Abstract
Background
High‐frequency oscillation (HFO) is an alternative to conventional mechanical ventilation that is sometimes used to treat people with acute respiratory distress syndrome, but effects on oxygenation, mortality and adverse clinical outcomes are uncertain. This review was originally published in 2004 and was updated in 2013 and again in 2015.
Objectives
To determine the effects of HFO compared to conventional mechanical ventilation on physiological outcomes, clinical outcomes, and mortality when used for the treatment of acute respiratory distress syndrome (ARDS).
Search methods
We electronically searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Ovid), MEDLINE (Ovid), EMBASE (Ovid), and ISI, from inception to December 2015. We conducted the original search in 2002. We manually searched reference lists from included studies and review articles; searched conference proceedings of the American Thoracic Society (1994 to 2015), Society of Critical Care Medicine (1994 to 2015), European Society of Intensive Care Medicine (1994 to 2015), and American College of Chest Physicians (1994 to 2015); contacted clinical experts in the field; and searched for unpublished and ongoing trials in clinicaltrials.gov and controlled‐trials.com.
Selection criteria
Randomized controlled trials (RCTs) comparing treatment using HFO with conventional mechanical ventilation for children and adults diagnosed with ARDS.
Data collection and analysis
Three review authors independently extracted data on clinical, physiological, and safety outcomes according to a predefined protocol. We contacted investigators of all included studies to clarify methods and obtain additional data. We used random‐effects models in the analyses.
Main results
We include 10 RCTs (n = 1850); almost all participants had moderate or severe ARDS. For the primary analysis, the risk of bias was low in three studies and unclear in five studies; the overall quality of evidence was very low due to imprecision, inconsistency, indirectness and methodologic limitations. In participants randomized to HFO, there was no significant difference in hospital or 30‐day mortality (risk ratio (RR) 0.92, 95% confidence interval (CI) 0.72 to 1.16; P = 0.46, I² = 66%; 8 trials, 1779 participants, 807 deaths) compared with conventional ventilation. One large multicentre RCT was terminated early because of increased mortality in participants randomized to HFO compared to mechanical ventilation with low tidal volume and high positive end expiratory pressure, with HFO reserved only as a rescue therapy. We found substantial between‐trial statistical heterogeneity (I² = 0% to 66%) for clinical outcomes, including mortality.
Authors' conclusions
The findings of this systematic review suggest that HFO does not reduce hospital and 30‐day mortality due to ARDS; the quality of evidence was very low. Our findings do not support the use of HFO as a first‐line strategy in people undergoing mechanical ventilation for ARDS.
Plain language summary
High‐frequency oscillation for the treatment of acute respiratory distress syndrome (ARDS)
Background
Acute respiratory distress syndrome (ARDS) is a life‐threatening condition. It is characterized by acute lung inflammation, stiff lungs that increase the work of breathing, and reduced ability of the lungs to adequately oxygenate the blood. Survivors can have a reduced quality of life. People with ARDS usually require an artificial respirator in order to prevent death. High‐frequency oscillation (HFO) ventilation differs from conventional ventilation in that very small breaths are delivered very rapidly (180 to 900 breaths per minute). HFO helps with the opening of collapsed lung tissue by providing constant positive pressure in a person's airway. We performed a systematic review to determine whether HFO improves clinical outcomes (including preventing deaths) when compared to conventional breathing machines for adults and children with ARDS.
Study characteristics
We included 10 randomized controlled trials (RCTs) enrolling 1850 participants in this updated review. One large trial was stopped early because of increased deaths among participants who were randomized to HFO. Four trials reported at least some funding from manufacturers of HFO ventilators.
Key results
HFO did not reduce the risk of death in hospital in eight trials enrolling 1779 participants. The ability of the lungs to oxygenate blood, measured at 24 to 72 hours of ventilation after randomization, was 18% to 26% better in participants receiving HFO. HFO had no effect on the length of time an artificial breathing machine was required. The risk of unwanted side effects, including low blood pressure or further injury to the lung due to high airway pressure, was not increased.
Quality of the evidence
We found substantial inconsistency among clinical trials which reported the effect of HFO on the risk of death in participants with ARDS. The quality of evidence is very low for outcomes that would be most important to patients. This is because of a lack of precision and consistency, and because in many cases the methods used by investigators during clinical trials were not of the highest standard. This indicates that there is considerable uncertainty regarding the effect of HFO on death. Additional randomized trials could change these findings.
Summary of findings
Summary of findings for the main comparison. High‐frequency oscillation versus conventional ventilation for the treatment of acute respiratory distress syndrome.
| Patient or population: people with Acute Respiratory Distress Syndrome Settings: Critical Care Unit Intervention: High‐fFrequency oscillation Comparison: Conventional mechanical ventilation | ||||||
| Outcomes** | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional Mechanical Ventilation | High Frequency Oscillation | |||||
| Mortality (at 30 days or until hospital discharge) | Medium risk population1 | RR 0.92 (0.72 to 1.16) | 1779 (8 studies) | ⊕⊝⊝⊝ very low2,3,4,5,6 | ||
| 470 per 1000 | 432 per 1000 (338 to 545) | |||||
| Mortality (at 6 months) | Medium risk population | RR 0.79 (0.58 to 1.08) | 148 (1 study) | ⊕⊝⊝⊝ very low2,4,5 | ||
| 590 per 1000 | 466 per 1000 (342 to 637) | |||||
| Treatment failure | Medium risk population1 | RR 0.64 (0.48 to 0.85) | 956 (6 studies) | ⊕⊝⊝⊝ very low2,4,5,7 | ||
| 190 per 1000 | 122 per 1000 (91 to 162) | |||||
| Duration of mechanical ventilation days. Scale from: 14 to 22 | The mean duration of mechanical ventilation in the control groups was 17.1 | The mean duration of mechanical ventilation in the intervention groups was 0.59 higher (1.09 lower to 2.28 higher) | 1142 (5 studies) | ⊕⊝⊝⊝ very low4,5 | ||
| Barotrauma | Medium risk population1 | RR 0.82 (0.51 to 1.32) | 951 (7 studies) | ⊕⊝⊝⊝ very low2,4,5,7 | ||
| 131 per 1000 | 121 per 1000 (67 to 173) | |||||
| Hypotension | Medium risk population1 | RR 1.02 (0.54 to 1.90) | 392 (4 studies) | ⊕⊝⊝⊝ very low2,4,5,7 | ||
| 381 per 1000 | 389 per 1000 (206 to 724) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; ** Not shown:ventilator‐free days (very low quality 2,3,4,5, pooled analysis not performed due to extreme heterogeneity), endotracheal tube obstruction (very low quality2,4,5, pooled analysis not performed because all events occurred in one study), non‐clinical endpoints (i.e. physiologic endpoints, for example mean airway pressure, because these are not patient‐important outcomes). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The basis of the assumed risk is the median risk in the control groups across trials. 2Downgraded for serious methodologic limitations (blinding was not possible in all studies; cross‐overs between treatment groups > 10% occurred in 3 trials; post‐randomization withdrawals ranging from 1.4% ‐ 17% occurred in 3 trials). 3Downgraded for inconsistency (moderate or high statistical heterogeneity). 4Downgraded for serious indirectness in the comparator (5 out of 10 trials included in this systematic review did not mandate low tidal volume conventional ventilation in participants who received conventional mechanical ventilation). 5Downgraded for imprecision (wide confidence intervals which include no benefit, and/or appreciable harm or benefit). With the exception of hospital or 30‐day mortality, the number of outcome events was low (i.e. fewer than 300). 6Although the event rate exceeded 300, and the sample size exceeded the optimal information size of 1290 (assuming alpha 0.05, beta 0.10, a baseline risk of death of 45%, and a 9% absolute reduction in risk), confidence intervals were wide and included no benefit (i.e. RR = 1) as well as both meaningful benefit and harm. We therefore downgraded this outcome due to serious imprecision. 7Downgraded for serious indirectness in the outcome (variable definition of treatment failures, hypotension, and barotrauma across studies).
Background
Description of the condition
Acute respiratory distress syndrome (ARDS) is a life‐threatening condition characterized by acute lung inflammation causing pulmonary congestion, hypoxaemia, and decreased pulmonary compliance. ARDS is defined as a syndrome characterized by acute onset (i.e. within seven days), bilateral pulmonary infiltrates on chest radiograph not fully explained by cardiac failure or fluid overload, and ratio of arterial oxygen tension to fraction of inspired oxygen (PaO₂/FiO₂) less than 300. People with mild ARDS (formerly called acute lung injury) have a PaO₂/FiO₂ between 200 and 300. Moderate and severe ARDS refer to the more severely ill subset of people in whom the PaO₂/FiO₂ ratio is less than or equal to 200 and 100 respectively (ARDS Definition Task Force 2012). ARDS has a fairly high incidence (Rubenfeld 2005) and is associated with substantial mortality (Phua 2009; Rubenfeld 2005), morbidity (Angus 2001; Herridge 2003; Herridge 2011), and costs (Cheung 2006).
Description of the intervention
High‐frequency oscillation (HFO) is an alternative ventilation technique in which very small tidal volumes are delivered at very high frequencies using an oscillatory pump while mean airway pressure is held constant. Humidified, oxygenated gas (bias flow) passes in front of a diaphragm which oscillates at high frequencies (3 to 15 Hz, or 180 to 900 breaths per minute). The mean airway pressure is determined by the rate of bias flow and by a resistance valve at the end of the bias flow circuit. Tidal volumes are much smaller during high‐frequency oscillation (1 to 4 mL/kg) compared to mechanical ventilation, and the constant mean airway pressure leads to smaller changes in alveolar pressure. Because tidal volumes during HFO may be smaller than anatomic dead space, gas exchange occurs through gas mixing as opposed to bulk flow. Mechanisms of gas exchange during HFO include direct ventilation of proximal alveoli, Taylor dispersion, asymmetric velocity profiles, pendelluft, and diffusion (Slutsky 2002).
How the intervention might work
Mechanical ventilation is usually required for adequate tissue oxygenation (Artigas 1998) but may also perpetuate lung injury by over‐distending and rupturing healthy alveoli, and by triggering a secondary inflammatory response that amplifies lung injury from repeatedly opening and collapsing lung units (Dreyfuss 1988; Gattinoni 2005; Muscedere 1994; Ranieri 1999). Lung‐protective ventilation seeks to limit alveolar distension, recruit non‐aerated alveoli, and prevent further alveolar collapse (Gattinoni 2006). Low tidal volumes with (Amato 1998; Meade 2008; Mercat 2008; Villar 2006) or without (ARDS Network 2000; Brochard 1998; Stewart 1998) high positive end‐expiratory pressure may reduce ventilator‐induced lung injury. Nevertheless, mortality in people with ARDS remains high (Phua 2009; Rubenfeld 2005). HFO theoretically meets the goals of a lung‐protective ventilation strategy (Rimensberger 2003), with extremely small tidal volumes (1 to 4 mL/kg) and constant lung recruitment.
Why it is important to do this review
Although HFO is used in some centres in people with ARDS who do not tolerate conventional mechanical ventilation (Chan 2005; Finkielman 2006; Mehta 2004), its role, especially outside the realm of ‘rescue’ therapy, remains controversial (Ferguson 2008; Kacmarek 2008). Several observational studies (Ferguson 2005; Fort 1997; Mehta 2001) show improved oxygenation when hypoxaemia seems refractory. Our earlier systematic review of randomized controlled trials (Sud 2013) included eight small trials, and found that HFO might reduce mortality, although several trials did not mandate optimal lung‐protective ventilation in control groups. Additional studies have subsequently become available. Furthermore, in the context of recent (Dominguez‐Cherit 2009; Kumar 2009) and future pandemics, there is a pressing need for evidence‐based syntheses of the effects of potentially life‐saving interventions for people with ARDS. We have therefore updated our previous systematic review and meta‐analysis of randomized controlled trials (Sud 2013) comparing HFO to conventional mechanical ventilation for adults and children with ARDS, to determine the effects of HFO on mortality, other clinical and physiological outcomes, and adverse events, to address these issues.
Objectives
To determine the effects of HFO compared to conventional mechanical ventilation on physiological outcomes, clinical outcomes, and mortality when used for the treatment of acute respiratory distress syndrome (ARDS).
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group, randomized controlled trials (RCTs) which compared HFO to conventional mechanical ventilation for the treatment of ARDS and reported at least one of our prespecified outcomes.
Types of participants
We included adults or children (greater than four weeks old and 42 weeks post‐conception) with ARDS who were receiving conventional mechanical ventilation. We accepted authors' definitions of ARDS. In trials enrolling participants with other forms of respiratory failure, we stipulated that a minimum of 70% of participants must have ARDS to meet the inclusion criteria. We included trials that enrolled both adults and children because we believed that the physiological benefits of lung recruitment and reduction in tidal volume that occur during high‐frequency oscillation would be similar for both adult and paediatric ARDS (Albuali 2007; ARDS Network 2000; Hanson 2006; Miller 2008).
Types of interventions
We included studies in which participants were randomly assigned to two or more groups, including an experimental group that received HFO and a control group that received conventional mechanical ventilation for ARDS. We also included trials in which a secondary intervention was delivered as part of HFO, such as tracheal gas insufflation or recruitment manoeuvres, since these are applied in association with HFO in some centres. We included trials in which the duration of HFO was 24 hours or less for physiological outcome analyses but excluded them from analyses of major clinical outcomes, such as mortality.
Types of outcome measures
Primary outcomes
Hospital or 30‐day mortality
Secondary outcomes
Six‐month mortality
Duration of mechanical ventilation (in days, as stated by the authors)
Ventilator‐free days to day 28 or 30 (in days, as stated by the authors)
Health‐related quality of life at one year
Treatment failure, leading to cross‐over to the other arm or discontinuation of the study protocol. We accepted authors’ definitions of treatment failure, which could include severe oxygenation failure, ventilation failure, hypotension, or barotrauma (pneumothorax, pneumomediastinum, subcutaneous emphysema)
The ratio of partial pressure of arterial oxygen (PaO₂) to inspired fraction of oxygen (FiO₂) (PaO₂/FiO₂ ratio) at 24, 48, and 72 hours after randomization
Oxygenation index ((OI), defined as 100 x mean airway pressure/(PaO₂/FiO₂ ratio)) measured at 24, 48, and 72 hours after randomization
Ventilation, measured by partial pressure of carbon dioxide (PaCO₂) at 24, 48, and 72 hours after randomization
Mean airway pressure 24, 48, and 72 hours after randomization
Barotrauma (as stated by the authors)
Hypotension (as stated by the authors)
Endotracheal tube obstruction due to secretions
Technical complications and equipment failure in participants treated with HFO (including unintentional system air leaks and problems with the oscillatory diaphragm, humidifier, and alarm systems) (Cartotto 2004; Finkielman 2006)
Search methods for identification of studies
We used systematic methods to identify published and unpublished RCTs comparing HFO to conventional mechanical ventilation in participants with ARDS, or other forms of hypoxaemic respiratory failure (Meade 1997).
For the previous version of this review (Sud 2013) we searched until 2011.
Electronic searches
To update our previous literature search, we:
electronically searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 11), MEDLINE (OvidSP) (1948 to December 2015), and EMBASE (OvidSP) (1980 to December 2015); please see Appendix 1 for search details; and
searched for unpublished and ongoing trials in clinicaltrials.gov and controlled‐trials.com.
Searching other resources
In addition to the electronic search, we:
manually searched reference lists from included studies and review articles;
searched conference proceedings of the American Thoracic Society (1994 to 2015), Society of Critical Care Medicine (1994 to 2015), European Society of Intensive Care Medicine (1994 to 2015), and American College of Chest Physicians (1994 to 2015); and
contacted clinical experts in the field.
Data collection and analysis
We collected and analysed data according to a prespecified protocol and analysis plan (Sud 2013; Differences between protocol and review). We assessed the quality of evidence for major clinical outcomes including mortality and treatment failure according to recommendations of the GRADE working group (Schünemann 2008).
Selection of studies
Two review authors (SS, MS) who were not blinded to study authors or results (Berlin 1997) independently evaluated study eligibility and resolved differences by consensus. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2015), and Characteristics of excluded studies tables. We did not impose any language restrictions.
Data extraction and management
Three review authors (SS, MS, JF), using a standardized spreadsheet, independently abstracted data on study methods, details of ventilation strategies, and study outcomes. We resolved any disagreements remaining after author contact by consensus.
Assessment of risk of bias in included studies
We abstracted data on methods of randomization and allocation concealment (Chalmers 1983), number of post‐randomization withdrawals and losses to follow‐up, cross‐overs between assigned groups, blinding of outcome assessors (Schulz 1995), and early stopping for benefit (Montori 2005). We summarized the risk of bias for individual studies using the Cochrane ‘Risk of bias’ instrument (Higgins 2011). Since blinding of caregivers, participants, and family members was impossible in these trials, we determined whether important co‐interventions (weaning, sedation, and paralysis) and use of rescue therapies for refractory respiratory failure (inhaled nitric oxide, prone positioning, steroids, and extracorporeal oxygenation) were standardized or equally applied in treatment groups.
Measures of treatment effect
We reported continuous outcomes using the mean difference (MD), a measure of absolute change, and binary outcomes as risk ratios (RRs). For continuous physiological outcomes we reported ratio of means (RoM), a measure of relative change (Friedrich 2008). We considered a (two‐sided) P value < 0.05 as statistically significant and report individual trial and summary results with 95% confidence intervals (CIs).
Unit of analysis issues
For clinical outcomes, the unit of analysis was the participant. We did not identify any issues of 'double‐counting', for example the reporting of number of events instead of the number of participants who experienced an event.
For physiologic outcomes, the unit of analysis was at the study level since investigators reported the mean, standard error or standard deviation, and the number of participants with measurements. Because several trials used repeated measurements for physiologic endpoints (for example PaO₂/FiO₂) we chose to perform a separate meta‐analysis for each of these endpoints at the prespecified times of 24, 48, and 72 hours after randomization.
Dealing with missing data
We contacted primary investigators of all included trials by email and fax to request additional data and to clarify methodology after careful review of each study. Primary investigators provided additional clinical (Demory 2007; Derdak 2002; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004) or physiologic data (Derdak 2002; Mentzelopoulos 2012; Samransamruajkit 2005; Shah 2004), or clarified data or methods (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013).
Assessment of heterogeneity
Potential sources of clinical and methodological heterogeneity between studies included participants, ventilation strategies, and outcome definitions. Because we felt that this diversity would not support the underlying assumptions of a fixed‐effect model, we used random‐effects models for all pooled analyses. Random‐effects models incorporate both within‐study and between‐study variation and typically provide wider CIs when heterogeneity is present. We assessed between‐study statistical heterogeneity for each outcome using the I² measure (Higgins 2002; Higgins 2003) and used published guidelines for low (I² = 25% to 49%), moderate (I² = 50% to 74%), and high (I² ≥ 75%) heterogeneity (Higgins 2003). We investigated potential sources of clinical and methodological heterogeneity through a priori subgroup analyses and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity; Sensitivity analysis).
Assessment of reporting biases
We tested for publication bias statistically using the Begg’s Rank Correlation test (Begg 1994) and Macaskill’s regression test (Macaskill 2001) as modified by Peters et al (Peters 2006).
Data synthesis
For each prespecified outcome, we employed random‐effects models to perform meta‐analysis with Review Manager 5 (RevMan 2014) where at least two trials with similar participants, interventions, and outcome definitions reported adequate data. We used the Mantel‐Haenszel variance estimator to obtain pooled risk ratios for dichotomous outcomes, and the generic inverse variance method to pool ratio‐of‐means outcomes. We generated forest plots to summarize our results and reported risk ratio or ratio of means with corresponding 95% CIs. We used Microsoft Excel with equations for the ratio of means to generate the ratio and standard error.
We used the metabias command in STATA 9.2 (Stata 9.2) for statistical tests of publication bias. Given the low statistical power of these tests, we assumed a more liberal level of significance (P value < 0.10) to indicate possible publication bias.
Subgroup analysis and investigation of heterogeneity
A priori, we planned subgroup analyses to explore potential heterogeneity for the primary outcome of hospital or 30‐day mortality and to test whether results were consistent for important subgroups of trials or participants.
First, we hypothesized that age might influence the benefit from HFO. Increasing age worsens the prognosis in ARDS and thus older participants might benefit less from HFO compared with younger participants (Brun‐Buisson 2004; Flori 2005). Conversely, HFO is commonly used for neonatal respiratory distress syndrome and thus children may benefit more from HFO compared to adults (Cools 2015). We therefore performed a subgroup analysis comparing effects of HFO in trials enrolling post‐neonatal children (weight ≤ 35 kg, as defined in the paediatric trials) versus adults.
Second, we hypothesized that the subset of participants with severe and life‐threatening hypoxaemia may benefit more from HFO, recognizing that most people with ARDS do not die of hypoxaemia (Montgomery 1985; Stapleton 2005). We therefore planned a subgroup analysis of participants with a higher (≥ 150) versus lower (< 150) baseline PaO₂/FiO₂ ratio, but insufficient data precluded this analysis.
We performed a post hoc subgroup analysis comparing trials which mandated lung‐protective ventilation in the control group, defined as ≤ 8 mL/kg of ideal or predicted body weight, to trials that did not mandate tidal volumes ≤ 8 mL/kg, to examine whether the benefit of HFO might be greater when compared to conventional ventilation with higher tidal volumes compared to conventional ventilation with smaller tidal volumes (ARDS Network 2000; Brochard 1998; Stewart 1998).
We assessed any subgroup effects using a z‐test for interaction.
Sensitivity analysis
A priori, we hypothesized that potential methodological biases (e.g. quasi‐randomization or unclear or unconcealed allocation) might influence the results of our meta‐analysis in favour of HFO, whereas excessive cross‐overs or losses to follow‐up might bias the results against HFO. We therefore performed a sensitivity analysis limited to trials at low risk of bias, across all domains of the Cochrane 'Risk of bias' tool.
We also performed a sensitivity analysis in which we excluded trials at risk of bias due to early termination for benefit or harm.
Finally, we performed additional sensitivity analyses to test the robustness of our findings under alternative assumptions where data were incomplete or missing after author contact (for example, censoring participants who were lost to follow‐up). These are described in the text (see Effects of interventions).
Results
Description of studies
Results of the search
In our original review (Sud 2013) we identified eight trials that were relevant to our topic of interest. We included eight RCTs (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004) and excluded seven studies (Carlon 1983; Dobyns 2002; Fessler 2008; Hurst 1984; Hurst 1990; Mentzelopoulos 2007a; Mentzelopoulos 2010).
In this updated version, we searched from the start of 2011 to December 2015; we identified 1111 citations from searches of electronic bibliographic databases and eight citations from our previous systematic review (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004). We retrieved 12 citations for detailed evaluation, of which 10 were unique RCTs (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013) which met the criteria for this review. One citation was an abstract for an RCT which was included after we obtained additional details from the primary investigator (Shah 2004), and one citation was for an RCT which did not meet our inclusion criteria (Vrettou 2014). Review authors were in perfect agreement for study inclusion.
See Figure 1.
1.
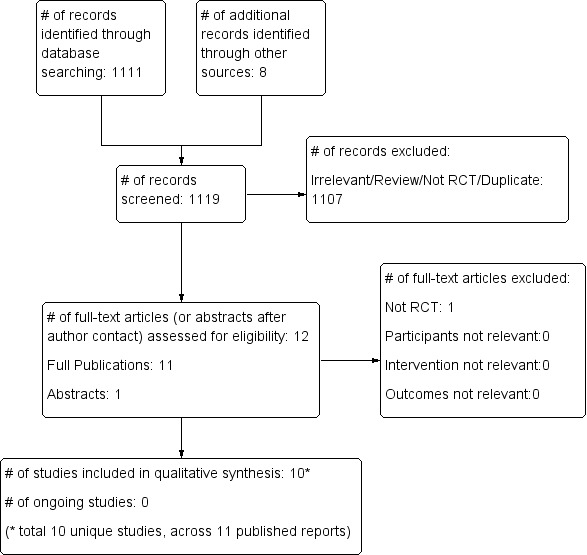
Study flow diagram for the updated literature search (from 2011 to Dec 2015, see also Results of the search)
Included studies
The 10 included trials (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013) (see Characteristics of included studies) enrolled 1850 participants with ARDS. Nine trials (Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013) enrolled participants exclusively with ARDS (n = 1780), and 86% of the participants in the 10th trial had ARDS (Arnold 1994). Two trials enrolled only children (Arnold 1994; Samransamruajkit 2005). One trial is currently published as an abstract (Shah 2004). All trials (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013) studied HFO as an initial ventilation strategy for ARDS as opposed to rescue therapy for refractory hypoxaemia. The trials enrolled participants within 48 (Demory 2007; Papazian 2005; Samransamruajkit 2005) or 72 (Mentzelopoulos 2012) hours of diagnosis of ARDS, or shortly after initiation of mechanical ventilation, mean of less than three days (Bollen 2005; Derdak 2002; Ferguson 2013; Young 2013) or five days (Arnold 1994; Shah 2004). All trials treated participants continuously with HFO except for one that applied HFO for six to 24 hours per day (most participants were treated for at least four days) according to a protocol until predefined criteria for resolution of severe ARDS had been met (Mentzelopoulos 2012). In two trials participants were treated for ≤ 24 hours (Demory 2007; Papazian 2005). The median baseline PaO₂/FiO₂ ratio was 113 (range 102 to 122) in nine trials (Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013).
Details of high frequency oscillation
Participants received HFO for a prespecified period (Demory 2007; Papazian 2005) (n = 54), until prespecified criteria for weaning from HFO were met (Arnold 1994; Bollen 2005; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Young 2013) (n = 1763), or until clinical resolution of ARDS (Shah 2004) (n = 33). All studies standardized HFO implementation (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004; Young 2013). HFO was initiated with a mean airway pressure 2 to 5 cm H₂O above mean airway pressure while on conventional ventilation (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Shah 2004) (n = 507), 5 cm H₂0 above plateau pressure (Young 2013) (n = 795), or at 30 cm H₂0 (Ferguson 2013) (n = 548). The initial frequency was set between 4 and 10 Hz. Pressure amplitude of oscillation was determined subjectively by chest wall vibration (Arnold 1994; Bollen 2005; Derdak 2002; Shah 2004) (n = 312), set according to arterial partial pressure of carbon dioxide (Demory 2007; Mentzelopoulos 2012; Papazian 2005) (n = 195), set at 10 cm H₂O above the peak inspiratory pressure during prerandomization conventional mechanical ventilation (Samransamruajkit 2005) (n = 16), or set at 90 cm H₂0 (Ferguson 2013) (n = 548). Many trials described adjunctive measures during HFO, including partial endotracheal tube cuff leak for hypercarbia (Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Shah 2004) (n = 908), tracheal gas insufflation (Mentzelopoulos 2012) (n = 125), and recruitment manoeuvres (Demory 2007; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005) (n = 727).
Details of conventional mechanical ventilation
All trials provided a description of conventional mechanical ventilation. Protocols for adjusting settings during conventional mechanical ventilation were described in six trials (Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Shah 2004) (n = 908). Five trials (Demory 2007; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Shah 2004) (n = 760) used low tidal volume ventilation in all participants (6 to 8 mL/kg predicted body weight (Demory 2007; Ferguson 2013; Mentzelopoulos 2012; Shah 2004) or ideal body weight (Papazian 2005)), and five trials (Demory 2007; Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Shah 2004) (n = 750) mandated plateau pressure below 35 cm H₂O. Eight trials (Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Shah 2004; Young 2013 ) (n = 1764) reported a mean positive end‐expiratory pressure of 11 to 18 cm H₂O during the first 72 hours of conventional mechanical ventilation.
Excluded studies
See Characteristics of excluded studies.
In our original review we excluded two studies (Carlon 1983; Hurst 1990) because they enrolled participants without ARDS, four studies (Dobyns 2002; Fessler 2008; Mentzelopoulos 2007a; Mentzelopoulos 2010) because they randomized on interventions other than HFO, and one study (Hurst 1984) which was a cross‐over trial. In this update, we excluded one additional trial (Vrettou 2014) which was a cross‐over trial.
Awaiting classification
We found no studies awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
Five trials (Demory 2007; Derdak 2002; Papazian 2005; Samransamruajkit 2005; Young 2013 ) had low risk of bias for our main outcome, hospital or 30‐day mortality, across all domains of the Cochrane 'Risk of bias' tool (except for blinding, which was not possible because of the nature of the intervention). The risk of bias was unclear in five studies (Arnold 1994; Bollen 2005; Ferguson 2013; Mentzelopoulos 2012; Shah 2004;) (see Characteristics of included studies; Figure 2; Figure 3).
2.
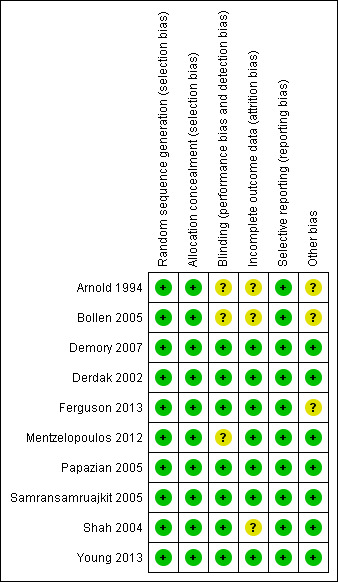
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
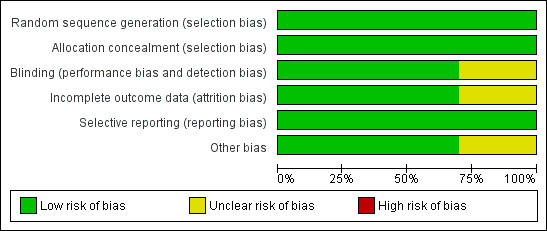
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
All trials concealed allocation and analysed clinical outcomes for participants by assigned group (Arnold 1994; Bollen 2005; Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Shah 2004; Young 2013) or provided enough information to perform analyses according to assigned group (Samransamruajkit 2005).
Blinding
Although no study could be completely blinded due to the nature of the intervention, the primary outcome for our systematic review was hospital or 30‐day mortality, which would be less susceptible to detection bias. Three studies reported partial blinding of: study investigators (Derdak 2002; Ferguson 2013); trial sponsors (Derdak 2002); and radiologists who assessed for barotrauma (Arnold 1994). All included trials used standardized protocols for the implementation of HFO, and six trials used protocols for adjusting settings during conventional mechanical ventilation (Demory 2007; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Shah 2004, n = 908) or provided guidelines to standardize conventional mechanical ventilation (Young 2013, n = 795), which would have reduced performance bias.
Incomplete outcome data
Seven trials (Bollen 2005; Demory 2007; Ferguson 2013; Mentzelopoulos 2012; Papazian 2005; Samransamruajkit 2005; Young 2013) reported no post‐randomization withdrawals; in three trials 1.4% (2/148) (Derdak 2002), 17% (12/70) (Arnold 1994), and 15% (5/33) (Shah 2004) of participants were withdrawn after randomization. After contacting investigators, we obtained mortality data for withdrawn participants in two trials (Derdak 2002; Shah 2004).
There was no loss to follow‐up in eight studies (Arnold 1994; Demory 2007; Derdak 2002; Ferguson 2013; Papazian 2005; Shah 2004; Samransamruajkit 2005; Young 2013) Two studies reported losses to follow‐up: intensive care unit (ICU) but not 30‐day mortality was available for 5% (3/61) of participants in one study (Bollen 2005); in another trial, one of 61 participants randomized to HFO was lost to follow‐up after being discharged to another institution on day 31 after randomization (Mentzelopoulos 2012).
Selective reporting
We found no evidence of selective reporting within in the included studies.
We found no evidence of publication bias. Neither Begg’s Rank Correlation test (P value = 0.71) nor Macaskill‐Peters' regression test (P value = 0.401) was significant.
Other potential sources of bias
Two trials were terminated early because of low recruitment (Bollen 2005) or after early stopping criteria for harm were nearly met (Ferguson 2013).
Seven trials (Arnold 1994; Bollen 2005; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Young 2013) reported cross‐overs between groups (range 1.3% to 52% of all randomized participants), which involved 0% to 19% of participants randomized to HFO (11/29 (Arnold 1994), 7/37 (Bollen 2005), 4/75 (Derdak 2002), 0/27 (Mentzelopoulos 2012), 0/6 (Samransamruajkit 2005) and 0/398 (Young 2013); and 2.5% to 65% of participants randomized to conventional ventilation (19/29 (Arnold 1994), 4/24 (Bollen 2005), 9/73 (Derdak 2002), 31/273 (Ferguson 2013), 2/27 (Mentzelopoulos 2012), 1/10 (Samransamruajkit 2005), and 10/397 (Young 2013)).
Effects of interventions
See: Table 1
Primary outcomes
1. Hospital or 30‐day mortality
In the primary analysis, including eight trials that treated participants with HFO until prespecified criteria for weaning or resolution of ARDS (Arnold 1994; Bollen 2005; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Shah 2004; Young 2013) (n = 1779), the median hospital or 30‐day mortality in the control group was 47% (range 33% to 67%). HFO did not reduce hospital or 30‐day mortality (RR 0.92, 95% CI 0.72 to 1.16; P = 0.46; Figure 4).
4.
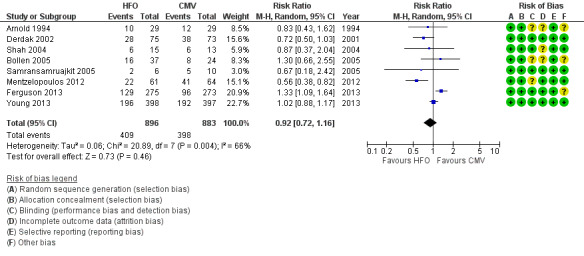
Forest plot of comparison: 1 Mortality, outcome: 1.1 Hospital or 30‐day mortality. (HFO = high frequency oscillation, CMV = conventional mechanical ventilation)
Mortality was determined at discharge from hospital (Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Young 2013) or at 30 days after randomization (Arnold 1994; Bollen 2005; Derdak 2002; Shah 2004). In two trials, 3/61 participants alive at discharge from the ICU (Bollen 2005) and 1/125 participants alive at the time of transfer to another facility (Mentzelopoulos 2012) were assumed to have survived; censoring these participants did not alter the results of the meta‐analysis (RR 0.92, 95% CI 0.73 to 1.16; P = 0.48; Analysis 1.2). Including three HFO and two conventional ventilation participants in one study (Shah 2004) who were withdrawn because they died less than 48 hours after randomization, but for whom no other baseline or outcome data were available after author contact, did not alter our findings ( RR 0.92, 95% CI 0.73 to 1.16, I2 = 66%, Analysis 1.3).
1.2. Analysis.
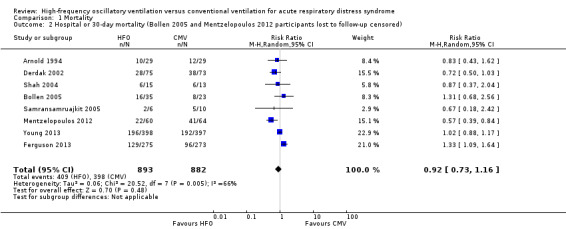
Comparison 1 Mortality, Outcome 2 Hospital or 30‐day mortality (Bollen 2005 and Mentzelopoulos 2012 participants lost to follow‐up censored).
1.3. Analysis.
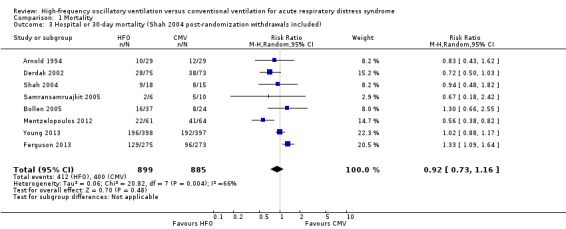
Comparison 1 Mortality, Outcome 3 Hospital or 30‐day mortality (Shah 2004 post‐randomization withdrawals included).
Subgroup analyses did not show significant differences in treatment effect among six adult (Bollen 2005; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Shah 2004; Young 2013) versus two paediatric (Arnold 1994; Samransamruajkit 2005) trials (P = 0.63 for interaction z‐test; Analysis 1.4). In a post hoc subgroup analysis, there was no significant difference in treatment effect among four trials (Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Shah 2004) that mandated tidal volumes ≤ 8 mL/kg in the control group and four trials (Arnold 1994; Bollen 2005; Derdak 2002; Young 2013) that permitted higher tidal volumes (z‐test for interaction P = 0.74; Analysis 1.5). Excluding one trial (Ferguson 2013) terminated early for harm did not alter our findings (RR 0.83, 95% CI 0.65 to 1.06; P = 0.08, I² = 48%). Similarly excluding five trials at unclear risk of bias (Arnold 1994; Bollen 2005; Ferguson 2013; Mentzelopoulos 2012; Shah 2004) did not alter our findings (RR 0.89, 95% CI 0.68 to 1.18; P = 0.42, I² = 41%, Analysis 1.6).
1.4. Analysis.
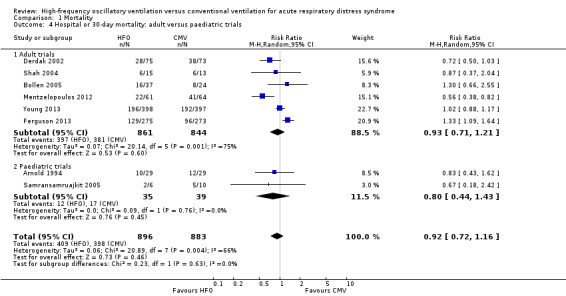
Comparison 1 Mortality, Outcome 4 Hospital or 30‐day mortality: adult versus paediatric trials.
1.5. Analysis.
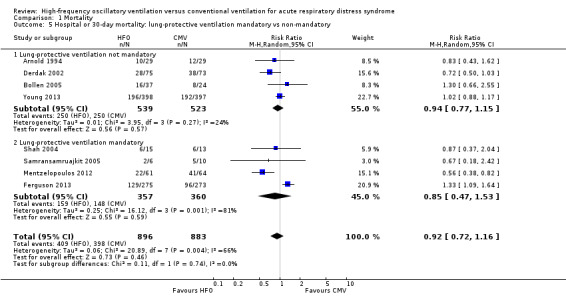
Comparison 1 Mortality, Outcome 5 Hospital or 30‐day mortality: lung‐protective ventilation mandatory vs non‐mandatory.
1.6. Analysis.
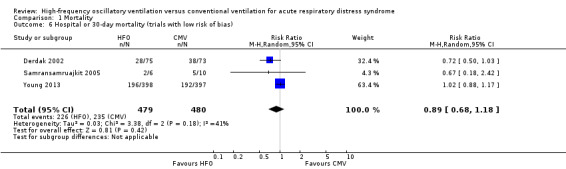
Comparison 1 Mortality, Outcome 6 Hospital or 30‐day mortality (trials with low risk of bias).
We found evidence of moderate statistical heterogeneity (I² = 66%) for our primary endpoint of hospital or 30‐day mortality, but statistical heterogeneity was low for secondary and adverse outcomes (I² = 0% ‐ 14%).
Secondary outcomes
2. Six‐month mortality
Only one trial (Derdak 2002) (n = 148) reported six‐month mortality, with no significant effect of HFO (RR 0.79, 95% CI 0.58 to 1.08; P = 0.14).
3. Duration of mechanical ventilation
The duration of mechanical ventilation (MD 0.59 days, 95% CI ‐1.09 to 2.28; P = 0.49; 5 trials, 1142 participants; Analysis 2.1) (Arnold 1994; Derdak 2002; Mentzelopoulos 2012; Samransamruajkit 2005; Young 2013) did not significantly differ between groups.
2.1. Analysis.
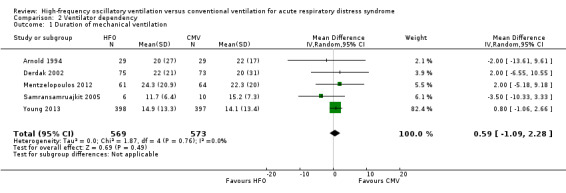
Comparison 2 Ventilator dependency, Outcome 1 Duration of mechanical ventilation.
4. Ventilator‐free days to day 28 or 30
We do not report a pooled result for ventilator‐free days because heterogeneity was extremely high ((I² = 95%) across two studies (Mentzelopoulos 2012; Young 2013) that reported this outcome.
5. Health‐related quality of life at one year
No trial reported health‐related quality of life. We identified one trial which collected health‐related quality of life at six months and one year; data are not yet available (Young 2013).
6. Treatment failure
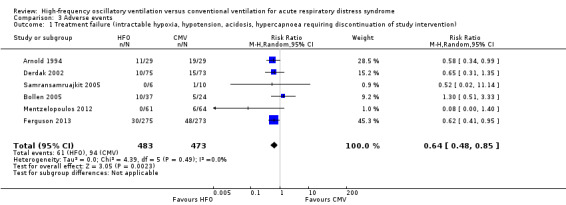
In six trials (Arnold 1994; Bollen 2005; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005) (n = 956) HFO reduced the risk of treatment failure compared with conventional mechanical ventilation (RR 0.64, 95% CI 0.48 to 0.85; P = 0.002; Figure 5). Three trials (Arnold 1994; Bollen 2005; Derdak 2002) (n = 267) reported treatment failure according to predefined criteria. Three trials (Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005) (n = 699) did not report this outcome but we obtained data directly from the authors (Mentzelopoulos 2012; Samransamruajkit 2005) or from outcomes in the published report (Ferguson 2013). In one trial, one participant randomized to conventional ventilation with early treatment failure who crossed over to HFO because of barotrauma was analysed as a treatment failure in the conventional mechanical ventilation group (Samransamruajkit 2005). When we did not count the two participants randomized to conventional ventilation in one trial (Mentzelopoulos 2012) who crossed over to HFO for only three and six hours as treatment failures, the pooled result remained significant (RR 0.64, 95% CI 0.48 to 0.86; P = 0.003). No trial reported blinding of outcome assessors or independent adjudication of treatment failure.
5.
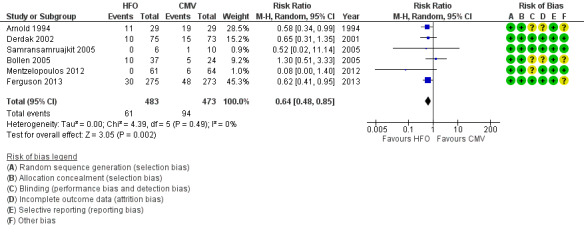
Forest plot of comparison: 2 Adverse events, outcome: 2.1 Treatment failure (intractable hypoxia, hypotension, acidosis, hypercapnoea requiring discontinuation of study intervention). (HFO = high frequency oscillation, CMV = conventional mechanical ventilation)
Physiologic outcomes
Physiological outcomes for day one, day two, and day three are summarized in Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; and Appendix 2.
4.1. Analysis.
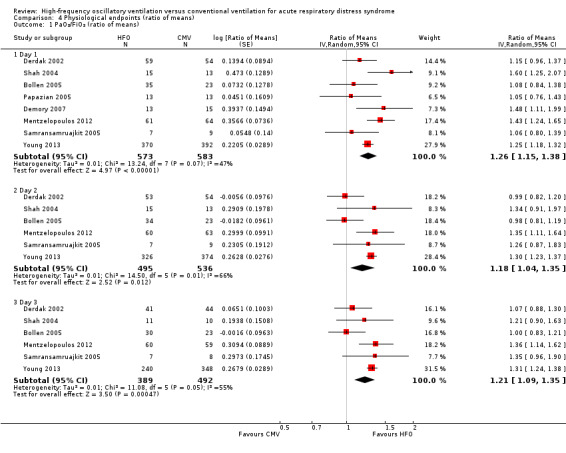
Comparison 4 Physiological endpoints (ratio of means), Outcome 1 PaO₂/FiO₂ (ratio of means).
4.2. Analysis.
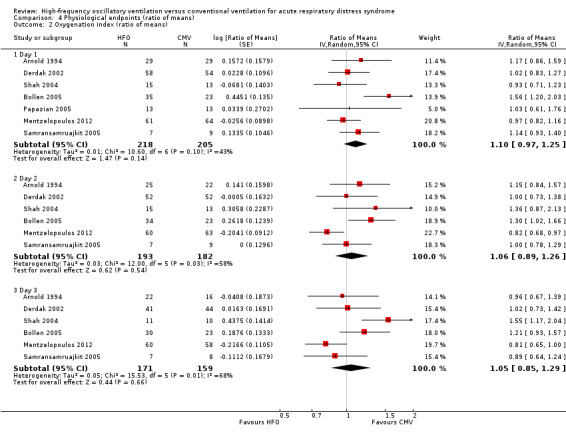
Comparison 4 Physiological endpoints (ratio of means), Outcome 2 Oxygenation index (ratio of means).
4.3. Analysis.
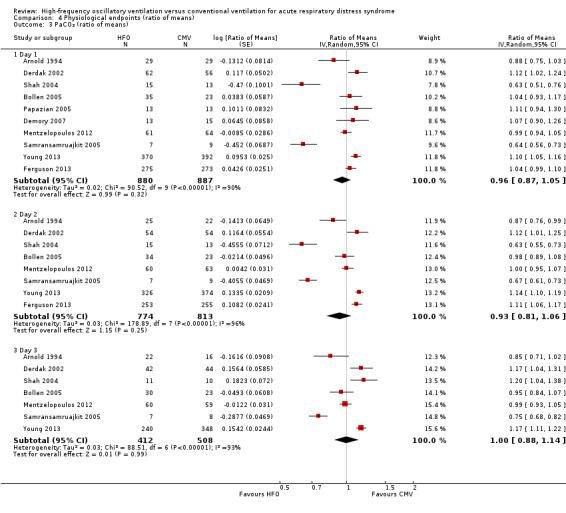
Comparison 4 Physiological endpoints (ratio of means), Outcome 3 PaCO₂ (ratio of means).
4.4. Analysis.
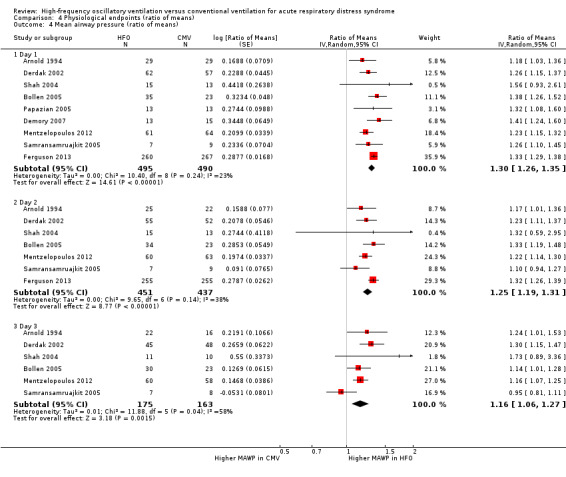
Comparison 4 Physiological endpoints (ratio of means), Outcome 4 Mean airway pressure (ratio of means).
Day one measurements were obtained at 24 hours except in two studies where they were obtained at 12 hours (Demory 2007; Papazian 2005). Analyses were by intention‐to‐treat except for one participant in one trial (Samransamruajkit 2005) who crossed over from conventional ventilation to HFO shortly after randomizations and was analysed as treated because we were unable to obtain sufficient data (after contacting the author) to permit an intention‐to‐treat analysis.
7. PaO₂/FiO₂ ratio at 24, 48, and 72 hours after randomization
At 24, 48, and 72 hours, HFO increased the PaO₂/FiO₂ ratio by 18% to 26% relative to conventional mechanical ventilation (see Analysis 4.1 and Appendix 2).
One trial included in our review combined tracheal gas insufflation and recruitment manoeuvres with HFO (Mentzelopoulos 2012). Because a separate randomized cross‐over trial (not included in this review due to cross‐over design) (Mentzelopoulos 2007a) showed that these co‐interventions may improve oxygenation, we performed a sensitivity analysis in which data from Mentzelopoulos 2012 were not included in the analysis of the effect of HFO on the PaO₂/FiO₂ ratio. Results were similar: ratio of means (RoM) of 1.23 (95% CI 1.11 to 1.35; P = 0.0001, I² = 39%) on day one, 1.15 (95% CI 0.98 to 1.35; P = 0.10, I² = 71%) on day two, and 1.18 (95% CI 1.03 to1.35; P = 0.02, I² = 62%) on day three.
8. Oxygenation index at 24, 48, and 72 hours after randomization
Effects on the oxygenation index did not significantly differ between HFO and conventional ventilation (see Analysis 4.2 and Appendix 2).
9. PaCO₂ at 24, 48, and 72 hours after randomization
We do not report a pooled result because heterogeneity was extremely high (I² > 90%) in all analyses (Analysis 4.3 and Appendix 2).
10. Mean airway pressure 24, 48, and 72 hours after randomization
At 24, 48, and 72 hours, HFO increased mean airway pressure by 16% to 30% relative to conventional mechanical ventilation (see Analysis 4.4).
Adverse events
11. Barotrauma
We found no significant differences in the risk of barotrauma (7 trials, 951 participants; Analysis 3.2) (Arnold 1994; Bollen 2005; Derdak 2002; Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005; Shah 2004). Included studies varied in definitions of barotrauma: one study reported only pneumothorax (Shah 2004), three studies reported any pulmonary air leak (Derdak 2002; Mentzelopoulos 2012; Samransamruajkit 2005), one study reported any pulmonary air leak that developed during the protocol (Ferguson 2013), and two studies reported severe air leak resulting in treatment failure (Arnold 1994; Bollen 2005).
3.2. Analysis.
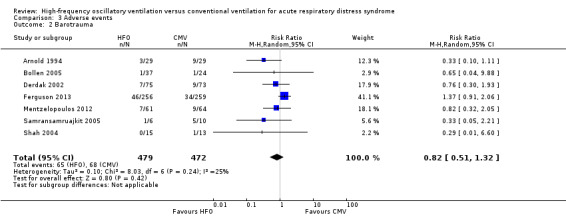
Comparison 3 Adverse events, Outcome 2 Barotrauma.
12. Hypotension
We found no significant differences in the risk of hypotension (4 trials, 392 participants; Analysis 3.3) (Arnold 1994; Bollen 2005; Derdak 2002; Mentzelopoulos 2012). Three studies reported intractable hypotension (Arnold 1994; Bollen 2005; Derdak 2002). One trial (Shah 2004) reported transient hypotension in one participant at the time of HFO initiation (1/15 in high‐frequency oscillation group and 0/13 in conventional ventilation group). Including these data minimally changed the pooled result (RR 1.01, 95% CI 0.61 to 1.66; P = 0.98, I² = 8%; Analysis 3.4).
3.3. Analysis.
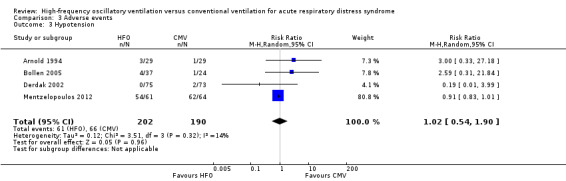
Comparison 3 Adverse events, Outcome 3 Hypotension.
3.4. Analysis.
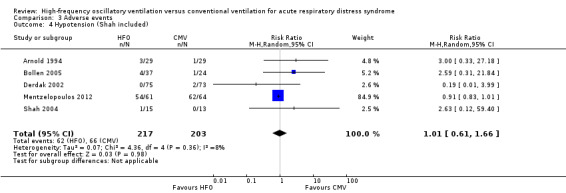
Comparison 3 Adverse events, Outcome 4 Hypotension (Shah included).
13. Endotracheal tube obstruction due to secretions
We found no significant differences in the risk of endotracheal tube obstruction (4 trials, 246 participants) (Derdak 2002; Mentzelopoulos 2012; Samransamruajkit 2005; Shah 2004). Although four trials reported on endotracheal tube obstruction, all events occurred in a single study (Derdak 2002), precluding a pooled analysis.
14. Technical complications and equipment failure in participants treated with HFO
There were no serious technical problems during HFO in the four trials that provided these data (Shah 2004; Samransamruajkit 2005; Derdak 2002; Mentzelopoulos 2012). One trial (n = 125) reported minor technical problems, including water accumulation in the circuit (number of instances not reported) and unintentional air leaks (21 instances in 223 uninterrupted sessions lasting six to 48 hours) and tearing of the ventilator diaphragm (seven instances in 223 uninterrupted sessions lasting six to 48 hours) (Mentzelopoulos 2012). A second trial (n = 148) reported four HFO device failures resulting from overheating, which was solved by switching to another Sensormedics 3100B HFO ventilator (Derdak 2002); the number of conventional ventilators which failed was not recorded.
Discussion
Summary of main results
The main finding of our systematic review and meta‐analysis is that HFO did not reduce hospital and 30‐day mortality in participants with ARDS compared to conventional mechanical ventilation. This finding was based on a small number of trials (eight for the primary analysis), participants (1779) and events (807), resulting in wide confidence intervals. The quality of evidence for the effect of HFO on mortality was very low, due to imprecision, inconsistency, indirectness and methodologic limitations (see Table 1). The risk of adverse events was similar.
Overall completeness and applicability of evidence
Our primary analysis was based on a small number of trials, some of which included relatively small numbers of participants and few outcome events, and have wide confidence intervals. Furthermore, although we found moderate heterogeneity for our primary outcome (hospital or 30‐day mortality), this may be an underestimate, given the small number of trials. A priori subgroup analyses did not adequately explain heterogeneity, although heterogeneity appeared to be slightly lower (I² = 41%) in three trials with low risk of bias (Derdak 2002; Samransamruajkit 2005; Young 2013). Nevertheless, HFO did not reduce mortality when only studies with low risk of bias were pooled (Analysis 1.6).
Although our meta‐analysis suggests HFO might reduce treatment failure, the criteria for treatment failure varied across trials (Arnold 1994; Bollen 2005; Derdak 2002) or were not predefined (Ferguson 2013; Mentzelopoulos 2012; Samransamruajkit 2005), and outcome assessors were not blinded. Therefore caution should be used when interpreting this finding, especially in view of the overall neutral effect of HFO on mortality in our systematic review, and the results of the second largest HFO trial to date (Ferguson 2013) which found that HFO increased mortality.
Limited data precluded subgroup analyses based on the degree of hypoxaemia and analyses of longer‐term mortality and health‐related quality of life. We found moderate to high heterogeneity for physiological endpoints, which limited their interpretability. Because of limited data, we were unable to analyse duration of mechanical ventilation separately for survivors and non‐survivors to address the possibility that differences in early mortality could drive overall differences in duration of mechanical ventilation. The risk of bias was low in five studies (Demory 2007; Derdak 2002; Papazian 2005; Samransamruajkit 2005; Young 2013) but remained unclear in five studies (Arnold 1994; Bollen 2005; Ferguson 2013; Mentzelopoulos 2012; Shah 2004). Thus, our findings do not support HFO for the treatment for ARDS. The overall quality of the evidence is very low for the most important outcomes to patients, such as mortality (see Table 1), indicating considerable uncertainty, and that future trials may impact our findings (Schünemann 2008).
Quality of the evidence
See Table 1.
The evidence for our main outcome of hospital of 30‐day mortality is drawn from three trials (Derdak 2002; Samransamruajkit 2005; Young 2013 ) with a low risk of bias and five trials (Arnold 1994; Bollen 2005; Ferguson 2013; Mentzelopoulos 2012; Shah 2004) with unclear risk of bias. Subgroup and sensitivity analyses did not provide a convincing explanation for observed variation between the results of the studies(seeAnalysis 1.4; Analysis 1.6; Analysis 1.5). We judged the quality of evidence for our main outcome of hospital or 30‐day mortality to be very low. We downgraded our assessment for the quality of evidence for serious indirectness in the comparator (four trials: Arnold 1994; Bollen 2005; Derdak 2002; Young 2013, n = 1062, did not mandate low tidal volume conventional ventilation in participants who received conventional mechanical ventilation); inconsistency (we detected moderate statistical heterogeneity, I² = 66%, for our primary outcome of hospital or 30‐day mortality); imprecision due to wide confidence intervals around our estimate of treatment effect; serious methodologic limitations (blinding was not possible in all studies; cross‐overs between treatment groups > 10% occurred in three trials (Arnold 1994; Bollen 2005; Derdak 2002); post‐randomization withdrawals ranging from 1.4% to 17% occurred in three trials (Arnold 1994; Derdak 2002; Shah 2004)).
Potential biases in the review process
Strengths of our review include methods to minimize bias, including a comprehensive literature search, duplicate data abstraction, and use of a predefined protocol outlining our hypotheses, methodological assessment of primary studies, and statistical analysis plan. We considered important clinical, physiological, and safety endpoints. Although blinding of participants, their families, and clinicians was not feasible, five of 10 trials had low risk of bias.
Our meta‐analysis may have overestimated the treatment effect of HFO because the control group of four studies (Arnold 1994; Bollen 2005; Derdak 2002; Young 2013), including the largest trial (Young 2013) which dominated the pooled analyses, was exposed to higher tidal volumes (> 6 to 8 mL/kg predicted body weight) than currently recommended (ARDS Network 2000). However, a sensitivity analysis showed no difference in trials that implemented lower tidal volume lung‐protective ventilation in the control group. Alternatively, the higher rate of cross‐overs due to treatment failure in participants randomized to conventional ventilation may have reduced the measured effect of HFO. In three studies (Arnold 1994; Bollen 2005; Ferguson 2013) more than 10% of participants crossed over from their assigned mode of ventilation.
Metholodical diversity in the primary studies may account for the inconsistency in some of the reported findings. In the second largest study (Ferguson 2013, n = 548), investigators reported an increase in mortality associated with early application of HFO in participants with ARDS. This trial, however, may have been susceptible to truncation bias due to early termination for harm. Furthermore, the control arm used a protocol incorporating recruitment manoeuvres and high positive end expiratory pressure, which may have conferred additional benefit over conventional ventilation with low tidal volumes in participants with ARDS (Briel 2010; Meade 2008). Conversely, Mentzelopoulos 2012 reported a statistically significant reduction in mortality associated with HFO; however this study differed from others because HFO was used intermittently and for a variable period of time each day based on a predefined protocol, and was combined with tracheal gas insufflation. Furthermore, participants who received protocolized HFO were cared for by selected experts (Mentzelopoulos 2012; personal correspondence), which was not the case for participants undergoing conventional ventilation, which may have led to performance bias. Finally, the largest trial (Young 2013, n = 795) utilized a different HFO ventilator (Novalung R100) which differed from the other studies which used HFO ventilators designed by Sensormedics.
Agreements and disagreements with other studies or reviews
Our findings differ from our previous systematic review (Sud 2013), which found reduced mortality and treatment failure in participants with ARDS receiving HFO. However, we have included two additional trials (Ferguson 2013; Young 2013) of HFO compared to conventional ventilation and unpublished data provided by primary investigators, which generated more reliable estimates of treatment effects through increases in sample size and outcome events.
The improvements in PaO₂/FiO₂ that we observed are consistent with observational studies (Ferguson 2005; Fort 1997; Mehta 2001). Although HFO increased PaO₂/FiO₂ compared to conventional ventilation, there was no difference in oxygenation index (defined as 100 x mean airway pressure/(PaO₂/FiO₂ ratio)) because of the higher mean airway pressure applied during HFO. Although high airway pressure during conventional ventilation is harmful to the lungs (Ranieri 1999), the importance of the higher mean airway pressure during HFO is unclear because of its incompletely characterized relationship to alveolar pressure, which is a more important determinant of lung injury than mean airway pressure in ARDS. Direct comparisons of mean airway pressure and oxygenation index between HFO and conventional ventilation may not be valid because, in contrast to conventional ventilation, mean airway pressures measured in the trachea during HFO are 6 to 8 cm H₂O lower than values displayed on the ventilator (Muellenbach 2007). From a clinical perspective, improved oxygenation may not always be associated with improved clinical outcomes in ARDS (Bernard 2008; Slutsky 2009), because death due to refractory hypoxaemia is relatively uncommon, compared with death due to multiple organ failure.
Our findings seem inconsistent with experimental studies in animals showing that HFO reduces histologic alveolar over‐distension compared to conventional mechanical ventilation (Sedeek 2003), possibly because of the delivery of smaller tidal volumes (Hager 2007), and by corollary, other clinical trials which have shown that smaller tidal volumes improve mortality in participants with ARDS (ARDS Network 2000). Although we found no increase in barotrauma or hypotension resulting from higher mean airway pressures employed during HFO in pooled analyses, in one trial (Ferguson 2013) HFO was associated with increased need for vasoactive medications, and increased use of sedatives and analgesics which may have offset the theoretical benefit of HFO.
Authors' conclusions
Implications for practice.
The risk of death due to ARDS is very high (Phua 2009; Rubenfeld 2005) and appears to have stabilized over the previous decade (Phua 2009). In our review, the median control group mortality in participants with ARDS was 47%. A recent observational study of H1N1 influenza demonstrated a substantial proportion of those with severe ARDS required inhaled nitric oxide, prone positioning, HFO, or extracorporeal membrane oxygenation (ECMO), usually for refractory hypoxaemia (Kumar 2009). These therapies have different risk‐benefit profiles (Adhikari 2007; Peek 2009; Sud 2008; Sud 2010a; Sud 2014). Inhaled nitric oxide is expensive, has not been shown to reduce mortality, and may increase renal dysfunction (Adhikari 2007). ECMO may reduce mortality but requires considerable technical expertise and is not widely available (Peek 2009). Mechanical ventilation in the prone position reduces mortality (Sud 2010a; Sud 2014) and is inexpensive, but is not without complications (Guérin 2013; Taccone 2009) and may interfere with other aspects of patient care (Bein 2007; Leonet 2002).
We found that the quality of evidence for the routine use of HFO in ARDS over conventional ventilation is very low. The role of HFO as rescue therapy when conventional mechanical ventilation has failed due to inadequate gas exchange or the inability to ventilate with acceptably low tidal volumes, is unclear because in several trials included in this review, participants randomized to conventional ventilation received HFO as rescue therapy. Which rescue therapy should be used when conventional ventilation fails likely depends on availability and centre‐specific expertise, although current evidence at this time indirectly favours the use of prone ventilation (Sud 2014) or ECMO (Peek 2009) before HFO. Because the effects on PaCO₂ were very inconsistent, it may be prudent to avoid HFO in intensive care units that admit many patients with raised intracranial pressure, which may be exacerbated by elevated PaCO₂ (e.g. neurocritical care units). Our results are based on a relatively modest number of trials with moderately heterogeneous findings, resulting in wide confidence intervals. As such, future trials may impact on our findings. Nevertheless, the use of HFO as a first‐line strategy for ARDS is not supported by the findings of our systematic review.
Implications for research.
Future research should focus on subgroups of patients that may benefit from the improvements in oxygenation during HFO, such as those with severe hypoxaemia at baseline or those who fail conventional ventilation due to refractory hypoxaemia, or that may require higher mean airway pressures in order to facilitate alveolar recruitment, such as those who are morbidly obese. An individual patient data meta‐analysis may be helpful to further explore the effects of HFO on mortality in such subgroups. Intermittent sessions of HFO employed in combination with tracheal gas insufflations according to a predefined protocol appeared to reduce mortality in one small single‐centre trial (Mentzelopoulos 2012, n = 125). While promising, these findings should be replicated in a larger multicentre RCT.
Participants who were randomized to HFO in one RCT that was stopped early for possible harm (Ferguson 2013) experienced slightly higher mean airway pressures immediately after randomization, compared to other studies in this review. However, because mean airway pressure required during HFO is a post‐randomization variable, and may be associated with illness severity, a relationship between the magnitude of mean airway pressure during HFO and mortality cannot be determined without additional data, such as a RCT comparing protocols for higher versus lower mean airway pressure for ARDS treated with HFO. An individual patient data meta‐analysis could also help elucidate other important prognostic factors which predict response to HFO. Recently, a small observational study demonstrated the feasibility of using an oesophageal balloon catheter during HFO to approximate transpulmonary pressure as an alternative means of titrating HFO. However further research is needed to determine whether this approach is clinically beneficial (Henderson 2015).
Another opportunity to improve the success of future trials of HFO would be to adopt consistent protocols for its optimal application (Fessler 2008). In our review, only two studies (Ferguson 2013; Mentzelopoulos 2012) routinely applied recruitment manoeuvres as part of the HFO technique, and only one trial (Ferguson 2013) attempted to maximize the frequency of oscillation in order to obtain the smallest possible tidal volume (Hager 2007).
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 2 March 2016 | New citation required and conclusions have changed | This is an update of the previous Cochrane systematic review, 'High‐frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome' (Sud 2013), which included 8 RCTs. We included 2 additional recently completed trials (Ferguson 2013; Young 2013) that met our inclusion criteria in this version. We included new data from a third recently published RCT (Mentzelopoulos 2012) that was previously unavailable. The updated review reaches new conclusions. Pooled results from 10 RCTs suggest that although high‐frequency oscillation improves oxygenation and reduces the risk of treatment failure (refractory hypoxaemia, hypercapnia, hypotension, or barotrauma), there is no difference in mortality compared with conventional mechanical ventilation in people with acute respiratory distress syndrome. The quality of evidence was very low for patient‐important outcomes due to imprecision, indirectness, inconsistency and methodological limitations. |
| 2 March 2016 | New search has been performed | In the previous version (Sud 2013) we searched the databases until March 2011. In this updated version we reran the searches until December 2015. We have included 'Risk of bias' and 'Summary of findings' tables, and updated the Background section in this updated version. |
Notes
December 2015:
We have included 'Risk of bias' and 'Summary of findings' tables, and updated the Background section in this updated version.
We have changed the title of the review to 'High‐frequency oscillatory ventilation versus conventional ventilation for treatment of acute respiratory distress syndrome' (previously 'High‐frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome'), as this better reflects the content of the review, and because the term acute lung injury has been removed from the Berlin Definition of ARDS.
Acknowledgements
We would like to acknowledge James Mapstone for contributions made to earlier versions of this systematic review (Wunsch 2004).
We would like to thank Mathew Zacharias (content editor), Marialena Trivella (statistical editor), Rodrigo Cavallazzi, Hansjoerg Waibel, Arash Afshari (peer reviewers) and Janet Wale (consumer editor) for their help and editorial advice during the preparation of a previous update of this systematic review (Sud 2013).
We would like to thank Javier Eslava‐Schmalbach (content editor), Nathan Pace (statistical editor), Andrew MacDuff, Ewan C Goligher, Antoine Roch (peer reviewers), Shunjie Chua (consumer referee) for their help and editorial advice during the preparation of the current update of this systematic review.
We thank all primary investigators who provided additional data for this review: Steven Derdak and Tom Bachman; Casper Bollen; Spyros Mentzelopoulos; Rujipat Samransamruajkit; and Sanjoy Shah.
Appendices
Appendix 1. Search strategies
MEDLINE (2011 to Dec 2, 2015)
1. Exp High‐Frequency Ventilation/
2. (high adj3 oscillat$).mp.
3. 1 or 2
4. clinical trial.mp. or clinical trial.pt. or random:.mp. or tu.xs.
5. 3 and 4 [NOTE this is the same as the following command: limit 3 to “therapy (sensitivity)”]
6. (animals not humans).sh.
7. 5 not 6
8. (Infant, newborn).sh.
9. 7 not 8
EMBASE (2011 to Dec 2, 2015) 1. Exp High Frequency Ventilation/
2. (high adj3 oscillat$).mp.
3. 1 or 2
4. random:.tw. or clinical trial:.mp. or exp health care quality/
5. 3 and 4 [NOTE this is the same as the following command: limit 3 to "treatment (2 or more terms high sensitivity)"]
6. (animal$ not human$).sh,hw.
7. 5 not 6
8. newborn/
9. 7 not 8
CENTRAL (Issue 11, 2015) 1. High Frequency Oscillat*
Web of Science (formerly ISI Web of Knowledge) (Dec 2, 2015)
1. High Frequency Oscillat* OR High Frequency Ventilat* (topic)
2. Random* or Random Alloc* OR Controlled Clinical Trial (topic)
Notes: ‘$’, ‘:’, and ‘*’ retrieve unlimited suffix variations; the .mp. extension includes the title, original title and abstract fields in MEDLINE and EMBASE; the .af. extension includes all searchable fields. Filters for MEDLINE (line 4) and EMBASE (line 4) are based on published sensitive strategies for retrieving randomized trials (Haynes 2005; Wong 2006). References from these four databases were combined and duplicates were removed manually.
Appendix 2. Physiologic outcomes on day one to three after randomization
|
Outcome |
No of trials |
No of participants |
Treatment Effect | Heterogeneity | ||
| Ratio of means* | (95% CI) | P value |
I² (%) |
|||
| Day one (24 hours) | ||||||
| PaO₂/FiO₂† | 8 | 1156 | 1.26 | (1.15 to 1.38) | < 0.00001 | 47 |
| Mean airway pressure | 9 | 985 | 1.30 | (1.26 to 1.35) | < 0.00001 | 23 |
| Oxygenation index | 7 | 423 | 1.10 | (0.97 to 1.25) | 0.14 | 43 |
| PaCO₂ | 10 | 1767 | 0.95 | (0.87 to 1.05) | 0.32 | 90 |
| Day two (48 hours) | ||||||
| PaO₂/FiO₂† | 6 | 1031 | 1.18 | (1.04 to 1.35) | 0.01 | 66 |
| Mean airway pressure | 7 | 888 | 1.25 | (1.19 to 1.31) | < 0.00001 | 38 |
| Oxygenation index | 6 | 375 | 1.06 | (0.89 to 1.26) | 0.54 | 58 |
|
PaCO₂ |
8 | 1587 | 0.93 | (0.81 to 1.06) | 0.25 | 96 |
| Day three (72 hours) | ||||||
| PaO₂/FiO₂† | 6 | 881 | 1.21 | (1.09 to 1.35) | 0.0005 | 55 |
| Mean airway pressure | 7 | 338 | 1.16 | (1.06 to 1.27) | 0.001 | 58 |
| Oxygenation index | 6 | 330 | 1.05 | (0.85 to 1.29) | 0.66 | 68 |
| PaCO₂ | 7 | 920 | 1.00 | (0.88 to 1.14) | 0.99 | 93 |
| Footnotes PaO₂/FiO₂ = ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; PaCO₂ = arterial partial pressure of carbon dioxide. †In one trial (Mentzelopoulos 2012) in which high‐frequency oscillation was used for variable duration according to a prespecified protocol, the mean (and standard error) of the average daily measurements for each participant were used in lieu of the mean of a single value for each participant measured at a similar time point. *Mean value in high‐frequency oscillation group divided by mean value in conventional ventilation group. Random‐effects models used for all meta‐analyses. | ||||||
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital or 30‐day mortality | 8 | 1779 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.16] |
| 2 Hospital or 30‐day mortality (Bollen 2005 and Mentzelopoulos 2012 participants lost to follow‐up censored) | 8 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 3 Hospital or 30‐day mortality (Shah 2004 post‐randomization withdrawals included) | 8 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 4 Hospital or 30‐day mortality: adult versus paediatric trials | 8 | 1779 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.16] |
| 4.1 Adult trials | 6 | 1705 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 4.2 Paediatric trials | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.44, 1.43] |
| 5 Hospital or 30‐day mortality: lung‐protective ventilation mandatory vs non‐mandatory | 8 | 1779 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.16] |
| 5.1 Lung‐protective ventilation not mandatory | 4 | 1062 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.77, 1.15] |
| 5.2 Lung‐protective ventilation mandatory | 4 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.47, 1.53] |
| 6 Hospital or 30‐day mortality (trials with low risk of bias) | 3 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.18] |
1.1. Analysis.
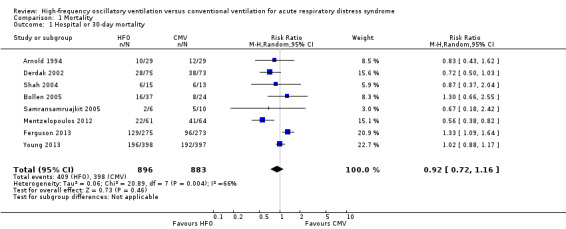
Comparison 1 Mortality, Outcome 1 Hospital or 30‐day mortality.
Comparison 2. Ventilator dependency.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation | 5 | 1142 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐1.09, 2.28] |
| 2 Ventilator‐free days | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
2.2. Analysis.
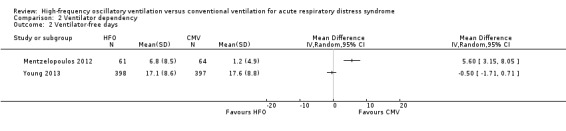
Comparison 2 Ventilator dependency, Outcome 2 Ventilator‐free days.
Comparison 3. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure (intractable hypoxia, hypotension, acidosis, hypercapnoea requiring discontinuation of study intervention) | 6 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.48, 0.85] |
| 2 Barotrauma | 7 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.32] |
| 3 Hypotension | 4 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.90] |
| 4 Hypotension (Shah included) | 5 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.61, 1.66] |
| 5 ETT obstruction | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3 Adverse events, Outcome 1 Treatment failure (intractable hypoxia, hypotension, acidosis, hypercapnoea requiring discontinuation of study intervention).
3.5. Analysis.
Comparison 3 Adverse events, Outcome 5 ETT obstruction.
Comparison 4. Physiological endpoints (ratio of means).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO₂/FiO₂ (ratio of means) | 8 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 1.1 Day 1 | 8 | 1156 | Ratio of Means (Random, 95% CI) | 1.26 [1.15, 1.38] |
| 1.2 Day 2 | 6 | 1031 | Ratio of Means (Random, 95% CI) | 1.18 [1.04, 1.35] |
| 1.3 Day 3 | 6 | 881 | Ratio of Means (Random, 95% CI) | 1.21 [1.09, 1.35] |
| 2 Oxygenation index (ratio of means) | 7 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 2.1 Day 1 | 7 | 423 | Ratio of Means (Random, 95% CI) | 1.10 [0.97, 1.25] |
| 2.2 Day 2 | 6 | 375 | Ratio of Means (Random, 95% CI) | 1.06 [0.89, 1.26] |
| 2.3 Day 3 | 6 | 330 | Ratio of Means (Random, 95% CI) | 1.05 [0.85, 1.29] |
| 3 PaCO₂ (ratio of means) | 10 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 3.1 Day 1 | 10 | 1767 | Ratio of Means (Random, 95% CI) | 0.96 [0.87, 1.05] |
| 3.2 Day 2 | 8 | 1587 | Ratio of Means (Random, 95% CI) | 0.93 [0.81, 1.06] |
| 3.3 Day 3 | 7 | 920 | Ratio of Means (Random, 95% CI) | 1.00 [0.88, 1.14] |
| 4 Mean airway pressure (ratio of means) | 9 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 4.1 Day 1 | 9 | 985 | Ratio of Means (Random, 95% CI) | 1.30 [1.26, 1.35] |
| 4.2 Day 2 | 7 | 888 | Ratio of Means (Random, 95% CI) | 1.25 [1.19, 1.31] |
| 4.3 Day 3 | 6 | 338 | Ratio of Means (Random, 95% CI) | 1.16 [1.06, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnold 1994.
| Methods | Multi‐centre RCT in 5 tertiary‐care paediatric ICUs in the United States from July 1990 to January 1994 | |
| Participants | 70 children (weight < 35 kg, mean age 2.8) with acute diffuse lung injury and impaired oxygenation Excluded: if < 40 weeks post‐conceptual age or former prematurity with residual chronic lung disease, obstructive airway disease, intractable septic or cardiogenic shock, non‐pulmonary terminal diagnosis |
|
| Interventions | 3100 high‐frequency oscillatory ventilator (SensorMedics). Initial settings of FiO₂: 1.0, frequency of 5 to 10 Hz, mPaw of CV+ (4 to 8) cm H₂O, pressure amplitude of oscillation set for “adequate chest wall movement” or according to transcutaneous PCO₂ sensor, bias gas flow 18 L/min Controls were ventilated with pressure‐limited conventional mechanical ventilation (Servo 900C, Siemens; Veolar, Hamilton Medical). Target blood gas values were the same as for HFO Cross‐over to the alternate ventilator was required if the participant met treatment failure criteria |
|
| Outcomes | Duration of mechanical ventilation, 30‐day mortality, supplemental oxygen at 30 days, neurological events. Other clinical and adverse outcomes including barotrauma, hypotension, and treatment failure were also reported. | |
| Notes | Lung‐protective ventilation was not mandated in the control group receiving conventional mechanical ventilation. Participants had ARDS (86%) or pulmonary barotrauma requiring chest tube (14%). 21/62 were < 1 year old. 12 participants excluded from the analysis due to: exclusion from the study within 8 hours of enrolment (n = 6); protocol violations (n = 4); transferred to other institution (n = 2). Open‐lung approach to achieve oxygenation targets used No specific use of lung‐volume recruitment manoeuvres Use of sedation and paralysis was not reported Use of rescue therapies or co‐interventions for ARDS was not reported Partial industry support (SensorMedics) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers (email correspondence, J Arnold, 5 June 2003) |
| Allocation concealment (selection bias) | Low risk | "Randomization was based on a serialized form which included the balanced block design, thus the assignment was blinded to the investigator when a patient was selected to be in the study" (email correspondence, J Arnold, 5 June 2003) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of caregivers and family members was not possible due to the nature of the intervention, which increases the risk of performance bias The primary outcome was death, which is less susceptible to detection bias. Radiologists who assessed chest radiographs were blinded to study treatment, which would have reduced detection bias for barotrauma |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were available for 58 of 70 randomized participants after author contact |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported |
| Other bias | Unclear risk | > 10% of participants (30/58) crossed over from assigned ventilator strategy |
Bollen 2005.
| Methods | Multi‐centre RCT in 5 ICUs in 4 European cities from October 1997 to March 2001 | |
| Participants | 61 adults (mean age 53) with ARDS Excluded: Patients with a non‐pulmonary terminal disease, severe chronic obstructive pulmonary disease or asthma and grade 3 or 4 air leak |
|
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Frequency of 5 Hz with inspiratory time of 33%, mPaw of CV+ 5 cm H₂O, pressure amplitude of oscillation set according to PaCO₂ and to achieve chest wall vibration Controls were ventilated with time‐cycled pressure‐controlled mechanical ventilation with mean tidal volume of 8 ‐ 9 mL/kg ideal body weight (calculated from mean tidal volume per kg of ideal body weight on day 1, 2, 3). General physiological targets were provided, including limitation of peak inspiratory pressure to 40 cm H₂O, but more detailed ventilation procedures and methods of weaning were according to standard protocols of the investigating centres. Cross‐over to the alternate ventilator was required if the participant met treatment failure criteria |
|
| Outcomes | Cumulative survival without mechanical ventilation or oxygen dependency at 30 days; mortality at 30 days; therapy failure; cross‐over rate; and persisting pulmonary problems defined as oxygen dependency or still being on a ventilator at 30 days. Data for ventilator settings and arterial blood gases were available for the first 3 days. Other clinical and adverse outcomes including barotrauma, hypotension, and treatment failure were also reported. | |
| Notes | Lung‐protective ventilation was not mandated in the control group receiving conventional mechanical ventilation 7/61 participants (5 HFO and 2 control) were reported lost to follow‐up at 30 days; ICU mortality, but not 30‐day mortality, was available for all participants lost to follow‐up except 3/61 (2 HFO and 1 control) participants after author contact Trial was terminated early for slow recruitment No specific use of lung‐volume recruitment manoeuvres Use of sedation and paralysis was not reported Use of rescue therapies or co‐interventions for ARDS was not reported Partial industry support (SensorMedics) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes(email correspondence, C. Bollen, June 26, 2009) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of caregivers and family members was not possible due to the nature of the intervention, which increases the risk of performance bias The primary outcome was death, which is less susceptible to detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30‐day mortality available for 54/61 participants; ICU mortality, but not 30‐day mortality, was available for 4/61 after author contact; vital status remains unknown for 3/61 participants |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Unclear risk | > 10% of participants (11/61) crossed over from assigned ventilator strategy |
Demory 2007.
| Methods | Single‐centre RCT in France from November 2003 to December 2004 | |
| Participants | 43 (28 included in this review) adults (mean age 49) with ARDS and PaO₂/FiO₂ < 150 and PEEP ≥ 5 cm H₂O | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings were FiO₂ 1.0, frequency of 5 Hz with inspiratory time of 33%, mPaw of CV+ 5 cm H₂O (but ≤ plateau pressure), pressure amplitude of oscillation = PaCO₂ during conventional mechanical ventilation (max 110) Controls were ventilated with volume‐assist control with tidal volume 6 ‐ 7 mL/kg predicted body weight. PEEP was adjusted according to the ARDSNet protocol Participants included in this review were randomized to receive conventional mechanical ventilation in the prone position for 12 hours prior to: supine HFO (intervention, n = 13), or supine conventional mechanical ventilation (control, n = 15). A third group (n = 15) was randomly assigned to conventional ventilation in the supine position for 12 hours, followed by HFO, and was not included in this review |
|
| Outcomes | Physiologic data including PaO₂/FiO₂, OI, venous admixture | |
| Notes | Duration of HFO was limited to 12 hours; therefore we included only physiologic data (PaO₂/FiO₂ and OI) in pooled analyses Recruitment manoeuvres were performed at HFO initiation, but not during conventional mechanical ventilation. Sedation and paralysis were applied equally to both treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers (email correspondence, L Papazian, 9 August 2011) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (email correspondence, L Papazian, 9 August 2011) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although blinding of caregivers and family members was not possible, the use of protocols, equal application of sedation and paralysis, and short study duration, likely minimizes the risk of performance bias The primary outcome, PaO₂/FiO₂, was measured from arterial blood in a standardized manner, and is therefore not likely to be susceptible to detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported; authors provided additional physiologic data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Derdak 2002.
| Methods | Multi‐centre (13 university‐affiliated medical centres) RCT in the United States and Canada from October 1997 to December 2000 | |
| Participants | 148 adults (mean age 49) with ARDS and PEEP > 10 | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of FiO₂ 0.80 ‐ 1.0, frequency of 5 Hz, mPaw of CV+5, pressure amplitude of oscillation set for “vibration down to level of mid‐thigh”, bias flow of 40 L/min. Switched back to CV when FiO₂ was 0.50 or less and mPaw was weaned to 24 cm H₂O or less with an SaO₂ of 88% or more Controls were ventilated using pressure control with an initial tidal volume of 6 ‐ 10 ml/kg actual body weight, RR adjusted for pH greater than 7.15, PEEP of 10, inspiratory time 33%. Subsequent adjustment of PEEP was according to study protocol (range 10 ‐ 14) |
|
| Outcomes | Survival without need for mechanical ventilation at 30 days from entry to study, 30‐day mortality, 6‐month mortality, need for mechanical ventilation at 30 days and 6 months. Physiologic endpoints and other clinical outcomes, including adverse events, were also obtained after author contact | |
| Notes | Lung‐protective ventilation was not mandatory in the control group receiving conventional mechanical ventilation. Designed as an equivalence trial Rescue therapies used in 9% of the HFO group (nitric oxide 4/75; prone position 2/75, high‐dose steroids 1/75) and 16% of the CV group (nitric oxide 8/73; prone position 3/73; high‐dose steroids 4/73) Lung‐volume recruitment manoeuvres were permitted, although not protocolized All participants who received HFO were paralysed; paralysis was not mandatory in participants receiving CV Partial industry support (SensorMedics) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomization with a balanced block design, balanced with respect to baseline oxygenation index > 40 (email correspondence, S Derdak, T Bachmann, 20 April 2009) |
| Allocation concealment (selection bias) | Low risk | Computerized randomization programme (email correspondence, S Derdak, T Bachmann, 20 April 2009) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study investigators and sponsors were blinded to overall results Although blinding of caregivers and family members was not possible due to the nature of the intervention, the use standardized ventilator settings in each study group reduces the risk of performance bias due to lack of blinding Because the primary outcome was death, detection bias was unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mortality data for withdrawn participants (2/148) obtained after author contact |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Ferguson 2013.
| Methods | Multi‐centre international RCT (39 centres in 5 countries) from July 2007 to August 2012 | |
| Participants | 548 adults with ARDS; 16 ‐ 85 years of age; acute onset of respiratory failure, with fewer than 2 weeks of new pulmonary symptoms; tracheal intubation; hypoxaemia ‐ defined as PaO₂/FiO₂ ≤ 200 mmHg on FiO₂ ≥ 0.5, regardless of PEEP; bilateral alveolar consolidation (airspace disease) seen on frontal chest radiograph | |
| Interventions | Intervention group: 3100B high‐frequency oscillatory ventilator using a lung‐open approach and an explicit protocol Control group: conventional ventilation using low tidal volumes (target tidal volume of 6 ml/kg, plateau pressure ≤ 35 cm H₂0) and a lung‐open approach according to an explicit protocol, with HFO only as rescue therapy | |
| Outcomes | Hospital mortality; also ICU and 28‐day mortality, quality of life at 6 months. Other clinical and adverse outcomes including barotrauma, and treatment failure were also available. | |
| Notes | After enrolment standardized ventilator settings were used for all participants: Tidal volume 6 ml/kg, FiO₂ of 0.60, PEEP of 10 cm H₂O or higher if needed for oxygenation. If PaO₂/FiO₂ remained ≤ 200 mmHg after 30 minutes, participants were randomized. Otherwise patients were reassessed daily for up to 72 hours Protocols for lung volume recruitment manoeuvres were used for both the HFO and CV group Steroids for ARDS were used in 93/275 and 96/273 of the HFO and CV groups respectively Paralysis was administered to 84/275 and 94/273 of the HFO and CV groups respectively 34 of 273 patients randomized to CV received "rescue" HFO for rescue hypoxaemia Partial industry support (CareFusion loaned 9 oscillators and provided technical support) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation schedule" (Study protocol, January 13 2010) |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed in undisclosed block sizes of 2 and 4 with the use of a central Web‐based randomisation system, stratified according to centre" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study investigators were blinded to overall results for the duration of the study Although blinding of caregivers, family members, and research co‐ordinators was not possible due to the nature of the intervention, the use of protocols for HFO and mechanical ventilation reduces the risk of performance bias due to lack of blinding Because the primary outcome was death, detection bias was unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported with the exception of quality of life at 6 months |
| Other bias | Unclear risk | The trial was terminated early because increased mortality was observed in the HFO group after an interim analysis of 500 participants (planned enrolment was 1200) |
Mentzelopoulos 2012.
| Methods | Single‐centre RCT in Greece from July 2006 to May 2009 | |
| Participants | 125 adults (mean age 52) with ARDS; PaO₂/FiO₂ <150, PEEP ≥ 8 cm H₂O | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of frequency of 4 Hz, mPaw of 3 above mean tracheal pressure measured distal to the endotracheal tube, pressure amplitude of oscillation set 30 above baseline PaCO₂ during CV. Participants received 6 ‐ 24 hours of HFO each day until PaO₂/FiO₂ ≥ 150 for > 12 hours on CV. All participants received tracheal gas insufflation with HFO Controls were ventilated using volume assist control according to a prespecified protocol with a target tidal volume of 5.5 to 7.5 ml/kg predicted body weight and target plateau pressure of ≤ 35 cm H₂0 |
|
| Outcomes | Hospital mortality. Additional physiologic and clinical outcomes, including adverse events, were obtained after author contact. | |
| Notes | Protocols for lung volume recruitment manoeuvres were used for both the HFO and CV group Steroids for ARDS were used in 40/61 and 39/64 of the HFO and CV groups respectively Paralysis was administered to 50/61 and 54/64 of the HFO and CV groups respectively In the CV group, 4 participants received HFO (without tracheal gas insufflation) and 2 participants received prone ventilation as rescue therapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers (email correspondence, SD Mentzelopoulus, 9 April 2009) |
| Allocation concealment (selection bias) | Low risk | Telephone (email correspondence, SD Mentzelopoulus, 9 April 2009) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of caregivers and family members was not possible due to the nature of the intervention Study investigators (with expertise in HFO and mechanical ventilation) were available to assist in the ventilatory management of participants randomized to HFO, but not those randomized to conventional ventilation (personal correspondence, S Mentzelopoulos, HFO investigators meeting London, UK, Feb 21 2013). This might introduce potential performance bias Because the primary outcome was death, detection bias was unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status of 1/61 participants randomized to HFO was not known because this participant was transferred to another hospital on day 31 post‐randomization. (However, this participant was assumed to have died in the primary analysis, which would slightly bias the overall results against HFO) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Papazian 2005.
| Methods | Single‐centre RCT in France | |
| Participants | 26 adults (mean age 51) with ARDS; PaO₂/FiO₂ ≤ 150, PEEP ≥ 5 cm H₂O | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of FiO₂ 1.0, frequency of 5 Hz with inspiratory time of 33%, bias flow of 20 L/min, mPaw of CV+ 5 cm H₂O, pressure amplitude of oscillation = PaCO₂ during conventional mechanical ventilation (max 110). All participants were ventilated in the prone position Controls were ventilated with volume‐assist control with tidal volume 6 mL/kg predicted body weight. PEEP was set to 2 cm H₂O above the lower inflection point of the pressure volume curve. All participants were ventilated in the prone position |
|
| Outcomes | Physiologic data (including PaO₂/FiO₂ and OI), haemodynamics, and inflammatory mediators in BAL fluid and blood | |
| Notes | All participants were ventilated in the prone position Recruitment manoeuvres (45 cm H₂O x 40 seconds) were performed at HFO initiation, but not during conventional mechanical ventilation Duration of HFO was limited to 12 hours; therefore we included only physiologic data (PaO₂/FiO₂ and OI) in pooled analyses Sedation and paralysis were applied equally to both treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers (email correspondence, L Papazian, 9 August 2011) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (email correspondence, L Papazian, 9 August 2011) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although blinding of caregivers and family members was not possible, the use of protocols, equal application of sedation and paralysis, and short study duration likely minimizes the risk of performance bias The primary outcome, PaO₂/FiO₂, was measured from arterial blood in a standardized manner, and is therefore not likely to be susceptible to detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Samransamruajkit 2005.
| Methods | Single‐centre RCT in Bangkok, Thailand from September 2000 to September 2002 | |
| Participants | 16 children (weight < 35 kg; mean age 5) with ARDS, PEEP > 5 cm H₂O; FiO₂ > 0.6 for 12 hours to keep SaO₂ > 92%; OI > 15 for ≥ 4 hours | |
| Interventions | SensorMedics 3100 high‐frequency oscillatory ventilator. Initial settings of frequency of 4 ‐ 10 Hz, mPaw of CV+ (2 or 3), pressure amplitude of oscillation set for 10 above peak inspiratory pressure during CV. Switched back to CV when mPaw was weaned to approximately 18 cm H₂O and participants were tolerating suctioning Controls were ventilated using pressure control with an initial tidal volume of 6 ‐ 10 mL/kg actual body weight, RR adjusted for pH greater than 7.15, PEEP of 10, inspiratory time 33%. PEEP was adjusted according to the ARDS Network protocol |
|
| Outcomes | Plasma sICAM‐1 measured by enzyme linked immunosorbent assay on days 1, 3, 5 and 7 of ARDS. Authors also reported duration of mechanical ventilation, daily OI and PaO₂/FiO₂, adverse events, and hospital mortality. | |
| Notes | No specific use of lung‐volume recruitment manoeuvres 1/7 and 0/9 children received inhaled nitric oxide in the HFO and CV groups respectively All participants were sedated and paralysed Not analysed by intention‐to‐treat. (1 participant who crossed over from CV to HFO shortly after randomization was analysed as treated; authors provided data which allowed analysis of this participant according to assigned group for clinical outcomes such as mortality and treatment failure, but not for physiologic outcomes such as OI and PaO₂/FiO₂) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers (email correspondence, R Samransamjuajkit, 3 March 2009) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (email correspondence, R Samransamjuajkit, 13 March 2009) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although blinding of caregivers and family members was not possible, the use of protocols, equal application of sedation and paralysis, and short study duration, likely minimizes the risk of performance bias The primary outcomes which were included in this review, were measured in a standardized manner in both groups (PaO₂/FiO₂, oxygenation index, duration of mechanical ventilation etc), or were not likely to be susceptible to detection bias (death). The risk of detection bias is therefore low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Shah 2004.
| Methods | Single‐centre RCT in the United Kingdom | |
| Participants | 33 adults (mean age 49) with ARDS | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of frequency of 5 Hz, mPaw of CV+5, pressure amplitude of oscillation set for “vibration down to level of mid‐thigh”. No specific criteria for transitioning to CV were reported but HFO was continued until "resolution of ARDS" Controls were ventilated with time‐cycled pressure‐controlled mechanical ventilation with mean tidal volume of 7 ‐ 8 mL/kg ideal body weight (calculated from mean tidal volume per kg of ideal body weight on day 1, 2, 3). Tidal volume and PEEP were adjusted according to the ARDS Network low tidal volume protocol |
|
| Outcomes | Changes in ventilatory parameters (PaO₂/FiO₂ and FiO₂) over the first 72 hours of HFO or CV. Data for 30‐day mortality and other outcomes, including adverse events, were also available after author contact | |
| Notes | No specific use of lung‐volume recruitment manoeuvres Protocols for sedation and paralysis were applied equally to HFO and CV groups. No use of rescue therapies or co‐interventions for ARDS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random draw ("sealed opaque envelopes which were drawn in a random manner by physician independent of the research team") (email correspondence, S Shah, 30 Nov 2007) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (email correspondence, S Shah, 30 Nov 2007) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although blinding of caregivers and family members was not possible due to the nature of the intervention, the use of protocols reduces the risk of performance bias due to lack of blinding. The primary outcomes which were included in this review, were measured in a standardized manner in both groups (e.g. PaO₂/FiO₂), or were not likely to be susceptible to detection bias (death). The risk of detection bias is therefore low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 (3 HFO and 2 conventional ventilation)/33 participants were withdrawn after randomization because they died within 48 hours; baseline data, physiologic and other clinical outcomes were not available for these participants even after author contact (email correspondence, S Shah, Nov 14 2014) |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Young 2013.
| Methods | Multi‐centre RCT (29 centres in UK) from December 2007 to July 2012 | |
| Participants | 795 adults with ARDS; age ≥ 16 years; Weight ≥ 35 kg; endotracheal intubation or tracheostomy; hypoxaemia defined as PaO₂/FiO₂ ratio ≤ 26.7 kPa (200 mmHg), with PEEP ≥ 5 cm H₂O, determined on 2 arterial blood samples 12 hours apart; bilateral infiltrates on chest radiograph; 1 or more risk factors for ARDS (including pneumonia, aspiration of gastric contents, inhalation injury, sepsis, major trauma, multiple transfusions, drug overdose, burn injury, acute pancreatitis, or shock); predicted to require at least 48 hours of artificial ventilation from the time of randomization | |
| Interventions | Intervention: Novalung R100 high‐frequency oscillatory ventilator, with initial frequency 10 Hz, mPaw 5 cm above the plateau airway pressure at enrolment, bias flow 20 L per minute, cycle volume of 100 ml, FiO₂ 1.0. Control: Conventional positive pressure ventilation, according to local practice in the participating ICUs |
|
| Outcomes | 30‐day mortality. An economic analysis is also planned. Duration of mechanical ventilation and physiologic data was also available. | |
| Notes | Some participants randomized to CV received higher tidal volumes (mean 8.2 to 8.3 ± 2.5 to 3.0 ml/kg of ideal body weight on days 1 ‐ 3) than currently recommended for ARDS (i.e. < 8 ml/kg) 10 out of 397 participants randomized to conventional ventilation received HFO |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by permuted block stratified according to study centre, PaO₂:FiO₂ ratio (≤113 mm Hg [15 kPa] or >113 mm Hg), age (≤55 years or >55 years), and sex" |
| Allocation concealment (selection bias) | Low risk | "Independent telephone randomisation system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although blinding of caregivers and family members was not possible due to the nature of the intervention, the use of protocols for HFO and standardized guidelines for conventional mechanical ventilation reduces the risk of performance bias due to lack of blinding. Because the primary outcome was death, detection bias was unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported with the exception of quality of life, which is not yet reported because data collection is ongoing |
| Other bias | Low risk | No other source of bias identified |
ARDS = acute respiratory distress syndrome BAL = bronchoalveolar lavage CV = conventional ventilation HFO =high frequency oscillation Hz = hertz ICU = intensive care unit mPaw = mean airway pressure OI = oxygenation index PEEP = positive end expiratory pressure RCT = randomized controlled trial RR = risk ratio sICAM = soluble intracellular adhesion molecule
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carlon 1983 | Participant population had “acute respiratory failure” from a variety of reasons and included many requiring mechanical ventilation who would not necessarily fit modern criteria for ARDS |
| Dobyns 2002 | Randomized on inhaled nitric oxide, not HFO |
| Fessler 2008 | Randomized on frequency of oscillation, not HFO |
| Hurst 1984 | Participants served as their own controls. Total of 9 participants randomized |
| Hurst 1990 | Participants in the study who received HFO were only “at risk” of developing ARDS |
| Mentzelopoulos 2007a | Randomized on tracheal gas insufflation, not HFO. Cross‐over design |
| Mentzelopoulos 2010 | Randomized on tracheal gas insufflation, not HFO. Cross‐over design |
| Vrettou 2014 | Cross‐over design |
ARDS = acute respiratory syndrome HFO = high‐frequency oscillation
Differences between protocol and review
| Original Protocol | Amended Protocol (March 27 2009; July 01 2013) | Reason (outcome #) |
|
Primary: Mortality (Intensive care unit (ICU), hospital, 30 days, 60 plus days) |
Primary outcomes: 1. Hospital or 30‐day mortality. |
Hospital mortality is the most common endpoint in critical care studies. ICU mortality is not a patient‐centred outcome. Hospital mortality and 30‐day mortality are considered equivalent. We included longer‐term mortality as a secondary outcome. |
|
Secondary: 1. Total length of mechanical ventilation (high‐frequency and conventional combined) 2. Length of stay in the intensive care unit 3. Length of hospital stay 4. Any long‐term quality of life measurements 5. Any long‐term cognitive measurements 6. Cost effectiveness. |
Secondary outcomes: 1. Six‐month mortality 2. Duration of mechanical ventilation (in days, as stated by the authors) 3. Ventilator‐free days to day 28 or 30 (in days, as stated by the authors) 4. Health‐related quality of life at one year 5. Treatment failure, leading to cross‐over to the other arm or discontinuation of the study protocol. We accepted authors’ definitions of treatment failure, which could include severe oxygenation failure, ventilation failure, hypotension, or barotrauma (pneumothorax, pneumomediastinum, subcutaneous emphysema) 6. The ratio of partial pressure of arterial oxygen (PaO₂) to inspired fraction of oxygen (FiO₂) (PaO₂/FiO₂ ratio) at 24, 48, and 72 hours after randomization 7. Oxygenation index (OI, defined as 100 x mean airway pressure/PaO₂/FiO₂ ratio) measured at 24, 48, and 72 hours after randomization 8. Ventilation, measured by partial pressure of carbon dioxide (PaCO₂) at 24, 48, and 72 hours after randomization 9. Mean airway pressure 24, 48, and 72 hours after randomization 10. Barotrauma (as stated by the authors) 11. Hypotension (as stated by the authors) 12. Endotracheal tube obstruction due to secretions, 13. Technical complications and equipment failure in participants treated with HFO (including unintentional system air leaks, and problems with the oscillatory diaphragm, humidifier, and alarm systems) |
Total duration of mechanical ventilation (1) is ambiguous, as it may be measured in two ways in critical care trials: days of mechanical ventilation or ventilator‐free days. Since these endpoints cannot be combined we analysed them separately. We did not analyse length of ICU stay or hospital length of stay (2, 3) as these were likely to be confounded by mortality (an intervention that improves survival will also increase ICU or hospital length of stay). Long‐term quality of life measurements (4) and long‐term cognitive measurements (5) were not precisely defined and unlikely to be reported in studies to date. We chose to analyse health‐related quality of life at one year, if reported. We did not analyse cost effectiveness (6) because it is unlikely that cost‐effectiveness studies would be performed, since this intervention has not been yet proven to be effective. We included several physiologic endpoints not in the original review in order to assess the effect of HFO on oxygenation (6, 7) and ventilation (8, 9). We included several additional safety endpoints prior to undertaking this update in order to assess potential complications of HFO (10 ‐ 13). |
|
Subgroup analyses: None |
Subgroup analyses: See Subgroup analysis and investigation of heterogeneity; Sensitivity analysis. |
See text |
|
Search strategy: See previous version: Wunsch 2004. |
Search strategy: See Appendix 1 |
We designed a more sensitive (but less specific) search strategy using published sensitive strategies for retrieving randomized trials (Haynes 2005; Wong 2006). We also improved the sensitivity of the search strategy by searching conference proceedings and contacting primary investigators. |
Contributions of authors
Sachin Sud (SS), Maneesh Sud (MS), Jan O Friedrich (JOF), Hannah Wunsch (HW), Maureen O Meade (MOM), Niall D Ferguson (NDF), Neill KJ Adhikari (NKJA): All authors contributed to the study concept and design, revised the manuscript for important intellectual content, and approved the final version. SS conceived the study, acquired data, analysed and interpreted data, and drafted the manuscript. MS, JOF, NKJA and HW acquired, analysed and interpreted the data. MOM, NDF, and HW interpreted the data. NKJA and JOF contributed equally to this study. SS, JOF, NKJA are guarantors.
Sources of support
Internal sources
-
New Source of support, Other.
This study received no specific funding or support
External sources
-
New Source of support, Other.
This study received no specific funding or support
Declarations of interest
All authors have completed the Cochrane online conflicts of interest form and declare the following:
Sachin Sud: nothing to declare. Maneesh Sud: nothing to declare. Jan O Friedrich: no financial interests to declare; Dr. Friedrich is a co‐investigators for the Canadian Institutes of Health Research (CIHR) funded OSCILLATE study, which was included in this review (Ferguson 2013). CareFusion (formerly SensorMedics) is providing study oscillators to some of the hospitals involved in the OSCILLATE study for the duration of the study. Hannah Wunsch: nothing to declare. Maureen O Meade:no financial interests to declare; Dr. Meade is a primary investigator for the Canadian Institutes of Health Research (CIHR) funded OSCILLATE study, which was included in this review (Ferguson 2013). CareFusion (formerly SensorMedics) is providing study oscillators to some of the hospitals involved in the OSCILLATE study for the duration of the study. Niall D Ferguson:no financial interests to declare; Dr. Ferguson is a primary investigator for the Canadian Institutes of Health Research (CIHR) funded OSCILLATE study, which was included in this review (Ferguson 2013). CareFusion (formerly SensorMedics) is providing study oscillators to some of the hospitals involved in the OSCILLATE study for the duration of the study. Neill KJ Adhikari: no financial interests to declare; Dr. Adhikari is a co‐investigators for the Canadian Institutes of Health Research (CIHR) funded OSCILLATE study, which was included in this review (Ferguson 2013). CareFusion (formerly SensorMedics) is providing study oscillators to some of the hospitals involved in the OSCILLATE study for the duration of the study.
Four of the seven authors (JOF, NKJA, NDF, MOM) co‐authored one of the included studies Ferguson 2013 and were not involved in the acquisition and interpretation of data, or the assessment of risk of bias for this study (which was performed by SS and MS). JOF verified the calculation of ratio of means (which was first performed independently by SS and MS), for physiologic outcomes for all studies, including Ferguson 2013.
Edited (no change to conclusions)
References
References to studies included in this review
Arnold 1994 {published and unpublished data}
- Arnold JH, Hanson JH, Toro‐Figuero LO, Gutierrez J, Berens RJ, Anglin DL. Prospective, randomized comparison of high‐frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Critical Care Medicine 1994;22(10):1530‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Bollen 2005 {published and unpublished data}
- Bollen CW, Well GT, Sherry T, Beale RJ, Shah S, Findlay G, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial. Critical Care 2005;9(4):R430‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demory 2007 {published and unpublished data}
- Demory D, Michelet P, Arnal JM, Donati S, Forel JM, Gainnier M, et al. High‐frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Critical Care Medicine 2007;35(1):106‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Derdak 2002 {published and unpublished data}
- Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, et al. High‐frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;166(6):801‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2013 {published data only}
- Ferguson N, Mehta S, Slutsky A, Stewart T, Hand L, Zhou Q, et al. Conversion from ventilation to high frequency oscillation ‐ physiologic responses in a pilot randomized trial. American Journal of Respiratory and Critical Care Medicine 2009;179:A3651. [Google Scholar]
- Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High‐frequency oscillation in early acute respiratory distress syndrome. New England Journal of Medicine 2013;368(9):795‐805. [DOI] [PubMed] [Google Scholar]
- Hand L, Matte A, Marie C, Ethier C, Lazosky L, Clarke F, et al. Results of a pilot randomized trial of High Frequency Oscillation in adults with Acute Respiratory Distress Syndrome. Critical Care Canada Forum (www.criticalcarecanada.com/abstracts/2009/high‐frequency‐oscillation.php). Toronto, Canada, 2009.
- Meade MO, Cook DJ, Mehta S, Arabi YM, Keenan S, Ronco JJ, et al. A multicentre pilot randomized trial of high frequency oscillation in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2009; Vol. 179:A1559.
Mentzelopoulos 2012 {published and unpublished data}
- Malachias S, Kokkoris S, Zakynthinos S, Mentzelopoulos SD. High frequency oscillation and tracheal gas insufflations for severe Acute Respiratory Distress Syndrome: Results from a single‐center, phase II, randomized controlled trial [NCT00416260]. Intensive Care Medicine 2009;35 Suppl 1:S6. [Google Scholar]
- Mentzelopoulos SD, Malachias S, Tzoufi M, Markaki V, Zervakis D, Pitaridis M. High frequency oscillation and tracheal gas insufflation for severe acute respiratory distress syndrome. Intensive Care Medicine 2007;33 Suppl 2:S142. [Google Scholar]
- Mentzelopoulos SD, Malachias S, Zintzaras E, Kokkoris S, Zakynthinos E, Makris D, et al. Intermittent recruitment with high‐frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. European Respiratory Journal 2012;39(3):635‐47. [DOI] [PubMed] [Google Scholar]
Papazian 2005 {published and unpublished data}
- Papazian L, Gainnier M, Marin V, Donati S, Arnal JM, Demory D, et al. Comparison of prone positioning and high‐frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Critical Care Medicine 2005;33(10):2162‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Samransamruajkit 2005 {published and unpublished data}
- Samransamruajkit R, Prapphal N, Deelodegenavong J, Poovorawan Y. Plasma soluble intercellular adhesion molecule‐1 (sICAM‐1) in pediatric ARDS during high frequency oscillatory ventilation: a predictor of mortality. Asian Pacific Journal of Allergy and Immunology 2005; Vol. 23, issue 4:181‐8. [MEDLINE: ] [PubMed]
- Samransamruajkit R, Prapphal N, Deerojanawong J, Vanapongtipagorn P, Poovorawan Y. Soluble Intercellular Adhesion Molecule‐1 (sICAM‐1) in pediatric ARDS during high frequency oscillatory ventilation; Randomized controlled trial, a predictor of mortality. American Journal of Respiratory and Critical Care Medicine 2003;167:A999. [Google Scholar]
Shah 2004 {unpublished data only}
- Shah SB, Findlay GP, Jackson SK, Smithies MN. Prospective study comparing HFOV versus CMV in patients with ARDS. Intensive Care Medicine 2004;30 Suppl 1:S84. [Google Scholar]
- Shah SB, Jackson SK, Findlay GP, Smithies MN. Prospective study comparing high frequency oscillatory ventilation (HFOV) versus conventional (CMV) in patients with acute respiratory distress syndrome (ARDS) [abstract]. Proceedings of the American Thoracic Society 2005;2:A847. [Google Scholar]
- Shah SB, Jackson SK, Findlay GP, Smithies MN. Ventilator induced lung injury comparing high frequency oscillator ventilation versus conventional mechanical ventilation [abstract]. Proceedings of the American Thoracic Society 2005;2:A251. [Google Scholar]
Young 2013 {published data only}
- Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High‐frequency oscillation for acute respiratory distress syndrome. New England Journal of Medicine 2013;368(9):806‐13. [DOI] [PubMed] [Google Scholar]
- Young JD. Conventional positive pressure ventilation or High Frequency Oscillatory Ventilation (HFOV) for adults with acute respiratory distress syndrome. www.isrctn.com/ISRCTN10416500 [accessed 1 August 2010].
References to studies excluded from this review
Carlon 1983 {published data only}
- Carlon GC, Howland WS, Ray C, Miodownik S, Griffin JP, Groeger JS. High‐frequency jet ventilation. A prospective randomized evaluation. Chest 1983;84(5):551‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dobyns 2002 {published data only}
- Dobyns EL, Anas NG, Fortenberry JD, Deshpande J, Cornfield DN, Tasker RC, et al. Interactive effects of high‐frequency oscillatory ventilation and inhaled nitric oxide in acute hypoxemic respiratory failure in pediatrics. Critical Care Medicine 2002;30(11):2425‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fessler 2008 {published data only}
- Fessler HE, Hager DN, Brower RG. Feasibility of very high‐frequency ventilation in adults with acute respiratory distress syndrome. Critical Care Medicine 2008;36(4):1043‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hurst 1984 {published data only}
- Hurst JM, DeHaven CB. Adult respiratory distress syndrome: improved oxygenation during high‐frequency jet ventilation/continuous positive airway pressure. Surgery 1984;96(4):764‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hurst 1990 {published data only}
- Hurst JM, Branson RD, Davis K, Jr, Barrette RR, Adams KS. Comparison of conventional mechanical ventilation and high‐frequency ventilation. A prospective, randomized trial in patients with respiratory failure. Annals of Surgery 1990;211(4):486‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mentzelopoulos 2007a {published data only}
- Mentzelopoulos SD, Roussos C, Koutsoukou A, Sourlas S, Malachias S, Lachana A, et al. Acute effects of combined high‐frequency oscillation and tracheal gas insufflation in severe acute respiratory distress syndrome. Critical Care Medicine 2007;35(6):1500‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mentzelopoulos 2010 {published data only}
- Mentzelopoulos SD, Malachias S, Kokkoris S, Roussos C, Zakynthinos SG. Comparison of high‐frequency oscillation and tracheal gas insufflation versus standard high‐frequency oscillation at two levels of tracheal pressure. Critical Care Medicine 2010;36(5):810‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vrettou 2014 {published data only}
- Vrettou CS, Zakynthinos SG, Malachias S, Mentzelopoulos SD. The effect of high‐frequency oscillatory ventilation combined with tracheal gas insufflation on extravascular lung water in patients with acute respiratory distress syndrome: a randomized, crossover, physiologic study. Journal of Critical Care 2014;29(4):568‐73. [DOI] [PubMed] [Google Scholar]
Additional references
Adhikari 2007
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ 2007;334(7597):779. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albuali 2007
Amato 1998
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi‐Filho G, et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine 1998;338(6):347‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Angus 2001
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde‐Zwirble WT, Dremsizov TT, et al. Quality‐adjusted survival in the first year after the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2001;163(6):1389‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ARDS Definition Task Force 2012
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526‐33. [DOI] [PubMed] [Google Scholar]
ARDS Network 2000
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. New England Journal of Medicine 2000;342(18):1301‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Artigas 1998
- Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, et al. The American‐European Consensus Conference on ARDS, part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1998;157(4 Pt 1):1332‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Bein 2007
- Bein T, Ritzka M, Schmidt F, Taeger K. Positioning therapy in intensive care medicine in Germany. Results of a national survey [German]. Anaesthesist 2007;56(3):226‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses? University of Pennsylvania Meta‐analysis Blinding Study Group. Lancet 1997;350(9072):185‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernard 2008
- Bernard GR. PEEP guided by esophageal pressure‐‐any added value?. New England Journal of Medicine 2008;359(20):2166‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briel 2010
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA 2010;303(9):865‐73. [DOI] [PubMed] [Google Scholar]
Brochard 1998
- Brochard L, Roudot‐Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez‐Mondejar E, et al. Tidal volume reduction for prevention of ventilator‐induced lung injury in acute respiratory distress syndrome. The Multicenter Trial Group on Tidal Volume reduction in ARDS. American Journal of Respiratory and Critical Care Medicine 1998;158(6):1831‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brun‐Buisson 2004
- Brun‐Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Medicine 2004;30(1):51‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cartotto 2004
- Cartotto R, Ellis S, Gomez M, Cooper A, Smith T. High frequency oscillatory ventilation in burn patients with the acute respiratory distress syndrome. Burns 2004;30(5):453‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H Jr. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309(22):1358‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2005
- Chan KP, Stewart TE. Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Critical Care Medicine 2005;33(3 Suppl):S170‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheung 2006
- Cheung AM, Tansey CM, Tomlinson G, Diaz‐Granados N, Matte A, Barr A, et al. Two‐year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2006;174(5):538‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cools 2015
- Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD000104.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dominguez‐Cherit 2009
- Dominguez‐Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa‐Perez L, Torre A, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009;302(17):1880‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dreyfuss 1988
- Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end‐expiratory pressure. American Review of Respiratory Disease 1988;137(5):1159‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2005
- Ferguson ND, Chiche JD, Kacmarek RM, Hallett DC, Mehta S, Findlay GP, et al. Combining high‐frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Critical Care Medicine 2005;33(3):479‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2008
- Ferguson ND, Slutsky AS. Point: High‐frequency ventilation is the optimal physiological approach to ventilate ARDS patients. Journal of Applied Physiology 2008;104(4):1230‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Finkielman 2006
- Finkielman JD, Gajic O, Farmer JC, Afessa B, Hubmayr RD. The initial Mayo Clinic experience using high‐frequency oscillatory ventilation for adult patients: a retrospective study. BMC Emergency Medicine 2006;6:2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flori 2005
- Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. American Journal of Respiratory and Critical Care Medicine 2005;171(9):995‐1001. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fort 1997
- Fort P, Farmer C, Westerman J, Johannigman J, Beninati W, Dolan S, et al. High‐frequency oscillatory ventilation for adult respiratory distress syndrome‐‐a pilot study. Critical Care Medicine 1997;25(6):937‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Friedrich 2008
- Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta‐analysis: a simulation study. BMC Medical Research Methodology 2008;8:32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattinoni 2005
- Gattinoni L, Pesenti A. The concept of "baby lung". Intensive Care Medicine 2005;31(6):776‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gattinoni 2006
- Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. New England Journal of Medicine 2006;354(17):1775‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guérin 2013
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. New England Journal of Medicine 2013;368(23):2159‐68. [DOI] [PubMed] [Google Scholar]
Hager 2007
- Hager DN, Fessler HE, Kaczka DW, Shanholtz CB, Fuld MK, Simon BA, et al. Tidal volume delivery during high‐frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Critical Care Medicine 2007;35(6):1522‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hanson 2006
- Hanson JH, Flori H. Application of the acute respiratory distress syndrome network low‐tidal volume strategy to pediatric acute lung injury. Respiratory Care Clinics of North America 2006;12(3):349‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haynes 2005
- Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 2005;330(7501):1179. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2015
- Henderson WR, Dominelli PB, Griesdale DE, Talmor D, Sheel AW. Airway pressure and transpulmonary pressure during high‐frequency oscillation for acute respiratory distress syndrome. Canadian Respiratory Journal 2014;21(2):107‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herridge 2003
- Herridge MS, Cheung AM, Tansey CM, Matte‐Martyn A, Diaz‐Granados N, Al‐Saidi F, et al. One‐year outcomes in survivors of the acute respiratory distress syndrome. New England Journal of Medicine 2003;348(8):683‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herridge 2011
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz‐Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine 2011;364(14):1293‐304. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kacmarek 2008
- Kacmarek RM. Counterpoint: High‐frequency ventilation is not the optimal physiological approach to ventilate ARDS patients. Journal of Applied Physiology 2008;104(4):1232‐3; discussion 1233‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009;302(17):1872‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leonet 2002
- Leonet S, Fontaine C, Moraine JJ, Vincent JL. Prone positioning in acute respiratory failure: survey of Belgian ICU nurses. Intensive Care Medicine 2002;28(5):576‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meade 1997
- Meade MO, Richardson WS. Selecting and appraising studies for a systematic review. Annals of Internal Medicine 1997;127(7):531‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meade 2008
- Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):637‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 2001
- Mehta S, Lapinsky SE, Hallett DC, Merker D, Groll RJ, Cooper AB, et al. Prospective trial of high‐frequency oscillation in adults with acute respiratory distress syndrome. Critical Care Medicine 2001;29(7):1360‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 2004
- Mehta S, Granton J, MacDonald RJ, Bowman D, Matte‐Martyn A, Bachman T, et al. High‐frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004;126(2):518‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mercat 2008
- Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):646‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2008
- Miller MP, Sagy M. Pressure characteristics of mechanical ventilation and incidence of pneumothorax before and after the implementation of protective lung strategies in the management of pediatric patients with severe ARDS. Chest 2008;134(5):969‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2015
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA‐P Group. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Systematic Reviews 2015;4(1):1. [PUBMED: 25554246] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1985
- Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. American Review of Respiratory Disease 1985;132(3):485‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montori 2005
- Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M, et al. Randomized trials stopped early for benefit: a systematic review. JAMA 2005;294(17):2203‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muellenbach 2007
- Muellenbach RM, Kredel M, Said HM, Klosterhalfen B, Zollhoefer B, Wunder C, et al. High‐frequency oscillatory ventilation reduces lung inflammation: a large‐animal 24‐h model of respiratory distress. Intensive Care Medicine 2007;33(8):1423‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muscedere 1994
- Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. American Journal of Respiratory and Critical Care Medicine 1994;149(5):1327‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peek 2009
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374(9698):1351‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peters 2006
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;295(6):676‐80. [DOI] [PubMed] [Google Scholar]
Phua 2009
- Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. American Journal of Respiratory and Critical Care Medicine 2009;179(3):220‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ranieri 1999
- Ranieri VM, Suter PM, Tortorella C, Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282(1):54‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rimensberger 2003
- Rimensberger PC. ICU cornerstone: high frequency ventilation is here to stay. Critical Care 2003;7(5):342‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubenfeld 2005
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. New England Journal of Medicine 2005;353(16):1685‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sedeek 2003
- Sedeek KA, Takeuchi M, Suchodolski K, Vargas SO, Shimaoka M, Schnitzer JJ, et al. Open‐lung protective ventilation with pressure control ventilation, high‐frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003;99(5):1102‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Slutsky 2002
- Slutsky AS, Drazen JM. Ventilation with small tidal volumes. New England Journal of Medicine 2002;347(9):630‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Slutsky 2009
- Slutsky AS. Improving outcomes in critically ill patients: the seduction of physiology. JAMA 2009;302(18):2030‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stapleton 2005
- Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest 2005;128(2):525‐32. [DOI] [PubMed] [Google Scholar]
Stata 9.2 [Computer program]
- StataCorp LP. Stata 9.2. College Station, TX: StataCorp LP, 2006.
Stewart 1998
- Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure‐ and Volume‐Limited Ventilation Strategy Group. New England Journal of Medicine 1998;338(6):355‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sud 2008
- Sud S, Sud M, Friedrich JO, Adhikari NK. Effect of prone positioning in patients with acute respiratory distress syndrome and high Simplified Acute Physiology Score II. Critical Care Medicine 2008;36(9):2711‐2; author reply 2712‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sud 2010a
- Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Medicine 2010;36(4):585‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sud 2014
- Sud S, Friedrich JO, Adhikari NK, Taccone P, Mancebo J, Polli F, et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: a systematic review and meta‐analysis. Canadian Medical Association Journal 2014;186(10):E381‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taccone 2009
- Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: A randomized controlled trial. JAMA 2009;302(18):1977‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Villar 2006
- Villar J, Kacmarek RM, Perez‐Mendez L, Aguirre‐Jaime A. A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical Care Medicine 2006;34(5):1311‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sud 2007
- Sud S, Adhikari N, Ferguson ND, Friedrich JO, Sud M, Meade MO. High‐frequency oscillatory ventilation for ARDS: a meta‐analysis. Critical Care Medicine 2007;35(12 Suppl):A225. [Google Scholar]
Sud 2010b
- Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta‐analysis. BMJ 2010;340:c2327. [1468‐5833: (Electronic)] [DOI] [PubMed] [Google Scholar]
Sud 2013
- Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, et al. High‐frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004085.pub3] [DOI] [PubMed] [Google Scholar]
Wunsch 2003
- Wunsch H, Mapstone, J. High‐frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004085] [DOI] [PubMed] [Google Scholar]
Wunsch 2004
- Wunsch H, Mapstone J. High‐frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004085.pub2] [DOI] [PubMed] [Google Scholar]


