Abstract
Background
Implant overdentures are one of the most common treatment options used to rehabilitate edentulous patients. Attachment systems are used to anchor the overdentures to implants. The plethora of attachment systems available dictates a need for clinicians to understand their prosthodontic and patient‐related outcomes.
Objectives
To compare different attachment systems for maxillary and mandibular implant overdentures by assessing prosthodontic success, prosthodontic maintenance, patient preference, patient satisfaction/quality of life and costs.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 24 January 2018); Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 24 January 2018); MEDLINE Ovid (1946 to 24 January 2018); and Embase Ovid (1980 to 24 January 2018). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials on 24 January 2018. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
All randomised controlled trials (RCTs), including cross‐over trials on maxillary or mandibular implant overdentures with different attachment systems with at least 1 year follow‐up.
Data collection and analysis
Four review authors extracted data independently and assessed risk of bias for each included trial. Several corresponding authors were subsequently contacted to obtain missing information. Fixed‐effect meta‐analysis was used to combine the outcomes with risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (95% CI). We used the GRADE approach to assess the quality of evidence and create 'Summary of findings' tables.
Main results
We identified six RCTs with a total of 294 mandibular overdentures (including one cross‐over trial). No trials on maxillary overdentures were eligible. Due to the poor reporting of the outcomes across the included trials, only limited analyses between mandibular overdenture attachment systems were possible.
Comparing ball and bar attachments, upon pooling the data regarding short‐term prosthodontic success, we identified substantial heterogeneity (I2 = 97%) with inconsistency in the direction of effect, which was unexplained by clinical or methodological differences between the studies, and accordingly we did not perform meta‐analyses for this outcome. Short‐term re‐treatment (repair of attachment system) was higher with ball attachments (RR 3.11, 95% CI 1.68 to 5.75; 130 participants; 2 studies; very low‐quality evidence), and there was no difference between both attachment systems in short‐term re‐treatment (replacement of attachment system) (RR 1.18, 95% CI 0.38 to 3.71; 130 participants; 2 studies; very low‐quality evidence). It is uncertain whether there is a difference in short‐term prosthodontic success when ball attachments are compared with bar attachments.
Comparing ball and magnet attachments, there was no difference between them in medium‐term prosthodontic success (RR 0.84, 95% CI 0.64 to 1.10; 69 participants; 1 study; very low‐quality evidence), or in medium‐term re‐treatment (repair of attachment system) (RR 1.75, 95% CI 0.65 to 4.72; 69 participants; 1 study; very low‐quality evidence). However, after 5 years, prosthodontic maintenance costs were higher when magnet attachments were used (MD ‐247.37 EUR, 95% CI ‐346.32 to ‐148.42; 69 participants; 1 study; very low‐quality evidence). It is uncertain whether there is a difference in medium‐term prosthodontic success when ball attachments are compared with magnet attachments.
One trial provided data for ball versus telescopic attachments and reported no difference in prosthodontic maintenance between the two systems in short‐term patrix replacement (RR 6.00, 95% CI 0.86 to 41.96; 22 participants; 1 study; very low‐quality evidence), matrix activation (RR 11.00, 95% CI 0.68 to 177.72; 22 participants; 1 study; very low‐quality evidence), matrix replacement (RR 1.75, 95% CI 0.71 to 4.31; 22 participants; 1 study; very low‐quality evidence), or in relining of the implant overdenture (RR 2.33, 95% CI 0.81 to 6.76; 22 participants; 1 study; very low‐quality evidence). It is uncertain whether there is a difference in short‐term prosthodontic maintenance when ball attachments are compared with telescopic attachments.
In the only cross‐over trial included, patient preference between different attachment systems was assessed after only 3 months and not for the entire trial period of 10 years.
Authors' conclusions
For mandibular overdentures, there is insufficient evidence to determine the relative effectiveness of different attachment systems on prosthodontic success, prosthodontic maintenance, patient satisfaction, patient preference or costs. In the short term, there is some evidence that is insufficient to show a difference and where there was no evidence was reported. It was not possible to determine any preferred attachment system for mandibular overdentures.
For maxillary overdentures, there is no evidence (with no trials identified) to determine the relative effectiveness of different attachment systems on prosthodontic success, prosthodontic maintenance, patient satisfaction, patient preference or costs.
Further RCTs on edentulous cohorts must pay attention to trial design specifically using the same number of implants of the same implant system, but with different attachment systems clearly identified in control and test groups. Trials should also determine the longevity of different attachment systems and patient preferences. Trials on the current array of computer‐aided designed/computer‐assisted manufactured (CAD/CAM) bar attachment systems are encouraged.
Plain language summary
Attachments for implant dentures
Review question
The aim of this review was to assess different attachments used for upper or lower jaw implant dentures with respect to their success, wear and tear, patient satisfaction, patient preference and cost.
Background
For adults with complete tooth loss, the modern approach is implant dentures with attachment systems connecting the implants to the undersurface of the dentures. It is important to do the review as the choice of the number of implants and the design of the attachments influences prosthesis success, the amount of wear and tear, patient satisfaction, preference and costs.
Study characteristics
Authors from Cochrane Oral Health carried out this review and the evidence is up to date to 24 January 2018. A total of six trials on adults with complete tooth loss were included with a total of 294 lower jaw dentures (anchored by one or more implants). The review looked at different attachment systems on the same implant systems. The six trials did not each evaluate the same attachment systems. There were no eligible trials with upper jaw implant dentures.
Key results
There is insufficient evidence to determine any significant differences between lower jaw implant denture attachment systems and an absence of evidence for upper jaw implant denture attachment systems. Further randomised controlled trials on people with complete tooth loss wearing dentures must pay specific attention to trial design using the same implant system and the same number of implants, but different attachment systems to determine their longevity and patient preferences.
Quality of the evidence
We judged the quality of the evidence to be very low. In all the included trials there were relatively few participants and few events, and there were serious limitations in the trial designs with data missing or not all outcomes reported.
Summary of findings
Background
Description of the condition
Edentulism according to the Glossary of Prosthodontic Terms (Ferro 2017; van Blarcom 2005) is defined as the state of being without any natural teeth. Complete tooth loss is an irreversible condition.
From an epidemiological perspective, there are numerous related risk factors leading to edentulism, with caries and periodontal disease seen as the main causes according to the World Health Organization (WHO) Global Oral Health databank (Brown 2009; Felton 2009; Petersen 2005a; Petersen 2005b). It is considered a disability by the WHO. Edentulism is also markedly affected by several factors including access to care, attitude towards dental hygiene, dentist/population ratios, oral health knowledge, education level, socioeconomic status, and lifestyle. Accordingly, its prevalence is known and varies considerably between countries and between different regions within the same country (Douglass 2002; Enami 2013; Mojon 2004; Petersen 2005a). It is higher in populations of lower income and educational levels, in rural areas, and in females; with the later being restricted to certain countries (Enami 2013; Felton 2009). Edentulism is always accompanied by reduction in the quality of life, due to it adversely affecting both oral and general health (Kapur 1964; Locker 1988; MacEntee 2003; Petersen 2005b). Tooth loss is associated with an increased risk of early mortality (Gupta 2018). Historically it has been identified to be a major oral disease entity (Atwood 1971).
From a prosthodontic perspective, the complete loss of either maxillary or mandibular teeth or both leads to adverse aesthetic and biomechanical sequelae including residual ridge resorption, degenerative changes, impaired masticatory function and loss of neuromuscular control (Hobkirk 2013; Zarb 2004).
Rehabilitation of edentulism improves quality of life and reduces morbidity. A recent systematic review on the rehabilitation of edentulism and mortality showed most of the included studies indicated a higher proportion of deceased edentulous patients not using dentures as compared to denture wearers (Gupta 2018).
Edentulism is managed through prosthodontic rehabilitation, either implant‐ or tissue‐supported, with a fixed or removable prosthesis (Ferro 2017; Gupta 2018; van Blarcom 2005).
Description of the intervention
An overdenture is a removable dental prosthesis that covers and rests on one or more remaining natural teeth, the roots of natural teeth, and/or dental implants (Ferro 2017; van Blarcom 2005). Removable implant overdentures are one standard of care (Feine 2002; Fitzpatrick 2006) used to resolve the problems of edentulism and the limitations of conventional complete dentures (Zarb 2004). Removable overdentures are connected to the implants using different attachment systems (Payne 2013; Preiskel 1996). An attachment system is defined as a mechanical device for the fixation, retention and stabilization of an overdenture (Ferro 2017; van Blarcom 2005). All attachment systems are comprised of two parts: the matrix being the receptacle component, and the patrix which closely fits into the matrix either mechanically, magnetically or by friction fit (Ferro 2017; Laney 2007; van Blarcom 2005). One part of the attachment system is connected to the implants and the reciprocal part incorporated within the undersurface of the overdenture.
Ball (stud‐shaped), magnetic and telescopic attachment systems are most commonly extra‐radicular (in which the male patrix element projects from the implant abutment) but also intra‐radicular (where the male patrix element forms part of the denture base and engages a depression on the implant abutment). Bar attachment systems can be divided into two groups, those allowing rotational movement between the components (termed bar joints ‐ where a spacer between the patrix and matrix is used) and comparatively rigid ones allowing no movement (termed bar units ‐ where no spacer between the patrix and matrix is used) (Preiskel 1996). Overdenture bars have historically been soldered, cast with milled designs, made using spark‐erosion or milled precision bars (Sadowsky 2007). Aligning with the path of insertion of the overdenture, the patrix and matrix can be made of metal alloys or plastic of various diameters and configurations and range from O‐rings to pillar‐shaped projections (telescopic attachment systems) with varying amount of retention (Laney 2007; Preiskel 1985; Preiskel 1996). All ball (or stud), magnetic, telescopic and bar attachment systems are adjustable or replaceable or both (Ferro 2017; Laney 2007; Preiskel 1996; van Blarcom 2005). Today, it is more common to see the use of computer‐aided designed/computer‐assisted manufactured (CAD/CAM) technology for both bar attachment systems alone or including additional ball (stud) attachments.
Mandibular overdentures (most commonly opposing complete maxillary dentures) are usually assisted by either one or two unsplinted implants with free‐standing ball (stud‐shaped), magnetic or telescopic attachment systems, or alternatively by two or as many as four splinted implants connected by bar joints or bar units (Alsabeeha 2009; Burns 2000; Carlsson 2003; Payne 2000a; Payne 2013).
Maxillary overdentures (most commonly opposing mandibular dentitions) have historically been assisted by at least four splinted implants using one of the bar attachment systems (Mericske‐Stern 2003; Payne 2013; Sadowsky 2007), but today can be assisted by as few as unsplinted two implants with ball or stud attachment systems (Zembic 2014).
Implant overdentures depend on their attachment systems for prosthodontic treatment outcomes, but are independent of peri‐implant marginal/crestal bone loss traditionally used in determining implant success or survival (Kim 2012; Klemetti 2008). Prosthodontic success is influenced by post‐insertion maintenance or technical complications, adjustments/repairs or aftercare of the attachment systems (Andreiotelli 2010; Bragger 2015; Cehreli 2010a; Kim 2012). Terminology and methodology of reporting prosthodontic outcomes varies with attachment system design and implant systems (Payne 2001a). Patient preference, patient satisfaction (including quality of life) and costs may also be independent of the attachment system used (Kim 2012).
How the intervention might work
The intervention is by way of implants, the attachment system and the overdentures. The attachment systems provide anchorage, retention and stability for implant overdentures. This facilitates improved masticatory function (Geertman 1999; van Kampen 2004), bite forces (Fontijin‐Tekamp 1998), food selection (Allen 2002) and quality of life (Awad 2003; Feine 1998).
There are four broad groups of attachment systems.
Ball/stud attachment systems: these are the simplest and the most widely used. They have a considerable stress‐breaking/stress‐relieving effect, provide adequate amount of retention and stability, are available in several vertical heights, and can be used with non‐parallel implants. Although the size of ball/stud attachments currently used are small, the choice of historical larger ball/stud attachment systems is influenced by inter‐arch space (Preiskel 1996) (Figure 1).
Bar attachment systems: these are made of single or multiple bars attached to and splinting the implants together, with the clips (riders) positioned in the undersurface of the overdenture. They offer high retentive capacities, reduce loading forces over the implants, and aid in correcting misaligned implants. However, the bulk of the attachments limits its application where there is limited inter‐arch space and minimal residual ridge resorption. If used with tapered arches it may encroach on the tongue space and influence speech. Gingival hyperplasia or mucosal enlargement can develop under the bars (Payne 2001b), and the plaque control and hygiene measures are more complicated (Preiskel 1996) (Figure 2).
Magnet attachment systems: these offer the advantage of self‐seating the prosthesis, which is especially suitable for elderly patients with limited manual dexterity or arthritis. Their fabrication procedures are relatively simple, can be used with maligned implants, and the amount of lateral loads transmitted by the retainer are less. Intraoral corrosion remains a main drawback, since it leads to rapid loss of retention and the replacement of the attachments becomes inevitable. Plaque tends to accumulate more around magnets, thus meticulous hygiene measures are required (Preiskel 1996) (Figure 3).
Telescopic attachment systems: these are composed of primary copings attached to implants and secondary telescopic crowns embedded in the overdenture. Hygiene measures are much easier and accessible in this system, and the secondary telescopic crowns add to the high retention and stability of the overdenture. Metal display of the primary crowns when the overdenture is removed can influence aesthetics (Langer 1980; Preiskel 1985) (Figure 3).
1.
Ball stud attachment systems. Copyright© 2018 Payne A, Zarb G. Implant overdentures. In: Zarb G, Hobkirk J, Eckert S, Jacob R, editor(s). Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant‐Supported Prostheses. 13th edition. St Louis, Missouri, USA: Mosby, 2013:330‐9 (Payne 2013): reproduced with permission.
2.
Bar attachment systems. Copyright© 2018 Payne A, Zarb G. Implant overdentures. In: Zarb G, Hobkirk J, Eckert S, Jacob R, editor(s). Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant‐Supported Prostheses. 13th edition. St Louis, Missouri, USA: Mosby, 2013:330‐9 (Payne 2013): reproduced with permission.
3.
Magnet telescopic attachment systems. Copyright© 2018 Professor Ralf Kohal, Germany; Professor Yoshinobu Maeda, Japan: reproduced with permission.
Why it is important to do this review
To determine whether any implant overdenture attachment system is more successful than others in terms of prosthodontic success, prosthodontic maintenance, patient preference, patient satisfaction and costs. Implant overdentures are used widely, but there are significant differences between some countries in their use related to healthcare systems which can favour or hinder their choice as a treatment option by clinicians (Carlsson 2004). A recent global survey completed by 116 prosthodontists from 33 countries showed that implant overdenture treatment for their edentulous patients is common, but there was great variation regarding the number of implants and the attachment systems used (Kronstrom 2017). The majority (84%) reported using two implants, while 13% used four implants for overdenture retention. Only one respondent used a single implant for retention of the mandibular overdenture, and two reported using three implants. There were great variations in the use of attachment systems for mandibular implant overdentures with as many as 10 different types listed in terms of usage, with the most common being the Locator® attachments system (Zest Anchors LLC, Espandido, CA, USA).
Clinicians usually rely on their expertise, personal preferences, dental technician preferences or most commonly commercial influence in attachment system selection (Naert 2003). Attachment systems wear and deteriorate over time causing prosthodontic maintenance events for clinicians and additional costs to patients in terms of re‐treatment. The constant evolution and changes in overdenture attachment systems principally driven by commercial implant companies, has resulted in some older designs being superseded by newer designs or alternatively subsequently withdrawn as problems develop with them in clinical practice (Walton 2006). In addition, there are pertinent aspects of sequential post‐treatment costs related to the prosthodontic maintenance of overdenture attachment systems which is different to fixed implant bridges.
Objectives
To compare different attachment systems for maxillary and mandibular implant overdentures by assessing prosthodontic success, prosthodontic maintenance, patient preference, patient satisfaction/quality of life and costs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) including cross‐over trials with at least 1 year follow‐up on attachment systems, and maxillary or mandibular overdentures or both, reporting prosthodontic and patient outcomes.
Types of participants
Edentulous adults receiving implant overdentures in one or both jaws to overcome problems with conventional complete dentures.
Types of interventions
The same number of implants of the same implant system comparing different attachment systems (more than one).
Ball/stud, magnetic, telescopic or bar attachment systems with mandibular 1‐, 2‐, 3‐, or 4‐implant overdentures using splinted or unsplinted prosthodontic designs.
Ball/stud, magnetic, telescopic or bar attachment systems with maxillary 1‐, 2‐, 3‐, 4‐, 5‐, or 6‐implant overdentures using splinted or unsplinted prosthodontic designs.
Types of outcome measures
Primary outcomes
Prosthodontic success by specific categorization (Appendix 1) and six‐field protocol (Appendix 2).
Prosthodontic maintenance by general categorization.
Secondary outcomes
Patient preference in cross‐over trials.
Patient satisfaction or quality of life assessed by a validated measure.
Cost (treatment time or material costs or both).
The outcomes would be assessed at the following time intervals:
Short term: 1 to 3 years.
Medium term: 3 to 5 years.
Long term: 5 to 10 years.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials without language or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 24 January 2018) (Appendix 3);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 24 January 2018) (Appendix 4);
MEDLINE Ovid (1946 to 24 January 2018) (Appendix 5);
Embase Ovid (1980 to 24 January 2018) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 24 January 2018) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 24 January 2018) (Appendix 7).
We wrote to authors of the identified RCTs, checked the bibliographies of all identified RCTs and used personal contacts in an attempt to identify unpublished or ongoing RCTs. We also contacted corresponding authors for further information to principally clarify aspects of the risk of bias tables, as well as unpublished data.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
We used methods which were specified in the review protocol (Payne 2009) and updated where necessary to conform with the latest Methodological Expectations of Cochrane Intervention Reviews (MECIR) (MECIR 2016).
Selection of studies
Four review authors (Alan Payne (AP), Nabeel Alsabeeha (NA), Momen A Atieh (MAA), and Marwah Anas El‐Wegoud (MAEW)) independently assessed studies for eligibility by initially screening titles, abstracts and keywords of every record retrieved through the electronic searches. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. We retrieved full‐text articles for further assessment when studies met the inclusion criteria. In the presence of more than one publication of the same trial, we reviewed all the publications. We linked together studies with multiple publications under a single study ID. Any disagreements were resolved by discussion and consultation with a fifth author. Where resolution was not possible, a sixth review author was consulted. We excluded any studies that had insufficient data.
Data extraction and management
We initially designed a form to extract data. For eligible studies, four review authors (AP, MAEW, MAA, and NA) independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a fifth person. We entered the data into Review Manager software (Review Manager 2014) and checked them for accuracy. When information was unclear, we contacted authors of the original reports to provide further details. The review authors were not blinded to the study authors' names, institutional affiliations, or journal of publication.
Assessment of risk of bias in included studies
Three review authors (AP, MAEW, MAA) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). There were no disagreements on the assessment of risk of bias in the included studies.
Random sequence generation (checking for possible selection bias)
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number): these studies were excluded as per the pre‐specified eligibility criteria; or
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We described for each included trial the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants and for personnel.
Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included trial, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
We categorized greater than 20% missing data as 'high' risk of bias.
Selective reporting (checking for reporting bias)
We described for each included trial how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the trial's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the trial's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; trial fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
Other bias
We described for each included trial any important concerns we have about other possible sources of bias.
We assessed whether each trial was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Overall risk of bias
We have made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to all domains above, we assessed the likely magnitude and direction of bias and whether we considered it likely to impact on the findings.
We assessed the methods as:
low risk of other bias (low risk of bias for all key domains);
high risk of other bias (high risk of bias for one or more key domains); or
unclear (unclear risk of bias for one or more key domains).
We planned to explore the impact of the level of bias through undertaking Sensitivity analysis.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (Review Manager 2014).
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) together with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI.
Unit of analysis issues
The statistical unit was the patient and the overdenture, not the number of implants, nor the type of implants used. In the analysis of cross‐over trials, we included data, if any, from the first period, if the duration of the period was at least 1 year.
Dealing with missing data
For included trials, we noted levels of attrition. We planned to explore the impact of including trials with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We have assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if I2 was greater than 30% and either Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more trials in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (Review Manager 2014). We have used fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and we judged the trials' populations and methods sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we explored this by sensitivity analysis followed by random‐effects if required.
Subgroup analysis and investigation of heterogeneity
Our planned subgroup analysis by the duration of follow‐up was not conducted as there were no data.
Sensitivity analysis
Our planned sensitivity analysis to explore the effect of trial quality assessed by omitting trials rated as high risk of bias and unclear when considering allocation concealment and incomplete outcome data was not conducted because the meta‐analysis of the primary outcomes included data from two trials only.
GRADE and 'Summary of findings' tables
We used the GRADE approach (Schünemann 2013) to create 'Summary of findings' tables (Table 1; Table 2; Table 3).
Summary of findings for the main comparison. Ball compared to bar attachment systems for implant overdentures in edentulous jaws.
| Interventions for replacing missing teeth: attachment systems for implant overdentures in edentulous jaws | ||||||
| Patient or population: edentulous adults receiving implant overdentures in one or both jaws to overcome problems with conventional complete dentures Setting: dental clinics (university clinics and/or private practice clinics) Intervention: ball attachment system of mandibular overdentures Comparison: bar attachment system of mandibular overdentures | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with bar attachment system | Risk with ball attachment system | |||||
| Success (short term) Follow‐up: range 1 year to 3 years | Study population | ‐ | 130 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1 | Considerable heterogeneity (I2 = 97%). Pooling of data was not done | |
| See comment | See comment | |||||
| Re‐treatment (repair) (short term) Follow‐up: range 1 year to 3 years | Study population | RR 3.11 (1.68 to 5.75) | 130 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 141 per 1000 | 437 per 1000 (236 to 809) | |||||
| Re‐treatment (replace) (short term) Follow‐up: range 1 year to 3 years | Study population | RR 1.18 (0.38 to 3.71) | 130 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 78 per 1000 | 92 per 1000 (30 to 290) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias (serious), inconsistency (very serious), imprecision (very serious); downgraded by 3 levels.
Summary of findings 2. Ball compared to telescopic attachment systems for implant overdentures in edentulous jaws.
| Interventions for replacing missing teeth: attachment systems for implant overdentures in edentulous jaws | ||||||
| Patient or population: edentulous adults receiving implant overdentures in one or both jaws to overcome problems with conventional complete dentures Setting: dental clinics (university clinics and/or private practice clinics) Intervention: ball attachment system of mandibular overdentures Comparison: telescopic attachment system of mandibular overdentures | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with telescopic attachment system | Risk with ball attachment system | |||||
| Patrix replaced (short term) Follow‐up: range 1 year to 3 years | Study population | RR 6.00 (0.86 to 41.96) | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 91 per 1000 | 545 per 1000 (78 to 1000) | |||||
| Matrix activated (short term) Follow‐up: range 1 year to 3 years | Study population | RR 11.00 (0.68 to 177.72) | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Matrix replaced (short term) Follow‐up: range 1 year to 3 years | Study population | RR 1.75 (0.71 to 4.31) | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 364 per 1000 | 636 per 1000 (258 to 1000) | |||||
| Reline of implant overdenture (short term) Follow‐up: range 1 year to 3 years | Study population | RR 2.33 (0.81 to 6.76) | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 273 per 1000 | 635 per 1000 (221 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias (serious), imprecision (very serious); downgraded by 3 levels.
Summary of findings 3. Ball compared to magnetic attachment systems for implant overdentures in edentulous jaws.
| Interventions for replacing missing teeth: attachment systems for implant overdentures in edentulous jaws | ||||||
| Patient or population: edentulous adults receiving implant overdentures in one or both jaws to overcome problems with conventional complete dentures Setting: dental clinics (university clinics and/or private practice clinics) Intervention: ball attachment system of mandibular overdentures Comparison: magnetic attachment system of mandibular overdentures | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with magnetic attachment system | Risk with ball attachment system | |||||
| Success (medium term) Follow‐up: range 3 years to 5 years | Study population | RR 0.84 (0.64 to 1.10) | 69 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 826 per 1000 | 694 per 1000 (529 to 909) | |||||
| Re‐treatment (repair) (medium term) Follow‐up: range 3 years to 5 years | Study population | RR 1.75 (0.65 to 4.72) | 69 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | ||
| 174 per 1000 | 304 per 1000 (113 to 821) | |||||
| Costs (medium term) Follow‐up: range 3 years to 5 years | The mean costs (medium term) was EUR 2286.34 | MD 247.37 EUR lower (346.32 lower to 148.42 lower) | ‐ | 69 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias (serious), imprecision (very serious); downgraded by 3 levels.
GRADEpro 2015 was used to import data from Review Manager (RevMan 2014) in order to create the 'Summary of finding' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (trial limitations, consistency of effect, indirectness, imprecision, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations in the five mentioned considerations.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our electronic searches identified a total of 876 references, coupled with 17 references detected by manual searches or monitoring e‐mail journal Table of Contents alerts. 421 references were assessed after duplicates were removed. Thereafter, 348 references were discarded after screening titles and abstracts leaving 73 references potentially eligible for inclusion. Assessment of full texts of these 73 references found a further 13 to be discarded as not meeting the criteria. The remaining 60 trials were scrutinized, leaving 23 (43 reports) being excluded studies, finally leaving six (17 reports) included studies (Figure 4).
4.
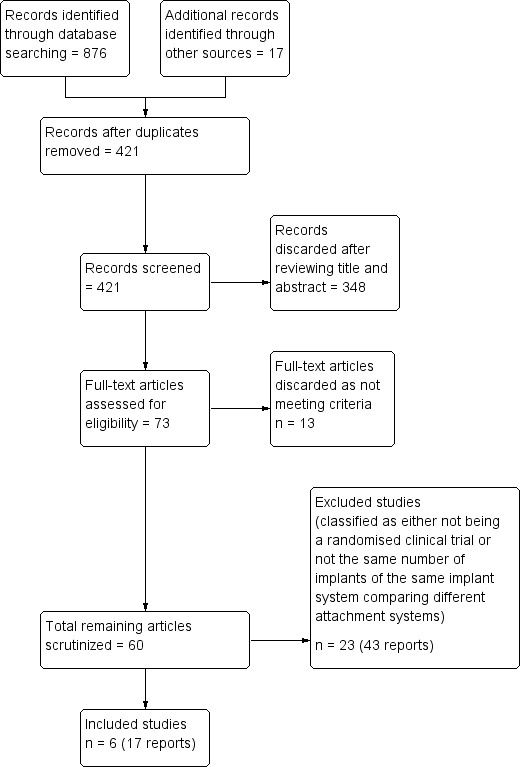
Study flow diagram.
Authors of all six included studies were contacted for additional information and we received replies from five of them (Cristache 2014; Cune 2010; Kappel 2016; Naert 1999; Walton 2002).
Authors of 17 excluded studies were contacted for additional information and we received replies from 12 of them (Burns 2011; de Souza 2015; ELsyad 2012; Krennmair 2006; Krennmair 2008; Krennmair 2012; Krennmair 2012b; Kronstrom 2010; Slot 2013; Slot 2014; Walton 2009; Wismeijer 1997).
It is relevant that clinical experience suggests that prosthodontic maintenance of a mechanical nature usually take more than 1 year to manifest themselves.
Included studies
Six trials were eligible to be included (Cepa 2017; Cristache 2014; Cune 2010; Kappel 2016; Naert 1999; Walton 2002). All were on mandibular overdentures, with no trials on maxillary overdentures being eligible.
Characteristics of the trial settings and design
Two trials were conducted in Germany (Cepa 2017; Kappel 2016), one conducted in the Netherlands (Cune 2010), one in Belguim (Naert 1999), one in Romania (Cristache 2014), and one in Canada (Walton 2002).
Five trials had a parallel group study design (Cepa 2017; Cristache 2014; Kappel 2016; Naert 1999; Walton 2002).
One trial had a cross‐over study design (Cune 2010).
Five trials declared industry support (Cepa 2017; Cristache 2014; Kappel 2016; Naert 1999; Walton 2002).
All trials were conducted in university clinics or research centres.
Study duration ranged between 2 years (Kappel 2016) and 10 years (Cune 2010; Naert 1999).
Characteristics of the interventions
By prosthodontic design of mandibular or maxillary overdentures
Ball/stud or bar attachment systems for mandibular 2‐implant overdentures using splinted and unsplinted prosthodontic designs: two trials (Kappel 2016; Walton 2002).
Ball/stud or telescopic attachment systems for mandibular 2‐implant overdentures using an unsplinted prosthodontic design: one trial (Cepa 2017).
Ball/stud, magnetic or bar attachment systems for mandibular 2‐implant overdentures using splinted and unsplinted prosthodontic designs: three trials (Cristache 2014; Cune 2010; Naert 1999).
No included trials for maxillary implant overdentures.
By attachment system of mandibular overdentures
Locator® attachments (Zest Anchors, Escondido, CA, USA) or an egg‐shaped bar (Dolder®, Sub‐TecWirobond® MI bar abutment and Dolder bar; BEGO Implant GmbH & Co KG, Germany) (Kappel 2016).
Branemark® 2.25 mm ball patrices and titanium alloy spring matrices; Branemark® 2 mm single round gold bar joint patrices and single clip matrices (Walton 2002).
Ankylos® (Dentsply Implants, Mannheim, Germany) ball attachments or prefabricated conus (telescopic) attachments with connecting matrices were polymerized into spacers of the metal framework using a self‐curing resin (Pattern Resin LS, GC Europe, Leuven, Belgium) (Cepa 2017).
Straumann® 2.25 mm ball patrices and Dalla Bona type gold alloy matrices; titanium alloy spring matrices; Locator® (ZEST Anchors, Escondido, CA 92029 USA) stud matrices and plastic patrices; magnetic attachments (Titanmagnetics® Steco system‐technick, Hamburg, Germany) (Cristache 2014).
Frialit‐2 (Friadent, Dentsply, Mannheim, Germany) 2.25 mm ball patrices and Dalla Bona type metallic matrices; single bar joint matrices and metal clips (Friadent); magnetic attachments (Dyna, Bergen, the Netherlands) (Cune 2010).
Branemark® 3.25 mm ball patrices and O‐ring matrices; Branemark® abutments with single egg‐shaped Dolder bar joint patrices and clip matrices (Cendres et Metaux, Biel/Bienne, Switzerland); Branemark® abutments with open field magnetic attachments (Dyna, Bergen, the Netherlands) (Naert 1999).
By attachment system of maxillary overdentures
No included trials for maxillary implant overdentures.
Characteristics of the outcome measures
The primary outcome of prosthodontic success by specific categorization was recorded in two trials (Cristache 2014; Walton 2002). Unpublished data on this outcome was provided by email by authors of Kappel 2016.
The primary outcome of prosthodontic maintenance by general categorization was recorded in six trials (Cepa 2017; Cristache 2014; Cune 2010; Kappel 2016; Naert 1999; Walton 2002).
The secondary outcome of patient preference in cross‐over trials, was not recorded in the only included cross‐over trial (Cune 2010).
The secondary outcome of patient satisfaction assessed by a validated measure was recorded in three trials (Cune 2010; Naert 1999; Walton 2002). However, we could not use any data from these studies. Quality of life as assessed by a validated measure was not recorded in any of the included trials.
The secondary outcome of cost analysis (treatment time and/or material costs), was recorded in two trials (Cristache 2014; Walton 2002).
Participants
For more details, see the Characteristics of included studies table.
Inclusion criteria
For mandibular overdentures:
healthy participants with adequate bone height; at least 1 year of experience wearing conventional complete dentures (Naert 1999; Walton 2002); persistent dissatisfaction/problems/complaints with their conventional dentures (Cepa 2017; Cune 2010; Naert 1999; Walton 2002); agreement for a follow‐up period (Cristache 2014); willing to consent and commit to participation in the trial (Kappel 2016; Naert 1999; Walton 2002).
For maxillary overdentures:
no included studies for maxillary implant overdentures.
Exclusion criteria
For mandibular overdentures:
general contraindications for oral surgical procedures (Cepa 2017; Cristache 2014; Kappel 2016);
insufficient bone volume to harbour two implants with a minimum length of 10 mm, unrealistic expectations of the prosthodontic treatment outcome, gag reflex, absence of maxillary complete denture, insufficient inter‐arch space, patients in Class IV classification (McGarry 1999) or Angle Class II relationship (Cristache 2014; Naert 1999);
participants were not enrolled in the trial after implant surgery because the prosthodontist found that the implants diverged more than 15 degrees from each other, or that the implants were located less than 20 mm or more than 35 mm apart, because of evidence that such an orientation and location of implants could disturb the stability and maintenance of the implant overdenture (Walton 2002).
For maxillary overdentures:
no studies included for maxillary implant overdentures.
Sample size
The number of participants in the included studies ranged between 18 (Cune 2010) and 100 (Walton 2002).
Excluded studies
The reasons for exclusion of 23 trials were.
For mandibular overdentures:
either not randomised controlled trials (Akca 2013; Karabuda 2008; Krennmair 2006; Krennmair 2012b; Mericske‐Stern 2009; Payne 2000b);
more than one implant system was used on one implant comparing different attachment systems (Alsabeeha 2011);
one implant system, different numbers of implants were used (Burns 2011; Wismeijer 1997);
the same number of implants used, two different implant systems were used each with different attachment systems (Cehreli 2010b);
different implant systems were used, different numbers of implants were used with different attachment systems (de Souza 2015);
two subtypes of bar attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) (ELsyad 2012);
did not use the same number of implants in all included patients (Gotfredsen 2000);
two subtypes of the ball attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) (Kleis 2010; Krennmair 2012);
two subtypes of the bar attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) (Krennmair 2008);
one implant system was used, different numbers of implants were used with only one attachment system (Kronstrom 2010; Walton 2009);
it was on both maxillary and mandibular overdentures with one implant system, using the same number of implants, there were both titanium and zirconia implants used as well as only one attachment system used per jaw. Different attachment systems were not used in the maxilla and mandible for comparison (Osman 2014);
more than one implant system was used on the same number of implants comparing different attachment systems (Watson 2002).
For maxillary overdentures:
more than one implant system was used on the same number of implants comparing different attachment systems (Al‐Zubeidi 2012a);
on maxillary overdentures and one implant system was used, different numbers of implants were used with only one attachment system (Slot 2013; Slot 2014).
Further details for the reasons for their exclusion are in the Characteristics of excluded studies table.
Risk of bias in included studies
We have provided detailed descriptions of the risk of bias in the included trials in the 'Risk of bias' tables. See Figure 5 and Figure 6 for a summary of 'Risk of bias' assessments.
5.
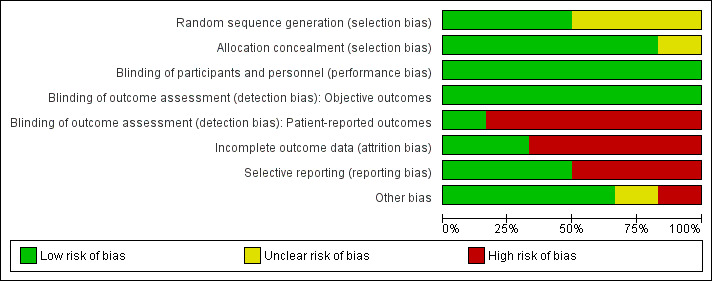
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
6.
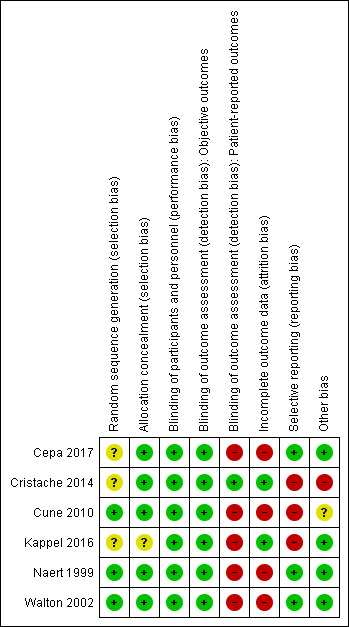
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the six included trials, only three noted adequate sequence generation (Cune 2010; Naert 1999; Walton 2002), while the other three trials did not provide any description of how the sequence was generated (Cepa 2017; Cristache 2014; Kappel 2016). All but one included trial (Kappel 2016 assessed as unclear) noted adequate allocation concealment and were at low risk of bias.
Blinding
Neither the participants nor the caregivers were blinded in the included trials due to the nature of the intervention, and since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel, we considered the risk of performance bias to be low. Regarding detection bias, we assessed blinding separately for different classes of outcomes as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and assessed the risk as low in objective outcomes (e.g. prosthodontic maintenance) and high in patient‐reported outcomes since lack of blinding can potentially introduce bias for this class of outcomes through multiple pathways (different expectations from the two groups and biased assessment of the effect).
Incomplete outcome data
For attrition bias, only two trials were at low risk (Cristache 2014; Kappel 2016), while the remaining four trials were at high risk due to reporting greater than 20% missing data (Cepa 2017; Cune 2010; Naert 1999), performing 'as treated' (Cune 2010) or 'per protocol' (Cepa 2017; Naert 1999) analyses, and presence of discrepancies between the reports of the same trial regarding the number of participants available for follow‐up and the reasons for dropouts (Walton 2002).
Selective reporting
Three of the included trials were at low risk of selective reporting of outcomes (Cepa 2017; Naert 1999; Walton 2002). The other three were at high risk either due to failure to report all the outcomes pre‐specified in their registered protocols (Cristache 2014; Kappel 2016), or due to failure to include results for key outcomes that would be expected to have been reported for such a study (Cune 2010).
Other potential sources of bias
The risk of other bias was low in four trials (Cepa 2017; Kappel 2016; Naert 1999; Walton 2002), and was high (Cristache 2014) and unclear (Cune 2010) in the other two trials.
Overall risk of bias
According to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), overall risk of bias of all the included studies was high, due to being assessed at high risk of bias for one or more key domains.
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2; Table 3.
The objectives of this review were to assess different attachment systems for maxillary and mandibular implant overdentures with respect to the outcomes measures of prosthodontic success, prosthodontic maintenance, patient preference, patient satisfaction (including quality of life), and costs.
By attachment system of maxillary overdentures
No maxillary overdenture trials were identified.
By attachment system of mandibular overdentures
Ball versus bar attachment systems
Two studies (Kappel 2016; Walton 2002) compared ball and bar attachments and reported results of 3 years of follow‐up.
Prosthodontic success by specific categorization
(See Appendix 1 for categorization and Appendix 2 for six‐field protocol.)
Kappel 2016 and Walton 2002 provided data that could be used and categorized into the six‐field protocol. Upon pooling the data obtained from the two trials regarding the outcome prosthodontic success, we identified substantial heterogeneity (I2 = 97%) with inconsistency in the direction of effect, which was unexplained by clinical or methodological differences between the studies, and accordingly we did not perform meta‐analysis as this could produce misleading results (Analysis 1.1). However, re‐treatment (repair) was higher in the ball attachment group (risk ratio (RR) 3.11, 95% confidence interval (CI) 1.68 to 5.75; 2 studies; 130 participants) (Analysis 1.2), and there was no difference between both systems in re‐treatment (replace) (RR 1.18, 95% CI 0.38 to 3.71; 2 studies; 130 participants) (Analysis 1.3). It is uncertain whether there is a difference in short‐term prosthodontic success when ball attachments are compared with bar attachments.
1.1. Analysis.
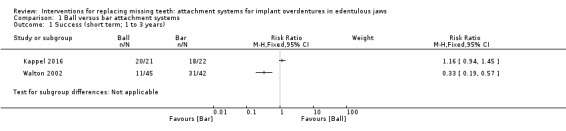
Comparison 1 Ball versus bar attachment systems, Outcome 1 Success (short term; 1 to 3 years).
1.2. Analysis.
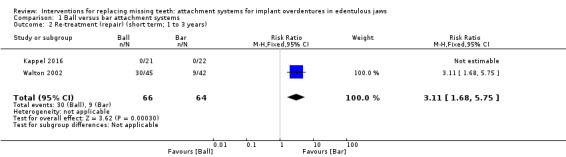
Comparison 1 Ball versus bar attachment systems, Outcome 2 Re‐treatment (repair) (short term; 1 to 3 years).
1.3. Analysis.
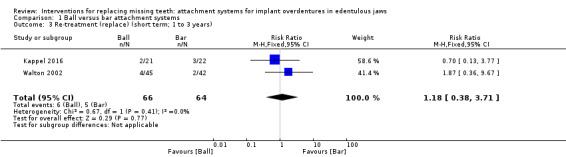
Comparison 1 Ball versus bar attachment systems, Outcome 3 Re‐treatment (replace) (short term; 1 to 3 years).
Prosthodontic maintenance by general categorization
Although this outcome was recorded by Kappel 2016 and Walton 2002, they did not provide usable data for inclusion in the review.
Patient satisfaction /quality of life assessed by a validated measure
Only Walton 2002 assessed patient satisfaction but reported narrative results and failed to provide any usable numbers. Quality of life as assessed by a validated measure was not recorded in any of the trials.
Cost (treatment time and/or material costs)
Walton 2002 reported costs but the data could not be used due to failure to report means and standard deviations and only providing the median of the costs.
Ball versus telescopic attachment systems
One trial compared ball and telescopic attachments (Cepa 2017).
Prosthodontic success by specific categorization
(See Appendix 1 for categorization and Appendix 2 for six‐field protocol.)
Cepa 2017 did not assess this outcome.
Prosthodontic maintenance by general categorization
Among the six included studies, only Cepa 2017 provided usable data for this outcome. This trial compared ball and telescopic attachments, and after 3 years of follow‐up reported no difference between both systems in patrix replacement (RR 6.00, 95% CI 0.86 to 41.96) (Analysis 2.1), matrix activation (RR 11.00, 95% CI 0.68 to 177.72) (Analysis 2.2), matrix replacement (RR 1.75, 95% CI 0.71 to 4.31) (Analysis 2.3), or in relining of the implant overdenture (RR 2.33, 95% CI 0.81 to 6.76) (Analysis 2.4). It is uncertain whether there is a difference in short‐term prosthodontic maintenance when ball attachments are compared with telescopic attachments.
2.1. Analysis.
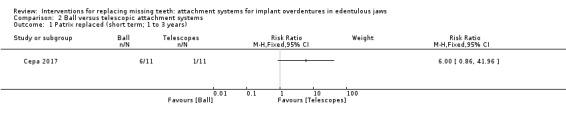
Comparison 2 Ball versus telescopic attachment systems, Outcome 1 Patrix replaced (short term; 1 to 3 years).
2.2. Analysis.
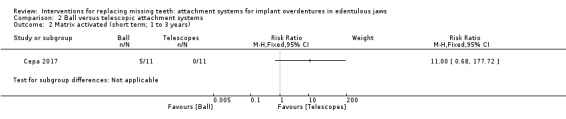
Comparison 2 Ball versus telescopic attachment systems, Outcome 2 Matrix activated (short term; 1 to 3 years).
2.3. Analysis.
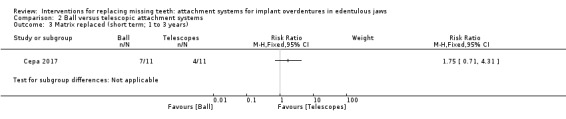
Comparison 2 Ball versus telescopic attachment systems, Outcome 3 Matrix replaced (short term; 1 to 3 years).
2.4. Analysis.
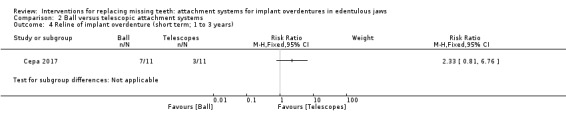
Comparison 2 Ball versus telescopic attachment systems, Outcome 4 Reline of implant overdenture (short term; 1 to 3 years).
Patient satisfaction /quality of life assessed by a validated measure
Cepa 2017 assessed patient satisfaction but did not use validated measures and we could not use the data. Quality of life as assessed by a validated measure was not recorded.
Cost (treatment time and/or material costs)
Cepa 2017 did not assess this outcome.
Ball, bar or magnetic attachment systems
Three trials compared ball, bar and magnetic attachment systems (Cristache 2014; Cune 2010; Naert 1999). Cune 2010 was the only included trial with a cross‐over design. We could not use Cune 2010 results because outcomes were reported only after 3 months which is not the time frame of interest to the review, and at 10 years which were results of "as treated analysis", and accordingly could not be used.
Prosthodontic success by specific categorization
(See Appendix 1 for categorization and Appendix 2 for six‐field protocol.)
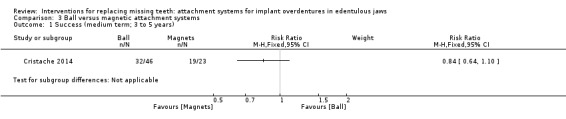
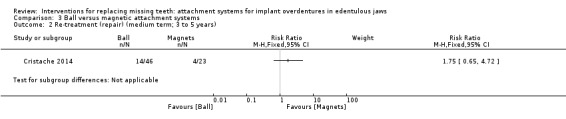
Only Cristache 2014 provided data that could be used and categorized into the six‐field protocol. Cristache 2014 compared ball and magnetic attachments and reported no difference between both systems in success (RR 0.84, 95% CI 0.64 to 1.10; 1 study; 69 participants) (Analysis 3.1) or re‐treatment (repair) (RR 1.75, 95% CI 0.65 to 4.72; 1 study; 69 participants) (Analysis 3.2) after a follow‐up of 5 years. It is uncertain whether there is a difference in medium‐term prosthodontic success when ball attachments are compared with magnet attachments.
3.1. Analysis.
Comparison 3 Ball versus magnetic attachment systems, Outcome 1 Success (medium term; 3 to 5 years).
3.2. Analysis.
Comparison 3 Ball versus magnetic attachment systems, Outcome 2 Re‐treatment (repair) (medium term; 3 to 5 years).
Prosthodontic maintenance by general categorization
Although this outcome was recorded by Cristache 2014; Cune 2010; Naert 1999, they did not provide usable data for inclusion in the review.
Patient preference in cross‐over trials
Among the six included trials, only one had a cross‐over design (Cune 2010). It did not assess patient preference between the attachments used during the trial's observation period.
Patient satisfaction /quality of life assessed by a validated measure
Two trials (Cune 2010; Naert 1999) reported patient satisfaction. However, we could not use any data from these studies. Quality of life as assessed by a validated measure was not recorded in any of the trials.
Cost (treatment time and/or material costs)
Cristache 2014 compared between total aftercare and costs of prosthodontic maintenance after 5 years with ball and magnet attachments, and reported higher costs when magnets were used (mean difference (MD) ‐247.37 EUR, 95% CI ‐346.32 to ‐148.42; 1 study; 69 participants) (Analysis 3.3).
3.3. Analysis.

Comparison 3 Ball versus magnetic attachment systems, Outcome 3 Costs (medium term; 3 to 5 years).
Discussion
Summary of main results
For mandibular overdentures, there is insufficient evidence to determine the relative effectiveness of different attachment systems on prosthodontic success, prosthodontic maintenance, patient satisfaction, patient preference or costs. In the short term, there is some evidence that is insufficient to show a difference and where there was no evidence was reported. It was not possible to determine any preferred attachment system for mandibular overdentures.
For maxillary overdentures, there is no evidence (with no trials identified) to determine the relative effectiveness of different attachment systems on prosthodontic success, prosthodontic maintenance, patient satisfaction, patient preference or costs.
There were six randomised controlled trials (RCTs) on mandibular overdentures (all opposing conventional complete maxillary dentures) included in this review evaluating ball/stud, magnetic, telescopic or bar attachment systems (Cepa 2017; Cristache 2014; Cune 2010; Kappel 2016; Naert 1999; Walton 2002). Two of the trials were over 2 years, one of 3 years, one over 5 years, and two over 10 years. There were no eligible trials on maxillary overdentures. Methodology of reporting the primary and secondary outcomes varied widely, but possibly less so in two trials (Cristache 2014; Walton 2002).
With regard to reporting the primary outcome of prosthodontic success by specific categorization (Appendix 1; Appendix 2), there is some evidence that there is no difference between ball and magnetic attachments and specifically re‐treatment (repair) up to 5 years. However, after 5 years, prosthodontic maintenance costs were higher when magnet attachments were used (Cristache 2014). In addition, with ball and bar attachments up to 3 years there appears to be no difference between both systems in re‐treatment (replace), however when looking at re‐treatment (repair) there is evidence that this is higher in the ball attachment group, which resulted in bar attachments having a higher prosthodontic success (Kappel 2016; Walton 2002). It is uncertain whether there is a difference in short‐term prosthodontic success when ball attachments are compared with bar attachments as well as when ball attachments are compared with magnet attachments.
Although prosthodontic maintenance by general categorization was evaluated in all six trials, there was also considerable variation in the way in which the prosthodontic maintenance or technical complications, adjustments/repairs or aftercare of the attachment systems were documented. Related to this prosthodontic maintenance by general categorization, there is some evidence that there is no difference between ball and telescopic attachments (Cepa 2017). It is uncertain whether there is a difference in short‐term prosthodontic maintenance when ball attachments are compared with telescopic attachments.
Related to patient preference between attachment systems in cross‐over trials, as well as patient satisfaction and quality of life using a validated measure, there is limited evidence from the single cross‐over trial (Cune 2010), with no possibility of performing any data analysis on different sets of data. This could have been possible if there had been more than one cross‐over trial.
Patient satisfaction measured in three of the six trials was with different validated measures (Cune 2010; Naert 1999; Walton 2002) and cost analysis (treatment time and/or material costs), measured in two trials (Cristache 2014; Walton 2002) was done in different manners. Although improved patient satisfaction is undoubtedly predictable regardless of the attachment system used for mandibular overdentures, a preferred attachment system related to prosthodontic success, prosthodontic maintenance, patient preference and costs could not be identified.
Related to cost (treatment time and/or material costs), there is some evidence that there are higher costs when using magnetic attachments, as opposed to ball attachments (Cristache 2014).
Overall completeness and applicability of evidence
The trials identified are not sufficient to address the review question. Although the participants and interventions investigated were of direct relevance to our review question, the pooled included trials did not provide a sufficient number of participants and did not report on the attachment systems with maxillary overdentures. In addition, the studies did not report on long‐term outcomes, and failed to include results for important key outcomes in usable numerical form to allow for meta‐analysis. Therefore, there can be no consensus on the attachment system to be used with maxillary or mandibular implant overdentures based on this review findings.
There are distinct difficulties in conducting randomised clinical trials in prosthodontics and especially determining true economic aspects of hardware maintenance longitudinally. This is of utmost relevance in older studies given the attachment systems included in those studies are no longer commercially available, except in some instances by custom fabrication by the commercial companies at a high cost to the clinician. In addition, although some of the older studies have a relevance, they could not be included as they were conducted not in a randomised clinical trial format, but rather in a prospective study format which was the usual study design at that time.
Quality of the evidence
The body of evidence identified do not allow for a robust conclusion regarding the objectives of the review. The evidence was of very low quality for all pre‐specified outcomes (Table 1; Table 2; Table 3). In all the included trials there were relatively few participants and few events, and most of the reported outcomes had wide confidence interval (CI) around the estimate of the effect that overlaps no effect and includes both appreciable benefit and appreciable harm. In addition, there were serious limitations in the trial design regarding incomplete outcome data and selective reporting of the outcomes. We evaluated the quality of evidence by using the GRADE approach. The evidence was of very low quality, we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect (Schünemann 2013).
Potential biases in the review process
The review authors followed the guidelines for conducting this systematic review under the strictest of conditions (Higgins 2011). We performed comprehensive searches to identify eligible trials, all abstracts were independently dual screened, and all references were assessed and had the risk of bias assessment carried out by at least two independent authors. All trials were subsequently reviewed and agreed by three of the review authors. Some corresponding authors did not reply to our requests of random sequence generation, allocation concealment, blinding of outcome assessment (detection bias), or additional primary outcome data on their trials and were therefore excluded from the analysis.
As the clinicians provided the evaluations related to the outcomes in the studies, there was a possibility of reporting bias and under‐reporting.
Agreements and disagreements with other studies or reviews
The present review included all the RCTs available to date. There have been five other related systematic reviews (Anas El‐Wegoud 2018; Assaf 2017; Cehreli 2010a; Kim 2012; Leão 2018).
Two of these were just on prosthodontic maintenance and complications of implant overdentures (Andreiotelli 2010; Cehreli 2010a) and both of these also included prospective studies as well as RCTs with a variation of 18 to 49 selected studies between them. Therefore direct comparisons with our findings could be misleading and difficult to interpret in these reviews. In addition, Kim 2012 also included prospective studies as well as RCTs up to August 2010, with duplication of the same studies at different time points in the 24 included studies. Implant survival was inaccurately attempted to be assessed simultaneously and lead to ambiguity. There were conflicting findings on prosthodontic maintenance between ball (stud) and bar attachments, and patient satisfaction was stated as "appearing" to be independent of attachment system. No meta‐analysis was performed.
The systematic review by Leão 2018 attempted to determine the influence of splinted and unsplinted overdenture attachment systems on prosthodontic maintenance and marginal bone loss and implant survival rate. Nine studies were included in the qualitative and quantitative analyses. A total of 984 implants were placed in 380 patients (mean age: 62.8 years). Splinted and unsplinted overdenture attachment systems achieved similar results with regard to prosthodontic maintenance. The meta‐analysis demonstrated no statistically significant differences between splinted and unsplinted attachment systems with regard to marginal bone loss (P = 0.39; mean difference (MD) ‐0.11, 95% confidence interval (CI) ‐0.37 to 0.14), complications (P = 0.31; risk ratio (RR) 1.26, 95% CI 0.80 to 1.99), and implant survival rate (P = 0.14; RR 0.37, 95% CI 0.10 to 1.36).
Similarly, Assaf 2017 from a total of 130 articles, found 33 studies that met the specified inclusion criteria for the review (14 RCTs, eight prospective clinical trials, three retrospective studies, and four systematic reviews). It was deduced that these articles provided evidence that a mean complication rate of prosthodontic maintenance was impossible to determine because of the multiplicity of contributing factors. No clear identification of the causes of prosthodontic maintenance were found, nor was there any clear evidence of superiority of any implant system or attachment design over another or both. It was concluded that prosthodontic maintenance with implant overdentures is inevitable. Further clinical studies were encouraged to achieve a constructive meta‐analysis that accounts for different parameters such as opposite arch, attachment functional variety, connection method, and prosthesis quality.
A more robust review was published recently by Anas El‐Wegoud 2018 who concluded that there was insufficient evidence to support bar or ball attachments being used with implant overdentures in edentulous patients to improve patient satisfaction and prosthesis retention. They included 10 trials (465 participants). After 5 years, one trial reported higher patient satisfaction when bar attachment was used (MD 1.30, 95% CI 0.20 to 2.40), and reported no difference between both systems in overdenture retention (MD ‐0.90, 95% CI ‐1.90 to 0.10). Two trials in Anas El‐Wegoud 2018 reported no implant failures after 1 and 5 years in both attachments and one of these (Naert 1999) was amongst the six included trials for this Cochrane Review. Downgrading of evidence was based on the unclear risk of bias of included studies and the wide confidence interval crossing the line of no effect.
Authors' conclusions
Implications for practice.
For mandibular overdentures, there is insufficient evidence to determine the relative effectiveness of different attachment systems on prosthodontic success, prosthodontic maintenance, patient satisfaction, preference or costs. It was not possible to determine any preferred attachment system for mandibular overdentures.
For maxillary overdentures, there is no evidence (with no trials identified) to determine the relative effectiveness of different attachment systems on prosthodontic success, prosthodontic maintenance, patient satisfaction, patient preference or costs.
Implications for research.
There is a profound need for further randomised controlled trials (especially on maxillary overdentures) with specific attention to trial design with the same number of implants of the same implant system, with different attachment systems (including those made with computer‐aided designed/computer‐assisted manufactured (CAD/CAM) technology clearly identified with control and test groups.
Acknowledgements
We wish to thank Anne Littlewood (Cochrane Oral Health) for her assistance with literature searching; Luisa Fernandez Mauleffinch and Philip Riley (Cochrane Oral Health) for their help with the preparation of this review; and RK Elswick (for David Burns), Andreea Didilescu (for Corina Christache), Marco Cune, Moustafa Elsyad, Stefanie Kappel, Ralf Kohal (for Sandy Cepa), Gerald Krennmair, Mats Kronstrom, Wim Slot, Joanne Walton and Daniel Wismeijer for providing us with additional information on their trials. We acknowledge in addition Anne‐Marie Glenny as well as David Moles for reviewing the draft protocol. We would like to thank the following for their peer review comments on the protocol: Professor P Finbarr Allen, in his previous appointment of Head of School, Cork University, Ireland (now Dean, Faculty of Dentistry at the National University of Singapore); Dr Yvette Solomons, Department of Prosthodontics, School of Oral Health Sciences, University of Witwatersrand, Johannesburg, South Africa. We thank again Professor P Finbarr Allen, Professor of Prosthodontics, Dean, Faculty of Dentistry at the National University of Singapore, as well as Professor Michael Fenlon, Professor of Prosthodontics, Kings College London, for their peer review comments on the review. Finally, we thank Professor Emeritus Joanne Walton, Division of Prosthodontics and Dental Geriatrics, Department of Oral Health Sciences, Faculty of Dentistry, University of British Columbia, Vancouver, Canada; and Helen Worthington (Cochrane Oral Health) for their contribution to the protocol and the early stages of this review.
Appendices
Appendix 1. Specific categories of prosthodontic maintenance for prosthodontic success
According to Payne 2001a.
Patrix loose. (Patrix here pertains to the patrix (ball abutment, retentive anchor, stud attachment, magnetic keeper) and/or its component screws as well as the patrix component screws of any related gold cylinders and all inter‐abutment and cantilever bars/superstructures (round, ovoid, U shaped, milled, spark eroded).)
Patrix activated (number of occasions).
Patrix replaced (number of occasions unilaterally or bilaterally).
Patrix fractured.
Dislodged, worn, or loose matrix or its respective housing. (Matrix here pertains to the matrix components (O ring, resilient cap, titanium spring, gold cap attachment, magnets) as well as all types of metal alloy or plastic retention clips (single sleeve or multiple sleeve) or permanent resilient lining material, connecting to inter‐abutment or cantilevered bars/superstructures.)
Matrix activated (number of occasions).
Matrix replaced (number of occasions unilaterally or bilaterally).
Matrix fractured.
Fractured implant overdenture, puncture fracture of acrylic resin over patrix, or fractured denture teeth.
Reline of implant overdenture.
New implant overdenture constructed.
Peri‐implant or inter‐abutment mucosal enlargement.
Appendix 2. 6‐field protocol for prosthodontic success for implant overdentures
| Field | Definition | Category (Appendix 1) |
| Success | Review of patient records during the study period reveals no evidence of re‐treatment except 1–8, 10, 12 for accepted maintenance.a Number of implants,b support differentiation,c and status of the opposing archd are identified |
1–8, 10, 12 |
| Survival | Patient cannot be examined directly, but the patient or another clinician confirms no evidence 1–8, 10, 12 of re‐treatment except that described for a successful outcome. Number of implants,b support differentiation,c and status of the opposing archd are identified |
1–8, 10, 12 |
| Unknown | Patient cannot be traced; surviving or successful implant overdenture removed to allow (lost to provision of a new overdenture, e.g. conversion to another overdenture design with additional follow‐up) implants or a fixed implant prosthesis using the same or additional implants | ‐ |
| Dead | Patient died during the study period regardless of whether successful or surviving criteria were experienced before death | ‐ |
| Re‐treatment (repair) |
Treatment of implant overdenture and/or mucosa where marginal integrity and associated patrices/1–10, 12 (repair) matrices are maintained irrespective of modifications as long as it continues as an implant overdenture. More than 2 replacements of either patrix or matrix in the first year or more than 5 replacements in the first 5 years. Includes replacement of worn or fractured overdenture teeth/fractured overdentures, relining of overdenture more than once in 5 years, or excision of patrix associated mucosal enlargement as a result of infringement on the shoulder/undersurface of the patrix. Number of implants,b support differentiation,c and status of the opposing archd are identified | 1–10, 12 |
| Re‐treatment (replace) |
Part or all of implant overdenture is no longer serviceable because of either loss of implants or 11 (replace) irreparable mechanical breakdown. A replacement prosthesis is indicated. Number of implants,b support differentiation,c and status of the opposing archd are identified | 11 |
a Includes patrix activation/repair/replacement, matrix activation/repair/replacement, and asymptomatic peri‐implant/interabutment mucosal enlargement not requiring excision. There is a limit of 2 replacements of either patrix or matrix in the first year and 5 replacements in 5 years, and 1 reline of the overdenture base in 5 years. b Designated as 1, 2, 3, 4, 5, or more than 5. Horseshoe designs for maxillary overdentures are identified. c Designated as implant only or implant and mucosa. d Designated as dentate, removable partial denture, complete denture, fixed implant prosthesis of any form, or implant overdenture. A combination of these designations is possible.
According to Payne 2001a.
Appendix 3. Cochrane Oral Health's Trials Register search strategy
#1 ((denture* and attachment*):ti,ab) AND (INREGISTER) #2 (((denture* or implant or attach*) and (ball* or bar* or magnet* or splint* or telescopic or "double crown*")):ti,ab) AND (INREGISTER) #3 ((attachment* and (prosthodontic* or prosthetic* or prosthes*)):ti,ab) AND (INREGISTER) #4 (#1 or #2 or #3) AND (INREGISTER) #5 ((dental and implant*)) AND (INREGISTER) #6 (("mandibular implant*" or "oral implant*"):ti,ab) AND (INREGISTER) #7 (#5 or #6) AND (INREGISTER) #8 ((overdenture* or "over denture*" or over‐denture*):ti,ab) AND (INREGISTER) #9 (("complete denture*" or "full‐upper denture*" or "full‐lower denture*" or "full lower denture*" or "full upper denture*" or "full denture*"):ti,ab) AND (INREGISTER) #10 (#8 or #9) AND (INREGISTER) #11 (#4 and #7 and #10) AND (INREGISTER)
Appendix 4. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 (denture* near/4 attachment*) #2 ((denture* or implant* or attach*) and (ball* or bar* or magnet* or splint* or telescopic or "double crown*")) #3 (attachment* and (prosthodontic* or prosthetic* or prothes*)) #4 #1 or #2 or #3 #5 [mh ^"Dental prosthesis, implant supported"] #6 [mh "Dental implantation"] #7 [mh "Dental implants"] #8 [mh ^"Mandibular prosthesis implantation"] #9 ((dental* and implant*) or "mandibular implant*" or "oral implant*") #10 #5 or #6 or #7 or #8 or #9 #11 [mh "Denture complete"] #12 (overdenture* or "over denture*" or over‐denture*) #13 ("complete denture*" or "full‐upper denture*" or "full‐lower denture*" or "full lower denture*" or "full upper denture*" or "full denture*") #14 #11 or #12 or #13 #15 #4 and #10 and #14
Appendix 5. MEDLINE Ovid search strategy
1. (denture$ adj4 attachment$).mp. 2. ((denture$ or implant$ or attach$) and (ball$ or bar$ or magnet$ or splint$ or telescopic or "double crown$")).mp. 3. (attachment$ and (prosthodontic$ or prosthetic$ or prosthes$)).mp. 4. or/1‐3 5. Dental prosthesis, implant‐supported/ 6. exp Dental implantation/ 7. exp Dental implants/ 8. Mandibular prosthesis implantation/ 9. ((dental$ and implant$) or "mandibular implant$" or "oral implant$").mp 10. or/5‐9 11. exp Denture complete/ 12. (overdenture$ or "over denture$" or over‐denture$).mp. 13. ("complete denture$" or "full‐upper denture$" or "full‐lower denture" or "full lower denture$" or "full upper denture$" or "full denture$").mp. 14. or/11‐13 15. 4 and 10 and 14
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐ maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 6. Embase Ovid search strategy
1. (denture$ adj4 attachment$).mp 2. ((denture$ or implant$ or attach$) and (ball$ or bar$ or magnet$ or splint$ or telescopic or "double crown$")).mp. 3. (attachment$ and (prosthodontic$ or prosthetic$ or prosthes$)).mp. 4. or/1‐3 5. Tooth implantation/ 6. Tooth implant/ 7. ((dental$ and implant$) or "mandibular implant$" or "oral implant$").mp. 8. or/5‐7 9. Complete denture/ 10. (overdenture$ or "over denture$" or over‐denture$).mp. 11. ("complete denture$" or "full‐upper denture$" or "full‐lower denture" or "full lower denture$" or "full upper denture$" or "full denture$").mp. 12. or/9‐11 13. 4 and 8 and 12
This subject search was linked to an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomisation/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy
implant AND attachment AND overdenture
Data and analyses
Comparison 1. Ball versus bar attachment systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success (short term; 1 to 3 years) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Re‐treatment (repair) (short term; 1 to 3 years) | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [1.68, 5.75] |
| 3 Re‐treatment (replace) (short term; 1 to 3 years) | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.38, 3.71] |
Comparison 2. Ball versus telescopic attachment systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patrix replaced (short term; 1 to 3 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Matrix activated (short term; 1 to 3 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Matrix replaced (short term; 1 to 3 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Reline of implant overdenture (short term; 1 to 3 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Ball versus magnetic attachment systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success (medium term; 3 to 5 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Re‐treatment (repair) (medium term; 3 to 5 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Costs (medium term; 3 to 5 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cepa 2017.
| Methods | Trial design: 3‐year follow‐up, randomised parallel group trial Parallel group randomised clinical trial in which the individuals were randomly assigned to the 2 trial groups consisting of ball and telescopic attachments. Randomisation was carried out by a statistician not involved in the trial using sealed envelopes. The envelope containing information on the abutments to be used was opened by a dental nurse |
|
| Participants | Inclusion criteria: participants recruited for the trial were dissatisfied with their conventional mandibular overdenture Exclusion criteria: general contraindications for oral surgical procedures such as infectious or metabolic diseases, cardiovascular disease and pregnancy; local contraindications (e.g. tumours, ulcers); alcohol abuse or smoking; psychological disease; non‐compliance and intolerance of pre‐ or post‐operative medication in the presence of chronic disease Age at baseline (years): mean 65.2 Gender (M/F): not specified Number of mandibular overdentures randomised: 25 (mandibular 2‐implant overdentures: 12 ball; 13 telescopic attachment) Number of mandibular overdentures evaluated at 1 year: 23 (12 ball; 11 telescopic attachment) Number of mandibular overdentures evaluated at 2 years: 21 (11 ball; 10 telescopic attachment) Number of mandibular overdentures evaluated at 3 years: 16 (11 ball; 5 telescopic attachment) |
|
| Interventions | Mandibular 2‐implant overdentures ‐ unsplinted prosthodontic design Test group 1 (n = 12): Ankylos® (Dentsply Implants, Mannheim, Germany) ball attachments with connecting matrices were polymerized into spacers of the metal framework using a self‐curing resin (Pattern Resin LS, GC Europe, Leuven, Belgium) Test group 2 (n = 13): prefabricated telescopic (conus) attachments with connecting matrices were polymerized into spacers of the metal framework using a self‐curing resin (Pattern Resin LS, GC Europe, Leuven, Belgium) |
|
| Outcomes | Prosthodontic maintenance by general categorization Patient satisfaction (trial did not use validated criteria for this outcome) Data collection was performed on the day of overdenture delivery and after 1, 2 and 3 years |
|
| Notes | Location: Germany Funding source: this investigation was supported by a grant from Dentsply Implants, Mannheim, Germany Patient satisfaction data presented did not use validated criteria Authors contacted by email (7 February 2017): requested full prosthodontic maintenance categorization to facilitate data analysis ‐ no reply received Details of ethical approval: Freiburg Regional Council and the Ethics Committee of the Medical‐Center, Freiburg, Germany (Votum number: 105/04) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to the two trial groups consisting of ball and telescopic attachments" Comment: the trial does not include any description of how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out by a statistician not involved in the study using sealed envelopes. The envelope containing information on the abutments to be used was opened by a dental nurse" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "As the prosthetic reconstruction was visible, blinding of the investigators was not possible" Comment: although participants and personnel were not blinded, we judge the risk of bias as low since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as low regarding prosthodontic maintenance due to being objective and is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | High risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as high regarding patient satisfaction due to being subjective and is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total losses: 9/25 (36%) Ball group losses: 1/12 Telescopic group losses: 8/13
In addition, 'per protocol' analysis was performed when analysing the patient satisfaction outcome |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available, but we judge the risk of bias as low due to reporting key outcomes that are expected to be reported for such a trial |
| Other bias | Low risk | None noted |
Cristache 2014.
| Methods | Trial design: 5‐year follow‐up, randomised parallel group trial Parallel group randomised trial. A dental assistant, not involved in this research project randomly assigned the participants to 1 of the 3 main groups. The random assigning was done using 69 sequentially numbered opaque sealed envelopes (SNOSE) |
|
| Participants | Inclusion criteria: complaints about the stability of the existing mandibular denture, Class I to III (McGarry 1999), acceptance of a mandibular overdenture retained by 2 implants; agreement for a 5‐year follow‐up period Exclusion criteria: insufficient bone volume (height and width) for inserting of at least a 10 mm implant (diameter 4.1), patients in Class IV classification (McGarry 1999), Angle Class II relationship, physical condition that will affect the minimal invasive surgical procedure or constitute a hindrance for a 5‐year follow‐up (e.g. immunosuppressive therapy, elderly patients in poor physical condition), history of radio‐/chemotherapy in the head and neck region, history of pre‐prosthetic surgery (including bone graft procedures) or previous implants Age at baseline (years): 42 to 84 Gender (M/F): not specified Number of mandibular overdentures randomised: 69 (mandibular 2‐implant overdentures) Number of mandibular overdentures evaluated at 5 years: 69 |
|
| Interventions | Mandibular 2‐implant overdentures ‐ unsplinted prosthodontic design Test group B1 (n = 12): Straumann® 2.25 mm ball patrices and Dalla Bona type gold alloy matrices Test group B2 (n = 11): Straumann® 2.25 mm ball patrices and titanium alloy spring matrices Test group M (n = 23): Titanmagnetics® magnetic attachments (patrices/matrices) Test group L (n = 23): Locator® stud matrices and plastic patrices |
|
| Outcomes | Prosthodontic success by specific categorization (Appendix 1) and 6‐field protocol (Appendix 2) Prosthodontic maintenance by general categorization Cost (treatment time and/or material costs) Data collection was performed at baseline assessment (1 week after insertion of the implant overdenture) (T0), 6 months (T) and annually thereafter for 5 years (T1–T5) |
|
| Notes | Location: Romania Funding source: supported by Grant Number 316/03 and Grant 507‐207 from the ITI Foundation for the Promotion of Oral Implantology, Switzerland Insufficient details on method of random sequence generation, allocation concealment, participant allocation, blinding of outcome assessment (detection bias) Authors contacted by email (23 August 2015): reply received 28 August 2015. Unpublished information supplied for risk of bias table Details of ethical approval: the use of human subjects in this trial was reviewed and approved by the Romanian Ministry of Health and written informed consent was obtained from all participants ClinicalTrials. gov Identifier: NCT01034930 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A dental assistant, not involved in this research project randomly assigned the participants to one of the three main groups" Comment: the trial does not include any description of how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "The random assigning was done using 69 sequentially numbered opaque sealed envelopes (SNOSE)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial did not report blinding, but we judge the risk of bias as low since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as low due to the outcomes being objective and are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition and exclusion reported |
| Selective reporting (reporting bias) | High risk | The trial protocol is available, and not all of the trial's pre‐specified primary outcomes have been reported (ClinicalTrials.gov Identifier: NCT01034930) |
| Other bias | High risk | Inconsistencies between the protocol and the trial report regarding recruitment and randomisation procedures Protocol: "Participants will be randomly assigned to one of two main groups balls and magnets, and these two groups will then be compared with 23 participants receiving Locator system abutments not originally included in the randomisation procedure" Trial: the participants were randomly assigned to 1 of the 3 main groups Accordingly, the Locator attachment system results were not included in any analysis |
Cune 2010.
| Methods | Trial design: 10‐year follow‐up, randomised, cross‐over trial Cross‐over randomised trial where the sequence in which the 3 attachments were applied was randomised |
|
| Participants | Inclusion criteria: healthy and fit participants, bone height in the inter‐foraminal > 15 mm Exclusion criteria: not reported Age at baseline (years): 33 to 56 Gender (M/F): 17/1 Number of mandibular overdentures randomised: 18 (mandibular 2‐implant overdentures) Number of mandibular overdentures evaluated at 10 years: 14 |
|
| Interventions | Mandibular 2‐implant overdentures ‐ unsplinted prosthodontic design (cross‐over study) 3 different attachment systems used; the attachment type was changed after 3 and 6 months respectively in random order and at the end of the trial at 1 year, the participants selected their attachment system. Of the 14 participants with complete data sets, evenly distributed between ball‐socket and bar‐clip groups Test group 1 (n = 7): Frialit‐2 2.25 mm ball abutment patrices and Dalla Bona type metallic matrices Test group 2 (n = 7): Frialit‐ single bar‐clip attachment system (round 2 mm bar) in conjunction with a metal omega‐shaped IMZ clip, Friadent Test group 3 (n = 0): magnetic attachment system (Dyna magnet ES, type extra strong; Dyna Dental Engineering, Bergen, the Netherlands) |
|
| Outcomes | Prosthodontic maintenance by general categorization Patient satisfaction assessed by a validated measure (4‐point scale and VAS scale) Patient preference (cross‐over trial) | |
| Notes | Location: the Netherlands Funding source: this trial was supported in part by the University Medical Center Utrecht, Military Hospital Utrecht, The Netherlands Institute for Dental Sciences and Friadent, Friedrichfeld, Germany. The laboratory procedures were performed by the Dental Laboratory of the University Clinic of Utrecht and the Zeister Tandtechnisch Laboraratorium, Zeist, the Netherlands Insufficient details on method of random sequence generation, allocation concealment, participant allocation, blinding of outcome assessment (detection bias) Authors contacted by email (23 August 2015): reply received 29 August 2015. Unpublished information supplied for risk of bias table Details of ethical approval: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clarification from trial authors: "The sequence was generated before the start of the trial by means of a randomisation protocol in SPSS, using the seed of the computer, ensuring that the different possible orders were equally distributed among the participants" |
| Allocation concealment (selection bias) | Low risk | Clarification from trial authors: "Consecutive participants fitting the inclusion and exclusion criteria who were willing to participate were enrolled in the trial. Cune was the supervising researcher. Whenever a new participant entered the trial, Cune communicated the order in which the attachment systems were to be provided to the treating surgeon (van Kampen)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clarification from trial authors: "Neither participants or examiners were blinded" Comment: we judge the risk of bias as low since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial did not report blinding of outcome assessor, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as low regarding prosthodontic maintenance due to being objective and is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | High risk | The trial did not report blinding of outcome assessor, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as high regarding patient satisfaction due to being subjective and is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total losses: 4/18 (22%) (after 10 years, 4 of the initial 18 participants were lost to follow‐up. No measures were made to follow the non‐respondents) In addition, 'as treated' analysis was performed in the 10 years reports (at the end of the initial trial, the dentures were fitted with the attachment type of the participant's choice, and the outcomes at 10 years were measured for the attachment of participant's preference) |
| Selective reporting (reporting bias) | High risk | The trial report failed to include results for key outcomes that would be expected to have been reported for such a trial |
| Other bias | Unclear risk | Insufficient duration of follow‐up to provide clinically meaningful data (3 months) |
Kappel 2016.
| Methods | Trial design: 2‐year follow‐up, randomised parallel group trial Parallel group randomised trial in which participants were randomly assigned to 1 of 2 attachment systems and special sealed sequentially numbered envelopes containing the randomised allocation were prepared at the beginning of the trial by an external source |
|
| Participants | Inclusion criteria: edentulous mandibles, adequate dimensions of the vertical and horizontal bone of the inter‐foramina regions (vertically and horizontally at least 1 mm of bone around each implant), and informed consent to participation Exclusion criteria: drug or alcohol abuse, inadequate vertical or horizontal bone dimensions or quality, less than 35 Ncm insertion torque, pregnant at the time of implant placement, and administration of intravenous bisphosphonates in the last 10 years Age at baseline (years): mean 69.4 Gender (M/F): 34:12 (73.9% male) Number of mandibular overdentures randomised: 46 ( mandibular 2‐implant overdentures) Number of mandibular overdentures evaluated at 1 year: not specified Number of mandibular overdentures evaluated at 2 years: 43 |
|
| Interventions | Mandibular 2‐implant overdentures ‐ unsplinted prosthodontic design Test group 1 (n = 23): Locator® attachments (Zest Anchors LLC, Espandido, CA, USA) Test group 2 (n = 23): egg‐shaped bar (Dolder®, Sub‐TecWirobond® MI bar abutment and Dolder bar joint (BEGO Implant GmbH & Co KG, Bremen, Germany) |
|
| Outcomes | Prosthodontic maintenance by general categorization
Patient satisfaction (trial did not use validated criteria for this outcome) Follow‐up examinations were performed after 3, 6, 12, and 24 months Unpublished data conforming to Appendix 1; Appendix 2 by authors via email |
|
| Notes | Location: Germany Funding source: BEGO Implant Systems GmbH & Co KG, Bremen, Germany: financial and material support Dr Stefanie Kappel was supported by the Olympia‐Morata programme of the Medical Faculty of the University of Heidelberg, Germany Patient satisfaction data presented did not use validated criteria ("Patients were asked whether they would recommend the treatment knowing what it entailed") Kappel 2016 is the primary trial since first report of Kappel 2015 was for less than 1 year of duration Authors contacted by email (07 February 2017): replies received (15 and 20 February 2017) providing participant numbers in test groups at year 2 plus full prosthodontic maintenance categorization from author (unpublished data) confirming to Appendix 1; Appendix 2 Details of ethical approval: the ethics committee of the Medical Faculty of the University of Heidelberg (S‐208/2010) approved the trial. Registered in the German Registry for Clinical Studies (DRKS 00004245) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were allocated to a treatment group on the basis of the content of the next envelope in the sequence" Comment: the trial does not include any description of how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed, sequentially numbered envelopes containing the randomised allocation were prepared by an external source before the trial began" Comment: it is not clear whether the envelopes were opaque or opened by an independent source not involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial did not report blinding, but we judge the risk of bias as low since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as low due to the outcomes being objective and are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | High risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as high regarding patient satisfaction due to being subjective and is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total losses: 3/46 (6.5%) 3 participants were lost to follow‐up because of death or serious illness |
| Selective reporting (reporting bias) | High risk | The trial protocol is available, and not all of the trial's pre‐specified outcomes have been reported (ISRCTN: DRKS00004245) |
| Other bias | Low risk | None noted |
Naert 1999.
| Methods | Trial design: 10‐year follow‐up randomised, parallel group trial Parallel group randomised trial. A randomised procedure, based on a lottery without replacement of the tags, allocated the patients into 3 groups of equal size |
|
| Participants | Inclusion criteria: all participants edentulous for at least more than 1 year and all complained about their existing mandibular dentures Exclusion criteria: insufficient bone volume to harbour 2 implants with a minimum length of 10 mm, Angle Class II jaw relationship, psychological problems with acceptance of a removable denture, gag reflex, absence of maxillary complete denture, administrative or physical considerations that would seriously affect the surgical procedure or longitudinal follow‐up Age at baseline (years): mean 63.7 (range 36 to 85) Gender (M/F): 17/19 Number of mandibular overdentures randomised: 36 ( mandibular 2‐implant overdentures) Number of mandibular overdentures evaluated at 5 years: 31 Number of mandibular overdentures evaluated at 10 years: 26 Note: some patients shifted from 1 group to another after allocation, hence the "intention‐to‐treat" principle was applied to prevent selection bias. Only those patients who maintained the same attachment system from the baseline ("pure group") were considered. 2 methods were applied, referring to the intention‐to‐treat group or the pure group |
|
| Interventions | Mandibular 2‐implant overdentures ‐ splinted and unsplinted prosthodontic design Baseline:
At 5 years:
At 10 years:
|
|
| Outcomes | Prosthodontic maintenance by general categorization (recorded at 4, 6, 24, 36, 48, 60, and 120 months after abutment connection) Patient satisfaction assessed by a validated measure (questionnaire) (recorded at 12, 60, and 120 months) | |
| Notes | Location: Belgium Funding source: NobelBiocare, Göteborg, Sweden, provided the ball attachments, Dyna Engineering, and Bergen of Zoom, the Netherlands, provided the magnet attachments Authors contacted by email (30 August 2015 and 20 September 2015): reply received 22 September 2015. Unpublished information supplied for risk of bias table Naert 1999 is 5‐year report which is same trial as Naert 2004 which is 10‐year report Details of ethical approval: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomised procedure, based on a lottery without replacement of the tags, allocated the patients into three groups of equal size" |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomised procedure, based on a lottery without replacement of the tags, allocated the patients into three groups of equal size" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial did not report blinding, but we judge the risk of bias as low since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as low regarding prosthodontic maintenance due to being objective and is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | High risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as high regarding patient satisfaction due to being subjective and is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total losses: 10/36 (28%) (9 participants died, and 1 was unable to complete 10 years of follow‐up because of severe illness (5, 2, and 3 participants had dropped out from the bar, magnet, and ball groups respectively)) Additionally, 'per protocol' analysis was performed for some of the outcomes (to evaluate clinical aspects, only those patients who maintained the same attachment system from the baseline, referred to as the "pure group" were considered) |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available, but we judge the risk of bias as low due to reporting key outcomes that are expected to be reported for such a trial |
| Other bias | Low risk | None noted |
Walton 2002.
| Methods | Trial design: 3‐year follow‐up, randomised parallel group trial Factorial design randomised trial where participants were randomly allocated to 1 of 4 treatment groups using balanced randomisation (retained by a ball or by a bar and clips, with or without a metal framework). The treatments were assigned randomly in blocks of 4 participants (via random permutations, generated in an Excel spreadsheet), using distinct randomisation within each of the 4 strata based jointly on gender and on ridge resorption |
|
| Participants | Inclusion criteria: edentulous participants with at least 1 year of experience wearing conventional complete dentures, medically and psychologically suited for implant surgery, able to complete the trial forms and to communicate orally in English, willing to commit to 2 years of participation in the trial after receiving new dentures (Walton 2002 and MacEntee 2005) but Walton 2003 states "be available for the duration of the trial" Exclusion criteria: received treatment previously with implants, need for additional pre‐prosthetic surgery, insufficient bone height for at least an 8.5 mm mandibular implant, or a history of systemic or neurologic disease or head and neck radiation, participants were not enrolled in the trial after implant surgery because the prosthodontist found that the implants diverged more than 15 degrees from each other, or that the implants were located less than 20 mm or more than 35 mm apart, because of evidence that such an orientation and location of implants could disturb the stability and maintenance of the implant overdenture Age at baseline (years): bar‐clip group: 61, ball‐spring: 63 (from MacEntee 2005); but Walton 2003 says 41 to 89 years (mean 65 years); and Walton 2002 says "ranged in age from 41.4 to 88.9 years, with a mean age of 64.4 years" Gender (M/F): bar‐clip group: 12/22, ball‐spring: 13/21 (from MacEntee 2005); Walton 2002 says "female participants (n = 41) outnumbered male participants (n = 23) by a ratio of almost 2:1" Number of mandibular overdentures randomised: 100 (mandibular 2‐implant overdentures) Number of mandibular overdentures evaluated at 1 year: 67 Number of mandibular overdentures evaluated at 2.98 years: 87 Note: number evaluated (participants): Walton 2002 says "of the 100 participants enrolled, 67 had their overdenture for a minimum of 1 year prior to the present analysis". 3 of these 67 were lost to follow‐up (1 died, 1 delayed treatment because of financial problems, and 1 was lost to follow‐up after implant placement surgery), leaving 64 continuing participants who had a mandibular overdenture for at least 1 year Note: MacEntee 2005 says 68/136; 87 participants had their overdentures for 2 to 4 years (mean 2.98) |
|
| Interventions | Mandibular 2‐implant overdentures ‐ splinted and unsplinted prosthodontic design Test group 1 (n = 34 at 1 year): Branemark® 2 mm single round gold bar joint patrices and single clip matrices Test group 2 (n = 34 at 1 year): Branemark® 2.25 mm ball patrices and titanium alloy spring matrices |
|
| Outcomes | Data were collected prior to implant surgery, and at 1 month, 1 year, 2 years, and 3 years after prosthesis placement, except for patient satisfaction which was not evaluated at year 3 Prosthodontic success by specific categorization Appendix 1 and 6‐field protocol Appendix 2 Prosthodontic maintenance by general categorization Patient satisfaction/quality of life assessed by a validated measure (VAS 100 mm scale after de Grandmont 1994) Cost (treatment time and/or material costs) measured in US dollars |
|
| Notes | Location: Canada Funding source: supported by Health Canada, National Health and Research Development Program (project number 6610‐2061‐403) and Nobel Biocare Canada Authors contacted by email (30 August 2015): reply received 14 September 2015. Unpublished information supplied for risk of bias table MacEntee 2005, Walton 2002, Walton 2003: all reports of same trial (Walton 2002) Details of ethical approval: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatments were assigned randomly in blocks of 4 participants (via random permutations, generated in an Excel spreadsheet)" |
| Allocation concealment (selection bias) | Low risk | Clarification from trial authors: "Statistician (Dr Ned Glick) completed the stratification and randomisation protocols, and provided sealed envelopes for participant allocation. The outer envelopes were each labelled with a stratum name (e.g. female, severe ridge resorption) and contained additional sealed envelopes specifying either number of implants or type of attachment system. Once a trial clinician had determined, according to pre‐defined criteria, the stratum to which the participant belonged, the trial dental assistant was instructed to confirm the stratum and then draw and open, in the presence of the clinician, an allocation envelope from that stratum. The assignment was then recorded on trial forms for that participant" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial did not report blinding, but we judge the risk of bias as low since it is an operative procedure and the outcomes are not likely to be influenced by lack of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as low regarding the following outcomes due to being objective and are not likely to be influenced by lack of blinding:
|
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | High risk | The trial did not report blinding, however blinding is not possible due to the nature of the intervention, but we judge the risk of bias as high regarding patient satisfaction due to being subjective and is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Discrepancies exist between the 3 trial reports regarding the number of participants available for follow‐up at 3 years and the reasons for dropouts MacEntee 2005:
Walton 2003:
|
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available, but we judge the risk of bias as low due to the reporting of key outcomes that are expected to be reported for such a trial |
| Other bias | Low risk | None noted |
F = female; IODs = implant‐retained overdentures; M = male; n = number; VAS = visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akca 2013 | Not an RCT ‐ no specific details on method of allocation concealment, participant allocation, blinding of outcome assessment (detection bias) |
| Al‐Zubeidi 2012a | Although this was an RCT on maxillary overdentures, more than 1 implant system was used on the same number of implants comparing different attachment systems |
| Alsabeeha 2011 | Although this was an RCT, more than 1 implant system was used on the same number of implants (1 implant only) comparing different attachment systems |
| Burns 2011 | Although this was an RCT using 1 implant system, different numbers of implants were used |
| Cehreli 2010b | Although this was termed as an RCT, and the same number of implants was used, 2 different implant systems were used each with different attachment systems Note: authors advised that this was a retrospective study as see excluded from Esposito 2014 |
| de Souza 2015 | Although this was an RCT, different implant systems were used, different numbers of implants were used with different attachment systems |
| ELsyad 2012 | The RCT compares between 2 subtypes of bar attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) listed under Types of interventions |
| Gotfredsen 2000 | The study did not use the same number of implants in all included participants. Study classified as an RCT, and even though termed a randomised prospective study, no details on method of random sequence generation, allocation concealment, participant allocation, or blinding of outcome assessment (detection bias) are included |
| Karabuda 2008 | Not an RCT. The study also used different implant systems and different number of implants Note: see also excluded from Esposito 2014 |
| Kleis 2010 | The study compares between 2 subtypes of the ball attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) listed under Types of interventions. Study classified as not an RCT although termed as a randomised, clinical prospective study |
| Krennmair 2012 | The study compares between 2 subtypes of the ball attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) listed under Types of interventions. Study classified as not a cross‐over RCT with consecutive participants admitted to the study |
| Krennmair 2006 | Not an RCT ‐ participants were randomised according to their clinician treating preference |
| Krennmair 2008 | The study compares between 2 subtypes of the bar attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) listed under Types of interventions |
| Krennmair 2012b | Not an RCT ‐ participants were randomly assigned according to clinician preference |
| Kronstrom 2010 | Although this was an RCT and 1 implant system was used, different numbers of implants were used with only 1 attachment system |
| Mericske‐Stern 2009 | Study classified as not a cross‐over RCT. The study compares between 2 subtypes of the bar attachment, and not between the main attachment system types (ball/stud, magnetic, telescopic or bar attachment systems) listed under Types of interventions |
| Osman 2014 | Although this was an RCT ‐ it was on maxillary and mandibular overdentures with 1 implant system, using the same number of implants, there were titanium and zirconia implants used as well as only 1 attachment system used per jaw. Different attachment systems were not used in the maxilla and mandible for comparison |
| Payne 2000b | Study not an RCT ‐ confirmed by author correspondence |
| Slot 2013 | Although this was an RCT on maxillary overdentures and 1 implant system was used, different numbers of implants were used with only 1 attachment system |
| Slot 2014 | Although this was an RCT on maxillary overdentures and 1 implant system was used, different numbers of implants were used with only 1 attachment system |
| Walton 2009 | Although this was an RCT and 1 implant system was used, different numbers of implants were used with only 1 attachment system |
| Watson 2002 | Although this was an RCT more than 1 implant system was used on the same number of implants comparing different attachment systems |
| Wismeijer 1997 | Although this was an RCT using 1 implant system, different numbers of implants were used |
RCT = randomised controlled trial.
Differences between protocol and review
The objective has been changed to "To compare different attachment systems for maxillary and mandibular implant overdentures by assessing the prosthodontic success, prosthodontic maintenance, patient preference, patient satisfaction/quality of life and costs." It was previously "To test the null hypothesis that there is no difference in the relative effectiveness of various attachment systems for mandibular or maxillary implant overdentures or both with respect to prosthesis (overdenture) success, prosthodontic maintenance, patient satisfaction, patient preference, implant success and costs." The justification is related to being more specific about what was assessed.
Under Types of interventions, we have specified the attachment systems of interest.
The sequence of primary and secondary outcomes has been changed, as well as additional secondary outcomes being added. The justifications were all to be more specific. The first primary outcome has been modified to "Prosthodontic success by specific categorization (Appendix 1) and six‐field protocol (Appendix 2)." It was only "prosthesis (overdenture) success" in the protocol. The second primary outcome has been modified to "Prosthodontic maintenance by general categorization." It was "Prosthodontic maintenance/technical complications/adjustments and repairs/aftercare" in the protocol. "Patient preference in cross‐over trials" is now the first secondary outcome. "Patient satisfaction or quality of life assessed by a validated measure" is now the second secondary outcome. The third secondary outcome has been changed to "cost (treatment time and/or material costs)". It was "costs" in the protocol. The protocol included "implant success" as the first secondary outcome and this has now been removed. The reason is that attachment systems are independent of the type of implants used and their peri‐implant marginal/crestal bone loss. We have now also specified the time intervals for outcome assessment (short term: 1 year to 3 years; medium term: 3 years to 5 years; and long term: 5 years to 10 years).
We have used fixed‐effect meta‐analysis for combining data in the review and not random‐effects as indicated in the protocol.
Contributions of authors
The specific tasks specified for the review authors are as follows.
| Task | Who has agreed to undertake the task? |
| Draft the protocol | Alan GT Payne, Joanne Walton, Nabeel HM Alsabeeha, Marco Esposito, Helen Worthington |
| Develop a search strategy | Alan GT Payne, Anne Littlewood |
| Search for trials | Alan GT Payne, Nabeel HM Alsabeeha, Momen A Atieh, Sunyoung Ma |
| Obtain copies of trials | Alan GT Payne, Nabeel HM Alsabeeha |
| Select which trials to include | Alan GT Payne, Nabeel HM Alsabeeha, Momen A Atieh, Marwah Anas El‐Wegoud |
| Extract data from trials | Alan GT Payne, Marwah Anas El‐Wegoud, Momen A Atieh, Nabeel HM Alsabeeha |
| Enter data into Review Manager | Marwah Anas El‐Wegoud |
| Carry out the analysis | Marwah Anas El‐Wegoud |
| Create GRADE 'Summary of findings' table | Marwah Anas El‐Wegoud |
| Interpret the analysis | Alan GT Payne, Marwah Anas El‐Wegoud, Momen A Atieh |
| Draft the final review | Alan GT Payne, Marwah Anas El‐Wegoud, Nabeel HM Alsabeeha, Momen A Atieh, Sunyoung Ma, Marco Esposito |
| Update the review | Alan GT Payne, Nabeel HM Alsabeeha, Marwah Anas El‐Wegoud |
Sources of support
Internal sources
The University of Manchester, UK.
Manchester Academic Health Sciences Centre (MAHSC) and NIHR Manchester Biomedical Research Centre, UK.
External sources
University of Otago, Dunedin, New Zealand.
Government of United Arab Emirates, United Arab Emirates.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Alan GT Payne: none known.
Nabeel HM Alsabeeha: none known.
Momen A Atieh: none known.
Marco Esposito: from April 2011 Marco Esposito became a full‐time freelance consultant in dentistry specialising in implantology. Therefore he receives funding for conducting clinical trials and presenting the results of trials/Cochrane Reviews at international dental meetings. This funding comes from universities, companies (in alphabetical order: Apollonia e Fama Implant, Biomax, Biomet 3i, Bioteck, Bone System, Branemark Integration, CMS Dental, Dentsply‐Friadent, Geistlich Pharma, Geass, Keystone Dental, MegaGen Implant, Mozo‐Grau, Nano Bridging molecules, Nobel Biocare, Ricerfarma, Saint Jude Medical, Southern Implants, Supercharched production, Techoss Dental Thommen Medical, Tutogen Medical, Zimmer Dental, Z‐Systems), scientific societies, publishing companies, and private dentists. This list of companies was provided by Marco on Friday 4 November 2011 and the funders will change all the time. This is to certify that: a) Marco does not own stock in companies that produce products included in the reviews of which he is an author; b) he does not have patents on any of the products included in the reviews; c) his salary will not be affected by company sales of any of the products included in the reviews. Marco's authorship has been authorised by The Cochrane Collaboration Funding Arbiter (reference 071111/057: Cochrane Oral Health).
Sunyoung Ma: none known.
Marwah Anas El‐Wegoud: none known.
New
References
References to studies included in this review
Cepa 2017 {published data only}
- Cepa S, Koller B, Spies BC, Stampf S, Kohal R‐J. Implant‐retained prostheses: ball vs conus attachments ‐ a randomised controlled clinical trial. Clinical Oral Implants Research 2017;28(2):177–85. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Blinding of outcome assessment (detection bias) used in trial; full details (manufacturer and make) of the ball attachments or the prefabricated coni (patrices) and the respective matrices used; full details of prosthodontic maintenance [personal communication]. Email to: R‐J Kohal. 7 February 2017.
Cristache 2014 {published data only}
- Cristache CM, Muntianu LA, Burlibasa M, Didilescu AC. Five‐year clinical trial using three attachment systems for implant overdentures. Clinical Oral Implants Research 2014;25(2):e171‐8. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias) and blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: AC Didilescu. 23 August 2015.
Cune 2010 {published data only}
- Cune M, Burgers M, Kampen F, Putter C, Bilt A. Mandibular overdentures retained by two implants: 10‐year results from a cross‐over clinical trial comparing ball‐socket and bar‐clip attachments. International Journal of Prosthodontics 2010;23(4):310‐7. [PubMed] [Google Scholar]
- Cune M, Kampen F, Bilt A, Bosman F. Patient satisfaction and preference with magnet, bar‐clip, and ball‐socket retained mandibular implant overdentures: a cross‐over clinical trial. International Journal of Prosthodontics 2005;18(2):99‐105. [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: M Cune. 23 August 2015.
- Kampen F, Cune M, Bilt A, Bosman F. Retention and post‐insertion maintenance of bar‐clip, ball and magnet attachments in mandibular implant overdenture treatment: an in vivo comparison after 3 months of function. Clinical Oral Implants Research 2003;14(6):720‐6. [DOI] [PubMed] [Google Scholar]
- Kampen FM, Bilt A, Cune MS, Bosman F. The influence of various attachment types in mandibular implant‐retained overdentures on maximum bite force and EMG. Journal of Dental Research 2002;81(3):170‐3. [DOI] [PubMed] [Google Scholar]
- Kampen FM, Bilt A, Cune MS, Fontijn‐Tekamp FA, Bosman F. Masticatory function with implant‐supported overdentures. Journal of Dental Research 2004;83(9):708‐11. [DOI] [PubMed] [Google Scholar]
- Bilt A, Burgers M, Kampen FM, Cune MS. Mandibular implant‐supported overdentures and oral function. Clinical Oral Implants Research 2010;21(11):1209‐13. [DOI] [PubMed] [Google Scholar]
- Bilt A, Kampen FM, Cune MS. Masticatory function with mandibular implant‐supported overdentures fitted with different attachment types. European Journal of Oral Sciences 2006;114(3):191‐6. [DOI] [PubMed] [Google Scholar]
Kappel 2016 {published and unpublished data}
- Kappel S, Eberhard L, Giannakopoulos NN, Rammelsberg P, Eiffler C. Immediate loading of two dental implants, in edentulous mandibles, with Locator® attachments or Dolder® bars: first results from a prospective randomized clinical study. Clinical Implant Dentistry and Related Research 2015;17(4):629‐38. [DOI] [PubMed] [Google Scholar]
- Kappel S, Giannakopoulos NN, Eberhard L, Rammelsberg P, Eiffler C. Immediate loading of dental implants in edentulous mandibles by use of Locator® attachments or Dolder® bars: two‐year results from a prospective randomized clinical study. Clinical Implant Dentistry and Related Research 2016;18(4):752‐61. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Allocation concealment (selection bias) used in trial; participants numbers in treatment groups at 2 years; full details of prosthodontic maintenance [personal communication]. Email to: S Kappel. 7 February 2017.
Naert 1999 {published data only}
- Naert I, Alsaadi G, Quirynen M. Prosthetic aspects and patient satisfaction with two‐implant‐retained mandibular overdentures: a 10‐year randomised clinical study. International Journal of Prosthodontics 2004;17(4):401‐10. [PubMed] [Google Scholar]
- Naert I, Gizani S, Vuylsteke M, Steenberghe D. A 5‐year prospective randomised clinical trial on the influence of splinted and unsplinted oral implants retaining a mandibular overdenture: prosthetic aspects and patient satisfaction. Journal of Oral Rehabilitation 1999;26(3):195‐202. [DOI] [PubMed] [Google Scholar]
- Naert I, Quirynen M, Hooghe M, Stennberghe D. A comparative prospective study of splinted and unsplinted Branemark implants in mandibular overdenture therapy: a preliminary report. Journal of Prosthetic Dentistry 1994;71(5):486‐92. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: I Naert. 30 August 2015.
Walton 2002 {published data only}
- MacEntee MI, Walton JN, Glick N. A clinical trial of patient satisfaction and prosthodontic needs with ball and bar attachments for implant‐retained complete overdentures: three‐year results. Journal of Prosthetic Dentistry 2005;93(1):28‐37. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias) used in trial [personal communication]. Email to: JN Walton. 30 August 2015.
- Walton JN. A randomised clinical trial comparing two mandibular implant overdenture designs: 3‐year prosthetic outcomes using a six‐field protocol. International Journal of Prosthodontics 2003;16(3):255‐60. [PubMed] [Google Scholar]
- Walton JN, MacEntee MI, Glick N. One‐year prosthetic outcomes with implant overdentures: a randomised clinical trial. International Journal of Oral & Maxillofacial Implants 2002;17(3):391‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Akca 2013 {published data only}
- Akca K, Cavusogiu Y, Sagirkaya E, Cehreli MC. Early loaded one stage implants retaining mandibular overdentures by two different mechanisms: 5‐year results. International Journal of Oral & Maxillofacial Implants 2013;28(3):824‐30. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: K Akca. 25 August 2015.
Alsabeeha 2011 {published data only}
- Alsabeeha NH, Payne AG, Silva RK, Thomson WM. Mandibular single‐implant overdentures: preliminary results of a randomised‐control trial on early loading with different implant diameters and attachment systems. Clinical Oral Implants Research 2011;22(3):330‐7. [DOI] [PubMed] [Google Scholar]
Al‐Zubeidi 2012a {published and unpublished data}
- Al‐Zubeidi MI, Alsabeeha NH, Thomson WM, Payne AG. Patient satisfaction with maxillary 3‐implant overdentures using different attachment systems opposing mandibular 2‐implant overdentures. Clinical Implant Dentistry and Related Research 2012;14 Suppl 1:e11‐9. [DOI] [PubMed] [Google Scholar]
- Ma S, Tawse‐Smith A, Silva RK, Atieh MA, Alsabeeha NHM, Payne AG. Maxillary 3‐implant overdentures opposing mandibular 2‐implant overdentures: 10‐year surgical outcomes of a randomised controlled trial. Clinical Implant Dentistry and Related Research 2016;18(3):527‐44. [DOI] [PubMed] [Google Scholar]
- Ma S, Waddell JN, Atieh MA, Alsabeeha NHM, Payne AG. Maxillary three‐implant overdentures opposing mandibular two‐implant overdentures: 10‐year prosthodontic outcomes. International Journal of Prosthodontics 2016;29(4):327‐36. [DOI] [PubMed] [Google Scholar]
- Payne AG, Tawse‐Smith A, Thomson WM, Duncan WD, Kumara R. One‐stage surgery and early loading of three implants for maxillary overdentures: a 1‐year report. Clinical Implant Dentistry and Related Research 2004;6(2):61‐74. [DOI] [PubMed] [Google Scholar]
Burns 2011 {published data only}
- Burns DR, Unger JW, Coffey JP, Waldrop TC, Elswick RK Jr. Randomized, prospective, clinical evaluation of prosthodontic modalities for mandibular implant overdenture treatment. Journal of Prosthetic Dentistry 2011;106(1):12‐22. [DOI] [PubMed] [Google Scholar]
- Burns DR, Unger JW, Elswick RK Jr, Beck DA. Prospective clinical evaluation of mandibular implant overdentures: part I ‐ retention, stability, and tissue response. Journal of Prosthetic Dentistry 1995;73(4):354‐63. [DOI] [PubMed] [Google Scholar]
- Burns DR, Unger JW, Elswick RK Jr, Giglio JA. Prospective clinical evaluation of mandibular implant overdentures: part II ‐ patient satisfaction and preference. Journal of Prosthetic Dentistry 1995;73(4):364‐9. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: DR Burns. 23 August 2015.
Cehreli 2010b {published data only}
- Cehreli MC, Uysal S, Akca K. Marginal bone level changes and prosthetic maintenance of mandibular overdentures supported by 2 implants: a 5‐year randomised clinical trial. Clinical Implant Dentistry and Related Research 2010;12(2):114‐21. [DOI] [PubMed] [Google Scholar]
de Souza 2015 {published data only}
- Payne AGT. Allocation concealment (selection bias) and blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: RF de Souza. 27 September 2015.
- Souza RF, Ribeiro AB, Della Vecchia MP, Costa L, Cunha TR, Reis AC, et al. Mini vs standard implants for mandibular overdentures: a randomised trial. Journal of Dental Research 2015;94(10):1376‐84. [DOI] [PubMed] [Google Scholar]
ELsyad 2012 {published data only}
- ELsyad MA. Prosthetic aspects and patient satisfaction with resilient liner and clip attachments for bar‐ and implant‐retained mandibular overdentures: a 3‐year randomised clinical study. International Journal of Prosthodontics 2012;25(2):148‐56. [PubMed] [Google Scholar]
- ELsyad MA, Ashmawy TM, Faramawy AG. The influence of resilient liner and clip attachments for bar‐implant‐retained mandibular overdentures on opposing maxillary ridge. A 5‐year randomised clinical trial. Journal of Oral Rehabilitation 2014;41(1):69‐77. [DOI] [PubMed] [Google Scholar]
- ELsyad MA, Shaheen NH, Ashmawy TM. Long‐term clinical and prosthetic outcomes of soft liner and clip attachments for bar/implant overdentures: a randomised controlled clinical trial. Journal of Oral Rehabilitation 2017;44(6):472‐80. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: MA ELsyad. 23 August 2015.
Gotfredsen 2000 {published data only}
- Gotfredsen K, Holm B. Implant‐supported mandibular overdentures retained with ball or bar attachments: a randomised prospective 5‐year study. International Journal of Prosthodontics 2000;13(2):125‐30. [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: K Gotfredsen. 23 August 2015.
Karabuda 2008 {published data only}
- Karabuda C, Yaltirik M, Bayraktar M. A clinical comparison of prosthetic complications of implant‐supported overdentures with different attachment systems. Implant Dentistry 2008;17(1):74‐81. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: C Karabunda. 23 August 2015.
Kleis 2010 {published data only}
- Kleis WK, Kämmerer PW, Hartmann S, Al‐Nawas B, Wagner W. A comparison of three different attachment systems for mandibular two‐implant overdentures: one‐year report. Clinical Implant Dentistry and Related Research 2010;12(3):209‐18. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: WK Kleis. 30 August 2015.
Krennmair 2006 {published data only}
- Krennmair G, Seemann R, Weinländer M, Piehslinger E. Comparison of ball and telescopic crown attachments in implant‐retained mandibular overdentures: a 5‐year prospective study. International Journal of Oral & Maxillofacial Implants 2011;26(3):598‐606. [PubMed] [Google Scholar]
- Krennmair G, Weinländer M, Krainhöfner M, Piehslinger E. Implant‐supported mandibular overdentures retained with ball or telescopic crown attachments: a 3‐year prospective study. International Journal of Prosthodontics 2006;19(2):164‐70. [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: G Krennmair. 19 March 2017; 26 March 2017.
Krennmair 2008 {published data only}
- Krennmair G, Krainhöfner M, Piehslinger E. The influence of bar design (round versus milled bar) on prosthodontic maintenance of mandibular overdentures supported by 4 implants: a 5‐year prospective study. International Journal of Prosthodontics 2008;21(6):514‐20. [PubMed] [Google Scholar]
Krennmair 2012 {published data only}
- Krennmair G, Seemann R, Fazekas A, Ewers R, Piehslinger E. Patient preference and satisfaction with implant‐supported mandibular overdentures retained with ball or locator attachments: a cross‐over clinical trial. International Journal of Oral & Maxillofacial Implants 2012;27(6):1560‐8. [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: G Krennmair. 30 August 2015.
Krennmair 2012b {published data only}
- Krennmair G, Sütö D, Seemann R, Piehslinger E. Removable four implant‐supported mandibular overdentures rigidly retained with telescopic crowns or milled bars: a 3‐year prospective study. Clinical Oral Implants Research 2012;23(4):481‐8. [DOI] [PubMed] [Google Scholar]
Kronstrom 2010 {published data only}
- Kronstrom M, Davis B, Loney R, Gerrow J, Hollender L. A prospective randomised study on the immediate loading of mandibular overdentures supported by one or two implants: a 12‐month follow‐up report. International Journal of Oral & Maxillofacial Implants 2010;25(1):181‐8. [PubMed] [Google Scholar]
- Kronstrom M, Davis B, Loney R, Gerrow J, Hollender L. A prospective randomised study on the immediate loading of mandibular overdentures supported by one or two implants; a 3‐year follow‐up report. Clinical Implant Dentistry and Related Research 2014;16(3):323‐9. [DOI] [PubMed] [Google Scholar]
- Kronstrom M, Davis B, Loney R, Gerrow J, Hollender L. Satisfaction and clinical outcomes among patients with immediately loaded mandibular overdentures supported by one or two dental implants: results of a 5‐year prospective randomized clinical trial. International Journal of Oral & Maxillofacial Implants 2017;32(1):128‐36. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias) used in trial [personal communication]. Email to: M Kronstrom. 29 August 2015.
Mericske‐Stern 2009 {published data only}
- Mericske‐Stern R, Probst D, Fahrländer F, Schellenberg M. Within‐subject comparison of two rigid bar designs connecting two interforaminal implants: patients' satisfaction and prosthetic results. Clinical Implant Dentistry and Related Research 2009;11(3):228‐37. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: R Merickse‐Stern. 30 August 2015.
Osman 2014 {published data only}
- Osman RB, Ma S. Prosthodontic maintenance of overdentures on zirconia implants: 1‐year results of a randomised controlled trial. International Journal of Prosthodontics 2014;27(5):461‐8. [DOI] [PubMed] [Google Scholar]
- Osman RB, Swain MV, Atieh M, Ma S, Duncan W. Ceramic implants (Y‐TZP): are they a viable alternative to titanium implants for the support of overdentures? A randomised clinical trial. Clinical Oral Implants Research 2014;25(12):1366‐77. [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Kieser JA, Silva RK, Thomson WM, Duncan WJ. Soft and hard tissue response to zirconia versus titanium one‐piece implants placed in alveolar and palatal sites: a randomised control trial. Clinical Implant Dentistry and Related Research 2015;17(3):483‐96. [DOI] [PubMed] [Google Scholar]
Payne 2000b {published data only}
- Payne AG, Solomons YF. Mandibular implant‐supported overdentures: a prospective evaluation of the burden of prosthodontic maintenance with 3 different attachment systems. International Journal of Prosthodontics 2000;13(3):246‐53. [PubMed] [Google Scholar]
Slot 2013 {published data only}
- Payne AGT. Request for additional information on random sequence generation (selection bias) and prosthodontic maintenance for Slot 2013 [personal communication]. Email to: W Slot. 16 August 2015.
- Slot W, Raghoebar GM, Cune MS, Vissink A, Meijer HJ. Maxillary overdentures supported by four or six implants in the anterior region: 5‐year results from a randomised controlled trial. Journal of Clinical Periodontology 2016;43(12):1180‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot W, Raghoebar GM, Vissink A, Meijer HJ. Maxillary overdentures supported by four or six implants in the anterior region; 1‐year results from a randomised controlled trial. Journal of Clinical Periodontology 2013;40(3):303‐10. [DOI] [PubMed] [Google Scholar]
Slot 2014 {published data only}
- Payne AGT. Request for additional information on random sequence generation (selection bias) and prosthodontic maintenance for Slot 2014 [personal communication]. Email to: W Slot. 16 August 2015.
- Slot W, Raghoebar GM, Vissink A, Meijer HJ. A comparison between 4 and 6 implants in the maxillary posterior region to support an overdenture; 1‐year results from a randomised controlled trial. Clinical Oral Implants Research 2014;25(5):560‐6. [DOI] [PubMed] [Google Scholar]
Walton 2009 {published data only}
- Bryant SR, Walton JN, MacEntee MI. A 5‐year randomised trial to compare 1 or 2 implants for implant overdentures. Journal of Dental Research 2015;94(1):36‐43. [DOI] [PubMed] [Google Scholar]
- Payne AGT. Allocation concealment (selection bias) and blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: JN Walton. 12 September 2015.
- Walton JN, Glick N, MacEntee MI. A randomized clinical trial comparing patient satisfaction and prosthetic outcomes with mandibular overdentures retained by one or two implants. International Journal of Prosthodontics 2009;22(4):331‐9. [PubMed] [Google Scholar]
Watson 2002 {published data only}
- Al‐Zubeidi MI, Alsabeeha NH, Thomson WM, Payne AG. Patient satisfaction and dissatisfaction with mandibular two‐implant overdentures using different attachment systems: 5‐year outcomes. Clinical Implant Dentistry and Related Research 2012;14(5):696‐707. Erratum in Clinical Implant Dentistry and Related Research 2013;15(2):309. [DOI] [PubMed] [Google Scholar]
- Ma S, Tawse‐Smith A, Thomson WM, Payne AG. Marginal bone loss with mandibular two‐implant overdentures using different loading protocols and attachment systems: 10‐year outcomes. International Journal of Prosthodontics 2010;23(4):321‐32. Erratum in International Journal of Prosthodontics 2010;23(5):462. [PubMed] [Google Scholar]
- Mackie A, Lyons K, Thomson WM, Payne AG. Mandibular two‐implant overdentures: prosthodontic maintenance using different loading protocols and attachment systems. International Journal of Prosthodontics 2011;24(5):405‐16. [PubMed] [Google Scholar]
- Watson GK, Payne AG, Purton DG, Thomson WM. Mandibular overdentures: comparative evaluation of prosthodontic maintenance of three different implant systems during the first year of service. International Journal of Prosthodontics 2002;15(3):259‐66. [PubMed] [Google Scholar]
Wismeijer 1997 {published data only}
- Payne AGT. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias) used in trial [personal communication]. Email to: D Wismeijer. 1 September 2015.
- Stoker GT, Wismeijer D, Waas MA. An eight‐year follow‐up to a randomised clinical trial of aftercare and cost‐analysis with three types of mandibular implant‐retained overdentures. Journal of Dental Research 2007;86(3):276‐80. [DOI] [PubMed] [Google Scholar]
- Timmerman R, Stoker GT, Wismeijer D, Oosterveld P, Vermeeren JI, Waas MA. An eight‐year follow‐up to a randomised clinical trial of participant satisfaction with three types of mandibular implant‐retained overdentures. Journal of Dental Research 2004;83(8):630‐3. [DOI] [PubMed] [Google Scholar]
- Wismeijer D, Waas MA, Vermeeren JI, Mulder J, Kalk W. Patient satisfaction with implant‐supported mandibular overdentures. A comparison of three treatment strategies with ITI‐dental implants. International Journal of Oral and Maxillofacial Surgery 1997;26(4):263‐7. [DOI] [PubMed] [Google Scholar]
- Wismeijer D, Waas MA, Mulder J, Vermeeren JI, Kalk W. Clinical and radiological results of patients treated with three treatment modalities for overdentures on implants of the ITI Dental Implant System. A randomised controlled clinical trial. Clinical Oral Implants Research 1999;10(4):297‐306. [DOI] [PubMed] [Google Scholar]
Additional references
Allen 2002
- Allen PF, McMillian A. Food selections and perceptions of chewing ability following provision of implant and conventional prostheses to complete denture wearers. Clinical Oral Implants Research 2002;13(3):320‐6. [DOI] [PubMed] [Google Scholar]
Alsabeeha 2009
- Alsabeeha N, Payne AG, Silva RK, Swain MV. Mandibular single‐implant overdentures: a review with surgical and prosthodontic perspectives of a novel approach. Clinical Oral Implants Research 2009;20(4):356‐65. [DOI] [PubMed] [Google Scholar]
Anas El‐Wegoud 2018
- Anas El‐Wegoud M, Fayyad A, Kaddah A, Nabhan A. Bar versus ball attachments for implant‐supported overdentures in complete edentulism: a systematic review. Clinical Implant Dentistry and Related Research 2018;20(2):243‐50. [DOI] [PubMed] [Google Scholar]
Andreiotelli 2010
- Andreiotelli M, Att W, Strub JR. Prosthodontic complications with implant overdentures: a systematic literature review. International Journal of Prosthodontics 2010;23(3):195‐203. [PubMed] [Google Scholar]
Assaf 2017
- Assaf A, Daas M, Boittin A, Eid N, Postaire M. Prosthetic maintenance of different mandibular implant overdentures: a systematic review. Journal of Prosthetic Dentistry 2017;118(2):144–52. [DOI] [PubMed] [Google Scholar]
Atwood 1971
- Atwood DA. Reduction of residual ridges: a major oral disease entity. Journal of Prosthetic Dentistry 1971;26(3):266‐79. [DOI] [PubMed] [Google Scholar]
Awad 2003
- Awad MA, Lund JP, Shapiro SH, Locker D, Klemetti E, Chehade A, et al. Oral health status and treatment satisfaction with mandibular implant overdentures and conventional dentures: a randomised clinical trial in a senior population. International Journal of Prosthodontics 2003;16(4):390‐6. [PubMed] [Google Scholar]
Bragger 2015
- Bragger U. Etiology and origin of hardware complications. In: Bragger U, Heitz‐Mayfield LJA editor(s). Biological and Hardware Complications in Implant Dentistry. ITI Treatment Guide Volume 8. Berlin: Quintessence Publishing Co, 2015:14, 36, 80, 109. [Google Scholar]
Brown 2009
- Brown DW. Complete edentulism prior to the age of 65 years is associated with all‐cause mortality. Journal of Public Health Dentistry 2009;69(4):260‐6. [DOI] [PubMed] [Google Scholar]
Burns 2000
- Burns DR. Mandibular implant overdenture treatment: consensus and controversy. Journal of Prosthodontics 2000;9(1):37‐46. [DOI] [PubMed] [Google Scholar]
Carlsson 2003
- Carlsson GE. Future directions. In: Feine JS, Carlsson GE editor(s). Implant Overdentures: The Standard of Care for Edentulous Patients. Chicago, Illinois, USA: Quintessence Publishing Co Inc, 2003:145‐54. [Google Scholar]
Carlsson 2004
- Carlsson GE, Kronström M, Baat C, Cune M, Davis D, Garefis P, et al. A survey of the use of mandibular implant overdentures in 10 countries. International Journal of Prosthodontics 2004;17(2):211‐7. [PubMed] [Google Scholar]
Cehreli 2010a
- Cehreli MC, Karasoy D, Kokat AM, Akca K, Eckert SE. Systematic review of prosthetic maintenance requirements for implant‐supported overdentures. International Journal of Oral & Maxillofacial Implants 2010;25(1):163‐80. [PubMed] [Google Scholar]
de Grandmont 1994
- Grandmont P, Feine JS, Tache R, Boudrias P, Donohue WB, Tanguay R, et al. Within‐subject comparisons of implant‐supported mandibular prostheses: psychometric evaluation. Journal of Dental Research 1994;73(5):1096‐104. [DOI] [PubMed] [Google Scholar]
Douglass 2002
- Douglass CW, Shih A, Ostry L. Will there be a need for complete dentures in the United States in 2020?. Journal of Prosthetic Dentistry 2002;87(1):5‐8. [DOI] [PubMed] [Google Scholar]
Enami 2013
- Emami E, Souza RF, Kabawat M, Feine JS. The impact of edentulism on oral and general health. International Journal of Dentistry 2013;2013:498305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2014
- Esposito M, Ardebili Y, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD003815.pub4] [DOI] [PubMed] [Google Scholar]
Feine 1998
- Feine JS, Dufresne E, Boudrias P, Lund JP. Outcome assessment of implant‐supported prostheses. Journal of Prosthetic Dentistry 1998;79(5):575‐9. [DOI] [PubMed] [Google Scholar]
Feine 2002
- Feine JS, Carlsson GE, Awad MA, Chehade A, Duncan WJ, Gizani S, et al. The McGill Consensus Statement on Overdentures. Montreal, Quebec, Canada. May 24‐25, 2002. International Journal of Prosthodontics 2002;15(4):413‐4. [PubMed] [Google Scholar]
Felton 2009
- Felton DA. Edentulism and comorbid factors. Journal of Prosthodontics 2009;18(2):88‐96. [DOI] [PubMed] [Google Scholar]
Ferro 2017
- Ferro KJ, Morgano SM, Driscoll CF, Guckes AD, Knoernschild KL, McGarry T. The Glossary of Prosthodontic Terms: Ninth Edition GPT‐9. Journal of Prosthetic Dentistry 2017;117(5 Suppl):e1‐e105. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2006
- Fitzpatrick B. Standard of care for the edentulous mandible: a systematic review. Journal of Prosthetic Dentistry 2006;95(1):71‐8. [DOI] [PubMed] [Google Scholar]
Fontijin‐Tekamp 1998
- Fontijin‐Tekamp FA, Slagter AP, van't Hof MA, Geertman ME, Kalk W. Bite forces with mandibular implant‐retained overdentures. Journal of Dental Research 1998;77:1832‐9. [DOI] [PubMed] [Google Scholar]
Geertman 1999
- Geertman ME, Slagter AP, van't Hof MA, Waas MA, Kalk W. Masticatory performance and chewing experience with implant‐retained overdentures. Journal of Oral Rehabilitation 1999;26(1):7‐13. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 27 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 2018
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hobkirk 2013
- Hobkirk JA, Zarb GA. The edentulous state. In: Zarb GA, Hobkirk JA, Eckert SE, Jacob RF editor(s). Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant‐Supported Prostheses. 13th Edition. St Louis, Missouri, USA: Mosby, 2013:1‐27. [Google Scholar]
Kapur 1964
- Kapur KK, Soman SD. Masticatory performance and efficiency in denture wearers. Journal of Prosthetic Dentistry 1964;14(4):687‐94. [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim HY, Lee JY, Shin SW, Bryant SR. Attachment systems for mandibular implant overdentures: a systematic review. Journal of Advanced Prosthodontics 2012;4(4):197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klemetti 2008
- Klemetti E. Is there a certain number of implants needed to retain an overdenture?. Journal of Oral Rehabilitation 2008;35 Suppl 1:80‐4. [DOI] [PubMed] [Google Scholar]
Kronstrom 2017
Laney 2007
- Laney WR, Broggini N, Cochran DL, Garcia LT, Giannobile MV, Hjorting‐Hansen E, et al. In: Laney WR editor(s). Glossary of Oral and Maxillo‐Facial Implants. 1st Edition. Chicago, Illinois, USA: Quintessence Publishing Co Inc, 2007:15‐16. [Google Scholar]
Langer 1980
- Langer A. Telescope retainers and their clinical application. Journal of Prosthetic Dentistry 1980;44(5):516‐22. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leão 2018
- Leão RS, Moraes SLD, Vasconcelos BCE, Lemos CAA, Pellizzer EP. Splinted and unsplinted overdenture attachment systems: a systematic review and meta‐analysis. Journal of Oral Rehabilitation 2018;45(8):647‐56. [DOI] [PubMed] [Google Scholar]
Locker 1988
- Locker D. Measuring oral health: a conceptual framework. Community Dental Health 1988;5(1):3‐18. [PubMed] [Google Scholar]
MacEntee 2003
- MacEntee M. The impact of edentulism on function and quality of life. In: Feine JS, Carlsson GE editor(s). Implant Overdentures: The Standard of Care for Edentulous Patients. Chicago, Illinois, USA: Quintessence Publishing Co Inc, 2003:23‐8. [Google Scholar]
McGarry 1999
- McGarry TJ, Nimmo A, Skiba JF, Ahlstrom RH, Smith CR, Koumjian JH. Classification system for complete edentulism. Journal of Prosthodontics 1999;8(1):27‐39. [DOI] [PubMed] [Google Scholar]
MECIR 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Cochrane: London, Version 1.02, 2016.
Mericske‐Stern 2003
- Mericske‐Stern R. Prosthodontic management of maxillary and mandibular overdentures. In: Feine JS, Carlsson GE editor(s). Implant Overdentures: The Standard of Care for Edentulous Patients. Chicago, Illinois, USA: Quintessence Publishing Co Inc, 2003:83‐98. [Google Scholar]
Mojon 2004
- Mojon P, Thomason JM, Walls AW. The impact of falling rates of edentulism. International Journal of Prosthodontics 2004;17(4):434‐40. [PubMed] [Google Scholar]
Naert 2003
- Naert I. The influence of attachment systems on implant‐retained mandibular overdentures. In: Feine JS, Carlsson GE editor(s). Implant Overdentures: The Standard of Care for the Edentulous Patients. Chicago, Illinois, USA: Quintessence Publishing Co Inc, 2003:99‐109. [Google Scholar]
Payne 2000a
- Payne AG, Solomons YF. The prosthodontic maintenance requirements of mandibular mucosa‐ and implant‐supported overdentures: a review of the literature. International Journal of Prosthodontics 2000;13(3):238‐43. [PubMed] [Google Scholar]
Payne 2001a
- Payne AG, Walton TR, Walton JN, Solomons YF. The outcome of implant overdentures from a prosthodontic perspective: proposal for a classification protocol. International Journal of Prosthodontics 2001;14(1):27‐32. [PubMed] [Google Scholar]
Payne 2001b
- Payne AGT, Solomons YF, Lownie JF, Tawse‐Smith A. Inter‐abutment and peri‐abutment mucosal enlargement with mandibular implant overdentures. Clinical Oral Implants Research 2001;13(2):179‐87. [DOI] [PubMed] [Google Scholar]
Payne 2013
- Payne A, Zarb G. Implant overdentures. In: Zarb G, Hobkirk J, Eckert S, Jacob R editor(s). Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant‐Supported Prostheses. 13th Edition. St Louis, Missouri, USA: Mosby, 2013:330‐9. [Google Scholar]
Petersen 2005a
- Petersen PE, Bourgeois D, Ogawa H, Estupinan‐Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bulletin of the World Health Organization 2005;83(9):661‐9. [PMC free article] [PubMed] [Google Scholar]
Petersen 2005b
- Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dentistry and Oral Epidemiology 2005;33(2):81‐92. [DOI] [PubMed] [Google Scholar]
Preiskel 1985
- Preiskel HW. Telescopic prostheses. In: Preiskel HW editor(s). Precision Attachments in Prosthodontics: Overdentures and Telescopic Prostheses. Vol. 2, Chicago, Illinois, USA: Quintessence Publishing Co Inc, 1985:307‐28. [Google Scholar]
Preiskel 1996
- Preiskel HW. In: Preiskel HW editor(s). Overdentures Made Easy: A Guide to Implant and Root Supported Prostheses. 1st Edition. Chicago, Illinois, USA: Quintessence Publishing Co Inc, 1996:81‐122. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sadowsky 2007
- Sadowsky S. Treatment considerations for maxillary overdentures: a systematic review. Journal of Prosthetic Dentistry 2007;97(6):340‐8. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE Handbook. The GRADE Working Group, 2013. [Google Scholar]
van Blarcom 2005
- Blarcom CW, Bello A, Eckert SE, Goodacre CJ, Morgano SM, Nathanson D, et al. The glossary of prosthodontic terms 8th edition. Journal of Prosthetic Dentistry 2005;94(1):10‐92. [DOI] [PubMed] [Google Scholar]
van Kampen 2004
- Kampen FM, Bilt A, Cune MS, Fontijn‐Tekamp FA, Bosman F. Masticatory function with implant‐supported overdentures. Journal of Dental Research 2004;83(9):708‐11. [DOI] [PubMed] [Google Scholar]
Walton 2006
- Walton TR. Too many cooks and not enough chefs!. International Journal of Prosthodontics 2006;19(6):532‐3. [PubMed] [Google Scholar]
Walton 2008
- Walton JN, MacEntee MI. Screening and enrolling subjects in a randomised clinical trial involving implant dentures. International Journal of Prosthodontics 2008;21(3):210–4. [PubMed] [Google Scholar]
Zarb 2004
- Zarb GA. The edentulous predicament. In: Zarb GA, Bolender CL, Eckert S, Jacob R, Fenton A, Mericske‐Stern R editor(s). Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant‐Supported Prostheses. 12th Edition. St Louis, Missouri, USA: Mosby, 2004:3‐5. [Google Scholar]
Zembic 2014
- Zembic A, Wismeijer D. Patient‐reported outcomes of maxillary implant‐supported overdentures compared with conventional dentures. Clinical Oral Implants Research 2014;25(4):441‐50. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Payne 2009
- Payne A, Walton J, Alsabeeha N, Worthington HV, Esposito M. Interventions for replacing missing teeth: attachment systems for implant overdentures in edentulous jaws. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008001] [DOI] [PMC free article] [PubMed] [Google Scholar]


