Abstract
Myeloproliferative neoplasms (MPNs) are rare, yet potentially life-threatening, disorders caused by overproliferation of bone marrow stem cells. The symptom burden experienced by patients with the BCR-ABL1-negative MPNs (also referred to as the classical MPNs, i.e., essential thrombocythemia [ET], polycythemia vera [PV] and myelofibrosis [MF]) can be significant and can negatively impact quality of life (QOL). Since patients with these MPNs can live for several years, thereby requiring long-term treatment and follow-up, nurses play an essential role in communicating with these patients, assessing their symptoms, and educating them on treatments and self-management strategies that can reduce their symptom burden. This article, which is the first of a two-part series, was developed to provide nurses and other healthcare professionals with a review of the diagnosis and treatment of the most common classical MPNs. The second article in this series (also available in this issue) will provide nurses with practical guidance for managing the symptom burden associated with MPNs in order to help enhance the overall health and well-being of patients living with these disorders.
Keywords: myeloproliferative neoplasms, essential thrombocythemia, polycythemia vera, myelofibrosis, diagnosis, treatment, nursing management
INTRODUCTION
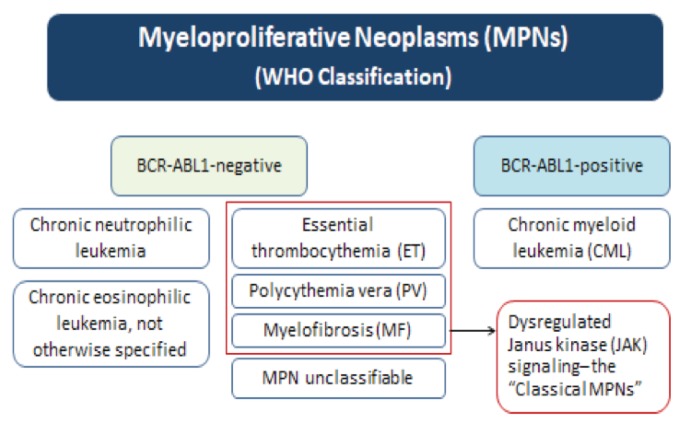
Myeloproliferative neoplasms (MPNs) are a closely related group of rare, yet potentially life-threatening, disorders caused by overproliferation of bone marrow stem cells. In these disorders, blood cell production (hematopoiesis) in the bone marrow becomes defective, resulting in the production of too many or too few blood cells. The World Health Organization (WHO) classifies MPNs as either BCR-ABL1-positive or negative, depending on the presence or absence of this fusion gene (see Figure 1) (Arber et al., 2016). Chronic myelogenous leukemia (CML) is the most common BCR-ABL1-positive MPN. Since there is extensive literature on CML management, this article will focus on the most common BCR-ABL1-negative MPNs. The severity of these BCR-ABL1-negative MPNs can range from mild to aggressive, and can severely affect patient quality of life (QOL) due to debilitating symptoms and an increased risk of thrombotic events. Since many patients with MPNs are well-informed about their disease and available treatment options, given the myriad of resources available online, it is important for nurses to have a solid understanding of these disorders, as well as therapeutic interventions for MPNs. This article—the first of a two-part series—was developed by a group of Canadian nurse practitioners and specialized hematology/oncology nurses to provide nurses and other healthcare professionals with a review of the characteristics, diagnosis and treatment of the most common BCR-ABL1-negative MPNs, as well as the nursing role in the treatment of these disorders. The second article in this series (also available in this issue) will provide nurses with practical guidance on managing the symptom burden associated with the “classical” MPNs.
Figure 1.
Overview of the MPNs
WHAT ARE THE MOST COMMON FORMS OF BCR-ABL1-NEGATIVE MPNS?
The most common BCR-ABL1-negative MPNs (also referred to as the classical MPNs; see Figure 1) are essential thrombocythemia (ET), polycythemia vera (PV) and myelofibrosis (MF). The key characteristics and clinical and molecular features of these classical MPNs are summarized in Table 1.
Table 1.
Characteristics and clinical and molecular features of the classical MPNs
| ET | PV | MF | |
|---|---|---|---|
| Characteristics |
|
|
|
| Molecular abnormalities/mutations |
|
|
|
| Clinical and/or laboratory findings |
|
|
|
ET: essential thrombocythemia; PV: polycythemia vera; MF: myelofibrosis; JAK2: Janus kinase 2; CALR: calreticulin
ET accounts for approximately 25% of the classical MPNs (Rollison et al., 2008) and is characterized by thrombocytosis (sustained elevation of platelets in the blood) and proliferation of enlarged, mature megakaryocytes (bone marrow cells responsible for the production of platelets) (Arber et al., 2016; Tefferi & Barbui, 2015). The excess production of platelets in ET can lead to thrombotic and hemorrhagic complications. Of the classical MPNs, ET is associated with the most favourable prognosis, with median survival ranging between 18 and 20 years, and tends to be more common in females than males (Wolanskyj et al., 2006; Radaelli et al., 2008).
PV is the most common MPN, accounting for approximately 45% of all MPN cases (Rollison et al., 2008). It is characterized by an increased red blood cell (RBC) volume, as indicated by elevated hemoglobin, hematocrit and red cell mass (Tonkin et al., 2012). It may also be associated with leukocytosis (elevated white blood cell counts) and/or thrombocytosis (elevated platelet count), as well as splenomegaly (an enlarged spleen) (Arber et al., 2016; Finazzi & Barbui, 2007). PV is associated with a high risk of thrombosis. The median survival for persons with PV is approximately 15–20 years; however, overall survival varies depending on whether certain prognostic factors are present that have been associated with an increased risk of thrombosis and death, such as age > 60 years, a history of venous thrombosis and leukocytosis (Tefferi et al., 2013). Approximately 10–20% of patients with a PV diagnosis will have their disease progress to MF, and 2–7% will progress to acute myeloid leukemia (AML) (Passamonti et al., 2011).
MF is characterized by debilitating symptoms, splenomegaly and progressive fibrosis or scarring of the bone marrow (Abdel-Wahab & Levine, 2009; Tonkin et al., 2012). This scarring impairs the patient’s ability to produce blood cells. MF can be classified as primary (PMF) or secondary to ET (post-ET MF) or PV (post-PV MF). The manifestations of PMF, post-PV MF and post-ET MF are virtually identical and treatment is generally the same for all three. Transformation to AML occurs most frequently in MF and, in general, patients with MF tend to have a much shorter life span than patients with PV or ET (Geyer & Mesa, 2014). However, prognosis varies depending on whether the patient is sub-classified as low, intermediate-1, intermediate-2, or high-risk based on various factors (see Table 2). At the time of diagnosis, the International Prognostic Scoring System (IPSS) may be used to estimate prognosis in patients with MF. After the diagnosis and for the remainder of the duration of the disease, prognosis can be estimated using the Dynamic IPPS (DIPPS) (Cervantes et al., 2009; Passamonti et al., 2010). The IPSS and DIPSS use the same risk factors for scoring, but differ slightly in how these factors are weighted. Recently, an updated DIPSS-PLUS algorithm has become available which incorporates other independent risk factors (Gangat et al., 2011) (see Table 2), however, it is not yet as commonly used as the DIPSS. While overall survival for MF patients in the low-risk (0 points) and intermediate-1 (1–2 points) categories is promising, it is greatly reduced in patients in the intermediate-2 and high-risk categories (see Table 3).
Table 2.
Prognostic scoring systems for MF in current clinical practice (Cervantes et al., 2009; Passamonti et al., 2010; Gangat et al., 2011)
| Variable | IPSS | DIPSS | DIPSS Plus |
|---|---|---|---|
| Age > 65 years | √ | √ | √ |
| Constitutional symptoms | √ | √ | √ |
| Hb: < 100 g/L | √ | √ | √ |
| Leukocyte count: > 25 × 109/L | √ | √ | √ |
| Circulating blasts: ≥ 1% | √ | √ | √ |
| Platelet count: < 100 × 109/L | √ | ||
| RBC transfusion need | √ | ||
| Unfavourable karyotype* | √ | ||
| Scoring | 1 point each | 1 point each, but Hb=2 | 1 pt for DIPSS int-1 2 pts for DIPSS int-2 3 pts for DIPSS high 1 point each for last three items |
Unfavorable karyotype = 8,7/7q, i(17q), inv(3),5/5q, 12p, or 11q23 rearrangements
IPSS: International Prognostic Scoring System; DIPSS: Dynamic
IPSS; Hb: hemoglobin; RBC: red blood cell
Table 3.
Survival according to DIPPS classification (Passamonti et al., 2010)
| Points | Median Survival (Months) | |
|---|---|---|
| Low | 0 | Not reached |
| Intermediate-1 | 1–2 | 170 |
| Intermediate-2 | 3–4 | 48 |
| High | 5–6 | 18 |
HOW ARE MPNS DIAGNOSED?
The diagnosis of MPNs is often challenging due to similarities in the pathogenesis and symptoms of MF, PV, and ET. The diagnosis is typically made by a hematologist who will generally order blood and molecular tests, as well as a bone marrow biopsy.
The differential diagnosis of MPNs is important as it guides patient management. The World Health Organization (WHO) diagnostic criteria for the classical MPNs were established to facilitate differential diagnosis in clinical practice (Arber et al., 2016). Table 4 provides a simplified schematic of the 2016 WHO criteria for the diagnosis of MPNs. A mutation of the tyrosine kinase Janus Kinase 2 (JAK2) gene, which is the gene involved in the formation of blood cells from haematopoietic stem cells in the bone marrow, may be used as a molecular marker of disease in patients with MPNs. The presence of a JAK2 mutation is found in more than 95% of patients with PV and approximately 50–60% of patients with ET and MF (Nangalia & Green, 2014). Therefore, JAK2 genotyping is commonly used as part of the diagnostic criteria for the classical MPNs.
Table 4.
Simplified 2016 WHO diagnostic criteria for the classical MPNs (Arber et al., 2016)
| ET | PV | MF |
|---|---|---|
Major criteria:
|
Major criteria:
|
Major criteria:
|
Minor criterion:
|
Minor criterion:
|
Minor criterion:
|
| Requirements for diagnosis: All 4 major OR first 3 major + the minor criterion | Requirements for diagnosis: All 3 major OR first 2 major + the minor criterion | Requirements for diagnosis: All 3 major and at least 1 minor criterion |
Red cell mass testing is currently not performed in many centres in Canada and, therefore, diagnosis is often based on hemoglobin, hematocrit or erythropoietin levels, as well as molecular testing.
ET: essential thrombocythemia; PV: polycythemia vera; PMF: primary myelofibrosis; MF: myelofibrosis; JAK2: Janus kinase 2; CALR: calreticulin; CML: chronic myelogenous leukemia; MDS: myelodysplastic syndrome; LDH: lactate dehydrogenase
HOW ARE MPNS TREATED AND WHAT ARE THE GOALS OF THERAPY?
The overarching goals of therapy for MPNs are to prevent thrombotic or hemorrhagic complications, provide the best possible symptom control, improve patient QOL, and prolong survival. Currently, the only curative approach for MF is allogeneic hematopoietic stem cell transplantation (HSCT). However, it carries a considerable risk of mortality and morbidity, and is generally reserved for patients with more advanced disease who are younger with few comorbidities.
Depending on the MPN, treatment may include pharmacological interventions such as acetylsalicylic acid (ASA), hydroxyurea, interferon-alpha, anagrelide, busulfan and ruxolitinib, and/or surgical procedures such as phlebotomy and splenectomy (see Table 5). Phlebotomy is used to control erythrocytosis (increase in red blood cell mass) by maintaining a hematocrit level of less than 45%. The side effects of phlebotomy are typically minimal, but local bruising, fatigue and feeling faint may be experienced by some patients (Tonkin et al., 2012). Splenectomy is a surgical procedure to remove the spleen; it is only recommended in select patients with splenomegaly. This section focuses primarily on pharmacological interventions since, with the exception of splenectomy and phlebotomy, little has been published on non-pharmacological modalities for the treatment of MPNs. All of the pharmacological interventions for MPNs discussed here are administered on an outpatient basis.
Table 5.
Common treatments for MPNs and nursing strategies to monitor/manage their associated side effects
| Treatment | Which MPNs is it used in? | Why is it used? | What are the adverse events/key safety issues to consider? | How can we monitor for/manage these treatment-associated adverse events? |
|---|---|---|---|---|
| Low-dose ASA (81 mg/day) |
|
|
|
|
| Hydroxyurea (HU) |
|
|
|
|
| Interferon |
|
|
Often significant and include:
|
|
| Anagrelide |
|
|
|
|
| Busulfan |
|
|
|
|
| Phlebotomy |
|
|
Minimal but may include:
|
|
| Ruxolitinib |
|
|
|
|
ET: essential thrombocythemia; PV: polycythemia vera; MF: myelofibrosis; CV: cardiovascular; HU: hydroxyurea; Hb: hemoglobin; ECG: electrocardiogram; RBCs: red blood cells; ASA: acetylsalicylic acid; TB: tuberculosis
ASA is used as an oral antiplatelet agent to reduce the risk of thrombosis in patients with PV and ET (Sirhan et al., 2015; Tefferi & Pardanani, 2015, Tefferi & Barbui, 2017; Tonkin et al., 2012; Mesa et al., 2016). It is typically prescribed at low doses, and is usually not required for the treatment of young patients with ET who are at low thrombotic risk and negative for the JAK2 mutation. The most common side effects of ASA include bleeding and indigestion with an increased risk of peptic ulceration. It may not be a suitable treatment option for patients who experience bleeding or who have low or high platelet counts (Tonkin et al., 2012).
Hydroxyurea (HU) is an oral agent that reduces the number of blood cells produced in the bone marrow by slowing cell division (Tonkin et al., 2012). It is generally recommended as the first-line cytoreductive treatment for PV and ET, and is also used to control symptomatic splenomegaly and thrombocytosis in patients with MF (Sirhan et al., 2015; Tefferi & Pardanani, 2015, Tefferi & Barbui, 2017; Tonkin et al., 2012; Mesa et al., 2016). HU is often used in combination with ASA in patients with ET, and with ASA and phlebotomy in PV. The most common side effects of HU include leg and/or mouth ulcers and cytopenias.
Interferon-alpha, which is administered subcutaneously, suppresses the overproduction of blood cells produced in the bone marrow, and it is often used as second- or third-line treatment in ET or PV (Sirhan et al., 2015; Tefferi & Barbui, 2015). However, because it is not contraindicated in pregnancy like HU, it is usually the preferred treatment for young adults who are pregnant or of child-bearing age. The side effects of interferon are often significant and include myelosuppression/infection, flu-like symptoms, depression, diarrhea, blurred vision, and asthenia.
Busulfan is an alkylating agent that interferes with the production of blood cells; oral busulfan is often used as second-line treatment in patients with BCR-ABL1-negative MPNs that are intolerant to or that develop side effects from HU (Tefferi & Barbui, 2015). Treatment with busulfan is associated with nausea, as well as profound and prolonged cytopenias, especially thrombocytopenia (Begna et al, 2016).
Originally developed as an anticoagulant, anagrelide (ANA) has potent and specific platelet-lowering activity; it reduces platelet production by inhibiting megakaryocyte (MK) colony development. ANA is an oral medication that is used primarily as a second-line treatment in ET for the prevention of thrombosis and hemorrhage (Birgegård, 2016). It does not appear to inhibit fibrosis development, and due to its anticoagulation properties, it may be associated with an increased risk of hemorrhage when combined with ASA. The most common side effects of ANA are headache and tachycardia, but these often subside within a few weeks of treatment.
Ruxolitinib is an oral Janus-associated kinase 1 (JAK1) and JAK2 inhibitor. It is a targeted therapy that interferes with the JAK/signal transducers and activators of transcription (STAT) pathway which regulates blood cell production and is known to play a key role in the underlying mechanisms of PV and MF. Ruxolitinib has been shown to reduce spleen volume, improve constitutional symptoms and QOL, and stabilize fibrosis; it may also improve survival (Verstovsek et al., 2012, 2016; Harrison et al., 2012; Harrison et al., 2016; Vannucchi et al., 2015; Passamonti et al., 2015). Currently in Canada, ruxolitinib is approved for the treatment of patients with PV who have had an inadequate response to or who are intolerant of HU, and for the treatment of splenomegaly and/or its associated symptoms in adult patients with primary MF, post-PV MF or post-ET MF (Ruxolitinib Product Monograph, 2017). The most common adverse events with ruxolitinib treatment include dose-dependent anemia and thrombocytopenia.
Other pharmacological treatments not discussed in detail here that may be used in MF include erythropoietin, corticosteroids and immunomodulators for the management of anemia.
WHAT IS THE NURSE’S ROLE IN THE TREATMENT OF MPNS?
Nurses play an important role in ensuring patient adherence to therapy and optimizing treatment outcomes by establishing therapeutic goals, educating patients on treatment administration and possible side effects, and monitoring and managing the adverse events of therapies for MPNs.
In patients using HU, nurses should monitor for signs of leg or mouth ulcers and refer to the physician for dose reductions or treatment discontinuation if these events occur. In those prescribed interferon, it is important to provide education on correct techniques for subcutaneous injections, as well as strategies to minimize flu-like symptoms, such as increasing fluid intake, taking analgesic and antipyretic medications, and maintaining bed rest until symptoms abate. In those undergoing phlebotomy, the nurse should ensure that the patient has not fasted prior to procedure and that he/she stays well hydrated post-procedure.
It is also important for nurses to monitor platelet counts in patients using ASA, busulfan or ruxolitinib to determine if dose reductions, treatment interruptions or discontinuation may be required. The hematologic events associated with ruxolitinib can generally be managed with dose modifications and temporary treatment interruptions, as well as RBC transfusions in the case of anemia (Mesa & Cortes, 2013).
Table 5 provides a summary of the treatments commonly used for the management of MPNs, their associated side effects and nursing strategies for the monitoring/management of these adverse events.
CONCLUSION
Nurses require a good understanding of the characteristics, diagnosis and treatment of MPNs since patients with these disorders are now well-informed given the numerous resources available online. As a primary patient contact, nurses play an essential role in communicating with patients with MPNs, educating them on treatment options, and monitoring and managing the adverse events of these therapies in order to optimize treatment adherence and outcomes. Ideally, all patients with MPNs should be managed in a shared-care model, with close collaboration between nurses, a community hematologist/oncologist and a tertiary-care centre with expertise in MPNs.
Footnotes
CONFLICT OF INTEREST
Sabrina Fowlkes has received honoraria from Novartis for speaking engagements, education program development and as a nurse consultant. Cindy Murray has received honoraria from Novartis for educational purposes. Adrienne Fulford has received honoraria from Novartis for speaking and a consultancy meeting. Tammy DeGelder has received honoraria from Novartis for speaking, education and consultancy. Nancy Siddiq has received honoraria from Novartis for educational activities. None of the authors received remuneration for writing of this article.
REFERENCES
- Abdel-Wahab OI, Levine RL. Primary myelofibrosis: Update on definition, pathogenesis, and treatment. Annual Review of Medicine. 2009;60:233–45. doi: 10.1146/annurev.med.60.041707.160528. [DOI] [PubMed] [Google Scholar]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- Begna K, Abdelatif A, Schwager S, Hanson C, Pardanani A, Tefferi A. Busulfan for the treatment of myeloproliferative neoplasms: The Mayo Clinic experience. Blood Cancer Journal. 2016;6:e427. doi: 10.1038/bcj.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgegård G. The use of anagrelide in myeloproliferative neoplasms, with focus on essential thrombocythemia. Current Hematologic Malignancy Reports. 2016;11(5):348–355. doi: 10.1007/s11899-016-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, Vannucchi AM, Tefferi A. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- Finazzi G, Barbui T. How I treat patients with polycythemia vera. Blood. 2007;109(12):5104–11. doi: 10.1182/blood-2006-12-038968. [DOI] [PubMed] [Google Scholar]
- Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, Van Dyke D, Tefferi A. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. Journal of Clinical Oncology. 2011;29(4):392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: When, which agent, and how? Blood. 2014;124(24):3529–37. doi: 10.1182/blood-2014-05-577635. [DOI] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. New England Journal of Medicine. 2012;366(9):787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, Cervantes F, Barbui T. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib versus best available therapy for myelofibrosis. Leukemia. 2016;30:1701–1707. doi: 10.1038/leu.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa RA, Cortes J. Optimizing the management of ruxolitinib in patients with myelofibrosis: the need for individualized dosing. Journal of Hematology & Oncology. 2013;6:79. doi: 10.1186/1756-8722-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa R, Jamieson C, Bhatia R, Deininger MW, Gerds AT, Gojo I, Gotlib J, Sundar H. Myeloproliferative Neoplasms, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2016;14(12):1572–1611. doi: 10.6004/jnccn.2016.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangalia J, Green TR. The evolving genomic landscape of myeloproliferative neoplasms. Hematology. American Society of Hematology. Education Program. 2014;2014:287–296. doi: 10.1182/asheducation-2014.1.287. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, Guglielmelli P, Tefferi A. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115(9):1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Maffioli M, Caramazza D, Cazzola M. Myeloproliferative neoplasms: from JAK2 mutations discovery to JAK2 inhibitor therapies. Oncotarget. 2011;2(6):485–490. doi: 10.18632/oncotarget.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti F, Vannucchi AM, Cervantes F, Harrison C, Morra E, Kantarjian H, Verstovsek S. Ruxolitinib and survival improvement in patients with myelofibrosis. Leukemia. 2015;29(3):739–40. doi: 10.1038/leu.2014.282. [DOI] [PubMed] [Google Scholar]
- Radaelli F, Onida F, Rossi FG, Zilioli VR, Colombi M, Usardi P, Calori R, Zanella A. Second malignancies in essential thrombocythemia (ET): A retrospective analysis of 331 patients with long-term follow-up from a single institution. Hematology. 2008;13(4):195–202. doi: 10.1179/102453308X316022. [DOI] [PubMed] [Google Scholar]
- Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- Ruxolitinib (Jakavi) Product Monograph. Novartis Pharmaceuticals Canada, Inc; Mar 2, 2017. [Google Scholar]
- Sirhan S, Busque L, Foltz L, Grewal K, Hamm C, Laferriere N, Laneuville P, Gupta V. Evolving therapeutic options for polycythemia vera: Perspectives of the Canadian Myeloproliferative Neoplasms Group. Clinical Lymphoma, Myeloma & Leukemia. 2015;15:715–27. doi: 10.1016/j.clml.2015.07.650. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. American Journal of Hematology. 2015;90(2):162–73. doi: 10.1002/ajh.23895. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. American Journal of Hematology. 2017;92(1):94–108. doi: 10.1002/ajh.24607. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncology. 2015;1(1):97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, Randi ML, Barbui T. Survival and prognosis among 1,545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin J, Francis Y, Pattinson A, Peters T, Taylor M, Thompson R, Wallis L. Myeloproliferative neoplasms: Diagnosis, management and treatment. Nursing Standard. 2012;26(51):44–51. doi: 10.7748/ns2012.08.26.51.44.c9243. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, Harrison CN, Verstovsek S. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. New England Journal of Medicine. 2015;372(5):426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. New England Journal of Medicine. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Vannucchi AM, Griesshammer M, Masszi T, Durrant S, Passamonti F, Harrison CN, Kiladjian JJ. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821–9. doi: 10.3324/haematol.2016.143644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clinic Proceedings. 2006;81(2):159–66. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]