Abstract
Background
Anaphylaxis is a serious hypersensitivity reaction that is rapid in onset and may cause death. Adrenaline (epinephrine) auto‐injectors are recommended as the initial, potentially life‐saving treatment of choice for anaphylaxis in the community, but they are not universally available and have limitations in their use.
Objectives
To assess the effectiveness of adrenaline (epinephrine) auto‐injectors in relieving respiratory, cardiovascular, and other symptoms during episodes of anaphylaxis that occur in the community.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 1), MEDLINE (Ovid SP) (1950 to January 2012), EMBASE (Ovid SP) (1980 to January 2012 ), CINAHL (EBSCO host) (1982 to January 2012 ), AMED (EBSCO host) (1985 to January 2012 ), LILACS, (BIREME) (1980 to January 2012 ), ISI Web of Science (1950 to January 2012 ). We adapted our search terms for other databases. We also searched websites listing on‐going trials: the World Health Organization International Clinical Trials Registry Platform, the UK Clinical Research Network Study Portfolio, and the meta Register of Controlled Trials; and contacted pharmaceutical companies who manufacture adrenaline auto‐injectors in an attempt to locate unpublished material.
Selection criteria
Randomized and quasi‐randomized controlled trials comparing auto‐injector administration of adrenaline with any control including no intervention, placebo, or other adrenergic agonists were eligible for inclusion.
Data collection and analysis
Two authors independently assessed articles for inclusion.
Main results
None of the 1328 studies that were identified satisfied the inclusion criteria.
Authors' conclusions
Based on this review, we cannot make any new recommendations on the effectiveness of adrenaline auto‐injectors for the treatment of anaphylaxis. Although randomized, double‐blind, placebo‐controlled clinical trials of high methodological quality are necessary to define the true extent of benefits from the administration of adrenaline in anaphylaxis via an auto‐injector, such trials are unlikely to be performed in individuals experiencing anaphylaxis because of ethical concerns associated with randomization to placebo. There is, however, a need to consider trials in which, for example, auto‐injectors of different doses of adrenaline and differing devices are compared in order to provide greater clarity on the dose and device of choice. Such trials would be practically challenging to conduct. In the absence of appropriate trials, we recommend that adrenaline administration by auto‐injector should still be regarded as the most effective first‐line treatment for the management of anaphylaxis in the community. In countries where auto‐injectors are not commonly used, it may be possible to conduct trials to compare administration of adrenaline via auto‐injector with adrenaline administered by syringe and ampoule, or comparing the effectiveness of two different types of auto‐injector.
Plain language summary
Adrenaline auto‐injectors for the treatment of anaphylaxis in the community
Anaphylaxis is a serious allergic reaction that is rapid in onset and may cause death. It is commonly triggered by a food, insect sting, medication, or natural rubber latex. The reaction typically occurs without warning and can be a frightening and life‐threatening experience for those at risk and for their families and friends. Adrenaline (epinephrine) is widely advocated as the main treatment in people experiencing anaphylaxis and many people at risk of anaphylaxis are prescribed adrenaline auto‐injectors (such as the Adrenaclick, Anapen, Epipen, Jext, and Twinject) so that they or their families can, if needed, administer adrenaline in the community. Potential problems with adrenaline auto‐injector use include failure to carry the auto‐injector at all times; failure to use it because the signs and symptoms of anaphylaxis are not recognized; a preference to use an oral antihistamine; or fear and panic in the emergency. In addition, in many countries adrenaline auto‐injectors are either not available or they are not affordable. The evidence base regarding the effectiveness of adrenaline auto‐injectors is unclear. We therefore conducted a systematic review of the literature, searching key databases for high quality published and unpublished material on the use of adrenaline auto‐injectors during episodes of anaphylaxis in the community. We also contacted the relevant pharmaceutical companies to see if they had any such information on the topic. Our searches found many studies relating to anaphylaxis and adrenaline auto‐injector use but no randomized controlled trials on this subject. We concluded that the use of adrenaline auto‐injectors in anaphylaxis is based on the best available information at present. There is no evidence from randomized controlled trials for the effectiveness of adrenaline auto‐injectors in the emergency treatment of anaphylaxis in the community. Such trials would ideally involve comparison of adrenaline with placebo; however, use of a placebo in anaphylaxis treatment would be unethical. We therefore recommend that auto‐injectors remain the medication of first choice in the treatment of anaphylaxis in the community.
Background
Description of the condition
Anaphylaxis is a serious allergic reaction that is rapid in onset and may cause death (Lieberman 2006; Sampson 2006). It is no longer considered to be a rare event (Lieberman 2006; Simons 2008a). The rate of occurrence is increasing, especially in young people (Decker 2008; Lin 2008; Sheikh 2008b). Many episodes of anaphylaxis occur in community settings rather than in hospital or healthcare settings (Decker 2008).
Description of the intervention
Adrenaline (epinephrine) is the initial medication of choice in the treatment of an acute anaphylaxis episode. It is recommended by the World Allergy Organization (WAO) (Kemp 2008; Simons 2011) and all anaphylaxis guidelines published in indexed, peer‐reviewed journals (Alrasbi 2007; Brown 2006; Lieberman 2010; Simons 2011; Worth 2010) including the recently updated, comprehensive United Kingdom guidelines (Soar 2008). The World Health Organization lists adrenaline (epinephrine) as an essential medication for the treatment of anaphylaxis (WHO 2009). Adrenaline auto‐injectors are the key components of emergency preparedness to treat anaphylaxis in the community (Simons 2009d). They provide a single, pre‐loaded dose of adrenaline to be self‐administered intramuscularly in an anaphylactic episode. The dose depends on body weight.
How the intervention might work
Through the alpha‐1 adrenergic receptor, adrenaline administration leads to vasoconstriction, increased peripheral vascular resistance, and decreased mucosal oedema. These actions are unique among the medications used for the treatment of anaphylaxis in community settings because they lead to prevention and relief of obstruction to airflow, both in the upper airway and in the lower airway, as well as to prevention and relief of hypotension and shock. Additional actions of adrenaline are also useful in anaphylaxis. These include its beta‐1 adrenergic agonist inotropic and chronotropic effects that increase the force and rate of cardiac contractions and its beta‐2 adrenergic agonist effects including bronchodilation and decreased release of chemical mediators of inflammation (Simons 2009a).
Cardiorespiratory arrest in the community from anaphylaxis occurs in a median time of 15 minutes from an insect sting and a median time of 30 minutes after ingestion of a food to which the patient is sensitized (Pumphrey 2000). Failure to inject adrenaline promptly in a patient with anaphylaxis potentially increases the risk of death from anaphylaxis (Bock 2007; Greenberger 2007; Pumphrey 2007). It also potentially increases the risk of biphasic anaphylaxis in which initial symptoms resolve only to recur one to 72 hours later despite no further exposure to the trigger (Lieberman 2005). People at risk of anaphylaxis in the community therefore need to be equipped and trained to self‐inject adrenaline.
Why it is important to do this review
A previous Cochrane review acknowledged that the efficacy and safety of adrenaline in anaphylaxis has never been documented in randomized controlled trials (Sheikh 2008a). It is nevertheless recommended that adrenaline administration by intramuscular injection should be regarded as the first‐line treatment in the management of anaphylaxis, based on 100 years of clinical experience and consensus opinion as well as on fatality reports in which individuals dying from anaphylaxis had not received prompt adrenaline treatment (Bock 2007; Greenberger 2007; Pumphrey 2007; Sheikh 2008a).
The recommended first‐aid dose of adrenaline for the treatment of acute anaphylaxis in a community setting is 0.01 mg/kg, up to a maximum dose of 0.5 mg in an adult, injected intramuscularly in the mid‐anterolateral thigh to rapidly achieve peak plasma and tissue concentrations (Sampson 2006; Simons 2010a; Soar 2008). At present, three fixed doses of adrenaline are available in auto‐injectors, 0.15 mg, 0.3 mg, and also 0.5 mg, introduced in 2010 (Simons 2010a). These adrenaline doses are lower than the initial 1 mg dose that is used in cardiopulmonary resuscitation (Worth 2010).
In some patients with severe or protracted uniphasic anaphylaxis the symptoms are not relieved by one injection. In addition, biphasic anaphylaxis, as defined previously, is reported in up to 23% of patients with anaphylaxis (Lieberman 2005; Scranton 2009). It seems to be less common in children (Jarvinen 2009; Lee 2000; Mehr 2009; Scranton 2009). Whether or not people in the community who are at risk of anaphylaxis should carry more than one adrenaline auto‐injector with them at all times is a subject of debate (Arkwright 2009; Worth 2010). The percentage of those experiencing anaphylaxis who are given a second injection of adrenaline appears to vary considerably, from 6% (Jarvinen 2009); 12% (Rudders 2010a); 13% (Manivannan 2009); 16% (Oren 2007; Rudders 2010b); 19% (Jarvinen 2008); through to 35% (Korenblat 1999). In cases of biphasic anaphylaxis where the first dose of adrenaline was given promptly, a second dose was not needed during the later phase (Confino‐Cohen 2010; Scranton 2009). An interval of five to 15 minutes between injections is generally recommended (Kemp 2008; Sampson 2006; Soar 2008), however this recommendation is based on clinical experience and consensus rather than on prospective studies. In most countries apart from the USA, it is not standard practice to recommend that people at risk of anaphylaxis in the community carry two adrenaline auto‐injectors with them at all times.
Currently, several types of adrenaline auto‐injectors are available. These include the EpiPen (most commonly used worldwide), the Anapen, the Adrenaclick, the Jext, and the Twinject. Each has limitations with regard to dose, needle length, shelf life, and a lack of user‐friendly design (Gallagher 2011; Simons 2010a). The first limitation is lack of options with regard to the adrenaline doses available. The 0.15 mg dose is too high for infants and young children weighing less than 15 kg. The 0.3 mg dose is too low for some children and for teens and adults, especially those who are overweight or obese (Simons 2001a; Simons 2002a; Simons 2002b). The second limitation is that, based on computed tomography scans of the distance from the skin to the surface of the vastus lateralis muscle in the thigh, adrenaline auto‐injector needles are not long enough to penetrate the subcutaneous tissue and to achieve intramuscular injection in many children let alone in overweight or obese teens and adults (Song 2005; Stecher 2009). The force of the adrenaline injection may compensate to some extent for the short needle on the adrenaline auto‐injector. The third limitation is the short shelf life of adrenaline auto‐injectors, which for Epipen and Twinject is only 12 to18 months and for Anapen and Jext is 21 to 24 months. After the expiry date has passed, gradual degradation of adrenaline to adrenochrome and melanin occurs over the ensuing months and years. These degradation products have no pharmacologic activity in anaphylaxis (Simons 2000a). The fourth limitation is that all users, including physicians and other healthcare professionals, must be trained (and re‐trained regularly) in how to use the auto‐injectors correctly and safely (Arkwright 2006; Huang 1998; Mehr 2007). The ability to correctly and safely inject adrenaline through an auto‐injector is not intuitive. Injuries after unintentional injection of the adrenaline into fingers, thumbs, and other body parts have been reported (Simons 2009b; Simons 2010b).
Failure to use an adrenaline auto‐injector for treatment of an anaphylaxis episode that occurs in the community has been reported (Simons 2009c). There are many reasons for this, including that the person at risk for anaphylaxis had not received a prescription for an adrenaline auto‐injector, the person used an antihistamine or an asthma inhaler, the allergic reaction was mild or transient, the person had an auto‐injector but it was not being carried or was not accessible at the time, they were afraid to inject adrenaline, they were concerned about potential adrenaline adverse effects, or the signs and symptoms of anaphylaxis were not recognized (Ben‐Shoshan 2008; Campbell 2011; Flokstra‐de Blok 2011; Gallagher 2011; Greenhawt 2009; Sheikh 2007; Simons 2009c).
Adrenaline auto‐injectors are not available in many countries and in some countries where they are available, their high cost makes them unaffordable (Simons 2009d). Randomized controlled clinical pharmacology studies of one auto‐injector (EpiPen 0.3 mg, EpiPen 0.15 mg) have been conducted in adults and in children (Simons 1998; Simons 2001b; Simons 2002a). Injection of adrenaline from this auto‐injector produces plasma, and presumably tissue, adrenaline levels that are likely to be associated with relief of systemic symptoms and are typically associated with mild, transient pharmacologic effects such as pallor, tremor, and palpitations (Simons 1998; Simons 2001b; Simons 2002a). Mechanical failure of auto‐injectors is uncommonly reported in the medical literature. Other adrenaline formulations cannot be depended on to rapidly produce high adrenaline plasma and tissue concentrations. These other formulations include adrenaline metered‐dose inhalers that are no longer widely available because they contain chlorofluorocarbon propellants; moreover, most adults find it difficult to inhale the 20 to 30 puffs (or for children 10 to 20 puffs) required to produce plasma and tissue adrenaline levels likely to be associated with relief of systemic anaphylaxis symptoms (Simons 2000b). People without medical training are significantly slower in drawing up a dose of adrenaline from an ampoule than are healthcare professionals, and lack of accuracy in the adrenaline doses drawn up by untrained individuals, especially the small doses needed for children, has been documented (Simons 2001c). The problem with syringes pre‐filled with adrenaline (as physicians in low resource countries commonly give to patients) is that an in vitro study has shown that the shelf life of adrenaline is only three to four months when dispensed in this manner (Rawas‐Qalaji 2009).
Existing adrenaline auto‐injectors have limitations and uncertainties with regard to their use. Although placebo‐controlled trials of adrenaline auto‐injectors would clearly be unethical, it might be possible to conduct randomized controlled trials comparing two different first‐aid doses of adrenaline in auto‐injectors, for example, 0.3 mg versus 0.5 mg in adults, or to compare administration of adrenaline in two different types of auto‐injectors (Simons 2008b; Simons 2009a).
In summary, at present there are no life‐saving alternatives for the first‐aid or pre‐hospital treatment of anaphylaxis. A systematic and critical review of the literature pertaining to adrenaline auto‐injectors is therefore needed in order to serve as the basis for future investigations, including randomized controlled trials.
Objectives
To assess the effectiveness of adrenaline auto‐injectors in relieving respiratory, cardiovascular, and other symptoms during episodes of anaphylaxis that occur in the community.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs) comparing auto‐injector administration of adrenaline with any control including no intervention, placebo, other adrenergic agonists, and different treatment approaches to the administration of adrenaline. We also planned to include quasi‐randomized trials, where the method of allocation was not truly random.
Types of participants
We were interested in participants of all ages (that is infants, children, and adults) where adrenaline had been administered by auto‐injector during an episode of anaphylaxis from any aetiology including idiopathic anaphylaxis for which no trigger had been identified.
Types of interventions
We were interested in studies where adrenaline had been administered in any setting by any type of auto‐injector (Adrenaclick, Anapen, EpiPen, or Twinject) compared to no treatment, placebo, alternative pharmacological agent, or adrenaline by an alternative route. This included adrenaline administered in a child or adult dose; self‐administered or administered by a lay caregiver, for example a parent, other relative, teacher; or administered by a healthcare professional. The adrenaline may have been administered in the community, hospital, other healthcare facility, or ambulance.
Types of outcome measures
Our outcome measures of interest were as follows.
Primary outcomes
1. Death
Secondary outcomes
2. Process outcomes: (a) proportion of participants carrying auto‐injector; b) proportion correctly using auto‐injector; c) proportion experiencing difficulty using auto‐injector; d) proportion that failed to use auto‐injector in a timely and appropriate manner
3. Resolution of airway obstruction
4. Improvement in arterial blood pressure (> 50 mm Hg systolic in an adult)
5. Hospital attendance or admission
6. Re‐presentation for treatment within 72 hours
7. Cost‐effectiveness
8. Adverse events due to treatment in either treatment arm, including long‐term morbidities such as neurological damage, stroke, hemiparesis; and including self‐injury or injury to the person administering adrenaline through the auto‐injector
Search methods for identification of studies
Electronic searches
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 1), see Appendix 1; MEDLINE (Ovid SP) (1950 to January 2012), see Appendix 2; EMBASE (Ovid SP) (1980 to January 2012), see Appendix 3; CINAHL (EBSCO host) (1982 to January 2012), see Appendix 4; AMED (EBSCO host) (1985 to January 2012), see Appendix 5; LILACS (BIREME) (1980 to January 2012), see Appendix 6); ISI Web of Science (1950 to January 2012), see Appendix 7. We adapted our search terms for other databases (see Appendix 8): KoreaMed, IndMed, PakMediNet, ZETOC, IMEMR (Index Medicus for WHO Eastern Mediterranean Region), IMSEAR (Index Medicus for WHO South‐East Asian Region). We did not apply any language restrictions.
We also planned to develop a database of first and last authors of potentially eligible studies and to search the Science Citation Index Expanded (SCI‐EXPANDED) (1945 to January 2012 ) using these authors' names for additional studies, and to search the bibliographies of identified studies.
Searching other resources
In an attempt to uncover additional relevant published data, grey literature, unpublished data, and research in progress, we searched the following websites listing on‐going and recently completed trials: the World Health Organization International Clinical Trials Registry Platform (ICTRP), the UK Clinical Research Network Study Portfolio, and the meta Register of Controlled Trials (mRCT). We also searched conference proceedings (ISI Web of Science). We contacted pharmaceutical companies which manufacture adrenaline auto‐injectors for any additional relevant information (Appendix 9).
Data collection and analysis
Four authors (AS, AW, VB, and FERS) undertook this review; AW co‐ordinated the review.
Selection of studies
Two authors (AW and VB) independently reviewed titles and abstracts identified from the literature searches for relevant trials. We planned to retrieve the full text copies of all potentially eligible studies and subject each to independent review using the inclusion criteria detailed above. We planned to resolve any disagreements by discussion between authors. In the case of consensus not being reached, we would have involved a third author (AS) to participate in discussions and, if necessary, to arbitrate.
Data extraction and management
We planned for two authors (AW and VB) to independently extract data using an adapted version of the data extraction form developed by the Cochrane Anaesthesia Review Group (Appendix 10). We intended to resolve any disagreements by discussion between the authors. In the case of consensus not being reached, we would have involved the third author (AS) to participate in discussions and, if necessary, to arbitrate. This did not prove to be necessary.
Assessment of risk of bias in included studies
We planned to assess the quality of included RCTs following the Cochrane approach, using the methods detailed in Section six of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We proposed the construction of risk of bias tables to support judgements of quality, obtained using the Cochrane 'Risk of bias' tool (Higgins 2011) with the following criteria.
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a risk of bias?
The judgement would have been be recorded as 'risk of bias' indicating that the study met that quality parameter, 'risk of bias' if it did not, or as 'unclear' if there was insufficient information to make a judgement either way.
We intended to analyse data obtained from quasi‐randomized controlled trials together with data derived from randomized controlled studies; however, given the inherent high risk of bias in quasi‐randomized designs, we stated we would undertake a sensitivity analysis by excluding such studies and thus focusing on the findings from the more methodologically robust studies.
We proposed that two authors (AW and VB) would independently assess study quality. The authors would not be masked to study details. The agreement of authors on methodological quality assessment would be assessed. We would resolve disagreements by discussion and, if necessary, with the involvement of a third author (AS).
Measures of treatment effect
We intended to provide a risk ratio (RR) for the primary outcome of each trial included, on the basis of an intention‐to‐treat analysis. An estimated pooled weight average for RRs would have been calculated using the Mantel‐Haenszel fixed‐effect or random‐effects model, as appropriate.
Unit of analysis issues
We anticipated that included studies would most likely follow a parallel group design, with individuals randomized to one of two arms of the trial. We did not anticipate identifying any cluster‐randomized trials in this review.
Dealing with missing data
In any studies with missing data, in line with recommendations in the Cochrane Handbook for Systematic Reviews of Interventions, Section 16.1.2 (Higgins 2011), we intended to contact the authors for further information. In the event of being unable to obtain such information, we would have reported this and discussed its potential impact, but we did not intend to impute the missing data.
Assessment of heterogeneity
We planned to test for heterogeneity using the I2 statistic and significant heterogeneity would have been assumed if I2 was greater than 40% (that is more than 40% of the variability in outcome between trials could not be explained by sampling variation) (Higgins 2002). We planned to undertake meta‐analysis using fixed‐effect or random‐effects modelling depending on whether or not data were found to be homogenous. In the absence of any statistically significant between‐study heterogeneity, we planned to report a pooled effect derived from the fixed‐effect model; if we had uncovered statistical heterogeneity, however, we would have investigated this (see below). If it had been still considered clinically appropriate to pool data, we would have report a pooled effect derived from the random‐effects model. In the absence of problematic clinical heterogeneity (such as trials enrolling infants versus those enrolling adults) we would, where possible, have tested for the source of this heterogeneity by undertaking subgroup analyses based on the age of the participants studied (that is children versus adults), the severity of the reaction (that is mild to moderate versus severe), and the type of adrenaline auto‐injector used.
Assessment of reporting biases
We planned to assess publication bias graphically using funnel plots and statistically using Begg and Egger tests (Egger 1997).
Data synthesis
We proposed that AS and AW would carry out data analysis. We planned to use Review Manager (RevMan 5.1) for data analysis and quantitative data synthesis. For dichotomous data, we would have calculated individual and pooled statistics as relative risks (RR) with 95% confidence intervals (CI). For continuous data, we would have calculated individual and pooled statistics as mean differences (MD) or standardized means differences (SMD) with 95% CIs. We planned to give consideration to the appropriateness of meta‐analysis in the presence of significant clinical or statistical heterogeneity. We would have carried out quantitative analyses of outcomes, wherever possible, on an intention‐to‐treat basis.
Subgroup analysis and investigation of heterogeneity
In the event of uncovering significant heterogeneity, we intended to investigate this by undertaking subgroup analyses. Our subgroups of interest were as follows.
Mild versus moderate versus severe anaphylaxis (Brown 2004).
Type of device used (for example Adrenaclick, Anapen, Epipen, Twinject).
Time from onset of first anaphylaxis symptom to receiving adrenaline.
Age (infants, children, adolescents, adults, elderly).
Sensitivity analysis
We planned to undertake sensitivity analysis for the allocation of missing data by best and worst case analysis and also to undertake sensitivity analysis on the basis of only including randomized studies, which would have allowed an assessment of the impact on the review conclusions of excluding studies judged to be at high risk of bias.
Results
Description of studies
Searching different databases up to January 2012 yielded 1586 citations. After removal of duplicates, 1328 citations remained (see Figure 1). After scrutiny of the abstracts of these studies, no studies fulfilled the inclusion criteria. We contacted pharmaceutical companies but were unable to identify any relevant published or unpublished studies. The search of the WHO International Clinical Trials Registry Platform, the UK Clinical Research Network Study Portfolio, and the meta Register of Controlled Trials identified no relevant studies.
1.
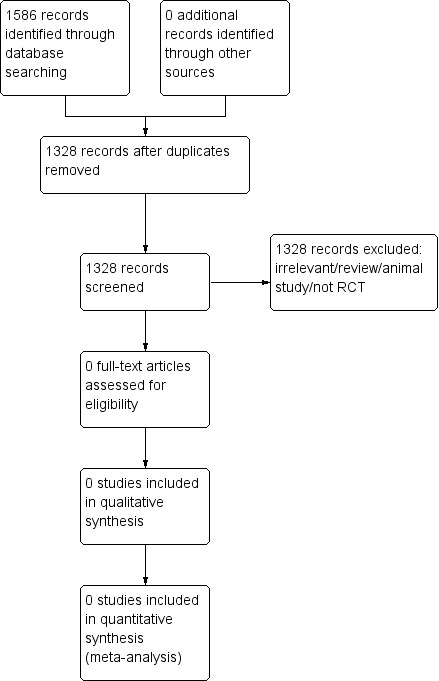
Study flow diagram.
Results of the search
There were no eligible studies.
Included studies
There were no eligible studies.
Excluded studies
All 1328 studies were excluded as they did not meet the inclusion criteria.
Risk of bias in included studies
For any included studies, we intended to construct a risk of bias summary table, assessing whether each study was low risk, high risk, or unclear in respect of the following potential sources of bias. See data extraction sheet for details (Appendix 10). There were no eligible studies.
Allocation
We intended to examine eligible studies for details of how participants were randomized or otherwise allocated, and to what extent and how treatment concealment was conducted and maintained. There were no eligible studies.
Blinding
We intended to examine eligible studies for evidence of blinding of participants, those undertaking assessments, and those undertaking the analysis. There were no eligible studies.
Incomplete outcome data
We intended to examine eligible studies for reporting of incomplete outcome data. There were no eligible studies.
Selective reporting
We intended to examine eligible studies for evidence of selective reporting by comparing the a priori stated outcomes with those detailed in the studies identified. There were no eligible studies.
Other potential sources of bias
We intended to examine included studies for evidence of other sources of bias, such as the funding source of trials and an assessment of the likely validity of trial findings. There were no eligible studies.
Effects of interventions
We intended to examine included studies for evidence of effectiveness in relation to our primary and secondary outcomes of interest. There were no eligible studies.
Discussion
Summary of main results
This review failed to uncover any evidence from prospective randomized or quasi‐randomized trials on the effectiveness of adrenaline auto‐injectors in relieving cardiovascular, respiratory and other related symptoms during episodes of anaphylaxis in the community.
Overall completeness and applicability of evidence
We found no relevant evidence for the effectiveness of adrenaline auto‐injector use in the treatment of anaphylaxis. We are, therefore, unable to make any new recommendations based on the findings of this review. Ideally, recommendations for adrenaline auto‐injector administration in anaphylaxis would be based on prospective randomized, double‐blinded, placebo‐controlled trials in individuals actually experiencing severe acute allergic reactions. Such placebo controlled clinical trials would, however, be unethical because prompt administration of adrenaline is the potentially life‐saving first‐line treatment in anaphylaxis. Moreover, the unpredictability of anaphylaxis and the likelihood of occurrence in unsupervised community settings make such trials difficult to conduct (Simons 2009a). We support existing guidelines (Simons 2011; Soar 2008) stating that intramuscular injection of adrenaline via auto‐injector is the recommended initial treatment of anaphylaxis in the community.
Auto‐injectors are under‐used in anaphylactic emergencies for a variety of reasons, including fear and panic, failure to recognise anaphylaxis, and uncertainty over technique (Bock 2007;Gallagher 2011; Kemp 2008;Simons 2009c;Simons 2010a). Many people at risk of anaphylaxis have not received training in auto‐injector use that adequately equips them to self‐administer adrenaline during an anaphylactic emergency (Gallagher 2011). The under‐use of adrenaline auto‐injectors in anaphylaxis is likely to contribute to fatalities (Bock 2007;Pumphrey 2007), and individuals who are at high risk of anaphylaxis should carry an adrenaline auto‐injector and receive training and support in how, when, and why to use it (Simons 2011; Soar 2008). A recent Swedish study in young people at risk of anaphylaxis recurrence has evaluated the impact on children’s anxiety of self‐administering adrenaline via auto‐injector with promising initial results (Hellstrom 2011).
Effective alternative routes of adrenaline administration which might be more acceptable to patients are not yet developed. An animal study concluded that a sublingual adrenaline tablet and adrenaline intramuscular injection can potentially produce similar plasma concentrations of adrenaline (Rawas‐Qalaji 2006). This has not yet been replicated in humans. An adrenaline metered‐dose inhaler has been found to be impractical with regard to rapid and adequate dosing (Simons 2000b).
There have been some recent developments with regard to adrenaline auto‐injectors. The EpiPen available in the US and Canada has been modified to include needle protection after injection. Another modified auto‐injector, Jext (ALK‐Abello, Berkshire, UK), introduced in Europe, has needle protection after injection, fewer restrictions on optimal storage conditions, and a 24‐month shelf life. A novel auto‐injector, e‐cue (Sanofi US, Bridgewater, NJ, USA), is approved for use in the USA and Canada. It has a compact design (credit card size and mobile phone thickness), voice prompting to help guide the user through the injection process, and needle protection before and after injection (Guerlain 2010a). The training materials for the e‐cue are reported to be safer and easier to use in comparison to the trainers for EpiPen and Twinject (Guerlain 2010a; Guerlain 2010b). Whether modified auto‐injectors or novel auto‐injectors, including others currently being developed, will truly be easier and safer for patients and caregivers to use needs to be tested in controlled studies (Frew 2011). Bioequivalence testing among auto‐injectors is now being conducted (Edwards 2011).
Quality of the evidence
We found no relevant trials on the effectiveness of adrenaline auto‐injector use in the treatment of anaphylaxis.
Potential biases in the review process
The multiple searches conducted minimised the chance of failure to identify relevant studies, although there remains a remote risk that the review authors may have missed some relevant studies. The absence of studies identified for inclusion in this review minimised the possibility of bias in the review process.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews on the effectiveness of adrenaline auto‐injectors. A previous systematic review of adrenaline for the treatment of anaphylaxis (Sheikh 2008a) also did not identify any relevant trials. The conclusions of this review align with the consensus in the literature and guidelines that adrenaline auto‐injectors remain the first‐line emergency treatment for anaphylaxis in the community (Simons 2011; Soar 2008).
Authors' conclusions
Implications for practice.
Currently, adrenaline auto‐injectors remain the best available treatment for first‐aid management of anaphylaxis in community settings, based on both pharmacological action and practical experience. Despite lack of high quality evidence, we agree with Simons that adrenaline auto‐injectors should remain a key component of emergency preparedness to treat anaphylaxis in the community (Simons 2009d).
Continued efforts should be made to ensure that more effective training in adrenaline auto‐injector use is given to people at risk of anaphylaxis and their care‐givers.
Implications for research.
Although placebo‐controlled trials of adrenaline auto‐injectors would clearly be unethical, there are key questions which could be addressed. In particular, there is currently no evidence‐based information on whether a first‐aid dose of 0.3 mg adrenaline is more effective than 0.5 mg for the treatment of anaphylaxis; it might be possible to conduct randomized controlled trials in adults comparing these two different doses of adrenaline in auto‐injectors, and this has been suggested (Simons 2008b). In countries where auto‐injectors are not commonly used, it may also be possible to conduct randomized controlled trials to compare administration of adrenaline via auto‐injector with adrenaline administered by syringe and ampoule; or comparing the effectiveness of two different types of auto‐injector.
Alternative methods of dose‐equivalent adrenaline delivery, such as the sublingual route (Rawas‐Qalaji 2006), should be explored further.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
| 18 June 2014 | Review declared as stable | This Cochrane review has been marked as ‘stable no longer being updated’ as there are no randomized controlled trials (RCTs) and for ethical reasons there are unlikely to be any RCTs in the future. If the situation changes, then the authors will update the review. |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 8, 2012
| Date | Event | Description |
|---|---|---|
| 29 January 2016 | Amended | Lead author's contact details updated |
| 15 December 2010 | Amended | Change to delaration of interest section. Previously Aziz Sheikh stated 'none known'. |
Acknowledgements
We would like to thank Mike Bennett (content editor); Nathan Pace (statistical editor); Hugh A Sampson, Richard Pumphrey (peer reviewers); Tracey Lloyd, Janet Wale and Vanitha Jagannath (representatives of the Cochrane Consumer Network); and Jane Cracknell from the Cochrane Anaesthesia Review Group for their help and editorial advice during the preparation of this systematic review. We would also like to thank Karen Hovhannisyan, Trials Search Co‐ordinator from the Cochrane Anaesthesia Review Group for advice and help with conducting the searches.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Anaphylaxis explode all trees #2 anaphyl* #3 acute near allerg* #4 (#1 OR #2 OR #3) #5 MeSH descriptor Sympathomimetics explode all trees #6 MeSH descriptor Catecholamines explode all trees #7 MeSH descriptor Epinephrine explode all trees #8 MeSH descriptor Norepinephrine explode all trees #9 catecholamine* or adrenalin* or epinephrine* or norepinephrine* #10 auto?injector* or EpiPen* or Anapen* or Minijet* or Jext #11 (#5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 (#4 AND #11)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Anaphylaxis/ or anaphyl*.ti,ab. or (acute adj3 allerg*).mp. 2. Sympathomimetics/ or Catecholamines/ or Epinephrine/ or Norepinephrine/ or (catecholamine* or adrenalin* or epinephrine* or norepinephrine*).ti,ab. or (auto?injector* or EpiPen* or Anapen* or Minijet* or Jext).af. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp ANAPHYLAXIS/ or exp ANAPHYLACTIC SHOCK/ or anaphyl*.ti,ab. or (acute adj3 allerg*).mp. 2. Sympathomimetics/ or Catecholamines/ or Epinephrine/ or Norepinephrine/ or (catecholamine* or adrenalin* or epinephrine* or norepinephrine*).ti,ab. or (auto?injector* or EpiPen* or Anapen* or Minijet* or Jext).af. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Search strategy for CINAHL (EBSCO host)
S1 (MM "Anaphylaxis") or anaphyl* or (acute and allerg*) S2 (MM "Sympathomimetics") OR (MM "Catecholamines") OR (MM "Epinephrine") OR (MM "Norepinephrine") or (catecholamine* or adrenalin* or epinephrine* or norepinephrine* ) or ( auto?injector* or EpiPen* or Anapen* or Minijet* or Jext) S3 S1 and S2 S4 (MM "Random Assignment") OR (MM "Randomized Controlled Trials") OR (MM "Double‐Blind Studies") OR (MM "Single‐Blind Studies") OR (MM "Triple‐Blind Studies") OR (MM "Placebos") OR (MM "Multicenter Studies") OR (MM "Prospective Studies") OR (MM "Clinical Trials") ) or ( random* or (controlled and trial*) S5 S3 and S4
Appendix 5. Search strategy for AMED (EBSCO host)
S1 anaphyl* or (acute and allerg*) S2 (catecholamine* or adrenalin* or epinephrine* or norepinephrine* or auto?injector* or EpiPen* or Anapen* or Minijet* or Jext) S3 S1 and S2
Appendix 6. Search strategy for LILACS (BIREME)
("ANAPHYLAXIS" or "ANAPHYLACTIC" or "anaphyl$") and ("SYMPATHOMIMETICS" or "CATECHOLAMINES" or "EPINEPHRINE" or "EPINEPHRYNE" or "NOREPINEPHRINE" or "ADRENALIN" or "ADRENALINA/" or "NORADRENALIN" or "auto‐injector$" or "EpiPen$" or "Anapen$" or "Minijet$" or "Jext")
Appendix 7. Search strategy for ISI Web of Science
# 1 TS=anaphyl* or TS=(acute SAME allerg*) # 2 TS=(catecholamine* or adrenalin* or epinephrine* or norepinephrine* or auto?injector* or EpiPen* or Anapen* or Minijet* or Jext) # 3 #2 AND #1 # 4 TS=(random* or multicenter* or prospective* or placebo*) or TS=(trial* SAME (controlled or clinical)) or TS=((blind* or mask*) SAME (single or double or triple or treble)) # 5 #4 AND #3
Appendix 8. Key words for other databases
Anaphylaxis
“Anaphylactic reaction”
“Anaphylactic shock”
“Anaphylactoid reaction”
Epinephrine
Adrenaline
EpiPen
Anapen
Twinject
Minijet
“Adrenaline auto‐injector”
“Epinephrine autoinjector”
Appendix 9. List of pharmaceutical companies contacted
ALK‐Abello
DeyPharma
IntellijectInc
International Medication Systems (UK) Ltd
Lincoln Medical Ltd
Meda Pharmaceuticals
Shionogi Inc
Appendix 10. Data extraction form
Study Selection, Quality Assessment & Data Extraction Form
| First author | Journal/Conference Proceedings etc | Year |
| |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* When authors may have taken measurements for particular outcomes, but not reported these within the paper(s), reviewers will contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study will be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study will then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below.
All references to a trial will be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers | ||
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Disease status / type, etc (if applicable) | |
| Other | |
Trial characteristics
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Low risk (Random) |
| High risk (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Low risk | |
| High risk | |
| Unclear | |
| Blinding | |
| Person responsible for participants' care | Yes / No |
| Participant | Yes / No |
| Outcome assessor | Yes / No |
| Other (please specify) | Yes / No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes No Not clear
Discuss if appropriate..................................................................................................................................
........................................................................................................................................................................
Data extraction
| Outcomes relevant to your review | |
| Reported in paper (circle) | |
| Death | Yes / No |
| Process outcomes (a. proportion carrying auto‐injector; b. proportion correctly using injector; c. proportion experiencing difficulty using auto‐injector; d. failure to use auto‐injector in a timely and appropriate manner) | Yes / No |
| Resolution of airway obstruction | Yes / No |
| Improvement in arterial blood pressure (greater than 50 mm Hg systolic in an adult) | Yes / No |
| Hospital attendance/admission | Yes / No |
| Re‐presentation for therapy within 72 hours | Yes / No |
| Cost‐effectiveness | Yes / No |
| Adverse events due to therapy, in either treatment arm, including long‐term morbidity such as neurological damage, stroke, hemiparesis and including self‐injury or injury to the person administering adrenaline through the auto‐injector | Yes / No |
| For Continuous data | ||||||||||||
| Code of paper |
Outcomes |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||||||
| A | Cost‐effectiveness | |||||||||||
| For Dichotomous data | ||||||||||||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
|||||||||
| A | Death | |||||||||||
| B | Process outcomes | |||||||||||
| C | Resolution of airway obstruction | |||||||||||
| D | Improvement in arterial blood pressure | |||||||||||
| E | Hospital attendance/admission | |||||||||||
| F | Re‐presentation for therapy within 72 hours | |||||||||||
| G | Adverse events due to therapy, in either treatment arm, including long‐term morbidity such as neurological damage, stroke, hemiparesis and including self‐injury or injury to the person administering adrenaline through the auto‐injector | |||||||||||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
| Trial characteristics | |
| Further details | |
| Single centre / multicentre | |
| Country / Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose / frequency of administration | |
| Duration of treatment (State weeks / months, etc, if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design (e.g. parallel / cross‐over*) | |
| Other | |
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data
Differences between protocol and review
The literature review has been updated. A new author has been added (VB).
Contributions of authors
Conceiving the review: AS, FERS Co‐ordinating the review: AW Undertaking manual searches: AW Screening search results: AW, VB Organizing retrieval of papers: AW Screening retrieved papers against inclusion criteria: AW, VB Appraising quality of papers: not applicable Abstracting data from papers: not applicable Writing to authors of papers for additional information: not applicable Providing additional data about papers: not applicable Obtaining and screening data on unpublished studies: AW Data management for the review: AW Entering data into Review Manager (RevMan 5.1): AW RevMan statistical data: not applicable Other statistical analysis not using RevMan: not applicable Double entry of data: not applicable Interpretation of data: not applicable Statistical inferences: not applicable Writing the review: AS, AW, FERS Securing funding for the review: not applicable Performing previous work that was the foundation of the present study: AS, FERS Guarantor for the review (one author): AS Person responsible for reading and checking review before submission: AS, FERS
Sources of support
Internal sources
No support, Other.
External sources
No sources of support supplied
Declarations of interest
Aziz Sheikh and Allison Worth: we have obtained funding from ALK‐Abello to support conference attendance by members of our research group. Aziz Sheikh has acted as a consultant to ALK‐Abello and Phadia.
F Estelle R. Simons: I have published more than 90 papers on epinephrine (adrenaline) and on anaphylaxis. This research has been funded mostly by the Manitoba Institute of Child Health, with the exception of one pharmaceutical grant totaling $23,000. I serve on Anaphylaxis Advisory Boards for ALK‐Abello, Pfizer, and Sanofi. I also serve on the US NIH National Institute of Allergy and Infectious Diseases Food Allergy Guidelines Expert Panel and on the Medical Advisory Board of the Food Allergy and Anaphylaxis Network. One of my roles on these Boards is to advocate for better treatment of anaphylaxis in community settings, including development of user‐friendly adrenaline auto‐injectors with a wider range of doses and improved safety features.
Victoria Barbour: none known.
Stable (no update expected for reasons given in 'What's new')
References
Additional references
Alrasbi 2007
- Alrasbi M, Sheikh A. Comparison of international guidelines for the emergency medical management of anaphylaxis. Allergy 2007;62:838‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arkwright 2006
- Arkwright PD, Farragher AJ. Factors determining the ability of parents to effectively administer intramuscular adrenaline to food allergic children. Pediatric Allergy and Immunology 2006;17:227‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arkwright 2009
- Arkwright PD. Automatic epinephrine device use in children with food allergies. Journal of Allergy and Clinical Immunology 2009;123:267‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ben‐Shoshan 2008
- Ben‐Shoshan M, Kagan R, Primeau MN, Alizadehfar R, Verreault N, Yu JW, et al. Availability of the epinephrine autoinjector at school in children with peanut allergy. Annals of Allergy, Asthma, and Immunology 2008;100:570‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bock 2007
- Bock SA, Munoz‐Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001‐2006. Journal of Allergy and Clinical Immunology 2007;119:1016‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 2004
- Brown SG. Clinical features and grading of anaphylaxis. Journal of Allergy and Clinical Immunology 2004;114:371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown SGA, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Medical Journal of Australia 2006;185:283‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campbell 2011
- Campbell RL, Hagan JB, Li JTC, Vukov SC, Kanthala AR, Smith VD, et al. Anaphylaxis in emergency department patients 50 or 65 years or older. Annals of Allergy, Asthma, and Immunology 2011;106:401‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Confino‐Cohen 2010
- Confino‐Cohen R, Goldberg A. Allergen immunotherapy‐induced biphasic systemic reactions: incidence, characteristics, and outcome: a prospective study. Annals of Allergy, Asthma, and Immunology 2010;104:73‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Decker 2008
- Decker WW, Campbell RL, Manivannan V, Luke A, Sauver JL, Weaver A, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. Journal of Allergy and Clinical Immunology 2008;122:1161‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2011
- Edwards E, Gunn R, Simons FER, Carr K, Chinchilli VM, Painter G, et al. Epinephrine 0.3 mg bioavailability following a single injection with a novel epinephrine auto‐injector, e‐cue, in healthy adults, with reference to a single injection using EpiPen 0.3 mg. Submitted for 2012 AAAAI Annual Meeting.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flokstra‐de Blok 2011
- Flokstra‐de Blok BMJ, Doriene van Ginkel C, Roerdink EM, Kroeze MAJM, Stel AA, Meulen GN, et al. Extremely low prevalence of epinephrine autoinjectors in high‐risk food‐allergic adolescents in Dutch high schools. Pediatric Allergy and Immunology 2011;22:374‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frew 2011
- Frew AJ. What are the ‘ideal’ features of an adrenaline (epinephrine) auto‐injector in the treatment of anaphylaxis?. Allergy 2011;66:15‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gallagher 2011
- Gallagher M, Worth A, Cunningham‐Burley S, Sheikh A. Epinephrine auto‐injector use in adolescents at risk of anaphylaxis: a qualitative study in Scotland, UK. Clinical and Experimental Allergy 2011;41:869‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Greenberger 2007
- Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Annals of Allergy, Asthma, and Immunology 2007;98:252‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Greenhawt 2009
- Greenhawt MJ, Singer AM, Baptist AP. Food allergy and food allergy attitudes among college students. Journal of Allergy and Clinical Immunology 2009;124:323‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guerlain 2010a
- Guerlain S, Wang L, Hugine A. Intelliject’s novel epinephrine autoinjector: sharps injury prevention validation and comparable analysis with Epipen and Twinject. Annals of Allergy, Asthma, and Immunology 2010;105:480‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerlain 2010b
- Guerlain S, Hugine A, Wang L. A comparison of 4 epinephrine autoinjector delivery systems: usability and patient preference. Annals of Allergy, Asthma, and Immunology 2010;104:172‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hellstrom 2011
- Hellstrom A, Eriksson K, Efraimsson EO, Svedmyr J, Borres MP. Assessment of self‐administered epinephrine during a training session. Acta Paediatrica 2011;100:e34–5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huang 1998
- Huang SW. A survey of EpiPen use in patients with a history of anaphylaxis. Journal of Allergy and Clinical Immunology 1998;102:525‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jarvinen 2008
- Jarvinen KM, Sicherer SH, Sampson HA, Nowak‐Wegrzyn A. Use of multiple doses of epinephrine in food‐induced anaphylaxis in children. Journal of Allergy and Clinical Immunology 2008;122:133‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jarvinen 2009
- Jarvinen KM, Amalanayagam S, Shreffler WG, Noone S, Sicherer SH, Sampson HA, et al. Epinephrine treatment is infrequent and biphasic reactions are rare in food‐induced reactions during oral food challenges in children. Journal of Allergy and Clinical Immunology 2009;124:1267‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 2008
- Kemp SF, Lockey RF, Simons FER. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008;63:1061‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Korenblat 1999
- Korenblat P, Lundie MJ, Dankner RE, Day JH. A retrospective study of epinephrine administration for anaphylaxis: how many doses are needed?. Allergy and Asthma Proceedings 1999;20:383‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2000
- Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics 2000;106:762‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lieberman 2005
- Lieberman P. Biphasic anaphylactic reactions. Annals of Allergy, Asthma, and Immunology 2005;95:217‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lieberman 2006
- Lieberman P, Camargo CA Jr, Bohlke K, Jick H, Miller RL, Sheikh A, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Annals of Allergy, Asthma, and Immunology 2006;97:596‐602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lieberman 2010
- Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. Journal of Allergy and Clinical Immunology 2010;126:477‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 2008
- Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990‐2006. Annals of Allergy, Asthma, and Immunology 2008;101:387‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manivannan 2009
- Manivannan V, Campbell RL, Bellolio F, Stead LG, Li JTC, Decker WW. Factors associated with repeated use of epinephrine for the treatment of anaphylaxis. Annals of Allergy, Asthma, and Immunology 2009;103:395‐400. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehr 2007
- Mehr S, Robinson M, Tang M. Doctor‐‐how do I use my EpiPen?. Pediatric Allergy and Immunology 2007;18:448‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehr 2009
- Mehr S, Liew WK, Tey D, Tang MLK. Clinical predictors for biphasic reactions in children presenting with anaphylaxis. Clinical and Experimental Allergy 2009;39:1390‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oren 2007
- Oren E, Banerji A, Clark S, Camargo CA. Food‐induced anaphylaxis and repeated epinephrine treatments. Annals of Allergy Asthma and Immunology 2007;99:429‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pumphrey 2000
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clinical and Experimental Allergy 2000;30:1144‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pumphrey 2007
- Pumphrey RSH, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999‐2006. Journal of Allergy and Clinical Immunology 2007;119:1018‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rawas‐Qalaji 2006
- Rawas‐Qalaji MM, Simons FER, Simons KJ. Sublingual epinephrine tablets versus intramuscular injection of epinephrine: Dose equivalence for potential treatment of anaphylaxis. Journal of Allergy and Clinical Immunology 2006;117:398‐403. [DOI] [PubMed] [Google Scholar]
Rawas‐Qalaji 2009
- Rawas‐Qalaji M, Simons FER, Simons KJ, Collins D. Long‐term stability of epinephrine dispensed in pre‐filled syringes for the first‐aid treatment of anaphylaxis. Annals of Allergy, Asthma, and Immunology 2009;102:500‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1
- The Nordic Cochrane Centre. The Cochrane Collaboration. Copenhagen: The Cochrane Collaboration, 2011. [Google Scholar]
Rudders 2010a
- Rudders SA, Banerji A, Corel B, Clark S, Camargo CA Jr. Multicenter study of repeat epinephrine treatments for food‐related anaphylaxis. Pediatrics 2010;125:e711‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudders 2010b
- Rudders SA, Banerji A, Katzman DP, Clark S, Camargo CA Jr. Multiple epinephrine doses for stinging insect hypersensitivity reactions treated in the emergency department. Annals of Allergy, Asthma, and Immunology 2010;105:85‐93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sampson 2006
- Sampson HA, Munoz‐Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report‐‐Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Journal of Allergy and Clinical Immunology 2006;117:391‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Scranton 2009
- Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. Journal of Allergy and Clinical Immunology 2009;123:493‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sheikh 2007
- Sheikh A, Broek V, Brown SGA, Simons FER. H1‐antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy 2007;62:830‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sheikh 2008a
- Sheikh A, Shehata YA, Brown SGA, Simons FER. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006312.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheikh 2008b
- Sheikh A, Hippisley‐Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. Journal of the Royal Society of Medicine 2008;101:139‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 1998
- Simons FER, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. Journal of Allergy and Clinical Immunology 1998;101:33‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2000a
- Simons FER, Gu X, Simons KJ. Outdated EpiPen and EpiPen Jr auto‐injectors: past their prime?. Journal of Allergy and Clinical Immunology 2000;105:1025‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2000b
- Simons FER, Gu X, Johnston L, Simons KJ. Can epinephrine inhalations be substituted for epinephrine injection in children at risk for systemic anaphylaxis?. Pediatrics 2000;106:1040‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2001a
- Simons FER, Peterson S, Black CD. Epinephrine dispensing for the out‐of‐hospital treatment of anaphylaxis in infants and children: a population‐based study. Annals of Allergy, Asthma, and Immunology 2001;86:622‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2001b
- Simons FER, Chan ES, Gu X, Simons KJ. Epinephrine for the out‐of‐hospital (first aid) treatment of anaphylaxis in infants: is the ampule/syringe/needle method practical?. Journal of Allergy and Clinical Immunology 2001;108:1040‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2001c
- Simons FER, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. Journal of Allergy and Clinical Immunology 2001;108:871‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2002a
- Simons FER, Gu X, Silver NA, Simons KJ. EpiPen Jr versus EpiPen in young children weighing 15‐30 kg at risk for anaphylaxis. Journal of Allergy and Clinical Immunology 2002;109:171‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2002b
- Simons FER, Peterson S, Black CD. Epinephrine dispensing patterns for an out‐of‐hospital population: A novel approach to studying the epidemiology of anaphylaxis. Journal of Allergy and Clinical Immunology 2002;110:647‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2008a
- Simons FER, Sampson HA. Anaphylaxis epidemic: fact or fiction?. Journal of Allergy and Clinical Immunology 2008;122:1166‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2008b
- Simons FER. Emergency treatment of anaphylaxis. Revised UK guidelines are a concise evidence‐based resource. BMJ 2008;336:1141‐2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 2009a
- Simons FER. Anaphylaxis: recent advances in assessment and treatment. Journal of Allergy and Clinical Immunology 2009;124:625‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2009b
- Simons FER, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from auto‐injectors: a systematic review. Annals of Allergy, Asthma, and Immunology 2009;102:282‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2009c
- Simons FER, Clark S, Camargo CA. Anaphylaxis in the community: learning from the survivors. Journal of Allergy and Clinical Immunology 2009;124:301‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2009d
- Simons FER, on behalf of the World Allergy Organization. Epinephrine auto‐injectors: first‐aid treatment still out of reach for many at risk of anaphylaxis in the community. Annals of Allergy, Asthma, and Immunology 2009;102:403‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2010a
- Simons FER, Simons KJ. Epinephrine and its use in anaphylaxis: current issues. Current Opinion in Allergy and Clinical Immunology 2010;10:354‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2010b
- Simons FER, Edwards ES, Read EJ, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto‐injectors. Journal of Allergy and Clinical Immunology 2010b;125:419‐23. [DOI: 10.1016/j.jaci.2009.10.056] [DOI] [PubMed] [Google Scholar]
Simons 2011
- Simons FER, Ardusso LRF, Bilo MB, El‐Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization anaphylaxis guideline: summary. Journal of Allergy and Clinical Immunology 2011;127:587‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soar 2008
- Soar J, Pumphrey R, Cant A, Clarke S, Corbett A, Dawson P, et al. Emergency treatment of anaphylactic reactions‐guidelines for healthcare providers. Resuscitation 2008;77:157‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2005
- Song TT, Nelson MR, Chang JH, Engler RJM, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Annals of Allergy, Asthma, and Immunology 2005;94:539‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stecher 2009
- Stecher D, Bulloch B, Sales J, Schaefer C, Keahey L. Epinephrine auto‐injectors: is needle length adequate for delivery of epinephrine intramuscularly?. Pediatrics 2009;124:65‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WHO 2009
- WHO Model List of Essential Medicines 16th edition, World Health Organization, Geneva, 2009. http://www.who.int/selection_medicines/committees/expert/17/sixteenth_adult_list_en.pdf (Accessed 15 November 2010).
Worth 2010
- Worth A, Soar J, Sheikh A. Management of anaphylaxis in the emergency setting. Expert Review of Clinical Immunology 2010;6:89‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sheikh 2011
- Sheikh A, Simons FER, Worth A. Adrenaline auto‐injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008935] [DOI] [PMC free article] [PubMed] [Google Scholar]


