced in Appendix 5, Appendix 6 and Appendix 7. The results have been calculated using Chi2 analysis for two by two tables in StatsDirect, version 6.2.8 (StatsDirect Ltd, Altricham, Cheshire 2010).
Bennett 2003 reported no difference in the proportion of participants who were completely recovered at discharge or six weeks later (at discharge: 59/84 (70%) in the placebo group versus 53/84 (63%) in the tenoxicam group; at six weeks: 64/80 (80%) with placebo versus 70/84 (83%) with tenoxicam). The analysis of the chance of recovery with tenoxicam as part of this review confirmed the lack of a significant effect (at discharge: relative risk (RR) for recovery with tenoxicam of 0.90, 95% confidence interval (CI) 0.72 to 1.11, P = 0.33); at six weeks: RR for recovery with tenoxicam 1.04, 95% CI 0.90 to 1.20, P = 0.58). However, this result was sensitive to the outcome of those lost to follow‐up, with a best case analysis suggesting that the chance of recovering completely at six weeks was improved with tenoxicam (RR 1.19, 95% CI 1.01 to 1.39, P = 0.03). See Appendix 5.
This trial reported a difference in the number of recompressions required to reach these outcomes. The placebo group required a median of three treatments (range one to eight) while the tenoxicam group required a median of two treatments (range one to six); this difference was reported as significant (P = 0.01, 95% CI 0 to 1). Analysis of the proportion of participants, suggested a benefit from the administration of tenoxicam (55/90 (61%) of the placebo group versus 35/90 (39%) of the tenoxicam group). The RR for requiring more than two treatments with tenoxicam was 0.64 (95% CI 0.46 to 0.86, P = 0.005). A stratified analysis by the severity grade of DCI on presentation suggested this treatment effect was present across the range of severities tested. This analysis suggested a need to treat five patients to reduce the number of compressions required for one extra patient (NNT 5, 95% CI 3 to 18). See Appendix 6.
Drewry 1994 reported that the proportion of participants requiring multiple recompressions was significantly smaller in the oxygen and helium group (heliox) (9/25 (36%) versus 20/31 (65%), P = 0.03). Analysis in this review identified that the chance of multiple recompressions was lower with heliox (RR 0..52, 95% CI 0.27 to 0.95, P = 0.03). This analysis suggested the need to treat four individuals with helium and oxygen in order to have one extra individual requiring only a single recompression (NNT = 4, 95% CI 2 to 31). See Appendix 7.
Adverse events were reported by Bennett 2003. Six participants had problems during initial recompression, three (one on tenoxicam, two on placebo) complained of aural barotrauma, two (one on tenoxicam, one on placebo) developed premonitory signs of cerebral oxygen toxicity, and one tenoxicam patient complained of nausea not resolved by removal from oxygen breathing at depth (pressure).
Discussion
Summary of main results
This review included only two trials that together enrolled a modest 268 patients. The proportion of participants requiring more than one recompression was significantly reduced by the use of an aggressive helium and oxygen recompression regimen in which treatment depth and duration were determined by symptom response (RR 0.52) (Drewry 1994). The addition of the NSAID tenoxicam to a standard recompression treatment also reduced the requirement for recompression by one treatment (RR 0.64) (Bennett 2003). This reduction in the number of compressions required to treat DCI is examined in the Table 1, along with a sensitivity analysis for the control group risk of a second treatment, assuming the RR is unchanged at different baseline risks. Our best estimate is that 391 extra compressions are avoided for each 1000 cases seen when the control group risk is 611 per 1000 cases (as for the control group in Bennett 2003). The median number of treatments required was 3 (range 1 to 8) in the recompression group versus 2 (range 1 to 6) in the tenoxicam and recompression group. Analysis suggested a modest treatment‐sparing effect with an NNT of five patients to reduce the number of recompression treatments required by at least one. A subgroup analysis by severity score suggested this benefit may extend across all severity grades, but it was underpowered to produce a definitive result.
Neither trial was designed to address our primary outcome of the benefit of recompression versus an alternative therapy for the treatment of decompression illness, and in neither trial was the ultimate success of treatment significantly influenced by treatment allocation.
There are a few major adverse effects of recompression (pulmonary barotrauma, acute cerebral oxygen toxicity, or death related to chamber fire) and short courses of non‐steroidal drugs (renal failure or significant gastric bleeding). While these are all rare enough not to be seen in the trials included in this review, they should be included in consideration of any benefit of these therapies. In practice, it is likely that a beneficial effect strong enough to be clearly identified in clinical trials would overwhelm the consideration of such rare events. There are, however, a number of more minor complications that may occur commonly and Bennett 2003 reported six individuals with minor adverse effects. None of the six were withdrawn from therapy.
Overall completeness and applicability of evidence
We did not find randomized controlled trial evidence to support or refute the effectiveness of recompression versus no recompression for the management of DCI. Recompression is a universally accepted therapy for DCI and, for ethical reasons, is most unlikely to be subject to randomized investigation against sham therapy in the future. The two trials considered in this review looked at alternative recompression strategies (Drewry 1994) and an NSAID drug as an adjunctive therapy to standard recompression (Bennett 2003), respectively. The results could not therefore be pooled for meta‐analysis.
The impact of the heliox regimen should be interpreted carefully in the context of local patient characteristics and the expected rate of multiple compressions. While calculation of the NNT with heliox using the control event rate in this study (65% required multiple compression) is four, this estimate is sensitive to the actual event rate in practice at other treatment facilities. For example, the data from 591 cases of DCI reported by the Divers Alert Network in 2001 suggest the proportion receiving multiple compressions is 50% (DAN 2001). Using this as the control event rate and an RR of 0.56 as our best estimate of effect suggests an NNT of five. Also of potential importance is the consideration that the treatment protocol was quite complex for both arms of the study and ultimately allowed for the participants to enter a saturation treatment that may have lasted for several days. This mode of treatment is unlikely to be a realistic prospect for most treatment facilities and the clinical relevance of this finding is therefore unclear. More information is needed on the actual profiles used and the clinical outcome of participants in this trial. This is important because it is possible that any benefit of heliox treatment may have arisen from an interaction with complex, long, high pressure recompression protocols that might be impractical in many hyperbaric units.
The methodology in Bennett 2003 is more directly applicable to clinical practice. These authors reported the dichotomous outcome 'one or two treatments versus more than two treatments' on the basis that the standard recompression approach in many Australasian institutions is to continue recompression treatments until resolution of symptoms plus one further recompression session, or until symptoms plateau for two consecutive recompression sessions. Thus, for many physicians two recompression sessions is a minimal treatment course. Prior to conducting this trial the median requirement in our unit was three sessions, and this is probably representative of common practice (personal knowledge). Similar considerations concerning the interpretation of NNT apply here as well as to Drewry 1994, particularly as world practice suggests that single recompression therapy remains common. Once again, using the DAN data for comparison (DAN 2001) and the effect estimate from the study (RR 0.65), only 30% of patients received more than two compressions, suggesting an NNT with tenoxicam of 10 rather than five.
An informal economic analysis based on the results of Bennett 2003 and using cost data from a contemporaneous cost analysis in the main contributing hyperbaric facility involved (Gomez‐Castillo 2005) suggests there may be modest cost savings associated with the administration of tenoxicam as an adjunctive measure for DCI. These data suggest a saving of AUD 720 (one session of HBOT for DCI) for every five patients treated for DCI (95% CI every 3 to 18 patients). This cost saving ignores the cost of the course of tenoxicam. While costs vary with the source of supply, a typical cost for 30 tablets in Australia is approximately AUD 13.00. The savings above would be reduced by approximately AUD 6.50 for each patient if they were issued with only a sufficient supply for the seven‐day course (MIMS 2006).
Quality of the evidence
The two trials included in this review involved a modest total of 268 patients. The Drewry 1994 trial was never reported at completion and is probably underpowered to find a clinically significant difference between the two recompression strategies. While a preliminary 1992 report on trial methodology (referenced here as a duplicate of Drewry 1994) suggests a sequential analysis strategy with a stopping rule including a demonstrated difference between the groups at one month (P = 0.05 or less), it is not clear this rule was invoked. We believe the trial was abandoned shortly after the Drewry 1994 report because of continuing protocol violations (personal communication). There is a significant difference in the reported number of participants enrolled in each arm of this study (25 versus 31) and although this may be due to chance we consider the potential for selection bias to be high. One further problem is that this trial reported only the proportion of participants who required multiple recompressions and there were no available data on the clinical health outcomes at any stage.
Bennett 2003 was powered to detect a difference between groups in the proportion of participants with complete resolution (30% placebo versus 20% tenoxicam predicted). This trial suggests that we can be reasonably confident that the addition of tenoxicam to recompression does not result in a clinically important improvement in the effectiveness of therapy.
We had also planned to perform subgroup analyses with respect to DCS type, time delay between symptoms and recompression, and dose of oxygen received. However, the paucity of eligible trials did not permit this approach. One further problem with research in this area is diagnostic uncertainty. There are no reliable diagnostic tests or clinical criteria for DCI and it is likely that all clinical trials will be contaminated by an unknown number of 'cases' that do not in fact suffer from a bubble‐related injury. In general, this will tend to minimize the apparent effectiveness of specific, targeted therapies while magnifying the effect of symptomatic therapies with broad, non‐specific activity. For the clinician, the included studies are pragmatic and likely to reflect the efficacy of interventions in the presence of this diagnostic uncertainty.
Potential biases in the review process
This review is constrained by the paucity of data in this area. Of the two studies included, one of the review authors (MB) is the first author of one (Bennett 2003) and it is possible this has introduced a bias in interpretation despite our methodology. However, our high level of involvement in this area and our comprehensive searching strategy give us confidence that we have located all relevant reports and data, both published and unpublished.
Agreements and disagreements with other studies or reviews
Recompression is universally accepted as the primary therapy for DCI and we are not aware of any previous reviews attempting to summarise the efficacy of recompression against alternative therapies. Our review does confirm the findings of previous authors that recompression is highly successful (DAN 2001; Moon 2003; Thalmann 1996). Non‐systematic reviews of adjunctive therapy have in general failed to identify any strategies that improve the success of recompression, with the exception of reference to the findings of Bennett 2003 (Moon 2000).
Authors' conclusions
Implications for practice.
Recompression therapy is universally accepted as standard practice for the treatment of DCI. While there is considerable evidence for good outcomes following recompression, this practice is not based on any randomized controlled trial evidence. There is some evidence that the addition of an NSAID to breathing 100% oxygen during recompression reduces the number of recompression sessions required to treat DCI, but there is no evidence for an improvement in the rate of complete recovery. Similarly, there is some evidence that helium and oxygen breathing during recompression may reduce recompression requirements, though the methodological problems in the single trial examining the use of helium and oxygen breathing should be noted. The use of an NSAID is likely to be associated with a modest reduction in the cost of therapy. Thus, the application of either of these strategies may be justified. The small number of studies and the modest numbers of patients included in this review demand a cautious interpretation. Given the lack of evidence for improved outcomes, benefits may be largely economic and an economic analysis should be undertaken.
Implications for research.
Given the natural history of severe DCI and the well‐documented clinical response to recompression, it is unlikely that any comparison of recompression therapy against a sham alternative can be justified. There is, however, a strong case for large randomized trials of high methodological rigour in order to define the extent of benefit (if any) from the use of different breathing gases and pressure profiles during recompression therapy. Specifically, information is required on the subset of disease severity that may justify the use of complex and expensive treatment tables. The diagnosis and classification of DCI is particularly problematic with the milder forms of the disease. Formal economic analysis is required to quantify the cost benefit of treatment with NSAIDs and heliox. Any future trials would need to consider the following, in particular.
Appropriate sample sizes with power to detect the expected differences generated by this review.
Careful definition and selection of target patients.
Appropriate treatment schedules (gas mixtures, pressure and time).
Appropriate supportive therapy to which recompression would be an adjunct.
Effective and explicit blinding of outcome assessors.
Appropriate outcome measures including all those listed in this review.
Careful elucidation of any adverse effects.
The cost utility of the therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 30 March 2012 | New citation required but conclusions have not changed | We have included study flow, risk of bias and summary of finding tables in this updated review. |
| 30 March 2012 | New search has been performed | We previously updated the review Bennett 2007) until October 2009. In this updated version we reran the searches until 6 October 2011. No new trials have been found. |
| 5 November 2009 | New search has been performed | We updated the search to October 23rd 2009. No new studies were found. We moved one ongoing study (Francis 2002) to the excluded studies section because the study did not continue and no data was supplied. We have removed one ongoing study from the review as it did not take place (Hink 2005). |
| 11 March 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank John Carlisle, Nathan Pace, Peter Kranke, Richard Moon, Tom S Neuman, James Vorosmarti, Robyn Walker, Janet Wale, Kathie Godfrey and Marcus Müllner for their help and editorial advice during the preparation of this review. We are also very grateful to Jane Cracknell of the Cochrane Anaesthesia Review Group for orchestrating the process.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Decompression Sickness explode all trees #2 MeSH descriptor Embolism, Air explode all trees #3 MeSH descriptor Diving explode all trees #4 MeSH descriptor Decompression explode all trees #5 (decompress* or (embolism near air) or diving):ti,ab #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Hyperbaric Oxygenation explode all trees #8 MeSH descriptor Oxygen Inhalation Therapy explode all trees #9 MeSH descriptor Oxygen, this term only #10 MeSH descriptor Atmospheric Pressure explode all trees #11 MeSH descriptor Atmosphere Exposure Chambers explode all trees #12 recompression or hyperbar* or HBO* or high pressure oxygen or 100% oxygen #13 ((monoplace or multiplace) near chamber*) #14 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 MeSH descriptor Anti‐Inflammatory Agents explode all trees #16 MeSH descriptor Adrenal Cortex Hormones explode all trees #17 MeSH descriptor Lidocaine explode all trees #18 MeSH descriptor Infusions, Intravenous, this term only #19 MeSH descriptor Benzodiazepines explode all trees #20 (#15 OR #16 OR #17 OR #18 OR #19) #21 (( #6 AND #14 ) OR ( #6 AND #20 ))
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. Decompression sickness/ or Embolism, Air/ or Diving/ or Decompression/ or (decompress* or (embolism adj3 air) or diving).ti,ab. 2. Hyperbaric Oxygenation/ or Oxygen Inhalation Therapy/ or Oxygen/ae, tu or Atmospheric Pressure/ or Atmosphere Exposure Chambers/ or recompression.mp. or (hyperbar* or HBO*).mp. or (high pressure oxygen or 100% oxygen).mp. or ((monoplace or multiplace) adj5 chamber$).mp. 3. Anti‐inflammatory Agents/ or Adrenal Cortex Hormones/ or Lidocaine/ or Infusions, Intravenous/ or Benzodiazepines/ 4. (1 and 2) or (1 and 3) 5. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 6. 4 and 5
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. decompression sickness/ or air embolism/ or diving/ or decompression/ or (decompress* or (embolism adj3 air) or diving).ti,ab. 2. hyperbaric oxygen/ or oxygen therapy/ or oxygen/ae, tu or atmospheric pressure/ or recompression.mp. or (hyperbar* or HBO*).mp. or (high pressure oxygen or 100% oxygen).mp. or ((monoplace or multiplace) adj5 chamber*).mp. 3. antiinflammatory agent/ or corticosteroid/ or lidocaine/ or intravenous drug administration/ or benzodiazepine derivative/ 4. (1 and 2) or (1 and 3) 5. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 6. 4 and 5
Appendix 4. Search strategy for CINAHL (EBSCO host)
S1 ( (MH "Decompression Sickness") OR (MH "Embolism, Air") OR (MH "Diving") OR (MH "Scuba Diving") ) OR ( decompress* or (embolism and air) or diving ) S2 ( (MH "Hyperbaric Oxygenation") OR (MH "Oxygen Therapy") OR (MH "Oxygen Therapy (Iowa NIC)") OR (MH "Oxygen") OR (MH "Atmospheric Pressure") ) OR ( recompression or hyperbar* or HBO* or high pressure oxygen or 100% oxygen ) OR ( ((monoplace or multiplace) and chamber*) ) S3 (MH "Antiinflammatory Agents") OR (MH "Adrenal Cortex Hormones") OR (MM "Lidocaine") OR (MH "Infusions, Intravenous") OR (MH "Antianxiety Agents, Benzodiazepine+") S4 ( (S1 and S2) ) OR ( (S1 and S3) )
Appendix 5. Results from Bennett 2003 ‐ complete recovery
| Outcome | Tenoxicam (n) | Placebo (n) | Risk ratio (95% CI) | ||
| Events | Total | Events | Total | ||
| 1.1 Complete recovery at discharge | 53 | 84 | 59 | 84 | 0.85 (0.64 to 1.18) |
| 1.2 best case scenario | 59 | 90 | 59 | 90 | 1.0 (0.74 to 1.38) |
| 1.3 worst case scenario | 53 | 90 | 65 | 90 | 0.75 (0.57 to 1.01) |
| 1.4 Complete recovery at six weeks | 70 | 84 | 64 | 80 | 1.12 (0.78 to 1.77) |
| 1.5 best case scenario | 76 | 90 | 64 | 90 | 1.55 (1.04 to 2.51) |
| 1.6 worst case scenario | 70 | 90 | 74 | 90 | 0.88 (0.65 to 1.27) |
Appendix 6. Results from Bennett 2003 ‐ more than two compressions required
| Outcome and stratum | Tenoxicam (n) | Placebo (n) | P value | ||
| More than two compressions required | Events | Total | Events | Total | |
| Grade 1 | 4 | 19 | 8 | 15 | 0.07 |
| Grade 2 | 25 | 56 | 34 | 57 | 0.12 |
| Grade 3,4,5 | 6 | 15 | 13 | 18 | 0.09 |
| Total | 35 | 90 | 55 | 90 | P = 0.005 |
Appendix 7. Results from Drewry 2004 ‐ more than two compressions required
| Outcome | Heliox (n) | Control (n) | Risk ratio (95% CI) | ||
| Events | Total | Events | Total | ||
| More than two compressions required | 9 | 25 | 20 | 31 | 0.52 (0.27 to 0.95) |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bennett 2003.
| Methods | Randomized controlled trial with allocation concealment, blinding of all participants and investigators. Analysed by intention to treat. Central computer code held by pharmacy. | |
| Participants | 180 participants with clinical DCI (excluding CAGE) from three centres. | |
| Interventions | Control: recompression on physician choice table (88% had USN TT6), placebo medication at first air break and daily for seven days, recompression as clinically indicated to plateau of symptoms or complete resolution plus one further treatment. Active: as above, but active medication with tenoxicam 20 mg per dose. | |
| Outcomes | Death, outcome functional score (see table 02), number of compression cycles required. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation was achieved using a computer generated, randomised schedule stratified by admission grade" |
| Allocation concealment (selection bias) | Low risk | Figure 1 indicates allocation after entry into study. "Allocation was achieved using a computer generated, randomised schedule stratified by admission grade." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Only the trial pharmacist knew the schedule, while the investigators, subjects, treating physicians and outcome assessors were all unaware of group allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ".. giving a loss at final follow‐up of 16 patients (8.9%)" |
| Selective reporting (reporting bias) | Low risk | All indicated outcomes were reported |
| Other bias | Unclear risk | Nil other bias detected |
Drewry 1994.
| Methods | Randomized controlled trial with blinding of investigators and participants. Sealed envelope method with stratification for presentation within 48 hours or more than 48 hours. | |
| Participants | 88 patients presenting with DCI (clinical diagnosis) and requiring recompression therapy. | |
| Interventions | Control: intravenous hydration and recompression breathing 100% oxygen at 18 msw. If 80% or more improvement after 45 minutes, then USN TT6 recompression table is completed. If less than 80% improvement, then proceeded to 30 msw breathing 50% oxygen with 50% nitrogen. Complex algorithm if there is still poor response, with maximum compression to 50 msw. Active: intravenous hydration and recompression breathing 50% oxygen and 50% helium at 18 msw. If 80% or more improvement after 45 minutes, then completed an 18 msw maximum depth table breathing heliox with no air breaks. If less than 80% improvement, then proceeded to 30 msw breathing 50% oxygen with 50% helium. Complex algorithm if there is still poor response, with maximum compression to 50msw breathing 20% oxygen and 80% helium. | |
| Outcomes | Proportion of participants requiring second recompression due to incomplete resolution of clinical symptoms or signs. | |
| Notes | Full trial only reported in abstract form. Not analysed by intention to treat (18 withdrawals due to protocol violations and 14 others with results not reported). The first report did not give any results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given "Patients are randomly allocated to receive either 50/50 oxygen‐helium or 100% oxygen". |
| Allocation concealment (selection bias) | High risk | Methods not described in detail, but delivered treatment is unbalanced between arms (31 versus 25). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients are randomly allocated to receive either 50/50 oxygen‐helium or 100% oxygen and compressed to 2.8 Bars abs. Treatment depth, duration and frequency is determined by symptom response. Patients are evaluated by medical examination and psychometric testing after the first treatment, before discharge, after one month and at one year. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Poor description of how the patient and staff were blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No indication that outcome assessors were unaware of allocation. "Patients are evaluated by medical examination and psychometric testing after the first treatment, before discharge, after one month and at one year." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High loss to follow up and unexplained different accrual rate in the two arms of the trial. "88 subjects have been entered, of whom 18 failed to meet the trial criteria (due to pregnancy, wrong diagnosis or failure to follow the study protocol). The one‐year follow‐up results for 56 subjects are available." |
| Selective reporting (reporting bias) | Unclear risk | Insufficent information available |
| Other bias | Unclear risk | It is possible that patients were selectively allocated to one arm of the trial in preference (31 and 25 in the two groups). |
DCI = Decompression illness CAGE = Cerebral arterial gas embolus HBOT = Hyperbaric oxygen therapy USN TT6 = United States Navy treatment table six = an 18 m maximum pressure table breathing 100% oxygen MSW = Metres of seawater = (a measure of treatment pressure, 10 msw = 1 atmosphere)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davis 1987 | Not investigating the treatment of DCI |
| Francis 2002 | Trial was abandoned before recruitment commenced |
| Hink 2001 | Trial was abandoned before recruitment commenced |
| Koltyn 1997 | Not investigating the treatment of DCI |
| Mitchell 2001 | Review only: no new data |
| Moon 1997 | Review only: no new data |
| Moon 1999 | Guidelines for therapy: no new data |
| Moon 2009 | A letter to the editor with no new data |
| Philp 1979 | Pre‐treatment (before diving) study for the prevention and amelioration of DCI |
| Saino 1992 | Not investigating the treatment of DCI |
| Shupak 1997 | Comparative trial with retrospective controls |
| Taylor 2000 | Not investigating the treatment of DCI |
| Taylor 2001 | Not investigating the treatment of DCI |
| Thorsen 1995 | Not investigating the treatment of DCI |
| Vann 2011 | A review of therapy for DCI |
DCI = Decompression illness RCT = Randomized controlled trial
Contributions of authors
Conceiving the review: Michael H Bennett (MB) Co‐ordinating the review: MB Undertaking manual searches:MB, Simon J Mitchel (SM) Screening search results: Bennett, SM Organizing retrieval of papers: MB, Jason Wasiak (JW) Screening retrieved papers against inclusion criteria: MB, SM, Jan P Lehm (JL) Appraising quality of papers: MB, SM, JL Abstracting data from papers: MB, SM JL Writing to authors of papers for additional information: MB, SM Providing additional data about papers: MB Obtaining and screening data on unpublished studies: MB Data management for the review: MB Entering data into Review Manager (RevMan 5.1): MB RevMan statistical data: MB Other statistical analysis not using RevMan: MB Double entry of data: (data entered by person one: MB; data entered by person two: SM) Interpretation of data: MB, SM, JL Statistical inferences: MB Writing the review: MB Securing funding for the review: none received Performing previous work that was the foundation of the present review: MB, SM, JL Guarantor for the review (one author): MB Person responsible for reading and checking review before submission: JL, JW
Sources of support
Internal sources
No internal support, Other.
External sources
No external support, Other.
Declarations of interest
Three of the authors of this review are diving physicians (MB, JL, SM) and both MB and SM are authors of trials included in this review. There are no known financial conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Bennett 2003 {published data only}
- Bennett M, Mitchell S, Dominguez A. Adjunctive treatment of decompression illness with a non‐steroidal anti‐inflammatory drug (tenoxicam) reduces compression requirement. Undersea & Hyperbaric Medicine 2003;30(3):195‐205. [1066‐2936] [PubMed] [Google Scholar]
Drewry 1994 {published data only}
- Drewry A, Gorman DF. A preliminary report on a prospective randomized, double‐blind, controlled study of oxygen and oxygen‐helium in the treatment of air‐diving decompression illness. South Pacific Underwater Medical Journal 1992;22(3):139‐43. [Google Scholar]
- Drewry A, Gorman DF. A progress report on the prospective randomised double blind controlled study of oxygen and oxygen‐helium in the treatment of air‐diving decompression illness. Undersea & Hyperbaric Medicine 1994;21 Suppl:98. [Google Scholar]
References to studies excluded from this review
Davis 1987 {published data only}
- Davis M. Scopoderm and diver performance. South Pacific Underwater Medicine Journal 1987;17(1):23‐4. [Google Scholar]
Francis 2002 {published data only}
- Francis T. A randomised prospective trial of lignocaine in the management of acute neurological decompression illness ‐ an update. South Pacific Underwater Medicine Journal 2002;32(2):97‐105. [Google Scholar]
Hink 2001 {unpublished data only}
- Hink J. The efficacy of Comex 30 He‐O2 vs. US Navy treatment tables for DCI. http://www.oxynet.org/02COSTinfo/protocol_dci.htm 2001.
Koltyn 1997 {published data only}
- Koltyn KF, Morgan WP. Influence of wet suit wear on anxiety responses to underwater exercise. Undersea & Hyperbaric Medicine 1997;24(1):23‐8. [PubMed] [Google Scholar]
Mitchell 2001 {published data only}
- Mitchell SJ. Lidocaine in the treatment of decompression illness: a review of the literature. Undersea & Hyperbaric Medicine 2001;28(3):165‐74. [MEDLINE: ] [PubMed] [Google Scholar]
Moon 1997 {published data only}
- Moon RE, Sheffield PJ. Guidelines for treatment of decompression illness. Aviation Space, and Environmental Medicine 1997;68(3):234‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Moon 1999 {published data only}
- Moon RE, Lisle Dear G, Stolp BW. Treatment of decompression illness and latrogenic gas embolism. Respiratory Care Clinics of North America 1999;5(1):93‐135. [MEDLINE: ] [PubMed] [Google Scholar]
Moon 2009 {published data only}
- Moon RE, Butler FK. [Letter]. Military Medicine 2009;174(12):xii. [PubMed] [Google Scholar]
Philp 1979 {published data only}
- Philp RB, Bennett PB, Andersen JC, Fields GN, McIntyre BA, Francey I, Briner W. Effects of aspirin and dipyridamole on platelet function, hematology, and blood chemistry of saturation divers. Undersea Biomedical Research 1979;6(2):127‐46. [PubMed] [Google Scholar]
Saino 1992 {published data only}
- Saino A, Perondi R, Alessio P, Gregorini L, Pomidossi G, Rimini A, et al. Coronary response to diving in subjects with mild and severe left coronary artery disease. European Heart Journal 1992;13(3):299‐303. [DOI] [PubMed] [Google Scholar]
Shupak 1997 {published data only}
- Shupak A, Melamed Y, Ramon Y, Bentur Y, Abramovich A, Kol S. Helium and oxygen treatment of severe air‐diving‐induced neurologic decompression sickness. Archives of Neurology 1997;54(3):305‐11. [DOI] [PubMed] [Google Scholar]
Taylor 2000 {published data only}
- Taylor D, O'Toole K, Auble T, Ryan C, Sherman D. The psychometric and cardiac effects of pseudoephedrine in the hyperbaric environment. Pharmacotherapy 2000;20(9):1045‐50. [DOI] [PubMed] [Google Scholar]
Taylor 2001 {published data only}
- Taylor D, O'Toole K, Auble T, Ryan C, Sherman D. The psychometric and cardiac effects of pseudoephedrine and antihistamines in the hyperbaric environment. South Pacific Underwater Medicine Society Journal 2001;31(1):50‐7. [Google Scholar]
Thorsen 1995 {published data only}
- Thorsen E, Risberg J, Segadal K, Hope A. Effects of venous gas microemboli on pulmonary gas transfer function. Undersea & Hyperbaric Medicine 1995;22(4):347‐53. [PubMed] [Google Scholar]
Vann 2011 {published data only}
- Vann RD, Butler FK, Mitchell SJ, Moon RE. Lancet 2011 Aug 8, 2012 Jan 8, 377(9760):153‐64, ISSN: 0099‐5355. Decompression illness. Lancet 2011;277(9760):153‐64. [DOI] [PubMed] [Google Scholar]
Additional references
Bennett 1995
- Bennett MH. The retrieval of diving injuries in New South Wales. South Pacific Underwater Medicine Society Journal 1995;25:142‐7. [Google Scholar]
Bennett 2004
- Bennett MH, Connor D. The Database of Randomised Controlled Trials in Hyperbaric Medicine (DORCTIHM). www.hboevidence.com 2004 (updated monthly).
Bourke 1998
- Bourke A, Verwork C, Dawson R. A study of diving practices and the incidence of decompression sickness in a population of indigenous diving fishermen in the Phillipines (ii Noc Nocan). Undersea & Hyperbaric Medicine 1998;25 Suppl:32. [Google Scholar]
Brubakk 1999
- Brubakk AO. The effect of bubbles on the living body. South Pacific Underwater Medicine Journal 1999;29:221‐7. [Google Scholar]
Brubakk 2003
- Brubakk AO, Neuman TS. In: Brubakk AO, Neuman TS editor(s). Bennett and Elliott's Physiology and Medicine of Diving. 5th Edition. Edinburgh: Saunders, 2003. [0‐7020‐2571‐2] [Google Scholar]
Cross 1998
- Cross MR, Dawson R. Diving diseases research centre's diving fishermen project. In: Lepawsky M, Wong R editor(s). Empirical Diving Techniques of Commercial Sea Harvesters: 50th Workshop of the Undersea and Hyperbaric Medical Society. Kensington: Undersea and Hyperbaric Medicine Society, 1998. [Google Scholar]
DAN 2001
- Divers Alert Network. The DAN annual review of recreational SCUBA diving injuries and fatalities based on 1999 data. In: Vann R, Uguccioni D editor(s). Report on Decompression Illness, Diving Fatalities and Project Dive Exploration. Durham: Divers Alert Network, 2001. [Google Scholar]
Dick 1985
- Dick AP, Massey EW. Neurologic presentation of decompression sickness and air embolism in sport divers. Neurology 1985;35:667‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Francis 1988
- Francis TJ, Pearson RR, Robertson AG, Hodgson M, Dutka AJ, Flynn ET. Central nervous system decompression sickness: latency of 1070 cases. Undersea Biomedical Research 1988;15:403‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Francis 2003
- Francis TJR, Mitchell SJ. Manifestations of decompression disorders. In: Brubakk AO, Neuman TS editor(s). Bennett and Elliott's Physiology and Medicine of Diving. 5th Edition. Edinburgh: Saunders, 2003:578‐9. [0‐7020‐2571‐2] [Google Scholar]
Gomez‐Castillo 2005
- Gomez‐Castillo JD, Bennett MH. The cost of hyperbaric therapy at the Prince of Wales Hospital, Sydney. South Pacific Underwater Medicine Journal 2005;35(4):194‐8. [Google Scholar]
Helps 1991
- Helps SC, Gorman DF. Air embolism of the brain in rabbits pretreated with mechlorethamine. Stroke 1991;22(3):351‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hills 1991
- Hills BA, James PB. Microbubble damage to the blood‐brain barrier: relevance to decompression sickness. Undersea Biomedical Research 1991;18(2):111‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Jain 2009
- Jain KK. In: Jain KK editor(s). Textbook of Hyperbaric Medicine. 5th Edition. Seattle: Hogrefe and Huber, 2009. [0‐88937‐203‐9] [Google Scholar]
Keays 1909
- Keays FL. Compressed air illness, with a report of 3,692 cases. Department of Medicine Publications, Cornell University Medical College 1909;2:1‐55. [Google Scholar]
Kindwall 2005
- Kindwall EP, Whelan HT. In: Kindwall EP, Whelan HT editor(s). Hyperbaric Medicine Practice. 3rd Edition. Flagstaff: Best Publishing Company, 2005. [0‐941332‐78‐0] [Google Scholar]
Ladd 2002
- Ladd G, Stepan V, Stevens L. The Abacus Project: Establishing the risk of recreational scuba death and decompression illness. South Pacific Underwater Medicine Society Journal 2002;32(3):124‐8. [0813‐1988] [Google Scholar]
Mathieu 2006
- Mathieu D. In: Mathieu D editor(s). Handbook on Hyperbaric Medicine. 2nd Edition. Milan: Springer, 2006. [3‐540‐75016‐9] [Google Scholar]
MIMS 2006
- MIMS Australia. MIMS Online. http://www.mims.com.au/ 2006.
Mitchell 1998
- Mitchell S, Holley T, Gorman D. A new system for scoring severity and measuring recovery in decompression illness. South Pacific Underwater Medicine Journal 1998;28(2):84‐94. [Google Scholar]
Moir 1896
- Moir EW. Tunnelling by compressed air. Journal of the Society of Arts 1896;44:567‐85. [Google Scholar]
Moon 2000
- UHMS Adjunctive Therapy Committee. Adjunctive therapy for decompression illness. 1. Kensington: UHMS, 2000. [Google Scholar]
Moon 2003
- Moon RE, Gorman DF. Treatment of the decompression disorders. In: Brubakk AO, Neuman TS editor(s). Bennett and Elliott's Physiology and Medicine of Diving. 5th Edition. Edinburgh: Saunders, 2003:600‐50. [0‐7020‐2571‐2] [Google Scholar]
Nossum 1999
- Nossum V, Koteng S, Brubakk AO. Endothelial damage by bubbles in the pulmonary artery of the pig. Undersea & Hyperbaric Medicine 1999;26(1):1‐8. [MEDLINE: ] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rudge 1991
- Rudge FW. Decompression sickness presenting as a viral syndrome. Aviation, Space and Environmental Medicine 1991;62(1):60‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Smith 1992
- Smith DH, Francis TJR, Pethybridge RJ. Concordance: a problem with the current classification of diving disorders. Undersea Biomedical Research 1992;18 Suppl:40. [Google Scholar]
Thalmann 1996
- Thalmann ED. Principles of US Navy recompression treatments for decompression sickness. In: Bennett PB, Moon RE editor(s). Diving Accident Management. Kensington: Undersea and Hyperbaric Medical Society, 1996:75‐95. [Google Scholar]
Thom 2009
- Thom S. Oxidative stress is fundamental to hyperbaric oxygen therapy. Journal of Applied Physiology 2009;106:988‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yarbrough 1939
- Yarbrough OD, Behnke AR. The treatment of compressed air illness using oxygen. The Journal of Industrial Hygrography and Toxicology 1939;21:213‐8. [Google Scholar]
References to other published versions of this review
Bennett 2007
- Bennett MH, Lehm JP, Mitchell SJ, Wasiak J. Recompression and adjunctive therapy for decompression illness. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005277.pub2] [DOI] [PubMed] [Google Scholar]


