Abstract
Background
The tubing (administration set) attached to both venous and arterial catheters may contribute to bacteraemia and other infections. The rate of infection may be increased or decreased by routine replacement of administration sets. This review was originally published in 2005 and was updated in 2012.
Objectives
The objective of this review was to identify any relationship between the frequency with which administration sets are replaced and rates of microbial colonization, infection and death.
Search methods
We searched The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 6), MEDLINE (1950 to June 2012), CINAHL (1982 to June 2012), EMBASE (1980 to June 2012), reference lists of identified trials and bibliographies of published reviews. The original search was performed in February 2004. We also contacted researchers in the field. We applied no language restriction.
Selection criteria
We included all randomized or controlled clinical trials on the frequency of venous or arterial catheter administration set replacement in hospitalized participants.
Data collection and analysis
Two review authors assessed all potentially relevant studies. We resolved disagreements between the two review authors by discussion with a third review author. We collected data for seven outcomes: catheter‐related infection; infusate‐related infection; infusate microbial colonization; catheter microbial colonization; all‐cause bloodstream infection; mortality; and cost. We pooled results from studies that compared different frequencies of administration set replacement, for instance, we pooled studies that compared replacement ≥ every 96 hours versus every 72 hours with studies that compared replacement ≥ every 48 hours versus every 24 hours.
Main results
We identified 26 studies for this updated review, 10 of which we excluded; six did not fulfil the inclusion criteria and four did not report usable data. We extracted data from the remaining 18 references (16 studies) with 5001 participants: study designs included neonate and adult populations, arterial and venous administration sets, parenteral nutrition, lipid emulsions and crystalloid infusions. Most studies were at moderate to high risk of bias or did not adequately describe the methods that they used to minimize bias. All included trials were unable to blind personnel because of the nature of the intervention.
No evidence was found for differences in catheter‐related or infusate‐related bacteraemia or fungaemia with more frequent administration set replacement overall or at any time interval comparison (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.67 to 1.69; RR 0.67, 95% CI 0.27 to 1.70). Infrequent administration set replacement reduced the rate of bloodstream infection (RR 0.73, 95% CI 0.54 to 0.98). No evidence revealed differences in catheter colonization or infusate colonization with more frequent administration set replacement (RR 1.08, 95% CI 0.94 to 1.24; RR 1.15, 95% CI 0.70 to 1.86, respectively). Borderline evidence suggested that infrequent administration set replacement increased the mortality rate only within the neonatal population (RR 1.84, 95% CI 1.00 to 3.36). No evidence revealed interactions between the (lack of) effects of frequency of administration set replacement and the subgroups analysed: parenteral nutrition and/or fat emulsions versus infusates not involving parenteral nutrition or fat emulsions; adult versus neonatal participants; and arterial versus venous catheters.
Authors' conclusions
Some evidence indicates that administration sets that do not contain lipids, blood or blood products may be left in place for intervals of up to 96 hours without increasing the risk of infection. Other evidence suggests that mortality increased within the neonatal population with infrequent administration set replacement. However, much the evidence obtained was derived from studies of low to moderate quality.
Plain language summary
Optimal timing for intravascular administration set replacement
The tubing (administration set) attached to venous and arterial catheters may contribute to bloodstream infection. The rate of infection may be increased or decreased by scheduled replacement of administration sets.
The objective of this review was to identify any association between the frequency with which administration sets were replaced and rates of microbial colonization, bloodstream infection and death.
We searched databases (CENTRAL, MEDLINE, CINAHL and EMBASE) to June 2012. We identified 16 studies with 5001 participants for inclusion in this updated review. No evidence suggests that bloodstream infection was more or less likely with more frequent changes, although the quality of included trials was low to moderate. Some evidence indicates that mortality was increased in neonates receiving parenteral nutrition when administration set replacement was less frequent.
Summary of findings
Summary of findings for the main comparison. Primary analysis: less versus more frequent for intravenous administration set replacement.
| Primary analysis: less versus more frequent for intravenous administration set replacement | ||||||
| Patient or population: patients with intravenous administration set replacement Settings: all acute care settings Intervention: primary analysis: less versus more frequent | ||||||
| Outcomes | Illustrative comparative risks | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
|
More frequent AS replacement |
Less frequent AS replacement |
|||||
|
Catheter‐related BSI as defined using criteria specified by Maki 2006; Mermel 2009; and O'Grady 2002 |
33 of 932 participants (3.5%) developed a catheter‐related BSI | 35 of 862 participants (4.1%) developed a catheter‐related BSI |
RR 1.06 (0.66 to 1.68) |
1794 (8 studies) |
⊕⊕⊝⊝ lowa,b | |
|
Infusate‐related BSI as defined using criteria specified by O'Grady 2002 |
9 of 945 participants (0.95%) developed an infusate‐related BSI | 11 of 902 participants (1.2%) developed an infusate‐related BSI | RR 0.69 (0.31 to 1.51) | 1847 (11 studies) | ⊕⊕⊝⊝ lowa,b | |
|
Infusate colonization any positive quantitative culture of infusate |
27 infusates, of a total of 808 (3.3%), were colonized | 29 infusates, of a total of 741 (3.9%), were colonized | RR 1.15 (0.7 to 1.86) | 1549 (8 studies) | ⊕⊕⊝⊝ lowa,b | |
|
Catheter colonization any positive semiquantitative or quantitative culture from the distal catheter segment |
240 catheters, of a total of 717 (33.4%), were colonized | 266 catheters, of a total of 731 (36.4%), were colonized | RR 1.08 (0.94 to 1.24) | 1448 (4 studies) | ⊕⊕⊕⊝ moderatea | |
|
All‐cause BSI any positive blood culture drawn from a peripheral vein taken whilst the IVD is in situ, or within 48 hours of removal (O'Grady 2002) |
82 of 1135 participants (7.2%) developed a BSI from any cause | 69 of 1162 participants (5.9%) developed a BSI from any cause | RR 0.82 (0.48 to 1.4) | 2297 (6 studies) | ⊕⊕⊝⊝ lowa,b | |
| Mortality | 11 of 303 neonatal ICU participants died (3.6%) during their admission to the hospital | 77 of 1052 neonatal ICU participants died (7.3%) during their admission to the hospital | RR 1.85 (1.01 to 3.38) | 1355 (2 studies) | ⊕⊕⊝⊝ lowa,c | |
| CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe proportion of information from studies at high or unclear risk of bias is sufficient to affect the interpretation of results. bBecause of the low rate of events and the wide confidence intervals of all studies. cStudies included were undertaken in a specific subgroup; not able to generalize results outside of this subgroup.
Background
Description of the condition
Intravascular catheters are plastic tubes inserted into a vein for the purpose of monitoring pressure, sampling blood or delivering fluid, nutrition and medication, or into an artery for the purpose of monitoring pressure or flow. Catheters are usually connected to an administration set that is an assembly of some or all of the following: tubing, fluid containers, pressure monitoring transducers, blood sampling ports, measuring burettes and extension tubing. Intravascular catheters break the skin and are the single most important cause of healthcare‐acquired bloodstream infection. About 250,000 to 500,000 catheter‐related infections occur each year in the United States alone, and 5000 cases are reported in Australia (Collignon 1994; Maki 2006). Bacteraemias and fungaemias are associated with increased mortality and substantially increased hospital stay by up to 20 days and costs by US$56,000 per episode (Al‐Rawajfah 2012; Maki 2006; Renaud 2001; Soufir 1999).
The type of catheter, infusate and patient may affect the rate and incidence of bloodstream infection. A systematic review of observational studies by Maki 2006 reported that rates and incidences of catheter‐related infection were lowest with peripheral intravenous catheters (0.1%, 0.5 per 1000 catheter‐days) and midline catheters (0.4%, 0.2 per 1000 catheter‐days), and that higher rates were seen with short‐term uncuffed and central venous catheters that do not have antimicrobial impregnation (4.4%, 2.7 per 1000 catheter‐days). Arterial catheters used for haemodynamic monitoring (0.8%, 1.7 per 1000 catheter‐days) and peripherally inserted central catheters (2.4%, 2.1 per 1000 catheter‐days) posed similar risks for those with short‐term conventional central catheters. Medicated, cuffed and tunnelled dual‐lumen central catheters were associated with considerably lower rates of catheter‐related infection.
Description of the intervention
Clinicians traditionally schedule administration set replacement every three or four days. Each replacement costs nursing time and up to US$275 for equipment (Rickard 2001). The frequency of set replacement has gradually fallen since 1971, as supporting research has been published.
How the intervention might work
Scheduled replacement of administration sets, contaminated through clinical use, may prevent patient infection. Catheter‐related bacteraemias (bacterial infections of the blood) are thought to stem from one of four sources: skin bacteria colonizing the catheter on or after insertion; administration fluid contaminated before connection to the patient; colonization of the hub connecting the catheter to the administration set; or bacteria contaminating the catheter (O'Grady 2011). Bacteraemias within seven days of catheter insertion probably arise from the patient's skin, after which time bacteraemias from hub contamination become more common (Crump 2000). Contaminated fluid and seeding via the blood are thought to be rare. Bacteria and fungi require time to reproduce and spread (Crump 2000), thus more frequent scheduled administration set replacement may prevent infection. However, the contact of clinicians during the scheduled administration set replacement provides an opportunity for contamination, supporting a counter‐argument for infrequent changes (O'Malley 1994).
The rate of infusate microbial colonization and the subsequent rate of bacteraemia or fungaemia (fungal infection of the blood) may be higher if the infusate supports microbial proliferation. Parenteral nutrition, with its high glucose content, has been associated with higher rates of catheter‐related infection in retrospective and prospective cohort studies (Ishizuka 2008; Moro 1994; Mulloy 1991; Perlman 2007). The schedule for set replacement that minimizes infection might be different for lipid emulsion infusates (e.g. propofol, vitalipid), as they are particularly well suited to the growth of a wide range of micro‐organisms (Gilbert 1986; Scott 1985; Sherertz 1992; Shiro 1995).
Why it is important to do this review
Intravascular catheters are associated with infection, which in turn may cause death (Renaud 2001; Soufir 1999) and significant cost (Al‐Rawajfah 2012). Less frequent administration set replacement saves nursing time and money and may not affect the rates of infection and death, or it might even reduce them. The Centers for Disease Control and Prevention (CDC) currently recommends that sets used to administer fluids (other than lipid, blood or blood products) should be routinely changed no more often than every 96 hours (O'Grady 2011).
An earlier version of this systematic review-'Timing of intravenous administration set changes: a systematic review'-was published in Infection Control and Hospital Epidemiology (Gillies 2004) and in the Cochrane Database of Systematic Reviews 2005, Issue 4 (Gillies 2005). This current update was done to evaluate more recent studies. The aim of this updated systematic review was to ascertain the optimal interval for replacement of administration sets, that is, the frequency with the lowest rate of infection.
Objectives
The objective of this review was to identify any relationship between the frequency with which administration sets are replaced and rates of microbial colonization, infection and death.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCT) and controlled clinical trials (CCTs) that allocated the frequency of administration set replacement. CCTs refer to studies in which the method of allocation is not considered strictly random (Lefebvre 2011).
Types of participants
We included hospitalized participants of any age who had a central or peripheral venous or arterial catheter in situ.
Types of interventions
We included studies that compared the frequency of intravascular administration set replacement.
Types of outcome measures
Primary outcomes
The rate of catheter‐related bloodstream infection, as defined by one of three criteria: bacteraemia or fungaemia with at least one positive blood culture from a peripheral vein, with no other identifiable source of infection other than the catheter, plus a positive semiquantitative (> 15 colony‐forming units (cfu)) or quantitative (>1 03 cfu) device culture, with the same organism (species and antibiogram) isolated from the device and blood (Maki 2006; O'Grady 2002); or two blood cultures (one drawn from the catheter and one from a peripheral vein) that grow the same organism, with the catheter colony count three‐fold the peripheral colony count, or with the catheter culture growing at least 2 hours before the peripheral culture; or two quantitative blood cultures drawn from two catheter lumens in which the colony counts differ three‐fold (Mermel 2009).
The rate of infusate‐related bloodstream infection: isolation of the same organism from a quantitative culture of the infusate and from separate percutaneous blood cultures, with no other identifiable source of infection (O'Grady 2002).
Secondary outcomes
Catheter‐related bloodstream infection per 1000 patient‐days: as previously defined but restricted to the first catheter per participant.
Infusate‐related bloodstream infection per 1000 patient‐days: as previously defined but restricted to the first catheter per participant.
Infusate colonization: any positive quantitative culture of infusate.
Catheter colonization: any positive semiquantitative or quantitative culture from the catheter tip (e.g. distal catheter segment).
All‐cause bloodstream infection (bacteraemia or fungaemia): any positive blood culture drawn from a peripheral vein taken with the catheter in situ or within 48 hours of removal (O'Grady 2002).
Mortality.
Cost.
Definitions
Administration set: tubing from the spike entering the fluid container to the hub of the catheter (Pearson 1996).
Central catheter: includes tunnelled and non‐tunnelled central venous catheters, central arterial catheters (pulmonary arterial and left atrial catheters), peripherally inserted central venous catheters and implanted subcutaneous intravascular devices (O'Grady 2011).
Parenteral nutrition: the infusion of basic nutrients through the venous system, with or without the infusion of lipids.
Peripheral catheter: a short catheter inserted into the veins or arteries (O'Grady 2011).
Search methods for identification of studies
Electronic searches
We searched the following.
The Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library, Issue 6, 2012) (Appendix 1).
MEDLINE (via Ovid) (1950 to June 2012) (Appendix 2).
EMBASE (via Ovid) (1980 to June 2012) (Appendix 3).
CINAHL (EBSCO host) (1982 to June 2012) (Appendix 4).
We increased the specificity of the topic search by combining it with the Cochrane Randomized Controlled Trial Search Strategy (Higgins 2011). No restrictions were applied for language, date of publication or study setting.
Searching other resources
We manually checked the reference lists of relevant studies and published reviews to identify trials missed by the electronic search strategy. We used the names of researchers known to have published on the topic as search terms. We contacted primary authors of identified trials to ask for more information, if required. We searched for ongoing trials at http://www.controlled‐trials.com; http://www.clinicaltrials.gov.
Data collection and analysis
Selection of studies
Two review authors (CMR, MLC) assessed all potentially relevant references for inclusion in the review. The PRISMA flow chart (Liberati 2009) illustrates the results of our search and the studies we selected (see Figure 1). We eliminated irrelevant studies. We resolved any disagreements regarding the selection of studies through consensus or, if necessary, by consultation with a third member of the review team (AD).
1.
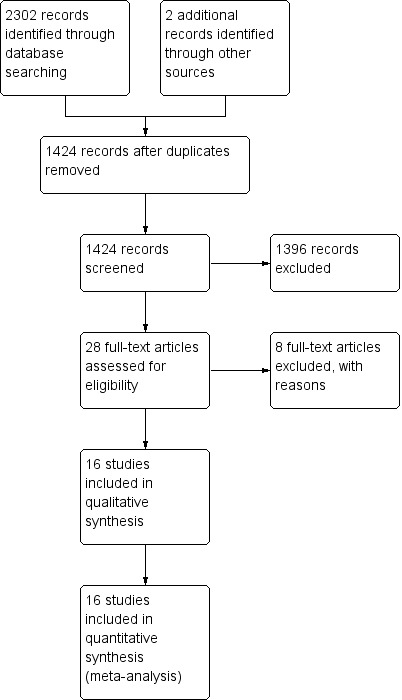
PRISMA study flow diagram (Liberati 2009).
Data extraction and management
For this updated review, we developed and piloted a data extraction form, using the template provided by the Cochrane Anaesthesia Review Group (CARG). Two members of the 2012 review team (CMR, MLC) used the data extraction form to independently extract methodological and outcome data from each newly identified study (Covey 1988; Luskin 1986; McLane 1998). As only newly identified studies required data extraction, CMR did not extract data for the study that she coauthored (Rickard 2004). The pair then met to compare results. Differences were resolved by consensus or by referral to a third member of the review team.
Assessment of risk of bias in included studies
Two review authors (CMR, MLC, or AD) independently used the Cochrane Collaboration tool to assess risk of bias (Higgins 2011) in all included studies. This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues that may potentially bias the study. Blinding and completeness of outcome data were assessed for each outcome separately. CMR did not assess for risk of bias the study that she coauthored (Rickard 2004). A risk of bias table was completed for each eligible study. Disagreements between review authors were resolved by consensus or by referral to a third review author. We attempted to contact investigators of included trials to resolve ambiguities. Assessment of risk of bias is discussed within the text and is presented as a 'Risk of bias summary figure' (see Figure 2), which cross‐tabulates judgements by study, and a 'Risk of bias summary graph' (see Figure 3), which summarizes judgements by domain.
2.
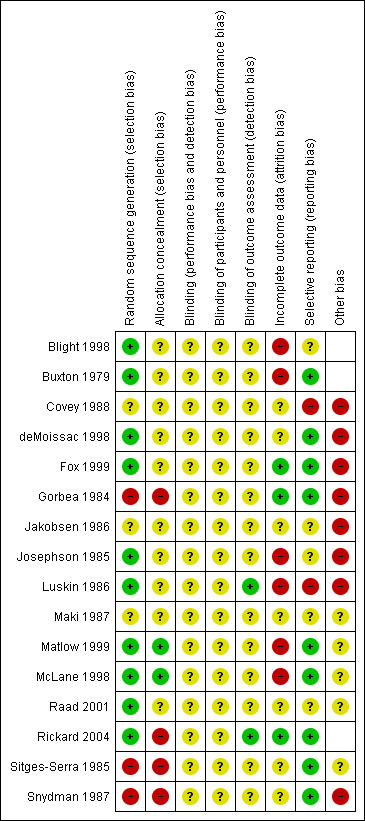
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
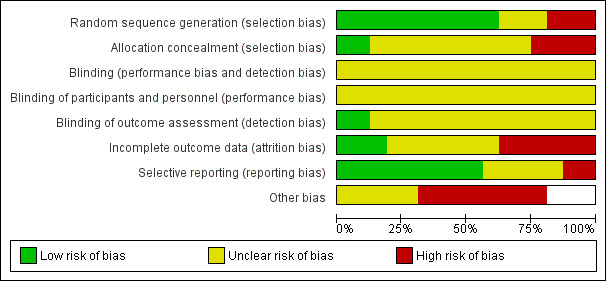
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We entered outcome data into RevMan 5.1 to generate meta‐analytical data and graphs. To generate clinically informative comparisons, we pragmatically divided time periods of administration set change into three different frequencies of administration set replacement (24 hours vs ≥ 48 hours; 48 hours vs ≥ 72 hours; 72 hours vs ≥ 96 hours). Event rates for binary outcomes (e.g. infection rates) were presented as risk ratios (RRs) and 95% confidence intervals (CIs). For continuous outcomes, we calculated differences in means with 95% CIs. For outcomes displaying incidence over a specified time period, we planned on calculating hazard ratios (HRs) with standard errors (SEs). We calculated pooled estimates for each outcome using a random‐effects model, chosen because of the substantial clinical heterogeneity noted between trials.
Unit of analysis issues
Three studies reported outcomes per administration set rather than per participant (deMoissac 1998; Maki 1987; Snydman 1987). One study (Fox 1999) reported outcomes per infusate sample. In future updates of this review, those studies that used administration sets as the unit of analysis will be included in a sensitivity analysis.
Dealing with missing data
All authors of included studies were emailed to ask for further information and clarification of key aspects of their study methods. Ten contact authors for fifteen of the studies responded (Blight 1998; Buxton 1979; Covey 1988; deMoissac 1998; Fox 1999; Gorbea 1984; Luskin 1986; McLane 1998; Rickard 2004; Sitges‐Serra 1985). One author was able to provide some information regarding study methods but was unable to provide further individual participant data (Blight 1998). Only one author (Rickard 2004) provided further information on both study methods and individual participant data. The remaining authors were unable to provide any study data (Blight 1998; Buxton 1979; Covey 1988; deMoissac 1998; Fox 1999; Gorbea 1984; Luskin 1986; Sitges‐Serra 1985).
Assessment of heterogeneity
We assessed evidence of statistical and clinical heterogeneity by visually inspecting the model, performing a Chi2 test and reviewing the I2 statistic (Deeks 2011). The Chi2 test examines the percentage of total variation across studies caused by heterogeneity rather than by chance. We planned to investigate heterogeneity (P < 0.10; I2 > 50%).
Assessment of reporting biases
We intended to use a funnel plot to identify small‐study effects, but studies were insufficient to allow this (Egger 1997). Any asymmetry of the funnel plot may indicate possible publication bias. We intended to explore other reasons for asymmetry, such as selection bias, methodological quality, heterogeneity, artefact or chance, as described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We performed a meta‐analysis when sufficient data were obtained from two or more studies that were similar in terms of population, intervention, comparison and outcomes.
Subgroup analysis and investigation of heterogeneity
We pre‐specified four subgroup analyses. These included the following.
Central versus peripheral catheters (catheter site).
Parenteral nutrition and/or lipid emulsions versus infusates not involving parenteral nutrition and/or lipid emulsions (type of infusate).
Neonatal (within 28 days of birth) versus adult participants (older than 16 years) age group.
Arterial versus venous catheters (vascular access).
Proof of differences between groups was obtained by using the method described in Deeks 2011 via RevMan 5.1 and by using fixed‐effect analyses based on the inverse‐variance method to determine risk ratios and associated 95% confidence intervals. Meta‐regression was not used as fewer than 10 studies were included in the meta‐analysis (Deeks 2011).
Sensitivity analysis
We intended to include in a sensitivity analysis studies that did not describe the method of randomization and quasi‐randomized studies (e.g. participants allocated by date of birth). If no substantive differences were noted within the primary outcomes, we intended to include these studies in the final analysis. If a substantive difference was identified, we planned to include only studies that clearly described methods of randomization. Results of the sensitivity analysis would be described within the text. We did not undertake a sensitivity analysis because insufficient studies reported primary outcome events.
Results
Description of studies
Results of the search
We identified 2087 references: we included 16 and excluded 10 studies. Figure 1 contains the PRISMA study flow diagram (Liberati 2009).
Included studies
Sixteen studies with 5001 participants met our 2012 inclusion criteria (see also 'Characteristics of included studies'): Blight 1998; Buxton 1979; Covey 1988; deMoissac 1998; Fox 1999; Gorbea 1984; Jakobsen 1986; Josephson 1985; Luskin 1986; Maki 1987; Matlow 1999; McLane 1998; Raad 2001; Rickard 2004; Sitges‐Serra 1985; Snydman 1987. We found an additional three studies from the previous version of the review because we broadened the inclusion criteria to include administration sets connected to peripheral arterial catheters (Covey 1988; Luskin 1986; McLane 1998).
We identified two additional references as duplicate publications of Maki 1987 and Raad 2001.
We collected data for seven outcomes and for three different frequencies of administration set replacement. We included five studies that reported data from participants with central catheters (Blight 1998; deMoissac 1998; Raad 2001; Rickard 2004; Sitges‐Serra 1985) and three that reported data from participants with peripheral catheters (Buxton 1979; Jakobsen 1986; Josephson 1985). In our 2012 update, we added three studies that reported data from participants with arterial lines (Covey 1988; Luskin 1986; McLane 1998).
We included data from three studies in which participants received parenteral nutrition (Fox 1999; Matlow 1999; Sitges‐Serra 1985). We excluded the data from the Raad study (Raad 2001), which combined data for participants receiving parenteral nutrition with data for participants receiving blood: The study authors could not provide separate data for participants receiving parenteral nutrition. Participants in three studies did not receive parenteral nutrition (Buxton 1979; Josephson 1985; Snydman 1987). Rickard 2004 reported separate data for participants who did and did not receive parenteral nutrition. No study singularly examined the use of different types of lipid emulsions such as vitalipid or propofol given separately.
Of the 16 included studies, four stipulated that participants had to be adults (Gorbea 1984; McLane 1998; Sitges‐Serra 1985; Snydman 1987), and two reported data for neonates only (Fox 1999; Matlow 1999). The other studies may have included neonates but did not provide separate data for neonates.
Excluded studies
We excluded one study because it did not address the review question (Robertson 1991). We excluded four studies because the participants were not randomly or systematically allocated (Band 1979; Cohen 1989; O'Malley 1994; Robathan 1995). We excluded four studies because of inadequate data (Alothman 1996; Chen 2000; Franceschi 1989; Trautmann 1997). Attempts were made to contact these authors for further information, but none could be obtained. In agreement with the previous version of this review, we excluded one further study because it defined catheter colonization as fever and positive tip culture, and catheter‐related bloodstream infection as fever and positive (but heterogeneous) blood and catheter cultures (Powell 1985). Please see the 'Characteristics of excluded studies' table for more information.
Risk of bias in included studies
Figure 2 and Figure 3 present overall risk of bias. The characteristics of individual studies are summarized in the 'Characteristics of included studies' table.
Allocation
Sequence generation
Of the 16 included studies, 10 described an adequate method of sequence generation (Blight 1998; Buxton 1979; deMoissac 1998; Fox 1999; Josephson 1985; Luskin 1986; Matlow 1999; McLane 1998; Raad 2001; Rickard 2004).
Allocation concealment
An adequate method of allocation concealment was reported in only two studies (Matlow 1999; McLane 1998).
Blinding
Blinding of personnel and participants
No study blinded personnel or participants.
Blinding of outcome assessor
Two studies blinded the outcome assessor (Luskin 1986; Rickard 2004): Both blinded laboratory or microbiology personnel, and a medical rater was also blinded in Rickard 2004.
Incomplete outcome data
Three studies reported complete outcome data (Fox 1999; Gorbea 1984; Rickard 2004). Seven studies (Covey 1988; deMoissac 1998; Jakobsen 1986; Maki 1987; Raad 2001; Sitges‐Serra 1985; Snydman 1987) provided incomplete outcome data.
Selective reporting
All of the included studies provided information on all of the outcomes that were pre‐specified in the paper (protocols were not available for any of the studies). Neither of our primary outcome measures was reported by all studies.
Other potential sources of bias
None of the studies was supported by manufacturer sponsorship. Seven studies described systematic differences between intervention and control groups other than the intervention (Covey 1988; deMoissac 1998; Gorbea 1984; Luskin 1986; McLane 1998; Sitges‐Serra 1985; Snydman 1987). Unit of analysis problems in four studies were reported per administration set rather than per participant (deMoissac 1998; Fox 1999; Luskin 1986; Maki 1987). A questionable randomization technique was used by Matlow 1999, in that the study randomly assigned approximately 10% of neonates a second time if a gap in administration set usage was noted. The outcomes reported by Matlow 1999 were analysed according to the first allocated intervention; however, interpretation of results is confounded by allocation to subsequent interventions.
Effects of interventions
See: Table 1
Overall Findings
The main results are displayed in 'Table 1'.
1.1 Catheter‐related bloodstream infection
No evidence showed an effect of the frequency of administration set replacement on catheter‐related bloodstream infection (RR 1.06, 95%CI 0.67 to 1.69; Analysis 1.1). Only eight studies reported this outcome, and the rate of catheter‐related infection was zero in five of these.
1.1. Analysis.
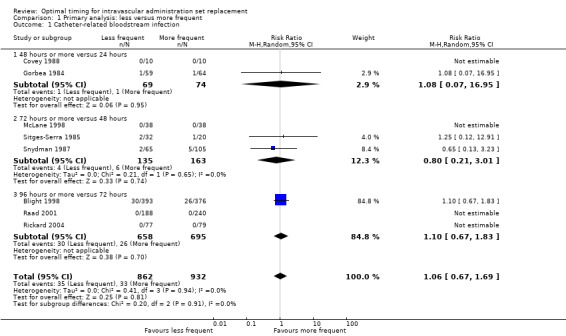
Comparison 1 Primary analysis: less versus more frequent, Outcome 1 Catheter‐related bloodstream infection.
1.2 Infusate‐related bloodstream infection
No evidence revealed an effect of the frequency of administration set replacement on infusate‐related bloodstream infection (RR 0.67, 95% CI 0.27 to 1.70; Analysis 1.2).
1.2. Analysis.
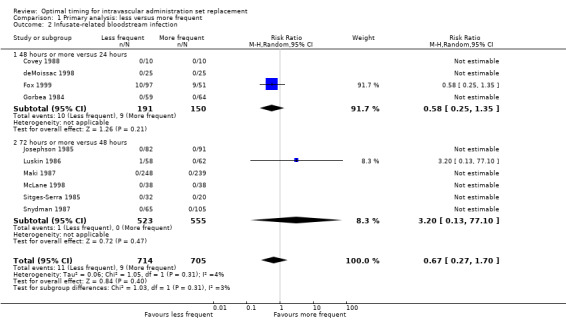
Comparison 1 Primary analysis: less versus more frequent, Outcome 2 Infusate‐related bloodstream infection.
Catheter‐related and infusate‐related bloodstream infections per 1000 days
We contacted all authors for data on this outcome. Only one study (Rickard 2004) provided data: The incidence of catheter‐related bloodstream infection was zero in both groups.
1.3 Catheter colonization
No evidence suggested an effect of the frequency of administration set replacement on infusate colonization (RR 1.08, 95% CI 0.94 to 1.24;Analysis 1.3).
1.3. Analysis.
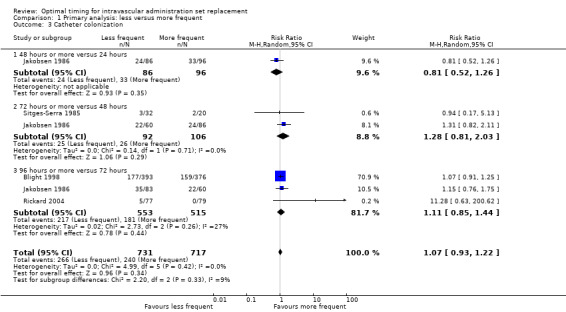
Comparison 1 Primary analysis: less versus more frequent, Outcome 3 Catheter colonization.
1.4 Infusate colonization
No evidence indicated an effect of the frequency of administration set replacement on infusate colonization (RR 1.15, 95% CI 0.70 to 1.86; Analysis 1.4).
1.4. Analysis.
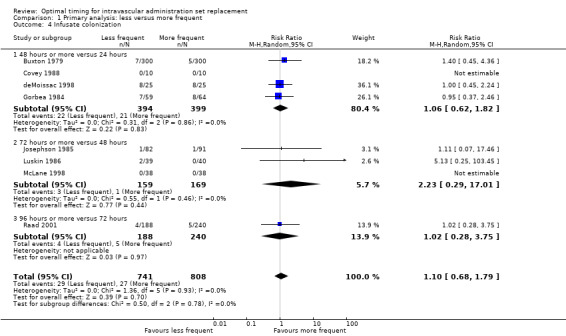
Comparison 1 Primary analysis: less versus more frequent, Outcome 4 Infusate colonization.
1.5 All‐cause bloodstream infection
Some evidence showed that less frequent replacement of administration sets reduced the rate of bloodstream infection from any cause (RR 0.72, 95% CI 0.54 to 0.98; Analysis 1.5). The point estimates of effect were less than one for all subcomparisons (24 hours vs ≥ 48 hours; 48 hours vs ≥ 72 hours; 72 hours vs ≥ 96 hours; I2 = 0%). The reduction in infection in one study, published in abstract form, was statistically significant (Blight 1998).
1.5. Analysis.
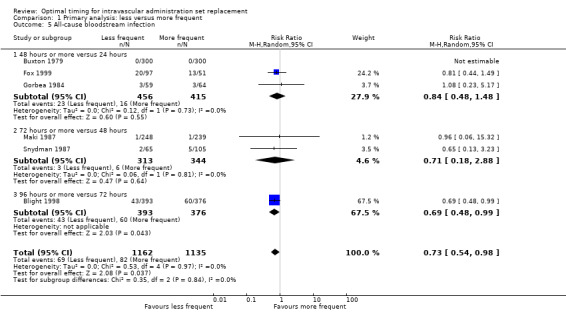
Comparison 1 Primary analysis: less versus more frequent, Outcome 5 All‐cause bloodstream infection.
1.6 Mortality
Some evidence indicated that less frequent replacement of administration sets increased the mortality rate within the neonatal population (RR 1.84, 95% CI 1.00 to 3.36; Analysis 1.6). Two studies of critically ill neonates given parenteral nutrition contributed to this result. One compared administration set replacement every 24 hours versus every 48 hours in 166 neonates (Fox 1999), and the other compared replacement every 24 hours versus every 72 hours in 1189 neonates (Matlow 1999). The composite result was dominated by Matlow 1999 (96% statistical weight). The risk of bias for mortality was not high, unlike the risk of bias for microbial sampling, which was undermined by a clinically significant imbalance in the weight of sampled neonates in the two groups.
1.6. Analysis.
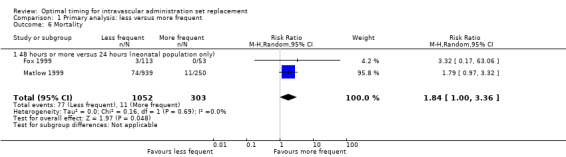
Comparison 1 Primary analysis: less versus more frequent, Outcome 6 Mortality.
Costs
We were unable to perform an economic analysis because of lack of data.
Subgroup Analyses
Catheter site
No data were available on any outcome from studies that recruited only participants with peripheral catheters.
2.1 Type of infusate
The only outcome that we could analyse for this subgroup was catheter‐related bloodstream infection, for which no evidence revealed an interaction between type of infusate and outcome (RR 0.8, 95% CI 0.21 to 3.01, P = 0.65; Analysis 2.1).
2.1. Analysis.
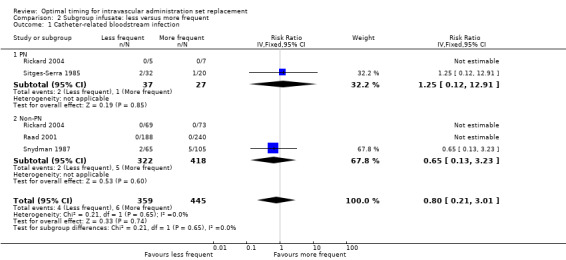
Comparison 2 Subgroup infusate: less versus more frequent, Outcome 1 Catheter‐related bloodstream infection.
3.1 Age group
The only outcome that we could analyse for this subgroup was infusate‐related bloodstream infection, for which no evidence showed an interaction between age group and outcome (RR 0.65, 95% CI 0.29 to 1.46, P = 0.30; Analysis 3.1).
3.1. Analysis.
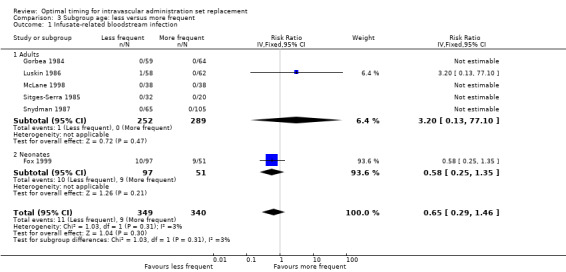
Comparison 3 Subgroup age: less versus more frequent, Outcome 1 Infusate‐related bloodstream infection.
4.1 Vascular access
The only outcome that we could analyse for this subgroup was infusate‐related bloodstream infection, for which no evidence suggested an interaction between vascular access and outcome (RR 0.65, 95% CI 0.29 to 1.46, P = 0.31; Analysis 4.1). This result was identical to that seen in the age subgroup.
4.1. Analysis.
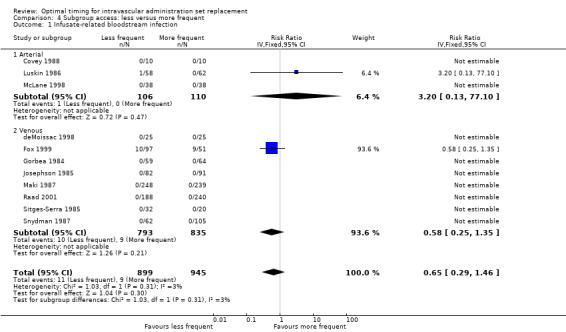
Comparison 4 Subgroup access: less versus more frequent, Outcome 1 Infusate‐related bloodstream infection.
Outcomes were statistically homogeneous, with I2 < 10%. We did not investigate statistical heterogeneity, as studies were insufficient.
Discussion
Summary of main results
Decreasing the frequency of administration set changes from 24 hours to intervals of ≥ 48 hours, from 48 hours to ≥ 72 hours, or from 72 hours to ≥ 96 hours did not appear to increase the incidence of catheter‐related bacteraemia or catheter colonization (see Table 1). It should be noted however, that power might not have been adequate to identify a clinically significant difference in the incidence of catheter‐related bloodstream infection in the 24 to ≥ 48 hour and 48 to ≥ 72 hour comparisons. The incidence of catheter‐related bacteraemia was 2% in the control group for the 24 to ≥ 48 hour comparison and 5% for the 48 to ≥ 72 hour and 72 to ≥ 96 hour comparisons. For a power of 0.8, 435 participants per group would be required to show an absolute difference of 5% in the incidence of catheter‐related bloodstream infection compared with the control group. The total number of participants was 143 for the 24 to ≥ 48 hour comparison, 298 for the 48 to ≥ 72 hour comparison and 1353 for the 72 to ≥ 96 hour comparison. Therefore power was probably adequate only for detecting a difference of 5% or more in the 72 to ≥ 96 hour comparison.
Although infusate colonization was identified in all cases where it was recorded as an outcome, only one study (Fox 1999), which reported data from neonates receiving parenteral nutrition in a neonatal intensive care unit, identified any cases of infusate‐related bacteraemia. Therefore the infusate colonization identified in most of these studies did not seem to result in bacteraemia.
It is difficult to draw conclusions regarding the risk of infection when administration sets contain parenteral nutrition and/or lipid emulsions. Although subgroup analyses of participants who received parenteral nutrition and/or lipid emulsions did not appear to differ from those of participants who were not given parenteral nutrition and/or lipid emulsions, or from the overall findings, few studies reported the incidence of bacteraemia in participants receiving parenteral nutrition or lipid emulsions (Fox 1999; Rickard 2004; Sitges‐Serra 1985). In addition, the number of participants in these studies was small, with the largest study reporting data for 148 participants (Fox 1999).
Evidence in relation to administration sets that contain fat emulsions is even weaker than for administration sets that contain parenteral nutrition. Apart from studies by Fox 1999, Matlow 1999 and Rickard 2004, which did include lipid emulsions, it was not clear whether the other studies included lipid emulsions in the parenteral nutrition administered to participants. It is notable that mortality risk was significantly increased when data from these two studies were pooled. However, at this stage, no evidence suggests that it is safe to extend the period of changing administration sets that contain lipids beyond an interval of 24 hours, which is generally accepted as best practice.
Although death was cited as a reason for a participant to be lost to follow‐up in two of the included studies (Blight 1998; Gorbea 1984), the death rate per group was reported in only two studies (Fox 1999; Matlow 1999). Some evidence suggests that less frequent replacement of administration sets increased mortality rate within the critically ill neonate population. When the outcome of mortality was examined, the composite result was dominated by Matlow 1999 (96% statistical weight), who used randomization techniques that may have been prone to bias. Investigators randomly assigned approximately 10% of neonates a second time, if a gap in administration set usage was noted. Mortality outcomes reported by Matlow 1999 were the result of the first random assignment; however, interpretation of results is confounded by allocation to subsequent interventions. The possibility that mortality might be increased with infrequent replacements, despite less bloodstream infection, should be of interest to researchers, clinicians and patients; however, current research has been conducted only within the neonatal population. Researchers would need to control for increased frequency of attendance by clinicians, which accompanies increased frequency of administration set replacement.
Overall completeness and applicability of evidence
Most of the studies included in this updated systematic review addressed either or both of the review's most important outcomes: catheter‐related and infusate‐related bacteraemia. However, some other outcomes, including mortality and costs, were poorly reported.
Of the 16 included studies, nine were conducted in an intensive care unit (Blight 1998; Covey 1988; Fox 1999; Gorbea 1984; Luskin 1986; Matlow 1999; McLane 1998; Rickard 2004; Snydman 1987). Two studies specifically included participants with cancer (deMoissac 1998; Raad 2001), and five studies (Buxton 1979; Jakobsen 1986; Josephson 1985; Maki 1987; Sitges‐Serra 1985) collected data from participants in a variety of settings. A particularly high rate of catheter‐related bacteraemia was reported in the study by Blight 1998 and of infusate‐related bacteraemia in the study by Fox 1999. As both of these studies were performed in intensive care units, it is possible that the high rate of infection was result of the severity of illness in these participants. However, findings in these studies were the same as in the overall analysis. The mortality meta‐analysis included only studies within the neonatal population.
Quality of the evidence
Risk of bias was difficult to assess in most of the studies because of poor reporting. It was not possible to blind personnel to the duration of administration sets, and this is a potential source of bias. However, blinding of outcome assessors for the primary outcomes was feasible but was adequately achieved and reported by only two of the studies (Luskin 1986; Rickard 2004). Allocation concealment was adequately achieved and reported by only two studies (Matlow 1999; McLane 1998). None of the trials disclosed receiving partial or full manufacturer sponsorship.
Potential biases in the review process
Clearly described procedures were followed to prevent potential bias in the review process. CMR was an author of one of the studies reviewed (Rickard 2004) but did not partake in any critique or data extraction for that study. A careful literature search was conducted, and the methods used are transparent and reproducible. None of the review authors has reported any conflict of interest.
To provide clinically informative comparisons, time periods of administration set change were pragmatically divided into three different frequencies of administration set replacement (24 hours versus ≥ 48 hours, 48 hours vs ≥ 72 hours, 72 hours vs ≥ 96 hours). An alternative technique would have been to use time ratios as the method of division of time periods, to highlight relative risks. Because bacteria and fungi require time for reproduction and spread, use of the time ratio technique would be in line with microbiological principles. However, investigators in the studies included in this review did not follow time ratios when choosing their interventional and control groups, thereby excluding this form of comparison.
Agreements and disagreements with other studies or reviews
The previous version of this review (Gillies 2005) also cautiously advocated changing intravascular administration sets that do not contain lipid emulsions, blood or blood products at an interval of up to 96 hours, having concluded that this does not affect the risk of catheter‐related or infusate‐related bacteraemia in participants with central or peripheral catheters. We now cautiously extend this finding to peripheral arterial catheters. The CDC advocates that intravascular administration sets used to administer fluids other than lipid emulsions, blood or blood products should be routinely changed no more frequently than every 96 hours (O'Grady 2011), and our review agrees with these recommendations. No evidence suggests that administration sets containing lipid emulsions should be changed less frequently than every 24 hours, as recommended by the CDC (O'Grady 2011).
Authors' conclusions
Implications for practice.
Overall, some evidence shows that changing intravascular administration sets that do not contain lipids, blood or blood products at an interval of up to 96 hours, does not affect the risk of infusate‐related or catheter‐related bacteraemia in participants with central or peripheral, venous or arterial catheters. Infrequent set replacement reduces nursing time and costs. Some evidence shows that mortality increased within the neonatal population with infrequent administration set replacement. However, many individual studies included in the review were at risk of bias. More data are required regarding the rates and incidences of infusate‐related and catheter‐related bacteraemia in participants who receive parenteral nutrition, in particular lipid emulsions.
Implications for research.
We think that future research in this area should focus on administration sets that contain parenteral nutrition, with and without lipid emulsions, and intervals longer than 96 hours between administration set changes.
Research should also include economic and survival analyses. Researchers should report catheter‐related and infusate‐related outcomes as rates per 1000 patient‐days. This facilitates comparisons between clinical settings.
Participants must be numerous enough to allow identification of clinically important differences in outcomes, with each participant being the unit of analysis, and with data presented separately when more than one type of administration set, infusate or frequency of replacement is studied. Researchers should plan their protocols so that the risk of bias in each domain is minimized and should report clearly in accordance with the CONSORT guidelines (Schulz 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 11 September 2013 | New citation required and conclusions have changed | A new authorship team consisting of previous authors, researchers and clinicians was established. We widened the review, and therefore the conclusions, to incorporate peripheral arterial administration sets, in addition to venous and central arterial giving sets. |
| 11 September 2013 | New search has been performed | An updated search was undertaken to June 2012, which led to the inclusion of Covey 1988; Luskin 1986; and McLane 1998 and the exclusion of O'Malley 1994. Additional outcome measures were developed to incorporate modern reporting strategies. Risk of bias summaries (Higgins 2011) were completed using updated criteria; GradePRO (Schunemann 2011) was used to provide 'Summary of findings' tables, and a PRISMA flow chart (Liberati 2009) was included to summarize study selection. |
| 1 August 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group) and Karen Hovhannisyan (Trial Search Co‐ordinator, Cochrane Anaesthesia Review Group) for their assistance in preparation of this updated review. We would like to thank John Carlisle (Content Editor), Cathal Walsh (Statistical Editor) and Mazen Bader, Nai An Lai and Peter Kruger (Peer Reviewers) for their help and editorial advice during the preparation of this updated systematic review. We would also like to thank the remaining authors of the original systematic review: Margaret Wallen, Karen Rankin, Anne Morrison and Sue Nagy (Gillies 2005).
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library
#1 MeSH descriptor Parenteral Nutrition, Total explode all trees #2 MeSH descriptor Parenteral Nutrition explode all trees #3 vamin or (transducer near (set* or tub*)) #4 MeSH descriptor Infusions, Intravenous explode all trees #5 (arterial or intravenous) near (therap* or infusion* or administrat* or catheter*) #6 MeSH descriptor Catheterization explode all trees #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 (timing or time‐frame) and ((line change*) or (set replacement)) #9 (#7 AND #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Parenteral Nutrition/ or Infusions‐Intravenous/ or Catheterization/ or Catheters, Indwelling/ or (parenteral adj5 nutrition).mp. or vamin*.mp. or ((arterial or intravenous) adj3 (therap* or infusion* or administrat* or catheter*)).ti,ab. or line change*.mp. or (transducer adj3 (set* or tub*)).mp. 2. (timing or time‐frame).mp. or period*.ti. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. parenteral‐nutrition/ or intravenous‐drug‐administration/ or exp artery catheter/ or indwelling catheter/ or CATHETERIZATION/ or (parenteral adj5 nutrition).mp. or vamin*.mp. or ((arterial or intravenous) adj3 (therap* or infusion* or administrat* or catheter*)).ti,ab. or line change*.mp. or (transducer adj3 (set* or tub*)).mp. 2. (timing or time‐frame).mp. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. Search strategy for CINAHL (EBSCO host)
S1 (MM "Arterial Catheters") OR (MM "Catheter Removal") OR (MH "Catheterization") S2 (MH "Parenteral Nutrition+") or (MM "Infusions, Intravenous") S3 (parenteral and nutrition) S4 (arterial or intravenous) and (therap* or infusion* or administrat* or catheter*) S5 line chang* S6 transducer and (set* or tub*) S7 S1 or S2 or S3 or S4 or S5 or S6 S8 TX ( timing or time‐frame ) or TI period S9 S7 and S8
Data and analyses
Comparison 1. Primary analysis: less versus more frequent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related bloodstream infection | 8 | 1794 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.69] |
| 1.1 48 hours or more versus 24 hours | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.07, 16.95] |
| 1.2 72 hours or more versus 48 hours | 3 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.21, 3.01] |
| 1.3 96 hours or more versus 72 hours | 3 | 1353 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.67, 1.83] |
| 2 Infusate‐related bloodstream infection | 10 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.70] |
| 2.1 48 hours or more versus 24 hours | 4 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.25, 1.35] |
| 2.2 72 hours or more versus 48 hours | 6 | 1078 | Risk Ratio (M‐H, Random, 95% CI) | 3.20 [0.13, 77.10] |
| 3 Catheter colonization | 4 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
| 3.1 48 hours or more versus 24 hours | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.52, 1.26] |
| 3.2 72 hours or more versus 48 hours | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.81, 2.03] |
| 3.3 96 hours or more versus 72 hours | 3 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.85, 1.44] |
| 4 Infusate colonization | 8 | 1549 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.68, 1.79] |
| 4.1 48 hours or more versus 24 hours | 4 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.62, 1.82] |
| 4.2 72 hours or more versus 48 hours | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [0.29, 17.01] |
| 4.3 96 hours or more versus 72 hours | 1 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.28, 3.75] |
| 5 All‐cause bloodstream infection | 6 | 2297 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 0.98] |
| 5.1 48 hours or more versus 24 hours | 3 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.48] |
| 5.2 72 hours or more versus 48 hours | 2 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.18, 2.88] |
| 5.3 96 hours or more versus 72 hours | 1 | 769 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.99] |
| 6 Mortality | 2 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.00, 3.36] |
| 6.1 48 hours or more versus 24 hours (neonatal population only) | 2 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.00, 3.36] |
Comparison 2. Subgroup infusate: less versus more frequent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related bloodstream infection | 4 | 804 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.21, 3.01] |
| 1.1 PN | 2 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.12, 12.91] |
| 1.2 Non‐PN | 3 | 740 | Risk Ratio (IV, Fixed, 95% CI) | 0.65 [0.13, 3.23] |
Comparison 3. Subgroup age: less versus more frequent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infusate‐related bloodstream infection | 6 | 689 | Risk Ratio (IV, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
| 1.1 Adults | 5 | 541 | Risk Ratio (IV, Fixed, 95% CI) | 3.20 [0.13, 77.10] |
| 1.2 Neonates | 1 | 148 | Risk Ratio (IV, Fixed, 95% CI) | 0.58 [0.25, 1.35] |
Comparison 4. Subgroup access: less versus more frequent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infusate‐related bloodstream infection | 11 | 1844 | Risk Ratio (IV, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
| 1.1 Arterial | 3 | 216 | Risk Ratio (IV, Fixed, 95% CI) | 3.20 [0.13, 77.10] |
| 1.2 Venous | 8 | 1628 | Risk Ratio (IV, Fixed, 95% CI) | 0.58 [0.25, 1.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blight 1998.
| Methods | Randomized controlled trial | |
| Participants | 769 ICU patients with a CVC containing crystalloids, PN, lipid or drugs. ICU, Australia | |
| Interventions | Administration set changes at 72 or 120 hours | |
| Outcomes |
|
|
| Notes | Exclusions: CVCs inserted by other institutions Central catheters: 100% PN: mixed (proportion unknown) Loss to follow‐up: 36% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation method; computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not discussed Quote: "Randomised on day 3/5 by an independent person" (abstract) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Minimal information discussed, "independent microbiologist" for primary and secondary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large dropout rate resulting around 30% of tips not cultured; 437/1206 The rate of attrition was similar in both groups |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract only, never published No pre‐protocol Lumen culture performed but not reported |
Buxton 1979.
| Methods | Randomized controlled trial | |
| Participants | 600 patients who had new infusions begun within the previous 24 hours. Four general medical wards (each with an ICU) and 4 general surgical wards at a general hospital, USA | |
| Interventions | Administration set changes at 24 or 48 hours | |
| Outcomes |
|
|
| Notes | Exclusions: IV sets in for < 48 hours; heparin locks, PN, participants previously in study, cannulation < 2 weeks, protocol failure Central catheters: 0% PN: 0% Loss to follow‐up: 39% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation used Quote: "used a random number table to assign each new patient" |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment (phlebitis) Quote: "a nurse or physician observer who had no knowledge of when each patient's AS was changed evaluated each patient daily for the presence of phlebitis" (p. 765) Not discussed whether IV fluid colonization outcome was assessed by blinded scientists No explanation about how BSIs were detected |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Minimal attrition information provided Quote: "A total of 987 patients were initially entered into the study, but 387 patient infusions were stopped in < 48 h" (p. 765) Possible participants treated at 24 hours had bacteraemia less than 48 hours, so intention to treat principle not upheld |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol All outcomes provided |
Covey 1988.
| Methods | Randomized controlled trial | |
| Participants | 30 critically ill patients requiring pressure monitoring (peripheral arterial and/or pulmonary artery catheters) | |
| Interventions | Random assignment to 24 or 48 hour AS replacement (and a third group who had a 24 hour solution change and a 48 hour tubing change) | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Minimal information provided regarding random sequence generation Quote: "Patients were randomly assigned to one of the following three groups" (p. 210) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcome measures; no mention of whether blinded or not Quote: "blood cultures taken as clinically indicated for unexplained fever or other signs of infection" (p. 211); bacteraemia was most likely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15/92 infusate cultures missing (not done), 7/39 tips not cultured No mention of numbers screened or consented or dropouts |
| Selective reporting (reporting bias) | High risk | Local infection and inflammation of the site defined in methods but not reported in results per group No pre‐protocol |
| Other bias | High risk | Baseline differences of clinical concern between groups (e.g. pre‐existing infection (group I 30%; group III 10%): Swan Ganz (group I 1; group II 6)) Both disposable and non‐disposable transducers used; confounding factor |
deMoissac 1998.
| Methods | Randomized controlled trial | |
| Participants | 50 cancer patients with a tunnelled CVC Large urban cancer centre, Canada | |
| Interventions | Administration set changes at 24 or 48 hours | |
| Outcomes |
|
|
| Notes | Exclusions: not stated Central catheters: 100% PN: 14.5% Loss to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "A table of random numbers was used to assign subjects" (p. 908) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, unfeasible because of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, unfeasible because of study design Quote: "colored labels added to IV AS" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Poor information regarding attrition provided 50 participants (25/25); results not reported per participant Quote: "In cases in which the study protocol for changing AS was broken, subjects were excluded from further analysis" (p. 909) n = 10 out of the 423 sets; doesn't say which groups were excluded Quote: "62% of subjects completed the 5 proposed infusate measurements" (p. 909) |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol |
| Other bias | High risk | Significant difference in chemotherapy and non‐antibiotic treatment between groups (p. 909) Results given per AS, not per participant |
Fox 1999.
| Methods | Randomized controlled trial | |
| Participants | 166 neonates receiving TPN. NICU, Canada | |
| Interventions | Administration set changes at 24 or 48 hours | |
| Outcomes |
|
|
| Notes | Exclusions: not stated Central catheters: > 30% PN: 100% Loss to follow‐up: 10.8%, nil for mortality | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "A random number table was used to allocate infants in a one to two ratio" (p. 150) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequately reported: n = 166 randomly assigned, 149 cultured infusate (therefore 14 not sent as PN ceased, 3 deceased, 1 crossed over) Roughly equates to equal attrition between groups |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol |
| Other bias | High risk | Did not monitor set interruptions Two types of administration sets in use. Quote: "determined by the type of IV pump in use" (p. 151) but not reported by group Quote: "Unit of measurement was infusate sample not patient" (p. 151), but study was randomized per participant |
Gorbea 1984.
| Methods | Alternately allocated controlled clinical trial | |
| Participants | 123 adult patients in a surgical ICU who required IV therapy for the required period of time. Hospital, USA | |
| Interventions | Administration set changes at 24 or 48 hours | |
| Outcomes |
|
|
| Notes | Exclusions: patients receiving PN through a central line. Confounders: The 24 hour group had a greater no. of catheters/participant and a greater proportion receiving antibiotics Central catheters: 38% PN: 0% (central), not stated for peripheral Loss to follow‐up: 22% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Poor random sequence generation; easily predictable Quote: "alternatively assigned" |
| Allocation concealment (selection bias) | High risk | Not feasible because of poor sequence generation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition adequately described 157 enrolled, 34 dropouts (15 in 24 hours, 19 in 48 hours) because of early discharge or death |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol |
| Other bias | High risk | Difference in baseline characteristics of concern
|
Jakobsen 1986.
| Methods | Randomized controlled trial | |
| Participants | 325 patients likely to receive IV therapy for at least 3 days. Three surgical and five medical centres, Denmark | |
| Interventions | Administration set changes at 24, 48, 72, 96 or 120 hours | |
| Outcomes | Catheter colonization | |
| Notes | Exclusions: cannula change due to extravasation; PN in main line Central catheters: 0% PN: mixed (not in main line) Loss to follow‐up: 27% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear randomization method Quote: "a prospective randomized trial" (p. 218) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed Quote: "All sampling was carried out by the physicians conducting the study" (p. 219) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information provided regarding attrition Quote: "If cannulae [were] removed because of extravasation, the patient was excluded from the study" (p. 218) Quote: "527 patient[s] admitted to the study; 140 were excluded for various reasons and as expected form the study design, the number excluded increased from group 1 to group 5" (p. 219) |
| Selective reporting (reporting bias) | Unclear risk | No pre‐protocol BSI not reported by group |
| Other bias | High risk | Quote: "The results were evaluated once a week and the study was designed to stop if a statistically significant difference in contamination rates of the cannulae and/or administration sets was found between any of the groups" (p. 219) No baseline statistics by group provided, just "no epidemiological difference was found" No correction for time at risk or multiple sampling |
Josephson 1985.
| Methods | Randomized controlled trial | |
| Participants | 173 medical patients who had an IV started in the previous 24 hours. 350 bed university hospital, USA | |
| Interventions | Administration set changes at 48 hours or no change for the remainder of the cannula placement (i.e. at least 72 hours) | |
| Outcomes |
|
|
| Notes | Exclusions: casein, fat emulsions or blood in lines or vascular line monitoring systems Central catheters: 0% PN: 0% Loss to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "were randomly assigned according to a table of random numbers" (abstract and p. 368) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalanced attrition Quote: "Patients whose IVs were in place for fewer than three days or whose tubing change protocol was broken prior to three days of therapy were dropped from the study". This occurred 166 times in the 48 hour group and 106 times in the no‐change group" (p. 368) |
| Selective reporting (reporting bias) | Unclear risk | No pre‐protocol Only 26% of tips were cultured (all were required, as it was a secondary endpoint) |
| Other bias | High risk | Multiple infusions per participant randomly assigned |
Luskin 1986.
| Methods | Randomized controlled trial | |
| Participants | 112 patients with a peripheral arterial or pulmonary arterial catheter started 36 to 48 hours ago, and it was anticipated that the catheter would remain in place for at least 72 hours | |
| Interventions | Transducer administration set changes at 48 hours or 192 hours, After a protocol amendment at n = 80, the 192 hour intervention was reduced to 96 hours for the remainder of the trial | |
| Outcomes |
|
|
| Notes | Exclusions: five catheters excluded post randomizations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "Patients were randomized in blocks of 20, based on a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of research design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded Quote: "Laboratory personnel had no knowledge of the group to which a patient was assigned" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Poor provision of attrition data Quote: “130 admissions (112 patients) were entered into the study” (p. 918) 112 participants were randomly assigned, and 120 participants were reported: “12 patients were re‐randomised” (p. 918). Not reported how many per group Difficult to ascertain because of overlap between participants and catheters Five transducers excluded post randomization: three catheters excluded because removed within six hours of intervention; one because time of transducer change not found, and one because it had not met inclusion criteria. Reason for removal not reported |
| Selective reporting (reporting bias) | High risk | No pre‐protocol Tip colonization and BSIs not reported per study group Infusate colonization not reported per participant or per device |
| Other bias | High risk | Clinical difference at baseline: non-statistically significant higher baseline infusate colonization in participants randomly assigned to the ≥ 96 hour group along with 9% higher incidence of dirty wounds Protocol changed at n = 80 and intervention changed (192 hour group changed to a 96 hour group) Non‐independence with 12 participants re‐randomly assigned (with new IV at another site) |
Maki 1987.
| Methods | Randomized controlled trial | |
| Participants | 487 patients in the general surgical, medical oncology, surgical ICU and Centre for Trauma and Life Support. Acute care hospital, USA | |
| Interventions | Administration set changes at 48 or 72 hours | |
| Outcomes |
|
|
| Notes | Exclusions: patients with granulocytopenia Central catheters: 56% PN: 12% Loss to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "was randomized" (p. 1778) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal attrition information provided; no mention of numbers randomly assigned or any missing data Inaccurate reporting in results section between Table 1 (participant number = 487; AS number = 1374) and text quote: "1479 patient IV infusions were studied" (p. 1778) |
| Selective reporting (reporting bias) | Unclear risk | No IR‐BSI reported No pre‐protocol |
| Other bias | Unclear risk | Not independent units of measure (i.e. per AS, not per participant) |
Matlow 1999.
| Methods | Randomized controlled trial | |
| Participants | 1189 neonates admitted to the NICU for whom IV lipid therapy was ordered. NICU of Paediatric Hospital, Canada | |
| Interventions | Administration set changes at 24 or 72 hours. Eight per cent of babies randomly assigned to study more than once | |
| Outcomes | Mortality | |
| Notes | Exclusions: patients receiving blood or blood products, disconnection of the set for longer than 4 hours or without sterile gauze coverage
Central catheters: mixed (proportion unknown)
PN: 100%
Loss to follow‐up: not clear, 45.9% of catheters were not sampled. Nil for mortality Data based on first randomization when babies were randomly assigned more than once to study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate method of random sequence generation Quote: "Infants requiring IV lipid treatment were randomly assigned to have IV sets changed on a 72 hour schedule, in a 3:1 ratio" (abstract) Quote: "Patients were randomized in pharmacy" (abstract) Quote: "Using a table of assignments by random number" (p. 488) Quote: "The ratio was altered to between 1:3 and 1:6 periodically when an imbalance in the numbers in the two treatment groups was observed" (p. 488) |
| Allocation concealment (selection bias) | Low risk | Randomly assigned in pharmacy, adequate concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate attrition data provided n = 1278 randomly assigned (979 = 72 hours, 250 = 24 hours). Only 500 of those assigned to 72 hours and 191 of those assigned to 24 hours completed the study |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol reported |
| Other bias | Unclear risk | Unequal group numbers |
McLane 1998.
| Methods | Randomized controlled trial | |
| Participants | 76 people with 49 arterial lines and/or 55 pulmonary artery catheters | |
| Interventions | 48 or 72 hour AS use | |
| Outcomes |
|
|
| Notes | Nil exclusions No ITT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "After informed consent was obtained, subjects were randomly allocated by coin toss to one of the following two groups" (p. 206) |
| Allocation concealment (selection bias) | Low risk | Coin toss after each participant would be adequate to conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate attrition data provided: not reported how many participants were screened, consented or were eliminated Quote: "Noncompliance with frequency of solution, stopcock or tubing changes, early catheter removal, or failure to obtain appropriate cultures eliminated the subjects from the study. Subject attrition was handled by entering another subject into the study" (p. 206) |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol |
| Other bias | Unclear risk | Quote: "Specimens were obtained for culture before each flush solution change per group assignment (48 or 72 h)" (p. 206) More frequent outcome measurements in 48 hour group |
Raad 2001.
| Methods | Randomized controlled trial | |
| Participants | 428 cancer patients requiring IV therapy. Tertiary university cancer centre, USA | |
| Interventions | Administration set changes at 72 or 96 to 168 hours | |
| Outcomes |
|
|
| Notes | Exclusions: not stated Central catheters: 100% PN: Data from PN participants couldn't be used, as they included participants receiving blood Loss to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "prospective randomized study" (abstract) Quote: "computer‐generated randomization list" (p. 136‐7) |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of any missing data or attrition |
| Selective reporting (reporting bias) | Unclear risk | No pre‐protocol Stopped early (n = 512) after interim analysis showed 3 IR‐BSI in 96 to 167 hour group (P = 0.09) |
| Other bias | Unclear risk | Outcome definition of contaminated tip was ≥ 10 cfu |
Rickard 2004.
| Methods | Randomized controlled trial | |
| Participants | 251 ICU patients with a CVC receiving any combination of crystalloids, lipids and non‐lipid PN | |
| Interventions | Administration set changes at 72 hours or no change up to 144 hours | |
| Outcomes |
|
|
| Notes | Fluid container bags were changed every 24 hours Central catheters: 100% PN: separate data available for participants receiving PN Loss to follow‐up: 5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation Quote: "A computerised random‐number generator randomized each CVC to either receive a routine set change or have the original administration set left intact for the duration of catheterization" (p. 651) |
| Allocation concealment (selection bias) | High risk | Not originally reported, after checking with author: not adequately concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, not feasible because of study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Main outcome: CR‐BSI concealed: laboratory staff were blinded to the study group Quote: "a blinded intensivist reviewed microbiological results .... and cases of systemic inflammatory response syndrome using strict definitions to diagnose" (p. 651) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "29 (7.1%) of the CVCs were not cultured due to autopsy or lost or contaminated specimens ... these were equally distributed between the groups" (p. 652) No information in manuscript re CR‐BSI missing outcomes; clarified with author, daily check of microbiological reporting system was undertaken |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol Primary outcome variables were specified and reported on |
Sitges‐Serra 1985.
| Methods | Randomized controlled trial | |
| Participants | 52 adult surgical patients undergoing PN through a subclavian catheter Appears to have been a hospital in Spain | |
| Interventions | Administration set changes at 48 or 96 hours | |
| Outcomes |
|
|
| Notes | Exclusions: not stated Central catheters: 100% PN: 100% Loss to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate random sequence generation; easily predictable Quote: "patients randomly assigned to one of two groups according to their hospital number" (p. 322) |
| Allocation concealment (selection bias) | High risk | Inadequate concealment; use of hospital number easily predictable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, but not feasible in the study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, but not feasible in the study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome blinding possible but not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal information provided regarding attrition; no mention of missing samples |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol |
| Other bias | Unclear risk | PN mean four days longer in group A (not statistically significant) No other demographic data presented |
Snydman 1987.
| Methods | Alternately allocated controlled clinical trial | |
| Participants | 170 adult patients admitted to the medical and surgical ICUs receiving IV therapy for the required period Medical and surgical ICUs, USA | |
| Interventions | Administration set changes at 48 or 72 hours | |
| Outcomes |
|
|
| Notes | Exclusions: previously enrolled in study or receiving blood products, PN or lipids. Confounders: The no. of burettes per line was statistically lower in the 72 hour group Central catheters: 27% PN: 0% Loss to follow‐up: 52% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Poor random sequence generation Quote: "alternatively assigned" (abstract) |
| Allocation concealment (selection bias) | High risk | Easily predictable, inadequate concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed, but not feasible in the study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, but not feasible in the study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Phlebitis measures were undertaken by staff blinded to culture results, but not known if they were blinded to the group Not discussed whether outcome assessors were blinded during cultures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No discussion of attrition and missed cultures Originally 356 enrolled (176/180) but (71/115) dropped as discharged/died < 48/72 hours Imbalanced groups n = 105 (48 hours), n = 62 (72 hours) |
| Selective reporting (reporting bias) | Low risk | No pre‐protocol |
| Other bias | High risk | Results given per AS, not per participant 1.5 days longer ICU stay for 72 hour group Higher antibiotic courses in the 48 hour group Imbalanced AS cultured (745/449) between groups |
AS: administration set, BSI: bloodstream infection, cfu: colony forming units, CR‐BSI: catheter‐related bloodstream infection, CVC: central venous catheter, h: hours, ICU: Intensive Care Unit, IR‐BSI: infusate‐related bloodstream infection, IV: intravenous, NICU: Neonatal Intensive Care Unit, no.: number, PN: parenteral nutrition.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alothman 1996 | No data could be obtained for the review |
| Band 1979 | Not an RCT or a CCT |
| Chen 2000 | No data could be obtained for the review |
| Cohen 1989 | Not an RCT or a CCT |
| Franceschi 1989 | Inadequate data for extraction |
| O'Malley 1994 | Not an RCT or a CCT |
| Powell 1985 | Outcomes did not correspond with the definitions used in this review |
| Robathan 1995 | Not an RCT or a CCT |
| Robertson 1991 | Does not investigate timing of administration set changes |
| Trautmann 1997 | No data could be obtained for the review |
CCT: controlled clinical trial; RCT: randomized clinical trial.
Differences between protocol and review
This updated review has incorporated methodological strategies as described in Higgins 2011. Risk of bias summaries (Higgins 2011) were completed using updated criteria; GradePRO (Schunemann 2011) was used to provide 'Summary of findings' tables, and the PRISMA flow chart (Liberati 2009) was included to summarize study selection.
Contributions of authors
Amanda J Ullman (AJU): extracted and entered data, performed analysis, wrote updated review
Marie L Cooke (MLC): extracted data, revised updated review
Donna Gillies (DG): developed and wrote original review, revised updated review
Nicole M Marsh (NMM): revised updated review
Azlina Daud (AD): extracted data, revised updated review
Matthew R McGrail (MRM): provided statistical assistance, revised updated review
Elizabeth O'Riordan (EO'R): developed and wrote original review, revised updated review
Claire M Rickard (CMR): extracted data, performed analysis and revised updated review
Sources of support
Internal sources
NHMRC Centre of Research Excellence in Nursing Interventions, Griffith University, Brisbane, Australia.
The Royal Brisbane and Women's Hospital, Brisbane, Australia.
The Children's Hospital at Westmead, Sydney, Australia.
Monash University, Churchill, Australia.
External sources
No sources of support supplied
Declarations of interest
Amanda J Ullman: none known
Marie L Cooke: none known
Donna Gillies: none known
Nicole M Marsh: none known
Azlina Daud: none known
Matthew R McGrail: none known
Elizabeth O'Riordan: none known
Claire M Rickard: was an author of one of the studies reviewed (Rickard 2004) but did not partake in any critique or data extraction for that study
Edited (no change to conclusions)
References
References to studies included in this review
Blight 1998 {unpublished data only}
- Blight I, Amadio J, Thomas P, Wilkinson I, Lusis I. Randomised prospective study of replacing intravenous administration sets at 72 hours versus 120 hour intervals in central venous catheters. 4th Nursing Practice Conference Book of Abstracts. Adelaide, Australia: Flinders University, 1998:3.
Buxton 1979 {published data only}
- Buxton AE, Highsmith AK, Garner JS, West CM, Stamm WE, Dixon RE, et al. Contamination of intravenous infusion fluid: effects of changing administration sets. Annals of Internal Medicine 1979;90(5):764‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Covey 1988 {published data only}
- Covey M, McLane C, Smith N, Matasic J, Holm K. Infection related to intravascular pressure monitoring: effects of flush and tubing changes. American Journal of Infection Control 1988;16(5):206‐13. [PUBMED: 3195780] [DOI] [PubMed] [Google Scholar]
deMoissac 1998 {published data only}
- deMoissac D, Jensen L. Changing IV administration sets: is 48 versus 24 hours safe for neutropenic patients with cancer?. Oncology Nursing Forum 1998;25(5):907‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Fox 1999 {published data only}
- Fox M, Molesky M, Aerde JE, Muttitt S. Changing parenteral nutrition administration sets every 24 h versus every 48 h in newborn infants. Canadian Journal of Gastroenterology 1999;13(2):147‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gorbea 1984 {published data only}
- Gorbea HF, Snydman DR, Delaney A, Stockman J, Martin WJ. Intravenous tubing with burettes can be safely changed at 48‐hour intervals. JAMA 1984;251(16):2112‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Jakobsen 1986 {published data only}
- Jakobsen CJ, Grabe N, Nielsen E, Hojbjerg T, Damm M, Lorentzen K, et al. Contamination of intravenous infusion systems-the effect of changing administration sets. Journal of Hospital Infection 1986;8(3):217‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Josephson 1985 {published data only}
- Josephson A, Gombert ME, Sierra MF, Karanfil LV, Tansino GF. The relationship between intravenous fluid contamination and the frequency of tubing replacement. Infection Control 1985;6(9):367‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Luskin 1986 {published data only (unpublished sought but not used)}
- Luskin R, Weinstein R, Nathan C, Chamberlin W, Sherwin A. Extended use of disposable pressure transducers. Journal of the American Medical Association 1986;255(7):916‐21. [PUBMED: 3944997] [PubMed] [Google Scholar]
Maki 1987 {published data only}
- Maki DG, Botticelli JT, LeRoy ML, Thielke TS. Prospective study of replacing administration sets for intravenous therapy at 48‐ vs 72‐hour intervals. 72 hours is safe and cost‐effective. JAMA 1987;258(13):1777‐81. [MEDLINE: ] [PubMed] [Google Scholar]
- Maki DG, Botticelli JT, LeRoy ML, Thielke TS. Prospective study of replacing administration sets for intravenous therapy at 48‐ vs 72‐hour intervals: 72 hours is safe and cost‐effective. CINA: Official Journal of the Canadian Intravenous Nurses Association 1990;6(4):12‐6. [PubMed] [Google Scholar]
Matlow 1999 {published data only}
- Matlow AG, Kitai I, Kirpalani H, Chapman NH, Corey M, Perlman M, et al. A randomised trial of 72 versus 24 hour intravenous tubing set changes in newborns receiving lipid therapy. Infection Control and Hospital Epidemiology 1999;20(7):487‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McLane 1998 {published data only}
- McLane C, Morris L, Holm K. A comparison of intravascular pressure monitoring system contamination and patient bacteremia with use of 48‐ and 72‐hour system change intervals. Heart and Lung May/June 1998;27(3):200‐8. [PUBMED: 9622407] [DOI] [PubMed] [Google Scholar]
Raad 2001 {published data only}
- Raad I, Hanna H, Richardson D, Abi‐Saia D, Awad A, Alrahwan A, et al. Optimal frequency of changing intravenous administration sets (IVAS): is it safe to prolong duration of use beyond three days?. Infection Control and Hospital Epidemiology 1998;19(Suppl 9):682. [Google Scholar]
- Raad I, Hanna HA, Awad A, Alrahwan A, Bivins C, Khan A, et al. Optimal frequency of changing intravenous administration sets: is it safe to prolong use beyond 72 hours?. Infection Control and Hospital Epidemiology 2001;22(3):136‐9. [PUBMED: 11310690] [DOI] [PubMed] [Google Scholar]
Rickard 2004 {published and unpublished data}
- Rickard CM, Lipman J, Courtney M, Siversen R, Daley P. Routine changing of intravenous administration sets does not reduce colonization or infection in central venous catheters. Infection Control and Hospital Epidemiology 2004;25(8):650‐5. [PUBMED: 15357156] [DOI] [PubMed] [Google Scholar]
Sitges‐Serra 1985 {published data only}
- Sitges‐Serra A, Linares J, Perez JL, Jaurrieta E, Lorente L. A randomized trial on the effect of tubing changes on hub contamination and catheter sepsis during parenteral nutrition. Journal of Parenteral and Enteral Nutrition 1985;9(3):322‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Snydman 1987 {published data only}
- Snydman DR, Donnelly‐Reidy M, Perry LK, Martin WJ. Intravenous tubing containing burettes can be safely changed at 72 hour intervals. Infection Control 1987;8(3):113‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alothman 1996 {published data only}
- Alothman A, Scharf S, Bryce EA. Extending central venous catheter tubing changes to five days: safety and cost effectiveness. Program and Abstracts of the IDSA 34th Annual Meeting. Arlington, Virginia: Infectious Diseases Society of America, 1996:861.
Band 1979 {published data only}
- Band JD, Maki DG. Safety of changing intravenous delivery systems at longer than 24‐hour intervals. Annals of Internal Medicine 1979;91(2):173‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen Y, Lin M, Lin MY, Wang FD, Liu JH, Chang MS. The central venous tubing change associated with catheter‐related infection. Infection Control and Hospital Epidemiology 2000;21(2):94. [Google Scholar]
Cohen 1989 {published data only}
- Cohen DM. A replication study: analysis of bacterial contamination of intravenous administration sets in use for 72 hours. Journal of the New York State Nurses Association 1989;20(3):12. [MEDLINE: ] [PubMed] [Google Scholar]
Franceschi 1989 {published data only}
- Franceschi D, Gerding RL, Phillips G, Fratianne RB. RIsk factors associated with intravascular catheter infections in burned patients: a prospective, randomized study. The Journal of Trauma 1989;29(6):811‐6. [PUBMED: 2738979] [DOI] [PubMed] [Google Scholar]
O'Malley 1994 {published data only}
- O'Malley M, Shame F, Cera F, McComb R. Value of routine pressure monitoring system changes after 72 hours of continuous use. Critical Care Medicine 1994;22(9):1424‐30. [PUBMED: 8062565] [DOI] [PubMed] [Google Scholar]
Powell 1985 {published data only}
- Powell CR, Traetow MJ, Fabri PJ, Kudsk KA, Ruberg RL. Op‐Site dressing study: a prospective randomized study evaluating povidone iodine ointment and extension set changes with 7‐day Op‐Site dressings applied to total parenteral nutrition subclavian sites. Journal of Parenteral and Enteral Nutrition 1985;9(4):443‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robathan 1995 {published data only}
- Robathan G, Woodger S, Merante D. A prospective study evaluating the effects of extending total parenteral nutrition line changes to 72 hours. Journal of Intravenous Nursing 1995;18(2):84‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Robertson 1991 {published data only}
- Robertson J. Changing central venous catheter lines: evaluation of a modification to clinical practice. Journal of Pediatric Oncology Nursing. 1991;8(4):173‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trautmann 1997 {published data only}
- Trautmann M, Zauser B, Wiedeck H, Buttenschon K, Marre R. Bacterial colonization and endotoxin contamination of intravenous infusion fluids. Journal of Hospital Infection 1997;37(3):225‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Rawajfah 2012
- Al‐Rawajfah OM, Hewitt JB, Stetzer F, Cheema J. Length of stay and charges associated with health care‐acquired bloodstream infections. American Journal of Infection Control 2012;40:227‐32. [DOI] [PubMed] [Google Scholar]
Collignon 1994
- Collignon PJ. Intravascular catheter associated sepsis: a common problem. The Australian study on intravascular catheter associated sepsis. The Medical Journal of Australia 1994;161(6):374‐8. [PubMed] [Google Scholar]
Crump 2000
- Crump JA, Collignon PJ. Intravascular catheter‐associated infections. European Journal of Clinical Microbiology and Infectious Diseases 2000;19:1‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPF, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.1. Chichester (UK): John WIley & Sons, 2011. [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 1986
- Gilbert M, Gallagher SC, Eads M, Elmore MF. Microbial growth patterns in a total parenteral nutrition formulation containing lipid emulsion. Journal of Parenteral and Enteral Nutrition 1986;10(5):494‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Stern JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.1. Chichester (UK): John WIley & Sons, 2011. [Google Scholar]
Ishizuka 2008
- Ishizuka M, Nagata H, Takagi K, Kubota K. Total parenteral nutrition is a major risk factor for central venous catheter‐related bloodstream infection in colorectal cancer patients receiving postoperative chemotherapy. European Surgical Research 2008;41(4):341‐5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville F. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.1. Chichester (UK): John Wiley & Sons, 2011. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ionnidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maki 2006
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proceedings 2006;81(9):1159‐71. [DOI] [PubMed] [Google Scholar]
Mermel 2009
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady N, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;49:1‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moro 1994
- Moro ML, Vigano EF, Cozzi Lepri A. Risk factors for central venous catheter‐related infections in surgical and intensive care units. Infection Control and Hospital Epidemiology 1994;15(4):253‐64. [PubMed] [Google Scholar]
Mulloy 1991
- Mulloy RH, Jadavji T, Russell ML. Tunneled central venous catheter sepsis: risk factors in a pediatric hospital. Journal of Parenteral and Enteral Nutrition 1991;15(4):460‐3. [DOI] [PubMed] [Google Scholar]
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report 2002;51([RR‐10]):1‐29. [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady N, Alexander M, Burns L, Dellinger E, Garland J, et al. and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter‐related infections. Clinical Journal of Infectious Diseases 2011 May;52(9):1087‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearson 1996
- Pearson ML. Special communication. HICPAC guideline for prevention of intravascular device‐related infections. Parts I and II. American Journal of Infection Control 1996;24(4):262‐93. [DOI] [PubMed] [Google Scholar]
Perlman 2007
- Perlman SE, Saiman L, Larson EL. Risk factors for late‐onset health care‐associated bloodstream infections in patients in neonatal intensive care units. American Journal of Infection Control 2007;35(3):177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Renaud 2001
- Renaud B, Brun‐Buisson C, ICU‐Bacteremia Study Group. Outcomes of primary and catheter‐related bacteremia. A cohort and case‐control study in critically ill patients. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1584‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.1 for Windows. Oxford, England: The Cochrane Collaboration, 2012.
Rickard 2001
- Rickard C, Daley P, Lipman J, Davie A, Koroloff N, Siversen R, et al. Routine administration set changes do not reduce central venous catheter infection (abstract). Anaesthesia and Intensive Care 2001;29(2):185. [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:726‐32. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann JH, Oxman AD, Higgins JPF, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.1. Chichester (UK): John WIley & Sons, 2011. [Google Scholar]
Scott 1985
- Scott EM, Gorman SP, Wyatt TD, Magill EA. Growth of microorganisms in total parenteral nutrition mixtures and related clinical observations. Journal of Clinical and Hospital Pharmacy 1985;10(1):79‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherertz 1992
- Sherertz RJ, Gledhill KS, Hampton KD, Pfaller MA, Givner LB, Abramson JS, et al. Outbreak of Candida bloodstream infections associated with retrograde medication administration in a neonatal intensive care unit. Journal of Pediatrics 1992;120(3):455‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shiro 1995
- Shiro H, Muller E, Takeda S, Tosteson TD, Goldmann DA, Pier GB. Potentiation of Staphylococcus epidermidis catheter‐related bacteremia by lipid infusions. Journal of Infectious Diseases 1995;171(1):220‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soufir 1999
- Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter‐related septicemia in critically ill patients: a matched, risk‐adjusted, cohort study. Infection Control and Hospital Epidemiology 1999;20(6):396‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gillies 2004
- Gillies D, O'Riordan L, Wallen M, Rankin K, Morrison A, Nagy S. Timing of intravenous administration set changes: a systematic review. Infection Control and Hospital Epidemiology 2004;25(3):240‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gillies 2005
- Gillies D, Wallen MA, Morrison AL, Rankin K, Nagy SA, O'Riordan E. Optimal timing for intravenous administration set replacement. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003588.pub2] [DOI] [PubMed] [Google Scholar]


