Abstract
Background
Intermediate hyperglycaemia (IH) is characterised by one or more measurements of elevated blood glucose concentrations, such as impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and elevated glycosylated haemoglobin A1c (HbA1c). These levels are higher than normal but below the diagnostic threshold for type 2 diabetes mellitus (T2DM). The reduced threshold of 5.6 mmol/L (100 mg/dL) fasting plasma glucose (FPG) for defining IFG, introduced by the American Diabetes Association (ADA) in 2003, substantially increased the prevalence of IFG. Likewise, the lowering of the HbA1c threshold from 6.0% to 5.7% by the ADA in 2010 could potentially have significant medical, public health and socioeconomic impacts.
Objectives
To assess the overall prognosis of people with IH for developing T2DM, regression from IH to normoglycaemia and the difference in T2DM incidence in people with IH versus people with normoglycaemia.
Search methods
We searched MEDLINE, Embase, ClincialTrials.gov and the International Clinical Trials Registry Platform (ICTRP) Search Portal up to December 2016 and updated the MEDLINE search in February 2018. We used several complementary search methods in addition to a Boolean search based on analytical text mining.
Selection criteria
We included prospective cohort studies investigating the development of T2DM in people with IH. We used standard definitions of IH as described by the ADA or World Health Organization (WHO). We excluded intervention trials and studies on cohorts with additional comorbidities at baseline, studies with missing data on the transition from IH to T2DM, and studies where T2DM incidence was evaluated by documents or self‐report only.
Data collection and analysis
One review author extracted study characteristics, and a second author checked the extracted data. We used a tailored version of the Quality In Prognosis Studies (QUIPS) tool for assessing risk of bias. We pooled incidence and incidence rate ratios (IRR) using a random‐effects model to account for between‐study heterogeneity. To meta‐analyse incidence data, we used a method for pooling proportions. For hazard ratios (HR) and odds ratios (OR) of IH versus normoglycaemia, reported with 95% confidence intervals (CI), we obtained standard errors from these CIs and performed random‐effects meta‐analyses using the generic inverse‐variance method. We used multivariable HRs and the model with the greatest number of covariates. We evaluated the certainty of the evidence with an adapted version of the GRADE framework.
Main results
We included 103 prospective cohort studies. The studies mainly defined IH by IFG5.6 (FPG mmol/L 5.6 to 6.9 mmol/L or 100 mg/dL to 125 mg/dL), IFG6.1 (FPG 6.1 mmol/L to 6.9 mmol/L or 110 mg/dL to 125 mg/dL), IGT (plasma glucose 7.8 mmol/L to 11.1 mmol/L or 140 mg/dL to 199 mg/dL two hours after a 75 g glucose load on the oral glucose tolerance test, combined IFG and IGT (IFG/IGT), and elevated HbA1c (HbA1c5.7: HbA1c 5.7% to 6.4% or 39 mmol/mol to 46 mmol/mol; HbA1c6.0: HbA1c 6.0% to 6.4% or 42 mmol/mol to 46 mmol/mol). The follow‐up period ranged from 1 to 24 years. Ninety‐three studies evaluated the overall prognosis of people with IH measured by cumulative T2DM incidence, and 52 studies evaluated glycaemic status as a prognostic factor for T2DM by comparing a cohort with IH to a cohort with normoglycaemia. Participants were of Australian, European or North American origin in 41 studies; Latin American in 7; Asian or Middle Eastern in 50; and Islanders or American Indians in 5. Six studies included children and/or adolescents.
Cumulative incidence of T2DM associated with IFG5.6, IFG6.1, IGT and the combination of IFG/IGT increased with length of follow‐up. Cumulative incidence was highest with IFG/IGT, followed by IGT, IFG6.1 and IFG5.6. Limited data showed a higher T2DM incidence associated with HbA1c6.0 compared to HbA1c5.7. We rated the evidence for overall prognosis as of moderate certainty because of imprecision (wide CIs in most studies). In the 47 studies reporting restitution of normoglycaemia, regression ranged from 33% to 59% within one to five years follow‐up, and from 17% to 42% for 6 to 11 years of follow‐up (moderate‐certainty evidence).
Studies evaluating the prognostic effect of IH versus normoglycaemia reported different effect measures (HRs, IRRs and ORs). Overall, the effect measures all indicated an elevated risk of T2DM at 1 to 24 years of follow‐up. Taking into account the long‐term follow‐up of cohort studies, estimation of HRs for time‐dependent events like T2DM incidence appeared most reliable. The pooled HR and the number of studies and participants for different IH definitions as compared to normoglycaemia were: IFG5.6: HR 4.32 (95% CI 2.61 to 7.12), 8 studies, 9017 participants; IFG6.1: HR 5.47 (95% CI 3.50 to 8.54), 9 studies, 2818 participants; IGT: HR 3.61 (95% CI 2.31 to 5.64), 5 studies, 4010 participants; IFG and IGT: HR 6.90 (95% CI 4.15 to 11.45), 5 studies, 1038 participants; HbA1c5.7: HR 5.55 (95% CI 2.77 to 11.12), 4 studies, 5223 participants; HbA1c6.0: HR 10.10 (95% CI 3.59 to 28.43), 6 studies, 4532 participants. In subgroup analyses, there was no clear pattern of differences between geographic regions. We downgraded the evidence for the prognostic effect of IH versus normoglycaemia to low‐certainty evidence due to study limitations because many studies did not adequately adjust for confounders. Imprecision and inconsistency required further downgrading due to wide 95% CIs and wide 95% prediction intervals (sometimes ranging from negative to positive prognostic factor to outcome associations), respectively.
This evidence is up to date as of 26 February 2018.
Authors' conclusions
Overall prognosis of people with IH worsened over time. T2DM cumulative incidence generally increased over the course of follow‐up but varied with IH definition. Regression from IH to normoglycaemia decreased over time but was observed even after 11 years of follow‐up. The risk of developing T2DM when comparing IH with normoglycaemia at baseline varied by IH definition. Taking into consideration the uncertainty of the available evidence, as well as the fluctuating stages of normoglycaemia, IH and T2DM, which may transition from one stage to another in both directions even after years of follow‐up, practitioners should be careful about the potential implications of any active intervention for people 'diagnosed' with IH.
Keywords: Humans; Blood Glucose; Blood Glucose/analysis; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/epidemiology; Diabetes Mellitus, Type 2/etiology; Disease Progression; Hyperglycemia; Hyperglycemia/blood; Hyperglycemia/complications; Incidence; Prediabetic State; Prediabetic State/blood; Prognosis; Prospective Studies
Plain language summary
Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia ('prediabetes')
Review question
We wanted to find out whether raised blood sugar ('prediabetes') increases the risk of developing type 2 diabetes and how many of these people return to having normal blood sugar levels (normoglycaemia). We also investigated the difference in type 2 diabetes development in people with prediabetes compared to people with normoglycaemia.
Background
Type 2 diabetes is often diagnosed by blood sugar measurements like fasting blood glucose or glucose measurements after an oral glucose tolerance test (drinking 75 g of glucose on an empty stomach) or by measuring glycosylated haemoglobin A1c (HbA1c), a long‐term marker of blood glucose levels. Type 2 diabetes can have bad effects on health in the long term (diabetic complications), like severe eye or kidney disease or diabetic feet, eventually resulting in foot ulcers.
Raised blood glucose levels (hyperglycaemia), which are above normal ranges but below the limit of diagnosing type 2 diabetes, indicate prediabetes, or intermediate hyperglycaemia. The way prediabetes is defined has important effects on public health because some physicians treat people with prediabetes with medications that can be harmful. For example, reducing the threshold for defining impaired fasting glucose (after an overnight fast) from 6.1 mmol/L or 110 mg/dL to 5.6 mmol/L or 100 mg/dL, as done by the American Diabetes Association (ADA), dramatically increased the number of people diagnosed with prediabetes worldwide.
Study characteristics
We searched for observational studies (studies where no intervention takes place but people are observed over prolonged periods of time) that investigated how many people with prediabetes at the beginning of the study developed type 2 diabetes. We also evaluated studies comparing people with prediabetes to people with normoglycaemia. Prediabetes was defined by different blood glucose measurements.
We found 103 studies, monitoring people over 1 to 24 years. More than 250,000 participants began the studies. In 41 studies the participants were of Australian, European or North American origin, in 7 studies participants were primarily of Latin American origin and in 50 studies participants were of Asian or Middle Eastern origin. Three studies had American Indians as participants, and one study each invited people from Mauritius and Nauru. Six studies included children, adolescents or both as participants.
This evidence is up to date as of 26 February 2018.
Key results
Generally, the development of new type 2 diabetes (diabetes incidence) in people with prediabetes increased over time. However, many participants also reverted from prediabetes back to normal blood glucose levels. Compared to people with normoglycaemia, those with prediabetes (any definition) showed an increased risk of developing type 2 diabetes, but results showed wide differences and depended on how prediabetes was measured. There were no clear differences with regard to several regions in the world or different populations. Because people with prediabetes may develop diabetes but may also change back to normoglycaemia almost any time, doctors should be careful about treating prediabetes because we are not sure whether this will result in more benefit than harm, especially when done on a global scale affecting many people worldwide.
Certainty of the evidence
The certainty of the evidence for overall prognosis was moderate because results varied widely. The certainty of evidence for studies comparing prediabetic with normoglycaemic people was low because the results were not precise and varied widely. In our included observational studies the researchers often did not investigate well enough whether factors like physical inactivity, age or increased body weight also influenced the development of type 2 diabetes, thus making the relationship between prediabetes and the development of type 2 diabetes less clear.
Summary of findings
Summary of findings for the main comparison. Summary of findings: overall prognosis of people with intermediate hyperglycaemia for developing T2DM.
| Outcome: development of T2DM Prognosis of people with intermediate hyperglycaemia | ||||||||
| Follow‐up (years) | Cumulative T2DM incidence % (95% CI) [no of studies; no of participants with intermediate hyperglycaemia] | Regression from intermediate hyperglycaemia to normoglycaemia % (95% CI) [no of studies; no of participants with intermediate hyperglycaemia] | Overall certainty of the evidence (GRADE)a | |||||
| IFG5.6 | IFG6.1 | IGT | IFG + IGT | HbA1c5.7 | HbA1c6.0 | |||
| 1 | — | — |
13 (5–23) [3; 671] |
29 (23–36) [1; 207] |
— | — |
59 (54–64) [2; 375] |
⊕⊕⊕⊝ Moderateb |
| 2 |
2 (1–2) [1; 1335] |
11 (8–14) [2; 549] |
16 (9–26) [9; 1998] |
— | — | — |
46 (36–55) [9; 2852] |
|
| 3 |
17 (6–32) [3; 1091] |
9 (2–20) [3; 927] |
22 (18–27) [3; 417] |
34 (28–41) [1; 209]— |
— |
7 (5–10) [1; 370] |
41 (24–69) [7; 1356] |
|
| 4 |
17 (13–22) [3; 800] |
30 (17–44) [2; 1567] |
22 (12–34) [5; 1042] |
— |
14 (7–23) [3; 5352] |
44 (40–48) [2; 627] |
33 (26–40) [3; 807] |
|
| 5 |
18 (10–27) [7; 3530] |
26 (19–33) [11; 3837] |
39 (25–53) [12; 3444] |
50 (37–63) [5; 478] |
25 (18–32) [4; 3524] |
38 (26–51) [3; 1462] |
34 (27–42) [9; 2603] |
|
| 6 |
22 (15–31) [4; 738] |
37 (31–43) [5; 279] |
29 (25–34) [7; 775] |
58 (48–67) [4; 106] |
17 (14–20) [1; 675] |
— |
23 (3–53) [5; 1328] |
|
| 7 |
18 (8–30) [5; 980] |
15 (0–45) [4; 434] |
19 (13–26) [5; 835] |
32 (20–45) [4; 753] |
21 (16–27) [1; 207] |
— |
41 (37–45) [4; 679] |
|
| 8 |
34 (27–40) [2; 1887] |
48 (31–66) [1;29] |
43 (37–49) [4; 1021] |
52 (47–57) [1; 356] |
— | — |
39 (33–44) [2; 328] |
|
| 9 |
38 (10–70) [3; 1356] |
— |
53 (45–60) [1; 163] |
84 (74–91) [1; 69] |
— | — |
17 (14–22) [1; 299] |
|
| 10 |
23 (14–33) [6; 1542] |
29 (17–43) [6; 537] |
26 (17–37) [6; 443] |
30 (17–44) [2; 49] |
31 (29–33) [2; 2854] |
— |
42 (22–63) [7; 894] |
|
| 11 | — |
38 (33–43) [1; 402] |
46 (43–49) [1; 1253] |
— | — | — |
28 (17–39) [2; 736] |
|
| 12 |
31 (19–34) [3; 433] |
31 (28–33) [1; 1382] |
41 (38–43) [2; 1552] |
70 (63–76) [2; 207] |
— | — | — | |
| 15 | — | — | — | — | — |
29 (19–40) [1; 70] |
— | |
| 20 | — | — |
60 (5–68) [1; 114] |
— | — | — | — | |
| CI: confidence interval; HbA1c5.7: glycosylated haemoglobin A1c, 5.7% threshold; HbA1c6.0: glycosylated haemoglobin A1c, 6.0% threshold; IFG5.6: impaired fasting glucose, 5.6 mmol/L threshold; IFG6.1: impaired fasting glucose, 6.1 mmol/L threshold; IGT: impaired glucose tolerance; T2DM: type 2 diabetes mellitus. | ||||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of imprecision (wide CIs for most intermediate hyperglycaemia definitions and the association with T2DM incidence and regression from intermediate hyperglycaemia)
Summary of findings 2. Summary of findings: risk of intermediate hyperglycaemia (IFG5.6 mmol/L definition) versus normoglycaemia for developing T2DM.
| Outcome: development of T2DM Prognostic factor: intermediate hyperglycaemia versus normoglycaemia as measured by IFG5.6 | ||||
| No of studies | No of participants with intermediate hyperglycaemia | Geographic region/special population | Estimated effect (95% CI) [95% prediction interval] | Overall certainty of the evidence (GRADE)a |
| HR: 4 IRR: 6 OR: 10 |
HR: 2385 IRR: 15,661 OR: 6359 |
Asia/Middle East |
HR: 5.07 (3.41–4.86) [1.07–24.02] IRR: 5.23 (3.77–7.25) [1.72–15.89] OR: 2.94 (1.77–4.86) [0.43–19.93] |
⊕⊕⊝⊝ Lowb |
| HR: 3 IRR: 3 OR: 9 |
HR: 5685 IRR: 6322 OR: 1949 |
Australia/Europe/North America |
HR: 4.15 (1.24–13.9) [N/M] IRR: 4.96 (3.25–7.57) [0.32–77.24] OR: 6.47 (3.81–11.00) [0.99–42.32] |
|
| HR: 0 IRR: 0 OR: 1 |
HR: 0 IRR: 0 OR: 65 |
Latin America |
HR: NA IRR: NA OR: 4.28 (3.21–5.71) |
|
| HR: 1 IRR: 1 OR: 1 |
HR: 947 IRR: 2374 OR: 947 |
American Indians/Islands |
HR: 2.38 (1.85–3.06) IRR: 2.74 (1.88–3.99) OR: 3.12 (2.31–4.21) |
|
| HR: 8 IRR: 10 OR: 21 |
HR: 9017 IRR: 24,357 OR: 9320 |
Overall |
HR: 4.32 (2.61–7.12) [0.75–25.0] IRR: 4.81 (3.67–6.30) [1.95–11.83] OR: 4.15 (2.75–6.28) [0.53–32.4] |
|
| CI: confidence interval; HR: hazard ratio;IFG5.6: impaired fasting glucose 5.6 mmol/L threshold; IRR: incidence rate ratio; NA: not applicable; N/M: fewer than 3 studies or calculation of the 95% prediction interval did not provide a meaningful estimate; OR: odds ratio; T2DM: type 2 diabetes mellitus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of study limitations (many studies did not adequately adjust for confounders, if at all) and by one level because of imprecision (CIs were wide) and inconsistency (wide 95% prediction intervals sometimes ranging from negative to positive prognostic factor to outcome associations)
Summary of findings 3. Summary of findings: risk of intermediate hyperglycaemia (IFG6.1 mmol/L definition) versus normoglycaemia for developing T2DM.
| Outcome: development of T2DM Prognostic factor: intermediate hyperglycaemia as measured by IFG6.1 | ||||
| No of studies | No of participants with intermediate hyperglycaemia | Geographic region/special population | Estimated effect (95% CI) [95% prediction interval] | Overall certainty of the evidence (GRADE)a |
| HR: 5 IRR: 2 OR: 7 |
HR: 1054 IRR: 1677 OR: 3317 |
Asia/Middle East |
HR: 10.55 (3.61–30.81) [N/M] IRR: 3.62 (1.67–7.83) [N/M] OR: 5.18 (2.32–11.53) [0.29–91.37] |
⊕⊕⊝⊝ Lowb |
| HR: 4 IRR: 4 OR: 7 |
HR: 1736 IRR: 3438 OR: 1240 |
Australia/Europe/North America |
HR: 3.30 (2.32–4.67) [0.84–12.99] IRR: 8.55 (6.37–11.48) [4.37–16.73] OR: 8.69 (4.95–15.24) [1.20–62.69] |
|
| HR: 0 IRR: 0 OR: 1 |
HR: 0 IRR: 0 OR: 17 |
Latin America |
HR: NA IRR: NA OR: 3.73 (2.18–6.38) |
|
| HR: 0 IRR: 0 OR: 0 |
HR: 0 IRR: 0 OR: 0 |
American Indians/Islands |
HR: NA IRR: NA OR: NA |
|
| HR: 9 IRR: 6 OR: 15 |
HR: 2818 IRR: 5115 OR: 4574 |
Overall |
HR: 5.47 (3.50–8.54) [1.09–27.56] IRR: 6.82 (4.53–10.25) [2.03–22.87] OR: 6.60 (4.18–10.43) [0.93–46.82] |
|
| CI: confidence interval; HR: hazard ratio;IFG6.1: impaired fasting glucose 6.1 mmol/L threshold; IRR: incidence rate ratio; NA: not applicable; N/M: fewer than 3 studies or calculation of the 95% prediction interval did not provide a meaningful estimate; OR: odds ratio; T2DM: type 2 diabetes mellitus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of study limitations (many studies did not adequately adjust for confounders, if at all) and by one level because of imprecision (CIs were wide) and inconsistency (wide 95% prediction intervals sometimes ranging from negative to positive prognostic factor to outcome associations)
Summary of findings 4. Summary of findings: risk of intermediate hyperglycaemia (IGT definition) versus normoglycaemia for developing T2DM.
| Outcome: development of T2DM Prognostic factor: intermediate hyperglycaemia as measured by IGT | ||||
| No of studies | No of participants with intermediate hyperglycaemia | Geographic region/special population | Estimated effect (95% CI) [95% prediction interval] | Overall certainty of the evidence (GRADE)a |
| HR: 3 IRR: 5 OR: 6 |
HR: 1780 IRR: 14,809 OR: 1226 |
Asia/Middle East |
HR: 4.48 (2.81–7.15) [N/M] IRR: 3.93 (3.03–5.10) [1.71–9.02] OR: 3.74 (2.83–4.94) [1.70–8.21] |
⊕⊕⊝⊝ Lowb |
| HR: 2 IRR: 5 OR: 11 |
HR: 2230 IRR: 2572 OR: 1481 |
Australia/Europe/North America |
HR: 2.53 (1.52–4.19) [N/M] IRR: 5.93 (4.11–8.57) [2.38–14.81] OR: 5.20 (3.62–7.45) [1.50–18.09] |
|
| HR: 0 IRR: 0 OR: 2 |
HR: 0 IRR: 0 OR: 381 |
Latin America |
HR: NA IRR: NA OR: 4.94 (3.15–7.76) [N/M] |
|
| IRR: 2 OR: 1 HR: 0 | IRR: 1087 OR: 51 HR: 0 | American Indians/Islands |
IRR: 4.46 (3.12–6.38) [N/M] OR: 3.60 (1.40–9.26) HR: NA |
|
| HR: 5 IRR: 12 OR: 20 |
HR: 4010 IRR: 18,468 OR: 3139 |
Overall |
HR: 3.61 (2.31–5.64) [0.69–18.97] IRR: 4.48 (3.59–5.44) [2.60–7.70] OR: 4.61 (3.76–5.64) [2.10–10.13] |
|
| CI: confidence interval; HR: hazard ratio;IGT: impaired glucose tolerance; IRR: incidence rate ratio; NA: not applicable; N/M: fewer than 3 studies or calculation of the 95% prediction interval did not provide a meaningful estimate; T2DM: type 2 diabetes mellitus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of study limitations (many studies did not adequately adjust for confounders, if at all) and by one level because of imprecision (CIs were wide) and inconsistency (wide 95% prediction intervals sometimes ranging from negative to positive prognostic factor to outcome associations)
Summary of findings 5. Summary of findings: risk of intermediate hyperglycaemia (combined IFG and IGT definition) versus normoglycaemia for developing T2DM.
| Outcome: development of T2DM Prognostic factor: intermediate hyperglycaemia as measured by combined IFG and IGT | ||||
| No of studies | No of participants with intermediate hyperglycaemia | Geographic region/special population | Estimated effect (95% CI) [95% prediction interval] | Overall certainty of the evidence (GRADE)a |
| HR: 3 IRR: 4 OR: 3 |
HR: 461 IRR: 3166 OR: 498 |
Asia/Middle East |
HR: 10.20 (5.45–19.09) [N/M] IRR: 11.20 (5.59–22.43) [N/M] OR: 6.99 (3.09–15.83) [N/M] |
⊕⊕⊝⊝ Lowb |
| HR: 1 IRR: 4 OR: 6 |
HR: 221 IRR: 699 OR: 154 |
Australia/Europe/North America |
HR: 3.80 (2.30–6.28) [N/M] IRR: 13.92 (9.99–19.40) [6.71–28.85] OR: 20.95 (12.40–35.40) [4.93–89.05] |
|
| HR: 0 IRR: 0 OR: 0 |
HR: 0 IRR: 0 OR: 0 |
Latin America |
HR: NA IRR: NA OR: NA |
|
| HR: 1 IRR: 1 OR: 0 |
HR: 356 IRR: 605 OR: 0 |
American Indians/Islands |
HR: 4.06 (3.05–5.40) IRR: 5.18 (3.42–7.83) OR: NA |
|
| HR: 5 IRR: 9 OR: 9 |
HR: 1038 IRR: 4470 OR: 652 |
Overall |
HR: 6.90 (4.15–11.45) [1.06–44.95] IRR: 10.94 (7.22–16.58) [2.58–46.46] OR: 13.14 (7.41–23.30) [1.84–93.66] |
|
| CI: confidence interval; HR: hazard ratio;IFG: impaired fasting glucose; IGT: impaired glucose tolerance; IRR: incidence rate ratio; NA: not applicable; N/M: fewer than 3 studies or calculation of the 95% prediction interval did not provide a meaningful estimate; OR: odds ratio; T2DM: type 2 diabetes mellitus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of study limitations (many studies did not adequately adjust for confounders, if at all) and by one level because of imprecision (CIs were wide) and inconsistency (wide 95% prediction intervals)
Summary of findings 6. Summary of findings: risk of intermediate hyperglycaemia (HbA1c5.7 definition) versus normoglycaemia for developing T2DM.
| Outcome: development of T2DM Prognostic factor: intermediate hyperglycaemia as measured by HbA1c5.7 | ||||
| No of studies | No of participants with intermediate hyperglycaemia | Geographic region/special population | Estimated effect (95% CI) [95% prediction interval] | Overall certainty of the evidence (GRADE)a |
| HR: 3 IRR: 1 OR: 1 |
HR: 3196 IRR: 1965 OR: 675 |
Asia/Middle East |
HR: 7.21 (5.14–10.11) [0.81–64.52] IRR: 6.62 (4.18–10.49) [N/M] OR: 4.54 (2.65–7.78) [N/M] |
⊕⊕⊝⊝ Lowb |
| HR: 1 IRR: 0 OR: 2 |
HR: 2027 IRR: 0 OR: 231 |
Australia/Europe/North America |
HR: 2.71 (2.48–2.96) [N/M] IRR: NA OR: 4.38 (1.36–14.15) [N/M] |
|
| HR: 0 IRR: 0 OR: 0 |
HR: 0 IRR: 0 OR: 0 |
Latin America |
HR: NA IRR: NA OR: NA |
|
| HR: 0 IRR: 0 OR: 0 |
HR: 0 IRR: 0 OR: 0 |
American Indians/Islands |
HR: NA IRR: NA OR: NA |
|
| HR: 4 IRR: 1 OR: 3 |
HR: 5223 IRR: 1965 OR: 906 |
Overall |
HR: 5.55 (2.77–11.12) [0.23–141.18] IRR: 6.62 (4.18–10.49) [N/M] OR: 4.43 (2.20–8.88) [N/M] |
|
| CI: confidence interval; HbA1c5.7: glycosylated haemoglobin A1c 5.7% threshold; HR: hazard ratio;IRR: incidence rate ratio; NA: not applicable; N/M: fewer than 3 studies or calculation of the 95% prediction interval did not provide a meaningful estimate; OR: odds ratio; T2DM: type 2 diabetes mellitus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of study limitations (many studies did not adequately adjust for confounders, if at all) and by one level because of imprecision (CIs were wide) and inconsistency (95% prediction intervals sometimes ranging from negative to positive prognostic factor to outcome associations)
Summary of findings 7. Summary of findings: risk of intermediate hyperglycaemia (HbA1c6.0 definition) versus normoglycaemia for developing T2DM.
| Outcome: development of T2DM Prognostic factor: intermediate hyperglycaemia as measured by HbA1c6.0 | ||||
| No of studies | No of participants with intermediate hyperglycaemia | Geographic region/special population | Estimated effect (95% CI) [95% prediction interval] | Overall certainty of the evidence (GRADE)a |
| HR: 2 IRR: 0 OR: 1 |
HR: 1040 IRR: 0 OR: 370 |
Australia/Europe/North America |
HR: 5.09 (1.69–15.37) [N/M] IRR: NA OR: 15.60 (6.90–35.27) [N/M] |
⊕⊕⊝⊝ Lowb |
| HR: 4 IRR: 0 OR: 1 |
HR: 3492 IRR: 0 OR: 1103 |
Asia/Middle East |
HR: 13.12 (4.10–41.96) [N/M] IRR: NA OR: 23.20 (18.70–28.78) [N/M] |
|
| HR: 0 IRR: 0 OR: 0 |
HR: 0 IRR: 0 OR: 0 |
Latin America |
HR: NA IRR: NA OR: NA |
|
| IRR: 0 OR: 1 HR: 0 | IRR: 0
OR: 121 HR: 0 |
American Indians/Islands |
IRR: NA OR: 5.89 (4.23–8.20) [N/M] HR: NA |
|
| HR: 6 IRR: 0 OR: 3 |
HR: 4532 IRR: 0 OR: 1594 |
Overall |
HR: 10.10 (3.59–28.43) [N/M] IRR: NA OR: 12.79 [4.56–35.85] [N/M] |
|
| CI: confidence interval; HbA1c6.0: glycosylated haemoglobin A1c 6.0% threshold; HR: hazard ratio;IRR: incidence rate ratio; NA: not applicable; N/M: fewer than 3 studies or calculation of the 95% prediction interval did not provide a meaningful estimate; OR: odds ratio; T2DM: type 2 diabetes mellitus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
aWith phase 2 explanatory studies aiming to confirm independent associations between the prognostic factor and the outcome, GRADE starts with 'high quality' (Huguet 2013). We assumed the GRADE factor publication bias was inherent with this type of research (phase 2 design), so we did not use it as a potential downgrading factor bDowngraded by one level because of study limitations (many studies did not adequately adjust for confounders, if at all) and by one level because of imprecision (most CIs were wide)
Background
For a glossary of terms please see Appendix 1.
'Prediabetes', 'borderline diabetes', 'prediabetic stage', 'high risk of diabetes', 'dysglycaemia' or 'intermediate hyperglycaemia' (IH) are terms used to characterise various measurements of elevated blood glucose concentrations, such as impaired fasting glucose (IFG), impaired glucose tolerance (IGT), elevated glycosylated haemoglobin A1c (HbA1c) or combinations of these conditions (WHO/IDF 2006). Elevated blood glucose levels that indicate hyperglycaemia are too high to be considered normal, but they are below the diagnostic threshold for type 2 diabetes mellitus (T2DM). Therefore, due to the continuous glycaemic spectrum from normal to the diabetic stage, a sound evidence base is needed to define glycaemic thresholds for people at high risk of T2DM, especially because dysglycaemia is commonly an asymptomatic condition, so naturally it often remains undiagnosed (CDC 2015). The various terms used to describe stages of hyperglycaemia may cause people to have marked emotional reactions. For example, the term prediabetes may imply (at least for non‐experts) that diabetes is unavoidable, whereas (high) risk of diabetes gives people the impression that they can possibly avoid the disease altogether. In addition to the disputable construct of intermediate health states termed 'predisease' (Viera 2011), many people may associate the label 'prediabetes' with dire consequences. Alternatively, any diagnosis of prediabetes may be an opportunity to reassess, for example, eating habits and physical activity levels, thus enabling affected individuals to actively change their health‐related behaviours.
Several institutional bodies like the American Diabetes Association (ADA) and the World Health Organization (WHO) have established commonly used criteria to define people who are at a high risk of developing T2DM.
In 1979, the National Diabetes Data Group (NDDG) described glucose intolerance as an intermediate metabolic state between normoglycaemia and diabetes (NDDG 1979). NDDG defined this IGT as an elevated plasma glucose concentration (7.8 mmol/L to 11.1 mmol/L or 140 mg/dL to 199 mg/dL) two hours after a 75 g glucose load on the oral glucose tolerance test (OGTT).
In 1997, the Expert Committe on the Diagnosis and Classification of Diabetes Mellitus and later the WHO defined two intermediate states of glucose regulation existing between regular glucose homeostasis and diabetes: IGT was diagnosed two hours after a 75 g OGTT by a plasma glucose level of 7.8 mmol/L to 11.1 mmol/L (140 mg/dL to 199 mg/dL) or by the concept of IFG (ADA 1997; WHO 1999). The initial definition of IFG was a fasting plasma glucose (FPG) level of 6.1 mmol/L to 6.9 mmol/L (110 mg/dL to 125 mg/dL). In 2003, the ADA reduced the lower threshold to 5.6 mmol/L (100 mg/dL) (ADA 2003). However, the WHO did not endorse this lower cut‐off point for IFG (WHO/IDF 2006).
More recently, an elevated HbA1c has been introduced to identify people at high risk of developing T2DM. In 2009, the International Expert Committee (IEC) proposed HbA1c measurements of 6.0% to 6.4% (42 mmol/mol to 46 mmol/mol) to identify people at a high risk of T2DM (IEC 2009). In 2010, the ADA re‐defined this HbA1c level as 5.7% to 6.4% (39 mmol/mol to 46 mmol/mol) (ADA 2010), a decision not endorsed by WHO, IEC or other organisations.
The various glycaemic tests do not identify the same people at risk, as there is an imperfect overlap among the glycaemic modalities available to define IH (Cheng 2006; Gosmanov 2014; Morris 2013; Selvin 2011). Unlike IFG and IGT, HbA1c reflects longer‐term glycaemic control, that is, how a person's blood glucose concentrations have been during the preceding two to three months (Inzucchi 2012). Compared with IFG and IGT measurements, HbA1c assessments have less intrapersonal variability when repeated. However, haemoglobin variants, genetic haemoglobinopathies, thalassemias and iron deficiency anaemia substantially influence HbA1c measurements (Mostafa 2011). The FPG thresholds of defining IFG and the question whether HbA1c is an adequate tool to diagnose IH are still a subject of debate (Buysschaert 2011; Buysschaert 2016). In studies investigating the risk of IH as measured by HbA1c, the association is probably underestimated if time‐dependent effects are not taken into account (Lind 2009). On the other hand, some investigators question whether HbA1c as such is the right outcome measure for studies of diabetes (Lipska 2017).
Also, IFG and IGT differ in their age and sex distribution, and both increase with advancing age (Nathan 2007), as glucose tolerance deteriorates with age (Gale 2013). 'Ethnicity' and geography are additional important features: the prevalence of elevated HbA1c in black people is twice as high as in non‐Hispanic white people, but the opposite is true for IGT (Selvin 2011; Ziemer 2010). The number of people with IH identified in South Asian compared with European cohorts and the associated cardiovascular disease (CVD) risk depend on how prediabetes is diagnosed (Eastwood 2016).
The increase in T2DM results from an interaction between genetic and environmental factors, reflecting behavioural changes over time such as decreased physical activity levels and increased body weight (DeFronzo 2011; Nathan 2007). Both IFG and IGT are insulin‐resistant states, and insulin resistance is thought to be the core defect in T2DM: people with (isolated) IFG predominantly have β‐cell dysfunction with impaired insulin secretion (DeFronzo 1989), plus moderate hepatic insulin resistance, but near‐normal muscle insulin sensitivity. The consequence is excessive fasting hepatic glucose production followed by elevated FPG. During an OGTT the early insulin response (0 to 30/60 min) is impaired, resulting in an excessive early rise in postload glucose (PG). The late insulin response (60 min to 120 min) appears intact and the two‐hour PG returns to its approximately starting FPG level (DeFronzo 2011; Nathan 2007). People with (isolated) IGT have normal to slightly reduced hepatic insulin sensitivity and moderate to severe muscle insulin resistance (Abdul‐Ghani 2006; Jensen 2002). During an OGTT both the early and the late insulin response are impaired. Hyperglycaemia is progressive and prolonged after the glucose load, and the two‐hour PG remains above its starting FPG level (DeFronzo 2011; Nathan 2007).
There are some known risk indicators for the development of T2DM, including a positive family history, gestational diabetes mellitus, obesity, 'ethnicity' (e.g. the risk of diabetes is thought to be higher among Asians, Hispanics, and 'black' people), polycystic ovarian syndrome, impaired insulin secretion and insulin resistance, abnormal coagulation factors and endothelial dysfunction. However, the evidence base for the weight of a single risk indicator and the interplay of various factors is still under investigation. Type 2 diabetes mellitus is a rather complex metabolic state and could be described as an asymptomatic risk factor for a future disease (Yudkin 2016), and hence prediabetes a risk factor for another risk factor (Nathan 2007).
Diabetes is a category, whereas IFG and IGT reflect a continuous variable with more or less arbitrarily chosen cut‐off points (Yudkin 1990; Yudkin 2014). The reduced lower threshold of 5.6 mmol/L (100 mg/dL) to define IFG by the ADA in 2003 substantially increased the prevalence of IFG with potentially significant public health and socioeconomic implications (Davidson 2003; Yudkin 2014; Yudkin 2016). Some authors have argued that substantial benefits might ensue even if it were only possible to delay the onset of diabetes by detecting and treating prediabetes (Cefalu 2016). Interestingly, some people with IH will not develop T2DM, and some people will return or 'regress' to normoglycaemia. In the Diabetes Prevention Program (DPP), the hazard ratio of developing T2DM was 0.44 (95% confidence interval 0.37 to 0.55) in people having at least one normal OGTT during the DPP compared with people who never regressed to normoglycaemia during the DPP (Perreault 2012; Perreault 2014). The ADA associated regression with remission and defined it as a partial or complete diabetes remission of glycaemic measurements for at least one year without pharmacological or surgical interventions (Buse 2009). This could have significant impact on "the therapeutic strategy from diabetes prevention and lifelong glucose‐lowering treatment to induction of regression and monitoring for relapse" (Yakubovich 2012).
Objectives
Objective 1: to assess the overall prognosis of people with IH for the development of T2DM and to assess how many people with IH revert back to normoglycaemia (regression).
With regard to objective 1 we established the following 'Population, Intervention, Outcome, Timing, Setting' (PICOTS) table (adapted according to the PICOTS system presented in Debray 2017).
| Item | Definition |
| Population | People with intermediate hyperglycaemia (defined by IFG, IGT or elevated HbA1c) |
| Intervention | None |
| Comparator | None |
| Outcome | Development of type 2 diabetes Regression to normoglycaemia |
| Timing | At least 1 year follow‐up |
| Setting | Outpatients |
| IFG: impaired fasting glucose; IGT: impaired glucose tolerance; HbA1c: glycosylated haemoglobin A1c | |
Objective 2: to assess the difference in T2DM incidence in people with IH versus people with normoglycaemia.
With regard to objective 2 we established the following PICOTS table (adapted according to the PICOTS system presented in Debray 2017).
| Item | Definition |
| Population | People with intermediate hyperglycaemia (defined by IFG, IGT or elevated HbA1c) |
| Intervention | Intermediate hyperglycaemia as a prognostic factor |
| Comparator | Normoglycaemia |
| Outcome | Development of type 2 diabetes |
| Timing | At least one year follow‐up |
| Setting | Outpatients |
| IFG: impaired fasting glucose; IGT: impaired glucose tolerance; HbA1c: glycosylated haemoglobin A1c | |
Methods
Criteria for considering studies for this review
Study design
Prospective cohort studies investigating either the overall prognosis of people with IH for developing T2DM or IH versus normoglycaemia as a prognostic factor for developing T2DM (Altman 2001).
Inclusion criteria
Types of participants
To study the overall prognosis of people with IH and regression from IH to normoglycaemia, we included cohort studies in people with IH at baseline, defined by impaired fasting glucose (IFG), impaired glucose tolerance (IGT), elevated glycosylated haemoglobin A1c (HbA1c) or any combination of these. IH had to be established by standard cut‐off values for IFG, IGT or elevated HbA1c, as defined by ADA or WHO (ADA 1997; ADA 2003; ADA 2010; ICH 1997; IEC 2009; WHO 1998; WHO/IDF 2006).
To study whether IH compared to normoglycaemia is a prognostic factor for developing T2DM, we included cohort studies in people with IH and normoglycaemia at baseline.
Definition of IH
We defined IH according to ADA and WHO descriptions.
IFG5.6 threshold, usually defined as a fasting plasma glucose level between 5.6 mmol/L and 6.9 mmol/L at baseline.
IFG6.1 threshold, usually defined as a fasting plasma glucose level between 6.1 mmol/L and 6.9 mmol/L at baseline.
IGT, usually defined as a plasma glucose level between 7.8 mmol/L and 11.1 mmol/L two hours after a 75 g OGTT at baseline.
Isolated IFG was defined as IFG5.6 or IFG6.1 only (without IGT), and isolated IGT was defined as IGT only (without IFG5.6 or IFG6.1).
HbA1c5.7 threshold, usually defined as HbA1c measurement between 5.7% and 6.4% at baseline.
HbA1c6.0 threshold, usually defined as HbA1c measurement between 6.0% and 6.4% at baseline.
Types of outcome measures
Our outcome of primary interest was the diagnosis of newly developed T2DM (T2DM incidence). T2DM incidence should have been diagnosed by blood glucose measurements such as fasting plasma glucose (FPG), two‐hour postload glucose (PG) or HbA1c. Diagnosis could have been combined with self‐reported diabetes, physician‐diagnosed diabetes or use of antihyperglycaemic medications such as oral hypoglycaemic drugs, insulin or both.
Exclusion criteria
Intervention trials and study designs other than prospective cohort studies.
People with comorbidities at baseline (e.g. people with coronary heart disease and IGT).
Missing data on transition from IH to T2DM.
Follow‐up period after baseline assessment not specified (not possible to associate T2DM incidence with length of follow‐up).
T2DM incidence evaluated by documents (e.g. hospital records, retrospective use of registers) or self‐report only.
Search methods for identification of studies
The fundamental challenge of this review question was to define the population of interest, that is, people with IH. We expected a great number of terms describing this population, such as people with prediabetes, mentions of IFG, IGT or HbA1c somewhere in the title or abstract of relevant publications, and terms like risk factors, predictors, prevalence, incidence and several other concepts which cannot be foreseen when developing a Boolean search strategy in a conceptual way.
One option to address this problem would have been to design a highly sensitive search strategy, which would have resulted in a yield of more than 15,000 references, which was unfeasible for fast human screening but could be addressed in the future with robust automated classification algorithms. Instead, we designed a more specific Boolean search approach based on text analysis and augmented by the following complementary search methods.
Identification of systematic reviews addressing our review question.
Careful checking of reference lists and Discussion sections of relevant studies.
A non‐human skill dependent search method based on PubMed's 'similar articles' algorithm.
Boolean search
We developed the search strategy using analytical text mining of 44 relevant publications (range of publication years 2008 to 2015, from 31 journals) already known to review author BR. We used the tools PubReMiner, TerMine and AntConc and applied the prognosis filters by the Hedges Team (Wilczynski 2004; Wilczynski 2005).
We searched the following sources from database inception to the specified date.
MEDLINE Ovid Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 15 December 2016 and then updated to 26 February 2018).
Embase Ovid (1974 to 2016 Week 50, last searched 15 December 2016).
ClinicalTrials.gov (searched 15 December 2016).
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch; searched 15 December 2016).
Before publication, we updated the MEDLINE search as reflected above. We restricted the update to MEDLINE because 98% of the publications of included studies identified up to the point of updating (on 26 February 2018) were indexed in MEDLINE.
The search strategy consisted of two tiers.
Prediabetes as predictor for cardiovascular disease (CVD), mortality, stroke, cancer, micro‐ and macrovascular complications.
Prediabetes as predictor for diabetes incidence.
We combined both strategies with the conjunction 'OR' because it was likely that search results for prediabetes as a predictor for complications also contained data on diabetes incidence. For details of all search strategies see Appendix 2.
Study extraction of relevant systematic reviews
In addition, we extracted relevant publications from 16 identified systematic reviews (Echouffo‐Tcheugui 2016; Erqou 2013; Ford 2010; Hope 2016; Huang 2014b; Huang 2014a; Huang 2016; Lee 2012; Morris 2013; Santos‐Oliveira 2011; Sarwar 2010; Schottker 2016; Twito 2015; Xu 2015; Zhang 2012a; Zhong 2016).
Reference checking of included studies
We extracted relevant publications after handsearching the full texts of included studies (Methods section, Discussion section, reference lists).
'Similar articles'‐based search method
On 15 March 2018 we ran PubMed's 'similar articles' algorithm with the 224 publications of included studies identified by our search methods so far ('seed publications' in Appendix 2). When using the 'similar articles' algorithm, search results in PubMed are retrieved and ranked according to pre‐calculated similarities of the seed publications. We downloaded the first 500 results (of 24,124), deduplicated them against the already identified seed publications and screened the resulting set.
Selection of studies
Two review authors (BR and BH) independently scanned the title, abstract, or both, of every record retrieved in the literature searches to determine which studies to assess further. We investigated the full text of all potentially relevant articles, resolving discrepancies through consensus or by recourse to a third review author (MIM). We prepared a flow diagram of the number of studies identified and excluded at each stage in accordance with the PRISMA flow diagram of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled our inclusion criteria, one review author (BR) extracted key study characteristics, inclusion and exclusion criteria of study participants, stated aim of the study, definitions of prognosis, prognostic factor and outcome (normoglycaemia, intermediate glycaemia and T2DM incidence), baseline characteristics of study participants and data on transition from IH (as defined by IFG, IGT, elevated HbA1c or combinations thereof) to T2DM. Another author (MIM) checked these data extractions, and we resolved any disagreements by discussion or, if required, by consultation with a third review author (BH). We used parts of the checklist for critical appraisal and data extraction for systematic reviews of prediction modelling studies (CHARMS), which helps to evaluate prediction modelling studies (Moons 2014), and we established our own context‐specific data extraction sheets after piloting data extraction for 15 studies.
Dealing with companion publications
In the event of companion publications or multiple reports of a prospective cohort study (e.g. because of different time points investigated) we focused on the analysis of the publication describing the longest follow‐up from baseline and extracted data from shorter follow‐ups in case some measures were not reported in the publication on the longest follow‐up (e.g. the most recent paper might have described the association between elevated HbA1c and T2DM incidence, but an older publication might have described the association between IGT and T2DM incidence). Companion publications or multiple reports of a primary study were listed as secondary references under the primary reference of the included, ongoing or excluded study.
Assessment of risk of bias in included studies
One review author (BR) assessed the risk of bias of each included study and another review author (MIM) checked the accuracy of this assessment. We resolved any disagreements by consensus, or by consultation with a third review author (BH). We used a tailored version of the Quality In Prognosis Studies (QUIPS) tool for assessing risk of bias in studies of the prognostic factor IH versus normoglycaemia (Dretzke 2014; Hayden 2013; see Appendix 3). Our tool consisted of six risk of bias domains: study participation, study attrition, glycaemic status measurement, outcome measurement, study confounding; and statistical analysis and reporting. The study participation domain consisted of five items: description of the source population or population of interest, description of the baseline study sample, adequate description of the sampling frame and recruitment, adequate description of the period and place of recruitment, and adequate description of inclusion and exclusion criteria. The study attrition domain consisted of four items: description of attempts to collect information on participants who dropped out, reasons for loss to follow‐up provided, adequate description of participants lost to follow‐up, and no important differences between participants who completed the study and those who did not. The glycaemic status measurement domain consisted of four items: provision of clear definition or description of the glycaemic status, adequately valid and reliable method of measuring glycaemic status, reporting of continuous variables or use of appropriate cut points, and use of same method and setting of measurement of glycaemic status in all study participants. The outcome measurement domain consisted of three items: provision of clear definition of the outcome, use of adequately valid and reliable method of outcome measurement, and use of same method and setting of outcome measurement in all study participants. The study confounding domain consisted of the seven items: measurement of all important confounders, provision of clear definitions of the important confounders measured, adequately valid and reliable measurement of all important confounders, use of same method and setting of confounding measurement in all study participants, appropriate imputation methods used for missing confounders (if applicable), important potential confounders accounted for in the study design, and important potential confounders accounted for in the analysis. The statistical analysis and reporting domain consisted of two items: sufficient presentation of data to assess the adequacy of the analytic strategy, and adequate statistical model for the design of the study. There is no recommended tool for assessing risk of bias in studies of overall prognosis. Therefore, we applied the tailored QUIPS tool to these studies as well but without the domains for study confounding and statistical analysis and reporting because these were not suitable to basic calculations of cumulative incidence. We planned to investigate the influence of low risk of bias (low risk of bias in all domains) versus unclear/high risk of bias (unclear or high risk of bias in at least one of these domains).
Measures of T2DM incidence and unit of analyses issues
If more than one group from the same cohort study was eligible for inclusion in the same meta‐analysis, we included the groups only if separate information was available (e.g. data on T2DM incidence for female and male participants). If more than one time point of T2DM was available for a study (e.g. cumulative incidence data) we included data in the appropriate meta‐analysis for each time point separately and did not pool data across different follow‐up periods.
Data synthesis
Our primary aim for overall prognosis in people with IH was to provide a transparent overview of the whole data matrix describing a wide variety of possible associations between various isolated and combined definitions of IH and incident T2DM in dissimilar populations covering diverse time periods. We also evaluated whether IH compared to normoglycaemia is a prognostic factor for developing T2DM.
First, we grouped studies on IH definitions, i.e. isolated IFG 5.6 mmol/L to 6.9 mmol/L (IFG5.6 threshold), isolated IFG 6.1 mmol/L to 6.9 mmol/L (IFG6.1 threshold), isolated IGT (glucose concentration 7.8 mmol/L to 11.1 mmol/L two hours after a 75 g glucose load on the OGTT), IFG and IGT combined, HbA1c 6.0% to 6.4% (HbA1c6.0 threshold), and HbA1c 5.7% to 6.4% (HbA1c5.7 threshold). Then we evaluated subgroups of different geographic locations/'ethnicities' for each IH definition.
We expected the following outcome measures.
Cases (cumulative incidence at follow‐up; e.g. 20 new diabetes cases out of 400 people with IFG at baseline (5%)) and cumulative incidence rates (cases per 1000 person‐years) for overall prognosis of people with IH.
Odds ratios (ORs), incidence rate ratios (IRRs), and hazard ratios (HRs) for IH versus normoglycaemia as a prognostic factor for developing T2DM.
We pooled incidence and incidence rate ratios (IRR) using a random‐effects model to account for between‐study heterogeneity. For meta‐analysis of incidence data, we used a method for pooling proportions which uses the Freeman‐Tukey Double Arcsine Transformation to stabilise the variances (Freeman 1950). The meta‐analysis was performed using the Stata software user written programme metaprop (Stata 2015). For the confidence intervals (CI) for individual studies shown on the forest plots for incidence, we used the Wilson approach (Newcombe 1998). For meta‐analysis of IRRs, we first computed the log IRRs and their approximate standard errors and then used an inverse variance weighted random‐effects model to pool the log IRRs (Hasselblad 1994; Higgins 2011b). We exponentiated the pooled log IRR to obtain the pooled IRR. The meta‐analysis of log IRRs was performed using the Stata user written programme metan.
If publications reported HRs with associated 95% CIs, we obtained standard errors from these CIs as described in chapter 7.7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and we performed meta‐analysis using the generic inverse‐variance method (RevMan 2014). When possible, we reported both adjusted and unadjusted HRs, but we primarily used adjusted HRs from multivariable models of studies incorporating similar covariates (Dretzke 2014).
Assessment of heterogeneity
We expected substantial clinical heterogeneity between studies because of geographical/'ethnic' and methodological diversity. We did not intend to address statistical heterogeneity (inconsistency) using the I2 statistic because this statistic does not indicate how much the effect size varies, which is what people want to know when asking about the implications of heterogeneity (Borenstein 2017a). Also, the I2 statistic is problematic in the context of prognosis studies because individual studies often have large sample sizes resulting in narrow CIs, which can result in high I2 values even if inconsistency between studies is moderate (Iorio 2015). Instead, when there were at least three studies, we reported the range of the effects of the random‐effects meta‐analyses using prediction intervals (Borenstein 2017b; Higgins 2009; IntHout 2016; Riley 2011; Riley 2015). In a random‐effects meta‐analysis, the prediction interval reflects the whole distribution of effects across study populations, including the effect expected in a future study (IntHout 2016; Riley 2015).
Certainty of the evidence
We created a 'Summary of findings' table using Review Manager 5 (RevMan 2014). We used an adapted version of the GRADE framework for prognostic factor research for describing the influence of IFG, IGT, elevated HbA1c and both IFG and IGT on the development of T2DM (Huguet 2013). We justified all decisions to downgrade the certainty of evidence using footnotes, and we made comments to aid the reader's understanding of this Cochrane Review where necessary.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by excluding:
studies at high or unclear risk of bias;
very long or large studies to establish the extent to which they dominate the results.
Subgroup analysis
Because we stratified the analyses by IH definition and geographical locations/'ethnicity', which we thought were the main sources of heterogeneity, we did not plan to perform subgroup analyses. However, if at least 10 studies specifying diabetes incidence data were included, we would have investigated age and sex by testing for interactions between subgroups.
If T2DM incidence data were available for children and adolescents, we reported the results separately.
Results
Description of studies
Results of the search
We identified a total of 8354 records through database searching and an additional 259 records from 16 systematic reviews. After excluding duplicates and non‐relevant records based on title and abstract screening, we assessed 450 full‐text records. Of these we excluded 213 full‐text articles; the remaining 237 articles were reports of 110 studies. Of the 110 studies, 4 were potentially relevant ongoing trials (NCT00786890; NCT02838693; NCT02958579; Vilanova 2017), and 3 are awaiting classification (Li 2001; Misnikova 2011; NCT00816608). Therefore, we included 103 studies. We added 86 new publications after handsearching the full texts of included studies, but these were all secondary publications of the included studies.
The complementary 'similar articles' algorithm search using our set of known publications yielded 263 publications for screening after deduplication. This resulted in 24 new publications after excluding irrelevant articles based on title and abstract screening. We did not identify new studies but found 13 secondary publications of studies we had already included.
Altogether, we included 103 prospective cohort studies (329 publications) in the review. After the initial search in four databases (in December 2016), we observed that 98% of all included publications were indexed in Ovid MEDLINE. Therefore, we decided to restrict the pre‐publication update search in February 2018 to Ovid MEDLINE.
For full details of search results see Figure 1.
1.
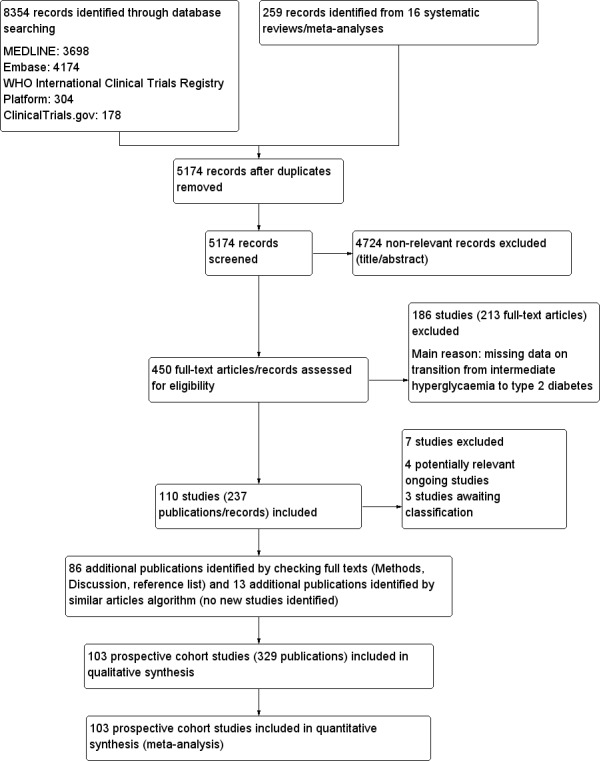
Study flow diagram
Included studies
For a detailed description of the characteristics of the included studies, see Characteristics of included studies; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16; and Appendix 17. The following is a succinct overview.
Source of data
The 103 studies took place in the following regions of the world.
Australia: 3 studies.
Latin America: 7 studies (Chile, 1 study; Columbia, 1 study; Mexico, 5 studies (2 studies with primarily Mexican Americans took place in the USA (Garcia 2016; Lorenzo 2003)).
North America: 12 studies (USA ,12 studies, with 4 studies in particular populations: Pima Indians/Native Americans, 3 studies (Vijayakumar 2017; Wang 2011; Wheelock 2016); and Japanese Americans, 1 study (McNeely 2003)).
Africa: 1 study (performed in South Africa but with a population consisting of South African Indians (Motala 2003)).
Middle East: 7 studies (Iran, 5 studies; Israel, 1 study; Jordan, 1 study).
Asia: 42 studies (China, 11 studies; India, 5 studies; Japan, 8 studies; Korea, 11 studies; Singapore, 2 studies; Taiwan, 2 studies; Thailand, 3 studies).
Islands: 2 studies (Mauritius, 1 study; Micronesia (Nauru), 1 study).
Europe: 29 studies (Denmark, 1 study; Finland, 5 studies; France, 3 studies; Germany, 3 studies; Greece, 1 study; Italy, 3 studies; Malta, 1 study; Spain, 3 studies; Sweden, 3 studies; Netherlands, 4 studies; UK, 2 studies). One study in the Netherlands included a mixed population of South‐Asian Surinamese participants, African Surinamese participants and "Ethnic Dutch" participants (Admiraal 2014).
Fifty‐eight studies contributed most of the data (Appendix 4).
Measurements of overall prognosis of people with IH and of the prognostic factor IH versus normoglycaemia
Of the 103 included studies, 17 evaluated the overall prognosis of people with IH for the development of type 2 diabetes mellitus without a normoglycaemic comparison group. Of these studies, six recruited participants with IFG at baseline (Baena‐Diez 2011; Gautier 2010; Lecomte 2007; Leiva 2014; Levitzky 2008; Sharifi 2013), six recruited participants with IGT at baseline (Kleber 2010; Kleber 2011; Ko 1999; Marshall 1994; Rajala 2000; Ramachandran 1986), two recruited a mixed IFG/IGT cohort (Rasmussen 2008; Toshihiro 2008), and three recruited participants with various definitions of IH (Kim 2014; Lee 2016; Song 2016a). In addition, 76 studies with a normoglycaemic comparison group contributed data to evaluate the overall prognosis of people with IH by means of cumulative incidence. Therefore, analysis of overall prognosis is based on 93 studies.
Fifty‐two studies assessed the prognostic effect of IH versus normoglycaemia for the development of type 2 diabetes mellitus and provided outcome measures as ratios (hazard ratio (HR), incidence rate ratio (IRR) and/or odds ratio (OR)). Forty‐seven studies explicitly defined normoglycaemia, often by a combination of FPG thresholds and two hour post‐load glucose thresholds (Anjana 2015; Baena‐Diez 2011; Bergman 2016; Chen 2003; Chen 2017; Coronado‐Malagon 2009; Den Biggelaar 2016; Derakhshan 2016; Dowse 1991; Forouhi 2007; Guerrero‐Romero 2006; Heianza 2012; Janghorbani 2015; Jaruratanasirikul 2016; Kim 2005; Ko 1999; Ko 2001; Larsson 2000; Lecomte 2007; Leiva 2014; Li 2003; Ligthart 2016; Lipska 2013; Liu 2014; Liu 2017; Lyssenko 2005; Magliano 2008; Man 2017; Meigs 2003; Motala 2003; Motta 2010; Mykkänen 1993; Nakanishi 2004; Peterson 2017; Qian 2012; Rajala 2000; Rathmann 2009; Rijkelijkhuizen 2007; Sasaki 1982; Soriguer 2008; Toshihiro 2008; Vaccaro 1999; Valdes 2008; Viswanathan 2007; Wang 2011; Wat 2001; Weiss 2005; Yeboah 2011). In the remaining studies, it was evident that normoglycaemia reflected the population with neither IH nor T2DM at baseline.
IH was commonly defined by the IFG5.6 threshold (FPG level 5.6 mmol/L to 6.9 mmol/L or 100 mg/dL to 125 mg/dL), IFG6.1threshold (FPG level 6.1 mmol/L to 6.9 mmol/L or 110 mg/dL to 125 mg/dL), IGT (plasma glucose concentration 7.8 mmol/L to 11.1 mmol/L or 140 mg/dL to 199 mg/dL two hours after a 75 g glucose load on the OGTT), or combinations of these criteria (Appendix 5; Appendix 6). Sixty‐six studies used an OGTT at baseline as part of the strategy to assess glycaemic status, and 46 studies used OGTT at baseline and follow‐up (Appendix 5).
Twelve studies defined IH by applying the HbA1c5.7 threshold (HbA1c 5.7% to 6.4% or 39 mmol/mol to 46 mmol/mol) (Bae 2011; Cederberg 2010; Han 2017; Heianza 2012; Kim 2014; Kim 2016a; Lee 2016; Lipska 2013; Man 2017; Nakagami 2016; Vijayakumar 2017; Warren 2017), and 10 studies used the HbA1c6.0 threshold (HbA1c 6.0% to 6.4% or 42 mmol/mol to 46 mmol/mol) (Bae 2011; Bonora 2011; Chamnan 2011; Han 2017; Heianza 2012; Kim 2016a; Nakagami 2016; Sato 2009; Wang 2011; Warren 2017).
Overview of study populations
Sixty‐nine studies (67%) started recruitment after 1990 (see Characteristics of included studies), and overall follow‐up ranged from 1 year in Bai 1999,Coronado‐Malagon 2009 and Kleber 2010 to 24 years in Bergman 2016 (see Characteristics of included studies; Appendix 7).
Depending on the phase of the study, the number of participants differed. The first phase of every study often constituted a large epidemiological investigation of, for example, the importance of various risk factors for cardiovascular health; in total, more than 250,000 participants began the studies (Appendix 8). The number of participants with IH depended on how the studies defined this condition at baseline and the way they measured the development of T2DM.
The overall prognosis of participants with IH at baseline and across all follow‐up times (1 to 20 years) was based on the following data (Table 8).
1. Overview: overall prognosis of people with intermediate hyperglycaemia and regression from intermediate hyperglycaemia to normoglycaemia.
| Follow‐up time (years) | % (95% CI) cumulative T2DM incidence [no of studies; no of participants with IH] | % (95% CI) regression from IH to normoglycaemia [no of studies; no of participants with IH] | ||||||
| IFG5.6 | IFG6.1 | IGT | IFG + IGT | HbA1c5.7 | HbA1c6.0 | |||
| 1 | — | — |
13 (5–23) [3; 671] |
29 (23–36) [1; 207] |
— | — |
59 (54–64) [2; 375] |
|
| 2 |
2 (1–2) [1; 1335] |
11 (8–14) [2; 549] |
16 (9–26) [9; 1998] |
— | — | — |
46 (36–55) [9; 2852] |
|
| 3 |
17 (6–32) [3; 1091] |
9 (2–20) [3; 927] |
22 (18–27) [3; 417] |
34 (28–41) [1; 209] |
— |
7 (5–10) [1; 370] |
41 (24–59) [7; 1356] |
|
| 4 |
17 (13–22) [3; 800] |
30 (17–44) [2; 1567] |
22 (12–34) [5; 1042] |
— |
14 (7–23) [3; 5352] |
44 (40–48) [2; 627] |
33 (26–40) [3; 807] |
|
| 5 |
18 (10–27) [7; 3530] |
26 (19–33) [11; 3837] |
39 (25–53) [12; 3444] |
50 (37–63) [5; 478] |
25 (18–32) [4; 3524] |
38 (26–51) [3; 1462] |
34 (27–42) [9; 2603] |
|
| 6 |
22 (15–31) [4; 738] |
37 (31–43) [5; 279] |
29 (25–34) [7; 775] |
58 (48–67) [4; 106] |
17 (14–20) [1; 675] |
— |
23 (3–53) [5; 1328] |
|
| 7 |
18 (8–30) [5; 980] |
15 (0–45) [4; 434] |
19 (13–26) [5; 835] |
32 (20–45) [4; 753] |
21 (16–27) [1; 207] |
— |
41 (37–45) [4; 679] |
|
| 8 |
34 (27–40) [2; 1887] |
48 (31–66) [1;29] |
43 (37–49) [4; 1021] |
52 (47–57) [1; 356] |
— | — |
39 (33–44) [2; 328] |
|
| 9 |
38 (10–70) [3; 1356] |
— |
53 (45–60) [1; 163] |
84 (74–91) [1; 69] |
— | — |
17 (14–22) [1; 299] |
|
| 10 |
23 (14–33) [6; 1542] |
29 (17–43) [6; 537] |
26 (17–37) [6; 443] |
30 (17–44) [2; 49] |
31 (29–33) [2; 2854] |
— |
42 (22–63) [7; 894] |
|
| 11 | — |
38 (33–43) [1; 402] |
46 (43–49) [1; 1253] |
— | — | — |
28 (17–39) [2; 736] |
|
| 12 |
31 (19–34) [3; 433] |
31 (28–33) [1; 1382] |
41 (38–43) [2; 1552] |
70 (63–76) [2; 207] |
— | — | — | |
| 15 | — | — | — | — | — |
29 (19–40) [1; 70] |
— | |
| 20 | — | — |
60 (5–68) [1; 114] |
— | — | — | — | |
CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0 (threshold 5.7% or 6.0%); IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG + IGT: both IFG and IGT; IH: intermediate hyperglycaemia; T2DM: type 2 diabetes mellitus
IFG5.6: 13,692 participants.
IFG6.1: 9943 participants.
IGT: 13,728 participants.
Both IFG and IGT: 2434 participants.
HbA1c5.7: 9758 participants.
HbA1c6.0: 2529 participants.
Follow‐up time across all measures of IH at baseline had the following number of participants per year of follow‐up (in parentheses, number of people with IH who regressed to normoglycaemia); see Table 8.
1 year: 878 (375) participants.
2 years: 3882 (2852) participants.
3 years: 3014 (1356) participants.
4 years: 9388 (807) participants.
5 years: 16,275 (2603) participants.
6 years: 2573 (1328) participants.
7 years: 3209 (679) participants.
8 years: 3293 (328) participants.
9 years: 1588 (299) participants.
10 years: 5425 (894) participants.
11 years: 1655 (736) participants.
12 years: 3574 (no data) participants.
15 years: 70 (no data) participants.
20 years: 114 (no data) participants.
Data on the prognostic factor IH versus normoglycaemia for the development of T2DM were based on the following number of participants with IH at baseline (Table 9). Data were reported by ratio measures (HR, IRR, OR).
2. Overview: intermediate hyperglycaemia versus normoglycaemia as a prognostic factor for the development of type 2 diabetes.
|
Ratio (95% CI)
95% prediction intervala,b [no of studies; no of participants with IH/no of participants with normoglycaemia] | |||||||
| Hazard ratio | |||||||
| Region | IFG5.6 cohort | IFG6.1 cohort | IGT cohort | IFG + IGT cohort | HbA1c5.7 cohort | HbA1c6.0 cohort | HbA1c5.7 + IFG5.6 cohort |
| Asia/Middle East |
5.07 (3.41‐7.53) 1.07–24.02 [4; 2385/12,837] |
10.55 (3.61–30.81) NAb [5; 1054/9756] |
4.48 (2.81–7.15) NAb [3; 1780/6695] |
10.20 (5.45–19.09) NAb [3; 461/6695] |
7.21 (5.14–10.11) 0.81–64.52 [3; 3196/13,609] |
13.12 (4.10–41.96) NAb [4; 3492/19,242] |
32.50 (23.00–45.92)c NAa [1; 410/4149] |
| Australia/Europe/North America |
4.15 (1.24–13.87) NAb [3; 5685/12,837] |
3.30 (2.32–4.67) 0.84–12.99 [4; 1736/8835] |
2.53 (1.52–4.19) NAa [2; 2230/5871] |
3.80 (2.30–6.28) NAa [1; 221/1429] |
2.71 (2.48–2.96) NAa [1: 2027/6215] |
5.09 (1.69–15.37) NAa [2; 1040/6925] |
— |
| Latin America | — |
2.06 (1.76–2.41) NAb [1; 28/66] |
— | — | — | — | — |
| American Indians/Islands |
2.38 (1.85–3.06) NAa [1; 947/595] |
— | — |
4.06 (3.05–5.40) NAa [1; 356/595] |
— | — | — |
| Overall |
4.32 (2.61–7.12) 0.75–25.01 [8; 9017/25,850] |
5.47 (3.50–8.54) 1.09–27.56 [9; 2818/18,591] |
3.61 (2.31–5.64) 0.69–18.97 [5; 4010/12,566] |
6.90 (4.15–11.45) 1.06–44.95 [5; 1038/8719] |
5.55 (2.77–11.12) 0.23–141.18 [4; 5223/19,824] |
10.10 (3.59–28.43) NAb [6; 4532/26,167] |
32.50 (23.00–45.92) NAa [1; 410/4149] |
| Incidence rate ratio | |||||||
| Region | IFG5.6 cohort | IFG6.1 cohort | IGT cohort | IFG + IGT cohort | HbA1c5.7 cohort | HbA1c6.0 cohort | HbA1c5.7 + IFG5.6 cohort |
| Asia/Middle East |
5.23 (3.77–7.25) 1.72–15.89 [6; 15,661/145,597] |
3.62 (1.67–7.83) NAa [2; 1677/36,334] |
3.93 (3.03–5.10) 1.71–9.02 [5; 14,809/73,128] |
11.20 (5.59–22.43) NAb [4; 3166/69,463] |
6.62 (4.18–10.49) NAa [1; 1965/19961] |
— |
40.72 (29.30–56.61) NAa [1; 1641/19,961] |
| Australia/Europe/North America |
4.96 (3.25–7.57) 0.32–77.24 [3; 6322/8062] |
8.55 (6.37–11.48) 4.37–16.73 [4; 3438/20,246] |
5.93 (4.11–8.57) 2.38–14.81 [5; 2572/22,329] |
13.92 (9.99–19.40) 6.71–28.85 [4; 699/18,966] |
— | — | — |
| Latin America | — | — | — | — | — | — | — |
| American Indians/Islands |
2.74 (1.88–3.99) NAa [1; 2374/1613] |
— |
4.46 (3.12–6.38) NAa [2; 1087/2952] |
5.18 (3.42–7.83) NAa [1; 605/1613] |
— | — | — |
| Overall |
4.81 (3.67–6.30) 1.95–11.83 [10; 24,357/155,272] |
6.82 (4.53–10.25) 2.03–22.87 [6; 5115/56,580] |
4.48 (3.69–5.44) 2.60–7.70 [12; 18,468/98,409] |
10.94 (7.22–16.58) 2.58–46.46 [9; 4470/90,072] |
6.62 (4.18–10.5) NAa [1; 1965/19961] |
— |
40.72 (29.30–56.61) NAa [1; 1641/19,961] |
| Odds ratio | |||||||
| IFG5.6 cohort | IFG6.1 cohort | IGT cohort | IFG + IGT cohort | HbA1c5.7 cohort | HbA1c6.0 cohort | HbA1c5.7 + IFG5.6 cohort | |
| Asia/Middle East |
2.94 (1.77–4.86) 0.43–19.93 [10; 6359/28,218] |
5.18 (2.32–11.53) 0.29–91.37 [7; 3317/25,604] |
3.74 (2.83–4.94) 1.70–8.21 [6; 1226/7417] |
6.99 (3.09–15.83) NAb [3; 498/3704] |
4.54 (2.65–7.78) NAa [1; 675/462] |
23.20 (18.70–28.78) NAa [1; 1103/10,763] |
46.70 (33.60–64.91) NAa [1; 1951/10,761] |
| Australia/Europe/North America |
6.47 (3.81–11.00) 0.99–42.32 [9; 1949/7920] |
8.69 (4.95–15.24) 1.20–62.69 [7; 1240/5094] |
5.20 (3.62–7.45) 1.50–18.09 [11; 1481/7684] |
20.95 (12.40–35.40) 4.93–89.05 [6; 154/5300] |
4.38 (1.36–14.15) NAa [2; 231/2100] |
15.60 (6.90–35.27) NAa [1; 370/5365] |
26.20 (16.30–41.11) NAa [1; 169/1125] |
| Latin America |
4.28 (3.21–5.71) NAa [1; 65/1594] |
3.73 (2.18–6.38) NAa [1; 17/1594] |
4.94 (3.15–7.76) NAa [2; 381/3097] |
— | — | — | — |
| American Indians/Islands |
3.12 (2.31–4.21) NAa [1; 947/595] |
— |
3.60 (1.40–9.26) NAa [1; 51/215] |
— | — |
5.89 (4.23–8.20) NAa [1; 121/595] |
— |
| Overall |
4.15 (2.75–6.28) 0.54–32.00 [21; 9320/38,327] |
6.60 (4.18–10.43) 0.93–46.82 [15; 4574/32,292] |
4.61 (3.76–5.64) 2.10–10.13 [20; 3139/18,413] |
13.14 (7.41–23.30) 1.84–93.66 [9; 652/9004] |
4.43 (2.20–8.88) NAb [3; 906/2562] |
12.8 [4.56–35.9] NAb [3; 1594/16,723] |
35.91 (20.43–63.12) NAa [2; 2120/11,886] |
CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0 (threshold 5.7% or 6.0%); HbA1c5.7 + IFG5.6: both HbA1c5.7 and IFG5.6; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG + IGT: both IFG and IGT; IH: intermediate hyperglycaemia; NA: not applicable; T2DM: type 2 diabetes mellitus; NR: not reported aWith fewer than 3 studies a prediction interval could not be calculated bCalculation of the 95% prediction interval did not provide a meaningful estimate cCombination of HbA1c6.0 plus IFG5.6 at baseline showed a hazard ratio for T2DM development of 53.7 (95% CI 38.4–75.1)
IFG5.6: 42,694 participants.
IFG6.1: 12,507 participants.
IGT: 25,617 participants.
Both IFG and IGT: 6160 participants.
HbA1c5.7: 8094 participants.
HbA1c6.0: 6126 participants.
Both HbA1c5.7 and IFG5.6: 3761 participants.
The mean age of adult participants at baseline ranged from 30 years to 77 years (Appendix 9). In two studies all the participants were female (De Abreu 2015; Larsson 2000), and in eight studies all the participants were male (Charles 1997; Lecomte 2007; Nakanishi 2004; Park 2006; Sato 2009; Stengard 1992; Toshihiro 2008; Zethelius 2004). The body mass index (BMI) of the participants at baseline ranged from 23.2 kg/m2 to 39.1 kg/m2. A family history of diabetes was reported in 3% to 100% of the study participants.
At baseline, 60 studies (58%) reported diastolic and systolic blood pressure; 43 studies (22%), smoking status; 66 studies (64%), FPG; 24 studies (23%), HbA1c; 44 studies (43%), two‐hour glucose measurements; 7 studies (7%), medications; 26 studies (25%), comorbidities; 20 studies (19%), hypertension; and 5 studies (5%), dyslipidaemia (Appendix 10).
Categorisation of studies
In order to address the complexity of our dataset with regard to factors potentially influencing the definition, detection and development of T2DM, such as genetics, environmental and social conditions, the way risk factors and T2DM incidence were measured, and access to health care (Avilés‐Santa 2016; De Rekeneire 2007; Herman 2012; Likhari 2010; Maruthur 2011; Parrinello 2016) – with all of these features interacting to some degree – we choose to provide the reader with a broad overview mainly focusing on geographic regions in the following way.
Groups consisted of participants from studies taking place in Australia, Europe or North America; people from Latin America; individuals from Asia or the Middle East; and American (Pima) Indians and Pacific/Indian Ocean islanders ('American Indians/Islands' group). The logic of grouping participants in the last cohort together resided in the fact that they shared some characteristics relevant to T2DM, including a considerable genetic background risk, historic isolation from outside communities with substantial influence from Western diets, or both (Hanson 2014; Jowett 2009; Nair 2015; Serjeantson 1983).
For 41 studies, we categorised the origin of participants as 'Australia/Europe/North America' (Admiraal 2014; Baena‐Diez 2011; Bonora 2011; Cederberg 2010; Chamnan 2011; Charles 1997; Cugati 2007; De Abreu 2015; Den Biggelaar 2016; Filippatos 2016; Forouhi 2007; Gautier 2010; Hanley 2005; Kleber 2010; Kleber 2011; Larsson 2000; Lecomte 2007; Levitzky 2008; Ligthart 2016; Lipska 2013; Lyssenko 2005; Magliano 2008; Marshall 1994; McNeely 2003; Meigs 2003; Motta 2010; Mykkänen 1993; Peterson 2017; Rajala 2000; Rasmussen 2008; Rathmann 2009; Rijkelijkhuizen 2007; Schranz 1989; Soriguer 2008; Stengard 1992; Vaccaro 1999; Valdes 2008; Warren 2017; Weiss 2005; Yeboah 2011; Zethelius 2004).
For seven studies, we categorised the origin of participants as 'Latin America' (Coronado‐Malagon 2009; Ferrannini 2009; Garcia 2016; Gomez‐Arbelaez 2015; Guerrero‐Romero 2006; Leiva 2014; Lorenzo 2003). Although Garcia 2016 and Lorenzo 2003 took place in the USA, they included primarily Mexican Americans, hence the rationale for this categorisation.
We categorised 50 studies as 'Asia/Middle East' (Aekplakorn 2006; Ammari 1998; Anjana 2015; Bae 2011; Bai 1999; Bergman 2016; Chen 2003; Chen 2017; Derakhshan 2016; Han 2017; Heianza 2012; Inoue 1996; Janghorbani 2015; Jaruratanasirikul 2016; Jeong 2010; Jiamjarasrangsi 2008a; Kim 2005; Kim 2008; Kim 2014; Kim 2016a; Ko 1999; Ko 2001; Latifi 2016; Lee 2016; Li 2003; Liu 2008; Liu 2014; Liu 2016; Liu 2017; Man 2017; Mohan 2008; Motala 2003; Nakagami 2016; Nakanishi 2004; Noda 2010; Park 2006; Qian 2012; Ramachandran 1986; Sadeghi 2015; Sasaki 1982; Sato 2009; Sharifi 2013; Shin 1997; Song 2015; Song 2016a; Toshihiro 2008; Viswanathan 2007; Wang 2007; Wat 2001; Wong 2003). Of these, 37 studies recruited participants from China, Japan, South Korea, Singapore, Taiwan and Thailand (Aekplakorn 2006; Bae 2011; Chen 2003; Chen 2017; Han 2017; Heianza 2012; Inoue 1996; Jaruratanasirikul 2016; Jeong 2010; Jiamjarasrangsi 2008a; Kim 2005; Kim 2008; Kim 2014; Kim 2016a; Ko 1999; Ko 2001; Lee 2016; Li 2003; Liu 2008; Liu 2014; Liu 2016; Liu 2017; Man 2017; Nakagami 2016; Nakanishi 2004; Noda 2010; Park 2006; Qian 2012; Sasaki 1982; Sato 2009; Shin 1997; Song 2015; Song 2016a; Toshihiro 2008; Wang 2007; Wat 2001; Wong 2003), 5 studies recruited participants from India (Anjana 2015; Bai 1999; Mohan 2008; Ramachandran 1986; Viswanathan 2007), 1 study involved Indian‐South African participants (Motala 2003), and 7 studies recruited participants from Iran, Israel and Jordan (Ammari 1998; Bergman 2016; Derakhshan 2016; Janghorbani 2015; Latifi 2016; Sadeghi 2015; Sharifi 2013).
We categorised the origin of participants as 'American Indians/Islands' in five studies. Three of the five studies had American Indians as participants (Vijayakumar 2017; Wang 2011; Wheelock 2016), one included Mauritians (Söderberg 2004), and the remaining study included Nauruans (Dowse 1991).
Six studies included black participants (Admiraal 2014; Bergman 2016; Hanley 2005; Söderberg 2004; Warren 2017; Yeboah 2011), representing 25% to 47% of all participants in these studies.
Six studies included children, adolescents or both as participants (Jaruratanasirikul 2016; Kleber 2010; Kleber 2011; Vijayakumar 2017; Weiss 2005; Wheelock 2016).
Measurement of the development of T2DM
Almost all studies combined criteria to define incident T2DM, using indicators such as FPG of 7.0 mmol/L or more, two‐hour postload glucose level of 11.1 mmol/L or more, HbA1c of 6.5% or more, receipt of antidiabetic medication, physician diagnosis or self‐report.
Of the 103 included studies, 64 included FPG of 7.0 mmol/L or more, and 52, two‐hour postload glucose level of 11.1 mmol/L or more, in their definition of incident T2DM. Eighteen studies used HbA1c as part of the definition of T2DM, typically an HbA1c level of 6.5% or more. One study defined T2DM incidence based only on an HbA1c level of 6.5% or more (Lee 2016). In 34 studies, antidiabetic treatment comprised part of the definition of T2DM, and in 15 studies physician diagnosis or self‐report was part of the T2DM incidence definition.
Risk of bias in included studies
For details on the QUIPS tool and the risk of bias of the included studies see Appendix 3 and Characteristics of included studies. The results are summarised below separately for studies that provided data on overall prognosis for people with IH and on IH versus normoglycaemia as a prognostic factor.
a) Overall prognosis of people with IH for the development of T2DM and b) regression from IH to normoglycaemia
There were 93 studies providing data on cumulative incidence. Figure 2 summarises the risk of bias results across all studies, while the results for each study are shown in Figure 3 and Figure 4 (split into two figures because of the large number of studies). We evaluated the first four risk of bias domains (i.e. study participation, study attrition, glycaemic status measurement, outcome measurement) of the QUIPS tool.
2.
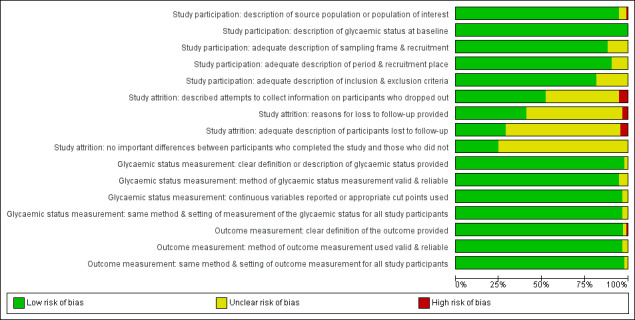
Risk of bias graph for studies of overall prognosis of people with intermediate hyperglycaemia for developing type 2 diabetes: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
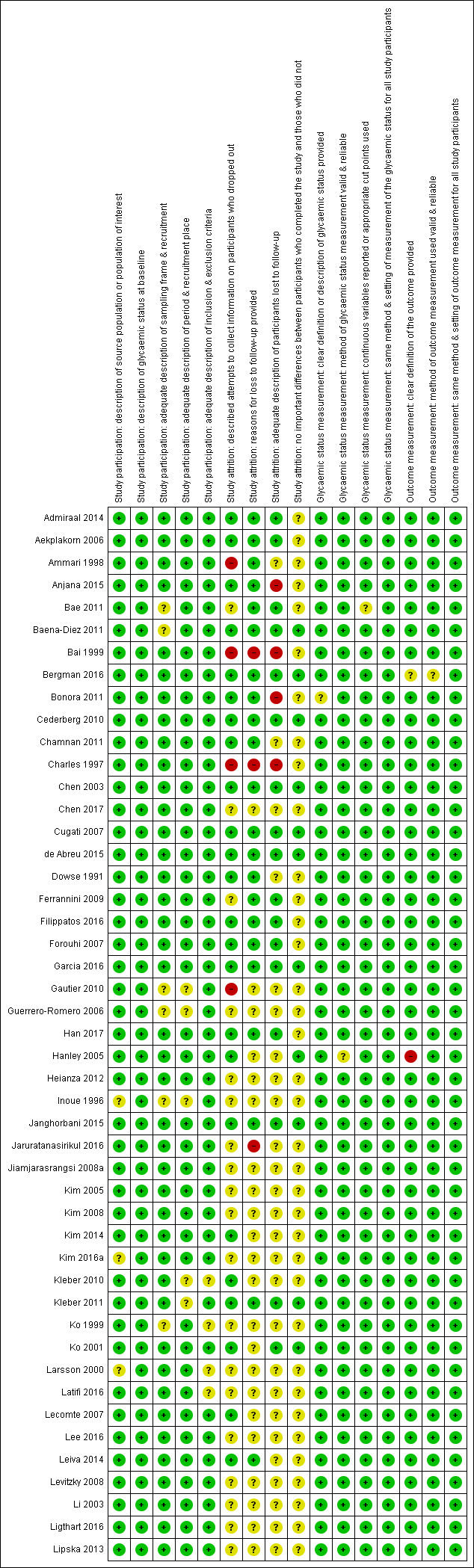
'Risk of bias' summary for studies of overall prognosis in people with intermediate hyperglycaemia for developing type 2 diabetes: review authors' judgements about each risk of bias item for each included study (part 1). The summary was split into part 1 (Figure 3) and part 2 (Figure 4) for better legibility
4.
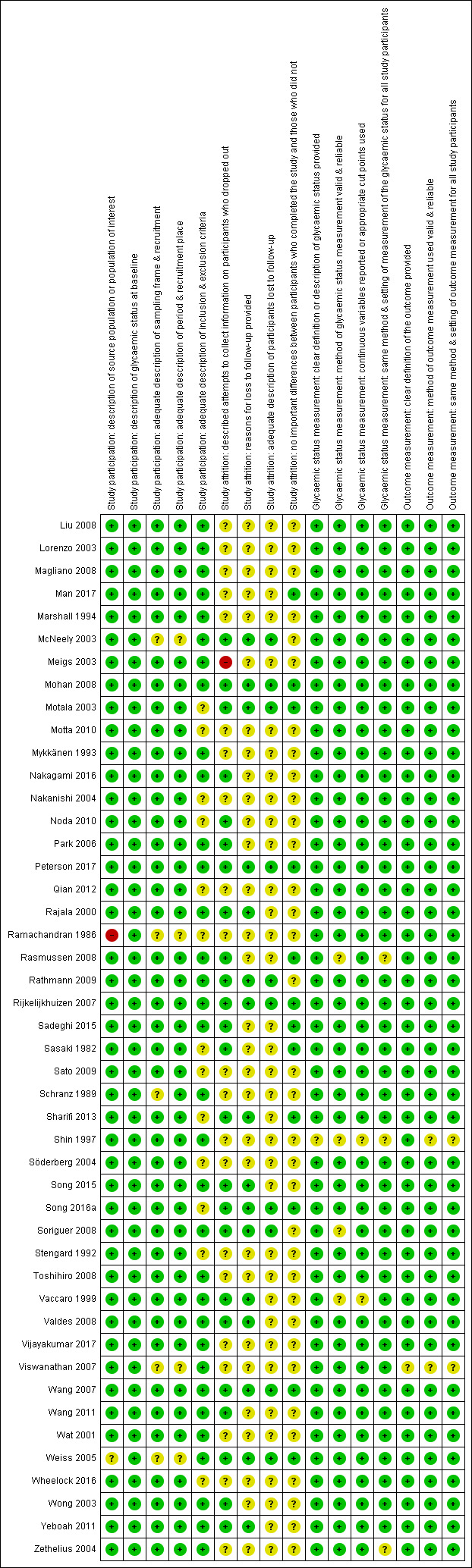
Risk of bias summary for studies of overall prognosis of people with intermediate hyperglycaemia for developing type 2 diabetes: review authors' judgements about each risk of bias item for each included study (part 2)
Study participation
Study authors described the five items in this domain sufficiently in most (65 studies; 70%) included studies. Eleven studies did not adequately characterise the sampling frame and/or recruitment procedures (Bae 2011; Baena‐Diez 2011; Gautier 2010; Guerrero‐Romero 2006; Inoue 1996; Ko 1999; McNeely 2003; Ramachandran 1986; Schranz 1989; Viswanathan 2007; Weiss 2005). One study was at high risk of bias for the item 'description of the source population or population of interest' (Ramachandran 1986).
Study attrition
Forty‐eight studies attempted to collect information on participants who were lost to follow‐up, while 40 studies were at unclear risk of bias and five studies were at high risk of bias (Ammari 1998; Bai 1999; Charles 1997; Gautier 2010; Meigs 2003).
In most (61 studies; 66%) of the studies we could not identify the reasons for loss to follow‐up or adequate descriptions of these participants. Five studies were at high risk of bias for one or both of the items (Anjana 2015; Bai 1999; Bonora 2011; Charles 1997; Jaruratanasirikul 2016).
Only 23 studies (25%) provided information on potentially important differences between participants who completed the studies and those who did not.
Glycaemic status measurement
Study authors described these items sufficiently in 85 studies (91%). One study did not describe three of the four items ('clear definition of the outcome provided', 'adequately valid and reliable method of measurement', and 'continuous variables reported or appropriate cut points used') in enough detail (Shin 1997).
Outcome measurement
Study authors described the three items sufficiently in 89 studies (96%). One study was at high risk of bias for the item 'provision of clear definition of the outcome' (Hanley 2005).
c) Development of T2DM in people with IH as compared to people with normoglycaemia
There were 52 studies comparing IH with normoglycaemia as a prognostic factor for T2DM. Figure 5 shows the results for the six domains summarised across studies, and the result for each study is shown in Figure 6.
5.
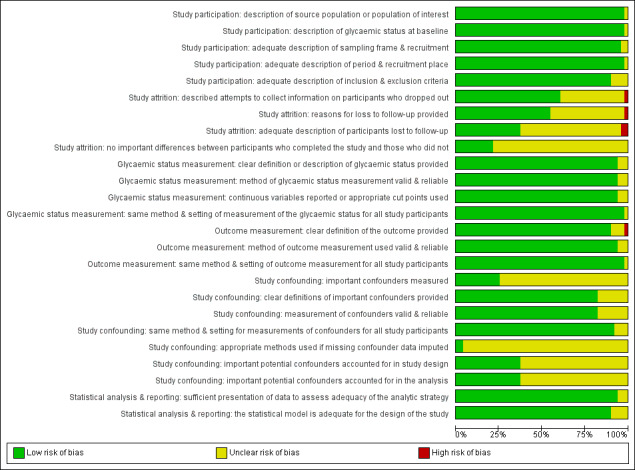
Risk of bias graph for studies of intermediate hyperglycaemia versus normoglycaemia as a prognostic factor for developing type 2 diabetes: review authors' judgements about each risk of bias item presented as percentages across all included studies
6.
Risk of bias summary for studies of intermediate hyperglycaemia versus normoglycaemia as a prognostic factor for developing type 2 diabetes: review authors' judgements about each risk of bias item for each included study
Fourteen studies provided data on multivariable HRs of T2DM incidence, adjusted for 2 to 13 covariates (Bae 2011; Bonora 2011; Forouhi 2007; Han 2017; Heianza 2012; Janghorbani 2015; Kim 2005; Li 2003; Liu 2016; Lyssenko 2005; Nakagami 2016; Wang 2011; Warren 2017; Yeboah 2011). Whenever possible, we used the reported model with the greatest number of covariates.
Study participation
Study authors described the items of this domain sufficiently in most (42 studies; 82%) of the included studies. Two studies did not adequately characterise the sampling frame and/or recruitment procedures (Bae 2011; Viswanathan 2007).
Study attrition
Study authors usually described these items sufficiently and attempted to collect information on participants who were lost to follow‐up. However, in most (32 studies; 63%) of the included studies we could not identify the reasons for losses to follow‐up or adequate descriptions of these participants. Only 10 studies (20%) provided information on potentially important differences between participants who completed the studies and those who did not. Two studies were at high risk of bias on one of the four items (Bonora 2011; Jeong 2010).
Glycaemic status measurement
Study authors described the items sufficiently in 40 (78%) studies.
Outcome measurement
Study authors described these items sufficiently in 46 studies (90%). One study had a high risk of bias for the item 'clear definition of the outcome provided' (Hanley 2005).
Study confounding
Only one study described all items sufficiently (Derakhshan 2016).
It was difficult to judge study confounding because the number of important covariates measured was limited. If studies analysed data by means of multivariable regression models, they often adjusted these analyses taking into account several covariates: age (43 out of 52 studies), anthropometric measures such as BMI (33 out of 52 studies), sex (31 out of 52 studies), family history of diabetes (24 out of 52 studies), smoking status (24 out of 52 studies), blood pressure/hypertension (19 out of 52 studies), triglycerides (18 out of 52 studies), cholesterol (17 out of 52 studies), physical activity (14 out of 52 studies), drinking status (12 out of 52 studies), socioeconomic status (8 out of 52 studies), 'ethnicity' (5 out 52 studies), medications (3 out of 52 studies) and renal function (1 study); for details see Appendix 16 and Appendix 17.
Twenty studies (39%) adjusted their analyses for age, sex and anthropometric measures (e.g. BMI or waist circumference) (Admiraal 2014; Bergman 2016; Bonora 2011; Chamnan 2011; Chen 2003; Derakhshan 2016; Forouhi 2007; Han 2017; Heianza 2012; Janghorbani 2015; Kim 2005; Kim 2016a; Li 2003; Man 2017; Sadeghi 2015; Soriguer 2008; Valdes 2008; Wang 2011; Warren 2017; Yeboah 2011). Six studies (12%) adjusted for age, sex, anthropometric measures and physical activity (Bonora 2011; Derakhshan 2016; Forouhi 2007; Han 2017; Kim 2016a; Yeboah 2011), and five studies (10%) also included smoking status (Bonora 2011; Derakhshan 2016; Forouhi 2007; Han 2017; Kim 2016a). When used, covariates were usually clearly defined and measured. However, only two studies reported an imputation method for missing confounders (Derakhshan 2016; Sadeghi 2015).
Statistical analysis and reporting
Study authors addressed this domain sufficiently in 44 studies (86%).
Development of T2DM in people with IH
In the following we report the results of the analyses for the overall prognosis of people with IH as well as regression from IH to normoglycaemia, and the effects of glycaemic status (IH versus normoglycaemia) as a prognostic factor for T2DM.
Definition of IH at baseline
Studies defined IH as follows.
IFG5.6 threshold, usually defined as a fasting plasma glucose level of 5.6 mmol/L to 6.9 mmol/L.
IFG6.1 threshold, usually defined as a fasting plasma glucose level of 6.1 mmol/L to 6.9 mmol/L.
IGT, usually defined as a plasma glucose level of 7.8 mmol/L to 11.1 mmol/L two hours after a 75 g OGTT.
Isolated IFG was defined as IFG5.6 or IFG6.1 alone, without IGT, and isolated IGT was defined as IGT alone, without IFG5.6 or IFG6.1.
HbA1c5.7 threshold, usually defined as HbA1c measurement of 5.7% to 6.4%.
HbA1c6.0 threshold, usually defined as HbA1c measurement of 6.0% to 6.4%.
Depending on how investigators measured IH, the following number of study cohorts provided information on T2DM incidence associated with glycaemic status at baseline (one study might have investigated several associations between glycaemic status and T2DM incidence within the same study, for example, one cohort with IFG5.6, another cohort with IFG6.1 and a third cohort with IGT).
IFG5.6/isolated IFG5.6: 27/10 study cohorts.
IFG6.1/isolated IFG6.1: 22/9 study cohorts.
IGT/isolated IGT: 39/18 study cohorts.
Combined IFG and IGT: 15 study cohorts.
HbA1c5.7: 7 study cohorts.
HbA1c6.0: 10 study cohorts.
Combined HbA1c5.7 and IFG5.6: 3 study cohorts.
a) Overall prognosis of people with IH for developing T2DM
Irrespective of the definition of IH at baseline, the cumulative incidence of T2DM seemed to increase with length of follow‐up, though there was no obvious linear trend. There was no clear pattern of differences between geographic regions.
IH defined by IFG5.6 mmol/L threshold
Diabetes incidence associated with IFG5.6 at baseline and follow‐up periods from 2 to 12 years showed pooled cumulative incidences of 2% to 38% (Figure 7; Figure 8).
7.
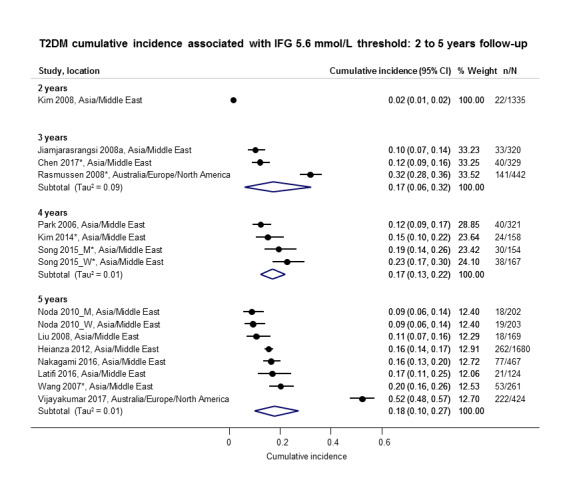
Impaired fasting glucose 5.6 mmol/L (IFG5.6) threshold: association with cumulative type 2 diabetes mellitus (T2DM) incidence over 2–5 years *Isolated IFG5.6 CI: confidence interval; M: men; n/N: events/number of participants; W: women
8.
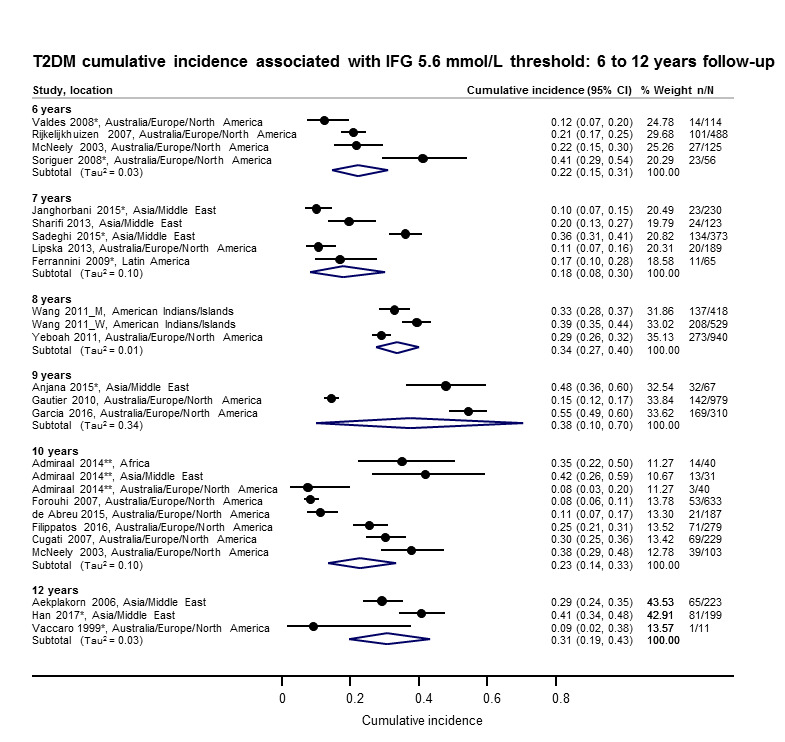
Impaired fasting glucose 5.6 mmol/L (IFG5.6) threshold: association with cumulative type 2 diabetes mellitus (T2DM) incidence over 6–12 years *Isolated IFG5.6 **'Africa': African Surinamese cohort, 'Asia': Asian Surinamese cohort, 'Australia/Europe/North America': 'ethnic Dutch' cohort. CI: confidence interval; M: men; n/N: events/number of participants; W: women
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
2 years' follow‐up: 1 study, 1335 participants, cumulative incidence 2% (95% confidence interval (CI) 1 to 2).
3 years' follow‐up: 3 studies, 1091 participants, cumulative incidence 17% (95% CI 6 to 32).
4 years' follow‐up: 3 studies, 800 participants, cumulative incidence 17% (95% CI 13 to 22).
5 years' follow‐up: 7 studies, 3530 participants, cumulative incidence 18% (95% CI 10 to 27).
6 years' follow‐up: 4 studies, 783 participants, cumulative incidence 22% (95% CI 15 to 31).
7 years' follow‐up: 5 studies, 980 participants, cumulative incidence 18% (95% CI 8 to 30).
8 years' follow‐up: 2 studies, 1887 participants, cumulative incidence 34% (95% CI 27 to 40).
9 years' follow‐up: 3 studies, 1356 participants, cumulative incidence 38% (95% CI 10 to 70).
10 years' follow‐up: 6 studies, 1542 participants, cumulative incidence 23% (95% CI 14 to 33).
12 years' follow‐up: 3 studies, 433 participants, cumulative incidence 31% (95% CI 19 to 34).
IH defined by IFG6.1 mmol/L threshold
Diabetes incidence, as associated with IFG6.1 at baseline and a follow‐up period of 2 to 15 years, showed pooled cumulative incidences of 9% to 48% (Figure 9; Figure 10).
9.

Impaired fasting glucose 6.1 mmol/L (IFG6.1) threshold: association with cumulative type 2 diabetes mellitus (T2DM) incidence over 2–5 years *Isolated IFG6.1 CI: confidence interval; M: men; n/N: events/number of participants; W: women
10.
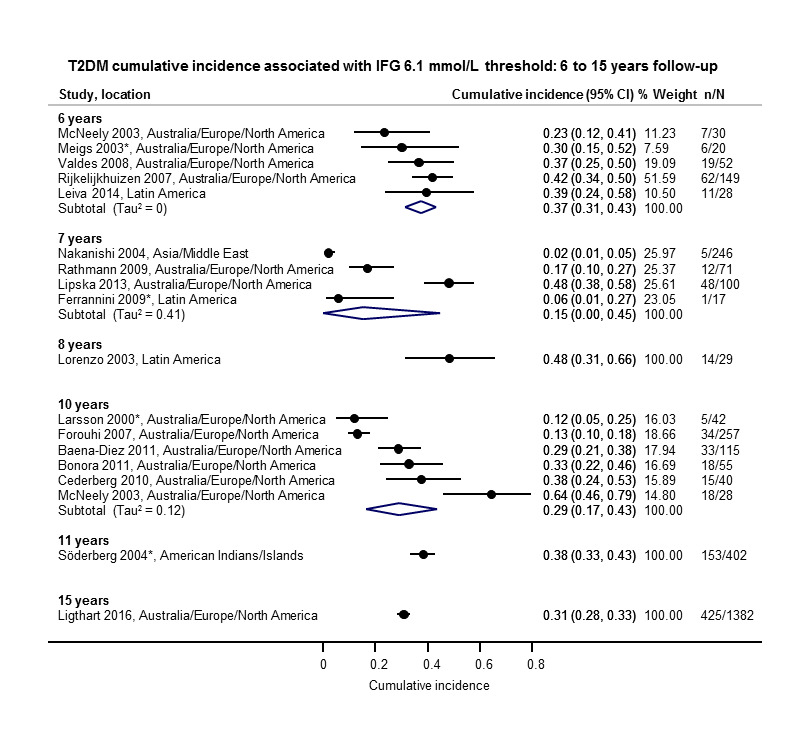
Impaired fasting glucose 6.1 mmol/L (IFG6.1) threshold: association with cumulative type 2 diabetes mellitus (T2DM) incidence over 6–15 years *Isolated IFG6.1 CI: confidence interval; n/N: events/number of participants
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
2 years' follow‐up: 2 studies, 549 participants, cumulative incidence 11% (95% CI 8 to 14).
3 years' follow‐up: 3 studies, 927 participants, cumulative incidence 9% (95% CI 2 to 20).
4 years' follow‐up: 2 studies, 1567 participants, cumulative incidence 30% (95% CI 17 to 44).
5 years' follow‐up: 11 studies, 3837 participants, cumulative incidence 26% (95% CI 19 to 33).
6 years' follow‐up: 5 studies, 279 participants, cumulative incidence 37% (95% CI 31 to 43).
7 years' follow‐up: 4 studies, 434 participants, cumulative incidence 15% (95% CI 0 to 45).
8 years' follow‐up: 1 study, 29 participants, cumulative incidence 48% (95% CI 31 to 66).
10 years' follow‐up: 6 studies, 537 participants, cumulative incidence 29% (95% CI 17 to 43).
11 years' follow‐up: 1 study, 402 participants, cumulative incidence 38% (95% CI 33 to 43).
15 years' follow‐up: 1 study, 1382 participants, cumulative incidence 31% (95% CI 28 to 33).
IH defined by IGT
Diabetes incidence associated with IGT at baseline showed pooled cumulative incidences of 13% to 60% after a follow‐up period of 1 to 20 years (Figure 11; Figure 12).
11.
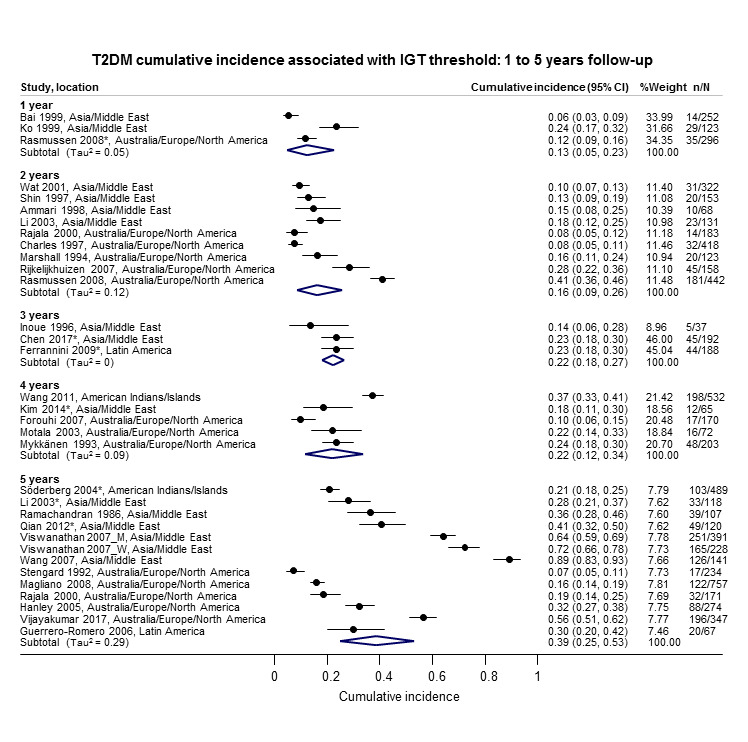
Impaired glucose tolerance (IGT): association with cumulative type 2 diabetes mellitus (T2DM) incidence over 1–5 years *Isolated IGT CI: confidence interval; n/N: events/number of participants
12.
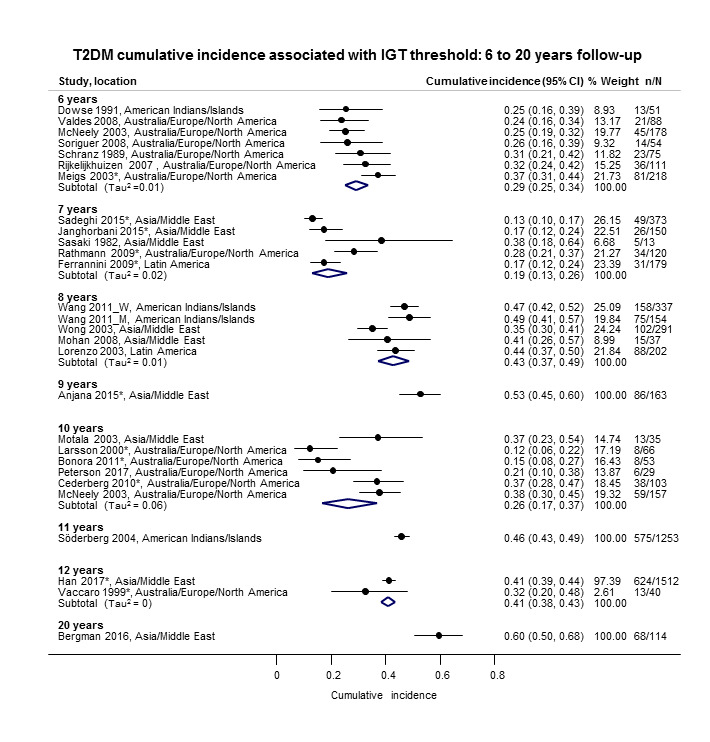
Impaired glucose tolerance (IGT): association with cumulative type 2 diabetes mellitus (T2DM) incidence over 6–20 years
*Isolated IGT CI: confidence interval; M: men; n/N: events/number of participants; W: women
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
1 year's follow‐up: 3 studies, 671 participants, cumulative incidence 13% (95% CI 5 to 23).
2 years' follow‐up: 9 studies, 1998 participants, cumulative incidence 16% (95% CI 9 to 26).
3 years' follow‐up: 3 studies, 417 participants, cumulative incidence 22% (95% CI 18 to 27).
4 years' follow‐up: 5 studies, 1042 participants, cumulative incidence 22% (95% CI 12 to 34).
5 years' follow‐up: 12 studies, 3444 participants, cumulative incidence 39% (95% CI 25 to 53).
6 years' follow‐up: 7 studies, 775 participants, cumulative incidence 29% (95% CI 25 to 34).
7 years' follow‐up: 5 studies, 835 participants, cumulative incidence 19% (95% CI 13 to 26).
8 years' follow‐up: 4 studies, 1021 participants, cumulative incidence 43% (95% CI 37 to 49).
9 years' follow‐up: 1 study, 163 participants, cumulative incidence 53% (95% CI 45 to 60).
10 years' follow‐up: 6 studies, 443 participants, cumulative incidence 26% (95% CI 17 to 37).
11 years' follow‐up: 1 study, 1253 participants, cumulative incidence 46% (95% CI 43 to 49).
12 years' follow‐up: 2 studies, 1552 participants, cumulative incidence 41% (95% CI 38 to 43).
20 years' follow‐up: 1 study, 114 participants, cumulative incidence 60% (95% CI 50 to 68).
IH defined by combined IFG and IGT
Diabetes incidence associated with the combination of both IFG and IGT at baseline showed pooled cumulative incidences of 29% to 84% at 1 to 12 years (Figure 13).
13.
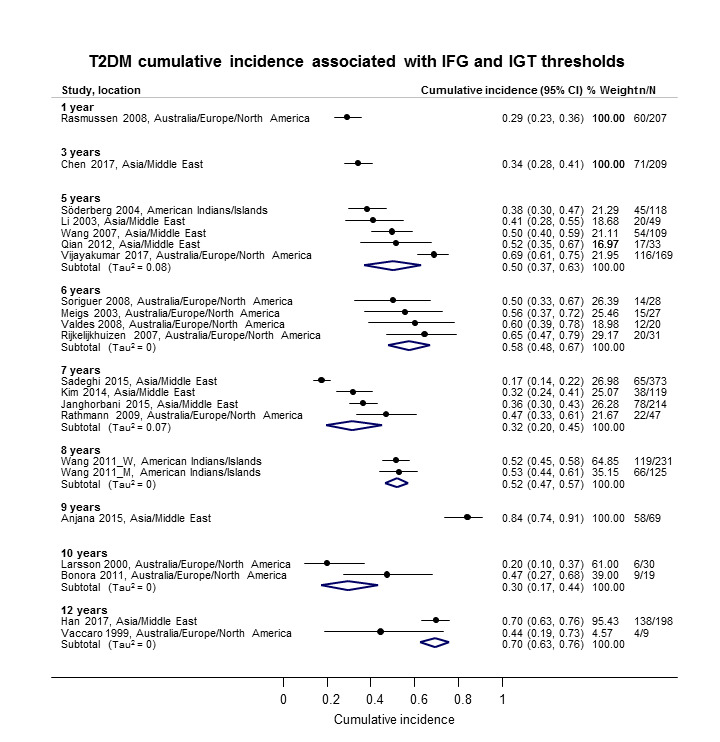
Combined impaired glucose tolerance (IGT) and impaired fasting glucose (IFG): association with cumulative type 2 diabetes mellitus (T2DM) incidence over 1–12 years CI: confidence interval; M: men; n/N: events/number of participants; W: women
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
1 year's follow‐up: 1 study, 207 participants, cumulative incidence 29% (95% CI 23 to 36).
3 years' follow‐up: 1 study, 209 participants, cumulative incidence 34% (95% CI 28 to 41).
5 years' follow‐up: 5 studies, 478 participants, cumulative incidence 50% (95% CI 37 to 63).
6 years' follow‐up: 4 studies, 106 participants, cumulative incidence 58% (95% CI 48 to 67).
7 years' follow‐up: 4 studies, 753 participants, cumulative incidence 32% (95% CI 20 to 45).
8 years' follow‐up: 1 study, 356 participants, cumulative incidence 52% (95% CI 47 to 57).
9 years' follow‐up: 1 study, 69 participants, cumulative incidence 84% (95% CI 74 to 91).
10 years' follow‐up: 2 studies, 49 participants, cumulative incidence 30% (95% CI 17 to 44).
12 years' follow‐up: 2 studies, 207 participants, cumulative incidence 70% (95% CI 63 to 76).
IH defined by HbA1c5.7 threshold
Diabetes incidence associated with HbA1c5.7 at baseline and a follow‐up period of 4 to 10 years showed pooled cumulative incidences of 14% to 31% (Figure 14).
14.
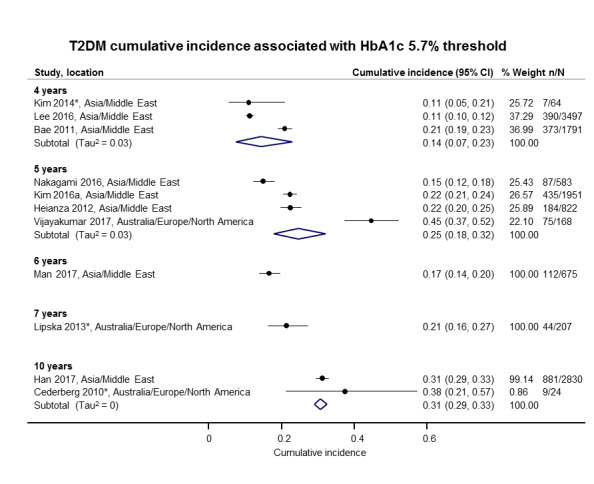
Elevated glycosylated haemoglobin A1c (HbA1c) 5.7% threshold: association with cumulative type 2 diabetes mellitus (T2DM) incidence over 4–10 years CI: confidence interval; n/N: events/number of participants
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
4 years' follow‐up: 3 studies, 5352 participants, cumulative incidence 14% (95% CI 7 to 23).
5 years' follow‐up: 4 studies, 3524 participants, cumulative incidence 25% (95% CI 18 to 32).
6 years' follow‐up: 1 study, 675 participants, cumulative incidence 17% (95% CI 14 to 20).
7 years' follow‐up: 1 study, 207 participants, cumulative incidence 21% (95% CI 16 to 27).
10 years' follow‐up: 2 studies, 2854 participants, cumulative incidence 31% (95% CI 29 to 33).
IH defined by HbA1c6.0 threshold
Most studies were undertaken in Asia. Diabetes incidence associated with HbA1c6.0 at baseline and a follow‐up period of 3 to 15 years showed pooled cumulative incidences of 7% to 44% (Figure 15).
15.
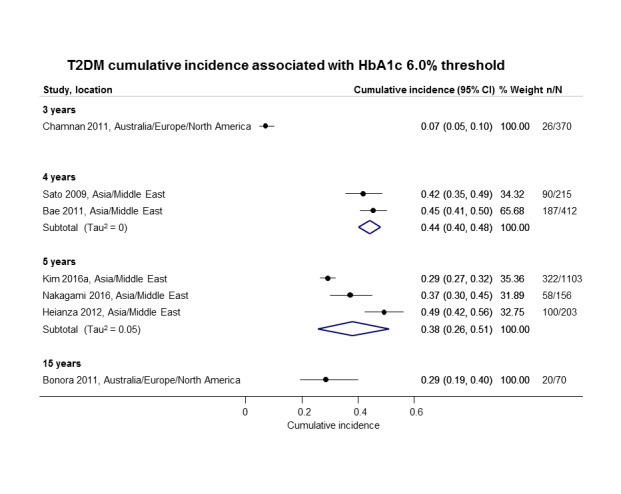
Elevated glycosylated haemoglobin A1c (HbA1c) 6.0% threshold: association with cumulative type 2 diabetes mellitus (T2DM) incidence over 3–15 years CI: confidence interval; n/N: events/number of participants
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
3 years' follow‐up: 1 study, 370 participants, cumulative incidence 7% (95% CI 5 to 10).
4 years' follow‐up: 2 studies, 627 participants, cumulative incidence 44% (95% CI 40 to 48).
5 years' follow‐up: 3 studies, 1462 participants, cumulative incidence 38% (95% CI 26 to 51).
15 years' follow‐up: 1 study, 70 participants, cumulative incidence 29% (95% CI 19 to 40).
Children and adolescents with IH (mostly IGT)
Diabetes incidence in children and adolescents, usually associated with IGT at baseline and with follow‐up of 1 to 10 years, showed pooled cumulative incidences of 1% to 56% (Figure 16). We did not observe any distinct pattern between T2DM incidence and geography.
16.
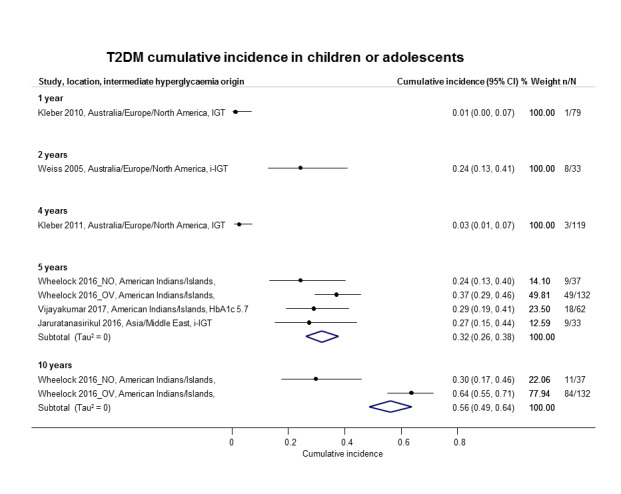
Cumulative type 2 diabetes mellitus (T2DM) incidence in children/adolescents over 1–10 years CI: confidence interval; HbA1c 5.7: glycosylated haemoglobin A1c 5.7% threshold; (i‐)IGT: (isolated) impaired glucose tolerance; n/N: events/number of participants; NO: non‐overweight; OV: overweight
The number of studies and participants, and the cumulative incidence of T2DM (pooled if more than one study) according to follow‐up period were as follows.
1 year's follow‐up: 1 study, 79 participants, cumulative incidence 1% (95% CI 0 to 7).
2 years' follow‐up: 1 study, 33 participants, cumulative incidence 24% (95% CI 13 to 41).
4 years' follow‐up: 1 study, 119 participants, cumulative incidence 3% (95% CI 1 to 7).
5 years' follow‐up: 3 studies, 264 participants, pooled cumulative incidence 32% (95% CI 26 to 38).
10 years' follow‐up: 1 study (2 subpopulations), 169 participants, cumulative incidence 56% (95% CI 49 to 64).
Special populations with IH
Studies involving black populations were scarce: one study reported a cumulative T2DM incidence of 35% in African Surinamese after 10 years of follow‐up in association with IFG5.6 at baseline (Admiraal 2014). Another study, which used IFG5.6 at baseline, reported a T2DM cumulative incidence of 33% in African Americans after 7.5 years of follow‐up (Yeboah 2011).
b) Regression from IH to normoglycaemia
Adults
In the 47 studies reporting data on regression from IH to normoglycaemia in adults within a follow‐up period of 1 to 11 years, pooled percentages ranged from 17% to 59% (Figure 17; Figure 18). Regression to normoglycaemia varied widely and showed neither a clear linear reduction or increase nor a distinct pattern associated with geography. Regression rates were often reported in association with IGT at baseline; however, there were no distinct differences in regression rates when compared with IFG5.6, IFG6.1 or HbA1c5.7 as IH risk factors.
17.
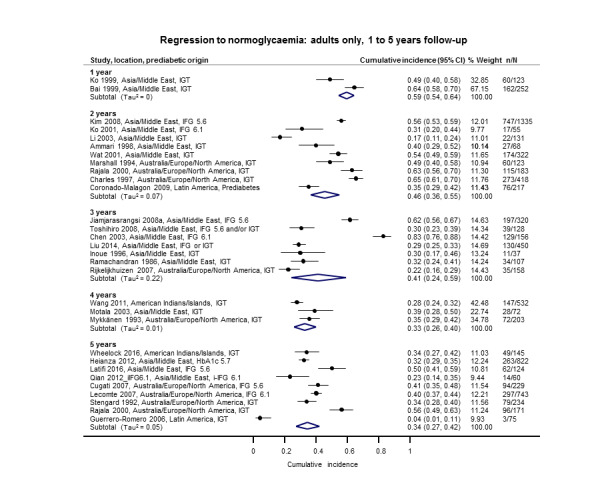
Regression from intermediate hyperglycaemia to normoglycaemia in adults over 1–5 years CI: confidence interval; HbA1c5.7: glycosylated haemoglobin A1c 5.7%; i‐IFG5.6/6.1: (isolated) impaired fasting glucose 5.6/6.1 mmol/L threshold;IGT: impaired glucose tolerance; n/N: events/number of participants
18.
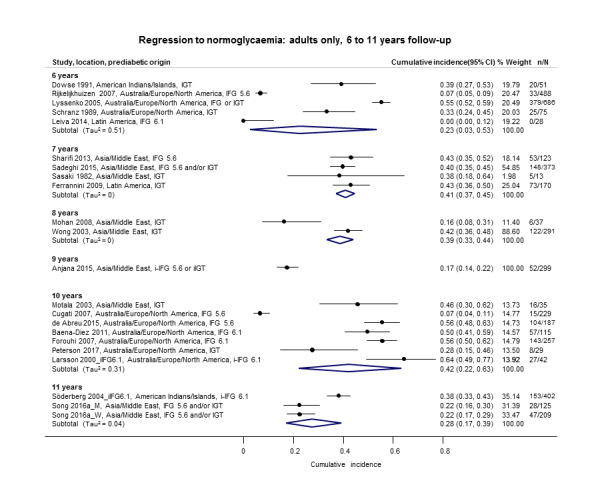
Regression from intermediate hyperglycaemia to normoglycaemia in adults over 6–11 years CI: confidence interval; i‐IFG5.6/6.1: (isolated) impaired fasting glucose 5.6/6.1 mmol/L threshold; i‐IGT: (isolated) impaired glucose tolerance; n/N: events/number of participants
The number of studies and participants, and the proportion regressing from IH to normoglycaemia (pooled if more than one study) according to follow‐up period were as follows.
1 year's follow‐up: 2 studies, 375 participants, regression to normoglycaemia 59% (95% CI 54 to 64).
2 years' follow‐up: 9 studies, 2852 participants, regression to normoglycaemia 46% (95% CI 36 to 55).
3 years' follow‐up: 7 studies, 1356 participants, regression to normoglycaemia 41% (95% CI 24 to 59).
4 years' follow‐up: 3 studies, 807 participants, regression to normoglycaemia 33% (95% CI 26 to 40).
5 years' follow‐up: nine studies, 2603 participants, regression to normoglycaemia 34% (95% CI 27 to 42).
6 years' follow‐up: 5 studies, 1328 participants, regression to normoglycaemia 23% (95% CI 3 to 53).
7 years' follow‐up: 4 studies, 679 participants, regression to normoglycaemia 41% (95% CI 37 to 45).
8 years' follow‐up: 2 studies, 328 participants, regression to normoglycaemia 39% (95% CI 33 to 44).
9 years' follow‐up: 1 study, 299 participants, regression to normoglycaemia 17% (95% CI 14 to 22)
10 years' follow‐up: 7 studies, 894 participants, regression to normoglycaemia 42% (95% CI 22 to 63).
11 years' follow‐up: 2 studies, 736 participants, regression to normoglycaemia 28% (95% CI 17 to 39).
Children and adolescents
Regression from IH to normoglycaemia in children and adolescents within a follow‐up period of one to four years showed percentages from 45% to 81% (Figure 19). There were no distinct patterns with regard to geography. IGT at baseline was often investigated as the IH risk factor.
19.
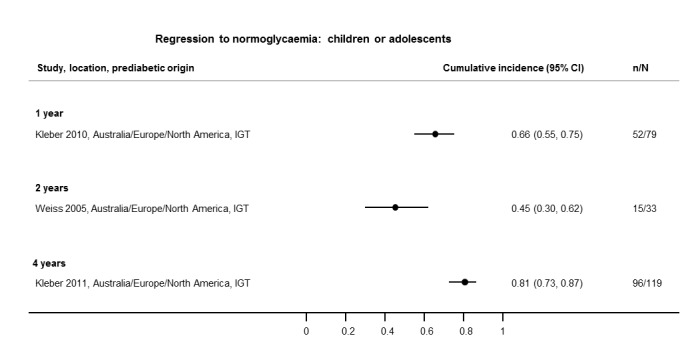
Regression from intermediate hyperglycaemia to normoglycaemia in children/adolescents over 1–4 years CI: confidence interval; IGT: impaired glucose tolerance; n/N: events/number of participants
The number of studies and participants, and the proportion regressing from IH to normoglycaemia according to follow‐up period were as follows.
1 year's follow‐up: 1 study, 79 participants, regression to normoglycaemia 66% (95% CI 55 to 75).
2 years' follow‐up: 1 study, 33 participants, regression to normoglycaemia 45% (95% CI 30 to 62).
4 years' follow‐up: 1 study, 119 participants, regression to normoglycaemia 81% (95% CI 73 to 87).
c) IH versus normoglycaemia as a prognostic factor for developing T2DM
Prognostic factor studies used various definitions for IH and different effect measures (IRR, OR and HR) to express the effect of glycaemic status on development of T2DM. The findings are presented below according to IH definition and effect measure. No data were available on the prognostic factor IH versus normoglycaemia for children or adolescents.
HR as the effect measure
IFG 5.6 mmol/L threshold
Eight studies reported HRs and the IFG5.6 threshold for IH at baseline (Analysis 1.1). The length of follow‐up ranged from 4 to 22 years (studies are ordered with ascending length of follow‐up in Analysis 1.1). The studies included 9017 participants with IH and 25,850 participants with normoglycaemia. The overall HR was 4.32 (95% CI 2.61 to 7.12). The 95% prediction interval ranged from 0.75 to 25.01
1.1. Analysis.
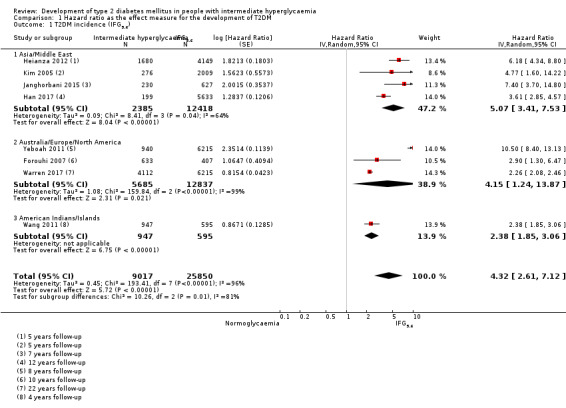
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 1 T2DM incidence (IFG5.6).
The comparison of geographic regions showed the following results (Analysis 1.1).
Asia/Middle East (4 studies, 2385 participants with IH and 12,418 participants with normoglycaemia, 5 to 12 years' follow‐up): the pooled HR was 5.07 (95% CI 3.41 to 7.53). The 95% prediction interval ranged from 1.07 to 24.02.
Australia/Europe/North America (3 studies, 5685 participants with IH and 12,837 participants with normoglycaemia, 8 to 22 years' follow‐up): the pooled HR was 4.15 (95% CI 1.24 to 13.87). Calculation of the 95% prediction interval did not provide a meaningful estimate.
American Indians/Islands (1 study, 947 participants with IH and 595 participants with normoglycaemia, 4 years' follow‐up): the HR was 2.38 (95% CI 1.85 to 3.06).
IFG 6.1 mmol/L threshold
Nine studies reported HRs and the IFG6.1 threshold for IH at baseline (Analysis 1.2). The length of follow‐up ranged from 5 to 22 years (studies are ordered by ascending length of follow‐up in Analysis 1.2). The studies included 2818 participants with IH and 18,591 participants with normoglycaemia. The overall HR was 5.47 (95% CI 3.50 to 8.54). The 95% prediction interval ranged from 1.09 to 27.56
1.2. Analysis.
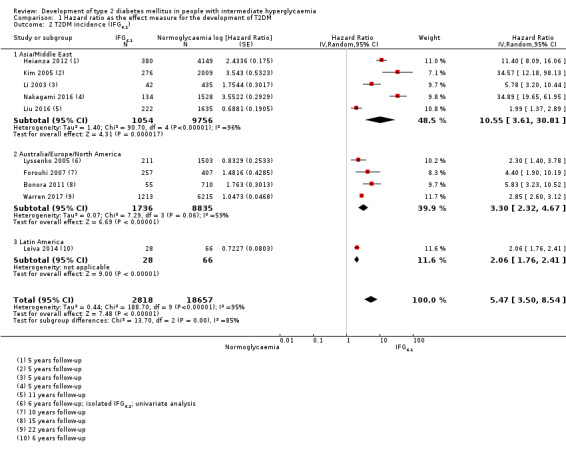
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 2 T2DM incidence (IFG6.1).
The comparison of geographic regions showed the following results (Analysis 1.2).
Asia/Middle East (5 studies, 1054 participants with IH and 9756 participants with normoglycaemia, 5 to 11 years' follow‐up): the pooled HR was 10.55 (95% CI 3.61 to 30.81). Calculation of the 95% prediction interval did not provide a meaningful estimate.
Australia/Europe/North America (4 studies, 1736 participants with IH and 8835 participants with normoglycaemia, 6 to 22 years' follow‐up): the pooled HR was 3.30 (95% CI 2.32 to 4.67). The 95% prediction interval ranged from 0.84 to 12.99.
Latin America (1 study, 28 participants with IH and 66 participants with normoglycaemia, 6 years' follow‐up): the HR was 2.06 (95% CI 1.76 to 2.41).
IGT
Five studies reported HRs and IGT for IH at baseline (Analysis 1.3). The length of follow‐up ranged from 5 to 16 years (studies are ordered by ascending length of follow‐up in Analysis 1.3). These studies included 4010 participants with IH and 12,566 participants with normoglycaemia. The overall HR was 3.61 (95% CI 2.31 to 5.64). The 95% prediction interval ranged from 0.69 to 18.97.
1.3. Analysis.
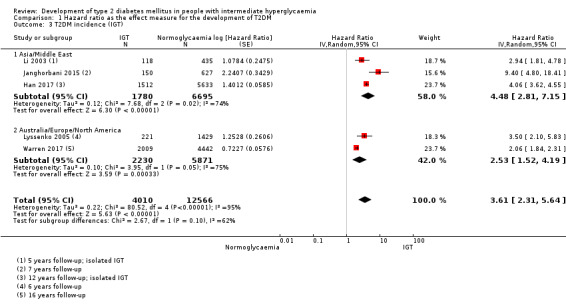
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 3 T2DM incidence (IGT).
The comparison of geographic regions showed the following results (Analysis 1.3).
Asia/Middle East (3 studies, 1780 participants with IH and 6695 participants with normoglycaemia, 5 to 12 years' follow‐up): the pooled HR was 4.48 (95% CI 2.81 to 7.15). Calculation of the 95% prediction interval did not provide a meaningful estimate.
Australia/Europe/North America (2 studies, 2230 participants with IH and 5871 participants with normoglycaemia, 6 to 16 years' follow‐up): the pooled HR was 2.53 (95% CI 1.52 to 4.19).
Combined IFG and IGT
Five studies reported HRs and used both IFG and IGT for defining IH at baseline (Analysis 1.4). The length of follow‐up ranged from 4 to 12 years (studies are ordered by ascending length of follow‐up in Analysis 1.4). These studies included 1038 participants with IH and 8719 participants with normoglycaemia. The overall HR was 6.90 (95% CI 4.15 to 11.45). The 95% prediction interval ranged from 1.06 to 44.95.
1.4. Analysis.
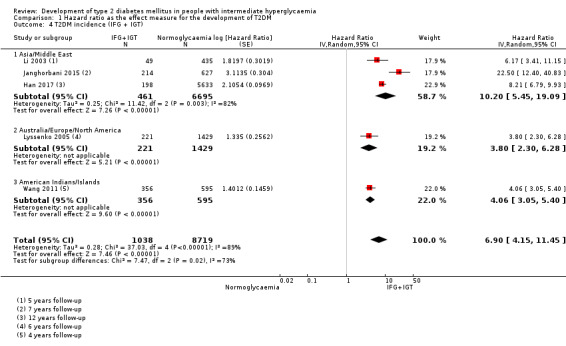
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 4 T2DM incidence (IFG + IGT).
The comparison of geographic regions showed the following results (Analysis 1.4).
Asia/Middle East (3 studies, 461 participants with IH and 6695 participants with normoglycaemia, 5 to 12 years' follow‐up): the pooled HR was 10.20 (95% CI 5.45 to 19.09). Calculation of the 95% prediction interval did not provide a meaningful estimate.
Australia/Europe/North America (1 study, 221 participants with IH and 1429 participants with normoglycaemia, 6 years' follow‐up): the HR was 3.80 (95% CI 2.30 to 6.28).
American Indians/Islands (1 study, 356 participants with both IFG and IGT and 595 participants with normoglycaemia, 4 years' follow‐up): the HR was 4.06 (95% CI 3.05 to 5.40).
HbA1c 5.7% threshold
Four studies reported HRs and the HbA1c5.7 threshold for IH at baseline (Analysis 1.5). The length of follow‐up ranged from 4 to 22 years (studies are ordered by ascending length of follow‐up in Analysis 1.5). The studies included 5223 participants with IH and 19,824 participants with normoglycaemia. The overall HR was 5.55 (95% CI 2.77 to 11.12). The 95% prediction interval ranged from 0.23 to 141.18.
1.5. Analysis.
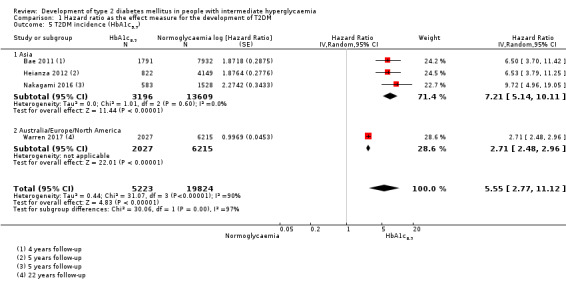
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 5 T2DM incidence (HbA1c5.7).
The comparison of geographic regions showed the following results (Analysis 1.5).
Asia/Middle East (3 studies, 3196 participants with IH and 13,609 participants with normoglycaemia, 4 to 5 years' follow‐up): the pooled HR was 7.21 (95% CI 5.14 to 10.11). The 95% prediction interval ranged from 0.81 to 64.52.
Australia/Europe/North America (1 study, 2027 participants with IH and 6215 participants with normoglycaemia, 22 years' follow‐up): the HR was 2.71 (95% CI 2.48 to 2.96).
HbA1c 6.0% threshold
Six studies reported HRs and the HbA1c6.0 threshold for IH at baseline (Analysis 1.6). The length of follow‐up ranged from 4 to 22 years (studies are ordered by ascending length of follow‐up in Analysis 1.6). The studies included 4532 participants with IH and 26,167 participants with normoglycaemia. The overall HR was 10.10 (95% CI 3.59 to 28.43). Calculation of the 95% prediction interval did not provide a meaningful estimate.
1.6. Analysis.
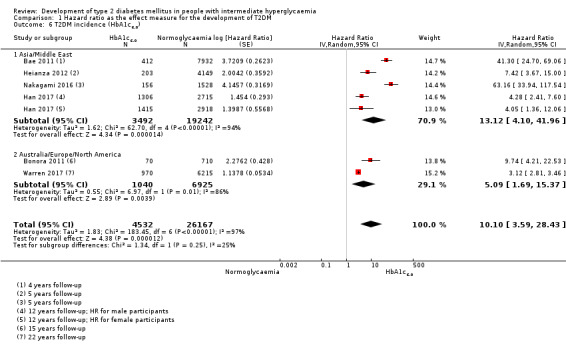
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 6 T2DM incidence (HbA1c6.0).
The comparison of geographic regions showed the following results (Analysis 1.6).
Asia/Middle East (4 studies, 3492 participants with IH and 19,242 participants with normoglycaemia, 4 to 12 years' follow‐up): the pooled HR was 13.12 (95% CI 4.10 to 41.96). Calculation of the 95% prediction interval did not provide a meaningful estimate.
Australia/Europe/North America (2 studies, 1040 participants with IH and 6925 participants with normoglycaemia, 15 to 22 years' follow‐up): the pooled HR was 5.09 (95% CI 1.69 to 15.37).
Both elevated HbA1c and IFG
One study in Japanese participants provided data on elevated HbA1c and IFG for defining IH at baseline and estimated the effect of IH versus normoglycaemia using the HR (Analysis 1.7). The combination of HbA1c5.7 plus IFG5.6 (410 participants) when compared with normoglycaemia (4149 participants) showed an HR of 32.50 (95% CI 23.00 to 45.92). The combination of HbA1c5.7 plus IFG6.1 (159 participants) when compared with normoglycaemia (5198 participants) showed an HR of 37.90 (95% CI 28.10 to 51.12). The combination of HbA1c6.0 plus IFG5.6 (135 participants) when compared with normoglycaemia (4493 participants) showed an HR of 53.70 (95% CI 38.40 to 75.09). The combination of HbA1c6.0 plus IFG6.1 (72 participants) when compared with normoglycaemia (5730 participants) showed an HR of 52.30 (95% CI 37.80 to 72.37).
1.7. Analysis.
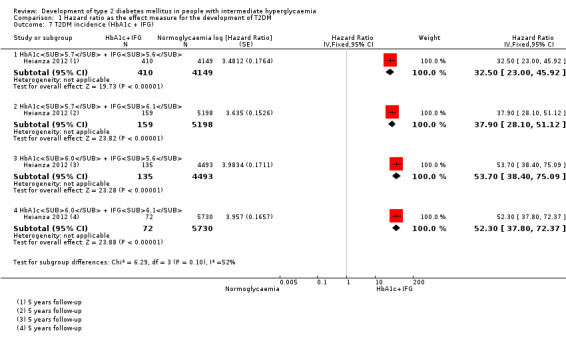
Comparison 1 Hazard ratio as the effect measure for the development of T2DM, Outcome 7 T2DM incidence (HbA1c + IFG).
IH in special populations
Data on black populations were scarce: one study in African Surinamese reported an adjusted OR of 5.1 (95% CI 2.0 to 13.3) for the association between IFG5.6 at baseline and T2DM incidence at 7.5 years' follow‐up (Admiraal 2014). Another study including a subgroup of African Americans reported the association of various measures of IH at baseline with the development of T2DM using HRs (Warren 2017): after 16 years of follow‐up the HR for IFG5.6 was 2.65 (95% CI 2.11 to 3.32); for IFG6.1, the HR was 3.16 (95% CI 2.47 to 4.06); and for IGT, the HR was 2.55 (95% CI 2.01 to 3.22). After 22 years' follow‐up, the HR for IFG5.6 was 2.05 (95% CI 1.75 to 2.40); for IFG6.1, the HR was 2.66 (95% CI 2.26 to 3.13); for HbA1c5.7, the HR was 2.24 (95% CI 1.92 to 2.61); and for HbA1c6.0, the HR was 2.60 (95% CI 2.21 to 3.05).
Incidence rate ratio as the effect measure
IFG 5.6 mmol/L threshold
Ten studies reported incidence rate ratios (IRRs) and used the IFG5.6 threshold for IH. The studies included 24,357 participants with IH and 155,272 participants with normoglycaemia (Figure 20). Of those with IH, 661 (2.7%) developed T2DM compared with 1270 (0.8%) in participants with normoglycaemia. The overall IRR was 4.81 (95% CI 3.67 to 6.30) with a 95% prediction interval ranging from 1.95 to 11.83.
20.
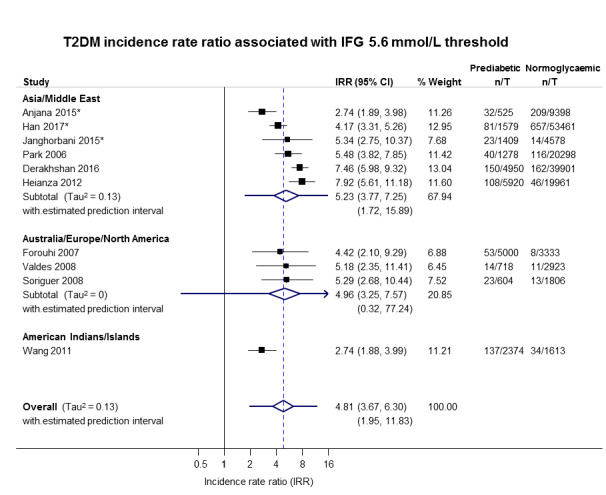
IFG: impaired fasting glucose; IRR: incidence rate ratio; n: number of cases; T: person‐time in years
The results for the geographic regions were as follows.
Asia/Middle East (6 studies): T2DM developed in 434/15,661 (2.8%) participants with IH and in 1204/145,597 (0.8%) participants with normoglycaemia. The pooled IRR was 5.23 (95% CI 3.77 to 7.25) with a 95% prediction interval ranging from 1.72 to 15.89.
Australia/Europe/North America (3 studies): T2DM developed in 90/6322 (1.4%) participants with IH and in 32/8062 (0.4%) participants with normoglycaemia. The pooled IRR was 4.96 (95% CI 3.25 to 7.57) with a 95% prediction interval ranging from 0.32 to 77.24.
American Indians/Islands (1 study): T2DM developed in 137/2374 (5.8%) participants with IH and in 34/1613 (2.1%) participants with normoglycaemia. The IRR was 2.74 (95% CI 1.88 to 3.99).
IFG 6.1 mmol/L threshold
Six studies reported IRRs and used an IFG6.1 threshold for IH. Thee studies included 5115 participants with IH, of whom 127 (2.5%) developed T2DM, plus 56,580 participants with normoglycaemia, of whom 188 (0.3%) developed T2DM (Figure 21). The overall IRR was 6.82 (95% CI 4.53 to 10.25) with a 95% prediction interval ranging from 2.03 to 22.87.
21.
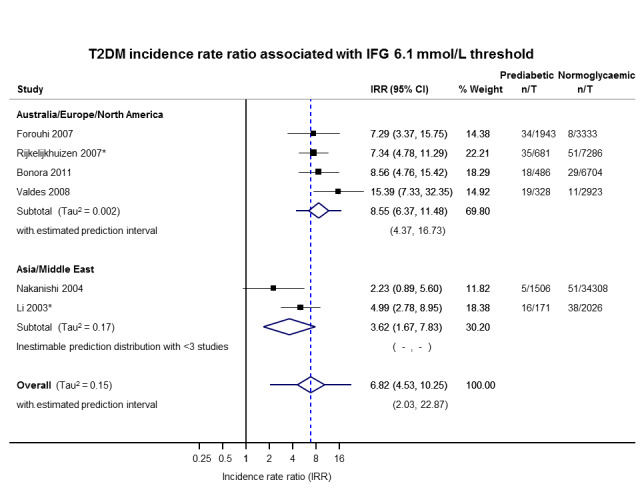
IFG: impaired fasting glucose; IRR: incidence rate ratio; n: number of cases; T: person‐time in years
The comparison of geographic regions showed a lower IRR for Asia/Middle East as follows.
Asia/Middle East (2 studies): T2DM developed in 21/1677 (1.3%) participants with IH and in 89/36,334 (0.2%) participants with normoglycaemia. The pooled IRR was 3.62 (95% CI 1.67 to 7.83).
Australia/Europe/North America (4 studies): T2DM developed in 106/3438 (3.1%) participants with IH and in 99/20,246 (0.5%) participants with normoglycaemia. The pooled IRR was 8.55 (95% CI 6.37 to 11.48) with a 95% prediction interval ranging from 4.37 to 16.73.
IGT threshold
Twelve studies reported IRRs and defined IH using IGT. The studies included 18,468 participants with IH and 98,409 participants with normoglycaemia (Figure 22). T2DM developed in 947 (5.1%) participants with IH compared to 1147 (1.2%) in participants with normoglycaemia. The overall IRR was 4.48 (95% CI 3.69 to 5.44) with a 95% prediction interval ranging from 2.60 to 7.70.
22.
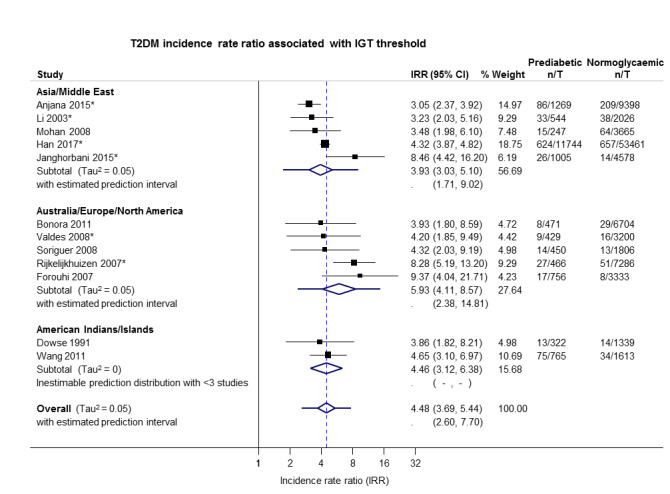
IGT: impaired glucose tolerance; IRR: incidence rate ratio; n: number of cases; T: person‐time in years
The findings according to geographic regions were as follows.
Asia/Middle East (5 studies): T2DM developed in 766/14,809 (5.2%) participants with IH and in 390/73,128 (0.5%) participants with normoglycaemia. The pooled IRR was 3.93 (95% CI 3.03 to 5.10) with a 95% prediction interval ranging from 1.71 to 9.02.
Australia/Europe/North America (5 studies): T2DM developed in 75/2572 participants with IH and in 117/22,329 (0.5%) participants with normoglycaemia. The pooled IRR was 5.93 (95% CI 4.11 to 8.57) with a 95% prediction interval ranging from 2.38 to 14.81.
American Indians/Islands (2 studies): T2DM developed in 88/1087 (8.1%) participants with IH and in 48/2952 (1.6%) participants with normoglycaemia. The pooled IRR was 4.46 (95% CI 3.12 to 6.38).
Combined IFG and IGT
Nine studies used both IFG and IGT to define IH and reported IRRs. Of the 4470 participants with IH included in the studies, 551 (12.3%) developed T2DM compared with 1091 of the 90,072 (1.2%) participants with normoglycaemia (Figure 23). The overall IRR was 10.94 (95% CI 7.22 to 16.58) with 95% prediction interval ranging from 2.58 to 46.46.
23.
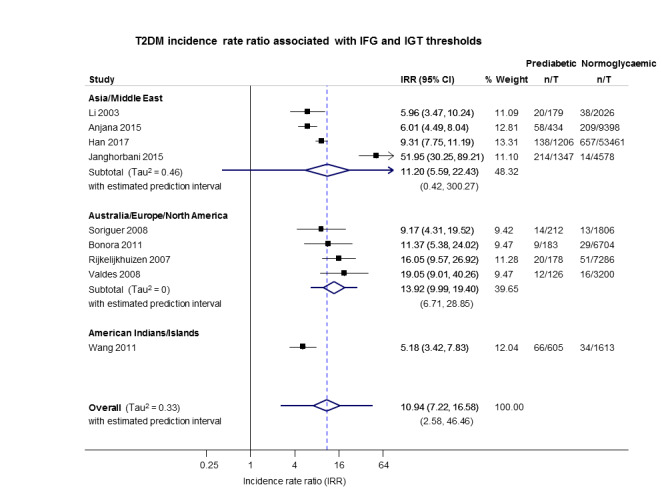
IFG: impaired fasting glucose; IGT: impaired glucose tolerance; IRR: incidence rate ratio; n: number of cases; T: person‐time in years
A lower pooled IRR was observed for the American Indians/Islands cohort compared to other cohorts as shown below.
Asia/Middle East (4 studies): T2DM developed in 430/3166 (13.6%) participants with IH and in 918/69,463 (1.3%) participants with normoglycaemia. The pooled IRR was 11.20 (95% CI 5.59 to 22.43). Calculation of the 95% prediction interval did not provide a meaningful estimate.
Australia/Europe/North America (4 studies): T2DM developed in 55/699 (7.9%) participants with IH and in 109/18,966 (0.6%) participants with normoglycaemia. The pooled IRR was 13.92 (95% CI 9.99 to 19.40) with a 95% prediction interval ranging from 6.71 to 28.85.
American Indians/Islands (1 study): T2DM developed in 66/605 (10.9%) participants with IH and in 34/1613 (2.1%) participants with normoglycaemia. The pooled IRR was 5.18 (95% CI 3.42 to 7.83).
HbA1c 5.7% threshold only and the combination of HbA1c 5.7% threshold with IFG 5.6 mmol/L threshold
One study, Heianza 2012, reported using HbA1c5.7 only or the combination of IFG5.6 plus HbA1c5.7 to define IH at baseline (Figure 24).
24.
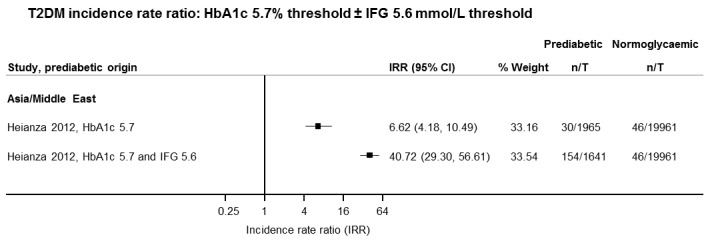
IFG: impaired fasting glucose; HbA1c: glycosylated haemoglobin A1c; IRR: incidence rate ratio; n: number of cases; T: person‐time in years
T2DM developed in 30/1965 (1.5%) participants with IH defined using HbA1c5.7 only compared with 46/19,961 (0.2%) in participants with normoglycaemia. The IRR was 6.62 (4.18 to 10.49).
In the cohort with both HbA1c5.7 and IFG5.6, T2DM developed in 154/1641 (9.4%) participants compared with 46/19961 (0.2%) in participants with normoglycaemia. The IRR was 40.72 (95% CI 29.30 to 56.61).
Odds ratio as the effect measure
IFG 5.6 mmol/L threshold
Twenty‐one studies reported ORs and the IFG5.6 threshold for IH (Analysis 2.1). The length of follow‐up ranged from 4 to 24 years (studies are ordered by ascending length of follow‐up in Analysis 2.1). The studies included 9320 participants with IH and 38,327 participants with normoglycaemia. The overall OR was 4.15 (95% CI 2.75 to 6.28). The 95% prediction interval ranged from 0.54 to 32.00.
2.1. Analysis.
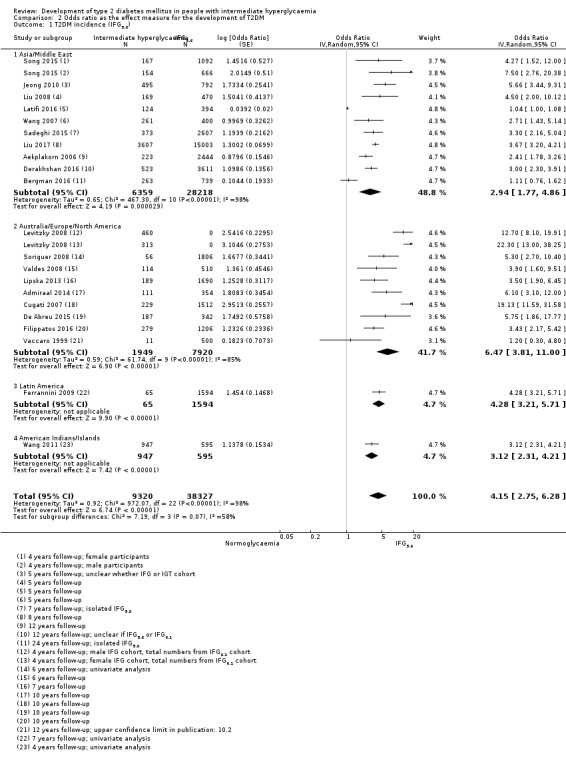
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 1 T2DM incidence (IFG5.6).
The comparison of geographic regions showed the following results (Analysis 2.1).
Asia/Middle East (10 studies, 6359 participants with IH and 28,218 participants with normoglycaemia, 4 to 24 years' follow‐up): the pooled OR was 2.94 (95% CI 1.77 to 4.86). The 95% prediction interval ranged from 0.43 to 19.93.
Australia/Europe/North America (9 studies, 1949 participants with IH and 7920 participants with normoglycaemia, 4 to 12 years' follow‐up): the pooled OR was 6.47 (95% CI 3.81 to 11.00). The 95% prediction interval ranged from 0.99 to 42.32.
Latin America (1 study, 65 participants with IH and 1594 participants with normoglycaemia, 7 years' follow‐up): the OR was 4.28 (95% CI 3.21 to 5.71).
American Indians/Islands (1 study, 947 participants with IH and 595 participants with normoglycaemia, 4 years' follow‐up): the OR was 3.12 (95% CI 2.31 to 4.21).
The test for subgroup differences did not indicate a significant subgroup effect (P = 0.07). However, two of the four subgroups had only one study each, so the validity of the analysis is uncertain. Furthermore, there is substantial heterogeneity between studies (Tau2 = 0.65 and 0.59) within each of the other two subgroups, and the subgroup analysis does not appear to have explained heterogeneity.
IFG 6.1 mmol/L threshold
Fifteen studies reported ORs and the IFG6.1 threshold for IH at baseline (Analysis 2.2). The length of follow‐up ranged from 3 to 24 years (studies are ordered by ascending length of follow‐up in Analysis 2.2). The studies included 4574 participants with threshold for IH and 32,292 participants with normoglycaemia. The overall OR was 6.60 (95% CI 4.18 to 10.43). The 95% prediction interval ranged from 0.93 to 46.82.
2.2. Analysis.
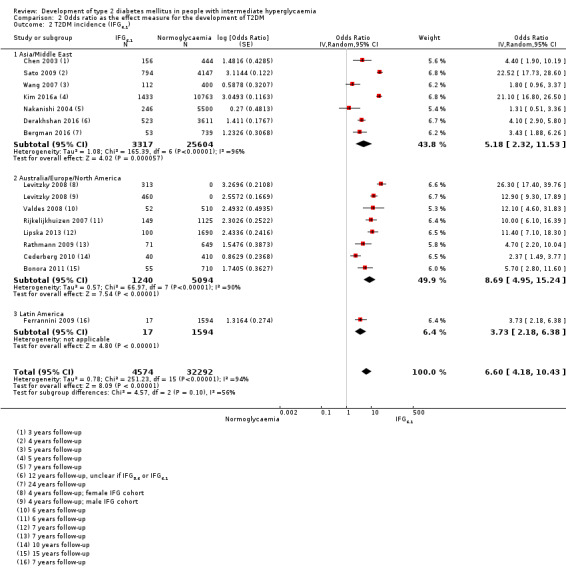
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 2 T2DM incidence (IFG6.1).
The comparison between geographic regions showed the following results (Analysis 2.2).
Asia/Middle East (7 studies, 3317 participants with IH and 25,604 participants with normoglycaemia, 3 to 24 years' follow‐up): the pooled OR was 5.18 (95% CI 2.32 to 11.53). The 95% prediction interval ranged from 0.29 to 91.37.
Australia/Europe/North America (7 studies, 1240 participants with IH and 5094 participants with normoglycaemia, 4 to 15 years' follow‐up): the pooled OR was 8.69 (95% CI 4.95 to 15.24). The 95% prediction interval ranged from 1.20 to 62.69.
Latin America (1 study, 17 participants with IH and 1594 participants with normoglycaemia, 7 years' follow‐up): the OR was 3.73 (95% CI 2.18 to 6.38).
The test for subgroup differences did not indicate a significant subgroup effect (P = 0.10). However, one of the three subgroups had only one study, and there is substantial heterogeneity between studies (Tau2 = 1.08 and 0.57) within each of the other two subgroups.
IGT
Twenty studies reported adjusted ORs and IGT for IH at baseline (Analysis 2.3). The length of follow‐up ranged from 5 to 24 years (studies are ordered by ascending length of follow‐up in Analysis 2.3). The studies included 3139 participants with IH and 18,413 participants with normoglycaemia. The overall OR was 4.61 (95% CI 3.76 to 5.64). The 95% prediction interval ranged from 2.10 to 10.13.
2.3. Analysis.
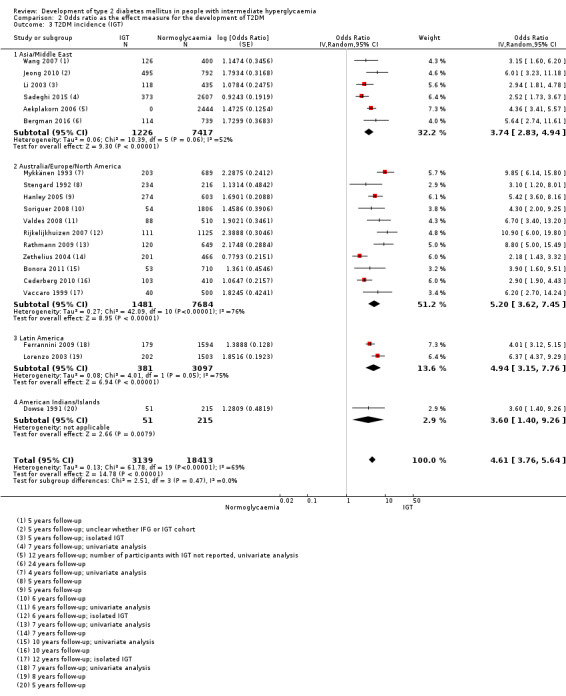
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 3 T2DM incidence (IGT).
The comparison of geographic regions showed the following results (Analysis 2.3).
Asia/Middle East (6 studies, 1226 participants with IH and 7417 participants with normoglycaemia, 5 to 24 years' follow‐up): the pooled OR was 3.74 (95% CI 2.83 to 4.94). The 95% prediction interval ranged from 1.70 to 8.21.
Australia/Europe/North America (11 studies, 1481 participants with IH and 7684 participants with normoglycaemia, 4 to 12 years' follow‐up): the pooled OR was 5.20 (95% CI 3.62 to 7.45). The 95% prediction interval ranged from 1.50 to 18.09.
Latin America (2 studies, 381 participants with IH and 3097 participants with normoglycaemia, 7 to 8 years' follow‐up): the pooled OR was 4.94 (95% CI 3.15 to 7.76).
American Indians/Islands (1 study, 51 participants with IH and 215 participants with normoglycaemia, 5 to 8 years' follow‐up): the OR was 3.60 (95% CI 1.40 to 9.26).
The test for subgroup differences did not indicate a significant subgroup effect (P = 0.47). However, two of the four subgroups had only one or two studies, so the validity of the analysis is uncertain.
Combined IFG and IGT
Nine studies reported ORs and used both IFG and IGT for defining IH at baseline (Analysis 2.4). The length of follow‐up ranged from 5 to 24 years (studies are ordered by ascending length of follow‐up in Analysis 2.4). The studies included 652 participants with IH and 9004 participants with normoglycaemia. The overall OR was 13.14 (95% CI 7.41 to 23.30). The 95% prediction interval ranged from 1.84 to 93.66.
2.4. Analysis.
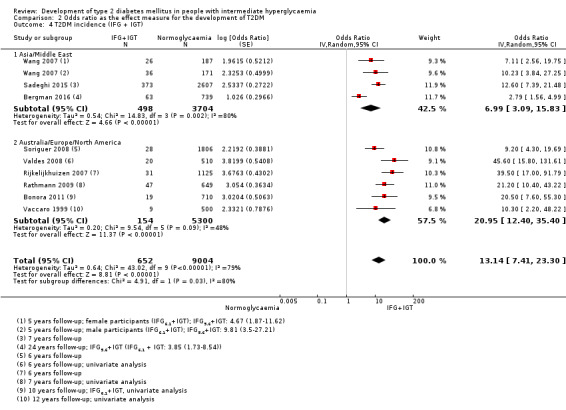
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 4 T2DM incidence (IFG + IGT).
The comparison of geographic regions showed the following results (Analysis 2.4).
Asia/Middle East (3 studies, 498 participants with IHT and 3704 participants with normoglycaemia, 5 to 24 years' follow‐up): the pooled OR was 6.99 (95% CI 3.09 to 15.83). Calculation of the 95% prediction interval did not provide a meaningful estimate.
Australia/Europe/North America (6 studies, 154 participants with IH and 5300 participants with normoglycaemia, 6 to 12 years' follow‐up): the pooled OR was 20.95 (95% CI 12.40 to 35.40). The 95% prediction interval ranged from 4.93 to 89.05.
The OR for the Australia/Europe/North America cohort of studies appeared to be higher compared with the Asia/Middle East cohort.
HbA1c 5.7% threshold
Three studies reported ORs and HbA1c5.7 threshold for IH at baseline (Analysis 2.5). The length of follow‐up ranged from 6 to 10 years (studies are ordered with ascending length of follow‐up in Analysis 2.5). The studies included 906 participants with IH and 2562 participants with normoglycaemia. The overall OR was 4.43 (95% CI 2.20 to 8.88). Calculation of the 95% prediction interval did not provide a meaningful estimate.
2.5. Analysis.
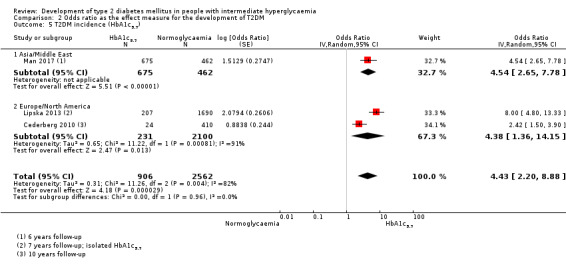
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 5 T2DM incidence (HbA1c5.7).
The results by geographic region are as follows (Analysis 2.5).
Asia/Middle East (1 study, 675 participants with IH and 462 participants with normoglycaemia, 6 years' follow‐up): the OR was 4.54 (95% CI 2.65 to 7.78).
Australia/Europe/North America (2 studies, 231 participants with IH and 2100 participants with normoglycaemia, 7 to 10 years' follow‐up): the pooled OR was 4.38 (95% CI 1.36 to 14.15).
HbA1c 6.0% threshold
Three studies reported ORs and the HbA1c6.0 threshold for IH at baseline (Analysis 2.6). The length of follow‐up ranged from three to five years. The studies included 1594 participants with IH and 16,723 participants with normoglycaemia. The overall OR was 12.79 (95% CI 4.56 to 35.85). Calculation of the 95% prediction interval did not provide a meaningful estimate.
2.6. Analysis.
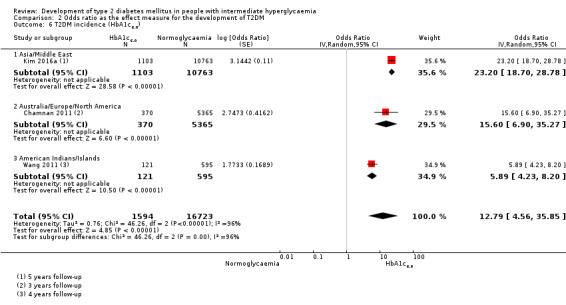
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 6 T2DM incidence (HbA1c6.0).
The comparison of geographic regions showed the following results (Analysis 2.6).
Asia/Middle East (1 study, 1103 participants with IH and 10,763 participants with normoglycaemia, 5 years' follow‐up): the OR was 23.20 (95% CI 18.70 to 28.78).
Australia/Europe/North America (1 study, 370 participants with IH and 5365 participants with normoglycaemia, 3 years' follow‐up): the OR was 15.60 (95% CI 6.90 to 35.27).
American Indians/Islands (1 study, 121 participants with IH and 595 participants with normoglycaemia, 4 years' follow‐up): the OR was 5.89 (95% CI 4.23 to 8.20).
The OR for the Asia/Middle East and Australia/Europe/North America studies appeared higher compared with the American Indians/Islands study.
Combination of HbA1c 5.7% threshold with IFG 5.6 mmol/L threshold
Two studies defined IH using a combination of HbA1c5.7 and IFG5.6 at baseline and reported ORs (Analysis 2.7). The length of follow‐up ranged from five to seven years (studies are ordered by ascending length of follow‐up in Analysis 2.7).The studies included 2120 participants with IH and 11,886 participants with normoglycaemia. The pooled OR was 35.91 (95% CI 20.43 to 63.12).
2.7. Analysis.
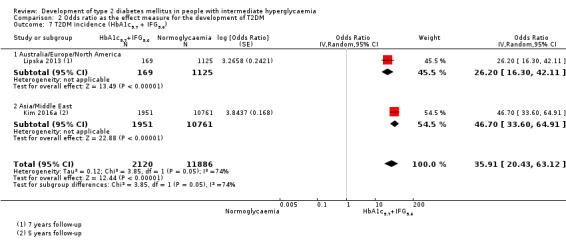
Comparison 2 Odds ratio as the effect measure for the development of T2DM, Outcome 7 T2DM incidence (HbA1c5.7 + IFG5.6).
The findings for each geographic region are as follows (Analysis 2.7).
Asia/Middle East (1 study, 1951 participants with IH and 10,761 participants with normoglycaemia, 5 years' follow‐up): the OR was 46.70 (95% CI 33.60 to 64.91).
Australia/Europe/North America (1 study, 169 participants with IH and 1125 participants with normoglycaemia, 7 years' follow‐up): the OR was 26.20 (95% CI 16.30 to 42.11).
Subgroup and sensitivity analyses
There were not enough data to perform subgroup analyses by age or sex. The special group of children and adolescents is reported under the headings corresponding to the association between IH and T2DM incidence and regression to normoglycaemia.
Sensitivity analyses for risk of bias were not meaningful because of the diversity in measurement of T2DM incidence, definitions of IH, and follow‐up periods. The analysis of adequate adjustment for confounding factors in studies reporting HRs may have provided interesting information, but there were not enough data to analyse the impact of at least four or five well‐known covariates influencing the relationship between prognostic factor and T2DM incidence. There were no very large studies including participants with IH at baseline.
Overview of complete data set and certainty of the evidence
Table 8 provides a succinct overview of the overall prognosis of people with IH as well as regression from IH to normoglycaemia over 1 to 20 years of follow‐up.
Table 9 provides a succinct overview of IH compared with normoglycaemia as a prognostic factor for developing T2DM according to geographic regions/special populations and type of outcome measurement.
Figure 25 shows the overall prognosis of IH as measured by cumulative incidence over different follow‐up periods and across all populations, as well as regression from IH to normoglycaemia.
25.
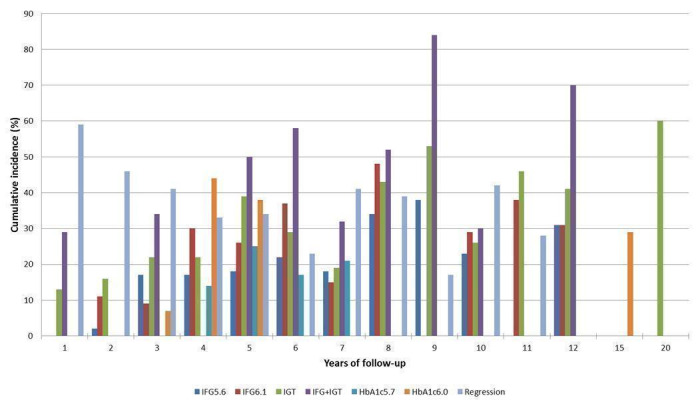
Overall prognosis of people with intermediate hyperglycaemia (cumulative type 2 diabetes incidence and regression to normoglycaemia) associated with measures of intermediate hyperglycaemia HbA1c5.7/HbA1c6.0: glycosylated haemoglobin A1c 5.7%/6.0% threshold; IFG5.6/6.1: impaired fasting glucose 5.6/6.1 mmol/L threshold; IGT: impaired glucose tolerance
Figure 26 shows IH versus normoglycaemia as a prognostic factor for developing T2DM measured by IRR, OR or HR across all populations.
26.
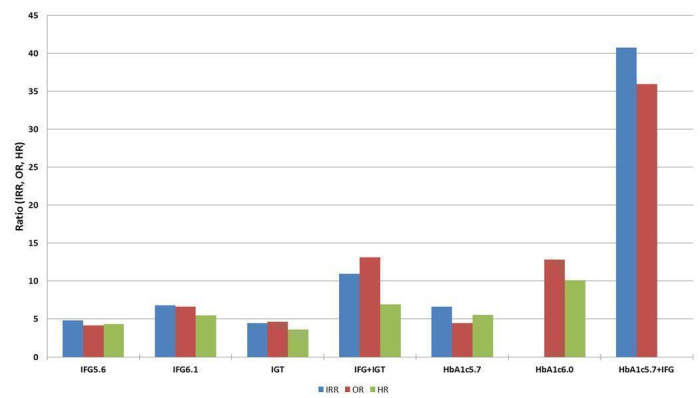
Intermediate hyperglycaemia versus normoglycaemia as a prognostic factor for developing type 2 diabetes (associated with different measures and relative risks of intermediate hyperglycaemia) HbA1c5.7/HbA1c6.0: glycosylated haemoglobin A1c 5.7%/6.0% threshold; IFG5.6/6.1: impaired fasting glucose 5.6/6.1 mmol/L threshold; IGT: impaired glucose tolerance; IRR: incidence rate ratio; OR: odds ratio; HR: hazard ratio
Taking into account all follow‐up times and all populations, the percentages of people with IH not developing T2DM over time (i.e. either regressing to normoglycaemia or remaining 'prediabetic') were as follows (see Appendix 11): IFG5.6 cohorts, 79.2%; IFG6.1 cohorts, 75.4%; IGT cohorts, 66.7%; combined IFG and IGT cohorts, 57.2%; HbA1c5.7 cohorts, 79.7%; and HbA1c6.0 cohorts, 69.0%.
For overall prognosis, we started with high‐certainty evidence because prospective cohort studies represent an adequate study design to investigate overall prognosis. However, we downgraded the certainty of the evidence to moderate because of imprecise results for most definitions of IH (Table 1).
We considered the overall certainty of the evidence for the prognostic factor IH versus normoglycaemia as low (Table 2; Table 3; Table 4; Table 5; Table 6; Table 7). We started with a high level of evidence because most included studies were phase 2 explanatory studies, defined as studies that aimed to confirm independent associations between the prognostic factor and the outcome (Huguet 2013). We downgraded the evidence for all IH measurements to low, first one level due to study limitations because many studies did not adequately adjust for confounders (only six studies used the covariate core set of age, sex, anthropometric measures and physical activity for adjustments in multivariable regression analyses ‐ Bonora 2011; Derakhshan 2016; Forouhi 2007; Han 2017; Kim 2016a; Yeboah 2011). Furthermore, we downgraded one level for imprecision/inconsistency (wide 95% CIs/wide 95% prediction intervals, sometimes ranging from negative to positive prognostic factor to outcome associations).
Discussion
Summary of main results
We included 103 prospective cohort studies from many parts of the world evaluating people with IH, usually defined using the IFG5.6 or IFG6.1 threshold, IGT, combined IFG/IGT or elevated HbA1c. However, we did not identify studies involving black Africans or Eastern Europeans. Participants were of Australian, European or North American origin in 41 studies; primarily of Latin American origin in 7 studies; Asian or Middle Eastern origin in 50 studies; American Indians in 3 studies; Mauritians in 1 study; and Nauruans in 1 study. Six studies included children, adolescents or both.
Ninety‐three studies contributed data to estimate the overall prognosis of people with IH, and 52 studies evaluated baseline glycaemic status as a prognostic factor by comparing an IH cohort with a normoglycaemic cohort.
Cumulative incidence of T2DM for the IFG5.6 threshold, the IFG6.1 threshold, IGT, combined IFG/IGT and elevated HbA1c, showed increasing percentages over follow‐up time; however, there was no clear linear increase over time. Regression rates to normoglycaemia, though decreasing over follow‐up, showed fluctuations and no clear linear decrease over time. The estimates of the prognostic effect of IH versus normoglycaemia were comparable when using HR, IRR or OR across the different definitions of IH. There was no clear pattern of risk differences between geographic regions.
Overall completeness and applicability of evidence
A limiting factor of our review was that most studies took place in Asia, the Middle East, Australia, Western Europe and North America, affecting the generalisability of findings to other populations residing in Africa and Eastern Europe. We are also aware that categorising the included studies based on region or 'ethnicity' has deficiencies with regard to clearly delineating study participants. The complicated interplay of factors like genetics, diets, and changing environmental and social conditions, among others, makes it virtually impossible to achieve a generally accepted categorisation. We chose an approach based primarily on geographic location because we thought that most readers would be interested in having a broad overview of any potential differences in T2DM incidence based on this characteristic. At the same time, we tried not to overload the reader with too much information by fragmenting our dataset into all the different countries or into more precisely defined 'ethnicities', since some investigators even reported several 'ethnic' subgroups within a single study cohort. However, we do provide detailed information, when available, in our appendices to enable the interested reader to identify studies according to whatever combination of factors seems of value to generate hypotheses of potential differences between the diverse study groups.
Only six studies addressed the overall prognosis of IH in 495 children or adolescents, with approximately 50% originating from high‐risk American Indian cohorts, also affecting the applicability of findings to other populations. No data were available on the prognostic factor of IH versus normoglycaemia for children or adolescents. Most studies determined the glycaemic status of participants at baseline and follow‐up on the basis of a single FPG, glucose tolerance test or HbA1c. Therefore, participants may have been misclassified at baseline, follow‐up or both in either direction. Interestingly, 93 studies provided data on overall prognosis of IH, but only 49 studies published information on regression from IH to normoglycaemia.
Certainty of the evidence
To our knowledge there is no validated risk of bias tool for studies addressing overall prognosis. Moreover, information on some applicable risk of bias domains of the QUIPS tools were limited. However, as illustrated in Figure 25, there was a wide fluctuation between the various definitions of IH as well as no linear increase in T2DM incidence over time of follow‐up. Of note, regression rates to normoglycaemia were also high, even after more than five years of follow‐up, emphasising that transition from IH to T2DM might be an intermediate state (Taylor 2017).
The certainty of the evidence for the overall prognosis of IH was moderate due to imprecise results for most IH definitions. The certainty of the evidence for the prognostic factor of IH versus normoglycaemia was low, mainly because most studies did not adjust for confounders known to be independently associated with T2DM incidence and due to substantial imprecision (wide 95% CIs) and inconsistency (wide 95% prediction intervals). However, the results of the six studies that adjusted for sex, anthropometric measures and physical activity were similar to the rest of the prospective cohort studies.
Limitations in the review process
As described in the Methods section, it was difficult to define a reliable search strategy, which probably holds true for many systematic reviews of prognostic studies. We noted that when checking other systematic reviews on the topic and the references of the included studies, around one third of our included studies were identified through reference checking. However, using PubMed's 'similar articles' algorithm did not yield new studies but did help us identify 13 secondary publications of studies we had already included. The 103 prospective cohort studies included in this review represent by far the largest amount of data synthesised on the overall prognosis of IH and the impact of IH versus normoglycaemia as a prognostic factor for T2DM development. We did not contact study authors for additional information, mainly for logistical reasons but also because we anticipated poor response, since many studies were published long ago. Moreover, retrieval of additional information, often demanding recalculations, would have imposed a considerable burden on study authors.
During the review process, the need to establish a database of cohort studies specifying details on prognostic factors and outcomes, amongst other things, became clear. Many large cohort studies investigate the association of a great number of prognostic factors with yet another large number of outcomes. These data may only be detected through a detailed analysis of the full text (especially tables and figures). It is evident that screening titles and abstracts will miss this information.
We did not include participants of randomised controlled trials. Though potentially some trials with longer time of follow‐up could provide additional data, we decided not to include information from intervention trials at this stage on theoretical grounds, as any intervention will interfere with peoples' lives, as opposed to demonstrating the natural progression of a disorder. In addition, we are conducting a series of Cochrane Reviews on interventions for people with IH and may integrate these data in a later update of this review (Hemmingsen 2016a; Hemmingsen 2016b; Hemmingsen 2016c).
Agreements and disagreements with other reviews
Gerstein 2007 is a widely cited review including 21 cohort studies and nine randomised controlled trials published between 1979 and 2004. The review authors annualised T2DM incidence rates, which varied from 5% to 10%. Their relative risks for T2DM incidence of 6.35 in people with IGT, 4.66 in people with IFG and 12.1 with both IFG and IGT were higher but comparable to our HR data. We did not annualise incidence rates because with pronounced fluctuations between regression and development of T2DM, assumptions to establish a model for annualising incidence data over prolonged period of times appeared too strong. Zhang 2010 examined ranges of HbA1c and also associated these with annualised diabetes incidences. The results of seven included studies reporting HbA1c categories showed an increase in T2DM incidence across an HbA1c range from 5.0% to 6.5%. No meta‐analysis was performed. Our results also showed increased T2DM incidence when the threshold of the HbA1c value at baseline was raised from 5.7% to 6.0%. Morris et al. performed a meta‐analysis of prospective observational studies in which participants had IH at baseline (Morris 2013). The review included 70 studies and estimated pooled incidence rates using IFG (35.5 incident cases per 1000 person‐years as defined by ADA and 47.4 incident cases per 1000 person‐years as defined by WHO, 11 and 34 studies, respectively), IGT (45.5 incident cases per 1000 person‐years, 46 studies) and IFG/IGT (70.4 incident cases per 1000 person‐years, 15 studies) definitions for IH. Elevated HbA1c was associated with a pooled incidence rate of 35.6 per 1000 person‐years. Similar to our results, the review found that progression rates to T2DM differed by definition of IH.
Authors' conclusions
Implications for practice
Our systematic review on the development of type 2 diabetes mellitus (T2DM) in people with intermediate hyperglycaemia (IH) or 'prediabetes' identified several uncertainties: glycaemic status can be measured in various ways, with IH usually defined by impaired fasting glucose (IFG) with cut‐off levels of 5.6 mmol/L or 6.1 mmol/L, by impaired glucose tolerance (IGT) or by elevated HbA1c levels with thresholds of 5.7% or 6.0%. These definitions imply specific settings and demands on resources. It is likely that the accuracy of information provided by the tests will need to be balanced against the time, effort and cost required to capture them. IFG measurement is cumbersome because of the need for overnight fasting. HbA1c measurement is resource intensive and must be standardised, taking into account potential interference factors like anaemia, haemoglobinopathy or renal insufficiency. IGT measurement is cumbersome and also resource intensive. Overall, the certainty of the evidence was low for IH versus normoglycaemia, mainly because many of the prospective cohort studies did not adequately investigate other factors or covariates which could have confounded or modified the prognostic effect of glycaemic status on T2DM incidence. Moreover, results varied widely, making it difficult to specify the best definition for IH. The certainty of the evidence for the overall prognosis of people with IH as well as regression from IH to normoglycaemia was moderate because of imprecise results for most intermediate hyperglycaemia definitions. With increasing years of follow‐up, T2DM incidence increased, but regression from IH to normoglycaemia was also high. There was no clear pattern of geographical differences; again, studies showed wide variation depending on the definition of IH, mode of measurement and length of follow‐up. Due to the fluctuating stages of normoglycaemia, IH and T2DM, which might show transition from one stage to another in both directions and even after years of follow‐up, practitioners should be careful about the potential implications of any active intervention for people 'diagnosed' with IH.
Implications for research
Future prospective cohort studies should address the consequences of IH to minimise secondary analyses of cohort studies where investigators synthetically form a subgroup of people with prediabetes, as such analyses are suboptimal. There is an urgent need for data from Eastern Europe and Africa to enable assessment of the prognostic value of IH in these regions, and for prospective cohort studies designed to examine the relationship between IH and normoglycaemia, T2DM incidence and the development of diabetic complications. The studies should adjust for confounding using important, well‐defined factors such as age, sex, 'ethnicity', anthropometric measures and physical activity. Also, studies should be adequately powered and analysed using suitable statistical techniques such as time‐dependent regression methods. There is a need for a database of cohort studies with details on all analysed prognostic factor to outcome associations because many cohort studies start with general questions like the influence of various risk factors on cardiovascular disease, and specific factors may only be identified by investigating the full text. The nature of these investigations means that search strategies basing their retrieval on titles and abstracts only will not be sufficient to identify these studies.
What's new
| Date | Event | Description |
|---|---|---|
| 26 November 2018 | Amended | Plain language summary: explanation on fasting blood sugar and oral glucose tolerance test corrected |
Acknowledgements
The World Health Organization (WHO) funded this review.
We thank Megan Harris for the excellent copy‐editing of our review. We thank Nuala Livingstone, Kerry Dwan, Toby Lasserson, Alex Sutton and especially Carl Moons for their distinguished peer‐reviewing which definitely raised the quality of our review.
Appendices
Appendix 1. Glossary of terms
| Abbreviation | Explanation |
| ADA | American Diabetes Association |
| ALAT | Alanine aminotransferase |
| ASAT | Aspartate transaminase |
| BG | Blood glucose |
| BMI | Body mass index |
| BW | Body weight |
| CI | Confidence interval |
| FG | Fasting glucose |
| FBG | Fasting blood glucose |
| FINDRISC | Finnish Diabetes Risk Score |
| FPG | Fasting plasma glucose |
| G6PD | Glucose‐6‐P‐dehydrogenase test |
| HbA1c | Glycosylated haemoglobin A1c |
| HbA1c5.7 | Intermediate hyperglycaemia with HbA1c level 5.7%‐6.4% at baseline (HbA1c 5.7% threshold) |
| HbA1c6.0 | Intermediate hyperglycaemia with HbA1c level 6.0%‐6.4% at baseline (HbA1c 6.0% threshold) |
| h‐CRP | High‐sensitivity C‐reactive protein |
| HOMA‐B(eta) | Homeostatic model assessment beta‐cell function |
| HOMA‐IR | Homeostatic model assessment for insulin resistance |
| HR | Hazard ratio |
| ICTRP | International Clinical Trials Registry Platform |
| IEC | International Expert Committee |
| IFG | Impaired fasting glucose |
| IFG5.6 | Intermediate hyperglycaemia with impaired fasting plasma glucose level 5.6–6.9 mmol/L at baseline (IFG 5.6 mmol/L threshold) |
| IFG6.1 | Intermediate hyperglycaemia with impaired fasting plasma glucose level 6.1–6.9 mmol/L at baseline (IFG 6.1 mmol/L threshold) |
| IFG/IGT | Combination of both IFG and IGT |
| i‐IFG | Isolated IFG |
| IGT | Impaired glucose tolerance (intermediate hyperglycaemia defined by IGT: plasma glucose 7.8–11.1 mmol/L 2 hours after a 75 g OGTT at baseline) |
| i‐IGT | Isolated IGT |
| IQR | Interquartile range |
| IRR | Incidence rate ratio |
| JDS | Japanese Diabetes Society |
| M | Men |
| NCEP | National cholesterol education program |
| NDDG | National Diabetes Data Group |
| NGSP | National Glycohemoglobin Standardization Program |
| NGT | Normal glucose tolerance |
| OGTT | Oral glucose tolerance test |
| OR | Odds ratio |
| PG | Postload glucose |
| QUIPS | Quality In Prognosis Studies tool |
| ROC | Receiver operating characteristics |
| RR | Risk ratio, relative risk |
| SD | Standard deviation |
| SE | Standard error |
| T2DM | Type 2 diabetes mellitus |
| W | Women |
| WHO | World Health Organization |
| γ‐GT | Gamma‐glutamyl transferase/transpeptidase |
Appendix 2. Search strategies
| Search strategy overview |
|
Tier 1: prediabetes as predictor for CVD, mortality, stroke, cancer, micro/macrovascular complications ( 1. Population block (prediabetes AND prognosis filter) OR 2. Prediabetes risk factors / diagnostic criteria block ((IFG, IGT, HbA1c) ADJ6 prognosis terms) ) AND 3. Outcomes block (diabetes complications, micro/macrovascular, mortality) Tier 2: prediabetes as predictor for diabetes incidence ( 1. Population block (prediabetes AND prognosis filter) OR 2. Prediabetes risk factors / diagnostic criteria block ((IFG, IGT, HbA1c) ADJ6 prognosis terms) ) AND 3. Outcomes block (diabetes incidence) |
| MEDLINE (Ovid SP) |
|
Whole strategy (combining tier 1: 'prediabetes' as predictor for cardiovascular disease, mortality, stroke, cancer, micro/macrovascular complications and tier 2: 'prediabetes' as predictor for diabetes incidence) 1. Prediabetic state/ 2. (prediabet* or pre diabet*).tw. 3. intermediate hyperglyc?emi*.tw. 4. or/1‐3 5. incidence.sh. or exp mortality/ or follow‐up studies.sh. or prognos*.tw. or predict*.tw. or course*.tw. [Wilczynski 2004: MEDLINE prognosis filter sensitivity maximizing] 6. prognosis/ or diagnosed.tw. or cohort*.mp. or predictor*.tw. or death.tw. or exp models, statistical/ [Wilczynski 2004: MEDLINE prognosis filter best balance] 7. or/5‐6 8. 4 and 7 [population block (prediabetes + prognosis filter)] 9. ((impaired fasting adj2 glucose) or IFG or (impaired adj FPG)).tw. 10. (impaired glucose tolerance or IGT).tw. 11. ("HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) adj h?emoglobin)).tw. 12. or/9‐11 13. (predict* or associa* or prognos*).tw. 14. ((prognostic or predict*) adj2 model?).tw. 15. predictive value?.tw. 16. (risk adj (predict* or factor? or score)).tw. 17. or/13‐16 18. (((impaired fasting adj2 glucose) or IFG or "impaired FPG" or impaired glucose tolerance or IGT or "HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) adj h?emoglobin)) adj3 (predict* or associa* or prognos* or ((prognostic or predict*) adj2 model?) or predictive value? or (risk adj (predict* or factor? or score)))).tw. [12 adj3 17 // risk factor block] 19. 8 or 18 [block 1 or block 2] 20. complication?.tw. 21. mortality.tw. 22. (CHD or CVD).tw. 23. (coronary adj2 disease).tw. 24. (coronar* adj (event? or syndrome?)).tw. 25. (heart adj (failure or disease? or attack? or infarct*)).tw. 26. (myocardial adj (infarct* or isch?emi*)).tw. 27. cardiac failure.tw. 28. angina.tw. 29. revasculari*.tw. 30. (stroke or strokes).tw. 31. cerebrovascular.tw. 32. ((brain* or cerebr*) adj (infarct* or isch?emi*)).tw. 33. apoplexy.tw. 34. ((vascular or peripheral arter*) adj disease?).tw. 35. cardiovascular.tw. 36. (neuropath* or polyneuropath*).tw. 37. (retinopath* or maculopath*).tw. 38. (nephropath* or nephrotic or proteinuri* or albuminuri*).tw. 39. ((kidney or renal) adj (disease? or failure or transplant*)).tw. 40. ((chronic or endstage or end stage) adj (renal or kidney)).tw. 41. (CRD or CRF or CKF or CRF or CKD or ESKD or ESKF or ESRD or ESRF).tw. 42. (microvascular or macrovascular or ((micro or macro) adj vascular)).tw. 43. (cancer or carcino* or neoplas* or tumo?r?).tw. 44. (amputation? or ulcer* or foot or feet or wound*).tw. 45. or/20‐44 [tier 1 strategy outcomes block] 46. 19 and 45 47. ((diabet* or type 2 or type II or T2D*) adj4 (progress* or inciden* or conversion or develop* or future)).tw. [tier 2 strategy outcomes block] 48. 19 and 47 49. 46 or 48 50. exp animals/ not humans/ 51. 49 not 50 52. (gestational or PCOS).tw. 53. 51 not 52 54. (comment or letter or editorial).pt. 55. 53 not 54 56. remove duplicates from 55 |
| Embase (Ovid SP) |
|
Whole strategy (combining tier 1: 'prediabetes' as predictor for cardiovascular disease, mortality, stroke, cancer, micro/macrovascular complications and tier 2: 'prediabetes' as predictor for diabetes incidence) 1. (prediabet* or pre diabet*).tw. 2. intermediate hyperglyc?emi*.tw. 3. or/1‐2 4. exp disease course or risk*.mp. or diagnos*.mp. or follow‐up.mp. or ep.fs. or outcome.tw. [Wilczynski 2005: Embase prognosis filter sensitivity maximizing] 5. follow‐up.mp. or prognos*.tw. or ep.fs. [Wilczynski 2005: Embase prognosis filter best balance] 6. or/4‐5 7. 3 and 6 [population block (prediabetes + prognosis filter)] 8. ((impaired fasting adj2 glucose) or IFG or (impaired adj FPG)).tw. 9. (impaired glucose tolerance or IGT).tw. 10. ("HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) adj h?emoglobin)).tw. 11. or/8‐10 12. (predict* or associa* or prognos*).tw. 13. ((prognostic or predict*) adj2 model?).tw. 14. predictive value?.tw. 15. (risk adj (predict* or factor? or score)).tw. 16. or/12‐15 17. (((impaired fasting adj2 glucose) or IFG or "impaired FPG" or impaired glucose tolerance or IGT or "HbA(1c)" or HbA1 or HbA1c or "HbA 1c" or ((glycosylated or glycated) adj h?emoglobin)) adj3 (predict* or associa* or prognos* or ((prognostic or predict*) adj2 model?) or predictive value? or (risk adj (predict* or factor? or score)))).tw. [12 adj3 17 // risk factor block] 18. 7 or 17 [block 1 or block 2] 19. complication?.tw. 20. mortality.tw. 21. (CHD or CVD).tw. 22. (coronary adj2 disease).tw. 23. (coronar* adj (event? or syndrome?)).tw. 24. (heart adj (failure or disease? or attack? or infarct*)).tw. 25. (myocardial adj (infarct* or isch?emi*)).tw. 26. cardiac failure.tw. 27. angina.tw. 28. revasculari*.tw. 29. (stroke or strokes).tw. 30. cerebrovascular.tw. 31. ((brain* or cerebr*) adj (infarct* or isch?emi*)).tw. 32. apoplexy.tw. 33. ((vascular or peripheral arter*) adj disease?).tw. 34. cardiovascular.tw. 35. (neuropath* or polyneuropath*).tw. 36. (retinopath* or maculopath*).tw. 37. (nephropath* or nephrotic or proteinuri* or albuminuri*).tw. 38. ((kidney or renal) adj (disease? or failure or transplant*)).tw. 39. ((chronic or endstage or end stage) adj (renal or kidney)).tw. 40. (CRD or CRF or CKF or CRF or CKD or ESKD or ESKF or ESRD or ESRF).tw. 41. (microvascular or macrovascular or ((micro or macro) adj vascular)).tw. 42. (cancer or carcino* or neoplas* or tumo?r?).tw. 43. (amputation? or ulcer* or foot or feet or wound*).tw. 44. or/19‐43 [tier 1 strategy outcomes block] 45. 18 and 44 46. ((diabet* or type 2 or type II or T2D*) adj4 (progress* or inciden* or conversion or develop* or future)).tw. [tier 2 strategy outcomes block] 47. 18 and 46 48. 45 or 47 [49‐53: TSC Portal filter for exclusion of animal references] 49. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 50. human/ or normal human/ or human cell/ 51. 49 and 50 52. 49 not 51 53. 48 not 52 54. (gestational or PCOS).tw. 55. 53 not 54 56. (comment or letter or editorial or conference).pt. 57. 55 not 56 58. remove duplicates from 57 |
| ClinicalTrials.gov (Expert search) |
| ( prediabetes OR prediabetic OR "pre diabetes" OR "pre diabetic" OR "intermediate hyperglycemia" OR "intermediate hyperglycaemia" OR "intermediate hyperglycemic" OR "intermediate hyperglycaemic" OR "impaired glucose tolerance" OR "impaired fasting glucose" ) AND ( complication OR complications OR mortality OR CHD OR CVD OR coronary OR heart OR myocardial OR infarct OR infarction OR infarcts OR infarctions OR ischemia OR ischemic OR ischaemia OR ischaemic OR failure OR angina OR revascularization OR revascularisation OR revascularizations OR revascularisations OR stroke OR strokes OR cerebrovascular OR apoplexy OR vascular or peripheral OR cardiovascular OR neuropathy OR neuropathies OR polyneuropathy OR polyneuropathies OR retinopathy OR retinopathies OR maculopathy OR maculopathies OR nephropathy OR nephropathies OR nephrotic OR proteinuria OR proteinuric OR albuminuria OR kidney OR renal OR CRD OR CRF OR CKF OR CRF OR CKD OR ESKD OR ESKF OR ESRD OR ESRF OR microvascular OR macrovascular OR "micro vascular" OR "macro vascular" OR cancer OR carcinoma OR neoplasm OR neoplasms OR tumor OR tumors OR tumour OR tumours OR amputation OR amputations OR ulcer OR foot OR feet OR wounds OR ( diabetes OR diabetic OR "type 2" OR "type II" OR T2D OR T2DM ) AND ( progress OR progression OR progressed OR incident OR incidence OR conversion OR developed OR development OR future ) ) [OUTCOME] |
| ICTRP Search Portal (Standard search) |
| prediabet* AND prognos* OR prediabet* AND predict* OR prediabet* AND inciden* OR prediabet* AND mortality OR prediabet* AND prevent* OR prediabet* AND progress* OR prediabet* AND develop* OR pre diabet* AND prognos* OR pre diabet* AND predict* OR pre diabet* AND inciden* OR pre diabet* AND mortality OR pre diabet* AND prevent* OR pre diabet* AND progress* OR pre diabet* AND develop* OR impaired glucose tolerance AND prognos* OR impaired glucose tolerance AND predict* OR impaired glucose tolerance AND inciden* OR impaired glucose tolerance AND mortality OR impaired glucose tolerance AND prevent* OR impaired glucose tolerance AND progress* OR impaired glucose tolerance AND develop* OR impaired fasting glucose AND prognos* OR impaired fasting glucose AND predict* OR impaired fasting glucose AND inciden* OR impaired fasting glucose AND mortality OR impaired fasting glucose AND prevent* OR impaired fasting glucose AND progress* OR impaired fasting glucose AND develop* OR HbA* AND prognos* OR HbA* AND predict* OR HbA* AND inciden* OR HbA* AND mortality OR HbA* AND prevent* OR HbA* AND progress* OR HbA* AND develop* |
| Seed publications (for PubMed's 'similar articles'‐algorithm) |
| 24355200[PMID] OR 16873795[PMID] OR 9705020[PMID] OR 25906786[PMID] OR 9363520[PMID] OR 21278140[PMID] OR 21676480[PMID] OR 21300382[PMID] OR 10862313[PMID] OR 18689695[PMID] OR 27596059[PMID] OR 12397006[PMID] OR 18673544[PMID] OR 21307378[PMID] OR 15220202[PMID] OR 22647753[PMID] OR 28258520[PMID] OR 10663216[PMID] OR 20573752[PMID] OR 20622160[PMID] OR 9300248[PMID] OR 2060716[PMID] OR 27459384[PMID] OR 12757990[PMID] OR 10414941[PMID] OR 21335372[PMID] OR 9653617[PMID] OR 20073428[PMID] OR 17309402[PMID] OR 17315136[PMID] OR 14025561[PMID] OR 10466767[PMID] OR 26273669[PMID] OR 28698884[PMID] OR 11311100[PMID] OR 14710970[PMID] OR 27933333[PMID] OR 27543801[PMID] OR 2035513[PMID] OR 12062857[PMID] OR 11978676[PMID] OR 11679461[PMID] OR 19224196[PMID] OR 14693710[PMID] OR 28278309[PMID] OR 17257284[PMID] OR 7859632[PMID] OR 2689122[PMID] OR 10937506[PMID] OR 27515749[PMID] OR 20484131[PMID] OR 26675051[PMID] OR 8866565[PMID] OR 17032347[PMID] OR 11686540[PMID] OR 26606421[PMID] OR 18282630[PMID] OR 8635647[PMID] OR 9243105[PMID] OR 8886564[PMID] OR 7589843[PMID] OR 9028719[PMID] OR 2407581[PMID] OR 28751960[PMID] OR 2912042[PMID] OR 28043048[PMID] OR 11916954[PMID] OR 16344402[PMID] OR 19531260[PMID] OR 19414206[PMID] OR 1216390[PMID] OR 22456865[PMID] OR 22510023[PMID] OR 22955996[PMID] OR 21705064[PMID] OR 21212932[PMID] OR 28768835[PMID] OR 9162608[PMID] OR 17000944[PMID] OR 25814432[PMID] OR 9406673[PMID] OR 11110508[PMID] OR 27740930[PMID] OR 24843430[PMID] OR 16518992[PMID] OR 18486512[PMID] OR 29133894[PMID] OR 29380232[PMID] OR 8894485[PMID] OR 28951335[PMID] OR 5226858[PMID] OR 27368062[PMID] OR 16100444[PMID] OR 15223223[PMID] OR 18452257[PMID] OR 27085081[PMID] OR 25245975[PMID] OR 6706044[PMID] OR 20827664[PMID] OR 20536946[PMID] OR 11606173[PMID] OR 10587859[PMID] OR 14967156[PMID] OR 7782724[PMID] OR 9754834[PMID] OR 11079739[PMID] OR 28004008[PMID] OR 17320447[PMID] OR 11772900[PMID] OR 2260546[PMID] OR 26885316[PMID] OR 25215305[PMID] OR 29074816[PMID] OR 18206734[PMID] OR 12590020[PMID] OR 26575606[PMID] OR 22640983[PMID] OR 24135387[PMID] OR 26840038[PMID] OR 24992623[PMID] OR 18485514[PMID] OR 27749572[PMID] OR 14578254[PMID] OR 15616025[PMID] OR 7748921[PMID] OR 17989310[PMID] OR 28371687[PMID] OR 8112189[PMID] OR 12610034[PMID] OR 12765960[PMID] OR 11784224[PMID] OR 9829346[PMID] OR 6702817[PMID] OR 3516770[PMID] OR 18697630[PMID] OR 11437858[PMID] OR 8612442[PMID] OR 8070301[PMID] OR 8454106[PMID] OR 9203444[PMID] OR 12519316[PMID] OR 19414203[PMID] OR 8335178[PMID] OR 1892482[PMID] OR 2261821[PMID] OR 27515716[PMID] OR 15036828[PMID] OR 15983331[PMID] OR 8875091[PMID] OR 8720611[PMID] OR 3751746[PMID] OR 20508383[PMID] OR 17914548[PMID] OR 7497867[PMID] OR 16600415[PMID] OR 23283714[PMID] OR 21738002[PMID] OR 8922541[PMID] OR 25624343[PMID] OR 7481176[PMID] OR 12414877[PMID] OR 11106838[PMID] OR 3527626[PMID] OR 17143605[PMID] OR 18060659[PMID] OR 12627316[PMID] OR 20002472[PMID] OR 17259503[PMID] OR 11068083[PMID] OR 29018885[PMID] OR 3054559[PMID] OR 25350916[PMID] OR 21107436[PMID] OR 7075915[PMID] OR 19131461[PMID] OR 17536075[PMID] OR 18316395[PMID] OR 2752891[PMID] OR 20855549[PMID] OR 20200384[PMID] OR 23497506[PMID] OR 24083174[PMID] OR 10097917[PMID] OR 9405904[PMID] OR 3542644[PMID] OR 20978739[PMID] OR 15189364[PMID] OR 25962707[PMID] OR 27239315[PMID] OR 18226046[PMID] OR 12777437[PMID] OR 12582008[PMID] OR 8314414[PMID] OR 8482427[PMID] OR 6507426[PMID] OR 18535192[PMID] OR 10333940[PMID] OR 16990660[PMID] OR 19046200[PMID] OR 10812323[PMID] OR 10480514[PMID] OR 17536076[PMID] OR 18249214[PMID] OR 20934897[PMID] OR 28632742[PMID] OR 27810987[PMID] OR 18405128[PMID] OR 8680609[PMID] OR 20578203[PMID] OR 16720024[PMID] OR 15451912[PMID] OR 15533586[PMID] OR 21270194[PMID] OR 10333943[PMID] OR 27863979[PMID] OR 11781759[PMID] OR 15175438[PMID] OR 15793193[PMID] OR 11194248[PMID] OR 26913636[PMID] OR 7712700[PMID] OR 14578234[PMID] OR 21718910[PMID] OR 15161800[PMID] |
Appendix 3. QUIPS tool signalling questions
| Study ID | |
| Signalling question | Authors' judgement for 'yes' |
| Study participation: yes/noa/unclearb/NAc | |
| a. Adequate participation in the study by eligible people | NA: usually participants with information on glycaemic status and follow‐up data providing information on development of type 2 diabetes are selected from a greater study cohort (e.g. study evaluating several cardiovascular risk factors) |
| b. Description of the source population or population of interest | Source population for cohort with intermediate hyperglycaemia is clearly described |
| c. Description of the baseline study sample | Number of people with intermediate hyperglycaemia at baseline is clearly described |
| d. Adequate description of the sampling frame and recruitment | Way of establishing the source population, selection criteria and key characteristics of the source population clearly described |
| e. Adequate description of the period and place of recruitment | Time period and place of recruitment for both baseline and follow‐up examinations are clearly described |
| f. Adequate description of inclusion and exclusion criteria | Definiton of people with normoglycaemia, intermediate hyperglycaemia or diabetes mellitus and description of other inclusion and exclusion criteria |
| Study participation: risk of bias rating (high/low/unclear) |
High: most items are answered with 'no'; Low: all items answered with 'yes'; Unclear: most items are answered with 'unclear' Note: potentially a single item may introduce a high risk of bias, depending on study specifics |
| Study attrition: yes/no/unclear/NA | |
| a. Adequate response rate for study participants | NA: usually participants with information on glycaemic status and follow‐up data providing information on development of type 2 diabetes are selected from a greater study cohort (e.g. study evaluating several cardiovascular risk factors) |
| b. Attempts to collect information on participants who dropped out described | Attempts to collect information on participants who dropped out are described (e.g. telephone contact, mail, registers) |
| c. Reasons for loss to follow‐up provided | Reasons on participants who dropped out are available (e.g. deceased participants between baseline and follow‐up, participants moving to another location) |
| d. Adequate description of participants lost to follow‐up | Key characteristics of participants lost to follow‐up are described (age, sex, glucose status at baseline, body mass index) |
| e. No important differences between participants who completed the study and those who did not | Study authors described differences between participants completing the study and those who did not as not important or information provided to judge the differences |
| Study attrition: risk of bias rating (high/low/unclear) |
High: most items are answered with 'no'; Low: all items answered with 'yes'; Unclear: most items are answered with 'unclear' Note: potentially a single item may introduce a high risk of bias, depending on study specifics |
| Glycaemic status measurement: yes/no/unclear/NA | |
| a. Clear definition or description provided | Measurements for glycaemic status are provided (e.g. IFG, IGT, elevated HbA1c) |
| b. Adequately valid and reliable method of measurement | Ideally measurements for glycaemic status are repeated to ensure diagnosis, single measurements are accepted as well; technique for glucose measurement or HbA1c measurement described |
| c. Continuous variables reported or appropriate cut points used | Standard categories for intermediate hyperglycaemia (FPG 5.6–6.9 mmol/L (IFG5.6), FPG 6.1–6.9 mmol/L (IFG6.1), 2‐h PG 7.8 to < 11.0 mmol/L (IGT), HbA1c 6.0–6.4% (HbA1c6.0), HbA1c 5.7–6.4% (HbA1c5.7)) |
| d. Same method and setting of measurement used in all study participants | Measurements of glycaemic status are the same for all study participants |
| e. Adequate proportion of the study sample had complete data | NA: usually participants with information on glycaemic status and follow‐up data providing information on development of type 2 diabetes are selected from a greater study cohort (e.g. study evaluating several cardiovascular risk factors) |
| f. Appropriate methods of imputation were used for missing data | NA: missing laboratory measurements for glycaemic status cannot be reliably imputed |
| Glycaemic status measurement: risk of bias rating (high/low/unclear) |
High: most items are answered with 'no'; Low: all items answered with 'yes'; Unclear: most items are answered with 'unclear' Note: potentially a single item may introduce a high risk of bias, depending on study specifics |
| Outcome measurement: yes/no/unclear | |
| a. Clear definition of the outcome provided | Measurement of type 2 diabetes mellitus has to be defined |
| b. Use of adequately valid and reliable method of outcome measurement | Measurement of type 2 diabetes mellitus: a glucose (FPG, PG) or HbA1c measurement has to be a part of the diagnosis (self‐reported diabetes alone will not be accepted) |
| c. Use of same method and setting of outcome measurement in all study participants | Measurements of type 2 diabetes mellitus are the same for all study participants |
| Outcome measurement: risk of bias rating (high/low/unclear) |
High: most items are answered with 'no'; Low: all items answered with 'yes'; Unclear: most items are answered with 'unclear' Note: potentially a single item may introduce a high risk of bias, depending on study specifics |
| Study confounding: yes/no/unclear | |
| a. Measurement of all important confounders | Important confounders are: age, sex, family history of diabetes, 'ethnicity', body mass index, blood pressure and hypertension, smoking and drinking status, socioeconomic status, comedications and comorbidities, physical activity |
| b. Provision of clear definitions of the important confounders measured | Measurement of confounders has to be clearly described |
| c. Adequately valid and reliable measurement of all important confounders | Measurement of confounders is valid and reliable |
| d. Use of same method and setting of confounding measurement in all study participants | Measurements of confounders are the same for all study participants |
| e. Appropriate imputation methods used for missing confounders (if applicable) | Strategy to impute missing confounder data is described |
| f. Important potential confounders were accounted for in the study design | Methods section of the publication describes strategy to account for confounders |
| g. Important potential confounders were accounted for in the analysis | Important confounders are accounted for in multivariable logistic regression and Cox proportional hazards models |
| Study confounding measurement: risk of bias rating (high/low/unclear) |
High: most items are answered with 'no'; Low: all items answered with 'yes'; Unclear: most items are answered with 'unclear' Note: potentially a single item may introduce a high risk of bias, depending on study specifics |
| Statistical analysis and reporting: yes/no/unclear/NA | |
| a. Sufficient presentation of data to assess the adequacy of the analytic strategy | Mean or median values, including confidence intervals or standard errors or standard deviations |
| b. Strategy for model building is appropriate and based on a conceptual framework or model | NA: we do not anticipate conceptual frameworks or explicit model building strategies for this type of research question (focusing on one prognostic factor only) |
| c. Statistical model is adequate for the study design | Mainly incidence rates, uni‐ and multivariate logistic regression, Cox proportional hazard model |
| d. No selective reporting of results | NA: development of type 2 diabetes mellitus and potentially regression to normoglycaemia from intermediate hyperglycaemia are the only outcomes; if missing the study will be excluded |
| Statistical analysis and reporting: risk of bias rating (high/low/unclear) |
High: most items are answered with 'no'; Low: all items answered with 'yes'; Unclear: most items are answered with 'unclear' Note: potentially a single item may introduce a high risk of bias, depending on study specifics |
|
aNo: no or no relevant information to answer the signalling question bUnclear: not enough information to answer signalling question with yes or no cNA (not applicable): signalling question not appropriate for this type of prognostic review FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; PG: postload glucose (after an oral glucose tolerance test) | |
Appendix 4. Major cohort studies
| Cohort study acronym | Full study name |
| ADDITION | Anglo‐Danish‐Dutch study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (Rasmussen 2008) |
| — | Ansung‐Ansan Cohort Study (part of the Korean Genome and Epidemiology Study (KoGES)) ‐ (Han 2017) |
| Asturias | Asturias Study (Valdes 2008) |
| ARIC | Atherosclerosis Risk in Communities study (Warren 2017) |
| ATTICA | Province of Attica, Greece Study (Filippatos 2016) |
| AusDiab | Australian Diabetes, Obesity and Lifestyle Study (Magliano 2008) |
| BLSA | Baltimore Longitudinal Study of Aging (Meigs 2003) |
| BLSA | Beijing Longitudinal Study on Aging (Liu 2016) |
| — | Beijing Project as part of the National Diabetes Survey (Wang 2007) |
| BMES | Blue Mountains Eye Study (Cugati 2007) |
| — | Botnia Study (Lyssenko 2005) |
| — | Bruneck Study (Bonora 2011) |
| CUPS‐19 | Chennai Urban Population Study‐19 (Mohan 2008) |
| CURES | Chennai Urban Rural Epidemiology Study (Anjana 2015) |
| ChinaMUCA | China Multicenter Collaborative Study of Cardiovascular Epidemiology (Liu 2017) |
| CODAM | Cohort on Diabetes and Atherosclerosis Maastricht (Den Biggelaar 2016) |
| DESIR | Data from an Epidemiological Study on the Insulin Resistance Syndrome (Gautier 2010) |
| — | Ely Study (Forouhi 2007) |
| EPIC‐Norfolk cohort | European Prospective Investigation of Cancer Norfolk cohort (Chamnan 2011) |
| — | Finnish Cohorts of the Seven Countries Study (Stengard 1992) |
| None | Framingham Heart Study (Levitzky 2008) |
| GOS | Geelong Osteoporosis Study (De Abreu 2015) |
| Health ABC | Health, Aging, and Body Composition Study (Lipska 2013) |
| — | Hoorn Study (Rijkelijkhuizen 2007) |
| None | Hong Kong Cardiovascular Risk Factor Prevalence Study (Wat 2001) |
| IRAS | Insulin Resistance Atherosclerosis Study (Hanley 2005) |
| ICS | Isfahan Cohort Study (baseline survey of the Isfahan Healthy Heart Program) (Sadeghi 2015) |
| IDPS | Isfahan Diabetes Prevention Study (Janghorbani 2015) |
| Israel GOH Study | Israel Study of Glucose Intolerance, Obesity and Hypertension (Bergman 2016) |
| ILSA | Italian Longitudinal Study on Aging (Motta 2010) |
| — | Japanese American Community Diabetes Study (McNeely 2003) |
| JPHC Study | Japanese Public‐Health Center‐based prospective (Diabetes) Study (Noda 2010) |
| — | Kansai Healthcare Study (Sato 2009) |
| — | Kinmen Study (Li 2003) |
| KORA S4/F4 | Kooperative Gesundheitsfroschung in der Region Augsburg (Rathmann 2009) |
| KoGES | Korean Genome Epidemiology Study‐Kangwha Study (Song 2015) |
| — | Kurihashi Lifestyle Cohort Study (Nakagami 2016) |
| — | Mexico City Diabetes Study (Ferrannini 2009) |
| MESA | Multi‐Ethnic Study of Atherosclerosis (Yeboah 2011) |
| — | Nauru Study (Dowse 1991) |
| — | Paris Prospective Study (Charles 1997) |
| — | Pima Indian Study (Gila River Indian Community) (Wheelock 2016) |
| — | Pizarra study (Soriguer 2008) |
| PIFRECV | Programa de Investigación de Factores de Riesgo de Enfermedad Cardiovascular (Leiva 2014) |
| — | Rotterdam study (Ligthart 2016) |
| SALSA | Sacramento Area Latino Study on Aging (Garcia 2016) |
| SAHS | San Antonio Heart Study (Lorenzo 2003) |
| — | San Luis Valley Diabetes Study (Marshall 1994) |
| — | Singapore Impaired Glucose Tolerance Follow‐up Study (Wong 2003) |
| SIMES | Singapore Malay Eye Study (Man 2017) |
| SDPP | Stockholm Diabetes Prevention Programme (Alvarsson 2009a) |
| SHS | Strong Heart Study (Wang 2011) |
| — | Study within the WHO‐assisted National Diabetes Programme (Schranz 1989) |
| SUNSET/HELIUS | Surinamese in the Netherlands: study on health and ethnicity/Healthy life in an urban setting (Admiraal 2014) |
| TLGS | Tehran Lipid and Glucose Study (Derakhshan 2016) |
| TOPICS | Toranomon Hospital Health Management Center Study (Heianza 2012) |
| — | Yonchon study (Shin 1997) |
| — | Zanjan Healthy Heart Study (Sharifi 2013) |
Appendix 5. Definition of normoglycaemia, intermediate hyperglycaemia and incident type 2 diabetes
| Study ID | Normoglycaemia (mmol/L or %) | Intermediate hyperglycaemia (mmol/L or %) | Incident type 2 diabetes (mmol/L or %) | OGTT measurement (glucose load) | OGTT at baseline | OGTT at follow‐up | Notes |
| Admiraal 2014 | — | IFG: FPG 5.7–6.9 | FPG ≥ 7.0; HbA1c ≥ 6.5; self‐reported diabetes | — | — | — | — |
| Aekplakorn 2006 | — | IFG: FPG ≥ 5.6 to < 7.0; IGT: 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11; diagnosis and/or receipt of antihyperglycaemic medication | 75 g | Yes | No | — |
| Ammari 1998 | — | IGT: 2‐h PG 7.8 to < 11.1 (WHO 1985) | 2‐h PG ≥ 11.1 (WHO 1985) | 75 g | Yes | Yes | — |
| Anjana 2015 | FPG < 5.6 and 2‐h PG < 7.8 | i‐IGT: 2‐h PG 7.8–11.0 and FPG > 5.6; i‐IFG: FPG 5.6–6.9 and 2‐h PG < 7.8; prediabetes: FPG 5.6–6.9 or 2‐h PG 7.8–11.0 (i‐IGT or i‐IFG or IFG/IGT) | FPG ≥ 7.0; 2‐h PG ≥ 11.1; diagnosed; antihyperglycaemic medication | 75 g | Yes | Unclear | — |
| Bae 2011 | — | HbA1c 5.7–6.4, HbA1c 6.0–6.4 | FPG ≥ 7.0; HbA1c ≥ 6.5; history of diabetes; antihyperglycaemic medication | None | None | None | — |
| Baena‐Diez 2011 | FPG < 6.1 | IFG: 6.1–6.9 | FPG ≥ 7.0 (measured twice) | — | — | — | — |
| Bai 1999 | — | IGT: 7.8 to < 11.1 (WHO 1985) | 2‐h PG ≥ 11.1 (WHO 1985) | 75 g | Yes | Yes | — |
| Bergman 2016 | FPG < 5.6 + and no antihyperglycaemic medication and 2‐h BG < 7.8 (if available) |
FPF 5.6–7.8 (7.7?); 2‐h BG 7.8–11.0 | FPG ≥ 7.8, 2‐h BG ≥ 11.1; self‐reported | 100 g | Yes | Unclear | — |
| Bonora 2011 | — | HbA1: 6.0–6.49; IFG: not defined, probably FPG 5.6–6.9 | FPG ≥ 7.0; HbA1c ≥ 6.5; diabetes treatment | 75 g | Yes | Unclear | — |
| Cederberg 2010 | — | IFG: 6.1–6.9, 2‐h PG < 7.8; IGT: FPG > 7.0, 2‐h PG 7.8 to < 11.1 (WHO 2009); elevated HbA1c: 5.7–6.4 | 2‐h PG: ≥ 11.1, confirmed by 2 OGTTs | — | — | — | Diabetes incidence and IFG/IGT not exactly defined |
| Chamnan 2011 | — | HbA1c 6.0–6.4 | HbA1c ≥ 6.5; reported physician‐diagnosed diabetes or diabetes medications; antihyperglycaemic medication; diagnosis through registers | — | — | — | — |
| Charles 1997 | — | IGT: 2‐h PG ≥ 7.8 to < 11.1 (WHO 1985) | 2‐h PG ≥ 11.1 (WHO 1985); physician diagnosed diabetes | 75 g | Yes | Yes | 2nd and 4th examination |
| Chen 2003 | FPG < 6.1 | IFG: FPG 6.1–7.0 | FPG ≥ 7.0 | — | — | — | — |
| Chen 2017 | FPG < 5.6 and 2‐h PG < 7.8 | IFG: FPG 5.6–6.9 + 2‐h PG ≤ 7.8; IGT: FPG < 5.6 + 2‐h PG 7.8–11.0; IFG/IGT: FPG 5.6–6.9 + 2‐h PG 7.8–11.0 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; previously diagnosed diabetes | 75 g | Yes | Unclear | — |
| Coronado‐Malagon 2009 | ADA 2007 | ADA 2007 (IFG/IGT: 5.6–6.9/7.8 to < 11.1) | ADA 2007 (≥ 7.0/≥ 11.1) | — | — | — | — |
| Cugati 2007 | — | IFG: FPG 5.6–6.9 (originally FPG ≥ 6.1 to < 7.0) | FPG ≥ 7.0; self‐reported diabetes history; antihyperglycaemic medication | — | — | — | — |
| De Abreu 2015 | — | IFG: 5.5–6.9 | FPG ≥ 7.0; self‐reported; antihyperglycaemic medication | — | — | — | — |
| Den Biggelaar 2016 | FPG < 6.1 and 2‐h PG < 7.8 | FPG 6.1–6.9; 2‐h PG 7.8–11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Unclear | — |
| Derakhshan 2016 | FPG ≤ 5.55 and 2‐h PG ≤ 7.77 | 5.55 ≤FPG < 7.0; 7.77 ≤ 2‐h PG ≤ 11.1; no antihyperglycaemic medication | FPG ≥ 7.0; 2‐h PG ≥ 11.1; antihyperglycaemic medication | 82.5 g | Yes | Unclear | Glucose monohydrate solution, equivalent to 75 g anhydrous glucose |
| Dowse 1991 | FPG and 2‐h PG < 7.8 | IGT: FPG < 7.8 and 2‐h PG ≥ 7.8 to < 11.1 | 2‐h PG ≥ 11.1 (WHO 1985); FPG ≥ 7.8 | 75 g | Yes | Yes | — |
| Ferrannini 2009 | — | IFG: FPG 6.1–6.9; IGT: FPG < 7.0 and 2‐h PG 7.8–11.1; i‐IFG6.1/i‐IFG5.6: 2‐h PG < 7.8 and FPG 6.1–6.9/5.6–6.1; i‐IGT/i‐IGT6.1/i‐IGT5.6 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | — |
| Filippatos 2016 | — | IFG5.6: FBG 5.6–6.9 | FBG > 6.9; antihyperglycaemic medication | None | None | None | — |
| Forouhi 2007 | FPG < 5.6 | IFG6.1: FPG 6.1–6.9 (FPG < 7.0 and 2‐h PG < 11.1) (all) IFG5.6: FPG 5.6–6.9 |
FPG ≥ 7.0; 2‐h PG ≥ 11.1; doctor diagnosis or treatment for diabetes | 75 g | Yes | Yes | — |
| Garcia 2016 | — | Prediabetes: FBG 5.6–6.9 | FPG ≥ 7.0; self‐reported; antihyperglycaemic medication; diabetes comedication of death | — | — | — | — |
| Gautier 2010 | — | IFG: FPG 5.6–6.9 | FPG ≥ 7.0; treatment for diabetes (at one of the 3‐yearly examinations) | — | — | — | — |
| Gomez‐Arbelaez 2015 | — | IFG: ≥ 5.6 to < 7.0; IGT: ≥ 7.8 to < 11.1; HbA1c ≥ 5.7 to ≤ 6.4 | FPG ≥ 7.0; OGTT ≥ 11.1; HbA1c ≥ 6.5 | OGTT | Yes | Yes | OGTTs from hospital's database |
| Guerrero‐Romero 2006 | FPG < 6.1 and 2‐h PG < 7.8 | IGT: 2‐h PG ≥ 7.8 to < 11.1 | 2‐h PG: ≥ 11.1 | OGTT | Yes | Yes | OGTT: as baseline and each year during the 5‐year follow‐up |
| Han 2017 | FPG < 5.6 and 2‐h PG < 7.8 | IFG: FPG 5.6–6.9 and no diagnosis of diabetes IGT: 2‐h PG 7.8 to < 11.1 i‐IFG5.6: IFG without IGT i‐IGT: IGT without IFG IGT, IGT: IFG + IGT 'Prediabetes': IFG or IGT |
FPG ≥ 7.0; 2‐h PG ≥ 11.1; HbA1c ≥ 6.5; current antihyperglycaemic treatment | 75 g | Yes | Yes | OGTT was performed every 2 years |
| Hanley 2005 | — | IFG,IGT (WHO 1999) | Unclear | 75 g | Yes | No | — |
| Heianza 2012 | Absence of IFG or elevated HbA1c | IFG: FPG 5.6–6.9 or FPG 6.1–6.9; HbA1c 5.7–6.4 or 6.0–6.4; IFG/HbA1c = 'prediabetes' | FPG ≥ 7.0; HbA1c ≥ 6.5%; self‐reported clinician‐diagnosed diabetes | — | — | — | — |
| Inoue 1996 | — | IGT: ≥ 7.8 to < 11.1 (presumed WHO 1985) | IGT: ≥ 11.1(presumed WHO 1985) | 75 g | Yes | Yes | OGGT was performed every year |
| Janghorbani 2015 | FPG < 5.6 and 2‐h PG < 7.8 | i‐IGT: FPG < 5.6 and 2‐h PG 7.8–11.1; i‐IFG: 5.6–6.9 and 2‐h PG < 7.8; IFG/IGT: 5.6–6.9 and 2‐h PG 7.8–11.1 | FPG ≥ 11.1; antihyperglycaemic medication; 2nd FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | — |
| Jaruratanasirikul 2016 | FPG < 5.6 | i‐IGT: FPG < 5.6 and 2‐h PG 7.8 to < 11.1 | FPG > 7.0; 2‐h PG ≥ 11.1 | 1.75 g/kg (maximum 75 g) glucose solution | Yes | No | — |
| Jeong 2010 | — | IFG: FPG ≥ 5.6 to < 7.0; IGT: 2‐h PG ≥ 7.8 to < 11.1: 'prediabetes': IFG or IGT | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | — |
| Jiamjarasrangsi 2008a | — | IFG: FPG ≥ 5.6 to < 7.0 | FPG ≥ 7.0 | — | — | — | — |
| Kim 2005 | FPG < 5.0 | IFG6.1: FPG 6.1 to < 7.0 (group 4, = 276) IFG5.6: FPG 5.6 to < 6.1 |
FPG ≥ 7.0; antihyperglycaemic treatment | — | — | — | — |
| Kim 2008 | — | IFG5.6: FPG 5.6–7.0; IFG6.1: FPG 6.1–7.0 | FPG ≥ 7.0 | — | — | — | — |
| Kim 2014 | — | i‐IFG: FPG 5.6–6.9 and 2‐h PG < 7.8; i‐IGT: 2‐h PG 7.8–11.1 and FPG < 5.6; IFG/IGT: combined glucose intolerance; HbA1c: 5.7–6.4 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; HbA1c ≥ 6.5 | 75 g | Yes | Unclear | — |
| Kim 2016a | — | FPG 5.6–6.9; HbA1c 5.7–6.4 | FPG ≥ 7.0; HbA1c ≥ 6.5; antihyperglycaemic medications | — | — | — | — |
| Kleber 2010 | — | IGT: 2‐h PG > 7.7: IFG: FPG ≥ 5.5 (WHO definition) | ADA 2000 | 1.75 g/kg body weight (maximum 75 g) flavoured glucose | Yes | — | |
| Kleber 2011 | — | IGT: not reported (presumed 7.8–11.1) | "ADA" (2000 criteria, 2‐h PG ≥ 11.1) | 1.75 g/kg body weight (max. 75 g) | Yes | Yes | |
| Ko 1999 | WHO/NDDG 1979 | WHO/NDDG 1979 | WHO/NDDG 1979 | — | Yes | Yes | — |
| Ko 2001 | FPG < 6.1 | IFG: FPG 6.1–6.9 | FPG ≥ 7.0 | 75 g | Yes | Yes | Annual OGTTs |
| Larsson 2000 | FPG < 5.3 and 2‐h BG < 7.8 | i‐IFG: BG 5.3–5.9 and 2‐h BG < 7.8; i‐IGT: FPG < 5.3 and 2‐h BG 7.8–11.0; IFG/IGT: BG 5.3–5.9 and 2‐h BG 7.8–11.0 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | NGT at baseline vs follow‐up: FPG < 5.3 vs < 6.1; FPG 5.3: 15% conversion factor as recommended by the WHO (blood glucose > plasma glucose) |
| Latifi 2016 | — | 5.6 ≤ FPG < 7.0 | FPG ≥ 7.0; antihyperglycaemic medication | — | — | — | — |
| Lecomte 2007 | FPG < 6.1; no personal history of diabetes; no hypoglycaemic treatment | IFG6.1: FPG 6.1–6.9; no personal history of diabetes; no hypoglycaemic treatment | FPG ≥ 7.0; personal history of diabetes; hypoglycaemic treatment | — | — | — | — |
| Lee 2016 | — | HbA1c 5.7–6.4 | HbA1c ≥ 6.5 | — | — | — | — |
| Leiva 2014 | — | IFG: 5.6–7.0 (low range: 5.6–6.1; high range: 6.1–6.9) | FPG ≥ 7.0 (2 cons. days), HbA1c ≥ 6.5 | — | — | — | — |
| Levitzky 2008 | — | IFG5.6: FPG 5.6–6.9; IFG6.1: FPG 6.1–6.9 | FPG ≥ 7.0; antihyperglycaemic medication | — | — | — | — |
| Li 2003 | FPG < 6.1 and 2‐h PG < 7.8 | i‐iFG:FPG 6.1–7.0 and 2‐h PG < 7.8; i‐IGT: FPG < 6.1 and 2‐h PG 7.8–11.1; IFG/IGT: FPG 6.1–7.0 and 2‐h PG 7.8–11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.0; antihyperglycaemic medications | 75 g | Yes | Yes | — |
| Ligthart 2016 | FBG ≤ 6.0 | FBG > 6.0 and < 7.0; non‐fasting BG > 7.7 and < 11.1 | FBG ≥ 7.0; non‐fasting BG ≥ 11.1; antihyperglycaemic medication | — | — | — | — |
| Lipska 2013 | FPG < 5.6 and HbA1c < 5.7 | i‐IFG: FPG 5.6–6.9 and HbA1c < 5.7; i‐HbA1c: 5.7–6.4 and FPG > 5.6; IFG and HbA1c: FPG 5.6–6.9 and HbA1c 5.7–6.4 | Single HbA1c ≥ 6.5 (years 2,6,7); self‐report of physician diagnosis (annually); antihyperglycaemic agent (years 1,2,4,6,7) | — | — | — | — |
| Liu 2008 | — | IFG 5.6–6.9 | FPG ≥ 7.0; 2‐h PG ≥ 11.0; antihyperglycaemic medication | — | — | — | — |
| Liu 2014 | WHO | IFG; IGT (WHO) | WHO | 75 g | Yes | Unclear | — |
| Liu 2016 | — | FPG 6.1–6.9 | FPG ≥ 7.0; self‐reported; antihyperglycaemic medication | — | — | — | — |
| Liu 2017 | FPG 3.9–5.5 | FG 5.6–6.9 | FG ≥ 7.0; using insulin/hypoglycaemic agents; self‐reported | — | — | — | — |
| Lorenzo 2003 | — | IFG: FPG 6.1–6.9; IGT: 2‐h PG 7.8 to < 11.1(WHO 1999) | FPG: ≥ 7.0; 2‐h PHG: ≥ 11.1 (WHO 1999/1985) | 75 g | Yes | Yes | — |
| Lyssenko 2005 | FPG < 6.1 | IFG: FPG ≥ 6.1; WHO 1999 criteria | WHO 1999 criteria | 75 g | Yes | Yes | — |
| Magliano 2008 | FPG < 6.1 and 2‐h PG < 7.8 | IFG: FPG 6.1–6.9 and 2‐h PG < 7.8; IGT: FPG < 7.0 and 2‐h PG ≤ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; current antihyperglycaemic medication | 75 g | Yes | Yes | — |
| Man 2017 | Not 'prediabetes', not diabetes | HbA1c 5.7–6.4; no self‐reported diabetes or antihyperglycaemic medication | Random glucose ≥ 11.1 or HbA1c > 6.4; self‐reported history or antihyperglycaemic medication | — | — | — | — |
| Marshall 1994 | — | IGT: 2‐h PG ≥ 7.8 to < 11.1 (WHO 1985) | 2‐h PG ≥ 11.1 (WHO 1985) | 75 g | Yes | Yes | — |
| McNeely 2003 | — | IFG: FPG ≥ 6.1 to < 7.0; IGT: 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; antihyperglycaemic medication prescribed by a physician | 75 g | Yes | Yes | — |
| Meigs 2003 | FPG < 6.1 and 2‐h PG ≤ 7.8 | IFG: FPG 6.1–6.9 and 2‐h PG ≤ 7.8; IGT: FPG < 6.1 and 2‐h PG 7.8–11.0; IFG/IGT | FPG ≥ 7.0; 2‐h PG ≥ 11.1 (IFG‐IGT person: diabetes defined by OGTT) | Before 07/1977: 1.75 g glucose/kg BW, average 143 g; from 07/1977: 40 g/kg body surface area, average 78 g (men) and 68 g (women) | Yes | Yes | Serial OGTTs over subsequent biennial examinations |
| Mohan 2008 | — | IFG: FPG ≥ 6.1 to < 7; IGT: 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | — |
| Motala 2003 | Both FPG and 2‐h PG < 7.8 (WHO 1985) | IGT: FPG < 7.8 and 2‐h PG 7.8 to < 11.1 (WHO 1985) | FPG ≥ 7.8; 2‐h PG ≥ 11.1 (WHO 1985) | 75 g glucose monohydrate dissolved in 250 mL of water (modified OGTT) | Yes | Yes | — |
| Motta 2010 | FPG < 6.1 | IFG: 6.1 to < 7.0 | FPG ≥ 7.0 | — | Yes | — | |
| Mykkänen 1993 | FPG and 2‐h PG < 7.8 | IGT: FPG < 7.8 and 2‐h PG 7.8–11.1 (WHO 1985) | FPG ≥ 7.8; 2‐h PG ≥ 11.1 (WHO 1985) | 75 g | Yes | Yes | — |
| Nakagami 2016 | — | HbA1c 5.7–6.4, FPG 5.5–6.9 | FPG ≥ 7.0, HbA1c ≥ 6.5; physician diagnosis of diabetes | — | — | — | — |
| Nakanishi 2004 | FPG < 6.1 | IFG: FPG 6.1–6.9 | FPG ≥ 7.0; antihyperglycaemic medication | — | — | — | — |
| Noda 2010 | — | Taken from table 2: FPG levels: IFG 5.6 and 6.1 | FPG ≥ 7.0; HbA1c ≥ 6.1%; self‐reported | — | — | — | — |
| Park 2006 | — | IFG: FPG ≥ 5.6 | FPG ≥ 7.0 | — | — | — | — |
| Peterson 2017 | FPG < 6.1 and 2‐h PG < 7.8 | IGT: FPG < 7.0 and 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | — | Yes | Yes | 2 standardised OGTT at baseline with about 1 week's interval to verify glucose status |
| Qian 2012 | FPG < 6.1 and 2‐h PG < 7.8 | i‐IFG: 6.1–6.9 and 2‐h PG < 7.8; i‐IGT: < 6.1 and 2‐h PG 7.8–11.0; IFG/IGT: 6.1–6.9 and 2‐h PG 7.8–11.0 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Unclear | — |
| Rajala 2000 | 2‐h PG < 7.8 | IGT: 2‐h PG 7.8 to < 11.1 | 2‐h PG ≥ 11.1; 2x FPG ≥ 6.7 | 75 g | Yes | Yes | New cases identified by OGTTs in 1994 and 1996–8 |
| Ramachandran 1986 | — | IGT: 7.8–11.0 (presumed NDDG 1979) | 2‐h PG > 11.0 (presumed NDDG 1979) | 75 g | Yes | Yes | — |
| Rasmussen 2008 | — | IFG (i‐IFG): FBG 5.6 to < 6.1 and 2‐h BG < 7.8; IGT (i‐IGT): FBG < 6.1 and 2‐h BG 7.8 to < 11.1; IFG/IGT | FBG ≥ 6.1 or 2‐h BG ≥ 11.1 | 75 g | Yes | Unclear | — |
| Rathmann 2009 | WHO 1999 | IFG: FPG 6.1–6.9; IGT: 2‐h PG 7.8 to < 11.1; 'prediabetes': i‐IFG, i‐IGT and IFG/IGT | FPG ≥ 7.0; 2‐h PG ≥ 11.1; validated physician diagnosis | 75 g | Yes | Yes | — |
| Rijkelijkhuizen 2007 | ADA 1997/2003 | IFG5.6: FPG 5.6–7.0; IFG6.1: FPG 6.1–7.0; IGT: 2‐h PG 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG: ≥ 11.1 | 75 g | Yes | Yes | — |
| Sadeghi 2015 | — | IFG: FPG ≥ 5.5 and < 7.0; IGT: 2‐h OGTT ≥ 7.8 and < 11.1 | FPG > 7.0; 2‐h OGTT > 11.1; IFG/IGT; antihyperglycaemic medication | — | Yes | Yes | — |
| Sasaki 1982 | FPG < 7.8 and 2‐h PG < 7.8 (WHO 1980) | IGT: FPG < 7.8 and 2‐h PG 7.8–11.1 (WHO 1980) | FPG ≥ 7.8 or 2‐h PG ≥ 11.1 (WHO 1980) | 50 g | Yes | Yes | — |
| Sato 2009 | — | (Table 1): IFG: FPG group 6.1–6.9; HbA1c‐group: 6.0–6.4 | FPG ≥ 7.0; antihyperglycaemic medication | — | — | — | — |
| Schranz 1989 | — | IGT: 2‐h PG ≥ 7.8 to < 11.1 (WHO 1985) | 2‐h PG ≥ 11.1 (WHO 1985) | OGTT | Yes | Yes | — |
| Sharifi 2013 | — | FPG 5.6–7.0 | FPG > 7.0 (2 measurements); diabetes diagnosis based on documents | OGTT | Yes (twice) | — | — |
| Shin 1997 | — | Assumed WHO 1985 criteria | "WHO criteria"; antihyperglycaemic medication | 75 g | Yes | Yes | — |
| Söderberg 2004 | — | IFG: FPG ≥ 6.1 to < 7.0 and 2‐h PG < 7.8; IGT: FPG < 7.0 and 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | — |
| Song 2015 | — | IFG: FPG 5.6–6.9 | FPG ≥ 7.0; HbA1c ≥ 6.5; antihyperglycaemic medication | — | — | — | — |
| Song 2016a | — | IFG: FG 5.6–6.9; IGT: 2‐h G 7.8–11.0 | FG ≥ 7.0; 2‐h G ≥ 11.0; HbA1c ≥ 6.5; self‐reported | 75 g | Yes | Yes | 100 g steamed bread at follow‐up |
| Soriguer 2008 | BG < 5.6 and 2‐h BG < 7.8 | IFG: BG 5.6–6.1 and 2‐h BG < 7.8; IGT: BG < 5.6 and 2‐h BG 7.8–11.1 | BG > 6.1 or 2‐h BG > 11.1 | 75 g | Yes | Yes | — |
| Stengard 1992 | — | IGT: 2‐h PG 7.8–11.1 | 2‐h PG ≥ 11.1 (WHO 1985); antihyperglycaemic medications | 75 g | Yes | Yes | — |
| Toshihiro 2008 | FPG < 6.1 and 2‐h PG < 7.8 | IFG: FPG 6.1–6.9 and 2‐h PG < 7.8; IGT: FPG < 7.0 and 2‐h PG 7.8–11.1 | FPG ≥ 7.0; 2‐h PG > 11.1; non‐fasting PG > 11.1 | 75 g | Yes | Yes | Annual OGTT during the observation period (3.2 years) |
| Vaccaro 1999 | FPG < 5.6; 2‐h PG < 6.7; 2‐h PG < 6.7 | IFG: FPG 5.6–6.0; IGT: 2‐h PG 6.7–9.9 | FPG> 6.1; antihyperglycaemic medications; 2‐h PG ≥ 10.0 | 75 g | Yes | No | Retrospective classification; note thresholds (whole blood) |
| Valdes 2008 | FPG < 5.6 | IFG5.6: 5.6–6.1; IFG6.1: 6.1–6.9 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; clinical diabetes diagnosis; antihyperglycaemic medication, diet | 75 g | Yes | Yes | — |
| Vijayakumar 2017 | — | FG 5.6–6.9; 2‐h PG 7.8–11.9; HbA1c 5.7–6.4 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; previous clinical diagnosis | 75 g | Yes | Yes | HbA1c new method = −0.1916 + (0.9829 × HbA1c old method) |
| Viswanathan 2007 | FPG and 2‐h PG < 6.1 and < 7.8 | IGT: 2‐h PG 7.8 to < 11.1 | Not defined, presumably by OGTT | 75 g | Yes | Yes | All participants underwent a second OGTT to confirm the diagnosis in order to be included in the study; follow‐up: a reminder letter was sent every 6 months to participants to undergo an OGTT |
| Wang 2007 | — | IFG: FPG 6.1–6.9; IGT: 2‐h PG 7.8–11.0 | FPG ≥ 7.0; 2‐h PG ≥ 11.1 | 75 g | Yes | Unclear | — |
| Wang 2011 | FPG < 5.6; HbA1c < 6.0; no FPG/HbA1c | IFG: 5.6 to < 7.0; HbA1c 6.0 to < 6.5 | Diabetes status: FPG ≥ 7.0; antihyperglycaemic medication; HbA1c ≥ 6.5, antihyperglycaemic medication; FPG/HbA1c: ≥ 6.5 or FPG ≥ 7.0 or antihyperglycaemic medication | — | — | — | — |
| Warren 2017 | — | FPG 5.6–6.9 (ADA); FG 6.1–6.9 (WHO); 2‐h 7.8–11.0 (ADA); HbA1c 5.7–6.4 (ADA); 6.0–6.4 (IEC) | Self‐report of physician diagnosis; antihyperglycaemic medication reported during a study visit or annual telephone call | 75 g | Yes (visit 4) | Unclear | — |
| Wat 2001 | FPG and 2‐h PG < 7.8 | IGT: FPG < 7.8 and 2‐h PG 7.8 to < 11.1 | FPG ≥ 7.8; 2‐h PG ≥ 11.1 | 75 g | Yes | Yes | — |
| Weiss 2005 | FPG < 5.6 and 2‐h PG < 7.8 | IGT: FPG < 5.6 and 2‐h PG 7.8–11.1 | FPG ≥ 7.0; 2‐h PG > 11.1; presentation of hyperglycaemia (more than 2 random glucose measurements > 11.1), glucosuria, polydipsia, and polyuria | 1.75 g/kg body weight flavoured glucose orally (up to a maximum of 75 g) | Yes | Yes | OGTT was repeated every 18–24 months |
| Wheelock 2016 | — | IGT: 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; previous diagnosis | 75 g | Yes | Unclear | Modified OGTT |
| Wong 2003 | — | IGT: 2‐h PG ≥ 7.8 to < 11.1 | FPG ≥ 7.0; 2‐h PG ≥ 11.1; physician diagnosed diabetes | 75 g | Yes | Yes | — |
| Yeboah 2011 | FPG < 5.6 | IFG: FPG 5.6–6.9 | FPG > 6.9; antihyperglycaemic medication during examinations 2,3, 4 | — | — | — | — |
| Zethelius 2004 | — | IGT: 2‐h PG 7.8 to < 11.1 | FPG ≥ 7.0; antihyperglycaemic medications | 75 g | Yes | No | — |
| BG: blood glucose; BW: body weight; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; i‐IFG: (isolated) impaired fasting glucose; i‐IGT: (isolated) impaired glucose tolerance; IFG/IGT: both impaired fasting glucose and impaired glucose tolerance; NDDG: National Diabetes Data Group; NGT: normal glucose tolerance; OGTT: oral glucose tolerance test; PG: postload glucose; WHO: World Health Organization | |||||||
Appendix 6. Number of participants with and without intermediate hyperglycaemia at baseline
| Study ID | N participants with/without IH | Definitions of IH at baseline | |||||
| 'Prediabetes'a (%) | Elevated HbA1c (%) | IFG (%) | IGT (%) | IFG/HbA1c (%) | IFG/IGT (%) | ||
| Admiraal 2014 | IFG5.6 total: 111/456 | — | — | IFG5.6: Total 24.3 South‐Asian Surinamese 34.4 African Surinamese 21.1 "Ethnic Dutch" 22.7 | — | — | — |
| Aekplakorn 2006 | IFG5.6: 223/2667 | — | — | IFG5.6: 8.4 | — | — | — |
| Ammari 1998 | IGT: 68 | — | — | — | 100 | — | — |
| Anjana 2015 | 'Prediabetes' (i‐IFG, i‐IGT or both): 299/1376 | 21.7 | — | i‐IFG5.6: 4.9 | i‐IGT: 11.8 | — | 5.0 |
| Bae 2011 | HbA1c5.7: 1791/9723; HbA1c6.0: 412/1791 | — | HbA1c5.7: 18.4 HbA1c6.0: 4.2 | — | — | — | — |
| Baena‐Diez 2011 | IFG6.1: 115 | — | — | IFG6.1: 100 | — | — | — |
| Bai 1999 | IGT: 252/696 | — | — | — | 36.2 | — | — |
| Bergman 2016 | IGT: 68/853 | — | — | — | 8 | — | — |
| Bonora 2011 | HbA1c6.0: 70/842 | — | 8.3 | — | — | — | — |
| Cederberg 2010 | IFG6.1: 40/553 IGT: 103/553 IFG/IGT: 15/553 | — | — | IFG6.1: 7.2 | 18.7 | — | 2.7 |
| Chamnan 2011 | HbA1c6.0: 370/5735 | — | HbA1c6.0: 6.5 | — | — | — | — |
| Charles 1997 | IGT: 418/4089; i‐IFG6.1: 476/5042 | — | — | i‐IFG6.1: 9.4 | 10.2 | — | — |
| Chen 2003 | IFG6.1: 156/600 | — | — | IFG6.1: 26 | — | — | — |
| Chen 2017 | i‐IFG5.6: 329/1347 i‐IGT: 192/1347 IFG/IGT: 209/1347 | — | — | i‐IFG5.6: 24.4 | i‐IGT: 14.2 | 15.5 | — |
| Coronado‐Malagon 2009 | 'Prediabetes': 217/656 | 33.1 | — | — | — | — | — |
| Cugati 2007 | IFG5.6: 244/2123 | — | — | IFG5.6: 11.5 | — | — | — |
| De Abreu 2015 | IFG5.6: 187/1167 | — | — | IFG5.6: 16 | — | — | — |
| Den Biggelaar 2016 | IFG6.1 and/or IGT: 122/476 | 25.6 | — | — | — | — | — |
| Derakhshan 2016 | IFG5.6 and/or IGT: 523/8231 | IFG5.6 and/or IGT: 6.4 | — | — | — | — | — |
| Dowse 1991 | IGT: 105/1201 | — | — | — | 8.7 | — | — |
| Ferrannini 2009 | i‐IFG5.6: 65/1941 i‐IFG6.1: 17/1941 IGT: 179/1941 i‐IGT(IFG5.6): 57/1941 i‐IGT(IFG6.1): 29/1941 | — | — | i‐IFG5.6: 3.3 i‐IFG6.1: 0.9 | IGT: 9.2 i‐IGT5.6: 2.9 i‐IGT6.1: 1.5 | — | — |
| Filippatos 2016 | IFG5.6: 279/1485 | — | — | IFG5.6: 18.8 | — | — | — |
| Forouhi 2007 | IFG6.1: 257/1040 IFG5.6: 633/1040 |
— | — | IFG5.6: 60.9 IFG6.1: 24.7 |
— | — | — |
| Garcia 2016 | IFG5.6: 310/1777 | — | — | IFG5.5: 17.5 | — | — | — |
| Gautier 2010 | IFG5.6: 979 | — | — | IFG5.6: 100 | — | — | — |
| Gomez‐Arbelaez 2015 | 'Prediabetes': 186/772 (Men: 61/772, women: 125/772) | 24.1 | — | — | — | — | — |
| Guerrero‐Romero 2006 | IGT: 75/375 | — | — | — | 20 | — | — |
| Han 2017 | i‐IFG5.6: 199/7542 i‐IGT: 1512/7542 IFG/IGT: 198/7542 |
— | — | i‐IFG5.6: 2.6 | i‐IGT: 20.0 | — | 2.6 |
| Hanley 2005 | IGT: 274/882 | — | — | — | 31.6 | — | — |
| Heianza 2012 | IFG5.6: 1680/6241 IFG6.1: 380/6241 HbA1c5.7: 822/6241 HbA1c6.0: 203/6241 IFG5.6/HbA1c5.7: 2092/6241 | — | HbA1c5.7: 13.2 HbA1c6.0: 3.3 | IFG5.6: 26.9 IFG6.1: 6.1 | — | 33.5 | — |
| Inoue 1996 | IGT: 37 | — | — | — | 100 | — | — |
| Janghorbani 2015 | i‐IFG5.6: 304/1530 i‐IGT: 198/1530 IFG/IGT: 268/1530 | — | — | i‐IFG5.6: 19.9 | i‐IGT: 12.9 | — | 17.5 |
| Jaruratanasirikul 2016 | i‐IGT: 27/177 | — | — | — | i‐IGT: 15.3 | — | — |
| Jeong 2010 | IFG5.6: 16% IGT: 5.3% | — | — | IFG5.6: 16 | 5.3 | — | — |
| Jiamjarasrangsi 2008a | IFG5.6: 320/2370 | — | — | IFG5.6: 13.5 | — | — | — |
| Kim 2005 | IFG6.1: 276/2964 | — | — | IFG6.1: 9.3 | — | — | — |
| Kim 2008 | IFG total: 1829/7211 IFG5.6: 1335/7211 IFG6.1: 494/7211 | — | — | IFG total: 25.4 IFG5.6: 18.5 IFG6.1: 6.9 | — | — | — |
| Kim 2014 | i‐IFG5.6: 158/406 i‐IGT: 65/406 IFG/IGT: 119/406 i‐HbA1c5.7: 64/406 | — | i‐HbA1c5.7: 15.8 | i‐IFG5.6: 38.9 | i‐IGT: 16 | — | 29.3 |
| Kim 2016a | IFG5.6: 3544/17971 HbA1c5.7: 1713/17971 IFG5.6/HbA1c5.7: 1951/17971 | — | HbA1c5.7: 9.5 | IFG5.6: 19.7 | — | 10.9 | — |
| Kleber 2010 | IGT: 79 | — | — | — | 100 | — | — |
| Kleber 2011 | IGT: 119 | — | — | — | 100 | — | — |
| Ko 1999 | IGT: 123 | — | — | — | 100 | — | — |
| Ko 2001 | IFG6.1: 55/319 | — | — | IFG6.1: 17.2 | — | — | — |
| Larsson 2000 | i‐IFG6.1: 42/265 i‐IGT: 66/265 IFG/IGT: 30/265 | — | — | i‐IFG6.1: 15.8 | i‐IGT: 24.9 | — | 11.3 |
| Latifi 2016 | IFG5.6: 124/593 | — | — | IFG5.6: 20.9 | — | — | — |
| Lecomte 2007 | IFG6.1: 743 | — | — | IFG6.1: 100 | — | — | — |
| Lee 2016 | HbA1c5.7: 3497 | — | HbA1c5.7: 100 | — | — | — | — |
| Leiva 2014 | IFG6.1: 28/94 | — | — | IFG6.1: 29.8 | — | — | — |
| Levitzky 2008 | Not reported | — | — | — | — | — | — |
| Li 2003 | i‐IFG6.1: 42/644 i‐IGT: 118/644 IFG/IGT: 49/644 | — | — | i‐IFG6.1: 6.5 | i‐IGT: 18.3 | — | 7.6 |
| Ligthart 2016 | IFG6.1: 1382/10,050 | — | — | IFG6.1: 13.8 | — | — | — |
| Lipska 2013 | IFG5.6: 189/1690 i‐HbA1c5.7: 207/1690 IFG/HbA1c: 169/1690 | — | i‐HbA1c: 12.2 | IFG5.6: 11.2 | — | 10.0 | — |
| Liu 2008 | IFG5.6: 169/1844 | — | — | IFG5.6: 9.2 | — | — | — |
| Liu 2014 | 'Prediabetes' (IFG or IGT): 450/2271 | 19.8 | — | — | — | — | — |
| Liu 2016 | IFG6.1: 222/1857 | — | — | IFG6.1: 12.0 | — | — | — |
| Liu 2017 | IFG5.6: 3607/18610 | — | — | IFG5.6: 19.4 | — | — | — |
| Lorenzo 2003 | IFG6.1: 29/1734 IGT: 202/1734 | — | — | IFG6.1: 1.7 | 11.6 | — | — |
| Lyssenko 2005 | i‐IFG6.1: 263/2115 i‐IGT: 250/2115 IFG/IGT: 173/2115 | — | — | i‐IFG6.1: 12.4 | i‐IGT: 11.8 | — | 8.2 |
| Magliano 2008 | Not reported | — | — | — | — | — | — |
| Man 2017 | HbA1c5.7: 675/1137 | — | HbA1c5.7: 59.4 | — | — | — | — |
| Marshall 1994 | IGT: 123 | — | — | — | 100 | — | — |
| McNeely 2003 | 5–6 years: IFG6.1: 30/465 IGT: 178/465 10 years: IFG6.1: 28/412 IGT: 157/412 | — | — | 5–6 years: IFG6.1: 6.5 10 years: IFG6.1: 6.8 | 5–6 years: 38.3 10 years: 38.1 | — | — |
| Meigs 2003 | i‐IFG5.6: 126/753
i‐IGT(IFG5.6): 115/753
IFG5.6/IGT: 103/753 i‐IFG6.1: 20/753 i‐IGT(IFG6.1): 218/753 IFG6.1/IGT: 27/753 |
— | — | i‐IFG5.6: 16.7 i‐IFG6.1: 2.7 | i‐IGT5.6: 15.3 i‐IGT6.1: 29 |
— | IFG5.6/IGT: 13.7 IFG6.1/IGT: 3.6 |
| Mohan 2008 | IGT: 37/513 | — | — | — | 7.2 | — | — |
| Motala 2003 | IGT: 35/563 | — | — | — | 6.2 | — | — |
| Motta 2010 | IFG6.1: 295/2603 | — | — | IFG6.1: 11.3 | — | — | — |
| Mykkänen 1993 | IGT: 203/892 | — | — | — | 22.8 | — | — |
| Nakagami 2016 | IFG5.6: 467/2267 IFG6.1: 134/2267 HbA1c5.7: 583/2267 HbA1c6.0: 156/2267 |
— | HbA1c5.7: 25.7 HbA1c6.0: 6.9 |
IFG5.6: 20.6 IFG6.1: 5.9 |
— | — | — |
| Nakanishi 2004 | IFG6.1: 246/5588 | — | — | IFG6.1: 4.4 | — | — | — |
| Noda 2010 | IGF5.6: 558/2207 IFG6.1: 153/2207 | — | — | IFG5.6: 25.3 IFG6.1: 6.9 | — | — | — |
| Park 2006 | IFG5.6: 321/5296 | — | — | IFG5.6: 6.1 | — | — | — |
| Peterson 2017 | IGT: 29/74 | — | — | — | 39.2 | — | — |
| Qian 2012 | i‐IFG6.1: 46/1042 i‐IGT: 120/1042 IFG/IGT: 33/1042 | — | — | i‐IFG6.1: 4.4 | i‐IGT:11.5 | — | 3.2 |
| Rajala 2000 | IGT: 100 | — | — | — | 100 | — | — |
| Ramachandran 1986 | IGT: 107 | — | — | — | 100 | — | — |
| Rasmussen 2008 | i‐IFG5.6: 607/1510 i‐IGT 903/1510 | — | — | i‐IFG5.6: 40.2 | i‐IGT: 59.8 | — | — |
| Rathmann 2009 | i‐IFG6.1: 71/887 i‐IGT: 120/887 IFG/IGT: 47/887 | — | — | i‐IFG6.1: 8 | i‐IGT: 13.5 | — | 5.3 |
| Rijkelijkhuizen 2007 | IFG5.6: 488/1428 IFG6.1: 149/1428 | — | — | IFG5.6: 34.2 IFG6.1: 10.4 | — | — | — |
| Sadeghi 2015 | 'Prediabetes' (IFG5.6 and/or IGT): 373/2980 | 12.5 | — | — | — | — | — |
| Sasaki 1982 | IGT: 13/207 | — | — | — | 6.3 | — | — |
| Sato 2009 | Unclear | — | — | — | — | — | — |
| Schranz 1989 | IGT: 75/2128 | — | — | — | 3.5 | — | — |
| Sharifi 2013 | IFG5.6: 123 | — | — | IFG5.6: 100 | — | — | — |
| Shin 1997 | IGT: 153/1193 | — | — | — | 12.8 | — | — |
| Söderberg 2004 | i‐IFG6.1: 87–98: 402/6690 87–92: 149/3193 92–98: 253/3437 IGT: 87–98: 1253/6690 87–92: 600/3193 92–98: 662/3437 | — | — | i‐IFG6.1: 87–98: 6 87–92: 4.7 92–98: 7.4 | 87–98: 18.9 87–92: 18.8 92–98: 19.3 | — | — |
| Song 2015 | IFG5.6: 321/2467 | — | — | IFG5.6: 13 | — | — | — |
| Song 2016a | 'Prediabetes': 344 | 100 | — | — | — | — | — |
| Soriguer 2008 | IFG5.6: 56/714 IGT: 54/714 IFG/IGT: 28/714 | — | — | IFG5.5: 7.8 | 7.6 | — | 3.9 |
| Stengard 1992 | IGT: 234/637 | — | — | — | 36.7 | — | — |
| Toshihiro 2008 | IFG6.1: 14/128 IFG and/or IGT: 114/128 | IFG and/or IGT: 89.1 | — | IFG6.1: 10.9 | — | — | — |
| Vaccaro 1999 | i‐IFG5.6: 36/1141 i‐IGT: 861141 IFG/IGT: 11/1141 | — | — | i‐IFG5.6: 3.1 | i‐IGT: 7.5 | — | 1.0 |
| Valdes 2008 | IFG5.6: 114/630 IFG6.1: 52/630 IGT: 50/630 |
— | — | IFG5.6: 18.1 IFG6.1: 8.3 | 7.9 | — | — |
| Vijayakumar 2017 | IFG5.6 adults: 423/2005 IFG5.6 children: 193/2095 HbA1c5.7 adults: 168/2005 HbA1c5.7 children: 62/2095 IGT adults: 347/2005 IGT children: 170/2095 IFG/IGT adults: 169/2005 IFG/IGT children: 53/2095 |
— | HbA1c5.7 adults: 8.4 HbA1c5.7 children: 3.0 | IFG5.6 adults: 21.1 IFG5.6 children: 9.2 | Adults: 17.3 Children: 8.1 | — | IFG/IGT adults: 8.4 IFG/IGT children: 2.5 |
| Viswanathan 2007 | IGT: 619/1659 | — | — | — | 37.3 | — | — |
| Wang 2007 | IGT: 141/541 | — | — | — | 26 | — | — |
| Wang 2011 | i‐IGT total: 135/10 i‐IGT men: 29/447 i‐IGT women: 106/635 |
— | — | — | i‐IGT total: 12.5 i‐IGT men: 6.5 i‐IGT women: 16.7 |
— | — |
| Warren 2017 | IFG5.6: 4112/10844
IFG6.1: 1213/10844
IGT: 2009/7194
HbA1c5.7: 2027/10844 HbA1c6.0: 970/10844 |
— | HbA1c5.7: 19 HbA1c6.0: 9 |
IFG5.6: 38 IFG6.1: 11 | 28 | — | — |
| Wat 2001 | IGT: 322 | — | — | — | 100 | — | — |
| Weiss 2005 | i‐IGT(IFG5.6): 33/117 | — | — | — | i‐IGT: 28.2 | — | — |
| Wheelock 2016 | IGT: 169/5532 | — | — | — | 3.1 | — | — |
| Wong 2003 | IGT: 291 | — | — | — | 100 | — | — |
| Yeboah 2011 | IFG5.6: 940/6753 | — | — | IFG5.6: 13.9 | — | — | — |
| Zethelius 2004 | IGT: 201/667 | — | — | — | 30.1 | — | — |
|
aTerm 'prediabetes' as used by study authors (usually defined by various combinations of glycaemic status measurements, e.g. IFG and/or IGT) FG: fasting glucose; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated;IFG 5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; PG: postload glucose;IH: intermediate hyperglycaemia; T2DM: type 2 diabetes mellitus | |||||||
Appendix 7. Follow‐up time and type of outcome measurement of the development of type 2 diabetes
| Study ID | Length of follow‐up | Time‐points of measurements | Outcome measurement of the development of T2DM | Notes |
| Admiraal 2014 | 10 years | Baseline, follow‐up | Incidence, odds ratio | Data for total population/South‐Asian Surinamese/African Surinamese/"Ethnic Dutch" |
| Aekplakorn 2006 | 12 years | Baseline, follow‐up | Incidence, odds ratio | — |
| Ammari 1998 | 2 years | Baseline, follow‐up | Incidence | — |
| Anjana 2015 | Median 9.1 years (IQR 2.6) | Baseline, follow‐up | Incidence, incidence rate | — |
| Bae 2011 | 4 years (mean 47.2 months) | Baseline, follow‐up (partially annually/biannually) | Incidence, incidence rate, hazard ratio | — |
| Baena‐Diez 2011 | 10 years | Baseline, follow‐up | Incidence | — |
| Bai 1999 | 1 year | Baseline, follow‐up | Incidence | — |
| Bergman 2016 | 24 years | Baseline, follow‐up | Incidence, odds ratio | Also adjusted for fasting blood glucose; 100 g OGTT |
| Bonora 2011 | 15 years | Baseline, follow‐up (5, 10, 15 years) | Incidence, incidence rate, hazard ratio | HbA1c category used: 6.0% to 6.49% |
| Cederberg 2010 | Mean 9.7 years (SD 0.7) | Baseline, follow‐up | Incidence, risk ratio | Total incident cases = mixture of isolated and combined intermediate glycaemic conditions |
| Chamnan 2011 | Median 3 years | Baseline, follow‐up | Incidence, odds ratio | Data for HbA1c 6.0% to 6.4% group, focus on clinically and/or biochemically diagnosed diabetes |
| Charles 1997 | 2 years | Baseline, follow‐up (5 annual clinical examinations) | Incidence | — |
| Chen 2003 | 3 years | Baseline, follow‐up | Incidence, odds ratio | Also adjusted for apolipoprotein B |
| Chen 2017 | 3 years | Baseline, follow‐up | Incidence | — |
| Coronado‐Malagon 2009 | 1 and 2 years | Baseline, follow‐up | Incidence, relative risk | Results are given for year 1/year 2 of follow‐up |
| Cugati 2007 | 10 years | Baseline, follow‐up (5 and 10 years) | Incidence, odds ratio | Odds‐ratio, age‐and sex‐adjusted |
| De Abreu 2015 | 10 years | Baseline, follow‐up | Incidence, incidence rate, odds ratio | Age‐standardised incidence rate; additional covariates: metabolic syndrome, fasting glucose at baseline |
| Den Biggelaar 2016 | 7 years | Baseline, follow‐up | Incidence | — |
| Derakhshan 2016 | Median 11.7 years (IQR 8.4–13.2) | Baseline, follow‐up | Incidence rate, hazard ratio | — |
| Dowse 1991 | Approx. 5 years | Baseline, follow‐up | Incidence, incidence rate, odds ratio | Incidence rates for the periods 1975/76–1982 and 1982–1987 |
| Ferrannini 2009 | 7 years | Baseline, follow‐up | Incidence, relative risk | — |
| Filippatos 2016 | 10 years | Baseline, follow‐up (intermediate 5 ‐year follow‐up) | Incidence, odds ratio | — |
| Forouhi 2007 | 10 years | Baseline, follow‐up | Incidence, incidence rate, hazard ratio | Cumulative incidence increased across increasing age groups and was higher in men than in women |
| Garcia 2016 | Approx. 9 years | Baseline, follow‐up (every 12–15 months, max. 6 follow‐ups) | Incidence | — |
| Gautier 2010 | 9 years | Baseline, follow‐up (3‐yearly examinations) | Incidence | — |
| Gomez‐Arbelaez 2015 | Approx. 2 years | Baseline, follow‐up | Incidence, incidence rate | Rate was given in terms of per 100 person‐years (recalculated to 1000 person‐years) |
| Guerrero‐Romero 2006 | 5 years | Baseline, follow‐up | Incidence, incidence rate | — |
| Han 2017 | 12 years | Baseline, follow‐up (biannually) | Incidence, incidence rate, hazard ratio | — |
| Hanley 2005 | Average 5.2 years (range 4.5–6.6) | Baseline, follow‐up | Incidence, odds ratio | — |
| Heianza 2012 | Median 5 years | Baseline, follow‐up (annual follow‐ups) | Incidence, incidence rate, hazard ratio | Adjusted odds ratios: mean age and sex‐adjusted |
| Inoue 1996 | 2.5 years | Baseline, follow‐up | Incidence | — |
| Janghorbani 2015 | Mean 6.8 years (SD 1.7) | Baseline, follow‐up (OGTT at 3‐year intervals) | Incidence, incidence rate, hazard ratio | Date for cohort without hypertension |
| Jaruratanasirikul 2016 | 3–6 years | Baseline, follow‐up | Incidence | — |
| Jeong 2010 | 5 years | Baseline, follow‐up | Odds ratio | Also adjusted for ALAT, ASAT, γ‐GT, h‐CRP |
| Jiamjarasrangsi 2008a | Mean 2.6 years (SD 0.97) | Baseline, follow‐up (annual follow‐ups, 1–4 years) | Incidence | — |
| Kim 2005 | 5 years | Baseline, follow‐up | Incidence, hazard ratio | — |
| Kim 2008 | 2 years | Baseline, follow‐up | Incidence | — |
| Kim 2014 | Median 46 months | Baseline, follow‐up (every 3–6 months, up to 9 years) | Incidence | 81 participants were diagnosed with diabetes with a conversion rate of 20% (81/406); conversion rates are given within prediabetes groups (e.g. 24/158 i‐IFG converters = 15.2%) |
| Kim 2016a | Mean 5.2 years (range 3.1–6.7) | Baseline, follow‐up | Incidence, odds ratio | — |
| Kleber 2010 | 1 year | Baseline, follow‐up | Incidence | — |
| Kleber 2011 | Mean 3.9 years (SD 0.6) | Baseline, follow‐up | Incidence | — |
| Ko 1999 | Mean 1.4 years (range 0.9–7.6) | Baseline, follow‐up (annual OGTTs) | Incidence | — |
| Ko 2001 | Median 1.7 years | Baseline, follow‐up (annual OGTTs) | Incidence | — |
| Larsson 2000 | Mean 10 years (SD 1 year 10 months) | Baseline, follow‐up | Incidence | — |
| Latifi 2016 | Median 5 years | Baseline, follow‐up | Incidence, incidence rate, odds ratio | — |
| Lecomte 2007 | 5 years | Baseline, follow‐up | Incidence | — |
| Lee 2016 | Mean 3.7 years (SD 2.3) | Baseline, follow‐up | Incidence | — |
| Leiva 2014 | 6 years | Baseline, follow‐up | Incidence, hazard ratio | — |
| Levitzky 2008 | 4 years | Baseline, follow‐up (approx. 4‐year intervals) | Incidence, odds ratio | — |
| Li 2003 | 5 years | Baseline, follow‐up (examination every 2 years) | Incidence, incidence rate, hazard ratio | Incidence rates for 5‐year cumulative incidence; further adjustments for HOMA‐IR and HOMA beta‐cell |
| Ligthart 2016 | 14.7 years | Baseline, follow‐up (blood glucose measures approx. every 4 years) | Incidence rate | — |
| Lipska 2013 | 7 years | Baseline (year 4), follow‐up (years 5,6,7) | Incidence, odds ratio | IFG6.1: sensitivity analysis, analysis for 'ethnicity', sex analysis |
| Liu 2008 | 5 years | Baseline, follow‐up | Incidence, incidence rate, relative risk | — |
| Liu 2014 | 3 years | Baseline, follow‐up | Incidence, incidence rate | No exact definition of 'prediabetes' and diabetes incidence |
| Liu 2016 | Median 10.9 years (IQR 8.0–15.3) | Baseline, follow‐up | Hazard ratio | Subdistribution hazard ratios; also adjusted for self‐rated health |
| Liu 2017 | 7.8 years | Baseline, follow‐up | Odds ratio | — |
| Lorenzo 2003 | 7–8 years | Baseline, follow‐up | Incidence, odds ratio | Also adjusted for NCEP metabolic syndrome definition, fasting insulin |
| Lyssenko 2005 | Median 6 years (range 2–12) | Baseline, follow‐up (every 2–3 years) | Incidence, hazard ratio | 1372 persons 1 visit, 392 persons 2 visits, 219 persons 3 visits, 132 persons 4 visits |
| Magliano 2008 | 5 years | Baseline, follow‐up | Incidence, incidence rate, odds ratio | 5‐year cumulative incidence rate was standardised to the 1998 Australian population (age and sex‐specific incidence rates) |
| Man 2017 | 6 years | Baseline, follow‐up | Incidence, incidence rate, risk ratio | Male: female, age standardised rate |
| Marshall 1994 | Mean 22.6 months (range 11–40) | Baseline, follow‐up | Incidence | — |
| McNeely 2003 | 10 years | Baseline, follow‐up (5–6 years and 10 years) | Incidence | — |
| Meigs 2003 | 5 years, 10 years | Baseline, follow‐up (3 to 10 biennial examinations) | Incidence, incidence rate | — |
| Mohan 2008 | Mean 8 years (SD 1.3) | Baseline, follow‐up | Incidence, incidence rate | — |
| Motala 2003 | 10 years | Baseline, follow‐up | Incidence | — |
| Motta 2010 | 3 years | Baseline, follow‐up | Incidence | — |
| Mykkänen 1993 | Mean 3.5 years (42 months (SD 4)) | Baseline, follow‐up | Incidence, odds ratio | — |
| Nakagami 2016 | 5 years | Baseline, follow‐up | Incidence, hazard ratio | — |
| Nakanishi 2004 | 7 years | Baseline, follow‐up (annual health examinations) | Incidence, incidence rate, relative risk | Also adjusted for all other components of the metabolic syndrome at study entry |
| Noda 2010 | 5 years | Baseline, follow‐up | Incidence | — |
| Park 2006 | Mean 4.1 years | Baseline, follow‐up (annual examinations) | Incidence, incidence rate | — |
| Peterson 2017 | 10 years | Baseline, follow‐up | Incidence | — |
| Qian 2012 | 5 years | Baseline, follow‐up | Incidence | — |
| Rajala 2000 | 4.6 years (1.9–6.4) | Baseline, follow‐up (including a separate cohort) | Incidence, incidence rate | — |
| Ramachandran 1986 | Reverters: 3.3 years (SD 2) Converters: 5.1 years (SD 3.5) |
Baseline, follow‐up ("periodically") | Incidence | All individuals were advised a calorie‐restricted high carbohydrate high‐fibre diet |
| Rasmussen 2008 | 3.5 years i‐IFG5.6: median 2.5 years i‐IGT: median 2.1 years | Baseline, follow‐up | Incidence, incidence rate | — |
| Rathmann 2009 | 7 years | Baseline, follow‐up | Incidence, incidence rate, odds ratio | — |
| Rijkelijkhuizen 2007 | Mean 6.4 years | Baseline, follow‐up | Incidence, incidence rate | — |
| Sadeghi 2015 | 7 years | Baseline, follow‐up (biannual) | Incidence, incidence rate | — |
| Sasaki 1982 | 7 years | Baseline, follow‐up | Incidence,odds ratio | — |
| Sato 2009 | 4 years | Baseline, follow‐up | Odds ratio | — |
| Schranz 1989 | 6 years | Baseline, follow‐up | Incidence | — |
| Sharifi 2013 | 7 years | Baseline, follow‐up | Incidence | — |
| Shin 1997 | 2 years | Baseline, follow‐up | Incidence | — |
| Söderberg 2004 | 11 years | Baseline, follow‐up | Incidence, incidence rate | Incidence rates are given for periods 1987–1992 and 1992–1998, stratified by men:women |
| Song 2015 | Median 3.97 years | Baseline, follow‐up | Incidence, relative risk | Also adjusted for glucose |
| Song 2016a | Mean 10.8 years (range 10.5–12) | Baseline, follow‐up (additional follow‐up 2014) | Incidence | — |
| Soriguer 2008 | Mean 6 years | Baseline, follow‐up | Incidence, incidence rate, relative risk | — |
| Stengard 1992 | 5 years | Baseline, follow‐up | Incidence, odds ratio | — |
| Toshihiro 2008 | Mean 3.2 years (SD 0.1) | Baseline, follow‐up (annual OGTT) | Incidence | — |
| Vaccaro 1999 | 11.5 years | Baseline, follow‐up | Incidence, odds ratio | Odds ratios probably unadjusted |
| Valdes 2008 | Mean 6.3 years (5.9–6.8) | Baseline, follow‐up | Incidence, incidence rate, odds ratio | Also adjusted for 2‐h PG |
| Vijayakumar 2017 | Adults median 4.6 years (IQR 2.8–7.9 ) Children: median 5.2 years (IQR 2.7–9.6) | Baseline, follow‐up (examinations every 2 years) | Incidence, incidence rate | Data for adults/children; incidence rate taken from figure 2 (boys:men; girls:women) |
| Viswanathan 2007 | Median 5 years | Baseline, follow‐up (reminder to undergo an OGTT every 6 months) | Incidence, odds ratio | Also adjusted for FPG and 2‐h PG |
| Wang 2007 | 5 years | Baseline, follow‐up | Incidence, risk ratio | — |
| Wang 2011 | 4 years | Baseline, follow‐up | Odds ratio | Unclear which confounders were used in the multivariate model |
| Warren 2017 | Cohort 1 (visit 2): 22 years Cohort 2 (visit 4): 16 years |
Baseline, follow‐up (3 visits every 3 years, 5th visit 2011–13) | Hazard ratio | Data for IFG5.6, IFG6.1, HbA1c5.7, HbA1c6.0, IGT (cohort 2 only) |
| Wat 2001 | 2 years | Baseline, follow‐up | Incidence | — |
| Weiss 2005 | Mean 20.4 months (SD 10.3) | Baseline, follow‐up (biannual) | Incidence | — |
| Wheelock 2016 | Median 12.4 years (IQR 6.0–22.9) | Baseline, follow‐up (approx. annual intervals for repeated OGTTs) | Incidence | Non‐overweight participants with IGT cohort and overweight participants with IGT group |
| Wong 2003 | 8 years | Baseline, follow‐up | Incidence | Odds ratios from Tai 2004 |
| Yeboah 2011 | 7.5 years | Baseline, follow‐up (3 examinations) | Incidence, hazard ratio | — |
| Zethelius 2004 | 7 years | Baseline, follow‐up | Odds ratio | Also adjusted for (split) proinsulin, intact insulin |
| ALAT: alanine aminotransferase; ASAT: aspartate transaminase; FG: fasting glucose; FPG: fasting plasma glucose; h‐CRP: high‐sensitivity C‐reactive protein; HOMA‐beta: homeostatic model assessment of beta‐cell function; HOMA‐IR: homeostatic model assessment of insulin resistance; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; IQR: interquartile range; NCEP: national cholesterol education program; OGTT: oral glucose tolerance test; PG: postload glucose; SD: standard deviation; T2DM: type 2 diabetes mellitus; γ‐GT: gamma‐glutamyl transferase/transpeptidase | ||||
Appendix 8. Baseline characteristics (I)
| Study ID | Setting | N participants in original cohort (several phases of the cohort study) | N study sample (several phases of the cohort study) | Notes |
| Admiraal 2014 | Amsterdam, The Netherlands | 2975 | 456 | Baseline data for total cohort included in the analyses (N = 456)/South‐Asian Surinamese (N = 90)/African Surinamese (N = 190)/"ethnic Dutch" (N = 176) |
| Aekplakorn 2006 | Bangkok, Thailand | 3499/3245 | 2667 | Baseline data for cohort becoming diabetic (N = 361) |
| Ammari 1998 | Jordan | Unclear | 121/68–200/144 (controls) | Few baseline data reported for study population (N = 212) |
| Anjana 2015 | Chennai, India | 26,001 | 3589/2207 | Baseline data for cohort becoming diabetic at follow‐up (N = 176) |
| Bae 2011 | South Korea | 10,959 | 9723 | Baseline data for the total cohort (N = 9723) |
| Baena‐Diez 2011 | Barcelona, Spain | 2248 | 168 | Baseline data for prediabetic cohort (N = 115) |
| Bai 1999 | Chennai, India | 4885/1082 | 1082/696 | Baseline data for the IGT cohort (N = 252) |
| Bergman 2016 | Israel | 1970 | 1037 | Baseline data for IGT cohort (N = 24) |
| Bonora 2011 | Bruneck (South Tyrol), Italy | 1000 | 936 | No baseline data (except white participants aged > 40 years, N = 919) |
| Cederberg 2010 | Finland | 593 | 553/499 | Baseline data for the cohort (total N = 553, men N = 223, women N = 330) |
| Chamnan 2011 | Norfolk (East Anglia), UK | 77,630/25,639 | 6372/5735 | Baseline data for HbA1c6.0‐6.4 cohort (N = 370) |
| Charles 1997 | Paris, France | Unclear | 7540 (2nd clinical examination)/4089 | Baseline data for individuals with IGT converting to T2DM (N = 32) |
| Chen 2003 | Penghu, Taiwan | 1601 | 1306/600 | Baseline data for cohort converting to T2DM (N = 26) |
| Chen 2017 | China | 8845 | 1374 | Baseline data for i‐IFG/i‐IGTand IFG/IGT across age groups < 40 years + > 60 years (data indicate range across groups) (i‐IFG < 40 years N = 51 and > 60 years N = 278; i‐IGT < 40 years N = 41 and > 60 years N = 151; IFG/IGT: < 40 years N = 34 and > 60 years N = 175) |
| Coronado‐Malagon 2009 | Mexico | 820 | 656 | Baseline characteristics for the prediabetic cohort (N = 217) |
| Cugati 2007 | Australia, Blue Mountains region | 4433/3654 | 2335 (5 years)/1952 (10 years)/2123 complete data (10 years) | Baseline data for people without diabetes (N = 3437) |
| De Abreu 2015 | Australia | Unclear | 1167/395 (IFG5.6) | Baseline data for IFG cohort at baseline (N = 187) |
| Den Biggelaar 2016 | The Netherlands | 574/491 | 476 | Baseline data for prediabetic group (N = 122) |
| Derakhshan 2016 | Tehran, Iran | 12808 | 8231 | Baseline data for prediabetes group with normal blood pressure |
| Dowse 1991 | Nauru, Micronesia | 1497/1201 | 830 (1982/1987‐including 143 nondiabetic person from 1975/76) | No baseline data provided |
| Ferrannini 2009 | Mexico | 3505 | 2282/1963 | Baseline characteristics: range across different definitions of prediabetes |
| Filippatos 2016 | Attica, Greece | 4056/3042/1875 | 1485 | Baseline data for IFG5.6 cohort (N = 343) |
| Forouhi 2007 | Ely (Cambridgeshire), UK | 1571/1122 (phase 1)/912 (phase 2) | 683 (phase 3) | Baseline data for IFG6.1 cohort (N = 257) |
| Garcia 2016 | Sacramento (CA), USA | 1789 | 1777 | Baseline data for prediabetic cohort (N = 310) |
| Gautier 2010 | France | 3817 | 979 | No baseline data |
| Gomez‐Arbelaez 2015 | Columbia | 2012 | 772 | Baseline data for the total cohort (N = 772) |
| Guerrero‐Romero 2006 | Durango, Mexico | Unclear | 375 | Baseline data for IGT cohort at baseline progressing to T2DM (N = 20); all individuals were counselled on the importance of diet and physical exercise (standard care for the whole cohort) |
| Han 2017 | Ansung‐Ansan, South Korea | 10,030 | 7542 | Baseline data for i‐IFG, i‐IGT and IFG/IGT cohort |
| Hanley 2005 | USA | 1625 | 822 | Baseline data for diabetic cohort at follow‐up (N = 131); participants were recruited from 2 population‐based studies: the San Antonio Heart Study and the San Luis Valley diabetes study |
| Heianza 2012 | Japan | 32057 | 6636/6241 | Baseline data for total cohort (N = 6241) |
| Inoue 1996 | Gunma (Gyeonggi), Japan | Unclear | Unclear | Baseline data for the IGT cohort (N = 37) |
| Janghorbani 2015 | Isfahan, Iran | 3370 | 1489 | Baseline data for i‐IFG, i‐IGT and IFG/IGT cohort at baseline (N = 770); first‐degree relatives of people with T2DM |
| Jaruratanasirikul 2016 | Thailand | 181 | 177 (157) | Baseline data for IGT cohort (N = 27) |
| Jeong 2010 | Dalseong County, South Korea | 1806/1599 | 1474 | 1287 participants were re‐evaluated in 2008 and 187 new participants "added to the study"; baseline data for participants with incident diabetes (N = 135) |
| Jiamjarasrangsi 2008a | Bangkok, Thailand | 3989 | 3243/2370 | Baseline data for total cohort becoming diabetic at follow‐up (N = 48) |
| Kim 2005 | Seoul, South Korea | 20,203/15,936 | 2964 | Baseline data for FPG group 4 (6.1–7.0) with baseline and follow‐up (N = 276) |
| Kim 2008 | Incheon, South Korea | 7510 | 7211 | Baseline data for IFG5.6/IFG6.1 cohort (N = 1335/494) |
| Kim 2014 | Seoul, South Korea | 418 | 418 | Baseline data for i‐IFG (N = 158)/i‐IGT (N = 65)/IFG/IGT (N = 119)/i‐HbA1c (N = 64); total (N = 406) |
| Kim 2016a | Seoul, South Korea | 19,356 | 17,971 | 2 baseline data cohorts: prediabetes by FPG only and HbA1c only (N = 3544 and N = 1713) |
| Kleber 2010 | Germany | 79 | 79 | Baseline data for IGT cohort (N = 79) |
| Kleber 2011 | Germany | 128 | 128 | Baseline data for IFG cohort (N = 128) |
| Ko 1999 | Hong Kong | 123 | 123 | Baseline data for the IGT cohort (N = 123) |
| Ko 2001 | Hong Kong | 657 | 319 | Baseline data for IFG cohort (N = 55) |
| Larsson 2000 | Sweden | 1843 | 265 | Baseline data for i‐IGT (N = 66)/i‐IFG (N = 42)/IFG/IGT (N = 30); 265 follow‐up participants were randomly sampled from each glucose tolerance group of the original cohort and invited for follow‐up |
| Latifi 2016 | Ahvaz (Khuzestan), Iran | 12,514/6640 | Unclear/593 | Baseline for prediabetic cohort becoming diabetic at follow‐up |
| Lecomte 2007 | France | 56,650 | 4532 | Baseline data for IFG cohort attending both examinations (N = 743) |
| Lee 2016 | South Korea | 6246 | 5528 | Baseline data for the total cohort (N = 3497) |
| Leiva 2014 | Chile | 1007 | 177 | Most baseline data for cohort becoming diabetic at follow‐up (N = 94 with IFG) |
| Levitzky 2008 | Framingham (MA), USA | Unclear | 3634 | Baseline data for individuals on first exam, free of cardiovascular disease (N = 4058) |
| Li 2003 | Kinmen, Taiwan | Unclear | 644 | Baseline data for i‐IGT (N = 118)/i‐IFG (N = 42)/IFG/IGT (N = 49) |
| Ligthart 2016 | Rotterdam, The Netherlands | 14,926/11,740 | 11,740/10,050 | Baseline data for prediabetic cohort (N = 1382) |
| Lipska 2013 | USA | 3075 | 1690 | Baseline data for i‐IFG (N = 189)/i‐HbA1c5.7 (N = 207)/IFG/HbA1c (N = 169) |
| Liu 2008 | Jiang Su province, China | 6400/5888 | 1844 | Baseline data for non‐diabetic participants (N = 1844); M (N = 788)/W (N = 1056) |
| Liu 2014 | Shanghai, China | 4556 | 3174 | Baseline data for the prediabetic cohort converting to T2DM (N = 78) |
| Liu 2016 | Beijing, China | 2101 | 1857 | Baseline data for participants without diabetes at baseline (N = 1857) |
| Liu 2017 | China | 27,020 | 23,626/18,610 | Baseline data for IFG cohort at baseline (N = 3607) |
| Lorenzo 2003 | San Antonio (TX), USA | 2941/2569 | 1734 | Baseline data for cohort converting to T2DM (N = 195) |
| Lyssenko 2005 | Finland | Unclear | 2115 | Baseline data for IFG‐IGT individuals who converted to T2DM (N = 86) |
| Magliano 2008 | Australia | 20,347/11,247 | 6537 | Baseline data for cohort becoming diabetic at follow‐up (N = 224) |
| Man 2017 | Singapore | 3280 | 1279/1137 | Baseline data for incident diabetes cohort (N = 127) |
| Marshall 1994 | Colorado, USA | 1321 | 173/134 | Baseline data for IGT cohort converting to T2DM (N = 20) |
| McNeely 2003 | Seattle (WA), USA | 518 | 465 (5 years)/412 (10 years) | Baseline data for cohort converting to T2DM at 5–6 years (N = 50) and 10 years (N = 74) |
| Meigs 2003 | Baltimore (MD) and Washington, D.C., USA | Unclear | 815/753 | Baseline data for the IFG‐IGT cohort (N = 265); follow‐up time: at least 6 years 77%, at least 10 years 44%, at least 16 years 16%, at least 20 years 4.5% |
| Mohan 2008 | Chennai, India | 1061 | 513 | Baseline data for cohort becoming diabetic at follow‐up (N = 64) |
| Motala 2003 | Durban (KwaZulu‐Natal), South Africa | 2479 | 563 | Baseline data for responders (both baseline and follow‐up examination) (N = 563) |
| Motta 2010 | Italy | 2603 | 2603 | No baseline data provided |
| Mykkänen 1993 | Kuopio (Northern Savonia), Finland | 1300 | 1054/892 | Baseline data for cohort developing T2DM (N = 69) |
| Nakagami 2016 | Japan | 6012 | 2770/2267 | Baseline data for cohort converting to T2DM (N = 99) |
| Nakanishi 2004 | Japan | Unclear/6812 | 5746 | Baseline characteristics for IFG cohort (N = 246) |
| Noda 2010 | Japan | 22387 | 2207 | Baseline characteristics for the total cohort (N = 2207) |
| Park 2006 | South Korea | 6305 | 5557 | Baseline data for incident diabetic participants with IFG at baseline (N = 40) |
| Peterson 2017 | Sweden | 119 | 87/74/29 | Baseline data for IGT cohort (N = 29) |
| Qian 2012 | Shanghai, China | 1869 | 1042 | Baseline data for cohort progressing to T2DM (N = 377) |
| Rajala 2000 | Oulo (North Ostrobothnia), Finland | 1008/768 | 183 (1st)/193 (2nd, other group) | Few baseline data for IGT cohort (N = 171) |
| Ramachandran 1986 | Madras, India | Unclear | 107 | Baseline data for the diabetic cohort at follow‐up (N = 39) |
| Rasmussen 2008 | Denmark | 1821 | 1510/1002 | Baseline data for IFG (N = 607)/IGT cohort (N = 903) |
| Rathmann 2009 | Augsburg (Bavaria), Germany | 2656 | 1202 | Baseline data for total cohort (follow‐up participants, age‐group 55–74 years, N = 887) |
| Rijkelijkhuizen 2007 | The Netherlands | 2484/1513 | 1428 | Baseline data for IFG6.1 (N = 149)/IFG5.6 (N = 488) |
| Sadeghi 2015 | Isfahan, Iran | 6323 | 2980 | Baseline data for prediabetic cohort becoming diabetic at follow‐up (N = 131) |
| Sasaki 1982 | Osaka, Japan | 507 | 207 | Baseline data for the IGT cohort (N = 13) |
| Sato 2009 | Japan | 12,647 | 9116/6804 | Baseline data for cohort becoming diabetic at follow‐up (N = 659) |
| Schranz 1989 | Malta | 2128 | 1422 | Baseline data for diabetic cohort at follow‐up (N = 166) |
| Sharifi 2013 | Zanjan, Iran | 2941 | 395 | Baseline data for active participants (N = 123) |
| Shin 1997 | Yonchon County, South Korea | 2520/2293 | 2248/1193 | Baseline data for individuals converting to T2DM (N = 67) |
| Söderberg 2004 | Mauritius | 5083/6616/6291 | Unclear | Baseline data for cohort 1987–1998 (N = 2631), 10 years follow‐up; 3 cohorts 1987–1992 (N = 3680), 1992–1998 (N = 4178), 1987–1998 (N = 2631) |
| Song 2015 | South Korea | 4899 | 2079 | Baseline data for prediabetic cohort (men N = 154; women N = 167; total N = 321) |
| Song 2016a | Shanghai, China | 2132 | 778/526 | Baseline data for prediabetic cohort (N = 334) |
| Soriguer 2008 | Pizarra (Andalusia), Spain | 1051 | 824 | Baseline data for final sample of follow‐up (N = 714) |
| Stengard 1992 | Finland | 1711 | 716/637 | Baseline data for IGT cohort converting to T2DM (N = 17) |
| Toshihiro 2008 | Japan | 732 | 128 | Baseline data for cohort becoming diabetic at follow‐up (N = 36); participants with IFG and/or IGT were given advice about lifestyle modifications once or twice a year |
| Vaccaro 1999 | Naples, Italy | 1285/1245 | 1141/560 | Baseline data for total cohort (follow‐up examination N = 560) |
| Valdes 2008 | Spain | 1626/1034 | 943/630 | Baseline data for IFG5.6–6.1 (N = 114)/IFG6.1–6.9 (N = 52) |
| Vijayakumar 2017 | Phoenix (AZ), USA | Unclear | 2095 (10–19 years)/2005 (20–39 years) | Baseline data for adults/children with HbA1c 5.7%‐6.4% (children N = 62, adults N = 168) |
| Viswanathan 2007 | India (probably Chennai) | 4084 | 1659 | Baseline data for IGT group (N = 619); participants were given advice on preventive measures such as dietary modifications and regular exercise |
| Wang 2007 | Beijing, China | 20,682/1566 | 902 | Baseline data for cohort with incident diabetes and no coronary heart disease (N = 67) |
| Wang 2011 | Arizona/North/South Dakota/Oklahoma, USA | Unclear | 2849/1670 (2nd exam) | No baseline data |
| Warren 2017 | USA, 4 communities | 15,792 | Cohort 1, N = 10844: 1990–1992 (FG, HbA1c) as baseline Cohort 2, N = 7194: 1996–1998 (FG, 2‐h glucose) as baseline |
2 different baseline cohorts; 4 prediabetes definitions (visit 2: IFG5.6–6.9 N = 4112; HbA1c5.7‐6.4 N = 2027; visit 4: IFG5.6–6.9 N = 2142; IGT N = 2009) |
| Wat 2001 | Hong Kong | 2900 | 434/322 | Baseline data for IGT cohort (N = 322) |
| Weiss 2005 | Conneticut, USA | 129 | 117 | Baseline data for IGT cohort (N = 33) |
| Wheelock 2016 | Arizona, USA | Unclear | 5532 | Baseline data for the full cohort (N = 5532); prediabetic cohort = non‐overweight (N = 37) + IGT group and overweight + IGT group (N = 132); 5–11 years/12–19 years |
| Wong 2003 | Singapore | 3568 | 469/291 | Baseline data for IGT group (N = 291) |
| Yeboah 2011 | USA | 6814 | 6814/6753 | Baseline data for IFG cohort (N = 940) |
| Zethelius 2004 | Uppsala, Sweden | 2322/1221/1010 | 840/667 | Baseline data for cohort converting to T2DM (N = 26) |
| FG: fasting glucose; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; PG: postload glucose; T2DM: type 2 diabetes mellitus | ||||
Appendix 9. Baseline characteristics (II)
| Study ID | Sex, % women | Age (SD), years | 'Ethnicity', % white | 'Ethnicity', % Arabian/Asian/(Pima) Indians | 'Ethnicity', % Hispanic | 'Ethnicity', % Black | Family history of diabetes, % | BMI (SD), kg/m2 | Notes |
| Admiraal 2014 | 59 57 68 51 | 45 44 44 47 | 39 | 20 | — | 42 | 55 77 59 38 | 26.4 25.7 27.4 25.6 | Total cohort South‐Asian Surinamese African Surinamese "Ethnic Dutch" (the Netherlands) |
| Aekplakorn 2006 | 19 | 43.6 (5.0) | — | 100 | — | — | 53 | 24.8 (3.2) | — |
| Ammari 1998 | — | 63% > 40 | — | 100 | — | — | 99 | — | — |
| Anjana 2015 | 61 | 47 (13.1) | — | 100 | — | — | 47 | 25.8 (4.3) | — |
| Bae 2011 | 25 | 44.7 (5.4) | — | 100 | — | — | — | 23.8 (2.8) | — |
| Baena‐Diez 2011 | 52 | 61.2 (11.8) | — | — | 100 | — | 26 | — | — |
| Bai 1999 | 35 | Mainly 40–60+ | — | 100 | — | — | — | — | — |
| Bergman 2016 | 38 | 50.5 (8.3) | 42 | 29 | — | 47 | — | Men: 26.5 (3.8) Women: 26.8 (5.2) | — |
| Bonora 2011 | — | — | 100 | — | — | — | — | — | — |
| Cederberg 2010 | — | — | 100 | — | — | — | — | Men: 27.6 (3.5) Women: 27.9 (4.5) | — |
| Chamnan 2011 | 54 | 62.4 (8.2) | 100 | — | — | — | 14 | 26.6 (4.0) | — |
| Charles 1997 | 0 | 48.8 (1.8) | 100 | — | — | — | — | 27 (4) | — |
| Chen 2003 | 49 | 59.6 | — | 100 | — | — | 21 | 25.7 (3.1) | — |
| Chen 2017 | 54–58 | 40–67 | — | 100 | — | — | 9–37 | 23.8–24.8 | — |
| Coronado‐Malagon 2009 | 10 | 47.9 (8.6) | — | — | 100 | — | — | 26.8 (3.0) | — |
| Cugati 2007 | 57 | 67.4 | 100 | — | — | — | 19 | 26 | — |
| De Abreu 2015 | 100 | 53.8 (IQR 44.0–64.4) | Mostly white Australians | — | — | — | — | 27.7 (IQR 24.3–31.4) | — |
| Den Biggelaar 2016 | 39 | 60.8 (IQR 55.3–64.9) | 100 | — | — | — | — | 28.0 (IQR 26.5–31.2) | — |
| Derakhshan 2016 | 56 | 42.8 (11.7) | — | 100 | — | — | — | 26.9 (4.1) | — |
| Dowse 1991 | — | — | — | 100 | — | — | — | — | — |
| Ferrannini 2009 | 52–70 | 47–50 | — | — | 100 | — | 27–45 | 29.1–30.5 | — |
| Filippatos 2016 | 35 | 46.4 (12.4) | 100 | — | — | — | 22 | 27.4 (4.7) | — |
| Forouhi 2007 | 44 | 55.5 (7.9) | 100 | — | — | — | — | 27.8 (4.6) | — |
| Garcia 2016 | — | 69.8 (6.9) | — | — | 49 | — | — | 31.1 (5.6) | — |
| Gautier 2010 | 31 | 30–64 | 100 | — | — | — | — | — | — |
| Gomez‐Arbelaez 2015 | 70 | 58 (12) | — | — | 100 | — | — | 27.4 (4.6) | — |
| Guerrero‐Romero 2006 | — | 38 | — | — | 100 | — | — | 32.9 (5.6) | — |
| Han 2017 | 28 60 33 |
50.4 (8.3) 53.1 (8.9) 52.4 (8.7) |
— | 100 100 100 |
— | — | 15 12 15 |
25.5 (3.4) 24.9 (3.2) 25.4 (3.2) |
i‐IFG5.6 i‐IGT IFG/IGT |
| Hanley 2005 | 60 | 56.2 (7.9) | 38 | — | 36 | 26 | — | — | — |
| Heianza 2012 | 25 | 49.9 (8.7) | — | 100 | — | — | — | 22.8 (2.8) | — |
| Inoue 1996 | — | — | — | 100 | — | — | — | 23.2 | — |
| Janghorbani 2015 | — | 44.4
42.9 44.1 |
— | 100 | — | — | 100 | 29.2 29.0 30.0 | i‐IFG i‐IGT IFG/IGT |
| Jaruratanasirikul 2016 | 37 | 12.4 (2.3) | — | 100 | — | — | — | 35.3 (5.8) BMI SDS: 3.66 (0.86) | — |
| Jeong 2010 | — | 61 (9) | — | 100 | — | — | 7 | 24.6 (3.2) | — |
| Jiamjarasrangsi 2008a | 67 | 49.5 (12) | — | 100 | — | — | 15 | 26.9 (0.6) | — |
| Kim 2005 | 15 | 50.7 (7.2) | — | 100 | — | — | 9 | 24.6 (2.2) | — |
| Kim 2008 | 7 5 | 41 43 | — | 100 | — | — | 9 8 | 24 25 | IFG5.6 IFG6.1 |
| Kim 2014 | 49 57 48 56 | 60.2 (11.3)
63.0 (11.0) 59.1 (10.1) 59.3 (10.1) |
— | 100 | — | — | 29 14 22 16 | 24.7 (3.0) 23.2 (3.5) 25.1 (3.3) 24.9 (4.7) | i‐IFG i‐IGT IFG/IGT i‐HbA1c |
| Kim 2016a | 24 47 | 49.5 51.2 | — | 100 | — | — | 22 22 | 24.4 23.9 | IFG HbA1c |
| Kleber 2010 | 51 | 13.1 (2.1) | 100 | — | — | — | — | 31.8 (6.3) BMI SDS: 2.56 (0.62) | — |
| Kleber 2011 | 53 | 13.5 (2.1) | 100 | — | — | — | — | 31.7 (6.1) | — |
| Ko 1999 | 88 | 22–26 | — | 100 | — | — | — | — | — |
| Ko 2001 | 84 | 37.4 (9.3) | — | 100 | — | — | 38 | 25.9 (4.0) | — |
| Larsson 2000 | 100 | 66 (2.3) | 100 | — | — | — | — | 24.6 26.2 26.7 | i‐IGT i‐IFG IFG/IGT (age at follow‐up) |
| Latifi 2016 | 38 | 46.6 (12.5) | — | 100 | — | — | 80 | — | — |
| Lecomte 2007 | 0 | 44.5 (7.5) | 100 | — | — | — | 3 | 26.4 (3.6) | — |
| Lee 2016 | 33 | 46.1 (8.5) | — | 100 | — | — | 24 | 24.8 (3.1) | — |
| Leiva 2014 | 57 | 25–80 | — | — | 100 | — | — | 33.1 (4.3) | — |
| Levitzky 2008 | 53 | Women: 48 Men: 49 |
Mainly white | — | — | — | — | Men: 27.3 (3.9) Women: 25.6 (5.4) |
— |
| Li 2003 | 57 36 53 | 56.1 48.4 58.9 | — | 100 | — | — | — | 24.8 23.8 25.5 | i‐IGT i‐IFG IFG/IGT |
| Ligthart 2016 | 51 | 66.6 (9.4) | 92 | — | — | — | — | 27.9 (4.2) | — |
| Lipska 2013 | 33 60 47 | 76.6 76.7 76.6 | 82 36 60 | — | — | — | — | 27.9 27.9 29.0 | i‐IFG i‐HbA1c IFG + HbA1c |
| Liu 2008 | 57 | Men: 52 Women: 50 | — | 100 | — | — | Men: 6 Women: 8 | — | — |
| Liu 2014 | 48 | 68.6 (6.7) | — | 100 | — | — | — | 23.5 (3.0) | — |
| Liu 2016 | ‐ | Men: 70 Women: 69 | — | 100 | — | — | — | — | — |
| Liu 2017 | 50 | 50.9 (9.7) | — | 100 | — | — | — | 24.2 (3.6) | — |
| Lorenzo 2003 | 61 | 47.7 (0.8) | 19 | — | 81 | — | 46 | 31.3 | — |
| Lyssenko 2005 | 50 | 52 (11) | 100 | — | — | — | 100 | — | — |
| Magliano 2008 | 49 | 55.8 (12.0) | 85 | — | — | — | 31 | Men: 29.3 (0.4) Women: 29.7 (0.6) | — |
| Man 2017 | 57 | 54.4 (9.7) | — | 100 | — | — | 39 | 28.5 (5.3) | — |
| Marshall 1994 | 75 | 58.6 | 40 | — | 60 | — | 53 | 29.2 | — |
| McNeely 2003 | 52 41 |
58.9 57.5 |
100 | — | — | 60 62 |
24.9 25.1 |
5–6 years follow‐up 10 years follow‐up | |
| Meigs 2003 | 28 | 61.8 (14) | 95 | — | — | — | 29 | ≥ 25: 60% | — |
| Mohan 2008 | — | 43 (14) | — | 100 | — | — | 28 | 24.4 (4.4) | — |
| Motala 2003 | 60 | 36.4 (13.9) | — | 100 | — | — | 45 | 22.6 (6.0) | — |
| Motta 2010 | — | 65–84 | 100 | — | — | — | — | — | — |
| Mykkänen 1993 | 57 | 68.6 | 100 | — | — | — | 29 | 29 | — |
| Nakagami 2016 | 27 | 53 (7) | — | 100 | — | — | 19 | 24.6 (3.5) | — |
| Nakanishi 2004 | 0 | 49 (5.8) | — | 100 | — | — | 16 | 24.6 (3.0) | — |
| Noda 2010 | 63 | Men: 62.4 Women: 61.5 |
— | 100 | — | — | — | Men: 24.1 (3.0) Women: 24.2 (3.2) |
— |
| Park 2006 | 0 | 36.4 (3.9) | — | 100 | — | — | — | 24.8 (3.0) | — |
| Peterson 2017 | 48 | 61.4 (0.8) | 100 | — | — | — | — | 26.9 (5.4) | — |
| Qian 2012 | — | 60 (13) | — | 100 | — | — | — | 24.9 (3.7) | — |
| Rajala 2000 | 58 | — | 100 | — | — | — | — | — | — |
| Ramachandran 1986 | 31 | 48 | — | 100 | — | — | 49 | 25.2 | — |
| Rasmussen 2008 | 43 56 | 59.9 61.2 | 100 | — | — | — | — | 29.1 29.6 | IFG IGT |
| Rathmann 2009 | 49 | 63.2 (5.4) | 100 | — | — | — | 23 | 28.1 (4.0) | — |
| Rijkelijkhuizen 2007 | 46 53 | 62.5 61.5 | 100 | — | — | — | — | 27.6 27.0 | IFG6.1 IFG5.6 |
| Sadeghi 2015 | 59 | 51.3 (9.8) | — | 100 | — | — | 20 | 29.4 (4.5) | — |
| Sasaki 1982 | 54 | 57.4 | — | 100 | — | — | — | — | — |
| Sato 2009 | 0 | 48.6 (4.2) | — | 100 | — | — | 20 | 24.7 (3.3) | — |
| Schranz 1989 | 56 | Women: 59.8 Men: 57.7 |
100 | — | — | — | — | — | — |
| Sharifi 2013 | 63 | 40 (14) | — | 100 | — | — | — | 27.5 (4) | — |
| Shin 1997 | 34 | 59.6 | — | 100 | — | — | 6 | 24.5 | — |
| Söderberg 2004 | 56 | 41.2 | — | 70 | — | 30 | — | 23.9 | — |
| Song 2015 | 52 | 56–57 | — | 100 | — | — | Men: 10 Women: 22 |
Men: 25.2 (2.7) Women: 25.8 (3.4) |
— |
| Song 2016a | 63 | 57.2 (10.0) | — | 100 | — | — | — | — | — |
| Soriguer 2008 | 65 | 45.0 (13.4) | 100 | — | — | — | 58 | 28.3 (5.2) | — |
| Stengard 1992 | 0 | 70.8 (4.8) | 100 | — | — | — | — | 26.1 (4.2) | — |
| Toshihiro 2008 | 0 | 50.5 (5.8) | — | 100 | — | — | — | 24.9 (3.3) | — |
| Vaccaro 1999 | 23 | 44.1 (4.0) | 100 | — | — | — | — | 26.9 (4.4) | — |
| Valdes 2008 | — | 54.8 56.7 | 100 | — | — | — | — | 28.2 29.8 | IFG5.6 IFG6.1 |
| Vijayakumar 2017 | 97 79 | 29.9 14 | — | 100 | — | — | — | 39.1 32.0 |
Adults Children |
| Viswanathan 2007 | 39 | 42.4 (9.8) | — | 100 | — | — | — | — | — |
| Wang 2007 | 46 | 47.9 (10.7) | — | 100 | — | — | — | 25.2 (3.5) | — |
| Wang 2011 | — | — | — | 100 | — | — | — | — | — |
| Warren 2017 | 48 | 57.6 (5.7) | — | — | — | 25 | 25 | 28.9 (5.2) | Data for cohort 1 (IFG5.6) |
| Wat 2001 | 57 | 51 | 100 | — | — | — | 25.6 | — | |
| Weiss 2005 | 73 | 12.5 (2.7) | 45 | 39 | 12 | — | — | 36.6 (8.7) BMI z score: 2.42 (0.41) | — |
| Wheelock 2016 | 53 | 11.4 (3.6) | 100 | 100 | — | — | — | Percentile: 87.6 | — |
| Wong 2003 | 53 | 43.8 | — | 100 | — | — | 28 | 25.2 | — |
| Yeboah 2011 | 44 | 64.2 (9.8) | 31 | 15 | 25 | 30 | — | 30.1 (5.7) | — |
| Zethelius 2004 | 0 | 77 | 100 | — | — | — | — | 26.7 (3.2) | — |
| BMI: body mass index; FG: fasting glucose; FPG: fasting plasma glucose; i‐HbA1c: (isolated) glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; IQR: interquartile range; SD: standard deviation; SDS: standard deviation score | |||||||||
Appendix 10. Baseline characteristics (III)
| Study ID | Mean (SD)/median (IQR)/range systolic BP, mmHg | Mean (SD)/median (IQR)/range diastolic BP (SD), mmHg | Smoking: current and/or past, % | Medications, % | Comorbidities, % | Mean (SD)/median (IQR)/range FPG, mmol/L | Mean (SD)/median (IQR)/range 2‐h glucose, mmol/L | Mean (SD)/median (IQR)/range HbA1c, % | Notes |
| Admiraal 2014 | — | — | 38 26 41 41 | — | Hypertension: 26 26 32 19 |
5.2 5.3 5.2 5.3 | — | — | Total cohort South‐Asian Surinamese African Surinamese "Ethnic Dutch" |
| Aekplakorn 2006 | — | — | 42 | — | Hypertension: 33 | — | — | — | — |
| Ammari 1998 | — | — | — | — | Hypertension: 47 | — | — | — | — |
| Anjana 2015 | 129 (21) | 78 (11) | 13 | — | — | 5.2 (0.6) | 8.7 (1.4) | 6.2 (0.7) | — |
| Bae 2011 | 113 (14) | 76 (10) | — | — | — | 5.3 (0.5) | — | 5.4 (0.3) | — |
| Baena‐Diez 2011 | — | — | 33 | — | Hypercholesterolaemia: 38 Hypertriglyceridaemia: 15 Hypertension: 55 | — | — | — | — |
| Bai 1999 | — | — | — | — | — | — | — | — | — |
| Bergman 2016 | 128 (16) | 84 (10) | 38 | — | — | 5.2 (0.5) | 8.6 (1.0) | — | — |
| Bonora 2011 | — | — | — | — | — | — | — | — | — |
| Cederberg 2010 | Men: 142 Women: 142 | Men: 80 Women: 79 | Men: 18 Women: 15 | — | — | Men: 5.0 Women: 5.0 | Men: 6.8 Women: 7.0 | — | — |
| Chamnan 2011 | 139 (17) | 84 (11) | 15 | BP lowering: 21 Corticosteroids: 4 | — | — | — | — | — |
| Charles 1997 | — | — | — | — | — | 6.6 (0.8) | 9.3 (0.9) | — | — |
| Chen 2003 | — | — | 38 | — | Hypertension: 46 | — | — | — | — |
| Chen 2017 | — | — | 12–24 | — | Hypertension: 28–55 | 5.1–6.1 | 5.9–9.2 | — | Range for i‐IFG, i‐IGT and IFG/IGT cohorts separated by < 40 years and > 60 years |
| Coronado‐Malagon 2009 | — | — | — | — | — | 5.9 (0.3) | — | — | — |
| Cugati 2007 | 146 | 83 | — | — | — | 5 | — | — | — |
| De Abreu 2015 | 128 (IQR 114–140) | 79 (IQR 72–86) | 13 | — | Hypertension: 43 | 5.3 (IQR 5.0–5.8) | — | — | — |
| Den Biggelaar 2016 | 141 (IQR 132–155) | 83 (IQR 78–92) | 18 | — | — | 6.0 (IQR 5.5–6.3) | 8.8 (IQR 7.8–9.9) | 5.8 (IQR 5.6–6.1) | — |
| Derakhshan 2016 | — | — | 26 | — | — | — | — | — | — |
| Dowse 1991 | — | — | — | — | — | — | — | — | — |
| Ferrannini 2009 | 118–128 | 71–78 | — | — | — | 4.9–6.4 | 6.7–9.5 | — | Range for i‐IFG5.6, i‐IFG6.1, i‐IGT, IGT5.6 and IGT6.1 cohorts |
| Filippatos 2016 | 127 (17) | 82 (10) | 62 | — | Hypertension: 36 Hypercholesterolaemia: 54 |
5.9 (0.3) | — | — | — |
| Forouhi 2007 | 136 (16) | 82 (10) | 52 | — | — | — | — | — | — |
| Garcia 2016 | — | — | 58 | — | — | — | — | — | — |
| Gautier 2010 | — | — | — | — | — | — | — | — | — |
| Gomez‐Arbelaez 2015 | — | — | — | — | — | 5.2 (0.7) | 6.0 (1.8) | 6.5 (1.3) | — |
| Guerrero‐Romero 2006 | — | — | — | — | Dyslipidaemia: 41 Hypertension: 24 | 6.4 (0.6) | — | — | — |
| Han 2017 | 120 (17) 119 (18) 124 (18) |
78 (12) 76 (12) 80 (11) |
64 34 59 |
— | Hypertension: 28 27 36 |
5.9 (0.3) 4.8 (0.4) 5.9 (0.3) |
6.1 (1.2) 8.9 (0.9) 9.3 (0.9) |
5.5 (0.4) 5.5 (0.4) 5.7 (0.4) |
i‐IFG5.6 i‐IGT IFG/IGT |
| Hanley 2005 | 132 (20) | 79 (10) | — | BP lowering: 38 Lipid lowering: 7 | — | 5.9 (0.7) | 8.5 (1.7) | — | — |
| Heianza 2012 | 125 (16) | 76 (11) | — | — | — | 5.3 (0.5) | 5.3 (0.3) | — | |
| Inoue 1996 | 142 (9) | 73 (7) | — | — | — | — | — | — | — |
| Janghorbani 2015 | 116–117 | 76–77 | — | — | Hypertension: 20–23 | 5.1–61 | 5.9–9.2 | 5.1–5.3 | Range for i‐IFG, i‐IGT and IFG/IGT cohorts |
| Jaruratanasirikul 2016 | 124 (15) | 77 (9) | — | — | — | — | — | — | — |
| Jeong 2010 | 139 (21) | 87 (12) | 43 | — | — | — | — | 5.7 (0.5) | — |
| Jiamjarasrangsi 2008a | — | — | 4 | — | — | — | — | — | — |
| Kim 2005 | — | — | — | — | — | 6.4 (0.2) | — | — | — |
| Kim 2008 | 128/132 | 80/83 | — | — | — | 5.8/6.4 | — | — | — |
| Kim 2014 | 127–129 | 78 | 20–31 | — | — | — | — | — | Range for i‐IFG, i‐IGT, IFG/IGT and i‐HbA1c cohorts |
| Kim 2016a | 116–120 | 72–75 | 24–25 | — | — | 5.1–5.9 | — | 5.3–5.8 | Range for IFG and HbA1c cohorts |
| Kleber 2010 | 120 (16) | 73 (13) | — | — | — | 5.1 (1.1) | 8.5 | 5.6 (0.7) | — |
| Kleber 2011 | 120 (14) | 73 (12) | — | — | — | 4.8 (0.4) | 8.4 (0.6) | — | — |
| Ko 1999 | — | — | — | — | — | — | — | — | — |
| Ko 2001 | 125 (21) | 78 (10) | 2 | — | — | 6.5 (0.3) | 9.1 (2.1) | 6.2 (0.6) | — |
| Larsson 2000 | — | — | — | — | — | 4.7/5.5/5.5 | 8.6/6.8/8.7 | — | — |
| Latifi 2016 | — | — | — | — | Hypertension: 40 | — | — | — | — |
| Lecomte 2007 | 135 (13) | 81 (10) | 23 | — | Hypertension: 48 | 6.4 (0.2) | — | — | — |
| Lee 2016 | 125 (15) | 81 (11) | 20 | — | Hypertension: 22 | — | — | 5.9 (0.2) | — |
| Leiva 2014 | 134 (16) | 77 (10) | — | — | — | — | — | — | — |
| Levitzky 2008 | Women: 122 Men: 127 | — | Women: 29 Men: 28 | Antihypertensives: Women: 14 Men: 16 | Hypertension: Women: 26 Men: 35 |
— | — | — | — |
| Li 2003 | 136–138 | 85–87 | — | — | — | 5.4–6.4 | 6.8–9.1 | — | Range for i‐IFG, i‐IGT and IFG/IGT cohorts |
| Ligthart 2016 | 145 (21) | 81 (12) | 50 | BP lowering: 33 Lipid lowering: 18 | Stroke: 3
CHD: 8 Hypertension: 64 |
— | — | — | — |
| Lipska 2013 | 140–143 | — | 54–65 | — | — | 5.1–6.1 | — | 5.3–5.9 | Range for i‐IFG, i‐HbA1c and IFG/HbA1c cohorts |
| Liu 2008 | Men: 126 Women: 124 | Men: 80 Women: 77 | — | — | — | Men: 5.3 Women: 5.4 | — | — | — |
| Liu 2014 | 132 (16) | 82 (8) | — | — | — | 5.8 (0.8) | 9.2 (1.2) | — | — |
| Liu 2016 | — | — | — | — | — | — | — | — | — |
| Liu 2017 | 128 (21) | 81 (11) | 37 | — | — | 5.9 (0.4) | — | — | — |
| Lorenzo 2003 | 124 | 75 | — | — | — | 5.3 | 7.6 | — | — |
| Lyssenko 2005 | 140 | 85 (11) | — | — | — | 6.3 (IQR 5.8–6.6) | 8.3 (1.6) | 5.7 (0.4) | — |
| Magliano 2008 | — | — | 48 | — | — | 6 | 8 | 5.5 | — |
| Man 2017 | 145 (20) | 80 (12) | 13 | — | Hypertension: 74 | — | — | — | — |
| Marshall 1994 | — | — | — | — | — | 6.1 | 9.5 | — | — |
| McNeely 2003 | 139 137 | 80 80 | — | — | — | 5.5 5.6 | 9.0 8.8 | — | 5–6 years follow‐up 10 years follow‐up |
| Meigs 2003 | — | — | — | — | — | — | — | — | — |
| Mohan 2008 | 127 (19) | 81 (11) | — | — | — | 4.5 (0.6) | — | — | — |
| Motala 2003 | 119 (19) | 78 (13) | — | — | — | 4.6 (1.8) | 6.2 (3.8) | — | — |
| Motta 2010 | — | — | — | — | — | — | — | — | — |
| Mykkänen 1993 | 159 | 84 | 1 | Antihypertensives: 24 | Hypertension: 47 | 6.2 | 8.4 | — | — |
| Nakagami 2016 | 134 (18) | 82 (12) | 35 | — | — | 6.0 (0.6) | — | 6.0 (0.3) | — |
| Nakanishi 2004 | 133 (16) | 81 (11) | 53 | — | Dyslipidaemia: 40 Proteinuria: 5 Hypertension: 35 | 6.4 (0.2) | — | — | — |
| Noda 2010 | — | — | — | — | — | Men: 5.4 Women: 5.2 | — | Men: 5.0 Women: 5.1 | — |
| Park 2006 | — | — | — | — | — | 6.0 (0.3) | — | — | — |
| Peterson 2017 | — | 75 (11) | — | — | — | — | — | 5.5 (0.4) | — |
| Qian 2012 | 126 (21) | 81 (12) | — | — | — | 5.2 (0.7) | 6.1 (1.5) | — | — |
| Rajala 2000 | — | — | — | — | Hypertension: 49 | — | — | — | — |
| Ramachandran 1986 | — | — | — | — | — | — | — | — | — |
| Rasmussen 2008 | 140–142 | — | — | — | — | — | — | — | Range for IFG and IGT cohorts |
| Rathmann 2009 | 133 (19) | 80 (10) | 49 | Lipid lowering: 11 | Hypertension: 49 | 5.5 (0.5) | 6.3 (1.7) | 5.6 (0.4) | — |
| Rijkelijkhuizen 2007 | 139–145 | 84–85 | — | — | — | — | — | — | Range for IFG5.6 and IFG6.1 cohorts |
| Sadeghi 2015 | 127 (21) | 81 (11) | 14 | — | — | 5.7 (0.7) | 8.4 (1.5) | — | — |
| Sasaki 1982 | — | — | — | — | — | 5.6 (0.9) | 9.0 (0.9) | — | — |
| Sato 2009 | — | — | 91 | — | — | 6.0 (0.6) | — | 5.6 (0.6) | — |
| Schranz 1989 | — | — | — | — | — | Women: 7.2 Men: 6.2 | Women: 10.8 Men: 9.7 | — | — |
| Sharifi 2013 | 130 (12) | 79 (8) | 5 | — | Hypertriglyceridaemia: 48 Hypertension: 25 | — | — | — | — |
| Shin 1997 | 130 | 84 | — | — | — | 6.1 | 6.7 | — | — |
| Söderberg 2004 | 125 | 77 | 27 | — | — | 5.5 | 6.5 | — | — |
| Song 2015 | 123–127 | 76–80 | 2–27 | — | Dyslipidaemia: 64–66 Hypertension: 35–44 | — | — | 5.7–5.8 | Ranges for male and female cohorts |
| Song 2016a | 134 (20) | 85 (12) | 23 | — | — | 6.0 (0.4) | 5.9 (1.6) | — | — |
| Soriguer 2008 | — | — | — | — | — | — | — | — | — |
| Stengard 1992 | 156 | 88 | — | — | Hypertension: 53 | 5.4 (1.1) | 9.7 (0.8) | — | — |
| Toshihiro 2008 | 126 (12) | 81 (10) | 47 | — | — | 6.1 (0.6) | 8.8 (1.3) | — | — |
| Vaccaro 1999 | — | — | — | — | — | 4.2 (0.8) | 4.5 (1.7) | — | — |
| Valdes 2008 | 135–144 | 84–92 | — | — | — | 5.8–6.4 | 6.4–7.3 | 4.9–5.1 | Ranges for IFG5.6 and IFG6.1 cohorts |
| Vijayakumar 2017 | — | — | — | — | — | A: 5.4/C: 5.2 | A: 6.7/C: 6.5 | A: 5.8/C: 5.7 | — |
| Viswanathan 2007 | — | — | — | — | — | 6.1 (0.7) | 8.9 (1.0) | — | — |
| Wang 2007 | 124 (19) | 78 (11) | 28 | — | Hypertension: 36 | 5.8 (0.9) | 7.4 (1.7) | — | — |
| Wang 2011 | — | — | — | — | — | — | — | — | — |
| Warren 2017 | — | — | 22 | — | Hypertension: 38 | 6.0 (0.4) | — | 5.6 (0.4) | Data for cohort 1 (IFG5.6) |
| Wat 2001 | 126 | 78 | — | — | — | 5.4 | 8.9 | — | — |
| Weiss 2005 | — | — | — | — | — | 5.2 | 8.9 | — | — |
| Wheelock 2016 | — | — | — | — | — | — | 5.4 (1.2) | — | — |
| Wong 2003 | 125 | 74 | 24 | — | — | 5.7 | 8.9 | — | — |
| Yeboah 2011 | 132 (21) | 74 (11) | 50 | BP lowering: 56 Lipid lowering (statins): 17 | — | 6.0 (0.4) | — | — | — |
| Zethelius 2004 | — | — | — | — | — | 5.7 (0.7) | 7.9 (1.9) | — | — |
| 2‐h: 2‐h measurement after an OGTT; BP: blood pressure; CHD: coronary heart disease; FG: fasting glucose; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L);IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; IQR: interquartile range; OGTT: oral glucose tolerance test; SD: standard deviation | |||||||||
Appendix 11. Cumulative incidence as the measurement for the development of T2DM
| Study ID (years of follow‐up) | Diabetes cumulative incidence | ||||||||
| NGT cohort | IFG5.6 cohort | i‐IFG5.6 cohort | IFG6.1 cohort | i‐IFG6.1 cohort | IGT cohort | i‐IGT cohort | IFG/IGT cohort | HbA1c cohort | |
| Admiraal 2014 (10) | Unclear/354 | Total cohort: 51/111 (45.9%) Asian 13/31 (41.9%) African 14/40 (35%) "Ethnic Dutch" 3/40 (7.5%) | — | — | — | — | — | — | — |
| Aekplakorn 2006 (12) | Unclear/2444 | 65/223 (29.1%) | — | — | — | — | — | — | — |
| Ammari 1998 (2) | 10/144 (6.9%) | — | — | — | — | 10/68 (14.7%) | — | — | — |
| Anjana 2015 (9.1) | 209/1077 (19.4%) | — | 32/67 (47.8%) | — | — | — | 86/163 (52.8%) | 58/69 (84.1%) | — |
| Bae 2011 (4) | 228/7932 (2.9%) | — | — | — | — | — | — | — | HbA1c5.7: 373/1791 (20.8%) HbA1c6.0: 187/412 (45.4%) |
| Baena‐Diez 2011 (10) | 0 (IFG cohort) | — | — | 33/115 (28.7%) | — | — | — | — | — |
| Bai 1999 (1) | 1/444 (0.2%) | — | — | — | — | 14/252 (5.6%) | — | — | — |
| Bergman 2016 (20) | 202/739 (27.3%) | — | — | — | — | 68/114 (59.6%) | — | — | — |
| Bonora 2011 (15) | 29/710 (4.1%) | — | 10 years: 18/55 (32.7%) | — | — | 10 years: 8/53 (15.1%) | 10 years: 9/19 (47.4%) | HbA1c6.0: 20/70 (28.6%) | |
| Cederberg 2010 (9.7) | 11/410 (2.7%) | — | — | 15/40 (37.8%) | 6.3% | 38/103 (37.1%) | 23.4% | — | HbA1c5.7: 9/24 (37.5%) |
| Chamnan 2011 (3) | 37/5365 (0.7%) | — | — | — | — | — | — | — | HbA1c6.0: 26/370 (7%) |
| Charles 1997 (2) | 27/3671 (0.7%) | — | — | — | 3 years: 15/476 (3.2%) |
2 years: 32/418 (7.7%) | — | — | — |
| Chen 2003 (3) | 11/444 (2.5%) | — | — | 15/156 (9.6%) | — | — | — | — | — |
| Chen 2017 (3) | 60/644 (9.3%) | — | 40/329 (12.2%) | — | — | — | 45/192 (23.4%) | 71/209 (34%) | — |
| Coronado‐Malagon 2009a (1, 2) | Year 1: 3/439 (0.7%) Year 2: 3/439 (0.6%) |
— | — | — | — | — | — | — | — |
| Cugati 2007 (10) | 108/1512 (7.1%) | 69/229 (30%) | — | — | — | — | — | — | — |
| De Abreu 2015 (10) | 11/342 (3.2%) | 21/187 (11.2%) | — | — | — | — | — | — | — |
| Den Biggelaar 2016b (7) | 17/294 (5.8%) | — | — | — | — | — | — | — | — |
| Derakhshan 2016c (11.7) | 162/3611 (4.5%) | — | — | — | — | — | — | — | — |
| Dowse 1991 (6.2) | 14/215 (6.5%) | — | — | — | — | 13/51 (25.5%) | — | — | — |
| Ferrannini 2009 (7) | 89/1594 (5.6%) | — | 11/65 (16.9%) | — | 1/17 (5.9%) | — | 31/179 (17.3%) 3 years: 44/188 (23.4%) |
— | — |
| Filippatos 2016 (10) | 120/1206 (10.0%) | 71/279 (25.4%) | — | — | — | — | — | — | — |
| Forouhi 2007 (10) | 8/407 (2%) | 53/633 (8.3%) | — | 34/257 (24.7%) | — | 4.4 years: 17/170 (10%) |
— | — | — |
| Garcia 2016 (9) | 132/881 (15.0%) | 169/310 (54.5%) | — | — | — | — | — | — | — |
| Gautier 2010 (9) | 0 (IFG cohort) | 142/979 (14.5%) | — | — | — | — | — | — | — |
| Gomez‐Arbelaez 2015d (2) | Unclear/586 | — | — | — | — | — | — | — | — |
| Guerrero‐Romero 2006 (5) | 1/272 (0.4%) | — | — | — | — | 20/67 (29.9%) | — | — | — |
| Han 2017 (12) | 657/5633 (11.7%) | — | 81/199 (40.7%) | — | — | — | 624/1512 (41.3%) | 138/198 (69.7%) | 10 years: HbA1c5.7: 881/2830 (31.1%) |
| Hanley 2005 (5.2) | 5 years: 47/603 (7.8%) | — | — | — | — | 88/274 (32.1%) 5 years: 101/303 (33.3%) |
— | — | — |
| Heianza 2012 (5) | 4.7 years: 34/4149 (0.8%) | 262/1680 (15.6%) | — | 155/380 (40.8%) | — | — | — | — | HbA1c5.7: 184/822 (22.4%)
HbA1c5.7 and IFG5.6: 292/2092 (14%) HbA1c6.0: 100/203 (49.3%) HbA1c6.0 and IFG5.6: 271/1748 (15.5%) |
| Inoue 1996 (2.5) | 1/22 (4.5%) | — | — | — | — | 5/37 (13.5%) | — | — | — |
| Janghorbani 2015 (6.8) | 14/627 (2.2%) | — | 23/230 (10%) | — | — | — | 26/150 (17.3%) | 78/214 (36.4%) | — |
| Jaruratanasirikul 2016 (3–6) | 12/108 (11.1%) | — | — | — | — | — | 9/33 (27.3%) | — | — |
| Jeong 2010e (5) | 228/792 (28.8%) | — | — | — | — | — | — | — | — |
| Jiamjarasrangsi 2008a (2.6) | 15/2050 (0.7%) | 33/320 (10.3%) | — | — | — | — | — | — | — |
| Kim 2005 (5) | Unclear/2009 | — | — | 15/276 (5.5%) | — | — | — | — | — |
| Kim 2008 (2) | 21/5382 (0.4%) | 22/1335 (1.6%) | — | 48/494 (9.7%) | — | — | — | — | — |
| Kim 2014 (3.8) | 0 (cohort with intermediate hyperglycaemia) | — | 24/158(15.2%) | — | — | — | 12/65 (18.5%) | 38/119 (31.9%) | i‐HbA1c5.7: 7/64 (10.9%) |
| Kim 2016a (5.2) | 43/10,763 (0.4%) | — | — | 357/1433 (24.9%) | — | — | — | — | HbA1c6.0: 322/1103 (29.2%) IFG5.6 and HbA1c5.7: 435/1951 (22.3%) |
| Kleber 2010 (1) | 0 (IGT cohort) | — | — | — | — | 1/79 (1.3%) | — | — | — |
| Kleber 2011 (3.9) | 0 (IGT cohort) | — | — | — | — | 3/119 (2.5%) | — | — | — |
| Ko 1999 (1.4) | 0 (IGT cohort) | — | — | — | — | 29/123 (23.6%) | — | — | — |
| Ko 2001 (1.7) | 13/264 (4.9%) | — | — | 14/55 (25.5%) | — | — | — | — | — |
| Larsson 2000 (10) | 5/127 (3.9%) | — | — | — | 5/42 (11.9%) | — | 8/66 (12.1%) | 6/30 (20.0%) | — |
| Latifi 2016 (5) | 25/394 (6.3%) | 21/124 (16.9%) | — | — | — | — | — | — | — |
| Lecomte 2007 (5) | 0 (IFG cohort) | — | — | 127/743 (17.1%) | — | — | — | — | — |
| Lee 2016 (3.7) | 0 (cohort with intermediate hyperglycaemia) | — | — | — | — | — | — | — | HbA1c5.7: 390/3497 (11.2%) |
| Leiva 2014 (6) | 0 (IFG cohort) | — | — | 11/28 (39.3%) | — | — | — | — | — |
| Levitzky 2008 (4) | 0 (IFG cohort) | — | — | Women: 87/313 (27.8%) Men: 92/460 (20.0%) |
— | — | — | — | — |
| Li 2003 (5) | 38/435 (8.7%) | — | — | — | 16/42 (38.1%) | 2 years: 23/131 (17.6%) |
33/118 (28%) | 20/49 (40.8%) | — |
| Ligthart 2016 (14.7) | Unclear/7462 | — | — | 425/1382 (30.8%) | — | — | — | — | — |
| Lipska 2013 (7) | 38/1690 (2.2%) | 20/189 (10.6%) | — | 48/100 (48%) | — | — | — | — | i‐HbA1c5.7: 44/207 (21.3%) IFG and HbA1c5.7: 81/169 (47.9%) |
| Liu 2008 (5) | 9/470 (1.9%) | 18/169 (10.7%) | — | — | — | — | — | — | — |
| Liu 2014f (3) | 153/1821 (8.4%) | — | — | — | — | — | — | — | — |
| Liu 2016 (10.9) | Unclear/1635 | — | — | — | — | — | — | — | — |
| Liu 2017 (7.8) | Unclear/15003 | — | — | — | — | — | — | — | — |
| Lorenzo 2003 (7–8) | Unclear/1503 | — | — | 14/29 (48.3%) | — | 88/202 (43.6%) | — | — | — |
| Lyssenko 2005g (6) | 41/1429 (2.9%) | — | — | — | — | — | — | — | — |
| Magliano 2008 (5) | 58/4715 (1.2%) | — | — | 44/370 (11.9%) | — | 122/757 (16.1%) | — | — | — |
| Man 2017 (6) | 15/462 (3.2%) | — | — | — | — | — | — | — | HbA1c5.7: 112/675 (16.6%) |
| Marshall 1994 (1.9) | 0 (IGT cohort) | — | — | — | — | 20/123 (16.3%) | — | — | — |
| McNeely 2003 (10) | 5–6 years: 5/277 (1.8%) 10 years: 13/277 (4.5%) |
5–6 years: 27/125 (21.6%) 10 years: 39/103 (37.9%) |
— | 5–6 years: 7/30 (23.3%) 10 years: 18/28 (64.3%) |
— | 5–6 years: 45/178 (25.3%) 10 years: 59/157 (37.6%) | — | — | — |
| Meigs 2003 (5, 10) | 6 (SD 5) years: 55/488 (11.3%) |
— | — | — | 6 (SD 5) years: 6/20 (30.0%) |
— | 6 (SD 5) years: 81/218 (37.1%) |
6 (SD 5) years: 15/27 (55.6%) | — |
| Mohan 2008 (8) | 64/476 (13.4%) | — | — | — | — | 15/37 (40.5%) | — | — | — |
| Motala 2003 (10) | 36/482 (7.5%) | — | — | — | — | 13/35 (37.1%) 4 years: 16/72 (22.2%) |
— | — | — |
| Motta 2010 (3) | 52/2018 (2.6%) | — | — | 50/295 (16.9%) | — | — | — | — | — |
| Mykkänen 1993 (3.5) | 21/689 (3.0%) | — | — | — | — | 48/203 (23.6%) | — | — | — |
| Nakagami 2016 (5) | 1528 | 77/467 (16.5%) | — | 50/134 (37.3) | — | — | — | — | HbA1c6.0: 58/156 (37.2%) HbA1c5.7: 87/583 (14.9%) |
| Nakanishi 2004 (7) | 51/5500 (0.9%) | — | — | 5/246 (2.0%) | — | — | — | — | — |
| Noda 2010 (5) | Total: 30/1649 (1.8%) Men: 13/540 (2.4%) Women: 17/1109 (6.4%) |
Total: 37/405 (9.1%) Men: 18/202 (8.9%) Women: 19/203 (9.4%) | — | Total: 58/153 (37.9%) Men: 25/79 (31.6%) Women: 33/74 (44.6%) | — | — | — | — | — |
| Park 2006 (4.1) | 116/4975 (2.3%) | 40/321 (12.5%) | — | — | — | — | — | — | — |
| Peterson 2017 (10) | 2/39 (5.1%) | — | — | — | — | 6/29 (20.7%) | — | — | — |
| Qian 2012 (5) | 59/843 (7.0%) | — | — | — | 17/46 (37%) | — | 49/120 (41%) | 17/33 (51%) | — |
| Rajala 2000 (4.6) | 0 (IGT cohort) | — | — | — | — | 32/171 (18.7%) 2.1 years: 14/183 (7.7%) |
— | — | — |
| Ramachandran 1986 (5.1) | 0 IGT cohort) | — | — | — | — | 39/107 (36.4%) | — | — | — |
| Rasmussen 2008 (3.5) (i‐IFG5.6: 2.5, IGT: 2.1 ) | 0 (IFG, IGT cohort) | — | 141/442 (32%) | — | — | 181/442 (41%) | 1 year: 35/296 (11.8%) | 1 year: 60/207 (29%) | — |
| Rathmann 2009 (7) | 25/649 (3.9%) | — | — | 12/71 (16.9%) | — | — | 34/120 (28.3%) | 22/47 (46.8%) | — |
| Rijkelijkhuizen 2007 (6.4) | 51/1125 (4.5%) | 101/488 (20.7%) | — | 62/149 (41.6%) | 35/106 (33%) | 36/111 (32.4%) 2 years: 45/158 (28.5%) |
27/80 (33.8%) | 20/31 (64.5%) | — |
| Sadeghi 2015 (7) | 141/2607 (5.4%) | — | 134/373 (35.9%) | — | — | — | 49/373 (13.1%) | 65/373 (17.4%) | — |
| Sasaki 1982 (7) | 7/161/4.3%) | — | — | — | — | 5/13 (38.5%) | — | — | — |
| Sato 2009 (4) | 118/4147 (2.9%) | — | — | 334/794 (42.1%) | — | — | — | — | HbA1c6.0: 90/215 (41.9%) |
| Schranz 1989 (6) | 54/1251 (4.3%) | — | — | — | — | 23/75 (30.7%) | — | — | — |
| Sharifi 2013 (7) | 0 (IFG cohort) | 24/123(19.5%) | — | — | — | — | — | — | — |
| Shin 1997 (2) | 47/1040 (4.5%) | — | — | — | — | 20/153 (13.1%) | — | — | — |
| Söderberg 2004 (11) | Unclear/2522 | — | — | 5 years: 32/148 (21.6%) |
153/402 (38%) | 575/1253 (45.9%) | 5 years: 103/489 (21.1%) |
5 years: 45/118 (38.1%) |
— |
| Song 2015 (4) | 74/1758 (4.2%) | — | 68/321 (21.2%) Men: 30/154 (19.5%) Women: 38/167 (22.8%) | — | — | — | — | — | — |
| Song 2016a (10.8) | 0 (cohort with intermediate hyperglycaemia) | — | — | — | — | — | — | — | — |
| Soriguer 2008 (6) | 13/1806 (0.7%) | — | 23/56 (41.1%) | — | — | 14/54 (25.9%) | — | 14/28 (50%) | — |
| Stengard 1992 (5) | 6/216 (2.8%) | — | — | — | — | 17/234 (7.3%) | — | — | |
| Toshihiro 2008 (3.2)h | 0 (cohort with IFG and/or IGT) | — | — | — | — | — | — | — | — |
| Vaccaro 1999 (11.5) | 36/500 (7.2%) | — | 1/11 (9.1%) | — | — | — | 13/40 (32.5%) | 4/9 (44.4%) | — |
| Valdes 2008 (6.3) | 16/510 (3.1%) | 14/114 (12.3%) | 7/32 (21.9%) | 19/52 (36.5%) | — | 21/88 (23.9%) | 9/68 (13.2%) | 12/20 (60%) | — |
| Vijayakumar 2017 (adults: 4.6,children: 5.2) | Adults: 58/1466 (3.9) Children: 26/1795 (1.4%) [estimated from figure 2] |
Adults: 222/424 (52.4%) Children: 52/193 (26.9%) | — | — | — | Adults: 196/347 (56.5%) Children: 55/169 (32.5%) | — | IFG5.6/IGT: Adults: 116/169 (68.7%) Children: 26/53 (49.1%) |
HbA1c5.7: adults: 75/168 (44.6%) HbA1c5.7: children: 18/62 (29%) |
| Viswanathan 2007 (5) | Total: 154/465 33.1%) M: 99/265 (37.4%) W: 55/200 (27.5%) |
— | — | — | — | Total: 416/619 (67.2%) M: 251/391 (64.2%) W: 165/228 (72.4%) |
— | — | — |
| Wang 2007 (5) | 51/358 (14.2%) | — | 53/261 (20%) | 28/112 (25%) | — | 126/141 (89.4%) | 31/95 (32.6%) | IFG5.6/IGT: 54/109 (49.5%) IFG6.1/IGT: 36/52 (69.2%) |
— |
| Wang 2011 (7.8) | 84/595 (14.1%) | Total:
345/947 (36.4%) Men: 137/418 (32.8%) Women: 208/529 (39.3%) |
— | — | — | Total: 233/491 (47.5%) Men: 75/154 (48.7%): Women: 158/337 (46.9%) 4 years: Total 198/532 (37.2%) |
— | Total: 185/356 (52%%) Men: 66/125 (52.8%) Women: 119/231 (51.5%) |
HbA1c6.0: 19/121 (15.7%) |
| Warren 2017 (cohort 1: 22, cohort 2: 16) | 22 years: 8322 16 years: 4772 |
— | — | — | — | — | — | — | — |
| Wat 2001 (2) | 4/333 (0.1%) | — | — | — | — | 31/322 (9.6%) | — | — | — |
| Weiss 2005 (1.7) | 8/84 (9.5%) | — | — | — | — | — | 8/33 (24.2%) | — | — |
| Wheelock 2016 (4.3) | Unclear/5363 | — | — | 5 years: 31% | — | Non‐overweight: 5 years: 9/37 (24%) 10 years: 11/37 (29.7%) Overweight: 5 years: 49/132 (37%) 10 years: 84/132 (63.6%) | — | 5 years: 41.2% | — |
| Wong 2003 (8) | 12/278 (4.3%) | — | — | — | — | 102/291 (35.1%) | — | — | — |
| Yeboah 2011 (7.5) | Unclear/4973 | 273/940 (29.0%) | — | — | — | — | — | — | — |
| Zethelius 2004 (7) | Unclear/466 | — | — | — | — | Not reported/201 | — | — | — |
|
aDevelopment of T2DM from 'prediabetes' (not defined) at year 1: 11/217 (5.1%), at year 2: 16/217 (7.6%). bDevelopment of T2DM from 'prediabetes' (IFG6.1 and/or IGT): 46/122 (37.7%). cDevelopment of T2DM from IFG5.6and/or IGT: 11.7 years150/523 (28.7%); 2.3 years: 121/911 (13.3%). dDevelopment of T2DM from IFG5.6or IGT or HbA1c5.7: 20/186 (10.8%). eDevelopment of T2DM from IFG or IGT: not reported. fDevelopment of T2DM from IFG or IGT: 78/450 (17.3%). gDevelopment of T2DM from IFG or IGT:86/686 (12.5%). hDevelopment of T2DM from IFG and/or IGT: 36/128 (28.1%). FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; NGT: normal glucose tolerance; PG: postload glucose; SD: standard deviation; T2DM: type 2 diabetes mellitus | |||||||||
Appendix 12. Diabetes incidence (cases per 1000 person‐years)
| Study ID | Rate (diabetes cases/1000 person‐years (95% CI)) | ||||||||
| Follow‐up (years) | NGT cohort | 'Prediabetes' cohort | IFG6.1 cohort | IFG5.6 cohort | IGT cohort | IFG/IGT cohort | Elevated HbA1c cohort | Elevated HbA1c/IFG cohort | |
| Anjana 2015 | 9.1 | 22.2 (19.4–25.4) | 78.9 (68.0–90.9) | — | 61.0 (42.1–85.0) | 67.8 (54.6–83.0) | 133.6 (103.1– 169.3) | — | — |
| Bae 2011 | 4 | — | — | — | — | — | — | Per 100 person‐years: HbA1c5.7: 5.6 HbA1c6.0: 14.0 |
— |
| Bonora 2011 | 15 | 10 years: 4.3 (2.7–5.9) | — | 10 years: 37.0 (20.2–53.8) | — | 10 years: 17.0 (5.3–28.7) | 10 years: 49.2 (17.9–80.5) | HbA1c6.0: 25.8 | — |
| De Abreu 2015 | 10 | — | — | — | 18.1 (10.7–28.2) | — | — | — | — |
| Derakhshan 2016 | 11.7 | — | 30.3 | 6.5 years: 69.4 (56.0–86.1) | 6.5 years: 39.5 (34.4–45.4) | 6.5 years: 41.6 (36.1–47.9) | — | — | — |
| Dowse 1991 | 6.2 | 10.5 | — | — | — | 40.4 | — | — | — |
| Forouhi 2007 | 10 | 2.4 (1.2–4.8) 4 years: 2.64 (1.23–4.05) |
— | 17.5 (12.5–24.5) | 10.6 (8.1–13.9) (IFG5.6: FPG 5.6–6.9) |
4 years: 22.5 (20.4–24.6) | — | — | — |
| Han 2017 | 12 | 12.3 | IFG or IGT: 58.0 | — | i‐IFG5.6: 51.3 | i‐IGT: 53.1 | 114.4 | 10 years: HbA1c5.7: 43.2 |
— |
| Heianza 2012 | 5 | 2.3 | — | 104 | 34.6 | — | — | HbA1c5.7: 51.0 HbA1c6.0: 129.2 |
HbA1c5.7 and IFG5.6: 30.6 HbA1c6.0 and IFG5.6: 34.4 |
| Janghorbani 2015 | 6.8 | 3.1 (1.5–4.7) 2.3 years: 4.6 (1.28–11.7) | — | — | 16.3 (10.3–24.4) 2.3 years: i‐IFG5.6: 50.7 (20.7–102.0) | 25.9 (17.0–37.7) 2.3 years: i‐IGT: 99.7 (77.1–126.0) |
57.9 (46.1–71.7) | — | — |
| Jiamjarasrangsi 2008a | 2.6 | — | — | — | 31.5 (11.4–86.8) | — | — | — | — |
| Latifi 2016 | 5 | 21.9 | — | — | 34.5 | — | — | — | — |
| Li 2003 | 5 | 18.8 | — | 93.7 | — | 60.7 | 117 | — | — |
| Ligthart 2016 | 14.7 | — | — | 43.0 (39.2–47.2) | — | — | — | — | — |
| Liu 2008 | 5 | 9 | — | — | 22.5 | — | — | — | — |
| Magliano 2008 | 5 | 0.2 (0.2–0.3) (incidence percent per years) | — | i‐IFG6.1: 2.6 (1.8–3.4) (incidence percent per years) |
— | i‐IGT: 3.5 (2.9–4.2) (incidence percent per years) |
— | — | — |
| Meigs 2003 | 5, 10 | Per 100 person‐years (annualised rate): FPG ≥ 7.0:
0.64 (0.32–1.13)
2‐h PG ≥ 11.1: 2.77 (2.01–3.71) |
— | — | — | — | Per 100 person‐years (annualised rate): IFG or IGT FPG ≥ 7.0: 0.98 (0.65–1.41) 2‐h PG ≥ 11.1: 4.61 (3.77–5.56) |
— | — |
| Mohan 2008 | 8 | 17.5 | — | — | — | 64.8 | — | — | — |
| Nakagami 2016 | 5 | — | — | 1 | — | — | — | — | — |
| Nakanishi 2004 | 7 | 1.5 | — | 3.3 | — | — | — | — | — |
| Park 2006 | 4.1 | 5.7 | — | — | 31.3 | — | — | — | — |
| Rajala 2000 | 4.6 | — | — | — | — | 41 (28–57) | — | — | — |
| Rasmussen 2008 | 3.5 (i‐IFG5.6: 2.5 , IGT: 2.1 ) | — | — | — | i‐IFG5.6: 11.8 (9.9–13.8) per 100 person‐years | 17.0 (14.9–19.1)
per 100 person‐years (i‐IGT: 11.8 (9.7–13.9) |
27 (22.5–31.7) per 100 person‐years | — | — |
| Rathmann 2009 | 7 | — | — | i‐IFG6.1: 24.2 (12.5–42.3) | — | i‐IGT: 42.0 (29.0–58.7) | 77.9 (48.8–117.9) | — | — |
| Rijkelijkhuizen 2007 | 6.4 | 7 | — | 66.5 (49.9–83.0) | 32.7 (26.3–39.1) | i‐IGT: 57.9 | 112.2 | — | — |
| Sadeghi 2015 | 7 | Total: 14.1 (12.5–15.9) Men: 12.8 (10.7–15.3) Women: 15.5 (13.1–18.3) |
— | — | Total: 48.4 (35.0–66.7) Men: 46.4 (28.9–74.7) Women: 50.1 (32.3–77.7) | Total: 40.3 (30.2–53.8) Men: 41.4 (25.7–66.6) Women: 39.6 (27.5–57.0) | Total: 137.6 (103.7–182.5) Men: 129.9 (83.0–203.7) Women: 143.1 (99.4–205.9) | — | — |
| Söderberg 2004 | 11 | — | — | 87–92:
Men: 54.1 (48.0–60.1)
Women: 35.1 (30.3–40.0) 92–98: Men: 60.5 (54.1–67.0) Women: 74–7 (67.8–81.8) |
— | 87–92:
Men: 60.7 (54.3–67.1)
Women: 47.9 (42.2–53.6) 92–98: Men: 119.6 (110.6–128.6) Women: 81.0 (73.6–88.4) |
— | — | — |
| Soriguer 2008 | 6 | 7.2 (4.2–12.4) | — | — | 38.1 (25.3–57.3) | 31.1 (18.4–52.5) | 66.0 (39.1–111.5) | — | — |
| Valdes 2008 | 6.3 | 3.8 (2.1–6.8) for i‐IGT and IFG/IGT: 5.0 (2.8–8) |
— | 58.0 (37–90.9) | 19.5 (11.5–32.9) | 37.9 (24.7–58.1) i‐IGT: 21 (10.9–40.4) |
95.2 (54.1–167.7) | — | — |
| Vijayakumar 2017 | Adults: 4.6 Children: 5.2 | — | — | — | Boys: 22 Men: 70 Girls: 55 Women: 101 | Boys: 38 Men: 94 Girls: 60 Women: 118 | — | Boys: 52 Men: 100 Girls: 100 Women: 118 | — |
| Wang 2011 | 7.8 | 21.1 | — | — | Total: 66.2 Men: 57.7 Women: 73.4 |
Total: 95.8 Men: 98.1 Women: 94.8 |
Total: 109 Men: 109 Women: 109 |
— | — |
| CI: confidence interval; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT;NGT: normal glucose tolerance; T2DM: type 2 diabetes mellitus | |||||||||
Appendix 13. T2DM cases and person‐time (for calculation incidence rate ratios)
| Study ID | Persons (cases) with diabetes with/without IH at baseline | ||||
| Follow‐up (years) | Cases in IH group | Person‐years for IH group | Cases in normoglycaemic group | Person‐years for normoglycaemic group | |
| Anjana 2015 | 9.1 | i‐IFG5.6: 32 i‐IGT: 86 IFG/IGT: 58 | i‐IFG5.6: 525 i‐IGT: 1269 IFG5.6/IGT: 434 | 209 | 9398 |
| De Abreu 2015 | 10 | IFG5.6: 21 | IFG5.6: 1768 | 11 | — |
| Bae 2011 | 4 | HbA1c5.7: 373 HbA1c6.0: 187 |
HbA1c5.7: 6594 HbA1c6.0: 1338 |
— | — |
| Bonora 2011 | 10 | IFG6.1: 18 IGT: 8 IFG/IGT: 9 |
IFG6.1: 486 IGT: 471 IFG/IGT: 183 |
29 | 6704 |
| Derakhshan 2016 | 11.7 | IFG5.6: 150 | IFG5.6: 4950 | 162 | 39,901 |
| Dowse 1991 | 6.2 | IGT: 13 | IGT: 322 | 14 | 1339 |
| Forouhi 2007 | 10 | IFG6.1: 34
IFG5.6: 53 4.44 years: IGT: 17 |
IFG6.1: 1943
IFG5.6: 5000 4.44 years: IGT: 756 |
8 4.44 years: 9 |
3333 4.44 years: 3409 |
| Guerrero‐Romero 2006 | 5 | IGT: 20 | IGT: 343 | 1 | 1388 |
| Han 2017 | 12 | i‐IFG5.6: 81 i‐IGT: 624 IFG/IGT: 138 |
i‐IFG5.6: 1579 i‐IGT: 11,744 IFG/IGT: 1206 |
657 | 53,461 |
| Heianza 2012 | 5 | IFG5.6: 108 HbA1c5.7: 30 HbA1c5.7/IFG5.6: 154 | IFG5.6: 5920 HbA1c5.7: 1965 HbA1c5.7/IFG5.6: 1641 | 46 | 19,961 |
| Janghorbani 2015 | 6.8 | i‐IFG5.6: 23 i‐IGT: 26 IFG/IGT: 214 | i‐IFG5.6: 1409 i‐IGT: 1005 IFG/IGT: 1347 | 14 | 4578 |
| Li 2003 | 5 | i‐IFG6.1: 16 i‐IGT: 33 IFG/IGT: 20 | i‐IFG6.1: 171 i‐IGT: 544 IFG/IGT: 179 | 38 | 2026 |
| Ligthart 2016 | 14.7 | IFG6.1: 425 | iFG6.1: 9884 | — | — |
| Meigs 2003 | 5, 10 | IFG or IGT T2DM measured by: FPG ≥ 7.0: 26 2‐h PG ≥ 11.1: 101 |
IFG or IGT T2DM measured by: FPG ≥ 7.0: 2647 2‐h PG ≥ 11.1: 2192 |
28 | 1539 |
| Mohan 2008 | 8 | IGT: 15 | IGT: 247 | 64 | 3665 |
| Nakanishi 2004 | 7 | IFG6.1: 5 | IFG6.1: 1506 | 51 | 34,308 |
| Park 2006 | 4.1 | IFG5.6: 40 | IFG5.6: 1278 | 116 | 20,298 |
| Rijkelijkhuizen 2007 | 6.4 | i‐IFG6.1: 35 i‐IGT: 27 IFG/IGT: 20 |
i‐IFG6.1: 681 i‐IGT: 466 IFG/IGT: 178 |
51 | 7286 |
| Soriguer 2008 | 6 | IFG5.6: 23 IGT: 14 IFG/IGT: 14 | IFG5.6: 604 IGT: 450 IFG/IGT: 212 | 13 | 1806 |
| Valdes 2008 | 6.3 | IFG5.6: 14
IFG6.1: 19 i‐IGT: 9 IFG/IGT: 12 |
IFG5.6: 718
IFG6.1:328 i‐IGT: 429 IFG/IGT: 126 |
11 (16 for i‐IGT and IFG/IGT) |
2923 (3200 for i‐IGT and IFG/IGT) |
| Wang 2011 | 7.8 | IFG5.6: 137 IGT: 75 IFG/IGT: 66 |
IFG5.6: 2374 IGT: 765 IFG/IGT: 605 |
34 | 1613 |
| FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; IH: intermediate hyperglycaemia;T2DM: type 2 diabetes mellitus | |||||
Appendix 14. Odds ratios and hazard ratios as the effect measures for the development of T2DM
| Study ID | Adjusted [unadjusted] ratios (95% CI) for the development of diabetes comparing IH with normoglycaemia at baseline | ||||||||
| Follow‐up (years) | IFG6.1 | IFG5.6 | IGT | 'Prediabetes' | IFG/IGT | HbA1c | HbA1c/IFG | Ratio | |
| Admiraal 2014 | 10 | — | Total cohort:
6.1 (3.1–12.1)
[5.7 (3.1–10.5)] South‐Asian Surinamese: 11.1 (3.0–40.8) [9.9 (2.9–34.3)] African Surinamese: 5.1 (2.0–13.3) [6.2 (2.6–14.9)] "Ethnic Dutch": 2.2 (0.5–10.2) [2.1 (0.5–9.3)] |
— | — | — | — | — | Odds ratio |
| Aekplakorn 2006 | 12 | — | [2.41 (1.78–3.28)] | [4.36 (3.41–5.57)] | — | — | — | — | Odds ratio |
| Bae 2011 | 4 | — | — | — | — | — | HbA1c5.7: 6.5 (3.7–10.2) HbA1c6.0:41.3 (24.7–69.2) [compared with HbA1c < 5.0] |
— | Hazard ratio |
| Bergman 2016 | 24 | 20 years: i‐IFG6.1: 3.43 (1.88–6.28) | 20 years: i‐IFG5.6: 1.11 (0.76–1.61) | 5.64 (2.74–12.33) 20 years: 3.03 (1.80–5.09) | — | IFG5.6 + IGT: 2.79 (1.56–5.00) IFG6.1 + IGT: 3.85 (1.73–8.54) |
— | — | Odds ratio |
| Bonora 2011 | 15 | 5.83 (3.23–10.54) 10 years: 5.7 (2.8–11.4) [3.9 (1.56–9.3)] |
10 years: [3.9 (1.6–9.3)] |
— | 10 years: [20.5 (7.6–55.3)] |
HbA1c6.0: 9.74 (4.21–22.56) | — | Hazard ratio, odds ratio (10 years) | |
| Cederberg 2010 | 9.7 | 2.37 (1.49–3.78) [2.56 (1.57–4.16)] | — | 2.90 (1.90–4.43) [2.98 (1.94–4.569] | — | — | HbA1c5.7: 2.42 (1.50–3.91) [2.78 (1.80–4.31)] | — | Risk ratio |
| Chamnan 2011 | 3 | — | — | — | — | — | HbA1c6.0: 15.6 (6.9–35.7) [15.5 (7.2–33.3)] | — | Odds ratio |
| Chen 2003 | 3 | 4.4 (1.9–10.6) | — | — | — | — | — | — | Odds ratio |
| Coronado‐Malagon 2009 | 1, 2 | — | — | — | [At 1 year: 7.7 (2.1–27.9)] | — | — | — | Relative risk |
| Cugati 2007 | 10 | [19.13 (11.59–31.66)] | — | — | — | — | — | — | Odds ratio |
| De Abreu 2015 | 10 | 5.75 (1.86–17.78) | — | — | — | — | — | — | Odds ratio |
| Derakhshan 2016 | 11.7 | 6.5 years: 4.1 (2.9–5.6) | 6.5 years: 3.0 (2.3–3.9) | — | IFG5.6 and/or IGT: 4.98 (4.08–6.07) | — | — | — | Hazard ratio, relative risk (6.5 years) |
| Dowse 1991 | 6.2 | — | — | [3.6 (1.4–9.1)] | — | — | — | — | Odds ratio |
| Ferrannini 2009 | 7 | [3.73 (2.18–6.39)] | [4.28 (3.21–5.71)] | [4.01 (3.12–5.14)] | — | — | — | — | Relative risk |
| Filippatos 2016 | 10 | — | 3.43 (2.17–5.44) | — | — | — | — | — | Odds ratio |
| Forouhi 2007 | 10 | 4.4 (1.9–10.0) | 2.9 (1.3–6.3) | — | — | — | — | — | Hazard ratio |
| Han 2017 | 12 | — | i‐IFG5.6: 3.61 (2.85–4.57) | i‐IGT: 4.06 (3.62–4.55) | — | 8.21 (6.79–9.94) | 6 years: HbA1c6.0: Men: 4.28 (2.41–7.58) Women: 4.05 (1.36–12.07) |
— | Hazard ratio |
| Hanley 2005 | 5.2 | — | — | 5.42 (3.60–8.17) | — | — | — | — | Odds ratio |
| Heianza 2012 | 5 | 11.4 (8.09–16.1) | 6.18 (4.34–8.80) | — | — | — | HbA1c5.7: 6.53 (3.79–9.64) HbA1c6.0: 7.42 (3.67–15.0) | HbA1c5.7 + IFG5.6: 32.5 (23.0–45.8) HbA1c5.7 + IFG6.1: 37.9 (28.1–51.1) HbA1c6.0 + IFG5.6: 53.7 (38.4–75.1) HbA1c6.0 + IFG6.1: 52.3 (37.8–72.3) |
Hazard ratio |
| Janghorbani 2015 | 6.8 | — | 7.4 (3.7–14.8) [8.2 (4.2–16.0)] | 9.4 (4.8–18.6) [10.0 (5.2–19.1)] | — | 22.5 (12.4–41.0) [26.7 (15.1–47.2)] | — | — | Hazard ratio |
| Jeong 2010 | 5 | — | 5.66 (3.44–9.31) | 6.01 (3.23–11.2) | — | — | — | — | Odds ratio |
| Kim 2005 | 5 | Total: 34.57 (12.18–98.10) Men: 76.02 (10.42–544.51) Women: 15.46 (4.08–58.61) | Total: 4.77 (1.60–14.15) Men: 9.5 (1.25–72.24) Women: 1.91 (0.45–8.21) |
— | — | — | — | — | Hazard ratio |
| Kim 2016a | 5.2 | 21.1 (16.8–26.3) | — | — | — | — | HbA1c6.0: 23.2 (18.7–28.7) | HbA1c5.7 + IFG5.6: 46.7 (33.5–64.9) |
Odds ratio |
| Latifi 2016 | 5 | — | 1.04 (1.00–1.07) | — | — | — | — | — | Odds ratio |
| Leiva 2014 | 6 | 2.06 (1.76‐5.14) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Odds ratio |
| Levitzky 2008 | 4 | Women: 26.3 (17.4–39.8) Men: 12.9 (9.3–18.1) | Women: 22.3 (13.0–38.1) Men: 12.7 (8.1–20.0) | — | — | — | — | — | Odds ratio |
| Li 2003 | 5 | 5.78 (3.20–10.43) | — | i‐IGT: 2.94 (1.81–4.76) | — | 6.17 (3.41–11.15) | — | — | Hazard ratio |
| Lipska 2013 | 7 | 11.4 (7.1–18.4) | IFG5.6:
Total: 3.5 (1.9–6.3) Men: 8.6 (3.4–21.9) Women: 1.5 (0.5–4.6) White: 3.2 (1.5–6.6) Black: 4.6 (1.6–13.3) |
— | — | — | i‐HbA1c5.7:
Total: 8.0 (4.8–13.2) Men: 24.2 (9.5–61.8) Women: 4.6 (2.4–8.7) White: 10.2 (5.0–20.8) Black: 5.8 (2.9–11.7) |
HbA1c5.7 + IFG5.6: Total: 26.2 (16.3–42.1) Men: 51.1 (21.2–123.2) Women: 20.4 (10.9–38.0) White: 34.9 (19.1–63.8) Black: 14.9 (6.8–32.6) |
Odds ratio |
| Liu 2008 | 5 | — | 4.5 (2.0–10.1) | — | — | — | — | — | Risk ratio |
| Liu 2016 | 10.9 | 1.99 (1.37–2.90) [2.12 (1.46–3.08)] | — | — | — | — | — | — | Hazard ratio |
| Liu 2017 | 7.8 | — | 3.67 (3.20–4.21) [4.36 (3.83–4.97)] |
— | — | — | — | — | Odds ratio |
| Lorenzo 2003 | 7–8 | — | — | 6.37 (4.37–9.28) | — | — | — | — | Odds ratio |
| Lyssenko 2005 | 6 | [i‐IFG6.1: 2.3 (1.4–3.7)] | — | [i‐IGT: 3.5 (2.1–5.8)] | — | [3.8 (2.3–6.2)] | — | — | Hazard ratio |
| Man 2017 | 6 | — | — | — | — | 4.54 (2.65–7.78) | — | — | Risk ratio |
| Mykkänen 1993 | 3.5 | — | — | [9.85 (6.14–15.8)] | — | — | — | — | Odds ratio |
| Nakagami 2016 | 5 | 34.89 (19.65–61.95) [37.85 (22.73–63.05)] | — | — | — | — | HbA1c6.0: [63.16 (33.94–117.52)] HbA1c5.7: 8.77(4.47–17.21) [9.72(4.96–19.05)] |
— | Hazard ratio |
| Nakanishi 2004 | 7 | 1.31 (0.51–3.34) | — | — | — | — | — | — | Risk ratio |
| Rathmann 2009 | 7 | [4.7 (2.2–10.0)] | — | [8.8 (5.0–15.6)] | — | [21.2 (10.4–43.3)] | — | — | Odds ratio |
| Rijkelijkhuizen 2007 | 6.4 | i‐IFG6.1: 10.0 (6.1–16.5) | — | i‐IGT: 10.9 (6.0–19.9) | — | 39.5 (17.0–92.1) | — | — | Odds ratio |
| Sadeghi 2015 | 7 | — | i‐IFG5.6: 3.30 (2.16–5.06) | i‐IGT: 2.52 (1.73–3.69) | — | 12.6 (7.39–21.4) | — | — | Odds ratio |
| Sato 2009 | 4 | 22.52 (17.73–28.60) | — | — | — | — | — | Odds ratio | |
| Song 2015 | 4 | — | Men: 7.50 (2.76–20.33) Women: 4.27 (1.52–12.00) | — | — | — | — | — | Relative risk |
| Soriguer 2008 | 6 | — | [5.3 (2.7–10.4)] | 4.3 (2.0–9.2) | — | 9.2 (4.3–19.5) | — | — | Relative risk |
| Stengard 1992 | 5 | — | — | 3.1 (1.2–8.2) | — | — | — | — | Odds ratio |
| Vaccaro 1999 | 11.5 | [i‐IFG6.1: 1.2 (0.3–10.2)] | [i‐IGT: 6.2 (2.7–13.8)] | — | [10.3 (2.2–46.8)] | — | — | Odds ratio | |
| Valdes 2008 | 6.3 | 12.1 (4.6–31.7) [11.5 (5.6–23.6] |
3.9 (1.6–9.8) | [6.7 (3.4–13.3)] [i‐IGT: 4.7 (1.9–11.7)] |
— | [45.6 (15.8–131.4)] | — | — | Odds ratio |
| Viswanathan 2007 | 5 | — | — | 1.57 | — | — | — | — | Odds ratio |
| Wang 2007 | 5 | 2.71 (1.43–5.16) Men: 2.29 (0.95–5.49) Women: 1.95 (0.83–4.61) |
1.80 (0.96–3.40) Men: 1.79 (0.70–4.57) Women: 2.08 (0.93–4.67) |
3.15 (1.60–6.19) i‐IGT(IFG6.1): Men: 7.33 (2.62–20.51) Women: 1.65 (0.76–3.60) i‐IGT(IFG5.): Men: 7.50 (1.62–34.63) Women: 2.21 (0.77–6.36) |
— | IGT/IFG6.1: Men: 10.23 (3.84–27.30) Women: 7.11 (2.56–19.72) IGT/IFG5.6: Men: 9.81 (3.5–27.21) Women: 4.67 (1.87–11.62) |
— | — | Risk ratio, odds ratio |
| Wang 2011 | 7, 8 | — | Total: 2.38 (1.85–3.05) [2.68 (2.25–3.63] Men: 2.10 (1.40–3.15) [2.78 (1.91–4.04)] Women: 2.46 (1.78–3.39) [ 2.92 (2.15–3.98] 4 years: [3.12 (2.31–4.22)] |
Total: 3.47 (2.64–4.55) [4.11 (3.20–5.27)] Men: 3.82 (2.41–6.04) [4.72 (3.15–7.09)] Women: 3.16 (2.26–4.43) [3.74 (2.72–5.14)] |
— | Total: 4.06 (3.05–5.40) [4.68 (3.62–6.07) ] Men: 4.44 (2.75–7.15) [5.28 (3.49–7.99)] Women: 3.80 (2.66–5.42) [4.30 (3.09–5.99)] |
4 years: HbA1c6.0: 5.89 (4.23–8.19) |
— | Hazard ratio, odds ratio (4 years) |
| Warren 2017 | Cohort 1: 22 Cohort 2: 16 |
Cohort 1:
2.85 (2.60–3.12) Black: 2.66 (2.26–3.13) White: 2.86 (2.57–3.19) Cohort 2: 3.41 (3.01–3.85) Black: 3.16 (2.47–4.06) White: 3.67 (3.18–4.23) |
Cohort 1:
2.26 (2.08–2.45)
Black: 2.05 (1.75–2.40)
White: 2.30 (2.10–2.53) Cohort 2: 2.70 (2.43–3.00) Black: 2.65 (2.11–3.32) White: 2.87 (2.54–3.23) |
Cohort 2:
2.06 (1.84‐2.31) Black: 2.55 (2.01–3.22) White: 1.95 (1.71–2.21) |
— | — | Cohort 1:
HbA1c5.7:
2.71 (2.48–2.95) Black: 2.24 (1.92–2.61) White: 2.91 (2.63–3.22) HbA1c6.0: 3.12 (2.81–3.46) Black: 2.60 (2.21–3.05) White: 3.64 (3.20–4.14) 6 years: HbA1c6.0: 9.24 (7.20–11.86) |
— | Hazard ratio |
| Yeboah 2011 | 7.5 | — | 10.5 (8.4–13.1) [13.2 (10.7–16.2)] | — | — | — | — | — | Hazard ratio |
| Zethelius 2004 | 7 | — | — | [2.18 (1.43–3.34)] | — | — | — | — | Odds ratio |
|
aUnreliable adjusted HbA1c6.0 interval in publication: 105.47 (29.30–101.86) CI: confidence interval; FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG; i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; IH: intermediate hyperglycaemia; T2DM: type 2 diabetes mellitus | |||||||||
Appendix 15. Regression from intermediate hyperglycaemia to normoglycaemia
| Study ID | Follow‐up (years) | Regression to normoglycaemia from IH at baseline |
| Ammari 1998 | 2 | IGT: 27/68 (39.7%) |
| Anjana 2015 | 9.1 | i‐IFG5.6 or i‐IGT: 52/299 (17.4%) |
| Baena‐Diez 2011 | 10 | IFG6.1: 57/115 (49.6%) |
| Bai 1999 | 1 | IGT: 162/252 (64.3%) |
| Charles 1997 | 2 | IGT: 273/418 (65.3%) |
| Chen 2003 | 3 | IFG6.1: 129/156 (82.6%) |
| Coronado‐Malagon 2009 | 1, 2 | 'Prediabetes': 76/217 (35%) |
| Cugati 2007 | 10 | IFG5.6: 5 years: 94/229 (27.9%); 10 years: 15/229 (6.6%) IFG6.1: 5 years: 34/50 (68%); 10 years: 2/50 (4%) |
| De Abreu 2015 | 10 | IFG5.6: 104/187 (55.6%) |
| Dowse 1991 | 6.2 | IGT: 20/51 (39%) |
| Ferrannini 2009 | 7 | IGT: 73/170 (42.9%) |
| Forouhi 2007 | 10 | IFG6.1: 143/257 (55.6%) |
| Guerrero‐Romero 2006 | 5 | IGT: 3/75 (4%) |
| Heianza 2012 | 5 | IFG5.6: 383/1680 (22.8%) IFG6.1: 101/380 (26.5%) HbA1c5.7: 263/822 (32%) HbA1c6.0: 63/203 (31.0%) HbA1c5.7/IFG5.6: 428/2092 (20.5%) HbA1c6.0/IFG5.6: 392/1748 (22.4%) |
| Inoue 1996 | 2.5 | IGT: 11/37 (29.7%) |
| Jiamjarasrangsi 2008a | 2.6 | IFG5.6: 197/320 (61.6%) |
| Kim 2008 | 2 | IFG total: 908/1829 (49.6%) IFG5.6: 747/1335 (56%) IFG6.1: 161/494 (32.6%) |
| Kleber 2010 | 1 | IGT: 52/79 (65.8%) |
| Kleber 2011 | 3.9 | IGT: 96/119 (80.1%) |
| Ko 1999 | 1.4 | IGT: 60/123 (48.8%) |
| Ko 2001 | 1.7 | IFG6.1: 17/55 (30.9%) |
| Larsson 2000 | 10 | i‐IFG6.1: 27/42 (64.3%) i‐IGT: 36/66 (54.6%) IFG/IGT: 17/30 (56.7%) |
| Latifi 2016 | 5 | IFG5.6: 62/124 (50%) |
| Lecomte 2007 | 5 | IFG6.1: 297/743 (44%) |
| Leiva 2014 | 6 | IFG6.1: 0/28 (0%) |
| Li 2003 | 2 | IGT: 22/131 (16.8%) |
| Liu 2014 | 3 | IFG or IGT: 130/450 (28.9%) |
| Lyssenko 2005 | 6 | IFG or IGT: 379/686 (55.2%) |
| Marshall 1994 | 1.9 | IGT: 60/123 (48.8%) |
| Mohan 2008 | 8 | IGT: 6/37 (16.2%) |
| Motala 2003 | 10 | IGT: 16/35 (45.7%) 4 years: IGT: 28/72 (38.9%) |
| Mykkänen 1993 | 3.5 | IGT: 72/203 (35.5%) |
| Peterson 2017 | 10 | IGT: 8/29 (27.6%) |
| Qian 2012 | 5 | i‐IFG6.1: 14/46 (30.4%) i‐IGT: 45/120 (37.5%) IFG/IGT: 8/33 (24.2%) |
| Rajala 2000 | 4.6 | IGT: 96/171 (56.1%) (2.1 years) IGT: 115/183 (62.8%) |
| Ramachandran 1986 | 3.3 | IGT: 34/107 (31.8%) |
| Rijkelijkhuizen 2007 | 6.4 | IFG6.1: 28/149 (18.8%)
IFG5.6: 33/488 (6.8%) (3 years) IGT: 35/158 (22.2%) |
| Sadeghi 2015 | 7 | IFG5.6and/or IGT: 148/373 (39.7%) |
| Sasaki 1982 | 7 | IGT: 5/13 (38.5%) |
| Schranz 1989 | 6 | IGT: 25/75 (33.3%) |
| Sharifi 2013 | 7 | IFG5.6: 53/123 (43.1%) |
| Söderberg 2004 | 11 | i‐IFG6.1: 153/402 (38%) IGT: 296/1253 (23.6%) |
| Song 2016a | 10.8 | Total: 75/334 (22.5%) Men: 28/125 (22.4%) Women: 47/209 (22.5%) |
| Stengard 1992 | 5 | IGT: 79/234 (33.8%) |
| Toshihiro 2008 | 3.2 | IFG and/or IGT: 39/128 (30.5%) |
| Wang 2011 | 4 | IGT: 147/532 (27.6%) |
| Wat 2001 | 2 | IGT: 174/322 (54%) |
| Weiss 2005 | 1.7 | i‐IGT: 15/33 (45.5%) |
| Wong 2003 | 8 | IGT: 122/291 (41.9%) |
| HbA1c: glycosylated haemoglobin A1c; HbA1c5.7/6.0: HbA1c threshold 5.7% or 6.0% (usually reflecting 5.7% to 6.4% and 6.0% to 6.4%, respectively); HbA1c/IFG: both HbA1c and IFG;i‐: isolated; IFG5.6/6.1: impaired fasting glucose (threshold 5.6 mmol/L or 6.1 mmol/L); IGT: impaired glucose tolerance; IFG/IGT: both IFG and IGT; IH: intermediate hyperglycaemia; IQR: interquartile range; SD: standard deviation | ||
Appendix 16. Confounder adjustment (I)
| Study ID | Age | Sex | Body mass index, waist circumference, waist‐to‐hip ratio | 'Ethnicity' | Site | Smoking status | Drinking status | Physical activity | Medications |
| Admiraal 2014 | Yes | Yes | Yes | No | No | No | No | No | No |
| Aekplakorn 2006 | No | No | No | No | No | No | No | No | No |
| Bae 2011 | Yes | Yes | No | No | No | No | No | No | No |
| Bergman 2016 | Yes | Yes | Yes | No | No | Yes | No | No | No |
| Bonora 2011 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No |
| Cederberg 2010 | No | Yes | Yes | No | No | Yes | Yes | Yes | No |
| Chamnan 2011 | Yes | Yes | Yes | No | No | Yes | No | No | Yes |
| Chen 2003 | Yes | Yes | Yes | No | No | No | No | No | No |
| Coronado‐Malagon 2009 | No | No | No | No | No | No | No | No | No |
| Cugati 2007 | Yes | Yes | No | No | No | No | No | No | No |
| De Abreu 2015 | Yes | No | Yes | No | No | Yes | Yes | Yes | No |
| Derakhshan 2016 | Yes | Yes | Yes | No | No | Yes | No | Yes | No |
| Dowse 1991 | No | No | No | No | No | No | No | No | No |
| Ferrannini 2009 | No | No | No | No | No | No | No | No | No |
| Filippatos 2016 | Yes | Yes | No | No | No | Yes | No | Yes | No |
| Forouhi 2007 | Yes | Yes | Yes | No | No | Yes | No | Yes | No |
| Han 2017 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No |
| Hanley 2005 | Yes | Yes | No | Yes | Yes | No | No | No | No |
| Heianza 2012 | Yes | Yes | Yes | No | No | Yes | No | No | No |
| Janghorbani 2015 | Yes | Yes | Yes | No | No | No | No | No | No |
| Jeong 2010 | No | No | Yes | No | No | No | No | No | No |
| Kim 2005 | Yes | Yes | Yes | No | No | No | No | No | No |
| Kim 2016a | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No |
| Latifi 2016 | Yes | No | Yes | Yes | No | No | No | No | No |
| Leiva 2014 | No | No | No | No | No | Yes | No | No | Yes |
| Levitzky 2008 | Yes | No | Yes | No | No | Yes | No | No | No |
| Li 2003 | Yes | Yes | Yes | No | No | No | No | No | No |
| Lipska 2013 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Liu 2008 | Yes | Yes | No | No | No | Yes | Yes | No | No |
| Liu 2016 | Yes | No | Yes | No | No | No | No | Yes | No |
| Liu 2017 | Yes | No | No | No | No | Yes | Yes | Yes | No |
| Lorenzo 2003 | Yes | Yes | No | No | No | No | No | No | No |
| Lyssenko 2005 | No | No | Yes | No | No | No | No | No | No |
| Man 2017 | Yes | Yes | Yes | No | No | Yes | No | No | No |
| Mykkänen 1993 | No | No | No | No | No | No | No | No | No |
| Nakagami 2016 | Yes | No | Yes | No | No | Yes | Yes | No | No |
| Nakanishi 2004 | Yes | No | No | No | No | Yes | Yes | No | No |
| Rathmann 2009 | Yes | Yes | No | No | Yes | No | No | No | No |
| Rijkelijkhuizen 2007 | Yes | Yes | No | No | No | No | No | No | No |
| Sadeghi 2015 | Yes | Yes | Yes | No | No | No | No | No | No |
| Sato 2009 | Yes | NA | Yes | No | No | Yes | Yes | Yes | No |
| Song 2015 | Yes | No | Yes | No | No | Yes | Yes | Yes | No |
| Soriguer 2008 | Yes | Yes | Yes | No | No | No | No | No | No |
| Stengard 1992 | Yes | No | Yes | No | No | No | No | No | No |
| Vaccaro 1999 | No | No | No | No | No | No | No | No | No |
| Valdes 2008 | Yes | Yes | Yes | No | No | No | No | No | No |
| Viswanathan 2007 | Yes | No | Yes | No | No | No | No | No | No |
| Wang 2007 | Yes | Yes | No | No | No | Yes | No | No | No |
| Wang 2011 | Yes | Yes | Yes | No | No | Yes | No | No | No |
| Warren 2017 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| Yeboah 2011 | Yes | Yes | Yes | Yes | No | No | No | Yes | No |
| Zethelius 2004 | Yes | No | Yes | No | No | No | No | No | No |
| 'No' denotes possible confounder but statistical analysis did not adjust for this covariate 'Yes' indicates that statistical analysis adjusted for this confounder NA: not applicable | |||||||||
Appendix 17. Confounder adjustment (II)
| Study ID | Cardiovascular disease | Glomerular filtration rate, albuminuria | Blood pressure, hypertension | Family history of diabetes | Socioeconomic status | Region | Depression | Triglycerides | Cholesterol |
| Admiraal 2014 | No | No | No | No | No | No | No | No | No |
| Aekplakorn 2006 | No | No | No | No | No | No | No | No | No |
| Bae 2011 | No | No | No | No | No | No | No | No | No |
| Bergman 2016 | Yes | No | Yes | No | No | No | No | Yes | Yes |
| Bonora 2011 | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Cederberg 2010 | No | No | No | No | No | No | No | No | No |
| Chamnan 2011 | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Chen 2003 | No | No | No | Yes | No | No | No | Yes | No |
| Coronado‐Malagon 2009 | No | No | No | No | No | No | No | No | No |
| Cugati 2007 | No | No | No | No | No | No | No | No | No |
| De Abreu 2015 | No | No | Yes | No | No | No | No | Yes | Yes |
| Derakhshan 2016 | No | No | No | Yes | Yes | No | No | Yes | Yes |
| Dowse 1991 | No | No | No | No | No | No | No | No | No |
| Ferrannini 2009 | No | No | No | No | No | No | No | No | No |
| Filippatos 2016 | No | No | Yes | No | No | No | No | Yes | Yes |
| Forouhi 2007 | No | No | No | Yes | No | No | No | No | No |
| Han 2017 | No | No | Yes | Yes | No | Yes | No | Yes | Yes |
| Hanley 2005 | No | No | No | No | No | No | No | No | No |
| Heianza 2012 | No | No | Yes | Yes | No | No | No | Yes | Yes |
| Janghorbani 2015 | No | No | No | No | No | No | No | Yes | Yes |
| Jeong 2010 | No | No | Yes | No | No | No | No | Yes | Yes |
| Kim 2005 | No | No | Yes | Yes | No | No | No | Yes | Yes |
| Kim 2016a | No | No | Yes | Yes | No | No | No | Yes | Yes |
| Latifi 2016 | No | No | Yes | Yes | No | No | No | No | No |
| Leiva 2014 | No | No | No | Yes | No | No | No | No | No |
| Levitzky 2008 | No | No | No | No | No | No | No | No | No |
| Li 2003 | No | No | No | No | No | No | No | No | No |
| Lipska 2013 | No | No | Yes | No | No | No | No | No | No |
| Liu 2008 | No | No | No | Yes | No | No | No | No | No |
| Liu 2016 | No | No | No | No | No | No | No | No | No |
| Liu 2017 | No | No | No | No | Yes | Yes | No | No | No |
| Lorenzo 2003 | No | No | No | Yes | No | No | No | No | No |
| Lyssenko 2005 | No | No | No | No | No | No | No | No | No |
| Man 2017 | No | No | Yes | Yes | Yes | No | No | No | Yes |
| Mykkänen 1993 | No | No | No | No | No | No | No | No | No |
| Nakagami 2016 | No | No | Yes | Yes | No | No | No | No | Yes |
| Nakanishi 2004 | No | No | No | Yes | No | No | No | No | No |
| Rathmann 2009 | No | No | Yes | No | No | No | No | No | No |
| Rijkelijkhuizen 2007 | No | No | No | No | No | No | No | No | No |
| Sadeghi 2015 | No | No | No | Yes | No | No | No | No | No |
| Sato 2009 | No | No | No | Yes | No | No | No | No | No |
| Song 2015 | No | No | Yes | Yes | No | No | No | Yes | No |
| Soriguer 2008 | No | No | Yes | Yes | No | No | No | Yes | No |
| Stengard 1992 | No | No | No | No | No | No | No | No | No |
| Vaccaro 1999 | No | No | No | No | No | No | No | No | No |
| Valdes 2008 | No | No | No | No | No | No | No | Yes | No |
| Viswanathan 2007 | No | No | No | Yes | No | No | No | No | No |
| Wang 2007 | No | No | No | Yes | Yes | No | No | No | Yes |
| Wang 2011 | No | No | Yes | Yes | No | No | No | Yes | Yes |
| Warren 2017 | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Yeboah 2011 | No | No | No | No | Yes | No | No | No | No |
| Zethelius 2004 | No | No | No | No | No | No | No | No | No |
| 'No' denotes possible confounder but statistical analysis did not adjust for this covariate 'Yes' indicates that statistical analysis adjusted for this confounder | |||||||||
Data and analyses
Comparison 1. Hazard ratio as the effect measure for the development of T2DM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 T2DM incidence (IFG5.6) | 8 | 34867 | Hazard Ratio (Random, 95% CI) | 4.32 [2.61, 7.12] |
| 1.1 Asia/Middle East | 4 | 14803 | Hazard Ratio (Random, 95% CI) | 5.07 [3.41, 7.53] |
| 1.2 Australia/Europe/North America | 3 | 18522 | Hazard Ratio (Random, 95% CI) | 4.15 [1.24, 13.87] |
| 1.3 American Indians/Islands | 1 | 1542 | Hazard Ratio (Random, 95% CI) | 2.38 [1.85, 3.06] |
| 2 T2DM incidence (IFG6.1) | 10 | 21475 | Hazard Ratio (Random, 95% CI) | 5.47 [3.50, 8.54] |
| 2.1 Asia/Middle East | 5 | 10810 | Hazard Ratio (Random, 95% CI) | 10.55 [3.61, 30.81] |
| 2.2 Australia/Europe/North America | 4 | 10571 | Hazard Ratio (Random, 95% CI) | 3.30 [2.32, 4.67] |
| 2.3 Latin America | 1 | 94 | Hazard Ratio (Random, 95% CI) | 2.06 [1.76, 2.41] |
| 3 T2DM incidence (IGT) | 5 | 16576 | Hazard Ratio (Random, 95% CI) | 3.61 [2.31, 5.64] |
| 3.1 Asia/Middle East | 3 | 8475 | Hazard Ratio (Random, 95% CI) | 4.48 [2.81, 7.15] |
| 3.2 Australia/Europe/North America | 2 | 8101 | Hazard Ratio (Random, 95% CI) | 2.53 [1.52, 4.19] |
| 4 T2DM incidence (IFG + IGT) | 5 | 9757 | Hazard Ratio (Random, 95% CI) | 6.90 [4.15, 11.45] |
| 4.1 Asia/Middle East | 3 | 7156 | Hazard Ratio (Random, 95% CI) | 10.20 [5.45, 19.09] |
| 4.2 Australia/Europe/North America | 1 | 1650 | Hazard Ratio (Random, 95% CI) | 3.80 [2.30, 6.28] |
| 4.3 American Indians/Islands | 1 | 951 | Hazard Ratio (Random, 95% CI) | 4.06 [3.05, 5.40] |
| 5 T2DM incidence (HbA1c5.7) | 4 | 25047 | Hazard Ratio (Random, 95% CI) | 5.55 [2.77, 11.12] |
| 5.1 Asia | 3 | 16805 | Hazard Ratio (Random, 95% CI) | 7.21 [5.14, 10.11] |
| 5.2 Australia/Europe/North America | 1 | 8242 | Hazard Ratio (Random, 95% CI) | 2.71 [2.48, 2.96] |
| 6 T2DM incidence (HbA1c6.0) | 6 | 30699 | Hazard Ratio (Random, 95% CI) | 10.10 [3.59, 28.43] |
| 6.1 Asia/Middle East | 4 | 22734 | Hazard Ratio (Random, 95% CI) | 13.12 [4.10, 41.96] |
| 6.2 Australia/Europe/North America | 2 | 7965 | Hazard Ratio (Random, 95% CI) | 5.09 [1.69, 15.37] |
| 7 T2DM incidence (HbA1c + IFG) | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 HbA1c5.7 + IFG5.6 | 1 | 4559 | Hazard Ratio (Fixed, 95% CI) | 32.50 [23.00, 45.92] |
| 7.2 HbA1c5.7 + IFG6.1 | 1 | 5357 | Hazard Ratio (Fixed, 95% CI) | 37.90 [28.10, 51.12] |
| 7.3 HbA1c6.0 + IFG5.6 | 1 | 4628 | Hazard Ratio (Fixed, 95% CI) | 53.70 [38.40, 75.09] |
| 7.4 HbA1c6.0 + IFG6.1 | 1 | 5802 | Hazard Ratio (Fixed, 95% CI) | 52.30 [37.80, 72.37] |
Comparison 2. Odds ratio as the effect measure for the development of T2DM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 T2DM incidence (IFG5.6) | 21 | 47647 | Odds Ratio (Random, 95% CI) | 4.15 [2.75, 6.28] |
| 1.1 Asia/Middle East | 10 | 34577 | Odds Ratio (Random, 95% CI) | 2.94 [1.77, 4.86] |
| 1.2 Australia/Europe/North America | 9 | 9869 | Odds Ratio (Random, 95% CI) | 6.47 [3.81, 11.00] |
| 1.3 Latin America | 1 | 1659 | Odds Ratio (Random, 95% CI) | 4.28 [3.21, 5.71] |
| 1.4 American Indians/Islands | 1 | 1542 | Odds Ratio (Random, 95% CI) | 3.12 [2.31, 4.21] |
| 2 T2DM incidence (IFG6.1) | 15 | 36866 | Odds Ratio (Random, 95% CI) | 6.60 [4.18, 10.43] |
| 2.1 Asia/Middle East | 7 | 28921 | Odds Ratio (Random, 95% CI) | 5.18 [2.32, 11.53] |
| 2.2 Australia/Europe/North America | 7 | 6334 | Odds Ratio (Random, 95% CI) | 8.69 [4.95, 15.24] |
| 2.3 Latin America | 1 | 1611 | Odds Ratio (Random, 95% CI) | 3.73 [2.18, 6.38] |
| 3 T2DM incidence (IGT) | 20 | 21552 | Odds Ratio (Random, 95% CI) | 4.61 [3.76, 5.64] |
| 3.1 Asia/Middle East | 6 | 8643 | Odds Ratio (Random, 95% CI) | 3.74 [2.83, 4.94] |
| 3.2 Australia/Europe/North America | 11 | 9165 | Odds Ratio (Random, 95% CI) | 5.20 [3.62, 7.45] |
| 3.3 Latin America | 2 | 3478 | Odds Ratio (Random, 95% CI) | 4.94 [3.15, 7.76] |
| 3.4 American Indians/Islands | 1 | 266 | Odds Ratio (Random, 95% CI) | 3.60 [1.40, 9.26] |
| 4 T2DM incidence (IFG + IGT) | 9 | 9656 | Odds Ratio (Random, 95% CI) | 13.14 [7.41, 23.30] |
| 4.1 Asia/Middle East | 3 | 4202 | Odds Ratio (Random, 95% CI) | 6.99 [3.09, 15.83] |
| 4.2 Australia/Europe/North America | 6 | 5454 | Odds Ratio (Random, 95% CI) | 20.95 [12.40, 35.40] |
| 5 T2DM incidence (HbA1c5.7) | 3 | 3468 | Odds Ratio (Random, 95% CI) | 4.43 [2.20, 8.88] |
| 5.1 Asia/Middle East | 1 | 1137 | Odds Ratio (Random, 95% CI) | 4.54 [2.65, 7.78] |
| 5.2 Europe/North America | 2 | 2331 | Odds Ratio (Random, 95% CI) | 4.38 [1.36, 14.15] |
| 6 T2DM incidence (HbA1c6.0) | 3 | 18317 | Odds Ratio (Random, 95% CI) | 12.79 [4.56, 35.85] |
| 6.1 Asia/Middle East | 1 | 11866 | Odds Ratio (Random, 95% CI) | 23.20 [18.70, 28.78] |
| 6.2 Australia/Europe/North America | 1 | 5735 | Odds Ratio (Random, 95% CI) | 15.60 [6.90, 35.27] |
| 6.3 American Indians/Islands | 1 | 716 | Odds Ratio (Random, 95% CI) | 5.89 [4.23, 8.20] |
| 7 T2DM incidence (HbA1c5.7 + IFG5.6) | 2 | 14006 | Odds Ratio (Random, 95% CI) | 35.91 [20.43, 63.12] |
| 7.1 Australia/Europe/North America | 1 | 1294 | Odds Ratio (Random, 95% CI) | 26.20 [16.30, 42.11] |
| 7.2 Asia/Middle East | 1 | 12712 | Odds Ratio (Random, 95% CI) | 46.70 [33.60, 64.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Admiraal 2014.
| Name of study | Surinamese in the Netherlands: study on health and ethnicity/healthy life in an urban setting (SUNSET/HELIUS) | |
| Inclusion criteria | Participants of 2 studies (SUNSET and HELIUS), Surinamese and ethnic Dutch, southeast Amsterdam, aged 35–60 years with completed interviews and medical examinations at baseline and follow‐up | |
| Exclusion criteria | Missing FPG data, diabetes | |
| Notes | Baseline data for total cohort included in the analyses (N = 456): South‐Asian Surinamese (N = 90), African Surinamese (N = 190), ethnic Dutch (N = 176) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Surinamese in the Netherlands study |
| Study participation: description of glycaemic status at baseline | Low risk | 456 participants available for analysis; table 1 specifies people with IFG5.7 |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Random sample of 2975 Surinamese and ethnic Dutch individuals, aged 35–60, drawn from the population register of 2 neighbourhoods in southeast Amsterdam |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria specified |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Those who were lost to follow‐up were younger, had a higher BMI and greater waist circumference, a higher FPG and more often had baseline IFG than those with follow‐up data available after 10 years |
| Study attrition: reasons for loss to follow‐up provided | Low risk | 777/1444 lost to follow‐up (moved outside of Amsterdam, declined to participate, died, non‐response); figure S1 |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Reported in Table S2 |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | See above |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | FPG measurement by G6PD test |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.7–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5; self‐reported T2DM |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Reliable measurement |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Limited number of confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Adjustment for sex, age, BMI and change in BMI after 10 years |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Unadjusted and adjusted analyses |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression |
Aekplakorn 2006.
| Name of study | None | |
| Inclusion criteria | Eymployees of the Electric Generation Authority Bangkok, Thailand aged ≥ 35 years ('exploratory cohort'); middle‐income social class | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for cohort becoming diabetic (N = 361) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Cohort study of employees of the Electric Generation Authority of Bangkok, Thailand |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | 3499 employees aged ≥ 35 years; mostly urban dwellers of middle‐income social class |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria specified |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Of 3254 participants without diabetes at baseline, 2667 took part in the 1997 survey |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Individuals lost to follow‐up were slightly older |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Unclear, limited data only |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | 2‐h OGTT after 75‐g glucose load |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Glucose oxidase method |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 5.6 to < 7.0; IGT: 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 or 2‐h glucose ≥ 11.1; development of T2DM during the follow‐up period until 1997 according to FPG or diagnosis and/or receipt of diabetes medication during follow‐up |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Limited number of confounders |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Age, sex, BMI, waist circumference, smoking status, drinking status, family history, hypertension |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes; IFG status (model 2) and IGT status (model 3) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariable logistic regression |
Ammari 1998.
| Name of study | None | |
| Inclusion criteria | Community‐based survey of cardiovascular risk factors in 4 Jordanian towns, individuals aged ≥ 25 years; follow‐up on one of the town (Sikhra) and matched control group with non‐IGT (normal) individuals from initial survey | |
| Exclusion criteria | Diabetes | |
| Notes | Few baseline data reported for total study population (N = 212) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | 4 community‐based survey of cardiovascular risk factors in 4 Jordanian towns |
| Study participation: description of glycaemic status at baseline | Low risk | Community‐based survey of cardiovascular risk factors in 4 Jordanian towns |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not described (some comparison of participants with non‐participants) |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not described |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | FPG and 2‐h 75 g OGTT |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG 7.8 to < 11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes (probably FPG and 2‐h OGTT was also measured at follow‐up) |
| Study confounding: important confounders measured | Unclear risk | Some baseline parameters were investigated (hypercholesterolaemia, hypertriglyceridaemia, obesity, hypertension, family history of diabetes) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Scarce data |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Scarce data |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Not reported |
Anjana 2015.
| Name of study | Chennai Urban Rural Epidemiology Study (CURES) | |
| Inclusion criteria | Representative sample from Chennai, ≥ 20 years of age | |
| Exclusion criteria | Diabetes at baseline, unknown glycaemic status | |
| Notes | Baseline data for cohort becoming diabetic at follow‐up (N = 176) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Chennai Urban Rural Epidemiology Study |
| Study participation: description of glycaemic status at baseline | Low risk | 299 with 'prediabetes' |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Representative sample from Chennai, ≥ 20 years |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria specified |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | High risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | i‐IFG, i‐IGT, IFG/IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | i‐IGT: 2‐h PG 7.8–11.0 and FPG > 5.6; i‐IFG: FPG 5.6–6.9 and 2‐h PG < 7.8; prediabetes: FPG 5.6–6.9 or 2‐h PG 7.8–11.0 (i‐IGT or i‐IFG or IFG/IGT) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; diagnosed; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | For IFG/IGT, several confounders measured as predictors for incident diabetes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cox proportional hazards model for various single factors |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Univariate analyses |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Cox proportional hazards model, univariate analyses for single variables |
Bae 2011.
| Name of study | None | |
| Inclusion criteria | Individuals who participated in comprehensive health check‐ups annually for 5 years | |
| Exclusion criteria | Anaemia with a haemoglobin level < 7.4 mmol/L; self‐reported diabetes and undiagnosed diabetes (FPG concentration 7.0 mmol/l or HbA1c 6.5%; absence of HbA1c data at any visit | |
| Notes | Baseline data for total cohort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Participants partially undergoing annual or biannual health check‐ups (Kangbuk Samsung Hospital Total,Healthcare Center) |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | HbA1c5.7 and HbA1c6.0 |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Unclear risk | Normal reference for HbA1c: < 5 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5; history of diabetes; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | 2 covariates measured: age and sex |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | 2 covariates included: age and sex |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | 2 covariates analysed: age and sex |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Kaplan‐Meier method, Cox proportional hazard analysis (2 covariates), ROC analysis |
Baena‐Diez 2011.
| Name of study | None | |
| Inclusion criteria | Participants aged > 18 years visiting a healthcare centre with impaired fasting glucose measured twice | |
| Exclusion criteria | Corticosteroid therapy, terminal illness, life expectancy of 1 year or less, diabetes | |
| Notes | Baseline data for cohort with intermediate hyperglycaemia (N = 115) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Healthcare centre in Barcelona, Spain, "Cohorta Zona Franca" |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria specified |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Quote: "no significant differences" |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | FPG measured twice |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: 6.1–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 (measured twice) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | FPG |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some variables (univariate analyses) associated with progression to diabetes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some confounders measured |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Univariate analyses for single variables |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Cox regression for other risk factors (e.g. obesity) associated with progression to diabetes |
Bai 1999.
| Name of study | None | |
| Inclusion criteria | Staff of the Indian Institute of Technology of Chennai, along with their family members, aged 20 years and over | |
| Exclusion criteria | Treatment for diabetes | |
| Notes | Baseline data for the IGT cohort (N = 252) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Staff of the Indian Institute of Technology of Chennai, along with their family members, aged 20 years and over |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | High risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | High risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 7.8 to < 11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Not reported, cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Not reported |
Bergman 2016.
| Name of study | Israel study of glucose intolerance, obesity and hypertension (Israel GOH study) | |
| Inclusion criteria | Survival until follow‐up with fasting blood glucose < 126 mg/dL (7.0 mmol/L) and 1‐ and 2‐h postload glucose values available at baseline | |
| Exclusion criteria | Individuals with diabetes | |
| Notes | Baseline data for IGT cohort (N = 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Israeli general population registry sample |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Comment: "no differences" between non‐participants and participants |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Comment: IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | Comment: FPG 5.6–7.8; 2‐h BG 7.8–11.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Unclear risk | Comment: FPG ≥ 7.8, 2‐h BG ≥ 11.1; reported diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Unclear risk | Non‐standard FPG thresholds |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Comment: some confounders were measured |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Comment: scarce data |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Comment: scarce data |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple multinomial logistic regression |
Bonora 2011.
| Name of study | Bruneck Study | |
| Inclusion criteria | White men and women, aged 40–79 years | |
| Exclusion criteria | Not reported | |
| Notes | No baseline data (except white participants aged > 40 years, N = 919) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Bruneck study, a long‐term prospective population‐based study of atherosclerosis and its risk factors |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | High risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Unclear risk | HbA1c categories, IFG (additional analyses) |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | HbA1: 6.0–6.49; IFG: not defined, probably FPG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5; diabetes treatment |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards models; additional models were run with updates variables (HbA1c and other variables were assessed every 5 years during follow‐up) |
Cederberg 2010.
| Name of study | None | |
| Inclusion criteria | All inhabitants of the city of Oulo, Finland, born in 1935 | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for the total cohort (N = 553), men (N = 223), women (N = 330) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Part of a longer follow‐up study assessing type 2 diabetes and IGT |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG, IGT, IFG/IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: 6.1–6.9; 2‐h PG < 7.8; IGT: FPG > 7.0; 2‐h PG 7.8 to < 11.1; elevated HbA1c: 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | Confirmed by 2 diabetic 75 g OGTTs (2‐h PG ≥ 11.1) and/or fasting values |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, risk ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Log‐binomial regression |
Chamnan 2011.
| Name of study | European Prospective Investigation of Cancer (EPIC)‐Norfolk cohort | |
| Inclusion criteria | Participants aged 40–74 years from the Norfolk region, UK; individuals with HbA1c measurements at baseline and the second health assessment | |
| Exclusion criteria | Diabetes at baseline, missing data | |
| Notes | Baseline data for HbA1c 6.0–6.4 cohort (N = 370) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Population‐based study monitoring individuals recruited from general practice in the Norfolk region, UK |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | HbA1c (50% of all participants had information on this measure at baseline); analyses were limited to these individuals |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | HbA1c 6.0–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | HbA1c ≥ 6.5; reported physician‐diagnosed diabetes or diabetes medications; antihyperglycaemic medication; diagnosis through medical records, registers or death certificates; results for clinically and/or biochemically diagnosed diabetes were used |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression (for every 0.5% increase in HbA1c as well as for different categories of HbA1c) |
Charles 1997.
| Name of study | Paris Prospective Study | |
| Inclusion criteria | Longitudinal epidemiologic study of cardiovascular risk factors in male employees of the Paris police, born in France between 1917–28 | |
| Exclusion criteria | No diabetes or cardiovascular disease | |
| Notes | Baseline data for individuals with IGT converting to T2DM (N = 32) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Longitudinal epidemiologic study of cardiovascular risk factors in male employees of the Paris |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | High risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | High risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG ≥ 7.8 to < 11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985); physician diagnosed diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes (see below) |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression (odds ratio for an increase of 1 SD in the population of participants with NGT or IGT) |
Chen 2003.
| Name of study | None | |
| Inclusion criteria | Residents of Penghu, Taiwan aged 40–79 years were selected for a baseline diabetes prevalence study | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for cohort converting to T2DM (N = 26) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Random sample of residents of Penghu, Taipei were selected for a baseline diabetes prevalence survey |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Quote: "the 600 persons who were re‐examined did not significantly differ from the others" |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–7.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Age‐sex adjusted odds ratio |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (selected risk factors) |
Chen 2017.
| Name of study | None | |
| Inclusion criteria | Participants with complete 3 year follow‐up and non‐pharmacological interventions | |
| Exclusion criteria | Participants aged 0–60 years, incomplete baseline data, diabetes at baseline | |
| Notes | Baseline data for i‐IFG/i‐IGTand IFG/IGT across age groups < 40 years + > 60 years (data indicate range across groups) (i‐IFG < 40 years: N = 51 and > 60 years: N = 278; i‐IGT < 40 years: N = 41 and > 60 years: N = 151; IFG/IGT: < 40 years: N = 34 and > 60 years: N = 175) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Permanent participants of Fujian province (China), part of the baseline survey from the REACTION study investigating the association between diabetes and cancer |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG, IGT, IFG/IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6–6.9 + 2‐h PG ≤ 7.8; IGT: FPG < 5.6 + 2‐h PG 7.8 to ≤ 11.0; IFG/IGT: FPG 5.6–6.9 + 2‐h PG 7.8 to ≤ 11.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; previously diagnosed diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Confounder adjustment for HOMA‐IR and HOMA‐B |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes (HOMA‐IR, HOMA‐B) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Stepwise multiple regression analysis (for HOMA‐IR or HOMA‐B) |
Coronado‐Malagon 2009.
| Name of study | None | |
| Inclusion criteria | Healthy Mexicans | |
| Exclusion criteria | Previous diabetes diagnosis, various diseases and medications affecting glucose metabolism | |
| Notes | Baseline characteristics for the prediabetic cohort (N = 217) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Personnel working for Petróleos Mexicanos with annual health‐checkups living in the metropolitan area of Mexico City |
| Study participation: description of glycaemic status at baseline | Unclear risk | Quote: "prediabetes" |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Unclear risk | IFG and IGT (ADA 2007), vague definition |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Unclear risk | IFG and IGT: 5.6–6.9 and 7.8 to < 11.1 (ADA 2007), vague definition |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Unclear risk | FPG ≥ 7.0 or 2‐h PG ≥ 11.1 (ADA 2007), vague definition |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Scarce data |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Scarce data |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Scarce data |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Scarce data |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Scarce data |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, relative risk |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression |
Cugati 2007.
| Name of study | Blue Mountains Eye Study (BMES) | |
| Inclusion criteria | Survey of vision and common eye diseases in 2 postcode areas west of Sydney; all permanent non‐institutionalised residents with birth date prior to January 1943 (aged 49+ at baseline) were invited to attend a detailed eye examination at a local clinic | |
| Exclusion criteria | Nursing home residents, diabetes at baseline, missing data | |
| Notes | Baseline data for BMES I study, people without diabetes (N = 3437/3654) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Older community within the geographically defined area west of Sydney, Australia; population‐based survey of vision and common eye diseases |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes, for most variables |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6 ‐6.9 (originally FPG ≥ 6.1 to < 7.0) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; self‐reported diabetes history; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Few variables (adjustment for age and sex) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Few variables |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Few variables |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate‐adjusted discrete logistic models, few variables |
De Abreu 2015.
| Name of study | Geelong Osteoporosis Study (GOS) | |
| Inclusion criteria | Female arm of the GOS | |
| Exclusion criteria | No FPG level or self‐report of antihyperglycaemic medication or diabetes status | |
| Notes | Baseline data for IFG cohort at baseline (N = 187) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Utilised data from the female arm of the Geelong Osteoporosis Study, Australia |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: 5.5–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; self‐reported; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes, also age‐standardised incidence rate and additional covariates reported (metabolic syndrome, fasting glucose at baseline) (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression |
Den Biggelaar 2016.
| Name of study | Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) | |
| Inclusion criteria | Individuals with an elevated risk of type 2 diabetes and cardiovascular disease | |
| Exclusion criteria | Previously diagnosed type 2 diabetes at baseline, who did not undergo an OGTT and incomplete OGTT data | |
| Notes | Baseline data for prediabetic group (N = 122) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Participants of the Cohort on Diabetes and Atherosclerosis Masstricht (CODAM) study on natural progression of glucose tolerance |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Analyses restricted individuals without T2DM who participated in the follow‐up measurements |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG and IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 6.1–6.9; 2‐h PG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Not reported, cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Discriminatory ability of beta‐cell functions indices to predict 'prediabetes' and T2DM by means of ROC curves |
Derakhshan 2016.
| Name of study | Tehran Lipid and Glucose Study (TLGS) | |
| Inclusion criteria | 3 separate analyses to investigate incidence of type 2 diabetes, hypertension and chronic kidney disease | |
| Exclusion criteria | Individuals aged < 20 years, type 2 diabetes at baseline, missing data, no follow‐ups | |
| Notes | Baseline data for 'prediabetes' group with normal blood pressure (IFG and/or IGT, N = 523) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Population‐based study on a representative sample of the population of Tehran to determine the prevalence and incidence of non‐communicable diseases and their risk factors |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Unclear risk | Quote: "prediabetes" (IFG and IGT) |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | 5.55 ≤ FPG < 7.0; 7.77 ≤ 2‐h PG ≤ 11.1; no antihyperglycaemic medication |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Low risk | Multiple imputation |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazard models |
Dowse 1991.
| Name of study | Nauru Study | |
| Inclusion criteria | All Nauruans aged 20 years and over; this survey included 266 individuals who were not diabetic in the combined 1975/76 survey; individuals who had previously attended either or both the 1975/76 and 1982 surveys; individuals with at least one parent identified as being of Nauruan heritage | |
| Exclusion criteria | Diabetes | |
| Notes | No baseline data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Nauruan population, persons of Micronesian ancestry |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Description of inclusion and exclusion criteria |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Some reasons provided |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 7.8 and 2‐h PG ≥ 7.8 ‐ < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985); FPG ≥ 7.8 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some confounders were measured |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression models |
Ferrannini 2009.
| Name of study | Mexico City Diabetes Study | |
| Inclusion criteria | Population‐based study of diabetes and cardiovascular risk factors in low‐income neighbourhoods in Mexico City, participants aged 35–64 years | |
| Exclusion criteria | Type 2 diabetes, type 1 diabetes, pregnant women | |
| Notes | Baseline characteristics provided for a range across different definitions of 'prediabetes' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Data were collected as part of the Mexico City Diabetes Study |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Description of inclusion and exclusion criteria |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Unclear, limited data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | (i)IFG, (i)IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9; IGT: FPG < 7.0 and 2‐h PG 7.8–11.1; i‐IFG6.1/i‐IFG5.6: 2‐h PG < 7.8 and FPG 6.1–6.9/5.6–6.1; i‐IGT/i‐IGT6.1/i‐IGT5.6 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Not for transition data (intermediate hyperglycaemia to T2DM) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Scarce data |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Scarce data |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, relative risk (multiple model odds ratios were calculated for 1 SD of the population value of that variable, in order to compare the relative importance of the variables (sex, familial diabetes, age, BMI, FPG, 2‐h PG) |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Logistic regression (for calculation of odds ratios, see above) |
Filippatos 2016.
| Name of study | ATTICA (province of Attica, Greece) | |
| Inclusion criteria | 1 participant per household, inhabitants from the Attica province | |
| Exclusion criteria | People living in institutions; people with CVD and of those with chronic viral infections | |
| Notes | Baseline data for IFG5.6 cohort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | ATTICA study |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described (participants with no diabetes and no CVD at baseline) |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes (85% participation rate) |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG5.6 |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FBG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FBG > 6.9; use of antidiabetic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some confounders included |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some confounders analysed |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression models |
Forouhi 2007.
| Name of study | Ely Study (Cambridgeshire, UK) | |
| Inclusion criteria | All adults free of known diabetes registered with a single practice serving Ely, adults aged 40–69 years | |
| Exclusion criteria | Diabetes | |
| Notes | Baseline data for the IFG6.1 cohort (N = 257) Cumulative incidence increased across increasing age groups and was higher in men than in women |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | The Ely Study (Cambridgeshire, UK) was a prospective study of the aetiology of T2DM |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG6.1: FPG 6.1–6.9 (FPG < 7.0 and 2‐h PG < 11.1) and IFG5.6: FPG 5.6–6.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; physician diagnosis or treatment for diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression (cumulative hazard curves by glucose status) |
Garcia 2016.
| Name of study | Sacramento Area Latino Study on Aging (SALSA) | |
| Inclusion criteria | Older Mexican Americans residing in the Sacramento metropolitan statistical area | |
| Exclusion criteria | Missing baseline diabetes status, certain neighbourhoods | |
| Notes | Baseline data for the IFG cohort (N = 310) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Participants were from the Sacramento Area Latino Study on Aging (SALSA), a longitudinal cohort study of physical and cognitive impairment and cardiovascular diseases in community‐dwelling older Mexican Americans residing in the Sacramento Metropolltan Statistical Area |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Not reported but only 12/1789 participants were excluded |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FBG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; self‐reported; antihyperglycaemic medication; diabetes comedication at death |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Multistate Markov models |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Multistate Markov models |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence (hazard ratio was calculated for the association between neighbourhood scocioeconomic position (NSEP) scores and transitions between various (pre)diabetic stages) |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multistate Markov models |
Gautier 2010.
| Name of study | Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort | |
| Inclusion criteria | Men and women aged 30–64 years recruited from volunteers who were offered periodic health examinations free of charge by the French Social Security at 10 health centres in western France | |
| Exclusion criteria | Diabetes at baseline, individuals with unknown diabetes status at the 9‐year examination | |
| Notes | No baseline data for cohort with intermediate hyperglycaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Participants of the Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort who had IFG at baseline |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Key characteristics unclear |
| Study participation: adequate description of period & recruitment place | Unclear risk | Time frame unclear |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IFG |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; treatment for diabetes (at 1 of the 3‐yearly examinations) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Some confounders measured |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes (see below) |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes (see below) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence (odds ratios for 9‐year incident diabetes per 1 SD change in waist circumference and weight in IFG) |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic models (for increases in waist circumference and weight) |
Gomez‐Arbelaez 2015.
| Name of study | None | |
| Inclusion criteria | Adults ≥ 35 years attending a general practitioner for any reason | |
| Exclusion criteria | Known diabetes, acute illness, pregnancy, use of antihyperglycaemic medication | |
| Notes | Baseline data for the total cohort (N = 772) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Longitudinal observational study conducted in a healthcare centre in Floridablanca, Colombia |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | The sub‐sample of people with intermediate hyperglycaemia was followed for diabetes incidence |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | High risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | High risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Intermediate hyperglycaemia as measured by FPG, OGTT, HbA1c; FINDRISC score |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: ≥ 5.6 to < 7.0; IGT: ≥ 7.8 to < 11.1; HbA1c ≥ 5.7 to ≤ 6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; OGTT ≥ 11.1; HbA1c ≥ 6.5 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Age and sex‐adjusted odds ratios for FINDRISC score |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | For FINDRISC score |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Age and sex‐adjusted odds ratios |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression for the association between the FINDRISC score and incident T2DM |
Guerrero‐Romero 2006.
| Name of study | None | |
| Inclusion criteria | Men and non‐pregnant women, aged 20–64 years, were recruited from the city of Durango, northern Mexico; with NGT or IGT | |
| Exclusion criteria | Participants who failed to attend 2 or more visits | |
| Notes | Baseline data for IGT cohort at baseline progressing to T2DM (N = 20); all individuals were counselled on the importance of diet and physical exercise (standard care for the whole cohort) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Cohort study in healthy Mexicans to determine predictors for the development of metabolic disorders |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Time frame unclear |
| Study participation: adequate description of period & recruitment place | Unclear risk | Period of recruitment unclear |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG: ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (for association between beta‐cell function and IGT/T2DM) (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | For beta‐cell function and IGT/T2DM |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some confounders measured |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression on relative risk of IGT or T2DM associated with beta‐cell function |
Han 2017.
| Name of study | Ansung‐Ansan cohort study, part of the Korean Genome and Epidemiology Study (KoGES), to investigate the trends in diabetes and associated risk factors | |
| Inclusion criteria | Urban (Ansan) and rural (Ansung) communities (within 60 km of Seoul) | |
| Exclusion criteria | Unknown glucose status, individuals with known diabetes, participants who were newly diagnosed with type 2 diabetes at baseline examination; persons with a history of malignant diseases, liver failure, end‐stage renal disease, rheumatological diseases and acute or chronic infectious diseases, individuals who had taken steroids in the previous 3 months; individuals who did not undergo any follow‐up examination after the baseline examination | |
| Notes | Baseline data for i‐IFG, i‐IGT and IFG/IGT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Ansung‐Ansan Cohort Study, part of the Korean Genome and Epidemiology Study (KoGES) |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes (follow‐up rate at 12 years 60.6%) |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6–6.9 and no diagnosis of diabetes; IGT: 2‐h PG 7.8 to < 11.1; i‐IFG5.6: IFG without IGT; i‐IGT: IGT without IFG; IGT/IGT: IFG+IGT; 'prediabetes': IFG or IGT |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; HbA1c ≥ 6.5; current antihyperglycaemic treatment |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate Cox proportional hazard model |
Hanley 2005.
| Name of study | Insulin Resistance Atherosclerosis Study (IRAS) | |
| Inclusion criteria | 4 clinical centres (Oakland, Los Angeles ‐ non‐Hispanic whites and blacks recruited from Kaiser Permanente) and San Antonio, San Luis Valley (non‐Hispanic whites and Hispanics): from 2 population‐based studies (San Antonio Heart Study and the San Luis Valley Diabetes study) | |
| Exclusion criteria | Participants with inflammatory diseases; diabetes; no information on metabolic variables of interest and follow‐up glucose tolerance status | |
| Notes | Baseline data for diabetic cohort at follow‐up (N = 131); participants were recruited from 2 population‐based studies: the San Antonio Heart Study and the San Luis Valley diabetes study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Observational study of the relationship between insulin resistance, cardiovascular disease and its known risk factors in different ethnic groups and varying states of glucose tolerance; the study was conducted at 4 clinical centres; report on individuals who were nondiabetic at baseline and for whom information was available on metabolic variables of interest and follow‐up glucose tolerance status |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Response rate 81% |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Unclear risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG, IGT (WHO 1999) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | High risk | Not specified |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates (age, sex, clinical centre, ethnicity) (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression |
Heianza 2012.
| Name of study | Toranomon Hospital Health Management Center Study (TOPICS) | |
| Inclusion criteria | Participants from the TOPICS: apparently healthy Japanese government employees who underwent annual multiphasic health screening examinations; the study attempted to elucidate the incidence of and risk factors for various diseases among the Japanese population | |
| Exclusion criteria | Diabetes at baseline, missing data at baseline | |
| Notes | Baseline data for the total cohort (N = 6241) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Healthy Japanese government employees who underwent annual examinations for health screening |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6–6.9 or FPG 6.1–6.9; HbA1c 5.7 ‐6.4 or 6.0–6.4; IFG/HbA1c = 'prediabetes' |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5%; self‐reported clinician‐diagnosed diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression, multivariate model |
Inoue 1996.
| Name of study | None | |
| Inclusion criteria | Non‐obese participants with IGT and 22 normal control persons were selected from the participants of a health screening programme | |
| Exclusion criteria | People with liver or kidney diseases | |
| Notes | Baseline data for the IGT cohort (N = 37) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Unclear risk | Participants of a health screening programme |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Unclear risk | Scarce data |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: ≥ 7.8 to < 11.1 (presumed WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | IGT: ≥ 11.1 (presumed WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Not reported, cumulative incidence data |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported, cumulative incidence data |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Kruskal‐Wallis test |
Janghorbani 2015.
| Name of study | Isfahan Diabetes Prevention Study (IDPS) | |
| Inclusion criteria | Participants with a family history of type 2 diabetes, being non‐diabetic | |
| Exclusion criteria | Type 1 diabetes, pregnancy | |
| Notes | Baseline data for i‐IFG, i‐IGT and IFG/IGT cohort (N = 770); first‐degree relatives of people with T2DM; data on the cohort without hypertension at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Ongoing cohort in central Iran to assess the various potential risk factors for diabetes in people with a family history of T2DM |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Description of inclusion and exclusion criteria |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | i‐IGT: FPG < 5.6 and 2‐h PG 7.8–11.1; i‐IFG: 5.6–6.9 and 2‐h PG < 7.8; IFG/IGT: 5.6–6.9 and 2‐h PG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 11.1; antihyperglycaemic medication; 2nd FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates measured (age, sex, BMI, triglycerides, total cholesterol) (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates measured (age, sex, BMI, triglycerides, total cholesterol) (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards model |
Jaruratanasirikul 2016.
| Name of study | None | |
| Inclusion criteria | Obese Thai children and adolescents aged 8–15 years, Pediatric Endocrine Clinic at Songklanagarind Hospital (Hat Yai, Songkhia Thailand) | |
| Exclusion criteria | No clinical findings of secondary obesity, not on corticosteroids | |
| Notes | Baseline data for IGT cohort (N = 27) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | High risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | (i)‐IGT: FPG < 5.6 and 2‐h PG 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG > 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression analysis for ROC curves (cut‐off FPG levels) |
Jeong 2010.
| Name of study | None | |
| Inclusion criteria | People older 20 years living in the rural area of Dalseong County near Daegu visiting community health centres | |
| Exclusion criteria | Not reported | |
| Notes | 1287 participants were re‐evaluated in 2008 and 187 new participants "added to the study"; baseline data for participants with incident diabetes (N = 135) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Population‐based survey to determine the prevalence and incidence of 'prediabetes' and diabetes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Several surveys plus new recruitment; follow‐up rate 80.5%; no description of dropouts |
| Study attrition: reasons for loss to follow‐up provided | High risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | High risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 5.6 to < 7.0; IGT: 2‐h PG ≥ 7.8 to < 11.1; 'prediabetes': IFG or IGT |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Several covariates were measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Unclear risk | Odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression models |
Jiamjarasrangsi 2008a.
| Name of study | None | |
| Inclusion criteria | Individuals 35 years or older participating in the annual physical checkup at least twice during the years 2001–2005 | |
| Exclusion criteria | People with diabetes | |
| Notes | Baseline data for total cohort becoming diabetic at follow‐up (N = 48) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | University hospital employees |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 5.6 to < 7.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Logistic regression on hepatic enzymes; incidence rate: few covariates (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Logistic regression on hepatic enzymes |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Logistic regression on hepatic enzymes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Logistic regression on hepatic enzymes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Logistic regression on hepatic enzymes |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | lLogistic regression on hepatic enzymes |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Logistic regression on hepatic enzymes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression (independent variables: hepatic enzymes) and Poisson regression analyses |
Kim 2005.
| Name of study | None | |
| Inclusion criteria | People visiting the Health Promotion Centre of Samsung Medical Center for a physical health check‐up | |
| Exclusion criteria | Diabetes | |
| Notes | Baseline data for FPG group 4 (6.1–7.0) with baseline and follow‐up (N = 276) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes (FPG categories) |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Participation rate 20.9% in group 4; scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1 to < 7.0 (group 4) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic treatment |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Several covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Scarce data |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Scarce data |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression analysis |
Kim 2008.
| Name of study | None | |
| Inclusion criteria | Individuals undergoing a medical examination at Inha University Hospital with a follow‐up medical examination 2 years later | |
| Exclusion criteria | Individuals diagnosed with diabetes at baseline | |
| Notes | Baseline data for IFG5.6/IFG6.1 cohort (N = 1335/N = 494) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Participants who underwent a medical examination at Inha University Hospital and had either NGT or IFG |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Participants diagnosed with diabetes in 2002 were excluded |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG5.6: FPG 5.6–7.0; IFG6.1: FPG 6.1–7.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Measurement of cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Measurement of cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | ROC curves for predicting the future onset of diabetes |
Kim 2014.
| Name of study | None | |
| Inclusion criteria | Pre‐screened individuals with 'prediabetes' visiting the diabetes clinic at Seoul National University Bundang Hospital (SNUB) in 2005/06 after they were diagnosed with prediabetes at their health check‐up or primary clinic | |
| Exclusion criteria | Taking oral hypoglycaemic agents or insulin | |
| Notes | Baseline data for i‐IFG (N = 158)/i‐IGT (N = 65)/IFG/IGT (N = 119)/i‐HbA1c (N = 64); total: N = 406 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Pres‐screened individuals with 'prediabetes' |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Pre‐defined participants with intermediate hyperglycaemia |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | i‐IFG: FPG 5.6–6.9 and 2‐h PG < 7.8; i‐IGT: 2‐h PG 7.8–11.1 and FPG < 5.6; IFG/IGT: combined glucose intolerance; HbA1c: 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; HbA1c ≥ 6.5 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | For C‐peptide |
| Study confounding: clear definitions of important confounders provided | Unclear risk | For C‐peptide |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | For C‐peptide |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | For C‐peptide |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | For C‐peptide |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | For C‐peptide |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression for association of T2DM development and C‐peptide levels |
Kim 2016a.
| Name of study | None | |
| Inclusion criteria | Medical examinations at the Health Screening and Promotion Center at Asan Medical Center (Seoul, Korea) | |
| Exclusion criteria | History of diabetes mellitus, taking antihyperglycaemic medications, FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% at baseline | |
| Notes | 2 baseline data cohorts: 'prediabetes' by FPG and HbA1c (N = 3544 and N = 1713) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Unclear risk | Participants who underwent medical examinations in a health screening and promotion centre |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 5.6–6.9; HbA1c 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5; antihyperglycaemic medications |
| Outcome measurement: clear definition of the outcome provided | Low risk | Yes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Several covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression |
Kleber 2010.
| Name of study | None | |
| Inclusion criteria | Obese children and adolescents aged 10‐17 years with IGT attending the outpatient centre (Department of Paediatric Nutrition Medicine, Witten/Herdecke Germany) | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for IGT cohort (N = 79) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Obese white children and adolescents with IGT attending an outpatient centre |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Unclear risk | Time of recruitment unclear |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | No exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Probably no dropouts |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG > 7.7: IFG: FPG ≥ 5.5 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | T2DM by ADA 2000 guidelines |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple linear regression |
Kleber 2011.
| Name of study | None | |
| Inclusion criteria | Obese white children with IGT without medication or endocrine/syndromal disorders, aged 10‐17 years not participating in the intervention part of the study | |
| Exclusion criteria | Children in the intervention part of the study | |
| Notes | Baseline data for IFG cohort (N = 128) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Obese children and adolescents with IGT not attending an intervention trial |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Unclear risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: not reported (presumed 7.8–11.1) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | "ADA" (2000 criteria ‐ 2‐h PG ≥ 11.1) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Measurement of cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Npt reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple linear regression |
Ko 1999.
| Name of study | None | |
| Inclusion criteria | Chinese participants with IGT | |
| Exclusion criteria | Not reported | |
| Notes | Letter to the editor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Chinese participants with IGT |
| Study participation: description of glycaemic status at baseline | Low risk | WHO/NDGG 1979 |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported (IGT cohort) |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported (IGT cohort) |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported (IGT cohort) |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not applicable (IGT cohort) |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | IGT (WHO/NDDG 1979 definition) |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | Yes |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | Assumed WHO/NDDG 1979 definition |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Cox regression analysis (to predict the progression to diabetes with age, sex, BMI, blood pressure, HbA1c, FPG, 1‐h PG and 2‐h PG as predictor variables) |
Ko 2001.
| Name of study | None | |
| Inclusion criteria | The Diabetes and Endocrine Centre of the prince of Wales Hospital in Hong Kong screened individuals with risk factors for glucose intolerance (family history of diabetes, history of gestational diabetes, overweight, hypertension) by OGTT | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for IFG cohort (N = 55) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Individuals with risk factors for glucose intolerance undergoing screening for diabetes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Measurement of cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | No ratios reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | No ratios reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Kaplan‐Meier analysis, Cox regression analysis (to predict the progression to diabetes with age, sex, BMI, blood pressure, FPG, gestational diabetes, HbA1c, smoking habit and IFG status being independent variables ‐ no hazard ratios provided) |
Larsson 2000.
| Name of study | None | |
| Inclusion criteria | Postmenopausal women aged 55–57 years in a health screening programme; random sample of 265/1843 invited for follow‐up (new OGTT); 1843 women were grouped according to WHO and ADA glucose tolerance criteria | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for (i)‐IGT (N = 66)/(i)‐IFG (N = 42)/IFG/IGT (N = 30); 265 follow‐up participants were randomly sampled from each glucose tolerance group of the original cohort and invited for follow‐up; NGT at baseline vs follow‐up: FPG < 5.3 vs < 6.1; FPG 5.3: 15% conversion factor as recommended by the WHO (blood glucose > plasma glucose) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Unclear risk | Postmenopausal women participating in a health screening programme; follow‐up: a random sample of the original cohort |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | No exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | (i)‐IFG: BG 5.3–5.9 and 2‐h BG < 7.8; (i)‐IGT: FPG < 5.3 and 2‐h BG 7.8–11.0; IFG/IGT: BG 5.3–5.9 and 2‐h BG 7.8–11.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Measurement of cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Chi‐squared test |
Latifi 2016.
| Name of study | None | |
| Inclusion criteria | Residents aged over 20 years | |
| Exclusion criteria | Not reported | |
| Notes | Baseline for prediabetic cohort becoming diabetic at follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | First phase of prevalence study of the metabolic syndrome and its related factors in Ahvaz Diabetes Research Centre, Iran |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | No exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | 5.6 ≤ FPG < 7.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Several covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Multiple logistic regression of factors affecting the incidence of diabetes and prediabetes among healthy people in phase 1 (baseline) |
Lecomte 2007.
| Name of study | None | |
| Inclusion criteria | People with IFG recruited from medical check‐ups by the French social security system in the 9 preventive health centres of IRSA (Institut Interrégional pur la Santé) | |
| Exclusion criteria | No personal history of diabetes, no hypoglycaemic drug treatment | |
| Notes | Baseline data for IFG cohort attending both examinations (N = 743) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Yes |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9; no personal history of diabetes; no hypoglycaemic treatment |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; personal history of diabetes; antihyperglycaemic treatment |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Measurement of cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Univariate analyses, some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates, univariate analyses (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression, univariate analyses on risk factors for developing diabetes |
Lee 2016.
| Name of study | None | |
| Inclusion criteria | Individuals undergoing health checkups at a single medical institution (Gangneung Asian Hospital) | |
| Exclusion criteria | Previously diagnosed with diabetes, history of diabetes medication use, only 1 measurement | |
| Notes | Baseline data for the total cohort (N = 3497) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | HbA1c 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | HbA1c ≥ 6.5 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Measurement of cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes for coffee consumption |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | 1 covariate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | No ratios reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Kaplan‐Meier survival analysis for progression to diabetes according to coffee consumption |
Leiva 2014.
| Name of study | Programa de Investigación de Factores de Riesgo de Enfermedad Cardiovascular (PIFRECV) | |
| Inclusion criteria | Study participants were recruited in 2005 by the 'Programa de Investigación de Factores de Riesgo de Enfermedad Cardiovascular' (PIFRECV); participants had to have an FPG 5.6–6.9 mmol/L | |
| Exclusion criteria | Diabetes, individuals on corticosteroid treatment, pregnant women, individuals with cardiovascular complications | |
| Notes | Most baseline data for cohort becoming diabetic at follow‐up (N = 94 with IFG) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: 5.6–7.0 (low range: 5.6–6.1; high range: 6.1–6.9) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 (on 2 consecutive days); HbA1c ≥ 6.5 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates were measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates planned (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression analysis (comparing 'high range' glycaemia (> 6.1 mmol/L) with 'low range' glycaemia (< 6.1 mmol/L) |
Levitzky 2008.
| Name of study | Framingham Heart Study | |
| Inclusion criteria | Participants were drawn from the Framingham Offspring cohort; participants who attended examinations (referred to as index examinations) | |
| Exclusion criteria | Participants with CHD or diabetes | |
| Notes | Baseline data for individuals on first exam, free of CVD (N = 4058) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG5.6: FPG 5.6–6.9; IFG6.1: FPG 6.1–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Pooled logistic regression, multivariable models |
Li 2003.
| Name of study | Kinmen Study (study in Kin‐Chen, Kinmen, Taiwan) | |
| Inclusion criteria | Individuals aged ≥ 30 years in Kin‐Chen; FPG 5.6–7.0 and 2‐h PG < 11.1 | |
| Exclusion criteria | Diabetes | |
| Notes | Baseline data for i‐IGT (N = 118)/i‐IFG (N = 42)/IFG/IGT (N = 49) cohorts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes, series of community‐based epidemiological surveys of diabetes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | i‐iFG: FPG 6.1–7.0 and 2‐h PG < 7.8; i‐IGT: FPG < 6.1 and 2‐h PG 7.8–11.1; IFG/IGT: FPG 6.1–7.0 and 2‐h PG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazard model (hazard ratios of T2DM for relative insulin resistance, beta‐cell dysfunction and varying degrees of glucose intolerance) |
Ligthart 2016.
| Name of study | Rotterdam study, targeting cardiovascular, endocrine, hepatic, neurological, ophthalmic, psychiatric, dermatological, oncological and respiratory diseases | |
| Inclusion criteria | Community dwelling population aged 45/55 years and older in Rotterdam, no diabetes at baseline | |
| Exclusion criteria | No valid baseline fasting glucose measurement, no informed consent | |
| Notes | Baseline data for prediabetic cohort (N = 1382) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FBG > 6.0 and < 7.0; non‐fasting BG > 7.7 and < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FBG ≥ 7.0; non‐fasting BG ≥ 11.1; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates for lifetime risk of diabetes (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | For lifetime risk of diabetes |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | For lifetime risk of diabetes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Modified version of survival analysis to calculate the lifetime risk of diabetes |
Lipska 2013.
| Name of study | Health, Aging, and Body Composition study (Health ABC) | |
| Inclusion criteria | Aged 70–79 years from Pittsburgh (PA) and Memphis (TN); no difficulty performing activities of daily living, walking 0.25 mile (402 m) or climbing 10 steps without resting; no reported need of assistive devices (e.g. cane, walker); no active treatment for cancer in the prior 3 years; no life‐threatening illness; and no plans to leave the area for 3 years | |
| Exclusion criteria | Not surviving baseline, diagnosed diabetes, missing HbA1c or FPG values at baseline, without adequate follow‐up after baseline | |
| Notes | Baseline data for i‐IFG (N = 189)/i‐HbA1c5.7 (N = 207)/IFG/HbA1c (N = 169) cohorts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | i‐IFG: FPG 5.6–6.9 and HbA1c < 5.7; i‐HbA1c: 5.7–6.4 and FPG > 5.6; IFG and HbA1c: FPG 5.6–6.9 and HbA1c 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | Single HbA1c ≥ 6.5 (years 2,6,7); self‐report of physician diagnosis (annually); antihyperglycaemic medication (years 1,2,4,6,7) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Multiple covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariable logistic regression |
Liu 2008.
| Name of study | None | |
| Inclusion criteria | Individuals from the JiangSu province of China, aged 35–74 years, to trace the incidence of CVD and diabetes; individuals participating twice in the study | |
| Exclusion criteria | Individuals suffering from cancer, severe disability, severe psychiatric disturbances; individuals with diabetes, missing data | |
| Notes | Baseline data for non‐diabetic participants (N = 1844); men (N = 788)/women (N = 1056) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, relative risk |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards regression |
Liu 2014.
| Name of study | None | |
| Inclusion criteria | Shanghai residents | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for the prediabetic cohort converting to T2DM (N = 78) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Unclear risk | "WHO criteria" |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Unclear risk | Scarce data |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Unclear risk | Scarce data; IFG or GT |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Unclear risk | "WHO criteria" |
| Outcome measurement: method of outcome measurement used valid & reliable | Unclear risk | Scarce data |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Analysis of variance |
Liu 2016.
| Name of study | Beijing Longitudinal Study on Aging (BLSA) | |
| Inclusion criteria | Chinese elders free of diabetes at baseline | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for participants without diabetes at baseline (N = 1857) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 6.1–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; self‐reported; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Subdistribution hazards model |
Liu 2017.
| Name of study | China Multicenter Collaborative Study of Cardiovascular Epidemiology (ChinaMUCA) | |
| Inclusion criteria | 2 studies: China Multicenter Collaborative Study of Cardiovascular Epidemiology (ChinaMUCA) study and the China Cardiovascular Health Study | |
| Exclusion criteria | Individuals with missing baseline glucose information, individuals from Deyang, Sichuan (earthquake) and individuals with ASCVD at baseline | |
| Notes | Baseline data for IFG cohort at baseline (N = 3607) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Participants lost to follow‐up e.g. were younger, had lower BMI levels and higher physical activity levels |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FBG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FBG ≥ 7.0; using insulin/antihyperglycaemic medications; self‐reported |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazard regression |
Lorenzo 2003.
| Name of study | San Antonio Heart Study (SAHS) | |
| Inclusion criteria | Mexican‐Americans and non‐Hispanic whites participating in a study of type 2 diabetes and cardiovascular disease | |
| Exclusion criteria | Phase 1 participants (waist circumference was not measured), and those in phase 2 with diabetes at baseline | |
| Notes | Baseline data for cohort converting to T2DM (N = 195) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9; IGT: 2‐h PG 7.8 to < 11.1 (WHO 1999) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG: ≥ 7.0; 2‐h PHG: ≥ 11.1 (WHO 1999/1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (diabetes risk of the metabolic syndrome and components of the metabolic syndrome) |
Lyssenko 2005.
| Name of study | Botnia Study | |
| Inclusion criteria | People with type 2 diabetes in western Finland were invited to participate together with their family members; nondiabetic individuals were invited (family members or 'controls' (spouses), aged 18–73 years; prospective visits every 2–3 years; at least 2 OGTTs | |
| Exclusion criteria | MODY, individuals with missing data | |
| Notes | Baseline data for IFG‐IGT individuals who converted to T2DM (N = 86) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Description of inclusion and exclusion criteria |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 6.1 (WHO 1999 criteria) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | WHO 1999 criteria |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Univariate analyses |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Univariate analyses |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Univariate analyses |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Univariate Cox proportional hazards model (adjusted for BMI) |
Magliano 2008.
| Name of study | Australian Diabetes, Obesity and Lifestyle Study (AusDiab) | |
| Inclusion criteria | National population‐based survey in adults aged ≥ 25 years | |
| Exclusion criteria | Participants refusing further contact, deceased, moved overseas or into a nursing facility classified for high care, had a terminal illness | |
| Notes | Baseline data for cohort becoming diabetic at follow‐up (N = 224/5842) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9 and 2‐h PG < 7.8; IGT: FPG < 7.0 and 2‐h PG ≤ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; current antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Multiple covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | ORs per SD changes in FPG and HbA1c |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate per year, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression (logFRPG and logHbA1c) |
Man 2017.
| Name of study | Singapore Malay Eye Study (SIMES) | |
| Inclusion criteria | Malay adults in Singapore aged 40–80 years; SIMES aims to assess the prevalence, incidence, progression, associated factors and impact of major eye disease as well as access to eye care by Asian Malays | |
| Exclusion criteria | Diabetes, missing data | |
| Notes | Baseline data for incident diabetes cohort (N = 127) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | HbA1c 5.7–6.4; no self‐reported diabetes or antihyperglycaemic medication |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | Random glucose ≥ 11.1 or HbA1c > 6.4; self‐reported history or antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, risk ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate analyses using modified Poission regression models to estimate adjusted risk ratios |
Marshall 1994.
| Name of study | San Luis Valley Diabetes Study | |
| Inclusion criteria | The San Luis Valley Diabetes Study determined the prevalence and incidence of NIDDM among Hispanic and non‐Hispanic white adults; sample without prior diabetes diagnosis aged 30–74 years; IGT at the initial visit | |
| Exclusion criteria | Unavailability of complete data | |
| Notes | Baseline data for IGT cohort converting to T2DM (N = 20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG ≥ 7.8 to < 11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (baseline dietary risk factors to predict the development of diabetes; glucose levels as continuous variables) |
McNeely 2003.
| Name of study | Japanese American Community Diabetes Study | |
| Inclusion criteria | Second‐generation (Nisei) and third‐generation (Sansei) Japanese‐American participants residing in Kong County, Washington | |
| Exclusion criteria | Individuals with diabetes at baseline | |
| Notes | Baseline data for cohort converting to T2DM at 5–6 years (N = 50)/10 years (N = 74) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Unclear risk | Scarce data |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Some difference reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 6.1 to < 7.0; IGT: 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; antihyperglycaemic medication prescribed by a physician |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression (ROC‐curves, clinical model) |
Meigs 2003.
| Name of study | Baltimore Longitudinal Study of Aging (BLSA) | |
| Inclusion criteria | Community dwelling volunteers, largely from the Baltimore (MD) and Washington, D.C. areas; primarily white middle‐ and upper‐middle socioeconomic class aged 21–96 years, being examined approximately every 2 years; open cohort design with dropouts replaced (around 1000 persons at each study cycle); attending at least 3 examinations and an OGTT within an 8‐year period | |
| Exclusion criteria | 2 or fewer OGTTs or > 4 years elapsed between any 2 OGTTs | |
| Notes | Baseline data for the IFG‐IGT cohort (N = 265); follow‐up time: at least 6 years 77%, at least 10 years 44%, at least 16 years 16%, at least 20 years 4.5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | High risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9 and 2‐h PG ≤ 7.8; IGT: FPG < 6.1 and 2‐h PG 7.8–11.0; IFG/IGT |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 (IFG‐IGT: diabetes defined by OGTT) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rates |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence, incidence rates |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence, incidence rates |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence, incidence rates |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence, incidence rates |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rates |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rates |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Kaplan‐Meier product limit estimates |
Mohan 2008.
| Name of study | Chennai Urban Population Study‐19 (CUPS‐19) | |
| Inclusion criteria | Participants of 2 residential colonies in Chennai, India, representing the middle and lower income groups ≥ 20 years of age | |
| Exclusion criteria | Individuals with diabetes | |
| Notes | Baseline data for cohort becoming diabetic at follow‐up (N = 64/476) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 6.1 to < 7; IGT: 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression analysis (effects of various risk factors but not intermediate hyperglycaemia on diabetes) |
Motala 2003.
| Name of study | None | |
| Inclusion criteria | South African Indians, mainly living in Durban (1984); survey to determine the prevalence of NIDDM among South African Indians; non‐pregnant participants > 15 years of age | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for responders (both baseline and follow‐up examination) (N = 563) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 7.8 and 2‐h PG 7.8 to < 11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.8; 2‐h PG ≥ 11.1 (WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (to evaluate the effect of various predictor variables for type 2 diabetes) |
Motta 2010.
| Name of study | Italian Longitudinal Study on Aging (ILSA) | |
| Inclusion criteria | Elderly participants aged 65–84 years involved in ILSA studies | |
| Exclusion criteria | Not reported | |
| Notes | No baseline characteristics provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: 6.1 to < 7.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | t‐test |
Mykkänen 1993.
| Name of study | None | |
| Inclusion criteria | Participants from Kuopio, Finland | |
| Exclusion criteria | Diabetes at baseline, incomplete OGTT at the follow‐up examination | |
| Notes | Baseline data for cohort developing T2DM (N = 69) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 7.8 and 2‐h PG 7.8–11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.8; 2‐h PG ≥ 11.1 (WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | ANCOVA, odds ratios (risk of developing diabetes associated with various risk factors) |
Nakagami 2016.
| Name of study | Kurihashi Lifestyle Cohort Study | |
| Inclusion criteria | Baseline health check‐ups at Kurihashi Hospital | |
| Exclusion criteria | People < 30 years or ≥ 80 years, diabetes at baseline, people with chronic diseases, missing covariate data | |
| Notes | Baseline data for cohort converting to T2DM (N = 99) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 5.5–6.9; HbA1c 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0, HbA1c ≥ 6.5; physician diagnosis of diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, hazard ratio (associated with a 1 SD increase in the levels of FPG or HbA1c) |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards models |
Nakanishi 2004.
| Name of study | None | |
| Inclusion criteria | Employees of Company A, one of the largest building contractors in Japan (in major cities around Japan); Japanese men aged 35–59 years with no prior history of coronary heart disease or stroke | |
| Exclusion criteria | Not participating in all the consecutive annual health examinations | |
| Notes | Baseline characteristics for IFG cohort (N = 246) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, relative risk (adjusted for all other components and clustering of components of the metabolic syndrome at study entry) |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards model |
Noda 2010.
| Name of study | Japanese Public‐Health Center‐based prospective (Diabetes) Study (JPHC Study) | |
| Inclusion criteria | All registered Japanese inhabitants in 11 public health center areas aged 40–59 years old in cohort I and 40–69 years old in cohort II; inhabitants who received annual health‐checkups; authors included those who were 51–70 years of age at the time of the baseline survey of diabetes | |
| Exclusion criteria | Missing data, casual blood samples in any of the 2 health check‐ups; known diabetes or an FPG of 125 mg/dL or more at baseline | |
| Notes | Baseline characteristics for the total cohort (N = 2207) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | Taken from table 2: FPG levels: IFG 5.6 and 6.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.1%; self‐reported |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Crude incidence, ROC curves |
Park 2006.
| Name of study | None | |
| Inclusion criteria | Korean men employed at a semiconductor manufacturing facility in Korea participating in an annual health examination at a university hospital | |
| Exclusion criteria | Diabetes, failing to undergo subsequent examinations within 2 years; missing data | |
| Notes | Baseline data for incident diabetic participants with IFG at baseline (N = 40) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 5.6 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards models (for sequential changes in FPG levels) |
Peterson 2017.
| Name of study | Follow‐up of a cohort originally from the population‐based Västerbotten Intervention Program (VIP), a strategy to reach all middle‐aged persons individually at ages 40, 50 and 60 years, by inviting them to participate in systematic risk factor screening and individual counselling about healthy lifestyle habits; neuropathy study part of the VIP | |
| Inclusion criteria | All individuals who became 40, 50 or 60 years and who belonged to the list for a specific primary care centre or lived within the area for that centre | |
| Exclusion criteria | People not participating in the neuropathy study | |
| Notes | Baseline data for IGT cohort (N = 29) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 7.0 and 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | Yes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | ANOVA, regression analyses |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Cumulative incidence |
Qian 2012.
| Name of study | None | |
| Inclusion criteria | Shanghai residents | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for cohort progressing to T2DM (N = 377) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | i‐IFG: 6.1–6.9 and 2‐h PG < 7.8; i‐IGT: < 6.1 and 2‐h PG 7.8–11.0; IFG/IGT: 6.1–6.9 and 2‐h PG 7.8–11.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression (to assess the potential contributing factors to diabetes incidence) |
Rajala 2000.
| Name of study | None | |
| Inclusion criteria | Inhabitants in Oulu (northern Finland) recruited from the official population register to investigate the prevalence of diabetes and IGT, reasons for early retirement and the prevalence of depression | |
| Exclusion criteria | Previoulsy diagnosed diabetic people | |
| Notes | Only few baseline data for IGT cohort (N = 171); new cases identified by OGTTs in 1994 and 1996–8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Prevalence of hypertension was higher among people lost to follow‐up |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1; 2 × FPG ≥ 6.7 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (for effects of hypertension and antihypertensive medications) |
Ramachandran 1986.
| Name of study | None | |
| Inclusion criteria | Indian individuals with IGT | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for the diabetic cohort at follow‐up (N = 39) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | High risk | Not reported |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Unclear risk | Scarce data |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 7.8–11.0 (presumed NDDG 1979) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG > 11.0 (presumed NDDG 1979) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Not reported |
Rasmussen 2008.
| Name of study | Anglo‐Danish‐Dutch study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (ADDITION) | |
| Inclusion criteria | Population‐based high‐risk screening and intervention study for type 2 diabetes; persons aged 40–69 years registered with the participating practices in 5 counties in Denmark with a risk score of 5 points or more; measurement of fasting capillary blood glucose and OGTT; annual glucose measurement recommended for individuals with IFG and IGT; individuals with 2 diabetic glucose values on separate days were included in the intervention programme | |
| Exclusion criteria | Severe concurrent illness, alcohol abuse or subsequently treated by general practitioners not in the addition study; individuals with diabetes | |
| Notes | Baseline data for IFG (N = 607)/IGT cohort (N = 903) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Unclear risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG (i‐IFG): FBG 5.6 to < 6.1 and 2‐h BG < 7.8; IGT (i‐IGT): FBG < 6.1 and 2‐h BG 7.8 to < 11.1; IFG/IGT |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Unclear risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FBG ≥ 6.1 or 2‐h BG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Regression models (for sequential changes in some covariates) |
Rathmann 2009.
| Name of study | Kooperative Gesundheitsforschung in der Region Augsburg (KORA S4/F4) | |
| Inclusion criteria | People living in Augsburg and surroundings; KORA was follow‐up of MONICA WHO‐Project (Monitoring Trends and determinants in Cardiovascular Disease); S1: 25–64 years, S2/S3/S4: 25–74 years | |
| Exclusion criteria | People with known diabetes | |
| Notes | Baseline characteristics for total cohort (participants of the follow‐up; age‐group 55–74 years; N = 887) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Some differences reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9; IGT: 2‐h PG 7.8 to < 11.1; 'prediabetes': i‐IFG, i‐IGT and IFG/IGT |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; validated physician diagnosis |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analyses (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression models |
Rijkelijkhuizen 2007.
| Name of study | Hoorn Study | |
| Inclusion criteria | General Dutch population (Hoorn) aged 50–75 years at baseline; participants completing both measurements in 1989 and 1996 | |
| Exclusion criteria | People using antihyperglycaemic medications or diet for diabetes were marked as known diabetes mellitus; missing information of plasma glucose values | |
| Notes | Baseline data for IFG6.1 (N = 149)/IFG5.6 (N = 488) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | No substantial differences |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG5.6: FPG 5.6–7.0; IFG6.1: FPG 6.1–7.0; IGT: 2‐h PG 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG: ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards models |
Sadeghi 2015.
| Name of study | Isfahan Cohort Study (ICS), baseline survey of the Isfahan Healthy Heart Program (IHHP) | |
| Inclusion criteria | Participants of the baseline survey of the Isfahan Healthy Heart Program, a community trial for prevention and control of CVD | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for prediabetic cohort at baseline becoming diabetic at follow‐up (N = 131) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 5.5 and < 7.0; IGT: 2‐h OGTT ≥ 7.8 and < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG > 7.0; 2‐h OGTT > 11.1; IFG/IGT; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Low risk | Stochastic regression methods |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression |
Sasaki 1982.
| Name of study | None | |
| Inclusion criteria | Epidemiological survey on diabetes mellitus in Osaka, Japan and follow‐up study | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for the IGT cohort (N = 13) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 7.8 and 2‐h PG 7.8–11.1 (WHO 1980) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.8 or 2‐h PG ≥ 11.1 (WHO 1980) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Scarce data |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Scarce data |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Scarce data |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Multiple logistic regression (standardised regression coefficients for single covariates) |
Sato 2009.
| Name of study | Kansai Healthcare Study | |
| Inclusion criteria | Japanese male employees of a company in the area of Kansai, aged 40–55 years, not taking an oral antihyperglycaemic or insulin at study entry and considered to be involved in sedentary jobs | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for cohort becoming diabetic at follow‐up (N = 659/6804); non‐standard categories for elevated HbA1c values were used (Table 1, p 645 of the publication) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | Table 1: IFG: FPG group 6.1–6.9; HbA1c‐group: 6.0–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (FPG, HbA1c categories) |
Schranz 1989.
| Name of study | Study within the WHO‐assisted National Diabetes Programme | |
| Inclusion criteria | Within the framework of the WHO‐assisted National Diabetes Programme a cohort of Maltese people was investigated | |
| Exclusion criteria | Known diabetic persons | |
| Notes | Baseline data for diabetic cohort at follow‐up (N = 166) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Yes |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG ≥ 7.8 to < 11.1 (WHO 1985) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985) |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Not reported |
Sharifi 2013.
| Name of study | Zanjan Healthy Heart Study | |
| Inclusion criteria | Participants from the Zanjan Healthy Heart Study, aged 21–75 years, individuals with IFG | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for active participants (N = 123) of the IFG cohort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | High attrition rate (> 50%) |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 5.6–7.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG > 7.0 (2 measurements); diabetes diagnosis based on documents |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Logistic regression (BMI and physical activity for prediction of diabetes) |
Shin 1997.
| Name of study | Yonchon study | |
| Inclusion criteria | Individuals living in Yonchon County (South Korea), free of diabetes aged ≥ 30 years | |
| Exclusion criteria | Diabetes | |
| Notes | Baseline data for individuals converting to T2DM (N = 67) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Unclear risk | Scarce data |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Unclear risk | Scarce data |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Unclear risk | Assumed WHO 1985 criteria |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Unclear risk | Scarce data |
| Outcome measurement: clear definition of the outcome provided | Low risk | "WHO criteria"; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Unclear risk | Scarce data |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Unclear risk | Scarce data |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression (1 mmol/L difference for FPG and 2‐h plasma glucose) |
Song 2015.
| Name of study | Korean Genome Epidemiology Study‐Kangwha Study (KoGES) | |
| Inclusion criteria | People aged ≥ 40 years | |
| Exclusion criteria | Missing key variables, history of stroke, angina pectoris or myocardial infarction, diabetes | |
| Notes | Baseline data for prediabetic cohort (men: N = 154; women: N = 167; total: N = 321); ranges for men ‐ women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Responders had relatively low FPG and HbA1c at baseline compared to non‐responders |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Unclear risk | Cumulative incidence, relative risk |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Generalised linear models |
Song 2016a.
| Name of study | None | |
| Inclusion criteria | Survey of the prevalence of T2DM in an urban community; eligible permanent inhabitants 15–74 years | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for prediabetic cohort (N = 334) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FG 5.6–6.9; IGT: 2‐h G 7.8–11.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | IFG ≥ 7.0; 2‐h G ≥ 11.0; HbA1c ≥ 6.5; self‐reported |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression models (sex‐related risk factors associated with the development of diabetes) |
Soriguer 2008.
| Name of study | Pizarra study, evaluating the prevalence of latent autoimmune diabetes of adults (LADA) in the context of the overall prevalence of diabetes in Southern Spain | |
| Inclusion criteria | People aged 18–65 years from Pizarra, Malaga | |
| Exclusion criteria | Institutionalised persons, pregnant women, severe clinical or psychological disorder | |
| Notes | Baseline data for final sample of follow‐up (N = 714); diabetes diagnosis according to capillary blood glucose levels > 6.1 mmol/L or post OGTT BG > 11.1 mmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Unclear risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: BG 5.6–6.1 and 2‐h BG < 7.8; IGT: BG < 5.6 and 2‐h BG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | BG > 6.1 or 2‐h BG > 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, relative risk |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression |
Stengard 1992.
| Name of study | Finnish Cohorts of the Seven Countries Study | |
| Inclusion criteria | Elderly Finnish men, survivors of the Finnish cohorts of the Seven‐Countries Study (studying mortality, morbidity and risk factor levels of cardiovascular diseases in different countries), aged 65–84 years at baseline | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for IGT cohort converting to T2DM (N = 17) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | 2‐h PG ≥ 11.1 (WHO 1985); antihyperglycaemic medications |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression |
Söderberg 2004.
| Name of study | None | |
| Inclusion criteria | Population based survey in Mauritius, 3 cohorts of nonpregnant participants aged 25–79 years with classifiable data from 2 separate surveys | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for cohort 1987–1998 (N = 2631), 10 years follow‐up; 3 cohorts 1987–1992 (N = 3680), 1992–1998 (N = 4178), 1987–1998 (N = 2631) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG ≥ 6.1 to < 7.0 and 2‐h PG < 7.8; IGT: FPF < 7.0 and 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Calculation of incidence rate ratios, Poisson regression analysis to estimate sex effects between 1987 and 1998 allowing for adjustments |
Toshihiro 2008.
| Name of study | None | |
| Inclusion criteria | Japanese mal workers of a railroad company receiving a health‐check at Nishimatsuzono Clinic, IFG and/or IGT cohort | |
| Exclusion criteria | People with type B or C hepatitis virus infections | |
| Notes | Baseline data for cohort becoming diabetic at follow‐up (N = 36/128);participants with IFG and/or IGT were given advice about lifestyle modifications once or twice a year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9 and 2‐h PG < 7.8; IGT: FPG < 7.0 and 2‐h PG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG > 11.1; non‐fasting PG > 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards model (multivariate analysis of independent risk factors and recovery factors) |
Vaccaro 1999.
| Name of study | None | |
| Inclusion criteria | Telephone company employees in the age range 40–59 years were screened in the province of Naples for major cardiovascular risk factors | |
| Exclusion criteria | Taking antihyperglycaemic medication, previous diabetes diagnosis | |
| Notes | Baseline data for total cohort (follow‐up examination; N = 560) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Those lost to follow‐up were older and more frequently women |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Unclear risk | Unusual thresholds |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Unclear risk | IFG: FPG 5.6–6.0; IGT: 2‐h PG 6.7–9.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Not reported |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Not reported |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio (probably unadjusted) |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Unclear risk | Quote: "standard methods" |
Valdes 2008.
| Name of study | Asturias Study (Asturias) | |
| Inclusion criteria | Survey of diabetes and cardiovascular risk factors in the principality of Asturias, northern Spain; participants from basic health area | |
| Exclusion criteria | Type 1 diabetes, pregnancy, severe disease, hospitalisation, use of diabetogenic drugs, missing data; diabetes | |
| Notes | Baseline data for IFG 5.6–6.1 (N = 114)/IFG 6.1–6.9 (N = 52) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG5.6: 5.6–6.1; IFG6.1: 6.1–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; clinical diabetes diagnosis; antihyperglycaemic medication, diet |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multivariate logistic regression |
Vijayakumar 2017.
| Name of study | None | |
| Inclusion criteria | Participants were 10–19 years of age at first examination without diabetes, and at least 1 follow‐up examination before the 40th birthday | |
| Exclusion criteria | History of possibly taking metformin at baseline | |
| Notes | Baseline data for adults (A)/children (C ) with HbA1c 5.7–6.4 (children: N = 62, adults: N = 168) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 5.6–6.9; 2‐h PG 7.8–11.9; HbA1c 5.7–6.4 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; previous clinical diagnosis |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence, incidence rate |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, incidence rate |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | ROC curves, increments in HbA1c and FPG or 2‐h PG to calculate 10‐year cumulative incidence |
Viswanathan 2007.
| Name of study | None | |
| Inclusion criteria | Programme on primary prevention of diabetes in the population and in high risk people (positive family history of diabetes); individuals with at least 2 follow‐up visits; participants were given advice on preventive measures such as dietary modifications and regular exercise | |
| Exclusion criteria | Known history of diabetes, newly diagnosed diabetes during screening | |
| Notes | Baseline data for IGT group (N = 619); participants were given advice on preventive measures such as dietary modifications and regular exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Unclear risk | Scarce data |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Unclear risk | Not defined, presumably by OGTT |
| Outcome measurement: method of outcome measurement used valid & reliable | Unclear risk | Scarce data |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Unclear risk | Scarce data |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Not reported |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Not reported |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression, Cox regression analysis |
Wang 2007.
| Name of study | Beijing Project as part of the National Diabetes Survey | |
| Inclusion criteria | Inhabitants of Beijing aged 25 years or older | |
| Exclusion criteria | Newly diagnosed diabetes or CHD at baseline, known diabetes | |
| Notes | Baseline data for cohort with incident diabetes and no CHD (N = 67) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | Yes |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | Yes |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 6.1–6.9; IGT: 2‐h PG 7.8–11.0 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, risk ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Multiple logistic regression |
Wang 2011.
| Name of study | Strong Heart Study (SHS) | |
| Inclusion criteria | Data collected from American Indians at the baseline and second exams from those participants who had HbA1c and FPG measured | |
| Exclusion criteria | Antihyperglycaemic medications, renal dialysis, kidney transplant | |
| Notes | No baseline data reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Those lost to follow‐up had lower BMI |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: 5.6 to < 7.0; HbA1c 6.0 to < 6.5 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; HbA1c ≥ 6.5; FPG/HbA1c: ≥ 6.5 or FPG ≥ 7.0; antihyperglycaemic medication |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression |
Warren 2017.
| Name of study | Atherosclerosis Risk in Communities study (ARIC) | |
| Inclusion criteria | Adults aged 45–64 years from the communities of Jackson, MS; Forsyth County, NC; suburban Minneapolis, MN; and Washington County, MD, USA | |
| Exclusion criteria | Participants with prevalent diabetes, chronic kidney disease, atherosclerotic cardiovascular disease, or peripheral arterial disease, those who were missing variables of interest, or those who fasted for < 10 h | |
| Notes | 2 different baseline cohorts; 4 prediabetes definitions (visit 2: IFG 5.6–6.9: N = 4112; HbA1c 5.7–6.4: N = 2027; visit 4: IFG 5.6–6.9: N = 2142; IGT: N = 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | FPG 5.6–6.9 (ADA); FG 6.1–6.9 (WHO); 2‐h 7.8–11.0 (ADA); HbA1c 5.7–6.4 (ADA); 6.0–6.4 (IEC) |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Unclear risk | Self‐report of physician diagnosis; antihyperglycaemic medication reported during a study visit or annual telephone call |
| Outcome measurement: method of outcome measurement used valid & reliable | Unclear risk | Missing lab measurements |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards models |
Wat 2001.
| Name of study | Hong Kong Cardiovascular Risk Factor Prevalence Study | |
| Inclusion criteria | Follow‐up of the Hong Kong Cardiovascular Risk Factor Prevalence Study in Hong Kong Chinese aged 25–74 years; persons with IGT (matched controls from the same population with normal glucose tolerance), investigation of the development of appropriate population‐wide coronary heart disease prevention strategies and monitoring their long‐term impact | |
| Exclusion criteria | Diabetes at baseline | |
| Notes | Baseline data for IGT cohort (N = 322) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 7.8 and 2‐h PG 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.8; 2‐h PG ≥ 11.1 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression (per unit increase for some covariates) |
Weiss 2005.
| Name of study | None | |
| Inclusion criteria | Obese children and adolescents aged 4–18 years were recruited from the Yale Pediatric Obesity Clinic (New Haven, Conneticut, USA) | |
| Exclusion criteria | Participants with medical conditions, using medications that may affect glucose metabolism before their first OGTT | |
| Notes | Baseline data for IGT cohort (N = 33) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Unclear risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Unclear risk | Scarce data |
| Study participation: adequate description of period & recruitment place | Unclear risk | Scarce data |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria reported |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | No dropouts |
| Study attrition: reasons for loss to follow‐up provided | Low risk | No dropouts |
| Study attrition: adequate description of participants lost to follow‐up | Low risk | No dropouts |
| Study attrition: no important differences between participants who completed the study and those who did not | Low risk | No dropouts |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: FPG < 5.6 and 2‐h PG 7.8–11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG > 11.1; presentation of hyperglycaemia (more than 2 random glucose measurements > 11.1), glucosuria, polydipsia, and polyuria |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Mann‐Whitney U test and linear regression (to identify predictors of 2‐h glucose on the second OGTT) |
Wheelock 2016.
| Name of study | Pima Indian Study (Gila River Indian Community ‐ near Phoenix, Arizona) | |
| Inclusion criteria | Gila River Indian Community in Arizona (mostly Pima or Tohono Indians); children and adolescents 5–19 years who were nondiabetic at baseline and had at least 1 follow‐up examination | |
| Exclusion criteria | Not reported | |
| Notes | Baseline data for the full cohort (N = 5532); prediabetic cohort = non‐overweight (N = 37) + IGT group and overweight + IGT group (N = 132); 5–11 years/12–19 years); age‐stratified incidence data on overweight participants + IGT or overweight and either hypertension or hypercholesterolaemia + IGT (metabolic set (MSet)) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Unclear risk | Only inclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Scarce data |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; previous diagnosis |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Cumulative incidence |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox regression model using each metabolic risk factor as a continuous variable; violation of the proportionality assumption was noted, therefore cumulative incidence rates were calculated from a Poisson regression model |
Wong 2003.
| Name of study | Singapore Impaired Glucose Tolerance Follow‐up Study | |
| Inclusion criteria | Representative sample of the Singapore population aged 18–69 years; persons with IGT and matched controls | |
| Exclusion criteria | Antihyperglycaemic medication, venepuncture failure; persons with IFG | |
| Notes | Baseline data for IGT group (N = 291) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Scarce data |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG ≥ 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; 2‐h PG ≥ 11.1; physician diagnosed diabetes |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Cumulative incidence |
| Study confounding: clear definitions of important confounders provided | Unclear risk | Cumulative incidence |
| Study confounding: measurement of confounders valid & reliable | Unclear risk | Cumulative incidence |
| Study confounding: same method & setting for measurements of confounders for all study participants | Unclear risk | Cumulative incidence |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Cumulative incidence |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Not reported |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | ANCOVA using general linear models (comparisons between continuous variables) |
Yeboah 2011.
| Name of study | Multi‐Ethnic Study of Atherosclerosis (MESA) | |
| Inclusion criteria | Persons without known CVD at baseline from 6 US communities aged 45–84 years | |
| Exclusion criteria | Persons with a history of physician‐diagnosed myocardial infarction, angina, heart failure, stroke, or transient ischaemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft surgery, angioplasty, valve replacement, pacemaker placement, or other vascular surgeries) | |
| Notes | Baseline data for IFG cohort (N = 940) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Low risk | Yes |
| Study attrition: reasons for loss to follow‐up provided | Low risk | Yes |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Scarce data |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Scarce data |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IFG: FPG 5.6–6.9 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Low risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG > 6.9; antihyperglycaemic medication during examinations 2,3,4 |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Low risk | Yes |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Low risk | Yes |
| Study confounding: important potential confounders accounted for in the analysis | Low risk | Yes |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Cumulative incidence, hazard ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Cox proportional hazards model |
Zethelius 2004.
| Name of study | None | |
| Inclusion criteria | All men residing in Uppsala were invited to a health survey in 1970; reinvestigation 20 years later (= baseline) at 70 years of age | |
| Exclusion criteria | Diabetes, antihyperglycaemic medications | |
| Notes | Baseline data for cohort converting to T2DM (N = 26) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study participation: description of source population or population of interest | Low risk | Yes |
| Study participation: description of glycaemic status at baseline | Low risk | Yes |
| Study participation: adequate description of sampling frame & recruitment | Low risk | Yes |
| Study participation: adequate description of period & recruitment place | Low risk | Yes |
| Study participation: adequate description of inclusion & exclusion criteria | Low risk | Inclusion and exclusion criteria described |
| Study attrition: description of attempts to collect information on participants who dropped out | Unclear risk | Not reported |
| Study attrition: reasons for loss to follow‐up provided | Unclear risk | Not reported |
| Study attrition: adequate description of participants lost to follow‐up | Unclear risk | Not reported |
| Study attrition: no important differences between participants who completed the study and those who did not | Unclear risk | Not reported |
| Glycaemic status measurement: provision of clear definition or description of glycaemic status | Low risk | Yes |
| Glycaemic status measurement: valid and reliable method of glycaemic status measurement | Low risk | Yes |
| Glycaemic status measurement: continuous variables reported or appropriate cut points used | Low risk | IGT: 2‐h PG 7.8 to < 11.1 |
| Glycaemic status measurement: same method and setting of measurement of the glycaemic status for all study participants | Unclear risk | Yes |
| Outcome measurement: clear definition of the outcome provided | Low risk | FPG ≥ 7.0; antihyperglycaemic medications |
| Outcome measurement: method of outcome measurement used valid & reliable | Low risk | Yes |
| Outcome measurement: same method & setting of outcome measurement for all study participants | Low risk | Yes |
| Study confounding: important confounders measured | Unclear risk | Some covariates measured (see Appendix 16 and Appendix 17) |
| Study confounding: clear definitions of important confounders provided | Low risk | Yes |
| Study confounding: measurement of confounders valid & reliable | Low risk | Yes |
| Study confounding: same method & setting for measurements of confounders for all study participants | Low risk | Yes |
| Study confounding: appropriate methods used if missing confounder data imputed | Unclear risk | Not reported |
| Study confounding: important potential confounders accounted for in study design | Unclear risk | Some covariates included (see Appendix 16 and Appendix 17) |
| Study confounding: important potential confounders accounted for in the analysis | Unclear risk | Some covariates analysed (see Appendix 16 and Appendix 17) |
| Statistical analysis & reporting: sufficient presentation of data to assess adequacy of the analytic strategy | Low risk | Odds ratio |
| Statistical analysis & reporting: the statistical model is adequate for the design of the study | Low risk | Logistic regression, multivariate models (adjusted for BMI, age at baseline and length of follow‐up) |
Note: for better readability all IFG/IGT and HbA1c measurements are reported in numerical format only (IFG and IGT were measured in mmol/L, HbA1c was measured in %)
ADA: American Diabetes Association; ANOVA: analysis of variance; BG: blood glucose; BMI: body mass index; CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; FG: fasting glucose; FBG: fasting blood glucose; FINDRISC: Finnish Diabetes Risk Score; FPG: fasting plasma glucose; G6PD: glucose‐6‐P‐dehydrogenase test; HbA1c: glycosylated haemoglobin A1c; HbA1c5.7: intermediate hyperglycaemia with HbA1c 5.7% as lower threshold (usually reflecting 5.7%–6.4%); HbA1c6.0: intermediate hyperglycaemia with HbA1c 6.0% as lower threshold (usually reflecting 6.0%–6.4%); HOMA‐B: homeostatic model assessment beta‐cell function; HOMA‐IR: homeostatic model assessment for insulin resistance; HR: hazard ratio; IEC: International Expert Committee; IFG: impaired fasting glucose; IFG5.6: impaired fasting glucose with 5.6 mmol/L as lower threshold; IFG6.1: impaired fasting glucose with 6.1 mmol/L as lower threshold; IFG/IGT: both IFG and IGT; i‐IFG: isolated IFG; IGT: impaired glucose tolerance; i‐IGT: isolated IGT; JDS: Japanese Diabetes Society; MSet: metabolic set; NDDG: National Diabetes Data Group; NGSP: National Glycohemoglobin Standardization Program; NGT: normal glucose tolerance; OGTT: oral glucose tolerance test; OR: odds ratio; PG: postload glucose; ROC: receiver operating characteristics; RR: risk ratio, relative risk; T2DM: type 2 diabetes mellitus; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdul‐Ghani 2011 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Alvarsson 2009 | Intervention study |
| Alyass 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Amoah 2002 | Not a prospective cohort study |
| Andreou 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes (prevalence data) |
| Bancks 2015 | Only self‐reported diabetes, frequency matched population |
| Birmingham Diabetes Survey Working Party 1976 | Non‐standard thresholds for intermediate hyperglycaemia |
| Bjornholt 2000 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Bodicoat 2017 | Long‐term follow‐up of an interventional study |
| Boned 2016 | Hypertensive cohort |
| Boucher 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Brantsma 2005 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Brateanu 2017 | Retrospective cohort study |
| Braun 1996 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Burchfiel 1995 | No cohort with intermediate hyperglycaemia |
| Chamukuttan 2016 | Intervention trial |
| Chang 2017 | Investigation of the association between thyroid function and the development of intermediate hyperglycaemia/diabetes |
| Chen 1995 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Cheng 2011 | Not a prospective cohort study |
| Cheung 2007 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Choi 2002 | Not a prospective cohort study |
| Cicero 2005 | No valid data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Cosson 2011 | Not a prospective cohort study |
| Costa 2005 | Study design paper |
| Cree‐Green 2013 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Cropano 2017 | Investigation of the association between gene variants and development of intermediate hyperglycaemia/diabetes |
| Dagogo‐Jack 2011 | Evaluation of the transition from normoglycaemia to intermediate hyperglycaemia |
| Daniel 1999 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Decode 2003 | Aggregate data of 22 cohorts; no data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Deedwania 2013 | No data on diabetes incidence |
| DeFina 2012 | Not a prospective cohort study |
| DeJesus 2016 | Not a prospective cohort study |
| Deschenes 2016 | Cohort with depressive symptoms |
| Dinneen 1998 | Not a prospective cohort study |
| Doi 2007 | No cohort with intermediate hyperglycaemia |
| Du 2016 | Cross‐sectional study, no cohort with intermediate hyperglycaemia |
| Edelman 2004 | Non‐standard thresholds for intermediate hyperglycaemia |
| Edelstein 1997 | Aggregated data on 6 prospective studies, no reliable additional data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Engberg 2010 | Intervention trial |
| Eskesen 2013 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Feizi 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Feskens 1989 | No cohort with intermediate hyperglycaemia |
| Festa 2003 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Folsom 2000 | No cohort with intermediate hyperglycaemia |
| Gil‐Montalban 2015 | Diagnosis of type 2 diabetes incidence by database only |
| Giraldez‐Garcia 2015 | No data on type 2 diabetes incidence |
| Glauber 2018 | Incidence established by register data |
| Gonzalez‐Villalpando 2014 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Gopinath 2013 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Gu 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes (database) |
| Gupta 2011 | Intervention trial, hypertensive cohort |
| Hackett 2014 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Haffner 1997 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Haffner 2000 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Hajat 2012 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Hanai 2005 | No data on transition from intermediate hyperglycaemia to type 2 diabetes, OGTTs were unit of analysis |
| He 2018 | Investigation of the association of glycaemic index diets and glycaemic load diets with development of type 2 diabetes |
| Helmrich 1991 | No cohort with intermediate hyperglycaemia |
| Henninger 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Holbrook 1990 | No cohort with intermediate hyperglycaemia |
| Hong 2016 | Not a prospective cohort study |
| Huang 2014c | Not a prospective cohort study (database) |
| Hulman 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Inoue 2008 | Retrospective cohort study |
| Invitti 2006 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Jallut 1990 | Not a prospective cohort study |
| James 1998 | No cohort with intermediate hyperglycaemia |
| Jansson 2015 | No cohort with intermediate hyperglycaemia |
| Jarrett 1979 | Intervention trial |
| Jarrett 1982 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Jeanne 2018 | No cohort with intermediate hyperglycaemia, investigation of the association between birth weight and physical activity and cardiometabolic health |
| Jiamjarasrangsi 2008b | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Joshipura 2017 | Diabetes incidence data for 'prediabetes' group only |
| Kadowaki 1984 | Non‐standard thresholds for intermediate hyperglycaemia |
| Kametani 2002 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kanauchi 2003 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kanaya 2005 | Investigation of a prediction model for development of diabetes |
| Kawahara 2015 | Not a prospective cohort study |
| Khan 2017 | Diabetes incidence defined by register data |
| Khang 2010 | Not a prospective cohort study |
| Kieboom 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kim 2012a | Not a prospective cohort study |
| Kim 2012b | Not a prospective cohort study |
| Kim 2013 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kim 2016b | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kim 2017a | Investigation of the association between sleep duration and development of type 2 diabetes |
| Kim 2017b | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Ko 2000 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kosaka 1996 | Non‐standard thresholds, no numerical data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Kowall 2013 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Krabbe 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Le Boudec 2016 | Withdrawn publication |
| Lee 2014 | No cohort with intermediate hyperglycaemia |
| Lee 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Leite 2009 | Intervention trial |
| Li 2011 | Evaluation of a diabetes risk tool |
| Liatis 2014 | Participants of a diabetes prevention programme |
| Libman 2008 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Liu 2017a | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Liu 2017b | Investigation of the association between the bone resorption marker CTX and incident intermediate hyperglycaemia/diabetes |
| Malmstrom 2018 | Type 2 diabetes incidence measured mainly through registers; nested case‐control study; no transition data |
| Manson 1992 | No cohort with intermediate hyperglycaemia |
| McNeill 2006 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| McPhillips 1990 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Medalie 1975 | No data on transition from intermediate hyperglycaemia to type 2 diabetes; no common thresholds for diagnosis of intermediate hyperglycaemia and type 2 diabetes |
| Metcalf 2017 | No cohort with intermediate hyperglycaemia |
| Miranda 2017 | Investigation of the association between advanced glycation end products (AGE) and their receptor (RAGE) and type 2 diabetes incidence |
| Mirbolouk 2016 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Monesi 2012 | No cohort with intermediate hyperglycaemia |
| Morrison 2012 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Nakagami 2017 | No cohort with intermediate hyperglycaemia |
| Nakasone 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Nano 2017 | Investigation of the association between liver transaminases and development of intermediate hyperglycaemia/type 2 diabetes |
| Nguyen 2014 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Nichols 2007 | Not a prospective cohort study |
| Nichols 2010 | Not a prospective cohort study |
| Nichols 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Njolstad 1998 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Norberg 2006 | Not a prospective cohort study |
| Nowicka 2011 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Ohlson 1987 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Oizumi 2011 | Non‐standard thresholds for intermediate hyperglycaemia |
| Okada 2017 | Diabetes incidence data for prediabetic cohort only (FPG 5.6–6.9 or HbA1c 5.7%–6.4%) |
| Onat 2007 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Onat 2013a | Non‐standard IFG/IGT definition |
| Onat 2013b | Non‐standard IFG/IGT definition |
| Osei 2004 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Paddock 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Perry 1995 | Type 2 diabetes mellitus incidence not established by glucose measurements (questionnaires, reviews of primary care records, reviews of death certificates) |
| Pinelli 2011 | Cross‐sectional study |
| Polakowska 2011 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Pradhan 2007 | Intervention trial (Women's Health Study) |
| Priya 2013 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Qiao 2003 | Not a prospective cohort study |
| Qiu 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Ramachandran 2012 | Not a prospective cohort study |
| Rauh 2017 | Development of a prediction model for HbA1c levels after 6 years in the non‐diabetic general population |
| Reynolds 2006 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Rimm 1995 | No cohort with intermediate hyperglycaemia |
| Sacks 2017 | Investigation of patient activation to predict the course of type 2 diabetes |
| Sai 2017 | No cohort with intermediate hyperglycaemia |
| Samaras 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Schmitz 2016 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Schottker 2011 | Diabetes incidence by self‐report only |
| Schulze 2008 | Evaluation of a diabetes risk score |
| Schwarz 2007 | No individuals with intermediate hyperglycaemia at baseline |
| Serrano 2013 | Study design paper |
| Shimazaki 2007 | Not a prospective cohort study |
| Song 2007 | Mix of old an new participants in 2 study phases, participants with with both IFG and IGT were combined into an IFG group |
| Song 2016b | Not a prospective cohort study |
| Sorgjerd 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Soria 2009 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Stampfer 1988 | No cohort with intermediate hyperglycaemia |
| Strauss 1974 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Suvitaival 2018 | Evaluation of a new biomarker ('plasma lipidome') model |
| Tabak 2009 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Tai 2004 | Aggregated data from several prevalence and incidence studies |
| Takkunen 2016 | Cohort from intervention trial, no data on cohort with intermediate hyperglycaemia |
| Tanabe 2009 | Not a prospective cohort study |
| Vaccaro 2005 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Vaidya 2016 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Vazquez 2000 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Vega‐Vázquez 2017 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Von Eckardstein 2000 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Wang 2010 | New diabetes cases were identified through hospital records only |
| Warram 1996 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Wei 1999 | Investigation of the association between cardiorespiratory fitness and intermediate hyperglycaemia/type 2 diabetes mellitus |
| Welborn 1979 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Wheeler 2017 | Investigation of genetic determinants of HbA1c on the development of type 2 diabetes |
| Wingard 1993 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Woo 2015 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Wu 2017a | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Wu 2017b | Intermediate hyperglycaemia determined through register data, retrospective study |
| Wu 2018 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Xu 2014 | Investigation of a prediction model for development of diabetes |
| Yang 2016 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Ye 2014 | No data on people with intermediate hyperglycaemia |
| Yi 2017 | No data on type 2 diabetes incidence |
| Yokota 2017 | Retrospective cohort study |
| Yoshinaga 1996 | Non‐standard thresholds for intermediate hyperglycaemia |
| Yoshinaga 1999 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Zargar 2001 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Zethelius 2008 | No data on transition from intermediate hyperglycaemia to type 2 diabetes, establishment of a predictive model |
| Zhang 2012b | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Zhang 2016 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
| Zimmet 1992 | No data on transition from intermediate hyperglycaemia to type 2 diabetes |
FPG: fasting plasma glucose; HbA1c: glycosylated haemoglobin A1c; IFG: impaired fasting glucose; IGT: impaired glucose tolerance.
Characteristics of studies awaiting assessment [ordered by study ID]
Li 2001.
| Study name | Model development of diabetes in adult Chinese |
| Starting date | 1986, follow‐up 6 years |
| Contact information | Guangwei Li, Department of Endocrinology, China‐Japan Friendship Hospital, Beijing 100029 China |
| Notes | Establishment of a model for type 2 diabetes and the roles of insulin resistance and insulin secretion impairment; needs translation |
Misnikova 2011.
| Study name | Risk of diabetes and cardiovascular events in persons with early glucose metabolism impairments |
| Starting date | 2006, follow‐up 3 years |
| Contact information | Misnikova IV, Endocrinology, Moscow Regional Research Clinical Institute, Russian Federation |
| Notes | Conference abstract, no publication available |
NCT00816608.
| Study name | The effect of maximum body weight in lifetime on the development of type 2 diabetes (MAXWEL) |
| Starting date | August 2006 |
| Contact information | Professor Soo Lim, Seoul National University Bundang Hospital |
| Notes | Study completion date: September 2013; no publication available |
Characteristics of ongoing studies [ordered by study ID]
NCT00786890.
| Trial name or title | A survey to evaluate the cardiovascular risk status of subjects with pre‐diabetes in Hong Kong (JADE‐HK2) |
| Starting date | November 2008 |
| Contact information | Juliana Chan, Professor, Chinese University of Hong Kong |
| Notes | Estimated study completion date: December 2018 |
NCT02838693.
| Trial name or title | Assessing progression to type‐2 diabetes (APT‐2D): a prospective cohort study expanded from BRITE‐SPOT (Bio‐bank and Registry for StratIfication and Targeted intErventions in the Spectrum Of Type 2 Diabetes) (APT‐2D) |
| Starting date | March 2016 |
| Contact information | Sue‐Anne Toh, MBBChir, MSc, MA; +65 67722195; mdcsates@nus.edu.sg |
| Notes | Estimated study completion date: December 2021 |
NCT02958579.
| Trial name or title | A population based study on metabolic syndrome complications, and mortality (MetSCoM) |
| Starting date | January 2005 |
| Contact information | Alireza Esteghamati, MD (esteghamati@tums.ac.ir); Zahra Aryan, MD, MPH (aryanzahra@yahoo.com) |
| Notes | Estimated study completion date: January 2020 |
Vilanova 2017.
| Trial name or title | Prevalence, clinical features and risk assessment of pre‐diabetes in Spain: the prospective Mollerussa cohort study |
| Starting date | August 2011 |
| Contact information | Dr Didac Mauricio, MD; didacmauricio@gmail.com |
| Notes | The Mollerussa study completed its recruitment phase in July 2014 and the 12 month follow‐up in July 2015. Participants will be followed up long‐term through annual extraction of data included in the individual's electronic medical records. |
Differences between protocol and review
We changed the title of the protocol from 'Intermediate hyperglycaemia as a predictor for the development of type 2 diabetes: prognostic factor exemplar review' to 'Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia' to fit the objectives of the review. We also modified the objectives from "to assess whether intermediate hyperglycaemia is a predictor for the development of type 2 diabetes mellitus (T2DM)" to objective 1 "to assess the overall prognosis of people with IH for the development of T2DM and to assess how many people with IH revert back to normoglycaemia (regression), and objective 2 "to assess the difference in T2DM incidence in people with IH versus people with normoglycaemia". Both changes reflect the fact that our review addresses two prognostic questions at the same time. First, if people have intermediate hyperglycaemia at baseline, how many individuals develop type 2 diabetes in the future? This research question investigates the cumulative incidence of type 2 diabetes over time and does not depend on a comparison with a group with normoglycaemia at baseline; it is also important to note how many people change back from intermediate hyperglycaemia to normoglycaemia. The second prognostic question is, how does glycaemic status (intermediate hyperglycaemia compared with normoglycaemia) at baseline affect the development of type 2 diabetes? In particular, we were interested in intermediate hyperglycaemia, defined using impaired fasting glucose, impaired glucose tolerance and elevated glycosylated haemoglobin A1c and combinations thereof.
We specified inclusion criteria in more detail to explain the difference between studies evaluating the overall prognosis of people with intermediate hyperglycaemia and studies evaluating intermediate hyperglycaemia versus normoglycaemia as a prognostic factor developing type 2 diabetes mellitus.
Regarding methods, we explained our exclusion criteria in more detail and deleted 'conference abstract' as an exclusion criterion (we moved one formerly excluded study, Misnikova 2011, to 'Studies awaiting classification').
Contributions of authors
All review authors read and approved the final review draft.
Bernd Richter (BR): protocol and review draft, search strategy development, acquisition of trial reports, trial selection, data extraction of all trials, data analysis, data interpretation and writing of drafts.
Maria‐Inti Metzendorf (MIM): search strategy development, trial selection, check of data extraction, review of drafts.
Bianca Hemmingsen (BH): protocol and review draft, trial selection, data interpretation and review of drafts.
Yemisi Takwoingi (YT): protocol and review draft, data analysis, data interpretation and review of drafts
Sources of support
Internal sources
No sources of support supplied
External sources
-
World Health Organization, Other.
This review is part of a series of reviews on predictors for the development of type 2 diabetes mellitus in people with intermediate hyperglycaemia and interventions for the prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus which is funded by the WHO (Hemmingsen 2016a; Hemmingsen 2016b; Hemmingsen 2016c)
Declarations of interest
BR: the World Health Organization (WHO) funded this review.
MIM: none known.
BH: none known.
YT: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Admiraal 2014 {published data only}
- Admiraal WM, Holleman F, Snijder MB, Peters RJ, Brewster LM, Hoekstra JB, et al. Ethnic disparities in the association of impaired fasting glucose with the 10‐year cumulative incidence of type 2 diabetes. Diabetes Research and Clinical Practice 2014;103(1):127‐32. [PUBMED: 24355200] [DOI] [PubMed] [Google Scholar]
- Agyemang C, Valkengoed I, Born B J, Stronks K. Prevalence and determinants of prehypertension among African Surinamese, Hindustani Surinamese, and White Dutch in Amsterdam, the Netherlands: the SUNSET study. European Journal of Cardiovascular Prevention and Rehabilitation 2007;14(6):775‐81. [DOI] [PubMed] [Google Scholar]
- Bindraban NR, Valkengoed IG, Mairuhu G, Holleman F, Hoekstra JB, Michels BP, et al. Prevalence of diabetes mellitus and the performance of a risk score among Hindustani Surinamese, African Surinamese and ethnic Dutch: a cross‐sectional population‐based study. BMC Public Health 2008;8:271. [PUBMED: 18673544] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker LH, Nicolaou M, A Dl, Busschers WB, Brewster LM, Snijder MB, et al. Sex differences in the association between serum ferritin and fasting glucose in type 2 diabetes among South Asian Surinamese, African Surinamese, and ethnic Dutch: the population‐based SUNSET study. Diabetes Care 2013;36(4):965‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aekplakorn 2006 {published data only}
- Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care 2006;29(8):1872‐7. [PUBMED: 16873795] [DOI] [PubMed] [Google Scholar]
- Sritara P, Cheepudomwit S, Chapman N, Woodward M, Kositchaiwat C, Tunlayadechanont S, et al. Twelve‐year changes in vascular risk factors and their associations with mortality in a cohort of 3499 Thais: the electricity generating authority of Thailand study. International Journal of Epidemiology 2003;32:461‐8. [PUBMED: 12777437] [DOI] [PubMed] [Google Scholar]
Ammari 1998 {published data only}
- Ajlouni K, Jaddou H, Batieha A. Obesity in Jordan. International Journal of Obesity and Related Metabolic Disorders 1998;22(7):624‐8. [PUBMED: 9705020] [DOI] [PubMed] [Google Scholar]
- Ammari F, Batieha A, Jaddou PH, Okashi M, Ajlouni K. A natural history of impaired glucose tolerance in North Jordan. Practical Diabetes International 1998;15(5):139‐40. [Google Scholar]
Anjana 2015 {published data only}
- Anjana RM, Shanthi Rani CS, Deepa M, Pradeepa R, Sudha V, Divya Nair H, et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10‐year follow‐up of the Chennai urban rural epidemiology study (CURES). Diabetes Care 2015;38(8):1441‐8. [PUBMED: 25906786] [DOI] [PubMed] [Google Scholar]
- Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, et al. The Chennai urban rural epidemiology study (CURES) ‐ study design and methodology (urban component) (CURES‐I). Journal of the Association of Physicians of India 2003;51:863‐70. [PUBMED: 14710970] [PubMed] [Google Scholar]
- Mohan D, Raj D, Shanthirani CS, Datta M, Unwin NC, Kapur A, et al. Awareness and knowledge of diabetes in Chennai ‐ the Chennai urban rural epidemiology study [CURES‐9]. Journal of the Association of Physicians of India 2005;53:283‐7. [PubMed] [Google Scholar]
- Mohan V, Deepa M, Farooq S, Datta M, Deepa R. Prevalence, awareness and control of hypertension in Chennai ‐ the Chennai urban rural epidemiology study (CURES‐52). Journal of the Association of Physicians of India 2007;55:326‐32. [PubMed] [Google Scholar]
- Mohan V, Deepa R, Pradeepa R, Vimaleswaran KS, Mohan A, Velmurugan K, et al. Association of low adiponectin levels with the metabolic syndrome ‐ the Chennai urban rural epidemiology study (CURES‐4). Metabolism 2005;54(4):476‐81. [DOI] [PubMed] [Google Scholar]
- Mohan V, Sandeep S, Deepa M, Gokulakrishnan K, Datta M, Deepa R. A diabetes risk score helps identify metabolic syndrome and cardiovascular risk in Indians ‐ the Chennai urban rural epidemiology study (CURES‐38). Diabetes, Obesity & Metabolism 2007;9(3):337‐43. [DOI] [PubMed] [Google Scholar]
- Radhika G, Sathya RM, Sudha V, Ganesan A, Mohan V. Dietary salt intake and hypertension in an urban south Indian population ‐ [CURES ‐ 53]. Journal of the Association of Physicians of India 2007;55:405‐11. [PubMed] [Google Scholar]
Bae 2011 {published data only}
- Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4‐year retrospective longitudinal study. Diabetes Care 2011;34(3):727‐9. [PUBMED: 21278140] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, et al. Optimal range of HbA1c for the prediction of future diabetes: a 4‐year longitudinal study. Diabetes Research and Clinical Practice 2011;93(2):255‐9. [PUBMED: 21676480] [DOI] [PubMed] [Google Scholar]
Baena‐Diez 2011 {published data only}
- Baena‐Diez JM, Bermudez‐Chillida N, Mundet X, Val‐Garcia JL, Munoz MA, Schroder H. Impaired fasting glucose and risk of diabetes mellitus at 10 years. Cohort study. Medicina Clinica 2011;136(9):382‐5. [PUBMED: 21300382] [DOI] [PubMed] [Google Scholar]
Bai 1999 {published data only}
- Bai PV, Krishnaswami CV, Chellamariappan M. Prevalence and incidence of type‐2 diabetes and impaired glucose tolerance in a selected Indian urban population. Journal of the Association of Physicians of India 1999;47(11):1060‐4. [PUBMED: 10862313] [PubMed] [Google Scholar]
Bergman 2016 {published data only}
- Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One‐hour post‐load plasma glucose level during the OGTT predicts dysglycemia: observations from the 24year follow‐up of the Israel study of glucose intolerance, obesity and hypertension. Diabetes Research and Clinical Practice 2016;120:221‐8. [PUBMED: 27596059] [DOI] [PubMed] [Google Scholar]
- Dankner R, Abdul‐Ghani MA, Gerber Y, Chetrit A, Wainstein J, Raz I. Predicting the 20‐year diabetes incidence rate. Diabetes/metabolism Research and Reviews 2007;23(7):551‐8. [PUBMED: 17315136] [DOI] [PubMed] [Google Scholar]
- Modan M, Halkin H, Karasik A, Lusky A. Effectiveness of glycosylated hemoglobin, fasting plasma glucose, and a single post load plasma glucose level in population screening for glucose intolerance. American Journal of Epidemiology 1984;119(3):431‐44. [PUBMED: 6702817] [DOI] [PubMed] [Google Scholar]
- Modan M, Karasik A, Halkin H, Fuchs Z, Lusky A, Shitrit A, et al. Effect of past and concurrent body mass index on prevalence of glucose intolerance and type 2 (non‐insulin‐dependent) diabetes and on insulin response. Diabetologia 1986;29(2):82‐9. [PUBMED: 3516770] [DOI] [PubMed] [Google Scholar]
Bonora 2011 {published data only}
- Bonora E, Kiechl S, Mayr A, Zoppini G, Targher G, Bonadonna RC, et al. High‐normal HbA1c is a strong predictor of type 2 diabetes in the general population. Diabetes Care 2011;34(4):1038‐40. [PUBMED: 21307378] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck study. Diabetes Care 2007;30(2):318‐24. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Population‐based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes 2004;53(7):1782‐9. [PUBMED: 15220202] [DOI] [PubMed] [Google Scholar]
Cederberg 2010 {published data only}
- Cederberg H, Saukkonen T, Laakso M, Jokelainen J, Harkonen P, Timonen M, et al. Postchallenge glucose, A1C, and fasting glucose as predictors of type 2 diabetes and cardiovascular disease: a 10‐year prospective cohort study. Diabetes Care 2010;33(9):2077‐83. [PUBMED: 20573752] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala U, Laakso M, Paivansalo M, Pelkonen O, Suramo I, Keinanen‐Kiukaanniemi S. Low insulin sensitivity measured by both quantitative insulin sensitivity check index and homeostasis model assessment method as a risk factor of increased intima‐media thickness of the carotid artery. Journal of Clinical Endocrinology and Metabolism 2002;87(11):5092‐7. [PUBMED: 12414877] [DOI] [PubMed] [Google Scholar]
Chamnan 2011 {published data only}
- Chamnan P, Simmons RK, Forouhi NG, Luben RN, Khaw KT, Wareham NJ, et al. Incidence of type 2 diabetes using proposed HbA1c diagnostic criteria in the European prospective investigation of Cancer‐Norfolk cohort: implications for preventive strategies. Diabetes Care 2011;34(4):950‐6. [PUBMED: 20622160] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC‐Norfolk: study design and characteristics of the cohort. European prospective investigation of cancer. British Journal of Cancer 1999;80(Suppl 1):95‐103. [PUBMED: 10466767] [PubMed] [Google Scholar]
- Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC‐Norfolk). BMJ 2001;322(7277):15‐8. [PUBMED: 11141143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charles 1997 {published data only}
- Balkau B, Forhan A, Eschwege E. Two hour plasma glucose is not unequivocally predictive for early death in men with impaired fasting glucose: more results from the Paris prospective study. Diabetologia 2002;45(9):1224‐30. [DOI] [PubMed] [Google Scholar]
- Charles MA, Eschwege E, Thibult N, Claude JR, Warnet JM, Rosselin GE, et al. The role of non‐esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris prospective study. Diabetologia 1997;40(9):1101‐6. [PUBMED: 9300248] [DOI] [PubMed] [Google Scholar]
- Charles MA, Fontbonne A, Thibult N, Warnet JM, Rosselin GE, Eschwege E. Risk factors for NIDDM in white population. Paris prospective study. Diabetes 1991;40(7):796‐9. [PUBMED: 2060716] [DOI] [PubMed] [Google Scholar]
- Eschwege E, Charles MA, Simon D, Thibult N, Balkau B. From policemen to policies: what is the future for 2‐h glucose? The Kelly West lecture, 2000. Diabetes Care 2001;24(11):1945‐50. [DOI] [PubMed] [Google Scholar]
- Eschwege E, Charles MA, Simon D, Thibult N, Balkau B. Reproducibility of the diagnosis of diabetes over a 30‐month follow‐up: the Paris prospective study. Diabetes Care 2001;24(11):1941‐4. [PUBMED: 11679461] [DOI] [PubMed] [Google Scholar]
Chen 2003 {published data only}
- Chen KT, Chen CJ, Gregg EW, Imperatore G, Narayan KMV. Impaired fasting glucose and risk of diabetes in Taiwan: follow‐up over 3 years. Diabetes Research and Clinical Practice 2003;60(3):177‐82. [PUBMED: 12757990] [DOI] [PubMed] [Google Scholar]
- Chen KT, Chen CJ, Gregg EW, Williamson DF, Narayan KM. High prevalence of impaired fasting glucose and type 2 diabetes mellitus in Penghu Islets, Taiwan: evidence of a rapidly emerging epidemic?. Diabetes Research and Clinical Practice 1999;44(1):59‐69. [PUBMED: 10414941] [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen G, Lin L, Chen L, Li L, Huang H, Wang W, et al. Comparison of insulin resistance and beta‐cell dysfunction between the young and the elderly in normal glucose tolerance and prediabetes population: a prospective study. Hormone and Metabolic Research 2017;49(2):135‐41. [DOI: 10.1055/s-0042-111325; PUBMED: 27459384] [DOI] [PubMed] [Google Scholar]
Coronado‐Malagon 2009 {published data only}
- Coronado‐Malagon M, Gomez‐Vargas JI, Espinoza‐Peralta D, Arce‐Salinas A. Progression toward type‐2 diabetes mellitus among Mexican pre‐diabetics. Assessment of a cohort. Gaceta Medica De Mexico 2009;145(4):269‐72. [PUBMED: 20073428] [PubMed] [Google Scholar]
Cugati 2007 {published data only}
- Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten‐year incidence of diabetes in older Australians: the Blue Mountains eye study. Medical Journal of Australia 2007;186(3):131‐5. [PUBMED: 17309402] [DOI] [PubMed] [Google Scholar]
- Mitchell P, Smith W, Wang JJ, Cumming RG, Leeder SR, Burnett L. Diabetes in an older Australian population. Diabetes Research and Clinical Practice 1998;41(3):177‐84. [PUBMED: 9829346] [DOI] [PubMed] [Google Scholar]
De Abreu 2015 {published data only}
- Abreu LLF, Holloway KL, Mohebbi M, Sajjad MA, Kotowicz MA, Pasco JA. All‐cause mortality risk in Australian women with impaired fasting glucose and diabetes. Journal of Diabetes Research 2017;2017:2042980. [PUBMED: 28698884] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Nicholson GC, Kotowicz MA. Cohort profile: Geelong osteoporosis study. International Journal of Epidemiology 2012;41(6):1565‐75. [PUBMED: 23283714] [DOI] [PubMed] [Google Scholar]
- Abreu L, Holloway KL, Kotowicz MA, Pasco JA. Dysglycaemia and other predictors for progression or regression from impaired fasting glucose to diabetes or normoglycaemia. Journal of Diabetes Research 2015;2015:373762. [DOI: 10.1155/2015/373762; PUBMED: 26273669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Den Biggelaar 2016 {published data only}
- Biggelaar LJ, Sep SJ, Eussen SJ, Mari A, Ferrannini E, Greevenbroek MM, et al. Discriminatory ability of simple OGTT‐based beta cell function indices for prediction of prediabetes and type 2 diabetes: the CODAM study. Diabetologia 2016;60(3):432‐41. [PUBMED: 27933333] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijshoop M, Feskens EJ, Blaak EE, Bruin TW. Validation of capillary glucose measurements to detect glucose intolerance or type 2 diabetes mellitus in the general population. Clinica Chimica Acta 2004;341(1‐2):33‐40. [PUBMED: 14967156] [DOI] [PubMed] [Google Scholar]
Derakhshan 2016 {published data only}
- Aghaei Meybodi HR, Azizi F. Changes in body weight and fat distribution; risk factors for abnormal glucose homeostasis? Tehran lipid and glucose study. Iranian Journal of Diabetes and Lipid Disorders 2009;8(1):1‐12. [Google Scholar]
- Bozorgmanesh M, Hadaegh F, Azizi F. A simple clinical model predicted diabetes progression among prediabetic individuals. Diabetes Research and Clinical Practice 2012;97(2):e34‐6. [PUBMED: 22647753] [DOI] [PubMed] [Google Scholar]
- Derakhshan A, Bagherzadeh‐Khiabani F, Arshi B, Ramezankhani A, Azizi F, Hadaegh F. Different combinations of glucose tolerance and blood pressure status and incident diabetes, hypertension, and chronic kidney disease. Journal of the American Heart Association 2016;5(8):pii: e003917. [DOI: 10.1161/JAHA.116.003917; PUBMED: 27543801] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan A, Sardarinia M, Khalili D, Momenan AA, Azizi F, Hadaegh F. Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow‐up: Tehran lipid and glucose study. PLOS ONE 2014;9(7):e102563. [PUBMED: 25029368] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan A, Tohidi M, Arshi B, Khalili D, Azizi F, Hadaegh F. Relationship of hyperinsulinaemia, insulin resistance and beta‐cell dysfunction with incident diabetes and pre‐diabetes: the Tehran lipid and glucose study. Diabetic Medicine 2015;32(1):24‐32. [PUBMED: 25131451] [DOI] [PubMed] [Google Scholar]
- Hadaegh F, Bozorgmanesh MR, Ghasemi A, Harati H, Saadat N, Azizi F. High prevalence of undiagnosed diabetes and abnormal glucose tolerance in the Iranian urban population: Tehran lipid and glucose study. BMC Public Health 2008;8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaegh F, Derakhshan A, Zafari N, Khalili D, Mirbolouk M, Saadat N, et al. Pre‐diabetes tsunami: incidence rates and risk factors of pre‐diabetes and its different phenotypes over 9 years of follow‐up. Diabetic Medicine 2017;34(1):69‐78. [PUBMED: 26606421] [DOI] [PubMed] [Google Scholar]
- Hadaegh F, Ghasemi Ar, Padyab M, Tohidi M, Azizi F. The metabolic syndrome and incident diabetes: assessment of alternative definitions of the metabolic syndrome in an Iranian urban population. Diabetes Research and Clinical Practice 2008;80(2):328‐34. [PUBMED: 18282630] [DOI] [PubMed] [Google Scholar]
- Harati H, Hadaegh F, Saadat N, Azizi F. Population‐based incidence of type 2 diabetes and its associated risk factors: results from a six‐year cohort study in Iran. BMC Public Health 2009;9:186. [DOI: 10.1186/1471-2458-9-186; PUBMED: 19531260] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harati H, Hadaegh F, Tohidi M, Azizi F. Impaired fasting glucose cutoff value of 5.6 mmol/l combined with other cardiovascular risk markers is a better predictor for incident type 2 diabetes than the 6.1 mmol/l value: Tehran lipid and glucose study. Diabetes Research and Clinical Practice 2009;85(1):90‐5. [PUBMED: 19414206] [DOI] [PubMed] [Google Scholar]
Dowse 1991 {published data only}
- Dowse GK, Zimmet PZ, Collins VR. Insulin levels and the natural history of glucose intolerance in Nauruans. Diabetes 1996;45(10):1367‐72. [DOI] [PubMed] [Google Scholar]
- Dowse GK, Zimmet PZ, Collins VR. Insulin levels and the natural history of glucose intolerance in Nauruans. Diabetes 1996;45(10):1367‐72. [PUBMED: 8826973] [DOI] [PubMed] [Google Scholar]
- Dowse GK, Zimmet PZ, Finch CF, Collins VR. Decline in incidence of epidemic glucose intolerance in Nauruans: implications for the "thrifty genotype". American Journal of Epidemiology 1991;133(11):1093‐104. [PUBMED: 2035513] [DOI] [PubMed] [Google Scholar]
- King H, Zimmet P, Raper LR, Balkau B. The natural history of impaired glucose tolerance in the Micronesian population of Nauru: a six‐year follow‐up study. Diabetologia 1984;26(1):39‐43. [PUBMED: 6706044] [DOI] [PubMed] [Google Scholar]
- Sicree RA, Zimmet PZ, King HOM, Coventry JS. Plasma‐insulin response among Nauruans ‐ prediction of deterioration in glucose‐tolerance over 6‐yr. Diabetes 1987;36(2):179‐86. [PUBMED: 3542644] [DOI] [PubMed] [Google Scholar]
Ferrannini 2009 {published data only}
- Ferrannini E, Massari M, Nannipieri M, Natali A, Lopez Ridaura R, Gonzales‐Villalpando C. Plasma glucose levels as predictors of diabetes: the Mexico City diabetes study. Diabetologia 2009;52(5):818‐24. [PUBMED: 19224196] [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes 2004;53(1):160‐5. [PUBMED: 14693710] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Gonzalez C, Mykkanen L, Stern M. Total immunoreactive proinsulin, immunoreactive insulin and specific insulin in relation to conversion to NIDDM: the Mexico City diabetes study. Diabetologia 1997;40(7):830‐7. [PUBMED: 9243105] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City diabetes study. Diabetes Care 1996;19(10):1138‐41. [PUBMED: 8886564] [DOI] [PubMed] [Google Scholar]
- Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, et al. Liver enzymes, the metabolic syndrome, and incident diabetes: the Mexico City diabetes study. Diabetes Care 2005;28(7):1757‐62. [PUBMED: 15983331] [DOI] [PubMed] [Google Scholar]
Filippatos 2016 {published data only}
- Filippatos TD, Panagiotakos DB, Georgousopoulou EN, Pitaraki E, Kouli GM, Chrysohoou C, et al. Mediterranean diet and 10‐year (2002‐2012) incidence of diabetes and cardiovascular disease in participants with prediabetes: the ATTICA study. Review of Diabetic Studies 2016;13(4):226‐35. [PUBMED: 28278309] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloverou E, Panagiotakos DB, Georgousopoulou EN, Grekas A, Christou A, Chatzigeorgiou M, et al. Dietary patterns and 10‐year (2002‐2012) incidence of type 2 diabetes: results from the ATTICA cohort study. Review of Diabetic Studies 2016;13(4):246‐56. [PUBMED: 28394951] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Grekas A, et al. Adherence to Mediterranean diet and 10‐year incidence (2002‐2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes/Metabolism Research and Reviews 2016;32(1):73‐81. [PUBMED: 26104243] [DOI] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Chrysohoou C, Stefanadis C. Epidemiology of cardiovascular risk factors in Greece: aims, design and baseline characteristics of the ATTICA study. BMC Public Health 2003;3:32. [PUBMED: 14567760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forouhi 2007 {published data only}
- Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990‐2000. Diabetic Medicine 2007;24(2):200‐7. [PUBMED: 17257284] [DOI] [PubMed] [Google Scholar]
- Simmons RK, Rahman M, Jakes RW, Yuyun MF, Niggebrugge AR, Hennings SH, et al. Effect of population screening for type 2 diabetes on mortality: long‐term follow‐up of the Ely cohort. Diabetologia 2011;54:312‐9. [PUBMED: 20978739] [DOI] [PubMed] [Google Scholar]
- Wareham NJ, Byrne CD, Williams R, Day NE, Hales CN. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care 1999;22(2):262‐70. [PUBMED: 10333943] [DOI] [PubMed] [Google Scholar]
- Williams DR, Wareham NJ, Brown DC, Byrne CD, Clark PM, Cox BD, et al. Undiagnosed glucose intolerance in the community: the Isle of Ely diabetes project. Diabetic Medicine 1995;12:30‐5. [PUBMED: 7712700] [DOI] [PubMed] [Google Scholar]
Garcia 2016 {published data only}
- Garcia L, Lee A, Al Hazzouri AZ, Neuhaus JM, Moyce S, Aiello A, et al. Influence of neighbourhood socioeconomic position on the transition to type II diabetes in older Mexican Americans: the Sacramento area longitudinal study on aging. BMJ Open 2016;6(8):e010905. [PUBMED: 27515749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautier 2010 {published data only}
- Balkau B, Lange C, Fezeu L, Tichet J, Lauzon‐Guillain B, Czernichow S, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the epidemiological study on the insulin resistance syndrome (DESIR). Diabetes Care 2008;31(10):2056‐61. [PUBMED: 18689695] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, et al. Use of HbA1c in predicting progression to diabetes in French men and women: data from an epidemiological study on the insulin resistance syndrome (DESIR). Diabetes Care 2006;29(7):1619‐25. [PUBMED: 16801588] [DOI] [PubMed] [Google Scholar]
- Gautier A, Roussel R, Ducluzeau PH, Lange C, Vol S, Balkau B, et al. Increases in waist circumference and weight as predictors of type 2 diabetes in individuals with impaired fasting glucose: influence of baseline BMI. Data from the DESIR study. Diabetes Care 2010;33(8):1850‐2. [PUBMED: 20484131] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulimane S, Simon D, Shaw J, Witte D, Zimmet P, Vol S, et al. HbA1c, fasting plasma glucose and the prediction of diabetes: Inter99, AusDiab and D.E.S.I.R. Diabetes Research and Clinical Practice 2012;96(3):392‐9. [PUBMED: 21741107] [DOI] [PubMed] [Google Scholar]
- Soulimane S, Simon D, Shaw JE, Zimmet PZ, Vol S, Vistisen D, et al. Comparing incident diabetes as defined by fasting plasma glucose or by HbA(1c). The AusDiab, Inter99 and DESIR studies. Diabetic Medicine 2011;28(11):1311‐8. [PUBMED: 21824186] [DOI] [PubMed] [Google Scholar]
Gomez‐Arbelaez 2015 {published data only}
- Gomez‐Arbelaez D, Alvarado‐Jurado L, Ayala‐Castillo M, Forero‐Naranjo L, Camacho PA, Lopez‐Jaramillo P. Evaluation of the Finnish diabetes risk score to predict type 2 diabetes mellitus in a Colombian population: a longitudinal observational study. World Journal of Diabetes 2015;6(17):1337‐44. [PUBMED: 26675051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerrero‐Romero 2006 {published data only}
- Guerrero‐Romer F, Rodriguez‐Moran M, Gonzalez‐Ortiz M, Martinez‐Abundis E. Insulin action and secretion in healthy Hispanic‐Mexican first‐degree relatives of subjects with type 2 diabetes. Journal of Endocrinological Investigation 2001;24:580‐6. [PUBMED: 11686540] [DOI] [PubMed] [Google Scholar]
- Guerrero‐Romero F, Rodriguez‐Moran M. Assessing progression to impaired glucose tolerance and type 2 diabetes mellitus. European Journal of Clinical Investigation 2006;36(11):796‐802. [PUBMED: 17032347] [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Moran M, Guerrero‐Romero F. Hyperinsulinemia and abdominal obesity are more prevalent in non‐diabetic subjects with family history of type 2 diabetes. Archives of Medical Research 2000;31:399‐403. [PUBMED: 11068083] [DOI] [PubMed] [Google Scholar]
Han 2017 {published data only}
- Choi SH, Kim TH, Lim S, Park KS, Jang HC, Cho NH. Hemoglobin A1c as a diagnostic tool for diabetes screening and new‐onset diabetes prediction: a 6‐year community‐based prospective study. Diabetes Care 2011;34(4):944‐9. [PUBMED: 21335372] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Kim HJ, Kim DJ, Lee KW, Cho NH. Incidence and predictors of type 2 diabetes among Koreans: a 12‐year follow up of the Korean genome and epidemiology study. Diabetes Research and Clinical Practice 2017;123:173‐80. [PUBMED: 28043048] [DOI] [PubMed] [Google Scholar]
- Jung DH, Byun YS, Kwon YJ, Kim GS. Microalbuminuria as a simple predictor of incident diabetes over 8 years in the Korean genome and epidemiology study (KoGES). Scientific Reports 2017;7(1):15445. [PUBMED: 29133894] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JY, Oh CM, Ryoo JH, Choi JM, Choi YJ, Ham W T, et al. The influence of prehypertension, hypertension, and glycated hemoglobin on the development of type 2 diabetes mellitus in prediabetes: the Korean genome and epidemiology study (KoGES). Endocrine 2018;59(3):593‐601. [PUBMED: 29380232] [DOI] [PubMed] [Google Scholar]
- Keun Park S, Ryoo JH, Oh CM, Choi JM, Choi YJ, Ok Lee K, et al. The risk of type 2 diabetes mellitus according to 2‐hour plasma glucose level: the Korean genome and epidemiology study (KoGES). Diabetes Research and Clinical Practice 2017 Aug 9 [Epub ahead of print]. [DOI: 10.1016/j.diabres.2017.08.002; PUBMED: 28951335] [DOI] [PubMed]
- Lim NK, Park SH, Choi SJ, Lee KS, Park HY. A risk score for predicting the incidence of type 2 diabetes in a middle‐aged Korean cohort: the Korean genome and epidemiology study. Circulation Journal 2012;76(8):1904‐10. [PUBMED: 22640983] [DOI] [PubMed] [Google Scholar]
Hanley 2005 {published data only}
- Festa A, D'Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 2004;53(6):1549‐55. [DOI] [PubMed] [Google Scholar]
- Festa A, D'Agostino R Jr, Rich SS, Jenny NS, Tracy RP, Haffner SM. Promoter (4G/5G) plasminogen activator inhibitor‐1 genotype and plasminogen activator inhibitor‐1 levels in blacks, Hispanics, and non‐Hispanic whites: the insulin resistance atherosclerosis study. Circulation 2003;107(19):2422‐7. [DOI] [PubMed] [Google Scholar]
- Haffner SM, D'Agostino R Jr, Goff D, Howard B, Festa A, Saad MF, et al. LDL size in African Americans, Hispanics, and non‐Hispanic whites : the insulin resistance atherosclerosis study. Arteriosclerosis, Thrombosis, and Vascular Biology 1999;19(9):2234‐40. [DOI] [PubMed] [Google Scholar]
- Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, et al. Increased insulin resistance and insulin secretion in nondiabetic African‐Americans and Hispanics compared with non‐Hispanic whites. The insulin resistance atherosclerosis study. Diabetes 1996;45(6):742‐8. [PUBMED: 8635647] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Howard G, Mayer E, Bergman RN, Savage PJ, Rewers M, et al. Insulin sensitivity and acute insulin response in African‐Americans, non‐Hispanic whites, and Hispanics with NIDDM: the insulin resistance atherosclerosis study. Diabetes 1997;46(1):63‐9. [DOI] [PubMed] [Google Scholar]
- Hanley AJ, D'Agostino RB Jr, Wagenknecht LE, Saad MF, Savage PJ, Bergman R, et al. Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the insulin resistance atherosclerosis study. Diabetes 2002;51(4):1263‐70. [PUBMED: 11916954] [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Karter AJ, Williams K, Festa A, D'Agostino RB Jr, Wagenknecht LE, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the insulin resistance atherosclerosis study. Circulation 2005;112(24):3713‐21. [PUBMED: 16344402] [DOI] [PubMed] [Google Scholar]
- Howard BV, Mayer‐Davis EJ, Goff D, Zaccaro DJ, Laws A, Robbins DC, et al. Relationships between insulin resistance and lipoproteins in nondiabetic African Americans, Hispanics, and non‐Hispanic whites: the insulin resistance atherosclerosis study. Metabolism 1998;47(10):1174‐9. [DOI] [PubMed] [Google Scholar]
- Karter AJ, Mayer‐Davis EJ, Selby JV, D'Agostino RB Jr, Haffner SM, Sholinsky P, et al. Insulin sensitivity and abdominal obesity in African‐American, Hispanic, and non‐Hispanic white men and women. The insulin resistance and atherosclerosis study. Diabetes 1996;45(11):1547‐55. [DOI] [PubMed] [Google Scholar]
- Mayer‐Davis EJ, Levin S, Bergman RN, D'Agostino RB Jr, Karter AJ, Saad MF, et al. Insulin secretion, obesity, and potential behavioral influences: results from the insulin resistance atherosclerosis study (IRAS). Diabetes/metabolism Research and Reviews 2001;17(2):137‐45. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Lugo L, Mayer‐Davis EJ, Howard G, Selby JV, Ayad MF, Rewers M, et al. Insulin sensitivity and intake of vitamins E and C in African American, Hispanic, and non‐Hispanic white men and women: the insulin resistance and atherosclerosis study (IRAS). American Journal of Clinical Nutrition 1997;66(5):1224‐31. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Annals of Epidemiology 1995;5(6):464‐72. [PUBMED: 8680609] [DOI] [PubMed] [Google Scholar]
Heianza 2012 {published data only}
- Heianza Y, Arase Y, Fujihara K, Hsieh SD, Saito K, Tsuji H, et al. Longitudinal trajectories of HbA1c and fasting plasma glucose levels during the development of type 2 diabetes: the Toranomon hospital health management center study 7 (TOPICS 7). Diabetes Care 2012;35(5):1050‐2. [PUBMED: 22456865] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heianza Y, Arase Y, Fujihara K, Tsuji H, Saito K, Hsieh SD, et al. High normal HbA(1c) levels were associated with impaired insulin secretion without escalating insulin resistance in Japanese individuals: the Toranomon hospital health management center study 8 (TOPICS 8). Diabetic Medicine 2012;29(10):1285‐90. [DOI] [PubMed] [Google Scholar]
- Heianza Y, Arase Y, Fujihara K, Tsuji H, Saito K, Hsieh SD, et al. Screening for pre‐diabetes to predict future diabetes using various cut‐off points for HbA(1c) and impaired fasting glucose: the Toranomon hospital health management center study 4 (TOPICS 4). Diabetic Medicine 2012;29(9):e279‐85. [PUBMED: 22510023] [DOI] [PubMed] [Google Scholar]
- Heianza Y, Arase Y, Hsieh SD, Saito K, Tsuji H, Kodama S, et al. Development of a new scoring system for predicting the 5 year incidence of type 2 diabetes in Japan: the Toranomon hospital health management center study 6 (TOPICS 6). Diabetologia 2012;55(12):3213‐23. [PUBMED: 22955996] [DOI] [PubMed] [Google Scholar]
- Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, et al. HbA1c 5.7‐6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011;378(9786):147‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heianza Y, Hara S, Arase Y, Saito K, Totsuka K, Tsuji H, et al. Low serum potassium levels and risk of type 2 diabetes: the Toranomon hospital health management center study 1 (TOPICS 1). Diabetologia 2011;54(4):762‐6. [PUBMED: 21212932] [DOI] [PubMed] [Google Scholar]
Inoue 1996 {published data only}
- Inoue I, Takahashi K, Katayama S, Harada Y, Negishi K, Ishii J, et al. A higher proinsulin response to glucose loading predicts deteriorating fasting plasma glucose and worsening to diabetes in subjects with impaired glucose tolerance. Diabetoc Medicine 1996;13(4):330‐6. [PUBMED: 9162608] [DOI] [PubMed] [Google Scholar]
Janghorbani 2015 {published data only}
- Amini M, Janghorbani M. Comparison of metabolic syndrome with glucose measurement for prediction of type 2 diabetes: the Isfahan diabetes prevention study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2009;3(2):84‐9. [Google Scholar]
- Haghighatdoost F, Amini M, Feizi A, Iraj B. Are body mass index and waist circumference significant predictors of diabetes and prediabetes risk: results from a population based cohort study. World Journal of Diabetes 2017;8(7):365‐73. [PUBMED: 28751960] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorbani M, Amini M. Normal fasting plasma glucose and risk of prediabetes and type 2 diabetes: the Isfahan diabetes prevention study. Review of Diabetic Studies 2011;8(4):490‐8. [PUBMED: 22580730] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorbani M, Amini M. Progression from optimal blood glucose and pre‐diabetes to type 2 diabetes in a high risk population with or without hypertension in Isfahan, Iran. Diabetes Research and Clinical Practice 2015;108(3):414‐22. [PUBMED: 25814432] [DOI] [PubMed] [Google Scholar]
Jaruratanasirikul 2016 {published data only}
- Jaruratanasirikul S, Thammaratchuchai S, Puwanant M, Mo‐Suwan L, Sriplung H. Progression from impaired glucose tolerance to type 2 diabetes in obese children and adolescents: a 3‐6‐year cohort study in southern Thailand. Journal of Pediatric Endocrinology & Metabolism 2016;29(11):1267‐75. [PUBMED: 27740930] [DOI] [PubMed] [Google Scholar]
Jeong 2010 {published data only}
- Jeong JY, Kim JG, Kim BW, Moon SS, Kim HS, Park KG, et al. Trend analysis of diabetic prevalence and incidence in a rural area of South Korea between 2003‐2008. Journal of Diabetes Investigation 2010;1(5):184‐90. [PUBMED: 24843430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiamjarasrangsi 2008a {published data only}
- Jiamjarasrangsi W, Aekplakorn W. Incidence and predictors of type 2 diabetes among professional and office workers in Bangkok, Thailand. Journal of the Medical Association of Thailand 2005;88(12):1896‐904. [PUBMED: 16518992] [PubMed] [Google Scholar]
- Jiamjarasrangsi W, Sangwatanaroj S, Lohsoonthorn V, Lertmaharit S. Increased alanine aminotransferase level and future risk of type 2 diabetes and impaired fasting glucose among the employees in a university hospital in Thailand. Diabetes & Metabolism 2008;34(3):283‐9. [PUBMED: 18486512] [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim DJ, Cho NH, Noh JH, Kim HJ, Choi YH, Jung JH, et al. Fasting plasma glucose cutoff value for the prediction of future diabetes development: a study of middle‐aged Koreans in a health promotion center. Journal of Korean Medical Science 2005;20(4):562‐5. [PUBMED: 16100444] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Cho NH, Noh JH, Lee MS, Lee MK, Kim KW. Lack of excess maternal transmission of type 2 diabetes in a Korean population. Diabetes Research and Clinical Practice 2004;65(2):117‐24. [PUBMED: 15223223] [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim SH, Shim WS, Kim EA, Kim EJ, Lee SH, Hong SB, et al. The effect of lowering the threshold for diagnosis of impaired fasting glucose. Yonsei Medical Journal 2008;49(2):217‐23. [PUBMED: 18452257] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim YA, Ku EJ, Khang AR, Hong ES, Kim KM, Moon JH, et al. Role of various indices derived from an oral glucose tolerance test in the prediction of conversion from prediabetes to type 2 diabetes. Diabetes Research and Clinical Practice 2014;106(2):351‐9. [PUBMED: 25245975] [DOI] [PubMed] [Google Scholar]
Kim 2016a {published data only}
- Kim CH, Kim HK, Kim EH, Bae SJ, Choe J, Park JY. Risk of progression to diabetes from prediabetes defined by HbA1c or fasting plasma glucose criteria in Koreans. Diabetes Research and Clinical Practice 2016;118:105‐11. [PUBMED: 27368062] [DOI] [PubMed] [Google Scholar]
Kleber 2010 {published data only}
- Kleber M, Lass N, Papcke S, Wabitsch M, Reinehr T. One‐year follow‐up of untreated obese white children and adolescents with impaired glucose tolerance: high conversion rate to normal glucose tolerance. Diabetic Medicine 2010;27(5):516‐21. [PUBMED: 20536946] [DOI] [PubMed] [Google Scholar]
Kleber 2011 {published data only}
- Kleber M, deSousa G, Papcke S, Wabitsch M, Reinehr T. Impaired glucose tolerance in obese white children and adolescents: three to five year follow‐up in untreated patients. Experimental and Clinical Endocrinology & Diabetes 2011;119(3):172‐6. [PUBMED: 20827664] [DOI] [PubMed] [Google Scholar]
Ko 1999 {published data only}
- Ko GT, Chan JC, Lau E, Woo J, Cockram CS. Fasting plasma glucose as a screening test for diabetes and its relationship with cardiovascular risk factors in Hong Kong Chinese. Diabetes Care 1997;20(2):170‐2. [DOI] [PubMed] [Google Scholar]
- Ko GT, Li JK, Cheung AY, Yeung VT, Chow CC, Tsang LW, et al. Two‐hour post‐glucose loading plasma glucose is the main determinant for the progression from impaired glucose tolerance to diabetes in Hong Kong Chinese. Diabetes Care 1999;22(12):2096‐7. [PUBMED: 10587859] [DOI] [PubMed] [Google Scholar]
Ko 2001 {published data only}
- Ko GT, Chan JC, Cockram CS. Change of glycaemic status in Chinese subjects with impaired fasting glycaemia. Diabetic Medicine 2001;18(9):745‐8. [PUBMED: 11606173] [DOI] [PubMed] [Google Scholar]
Larsson 2000 {published data only}
- Larsson H, Ahren B, Lindgarde F, Berglund G. Fasting blood glucose in determining the prevalence of diabetes in a large, homogeneous population of Caucasian middle‐aged women. Journal of Internal Medicine 1995;237(6):537‐41. [PUBMED: 7782724] [DOI] [PubMed] [Google Scholar]
- Larsson H, Berglund G, Lindgarde F, Ahren B. Comparison of ADA and WHO criteria for diagnosis of diabetes and glucose intolerance. Diabetologia 1998;41(9):1124‐5. [PUBMED: 9754834] [DOI] [PubMed] [Google Scholar]
- Larsson H, Lindgärde F, Berglund G, Ahrén B. Prediction of diabetes using ADA or WHO criteria in post‐menopausal women: a 10‐year follow‐up study. Diabetologia 2000;43(10):1224‐8. [PUBMED: 11079739] [DOI] [PubMed] [Google Scholar]
Latifi 2016 {published data only}
- Latifi SM, Karandish M, Shahbazian H, Hardani Pasand L. Incidence of prediabetes and type 2 diabetes among people aged over 20 years in Ahvaz: a 5‐year perspective study (2009‐2014). Journal of Diabetes and Research 2016;2016:4908647. [PUBMED: 28004008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian H, Latifi SM, Jalali MT, Shahbazian H, Amani R, Nikhoo A, et al. Metabolic syndrome and its correlated factors in an urban population in South West of Iran. Journal of Diabetes & Metabolic Disorders 2013;12(1):11. [PUBMED: 23497506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lecomte 2007 {published data only}
- Lecomte P, Vol S, Cacès E, Born C, Chabrolle C, Lasfargues G, et al. Five‐year predictive factors of type 2 diabetes in men with impaired fasting glucose. Diabetes & Metabolism 2007;33(2):140‐7. [PUBMED: 17320447] [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee JH, Lim JT, Kim HG, Oh MK, Lee WJ. Effect of coffee consumption on the progression of type 2 diabetes mellitus among prediabetic individuals. Korean Journal of Family Medicine 2016;37(1):7‐13. [PUBMED: 26885316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leiva 2014 {published data only}
- Leiva E, Mujica V, Orrego R, Wehinger S, Soto A, Icaza G, et al. Subjects with impaired fasting glucose: evolution in a period of 6 years. Journal of Diabetes Research 2014;2014:710370. [DOI: 10.1155/2014/710370; PUBMED: 25215305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo GI, Icaza NG, Mujica EV, Nunez FL, Leiva ME, Vasquez RM, et al. Prevalence of cardiovascular risk factors in adult from Talca, Chile [Prevalencia de factores de riesgo cardiovascular clásicos en población adulta de Talca, Chile, 2005]. Revista Medica de Chile 2007;135(7):904‐12. [PUBMED: 17914548] [DOI] [PubMed] [Google Scholar]
Levitzky 2008 {published data only}
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Annals of the New York Academy of Sciences 1963;107:539‐56. [PUBMED: 14025561] [DOI] [PubMed] [Google Scholar]
- Hruby A, Ma J, Rogers G, Meigs JB, Jacques PF. Associations of dairy intake with incident prediabetes or diabetes in middle‐aged adults vary by both dairy type and glycemic status. Journal of Nutrition 2017;147(9):1764‐75. [PUBMED: 28768835] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A, Daya N, Porneala B, Devlin JJ, Shiffman D, McPhaul MJ, et al. Prediction of type 2 diabetes by hemoglobin A1c in two community‐based cohorts. Diabetes Care 2018;41(1):60‐8. [PUBMED: 29074816] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, et al. Impact of impaired fasting glucose on cardiovascular disease: the Framingham heart study. Journal of the American College of Cardiology 2008;51(3):264‐70. [PUBMED: 18206734] [DOI] [PubMed] [Google Scholar]
- Wilson PW, Anderson KM, Kannel WB. Epidemiology of diabetes mellitus in the elderly. The Framingham study. American Journal of Medicine 1986;80(5A):3‐9. [PUBMED: 3706388] [DOI] [PubMed] [Google Scholar]
- Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RBSr. Prediction of incident diabetes mellitus in middle‐aged adults: the Framingham offspring study. Archives of Internal Medicine 2007;167(10):1068‐74. [PUBMED: 17533210] [DOI] [PubMed] [Google Scholar]
Li 2003 {published data only}
- Chou PS, Li CL, Wu GS, Tsai ST. Progression to type 2 diabetes among high‐risk groups in Kin‐Chen, Kinmen ‐ exploring the natural history of type 2 diabetes. Diabetes Care 1998;21(7):1183‐7. [PUBMED: 9653617] [DOI] [PubMed] [Google Scholar]
- Li CL, Tsai ST, Chou P. Comparison of the results between two diagnostic criteria by ADA and WHO among subjects with FPG 5.6‐7.8 mmol/l in Kin‐Hu and Kin‐Chen, Kinmen, 1991‐94. Diabetes Research and Clinical Practice 1999;45(1):51‐9. [DOI] [PubMed] [Google Scholar]
- Li CL, Tsai ST, Chou P. Persistent impaired glucose tolerance, insulin resistance, and beta‐cell dysfunction were independent predictors of type 2 diabetes. Journal of Clinical Epidemiology 2005;58(7):728‐32. [DOI] [PubMed] [Google Scholar]
- Li CL, Tsai ST, Chou P. Relative role of insulin resistance and beta‐cell dysfunction in the progression to type 2 diabetes ‐ the Kinmen study. Diabetes Research and Clinical Practice 2003;59(3):225‐32. [PUBMED: 12590020] [DOI] [PubMed] [Google Scholar]
- Tsai ST, Li CL, Chen CH, Chou P. Community‐based epidemiological study of glucose tolerance in Kin‐Chen, Kinmen: support for a new intermediate classification. Journal of Clinical Epidemiology 2000;53(5):505‐10. [PUBMED: 10812323] [DOI] [PubMed] [Google Scholar]
Ligthart 2016 {published data only}
- Brahimaj A, Ligthart S, Ghanbari M, Ikram MA, Hofman A, Franco OH, et al. Novel inflammatory markers for incident pre‐diabetes and type 2 diabetes: the Rotterdam Study. European Journal of Epidemiology 2017;32(3):217‐26. [PUBMED: 28258520] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman A, Darwish Murad S, Duijn CM, Franco OH, Goedegebure A, Ikram MA, et al. The Rotterdam study: 2014 objectives and design update. European Journal of Epidemiology 2013;28(11):889‐926. [DOI] [PubMed] [Google Scholar]
- Ligthart S, Herpt TT, Leening MJ, Kavousi M, Hofman A, Stricker BH, et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes & Endocrinology 2016;4(1):44‐51. [PUBMED: 26575606] [DOI] [PubMed] [Google Scholar]
- Schaft N, Brahimaj A, Wen KX, Franco OH, Dehghan A. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: the Rotterdam study. PLOS ONE 2017;12(6):e0179482. [PUBMED: 28632742] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipska 2013 {published data only}
- Lipska KJ, Inzucchi SE, Ness PH, Gill TM, Strotmeyer ES, Koster A, et al. Elevated HbA1c and fasting plasma glucose in predicting diabetes incidence among older adults: are two better than one?. Diabetes Care 2013;36(12):3923‐9. [PUBMED: 24135387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmeyer ES, Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the health, aging, and body composition (Health ABC) study. Diabetes Care 2008;31(9):1767‐72. [PUBMED: 18535192] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu SJ, Guo ZR, Hu XS, Wu M, Chen FM, Kang GD, et al. Risks for type‐2 diabetes associated with the metabolic syndrome and the interaction between impaired fasting glucose and other components of metabolic syndrome. Diabetes Research and Clinical Practice 2008;81(1):117‐23. [PUBMED: 18485514] [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu J, Wu YY, Huang XM, Yang M, Zha BB, Wang F, et al. Ageing and type 2 diabetes in an elderly Chinese population: the role of insulin resistance and beta cell dysfunction. European Review for Medical and Pharmacological Sciences 2014;18(12):1790‐7. [PUBMED: 24992623] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu X, Fine J P, Chen Z, Liu L, Li X, Wang A, et al. Prediction of the 20‐year incidence of diabetes in older Chinese: application of the competing risk method in a longitudinal study. Medicine 2016;95(40):e5057. [PUBMED: 27749572] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Wang HX, Meng C, Wu XG, Ericsson K, Winblad B, et al. The prevalence of functional disability in activities of daily living and instrumental activities of daily living among elderly Beijing Chinese. Archives of Gerontology and Geriatrics 1999;29(2):115‐25. [DOI] [PubMed] [Google Scholar]
- Tang Z, Zhou T, Luo Y, Xie C, Huo D, Tao L, et al. Risk factors for cerebrovascular disease mortality among the elderly in Beijing: a competing risk analysis. PLOS ONE 2014;9(2):e87884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2017 {published data only}
- He J, Neal B, Gu D, Suriyawongpaisal P, Xin X, Reynolds R, et al. International collaborative study of cardiovascular disease in Asia: design, rationale, and preliminary results. Ethnicity & Disease 2004;14(2):260‐8. [PubMed] [Google Scholar]
- Liu FC, Yang XL, Li JX, Cao J, Chen JC, Li Y, et al. Association of fasting glucose levels with incident atherosclerotic cardiovascular disease: an 8‐year follow‐up study in a Chinese population. Journal of Diabetes 2017;9(1):14‐23. [PUBMED: 26840038] [DOI] [PubMed] [Google Scholar]
Lorenzo 2003 {published data only}
- Abdul‐Ghani MA, Williams K, DeFronzo R, Stern M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006;29(7):1613‐8. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Bowsher RR, Mykkänen L, Hazuda HP, Mitchell BD, Valdez RA, et al. Proinsulin and specific insulin concentration in high‐ and low‐risk populations for NIDDM. Diabetes 1994;43(12):1490‐3. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7‐year risk of NIDDM in Mexican‐Americans. Diabetes 1995;44(12):1386‐91. [PUBMED: 7589843] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Stern M P. The homeostasis model in the San Antonio heart study. Diabetes Care 1997;20(7):1087‐92. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Stern MP. Are risk factors for conversion to NIDDM similar in high and low risk populations?. Diabetologia 1997;40(1):62‐6. [PUBMED: 9028719] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body‐fat distribution. Diabetes 1990;39(3):283‐8. [PUBMED: 2407581] [DOI] [PubMed] [Google Scholar]
- Hazuda HP, Haffner SM, Stern MP, Eifler CW. Effects of acculturation and socioeconomic status on obesity and diabetes in Mexican Americans. The San Antonio heart study. American Journal of Epidemiology 1988;128(6):1289‐301. [DOI] [PubMed] [Google Scholar]
- Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner S M. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 2003;26(11):3153‐9. [PUBMED: 14578254] [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Stern MP, Haffner SM, Hazuda HP, Patterson JK. Risk factors for cardiovascular mortality in Mexican Americans and non‐Hispanic whites. San Antonio heart study. American Journal of Epidemiology 1990;131(3):423‐33. [DOI] [PubMed] [Google Scholar]
- Stern MP, Morales PA, Valdez RA, Monterrosa A, Haffner SM, Mitchell BD, et al. Predicting diabetes. Moving beyond impaired glucose tolerance. Diabetes 1993;42(5):706‐14. [PUBMED: 8482427] [DOI] [PubMed] [Google Scholar]
- Stern MP, Rosenthal M, Haffner SM, Hazuda HP, Franco LJ. Sex difference in the effects of sociocultural status on diabetes and cardiovascular risk factors in Mexican Americans. The San Antonio heart study. American Journal of Epidemiology 1984;120(6):834‐51. [PUBMED: 6507426] [DOI] [PubMed] [Google Scholar]
Lyssenko 2005 {published data only}
- Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissen M, et al. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex‐specific parental effects. Diabetes 1996;45(11):1585‐93. [PUBMED: 8866565] [DOI] [PubMed] [Google Scholar]
- Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M, et al. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54(1):166‐74. [PUBMED: 15616025] [DOI] [PubMed] [Google Scholar]
- Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 2000;49(6):975‐80. [DOI] [PubMed] [Google Scholar]
Magliano 2008 {published data only}
- Al Salmi I, Hoy WE, Kondalsamy‐Chennakesavan S, Wang Z, Gobe GC, Barr EL, et al. Disorders of glucose regulation in adults and birth weight: results from the Australian diabetes, obesity and lifestyle (AUSDIAB) study. Diabetes Care 2008;31(1):159‐64. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Zimmet PZ, Welborn TA, Cameron AJ, Shaw J, Courten M, et al. The Australian diabetes, obesity and lifestyle study (AusDiab) ‐ methods and response rates. Diabetes Research and Clinical Practice 2002;57:119‐29. [PUBMED: 12062857] [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Zimmet PZ, Welborn TA, Courten MP, Cameron AJ, Sicree RA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian diabetes, obesity and lifestyle study. Diabetes Care 2002;25:829‐34. [PUBMED: 11978676] [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Barr ELM, Zimmet PZ, Cameron AJ, Dunstan DW, Colagiuri S, et al. Glucose indices, health behaviors, and incidence of diabetes in Australia: the Australian diabetes, obesity and lifestyle study. Diabetes Care 2008;31(2):267‐72. [PUBMED: 17989310] [DOI] [PubMed] [Google Scholar]
- Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE. Differences in height explain gender differences in the response to the oral glucose tolerance test‐ the AusDiab study. Diabetic Medicine 2008;25(3):296‐302. [DOI] [PubMed] [Google Scholar]
- Soulimane S, Simon D, Shaw JE, Zimmet PZ, Vol S, Vistisen D, et al. Comparing incident diabetes as defined by fasting plasma glucose or by HbA(1c). The AusDiab, Inter99 and DESIR studies. Diabetic Medicine 2011;28(11):1311‐8. [PUBMED: 21824186] [DOI] [PubMed] [Google Scholar]
- Williams ED, Magliano DJ, Tapp RJ, Oldenburg BF, Shaw JE. Psychosocial stress predicts abnormal glucose metabolism: the Australian diabetes, obesity and lifestyle (AusDiab) study. Annals of Behavioral Medicine 2013;46(1):62‐72. [PUBMED: 23389687] [DOI] [PubMed] [Google Scholar]
Man 2017 {published data only}
- Foong AW, Saw SM, Loo JL, Shen S, Loon SC, Rosman M, et al. Rationale and methodology for a population‐based study of eye diseases in Malay people: the Singapore Malay eye study (SiMES). Ophthalmic Epidemiology 2007;14(1):25‐35. [DOI] [PubMed] [Google Scholar]
- Man RE, Charumathi S, Gan AT, Fenwick EK, Tey CS, Chua J, et al. Cumulative incidence and risk factors of prediabetes and type 2 diabetes in a Singaporean Malay cohort. Diabetes Research and Clinical Practice 2017;127:163‐71. [PUBMED: 28371687] [DOI] [PubMed] [Google Scholar]
Marshall 1994 {published data only}
- Baxter J, Hamman RF, Lopez TK, Marshall JA, Hoag S, Swenson CJ. Excess incidence of known non‐insulin‐dependent diabetes mellitus (NIDDM) in Hispanics compared with non‐Hispanic whites in the San Luis Valley, Colorado. Ethnicity & Disease 1993;3(1):11‐21. [PubMed] [Google Scholar]
- Boyko EJ, Keane EM, Marshall JA, Hamman RF. Higher insulin and C‐peptide concentrations in Hispanic population at high risk for NIDDM. San Luis Valley diabetes study. Diabetes 1991;40(4):509‐15. [DOI] [PubMed] [Google Scholar]
- Hamman RF, Marshall JA, Baxter J, Kahn LB, Mayer EJ, Orleans M, et al. Methods and prevalence of non‐insulin‐dependent diabetes mellitus in a bi‐ethnic Colorado population. The San Luis Valley diabetes study. American Journal of Epidemiology 1989;129(2):295‐311. [PUBMED: 2912042] [DOI] [PubMed] [Google Scholar]
- Marshall JA, Hoag S, Shetterly S, Hamman RF. Dietary fat predicts conversion from impaired glucose tolerance to NIDDM: the San Luis Valley diabetes study. Diabetes Care 1994;17(1):50‐6. [PUBMED: 8112189] [DOI] [PubMed] [Google Scholar]
- Nelson TL, Bessesen DH, Marshall JA. Relationship of abdominal obesity measured by DXA and waist circumference with insulin sensitivity in Hispanic and non‐Hispanic white individuals: the San Luis Valley diabetes study. Diabetes/metabolism Research and Reviews 2008;24(1):33‐40. [DOI] [PubMed] [Google Scholar]
McNeely 2003 {published data only}
- Bergstrom RW, Newell‐Morris LL, Leonetti DL, Shuman WP, Wahl PW, Fujimoto WY. Association of elevated fasting C‐peptide level and increased intra‐abdominal fat distribution with development of NIDDM in Japanese‐American men. Diabetes 1990;39(1):104‐11. [DOI] [PubMed] [Google Scholar]
- Fujimoto WY, Bergstrom RW, Boyko EJ, Kinyoun JL, Leonetti DL, Newell‐Morris LL, et al. Diabetes and diabetes risk factors in second‐ and third‐generation Japanese Americans in Seattle, Washington. Diabetes Research and Clinical Practice 1994;24(Suppl):S43‐52. [PUBMED: 7859632] [DOI] [PubMed] [Google Scholar]
- Fujimoto WY, Bergstrom RW, Newell‐Morris L, Leonetti D L. Nature and nurture in the etiology of type 2 diabetes mellitus in Japanese Americans. Diabetes/metabolism Reviews 1989;5(7):607‐25. [PUBMED: 2689122] [DOI] [PubMed] [Google Scholar]
- Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Bergstom RW, Fujimoto WY. Proinsulin levels predict the development of non‐insulin‐dependent diabetes mellitus (NIDDM) in Japanese‐American men. Diabetic Medicine 1996;13(9 Suppl 6):S63‐6. [PUBMED: 8894485] [PubMed] [Google Scholar]
- Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Bergstrom RW, Fujimoto WY. Proinsulin as a marker for the development of NIDDM in Japanese‐American men. Diabetes 1995;44(2):173‐9. [DOI] [PubMed] [Google Scholar]
- McNeely MJ, Boyko EJ, Leonetti DL, Kahn SE, Fujimoto WY. Comparison of a clinical model, the oral glucose tolerance test, and fasting glucose for prediction of type 2 diabetes risk in Japanese Americans. Diabetes Care 2003;26(3):758‐63. [PUBMED: 12610034] [DOI] [PubMed] [Google Scholar]
- Rodriguez BL, Abbott RD, Fujimoto W, Waitzfelder B, Chen R, Masaki K, et al. The American Diabetes Association and World Health Organization classifications for diabetes ‐ their impact on diabetes prevalence and total and cardiovascular disease mortality in elderly Japanese‐American men. Diabetes Care 2002;25(6):951‐5. [PUBMED: 12032097] [DOI] [PubMed] [Google Scholar]
Meigs 2003 {published data only}
- Blake DR, Meigs JB, Muller DC, Najjar SS, Andres R, Nathan DM. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: results from the Baltimore longitudinal study on aging. Diabetes 2004;53(8):2095‐100. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore longitudinal study of aging. Diabetes 2003;52(6):1475‐84. [PUBMED: 12765960] [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore longitudinal study of aging. Metabolism 2005;54(4):542‐7. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, Aremberg D, Costa PT, Lakatta EG, Tobin JD. Normal human aging: the Baltimore longitudinal study of aging. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Aging, Gerontology Research Center, Baltimore City Hospitals; 1984. Report No.: NIH‐84‐2450.
- Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2‐h postchallenge plasma glucose concentrations to mortality: data from the Baltimore longitudinal study of aging with a critical review of the literature. Diabetes Care 2005;28(11):2626‐32. [DOI] [PubMed] [Google Scholar]
Mohan 2008 {published data only}
- Deepa R, Shanthi Rani S, Premalatha G, Mohan V. Comparison of ADA 1997 and WHO 1985 criteria for diabetes in south Indiansb ‐ the Chennai urban population study. Diabetic Medicine 2000;17(12):872‐4. [DOI] [PubMed] [Google Scholar]
- Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre‐diabetes in a selected urban south Indian population (CUPS‐19). Journal of the Association of Physicians of India 2008;56:152‐7. [PUBMED: 18697630] [PubMed] [Google Scholar]
- Mohan V, Gokulakrishnan K, Deepa R, Shanthirani CS, Datta M. Association of physical inactivity with components of metabolic syndrome and coronary artery disease ‐ the Chennai urban population study (CUPS no. 15). Diabetic Medicine 2005;22(9):1206‐11. [DOI] [PubMed] [Google Scholar]
- Mohan V, Shanthirani CS, Deepa M, Deepa R, Unnikrishnan RI, Datta M. Mortality rates due to diabetes in a selected urban south Indian population ‐ the Chennai urban population study [CUPS‐16]. Journal of the Association of Physicians of India 2006;54:113‐7. [PubMed] [Google Scholar]
- Mohan V, Shanthirani CS, Deepa R. Glucose intolerance (diabetes and IGT) in a selected South Indian population with special reference to family history, obesity and lifestyle factors ‐ the Chennai urban population study (CUPS 14). Journal of the Association of Physicians of India 2003;51:771‐7. [PubMed] [Google Scholar]
- Mohan V, Shanthirani S, Deepa R, Premalatha G, Sastry NG, Saroja R, et al. Intra‐urban differences in the prevalence of the metabolic syndrome in southern India ‐ the Chennai urban population study (CUPS No. 4). Diabetic Medicine 2001;18:280‐7. [PUBMED: 11437858] [DOI] [PubMed] [Google Scholar]
- Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, et al. A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care 2010;33(3):515‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeepa R, Deepa R, Rani SS, Premalatha G, Saroja R, Mohan V. Socioeconomic status and dyslipidaemia in a South Indian population: the Chennai urban population study (CUPS 11). National Medical Journal of India 2003;16(2):73‐8. [PubMed] [Google Scholar]
- Premalatha G, Shanthirani S, Deepa R, Markovitz J, Mohan V. Prevalence and risk factors of peripheral vascular disease in a selected South Indian population: the Chennai urban population study. Diabetes Care 2000;23(9):1295‐300. [DOI] [PubMed] [Google Scholar]
- Shanthirani CS, Pradeepa R, Deepa R, Premalatha G, Saroja R, Mohan V. Prevalence and risk factors of hypertension in a selected South Indian population ‐ the Chennai urban population study. Journal of the Association of Physicians of India 2003;51:20‐7. [PubMed] [Google Scholar]
Motala 2003 {published data only}
- Motala AA, Omar MA. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Diabetes Research and Clinical Practice 1994;23(2):103‐9. [PUBMED: 8070301] [DOI] [PubMed] [Google Scholar]
- Motala AA, Omar MA. Evidence for impaired pancreatic beta cell function in South African Indians with impaired glucose tolerance. Diabetic Medicine 1994;11(5):437‐44. [DOI] [PubMed] [Google Scholar]
- Motala AA, Omar MA, Gouws E. High risk of progression to NIDDM in South‐African Indians with impaired glucose tolerance. Diabetes 1993;42(4):556‐63. [PUBMED: 8454106] [DOI] [PubMed] [Google Scholar]
- Motala AA, Omar MA, Gouws E. Transient impaired glucose tolerance in South African Indians does not carry a risk for progression to NIDDM. Diabetes Care 1997;20(7):1101‐7. [PUBMED: 9203444] [DOI] [PubMed] [Google Scholar]
- Motala AA, Pirie FJ, Gouws E, Amod A, Omar MA. High incidence of type 2 diabetes mellitus in South African Indians: a 10‐year follow‐up study. Diabetic Medicine 2003;20(1):23‐30. [PUBMED: 12519316] [DOI] [PubMed] [Google Scholar]
- Omar MA, Seedat MA, Dyer RB, Motala AA, Knight LT, Becker PJ. South African Indians show a high prevalence of NIDDM and bimodality in plasma glucose distribution patterns. Diabetes Care 1994;17(1):70‐3. [DOI] [PubMed] [Google Scholar]
Motta 2010 {published data only}
- Anonymous. Prevalence of chronic diseases in older Italians: comparing self‐reported and clinical diagnoses. The Italian longitudinal study on aging working group. International Journal of Epidemiology 1997;26(5):995‐1002. [PUBMED: 9363520] [DOI] [PubMed] [Google Scholar]
- Maggi S, Zucchetto M, Grigoletto F, Baldereschi M, Candelise L, Scarpini E, et al. The Italian longitudinal study on aging (ILSA): design and methods. Aging 1994;6(6):464‐73. [PUBMED: 7748921] [DOI] [PubMed] [Google Scholar]
- Motta M, Bennati E, Cardillo E, Ferlito L, Malaguarnera M. The value of glycosylated hemoglobin (HbA1c) as a predictive risk factor in the diagnosis of diabetes mellitus (DM) in the elderly. Archives of Gerontology and Geriatrics 2010;50(1):60‐4. [DOI] [PubMed] [Google Scholar]
- Motta M, Bennati E, Cardillo E, Ferlito L, Passamonte M, Vacante M, et al. A combination of glycosylated hemoglobin, impaired fasting glucose and waist circumference is effective in screening for individuals at risk for future type 2 diabetes. Archives of Gerontology and Geriatrics 2010;50(1):105‐9. [PUBMED: 19414203] [DOI] [PubMed] [Google Scholar]
Mykkänen 1993 {published data only}
- Mykkänen L, Kuusisto J, Pyorala K, Laakso M. Cardiovascular‐disease risk‐factors as predictors of type‐2 (non‐insulin‐dependent) diabetes‐mellitus in elderly subjects. Diabetologia 1993;36(6):553‐9. [PUBMED: 8335178] [DOI] [PubMed] [Google Scholar]
- Mykkänen L, Laakso M, Penttila I, Pyorala K. Asymptomatic hyperglycemia and cardiovascular risk factors in the elderly. Atherosclerosis 1991;88:153‐61. [PUBMED: 1892482] [DOI] [PubMed] [Google Scholar]
- Mykkänen L, Laakso M, Uusitupa M, Pyorala K. Prevalence of diabetes and impaired glucose tolerance in elderly subjects and their association with obesity and family history of diabetes. Diabetes Care 1990;13:1099‐105. [PUBMED: 2261821] [DOI] [PubMed] [Google Scholar]
- Wang J, Ruotsalainen S, Moilanen L, Lepisto P, Laakso M, Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: a 13‐year follow‐up study in elderly non‐diabetic Finns. European Heart Journal 2007;28(7):857‐64. [DOI] [PubMed] [Google Scholar]
Nakagami 2016 {published data only}
- Nakagami T, Tanaka Y, Oya J, Kurita M, Isago C, Hasegawa Y, et al. Associations of HbA1c and fasting plasma glucose with incident diabetes: implications for pre‐diabetes thresholds in a Japanese population. Primary Care Diabetes 2016;10(6):407‐14. [PUBMED: 27515716] [DOI] [PubMed] [Google Scholar]
Nakanishi 2004 {published data only}
- Nakanishi N, Takatorige T, Fukuda H, Shirai K, Li W, Okamoto M, et al. Components of the metabolic syndrome as predictors of cardiovascular disease and type 2 diabetes in middle‐aged Japanese men. Diabetes Research and Clinical Practice 2004;64(1):59‐70. [PUBMED: 15036828] [DOI] [PubMed] [Google Scholar]
Noda 2010 {published data only}
- Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large‐scale population‐based cohort study in Japan. Archives of Internal Medicine 2006;166(17):1871‐7. [PUBMED: 17000944] [DOI] [PubMed] [Google Scholar]
- Kato M, Takahashi Y, Matsushita Y, Mizoue T, Inoue M, Kadowaki T, et al. Diabetes mellitus defined by hemoglobin A1c value: risk characterization for incidence among Japanese subjects in the JPHC diabetes study. Journal of Diabetes Investigation 2011;2(5):359‐65. [PUBMED: 24843514] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Kato M, Takahashi Y, Matsushita Y, Mizoue T, Inoue M, et al. Fasting plasma glucose and 5‐year incidence of diabetes in the JPHC diabetes study ‐ suggestion for the threshold for impaired fasting glucose among Japanese. Endocrine Journal 2010;57(7):629‐37. [PUBMED: 20508383] [DOI] [PubMed] [Google Scholar]
Park 2006 {published data only}
- Park YW, Chang Y, Sung KC, Ryu S, Sung E, Kim WS. The sequential changes in the fasting plasma glucose levels within normoglycemic range predict type 2 diabetes in healthy, young men. Diabetes Research and Clinical Practice 2006;73(3):329‐35. [PUBMED: 16600415] [DOI] [PubMed] [Google Scholar]
- Ryu S, Shin H, Chang Y, Sung KC, Song J, Lee S J. Should the lower limit of impaired fasting glucose be reduced from 110 mg/dL in Korea?. Metabolism 2006;55(4):489‐93. [DOI] [PubMed] [Google Scholar]
Peterson 2017 {published data only}
- Norberg M, Wall S, Boman K, Weinehall L. The Västerbotten intervention programme: background, design and implications. Global Health Action 2010;3(1):4643. [PUBMED: 20339479] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M, Pingel R, Lagali N, Dahlin LB, Rolandsson O. Association between HbA1c and peripheral neuropathy in a 10‐year follow‐up study of people with normal glucose tolerance, impaired glucose tolerance and type 2 diabetes. Diabetic Medicine 2017;34(12):1756‐64. [PUBMED: 28929513] [DOI] [PubMed] [Google Scholar]
- Pourhamidi K, Dahlin LB, Boman K, Rolandsson O. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia 2011;54(12):3143‐9. [PUBMED: 21909836] [DOI] [PubMed] [Google Scholar]
Qian 2012 {published data only}
- Feng B, Li X, Huang YW. A survey of diabetes mellitus and its risk factors among permanent inhabitants in Shanghai Pudong new economic area. Chinese Journal of Diabetes 2004;12:187‐90. [Google Scholar]
- Qian Q, Li X, Huang X, Fu M, Meng Z, Chen M, et al. Glucose metabolism among residents in Shanghai: natural outcome of a 5‐year follow‐up study. Journal of Endocrinological Investigation 2012;35(5):453‐8. [PUBMED: 21738002] [DOI] [PubMed] [Google Scholar]
Rajala 2000 {published data only}
- Qiao Q, Keinanen‐Kiukaanniemi S, Rajala U, Uusimaki A, Kivela SL. Risk for diabetes and persistent impaired glucose tolerance among middle‐aged Finns. Diabetes Research & Clinical Practice 1996;33(3):191‐8. [PUBMED: 8922541] [DOI] [PubMed] [Google Scholar]
- Rajala U, Keinanen‐Kiukaanniemi S, Uusimaki A, Reijula K, Kivela SL. Prevalence of diabetes mellitus and impaired glucose tolerance in a middle‐aged Finnish population. Scandinavian Journal of Primary Health Care 1995;13(3):222‐8. [PUBMED: 7481176] [DOI] [PubMed] [Google Scholar]
- Rajala U, Qiao Q, Laakso M, Keinänen‐Kiukaanniemi S. Antihypertensive drugs as predictors of type 2 diabetes among subjects with impaired glucose tolerance. Diabetes Research and Clinical Practice 2000;50(3):231‐9. [PUBMED: 11106838] [DOI] [PubMed] [Google Scholar]
Ramachandran 1986 {published data only}
- Ramachandran A, Snehalatha C, Naik RA, Mohan V, Shobana R, Viswanathan M. Significance of impaired glucose tolerance in an Asian Indian population: a follow‐up study. Diabetes Research and Clinical Practice 1986;2(3):173‐8. [PUBMED: 3527626] [DOI] [PubMed] [Google Scholar]
Rasmussen 2008 {published data only}
- Rasmussen SS, Glumer C, Sandbaek A, Lauritzen T, Borch‐Johnsen K. Determinants of progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high‐risk screened population: 3 year follow‐up in the ADDITION study, Denmark. Diabetologia 2008;51(2):249‐57. [PUBMED: 18060659] [DOI] [PubMed] [Google Scholar]
- Rasmussen SS, Glumer C, Sandbaek A, Lauritzen T, Borch‐Johnsen K. Progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high‐risk screening programme in general practice: the ADDITION study, Denmark. Diabetologia 2007;50(2):293‐7. [PUBMED: 17143605] [DOI] [PubMed] [Google Scholar]
Rathmann 2009 {published data only}
- Herder C, Kannenberg JM, Carstensen‐Kirberg M, Huth C, Meisinger C, Koenig W, et al. Serum levels of interleukin‐22, cardiometabolic risk factors and incident type 2 diabetes: KORA F4/FF4 study. Cardiovascular Diabetology 2017;16(1):17. [PUBMED: 28143481] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall B, Rathmann W, Strassburger K, Meisinger C, Holle R, Mielck A. Socioeconomic status is not associated with type 2 diabetes incidence in an elderly population in Germany: KORA S4/F4 cohort study. Journal of Epidemiology & Community Health 2011;65(7):606‐12. [PUBMED: 20693490] [DOI] [PubMed] [Google Scholar]
- Meisinger C, Doring A, Heier M. Blood pressure and risk of type 2 diabetes mellitus in men and women from the general population: the monitoring trends and determinants on cardiovascular diseases/cooperative health research in the region of Augsburg cohort study. Journal of Hypertension 2008;26(9):1809‐15. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Doring A, Thorand B, Heier M, Lowel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. American Journal of Clinical Nutrition 2006;84(3):483‐9. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Thorand B, Schneider A, Stieber J, Doring A, Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Archives of Internal Medicine 2002;162(1):82‐9. [PUBMED: 11784224] [DOI] [PubMed] [Google Scholar]
- Rathmann W, Haastert B, Icks A, Lowel H, Meisinger C, Holle R, et al. High prevalence of undiagnosed diabetes mellitus in Southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia 2003;46(2):182‐9. [PUBMED: 12627316] [DOI] [PubMed] [Google Scholar]
- Rathmann W, Meisinger C. How prevalent is type 2 diabetes in Germany? Results from the MONICA/KORA studies [Wie häufig ist Typ‐2‐Diabetes in Deutschland?]. Diabetologe 2010;6(3):170‐6. [Google Scholar]
- Rathmann W, Strassburger K, Heier M, Holle R, Thorand B, Giani G, et al. Incidence of type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabetic Medicine 2009;26(12):1212‐9. [PUBMED: 20002472] [DOI] [PubMed] [Google Scholar]
Rijkelijkhuizen 2007 {published data only}
- Heine RJ, Nijpels G, Mooy JM. New data on the rate of progression of impaired glucose tolerance to NIDDM and predicting factors. Diabetic Medicine 1996;13(3 Suppl 2):S12‐4. [PubMed] [Google Scholar]
- Mooy JM, Grootenhuis PA, Vries H, Valkenburg HA, Bouter LM, Kostense PJ, et al. Prevalence and determinants of glucose intolerance in a Dutch Caucasian population. The Hoorn study. Diabetes Care 1995;18:1270‐3. [PUBMED: 8612442] [DOI] [PubMed] [Google Scholar]
- Nijpels G, Popp‐Snijders C, Kostense P J, Bouter LM, Heine RJ. Fasting proinsulin and 2‐h post‐load glucose levels predict the conversion to NIDDM in subjects with impaired glucose tolerance: the Hoorn study. Diabetologia 1996;39(1):113‐8. [PUBMED: 8720611] [DOI] [PubMed] [Google Scholar]
- Nijpels G, Popp‐Snijders C, Kostense PJ, Bouter LM, Heine RJ. Cardiovascular risk factors prior to the development of non‐insulin‐dependent diabetes mellitus in persons with impaired glucose tolerance: the Hoorn Study. Journal of Clinical Epidemiology 1997;50(9):1003‐9. [DOI] [PubMed] [Google Scholar]
- Rijkelijkhuizen JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Dekker JM. High risk of cardiovascular mortality in individuals with impaired fasting glucose is explained by conversion to diabetes: the Hoorn study. Diabetes Care 2007;30(2):332‐6. [PUBMED: 17259503] [DOI] [PubMed] [Google Scholar]
- Ruijgrok C, Dekker JM, Beulens JW, Brouwer IA, Coupe VMH, Heymans MW, et al. Size and shape of the associations of glucose, HbA1c, insulin and HOMA‐IR with incident type 2 diabetes: the Hoorn study. Diabetologia 2018;61(1):93‐100. [PUBMED: 29018885] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn study. JAMA 2001;285(16):2109‐13. [PUBMED: 11311100] [DOI] [PubMed] [Google Scholar]
- Vegt F, Dekker JM, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Similar 9‐year mortality risks and reproducibility for the World Health Organization and American Diabetes Association glucose tolerance categories: the Hoorn study. Diabetes Care 2000;23(1):40‐4. [DOI] [PubMed] [Google Scholar]
- Vegt F, Dekker JM, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. The 1997 American Diabetes Association criteria versus the 1985 World Health Organization criteria for the diagnosis of abnormal glucose tolerance: poor agreement in the Hoorn study. Diabetes Care 1998;21(10):1686‐90. [DOI] [PubMed] [Google Scholar]
Sadeghi 2015 {published data only}
- Hosseini N, Talaei M, Dianatkhah M, Sadeghi M, Oveisgharan S, Sarrafzadegan N. Determinants of incident metabolic syndrome in a Middle Eastern population: Isfahan cohort study. Metabolic Syndrome and Related Disorders 2017;15(7):354‐62. [PUBMED: 28677982] [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Talaei M, Parvaresh RE, Dianatkhah M, Oveisgharan S, Sarrafzadegan N. Determinants of incident prediabetes and type 2 diabetes in a 7‐year cohort in a developing country: the Isfahan cohort study. Journal of Diabetes 2015;7(5):633‐41. [PUBMED: 25350916] [DOI] [PubMed] [Google Scholar]
- Sarrafzadegan N, Talaei M, Sadeghi M, Kelishadi R, Oveisgharan S, Mohammadifard N, et al. The Isfahan cohort study: rationale, methods and main findings. Journal of Human Hypertension 2011;25(9):545‐53. [PUBMED: 21107436] [DOI] [PubMed] [Google Scholar]
Sasaki 1982 {published data only}
- Sasaki A, Suzuki T, Horiuchi N. Development of diabetes in Japanese subjects with impaired glucose tolerance: a seven year follow‐up study. Diabetologia 1982;22(3):154‐7. [PUBMED: 7075915] [DOI] [PubMed] [Google Scholar]
- Sasaki A, Suzuki T, Horiuchi N. Survival rate and causes of death in Japan. A 10‐year follow‐up study. Journal of Chronic Diseases 1980;33:341‐6. [DOI] [PubMed] [Google Scholar]
Sato 2009 {published data only}
- Sato KK, Hayashi T, Harita N, Yoneda T, Nakamura Y, Endo G, et al. Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes: the Kansai healthcare study. Diabetes Care 2009;32(4):644‐6. [PUBMED: 19131461] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato KK, Hayashi T, Kambe H, Nakamura Y, Harita N, Endo G, et al. Walking to work is an independent predictor of incidence of type 2 diabetes in Japanese men: the Kansai healthcare study. Diabetes Care 2007;30(9):2296‐8. [PUBMED: 17536075] [DOI] [PubMed] [Google Scholar]
- Sato KK, Hayashi T, Nakamura Y, Harita N, Yoneda T, Endo G, et al. Liver enzymes compared with alcohol consumption in predicting the risk of type 2 diabetes: the Kansai healthcare study. Diabetes Care 2008;31:1230‐6. [PUBMED: 18316395] [DOI] [PubMed] [Google Scholar]
Schranz 1989 {published data only}
- Katona G, Aganovic I, Vuksan V, Skrabalo Z. National Diabetes Programme in Malta: Phase I and II Final Report. Valletta: WHO, 1983. [Google Scholar]
- Schranz AG. Abnormal glucose tolerance in the Maltese. A population‐based longitudinal study of the natural history of NIDDM and IGT in Malta. Diabetes Research and Clinical Practice 1989;7(1):7‐16. [PUBMED: 2752891] [DOI] [PubMed] [Google Scholar]
Sharifi 2013 {published data only}
- Sharifi F, Jaberi Y, Mirzamohammadi F, Mirzamohammadi H, Mousavinasab N. Determinants of developing diabetes mellitus and vascular complications in patients with impaired fasting glucose. Indian Journal of Endocrinology and Metabolism 2013;17(5):899‐905. [PUBMED: 24083174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 1997 {published data only}
- Park Y, Lee H, Koh CS, Min H, Yoo K, Kim Y, et al. Prevalence of diabetes and IGT in Yonchon county, South Korea. Diabetes Care 1995;18:545‐8. [PUBMED: 7497867] [DOI] [PubMed] [Google Scholar]
- Shin CS, Lee HK, Koh CS, Kim YI, Shin YS, Yoo KY, et al. Risk factors for the development of NIDDM in Yonchon county, Korea. Diabetes Care 1997;20(12):1842‐6. [PUBMED: 9405904] [DOI] [PubMed] [Google Scholar]
Söderberg 2004 {published data only}
- Boyko EJ, Shaw JE, Zimmet PZ, Chitson P, Tuomilehto J, Alberti KG. A prospective study of glycemia, body size, insulin resistance and the risk of hypertension in Mauritius. Journal of Hypertension 2008;26(9):1742‐9. [DOI] [PubMed] [Google Scholar]
- Dowse GK, Zimmet PZ, Gareeboo H, George K, Alberti MM, Tuomilehto J, et al. Abdominal obesity and physical inactivity as risk factors for NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians. Diabetes Care 1991;14(4):271‐82. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Zimmet PZ, Courten M, Dowse GK, Chitson P, Gareeboo H, et al. Impaired fasting glucose or impaired glucose tolerance ‐ what best predicts future diabetes in Mauritius?. Diabetes Care 1999;22(3):399‐402. [PUBMED: 10097917] [DOI] [PubMed] [Google Scholar]
- Söderberg S, Zimmet P, Tuomilehto J, Courten M, Dowse GK, Chitson P, et al. High incidence of type 2 diabetes and increasing conversion rates from impaired fasting glucose and impaired glucose tolerance to diabetes in Mauritius. Journal of Internal Medicine 2004;256(1):37‐47. [PUBMED: 15189364] [DOI] [PubMed] [Google Scholar]
- Söderberg S, Zimmet P, Tuomilehto J, Courten M, Dowse GK, Chitson P, et al. Increasing prevalence of type 2 diabetes mellitus in all ethnic groups in Mauritius. Diabetic Medicine 2005;22(1):61‐8. [DOI] [PubMed] [Google Scholar]
- Williams JW, Zimmet PZ, Shaw JE, Courten MP, Cameron AJ, Chitson P, et al. Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius. Does sex matter?. Diabetic Medicine 2003;20(11):915‐20. [DOI] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Kim Y, Han BG. Cohort profile: the Korean genome and epidemiology study (KoGES) consortium. International Journal of Epidemiology 2017;46(2):e20. [PUBMED: 27085081] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Han BG. Cohort profile: the Korean genome and epidemiology study (KoGES) consortium [Erratum]. International Journal of Epidemiology 2017;46(4):1350. [PUBMED: 28938752] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BM, Kim HC, Lee JY, Lee JM, Kim DJ, Lee YH, et al. Performance of HbA1c for the prediction of diabetes in a rural community in Korea. Diabetic Medicine 2015;32(12):1602‐10. [PUBMED: 25962707] [DOI] [PubMed] [Google Scholar]
Song 2016a {published data only}
- Qiu M, Shen W, Song X, Ju L, Tong W, Wang H, et al. Effects of prediabetes mellitus alone or plus hypertension on subsequent occurrence of cardiovascular disease and diabetes mellitus: longitudinal study. Hypertension 2015;65(3):525‐30. [PUBMED: 25624343] [DOI] [PubMed] [Google Scholar]
- Song X, Qiu M, Zhang X, Wang H, Tong W, Ju L, et al. Gender‐related affecting factors of prediabetes on its 10‐year outcome. BMJ Open Diabetes Research & Care 2016;4(1):e000169. [PUBMED: 27239315] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JY, Cheng Q, Song XM, Li G, Jiang GX, Gu YY, et al. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. European Journal of Endocrinology/European Federation of Endocrine Societies 2006;155(4):601‐7. [PUBMED: 16990660] [DOI] [PubMed] [Google Scholar]
Soriguer 2008 {published data only}
- Soriguer F, Rojo‐Martínez G, Almaraz MC, Esteva I, Ruiz de Adana MS, Morcillo S, et al. Incidence of type 2 diabetes in southern Spain (Pizarra study). European Journal of Clinical Investigation 2008;38(2):126‐33. [PUBMED: 18226046] [DOI] [PubMed] [Google Scholar]
- Soriguer‐Escofet F, Esteva I, Rojo‐Martinez G, Ruiz de Adana S, Catala M, Merelo MJ, et al. Prevalence of latent autoimmune diabetes of adults (LADA) in Southern Spain. Diabetes Research and Clinical Practice 2002;56(3):213‐20. [DOI] [PubMed] [Google Scholar]
Stengard 1992 {published data only}
- Keys A, Aravanis C, Blackburn HW, Buchem FS, Buzina R, Djordjevic BD, et al. Epidemiological studies related to coronary heart disease: characteristics of men aged 40‐59 in seven countries. Acta Medica Scandinavica. Supplementum 1966;460:1‐392. [MEDLINE: ] [PubMed] [Google Scholar]
- Nissinen A, Kivela SL, Pekkanen J, Tuomilehto J, Kostiainen E, Piippo H, et al. Levels of some biological risk indicators among elderly men in Finland. Age and Ageing 1986;15(4):203‐11. [PUBMED: 3751746] [DOI] [PubMed] [Google Scholar]
- Stengård JH, Pekkanen J, Tuomilehto J, Kivinen P, Kaarsalo E, Tamminen M, et al. Changes in glucose tolerance among elderly Finnish men during a five‐year follow‐up: the Finnish cohorts of the seven countries study. Diabete & Metabolisme 1992;19(1 Pt 2):121‐9. [PUBMED: 8314414] [PubMed] [Google Scholar]
Toshihiro 2008 {published data only}
- Toshihiro M, Saito K, Takikawa S, Takebe N, Onoda T, Satoh J. Psychosocial factors are independent risk factors for the development of type 2 diabetes in Japanese workers with impaired fasting glucose and/or impaired glucose tolerance. Diabetic Medicine 2008;25(10):1211‐7. [PUBMED: 19046200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaccaro 1999 {published data only}
- Vaccaro O, Ruffa G, Imperatore G, Iovino V, Rivellese AA, Riccardi G. Risk of diabetes in the new diagnostic category of impaired fasting glucose: a prospective analysis. Diabetes Care 1999;22(9):1490‐3. [PUBMED: 10480514] [DOI] [PubMed] [Google Scholar]
Valdes 2008 {published data only}
- Valdes S, Botas P, Delgado E, Alvarez F, Cadorniga FD. Population‐based incidence of type 2 diabetes in northern Spain: the Asturias study. Diabetes Care 2007;30(9):2258‐63. [PUBMED: 17536076] [DOI] [PubMed] [Google Scholar]
- Valdes S, Botas P, Delgado E, Alvarez F, Diaz‐Cadorniga F. HbA(1c) in the prediction of type 2 diabetes compared with fasting and 2‐h post‐challenge plasma glucose: the Asturias study (1998‐2005). Diabetes & Metabolism 2011;37(1):27‐32. [PUBMED: 20934897] [DOI] [PubMed] [Google Scholar]
- Valdés S, Botas P, Delgado E, Álvarez F, Cadórniga FD. Does the new American Diabetes Association definition for impaired fasting glucose improve its ability to predict type 2 diabetes mellitus in Spanish persons? The Asturias study. Metabolism 2008;57(3):399‐403. [PUBMED: 18249214] [DOI] [PubMed] [Google Scholar]
Vijayakumar 2017 {published data only}
- Vijayakumar P, Nelson R G, Hanson R L, Knowler W C, Sinha M. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care 2017;40(1):16‐21. [PUBMED: 27810987] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viswanathan 2007 {published data only}
- Viswanathan V, Clementina M, Nair BM, Satyavani K. Risk of future diabetes is as high with abnormal intermediate post‐glucose response as with impaired glucose tolerance. Journal of the Association of Physicians of India 2007;55:833‐7. [PUBMED: 18405128] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang JJ, Li HB, Kinnunen L, Hu G, Jarvinen TM, Miettinen ME, et al. How well does the metabolic syndrome defined by five definitions predict incident diabetes and incident coronary heart disease in a Chinese population?. Atherosclerosis 2007;192(1):161‐8. [PUBMED: 16720024] [DOI] [PubMed] [Google Scholar]
- Wang JJ, Qiao Q, Miettinen ME, Lappalainen J, Hu G, Tuomilehto J. The metabolic syndrome defined by factor analysis and incident type 2 diabetes in a Chinese population with high postprandial glucose. Diabetes Care 2004;27(10):2429‐37. [PUBMED: 15451912] [DOI] [PubMed] [Google Scholar]
- Wang JJ, Yuan SY, Zhu LX, Fu HJ, Li HB, Hu G, et al. Effects of impaired fasting glucose and impaired glucose tolerance on predicting incident type 2 diabetes in a Chinese population with high post‐prandial glucose. Diabetes Research and Clinical Practice 2004;66(2):183‐91. [PUBMED: 15533586] [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, et al. Rising tide of cardiovascular disease in American Indians. The strong heart study. Circulation 1999;99(18):2389‐95. [DOI] [PubMed] [Google Scholar]
- Lee ET, Howard BV, Go O, Savage PJ, Fabsitz RR, Robbins DC, et al. Prevalence of undiagnosed diabetes in three American Indian populations. A comparison of the 1997 American Diabetes Association diagnostic criteria and the 1985 World Health Organization diagnostic criteria: the strong heart study. Diabetes Care 2000;23(2):181‐6. [DOI] [PubMed] [Google Scholar]
- Lee ET, Howard BV, Savage PJ, Cowan LD, Fabsitz RR, Oopik AJ, et al. Diabetes and impaired glucose tolerance in three American Indian populations aged 45‐74 years. The strong heart study. Diabetes Care 1995;18(5):599‐610. [DOI] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Cowan LD, Wang W, Rhoades DA, Devereux R, et al. Incidence of diabetes in American Indians of three geographic areas: the strong heart study. Diabetes Care 2002;25(1):49‐54. [PUBMED: 11772900] [DOI] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The strong heart study. A study of cardiovascular disease in American Indians: design and methods. American Journal of Epidemiology 1990;132(6):1141‐55. [PUBMED: 2260546] [DOI] [PubMed] [Google Scholar]
- Lu W, Resnick HE, Jain AK, Adams‐Campbell LL, Jablonski KA, Gottlieb AM, et al. Effects of isolated post‐challenge hyperglycemia on mortality in American Indians: the strong heart study. Annals of Epidemiology 2003;13(3):182‐8. [DOI] [PubMed] [Google Scholar]
- Wang H, Shara N M, Calhoun D, Umans JG, Lee ET, Howard BV. Incidence rates and predictors of diabetes in those with prediabetes: the strong heart study. Diabetes/metabolism Research and Reviews 2010;26(5):378‐85. [PUBMED: 20578203] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lee ET, Fabsitz R, Welty TK, Howard BV. Using HbA(1c) to improve efficacy of the American Diabetes Association fasting plasma glucose criterion in screening for new type 2 diabetes in American Indians: the strong heart study. Diabetes Care 2002;25(8):1365‐70. [DOI] [PubMed] [Google Scholar]
- Wang W, Lee ET, Howard BV, Fabsitz RR, Devereux RB, Welty TK. Fasting plasma glucose and hemoglobin A1c in identifying and predicting diabetes: the strong heart study. Diabetes Care 2011;34(2):363‐8. [PUBMED: 21270194] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone G, Devereux RB, Chinali M, Best LG, Lee ET, Galloway JM, et al. Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the strong heart study. Diabetes Care 2007;30(7):1851‐6. [DOI] [PubMed] [Google Scholar]
Warren 2017 {published data only}
- Leong A, Daya N, Porneala B, Devlin JJ, Shiffman D, McPhaul MJ, et al. Prediction of type 2 diabetes by hemoglobin A1c in two community‐based cohorts. Diabetes Care 2018;41(1):60‐8. [PUBMED: 29074816] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, et al. Identifying individuals at high risk for diabetes ‐ the atherosclerosis risk in communities study. Diabetes Care 2005;28(8):2013‐8. [PUBMED: 16043747] [DOI] [PubMed] [Google Scholar]
- Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the atherosclerosis risk in communities (ARIC) study. Lancet Diabetes & Endocrinology 2014;2(4):279‐88. [PUBMED: 24703046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Steffes M W, Gregg E, Brancati F L, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care 2011;34(1):84‐9. [PUBMED: 20855549] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. New England Journal of Medicine 2010;362(9):800‐11. [PUBMED: 20200384] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B, Pankow J S, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the atherosclerosis risk in communities (ARIC) study. Lancet Diabetes & Endocrinology 2017;5(1):34‐42. [PUBMED: 27863979] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton SP, McEvoy JW, Lazo M, Coresh J, Ballantyne CM, Selvin E. High‐sensitivity cardiac troponin T (hs‐cTnT) as a predictor of incident diabetes in the atherosclerosis risk in communities study. Diabetes Care 2017;40(2):261‐9. [PUBMED: 28108537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wat 2001 {published data only}
- Janus ED. Epidemiology of cardiovascular risk factors in Hong Kong. Clinical and Experimental Pharmacology & Physiology 1997;24(12):987‐8. [PUBMED: 9406673] [DOI] [PubMed] [Google Scholar]
- Janus ED, Watt NM, Lam KS, Cockram CS, Siu ST, Liu LJ, et al. The prevalence of diabetes, association with cardiovascular risk factors and implications of diagnostic criteria (ADA 1997 and WHO 1998) in a 1996 community‐based population study in Hong Kong Chinese. Diabetic Medicine 2000;17(10):741‐5. [PUBMED: 11110508] [DOI] [PubMed] [Google Scholar]
- Tan KC, Wat NM, Tam SC, Janus ED, Lam TH, Lam KS. C‐reactive protein predicts the deterioration of glycemia in Chinese subjects with impaired glucose tolerance. Diabetes Care 2003;26(8):2323‐8. [DOI] [PubMed] [Google Scholar]
- Wat NM, Lam TH, Janus ED, Lam KS. Central obesity predicts the worsening of glycemia in southern Chinese. International Journal of Obesity and Related Metabolic Disorders 2001;25(12):1789‐93. [PUBMED: 11781759] [DOI] [PubMed] [Google Scholar]
Weiss 2005 {published data only}
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. New England Journal of Medicine 2004;350(23):2362‐74. [PUBMED: 15175438] [DOI] [PubMed] [Google Scholar]
- Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 2005;28(4):902‐9. [PUBMED: 15793193] [DOI] [PubMed] [Google Scholar]
Wheelock 2016 {published data only}
- Wheelock KM, Sinha M, Knowler WC, Nelson RG, Fufaa GD, Hanson RL. Metabolic risk factors and type 2 diabetes incidence in American Indian children. Journal of Clinical Endocrinology & Metabolism 2016;101(4):1437‐44. [PUBMED: 26913636] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2003 {published data only}
- Tan CE, Emmanuel SC, Tan BY, Jacob E. Prevalence of diabetes and ethnic differences in cardiovascular risk factors. The 1992 Singapore national health survey. Diabetes Care 1999;22(2):241‐7. [PUBMED: 10333940] [DOI] [PubMed] [Google Scholar]
- Wong MS, Gu K, Heng D, Chew SK, Chew LS, Tai ES. The Singapore impaired glucose tolerance follow‐up study: does the ticking clock go backward as well as forward?. Diabetes Care 2003;26(11):3024‐30. [PUBMED: 14578234] [DOI] [PubMed] [Google Scholar]
Yeboah 2011 {published data only}
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi‐ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology 2002;156(9):871‐81. [PUBMED: 12397006] [DOI] [PubMed] [Google Scholar]
- Yeboah J, Bertoni A G, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (multi‐ethnic study of atherosclerosis). Journal of the American College of Cardiology 2011;58(2):140‐6. [PUBMED: 21718910] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zethelius 2004 {published data only}
- Byberg L, McKeigue PM, Zethelius B, Lithell HO. Birth weight and the insulin resistance syndrome: association of low birth weight with truncal obesity and raised plasminogen activator inhibitor‐1 but not with abdominal obesity or plasma lipid disturbances. Diabetologia 2000;43(1):54‐60. [PUBMED: 10663216] [DOI] [PubMed] [Google Scholar]
- Hedstrand H. A study of middle‐aged men with particular reference to risk factors for cardiovascular disease. Upsala Journal of Medical Sciences 1975;Suppl 19:1‐61. [PUBMED: 1216390] [PubMed] [Google Scholar]
- Zethelius B, Hales CN, Lithell HO, Berne C. Insulin resistance, impaired early insulin response, and insulin propeptides as predictors of the development of type 2 diabetes: a population‐based, 7‐year follow‐up study in 70‐year‐old men. Diabetes Care 2004;27(6):1433‐8. [PUBMED: 15161800] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdul‐Ghani 2011 {published data only}
- Abdul‐Ghani MA, Abdul‐Ghani T, Muller G, Bergmann A, Fischer S, Bornstein S, et al. Role of glycated hemoglobin in the prediction of future risk of T2DM. Journal of Clinical Endocrinology and Metabolism 2011;96(8):2596‐600. [DOI] [PubMed] [Google Scholar]
Alvarsson 2009 {published data only}
- Alvarsson M, Hilding A, Ostenson CG. Factors determining normalization of glucose intolerance in middle‐aged Swedish men and women: a 8‐10‐year follow‐up. Diabetic Medicine 2009;26(4):345‐53. [DOI] [PubMed] [Google Scholar]
- Andersson CM, Bjaras GE, Ostenson CG. A stage model for assessing a community‐based diabetes prevention program in Sweden. Health Promotion International 2002;17(4):317‐27. [DOI] [PubMed] [Google Scholar]
- Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG. Psychological distress and risk of pre‐diabetes and type 2 diabetes in a prospective study of Swedish middle‐aged men and women. Diabetic Medicine 2008;25(7):834‐42. [DOI] [PubMed] [Google Scholar]
- Eriksson K F, Lindgärde F. Poor physical fitness, and impaired early insulin response but late hyperinsulinaemia, as predictors of NIDDM in middle‐aged Swedish men. Diabetologia 1996;39(5):573‐9. [DOI] [PubMed] [Google Scholar]
Alyass 2015 {published data only}
- Alyass A, Almgren P, Akerlund M, Dushoff J, Isomaa B, Nilsson P, et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia 2015;58(1):87‐97. [DOI] [PubMed] [Google Scholar]
Amoah 2002 {published data only}
- Amoah AG. Undiagnosed diabetes and impaired glucose regulation in adult Ghanaians using the ADA and WHO diagnostic criteria. Acta Diabetologica 2002;39(1):7‐13. [DOI] [PubMed] [Google Scholar]
Andreou 2017 {published data only}
- Andreou E, Papandreou D, Hajigeorgiou P, Kyriakou K, Avraam T, Chappa G, et al. Type 2 diabetes and its correlates in a first nationwide study among Cypriot adults. Primary Care Diabetes 2017;11(2):112‐8. [DOI] [PubMed] [Google Scholar]
Bancks 2015 {published data only}
- Bancks MP, Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Glycated hemoglobin and incident type 2 diabetes in Singaporean Chinese adults: the Singapore Chinese health study. PLOS ONE 2015;10(3):e0119884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birmingham Diabetes Survey Working Party 1976 {published data only}
- Birmingham Diabetes Survey Working Party 1976. Ten‐year follow‐up report on Birmingham diabetes survey of 1961. Report by the Birmingham diabetes survey working party. British Medical Journal 1976;2(6026):35‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjornholt 2000 {published data only}
- Bjornholt JV, Erikssen G, Liestol K, Jervell J, Thaulow E, Erikssen J. Type 2 diabetes and maternal family history: an impact beyond slow glucose removal rate and fasting hyperglycemia in low‐risk individuals? Results from 22.5 years of follow‐up of healthy nondiabetic men. Diabetes Care 2000;23(9):1255‐9. [DOI] [PubMed] [Google Scholar]
Bodicoat 2017 {published data only}
- Bodicoat DH, Khunti K, Srinivasan BT, Mostafa S, Gray LJ, Davies MJ, et al. Incident type 2 diabetes and the effect of early regression to normoglycaemia in a population with impaired glucose regulation. Diabetic Medicine 2017;34(3):396‐404. [PUBMED: 26871995] [DOI] [PubMed] [Google Scholar]
Boned 2016 {published data only}
- Boned Ombuena P, Rodilla Sala E, Costa Munoz JA, Pascual Izuel JM. Arterial hypertension and prediabetes. Medicina Clinica 2016;147(9):387‐92. [DOI] [PubMed] [Google Scholar]
Boucher 2015 {published data only}
- Boucher AB, Adesanya EA, Owei I, Gilles AK, Ebenibo S, Wan J, et al. Dietary habits and leisure‐time physical activity in relation to adiposity, dyslipidemia, and incident dysglycemia in the pathobiology of prediabetes in a biracial cohort study. Metabolism 2015;64(9):1060‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brantsma 2005 {published data only}
- Brantsma AH, Bakker SJL, Hillege HL, Zeeuw D, Jong PE, Gansevoort RT. Urinary albumin excretion and its relation with C‐reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care 2005;28(10):2525‐30. [DOI] [PubMed] [Google Scholar]
Brateanu 2017 {published data only}
- Brateanu A, Barwacz T, Kou L, Wang S, Misra‐Hebert AD, Hu B, et al. Determining the optimal screening interval for type 2 diabetes mellitus using a risk prediction model. PLOS ONE 2017;12(11):e0187695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 1996 {published data only}
- Braun B, Zimmermann MB, Kretchmer N, Spargo RM, Smith RM, Gracey M. Risk factors for diabetes and cardiovascular disease in young Australian aborigines. A 5‐year follow‐up study. Diabetes Care 1996;19(5):472‐9. [DOI] [PubMed] [Google Scholar]
Burchfiel 1995 {published data only}
- Burchfiel CM, Curb JD, Rodriguez BL, Yano K, Hwang LJ, Fong KO, et al. Incidence and predictors of diabetes in Japanese‐American men. The Honolulu heart program. Annals of Epidemiology 1995;5(1):33‐43. [DOI] [PubMed] [Google Scholar]
Chamukuttan 2016 {published data only}
- Chamukuttan S, Ram J, Nanditha A, Shetty AS, Sevick MA, Bergman M, et al. Baseline level of 30‐min plasma glucose is an independent predictor of incident diabetes among Asian Indians: analysis of two diabetes prevention programmes. Diabetes‐Metabolism Research and Reviews 2016;32(7):762‐7. [DOI] [PubMed] [Google Scholar]
Chang 2017 {published data only}
- Chang CH, Yeh YC, Shih SR, Lin JW, Chuang LM, Caffrey JL, et al. Association between thyroid dysfunction and dysglycaemia: a prospective cohort study. Diabetic Medicine 2017;34(11):1584‐90. [DOI] [PubMed] [Google Scholar]
Chen 1995 {published data only}
- Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell‐Morris L, Wahl PW, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM: 5‐year follow‐up of initially nondiabetic Japanese‐American men. Diabetes Care 1995;18(6):747‐53. [DOI] [PubMed] [Google Scholar]
Cheng 2011 {published data only}
- Cheng P, Neugaard B, Foulis P, Conlin PR. Hemoglobin A1c as a predictor of incident diabetes. Diabetes Care 2011;34(3):610‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2007 {published data only}
- Cheung BM, Wat NM, Man YB, Tam S, Thomas GN, Leung GM, et al. Development of diabetes in Chinese with the metabolic syndrome: a 6‐year prospective study. Diabetes Care 2007;30(6):1430‐6. [DOI] [PubMed] [Google Scholar]
Choi 2002 {published data only}
- Choi KM, Lee J, Kim DR, Kim SK, Shin DH, Kim NH, et al. Comparison of ADA and WHO criteria for the diagnosis of diabetes in elderly Koreans. Diabetic Medicine 2002;19(10):853‐7. [DOI] [PubMed] [Google Scholar]
Cicero 2005 {published data only}
- Cicero AF, Derosa G, Rosticci M, D'Addato S, Agnoletti D, Borghi C, et al. Long‐term predictors of impaired fasting glucose and type 2 diabetes in subjects with family history of type 2 diabetes: a 12‐years follow‐up of the Brisighella heart study historical cohort. Diabetes Research and Clinical Practice 2014;104(1):183‐8. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Dormi A, Nascetti S, Panourgia MP, Grandi E, D'Addato S, et al. Relative role of major risk factors for type 2 diabetes development in the historical cohort of the Brisighella heart study: an 8‐year follow‐up. Diabetic Medicine 2005;22(9):1263‐6. [DOI] [PubMed] [Google Scholar]
Cosson 2011 {published data only}
- Cosson E, Nguyen MT, Hamo‐Tchatchouang E, Banu I, Chiheb S, Charnaux N, et al. What would be the outcome if the American Diabetes Association recommendations of 2010 had been followed in our practice in 1998‐2006?. Diabetic Medicine 2011;28(5):567‐74. [DOI] [PubMed] [Google Scholar]
Costa 2005 {published data only}
- Costa B, Vizcaino J, Pinol J, Martin F, Cabre J J, Basora J, et al. The RECORD project. continuous blood glucose monitoring among high risk subjects for developing diabetes in Spanish primary health care. Atencion Primaria 2005;35(2):99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cree‐Green 2013 {published data only}
- Cree‐Green M, Triolo TM, Nadeau KJ. Etiology of insulin resistance in youth with type 2 diabetes. Current Diabetes Reports 2013;13(1):81‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cropano 2017 {published data only}
- Cropano C, Santoro N, Groop L, Dalla Man C, Cobelli C, Galderisi A, et al. The rs7903146 variant in the TCF7L2 gene increases the risk of prediabetes/type 2 diabetes in obese adolescents by impairing beta‐cell function and hepatic insulin sensitivity. Diabetes Care 2017;40(8):1082‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dagogo‐Jack 2011 {published data only}
- Dagogo‐Jack S, Edeoga C, Ebenibo S, Chapp‐Jumbo E. Pathobiology of prediabetes in a biracial cohort (POP‐ABC) study: baseline characteristics of enrolled subjects. Journal of Clinical Endocrinology and Metabolism 2013;98(1):120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagogo‐Jack S, Edeoga C, Ebenibo S, Nyenwe E, Wan J. Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 diabetes: the pathobiology of prediabetes in a biracial cohort (POP‐ABC) study. Journal of Clinical Endocrinology and Metabolism 2014;99(6):E1078‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagogo‐Jack S, Edeoga C, Nyenwe E, Chapp‐Jumbo E, Wan J. Pathobiology of prediabetes in a biracial cohort (POP‐ABC): design and methods. Ethnicity & Disease 2011;21(1):33‐9. [PMC free article] [PubMed] [Google Scholar]
- Edeoga C, Owei I, Siwakoti K, Umekwe N, Ceesay F, Wan J, et al. Relationships between blood pressure and blood glucose among offspring of parents with type 2 diabetes: prediction of incident dysglycemia in a biracial cohort. Journal of Diabetes and Its Complications 2017;31(11):1580‐6. [DOI] [PubMed] [Google Scholar]
- Owei I, Umekwe N, Wan J, Dagogo‐Jack S. Plasma lipid levels predict dysglycemia in a biracial cohort of nondiabetic subjects: potential mechanisms. Experimental Biology and Medicine 2016;241(17):1961‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniel 1999 {published data only}
- Daniel M, Rowley KG, McDermott R, Mylvaganam A, O'Dea K. Diabetes incidence in an Australian aboriginal population. An 8‐year follow‐up study. Diabetes Care 1999;22(12):1993‐8. [DOI] [PubMed] [Google Scholar]
Decode 2003 {published data only}
- Decode Study Group European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases?. Diabetes Care 2003;26(3):688‐96. [DOI] [PubMed] [Google Scholar]
Deedwania 2013 {published data only}
- Deedwania P, Patel K, Fonarow GC, Desai RV, Zhang Y, Feller MA, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population‐based cohort study. International Journal of Cardiology 2013;168(4):3616‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeFina 2012 {published data only}
- DeFina LF, Vega GL, Leonard D, Grundy SM. Fasting glucose, obesity, and metabolic syndrome as predictors of type 2 diabetes: the Cooper center longitudinal study. Journal of Investigative Medicine 2012;60(8):1164‐8. [DOI] [PubMed] [Google Scholar]
DeJesus 2016 {published data only}
- DeJesus RS, Breitkopf CR, Rutten LJ, Jacobson DJ, Wilson PM, Sauver JS. Incidence rate of prediabetes progression to diabetes: modeling an optimum target group for intervention. Population Health Management 2016;30:30. [DOI] [PubMed] [Google Scholar]
Deschenes 2016 {published data only}
- Deschenes SS, Burns RJ, Graham E, Schmitz N. Prediabetes, depressive and anxiety symptoms, and risk of type 2 diabetes: a community‐based cohort study. Journal of Psychosomatic Research 2016;89:85‐90. [DOI] [PubMed] [Google Scholar]
Dinneen 1998 {published data only}
- Dinneen SF, Maldonado D, Leibson CL, Klee GG, Li H, Melton LJ, et al. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes Care 1998;21(9):1408‐13. [DOI] [PubMed] [Google Scholar]
Doi 2007 {published data only}
- Doi Y, Kubo M, Yonemoto K, Ninomiya T, Iwase M, Tanizaki Y, et al. Liver enzymes as a predictor for incident diabetes in a Japanese population: the Hisayama study. Obesity 2007;15(7):1841‐50. [DOI] [PubMed] [Google Scholar]
- Mukai N, Doi Y, Ninomiya T, Hata J, Hirakawa Y, Fukuhara M, et al. Cut‐off values of fasting and post‐load plasma glucose and HbA1c for predicting type 2 diabetes in community‐dwelling Japanese subjects: the Hisayama study. Diabetic Medicine 2012;29(1):99‐106. [DOI] [PubMed] [Google Scholar]
Du 2016 {published data only}
- Du TT, Yuan G, Zhou XR, Sun XX. Sex differences in the effect of HbA1c‐defined diabetes on a wide range of cardiovascular disease risk factors. Annals of Medicine 2016;48(1‐2):34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelman 2004 {published data only}
- Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Utility of hemoglobin A1c in predicting diabetes risk. Journal of General Internal Medicine 2004;19(12):1175‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelstein 1997 {published data only}
- Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett‐Connor EL, Dowse GK, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46(4):701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engberg 2010 {published data only}
- Engberg S, Glumer C, Witte D R, Jorgensen T, Borch‐Johnsen K. Differential relationship between physical activity and progression to diabetes by glucose tolerance status: the Inter99 Study. Diabetologia 2010;53(1):70‐8. [DOI] [PubMed] [Google Scholar]
- Engberg S, Vistisen D, Lau C, Glumer C, Jorgensen T, Pedersen O, et al. Progression to impaired glucose regulation and diabetes in the population‐based Inter99 study. Diabetes Care 2009;32(4):606‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glumer C, Jorgensen T, Borch‐Johnsen K. Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 2003;26(8):2335‐40. [DOI] [PubMed] [Google Scholar]
- Jorgensen T, Borch‐Johnsen K, Thomsen TF, Ibsen H, Glumer C, Pisinger C. A randomized non‐pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Europan Journal of Cardiovascular Prevention and Rehabilitation 2003;10(5):377‐86. [DOI] [PubMed] [Google Scholar]
- Soulimane S, Simon D, Shaw JE, Zimmet PZ, Vol S, Vistisen D, et al. Comparing incident diabetes as defined by fasting plasma glucose or by HbA(1c). The AusDiab, Inter99 and DESIR studies. Diabetic Medicine 2011;28(11):1311‐8. [DOI] [PubMed] [Google Scholar]
Eskesen 2013 {published data only}
- Eskesen K, Jensen MT, Galatius S, Vestergaard H, Hildebrandt P, Marott JL, et al. Glycated haemoglobin and the risk of cardiovascular disease, diabetes and all‐cause mortality in the Copenhagen city heart study. Journal of Internal Medicine 2013;273(1):94‐101. [DOI] [PubMed] [Google Scholar]
Feizi 2017 {published data only}
- Feizi A, Meamar R, Eslamian M, Amini M, Nasri M, Iraj B. Area under the curve during OGTT in first‐degree relatives of diabetic patients as an efficient indicator of future risk of type 2 diabetes and prediabetes. Clinical Endocrinology 2017;87(6):696‐705. [DOI] [PubMed] [Google Scholar]
Feskens 1989 {published data only}
- Feskens EJ, Kromhout D. Cardiovascular risk factors and the 25‐year incidence of diabetes mellitus in middle‐aged men. The Zutphen study. American Journal of Epidemiology 1989;130(6):1101‐8. [DOI] [PubMed] [Google Scholar]
Festa 2003 {published data only}
- Festa A. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation 2003;108(15):1822‐30. [DOI] [PubMed] [Google Scholar]
Folsom 2000 {published data only}
- Folsom AR, Kushi LH, Hong CP. Physical activity and incident diabetes mellitus in postmenopausal women. American Journal of Public Health 2000;90(1):134‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gil‐Montalban 2015 {published data only}
- Gil‐Montalban E, Martin‐Rios MD, Ortiz‐Marron H, Zorrilla‐Torras B, Martinez‐Cortes M, Esteban‐Vasallo MD, et al. Incidence of type 2 diabetes and associated factors in the adult population of the community of Madrid. PREDIMERC cohort. Revista Clinica Espanola 2015;215(9):495‐502. [DOI] [PubMed] [Google Scholar]
Giraldez‐Garcia 2015 {published data only}
- Giraldez‐Garcia C, Sangros FJ, Diaz‐Redondo A, Franch‐Nadal J, Serrano R, Diez J, et al. Cardiometabolic risk profiles in patients with impaired fasting glucose and/or hemoglobin A1c 5.7% to 6.4%: evidence for a gradient according to diagnostic criteria: the PREDAPS study. Medicine 2015;94(44):e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glauber 2018 {published data only}
- Glauber H, Vollmer WM, Nichols GA. A simple model for predicting two‐year risk of diabetes development in individuals with prediabetes. Permanente Journal 2018;22:17‐050. [DOI: 10.7812/TPP/17-050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Villalpando 2014 {published data only}
- Gonzalez‐Villalpando C, Davila‐Cervantes CA, Zamora‐Macorra M, Trejo‐Valdivia B, Gonzalez‐Villalpando ME. Risk factors associated to diabetes in Mexican population and phenotype of the individuals who will convert to diabetes. Salud Publica de Mexico 2014;56(4):317‐22. [DOI] [PubMed] [Google Scholar]
Gopinath 2013 {published data only}
- Gopinath B, Rochtchina E, Flood VM, Mitchell P. Diet quality is prospectively associated with incident impaired fasting glucose in older adults. Diabetic Medicine 2013;30(5):557‐62. [DOI] [PubMed] [Google Scholar]
Gu 2015 {published data only}
- Gu Y, Warren J, Kennelly J, Walker N, Harwood M. Incidence rate of prediabetes: an analysis of New Zealand primary care data. Studies in Health Technology and Informatics 2015;214:81‐6. [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta AK, Prieto‐Merino D, Dahlof B, Sever PS, Poulter NR. Metabolic syndrome, impaired fasting glucose and obesity, as predictors of incident diabetes in 14 120 hypertensive patients of ASCOT‐BPLA: comparison of their relative predictability using a novel approach. Diabetic Medicine 2011;28(8):941‐7. [DOI] [PubMed] [Google Scholar]
Hackett 2014 {published data only}
- Hackett RA, Kivimaki M, Kumari M, Steptoe A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II cohort study. Journal of Clinical Endocrinology and Metabolism 2016;101(2):619‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. Journal of Clinical Endocrinology and Metabolism 2014;99(12):4625‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haffner 1997 {published data only}
- Haffner SM, Miettinen H, Stern MP. Relatively more atherogenic coronary heart disease risk factors in prediabetic women than in prediabetic men. Diabetologia 1997;40(6):711‐7. [DOI] [PubMed] [Google Scholar]
Haffner 2000 {published data only}
- Haffner SM, Mykkanen L, Festa A, Burke JP, Stern MP. Insulin‐resistant prediabetic subjects have more atherogenic risk factors than insulin‐sensitive prediabetic subjects ‐ implications for preventing coronary heart disease during the prediabetic state. Circulation 2000;101(9):975‐80. [DOI] [PubMed] [Google Scholar]
Hajat 2012 {published data only}
- Hajat C, Shather Z. Prevalence of metabolic syndrome and prediction of diabetes using IDF versus ATPIII criteria in a Middle East population. Diabetes Research & Clinical Practice 2012;98(3):481‐6. [DOI] [PubMed] [Google Scholar]
Hanai 2005 {published data only}
- Hanai K, Kiuchi Y, Wasada T. Prevalence and progression of impaired glucose homeostasis assessed by the different criteria for IFG in Japanese adults. Diabetologia 2005;48(4):799‐800. [DOI] [PubMed] [Google Scholar]
He 2018 {published data only}
- He F. Diets with a low glycaemic load have favourable effects on prediabetes progression and regression: a prospective cohort study. Journal of Human Nutrition and Dietetics 2018;23:23. [DOI] [PubMed] [Google Scholar]
Helmrich 1991 {published data only}
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1991;325(3):147‐52. [DOI] [PubMed] [Google Scholar]
Henninger 2015 {published data only}
- Henninger J, Hammarstedt A, Rawshani A, Eliasson B. Metabolic predictors of impaired glucose tolerance and type 2 diabetes in a predisposed population ‐ a prospective cohort study. BMC Endocrine Disorders 2015;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holbrook 1990 {published data only}
- Holbrook TL, Barrett‐Connor E, Wingard DL. A prospective population‐based study of alcohol use and non‐insulin‐dependent diabetes mellitus. American Journal of Epidemiology 1990;132(5):902‐9. [DOI] [PubMed] [Google Scholar]
Hong 2016 {published data only}
- Hong JL, McNeill AM, He JH, Chen Y, Brodovicz KG. Identification of impaired fasting glucose, healthcare utilization and progression to diabetes in the UK using the clinical practice research datalink (CPRD). Pharmacoepidemiology and Drug Safety 2016;25(12):1375‐86. [DOI] [PubMed] [Google Scholar]
Huang 2014c {published data only}
- Huang CL, Iqbal U, Nguyen PA, Chen ZF, Clinciu DL, Hsu YHE, et al. Using hemoglobin A1C as a predicting model for time interval from pre‐diabetes progressing to diabetes. PLOS ONE 2014;9(8):e104263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hulman 2017 {published data only}
- Hulman A, Gujral UP, Narayan KMV, Pradeepa R, Mohan D, Anjana RM, et al. Glucose patterns during the OGTT and risk of future diabetes in an urban Indian population: the CARRS study. Diabetes Research and Clinical Practice 2017;126:192‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 2008 {published data only}
- Inoue K, Matsumoto M, Akimoto K. Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabetic Medicine 2008;25(10):1157‐63. [DOI] [PubMed] [Google Scholar]
- Kashima S, Inoue K, Matsumoto M, Akimoto K. Low serum creatinine is a type 2 diabetes risk factor in men and women: the Yuport health checkup center cohort study. Diabete & Metabolisme 2017;43(5):460‐4. [DOI] [PubMed] [Google Scholar]
Invitti 2006 {published data only}
- Invitti C, Gilardini L, Pontiggia B, Morabito F, Mazzilli G, Viberti G. Period prevalence of abnormal glucose tolerance and cardiovascular risk factors among obese children attending an obesity centre in Italy. Nutrition, Metabolism & Cardiovascular Diseases 2006;16(4):256‐62. [DOI] [PubMed] [Google Scholar]
Jallut 1990 {published data only}
- Jallut D, Golay A, Munger R, Frascarolo P, Schutz Y, Jequier E, et al. Impaired glucose tolerance and diabetes in obesity: a 6‐year follow‐up study of glucose metabolism. Metabolism 1990;39(10):1068‐75. [DOI] [PubMed] [Google Scholar]
James 1998 {published data only}
- James SA, Jamjoum L, Raghunathan TE, Strogatz DS, Furth ED, Khazanie PG. Physical activity and NIDDM in African‐Americans. The Pitt county study. Diabetes Care 1998;21(4):555‐62. [DOI] [PubMed] [Google Scholar]
Jansson 2015 {published data only}
- Jansson SP, Fall K, Brus O, Magnuson A, Wandell P, Ostgren CJ, et al. Prevalence and incidence of diabetes mellitus: a nationwide population‐based pharmaco‐epidemiological study in Sweden. Diabetic Medicine 2015;32(10):1319‐28. [DOI] [PubMed] [Google Scholar]
Jarrett 1979 {published data only}
- Jarrett RJ, Keen H, Fuller JH, McCartney M. Worsening to diabetes in men with impaired glucose tolerance ("borderline diabetes"). Diabetologia 1979;16(1):25‐30. [DOI] [PubMed] [Google Scholar]
Jarrett 1982 {published data only}
- Jarrett RJ, McCartney P, Keen H. The Bedford survey: ten year mortality rates in newly diagnosed diabetics, borderline diabetics and normoglycaemic controls and risk indices for coronary heart disease in borderline diabetics. Diabetologia 1982;22(2):79‐84. [DOI] [PubMed] [Google Scholar]
Jeanne 2018 {published data only}
- Jeanne TL, Hooker ER, Nguyen T, Messer LC, Sacks RM, Andrea SB, et al. High birth weight modifies association between adolescent physical activity and cardiometabolic health in women and not men. Preventive Medicine 2018;108:29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiamjarasrangsi 2008b {published data only}
- Jiamjarasrangsi W, Lohsoonthorn V, Lertmaharit S, Sangwatanaroj S. Incidence and predictors of abnormal fasting plasma glucose among the university hospital employees in Thailand. Diabetes Research and Clinical Practice 2008;79(2):343‐9. [DOI] [PubMed] [Google Scholar]
Joshipura 2017 {published data only}
- Joshipura KJ, Munoz‐Torres FJ, Campos M, Rivera‐Diaz AD, Zevallos JC. Association between within‐visit systolic blood pressure variability and development of pre‐diabetes and diabetes among overweight/obese individuals. Journal of Human Hypertension 2017;32(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadowaki 1984 {published data only}
- Kadowaki T, Miyake Y, Hagura R, Akanuma Y, Kajinuma H, Kuzuya N, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia 1984;26(1):44‐9. [DOI] [PubMed] [Google Scholar]
Kametani 2002 {published data only}
- Kametani T, Koshida H, Nagaoka T, Miyakoshi H. Hypertriglyceridemia is an independent risk factor for development of impaired fasting glucose and diabetes mellitus: a 9‐year longitudinal study in Japanese. Internal Medicine 2002;41(7):516‐21. [DOI] [PubMed] [Google Scholar]
Kanauchi 2003 {published data only}
- Kanauchi M, Nakajima M, Saito Y, Kanauchi K. Pancreatic beta‐cell function and insulin sensitivity in Japanese subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes mellitus. Metabolism 2003;52(4):476‐81. [DOI] [PubMed] [Google Scholar]
Kanaya 2005 {published data only}
- Kanaya AM, Wassel Fyr CL, Rekeneire N, Shorr RI, Schwartz AV, Goodpaster BH, et al. Predicting the development of diabetes in older adults: the derivation and validation of a prediction rule. Diabetes Care 2005;28(2):404‐8. [DOI] [PubMed] [Google Scholar]
Kawahara 2015 {published data only}
- Kawahara T, Imawatari R, Kawahara C, Inazu T, Suzuki G. Incidence of type 2 diabetes in pre‐diabetic Japanese individuals categorized by HbA1c levels: a historical cohort study. PLOS ONE 2015;10(4):e0122698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2017 {published data only}
- Khan T, Tsipas S, Wozniak G. Medical care expenditures for individuals with prediabetes: the potential cost savings in reducing the risk of developing diabetes. Population Health Management 2017;20(5):389‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khang 2010 {published data only}
- Khang YH, Cho Sl, Kim HR. Risks for cardiovascular disease, stroke, ischaemic heart disease, and diabetes mellitus associated with the metabolic syndrome using the new harmonised definition: findings from nationally representative longitudinal data from an Asian population. Atherosclerosis 2010;213(2):579‐85. [DOI] [PubMed] [Google Scholar]
Kieboom 2017 {published data only}
- Kieboom BCT, Ligthart S, Dehghan A, Kurstjens S, Baaij JHF, Franco OH, et al. Serum magnesium and the risk of prediabetes: a population‐based cohort study. Diabetologia 2017;60(5):843‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012a {published data only}
- Kim TN, Park MS, Lee SK, Yang SJ, Lee KW, Nam M, et al. Elevated A1C is associated with impaired early‐phase insulin secretion rather than insulin resistance in Koreans at high risk for developing diabetes. Endocrine 2012;42(3):584‐91. [DOI] [PubMed] [Google Scholar]
Kim 2012b {published data only}
- Kim HK, Bae SJ, Choe J. Impact of HbA1c criterion on the detection of subjects with increased risk for diabetes among health check‐up recipients in Korea. Diabetes & Metabolism Journal 2012;36(2):151‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim JY, Goran MI, Toledo‐Corral CM, Weigensberg MJ, Choi M, Shaibi GQ. One‐hour glucose during an oral glucose challenge prospectively predicts beta‐cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care 2013;36(6):1681‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016b {published data only}
- Kim JD, Kang SJ, Lee MK, Park SE, Rhee EJ, Park CY, et al. C‐peptide‐based index is more related to incident type 2 diabetes in non‐diabetic subjects than insulin‐based index. Endocrinology and Metabolism 2016;31(2):320‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2017a {published data only}
- Kim CW, Chang Y, Sung E, Ryu S. Sleep duration and progression to diabetes in people with prediabetes defined by HbA1c concentration. Diabetic Medicine 2017;34(11):1591‐8. [DOI] [PubMed] [Google Scholar]
Kim 2017b {published data only}
- Kim NH, Kwon TY, Yu S, Kim NH, Choi KM, Baik SH, et al. Increased vascular disease mortality risk in prediabetic Korean adults is mainly attributable to ischemic stroke. Stroke 2017;48(4):840‐5. [DOI] [PubMed] [Google Scholar]
Ko 2000 {published data only}
- Ko GT, Chan JC, Tsang LW, Cockram CS. Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care 2000;23(12):1770‐3. [DOI] [PubMed] [Google Scholar]
Kosaka 1996 {published data only}
- Kosaka K, Kuzuya T, Yoshinaga H, Hagura R. A prospective study of health check examinees for the development of non‐insulin‐dependent diabetes mellitus: relationship of the incidence of diabetes with the initial insulinogenic index and degree of obesity. Diabetic Medicine 1996;13(9 Suppl 6):S120‐6. [PubMed] [Google Scholar]
Kowall 2013 {published data only}
- Kowall B, Rathmann W, Giani G, Schipf S, Baumeister S, Wallaschofski H, et al. Random glucose is useful for individual prediction of type 2 diabetes: results of the study of health in Pomerania (SHIP). Primary Care Diabetes 2013;7(1):25‐31. [DOI] [PubMed] [Google Scholar]
Krabbe 2017 {published data only}
- Krabbe CEM, Schipf S, Ittermann T, Dorr M, Nauck M, Chenot JF, et al. Comparison of traditional diabetes risk scores and HbA1c to predict type 2 diabetes mellitus in a population based cohort study. Journal of Diabetes and Its Complications 2017;31(11):1602‐7. [DOI] [PubMed] [Google Scholar]
Le Boudec 2016 {published data only}
- Boudec J, Marques‐Vidal P, Cornuz J, Clair C. Smoking cessation and the incidence of pre‐diabetes and type 2 diabetes: a cohort study. Journal of Diabetes and Its Complications 2016;30(1):43‐8. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju metabolic disease cohort (CMC) study. PLOS ONE 2014;9(2):e90430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee EY, Lee YH, Yi SW, Shin SA, Yi JJ. BMI and all‐cause mortality in normoglycemia, impaired fasting glucose, newly diagnosed diabetes, and prevalent diabetes: a cohort study. Diabetes Care 2017;40(8):1026‐33. [DOI] [PubMed] [Google Scholar]
Leite 2009 {published data only}
- Leite SA, Anderson RL, Kendall DM, Monk AM, Bergenstal RM. A1C predicts type 2 diabetes and impaired glucose tolerance in a population at risk: the community diabetes prevention project. Diabetology & Metabolic Syndrome 2009;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li CI, Chien L, Liu CS, Lin WY, Lai MM, Lee CC, et al. Prospective validation of American Diabetes Association risk tool for predicting pre‐diabetes and diabetes in Taiwan‐Taichung community health study. PLOS ONE 2011;6(10):e25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liatis 2014 {published data only}
- Liatis S, Sfikakis PP, Tsiakou A, Stathi C, Terpos E, Katsilambros N, et al. Baseline osteocalcin levels and incident diabetes in a 3‐year prospective study of high‐risk individuals. Diabetes & Metabolism 2014;40(3):198‐203. [DOI] [PubMed] [Google Scholar]
Libman 2008 {published data only}
- Libman IM, Barinas‐Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. Journal of Clinical Endocrinology Metabolism 2008;93(11):4231‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2017a {published data only}
- Liu M, Wang J, Zeng J, Cao X, He Y. Association of NAFLD with diabetes and the impact of BMI changes: a 5‐year cohort study based on 18,507 elderly. Journal of Clinical Endocrinology and Metabolism 2017;102(4):1309‐16. [DOI] [PubMed] [Google Scholar]
Liu 2017b {published data only}
- Liu TT, Liu DM, Xuan Y, Zhao L, Sun LH, Zhao DD, et al. The association between the baseline bone resorption marker CTX and incident dysglycemia after 4 years. Bone Research 2017;5:17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malmstrom 2018 {published data only}
- Malmstrom H, Walldius G, Carlsson S, Grill V, Jungner I, Gudbjornsdottir S, et al. Elevations of metabolic risk factors 20 years or more before diagnosis of type 2 diabetes: experience from the AMORIS study. Diabetes, Obesity & Metabolism 2018;5:05. [DOI] [PubMed] [Google Scholar]
Manson 1992 {published data only}
- Ajani UA, Hennekens CH, Spelsberg A, Manson JE. Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Archives of Internal Medicine 2000;160(7):1025‐30. [DOI] [PubMed] [Google Scholar]
- Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 1992;268(1):63‐7. [PubMed] [Google Scholar]
McNeill 2006 {published data only}
- McNeill AM, Katz R, Girman CJ, Rosamond WD, Wagenknecht LE, Barzilay JI, et al. Metabolic syndrome and cardiovascular disease in older people: the cardiovascular health study. Journal of the American Geriatrics Society 2006;54(9):1317‐24. [DOI] [PubMed] [Google Scholar]
McPhillips 1990 {published data only}
- McPhillips JB, Barrett‐Connor E, Wingard DL. Cardiovascular disease risk factors prior to the diagnosis of impaired glucose tolerance and non‐insulin‐dependent diabetes mellitus in a community of older adults. American Journal of Epidemiology 1990;131(3):443‐53. [DOI] [PubMed] [Google Scholar]
Medalie 1975 {published data only}
- Herman JB, Medalie JH, Kahn HA, Neufeld HN, Riss E, Perlstein T. Diabetes incidence: a two‐year follow‐up of 10,000 men in a survey of ischemic heart disease in Israel. Diabetes 1970;19(12):938‐43. [DOI] [PubMed] [Google Scholar]
- Kahn HA, Herman JB, Medalie JH, Neufeld HN, Riss E, Goldbourt U. Factors related to diabetes incidence: a multivariate analysis of two years observation on 10,000 men. The Israel ischemic heart disease study. Journal of Chronic Diseases 1971;23(9):617‐29. [DOI] [PubMed] [Google Scholar]
- Medalie JH, Papier C, Herman JB, Goldbourt U, Tamir S, Neufeld HN, et al. Diabetes mellitus among 10,000 adult men. I. Five‐year incidence and associated variables. Israel Journal of Medical Sciences 1974;10(7):681‐97. [PubMed] [Google Scholar]
- Medalie JH, Papier CM, Goldbourt U, Herman JB. Major factors in the development of diabetes mellitus in 10,000 men. Archives of Internal Medicine 1975;135(6):811‐7. [PubMed] [Google Scholar]
Metcalf 2017 {published data only}
- Metcalf PA, Kyle C, Kenealy T, Jackson RT. HbA1c in relation to incident diabetes and diabetes‐related complications in non‐diabetic adults at baseline. Journal of Diabetes and Its Complications 2017;31(5):814‐23. [DOI] [PubMed] [Google Scholar]
Miranda 2017 {published data only}
- Miranda ER, Somal VS, Mey JT, Blackburn BK, Wang E, Farabi S, et al. Circulating soluble RAGE isoforms are attenuated in obese, impaired‐glucose‐tolerant individuals and are associated with the development of type 2 diabetes. American Journal of Physiology. Endocrinology and Metabolism 2017;313(6):E631‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirbolouk 2016 {published data only}
- Mirbolouk M, Hajebrahimi MA, Akbarpour S, Tohidi M, Azizi F, Hadaegh F. Different glucose tolerance status and incident cardiovascular disease and all‐cause mortality among elderly Iranians. Geriatrics & Gerontology International 2016;16:1263‐71. [DOI] [PubMed] [Google Scholar]
Monesi 2012 {published data only}
- Monesi L, Baviera M, Marzona I, Avanzini F, Monesi G, Nobili A, et al. Prevalence, incidence and mortality of diagnosed diabetes: evidence from an Italian population‐based study. Diabetic Medicine 2012;29(3):385‐92. [DOI] [PubMed] [Google Scholar]
Morrison 2012 {published data only}
- Morrison JA, Glueck CJ, Wang P. Childhood risk factors predict cardiovascular disease, impaired fasting glucose plus type 2 diabetes mellitus, and high blood pressure 26 years later at a mean age of 38 years: the Princeton‐lipid research clinics follow‐up study. Metabolism: Clinical and Experimental 2012;61(4):531‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakagami 2017 {published data only}
- Nakagami T, Takahashi K, Suto C, Oya J, Tanaka Y, Kurita M, et al. Diabetes diagnostic thresholds of the glycated hemoglobin A1c and fasting plasma glucose levels considering the 5‐year incidence of retinopathy. Diabetes Research and Clinical Practice 2017;124:20‐9. [DOI] [PubMed] [Google Scholar]
Nakasone 2017 {published data only}
- Nakasone Y, Miyakoshi T, Sato Y, Yamauchi K, Hashikura R, Takayama M, et al. Impact of weight gain on the evolution and regression of prediabetes: a quantitative analysis. European Journal of Clinical Nutrition 2017;13(71):206‐11. [DOI] [PubMed] [Google Scholar]
Nano 2017 {published data only}
- Nano J, Muka T, Ligthart S, Hofman A, Darwish Murad S, Janssen HLA, et al. Gamma‐glutamyltransferase levels, prediabetes and type 2 diabetes: a Mendelian randomization study. International Journal of Epidemiology 2017;46(5):1400‐9. [DOI] [PubMed] [Google Scholar]
Nguyen 2014 {published data only}
- Nguyen QC, Whitsel EA, Tabor JW, Cuthbertson CC, Wener MH, Potter AJ, et al. Blood spot‐based measures of glucose homeostasis and diabetes prevalence in a nationally representative population of young US adults. Annals of Epidemiology 2014;24(12):903‐9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 2007 {published data only}
- Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glucose to type 2 diabetes. Diabetes Care 2007;30(2):228‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 2010 {published data only}
- Nichols Gregory A, Moler Edward J. Diabetes incidence for all possible combinations of metabolic syndrome components. Diabetes Research and Clinical Practice 2010;90(1):115‐21. [DOI] [PubMed] [Google Scholar]
Nichols 2015 {published data only}
- Nichols GA, Schroeder EB, Karter AJ, Gregg EW, Desai J, Lawrence JM, et al. Trends in diabetes incidence among 7 million insured adults, 2006‐2011: the SUPREME‐DM project. American Journal of Epidemiology 2015;181(1):32‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Njolstad 1998 {published data only}
- Njolstad I, Arnesen E, Lund‐Larsen P G. Sex differences in risk factors for clinical diabetes mellitus in a general population: a 12‐year follow‐up of the Finnmark Study. American Journal of Epidemiology 1998;147(1):49‐58. [DOI] [PubMed] [Google Scholar]
Norberg 2006 {published data only}
- Norberg M, Eriksson JW, Lindahl B, Andersson C, Rolandsson O, Stenlund H, et al. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. Journal of Internal Medicine 2006;260(3):263‐71. [DOI] [PubMed] [Google Scholar]
Nowicka 2011 {published data only}
- Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34(6):1306‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohlson 1987 {published data only}
- Ohlson LO, Larsson B, Bjorntorp P, Eriksson H, Svardsudd K, Welin L, et al. Risk factors for type 2 (non‐insulin‐dependent) diabetes mellitus. Thirteen and one‐half years of follow‐up of the participants in a study of Swedish men born in 1913. Diabetologia 1988;31(11):798‐805. [DOI] [PubMed] [Google Scholar]
- Ohlson LO, Larsson B, Eriksson H, Svardsudd K, Welin L, Tibblin G. Diabetes mellitus in Swedish middle‐aged men. The study of men born in 1913 and 1923. Diabetologia 1987;30(6):386‐93. [DOI] [PubMed] [Google Scholar]
Oizumi 2011 {published data only}
- Nakagami T, Tominaga M, Nishimura R, Yoshiike N, Daimon M, Oizumi T, et al. Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Research and Clinical Practice 2007;76(2):251‐6. [DOI] [PubMed] [Google Scholar]
- Oizumi T, Daimon M, Karasawa S, Kaino W, Takase K, Jimbu Y, et al. Assessment of plasma glucose cutoff values to predict the development of type 2 diabetes in a Japanese sample: the Funagata study. Diabetology International 2011;2(1):26‐31. [Google Scholar]
Okada 2017 {published data only}
- Okada R, Tsushita K, Wakai K, Ishizaka Y, Kato K, Wada T, et al. Lower risk of progression from prediabetes to diabetes with health checkup with lifestyle education: Japan Ningen Dock study. Nutrition, Metabolism & Cardiovascular Diseases 2017;27(8):679‐87. [DOI] [PubMed] [Google Scholar]
Onat 2007 {published data only}
- Onat A, Hergenc G, Can G. Prospective validation in identical Turkish cohort of two metabolic syndrome definitions for predicting cardiometabolic risk and selection of most appropriate definition. Anadolu Kardiyoloji Dergisi ‐ the Anatolian Journal of Cardiology 2007;7(1):29‐34. [PubMed] [Google Scholar]
Onat 2013a {published data only}
- Onat A, Can G, Cicek G, Ayhan E, Dogan Y, Kaya H. Fasting, non‐fasting glucose and HDL dysfunction in risk of pre‐diabetes, diabetes, and coronary disease in non‐diabetic adults. Acta Diabetologica 2013;50(4):519‐28. [DOI] [PubMed] [Google Scholar]
Onat 2013b {published data only}
- Onat A, Aydin M, Can G, Cakmak HA, Koroglu B, Kaya A, et al. Impaired fasting glucose: pro‐diabetic, "atheroprotective" and modified by metabolic syndrome. World Journal of Diabetes 2013;4(5):210‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osei 2004 {published data only}
- Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre‐diabetic African Americans: implications for primary diabetes prevention. Diabetes Care 2004;27(6):1439‐46. [DOI] [PubMed] [Google Scholar]
Paddock 2017 {published data only}
- Paddock E, Hohenadel MG, Piaggi P, Vijayakumar P, Hanson RL, Knowler WC, et al. One‐hour and two‐hour postload plasma glucose concentrations are comparable predictors of type 2 diabetes mellitus in Southwestern Native Americans. Diabetologia 2017;60(9):1704‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 1995 {published data only}
- Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non‐insulin dependent diabetes in middle aged British men. BMJ 1995;310(6979):560‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, Craen AJ, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet 2008;371(9628):1927‐35. [DOI] [PubMed] [Google Scholar]
- Shaper AG, Pocock SJ, Walker M, Cohen NM, Wale CJ, Thomson AG. British regional heart study: cardiovascular risk factors in middle‐aged men in 24 towns. British Medical Journal 1981;283(6285):179‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG. The metabolic syndrome and cardiovascular risk in the British regional heart study. International Journal of Obesity 2008;32(Suppl 2):S25‐9. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Papacosta O, Whincup PH, Thomas MC, Carson C, Lawlor DA, et al. The potential for a two‐stage diabetes risk algorithm combining non‐laboratory‐based scores with subsequent routine non‐fasting blood tests: results from prospective studies in older men and women. Diabetic Medicine 2011;28(1):23‐30. [DOI] [PubMed] [Google Scholar]
Pinelli 2011 {published data only}
- Pinelli NR, Jantz AS, Martin ET, Jaber LA. Sensitivity and specificity of glycated hemoglobin as a diagnostic test for diabetes and prediabetes in Arabs. Journal of Clinical Endocrinology & Metabolism 2011;96(10):E1680‐3. [DOI] [PubMed] [Google Scholar]
Polakowska 2011 {published data only}
- Polakowska M, Piotrowski W. Incidence of diabetes in the Polish population: results of the multicenter Polish population health status study ‐ WOBASZ. Polskie Archiwum Medycyny Wewnetrznej 2011;121(5):156‐63. [PubMed] [Google Scholar]
Pradhan 2007 {published data only}
- Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. American Journal of Medicine 2007;120(8):720‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Priya 2013 {published data only}
- Priya M, Anjana RM, Chiwanga FS, Gokulakrishnan K, Deepa M, Mohan V. 1‐hour venous plasma glucose and incident prediabetes and diabetes in Asian Indians. Diabetes Technology & Therapeutics 2013;15(6):497‐502. [DOI] [PubMed] [Google Scholar]
Qiao 2003 {published data only}
- Qiao Q, Lindstrom J, Valle TT, Tuomilehto J. Progression to clinically diagnosed and treated diabetes from impaired glucose tolerance and impaired fasting glycaemia. Diabetic Medicine 2003;20(12):1027‐33. [DOI] [PubMed] [Google Scholar]
Qiu 2015 {published data only}
- Qiu M, Shen W, Song X, Ju L, Tong W, Wang H, et al. Effects of prediabetes mellitus alone or plus hypertension on subsequent occurrence of cardiovascular disease and diabetes mellitus: longitudinal study. Hypertension 2015;65(3):525‐30. [DOI] [PubMed] [Google Scholar]
Ramachandran 2012 {published data only}
- Ramachandran A, Snehalatha C, Samith Shetty A, Nanditha A. Predictive value of HbA1c for incident diabetes among subjects with impaired glucose tolerance ‐ analysis of the Indian diabetes prevention programmes. Diabetic Medicine 2012;29(1):94‐8. [DOI] [PubMed] [Google Scholar]
Rauh 2017 {published data only}
- Rauh SP, Heymans MW, Koopman AD, Nijpels G, Stehouwer CD, Thorand B, et al. Predicting glycated hemoglobin levels in the non‐diabetic general population: development and validation of the DIRECT‐DETECT prediction model ‐ a DIRECT study. PLOS ONE 2017;12(2):e0171816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 2006 {published data only}
- Reynolds SS, Yanek LR, Vaidya D, Mora S, Moy TF, Saudek CD, et al. Glucose levels in the normal range predict incident diabetes in families with premature coronary heart disease. Diabetes Research and Clinical Practice 2006;74(3):267‐73. [DOI] [PubMed] [Google Scholar]
Rimm 1995 {published data only}
- Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ 1995;310(6979):555‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacks 2017 {published data only}
- Sacks RM, Greene J, Hibbard J, Overton V, Parrotta CD. Does patient activation predict the course of type 2 diabetes? A longitudinal study. Patient Education and Counselling 2017;100(7):1268‐75. [DOI] [PubMed] [Google Scholar]
Sai 2017 {published data only}
- Sai Prasanna N, Amutha A, Pramodkumar TA, Anjana RM, Venkatesan U, Priya M, et al. The 1h post glucose value best predicts future dysglycemia among normal glucose tolerance subjects. Journal of Diabetes and Its Complications 2017;31(11):1592‐6. [DOI] [PubMed] [Google Scholar]
Samaras 2015 {published data only}
- Samaras K, Crawford J, Lutgers HL, Campbell LV, Baune BT, Lux O, et al. Metabolic burden and disease and mortality risk associated with impaired fasting glucose in elderly adults. Journal of American Geriatrics Society 2015;63(7):1435‐42. [DOI] [PubMed] [Google Scholar]
Schmitz 2016 {published data only}
- Schmitz N, Deschenes SS, Burns RJ, Smith KJ, Lesage A, Strychar I, et al. Depression and risk of type 2 diabetes: the potential role of metabolic factors. Molecular Psychiatry 2016;21(12):1726‐32. [DOI] [PubMed] [Google Scholar]
Schottker 2011 {published data only}
- Schottker B, Raum E, Rothenbacher D, Muller H, Brenner H. Prognostic value of haemoglobin A1c and fasting plasma glucose for incident diabetes and implications for screening. European Journal of Epidemiology 2011;26(10):779‐87. [DOI] [PubMed] [Google Scholar]
Schulze 2008 {published data only}
- Schulze MB, Boeing H, Haring HU, Fritsche A, Joost HG. Validation of the German diabetes risk score with metabolic risk factors for type 2 diabetes [Validierung des Deutschen Diabetes‐Risiko‐Scores mit metabolischen Risikofaktoren für Typ‐2‐Diabetes]. Deutsche Medizinische Wochenschrift 2008;133(17):878‐83. [DOI] [PubMed] [Google Scholar]
Schwarz 2007 {published data only}
- Schwarz PEH, Bornstein SR, Hanefeld M. Elevated fasting glucose levels predicts IGT and diabetes also in middle‐age subjects. Diabetes Research and Clinical Practice 2007;77(1):148‐50. [DOI] [PubMed] [Google Scholar]
Serrano 2013 {published data only}
- Serrano R, Garcia‐Soidan FJ, Diaz‐Redondo A, Artola S, Franch J, Diez J, et al. Cohort study in primary health care on the evolution of patients with prediabetes (PREDAPS): basis and methodology. Revista Española Salud Publica 2013;87(2):121‐35. [DOI] [PubMed] [Google Scholar]
Shimazaki 2007 {published data only}
- Shimazaki T, Kadowaki T, Ohyama Y, Ohe K, Kubota K. Hemoglobin A1c (HbA1c) predicts future drug treatment for diabetes mellitus: a follow‐up study using routine clinical data in a Japanese university hospital. Translational Research 2007;149(4):196‐204. [DOI] [PubMed] [Google Scholar]
Song 2007 {published data only}
- Song KH, Nam‐Goomg IS, Han SM, Kim MS, Lee EJ, Lee YS, et al. Change in prevalence and 6‐year incidence of diabetes and impaired fasting glucose in Korean subjects living in a rural area. Diabetes Research and Clinical Practice 2007;78(3):378‐84. [DOI] [PubMed] [Google Scholar]
Song 2016b {published data only}
- Song YS, Hwang YC, Ahn HY, Park CY. Comparison of the usefulness of the updated homeostasis model assessment (HOMA2) with the original HOMA1 in the prediction of type 2 diabetes mellitus in Koreans. Diabetes & Metabolism Journal 2016;40(4):318‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorgjerd 2015 {published data only}
- Sorgjerd EP, Thorsby PM, Torjesen PA, Skorpen F, Kvaloy K, Grill V. Presence of anti‐GAD in a non‐diabetic population of adults; time dynamics and clinical influence: results from the HUNT study. BMJ Open Diabetes Research and Care 2015;3:e000076. [DOI: 10.1136/bmjdrc-2014-000076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soria 2009 {published data only}
- Soria ML, Sy RG, Vega BS, Ty‐Willing T, Abenir‐Gallardo A, Velandria F, et al. The incidence of type 2 diabetes mellitus in the Philippines: a 9‐year cohort study. Diabetes Research and Clinical Practice 2009;86(2):130‐3. [DOI] [PubMed] [Google Scholar]
Stampfer 1988 {published data only}
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non‐insulin‐dependent diabetes mellitus in women. Lancet 1991;338(8770):774‐8. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, et al. Cigarette smoking and the risk of diabetes in women. American Journal of Public Health 1993;83(2):211‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Arky RA, Hennekens CH, et al. A prospective study of moderate alcohol drinking and risk of diabetes in women. American Journal of Epidemiology 1988;128(3):549‐58. [DOI] [PubMed] [Google Scholar]
Strauss 1974 {published data only}
- Strauss WT, Hales CN. Plasma insulin in minor abnormalities of glucose tolerance: a 5 year follow‐up. Diabetologia 1974;10(3):237‐43. [DOI] [PubMed] [Google Scholar]
Suvitaival 2018 {published data only}
- Suvitaival T, Bondia‐Pons I, Yetukuri L, Poho P, Nolan JJ, Hyotylainen T, et al. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism 2018;78:1‐12. [DOI] [PubMed] [Google Scholar]
Tabak 2009 {published data only}
- Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373(9682):2215‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tai 2004 {published data only}
- Tai ES, Goh SY, Lee JJ, Wong MS, Heng D, Hughes K, et al. Lowering the criterion for impaired fasting glucose: impact on disease prevalence and associated risk of diabetes and ischemic heart disease. Diabetes Care 2004;27(7):1728‐34. [DOI] [PubMed] [Google Scholar]
Takkunen 2016 {published data only}
- Takkunen MJ, Schwab US, Mello VD, Eriksson JG, Lindstrom J, Tuomilehto J, et al. Longitudinal associations of serum fatty acid composition with type 2 diabetes risk and markers of insulin secretion and sensitivity in the Finnish diabetes prevention study. European Journal of Nutrition 2016;55(3):967‐79. [DOI] [PubMed] [Google Scholar]
Tanabe 2009 {published data only}
- Tanabe N, Saito K, Yamada Y, Takasawa T, Seki N, Suzuki H. Risk assessment by post‐challenge plasma glucose, insulin response ratio, and other indices of insulin resistance and/or secretion for predicting the development of type 2 diabetes. Internal Medicine 2009;48(6):401‐9. [DOI] [PubMed] [Google Scholar]
Vaccaro 2005 {published data only}
- Vaccaro O, Riccardi G. Changing the definition of impaired fasting glucose: impact on the classification of individuals and risk definition. Diabetes Care 2005;28(7):1786‐8. [DOI] [PubMed] [Google Scholar]
Vaidya 2016 {published data only}
- Vaidya A, Cui L, Sun L, Lu B, Chen S, Liu X, et al. A prospective study of impaired fasting glucose and type 2 diabetes in China: the Kailuan study. Medicine 2016;95(46):e5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vazquez 2000 {published data only}
- Vazquez JA, Gaztambide S, Soto‐Pedre E. 10‐year prospective study on the incidence and risk factors for type 2 diabetes mellitus. Medicina Clinica 2000;115(14):534‐9. [PubMed] [Google Scholar]
Vega‐Vázquez 2017 {published data only}
- Vega‐Vázquez MA, Ramírez‐Vick M, Muñoz‐Torres FJ, González‐Rodríguez LA, Joshipura K. Comparing glucose and hemoglobin A1c diagnostic tests among a high metabolic risk Hispanic population. Diabetes/Metabolism Research and Reviews 2017;33(4):e2874. [DOI: 10.1002/dmrr.2874] [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Eckardstein 2000 {published data only}
- Eckardstein A, Schulte H, Assmann G. Risk for diabetes mellitus in middle‐aged Caucasian male participants of the PROCAM study: implications for the definition of impaired fasting glucose by the American Diabetes Association. Journal of Clinical Endocrinology and Metabolism 2000;85(9):3101‐8. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang Z, Hoy W E, Si D. Incidence of type 2 diabetes in Aboriginal Australians: an 11‐year prospective cohort study. BMC Public Health 2010;10:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warram 1996 {published data only}
- Warram JH, Sigal RJ, Martin BC, Krolewski AS, Soeldner JS. Natural history of impaired glucose tolerance: follow‐up at Joslin Clinic. Diabetic Medicine 1996;13(9 Suppl 6):S40‐5. [PubMed] [Google Scholar]
Wei 1999 {published data only}
- Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Annals of Internal Medicine 1999;130(2):89‐96. [DOI] [PubMed] [Google Scholar]
Welborn 1979 {published data only}
- Welborn TA, Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care 1979;2(2):154‐60. [DOI] [PubMed] [Google Scholar]
Wheeler 2017 {published data only}
- Wheeler E, Leong A, Liu CT, Hivert MF, Strawbridge RJ, Podmore C, et al. Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome‐wide meta‐analysis. PLOS Medicine 2017; Vol. 14:e1002383. [DOI] [PMC free article] [PubMed]
Wingard 1993 {published data only}
- Wingard DL, Barrett‐Connor EL, Scheidt‐Nave C, McPhillips JB. Prevalence of cardiovascular and renal complications in older adults with normal or impaired glucose tolerance or NIDDM. A population‐based study. Diabetes Care 1993;16(7):1022‐5. [DOI] [PubMed] [Google Scholar]
Woo 2015 {published data only}
- Woo YC, Cheung BM, Yeung CY, Lee CH, Hui EY, Fong CH, et al. Cardiometabolic risk profile of participants with prediabetes diagnosed by HbA1c criteria in an urban Hong Kong Chinese population over 40 years of age. Diabetic Medicine 2015;32(9):1207‐11. [DOI] [PubMed] [Google Scholar]
Wu 2017a {published data only}
- Wu J, Gong L, Li Q, Hu J, Zhang S, Wang Y, et al. A novel visceral adiposity index for prediction of type 2 diabetes and pre‐diabetes in Chinese adults: a 5‐year prospective study. Scientific Reports 2017;7(1):13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2017b {published data only}
- Wu J, Ward E, Threatt T, Lu ZK. Progression to type 2 diabetes and its effect on health care costs in low‐income and insured patients with prediabetes: a retrospective study using Medicaid claims data. Journal of Managed Care & Speciality Pharmacy 2017;23(3):309‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2018 {published data only}
- Wu F, Juonala M, Pitkanen N, Jula A, Lehtimaki T, Sabin MA, et al. Both youth and long‐term vitamin D status is associated with risk of type 2 diabetes mellitus in adulthood: a cohort study. Annals of Medicine 2018;50(1):74‐82. [DOI] [PubMed] [Google Scholar]
Xu 2014 {published data only}
- Xu L, Jiang CQ, Schooling CM, Zhang WS, Cheng KK, Lam TH. Prediction of 4‐year incident diabetes in older Chinese: recalibration of the Framingham diabetes score on Guangzhou biobank cohort study. Preventive Medicine 2014;69:63‐8. [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang HK, Ha HS, Rhee M, Lee JH, Park YM, Kwon HS, et al. Predictive value of glucose parameters obtained from oral glucose tolerance tests in identifying individuals at high risk for the development of diabetes in Korean population. Medicine 2016;95(10):e3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2014 {published data only}
- Ye X, Zong G, Liu X, Liu G, Gan W, Zhu J, et al. Development of a new risk score for incident type 2 diabetes using updated diagnostic criteria in middle‐aged and older Chinese. PLOS ONE 2014;9(5):e97042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yi 2017 {published data only}
- Yi SW, Park S, Lee YH, Park HJ, Balkau B, Yi JJ. Association between fasting glucose and all‐cause mortality according to sex and age: a prospective cohort study. Scientific Reports 2017;7(1):8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yokota 2017 {published data only}
- Yokota N, Miyakoshi T, Sato Y, Nakasone Y, Yamashita K, Imai T, et al. Predictive models for conversion of prediabetes to diabetes. Journal of Diabetes and Its Complications 2017;31(8):1266‐71. [DOI] [PubMed] [Google Scholar]
Yoshinaga 1996 {published data only}
- Yoshinaga H, Kosaka K. High glycosylated hemoglobin levels increase the risk of progression to diabetes mellitus in subjects with glucose intolerance. Diabetes Research and Clinical Practice 1996;31(1‐3):71‐9. [DOI] [PubMed] [Google Scholar]
Yoshinaga 1999 {published data only}
- Yoshinaga H, Kosaka K. Heterogeneous relationship of early insulin response and fasting insulin level with development of non‐insulin‐dependent diabetes mellitus in non‐diabetic Japanese subjects with or without obesity. Diabetes Research & Clinical Practice 1999;44(2):129‐36. [DOI] [PubMed] [Google Scholar]
Zargar 2001 {published data only}
- Zargar AH, Masoodi SR, Khan AK, Bashir MI, Laway BA, Wani AI, et al. Impaired fasting glucose and impaired glucose tolerance ‐ lack of agreement between the two categories in a North Indian population. Diabetes Research and Clinical Practice 2001;51(2):145‐9. [DOI] [PubMed] [Google Scholar]
Zethelius 2008 {published data only}
- Zethelius B, Berglund L, Hanni A, Berne C. The interaction between impaired acute insulin response and insulin resistance predicts type 2 diabetes and impairment of fasting glucose. Upsala Journal of Medical Sciences 2008;113(2):117‐29. [DOI] [PubMed] [Google Scholar]
Zhang 2012b {published data only}
- Zhang M, Gao Y, Chang H, Wang X, Liu D, Zhu Z, et al. Hypertriglyceridemic‐waist phenotype predicts diabetes: a cohort study in Chinese urban adults. BMC Public Health 2012;12:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang T, Li Y, Zhang HJ, Sun DJY, Li SX, Fernandez C, et al. Insulin‐sensitive adiposity is associated with a relatively lower risk of diabetes than insulin‐resistant adiposity: the Bogalusa heart study. Endocrine 2016;54(1):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmet 1992 {published data only}
- Zimmet PZ, Collins VR, Dowse GK, Knight LT. Hyperinsulinaemia in youth is a predictor of type 2 (non‐insulin‐dependent) diabetes mellitus. Diabetologia 1992;35(6):534‐41. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Li 2001 {published data only}
- Li G, Wang J, Chen C. [Model of development of diabetes mellitus in adult Chinese]. Zhonghua Yi Xue Za Zhi [National Medical Journal of China] 2001;81(15):914‐7. [PubMed] [Google Scholar]
Misnikova 2011 {published data only}
- Misnikova IV, Dreval AV, Barsukov IA, Dzebisashvili TG. Risk of diabetes and cardiovascular events in persons with early glucose metabolism impairments. Diabetologia 2011;54(Suppl 1):S119. [Google Scholar]
NCT00816608 {published data only}
- NCT00816608. The effect of maximum body weight in lifetime on the development of type 2 diabetes (MAXWEL) [Study of MAXimum Weight in Lifetime on glucose homeostasis (MAXWEL)]. clinicaltrials.gov/show/NCT00816608 (first received 1 January 2009).
References to ongoing studies
NCT00786890 {unpublished data only}
- NCT00786890. A survey to evaluate the cardiovascular risk status of subjects with pre‐diabetes in Hong Kong (JADE‐HK2). clinicaltrials.gov/show/NCT00786890 (accessed 1 November 2017).
NCT02838693 {unpublished data only}
- NCT02838693. Assessing progression to type‐2 diabetes (APT‐2D): a prospective cohort study expanded from BRITE‐SPOT (bio‐bank and registry for stratIfication and targeted interventions in the spectrum of type 2 diabetes) (APT‐2D). clinicaltrials.gov/show/NCT02838693 (accessed 1 November 2017).
NCT02958579 {unpublished data only}
- NCT02958579. A population based study on metabolic syndrome complications, and mortality (MetSCoM). clinicaltrials.gov/show/NCT02958579 (accessed 1 November 2017).
Vilanova 2017 {published data only}
- Vilanova MB, Falguera M, Marsal JR, Rubinat E, Alcubierre N, Castelblanco E, et al. Prevalence, clinical features and risk assessment of pre‐diabetes in Spain: the prospective Mollerussa cohort study. BMJ Open 2017;7(6):e015158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdul‐Ghani 2006
- Abdul‐Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans administration genetic epidemiology study. Diabetes 2006;55(5):1430‐5. [PUBMED: 16644701] [DOI] [PubMed] [Google Scholar]
ADA 1997
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 2003
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5‐20. [DOI] [PubMed] [Google Scholar]
ADA 2010
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62‐9. [PUBMED: 20042775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2001
- Altman D G. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avilés‐Santa 2016
- Avilés‐Santa ML, Hsu LL, Arredondo M, Menke A, Werner E, Thyagarajan B, et al. Differences in hemoglobin A1c between Hispanics/Latinos and non‐Hispanic whites: an analysis of the Hispanic Community Health Study/Study of Latinos and the 2007‐2012 National Health and Nutrition Examination Survey. Diabetes Care 2016;39(6):1010‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2017a
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. [DOI] [PubMed] [Google Scholar]
Borenstein 2017b
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 March 2018).
Buse 2009
- Buse JB, Caprio S, Cefalu WT, Ceriello A, Prato S, Inzucchi SE, et al. How do we define cure of diabetes?. Diabetes Care 2009;32:2133‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buysschaert 2011
- Buysschaert M, Bergman M. Definition of prediabetes. Medical Clinics of North America 2011;95:289‐97, vii. [DOI] [PubMed] [Google Scholar]
Buysschaert 2016
- Buysschaert M, Medina JL, Buysschaert B, Bergman M. Definitions (and current controversies) of diabetes and prediabetes. Current Diabetes Reviews 2016;12:8‐13. [DOI] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. 2014 National Diabetes Statistics Report. www.cdc.gov/diabetes/pdfs/data/2014‐report‐estimates‐of‐diabetes‐and‐its‐burden‐in‐the‐united‐states.pdf (accessed 3 March 2018).
Cefalu 2016
- Cefalu WT. "Prediabetes": Are there problems with this label? No, we need heightened awareness of this condition!. Diabetes Care 2016;39:1472‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2006
- Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism: Clinical and Experimental 2006;55(4):434‐8. [PUBMED: 16546472] [DOI] [PubMed] [Google Scholar]
Davidson 2003
- Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care 2003;26:3329‐30. [DOI] [PubMed] [Google Scholar]
De Rekeneire 2007
- Rekeneire N, Rooks RN, Simonsick EM, Shorr RI, Kuller LH, Schwartz AV, et al. Racial differences in glycemic control in a well‐functioning older diabetic population: Findings from the Health, Aging and Body Composition study. Diabetes Care 2007;26:1986‐92. [DOI] [PubMed] [Google Scholar]
Debray 2017
- Debray TP, Damen JA, Snell KI, Ensor J, Hooft L, Reitsma JB, et al. A guide to systematic review and meta‐analysis of prediction model performance. BMJ 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
DeFronzo 1989
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387‐95. [PUBMED: 2657323] [DOI] [PubMed] [Google Scholar]
DeFronzo 2011
- DeFronzo RA, Abdul‐Ghani MA. Preservation of beta‐cell function: the key to diabetes prevention. Journal of Clinical Endocrinology and Metabolism 2011;96:2354‐66. [DOI] [PubMed] [Google Scholar]
Dretzke 2014
- Dretzke J, Ensor J, Bayliss S, Hodgkinson J, Lordkipanidze M, Riley RD, et al. Methodological issues and recommendations for systematic reviews of prognostic studies: an example from cardiovascular disease. Systematic Reviews 2014;3:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eastwood 2016
- Eastwood SV, Tillin T, Mayet J, Shibata DK, Wright A, Heasman J, et al. Ethnic differences in cross‐sectional associations between impaired glucose regulation, identified by oral glucose tolerance test or HbA1c values, and cardiovascular disease in a cohort of European and South Asian origin. Diabetic Medicine 2016;33:340‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Echouffo‐Tcheugui 2016
- Echouffo‐Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta‐analysis. Diabetic Medicine 2016;33(12):1615‐24. [DOI] [PubMed] [Google Scholar]
Erqou 2013
- Erqou S, Lee CT, Suffoletto M, Echouffo‐Tcheugui JB, Boer RA, Melle JP, et al. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta‐analysis. European Journal of Heart Failure 2013;15(2):185‐93. [DOI] [PubMed] [Google Scholar]
Ford 2010
- Ford ES, Zhao G, Li C. Pre‐diabetes and the risk for cardiovascular disease: a systematic review of the evidence. Journal of the American College of Cardiology 2010;55(13):1310‐7. [DOI] [PubMed] [Google Scholar]
Freeman 1950
- Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals of Mathematical Statistics 1950;21:607‐11. [Google Scholar]
Gale 2013
- Gale EA. Can NICE prevent diabetes?. Heart 2013;99:824‐6. [DOI] [PubMed] [Google Scholar]
Gerstein 2007
- Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta‐analysis of prospective studies. Diabetes Research and Clinical Practice 2007;78(3):305‐12. [DOI] [PubMed] [Google Scholar]
Gosmanov 2014
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. American Journal of the Medical Sciences 2014;348(3):191‐4. [PUBMED: 24556928] [DOI] [PubMed] [Google Scholar]
Hanson 2014
- Hanson RL, Muller YL, Kobes S, Guo T, Bian L, Ossowski V, et al. A genome‐wide association study in American Indians implicates DNER as a susceptibility locus for type 2 diabetes. Diabetes 2014;63(1):369‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasselblad 1994
- Hasselblad VV, McCrory DCD. Meta‐analytic tools for medical decision making: a practical guide. Medical Decision Making 1994;15(1):81‐96. [DOI] [PubMed] [Google Scholar]
Hayden 2013
- Hayden JA, Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Annals of Internal Medicine 2013;158(4):280‐6. [DOI] [PubMed] [Google Scholar]
Hemmingsen 2016a
- Hemmingsen B, Krogh J, Metzendorf MI, Richter B. Sodium‐glucose cotransporter (SGLT) 2 inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012106.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemmingsen 2016b
- Hemmingsen B, Krogh J, Metzendorf MI, Richter B. Dipeptidyl‐peptidase (DPP)‐4 inhibitors or glucagon‐like peptide (GLP)‐1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemmingsen 2016c
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012151.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 2012
- Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. Journal of Clinical Endocrinology & Metabolism 2012;97(4):1067‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172:137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Section 9.4.8: Meta‐analysis of counts and rates. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hope 2016
- Hope C, Robertshaw A, Cheung KL, Idris I, English E. Relationship between HbA1c and cancer in people with or without diabetes: a systematic review. Diabetic Medicine 2016;33(8):1013‐25. [DOI] [PubMed] [Google Scholar]
Huang 2014a
- Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta‐analysis. Diabetologia 2014;57(11):2261‐9. [DOI] [PubMed] [Google Scholar]
Huang 2014b
- Huang Y, Cai X, Chen P, Mai W, Tang H, Huang Y, et al. Associations of prediabetes with all‐cause and cardiovascular mortality: a meta‐analysis. Annals of Medicine 2014;46(8):684‐92. [DOI] [PubMed] [Google Scholar]
Huang 2016
- Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta‐analysis. BMJ 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huguet 2013
- Huguet A, Hayden JA, Stinson J, McGrath PJ, Chambers CT, Tougas ME, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Systematic Reviews 2013;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. PA 19063‐2043 USA: Barnett International/PAREXEL, 1997.
IEC 2009
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32(7):1327‐34. [PUBMED: 19502545] [DOI] [PMC free article] [PubMed] [Google Scholar]
IntHout 2016
- IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inzucchi 2012
- Inzucchi SE. Clinical practice. Diagnosis of diabetes. New England Journal of Medicine 2012;367(6):542‐50. [PUBMED: 22873534] [DOI] [PubMed] [Google Scholar]
Iorio 2015
- Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
Jensen 2002
- Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta‐cell function is a major contributor to oral glucose tolerance in high‐risk relatives of four ethnic groups in the U.S. Diabetes 2002;51(7):2170‐8. [PUBMED: 12086947] [DOI] [PubMed] [Google Scholar]
Jowett 2009
- Jowett JB, Diego VP, Kotea N, Kowlessur S, Chitson P, Dyer TD, et al. Genetic influences on type 2 diabetes and metabolic syndrome related quantitative traits in Mauritius. Twin Research and Human Genetics 2009;12(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012
- Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre‐diabetes on future risk of stroke: meta‐analysis. BMJ 2012;344:e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLOS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Likhari 2010
- Likhari T, Gama R. Ethnic differences in glycated haemoglobin between White subjects and those of South Asian origin with normal glucose tolerance. Journal of Clinical Pathology 2010;63:278‐80. [DOI] [PubMed] [Google Scholar]
Lind 2009
- Lind M, Oden A, Fahlen M, Eliasson B. The true value of HbA1c as a predictor of diabetic complications: simulations of HbA1c variables. PLOS ONE 2009;4:e4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipska 2017
- Lipska KJ, Krumholz HM. Is hemoglobin A1c the right outcome for studies of diabetes?. JAMA 2017;317:1017‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maruthur 2011
- Maruthur NM, Kao WH, Clark JM, Brancati FL, Cheng CY, Pankow JS, et al. Does genetic ancestry explain higher values of glycated hemoglobin in African Americans?. Diabetes 2011;60(9):2434‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moons 2014
- Moons KG, Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Medicine 2014;11(10):e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2013
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0‐6.4% and other prediabetes definitions to type 2 diabetes: a meta‐analysis. Diabetologia 2013;56(7):1489‐93. [PUBMED: 23584433] [DOI] [PubMed] [Google Scholar]
Mostafa 2011
- Mostafa SA, Khunti K, Srinivasan BT, Webb D, Davies MJ. Detecting type 2 diabetes and impaired glucose regulation using glycated hemoglobin in different populations. Diabetes Management 2011;1:77‐97. [Google Scholar]
Nair 2015
- Nair AK, Baier LJ. Complex genetics of type 2 diabetes and effect size: what have we learned from isolated populations?. Review of Diabetic Studies 2015;12(3‐4):299‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2007
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753‐9. [DOI] [PubMed] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28(12):1039‐57. [PUBMED: 510803] [DOI] [PubMed] [Google Scholar]
Newcombe 1998
- Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine 1998;17:857‐72. [DOI] [PubMed] [Google Scholar]
Parrinello 2016
- Parrinello CM, Sharrett AR, Maruthur NM, Bergenstal RM, Grams ME, Coresh J, et al. Racial differences in and prognostic value of biomarkers of hyperglycemia. Diabetes Care 2016;39:4589‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perreault 2012
- Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, et al. Effect of regression from prediabetes to normal glucose regulation on long‐term reduction in diabetes risk: results from the diabetes prevention program outcomes study. Lancet 2012;379:2243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perreault 2014
- Perreault L, Temprosa M, Mather KJ, Horton E, Kitabchi A, Larkin M, et al. Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the diabetes prevention program outcomes study. Diabetes Care 2014;37:2622‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Riley 2015
- Riley RD, Elia EG, Malin G, Hemming K, Price MP. Multivariate meta‐analysis of prognostic factor studies with multiple cut‐points and/or methods of measurement. Statistics in Medicine 2015;34:2481‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos‐Oliveira 2011
- Santos‐Oliveira R, Purdy C, Silva MP, dos Anjos Carneiro‐Leao AM, Machado M, Einarson TR. Haemoglobin A1c levels and subsequent cardiovascular disease in persons without diabetes: a meta‐analysis of prospective cohorts. Diabetologia 2011;54(6):1327‐34. [DOI] [PubMed] [Google Scholar]
Sarwar 2010
- Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, Forouhi NG, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLOS Medicine 2010;7(5):e1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schottker 2016
- Schottker B, Rathmann W, Herder C, Thorand B, Wilsgaard T, Njolstad I, et al. HbA1c levels in non‐diabetic older adults ‐ No J‐shaped associations with primary cardiovascular events, cardiovascular and all‐cause mortality after adjustment for confounders in a meta‐analysis of individual participant data from six cohort studies. BMC Medicine 2016;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selvin 2011
- Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross‐sectional analysis of community‐based data. Annals of Internal Medicine 2011;154(5):303‐9. [PUBMED: 21357907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Serjeantson 1983
- Serjeantson SW, Owerbach D, Zimmet P, Nerup J, Thoma K. Genetics of diabetes in Nauru: effects of foreign admixture, HLA antigens and the insulin‐gene‐linked polymorphism. Diabetologia 1983;25(1):13‐7. [DOI] [PubMed] [Google Scholar]
Stata 2015 [Computer program]
- StataCorp LP. Stata. Version 14. College Station (TX): StataCorp LP, 2015.
Taylor 2017
- Taylor R, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Clinical and metabolic features of the randomised controlled Diabetes Remission Clinical Trial (DiRECT) cohort. Diabetologia 2017;30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Twito 2015
- Twito O, Frankel M, Nabriski D. Impact of glucose level on morbidity and mortality in elderly with diabetes and pre‐diabetes. World Journal of Diabetes 2015;6(2):345‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viera 2011
- Viera AJ. Predisease: when does it make sense?. Epidemiologic Reviews 2011;33:122‐34. [DOI] [PubMed] [Google Scholar]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539‐53. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. WHO, 1999. [Google Scholar]
WHO/IDF 2006
- World Health Organization/ International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. WHO, 2006. Available from www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf Vol. (assessed 3 March 2018).
Wilczynski 2004
- Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. BMC Medicine 2004;2(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilczynski 2005
- Wilczynski NL, Haynes RB. Optimal search strategies for detecting clinically sound prognostic studies in EMBASE: an analytic survey. Journal of the American Medical Informatics Association 2005;12(4):481‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2015
- Xu T, Liu W, Cai X, Ding J, Tang H, Huang Y, et al. Risk of coronary heart disease in different criterion of impaired fasting glucose: a meta‐analysis. Medicine 2015;94(40):e1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yakubovich 2012
- Yakubovich N, Gerstein HC. Is regression to normoglycaemia clinically important?. Lancet 2012;379:2216‐8. [DOI] [PubMed] [Google Scholar]
Yudkin 1990
- Yudkin JS, Alberti KG, McLarty DG, Swai AB. Impaired glucose tolerance. BMJ 1990;301:397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yudkin 2014
- Yudkin JS, Montori VM. The epidemic of pre‐diabetes: the medicine and the politics. BMJ 2014;349:g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yudkin 2016
- Yudkin JS. "Prediabetes": Are there problems with this label? Yes, the label creates further problems!. Diabetes Care 2016;39:1468‐71. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang X, Gregg EW, Williamson DF, Barker L E, Thomas W, Bullard KM, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care 2010;33(7):1665‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012a
- Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta‐analysis. PLOS ONE 2012;7(8):e42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhong 2016
- Zhong GC, Ye MX, Cheng JH, Zhao Y, Gong JP. HbA1c and risks of all‐cause and cause‐specific death in subjects without known diabetes: a dose‐response meta‐analysis of prospective cohort studies. Scientific Reports 2016;6:24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziemer 2010
- Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose‐independent, black‐white differences in hemoglobin A1c levels: a cross‐sectional analysis of 2 studies. Annals of Internal Medicine 2010;152:770‐7. [DOI] [PubMed] [Google Scholar]


