Abstract
Background
Severe sepsis and septic shock have recently emerged as particularly acute and lethal challenges amongst critically ill patients presenting to the emergency department (ED). There are no existing data on the current practices of management of patients with severe sepsis comparing early versus late administration of appropriate broad spectrum antibiotics as part of the early goal‐directed therapy that is commenced in the first few hours of presentation.
Objectives
To assess the difference in outcomes with early compared to late administration of antibiotics in patients with severe sepsis in the pre‐intensive care unit (ICU) admission period. We defined early as within one hour of presentation to the ED.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2009); MEDLINE (1990 to February 2010); EMBASE (1990 to February 2010); and ISI web of Science (February 2010). We also searched for relevant ongoing trials in specific websites such as www.controlled‐trials.com; www.clinicalstudyresults.org; and www.update‐software.com. We searched the reference lists of articles. There were no constraints based on language or publication status.
Selection criteria
We planned to include randomized controlled trials of early versus late broad spectrum antibiotics in adult patients with severe sepsis in the ED, prior to admission to the intensive care unit.
Data collection and analysis
Two authors independently assessed articles for inclusion.
Main results
We found no studies that satisfied the inclusion criteria.
Authors' conclusions
Based on this review we are unable to make a recommendation on the early or late use of broad spectrum antibiotics in adult patients with severe sepsis in the ED pre‐ICU admission. There is a need to do large prospective double blinded randomized controlled trials on the efficacy of early (within one hour) versus late broad spectrum antibiotics in adult severe sepsis patients. Since it makes sense to start antibiotics as soon as possible in this group of seriously ill patients, administering such antibiotics earlier as opposed to later is based on anecdotal suboptimal evidence.
Plain language summary
To assess the optimal of timing of administering antibiotics to sepsis patients in the emergency department
Sepsis is a serious medical condition characterized by an inflammatory response to an infection that can affect the whole body. The patient may develop an inflammatory response to microbes in their blood, urine, lungs, skin, or other tissues. Sepsis is a serious condition with a very high death rate if left untreated. Most sepsis patients require antibiotics and admission to an intensive care unit (ICU). How soon broad spectrum antibiotics should be delivered is as yet unclear. Broad spectrum antimicrobial treatment is defined as a combination of antibiotics which act against a wide range of disease‐causing bacteria, used to reduce mortality rates in patients with sepsis, severe sepsis or septic shock. We carried out a systematic review of the literature by searching key databases for high quality published and unpublished material on the timing of antibiotics in the emergency department prior to ICU admission. Our searches revealed no randomized controlled trials (RCTs) on the timing of broad spectrum antibiotic treatment in this population. We conclude that there is a need to carry out observational cohort studies, in the absence of RCTs, even if they lack the precision of RCTs. We also conclude that the earlier the antibiotics are administered the better. It is important to realize that the clock starts ticking when the patient arrives in the ED and stops once the antibiotic is started. The pre‐intensive care unit period is the time spent in the ward or ED prior to being admitted to the ICU, where most patients with severe sepsis are admitted. The review was purposefully very specific as it is focused only on patients with severe sepsis and in finding only RCTs. The absence of these may imply, in itself, the complicated nature of the study question as it may be ethically wrong to randomize such patients to a seemingly inferior treatment arm.
Background
The mortality rate for patients with severe sepsis is generally high. The principles of treatment and the approach to sepsis are timely recognition and early treatment. Appropriate antibiotics and source control are the cornerstones of treatment.
Patients who are admitted to the emergency department (ED) with severe sepsis are often not given appropriate broad spectrum antibiotics, as set out in the Surviving Sepsis Guidelines (Dellinger 2008) developed by the Society of Critical Care Medicine. These patients subsequently arrive in the intensive care unit (ICU) in a moribund state with profound shock and multi‐organ failure (Besselink 2009). A variety of factors may contribute to this state. These include how long the infection has been present, other host factors (such as age, general health status etc.) and how long it takes to reach an ED. Better understanding of the pathophysiology of sepsis has led to several recommendations aimed at early and aggressive management as an integral part of improving outcomes (Nguyen 2006). The biggest challenge with complicated infections is early recognition of the problem (Bochud 2004).
Standardized regimens using either monotherapy or combination therapy have proven their efficacy, as shown in recent recommendations from large‐scale prospective studies done in western countries (Adler 2005; Blot 2005). More recent literature points at worse outcomes when appropriate broad spectrum antibiotics are delayed, that is after the first hour of admission (Kumar 2006). This evidence is for septic shock patients, however, and not those with severe sepsis. We set out to review the literature for evidence in patients with severe sepsis. Patients who are admitted with severe sepsis may eventually arrive in ICU in a non‐salvageable state, partly due to a delay in administration of antibiotics (Tsolia 2003). The Surviving Sepsis Campaign (Dellinger 2008; Rivers 2006) was created in 2004 and incorporated evidence‐based guidelines to reduce mortality from severe sepsis and septic shock (Morimatsu 2004). Our review specifically looked at the timing of administration of antibiotics.
As the mechanisms of sepsis become better defined, many evidence‐based recommendations centred on pathophysiological principles indicate that early, systematic supportive therapy can improve outcomes, most notably goal‐directed therapy that includes early identification, aggressive fluid resuscitation and broad spectrum antibiotics (Morimatsu 2004). The recommendations for low‐dose hydrocortisone and activated protein C (Grimaldi 2004) to disrupt inflammatory cascades and favourably influence the course of the disease may be impractical and expensive in developing countries. Efforts are required to better define nationwide epidemiology and treatment practices for severe sepsis (Dellinger 2004). We planned to look at the timing of administration of broad spectrum antibiotics in the pre‐intensive care setting to see if early (within one hour of presentation), appropriate broad spectrum antibiotics changed the mortality rate of patients with severe sepsis (Fadel 2008; Kula 2009). The economic aspect of such studies and decisions are also an integral part of the outcomes for this review.
Objectives
The objective of this review was to assess the difference in outcomes with early compared to late administration of antibiotics in patients with severe sepsis. We defined 'early' as within one hour of presentation to the emergency department (ED).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs).
Only those studies in which the difference between the two arms was the timing of antibiotics, and not any other aspect of intervention, were to be included.
Types of participants
We planned to include all patients above the age of 16 years presenting to ED with 'severe sepsis' diagnosed according to the current Society of Critical Care Medicine (SCCM) definitions (Montravers 2006), that is the systemic inflammatory response syndrome (SIRS) criteria plus organ failure or haemodynamic instability.
We planned to exclude paediatric patients and pregnant women as other confounding factors such as microbial heterogeneity may obscure the results. Also, the surviving sepsis guidelines differ for a paediatric population. We also excluded 'septic shock' and 'early sepsis' patients as early broad spectrum antibiotics are a foregone conclusion in one and not recommended so soon in another.
Types of interventions
Early (within one hour of ED admission) versus late (defined as greater than one hour after admission) administration of broad spectrum antibiotics.
Late administration of antibiotics was independent of microbiology cultures, waiting for cultures, or both.
We did not study the types of antibiotics used.
Types of outcome measures
Primary outcomes
1. Mortality at 28 days 2. Length of stay in hospital
Secondary outcomes
1. Development of multi‐organ failure (such as renal failure, hepatic insufficiency, coma etc) 2. Length of ICU stay
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 6), see Appendix 1; Ovid MEDLINE (from 1990 to February 2010), see Appendix 2; EMBASE via Ovid (1990 to February 2010), see Appendix 3; and the ISI Web of Science (1990 to February 2010) see Appendix 4. We started the searches from 1990 as the 'Surviving Sepsis' campaign formulated the guidelines of early, goal‐directed therapy then, which included early antibiotics. Literature from before then would have been out of date with regard to current guidelines.
For our subject search we combined the "terms for severe sepsis" AND "terms for antibiotics" AND "timing" and "terms for ICU" AND (RCT filter). We combined the MEDLINE search strategy with the Cochrane highly sensitive search strategy for identifying RCTs, contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
We searched for relevant ongoing trials on specific websites: 1) www.controlled‐trials.com; 2) www.clinicalstudyresults.org; 3) www.update‐software.com.
Searching other resources
We identified trials by manually searching abstracts of relevant conference proceedings; checking the reference list of relevant articles; contacting relevant trial authors to identify any additional studies; and contacting relevant hospitals and pharmaceutical companies for published and unpublished reports.
We did not apply language or publication restrictions.
Data collection and analysis
Trial identification
We (SS and JR) independently scanned the titles and abstracts of reports identified by the electronic searches, manual handsearching, and through contact with experts and trial authors.
Trial selection
We independently selected trials that met the inclusion criteria using a checklist designed specifically for this purpose.
Quality assessment
We judged the quality of trials by following the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), on the basis of the following.
1. Method of randomization. 2. Method of allocation concealment:
i. low risk of bias ‐ adequate allocation concealment i.e. central randomization or equivalent, described fully; ii. moderate risk of bias ‐ unclear allocation concealment, the author did not give details of concealment (randomization); iii. high risk of bias ‐ inadequate allocation concealment, such as changes to randomization.
3. Blinding of treatment or outcome assessment. 4. Completeness of follow up.
Data extraction
We (SS and JR) planned to independently extract data using the data extraction form given in Grimaldi 2004 (see Appendix 5). We planned to resolve any disagreements by discussion. We were not blinded with regard to the names of the study authors, investigators, institutions and results. We intended to identify the excluded trials and list the reasons for exclusion.
Statistics
With help from the Statistics Department of Aga Khan University, Pakistan, we were to review the data extracted from the trials on population, intervention and outcomes first qualitatively and then quantitatively using the Cochrane Collaboration's software Review Manager (RevMan 5.0).
We planned to base quantitative analyses on intention‐to‐treat results. In the case of clinical heterogeneity we would not pool the results.
We planned to explore clinical and statistical heterogeneity (the latter using the I2 statistic) (Higgins 2008). We planned to perform a meta‐analysis with a sensitivity analysis based on trial quality. We planned to undertake the following subgroup analyses based on:
1. site of infection; 2. type of pathogen; 3. whether patients had already received antibiotics outside the hospital;
4. comorbidities that lead to immunosuppression, such as diabetes mellitis.
Patients with intra‐abdominal sepsis are often taken to the operating room straight away and so may not receive antibiotics until during the procedure. Patients who received antibiotics outside the hospital may not be given a repeat dose or may have microbiological cultures that are misrepresentative.
For dichotomic outcomes we intended to calculate relative risk (RR) and 95% confidence intervals (CIs). We planned to calculate the standardized difference in means for continuous outcomes.
Results
Description of studies
We searched the databases (to February 22nd 2010). Our search yielded 590 hits (see Figure 1). After scrutiny of the study abstracts, no study fulfilled the inclusion criteria. They were either not relevant or not RCTs. We looked at newspapers and conference proceedings but this showed only 10 hits, which failed to contribute any relevant published or unpublished studies. The search of the Internet (PubMed, Yahoo and Google) using 'severe sepsis' and 'antibiotics' and 'timing' as a keyword identified 100 articles, all of which were excluded as they were either not RCTs or were not relevant.
1.
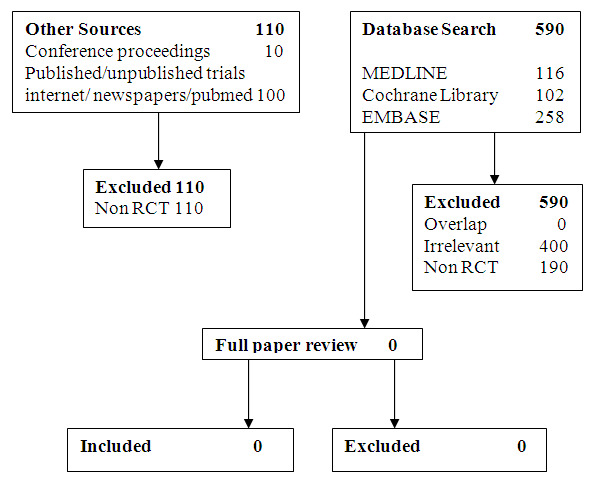
Searching flow diagram
Risk of bias in included studies
There were no eligible studies.
Effects of interventions
There were no eligible studies.
Discussion
This review failed to uncover any evidence from prospective, randomized or quasi‐randomized trials on the effectiveness of early broad spectrum antibiotics in adult patients with severe sepsis, before admission into the ICU. This is an important question to review, to see if the literature exists to support a recommendation for the use of pre‐intensive care broad spectrum antibiotics and to determine if there is a need for such literature, as is clearly demonstrated by our review. Given the severity of the condition, the high mortality rate (Fadel 2008) and the haste in delivering antibiotics, this lack of evidence on the timing of antibiotics is perhaps unsurprising (Besselink 2009). Despite modern day advances in therapeutics and diagnostic tests, severe sepsis remains a serious condition with a high mortality rate. Treatment focuses on early goal‐directed therapy, with antibiotics as part of source control. However, the optimal timing of antibiotic delivery is yet to be ascertained. It makes sense to administer them as early as possible but other considerations, whilst awaiting objective evidence such as microbial cultures, such as spread of resistance with unnecessary broad spectrum antibiotics, cost and side effects as well as reduction in immunity may hamper this hypothesis. We endeavoured to find level 1 evidence from randomized controlled double blinded trials in this field, however we could not find any such studies.
Initiating effective antibiotic therapy is proven to lead to better outcomes in severe sepsis and shock. The Surviving Sepsis Campaign, developed in 2004, incorporated evidence‐based guidelines to reduce mortality from severe sepsis and septic shock (Dellinger 2008). These guidelines include the early initiation of broad spectrum antimicrobials, that is within one hour of recognition of sepsis (Kumar 2006). However, too early use of antibiotics can lead to excessive, unnecessary use as many other disease states such as myocardial infarction or pulmonary embolism can mimic severe sepsis. The biggest challenge in sepsis is early recognition of the problem. The presentation of severe sepsis and septic shock can initially be non‐specific but can progress within hours to fulminant multiple organ failure and death. Patients presenting to the emergency department with sepsis may not receive timely or appropriate antibiotics since the diagnoses of systemic inflammatory response syndrome (SIRS) and sepsis are often missed. Delays in the identification, transfer and management of critically ill patients during the first six hours after admission have been associated with higher mortality rates and increased utilization of hospital resources (Ruokonen 2009). Antimicrobial selection is often random and erratic.
Delaying antibiotic administration may be related to worsened clinical outcomes. Patients eventually arrive in the intensive care unit in a moribund state with profound shock and multi‐organ failure. Better understanding of the pathophysiology of sepsis has led to recommendations that target both early and goal‐directed management to improve outcomes. The timeliness of treatment became apparent after Rivers et al showed a significant mortality benefit when haemodynamic optimization was provided within the first few hours of disease presentation (Rivers 2006). These ideals have been incorporated into the Surviving Sepsis Campaign, a multi‐national initiative which recommends a 24‐hour sepsis pathway that includes a critical six‐hour course of action. Results of studies, predominantly from Europe and North America, document the mortality and morbidity benefits of both early and appropriate antimicrobials. Kollef et al, in their landmark paper on 2000 patients with both community‐acquired and nosocomial infections, demonstrated that inadequate antimicrobial treatment of infection was the most important independent determinant of hospital mortality for the entire patient cohort (adjusted odds ratio 4.27; 95% CI 3.35 to 5.44; P < 0.001) (Kumar 2006 ). Observational studies suggest a significant reduction in mortality when antibiotics are administered within four to eight hours of hospital presentation (P < 0.01). In these studies they were able to demonstrate a statistically significant relationship between early administration of antibiotics and survival, the crude odds ratio of 0.70 (95% CI 0.63 to 0.98; P = 0.04) indicated a protective effect when antibiotics were given early. Kumar conducted a study (Kumar 2006) which showed a 5% to 10% mortality. This was a multi‐centre, retrospective study which only looked at hypotensive patients. Since our review focuses on all severe sepsis patients, and not just patients in shock, the Kumar study may not encompass our cohort. The basis for the timing of antibiotics in the Surviving Sepsis Guidelines (Dellinger 2008) is this study, which is for septic shock patients and is appropriate given the ethics involved and the urgency of the situation. The guidelines for severe sepsis state 'within six hours of presentation' and are based on non‐RCT studies.
Unfortunately guidelines are not always immediately incorporated. There are delays in recognition of disease states and in institution of therapy, especially in the emergency room setting where patient volumes and time constraints put additional burdens on the care providers. Since there may be serious ethical issues involved with implementing an RCT on this topic, as randomizing a patient to a potentially harmful intervention (late administration of antibiotics) in a potentially fatal condition such as severe sepsis may not be a feasible option, we suggest a review of non‐randomized, observational cohort trials in the future. There are many barriers to commencement of treatment for such patients, such as physician failure, system delays and work‐up delays as well as recognition of the pathophysiology. Treatment should ideally be started prior to admission in order to save time. Timely intervention is key, thereby decreasing mortality and morbidity.
Authors' conclusions
Implications for practice.
We found no relevant evidence for early use of broad spectrum antibiotics in severe sepsis. We are, therefore, unable to make any recommendations based on the findings of this review.
Implications for research.
Given the seriousness of the condition and high associated mortality, there is a need for further studies. Non‐RCTs may be acceptable to show any improvement in outcomes. Any future trials would need to look at the appropriate sample size, ethics of randomization and appropriate outcome measures. We also recommend research on quality improvement strategies to facilitate timely administration of antibiotics in the emergency department. Research on implementing the sepsis bundle of care is the other direction for research into.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 17 April 2012 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Dr Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia Review Group (CARG)) for help in running the searches and Jane Cracknell (Managing Editor, CARG) for her expert guidance.
We would like to thank Mr Iqbal Azam of the Department of Statistics, Aga Khan University in Pakistan, who will help with statistical analyses not included in Review Manager and will be available for statistical help with the meta‐analysis, such as calculating relative risks, confidence intervals and odds ratios.
We would like to thank Dr Nicola Petrucci (content editor), Dr Marissa M Alejandria, Dr Mazen Bader (peer reviewers), Nathan Grimm and Karl Gallegos (Cochrane Consumer Network) for their help and editorial advice during the preparation of the systematic review.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 . MeSH descriptor Antibiotic Prophylaxis explode all trees #2 . (broad spectrum) near antibiotic* #3 . MeSH descriptor Anti‐Bacterial Agents, this term only #4 . MeSH descriptor Cephalosporins, this term only #5 . MeSH descriptor Aminoglycosides, this term only #6 . MeSH descriptor Vancomycin explode all trees #7 . MeSH descriptor Carbapenems explode all trees #8 . carbapenem* or vancomycin #9 . (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 . MeSH descriptor Sepsis explode all trees #11 . MeSH descriptor Shock, Septic explode all trees #12 . MeSH descriptor Systemic Inflammatory Response Syndrome explode all trees #13 . (septic* or sepsis*):ti,ab #14 . Severe near sepsis #15 . shock near septic #16 . (#1o OR #11 OR #12 OR #13 OR #14 OR #15) #17 . (#9 AND #16) #18 . MeSH descriptor Intensive Care explode all trees #19 . MeSH descriptor Intensive Care Units explode all trees #20 . MeSH descriptor Emergency Medical Services explode all trees #21 . MeSH descriptor Emergency Medicine explode all trees #22 . MeSH descriptor Emergency Service, Hospital explode all trees #23 . (emergency near department*) #24 . (intensive near (care or unit)) #25 . (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 . (#17 AND #25) #27 . ("child*" not ("adult*" and "child*") #28 . (#26 AND NOT #27)
Appendix 2. MEDLINE (Ovid SP) search strategy
1 . exp Antibiotic Prophylaxis/ 2 . (broad spectrum adj3 antibiotic*).mp. 3 . Anti‐Bacterial‐Agents/ 4 . Cephalosporins/ or Aminoglycosides/ 5 . exp Vancomycin/ or vancomycin.mp. 6 . exp Carbapenems/ or carbapenem*.mp. 7 . 1 or 2 or 3 or 4 or 5 or 6 8 . exp Sepsis/ or exp Shock, Septic/ or exp Sepsis‐Syndrome/ or exp Septicemia/ 9 . (septic* or sepsis*).ti,ab. 10 . (Severe adj3 sepsis).mp. 11 . (shock adj3 septic).mp. 12 . 8 or 11 or 10 or 9 13 . 7 and 12 14 . exp Intensive‐Care/ or exp Intensive‐Care‐Units/ or exp Emergency‐Medical‐Services/ or Emergency‐Medicine/ or Emergency‐Service‐Hospital/ 15 . (emergency adj3 department*).mp. 16 . (intensive adj3 (care or unit)).mp. 17 . 16 or 15 or 14 18 . 13 and 17 19 . ("child*" not ("adult*" and "child*")).sh,kw. 20 . 18 not 19 21 . ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 22 . 21 and 20
Appendix 3. Search strategy for EMBASE (Ovid SP)
1 . exp Antibiotic Prophylaxis/ 2 . (broad spectrum adj3 antibiotic*).mp. 3 . Antiinfective Agent/ 4 . Cephalosporins/ or Aminoglycosides/ 5 . exp Vancomycin/ or vancomycin.mp. 6 . exp Carbapenems/ or carbapenem*.mp. 7 . 1 or 2 or 3 or 4 or 5 or 6 8 . exp Sepsis/ 9 . exp Septic Shock/ 10 . exp Septicemia/ 11 . (septic* or sepsis*).ti,ab. 12 . (Severe adj3 sepsis).mp. 13 . (shock adj3 septic).mp. 14 . 8 or 11 or 13 or 10 or 9 or 12 15 . 7 and 14 16 . exp Intensive Care/ 17 . exp Intensive Care Unit/ 18 . exp Emergency Health Service/ 19 . exp Emergency Medicine/ 20 . (emergency adj3 department*).mp. 21 . (intensive adj3 (care or unit)).mp. 22 . 21 or 18 or 19 or 16 or 17 or 20 23 . 22 and 15 24 . ("child*" not ("adult*" and "child*")).sh,tw. 25 . 23 not 24 26 . (RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/ or (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER* or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab.) not (animals not humans and animals).sh. 27 . 25 and 26
Appendix 4. Search strategy for ISI Web of Science
#1. TS=(Antibiotic SAME Prophylaxis) or TS=((broad spectrum) SAME antibiotic*) or TS=(Cephalosporin* or Aminoglycoside* or vancomycin or carbapenem*) #2. TS=(septic* or sepsis*) #3. #2 AND #1 #4. TS=(intensive SAME (care or unit)) or TS=emergency #5. #4 AND #3 #6. TS=child* NOT TS=(adult* and child*) #7. #5 NOT #6 #8. (TS=random* or TS=((clinical or controlled) SAME trial) or TS=((CONTROLLED or MULTICENTER) SAME STUDY) or TS=(CROSSOVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) or TS=((SINGL* or DOUBL* or TREBL* or TRIPL*) SAME (BLIND* or MASK*))) NOT (TS=(animal* not (human* and animal*))) #9. #8 AND #7
Appendix 5. Data extraction form
MEDLINE Journal ID: (as per article)
Year of publication Language: Type of study: RCT CCT CS Comments on design of study: Quality of randomization/concealment: D Allocation was not concealed (e.g. quasi randomization) C Allocation concealment was inadequate B Methods of concealment not stated clearly A Concealment adequate (e.g. numbered, sealed opaque envelopes drawn non consecutively, or computerized block randomization). Inclusion/ exclusion criteria not clearly defined in methods. Yes/ no Outcomes of patients dropped or excluded after allocation were stated separately OR included in intention to treat analysis OR the text stated there were no exclusions. Yes/ no Treatment and control groups were NOT adequately described at the beginning Yes/ no A minimum of 4 admission details were described Yes/no Methods: Yes/No/Unclear Subject blinded Physician blinded Outcome assessor blinded Participants: No. of eligible participants:No. enrolled in study: No. of males:No. of females: Severity of sepsis: SIRS severe sepsis septic shock Intervention: abxdose time of administration route (hrs) Early antibiotics group (treatment group 1) <1 hour Late treatment group (treatment group 2) > 1 hour Comment on treatment and timing: (Prior antibiotic use Yes/No) Withdrawals: Yes No Unclear Outcomes: LOS (days, mean)Mortality (n, %) Morbidity (n, %) Cost‐ if given ($) Treatment Group 1 Treatment Group 2 Changes in protocol: Yes / No Contact with author: Yes/ No Other comments: Data extraction form DY: Quality of randomization/concealment: D Allocation was not concealed (e.g. quasi randomization) C Allocation concealment was inadequate B Methods of concealment not stated clearly A Concealment adequate (e.g. numbered, sealed opaque envelopes drawn non consecutively, or computerized block randomization). Inclusion/ exclusion criteria not clearly defined in methods. Yes/ no Outcomes of patients dropped or excluded after allocation were stated separately OR included in intention to treat analysis OR the text stated there were no exclusions. Yes/ no Treatment and control groups were NOT adequately described at the beginning Yes/ no A minimum of 4 admission details were described Yes/no
Differences between protocol and review
We did not search the LILACS database as stated in the published protocol. This was due to the difficulty in accessing it.
Contributions of authors
Conceiving the review: Shahla Siddiqui (SS), Junaid Razzak (JR) Designing the review: SS, JR Co‐ordinating the review: SS Undertaking manual searches: SS, JR Screening search results: SS Organizing retrieval of papers: SS Screening retrieved papers against inclusion criteria: SS Appraising quality of papers: SS, JR Abstracting data from papers: SS, JR Writing to authors of papers for additional information: SS Providing additional data about papers: SS Obtaining and screening data on unpublished studies: SS Data management for the review: SS Entering data into Review Manager (RevMan 5.0): SS, JR RevMan statistical data: SS Other statistical analysis not using RevMan: MrIqbal Azam, Dept of Statistics, AKUH, Pakistan Double entry of data: (data entered by person one: SS) Interpretation of data: SS, JR Statistical analysis: SS, MrIqbal Azam, Dept of Statistics, AKUH Writing the review: SS Securing funding for the review: SS, JR Performing previous work that was the foundation of the present study: SS Guarantor for the review (one author): SS Person responsible for reading and checking review before submission: SS, JR
Sources of support
Internal sources
Department of Anaesthesia and Critical Care, AKUH, Karachi, Pakistan.
Mr Iqbal Azam, department of statistics, AKUH, Karachi, Pakistan.
External sources
-
The Aga Khan University, Pakistan.
Non‐monetary support in the form of computer access, personnel for help and software access.
Declarations of interest
None known
Edited (no change to conclusions)
References
Additional references
Adler 2005
- Adler G, Woehrle H. Diagnosis and therapy of acute pancreatitis. Internist 2005;46(2):131‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Besselink 2009
- Besselink MG, Santvoort HC, Boermeester MA, Nieuwenhuijs VB, Goor H, Dejong CH, et al. Timing and impact of infections in pancreatitis. The British Journal of Surgery 2009;96(3):267‐73. [DOI] [PubMed] [Google Scholar]
Blot 2005
- Blot S, DeWaele JJ. Critical issues in the clinical management of complicated intra‐abdominal infections. Drugs 2005;65(12):1611‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bochud 2004
- Bochud PY, Bonten M, Marchetti O, Calandra T. Antimicrobial therapy for patients with severe sepsis and septic shock: an evidence‐based review. Critical Care Medicine Nov 2004;32:495‐512. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dellinger 2004
- Dellinger RP, Carlet JM, Gerlach H, Ramsey G, Levy M. The surviving sepsis guidelines: not another "groundhog day". Intensive Care Medicine 2004;32:1601‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dellinger 2008
- Dellinger RP, Vincent JL. The Surviving Sepsis Campaign: sepsis change bundles and clinical practice. Critical Care 2008;9:653‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fadel 2008
- Fadel MV, Repka JC, Cunha CL, Leão MT. Inadequate timing between corticosteroid and antibiotics applications increases mortality due to sepsis. The Brazilian Journal of infectious Diseases 2008;12(5):416‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grimaldi 2004
- Grimaldi D, Caille V, Vieillard‐Baron A, Bossi P. [Treatment of severe sepsis and septic shock]. La Presse Médicale 2004;33(4):265‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kula 2009
- Kula R, Chylek V, Szturz P, Sklienka P, Sukenik P, Tichy J, et al. A response to infection in patients with severe sepsis‐‐do we need a "stage‐directed therapy concept"?. Bratislavské Lekárske Listy 2009;110‐8:459‐64. [MEDLINE: ] [PubMed] [Google Scholar]
Kumar 2006
- Kumar A, Roberts D, Wood K E, Light B, Parrillo J E, Sharma S, et al. Duration of hypotension before initiation of effective. Critical Care Medicine 2006;34(6):1589‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montravers 2006
- Montravers P, Quintard H, Piednoir P. [Activated proteine C]. Annales Françaises d'Anesthèsie et de Rèanimation 2006;25:270‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morimatsu 2004
- Morimatsu H, Singh K, Uchino S, Bellomo R, Hart G. Early and exclusive use of norepinephrine in septic shock. Resuscitation 2004;62(2):249‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nguyen 2006
- Nguyen HB, Corbett SW, Menes K, Cho T, Daugharthy J, Klein W, et al. Early goal‐directed therapy, corticosteroid, and recombinant human activated protein C for the treatment of severe sepsis and septic shock in the emergency department. Academic Emergency Medicine 2006;13(1):109‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.0 [Computer program]
- Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: Cochrane Collaboration, 2008.
Rivers 2006
- Rivers EP, McIntyre, L Morro DC, Rivers KK. The outcome of patients presenting to the emergency department with severe sepsis or septic shock. Critical Care 2006;10(4):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruokonen 2009
- Ruokonen E, Hovilehto S, Loisa P, Perttilä J, Pettilä V, Puurunen M, et al. Update on current care guidelines. Treatment of severe sepsis in adults. Duodecim 2009;125:2402‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Tsolia 2003
- Tsolia MN, Kafetzis D, Danelatou K, Astral H, Kallergi K, Spyridis P, et al. Epidemiology of respiratory syncytial virus bronchiolitis in hospitalized infants in Greece. European Journal of Epidemiology 2003;18(1):55‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


