Abstract
Background
Wrist fractures, involving the distal radius, are the most common fractures in children. Most are buckle fractures, which are stable fractures, unlike greenstick and other usually displaced fractures. There is considerable variation in practice, such as the extent of immobilisation for buckle fractures and use of surgery for seriously displaced fractures.
Objectives
To assess the effects (benefits and harms) of interventions for common distal radius fractures in children, including skeletally immature adolescents.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group's Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, trial registries and reference lists to May 2018.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing interventions for treating distal radius fractures in children. We sought data on physical function, treatment failure, adverse events, time to return to normal activities (recovery time), wrist pain, and child (and parent) satisfaction.
Data collection and analysis
At least two review authors independently performed study screening and selection, 'Risk of bias' assessment and data extraction. We pooled data where appropriate and used GRADE for assessing the quality of evidence for each outcome.
Main results
Of the 30 included studies, 21 were RCTs, seven were quasi‐RCTs and two did not describe their randomisation method. Overall, 2930 children were recruited. Typically, trials included more male children and reported mean ages between 8 and 10 years. Eight studies recruited buckle fractures, five recruited buckle and other stable fractures, three recruited minimally displaced fractures and 14 recruited displaced fractures, typically requiring closed reduction, typically requiring closed reduction. All studies were at high risk of bias, mainly reflecting lack of blinding. The studies made 14 comparisons. Below we consider five prespecified comparisons:
Removable splint versus below‐elbow cast for predominantly buckle fractures (6 studies, 695 children) One study (66 children) reported similar Modified Activities Scale for Kids ‐ Performance scores (0 to 100; no disability) at four weeks (median scores: splint 99.04; cast 99.11); low‐quality evidence. Thirteen children needed a change or reapplication of device (splint 5/225; cast 8/219; 4 studies); very low‐quality evidence. One study (87 children) reported no refractures at six months. One study (50 children) found no between‐group difference in pain during treatment; very low‐quality evidence. Evidence was absent (recovery time), insufficient (children with minor complications) or contradictory (child or parent satisfaction). Two studies estimated lower healthcare costs for removable splints.
Soft or elasticated bandage versus below‐elbow cast for buckle or similar fractures (4 studies, 273 children) One study (53 children) reported more children had no or only limited disability at four weeks in the bandage group; very low‐quality evidence. Eight children changed device or extended immobilisation for delayed union (bandage 5/90; cast 3/91; 3 studies); very low‐quality evidence. Two studies (139 children) reported no serious adverse events at four weeks. Evidence was absent, insufficient or contradictory for recovery time, wrist pain, children with minor complications, and child and parent satisfaction. More bandage‐group participants found their treatment convenient (39 children).
Removal of casts at home by parents versus at the hospital fracture clinic by clinicians (2 studies, 404 children, mainly buckle fractures)
One study (233 children) found full restoration of physical function at four weeks; low‐quality evidence. There were five treatment changes (home 4/197; hospital 1/200; 2 studies; very low‐quality evidence). One study found no serious adverse effects at six months (288 children). Recovery time and number of children with minor complications were not reported. There was no evidence of a difference in pain at four weeks (233 children); low‐quality evidence. One study (80 children) found greater parental satisfaction in the home group; low‐quality evidence. One UK study found lower healthcare costs for home removal.
Below‐elbow versus above‐elbow casts for displaced or unstable both‐bone fractures (4 studies, 399 children)
Short‐term physical function data were unavailable but very low‐quality evidence indicated less dependency when using below‐elbow casts. One study (66 children with minimally displaced both‐bone fractures) found little difference in ABILHAND‐Kids scores (0 to 42; no problems) (mean scores: below‐elbow 40.7; above‐elbow 41.8); very low‐quality evidence. Overall treatment failure data are unavailable, but nine of the 11 remanipulations or secondary reductions (366 children, 4 studies) were in the above‐elbow group; very low‐quality evidence. There was no refracture or compartment syndrome at six months (215 children; 2 studies). Recovery time and overall numbers of children with minor complications were not reported. There was little difference in requiring physiotherapy for stiffness (179 children, 2 studies); very low‐quality evidence. One study (85 children) found less pain at one week for below‐elbow casts; low‐quality evidence. One study found treatment with an above‐elbow cast cost three times more in Nepal.
Surgical fixation with percutaneous wiring and cast immobilisation versus cast immobilisation alone after closed reduction of displaced fractures (5 studies, 323 children)
Where reported, above‐elbow casts were used. Short‐term functional outcome data were unavailable. One study (123 children) reported similar ABILHAND‐Kids scores indicating normal physical function at six months (mean scores: surgery 41.9; cast only 41.4); low‐quality evidence. There were fewer treatment failures, defined as early or problematic removal of wires or remanipulation for early loss in position, after surgery (surgery 20/124; cast only 41/129; 4 studies; very low‐quality evidence). Similarly, there were fewer serious advents after surgery (surgery 28/124; cast only 43/129; 4 studies; very low‐quality evidence). Recovery time, wrist pain, and satisfaction were not reported. There was lower referral for physiotherapy for stiffness after surgery (1 study); very low‐quality evidence. One USA study found similar treatment costs in both groups.
Authors' conclusions
Where available, the quality of the RCT‐based evidence on interventions for treating wrist fractures in children is low or very low. However, there is reassuring evidence of a full return to previous function with no serious adverse events, including refracture, for correctly‐diagnosed buckle fractures, whatever the treatment used. The review findings are consistent with the move away from cast immobilisation for these injuries. High‐quality evidence is needed to address key treatment uncertainties; notably, some priority topics are already being tested in ongoing multicentre trials, such as FORCE.
Plain language summary
Interventions for treating wrist fractures (broken wrists) in children
Background and aim
Wrist fractures are the most common bone injury in children. Most are buckle (or torus) fractures, where the bone surface bulges out. These minor fractures heal well. They are often treated with a wrist splint or a below‐elbow plaster cast. More serious fractures are where the bone breaks, generally resulting in displacement of the bone parts. Usually the bone is manipulated back into place ('reduction'), followed by cast immobilisation, often with an above‐elbow cast including the elbow. When considered, surgery generally involves placing wires through the skin and into the bone (percutaneous wiring).
We aimed to assess the best‐quality evidence for different treatments of wrist fractures in children.
Results of the search
We searched medical databases up to May 2018 and included 30 studies with 2930 children. Studies included more male children and reported mean ages between eight and 10 years. We summarise the results from five key comparisons.
Key results
Six studies compared a removable splint with a below‐elbow cast for buckle fractures. One study found there may be little or no difference between the two devices in physical function at four weeks. Few children needed a change or reapplication of either splint or cast (4 studies). There were no refractures. We are uncertain whether there is any difference in pain during device use. There was insufficient evidence to evaluate time to return to former activities (recovery time), minor complications, and child or parent satisfaction. Two studies found lower healthcare costs for splints.
Four studies compared a soft or elasticated bandage with a below‐elbow cast for buckle fractures. We are uncertain if there is less disability at four weeks after bandaging. Few children changed device or needed extended immobilisation (3 studies). There were no serious adverse events. There was insufficient evidence to evaluate recovery time, wrist pain, minor complications, and satisfaction. Children found the bandage more convenient (1 study).
Two studies (mainly buckle fractures) compared cast removal at home by parents versus at the hospital fracture clinic by clinicians (a cast saw was not required for home removal). All had recovered function at four weeks (1 study). There were few treatment changes and no serious adverse effects. Recovery time and number of children with minor complications were not reported. There may be no difference in pain at four weeks (1 study). There may be greater parental satisfaction for cast removal at home (1 study). One study found lower healthcare costs for home removal.
Four studies compared below‐elbow versus above‐elbow casts in usually displaced fractures. We are uncertain if children are less dependent on help when using below‐elbow casts. We are uncertain if there is a difference between the two casts in physical function at six months (1 study). We are uncertain about the finding that all children with above‐elbow casts needed another fracture reduction. There were no serious adverse events. Recovery time and minor complications were not reported. There may be little difference in needing physiotherapy for stiffness. Pain at one week may be less for below‐elbow casts (1 study). One study found lower healthcare costs for below‐elbow casts.
Five studies compared percutaneous wiring and above‐elbow cast immobilisation versus above‐elbow cast immobilisation alone after closed reduction of displaced fractures. Short‐term physical function was not reported. There may be no between‐group difference in function at six months (1 study). We are uncertain whether surgery reduces the risk of treatment failure, defined as early or difficult removal of wires, and remanipulation for loss in position. We are uncertain whether there are fewer serious adverse events with surgery. Recovery time, wrist pain, and satisfaction were not reported. There may be less need for physiotherapy after surgery. One USA study found treatment costs were similar.
Quality of the evidence
All 30 studies had weaknesses that could affect the reliability of their results. We considered the evidence for all outcomes to be low or very low quality.
Conclusion
There is not enough evidence to determine the best ways of treating different types of wrist fractures in children. However, the review findings are consistent with the move away from cast immobilisation for buckle fractures.
Summary of findings
Background
Description of the condition
The two forearm bones are the radius and the ulna. Wrist fracture is often used to describe breaks in the distal parts (roughly the distal third) of these bones. Most fractures involve the distal radius, which is the focus of this review. Sometimes they can be accompanied by an adjacent fracture of the ulna. Isolated distal ulna fractures are rare and not considered further here.
Distal radius fractures are the most common fractures in children, amounting to around a quarter to a third of all paediatric fractures (Hedström 2010). Annual incidences of 30 per 10,000 children (aged 0 to 17 years) have been reported in the USA during 2009 (Karl 2015). The mean age of children (aged up to 16 years) presenting with these injuries in 2000 at two Edinburgh hospitals was 9.9 years, and 55% were boys (Rennie 2007). The distribution of fractures is unimodal for both sexes (Rennie 2007); Hedström 2010 reported peaks at 11 years for girls and 14 years for boys.
Distal radius fractures most commonly result from a fall on an outstretched hand. They vary in severity, complexity and location in relation to the growth plate (physis) and the age of the child. Growth plates are areas of cartilage near the end (epiphysis) of the long bones in children and adolescents. Fractures involving the growth plate are also called physeal fractures. Growth‐plate fractures of the distal radius are more common in older children (Mizuta 1987). The most frequently used classification of physeal injuries is that of Salter and Harris (Salter 1963).
The other three categories of paediatric distal radius fractures commonly described in the literature are: 'buckle' or 'torus' fractures, 'greenstick' fractures, and complete or 'off‐ended' fractures. These 'metaphyseal' fractures occur in the metaphysis, the area that lies between the shaft (diaphysis) and the growth plate.
Buckle or torus fractures involve compression of only part of the circumference of the cortex (outside part) of the bone. This results in a deformity but not a complete break in the cortex. Buckle fractures are considered stable fractures, with little risk of subsequent deformity (Macnicol 2010; Randsborg 2012; Slongo 2007). They are by far the most common distal radius fracture (Randsborg 2012; Thimmaiah 2012).
Greenstick fractures are where the bone is broken on one side but only bowed (plastically deformed) on the opposite side. This fracture pattern occurs predominantly in the shaft and, strictly speaking, greenstick fractures are not metaphyseal fractures. However, variation in the definition of where distal forearm fractures start can mean that shaft fractures are also included. Greenstick fractures, which are unstable fractures, can occur in all immature bones. Like buckle fractures, they occur in younger children (Randsborg 2009). They can be challenging to treat in older children (over 10 years of age) because they take a long time to heal.
Complete metaphyseal fractures are fractures across the bone where both sides of the cortex are disrupted; if displaced, the fractured end fragment is usually displaced dorsally relative to the rest of the bone. These are unstable fractures.
A distal radius fracture is painful, with local tenderness and swelling. There is often deformity in the case of displaced fractures, and movement restriction can result. The great majority of distal radius fractures are closed fractures, where the overlying skin and tissues are intact. Open fractures, where the bone has been exposed, are always treated as serious injuries. The presence and type of fracture is determined by X‐rays. Most children are treated in emergency care or as outpatients, with around 3% being admitted to hospital (Shah 2015).
Children's bones, especially in younger children, are softer and more pliable than those of adults. This results in distinct fracture patterns in children, such as the buckle and greenstick fractures, where the bone distorts or bends rather than breaking at all or completely. Growth‐plate fractures are also specific to children. Conversely, intra‐articular fractures (involving disruption of the joint surface) and comminuted (multiple fragmented) fractures are rare at the wrist in children (Randsborg 2012). Children's bones heal faster than adults' bones and the distal radius has a significant remodelling capacity that occurs with growth of the bone over time. This means that some residual angular deformity and displacement after the fracture has healed can be acceptable in children, as the bone will return to a normal shape as it grows over the years. An angulation of 30 º will fully remodel within five years in young children (Wilkins 2005), but this capacity is much reduced in older children (Macnicol 2010). Growth‐plate fractures of the distal radius also have a large capacity for remodelling (Wilkins 2005). Fractures may also result in overgrowth of the bone. Conversely, damage to the growth plate may result in premature growth‐plate closure, but this is uncommon in wrist fractures. Surgery may be required to correct deformity resulting from abnormal bone growth (Macnicol 2010; Williams 2005).
Given the preponderance of distal radius buckle fractures, the rapid healing and good remodelling capacity of children's distal forearm bones, the vast majority of children with distal radius fractures have a good prognosis with a complete recovery.
Description of the intervention
Treatment for most children with these fractures is non‐surgical (Mellstrand‐Navarro 2014). Non‐surgical treatment primarily involves splintage ranging from support with a simple bandage to full immobilisation in a complete (encircles arm) rigid cast, that may sometimes include the elbow joint. Rigid casts are usually made from materials such as plaster of Paris or one of the forms of fibreglass. Some casts (backslabs) are incomplete, involving only part of the circumference of the arm; these are often applied initially to allow for swelling to subside. More recently, casts can be made of softer more flexible materials. Other types of non‐rigid supports, often removable, consist of splints (also called orthoses). Some devices are 'off the shelf', whereas others, such as rigid casts, are 'custom‐made', being tailored to the child and requiring specialist application and removal. The duration of splintage varies but is typically around three weeks for stable fractures.
When fractures are displaced beyond a tolerable limit (see How the intervention might work), closed reduction is generally performed, where the displaced parts are manipulated externally to restore the correct anatomy. Reduction is usually performed under sedation with analgesia, regional anaesthesia or general anaesthetic. Most fractures can be reduced closed and this reduction will be followed by immobilisation in a suitably rigid cast for four to six weeks. In other cases, surgical fixation of the fragments is performed, to prevent re‐displacement in the cast (Proctor 1993). This usually comprises percutaneous pinning, where one or two wires are inserted through small incisions in the skin into the bones to secure the bones and stabilise the fracture. This is followed by splintage, typically cast immobilisation.
Surgical open reduction of children's distal radius fractures is rarely performed, being reserved for the most serious and rare injuries such as open fractures, neurovascular injuries, complex intra‐articular fractures and some fractures at the metaphyseal‐diaphyseal junction.
Metalwork inserted into children's distal radius fractures is generally removed. Percutaneous wires are mostly left outside the skin to facilitate removal in the clinic. If buried, a further anaesthetic is required for removal.
Aside from visits to a fracture clinic for monitoring purposes and for removal of rigid casts, children do not usually need rehabilitation interventions, such as physiotherapy. Longer‐term follow‐up may be recommended for displaced growth‐plate fractures to check that growth is proceeding normally.
How the intervention might work
The choice of intervention is influenced primarily by an assessment of the stability and the degree of displacement of the distal radius fracture, taking into account the age of the child and the potential for remodelling. In particular, the concept of tolerable displacement (angulation or linear displacement, or both) is useful in children's fracture practice; it describes an amount of displacement that will reliably remodel to a normal‐shaped and ‐sized bone (Schneidmüller 2011).
For stable fractures (predominantly buckle fractures) the main aim of treatment is pain relief and protection, including from re‐injury. This can be provided with a variety of devices such as a simple bandage, a wrist brace or orthosis, a backslab or a complete cast. One key issue is whether a rigid cast is required or whether it represents over‐treatment. Other types of support, which can often be removed at home, may be preferable in terms of convenience and cost‐saving. Attendance for removal of casts and the need for routine follow‐up are additional considerations in the management of these minor fractures.
All splints aim to hold the fracture in place while healing occurs. They also provide pain relief and protection from further injury. However, rigid casts are cumbersome and inconvenient; in particular, casts need to be kept dry. There is a risk of complications, such as skin problems, especially from poorly fitted casts. The removal of casts using a cast saw can be distressing; injuries are rare, even if a source of litigation (Atrey 2010). There is often short‐term stiffness of immobilised joints upon cast removal. The inclusion of the elbow in above‐elbow casts increases this risk, but may enhance fracture stability for more unstable fractures. Extent and position of cast immobilisation are sources of variation in practice (Webb 2006).
Unstable fractures, whether undisplaced or minimally displaced initially or following reduction or surgery, are considered to require immobilisation to prevent later displacement and deformity. As well as rigid casts made from plaster of Paris or fibreglass, softer casting materials may be used when reinforced at vital points in the cast. Splints could also be used if specifically designed for preventing displacement. A preliminary plaster backslab may be applied to allow for swelling to subside.
Closed reduction of the displaced (angular or translated) fracture aims to restore the anatomy of the bone. While painful and often requiring anaesthesia, closed reduction may reduce deformity and restore function. However, given the remodelling capabilities of younger children's bone, reduction of less severe angulation or translation may be unnecessary for a successful long‐term outcome. Indeed, tolerable displacement may be very extensive; full dorsal displacement of a distal radius fracture in a child aged under 10 years can be successfully treated by immobilisation without reduction because of reliable modelling of the radius (Crawford 2012). However, the extent of what is an 'acceptable' deformity will also depend on child, parental and clinician perception, even if eventual correction through remodelling is very likely.
When deemed necessary for stability, supplemental surgical fixation involving metalwork also comes at the risk of complications, such as infection and iatrogenic injuries to nerves, tendons and blood vessels. Wire removal (unless buried) is usually done in a fracture clinic at the same time as removal of the plaster cast. The indications for closed reduction or metalwork insertion (or both) in the context of the good healing and remodelling capabilities of children's distal radius bones are sources of debate (Crawford 2012; Proctor 1993).
Why it is important to do this review
Although distal radius fractures in children have a good prognosis and the vast majority can be treated without surgery, the societal impact is huge, given the large numbers involved. A National Institute for Health and Care Excellence (NICE) guideline published in 2016 estimated that buckle fractures "account for an estimated 500,000 emergency department attendances a year in the UK" (NICE 2016). As well as affecting the child, the impact, including financial, on families can be considerable where caring for the injured child or attendance at hospital requires time off work or making other arrangements (Morris 2006).
There is also considerable variation in practice, such the use of removable splints versus casts for buckle fractures in Canada (Boutis 2014), and of different types of removable splints and bandages in the UK (NICE 2016).
A previous Cochrane Review on this topic, which searched the literature up to October 2007, included 10 trials involving 827 children (Abraham 2008). It reported finding only "limited evidence" to inform on the use of removable splintage for buckle fractures, and on the use of above‐elbow casts and use of surgical fixation with percutaneous wiring for displaced fractures. NICE 2016, which searched up to April 2015, reported finding only low or very low‐quality evidence to inform management decisions for buckle fractures, and concluded that the "evidence suggested that soft casts and bandaging were probably the optimal approaches out of the four considered [bandage, soft cast, removable splint and rigid cast]". Interestingly, simple removable splints are more commonly used in the UK. Given the suggested limitations in the evidence so far, it was important to produce an update of the evidence for buckle and other distal radius fractures in children, to inform practice and the research agenda.
Objectives
To assess the effects (benefits and harms) of interventions for common distal radius fractures in children, including skeletally immature adolescents.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised controlled trials (method of allocating participants to a treatment that is not strictly random, e.g. by hospital number) that assess interventions for treating distal radius fractures in children.
Types of participants
We included trials of children with an open distal radius physis who were being treated for an acute distal radius fracture with or without ulna fracture. This also included skeletally immature adolescents (typically aged under 16 years) with these fractures. This review focuses on the more common types of these fractures. We did not include Galeazzi fractures, which are fractures of the distal radius with disruption of the distal radio‐ulnar joint.
While we excluded trials exclusively on forearm diaphyseal (shaft) fractures, we gave some consideration to the inclusion of mixed populations (shaft and distal radius fracture) in the context of the comparison under test and relative proportions of the two types of fracture.
Types of interventions
We included all trials testing conservative treatments such as rigid non‐removable casts (plaster of Paris; fibreglass) and removable splints, and surgery, primarily involving wire fixation. In setting out comparisons of conservative splintage or casts, our general rule was to make the control group the more traditional treatment, which typically would be the more cautious and restrictive intervention, such as rigid plaster casts.
We set out the following main comparisons:
non‐rigid or removable splintage (e.g. splints, non‐rigid complete cast, backslab or bandages) or 'no splintage' (analgesia only) versus rigid complete casts for treating buckle and minimally displaced (stable) fractures. We surmised that individual trials in the category were likely to compare single interventions such as bandage versus below‐elbow cast. Although we categorised these into different sub‐comparisons under the umbrella comparison, we analysed separately the two main interventions, removable splints, including backslabs, and bandages, that were reported in the included trials;
bandages and 'off the shelf' removable splints versus backslab and other custom‐made devices requiring application by trained, typically clinical, personnel for treating buckle and minimally displaced (stable) fractures. We planned to stratify by the different types of splintage in the two categories tested in the individual trials;
below‐elbow versus above‐elbow casts after reduction of displaced fractures;
closed reduction, wire fixation and immobilisation versus closed reduction and cast alone for the treatment of displaced fractures.
We planned to perform the following secondary comparisons and any other comparisons of definitive treatment (splints, closed reduction, surgical fixation) tested by RCTs identified by the search:
different types of non‐rigid splintage, including 'no splintage', for buckle and other stable fractures;
different durations of cast or splint immobilisation (longer duration will be the control group);
rigid casts of materials other than plaster of Paris versus plaster of Paris casts;
above‐elbow casts with forearm in supination versus neutral versus pronation;
removal of splintage at home versus at fracture clinic; this may link with delivery of care methods: optional consultation versus fixed formal follow‐up at fracture clinic;
different methods of percutaneous pinning (wire fixation).
We excluded trials comparing different methods of anaesthesia, analgesia or diagnosis.
Types of outcome measures
Primary outcomes
Physical function using validated measures, such as the Activities Scale for Kids (performance version) (Young 2000), or Paediatric Outcome Data Collection Instrument (PODCI) (Daltroy 1998)
Treatment failure (a composite outcome defined as either the need for a second procedure (further immobilisation, unscheduled change in device such as reapplication of a cast, reduction or surgical intervention) or the presence of a symptomatic malunion/unacceptable anatomy (deformity))
Serious adverse effects (these are partly comparison‐dependent): major sustained loss of elbow or wrist (or both) range of movement, infection, nerve or tendon injury, complex regional pain syndrome type 1, compartment syndrome, refracture
Secondary outcomes
Time to return to normal activities (or interim stages of recovery)
Wrist pain (visual analogue scale (VAS) or Faces Pain Scale (Bieri 1990))
Minor complications (e.g. short‐term wrist or elbow stiffness; skin breakage) and non‐routine treatment adjustments (e.g. cast slippage)
Child (and parent) satisfaction with outcome
Child (and parent) satisfaction with treatment; this may be collected in response to the question of whether they would choose the same treatment again
Where it seemed appropriate, we grouped outcomes under short‐term (less than three months), medium‐term (three months to less than 12 months) and longer‐term (12 months or longer) follow‐up.
We also recorded resource use (e.g. number of outpatient visits and routine cast changes; duration of hospitalisation), other costs and findings of included trials reporting cost‐effectiveness analysis.
Outcomes used in NICE 2016 guidelines for torus fractures
NICE 2016 set out the following review question: "What is the most clinically and cost‐effective management strategy for children with torus fractures of the forearm". They established the following outcomes.
Critical: pain/discomfort; patient experience; return to normal activities; health‐related quality of life; skin problems; refracture.
Important: number of outpatients visits; cast changes.
We prepared a summary table of the results for these outcomes for each comparison, focusing on these fractures.
Search methods for identification of studies
Electronic searches
We searched:
Cochrane Bone, Joint and Muscle Trauma Group's Specialised Register (9 May 2018);
Cochrane Central Register of Controlled Trials (CENTRAL) (2018, Issue 5);
MEDLINE (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE) (1946 to 4 May 2018);
Embase (1974 to 9 May 2018).
We also searched the World Health Organization International Clinical Trials Registry Platform Search Portal (WHO ICTRP) and Clinicaltrials.gov for ongoing and recently completed trials (9 May 2018).
In MEDLINE, we combined subject‐specific terms with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). We report the search strategies for all databases in Appendix 1.
We did not apply any language or publication status restrictions.
Searching other resources
We searched the reference lists of all included studies. We also checked the reference lists of other articles, including guidelines (NICE 2016), a previous Cochrane Review (Abraham 2008) and other systematic reviews. We also searched abstracts of the American Academy of Orthopaedic Surgeons (AAOS) annual meetings (2009 to 2018), the Orthopaedic Trauma Association (OTA) annual meetings (1996 to 2017), the Bone and Joint Journal (BJJ) Orthopaedic Proceedings (9 May 2018), the British Society for Surgery of the Hand (BSSH) scientific meetings (2012 to October 2017), and the British Trauma Society (BTS) annual scientific meetings (2014, 2015, 2016, 2018).
Data collection and analysis
We performed data collection and analysis in accordance with methods specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Pairs of review authors (from JE, HH and ZIE) independently screened all titles and abstracts for potentially eligible studies using Covidence. We obtained full‐text reports where appropriate. The same three review authors independently performed study selection. We resolved any disagreements about the inclusion or exclusion of individual studies by discussion. We contacted authors of articles published since 2006 where we needed clarification to inform study selection. All three review authors discussed and decided on the final study selection to ensure a consensus. We did not mask the source and authorship of the trial reports.
Data extraction and management
Pairs of the same three review authors performed independent data extraction of the included trials, using a piloted data collection form. The data collected included information on study design, study population, interventions and outcomes measurement, and results. We resolved any discrepancies in data extraction either by discussion between the two authors or with involvement with another review author. Three review authors (HH, JE and ZIE) entered initial data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
Pairs of the same three review authors performed independent 'Risk of bias' assessment of the same included trials for which they collected data. We used the Cochrane 'Risk of bias' tool (Higgins 2011), resolving inter‐rater differences by discussion or by involvement by a third review author. We assessed the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
completeness of outcome data;
selective reporting;
other potential sources of bias.
We considered subjective and functional outcomes (e.g. physical function, pain, satisfaction) and 'hard' outcomes (complications, treatment failure) separately in our assessment of blinding and completeness of outcome data. We assessed two additional sources of other bias: bias resulting from major imbalances in key baseline characteristics (e.g. age, gender, type of fracture); and performance bias such as resulting from lack of comparability in the experience of care providers.
We judged studies to be at 'high', 'low' or 'unclear' risk of bias for each domain assessed. We judged the risk of bias across studies as follows:
'low' risk of bias (plausible bias unlikely to seriously alter the results) if all domains are at low risk of bias;
'unclear' risk of bias (plausible bias that raises some doubt about the results) if one or more domains are at unclear risk of bias;
'high' risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains are at high risk of bias.
Measures of treatment effect
For dichotomous outcomes, we expressed treatment effect as risk ratios (RRs) and 95% confidence intervals (CIs), and presented continuous outcomes as mean differences (MDs) and 95% CIs. Where studies reported the same continuous outcome measured in different ways or scales, we planned to use the standardised mean difference (SMD) when pooling their data. For continuous outcomes, we presented final scores in preference to change scores.
Unit of analysis issues
As we anticipated, the individual child was the unit of randomisation and analysis in all included studies; children with bilateral distal radius fractures are typically very rare. Should potential unit‐of‐analysis issues have arisen from the inclusion of children with bilateral fractures and where appropriate adjustments had not been made, where practical we would have conducted sensitivity analyses to explore the potential effects of the incorrect analysis, including where pooled with data from other trials. We were alert to the unit‐of‐analysis issues relating to outcome reporting at different follow‐up times and the presentation of outcomes, such as total complications, by the number of outcomes rather than participants with these outcomes.
Dealing with missing data
We contacted study authors of reports available since 2006 for missing data, such as for missing denominators and standard deviations. We used intention‐to‐treat analysis where possible. Where feasible, we calculated missing standard deviations from other data (standard errors, 95% CIs, exact P values). We did not impute missing standard deviations. We have noted instances where we extracted data from graphs.
Assessment of heterogeneity
The decision to pool the results of individual studies depended on an assessment of clinical and methodological heterogeneity. If we considered studies sufficiently homogeneous for data pooling, we assessed statistical heterogeneity by visual inspection of the forest plots, and by using the Chi2 test with a significance level of P value less than 0.1, and the I2 statistic. We based our interpretation of the I2 statistic results on those suggested by Higgins 2011 (Section 9.5.2):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%; may represent substantial heterogeneity;
75% to 100%: considerable (very substantial) heterogeneity.
Assessment of reporting biases
We attempted to reduce the impact of reporting bias by conducting an extensive literature search that included inspection of unpublished trials, including conference abstracts and trial registries. If there had been more than 10 studies included in a meta‐analysis, we would have considered exploring potential publication bias by generating a funnel plot. We would have initially determined the magnitude of publication bias by visual inspection of the asymmetry of the funnel plot. If this appeared asymmetric, we would have performed a linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Egger 1997). A P value of less than 0.1 could have been an indication of a publication bias or small‐study effects.
Data synthesis
Where appropriate, we pooled results of comparable studies using both fixed‐effect and random‐effects models. We decided on the choice of the model to report by careful consideration of the extent of heterogeneity and whether it can be explained, in addition to other factors, such as the number and size of included studies. We used 95% confidence intervals (CIs) throughout. We considered not pooling data where there was considerable heterogeneity (I2 statistic value greater than 75%) that could not be explained by the diversity of methodological or clinical features among trials. Where it was inappropriate to pool data, we present trial data in the analyses or tables for illustrative purposes, and report these in the text.
Where possible, we planned to stratify by basic fracture type where trial populations include several categories of distal radius fracture. Similarly, we planned to stratify by different categories of splintage or 'no splintage', where appropriate. We usually did not implement this, given the insufficient data.
Subgroup analysis and investigation of heterogeneity
We had planned to investigate the influence of effect modifiers on results using the following subgroup analyses. However, we did not perform these as insufficient data were available.
-
Type of fracture: this will depend partly on the comparison. Planned subgroups were:
incomplete metaphyseal fractures (buckle and torus);
undisplaced complete metaphyseal fractures (this may contain some fractures classified by authors as 'greenstick');
displaced complete metaphyseal fractures (this may contain some fractures classified by authors as 'greenstick');
physeal fractures (Salter‐Harris I and II);
articular fractures (Salter‐Harris III and IV).
Fracture of distal radius only versus fracture of distal radius and associated ulna fracture.
Age: up to five years, six to 10 years and 11 years and over.
Different categories of splintage, including 'no splintage'. We anticipated that this would depend on the comparison. We envisaged that the categorisation for the intervention group for the first comparison would be 'no splintage', bandage, soft casts, and removable splints.
We had planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of CIs and performing the test for subgroup differences available in Review Manager 5 (RevMan 2014).
Sensitivity analysis
In our protocol, we set out the following sensitivity analyses to assess whether the results of the review were robust to the decisions made during the review process. However, while we undertook some exploratory analyses, the number of trials available for all comparisons were too few for formal testing of the effects of excluding trials, where the criteria applied, and data were not available for appropriate testing of missing data and potential unit‐of‐analyses issues. We always took a conservative approach to analysis and interpretation. The listed sensitivity analyses were:
excluding trials at high or unclear risk of bias, either overall or selection bias, reflecting inadequate or lack of allocation concealment;
excluding trials reported in abstracts only;
excluding trials not reporting radiographic confirmation of buckle or other undisplaced fractures;
excluding mixed‐population trials with data from radial shaft fractures;
adjusting for missing data;
different interpretations of data where there are potential or known unit‐of‐analysis issues; and
using fixed‐effect versus random‐effects models for pooling.
Assessing the quality of the evidence and 'Summary of findings' tables
We used the GRADE approach to assess the quality of evidence related to all outcomes listed in the Types of outcome measures (Schünemann 2011). The four levels of evidence certainty are 'high', 'moderate', 'low' or 'very low'. Quality may be downgraded due to study limitations (risk of bias), imprecision, inconsistency, indirectness or publication bias.
Where there was sufficient evidence, we prepared 'Summary of findings' tables for our main comparisons. As planned, we presented the results for each primary outcome and the first three listed secondary outcomes. We presented functional outcome at short term and either medium or long term, depending on data availability. Two review author produced 'Summary of findings' tables using those generated in RevMan.
We adjusted our selection of outcomes for presentation in the 'Summary of findings' tables at the review stage for 'stable', predominantly buckle (torus) fractures. Given the generally speedy and full recovery associated with these fractures, we decided that we would remove medium‐ or long‐term functional outcomes, as they are very unlikely to reflect differences in treatment effect. Instead, often in parallel with trials on these fractures, we increased our focus on the acceptability of treatment by adding in child or parent satisfaction with treatment.
Results
Description of studies
Results of the search
We screened a total of 2417 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (71); CENTRAL (414), MEDLINE (701), Embase (776), the WHO ICTRP (69), Clinicaltrials.gov (107) and the BJJ Orthopaedic Proceedings (279). We also found one potentially eligible study after searching for references to an outcome scale (Krishnan 2014), three other records relating to two unpublished trials (Clarke 2007; Jones 2001) from a search of a personal database of one author (HH), and two reports of the ongoing FORCE 2018 trial (NIHR projects database and NDORMS Current trials and studies).
The search identified a total of 128 records for potential inclusion. Where possible, we obtained full‐text copies of these records and linked any references pertaining to the same study under a single study ID. These 128 records represented 93 studies. Upon further analysis, we included 30 studies (Included studies), excluded 53 (Excluded studies), and four were ongoing studies (Adrian 2015; NCT03248687; FORCE 2018; NCT03297047). A further six studies (ACTRN12611000101987; Bae 2015; Baldwin 2017; NCT02670629; NCT03097757; NTR2508) await classification.
A flow diagram summarising the study selection process is shown in Figure 1.
1.
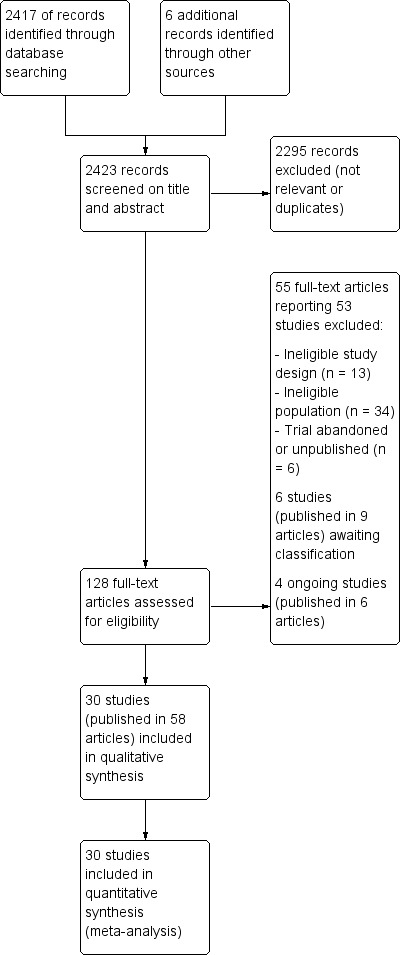
Study flow diagram
Included studies
We included 30 studies in this review; 28 were published as full reports in journal articles (date range 1990 to 2016) and two were reported only as conference abstracts (Ghoneem 2003; Jones 2001). We were able to find a trial registry number for nine studies (Boutis 2010; Colaris 2012; Colaris 2013a; Hamilton 2013; Khan 2010; Kropman 2010; Oakley 2008; Silva 2016; Williams 2013).
We requested additional information from trialists for 11 trials (Derksen 2011; Gibbons 1994; Hamilton 2013; Inglis 2013; Karimi 2013; Paneru 2010; Pountos 2010; Schulte 2014; Silva 2016; Stevenson 2013; Williams 2013), and were successful in two cases (Schulte 2014; Stevenson 2013).
We provide details of study methods, participants, interventions and outcome measurement for the individual studies in the Characteristics of included studies, and we summarise them below.
Design
Twenty‐one trials were confirmed RCTs; however, data were included for nine participants who had declined participation and were treated according to the surgeon's preference in Miller 2005 (results for 42 children). Seven trials were quasi‐RCTs, and two trials did not report the method of randomisation (Ghoneem 2003; Jones 2001). One study used an inappropriate cross‐over design but we only used first‐period data (Silva 2016); the remaining studies all used a parallel design.
Sample sizes
The 30 trials enrolled a total of 2930 participants, with sample sizes ranging from 23 (Gibbons 1994) to 317 (Hamilton 2013).
Setting
Twenty‐eight trials were conducted at a single centre in 10 different countries: Australia (3); Canada (3); Iran (1); Kuwait (1); Nepal (1); Netherlands (2); Saudi Arabia (1); Switzerland (1); UK (8); and USA (7). The remaining two trials were multicentre trials conducted in four hospitals in the Netherlands (Colaris 2012; Colaris 2013a).
The dates for the recruitment period were provided for 24 trials, the length of recruitment for three trials, and no information for the other three trials. The earliest known start date was reported as 1991 (Gibbons 1994; McLauchlan 2002) and the most recent trial began in 2014 (Silva 2016). The longest period of recruitment was three years and one month (Williams 2013).
Participants
The range of mean ages of participants in the 28 trials reporting this was between 6.2 years (Jones 2001) and 12.4 years (Miller 2005), with most means lying between eight and 10 years. Twenty‐six trials recruited more male than female participants; the percentage of male children ranged from 53% (Davidson 2001) to 91% (Miller 2005). Only one trial had a higher proportion of female participants (60%) (Derksen 2011). No data on gender were provided for the remaining three trials (Ghoneem 2003; Jones 2001; West 2005).
Eight trials aimed to recruit children with buckle fractures only (Davidson 2001; Jones 2001; Karimi 2013; Oakley 2008; Plint 2006; Symons 2001; West 2005; Williams 2013). Notably, Plint 2006 checked for misdiagnosis within 24 hours of admission and, implementing an a priori plan of action, withdrew 16 trial participants from the study who were found post‐randomisation to have a fracture other than a buckle fracture. Most fractures were buckle fractures in four other trials: Hamilton 2013, which also included greenstick and epiphyseal fractures; Derksen 2011, which also included isolated greenstick fractures; Pountos 2010, which also included "undisplaced" greenstick fractures; and Silva 2016, which also included non‐ or minimally displaced fractures. Kropman 2010 included "impacted" greenstick fractures, which are also, unlike typical greenstick fractures, likely to be stable. The fractures in three trials were described as minimally displaced (or angulated) (Boutis 2010; Colaris 2012; Stevenson 2013). Thirteen trials specified the inclusion of displaced fractures necessitating fracture reduction (Bohm 2006; Boyer 2002; Colaris 2013a; Ghoneem 2003; Gibbons 1994; Inglis 2013; Khan 2010; Levy 2015; McLauchlan 2002; Miller 2005; Paneru 2010; Schulte 2014; Webb 2006). All fractures were completely displaced in McLauchlan 2002; and 20% (23/113) were in Webb 2006. Gupta 1990 included solitary displaced greenstick fractures, 42% (25/60) of which were reduced as angulation was 20 º or more.
Most trials included children with either a radius or ulna fracture, or with fractures to both bones (Bohm 2006; Boutis 2010; Boyer 2002; Hamilton 2013; Inglis 2013; Karimi 2013; Khan 2010; Kropman 2010; Levy 2015; McLauchlan 2002; Oakley 2008; Plint 2006; Silva 2016; Webb 2006). Three trials stipulated that the ulna needed to be intact (Derksen 2011; Gibbons 1994; Gupta 1990), and two studies only included participants with fractures to both radius and ulna (Colaris 2012; Colaris 2013a). A further nine trials did not report whether ulna fractures were included or excluded (Davidson 2001; Ghoneem 2003; Jones 2001; Pountos 2010; Schulte 2014; Stevenson 2013; Symons 2001; West 2005; Williams 2013).
Two trials recruited children with displaced forearm fractures (Inglis 2013; Schulte 2014). Separate data on the distal radius fracture subgroup were available from Abson 2016 for Inglis 2013, and were obtained after author contact for Schulte 2014.
No trial exclusively recruited children with growth‐plate fractures and the inclusion criteria of most trials imply that physeal fractures were not included. Boutis 2010 explicitly excluded growth‐plate fractures, while Stevenson 2013 reported after author contact that an unknown number of Salter‐Harris II fractures were included among the minimally displaced fractures. Three trials involving displaced fractures explicitly excluded Salter‐Harris type III and IV fractures (Bohm 2006; Hamilton 2013; Levy 2015). Three other trials quantified the number of fractures involving the growth‐plate without defining the type: 17 (17%) in Khan 2010; 12 (12%) in Schulte 2014; and 17 (15%) in Webb 2006.
Comparisons
Most trials had two intervention groups, with the exceptions of Boyer 2002, Gupta 1990 and Pountos 2010, which all had three arms.
We have grouped the 30 included trials below according to the comparisons addressed by each trial. Five of the 14 comparisons for which trials were identified pertained to children with exclusively or predominantly buckle fractures.
In the following, we report on the number and main characteristics of trials for the comparisons listed in Types of interventions, starting with the four main comparisons. This is followed by the extra comparisons tested by the included trials for which trials were identified.
Non‐rigid or removable splintage (e.g. splints, non‐rigid complete cast, backslab or bandages) or 'no splintage' (analgesia only) versus rigid complete casts for buckle and minimally displaced (stable) fractures
No trials tested 'no splintage', non‐rigid complete casts (soft casts) or traditional backslabs. All casts were below‐elbow casts; two trials reported that a backslab plaster cast was applied for one week before conversion to a complete cast (Kropman 2010; West 2005). The remaining trials tested either removable splints, bandages or both in the case of one three‐group trial (Pountos 2010). We split these trials into two main comparisons.
Removable splint versus below‐elbow cast
Six trials compared a removable splint with a below‐elbow cast in 695 children with distal radius fractures (Table 6). Four trials used commercially available splints (Davidson 2001; Karimi 2013; Pountos 2010; Williams 2013), whereas Oakley 2008 used a fibreglass volar slab secured by an elasticated bandage and Plint 2006 used an individually‐fitted plaster splint attached with a tensor bandage. Five studies included 645 children with buckle fractures (Davidson 2001; Karimi 2013; Oakley 2008; Plint 2006; Williams 2013). Pountos 2010 provided results for 50 children with buckle or undisplaced greenstick fractures (Pountos 2010).
1. Removable splintage versus below‐elbow cast for buckle or minimally displaced (stable) fractures: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Removable splint (or backslab) | Cast | Duration of use |
| Davidson 2001 | 201 Mean: 8.9 years; range 2 to 15 years |
Buckle fractures of the distal radius | Futura‐type wrist splint | Standard full ‘Colles type’ (full below‐elbow) plaster of Paris cast | 3 weeks Discharged if no complications after removal of splint or cast and clinical examination and radiograph and questioning |
| Karimi 2013 | 142 Mean: 9.5 years; range 1.2 to 17 years |
Distal forearm buckle fractures | Removable wrist splint | Short arm cast | 3 weeks |
| Oakley 2008 | 95 Mean: 8.5 years; range 9 months to 15 years | Buckle fracture of the distal radius and or ulna. Radius only: 71 Radius and ulna: 13 |
Fibreglass volar slab (backslab) secured with an elasticised bandage | Encircling (full) below‐elbow Plaster‐of‐Paris cast | 12 to 16 days, extended by 2 weeks if significant tenderness or discomfort remained |
| Plint 2006 | 113 Mean: 9.72 years; range 6 to 15 years (eligible) |
Distal radius and/or ulna buckle fractures Radius only: 87 Radius and ulna: 7 |
Individually fitted plaster splint (composed of 12 plaster layers) that was attached with a tensor bandage | Below‐elbow (short arm) plaster cast. | 3 weeks |
| Pountos 2010a | 50 (in analysis) Mean: 9 years; range 2 to 16 years) |
Undisplaced greenstick and buckle fractures of the distal radius | Futuro wrist splint | Plaster of Paris cast; below‐elbow implied | 4 to 6 weeks (probably) |
| Williams 2013 | 94 Median: 9.5 years (splint) and 9 years (cast); range 2 to 16 years |
Distal radial buckle fracture | Prefabricated, cock‐up wrist splint (if an appropriately sized, prefabricated splint was not available, a custom splint was made from plaster) | Fibreglass short‐arm cast with protective layers of stockinette and webril underneath | 3 weeks |
aPountos 2010 was a three‐arm trial comparing Futuro splint, double Tubi‐grip and plaster cast in 90 children. The numbers allocated to each group were not reported.
Bandage versus below‐elbow cast
Four trials compared a soft or elasticated bandage with a below‐elbow cast in 237 children with distal radius fractures. Two studies included 92 children with buckle fractures (Jones 2001; West 2005); Kropman 2010 included 92 children with impacted greenstick fractures; and Pountos 2010 provided results for 53 children with either a buckle or an undisplaced greenstick fracture (Table 7).
2. Bandage versus below‐elbow cast for buckle or similar fractures: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Bandage | Cast | Duration of use |
| Jones 2001 | 50 Mean: 6.2 years; range 3 to 10 years |
Buckle fracture of the distal radius | Wool and crepe bandage | Below‐elbow plaster of Paris cast (back slab?) | 3 weeks |
| Kropman 2010 | 92 Mean: 10 years; range 4 to 12 years) |
Impacted greenstick fracture of the distal radius or ulna Radius injured: 89 Radius and ulna injured: 8 |
Soft bandage: layer of wool covered with a layer of commercial cotton crepe bandage supported by a sling. After 1 week, a Tubi‐grip was placed for 3 weeks |
Below‐elbow backslab plaster cast. After 1 week, the cast was made circular and continued for another 3 weeks | 4 weeks |
| Pountos 2010a | 53 (in analysis) Mean: 9 years; range 2 to 16 years) |
Undisplaced greenstick and buckle fractures of the distal radius | Double Tubi‐grip | Plaster of Paris cast; below‐elbow implied | 4 to 6 weeks (probably) |
| West 2005 | 42 < 5 years: 1 5 to 10 years: 26 > 10 years: 12 |
Buckle fractures of the distal radius | Bandage: a layer of orthopaedic wool was applied. This was then covered with a layer of ordinary commercial cotton crepe bandage, which was held with tape. Participants also seen at 2 and 3 weeks |
Plaster cast, these participants were initially placed into a below‐elbow backslab cast. At 1 week, the cast was converted to a full below‐elbow polymer cast | 4 weeks (however, all participants in the bandage group had removed their bandage after 2 weeks) |
aPountos 2010 was a three‐arm trial comparing Futuro splint, double Tubi‐grip and plaster cast in 90 children. The numbers allocated to each group were not reported.
Bandages and 'off the shelf' removable splints versus backslab and other custom‐made devices that require specialist application for treating buckle and minimally displaced (stable) fractures
None of the included trials made this comparison.
Below‐elbow versus above‐elbow casts
Four trials compared below‐elbow versus above‐elbow casts. In three trials (333 participants) casts were applied after closed reduction of displaced distal radius or both radius and ulna fractures (Bohm 2006; Paneru 2010; Webb 2006), whereas the 66 participants in Colaris 2012 had minimally displaced metaphyseal fracture of the radius and ulna (Table 8). The casts were full in three trials but were non‐circumferential in Colaris 2012.
3. Below‐elbow versus above‐elbow casts trials: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Below‐elbow cast | Above‐elbow cast | Duration of use |
| Bohm 2006 | 117 Mean: 8.6 years; range 4 to 12 years (eligible) |
Displaced closed fracture of the distal third of the forearm (radial or radial and ulnar; no isolated distal ulnar fractures). Manual reduction | Full below‐elbow plaster cast | Above‐elbow plaster cast (below‐elbow applied first; then cast extended). | Casts removed after 6 weeks |
| Colaris 2012 | 66 Mean: 7.1 years; < 16 years |
Minimally displaced metaphyseal fracture of the radius and ulna | Below‐elbow plaster cast (non‐circumferential). | Above‐elbow plaster cast (non‐circumferential) | Casts removed after 4 weeks |
| Paneru 2010 | 89 Mean: 8.4 years; range 4 to 12 years (eligible) |
Displaced closed distal forearm fractures (combined radius and ulna fracture) Closed reduction | Full below‐elbow plaster cast | Above‐elbow plaster cast (below‐elbow applied first; then cast extended) | Not stated, probably casts removed after 6 to 8 weeks dependent on detection of union |
| Webb 2006 | 127 Mean: 9.8 years; range 4 to 12 years |
Displaced (partially or completely) closed fracture of the distal third of the forearm (radial or radial and ulnar; no isolated distal ulnar fractures) Manual reduction | Full below‐elbow plaster cast | Above‐elbow plaster cast (below‐elbow applied first; then cast extended). | Casts removed after 4 weeks if evidence of healing. Otherwise extended 2 weeks (above‐elbow casts cut to below‐elbow) |
Percutaneous wire fixation and cast immobilisation versus cast alone after closed reduction of displaced fractures
All five trials (323 participants) comparing surgical fixation and cast immobilisation versus cast immobilisation alone after closed reduction of displaced fractures used percutaneous wiring (Colaris 2013a; Ghoneem 2003; Gibbons 1994; McLauchlan 2002; Miller 2005). The use of above‐elbow casts was confirmed in four trials (Table 9). Ghoneem 2003, which was reported only in a conference abstract, provided no details of the cast immobilisation nor of bone involvement. Both‐bone fractures were present in all 128 participants of Colaris 2013a, and in most participants (88%: 60/68) in McLauchlan 2002. Conversely, all participants had isolated distal radius fracture in Gibbons 1994 and there was no mention of ulna fractures in Miller 2005.
4. Surgery versus not‐surgery trials: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Surgery | Not surgery | Duration of use |
| Colaris 2013a | 128 Mean: 8.8 years; < 16 years. |
Displaced metaphyseal both bone fractures
Radius: complete or greenstick Stable post‐reduction |
Closed reduction
Percutanous K‐wire (1 or 2 wires) Above‐elbow cast Wire removed with cast |
Above‐elbow cast | Cast and wires removed after 4 weeks |
| Ghoneem 2003 | 70 "in children", age not reported |
Displaced distal forearm fractures | Closed reduction Percutaneous wire fixation Plaster cast |
Closed reduction Plaster cast |
No details |
| Gibbons 1994 | 23 Mean: 8.5 years; range 5 to 14 years |
Isolated displaced distal radius fracture (intact ulna) | Manipulation Percutaneous (stab incision) wire fixation (1 wire) Above‐elbow plaster cast and wire removed after 3 weeks, then below‐elbow cast | Manipulation Above‐elbow plaster cast | 4 weeks for surgery group. Timing not stated for not‐surgery group |
| McLauchlan 2002 | 68 Mean: 7.6 years; range 4 to 14 years |
Completely displaced metaphyseal fracture of distal radius with or without ulna fracture Intact ulna: 8 |
Closed reduction Single percutaneous Kirschner wire fixation Above‐elbow cast (probably plaster). Wire removed at 3 weeks and cast changed |
Closed reduction Above‐elbow cast (probably plaster) |
Casts removed between 4 and 6 weeks after injury depending on age of child |
| Miller 2005 | 34 (9 not randomised) Mean: 12.4 years; range 10 to 14 years |
Closed displaced metaphyseal fracture of distal radius (No mention of ulna fracture) |
Closed reduction Percutaneous wire fixation (1 or 2 wires) Above‐elbow cast. Wires removed at 4 weeks, then below‐elbow cast. |
Closed reduction Above‐elbow cast (plaster cast over‐wrapped with fibreglass casting material). All participants had above‐elbow cast for 4 weeks and then below‐elbow cast |
Above‐elbow cast for 4 weeks, then 2 weeks in a below‐elbow cast |
Different types of non‐rigid splintage, including 'no splintage', for buckle and other stable fractures: bandage versus removable splint
The sole trial in this category compared an elasticated bandage versus removable splint (Pountos 2010). Results were reported for 55 children with either a buckle or an undisplaced greenstick fracture; see entries for the two interventions in Table 6.
Different durations of cast or splint immobilisation
None of the included trials made this comparison.
Rigid casts of materials other than plaster of Paris versus plaster of Paris casts: fibreglass versus plaster cast
Inglis 2013 compared a fibreglass cast (80% were above‐elbow) versus plaster cast (90% were above‐elbow) in 201 children with a displaced fracture of the forearm (radius or ulna or both) requiring closed reduction and immobilisation (Table 10). Limited results for the subgroup of 143 children with distal radius fracture only (epiphyseal and metaphyseal) were reported in Abson 2016, a report that was otherwise focused on the effects on fracture reduction of treatment by either a resident versus an attending surgeon; data extracted from case notes.
5. Fibreglass versus plaster cast: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Fibreglass cast | Plaster cast | Duration of use |
| Inglis 2013 | 201 forearm fractures (143 with distal radius fractures) Mean: 9.7 years; range 1.4 to 17.5 years |
Displaced fracture of the forearm (radius or ulna or both) | Closed reduction then fibreglass cast: 80% had above‐elbow and 20% below‐elbow |
Closed reduction then plaster cast: 90% had above‐elbow and 10% below‐elbow |
Casts removed at 6 weeks |
Position of arm in above‐elbow cast (forearm supinated versus pronated versus neutral)
Two quasi‐randomised trials assessed the effect of the forearm position (supinated versus pronated versus neutral) held by an above‐elbow cast (Boyer 2002; Gupta 1990) (Table 11). In Boyer 2002, the cast was applied after reduction under general anaesthesia in 109 children presenting with displaced or angulated fractures, either radius only or both radius and ulna. All 60 participants in Gupta 1990 had a dorsally angulated solitary metaphyseal greenstick fracture, 25 of which met the criterion (greater or equal to 20 º dorsal angulation) for reduction before cast application.
6. Position of arm in above‐elbow cast (forearm supinated versus pronated versus neutral): participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Forearm supinated | Forearm pronated | Forearm in neutral | Duration of use |
| Boyer 2002 | 109 Mean: 7.8 years |
Displaced or angulated fractures, either radius only or both radius and ulna | Closed reduction Forearm supinated in above‐elbow cast (plaster below elbow and fibreglass above elbow) | Closed reduction Forearm pronated in above‐elbow cast (plaster below elbow and fibreglass above elbow) | Closed reduction Forearm neutral in above‐elbow cast (plaster below elbow and fibreglass above elbow) | Not stated but probably removed at fracture union (6 to 8 weeks) |
| Gupta 1990 | 60 Mean: 8.3 years |
Dorsally angulated solitary metaphyseal greenstick fracture. Closed reduction in 25 | Closed reduction if ≥ 20 º dorsal angulation. Forearm supinated in above‐elbow plaster cast |
Closed reduction if ≥ 20º dorsal angulation. Forearm pronated in above‐elbow plaster cast | Closed reduction if ≥ 20º dorsal angulation. Forearm neutral in above‐elbow plaster cast | Cast removed at 6 weeks |
Home versus hospital‐clinic removal of casts for predominantly buckle fractures
Two trials, involving 404 children with stable, predominantly buckle fractures, compared removal at three weeks of casts at home by parents versus removal at the hospital fracture clinic by clinicians (Hamilton 2013; Symons 2001) (Table 12). In Hamilton 2013, home removal was facilitated by using a flexible cast instead of a standard fibreglass cast. A plaster backslab was used for all 87 children in Symons 2001.
7. Home versus hospital‐clinic removal of casts: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Home removal | Hospital removal | Duration of use |
| Hamilton 2013 | 317 Mean: 9.4 years; range 2 to 16 years (eligible) |
Stable distal forearm fractures.
Buckle (194: 61%); greenstick (63: 20%); epiphyseal (60: 19%). Both bones: 30 (9.5%) |
Flexible cast that allowed home removal |
'Standard' fibreglass cast Removed at fracture clinic |
3 weeks |
| Symons 2001 | 87 Mean: 9.2 years |
Buckle fractures | Below‐elbow backslab Instructions for removal by parents |
Below‐elbow backslab. Removed at fracture clinic |
3 weeks |
Different methods of percutaneous pinning (wire fixation)
None of the included trials made this comparison.
Additional comparisons
Seven trials made one of the following six comparisons.
Removable splintage versus rigid complete casts for minimally displaced but potentially unstable fractures
Boutis 2010 compared a commercially available removable splint with a below‐elbow cast in 100 children with minimally angulated or a minimally displaced acute greenstick or transverse fractures, which are potentially unstable fractures (Table 13).
8. Removable splintage versus below‐elbow cast for minimally displaced but potentially unstable fractures: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Removable splint (or backslab) | Cast | Duration of use |
| Boutis 2010 | 100 Mean: 9.3 years; range 5 to 12 (eligible) |
Minimally angulated or a minimally displaced acute greenstick or transverse fracture of the metaphyseal portion of the distal radius Radius (distal metaphyseal) Greenstick : 55 (57%); transverse: 41 (43%) Associated ulna (distal metaphyseal); buckle 22 (23%) ulnar styloid 5 (5%) |
Prefabricated splint | Fibreglass below‐elbow (short arm) cast | Duration of immobilisation: 4 weeks Six children (3 in each group) had to wear the immobilisation device for 6 weeks because their fracture angulation had progressed to 25 ° at the 4‐week visit |
Waterproof versus 'traditional' non‐waterproof casts for predominantly buckle fractures
Two trials compared a waterproof cast versus a more traditional non‐waterproof cast in 95 children, over 80% of whom had buckle fractures of the distal radius (Derksen 2011; Silva 2016) (Table 14). Silva 2016 used a cross‐over design, in which the alternative cast was applied after two weeks.
9. Waterproof cast versus "traditional" cast: participant and intervention characteristics.
| Study ID | No. participantsAge | Fracture type | Waterproof cast | Traditional cast | Duration of use |
| Derksen 2011 | 68 Mean: 9.8 years; range 5 to 15 years (eligible) |
Isolated greenstick or buckle fracture of the distal radius (56 (82%) were buckle fractures) | "Swim cast": air‐ventilating semi‐flexible polyester cast manufactured without the use of a synthetic wool liner and thus applied directly over the protective stocking | Traditional cast (made of polyurethane material, with a cotton liner). Participants receiving the traditional cast were advised to use a protective plastic bag when taking a shower or going for a swim | Both types of casts were worn for 2 to 3 weeks. Children younger than 8 years were immobilised for a total of 3 weeks and children 8 years and older were immobilised for a total of 4 weeks, |
| Silva 2016 | 27 Mean: 9.4 years; range 6 to 13 years |
Nondisplaced or minimally angulated (< 15 °) fracture of the distal radius (23 (85%) were buckle fractures) | "Waterproof cast": below‐elbow cast made of the waterproof hybrid mesh material with a waterproof skin protector | Non‐waterproof cast: below‐elbow cast of traditional fibreglass material with a non‐waterproof lining material. Participants with the traditional cast were asked to keep it dry | Cross‐over trial. Casts were replaced by the alternative cast at 2 weeks post‐cast application |
Split versus closed circumferential synthetic semi‐rigid above‐elbow cast
Schulte 2014 compared a split versus closed circumferential synthetic semi‐rigid above‐elbow cast in 100 children, of which 40 had displaced distal radius fractures (including 12 that involved the growth plate). Cast removal was at four weeks (Table 15).
10. Split versus closed circumferential synthetic semi‐rigid above‐elbow cast: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Split cast | Complete cast | Duration of use |
| Schulte 2014 | 40 children with displaced distal radius fractures (out of 100 children with displaced closed forearm fractures). Mean: 9.1 years (all participants) |
Closed displaced distal radius fracture; 12 fractures involved the growth plate. Number of distal both bone fractures unknown, 52 both bone fractures in the whole sample | Closed reduction. Split circumferential synthetic semi‐rigid above‐elbow cast | Closed reduction. Closed circumferential synthetic semi‐rigid above‐elbow cast | 4 weeks |
Double‐sugar‐tong splint extended at one week to an above‐elbow cast versus above‐elbow bivalved cast
Levy 2015 compared a double‐sugar‐tong splint extended at one week with an above‐elbow cast versus an above‐elbow bivalved cast in 71 children with displaced distal radius or distal both‐bone forearm fractures. Cast removal was at six or eight weeks (Table 16).
11. Double‐sugar‐tong splint extended after 1 week to above‐elbow cast versus long arm bivalved cast: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Splint | Cast | Duration of use |
| Levy 2015 | 71 Mean: 8.7 years; range 4 to 12 years |
Displaced distal radius or distal both‐bone forearm fractures Radius only: 28 Radius and ulna: 43 |
Closed reduction Double–sugar‐tong splint (elbow enclosed) The splint was overwrapped into an above‐elbow cast after a week. Splint overwrap was changed to below‐elbow cast at 4 or 6 weeks |
Closed reduction Above‐elbow bivalved cast, changed to below‐elbow cast at 4 or 6 weeks (optional) |
6 or 8 weeks |
Comparison of two different water‐resistant cast liners (minimally displaced fractures)
One trial compared two different types of water‐resistant cast liner, Wet or Dry® versus Delta Dry®, in 105 children with minimally displaced distal radius fracture, including both metaphyseal and physeal fractures (Stevenson 2013) (Table 17). The below‐elbow casts were removed at around five weeks.
12. Comparison of two different water‐resistant cast liners: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Cast liner | Cast liner | Duration of use |
| Stevenson 2013 | 105 Mean: 10 years; < 18 years |
Minimally displaced distal radius fracture ("mixture", including metaphyseal and Salter Harris II fractures) | Initial above‐elbow slab then below‐elbow cast with Wet or Dry® undercast lining | Initial above‐elbow slab then below‐elbow cast with Delta Dry® water‐resistant undercast lining | Around 5 weeks |
Closed reduction by Paediatric Emergency Physician (EP) versus Orthopaedic Resident
One single‐centre trial in USA with 104 participants compared closed reduction of displaced or angulated distal forearm fractures (70% involved both bones) by one of two pre‐trained paediatric emergency physicians versus closed reduction by postgraduate year 3 or year 4 orthopaedic residents (Khan 2010) (Table 18).
13. Closed reduction by Paediatric Emergency Physician (EP) versus Orthopaedic Resident: participant and intervention characteristics.
| Study ID |
No. participants Age |
Fracture type | Paediatric Emergency Physicians | Orthopaedic Residents | Type and duration of immobilisation |
| Khan 2010 | 104 Mean: 9.4 years |
Closed displaced or angulated distal forearm fractures (70% involved both bones) | Closed reduction by pre‐trained paediatric emergency physicians. Manipulation with aid of portable fluoroscopy | Closed reduction by postgraduate year 3 or 4 orthopaedic residents. Manipulation with aid of portable fluoroscopy | Not stated. Standard follow‐up at 6 to 8 weeks |
Outcome measurement
Details of the follow‐up schedules and the outcomes measured in individual studies are provided in the Characteristics of included studies tables. The follow‐up period ranged from three weeks, such as in Davidson 2001, to a mean of 7.7 months in Webb 2006. We comment only on the primary outcomes below.
Seven studies assessed physical function using validated measures, although not for wrist fracture: ABILHAND‐Kids score (Colaris 2012; Colaris 2013a); Activities Scale for Kids ‐ Performance version (ASK‐P) (Boutis 2010; Plint 2006; Silva 2016); Childhood Health Assessment Questionnaire (CHAQ) index (Hamilton 2013), and, while not reported, the Peds QL questionnaire (Williams 2013).The modified version of the ASK‐P used in Boutis 2010 and Plint 2006 included eight additional questions related more specifically to activity of the wrist.
Aspects of treatment failure, such as the need for a change in procedure or further immobilisation, were commonly reported, but the composite outcome (number of participant with treatment failure) was generally not stated. This applied also to serious adverse effects and complications. The differences in the reasons for treatment failure or intervention‐specific complications was particularly notable for the comparison of percutaneous pinning versus cast only as detailed in the Effects of interventions.
Funding and conflicts of interest
Five trials reported the source of funding, seven trials stated that they did not receive any funding, and 18 did not publish the source of funding.
No trials explicitly declared any conflicts of interest: 16 studies stated that there were no conflicts of interest and the remaining 14 studies did not mention conflicts of interest.
Excluded studies
Fifty‐three studies failed to meet the inclusion criteria and were excluded, three after receiving further information from the trial investigators (ISRCTN25187648; ISRCTN34857372; NCT01493167). Reasons for exclusion are detailed in the Characteristics of excluded studies table and summarised below:
Ineligible study design: 13 studies were either narrative reviews (Bae 2012; Parsch 2002), non‐randomised comparative studies (Bhaskar 2000; Dresing 2009; Khan 2007; Krishnan 2014; Lidstrom 1959; Robert 2011; Sutherland 2011; Witney‐Lagen 2013; Zhao 2015), a case report (Pritchett 1994), or a cohort study (NCT00398268).
Ineligible population: 29 studies assessed an adult population (Abramo 2009; Basdekis 2006; Cohen 1997; Delattre 1994; Egol 2008; Fikry 1998; Franke 2013; Gradl 2014; Gupta 1991; Hahnloser 1999; Kasapinova 2009; Kavouriadis 2012; Krishnan 2003; NCT01493167; McQueen 1996; Mitsukane 2015; Mullett 2002; Murphy 2010; Pieske 2008; Pieske 2011; Saddiki 2011; Schønnemann 2011; Serrano‐Fernandez 2008; Sha 2015; Tamblyn 2010; Vang Hansen 1998; Van Manen 2008; Walker 2003; Young 2003), two studies had a mixed population of adults and children (Hargreaves 2004; Hutchinson 1995) but did not separate out the results for the two subpopulations, and three studies included shaft fractures only (Colaris 2013b; Ho 2010; Lu 2014).
Other reasons: three trials were abandoned (Clarke 2007; Duncan 2006; NCT01762605), two studies were never written up or published (ISRCTN25187648; Yousef 2006) and a co‐investigator of one study (ISRCTN34857372) failed to respond to requests to share the study data.
Studies awaiting classification
There are six RCTs awaiting classification. Details have been reported in the Characteristics of studies awaiting classification table and briefly summarised below. We received additional information from the trialists of two studies (Bae 2015; NCT02670629). All six studies are RCTs that recruited children between two and 15 years old in four studies (Baldwin 2017; NTR2508; ACTRN12611000101987; NCT02670629), a mean age of 10 years in one study (Bae 2012) and aged up to 21 years in another study (NCT03097757). The studies made the following comparisons; the reason for each study being in this category is also given in brackets.
Bae 2015 (n = 202 participants): bivalved cast versus circumferential cast (no response to request for separate data on wrist fractures).
Baldwin 2017 (n = 60 participants): bivalved versus univalved versus intact (no value) above‐elbow fibreglass cast (no response to request for separate data on wrist fractures).
NTR2508 (n = not stated): Mitella sling versus plaster cast (unable to contact authors to establish trial status).
ACTRN12611000101987 (n = 100 participants): sugar‐tong plaster of Paris splint with an elastic bandage versus above‐elbow circumferential plaster of Paris cast (full text not available; no response to request for information).
NCT02670629 (n = 60 participants): closed anatomic reduction under anaesthesia and short cast versus closed overriding alignment and short cast with oral medications only (although the full publication is pending, a conference abstract was identified by JH subsequent to the search and so has not been included in the results of the search (Hernandez 2018)).
NCT03097757 (n = 112 participants): ultrasound guided fracture reduction versus standard of care fracture reduction (mixed fracture trial; awaiting publication).
Ongoing studies
There are four studies listed as ongoing, and we will include them in the next update if data are available. Details have been reported in the Characteristics of ongoing studies table and summarised below. The target number of participants is given for each trial.
Adrian 2015 (n = 742 children with angulated distal radius or distal bones fractures): Plaster immobilisation without reduction versus closed reduction under anaesthesia and percutaneous K‐wire osteosynthesis with one or two wires (estimated date of completion is March 2018).
NCT03248687 (n = 125 children with a distal radius buckle fracture): removable splint with scheduled primary care physician versus removable splint without scheduled primary care physician (estimated date of completion is June 2018).
FORCE 2018 (n = 696 children with a distal radius buckle fracture): soft bandage and immediate discharge versus current treatment with rigid immobilisation (recruitment due to open in November 2018 and follow‐up to be completed by February 2020).
NCT03297047 (n = 120 children with a distal radius or forearm fracture): forearm combi‐cast versus upper‐arm combi‐cast (estimated date of completion is June 2019).
Risk of bias in included studies
The 'Risk of bias' judgements on nine items for the individual trials are summarised in Figure 2 and described in the 'Risk of bias' tables in the Characteristics of included studies. A 'Yes' (+) judgement means that the review authors considered there was a low risk of bias associated with the item, whereas a 'No' (‐) means that there was a high risk of bias. Frequently assessments resulted in an 'Unclear' (?) verdict; this often reflected a lack of information upon which to judge the item (see Figure 3). However, we usually took a lack of information on blinding to imply that there was no blinding, and rated it as a 'No'.
2.
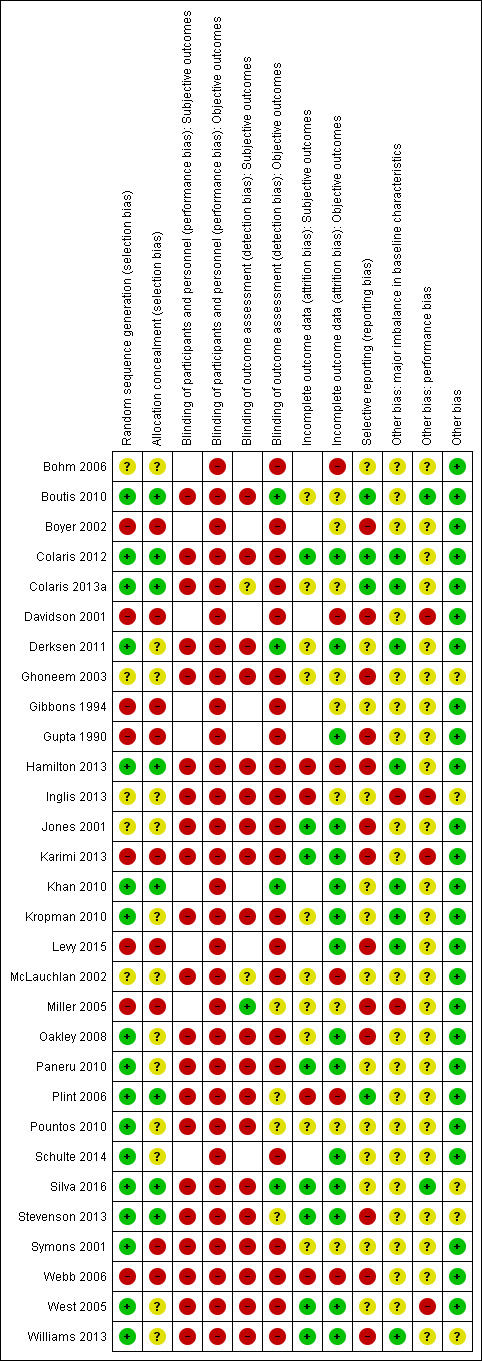
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
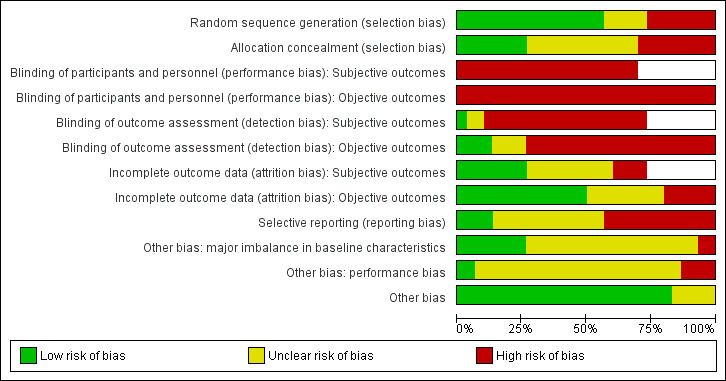
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
All trials were at high risk of bias, invariably performance bias that for most trials reflected the impracticality of blinding care providers or participants to the treatment allocation, and generally detection bias, although a few trials did succeed in blinding of some outcome assessment. Most trials were at high risk of bias for other domains, notably the nine trials, eight of which were quasi‐randomised, at high risk of selection bias, reflecting lack of allocation concealment.
Allocation
Overall, we rated eight trials at low risk of selection bias as they had random sequence generation and allocation concealment (Boutis 2010; Colaris 2012; Colaris 2013a; Hamilton 2013; Khan 2010; Plint 2006; Silva 2016; Stevenson 2013). We judged eight studies as having a high risk of selection bias as they were, or were likely to be, quasi‐randomised (Boyer 2002; Davidson 2001; Gibbons 1994; Gupta 1990; Karimi 2013; Levy 2015; Webb 2006) or had included non‐ or quasi‐randomised participants (Miller 2005). The remaining 14 studies provided insufficient or no information on safeguards for allocation concealment and, in five studies, insufficient or no information on random sequence generation.
Random sequence generation
Information on the method of randomisation either confirmed (e.g. computer‐generated sequence) or implied an adequate method of generating a sequence in 17 studies, which we therefore judged to be at low risk of selection bias for sequence generation (Boutis 2010; Colaris 2012; Colaris 2013a; Derksen 2011; Hamilton 2013; Khan 2010; Kropman 2010; Oakley 2008; Paneru 2010; Plint 2006; Pountos 2010; Schulte 2014; Silva 2016; Stevenson 2013; Symons 2001; West 2005; Williams 2013). We rated eight studies as having a high risk of selection bias as they were, or were likely to be, quasi‐randomised or had a non‐ or quasi‐randomised component (Boyer 2002; Davidson 2001; Gibbons 1994; Gupta 1990; Karimi 2013; Levy 2015; Miller 2005; Webb 2006). Five studies provided no or inadequate information on the sequence generation and were deemed unclear (Bohm 2006; Ghoneem 2003; Jones 2001; Inglis 2013; McLauchlan 2002).
Allocation concealment
Allocation of the interventions was adequately concealed in eight studies that we judged to have low risk of bias (Boutis 2010; Colaris 2012; Colaris 2013a; Hamilton 2013; Khan 2010; Plint 2006; Silva 2016; Stevenson 2013). Thirteen studies had an unclear risk of bias, reflecting lack of clarity on methods including insufficient information on safeguards, the potential for predictability, and no mention of sequential numbering for randomisation involving envelopes (Bohm 2006; Derksen 2011; Ghoneem 2003; Inglis 2013; Jones 2001; Kropman 2010; McLauchlan 2002; Oakley 2008; Paneru 2010; Pountos 2010; Schulte 2014; West 2005; Williams 2013). We judged nine studies to have a high risk of bias, in eight due to having a predictable sequence (Boyer 2002; Davidson 2001; Gibbons 1994; Gupta 1990; Karimi 2013; Levy 2015; Miller 2005; Webb 2006) and in one study because it used an open list (Symons 2001).
Blinding
We assessed the risk of performance and detection biases separately for subjective and objective outcomes. Overall, there was little attempt at blinding in the included studies, with limited but probably effective blinding of outcome assessment being reported in only four trials (Boutis 2010; Derksen 2011; Khan 2010; Silva 2016) and ineffective or very limited blinding of outcome assessment being reported in three others (Bohm 2006; Schulte 2014; Stevenson 2013).
Blinding of participants and personnel (performance bias)
Blinding of participants (children and parents) and personnel providing care was generally not feasible and no trial reported attempting this.
We judged all 21 trials reporting subjective outcomes to be at high risk of performance bias relating to these outcomes. We rated the other nine trials not reporting these outcomes at unclear risk (Bohm 2006; Boyer 2002; Davidson 2001; Gibbons 1994; Gupta 1990; Khan 2010; Miller 2005; Levy 2015; Schulte 2014). We judged all 30 trials at high risk of performance bias relating to objective outcomes.
Blinding of outcome assessment (detection bias)
Subjective outcomes
We judged one trial to be at low risk of bias because of the unlikely risk of bias for the few subjective outcomes collected at an average of 2.8 years follow‐up (Miller 2005). Of the 11 studies at unclear risk of bias, the lack of blinding of the participants may have been moderated to some extent by the involvement of a blinded or independent assessor of function and recovery in two trials (Boutis 2010; McLauchlan 2002) or longer‐term follow‐up at six months in Colaris 2013a. The other eight studies in this group did not measure subjective outcomes (Bohm 2006; Boyer 2002; Davidson 2001; Gibbons 1994; Gupta 1990; Karimi 2013; Levy 2015; Schulte 2014). A high risk of bias reflecting no reporting or indication of blinding was likely in 18 trials.
Objective outcomes
We rated four trials reporting effective blinding of key objective outcomes, mainly assessment of complications, to be at low risk of bias (Boutis 2010; Derksen 2011; Khan 2010; Silva 2016). A high risk of bias reflecting no reporting of blinding, or in two studies very partial blinding, was likely in 22 studies. Of the four studies judged at unclear risk of detection bias of objective outcomes, we assessed the risk of bias may have been reduced through the involvement of independent assessment in two studies (Pountos 2010; Stevenson 2013), and the reduced vulnerability to detection bias of the outcomes assessed in the other two studies (Miller 2005; Plint 2006).
Incomplete outcome data
We assessed the risk of attrition bias separately for subjective and objective outcomes.
Subjective outcomes
We considered eight trials to be at low risk of bias from the incompleteness of data on subjective outcomes (Colaris 2012; Jones 2001; Karimi 2013; Paneru 2010; Silva 2016; Stevenson 2013; West 2005; Williams 2013). We rated four trials at high risk of bias, reflecting large losses to follow‐up, post‐randomisation exclusions and difference in losses between groups (Hamilton 2013; Inglis 2013; Plint 2006; Webb 2006). Of the 18 trials rated at unclear risk of attrition bias for subjective outcomes, eight did not report on these outcomes (Bohm 2006; Boyer 2002; Davidson 2001; Gibbons 1994; Gupta 1990; Khan 2010; Levy 2015; Schulte 2014).
Objective outcomes
We judged 15 trials to be at low risk of bias from the incompleteness of data on objective outcomes (Colaris 2012; Derksen 2011; Gupta 1990; Jones 2001; Karimi 2013; Khan 2010; Kropman 2010; Levy 2015; Oakley 2008; Paneru 2010; Schulte 2014; Silva 2016; Stevenson 2013; West 2005; Williams 2013). We judged six trials to be at high risk of bias, usually reflecting large losses to follow‐up, post‐randomisation exclusions and difference in losses between groups (Bohm 2006; Davidson 2001; Hamilton 2013; McLauchlan 2002; Plint 2006; Webb 2006). We rated the remaining nine trials at unclear risk of attrition bias.
Selective reporting
Trial registration documentation was available for 10 trials (Boutis 2010; Colaris 2012; Colaris 2013a; Hamilton 2013; Jones 2001; Khan 2010; Kropman 2010; Oakley 2008; Silva 2016; Williams 2013) but was retrospective in three of these (Khan 2010; Oakley 2008; Williams 2013). The outcomes at trial registration were minimally described for some trials, such as Colaris 2012 and Colaris 2013a, and there were also some discrepancies in the intervention described at trial registration and in the conference abstract report of Jones 2001. We found no published protocols.
We judged 13 trials to be at high risk of selective reporting bias, typically because of incomplete reporting of outcome including at final follow‐up (Boyer 2002; Davidson 2001; Ghoneem 2003; Gupta 1990; Hamilton 2013; Jones 2001; Karimi 2013; Levy 2015; Miller 2005; Oakley 2008; Stevenson 2013; Webb 2006; Williams 2013). We judged 13 trials to be at unclear risk of bias, often because function was not reported. We judged the remaining four trials at low risk of selective reporting bias (Boutis 2010; Colaris 2012; Colaris 2013a; Plint 2006).
Other potential sources of bias
We specifically assessed other bias resulting from major imbalances in baseline characteristics and bias resulting from differences in care provision, including in the potential expertise of care providers, other than the interventions being compared. Finally we noted if there were other noteworthy potential sources of bias additional to those already covered.
We judged two studies to be at high risk of bias resulting from major imbalances in baseline characteristics: this pertained to sex and fracture characteristics in Inglis 2013, and fracture characteristics in Miller 2005. There were no obvious baseline imbalances in the eight studies at low risk of bias for this item (Colaris 2012; Colaris 2013a; Derksen 2011; Hamilton 2013; Khan 2010; Kropman 2010; Levy 2015; Williams 2013). We judged the other 20 studies to be at unclear risk of bias, usually because there were insufficient or no baseline characteristics data for all participants split by treatment group.
We judged four studies to be at high risk of other performance bias: Davidson 2001 mainly because of the probable between‐groups difference in the provision of written instructions between the two groups; Inglis 2013 because of between‐group differences in the use of below‐elbow casts and general anaesthesia; and Karimi 2013 and West 2005 because of between‐group differences in the follow‐up schedules. We rated just two studies at low risk of bias (Boutis 2010; Silva 2016). We considered the remaining 24 studies to be at unclear risk of other performance bias, typically because of a lack of details of the care providers.
We judged five studies to be at unclear risk of other potential sources of bias: Ghoneem 2003 because of very minimal reporting, even for a conference abstract; Inglis 2013 because of discrepancies in the reporting of the duration of the study; Silva 2016 because of the potential impact of the inappropriate cross‐over design; Stevenson 2013 because of the potential for data‐driven stopping of the trial; and Williams 2013 because of the premature follow‐up at three weeks. We found no other sources of potential bias for the other 25 trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Summary of findings. Removable splintage versus cast for buckle and other stable fractures.
| Removable splintage versus below‐elbow cast for buckle or minimally displaced fracture in children | ||||||
|
Patient or population: children with stable wrist fracture, predominantly buckle (torus) fracturesa Settings: hospital clinic Intervention: Removable splintb for 2 to 6 weeks Comparison: Below‐elbow cast for 2 to 6 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Below‐elbow cast | Removable splint | |||||
| Physical function (short‐term): measured using the Modified Activities Scale for Kids ‐ performance version (0 to 100; best function; no disability) (4 weeks follow‐up) | See comment. The median score in the study control group was 99.11 (IQR 96.42 to 100.00) | See comment. The median score in the intervention group was 99.04 (IQR 95.29 to 100.00) | ‐ | 65 children (1 study) | ⊕⊕⊝⊝ lowc | The data for the final scores are shown here for illustrative purposes; with no evidence of a MCID between the 2 groups (set at 15 in the study for sample size calculation) Another study (50 children) found little between‐group difference at 4 to 6 weeks in the numbers with no or only limited disability.d |
| Treatment failure (4 weeks follow‐up) |
36 per 1000e | 26 per 1000 (10 to 68) | RR 0.71 (0.26 to 1.89) | 444 children (4 studies) |
⊕⊝⊝⊝ very lowf | The data for this outcome were based on change or replacement of device for problems: pain, intolerance, increased deformity (missed greenstick fractures) in the splint group (5 events); cast replacement for broken or wet cast, lodged pencil in the cast group (8 events)g |
| Serious adverse events (6 months follow‐up) |
See comment | See comment | Not estimable | 87 children (1 study) | See comment | One study reported there had been no refractures. This is consistent with other evidence, including from other included trials with buckle fractures that explicitly reported the absence of serious adverse events (139 children from 2 studies comparing bandage versus cast; 288 children from 1 study comparing home versus hospital removal of casts) |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported.h |
| Pain VAS (0 to 10; worst pain) during device use (4 ‐ 6 weeks follow‐up) |
The mean score in the study control group was 2.92 | The mean score in the intervention group was 0.20 higher (1.10 lower to 1.50 higher) | ‐ | 50 children (1 study) | ⊕⊝⊝⊝ very lowi | A 0.2 difference is minute and clinically unimportant. Overall, 5 trials provided data on pain, using different measures and timings. The 2 trials (161 children) reporting pain at 1 week found higher median pain scores in the splint group but neither of the differences between the 2 groups reached statistical significance; moreover, the difference in 1 trial was also unlikely to be clinically important. Most children in these 2 trials had no or very little pain by the end of 2 or 3 weeks immobilisation |
| Minor complications (3 to 6 weeks follow‐up) |
See comment | See comment | Not estimable | 500 children
(5 studies) (individual complications) |
See comment | The numbers of participants with complications were not reported. There was a large variety of probably minor complications or problems. Other than those described under treatment failure, these included slightly increased deformity (splint 1 case; cast 1 case); skin problems (splint: rash 11 cases); oedema (cast: 5 cases); stiffness (cast: 3 cases); subnormal grip strength (cast: 9 cases); medical attention sought by concerned parents (10 cases) and minor device problems (33 cases)j |
| Participant satisfaction: child and/or parent preference for same device in future (3 to 6 weeks follow‐up) | See comment | See comment | Not estimable | 178 children (2 studies) | See comment | Results (1 indicating no difference, 1 favouring the splint) not pooled: clinically (e.g. different types of splint) and statistically heterogeneous (I2 = 83%)k |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IQR: Interquartile range; MCID: Minimal clinically important difference; RR: Risk ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aChildren had buckle fractures in five studies and either buckle or an "undisplaced greenstick" fracture in one study. bSix studies made this comparison. Four probably used commercially available splints: one reported using prefabricated splints, the illustration in one also indicated a prefabricated splint; and two reported using futuro or futura type splints. Of the other two trials, one reported using a fibreglass volar slab secured by an elasticated bandage, and the other reported an individually‐fitted plaster splint attached with a tensor bandage. cWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection biases) and attrition bias. We did not downgrade for imprecision, given the minimal differences and small IQRs in relation to the MCID, and the consistency of these results with other data from this study and from another study that also reflected the lack of important differences shown by this result. d'Limited disability' applied to one of five areas: interference with play; help needed with feeding; help needed with washing and dressing; sleep disturbance; missed days of school. eControl group risk is derived from the median control group risk across studies. fWe downgraded the evidence by one level for serious risk of bias, mainly reflecting lack of blinding (performance and detection biases), by one level for serious indirectness for an incompletely reported outcome measure (see footnote 'g'), and by one level for serious imprecision (wide confidence interval and few events). gOne study (84 children) also provided data for extended immobilisation (also defined as 'treatment failure') for pain and discomfort (6/42 versus 3/42; RR 2.00, 95% CI 0.54 to 7.47; very low‐quality evidence). These data could not be pooled with the data on change in or reapplication of devices because of the high risk of a unit‐of‐analysis error. hAlthough this outcome was not reported, return to sporting or normal physical activities by four weeks in one trial (60 children) was greater in the splint group (25/26 versus 23/34; RR 1.42, 95% CI 1.11 to 1.82). However, there were contradictory and considerably heterogeneous findings (I2 = 92%) in the return to normal activities between this trial (at 20 days), which favoured the splint group, and another trial (at 14 days) that favoured the cast group. iWe downgraded by one level for serious risk of bias, reflecting lack of blinding (performance and detection biases), by one level for very serious imprecision, given the data for this outcome from two other studies were unavailable for pooling and the wide confidence interval, and by one level for indirectness, given the measure was poorly defined. jThere was no indication of revised treatment for these less serious complications. All individual complications were reported by single trials only. kEach result was assessed as very low‐quality evidence, downgraded by two levels for very serious risk of bias reflecting lack of blinding (blinding and performance biases) and selective reporting bias, and by one level for serious imprecision reflecting the small sample size.
Summary of findings 2. Summary of findings. Bandage versus cast.
| Bandage versus below‐elbow cast for buckle or minimally displaced fracture in children | ||||||
|
Patient or population: children with stable wrist fracture, predominantly buckle (torus) fracturesa Settings: hospital clinic Intervention: Soft or elasticated bandageb for 3 to 4 weeks Comparison: Below‐elbow cast for 3 to 4 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Below‐elbow cast | Bandage | |||||
| Physical function (short‐term): no problems or only 'limited disability' (see Comments) (4 to 6 weeks follow‐up) | 500 per 1000c | 895 per 1000 (590 to 1000) | RR 1.79 (1.18 to 2.73) | 53 (1 study) | ⊕⊝⊝⊝ very lowd | 'Limited disability' applied to 1 of 5 areas: interference with play; help needed with feeding; help needed with washing and dressing; sleep disturbance; missed days of school |
| Treatment failure: (3 to 6 weeks follow‐up) |
33 per 1000e | 51 per 1000 (15 to 176) | RR 1.53 (0.44 to 5.32) | 181 children (3 studies) |
⊕⊝⊝⊝ very lowf | Parents of 4 children (4.4%) requested a change from bandage to cast; 3 because they were sore from overuse and 1 "special needs" child. There were no requests for change in the cast group. 1 trial reported 4 cases (1 in the bandage group versus 3 in the cast group) of delayed union requiring an extra week |
| Serious adverse events (3 to 4 weeks follow‐up) |
See comment | See comment | Not estimable | 139 children (2 studies) | See comment | No children developed a serious adverse event, including refracture, in these 2 studies. This is consistent with other evidence, including from other included trials with buckle fractures that explicitly reported the absence of serious adverse events (87 children from 1 study comparing removable splint versus cast; 288 children from 1 study comparing home versus hospital removal of casts) |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported |
| Pain with VAS (0 to 100; worst pain) at 1 week | The mean score in the study control group was 20 | The mean score in the intervention group was 6 higher (1.31 lower to 13.31 higher) | ‐ | 89 children (1 study) | ⊕⊝⊝⊝ very lowg | The 95% CI is unlikely to include a clinically important effect. There was also very low‐quality evidence of less pain in the bandage group in 1 study (39 children), and little difference in pain during device use or requirement for analgesic in another study (53 participants)h |
| Minor complications (3 to 6 weeks follow‐up) |
See comment | See comment | Not estimable | 92 children
(2 studies) (individual complications) |
See comment | Complications reported were skin problems, increased deformity (minimal) and stiffnessi. Where reported, there was very low‐quality evidence of little or no difference between groups in the individual complications |
| Participant satisfaction: children found treatment was convenient (4 weeks follow‐up) |
143 per 1000c | 946 per 1000 (331 to 1000) | RR 6.61 (2.31 to 18.96) |
39 children (1 study) |
⊕⊝⊝⊝ very lowj | In this study, all 18 participants followed up in the bandage group had removed their bandage by 2 weeks |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aChildren had buckle fractures in two studies, "impacted greenstick" fractures in one study and either buckle or an "undisplaced greenstick" fracture in one study. bThe soft bandage was a wool layer covered with a cotton crepe bandage. The elasticated bandage was a tubigrip. cControl group risk is derived from the study data. dWe downgraded the evidence by two levels for very serious risk of bias, mainly reflecting lack of blinding (performance and detection biases), and by one level for serious indirectness for an inadequately reported outcome measure. eControl group risk is derived from the mean, since the median control risk (as in 2 studies) = 0. fWe downgraded the evidence by two levels for very serious risk of bias, mainly reflecting lack of blinding (performance and detection biases), and by two levels for very serious imprecision (few events, wide confidence interval). gWe downgraded by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection biases), and by one level for imprecision for wide confidence intervals. hPain was measured in different ways: one study referred to a "semantic scale", one used a VAS and also reported in terms of requiring analgesics. iOne study (39 children) reported an absence of skin problems; and one study (53 children) reported one child in each group had slightly increased deformity; the same trial found three children in the cast group had stiffness after cast removal. The data from one study (49 children) reporting four cases of delayed union requiring extended treatment are included under treatment failure. jWe downgraded by two levels for very serious risk of bias, reflecting lack of blinding (blinding and performance biases), by one level for serious imprecision reflecting the small sample size, and by one level for serious indirectness as the outcome was not a full measure of satisfaction.
Summary of findings 3. Summary of findings. Below‐elbow versus above‐elbow cast.
| Below‐elbow compared with above‐elbow cast for distal forearm fractures in children | ||||||
|
Patient or population: children with either displaced distal radius fracture with intact or displaced ulna fracture (both‐bone fracture), or minimally displaced bone metaphyseal fracture Settings: hospital Intervention: below‐elbow cast, after closed reduction if displaced fracture Comparison: above‐elbow cast, after closed reduction if displaced fracture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Above‐elbow cast | Below‐elbow cast | |||||
| Physical function (short‐term; under 3 months) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reporteda |
| Physical function (medium‐term; 3 to 12 months): measured using the ABILHAND‐Kids score (0 to 42: no problems) (6 months follow‐up; mean 7 months) | The mean score in the study control group was 41.8 | The mean score in the intervention group was 1.1 lower (3.47 lower to 1.27 higher) | ‐ | 66 children (1 study)b | ⊕⊝⊝⊝ very lowc | It is unknown whether (including the 95% CI) this difference is clinically important and there is a strong possibility of a non‐normal distribution or ceiling effect No data were available from the 3 trials that included reduced displaced fractures |
| 'Treatment failure': secondary procedures such as remanipulation for early loss of position (only data for this used here); and change in treatment (Follow‐up 4 weeks) |
59 per 1000d | 16 per 1000 (5 to 63) | RR 0.27 (0.07 to 1.06) |
366 participants (4 studies) | ⊕⊝⊝⊝ very lowe | Overall treatment failure data were not available. One trial also reported change of cast typeg |
| Serious adverse events (Follow‐up up to 6 months) | See comment | See comment | Not estimable | 215 participants (2 studies) | See comment | Where reported, there was an absence of serious adverse events: refracture at 6 months (113 participants); compartment syndrome (102 participants) |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reportedh |
| Pain after 1 week in cast: VAS (0 to 10: worst pain) (Follow‐up 1 week) |
The mean score in the study control group was 2.24 | The mean score in the intervention group was 1.91 lower (1.27 to 2.55 lower) | ‐ | 85 participants (1 study) | ⊕⊕⊝⊝ lowi | ‐ |
| Minor complications.,j,k Here represented by Referral for physical therapy for range of motion limitation (post‐immobilisation after 4 weeks) |
57 per 1000d | 42 per 1000 (11 to 162) | RR 0.73 (0.19 to 2.84) |
179 participants (2 studies) | ⊕⊝⊝⊝ very lowl | Other complications reported included cast splitting, cast reinforcement, cast change, skin abrasion and transient neuropraxiam |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDifficulties with activities of daily living during cast use was quantified in one quasi‐randomised trial (107 participants), where 10 times fewer children in the below‐elbow group needed help during cast use (3/49 (6%) versus 35/58; RR 0.10, 95% CI 0.03 to 0.31). This subsidiary evidence, while consistent with expectations, was downgraded by two levels for very serious risk of bias refecting various biases, including selection bias (quasi‐randomised trial), lack of blinding (performance bias and detection bias) and attrition bias; and by one level for serious indirectness reflecting the vague description and timing of the outcome. bAll fractures in this trial were minimally displaced both‐bone metaphyseal fractures. Additionally, non‐circumferential plaster casts were used. cWe downgraded the evidence by one level for serious risk of bias reflecting lack of blinding (performance bias and detection bias), by one level for indirectness (the scoring system is validated for children with cerebral palsy), and by one level for imprecision (small single study with sufficiently wide confidence intervals that may or may not include a clinically important difference). The fracture population and interventions used should be noted; see footnote 'b'. dThe assumed risk is calculated from the median control group risk across studies. eWe downgradedthe evidence by one level for serious risk of bias, primarily reflecting lack of blinding (performance bias), by one level for indirectness (as well as variation in the type of fracture, decisions and decision criteria for remanipulation varied), and by one level for imprecision (wide confidence intervals, few events). gAfter remanipulation in one trial (102 participants), three children in the below‐elbow group were given above‐elbow casts. This trial also reported change of cast type for six participants: one below‐elbow cast had fallen off and five above‐elbow casts were changed to below‐elbow cast for comfort at three weeks. hChildren allocated below‐elbow casts in two trials had on average fewer days off school (4.19 days versus 10.43 days (85 participants); 0.56 days versus 1.6 days (113 participants)). We downgraded this very low‐quality subsidiary evidence by two levels for very serious risk of bias and by one level for indirectness, given the absence of a link between this measure and return to former activities. iWe downgraded the evidence by one level for serious risk of bias, mainly reflecting lack of blinding, and by one level for serious indirectness, given these data were from one small trial only. jThe data for total number of participants with less serious complications were not available; there was a strong possibility of unit‐of‐analysis issues. kConsideration of whether secondary displacement is a 'complication', reported by three trials, will depend on the extent to which the decision to act on loss of position may be guided by criteria or personal judgement, or both. Pooled data for secondary displacement favoured the below‐elbow group (21/133 versus 42/146; RR 0.56, 95% CI 0.36 to 0.87; 279 participants, 3 studies; I2 = 18%; low‐quality evidence downgraded by two levels for serious risk of bias). However, we focused only on displacements that had resulted in a secondary procedure; reported as 'treatment failure'. lWe downgraded the evidence by two levels for very serious risk of bias, reflecting in particular lack of blinding (performance bias) in two trials and selection bias in one quasi‐randomised trial, and by one level for imprecision (wide confidence interval, few events). mNon‐routine adjustments of casts included cast splitting for swelling (3/89 versus 6/98; RR 0.61, 95% CI 0.18 to 2.10; 187 participants, 2 studies); cast reinforced for 'breakdown' (4/89 versus 20/98; RR 0.25, 95% CI 0.10 to 0.65; 187 participants, 2 studies; I2 = 56%); and cast changed for loosening or breakdown (10/89 versus 7/98; RR 1.61, 95% CI 0.67 to 3.84; 187 participants, 2 studies; I2 = 75%). One trial (66 participants) also reported two cases of skin abrasion at the elbow and two cases of transient neuropraxia at the elbow; all were in the above‐elbow group. The evidence for all individual complications was very low quality, downgraded for serious risk of bias and, variously, for serious or very serious imprecision (few events, wide confidence intervals) and for serious inconsistency reflecting heterogeneous results.
Summary of findings 4. Summary of findings. Surgery (percutaneous wire fixation) versus not surgery (cast only).
| Surgery (percutaneous wire fixation) compared with cast alone after closed reduction for displaced distal forearm fractures in children | ||||||
|
Patient or population: children with displaced distal radius fracture with intact or involved or displaced ulna fracture (both‐bone fracture) Settings: hospital Intervention: surgery (percutaneous wire fixation) and cast (typically above‐elbow) cast after closed reduction Comparison: cast (typically above‐elbow) cast after closed reduction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Not surgery (cast alone) | Surgery (percutaneous wiring then cast) | |||||
| Physical function (short‐term; under 3 months) | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported. |
| Physical function (medium‐term; 3 to 12 months) measured using the ABILHAND‐Kids score (0 to 42: no problems) (6 months follow‐up; mean 7 months) |
The mean score in the study control group was 41.5 | The mean score in the intervention group was 0.4 higher (0.01 lower to 0.81 higher) | ‐ | 123 children (1 study)a | ⊕⊕⊝⊝ lowb | It is unlikely that this difference is clinically important and there is a strong possibility of a non‐normal distribution or ceiling effect. 3 other trials reported there was no functional deficit at 3 months (56 participants), 4 months (70 participants) and 2.8 years (25 participants) (very low‐quality evidence)c |
| 'Treatment failure': various secondary procedures such as remanipulation for early loss of position; early or more complex wire removal (Follow‐up 3 to 6 months) |
322 per 1000d | 168 per 1000 (107 to 268) | RR 0.52 (0.33 to 0.83) | 253 participants (4 studies) | ⊕⊝⊝⊝ very lowe | Most secondary procedures took place for early complications (up to 4 weeks). Procedures were mainly wire‐related (e.g. migration, infection) in the surgery group and for loss in position in the cast‐only groupf |
| Serious adverse events (typically more serious complications) (Follow‐up 3 to 6 months) | 445 per 1000d | 303 per 1000 (201 to 454) | RR 0.68 (0.45 to 1.02) | 253 participants (4 studies) | ⊕⊝⊝⊝ very lowg | Most complications occurred early and within 4 weeks (i.e. before cast removal). Adverse effects were mainly wire‐related (e.g. migration, infection, scar at K‐wire insertion point) and treatment for loss in position in the cast‐only grouph Where reported, there were no incidences of early physeal closure (2 studies, 57 children) or compartment syndrome (1 study; 34 children) |
| Time to return to former activities | See comment | See comment | Not estimable | See comment | This outcome was not reported | |
| Wrist pain | See comment | See comment | Not estimable | See comment | This outcome was not reportedi | |
| Minor complications (less serious).j Here represented by referral for physical therapy for range of motion limitation (post‐immobilisation after 4 weeks) |
546 per 1000k | 355 per 1000 (241 to 530) | RR 0.65 (0.44 to 0.97) | 128 participants (1 study) | ⊕⊝⊝⊝ very lowl | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAll fractures in this trial were displaced both‐bone metaphyseal fractures that appeared stable after reduction. bWe downgraded the evidence by one level for serious risk of bias, reflecting lack of blinding (performance bias), and by one level for indirectness (the scoring system is validated for children with cerebral palsy). We did not downgrade for imprecision, given the results of little between‐group difference, which was unlikely to be clinically important, the small 95% CI and values indicating minimal functional deficit in both groups that were consistent with the findings of three other trials. cThis subsidiary evidence, while reassuring, was downgraded by two levels for very serious risk of bias, refecting various biases including selection bias (allocation concealment unknown or not done) and lack of blinding (performance bias and detection bias), and by one level for serious indirectness, reflecting the vague description of the outcome and results. dThe assumed risk is calculated from the median control group risk across studies. eWe downgradedthe evidence by two levels for very serious risk of bias, primarily reflecting selection bias and lack of blinding (performance and detection biases), and by one level for serious inconsistency reflecting moderate heterogeneity (I2 = 58%). fThe percentage of secondary procedures, usually resulting in re‐reduction but some had wire fixation, for loss of position in the cast‐only group ranged from 21% to 91%. The criteria for this varied among the trials, with current trends towards accepting some displacement. Not included is the routine removal of K‐wires, nowadays typically carried out in the clinic. However, in one trial (conducted in 1997), this routinely involved general anaesthesia in the operating theatre. gWe downgraded the evidence by two levels for very serious risk of bias, primarily reflecting selection bias and lack of blinding (performance and detection biases), and by one level for serious indirectness, reflecting variation or lack of definitions of some complications, including redisplacement. hConsideration of whether redisplacement is a 'complication' will depend on extent to which the decision to act on loss of position may be guided by criteria or personal judgement, or both. The proportion of displacement in the cast‐only group of the four studies ranged from 39% to 91%, but we included only those for which a remedial procedure was undertaken (see footnote 'f'). iOne study reported five children (9% of 56; groups not identified) complained of minor pain upon strenuous activity at three months clinical review. jThese included items such as short‐term wrist or elbow stiffness, skin breakage, and non‐routine treatment adjustments. The only report was of range of motion restrictions that prompted physiotherapy in one trial. kBased on study control group data. lWe downgraded the evidence by two levels for serious risk of bias, reflecting lack of blinding (performance bias and detection bias), and by one level for serious indirectness (the evidence was not available for the other potential complications).
Summary of findings 5. Summary of findings. Home versus hospital‐clinic removal of casts.
| Home compared with hospital‐clinic removal of casts for stable wrist fractures in children | ||||||
|
Patient or population: children with stable wrist fracture, predominantly buckle (torus) fractures Settings: hospital clinic or home Intervention: home removal of casta (at 3 weeks) Comparison: hospital‐clinic removal of cast (at 3 weeks) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hospital‐clinic removal of cast | Removal of cast at home by parent | |||||
| Physical function (short‐term) measured using the Childhood Health Assessment Questionnaire (CHAQ) Index change scores from pre‐injury at 4 weeks ‐ VAS (probably 0 to 100; worst) | The mean change score in the study control group was −0.48 | The mean change score in the intervention group was 0.96 higher (0.21 lower to 2.13 higher) | ‐ | 233 children (1 study) | ⊕⊕⊝⊝ lowb | These scores indicate restoration of pre‐injury function in both groups No participant had difficulties in activities of daily living at 6 weeks in another study (80 children) |
| Treatment failure (change in treatment) (6 weeks follow‐up) |
5 per 1000c | 16 per 1000 (3 to 100) | RR 3.16 (0.50 to 19.93) | 397 children (2 studies) |
⊕⊝⊝⊝ very lowd | Details of the 5 changes to treatment are given in the footnotese |
| Serious adverse events (6 months follow‐up) |
See comment | See comment | Not estimable | 288 children (1 study) | See comment | No participants developed a long‐term serious adverse event in this study. This is consistent with other evidence, including from other included trials with buckle fractures that explicitly reported the absence of serious adverse events (87 children from 1 study comparing removable splint versus cast; 139 children from 2 studies comparing bandage versus cast) |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reportedf |
| Pain (CHAQ) via VAS (0 to 100; worst pain) at 4 weeks | The mean score in the study control group was 5.55 | The mean score in the intervention group was 0.43 lower (3.88 lower to 3.02 higher) | ‐ | 233 children (1 study) | ⊕⊕⊝⊝ lowb | The 95% CI does not include a clinically important effect |
| Minor complications (4 to 6 weeks follow‐up) |
See comment | See comment | Not estimable | 80 participants (1 study) |
See comment | Overall numbers with minor complications were not reported. Where reported, the few symptoms and problems with the backslab were minor and none resulted in further treatmentg |
| Participant satisfaction: Parents would not choose the same treatment again (6 weeks follow‐up) |
643 per 1000c | 103 per 1000 (39 to 277) | RR 0.16 (0.06 to 0.43) |
80 children (1 study) |
⊕⊕⊝⊝ lowh | This was reflected in the greater proportion of parental complaints related to the inconvenience and costs of attending the hospital clinici |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aTwo trials conducted in the UK tested this comparison. In one trial, home removal was facilitated by using a flexible cast instead of a standard fibreglass cast. A plaster backslab was used for all children in the second trial; this was precut in readiness for the home removal group. bWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection bias) and large and imbalanced loss to follow‐up (attrition bias). We did not downgrade for imprecision, given evidence of restoration of pre‐injury function in both groups of this study and in a second study; there was also minimal between‐group difference and a narrow 95% confidence interval. cControl group risk is derived from the study data. dWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection bias), and by one level for serious imprecision (few events and wide confidence interval). eTwo children had their cast changed at 1 week due to pain in the trial that used different casts in the two groups. Change to treatment reflected non‐compliance to planned cast removal at three weeks at the assigned locations in the other trial: this featured one early cast removal by participant and one delayed removal due to parental anxiety in the home group, and one removal by parent to avoid loss of earnings in the hospital group. fAlthough this outcome was not reported, at six weeks no children had difficulties in activities of daily living in one trial (80 participants) and average CHAQ changes scores for activities compared with pre‐injury scores at 4 weeks were small, with little difference between the two groups in another trial (233 participants). gOne trial (233 participants) reported there was no difference between the two groups in the number of casts that needed replacing or the number of additional plaster room visits. hWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of allocation concealment (selection bias) and lack of blinding (performance and detection bias). We did not downgrade for imprecision, given the corroborative evidence within the trial and consideration of the impact of the extra inconvenience of needing to return to clinic for cast removal. iParental complaints (14: 33% of 42) in the hospital group included 10 complaints about hospital waiting times, five about difficulties in getting time off work, three about transport problems and two about hospital parking. Some (7: 18% of 38) of the home group would have liked an extra bandage. In the other trial, 70 (67% of 104) children had to miss school to attend the appointment, with 52 carers taking time off work and nine of these losing pay as a result.
1. Non‐rigid or removable splintage (e.g. splints, non‐rigid complete cast, backslab or bandages) or 'no splintage' (analgesia only) versus rigid complete casts for buckle and minimally displaced (stable) fractures
Of the nine trials making this comparison, six trials compared splint versus below‐elbow cast and four compared a bandage versus a below‐elbow cast. No trials tested 'no splintage' or non‐rigid complete casts. One three‐group trial tested both comparisons (Pountos 2010).
Removable splint versus below‐elbow cast
Six trials compared a removable splint with a below‐elbow cast in 695 children with distal radius fractures (Table 6). Children had buckle fractures in five studies (Davidson 2001; Karimi 2013; Oakley 2008; Plint 2006; Williams 2013), and buckle or undisplaced greenstick fractures in Pountos 2010. Final follow‐up was at the end of treatment at three weeks in three trials (Davidson 2001; Karimi 2013; Williams 2013), at four to six weeks in Oakley 2008, at 12 weeks in Pountos 2010, and at six months in Plint 2006. We summarise the evidence for this comparison in Table 1. Appendix 2 shows a separate 'Summary of findings' table for this comparison that draws on the outcomes considered in the NICE 2016 guidelines.
Primary outcomes
Plint 2006 reported on function using a modified version of the Activities Scale for Kids ‐ Performance scores (ASK‐P 0 to 100: best function) at days 7, 14, 20 and 28 (see Analysis 1.1). There was low‐quality evidence of little or no difference between the two groups at four weeks (reported median difference 0.00, interquartile range (IQR) −2.13 to 0.86). None of the IQRs of the differences at the four time points included the minimal clinically important difference (MCID) of 15 chosen by Plint 2006 for their sample size calculation. Based on a poorly‐defined composite measure, there was very low‐quality evidence (downgraded for very serious risk of bias and serious indirectness) of little between‐group difference reported by Pountos 2010 in the number of children with no problems or 'limited disability' (applied to one of five areas: interference with play; help needed with feeding; help needed with washing and dressing; sleep disturbance; missed days of school) at 4 to 6 weeks follow‐up (17/26 versus 12/24; risk ratio (RR) 1.31, 95% confidence interval (CI) 0.80 to 2.13; Analysis 1.2). Pountos 2010 did not report on the findings of an appointment at 12 weeks to check return to full function. Other data from Plint 2006 on the numbers of children with moderate or severe difficulty in five activities confirmed a lack of differences at four weeks follow‐up (Analysis 1.3). As expected, the activities during splint or cast use favoured the splint group in this trial, particularly with bathing and showering; the results for 14 days are shown in Analysis 1.3 (8/32 versus 26/40; RR 0.38, 95% CI 0.20 to 0.73; 72 children).
1.1. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 1 Modified Activities Scale for Kids ‐ performance version (ASK‐P) (0 to 100: best function)(median, IQR (interquartile range)); higher scores = worse pain.
| Modified Activities Scale for Kids ‐ performance version (ASK‐P) (0 to 100: best function)(median, IQR (interquartile range)); higher scores = worse pain | |||||||
|---|---|---|---|---|---|---|---|
| Study | Follow‐up | Splint: median (IQR) | Splint: N | Cast: median (IQR) | Cast: N | Difference median (IQR) | Reported P |
| Plint 2006 | Day 7 | 83.48 (75.67 to 93.37) | 38 | 88.67 (78.02 to 92.98) | 44 | −2.70 (−8.44 to 2.41) | P = 0.331 |
| Plint 2006 | Day 14 | 93.77 (87.26 to 99.15) | 38 | 89.29 (82.33 to 95.69) | 45 | 2.97 (0.00 to 6.90) | P = 0.041 |
| Plint 2006 | Day 20 | 96.55 (92.45 to 100) | 34 | 92.97 (85.66 to 98.06) | 40 | −1.72 (−0.31 to 5.31) | P = 0.091 |
| Plint 2006 | Day 28 | 99.04 (95.29 to 100) | 28 | 99.11 (96.42 to 100) | 37 | 0.00 (−2.13 to 0.86) | P = 0.934 |
1.2. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 2 Functional disabilities at 4 to 6 weeks.
1.3. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 3 Moderate or severe difficulties in performing activities.
Complete data for treatment failure were not available, partly because it was not clear whether those children who had an early second procedure (e.g. splint to cast or cast change) had also required further immobilisation (extended treatment). There was very low‐quality evidence of little or no difference between groups in treatment failure, defined by change or replacement of the splint or cast for problems such as pain, rash, increased deformity (missed greenstick fractures) and cast replacement for broken or wet casts, and lodged pencil in the cast (5/225 versus 8/219; RR 0.71, 95% CI 0.26 to 1.89; 444 participants, 4 studies; Analysis 1.4). Oakley 2008 (84 children) also provided data for extended immobilisation (also defined as 'treatment failure') for pain and discomfort (6/42 versus 3/42; RR 2.00, 95% CI 0.54 to 7.47; very low‐quality evidence of little or no difference). Also shown in Analysis 1.4 are the results for any change in treatment (i.e. splint to cast) or reapplication of a cast; these show no evidence of a difference between the two groups (7/225 versus 8/219; RR 0.94, 95% CI 0.38 to 2.32; very low‐quality evidence). When we added data for treatment change for children excluded after randomisation from Oakley 2008 and Plint 2006, primarily because they were not buckle fractures, the results begin to favour the cast group, as cast is the default treatment for these less stable fractures. In total, there were reports of 22 incorrectly‐diagnosed fractures, distributed among the trials as follows: Davidson 2001 had one greenstick fracture (0.5%) found at three weeks follow‐up; Oakley 2008, which had four fractures (5%), excluded two complete radius fractures allocated splint but treated with an above‐elbow cast, and reported on two greenstick fractures allocated a splint but then changed to a cast; Plint 2006 excluded 16 non‐buckle fractures (14%) of which 15 were greenstick fractures, all diagnosed within 24 hours of randomisation, with the seven allocated splint being revised to a cast; and finally one child (1%) allocated splint in Williams 2013 was given a cast when diagnosed with a transverse radius fracture.
1.4. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 4 Complications and treatment failure.
Explicit and reliable mention of serious adverse events was only made in Plint 2006 (87 children), which reported no child had a refracture by six months follow‐up. Although Karimi 2013 stated "there were no adverse events or skin problems", they contradicted this statement with reports of oedema and skin rash.
Secondary outcomes
Time to return to normal activities (or interim stages of recovery) was not reported but data on the return to normal activities by set times were available from two studies (Oakley 2008; Plint 2006), as shown in Analysis 1.5. Return to sporting or normal physical activities by four weeks in Plint 2006 (60 children) was greater in the splint group (25/26 versus 23/34; RR 1.42, 95% CI 1.11 to 1.82; very low‐quality evidence downgraded for very serious risk of bias, serious indirectness in relation to time to return to normal activities and serious imprecision, given the data were from one trial only). There were contradictory and considerably heterogeneous findings (I2 = 92%; results not pooled) in the return to normal activities between Plint 2006 (at 20 days), which favoured the splint group, and Oakley 2008 (at 14 days), which favoured the cast group.
1.5. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 5 Return to normal activities.
Wrist pain was measured and reported in various ways in five studies, but was not reported in Davidson 2001. We selected the pain score during device use (visual analogue scale (VAS) 0 to 10; worst pain) as reported in Pountos 2010 for presentation in Table 1: MD 0.20, 95% CI −1.10 to 1.50; 50 children; very low‐quality evidence; Analysis 1.6). The two other trials (Plint 2006; Williams 2013) reporting pain at one week found higher median pain scores in the splint group but neither of the differences between the two groups reached statistical significance and, notably, that in Plint 2006 was also unlikely to be clinically important (161 children, very low‐quality evidence; Analysis 1.7). Other pain data shown in Analysis 1.7 show little difference in pain at three weeks (Plint 2006; Williams 2013; 159 children) or pain intensity when in pain (Oakley 2008; 84 participants). However, Williams 2013 noted that the initial application of the cast reduced pain to zero but pain was still high in the splint group (94 children). Other summaries of pain during device use shown in Analysis 1.8 are numbers reporting mild to moderate pain during activity (Karimi 2013); pain lasting more than six days (Oakley 2008) and numbers requiring regular analgesics in Pountos 2010; all are very low‐quality evidence, downgraded by two levels for very serious risk of bias and by one level for serious imprecision.
1.6. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 6 Pain VAS (0 to 10; higher = worse pain) during device use.
1.7. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 7 Non‐parametric pain scores (median, IQR (interquartile range)); higher scores = worse pain.
| Non‐parametric pain scores (median, IQR (interquartile range)); higher scores = worse pain | ||||||
|---|---|---|---|---|---|---|
| Study | Measure | Splint: median (IQR) | Splint: N | Cast: median (IQR) | Cast: N | Reported P |
| Just after application | ||||||
| Williams 2013 | 0 to 9 point scale | 3.0 | 43 | 0 | 51 | P < 0.005 |
| At 1 week | ||||||
| Plint 2006 | VAS: 0 to 100 | 14.5 (2.75 to 35.00) | 30 | 7.00 (0.00 to 23.00) | 37 | P = 0.92 |
| Williams 2013 | 0 to 9 point scale | 2.5 | 43 | 1.0 | 51 | NS (not significant) |
| At 3 weeks | ||||||
| Plint 2006 | VAS: 0 to 100 | 0.00 (0.00 to 1.00) | 27 | 0.00 (0.00 to 1.00) | 38 | P = 0.926 |
| Williams 2013 | 0 to 9 point scale | 1.0 | 43 | 0 | 51 | NS |
| Pain intensity when in pain | ||||||
| Oakley 2008 | VAS: 0 to 100 | 39 (30.0 to 59.0) | 42 | 35 (25.0 to 51.0) | 42 | P = 0.48 |
1.8. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 8 Pain during use of splint or cast.
Although five trials reported on the numbers of children with individual complications, other than those described under treatment failure, none reported the numbers of participants with any minor complication. There were a variety of individual complications or problems reported (see Analysis 1.4). These were one case in each group of slightly increased deformity reported in Pountos 2010; 11 cases of rash in the splint group and five cases of oedema in the cast group of Karimi 2013; and three cases of stiffness and nine cases of subnormal grip strength, all in the cast group of Pountos 2010. Medical attention was sought more often in the cast group in Oakley 2008, but it was unclear whether any of the 10 cases resulted in anything more substantive than advice: 2/42 versus 8/42; RR 0.25, 95% CI 0.06 to 1.11; very low‐quality evidence. There were 33 reports in Oakley 2008 of minor device problems (bandage holding volar slab requiring replacement and cast softening or breaking round the rim); see Analysis 1.4.
Four trials reported satisfaction data assessed by the child, the parent, or both of them (Analysis 1.9); two trials asked whether participants would choose the same treatment in future (Oakley 2008; Williams 2013). We did not pool the results from these two trials because of the substantial statistical heterogeneity (I2 = 83%), plausibly reflecting clinical heterogeneity such as the different splintage used. There was very low‐quality evidence of no difference in child and carer satisfaction between a splint comprising a fibreglass volar slab secured with an elasticised bandage versus a cast in Oakley 2008. In contrast, there was very low‐quality evidence in favour of the prefabricated splint used in Williams 2013. Karimi 2013 (142 children) found little difference between groups in the child's assessment of treatment convenience, but Williams 2013 found much lower scores for convenience (0 to 9; extremely convenient) at three weeks in the cast group (median score 9.0 in the splint group versus 3.2 in the cast group; reported P < 0.001). Plint 2006 separately asked if children or parents would prefer a splint in future; this question is potentially biased and the findings in favour of the splint may reflect this (Analysis 1.9).
1.9. Analysis.
Comparison 1 Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures, Outcome 9 Patient satisfaction, preference and convenience.
Four trials provided some data relating to the use of the splint. Karimi 2013 found the timing of splint removal was similar to that of the cast (3.15 weeks versus 3.14 weeks). In Oakley 2008, the duration of splint use was one day more in the splint group (17.0 days versus 15.8 days). Oakley 2008 reported that 37 (88.1%) had removed their splint during the immobilisation period; on average, participants had removed their splint 7.24 times, mainly to shower. Plint 2006 reported that splints were used for at least some part of the day or night for an average of 13.7 days and that continuous use rapidly declined from 28% at seven days to 10% at 20 days. Finally, Williams 2013 reported that two children (5%) did not remove their splint at three weeks and 25 (58%) removed their splint one of more times, leaving, by deduction, 16 children (37%) who were no longer using the splint at this time. Also of note is that seven very young children attempted to remove their splint prematurely; two children (5%) in Davidson 2001 and five (8%) in Karimi 2013. Both trials observed that both parent and child liked that the splint could be removed for bathing and several parents had said that their child had removed their splint before the end of three weeks after the pain had settled. Lastly, the number of participants who failed to attend the three‐week follow‐up in Davidson 2001 was over three times greater in the splint group (18/116 (15.5%) versus 4/85 (4.7%)), perhaps reflecting that a clinic visit is unnecessary for splint removal.
Economic data
Davidson 2001 (based in the UK) and Karimi 2013 (based in Iran) reported cost‐benefit analyses based on costs from their respective hospital contracts departments. Both found lower healthcare costs for removable splints; the estimated cost saving per patient was GBP 51 (prices probably applying to year 2000) in Davidson 2001, and USD 6 in Iran (probably 2010 prices) in Karimi 2013 (Appendix 3). Neither healthcare nor societal costs were quantified in Oakley 2008, based in Australia. However, although nine parents in each group of Oakley 2008 took time off work, the total number of days off work were nearly twice as high in the splint (volar slab) group as in the cast group (21 days versus 11 days). Similarly, almost equal numbers of children had time off school in the two groups (15 in splint group versus 14 in the cast group), but the number of days off school were almost three times more in the splint group (45 days versus 16 days).
2. Bandage versus below‐elbow cast
Four trials compared a soft or elasticated bandage with a below‐elbow cast in 237 children with distal radius fractures. Children had buckle fractures in two studies (Jones 2001; West 2005), impacted greenstick fractures in Kropman 2010, and either buckle or an undisplaced greenstick fracture in Pountos 2010 (Table 7). Follow‐up was at the end of treatment at three or four weeks in Jones 2001 and West 2005, at six weeks in Kropman 2010, and at 12 weeks in Pountos 2010. We present the evidence for this comparison in Table 2. Appendix 4 shows a separate 'Summary of findings' table for this comparison that draws on the outcomes considered in the NICE 2016 guidelines.
Primary outcomes
Only Pountos 2010 reported on physical function. Based on a poorly‐defined composite measure, there was very low‐quality evidence (downgraded for very serious risk of bias and serious indirectness) that more children in the bandage group had no problems or only 'limited disability' at follow‐up (applied to one of five areas: interference with play; help needed with feeding; help needed with washing and dressing; sleep disturbance; missed days of school) (26/29 versus 12/24; RR 1.79, 95% CI 1.18 to 2.73; Analysis 2.1). Despite the reporting of an appointment at 12 weeks to check return to full function, Pountos 2010 did not report on the outcome.
2.1. Analysis.
Comparison 2 Bandage versus below‐elbow cast for buckle or minimally‐displaced fractures, Outcome 1 Functional disabilities at 4 to 6 weeks.
There was very low‐quality evidence of little between‐group differences in treatment failure, comprising four cases of change from bandage to cast at the request of the parent, and four cases of treatment extended by one week for delayed union (5/90 versus 3/91; RR 1.53, 95% CI 0.44 to 5.32; 3 studies; 181 children; I2 = 33%; Analysis 2.2). Two studies (139 children) reported no serious adverse event in either group.
2.2. Analysis.
Comparison 2 Bandage versus below‐elbow cast for buckle or minimally‐displaced fractures, Outcome 2 Complications and treatment failure.
Secondary outcomes
None of the trials reported on time to return to normal activities (or interim stages of recovery).
Wrist pain was measured and reported in various ways in three studies (Kropman 2010; Pountos 2010; West 2005); see Analysis 2.3 and Analysis 2.4. Kropman 2010 (89 children) found no clinically important difference between the two groups in wrist pain at one week, measured using VAS (0 to 100; worst pain): MD 6.00, 95% CI −1.31 to 13.31, very low‐quality evidence; Analysis 2.3). There was also very low‐quality evidence of less pain and discomfort in the bandage group in West 2005 (39 children), and of little difference in pain during device use or requirement for analgesic in Pountos 2010 (53 participants). Kropman 2010 assessed discomfort on a weekly basis by a participant questionnaire in which the child recorded how often they had itching, neck pain or had found the bandage or cast too heavy, too loose or too tight. Although the data were unavailable for use in this review, being presented separately in a graph for each aspect and for each of the three weeks of usage, it was clear that itching was the prime source of discomfort for all three weeks, being reported a total of 140 times in the bandage group versus 219 times in the cast group (reported P < 0.001).
2.3. Analysis.
Comparison 2 Bandage versus below‐elbow cast for buckle or minimally‐displaced fractures, Outcome 3 Pain VAS 0 to 100 (higher = worse pain).
2.4. Analysis.
Comparison 2 Bandage versus below‐elbow cast for buckle or minimally‐displaced fractures, Outcome 4 Pain or discomfort during use of bandage or cast.
Two studies reported on minor complications (delayed union requiring a treatment extension is covered under treatment failure). West 2005 reported there were no skin problems in either group. Pountos 2010 (53 participants) reported three children in the cast group had stiffness at four to six weeks (RR 0.12, 95% CI 0.01 to 2.20; very low‐quality evidence) and one child in each group had a slight increase in deformity (Analysis 2.2).
Jones 2001 reported that all parents were happy with either treatment. Although none of the trials reported on child or parent satisfaction with outcome, West 2005 found that more children In the bandage group found their treatment convenient (17/18 versus 3/21; RR 6.61, 95% CI 2.31 to 18.96; very low‐quality evidence; Analysis 2.5); all 18 children followed up in the bandage group were no longer using a bandage after two weeks.
2.5. Analysis.
Comparison 2 Bandage versus below‐elbow cast for buckle or minimally‐displaced fractures, Outcome 5 Patient satisfaction: treatment was convenient.
Range of wrist motion was assessed in two trials (Kropman 2010; West 2005); unsurprisingly, both confirmed that the range of movement at four weeks, at the end of treatment, was significantly greater in the bandage group (median range of flexion‐extension movement in Kropman 2010: 154 º versus 121 º; in West 2005: 162 º versus 126 º). Kropman 2010 reported there was no difference between the groups at six weeks (median flexion‐extension: 165 º versus 163 º).
3. Below‐elbow versus above‐elbow casts
Table 8 presents brief details of the four trials comparing below‐elbow versus above‐elbow casts (Bohm 2006; Colaris 2012; Paneru 2010; Webb 2006). Notably, complete casts were applied after closed reduction of the displaced fracture or fractures in three trials (333 participants), whereas non‐circular casts were applied to minimally displaced metaphyseal fracture of the radius and ulna in Colaris 2012 (66 participants). Follow‐up data were available at six weeks in Bohm 2006, at six months in Colaris 2012 and Paneru 2010, and at an average of 7.7 months (for refracture) in Webb 2006. We present the main results for this comparison in Table 3 .
Primary outcomes
There were no data available for functional outcome based on validated measures in the short term (up to three months) or long term (12 months or longer). However, as would be expected, children in the below‐elbow group reported less need for help with various activities including overall activities of daily living during four weeks of cast immobilisation (3/49 versus 35/58; RR 0.10, 95% CI 0.03 to 0.31; 107 participants, 1 study; low‐quality evidence downgraded by two levels for serious risk of bias; Analysis 3.1). Colaris 2012 found little difference between the two groups in the ABILHAND‐Kids score (0 to 42: no problems) at six months (MD −1.10, 95% CI −3.47 to 1.27; 66 participants; very low‐quality evidence downgraded by one level for serious risk of bias, by one level for serious indirectness and by one level for imprecision; Analysis 3.2).
3.1. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 1 Limitations in activities of daily living during cast use.
3.2. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 2 ABILHAND‐Kids score (0 to 42: no problems) at 6 months.
Overall treatment failure data were not available. Remanipulation or secondary reduction, as in Colaris 2012, was less frequent in the below‐elbow group (2/177 versus 9/189; RR 0.27, 95% CI 0.07 to 1.06; 366 participants; 4 studies; very low‐quality evidence downgraded for serious risk of bias, serious indirectness and serious imprecision; Analysis 3.3). All four children given remanipulation in Bohm 2006 were then treated with an above‐elbow cast. Bohm 2006 also reported change of cast type for six participants: one below‐elbow cast had fallen off and five above‐elbow casts were changed to below‐elbow cast for comfort at three weeks; Analysis 3.4. Where reported, there was an absence of serious adverse events: no refracture at six months (113 participants; Webb 2006); and no compartment syndrome (102 participants; Bohm 2006).
3.3. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 3 Subsequent (secondary) fracture displacement or reduction.
3.4. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 4 Complications.
Secondary outcomes
Time to return to former activities was not reported. As noted above, more children were recorded as requiring help with activities during cast use in the above‐elbow group in Webb 2006 (Analysis 3.1). Children allocated below‐elbow casts in two trials had on average fewer days off school compared with those in above‐elbow casts (Paneru 2010: 4.19 days versus 10.43 days; Webb 2006: 0.56 days versus 1.6 days; Analysis 3.5). Only Paneru 2010 reported on pain, measured on a VAS (0 to 10; higher score = worse pain) after one week of cast immobilisation. They found participants in the below‐elbow group had lower pain scores at this time (MD −1.91, 95% CI −2.55 to −1.27; 85 participants; low‐quality evidence downgraded by one level for risk of bias and by one level for indirectness, given these data were from one small trial only; Analysis 3.6).
3.5. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 5 Days off school.
3.6. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 6 Pain after 1 week in cast: VAS 0 to 10 (higher = worse pain).
Overall numbers of participants in the two groups with less serious complications such as non‐routine cast adjustments were not provided by any of the trials. Pooled data for secondary displacement favoured the below‐elbow group (21/133 versus 42/146; RR 0.56, 95% CI 0.36 to 0.87; 279 participants, 3 studies; I2 = 18%; low‐quality evidence downgraded by two levels for serious risk of bias; Analysis 3.3). However, we focused only on displacements that had resulted in a secondary procedure, reported above as treatment failure. We selected physiotherapy for restricted range of motion post‐immobilisation as a representative complication; there was very low‐quality evidence of little or no difference between the two groups (3/131 versus 6/133; RR 0.54, 95% CI 0.16 to 1.80; 264 participants, 3 studies; very low‐quality evidence downgraded for very serious risk of bias and imprecision; Analysis 3.4). Other complications reported included cast splitting for swelling (3/89 versus 6/98; RR 0.61, 95% CI 0.18 to 2.10; 187 participants, 2 studies), cast reinforcement for 'breakdown' (4/89 versus 20/98; RR 0.25, 95% CI 0.10 to 0.65; 187 participants, 2 studies; I2 = 56%); cast changed for loosening or breakdown (10/89 versus 7/98; RR 1.61, 95% CI 0.67 to 3.84; 187 participants, 2 studies; I2 = 75%), skin abrasion (2 cases in the above‐elbow group) and transient neuropraxia at the elbow (2 cases in the above‐elbow group); see Analysis 3.4. The evidence for all individual complications was very low quality, downgraded for serious risk of bias and, variously, for serious or very serious imprecision (few events, wide confidence intervals) and for serious inconsistency, reflecting heterogeneous results.
There was no report of child (or parent) satisfaction with outcome or treatment. Using a VAS scale to assess cosmetic appearance (0 to 10; same as fractured arm), Colaris 2012 found no evidence of a difference at six months between the two groups when rated by the parents (MD 0.00, 95% CI −0.47 to 0.47; 63 participants) nor when rated by an orthopaedic surgeon (MD 0.10, 95% CI −0.22 to 0.42; 63 participants); Analysis 3.7. This very low‐quality evidence was downgraded by one level for serious risk of bias, by one level for serious indirectness, and by one level for serious imprecision.
3.7. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 7 Cosmetic appearance at 6 months (VAS 0 to 10: best cosmetics).
Included for completeness are the range of motion data at cast removal and final follow‐up (this was between 8 to 10 weeks in Webb 2006, and six months in Colaris 2012); see Analysis 3.8. Results at cast removal favoured the below‐elbow group, with confirmation of the expected restrictions in elbow motion (MD −32.54 º, 95% CI −36.26 º to −28.82 º; 108 participants, 2 studies; I2 = 94%; low‐quality evidence, downgraded by one level for serious risk of bias and by one level for serious inconsistency). At final follow‐up, there was little or no clinically important difference found for wrist motion, elbow motion or forearm pronation and supination; low‐quality evidence, downgraded by one level for serious risk of bias and by one level for serious imprecision). Based on a participant questionnaire, restoration of range of motion took on average 10 fewer days in the below‐elbow group in Webb 2006 (−10.00 days, 95% CI −12.53 to −7.47 days; 113 participants; very low‐quality evidence, downgraded by two levels for very serious risk of bias and by one level for serious indirectness, as the question addressed in the questionnaire is not known; Analysis 3.9).
3.8. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 8 Ranges of wrist and elbow movement (degrees).
3.9. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 9 Time to regain range of motion (days).
Economic data
Paneru 2010 reported that the mean cost of treatment (2007 to 2008 data in Nepal) in the below‐elbow group was approximately a third of that in the above‐elbow group (NPR 358 versus NPR 1144; MD NPR −785.86, 95% CI NPR −881.89 to NPR −689.83; 85 participants; Analysis 3.10). A breakdown of the components was not given. As also pointed out in Paneru 2010, the number of school days lost was also significantly higher in the above‐elbow group (Analysis 3.5).
3.10. Analysis.
Comparison 3 Below elbow versus above elbow cast, Outcome 10 Overall treatment cost (rupees, Nepal).
4. Percutaneous wire fixation and cast immobilisation versus cast alone after closed reduction of displaced fractures
Table 9 presents brief details of the five trials (323 participants) comparing percutaneous wire fixation and, where detailed, above‐elbow cast immobilisation versus above‐elbow cast alone after closed reduction of displaced fractures. Participants of two trials had exclusively or mainly both‐bone fractures (Colaris 2013a; McLauchlan 2002), whereas all participants had isolated distal radius fractures in Gibbons 1994. Neither Ghoneem 2003 nor Miller 2005 provided details of ulna involvement. Follow‐up was six months in Colaris 2013a, Gibbons 1994 and Miller 2005; four months or union in Ghoneem 2003; and three months in McLauchlan 2002. Miller 2005 also reported on outcome at 2.8 years. We present the main results for this comparison in Table 5.
Primary outcomes
There were no data available for functional outcome in the short term (up to three months) or long term (12 months or longer). Colaris 2013a found little difference between the two groups in the ABILHAND‐Kids score (0 to 42: no problems) at six months (MD 0.40, 95% CI −0.01 to 0.81; 123 participants; low‐quality evidence downgraded by one by level for serious risk of bias and by one level for serious indirectness; Analysis 8.1). It is unlikely that this difference is clinically important and there is a strong possibility of a ceiling effect. There was very low‐quality evidence in support of this finding from three other trials that reported no functional deficit at three months (McLauchlan 2002; 56 participants), four months (Ghoneem 2003; 70 participants) and 2.8 years (Miller 2005; 25 participants).
8.1. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 1 ABILHAND‐Kids score (0 to 42: no problems) at 6 months.
Several contrasting outcomes that occur at different times could contribute to the composite outcomes of treatment failure and serious adverse events for this comparison. Some of these would be more expected in the cast‐only group, such as fracture redisplacement and remanipulation for fracture redisplacement, and others are typically exclusive to surgery, such as pin‐site infection and wire migration. Secondary procedures depicting treatment failure and adverse events are reported in four trials that are shown in Analysis 8.2. There is very low‐quality evidence (downgraded by two levels for very serious risk of bias and by one level for serious inconsistency) that surgery reduces the risk of treatment failure (20/124 versus 41/129; RR 0.52, 95% CI 0.33 to 0.83; 253 participants; 4 studies; I2 = 58%; Analysis 8.3). We note that routine wire removal is not treatment failure. However, although it is typically carried out in the clinic nowadays, it can sometimes involve another operation under general anaesthesia, as took place for all wire removal in McLauchlan 2002. There is very low‐quality evidence (downgraded by two levels for very serious risk of bias and by one level for serious indirectness) that surgery reduces the overall risk of the more serious adverse events (28/124 versus 43/129; RR 0.68, 95% CI 0.45 to 1.02; 253 participants; 4 studies; I2 = 48%; Analysis 8.3). These outcomes were mainly wire‐related (e.g. migration, infection, scar at K‐wire insertion point) in the surgery group and treatment for loss in position (redisplacement or malunion) in the cast‐only group. There was a small but unavoidable risk of a unit‐of‐analysis error for the data from Colaris 2013a in which some children may have incurred more than one complication.
8.2. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 2 Complications and secondary treatment.
| Complications and secondary treatment | |||||
|---|---|---|---|---|---|
| Study | Surgery Secondary treatment (failure) | Surgery no. | Not‐surgery Secondary treatment (failure) | Not‐surgery no. | Comments |
| Complications | |||||
| Colaris 2013a |
|
61 |
|
67 | Unit of analysis problems ‐ thus one or more children having more than one complication ‐ cannot be ruled out |
| Gibbons 1994 |
|
12 |
|
11 | ‐ |
| McLauchlan 2002 |
|
35 |
|
33 | ‐ |
| Miller 2005 |
|
16 |
|
18 | ‐ |
| Secondary treatment | |||||
| Colaris 2013a |
|
61 |
|
67 | Potential unit of analysis issue ‐ thus one or more children having more than one complication requiring treatment ‐ cannot be ruled out. * Actual treatment not specified for these but can be assumed. |
| Gibbons 1994 |
|
12 |
|
11 | ‐ |
| McLauchlan 2002 |
|
35 |
|
33 | Corrective osteotomy at 6 months was not counted as follow‐up was 3 months; it restored function. All participants in the surgery group had a general anaesthetic (another operation) for routine wire removal. This was the standard hospital procedure (Edinburgh, 1997) |
| Miller 2005 |
|
16 |
|
18 | ‐ |
8.3. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 3 Overall treatment failure and adverse events.
As shown in Analysis 8.4, surgery reduces the risk of fracture redisplacement (6/159 versus 69/164; RR 0.11, 95% CI 0.05 to 0.23; 323 participants, 5 studies; I2 = 0%) and treatment (typically remanipulation) for loss of fracture position (1/124 versus 40/129; RR 0.06, 95% CI 0.02 to 0.22; 253 participants; 4 studies; I2 = 0%). For both outcomes the evidence is of low quality, downgraded by two levels for very serious risk of bias. Analysis 8.5 presents the sparse data, often reported just by one or two trials for individual outcomes. It is notable that, where reported, there were no incidences of early physeal closure (2 studies, 57 children) or compartment syndrome (1 study; 34 children). Miller 2005 found no deformity in 25 children followed up for an average of 2.8 years.
8.4. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 4 Fracture redisplacement and rereduction.
8.5. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 5 Complications (not redisplacement or re‐manipulation).
Secondary outcomes
Neither time to return to former activities nor pain were reported. McLauchlan 2002 noted that five children (9% of 56 followed up at clinical review; groups not identified) complained of minor pain upon strenuous activity at three months.
More minor complications such as short‐term wrist or elbow stiffness, skin breakage, and non‐routine treatment adjustments were under‐reported. The only report was of range of motion restrictions that prompted physiotherapy in Colaris 2013a, which found fewer children in the surgery group were referred for physiotherapy at clinical review (22/62 versus 36/66; RR 0.65, 95% CI 0.44 to 0.97; 128 participants; very low‐quality evidence downgraded by two levels for serious risk of bias reflecting lack of blinding, and by one level for serious indirectness).
There was no report of child (or parent) satisfaction with outcome or treatment.
Using a VAS scale to assess cosmetic appearance (0 to 10; same as fractured arm), Colaris 2013a found little evidence of a difference at six months between the two groups when rated by the parents (MD −0.50, 95% CI −1.21 to 0.21; 123 participants), although the result slightly favoured the cast group when rated by an orthopaedic surgeon (MD −0.50, 95% CI −0.94 to −0.06; 123 participants); Analysis 8.6. The low‐quality evidence was downgraded by one level for serious risk of bias and by one level for serious indirectness.
8.6. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 6 Cosmetic appearance at 6 months: VAS (0 to 10: same as non‐fractured arm).
Included for completeness are the low‐quality range of motion data at six months from Colaris 2013a (123 participants); see Analysis 8.7 and Analysis 8.8. These illustrate that there were differences, favouring surgery, between the two groups in the limitations in pronation and supination. It is notable that this is a proxy measure for function, and the limitations noted at this time were not reflected in the overall ABILHAND‐Kids scores. Colaris 2013a noted that six of the 14 children with 30 º or more restriction in pronation and supination had malunion, but did not identify the group.
8.7. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 7 Range of motion limitations at 6 months (degrees).
8.8. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 8 Restricted pronation and supination at 6 months.
Economic data
Miller 2005, recruiting between June 1995 and July 1997 in the USA, performed a retrospective cost analysis based on operating room, anaesthesia, surgery, radiology and ambulatory visits charges, including materials and cast technician services. They found "no significant difference" between the two groups in average treatment costs (USD 3347.2 versus USD 3831.0; cost period not stated). Miller 2005 noted that the costs in the cast group were lower for the initial procedure but became higher subsequently in this group because of further intervention resulting from loss of reduction. This is very low‐quality evidence, reflecting the very serious risk of bias and serious imprecision. Colaris 2013a (128 children) found low‐quality evidence of little between‐group differences in duration of cast use (MD −1.20 days, 95% CI −3.80 to 1.40) or visits to physiotherapy (MD −1.30 visits, 95% CI −3.62 to 1.02), although both favoured the surgery group; Analysis 8.9.
8.9. Analysis.
Comparison 8 Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures, Outcome 9 Days in cast and physiotherapy visits.
5. Different types of non‐rigid splintage, including 'no splintage', for buckle and other stable fractures: bandage versus removable splint
The sole trial in this category compared an elasticated bandage versus removable splint (Pountos 2010); see entries for the two interventions in Table 6. Results were reported for 55 children with either a buckle or an undisplaced greenstick fracture who were available at four to six weeks follow‐up. Pountos 2010 reported having a final appointment at 12 weeks to check return to full function but did not report this outcome, treatment failure, serious adverse events, complications with the interventions, time to return to former activities, or satisfaction. The evidence for all reported outcomes from this trial was of very low quality, downgraded by one or two levels for serious or very serious risk of bias, primarily reflecting a high risk of performance bias, by one or two levels for serious or very serious imprecision reflecting few events and wide confidence intervals, and for all outcomes by one level for serious indirectness relating to the suboptimal outcome measures.
Based on a poorly‐defined composite measure, there was very low‐quality evidence that fewer children in the bandage group had no problems or 'limited disability' at follow‐up (applied to one of five areas: interference with play; help needed with feeding; help needed with washing and dressing; sleep disturbance; missed days of school) (26/29 versus 17/26; RR 1.37, 95% CI 1.01 to 1.86; Analysis 5.1). There was very low‐quality evidence of little or no difference between groups in pain (VAS 0 to 100; higher score = worst pain; data derived from a histogram) during device use (MD −7.80, 95% CI −19.17 to 3.57; Analysis 5.2) nor in the regular use of analgesics (2/29 versus 3/26; Analysis 5.3). Three children had a marginal increase in deformity (1/29 versus 2/26); and there were three cases of slightly abnormal grip strength and three cases of stiffness (> 15 º) in the splint group at follow‐up (very low‐quality evidence, Analysis 5.4).
5.1. Analysis.
Comparison 5 Bandage versus removable splint for buckle or minimally‐displaced fractures, Outcome 1 Functional disabilities at 4 to 6 weeks.
5.2. Analysis.
Comparison 5 Bandage versus removable splint for buckle or minimally‐displaced fractures, Outcome 2 Pain VAS 0 to 100 (higher = worse pain) during device use.
5.3. Analysis.
Comparison 5 Bandage versus removable splint for buckle or minimally‐displaced fractures, Outcome 3 Pain: regular analgesic required.
5.4. Analysis.
Comparison 5 Bandage versus removable splint for buckle or minimally‐displaced fractures, Outcome 4 Complications.
6. Fibreglass versus plaster casts
One trial (Inglis 2013) compared fibreglass cast versus plaster cast immobilisation after closed reduction in 201 children with displaced forearm fractures (Table 10). Mainly above‐elbow casts were applied but to different extents in the two groups (80% in the fibreglass group versus 90% in the plaster‐cast group). Follow‐up was for six weeks, at cast removal.
Inglis 2013 did not report on function, recovery or pain and only the results for repeat reduction were available for 130 of the 143 children with distal radius fracture (2/71 versus 3/59; RR 0.55, 95% CI 0.10 to 3.21; very low‐quality evidence, downgraded by two levels for very serious risk of bias and by one level for serious imprecision; Analysis 6.1). Results for the whole trial population (199 children) for remanipulation were similar (Analysis 6.1). Very low‐quality evidence, downgraded by two levels for very serious risk of bias and by one level for indirectness, reflecting the mixed population and inadequate description of outcome measurement, was available for several other outcomes for all fractures. This indicated the need for a new cast without remanipulation (1/110 versus 8/89; RR 0.10, 95% CI 0.01 to 0.79) or cast reinforcement (4/110 versus 20/89; RR 0.16, 95% CI 0.06 to 0.46) because softening or breakage was less in the fibreglass group for the whole population (Analysis 6.1). Extra but non‐quantified costs were reported for the plaster‐cast group in relation to the further care and clinic attendances involved. The two minor skin complaints in the fibreglass group did not require further treatment. A graph showing the means and standard errors for seven five‐point ordinal scales (1 to 5; best outcome = highest score) used to measure participant and parent satisfaction overall and with various aspects (such as comfort, first application, weight, itchiness, heat and smell) consistently showed greater satisfaction with fibreglass casts. However, while the differences in the means for the seven types of satisfaction could be estimated, the interpretation is hampered by the ordinal and uneven nature of the scale. The difference in the means for overall satisfaction (4.4 versus 3.2) indicates a distinction between "very comfortable" in the fibreglass group and "good overall comfort" in the plaster‐cast group.
6.1. Analysis.
Comparison 6 Fibreglass versus plaster cast, Outcome 1 Complications.
7. Position of arm in above‐elbow cast (forearm supinated versus pronated versus neutral)
Two quasi‐randomised three‐group trials, reporting results for 159 children, assessed the effect of the forearm position (supinated versus pronated versus neutral) held by an above‐elbow cast (Boyer 2002; Gupta 1990); Table 11. Casts were applied after reduction under general anaesthesia in all participants in Boyer 2002, whereas only 42% (25/60 children) had reduction before cast application in Gupta 1990. The outcome‐reporting in both trials was restricted to subsequent reduction and final angulation post‐immobilisation at six weeks (Boyer 2002) or change in angulation between two and six weeks (Gupta 1990). Neither trial found a difference between any two positions in subsequent reduction (very low‐quality evidence, downgraded by two levels for very serious risk of bias and by one level for serious imprecision; Analysis 7.1). Similarly, there was very low‐quality evidence of minimal between‐group differences in final angulation: Boyer 2002 reported there was no significant effect (P > 0.05) on angular deformity at final follow‐up (overall mean = 7 º), and Gupta 1990 found minimal change (all less than 0.6 º) in dorsal angulation in any of the three groups.
7.1. Analysis.
Comparison 7 Above‐elbow cast (forearm pronated versus neutral versus supinated) for displaced fractures, Outcome 1 Second or subsequent reduction for unacceptable loss of alignment.
8. Home versus hospital‐clinic removal of casts
Table 12 presents brief details of the two trials, involving 404 children with stable, predominantly buckle fractures, that compared home removal of casts versus removal at the hospital fracture clinic at three weeks (Hamilton 2013; Symons 2001). Hamilton 2013 used two different casts for their comparison, whereas Symons 2001 used the backslab in both groups with a rewrapping of the backslab done in front of the parents of the home‐removal group while explaining the method of removal. Although Hamilton 2013 had a six‐month follow‐up, they only reported the quantitative results at four‐week follow‐up. Symons 2001 reported results at six‐week follow‐up. We present the main results for this comparison in Table 5. Appendix 5 shows a separate 'Summary of findings' table for this comparison that draws on the outcomes considered in the NICE 2016 guidelines.
Hamilton 2013 found no "significant differences" in the Childhood Health Assessment Questionnaire (CHAQ) index change scores ('Health status VAS': 0 to 100; worst outcome) at one week post‐cast removal (MD 0.96 favours hospital, 95% CI −0.21 to 2.13; 233 participants; low‐quality evidence downgraded by two levels for very serious risk of bias; Analysis 4.1) or at six‐month follow‐up (no data provided). A similar finding applied for EuroQol 5‐Dimensions data (not shown for four weeks; not reported for six months). Change scores at four weeks for eight domains of the CHAQ shown in Analysis 4.1 also support this finding. None of the 80 children followed up in Symons 2001 had difficulties with writing, where appropriate, or activities of daily living (Analysis 4.2). Fewer children in the home group avoided some hobbies (3/38 versus 7/42; RR 0.47, 95% CI 0.13 to 1.70; very low‐quality evidence downgraded by two levels for very serious risk of bias, by one level for serious imprecision and by one level for indirectness, given the vague definition of this outcome; Analysis 4.2). Five children had a change in treatment (4/197 versus 1/200; RR 3.16, 95% CI 0.50 to 19.93; 397 participants, 2 studies; very low‐quality evidence downgraded by two levels for very serious risk of bias and by one level for serious imprecision; Analysis 4.3). There were two flexible‐cast changes at one week because of pain in Hamilton 2013. Of the three cases of non‐adherence to treatment in Symons 2001, one child removed their backslab prematurely and one parent delayed removal of the backslab until six weeks in the home‐removal group; one parent in the hospital group successfully removed their child's backslab at home to avoid loss of earnings. No serious adverse effects, including refractures, were reported at six‐month follow‐up in Hamilton 2013 (288 children).
4.1. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 1 Childhood Health Assessment Questionnaire change scores from pre‐injury at 4 weeks.
4.2. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 2 Functional activity at 6 weeks.
4.3. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 3 Change to allocated treatment.
Neither trial reported on time to return to former activities, but the CHAQ findings for Hamilton 2013 at four weeks and the lack of children with activities of daily living difficulties at six weeks in Symons 2001 indicate ready restoration of pre‐injury activities. Hamilton 2013 found no difference in pain at four weeks (CHAQ pain VAS (0 to 100; higher means worse pain): MD −0.43, 95% CI −3.88 to 3.02; 233 participants; low‐quality evidence downgraded by two levels for very serious risk of bias; Analysis 4.1). All complications reported at six weeks in Symons 2001 were minor, with one case of 'mild' swelling, six cases of 'mild' tenderness and six cases where the backslab had become soft; none had necessitated a return to hospital (Analysis 4.4; Analysis 4.5). More children in the home‐removal group of Hamilton 2013 reported that their casts had become loose than in the hospital‐removal group (27/123 versus 10/91 (denominators calculated from percentages); RR 2.00, 95% CI 1.02 to 3.92; low‐quality evidence downgraded by two levels for very serious risk of bias; Analysis 4.5). However, Hamilton 2013 (233 participants) reported without providing data that "there was no difference" between the two groups "in the number of casts that needed replacing or number of additional plaster room visits". Fewer participants in the home‐removal group in Symons 2001 reported problems with fracture care (5/38 versus 14/42; RR 0.39, 95% CI 0.16 to 0.99; low‐quality evidence downgraded by two levels for very serious risk of bias). The complaints in the hospital‐group parents related to hospital waiting times (10 complaints), having to take time off work (5), transport difficulties (3) and hospital parking (2). In contrast, more parents in the home group would have liked a spare bandage (7/38 versus 2/42; RR 7.74, 95% CI 1.00 to 60.03; very low‐quality evidence downgraded by two levels for very serious risk of bias and by one level for serious imprecision; Analysis 4.5). No deformity was reported at six weeks in either group of Symons 2001, although this was confirmed radiologically in only 33 children. Hamilton 2013 reported there was no secondary displacement at six months. Significantly more parents in the hospital group of Symons 2001 indicated that they would not opt for the same treatment again (4/38 versus 27/42; RR 0.16, 95% CI 0.06 to 0.43; Analysis 4.6). In contrast, in addressing a different question relating to the care received, most of the parents of Hamilton 2013 indicated they were always or almost always happy with the treatment received (120/126 versus 103/106; RR 0.98, 95% CI 0.93 to 1.03; low‐quality evidence downgraded two levels for very serious risk of bias; Analysis 4.7).
4.4. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 4 Complications.
4.5. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 5 Parents or children reporting problems with cast or care of fracture.
4.6. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 6 Parents would not choose the same treatment again.
4.7. Analysis.
Comparison 4 Home versus hospital clinic removal of casts for stable, mainly buckle, fractures, Outcome 7 Parent satisfaction with treatment (always or almost always happy).
Healthcare cost analysis (UK NHS unit costs 2010 and 2011) conducted by Hamilton 2013 showed that, while the flexible casts for home removal were more expensive compared with the standard casts (GBP 8.13 versus GBP 2.87), the overall cost of treating a stable paediatric forearm fracture with a cast that was removed at home was significantly less (reported P < 0.001) compared with one that was removed in a hospital clinic (GBP 150.88 versus GBP 251.62); the follow‐up appointment took up most of the cost. Hamilton 2013 found that the mean distance of travel to attend cast removal in the hospital group was 11.7 miles; 70 children (67% of 104) missed school for this and 52 carers (50%) had to take time off work, with nine losing pay as a result.
9. Removable splintage versus rigid complete casts for minimally displaced but potentially unstable fractures
Boutis 2010 compared a commercially available removable splint with a below‐elbow cast in 100 children with minimally angulated or a minimally displaced acute greenstick or transverse fractures (Table 13). Final follow‐up was at three months.
Primary outcomes
Boutis 2010 found no clinically important between‐group difference in the modified ASK‐P scores (0 to 100: best function; no disability) results at six weeks (MD 1.40, 95% CI −1.79 to 4.59; 92 participants; low‐quality evidence downgraded by one level for serious risk of bias, mainly reflecting lack of blinding and by one level for imprecision reflecting data availability from one small trial; Analysis 9.1). Boutis 2010 based their sample calculation on a difference of at least seven points on the ASK‐P at six weeks; the seven‐point difference was chosen as it was half the difference between average scores of children with normal ability and those considered to be mildly disabled. Boutis 2010 noted a full resumption of activities at three‐month follow‐up.
9.1. Analysis.
Comparison 9 Removable splintage versus below‐elbow cast for minimally‐displaced but potentially unstable fractures, Outcome 1 Modified Activities Scale for Kids ‐ performance version (ASK‐P) (0 to 100: best function) at 6 weeks.
Complete data for treatment failure were not available, partly because it was not clear whether those children who had an early second procedure (e.g. splint to cast or cast change) had also required further immobilisation (extended treatment). There was very low‐quality evidence of little or no difference between groups in treatment failure, defined by change or replacement of the splint or cast for the reported problems of rash and cast breakage (1/46 versus 3/50; RR 0.36, 95% CI 0.04 to 3.36; evidence downgraded by one level for serious risk of bias, by one level for serious indirectness for an incompletely reported outcome measure, and by one level for serious imprecision (wide confidence interval and few events); Analysis 9.2). Also shown in Analysis 9.2, are the results for any change in treatment (i.e. splint to cast) or reapplication of a cast that also include four children from the splint group who received a cast because they had either a displaced transverse fracture (angulation > 25 º) or a Salter‐Harris II fracture (5/50 versus 3/50; RR 1.67, 95% CI 0.42 to 6.60). Three children in each group had extended immobilisation for re‐angulation (RR 1.09, 95% CI 0.23 to 5.12; very low‐quality evidence).
9.2. Analysis.
Comparison 9 Removable splintage versus below‐elbow cast for minimally‐displaced but potentially unstable fractures, Outcome 2 Complications and treatment failure.
Boutis 2010 (96 children) explicitly reported that no child had incurred a serious adverse event by three‐month follow‐up.
Secondary outcomes
Time to return to normal activities (or interim stages of recovery) was not reported.
There was very low‐quality evidence from Boutis 2010 of little or no between‐group differences in pain at one, four or six weeks follow‐up measured with the revised Faces pain scale (0 to 5; worst pain); Analysis 9.3. There was very low‐quality evidence of little or no difference between the groups in the numbers reporting pain (6/42 versus 7/47; RR 0.96, 95% CI 0.35 to 2.63) or discomfort (8/42 versus 12/47; RR 0.75, 95% CI 0.34 to 1.65) during device use; Analysis 9.4.
9.3. Analysis.
Comparison 9 Removable splintage versus below‐elbow cast for minimally‐displaced but potentially unstable fractures, Outcome 3 Faces Pain Scale (0 to 5; higher = worse pain).
9.4. Analysis.
Comparison 9 Removable splintage versus below‐elbow cast for minimally‐displaced but potentially unstable fractures, Outcome 4 Pain or discomfort during use of splint or cast.
Boutis 2010 reported on a variety of individual complications or problems but did not report the numbers of participants with one or more minor complications. Boutis 2010 reported three cases of increased deformity in each group, all treated with increased device use; but no cases of clinically‐observed deformity at four‐week follow‐up. Based on parent reports, there were more cases of skin sores, irritation and itching during device use in the splint group, but the 95% CI intervals overlapped the line of no effect for all three outcomes and we rated the evidence as very low‐quality, downgraded by one level each for risk of bias, indirectness and imprecision (Analysis 9.2).
Child and parental satisfaction, based on those who would choose the same treatment in future, favoured the splint group, with over twice as many preferring the splint to the cast (child preference: 27/42 versus 15/47; RR 2.76, 95% CI 1.79 to 4.25; very low‐quality evidence, downgraded by one level for serious risk of bias relating to lack of blinding, by one level for indirectness, given that the results were deduced from data indicating preference for the other device, and by one level for imprecision, given the data are from one small trial; Analysis 9.5). Boutis 2010 (89 children) also reported that all parents were satisfied with wrist appearance at six weeks.
9.5. Analysis.
Comparison 9 Removable splintage versus below‐elbow cast for minimally‐displaced but potentially unstable fractures, Outcome 5 Patient and parent satisfaction: preference for the same device.
Boutis 2010 found all children were still using their splint at four weeks, but the percentage always wearing their splint had dropped from 94% at one week to 57% at four weeks.
Boutis 2010 also reported no difference between the two groups in angulation at one and four weeks (mean 9.85 º versus 8.20 º; MD 1.65, 95% CI −1.82 to 5.11); in six range‐of‐motion measures or in grip strength at six weeks (mean 26.6 lb versus 28.8 lb; MD −2.16 lb, 95% CI −7.34 to 3.02).
Economic data
Boutis 2010, based in Canada, performed a cost‐effectiveness analysis, which is reported in Von Keyserlingk 2011. It estimated the average healthcare costs were CAD 97.56 lower in the splint group (2009 prices); Appendix 3. Although Boutis 2010 found higher societal costs reflecting higher productivity cost (loss in work hours) in the splint group, the total costs still favoured the splint group. Boutis 2010 identified the main differences between the splint and the cast groups related to the number of unscheduled outpatient visits to see an orthopaedic surgeon and to have an X‐ray (five visits in the splint group versus none in the cast group), the number of additional wrist support devices used (three casts and five splints in the cast group versus one cast in the splint group), and an additional cast removal and replacement for assessment purposes at week 1.
10. Waterproof versus 'traditional' non‐waterproof casts
Table 14 presents brief details of the two trials comparing waterproof versus more traditional non‐waterproof casts in 95 children, most of whom had buckle fractures (Derksen 2011; Silva 2016). We used data only at the first cast removal after two weeks in Silva 2016; at this time, participants were crossed over to the alternative cast. In Derksen 2011, children and parents were advised they could shower and swim in the traditional‐cast group provided they covered the cast with a protective plastic bag, whereas participants were advised to keep the non‐waterproof cast dry in Silva 2016. These and other differences meant that we decided against pooling data for the relatively few outcomes the two trials had in common. Silva 2016 found better ASK‐P scores (Activities Scale for Kids ‐ Performance scores: 0 to 100; higher scores = better performance) in the waterproof‐cast group at the two week cross‐over time (MD 16.90, 95% CI 6.87 to 26.93; 26 participants; very low‐quality evidence downgraded by two levels for very serious risk of bias and by one level for serious indirectness; Analysis 10.1). Neither trial reported any redisplacement, but one child with a greenstick fracture in Derksen 2011 required an extra two weeks cast immobilisation for delayed fracture healing (Analysis 10.2; very low‐quality evidence downgraded for serious risk of bias and very serious imprecision). Derksen 2011 found, as would be expected, that all children issued waterproof plasters had showers and more went for a swim compared with those in the other cast group that needed to use a protective covering (Analysis 10.3). Silva 2016 found no clinically important difference in pain scores at two weeks (Analysis 10.4; very low‐quality evidence downgraded for very serious risk of bias and imprecision). Silva 2016 reported there were no complications (need for non‐routine cast change; skin changes) at two weeks; both groups reported similarly very low levels of itching (very low‐quality evidence downgraded for very serious risk of bias and imprecision). Derksen 2011 reported the incidences of several types of skin conditions but did not report on their severity or the number of participants with any skin condition; there was very low‐quality evidence of little difference between the two groups in the individual skin conditions, and notably there were no macerations relating to soaking of the skin (Analysis 10.5; downgraded by one level for serious risk of bias and by two levels for very serious imprecision). Silva 2016 found no clinically important difference in satisfaction with treatment scores at two weeks (Analysis 10.6; very low‐quality evidence downgraded for very serious risk of bias and imprecision). Both children and parents of the waterproof‐cast group recorded greater satisfaction at cast removal; however, the clinical important of the mean differences is not established (Analysis 10.6; low‐quality evidence downgraded for serious risk of bias and indirectness). Derksen 2011, based in the Netherlands, reported the "swim cast was around 50% cheaper"; whereas Silva 2016, based in the USA, noted that waterproof casting materials "are usually more expensive".
10.1. Analysis.
Comparison 10 Waterproof versus non‐waterproof cast, Outcome 1 Activities Scale for Kids ‐ Performance (0 to 100: best function).
10.2. Analysis.
Comparison 10 Waterproof versus non‐waterproof cast, Outcome 2 Fracture redisplacement, reduction or delayed healing.
10.3. Analysis.
Comparison 10 Waterproof versus non‐waterproof cast, Outcome 3 Water activities during cast use.
10.4. Analysis.
Comparison 10 Waterproof versus non‐waterproof cast, Outcome 4 Faces Pain Scale (0 to 10: worst pain).
10.5. Analysis.
Comparison 10 Waterproof versus non‐waterproof cast, Outcome 5 Complications.
10.6. Analysis.
Comparison 10 Waterproof versus non‐waterproof cast, Outcome 6 Satisfaction at cast removal (child or parent).
11. Split versus closed circumferential synthetic semi‐rigid above‐elbow cast
Schulte 2014 compared a split versus a closed circumferential synthetic semi‐rigid above‐elbow cast in 40 children with displaced distal radius fractures (Table 15). The main follow‐up was between four and six weeks. Schulte 2014 did not report on function, time to return to former activities, pain or satisfaction. We judged the quality of the reported evidence to be very low, with serious risk of bias (downgraded by one level) and very serious imprecision (downgraded by two levels).
There was very low‐quality evidence of little or no between‐group differences in treatment failure (2/17 versus 4/23; RR 0.68, 95% CI 0.14 to 3.28), comprising redisplacement needing surgery (2/17 versus 3/23) and secondary splitting of cast due to reversible lymphoedema (0/17 versus 1/23); Analysis 11.1. There was one report of skin breakdown in each group (1/17 versus 1/23) and no reports of compartment syndrome, neurovascular syndrome or cast saw burns (deduced).
11.1. Analysis.
Comparison 11 Split versus closed circumferential synthetic semi‐rigid above‐elbow cast, Outcome 1 Treatment failure and complications.
12. Double‐sugar‐tong splint versus above‐elbow bivalved cast
Levy 2015 compared a double‐sugar‐tong splint extended at one week with an above‐elbow cast versus an above‐elbow bivalved cast in 71 children with displaced fractures (Table 16). Follow‐up was eight to 12 weeks, which was two to four weeks after cast removal at six to eight weeks. Levy 2015 did not report on function, time to return to former activities, pain or satisfaction. The evidence for all reported outcomes was very low quality, downgraded by two levels for very serious risk of bias, and by two levels for very serious imprecision, reflecting few events and wide confidence intervals.
There was very low‐quality evidence of little or no difference between treatment failure, either remanipulation or cast conversion due to loosening or damage at one week (3/34 versus 4/37; RR 0.82, 95% CI 0.20 to 3.39); fracture redisplacement (5/34 versus 10/37; RR 0.54, 95% CI 0.21 to 1.43); or remanipulation (1/34 versus 3/37; RR 0.36, 95% CI 0.04 to 3.32); Analysis 12.1. There was no non‐union or subsequent surgery.
12.1. Analysis.
Comparison 12 Double‐sugar‐tong splint versus above‐elbow bivalved cast, Outcome 1 Treatment failure and complications.
13. Comparison of two different water‐resistant cast liners
Stevenson 2013 compared two different types of water‐resistant below‐elbow cast liner, Wet or Dry® versus Delta Dry®, in 105 children with minimally displaced distal radius fracture in Australia (Table 17). Follow‐up was at the time of cast removal at around five weeks. Stevenson 2013 did not report on function, treatment failure, time to return to former activities or pain. The evidence for all reported outcomes was very low quality, downgraded by one level for serious risk of bias, by one or two levels for serious or very serious indirectness, reflecting the inadequate description of outcomes, and by one or two levels for serious or very serious imprecision, reflecting few events and wide confidence intervals.
There was no skin ulceration or obvious dermatitis; one case of 'skin damp or maceration' occurred in each group (RR 1.06, 95% CI 0.07 to 16.48; 105 participants; Analysis 13.1). There was very low‐quality evidence of more skin complaints (mainly skin irritation or reddening in the Wet or Dry group, 44/51 versus 26/54; RR 1.79, 95% CI 1.33 to 2.41; Analysis 13.1). There was very low‐quality evidence of little difference between groups in child satisfaction (excellent or very comfortable cast: 43/51 versus 39/54; RR 1.17, 95% CI 0.95 to 1.43; Analysis 13.2). All participants except one in the Wet or Dry group found the liner was excellent, comfortable or good overall. The technicians' impression of overall cast padding quality favoured the Delta Dry liners, with more complaints in the Wet or Dry cast liner group (9/51 versus 4/53; RR 2.34, 95% CI 0.77 to 7.12; Analysis 13.3). All these results were derived from categorical data provided by the trialists (Appendix 6).
13.1. Analysis.
Comparison 13 'Wet or dry' versus 'Delta dry' water‐resistant cast liner (below‐elbow cast), Outcome 1 Complications.
13.2. Analysis.
Comparison 13 'Wet or dry' versus 'Delta dry' water‐resistant cast liner (below‐elbow cast), Outcome 2 Patient satisfaction with liner.
13.3. Analysis.
Comparison 13 'Wet or dry' versus 'Delta dry' water‐resistant cast liner (below‐elbow cast), Outcome 3 Cast technician's impression: below average or worse.
Stevenson 2013 reported several other outcomes relating to technician‐ or participant‐ or caregiver‐reported outcomes relating to the use and satisfaction withe the cast, such as itchiness, smell, water resistance and how long it took to dry. However, these were inadequately measured using self‐designed non‐validated scoring systems, which hinder interpretation.
14. Closed reduction by Paediatric Emergency Physician (EP) versus Orthopaedic Resident
Khan 2010 compared closed reduction of displaced or angulated distal forearm fractures by specifically trained paediatric emergency physicians versus closed reduction by orthopaedic residents in 104 children in the USA (Table 18). The type and duration of immobilisation was not reported, but a standard follow‐up at six to eight weeks was applied. Khan 2010 did not report on function or recovery but observed no significant pain or limitation in range of motion at final follow‐up in 96 participants. There is very low‐quality evidence of little difference between the two groups in the need for remanipulation (4/48 versus 6/48; RR 0.67, 95% CI 0.20 to 2.21; evidence downgraded by one level for serious risk of bias and by two levels for very serious imprecision; Analysis 14.1). None of the trial participants needed hospital admission or developed compartment syndrome. There is very low‐quality evidence of few between‐group differences in cast‐related complications (6/51 versus 4/52; RR 1.53, 95% CI 0.46 to 5.10) or inadequate fracture alignment at final follow‐up (3/48 versus 7/48; RR 0.43, 95% CI 0.12 to 1.56). The length of stay in the emergency department was on average half an hour less in the emergency physicians group (mean 4.5 versus 5.0 hours, MD −0.50 hours, 95% CI −1.33 to 0.33; very low‐quality evidence downgraded by one level for serious risk of bias and by two levels for serious imprecision; Analysis 14.2).
14.1. Analysis.
Comparison 14 Closed reduction by Paediatric Emergency Physician (EP) versus Orthopaedic Resident, Outcome 1 Complications.
14.2. Analysis.
Comparison 14 Closed reduction by Paediatric Emergency Physician (EP) versus Orthopaedic Resident, Outcome 2 Length of stay in Emergency Department (hours).
Discussion
Summary of main results
Of the 30 included trials, 21 were confirmed as RCTs, seven were quasi‐RCTs and two did not report on their method of randomisation. The 30 trials recruited a total of 2930 children. With one exception, trials included more male children and typically reported mean ages between eight and 10 years. Thirteen trials recruited predominantly stable fractures; buckle fractures were exclusively recruited in eight of these and formed the majority in four others. Fractures were minimally displaced in three trials, and displaced, typically requiring closed reduction, in the other 14 trials.
The trials made a total of 14 comparisons, seven of which were tested by one trial only. Of our prestated comparisons, none of the included trials tested: (a) 'no splintage', non‐rigid complete casts (soft casts) or traditional backslabs versus below‐elbow casts for buckle and minimally displaced (stable) fractures; (b) bandages and 'off the shelf' removable splints versus backslab and other custom‐made devices that require specialist application for treating buckle and stable fractures; (c) different durations of cast or splint immobilisation; and (d) different methods of percutaneous pinning (wire fixation). We summarise the evidence available to this review below. We rated all of the evidence as either low quality or low certainty about the results, or very low quality, which indicates that we are uncertain of the findings.
Removable splintage versus below‐elbow cast for buckle and minimally displaced (stable) fractures
Six trials compared a removable splint with a below‐elbow cast in 695 children with stable, predominantly buckle, distal radius fractures. We present the evidence for this comparison in Table 1. There is low‐quality evidence from one trial of no clinically important between‐group difference in physical function, assessed using the modified Activites Scale for Kids ‐ Performance score (ASK‐P) at 7, 14, 20 and 28 days follow‐up. There is very low‐quality evidence of little or no difference between groups in treatment failure, defined by change or replacement of the splint or cast for various problems such as pain and broken or wet casts. A similar finding applied to extended immobilisation (also defined as 'treatment failure') for pain and discomfort. The only trial reporting on serious adverse events explicitly reported no child had a refracture by six‐month follow‐up. This finding is consistent with other evidence, including from other included trials with buckle fractures, that explicitly reported the absence of serious adverse events: 139 children from two studies comparing bandage versus cast, and 288 children from one study comparing home versus hospital removal of casts. Time to return to former activities was not reported. There is very low‐quality evidence of no difference between groups in pain during device use; most children had no or very little pain after the first week. The number of children with minor complications was not reported, although five trials reported on a variety of minor complications or short‐term problems such as skin rash, oedema and stiffness. Of two trials reporting satisfaction data based on whether participants or their parents would choose the same treatment in future, one testing a splint comprising a fibreglass volar slab secured with an elasticised bandage provided very low‐quality evidence of no between‐group difference, whereas the other provided very low‐quality evidence in favour of a prefabricated splint. Two trials, based in the UK and Iran, reported lower healthcare costs for removable splints.
Bandage versus below‐elbow cast for buckle and minimally displaced (stable) fractures
Four trials compared a soft or elasticated bandage with a below‐elbow cast in 237 children with distal radius fractures, which were either buckle fractures or so‐called impacted or undisplaced greenstick fractures of probably comparable prognosis. We present the evidence for this comparison in Table 2. There is very low‐quality evidence from one trial of less disability (better function) in the bandage group at four weeks. There is very low‐quality evidence of little or no between‐group difference in treatment failure, which comprised four changes from bandage to cast upon parental request, mainly for pain, and four increased immobilisation for delayed union (three in the cast group). Two studies reported no serious adverse events in either group. This finding is consistent with other evidence, including from other included trials with buckle fractures, that explicitly reported the absence of serious adverse events: 87 children from one study comparing removable splint versus cast, and 288 children from one study comparing home versus hospital removal of casts. Time to return to normal activities was not reported. There is very low‐quality and contradictory evidence from three studies on wrist pain, two of which found no clinically important between‐group difference in wrist pain and one of which found less pain in the bandage group. The number of children with minor complications was not reported; there was very low‐quality evidence of little of no between‐group difference in the few reports of complications, such as stiffness after cast removal (three cases). Participant satisfaction was not reported, but one trial provided very low‐quality evidence that, unsurprisingly, more bandage‐group participants found their treatment was convenient.
Below‐elbow versus above‐elbow casts
Four trials compared below‐elbow versus above‐elbow casts; three of them (333 children) applied complete casts after closed reduction of the displaced fractures and one of them (66 children) applied non‐circular casts to minimally displaced metaphyseal fracture of the radius and ulna. We present the evidence for this comparison in Table 3. Short‐term functional outcome data based on validated measures in the short term (up to three months) were not available; there is very low‐quality evidence that nonetheless correlates with expectations of less dependency in the below‐elbow group during cast use. There is very low‐quality evidence from the trial that included children with minimally displaced forearm bone fractures of little difference in function between the two groups at six months. Overall treatment failure data are not available; however, nine of the 11 remanipulations or secondary reductions were in the above‐elbow group (very low‐quality evidence); while cast changes specifically for loosening or breakdown were similar in the two groups (very low‐quality evidence). There was an absence of the named serious adverse events of refracture and compartment syndrome at six months in two trials. Time to return to former activities was not reported. Low‐quality evidence from one trial indicated that pain at one week may be less for below‐elbow casts. Overall numbers of participants in the two groups with less serious (minor) complications such as non‐routine cast adjustments were not reported; there was very low‐quality evidence on individual complications with little or no between‐groups difference in physiotherapy for restricted range of motion post‐immobilisation, selected as a representative complication. There was no report of child or parent satisfaction with outcome or treatment. One trial, based in Nepal, found the mean cost of treatment in the below‐elbow group was approximately a third of that in the above‐elbow group; the below‐elbow group also had fewer days off school.
Percutaneous wire fixation and cast immobilisation versus cast alone after closed reduction of displaced fractures
Five trials (323 participants) compared surgical fixation with percutaneous wiring and cast immobilisation versus cast immobilisation alone after closed reduction of displaced fractures. Four trials confirmed the use of above‐elbow casts. We present the evidence for this comparison in Table 4. Short‐term functional outcome data based on validated measures in the short term (up to three months) were not available. There is low‐quality evidence from a trial that included children with displaced both‐bone fractures that there may be no difference in function between the two groups at six months. This finding is supported by reports in two trials of no children with functional deficit at three and four months. There is very low‐quality evidence that surgery halves the risk of treatment failure, where this is defined as early or more complex removal of wires and remanipulation for early loss in position. There is very low‐quality evidence that surgery reduces the overall risk of the more serious adverse events, such wire migration and infection in the surgery group and remanipulation for loss in position in the cast‐only group. In calculating the totals for the cast‐only group in each study, we included only redisplacement or malunion that had been treated. Notably, where reported, there were no incidences of early physeal closure (two studies) or compartment syndrome (one study). Neither time to return to former activities nor pain were reported. More minor complications such as short‐term wrist or elbow stiffness, skin breakage, and non‐routine treatment adjustments were under‐reported. There is very low‐quality evidence of lower referral for physiotherapy for restricted range of motion in the surgical group. There was no report of child or parent satisfaction with outcome or treatment. A retrospective cost analysis conducted by one trial based in the USA provided very low‐quality evidence of little or no difference in treatment costs between the two groups. We note that the additional cost for remanipulation in the cast‐alone group offset the lower initial treatment costs in this group.
Different types of non‐rigid splintage, including 'no splintage', for buckle and other stable fractures: bandage versus removable splint
The trial (55 children) comparing an elasticated bandage versus removable splint for either a buckle or an undisplaced greenstick fracture did not report on treatment failure, serious adverse events, complications with the interventions, time to return to former activities or satisfaction. It provided very low‐quality evidence of less disability (better function) in the bandage group at four weeks, of little or no difference between groups in pain during device use or in the low incidence of minor complications, most of which occurred in the splint group.
Rigid casts of materials other than plaster of Paris versus plaster of Paris casts: fibreglass versus plaster cast
One trial (143 of 201 participants had distal radius fracture) compared a fibreglass cast (80% were above‐elbow) versus plaster cast (90% were above‐elbow) for displaced fractures of the forearm (radius or ulna or both) requiring closed reduction and immobilisation. The trial did not report on function, recovery or pain. There is very low‐quality evidence of little or no difference in repeat reduction, which occurred in five cases with distal radius fracture. The other reported results were for the whole population. There is very low‐quality evidence of less need for a new cast or cast reinforcement in the fibreglass group because of fewer cast softenings and breakages. The two minor skin complaints in the fibreglass group did not require further treatment. Graphically‐shown data for a suboptimal measure of satisfaction consistently showed greater satisfaction with fibreglass casts. Extra but non‐quantified costs were reported for the plaster‐cast group in relation to the further care and clinic attendances involved.
Position of arm in above‐elbow cast (forearm supinated versus pronated versus neutral)
Two quasi‐randomised three‐group trials, reporting results for 159 children, assessed the effect of the forearm position (supinated versus pronated versus neutral) held by an above‐elbow cast. These provided very low‐quality evidence of little or no difference between any two positions in subsequent reduction or final angulation. Neither trial reported on function or the other outcomes sought in this review.
Home versus hospital‐clinic removal of casts for buckle and other stable fractures
Two trials, involving 404 children with stable, predominantly buckle fractures, compared removal at three weeks of casts at home by parents versus removal at the hospital fracture clinic by clinicians. We present the evidence for this comparison in Table 5. There was low‐quality evidence of no between‐group difference in function at four weeks, assessed using the Childhood Health Assessment Questionnaire. There were five changes in treatment, with very low‐quality evidence of little difference between the two groups. One study found no serious adverse effects, including deformity or refractures, at six months. This finding is consistent with evidence from other included trials with buckle fractures that explicitly reported the absence of serious adverse events: 87 children from one study comparing removable splint versus cast, and 139 children from two studies comparing bandage versus cast. Neither trial reported on time to return to former activities. There was low‐quality evidence of no difference in pain at four weeks. Overall data for children with minor complications were not available; none of the individual complications or problems reported had necessitated a return to hospital in one trial, whereas the other trial reported only that there was no difference in the numbers of casts needing to be replaced or additional plaster room visits. There was low‐quality evidence of greater parental satisfaction in the home removal of the cast group in one trial. Healthcare cost analysis (UK NHS unit costs 2010 and 2011) conducted by Hamilton 2013 showed that the overall cost of treating a stable paediatric forearm fracture with a cast that was removed at home was around GBP 100 less compared with one that was removed in a hospital clinic.
Removable splintage versus rigid complete casts for minimally displaced but potentially unstable fractures
One trial compared a commercially available removable splint with a below‐elbow cast in 100 children with minimally angulated or a minimally displaced acute greenstick or transverse fracture. It provided low‐quality evidence of no clinically important between‐group difference in function at six weeks, assessed by the modified ASK‐P scores; all children had fully resumed activities at three months. There is very low‐quality evidence of little or no difference between groups in treatment failure defined by change or replacement of the splint or cast for the reported problems of rash and cast breakage (four cases). Three children in each group had extended immobilisation for re‐angulation. No child had incurred a serious adverse event, including clinical deformity, by three‐month follow‐up. Time to return to normal activities (or interim stages of recovery) was not reported. There is very low‐quality evidence of little or no between‐group differences in pain. The numbers of children with minor complications were not reported. Based on parental reports, there were more cases of skin sores, irritation and itching during device use in the splint group, but the 95% confidence intervals overlapped the line of no effect for all three outcomes and we rated the evidence as very low quality. There was very low‐quality evidence of greater child and parental satisfaction in the splint group. The cost‐effectiveness analysis of this Canadian trial estimated the average healthcare costs were CAD 97.56 lower in the splint group (2009 prices); this difference mainly related to the extra unscheduled outpatient visits to see an orthopaedic surgeon and to have an X‐ray.
Waterproof versus 'traditional' non‐waterproof casts
Two trials compared waterproof versus more traditional non‐waterproof casts in 95 children, most of whom had buckle fractures. Diiferences in the interventions and study design meant that the trials were reported separately. There was very low‐quality evidence from Silva 2016 of better function (higher ASK‐P scores) in the waterproof‐cast group at two weeks. Neither trial reported any redisplacement; but one child with a greenstick fracture in Derksen 2011 had an extra two weeks of cast immobilisation for delayed fracture healing. There was very low‐quality evidence from Silva 2016 of no clinically important difference in pain scores at two weeks. Silva 2016 reported there were no complications at two weeks and only low levels of itching in both groups. There was very low‐quality evidence from Derksen 2011 of little or no difference between the two groups in the individual skin conditions and, notably, no macerations relating to soaking of the skin. There was very low‐quality evidence from Silva 2016 of no clinically important difference in satisfaction with treatment at two weeks. Derksen 2011 reported the "swim cast was around 50% cheaper", whereas, Silva 2016 noted that waterproof casting materials "are usually more expensive".
Split versus closed circumferential synthetic semi‐rigid above‐elbow cast
One trial, comparing a split versus closed circumferential synthetic semi‐rigid above‐elbow cast in 40 children with displaced distal radius fractures, did not report on function, time to return to former activities, pain or satisfaction. It provided very low‐quality evidence of little or no between‐group differences in treatment failure (five cases). There was one report of skin breakdown in each group and no reports of compartment syndrome, neurovascular syndrome or cast saw burns.
Double‐sugar‐tong splint versus above‐elbow bivalved cast
One trial compared a double‐sugar‐tong splint extended at one week with an above‐elbow cast versus an above‐elbow bivalved cast in 71 children with displaced fractures. The trial did not report on function, time to return to former activities, pain or satisfaction. There was very low‐quality evidence of little or no difference between treatment failure, either remanipulation (four cases) or cast conversion due to loosening or damage at one week (three cases), or fracture redisplacement (15 cases). There was no non‐union or subsequent surgery.
Comparison of two different water‐resistant cast liners
One trial (105 children) compared two different types of water‐resistant below‐elbow cast liner, Wet or Dry® versus Delta Dry®, for children with minimally displaced distal radius fracture. There was no report of function, treatment failure, time to return to former activities or pain. There is very low‐quality evidence for more skin complaints (such as skin irritation) in the Wet or Dry group. There is very low‐quality evidence of little difference between groups in child satisfaction, although there were more complaints by technicians with the Wet or Dry cast liner.
Closed reduction by paediatric emergency physician (EP) versus orthopaedic resident
One trial in the USA (104 children) compared closed reduction of displaced or angulated distal forearm fractures by specifically trained paediatric emergency physicians versus closed reduction by orthopaedic residents. The trial did not report on function or recovery but observed no significant pain or limitation in range of motion at six‐ to eight‐week follow‐up. There is very low‐quality evidence of little or no between‐group differences in the need for remanipulation, in cast‐related complications, in inadequate fracture alignment at follow‐up, or length of stay in the emergency department. None of the trial participants needed hospital admission or developed compartment syndrome.
Overall completeness and applicability of evidence
As indicated above, there was a lack of trials for several of our prestated comparisons, and evidence from small single trials only for several other comparisons. A maximum of six trials, including 695 children, made the same comparison, i.e. removable splint versus cast for buckle and other stable fractures. Furthermore, the evidence for all comparisons was incomplete; for example, only a few trials reported participant‐reported measures of function; and the variety of outcome measures used, such as for pain, plus inadequacies in reporting restricted our meta‐analyses. The largest meta‐analysis, which included four trials and 444 children, had only 13 events (treatment failure). Thus, the overall sparseness of the data is clear. For buckle fractures in particular the absence of serious adverse events, where reported, confirms expectations and a more general message is possible.
To inform consideration of the applicability of the evidence, we give quite comprehensive details of individual trials in the Characteristics of included studies. We supplement this by additional tables summarising the key characteristics of the study populations contributing evidence to the individual comparisons. Poor reporting of these characteristics clearly hampers our assessment of applicability, which particularly applies to both trials only reported as abstracts (Ghoneem 2003; Jones 2001).
Study populations
On the whole, the study populations appear representative in terms of mean age, the greater numbers of male children, and the fracture populations. The latter includes the overall correspondence of the fracture type with the comparisons under test, such as bandage versus cast for buckle fractures. However, a particular challenge of this review is that the terminology used by authors and clinicians varies. For example, descriptions of “impacted” or “undisplaced” greenstick as used in Kropman 2010 and Pountos 2010 do not correspond to the description of a true greenstick fracture, with the inherent instability of that fracture configuration. This distinction was recognised in Kropman 2010, which clarified that "An impacted greenstick fracture, torus fracture, or buckle fracture is defined as a specific type of greenstick fracture in which the cortex has become impacted". Conversely, misdiagnosis resulting in the incorrect inclusion of greenstick or, less commonly, transverse fractures in trials of buckle fractures was reported for a total of 22 fractures in four trials comparing removable splints versus casts. The largest number (14% of the study population) occurred in Plint 2006, which set out an a priori but incorrect intention to exclude such fractures from the analyses. Plint 2006 urged treating physicians "to be careful about the distinction between greenstick and buckle fractures and not to extrapolate to greenstick fractures the results of this trial without further study". To some extent, with the inclusion of minimally angulated or minimally displaced greenstick or transverse fracture, Boutis 2010 has moved this question along; however, the results from this trial serve mainly to strengthen the findings of trials focused on buckle fractures.
A commentary on Symons 2001 reinforces the dependency on proper classification of buckle fractures for trials on and treatment of buckle fractures. Mehlman 2002 refers to a US study, available as a conference abstract in 1997, that reported that as many as 16% of fractures labelled 'buckle fracture' by paediatric radiologists "involve complete cortical disruption". Mehlman 2002 estimated that around 2.5% of buckle fractures will displace with an average angulation of 11 º, but surmised that "it is likely that thousands of buckle fractures would need to be examined before one was found to be displaced enough to warrant reduction or other corrective procedures".
The second category of displaced fractures is more varied but although the criteria for closed reduction were often missing, pragmatically the use of closed reduction is indicative of the sort of fractures for which more extensive immobilisation using above‐elbow casts or indeed surgery are considered. The pooling of Colaris 2012, which included minimally displaced both‐bone fractures that did not warrant reduction, with other trials of displaced fractures for the above‐elbow versus below‐elbow comparison may initially seem incorrect. However, the high rate of secondary displacement in Colaris 2012 is comparable to those for redisplacement in the other three trials, and helps to support this decision. There is also variation in the inclusion criteria in trials in the surgery versus non‐surgery comparison (Table 9); nonetheless, we consider that these represent the fractures, such as completely displaced fractures in McLauchlan 2002, that many clinicians would consider may benefit from surgery.
Interventions/comparisons
As is evident from the Additional tables for the different comparisons, there is variation such as duration of treatment, type of splint or bandage, in the management of these injuries. The variation is accentuated for the timing and scope of treatment; thus for the removable splint versus cast comparison, the duration of cast use is around two weeks in Oakley 2008 but twice as long (four to six weeks) in Pountos 2010. Other variation in management, whether reported or not, is commonplace, as illustrated in Dua 2017. Treatment protocols or algorithms, particularly for buckle fractures, have been developed; for example, Biag 2017 and various patient information resources.
Inevitably, the cost, practicality and availability of treatment varies with location. A formerly published letter (Deshpande 2014), lost from the reconfigured Bone and Joint Journal website in early 2018, from Deshpande and Nadkarni in India, in response to Inglis 2013, pointed to the disadvantages of synthetic casting in terms of additional cost, practicality of application and the need for the use of an electric saw for removal (Appendix 7). Cost and availability of splints will also vary; Davidson 2001 reported that contact with the manufacturer resolved the problem of not having splints to fit very young children.
Outcomes
We have already noted above the dearth of data for several outcomes, and have listed them for the separate comparisons. In the frequent absence of specific data, determining the total numbers of children with treatment failure and with minor complications in each group was often difficult. As well as potential unit‐of‐analysis problems where the numbers with individual complications are summed, information on the severity or consequences of complications were often not provided. The treatment of secondary displacement and redisplacement is a particular issue for these fractures, where a clinician's judgement will vary in relation to the perceived capacity for bone remodelling, acceptability of displacement (angulation) and burden on the child and parents. We adopted a pragmatic approach in using data as reported, while noting that adherence to the trial's criteria for manipulation or remanipulation, where stipulated as in Colaris 2013a, may not have occurred. When assessing the directness (applicability) component of GRADE, we sometimes downgraded where the outcome presented was not a direct or sufficient substitute for the desired outcome measure (for example, convenience rather than satisfaction in Table 2), or was poorly defined (such as pain in Table 1). The use of study‐specific composite outcome measures of function, such as used and reported in Pountos 2010, or measures such as the ABILHAND‐Kids, validated for cerebral palsy, also give rise to concerns about applicability.
Quality of the evidence
Where data were available, the quality or certainty of the evidence for all outcomes in all comparisons was either low or more usually very low.
We downgraded all evidence for risks of bias, which we deemed either serious or very serious. In particular, this reflects the susceptibility to performance and detection biases for most outcomes where blinding to the allocated intervention is not possible. Other common sources of bias were selection bias (in nine trials, the allocation sequence was predictable or based on an open list) and selective reporting bias, typically because of incomplete reporting of outcome including at final follow‐up.
For some outcomes, we downgraded the quality for indirectness, usually relating to outcome assessment. Examples shown in Table 4 include the use of a scoring system that was validated for children with cerebral palsy, and poor descriptions of complications.
Downgrading for inconsistency, reflecting substantial heterogeneity, was rare. This is likely in part to reflect of the lack of data for pooling for most of the outcomes.
Conversely, downgrading for imprecision was common. This reflected wide confidence intervals but also the problems relating to small sample sizes and often small numbers of events. The need to be wary of the results from small single‐centre trials was often behind a decision to downgrade for imprecision. However, we did not apply this indiscriminately but considered the results of single trials in the context of properties of the measure used, including distinguishing between binary and continuous measures, and other evidence. Hence, for physical function in Table 1, we did not downgrade for imprecision based on the following reasons: the difference was minimal and the interquartile ranges (IQRs) were small, particularly in relation to the minimal clinically important difference (MCID); and the median scores in both groups indicated no disability, which was consistent with the findings of another trial for the same comparison, and the natural course of buckle fractures where children would be expected to have recovered their former physical function within four weeks.
We did not downgrade for publication bias; indeed, constructing funnel plots to explore the possibility of publication bias was not viable, given the few trials. This does not, however, mean that we can discount the possibility of publication bias.
Potential biases in the review process
Overall we adhered to our protocol and have noted the main changes in methods when undertaking our review under Differences between protocol and review. In this section, we discuss potential sources of bias in relation to trial searching and selection, outcome reporting and decisions for pooling.
Our search for trials was comprehensive, and screening and study selection were performed systematically and according to protocol. The possibility of unpublished trials, such as conference abstracts, remains, but as the potential contribution of these to the evidence is likely to be very limited we do not think this is an important source of bias.
The frequent mismatch between the outcomes sought in the review and the outcomes reported in the included trials can prove challenging in terms of judgements of whether a reported outcome is sufficiently representative of a sought outcome, or, for outcomes such as pain, which one(s) of a number of different outcomes can best represent the sought outcome. While we do not perceive our judgements as a serious source of bias, our review would have been stronger for setting out the possibility of some variation in the outcomes listed in Types of outcome measures that reflected the differences in the prognosis of different fracture types, especially buckle and displaced metaphyseal fractures, and variation in the different comparisons. We consider that we have avoided selective reporting bias, whilst still making use of some of the extra data available from the trials.
Decisions for placing trials under the same comparison and subsequently pooling data can be open to question. Clinical considerations were key to the first decision and where there was a clear indication of clinical heterogeneity, such as in the below‐elbow versus above‐elbow comparison, we have considered the implications of this in our interpretation of the results. While we took care to avoid pooling clearly statistically heterogeneous results, where we did so we downgraded for inconsistency in the GRADE assessment of the quality of the evidence.
Lastly, GRADE is a blunt instrument and downgrading by whole levels can rapidly result in a very low‐quality rating depicting "uncertainty about the estimate". This can indeed apply to the evidence available for the specific comparison but other evidence and insight can support a finding, for instance, whatever the quality rating of the evidence on elbow stiffness for trials comparing below‐elbow versus above‐elbow casts, some or greater elbow stiffness after wearing an above‐elbow cast is to be expected. Thus, we have sometimes moderated our interpretation of the results with this sort of consideration in mind.
Agreements and disagreements with other studies or reviews
We did not identify any systematic review with a similar scope to ours. Appendix 8 presents summaries and brief commentaries on seven systematic reviews, five of which focused on removable splints or bandages versus casts for buckle fractures (Hill 2016; Howes 2008; Jiang 2016; Kennedy 2010; Li 2014), one which compared below‐elbow versus above‐elbow casts for displaced fractures (Hendrickx 2011), and one which investigated K‐wiring for displaced fractures (Khandekar 2016). Although based on different mixtures of trials and studies, with only one presenting a meta‐analysis (incidence of complications in Jiang 2016), all five reviews on buckle fractures concluded in favour of non‐rigid or removable splintage. Hendrickx 2011 argued that, given the limited evidence did not show below‐elbow casts were inferior to above‐elbow casts, below‐elbow casts are a valid option for displaced fractures. They also pointed to the desirability of future high‐quality RCTs testing this comparison. Khandekar 2016, which summarised data from published studies on indications for wiring, K‐wiring technique, type of cast, incidence of redisplacement, relative incidences of complications, and timing of K‐wire removal, also suggested the need for a multicentre RCT for managing displaced distal radius fractures in children.
Systematic review of the evidence relating to bandaging, removable splints, soft casts, rigid non‐removable casts was also performed for the preparation of the NICE 2016 guidelines. The data available, outcomes with GRADE ratings and direction of effect, consideration of the 'trade‐off between clinical benefits and harms', comments in relation to our review and recommendations are summarised in Table 19. Subsequent to publication of our protocol, we established a set of alternative outcomes based on those listed in NICE 2016 (see Differences between protocol and review). We did this to explore the potential for different messages when different outcomes are used and also prepared a second 'Summary of findings' table for each comparison, focusing on interventions for treating buckle or other stable fractures (Appendix 2; Appendix 4; Appendix 5). As well as our review having more trials available for the removable splint versus cast and bandage versus cast comparisons, it is noteworthy that NICE 2016 strictly applied the exclusion of trials that included greenstick fractures (Davidson 2001; Pountos 2010; see Table 19). Our GRADE ratings for the evidence are generally lower, particularly with greater suspicion of the results from single‐centre trials at high risk of bias such as Karimi 2013 and West 2005, which in turn formed the evidence base for half or all of these two comparisons.
14. Summary of NICE 2016 guideline on torus fractures of the distal radius.
| Comparison | Rigid cast versus removable splint | Rigid cast versus soft cast | Rigid cast versus bandage |
| Included trials | Karimi 2013; Oakley 2008; Plint 2006; Williams 2013 | Used Khan 2007; labelled an RCT | West 2005 |
| Trials excluded but included in our review |
Davidson 2001: no relevant outcomes (used for cost analysis) Pountos 2010: included greenstick with no subgrouping by fracture type |
‐ | Pountos 2010: included greenstick with no subgrouping by fracture type |
| Outcomes with GRADE rating (L = low; VL = very low) | Pain on activity (VL; favoured cast) Found treatment convenient (L; no difference) Skin problems (L: favoured cast) Oedema (VL; favoured removable splint) Would use treatment again (VL; favoured removable splint)* Resumed normal activities at 2 weeks (L; favoured cast) Required re‐immobilisation at 2 weeks (VL; no difference) Adverse events: refractures (L; no difference (0 events)) * data from 3 trials | Parental problems with casts (VL; no difference) Would use treatment again (L; favoured soft cast) Cast complications at 3 weeks (VL; favoured soft‐cast) | Pain at 4 weeks (L; favoured bandage) Pain 2 or more days at 4 weeks (L; favoured bandage) Discomfort during treatment (L; favoured bandage) Found treatment convenient (L; favoured bandage) |
| Trade‐off between clinical benefits and harms | Rigid casts had a relative benefit in terms of pain, a return to normal activities, and the adverse events of skin problems. However, this was partially offset by a relative harm for rigid casts in terms of the proportion who would choose to continue the therapy in future, and the adverse event of oedema. Overall, however, the benefits of rigid casts over removable splints were deemed to outweigh the harms | There were no benefits of using rigid casts over soft casts, and thus the relative harms for rigid casts (parents not wishing to choose that treatment in future and cast complications) were unopposed. Overall, then, soft casts were deemed preferable to rigid casts | There were no benefits of using rigid casts over bandaging, and thus the relative harms for rigid casts (parents not wishing to choose that treatment in future, pain, and inconvenience) were unopposed. Overall, then, bandaging was deemed preferable to rigid casts |
| Comments | See: Appendix 2 Where data for the NICE outcomes were available, we gave very low GRADE ratings: Pain and Patient experience (would use same treatment in future) | Khan 2007 was referred to as an RCT in the guideline but excluded from our review as the 2 groups are not concurrent: essentially it is a before‐and‐after cohort comparison) | See: Appendix 4 Where data for the NICE outcomes were available, we gave very low GRADE ratings: Pain, Discomfort and Patient experience (treatment was convenient) |
| Recommentations for practice |
|
||
| Key research recommendation |
|
||
The selection of outcomes for a topic can involve considerable debate when formulating a protocol. Although the order differs, there is good correspondence between those selected by us and those by NICE 2016. Additionally, we have taken pragmatic decisions in the context of the actual outcomes reported by the trials in both cases. The final picture for each comparison, shown in the corresponding 'Summary of findings' tables (Table 1 versus Appendix 2; Table 2 versus Appendix 4; and Table 5 versus Appendix 5), is much the same. However, this might not remain the case when more data become available. The definition of outcomes in a future update of the NICE guideline might be refined by those in the recently funded FORCE 2018 trial, whose primary outcome is pain (Wong Baker FACES Pain Scale measured at three days). FORCE 2018 in its comparison of soft bandage and immediate discharge versus current treatment with rigid splint immobilisation for torus fractures of the distal radius in children was prompted by the recommendation for future research in NICE 2016 listed in Table 19.
Authors' conclusions
Implications for practice.
Overall there is insufficient evidence from RCTs to inform the best ways of treating different types of wrist fractures in children. There is a lack of evidence on several key comparisons, as detailed below. Where evidence is available for the main outcomes for the prespecified comparisons or the additional comparisons included in the review, the quality or 'certainty' assessed using GRADE is either low or very low. However, taking into account the evidence across comparisons, there is reassuring evidence of a full return to previous function with no serious adverse events, including refracture, for correctly‐diagnosed buckle fractures, whatever the treatment used. The absence of serious adverse events at three months in a trial including potentially unstable minimally displaced fractures that compared removable splintage versus cast also provides support (Boutis 2010). These review findings are consistent with the move away from cast immobilisation for buckle fractures.
In the following, we focus on our prespecified comparisons and primary outcomes.
For children with buckle fractures
Using a removable splint compared with a below‐elbow cast may result in no difference in physical function at four weeks. We are uncertain of the findings of little or no between‐group difference in treatment failure (few children needed a change or reapplication of device). There were no refractures reported at six months.
We are uncertain whether using a bandage compared with a below‐elbow cast results in less physical disability at four weeks. We are uncertain of the findings of little or no difference in treatment failure (few children needed a change of device or extension of immobilisation). There were no serious adverse events reported at one month.
There is no RCT evidence available on no‐splintage, soft casts or traditional backslabs versus rigid casts.
We are uncertain of the relative effects of using a bandage versus a splint on physical function at four weeks. There is no evidence available on treatment failure or adverse events for this comparison.
There is no RCT evidence available on the relative effects of off‐the‐shelf versus custom‐made devices.
There is no RCT evidence available on the relative effects of different durations of cast or splint immobilisation.
The removal at three weeks of casts at home by parents versus removal at the hospital fracture clinic by clinicians may result in no difference in physical function at four weeks. We are uncertain of the findings of little or no difference in treatment failure (few cases of change from protocol). There were no serious adverse events reported at six months.
For children with displaced distal radius fractures
There may be no difference in physical function at six months in children with minimally displaced both‐bone fractures treated with below‐elbow casts compared with those treated with above‐elbow casts. Functional outcome in the short term was not reported, but there was very low‐quality evidence, yet consistent with expectations, of fewer children needing help when wearing a below‐elbow cast. Overall treatment failure data were not available; we are uncertain of the findings of greater remanipulation in the above‐elbow group (few children involved). There were no reports of refracture or compartment syndrome at six months.
There is no RCT evidence available on the relative effects of different durations of cast (or splint) immobilisation.
We are uncertain of the relative effects of a fibreglass cast versus a plaster cast (most were above‐elbow casts) on the need for further reduction (few events). There is no evidence available on physical function or serious adverse events.
We are uncertain of the relative effects of different forearm positions (supinated versus pronated versus neutral) held by an above‐elbow cast on the need for reduction or further reduction (few events). There is no evidence available on physical function or serious adverse events.
There may be no difference between percutaneous wiring and cast immobilisation versus cast immobilisation alone (probably all were above‐elbow casts) in physical function at six months in children with reduced displaced fractures. There is no evidence available on short‐term physical function. There is very low‐quality evidence that surgery halves the risk of treatment failure (early or more complex removal of wires versus remanipulation for early loss in position) and reduces the overall risk of the more serious adverse events (there were no cases of early physeal closure or compartment syndrome).
There is no RCT evidence available on the relative effects of different methods of percutaneous pinning.
Implications for research.
There is a need for high‐quality evidence, primarily from sufficiently powered multicentre randomised trials, to help address key treatment uncertainties about these fractures. Prompted by a key research recommendation in the NICE 2016 guidelines, the FORCE 2018 trial has been established to evaluate the effectiveness and cost effectiveness of soft bandage and immediate discharge versus rigid splint immobilisation for buckle fractures of the distal radius in children. We consider this publicly‐funded multicentre trial covers the key question for these very common minor bone injuries and we also point to its emphasis on patient‐important outcomes and patient and parent involvement in study design and materials. This trial aims to provide definitive evidence on this question. Should other research groups consider testing the question in different settings, we encourage collaboration and adoption, where they see fit, of similar methods and outcomes to facilitate pooling of results.
Another key research question, already covered by an ongoing multicentre trial, is whether rough alignment with no formal reduction plus cast for distal displaced (angulated) forearm fractures gives as good a result as closed reduction and percutaneous K‐wire pinning then cast (Adrian 2015). This trial, conducted in Germany, Austria and Switzerland, has stopped recruiting but has a two‐year follow‐up. Another trial testing a similar comparison but with some scope and design differences is under consideration (Hunter 2018). This draws on a cohort study by Crawford 2012, which reported a good outcome for a rough correction of angulation, but not of shortening, of closed overriding metaphyseal fracture of the distal radius during application of a below‐elbow cast.
The identification of other priority topics warranting consideration for multicentre randomised trials that evaluate both effectiveness and cost effectiveness requires input from others, including patients and parents. Accurate fracture pattern identification and reporting is important for interpretation of trial results, especially of displaced fractures; the use of validated classification systems, such as the AO paediatric classification system, would be helpful in this regard (Randsborg 2012). The evidence would benefit also from agreement on a common set of outcome measures, including optimal reporting times, for these more serious fractures. Consideration should also be given to prospective collection of longer‐term data to establish the risk profile for serious sequelae of the more severe fracture types.
When designing trials that evaluate interventions for mixed fracture populations, such as all forearm fractures, we recommend stratification at randomisation and reporting by fracture location.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | Amended | Acknowledgements and External sources of support updated |
Notes
Editorial management and appraisal for this review were conducted by the Cochrane Fast‐Track Service (Managing Editor: Helen Wakeford; Associate Editor: Liz Bickerdike; Information Specialist Advisor: Ruth Foxlee), with approval for publication given by Michael Brown, the Senior Editor of the Cochrane Acute and Emergency Care Network. This review was copy‐edited by Kate Cahill.
Acknowledgements
We are very grateful for helpful feedback from Liz Bickerdike, Kathy Boutis, Christopher Hill, Liz O'Neill and Alysia Sengab on drafts of the review. We also thank Lindsey Elstub and Helen Wakeford for editorial support on the review and Kate Cahill for copy‐editing.
We thank Daniel Garcia for providing additional data on Schulte 2014 and Georgia Antonio for providing additional data on Stevenson 2013. We also thank the following people for providing information on the status and eligibility of their trials: Donald Bae (Bae 2015); Adriana Hernandez (NCT02670629); Anthony Hudson (ISRCTN25187648); Michelle Jacobs (ISRCTN34857372); and Eija Pirhonen (NCT01493167).
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. This review was also supported via the NIHR Cochrane Incentive Scheme 2017, project reference 17/62/04.
Appendices
Appendix 1. Search strategies
CENTRAL (Cochrane Register of Studies Online)
#1 MESH DESCRIPTOR Radius Fractures EXPLODE ALL TREES #2 MESH DESCRIPTOR Ulna Fractures EXPLODE ALL TREES #3 (distal or metaphys* or epiphys* or torus or wrist):TI,AB,KY #4 (#1 or #2) and #3 #5 MESH DESCRIPTOR Wrist Injuries EXPLODE ALL TREES #6 MESH DESCRIPTOR Forearm Injuries EXPLODE ALL TREES #7 #5 OR #6 #8 MESH DESCRIPTOR Fractures, Bone EXPLODE ALL TREES #9 fracture*:TI,AB,KY #10 #8 OR #9 #11 #7 AND #10 #12 (ulna* or radius or radial or forearm*):TI,AB,KY #13 #3 AND #10 AND #12 #14 (wrist* or buckle or torus):TI,AB,KY #15 #10 AND #14 #16 #4 OR #11 OR #13 OR #15 #17 infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or minors or minors* or boy or boys or boyfriend or boyhood or girl* or kid or kids or child or child* or children* or schoolchild* or schoolchild or school child* or adolescen* or juvenil* or youth* or teen* or pubescen* or pediatric* or paediatric* or peadiatric* or school* or prematur* or preterm* #18 #16 AND #17 #19 forearm*:TI #20 #10 AND #19 #21 #18 OR #20
Line 17: modified version of the paediatric search filter developed and validated by Leclercq 2013.
MEDLINE (Ovid Online)
1 exp Radius Fractures/ or exp Ulna Fractures/ 2 (distal or metaphys* or epiphys* or torus or wrist).tw. 3 1 and 2 4 Wrist Injuries/ or Forearm Injuries/ 5 exp Fractures, Bone/ 6 fracture*.tw. 7 5 or 6 8 4 and 7 9 (ulna* or radius or radial or forearm*).tw. 10 2 and 7 and 9 11 (wrist* or buckle or torus).tw. 12 7 and 11 13 3 or 8 or 10 or 12 14 (Infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or minors or minors* or boy or boys or boyfriend or boyhood or girl* or kid or kids or child or child* or children* or schoolchild* or schoolchild or school child* or adolescen* or juvenil* or youth* or teen* or pubescen* or pediatric* or paediatric* or peadiatric* or school* or prematur* or preterm*).mp,jn. 15 13 and 14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomized.ab. 19 placebo.ab. 20 drug therapy.fs. 21 randomly.ab. 22 trial.ab. 23 groups.ab. 24 or/16‐23 25 exp Animals/ not Humans.sh. 26 24 not 25 27 15 and 26 28 forearm*.ti. 29 7 and 26 and 28 30 27 or 29
Embase (Ovid Online)
1 Wrist Fracture/ 2 exp Radius Fracture/ or Ulna Fracture/ 3 (distal or metaphys* or epiphys* or torus or wrist).tw. 4 2 and 3 5 Wrist Injury/ or Arm Injury/ 6 exp Fracture/ 7 fracture*.tw. 8 6 or 7 9 5 and 8 10 (ulna* or radius or radial or forearm*).tw. 11 3 and 8 and 10 12 (wrist* or buckle or torus).tw. 13 8 and 12 14 1 or 4 or 9 or 11 or 13 15 (infan* or newborn* or new‐born* or perinat* or neonat* or baby or baby* or babies or toddler* or minors or minors* or boy or boys or boyfriend or boyhood or girl* or kid or kids or child or child* or children* or schoolchild* or schoolchild or school child* or adolescen* or juvenil* or youth* or teen* or pubescen* or pediatric* or paediatric* or peadiatric* or school* or prematur* or preterm*).mp,jn. 16 14 and 15 17 Randomized controlled trial/ 18 Clinical trial/ 19 Controlled clinical trial/ 20 Randomization/ 21 Single blind procedure/ 22 Double blind procedure/ 23 Crossover procedure/ 24 Placebo/ 25 Prospective study/ 26 randomi#ed.tw. 27 ((clinical or controlled or comparative or placebo or prospective*) adj3 (trial or study)).tw. 28 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. 29 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. 30 (cross?over* or (cross adj1 over*)).tw. 31 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. 32 RCT.tw. 33 or/17‐32 34 16 and 33 35 forearm*.ti. 36 8 and 33 and 35 37 34 or 36
WHO ICTRP
1. buckle AND fracture* 2. torus AND fracture* 3. wrist AND fracture* AND child* 4. wrist AND fracture* AND paediatric* 5. forearm AND fracture* AND child* 6. forearm AND fracture* AND paediatric* 7. wrist AND fracture* AND pediatric* 8. forearm AND fracture* AND pediatric* 9. distal* AND radi* AND fracture* AND child* 10. metaphys* AND radi* AND fracture* and child* 11. distal* AND radi* AND fracture* AND paediatric* 12. metaphys* AND radi* AND fracture* and paediatric* 13. distal* AND radi* AND fracture* AND pediatric* 14. metaphys* AND radi* AND fracture* AND pediatric*
ClinicalTrials.gov
1. (wrist OR forearm) AND fracture AND (child OR children OR paediatric OR pediatric) 2. (buckle OR torus) AND fracture 3. (distal OR metaphysis OR epiphysis) AND radius AND fracture AND (child OR children OR paediatric OR pediatric)
Bone & Joint Journal Orthopaedic Proceedings
Strategy 1 (search ran 15 December 2016)
1. abstract or title "distal radius fracture" (match all words) and full text or abstract or title "randomised randomized trial randomly" (match whole any) 2. abstract or title "distal radial fracture" (match all words) and full text or abstract or title "randomised randomized trial randomly" (match whole any) 3. abstract or title "wrist fracture" (match all words) and full text or abstract or title "randomised randomized trial randomly" (match whole any) 4. abstract or title "forearm fracture" (match all words) and full text or abstract or title "randomised randomized trial randomly" (match whole any) 5. abstract or title "torus fracture" (match all words) and full text or abstract or title "randomised randomized trial randomly" (match whole any) 6. abstract or title "buckle fracture" (match all words) and full text or abstract or title "randomised randomized trial randomly" (match whole any) 7. abstract or title "wrist fracture" (match all words) and full text or abstract or title "child children paediatric pediatric" (match whole any) 8. abstract or title "radial fracture" (match all words) and full text or abstract or title "child children paediatric pediatric" (match whole any) 9. abstract or title "radius fracture" (match all words) and full text or abstract or title "child children paediatric pediatric" (match whole any) 10. abstract or title "forearm fracture" (match all words) and full text or abstract or title "child children paediatric pediatric" (match whole any)
Strategy 2 (search update ran 9 May 2018)
1. radius OR radial OR wrist OR buckle OR torus OR forearm [Anywhere] AND random* [Anywhere] Nov 2016 to May 2018 [Cutsom range]
2.radius OR radial OR wrist OR buckle OR torus OR forearm [Anywhere] AND child* OR paediatric OR pediatric [Anywhere] Nov 2016 to May 2018 [Cutsom range]
Appendix 2. NICE outcomes. Extra summary of findings: removable splintage versus cast
| Removable splintage versus below‐elbow cast for buckle or minimally displaced fracture in children | ||||||
|
Patient or population: children with stable wrist fracture, predominantly buckle (torus) fracturesa Settings: hospital clinic Intervention: Removable splintb for 2 to 6 weeks Comparison: Below‐elbow cast for 2 to 6 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Below‐elbow cast | Removable splint | |||||
| Pain VAS (0 to 10; worst pain) during device use (4 ‐ 6 weeks follow‐up) |
The mean score in the study control group was 2.92 | The mean score in the intervention group was 0.20 higher (1.10 lower to 1.50 higher) | ‐ | 50 children (1 study) | ⊕⊝⊝⊝ very lowc | A 0.2 difference is minute and clinically unimportant. Overall, 5 trials provided data on pain, using different measures and timings. The 2 trials (161 children) reporting pain at 1 week found higher median pain scores in the splint group but neither of the differences between the 2 groups reached statistical significance; moreover, the difference in 1 trial was also unlikely to be clinically important. Most children in these 2 trials had no or very little pain by the end of 2 or 3 weeks immobilisation |
| Discomfort during use of device | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported |
| Participant satisfaction: child and/or parent preference for same device in future (3 to 6 weeks follow‐up) | See comment | See comment | Not estimable | 178 children (2 studies) | See comment | Results (1 indicating no difference, 1 favouring the splint) not pooled: clinically (e.g. different types of splint) and statistically heterogeneous (I2 = 83%)d |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reportede |
| Skin problems (rash) (3 weeks follow‐up) | See comment | See comment | Not estimable | ‐ | See comment | 1 study reported 11 cases of rash (17% of 64) in the splint group but none in the cast group (73 children). Conversely it reported 5 cases of oedema (7% of 73) in the cast group. The severity of both complications was not stated and the trial also made a contradictory statement that there were no adverse events or skin problems |
| Serious adverse events: refracture (6 months follow‐up) |
See comment | See comment | Not estimable | 87 children (1 study) | See comment | This study reported there had been no refractures |
| Health‐related quality of life Modified Activities Scale for Kids ‐ performance version (0 to 100; best function; no disability) (4 weeks follow‐up) | See comment. The median score in the study control group was 99.11 (IQR 96.42 to 100.00) | See comment. The median score in the intervention group was 99.04 (IQR 95.29 to 100.00) | ‐ | 65 children (1 study) | ⊕⊝⊝⊝ very lowf | This outcome assesses physical function rather than quality of life but has been used as a basis for cost‐effectiveness analysis in Boutis 2010. The data for the final scores are shown here for illustrative purposes; with no evidence of a clinically important difference between the two groups (MCID set at 15 in the study for sample size calculation). |
| Cast changes & number of outpatient visits (3 to 6 weeks follow‐up) | See comment | See comment | Not estimable | ‐ | See comment | Data for this outcome, which serves primarily as an indication of health care resource utilisation, were not available. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Footnotes
aChildren had buckle fractures in five studies and either buckle or an "undisplaced greenstick" fracture in one study. bSix studies made this comparison. Four probably used commercially available splints: one reported using prefabricated splints, the illustration in one also indicated a prefabricated splint; and two reported using futuro or futura type splints. Of the other two trials, one reported using a fibreglass volar slab secured by an elasticated bandage, and the other reported an individually‐fitted plaster splint attached with a tensor bandage. cWe downgraded by one level for serious risk of bias, reflecting lack of blinding (performance and detection biases), by one level for very serious imprecision, given the data for this outcome from two other studies were unavailable for pooling and the wide confidence interval, and by one level for indirectness, given the measure used. dEach result was assessed as very low‐quality evidence, downgraded by two levels for very serious risk of bias, reflecting lack of blinding (blinding and performance biases) and selective reporting bias, and by one level for serious imprecision, reflecting the small sample size. eAlthough this outcome was not reported, return to sporting or normal physical activities by four weeks in one trial (60 children) was greater in the splint group (25/26 versus 23/34; RR 1.42, 95% CI 1.11 to 1.82). However, there were contradictory and considerably heterogeneous findings (I2 = 92%) in the return to normal activities between this trial (at 20 days), which favoured the splint group, and another trial (at 14 days) that favoured the cast group. fWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection biases) and attrition bias, and by one level for serious indirectness, as this outcome assesses function rather than quality of life.
Appendix 3. Removable splint versus cast: economic data
| Study ID | Country / cost period, currency | Outcome | Results | Difference | Comment |
| Buckle fractures | |||||
| Davidson 2001 | UK 2000? Pounds sterling (GBP) | Estimate of treatment costs | Splint: GBP 65.75 Cast: GBP 116.78 | MD −GBP 51.03 | “Cost‐benefit analysis” Unit costs from hospital contracts department Radiograph, clinic attendance, full PoP cast including materials and technician's time, PoP backslab, Futura splint, temporary splint |
| Karimi 2013 | Iran
2010? US Dollars (USD) in Iran |
Estimate of treatment costs | Splint: USD 9.3 Cast: USD 15.3 | MD −USD 6.0 | “Cost‐benefit analysis” Unit costs from hospital contracts department Screening visits, radiography in ED, visits to fracture clinic, resources for application, cast removal and radiography |
| Minimally angulated or a minimally displaced acute greenstick or transverse fractures | |||||
| Boutis 2010 | Canada 2009 Canadian dollars (CAD) | Mean total cost | Splint: CAD 877.58 Cast: CAD 950.35 | MD −CAD 72.76 (SE 45.88) | Formal cost‐effectiveness analysis. The ASK‐P was used as the basis for the cost‐effectiveness analysis, where a threshold value of CAD 20 per unit gain in the ASK‐P score was used Parents completed expense diary Unit costs also from provincial statistical reports and local administrative data sources Other societal resources included participant and family resources and productivity costs |
| Mean total healthcare cost | Splint: CAD 670.66 Cast: CAD 768.22 | MD −CAD 97.56 (SE 9.24). | |||
| Mean societal costs | Splint: CAD 206.92 Cast: CAD 182.13 | MD CAD 24.79 (SE 37.52) | |||
Appendix 4. NICE outcomes. Extra 'Summary of findings': bandage versus cast
| Bandage versus below‐elbow cast for buckle or minimally displaced fracture in children | ||||||
|
Patient or population: children with stable wrist fracture, predominantly buckle (torus) fracturesa Settings: hospital clinic Intervention: Soft or elasticated bandageb for 3 to 4 weeks Comparison: Below‐elbow cast for 3 to 4 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Below‐elbow cast | Bandage | |||||
| Pain with VAS (0 to 100; worst pain) at 1 week | The mean score in the study control group was 20 | The mean score in the intervention group was 6 higher (1.31 lower to 13.31 higher) | ‐ | 89 children (1 study) | ⊕⊝⊝⊝ very lowc | The 95% CI is unlikely to include a clinically important effect. There was also very low‐quality evidence of less pain in the bandage group in 1 study (39 children), and little difference in pain during device use or requirement for analgesic in another study (53 participants)d |
| Discomfort during use of device (up to 4 weeks) | 572 per 1000e | 58 (6 to 389) | RR 0.10 (0.01 to 0.68) | 39 children (1 study) |
⊕⊝⊝⊝ very lowf | Another study (89 participants at 1 week) also reported "significantly less" discomfort in the bandage group; mainly in relation to itchingg |
| Patient experience: children found treatment was convenient (4 weeks follow‐up) |
143 per 1000e | 946 per 1000 (331 to 1000) | RR 6.61 (2.31 to 18.96) |
39 children (1 study) |
⊕⊝⊝⊝ very lowh | In this study, all 18 participants followed up in the bandage group had removed their bandage by 2 weeks |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported |
| Skin problems | See comment | See comment | Not estimable | ‐ | See comment | 1 trial (39 participants) reported no skin problems |
| Serious adverse events: refracture | See comment | See comment | Not estimable | ‐ | See comment | No children developed a serious adverse event in the 2 studies (139 children) followed up at 3 to 4 weeks |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported |
| Cast changes and number of outpatient visits (3 to 6 weeks follow‐up) | See comment | See comment | Not estimable | ‐ | See comment | Data not provided. 3 studies (181 children) reported on treatment failure (treatment change or extended use due to delayed union)i |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Footnotes
aChildren had buckle fractures in two studies, "impacted greenstick" fractures in one study and either buckle or an "undisplaced greenstick" fracture in one study. bThe soft bandage was a wool layer covered with a cotton crepe bandage. The elasticated bandage was a tubigrip.
cDowngraded by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection biases), and by one level for imprecision for wide confidence intervals. dPain was measured in different ways: one study referred to a "semantic scale", one used a VAS and also reported in terms of requiring analgesics. eControl group risk is derived from the study data. fWe downgraded the evidence by two levels for very serious risk of bias, mainly reflecting lack of blinding (performance and detection biases), and by one level for serious imprecision, reflecting these data were from one small trial. gDiscomfort was assessed on a weekly basis by participants recording how often they had itching, neck pain, or had found the bandage or cast too heavy, too loose or too tight. Although the data were unavailable for use in the review, being presented separately in a graph for each aspect and for each of the three weeks of usage, it was clear that itching was the prime source of discomfort for all three weeks, being reported a total of 140 times in the bandage group versus 219 times in the cast group (reported P < 0.001). hDowngraded by two levels for very serious risk of bias, mainly reflecting lack of blinding (blinding and performance biases), by one level for serious imprecision reflecting the small sample size, and by one level for serious indirectness, as the outcome was not a full measure of participant experience. iParents of four children (4.4%) requested a change from bandage to cast; three because they were sore from overuse and one "special needs" child. There were no requests for change in the cast group. One trial reported four cases (one in the bandage group versus three in the cast group) of delayed union requiring an extra week was reported in one trial.
Appendix 5. NICE outcomes. Extra summary of findings: home versus hospital‐clinic removal of casts
| Home compared with hospital‐clinic removal of casts for stable wrist fractures in children | ||||||
|
Patient or population: children with stable wrist fracture, predominantly buckle (torus) fractures Settings: hospital clinic or home Intervention: home removal of casta (at 3 weeks) Comparison: hospital‐clinic removal of cast (at 3 weeks) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hospital‐clinic removal of cast | Removal of cast at home by parent | |||||
| Pain/discomfort | See comment | See comment | Not estimable | 317 children (1 study) | See comment | This was not reported. Two children (1.3% of 159) in the removable cast group of Hamilton 2013 required a cast change (to the non‐removable fibreglass cast) because of pain in the first week |
| Pain (CHAQ) by VAS (0 to 100; worst pain) at 4 weeks | The mean score in the study control group was 5.55 | The mean score in the intervention group was 0.43 lower (3.88 lower to 3.02 higher) | ‐ | 233 children (1 study) | ⊕⊕⊝⊝ lowb | The 95% CI does not include a clinically important effect |
| 'Patient experience'
Parents would not choose the same treatment again (6 weeks follow‐up) |
643 per 1000c | 103 per 1000 (39 to 277) | RR 0.16 (0.06 to 0.43) |
80 children (1 study) |
⊕⊝⊝⊝ very lowd | This outcome was not reported. A proxy outcome, downgraded for indirectness, reflecting parental experience is given instead This was reflected in the greater proportion of parental complaints related to the inconvenience and costs of attending the hospital clinice |
| Time to return to former activities | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not reported.f |
| Childhood Health Assessment Questionnaire (CHAQ) Index change scores from pre‐injury at 4 weeks ‐ VAS (probably 0 to 100; worst) | The mean change score in the study control group was −0.48 | The mean change score in the intervention group was 0.96 higher (0.21 lower to 2.13 higher) | ‐ | 233 children (1 study) | ⊕⊕⊝⊝ lowb | These scores indicate restoration of pre‐injury function in both groups No participant had difficulties in activities of daily living at 6 weeks in another study (80 children) |
| Skin problems | See comment | See comment | Not estimable | 0 studies | See comment | This outcome was not reported |
| Serious adverse events: re‐fracture (6 months follow‐up) |
See comment | See comment | Not estimable | 288 children (1 study) | See comment | No participants developed a long‐term serious adverse event in this study |
| Cast changes and number of outpatient visitsg | See comment | See comment | Not estimable | 313 children (2 studies) | See comment |
Hamilton 2013 (233 children) reported without providing data that "there was no difference" between the 2 groups "in the number of casts that needed replacing or number of additional plaster room visits". In Symons 2001 (80 children), none of the minor complications reported for the backslab resulted in further treatment. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Footnotes
aTwo trials conducted in the UK tested this comparison. In one trial, home removal was facilitated by using a flexible cast instead of a standard fibreglass cast. A plaster backslab was used for all children in the second trial; this was precut in readiness for the home removal group. bWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of blinding (performance and detection bias) and large and imbalanced loss to follow‐up (attrition bias). cControl group risk is derived from the study data. dWe downgraded the evidence by two levels for very serious risk of bias, reflecting lack of allocation concealment (selection bias) and lack of blinding (performance and detection bias), and by one level for serious indirectness, given that children may have a different perspective. eParental complaints (14: 33% of 42) in the hospital group included 10 complaints about hospital waiting times, five about difficulties in getting time off work, three about transport problems and two about hospital parking. Some of the home group (7: 18% of 38) would have liked an extra bandage. In the other trial, 70 children (67% of 104) had to miss school to attend the appointment, with 52 carers taking time off work and nine of these losing pay as a result. fAlthough this outcome was not reported, at six weeks no children had difficulties in activities of daily living in one trial (80 participants) and average CHAQ changes scores for activities compared with pre‐injury scores at four weeks were small, with little difference between the two groups in another trial (233 participants). gThese primarily cost outcomes are linked and thus considered together. The focus of this comparison was on whether an additional outpatient appointment for cast removal could be avoided. The healthcare cost analysis (UK NHS unit costs 2010 and 2011) conducted by Hamilton 2013 showed that, while the flexible casts for home removal were more expensive compared with the standard casts (GBP 8.13 versus GBP 2.87), the overall cost of treating a stable paediatric forearm fracture with a cast that was removed at home was significantly less (reported P < 0.001) compared with one that was removed in a hospital clinic (GBP 150.88 versus GBP 251.62); the follow‐up appointment took up most of the cost.
Appendix 6. Additional categorical data for Stevenson 2013 (received from Georgia Antonio 28.04.17)
| Outcome |
Wet or Dry cast liner Category/score; Description; (number of participants) |
Delta Dry cast liner Category/score; Description; (number of participants) |
| Participant/caregiver overall score for satisfaction (comfort) at cast removal | 5 Excellent, I would recommend this cast to friends (n = 29) 4 Very comfortable (n =14) 3 Good overall. Comfort was satisfactory most of the time (n = 7) 2 Only just OK, not as easy as I imagined (n = 1) 1 Awful, the cast was intolerable (n = 0) |
5 Excellent, I would recommend this cast to friends (n = 28) 4 Very comfortable (n = 11) 3 Good overall. Comfort was satisfactory most of the time (n = 15) 2 Only just OK, not as easy as I imagined (n = 0) 1 Awful, the cast was intolerable (n = 0) |
| Technician's rating of skin condition at cast removal | 5 Excellent (n = 7) 4 Minor skin irritation, skin flaky (n = 21) 3 Skin reddened in places due to the padding (n = 22) 2 Skin damp or macerated (n = 1) 1 Ulceration or obvious dermatitis (n = 0) |
5 Excellent (n = 28) 4 Minor skin irritation, skin flaky (n =16) 3 Skin reddened in places due to the padding (n = 9) 2 Skin damp or macerated (n = 1) 1 Ulceration or obvious dermatitis (n = 0) |
| Technician's impression of overall cast padding quality | 5 Impressed with the padding and ease of use (n = 5) 4 Pleased (n = 6) 3 Satisfactory (n = 31) 2 Below average (n = 8) 1 Lots of complaints (n = 1) |
5 Impressed with the padding and ease of use (n = 12) 4 Pleased (n = 27) 3 Satisfactory (n = 10) 2 Below average (n = 4) 1 Lots of complaints (n = 0) Missing (n = 1) |
Appendix 7. Copy of letter commenting on Inglis 2013
Letter on Inglis 2013 former url: bjj.boneandjoint.org.uk/content/95‐B/9/1285.e‐letters Accessed: 02 June 2017
9 April 2014 Disadvantages of the synthetic cast Milind M Deshpande, Orthopaedic Surgeon Other Contributers: S Nadkarni
Sir,
In response to the article by Inglis et al, we would like to point out that synthetic casting materials have a number of disadvantages. First, in India each roll cost about three to five times as much as plaster of Paris, making it cost ineffective, especially when the time for which the cast is applied may be as little as three weeks in many cases.
Second, its poor moulding qualities make the casting procedure cumbersome.
Third, the fact that it sets quickly puts the surgeon at risk of hurrying the procedure.
Fourth, the translation between synthetic cast padding and the synthetic cast displaces the padding, and the stockinet within is incapable for preventing friction sores over bony prominences, particularly the patella and olecranon.
Finally, the electric saw used to remove the cast is the final nail in the coffin for both child and parents and may make them wish that they had opted for plaster.
M. Deshpande, Orthopaedic Surgeon, S. Nadkarni, Goa Medical College, Goa, India
Appendix 8. Summaries of other systematic reviews on paediatric wrist fractures
| Review ID | Search date | Studies | Outcomes | Conclusion | Comment |
| Systematic reviews of bandage and/or splint versus cast for buckle fractures | |||||
| Hill 2016 | November 2013 | 8 trials (825 children): Davidson 2001; Karimi 2013; Khan 2010; Kropman 2010; Oakley 2008; Plint 2006; West 2005; Williams 2013 |
|
"The evidence endorses the use alternative splinting [bandage or removable splint] over casting in paediatric wrist‐buckle fractures." | No pooling of data or meta‐analysis. No quantitative results presented |
| Howes 2008 | November 2007 | 2 trials (266 children): Davidson 2001; Plint 2006 |
|
"Removable braces [splints] support healing as much as casts and promote earlier functional recovery in children with buckle fractures" | Dated "short‐cut" review geared towards giving a 'Clinical bottom line' |
| Jiang 2016 | December 2013 | 8 trials (781 children): Davidson 2001; Khan 2010; Oakley 2008; Plint 2006; Pountos 2010; Symons 2001; West 2005; Williams 2013 |
|
"Nonrigid immobilization methods [soft cast, splint, bandage, and slab] have more advantages than rigid cast for immobilization of pediatric forearm torus fracture" | Methodological assessment was conducted using the
modified Jadad scale; which still uses a score‐based approach. Only outcome data on incidence of complications were pooled |
| Kennedy 2010 | March 2007 | 5 studies:
3 RCTs and 1 quasi‐RCT: Davidson 2001; Plint 2006; Symons 2001; West 2005 1 case series |
|
"..treatment in a removable splint [splint or bandage] does not increase risk of refracture or late displacement during the treatment period for buckle fractures of the distal forearm. Long‐term data on refracture rate is limited. There tends to be improved function, patient acceptance, and caregiver satisfaction with the use of removable splints." "Further study is needed to determine whether there are differences for longer periods of follow‐up on a population basis." |
No pooling of data or meta‐analysis. There was no refracture in 455 participants but only Plint 2006 reported for 6 months. The references used to justify the focus on refracture do not appear to support this for buckle fractures; e.g. none of the 10 refractures (1.9%) of the sample of 529 metaphyseal fractures were after buckle fracture (Bould 1999). |
| Li 2014 | April 2011 | 2 studies (314 children): 1 RCT and 1 quasi‐RCT Identities of studies not known. |
|
"The results indicate that the pain score of the patients with distal forearm buckle fracture in children do not improved after treated with splint and plaster cast, but splint fixation is better than plaster cast fixation in maintaining the bathing and regular exercise participation ability with good safety." Well‐designed, large sample and multi‐centre randomised controlled trials are needed for validation. |
Limited to 2 studies; very basic quality assessment (A,B,C). Abstract used only: full report, in Chinese, not sought |
| Meta‐analysis of below‐elbow versus above‐elbow casts for distal third forearm fractures | |||||
| Hendrickx 2011 | August 2010 | 3 trials (300 children): Bohm 2006; Paneru 2010; Webb 2006 |
|
"Due to heterogeneity, the trials are not fully compared. Based on the presented meta‐analysis, we conclude that BEC [below‐elbow cast] is not inferior to AEC [above‐elbow cast] so that this is a valid treatment option for distal third forearm fractures." "Future high quality randomized clinical trials, preferably multicentre, are desirable in this field..." |
Unlike our review did not include Colaris 2012. Used previous BJMT quality assessment tool that produced a score; now recognised as an inappropriate approach. Otherwise were aware of methodological limitations of the included trials |
| Systematic review of Kirshner wiring for displaced distal radius fractures | |||||
| Khandekar 2016 | December 2013 | 14 studies (527 children): 3 RCTs: Colaris 2013a; McLauchlan 2002; Miller 2005, 1 prospective cohort study; 10 retrospective studies |
|
Commonest indications: complete fracture displacement and translation more than 50% Commonest technique: Kirschner wiring with 2 retrograde wires in non‐Kapandji fashion Above‐elbow casts favoured over below‐elbow casts Minimal risk of fracture re‐displacement after K‐wiring Superficial pin tract infection is the commonest complication (If wires not buried, removed at 3 to 4 weeks after insertion) "Need for a multicenter randomized controlled trial to define protocols for management of displaced distal radius fractures in children." |
No quality assessment Focused on wiring rather than comparing wiring versus cast only |
Data and analyses
Comparison 1. Removable splintage versus below‐elbow cast for buckle or minimally‐displaced (stable) fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Activities Scale for Kids ‐ performance version (ASK‐P) (0 to 100: best function)(median, IQR (interquartile range)); higher scores = worse pain | Other data | No numeric data | ||
| 2 Functional disabilities at 4 to 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 No problems reported | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 No problems or only limited disability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Moderate or severe difficulties in performing activities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Difficulty in printing or writing at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Difficulty in drawing at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Difficulty in self‐feeding at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Difficulty in grooming at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Difficulty in bathing/showering at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Difficulty in bathing/showering at 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Complications and treatment failure | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Treatment failure | 4 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.26, 1.89] |
| 4.2 Change in treatment or reapplication | 4 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.38, 2.32] |
| 4.3 Change in treatment or reapplication; + excluded fractures | 4 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.97, 4.45] |
| 4.4 Extended immobilisation | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.54, 7.47] |
| 4.5 Serious adverse events | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Increase in deformity | 3 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.34, 13.61] |
| 4.7 Skin problems | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 26.18 [1.57, 435.69] |
| 4.8 Oedema (under device) | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.84] |
| 4.9 Grip strength "not quite normal" at 4 to 6 weeks | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.72] |
| 4.10 Stiffness at 4 to 6 weeks | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.20] |
| 4.11 Medical attention sought | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.70] |
| 4.12 Device problems noted | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.24, 0.80] |
| 5 Return to normal activities | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 By 2 or 3 weeks (end of device use) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Return to sporting or normal physical activities at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pain VAS (0 to 10; higher = worse pain) during device use | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Non‐parametric pain scores (median, IQR (interquartile range)); higher scores = worse pain | Other data | No numeric data | ||
| 7.1 Just after application | Other data | No numeric data | ||
| 7.2 At 1 week | Other data | No numeric data | ||
| 7.3 At 3 weeks | Other data | No numeric data | ||
| 7.4 Pain intensity when in pain | Other data | No numeric data | ||
| 8 Pain during use of splint or cast | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Mild to moderate pain during activity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 More than 6 days pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Regular analgesic required | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Patient satisfaction, preference and convenience | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Preference for same device | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Found treatment convenient | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Child preference for splint in future | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Parent preference for splint in future | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Bandage versus below‐elbow cast for buckle or minimally‐displaced fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional disabilities at 4 to 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 No problems reported | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 No problems or only limited disability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Complications and treatment failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment failure | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.44, 5.32] |
| 2.2 Delayed union | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.11] |
| 2.3 Serious adverse events | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Skin problems | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Slight increase in deformity | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 12.54] |
| 2.6 Grip strength "not quite normal" at 4 to 6 weeks | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.72] |
| 2.7 Stiffness at 4 to 6 weeks | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.20] |
| 3 Pain VAS 0 to 100 (higher = worse pain) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 During device use | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain or discomfort during use of bandage or cast | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Discomfort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Regular analgesic required | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Patient satisfaction: treatment was convenient | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Below elbow versus above elbow cast.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Limitations in activities of daily living during cast use | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Needed help dressing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Unable to shower | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Needed help using toilet | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Needed help eating | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Needed help at school | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Unable to write | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Patient reported help required because of difficulties with activities of daily living | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ABILHAND‐Kids score (0 to 42: no problems) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Subsequent (secondary) fracture displacement or reduction | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Secondary displaced fracture | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.87] |
| 3.2 Reangulation greater than 15 degrees or > 30% redisplacement | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.05] |
| 3.3 Remanipulation or secondary reduction | 4 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.07, 1.06] |
| 4 Complications | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Refracture | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Change of cast type (for comfort or other problems) | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.01] |
| 4.3 Compartment syndrome | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Cast split for swelling | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.10] |
| 4.5 Cast reinforced for 'breakdown' | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.65] |
| 4.6 Cast changed for loosening or breakdown | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.67, 3.84] |
| 4.7 Cast fell off | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.33, 29.12] |
| 4.8 Delayed union | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 70.00] |
| 4.9 Referral for physical therapy for range of motion limitation | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.16, 1.80] |
| 4.10 Skin abrasion at elbow | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.57] |
| 4.11 Transient neuropraxia | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.57] |
| 5 Days off school | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pain after 1 week in cast: VAS 0 to 10 (higher = worse pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Cosmetic appearance at 6 months (VAS 0 to 10: best cosmetics) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Rated by parents | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Rated by orthopaedic surgeon | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Ranges of wrist and elbow movement (degrees) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Wrist motion at cast removal | 2 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐6.67 [‐11.82, ‐1.52] |
| 8.2 Final wrist motion (flexion‐extension arc) | 2 | 179 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐1.43, 1.80] |
| 8.3 Elbow motion at cast removal | 2 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐32.54 [‐36.26, ‐28.82] |
| 8.4 Final elbow motion (flexion‐extension arc) | 2 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐2.94, ‐0.74] |
| 8.5 Final limitation of pronation and supination (6 months) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐5.35, 2.55] |
| 9 Time to regain range of motion (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Overall treatment cost (rupees, Nepal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Home versus hospital clinic removal of casts for stable, mainly buckle, fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Childhood Health Assessment Questionnaire change scores from pre‐injury at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Health status VAS (probably 0 to 100; worst) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Dressing/grooming (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Arising (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Eating (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Walking (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Hygiene (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Reach (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Grip (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Activities (0 to 3; unable) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Pain VAS (0 to 100; worst) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional activity at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Difficulties with writing or ADL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Avoidance of some hobbies | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change to allocated treatment | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.50, 19.93] |
| 3.1 Non‐compliance/adherence to cast removal at 3 weeks | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.21, 23.41] |
| 3.2 Change in treatment at 1 week (due to pain) | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [0.24, 102.68] |
| 4 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Change in treatment at 1 week (due to pain) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Swelling (mild) at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Tenderness (mild) at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Deformity at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Parents or children reporting problems with cast or care of fracture | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Parent: problems with fracture care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Parent: Would have liked spare bandage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Parent: cast became soft | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Child: cast become loose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Parents would not choose the same treatment again | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Would not choose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Would never choose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Parent satisfaction with treatment (always or almost always happy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Bandage versus removable splint for buckle or minimally‐displaced fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional disabilities at 4 to 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 No problems reported | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 No problems or only limited disability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain VAS 0 to 100 (higher = worse pain) during device use | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pain: regular analgesic required | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Increase in deformity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Grip strength "not quite normal" at 4 to 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Stiffness at 4 to 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Fibreglass versus plaster cast.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Remanipulation: distal radius fractures only | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Remanipulation: all fractures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 New cast (no remanipulation): all fractures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Cast reinforcement: all fractures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Skin irritation or pressure area: all fractures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Above‐elbow cast (forearm pronated versus neutral versus supinated) for displaced fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Second or subsequent reduction for unacceptable loss of alignment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Supination versus pronation | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.20, 9.99] |
| 1.2 Supination versus neutral position | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.15, 3.51] |
| 1.3 Pronation versus neutral position | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.12, 2.80] |
Comparison 8. Percutaneous wire fixation and above‐elbow cast versus above‐elbow cast alone for displaced fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ABILHAND‐Kids score (0 to 42: no problems) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Complications and secondary treatment | Other data | No numeric data | ||
| 2.1 Complications | Other data | No numeric data | ||
| 2.2 Secondary treatment | Other data | No numeric data | ||
| 3 Overall treatment failure and adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Overall complications (includes redisplacement) | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.37, 0.74] |
| 3.2 Overall complications (any redisplacement / malunion had to be treated) | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.45, 1.02] |
| 3.3 Treament failure (secondary procedures: early wire removal, rereduction etc) | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.83] |
| 4 Fracture redisplacement and rereduction | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Redisplaced fracture | 5 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.23] |
| 4.2 Remanipulation (and secondary procedure for loss of position) | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.22] |
| 5 Complications (not redisplacement or re‐manipulation) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Failed reduction | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.55] |
| 5.2 Failed insertion of wire | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.14, 79.28] |
| 5.3 Pain resulting from wire | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.12, 67.19] |
| 5.4 Pin site or superficial infection | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.54 [0.67, 45.89] |
| 5.5 Pin migration (wires removed) | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [0.49, 35.21] |
| 5.6 Operation to remove subcutaneous wires | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.45 [0.96, 282.13] |
| 5.7 Nerve damage or irritation | 3 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.16, 7.60] |
| 5.8 Tendon irritation | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.15, 76.93] |
| 5.9 Compartment syndrome | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.10 Non‐union | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.11 Malunion | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.93] |
| 5.12 Prominent scar at K‐wire insertion site | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.74 [0.44, 32.03] |
| 5.13 Early physeal closure | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.14 Referral for physical therapy for range of motion limitation | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.97] |
| 5.15 Refracture | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.68 [0.40, 145.68] |
| 6 Cosmetic appearance at 6 months: VAS (0 to 10: same as non‐fractured arm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Rated by parents | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Rated by orthopaedic surgeon | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Range of motion limitations at 6 months (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Limitation of wrist flexion‐extension | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Limitation of elbow flexion‐extension | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Limitation of pronation and supination | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Restricted pronation and supination at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Limitation > 31 degrees | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Limitation >/= 30 degrees | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Days in cast and physiotherapy visits | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Days in cast | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Visits to physiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Removable splintage versus below‐elbow cast for minimally‐displaced but potentially unstable fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Activities Scale for Kids ‐ performance version (ASK‐P) (0 to 100: best function) at 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Complications and treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Change in treatment or reapplication; including wrong diagnoses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Extended immobilisation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Increase in deformity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Clinical deformity (4 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Irritation during device use | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Skin problems (sores) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Itching during device use | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Faces Pain Scale (0 to 5; higher = worse pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain or discomfort during use of splint or cast | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Pain during device use | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Discomfort | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Patient and parent satisfaction: preference for the same device | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Child preference | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Parental preference | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Waterproof versus non‐waterproof cast.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities Scale for Kids ‐ Performance (0 to 100: best function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Fracture redisplacement, reduction or delayed healing | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Remanipulation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Redisplacement | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Extended immobilisation for non‐healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Water activities during cast use | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Took a shower | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Went for a swim | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Faces Pain Scale (0 to 10: worst pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Non‐routine cast change | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Skin changes at cast removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Skin: raised, itchy rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Skin: redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Skin: peeling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Skin: pressure sores | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Skin: maceration (breakdown) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Skin: inflammation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Satisfaction at cast removal (child or parent) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 With treatment at 2 weeks (0 to 100: best satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At around 4 weeks for child (0 to 10: best satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 At around 4 weeks for parent (0 to 10: best satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Split versus closed circumferential synthetic semi‐rigid above‐elbow cast.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure and complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Any treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Redisplacement needing surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Secondary splitting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Compartment syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Neurovascular compromise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Skin breakdown | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Double‐sugar‐tong splint versus above‐elbow bivalved cast.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure and complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Cast change at one week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Redisplaced fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Remanipulation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Nonunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. 'Wet or dry' versus 'Delta dry' water‐resistant cast liner (below‐elbow cast).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 'Skin damp or macerated' | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ulceration or obvious dermatitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Any skin complaint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Patient satisfaction with liner | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Excellent or very comfortable | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Excellent, very comfortable or good | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Cast technician's impression: below average or worse | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Closed reduction by Paediatric Emergency Physician (EP) versus Orthopaedic Resident.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Need for remanipulation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Compartment syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Required admission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Cast‐related complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Inadequate alignment at 6 to 8 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Significant limitation of motion or pain at 6 to 8 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Length of stay in Emergency Department (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bohm 2006.
| Methods | Randomised trial Study period: July 1999 to January 2003 |
|
| Participants | Royal University Hospital, Saskatoon, Saskatchewan, Canada 117 children, aged between 4 and 12 years, with closed fracture of the distal third of the forearm (radial or radial and ulnar; no isolated distal ulnar fractures) that required reduction Exclusion: open fracture or Salter Harris type III and IV fractures Sex: 61 male (60% of 102) Age: mean 8.6 years Fracture type: radius only (33); combined radius and ulna (69) Assigned: ? (below‐elbow) / ? (above‐elbow) Post‐exclusion (see Notes): 56 / 46 Analysed: 56 / 46 (at 6 weeks follow‐up) (see Notes) |
|
| Interventions | Closed reduction under conscious sedation in emergency department (within 4 hours of presentation) ‐ performed or supervised by senior orthopaedic residents ‐ or general anaesthesia in operating theatre (within 24 hours) ‐ reduction by residents supervised by attending orthopaedic surgeon 1. Below‐elbow plaster cast (3‐point moulding) 2. Above‐elbow plaster cast. Once hard, the below‐elbow cast was extended to above the elbow. Follow‐up visit to fracture clinic every week for 3 weeks. Cast removal at clinic at 6 weeks after injury. Hospital discharge with a sling and analgesia |
|
| Outcomes | Length of follow‐up: 18 weeks, but data apply to 6‐week follow‐up; also 1, 2 and 3 weeks
Function data: not reported, no formal data collection Redisplacement according to prespecified criteria and remanipulation Complications: reinforced or changed cast, cast split because of swelling, compartment syndrome (none) Conversion to other cast Radiological outcomes: angular deformity (radial and ulnar) |
|
| Funding and declarations of interest | Funding source: "One or more of the authors received grants or outside funding from The Canadian Orthopedic Foundation". No funding from commercial entity. Declarations of interest: reported, none |
|
| Notes | 15 patients were excluded after enrolment: 9 did not require fracture reduction, 2 had wrong cast applied, 2 were of the wrong age, 1 had wrong fracture type, and 1 had surgery. Group allocation not stated Radiographs were inadequate for 2 participants (1 versus 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was accomplished with use of a sealed envelope.” No details of sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was accomplished with use of a sealed envelope.” Quote: “Blinding of the patient and surgeon was maintained until the time of cast application;” The reasons for post‐randomisation exclusions (no reduction required) and lack of clarity when randomisation occurred adds some doubt to this statement |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding of participants, their parents and care providers not practical |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: “single blinding (of the outcome assessor) was maintained after cast application.” Although blinding of radiographic measurement (basis for assessing need for remanipulation) was attempted: Quote: “Complete blinding of the radiographic assessor was not always possible because the type of cast was sometimes identifiable on the radiograph.” No other blinding done, including for remanipulation |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | The allocated groups of the 15 children (13% of 117) excluded post‐randomisation were not declared. It is possible that this differed importantly between the 2 groups:
Quote: “There was an unequal distribution of patients in the two groups, with fifty‐six patients in the above‐the‐elbow group and forty‐six in the below‐the‐elbow group. This was because we did not use a block randomisation process and, initially, some children with nondisplaced fractures were erroneously enrolled. These children were excluded from the analysis presented here.” Only 1 child was missing from each group for those in the final analyses |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol but all outcomes in methods reported. However, data relating to participant recovery and function were not reported although clinically these would have been collected. Additionally, follow‐up was for 18 weeks but outcomes appear to apply to the time of cast removal at 6 weeks |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics not provided for all 117 randomised participants. Some imbalance in types of fracture (radius fracture only; combined radius + ulna) but not statistically significant |
| Other bias: performance bias | Unclear risk | Reduction method, length of cast use, and post‐cast care comparable. Also, mean cast index indicating similar fit of casts Equivalence in location of care (operating room versus emergency department), including assess to fluoroscopy and type of anaesthesia, was not established |
| Other bias | Low risk | No other source of bias identified |
Boutis 2010.
| Methods | Randomised trial Study period: April 2007 to September 2009 |
|
| Participants | The Hospital for Sick Children, Toronto, Ontario province, Canada 100 children with a minimally angulated or a minimally displaced acute greenstick or transverse fracture of the metaphyseal portion of the distal radius Inclusion: children between 5 and 12 years of age with open growth plates and presenting to the emergency department with a minimally angulated or a minimally displaced acute greenstick or transverse fracture of the metaphyseal portion of the distal radius. Definition of minimal angulation was a fracture with angulation of 15 ° or less in the sagittal plane of the radiograph. Minimal displacement was defined as translational displacement of 5 mm or less on the frontal plane. Informed consent Exclusion: children whose injuries were older than 5 days and those with a buckle (torus), growth‐plate or open fracture. Children at risk of pathologic fractures, those with congenital anomalies of the wrists, coagulopathies, multisystem trauma or multiple injuries to the same limb, and those with developmental delay Sex: 63 male (66% of 96) Age: mean 9.3 years Fracture type (of 96): minimally displaced acute greenstick (55) or transverse fracture (41) of distal radius; distal radius only (69), associated distal ulna fracture (27 : 22 buckle, 5 ulnar styloid) Assigned: 50 (splint) / 50 (cast) Analysed: 43 / 49 (at 3 months) |
|
| Interventions | 1. Prefabricated splint (W‐312 Pediatric Thermoplastic Wrist Support, Benik Corporation, Silverdale, USA). Children in the splint group were instructed to always wear the splint except for removal as needed for hygiene reasons 2. Fibreglass short arm cast applied by cast technician Splints and casts were applied by certified cast technicians. Apart from specific instructions about care of the cast or the splint, both study groups received identical instructions. The participants wore the immobilisation device for four weeks and were advised to avoid activities that could re‐injure the wrist for a further 2 weeks. (6 children wore the immobilization device for 6 weeks because their fracture angulation had progressed to 25 ° at the 4‐week visit) All participants attended the fracture clinic at the study hospital at 1 and 4 weeks after the injury. 6 weeks after the injury, participants were visited at home by a research physiotherapist |
|
| Outcomes | Length of follow‐up: 3 months, also 1, 4 and 6 weeks Function: modified Activities Scale for Kids‐performance version (ASK‐P) at 6 weeks and status at 3 months (included 8 additional questions related more specifically to activity of the wrist) Complications: extended immobilisation because fracture angulation had progressed to 25 ° at the 4‐week visit; required surgery (3 months); complications resulting in cast or splint change; minor complications recorded by parents (irritation, pain, sores, itching, discomfort) Clinical deformity (4 weeks) Grip strength (6 weeks) Range of motion (6 weeks) Wrist pain (1, 4, 6 weeks) Participant and parent satisfaction and preference Radiological: fracture angulation (1, 4 weeks) Splint use (1, 4 weeks) Cost effectiveness analysis |
|
| Funding and declarations of interest | Funding source: "The study was funded by the SickKids Foundation (grant no.XG 07‐001)". Declarations of interest: reported, none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed allocation of treatment was provided by an online program (www.randomize.net) using block randomisation with random block sizes of three and six.” |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed allocation of treatment was provided by an online program (www.randomize.net) using block randomisation with random block sizes of three and six.” Safeguards not mentioned but computer package was independent |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants and treatment providers (cast technicians) not possible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding of participants and treatment providers (cast technicians) not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: “Because families were aware of treatment allocation, this may have introduced bias in the measurement of our outcomes.”
Most outcomes come under the above category Quote: “Three months after the injury, parents were telephoned by a research assistant unaware of treatment allocation to assess recovery and any subsequent complications.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “a radiograph of the wrist was obtained and examined by a staff orthopedic surgeon who was unaware of the treatment allocation.” Quote: “To ensure consistency and accuracy of initial diagnoses, a pediatric musculoskeletal radiologist (P.B.), who was unaware of the treatment allocation, reviewed the radiographs obtained at baseline and at one and four weeks after injury” Quote: “Six weeks after the injury, patients were visited at home by a research physiotherapist unaware of the treatment allocation. To preserve blinding, families were instructed not to reveal which immobilization device had been used, and patients were provided with an opaque stocking that was placed over the affected arm before the physiotherapist’s assessment to hide any indications of which device had been used.” Quote: “for 90 of the 92 patients the physiotherapist could not ascertain which immobilization device had been used.” |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Slight discrepancy with Howard abstract which reports “93 of the 97 completed full clinical, radiographic, and patient determined followup” Quote: “Follow‐up of the primary outcome at six weeks was completed in 92 (96%) of the 96 children.” 4 excluded participants (8%) from splint group incorrectly diagnosed Lost to follow‐up: 3 participants uncontactable from splint group and 1 from cast group |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Slight discrepancy with Howard abstract which reports “93 of the 97 completed full clinical, radiographic, and patient determined followup” Quote: “Follow‐up of the primary outcome at six weeks was completed in 92 (96%) of the 96 children.” 4 excluded participants (8%) from splint group incorrectly diagnosed Lost to follow‐up: 3 participants uncontactable from splint group and 1 from cast group |
| Selective reporting (reporting bias) | Low risk | Trial registration available. All outcomes reported in full article |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics reported and appear comparable. However, those for the 4 children excluded from the splint group are missing |
| Other bias: performance bias | Low risk | Quote: "A certified research cast technician placed either the fibreglass cast or the splint" Both groups received comparable instructions aside from specific instructions regarding the care of the splint or cast |
| Other bias | Low risk | None detected |
Boyer 2002.
| Methods | Quasi‐randomised trial Study period: May 1995 to September 1996 |
|
| Participants | Children’s Hospital Medical Center of Akron, Akron, Ohio, USA 109 children with displaced (or angulated) fractures of the distal third of forearm (distal radius or radius and ulna) requiring closed reduction (based on judgement of attending physician). (Mention of criteria for displacement / angulation.) Informed consent Exclusion: closed physes Sex: 71 male (65%) Age: mean 8.7 years Fracture: 59 "displaced"; 40 "angulated" Assigned: ? (supinated) / ? (pronated) / ? (neutral) Analysed: 35 / 26 / 38 (at minimum 6 weeks follow‐up ) (see Notes) |
|
| Interventions | All participants had a closed reduction under general anaesthesia. A below‐elbow plaster cast was then applied. After confirmation of the reduction with fluoroscopy, fibreglass casting material was used to complete an above‐elbow cast. The forearm was positioned in 1 of 3 positions: 1. Supinated forearm position 2. Pronated forearm position 3. Neutral forearm position Routine clinical and radiographic follow‐up; first check at 1 week. Duration of splintage was not stated but assumed to be at fracture union (6 to 8 weeks) |
|
| Outcomes | Length of follow‐up: minimum 6 weeks (6 to 8 weeks)
Clinical and radiological union (no report)
Residual fracture angulation
Secondary reduction (for unacceptable loss of alignment) Routine cast changes (data not collected) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | 10 children were excluded from the analyses because of insufficient X‐rays | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “According to the child’s birth date, each patient was randomised for the position of immobilization.” Quasi‐randomised – sequence was not random |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised Predictable sequence |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not feasible and compliance with positioning not stated |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Decisions for secondary reduction were probably discretionary and thus susceptible to bias |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | 10 excluded but the distribution in the groups was not stated – nor were the numbers randomised into the 3 groups |
| Selective reporting (reporting bias) | High risk | No trial registration or protocol. Incompletely reported including absence of separate data for outcomes except secondary reduction. No reporting of child function or recovery |
| Other bias: major imbalance in baseline characteristics | Unclear risk | No data on which to judge this. No assurance given that the baseline characteristics were balanced |
| Other bias: performance bias | Unclear risk | No information on clinicians or their experience. Slightly high mean cast index (0.80) but no separate data by allocated group Decision for first reduction was at discretion of the attending physician |
| Other bias | Low risk | No other source detected |
Colaris 2012.
| Methods | Randomised controlled trial Study period: January 2006 to August 2010 |
|
| Participants | Multicentre study at 4 participating hospitals (Erasmus Medical Center (Rotterdam), HAGA Hospital (The Hague), Reinier de Graaf Hospital (Delft) and Sint Franciscus Hospital (Rotterdam)) in The Netherlands 66 children, aged under 16 years, with minimally displaced metaphyseal fracture of the radius and ulna Exclusion: buckle fractures of both the ulna and radius, fracture sustained longer than 1 week, a severe open fracture (Gustilo II and III), a relapse fracture in the same location, and need for reduction according to a priori defined criteria (age dependent: < 10; 10 to 16); see Colaris 2013a inclusion criteria Sex: 37 male (56%) Age: mean 7.1 years Fracture type: combined radius and ulna; fracture in either bone could be torus (5 radius), greenstick (56 radius) or complete (5 radius) Assigned: 35 (below‐elbow) / 31 (above‐elbow) Analysed: 35 / 31 (at 6 months follow‐up) (see Notes) |
|
| Interventions | Treatment in the emergency department. 1. Below‐elbow plaster cast (non‐circumferential) 2. Above‐elbow plaster cast (non‐circumferential) All casts applied in the neutral position. All children received a sling for at least 1 week. The children were clinically and radiologically evaluated at 1 and 4 weeks after initial trauma by a resident, supervised by an attending orthopaedic or trauma surgeon. A specialist in plaster revised the cast after 1 week. Where necessary, the fractures were reduced during the period of casting according to initial reduction criteria. The cast was removed 4 weeks after initial treatment Physical therapy was prescribed for participants with at least 30 degrees of functional impairment at the 2‐month examination |
|
| Outcomes | Length of follow‐up: outpatient follow‐up at 6 months; also 1 and 4 weeks and 2 months ABILHAND‐Kids score at 6 months Limitation of pronation and supination at 2 and 6 months Limitation of wrist and elbow flexion‐extension (6 months?) Comfort of cast (VAS) Cosmetics of fractured arm rated by parents (VAS) Complications: displacement in cast, cast fell off, excoriation (skin abrasion) in elbow crease, transient neuropraxia of superficial radial nerve; need for physiotherapy Radiological outcomes: angulation (radial and ulnar) |
|
| Funding and declarations of interest | Funding source: "The corresponding author received a grant of 10,800 euro from the Anna Foundation, the Netherlands." Declarations of interest: reported, none |
|
| Notes | 3 participants missed the 2‐month assessment (1 below‐elbow and 2 above‐elbow) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “An independent physician randomised the children by sealed envelopes with varied block sizes.” Very likely to be a randomised sequence |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent physician randomised the children by sealed envelopes with varied block sizes.” Very likely that allocation concealment was achieved |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “A surgeon treated the children during the first 4 weeks without masking.” Children, their parents and personnel could not be blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: “A surgeon treated the children during the first 4 weeks without masking.” Children, their parents and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding for listed outcomes. Although: “One independent orthopaedic surgeon examined all children 2 and 6 months after the initial trauma without masking”, this was a multi‐centre trial, with decisions taken by other clinicians However, the risk was probably low for radiographic outcomes: Quote: “Both the orthopaedic surgeon and independent trauma surgeon measured the radiographs with masking.” |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Participant flow clearly shown. 3 participants (1 versus 2) missing from 2‐month follow‐up |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Participant flow clearly shown. 3 participants (1 versus 2) missing from 2‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | Trial registration available, although outcomes only minimally described. All outcomes listed in Methods are reported in the Results |
| Other bias: major imbalance in baseline characteristics | Low risk | No significant differences between the 2 groups |
| Other bias: performance bias | Unclear risk | Follow‐up and post‐cast procedures comparable. However, no detail on expertise of the surgeons applying casts. There is a suggestion in the Discussion that the failure of the above‐elbow cast to prevent fracture displacement “might be due to less moulding around the lower arm caused by a more difficult to apply above‐elbow cast.” |
| Other bias | Low risk | None detected |
Colaris 2013a.
| Methods | Randomised controlled trial Study period: January 2006 to August 2010 |
|
| Participants | Multicentre study at 4 participating hospitals (Erasmus Medical Center (Rotterdam), HAGA Hospital (The Hague), Reinier de Graaf Hospital (Delft) and Sint Franciscus Hospital (Rotterdam)) in The Netherlands. 128 children, aged under 16 years, with displaced metaphyseal fracture of the radius and ulna that was stable after closed reduction in the operating room. Indications for reduction occurred: age < 10 years: angulation > 15 º; age 10 to 16 years: angulation > 10 º; translation > half bone diameter; any rotation of radius or ulna. Exclusion: fractures older than 1 week, severe open fractures (Gustilo II and III) and re‐fractures Sex: 83 male (66% of 126) Age: mean 8.8 years Fracture type (all both bones): torus (0 radius, 9 ulna); greenstick (28 radius, 60 ulna); complete (100 radius, 59 ulna) Assigned: 61 (wire) / 67 (cast only) Analysed: 60 / 63 (at 6 months follow‐up) |
|
| Interventions | Closed reduction under general anaesthesia with fluoroscopic guidance 1. Percutaneous wire fixation. Small incision made over radial styloid. Wire directed proximally and ulnarly across fracture site engaging in opposite cortex. Optional second wire inserted through small dorsal incision. The K wires were bent, cut and left transcutaneous. Above‐elbow cast applied by surgeon in the operating room. Primarily, a stockinet and a layer of wool were applied for protection, secondarily a well‐fitted plaster slab was applied, which covered approximately ⅔ of the circumference of the arm. Finally, a bandage was wrapped around the arm. The elbow was set in 90 ° of flexion and the wrist in neutral position. All children received a sling for at least 1 week 2. Above‐elbow cast alone. Applied as described above Fractures were evaluated clinically and radiologically at 1, 2 and 4 weeks after initial trauma and casts were revised and renewed where necessary. Re‐displaced fractures required a second reduction with percutaneous pinning in the operating room. Both the cast and the K wires were removed in the outpatient clinic after 4 weeks |
|
| Outcomes | Length of follow‐up: outpatient follow‐up at 6 months (mean 7.1 months); also 1, 2 and 4 weeks ABILHAND‐Kids score at 6 months Redisplacement according to predefined criteria and re‐reduction (according to the Methods this may have involved percutaneous pinning but not clear in results) Limitation of pronation and supination at 2 and 6 months Limitation of wrist and elbow flexion–extension at 6 months Complications: subcutaneous K wires, refractures, superficial infections, failed insertion of K wire, transient neuropraxia, need for physiotherapy Days in cast Cosmetics of fractured arm rated by parents and surgeon (VAS) Radiological outcomes: angulation (radial and ulnar) |
|
| Funding and declarations of interest | Funding source: grant from the Anna Foundation, The Netherlands Declarations of interest: reported, none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “An independent physician randomised the children by sealed envelopes with varied block sizes.” Very likely to be a randomised sequence |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent physician randomised the children by sealed envelopes with varied block sizes.” Very likely that allocation concealment was achieved. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “The children, parents and clinicians were not blinded for randomisation.” |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: “The children, parents and clinicians were not blinded for randomisation.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote: “The children, parents and clinicians were not blinded for randomisation.” Not blinded but at 6 months lack of blinding may be less potent by then |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: “The children, parents and clinicians were not blinded for randomisation.” Not blinded and treatment decisions are not blinded |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Participant flow shown with little difference in the small losses at final follow‐up (1 versus 4) However, some rounding errors in data provided as percentages |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Participant flow shown with little difference in the small losses at final follow‐up (1 versus 4) However, some rounding errors in data provided as percentages |
| Selective reporting (reporting bias) | Low risk | Trial registration available, although outcomes only minimally described. All outcomes listed in Methods are reported in the Results |
| Other bias: major imbalance in baseline characteristics | Low risk | No significant differences between the 2 groups |
| Other bias: performance bias | Unclear risk | Follow‐up and post‐treatment procedures comparable. However, no detail on expertise of surgeons and although standardisation of some techniques, some suboptimal surgery was reported |
| Other bias | Low risk | None detected |
Davidson 2001.
| Methods | Quasi‐randomised controlled trial Study period: 6 month period, before June 2000 |
|
| Participants | Alder Hey Children’s Hospital, Liverpool, UK 201 children with torus fractures of the distal radius Exclusion: None described Sex: 107 male (53.2%) Age: mean 8.9 years (2 to 15) Fracture type: torus Assigned: 116 (splint) / 85 (cast) Analysed: 98 / 81 (ignores the 2 excluded, 1 of which was in the splint group) |
|
| Interventions | 1. Futura‐type wrist splint sized and fitted by doctor or nurse. Written instructions provided to participants and parents, including removal for bathing and reapplication of splint; advice for use with discharge after first visit; removal at follow‐up clinic 2. Standard full ‘Colles type’ (full below‐elbow) plaster of Paris cast applied by plaster technician; removal at follow‐up clinic Seen at A&E department, radiograph diagnosis. Fracture immobilised by a metal splint held in place by a crepe bandage and participant referred to fracture clinic. Follow‐up appointment at 3 weeks; discharged if no complications after removal of splint / cast and clinical examination and radiograph and questioning |
|
| Outcomes | Length of follow‐up: 3 weeks Function data: not reported, no formal data collection Non‐union or loss of position: 3 weeks Compliance Protocol violation Costs |
|
| Funding and declarations of interest | Funding source: authors stated that no benefits in any form were received from a commercial party related directly or indirectly to the subject of the article Declarations of interest: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “They were randomised into two groups depending on the day on which they attended clinic, which was usually the day after injury.” Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Quote: “They were randomised into two groups depending on the day on which they attended clinic, which was usually the day after injury.” No allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Difference in losses between groups: 18/116 (15.5%) versus 4/85 (4.7%) |
| Selective reporting (reporting bias) | High risk | No protocol or trial registration. Insufficient detail on outcome recording and reporting. No mention of function |
| Other bias: major imbalance in baseline characteristics | Unclear risk | No data provided |
| Other bias: performance bias | High risk | Insufficient details on care personnel to make a judgement. No mention of written instructions for plaster cast use |
| Other bias | Low risk | None detected |
Derksen 2011.
| Methods | Randomised controlled trial Study period: 1 year period – not stated |
|
| Participants | Red Cross Hospital, Vondellaan, Beverwijk, The Netherlands 68 children, aged 5 to 15 years, with an isolated greenstick or torus fracture of the distal radius Exclusion: polytrauma, osteogenesis imperfecta or other bone diseases, pre‐existent fractures or deformity of the injured forearm (congenital or acquired), or concurrent participation in another study Sex: 27 male (40%) Age: mean 9.8 years Fracture type: isolated greenstick (12) or torus (56) fracture of the distal radius Assigned: 34 (swim cast) / 34 (traditional cast) Analysed: 34 / 34 (at 4 weeks follow‐up) |
|
| Interventions | Initial application of a plaster of Paris splint at the emergency department. After a week, applied 1 of 2 casts. 1. Swim cast: air‐ventilating semi‐flexible Polyester cast manufactured without the use of a synthetic wool liner and thus applied directly over the protective stocking: only a single layer of synthetic cast was applied after a "reinforcing longuette" of the same cast material was used on the ulnar side. MOKcast technique used (Wierzimok 2017). Participants were instructed not to shower or swim just before going to bed but no additional instructions were given about avoiding swimming or going to the beach. 2. Traditional cast (made of polyurethane material, with a cotton liner). Participants receiving the traditional cast were advised to use a protective plastic bag when taking a shower or going for a swim Both types of casts were worn for 2 to 3 weeks. Children younger than 8 years were immobilised for a total of 3 weeks and children 8 years and older were immobilised for a total of 4 weeks, in accordance with hospital protocol. The cast was removed by employees of the plaster room. Control radiographs (anterior‐posterior and lateral) were made (additional to usual treatment) |
|
| Outcomes | Length of follow‐up: 4 weeks Function data: not reported Secondary displacement and radiological bone healing Complications: skin lesions (urticaria, redness, desquamation, pressure sores, maceration, inflammation); non‐union Participant and parent satisfaction (questionnaire) Activities during cast use: taking a shower, going for a swim Comfort (not reported) Costs (no data) |
|
| Funding and declarations of interest | Funding source: "None of the authors received financial or grant support for this study." Declarations of interest: reported, none |
|
| Notes | Authors gave a different number of people in the swim cast group (32 rather than 34) in the Discussion. The authors do not make clear whether all participants and their parents answered all the questionnaire questions Sent email to R Derksen 21.04.17. Asked for 1) more details on the traditional cast, in particular, its composition (polyester and polyurethane are both mentioned in the article); 2) if there were missing data for any of the outcomes and, if so, to supply the denominators for each group; 3) to provide an estimate of the costs of each of the casts? Email bounced so emails sent to co‐authors Dr Deij and Dr Breederveld 21.04.17. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was by the drawing of an envelope in the presence of employees of the plaster room who guided this process and occurred in blocks of ten patients.” No details but seems likely |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was by the drawing of an envelope in the presence of employees of the plaster room who guided this process and occurred in blocks of ten patients.” Insufficient information. Some potential for predictability with fixed block size |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded: care providers, children and their parents |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Not blinded: care providers, children and their parents |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Children and their parents knew what interventions they had |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinded assessment of clinical bone healing and for skin lesions. Quote: “Both the radiologist and the surgery resident [there were three of these] were blinded to the type of cast that was administered.” |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | No explicit report on whether there were missing questionnaire data. Small discrepancy in the Discussion |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Not reported. However, all percentages related closely to whole numbers |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol. Outcomes in Methods reported except for 1 on cast comfort |
| Other bias: major imbalance in baseline characteristics | Low risk | No major baseline imbalances |
| Other bias: performance bias | Unclear risk | No mention of clinician expertise |
| Other bias | Low risk | No other bias detected |
Ghoneem 2003.
| Methods | Randomised trial Study period: not reported (before 2003) |
|
| Participants | Location of trial not reported but author based in Dr. Fakhry & Al‐Mouhawis Hospital, Al‐Khobar, Saudia Arabia 70 displaced distal forearm fractures Exclusion: not reported Sex: not reported Age: not reported Fracture type: not reported Assigned: 35 (wire and cast) / 35 (cast only) Analysed: ? / ? 59 patients (84%) were reviewed at 4 months |
|
| Interventions | Closed reduction 1. Percutaneous wire fixation and then cast 2. Plaster cast only Follow‐up until union occurred |
|
| Outcomes | Length of follow‐up: 4 months and union Functional deficit Redisplacement Quality of reduction |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | Trial published as an abstract; no full report available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “These children were randomly allocated to one of two treatment groups”. No details to inform judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: “These children were randomly allocated to one of two treatment groups”. No details to inform judgement |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of clinicians or participants. (Unclear how functional results assessed) |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of clinicians or participants |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of clinicians or participants. (Unclear how functional results assessed) |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding of clinicians or participants |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | 11/70 (16%) lost at 4 months. Unclear how functional deficit was assessed |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Unclear criteria for redisplacement or quality of reduction |
| Selective reporting (reporting bias) | High risk | Very minimally reported abstract. No trial registration or protocol identified. No mention of complications |
| Other bias: major imbalance in baseline characteristics | Unclear risk | No data on baseline characteristics or statement on whether these were balanced between groups |
| Other bias: performance bias | Unclear risk | No details on which to judge relative expertise of care providers or whether there were between‐group differences in the care programme |
| Other bias | Unclear risk | Study reported briefly and incompletely in a conference abstract |
Gibbons 1994.
| Methods | Quasi‐randomised controlled trial Study period: January 1991 to 30 June 1992 |
|
| Participants | John Radcliffe Hospital, Oxford, UK 23 children, aged < 15 years, requiring manipulation for isolated distal radius fracture. Indications for manipulation: > 15 º angulation for children under 10 years or > 10 º angulation if age > 10 years Exclusion: incomplete, greenstick, or undisplaced fracture of the distal radius, or displaced ulna fracture Sex: 15 male (65% of 23) Age: mean 9 years, range 5 to 14 years Fracture type: complete displacement (16); open fracture (1); number of both‐bone fractures (ulna would be intact) unknown Assigned: 12 (wire) / 11 (cast only) Analysed: 12 / 11 (6 months: numbers assumed) |
|
| Interventions | Manipulation under general anaesthesia involving surgeon and assistant 1. Percutaneous (stab incision) Kirschner wire inserted from the radial styloid. Use of fluoroscopy. Above‐elbow plaster cast. Wire removed under sedation or general anaesthesia after 3 weeks, then below‐elbow cast applied for a further week 2. Above‐elbow plaster cast. Longitudinal traction applied across the fracture with assistant applying counter‐traction to the flexed elbow. Arm placed in an above‐elbow, moulded cast. (Duration in cast not stated) All participants kept overnight in hospital and then monitored with weekly radiographs at fracture clinic |
|
| Outcomes | Length of follow‐up: 6 months Loss of reduction and remanipulation Non‐union Superficial radial nerve damage Early physeal closure Hypertrophic scar |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | Email sent to Gibbons 26.04.17. No reply. Email sent to P Worlock (via Newcastle Hospitals website) on 04.05.17 "1. Your paper reports that "Children were allocated to treatment groups according to which consultant was responsible for their care". It would be very helpful to have details of the exact method of allocation, including how soon after presentation the allocation took place. 2. If you still have access to your study records, or can recall, how many of the 23 study participants were available for review at 6 months (final follow‐up)? Group A: manipulation and casting alone n = Group B: manipulation, percutaneous K wiring and casting n = 3. You state that there were "no cases of early physeal closure", which I presume was checked for at 6 months. Can you recall or identify any other concerns (aside from the child with the hypertrophic scar) logged at six months? 4. Do you have any records on the timing of clinical union for these fractures. Was there any difference between the two groups?" No reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Probably quasi‐RCT: Quote: "Children were allocated to treatment groups according to which consultant was responsible for their care: two consultants treated their patients by manipulation and casting, and two by manipulation, percutaneous Kirscher wiring, and casting" |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | No participant flow, including no indication if all participants were successfully contacted at 6‐month review |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol. No report of child function or recovery |
| Other bias: major imbalance in baseline characteristics | Unclear risk | 1 open fracture in the pinning group; differences in the initial dorsal angulation with more extreme cases in the pinning group (mean 26.4 º versus 13.4 º) |
| Other bias: performance bias | Unclear risk | Insufficient details to confirm |
| Other bias | Low risk | None detected |
Gupta 1990.
| Methods | Quasi‐randomised trial Study period: not stated |
|
| Participants | Hospital linked with Kuwait University, Kuwait 60 children with solitary greenstick fractures at the junction of the metaphysis and diaphysis of the distal radius Exclusion: displaced fractures of radius or fractures combined with ulna fracture Sex: 48 male (80%) Age: mean 8.33 years Fracture: solitary greenstick fractures; 25/60 with ≥ 20 º dorsal angulation Assigned: 20 (supinated) / 20 (pronated) / 20 (neutral) Analysed: 20 / 20 / 20 (at minimum 6‐week follow‐up) (see Notes) |
|
| Interventions | Above‐elbow cast with forearm positioned in 1 of 3 positions: 1. Supinated forearm position 2. Pronated forearm position 3. Neutral forearm position Manipulation at day 1 before immobilisation if dorsal angulation ≥ 20 º Manipulation at 2 weeks if dorsal angulation ≥ 20 º Cast removed at 6 weeks |
|
| Outcomes | Length of follow‐up: minimum 2 weeks (3 and 6 weeks)
Reduction / manipulation at 2 weeks Change in dorsal angulation day 1 to 2 weeks (degrees) Change in dorsal angulation day 2 to 6 weeks (degrees) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Every third patient (20 in each group) was treated with the forearm in a pronated, neutral or supinated position.” Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Alternation Predictable sequence |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | There was no blinding |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Very likely none had been lost to follow‐up |
| Selective reporting (reporting bias) | High risk | No trial registration or published protocol. Very limited outcomes and poorly reported |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Gender imbalance (no girls in the supination group). Unsure of the potential effect on trial result. Balances in other characteristics M/F ratios: supinated 20/0; pronated 15/5; neutral 13/7 |
| Other bias: performance bias | Unclear risk | No information on who treated the participants or their prior experience. No information on other care |
| Other bias | Low risk | None detected |
Hamilton 2013.
| Methods | Randomised controlled trial Study period: May 2008 and March 2011 |
|
| Participants | John Radcliffe Hospital, Oxford, UK 317 children aged between 2 and 16 years with a stable distal forearm fracture presenting to clinic within 72 hours of injury. Torus fractures and greenstick fractures < 15 ° angulated in the sagittal plane and Salter–Harris I and II epiphyseal fractures < 5 mm displacement with translation were included Exclusion: Other fractures of the upper limb, multi‐limb trauma and injuries requiring admission; history of previous surgery or significant injury to the affected arm, developmental delay, failure to thrive (failure of expected normal physiological development), a musculoskeletal disease affecting the upper limb; suspicion of non‐accidental injury; use of medications that influenced bone metabolism or living outside the hospital catchment area Sex: 177 male (56% of 317) Age: mean 9.4 years Fracture type: buckle (torus) 194 (61%); greenstick 63 (20%); stable epiphyseal 60 (19%). Radius only 286 (90%); ulna only (a stable epiphyseal) 1; both bones 30 (9.5%) Assigned: 159 (home) / 158 (hospital) Analysed: 129 / 104 (at 4 weeks); 140 / 148 (at 6 months) |
|
| Interventions | 1. Home cast removal. Flexible cast (3M Scotchcast Soft Cast casting tape; 3M Healthcare) removed at home at 3 weeks. No further appointment given, but could request one if required 2. Hospital cast removal. Fibreglass cast (3M Scotchcast Poly Plus casting tape; 3M Healthcare) removed in the clinic under clinical review at 3 weeks |
|
| Outcomes | Length of follow‐up: 6 months, also at 4 weeks (1 week post‐cast removal) Childhood Health Assessment Questionnaire (Index) Childhood Health Assessment Questionnaire (VAS 15 cm: 0 to 100: worst pain) at 4 weeks EuroQol 5‐Dimensions (EQ‐5D) quality of life questionnaire Change in treatment (at 1 week) Serious adverse events Subsequent need for further care Complications: loose cast, cast needed replacing, cast difficult to remove Satisfaction Inconvenience and additional healthcare contacts Costs and societal costs |
|
| Funding and declarations of interest | Funding source: no funding from a relevant commercial source Declarations of interest: reported, none |
|
| Notes | Sent request for information and data to Mr Hamilton 26 April 2017. (Repeated 5 June 2017). No response received. Asked for:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomisation was performed using sequentially numbered opaque envelopes that contained a computer generated random allocation sequence prepared by the Warwick Clinical Trials Unit.” Clearly random |
| Allocation concealment (selection bias) | Low risk | Quote: “randomisation was performed using sequentially numbered opaque envelopes that contained a computer generated random allocation sequence prepared by the Warwick Clinical Trials Unit.” Allocation was concealed |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding of participants or care providers |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding of participants or care providers |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessment (Bias is likely to be less at 6 months follow‐up: Unclear) |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding of outcome assessment. These outcomes were usually reported by parents. (Bias is likely to be less at 6 months follow‐up: Unclear) |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Missing data. Difference in loss to follow‐up between the 2 groups at 4 weeks; 30/159 (19%) versus 44/158 (30%) |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Missing data. Difference in loss to follow‐up between the 2 groups at 4 weeks; 30/159 (19%) versus 44/158 (30%) |
| Selective reporting (reporting bias) | High risk | Discrepancies between trial registration and report; including sample size calculation and some outcome measures. No data provided for 6‐month follow‐up |
| Other bias: major imbalance in baseline characteristics | Low risk | No major differences in baseline characteristics |
| Other bias: performance bias | Unclear risk | Insufficient details of care in cast and of how to remove for the flexible cast group. No details of care providers |
| Other bias | Low risk | None detected |
Inglis 2013.
| Methods | Randomised trial Study period: March 2009 to August 2011 (main trial report: Inglis 2013) Study period: February 2009 to December 2011 (Abson 2016; see Notes) |
|
| Participants | Women’s and Children’s Hospital, Adelaide, Australia 201 children with a displaced fracture of the forearm (radius or ulna or both) requiring closed reduction and immobilisation. 143 children with distal radius fracture subgroup selected in Abson 2016 Inclusion: Children with a displaced fracture of the forearm (radius or ulna or both) requiring closed reduction and immobilisation. Informed consent Exclusion: Pathological or open fractures, fractures requiring internal fixation and patients who were not available for local follow‐up Sex: 132 male (66% of 199); Abson 2016: 85 (65% of 130) Age: mean 9.7 (range 1.4 to 17.5) years; Abson 2016: Fibreglass: median 11.1 (4.1 to 17.5), PoP: median 10.6 (4.2 to 15.5) years Fracture type of 130 DRF in Abson 2016: 32 epiphyseal and 98 metaphyseal Assigned: 111 (fibreglass)/ 90 (PoP); Abson 2016: 77 (fibreglass)/ 66 (PoP) Analysed (at 6 weeks): 110 (fibreglass) / 89 (PoP); Abson 2016: 71 (fibreglass) / 59 (PoP) |
|
| Interventions | 1. Fibreglass: synthetic (Scotchcast Plus; 3M, St Paul, Minnesota) group. 22 (20% of 110) had below‐elbow cast, the rest had above‐elbow cast. Cast removed at 6 weeks. 2. Plaster of Paris group (Gypson; BSN Medical Pty Ltd, Mt Waverly, Australia). 9 (10.1% of 89) had below‐elbow cast, the rest had above‐elbow cast. Cast removed at 6 weeks. The participants underwent a standardised closed reduction and full‐cast immobilisation dependent on the configuration by a consultant, accredited orthopaedic registrar or a resident. Casts were not split prophylactically. All participants had the same padding under the cast: Wet n’ Dry (3M). Routine follow‐up was undertaken at 1 and 6 weeks. Management was supervised by 7 orthopaedic consultants (including 1 author, PJC) or attending surgeons who were blinded to the participant’s involvement in the study |
|
| Outcomes | Length of follow‐up: 6 weeks, also 1 week
Function data: not reported or collected
Re‐manipulation Complications: further care of cast, skin complications Participant satisfaction (1 lowest to 5 highest) Cost |
|
| Funding and declarations of interest | Funding source: The authors report that "no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article". Declarations of interest: Recorded, none |
|
| Notes | Commentary on practicality of synthetic cast in India: Deshpande 2014. There is a separate publication, using data extracted from case notes, comparing the results of residents versus attending surgeons in 143 distal radius fractures (130 with data) from the trial (Abson 2016). Sent request for data (baseline, use of below‐elbow casts, and results (complications) to Dr Inglis on 5 June 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were enrolled into the study on presentation to the Emergency Department”. Quote: “The patients were randomised using a sealed envelope..” No details of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The patients were randomised using a sealed envelope..” Insufficient information on safeguards |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “Management was supervised by seven orthopaedic consultants (including one author, PJC) or attending surgeons who were blinded to the patient’s involvement in the study.” However, the participants were not blinded. Not feasible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: “Management was supervised by seven orthopaedic consultants (including one author, PJC) or attending surgeons who were blinded to the patient’s involvement in the study.” However, the participants were not blinded. Not feasible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: “One author (MI) performed all radiological measurements” Not blinded |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | High and imbalanced loss to follow‐up for participant questionnaires: 25% (28/111) versus 37% (33/90) |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Although balanced, 9% excluded with insufficient data for Abson 2016 |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol but outcomes reported. However, failure to report on child function or recovery |
| Other bias: major imbalance in baseline characteristics | High risk | Difference in numbers randomised (111 versus 90); differences in gender (males: 60.6% versus 71.2% in Abson 2016); differences in fracture distribution (epiphyseal: 19.7% versus 30.5% in Abson 2016). Ages similar, however. |
| Other bias: performance bias | High risk | Differences in use of below‐elbow casts (20% in synthetic cast versus 10.1% in PoP cast) and in general anaesthesia (87% versus 76%) Some assurance regarding effect of surgeon experience: performed by consultant (28 casts); registrar (144 casts); 1 resident (16 casts) including a sufficiently similar cast index |
| Other bias | Unclear risk | Discrepancies in duration of study in Inglis 2013 and Abson 2016. May have a slight impact? |
Jones 2001.
| Methods | Randomised trial Study period: not reported; trial registration document suggests October 1998 to April 1999 |
|
| Participants | Location of trial: probably Gwynedd Hospitals NHS Trust, Ysbyty Gwynedd, Bangor, Wales, UK 50 children with a distal radius buckle fracture Exclusion: not reported Sex: not reported Age: mean 6.2 years (3 to 10) Fracture type: buckle fracture Assigned: 25 (bandage) / 25 (cast) Analysed: 24 / 25 (at 3 to 4 weeks) |
|
| Interventions | 1. Wool and crepe bandage for 3 weeks 2. Below‐elbow (short arm) POP cast for 3 weeks and thereafter mobilisation. (Described as "standard POP back slab" in probable trial registration document) Weekly review |
|
| Outcomes | Length of follow‐up: 3 to 4 weeks (end of treatment) Function data: not reported, no formal data collection Clinical union Delayed healing (treatment extended1 week) Adverse events (1 unrelated injury to contralateral elbow reported; not included in review) Parent satisfaction |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | Trial published as a poster abstract; no full report available. Linked to a National Research Register entry on 1 of the review author's (HH) files but with some unexplained discrepancies. Neither the abstract nor the trial registration documentation are now available online | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Twenty‐five patients were randomised to each group” No information on method |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding not feasible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not feasible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unlikely to be blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Unlikely to be blinded |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | 1 participant withdrawn from wool and crepe group (4% of 25 participants). Unlikely to be a problem |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 1 participant withdrawn from wool and crepe group (4% of 25 participants) Unlikely to be a problem |
| Selective reporting (reporting bias) | High risk | Incompletely reported only in a poster abstract |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics not reported by group |
| Other bias: performance bias | Unclear risk | No information available |
| Other bias | Low risk | None apparent |
Karimi 2013.
| Methods | Likely to be a quasi‐randomised controlled trial Study period: July to December 2010 |
|
| Participants | Orthopaedic Clinic of Shahid Bahonar Hospital, Iran 142 children with distal forearm torus fractures Inclusion: children with distal forearm torus fractures Exclusion: exclusion criteria not reported Sex: 103 male (72.5% of 142) Age: mean 9.5 (range 1.2 to 17) years Fracture type: isolated radius (114: 80.3%); isolated ulna (2: 1.4%); radius and ulna (26: 18.3%) Assigned: 65 (splint) / 77 (cast) Analysed: 64 (splint) / 73 (cast) (at 3 weeks) |
|
| Interventions | 1. Removable wrist splint. Full verbal and written instructions provided to parents at first visit to fracture clinic. Splint removed at home at 3 weeks. Participants were followed up by phone upon termination of their treatment period (Instructions included that the splint could be removed for washing; implied in report's Discussion) 2. Short arm cast. Patients visited clinic at 3 weeks for cast removal, radiography and completion of follow‐up forms |
|
| Outcomes | Length of follow‐up: 3 weeks Function data: not reported, no formal data collection Bone healing without loss of position (only assessed in cast group) Complications: adverse events including rash and oedema; difficulties removing splint; broken or soft cast Pain Compliance with treatment Convenience of treatment, satisfaction with one‐stop treatment (splint group only) Cost‐benefit analysis |
|
| Funding and declarations of interest | Funding source: reported, none declared Declarations of interest: reported, none |
|
| Notes | Similarities with excluded trial Krishnan 2014 detected HH sent email to Ali Nemati 22.07.17 asking for:
No response received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were “randomly divided into two groups on the day of attendance in the clinic”. Likely to be quasi‐randomised controlled trial |
| Allocation concealment (selection bias) | High risk | Participants were “randomly divided into two groups on the day of attendance in the clinic”. Likely to be quasi‐randomised controlled trial |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Blinding not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Blinding not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Loss to follow‐up is small: 1 (1.5% of 65) from splint and 4 (5.2% of 77) from cast |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Loss to follow‐up is small: 1 (1.5% of 65) from splint and 4 (5.2% of 77) from cast |
| Selective reporting (reporting bias) | High risk | No protocol or trial registration. No reporting of function. The Methods state that satisfaction of the one‐stop splint treatment was measured via the “Verhaar scale”, but this was not reported in the results |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Data not given for individual treatment groups |
| Other bias: performance bias | High risk | Differences in follow‐up: Quote: “The duration of treatment was three weeks for both groups. Appointments were made, for three weeks later, for the SAC group for cast removal, control radiography and filling the follow‐up form. The patients in the RWS group were followed up by phone upon termination of their treatment period”. Informed consent obtained only for splint group |
| Other bias | Low risk | None identified |
Khan 2010.
| Methods | Randomised controlled trial Study period: June 2008 to July 2009 |
|
| Participants | Le Bonheur Children’s Hospital, Memphis, Tennessee, USA 104 children with isolated, closed, distal forearm fractures requiring manipulation (1 child excluded in final report; see Notes) Inclusion: patients with isolated, closed angulated or displaced fractures of the distal forearm (distal third of the radius or ulna) meeting standardised criteria for manipulation (angulation in the dorsovolar plane and radiulnar plane of > 20 º and ⁄ or > 15 º, respectively for children younger than 9 years; > 10 to 15 º and > 5 º for children 9 – 13 years; and > 5 to 10 º and > 5 º in patients older than 13 years and any degree of shortening). Exclusion: open fractures, polytrauma, neurovascular compromise, or a previous reduction attempt before arriving at hospital emergency room Sex: 75 male (73% of 103) Age: 9.4 years (range 6 months to 18 years; trial registration document) Fracture type (of 103): isolated radius (30: 30%); radius and ulna (71: 70%); involved growth plate (17: 17%) Assigned: 51 (emergency physician) / 52 (orthopaedic resident) Analysed: 48 (emergency physician) / 48 (orthopaedic resident) (at 6 to 8 weeks) |
|
| Interventions | 1. Closed manipulation and cast immobilisation by a paediatric emergency physician (the principal investigator or co‐investigator) who had received focused training in forearm fracture reduction by a paediatric orthopaedist, who supervised 5 reductions before the trial. No orthopaedic consultation. Sedation or analgesia or both were provided by the treating emergency physician assigned to the participant; this could be another paediatric emergency physician, fellow or general paediatrician "with sedation privileges" 2. Closed manipulation and cast immobilisation by an unsupervised postgraduate year 3 or 4 orthopaedic resident. Although not made clear, it seems very likely that the above procedures for sedation or analgesia or both applied also to this group All participants had their fractures manipulated with the aid of portable fluoroscopy All participants were discharged with orthopaedic follow‐up arranged within 5 to 7 days of injury The 6‐ to 8‐week follow‐up was also a standard of care |
|
| Outcomes | Length of follow‐up: 6 to 8 weeks, also at 5 to 7 days Function data: not reported, no formal data collection Limitation or pain at final follow‐up (observation) Complications: need for remanipulation, cast‐related complications (tight or wet cast), compartment syndrome (no cases) Radiographic: adequacy of alignment, healing (also functional healing mentioned) Back‐up orthopaedic consultation required by emergency physician Length of stay during the initial emergency department encounter Complaints against emergency physicians or orthopaedic residents (anecdotal) Facility charges (mention in registration document) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | Retrospective trial registration reports 104 participants. It is very likely that 1 fracture found not to require manipulation had originally been randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequence was generated a priori using blocks of four and six and maintained in sealed numbered envelopes under lock and key.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation sequence was generated a priori using blocks of four and six and maintained in sealed numbered envelopes under lock and key.” Does not specifically report that envelopes were opaque but other safeguards suggest risk of selection bias was low |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: “To ensure blinding, no reference was made to the study in the electronic patient record. Apart from the collaborating pediatric orthopedic investigator, none of the other orthopaedic attending and resident physicians assigned to the fracture clinic were aware of study initiation and closure. Hence, the orthopedic surgeon treating the patient at follow‐up was unaware of the group to which the subject had been assigned.” However, the 2 paediatric emergency medicine clinical investigators, who had received focused instruction in fracture manipulation, were not blinded. Nor were the participants and their parents |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “One of three board‐certified pediatric orthopedic surgeons assessed patients at follow‐up. To ensure blinding, no reference was made to the study in the electronic patient record. Apart from the collaborating pediatric orthopedic investigator, none of the other orthopaedic attending and resident physicians assigned to the fracture clinic were aware of study initiation and closure. Hence, the orthopedic surgeon treating the patient at follow‐up was unaware of the group to which the subject had been assigned.” |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Loss to follow‐up was low and balanced between the 2 groups (3/51 (5.9%) versus 4/52 (7.7%)). Worst‐case scenario analysis presented in the paper did not result in a important difference in outcome |
| Selective reporting (reporting bias) | Unclear risk | Retrospective trial registration and no published protocol. All outcomes listed in Methods reported; however, no recording of self‐reported function |
| Other bias: major imbalance in baseline characteristics | Low risk | Quote: “Patients in the two groups were similar in age, involvement of the physes, degree of angulation, percentage of displacement, and need for procedural sedation (Table 1).” |
| Other bias: performance bias | Unclear risk | Incomplete information on immobilisation post‐reduction. Casts applied by 1 of 2 emergency physicians or by orthopaedic residents |
| Other bias | Low risk | None detected |
Kropman 2010.
| Methods | Randomised trial Study period: September 2005 to October 2006 | |
| Participants | St Antonius Hospital, Nieuwegein, The Netherlands 92 children with impacted greenstick fracture of the distal radius or ulna Inclusion: impacted greenstick fracture, which comprised ⅓ of the distal radius or ulna, age between 4 and 13 years; signed informed consent Exclusion: complicated fractures or the necessity to reposition the fracture. Patients with a typical greenstick fracture Sex: 53 male (59% of 90) Age: mean 10 (range 4 to 12) years Fracture type (of 90): radius only (81: 90%), ulna only (1: 1%, by deduction); both radius and ulna 8 (9%) Assigned: 46 (bandage) / 46 (cast) Analysed: 44 (bandage) / 44 (cast) (at 6 weeks) |
|
| Interventions | 1. Soft bandage (layer of wool, which was covered with a layer of commercial cotton crepe bandage) supported by a sling. After 1 week, a tubigrip was placed for 3 weeks. The group participants were given verbal and written instructions on handling the bandage and removing the bandage for comfort only, or removing for desired activities, and discontinue completely when desired 2. Below‐elbow back‐slab cast. After 1 week, the cast was made circular and continued for another 3 weeks. The group participants were given the usual verbal and written cast‐care instructions (e.g. avoid getting the cast wet) All participants were instructed to avoid contact sports until 4 weeks after treatment. Participants were seen after 1, 4, and 6 weeks |
|
| Outcomes | Length of follow‐up: 6 weeks, also at 1 and 4 weeks Wrist function: not reported Complications: secondary angulation (none), refractures (none), change in treatment (conversion from bandage to cast at request of parents (within 1 week)), problems removing bandage Fracture displacement Pain Range of motion Discomfort (itching, neck pain, too heavy, too loose, too tight) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | Clarification provided in the text of the fracture population: "An impacted greenstick fracture, torus fracture, or buckle fracture is defined as a specific type of greenstick fractures in which the cortex has become impacted." Although 92 children were recruited, one child in each group was excluded early on because incorrect diagnosis: one had a contusion of the distal forearm and the other had a Salter Harris II fracture. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The enrolled patients were thereafter prospectively randomised between soft bandage and CT using a randomisation plan from www.randomization.com". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation scheme was obtained by using sealed envelopes containing the indication to BT or CT that were put into a container in 15 blocks (3 BT, 3 CT)." The envelopes were extracted by the physician in the emergency department. However, safeguards (the envelopes were not described as opaque) are not mentioned and it is possible that the sequence may have been partly predictable |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding not feasible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not feasible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Participant flow diagram provided but incomplete account of losses to follow‐up. The denominators of group participants for participant‐/parent‐recorded pain and discomfort were not provided. In particular the allocation of 5 participants whose VAS and discomfort form data were lost at 2 and 3 weeks was not provided. Intention‐to‐treat analysis was conducted including in the retention in the bandage group of 3 participants who switched from bandage to cast therapy |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Participant flow diagram provided and loss to follow‐up (1 excluded and 1 loss to follow‐up in each group) provided for these outcomes. (The 3 participants who switched from bandage to cast therapy were kept in the bandage group for analysis purposes) |
| Selective reporting (reporting bias) | Unclear risk | Trial registered prospectively in the Netherlands Trial Register. However, function was assessed only in terms of range of motion and the data for pain and discomfort were incompletely linked with the recording of these |
| Other bias: major imbalance in baseline characteristics | Low risk | Quote: “Between the two randomisation groups no statistical significant difference was found in the demographic data” |
| Other bias: performance bias | Unclear risk | No detail on expertise of healthcare personnel |
| Other bias | Low risk | None detected |
Levy 2015.
| Methods | Quasi‐randomised controlled trial Study period: February 2010 to November 2012 |
|
| Participants | Tripler Army Medical Center, Honolulu (likely), Haiwaii, USA 71 children with distal radius or distal both‐bone forearm fractures Inclusion: children aged 4 to 12 years, and distal radius fractures both with and without an associated distal ulna fracture. Informed consent Exclusion: fractures not requiring reduction, Salter Harris III/IV fractures, forearm fractures proximal to the distal radial metaphyseal‐diaphyseal junction, operative cases, open fractures, children with metabolic defects, pathologic fractures, or those with a previous fracture in the same location Sex: 43 male (61% of 71) Age: mean 8.7 (range 4 to 12) years Fracture type: radius only (28: 39%); both radius and ulna (43: 61%) Assigned: 34 (splint) / 37 (cast) Analysed: 33 (splint) / 36 (cast) (at 6 to 8 weeks) |
|
| Interventions | Fractures were manipulated and reduced by orthopaedic residents after appropriate analgesia or sedation or both were provided. Finger traps were used to assist with reduction at the discretion of the treating provider 1. Double‐sugar‐tong splint. The splint was overwrapped into an above‐elbow cast after a week (see Notes). The double‐sugar‐tong splint overwrap was changed to below‐elbow cast at 4 or 6 weeks 2. Above‐elbow bivalved cast. Cast changed to below‐elbow cast at 4 or 6 weeks (optional) Initial immobilisation used plaster for both the double‐sugar‐tong splint and above‐elbow cast groups. Application of a short‐arm portion first, versus uniformly applying an entire long‐arm cast, was performed at the preference of the provider. Casts were removed at 6 to 8 weeks. Fractures were remanipulated at the discretion of the treating provider if the alignment did not meet acceptable radiographic parameters |
|
| Outcomes | Length of follow‐up: 8 to 12 weeks, also 1, 2, 3, 4 weeks and after cast removal (6 to 8 weeks)
Function: not reported Loss of reduction, remanipulation required (criteria met ‐ unacceptable displacement ‐ for remanipulation) Complications: non‐union (none), surgical intervention required (none) Change of treatment: conversion from double‐sugar‐tong splint to long‐arm cast; conversion from plaster long‐arm cast to fibreglass long‐arm cast Radiological outcomes: sagittal alignment, coronal alignment, apposition, displacement |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | Authors acknowledge that they did not account for the layers of plaster used in making the initial casts or fibreglass used in overwrapping them | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Randomization was performed utilizing the last digit of the medical record number. Even numbers were randomised to a LAC and odd to the DSTS.” Quasi‐randomised – sequence was not random |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not feasible |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 2 participants (1 in each group) only included in analysis up to last follow‐up. Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | High risk | No trial registration or protocol. Functional outcomes not reported. Outcomes not well reported, including results at final follow‐up |
| Other bias: major imbalance in baseline characteristics | Low risk | Quote: “there were no differences between the 2 groups in terms of age, sex, anaesthesia, DR, or both‐bone fractures, or fracture type.” |
| Other bias: performance bias | Unclear risk | Variation in practice. For example, 13 orthopaedic junior residents were involved in the care of the trial participants, with no details on analgesia and/or sedation. Also the method of constructing the long‐arm cast (short‐arm portion first or entire cast was according to preference) and the numbers of layers of plaster for initial casts and splints and fibreglass subsequently were not accounted for |
| Other bias | Low risk | None detected |
McLauchlan 2002.
| Methods | Randomised trial Study period: May 1997 to October 1999 |
|
| Participants | Royal Hospital for Sick Children, Edinburgh, UK
68 with completely displaced metaphyseal fractures of the distal radius, with of without ulnar fracture
Exclusion: physeal injuries
Sex: 42 male (62%)
Age: mean 7.9 years, range 4 to 14 years Fracture type: both bones 60; "intact ulna" 8; 1 grade 1 open injury Assigned: 35 (wire) / 33 (cast only) Analysed: 34 / 31 (radiological review); 56 for clinical review (3 months) (see Notes) |
|
| Interventions | Reduction under general anaesthesia within 18 hours of admission, checked with image intensifier 1. Single percutaneous Kirschner wire introduced across the fracture to the radial side of Lister's tubercule. Then above‐elbow cast (probably plaster). Review at 3 weeks when wire removed and cast changed 2. Above‐elbow cast (probably plaster). Weekly radiological review for 3 weeks Casts removed between 4 and 6 weeks after injury, depending on age of child |
|
| Outcomes | Length of follow‐up: 3 months, also 3 weeks, and 4 to 6 weeks (at cast removal) Functional deficit Loss of position and secondary procedure Pain requiring early wire removal Prominant scarring Wire migration Malunion Residual pain Grip strength Range of motion (flexion, extension, radial and ulnar deviation, supination, pronation) Angular deformity |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | Paper did not provide the numbers of participants in the 2 groups available for clinical review Corrective osteotomy was performed at 6 months in 1 participant of the cast‐only group Small discrepancies between abstract and full reports of the trial 7 children whose parents refused consent for trial inclusion were treated conservatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “A sealed envelope was then opened to determine whether it was to be managed in a long‐arm cast alone or with an additional single percutaneous K‐wire.” (Opened after closed reduction) Insufficient information |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded. Obvious differences in the 2 groups |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Not blinded. Obvious differences in the 2 groups |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote: “At three months after injury assessment of the function of the wrist was carried out independently by the same physiotherapist (BC) to avoid interobserver error.” Quote: “Final radiographs, taken at the time of clinical assessment, were evaluated by one surgeon.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Not blinded. Including interim assessment of deformity |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | 56 returned for clinical review. The allocation of the 12 (18%) lost to follow‐up was not stated Paper did not provide the numbers of participants in the 2 groups available for clinical review |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | 56 returned for clinical review. The allocation of the 12 (18%) lost to follow‐up was not stated Additionally, radiological records were incomplete in 3 records – these were probably in addition to the 12 already missing from follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol or trial registration. Small discrepancies between abstract and full report (e.g. 8 cast‐only required a second procedure to correct deformity compared with 7 in full report) Incomplete reporting on function |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Insufficiently reported |
| Other bias: performance bias | Unclear risk | After enrolment “the patient was under the care of one of four consultations and any further management followed the consultant’s normal practice.” |
| Other bias | Low risk | No other sources of bias identified |
Miller 2005.
| Methods | Randomised trial for consenting participants (25), but a further patients enrolled and treated according to surgeon's preference (see Notes) Study period: June 1995 to July 1997 |
|
| Participants | Children's Hospital, Boston, MA, USA
34 children with closed displaced metaphyseal fractures of the distal radius. Aged 10 years or over. Angulation > 30 º or complete fracture displacement
Exclusion: open fracture, history of injury or surgery of the affected wrist, fractures requiring open reduction, swelling or neurovascular compromise precluding circumferential cast immobilisation. Skeletal maturity
Sex: male 31 (91%)
Age: mean 12.4 years, range 10 to 14 years Fracture type: displaced metaphyseal fractures; no information on ulna involvement Assigned: 16 (wire) / 18 (cast only) Analysed: ? / ? (25 followed up at mean 2.8 years) |
|
| Interventions | Closed reduction under general anaesthesia with fluoroscopic guidance 1. Percutaneous wire fixation. Small incision made over radial styloid. Wire directed proximally and ulnarly across fracture site engaging in opposite cortex. Optional second wire inserted through small dorsal incision. 6 participants (37.5%) required double‐pin fixation and 2 (12.5%) required transphyseal pin fixation.Then above‐elbow cast. Wires removed at 4 weeks 2. Above‐elbow cast. Above‐elbow cast comprised plaster cast overwrapped with fibreglass casting material All participants had above‐elbow cast for 4 weeks and then a further 2 weeks in a below‐elbow cast Repeat reduction was performed if participants showed > 25 º of angulation or complete loss of cortical contact at follow‐up |
|
| Outcomes | Length of follow‐up: 6 months (average 10½ weeks). Also 1, 2, 4 and 6 weeks (clinical evaluation and radiographs) Long‐term follow‐up: mean 2.8 years (numbers in each group not stated)
Long‐term pain, limitations in range of motion, strength, or activities (none noted) Long‐term neurovascular compromise, growth arrest or deformity (none noted) Fracture alignment (post‐initial treatment and change between weeks 1 and 4) Loss of reduction and secondary procedures Nerve hyperaesthesia Tendon (extensor carpi ulnaris) irritation Wire migration Pin‐site (wire‐site) infection Failed closed reduction Non‐union Permanent nerve damage Compartment syndrome Cost analysis (see Notes) |
|
| Funding and declarations of interest | Funding source: "None of the authors received financial support for this study." Declarations of interest: reported, none |
|
| Notes | Separate data were not provided for the 9 children treated according to the surgeon's preference. Discrepancies between the 2 groups in initial dorsal angulation and shortening may have reflected some bias in the surgeon preference group A retrospective cost analysis was based on charges for operating room, anaesthesia services, orthopaedic surgery, office visits, radiology, plaster cast services | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Patients who agreed to study participation were randomised to a treatment group via the drawing of sealed envelopes. Patients who declined participation were treated according to the preference of the attending pediatric orthopaedic surgeon on call. Of the eight participating surgeons, four treated their patients primarily by closed reduction and casting, the other four primarily by pin fixation.” Separate data not available for the 25 properly randomised participants; thus, based this on the, at best, quasi‐randomised allocation for the other 9 participants |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Not blinded – interventions obvious |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Limited subjective outcomes at long‐term follow‐up. Unlikely to be affected at long‐term follow‐up |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not blinded but criteria stated for displacement; other complications would have been self‐evident Long term was probably ‘low risk’ |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Just applies to the long term (2.8 years). However, loss to follow‐up (9: 26% of 34) of the already mixed population, with no separate denominators for the 2 groups |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Mixed population of randomised and non‐randomised. Although no losses to follow‐up, the flawed data handling is the key issue |
| Selective reporting (reporting bias) | High risk | No trial registration or published protocol. No explicit functional recovery data. The merging of data from the randomised and non‐randomised groups is inappropriate, even though it appears to have been approved by ethics |
| Other bias: major imbalance in baseline characteristics | High risk | Differences in the displacement: pinning lower dorsal angulation (17 º versus 30 º) but less shortening (6.9 versus 16.4 mm) Quote: “more patients in the pinning group had bayonet apposition of the fracture site; this reflected the element of the randomisation process based upon surgeon preference.” Bayonet apposition is where the 2 fracture fragments lie next to each other rather than in end‐to‐end contact |
| Other bias: performance bias | Unclear risk | No mention of expertise of clinicians |
| Other bias | Low risk | The issues relating to the inappropriate use of non‐RCT data are already covered in the above ratings |
Oakley 2008.
| Methods | Randomised controlled trial Study period: March 2002 to March 2003 |
|
| Participants | The Royal Children’s Hospital, Melbourne, Australia 95 children with torus fractures of the distal forearm Inclusion: patients up to the age of 18 years presenting to the ED with a torus fracture of the distal radius or ulna or both Exclusion: other upper limb injury, other serious injury, English was inadequate to complete the patient diary Sex: 54 male (64% of 84) Age: mean 8.5 years (9 months to 15 years) Fracture type: radius only (71); both radius and ulna (13) Assigned: 48 (fibreglass) / 47 (plaster cast) Analysed: 42 / 42 |
|
| Interventions | 1. Fibreglass volar slab (Dynacast Prelude (Smith + Nephew) volar slab). The slab was secured with an elasticised bandage. Participants were advised that they could remove the slab for periods to use or clean the arm if desired 2. Encircling (full) below‐elbow plaster‐of‐Paris cast All participants were placed in a broad arm sling and given information on home care of the plaster or slab Radiological diagnosis was confirmed with a radiologist within 24 hours. Clinical review at 12 to 16 days post‐application of plaster by one of the investigators. The plaster or slab was removed, and an X‐ray was performed. If there was minimal or no tenderness and an acceptable position on X‐ray, the arm was mobilised. If significant tenderness or discomfort remained, the arm was re‐immobilised in the same type of immobilisation with a review in the ED for a further 2 weeks. If there was displacement of the fracture, the participant was referred for orthopaedic review |
|
| Outcomes | Length of follow‐up: 4 to 6 weeks, also 12 to 16 days post‐application, and telephone review at 2 weeks post‐immobilisation Function (at 1 to 2 days) and return to normal activity (at 2 weeks) Complications: plaster or slab problem, replaced, removal; medical attention sought at 2 to 5 days post‐immobilisation; displaced fractures; re‐immobilisation at 2 weeks Pain: VAS (0 to 100 mm; worst pain), duration of pain (days); > 6 days of pain; pain post‐immobilisation; medication use (days) Duration of immobilisation (days) Participant time off school or day care; parental time off work (at 2 weeks) Satisfaction: happy to use the same method in future (at 2 weeks) |
|
| Funding and declarations of interest | Funding source: not stated, however, the authors acknowledge the donation of the Dynacast Prelude material used for the volar slab by Smith and Nephew Declarations of interest: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was by opaque envelope, and the sequence was generated by a computer program in blocks of 6.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was by opaque envelope, and the sequence was generated by a computer program in blocks of 6.” Insufficient details of safeguards |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “None of the researcher, clinician, patient, or family was blinded to the intervention.” |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: “None of the researcher, clinician, patient, or family was blinded to the intervention.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: “None of the researcher, clinician, patient, or family was blinded to the intervention.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: “None of the researcher, clinician, patient, or family was blinded to the intervention.” |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | 12% loss to follow‐up; only a small difference between groups: 12.5% versus 10.6%. Some worst‐case/best‐case analysis reported in the trial report found reduction in effect sizes for pain scores; and duration of pain |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Only a small difference between groups: 12.5% versus 10.6%. Unlikely that participants with bad outcomes would have been lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Trial registration was retrospective. No published protocol. Various outcomes not reported (e.g. VAS for function) or reported in different ways (e.g. pain) than described in Methods. Trial registration refers to “minimally displaced greenstick fractures” |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics were provided only for the 84 followed up. Some imbalance in initial pain could have affected results |
| Other bias: performance bias | Unclear risk | Care provision seemed comparable. No information on care provider expertise |
| Other bias | Low risk | None detected “The authors thank Smith and Nephew for the donation of the Dynacast Prelude material used for the volar slab.” We anticipate that this did not affect the results |
Paneru 2010.
| Methods | Randomised controlled trial Study period: June 2007 to May 2008 |
|
| Participants | Emergency or outpatient at Hospital, Nepal 89 children with displaced distal forearm fractures, aged between 4 and 12 years. Not explicitly stated in inclusion criteria but restriction to both‐bone fractures implied in report Exclusion: open fractures, previous manipulations Sex: 66 male (78% of 89) Age: mean 8.4 years Fracture type: combined radius and ulna Assigned: 45 (below‐elbow)/ 44 (above‐elbow) Analysed: 43 / 42 (at 6 to 8 weeks?) (see Notes) |
|
| Interventions | Closed reduction under analgesia and sedation 1. Below‐elbow plaster cast 2. Above‐elbow plaster cast. Below‐elbow cast applied and moulded, then extended above the elbow Next day, inspection of swelling of hand and fingers and distal neurovascular assessment Instructions to participants and family on strict arm elevation for first 24 to 48 hours and advice on warning signs for consultation. Cast duration not stated, probably 6 to 8 weeks dependent on detection of union. Physical therapy was prescribed for elbow stiffness at 8 weeks follow‐up |
|
| Outcomes | Length of follow‐up: 6 months; also at 1 day, and 1, 2, 4, 6, 8 and 12 weeks post casting Function: No patient‐reported outcome measure of function reported Need for remanipulation (lost reduction in cast; prespecified criteria) Swelling: associated with pain or movement limitation at 1 week Pain at 1 week (VAS) Wrist and elbow mobility after cast removal (6 to 8 weeks) Days missed at school Complications: plaster reinforcement or cast change required, stiff elbow requiring physical therapy Radiological outcomes: time to fracture union, translation, angulation and overriding (6 to 8 weeks) Direct costs of treatment |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | 4 participants were lost to follow‐up (2 from each group). It is not clear whether this loss also applied to later follow‐ups JE emailed R Rijal 8 March 2017 to ask:
JE sent follow‐up email to R Rijal 11.04.17. No response received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Random allocation of the patient was done on the basis of a computer‐based random number generation technique.” |
| Allocation concealment (selection bias) | Unclear risk | No details of safeguards for allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding not possible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Not blinded. Although efforts made to standardise criteria for requiring reduction and for fracture union |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Loss to follow‐up was small and equally distributed in both groups (2 vs 2) |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Loss to follow‐up was small and equally distributed in both groups (2 vs 2) |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol. Also, no data for 6‐month follow‐up. Otherwise, no indication of reporting bias |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Time since injury to manipulation was statistically significantly different but amounted to a mean difference of 1.6 hours less in the below‐elbow group Data not provided for 4 children, 2 in each group, who were lost to follow‐up |
| Other bias: performance bias | Unclear risk | Cast index equivalent in both groups, values showing adequate cast moulding done during cast application No information on clinicians or experience of clinicians applying the cast |
| Other bias | Low risk | No concerns |
Plint 2006.
| Methods | Randomised controlled trial Study period: August 2002 to September 2003 |
|
| Participants | Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada 113 children with a distal radius or ulna buckle fractures or both, attending emergency department Inclusion: Children aged 6 to 15 years who presented to the ED with a buckle fracture of the distal radius or ulna. (A buckle fracture was defined as compression of the bony cortex with the opposite cortex intact and confirmed by a paediatric radiologist.) Written informed consent and assent from parents and children Exclusion: Children with another fracture of the same limb requiring immobilisation, fractures of both wrists, evidence of metabolic bone disease, a language barrier, or who lived outside the hospital catchment area Sex: 57 male (66% of 87) Age: mean 9.72 years Fracture type: radius only (80) and radius and ulna (7) Assigned: 57 (splint) / 56 (cast) Analysed: 42 / 45; 34 / 41 (6 months) |
|
| Interventions | 1. Removable splinting. Individually fitted plaster split (composed of 12 plaster layers) that was attached with a tensor bandage
Splint applied by research assistant or ED medical staff. Participants in the splint group were given verbal and written instructions to use the splint for comfort only, remove as desired for activities, and discontinue completely when desired 2. Below‐elbow (short arm) plaster cast. Participants in the cast group were given the usual verbal and written cast‐care instructions (e.g. avoid getting wet, etc) Initial diagnosis made by the emergency physician who referred the participant to the study. Radiographs were reviewed by a paediatric radiologist within 24 hours but sometimes after study inclusion. All of the participants were instructed to avoid contact sports (such as competitive hockey) until clinic follow‐up. All of the participants were asked to return to the orthopaedic clinic at 21 days after injury for assessment and cast removal |
|
| Outcomes | Length of follow‐up: 6 months (telephone and hospital charts reviewed), also 21 days, and questionnaires at 7, 14, 21 and 28 days Function: Modified ASK‐P (includes 8 extra questions related to upper limb functioning) Refracture: 6 months Complications Pain: VAS score (0 to 100; worst pain): questionnaire at 7, 14, 21 and 28 days Length of immobilisation Preference for splint in future for same injury: asked at 28 days Difficulties in performing different activities: moderate or severe difficulty at 7, 14, 21, 28 days Return to regular sporting/physical play activities |
|
| Funding and declarations of interest | Funding source: supported by a grant from the Physician Services Incorporated Foundation Declarations of interest: reported, no financial relationships relevant to the article to disclose |
|
| Notes | As described above, radiographs were reviewed by a paediatric radiologist within 24 hours but sometimes after study inclusion. The study protocol set out an a priori intention "to withdraw patients from the study who were subsequently determined to have a fracture other than a buckle fracture". Of the 16 children removed from the study for this reason, the 7 assigned to a splint had their treatment changed to a cast, whereas the 9 assigned to a cast remained in a cast | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequence was computer generated with a block size of 4.” Random sequence generated |
| Allocation concealment (selection bias) | Low risk | Quote: “Initially, a Web‐based allocation program was used for group allocation. However, because of problems with timely access to the program, sealed opaque envelopes containing the group assignment were used from November 2003 [2002?] onward. The research assistant accessed either the Web‐based allocation program or used the next envelope to determine to which group the patient was assigned.” Independent allocation. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding practical |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding practical |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: “The research assistant making the telephone follow‐up calls was not blinded to the group intervention, because they needed to ask about splint usage depending on patient group assignment.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not blinded but less likely these outcomes would be affected |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | As well as exclusions (mainly non‐buckle fractures) and losses from follow‐up, there were losses in functional data reported. These differed between the 2 groups. Thus ASK‐P at 28 days: losses 51% (29/57) versus 34% (19/56) |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | While exclusion for non‐buckle fractures (16) was comparable between the 2 groups, the losses were high and different between the 2 groups (15/57 (26%) versus 11/56 (20%)) |
| Selective reporting (reporting bias) | Low risk | No trial registration or protocol available. However, all outcomes, including function, recorded were reported |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics appeared adequately balanced for those included in the analysis but were missing for 26 randomised participants. (Notably, the participants excluded for non‐buckle (greenstick fracture) were balanced between the groups: 7 versus 9) |
| Other bias: performance bias | Unclear risk | Insufficient information on which to judge clinical expertise of the treatment providers. However, both groups received similar care, including written instructions |
| Other bias | Low risk | None detected |
Pountos 2010.
| Methods | Randomised controlled trial Study period: dates not reported but described as "Over a period of ten months." |
|
| Participants | University Hospitals of Coventry and Warwickshire, Coventry, UK 90 children with undisplaced greenstick or buckle fractures of the distal radius Inclusion: Children aged 0 to 16 years sequentially attending treatment centre, who had sustained a minimally angulated greenstick or torus fracture of the distal third of the radius, as confirmed by either X‐ray or ultrasound. The term ‘‘minimally angulated’’ was defined as a complete absence of any discernible clinical deformity, which on a plain X‐ray would be less than 10 º of angulation in any plane. Exclusion: none reported Sex: 47 male (59% of 79) Age (of 79): mean 9 (range 2 to 16) years Fracture type: undisplaced greenstick and buckle Assigned: not reported (90 in all) Analysed: 26 (splint) / 29 (Tubigrip) / 24 (cast) |
|
| Interventions | 1. Futuro wrist splint 2. Bandage: double Tubigrip 3. Plaster of Paris cast (below‐elbow implied) All received plain X‐ray in A&E department and, as part of the study, an ultrasound scan within 2 to 3 days (mean 1.4 days) of injury. Once applied, the parent and child were given advice, and a follow‐up appointment within 4 to 6 weeks |
|
| Outcomes | Length of follow‐up: 12 weeks, also 4 to 6 weeks Function: final appointment at 12 weeks to check return to full function (not confirmed) Paediatric disability score (0 to 10; worst outcome): interference with play; help needed with feeding; help needed with washing and dressing; sleep disturbance; missed days of school Pain during device use (VAS score of 0 to 10; worst pain); data calculated from histogram Analgesic use during 4 to 6 weeks (none, occasional, regular) Increase in deformity at 4 to 6 weeks Grip strength at 4 to 6 weeks Stiffness at 4 to 6 weeks Radiologically visible signs of healing |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | JE emailed Dr Pountos 12 September 2017 for the following data: 1. With regards to randomisation, were there any safeguards in place to ensure that only one attempt at sweet selection was possible? 2. How many patients were randomised into the 3 groups? 3. How many had torus fractures in each group: (a) At randomisation? (b) At follow‐up? 4. Who applied the interventions? 5. Was written information provided to all groups? 6. Were the patients / parents advised that they could remove the Futuro splint or Tubigrip and how to reapply these? 7. What type / types of casts were applied (e.g. were these all below‐elbow casts)? No response received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The child was offered an opaque pot containing equal numbers of sweets of three different colours in such a way that they could not see what colour they were picking. Each colour was a code for the treatment that would be applied to the child, be it a plaster cast, a Futuro wrist splint, or a double Tubigrip." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The child was offered an opaque pot containing equal numbers of sweets of three different colours in such a way that they could not see what colour they were picking. Each colour was a code for the treatment that would be applied to the child, be it a plaster cast, a Futuro wrist splint, or a double Tubigrip." Unclear safeguards to second attempts at picking a sweet. Trial described as being “randomised (single blindly)”. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Not blinded. Most of these outcomes were not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: “At the follow‐up appointment, a single observer carried out a clinical and radiological assessment after the treatment device had been removed in a separate room and the child had been sent for an X‐ray.” Most of these outcomes were not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | 11 participants were lost to follow‐up due to non‐attendance at the follow‐up clinic; no report of which groups they were in. There was an equal number of coloured sweets for selection and so if the numbers were balanced at randomisation, there is a possibility of important differences in attrition rates in the 3 groups (i.e. 4 from splint, 1 from tubigrip and 6 from cast) |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | 11 participants were lost to follow‐up due to non‐attendance at the follow‐up clinic. It is not reported which groups they were in but (see above) possibly 4 from splint, 1 from tubigrip and 6 from cast Most of these outcomes were not reported |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol No adverse events reported. Results of the author’s ‘paediatric disability score’ were also inadequately reported and inappropriately analysed |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Balance characteristics in the 3 groups, even for those available at follow‐up, were not reported |
| Other bias: performance bias | Unclear risk | Details of healthcare professionals applying immobilisation device were not reported |
| Other bias | Low risk | None detected |
Schulte 2014.
| Methods | Randomised controlled trial Study period: June 2008 to September 2009 |
|
| Participants | Department of Emergency Medicine, University Children’s Hospital, Zurich, Switzerland 40 children with displaced distal radius fracture (out of 100 children with displaced closed forearm fractures needing reduction) Inclusion: Children younger than 16 years presenting to the emergency department with a closed fracture of the forearm needing reduction; Informed consent obtained Exclusion: Pre‐existing ailments such as skin infection of the affected limb, buckle fractures, compound fractures, fractures needing open reduction or wire fixation, and pathologic fractures Sex: 28 male (70% out of 40) Age: mean 9.1 years (for whole study population) Fracture type: distal forearm (40, of which 12 involved growth plate); number of both‐bone fractures not known but 52 of the 100 children in study had both‐bone fractures Assigned: 17 (split) / 23 (closed) Analysed: 17 (split) / 23 (closed) |
|
| Interventions | Standardised closed reduction in ED (performed or supervised by senior emergency physician) 1. Split circumferential synthetic semi‐rigid above‐elbow cast. According to the protocol, the casts were split using cast scissors or cast saw or both; time of sedation extended for this group 2. Closed circumferential synthetic semi‐rigid above‐elbow cast Participants had casts on for 4 weeks. Radiological diagnosis was confirmed with a radiologist within 24 hours. All cast applications and manipulations were performed by specialised casting nurses. All participants and their parents were given standardised post‐op instructions and analgesia. Cast removal was performed with a cast scissor or saw or both at the discretion of the casting nurse |
|
| Outcomes | Length of follow‐up: 4 or 6 weeks (or 3 months if delayed union); also on day 1, 5 and 10 Function not reported Fracture redisplacement requiring surgery Secondary splitting due to reversible lymphoedema Cast‐related soft tissue problems: compartment syndrome, neurovascular compromise, saw burns, skin breakdown (< 2 cm2) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | JE emailed Daniel Garcia 21.06.17 for additional data. Garcia replied 27.06.17 with data. Upon a further request by JE for data on relevant secondary displacement necessitating surgical treatment for split and closed cast, Garcia confirmed on 09.08.17 that they had "3 secondary surgeries regarding distal fractures in the closed cast group and 2 in the split cast group". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was generated by a computer in blocks of 10 |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was performed by sealed opaque envelope” There is no mention of sequential numbering of the envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "Apart from the radiologist assessing the fracture alignment, none of the other researchers, clinicians, patients or families were blinded to the intervention" |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "Apart from the radiologist assessing the fracture alignment, none of the other researchers, clinicians, patients or families were blinded to the intervention" Fracture alignment was measured by a blinded radiologist |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published/protocol. Separate data for DRF obtained from authors. However, no reporting of child function or recovery |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Quote: “Demographic and fracture characteristics were similar in both groups” (Table 1) However, little data for distal radius fractures, with an imbalance between groups in the sexes: 17/17 (100%) male in the split cast group and 11/23 (48%) in the closed cast group |
| Other bias: performance bias | Unclear risk | Quote: “All casts applications and manipulations were performed by specialized casting nurses.” Quote: “Cast removal was performed with a cast scissor and/or saw at the discretion of the casting nurse.” No data on cast index. Insufficient information reported |
| Other bias | Low risk | None detected |
Silva 2016.
| Methods | Randomised controlled cross‐over trial (cross‐over at 2 weeks) Study period: October 2014 to January 2015 |
|
| Participants | Orthopaedic Institute for Children, Los Angeles, California, USA 27 children with non‐displaced or minimally angulated (< 15 °) fracture of the distal radius Exclusion: skeletally mature patients (closed physis), any associated generalised condition that affected the forearm or wrist range of motion, history of injury or surgery to the affected or contralateral forearm or wrist, open fractures, neurovascular abnormalities or suspicion of a compartment syndrome, or established skin irritating conditions (i.e. eczema) Sex: 15 male (58% of 26) Age: mean 9.4 years, range 6 to 13 years Fracture type: nondisplaced or minimally angulated (< 15 °) fracture of the distal radius (23 buckle and 3 greenstick fractures) Assigned: 12 (+1?) (waterproof cast) / 14 (+1?) (traditional fibreglass cast) (see Notes) Analysed: 12 / 14 (at 2 weeks before cross‐over) |
|
| Interventions | At Urgent Care facility, cast applied after radiographs 1. Waterproof cast: below‐elbow cast made of the waterproof hybrid mesh material with a waterproof skin protector. Participants with the waterproof cast were asked to shower and get the waterproof cast as wet as they desired 2. Non‐waterproof cast: below‐elbow cast of traditional fibreglass material with a non‐waterproof lining material. Participants with the traditional cast were asked to keep it dry Participants returned for a clinical and radiological evaluation 1 week after cast application to ensure that no further displacement of the fracture had occurred and to evaluate the overall level of comfort The cross‐over (casts were replaced by the alternative cast) took place at 2 weeks post‐cast application, at which time a new clinical and radiological evaluation was performed. Cast removal was achieved in both groups using a cast saw and standard techniques. No physical therapy was prescribed to any participant. After cast removal (4 weeks), all participants were advised to avoid physical education, contact sports, and strenuous activities until week 8 to avoid refractures |
|
| Outcomes | Length of follow‐up: 8 weeks, also 1, 2 and 4 weeks (but only data from the first 2 weeks were evaluated for this review) Function: Activities Scale for Kids – Performance (ASK‐P) (questionnaire) Redisplacement Complications or non‐routine cast changes; skin changes at the time of cast removal Pain: Faces Pain Scale – Revised version Itching (VAS) Participant satisfaction with the treatment at cast removal (survey) Costs (comments) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | 1 participant was excluded because they sought care at a different facility after the initial cast immobilisation. JE sent an email to Dr Silva 11.04.17 asking: 1. Please could you tell us the mean age, the number of males/females, and the fracture types in each group? Allocated waterproof cast (n = 12). Mean age: ; number of males: ; number of buckle fractures: Allocated non‐waterproof cast (n = 14). Mean age: ; number of males: ; number of buckle fractures: 2. Did you provide any advice on drying the waterproof cast; for example, after showering? 3. Please can you provide the standard deviations for the 2 groups at Week 0‐2 for the following: ‐ Total ASK‐P score. Waterproof cast: ; Nonwaterproof cast: ‐ Pain score. Waterproof cast: ; Nonwaterproof cast: ‐ Itchiness score. Waterproof cast: ; Nonwaterproof cast: ‐ Patient satisfaction. Waterproof cast: ; Nonwaterproof cast: 4. Please could you provide the cost of each type of cast: Waterproof cast (HM cast): Nonwaterproof (Scotchcast): No response received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed using sealed, sequentially numbered envelopes, in which the randomisation sequence was concealed.” |
| Allocation concealment (selection bias) | Low risk | Quote: “single‐center, randomised, controlled, cross‐over” Quote: “Randomization was performed using sealed, sequentially numbered envelopes, in which the randomisation sequence was concealed” This applied to first 2 weeks; after this treatment was crossed over to the other cast |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of personnel, children or parents not possible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding of personnel, children or parents not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The identification of skin changes was performed by an independent observer, who was unaware of the type of cast that had been removed, by analysis of digital photographs of the front and back of the forearm" No blinding for the other aspects but only 2 weeks data used in the review |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | 1 participant (group not identified) excluded as they sought care elsewhere. Unlikely to have impacted on the results |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 1 participant (group not identified) excluded as they sought care elsewhere. Unlikely to have impacted on the results |
| Selective reporting (reporting bias) | Unclear risk | Although prospectively registered, the cross‐over study design was inappropriate and has meant that the follow‐up data available for this review was curtailed to 2 weeks |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Separate data for the 2 initially allocated groups were not reported |
| Other bias: performance bias | Low risk | Quote: “All casting procedures were performed by the on‐call orthopedic staff member, with the assistance of an experienced cast technician” |
| Other bias | Unclear risk | The cross‐over design of the trial may have influenced the results, even at 2 weeks |
Stevenson 2013.
| Methods | Randomised controlled trial Study period: May 2010 to June 2011 |
|
| Participants | Women’s and Children’s Hospital, Adelaide, Australia 105 children with minimally displaced traumatic distal radius fracture (metaphyseal and physeal) Exclusion: open injuries Sex: 63 male (60% of 105) Age: mean 10 years (range 3 to 17 years) Fracture type: minimally displaced distal radius fracture ("mixture", including metaphyseal and Salter Harris II fractures) Assigned: 51 (Wet or Dry®)/ 54 (Delta Dry®) Analysed: 51 / 54 (at cast removal) |
|
| Interventions | All participants had an initial above‐elbow slab applied in the emergency department before referral to the fracture clinic 1. Below‐elbow cast with Wet or Dry® undercast padding 2. Below‐elbow cast with Delta Dry® undercast padding The synthetic below‐elbow cast was made of 3M ScotchcastTM plus fibreglass. A single senior cast technician applied all casts. Participants and their parents were provided with written instructions advising and encouraging water exposure. Cast was removed by a clinical nurse. Duration of cast use: around 5 weeks |
|
| Outcomes | Length of follow‐up: at cast removal (around 5 weeks) Function: not reported Complications: adverse events; skin complications Satisfaction: participant and caregiver; cast technician Outcome assessment was questionnaire‐based, with individual components (e.g. comfort) scored on a 3‐ or 5‐point scale: Participant/caregiver report at cast removal (questionnaire): comfort, weight, itchiness, hot and sweaty, smell, water resistance, time to dry, overall satisfaction Technician‐reported outcomes (questionnaire):
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | Additional information and data received from Georgia Antonio 28.04.17 1. Types of distal radius fracture: "Not specified ‐ a mixture of distal 1/3 radius, metaphyseal and Salter Harris II fractures. There was no discrimination of type of fracture and type of cast liner." 2. Age range in the two groups: Wet or Dry cast liners: 3 to 16 years; Delta Dry cast liners: 4 to 17 years 3. Duration of cast use: "Not recorded for individual cases however the forearm fracture pathway dictated 5 weeks (give or take a few days depending on clinic appointment times/public holidays etc). There was no differentiation in times due to padding type used." 4. Categorical outcome data given in Appendix 6. Iinteringly, the company instructions for Wet or Dry® liner are contrary to those advised in the trial: multimedia.3m.com/mws/media/378580O/scotchcast‐wet‐or‐dry‐cast‐padding‐clinician‐sheet.pdf From instructions: “It is not recommended that an infant get a cast wet since it is difficult to drain water from an infant’s cast.” “After wetting, most casts feel dry within one to three hours.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Two hundred allotments with equal numbers of both interventions were made and placed in unmarked envelopes in a cardboard box in a random sequence.” Not clear how the random sequence was generated but probably OK as it was then supplemented by ‘random’ selection of an envelope |
| Allocation concealment (selection bias) | Low risk | Quote: “Opaque envelopes were used”; “After consent and recruitment, the plaster technician randomly picked an envelope from the box and the listed intervention was assigned.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Personnel and participants were not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Personnel and participants were not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | There was no blinding for these outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: “Following cast removal by a clinical nurse, an assessment of skin condition was completed by the cast technician. The cast technician assessing the skin condition was, thus, blinded to the type of liner; however, the authors are aware that the pattern of liner on the skin may have indicated the type of padding used, thus, inducing some observer bias.” The effectiveness of the blinding is not certain |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | There was no loss to follow‐up overall and there were complete data for subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | There was no loss to follow‐up overall and there were complete data for the reported objective outcomes, except for duration of exposure of cast to water |
| Selective reporting (reporting bias) | High risk | No trial registration or published protocol. Non‐validated measures used; incompletely reported results with no numbers reported for individual categories. Child function not reported |
| Other bias: major imbalance in baseline characteristics | Unclear risk | No quantification of types of fracture. Otherwise, baseline characteristics seemed balanced |
| Other bias: performance bias | Unclear risk | Quote: “A single senior cast technician applied all casts.” Discussion: “The possibility of bias due to prior experience of the technician and the learning curve is inherent to the study design.” |
| Other bias | Unclear risk | Quote: “A sample size of convenience was taken, with a total of 105 patients.” While an interim analysis was planned at 100 participants, data‐driven stopping may be a source of bias |
Symons 2001.
| Methods | Randomised trial Study period: September 1997 to May 1998 |
|
| Participants | Leicester Royal Infirmary, Leicester, UK 87 children with buckle fractures presenting to A&E department Exclusion: pathological fractures, previous problems with the wrist on the side of the fracture, bicortical fractures, did not understand or unwilling to enter the study Sex: 47 male (59% of 80) Age: mean 9.2 years Fracture type: buckle fractures; associated symptoms (of 80): deformity (2), moderate or severe swelling (51), immediate severe/mild or moderate (63 / 17) Assigned: 40 (home) / 47 (hospital) Analysed: 38 / 42 (at 6 weeks follow‐up) |
|
| Interventions | All received a below‐elbow backslab for 3 weeks 1. Home cast removal on given date 3 weeks after injury. To aid safe removal by parents at home, backslabs were applied, dried and cut but not removed, and then rewrapped with a bandage by the nursing staff. This procedure was watched by the attending parent and clear explanation of removal of backslabs was given. Emphasis on returning if problems or concern regarding their child's fracture 2. Hospital cast removal on return to fracture clinic 3 weeks after injury by nursing staff Reviewed at 6 weeks and discharged if there were no adverse clinical features |
|
| Outcomes | Length of follow‐up: 6 weeks Function: difficulty with writing / ADL; avoiding some hobbies Complications: swelling; tenderness; deformity Range of movement (wrist and forearm: dorsiflexion, palmar flexion, radial deviation, ulnar deviation, pronation, supination) Problems with their child's fracture care Had backslab remained supportive for 3 weeks? Complaints and feedback (e.g. would have liked a spare bandage to care better) Treatment difference from planned Parent and child satisfaction (VAS) Future choice of home or hospital removal (VAS scale 0 to 6) Bone healing |
|
| Funding and declarations of interest | Funding source: no mention Declarations of interest: reported, none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomised either to home (study) or hospital (control) groups using a computer‐generated random‐number sheet.” Random sequence |
| Allocation concealment (selection bias) | High risk | Quote: “a computer‐generated random‐number sheet.” No mention of allocation concealment. Seems to be an open list, hence not concealed |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding; clear difference between interventions |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding; clear difference between interventions |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Although slightly fewer home participants were lost to follow‐up at 6 weeks (2 (5%) vs 5 (11%)), it is unlikely that these would not have returned if there had been problems Incomplete reporting, e.g. no data for satisfaction |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Although slightly fewer home participants were lost to follow‐up at 6 weeks (2 (5%) vs 5 (11%)), it is unlikely that these would not have returned if there had been problems Some lack of clarity on the definition of outcome measures |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration. Some incomplete reporting of results but it does not appear to be selective reporting |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics were balanced for the 80 reviewed at 6 weeks; but missing for the 7 who did not attend |
| Other bias: performance bias | Unclear risk | Additional demonstration of the backslab procedure given to home‐removal parents. No mention of expertise of those applying the casts. However, similar follow‐up |
| Other bias | Low risk | None noted |
Webb 2006.
| Methods | Quasi‐randomised trial Study period: April 2002 to December 2003 |
|
| Participants | The Women’s and Children’s Hospital of Buffalo, Buffalo, New York, USA 127 children with displaced fractures of the distal third of the forearm Exclusion: age under 4 years, open fracture, pathologic fracture, a refracture through pre‐existing fracture lines, closed physes Sex: 85 male (75% of 113) Age: mean 9.8 years, range 4 to 16 years Fracture type: partially or completely displaced fractures of radius only (49 including 17 physeal fractures) or combined radius and ulna (64); 23 complete radius fractures Assigned: 63 (below‐elbow) / 64 (above‐elbow) Analysed: 53 / 60 (at 8 to 10 weeks); 104 (92%) were followed up at 7.7 months (see Notes) |
|
| Interventions | Manipulation and reduction (manual method) by orthopaedic resident at emergency department with analgesia and sedation provided. The hand was held by an assistant while a circumferential plaster cast was applied; if assistant not available fingertraps were applied but the arm was not suspended until after manipulation 1. Below‐elbow plaster cast 2. Above‐elbow plaster cast: The short‐arm portion was applied first and moulded and then the plaster was extended above the elbow Strict elevation for first 24 to 48 hours. First follow‐up visit at 7 to 10 days; with the intention of a remanipulation under general anaesthesia if unacceptable alignment. At 4 weeks, cast was removed if radiological and clinical evidence of healing and participants instructed to perform range‐of‐motion exercises at home. Otherwise, casts left in place for another 2 weeks but above‐elbow casts were cut down to below‐elbow casts. Clinical examination at 8 to 10 weeks and physical therapy prescribed if restricted mobility |
|
| Outcomes | Length of follow‐up: mean 7.7 months (3.5 to 11 months) (telephone interview); also at 7 to 10 days, 4 weeks and 8 to 10 weeks (questionnaire on impact of cast on ADLs) Function: ADL during cast use (questionnaire at 8 to 10 weeks) Redisplacement (lost reduction in cast) and remanipulation (some criteria reported but not clear if applied) Duration in cast Complications: refractures (none); stiff elbow requiring physical therapy Range of elbow and wrist motion (cast removal around 6 weeks and 8 to 10 weeks) Time to regain range of motion (questionnaire at 8 to 10 weeks) Days missed school Radiological outcomes: displacement, angulation, deviation |
|
| Funding and declarations of interest | Funding source: "The authors did not receive grants or outside funding in support of their research for or preparation of this manuscript." Declarations of interest: reported, none |
|
| Notes | Of 10 children in the below‐elbow cast group excluded from the analyses, 7 were lost to follow‐up and 3 were excluded because of surgery. Of 4 children in the above‐elbow cast group excluded from the analyses, 3 were lost to follow‐up and 1 was excluded because of surgery. No results explicitly provided for the 104 participants (numbers in each group not reported) followed‐up via telephone interview at 7.7 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote; “Patients were then randomised to be treated with either a short or a long arm cast on the basis of whether the last digit of their medical record number was odd or even.” Quasi‐randomised: sequence generation is not random |
| Allocation concealment (selection bias) | High risk | Quote: “Patients were then randomised to be treated with either a short or a long arm cast on the basis of whether the last digit of their medical record number was odd or even.” Predictable sequence: no allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants, their parents and care providers not practical |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding of participants, their parents and care providers not practical |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Differences between the 2 groups in losses (exclusions and losses): below‐elbow 10/63 (16%) versus above‐elbow 4/64 (6%) Additionally, greater losses relating to missing questionnaire responses at 10 weeks, e.g. losses for difficulties with ADLs were 14/63 (22%) versus 6/64 (9%) |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Differences between the two groups in losses (exclusions and losses): below‐elbow 10/63 (16%) versus above‐elbow 3/64 (5%). Additionally, greater losses relating to missing questionnaire responses at 10 weeks |
| Selective reporting (reporting bias) | High risk | No trial registration or published protocol. Insufficent details of the ADL questionnaire at 10 weeks and no details of telephone interview in Methods or Results |
| Other bias: major imbalance in baseline characteristics | Unclear risk | Baseline characteristics only provided for 113 participants in the analysis, not the 127 randomised participants No major imbalances (upon statistical testing) but notably more radius‐only fractures in the below‐elbow group (27/53 (51%)) than in the above‐elbow group (22/60 (37%)), and thus conversely more combined radius and ulna fractures in the above‐elbow group. This distribution might reflect some selection bias. This issue was highlighted in a letter commenting on this trial (Kumar 2006) |
| Other bias: performance bias | Unclear risk | All of the “orthopaedic residents,… had been fully trained in the proper application of plaster casts.” In Discussion: Quote: "Our casts were all applied by orthopedic residents in their third or fourth year of training, with varied amounts of experience in pediatric orthopaedics. There is a learning curve in the application of a well‐molded cast, and the majority of poorly molded casts were applied by residents early in their pediatric orthopaedic training." There was no difference between the 2 groups in the mean cast index |
| Other bias | Low risk | No other source of bias identified |
West 2005.
| Methods | Randomised trial Study period: not stated (ethical approval July 1999) |
|
| Participants | Royal Gwent Hospital, Newport, South Wales, UK 42 children with buckle fractures of the distal radius Exclusion: not stated, no written consent Sex: not stated Age (< 5 / 5 to 10 / > 10 years): 1/26/12 (39 analysed) Fracture type: buckle fracture Assigned: 21 (bandage)/ 21 (cast) Analysed: 18 / 21 (according to the primary trial); 17 / 20 (as stated in 2 abstracts) |
|
| Interventions | 1. Bandage: a layer of orthopaedic wool was applied. This was then covered with a layer of ordinary commercial cotton crepe bandage, which was held with tape. Participants seen every week. Bandage was removed and then reapplied after measuring range of movement each week. Participants were encouraged to report adverse incidents and advised they could convert to a cast at any time 2. Plaster cast: initially, a below‐elbow back‐slab cast. At 1 week the cast was converted to a full below‐elbow polymer cast. Seen at 1 week and then at 4 weeks, when cast was removed At A&E on day of presentation, given an information booklet that set out in a question‐and‐answer format information on the 2 treatments provided prior to consent. Discharged at 4 weeks |
|
| Outcomes | Questionnaire at 4 weeks; also 1 week for both groups Function data: not reported, no formal data collection Adverse events or skin problems Cross‐over (protocol deviation; parents requested that a special‐needs child had bandage changed to a cast) Pain and comfort at 4 weeks Early bandage removal at first week or second week Convenience of treatment Range of movement: 1, 2, 3, 4 weeks in bandage group and 4 weeks in cast group |
|
| Funding and declarations of interest | Funding source: authors received no financial support for study Declarations of interest: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomised using a set of presealed envelopes, of which there were equal numbers to direct patients to either bandage or cast. Patients selected the envelope themselves.” Probably random |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomised using a set of presealed envelopes, of which there were equal numbers to direct patients to either bandage or cast. Patients selected the envelope themselves.” Probable allocation concealment but not quite enough to stop meddling |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | 3 withdrawals from the bandage group; 2 confirmed to have no problems |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | 3 withdrawals from the bandage group; 2 confirmed to have no problems |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol, outcomes insufficiently reported (e.g. pain and function). Also 2 conference abstracts refer to power calculations based on a difference of 5 º at 3 weeks (rather than 4 weeks) and target sample size of 46 participants |
| Other bias: major imbalance in baseline characteristics | Unclear risk | No information. Only provided for age ranges for 39 or 42 participants |
| Other bias: performance bias | High risk | The extra follow‐ups for the bandage group added an important co‐intervention. No details on clinician expertise |
| Other bias | Low risk | None detected |
Williams 2013.
| Methods | Randomised controlled trial Study period: April 2006 to May 2009 |
|
| Participants | St Louis Children’s Hospital, St Louis, Missouri, USA 94 children with a radiologically confirmed distal radial buckle fracture Exclusion: skeletally mature, previous distal radial buckle fractures, or concurrent other fractures except for an ipsilateral ulnar buckle fracture. Patients with osteogenesis imperfecta or other metabolic bone diseases. Sex: 51 male (54% of 94) Age: median 9.5 years (splint); median 9 years (cast); range 2 to 16 years Fracture type: buckle fracture Assigned: 43 (splint)/ 51 (cast) Analysed: 43 (splint)/ 51 (cast) |
|
| Interventions | 1. Prefabricated wrist splint. In the event that an appropriately‐sized, prefabricated cock‐up splint was not available, a custom splint was made from plaster. Children were advised to wear the splint as much as possible. However, parents were told that it was likely the child would remove the splint more frequently as pain improved. Trial registration indicates the Velcro volar splints were "Biomed Volar Splint" and that these were to be worn until follow‐up at 3 weeks 2. Short‐arm fibreglass cast with protective layers of stockinette and webril underneath. Children were given standard cast‐care instructions, such as keeping the cast dry and returning for any concerns with the cast Cast or splint application was performed or supervised by an attending physician or paediatric emergency medicine fellow in the paediatric emergency department. There were no stated limitations on activities for either group. Both groups were advised to follow up with the paediatric orthopaedic department in 3 weeks for a re‐evaluation |
|
| Outcomes | Length of follow‐up: 3 weeks; phone calls on day 1, 3, 7 and 21 and 21 day follow‐up visit Function (Peds QL questionnaire): primary outcome listed in the trial registration but not reported in full article. Satisfaction and convenience Pain Parental preference for same immobilisation device in future Resource utilisation (assistant required, median time for immobilisation, physician delay) Treatment concerns Number of times splint removed each day (at 1 and 3 weeks) |
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: reported, none |
|
| Notes | JE sent Williams an email 03.08.17 checking link with trial registration (NCT01010347); and depending on the answer: his plans for reporting either the listed primary outcome (Peds QL) or the other trial. Also checks on loss to follow‐up at 3 weeks, how many children were still using their splint at 3 weeks and how many children in the cast group had their cast removed at 3 weeks. No response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequence was computer‐generated with a block size of 10 ..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The randomisation sequence was computer‐generated with a block size of 10, and sealed, opaque envelopes were included in each study packet.” No mention of sequential numbering |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding not feasible |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Blinding not feasible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | No blinding reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No loss to follow‐up reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | High risk | Retrospective trial registration and no published protocol. No participant‐reported measures of function, but daily function assessed with the “Peds QL” questionnaire was the primary outcome listed in the trial registration. Outcomes not measured until or after cast and splint removal; timing of these not reported. Some data discrepancies |
| Other bias: major imbalance in baseline characteristics | Low risk | Quote: “There were no significant differences for gender, age, ethnicity, right‐hand dominance, fracture location, or history of prior cast or splint”. Baseline preferences favoured splint use but this is already considered under blinding |
| Other bias: performance bias | Unclear risk | Quote: “The application of the cast or splint was performed or supervised by an attending physician or pediatric emergency medicine fellow in the pediatric ED.” Level of training of physician was recorded but not reported Quote: “At the 3‐week follow‐up visit with orthopedics, a cast technician or nurse practitioner assessed the integrity of the immobilization technique and recorded the findings on a data sheet.” No information on timing of removal of cast |
| Other bias | Unclear risk | Only 3 weeks follow‐up |
ADL: activities of daily living; A&E: Accident and Emergency department; ASK: Activities Scale for Kids; ASP‐P: Activities Scale for Kids – Performance (also written as ASKp); N/A: not applicable or available; POP: plaster of Paris; VAS: visual analogue score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramo 2009 | Adult population |
| Bae 2012 | Narrative review of RCTs |
| Basdekis 2006 | Adult population |
| Bhaskar 2000 | Non‐randomised comparative study |
| Clarke 2007 | Trial abandoned: "The EPOS trial proved impossible to complete because of difficulties in consent and numbers." email from Prof NMP Clarke (10 September 2007) |
| Cohen 1997 | Adult population |
| Colaris 2013b | Shaft fracture |
| Delattre 1994 | Adult population |
| Dresing 2009 | Non‐randomised comparative study |
| Duncan 2006 | Trial abandoned |
| Egol 2008 | Adult population |
| Fikry 1998 | Adult population |
| Franke 2013 | Adult population |
| Gradl 2014 | Adult population |
| Gupta 1991 | Adult population |
| Hahnloser 1999 | Adult population |
| Hargreaves 2004 | Mixed population of adults and children ‐ results not separated by age |
| Ho 2010 | Shaft fracture |
| Hutchinson 1995 | Mixed population of adults and children‐ results not separated by age |
| ISRCTN25187648 | Email from Antony Hudson on 22.03.17 reported: "This [trial] was never analysed or written up as the lack of patients recruited, inconsistent and non consecutive recruitment and poor completion of outcome assessments meant that the study was meaningless" |
| ISRCTN34857372 | Dr Jacobs (primary contact for the trial) confirmed that this trial has not been published and that a co‐investigator has not responded to requests to share the data |
| Kasapinova 2009 | Adult population |
| Kavouriadis 2012 | Adult population |
| Khan 2007 | Non‐randomised comparative study despite claims. "Patients were randomised into two groups on the basis of the month in which they attended the fracture clinic. The children with buckle fractures attending in July and August 2004 were treated with below‐elbow soft cast (Cellacast) and those attending in September and October 2004 were treated with below‐elbow rigid cast." |
| Krishnan 2003 | Adult population |
| Krishnan 2014 | The reporting of the methods of this poorly reported study of torus fractures, which had curious similarities in design, reporting and results to Karimi 2013 (not cited in the report), was contradictory and it is very unlikely that this was a randomised or quasi‐randomised trial. If it is an authentic report of a prospective study (participants were, however, “reviewed retrospectively”) then there are several aspects that are surprising, such as the choice and reporting of the clinician‐rated Mayo score (usually used for adults) for the splint group who were only followed up by phone |
| Lidstrom 1959 | Non‐randomised comparative study |
| Lu 2014 | Shaft fracture |
| McQueen 1996 | Adult population |
| Mitsukane 2015 | Adult population |
| Mullett 2002 | Adult population |
| Murphy 2010 | Adult population. Although not explicitly stated in the abstract reports of this trial, the treatment regimen and complications are more typical of an adult population |
| NCT00398268 | Cohort study |
| NCT01493167 | Email from Eija Pirhonen of sponsor Onbone Oy (manufacturer of the wood‐based cast) on 23.03.17 confirmed that the study recruited adults only and that distal radius fractures were not included |
| NCT01762605 | Study was terminated at 11 participants due to “inadequate enrolment” |
| Parsch 2002 | Narrative review (abstract) |
| Pieske 2008 | Adult population |
| Pieske 2011 | Adult population |
| Pritchett 1994 | Case report |
| Robert 2011 | Non‐randomised comparative study |
| Saddiki 2011 | Adult population |
| Schønnemann 2011 | Adult population |
| Serrano‐Fernandez 2008 | Adult population |
| Sha 2015 | Adult population |
| Sutherland 2011 | Non‐randomised comparative study |
| Tamblyn 2010 | Adult population |
| Van Manen 2008 | Adult population |
| Vang Hansen 1998 | Adult population |
| Walker 2003 | Adult population |
| Witney‐Lagen 2013 | Non‐randomised comparative study |
| Young 2003 | Adult population |
| Yousef 2006 | Contacted co‐author Tracy Horton by email (22 May 2017), who confirmed that the study has never been published and no data are available |
| Zhao 2015 | Non‐randomised comparative study |
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12611000101987.
| Methods | Randomised controlled trial: randomisation generated by computer and participants allocated to treatment group by numbered sealed opaque envelopes |
| Participants | 100 participants (target), aged 3 to 14 years (eligible) with distal third radius fractures Inclusion criteria:
Exclusion criteria:
|
| Interventions | 1. Sugar tong plaster of Paris splint with an elastic bandage. Splint changed to an above‐elbow cast at 2 weeks if no loss of reduction. Cast duration: 3 to 4 weeks 2. Above‐elbow circumferential plaster of paris cast. Cast duration: 5 to 6 weeks |
| Outcomes | Follow‐up: 1, 2 and 6 weeks Primary: remanipulation rates, determined by X‐rays Secondary: cast complications, determined by questionnaire at 6‐week visit |
| Notes | Full text not available. Included based on trial registration ACTRN12611000101987, Date of registration: 31 January 2011 JE sent email to K Huh 2 March 2017 asking for information. A follow‐up email was sent on 22 March 2017 JE sent email to Starship Hospital (sponsor) 30 March 2017 asking for information. No response received. |
Bae 2015.
| Methods | Randomised clinical trial: treatment allocation was determined by drawing from prepackaged, sealed envelopes with assignments made based on an age‐stratified randomised block design |
| Participants | 202 children with displaced forearm fractures, mean age 10 ± 3 years Inclusion criteria: displaced distal or mid‐diaphyseal radius or ulna fractures or both, requiring closed reduction and cast immobilisation 75/101 participants in bivalved group had fracture in distal third of radius and 76/101 participants in circumferential group had fracture in distal third of radius 44/101 participants in bivalved group had fracture in distal third of ulna and 43/101 participants in circumferential group had fracture in distal third of ulna |
| Interventions | 1. Bivalved cast, with cuts made on the medial and lateral aspects along the entire length of the cast. Adhesive tape was applied externally to prevent loosening of cast components 2. Circumferential cast Casts were mostly fibreglass with cotton undercast padding. Long‐arm, above‐elbow casts were initially applied and then changed to short‐arm circumferential casts 4 weeks after injury |
| Outcomes | Follow‐up: 1, 2, 4,and 6 weeks post‐reduction Primary outcome: radiographic loss of reduction by 4 weeks, based on age and fracture criteria Secondary outcomes: need for remanipulation or surgical intervention or both by 4 weeks; complications (including compartment syndrome, neurovascular compromise or cast saw injuries) |
| Notes | Separate data on DRF not reported. JE sent email to Dr Bae 7 March 2017 to ask if trial results have been published (donald.bae@childrens.harvard.edu). Dr Bae replied 7 March 2017 with reference to full report. JE sent email to Dr Bae 22 March 2017 asking for separate data on DRF. A follow‐up email was sent 11 April 2017. No reply. |
Baldwin 2017.
| Methods | Randomised controlled trial, non‐blinded. Participants randomised using a card‐draw method |
| Participants | 60 participants, aged 3 to 13 years with closed shaft or distal third radius and ulna fractures Inclusion criteria: radius and ulnar shaft or distal radius and ulna fracture necessitating reduction under sedation Exclusion criteria:
|
| Interventions | 1. Intact long‐arm fibreglass cast with no valve 2. Long‐arm fibreglass cast with a single dorsal or volar valve (univalve) 3. Long‐arm fibreglass cast with a dorsal and volar valve (bivalve) |
| Outcomes | Follow‐up: 6 weeks Primary outcome: cast‐related complications (frequency of neurovascular injury; cast saw injury; unplanned office visits; cast modifications; need for operative intervention) Secondary outcome: pain (Wong‐Baker FACES visual pain rating scale) |
| Notes | JE emailed Dr Mark Lee 8 March 2017 for current status. Follow‐up email sent 11 April 2017. Dr Matt Solomito replied 13 April 2017 to say "The trial is currently completed and we are finishing up the analysis and manuscript for the study. We had 31 patients with distal radius/ulna fractures and an additional 14 patients with distal third radius and ulna fractures. The remainder of the patients were either midshaft or proximal third." HH contacted Dr Lee 27 November 2017 for separate results for the 45 children with distal fractures Unclear if separate analyses for distal radius fracture is planned. |
NCT02670629.
| Methods | Randomised, double‐blind controlled trial Study period January 2013 to December 2015 |
| Participants | 60 participants, aged 2 to 11 years (eligible) with completely displaced distal radius fractures with or without distal ulna fractures |
| Interventions | 1. Closed anatomic reduction under anaesthesia and short cast 2. Closed overriding alignment and short cast. Oral medications only The cast was removed after 6 weeks and rehabilitation in‐house was started as soon as the pain was over |
| Outcomes | Follow‐up: Weeks 1, 3, 6 and 10 Primary outcomes: Residual radiographic deformities, radial tilt, radial shortening and radial variation Secondary outcomes: Pain (VAS), residual functional deficits assessed by the Upper Extremity Functional Index, aesthetic results measured by clinical radial alignment |
| Notes | JE sent email to Dr Acosta‐Olivo (acostaolivocarlos@gmail.com) 8 March 2017 asking for current plans for publishing the trial results and permission to access individual patient data file on the trial registration website. Reply received from Dr Adriana Hernandez 8 March 2017 confirming that they are planning to publish the results and that permission would need to be granted from the University in Mexico to share the data. Data from the University in Mexico not provided. Awaiting publication of trial report. A conference abstract was identified by JH subsequent to the search and so has not been included in the results of the search (Hernandez 2018) |
NCT03097757.
| Methods | Randomised, single‐blind, controlled trial Sudy start date: January 2017, estimated completion date: June 2018 |
| Participants | 112 participants, aged up to 21 years (eligible) with displaced forearm fracture Inclusion criteria: confirmed displaced forearm fracture that will require reduction by orthopedic surgery in the Pediatric Emergency Department Exclusion criteria:
|
| Interventions | 1. Ultrasound‐guided fracture reduction 2. Standard of care fracture reduction (closed fracture reduction without real‐time imaging, or with c‐arm or portable X‐ray) |
| Outcomes | Primary outcome: number of participants requiring repeat reduction procedure Secondary outcomes:
|
| Notes | Pending publication. Mixed fracture population, unclear whether separate analysis for DRF is planned. |
NTR2508.
| Methods | Randomised, single‐blind controlled trial Planned start date: 1 November 2010; planned closing date: 1 March 2012 |
| Participants | Distal radius torus fracture, aged 5 ‐ 15 years (eligible) |
| Interventions | 1. Mitella sling 2. Plaster cast The children will be treated for 2 weeks with the sling or the cast. The study will last 6 weeks |
| Outcomes | Follow‐up: "Day 0, day 4, 1‐2‐6 weeks". Primary outcome: pain (VAS, day 1 ‐ 4) Secondary outcomes:
|
| Notes | JE sent email to Dr Brusse (cindyzpd@hotmail.com) 8 March 2017 requesting information on current status of the trial, how many patients were recruited, and if there are any plans for publication of the results. Email address failed |
Characteristics of ongoing studies [ordered by study ID]
Adrian 2015.
| Trial name or title | Official title: "A comparison of intervention and conservative treatment for angulated fractures of the distal forearm in children (AFIC): study protocol for a randomised controlled trial |
| Methods | Multicentre randomised controlled trial. 'Online‐based randomisation' by the Interdisciplinary Center for Clinical Trials, University Medical Centre of Mainz. Observer‐blinded |
| Participants | Target: 742 participants with angulated fractures of the distal forearm Inclusion criteria:
Exclusion criteria:
|
| Interventions | 1. Plaster immobilisation without any reduction for 4 weeks; plaster kind to be determined by treating clinic 2. Closed reduction under anaesthesia, percutaneous K‐wire osteosynthesis with or 1 or 2 wires, plaster to be determined by the treating clinic |
| Outcomes | Follow‐up: 3, 12 and 24 months Primary outcome: Cooney score after 24 months Secondary outcome:
|
| Starting date | April 2014 Estimated date of completion: March 2018 |
| Contact information | Miriam Adrian Clinic for Pediatric Surgery, University Hospital Mannheim, Faculty of Heidelberg, Mannheim, Germany Email: miriam.adrian@umm.de |
| Notes | Trial registration: DRKS00004874 At the time of submission of protocol, 30 trauma centres had been initiated and 42 participants included. Centres in Austria and Switzerland were preparing for initiation |
FORCE 2018.
| Trial name or title | FORCE The FOrearm fracture Recovery in Children Evaluation. A multi‐centre prospective randomised equivalence trial of a soft bandage and immediate discharge versus current treatment with rigid immobilisation for torus fractures of the distal radius in children |
| Methods | A UK multi‐centre prospective randomised equivalence trial (minimum of 10 centres) |
| Participants | Target: 696 children with a torus fracture of the distal radius (minimum of 348 in the 4‐ to 7‐year age group and 348 in the 8‐ to 16‐year age group) Inclusion criteria: age 4 to 16 years, torus fracture of the distal radius Exclusion criteria: unknown |
| Interventions | 1. Treatment with soft bandage, simple analgesia and immediate discharge with no hospital follow‐up 2. Rigid splint immobilisation and usual follow‐up |
| Outcomes | Follow‐up: 3 days and 6 weeks Primary outcome: pain (Wong Baker FACES Pain Scale measured at 3 days) Secondary outcomes:
Text messages (with hyperlinks) will be sent to parents/ children at days 1, 3, 7, 21 and 42, with slightly different information collected at each time point |
| Starting date | Open to recruitment: November 2018 Recruitment end: December 2019 Estimated date of follow‐up completion: February 2020 |
| Contact information | Associate Professor Daniel Perry University of Oxford |
| Notes | The trial will take place over 24 months: 4 months set‐up, 4 months internal pilot, 8 months recruitment, 3 months follow‐up respectively, and 5 months for data analysis and reporting NIHR funding, project 17/23/02 Information on trial recruitment available at the trial's website |
NCT03248687.
| Trial name or title | Official title: "Home management versus primary care physician follow up in children with distal radius fractures: A randomised control trial. |
| Methods | Randomised controlled trial. Single blind (outcomes assessor) |
| Participants | Target: 125 participants with a distal radius buckle fracture Inclusion criteria: age 5 to 17 years who present to the study emergency department within 3 days of a wrist injury that is diagnosed as a distal radius buckle fracture with or without an associated buckle/styloid fracture of the distal ulna Exclusion criteria:
|
| Interventions | 1. Removable splint with discharge instructions and anticipatory guidance with scheduled primary care physician follow up at 1 ‐ 2 weeks post‐visit to the emergency department 2. Removable splint with discharge instructions and anticipatory guidance without any scheduled physician follow‐up |
| Outcomes | Primary outcome: physical function (Activity Scales for Kids ‐ Performance Version) Secondary outcomes:
|
| Starting date | February 2018 Estimated date of completion: June 2020 |
| Contact information | Kathy Boutis Staff Physician and Sr. Associate Scientist The Hospital for Sick Children Canada |
| Notes |
NCT03297047.
| Trial name or title | Official title: Randomized controlled trial comparing forearm and upper arm combi cast for immobilization after closed reduced distal forearm fractures in children |
| Methods | Randomised controlled trial. Open‐label |
| Participants | Target: 120 participants with distal radial or forearm fractures Inclusion criteria:
Exclusion criteria:
|
| Interventions | 1. Forearm combi cast 2. Upper arm combi cast |
| Outcomes | Follow‐up: 5, 10, 28 days, 4 weeks and 7 weeks Primary outcome: secondary displacement of the fracture Secondary outcomes:
|
| Starting date | October 2017 Estimated date of completion: June 2019 |
| Contact information | Dr Gerog Staubli georg.staubli@kispi.uzh.ch Dr Michelle Seiler michelle.seiler@kispi.uzh.ch University Children's Hospital Zurich |
| Notes |
Differences between protocol and review
Types of interventions
Originally we had planned to present splint versus cast and bandage versus cast for buckle and other stable fractures under an umbrella comparison; this implied that we would present these two sub‐comparisons under the same analysis. Partly reflecting the deficiency in the evidence to support subgroup analysis, we considered that the two interventions ‐ removable splint and bandage ‐ were different enough to warrant separate analyses.
Types of outcome measures
We added an unscheduled change in device such as reapplication of a cast as an example of treatment failure.
We added refracture as a specific example of a serious adverse event.
'Summary of findings' tables
For convenience, we produced these using the facility in RevMan instead of using GRADEpro GDT software (GRADEpro GDT 2015). Depending on availability, the tables were produced by either HH or JE, and then checked by the other review author.
Outcomes for 'Summary of findings' tables
We adjusted our selection of outcomes for presentation in the 'Summary of findings' tables at the review stage for 'stable', predominantly buckle (torus) fractures. We removed medium‐ or long‐term functional outcome, as it is very unlikely to reflect differences in treatment effect. Instead, we increased our focus on acceptability of treatment by adding in parent or child satisfaction with treatment.
Outcomes for alternative 'Summary of findings' tables for buckle fractures
To explore the potential for different messages by guideline producers and our review, we produced a second 'Summary of findings' table for each comparison, focusing on interventions for treating buckle fractures (or other stable fractures) based on the outcomes listed in NICE 2016; see Types of outcome measures.
Contributions of authors
HH screened and selected studies, performed 'Risk of bias' assessment and extracted data from included studies, contributed to the 'Characteristics' tables and description of the studies, prepared most of the analyses, performed GRADE assessment, compiled and checked 'Summary of findings' tables and drafted most of the results and subsequent text. HH is the guarantor of the review.
JE co‐ordinated and performed searching, screening and study selection, data extraction and assessment, contributed to the 'Characteristics' tables, produced study characteristics and 'Risk of bias' summaries and drafted the related text, prepared the analyses for some comparisons, performed GRADE assessment, and compiled and checked 'Summary of findings' tables.
ZIE screened and selected studies, performed 'Risk of bias' assessment and extracted data from included studies and contributed to the 'Characteristics' tables and description of the studies.
AK and JH provided clinical oversight and revised the review for clinical content.
All authors commented on drafts and approved the final version.
Contributions at the protocol stage are listed in Handoll 2016.
Sources of support
Internal sources
University of Manchester, Manchester, UK.
Teesside University, Middlesbrough, UK, Other.
External sources
NIHR Cochrane Incentive Scheme 2017, project reference 17/62/04, UK.
Declarations of interest
Helen HG Handoll: None known. Joanne Elliott: None known. Zipporah Iheozor‐Ejiofor: None known. James Hunter: None known. Alexia Karantana: None known
Edited (no change to conclusions)
References
References to studies included in this review
Bohm 2006 {published data only}
- Bohm ER, Bubbar V, Yong Hing K, Dzus A. Above and below‐the‐elbow plaster casts for distal forearm fractures in children. A randomized controlled trial. Journal of Bone and Joint Surgery. American Volume 2006;88(1):1‐8. [DOI] [PubMed] [Google Scholar]
Boutis 2010 {published data only}
- Boutis K. Cast versus splint in children with acceptably angulated wrist fractures. clinicaltrials.gov/ct2/show/NCT00610220 (first received 7 February 2008).
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. Canadian Medical Association Journal 2010;182(14):1507‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard W, Willan A, Boutis K. A randomised controlled trial of cast versus wrist splint in children with acceptably angulated wrist fractures [abstract]. Journal of Bone and Joint Surgery. British Volume 2011;93(SUPP IV):578. [Google Scholar]
- Price CT. A splint was not inferior to a cast for distal radial fracture in children: Commentary. Journal of Bone and Joint Surgery 2011;93(10):970. [DOI] [PubMed] [Google Scholar]
- Keyserlingk C, Boutis K, Willan AR, Hopkins RB, Goeree R. Cost‐effectiveness analysis of cast versus splint in children with acceptably angulated wrist fractures. International Journal of Technology Assessment in Health Care 2011;27(2):101‐7. [DOI] [PubMed] [Google Scholar]
Boyer 2002 {published data only}
- Boyer BA, Overton B, Schrader W, Riley P, Fleissner P. Position of immobilization for pediatric forearm fractures. Journal of Pediatric Orthopaedics 2002;22(2):185‐7. [PubMed] [Google Scholar]
Colaris 2012 {published data only}
- Colaris J. Undislocated distal both‐bone forearm fractures in children. clinicaltrials.gov/ct2/show/NCT00397995 (first received 10 November 2006).
- Colaris JW, Biter LU, Allema JH, Bloem RM, Ven CP, Vries MR, et al. Below‐elbow cast for distal fractures of radius and ulna: A multicenter randomized study in children. Nederlands Tijdschrift Voor Geneeskunde 2012;156:1107‐11. [Google Scholar]
- Colaris JW, Biter LU, Allema JH, Bloem RM, Ven CP, Vries MR, et al. Below‐elbow cast for metaphyseal both‐bone fractures of the distal forearm in children: A randomised multicentre study. Injury 2012;43(7):1107‐11. [DOI] [PubMed] [Google Scholar]
Colaris 2013a {published data only}
- Colaris J. Dislocated stable distal both‐bone forearm fractures in children. clinicaltrials.gov/ct2/show/NCT00397852 (first received 10 November 2006).
- Colaris JW, Allema JH, Biter LU, Vries MR, Ven CP, Bloem RM, et al. Re‐displacement of stable distal both‐bone forearm fractures in children: a randomised controlled multicentre trial. Injury 2013;44(4):498‐503. [DOI] [PubMed] [Google Scholar]
Davidson 2001 {published data only}
- Davidson JS, Brown DJ, Barnes SN, Bruce CE. Simple treatment for torus fractures of the distal radius. Journal of Bone and Joint Surgery. British Volume 2001;83(8):1173‐5. [DOI] [PubMed] [Google Scholar]
Derksen 2011 {published data only}
- Derksen RJ, Commandeur JP, Deij R, Breederveld RS. Swim cast versus traditional cast in pediatric distal radius fractures: a prospective randomized controlled trial. Journal of Children's Orthopaedics 2013;7(2):117‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen RJ, Commandeur JP, Walet W, Deij R, Breederveld RS. Swimcast wrist immobilization (SWIM) trial. Comparison of waterproof versus conventional cast for pediatric distal radius fractures [abstract]. European Journal of Trauma and Emergency Surgery 2011;37:S135. [Google Scholar]
Ghoneem 2003 {published data only}
- Ghoneem H. Pediatric displaced distal forarm fractures: avoiding failure of treatment (abstract). Journal of Bone and Joint Surgery. British Volume 2003;85(SUPP III):262‐3. [Google Scholar]
Gibbons 1994 {published data only}
- Gibbons CL, Woods DA, Pailthorpe C, Carr AJ, Worlock P. The management of isolated distal radius fractures in children. Journal of Pediatric Orthopaedics 1994;14(2):207‐10. [DOI] [PubMed] [Google Scholar]
- Gibbons CL, Woods DA, Pailthorpe C, Carr AJ, Worlock P. The management of isolated distal radius fractures in children [abstract]. Journal of Bone and Joint Surgery. British Volume 1994;76(Suppl):38. [DOI] [PubMed] [Google Scholar]
Gupta 1990 {published data only}
- Gupta RP, Danielsson LG. Dorsally angulated solitary metaphyseal greenstick fractures in the distal radius: Results after immobilization in pronated, neutral, and supinated position. Journal of Pediatric Orthopaedics 1990;10(1):90‐2. [PubMed] [Google Scholar]
Hamilton 2013 {published data only}
- Hamilton TW, Hutchings L, Alsousou J, Tutton E, Hodson E, Smith CH, et al. The treatment of stable paediatric forearm fractures using a cast that may be removed at home: comparison with traditional management in a randomised controlled trial. Bone & Joint Journal 2013;95‐B(12):1714‐20. [DOI] [PubMed] [Google Scholar]
- Willett K. Self cast removal at the child's home. www.isrctn.com/ISRCTN55089523 (first received 13 August 2008).
Inglis 2013 {published data only}
- Abson S, Williams N, Inglis M, Antoniou G, Cundy P. Resident versus attending surgeons in achieving and maintaining fracture reduction in pediatric distal radius fractures. Journal of Pediatric Orthopaedics 2016;36(5):478‐82. [DOI] [PubMed] [Google Scholar]
- Inglis M, McCelland B, Sutherland L, Cundy P. Fiberglass versus plaster of paris casts for paediatric forearm fractures. an investigation of clinical outcomes and patient satisfaction [abstract]. Journal of Bone and Joint Surgery.British Volume 2012;94(SUPP XXIII):140. [Google Scholar]
- Inglis M, McClelland B, Sutherland LM, Cundy PJ. Synthetic versus plaster of Paris casts in the treatment of fractures of the forearm in children: a randomised trial of clinical outcomes and patient satisfaction. Bone & Joint Journal 2013;95‐B(5):1285‐9. [DOI] [PubMed] [Google Scholar]
Jones 2001 {unpublished data only}
- Jones S, Smith I, Jones MW. Treatment of distal radius buckle fractures [Poster abstract 41]. British Orthopaedic Congress 2001.
- Smith I. The management of torus fractures of the distal radius. National Research Register 2001, issue 2.
Karimi 2013 {published data only}
- Karimi Mobarakeh M, Nemati A, Noktesanj R, Fallahi A, Safari S. Application of removable wrist splint in the management of distal forearm torus fractures. Trauma Monthly 2013;17(4):370‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2010 {published data only}
- Khan S. Closed reduction of distal forearm fractures by pediatric emergency medicine physicians: a prospective study. clinicaltrials.gov/ct2/show/NCT01101607 (first received 12 April 2010).
- Khan S, Sawyer J, Pershad J. Closed manipulation of distal radial fractures by pediatric emergency physicians. Pediatric Emergency Care 2009; American Academy of Pediatrics, Section on Emergency Medicine, Scientific Abstract Presentations, AAP National Conference and Exhibition; 2009 Oct 16; Washington (DC). American Academy of Pediatrics, 2009; Vol. 709.
- Khan S, Sawyer J, Pershad J. Closed reduction of distal forearm fractures by pediatric emergency physicians. Academic Emergency Medicine 2010;17:1169‐74. [DOI] [PubMed] [Google Scholar]
Kropman 2010 {published data only}
- Hammacher ER. Treatment of greenstick forearm fractures in children using bandages or cast therapy. apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR393 (first received 21 September 2005).
- Kropman RH, Bemelman M, Segers MJ, Hammacher ER. Treatment of impacted greenstick forearm fractures in children using bandage or cast therapy: a prospective randomized trial. Journal of Trauma 2010;68(2):425‐8. [DOI] [PubMed] [Google Scholar]
Levy 2015 {published data only}
- Levy J, Ernat J, Song D, Cook JB, Judd D, Shaha S. Outcomes of long‐arm casting versus double‐sugar‐ tong splinting of acute pediatric distal forearm fractures. Journal of Pediatric Orthopaedics 2015;35(1):11‐7. [DOI] [PubMed] [Google Scholar]
McLauchlan 2002 {published data only}
- Gambhir AK, Fischer J, Waseem M, Robb JE, Maclauchlan GJ, Annan IH. Management of completely displaced metaphyseal fractures of the distal radius in children (multiple letters). Journal of Bone and Joint Surgery. British Volume 2003;85:463. [PubMed] [Google Scholar]
- McLauchlan GJ, Cowan B, Annan IH, Macnicol MF, Robb JE. A randomised trial of treatment of completely displaced distal radial fractures in children [abstract]. Journal of Bone and Joint Surgery. British Volume 2000;82(Suppl 1):63. [Google Scholar]
- McLauchlan GJ, Cowan B, Annan IH, Robb JE. Management of completely displaced metaphyseal fractures of the distal radius in children. A prospective, randomised controlled trial. Journal of Bone and Joint Surgery. British Volume 2002;84:413‐7. [DOI] [PubMed] [Google Scholar]
- McLauchlan GJ, Cowan B, Annan IH, Robb JE, Price CT. The addition of a Kirschner wire to an above‐the‐elbow cast prevented loss of position in displaced fractures of the distal radius in children. Journal of Bone and Joint Surgery. American Volume 2002;84:2109. [DOI] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller B, Waters PM, Taylor B. A prospective, randomized study of displaced pediatric metaphyseal distal radius fracture: cast immobilization versus percutaneous pin fixation [abstract]. American Academy of Orthopaedic Surgeons 67th Annual Meeting; 2000; Orlando (FL). 2000.
- Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. Journal of Pediatric Orthopaedics 2005;25(4):490‐4. [DOI] [PubMed] [Google Scholar]
Oakley 2008 {published data only}
- Oakley E. Comparison of two methods of immobilising torus fractures of the distal forearm. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12606000126516 (first received 6 April 2006).
- Oakley EA, Ooi KS, Barnett PL. A randomized controlled trial of 2 methods of immobilizing torus fractures of the distal forearm. Pediatric Emergency Care 2008;24(2):65‐70. [DOI] [PubMed] [Google Scholar]
Paneru 2010 {published data only}
- Paneru SR, Rijal R, Shrestha BP, Nepal P, Khanal GP, Karn NK, et al. Randomized controlled trial comparing above‐ and below‐elbow plaster casts for distal forearm fractures in children. Journal of Children's Orthopaedics 2010;4(3):233‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plint 2006 {published data only}
- Anonymous. Splint equal to cast for wrist buckle fracture in children [POEM]. Journal of Family Practice 2006;55(6):476. [PubMed] [Google Scholar]
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawton L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics 2006;117(3):691‐7. [DOI] [PubMed] [Google Scholar]
Pountos 2010 {published data only}
- Pountos I, Clegg J, Siddiqui A. Diagnosis and treatment of greenstick and torus fractures of the distal radius in children: a prospective randomised single blind study. Journal of Children's Orthopaedics 2010;4(4):321‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulte 2014 {published and unpublished data}
- Garcia D. Additional data [personal communication]. Email to: J Elliott 21 June 2017 and 9 August 2017.
- Schulte D, Habernig S, Zuzak T, Staubli G, Altermatt S, Horst M, et al. Forearm fractures in children: split opinions about splitting the cast. European Journal of Pediatric Surgery 2014;24(2):163‐7. [DOI] [PubMed] [Google Scholar]
Silva 2016 {published data only}
- Silva M. Waterproof casting for pediatric distal radius fractures. clinicaltrials.gov/ct2/show/NCT02095106 (first received 24 March 2014).
- Silva M, Avoian T, Warnock RS, Sadlik G, Ebramzadeh E. It is not just comfort: waterproof casting increases physical functioning in children with minimally angulated distal radius fractures. Journal of Pediatric Orthopaedics. Part B 2017;26:417‐23. [DOI] [PubMed] [Google Scholar]
Stevenson 2013 {published and unpublished data}
- Antoniou G. Additional data [personal communication]. Email to: J Elliott 28 April 2017.
- Stevenson AW, Gahukamble AD, Antoniou G, Pool B, Sutherland LM, Cundy P. Waterproof cast liners in paediatric forearm fractures: a randomized trial. Journal of Childrens Orthopaedics 2013;7(2):123‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Symons 2001 {published data only}
- Mehlman CT. Home removal of a backslab 3 weeks after buckle fracture of the distal radius was as safe as removal at a fracture clinic. Journal of Bone and Joint Surgery. American Volume 2002;84(5):883. [Google Scholar]
- Symons S, Rowsell M, Bhowal B, Dias JJ. Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomised trial. Journal of Bone and Joint Surgery. British Volume 2001;83:556‐60. [DOI] [PubMed] [Google Scholar]
Webb 2006 {published data only}
- Kumar G. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children (letter). Journal of Bone and Joint Surgery. American Volume 2006;88(8):1888‐9. [DOI] [PubMed] [Google Scholar]
- Webb GR, Galpin RD, Armstrong DG. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children. Journal of Bone and Joint Surgery. American Volume 2006;88:9‐17. [DOI] [PubMed] [Google Scholar]
West 2005 {published data only}
- West S, Andrews J, Alderman P. Treatment of buckle fractures in bandage versus plaster: a randomised prospective trial [abstract]. Journal of Bone and Joint Surgery. British Volume 2003;85(Suppl III):276. [Google Scholar]
- West S, Andrews J, Bebbington A, Ennis O, Alderman P. Buckle fractures of the distal radius are safely treated in a soft bandage: a randomized prospective trial of bandage versus plaster cast. Journal of Pediatric Orthopaedics 2005;25(3):322‐5. [DOI] [PubMed] [Google Scholar]
- West S, Andrews J, Bebbington A, Ennis O, Alderman P. The treatment of buckle fractures in a bandage: a prospective randomised trial [abstract]. Journal of Bone and Joint Surgery. British Volume 2004;86(Suppl III):286. [Google Scholar]
Williams 2013 {published data only}
- Williams K. A comparison of casting and splinting in pediatric radial buckle fractures. clinicaltrials.gov/ct2/show/NCT01010347 (first received 10 November 2009).
- Williams KG, Smith G, Luhmann SJ, Mao J, Gunn JD 3rd, Luhmann JD. A randomized controlled trial of cast versus splint for distal radial buckle fracture: An evaluation of satisfaction, convenience, and preference. Pediatric Emergency Care 2013;29(5):555‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abramo 2009 {published data only}
- Abramo A, Kopylov P, Geijer M, Tägil M. Open reduction and internal fixation compared to closed reduction and external fixation in distal radial fractures: a randomized study of 50 patients. Acta Orthopaedica 2009;80(4):478‐85. [DOI: 10.3109/17453670903171875] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bae 2012 {published data only}
- Bae DS, Howard AW. Distal radius fractures: What is the evidence?. Journal of Pediatric Orthopaedics 2012;32:S128‐S130. [DOI] [PubMed] [Google Scholar]
Basdekis 2006 {published data only}
- Basdekis GK, Varitimidis S, Dailiana ZH, Hantes ME, Bargiotas K, Malizos KN. Intra‐articular distal radius fractures: fluoroscopic or arthroscopic reduction?. Journal of Bone and Joint Surgery. British Volume 2006;88‐B(SUPP I):187. [DOI] [PubMed] [Google Scholar]
Bhaskar 2000 {published data only}
- Bhaskar AR, Roberts JA. Treatment of unstable forearm fractures in children comparison of both bones versus single bone plating [abstract]. Journal of Bone and Joint Surgery. British Volume 2000;82 Suppl 1(CCT):63. [DOI] [PubMed] [Google Scholar]
Clarke 2007 {published data only}
- Bell M. EPOS Randomised controlled trial of management of distal radial fractures: A randomised controlled trial comparing Kirschner wire stabilisation versus manipulation and plaster. National Research Register 2007, issue 3.
- Bell M. Study status [personal communication]. Email to: H Handoll 4 September 2007.
- Clarke NM. EPOS Randomised controlled trial of management of distal radial fractures: A randomised controlled trial comparing Kirschner wire stabilisation versus manipulation and plaster. National Research Register 2002, issue 3.
- Clarke NM. Study status [personal communication]. Email to: H Handoll 10 September 2007.
Cohen 1997 {published data only}
- Cohen MS, Frillman T. Distal radius fractures: A prospective randomized comparison of fibreglass tape with QuickCast(TM). Injury 1997;28(4):305‐9. [DOI] [PubMed] [Google Scholar]
Colaris 2013b {published data only}
- Colaris JW, Reijman M, Allema JH, Biter LU, Bloem RM, Ven CP, et al. Early conversion to below‐elbow cast for non‐reduced diaphyseal both‐bone forearm fractures in children is safe: preliminary results of a multicentre randomised controlled trial. Archives of Orthopaedic and Trauma Surgery 2013;133(10):1407‐14. [DOI: 10.1007/s00402-013-1812-8] [DOI] [PubMed] [Google Scholar]
Delattre 1994 {published data only}
- Delattre O, Saillant G, Lemoine J, Benazet JP, Roy‐Camille R. Reduction and osteosynthesis with pin fixation of wrist fractures. A comparative study between Kapandji's and Py's techniques. Revue de Chirurgie Orthopedique et Reparatrice de l'appareil Moteur 1994;80(2):94‐107. [PMID: 7899635] [PubMed] [Google Scholar]
Dresing 2009 {published data only}
- Dresing K, Schleikis A, Sturmer KM. Primary definitive cast therapy on the upper and lower extremities : IIndications and cost analysis. Der Chirurg; Zeitschrift fur alle Gebiete der Operativen Medizen 2009;80(3):223‐30. [DOI: 10.1007/s00104-008-1636-1] [DOI] [PubMed] [Google Scholar]
Duncan 2006 {published data only}
- Duncan RD. Pinning or plaster for the unstable wrist fracture in children?. National Research Register 2006.
Egol 2008 {published data only}
- Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner‐wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. Journal of Bone and Joint Surgery. British Volume 2008;90(9):1214‐21. [DOI: 10.1302/0301-620X.90B9.20521] [DOI] [PubMed] [Google Scholar]
Fikry 1998 {published data only}
- Fikry T, Fadili M, Harfoui A, Dkhissi M, Zryouil B. Metaphysis fracture of the distal radius: Kapandji's or Py's pinning? [Fractures metaphysaires du radius distal: embrochage de Kapandji ou de Py?]. Annales de Chirurgie de la Main et du Membre Superieur 1998;17(1):31‐40. [PMID: 10941382] [DOI] [PubMed] [Google Scholar]
Franke 2013 {published data only}
- Franke J, Vetter SY, Mühlhäuser I, Grützner PA, Recum J. Virtual implant planning system: first clinical results. Bone and Joint Journal 2013;95(SUPP 28):76. [Google Scholar]
Gradl 2014 {published data only}
- Gradl G, Mielsch N, Wendt M, Falk S, Mittlmeier T Gierer P, et al. Intramedullary nail versus volar plate fixation of extra‐articular distal radius fractures. Two year results of a prospective randomized trial. Injury 2014;45(Suppl 1):S3‐8. [DOI: 10.1016/j.injury.2013.10.045] [DOI] [PubMed] [Google Scholar]
Gupta 1991 {published data only}
- Gupta A. The treatment of Colles' fracture. Immobilisation with the wrist dorsiflexed. Journal of Bone and Joint Surgery. British Volume 1991;73(2):312‐5. [PMID: 2005163] [DOI] [PubMed] [Google Scholar]
Hahnloser 1999 {published data only}
- Hahnloser D, Platz A, Amgwerd M, Trentz O. Internal fixation of distal radius fractures with dorsal dislocation: pi‐plate or two 1/4 tube plates? A prospective randomized study. Journal of Trauma 1999;47(4):760‐5. [DOI: 10.1097/00005373-199910000-00024] [DOI] [PubMed] [Google Scholar]
Hargreaves 2004 {published data only}
- Hargreaves DG, Drew SJ, Eckersley R. Kirschner wire pin tract infection rates: a randomized controlled trial between percutaneous and buried wires. Journal of Hand Surgery 2004;29(4):374‐6. [DOI: 10.1016/j.jhsb.2004.03.003] [DOI] [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho CA, Wilson PL. A comparison of fracture reductions performed by physician extenders and orthopaedic residents in the acute pediatric orthopaedic practice. Journal of Orthopaedic Trauma 2010;24(4):244‐9. [DOI: 10.1097/BOT.0b013e3181bd5863] [DOI] [PubMed] [Google Scholar]
Hutchinson 1995 {published data only}
- Hutchinson DT, Strenz GO, Cautilli RA. Pins and plaster vs external fixation in the treatment of unstable distal radial fractures. A randomized prospective study. Journal of Hand Surgery (Edinburgh, Scotland) 1995;20(3):365‐72. [PMID: 7561414] [DOI] [PubMed] [Google Scholar]
ISRCTN25187648 {published data only}
- Hudson A. Forearm buckle fracture treatment study. www.isrctn.com/ISRCTN25187648 (first received 30 September 2005).
- Hudson A. Study status [personal communication]. Email to: J Elliott 22 March 2017.
ISRCTN34857372 {published data only}
- Jacobs M. Distal forearm torus fractures ‐ do they need a splint?. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN34857372 (first received 28 September 2007).
- Jacobs M. Study status [personal communication]. Email to: J Elliott 6 March 2017.
Kasapinova 2009 {published data only}
- Kasapinova K, Kamiloski V. Pain and disability during six months in patients with a distal radius fracture. Makedonska Akademija na Naukite i Umetnostite Oddelenie za Bioloshki i Meditsinski Nauki Prilozi 2009;30(2):185‐96. [PubMed] [Google Scholar]
Kavouriadis 2012 {published data only}
- Kavouriadis V, O'Gorman A, Bain G, Ashwood N. Is external fixation a better way than plaster to supplement k‐wires in noncomminuted distal radius fractures? A prospective randomised surgeon independent study. Journal of Bone and Joint Surgery. British Volume 2012;94(SUPP III):135. [Google Scholar]
Khan 2007 {published data only}
- Khan KS, Grufferty A, Gallagher O, Moore DP, Fogarty E, Dowling F. A randomized trial of 'soft cast' for distal radius buckle fractures in children. Acta Orthopaedica Belgica 2007;73(5):594‐7. [PMID: 18019914] [PubMed] [Google Scholar]
Krishnan 2003 {published data only}
- Krishnan J, Wigg AE, Walker RW, Slavotinek J. Intra‐articular fractures of the distal radius: a prospective randomised controlled trial comparing static bridging and dynamic non‐bridging external fixation. Journal of Hand Surgery 2003;28(5):417‐21. [DOI: 10.1016/S0266-7681(03)00083-4] [DOI] [PubMed] [Google Scholar]
Krishnan 2014 {published data only}
- Krishnan M, Jacob C. Splints are an overall better alternative to casting for torus fractures in children in rural hospitals. International Journal of Recent Trends in Science And Technology 2014;12(3):446‐8. [Google Scholar]
Lidstrom 1959 {published data only}
- Lidstrom A. Fractures of the distal end of the radius. A clinical and statistical study of end results. Acta Orthopaedica Scandinavica 1959;Supplementum 41:1‐118. [PubMed] [Google Scholar]
Lu 2014 {published data only}
- Lu B, Liu P, Wang Y. Application of elastic intramedullary nail in the treatment of both ulna and radius fractures in children. Zhonghua Yi Xue Za Zhi 2014;94(24):1882‐5. [PubMed] [Google Scholar]
McQueen 1996 {published data only}
- McQueen MM, Hajducka C, Court‐Brown CM. Redisplaced unstable fractures of the distal radius. Journal of Bone and Joint Surgery. British Volume 1996;78(3):404‐9. [PubMed] [Google Scholar]
Mitsukane 2015 {published data only}
- Mitsukane M, Sekiya N, Himei S, Oyama K. Immediate effects of repetitive wrist extension on grip strength in patients with distal radial fracture. Archives of Physical Medicine and Rehabilitation 2015;96(5):862‐8. [DOI] [PubMed] [Google Scholar]
Mullett 2002 {published data only}
- Mullett H, O’Connor D, Doyle M, Kutty S, Laing A, O’Sullivan M. Plaster cast vs futura splint: a prospective randomised trial in the treatment of distal radial fractures. Journal of Bone and Joint Surgery. British Volume 2002;84(SUPP I):11. [Google Scholar]
Murphy 2010 {published data only}
- Murphy M, Flannery O, Kenny P, Keogh P, Lui D, Hugh G, et al. Outcome following buried versus exposed kirschner wiring of distal radius fractures: a randomised controlled trial. Journal of Bone and Joint Surgery. British Volume 2010;92((SUPP IV)):581‐2. [Google Scholar]
- Murphy M, Flannery O, McHugh G, Lui D, Kenny P, Keogh P, et al. Outcome following buried versus exposed kirschner wiring of distal radius fractures: a randomised controlled trial. Journal of Bone and Joint Surgery ‐ British Volume 2010;92(SUPP I):50‐1. [Google Scholar]
NCT00398268 {published data only}
- Colaris JW. Dislocated unstable distal both‐bone forearm fractures in children. clinicaltrials.gov/ct2/show/NCT00398268 (first received 10 November 2006).
NCT01493167 {published data only}
- Lindfors NC. Study on wood‐plastic composite for circumferential casting. clinicaltrials.gov/ct2/show/NCT01493167 (first received 15 December 2011).
- Pirhonen E. Study population and status [personal communication]. Email to: J Elliott 23 March 2017.
NCT01762605 {published data only}
- Gardner A. Treatment of distal radius buckle fractures. clinicaltrials.gov/ct2/show/NCT01762605 (first received 8 January 2013).
Parsch 2002 {published data only}
- Parsch K. Radius and ulna fractures in children. Journal of Bone and Joint Surgery. British Volume 2002;84(SUPP III):360‐1. [Google Scholar]
Pieske 2008 {published data only}
- Pieske O, Geleng P, Zaspel J, Piltz S. Titanium alloy pins versus stainless steel pins in external fixation at the wrist: a randomized prospective study. Journal of Trauma 2008;64(5):1275‐80. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pieske 2011 {published data only}
- Pieske O, Pichlmaier L, Kaltenhauser F, Schramm N, Rubenbauer B Greiner A, et al. Hydroxyapatite‐coated pins versus titanium alloy pins in external fixation at the wrist: a controlled cohort study. Journal of Trauma 2011;70(4):845‐51. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pritchett 1994 {published data only}
- Pritchett JW. Does pinning cause distal radial growth plate arrest?. Orthopedics 1994;17(6):550‐2. [DOI] [PubMed] [Google Scholar]
Robert 2011 {published data only}
- Robert CE, Jiang JJ, Khoury JG. A prospective study on the effectiveness of cotton versus waterproof cast padding in maintaining the reduction of pediatric distal forearm fractures. Journal of Paediatric Orthopaedics 2011;31(2):144‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Saddiki 2011 {published data only}
- Saddiki R, Harisboure A, Hemery X, Ohl X, Kabbaj R, Dehoux É. Comparative studies of the py and Kapandji techniques for fractures of the distal radius with posterior displacement. Journal of Bone and Joint Surgery. British Volume 2011;93(SUPP IV):526. [Google Scholar]
Schønnemann 2011 {published data only}
- Schønnemann JO, Hansen TB, Søballe K. Randomised study of non‐bridging external fixation compared with intramedullary fixation of unstable distal radial fractures. Journal of Plastic Surgery and Hand Surgery 2011;45(4‐5):232‐7. [DOI] [PubMed] [Google Scholar]
Serrano‐Fernandez 2008 {published data only}
- Serrano‐Fernandez JM, Espejo‐Baena A, Martin‐Castilla B, Torre‐Solis F, Mariscal‐Lara J, Merino‐Ruiz ML. Bridging external fixation and supplementary Kirschner‐wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. Journal of Bone and Joint Surgery. British Volume 2008;90(9):1214‐21. [DOI] [PubMed] [Google Scholar]
Sha 2015 {published data only}
- Sha L, Chen Q, Sun L, Dong B, Li L. Effectiveness comparison of external fixation and volar locking compression plate in treatment of distal radius fractures of type c. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi [Chinese Journal of Reparative and Reconstructive Surgery] 2015;29(6):683‐7. [PubMed] [Google Scholar]
Sutherland 2011 {published data only}
- Sutherland M. Casting vs. splinting for wrist fractures in children. American Family Physician 2011;83(9):83 (9) (no pagination). [Google Scholar]
Tamblyn 2010 {published data only}
- Tamblyn P, McCamley C, Kimber C, Jaarsma R. Volar locking plate for distal radius fractures: a prospective randomised controlled study. Journal of Bone and Joint Surgery. British Volume 2010;92(SUPP I):214. [Google Scholar]
Vang Hansen 1998 {published data only}
- Vang Hansen F, Staunstrup H, Mikkelsen S. A comparison of 3 and 5 weeks immobilization for older type 1 and 2 Colles' fractures. Journal of Hand Surgery 1998;23(3):400‐1. [DOI] [PubMed] [Google Scholar]
Van Manen 2008 {published data only}
- Manen CJ, Dekker ML, Eerten PV, Rhemrev SJ, Olden GD, Elst M. Bio‐resorbable versus metal implants in wrist fractures: a randomised trial. Archives of Orthopaedic and Trauma Surgery 2008;128(12):1413‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Walker 2003 {published data only}
- Walker RW, Wigg A, Krishnan J, Slavotinek J. Intra‐articular fractures of the distal radius: a prospective randomised trial comparing bridging and non‐bridging external fixation. Journal of Bone and Joint Surgery. British Volume 2003;85(SUPP I):27‐8. [Google Scholar]
Witney‐Lagen 2013 {published data only}
- Witney‐Lagen C, Smith C, Walsh G. Soft cast versus rigid cast for treatment of distal radius buckle fractures in children. Injury 2013;44(4):508‐13. [DOI: ] [DOI] [PubMed] [Google Scholar]
Young 2003 {published data only}
- Young CF, Nanu AM, Checketts RG. Seven year outcome study of colles’ type distal radial fractures. Journal of Bone and Joint Surgery. British Volume 2003;85(SUPP I):27. [Google Scholar]
Yousef 2006 {published data only}
- Yousef A, Al‐Jafari N, Horton T. Soft cast verses plaster of Paris treatment of greenstick forearm fractures in children [abstract]. Journal of Bone and Joint Surgery. British Volume 2006;88(Suppl 2):278. [Google Scholar]
Zhao 2015 {published data only}
- Zhao C. Outcomes of different operations for distal forearm fractures in children. Chinese Medical Journal 2015;95(15):1168‐70. [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12611000101987 {published data only}
- Huh K. Long arm casting versus splinting after manipulation of distal third radius fractures in children. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611000101987 (first received 31 January 2011).
Bae 2015 {published data only}
- Bae D. Randomized trial of casting techniques for displaced forearm fractures. clinicaltrials.gov/ct2/show/NCT00823823?term=NCT00823823&rank=1 (first received 16 January 2009).
- Bae D. Study status [personal communication]. Email to: J Elliott 7 March 2017.
- Bae D S, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. Journal of Pediatric Orthopedics 2017;37:239‐46. [DOI] [PubMed] [Google Scholar]
- Bae DS, Valim C, Connell P, Brustowicz K, Waters PM. Circumferential vs bivalved cast immobilization for displaced forearm fractures: A randomized clinical trial to assess safety and efficacy: Level 1 evidence (Abstract). Journal of Hand Surgery 2012;8 Suppl:41‐2. [Google Scholar]
Baldwin 2017 {published data only (unpublished sought but not used)}
- Baldwin PC 3rd, Han E, Parrino A, Solomito MJ, Lee MC. Valve or no valve: a prospective randomized controlled trial of casting options for pediatric forearm fractures. Orthopedics 2017;40:e849‐54. [DOI] [PubMed] [Google Scholar]
- Lee M, Solomito M. Is univalving or bivalving of long arm casts for forearm fractures necessary?. clinicaltrials.gov/ct2/show/NCT02614690 (first received 25 November 2015).
- Solomito M. Trial status [personal communication]. Email to: J Elliott 12 April 2017.
NCT02670629 {published data only}
- Hernandez A. Confirmation that the trial will be published shortly [personal communication]. Email to: J Elliott 8 March 2017.
- Lemus OF. Management of distal radius fractures in children younger than 11 years old. clinicaltrials.gov/ct2/show/NCT02670629 (first received 2 February 2016).
NCT03097757 {published data only}
- Rabiner J. Randomized controlled trial of ultrasound guidance for reduction of pediatric forearm fractures. clinicaltrials.gov/ct2/show/record/NCT03097757 (first received 31 March 2017).
NTR2508 {published data only}
- Brusse C. Paediatric distal radius torus fracture treatment comparison in a prospective randomized control trial: Mitella versus plaster cast. apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR2508 (first received 6 September 2010).
References to ongoing studies
Adrian 2015 {published data only}
- Adrian M, Wachtlin D, Kronfeld K, Sommerfeldt D, Wessel LM. A comparison of intervention and conservative treatment for angulated fractures of the distal forearm in children (AFIC): study protocol for a randomized controlled trial. Trials 2015;16:437. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel LM. Angulated fractures of the distal forearm in children: is remodeling a therapeutic option?. apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00004874 (first received 30 October 2013).
FORCE 2018 {published data only}
- FORCE. www.ndorms.ox.ac.uk/clinical‐trials/current‐trials‐and‐studies/force (accessed 23 October 2018).
- Perry D. FORCE The FOrearm fracture Recovery in Children Evaluation. A multi‐centre prospective randomized equivalence trial of a soft bandage and immediate discharge versus current treatment with rigid immobilisation for torus fractures of the distal radius in children. Health Technology Assessment Research Projects www.journalslibrary.nihr.ac.uk/programmes/hta/172302/#/ (accessed 12 July 2018). [DOI] [PMC free article] [PubMed]
NCT03248687 {published data only}
- Boutis K. Home management versus primary care physician follow up in children with distal radius fractures: A randomized control trial. clinicaltrials.gov/ct2/show/record/NCT03248687 (first received 14 August 2017).
NCT03297047 {published data only}
- Staubli G. Randomized controlled trial comparing forearm and upper arm combi cast for immobilization after closed reduced distal forearm fractures in children. clinicaltrials.gov/ct2/show/record/NCT03297047 (first received 29 September 2017).
Additional references
Abson 2016
- Abson S, Williams N, Inglis M, Antoniou G, Cundy P. Resident versus attending surgeons in achieving and maintaining fracture reduction in pediatric distal radius fractures. Journal of Pediatric Orthopaedics 2016;36(5):478‐82. [DOI] [PubMed] [Google Scholar]
Atrey 2010
- Atrey A, Nicolaou N, Katchburian M, Norman‐Taylor F. A review of reported litigation against English health trusts for the treatment of children in orthopaedics: present trends and suggestions to reduce mistakes. Journal of Children's Orthopaedics 2010;4(5):471‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biag 2017
- Baig MN, Egan C. Improving management of paediatric buckle fracture in orthopaedic outpatients: A completed audit loop. Cureus 2017;8(9(11)):e1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bieri 1990
- Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990;41(2):139‐50. [DOI] [PubMed] [Google Scholar]
Bould 1999
- Bould M, Bannister GC. Refractures of the radius and ulna in children. Injury 1999;30(9):583‐6. [DOI] [PubMed] [Google Scholar]
Boutis 2014
- Boutis K, Howard A, Constantine E, Cuomo A, Narayanan U. Evidence into practice: emergency physician management of common pediatric fractures. Pediatric Emergency Care 2014;30(7):462‐8. [DOI] [PubMed] [Google Scholar]
Covidence
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Crawford 2012
- Crawford SN, Lee LS, Izuka BH. Closed treatment of overriding distal radial fractures without reduction in children. Journal of Bone and Joint Surgery. American Volume 2012;94(3):246‐52. [DOI] [PubMed] [Google Scholar]
Daltroy 1998
- Daltroy LH, Liang MH, Fossel AH, Goldberg MJ, Pediatric Orthopaedic Society of North America. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. Journal of Pediatric Orthopaedics 1998;18(5):561‐71. [DOI] [PubMed] [Google Scholar]
Deshpande 2014
- Despande MM, Nadkarni S. Disadvantages of the synthetic cast (letter). Bone and Joint Journal 2014:bjj.boneandjoint.org.uk/content/95‐B/9/1285.e‐letters.
Dua 2017
- Dua K, Stein MK, O'Hara NN, Brighton BK, Hennrikus WL, Herman MJ, et al. Variation among pediatric orthopaedic surgeons when diagnosing and treating pediatric and adolescent distal radius fractures. Journal of Pediatric Orthopedics 2017 Feb 27;[Epub ahead of print]:doi: 10.1097/BPO.0000000000000954. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;317(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Hedström 2010
- Hedström EM, Svensson O, Bergström U, Michno P. Epidemiology of fractures in children and adolescents: increased incidence over the past decade: a population‐based study from northern Sweden. Acta Orthopaedica 2010;81(1):148‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendrickx 2011
- Hendrickx RP, Campo MM, Lieshout AP, Struijs PA, Bekerom MP. Above‐ or below‐elbow casts for distal third forearm fractures in children? A meta‐analysis of the literature. Archives of Orthopaedic and Trauma Surgery 2011;131(12):1663‐71. [DOI: 10.1007/s00402-011-1363-9] [DOI] [PubMed] [Google Scholar]
Hernandez 2018
- Hernandez A, Martinez A. Displaced distal radius fractures in children younger than 11 years old: a randomised controlled trial comparing two treatment methods. Orthopaedic Trauma Association Annual Meeting, 2018 October 17–20; Kissimmee (FL). 2018.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2016
- Hill CE, Masters JP, Perry DC. A systematic review of alternative splinting versus complete plaster casts for the management of childhood buckle fractures of the wrist. Journal of Pediatric Orthopaedics B 2016;25(2):183‐90. [DOI: 10.1097/BPB.0000000000000240] [DOI] [PubMed] [Google Scholar]
Howes 2008
- Howes MC, Cutting P, Thomas M. Splinting of buckle fractures of the distal radius in children. Emergency Medicine Journal 2008;25(4):222‐3. [DOI: 10.1136/emj.2008.058172] [DOI] [PubMed] [Google Scholar]
Hunter 2018
- Hunter J. Information on the proposed CRAFT trial [personal communication]. Email to: H Handoll 24 September 2018.
Jiang 2016
- Jiang N, Cao ZH, Ma YF, Lin Z, Yu B. Management of pediatric forearm torus fractures: a systematic review and meta‐analysis. Pediatric Emergency Care 2016;32(11):773‐8. [DOI] [PubMed] [Google Scholar]
Karl 2015
- Karl JW, Olson PR, Rosenwasser MP. The epidemiology of upper extremity fractures in the United States, 2009. Journal of Orthopaedic Trauma 2015;29(8):e242‐4. [DOI] [PubMed] [Google Scholar]
Kennedy 2010
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture?. Journal of Pediatric Orthopaedics Part B 2010;19(1):77‐81. [DOI] [PubMed] [Google Scholar]
Khandekar 2016
- Khandekar S, Tolessa E, Jones S. Displaced distal end radius fractures in children treated with Kirschner wires ‐ A systematic review. Acta Orthopaedica Belgica 2016;82(4):681‐9. [PubMed] [Google Scholar]
Kumar 2006
- Kumar G. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children (letter). Journal of Bone and Joint Surgery. American Volume 2006;88(8):1888; author reply 1888‐9. [DOI] [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2014
- Li X‐L, Li G‐H. Splint versus plaster cast external fixator for the treatment of distal forearm buckle fracture in children: Systematic review. Chinese Journal of Tissue Engineering Research 2014;18(13):2113‐8. [DOI: 10.3969/j.issn.2095-4344.2014.13.024] [DOI] [Google Scholar]
Macnicol 2010
- Macnicol MF, Parsch K. Chapter 45: wrist and hand fractures. In: Benson M, Fixsen J, Macnicol M, Parsch K editor(s). Children's Orthopaedics and Fractures. London: Springer‐Verlag, 2010:743‐50. [Google Scholar]
Mehlman 2002
- Mehlman CT. Home removal of a backslab 3 weeks after buckle fracture of the distal radius was as safe as removal at a fracture clinic. Journal of Bone and Joint Surgery. American Volume 2002;84(5):883. [Google Scholar]
Mellstrand‐Navarro 2014
- Mellstrand‐Navarro C, Pettersson HJ, Tornqvist H, Ponzer S. The operative treatment of fractures of the distal radius is increasing: results from a nationwide Swedish study. Bone and Joint Journal 2014;96(7):963‐9. [DOI] [PubMed] [Google Scholar]
Mizuta 1987
- Mizuta T, Benson WM, Foster BK, Paterson DC, Morris LL. Statistical analysis of the incidence of physeal fractures. Journal of Pediatric Orthopedics 1987;7(5):518‐23. [DOI] [PubMed] [Google Scholar]
Morris 2006
- Morris MW, Bell MJ. The socio‐economical impact of paediatric fracture clinic appointments. Injury 2006;37(5):395‐7. [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Care Excellence. Fractures (non‐complex): assessment and management. NICE guidelines [NG38], 2016. www.nice.org.uk/guidance/ng38/evidence/full‐guideline‐2358460765 (accessed 19 May 2016).
Proctor 1993
- Proctor MT, Moore DJ, Paterson JM. Redisplacement after manipulation of distal radial fractures in children. Journal of Bone and Joint Surgery. British Volume 1993;75(3):453‐4. [DOI] [PubMed] [Google Scholar]
Randsborg 2009
- Randsborg PH, Sivertsen EA. Distal radius fractures in children: substantial difference in stability between buckle and greenstick fractures. Acta Orthopaedica 2009;80(5):585‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Randsborg 2012
- Randsborg PH, Sivertsen EA. Classification of distal radius fractures in children: good inter‐ and intraobserver reliability, which improves with clinical experience. BMC Musculoskeletal Disorders 2012;13:6. [DOI: 10.1186/1471-2474-13-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rennie 2007
- Rennie L, Court‐Brown CM, Mok JY, Beattie TF. The epidemiology of fractures in children. Injury 2007;38(8):913‐22. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salter 1963
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. Journal of Bone and Joint Surgery. American Volume 1963;45(3):587‐622. [Google Scholar]
Schneidmüller 2011
- Schneidmüller D, Röder C, Kraus R, Marzi I, Kaiser M, Dietrich D, et al. Development and validation of a paediatric long‐bone fracture classification. A prospective multicentre study in 13 European paediatric trauma centres. BMC Musculoskeletal Disorders 2011;12:89. [DOI: 10.1186/1471-2474-12-89] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shah 2015
- Shah NS, Buzas D, Zinberg EM. Epidemiologic dynamics contributing to pediatric wrist fractures in the United States. Hand 2015;10(2):266‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slongo 2007
- Slongo T, Audigé L, AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO pediatric comprehensive classification of long bone fractures (PCCF). Journal of Orthopaedic Trauma 2007;21 Suppl 10:135‐60. [DOI] [PubMed] [Google Scholar]
Thimmaiah 2012
- Thimmaiah R, Bass A. Paediatric fracture clinic referrals: what does it consist of?. British Journal of Medical Practitioners 2012;5(2):a521. [Google Scholar]
Von Keyserlingk 2011
- Keyserlingk C, Boutis K, Willan AR, Hopkins RB, Goeree R. Cost‐effectiveness analysis of cast versus splint in children with acceptably angulated wrist fractures. International Journal of Technology Assessment in Health Care 2011;27(2):101‐7. [DOI] [PubMed] [Google Scholar]
Wierzimok 2017
- Wierzimok AA. Conservative plaster‐free fracture treatment with definitive cast bandage / MOKcast. www.mokcast.com/index_e.html (accessed 20 April 2017).
Wilkins 2005
- Wilkins KE. Principles of fracture remodelling in children. Injury 2005;36 Suppl 1:A3‐11. [DOI] [PubMed] [Google Scholar]
Williams 2005
- Williams AA, Szabo RM. Case report: radial overgrowth and deformity after metaphyseal fracture fixation in a child. Clinical Orthopaedics and Related Research 2005;(435):258‐62. [DOI] [PubMed] [Google Scholar]
Young 2000
- Young NL, Wiliams JI, Joshida KK, Wright JG. Measurement properties of the Activities Scale for Kids. Journal of Clinical Epidemiology 2000;53(2):125‐37. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abraham 2008
- Abraham A, Handoll HH, Khan T. Interventions for treating wrist fractures in children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004576.pub2] [DOI] [PubMed] [Google Scholar]
Handoll 2016
- Handoll HH, Elliott J, Iheozor‐Ejiofor Z, Hunter J, Karantana A. Interventions for treating wrist fractures in children. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD012470] [DOI] [PMC free article] [PubMed] [Google Scholar]


