Abstract
Background
Herpes zoster, also known as 'shingles', is a neurocutaneous disease characterised by the reactivation of the latent varicella zoster virus (VZV), the virus that causes chickenpox when immunity to VZV declines. It is an extremely painful condition that can last many weeks or months and it can significantly compromise the quality of life of affected individuals. The natural process of aging is associated with a reduction in cellular immunity and this predisposes older people to herpes zoster. Vaccination with an attenuated form of VZV activates specific T cell production avoiding viral reactivation. The Food and Drug Administration has approved a herpes zoster vaccine with an attenuated active virus for clinical use among older adults, which has been tested in large populations. A new adjuvanted recombinant VZV subunit zoster vaccine has also been tested. It consists of recombinant VZV glycoprotein E and a liposome‐based AS01B adjuvant system. This new vaccine is not yet available for clinical use.
Objectives
To evaluate the effectiveness and safety of vaccination for preventing herpes zoster in older adults.
Search methods
For this 2015 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 9), MEDLINE (1948 to the 3rd week of October 2015), EMBASE (2010 to October 2015), CINAHL (1981 to October 2015) and LILACS (1982 to October 2015).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing zoster vaccine with placebo or no vaccine, to prevent herpes zoster in older adults (mean age > 60 years).
Data collection and analysis
Two review authors independently collected and analysed data using a data extraction form. They also performed 'Risk of bias' assessment.
Main results
We identified 13 studies involving 69,916 participants. The largest study included 38,546 participants. All studies were conducted in high‐income countries and included only healthy Caucasian individuals ≥ 60 years of age without immunosuppressive comorbidities. Ten studies used live attenuated varicella zoster virus (VZV) vaccines. Three studies tested a new type of vaccine not yet available for clinical use. We judged five of the included studies to be at low risk of bias.
The incidence of herpes zoster, at up to three years of follow‐up, was lower in participants who received the vaccine than in those who received a placebo: risk ratio (RR) 0.49; 95% confidence interval (CI) 0.43 to 0.56, risk difference (RD) 2%, number needed to treat to benefit (NNTB) 50; GRADE: moderate quality evidence. The vaccinated group had a higher incidence of mild to moderate intensity adverse events. These date came from one large study that included 38,546 people aged 60 years or older.
A study including 8122 participants compared the new vaccine (not yet available) to the placebo; the group that received the new vaccine had a lower incidence of herpes zoster at 3.2 years of follow‐up: RR 0.04, 95% CI 0.02 to 0.10, RD 3%, NNTB 33; GRADE: moderate quality evidence. The vaccinated group had a higher incidence of adverse events but most them were of mild to moderate intensity.
All studies received funding from the pharmaceutical industry.
Authors' conclusions
Herpes zoster vaccine is effective in preventing herpes zoster disease and this protection can last three years. In general, zoster vaccine is well tolerated; it produces few systemic adverse events and injection site adverse events of mild to moderate intensity.
There are studies of a new vaccine (with a VZV glycoproteic fraction plus adjuvant), which is currently not yet available for clinical use.
Keywords: Aged; Humans; Middle Aged; Herpes Zoster; Herpes Zoster/prevention & control; Herpes Zoster Vaccine; Herpes Zoster Vaccine/adverse effects; Herpes Zoster Vaccine/therapeutic use; Randomized Controlled Trials as Topic; Vaccines, Attenuated; Vaccines, Attenuated/adverse effects; Vaccines, Attenuated/therapeutic use
Vaccines for preventing herpes zoster (shingles) in older adults
Review question There is a vaccine to prevent shingles. Our objective was to evaluate the effectiveness and safety of the vaccine to prevent shingles in healthy older people.
Background The varicella zoster virus causes chickenpox and can remain dormant inside nerve cells. After many years, it can reactivate, travel through the nerve to the skin and produce blisters along the nerve path. This is called herpes zoster or shingles. It affects people with low immunity such as older people. Before the blisters, the person may feel itching, numbness, tingling or local pain. Herpes zoster causes inflammation of the nerves and severe pain, which can affect quality of life. There are about 5.22 episodes of herpes zoster for every 1000 older people. This is increasing, in part because people are living longer.
Study characteristics Our evidence is current to 26 October 2015. We found 13 randomised controlled trials including 69,917 healthy older adults. Only five of the 13 trials were of high quality and had a low risk of bias. Pharmaceutical companies that produce the vaccines funded all of the included studies.
Key results and quality of the evidence All included studies were conducted in high‐income countries and included only healthy elderly Caucasians (> 60 years) without any immunosuppressive problems.
One big study included 38,546 persons 60 years of age or older. It compared the vaccine with a placebo (fake vaccine). It was a high quality study, which showed that the vaccine is effective in preventing shingles at three years (moderate quality evidence). Adverse effects caused by the vaccine were mostly mild to moderate symptoms at the injection site. Refrigerated vaccines caused fewer injection site adverse effects than frozen vaccines. The injection of the vaccine into the muscle caused fewer adverse effects when it was injected under the skin (subcutaneously). The herpes zoster vaccine caused fewer adverse effects than the 'pneumo 23' vaccine.
A new vaccine, not yet available for clinical use, is being tested. This vaccine contains a small part of varicella zoster virus plus substances that boost the immune response of the body. A study including 8122 participants who were randomised to receive either the new vaccine or a placebo vaccine showed that those in the new vaccine group had fewer episodes of herpes zoster and more mild to moderate adverse events than those in the placebo group (moderate quality evidence).
Summary of findings
Summary of findings for the main comparison.
Available live attenuated VZV zoster vaccine versus placebo for preventing herpes zoster in older adults
| Available live attenuated VZV zoster vaccine versus placebo for preventing herpes zoster in older adults | ||||||
|
Patient or population: healthy older adults Settings: outpatients Intervention: available live attenuated VZV zoster vaccine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Available live attenuated VZV zoster vaccine | |||||
|
Incidence of herpes zoster Clinical and laboratory criteria Follow‐up: median 3.1 years |
Study population | RR 0.49 (0.43 to 0.56) | 38,546 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for available live attenuated VZV zoster vaccine = 1.6% Absolute risk for placebo group = 3.3% |
|
| 33 per 1000 | 16 per 1000 (14 to 19) | |||||
|
Participants with AEs: ≥ 1 serious AE regardless of type of storage of the vaccine Clinical and laboratory criteria Follow‐up: median 3.1 years |
Study population | RR 1.08 (0.96 to 1.2) | 50,896 (4 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk for available live attenuated VZV zoster vaccine = 2.3% Absolute risk for placebo group = 2.2% |
|
| 22 per 1000 | 23 per 1000 (21 to 26) | |||||
|
Participants with AEs: hospitalised Number of participants hospitalised Follow‐up: median 3.1 years |
Study population | RR 1.00 (0.93 to 1.07) | 6616 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for available live attenuated VZV zoster vaccine = 34.1% Absolute risk for placebo group = 34.1% |
|
| 341 per 1000 | 341 per 1000 (317 to 365) | |||||
|
Participants with AEs: injection site AEs Clinical and laboratory criteria Follow‐up: median 3.1 years |
Study population | RR 2.99 (2.75 to 3.26) | 6986 (3 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk for available live attenuated VZV zoster vaccine = 47.9% Absolute risk for placebo group = 16.0% |
|
| 160 per 1000 | 479 per 1000 (440 to 521) | |||||
|
Drop‐outs: death Number of deaths Follow‐up: median 3.1 years |
Study population | RR 1.01 (0.92 to 1.11) | 50,687 (3 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk for available live attenuated VZV zoster vaccine = 3.3% Absolute risk for placebo group = 3.2% |
|
| 32 per 1000 | 33 per 1000 (30 to 36) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Did not describe random sequence generation.
Summary of findings 2.
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo for preventing herpes zoster in older adults
| Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo for preventing herpes zoster in older adults | ||||||
|
Patient or population: healthy older adults Settings: outpatients Intervention: adjuvanted recombinant VZV subunit zoster vaccine (not yet available) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo | |||||
|
Incidence of herpes zoster 3.2 years follow‐up (≥ 60 yo) Clinical and laboratory criteria Follow‐up: mean 3.2 years |
Study population | RR 0.04 (0.02 to 0.1) | 8122 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for adjuvanted recombinant VZV subunit zoster vaccine (not yet available) = 0.2% Absolute risk for placebo group = 3.4% |
|
| 34 per 1000 | 2 per 1000 (1 to 3) | |||||
|
Participants with AEs: any local symptom Clinical criteria Follow‐up: mean 3.2 years |
Study population | RR 6.83 (6.30 to 7.42) | 8759 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for adjuvanted recombinant VZV subunit zoster vaccine (not yet available) = 81.5% Absolute risk for placebo group = 11.9% |
|
| 119 per 1000 | 815 per 1000 (751 to 885) | |||||
|
Participants with AEs: serious AEs Clinical and laboratory criteria Follow‐up: mean 3.2 years |
Study population | RR 1.01 (0.91 to 1.1) | 15,411 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for adjuvanted recombinant VZV subunit zoster vaccine (not yet available) = 9.0% Absolute risk for placebo group = 8.9% |
|
| 89 per 1000 | 90 per 1000 (81 to 99) | |||||
|
Participants with AEs: potential immune‐mediated disease Clinical and laboratory criteria Follow‐up: mean 3.2 years |
Study population | RR 0.81 (0.06 to 1.08) | 15,411 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for adjuvanted recombinant VZV subunit zoster vaccine (not yet available) = 1.0% Absolute risk for placebo group = 1.3% |
|
| 13 per 1000 | 10 per 1000 (1 to 14) | |||||
|
Participants with AEs: deaths Number of deaths Follow‐up: mean 3.2 years |
Study population | RR 0.96 (0.78 to 1.19) | 15,411 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk for adjuvanted recombinant VZV subunit zoster vaccine (not yet available) = 2.2% Absolute risk for placebo group = 2.3% |
|
| 23 per 1000 | 22 per 1000 (18 to 27) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Did not describe allocation concealment and participant flow not clear.
Background
Description of the condition
Herpes zoster, or shingles, is a neurocutaneous disease that can be extremely painful. Frequently, the symptoms can last for many weeks or months after complete healing of the lesions (Gilden 2000). It is caused by the reactivation of the varicella zoster virus (VZV) when immunity to VZV declines.
The geographical distribution of VZV indicates that it is a common human pathogen with a worldwide occurrence (Cohen 2007). Although varicella occurs worldwide, the epidemiology of the disease is markedly different in tropical and temperate countries. In temperate countries such as the United Kingdom (UK) and the United States (US), most people have seroconverted to VZV by adolescence (this means that they have had prior contact with the virus and developed antibodies against it). Serological studies of resident tropical populations and of immigrants from tropical countries indicate that seroconversion generally occurs in late adolescence and adulthood (Lee 1998).
The VZV is a highly contagious agent and in the first contact with the virus, usually in childhood, the individual develops chickenpox (varicella). After this, the VZV can remain dormant for years in the dorsal sensory ganglia of the spinal cord. The latency of the virus is maintained by cellular immunity, which inhibits viral replication. Years later, during periods of decreased cell‐mediated immunity or simply because of aging, the virus can replicate in the dorsal sensory ganglia of the spinal cord and migrate along sensory nerves. Prodromal symptoms of viral reactivation include itching, numbness, tingling or severe localised pain, which precede the appearance of skin lesions by one to five days. The typical cutaneous manifestations of an acute herpes zoster episode include clusters of vesicles that spread in a linear pattern along the path of nerves and do not cross the midline of the body (Cohen 2007; Moffat 2007). Within three to five days, these lesions progress to pustules, ulcerations and crusting and go on to heal spontaneously within two to four weeks (Gnann 2002). This disease causes substantial morbidity and has a significant impact on the quality of life of patients (Gnann 2002; Partridge 2009; Sampathkumar 2009). Schmader 2007 conducted a prospective, observational study of 165 outpatients with acute herpes zoster who were enrolled within 14 days of onset of rash. The pain was moderate to severe and discomfort was common during the acute rash phase. Acute herpetic neuralgia was associated with sleep disruption and impaired general activities and enjoyment of life, especially after the onset of the rash, and had a significant impact on the quality of life of the patients.
Older adults (aged > 60 years old) have an increased risk of developing herpes zoster disease (Arvin 1996; Cho 2007; Heymann 2008; Jih 2009; Thomas 2004). Although familial history of herpes zoster suggests possible genetic predisposition to the disease (Cho 2007; Haanpaa 2002), results from available case‐control studies are conflicting (Gatti 2010; Hicks 2008). Due to lengthening lifespans, there are increasing concerns about quality of life for older adults, a growing segment of the population, especially in high‐income countries. In the United States, the annual incidence of herpes zoster increased from 3.10 episodes per 1000 in older adults in 2000 to 5.22 in 2007 (Rimland 2010).
Description of the intervention
Vaccination with an attenuated form of VZV activates specific T cell production, therefore avoiding viral reactivation. A herpes zoster vaccine with an active virus has been approved for clinical use among older adults by the Food and Drug Administration (FDA) and has been tested in large populations (Oxman 2005). A new adjuvanted recombinant VZV subunit zoster vaccine, not yet available for clinical use, has also been tested. It is composed of recombinant VZV glycoprotein E plus a liposome‐based AS01B adjuvant system (Lal 2015).
Available live attenuated VZV zoster vaccine: this vaccine contains the same live attenuated virus used in the chickenpox vaccine but it has over 14‐fold more plaque‐forming units (PFUs) of the attenuated virus per dose. Therefore the two vaccines are not interchangeable (Oxman 2005).
Adjuvanted recombinant subunit zoster vaccine (not yet clinically available): this other type of vaccine has recently been tested (Leroux‐Roels 2012). It does not contain the live attenuated virus but a small fraction of the virus, which cannot replicate but can boost immunogenicity. This vaccine contains antigen gE (glycoprotein E), which is the most abundant antigen in VZV‐infected cells and the main target for VZV‐specific CD4 + T‐cell response (Arvin 1986). This vaccine also includes adjuvant AS01, which is a liposome‐based adjuvant system containing immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) plus saponin QS‐21 (Quillaja saponaria Molina, fraction 21) (Baldridge 2004; Kensil 1991). It has not yet been approved for clinical use.
How the intervention might work
Primary infection with VZV induces the production of specific memory T cells in sufficient numbers to keep the virus in its latent form. Host factors such as aging, or other conditions that affect cellular immunity, may reduce T cells to levels that can no longer inhibit viral replication therefore increasing the likelihood of clinical manifestations of the disease.
Available live attenuated VZV zoster vaccine: this vaccine, which consists of live attenuated VZV, activates specific T cell production, thus increasing existing immunity and avoiding reactivation of viral replication (Arvin 2005). Several randomised controlled trials (RCTs) have evaluated the efficacy and safety of live attenuated virus vaccine in preventing herpes zoster (Gilderman 2008; Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012).
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available): this new vaccine contains antigen gE (glycoprotein E), which is the most abundant antigen in VZV‐infected cells and the main target for VZV‐specific immunity CD4 + T‐cell response (Arvin 1986). This vaccine also includes adjuvant AS01, which is a liposome‐based adjuvant system containing immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) plus saponin QS‐21 (Quillaja saponaria Molina, fraction 21) (Baldridge 2004; Kensil 1991). The adjuvant component is important because it helps to elicit an early, high and long‐lasting immune response with less antigen (Rajesh 1995); consequently this leads to additional stimulation of the immune system when it is given with the gE antigen. The new adjuvanted recombinant zoster vaccine improves immune stimulation against VZV and its efficacy and safety have been tested in several RCTs (Chlibek 2013; Chlibek 2014; Lal 2015; Leroux‐Roels 2012).
Why it is important to do this review
Although the incidence of herpes zoster increases with age, prevalence rates differ worldwide (Choi 2010; Hope‐Simpson 1965; Jih 2009; Rimland 2010; Schmader 2008). Every year more than one million new cases are diagnosed in the US (Weaver 2007). The acute episode of herpes zoster can significantly affect the quality of life of affected individuals due to pain, increased risk of depression, anxiety and significantly lower emotional well‐being (Katz 2004).
Herpes zoster also has a significant impact on the health system, particularly among older adults. In addition, the effectiveness of some treatments for herpes zoster is relatively uncertain (Hornberger 2006). Several randomised controlled trials (RCTs) have evaluated the efficacy and safety of vaccines in preventing herpes zoster (Gilderman 2008; Oxman 2005). Recent trials have tested a new adjuvanted recombinant VZV vaccine (Chlibek 2013; Chlibek 2014; Leroux‐Roels 2012). If it is proved that this vaccine is safe and effective, it could be given to immunocompromised people who frequently have herpes zoster (Dolin 1978). Therefore, it is necessary to conduct a systematic review of these trials to critically appraise and synthesise the best available evidence.
This is an update of a Cochrane review first published in 2012 (Gagliardi 2012).
Objectives
To evaluate the effectiveness and safety of vaccination for preventing herpes zoster in older adults.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs, regardless of publication date or language.
Types of participants
We included studies involving older adults (mean age ≥ 60 years). We excluded trials involving participants with immunosuppressive disorders.
Types of interventions
We included clinical trials that compared herpes zoster vaccine, of any dose and potency, with at least one of the following comparison groups.
Any other type of intervention (for example, varicella vaccine, antiviral medication).
Placebo.
Nothing (no vaccine).
Types of outcome measures
Primary outcomes
Incidence of herpes zoster, diagnosed according to the criteria (clinical and/or laboratory) established by the primary studies.
Secondary outcomes
Adverse events: local or systemic reactions (for example, pain, pruritus, swelling, headache) occurring at any time after vaccination.
Drop‐outs.
Search methods for identification of studies
Electronic searches
In this 2015 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 9), MEDLINE (1948 to October week 3 2015), EMBASE (2010 to October 2015), CINAHL (1981 to October 2015) and LILACS (1982 to October 2015).
We used the search strategy in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (Appendix 2), LILACS (Appendix 3) and CINAHL (Appendix 4). We imposed no language or publication restrictions.
Searching other resources
We searched two trial registries, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov, for completed and ongoing studies (latest search 26 October 2015).
We checked the reference lists of relevant studies. We contacted trial authors for additional information and unpublished studies. We checked conference proceedings and thesis banks for unpublished studies. We also contacted vaccine manufacturers for unpublished data.
Data collection and analysis
Selection of studies
Two review authors (AG, BNGS) independently assessed titles and abstracts of all retrieved citations according to our inclusion criteria. We used the Kappa coefficient to test concordance among review authors (Latour 1997). We resolved discrepancies through consensus and consulted a third review author (MRT) in case of disagreements.
Data extraction and management
We created a specific data extraction form for this review to collect relevant information such as study methods, participants, intervention group, control group and outcomes.
Assessment of risk of bias in included studies
We evaluated the methodological quality of each included study in accordance with the criteria established by the Cochrane tool for assessing risk of bias (Higgins 2011). We evaluated the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
We classified each of these domains as 'low risk of bias', 'uncertain risk of bias' or 'high risk of bias'.
Measures of treatment effect
Dichotomous data
For binary data, we calculated the results for each study using the risk ratio (RR) with 95% confidence interval (CI) and number needed to treat for an additional beneficial outcome (NNTB) for efficacy and number needed to treat for an additional harmful outcome (NNTH) for adverse events, where there were statistically significant differences. We entered the data into the Cochrane Review Manager software (RevMan 2014), and conducted meta‐analyses using a random‐effects model.
Continuous data
For outcomes presented in other forms (for example, reported as medians, quartiles, etc.) or without consistent statistical information (despite requests to the trial authors) (for example, standard deviations (SDs), number of patients, etc.), we inserted these data into an additional table.
Unit of analysis issues
The patient was the unit of analysis, including patients undergoing more than one intervention in a cross‐over trial.
Dealing with missing data
For dichotomous data, we performed intention‐to‐treat (ITT) analyses to include all participants randomised to the intervention groups. We contacted trial authors to supply any missing data from the included studies. In studies that did not explain the reasons for withdrawal, we analysed data assuming the worst possible outcome, since imputation of data is a matter of personal judgement (Higgins 2011).
Assessment of heterogeneity
We assessed the consistency of results through visual inspection of the forest plots and by calculating the I2 statistic (Higgins 2003), which estimates the proportion of variation in point estimates that is due to heterogeneity rather than sampling error. We assumed substantial (significant) heterogeneity when the I2 statistic was > 50%. We analysed data using a fixed‐effect model, but if there was significant heterogeneity between studies, we used the random‐effects model.
Assessment of reporting biases
It was not necessary to prepare a funnel plot since we included fewer than 10 studies in the meta‐analysis.
Data synthesis
For dichotomous variables we calculated the RR and for continuous variables we calculated the mean difference (MD), when studies reported their results in the same units of measurement. When continuous data were reported in different units, we pooled the data through standardised mean differences (SMDs). For all statistical methods used to pool data, we used 95% CIs.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: incidence of herpes zoster, adverse events and drop‐outs. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) (Atkins 2004), in order to assess the quality of the body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Guyatt 2006a; Guyatt 2006b). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Factors that can reduce the quality of the evidence (downgrade) include:
limitations in study design or execution (risk of bias): lower by one or two levels;
inconsistency of results: lower by one or two levels;
indirectness of evidence: lower by one or two levels;
imprecision: lower by one or two levels;
publication bias: lower by one or two levels.
Factors that can increase the quality of the evidence (upgrade) include:
large magnitude of effect: upgrade by one or two levels;
all plausible confounding that would reduce the demonstrated effect or increase the effect if no effect was observed: upgrade by one level;
dose‐response gradient: upgrade by one level.
Based on those factors, for each outcome, the quality of evidence is classified as: 'high quality evidence', ' moderate quality evidence', 'low quality evidence' or 'very low quality evidence' (Schünemann 2011):
high quality evidence: RCTs or double‐upgraded observational studies;
moderate quality evidence: downgraded RCTs or upgraded observational studies;
low quality evidence: double‐downgraded RCTs or observational studies;
very low quality evidence: triple‐downgraded RCTs or downgraded observational studies; or case series/case reports.
Subgroup analysis and investigation of heterogeneity
We grouped results from studies according to methodological and clinical aspects, such as vaccine dosage (plaque‐forming units (pfu) per dose), vaccine conservation method (refrigerated or frozen), participant age, previous episode of herpes zoster and simultaneous administration of other vaccines.
Sensitivity analysis
Where possible, we performed sensitivity analyses. We investigated the impact of quasi‐RCTs, studies with lower methodological quality and unpublished data on the results of the review.
Results
Description of studies
In this 2015 review update, we included 13 RCTs published in 20 papers (Berger 1998; Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Gilderman 2008; Lal 2015; Levin 2000; Mills 2010; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013). Only Mills 2010 used a cross‐over design and reported data separately for patients 50 to 59 years and 60 or older; we only included data pertaining to the older participants of this study. The Lal 2015 study presented efficacy data by age and in theory we would be able to use these data for participants aged 60 or over. However, the authors replied that safety data per age were not yet available and we therefore used the data provided for participants 50 years of age or more.
Results of the search
In the first publication of this review, we searched five databases (CENTRAL, MEDLINE, EMBASE, CINAHL and LILACS) and identified 467 citations, which reduced to 328 after excluding duplicates (Gagliardi 2012). Of these, we selected 19 citations for full‐text reading, which reported on 14 RCTs. We excluded six of these trials and included eight in the review (corresponding to 13 published references). In the clinical trials registry platforms, we identified three ongoing studies as of 25 June 2012.
In this 2015 update we searched the same five databases: CENTRAL (2015, Issue 3); MEDLINE (1948 to October week 3 2015), EMBASE (2010 to October 2015), CINAHL (1981 to October 2015) and LILACS (1982 to October 2015) and we identified a total of 101 references. After excluding the references examined in the initial search and duplicated references, we identified 72 newly published records. After analysis of titles and abstracts, we excluded 65 records and selected seven for full‐text reading: we included six of these and excluded one because it did not involve older people (Leroux‐Roels 2012). One of the newly included studies had two publications (European Geriatric Medicine 2013;4 (Suppl):81‐141 and Vaccine 2015;33(6):789‐95) (Diez‐Domingo 2015). Since we considered both publications as being one study, a total of five new studies are included in this update (Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Lal 2015; Vesikari 2013). Figure 1 depicts the complete process of study identification and selection of all studies (including those included in the first publication of this review).
Figure 1.

Study flow diagram 2015 update
We identified 11 ongoing studies in the trial registry platforms (ClinicalTrials.gov site and the International Clinical Trials Registry Platform (ICTRP)) on 15 November 2015. The detailed steps of the whole process of selection of studies are shown in Figure 1.
Included studies
The 13 included trials enrolled a total of 69,916 participants.
Available live attenuated VZV zoster vaccine
We included 10 trials (53,381 participants) reporting on the live attenuated VZV zoster vaccine. All of them assessed the safety of the vaccine and only Oxman 2005 also evaluated its efficacy. Four studies compared the vaccine with placebo (Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012), one study compared it with pneumo 23 vaccine (Berger 1998), and another study compared different routes of administration (intramuscular versus subcutaneous; Diez‐Domingo 2015). One study assessed different forms of vaccine conservation (refrigerated and frozen; Gilderman 2008); another study compared live versus inactivated virus (Levin 2000). One trial tested different amounts of the virus (higher‐potency zoster vaccine to lower‐potency zoster vaccine; Tyring 2007), and another compared two doses of a zoster vaccine versus a single dose and also two doses given at different intervals (Vesikari 2013). The most important study was Oxman 2005, which included 38,546 participants and evaluated the efficacy and safety of zoster vaccine versus placebo and performed a more detailed safety investigation, with voluntary (not randomised) participation of patients. This study followed participants for an average of five years.
Investigators reported adverse events at various time intervals after inoculation of the zoster vaccine: 28 days (Gilderman 2008; Mills 2010; Vesikari 2013), 35 days (Diez‐Domingo 2015), 42 days (Berger 1998; Oxman 2005; Tyring 2007; Vermeulen 2012), and serious side effects until 182 days after the vaccination (Murray 2011). Vermeulen 2012 reported adverse events within six months after the second vaccination.
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available)
We included three studies on a new zoster vaccine that it not yet available for clinical use. These studies involved a total of 16,535 participants (Chlibek 2013; Chlibek 2014; Lal 2015). Both Chlibek 2013 and Chlibek 2014 evaluated adverse effects. The first study compared four groups that received either lower or higher volumes of adjuvants plus gE subunit VZV or unadjuvanted gE or saline injections. The second trial compared adverse events in three groups of VZV plus gE in three different quantities, one group that received unadjuvanted gE and one group that received only saline. The third study assessed the efficacy and safety of the new vaccine versus placebo (Lal 2015).
The adverse effects were monitored for approximately one year after last vaccination (Chlibek 2013), and 36 months after last dose (Chlibek 2014). Lal 2015 is a ongoing study.
Excluded studies
We excluded the following seven studies.
Hayward 1994, Hayward 1996 and Patterson‐Bartlett 2007: RCTs evaluating zoster vaccine focused on immunogenicity, without any clinical outcomes.
Irwin 2007: a RCT that tested another intervention (Tai Chi) and not the zoster vaccine.
Kerzner 2007: a RCT evaluating zoster vaccine administered concomitantly with influenza vaccine.
Leroux‐Roels 2012: a RCT evaluating zoster vaccine, but including participants outside the age range of interest (55 to 57 years).
Macaladad 2007: a RCT evaluating zoster vaccine, but including participants outside the age range of interest (adults less than 60 years).
Risk of bias in included studies
Details of the 'Risk of bias' assessment for each trial are shown in the Characteristics of included studies section. The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3. We categorised Chlibek 2013, Diez‐Domingo 2015, Lal 2015, Oxman 2005, Vermeulen 2012 and Vesikari 2013 as having a low risk of bias. All of these studies had at least five of the eight domains categorised as 'low risk of bias', thus fulfilling the criteria recommended by Cochrane for establishing that a study is at low risk of bias.
Figure 2.
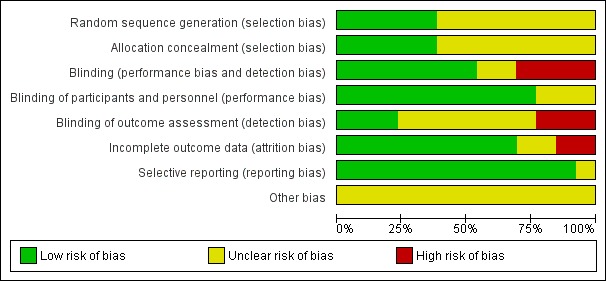
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
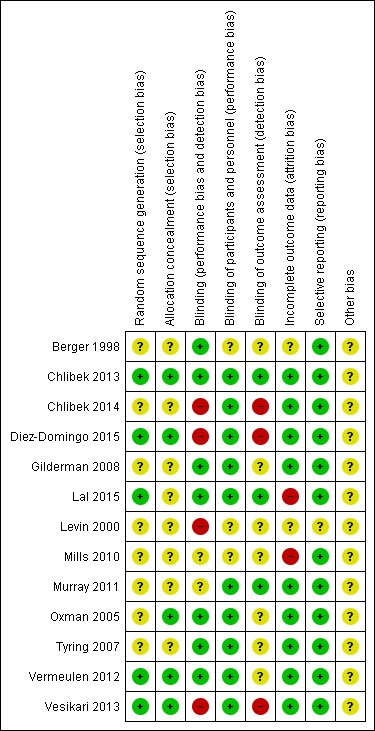
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
See Table 13 for the complete evaluation of the risk of bias of included studies.
Table 1.
Comprehensive risk of bias
| Domain | Risk of bias |
| Allocation (selection bias): randomisation criteria | We graded 5 studies as having a low risk of bias for random sequence generation (selection bias) because they described how the randomisation was done (Chlibek 2013; Diez‐Domingo 2015; Lal 2015; Vermeulen 2012; Vesikari 2013). Chlibek 2013 stated that "Randomization was made using an algorithm that stratified by country, minimized for age, and included a block size of 11". In Diez‐Domingo 2015: "The subjects were randomised using an electronic case report form (e‐CRF)". Lal 2015 stated that "We randomly assigned participants in a 1:1 ratio to receive either vaccine or placebo using an online centralized randomization system". Vermeulen 2012 stated that "Subjects were randomised in a 1:1 ratio to receive two doses of either zoster vaccine or placebo, according to a computer‐generated, study‐centre specific allocation schedule". Vesikari 2013 used "blocks of randomisation, with stratification by age (70–79 y and ≥ 80 y) and country". The other 8 trials provided no details on the randomisation process and we therefore classified them as having an unclear risk of bias for this domain (Berger 1998; Chlibek 2014; Gilderman 2008; Levin 2000; Mills 2010; Murray 2011; Oxman 2005; Tyring 2007). |
| Allocation (selection bias): allocation criteria | We classified Chlibek 2013, Diez‐Domingo 2015, Lal 2015, Oxman 2005, Vermeulen 2012 and Vesikari 2013 as having low risk of bias because of adequate allocation concealment described by the trial authors as follows. Chlibek 2013: "Treatments were allocated at each site using a central randomisation system on the Internet". Diez‐Domingo 2015: "Allocation schedules were generated using a 1:1 ratio with permuted blocks of 4‐6". Lal 2015: "Participants were stratified according to region and age group (50 to 59, 60 to 69, and ≥70 years)". Oxman 2005: "Each study site received randomly ordered vials of zoster vaccine and placebo in separate boxes for each age stratum". Vermeulen 2012: "Allocation numbers were assigned sequentially by the study site personnel to subjects who met the study eligibility criteria, beginning with the lowest number available at the study centre, after informed consent and medical history had been obtained. The allocation schedule was generated by a sponsor statistician not otherwise associated with the zoster vaccine program". Vesikari 2013: "The allocation schedule was generated using balanced permuted blocks of randomisation" Berger 1998, Chlibek 2014, Gilderman 2008, Levin 2000, Mills 2010, Murray 2011 and Tyring 2007 did not report details of allocation concealment and we therefore classified these trials as having an 'unclear' risk of bias for this domain. |
| Blinding (performance bias and detection bias) | 8 trials were double‐blind and we considered them at low risk for this domain (Berger 1998; Chlibek 2013; Gilderman 2008; Lal 2015; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012). The Berger 1998 trial had 4 arms: 3 received different concentrations of a live attenuated VZV/Oka vaccine under double‐blind conditions. The 4th arm used a pneumococcal polysaccharide vaccine as a control for reactogenicity and immune response, under single‐blind conditions Chlibek 2013 stated that "Both vaccine recipients and observers responsible for evaluations were blinded to which formulation was administered". Gilderman 2008 included the following comment: "Double‐blind, with in‐house blinding. The vaccine and placebo were indistinguishable from each other." Lal 2015 reported "Because the appearance of the reconstituted HZ/su vaccine differed from the placebo solution, injections were prepared and administered by study staff who did not participate in any study assessment." In Murray 2011, the authors reported that "The zoster vaccine and placebo were reconstituted with sterile diluent immediately prior to administration, and were indistinguishable from each other in appearance. Placebo was the vaccine stabiliser of zoster vaccine with no live virus. An independent Data Monitoring Committee was established for continuous safety oversight during the study." Oxman 2005 provided the following statement: "Since the reconstituted zoster vaccine had a different appearance from the placebo, reconstitution and administration were performed by technicians who did not otherwise interact with subjects, evaluate outcomes or adverse events, answer the telephone or enter study data." Tyring 2007 states "Blinded subject, investigator and sponsor. The 2 potency formulations were indistinguishable in appearance". Vermeulen 2012 declares that "The subject, investigator, clinical study site personnel, and sponsor personnel directly involved in the study were blinded to whether the subject received zoster vaccine or placebo. They remained blinded until all subjects completed the study. The clinical materials were prepared by an unblinded vaccine coordinator at each clinical site, because of differences in the turbidity of the study vaccine and placebo. Each vial of vaccine or placebo was labelled with a subject‐specific allocation number. The unblinded vaccine coordinator reconstituted the study vaccine/placebo and wrapped the syringe in an opaque label containing subject allocation number and time of reconstitution. The unblinded vaccine coordinator did not have any contact with the subject and did not disclose the contents of the syringe to the person administering the study vaccine/placebo." We classified 3 trials as having a 'low risk of bias' only for the domain "blinding of participants and personnel (performance bias)" although "personnel were not blinded" because the participants themselves were blinded and they were the ones who described adverse events in diary cards (Chlibek 2014; Diez‐Domingo 2015; Vesikari 2013). Please see below: Chlibek 2014 described: "solicited local reactions (pain, redness and swelling) and general reactions (fatigue, fever, headache and myalgia) were recorded by subjects on diary cards for seven days after each vaccination". Diez‐Domingo 2015 stated: "Between visit 1 and 2, the participants were given a diary card to record their temperature if they were febrile (oral temperature ≥38.3 ◦C), occurrence of any solicited injection‐site (erythema, swelling and pain) adverse reactions (Days 0–4) and any unsolicited injection‐site adverse reactions, varicella, varicella‐like rashes, HZ and zoster‐like rashes and other systemic adverse events (AEs) (Days 0–28). They were also asked to report any serious AEs (SAEs) that occurred at any time during the study". Vesikari 2013 provided the following description: "Solicited injection‐site reactions (erythema, swelling, and pain) occurring within 4 days of vaccination were recorded by participants in a diary card. Other injection‐site reactions and systemic AEs were recorded in the diary card for up to 28 d following each vaccination." 1 trial was an open study and we considered it to be at high risk of bias for blinding (Levin 2000). We classified Mills 2010 as 'unclear risk of bias' because the authors did not provide any information on blinding. |
| Incomplete outcome data (attrition bias) | We classified Chlibek 2013, Chlibek 2014, Diez‐Domingo 2015, Gilderman 2008, Murray 2011, Oxman 2005, Tyring 2007, Vermeulen 2012 and Vesikari 2013 as 'low risk' in this domain because the flow of patients was clear. Mills 2010 had no data on the first arm of the cross‐over study and we therefore classified it as 'high risk'. We also classified Lal 2015 as high risk of bias because the patient flow is not clear. We classified Berger 1998 and Levin 2000 as 'unclear risk' as they did not provide any information for this domain. |
| Selective reporting (reporting bias) | We classified the following studies as 'low risk' in this domain. In Berger 1998, the adverse events originally defined by the authors were presented for all groups. Chlibek 2013 presented the adverse events originally defined by the authors in all groups that received 2 doses of 2 different amounts of adjuvant plus gE subunit VZV, unadjuvanted gE or saline. Chlibek 2014 also presented the adverse events associated with 2 doses of different amounts of adjuvanted gE, unadjuvanted gE or saline. Diez‐Domingo 2015 presented all adverse events proposed in the methodology in both groups (intramuscular versus subcutaneous zoster vaccine). Gilderman 2008 reported all adverse events that the investigators selected, for both groups (refrigerated versus frozen zoster vaccines). In Lal 2015, the data for efficacy and safety of the adjuvanted recombinant zoster vaccine proposed in the methods were described in the results. Mills 2010 described in the results all of the adverse events listed in the methods. Murray 2011 presented in the results all the serious adverse events that were defined in the methods section. Oxman 2005 reported in the results all the data on effectiveness and adverse events that the authors proposed in their methodology. Tyring 2007 provided in the results all the adverse events defined in the methods section, for both higher‐potency and lower‐potency zoster vaccines. Vermeulen 2012 described in their results all adverse events listed by the authors in the methods for both groups and Vesikari 2013 reported all the data that had been proposed in their methodology in the results section, for the 3 groups who received 2 doses of zoster vaccines given at different times or a single dose. We classified Levin 2000 as having an 'unclear' risk of bias for this domain because it was basically a study that analysed immune response. |
| Other potential sources of bias | We did not identify any significant aspects pertaining to this domain. |
Allocation
Randomisation criteria
We graded five studies as having a low risk of bias for random sequence generation (selection bias) because they described how the randomisation was done (Chlibek 2013; Diez‐Domingo 2015; Lal 2015; Vermeulen 2012; Vesikari 2013). See Table 13 for more details.
Allocation criteria
We classified Chlibek 2013, Diez‐Domingo 2015, Lal 2015, Oxman 2005, Vermeulen 2012 and Vesikari 2013 as having a low risk of bias because of adequate allocation concealment described by the trial authors. See Table 13 for more details.
Blinding
Seven trials were double‐blind and we considered them at low risk for this domain (Berger 1998; Chlibek 2013; Gilderman 2008; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012). See Table 13 for more details.
Incomplete outcome data
We classified Chlibek 2013, Chlibek 2014, Diez‐Domingo 2015, Gilderman 2008, Murray 2011, Oxman 2005, Tyring 2007, Vesikari 2013 and Vermeulen 2012 as 'low risk' in this domain because the flow of patients was clear. Mills 2010 had no data on the first arm of the cross‐over study and we therefore classified it as 'high risk'. We classified Berger 1998 and Levin 2000 as 'unclear risk' as they did not provide any information for this domain.
Selective reporting
We classified the following studies as 'low risk' in this domain: Berger 1998; Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Gilderman 2008; Lal 2015; Mills 2010; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013. See Table 13 for more details. We classified Levin 2000 as having an 'unclear' risk of bias for this domain because it was basically a study that analysed immune response.
Other potential sources of bias
We did not identify any significant aspects pertaining to this domain.
Quality of evidence
In the comparison between available live attenuated zoster vaccine versus placebo (Oxman 2005), the overall quality of the evidence for the main effectiveness outcome ('incidence of herpes zoster' up to three years of follow‐up) (Types of outcome measures) was moderate. The reason for downgrading the evidence was due to the risk of bias of this study, because it did not describe random sequence generation (Table 1).
We classified the quality of the evidence for safety outcomes up to three years of follow‐up (hospital admissions or participants with injection site adverse effects) as moderate. We downgraded by one point because of risk of bias due to the lack of description of random sequence generation (Table 1).
Effects of interventions
Primary outcome
1. Incidence of herpes zoster
Available live attenuated varicella zoster virus (VZV) vaccine versus placebo
Oxman 2005 evaluated the effectiveness of zoster vaccine versus placebo in reducing the incidence of herpes zoster with a median surveillance of 3.1 years and reported a significant reduction for this outcome in the vaccinated group: risk ratio (RR) 0.49, 95% confidence interval (CI) 0.43 to 0.56 (Analysis 1.1.1). Although this was a significant difference in favour of the intervention, the magnitude of this effect was a risk difference (RD) of 2% and the number needed to treat for an additional beneficial outcome (NNTB) was 50. The quality of the evidence was moderate due to one downgrade because of risk of bias (no description of the randomisation process) (Table 1).
Analysis 1.1.
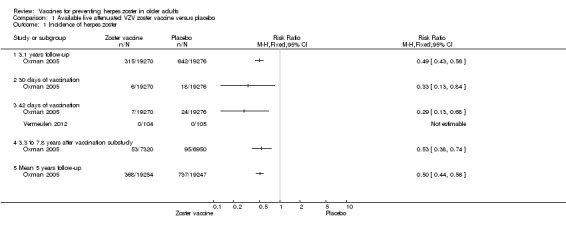
Comparison 1 Available live attenuated VZV zoster vaccine versus placebo, Outcome 1 Incidence of herpes zoster.
The vaccinated group had a reduced incidence of herpes zoster as early as 30 days post‐vaccination: RR 0.33, 95% CI 0.13 to 0.84 (Analysis 1.1.2). These cases were excluded from the final intention‐to‐treat (ITT) analysis. At 42 days post‐vaccination, the benefits of vaccination are clear, with a RR of 0.29 (95% CI 0.13 to 0.68) (Analysis 1.1.3).
The continuation of the Oxman 2005 study was published in 2012 (Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R et al. Clinical Infectious Diseases 2012;55(10): 1320–8) and evaluated the effectiveness of the vaccine five years after the individuals had been vaccinated. However, the published data report different dates for the collection of outcomes in the intervention and the placebo groups. The data from the zoster vaccine group are from December 2004 to March 2006 (16 months). In the placebo group, data were reported from December 2004 to September 2005 (10 months), since in October 2005 the zoster vaccine was also offered to participants in the placebo group, as stated by the authors: "Beginning in October 2005, open‐label zoster vaccine was offered without charge to Shingles Prevention Study placebo recipients." We contacted the authors of this study asking for the data corresponding to the period from December 2004 to September 2005 (10 months) for both groups (vaccine and placebo). They replied to our request but did not provide this information and suggested instead that we should assume a uniform rate of events and calculate the estimated number of cases from that. According to their suggestion, we calculated that the inferred rate of incidence of herpes zoster (from December 2004 to September 2005) would be 53 in the vaccine group at 10 months (total number of herpes zoster cases in the vaccine group 84 in 16 months, therefore 53 in 10 months) and the incidence of herpes zoster would be 95 cases in 10 months in the placebo group. The resulting RR was 0.53, 95% CI 0.38 to 0.74, RD ‐0.01, 95% CI ‐0.01 to ‐0.00 and NNTB 100, in favour of the vaccinated group (Analysis 1.1.4). By the same reasoning, when considering the follow‐up period of five years, there was a significant decrease in the incidence of herpes zoster in the vaccine group compared to the placebo group: RR 0.50, 95% CI 0.44 to 0.56; RD ‐0.02, 95% CI ‐0.02 to ‐0.02 and NNTB 50 (Analysis 1.1.5). We did not include these data in Table 1 since they are inferred data.
The interference of herpes zoster in activities of daily life (ADL) was measured by the zoster brief pain inventory (ZBPI ADL), in which scores greater than or equal to 300 indicate significant pain‐related interference in daily life and quality of life (Coplan 2004). There were no significant differences between the vaccinated and placebo groups for this outcome in the study by Oxman 2005 (RR 0.63, 95% CI 0.34 to 1.16) (Analysis 1.2).
Analysis 1.2.

Comparison 1 Available live attenuated VZV zoster vaccine versus placebo, Outcome 2 Incidence of herpes zoster with ZBPI ADL. Severity of interference scores of 300 or greater (high score is worse).
Higher‐potency versus lower‐potency zoster vaccine
Tyring 2007 compared higher‐potency zoster vaccine with lower‐potency zoster vaccine and reported a higher incidence of herpes zoster (the polymerase chain reaction was positive for wild type of VZV in two cases) in the first group but this difference was not significant (RR 2.55, 95% CI 0.12 to 52.99) (Analysis 2.1).
Analysis 2.1.
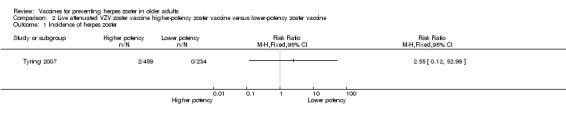
Comparison 2 Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine, Outcome 1 Incidence of herpes zoster.
Live versus inactivated zoster vaccine
One study, Levin 2000, compared live zoster vaccine with an inactivated zoster vaccine and reported no differences in the incidence of herpes zoster (RR 0.96, 95% CI 0.06 to 15.17) (Analysis 4.1).
Analysis 4.1.

Comparison 4 Live attenuated VZV zoster vaccine versus inactivated zoster vaccine, Outcome 1 Incidence of herpes zoster.
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available)
The efficacy of the new recombinant adjuvanted VZV subunit vaccine was tested by Lal 2015. During the follow‐up of 3.2 years, there was a decrease in the incidence of herpes zoster in vaccinated participants compared to those who received a placebo: RR 0.04, 95% CI 0.02 to 0.10 (Analysis 10.1), RD 3% and NNTB 33. We classified the evidence as being of moderate quality because we downgraded the score due to lack of information on allocation concealment and because the flow of the participants was not clear (Table 2).
Analysis 10.1.
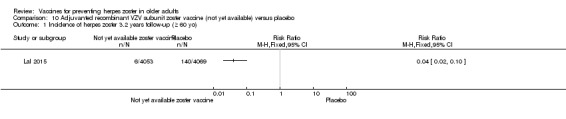
Comparison 10 Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo, Outcome 1 Incidence of herpes zoster 3.2 years follow‐up (≥ 60 yo).
Secondary outcomes
1. Adverse events
Available live attenuated VZV zoster vaccine versus placebo
Four studies compared herpes zoster vaccine to placebo and presented safety data that could be pooled into a meta‐analysis (Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012). Oxman 2005 presented more detailed assessment of safety only in a subgroup of patients (zoster vaccine N = 3345; placebo N = 3271). Murray 2011 assessed only serious adverse events.
The main findings for adverse events are:
Participants receiving the active agent had a higher risk of adverse events than those receiving placebo. When we pooled data from studies reporting the number of participants with one or more adverse events (Mills 2010; Oxman 2005; Vermeulen 2012), we observed an increased risk in the vaccine group: RR 1.70, 95% CI 1.61 to 1.80, RD 0.24, 95% CI 0.22 to 0.26 and number needed to treat to harm (NNTH) 4.1, 95% CI 3.8 to 4.5 (Analysis 1.3.1).
Analysis 1.3.
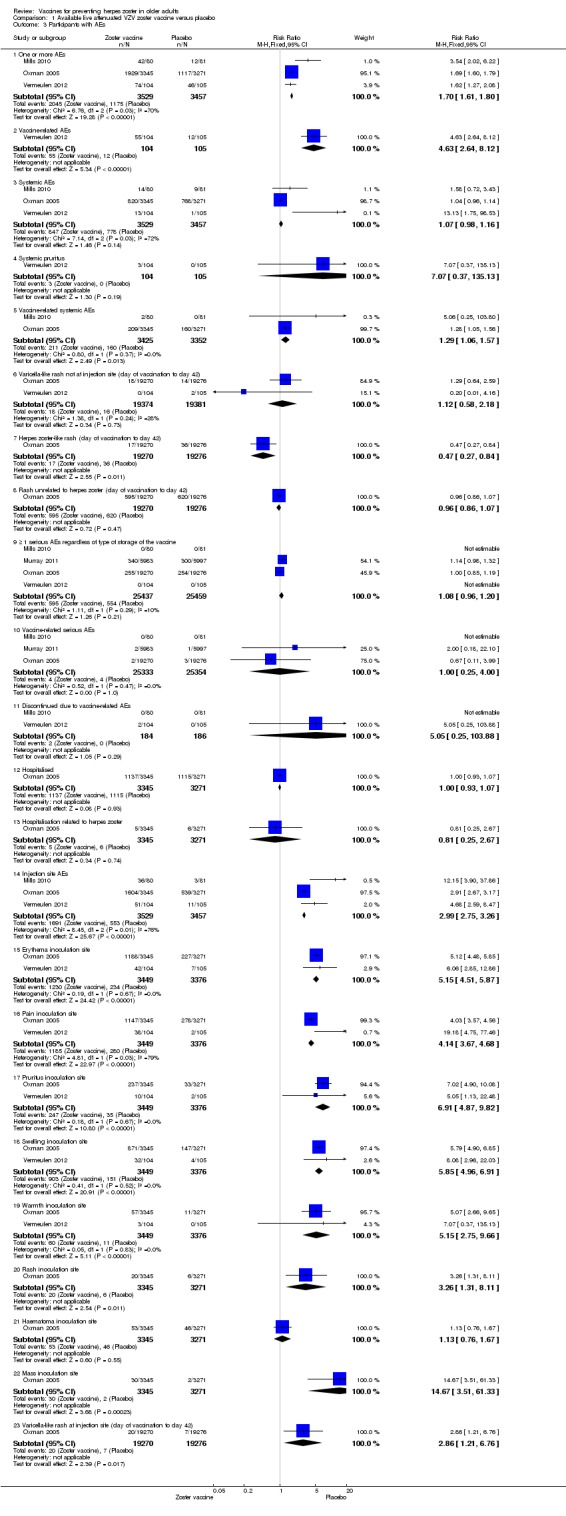
Comparison 1 Available live attenuated VZV zoster vaccine versus placebo, Outcome 3 Participants with AEs.
As expected, vaccine‐related adverse events were more frequent in the vaccinated group than in the placebo group (RR 4.63, 95% CI 2.64 to 8.12; RD 0.41, 95% CI 0.30 to 0.53 and NNTH 2.4, 95% CI 1.9 to 3.3) (Analysis 1.3.2) (Vermeulen 2012).
Vaccine‐related systemic adverse events were more frequent in the vaccinated group than in the placebo group: pooled data RR 1.29, 95% CI 1.06 to 1.57, RD 0.01, 95% CI ‐0.01 to 0.02 (Mills 2010; Oxman 2005) (Analysis 1.3.5).
There were no significant differences between the groups receiving zoster vaccine or placebo for: one or more serious adverse event (including death) (Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012); vaccine‐related serious adverse events (Mills 2010; Murray 2011; Oxman 2005); discontinuation due to a vaccine‐related adverse event (Mills 2010; Vermeulen 2012).
The vaccinated group had a higher risk of injection site adverse events than the placebo group, with a pooled RR of 2.99 (95% CI 2.75 to 3.26), a RD of 0.32 (95% CI 0.30 to 0.34) and a NNTH of 3.1 (95% CI 2.9 to 3.3) (Analysis 1.3.14) (Mills 2010; Oxman 2005; Vermeulen 2012).
Specific injection site adverse events were more frequent in the vaccinated group but mild to moderate in intensity.
In Table 1 we present the most important adverse events: serious adverse events, hospitalisation, injection site adverse events and death. Although the vaccinated groups had a higher rate of injection site adverse events, this was not detected for serious adverse events, hospitalisation or deaths.
See Table 14 for details of adverse events.
Table 2.
Adverse events of available live attenuated VZV zoster vaccine
| Comparison (studies) | Results |
|
Available live attenuated VZV zoster vaccineversus placebo (Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012) |
The risk of herpes zoster‐like rash up to 42 days post‐vaccination (Oxman 2005) was lower in the vaccinated group (RR 0.47, 95% CI 0.27 to 0.84) than the placebo group but without a significant RD (Analysis 1.3.7). The following systemic AEs were not significantly different between the groups receiving zoster vaccine or placebo: systemic AEs (Mills 2010; Oxman 2005; Vermeulen 2012), systemic pruritus (Vermeulen 2012), varicella‐like rash not at injection site (from day of vaccination to day 42) (Oxman 2005; Vermeulen 2012), rash unrelated to HZ (from day of vaccination to day 42) (Oxman 2005), 1 or more SAE (including death) (Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012), vaccine‐related SAEs (Mills 2010; Murray 2011; Oxman 2005), discontinuation due to a vaccine‐related AE (Mills 2010; Vermeulen 2012), hospitalisation (Oxman 2005), and hospitalisation related to HZ (Oxman 2005). Specific injection site AEs were more frequent in the vaccinated group. Specific risks for individual AEs were:
Varicella‐like rash at injection site (up to day 42) was also more frequent in the vaccinated group: RR 2.86, 95% CI 1.21 to 6.76 (Analysis 1.3.23) (Oxman 2005), but without a significant RD due to the small number of events. Duration of injection site AEs In general, injection site AEs lasted longer in the zoster vaccine group. There were significant differences with respect to the duration of the following local AEs: erythema, with a mean difference (MD) of 2.40 days (95% CI 1.56 to 3.24) (Analysis 1.4.1), swelling MD 1.90 days (95% CI 1.35 to 2.45) (Analysis 1.4.2) and pruritus MD 2.40 days (95% CI 1.32 to 3.48) (Analysis 1.4.5). The duration of pain and haematoma did not differ significantly between the groups, MD 1.00 (95% CI ‐0.10 to 2.10) (Analysis 1.4.3) and MD ‐0.50 (95% CI ‐5.52 to 4.52) (Analysis 1.4.6) respectively. The duration of rash was longer in the placebo compared to the vaccine group: RR ‐16.60 (95% CI ‐33.68 to 0.48) (Analysis 1.4.4). |
| High‐potency versus low‐potency zoster vaccine (Tyring 2007) | The comparison of high versus low‐potency zoster vaccine yielded no significant differences between groups for the following AEs: vaccine‐related AEs, systemic vaccine‐related AEs and vaccine‐related serious AEs (death). |
|
Refrigerated versus frozen zoster vaccine (Gilderman 2008) |
Compared refrigerated versus frozen zoster vaccine and reported no significant differences between groups for the following AEs: 1 or more AEs, vaccine‐related AEs, systemic AEs, systemic vaccine‐related AEs, serious AEs, vaccine‐related serious AEs or death. However, there were more injection site AEs in the group receiving frozen vaccines (RR 0.77, 95% CI 0.60 to 0.98) (Analysis 3.1.8). |
|
Zoster vaccine versus pneumo 23 (Berger 1998) |
One study compared 3 different concentrations of plaque‐forming units (pfu) of live attenuated VZV and presented the following adverse events: 3200 pfu VZV/dose versus pneumo 23 There was a lower incidence of 1 or more injection site reactions in the group vaccinated with the 3200 pfu/dose zoster vaccine (RR 0.61, 95% CI 0.41 to 0.91) (Analysis 5.1.1) as well as pain at the injection site (RR 0.49, 95% CI 0.30 to 0.81) (Analysis 5.1.3). There were no significant differences between the 3200 pfu/dose zoster vaccine and the pneumo 23 vaccine for the following local adverse events: induration (≥ 2 cm diameter injection site), probably vaccine‐related injection site pain, redness (≥ 2 cm diameter injection site), pruritus or vesicles (no patients had vesicles in the 3200 pfu/dose zoster vaccine nor the pneumo 23 groups). 8500 pfu VZV/dose versus pneumo 23 There was a lower incidence of 1 or more injection site reaction in the group vaccinated with the 8500 pfu/dose zoster vaccine (RR 0.63, 95% CI 0.43 to 0.93) (Analysis 5.2.1). There were no significant differences for the following injection site AEs between participants who received the 8500 pfu/dose VZV vaccine and those who received the pneumo 23 vaccine: induration (≥ 2 cm diameter injection site), pain (injection site), probably vaccine‐related injection site pain, redness, pruritus and vesicles. 41,650 pfu VZV/dose VZV versus pneumo 23 Participants receiving the 41,650 pfu/dose zoster vaccine had significantly lower rates of one or more injection site reaction (RR 0.41, 95% CI 0.24 to 0.68) (Analysis 5.3.1) and pain at injection site (RR 0.43, 95% CI 0.25 to 0.74) (Analysis 5.3.3) than those receiving the pneumo 23 vaccine. There were no significant differences between the groups for the following injection site AEs: induration (≥ 2 cm diameter injection site), probably vaccine‐related injection site pain, redness (≥ 2 cm diameter injection site), pruritus and vesicles (no patients had vesicles in the 41,650 pfu/dose zoster vaccine nor the pneumo 23 vaccine groups). |
|
Zoster vaccine intramuscular route versus zoster vaccine subcutaneous route (Diez‐Domingo 2015) |
Compared intramuscular (IM) versus subcutaneous (SC) zoster vaccine and reported that compared to the IM group, participants who received SC vaccines had a significantly higher incidence of the following AEs:
There were no significant differences between groups for the following AEs: all systemic AEs: RR 1.03, 95% CI 0.70 to 1.51 (Analysis 6.1.3); vaccine‐related systemic AE: RR 0.93, 95% CI 0.44 to 1.98 (Analysis 6.1.4); headache considered as vaccine‐related by the investigator: RR 0.75, 95% CI 0.17 to 3.32 (Analysis 6.1.5); unsolicited injection site reaction: RR 0.65 95% CI 0.29 to 1.45 (Analysis 6.1.7); severe injection site erythema (> 10 cm): RR 0.67 95% CI 0.11 to 3.96 (Analysis 6.1.9); severe injection site pain (inability to work or usual activity): RR 1.01, 95% CI 0.14 to 7.06 (Analysis 6.1.11); severe injection site swelling (> 10 cm): RR 0.25, 95% CI 0.03 to 2.23 (Analysis 6.1.13). No participant withdrew from the trial because of AE (Analysis 6.1.15). |
|
2 doses of a zoster vaccine versus a single dose and also 2 doses given at different intervals (Vesikari 2013) |
Zoster vaccine 1‐month schedule versus zoster vaccine 3‐month schedule There was no statistical difference between participants who received the doses of zoster vaccine 2 months apart compared to those receiving the doses 3 months apart: AE RR 1.10, 95% CI 0.91 to 1.31 (Analysis 7.1.1), vaccine‐related AE RR 1.00, 95% CI 0.81 to 1.24 (Analysis 7.1.2); serious AE RR 0.95, 95% CI 0.14 to 6.70 (Analysis 7.1.3); withdrawal due to AE RR 2.86, 95% CI 0.12 to 69.80 (Analysis 7.1.5); systemic AE RR 1.34, 95% CI 0.90 to 2.00 (Analysis 7.1.8); vaccine‐related systemic AE RR 1.27, 95% CI 0.45 to 3.60 (Analysis 7.1.9); rash of interest non‐injection site rashes RR 0.95, 95% CI 0.06 to 15.14 (Analysis 7.1.10); varicella/varicella‐like rash RR 0.95, 95% CI 0.06 to 15.14 (Analysis 7.1.11); injection site reaction RR 0.99, 95% CI 0.80 to 1.23 (Analysis 7.1.13); solicited injection site reaction RR 1.00, 95% CI 0.81 to 1.25 (Analysis 7.1.14); unsolicited injection site reaction RR 0.41, 95% CI 0.11 to 1.56 (Analysis 7.1.15); erythema injection site RR 1.01, 95% CI 0.80 to 1.27 (Analysis 7.1.16); pain injection site RR 0.84, 95% CI 0.57 to 1.25 (Analysis 7.1.17); swelling injection site RR 1.05, 95% CI 0.75 to 1.47 (Analysis 7.1.18). No participants, from either group, reported the following AE: vaccine‐related serious AE (Analysis 7.1.4); vaccine‐related withdrawal due to AE (Analysis 7.1.6); non‐serious vaccine‐related withdrawal due to AE (Analysis 7.1.7) and herpes zoster/zoster‐like rash (Analysis 7.1.12). Zoster vaccine 1 month schedule versus zoster vaccine single dose Only participants with systemic AE: there were significant differences in favour of the 2 doses 1 month apart, with a higher incidence in the single dose group: RR 0.74, 95% CI 0.56 to 0.97, RD ‐0.07, 95% CI ‐0.13 to ‐0.01 and NNTH 14.3, 95% CI 7.6 to 100 (Analysis 7.2.8). For most AEs, there was no statistical difference: AE RR 0.92, 95% CI 0.80 to 1.05 (Analysis 7.2.1), vaccine‐related AE RR 0.91, 95% CI 0.77 to 1.08 (Analysis 7.2.2); serious AE RR 0.72, 95% CI 0.16 to 3.30 (Analysis 7.2.3); withdrawal due to AE RR 0.36, 95% CI 0.05 to 2.82 (Analysis 7.1.5); vaccine‐related withdrawal due to AE RR 0.21, 95% CI 0.01 to 3.74 (Analysis 7.2.6); non‐serious vaccine‐related withdrawal due to AE RR 0.21, 95% CI 0.01 to 3.74 (Analysis 7.2.7); vaccine‐related systemic AE RR 0.54, 95% CI 0.26 to 1.12 (Analysis 7.2.9); rash of interest non‐injection site rashes RR 1.61, 95% CI 0.15 to 17.72 (Analysis 7.2.10); varicella/varicella‐like rash RR 9.66, 95% CI 0.39 to 236.25 (Analysis 7.2.11); herpes zoster/zoster‐like rash RR 0.64, 95% CI 0.03 to 13.36 (Analysis 7.2.12); injection site reaction RR 0.93, 95% CI 0.78 to 1.10 (Analysis 7.2.13); solicited injection site reaction RR 0.94, 95% CI 0.79 to 1.11 (Analysis 7.2.14); unsolicited injection site reaction RR 0.35, 95% CI 0.11 to 1.13 (Analysis 7.2.15); injection site erythema RR 0.98, 95% CI 0.81 to 1.17 (Analysis 7.2.16); injection site pain RR 0.74, 95% CI 0.54 to 1.01 (Analysis 7.2.17); injection site swelling RR 1.08, 95% CI 0.82 to 1.41 (Analysis 7.2.18). There were no participants with vaccine‐related serious AE in either group (Analysis 7.2.4). Zoster vaccine 3 month schedule versus zoster vaccine single dose The participants in the group that received a single dose had a higher incidence of the following AE in comparison to those in the group that received 2 doses, 3 months apart: AEs RR 0.84, 95% CI 0.72 to 0.97; RD ‐0.09; 95% CI ‐0.17 to ‐0.02 and NNTH 11.1, 95% CI 5.9 to 50 (Analysis 7.3.1), systemic AEs RR 0.55, 95% CI 0.39 to 0.76, RD ‐0.13, 95% CI ‐0.18 to ‐0.07 and NNTH 7.6, 95% CI 5.6 to 14.3 (Analysis 7.3.8) and vaccine‐related systemic AE RR 0.42, 95% CI 0.18 to 0.98), RD ‐0.04, 95% CI ‐0.06 to ‐0.01 and NNTH 25.0, 95% CI 16.6 to 100 (Analysis 7.3.9). There were no significant differences between these groups in relation to the following AEs: vaccine‐related AE RR 0.91, 95% CI 0.77 to 1.08 (Analysis 7.3.2); serious AE RR 0.75, 95% CI 0.16 to 3.46 (Analysis 7.3.3); withdrawal due to AE RR 0.18, 95% CI 0.01 to 3.04 (Analysis 7.3.5); vaccine‐related withdrawal due to AE RR 0.23, 95% CI 0.01 to 3.93 (Analysis 7.3.6); non‐serious vaccine‐related withdrawal due to AE RR 0.23, 95% CI 0.01 to 3.93 (Analysis 7.3.7); rash of interest non‐injection site rashes RR 1.69, 95% CI 0.15 to 18.60 (Analysis 7.3.10); varicella/varicella‐like rash RR 10.14, 95% CI 0.41 to 247.92 (Analysis 7.3.11); herpes zoster/zoster‐like rash RR 0.68, 95% CI 0.03 to 14.02 (Analysis 7.3.12); injection site reaction RR 1.10, 95% CI 0.79 to 1.11 (Analysis 7.3.13); solicited injection site reaction RR 0.93, 95% CI 0.78 to 1.11 (Analysis 7.3.14); unsolicited injection site reaction RR 0.85, 95% CI 0.38 to 1.91 (Analysis 7.3.15); injection site erythema RR 0.97, 95% CI 0.80 to 1.17 (Analysis 7.3.16); injection site pain RR 0.87, 95% CI 0.65 to 1.17 (Analysis 7.3.17); injection site swelling RR 1.03, 95% CI 0.77 to 1.36 (Analysis 7.3.18). There were no participants with vaccine‐related serious AE in either group (Analysis 7.3.4). |
AE: adverse event CI: confidence interval HZ: herpes zoster RD: risk difference RR: risk ratio SC: subcutaneous VZV: varicella zoster virus
Analysis 1.4.
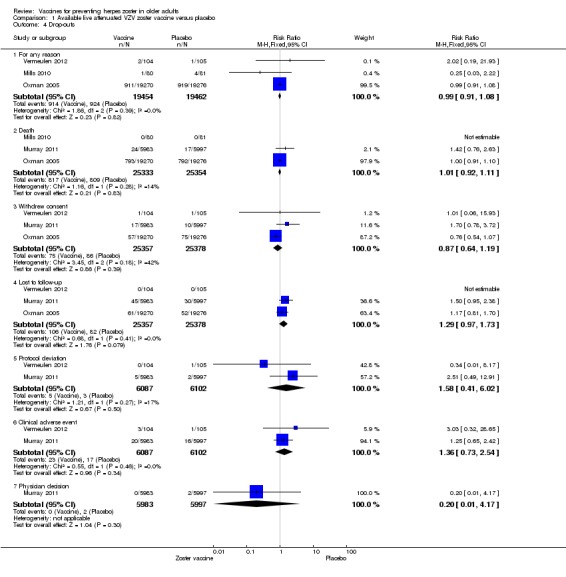
Comparison 1 Available live attenuated VZV zoster vaccine versus placebo, Outcome 4 Drop‐outs.
Analysis 3.1.
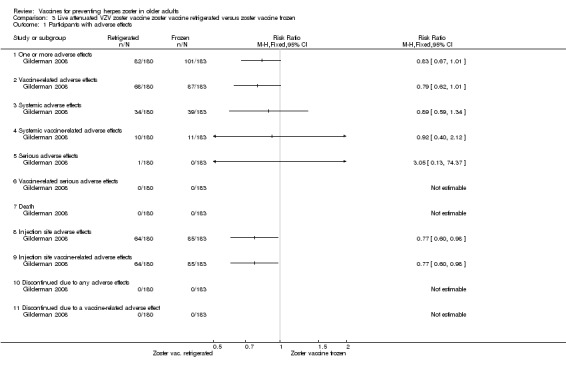
Comparison 3 Live attenuated VZV zoster vaccine zoster vaccine refrigerated versus zoster vaccine frozen, Outcome 1 Participants with adverse effects.
Analysis 5.1.
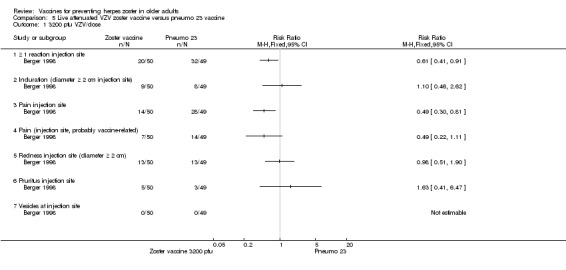
Comparison 5 Live attenuated VZV zoster vaccine versus pneumo 23 vaccine, Outcome 1 3200 pfu VZV/dose.
Analysis 5.2.
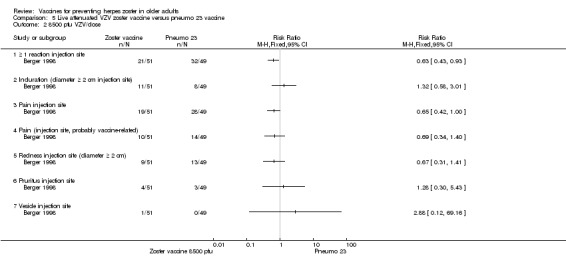
Comparison 5 Live attenuated VZV zoster vaccine versus pneumo 23 vaccine, Outcome 2 8500 pfu VZV/dose.
Analysis 5.3.
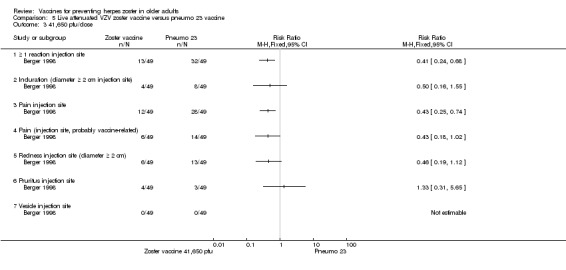
Comparison 5 Live attenuated VZV zoster vaccine versus pneumo 23 vaccine, Outcome 3 41,650 pfu/dose.
Analysis 6.1.
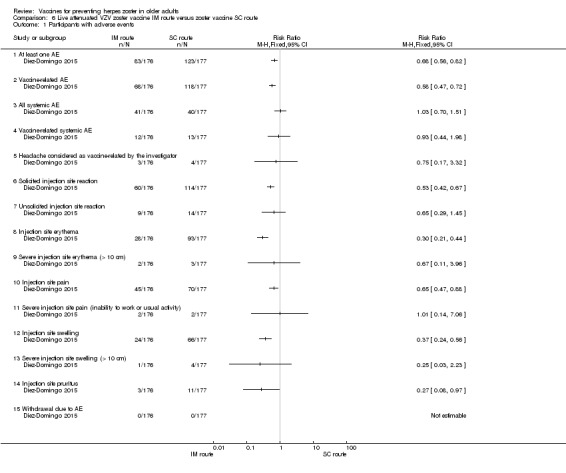
Comparison 6 Live attenuated VZV zoster vaccine IM route versus zoster vaccine SC route, Outcome 1 Participants with adverse events.
Analysis 7.1.
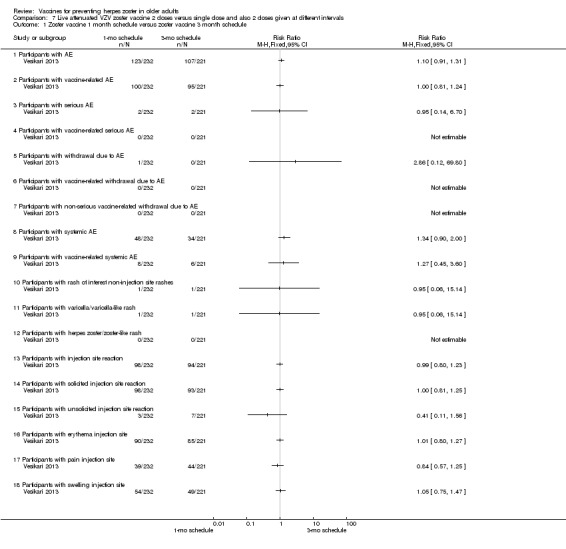
Comparison 7 Live attenuated VZV zoster vaccine 2 doses versus single dose and also 2 doses given at different intervals, Outcome 1 Zoster vaccine 1 month schedule versus zoster vaccine 3 month schedule.
Analysis 7.2.
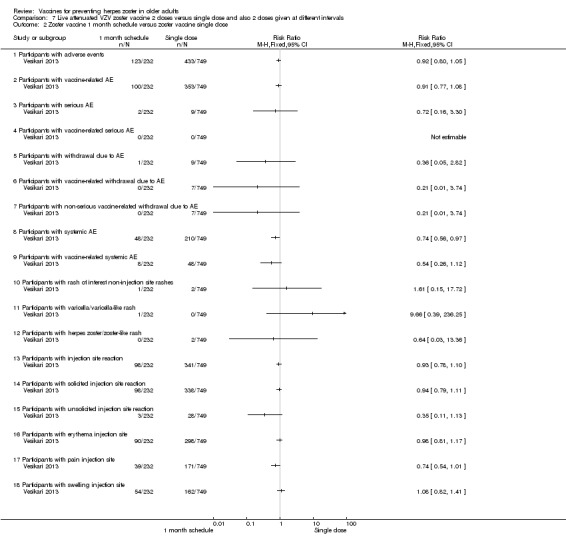
Comparison 7 Live attenuated VZV zoster vaccine 2 doses versus single dose and also 2 doses given at different intervals, Outcome 2 Zoster vaccine 1 month schedule versus zoster vaccine single dose.
Analysis 7.3.
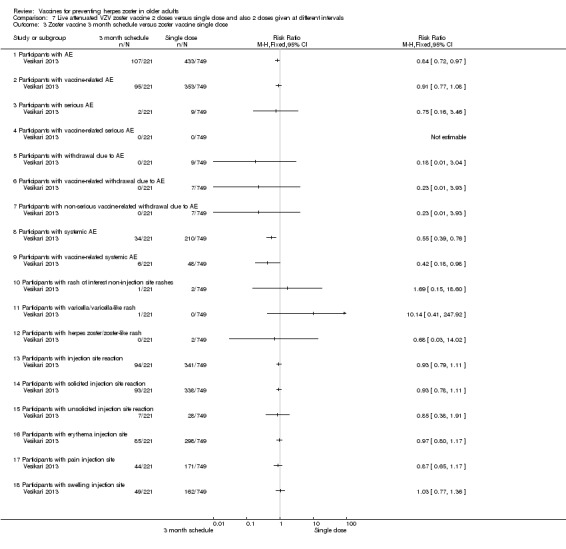
Comparison 7 Live attenuated VZV zoster vaccine 2 doses versus single dose and also 2 doses given at different intervals, Outcome 3 Zoster vaccine 3 month schedule versus zoster vaccine single dose.
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available)
Lower or higher volumes of adjuvants plus gE subunit VZV or unadjuvanted gE or saline
Chlibek 2013 compared adverse events in the four groups that received two doses two months apart: two groups with different amounts of adjuvants with the same amount of antigen (50 gE/AS01B and 50 gE/AS01E), one group receiving 50 µg gE plus saline and one group receiving only saline (placebo).
General and local reactions to vaccination were more frequent with both adjuvanted candidate herpes zoster vaccines and were most frequent with the groups that received higher amounts of adjuvant (gE/AS01B). The participants who received gE/AS01B had a significantly higher incidence of adverse events: any symptom, general reaction (fatigue, headache) and local reaction (any symptom, pain and redness). However, all adverse events were generally mild to moderate and transient. No vaccine‐related severe adverse events were reported.
Three groups of VZV subunit gE in three different quantities versus unadjuvanted gE or saline
Chlibek 2014 compared adverse events in five groups that received two doses two months apart: three groups received vaccines, each one with different amounts of antigen (25 μg gE, 50 μg gE and 100 μg gE) but the same amount of adjuvant AS01B; one group received one dose of saline + one dose 100 µg gE two months later; and one group received100 µg gE/saline (unadjuvanted gE).
All adverse events were common in the three different formulations of gE/AS01B and more frequent than with the unadjuvanted gE/saline. In the comparison between the three different amounts of gE antigen, there were no differences in the incidence of adverse events except for any myalgia in which there was a slightly higher incidence in the group receiving 100 µg compared with 50 µg: RR 1.26, 95% CI 1.01 to 1.59, RD 0.11 95% CI 0.00 to 0.22 and NNTH 9.0 95% CI 0 to 4.5 (Analysis 9.3.7). Thre was no difference between groups for more important myalgia that prevents normal everyday activities.
Analysis 9.3.
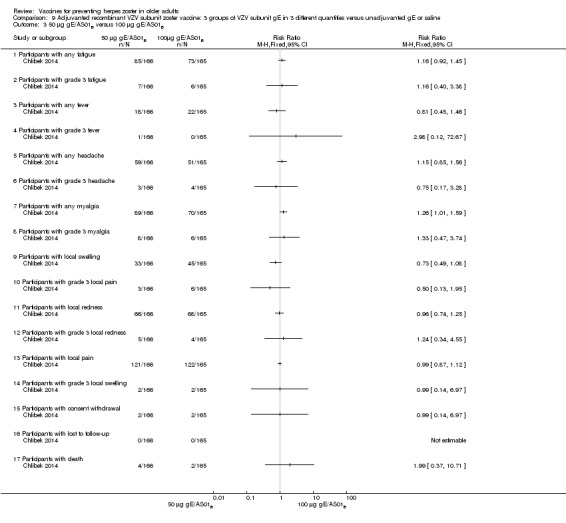
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 3 50 µg gE/AS01B versus 100 µg gE/AS01B.
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo
We did the analysis of adverse events in patients aged 50 years or more because the data for adverse events by specific age groups were not available. We performed intention‐to‐treat (ITT) analyses for adverse events that did not include all randomised participants. In other words, we considered the worst case scenario for the intervention group (we assumed that the participants with missing information had adverse events) and the best case scenario for the placebo group (we assumed that the participants with missing information did not have adverse events). In this analysis, we detected no differences between the groups. Therefore, we decided to present the results for adverse events as they were published.
In the comparison between the new adjuvanted recombinant VZV subunit zoster vaccine versus placebo, the vaccinated group had a higher incidence of the following adverse events: systemic symptoms (myalgia, fatigue, headache, shivering, fever and gastrointestinal symptoms) and injection site adverse events (pain, redness and swelling) but most symptoms were of mild to moderate intensity. The most important difference between the adverse events was injection site events with an absolute risk of 81.5% in comparison to placebo, which was 11.9% (Table 2).
There was no significant difference between groups for serious adverse events, potential immune‐mediated disease and deaths (Table 2).
See Table 15 for details of adverse events for these comparisons between the new adjuvanted recombinant VZV subunit zoster vaccine versus placebo.
Table 3.
Adverse events of adjuvanted recombinant VZV subunit zoster vaccine
| Comparison (studies) | Results |
|
Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline (Chlibek 2013) |
Compared 4 groups that received either lower (AS01E) or higher (AS01B) volumes of adjuvants plus gE subunit VZ or unadjuvanted gE or saline injections. 50 μg gE/AS01E versus 50 μg gE/AS01B There was a significantly higher incidence of AEs in the participants who received a higher quantity of adjuvant (AS01B):
There were no significant differences between groups for all other AEs: any grade 3 symptom; any general symptom, any general grade 3 symptom, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, grade 3 headache, myalgia, grade 3 myalgia, any grade 3 local symptom, local grade 3 pain, local grade 3 redness, local swelling and local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AE. No participants had grade 3 fever in either group. 50 μg gE/AS01E versus 50 μg gE/saline (unadjuvanted)
All these AE differences were favourable to the unadjuvanted gE group. There were no significant differences between the groups for the following AEs: any grade 3 symptom, any general grade 3 symptom, fatigue, grade 3 fatigue, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local grade 3 redness and local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AE. No participants had grade 3 fever or grade 3 headache in either group. 50 μg gE/AS01B versus 50 μg gE/saline (unadjuvanted)
All these AE differences were favourable to unadjuvanted gE. There were no significant differences between the groups for the following AEs: any grade 3 symptom, any general grade 3 symptom, grade 3 fatigue, gastrointestinal symptoms, grade 3 headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local grade 3 redness and local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AE. No participant had grade 3 fever or grade 3 gastrointestinal symptoms in either group. 50 μg gE/AS01E versus saline
All differences in these AEs were favourable to the saline group. There were no significant differences in the following AEs between the groups:any grade 3 symptom, any general grade 3 symptom, fatigue, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, headache, grade 3 headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local redness, local grade 3 redness, local swelling and local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AE No participants had grade 3 fever or grade 3 headache in either group. 50 μg gE/AS01B versus saline
All AE differences were favourable to saline. There was no significant difference in AEs between groups for the following: any grade 3 symptom, any general grade 3 symptom, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, grade 3, headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local grade 3 redness, local swelling and local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever in either group. 50 μg gE/saline (unadjuvanted) versus saline
There were no significant differences between groups for the following AEs: any grade 3 symptom, any general symptom, any general grade 3 symptom, fatigue, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, headache, myalgia, grade 3 myalgia, any local symptom, local pain, local redness and local swelling or consent withdrawal. No participant, in either group had grade 3 fever, grade 3 headache, any local grade 3 symptom, local grade 3 pain, local grade 3 redness, local grade 3 swelling, loss to follow‐up and serious AE. |
|
Adjuvanted recombinant VZV subunit zoster vaccine: three groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline (Chlibek 2014) |
3 groups of VZV plus gE were compared in 3 different quantities, 1 group that received unadjuvanted gE and 1 group that received only saline 25 µg gE/AS01B versus 50 µg gE/AS01B There was no difference in the incidence of the following AEs: any fatigue, grade 3 fatigue, any fever, grade 3 fever, any headache, grade 3 headache, any myalgia, grade 3 myalgia, local pain, local grade 3 pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. 25 µg gE/AS01B versus 100 µg gE/AS01B There were no differences in the incidence of the following AEs: any fatigue, grade 3 fatigue, any fever, any headache, grade 3 headache, any myalgia, grade 3 myalgia, local pain, grade 3 local pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. 50 µg gE/AS01B versus 100 µg gE/AS01B
There were no differences in the incidence of all the others AEs: any fatigue, grade 3 fatigue, any fever, grade 3 fever, any headache, grade 3 headache, grade 3 myalgia, local pain, local grade 3 pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal and serious AEs 25 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All these differences in AEs were favourable to unadjuvanted gE. There were no differences in the incidence of the following AEs: grade 3 fatigue, any fever, any headache, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever in either of the groups. 50 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All these differences of AEs were favourable to unadjuvanted gE. There were no differences in the incidence of the following AEs: grade 3 fatigue, any fever, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever in either of the groups 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All these differences in AEs were favourable to unadjuvanted gE. There were no differences in the incidence of the following AEs: grade 3 fatigue, any fever, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever in either of the groups. 25 µg gE/AS01B versus saline + 100 µg gE/AS01B
All differences in AEs were favourable to saline + 100 µg gE/AS01B. There were no differences in the incidence of the following AEs:, any fatigue, grade 3 fever, any headache, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever in either of the groups. 50 µg gE/AS01B versus saline + 100 µg gE/AS01B
All differences in AEs were favourable to saline + 100 µg gE/AS01B. There were no differences in the incidence of the following AEs: grade 3 fatigue, any fever, grade 3 fever, grade 3 headache, grade 3 myalgia, local grade 3 pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. 100 µg gE/AS01B versus saline + 100 µg gE/AS01B
All differences in AEs were favourable to saline + 100 µg gE/AS01B. There were no difference in the incidence of the following AEs: grade 3 fatigue, headache, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever in either of the groups. Saline + 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All differences in AEs were favourable to 100 µg gE/saline. There were no differences in the incidence of the following AEs: any fatigue, grade 3 fatigue, any fever, any headache, any myalgia, grade 3 myalgia, local grade 3 pain, local grade 3 redness, consent withdrawal, loss to follow‐up and serious AEs. No participant had grade 3 fever, grade 3 headache and local grade 3 swelling in either of the groups. |
| Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo (Lal 2015) | The AEs related the comparison between adjuvanted recombinant VZV subunit zoster vaccine (not yet available) and placebo are shown below:
|
AEs: adverse events CI: confidence interval HZ: herpes zoster NNTH: number needed to treat for an additional harmful outcome RD: risk difference RR: risk ratio VZV: varicella zoster virus
Analysis 8.1.
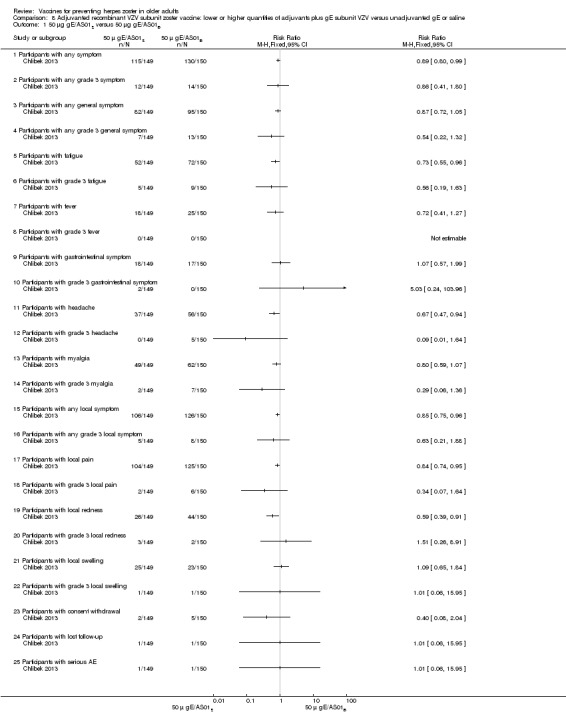
Comparison 8 Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline, Outcome 1 50 μg gE/AS01E versus 50 μg gE/AS01B.
Analysis 8.2.
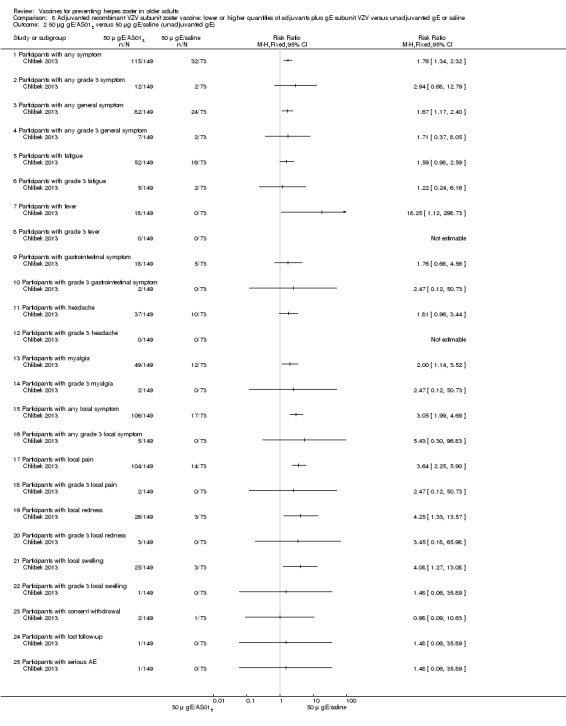
Comparison 8 Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline, Outcome 2 50 μg gE/AS01E versus 50 μg gE/saline (unadjuvanted gE).
Analysis 8.3.
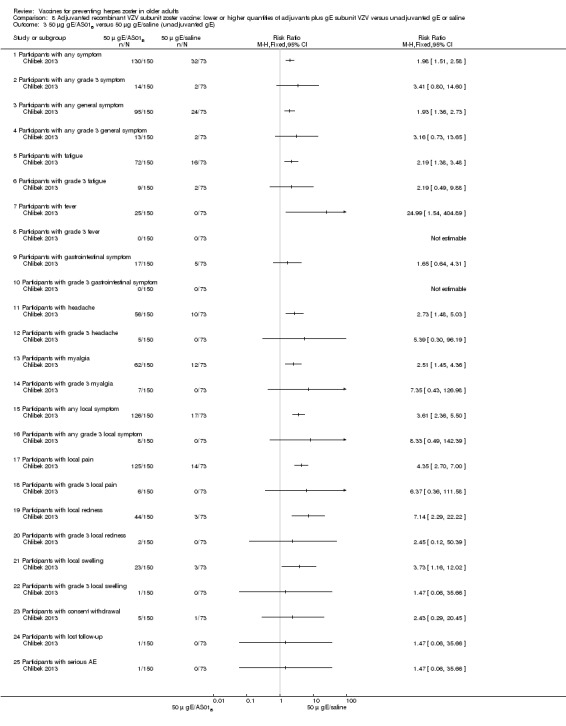
Comparison 8 Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline, Outcome 3 50 μg gE/AS01B versus 50 μg gE/saline (unadjuvanted gE).
Analysis 8.4.
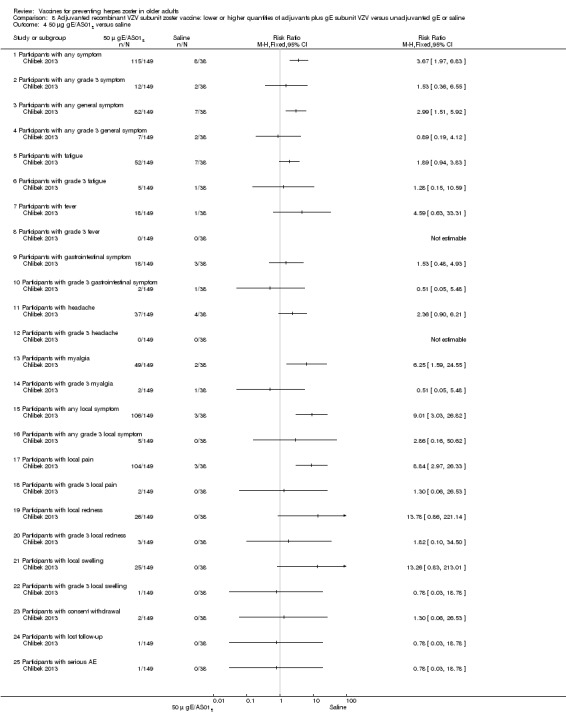
Comparison 8 Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline, Outcome 4 50 μg gE/AS01E versus saline.
Analysis 8.5.
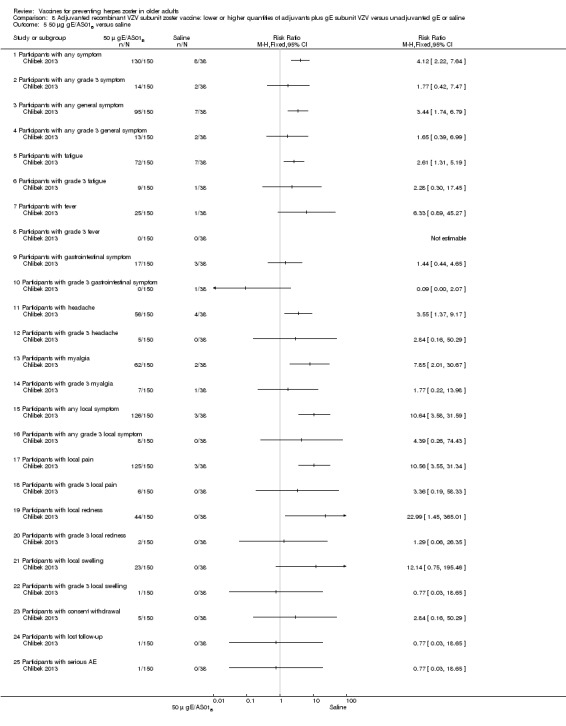
Comparison 8 Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline, Outcome 5 50 μg gE/AS01B versus saline.
Analysis 8.6.
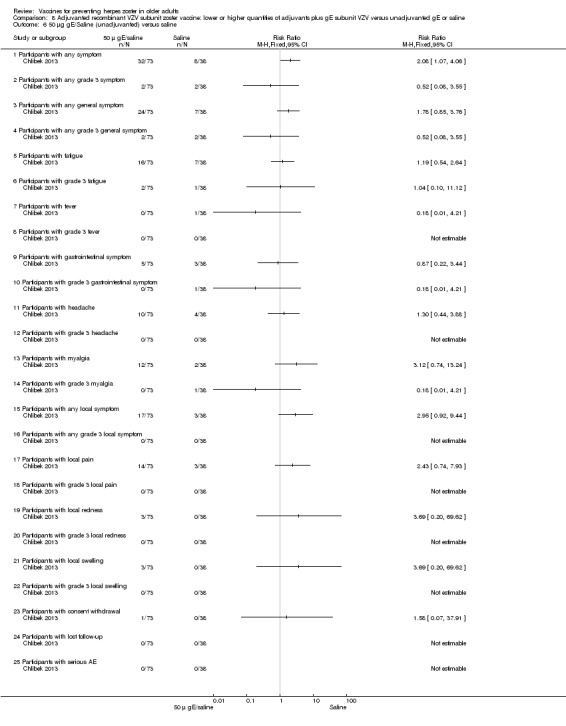
Comparison 8 Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline, Outcome 6 50 μg gE/Saline (unadjuvanted) versus saline.
Analysis 9.4.
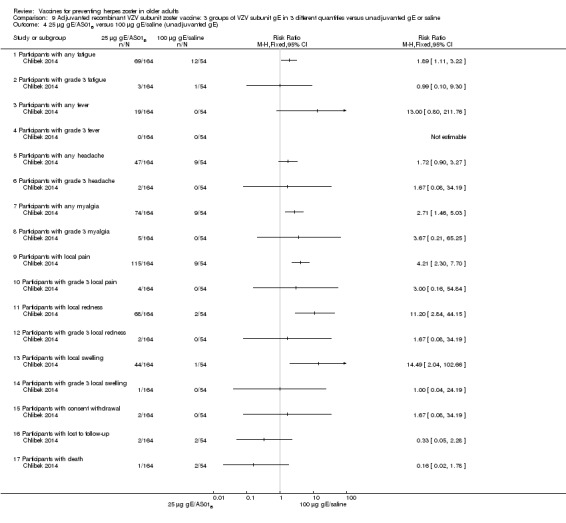
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 4 25 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE).
Analysis 9.5.
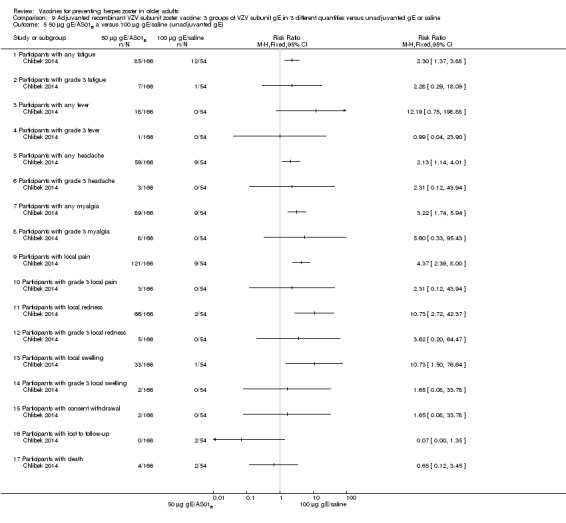
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 5 50 µg gE/AS01B a versus 100 µg gE/saline (unadjuvanted gE).
Analysis 9.6.
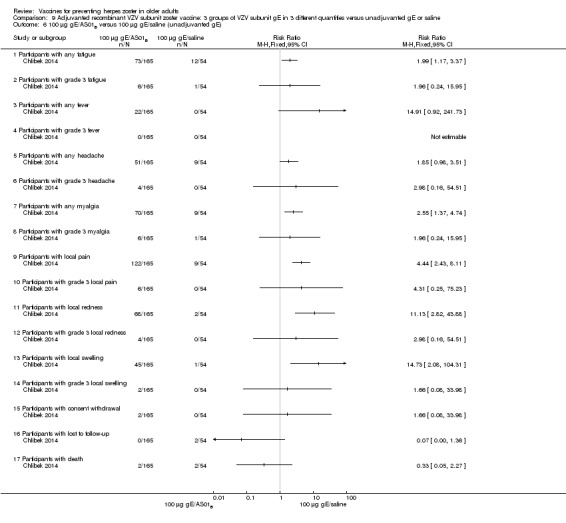
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 6 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE).
Analysis 9.7.
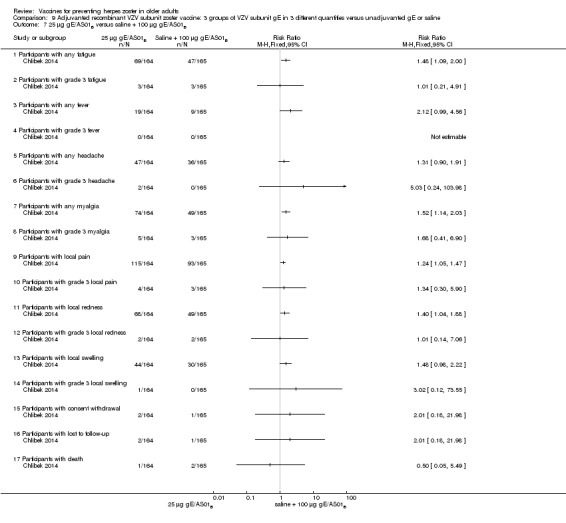
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 7 25 µg gE/AS01B versus saline + 100 µg gE/AS01B.
Analysis 9.8.
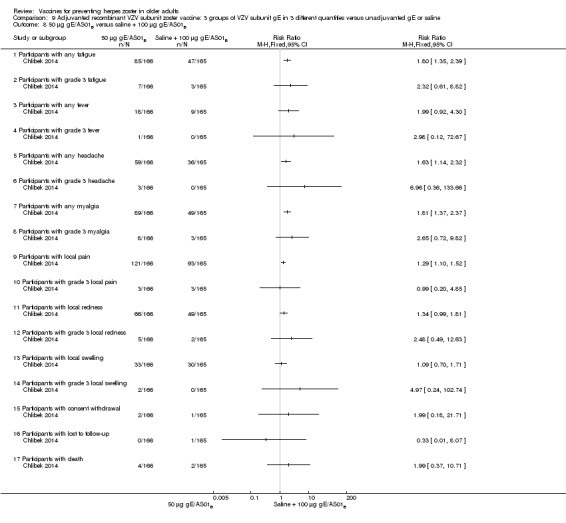
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 8 50 µg gE/AS01B versus saline + 100 µg gE/AS01B.
Analysis 9.9.
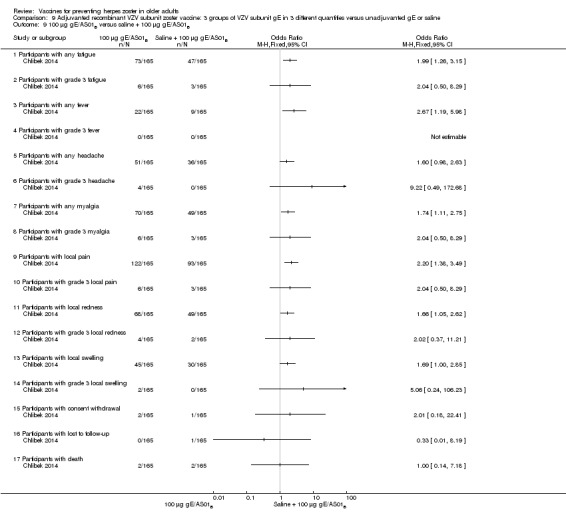
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 9 100 µg gE/AS01B versus saline + 100 µg gE/AS01B.
Analysis 9.10.
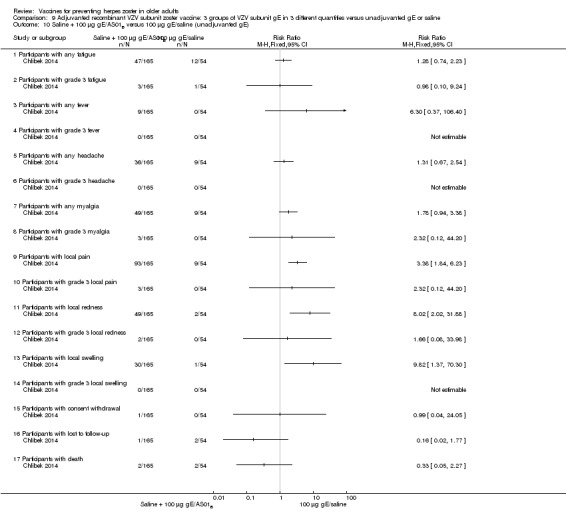
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 10 Saline + 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE).
Analysis 10.2.
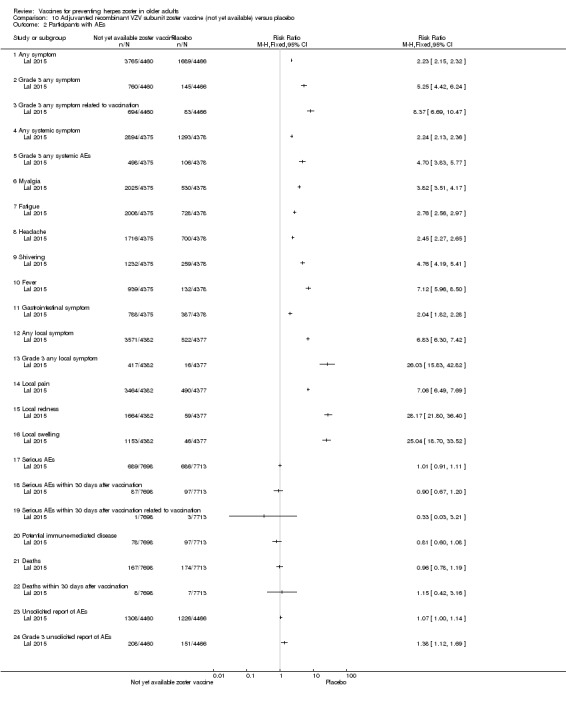
Comparison 10 Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo, Outcome 2 Participants with AEs.
2. Drop‐outs
There were no important differences in the reasons for drop‐outs in the two main studies that assessed the incidence of herpes zoster between vaccinated and placebo groups, regardless of the type of vaccine (live attenuated VZV zoster vaccine or adjuvanted recombinant VZV subunit zoster vaccine).
Lal 2015 described three reasons for drop‐out: not receiving vaccine according to protocol, receiving the wrong vaccine and a diagnosis of herpes zoster less than 30 days after dose 2. This last outcome had a RR of 0.29, 95% CI 0.09 to 0.87 but no RD. We considered it as a drop‐out and did not put it in the incidence outcome since it was reported for participants aged 50 years or more and not specifically for participants 60 years or more, who were our group of interest.
See Table 16 for details on all the comparisons of drop‐outs in all of the included studies.
Table 4.
Drop‐outs
| Drop‐outs of all included studies |
Available live attenuated VZV zoster vaccine versus placebo The pooled data from the studies that compared zoster vaccine and placebo showed no differences in the reasons for drop‐outs (Analysis 1.4): for any reason (RR 0.99, 95% CI 0.91 to 1.08) (Analysis 1.4.1) (Mills 2010; Oxman 2005; Vermeulen 2012), for death (RR 1.01, 95% CI 0.92 to 1.11) (Analysis 1.4.2) (Mills 2010; Murray 2011; Oxman 2005), for withdrawal of consent (RR 0.87, 95% CI 0.64 to 1.19) (Analysis 1.4.3) (Murray 2011; Oxman 2005; Vermeulen 2012), for loss to follow‐up (RR 1.29, 95% CI 0.97 to 1.73) (Analysis 1.4.4) (Mills 2010; Murray 2011; Oxman 2005; Vermeulen 2012), for protocol deviation (RR 1.58, 95% CI 0.41 to 6.02) (Analysis 1.4.5) (Murray 2011; Vermeulen 2012), for clinical AE (RR 1.36, 95% CI 0.73 to 2.54) (Analysis 1.4.6) (Murray 2011; Vermeulen 2012) and for physician decision (RR 0.20, 95% CI 0.01 to 4.17) (Analysis 1.4.7) (Murray 2011). In Mills 2010, Oxman 2005 and Vermeulen 2012 consent was withdrawn after the intervention. In Murray 2011, some patients apparently withdrew consent after randomisation, but the trial authors do not describe the exact number who withdrew consent after the intervention. The pooled data from the studies that compared zoster vaccine and placebo (Mills 2010; Murray 2011; Oxman 2005) showed no differences in the reasons for participants with no follow‐up (Analysis 1.5). High‐potency versus low‐potency zoster vaccine: There were no differences between the groups (Analysis 2.6). Refrigerated versus frozen zoster vaccine: There were no differences between the groups (Analysis 3.2). Zoster vaccine IM route versus zoster vaccine SC route: There were no withdrawals due to AE in either group (Analysis 6.1.15). 2 doses of a zoster vaccine versus a single dose and also 2 doses given at different intervals: There were no differences between the groups for participants with withdrawal due to AE (Analysis 7.1.5; Analysis 7.2.5; Analysis 7.3.5) (Vesikari 2013). Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) ‐ lower or higher volumes of adjuvants plus gE subunit VZV or unadjuvanted gE or saline injections: There were no differences between the groups for the following reasons of drop‐out: participants with consent withdrawal and participants with loss to follow‐up (Chlibek 2013). 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline: There were no differences between groups for participants with withdrawal of consent or participants with loss to follow‐up for all comparisons provided (Chlibek 2014). Adjuvanted recombinant VZV subunit zoster vaccine not yet available versus placebo:Lal 2015 described 3 reasons to drop‐out: did not receive vaccine according to protocol (Analysis 10.3.1), received wrong vaccine (Analysis 10.3.2) and had diagnosis of HZ less than 30 days after dose 2 (Analysis 10.3.3). For the first 2 there were no differences between the groups. The last outcome had a RR of 0.29 (95% CI 0.09 to 0.87) but no RD and we considered it as drop‐out and not an incidence outcome since it is related to participants aged > 50 years old and not with our age group of interest (participants 60 years old or more). |
AE: adverse event CI: confidence interval HZ: herpes zoster IM: intramuscular RD: risk difference RR: risk ratio SC: subcutaneous VZV: varicella zoster virus
Analysis 1.5.
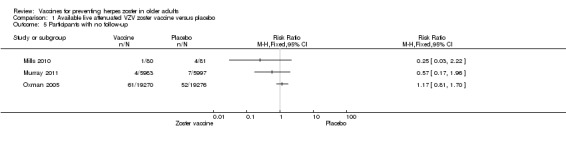
Comparison 1 Available live attenuated VZV zoster vaccine versus placebo, Outcome 5 Participants with no follow‐up.
Analysis 2.6.
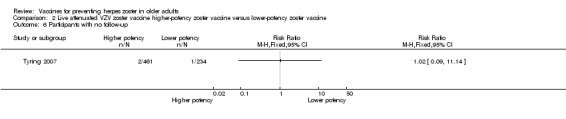
Comparison 2 Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine, Outcome 6 Participants with no follow‐up.
Analysis 3.2.

Comparison 3 Live attenuated VZV zoster vaccine zoster vaccine refrigerated versus zoster vaccine frozen, Outcome 2 Participants with no follow‐up.
Analysis 10.3.
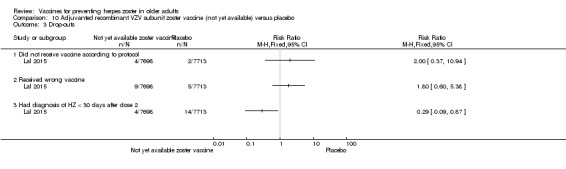
Comparison 10 Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo, Outcome 3 Drop‐outs.
Discussion
Summary of main results
Available live attenuated varicella zoster virus (VZV) vaccine
For this vaccine we included a total of 10 clinical trials that had clinical outcomes (herpes zoster cases, adverse events and drop‐outs) (Berger 1998; Diez‐Domingo 2015; Gilderman 2008; Levin 2000; Mills 2010; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013). We excluded a total of six trials: three with only immunological outcomes (Hayward 1994; Hayward 1996; Patterson‐Bartlett 2007), one RCT that tested another intervention (Irwin 2007), one RCT evaluating zoster vaccine administered concomitantly with another vaccine (Kerzner 2007), and one trial that did not fulfil our age criteria (Macaladad 2007).
We considered four of these 10 studies to be at low risk of bias (Diez‐Domingo 2015; Oxman 2005; Vermeulen 2012; Vesikari 2013). Data from a major randomised controlled trial (RCT), the Shingles Prevention Study (Oxman 2005), which included 38,546 participants, confirm its effectiveness when compared to placebo in the elderly population, for at least for 3.1 years. The continuation of this study was the study with the longest duration of follow‐up, reporting an average five years of herpes zoster surveillance in individuals aged 60 or older. The available data suggest that the vaccine works for an average of five years to prevent herpes zoster in individuals over 60 years of age. However, these long‐term effect estimates for the outcome incidence of herpes zoster should be interpreted with caution since they were derived from inferred data.
A previous review on zoster vaccine highlighted that individuals in the Shingles Prevention Study who developed herpes zoster despite vaccination had a lower duration and severity of symptoms than those in the placebo group (Sanford 2010).
The impact of zoster episodes on daily life activities was assessed. Despite the lower incidence of cases in the vaccinated population, there were no significant differences for this outcome when compared to the placebo group.
According to a few observational studies acute herpes zoster pain can have an important negative impact on the lives of a significant proportion of affected individuals (Katz 2004; Lydick 1995; Schmader 2007). However, one randomised study did not detect significant differences in the health‐related quality of life of herpes zoster patients treated with placebo compared to analgesics (Dworkin 2009). Only one of the studies included in our review addressed this issue and did not detect significant differences between the zoster vaccine versus the placebo groups (Oxman 2005). The advantage of the vaccine is that it reduces the risk of developing herpes zoster, a disease that can potentially affect the quality of life of patients. In our review, 13 participants in the vaccine group and 42 in the placebo group had severe impairment in their quality of life due to acute herpes zoster pain.
Data from other studies included in this review failed to detect any significant differences in relevant outcomes for higher‐ versus lower‐potency zoster vaccines and live versus inactivated zoster vaccines. It should be noted that there were no cases of herpes zoster caused by attenuated live zoster vaccines.
The vaccine proved to be safe and well tolerated with a low incidence of systemic adverse events. Although systemic adverse events were more frequent in the vaccinated group than in the placebo group, the number needed to treat for an additional harmful outcome (NNTH) for any systemic adverse event is 100. Serious adverse events and vaccine‐related serious adverse events had similar frequencies in both groups.
Although the rate of adverse events was higher in the group receiving the zoster vaccine, the rates of drop‐outs were similar in the vaccine and placebo group, suggesting that these adverse events did not have important repercussions.
Diez‐Domingo 2015 compared rates of adverse events with the intramuscular versus the subcutaneous route using zoster vaccines. These authors reported a higher incidence of adverse events, mainly injection site reactions (erythema, pain and swelling), in the group vaccinated by the subcutaneous route. For every four patients receiving the vaccine by the subcutaneous route, there was one additional individual who had an adverse event when compared to participants who received the vaccine by the intramuscular route. However, there were no differences in the rate of severe injection site, systemic and vaccine‐related systemic adverse events.
In the Vesikari 2013 study we used only data for the single dose.
Injection site adverse events were less frequent in participants inoculated with refrigerated herpes zoster vaccine than in those receiving the frozen vaccine.
Even with this unfavourable safety profile, it is of note that the majority of the adverse events were of mild to moderate intensity. This is clearly reported in the adverse event sub‐study of Oxman 2005.
A previously published review pooled data from two studies to evaluate the safety of zoster vaccines and concluded that tolerability was good and that safety was not a major concern (Sutradhar 2009). Berger 1998 compared different dosages of zoster vaccines and pneumo 23 and also reported that zoster vaccine produced fewer injection site adverse events than the pneumo 23 vaccine.
The Food and Drug Administration (FDA) approved zoster vaccine for older adults (60 years and over) in May 2006 (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm132873.htm).
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available)
We included three trials that tested this new vaccine on clinical outcomes (efficacy, adverse events and drop‐out) and we considered two of them as having a low risk of bias (Chlibek 2013; Lal 2015). The main study, Lal 2015, evaluated the incidence of herpes zoster in a vaccinated group versus a placebo group during an average of 3.2 years of follow‐up and observed a significant decrease in this outcome in the vaccinated group. This new vaccine also proved to be safe since there was no difference in serious adverse events between the vaccinated and placebo groups. Although systemic and injection sites adverse events were more frequent in the vaccinated group, these were transient.
All studies received funding from the pharmaceutical industry.
Overall completeness and applicability of evidence
All included studies except one enrolled healthy elderly participants with previous VZV contact but without a history of herpes zoster. Only Mills 2010 enrolled participants with a history of herpes zoster. Most (> 68%) of the participants in the primary studies were Caucasian and their mean/median age was 60 to 70 years. One study included individuals aged ≥ 70 years (Vesikari 2013).
All studies were conducted in high‐income countries. Three studies were conducted in the United States (Gilderman 2008; Mills 2010; Oxman 2005), and one in Switzerland (Berger 1998). The others were multi‐country studies: Chlibek 2013 recruited participants in the Czech Republic, Spain and the United States, Chlibek 2014 enrolled participants in the Czech Republic, Germany, The Netherlands and Sweden, Diez‐Domingo 2015 recruited participants in Germany and Spain, Murray 2011 enrolled patients in Canada, Germany, Spain, the United Kingdom and the United States, Tyring 2007 recruited participants in the United States, Canada, the United Kingdom, Germany and Belgium, and Vermeulen 2012 enrolled participants in the United States and the Netherlands.
Despite the wide geographic diversity of the primary studies, we consider the external validity to be low due to the homogeneous characteristics of the participants enrolled in the primary studies.
Quality of the evidence
We classified Chlibek 2013 as having a low risk of bias in six domains of the Cochrane 'Risk of bias' tool: random sequence generation, allocation concealment, blinding (performance bias and detection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data and freedom from selective reporting. We judged it to have an unclear risk of bias for the 'other bias' domain because it lacked details for this domain.
We classified Diez‐Domingo 2015 and Vesikari 2013 as being at low risk of bias for the domains random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias).
We classified Oxman 2005 and Vermeulen 2012 as having a low risk of bias in four of the domains of the Cochrane 'Risk of bias' tool: allocation concealment, blinding, incomplete outcome data and freedom from selective reporting. We classified Murray 2011 as having a low risk of bias in the following four domains: blinding, blinding of outcome assessment, incomplete outcome data and freedom from selective reporting.
Chlibek 2014 had a low risk of bias in three domains (blinding of participants and personnel (performance bias), incomplete outcome data and freedom from selective reporting).
Only Levin 2000 had an unclear risk of bias for selective reporting, while we classified all other studies as having a low risk of bias for this domain (Berger 1998; Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Gilderman 2008; Mills 2010; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013).
Berger 1998, Chlibek 2013, Gilderman 2008, Murray 2011, Oxman 2005, Tyring 2007 and Vermeulen 2012 had a low risk of bias for blinding.
Nine studies were at low risk of attrition bias (Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Gilderman 2008; Murray 2011; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013). We classified Berger 1998 and Levin 2000 as having an unclear risk of bias for this domain and we considered Mills 2010 to have a high risk of bias for this domain.
Potential biases in the review process
Due to the existence of ongoing but unfinished studies, the results currently described in this review may be underestimated (NCT00886613; NCT01165177; NCT01165229; NCT01385566; NCT01505647; NCT01751165; NCT01777321; NCT02075515; NCT02114333; NCT02180295; NCT02526745).
Agreements and disagreements with other studies or reviews
A cohort study followed 766,330 individuals of 65 years of age or more (a 5% random sample of Medicare patients) who had received and not received zoster vaccines between 1 January 2007 and 31 December 2009. Overall, the incidence rate of herpes zoster in the vaccinated participants was 5.4 (95% confidence interval (CI) 4.6 to 6.4) per 1000 person‐years compared to 10.0 (95% CI 9.8 to 0.2) per 1000 person‐years in those not vaccinated (Langan 2013).
Although the primary studies did not assess adverse events associated with autoimmune diseases, a matched case‐control study that collected data from May 2006 to November 2014 was conducted by the Vaccine Adverse Event Reporting System (a national vaccine safety surveillance database maintained jointly by the United States Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA)) to clarify severe autoimmune adverse events post live attenuated herpes zoster vaccine. The adverse events assessed were arthritis, vasculitis, systemic lupus erythematosus, thrombocytopenia, alopecia, Guillain‐Barre syndrome, optic neuritis and multiple sclerosis. That study reported a higher incidence of arthritis and alopecia, after vaccination. Compared to the unexposed, patients with zoster vaccination had 2.2 and 2.7 times the odds of developing arthritis and alopecia, respectively (P value < 0.001 and P value = 0.015, respectively) (Lay 2015).
Our main findings are in concordance with the previous review by Sanford 2010 regarding both the effectiveness and tolerability of herpes zoster vaccines and we have completed their data with additional studies.
Authors' conclusions
There is a clear benefit in vaccinating elderly people with the herpes zoster vaccine, with no major safety/tolerability concerns. Herpes zoster is more frequent among elderly individuals than in other adults and its main clinical feature is pain; therefore prevention of this disease is desirable. Moderate quality evidence suggests that in persons of 60 years of age or more the zoster vaccine can reduce the incidence of herpes zoster for at least three years post‐vaccination.
There are studies of a new vaccine (with a VZV glycoproteic fraction plus adjuvant), which is currently not yet available for clinical use.
The effectiveness of vaccines with lower concentrations (< 18,700 plaque‐forming units/dose – the minimum dose used in Oxman 2005) of VZV should be tested to optimise the amount of virus used in each dose and therefore reduce costs, thus making more vaccine available to everyone who can benefit from it.
According to www.clinicaltrials.gov, https://eudract.ema.europa.eu/ and http://www.who.int/ictrp/en/ there are several ongoing studies:
V212/heat‐treated VZV vaccine or with live zoster vaccine or placebo in healthy volunteers 60 years of age or older (NCT00886613).
Different routes of administration: a randomised controlled trial (RCT) on the immunogenicity and safety of intradermal administration of Zostavax™ (available live attenuated VZV vaccine) (NCT01385566).
Different formulations of Zostavax™ (AMP): Zostavax™ manufactured with an alternative process compared with Zostavax™ manufactured with the current process (NCT01505647).
There are also several ongoing studies of the as yet unavailable vaccine candidate with adjuvanted recombinant subunit glycoproteic gE:
In one the participants will receive intramuscular herpes zoster vaccine GSK1437173A versus an intramuscular placebo (NCT01165177).
In another, adults aged > 70 years will receive intramuscular herpes zoster vaccine GSK1437173A versus an intramuscular placebo (NCT01165229).
A study will evaluate the safety and immunogenicity of GlaxoSmithKline (GSK) Biologicals' herpes zoster vaccine GSK1437173A in adults aged ≥ 50 years, given as two doses in three different schedules: 0 and 2 months schedule; 0 and 6 months schedule and 0 and 12 months schedule (NCT01751165).
A study is comparing herpes zoster subunit (HZ/su) vaccine given subcutaneously at 0 and 2 months versus the same vaccine given by the intramuscular route at 0 and 2 months (NCT01777321).
A study is comparing herpes zoster vaccine GSK1437173A in two different lots (Lot A and Lot B), with two doses given intramuscularly (NCT02075515).
A study is assessing the immunogenicity and safety of available live attenuated VZV zoster vaccine and adjuvanted recombinant VZV subunit zoster vaccine, which is not yet available (NCT02114333).
One study has been withdrawn prior to enrolment (NCT02180295).
The study NCT02526745 is testing different amounts of VZV in Chinese individuals of 50 years of age or more.
Feedback
Seeking efficacy and safety information for autoimmune cohort, 9 May 2018
Summary
Possibly the Institute, in consideration of recent developments in knowledge of immunology and adjuvants, may update, on behalf of millions of people diagnosed with autoimmune syndromes, the Institute's herpes zoster vaccine page, in consideration of more recent medical research into adjuvant‐induced autoimmunity, and the new herpes zoster vaccine, Shingrix, with the QS‐21 adjuvant, in view of current research, e.g., "The Autoimmune/inflammatory syndrome induced by adjuvants (ASIA), Descriptive Analysis of 300 Patients from the International Asia syndrome Registry," Watad , Quaresma M, Bragazzi NL, Cervera R, Tervaer, Amital, Shoenfeld, for a current review of Shingrix, which uses a markedly powerful immune stimulant called QS‐21 Quillaja saponaria ‐ GlaxoKlineSmith in their 2016 application to the FDA states they excluded "immunosuppressed" patients from their studies. Given the use of QS‐21 adjuvant in their Shingrix vaccine, it is unlikely GKS has funded no research of the autoimmune patient response to Shingrix.
Given the Shingrix use of this powerful immune stimulant, of interest is both GKS's use of the term, "immunosuppressed," rather than "immune‐compromised," and what does not appear are studies of the Shingrix use in autoimmune patients and varying potential in this population of millions of people, for QS‐21‐ induced autoimmunity. Some of these syndromes can be catastrophic. The lack of knowledge of, for example, non‐thrombotic antiphospholipid syndrome pathophysiology, prognosis, treatment, is very difficult for patients and doctors. Thank you for considering this suggestion.
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Suzanne Gorenfeld
Acknowledgements
The authors would like to thank the Acute Respiratory Infections (ARI) Group for their support through the editorial process: Elizabeth Dooley for all the support she gave us and Sarah Thorning and Justin Clark for the searches of the databases. We would also like to thank the following persons for commenting on the draft protocol: John Holden, Mary Cadogan, Erin Adams, Anne Lyddiatt and Teresa Neeman. We also thank Peg Ford, Theresa Wrangham, Mary Cadogan, Teresa Neeman and John Holden for commenting on the draft review. We are very grateful to the referees of this update Julie Gildie, Marian Nicholson, Mary Cadogan and Terry Neeman. We are very grateful to the editorial group for its generous help and support throughout the stages of this review.
We want to thank Dr. Myron J. Levin for his willingness and readiness to answer our e‐mails, which was a valuable contribution to the quality of this review.
We want to thank Stéphane Thomas for her willingness and readiness to answer our e‐mails.
Appendices
Appendix 1. CENTRAL and MEDLINE search strategy
MEDLINE (Ovid)
1 exp Herpes Zoster/ 2 Herpesvirus 3, Human/ 3 shingles.tw. 4 zoster.tw. 5 (varicella adj3 virus*).tw. 6 Varicellovirus/ 7 varicellovir*.tw. 8 (hhv3 or hhv‐3).tw. 9 or/1‐8 10 exp Vaccines/ 11 exp Immunization/ 12 Vaccination/ 13 (vaccin* or immuni* or inocul*).tw. 14 or/10‐13 15 9 and 14 16 Herpes Zoster Vaccine/ 17 ((zoster or shingles) adj3 vaccin*).tw. 18 zostavax.tw,nm. 19 or/15‐18
Appendix 2. EMBASE.com search strategy
#22. #18 AND #21 228 #21. #19 OR #20 856,507 #20. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 816,906 #19. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 241,010 #18. #14 OR #15 OR #16 OR #17 3,723 #17. zostavax:ab,ti AND [embase]/lim 22 #16. ((zoster OR shingles) NEAR/3 vaccin*):ab,ti AND [embase]/lim 425 #15. 'varicella zoster vaccine'/de AND [embase]/lim 1,065 #14. #8 AND #13 3,486 #13. #9 OR #10 OR #11 OR #12 375,972 #12. vaccin*:ab,ti OR immuni*:ab,ti OR inocul*:ab,ti AND [embase]/lim 315,836 #11. 'vaccination'/de AND [embase]/lim 60,243 #10. 'immunization'/exp AND [embase]/lim 127,614 #9. 'vaccine'/exp AND [embase]/lim 146,730 #8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 17,850 #7. hhv3:ab,ti OR 'hhv‐3':ab,ti AND [embase]/lim 6 #6. varicellovir*:ab,ti AND [embase]/lim 31 #5. 'varicellovirus'/de AND [embase]/lim 8 #4. (varicella NEAR/3 virus*):ab,ti AND [embase]/lim 5,290 #3. shingles:ab,ti OR zoster:ab,ti AND [embase]/lim 10,726 #2. 'varicella zoster virus'/de AND [embase]/lim 8,085 #1. 'herpes zoster'/exp AND [embase]/lim 10,650
Appendix 3. LILACS (BIREME VHL) search strategy
((MH:"herpes zoster" OR "herpes zoster" or shingles or zona or zoster OR Cobreiro OR Cobrelo OR MH:C02.256.466.423$ OR MH:"Herpesvirus 3, Human"OR "Herpesvirus Humano 3" OR "Varicella‐Zoster Virus" OR "Human herpesvirus 3" OR "Herpesvirus varicellae" OR "Virus de la Varicella‐Zoster" OR "Herpesvirus Humano Tipo 3" OR "Virus del Herpes Zoster" OR "Virus de la Varicela" OR "Vírus da Varicela" OR varicella OR varicela OR MH:varicellovirus OR hhv3 OR "hhv‐3") AND (MH:vaccines OR vacunas OR vacinas OR MH:D20.215.894$ OR MH:immunization OR Inmunización OR Imunização OR MH:E02.095.465.425.400$ OR MH:E05.478.550$ OR MH:N02.421.726.758.310$ OR MH:N06.850.780.200.425$ OR MH:N06.850.780.680.320$ OR MH:SP2.026.182.113$ OR MH:SP4.001.002.015.049$ OR MH:SP8.946.819.838$ OR MH:vaccination OR Vacunación OR Vacinação OR vaccin$ OR immuni$ OR inocul$)) OR (MH:"Herpes Zoster Vaccine" OR "Vacuna contra el Herpes Zoster" OR "Vacina contra Herpes Zoster" OR "shingles vaccine" OR "zoster vaccine" OR zostavax OR "Vacina contra Cobrelo") > clinical_trials
Appendix 4. CINAHL (Ebsco) search strategy
S26 S16 and S25 S25 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 (MH "Quantitative Studies") S23 TI placebo* or AB placebo* S22 (MH "Placebos") S21 TI random* or AB random* S20 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) or AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) S19 TI clinic* trial* or AB clinic* trial* S18 PT clinical trial S17 (MH "Clinical Trials+") S16 S11 or S12 or S13 or S14 or S15 S15 TI zostavax or AB zostavax S14 TI zoster N3 vaccin* or AB zoster N3 vaccin* Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost Search Screen ‐ Advanced Search Database ‐ CINAHL 123 Edit S14 S13 TI shingles N3 vaccin* or AB shingles N3 vaccin* Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost Search Screen ‐ Advanced Search Database ‐ CINAHL 52 Edit S13 S12 TI herpes zoster vaccin* or AB herpes zoster vaccin* S11 S6 and S10 S10 S7 or S8 or S9 S9 TI (vaccin* or immuni* or inocul*) or AB (vaccin* or immuni* or inocul*) S8 (MH "Immunization+") S7 (MH "Vaccines+") S6 S1 or S2 or S3 or S4 or S5 S5 TI (hhv3 or hhv‐3) or AB (hhv3 or hhv‐3) S4 TI varicella N3 virus* or AB varicella N3 virus* S3 TI zoster or AB zoster S2 TI shingles or AB shingles S1 (MH "Herpes Zoster+")
Appendix 5. Study selection, quality assessment and data extraction form
| First author | Journal/conference proceedings, etc. | Year |
| |
Study eligibility
| RCT/quasi‐RCT | Sample mean age ≥ 60 years | Vaccine for herpes zoster | Relevant outcomes |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/No*/Unclear |
| Do not proceed if any of the above answers are 'No'. If study to be included in 'Excluded studies' section of the review, record below the information to be inserted into 'Table of excluded studies'. |
|
Freehand space for comments on study design and treatment: |
References to trial (secondary references)
Check other references identified in searches. If there are further references to this trial link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/conference proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers |
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers/%, etc) | |
| Disease status/type, etc (if applicable) | |
| Underlying disease | |
| Setting | |
| Other | |
Trial characteristics
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate | |
| Inadequate | |
| Unclear | |
| Blinding | |
| Person responsible for participants' care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as intention‐to‐treat | |
| Unclear | |
Were withdrawals described? Yes?/No?/Not clear?
Discuss if appropriate
Data extraction
|
Outcomes relevant to your review Copy and paste from 'Types of outcome measures' | |
| Reported in paper (circle) | |
| Primary outcomes | |
| 1) Incidence of herpes zoster at any time point | Yes/No |
| Secondary outcomes | |
| 1) Adverse events ‐ local, systemic or both (e.g. pain, pruritus, swelling, headache) | Yes/No |
| For continuous data | |||||||
| Code of paper | Outcomes (rename) | Unit of measurement | Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A etc | 1) Mean duration of vaccine protection | ||||||
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| Trial characteristics | |
| Further details | |
| Single centre/multicentre | |
| Country/countries | |
| How was participant eligibility defined? |
|
| How many people were randomised? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Vaccine used | |
| Dose | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data.
Data and analyses
Comparison 1.
Available live attenuated VZV zoster vaccine versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes zoster | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 3.1 years follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 days of vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 42 days of vaccination | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 3.3 to 7.8 years after vaccination substudy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Mean 5 years follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of herpes zoster with ZBPI ADL. Severity of interference scores of 300 or greater (high score is worse) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Participants with AEs | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 One or more AEs | 3 | 6986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.61, 1.80] |
| 3.2 Vaccine‐related AEs | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.63 [2.64, 8.12] |
| 3.3 Systemic AEs | 3 | 6986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.16] |
| 3.4 Systemic pruritus | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.07 [0.37, 135.13] |
| 3.5 Vaccine‐related systemic AEs | 2 | 6777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.57] |
| 3.6 Varicella‐like rash not at injection site (day of vaccination to day 42) | 2 | 38755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.58, 2.18] |
| 3.7 Herpes zoster‐like rash (day of vaccination to day 42) | 1 | 38546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.84] |
| 3.8 Rash unrelated to herpes zoster (day of vaccination to day 42) | 1 | 38546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 3.9 ≥ 1 serious AEs regardless of type of storage of the vaccine | 4 | 50896 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.96, 1.20] |
| 3.10 Vaccine‐related serious AEs | 3 | 50687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 4.00] |
| 3.11 Discontinued due to vaccine‐related AEs | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.25, 103.88] |
| 3.12 Hospitalised | 1 | 6616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 3.13 Hospitalisation related to herpes zoster | 1 | 6616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.25, 2.67] |
| 3.14 Injection site AEs | 3 | 6986 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [2.75, 3.26] |
| 3.15 Erythema inoculation site | 2 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.15 [4.51, 5.87] |
| 3.16 Pain inoculation site | 2 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [3.67, 4.68] |
| 3.17 Pruritus inoculation site | 2 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.91 [4.87, 9.82] |
| 3.18 Swelling inoculation site | 2 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.85 [4.96, 6.91] |
| 3.19 Warmth inoculation site | 2 | 6825 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.15 [2.75, 9.66] |
| 3.20 Rash inoculation site | 1 | 6616 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [1.31, 8.11] |
| 3.21 Haematoma inoculation site | 1 | 6616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.76, 1.67] |
| 3.22 Mass inoculation site | 1 | 6616 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.67 [3.51, 61.33] |
| 3.23 Varicella‐like rash at injection site (day of vaccination to day 42) | 1 | 38546 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.21, 6.76] |
| 4 Drop‐outs | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 For any reason | 3 | 38916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 4.2 Death | 3 | 50687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 4.3 Withdrew consent | 3 | 50735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.64, 1.19] |
| 4.4 Lost to follow‐up | 3 | 50735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.97, 1.73] |
| 4.5 Protocol deviation | 2 | 12189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.41, 6.02] |
| 4.6 Clinical adverse event | 2 | 12189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.73, 2.54] |
| 4.7 Physician decision | 1 | 11980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.17] |
| 5 Participants with no follow‐up | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2.
Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes zoster | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Vaccine‐related adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Vaccine‐related systemic adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Vaccine‐related serious adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Injection site vaccine‐related adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Participants with no follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Analysis 2.2.
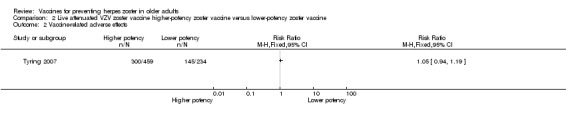
Comparison 2 Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine, Outcome 2 Vaccine‐related adverse effects.
Analysis 2.3.
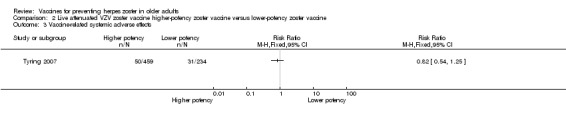
Comparison 2 Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine, Outcome 3 Vaccine‐related systemic adverse effects.
Analysis 2.4.
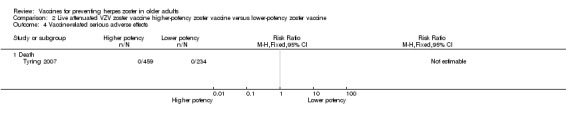
Comparison 2 Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine, Outcome 4 Vaccine‐related serious adverse effects.
Analysis 2.5.
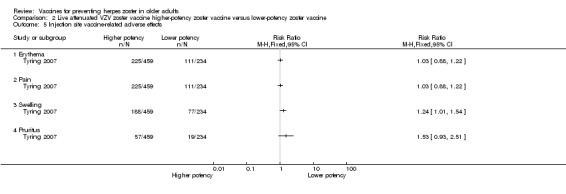
Comparison 2 Live attenuated VZV zoster vaccine higher‐potency zoster vaccine versus lower‐potency zoster vaccine, Outcome 5 Injection site vaccine‐related adverse effects.
Comparison 3.
Live attenuated VZV zoster vaccine zoster vaccine refrigerated versus zoster vaccine frozen
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 One or more adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Vaccine‐related adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Systemic adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Systemic vaccine‐related adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Serious adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Vaccine‐related serious adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Injection site adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Injection site vaccine‐related adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Discontinued due to any adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Discontinued due to a vaccine‐related adverse effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participants with no follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4.
Live attenuated VZV zoster vaccine versus inactivated zoster vaccine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes zoster | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5.
Live attenuated VZV zoster vaccine versus pneumo 23 vaccine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 3200 pfu VZV/dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 ≥ 1 reaction injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Induration (diameter ≥ 2 cm injection site) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pain injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Pain (injection site, probably vaccine‐related) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Redness injection site (diameter ≥ 2 cm) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Pruritus injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Vesicles at injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 8500 pfu VZV/dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 ≥ 1 reaction injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Induration (diameter ≥ 2 cm injection site) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pain injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Pain (injection site, probably vaccine‐related) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Redness injection site (diameter ≥ 2 cm) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Pruritus injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Vesicle injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 41,650 pfu/dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 ≥ 1 reaction injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Induration (diameter ≥ 2 cm injection site) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Pain injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Pain (injection site, probably vaccine‐related) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Redness injection site (diameter ≥ 2 cm) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Pruritus injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Vesicle injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Duration in days of adverse effects | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Erythema | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Swelling | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Rash | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Pruritus | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Haematoma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 5.4.
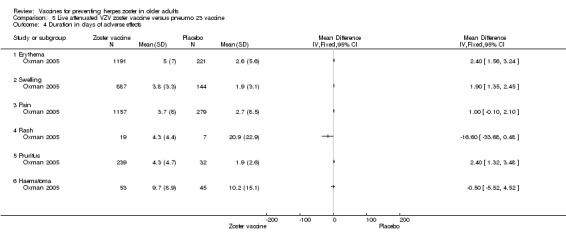
Comparison 5 Live attenuated VZV zoster vaccine versus pneumo 23 vaccine, Outcome 4 Duration in days of adverse effects.
Comparison 6.
Live attenuated VZV zoster vaccine IM route versus zoster vaccine SC route
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At least one AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Vaccine‐related AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 All systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Vaccine‐related systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Headache considered as vaccine‐related by the investigator | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Solicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Unsolicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Injection site erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Severe injection site erythema (> 10 cm) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Injection site pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Severe injection site pain (inability to work or usual activity) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Injection site swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Severe injection site swelling (> 10 cm) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Injection site pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 Withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7.
Live attenuated VZV zoster vaccine 2 doses versus single dose and also 2 doses given at different intervals
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Zoster vaccine 1 month schedule versus zoster vaccine 3 month schedule | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participants with AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Participants with vaccine‐related AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Participants with vaccine‐related serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Participants with withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Participants with vaccine‐related withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Participants with non‐serious vaccine‐related withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Participants with systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Participants with vaccine‐related systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Participants with rash of interest non‐injection site rashes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Participants with varicella/varicella‐like rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Participants with herpes zoster/zoster‐like rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Participants with injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Participants with solicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 Participants with unsolicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Participants with erythema injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Participants with pain injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.18 Participants with swelling injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Zoster vaccine 1 month schedule versus zoster vaccine single dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Participants with adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Participants with vaccine‐related AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Participants with vaccine‐related serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Participants with withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Participants with vaccine‐related withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Participants with non‐serious vaccine‐related withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Participants with systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Participants with vaccine‐related systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Participants with rash of interest non‐injection site rashes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Participants with varicella/varicella‐like rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Participants with herpes zoster/zoster‐like rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Participants with injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Participants with solicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Participants with unsolicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Participants with erythema injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.17 Participants with pain injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.18 Participants with swelling injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Zoster vaccine 3 month schedule versus zoster vaccine single dose | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Participants with AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Participants with vaccine‐related AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Participants with vaccine‐related serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Participants with withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Participants with vaccine‐related withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Participants with non‐serious vaccine‐related withdrawal due to AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Participants with systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Participants with vaccine‐related systemic AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Participants with rash of interest non‐injection site rashes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Participants with varicella/varicella‐like rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Participants with herpes zoster/zoster‐like rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Participants with injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Participants with solicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Participants with unsolicited injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Participants with erythema injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Participants with pain injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Participants with swelling injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8.
Adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50 μg gE/AS01E versus 50 μg gE/AS01B | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participants with any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Participants with any grade 3 symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Participants with any general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Participants with any grade 3 general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Participants with fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Participants with fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Participants with gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Participants with grade 3 gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Participants with headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Participants with myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 Participants with any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Participants with any grade 3 local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.18 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.19 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.20 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.21 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.22 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.23 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.24 Participants with lost follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.25 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 50 μg gE/AS01E versus 50 μg gE/saline (unadjuvanted gE) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Participants with any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Participants with any grade 3 symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Participants with any general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Participants with any grade 3 general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Participants with fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Participants with fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Participants with gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Participants with grade 3 gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Participants with headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Participants with myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Participants with any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Participants with any grade 3 local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.17 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.18 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.19 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.20 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.21 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.22 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.23 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.24 Participants with lost follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.25 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 50 μg gE/AS01B versus 50 μg gE/saline (unadjuvanted gE) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Participants with any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Participants with any grade 3 symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Participants with any general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Participants with any grade 3 general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Participants with fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Participants with fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Participants with gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Participants with grade 3 gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Participants with headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Participants with myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Participants with any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Participants with any grade 3 local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.22 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.23 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.24 Participants with lost follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.25 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 50 μg gE/AS01E versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Participants with any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Participants with any grade 3 symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Participants with any general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Participants with any grade 3 general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Participants with fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Participants with fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Participants with gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Participants with grade 3 gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Participants with headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Participants with myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 Participants with any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Participants with any grade 3 local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.22 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.23 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.24 Participants with lost follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.25 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 50 μg gE/AS01B versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Participants with any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Participants with any grade 3 symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Participants with any general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Participants with any grade 3 general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Participants with fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Participants with fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.9 Participants with gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.10 Participants with grade 3 gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.11 Participants with headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.12 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.13 Participants with myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.14 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.15 Participants with any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.16 Participants with any grade 3 local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.17 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.18 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.19 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.20 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.21 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.22 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.23 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.24 Participants with lost follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.25 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 50 μg gE/Saline (unadjuvanted) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Participants with any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Participants with any grade 3 symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Participants with any general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Participants with any grade 3 general symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Participants with fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Participants with fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.9 Participants with gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.10 Participants with grade 3 gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.11 Participants with headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.12 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.13 Participants with myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.14 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.15 Participants with any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.16 Participants with any grade 3 local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.17 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.18 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.19 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.20 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.21 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.22 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.23 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.24 Participants with lost follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.25 Participants with serious AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9.
Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 25 µg gE/AS01B versus 50 µg gE/AS01B | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 25 µg gE/AS01B versus 100 µg gE/AS01B | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 50 µg gE/AS01B versus 100 µg gE/AS01B | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 25 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 50 µg gE/AS01B a versus 100 µg gE/saline (unadjuvanted gE) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 25 µg gE/AS01B versus saline + 100 µg gE/AS01B | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 50 µg gE/AS01B versus saline + 100 µg gE/AS01B | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 100 µg gE/AS01B versus saline + 100 µg gE/AS01B | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Participants with any fatigue | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Participants with grade 3 fatigue | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Participants with any fever | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Participants with grade 3 fever | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Participants with any headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Participants with grade 3 headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 Participants with any myalgia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.8 Participants with grade 3 myalgia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.9 Participants with local pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.10 Participants with grade 3 local pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.11 Participants with local redness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.12 Participants with grade 3 local redness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.13 Participants with local swelling | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.14 Participants with grade 3 local swelling | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.15 Participants with consent withdrawal | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.16 Participants with lost to follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.17 Participants with death | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Saline + 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Participants with any fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Participants with grade 3 fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Participants with any fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Participants with grade 3 fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Participants with any headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 Participants with grade 3 headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.7 Participants with any myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.8 Participants with grade 3 myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.9 Participants with local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.10 Participants with grade 3 local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.11 Participants with local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.12 Participants with grade 3 local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.13 Participants with local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.14 Participants with grade 3 local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.15 Participants with consent withdrawal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.16 Participants with lost to follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.17 Participants with death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 9.1.
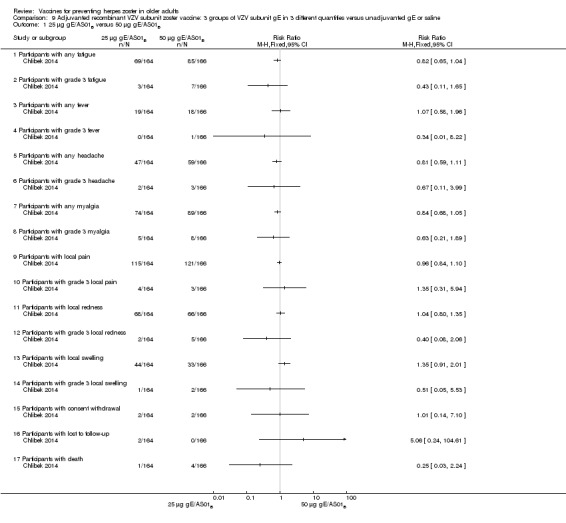
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 1 25 µg gE/AS01B versus 50 µg gE/AS01B.
Analysis 9.2.
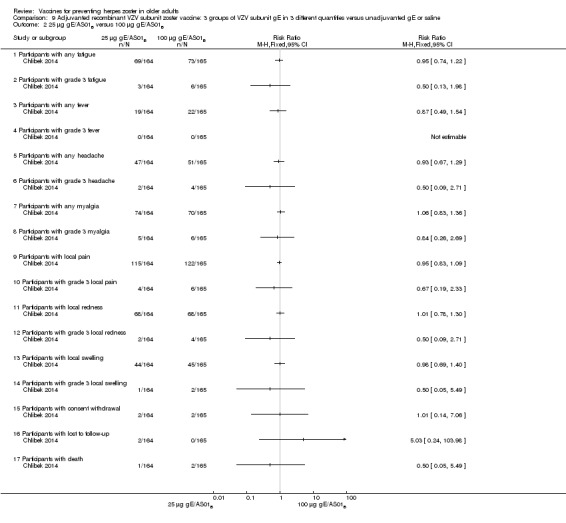
Comparison 9 Adjuvanted recombinant VZV subunit zoster vaccine: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline, Outcome 2 25 µg gE/AS01B versus 100 µg gE/AS01B.
Comparison 10.
Adjuvanted recombinant VZV subunit zoster vaccine (not yet available) versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes zoster 3.2 years follow‐up (≥ 60 yo) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Participants with AEs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Grade 3 any symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Grade 3 any symptom related to vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Any systemic symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Grade 3 any systemic AEs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Shivering | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Gastrointestinal symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Grade 3 any local symptom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Local pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Local redness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Local swelling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.17 Serious AEs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.18 Serious AEs within 30 days after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.19 Serious AEs within 30 days after vaccination related to vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.20 Potential immune‐mediated disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.21 Deaths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.22 Deaths within 30 days after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.23 Unsolicited report of AEs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.24 Grade 3 unsolicited report of AEs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Drop‐outs | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Did not receive vaccine according to protocol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Received wrong vaccine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Had diagnosis of HZ < 30 days after dose 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2018 | Feedback has been incorporated | Feedback comment published |
History
Protocol first published: Issue 12, 2010 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 26 October 2015 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 26 October 2015 | New search has been performed | In this 2015 update we included five new trials (Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Lal 2015; Vesikari 2013), and we excluded one new trial (Leroux‐Roels 2012). A new vaccine that contains a varicella zoster virus glycoproteic fraction plus adjuvant is under study. |
Differences between protocol and review
We deleted the secondary outcome 'mean duration of vaccine protection'.
We added 'drop‐outs' as a secondary outcome as this relates to the safety of the intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, double‐blind Duration: 42 days post‐vaccination | |
| Participants | 200 older adult participants Age range 55 to 88 years ˜59% male ˜66 yo Previous history of varicella confirmed by positive serology to VZV and a competent immune system (no signs of immunodeficiency) | |
| Interventions | 1. A live attenuated VZV/Oka vaccine 3200 pfu/dose SC (frozen); N = 49 2. A live attenuated VZV/Oka vaccine 8500 pfu/dose SC (frozen); N = 51 3. A live attenuated VZV/Oka vaccine 41,650 pfu/dose SC (frozen); N = 49 4. Pneumococcal polysaccharide vaccine (pneumo 23) SC (refrigerated); N = 49 |
|
| Outcomes | Local adverse reaction during 42 days (6 weeks): none, ≥ 1 reaction, induration (diameter ≥ 2 cm), pain (all), pain (probably vaccine‐related), redness (diameter ≥ 2 cm), pruritus and vesicles | |
| Purpose of the Study | "To evaluate the cell‐mediated and humoral immunogenicity and the safety of 1 of 3 doses of a live attenuated varicella‐zoster virus vaccine/OKA compared with a control vaccine" | |
| Funding sources | Pasteur Mérrieux Connaught, Lyon, France | |
| Notes | No participants had fever during the 72 hours following vaccination 1 participant in the 8500 pfu VZV group presented with a mild vesicular rash after vaccination, which lasted 7 days Analysis of the vesicular fluid was negative for VZV (polymerase chain reaction (PCR) analysis) No intention‐to‐treat analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Three groups of different concentrations of a live attenuated VZV/Oka vaccine under double‐blind conditions. 1 group of pneumococcal polysaccharide vaccine under single‐blind conditions and used as a control for a reactogenicity and immune response" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented for all groups |
| Other bias | Unclear risk | Not described |
| Methods | RCT phase II, parallel‐group, placebo‐controlled, double‐blind 12 centres (1 centre in the Czech Republic, 4 in Spain and 7 in the United States) Duration: 1 year after the last vaccination (14 months) |
|
| Participants | N = 410 participants aged > 50 years Participants were excluded if they were using any investigational or non‐registered drug or vaccine within 30 days preceding the first dose of study vaccine or any non‐replicating vaccines within 2 weeks of enrolment, were receiving chronic (> 14 consecutive days) immunosuppressants or other immune‐modifying drugs within 3 months prior to enrolment (for corticosteroids, ≥0.5 mg/kg/day prednisone or equivalent), were previously vaccinated against HZ or varicella, had a history of HZ, allergic disease or reactions likely to be exacerbated by any component of the vaccine, had a confirmed or suspected immunosuppressive or immunodeficient condition, were administered immunoglobulins or any blood products within the 3 months preceding the first injection of study vaccine or planned to receive them during the study period, or had an acute disease at enrolment. In addition, women could not be pregnant or had to be using birth control or be of non‐childbearing potential Mean age ˜65 years Just over half of the participants were women The population was predominantly Caucasian |
|
| Interventions | 1. 2 doses 2 months apart 50 μg purified gE/AS01B (1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL and 50 μg QS‐21) 0.5 mL IM N = 150 2. 2 doses 2 months apart 50 μg purified gE/AS01E (500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL and 25 μg QS‐21) 0.5 mL IM N = 149 3. 2 doses 2 months apart 50 μg purified gE/saline (unadjuvanted gE) 0.5 mL IM N = 73 4. 2 doses 2 months apart saline 0.5 mL IM N = 38 |
|
| Outcomes | 1. Participants with solicited general solicited symptoms (fatigue, fever (recorded as temperature), headache, gastrointestinal symptoms, and myalgia) between days 0 and 6 2. Participants with solicited local reactions (pain, redness and swelling at the injection site) between days 0 and 6 3. Participants with unsolicited symptoms between days 0 and 29 after each dose 4. Participants with temperature was scored grade 3 (> 39.0°C) 5. Participants with other symptoms were scored grade 3 for prevents normal activity 6. Participants with redness and swelling at the injection site were scored grade 3 (> 100 mm) 7. Severe adverse events (SAEs) were collected for 1 year after the last vaccination and were defined as events that resulted in death, were life‐threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in disability/incapacity, caused a congenital anomaly/birth defect in the child of a study participant, or could have jeopardised the participant or required medical or surgical intervention |
|
| Purpose of the Study | Immunogenicity and reactogenicity of recombinant gE in a representative older adult population | |
| Funding sources | GlaxoSmithKline Biologicals SA, Belgium | |
| Notes | "Of the 410 subjects, 395 completed the study. Of the 15 participants who discontinued the study early, 2 withdrew due to treatment related AEs (1 participants each in the gE/AS01E and gE/AS01B groups) and 2 withdrew for SAEs not considered treatment related (digestive tract haemorrhage in the gE/AS01E group and myocardial infarction in the gE/AS01B group), 2 vaccine‐related adverse events led to withdrawal from the study: 1 subject treated with gE/AS01B withdrew due to malaise beginning on the day of vaccination, and 1 participants treated with gE/AS01E withdrew due to injection site redness that lasted > 2 weeks. 2 lost to follow‐up (gE/AS01B), 8 consent withdrawal (4 in the gE/AS01B, 2 in the gE/AS01E, 1 in the gE/saline and 1 after second dose of vaccine in the group gE/AS01B). 1 protocol violation (gE/AS01E)" The only unsolicited symptom reported by > 3% of participants in any group was chills, which was reported by 5% (8/150) of participants treated with gE/AS01B and 2% (3/149) of those treated with gE/AS01E; it was not reported in participants treated with gE/ saline or saline alone No vaccine‐related SAEs and no cases of HZ were reported through month 14 of the study We had asked to authors about the AEs by age or by vaccination but they have answered us only the published data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was made using an algorithm that stratified by country, minimized for age, and included a block size of 11" |
| Allocation concealment (selection bias) | Low risk | "Treatments were allocated at each site using a central randomisation system on the Internet" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The person in charge of the vaccination accessed the randomisation system on Internet using the subject number and age" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both vaccine recipients and observers responsible for evaluations were blinded to which formulation was administered" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both vaccine recipients and observers responsible for evaluations were blinded to which formulation was administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The patient flow is clear |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented for all groups |
| Other bias | Unclear risk | We found no more details on this topic |
| Methods | RCT phase II, randomised, controlled, single‐blind (participants) 11 centres in the Czech Republic, Germany, The Netherlands and Sweden Duration: 36 months after first vaccination |
|
| Participants | 714 healthy participants aged ≥ 60 years Participants were excluded if they had a history of HZ; were previously vaccinated against HZ or with any vaccine containing 3‐O‐desacyl‐ 4‐monophosphoryl lipid A(MPL) or Quillaja saponaria Molina, fraction 21 (QS21), were allergic to any of the vaccine components, had received a vaccine (except influenza) within 2 weeks, an investigational or non‐registered product, chronic immunosuppressants, corticosteroids within 30 days, or immunoglobulins or a blood product within 3 months before the first study vaccine dose, or had a history of drug or alcohol abuse The mean age was ˜69.9 years ˜60% female Predominantly Caucasian (99.3%) |
|
| Interventions | 1. 2 doses 2 months apart 25 µg gE/AS01B 0.5 mL IM N = 164 2. 2 doses 2 months apart 50 µg gE/AS01B 0.5 mL IM N = 166 3. 2 doses 2 months apart 100 µg gE/AS01B 0.5 mL IM N = 165 4. 1 dose saline + 1 dose 100 µg gE 2 months later 0.5 mL IM N = 165 5. 2 doses 2 months apart 100 µg gE/saline (unadjuvanted gE) 0.5 mL IM N = 54 |
|
| Outcomes | 1. Participants with solicited general reactions (fatigue, fever, headache and myalgia): recorded by participants on diary cards for 7 days after each vaccination 2. Participants with solicited local reactions (pain, redness and swelling at the injection site) 3. Participants with unsolicited adverse events (AEs): recorded for 30 days after each vaccination 4. Participants with serious adverse events (SAEs): recorded over the entire study period (36 months) Intensity of the solicited reactions was scored on a scale from 0 (absent) to 3 (severe). All solicited local reactions were considered vaccination‐related and causality of the solicited general reactions, unsolicited AEs and SAEs was assessed by the investigators |
|
| Purpose of the Study | "The aim of the current study is to evaluate the safety and immunogenicity of different schedules and formulations of gE/AS01B in adults ≥ 60 years of age" | |
| Funding sources | GlaxoSmithKline Biologicals SA, Belgium | |
| Notes | 715 participants were enrolled but 714 vaccinated 701 completed the study through month 3 Most solicited reactions were transient (1.1 to 3.5 days on average) and were of mild to moderate intensity (grade 1 or 2), with ≤ 4.8% of participants in each group reporting grade 3 reactions A total of 349 SAEs were reported in 205 participants during the study. 14 participants died due to a SAE, most of which were due to cancer or heart failure. No SAEs were considered related to the study vaccines by the investigators 47 participants (6.6%) were excluded from the according‐to‐protocol immunogenicity cohort. The most common reasons for exclusion were non‐compliance with the blood sampling schedule (N = 27) and the absence of essential serological data (N = 9) Of the 714 vaccinated participants, 685 (95.9%) were followed through month 12, 665 (93.1%) through month 24, and 646 (90.5%) through month 36 8 were withdrawn from 25 µg gE/AS01B group (3 not eligible, 2 lost to follow‐up, 2 consent withdrawal and 1 death); 7 were withdrawn from the 50 µg gE/AS01B group (1 not eligible, 2 consent withdrawal and 4 deaths); 6 were withdrawn from the 100 µg gE/AS01B group (2 not eligible, 2 consent withdrawal and 2 deaths); 4 were withdrawn from the saline + 100 µg gE/AS01B group (1 lost to follow‐up, 1 consent withdrawal and 2 deaths) and 4 were withdrawn from the 100 µg gE/saline group (2 lost to follow‐up and 2 deaths) "The proportion of subjects with solicited reactions was higher for groups receiving two doses of gE/AS01B but the proportion did not increase between the first and the second vaccination (data not shown)" We had asked the authors for information about the AEs by age or vaccination but they have only provided the published data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were stratified by age (60–69years and ≥70 years in a 1:4 ratio) and randomised"; the method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | No information was found about this domain |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no mention of whether the prepared injections were indistinguishable in all aspects of their outward appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind (only for participants) but the participants themselves completed their diary cards as described "solicited local reactions (pain, redness and swelling) and general reactions (fatigue, fever, headache and myalgia) were recorded by subjects on diary cards for seven days after each vaccination" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although the participants themselves completed their diary cards the other AEs were not blinded for the evaluator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The patient flow is clear |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented |
| Other bias | Unclear risk | We found no more details on this topic |
| Methods | Phase 3, open‐label, randomised, comparative, 2‐arm, multicentre study 10 centres in Germany and Spain Duration: participants were followed up for a maximum of 35 days post‐vaccination | |
| Participants | 353 participants of either gender aged ≥ 50 years on day of vaccination, varicella history‐positive or residence for > 30 years in a country with endemic VZV infection Mean age of the 354 participants was 62.6 years ˜55% were female |
|
| Interventions | 1. Intramuscular (IM) route: zoster vaccine (refrigerated) 0.65 mL containing not less than 19,400 plaque‐forming units (pfu) of VZV per dose by IM route; N = 176 2. Subcutaneous (SC) route: zoster vaccine (refrigerated): 0.65 mL containing not less than 19,400 pfu of VZV per dose by SC route; N = 177 |
|
| Outcomes | 1. Injection site adverse reactions (ISRs): injection site erythema, injection site swelling and injection site pain were collected from day 0 to day 4 post‐vaccination ISRs were mainly mild (< 5 cm in size or defined as awareness of sign or symptom but easily tolerated) or moderate (5 cm to < 10 cm in size or defined as discomfort enough to cause interference with usual activity) in intensity. Few participants reported severe ISRs (> 10 cm or defined as incapacitating with inability to work or do usual activity) 2. Fever ‐ temperature > 38.3°C (day 0 to day 28 post‐vaccination) 3. Unsolicited injection site adverse reactions and systemic adverse events and rashes of interest (i.e. varicella, varicella‐like rashes, herpes zoster or shingles and herpes zoster‐like rashes) were collected from day 0 to day 28 post‐vaccination 4. Serious adverse events were collected any time during the study (day 0 to day 35 post‐vaccination) |
|
| Purpose of the Study | "To evaluate the immunogenicity as measured by VZV antibody titres (gpELISA) at 4 weeks following ZOSTAVAX® administered by IM or SC route" "To evaluate the immune response as measured by a second assay, the VZV Interferon‐gamma (IFN‐Ȗ)‐ELISPOT at 4 weeks following ZOSTAVAX® administered by IM or SC route" "To describe the safety profile of ZOSTAVAX® administered by IM or SC route" |
|
| Funding sources | Sanofi Pasteur MSD | |
| Notes | This was basically an immunogenicity study and we only used the safety data More detailed unpublished data were kindly provided by Sanofi Pasteur MSD SNC Data by age were not available One participant reported in Group 1 (IM route) a zoster‐like rash (right thoracic dermatome) of mild intensity that occurred on day 12 after vaccine administration and lasted 6 days. No specimen was obtained for PCR testing. No participant was withdrawn due to an AE at any time after vaccine administration. No deaths were reported. 3 participants reported a SAE: 1 participant (hernia obstructive) in Group 1 (IM route) and 2 participants (humerus fracture and deep vein thrombosis) in Group 2 (SC route). None were assessed as vaccine‐related by the investigator No participant was withdrawn due to an AE at any time after vaccine administration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomised using an electronic case repot form (e‐CRF)" |
| Allocation concealment (selection bias) | Low risk | "Allocation schedules were generated using a 1:1 ratio with permuted blocks of 4‐6" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Between visit 1 and 2, the participants were given a diary card to record their temperature if they were febrile (oral temperature ≥38.3 ◦C), occurrence of any solicited injection site (erythema, swelling and pain) adverse reactions (Days 0–4) and any unsolicited injection site adverse reactions, varicella, varicella‐like rashes, HZ and zoster‐like rashes and other systemic adverse events (AEs) (Days 0–28). They were also asked to report any serious AEs (SAEs) that occurred at any time during the study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The participants did not put any serious AEs (SAEs) in their diary cards themselves, therefore this was not blinded for the staff. "They were also asked to report any serious AEs (SAEs) that occurred at any time during the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on adverse events that the authors proposed in their methodology were described in the results for both groups |
| Other bias | Unclear risk | We found no more details on this topic |
| Methods | RCT, double‐blind, multicentre, USA Duration: 28 days post‐vaccination | |
| Participants | 368 participants (367 analysed) ˜55% female ˜63 yo 68.1% white participants Immunocompetent with a history of varicella or residence in a country where VZV infection is endemic |
|
| Interventions | 1. Zoster vaccine refrigerated SC; N = 182 2. Zoster vaccine frozen SC; N = 185 |
|
| Outcomes | Participants with follow‐up, participants with 1 or more adverse events (AEs), participants with serious AEs, vaccine‐related serious AEs, death, participants who discontinued due to any AE, participants who discontinued due to a vaccine‐related AE | |
| Purpose of the Study | "To support the development of a refrigerator‐stable formulation of Zostavax with a confirmatory clinical trial with varicella‐zoster virus antibody‐seropositive adults ≥50 years of age" | |
| Funding sources | Merck & Co., Inc | |
| Notes | 1 patient withdrew consent prior to intervention No intention‐to‐treat analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, with in‐house blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The formulations were visually indistinct, supplied in identical glass vials |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | The adverse events that the investigators selected were reported in the results section, for both refrigerated and frozen zoster vaccines |
| Other bias | Unclear risk | Not described |
| Methods | Randomised, placebo‐controlled study conducted in 18 countries in Europe, North America, Latin America, Asia and Australia Mean follow‐up of 3.2 years and ongoing (it is expected to be approximately 60 months) | |
| Participants | 15,411 participants, 50 years of age or older, with no history of herpes zoster, not previously vaccinated against varicella or herpes zoster, and no immunosuppressive condition Mean age ˜62.4 years ˜61.2% were female ˜71.5% of white race The majority from Europe: 51.2% | |
| Interventions | 1. Recombinant zoster vaccine (2 doses: first dose month 0 and second dose month 2); N = 7698 2. Placebo (2 doses: first dose month 0 and second dose month 2); N = 7713 |
|
| Outcomes | Cases of herpes zoster A reactogenicity subgroup ‐ 7 days after each vaccination: systemic reactions (fatigue, fever, gastrointestinal symptoms, headache, myalgia and shivering) and solicited injection site reactions (pain, redness and swelling) Serious adverse events were recorded in all participants for up to 12 months after the second dose Death Potentially immune‐mediated diseases |
|
| Purpose of the Study | "The primary objective of the study was to evaluate overall vaccine efficacy in reducing the risk of herpes zoster, as compared with placebo. Secondary objectives included determining the vaccine efficacy in reducing the incidence of herpes zoster in each age group (50 to 59 years, 60 to 69 years, and ≥70 years) and HZ/su safety and reactogenicity profiles." | |
| Funding sources | Supported by GlaxoSmithKline Biologicals | |
| Notes | We used the available data for efficacy by age ≥ 60 y (a total of 8122 participants) and we contacted the authors asking for AEs by age but the data were not provided; therefore we used the AEs published for ≥ 50 y A total of 16,160 participants were enrolled. Of these participants, 749 were excluded from the efficacy analyses, mostly owing to deviations from Good Clinical Practice standards at 2 study centres (involving 726 patients) The remaining 15,411 participants constituted the total vaccinated cohort for analysis; of these participants, 14,759 (95.8%) were included in the modified vaccinated cohort but we did not consider this last cohort since we used ITT analysis Most participants received two doses of the study vaccines (95.6% of HZ/su recipients and 96.4% of placebo recipients) "A reactogenicity subgroup of participants. This subgroup included all participants who were 70 years of age or older and randomly selected participants in the two other age groups (50 to 59 years and 60 to 69 years). The participants rated the intensity of the solicited reactions on a scale from 0 (absent) to 3 (preventing normal everyday activities). Unsolicited adverse events were recorded for 30 days after each dose. Serious adverse events were recorded in all participants for up to 12 months after the second dose. Such events that were considered to be related to the study vaccine or study participation, any events resulting in death, and potentially immune‐mediated diseases were evaluated in all participants over the entire study period. (A full list of potentially immune‐mediated diseases is provided in the Supplementary Appendix.)" We contacted the authors of this study asking for details about the reason why the participants did not receive dose 2. They replied to our email but could not provide this information because "the ZOE‐50 study, which was the subject of the NEJM report, is still ongoing and consequently blinded at the subject level. Therefore, information on the specific reasons for non‐receipt of the second vaccine or placebo dose is not presently available." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly assigned participants in a 1:1 ratio to receive either vaccine or placebo using an online centralized randomization system" |
| Allocation concealment (selection bias) | Unclear risk | Despite the sequence and random number generation being appropriate, there were no details about allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Because the appearance of the reconstituted HZ/su vaccine differed from the placebo solution, injections were prepared and administered by study staff who did not participate in any study assessment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Because the appearance of the reconstituted HZ/su vaccine differed from the placebo solution, injections were prepared and administered by study staff who did not participate in any study assessment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigators, participants, and those who were responsible for the evaluation of any study end point were unaware of whether vaccine or placebo had been administered" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No clear participant flow; the number of patients randomised to each group is not described for all outcomes |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were described in the results |
| Other bias | Unclear risk | Not described |
| Methods | RCT, non‐blinded USA Duration: 36 months post‐vaccination | |
| Participants | 167 participants ˜55% female ˜65 yo (age range 55 to 89 years) Healthy people free from immunosuppressive illness or medication, with a history of varicella but not HZ | |
| Interventions | 1. Live zoster vaccine SC (not specified if frozen); N = 85 2. Inactivated zoster vaccine (live vaccine heated at 56 ºC for 7 days) SC; N = 82 |
|
| Outcomes | Confirmed HZ | |
| Purpose of the Study | "To compare a live attenuated varicella vaccine versus heat‐inactivated varicella vaccine in relation the confirmed cases of HZ and immunogenicity in individuals aged 55‐89 years" | |
| Funding sources | Merck Research Laboratories, West Point, PA, USA | |
| Notes | Author answered our e‐mail and provided data for 1 clinical outcome. Most outcomes evaluated were immunologic There is a misspelling of an author name on the paper, where Dr Levin was referenced as Dr. Levine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
| Methods | RCT, cross‐over, multicentre (9 centres in USA) | |
| Participants | N = 101 healthy participants with physician‐documented history of HZ ˜60% female Mean age in the intervention group was 68.3 years and in the placebo group 67.4 years Data collected for 28 days after each injection | |
| Interventions | 1. Lyophilised (frozen) zoster vaccine SC; N = 51 2. Placebo SC; N = 50 |
|
| Outcomes | In participants ≥ 60 yo 1. Adverse events (AEs): 1 or more AE, injection site AEs, systemic and vaccine‐related systemic AEs 2. Drop‐outs |
|
| Purpose of the Study | "To determine the safety profile and immunogenicity of zoster vaccine in individuals who experienced a prior episode of herpes zoster" | |
| Funding sources | Merck & Co., Inc | |
| Notes | We only used the data for participants 60 years or older Data were analysed with pooled data from cross‐over arms Author contacted and answered our message. There was no separate analysis for the first arm, prior to cross‐over |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but not explained how |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data from the first arm of this cross‐over study were reported |
| Selective reporting (reporting bias) | Low risk | All of the adverse events listed in the methods section were described in the results |
| Other bias | Unclear risk | Not described |
| Methods | Randomised, double‐blind, placebo‐controlled, age‐stratified study Multicentre at 46 sites in Canada, Germany, Spain, the UK and the US Duration: 182 days post‐vaccination | |
| Participants | 11,980 afebrile participants ≥ 60 years of age; no prior receipt of any varicella or zoster vaccine; no intercurrent illness that might interfere with the interpretation of the study or prevent the participant from completion of the study; no immune dysfunction caused by a medical condition; no use of immunosuppressive therapy; no concomitant use of systemic antiviral therapy with activity against herpes viruses Median age in both group was 69 years Female ˜58.7% ˜96.2% white participants | |
| Interventions | 1. Zoster vaccine (refrigerated) SC; N = 5983 2. Placebo SC; N = 5997 |
|
| Outcomes | 1 or more serious side effect(s) occurring 26 weeks (182 days) after the vaccination; vaccine‐related serious side effects, death, injection site adverse events, systemic adverse events; rashes and temperature were only reported if they were considered serious | |
| Purpose of the Study | "To evaluate the general safety of zoster vaccine in adults ≥ 60 years old" | |
| Funding sources | Merck Sharp Dohme Corp. | |
| Notes | Non‐serious adverse events were not reported The study reported 1 or more serious side effect(s) occurring 6 weeks (42 days) and 26 weeks (182 days) after vaccination. In our analyses, we included only the data reported for the second monitoring period, i.e. serious adverse event(s) detected at 182 days after vaccination 36 participants discontinued because of adverse events, 27 participants withdrew consent, 75 participants were lost to follow‐up, 7 participants discontinued because of protocol deviation and 2 participants were discontinued following physician's decision (both were in the placebo group) ITT analysis "For all analyses, cross‐treated (i.e. randomised to ZV and received placebo, or randomised to placebo and received ZV) participants were considered according to the vaccine received and not the vaccine assigned" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The ZV and placebo were reconstituted with sterile diluent immediately prior to administration, and were indistinguishable from each other in appearance. Placebo was the vaccine stabiliser of ZV with no live virus." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent data monitoring committee was established for continuous safety oversight during the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | The serious adverse events that were defined in the methods section were presented in the results |
| Other bias | Unclear risk | Not described |
| Methods | Randomised, placebo‐controlled, double‐blind study at 22 sites in the US Time of follow‐up: at least 7 years of surveillance for HZ | |
| Participants | N = 38,546 participants 60 years of age or older, with history of varicella or had resided in the continental United States for at least 30 years Median age in both groups was 69 years ˜59% male 95.4% white race | |
| Interventions | 1. Zoster vaccine (frozen) (18,700 to 60,000 plaque‐forming units per dose (pfu/dose) and more than 90% of vaccinated participants received 32,300 pfu or less) SC; N = 19,270 2. Placebo SC; N = 19,276 |
|
| Outcomes | Confirmed cases of HZ, cases of HZ within 30 days of vaccination, confirmed HZ cases and all adverse events occurring within 42 days after vaccination and during the whole study Participants with follow‐up, participants with 1 or more AEs (systemic or injection site), participants with serious AEs, vaccine‐related AEs (systemic or injection site), death, varicella‐like rash at injection site and not at injection site, herpes zoster‐like rash, rash unrelated to HZ, participants hospitalised, hospitalisation related to HZ |
|
| Purpose of the Study | "To determine whether vaccination with a live attenuated varicella‐zoster virus vaccine would decrease the incidence, severity, or both of HZ and postherpetic neuralgia in adults 60 years of age or older" | |
| Funding sources | "Supported by the Cooperative Studies Program, Department of Veterans Affairs, Office of Research and Development; by a grant from Merck (to the Cooperative Studies Program); and by a grant from the James R. and Jesse V. Scott Fund for Shingles Research (to Dr. Oxman). The vaccine and placebo used for the study were supplied by Merck; famciclovir was supplied by SmithKline Beecham and Novartis Pharmaceuticals" | |
| Notes | "Zoster vaccine and placebo were lyophilised, held frozen at ‐15°C until reconstituted with sterile water, and administered within 30 minutes" 132 participants withdrew from the study and 113 were lost to follow‐up 1588 participants died during the study, but it was not described whether these were related to the protocol or not Only a subgroup of patients had a safety assessment (zoster vaccine N = 3345; placebo N = 3271), being the adverse event sub‐study This study performed 2 ITT analyses, with all individuals developing HZ and only with those who developed after 30 days from the vaccine injection (modified ITT). For the meta‐analysis we considered the modified ITT There was a break in surveillance for cases of HZ of approximately 15 months between the completion of the Shingles Prevention Study surveillance in September 2003 and resumption of follow‐up in the Short‐Term Persistence Substudy in December 2004. Beginning in October 2005, open‐label zoster vaccine was offered without charge to Shingles Prevention Study placebo recipients. Placebo recipients enrolled in the Short‐Term Persistence Substudy completed the study upon receiving zoster vaccine, since they could then no longer serve as unvaccinated controls. The Short‐Term Persistence Substudy participants who were zoster vaccine recipients in the Shingles Prevention Study continued to be followed until the initiation of the Long‐Term Persistence Substudy in March 2006 The 2012 publication evaluated the effectiveness of the vaccine for up to 7 years after the participants had been vaccinated. However, the data available in this publication report different dates for the collection of outcomes in the intervention and in the placebo groups. The data from the zoster vaccine group are from December 2004 to March 2006 (16 months). In the placebo group, data are reported only from December 2004 to September 2005 (10 months), because in October 2005 the zoster vaccine was also offered to participants in the placebo group, as stated by the authors reported above We contacted the authors of this study asking for the data corresponding to the period from December 2004 to September 2005 (10 months) for both groups (vaccine and placebo). They replied to our email but did not provide this information and suggested instead that we should "assume a uniform rate of events and calculate the estimated number of cases from that" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Each study site received randomly ordered vials of zoster vaccine and placebo in separate boxes for each age stratum" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All other study personnel were blinded to study treatment assignments" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Since the reconstituted zoster vaccine had a different appearance from the placebo, reconstitution and administration were performed by technicians who did not otherwise interact with participants, evaluate outcomes or adverse events, answer the telephone or enter study data." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on effectiveness and adverse events that the authors proposed in their methodology were described in the results for both groups |
| Other bias | Unclear risk | Not described |
| Methods | Randomised clinical trial, blinded to participant, investigator and sponsor 18 sites in the United States, Canada, United Kingdom, Germany and Belgium Duration: 42 days post‐vaccination | |
| Participants | 698 healthy participants, varicella history‐positive (or resident for more 30 years in a country with endemic VZV infection), HZ history‐negative, men and women 50 or more years of age Median age in zoster vaccine higher‐potency group was 64 years and median age of zoster vaccine lower‐potency group was 65 years ˜59.25% female (61.2% in the higher‐potency group and 57.3% in the lower‐potency group) 92.6% white participants | |
| Interventions | 1. Higher‐potency zoster vaccine (frozen) SC (˜207,000 pfu/0.65 mL dose); N = 459 2. Lower‐potency zoster vaccine (frozen) SC (˜58,000 pfu/0.65 mL dose); N = 233 |
|
| Outcomes | Herpes zoster or HZ‐like rash, varicella or varicella‐like rash, local and systemic clinical adverse events and tolerability of both | |
| Purpose of the Study | "To compare the safety and tolerability profile of a higher potency zoster vaccine (˜207,000 plaque forming units (PFU)/0.65‐mL dose) with that of a lower potency vaccine (˜58,000 PFU/0,65‐mL dose)" | |
| Funding sources | Merck Research Laboratories | |
| Notes | Lower‐potency zoster vaccine in this study was similar to vaccine potencies studied in Oxman 2005 Randomised 2:1 ratio to receive 1 injection of each 3 participants were discontinued from the study. 2 participants lost to follow‐up in the higher‐potency zoster vaccine group and 1 participant belonging to the lower‐potency zoster vaccine group withdrew consent prior to completion of the follow‐up period, but was included in the safety analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded participants, investigator and sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The 2 potency formulations were indistinguishable in appearance. All participants received a single 0.65 mL subcutaneous injection of either the higher‐potency zoster vaccine or the lower‐potency zoster vaccine |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | The adverse events defined in the methods section were reported in the results for both higher‐potency and lower‐potency zoster vaccines |
| Other bias | Unclear risk | Not described |
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre study: United States (5 sites) and the Netherlands (1 site) Duration: 6 months after the second vaccination | |
| Participants | N = 209 healthy participants ≥ 60 years with a history of varicella and no prior HZ The mean age at enrolment was 68.7 years for the ZV group and 70.7 years for the placebo group, ˜48% ≥ 70 years old and 8% ≥ 80 years old > 60% women Almost all white participants (97.1% in both groups) | |
| Interventions | 1. Lyophilised zoster vaccine (frozen) SC (∼23,000 pfu); N = 104 2. Placebo SC; N = 105 |
|
| Outcomes | Adverse events (AEs), both injection site and/or systemic. Swelling, redness, pain or tenderness or rash at the injection site, or varicella(‐like) rash or HZ(‐like) rash, any serious AEs (SAEs) | |
| Purpose of the Study | "To examine the safety, tolerability and immunogenicity after 1 and 2 doses of zoster vaccine in adults 60 years of age and older" | |
| Funding sources | Merck Sharp Dohme Corp | |
| Notes | The first and second doses were administered 42 days apart (post‐vaccination 1 and post‐vaccination 2) 1 participant withdrew consent before vaccination in the vaccine group Discontinued after first vaccination vaccine group: clinical AE = 3, withdrew consent = 1, no participants lost follow‐up or due to protocol deviation, other = 2 Discontinued after first vaccination placebo group: 1 participant due to clinical AE, no participants lost to follow‐up, 1 withdrew consent, 1 participant due to protocol deviation and 1 for other reason Discontinued after second vaccination vaccine group: only 1 participant due to clinical AE Discontinued after second vaccination placebo group: 1 to lost follow‐up and 2 for other reasons No ITT analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomised in a 1:1 ratio to receive 2 doses of either ZV or placebo, according to a computer‐generated, study‐centre specific allocation schedule" |
| Allocation concealment (selection bias) | Low risk | "Allocation numbers were assigned sequentially by the study site personnel to subjects who met the study eligibility criteria, beginning with the lowest number available at the study centre, after informed consent and medical history had been obtained. The allocation schedule was generated by a sponsor statistician not otherwise associated with the ZV program" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The subject, investigator, clinical study site personnel, and sponsor personnel directly involved in the study were blinded to whether the subject received zoster vaccine or placebo. They remained blinded until all subjects completed the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The clinical materials were prepared by an unblinded vaccine coordinator at each clinical site, because of differences in the turbidity of the study vaccine and placebo. Each vial of vaccine or placebo was labelled with a subject‐specific allocation number. The unblended vaccine coordinator reconstituted the study vaccine/placebo and wrapped the syringe in an opaque label containing subject allocation number and time of reconstitution. The unblinded vaccine coordinator did not have any contact with the subject and did not disclose the contents of the syringe to the person administering the study vaccine/placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All adverse events listed by the authors were described in their results for both vaccinations |
| Other bias | Unclear risk | Not described |
| Methods | Phase 3, open‐label, randomised, 24 centres: Finland (6 centres), Germany (13 centres), Italy (2 centres), Spain (2 centres) and The Netherlands (1 centre) Time of follow‐up: 12 months after the last dose | |
| Participants | 759 individuals randomised aged ≥ 70 y with either a history of varicella or > 30 y residency in a country with endemic VZV infection were enrolled Individuals were excluded if they had: a history of HZ, previous varicella or HZ vaccination, exposure to varicella or HZ during the preceding 4 weeks, fever (oral temperature 38.3°C) during the preceding 72 hours, live virus vaccination during the preceding 4 weeks and inactivated vaccination during the preceding 2 weeks 509 (67.2%) were aged 70 to 79 years and 248 (32.8%) were aged > 80 years (total = 757) ˜56% female |
|
| Interventions | 1. Refrigerated live attenuated HZ vaccine single dose SC; N = 749 2. Refrigerated live attenuated HZ vaccine 2 doses 1 month apart schedule: 1 month after first dose SC; N = 242 3. Refrigerated live attenuated HZ vaccine 2 doses 3 months apart schedule: 3 months after first dose SC; N = 246 |
|
| Outcomes | AEs, immediate and not immediate, both at injection site and/or systemic: 1. Erythema, swelling and pain within 4 days of vaccination and other injection site reactions were recorded by participants in a diary card 2. Other injection site reaction and systemic AEs were recorded in the diary card for up to 28 days following each vaccination 3. Vaccine‐related serious AEs, deaths and occurrences of HZ, varicella, or zoster‐like and varicella‐like rashes were recorded by the investigators until the study was stopped (1 year) 4. Varicella(‐like) rash or HZ(‐like) rash, any SAEs, vaccine‐related AEs |
|
| Purpose of the Study | "The primary objective of the study was to demonstrate that a second dose of HZ vaccine, administered 1 mo or 3 mo after the first dose, elicits superior VZV antibody titres 4 weeks after vaccination compared with the first dose" "Secondary objectives of the study were to compare VZV antibody titres 12 mo after completion of each two‐dose schedule with those 12 mo after a single dose, and to describe the safety profile of all three HZ vaccination schedules" |
|
| Funding sources | Sanofi Pasteur MSD | |
| Notes | This was an immunogenicity study. For safety analyses, 1 patient randomised to the 1 mo between doses was analysed as receiving the 3 mo schedule More detailed unpublished data were kindly provided by Sanofi Pasteur MSD SNC For the period of first vaccination, the data for the 3 groups were pooled Randomised 1:1:1 ratio to receive: 1 injection only; 2 injections with 1 month between the doses (day 28 to 35) and 2 injections with 3 months between the doses (day 81 to 97) For safety analyses, 1 patient randomised to the 1 month between doses was analysed as receiving the 3 months schedule "Seventeen participants withdrew from study due to adverse events, of whom ten withdrew within 28 d after vaccination" The injection site reactions were generally mild to moderate in intensity and resolved in 3 to 7 d 19 participants reported serious AEs between screening and 12 mo after the last vaccine dose 2 serious AEs were reported by 1 participant None of the serious AEs was considered by the investigator to be vaccine‐related Serious AEs occurred within 28 d of the first vaccine dose in 1.2% of participants (n = 9), and within 28 d of the second dose in 0.9% of participants (n = 4) In 7 participants serious AEs occurred between 28 d and 12 mo after the last dose Until the study was stopped, 12 participants died, 7 within 12 mo of the last vaccination and 5 > 12 mo after the last vaccination No intention‐to‐treat analysis We asked the authors for the outcomes by age but they kindly answered that there was no analysis of safety by age group We used only the data for single doses since the authors state in their conclusion "The results of this study demonstrate that there is no apparent advantage to administering a second dose of Zostavax on a one month or three month schedule among individuals aged ≥ 70 years." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used "blocks of randomisation" |
| Allocation concealment (selection bias) | Low risk | "The allocation schedule was generated using balanced permuted blocks of randomisation" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Solicited injection‐site reactions (erythema, swelling, and pain) occurring within 4 d of vaccination were recorded by participants in a diary card. Other injection‐site reactions and systemic AEs were recorded in the diary card for up to 28 d following each vaccination" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although participants completed their diary cards themselves the other AEs were not blinded for the evaluator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were described in the results |
| Other bias | Unclear risk | We found no more details on this topic |
AE: adverse event AS01: liposome‐based adjuvant system containing the immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and the saponin QS‐21 (Quillaja saponaria Molina, fraction 21) Adjuvanted gE/AS01B: 50 μg purified gE with adjuvant B (1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol 50 μg MPL and 50 μg QS‐21) Adjuvanted gE/AS01E: 50 μg purified gE with adjuvant E (500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL and 25 μg QS‐21) AS01B: adjuvant B composed of 1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol 50 μg MPL and 50 μg QS‐21 AS01E: adjuvant E composed of 500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL and 25 μg QS‐21 d: days Elderly or older adults: aged ≥ 60 years old Frozen: ‐15 °C or colder gE: recombinant subunit VZV composed of glycoprotein E gE/saline: unadjuvanted gE HZ: herpes zoster ID: identification IM: intramuscular ITT: intention‐to‐treat mo: month MPL: immunoenhancer 3‐O‐desacyl‐4′‐monophosphoryl lipid A µg: micrograms N: number NNTB: number needed to treat for an additional beneficial outcome NNTH: number needed to treat for an additional harmful outcome pfu: plaque‐forming units QS‐21: immunoenhancer saponin quillaja saponaria Molina, fraction 21 Refrigerated: 2 °C to 8 °C Recombinant vaccine: the HZ/su vaccine contains 50 µg of recombinant VZV glycoprotein E and the liposome‐based AS01B adjuvant system contains 50 µg of 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and 50 µg of Quillaja saponaria Molina, fraction 21 (QS21, Antigenics, a wholly owned subsidiary of Agenus) SAEs: serious adverse events SC: subcutaneously UK: United Kingdom US: United States VZV: varicella zoster virus y: year yo: years old ZV: zoster vaccine Zoster vaccine 1‐mo schedule: ZV 2 doses given 1 month apart Zoster vaccine 3‐mo schedule: ZV 2 doses given 3 months apart
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hayward 1994 | RCT, evaluating zoster vaccine, with no clinical outcome: focus on immunogenicity |
| Hayward 1996 | RCT, evaluating zoster vaccine, with no clinical outcome: focus on immunogenicity |
| Irwin 2007 | RCT: intervention tested was Tai Chi, not the zoster vaccine |
| Kerzner 2007 | RCT, evaluating zoster vaccine when administered concomitantly with influenza vaccine |
| Leroux‐Roels 2012 | RCT, evaluating zoster vaccine but the mean of age was outside our inclusion criteria (means ranged from 55 to 57 years) |
| Macaladad 2007 | RCT, evaluating zoster vaccine but the age was outside the range of interest: adults ≥ 30 years of age (adults less than 60 years of age) |
| Patterson‐Bartlett 2007 | RCT, evaluating zoster vaccine, with no clinical outcome: focus on immunogenicity |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 'A double‐blind, randomised, placebo‐controlled, parallel group study to evaluate biomarkers of immunity to varicella zoster virus following immunisation with V212/heat‐treated varicella‐zoster virus (VZV) vaccine or with ZOSTAVAX in healthy volunteers' |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants | 120 healthy participants, 60 years and older, both genders |
| Interventions | 1. V212 (heat‐treated VZV vaccine) 2. Live zoster vaccine 3. Placebo |
| Outcomes | Immunogenicity (skin tests) and safety (adverse events) |
| Starting date | March 2009 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT00886613 |
| Notes | This study has been completed. No publications provided |
| Trial name or title | 'Efficacy, safety, and immunogenicity study of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged ≥ 50 years' |
| Methods | Allocation: randomised Endpoint classification: efficacy Study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | 16,256 healthy volunteers, 50 years and older, both genders |
| Interventions | 1. Participants will receive herpes zoster vaccine GSK1437173A according to a 0, 2‐month schedule, intramuscular injection 2. Participants will receive NaCl solution placebo according to a 0, 2‐month schedule, intramuscular injection |
| Outcomes | Confirmed HZ cases, incidence of PHN, duration of severe 'worst' HZ‐associated pain, incidence of overall and HZ‐related mortality, incidence of HZ complications in participants with confirmed HZ, incidence of overall and HZ‐related hospitalisations, duration of pain medication administered for HZ in participants with confirmed HZ, occurrence of solicited local and general symptoms in a subset of participants, occurrence of unsolicited adverse events (AEs), occurrence of serious adverse events (SAEs), occurrence of SAEs related to study participation or to a concurrent GSK medication/vaccine in all participants, occurrence of fatal SAEs, occurrence and relationship to vaccination of any potential immune‐mediated diseases (pIMDs) in all participants, occurrence and relationship to vaccination of any potential immune‐mediated diseases (pIMDs) in all participants |
| Starting date | August 2010 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT01165177 |
| Notes | It has been published but remains ongoing |
| Trial name or title | 'Efficacy, safety and immunogenicity of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged >= 70 years' |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | 14,512 healthy participants, 70 years and older, both genders |
| Interventions | 1. Herpes zoster vaccine intramuscular injection 2. Placebo intramuscular injection |
| Outcomes | Confirmed HZ cases, occurrence of overall postherpetic neuralgia, safety: occurrence of adverse events (AEs) |
| Starting date | August 2010 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT01165229 |
| Notes | This study is ongoing, but not recruiting participants. No publications provided Secondary ID: EudraCT number 2009‐015791‐94 |
| Trial name or title | 'A partially blinded randomised clinical trial to study the immunogenicity and safety of intradermal administration of ZOSTAVAX™ (V211)' |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: single‐Blind (participant) Primary purpose: prevention |
| Participants | 223 healthy volunteers, 50 years and older, both genders |
| Interventions | 1. Active comparator: full dose subcutaneous. Participants will receive a full dose of Zostavax™ administered subcutaneously on Day 1 of the study. 9 participants in this group will also receive saline placebo intradermally in the alternate limb on Day 1 2. Experimental: 1/3 dose subcutaneous. Participants will receive a 1/3 dose of Zostavax™ administered subcutaneously on Day 1 of the study. 6 participants in this group will also receive saline placebo intradermally in the alternate limb on Day 1. Participants will have the option to receive a full subcutaneous dose of Zostavax™ after completion of the study. 3. Experimental: full dose intradermal. Participants will receive a full dose of Zostavax™ administered intradermally on Day 1 of the study. 6 participants in this group will also receive saline placebo intradermally in the alternate limb on Day 1. Participants will have the option to receive a full subcutaneous dose of Zostavax™ after completion of the study. 4. Experimental: 1/3 dose intradermal. Participants will receive a 1/3 dose of Zostavax™ administered intradermally on Day 1 of the study. 6 participants in this group will also receive saline placebo intradermally in the alternate limb on Day 1. Participants will have the option to receive a full subcutaneous dose of Zostavax™ after completion of the study. 5. Experimental: 1/10 dose intradermal. Participants will receive a 1/10 dose of Zostavax™ administered intradermally on Day 1 of the study. 6 participants in this group will also receive saline placebo intradermally in the alternate limb on Day 1. Participants will have the option to receive a full subcutaneous dose of Zostavax™ after completion of the study. 6. Experimental: 1/27 dose intradermal. Participants will receive a 1/27 dose of Zostavax™ administered intradermally on Day 1 of the study. 6 participants in this group will also receive saline placebo intradermally in the alternate limb on Day 1. Participants will have the option to receive a full subcutaneous dose of Zostavax™ after completion of the study. |
| Outcomes | Geometric mean fold change from baseline in varicella zoster virus (VZV)‐specific antibodies, number of participants reporting an adverse experience (AE), number of participants reporting a serious adverse experience (SAE), number of participants reporting specific local injection site adverse experiences, number of participants reporting a non‐injection site rash |
| Starting date | September 2011 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT01385566 |
| Notes | This study has been completed. No publications provided |
| Trial name or title | 'A phase III double‐blinded, randomised, multicenter, controlled study to evaluate the safety, tolerability, and immunogenicity of ZOSTAVAX™ made with an alternative manufacturing process (AMP)' |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants | 498 healthy volunteers, 50 years and older, both genders |
| Interventions | 1. Experimental: Zostavax™ (AMP) Zostavax™ manufactured with an alternative process 2. Active comparator: Zostavax™ manufactured with the current process |
| Outcomes | Geometric mean titre (GMT) of varicella zoster virus (VZV) antibody, geometric mean fold rise (gmfr) in VZV antibody titres, number of participants with 1 or more adverse experiences (AEs), number of participants with 1 or more serious adverse experience (SAE) day 1 to 42 post‐vaccination, number of participants with 1 or more serious adverse experience day 1 to 182 post‐vaccination |
| Starting date | April 2012 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT01505647 |
| Notes | This study has been completed. No publications provided |
| Trial name or title | 'Open‐label study to evaluate the safety and immunogenicity of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged ≥ 50 years' |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants | 354 healthy volunteers, 50 years and older, both genders |
| Interventions | 1. HZ/su‐0,2 Group. Participants will receive HZ/su vaccine on a 0.2 month schedule 2. HZ/su‐0,6 Group. Participants will receive HZ/su vaccine on a 0.6 month schedule 3. HZ/su‐0,12 Group. Participants will receive HZ/su vaccine on a 0.12 month schedule |
| Outcomes | Anti‐gE humoral immunogenicity in terms of antibody concentration, occurrence of solicited local and general symptoms, occurrence of unsolicited symptoms, occurrence of serious adverse events (SAEs), occurrence of AEs of specific interest |
| Starting date | March 2013 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT01751165 |
| Notes | This study is ongoing, but not recruiting participants. No publications provided Secondary ID: EudraCT number 2012‐004456‐11 or Study ID: 116697 |
| Trial name or title | 'Safety and immunogenicity study of GSK Biologicals' herpes zoster subunit (HZ/su) vaccine GSK1437173A when administered subcutaneously vs. intramuscularly in adults aged ≥ 50 years' |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: prevention |
| Participants | 60 healthy volunteers, 50 years and older, both genders |
| Interventions | 1. Experimental: subcutaneus HZ/su Group 0.2 month schedule 2. Active comparator: intramuscular HZ/su Group 0.2 month schedule |
| Outcomes | Evaluation of gE‐specific antibody concentrations, occurrence of solicited local and general symptoms, occurrence of unsolicited symptoms, occurrence of serious adverse events (SAEs), occurrence of adverse events (AEs) of specific interest |
| Starting date | June 2013 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT01777321 |
| Notes | This study has been completed. No publications provided |
| Trial name or title | 'Consistency, immunogenicity and safety study of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults ≥ 50 years of age' |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, caregiver, investigator) Primary purpose: prevention |
| Participants | 651 healthy volunteers, 50 years and older, both genders |
| Interventions | 1.HZ/su Lot A vaccine, 2 doses administered intramuscularly 2. HZ/su Lot B vaccine, 2 doses administered intramuscularly 3. HZ/su Lot C vaccine, 2 doses administered intramuscularly |
| Outcomes | Anti‐gE humoral immunogenicity, occurrence of solicited local and general symptoms, occurrence of unsolicited symptoms, occurrence of serious adverse events (SAEs), occurrence of AEs of specific interest |
| Starting date | August 2014 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT02075515 |
| Notes | This study is ongoing, but not recruiting participants. No publications provided Secondary ID: EudraCT number: 2013‐000373‐76 or Study ID: 117177 |
| Trial name or title | 'A comparison of the immunogenicity and descriptive safety of a live attenuated herpes zoster vaccine and the GSK herpes zoster recombinant HZ/su candidate vaccine in 50 to 59 year old and 70 to 85 year old vaccine recipients' |
| Methods | Allocation: randomised Endpoint classification: pharmacodynamics study Intervention model: parallel assignment Masking: single‐blind (participant) Primary purpose: basic science |
| Participants | 160 healthy volunteers aged 50 years to 85 years, both genders |
| Interventions | 1. No previous zoster vaccine: live zoster vaccine subcutaneous and second dose placebo, normal saline subcutaneous 2. No previous zoster vaccine: recombinant vaccine HZ/su intramuscular and second dose recombinant vaccine intramuscular 3. 1 previous dose of zoster vaccine at least 5 years previously: live zoster vaccine subcutaneous and second dose placebo, normal saline subcutaneous 4. 1 previous dose of zoster vaccine at least 5 years previously: recombinant vaccine HZ/su intramuscular and second dose recombinant vaccine intramuscular |
| Outcomes | Unsolicited adverse events, interferon gamma/ Interleukin 2 (IFNg/IL2) dual colour fluorospot number, glycoprotein‐based enzyme‐linked immunosorbent assay (gpELISA) |
| Starting date | May 2014 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT02114333 |
| Notes | This study is currently recruiting participants. No publications provided |
| Trial name or title | 'A phase III, double‐blind, lot‐to‐lot consistency clinical trial to evaluate the safety, tolerability and immunogenicity of V212 in healthy adults' |
| Methods | Allocation: randomised Endpoint classification: safety study Intervention model: parallel assignment Masking: double‐blind (participant, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | 0 healthy volunteers, 50 years and older, both genders |
| Interventions | 1. Biological: V212 Lot 1. Approximately7.5 units/0.5 mL subcutaneous injection administered in a 4‐dose regimen given approximately 30 days apart 2. Biological: V212 Lot 2. Approximately 7.5 units/0.5 mL subcutaneous injection administered in a 4‐dose regimen given approximately 30 days apart 3. Biological: V212 Lot 3. Approximately 7.5 units/0.5 mL subcutaneous injection administered in a 4‐dose regimen given approximately 30 days apart |
| Outcomes | Geometric mean titre of VZV glycoprotein enzyme‐linked immunosorbent assay (gpELISA) antibody titres, number or percentage of participants with a serious adverse experience (time frame: up to 28 days post dose 4) |
| Starting date | July 2014 |
| Contact information | Please refer to this study by its ClinicalTrials.gov identifier: NCT02180295 |
| Notes | This study has been withdrawn prior to enrolment. No publications provided |
| Trial name or title | 'Safety and immunogenicity study of live attenuated vaccine against herpes zoster in Chinese adults aged 50 years and older' |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants | 440 participants. Aged 50 to 80 years, both gender, accepts healthy volunteers |
| Interventions | 1. Vaccine with low dose of virus content, between 4.7 to 5.0 lg PFU 2.Vaccine with high dose of virus content, between 4.3 to 5.0 lg PFU 3. Vaccine with middle dose of virus content, between 4.3 to 5.0 lg PFU 4. Vaccine with very low dose of virus content, between 4.3 to 5.0 lg PFU 5. Placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2015 |
| Contact information | Beijing Chaoyang District Centre for Disease Control and Prevention Please refer to this study by its ClinicalTrials.gov identifier: NCT02526745 |
| Notes | This study evaluates the safety and immunogenicity of live attenuated vaccine in adults aged 50 years and older. Half of participants will receive high doses of the vaccine,while the other half will receive low doses of the vaccine in phase I clinical trial. At the phase II clinical trial, participants will be distributed equally to four groups (low, middle, high doses of the vaccine and placebo) |
AE: adverse event GSK: GlaxoSmithKline HZ: herpes zoster PFU: plaque‐forming units PHN: postherpetic neuralgia pIMDs: potential immune‐mediated diseases SAE: serious adverse event VZV: varicella zoster virus
Contributions of authors
Conceived the idea for the review: Anna Gagliardi (AG), Maria Regina Torloni (MT) and Brenda Nazaré Gomes Silva (BNGS) Co‐ordinating the review: AG Screening search results: AG, MT, BNGS Organising retrieval of papers: AG, BS Screening retrieved papers against inclusion criteria: AG, BS, MT Appraising quality of papers: AG, BNGS, MT Extracting data from papers: AG, BNGS, BS Writing to authors of papers for additional information: AG, BNGS, MT Providing additional data about papers: AG, BS Obtaining and screening data on unpublished studies: AG, MT Data management for the review: AG, BNGS, MT Entering data into Review Manager (RevMan): AG, BNGS, MT RevMan statistical data: AG, BNGS Other statistical analysis not using RevMan: MT Interpretation of data: AG, BNGS, MT, BS Statistical inferences: AG, BNGS, MT Writing the review: AG, BNGS, MT, BS Guarantor for the review: AG Responsible for reading and checking the review before submission: AG, BNGS, BS, MT
Declarations of interest
Anna MZ Gagliardi: none known Brenda NG Andriolo: none known Maria R Torloni: none known Bernardo GO Soares: none known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
- Berger R, Trannoy E, Holländer G, Bailleux F, Rudin C, Creusvaux H. A dose‐response study of a live attenuated varicella‐zoster virus (Oka strain) vaccine administered to adults 55 years of age and older. Journal of Infectious Diseases 1998;178(Suppl 1):99‐103. [0022‐1899/98/78S1‐0022$02.00] [DOI] [PubMed] [Google Scholar]; Trannoy E, Berger R, Holländer G, Bailleux F, Heimendinger P, Vuillier D, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose‐response trial. Vaccine 2000;18:1700‐6. [PII: S0264‐410X (99) 00510‐1] [DOI] [PubMed] [Google Scholar]
- Chlibek R, Bayas JM, Collins H, Pinta MLR, Ledent E, Johann F, et al. Safety and immunogenicity of an AS01 adjuvanted varicella‐zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. Journal of Infectious Diseases 2013;208:1953–61. [DOI] [PubMed] [Google Scholar]
- Chlibek R, Smetana J, Pauksens K, Rombo L, Hoek JA, Richardus JH, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella‐zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014;32(15):1745‐53. [DOI] [PubMed] [Google Scholar]
- Diez‐Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live‐attenuated vaccine in adults aged ≥50 years: a randomised non‐inferiority clinical trial. Vaccine 2015;33(6):789‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Diez‐Domingo J, Weinke T, Kieninger‐Baum D, Eymin C, Thomas S, Sadorged C. A clinical study of a shingles (herpes zoster) vaccine (live) administered by intramuscular or subcutaneous routes in adults aged ≥ 50 years. European Geriatric Medicine 2013;4(Suppl):81–141. [Google Scholar]
- Gilderman LI, Lawless JF, Nolen TM, Sterling T, Rutledge RZ, Fernsler DA, et al. A double‐blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator‐stable formulation of Zostavax. Clinical and Vaccine Immunology 2008;15(2):314‐9. [DOI: 10.1128/CVI.00310-07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez‐Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New England Journal of Medicine 2015;372(22):2087‐96. [DOI: 10.1056/NEJMoa1501184] [DOI] [PubMed] [Google Scholar]
- Levine MJ, Ellison MC, Zerbe GO, Barber D, Chan C, Stinson D, et al. Comparison of a live attenuated and an inactivated varicella vaccine to boost the varicella‐specific immune response in seropositive people 55 years of age and older. Vaccine 2000;18(25):2915‐20. [PUBMED: 10812235] [DOI] [PubMed] [Google Scholar]
- Mills R, Tyring SK, Levin MJ, Parrino J, Li X, Coll KE, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine 2010;28(25):4204‐9. [DOI: 10.1016/j.vaccine.2010.04.003] [DOI] [PubMed] [Google Scholar]
- Murray AV, Reisinger KS, Kerzner B, Stek JE, Sausser TA, Xu J, et al. Safety and tolerability of zoster vaccine in adults ≥ 60 years old. Human Vaccines 2011;7(11):1130‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella‐zoster virus‐specific immune responses in elderly recipients of a herpes zoster vaccine. Journal of Infectious Diseases 2008;197:825‐35. [DOI: 10.1086/528696] [DOI] [PMC free article] [PubMed] [Google Scholar]; Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. New England Journal of Medicine 2005;352(22):2271‐84. [PUBMED: 15930418] [DOI] [PubMed] [Google Scholar]; Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WWB, Zhang JH, et al. Effect of a zoster vaccine on herpes zoster‐related interference with functional status and health‐related quality‐of‐life measures in older adults. Journal American Geriatrics Society 2010;58(9):1634‐41. [DOI: 10.1111/j.1532-5415.2010.03021.x] [DOI] [PMC free article] [PubMed] [Google Scholar]; Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short‐term persistence substudy. Clinical Infectious Diseases 2012;55(10):1320–8. [DOI: 10.1093/cid/cis638] [DOI] [PMC free article] [PubMed] [Google Scholar]; Simberkoff MS, Arbeit RD, Johnson GR, Oxman MN, Boardman KD, Williams HM, et al. Safety of herpes zoster vaccine in the shingles prevention study a randomized trial. Annals of Internal Medicine 2010;152(9):545‐54. [PUBMED: 20439572] [DOI] [PubMed] [Google Scholar]; Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caufield MJ, et al. Varicella‐zoster virus‐specific immune response to herpes zoster in elderly participants of a clinically effective zoster vaccine. Journal of Infectious Diseases 2009;200(7):1068‐77. [DOI: 10.1086/605611] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring SK, Diaz‐Mitoma F, Padget LG, Nunez M, Poland G, Cassidy WM, et al. Safety and tolerability of a high‐potency zoster vaccine in adults ≥ 50 years of age. Vaccine 2007;25:1877‐83. [DOI] [PubMed] [Google Scholar]
- Vermeulen JN, Lange JMA, Tyring SK, Peters PH, Nunez M, Poland G, et al. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults ≥60 years of age. Vaccine 2012;30(5):904‐10. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Hardt R, Rümke HC, Icardi G, Montero J, Thomas S, et al. Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax®) in individuals aged ≥ 70 years. A randomized study of a single dose vs. two different two‐dose schedules. Human Vaccines & Immunotherapeutics 2013;9(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or Inactivated VZV vaccine in elderly adults. Viral Immunology 1994;7(1):31‐6. [PUBMED: 7986334] [DOI] [PubMed] [Google Scholar]
- Hayward AR, Buda K, Jones M, White C Jo, Levin MJ. Varicella zoster virus specific cytotoxicity following secondary immunization with live or killed vaccine. Viral Immunology 1996;9(4):241‐5. [PUBMED: 8978020] [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of tai chi. Journal American Geriatrics Society 2007;55(4):511‐7. [DOI: 10.1111/j.532-5415.2007.01109.x] [DOI] [PubMed] [Google Scholar]
- Kerzner B, Murray AV, Gheng E, Ifle R, Harvey PR, Tomlinson M, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. Journal of the American Geriatrics Society 2007;55(10):1499‐507. [DOI: 10.1111/j.1532-5415.2007.01397.x] [DOI] [PubMed] [Google Scholar]
- Leroux‐Roels I, Leroux‐Roels G, Frédéric Clement F, Vandepapelière P, Vassilev V, Ledent E, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. Journal of Infectious Diseases 2012;206:1280–90. [DOI] [PubMed] [Google Scholar]
- Macaladad N, Marcano T, Guzman M, Moya J, Jurado F, Thompson M, et al. Safety and immunogenicity of a zoster vaccine in varicella‐zoster virus seronegative and low‐seropositive healthy adults. Vaccine 2007;25:2139‐44. [DOI: 10.1016/j.vaccine.2006.11.011] [DOI] [PubMed] [Google Scholar]
- Patterson‐Bartlett J, Levin MJ, Lang N, Schödel FP, Vessey R, Weingerg A. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine 2007;25(41):7087‐93. [PUBMED: 17766015] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- 'A double‐blind, randomised, placebo‐controlled, parallel group study to evaluate biomarkers of immunity to varicella zoster virus following immunisation with V212/heat‐treated varicella‐zoster virus (VZV) vaccine or with ZOSTAVAX in healthy volunteers'. Ongoing studyMarch 2009.
- 'Efficacy, safety, and immunogenicity study of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged ≥ 50 years'. Ongoing studyAugust 2010.
- 'Efficacy, safety and immunogenicity of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged >= 70 years'. Ongoing studyAugust 2010.
- 'A partially blinded randomised clinical trial to study the immunogenicity and safety of intradermal administration of ZOSTAVAX™ (V211)'. Ongoing studySeptember 2011.
- 'A phase III double‐blinded, randomised, multicenter, controlled study to evaluate the safety, tolerability, and immunogenicity of ZOSTAVAX™ made with an alternative manufacturing process (AMP)'. Ongoing studyApril 2012.
- 'Open‐label study to evaluate the safety and immunogenicity of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults aged ≥ 50 years'. Ongoing studyMarch 2013.
- 'Safety and immunogenicity study of GSK Biologicals' herpes zoster subunit (HZ/su) vaccine GSK1437173A when administered subcutaneously vs. intramuscularly in adults aged ≥ 50 years'. Ongoing studyJune 2013.
- 'Consistency, immunogenicity and safety study of GSK Biologicals' herpes zoster vaccine GSK1437173A in adults ≥ 50 years of age'. Ongoing studyAugust 2014.
- 'A comparison of the immunogenicity and descriptive safety of a live attenuated herpes zoster vaccine and the GSK herpes zoster recombinant HZ/su candidate vaccine in 50 to 59 year old and 70 to 85 year old vaccine recipients'. Ongoing studyMay 2014.
- 'A phase III, double‐blind, lot‐to‐lot consistency clinical trial to evaluate the safety, tolerability and immunogenicity of V212 in healthy adults'. Ongoing studyJuly 2014.
- 'Safety and immunogenicity study of live attenuated vaccine against herpes zoster in Chinese adults aged 50 years and older'. Ongoing studyNovember 2015.
Additional references
- Arvin A. Ageing, immunity, and the varicella‐zoster virus. New England Journal of Medicine 2005;352(22):2266‐7. [PUBMED: 15930416] [DOI] [PubMed] [Google Scholar]
- Arvin AM, Kinney‐Thomas E, Shriver K, Grose C, Koropchak CM, Scranton E, et al. Immunity to varicella‐zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. Journal of Immunology 1986;137(4):1346‐51. [PMID: 3016094] [PubMed] [Google Scholar]
- Arvin AM. Varicella‐zoster virus. Clinical Microbiology Reviews 1996;9(3):361‐81. [PUBMED: 8809466 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, el al. Taking a toll on human disease: toll‐like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opinion on Biological Therapy 2004;4:1129‐38. [PMID: 15268679] [DOI] [PubMed] [Google Scholar]
- Cho JW, Shin DH, Lee KS. Polymorphism of the IL‐10 gene is associated with susceptibility to herpes zoster in Korea. Journal of Dermatological Science 2007;45(3):213‐5. [PUBMED: 17204399] [DOI] [PubMed] [Google Scholar]
- Choi WS, Noh JY, Huh JY, Jo YM, Lee J, Song JY, et al. Disease burden of herpes zoster in Korea. Journal of Clinical Virology 2010;47(4):325‐9. [PUBMED: 20181512] [DOI] [PubMed] [Google Scholar]
- Cohen JI, Straus SE, Arvin AM. Varicella‐zoster virus replication, pathogenesis, and management. In: Knipe DM editor(s). Fields’ Virology. 5th Edition. Philadelphia: Lippincott Williams and Wilkins, 2007:2773‐818. [Google Scholar]
- Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the Brief Pain Inventory. Journal of Pain 2004;5(6):344‐56. [DOI: 10.1016/j.jpain.2004.06.001] [DOI] [PubMed] [Google Scholar]
- Dolin R, Reichman RC, Mazur MH, Whitkey RJ. Herpes zoster‐varicella infections in immunosuppressed patients. Annals of Internal Medicine 1978;89(3):375‐88. [DOI: 10.7326/0003-4819-89-3-375] [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Barbano RL, Tyring SK, Betts RF, McDermott MP, Pennella‐Vaughan J, et al. A randomized, placebo‐controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009;142(3):209‐17. [PUBMED: 19195785] [DOI] [PubMed] [Google Scholar]
- Gatti A, Pica F, Boccia MT, Antoni F, Sabato AF, Volpi A. No evidence of family history as a risk factor for herpes zoster in patients with post‐herpetic neuralgia. Journal of Medical Virology 2010;82(6):1007‐11. [PUBMED: 20419815] [DOI] [PubMed] [Google Scholar]
- Gilden DH, Kleinschmidt‐DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella zoster virus. New England Journal of Medicine 2000;342(9):635–45. [PUBMED: 10699164] [DOI] [PubMed] [Google Scholar]
- Gnann JW Jr, Whitley RJ. Clinical practice: herpes zoster. New England Journal of Medicine 2002;347(5):340‐6. [12151472] [DOI] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
- Guyatt G, Gutterman D, Baumann MH, Addrizzo‐Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 2006;129:174‐81. [PUBMED: 16424429] [DOI] [PubMed] [Google Scholar]
- Guyatt G, Vist G, Falck‐Ytter Y, Kunz R, Magrini N, Schünemann H. An emerging consensus on grading recommendations?. ACP Journal Club 2006;144:A8‐9. [PUBMED: 16388549] [PubMed] [Google Scholar]
- Haanpaa M, Nurmikko T, Hurme M. Polymorphism of the IL‐10 gene is associated with susceptibility to herpes zoster. Scandinavian Journal of Infectious Diseases 2002;34:112‐4. [PUBMED: 11928840] [DOI] [PubMed] [Google Scholar]
- Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, et al. Diabetes as a risk factor for herpes zoster infection: results of a population‐based study in Israel. Infection 2008;36(3):226‐30. [PUBMED: 18454342] [DOI] [PubMed] [Google Scholar]
- Hicks LD, Cook‐Norris RH, Mendoza N, Madkan V, Arora A, Tyring SK. Family history as a risk factor for herpes zoster: a case‐control study. Archives of Dermatology 2008;144(5):603‐8. [PUBMED: 18490586] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hope‐Simpson E. The nature of herpes zoster: a long‐term study and a new hypothesis. Proceedings of the Royal Society of Medicine 1965;58(1):9‐12. [PMC 1898279] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger J, Robertus K. Cost‐effectiveness of a vaccine to prevent herpes zoster and post herpetic neuralgia in older adults. Annals of Internal Medicine 2006;145(5):317‐25. [PUBMED: 16954357] [DOI] [PubMed] [Google Scholar]
- Jih JS, Chen YJ, Lin MW, Chen YC, Chen TJ, Huang YL, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Dermato‐Venereologica 2009;89(6):612‐6. [PUBMED: 19997693 ] [DOI] [PubMed] [Google Scholar]
- Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health‐related quality of life. Clinical Infectious Diseases 2004;39:342–8. [PUBMED: 15307000] [DOI] [PubMed] [Google Scholar]
- Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molinacortex. Journal of Immunology 1991;146:431‐7. [PMID: 1987271] [PubMed] [Google Scholar]
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post‐herpetic neuralgia in an older US population: a cohort study. PLoS Medicine 2013;10(4):1‐11. [PUBMED: 23585738] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour J, Abraira V, Cabello JB, López Sánchez J. Investigation methods in clinical cardiology (IV) clinical measurements in cardiology: validity and errors of measurement. Revista Española de Cardiología 1997;50(2):117‐28. [PUBMED: 9091999 ] [DOI] [PubMed] [Google Scholar]
- Lai YC, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case‐control study of adverse events in a national database. Journal of Drugs in Dermatology 2015;14(7):681‐4. [PUBMED: 26151783] [PubMed] [Google Scholar]
- Lee BW. Review of varicella zoster soroepidemiology in India and South‐East Asia. Tropical Medicine & International Health 1998;3(11):886‐90. [DOI: 10.1046/j.1365-3156.1998.00316.x; PUBMED: 9855401] [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: a self‐limited disease with severe impact. Neurology 1995;45(Suppl 8):52‐3. [PUBMED: 8545021] [DOI] [PubMed] [Google Scholar]
- Moffat J, Ku CC, Zerboni L, Sommer M, Arvin A. Pathogenesis and the disease consequences of primary infection. In: Arvin A, Campadelli‐Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al. editor(s). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [PUBMED: 21348076 ] [Google Scholar]
- Partridge DG, McKendrick MW. The treatment of varicella‐zoster virus infection and its complications. Expert Opinion on Pharmacotherapy 2009;10(5):797‐812. [PUBMED: 19351229] [DOI] [PubMed] [Google Scholar]
- Rajesh K, Gupta RK, Siber GR. Adjuvants for human vaccines ‐ current status, problems and future prospects. Vaccine 1995;13(14):1263–76. [DOI: 10.1016/0264-410X(95)00011-O] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clinical Infectious Diseases 2010;50(7):1000‐5. [PUBMED: 20178416] [DOI] [PubMed] [Google Scholar]
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proceedings 2009;84(3):274‐80. [PUBMED: 19252116 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M, Keating GM. Zoster vaccine (Zostavax®) a review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs and Aging 2010;27(2):159‐76. [1170‐229X/10/0002‐0159/$49.95/0] [DOI] [PubMed] [Google Scholar]
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. Herpes zoster pain and discomfort on functional status and quality of life in older adults. Clinical Journal of Pain 2007;23(6):490‐6. [PUBMED: 17575488] [DOI] [PubMed] [Google Scholar]
- Schmader K, Gnann JW Jr, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. Journal of Infectious Diseases 2008;197(Suppl):207–15. [PUBMED: 18419399] [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Sutradhar SC, Wang WWB, Schlienger K, Stek JE, Xu J, Chan ISF, et al. Comparison of the levels of immunogenicity and safety of Zostavax in adults 50 to 59 years old and in adults 60 years old or older. Clinical and Vaccine Immunology 2009;16:646‐52. [DOI: 10.1128/CVI.00407-08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster?. Lancet Infectious Diseases 2004;4(1):26‐33. [PUBMED: 14720565] [DOI] [PubMed] [Google Scholar]
- Weaver BA. The burden of herpes zoster and postherpetic neuralgia in the United States. Journal of the American Osteopathic Association 2007;107(Suppl 1):2‐7. [PUBMED: 17488884] [PubMed] [Google Scholar]
References to other published versions of this review
- Gagliardi AMZ, Gomes SBN, Torloni MR, Soares BGO. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008858] [DOI] [Google Scholar]
- Gagliardi AMZ, Silva BNG, Torloni MR, Soares BGO. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008858.pub2] [DOI] [PubMed] [Google Scholar]


