Abstract
Background
Variceal haemorrhage that is refractory or recurs after pharmacologic and endoscopic therapy requires a portal decompression shunt (either surgical shunts or radiologic shunt, transjugular intrahepatic portosystemic shunt (TIPS)). TIPS has become the shunt of choice; however, is it the preferred option? This review assesses evidence for the comparisons of surgical portosystemic shunts versus TIPS for variceal haemorrhage in people with cirrhotic portal hypertension.
Objectives
To assess the benefits and harms of surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt (TIPS) for treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, LILACS, Science Citation Index Expanded, and Conference Proceedings Citation Index – Science. We also searched on‐line trial registries, reference lists of relevant articles, and proceedings of relevant associations for trials that met the inclusion criteria for this review (date of search 8 March 2018).
Selection criteria
Randomised clinical trials comparing surgical portosystemic shunts versus TIPS for the treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension.
Data collection and analysis
Two review authors independently assessed trials and extracted data using methodological standards expected by Cochrane. We assessed risk of bias according to domains and risk of random errors with Trial Sequential Analysis (TSA). We assessed the certainty of the evidence using the GRADE approach.
Main results
We found four randomised clinical trials including 496 adult participants diagnosed with variceal haemorrhage due to cirrhotic portal hypertension. The overall risk of bias in all the trials was judged at high risk. All the trials were conducted in the United States of America (USA). Two of the trials randomised participants to selective surgical shunts versus TIPS. The other two trials randomised participants to non‐selective surgical shunts versus TIPS. The diagnosis of liver cirrhosis was by clinical and laboratory findings. We are uncertain whether there is a difference in all‐cause mortality at 30 days between surgical portosystemic shunts compared with TIPS (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.44 to 1.99; participants = 496; studies = 4). We are uncertain whether there is a difference in encephalopathy between surgical shunts compared with TIPS (RR 0.56, 95% CI 0.27 to 1.16; participants = 496; studies = 4). We found evidence suggesting an increase in the occurrence of the following harms in the TIPS group compared with surgical shunts: all‐cause mortality at five years (RR 0.61, 95% CI 0.42 to 0.90; participants = 496; studies = 4); variceal rebleeding (RR 0.18, 95% CI 0.07 to 0.49; participants = 496; studies = 4); reinterventions (RR 0.13, 95% CI 0.06 to 0.28; participants = 496; studies = 4); and shunt occlusion (RR 0.14, 95% CI 0.04 to 0.51; participants = 496; studies = 4). We could not perform an analysis of health‐related quality of life but available evidence appear to suggest improved health‐related quality of life in people who received surgical shunt compared with TIPS. We downgraded the certainty of the evidence for all‐cause mortality at 30 days and five years, irreversible shunt occlusion, and encephalopathy to very low because of high risk of bias (due to lack of blinding); inconsistency (due to heterogeneity); imprecision (due to small sample sizes of the individual trials and few events); and publication bias (few trials reporting outcomes). We downgraded the certainty of the evidence for variceal rebleeding and reintervention to very low because of high risk of bias (due to lack of blinding); imprecision (due to small sample sizes of the individual trials and few events); and publication bias (few trials reporting outcomes). The small sample sizes and few events did not allow us to produce meaningful trial sequential monitoring boundaries, suggesting plausible random errors in our estimates.
Authors' conclusions
We found evidence suggesting that surgical portosystemic shunts may have benefit over TIPS for treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension. Given the very low‐certainty of the available evidence and risks of random errors in our analyses, we have very little confidence in our review findings.
Plain language summary
Surgical shunts versus radiologic shunt for variceal haemorrhage in people with chronic disease of the liver
Background
Varices are enlarged thin walled vessels found in the walls of the oesophagus and stomach of people with high blood pressure in the portal circulation (blood vessels supplying the liver with blood from the bowels). Bleeding from varices are not uncommon and maybe life‐threatening. The first‐line treatment of this bleeding is with medications and endoscopy (use of a long tube fitted with camera to locate and occlude the varices with elastic bands). Most people will respond to the first‐line treatment, but a few will continue to bleed or have repeat bleeding. This later group will require further treatments in the form of shunts (tubes that divert blood from portal circulation direct to the heart). There are two types of shunts; one that is created through a surgical operation and called surgical shunt, the other is created with the help of an ultrasound machine and called radiologic shunt. Both types of shunts have their benefits and harms. This review was done to determine whether surgical shunts are better than radiologic shunt in treating persistent and repeat bleeding due to varices in people with cirrhosis (chronic disease of the liver in which normal liver cells are replaced by hard scar).
Study characteristics
We found four randomised clinical trials in which 496 adult participants were allowed to receive either a surgical shunt or a radiologic shunt. There were problems with the design of the trials as they had small number of participants and used different shunt types. We judged all four trials at high risk of bias (trials may have overestimated the true effect of shunts treatment).
Key results
We found no difference in the number of participants who died within 30 days of treatment, and the number that developed encephalopathy (disease of the brain due to toxins bypassing the liver to reach the brain), when surgical shunts were compared with radiologic shunt. We found evidence suggesting more harms with radiologic shunt when we considered the number of participants that died five years after treatment; or had repeat bleeding; or required repeated treatment; or had shunt blockage; that appeared to be more in the radiologic shunt group.
Conclusions
Surgical shunts appear to be better than radiologic shunt for treating persistent and repeated bleeding due to varices in people with liver cirrhosis. Given the very low certainty of the evidence due to problems with design of the trials and inadequate number of participants, we are unsure if our conclusion is correct. Future trials with better design and adequate number of participants will likely produce results that are reliable.
Summary of findings
Summary of findings for the main comparison. Surgical portosystemic shunts compared to transjugular intrahepatic portosystemic shunt (TIPS) for variceal haemorrhage.
| Surgical portosystemic shunts compared to transjugular intrahepatic portosystemic shunt (TIPS) for variceal haemorrhage in people with cirrhotic portal hypertension | ||||||
| Patient or population: people with cirrhotic portal hypertension Setting: health institutions in USA Intervention: surgical portosystemic shunts Comparison: transjugular intrahepatic portosystemic shunt (TIPS) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with TIPS | Risk with Surgical portosystemic shunts | |||||
| All‐cause mortality at 30 days | Study population | RR 0.94 (0.44 to 1.99) | 496 (4 RCTs) | ⊕⊝⊝⊝ Very low a b c d | ‐ | |
| 178 per 1000 | 167 per 1000 (78 to 354) | |||||
| All‐cause mortality at 5 years | Study population | RR 0.61 (0.42 to 0.90) | 496 (4 RCTs) | ⊕⊝⊝⊝ Very low a b c d | ‐ | |
| 551 per 1000 | 336 per 1000 (231 to 496) | |||||
| Number of participants with Variceal rebleeding episodes at 30 days | Study population | RR 0.18 (0.07 to 0.49) | 496 (4 RCTs) | ⊕⊝⊝⊝ Very low a c d | ‐ | |
| 117 per 1000 | 21 per 1000 (8 to 58) | |||||
| Number of participants with reintervention at 5 years | Study population | RR 0.13 (0.06 to 0.28) | 496 (4 RCTs) | ⊕⊝⊝⊝ Very low a c d | ‐ | |
| 518 per 1000 | 67 per 1000 (31 to 145) | |||||
| Irreversible shunt occlusion at 5 years | Study population | RR 0.14 (0.04 to 0.51) | 496 (4 RCTs) | ⊕⊝⊝⊝ Very low a b c d | ‐ | |
| 271 per 1000 | 38 per 1000 (11 to 138) | |||||
| Number of participants with encephalopathy at 5 years | Study population | RR 0.56 (0.27 to 1.16) | 496 (4 RCTs) | ⊕⊝⊝⊝ Very low a b c d | ‐ | |
| 385 per 1000 | 215 per 1000 (104 to 446) | |||||
| Health‐related quality of life | See comment | See comment | ‐ | 210 (2 RCTs) | ‐ | One trial provided data on health‐related quality of life with median score, while the other trial provided data on health‐related quality of life in a narrative way. Both studies appear to suggest improved health‐related quality of life in people who received surgical shunts compared with TIPS. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio; TIPS: transjugular intrahepatic portosystemic shunt | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for risk of bias due to lack of blinding. bDowngraded one level for inconsistency due to significant heterogeneity. cDowngraded two levels for imprecision due to small sample size and few events. dDowngraded one level for publication bias due to few trials reporting outcome.
Background
Description of the condition
Variceal haemorrhage is the most life‐threatening complication of portal hypertension. By definition, there is a pathological increase in portal venous pressure that exceeds 8 mm mercury (Hg), and resulting in complications such as varices, encephalopathy, and new‐onset, or worsening of pre‐existing ascites (Goff 1993; Sanyal 2008). The pathogenesis of portal hypertension is complex, but involves an increase in portal outflow resistance as well as increased portal venous inflow. An imbalance in vasoactive mediators (endothelin‐1, angiotensinogen, eicosanoids, and nitric oxide) that results in intrahepatic vasoconstriction has also been described (Sharara 2001; Moore 2004). Varices develop when natural portosystemic collateral vessels enlarge to decompress the elevated portal pressure. This develops commonly at the oesophagogastric junction in approximately half of people with liver cirrhosis, of these, 15% to 20% will bleed requiring an intervention to stop bleeding (Amitrano 2012). Oesophagogastric variceal haemorrhage occurs when portal venous pressure gradient is greater than 12 mm Hg (Garcia‐Tsao 1985). An interplay between variceal pressure, variceal wall tension, and variceal size according to Laplace's law, explains how variceal rupture occurs when wall tension exceeds the elastic limit of the variceal wall (Rigau 1989). Due to the advancement in medical management of acute variceal haemorrhage, as well as primary prevention for high‐risk varices (Jamal 2008a; Jamal 2008b), mortality from the first bleeding episode has reduced from approximately 50% to 15% (DeDombal 1986; Chalasani 2003; Carbonell 2004). Yet, the greatest morbidity is rebleeding, approaching 70% over two years without secondary prevention (D'Amico 2003). The risk of rebleeding is highest within the first few days after an index bleed (Smith 1982), with mortality similar to the first bleeding episode. Surveillance upper gastrointestinal endoscopy in people with liver cirrhosis can be used to establish diagnosis, and identify high‐risk varices (i.e. medium to large size varices and red wale sign) (Garcia‐Tsao 1985; de Franchis 2010). Mortality in portal hypertension is largely determined by the patient's underlying liver function (Graham 1981). Scoring systems such as the Child‐Pugh and model for end‐stage liver disease (MELD) scores are used to assess the degree of liver dysfunction and extrapolate this to predict survival (Child 1964; Pugh 1973). Patients with the best score of a Child‐Pugh A5 have a predicted life expectancy of 15 years to 20 years as opposed to the worst score of a Child‐Pugh C15, with a predicted life expectancy of one to three years (Kaplan 2015).
Description of the intervention
Variceal haemorrhage can be controlled effectively using a combination of pharmacologic as well as endoscopic therapy. However, about 20% to 30% of people treated with this modality will rebleed or have refractory bleeding despite the medical therapy (Sharara 2001). These people will require liver transplantation, or decompression shunting (either surgical shunts or radiologic shunt). As availability of donor organs is in short supply, transjugular intrahepatic portosystemic shunt (TIPS) is often proposed as an attractive option when medical therapy fails because it is less invasive and could serve as a bridge to transplantation (de Franchis 2010; Rosemurgy 2012). However, only 3% to 14% of people with cirrhosis who bleed from their varices eventually receive transplantation post‐TIPS (Stanley 1996; Tripathi 2004; Rössle 2006; Rosemurgy 2012; Toomey 2013). When considering both methods of shunting, procedure‐related mortality rates for elective surgical shunts are less than 10% (Cowgill 2006). Although TIPS is a less invasive procedure, it appears to have more complications and requires more reinterventions. The incidence of occlusion for TIPS is 17% compared to 9% for surgical shunts (Rosemurgy 2012); post‐shunt encephalopathy is 18% to 45% for TIPS (Rössle 1994), compared to 25% for surgical shunts (Zervos 1998); and rebleeding occurs in 20% to 30% of patients who received TIPS compared with less than 10% in patients who received a surgical shunt. Median survival is 26 months in patients who received TIPS compared with 52 months in patients who received a surgical shunt (Rikkers 1998; Costa 2010; Rosemurgy 2012). In addition, TIPS requires more intensive long‐term surveillance than surgical shunts due to the high likelihood for occlusion requiring reinterventions (up to 21% for TIPS compared to 6% for surgical shunts; Toomey 2013). This poses a significant burden on the healthcare system in terms of costs and resource utilisation. Another concern is that TIPS may divert nutrient‐effective hepatic blood flow away from already compromised hepatocytes, potentially accelerating hepatic decompensation (Rosemurgy 2003). This contrasts with surgical shunts that may improve nutrient portal blood flow, thus resulting in less postprocedural liver failure (Rosemurgy 2004).
TIPS is created by an interventional radiologist using fluoroscopic guidance. Under conscious sedation or general anaesthesia, a jugular vein is cannulated and a guidewire is fed into the liver via a hepatic vein. A special needle known as a Colapinto is advanced over the guidewire, through the liver parenchyma into a portal vein. The tract is then dilated with an angioplasty balloon. A covered or uncovered metal stent is then placed through this tract creating an open channel between the hepatic vein and portal vein (LaBerge 1993).
Surgical portosystemic shunts are divided into non‐selective and selective shunts. Non‐selective shunts divert all portal circulation into the systemic circulation. This includes the portocaval shunts (either side‐to‐side or end‐to‐side) (Orloff 2007); central splenorenal shunts (constructed by anastomosing proximal splenic vein to left renal vein); and the large diameter H‐graft shunts constructed with 16 mm polytetrafluoroethylene (PTFE) prosthesis (Sarfeh 1986). Selective shunts can maintain some hepatic perfusion while providing adequate portal decompression. Selective shunts include the distal splenorenal shunt (DSRS) and the small diameter H‐graft shunt constructed with 8 mm PTFE prosthesis (Sarfeh 1983; Rosemurgy 1994). The DSRS is constructed by anastomosing the distal splenic vein (portal venous system) to the left renal vein (systemic venous system) with or without splenopancreatic and gastric disconnection (Warren 1967). A review showed that there is no difference in overall survival and rebleeding between non‐selective and selective surgical shunts (Wolff 2003).
The greatest concern with invasive (surgical) shunts are the perceived increased periprocedural morbidity and mortality rates of surgical procedures in patients with advanced liver cirrhosis (Garbuzenko 2016). Three months after a patient has survived their acute variceal bleed their expected survival returns to the survival rate of a comparable cirrhotic who has not bled (Carbonell 2004). Furthermore, acutely deteriorating liver function, which may be precipitated by a variceal bleed, is also associated with poorer surgical morbidity and mortality (Shalimar 2016). Hence the need for this review to determine whether or not surgical shunts compared with TIPS are safe as well as effective.
How the intervention might work
Surgical portosystemic shunts bypass blood flow from the portal venous circulation into the systemic venous circulation, thus decreasing the blood pressure within the portal system. This will ultimately reduce portal venous pressure gradient and consequently decrease the likelihood of variceal rebleeding.
Why it is important to do this review
An evidence‐based approach should be developed to guide the treatment of people with hepatic cirrhosis complicated by potentially life‐threatening refractory or recurrent variceal haemorrhage who require portal decompression. This review attempted to provide evidence for which type of shunting procedure to use in people with refractory or recurrent variceal haemorrhage due to cirrhotic portal hypertension.
Objectives
To assess the benefits and harms of surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt (TIPS) for the treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomised clinical trials in which surgical portosystemic shunts were compared with transjugular intrahepatic portosystemic shunt for treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension. For assessment of harms, we intended to include quasi‐randomised studies and observational studies identified during our search for randomised clinical trials. We are aware that this approach increases the risk of overlooking harms of the interventions. We did not place any restrictions regarding language, year of publication, country of origin, or institution of publication in selecting studies for this review.
Types of participants
We included participants with cirrhotic portal hypertension diagnosed by clinical and laboratory data, irrespective of age and sex, that had documented refractory or recurrent variceal haemorrhage following pharmacologic and endoscopic interventions. We excluded participants with non‐cirrhotic portal hypertension and participants who had non‐shunt surgical interventions.
Types of interventions
We considered all types of surgical portosystemic shunt interventions as the experimental intervention. We divided the surgical shunt interventions into two groups as follows.
Surgical shunts
Selective shunts
Small diameter (8 mm) H‐graft shunt (externally reinforced polytetrafluoroethylene (PTFE)) either as mesocaval or portocaval shunt); and distal splenorenal shunt (DSRS; connecting the distal splenic vein and left renal vein).
Non‐selective shunts
Portocaval shunts (connecting the portal vein and vena cava); mesocaval shunts (connecting the mesenteric vein and vena cava); central splenorenal shunt (connecting proximal splenic vein and left renal vein); and 16 mm H‐graft shunt (externally reinforced PTFE shunt either as mesocaval or portocaval shunt).
Non‐surgical shunts
We considered the radiologic, transjugular intrahepatic portosystemic shunt (TIPS) as the control intervention.
Types of outcome measures
Primary outcomes
All‐cause mortality at 30 days, and five years
Serious adverse events. We defined serious adverse events as any untoward medical occurrence that was life‐threatening, resulted in death or persistent or significant disability, or any medical event that may have jeopardised the person or required intervention to prevent it (ICH‐GCP 1997). We considered all other adverse events (i.e. any medical occurrence not necessarily having a causal relationship with the treatment, yet causing a dose reduction or discontinuation of treatment) as non‐serious (see below).
Health‐related quality of life. We defined health‐related quality of life as any deviation from a person's usual or expected physical, emotional, and social well‐being that is due to an intervention.
Secondary outcomes
Variceal rebleed‐related mortality
Number of participants with variceal rebleeding episodes at 30‐days (diagnosed by endoscopy or identification of haematemesis, melena, or gastric aspirate containing blood)
Non‐serious adverse events
Exploratory outcomes
Number of participants who were bridged to liver transplantation and were transplanted
Number of participants with reintervention at five years
Irreversible shunt occlusion at five years, identified by follow‐up Doppler examination or abdominal computer tomography scan and failed reintervention
Number of participants with encephalopathy (persistent or new‐onset) at five years. Encephalopathy is defined by any of the following: classical signs detected on physical examination; signs unequivocally described by person's relatives; psychometric testing; and electroencephalogram
Number of participants with clinically significant ascites at five years (new‐onset or persistent) detected clinically or radiologically
Search methods for identification of studies
Electronic searches
We performed electronic searches for relevant trials in the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2018; 8 March 2018); Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 2); MEDLINE Ovid (1946 to 8 March 2018); Embase Ovid (1974 to 8 March 2018); LILACS (Latin American and Caribbean HealthSciences Literature) (BIREME; 1982 to 8 March 2018); Science Citation Index Expanded (Web of Science; 1900 to 8 March 2018); and Conference Proceedings Citation Index ‐ Science (Web of Science; 1990 to 8 March 2018) (Royle 2003). The search strategies and the time spans of the searches are listed in Appendix 1.
Searching other resources
We handsearched reference lists of identified studies for further relevant trials.
We also searched conference/meeting proceedings and abstracts published by the International Hepato‐Pancreato Biliary Association (IHPBA) (1994 to 8 March 2018); the American Association for the Study of Liver Diseases (AASLD) (1994 to 8 March 2018); and other relevant organisations.
We also searched on‐line trial registries such as ClinicalTrials.gov (clinicaltrials.gov), the European Medicines Agency (EMA) (ema.europa.eu), the World Health Organization, International Clinical Trials Registry Platform (who.int/ictrp), and the Food and Drug Administration (FDA; fda.gov), for ongoing or unpublished trials on 8 March 2018.
Data collection and analysis
Selection of studies
Two review authors (CJE and LP) independently screened the list of titles and abstracts retrieved by the search in order to identify potentially eligible studies. We retrieved the full‐text articles of those studies deemed potentially eligible, and two review authors (CJE and LP) reviewed the full‐text articles for inclusion in the review. We resolved any areas of disagreement through discussion with MB. We planned to obtain unpublished data by writing to trial authors but we could not find any unpublished data through our database search. We used a flow diagram to summarise study selection according to the PRISMA statement (Liberati 2009)
Data extraction and management
Two review authors (CJE and LP) independently extracted the following data from included trials.
Basic characteristics of each study including: name of first author; date of trial publication; country of trial; type of institution; time from bleeding episode to randomisation; duration of maximal follow‐up; inclusion and exclusion criteria; number of participants randomised; number of participants excluded; and type of interventions.
Participant information including: age; aetiology of cirrhosis, methods for diagnosis of cirrhosis; endoscopic findings and Child‐Pugh criteria.
Outcomes: proportion of participants with events for dichotomous outcome and the mean events with standard deviation for continuous outcomes.
Assessment of risk of bias.
Assessment of risk of bias in included studies
Two review authors (CJE and LP) independently assessed the risk of bias of each included trial using the domains as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the Cochrane Hepato‐Biliary group Module (Gluud 2018), and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Hrobjartsson 2012; Savović 2012a; Savović 2012b; Hrobjartsson 2013; Hrobjartsson 2014a; Hrobjartsson 2014b; Lundh 2017; Savović 2018). We judged trials to be at an overall low risk of bias if they were assessed at 'low risk of bias' in all 'Risk of bias' domains. We judged trials to be at an overall high risk of bias if they were assessed to be at 'unclear risk of bias' or 'high risk of bias' in one or more of the 'Risk of bias' domains. We used the following definitions to assess the risk of bias in included trials.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We only used such studies for the assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants. These studies were only considered for the assessment of harms.
Blinding of participants and personnel
Low risk of bias (any of the following): no blinding or incomplete blinding but the review authors judged that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias (any of the following): insufficient information to permit judgement of 'low risk'; or 'high risk'; or the trial did not address this outcome.
High risk of bias (any of the following): no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment
Low risk of bias (any of the following): no blinding of outcome assessment, but the review authors judged that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias (any of the following): insufficient information to permit judgement of 'low risk'; or 'high risk'; or the trial did not address this outcome.
High risk of bias (any of the following): no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: no missing outcome data or missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk: the trial reported the predefined outcomes in the method section. If the original trial protocol was available; the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, we will not consider those outcomes to be reliable.
Unclear risk: the study authors did not report all predefined outcomes fully, or it is unclear whether the study authors recorded data on these outcomes.
High risk: the study authors did not report one or more predefined outcomes.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that could manipulate the trial design, conductance, or results of the trial. (Industry‐sponsored trials overestimate the efficacy by about 25%) (Lundh 2017).
Unclear risk of bias: the trial may or may not be free of for‐profit bias as the trial did not provide any information on clinical trial support or sponsorship.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other factors that could put it at risk of bias.
Unclear risk of bias: the trials may or may not have been free of other factors that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall risk of bias
We judged trials to be at an overall low risk of bias if they were assessed at 'low risk of bias' in all 'Risk of bias' domains. We judged trials to be at an overall high risk of bias if they were assessed to be at 'unclear risk of bias' or 'high risk of bias' in one or more of the 'Risk of bias' domains.
Measures of treatment effect
We measured intervention effects for dichotomous outcomes using risk ratio (RR) with 95% confidence intervals (CIs), and for continuous outcomes we planned to use mean difference (MD) with 95% CIs. We planned to report Trial Sequential Analysis‐adjusted confidence intervals.
Unit of analysis issues
The unit of analysis was trial participants as randomised to the trial groups. We did not expect that cross‐over trials or cohort, cluster‐randomised trials were performed.
Dealing with missing data
We performed an intention‐to‐treat analysis.
Assessment of heterogeneity
We assessed heterogeneity between the trials using the Chi2 test and I2 test. The degree of heterogeneity observed was measured using the I2 statistic (Higgins 2002; Sterne 2011). The values of the I2 statistic were:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: represent considerable heterogeneity.
We considered an I2 statistic above 50% as significant, and we explored the possible cause of heterogeneity further in a sensitivity analysis.
Assessment of reporting biases
We planned to investigate reporting bias by visual inspection of funnel plot asymmetry if we identified at least 10 trials. For dichotomous outcomes we planned to use the Harbord test for asymmetry (Harbord 2006). For continuous outcome we planned to use the regression asymmetry test (Egger 1997), and the adjusted rank correlation test (Begg 1994).
Data synthesis
Meta‐analysis
We performed meta‐analysis according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Hepato‐Biliary Module (Gluud 2018). We meta‐analysed data according to the eight‐step procedure for validation of meta‐analytic results in systematic reviews, as suggested by Jakobsen and colleagues (Jakobsen 2014). We used the software packages Review Manager 5 provided by Cochrane (Review Manager 2014), and Trial Sequential Analysis version 0.9.5.10 Beta provided by the Copenhagen Trial Unit for our data analysis (Thorlund 2011; TSA 2011). We used both the fixed‐effect and the random‐effects meta‐analyses, and presented the data with the most conservative estimate of the two. The most conservative estimate of the two is the one closest to 1 for dichotomous outcomes or 0 (zero) for continuous outcomes (Jakobsen 2014). If the two point estimates were equal, we used the estimate with the widest CI as our main result of the two analyses (Jakobsen 2014). We presented heterogeneity using the I2 statistic (Higgins 2002). Where data are only available from one trial, we planned to use Fischer's test for dichotomous data (Fisher 1922), and used Student's t‐test for continuous data to present the results in a narrative way (Student 1908).
Trial Sequential Analysis
Cumulative meta‐analysis can introduce random error because of sparse data and repetitive testing of accumulating data (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Higgins 2011; Wetterslev 2017); hence we used Trial Sequential Analysis in this review to control for random errors (Thorlund 2011; TSA 2011). We calculated the diversity‐adjusted required information size (DARIS) for all outcomes in order to control for random errors (Wetterslev 2008; Wetterslev 2009). The DARIS calculation took into account the following: control group event proportion observed in the meta‐analysis; a plausible relative risk reduction of 20%; a risk of type I error of 2.5% due to three primary outcomes and three secondary outcomes; a risk of type II error of 10% (Castellini 2017; Wetterslev 2017); and the adjusted diversity from the meta‐analysis (Wetterslev 2008; Wetterslev 2009; Jakobsen 2014; Wetterslev 2017). We also planned to calculate and report the Trial Sequential Analysis‐adjusted CI (Thorlund 2011). We assumed that testing for statistical significance was performed with each new trial added to the trial sequential meta‐analysis. On the basis of the calculated DARIS we planned to construct trial sequential monitoring boundaries. If the Z‐curve crossed the trial sequential monitoring boundary for benefit or harm before the DARIS was reached, we planned to conclude evidence of benefit or harm of the intervention provided that bias could be excluded. In contrast, if the boundary was not surpassed, we planned to conclude the need to conduct further trials in order to attain the true intervention effect. However, where the Z‐curve crossed the monitoring boundary for futility, we planned to conclude non‐superiority, or non‐inferiority of intervention, or both (Thorlund 2011; Wetterslev 2008; Wetterslev 2017).
A more detailed description of Trial Sequential Analysis can be found at www.ctu.dk/tsa/ (Thorlund 2011).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Trials at low risk of bias compared to trials at high risk of bias.
Child‐Pugh classes A compared to class B compared to class C (Child 1964); or model for end‐stage liver disease score less than or equal to 18 compared to greater than 18 (Dhiman 2007).
Selective shunts versus TIPS compared to non‐selective shunts versus TIPS.
Surgical shunts versus TIPS with bare stent compared to surgical shunts versus TIPS with covered stents.
Surgical shunts by experienced surgeon compared to TIPS by experienced radiologist.
Sensitivity analysis
We planned sensitivity analysis of search methods for inclusion of trials; exclusion of trials; type of data analysed; process of data analysis; and measurement of intervention outcomes at six and 12 months. We also planned to compare our GRADE imprecision assessments to that conducted with Trial Sequential Analysis (Jakobsen 2014).
'Summary of findings' table
We designed one 'Summary of findings' table for our review comparison using GRADEpro software (community.cochrane.org/tools/review‐production‐tools/gradepro‐gdt). Using GRADE (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Mustafa 2013; Guyatt 2017), we graded the certainty of evidence for our Primary outcomes: all‐cause mortality; serious adverse events (reported individually as reintervention, irreversible shunt occlusion, and encephalopathy following ICH‐GCP 1997 definition of serious adverse events); and health‐related quality of life. We also graded the certainty of evidence for variceal rebleeding as Secondary outcomes. We based the grading of the certainty of evidence on five domains: risk of bias; indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of result, and a high probability of publication bias. We defined the levels of evidence as 'high', 'moderate', 'low', or 'very low'. We followed the recommendations of section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
These grades are defined as follows.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect
Results
Description of studies
We identified 2512 references through the electronic database searches (Figure 1). After removing 904 duplicates, we were left with 1608 references that we screened by reading through their titles and abstracts, we excluded 1600 references. Of the remaining eight full‐text articles, three were retrospective studies (Faust 1997; Zacks 1999; Helton 2001), and one was a prospective non‐randomised study that we considered for harms (Khaitiyar 2000) (Characteristics of excluded studies). We selected four studies for meta‐analysis (Henderson 2006; Orloff 2012; Rosemurgy 2012; Orloff 2015). We identified no other references of interest through other sources or through screening the reference lists of the identified randomised clinical trials.
1.
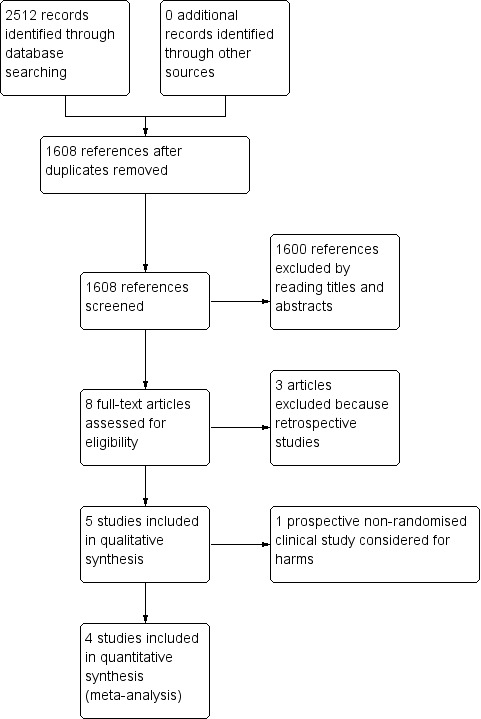
Study flow diagram. A total of 2512 records identified through electronic database search. After removing 904 duplicates, a total of 1608 references were screened for titles and abstracts, eight articles were selected for full‐text review. Of these, three articles were retrospective studies and one article was a prospective non‐randomised study. Four studies were selected for final meta‐analysis.
Included studies
The four randomised clinical trials that we selected for meta‐analysis are presented in Characteristics of included studies tables. Henderson 2006, Orloff 2012, Rosemurgy 2012, and Orloff 2015 randomised 496 participants, irrespective of sex, who were diagnosed with variceal haemorrhage secondary to liver cirrhosis into two interventions (surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt (TIPS)). In all the trials, diagnosis of liver cirrhosis was established by clinical and laboratory findings, then confirmed by liver biopsy at time of intervention. Participants in Henderson 2006 were Child‐Pugh class A or B liver disease. Orloff 2012, Rosemurgy 2012, and Orloff 2015 included participants with Child‐Pugh class A, B, or C liver disease. The predominant cause of variceal haemorrhage in all trials was alcohol‐related liver cirrhosis. All the trials were conducted in the USA, and an intention‐to‐treat principle was used for data analyses. Henderson 2006 randomised 140 participants into a selective surgical shunt (distal splenorenal shunt; DSRS) versus TIPS. Orloff 2012 randomised 154 participants with oesophageal variceal haemorrhage into a non‐selective surgical shunt (portocaval shunt) versus TIPS. Rosemurgy 2012 randomised 132 participants into a selective surgical shunt (8 mm H‐graft shunt) versus TIPS, while Orloff 2015 randomised 70 participants with gastric variceal haemorrhage into a non‐selective surgical shunt (portocaval shunt) versus TIPS. The mean age of the participants in Henderson 2006 was 53 ± 10 years, in Orloff 2012 it was 49 years, in Rosemurgy 2012 it was 55 ± 12 years, and in Orloff 2015 it was 50 years. Participants were followed up for a maximum period of eight years in Henderson 2006, 15 years in Orloff 2012, 18 years in Rosemurgy 2012, and 10 years in Orloff 2015. All the trials were funded by institutional grants.
Excluded studies
We excluded four studies from this meta‐analysis with reasons (Faust 1997; Zacks 1999; Khaitiyar 2000; Helton 2001) (Characteristics of excluded studies). Of these, three were retrospective studies (Faust 1997; Zacks 1999; Helton 2001), while one was a prospective non‐randomised study that grouped participants into distal splenorenal shunt compared to TIPS, that we considered only for harms (Khaitiyar 2000).
Risk of bias in included studies
The risks of bias in included trials have been summarised in Figure 2 and Figure 3. We considered all the trials to be at overall high risk of bias, due to lack of blinding of participants, personnel, or outcome assessors.
2.
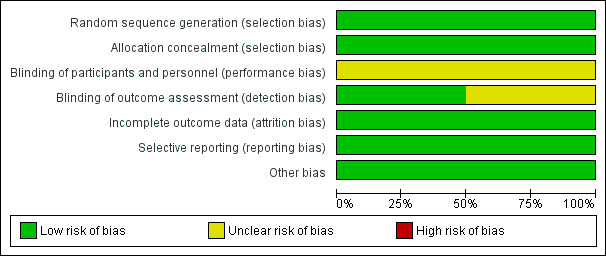
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
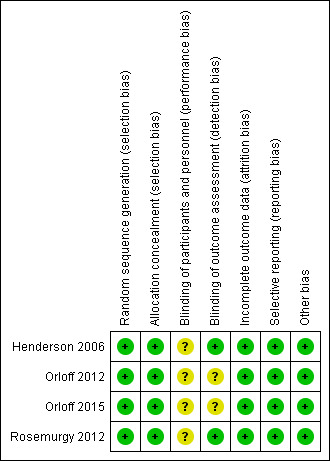
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation (selection bias)
We considered random sequence generation and allocation concealment to be adequate for all four trials included in our meta‐analysis. So review authors judged all four trials at low risks of selection bias.
Blinding (Performance and detection bias)
Participants and personnel were not blinded in any of the four trials (Henderson 2006; Orloff 2012; Orloff 2015, Rosemurgy 2012). Given the nature of the interventions, it was unrealistic to blind participants and personnel to the interventions. Lack of blinding in randomised clinical trials could overestimate intervention effects (Savović 2018). Given that in all four trials most outcomes were objective we judged all four trials to be at unclear risk of performance bias. We judged two trials that blinded outcome assessors to be at low risk of detection bias (Henderson 2006; Rosemurgy 2012). We judged two other trials that did not specify blinding of outcome assessors to be at unclear risk of detection bias (Orloff 2012; Orloff 2015).
Incomplete outcome data (attrition bias)
We observed that data analyses in all four trials were performed using an intention‐to‐treat principle, so we judged all trials at low risk of attrition bias.
Selective reporting (reporting bias)
We found published protocols for three trials in the clinicaltrials.gov registry (Henderson 2006; Orloff 2012; Orloff 2015). We could not find a published protocol for one trial but all prespecified outcomes in the methods were reported (Rosemurgy 2012). Review authors judged all trials to be at low risk of reporting bias.
Other potential sources of bias
For‐profit bias
We assessed all four trials for possible sources of funding and it was clear they were all funded by institutional grants. So we judged all trials at low risk for‐profit bias.
Effects of interventions
See: Table 1
Surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt
All‐cause mortality
Four trials provided data on all‐cause mortality (Henderson 2006; Orloff 2012; Orloff 2015; Rosemurgy 2012). We have analysed all‐cause mortality at 30 days and five years. We found no evidence of a difference in all‐cause mortality at 30 days between surgical shunts (analysed together) versus TIPS (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.44 to 1.99; participants = 496; studies = 4; I2 = 64%; Analysis 1.1). We found evidence suggesting a difference in all‐cause mortality at five years between surgical shunts (analysed together) versus TIPS (RR 0.61, 95% CI 0.42 to 0.90; participants = 496; studies = 4; I2 = 64%; Analysis 1.2).
1.1. Analysis.
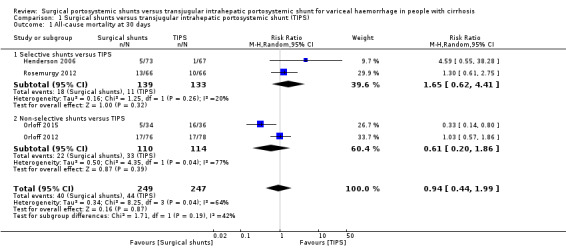
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 1 All‐cause mortality at 30 days.
1.2. Analysis.
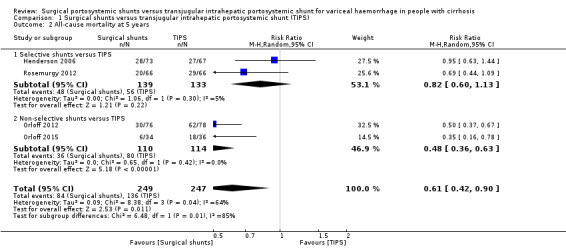
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 2 All‐cause mortality at 5 years.
Serious adverse events
None of the trial authors reported adverse events as a composite outcome. Following the ICH‐GCP 1997 definition of serious adverse events, we determined that, mortality, reinterventions, irreversible shunt occlusion, and encephalopathy were serious adverse events, and we presented data individually.
Health‐related quality of life
Two trials provided data on health‐related quality of life (Henderson 2006; Orloff 2015). Henderson 2006 used Short Form (SF)‐36 questionnaire and reported no difference in the median score for health‐related quality of life between surgical shunts versus TIPS. Orloff 2015 defined health‐related quality of life as: freedom from encephalopathy, long‐term shunt patency, abstinence from alcohol, improvement in liver function, improvement in Child‐Pugh class, return to work or housekeeping, and avoidance of the need for liver transplantation. We could not perform a meta‐analysis for this outcome. Based on these criteria, review authors concluded improved health‐related quality of life in the surgical shunts group compared with the TIPS group.
Variceal rebleed‐related mortality
Three trials provided data on variceal rebleed‐related mortality (Henderson 2006; Orloff 2012; Rosemurgy 2012). We found evidence of a difference in variceal rebleed‐related mortality at 30 days between surgical shunts (analysed together) versus TIPS (RR 0.18, 95% CI 0.04 to 0.82; participants = 426; studies = 3; I2 = 0%; Analysis 1.3).
1.3. Analysis.
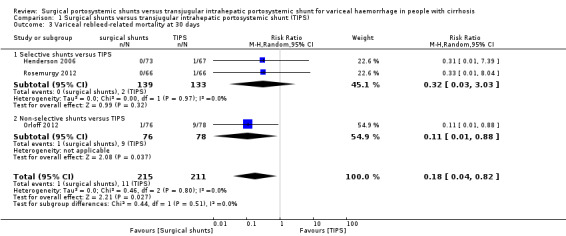
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 3 Variceal rebleed‐related mortality at 30 days.
Number of participants with variceal rebleeding episodes
Four trials provided data on variceal rebleeding episodes (Henderson 2006; Orloff 2012; Rosemurgy 2012; Orloff 2015). We considered variceal rebleeding occurring within 30 days of intervention as related to failure of intervention rather than disease progression. We found evidence suggesting a difference in variceal rebleeding episodes at 30 days between surgical shunts (analysed together) versus TIPS (RR 0.18, 95% CI 0.07 to 0.49; participants = 496; studies = 4; I2 = 0%; Analysis 1.4).
1.4. Analysis.
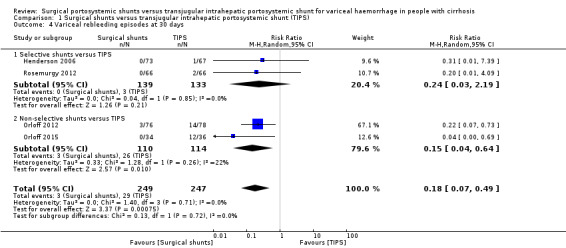
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 4 Variceal rebleeding episodes at 30 days.
Non‐serious adverse events
None of the four trials provided data on adverse events as a composite outcome.
Number of participants bridged to liver transplantation and were transplanted
We planned to report the number of participants who were bridged to liver transplantation and were transplanted. None of the trials bridged participants to liver transplantation.Two trials provided data only for participants who received liver transplantation as a definitive therapy (Henderson 2006; Rosemurgy 2012). We found no evidence of a difference in number of participants who received liver transplantation as a definitive therapy at 10 years between selective shunts versus TIPS (RR 0.46, 95% CI 0.13 to 1.65; participants = 272; studies = 2; I2 = 33%; Analysis 1.5).
1.5. Analysis.
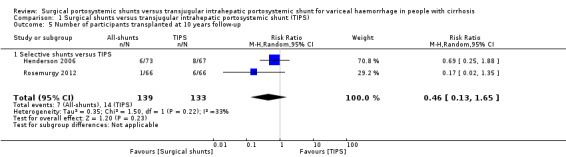
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 5 Number of participants transplanted at 10 years follow‐up.
Number of participants with reintervention
All four trials provided data on number of participants who required reintervention at five years. We found evidence of a difference in number of participants with reintervention at five years between surgical shunts (analysed together) versus TIPS (RR 0.13, 95% CI 0.06 to 0.28; participants = 496; studies = 4; I2 = 42%; Analysis 1.6).
1.6. Analysis.
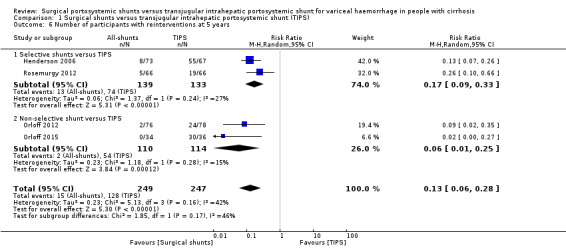
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 6 Number of participants with reinterventions at 5 years.
Irreversible shunt occlusion
All four trials provided data on irreversible shunt occlusion at five years. We found evidence of a difference in number of participants with irreversible shunt occlusion at five years between surgical shunts (analysed together) versus TIPS (RR 0.14, 95% CI 0.04 to 0.51; participants = 496; studies = 4; I2 = 59%; Analysis 1.7).
1.7. Analysis.
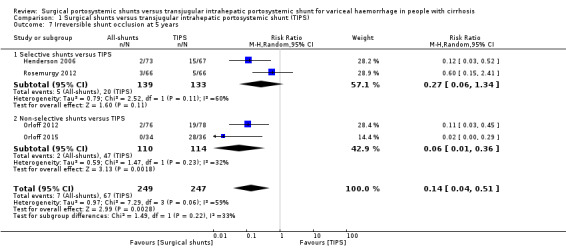
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 7 Irreversible shunt occlusion at 5 years.
Number of participants with encephalopathy
All four trials provided data on persistent or new‐onset encephalopathy at five years postintervention. We found no evidence of a difference in number of participants with persistent or new‐onset encephalopathy at five years (RR 0.56, 95% CI 0.27 to 1.16; participants = 496; studies = 4; I2 = 79%; Analysis 1.8).
1.8. Analysis.
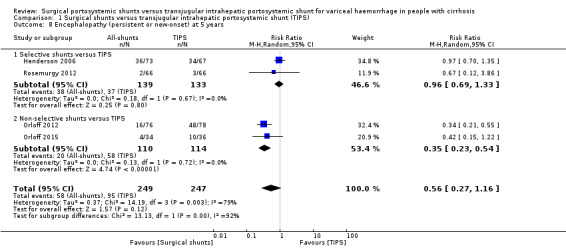
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 8 Encephalopathy (persistent or new‐onset) at 5 years.
Number of participants with clinically significant ascites
Two trials provided data on persistent or new‐onset ascites at five years (Henderson 2006; Rosemurgy 2012). We found no evidence of a difference in persistent or new‐onset ascites at five years between selective shunts versus TIPS (RR 0.93, 95% CI 0.60 to 1.44; participants = 272; studies = 2; I2 = 12%; Analysis 1.9).
1.9. Analysis.
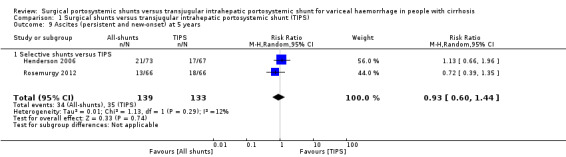
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 9 Ascites (persistent and new‐onset) at 5 years.
Subgroup analysis
We performed a subgroup analysis of selective shunts versus TIPS compared to non‐selective shunts versus TIPS for all outcomes. We observed no change in our overall estimates of all‐cause mortality at 30 days. The test for subgroup difference showed no difference for all‐cause mortality at 30 days, P = 0.19 (Analysis 1.1). We also observed no subgroup difference in variceal rebleed‐related mortality, P = 0.51 (Analysis 1.3); variceal rebleeding, P =0.72 (Analysis 1.4); reinterventions, P = 0.17 (Analysis 1.6); and irreversible shunt occlusion, P = 0.79 (Analysis 1.7). We observed a subgroup difference in estimates for all‐cause mortality at five years, P = 0.01 (Analysis 1.2); and encephalopathy, P = 0.0003 when selective shunts versus TIPS analysed separately was compared to non‐selective shunts versus TIPS analysed separately (Analysis 1.8). We observed that in performing subgroup analysis of selective shunts versus TIPS compared to non‐selective shunts versus TIPS, the significant heterogeneity observed in estimates of all‐cause mortality at 30 days and five years; irreversible shunt occlusion, and encephalopathy were eliminated. We also performed a subgroup analysis of survival at five years for Child‐Pugh risk class A, versus class B, versus class C between surgical shunts compared with TIPS. Three trials provided data on survival based on Child‐Pugh risk classes (Orloff 2012; Rosemurgy 2012; Orloff 2015). We found no evidence of a difference in survival at five years between surgical shunts versus TIPS for participants in Child‐Pugh class A versus B or C from two trials (Orloff 2012; Orloff 2015;Analysis 1.10). We did not include Rosemurgy 2012 in our subgroup analysis of survival at five years for Child‐Pugh risk class as the trial authors provided data as mean survival in months. Due to few trials we could not conduct the other prespecified subgroup analyses.
1.10. Analysis.
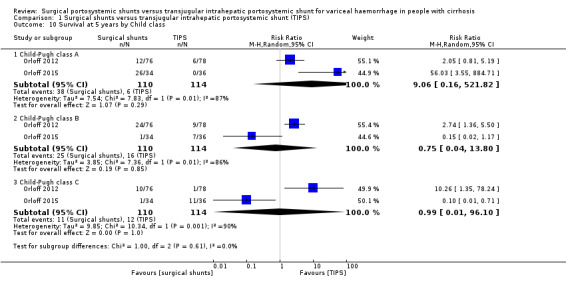
Comparison 1 Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS), Outcome 10 Survival at 5 years by Child class.
Sensitivity analysis
We investigated the source of significant heterogeneity in our estimates of all‐cause mortality (at 30 days and 5 years), and encephalopathy by excluding from our analysis two trials from the same authors. We observed that by doing this, the significant heterogeneity that occurred in our effect estimates was eliminated. Due to only four included trials, we did not perform planned sensitivity analysis of search methods for inclusion of trials; exclusion of trials; type of data analysed; process of data analysis; and measurement of intervention outcomes at six and 12 months.
Trial Sequential Analysis
We performed Trial Sequential Analysis for all our review outcomes. Due to few included trials with small sample sizes, the alpha spending boundary could not be drawn for all‐cause mortality at 30 days, variceal rebleed‐related mortality, number of participants who received liver transplantation, encephalopathy, and ascites. We calculated diversity‐adjusted required information size (DARIS) by taking into consideration the control group event proportion observed in the meta‐analysis; a plausible relative risk reduction of 20%; a risk of type I error of 2.5% due to three primary outcomes and three secondary outcomes; a risk of type II error of 10%; and the adjusted diversity from the meta‐analysis. We calculated a DARIS of 3400 participants for all‐cause mortality at five years (Figure 4); 8304 participants for rebleeding episodes (Figure 5); 2594 for number of participants with reinterventions (Figure 6); and 7438 for irreversible shunt occlusion. Due to few trials with small sample sizes, the cumulative Z‐curves did not cross the alpha spending boundaries for all‐cause mortality, rebleeding, reinterventions, and shunt occlusion, suggesting the need to conduct further trials to attain the true intervention effect.
4.
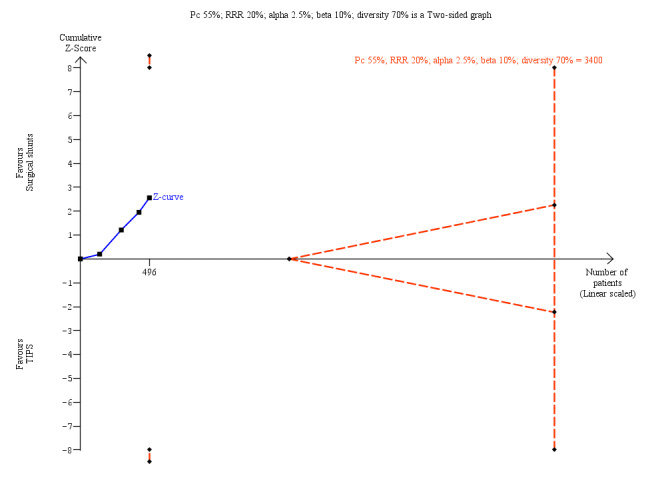
Trial Sequential Analysis for all‐cause mortality at 5 years calculated based on control event rate of 55%; a RRR of 20%; a type I error of 2.5%, a type II error of 10%; and diversity of 70%. The DARIS of 3400 was not achieved and Z‐curve did not cross the alpha spending boundary, (RR 0.61, alpha spending boundary adjusted CI 0.13 to 2.86).
5.
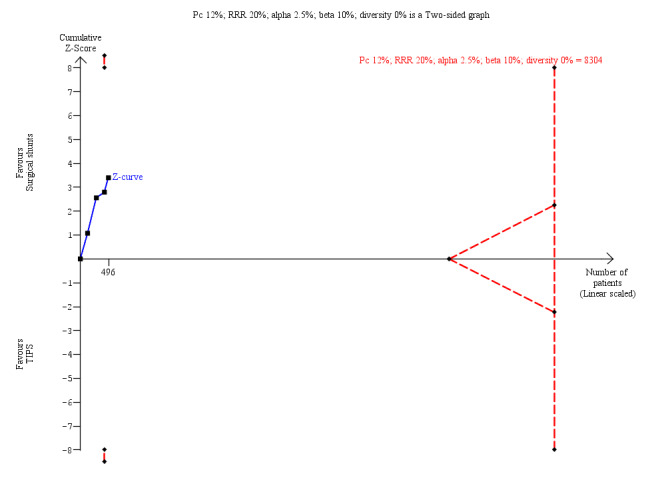
Trial Sequential Analysis for variceal rebleeding episodes at 30 days is calculated based on control event rate of 12%; a RRR of 20%; a type I error of 2.5%, a type II error of 10%; and diversity of 0%. The DARIS of 8304 was not achieved and Z‐curve did not cross the alpha‐spending boundary (RR 0.18, alpha‐spending boundary‐adjusted CI 0.00 to 10.31).
6.
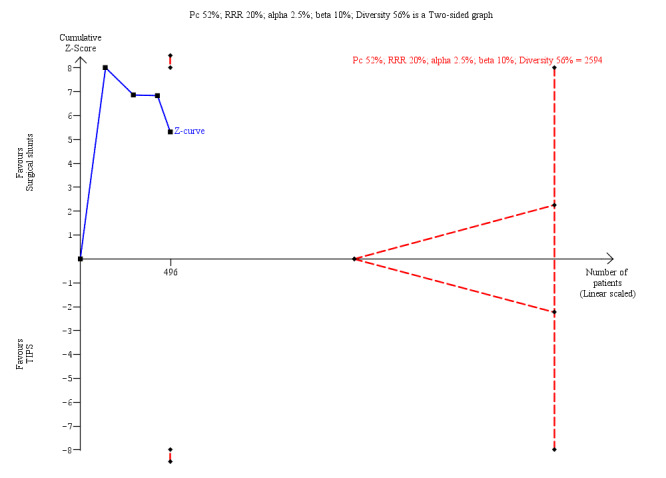
Trial Sequential Analysis for number of participants with reinterventions at 5 years is calculated based on control event rate of 52%; a RRR of 20%; a type I error of 2.5%, a type II error of 10%; and diversity of 56%. The DARIS of 2594 was not achieved and Z‐curve did not cross the alpha‐spending boundary (RR 0.13, alpha‐spending boundary‐adjusted CI 0.01 to 2.80).
Certainty of the evidence
We have presented the certainty of the evidence in Table 1. We judged the certainty of the evidence for all‐cause mortality (at 30 days and 5 years), irreversible shunt occlusion, and encephalopathy as very low because of overall high risk of bias (downgraded one level due to lack of blinding), inconsistency (downgraded one level due to heterogeneity); imprecision (downgraded two levels due to small sample sizes and few events); and publication bias (downgraded one level due to few trials). We judged the certainty of the evidence for variceal rebleeding and reinterventions as very low because of overall high risk of bias (downgraded one level due to lack of blinding), imprecision (downgraded one level due to small sample sizes and few events), and publication bias (downgraded one level due to few trials).
Discussion
Summary of main results
We identified only four small single‐centre randomised clinical trials at high risk of bias that compared surgical portosystemic shunts with transjugular intrahepatic portosystemic shunt for treatment of refractory or recurrent variceal haemorrhage in 496 people with cirrhotic portal hypertension. The trials were all conducted in the USA. Two of the trials compared selective portosystemic shunts (distal splenorenal shunt (DSRS) and 8‐mm H‐graft stent) to TIPS in elective setting. The other two trials compared non‐selective shunt (portocaval shunt) to TIPS in emergency setting. We found no evidence of a difference between surgical shunt versus TIPS for all‐cause mortality at 30 days and encephalopathy at five years of follow‐up. There appeared to be very low‐certainty of evidence suggesting an increase in the development of all‐cause mortality at five years; variceal‐bleed related mortality; variceal rebleeding; reintervention; and irreversible shunt occlusion in the TIPS group compared with surgical shunts (considered together). Two trials provided data on health‐related quality of life that appeared to suggest better health‐related quality of life after portocaval shunts compared with TIPS. We identified one small prospective non‐randomised study from India, including 67 participants with cirrhotic portal hypertension that received DSRS (n = 32); and TIPS (n = 35). This study was considered for harms (Khaitiyar 2000; Table 2). The result from this study appears to suggest an increase in the development of adverse events with TIPS compared to surgical shunts. Our sensitivity analyses showed that significant heterogeneity observed in effect estimates for all‐cause mortality (at 30 days and five years), and encephalopathy corrected when two trials conducted by the same authors were eliminated in the meta‐analyses. This could be due to the type of surgical shunts used and the emergency setting that these shunts were placed. Because of the very low‐certainty of the evidence for all outcomes encompassing high risk of bias (due to lack of blinding), inconsistency (due to heterogeneity), imprecision (due to small sample size and few events), and publication bias (due to few trials reporting outcomes), we are uncertain in our estimates of intervention effects. Furthermore, the small sample sizes and few events did not allow us to produce meaningful trial sequential monitoring boundaries, suggesting a plausible random error in our estimates.
1. Harms of interventions from Khaitiyar 2000.
| No | Outcomes | DSRS (N = 32) | TIPS (N = 35) | Fisher exact test |
| 1 | All‐cause mortality at 30 days | 2 | 2 | P = 1 |
| 2 | All‐cause mortality at 2 years | 6 | 7 | P = 1 |
| 2 | Rebleed‐related mortality at 2 years | 2 | 9 | P = 0.05 |
| 3 | Variceal rebleeding episodes at 2 years | 2 | 9 | P = 0.05 |
| 4 | Shunt occlusion at 2 years | 2 | 24 | P = 0.00001 |
| 5 | Encephalopathy at 2 years | 6 | 15 | P = 0.04 |
| 6 | Ascites at 2 years | 4 | 13 | P = 0.03 |
DSRS: distal splenorenal shunt; TIPS: transjugular intrahepatic portosystemic shunt
Overall completeness and applicability of evidence
Our confidence in estimates of intervention effects is very low because of a small number of available trials including few participants, and risk of random error in our estimates. The participants included in all the trials had cirrhotic portal hypertension confirmed by histopathological investigation and so the findings of this review are applicable to people with variceal haemorrhage due to cirrhotic portal hypertension. Two of the trials included participants who received interventions in the emergency setting (the intervention was performed within 24 hours of contact with the trial team). It appeared that the results of these two trials introduced heterogeneity in our estimates. Considering that these trials were conducted by the same authors with vast experience in emergency portocaval shunts, their results may not be applicable in a different setting. All the trials were performed at the time when TIPS with polytetrafluoroethylene (PTFE)‐covered stents were not common. So the findings of this review may not be applicable to TIPS with PTFE‐covered stents which have been shown to improve patency and require less interventions than TIPS with bare metal stents (Clark 2011; Perarnau 2014).
Certainty of the evidence
We judged the four trials at high risk of bias. There was significant heterogeneity in the analysis of all‐cause mortality and encephalopathy, mainly because of two trials from the same authors that compared non‐selective surgical shunt versus TIPS in the emergency setting. We downgraded the certainty of the evidence for all‐cause mortality at 30 days and five years, irreversible shunt occlusion, and encephalopathy to very low because of high risk of bias (due to lack of blinding), inconsistency (due to heterogeneity), imprecision (due to small sample sizes of the individual trials and few events), and publication bias (few trials reporting outcomes). We graded the certainty of the evidence for variceal rebleeding and reintervention as very low because of high risk of bias (due to lack of blinding), imprecision (due to small sample sizes of the individual trials and few events), and publication bias (few trials reporting outcomes).
Potential biases in the review process
We performed an extensive search of databases according to the recommendation of Cochrane. We searched electronic databases for any randomised clinical trials that had included participants with variceal haemorrhage due to non‐cirrhotic portal hypertension. We considered participants treated with surgical portosystemic shunts or TIPS either in the elective or emergency setting. Although our search strategies were very broad, we only retrieved four randomised clinical trials that fulfilled the inclusion criteria of our review. A reason for the paucity of the trials of interest to our review could be the wide availability and popularity of non‐surgical interventions, such as endoscopy. Among the retrieved study references was one small size prospective non‐randomised study that included the following harms: all‐cause mortality, rebleed‐related mortality, variceal rebleeding, shunt occlusion, reinterventions, encephalopathy, and ascites. The respective references to the included trials did not provide any further references to the topic of our review. We found no relevant observational studies reporting on harms among the search result for randomised clinical trials, and this is a known limitation for meta‐analyses with randomised clinical trials alone. Because of the very small number of trials with small sizes, we could not produce meaningful Trial Sequential Analysis monitoring boundaries, and this underlines a high risk of random error in our review analysis. We could not construct funnel plots in order to look for publication bias. Thus, the risk of random errors because of paucity of trials and very low‐certainty of the evidence contributed to the uncertainty in our review findings.
Agreements and disagreements with other studies or reviews
We found two systematic reviews that compared surgical portosystemic shunts versus TIPS for treatment of variceal haemorrhage in people with cirrhotic portal hypertension (Clark 2010; Huang 2015). Clark 2010 included 314 participants of Child‐Pugh risk class A and class B from four studies in their review. Two of the studies were randomised clinical trials while the other two were a prospective non‐randomised study and a retrospective study. Although the authors reported their findings of two‐year follow‐up, there was no difference in all‐cause mortality at 30 days between surgical shunts compared with TIPS. There was an increase in all‐cause mortality at two years, and the number of shunt failures in the TIPS group compared with surgical shunts is very similar to our systematic review analysis. Huang 2015 included 493 participants, irrespective of Child‐Pugh risk class, from four studies in their review. One of the studies was a prospective non‐randomised study and the other three were randomised clinical trials included in our review. There was a significant heterogeneity in their analyses similar to our review, and the authors did not grade the quality of their evidence. The results of their meta‐analyses showed there was no evidence of a difference in mortality at 30 days between surgical shunts and TIPS. There was evidence of an increase in occurrence of variceal rebleeding, shunt occlusion, and encephalopathy in the TIPS group compared with surgical shunt that is very similar to our review findings.
Authors' conclusions
Implications for practice.
We found evidence suggesting that surgical portosystemic shunts may have benefits over transjugular intrahepatic portosystemic shunt (TIPS) for treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension. Given the very low‐certainty of the available evidence and risk of random error in our analyses, we have very little confidence in our review findings.
Implications for research.
Future randomised clinical trials including a large number of participants are required to address bias issues as well as play of chance due to random errors. Such trials should be multicentred in order to achieve sufficient statistical power to produce true intervention effects. TIPS intervention should include covered stents to assess benefit or harm of TIPS with covered stents compared to TIPS with bare metal stents in subgroup analyses. These trials should be registered and be given open access (Skoog 2015), with their protocols drafted according to the SPIRIT statement (Chan 2013), and their reporting according to the CONSORT statement (Schulz 2010).
Notes
CJ Ede joined the authors after the protocol was published.
Acknowledgements
S Khan et al. drafted a protocol in 1998 for this review title (Khan 1998). We used the original protocol as the starting point for the current review.
We would like to thank Dimitrinka Nikolova for her help during the preparation of the protocol and review, and Sarah Klingenberg for assistance with development of the search strategies.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers: Liu Fuquan, China; Jean‐Marc Perarnau, France; Naimish Mehta, India. Contact editors: Stefano Trastulli, Italy. Sign‐off editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | 8 March 2018 | ((portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt* or anastomos*)) AND (varic* and (hemorrhag* or Haemorrhage* or bleed* or rebleed*)) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2018, Issue 2 | #1 MeSH descriptor: [Portasystemic Shunt, Surgical] explode all trees #2 (portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt* or anastomos*) #3 'dean warren shunt*' or H‐shunt* or TIPS or PSS #4 #1 or #2 or #3 #5 MeSH descriptor: [Esophageal and Gastric Varices] explode all trees #6 MeSH descriptor: [Liver Cirrhosis] explode all trees #7 varic* and (h*emorrhag* or bleed* or rebleed*) #8 #5 or #6 or #7 #9 #4 and #8 |
| MEDLINE Ovid | 1946 to 8 March 2018 | 1. exp Portasystemic Shunt, Surgical/ 2. ((portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt* or anastomos*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 3. ('dean warren shunt*' or H‐shunt* or TIPS or PSS).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 4. 1 or 2 or 3 5. exp "Esophageal and Gastric Varices"/ 6. exp Liver Cirrhosis/ 7. (varic* and (h*emorrhag* or bleed* or rebleed*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 8. 5 or 6 or 7 9. 4 and 8 10. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 11. 9 and 10 |
| Embase Ovid | 1974 to 8 March 2018 | 1. exp portosystemic anastomosis/ 2. ((portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt* or anastomos*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. ('dean warren shunt*' or H‐shunt* or TIPS or PSS).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 4. 1 or 2 or 3 5. exp esophagus varices/ 6. exp liver cirrhosis/ 7. (varic* and (h*emorrhag* or bleed* or rebleed*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 8. 5 or 6 or 7 9. 4 and 8 10. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 11. 9 and 10 |
| Science Citation Index Expanded (Web of Science) |
1900 to 8 March 2018 | #7 #6 AND #5 #6 TS=(random* or blind* or placebo* or meta‐analys*) #5 #4 AND #3 #4 TS=(varic* and (h*emorrhag* or bleed* or rebleed*)) #3 #2 OR #1 #2 TS=('dean warren shunt*' or H‐shunt* or TIPS or PSS) #1 TS=((portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt* or anastomos*)) |
| Conference Proceedings Citation Index ‐ Science (Web of Science) | 1990 to 8 March 2018 | #7 #6 AND #5 #6 TS=(random* or blind* or placebo* or meta‐analys*) #5 #4 AND #3 #4 TS=(varic* and (h*emorrhag* or bleed* or rebleed*)) #3 #2 OR #1 #2 TS=('dean warren shunt*' or H‐shunt* or TIPS or PSS) #1 TS=((portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt* or anastomos*)) |
| LILACS (Bireme) | 1982 to 8 March 2018 | ((portosystemic or portasystemic or portacaval or mesocaval or splenorenal or surgical or radiological or intrahepatic or selective or non‐selective or partial or total) and (shunt$ or anastomos$)) [Words] and (dean warren shunt$ or H‐shunt$ or TIPS or PSS) [Words] and (varic$ and (h$morrhag$ or bleed$ or rebleed$)) [Words] |
Data and analyses
Comparison 1. Surgical shunts versus transjugular intrahepatic portosystemic shunt (TIPS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality at 30 days | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.44, 1.99] |
| 1.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.62, 4.41] |
| 1.2 Non‐selective shunts versus TIPS | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.20, 1.86] |
| 2 All‐cause mortality at 5 years | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.90] |
| 2.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.60, 1.13] |
| 2.2 Non‐selective shunts versus TIPS | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.36, 0.63] |
| 3 Variceal rebleed‐related mortality at 30 days | 3 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.82] |
| 3.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.03] |
| 3.2 Non‐selective shunts versus TIPS | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.88] |
| 4 Variceal rebleeding episodes at 30 days | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.07, 0.49] |
| 4.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 2.19] |
| 4.2 Non‐selective shunts versus TIPS | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.64] |
| 5 Number of participants transplanted at 10 years follow‐up | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.13, 1.65] |
| 5.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.13, 1.65] |
| 6 Number of participants with reinterventions at 5 years | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.06, 0.28] |
| 6.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.33] |
| 6.2 Non‐selective shunt versus TIPS | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.25] |
| 7 Irreversible shunt occlusion at 5 years | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.51] |
| 7.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.06, 1.34] |
| 7.2 Non‐selective shunts versus TIPS | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.36] |
| 8 Encephalopathy (persistent or new‐onset) at 5 years | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.27, 1.16] |
| 8.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.69, 1.33] |
| 8.2 Non‐selective shunts versus TIPS | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.54] |
| 9 Ascites (persistent and new‐onset) at 5 years | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.44] |
| 9.1 Selective shunts versus TIPS | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.44] |
| 10 Survival at 5 years by Child class | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Child‐Pugh class A | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 9.06 [0.16, 521.82] |
| 10.2 Child‐Pugh class B | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.04, 13.80] |
| 10.3 Child‐Pugh class C | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.01, 96.10] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Henderson 2006.
| Methods | Randomised clinical trial (DIVERT Study) | |
| Participants | Country: USA Number randomised: 140 participants Analysis: intention‐to‐treat Age: mean age 53 ± 10 years for DSRS, and 52 ± 1 years for TIPS Initial endoscopic assessment, including therapy before randomisations Duration of follow‐up: 8 years Inclusion criteria
Exclusion criteria
Prominent cause of liver cirrhosis: alcohol‐related |
|
| Interventions | Distal splenorenal shunt (n = 73) versus TIPS (n = 67) Only uncovered stents of variable sizes from 8 mm to 12 mm used for TIPS Intervention performed within five days of randomisation |
|
| Outcomes |
Primary outcomes: variceal rebleeding, and encephalopathy Secondary outcomes: mortality, ascites, shunt stenosis and thrombosis, need for liver transplantation, liver tests, quality of life, and cost |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised. 'Randomisation' was controlled by a central data co‐ordinating centre |
| Allocation concealment (selection bias) | Low risk | Treatment allocation achieved with use of "a variable 2 or 4 permuted block size design, stratifying by Child‐Pugh class A and B, and alcoholic and nonalcoholic patients". By randomly varying the block sizes, treatment allocation could not be predicted towards the end of a block. |
| Blinding of participants and personnel (performance bias) All‐cause mortality; serious adverse events, variceal rebleed‐related mortality, variceal rebleeding, non‐serious adverse events. | Unclear risk | Participants and personnel were not blinded to the interventions received. Given the nature of the interventions, it was unrealistic to blind participants and study personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The Clinical Endpoint Review Committee received summary data on all outcomes in a blinded manner from the data co‐ordinating centre. Blinding of outcome assessors judged as adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published (NCT00006161). Reported all protocol predefined outcomes |
| Other bias | Low risk | Study sponsored by institutional grant |
Rosemurgy 2012.
| Methods | Randomised clinical trials | |
| Participants | Country: USA Number randomised: 132 participants Intention‐to‐treat analysis Mean age 54 ± 14 years for H‐shunt and 55 ± 12 years for TIPS Maximal duration of follow‐up: 18 years Inclusion criteria Child‐Pugh A, B, C cirrhosis and portal hypertension with variceal or portal gastropathy bleeding who have failed or not candidates for endoscopic therapy. Median MELD score 13 Exclusion criteria Participants with unfavourable anatomy or unlikely to survive because of profound ill‐health or hepatic dysfunction were excluded. |
|
| Interventions | TIPS (66), H‐graft shunt 8mm (66) Intervention performed as a definitive procedure, never as a bridge to transplantation |
|
| Outcomes | Mortality, variceal rebleeding, shunt occlusion, liver failure requiring transplantation, inability to accomplish shunting, ascites, and encephalopathy | |
| Notes | Shunt failure was referred to as mortality, variceal rebleeding, irreversible shunt occlusion, liver failure requiring transplantation, and inability to accomplish shunting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by parallel allocation |
| Allocation concealment (selection bias) | Low risk | Computer generated sequential allocation |
| Blinding of participants and personnel (performance bias) All‐cause mortality; serious adverse events, variceal rebleed‐related mortality, variceal rebleeding, non‐serious adverse events. | Unclear risk | Participants were blinded to the intervention received. The surgeon was only blinded to the type of shunt to be assigned. Given the nature of the interventions, it was unrealistic to blind participants to the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel were blinded to the intervention received. "A senior faculty hepatologist/gastroenterologist was "blinded" to the intervention received and evaluated participants for encephalopathy during the clinic visits". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat principle used for data analysis |
| Selective reporting (reporting bias) | Low risk | Trial protocol was not found but predetermined outcomes in methods section were all reported |
| Other bias | Low risk | Trial supported by institutional grant |
Orloff 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 154 participants Analysis: intention‐to‐treat Age: mean age 49 years for both groups Initial combined endoscopic sclerotherapy and pharmacotherapy before randomisations Duration of follow‐up: 15 years Inclusion criteria
Exclusion criteria
Prominent cause of liver cirrhosis: alcohol‐related |
|
| Interventions | Emergency portocaval shunt (n = 76) versus emergency TIPS (n = 78) Either side‐to‐side or end‐to‐side portocaval shunt performed Only uncovered stents of variable sizes from 12 mm and above were used for TIPS Interventions performed within 24 hours of contact with study personnel Biopsy of liver performed during intervention |
|
| Outcomes | Survival, variceal rebleeding, encephalopathy, health‐related quality of life, and economic costs | |
| Notes | Treatment failure was defined as: variceal bleeding requiring six or more units of blood over and above normal transfusion requirements in first 8 days after study entry; variceal rebleeding that required eight units of blood transfusion during any 12‐month period; or variceal rebleeding after participant deemed cured following endoscopy. Rescue therapy was defined as use of alternative intervention when the primary therapy was declared a failure. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation achieved by a central computer‐generated random blocks |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed to investigators by "drawing an instruction card from a serially numbered, opaque, sealed envelope". |
| Blinding of participants and personnel (performance bias) All‐cause mortality; serious adverse events, variceal rebleed‐related mortality, variceal rebleeding, non‐serious adverse events. | Unclear risk | Participants and personnel were not blinded to the intervention received. Given the nature of the interventions, it was unrealistic to blind participants and study personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This information is not clear from the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat principle applied |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published (NCT00734227). Reported protocol's predetermined outcomes |
| Other bias | Low risk | Study sponsored by institutional grant |
Orloff 2015.
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised:70 participants representing a subset in an extended trial Analysis: intention‐to‐treat Age: mean age 50 years for both groups Initial endoscopic therapy before randomisations Duration of follow‐up: 10.5 years Inclusion criteria
Exclusion criteria
Prominent cause of liver cirrhosis: alcohol‐related |
|
| Interventions | Emergency portocaval shunt (n = 34 ) versus emergency TIPS (n = 36 ) Either side‐to‐side or end‐to‐side portocaval shunt performed Only uncovered stents of variable sizes from 10 mm and above were used for TIPS Interventions performed within 24 hours of contact with study personnel Biopsy of liver performed during intervention |
|
| Outcomes | Mortality, variceal rebleeding, encephalopathy, health‐related quality of life, and economic costs | |
| Notes | Treatment failure was defined as persistent or recurrent portal hypertension‐related bleeding: requiring transfusion of 4 or more units of packed red blood cells or whole blood during the first 7 days after intervention; requiring transfusion of 8 or more units after the first 7 days; or requiring transfusion of 8 or more units of blood during any 12‐month period; after the attending faculty endoscopist had previously declared the gastric varices obliterated or gone. Crossover rescue therapy not included in protocol but, "whenever possible, every effort was made to facilitate rescue treatment". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation achieved by a central computer‐generated random blocks |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed to investigators by "drawing an instruction card from a serially numbered, opaque, sealed envelope". |
| Blinding of participants and personnel (performance bias) All‐cause mortality; serious adverse events, variceal rebleed‐related mortality, variceal rebleeding, non‐serious adverse events. | Unclear risk | Participants and personnel were not blinded to the intervention received. Given the nature of the interventions, it was unrealistic to blind participants and study personnel to the intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This information is not clear from the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | Trial protocol was published (NCT00820781). Reported protocol's predetermined outcomes |
| Other bias | Low risk | Study sponsored by institutional grant |
DSRS ‐ distal splenorenal shunt; MELD ‐ model for end‐stage liver disease; TIPS ‐ transjugular intrahepatic portosystemic shunt
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
|---|---|
| Helton 2001 | Retrospective case control study |
| Khaitiyar 2000 | Prospective non‐randomised study |
| Faust 1997 | Retrospective case control study |
| Zacks 1999 | Retrospective case control study |
Differences between protocol and review
We improved the wording of the review objectives as follows: 'To assess the benefits and harms of surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt (TIPS) for the treatment of refractory or recurrent variceal haemorrhage in people with cirrhotic portal hypertension'. (The protocol objectives read: 'To compare the benefits and harms of surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt (TIPS) in the treatment of chronic variceal haemorrhage').
We analysed outcomes at fixed time points instead of the protocol's prespecified maximal follow‐up. This is because of wide variations in maximal follow‐up in the trials.
We added an exploratory outcome: 'irreversible shunt occlusion' to identify participants who had shunt failure despite reinterventions.
We included emergency shunt procedures in the review, as one trial was specifically designed to investigate shunts in the management of acute variceal haemorrhage (Orloff 2012), and another trial included both elective and emergency shunt procedures and we were not able to differentiate the results of these two groups from their manuscript (Rosemurgy 2012).
CJ Ede was added as an author for his contributions to this review
Contributions of authors
MB and LP drafted the current protocol. CJE and LP completed the trial selection. CJE and LP completed the data extraction and entry into RevMan. CJE performed the meta‐analyses, Trial Sequential Analysis, and GRADE. MB, CJE and LP agreed on the manuscript.
Sources of support
Internal sources
Department of Surgery, University of the Witwatersrand, South Africa.
External sources
No sources of support supplied
Declarations of interest
MB has on conflict of interest to declare LP has no conflict of interest to declare CJE has no conflict of interest to declare
New
References
References to studies included in this review
Henderson 2006 {published data only}
- Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006;130(6):1643‐51. [PUBMED: 16697728] [DOI] [PubMed] [Google Scholar]
Orloff 2012 {published data only}
- Orloff MJ, Vaida F, Haynes KS, Hye RJ, Isenberg JI, Jinich‐Brook H. Randomized controlled trial of emergency transjugular intrahepatic portosystemic shunt versus emergency portacaval shunt treatment of acute bleeding esophageal varices in cirrhosis. Journal of Gastrointestinal Surgery 2012;16(11):2094‐111. [PUBMED: 23007280] [DOI] [PubMed] [Google Scholar]
Orloff 2015 {published data only}
- Orloff MJ, Hye RJ, Wheeler HO, Isenberg JI, Haynes KS, Vaida F, et al. Randomized trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt versus portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis. Surgery 2015;157(6):1028‐45. [PUBMED: 25957003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosemurgy 2012 {published data only}
- Rosemurgy AS, Frohman HA, Teta AF, Luberice K, Ross SB. Prosthetic H‐graft portacaval shunts vs transjugular intrahepatic portasystemic stent shunts: 18‐year follow‐up of a randomized trial. Journal of the American College of Surgeons 2012;214(4):445‐53; discussion 453‐5. [PUBMED: 22463885] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Faust 1997 {published data only}
- Faust TW, Sorrell MF. Pre‐liver transplant: TIPS versus distal splenorenal shunt. HPB Surgery 1997;10(4):261‐5. [PUBMED: 9184884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helton 2001 {published data only}
- Helton WS, Maves R, Wicks K. Transjugular intrahepatic portosystemic shunt vs surgical shunt in good‐risk cirrhotic patients: a case control study. Archives of Surgery 2001;136:17‐20. [DOI] [PubMed] [Google Scholar]
Khaitiyar 2000 {published data only}
- Khaitiyar JS, Luthra SK, Prasad N, Ratnakar N, Daruwala DK. Transjugular intrahepatic portosystemic shunt versus distal splenorenal shunt ‐ a comparative study. Hepato‐gastroenterology 2000;47(32):492‐7. [PUBMED: 10791220] [PubMed] [Google Scholar]
Zacks 1999 {published data only}
- Zacks SL, Sandler RS, Biddle AK, Mauro MA, Brown RS Jr. Decision‐analysis of transjugular intrahepatic portosystemic shunt versus distal splenorenal shunt for portal hypertension. Hepatology (Baltimore, Md.) 1999;29(5):1399‐405. [PUBMED: 10216122] [DOI] [PubMed] [Google Scholar]
Additional references
Amitrano 2012
- Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short‐term prognosis and risk factors. American Journal of Gastroenterology 2012;107(12):1872‐8. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Carbonell 2004
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology (Baltimore, Md.) 2004;40(3):652‐9. [DOI] [PubMed] [Google Scholar]
Castellini 2017
- Castellini G, Nielsen EE, Gluud C. Comment on: "Cell therapy for heart disease: trial sequential analyses of two Cochrane reviews". Clinical Pharmacology and Therapeutics 2017;102(1):21‐4. [DOI] [PubMed] [Google Scholar]
Chalasani 2003
- Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. American Journal of Gastroenterology 2003;98(3):653‐9. [PUBMED: 12650802] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ (Clinical research ed.) 2013;346:e7586. [PUBMED: 23303884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Child 1964
- Child CG, Turcotte JG. Surgery and portal hypertension. In: Child CG editor(s). The Liver and Portal Hypertension. Philadelphia: W.B. Saunders Co, 1964:50. [Google Scholar]
Clark 2010
- Clark W, Hernandez J, McKeon B, Villadolid D, Al‐Saadi S, Mullinax J, et al. Surgical shunting versus transjugular intrahepatic portosystemic shunting for bleeding varices resulting from portal hypertension and cirrhosis: a meta‐analysis. American Surgeon 2010;76(8):857‐64. [PubMed] [Google Scholar]
Clark 2011
- Clark W, Golkar F, Luberice K, Toomey P, Paul H, Marcadis A, et al. Uncovering the truth about covered stents: is there a difference between covered versus uncovered stents with transjugular intrahepatic portosystemic shunts?. American Journal of Surgery 2011;202(5):561‐4. [DOI] [PubMed] [Google Scholar]
Costa 2010
- Costa G, Cruz RJ Jr, Abu‐Elmagd KM. Surgical shunt versus TIPS for treatment of variceal hemorrhage in the current era of liver and multivisceral transplantation. Surgical Clinics of North America 2010;90:891‐905. [DOI] [PubMed] [Google Scholar]
Cowgill 2006
- Cowgill SM, Carey E, Villadolid D, Al‐Saadi S, Zervos EE, Rosemurgy AS. Preshunt liver function remains the prominent determinant of survival after portasystemic shunting. American Journal of Surgery 2006;192:617‐21. [DOI] [PubMed] [Google Scholar]
D'Amico 2003
- D'Amico G, Franchis R, Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology (Baltimore, Md.) 2003;38(3):599‐612. [DOI] [PubMed] [Google Scholar]
de Franchis 2010
- Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of Hepatology 2010;53:762‐8. [DOI] [PubMed] [Google Scholar]
DeDombal 1986
- DeDombal FT, Clarke JR, Clamp SE, Malizia G, Kotwal MR, Morgan AG. Prognostic factors in upper GI bleeding. Endoscopy 1986;18(Suppl 2):6‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dhiman 2007
- Dhiman RK, Jain S, Maheshwari U, Bhalla A, Sharma N, Ahluwalia J, et al. Early indicators of prognosis in fulminant hepatic failure: an assessment of the model for end‐stage liver disease (MELD) and King's College Hospital criteria. Liver Transplantation 2007;13:814‐21. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 1922
- Fisher RA. On the Interpretation of X2 from Contingency Tables, and the Calculation of P. Journal of the Royal Statistical Society 1922;85(1):94‐87. [Google Scholar]
Garbuzenko 2016
- Garbuzenko DV. Current approaches to the management of patients with liver cirrhosis who have acute esophageal variceal bleeding. Current Medical Research Opinion 2016;32(3):467‐75. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 1985
- Garcia‐Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology (Baltimore, Md.) 1985;5(3):419‐24. [PUBMED: 3873388] [DOI] [PubMed] [Google Scholar]
Gluud 2018
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2018, Issue 3. Art. No.: LIVER.
Goff 1993
- Goff JS. Gastroesophageal varices: pathogenesis and therapy of acute bleeding. Gastroenterology Clinics of North America 1993;187:413‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Graham 1981
- Graham DY, Smith JL. The course of patients after variceal haemorrhage. Gastroenterology 1981;80:800‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ risk of bias. Journal of Clinical Epidemiology 2011;64(4):407‐15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing Summary of Findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [DOI] [PubMed] [Google Scholar]
Guyatt 2013d
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso‐Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14‐22. [PUBMED: 28529188] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hrobjartsson 2012
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ (Clinical research ed.) 2012;344:e1119. [PUBMED: 22371859] [DOI] [PubMed] [Google Scholar]
Hrobjartsson 2013
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ : Canadian Medical Association Journal 2013;185(4):E201‐11. [PUBMED: 23359047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrobjartsson 2014a
- Hrobjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub‐studies. International Journal of Epidemiology 2014a;43(4):1272‐83. [PUBMED: 24881045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrobjartsson 2014b
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Rasmussen JV, Hilden J, et al. Observer bias in randomized clinical trials with time‐to‐event outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. International Journal of Epidemiology 2014b;43(3):937‐48. [PUBMED: 24448109] [DOI] [PubMed] [Google Scholar]
Huang 2015
- Huang L, Yu QS, Zhang Q, Liu JD, Wang Z. Transjugular intrahepatic portosystemic shunt versus surgical shunting in the management of portal hypertension. Chinese Medical Journal 2015;128(6):826‐34. [PUBMED: 25758281] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamal 2008a
- Jamal MM, Samarasena JB, Hashemzadeh M, Vega KJ. Declining hospitalization rate of esophageal variceal bleeding in the United States. Clinical Gastroenterology and Hepatology 2008;6(6):689‐95. [DOI] [PubMed] [Google Scholar]
Jamal 2008b
- Jamal MM, Samarasena JB, Hashemzadeh M. Decreasing in‐hospital mortality for oesophageal variceal hemorrhage in the USA. Eurupean Journal of Gastroenterology and Hepatology 2010;20(10):947‐55. [DOI] [PubMed] [Google Scholar]
Kaplan 2015
- Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, et al. VOCAL Study Group. Development and performance of an algorithm to estimate the Child‐Turcotte‐Pugh score from a national electronic healthcare database. Clinical Gastroenterology and Hepatology 2015;13(13):2333‐41.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [DOI] [PubMed] [Google Scholar]
LaBerge 1993
- LaBerge JM, Ring EJ, Gordon RL, Lake JR, Doherty MM, Somberg KA, et al. Creation of transjugular intrahepatic portosystemic shunts with the wall stent endoprosthesis: results in 100 patients. Radiology 1993;187:413‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed.) 2009;339:b2700. [PUBMED: 19622552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3; PUBMED: 28207928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moore 2004
- Moore K. Endothelin and vascular function in liver disease. Gut 2004;53(2):159‐61. [PUBMED: 14724140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Orloff 2007
- Orloff MJ, Orloff MS, Orloff SL. Portacaval shunts: side‐to‐side and end‐to‐side. In: Clavien PA, Sarr MG, Fong Y, Georgiev P editor(s). Atlas of Upper Gastrointestinal and Hepato‐Pancreato‐Biliary Surgery. Berlin, Heidelberg: Springer, 2007:687‐702. [Google Scholar]
Perarnau 2014
- Perarnau JM, Gouge A, Nicolas C, d'Alteroche L, Borentain P, Saliba F, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. Journal of Hepatology 2014;60(5):962‐8. [PUBMED: 24480619] [DOI] [PubMed] [Google Scholar]
Pugh 1973
- Pugh RNH, Murray‐Lyon IM, Dawson JL, Pietoni MC, Williams R. Transection of the esophagus for bleeding esophageal varices. British Journal of Surgery 1973;60(8):648‐52. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rigau 1989
- Rigau J, Bosch J, Bordas JM, Navasa M, Mastai R, Kravetz D, et al. Endoscopic measurement of variceal pressure in cirrhosis: correlation with portal pressure and variceal hemorrhage. Gastroenterology 1989;96(3):873‐80. [PUBMED: 2783677] [PubMed] [Google Scholar]
Rikkers 1998
- Rikkers LF. The changing spectrum of treatment for variceal bleeding. Annals of Surgery 1998;228:536‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosemurgy 1994
- Rosemurgy AS, McAllister EW. Small‐diameter prosthetic H‐graft portacaval shunt. Surgery Annual 1994;26:101‐13. [PUBMED: 8303516] [PubMed] [Google Scholar]
Rosemurgy 2003
- Rosemurgy AS, Zervos EE, Bloomston M, Durkin AJ, Clark WC, Goff S. Post‐shunt resource consumption favors small‐diameter prosthetic H‐graft portacaval shunt over TIPS for patients with poor hepatic reserve. Annals of Surgery 2003;237(6):820‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosemurgy 2004
- Rosemurgy AS, Thometz DP, Zervos EE. Portal blood flow, effective hepatic blood flow, and outcome after partial portal decompression. Journal of Surgical Research 2004;117:64‐70. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology, Assessment and Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rössle 1994
- Rössle M, Haag K, Ochs A, Sellinger M, Noldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic stent‐shunt procedure for variceal bleeding. New England Journal of Medicine 1994;330:165‐71. [DOI] [PubMed] [Google Scholar]
Rössle 2006
- Rössle M, Siegerstetter V, Euringer W, Olschewski M, Kromeier J, Kurz K, et al. The use of a polytetrafluoroethylene‐covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): long‐term follow‐up of 100 patients. Acta Radiologica 2006;47:660‐6. [DOI] [PubMed] [Google Scholar]
Sanyal 2008
- Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology 2008;134(6):1715‐28. [PUBMED: 18471549] [DOI] [PubMed] [Google Scholar]
Sarfeh 1983
- Sarfeh IJ, Rypins EB, Conroy RM, Mason GR. Portacaval H‐graft: relationships of shunt diameter, portal flow patterns and encephalopathy. Annals of Surgery 1983;197(4):422‐6. [PUBMED: 6600914] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarfeh 1986
- Sarfeh IJ, Rypins EB, Mason GR. A systematic appraisal of portacaval H‐graft diameters. Clinical and hemodynamic perspectives. Annals of Surgery 1986;204(4):356‐63. [PUBMED: 3490229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane Reviews: The ROBES Meta‐Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [PUBMED: 20334633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shalimar 2016
- Shalimar, Kumar D, Vadiraja PK, Nayak B, Thakur B, Das P, et al. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. Journal of Gastroenterology and Hepatology 2016;31(4):856‐64. [DOI] [PubMed] [Google Scholar]
Sharara 2001
- Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. New England Journal of Medicine 2001;345(9):669‐81. [PUBMED: 11547722] [DOI] [PubMed] [Google Scholar]
Skoog 2015
- Skoog M, Saarimäki JM, Gluud C, Scheinin M, Erlendsson K, Aamdal S, et al. The Nordic Trial Alliance Working Group on Transparency and Registration. Transparency and registration in clinical research in the Nordic countries. nta.nordforsk.org/projects/nta_transparency_report.pdf (assessed 17 March 2018).
Smith 1982
- Smith JL, Graham DY. Variceal haemorrhage, a critical evaluation of survival analysis. Gastroenterology 1982;82:968‐73. [MEDLINE: ] [PubMed] [Google Scholar]
Stanley 1996
- Stanley AJ, Jalan R, Forrest EH, Redhead DN, Hayes PC. Long term follow up of transjugular intrahepatic portosystemic stent shunt (TIPSS) for the treatment of portal hypertension: results in 130 patients. Gut 1996;39:479‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Student 1908
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25. [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Gyuatt G, Ioannidis JPA, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 19 July 2018).
Toomey 2013
- Toomey PG, Ross SB, Golkar FC, Hernandez JM, Clark WC, Luberice K, et al. Outcomes after transjugular intrahepatic portosystemic stent shunt: a "bridge" to nowhere. American Journal of Surgery 2013;205:441‐6. [DOI] [PubMed] [Google Scholar]
Tripathi 2004
- Tripathi D, Helmy A, Macbeth K, Balata S, Lui HF, Stanley AJ, et al. Ten years' follow‐up of 472 patients following transjugular intrahepatic portosystemic stent‐shunt insertion at a single centre. European Journal of Gastroenterology & Hepatology 2004;16:9‐18. [DOI] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Warren 1967
- Warren WD, Zeppa R, Fomon JJ. Selective trans‐splenic decompression of gastroesophageal varices by distal splenorenal shunt. Annals of Surgery 1967;166(3):437‐55. [PUBMED: 6068492] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [PUBMED: 28264661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 2003
- Wolff M, Hirner A. Current state of portosystemic shunt surgery. Langenbeck's archives of surgery 2003;388(3):141‐9. [PUBMED: 12942328] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zervos 1998
- Zervos EE, Goode SE, Rosemurgy AS. Immediate and long‐term portal hemodynamic consequences of small‐diameter H‐graft portacaval shunt. Journal of Surgical Research 1998;74:71‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Khan 1998
- Khan SA, Sutton R. Surgical portosystemic shunts versus transjugular intrahepatic portosystemic shunt for variceal haemorrhage. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD001023] [DOI] [PMC free article] [PubMed] [Google Scholar]


