Abstract
Background
The para‐aortic lymph nodes (located along the major vessels in the mid and upper abdomen) are a common place for disease recurrence after treatment for locally advanced cervical cancer. The para‐aortic area is not covered by standard pelvic radiotherapy fields and so treatment to the pelvis alone is inadequate for women at a high risk of occult cancer within para‐aortic lymph nodes. Extended‐field radiotherapy (RT) widens the pelvic RT field to include the para‐aortic lymph node area. Extended‐field RT may improve outcomes in women with locally advanced cervical cancer by treating occult disease in para‐aortic nodes not identified at pretreatment imaging. However, RT treatment of the para‐aortic area can cause severe adverse effects, so may increase harms.
Studies of pelvic chemoradiotherapy (CRT) demonstrated improved survival rates compared to pelvic RT alone. CRT is now the standard of care in the treatment of locally advanced cervical cancer. Studies comparing pelvic RT alone (without concurrent chemotherapy) with extended‐field RT should therefore be viewed with caution, since they compare treatments against what is now substandard treatment (pelvic RT alone). This review should therefore be read with this in mind and comparisons with pelvic RT cannot be extrapolated to pelvic CRT.
Objectives
To evaluate the effectiveness and toxicity of extended‐field radiotherapy in women undergoing first‐line treatment for locally advanced cervical cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7), MEDLINE via Ovid (1946 to August week 4, 2018), and Embase via Ovid (1980 to 2018, week 35). We checked registers of clinical trials, grey literature, conference reports, and citation lists of included studies to August 2018.
Selection criteria
We included randomised controlled trials (RCTs) evaluating the effectiveness and toxicity of extended‐field RT for locally advanced cervical cancer.
Data collection and analysis
Two review authors independently selected potentially relevant RCTs, extracted data, assessed risk of bias, compared results, and made judgements on the quality and certainty of the evidence for each outcome. Any disagreements were resolved by discussion or consultation with a third review author.
Main results
Five studies met the inclusion criteria. Three included studies compared extended‐field RT versus pelvic RT, one included study compared extended‐field RT with pelvic CRT, and one study compared extended‐field CRT versus pelvic CRT.
Extended‐field radiotherapy versus pelvic radiotherapy alone Compared to pelvic RT, extended‐field RT probably reduces the risk of death (hazard ratio (HR) 0.67, 95% confidence interval (CI) 0.48 to 0.94; 1 study; 337 participants; moderate‐certainty evidence) and para‐aortic lymph node recurrence (risk ratio (RR) 0.36, 95% CI 0.18 to 0.70; 2 studies; 477 participants; moderate‐certainty evidence), although there may or may not have been improvement in the risk of disease progression (HR 0.92, 95% CI 0.69 to 1.22; 1 study; 337 participants; moderate‐certainty evidence) and severe adverse events (RR 1.05, 95% CI 0.79 to 1.41; 2 studies; 776 participants; moderate‐certainty evidence).
Extended‐field radiotherapy versus pelvic chemoradiotherapy In a comparison of extended‐field RT versus pelvic CRT, women given pelvic CRT probably had a lower risk of death (HR 0.50, 95% CI 0.39 to 0.64; 1 study; 389 participants; moderate‐certainty evidence) and disease progression (HR 0.52, 95% CI 0.37 to 0.72; 1 study; 389 participants; moderate‐certainty evidence). Participants given extended‐field RT may or may not have had a lower risk of para‐aortic lymph node recurrence (HR 0.44, 95% CI 0.20 to 0.99; 1 study; 389 participants; low‐certainty evidence) and acute severe adverse events (RR 0.05, 95% CI 0.02 to 0.11; 1 study; 388 participants; moderate‐certainty evidence). There were no clear differences in terms of late severe adverse events among the comparison groups (RR 1.06, 95% CI 0.69 to 1.62; 1 study; 386 participants; moderate‐certainty evidence).
Extended‐field chemoradiotherapy versus pelvic chemoradiotherapy Very low‐certainty evidence obtained from one small study (74 participants) showed that, compared to pelvic CRT, extended‐field CRT may or may not have reduced risk of death (HR 0.37, 95% CI 0.14 to 0.96) and disease progression (HR 0.25, 95% CI 0.07 to 0.87). There were no clear differences between the groups in the risks of para‐aortic lymph node recurrence (RR 0.19, 95% CI 0.02 to 1.54; very low‐certainty evidence) and severe adverse events (acute: RR 0.95, 95% CI 0.20 to 4.39; late: RR 0.95, 95% CI 0.06 to 14.59; very low‐certainty evidence).
Authors' conclusions
Moderate‐certainty evidence shows that, compared with pelvic RT alone, extended‐field RT probably improves overall survival and reduces risk of para‐aortic lymph node recurrence. However, pelvic RT alone would now be considered substandard treatment, so this result cannot be extrapolated to modern standards of care. Low‐ to moderate‐certainty evidence suggests that pelvic CRT may increase overall and progression‐free survival compared to extended‐field RT, although there may or may not be a higher rate of para‐aortic recurrence and acute adverse events. Extended‐field CRT versus pelvic CRT may improve overall or progression‐free survival, but these findings should be interpreted with caution due to very low‐certainty evidence.
High‐quality RCTs, comparing modern treatment techniques in CRT, are needed to more fully inform treatment for locally advanced cervical cancer without obvious para‐aortic node involvement.
Plain language summary
Does extended‐field radiotherapy reduce death from locally advanced cervical cancer and what are the side effects?
The issue Radiotherapy (RT) to the pelvis is used to treat cervical cancer. However, pelvic RT will not treat cancer that has spread to para‐aortic lymph nodes (lymph nodes lying along main blood vessels in the mid and upper stomach), since these are outside the target area (field) of RT. Extended‐field RT targets areas containing both pelvic and para‐aortic lymph nodes. Widening the RT field to include the para‐aortic area may reduce the risk of cancer returning.
Chemotherapy is now normally given at the same time as RT for the treatment of cervical cancer (concurrent chemotherapy) and the combine treatment is called chemoradiotherapy (CRT). This is now standard treatment because studies have shown that addition of chemotherapy during RT improved survival for women with cervical cancer thought to be confined to the pelvis. Older studies, which compared treatments with pelvic RT alone, would not now be considered the standard of care for women well enough to have CRT. We cannot assume that results from studies which compared extended‐field RT with pelvic RT apply to modern CRT treatments.
The aim of this review In women with locally advanced cervical cancer, does extending the RT field to cover the para‐aortic area reduce the risk of death from cervical cancer and what are the harms?
Study characteristics We searched databases from their inception to August 2018 and found five studies that met the inclusion criteria. Three studies compared extended‐field RT versus pelvic RT. None of these three studies compared against the current gold‐standard of pelvic CRT. One study compared extended‐field RT versus pelvic CRT and one study compared extended‐field CRT versus pelvic CRT.
What were the main findings? Compared with pelvic RT alone, women given extended‐field RT may have been less likely to die and probably were less likely to have a cervical cancer come back (recurrence) in the para‐aortic lymph nodes. However, extended‐field RT may have made little or no difference to how often their cancer recurred elsewhere and how often they experience severe side effects.
Pelvic CRT is the modern standard of treatment for locally advanced cervical cancer. In a comparison of extended‐field RT alone versus pelvic CRT, women given pelvic CRT were probably less likely to die or have recurrence of their cancer. Women given extended‐field RT alone may have been less likely to experience a recurrence within the para‐aortic lymph nodes and have had adverse events during or shortly after treatment. There were no clear differences regarding the late adverse events between the two groups.
Women given extended‐field CRT may or may not have been less likely to die or have cancer progression than those women pelvic CRT. There were no clear differences in the chances of experiencing a cancer recurrence in the para‐aortic lymph nodes and severe side effects between the groups.
Certainty of the evidence The evidence for outcomes in the comparison of extended‐field RT alone versus pelvic RT alone were of moderate certainty. In the comparison of extended‐field RT versus pelvic CRT, the evidence regarding the survival and side effects were of moderate certainty. The evidence for para‐aortic recurrence was of low certainty. The evidence for all outcomes in a comparison of extended‐field CRT versus pelvic CRT were of very‐low certainty because of concerns regarding the high risk of bias and results coming from a single trial of very few women.
What were the conclusions? We are moderately certain that, compared with pelvic RT alone, extended‐field RT probably improves overall survival and reduces risk of para‐aortic lymph node recurrence. However, pelvic RT alone would now not be considered the standard of care in women well enough to receive CRT, so these results should be reviewed with caution and cannot be extrapolated to modern treatment techniques.
Low‐ to moderate‐certainty evidence supports the use of pelvic CRT rather than extended‐field RT alone, as it appears to reduce the risk of death and cancer progression. The likelihood of experiencing unwanted side effects during treatment was higher among women receiving pelvic CRT than extended‐field RT. Evidence comparing extended‐field CRT to pelvic CRT was very low certainty regarding outcomes and it may or may not improve survival.
Summary of findings
Background
Description of the condition
Cervical cancer is the most common gynaecological cancer affecting women worldwide, with an estimated 528,000 new cases, and 266,000 cervical cancer deaths, globally in 2012 (GLOBOCAN 2012). Approximately 70% of all cervical cancer cases, and almost 90% of cervical cancer‐related deaths, occur in low‐income countries. The high death rate among women residing in low‐income countries is because most women present with advanced disease (Pisani 1999). A woman's risk of developing cervical cancer before the age of 75 years is estimated to range from 0.9% in high‐income to 1.9% in low‐income countries (Jemal 2011). This discrepancy in the incidence, risk, and stage at diagnosis between high and low‐income countries is due to lack of access to effective screening programmes that facilitate early diagnosis and treatment.
A summary of the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer is displayed in Appendix 1 (FIGO Committee 2014). Women with FIGO stage IA1 are normally treated with local excision or simple hysterectomy (surgery to remove the uterus and the neck of the uterus). FIGO stage IA2 to IB1 cervical cancers have an increased risk of pelvic lymph node involvement (3.4% to 15.3%) (Mahawerawat 2013; Sakuragi 1999; Spirtos 2002; Suprasert 2010), and are usually treated with radical hysterectomy (the removal of the uterus, the cervix, the upper part of the vagina, and the tissues around the cervix), and pelvic lymph node dissection. However, treatment outcomes of pelvic radiotherapy (RT) are comparable to those of women who undergo surgical treatment (Landoni 1997). Therefore, decision‐making for early‐stage cervical cancer should be tailored based on individual patient characteristics, weighing up the risks of surgery with the longer‐term risks of radical RT. Pelvic RT was the principal treatment for more advanced cervical cancer, since the rate of positive pelvic lymph nodes is higher (22.7% to 71.4%) (Marnitz 2005; Sakuragi 1999; Srisomboon 2011; Suprasert 2010), although some clinicians recommend extended radical hysterectomy for locally advanced disease (Höckel 2008).
Randomised controlled trial (RCT) data demonstrated that giving chemotherapy at the same time as pelvic RT as a radiosensitiser, or the so‐called 'concurrent chemoradiation' or chemoradiotherapy (CRT) significantly improved the rates of local and distant disease control for women with all stages of cervical cancer compared to RT alone (Keys 1999; Morris 1999; Peters 2000; Rose 1999; Whitney 1999). Pelvic concurrent CRT has been recommended as the treatment of choice instead of pelvic RT alone in locally advanced cervical cancer, if clinically feasible (National Cancer Institute 1999). One Cochrane Review also endorsed the use of pelvic CRT versus pelvic RT alone for women with cervical cancer based on evidence obtained from individual patient data meta‐analysis (CCCMAC 2010).
Description of the intervention
Conventionally, whole pelvis external‐beam RT treatment field covers the space between L4 (fourth lumbar spine) to L5 (fifth lumbar spine) to the mid of pubis, or to a line 4 cm below the most distal vaginal or cervical site of disease. Lateral fields are designed to encompass S3 (third sacral spine) posteriorly, with a margin of at least 3 cm from the primary cervical tumour. The RT schedule is about 1.6 Gy to 2.0 Gy (unit of irradiation dose) per day, five days per week, with a total radiation dose of 45 Gy to 50 Gy. The prescribed dose is delivered to the mid‐depth on the central axis of the beams. Intracavitary brachytherapy is performed after the completion of pelvic RT and a total cumulative dose to point A is at least 80 Gy (Eifel 2004; Lim 2011; Rotman 1978; Rotman 1990).
Extended‐field or para‐aortic RT is the extension of whole pelvis external‐beam RT to also cover the para‐aortic lymph nodes (the nodes lying adjacent to the aorta and vena cava, the major vessels in the mid and upper abdomen). This additional RT is delivered in the same setting with the conventional whole pelvis external‐beam RT. The RT schedule and total dose of the two interventions are the same, but the extended‐field RT covers a wider area (Eifel 2004; Rotman 1978; Rotman 1990).
Severe acute and late morbidities (adverse effects and complications of treatment) seem to be fundamental reasons limiting the use of extended‐field RT for cervical cancer, particularly among women receiving CRT. Severe acute toxicities in women who had undergone extended‐field RT with concomitant chemotherapy occur at approximately 33% to 80% and severe late toxicities (grade 3 or higher) occur in 10% to 15% (Kim 2009; Sood 2003; Varia 1998). Approximately 10% of women receiving extended‐field RT develop severe acute toxicities and 6% of women develop severe late toxicities, following extended‐field RT alone (Sood 2003).
How the intervention might work
Occult para‐aortic lymph node disease can occur in women thought to have locally advanced cervical cancer, which is confined to the pelvis. From a previous literature review, which was undertaken to evaluate the impact of pretreatment surgical para‐aortic lymph node staging on treatment outcomes of cervical cancer, para‐aortic lymph node metastasis were noted in 11% of women with stage I disease, 13% to16% with stage II, 29% with stage III, and 36% with stage IVA (Smits 2014). In one review of the literature, despite having normal findings on positron emission tomography (PET) or positron emission tomography‐computed tomography (PET‐CT), 4% to 15% of women thought to have negative para‐aortic lymph node on PET and PET‐CT had para‐aortic lymph node metastasis (Smits 2014).
Standard radical pelvic RT is likely to be inadequate for women with para‐aortic lymph node metastasis, since the para‐aortic area is outside of standard fields of traditional pelvic RT techniques. Para‐aortic lymph node recurrence is a frequent cause of extrapelvic failure among women with locally advanced cervical cancer who had undergone the standard technique of pelvic RT (21.6% to 57.1%) (Hong 2004; Sakurai 2001). Even in the era of concomitant CRT, para‐aortic lymph node metastasis has been noted in approximately 7% to 15% of women undergoing concomitant CRT for cervical cancer (Eifel 2004; Pearcey 2002; Toita 2012).
Previous studies have observed that extended‐field RT prolonged the survival of women with endometrial cancer (Rose 1992), and cervical cancer (Marnitz 2015; Varia 1998) who had para‐aortic lymph node metastasis. In addition, prophylaxis extended‐field RT to the para‐aortic region improved overall survival in women with cervical cancer (Rotman 1995). Based on these findings, delivering extended‐field RT to cover para‐aortic regions aimed at eliminating unrecognised metastatic lesions might be effective for reducing treatment failure at this region and thus improving treatment outcomes of women with cervical cancer.
Why it is important to do this review
The prognosis for women with recurrent cervical cancer is poor and para‐aortic lymph node recurrence is one of the most common sites of treatment failure in women with cervical cancer (Hong 2004; Sakurai 2001). However, there is significant morbidity from extended‐field RT (Kim 2009; Sood 2003; Varia 1998). Evidence for an effective strategy in reducing para‐aortic lymph node recurrence is therefore required to establish whether extended‐field RT may decrease the risk of para‐aortic recurrence. To date there has been no systematic review evaluating the impact of extended‐field RT on the treatment outcomes of women with locally advanced cervical cancer.
Objectives
To evaluate the effectiveness and toxicity of extended‐field radiotherapy in women undergoing first‐line treatment for locally advanced cervical cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs. We excluded quasi‐randomised trials as these may have been subject to bias.
Types of participants
Women aged 18 years or older undergoing first‐line treatment for locally advanced cervical cancer. Cervical cancers with the largest diameter of 4 cm or larger or FIGO stage IIB to IVA (or both) were considered locally advanced. Details of the FIGO staging classification of cervical cancer are shown in Appendix 1 (FIGO Committee 2014).
Types of interventions
We compared extended‐field versus standard radical pelvic RT as follows:
extended‐field RT versus pelvic RT alone;
extended‐field RT versus pelvic CRT;
extended‐field CRT versus pelvic CRT.
Types of outcome measures
Primary outcomes
Overall survival (OS): survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Para‐aortic lymph node recurrence: any relapse or persistent disease at the para‐aortic region.
Secondary outcomes
Progression‐free survival (PFS) or disease progression: survival until appearance of a new lesion. Survival was assessed from the time when women were enrolled in the study.
Non‐para‐aortic recurrence: recurrent disease other than the para‐aortic region was classified as locoregional and distant recurrences.
-
Adverse events: which were classified as:
acute complications including digestive complications (e.g. nausea/vomiting, diarrhoea); urological complications (e.g. cystitis); haematological complications (e.g. anaemia, leukopenia, neutropenia, and thrombocytopenia); and cardiovascular and thromboembolic complications (e.g. myocardial infarction, arterial thrombosis, venous thrombosis, pulmonary embolism);
late complications including digestive complications (e.g. proctitis, sigmoiditis, intestinal obstruction, and fistular formation); and urological complications (e.g. chronic cystitis, incontinence, ureteral stenosis, and fistula) (Cox 1995).
Quality of life: using validated scales (i.e. European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐CX24; Greimel 2006).
Cost‐effectiveness: using a scale that was validated and reported in a peer‐reviewed publication (i.e. European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO‐MCBS; Cherny 2015).
We presented a 'Summary of findings' tables reporting the following outcomes listed in order of priority (see Data synthesis).
Overall survival.
Para‐aortic lymph node recurrence.
Progression‐free survival.
Non‐para‐aortic recurrence.
Adverse events.
Quality of life.
Cost‐effectiveness.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7) in the Cochrane Library;
MEDLINE via Ovid (1946 to August week 4, 2018);
Embase via Ovid (1980 to 2018, week 35).
Appendix 2; Appendix 3; Appendix 4 display the search strategies for CENTRAL, MEDLINE, and Embase.
Searching other resources
Ongoing studies and grey literature
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/) and ClinicalTrials.gov to identify any ongoing trials. If we had identified ongoing trials that had not been published, we planned to approach the principal investigators, and major co‐operative groups active in this area, to ask for relevant data.
We searched the following databases for grey literature: Open‐Grey (www.opengrey.eu/) and Index to theses (ProQuest Dissertations & Theses: UK & Ireland).
Handsearching
We handsearched the citation lists of included studies and key textbooks, and contacted experts in the field to identify further reports of trials. We also handsearched the reports of conferences in the following sources.
Annual Meeting of the American Society of Gynecologic Oncology (ASGO).
Annual Meeting of the American Society of Radiation Oncology (ASTRO).
Annual Meeting of European Society of Radiotherapy and Oncology (ESTRO).
Annual Meeting of European Society of Medical Oncology (ESMO).
Annual Meeting of the American Society of Clinical Oncology (ASCO).
Annual Meeting of the British Gynaecological Cancer Society (BGCS).
Biennial Meeting of the Asian Society of Gynecologic Oncology (ASGO).
Biennial Meeting of Asia and Oceania Federation of Obstetrics and Gynaecology (AOFOG).
Biennial Meeting of the European Society of Gynaecological Oncology (ESGO).
Biennial Meeting of the International Gynecologic Cancer Society (IGCS).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (Endnote). After removal of duplicates, we transferred these data to Covidence (www.covidence.org). Two review authors (KT and NS) independently examined the remaining references. We excluded studies that clearly did not meet the inclusion criteria, and we obtained copies of the full‐text of potentially relevant references. Two review authors (KT and NS) independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreements through discussion or, when required, we consulted a third review author (CK). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We used the details regarding the selection process in Covidence 2018 to complete a PRISMA flow diagram and Characteristics of excluded studies table (Liberati 2009).
Data extraction and management
Two review authors (KT and NS) independently extracted study characteristics and outcome data from included studies using Covidence 2018. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (PL or CK). A second review author (CK) checked study characteristics for accuracy against the trial report.
For included studies, we extracted the following data using a piloted data collection form.
Author, year of publication, and journal citation (including language).
Country.
Setting.
Inclusion and exclusion criteria.
Study methodology.
-
Study population and characteristics:
total number enrolled;
participant characteristics;
age;
co morbidities;
other baseline characteristics;
FIGO stage of cervical cancer;
tumour size (largest diameter);
histopathological type of cervical cancer;
status of para‐aortic lymph node.
-
Intervention details:
any regimens of pelvic RT or CRT plus extended‐field RT;
RT technique;
dose;
duration;
schedule.
-
Comparison:
any regimens of pelvic RT or CRT.
Risk of bias in study (see below).
Duration of follow‐up.
Outcomes: for each outcome, we extracted the outcome definition and unit of measurement. For adjusted estimates, we planned to record variables adjusted for in analyses.
Results: we extracted the number of participants allocated to each intervention group, the total number analysed for each outcome, and the missing participants.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Results were extracted as follows.
For time to event data (survival and disease progression), we extracted the log of the hazard ratio [log(HR)] and its standard error (SE) from trial reports. If these were not reported, we estimated the log (HR) and its SE using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events or deaths, if it was not possible to use the HR), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR) with 95% confidence interval (CI).
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in groups to which they were assigned.
Assessment of risk of bias in included studies
We assessed and reported on the methodological quality and risk of bias in included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which recommends the explicit reporting of the following individual elements for RCTs.
Selection bias: random sequence generation and allocation concealment.
Performance bias: blinding of participants and personnel (participants and treatment providers).
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data.
Reporting bias: selective reporting of outcomes.
Other possible bias.
Two review authors (NS and CK) independently applied the Cochrane 'Risk of bias' tool and resolved differences by discussion or by appeal to a third review author (PL). We judged each item as being at high, low, or unclear risk of bias as set out in the criteria displayed in Appendix 5 (Higgins 2011). We provided a quote from the study report or a statement as justification for the judgement for each item (or both a quote and statement) in the 'Risk of bias' table. We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. When interpreting treatment effects and meta‐analyses, we took into account the risk of bias for the studies that contributed to that outcome. Where information on risk of bias related to unpublished data or correspondence with a trial authors, we noted this in the table.
Measures of treatment effect
We used the following measures of the effect of treatment.
For survival outcomes (e.g. OS and PFS), we used HR and 95% CI.
For dichotomous outcomes (e.g. adverse events, recurrences, and death), we analysed data based on the number of events and the number of people assessed in the intervention and comparison groups. We planned to use these to calculate the RR and 95% CI.
For continuous outcomes (e.g. quality of life and cost‐effectiveness measures), we planned to analyse data based on the mean, standard deviation (SD), and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) between treatment arms with a 95% CI. If the MD was reported without individual group data, we planned to use this to report the study results. If studies measured the same outcome using different tools, we planned to calculate the standardised mean difference (SMD) and 95% CI using the inverse variance method.
Unit of analysis issues
We planned to include studies where individual participants were randomised and cluster‐randomised studies. For studies that used a cluster‐randomised design but did not have any information related to the design effect, we planned to estimate the design effect based on a fairly large assumed intracluster correlation of 0.10. We planned to base this assumption by analogy on studies about implementation research (Campbell 2000; Ukoumunne 1999). We intended to follow the methods stated in the Cochrane Handbook for Systematic Reviews of Interventions for determining the calculations (Higgins 2011). In a study with multiple intervention groups, where possible, we planned to combine all relevant experimental intervention groups into a single group to create a single pair‐wise comparison (Higgins 2011).
Only studies that randomised individual participants into one of two comparison groups met our inclusion criteria. There was no requirement to review unit of analysis issues. This may be required in updates of this review, should additional studies become available.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies by visually inspecting forest plots, estimating the percentage of heterogeneity (I² statistic) and Chi² test between trials that could not be ascribed to sampling variation (Higgins 2003; Higgins 2011), and performing a formal statistical test of the significance of identified heterogeneity (Deeks 2001), and, where possible, by subgroup analyses. If there was evidence of substantial heterogeneity (I² greater than 60%), we investigated and reported the possible reasons for this.
Assessment of reporting biases
We planned to construct funnel plots to determine the possibility of publication bias. In future updates of this review, we will construct funnel plots corresponding to meta‐analysis of the primary outcomes to assess the potential for small‐study effects such as publication bias, if we identify more than 10 studies. We plan to assess funnel plot asymmetry visually (Sterne 2011).
We did not determine the potential of reporting bias, as there were too few studies available for this review.
Data synthesis
We pooled the results in a meta‐analyses. We used the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986). We performed statistical analysis using Review Manager 5 (Review Manager 2014).
For time‐to‐event data, we pooled HRs using the generic inverse variance.
For dichotomous outcomes, we calculated the RRs for each study and pooled them.
For continuous outcomes, we planned to pool the MDs between the treatment arms, if trials measured the outcome on the same scale; if trials measured the outcome on a different scale, we planned to pool SMDs.
We prepared 'Summary of findings' tables to display the results of the meta‐analysis, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented the results of the meta‐analysis for the outcomes and harms as outlined in the Types of outcome measures section. If we are unable to pool the results using meta‐analysis methods, we planned to conduct a narrative review of the available results.
Main outcomes of 'Summary of findings' tables for assessing the certainty of the evidence
We presented the overall certainty of evidence for each outcome according to the GRADE approach, which takes into account issues relating to internal validity (risk of bias, inconsistency, imprecision, publication bias), and external validity such as directness of results (Langendam 2013). We created 'Summary of findings' tables based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and using GRADEpro GDT. We used the GRADE checklist and GRADE Working Group definitions (Meader 2014). We downgraded the evidence from high certainty by one level for serious (or by two for very serious) limitations. See Table 1; Table 2; Table 3.
Summary of findings for the main comparison. Extended‐field radiotherapy compared to pelvic radiotherapy for locally advanced cervical cancer.
| Extended‐field radiotherapy versus pelvic radiotherapy for locally advanced cervical cancer | ||||||
| Participant: women with locally advanced cervical cancer undergoing radiotherapy as a primary treatment Setting: specialised hospital Intervention: extended‐field radiotherapy Comparison: pelvic radiotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with pelvic radiotherapy | Risk with extended‐field radiotherapy | |||||
| Overall survival | — | — | HR 0.67 (0.48 to 0.94) | 337 (1 study) |
⊕⊕⊕⊝ Moderatea |
As a result of the way HR was calculated, assumed and corresponding risks were not estimated. |
| Para‐aortic lymph node recurrence | 126 per 1000 | 45 per 1000 (23 to 88) |
RR 0.36 (0.18 to 0.70) |
477 (2 studies) | ⊕⊕⊕⊝ Moderatea |
— |
| Progression‐free survival | — | — | HR 0.92 (0.69 to 1.22) | 377 (1 study) |
⊕⊕⊕⊝ Moderateb |
As a result of the way, HR was calculated, assumed and corresponding risks were not estimated. |
| Adverse events | 357 per 1000 | 375 per 1000 (282 to 503) | RR 1.05 (0.79 to 1.41) | 776 (2 study) | ⊕⊕⊕⊝ Moderateb |
See Differences between protocol and review. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to a wide 95% confidence interval. bDowngraded one level due to wide confidence intervals crossing the line of no effect.
Summary of findings 2. Extended‐field radiotherapy compared to pelvic chemoradiotherapy for locally advanced cervical cancer.
| Extended‐field radiotherapy versus pelvic CRT for locally advanced cervical cancer | ||||||
| Participant: women with locally advanced cervical cancer undergoing radiotherapy as a primary treatment Setting: specialised hospital Intervention: extended‐field radiotherapy Comparison: pelvic CRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with pelvic chemoradiotherapy | Risk with extended‐field radiotherapy | |||||
| Overall survival | — | — | HR 0.50 (0.39 to 0.64) | 389 (1 study) |
⊕⊕⊕⊝ Moderatea |
As a result of the way HRs are calculated, assumed and corresponding risks were not estimated. |
| Para‐aortic lymph node recurrence | 93 per 1000 | 41 per 1000 (19 to 92) | RR 0.44 (0.20 to 0.99) | 389 (1 study) | ⊕⊕⊝⊝ Lowa,b |
— |
| Progression‐free survival | — | — | HR 0.52 (0.37 to 0.72) | 389 (1 study) |
⊕⊕⊕⊝ Moderatea |
As a result of the way HRs are calculated, assumed and corresponding risks were not estimated. |
| Acute adverse events | 646 per 1000 | 32 per 1000 (13 to 71) | RR 0.05 (0.02 to 0.11) | 388 (1 study) | ⊕⊕⊕⊝ Moderatea |
— |
| Late adverse events | 176 per 1000 | 187 per 1000 (122 to 285) | RR 1.06 (0.69 to 1.62) | 386 (1 study) | ⊕⊕⊕⊝ Moderatea |
— |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to imprecision, as indicated by a 95% CI. bDowngraded two levels level due to serious imprecision: wide 95% CI with few events.
Summary of findings 3. Extended‐field chemoradiotherapy compared to pelvic chemoradiotherapy for locally advanced cervical cancer.
| Extended‐field CRT versus pelvic CRT for locally advanced cervical cancer | ||||||
| Participant: women with locally advanced cervical cancer undergoing CRT as a primary treatment Setting: specialised hospital Intervention: extended‐field CRT Comparison: pelvic CRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with pelvic CRT | Risk with extended‐field CRT | |||||
| Overall survival | — | — | HR 0.37 (0.14 to 0.96) | 74 (1 study) |
⊕⊝⊝⊝ Very lowa,b |
As a result of the way HRs are calculated, assumed, and corresponding risks were not estimated. |
| Para‐aortic lymph node recurrence | 139 per 1000 | 26 per 1000 (3 to 214) | RR 0.19 (0.02 to 1.54) | 74 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
— |
| Progression‐free survival | — | — | HR 0.25 (0.07 to 0.87) | 74 (1 study) |
⊕⊝⊝⊝ Very lowa,b |
As a result of the way HRs are calculated, assumed and corresponding risks were not estimated |
| Acute adverse events | 83 per 1000 | 79 per 1000 (17 to 366) | RR 0.95 (0.20 to 4.39) | 74 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
— |
| Late adverse events | 28 per 1000 | 26 per 1000 (2 to 405) | RR 0.95 (0.06 to 14.59) | 74 (1 study) | ⊕⊝⊝⊝ Very lowa,b |
— |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CRT: chemoradiotherapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to serious risk of bias: the included study had a high risk of bias in three key domains assessed. bDowngraded two levels due to serious imprecision: small sample size and few events.
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very‐low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for the following factors:
type of treatment (CRT versus RT only);
status of para‐aortic lymph node (unknown versus negative on radiological imaging).
However, we did not perform subgroup analysis, as most analyses were based on only one or two included studies. Nevertheless, we acknowledged these factors in the interpretation of review findings.
Sensitivity analysis
We were unable to conduct sensitivity analysis because of the small number of studies. If more studies are included in future review updates, we will perform sensitivity analysis to assess the effect of the following factors.
Repeating the analysis excluding unpublished studies, if any.
Repeating the analysis excluding studies judged at high or unclear risk of bias for allocation concealment.
Results
Description of studies
Results of the search
A broad search of the literature databases in August 2018 yielded the following results; CENTRAL (99 references), MEDLINE (115 references), and Embase (159 references). Searching other bases yielded one congress abstract. After deduplication, we screened titles and abstracts of 252 references and excluded 235 that obviously did not meet the review inclusion criteria. The search found no ongoing trials. Of the 17 references that potentially met the review inclusion, we excluded 10 reports after reviewing the full texts (see Characteristics of excluded studies table). Figure 1 shows the PRISMA flow chart for study selection. We included five studies (seven reports).
1.

Study flow diagram. RCT: randomised controlled trial; RT: radiotherapy.
Included studies
Five studies (seven reports) met the inclusion criteria (Asiri 2014; Chatani 1995; Haie 1988; Morris 1999; Rotman 1990). Rotman 1995 reported 10‐year treatment results of Rotman 1990. Eifel 2004 described updated results of Morris 1999. See Characteristics of included studies table for details of each study.
Participants
Haie 1988 was a two‐armed parallel RCT conducted by the Radiotherapy Cooperative Group of EORTC from November 1977 to July 1981. Participants were previously 441 untreated women with histologically confirmed carcinoma of the cervix with high risk of subclinical para‐aortic node metastases. Women were considered ineligible if they had para‐aortic lymph node involvement confirmed either by surgery or diagnosed on lymphangiography.
Rotman 1990 was a two‐armed parallel RCT conducted by the Radiation Therapy Oncology Group (RTOG) from November 1979 to October 1986. This study analysed 335 women who had cervical cancer stage IIB without clinical evidence of para‐aortic nodal involvement or stages IB and IIA with tumour size of 4 cm or greater in lateral dimension. Women were considered eligible if they had no clinical evidence of para‐aortic lymph node metastases. However, approximately one‐third (37%) did not undergo any para‐aortic lymph node evaluation. Of the remaining 63% of participants, 50% were treated by non‐surgical procedures and 13% were treated by surgical procedures. The median follow‐up time was six years. Updated data of this report were subsequently published in Rotman 1995.
Chatani 1995 conducted an RCT study at University Hospital in Japan between November 1986 and October 1990. Ninety‐three women with cervical carcinoma were randomly allocated for treatment with either pelvic RT or extended‐field RT. Thirty‐six participants received RT as a primary therapy and the remaining 57 participants received RT as an adjuvant treatment following extended radical hysterectomy. Based on the review inclusion criteria, only data obtained from the RT arm in which 18 participants were randomly allocated to each pelvic RT group and extended‐field RT group were extracted for meta‐analyses.
Morris 1999 was a two‐armed parallel, RCT conducted by RTOG between 1990 and 1997. Participants were 388 women who had FIGO stages IIB to IVA, squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the cervix or stage IB or IIA of one of these cancers with a tumour diameter of at least 5 cm or biopsy‐confirmed metastasis to pelvic lymph nodes. All participants underwent para‐aortic lymph node evaluation by bipedal lymphangiogram or retroperitoneal surgical exploration. Women who had cancer spreading to para‐aortic lymph nodes were ineligible. Median follow‐up time was 43 months. The results of this study were updated in Eifel 2004.
Asiri 2014 was a two‐armed parallel, RCT conducted between July 2007 and April 2008. Participants were 74 women with histopathologically confirmed squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma, and radiologically negative para‐aortic lymph node cervical cancer stage IIB to IVA.
Interventions
Chatani 1995; Haie 1988; and Rotman 1990 compared pelvic RT versus extended‐field RT.
Morris 1999 compared pelvic CRT (with fluorouracil and cisplatin concurrent chemotherapy) versus extended‐field RT alone.
Asiri 2014 compared pelvic CRT (with weekly cisplatin concurrent chemotherapy) versus extended‐field CRT (with weekly cisplatin concurrent chemotherapy).
Outcomes
Haie 1988 reported disease‐free survival, rates of locoregional and distant recurrences, and severe adverse events
Asiri 2014; Morris 1999; and Rotman 1990, reported OS, disease‐free survival, rates of locoregional and distant recurrences, and severe adverse events.
Chatani 1995 reported the three‐year cause‐specific survival, rates of locoregional and distant recurrences. The authors reported rate of adverse event but did not state its severity.
Rotman 1995 updated the results of Rotman 1990. In Rotman 1995, every participant could have been followed up for at least eight years, and 55% of the participants could have been followed up for at least 10 years.
Eifel 2004 reported an updated results of Morris 1999. The median follow‐up time of the early and updated reports was 43 months (Morris 1999) and 6.6 years (Eifel 2004).
Asiri 2014; Chatani 1995; Eifel 2004; and Haie 1988 reported rate of para‐aortic recurrence.
None of the studies reported quality of life and cost‐effectiveness.
Excluded studies
We excluded seven studies that were not RCTs (Du 2010; Liang 2014; Sood 2003; Vargo 2014; Varia 1998; Wakatsuki 2015; Yoon 2014).
We excluded two studies because the interventions applied were not applicable to this review (Lin 2015; Tsai 2010).
One study divided participants into two comparison groups by the status of CA9 immunohistochemical staining (CA9‐positive and CA9‐negative) and then were randomly allocated to extended‐field RT and pelvic RT arms (Kim 2016). This was excluded because the results were reported according to the status of CA9 expression. There were no data available for comparing extended‐field RT and pelvic RT, which was the comparison that this review aimed to evaluate (see Characteristics of excluded studies table).
Risk of bias in included studies
See Characteristics of included studies table; Figure 2; and Figure 3 for full details.
2.
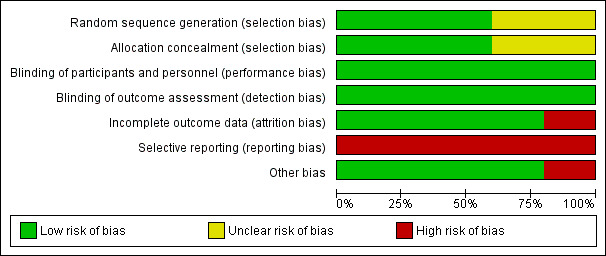
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Haie 1988 stated that the participants were randomised by drawing a sealed envelope which contained computer‐generated random numbers thus indicating a low risk of selection bias.
Asiri 2014 and Chatani 1995 did not state the methods of random sequence generation and allocation concealment. Therefore, these two included studies had an unclear risk of selection bias.
There was no information regarding the sequence generation process and method applied for allocation concealment provided in Morris 1999 and Rotman 1990. By personal communication, however, to Kathryn Winter, Co‐Director, Division of Biostatistics and Science, NRG Oncology SDMC/RTOG and Senior Director of Statistics, American College of Radiology, treatment allocations in either RTOG 7902 (Rotman 1990) or RTOG 9001 (Morris 1999) were made centrally by the RTOG Statistical Center using an algorithm that implemented a permuted block randomisation scheme (Winter 2017 [pers comm]). Therefore, Morris 1999 and Rotman 1990 had low risk of selection bias.
Blinding
None of the studies provided statements regarding blinding of participants, personnel, and outcome assessor. Although blinding of participants and personnel is not easily applicable in this study setting, the outcomes of interest, including survival, cancer recurrence, and grade 3 or 4 complications we deemed it unlikely to be affected by lack of blinding of participants and personnel. Therefore, blinding was at a low risk of bias in all included studies.
Incomplete outcome data
Asiri 2014 excluded relatively high numbers of participants (28 participants, 27.5%) after randomisation due to incomplete treatment protocol (11 participants), incomplete staging (seven participants), irregular follow‐up (six participants), and social reasons (four participants), leaving only 74 participants for analyses (36 in pelvic CRT group and 38 in extended‐field CRT group). Therefore, Asiri 2014 was at high risk of attrition bias.
Other included studies had low percentages of withdrawals and dropouts indicating a low risk of attrition bias (Chatani 1995; Haie 1988; Morris 1999; Rotman 1990; see Characteristics of included studies table for the proportion of participants analysed).
Selective reporting
None of the included studies reported quality of life and cost‐effectiveness. Chatani 1995 and Haie 1988 did not report OS, which is an important oncological outcome. Chatani 1995 did not report the severity of adverse events. So all included studies were at high risk of reporting bias.
Other potential sources of bias
The statistical analyses carried out in all included studies followed an intention‐to‐treat basis except Asiri 2014. Additionally, there was imbalance of allocation in Asiri 2014. Participants assigned to the extended‐field CRT group had a higher rate of common iliac node involvement than participants in the pelvic CRT group (36.8% with extended field RT versus 5.6% with pelvic CRT). Asiri 2014 applied a difference technique of RT between the groups. In pelvic CRT, all participants underwent a three‐dimensional conformal radiotherapy technique (3D‐CRT). However, approximately 20% of participants allocated to extended‐field CRT underwent the intensity‐modulated radiotherapy technique (IMRT) while the remaining 80% of participants underwent 3D‐CRT. For this domain, this indicated a high risk of bias in Asiri 2014, and low risk of bias in the other studies (Chatani 1995; Haie 1988; Morris 1999; Rotman 1990).
Effects of interventions
See: Table 1; Table 2; Table 3
In an attempt to cover a broad range of outcome measures, we added the rates of locoregional, distant, combined locoregional recurrences as additional secondary outcomes (see Differences between protocol and review). None of the studies reported the rate of non‐para‐aortic recurrence, quality of life, and cost‐effectiveness. HRs were estimated indirectly from published data.
Chatani 1995, Haie 1988, and Rotman 1990 compared extended‐field RT with pelvic RT. Morris 1999 compared extended‐field RT with pelvic CRT. Asiri 2014 compared extended‐field CRT versus pelvic CRT.
Extended‐field radiotherapy versus pelvic radiotherapy alone
Three studies compared extended‐field radiotherapy versus pelvic radiotherapy (Chatani 1995; Haie 1988; Rotman 1990).
Primary outcomes
Overall survival
Rotman 1990 reported OS and assessed 337 participants. We estimated HRs indirectly from published data of update report. Extended‐field RT reduced risk of death when compared to pelvic RT (HR 0.67, 95% CI 0.48 to 0.94; Analysis 1.1).
1.1. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 1 Overall survival.
Para‐aortic lymph node recurrence
Chatani 1995 and Haie 1988 reported the rate of para‐aortic lymph node recurrence. Meta‐analysis assessing 477 participants showed a lower risk of para‐aortic recurrence among participants given extended‐field RT than those given pelvic RT (RR 0.36, 95% CI 0.18 to 0.70; Analysis 1.2). The percentage of variability in effect estimates due to heterogeneity rather than to chance was not important (I² = 0%).
1.2. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 2 Para‐aortic lymph node recurrence.
Secondary outcomes
Progression‐free survival
Haie 1988 reported four‐year PFS. Four‐year PFS of participants allocated to extended‐field RT was 53.3%, which was slightly higher than that of 49.8% in the pelvic RT group. However, we could not estimate HR and the confidence in this data due to insufficient published data.
Updated data of Rotman 1990 reported the five‐year and 10‐year PFS (Rotman 1995). This study showed that there may have been little or no difference in risk of disease progression among participants given extended‐field RT compared to those given pelvic RT (HR 0.92, 95% CI 0.69 to 1.22; Analysis 1.3).
1.3. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 3 Progression‐free survival.
Locoregional recurrence
Meta‐analysis of three included studies, assessing 814 participants, showed no difference in risk of locoregional recurrence between participants who underwent extended‐field RT and those who underwent pelvic RT (RR 1.16, 95% CI 0.91 to 1.48; Analysis 1.4) (Chatani 1995; Haie 1988; Rotman 1990). The percentage of variability in effect estimates due to heterogeneity rather than to chance was not important (I² = 0%).
1.4. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 4 Locoregional recurrence.
Distant recurrence
Meta‐analysis of three studies, assessing 814 participants, showed that there may be little or no differences in risk of distant recurrence between participants who underwent extended‐field RT and those who underwent pelvic RT (RR 0.90, 95% CI 0.65 to 1.24; Analysis 1.5) (Chatani 1995; Haie 1988; Rotman 1990). The percentage of variability in effect estimates due to heterogeneity rather than to chance was not important (I² = 0%).
1.5. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 5 Distant recurrence.
Combined locoregional and distant recurrences
Rotman 1990, assessing 337 participants, found that participants given extended‐field RT had a lower risk of combined locoregional and distant metastasis than those given pelvic RT (RR 0.14, 95% CI 0.03 to 0.61; Analysis 1.6).
1.6. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 6 Combined locoregional/distant recurrence.
Adverse events
Both Haie 1988 and Rotman 1990 reported grade 3 or 4 perioperative complications but they did not specify the onset of these complications (see Differences between protocol and review). Meta‐analysis, assessing 776 participants, revealed that there may have been little or no difference in risk of severe complications between participants who underwent extended‐field RT and those who underwent pelvic RT (RR 1.05, 95% CI 0.79 to 1.41; Analysis 1.7). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance may have represented moderate heterogeneity (I² = 41%).
1.7. Analysis.
Comparison 1 Extended‐field radiotherapy (RT) versus pelvic RT, Outcome 7 Adverse events (onset not specified).
Chatani 1995 did not state the severity of complications observed in this study. We thus excluded Chatani 1995 from the analysis for this outcome.
Quality of life
None of the studies reported quality of life.
Cost‐effectiveness
None of the studies reported cost‐effectiveness.
Extended‐field radiotherapy versus pelvic chemoradiotherapy
One study compared extended‐field radiotherapy versus pelvic CRT (Morris 1999).
Primary outcomes
Overall survival
Updated data of Morris 1999, assessing 389 participants, reported a probable lower risk of death in participants undergoing pelvic CRT (HR 0.50, 95% CI 0.39 to 0.64; Analysis 2.1).
2.1. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 1 Overall survival.
Para‐aortic lymph node recurrence
Participants given extended‐field RT probably carried a lower risk of para‐aortic recurrence than those given pelvic CRT (RR 0.44, 95% CI 0.20 to 0.99; Analysis 2.2).
2.2. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 2 Para‐aortic lymph node recurrence.
Secondary outcomes
Progression‐free survival
An update data of Morris 1999 showed that there was a probable lower risk of disease progression among participants given pelvic CRT (HR 0.52, 95% CI 0.37 to 0.72; Analysis 2.3).
2.3. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 3 Progression‐free survival.
Locoregional recurrence
Participants undergoing extended‐field RT had higher risk of locoregional recurrence than those given pelvic CRT (RR 1.79, 95% CI 1.08 to 2.98; Analysis 2.4).
2.4. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 4 Locoregional recurrence.
Distant recurrence
There may have been little or no difference in the risk of distant recurrence between participants undergoing extended‐field RT and those who received pelvic CRT (RR 1.36, 95% CI 0.81 to 2.27; Analysis 2.5).
2.5. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 5 Distant recurrence.
Combined locoregional and distant recurrences
The risk of encountering combined locoregional and distant recurrences was probably higher among participants given extended‐field RT compared to those given pelvic CRT (RR 2.45, 95% CI 1.33 to 4.52; Analysis 2.6).
2.6. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 6 Combined locoregional/distant recurrence.
Adverse events
Acute complications
Participants undergoing extended‐field RT probably had a lower rate of acute grade 3 or 4 complications than those undergoing pelvic CRT (RR 0.05, 95% CI 0.02 to 0.11; Analysis 2.7).
2.7. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 7 Acute adverse events.
Late complications
There was little or no difference in the risk of late complications between participants undergoing extended‐field RT and those who received pelvic CRT (RR 1.06, 95% CI 0.69 to 1.62; Analysis 2.8).
2.8. Analysis.
Comparison 2 Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT), Outcome 8 Late adverse events.
Quality of life
The study did not report quality of life.
Cost‐effectiveness
The study did not report cost‐effectiveness.
Extended‐field chemoradiotherapy versus pelvic chemoradiotherapy
One small trial with relatively few events in each arm compared extended‐field CRT versus pelvic CRT (Asiri 2014).
Primary outcomes
Overall survival
Asiri 2014, assessing 74 participants, reported a probable decreased risk of death in participants receiving extended‐field CRT (HR 0.37, 95% CI 0.14 to 0.96; Analysis 3.1).
3.1. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 1 Overall survival.
Para‐aortic lymph node recurrence
There may have been little or no difference in the risk of para‐aortic recurrence between the two comparison groups (RR 0.19, 95% CI 0.02 to 1.54; Analysis 3.2).
3.2. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 2 Para‐aortic lymph node recurrence.
Secondary outcomes
Progression‐free survival
Extended‐field CRT lowered the risk of death when compared to pelvic CRT (HR 0.25, 95% CI 0.07 to 0.87; Analysis 3.3).
3.3. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 3 Progression‐free survival.
Locoregional recurrence
There may have been little or no difference in the risk of locoregional recurrence between participants undergoing extended‐field CRT and those who received pelvic CRT (RR 0.95, 95% CI 0.20 to 4.39; Analysis 3.4).
3.4. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 4 Locoregional recurrence.
Distant recurrence
There may have been little or no difference in the risk of distant recurrence between participants undergoing extended‐field CRT and those who received pelvic CRT (RR 0.54, 95% CI 0.17 to 1.69; Analysis 3.5).
3.5. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 5 Distant recurrence.
Combined locoregional and distant recurrences
There may have been little or no difference in the risk of developing combined locoregional and distant recurrences between participants undergoing extended‐field CRT and those who received pelvic CRT (RR 0.24, 95% CI 0.03 to 2.02; Analysis 3.6).
3.6. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 6 Combined locoregional/distant recurrence.
Adverse events
Acute complications
There may have been little or no difference in the risk of acute complications between participants undergoing extended‐field CRT and those who underwent pelvic CRT (RR 0.95, 95% CI 0.20 to 4.39; Analysis 3.7).
3.7. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 7 Acute adverse events.
Late complications
There may have been little or no difference in the risk of late complications between participants receiving extended‐field CRT and those who received pelvic CRT (RR 0.95, 95% CI 0.06 to 14.59; Analysis 3.8).
3.8. Analysis.
Comparison 3 Extended‐field chemoradiotherapy (CRT) versus pelvic CRT, Outcome 8 Late adverse events.
Quality of life
The study did not report quality of life.
Cost‐effectiveness
The study did not report cost‐effectiveness.
Discussion
Summary of main results
The findings of this review were based on five RCTs (seven reports). Three included studies compared extended‐field RT versus pelvic RT (Chatani 1995; Haie 1988; Rotman 1990). One included study compared extended‐field RT versus pelvic CRT (Morris 1999). One study compared extended‐field CRT to pelvic CRT (Asiri 2014). Four included studies had participants who were only recruited after imaging or surgical lymph node excision to exclude para‐aortic lymph node disease (Asiri 2014; Chatani 1995; Haie 1988; Morris 1999). In Rotman 1990, approximately 37% of participants did not undergo any para‐aortic lymph node status evaluation.
We observed the following main findings.
Compared with pelvic RT alone, extended‐field RT probably lowered the risk of death (HR 0.67, 95% CI 0.48 to 0.94; 1 study; 337 participants; moderate‐certainty evidence; Analysis 1.1), rates of para‐aortic lymph node recurrence (RR 0.36, 95% CI 0.18 to 0.70; 2 studies; 477 participants; moderate‐certainty evidence; Analysis 1.2), with probably little or no difference in the risks of disease progression (HR 0.92, 95% CI 0.69 to 1.22; 1 study; 337 participants; moderate‐certainty evidence; Analysis 1.3) and severe adverse events (RR 1.05, 95% CI 0.79 to 1.41; 2 studies; 776 participants; moderate‐certainty evidence; Analysis 1.7).
In a comparison of extended‐field RT versus pelvic CRT, participants undergoing pelvic CRT probably carried lower risks of death (HR 0.50, 95% CI 0.39 to 0.64; 1 study; 389 participants; moderate‐certainty evidence; Analysis 2.1), and disease progression (HR 0.52, 95% CI 0.37 to 0.72; 1 study; 389 participants; moderate‐certainty evidence; Analysis 2.3). Participants given extended‐field RT probably had lower risk of para‐aortic lymph node recurrence (HR 0.44, 95% CI 0.20 to 0.99; 1 study; 389 participants; low‐certainty evidence; Analysis 2.2) and severe acute adverse events (RR 0.05, 95% CI 0.02 to 0.11; 1 study; 388 participants; moderate ‐certainty evidence; Analysis 2.7). There may have been little or no difference in term of severe late adverse events among the comparison groups (RR 1.06, 95% CI 0.69 to 1.62; 1 study; 386 participants; moderate‐certainty evidence; Analysis 2.8).
Compared to pelvic CRT, extended‐field CRT may have lowered the risks of death (HR 0.37, 95% CI 0.14 to 0.96; 1 study; 74 participants; very low‐certainty evidence; Analysis 3.1) and disease progression (HR 0.25, 95% CI 0.07 to 0.87; 1 study; 74 participants; very low certainty evidence; Analysis 3.3). There may have been little or no difference in the risks of para‐aortic lymph node recurrence (RR 0.19, 95% CI 0.02 to 1.54; 1 study; 74 participants; very low‐certainty evidence; Analysis 3.2) and severe adverse events (acute: RR 0.95, 95% CI 0.20 to 4.39; 1 study; 74 participants; very low‐certainty evidence; Analysis 3.7; late: RR 0.95, 95% CI 0.06 to 14.59; 1 study; 74 participants; very low‐certainty evidence; Analysis 3.8).
Overall completeness and applicability of evidence
We identified seven published reports from five RCTs that included comparisons of extended‐field RT/CRT with other interventions. Primary outcomes of this review included OS and para‐aortic lymph node recurrence, which three studies reported. Secondary outcomes of this review were PFS, recurrence outside of the para‐aortic area (non‐para‐aortic), acute and late severe adverse events, quality of life, and cost‐effectiveness. All included studies reported PFS, but data from one included study were insufficient for calculating an HR.
We planned in the protocol to classify cancer recurrence into two groups including para‐aortic lymph node recurrence and non‐para‐aortic recurrence (Thamronganantasakul 2016). However, all included studies reported the patterns of cancer recurrences by locoregional, distant, and combined locoregional and distant recurrences. None of included studies specifically reported the rate of non‐para‐aortic recurrence. Therefore, we analysed this outcome by those that were reported in the studies.
In addition, the review protocol stated that we intended to analyse severe adverse events by timing of onset; early and late onset (Thamronganantasakul 2016). Two included studies, which compared extended‐field RT versus pelvic RT alone, only reported overall severe adverse event rates. Therefore, the rate of severe adverse events in this comparison was assessed as one composite outcome. None of the studies reported data on quality of life and cost‐effectiveness.
The following findings have to be kept in mind when considering the applicability of the evidence presented in this review.
Most participants were diagnosed with squamous cell carcinoma or adenocarcinoma of the cervix, thus limiting the applicability of the existing results to women with other less common histological types.
The main methods used for evaluating the status of para‐aortic lymph nodes in the included studies were older radiological imaging techniques including computed tomography (CT), magnetic resonance imaging (MRI), or lymphangiogram. Only a few participants underwent evaluation of para‐aortic lymph node by surgery. Therefore, the evidence presented may not be fully applicable to a group of women undergoing an assessment of para‐aortic lymph node status done by surgery or more advanced imaging techniques, such as a PET scan.
The benefit in terms of improving OS noted among participants undergoing an extended‐field RT compared to those given pelvic RT alone was obtained from one study in which approximately 37% of participants did not undergo any para‐aortic lymph node evaluation. There were no results of subgroup analysis according to the status of para‐aortic lymph node evaluation. Extrapolation of this result in a cohort of participants given an adequate assessment of para‐aortic lymph node status would be questionable.
In regard to RT technique, RT given in the comparisons of extended‐field RT versus pelvic RT alone and extended‐field RT versus pelvic CRT was accomplished through conventional techniques using megavoltage equipment. Generalisation of the findings obtained from these two comparisons to a group of participants undergoing more advanced RT techniques (i.e. 3D‐CRT or IMRT), which better focus the RT beam and cause less damage to normal tissues, should be done with great caution.
In a comparison of extended‐field RT versus pelvic CRT, participants who received pelvic CRT experienced a higher rate of acute severe adverse events than would have been expected in current clinical practice. This finding should be viewed with extreme caution, especially because chemotherapy given in this study was a combination of cisplatin and fluorouracil. Therefore, the results should not be extrapolated to women receiving another chemotherapy regimen, particularly among those given single weekly cisplatin, which is the current gold‐standard concurrent chemotherapy regimen used in CRT for treating cervical cancer.
Quality of the evidence
It was planned in the protocol to assess the certainty of evidence by seven relevant outcomes including: OS, PFS, para‐aortic recurrence, non‐para‐aortic recurrence, severe adverse events, quality of life, and cost‐effectiveness (Thamronganantasakul 2016). None of the studies reported data for non‐para‐aortic recurrences, quality of life, and cost‐effectiveness. A relatively high number of participants (27.5%) in Asiri 2014 were excluded after randomisation, thus indicating a high risk of incomplete outcome data. Other included studies had low percentages of withdrawals and dropouts, indicating a low risk of bias in this domain (see Characteristics of included studies table for proportion of participants analysed).
As the review included only five studies, evidence was derived from relatively few studies and participants.
Using GRADE assessments, we graded the certainty of evidence is as follows.
Extended‐field RT versus pelvic RT: the evidence for all outcomes was of moderate certainty. The certainty of evidence was downgraded because of concerns regarding an imprecise estimates, as indicated by the 95% CIs.
Extended‐field RT versus pelvic CRT: the evidence regarding OS, PFS, and late adverse events were of moderate certainty. The certainty of evidence for these outcomes was downgraded due to concerns regarding imprecision. The evidence for rate of para‐aortic recurrence and acute adverse events were downgraded to moderate‐certainty because of concerns regarding the serious imprecision of the pooled estimates.
Extended‐field CRT versus pelvic CRT: the evidence for all outcomes was of very‐low certainty because of concerns regarding the high risk of bias and serious imprecision of the pooled results.
Potential biases in the review process
With assistance from the Information Specialist of the Cochrane Gynaecological, Neuro‐oncology & Orphan Cancers, we were able to conduct a comprehensive literature search, including a search of the grey literature, conference proceedings and abstracts, citation lists of included studies, and registered databases of ongoing trials. In addition, bias was minimised in terms of the overall review process recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
As this review included only five studies, there remains the possibility of publication bias. We did not perform a funnel plot, as the analyses were limited to only one or two included studies. Because of a few included studies, we did not carry out sensitivity and subgroup analyses as previously stated in the review protocol (Thamronganantasakul 2016).
There were no issues associated to bias secondary to conflicts of interests of the authors of this review.
Agreements and disagreements with other studies or reviews
Sapienza 2017 conducted a systematic review investigating the impact of extended‐field RT on the risks of distant metastasis and cancer‐related death in women with advanced‐stage cervical cancer. This review included four RCTs with several comparisons. Three included studies compared extended‐field RT versus pelvic RT alone, which were also included in the review. One RCT that Sapienza 2017 included compared preoperative 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) with those not given the tomography (Tsai 2010). Participants with extrapelvic metastasis on FDG‐PET received pelvic RT alone and the remainder of participants received extended‐field RT. Chatani 1995, which was also included in Sapienza 2017, compared extended‐field RT and pelvic RT in 36 women who underwent RT as a primary treatment and 57 women who received RT following radical hysterectomy. Sapienza 2017 pooled the results of four RCTs for para‐aortic lymph node recurrence and distant recurrence and two RCTs for cancer‐related death. They noted that extended‐field RT reduced the rate of para‐aortic recurrence (HR 0.53, 95% CI 0.19 to 0.64) and other distant metastases (HR 0.69, 95% CI 0.50 to 0.96), but conferred no clear difference in the risk of cancer‐related death between the comparison groups (HR 0.68, 95% CI 0.45 to 1.01).
There are important differences between the review by Sapienza 2017 and the present review. Firstly, the outcomes to each comparison type were examined and the effects of extended‐field RT more thoroughly separately analysed and we assessed the impact on OS, PFS, locoregional recurrence, and severe adverse events. Secondly, the present review question was more specific. The review was only interested in assesing if there was any clinical benefit and the possible harm of extended‐field RT given as a primary treatment for locally cervical cancer. Therefore, data for only 36 participants who were assigned to receive RT as a primary treatment in Chatani 1995 were extracted. Thirdly, we excluded Tsai 2010 as the participants were not randomly assigned to the types of RT given.
However, we do agree with Sapienza 2017 that applicability of the current evidence to the modern day practice may be limited. The RT techniques and chemotherapy regimens used in the previous studies are now outmoded for many clinical settings.
Authors' conclusions
Implications for practice.
The studies in this review largely used now non‐standard radiotherapy (RT) and chemotherapy regimens. Therefore, it is difficult to draw any firm conclusions. However, based on existing evidence presented in this review:
pelvic chemoradiotherapy (CRT) probably has a lower risk of death and disease progression than extended‐field RT in treatment of women with locally advanced cervical cancer, despite a probable increase in acute severe adverse events;
extended‐field RT may improve survival in women who are unable to have concurrent chemotherapy. This is likely due to better control of the para‐aortic area when compared to pelvic RT alone. However, this finding should be interpreted with caution as it was obtained from one study in which approximately one‐third of women did not undergo any para‐aortic lymph node evaluation;
although extended‐field CRT may improve overall and progression‐free survival when compared to pelvic CRT, the benefits and harm of extended‐field CRT should be regarded with caution due to a small number of reported adverse events and serious imprecision of data noted in one small included study. Further research is highly likely to have an important impact on the estimates of treatment effects and may alter existing estimates in this comparison.
Implications for research.
High‐quality randomised controlled trials (RCTs) are needed to inform treatment of locally advanced cervical cancer in the era of modern RT techniques. Although CRT could improve survival, some women may not be able to tolerate this treatment combination. There is a need for an adequately sized RCT for evaluating the effectiveness and safety of an extended‐field RT given in a selected group of women suitable for RT treatment alone who have undergone adequate assessment of para‐aortic lymph nodes.
Due to the lack of sufficient data, further high‐quality RCTs are required to confirm the effectiveness and safety of an extended‐field CRT compared to standard pelvic CRT before it can be considered for use outside of clinical trials.
In addition, the benefits and harms of extended‐field RT need to be reassessed in further randomised studies using modern chemotherapy regimens and modern RT techniques (e.g. volumetric‐modulated arc therapy (VMAT) and tomotherapy which are likely to allow extended‐field RT to be better tolerated). Importantly, any further studies should include a mandatory assessment on the impact on quality of life and cost‐effectiveness.
Acknowledgements
We thank Jo Morrison for clinical and editorial advice; Jo Platt for designing the search strategy; and Gail Quinn, Clare Jess, and Tracey Harrison for their contribution to the editorial process. We would like to acknowledge Emeritus Professor Dr James A Will, University of Wisconsin, Madison, WI, for editing the manuscript via Publication Clinic KKU, Thailand.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National health Service, or the Department of Health.
Appendices
Appendix 1. Staging classification of cervical cancer
| FIGO Stage | Description | ||
| I | — | — | Carcinoma confined to the cervix. |
| — | IA | — | Invasive cancer identified only microscopically. (All gross lesions even with superficial invasion are stage IB cancers.) Invasion is limited to measured stromal invasion with a maximum depth of 5 mm and no wider than 7 mm. |
| — | — | IA1 | Measured invasion of stroma ≤ 3 mm in depth and ≤ 7 mm width. |
| — | — | IA2 | Measured invasion of stroma > 3 mm and < 5 mm in depth and ≤ 7 mm width. |
| — | IB | — | Clinical lesions confined to the cervix, or preclinical lesions greater than stage IA. |
| — | — | IB1 | Clinical lesions no greater than 4 cm in size. |
| — | — | IB2 | Clinical lesions > 4 cm in size. |
| II | — | — | Carcinoma extends beyond the uterus, but has not extended onto the pelvic wall or to the lower third of vagina. |
| — | IIA | — | Involvement of up to the upper 2/3 of the vagina. No obvious parametrial involvement. |
| — | — | IIA1 | Clinically visible lesion ≤ 4 cm. |
| — | — | IIA2 | Clinically visible lesion > 4 cm. |
| — | IIB | — | Parametrial involvement but not onto the pelvic sidewall. |
| III | — | — | Carcinoma has extended onto the pelvic sidewall. On rectal examination, there is no cancer‐free space between the tumour and pelvic sidewall. The tumour involves the lower 1/3 of the vagina. All cases of hydronephrosis or non‐functioning kidney should be included unless they are known to be due to other causes. |
| — | IIIA | — | Involvement of the lower vagina but no extension onto pelvic sidewall. |
| — | IIIB | — | Extension onto the pelvic sidewall, or hydronephrosis/non‐functioning kidney. |
| IV | — | — | Carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum (or both). |
| — | IVA | — | Spread to adjacent pelvic organs. |
| — | IVB | — | Spread to distant organs. |
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Uterine Cervical Neoplasms] this term only #2 cervi* near/5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*) 3 #1 or #2 #4 MeSH descriptor: [Radiotherapy] explode all trees #5 radiotherap* #6 irradiat* or radiat* #7 Any MeSH descriptor with qualifier(s): [Radiotherapy ‐ RT] #8 #4 or #5 or #6 or #7 #9 para‐aortic or paraaortic or peri‐aortic or periaortic 10 extended field #11 #9 or #10 #12 #3 and #8 and #11
Appendix 3. MEDLINE Ovid search strategy
1. Uterine Cervical Neoplasms/ 2. (cervi* adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3. 1 or 2 4. exp Radiotherapy/ 5. radiotherap*.mp. 6. (irradiat* or radiat*).mp. 7. radiotherapy.fs. 8. 4 or 5 or 6 or 7 9. (para‐aortic or paraaortic or peri‐aortic or periaortic).mp 10. extended field.mp. 11. 9 or 10 12. 3 and 8 and 11 13. randomized controlled trial.pt. 14. controlled clinical trial.pt. 15. randomized.ab. 6. placebo.ab. 17. clinical trials as topic.sh. 18. randomly.ab. 19. trial.ab. 20. 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 12 and 20
Key: mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier
sh=subject heading; ab=abstract; pt=publication type
Appendix 4. Embase Ovid search strategy
1. exp uterine cervix tumor/ 2. (cervi* adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3. 1 or 2 4. exp radiotherapy/ 5. radiotherap*.mp. 6. (irradiat* or radiat*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 7. rt.f 8. 4 or 5 or 6 or 7 9. (para‐aortic or paraaortic or peri‐aortic or periaortic).mp. 10. extended field.mp. 11. 9 or 10 12. 3 and 8 and 11 13. crossover procedure/ 14. double‐blind procedure/ 15. randomized controlled trial/ 16. single‐blind procedure/ 17. random*.mp. 18. factorial*.mp. 9. (crossover* or cross over* or cross‐over*).mp. 20. placebo*.mp. 21. (double* adj blind*).mp. 22. (singl* adj blind*).mp. 23. assign*.mp. 24. allocat*.mp. 25. volunteer*.mp. 26. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. 12 and 26
Key: mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 5. 'Risk of bias' assessment
Assessment of risks of bias were based on Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Random sequence generation
Low risk of bias (e.g. participants assigned to treatments on the basis of a computer‐generated random sequence or a table of random numbers).
High risk of bias (e.g. participants assigned to treatments on the basis of date of birth, clinic identity number or surname, or no attempt to randomise participants).
Unclear risk of bias (e.g. not reported, information not available).
-
Allocation concealment
Low risk of bias (e.g. where the allocation sequence could not be foretold).
High risk of bias (e.g. allocation sequence could be foretold by participants, investigators, or treatment providers).
Unclear risk of bias (e.g. not reported).
-
Blinding of participants and personnel
Low risk of bias if participants and personnel were adequately blinded
High risk of bias if participants were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
-
Blinding of outcomes assessors
Low risk of bias if outcome assessors were adequately blinded.
High risk of bias if outcome assessors were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or is unclear.
-
Incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded a satisfactory level of loss to follow‐up for each outcome as:
low risk of bias (e.g. if less than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms);
high risk of bias (e.g. if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms);
unclear risk of bias (e.g. if loss to follow‐up was not reported).
-
Selective reporting of outcomes
Low risk of bias (e.g. review reported all outcomes specified in the protocol).
High risk of bias (e.g. study is suspected to have outcomes that have been selectively reported).
Unclear risk of bias (e.g. unclear whether outcomes have been selectively reported).
-
Other bias
Low risk of bias (e.g. the review authors did not suspect any other source of bias and the trial appeared to be methodologically sound).
High risk of bias (e.g. the review authors suspected that the trial was prone to an additional bias).
Unclear risk of bias (e.g. the review authors were uncertain whether an additional bias may have been present).
Data and analyses
Comparison 1. Extended‐field radiotherapy (RT) versus pelvic RT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Para‐aortic lymph node recurrence | 2 | 477 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.18, 0.70] |
| 3 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Locoregional recurrence | 3 | 814 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.91, 1.48] |
| 5 Distant recurrence | 3 | 814 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.65, 1.24] |
| 6 Combined locoregional/distant recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Adverse events (onset not specified) | 2 | 776 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.79, 1.41] |
Comparison 2. Extended‐field radiotherapy (RT) versus pelvic chemoradiotherapy (CRT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Para‐aortic lymph node recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Locoregional recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Distant recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Combined locoregional/distant recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Acute adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8 Late adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 3. Extended‐field chemoradiotherapy (CRT) versus pelvic CRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Para‐aortic lymph node recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Locoregional recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Distant recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Combined locoregional/distant recurrence | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Acute adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8 Late adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asiri 2014.
| Methods | 2‐armed parallel, RCT Study duration: July 2007 and April 2008 |
|
| Participants | 74 women with histopathologically confirmed squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma, and radiologically negative para‐aortic lymph node cervical cancer stage IIB‐IVA. WP‐CCRT (50 participants) vs EF‐CCRT (52 participants), followed by HDR brachytherapy. Eligibility criteria: histologically confirmed squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the cervix uteri; clinical and radiological FIGO stage IIB–IVA, with no other evidence of distant metastasis outside the pelvis; ECOG performance status 0–2; negative para‐aortic lymph node on imaging CT, MRI, or FDG‐PET; and normal haematological, hepatic, and renal functions tests (white blood cell count ≥ 4000/mm³, absolute neutrophil count ≥ 1500/mm³, platelet count ≥ 100,000/mm³, total bilirubin ≤ 1.5 mg/dL, alanine transaminase ≤ 2 × normal, creatinine ≤ 1.5 mg/dL). Exclusion criteria: history of hysterectomy, retroperitoneal surgery, abdominal or pelvic RT, prior chemotherapy, pregnancy, or positive para‐aortic nodes on imaging or biopsy‐confirmed para‐aortic lymph node metastasis. 66% of participants were diagnosed with stage IIB. 38 (51.4%) participants had iliac/common iliac nodal involvement detected by pretreatment MRI. |
|
| Interventions | Control arm: WP‐CCRT Intervention arm: EF‐CCRT RT technique: all participants were scanned, for simulation, on a CT simulator. 91.7% of participants; equally spaced, coplanar 3D‐CRT field plans (box‐field) were applied; however, IMRT was also generated in 8.3% of participants to achieve better dose distribution. Pelvic RT technique applied in EF‐CCRT was similar to those for the WP‐CCRT group, and para‐aortic fields were added as a continuous area or with a half‐beam block, with a superior field border at the junction of T12/L1, to cover para‐aortic lymph node up to the level of the renal hila. HDR‐brachytherapy with iridium‐192 sources was given once per week, following 45 Gy of EBRT. A dose of 7 Gy per fraction, using 3 insertions to point A with a total dose of 21 Gy, was delivered, based on the dose limit derived from the treatment plan for the rectum and bladder. Both groups of participants received weekly cisplatin 40 mg/m² before 6 doses of RT. |
|
| Outcomes | Primary endpoint: locoregional recurrence (pelvic and paraaortic control) Other endpoints: distant metastasis, survival outcomes (disease‐free survival and overall survival), treatment‐related toxicities |
|
| Notes | 102 participants randomised. However, 28 (27.5%) participants were excluded after randomisation due to incomplete treatment protocol (11 participants), incomplete staging (7 participants), irregular follow‐up (6 participants), and social reason (4 participants), leaving 74 participants for analyses (36 in WP‐CCRT and 38 in EF‐CCRT). Imbalance of participant allocation was noted. Participants assigned to EF‐CCRT group had a higher rate of common iliac node involvement determined by pretreatment MRI than those allocated to WP‐CCRT group (36.8% with EF‐CCRT vs 5.6% with WP‐CCRT). Technique of RT was different between the groups. In WP‐CCRT, all participants underwent 3D‐CRT. Approximately 20% of participants allocated to EF‐CCRT, however, underwent IMRT technique while the remaining 80% of participants underwent 3D‐CRT which could have resulted in a differences of treatment‐related toxicity. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement regarding method of random sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | No statement regarding allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No statement regarding blinding of participants and personnel. However, the outcomes of interest were unlikely to be affected by lack of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No statement regarding blinding of outcome assessment. However. the outcomes of interest were unlikely to be affected by lack of blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 102 participants randomised. 28 (27.5%) participants excluded after randomisation due to incomplete treatment protocol (11 participants), incomplete staging (7 participants), irregular follow‐up (6 participants), and social reason (4 participants), leaving 74 participants for final analyses (36 in WR‐CCRT group and 38 in EF‐CCRT group). |
| Selective reporting (reporting bias) | High risk | No data on quality of life and cost‐effectiveness reported. |
| Other bias | High risk | Participants assigned to EF‐CCRT group had a higher rate of common iliac node involvement determined by pretreatment MRI than those allocated to WP‐CCRT group (36.8% with EF‐CCRT vs 5.6% with WP‐CCRT). |
Chatani 1995.
| Methods | Randomised study conducted at a University Hospital in Japan. Study duration: November 1986 and October 1990. |
|
| Participants | 93 women with cervical carcinoma randomly allocated. 36 participants received external RT and intracavitary brachytherapy as a primary therapy and 57 participants received RT as an adjuvant treatment following extended radical hysterectomy and external RT. All participants had histologically confirmed carcinoma of the uterine cervix with negative para‐aortic lymph node status detected by CT. Positive para‐aortic status defined as a single node of ≥ 15 mm and multiple nodes of ≥ 10 mm in diameter. This study excluded women who had RTCOG status > 3, and aged > 75 years, and women with active gastric and duodenal ulcer. The distribution of pretreatment characteristics of the participants including stage of disease, histological subtypes of cancer, age, and performance status were well balance between groups. |
|
| Interventions | Control arm: pelvic RT Intervention arm: extended‐field RT RT technique: source of beam energy for external RT was a megavoltage machine. Brachytherapy was conducted following external RT using 60Co HDR remote after loading. Extended‐field RT was delivered through anterior‐posterior fields. |
|
| Outcomes | 3‐year cause‐specific survival, local failure, distant metastasis, and complications | |
| Notes | No data regarding overall and progression‐free survival. In addition, the authors did not state the severity of complications observed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement regarding the method used to generate the allocation sequence. The authors only stated that participants were randomised based on a randomised scheme of Peto 1977. |
| Allocation concealment (selection bias) | Unclear risk | No statement regarding the method used to conceal the allocation sequence. The authors only stated that participants were randomised based on a randomised scheme of Peto 1977. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No statement regarding the blinding of participants and personnel. However, outcomes of interest were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No statement regarding blinding of outcome assessment. However, outcomes of interest were unlikely to be affected by lack of blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analyses based on all participants. |
| Selective reporting (reporting bias) | High risk | No data regarding overall, progression‐free survival, quality of life, and cost‐effectiveness. In addition, the authors did not state the severity of complications observed in this study. |
| Other bias | Low risk | Analyses based on an intention‐to‐treat basis. |
Haie 1988.
| Methods | Multicentre, 2‐armed parallel, RCT conducted by the Radiotherapy Cooperative Group of EORTC. Study duration: November 1977 to July 1981 |
|
| Participants | 441 previously untreated women with histologically confirmed carcinoma of the cervix with high risk of subclinical para‐aortic node metastases. 3 high‐risk groups of para‐aortic node metastasis applied in this study including: group 1: stages I and IIB proximal, i.e. with proximal vaginal or parametrial (or both) involvement, with histologically positive pelvic lymph nodes after surgery; group 2: stages I and IIB proximal without surgery and with positive pelvic lymph nodes on lymphangiogram; group 3: stages IIB distal, i.e. with distal vaginal or parametrial (or both) involvement and IIIb regardless of pelvic nodal status on lymphangiogram. Exclusion criteria: para‐aortic node involvement confirmed either by surgery or diagnosed on lymphangiogram; no lymphangiogram results or with a non‐interpretable lymphangiogram; urinary tract obstruction; higher risk of postoperative RT complications, i.e. previous abdominopelvic surgery or inflammatory bowel disease; previous history of other primary cancer, excluding basal cell carcinoma of the skin; pregnancy; and aged > 75 years. |
|
| Interventions | Control arm: pelvic RT. The upper limit of the field was the lower border of L4. Intervention arm: pelvic and para‐aortic RT. The pelvic field was extended to cover the para‐aortic nodes to the upper border of L1. RT technique: either 2 fields or 4 fields (box technique) for field configuration during EBRT. Source of beam energy was a megavoltage machine. The dose delivered to the pelvis was 40–50 Gy over 4–6 weeks using megavoltage machines. Further pelvic RT was given either by intracavitary brachytherapy or localised EBRT depending on each centre's practice. The external beam dose to the para‐aortic area was fixed at 45 Gy in 5 weeks. No chemotherapy administered. |
|
| Outcomes | Primary endpoint: 4‐year disease‐free survival Other outcomes: pelvic failure (no complete disappearance of all detectable pelvic disease or local recurrence); para‐aortic metastasis; distant metastasis other than para‐aortic metastasis; and 4‐year severe complication (grade 3 or 4) rate. However, this study did not report overall survival which is the most important outcome in research on cancer treatment. |
|
| Notes | Analyses based on an intention‐to‐treat basis. The distributions of important baseline characteristics of participants including participant age, histological subtypes, and FIGO stage were similar between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors stated that order of treatment allocation was determined by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Authors stated that participants were randomised by drawing a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No statement regarding blinding of participants and personnel; however, outcomes of interest were unlikely to be affected by lack of blinding of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No statement regarding blinding of outcome assessor; however, outcomes of interest were unlikely to be affected by lack of blinding of outcome assessment. There was a well‐defined protocol for post‐treatment surveillance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 214 (94%) participants in the pelvic RT group and 201 (94%) in the extended‐field RT group were followed for a minimum of 4 years and available for analysis of primary outcome. |
| Selective reporting (reporting bias) | High risk | No overall survival, quality of life, and cost‐effectiveness were reported which are important oncological outcomes. |
| Other bias | Low risk | Analyses based on an intention‐to‐treat basis. |
Morris 1999.
| Methods | 2‐armed parallel, RCT conducted by the RTOG; study number RTOG 90‐01. Study duration: 1990–1997 |
|
| Participants | 388 women with FIGO stages IIB–IVA, squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the cervix or stage IB or IIA of 1 of these cancers with a tumour diameter ≥ 5 cm or biopsy‐confirmed metastasis to pelvic lymph nodes. Other inclusion criteria included Karnofsky performance score ≥ 60 and normal results of full blood counts and serum levels of urea, creatinine, and bilirubin. Exclusion criteria: disease outside the pelvic area or spread to para‐aortic lymph nodes; prior cancer other than cutaneous basal‐cell carcinoma; medical contraindications to chemotherapy; other rare histological subtypes; and prior hysterectomy or transperitoneal staging procedure for cervical cancer, pelvic RT, or systemic chemotherapy. Participants in each treatment group were stratified according to the tumour stage (IB, IIA, or IIB vs III or IVA) and the staging method used for para‐aortic lymph nodes (clinical vs surgical). 73.7% of participants underwent lymphangiography for para‐aortic lymph node evaluation. Approximately 40% of participants had cervical cancer stage III–IVA. Median tumour diameter in participants with stage IB and IIA was 6 cm. Allocations of participants were well balance between groups determined by equal distribution of characteristics of participants. |
|
| Interventions | Intervention arm: 45 Gy of EBRT given to the pelvis and para‐aortic lymph nodes. Control arm: 45 Gy of EBRT given to the pelvis alone plus 3 cycles of fluorouracil and cisplatin ( intravenous infusion of cisplatin 75 mg/m² of body‐surface area over a 4‐hour period followed by an intravenous infusion of fluorouracil 4000 mg/m² over a 96‐hour period; days 1–5; days 22–26 of RT; and during the time of the second intracavitary RT session). RT technique: either 2 fields or 4 fields (box technique) for field configuration during EBRT. Source of beam energy was a megavoltage machine. Brachytherapy using low‐dose‐rate intracavitary RT was performed within 2 weeks (preferably < 1 week) after the completion of pelvic radiation. For participants given RT plus systemic chemotherapy, the treatment field began from the space between L4 and L5 to the mid pubis or to a line 4 cm below the most distal vaginal or cervical lesion. Lateral fields were designed to encompass S3 posteriorly, with a margin of ≥ 3 cm from primary lesion. Shielding was designed to cover the areas of the pelvic lymph nodes, with a margin of ≥ 1–1.5 cm. For participants given RT alone, the pelvic and para‐aortic areas were treated as a continuous area, with a superior field border at the space between L1 and L2. The radiation dose was calculated at the midplane in the central ray of the field (for anteroposterior‐posteroanterior fields) or to the isocentre of the beams. |
|
| Outcomes | Primary endpoint: 5‐year and 8‐year overall survival Other study endpoints: 5‐year and 8‐year disease‐free survival rates of locoregional failure, para‐aortic failure, and distance metastasis, and grade 3–4 treatment‐related adverse effects within 60 days |
|
| Notes | The second round of interim analysis of this study, which was conducted when achieving the enrolment goal (July 1998), found the significant difference in terms of survival between groups that met the requirement for early reporting. The results presented in this study were updated using the results received by 11 November 1998. Median follow‐up time in Morris 1999 was 43 months. Eifel 2004 is an updated result of Morris 1999. The median follow‐up time of the participants in Eifel 2004 was 6.6 years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No statement regarding the method applied for random sequence generation provided in the published article. Kathryn Winter, Co‐Director, Division of Biostatistics and Science, NRG Oncology SDMC/RTOG and Senior Director of Statistics, American College of Radiology, confirmed that this study applied an algorithm that implemented a permuted block randomisation scheme (Winter 2017 [pers comm]). |
| Allocation concealment (selection bias) | Low risk | No statement regarding allocation concealment provided in the published article. Kathryn Winter, Co‐Director, Division of Biostatistics and Science, NRG Oncology SDMC/RTOG and Senior Director of Statistics, American College of Radiology, confirmed that central allocation was applied in this study (RTOG 9001) (Winter 2017 [pers comm]). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No statement regarding blinding of participants and personnel; however, outcomes of interest were unlikely to be affected by lack of blinding of outcome assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No statement regarding blinding of outcome assessor; however, outcomes of interest were unlikely to be affected by lack of blinding of outcome assessment. There was a well‐defined protocol for post‐treatment surveillance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 15 (4%) participants were excluded after randomisation (6 in chemoradiation group and 9 in extended‐field RT group). Data used during the analyses of primary outcomes were obtained from approximately 96.5% of participants. |
| Selective reporting (reporting bias) | High risk | No data on quality of life and cost‐effectiveness reported. |
| Other bias | Low risk | Data analysed according to an intention‐to‐treat basis. The distributions of baseline characteristics of the participants which have had an impact on the treatment outcomes including age, performance status, histological subtypes, FIGO stage, and pelvic lymph node involvement were balanced between groups. |
Rotman 1990.
| Methods | Multicentre, 2‐armed parallel, RCT conducted by the RTOG; study number RTOG 79‐20 Study duration: November 1979 to October 1986 |
|
| Participants | Enrolled 367 women to receive either pelvic RT alone or pelvic + para‐aortic radiation. However, 23 participants had insufficient data for any evaluation, leaving 335 participants for final analysis. Inclusion criteria: cervical cancer stage IIB without clinical evidence of para‐aortic nodal involvement; and people with stages IB and IIA who had large tumour sizes (≥ 4 cm in lateral dimension) determined by digital examination. Exclusion criteria: Karnofsky performance status < 40; inadequate bone marrow reserve; poor renal function (urea > 30 mg%); and prior curative surgery. 73.1% of participants were diagnosed with cervical cancer stage IIB. The distribution of pretreatment characteristics of the participants including stage of disease, histological subtypes of cancer, proportion of participants undergoing para‐aortic node evaluation, age, and performance status were well balanced between groups. |
|
| Interventions | Control arm: pelvic RT Intervention arm: pelvic and para‐aortic RT RT technique: RT to the pelvis was accomplished through megavoltage equipment with energy of ≥ 60Co delivering a total mid‐depth dose of 40–50 Gy in 4.5–6.5 weeks. The dose rate was 1.6–1.8 Gy per day for 5 days per week. Either 2 fields or 4 fields (box technique) for field configuration during EBRT used. Maximum of 30 Gy was allowed to be delivered to the pelvis through opposing anterior‐posterior portals. Minimum of 20 Gy was to be delivered through opposing lateral portals to the mid depth on the central axis of the fields. Opposing fields were treated daily throughout the course of treatment. External RT was delivered prior to intracavitary applications. For participants given para‐aortic node RT, 44–45 Gy was delivered in 4.5–5 weeks. After completion of EBRT, all participants received additional RT delivered by intracavitary brachytherapy using radium or caesium sources delivering 30–40 Gy to Point A. No chemotherapy administered. |
|
| Outcomes | Primary endpoints: 2‐year and 5‐year overall survival Other endpoints: rate of complete response, locoregional control, time to distant metastases, disease‐free survival, and rate of severe (grade ≥ 3) complications |
|
| Notes | Median follow‐up time of the early report was 6 years. Rotman 1995 is updated results of Rotman 1990. In this updated report, every participant could have been followed up for at least 8 years, and 55% of the participants could have been followed up for at least 10 years. In Rotman 1995, the primary endpoints were the 5‐year and 10‐year overall survival. Other endpoints were rate of complete response, locoregional control, time to distant metastases, disease‐free survival, and rate of severe (grade ≥ 3) complications. The final analyses for this updated report were carried out among 337 participants (167 in pelvic only RT and 170 in pelvic plus para‐aortic RT). 30 cases that were included in the first report were subsequently excluded from all analyses in the updated report due to a withdrawal of 1 participating site and incomplete data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No methods of random sequence generation provided in the published article. Kathryn Winter, Co‐Director, Division of Biostatistics and Science, NRG Oncology SDMC/RTOG and Senior Director of Statistics, American College of Radiology; however, confirmed that this RCT (RTOG 7902) applied an algorithm that implemented a permuted block randomisation scheme (Winter 2017 [pers comm]). |
| Allocation concealment (selection bias) | Low risk | Central allocation using a telephone call to RTOG Headquarters was made where eligibility was confirmed to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No statement regarding blinding of participants and personnel; however, outcomes of interest were unlikely to be affected by lack of blinding of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No statement regarding blinding of outcome assessor; however, outcomes of interest were unlikely to be affected by lack of blinding of outcome assessment. There was a well‐defined protocol for post‐treatment surveillance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 30 (8.2%) participants included in this report were subsequently excluded from all analyses in this updated report due to a withdrawal of 1 participating site and incomplete data. |
| Selective reporting (reporting bias) | High risk | No data on quality of life and cost‐effectiveness reported. |
| Other bias | Low risk | Data analysed according to an intention‐to‐treat basis. |
3D‐CRT: 3‐dimensional conformal radiotherapy; CT: computed tomography; EBRT: external‐beam radiotherapy; ECOG: Eastern Cooperative Oncology Group; EF‐CCRT: extended‐field concurrent chemoradiation; EORTC: European Organisation for Research and Treatment of Cancer; FDG‐PET: 18F‐fluorodeoxyglucose positron emission tomography; FIGO: Federation of Gynecology and Obstetrics; HDR: high‐dose rate; IMRT: intensity‐modulated radiotherapy; MRI: magnetic resonance imaging; RCT: randomised controlled trial; RT: radiotherapy; RTOG: Radiation Therapy Oncology Group; WP‐CCRT: whole‐pelvis concurrent chemoradiation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Du 2010 | Prospectively examined 60 women with cervical cancer who had para‐aortic lymph node metastasis and who were undergoing whole‐pelvis radiotherapy followed by brachytherapy. The results of participants undergoing extended‐field radiotherapy using IMRT were compared with those given the 3D technique. Not a randomised study. |
| Kim 2016 | Randomised phase II multi‐institutional study conducted by the Korean Radiation Oncology Group to determine the survival benefit of prophylactic extended‐field radiotherapy for locally advanced cervical cancer relative to the hypoxic level. Participants were divided into 2 comparison groups by the result of CA9 immunohistochemical staining (CA9‐positive and CA9‐negative) and then were further randomly allocated to extended‐field radiotherapy and pelvic radiotherapy arms. However, excluded because the results were reported according to the status of CA9 expression. No data available for comparing extended‐field radiotherapy and pelvic radiotherapy. |
| Liang 2014 | Prospectively included 32 women with stage IB2–IIIB cervical cancer with positive para‐aortic lymph node and negative para‐aortic lymph node. All participants underwent low‐dose prophylactic extended‐field, IMRT + concurrent weekly cisplatin. Non‐randomised study design. |
| Lin 2015 | Updated report of a previously published randomised trial (Tsai 2010) that was undertaken to assess the impact of FDG‐PET on the detection of extrapelvic disease and improvement of survival among people with stage I–IVA cervical cancer who had enlarged pelvic lymph node on MRI. Pelvic radiotherapy was given to participants found to have no extrapelvic findings on PET. Extended‐field radiotherapy was given for the remainder of the participants. All participants received 6 cycles of weekly intravenous infusion of cisplatin (50 mg/m² body surface area) as a concurrent chemotherapy. Thus, the comparisons in this randomised study were not the comparisons that this review aimed to evaluate. |
| Sood 2003 | Included 54 women with biopsy‐confirmed carcinoma of the cervix using extended‐field radiotherapy and high‐dose‐rate brachytherapy with or without concomitant chemotherapy. Non‐randomised study design containing a single‐arm study without a comparator arm. |
| Tsai 2010 | Randomised trial to determine the usefulness of FDG‐PET for assessing extra‐pelvic metastases, designed radiotherapy field technique, and improved survival among people with stage I–IVA cervical cancer who had enlarged pelvic lymph node on MRI. Pelvic radiotherapy was given for participants who found to have no extrapelvic findings on PET. An extended‐field radiotherapy was given for the remainder of the participants. All participants received 6 cycles of weekly intravenous infusion of cisplatin (50 mg/m² body surface area) as a concurrent chemotherapy. Thus, the comparison in this randomised study was not the comparison that this review aimed to evaluate. |
| Vargo 2014 | 61 women with cervical cancer (stage IB1–IVA) who had PET‐positive para‐aortic nodes treated with extended‐field intensity modulated radiation therapy (IMRT). Non‐randomised study design. |
| Varia 1998 | Evaluated efficacy and safety of extended‐field radiotherapy with 5‐FU and cisplatin among 86 participants with biopsy‐confirmed para‐aortic node metastases from cervical carcinoma. It was excluded because of a non‐randomised study design. All participants underwent extended‐field radiotherapy. |
| Wakatsuki 2015 | Evaluated efficacy and the toxicity of prophylactic extended‐field carbon‐ion radiotherapy among 26 women with locally advanced squamous cell carcinoma of the uterine cervix. Non‐randomised study design. All participants underwent extended‐field carbon‐ion radiotherapy. |
| Yoon 2014 | Conducted to assess the efficacy and toxicity of extended‐field radiotherapy for 101 women with stage IB–IVA cervical cancer and positive para‐aortic nodes. Retrospective descriptive study. All women underwent extended‐field radiotherapy. |
3D: 3 dimensional; 5‐FU: 5‐fluorouracil; FDG‐PET: 18F‐fluorodeoxyglucose positron emission tomography; IMRT: intensity‐modulated radiation therapy; MRI: magnetic resonance imaging; PET: positron emission tomography.
Differences between protocol and review
Title
We changed the title from 'Extended‐field irradiation for locally advanced cervical cancer' to 'Extended‐field radiotherapy for locally advanced cervical cancer' to obtain a better understanding.
Types of outcome measures
We defined the rate of non‐para‐aortic recurrences as a secondary outcomes in the initial protocol, but none of the included studies reported this. In an attempt to cover a broad range of outcome measures, we added types of cancer recurrences including locoregional, distant, and combined locoregional recurrences as additional secondary outcomes.
Furthermore, the review protocol stated that adverse events would be categorised as acute and late adverse events. However, the authors of some included studies reported only the rates of adverse events, but did not specify the onset of occurrence. Therefore, we added adverse events that were not specified onset of occurrence as an additional secondary outcome in some comparisons.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analysis for the following factors: type of treatment (concurrent CRT versus radiotherapy only) and status of para‐aortic lymph node (unknown versus negative on radiological imaging). However, it was not performed but acknowledged as most analyses were based on only one or two included studies. Nevertheless, these factors are important in the interpretation of review findings.
Contributions of authors
KT: conceived the review question, developed and completed the review. NS: conceived the review question, developed and completed the review. CK: conceived the review question, developed and completed the review. PP: edited the review and provided advice throughout. PL: edited the review and provided advice throughout.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, Faculty of Medicine, Khon Kaen University, Thailand.
Department of Radiology, Faculty of Medicine, Khon Kaen University, Thailand.
Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Thailand.
Cochrane Thailand, Thailand.
External sources
Thailand Research Fund (Distinguished Professor Award), Thailand.
Long‐term Institutional Development HUBs (LID‐HUBs), the Human Reproduction Programme (HRP) Alliance for Research Capacity Strengthening, Department of Reproductive Health and Research, World Health Organization, Switzerland.
Declarations of interest
KT: none known. NS: none known. CK: none known. PP: none known. PL: none known.
New
References
References to studies included in this review
Asiri 2014 {published data only}
- Asiri MA, Tunio MA, Mohamed R, Bayoumi Y, Alhadab A, Saleh RM, et al. Is extended‐field concurrent chemoradiation an option for radiologic negative paraaortic lymph node, locally advanced cervical cancer?. Cancer Management and Research 2014;6:339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatani 1995 {published data only}
- Chatani M, Matayoshi Y, Masaki N, Narumi Y, Teshima T, Inoue T. Prophylactic irradiation of para‐aortic lymph nodes in carcinoma of the uterine cervix. A prospective randomized study. Strahlentherapie Und Onkologie 1995;171(11):655‐60. [PubMed] [Google Scholar]
Haie 1988 {published data only}
- Haie C, Pejovic MH, Gerbaulet A, Horiot JC, Pourquier H, Delouche J, et al. Is prophylactic para‐aortic irradiation worthwhile in the treatment of advanced cervical carcinoma? Results of a controlled clinical trial of the EORTC radiotherapy group. Radiotherapy and Oncology 1988;11(2):101‐12. [DOI] [PubMed] [Google Scholar]
Morris 1999 {published data only}
- Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para‐aortic irradiation for high‐risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90‐01. Journal of Clinical Oncology 2004;22(5):872‐80. [DOI] [PubMed] [Google Scholar]
- Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para‐aortic radiation for high‐risk cervical cancer. New England Journal of Medicine 1999;340(15):1137‐43. [DOI] [PubMed] [Google Scholar]
Rotman 1990 {published data only}
- Rotman M, Choi K, Guse C, Marcial V, Hornback N, John M. Prophylactic irradiation of the para‐aortic lymph node chain in stage IIB and bulky stage IB carcinoma of the cervix, initial treatment results of RTOG 7920. International Journal of Radiation Oncology, Biology, Physics 1990;19(3):513‐21. [DOI] [PubMed] [Google Scholar]
- Rotman M, Pajak TF, Choi K, Clery M, Marcial V, Grigsby PW, et al. Prophylactic extended‐field irradiation of para‐aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten‐year treatment results of RTOG 79‐20. JAMA 1995;274(5):387‐93. [PubMed] [Google Scholar]
References to studies excluded from this review
Du 2010 {published data only}
- Du XL, Sheng XG, Jiang T, Yu H, Yan YF, Gao R, et al. Intensity‐modulated radiation therapy versus para‐aortic field radiotherapy to treat para‐aortic lymph node metastasis in cervical cancer: prospective study. Croatian Medical Journal 2010;51(3):229‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim JH, Kim JY, Yoon MS, Kim YS, Lee JH, Kim HJ, et al. Prophylactic irradiation of para‐aortic lymph nodes for patients with locally advanced cervical cancers with and without high CA9 expression (KROG 07‐01): a randomized, open‐label, multicenter, phase 2 trial. Radiotherapy and Oncology 2016;120(3):383‐9. [DOI] [PubMed] [Google Scholar]
Liang 2014 {published data only}
- Liang JA, Chen SW, Hung YC, Yeh LS, Chang WC, Lin WC, et al. Low‐dose, prophylactic, extended‐field, intensity‐modulated radiotherapy plus concurrent weekly cisplatin for patients with stage IB2–IIIB cervical cancer, positive pelvic lymph nodes, and negative para‐aortic lymph nodes. International Journal of Gynecological Cancer 2014;24(5):901‐7. [DOI] [PubMed] [Google Scholar]
Lin 2015 {published data only}
- Lin SY, Tsai CS, Chang YC, Ng KK, Chang TC, Kao WH, et al. The role of pretreatment FDG‐PET in treating cervical cancer patients with enlarged pelvic lymph node(s) shown on MRI: a phase 3 randomized trial with long‐term follow‐up. International Journal of Radiation Oncology, Biology, Physics 2015;92(3):577‐85. [DOI] [PubMed] [Google Scholar]
Sood 2003 {published data only}
- Sood BM, Gorla GR, Garg M, Anderson PS, Fields AL, Runowicz CD, et al. Extended‐field radiotherapy and high‐dose‐rate brachytherapy in carcinoma of the uterine cervix: clinical experience with and without concomitant chemotherapy. Cancer 2003;97(7):1781‐8. [DOI] [PubMed] [Google Scholar]
Tsai 2010 {published data only}
- Tsai CS, Lai CH, Chang TC, Yen TC, Ng KK, Hsueh S, et al. A prospective randomized trial to study the impact of pretreatment FDG‐PET for cervical cancer patients with MRI‐detected positive pelvic but negative para‐aortic lymphadenopathy. International Journal of Radiation Oncology, Biology, Physics 2010;76(2):477‐84. [DOI] [PubMed] [Google Scholar]
Vargo 2014 {published data only}
- Vargo JA, Kim H, Choi S, Sukumvanich P, Olawaiye AB, Kelley JL, et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node‐positive cervical cancer: analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. International Journal of Radiation Oncology, Biology, Physics 2014;90(5):1091‐8. [DOI] [PubMed] [Google Scholar]
Varia 1998 {published data only}
- Varia MA, Bundy BN, Deppe G, Mannel R, Averette HE, Rose PG, et al. Cervical carcinoma metastatic to para‐aortic nodes: extended field radiation therapy with concomitant 5‐fluorouracil and cisplatin chemotherapy: a Gynecologic Oncology Group study. International Journal of Radiation Oncology, Biology, Physics 1998;42(5):1015‐23. [DOI] [PubMed] [Google Scholar]
Wakatsuki 2015 {published data only}
- Wakatsuki M, Kato S, Kiyohara H, Ohno T, Karasawa K, Tamaki T, et al. Clinical trial of prophylactic extended‐field carbon‐ion radiotherapy for locally advanced uterine cervical cancer (protocol 0508). PloS One 2015;10(5):e0127587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2014 {published data only}
- Yoon H, Cha J, Kim G, Chung Y, Kim Y. The long‐term follow‐up result of extended field radiation therapy for uterine cervical cancer with positive para‐aortic lymph nodes: does the addition of chemotherapy have survival benefit?. International Journal of Radiation Oncology, Biology, Physics 2014;S:479. [Google Scholar]
Additional references
Campbell 2000
- Campbell MJ. Cluster randomized trials in general (family) practice research. Statistical Methods in Medical Research 2000;9(2):81‐94. [DOI] [PubMed] [Google Scholar]
CCCMAC 2010
- Chemoradiotherapy for Cervical Cancer Meta‐analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta‐analysis. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherny 2015
- Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti‐cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO‐MCBS). Annals of Oncology 2015;26(8):1547‐73. [DOI] [PubMed] [Google Scholar]
Covidence 2018
- Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Cox 1995
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). International Journal Of Radiation Oncology Biology Physics 1995;31(5):1341‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Eifel 2004
- Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para‐aortic irradiation for high‐risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90‐01. Journal of Clinical Oncology 2004;22(5):872‐80. [DOI] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomas Reuters. Endnote Version X7. New York: Thomas Reuters, 2015.
FIGO Committee 2014
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. International Journal of Gynaecology & Obstetrics 2014;125(2):97‐8. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC Cancer Base No. 11, 2013. Lyon, France: International Agency for Research on Cancer, Available from: globocan.iarc.fr. [Google Scholar]
Greimel 2006
- Greimel ER, Kuljanic Vlasic K, Waldenstrom AC, Duric VM, Jensen PT, Singer S, et al. The European Organization for Research and Treatment of Cancer (EORTC) Quality‐of‐Life questionnaire cervical cancer module: EORTC QLQ‐CX24. Cancer 2006;107(8):1812‐22. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hong 2004
- Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. International Journal of Radiation Oncology, Biology, Physics 2004;60(1):249‐57. [DOI] [PubMed] [Google Scholar]
Höckel 2008
- Höckel M. Laterally extended endopelvic resection (LEER) – principles and practice. Gynecologic Oncology 2008;111(2 Suppl):S13‐7. [DOI] [PubMed] [Google Scholar]
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians 2011;61(2):69‐90. [DOI] [PubMed] [Google Scholar]
Keys 1999
- Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New England Journal of Medicine 1999;340(15):1154‐61. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim YS, Kim JH, Ahn SD, Lee SW, Shin SS, Nam JH, et al. High‐dose extended‐field irradiation and high‐dose‐rate brachytherapy with concurrent chemotherapy for cervical cancer with positive para‐aortic lymph nodes. International Journal of Radiation Oncology, Biology, Physics 2009;74(5):1522‐8. [DOI] [PubMed] [Google Scholar]
Landoni 1997
- Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet 1997;350(9077):535‐40. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gotzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2011
- Lim K, Small W Jr, Portelance L, Creutzberg C, Jürgenliemk‐Schulz IM, Mundt A, et al. Gyn IMRT Consortium. Consensus guidelines for delineation of clinical target volume for intensity‐modulated pelvic radiotherapy for the definitive treatment of cervix cancer. International Journal of Radiation Oncology, Biology, Physics 2011;79(2):348‐55. [DOI] [PubMed] [Google Scholar]
Mahawerawat 2013
- Mahawerawat S, Charoenkwan K, Srisomboon J, Khunamornpong S, Suprasert P, Sae‐Teng CT. Surgical outcomes of patients with stage IA2 cervical cancer treated with radical hysterectomy. Asian Pacific Journal of Cancer Prevention 2013;14(9):5375‐8. [DOI] [PubMed] [Google Scholar]
Marnitz 2005
- Marnitz S, Köhler C, Roth C, Füller J, Hinkelbein W, Schneider A. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer?. Gynecologic Oncology 2005;99(3):536‐44. [DOI] [PubMed] [Google Scholar]
Marnitz 2015
- Marnitz S, Schram J, Budach V, Sackerer I, Vercellino GF, Sehouli J, et al. Extended field chemoradiation for cervical cancer patients with histologically proven para‐aortic lymph node metastases after laparoscopic lymphadenectomy. Strahlentherapie und Onkologie 2015;191(5):421‐8. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Cancer Institute 1999
- National Cancer Institute. NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival, 1999. www.nih.gov/news/pr/feb99/nci22.htm (accessed prior to 20 October 2018).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pearcey 2002
- Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. Journal of Clinical Oncology 2002;20(4):966‐72. [DOI] [PubMed] [Google Scholar]
Peters 2000
- Peters WA, Lui PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high risk early stage cancer of the cervix. Journal of Clinical Oncology 2000;18(8):1606‐13. [DOI] [PubMed] [Google Scholar]
Peto 1977
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. British Journal of Cancer 1977;35(1):1‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisani 1999
- Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. International Journal of Cancer 1999;83(1):18‐29. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 1992
- Rose PG, Cha SD, Tak WK, Fitzgerald T, Reale F, Hunter RE. Radiation therapy for surgically proven para‐aortic node metastasis in endometrial carcinoma. International Journal of Radiation Oncology, Biology, Physics 1992;24(2):229‐33. [DOI] [PubMed] [Google Scholar]
Rose 1999
- Rose PG, Bundy BN, Watkins EB, Thigpen T, Deppe G, Maiman MA, et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine 1999;340(15):1144‐53. [DOI] [PubMed] [Google Scholar]
Rotman 1978
- Rotman M, Moon S, John M, Choi K, Sall S. Extended field para‐aortic radiation in cervical carcinoma: the case for prophylactic treatment. International Journal of Radiation Oncology, Biology, Physics 1978;4(9‐10):795‐9. [DOI] [PubMed] [Google Scholar]
Rotman 1995
- Rotman M, Pajak TF, Choi K, Clery M, Marcial V, Grigsby PW, et al. Prophylactic extended‐field irradiation of para‐aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten‐year treatment results of RTOG 79‐20. JAMA 1995;274(5):387‐93. [PubMed] [Google Scholar]
Sakuragi 1999
- Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stages IB, IIA, and. Cancer 1999;85(7):1547‐54. [DOI] [PubMed] [Google Scholar]
Sakurai 2001
- Sakurai H, Mitsuhashi N, Takahashi M, Akimoto T, Muramatsu H, Ishikawa H, et al. Analysis of recurrence of squamous cell carcinoma of the uterine cervix after definitive radiation therapy alone: patterns of recurrence, latent periods, and prognosis. International Journal of Radiation Oncology, Biology, Physics 2001;50(5):1136‐44. [DOI] [PubMed] [Google Scholar]
Sapienza 2017
- Sapienza LG, Gomes MJ, Calsavara VF, Leitao MM Jr, Baiocchi G. Does para‐aortic irradiation reduce the risk of distant metastasis in advanced cervical cancer? A systematic review and meta‐analysis of randomized clinical trials. Gynecologic Oncology 2017;144(2):312‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Smits 2014
- Smits RM, Zusterzeel PL, Bekkers RL. Pretreatment retroperitoneal para‐aortic lymph node staging in advanced cervical cancer: a review. International Journal of Gynecological Cancer 2014;24(6):973‐83. [DOI] [PubMed] [Google Scholar]
Spirtos 2002
- Spirtos NM, Eisenkop SM, Schlaerth JB, Ballon SC. Laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy in patients with stage I cervical cancer: surgical morbidity and intermediate follow‐up. American Journal of Obstetrics & Gynecology 2002;187(2):340‐8. [DOI] [PubMed] [Google Scholar]
Srisomboon 2011
- Srisomboon J, Kietpeerakool C, Suprasert P, Manopanya M, Siriaree S, Charoenkwan K, et al. Survival and prognostic factors comparing stage IB 1 versus stage IB 2 cervical cancer treated with primary radical hysterectomy. Asian Pacific Journal of Cancer Prevention 2011;12(7):1753‐6. [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Sutton A, Loannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Suprasert 2010
- Suprasert P, Srisomboon J, Charoenkwan K, Siriaree S, Cheewakriangkrai C, Kietpeerakool C, et al. Twelve years experience with radical hysterectomy and pelvic lymphadenectomy in early stage cervical cancer. Journal of Obstetrics and Gynaecology 2010;30(3):294‐8. [DOI] [PubMed] [Google Scholar]
Toita 2012
- Toita T, Kitagawa R, Hamano T, Umayahara K, Hirashima Y, Aoki Y, et al. Phase II study of concurrent chemoradiotherapy with high‐dose‐rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: efficacy and toxicity of a low cumulative radiation dose schedule. Gynecologic Oncology 2012;126(2):211‐6. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Whitney 1999
- Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea in stage IIB/IVA carcinoma of the cervix. Journal of Clinical Oncology 1999;17(5):1339‐48. [DOI] [PubMed] [Google Scholar]
Winter 2017 [pers comm]
- Winter K. Request for information of published article [personal communication]. Email to: Kathryn Winter 2017 11 August 2017.
References to other published versions of this review
Thamronganantasakul 2016
- Thamronganantasakul K, Supakalin N, Kietpeerakool C, Pattanittum P, Lumbiganon P. Extended‐field irradiation for locally advanced cervical cancer. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012301] [DOI] [PMC free article] [PubMed] [Google Scholar]


