Abstract
Background
This is the first update of a Cochrane Review first published in 2015. Renin angiotensin system (RAS) inhibitors include angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) and renin inhibitors. They are widely prescribed for treatment of hypertension, especially for people with diabetes because of postulated advantages for reducing diabetic nephropathy and cardiovascular morbidity and mortality. Despite widespread use for hypertension, the efficacy and safety of RAS inhibitors compared to other antihypertensive drug classes remains unclear.
Objectives
To evaluate the benefits and harms of first‐line RAS inhibitors compared to other first‐line antihypertensive drugs in people with hypertension.
Search methods
The Cochrane Hypertension Group Information Specialist searched the following databases for randomized controlled trials up to November 2017: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. The searches had no language restrictions.
Selection criteria
We included randomized, active‐controlled, double‐blinded studies (RCTs) with at least six months follow‐up in people with elevated blood pressure (≥ 130/85 mmHg), which compared first‐line RAS inhibitors with other first‐line antihypertensive drug classes and reported morbidity and mortality or blood pressure outcomes. We excluded people with proven secondary hypertension.
Data collection and analysis
Two authors independently selected the included trials, evaluated the risks of bias and entered the data for analysis.
Main results
This update includes three new RCTs, totaling 45 in all, involving 66,625 participants, with a mean age of 66 years. Much of the evidence for our key outcomes is dominated by a small number of large RCTs at low risk for most sources of bias. Imbalances in the added second‐line antihypertensive drugs in some of the studies were important enough for us to downgrade the quality of the evidence.
Primary outcomes were all‐cause death, fatal and non‐fatal stroke, fatal and non‐fatal myocardial infarction (MI), fatal and non‐fatal congestive heart failure (CHF) requiring hospitalizations, total cardiovascular (CV) events (fatal and non‐fatal stroke, fatal and non‐fatal MI and fatal and non‐fatal CHF requiring hospitalization), and end‐stage renal failure (ESRF). Secondary outcomes were systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR).
Compared with first‐line calcium channel blockers (CCBs), we found moderate‐certainty evidence that first‐line RAS inhibitors decreased heart failure (HF) (35,143 participants in 5 RCTs, risk ratio (RR) 0.83, 95% confidence interval (CI) 0.77 to 0.90, absolute risk reduction (ARR) 1.2%), and that they increased stroke (34,673 participants in 4 RCTs, RR 1.19, 95% CI 1.08 to 1.32, absolute risk increase (ARI) 0.7%). Moderate‐certainty evidence showed that first‐line RAS inhibitors and first‐line CCBs did not differ for all‐cause death (35,226 participants in 5 RCTs, RR 1.03, 95% CI 0.98 to 1.09); total CV events (35,223 participants in 6 RCTs, RR 0.98, 95% CI 0.93 to 1.02); and total MI (35,043 participants in 5 RCTs, RR 1.01, 95% CI 0.93 to 1.09). Low‐certainty evidence suggests they did not differ for ESRF (19,551 participants in 4 RCTs, RR 0.88, 95% CI 0.74 to 1.05).
Compared with first‐line thiazides, we found moderate‐certainty evidence that first‐line RAS inhibitors increased HF (24,309 participants in 1 RCT, RR 1.19, 95% CI 1.07 to 1.31, ARI 1.0%), and increased stroke (24,309 participants in 1 RCT, RR 1.14, 95% CI 1.02 to 1.28, ARI 0.6%). Moderate‐certainty evidence showed that first‐line RAS inhibitors and first‐line thiazides did not differ for all‐cause death (24,309 participants in 1 RCT, RR 1.00, 95% CI 0.94 to 1.07); total CV events (24,379 participants in 2 RCTs, RR 1.05, 95% CI 1.00 to 1.11); and total MI (24,379 participants in 2 RCTs, RR 0.93, 95% CI 0.86 to 1.01). Low‐certainty evidence suggests they did not differ for ESRF (24,309 participants in 1 RCT, RR 1.10, 95% CI 0.88 to 1.37).
Compared with first‐line beta‐blockers, low‐certainty evidence suggests that first‐line RAS inhibitors decreased total CV events (9239 participants in 2 RCTs, RR 0.88, 95% CI 0.80 to 0.98, ARR 1.7%), and decreased stroke (9193 participants in 1 RCT, RR 0.75, 95% CI 0.63 to 0.88, ARR 1.7% ). Low‐certainty evidence suggests that first‐line RAS inhibitors and first‐line beta‐blockers did not differ for all‐cause death (9193 participants in 1 RCT, RR 0.89, 95% CI 0.78 to 1.01); HF (9193 participants in 1 RCT, RR 0.95, 95% CI 0.76 to 1.18); and total MI (9239 participants in 2 RCTs, RR 1.05, 95% CI 0.86 to 1.27).
Blood pressure comparisons between first‐line RAS inhibitors and other first‐line classes showed either no differences or small differences that did not necessarily correlate with the differences in the morbidity outcomes.
There is no information about non‐fatal serious adverse events, as none of the trials reported this outcome.
Authors' conclusions
All‐cause death is similar for first‐line RAS inhibitors and first‐line CCBs, thiazides and beta‐blockers. There are, however, differences for some morbidity outcomes. First‐line thiazides caused less HF and stroke than first‐line RAS inhibitors. First‐line CCBs increased HF but decreased stroke compared to first‐line RAS inhibitors. The magnitude of the increase in HF exceeded the decrease in stroke. Low‐quality evidence suggests that first‐line RAS inhibitors reduced stroke and total CV events compared to first‐line beta‐blockers. The small differences in effect on blood pressure between the different classes of drugs did not correlate with the differences in the morbidity outcomes.
Plain language summary
Renin angiotensin system inhibitors versus other types of medicine for hypertension
Review question
We determined how RAS (renin angiotensin system) inhibitors compared as first‐line medicines for treating hypertension with other types of first‐line medicines (thiazide diuretics, beta‐blockers, CCBs, alpha‐blockers, or central nervous system (CNS) active drugs) for hypertension.
Background
Hypertension is a long‐lasting medical condition and associated with cardiovascular mortality and morbidity such as coronary artery disease, cerebrovascular disease, and peripheral vascular disease, which will reduce quality of life. RAS inhibitors have become a focus of interventions for hypertension in recent years and have been widely prescribed for treatment of hypertension. However, it remains unclear whether RAS inhibitors are superior to other antihypertensive drugs in terms of clinically relevant outcomes.
Search date
We searched for evidence up to November 2017.
Study characteristics
We included randomized, double‐blind, parallel design RCTs for the present review. 45 trials with 66,625 participants who were followed‐up for between 0.5 year and 5.6 years were included. The participants had an average age of 66 years.
Key results
We found that first‐line RAS inhibitors caused more heart failure and stroke than first‐line thiazides. When compared to first‐line CCBs, first‐line RAS inhibitors showed superiority in preventing heart failure but were inferior in preventing stroke, with greater absolute risk reduction in heart failure than increase in stroke. When compared to first‐line beta‐blockers, RAS inhibitors reduced total cardiovascular events and stroke. Small differences on efficacy for lowering blood pressure were detected, but these did not to seem to be related to the number of heart attacks, strokes or kidney problems.
Certainty of evidence
Overall, certainty of evidence was assessed as low to moderate according to the GRADE assessment. Moderate‐certainty evidence demonstrated superiority of first‐line thiazides to first‐line RAS inhibitors in preventing heart failure and stroke. The certainty of evidence was assessed moderate for comparison between RAS inhibitors and CCBs. The certainty of evidence was low for comparison between RAS inhibitors and beta‐blockers on total cardiovascular events and stroke since the results were based primarily on one large trial with moderate to high risk of bias.
Summary of findings
Background
Description of the condition
Hypertension is a worldwide health problem and has become a heavy burden on healthcare systems. Hypertension is associated with cardiovascular (CV) mortality and morbidity such as coronary artery disease, cerebrovascular disease, and peripheral vascular disease. Blood pressure (BP) is elevated in many people with type 2 diabetes, which is a major health problem worldwide. The increasing prevalence of diabetes mellitus (DM) is primarily due to the increase in type 2 diabetes mellitus (T2DM; Inzucchi 2005). A survey of US adults with diabetes showed that 71.0% had elevated BP, defined as BP that equals or exceeds 130/85 mmHg, or current use of prescription medication for hypertension (Geiss 2002). Elevated BP is associated with a spectrum of later health problems in people with diabetes, notably CV disease and kidney damage (nephropathy). Elevated BP has been identified as a major risk factor in progression of diabetic nephropathy (Aurell 1992). The risk of CV morbidity and mortality is also doubled in hypertensive people when diabetes is present (DeStefano 1993). Review of the evidence base on this topic is covered among guidelines primarily addressing diabetes or hypertension (CPG 2013; JNC‐8 2014, respectively). Antihypertensive agents used as first‐line drugs include angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), beta‐blockers and diuretics.
Description of the intervention
In the past 10 years, antagonism of the renin angiotensin system (RAS) has become a focus of therapeutic interventions for hypertension. The guidelines that recommend the use of ACE inhibitors or ARBs in hypertensive people with diabetes or renal disease base their recommendations on the results of placebo‐controlled studies, which have been interpreted to show that ACE inhibitors and ARBs have specific renoprotective effects beyond those resulting from lowering blood pressure (ADA 2013; JNC‐8 2014). Blood pressure‐independent beneficial effects of ACE inhibitors and ARBs on CV outcomes have also been proposed, based on the results of several large, multicenter, placebo‐controlled studies, especially the HOPE 2000, PROGRESS 2001 and RENAAL 2001 studies. A recent meta‐analysis has suggested that in people with DM, treatment with tissue‐specific ACE inhibitors (ramipril 1.25 mg/day or 10 mg/day; perindopril 4 mg/day or 8 mg/day) when compared to placebo significantly reduces the risk of CV mortality by 14.9% (P = 0.022), myocardial infarction (MI) by 20.8% (P = 0.002) and the need for invasive coronary revascularization by 14% (P = 0.015); but not all‐cause death (risk ratio (RR) 0.913, 95% confidence intervals (CI) 0.825 to 1.011; Saha 2008). A Cochrane Review (Strippoli 2006), that included 13 randomized controlled trials (RCTs) with 10,070 participants, showed a significant reduction in the risk of end‐stage renal failure (ESRF) with ACE inhibitors or ARBs compared to placebo or no treatment (RR 0.60, 95% CI 0.39 to 0.93; RR 0.78, 95% CI 0.67 to 0.91, respectively). Furthermore, 10 studies with 2034 participants showed that ACE inhibitors, at the maximum tolerable dose, significantly reduce the risk of all‐cause death in placebo‐controlled studies (RR 0.78, 95% CI 0.61 to 0.98, absolute risk reduction (ARR) 0.04, number needed to treat for an additional beneficial outcome (NNTB) 25).
The evidence of benefit in terms of mortality and morbidity using ACE inhibitors or ARBs versus other antihypertensive agents is not clear. Some studies suggest that RAS inhibitors might prevent or delay CV events in some subgroups, but their role in the broader group of people with hypertension remains unknown (CAPPP 2001; LIFE 2002). Some studies provided evidence of benefit of RAS inhibitors on renal function over other antihypertensive drugs (ABCD‐HT 2000; LIFE 2002; MARVAL 2002), but did not examine clinically relevant outcomes such as combined renal dysfunction or renal failure.
Other systematic reviews related to this review are summarized below in chronological order by date of publication.
A systematic review and Bayesian network meta‐analysis of 63 randomized clinical trials assessed the effects of different classes of antihypertensive treatments (monotherapy and their combinations) on survival and major renal outcomes in people with diabetes (Wu 2013). This review examined clinical endpoints that included all‐cause mortality, requirement for dialysis and doubling of serum creatinine levels. When compared with placebo, ARBs showed no reduction in any of the three outcomes, and ACE inhibitors only reduced the doubling of serum creatinine levels compared with placebo (odds ratio (OR) 0.58, 95% CI 0.32 to 0.90). Although ACE inhibitors did not show other beneficial effects compared with other drugs, the researchers supported the use of ACE inhibitors as the first‐line antihypertensive agent in people with diabetes. However, all the suggestions were based on the results of Bayesian network meta‐analysis, which not only included the results of direct comparisons, but also incorporated indirect comparisons. The review did not report the proportion of hypertensive people, and the indirect comparisons could affect the applicability of this evidence.
A systematic review and meta‐analysis by Casas et al assessed the effect of RAS inhibitors and other antihypertensive drugs on renal outcomes (Casas 2005). In this review, the effects of ACE inhibitors or ARBs in placebo‐controlled studies were indirectly compared to the effects of other antihypertensive drugs in people with type 1 or type 2 diabetes or without diabetes. For those with diabetic nephropathy, comparative studies of ACE inhibitors or ARBs showed no benefit on ESRF, glomerular filtration rate (GFR), or creatinine levels. Placebo‐controlled studies of ACE inhibitors or ARBs decreased all renal outcomes, and also reduced BP.
A Cochrane Review of RCTs compared any antihypertensive agent with placebo or another agent in hypertensive or normotensive people with diabetes and no kidney disease (Strippoli 2005). This review assessed the renal outcomes and all‐cause and CV mortality. It showed that compared to placebo, ACE inhibitors significantly reduced the development of microalbuminuria (six trials, 3840 participants: RR 0.60, 95% CI 0.43 to 0.84, ARR 0.03 and NNTB 33), but not doubling of creatinine or ESRF or all‐cause death. Compared to CCBs, ACE inhibitors significantly reduced progression to microalbuminuria (four trials,1210 participants: RR 0.58, 95% CI 0.40 to 0.84, ARR 0.05 and NNTB 20).
A meta‐analysis of double‐blinded RCTs by Siebenhofer et al compared ARBs to placebo or standard antihypertensive treatment in T2DM (three studies, 4423 participants) and examined clinical endpoints (all‐cause death, CV morbidity and mortality, and ESRF; Siebenhofer 2004). The only statistically significant benefit of ARBs was the reduction of ESRF compared with placebo (OR 0.73, 95% CI 0.60 to 0.89, ARR 0.05 and NNTB 20). ARBs failed to show superiority to standard antihypertensive treatment (CCBs, beta‐blockers) for total mortality and CV morbidity and mortality. However, ACE inhibitors were not included in this meta‐analysis.
A systematic review and meta‐analysis by Pahor et al assessed therapeutic benefits of ACE inhibitors and other antihypertensive drugs in people with T2DM and hypertension (Pahor 2000). This meta‐analysis showed a significant benefit of ACE inhibitors compared with alternative treatments (CCBs, beta‐blockers, diuretics) on acute MI (63% reduction, P < 0.001, ARR 0.06 and NNTB 17), CV events (51% reduction, P < 0.001, ARR 8% and NNTB 13), and all‐cause death (62% reduction, P = 0.010, ARR 0.02 and NNTB 40), but not stroke. However, ARBs were not included in this review. Renal outcomes (ESRF, GFR, serum creatinine or albuminuria) were not reported.
A meta‐analysis of 100 controlled and uncontrolled studies in 2494 participants with diabetes assessed the effect on proteinuria of different classes of antihypertensive agents (ACE inhibitors, CCBs, beta‐blockers and control; Kasiske 1993). This review showed that ACE inhibitors produced the greatest reductions in urine albumin and protein excretion compared with other antihypertensive agents (P < 0.05 versus CCBs; P < 0.05 versus control). ACE inhibitors achieved these beneficial effects on renal function independent of changes in blood pressure. This meta‐analysis examined surrogate markers rather than clinically relevant endpoints (such as ESRF, all‐cause death).
How the intervention might work
The RAS is a potentially pathophysiologic mechanism that causes diabetic heart disease. Angiotensin II (Ang II) is thought to play an important role in the pathogenesis of CV complications (Dzau 2001). RAS inhibitors have been proven to have additional antiproteinuric and renoprotective benefits on diabetic nephropathy (Kocks 2002).
Drugs inhibiting the RAS include: renin inhibitors, ACE inhibitors and ARBs, which inhibit the enzymatic action of renin, the conversion of angiotensin I (Ang I) to Ang II and block the Ang II receptors, respectively.
ACE inhibitors and ARBs block the RAS further downstream than renin inhibitors, which prevent the formation of renin. Renin is the substrate responsible for all downstream events that lead to production of Ang II and subsequent stimulation of its receptors. It has been proposed that renin inhibitors might provide a more effective means of blockade of the RAS than ACE inhibitors or ARBs (Duprez 2006).
Why it is important to do this review
RAS inhibitors are widely prescribed for treatment of hypertension. ACE inhibitors and ARBs are specifically promoted for people with diabetes on the basis of postulated advantages for the reduction of diabetic nephropathy and CV morbidity and mortality. Despite widespread use of ACE inhibitors and ARBs for diabetes, their efficacy compared to other antihypertensive drugs is still unclear. A systematic review is needed in order to establish the benefits and harms of clinically relevant outcomes (especially all‐cause death and morbidity, renal and CV outcomes) of RAS inhibitors compared to other antihypertensive drugs in people with elevated blood pressure.
Objectives
To evaluate the benefits and harms of first‐line RAS inhibitors compared to other first‐line antihypertensive drugs in people with hypertension.
Methods
Criteria for considering studies for this review
Types of studies
Study design must meet the following criteria:
RCTs with parallel design;
double‐blind;
minimum follow‐up of six months.
Types of participants
People with primary elevated BP (that equals or exceeds 130/85 mmHg). We chose this BP threshold, lower than the standard 140/90 mmHg, to include more people and to be consistent with the recommended lower targets for people with hypertension and diabetes. We excluded people with proven secondary hypertension.
Types of interventions
RAS inhibitors including ACE inhibitors, ARBs or renin inhibitors:
ACE inhibitors include: alacepril, altiopril, benazepril, captopril, ceronapril, cilazapril, delapril, derapril, enalapril, enalaprilat, fosinopril, idapril, imidapril, lisinopril, moexipril, moveltipril, pentopril, perindopril, quinapril, ramipril, spirapril, temocapril, trandolapril, and zofenopril.
ARBs include: candesartan, eprosartan, irbesartan, losartan, olmesartan, tasosartan, telmisartan, valsartan, and KT3‐671.
Renin inhibitors include: aliskiren, remikiren.
Comparators
Any other antihypertensive drug class including: thiazides, beta‐blockers, CCBs, alpha‐blockers, or central nervous system (CNS) active drugs.
Types of outcome measures
Primary outcomes
All‐cause death.
All‐cause serious morbidity (non‐fatal serious adverse events).
-
Total CV events:
fatal and non‐fatal MI;
fatal and non‐fatal stroke;
fatal and non‐fatal congestive heart failure (CHF) requiring hospitalizations.
-
Renal outcomes:
ESRF (defined as a requirement for maintenance dialysis).
Secondary outcomes
Change in or end‐point systolic and diastolic BP.
Change in or end‐point heart rate.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Group Information Specialist conducted systematic searches in the following databases for randomized controlled trials without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 22 November 2017);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11) via Wiley (searched 22 November 2017);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 20 November 2017);
Embase Ovid (searched 20 November 2017);
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 20 November 2017);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 22 November 2017).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies are provided in Appendix 1.
Searching other resources
The Cochrane Hypertension Group Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches of CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses and Web of Knowledge.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
Data collection and analysis
We performed the initial search of all the databases to identify citations with potential relevance. In our initial screen of these abstracts we excluded articles whose titles or abstracts, or both, were clearly irrelevant. We retrieved the full text of the remaining articles (and translated into English where required) to assess whether the trials met the prespecified inclusion criteria. We searched the bibliographies of pertinent articles, reviews and texts for additional relevant citations. Two independent review authors assessed the eligibility of the trials using a study selection form. A third review author resolved discrepancies.
Selection of studies
We imported references and abstracts of search results into Reference Manager software. We based selection of studies on the criteria listed above.
Data extraction and management
Two review authors independently extracted data using a standard form, and then cross‐checked them. All numeric calculations and graphic interpolations were confirmed by a second person.
Assessment of risk of bias in included studies
We assessed the risk of bias for each trial according to Cochrane 'Risk of bias' guidelines using the following six domains (Higgins 2011):
sequence generation;
allocation concealment;
blinding or objective assessment of primary outcomes;
incomplete outcome data;
selective outcome reporting;
other biases.
We used the overall risk of bias in the GRADE assessment in the 'Summary of findings' table. We conducted GRADE assessment according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We based quantitative analysis of outcomes on intention‐to‐treat principles as much as possible. For dichotomous outcomes, we expressed results as the risk ratio (RR) with a 95% confidence interval (CI). For combining continuous variables (systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR)), we used the mean difference (with 95% CI).
Dealing with missing data
If the included studies had missing information, we contacted investigators (using email, letter or fax or both) to obtain the missing information.
When studies did not report a within‐study variance for the effect change of continuous data, we imputed the standard deviation (SD) using the following hierarchy:
pooled SD calculated either from the t‐statistic corresponding to an exact P value reported or from the 95% CI of the mean difference between treatment group and comparative group;
SD at the end of treatment;
SD at baseline;
weighted mean SD of change calculated from at least three other trials using the same dose regimen;
weighted mean SD of change calculated from other trials using any dose.
Assessment of heterogeneity
We used Chi² and I² statistics to test for heterogeneity of treatment effect among trials. We consider a Chi² value P < 0.1 or I² value > 50% indicative of heterogeneity.We used the fixed‐effect model when there was homogeneity and used the random‐effects model to test for statistical significance where there was heterogeneity.
Assessment of reporting biases
We used funnel plots to investigate publication reporting bias when suspected. As a rule of thumb, tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta‐analysis, because when there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry.
Data synthesis
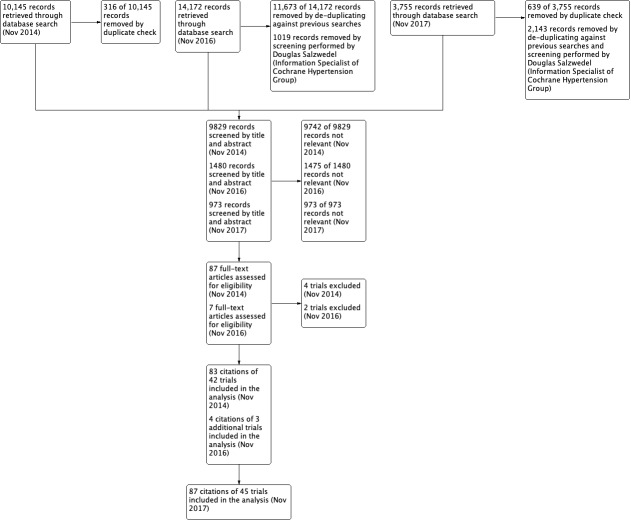
We performed data synthesis and analyses using the Cochrane Review Manager software, RevMan 5.3 (RevMan 2014). We described data results in tables and forest plots according to Cochrane guidelines. In addition we gave full details for all studies we included and excluded. We have included a standard PRISMA flow diagram (Figure 1).
1.
PRISMA Study flow diagram
Subgroup analysis and investigation of heterogeneity
Where appropriate, we performed the following subgroup analyses:
-
Heterogeneity among participants could be related to:
gender;
age;
presence of diabetes at initiation of antihypertensive treatment (time of trial entry);
baseline blood pressure;
previous renal disease;
previous CV disease.
-
Heterogeneity in treatments could be related to:
dose of drugs;
duration of therapy.
Sensitivity analysis
We tested the robustness of the results using several sensitivity analyses, including:
trials that were industry‐sponsored versus non‐industry‐sponsored;
trials with reported standard deviations of effect change versus imputed standard deviations;
trials that have a high risk of bias versus those with a low risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Up to November 2017, the search strategy identified 15,145 citations (Figure 1). After excluding all the studies that did not meet the inclusion criteria or those we have included before, we performed full‐text assessment of five potentially eligible studies and identified three new trials (NESTOR 2015; SILVHIA 2001; Xiao 2016) (four citations) that we included in the review update. This update includes 45 RCTs with 87 citations, i.e. the three new RCTs and the 42 RCTs (83 citations) in the first publication of this review (Xue 2015).
Included studies
The 45 included studies involved 66,625 participants with a mean age of 66 years. Participants in nine studies were under 50 years old (Buus 2004; Buus 2007; Dahlöf 1993; Pedersen 1997; Schiffrin 1994; Sørensen 1998; Tarnow 1999; Xiao 2016; Zeltner 2008); in 22 studies participants were between 50 and 59 years old (Ariff 2006; Dahlöf 2002; Dalla 2004; Derosa 2004; Derosa 2005; Derosa 2014; Esnault 2008; Estacio 1998; Gottdiener 1998; Hauf‐Zachariou 1993; Hughes 2008; IDNT 2001; Malmqvist 2002; Parrinello 2009; Petersen 2001; Roman 1998; Schmieder 2009; Schneider 2004; Seedat 1998; SILVHIA 2001; Tedesco 1999; TOHMS 1993); and over 60 years old in the remaining 14 studies (ALLHAT 2002; BENEDICT 2004; Devereux 2001; Fogari 2012; Gerritsen 1998; Hajjar 2013; Hayoz 2012; Himmelmann 1996; LIFE 2002; NESTOR 2015; Ostman 1998; Schram 2005; Terpstra 2004; VALUE 2004). The mean duration of therapy was 1.9 years, ranging from 0.5 to 5.6 years. The number of participants who received RAS inhibitors was 25,421, while 5,525 received beta‐blockers, 19,040 CCBs, 16,316 thiazides, 240 alpha‐blockers, and 83 CNS active drugs. Three studies contained multiple different drug groups: Gottdiener 1998 contained six, TOHMS 1993 contained five, and ALLHAT 2002 contained three, so the numbers of studies comparing RAS inhibitors with other drug classes were 17 for beta‐blockers, 22 for CCBs ‐ within which there were two studies that used non‐dihydropyridine (BENEDICT 2004; Gottdiener 1998), and 20 studies that used dihydropyridine, 10 for thiazides, 3 for alpha‐blockers, and 1 for CNS active drugs.
Most of the included studies were industry‐sponsored (28/45). Participants with diabetes were involved in 14 studies, while one study included participants with impaired fasting glucose; participants with decreased renal function in seven studies, and seven studies contained participants with at least one risk factor for CV diseases. Three studies recruited only men (Dahlöf 1993; Gottdiener 1998; Schiffrin 1994). One study included only women, as it focused on postmenopausal women (Hayoz 2012). All 87 included citations were published in English with publication years ranging from 1993 to 2016.
Most participants (30 studies) were recruited from European countries; seven studies included participants from North America; two studies included participants from North America, Europe, and Asia (Dahlöf 2002; VALUE 2004); one study included participants from North America, South America, Europe, Asia and Australia (IDNT 2001); NESTOR 2015 included participants from North America, South America, Europe and Asia; one study included participants from North America and Europe (LIFE 2002); Devereux 2001 included participants from Europe and Asia; Seedat 1998 included participants from South Africa; and Xiao 2016 included participants from Asia. Fifteen of the 45 included studies reported ethnicity; the percentages of white, Hispanic, Asian, Black and other race participants were 71.0%, 0.3%, 1.7%, 23.7% and 3.3%, respectively.
In terms of baseline comorbidities, nine studies stated that they would not include people with a history of prior MI or stroke; 14 studies allowed participants with a history of prior MI or stroke if this had not occurred within the previous three or six months); the other 22 studies made no clear statement, but in general the proportion of participants without cardiac‐cerebral vascular comorbidities was high. Overall, this review represents treatment effects for primary prevention.
A stepwise therapeutic regimen was used in 34 studies, in which add‐on drugs were allowed to achieve BP goals. The second‐line drugs included open‐label, non‐study agents such as CCBs, thiazides, or beta‐blockers. The remaining eleven studies restricted the therapeutic regimens to monotherapy (Dahlöf 1993; Derosa 2004; Derosa 2014; Gottdiener 1998; Himmelmann 1996; Hauf‐Zachariou 1993; Sørensen 1998; Tedesco 1999; Terpstra 2004; TOHMS 1993; Xiao 2016).
With regard to the clinical classification of hypertension (see ESH/ESC 2013), we classified mean blood pressure of participants at baseline into two groups: 30 studies included Grade 1 hypertensive participants (SBP: 140 mmHg to 159 mmHg); 15 studies included Grade 2 hypertensive participants (SBP: 160 mmHg to 179 mmHg). Baseline untreated mean BP was 156/89 mmHg (SBP/DBP) for RAS inhibitors; 151/86 mmHg for CCBs; 172/98 mmHg for beta‐blockers; 146/85 mmHg for thiazides; 150/96 mmHg for alpha‐blockers; 152/99 mmHg for CNS active drugs.
For details, see Characteristics of included studies.
Excluded studies
Full‐text screening according to the prespecified inclusion criteria led to us excluding three of the seven citations during the update, in addition to the four citations excluded in the previous version of the review. In total, we excluded seven citations of six studies in the updated review. The reasons for each study's exclusion are described in Characteristics of excluded studies.
Risk of bias in included studies
The data extraction forms for each included study contained the details of study design, randomization, allocation, blinding, duration of treatment, funding, diagnosis, number of participants, age of participants, gender of participants, history of participants, inclusion and exclusion criteria, outcomes and intervention. We assessed the risk of bias for each included study (Figure 2), and all included studies (Figure 3), in detail (see Characteristics of included studies).
2.
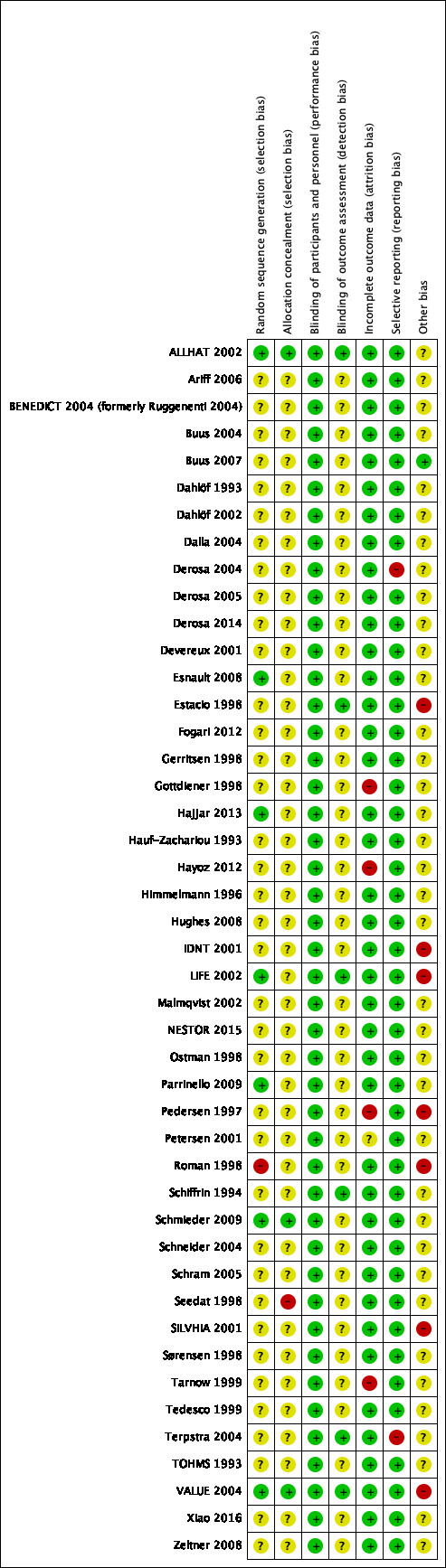
Risk of bias summary: review authors' judgements about each risk of bias item for each included citations
3.
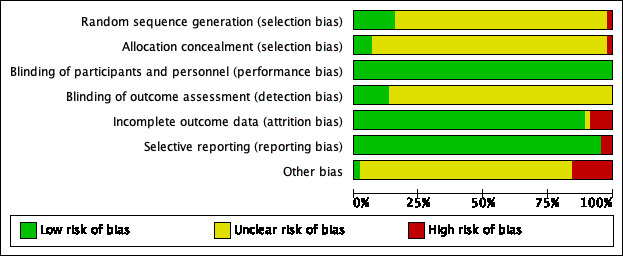
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included citations
Allocation
We assessed seven of the 45 studies as being at low risk of bias for reporting the method for generation of random sequence and one study as being at high risk (Roman 1998); in the remaining 37 studies the risk of bias for this domain was unclear. We assessed three of the 45 studies as being at low risk for allocation concealment, one study as being at high risk, and 41 studies as being at unclear risk. The three studies at low risk used either a central office allocation (ALLHAT 2002; VALUE 2004), or were strictly confidential until unblinding time (Schmieder 2009); one study reported using alternate allocation, which is a high risk method (Seedat 1998); two studies at unclear risk of selection bias reported the allocation concealment by using an envelope to maintain the random number (Derosa 2004; Derosa 2005); however, it was not clear whether the envelope was transparent or opaque.
Blinding
All the 45 included studies were at low risk of performance bias as they were all double‐blinded and met the inclusion criteria. In terms of detection bias, we judged only six studies to be at low risk due to the use of blinding for outcome assessment for BP or HR, which was critical for the control of detection bias; the risk of bias for this domain was unclear for the remaining 39 studies. We thought that unblinded assessment of outcomes like MI, stroke, HF, CV events, all‐cause death, and ESRF was not as critical as it would be for BP and HR.
Incomplete outcome data
We judged the risk of attrition bias in 40 of the 45 studies included in the review to be low because missing data were unlikely to have an impact because of low or equal numbers of dropouts between arms. In one study this risk was unclear (Petersen 2001); and in the other four studies we judged it to be high. Among these four studies with a high risk of attrition bias, Gottdiener 1998 only included participants with a left atrial dimension measurement (a small proportion of all participants) in the analysis. Pedersen 1997 and Tarnow 1999 had many participants lost to follow‐up at the end of study and Hayoz 2012 reported inconsistent numbers of participants in Figure 1 and Table 2.
Selective reporting
In this review, we judged 43 included studies to have a low risk of reporting bias; we judged that two studies had a high risk of selective reporting as they did not report HR, which was a prespecified outcome in their 'Methods' sections (Derosa 2004; Terpstra 2004).
Other potential sources of bias
Seven studies had a high risk of other potential sources of bias. Pedersen 1997 and Roman 1998 had unbalanced baseline characteristics. VALUE 2004 had an unbalanced proportion of monotherapy and highest dose between the two groups (including HCTZ and other non‐study add‐on drugs); Estacio 1998 had an unbalanced proportion of monotherapy. LIFE 2002 was evaluated as being at high risk as it was funded and conducted by the pharmaceutical company Merck. Similarly, many of the authors of IDNT 2001 had received research grants from Bristol‐Myers Squibb. Numbers of participants reported for different outcomes were not consistent in SILVHIA 2001.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. RAS inhibitors compared to CCBs for hypertension.
| First‐line RAS inhibitors compared to first‐line CCBs for hypertension | |||||||
| Patient or population: people with hypertension Settings: outpatients with mean follow‐up of 4.5 years Intervention: First‐line RAS inhibitors Comparison: First‐line CCBs | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | ||
| Assumed risk | Corresponding risk | ||||||
| CCBs | RAS inhibitors | ||||||
| All‐cause death | 124 per 1000 | 127 per 1000 (121 to 135) | RR 1.03 (0.98 to 1.09) | 35,226 (5) | ⊕⊕⊕⊝ moderate1 | ||
| Total cardiovascular events | 178 per 1000 | 174 per 1000 (166 to 182) | RR 0.98 (0.93 to 1.02) | 35,223 (6) | ⊕⊕⊕⊝ moderate1 | ||
| Death or hospitalization for heart failure | 72 per 1000 | 60 per 1000 (55 to 65) | RR 0.83 (0.77 to 0.90) | 35,143 (5) | ⊕⊕⊕⊝ moderate1 | ARR = 1.2% NNTB = 83 |
|
| Total myocardial infarction | 68 per 1000 | 69 per 1000 (63 to 74) | RR 1.01 (0.93 to 1.09) | 35,043 (5) | ⊕⊕⊕⊝ moderate1 | ||
| Total stroke | 39 per 1000 | 46 per 1000 (42 to 51) | RR 1.19 (1.08 to 1.32) | 34,673 (4) | ⊕⊕⊕⊝ moderate1 | ARI = 0.7% NNTH = 143 |
|
| End stage renal failure | 25 per 1000 | 22 per 1000 (19 to 26) | RR 0.88 (0.74 to 1.05) | 19,551 (4) | ⊕⊕⊝⊝ low1, 2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; ARR: absolute risk reduction; ARI: absolute risk increase; NNTB: number needed to treat to prevent one adverse outcome; NNTH: number needed to treat to cause one adverse outcome | |||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | |||||||
1Downgraded because we judged some of the included trials to be at high risk of bias. 2Downgraded because of wide confidence intervals which include a clinically important benefit.
Summary of findings 2. RAS inhibitors compared to thiazides for hypertension.
| First‐line RAS inhibitors compared to first‐line thiazides for hypertension | ||||||
|
Patient or population: people with hypertension
Settings: outpatients with mean follow‐up of 4.9 years
Intervention: First‐line RAS inhibitors Comparison: First‐line thiazides | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Thiazides | RAS inhibitors | |||||
| All‐cause death | 144 per 1000 |
144 per 1000 (135 to 154) |
RR 1.00 (0.94 to 1.07) |
24,309 (1) |
⊕⊕⊕⊝ moderate1 | |
|
Total cardiovascular events |
194 per 1000 | 204 per 1000 (194 to 215) | RR 1.05 (1.00 to 1.11) | 24,379 (2) | ⊕⊕⊕⊝ moderate1 | |
| Death or hospitalization for heart failure | 57 per 1000 |
68 per 1000 (61 to 75) |
RR 1.19 (1.07 to 1.31) |
24,309 (1) |
⊕⊕⊕⊝ moderate1 | ARI = 1.1% NNTH = 91 |
| Total myocardial infarction | 93 per 1000 | 86 per 1000 (80 to 94) | RR 0.93 (0.86 to 1.01) | 24,379 (2) | ⊕⊕⊕⊝ moderate1 | |
| Total stroke | 44 per 1000 |
50 per 1000 (45 to 56) |
RR 1.14 (1.02 to 1.28) |
24,309 (1) |
⊕⊕⊕⊝ moderate1 | ARI = 0.6% NNTH = 167 |
|
End stage renal failure Follow‐up: mean 4.9 years |
13 per 1000 |
14 per 1000 (11 to 18) |
RR 1.10 (0.88 to 1.37) |
24,309 (1) |
⊕⊕⊝⊝ low1, 2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; ARI; absolute risk increase. NNTH: number needed to treat to cause one adverse outcome | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
1Based on one large trial (ALLHAT 2002). 2Downgraded due to wide confidence intervals.
Summary of findings 3. RAS inhibitors compared to beta‐blockers for hypertension.
| First‐line RAS inhibitors compared to first‐line beta‐blockers for hypertension | ||||||
| Patient or population: people with hypertension Settings: outpatients with mean follow‐up of 4.8 years Intervention: First‐line RAS inhibitors Comparison: First‐line beta‐blockers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Β‐blockers | RAS inhibitors | |||||
| All‐cause death | 94 per 1000 | 84 per 1000 (73 to 95) |
RR 0.89 (0.78 to 1.01) |
9193 (1) |
⊕⊕⊝⊝ low1, 2 | |
|
Total cardiovascular events |
143 per 1000 | 126 per 1000 (114 to 140) | RR 0.88 (0.80 to 0.98) | 9239 (2) | ⊕⊕⊝⊝ low1, 2 | ARR = 1.7% NNTB = 59 |
| Total heart failure | 35 per 1000 |
33 per 1000 (27 to 41) |
RR 0.95 (0.76 to 1.18) |
9193 (1) |
⊕⊕⊝⊝ low1, 2 | |
|
Total myocardial infarction |
41 per 1000 | 43 per 1000 (35 to 52) | RR 1.05 (0.86 to 1.27) | 9239 (2) | ⊕⊕⊝⊝ low1, 2 | |
| Total stroke | 67 per 1000 |
50 per 1000 (42 to 59) |
RR 0.75 (0.63 to 0.88) |
9193 (1) |
⊕⊕⊝⊝ low1, 2 | ARR = 1.7% NNTB = 59 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; ARR: absolute risk reduction. NNTB: number needed to treat to prevent one adverse outcome | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
1Based primarily on one moderate‐sized trial (LIFE 2002). 2Wide confidence intervals and moderate to high risk of bias.
First‐line RAS inhibitors versus first‐line CCBs
Compared with CCBs, RAS inhibitors decreased HF (5 RCTs, 35,143 participants, RR 0.83, 95% CI 0.77 to 0.90; Analysis 1.3), and increased stroke (4 RCTs, 34,673 participants, RR 1.19, 95% CI 1.08 to 1.32; Analysis 1.5), but were not significantly different for all‐cause death (5 RCTs, 35,226 participants, RR 1.03, 95% CI 0.98 to 1.09; Analysis 1.1), total CV events (6 RCTs, 35,223 participants, RR 0.98, 95% CI 0.93 to 1.02; Analysis 1.2), total MI (5 RCTs, 35,043 participants, RR 1.01, 95% CI 0.93 to 1.09; Analysis 1.4), and ESRF (4 RCTs, 19,551 participants, RR 0.88, 95% CI 0.74 to 1.05; Analysis 1.6). CCBs lowered SBP and DBP to a greater degree than RAS inhibitors (SBP: 20 RCTs, 36,437 participants, MD 1.23, 95% CI 0.90 to 1.56; Analysis 1.7; DBP: 20 RCTs, 36,437 participants, MD 0.98, 95% CI 0.79 to 1.18; Analysis 1.8). There was no difference in the effect of CCBs and RAS inhibitors on HR (5 RCTs, 540 participants, MD 0.30, 95% CI ‐1.63 to 2.22; Analysis 1.9).
1.3. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 3 Total HF.
1.5. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 5 Total stroke.
1.1. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 1 All‐cause death.
1.2. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 2 Total CV events.
1.4. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 4 Total MI.
1.6. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 6 ESRF.
1.7. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 7 SBP.
1.8. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 8 DBP.
1.9. Analysis.
Comparison 1 RAS inhibitors vs CCBs, Outcome 9 HR.
First‐line RAS inhibitors versus first‐line thiazides
Compared with thiazides, RAS inhibitors increased HF (1 RCT, 24,309 participants, RR 1.19, 95% CI 1.07 to 1.31; Analysis 2.3), and increased stroke (1 RCT, 24,309 participants, RR 1.14, 95% CI 1.02 to 1.28; Analysis 2.5), but were not significantly different for all‐cause death (1 RCT, 24,309 participants, RR 1.00, 95% CI 0.94 to 1.07; Analysis 2.1), total CV events (2 RCTs, 24,379 participants, RR 1.05, 95% CI 1.00 to 1.11; Analysis 2.2), total MI (2 RCTs, 24,379 participants, RR 0.93, 95% CI 0.86 to 1.01; Analysis 2.4), and ESRF (1 RCT, 24,309 participants, RR 1.10, 95% CI 0.88 to 1.37; Analysis 2.6). Thiazides lowered SBP to a greater degree than RAS inhibitors (10 RCTs, 26,382 participants, MD 1.60, 95% CI 1.20 to 1.99; Analysis 2.7), but had a similar effect to RAS inhibitors on DBP (9 RCTs, 26,335 participants, MD ‐0.12, 95% CI ‐0.36 to 0.13; Analysis 2.8). There was also no difference in the effect on HR, but only two small trials reported this outcome (2 RCTs, 84 participants, MD 0.66, 95% CI ‐2.87 to 4.19; Analysis 2.9).
2.3. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 3 Total HF.
2.5. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 5 Total stroke.
2.1. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 1 All‐cause death.
2.2. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 2 Total CV events.
2.4. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 4 Total MI.
2.6. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 6 ESRF.
2.7. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 7 SBP.
2.8. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 8 DBP.
2.9. Analysis.
Comparison 2 RAS inhibitors vs thiazides, Outcome 9 HR.
First‐line RAS inhibitors versus first‐line beta‐blockers
Compared with beta‐blockers, RAS inhibitors decreased total CV events (2 RCTs, 9,239 participants, RR 0.88, 95% CI 0.80 to 0.98; Analysis 3.2) and decreased stroke (1 RCT, 9,193 participants, RR 0.75, 95% CI 0.63 to 0.88; Analysis 3.5). Beta‐blockers and RAS inhibitors were not significantly different for all‐cause death (1 RCT, 9,193 participants, RR 0.89, 95% CI 0.78 to 1.01; Analysis 3.1), HF (1 RCT, 9,193 participants, RR 0.95, 95% CI 0.76 to 1.18; Analysis 3.3), or MI (2 RCTs, 9.239 participants, RR 1.05, 95% CI 0.86 to 1.27; Analysis 3.4). The effect on ESRF could not be assessed because there was only one small trial that examined this outcome (1 RCT, 46 participants, RR 0.33, 95% CI 0.01 to 7.78; Analysis 3.6). Beta‐blockers lowered DBP and HR more than RAS inhibitors (DBP: 16 RCTs, 10,905 participants, MD 0.48, 95% CI 0.14 to 0.83; Analysis 3.8; HR: 10 RCTs, 9,979 participants, MD 6.05, 95% CI 5.59 to 6.50; Analysis 3.9). The effect on SBP did not differ between the two classes of drug (16 RCTs, 10,905 participants, MD ‐0.55, 95% CI ‐1.22 to 0.11; Analysis 3.7).
3.2. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 2 Total CV events.
3.5. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 5 Total stroke.
3.1. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 1 All‐cause death.
3.3. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 3 Total HF.
3.4. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 4 Total MI.
3.6. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 6 ESRF.
3.8. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 8 DBP.
3.9. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 9 HR.
3.7. Analysis.
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 7 SBP.
First‐line RAS inhibitors versus first‐line alpha‐blockers
RAS inhibitors lowered SBP more than alpha‐blockers did (3 small RCTs, 380 participants, MD ‐2.38, 95% CI ‐3.98 to ‐0.78; Analysis 4.1), but did not differ in their effect on DBP and HR (DBP 3 small RCTs, 380 participants, MD ‐0.12, 95% CI ‐1.09 to 0.85; Analysis 4.2; HR: 1 small RCT, 44 participants, MD 3.10, 95% CI ‐2.41 to 8.61; Analysis 4.3).
4.1. Analysis.
Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 1 SBP.
4.2. Analysis.
Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 2 DBP.
4.3. Analysis.
Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 3 HR.
First‐line RAS inhibitors versus first‐line CNS active drugs
When compared with CNS active drugs in one small trial, RAS inhibitors did not differ in their effect on SBP (1 RCT, 56 participants, MD 1.30, 95% CI ‐6.01 to 8.61; Analysis 5.1), DBP (1 RCT, 56 participants, MD ‐0.30, 95% CI ‐1.85 to 1.25; Analysis 5.2), or HR (1 RCT, 56 participants, MD 1.50, 95% CI ‐4.13 to 7.13; Analysis 5.3).
5.1. Analysis.
Comparison 5 RAS inhibitors vs CNS active drug, Outcome 1 SBP.
5.2. Analysis.
Comparison 5 RAS inhibitors vs CNS active drug, Outcome 2 DBP.
5.3. Analysis.
Comparison 5 RAS inhibitors vs CNS active drug, Outcome 3 HR.
Subgroup analysis and investigation of heterogeneity
In this review, when the result was significant and the value of I2 was greater than 50%, we tested whether the result was still significant using the random‐effects model. However, in presenting the data we use the fixed‐effect model, as it weights the contributing trials more appropriately.
In an attempt to explore the heterogeneity of RAS inhibitors versus CCBs on HF (I2 of 68%) we analyzed the trials according to whether or not the participants had decreased renal function. In those with decreased renal function the RR was 0.55, 95% CI 0.43 to 0.70, without heterogeneity (Dalla 2004; IDNT 2001); while in those without decreased renal function the RR was 0.87, 95% CI 0.80 to 0.95, without heterogeneity (ALLHAT 2002; Estacio 1998; VALUE 2004). Subgroup analysis thus provided a possible explanation for the variation of effect sizes across the studies. The magnitude of the effect of RAS inhibitors for decreasing HF, when compared to CCBs, was greater in hypertensive participants with kidney dysfunction than in those with normal renal function.
The key results on the clinically important outcomes and grading of the evidence quality are presented in the 'Summary of findings' tables, which we created by using the software GRADEpro 3.6. (Atkins 2004) (Table 1; Table 2; Table 3) These tables provide the absolute effects as well as the relative effects.
Discussion
Summary of main results
This first update of the review provides no change in primary outcomes, because the three new RCTs added only provided blood pressure and heart rate data. Compared with first‐line CCBs, first‐line RAS inhibitors reduce death or hospitalizations for HF, increase fatal and non‐fatal stroke, and are similar for all‐cause death, total CV events and ESRF events. Compared with first‐line thiazides, first‐line RAS inhibitors increase death or hospitalizations for HF and increase fatal and non‐fatal stroke. RAS inhibitors are similar to thiazides for all‐cause death, total CV events, fatal and non‐fatal MI and ESRF events. Compared with first‐line beta‐blockers, first‐line RAS inhibitors reduce total CV events and fatal and non‐fatal stroke and are similar for all‐cause death, HF, MI and ESRF. There were no RCTs that compared first‐line RAS inhibitors with any other classes of drugs that reported mortality and morbidity outcomes.
These results demonstrate that first‐line RAS inhibitors are an inferior choice to first‐line thiazides, because first‐line RAS inhibitors increase both death and hospitalizations for HF and fatal and non‐fatal stroke events compared to thiazides.
The results also suggest that first‐line RAS inhibitors are a better choice than first‐line CCBs, because the absolute reduction in death or hospitalizations for HF of 1.2% found with RAS inhibitors is greater than the increase in fatal and non‐fatal stroke of 0.7%. These findings confirm and extend the findings of the Cochrane Review of first‐line CCBs versus other classes of drugs (Chen 2010).
The results also suggest that RAS inhibitors are a better first‐line choice than first‐line beta‐blockers for hypertension, confirming the conclusions of two other Cochrane Reviews (Wiysonge 2017; Wright 2009).
For the blood pressure and heart rate outcomes, the small but statistically significant greater reduction in SBP of 1.6 mmHg with first‐line thiazides compared to RAS inhibitors could have contributed to the improved outcomes with first‐line thiazides, but is unlikely to be the only explanation. The fact that BP is not the only explanation is demonstrated by the fact that first‐line beta‐blockers, which lowered HR and diastolic BP more than first‐line RAS inhibitors, had worse morbidity outcomes.
Overall completeness and applicability of evidence
The number of trials and participants contributing to the three main comparisons in this review provide sufficient evidence regarding the outcomes that are important to patients to make first‐line thiazides the optimal first‐line drug for hypertension and to make RAS inhibitors the second best first‐line choice for hypertension. This result is based on moderate‐quality evidence demonstrating that first‐line thiazides decrease HF and stroke by about 1.7% over 4.9 years when compared to first‐line RAS inhibitors, meaning that for every 59 people treated for five years one event can be prevented. First‐line RAS inhibitors are the second best first‐line drug according to low‐quality evidence that first‐line RAS inhibitors reduce stroke by 1.7% compared to beta‐blockers and moderate‐quality evidence that they decrease overall CV events by 0.5% compared to CCBs, due to a reduction in HF events.
The evidence in this review is mostly relevant to primary prevention in patients, but is also relevant to people with hypertension and comorbidities such as T2DM, left ventricular hypertrophy, or diabetic nephropathy.
It is also important to note that the mortality and morbidity comparisons studied here involved predominately ACE inhibitors versus thiazides (ALLHAT 2002) and ARBs versus beta‐blockers (LIFE 2002). The comparison with CCBs involved both ACE inhibitors and ARBs. Subgroup comparisons based on either ACE inhibitors or ARBs compared to CCBs showed that the results were similar for HF and stroke. In addition, it is important to appreciate that in 12 of 17 studies using beta‐blockers, atenolol was the study drug, so that it is possible that the worse outcomes seen with beta‐blockers are limited to atenolol.
Sensitivity analysis
In this review, we used several analyses to test the robustness of the results. The specific sensitivity analyses done are described below.
Studies with reported standard deviations (SDs) of effect change versus those with imputed SDs
In this review, three studies did not report a within‐study variance for change in BP and we imputed SDs using the weighted mean SD from other trials (Esnault 2008; Fogari 2012; Roman 1998). When we excluded these three trials from the analysis, the BP estimates were not changed significantly.
Studies with a high risk of bias versus those with a low risk of bias
We judged four of the included studies that contributed data to the primary outcomes analyses to be at a high risk of 'other' bias (Estacio 1998; IDNT 2001; LIFE 2002; VALUE 2004). Three of these four studies compared RAS inhibitors with CCBs; their high risk of bias resulted from an unbalanced proportion of monotherapy and use of higher doses in one of the two treatment groups in the VALUE 2004 study, an unbalanced proportion of monotherapy in the Estacio 1998 study, and many authors receiving research grants form Bristol‐Myers Squibb in the IDNT 2001 study. When we dropped these three studies from the analysis, the results did not change significantly. Another high‐risk trial was funded and conducted by Merck (LIFE 2002), but this RCT was the only one providing data for the comparison of RAS inhibitors and beta‐blockers, and we therefore could not perform a sensitivity analysis.
In terms of the secondary outcomes (SBP, DBP and HR), when we excluded the studies with a high risk of other bias from the analysis in comparison of RAS inhibitors with CCBs (Pedersen 1997; VALUE 2004), the results did not change significantly. In the comparison of RAS inhibitors with beta‐blockers, when we dropped the studies with a high risk of other bias from the analysis (LIFE 2002; SILVHIA 2001), SBP decreased more in beta‐blockers (14 RCTs, MD 1.37, 95% CI 0.02 to 2.71) than with RAS inhibitors, with little clinical significance. The results did not change significantly in the comparison of RAS inhibitors with thiazides when we excluded Roman 1998 with a high risk of 'other' bias.
Potential biases in the review process
One potential bias that deserves attention is combination medication. For most long‐term and large‐scale studies, it is impossible to maintain single first‐line drug treatment, as a single drug frequently does not adequately lower the BP to an acceptable level. In most cases in the included studies, physicians were permitted to add other non‐study drugs to attempt to reach the target BP. In these RCTs and in this review, we hope that the add‐on drugs were balanced between the different treatment groups and, therefore, that any differences in outcomes were due to the first‐line drugs. The fact that we include only double‐blinded trials in this review decreases this possible bias, but there was no way of verifying that this was the case in all the trials. A potential limitation of this review is the possibility that there are differences in the effect of ACE inhibitors and ARBs on morbidity and mortality. This would have to be answered by specific head‐to‐head RCTs comparing the subclasses of drugs that inhibit the renin angiotensin system. A Cochrane Review comparing first‐line ACE inhibitors with first‐line ARBs suggests no difference in total mortality and total cardiovascular events (Li 2014), but more evidence is needed.
Unfortunately, there were not enough trials contributing to the primary outcomes to allow us to assess for publication bias. The BP and HR estimates cannot be used to estimate the BP‐lowering capacity of the first‐line drug, as other drugs could be added. The small statistically significant differences in BP lowering therefore cannot be entirely attributed to the first‐line drug. They may represent real differences in BP‐lowering capacity, but other systematic reviews specifically designed to assess BP will be needed to confirm these results.
Agreements and disagreements with other studies or reviews
The results of comparison between RAS inhibitors and CCBs are in agreement with those in the Chen 2010 Cochrane Review for the outcomes of MI, stroke, HF, CV events, and all‐cause death, as well as SBP and DBP. Likewise, the results in this review for first‐line RAS inhibitors compared with first‐line beta‐blockers are similar to those reported by another review (Wiysonge 2017) for morbidity and mortality outcomes.
Authors' conclusions
Implications for practice.
Compared to first‐line renin angiotensin system (RAS) inhibitors, first‐line thiazides reduce heart failure (HF) and stroke. Compared with calcium channel blockers (CCBs), RAS inhibitors reduce HF, but increase stroke; the magnitude of the reduction in HF outweighs the increase in stroke. The lower incidence of cardiovascular events and stroke that we found with RAS inhibitors relative to beta‐blockers may change with additional trials. In this updated review, only the data for blood pressure are changed by a small amount. The small differences in effect on blood pressure between the different classes of drugs did not necessarily correlate with the differences in the primary outcomes.
Implications for research.
Most of the data in this review come from the ALLHAT 2002 and LIFE 2002 trials. More large long‐term trials are needed to compare first‐line RAS inhibitors with other classes of drugs, particularly in subgroups of patients such as those with type 2 diabetes mellitus or early renal failure.
It is possible that first‐line angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers and renin inhibitors could have different mortality and morbidity outcomes, so more randomized controlled trials comparing them are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2018 | New citation required but conclusions have not changed | This review includes an updated search conducted in November 2017. Three new studies met the inclusion criteria, making the number of included RCTs 45 in total. Three additional authors contributed to the update: Song Jia Yang, Qiu Ru and Li Qian. |
| 15 October 2018 | New search has been performed | No data for the primary outcomes were reported in the three new RCTs, so the evidence on all‐cause death, total CV events, total HF, total MI, total stroke and ESRF remain the same. Data on blood pressure were updated in the three main comparisons: RAS inhibitors versus CCBs, thiazides, and beta‐blockers. However, we found little change in blood pressure. In addition, data on heart rate were updated in the comparison of RAS versus beta‐blocker, with no change to that outcome either. The formerly "Ruggenenti 2004" trial was renamed "BENEDICT 2004" in this version, The abbreviation "BENEDICT" stands for "Bergamo Nephrologic Diabetes Complications Trial" and was given by the study group. We regard it necessary to make the change for the readers to identify this trial easier by its official abbreviation. |
Acknowledgements
The authors would like to acknowledge the help provided by the Cochrane Hypertension Group.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 20 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Angiotensin‐Converting Enzyme Inhibitors/ 2 ((angiotensin$ or dipeptidyl$ or kininase ii) adj3 (convert$ or enzyme or inhibit$ or recept$ or block$)).tw,kf. 3 (ace adj2 inhibit$).tw,kf. 4 acei.tw,kf. 5 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw,kf. 6 or/1‐5 7 exp Angiotensin Receptor Antagonists/ 8 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw,kf. 9 (arb or arbs).tw,kf. 10 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan).tw,kf. 11 or/7‐10 12 renin/ai 13 (aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren).mp. 14 ((RAS or renin) adj2 inhibit$).tw,kf. 15 or/12‐14 16 6 or 11 or 15 17 exp calcium channel blockers/ 18 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw,kf. 19 (calcium adj2 (antagonist? or block$ or inhibit$)).tw,kf. 20 or/17‐19 21 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 22 (reserpine or serpentina or rauwolfia or serpasil).mp. 23 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 24 exp hydralazine/ 25 (dihydralazine or hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw,kf. 26 or/21‐25 27 exp adrenergic beta‐antagonists/ 28 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw,kf. 29 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw,kf. 30 or/27‐29 31 exp adrenergic alpha antagonists/ 32 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw,kf. 33 (adrenergic adj2 (alpha or antagonist?)).tw,kf. 34 ((adrenergic or alpha or receptor?) adj2 block$).tw,kf. 35 or/31‐34 36 exp thiazides/ 37 exp sodium potassium chloride symporter inhibitors/ 38 ((loop or ceiling) adj diuretic?).tw,kf. 39 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw,kf. 40 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw,kf. 41 or/36‐40 42 hypertension/ 43 hypertens$.tw,kf. 44 ((high or elevat$ or rais$) adj2 blood pressure).tw,kf. 45 or/42‐44 46 randomized controlled trial.pt. 47 controlled clinical trial.pt. 48 randomized.ab. 49 placebo.ab. 50 drug therapy.fs. 51 randomly.ab. 52 trial.ab. 53 groups.ab. 54 or/46‐53 55 animals/ not (humans/ and animals/) 56 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 57 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. 58 54 not (55 or 56 or 57) 59 16 and 45 and 58 and (20 or 26 or 30 or 35 or 41)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web) Search Date: 22 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ((angiotensin or dipeptidyl or kininase ii) near3 (convert* or enzyme or inhibit* or recept* or block*)) AND INSEGMENT #2 (ace near2 inhibit*) AND INSEGMENT #3 acei AND INSEGMENT #4 ((alacepril or altiopril or benazepril or captopril or ceronapril or cilazapril or delapril or enalapril or fosinopril or idapril or imidapril or lisinopril or moexipril or moveltipril or pentopril or perindopril or quinapril or ramipril or spirapril or temocapril or trandolapril or zofenopril)) AND INSEGMENT #5 #1 OR #2 OR #3 OR #4 AND INSEGMENT #6 (angiotensin near3 (receptor antagon* or receptor block*)) AND INSEGMENT #7 (arb or arbs) AND INSEGMENT #8 ((abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan)) AND INSEGMENT #9 #6 OR #7 OR #8 AND INSEGMENT #10 MESH DESCRIPTOR Renin WITH QUALIFIER AI AND INSEGMENT #11 ((aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren)) AND INSEGMENT #12 (RAS or renin) near2 inhibit* AND INSEGMENT #13 #10 OR #11 OR #12 AND INSEGMENT #14 #5 OR #9 OR #13 AND INSEGMENT #15 ((amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM)) AND INSEGMENT #16 (calcium near2 (antagonist* or block* or inhibit*)) AND INSEGMENT #17 #15 OR #16 AND INSEGMENT #18 ((methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa)) AND INSEGMENT #19 ((clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets)) AND INSEGMENT #20 ((dihydralazine or hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat)) AND INSEGMENT #21 #18 OR #19 OR #20 AND INSEGMENT #22 ((acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol)) AND INSEGMENT #23 (beta near2 (antagonist* or receptor* or adrenergic* next block*)) AND INSEGMENT #24 #22 OR #23 AND INSEGMENT #25 ((alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin)) AND INSEGMENT #26 (adrenergic near2 (alpha or antagonist*)) AND INSEGMENT #27 ((adrenergic or alpha or receptor*) near2 block*:ti,ab,kw) AND INSEGMENT #28 #25 OR #26 OR #27 AND INSEGMENT #29 ((loop or ceiling) next diuretic*) AND INSEGMENT #30 ((amiloride or benzothiadiazine* or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*)) AND INSEGMENT #31 ((chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide)) AND INSEGMENT #32 (Sodium Potassium Chloride Symporter Inhibitor*) AND INSEGMENT #33 #29 OR #30 OR #31 OR #32 AND INSEGMENT #34 #14 AND (#17 OR #21 OR #24 OR #28 OR #33) AND INSEGMENT #35 RCT:DE AND INSEGMENT #36 Review:MISC2 AND INSEGMENT #37 #35 OR #36 AND INSEGMENT #38 #34 AND #37 AND INSEGMENT ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search Date: 22 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (angiotensin or dipeptidyl or kininase ii) near3 (convert* or enzyme or inhibit* or recept* or block*) AND CENTRAL:TARGET #2 (ace near2 inhibit*) AND CENTRAL:TARGET #3 acei:ti,ab,kw AND CENTRAL:TARGET #4 (alacepril or altiopril or benazepril or captopril or ceronapril or cilazapril or delapril or enalapril or fosinopril or idapril or imidapril or lisinopril or moexipril or moveltipril or pentopril or perindopril or quinapril or ramipril or spirapril or temocapril or trandolapril or zofenopril) AND CENTRAL:TARGET #5 #1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET #6 (angiotensin) near3 (receptor antagon* or receptor block*) AND CENTRAL:TARGET #7 (arb or arbs) AND CENTRAL:TARGET #8 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan) AND CENTRAL:TARGET #9 #6 OR #7 OR #8 AND CENTRAL:TARGET #10 MESH DESCRIPTOR Renin WITH QUALIFIER AI AND CENTRAL:TARGET #11 (aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren) AND CENTRAL:TARGET #12 (RAS or renin) near2 inhibit* AND CENTRAL:TARGET #13 #10 OR #11 OR #12 AND CENTRAL:TARGET #14 #5 OR #9 OR #13 AND CENTRAL:TARGET #15 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM) AND CENTRAL:TARGET #16 (calcium near2 (antagonist* or block* or inhibit*)) AND CENTRAL:TARGET #17 #15 OR #16 AND CENTRAL:TARGET #18 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa) AND CENTRAL:TARGET #19 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets) AND CENTRAL:TARGET #20 (dihydralazine or hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat) AND CENTRAL:TARGET #21 #18 OR #19 OR #20 AND CENTRAL:TARGET #22 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) AND CENTRAL:TARGET #23 (beta near2 (antagonist* or receptor* or adrenergic* next block*)) AND CENTRAL:TARGET #24 #22 OR #23 AND CENTRAL:TARGET #25 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin) AND CENTRAL:TARGET #26 (adrenergic near2 (alpha or antagonist*)) AND CENTRAL:TARGET #27 (adrenergic or alpha or receptor*) near2 block* AND CENTRAL:TARGET #28 #25 OR #26 OR #27 AND CENTRAL:TARGET #29 MESH DESCRIPTOR Thiazides Explode ALL AND CENTRAL:TARGET #30 MESH DESCRIPTOR Sodium Potassium Chloride Symporter Inhibitors Explode ALL AND CENTRAL:TARGET #31 ((loop or ceiling) next diuretic*) AND CENTRAL:TARGET #32 (amiloride or benzothiadiazine* or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*) AND CENTRAL:TARGET #33 ((chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide)) AND CENTRAL:TARGET #34 #29 OR #30 OR #31 OR #32 OR #33 AND CENTRAL:TARGET #35 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #36 (antihypertens* OR hypertens*):TI,AB AND CENTRAL:TARGET #37 (high or elevat* or rais*) NEAR2 "blood pressure":TI,AB,KW AND CENTRAL:TARGET #38 #35 or #36 or #37 AND CENTRAL:TARGET #39 #14 and #38 and (#17 or #21 or #24 or #28 or #34) AND CENTRAL:TARGET ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2017 November 17> Search Date: 20 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp dipeptidyl carboxypeptidase inhibitor/ 2 ((angiotensin$ or dipeptidyl$ or kininase ii) adj3 (convert$ or enzyme or inhibit$ or recept$ or block$)).tw. 3 (ace adj2 inhibit$).tw. 4 acei.tw. 5 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. 6 or/1‐5 7 exp angiotensin receptor antagonist/ 8 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. 9 (arb or arbs).tw. 10 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan).tw. 11 or/7‐10 12 exp renin inhibitor/ 13 (aliskiren or ciprokiren or ditekiren or enalkiren or remikiren or rasilez or tekturna or terlakiren or zankiren).tw. (2012) 14 ((RAS or renin) adj2 inhibit$).tw. (7669) 15 or/12‐14 16 6 or 11 or 15 17 calcium channel blocking agent/ 18 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. 19 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 20 or/17‐19 21 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 22 (reserpine or serpentina or rauwolfia or serpasil).mp. 23 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 24 hydralazine/ 25 (dihydralazine or hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. 26 or/21‐25 27 exp beta adrenergic receptor blocking agent/ 28 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. 29 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 30 or/27‐29 31 exp alpha adrenergic receptor blocking agent/ 32 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. 33 (adrenergic adj2 (alpha or antagonist?)).tw. 34 ((adrenergic or alpha or receptor?) adj2 block$).tw. 35 or/31‐34 36 exp thiazide diuretic agent/ 37 exp loop diuretic agent/ 38 ((loop or ceiling) adj diuretic?).tw. 39 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. 40 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. 41 or/36‐40 42 exp hypertension/ 43 (hypertens$ or antihypertens$).tw. 44 ((high or elevat$ or rais$) adj2 blood pressure).tw. 45 or/42‐44 46 randomized controlled trial/ 47 crossover procedure/ 48 double‐blind procedure/ 49 (randomi?ed or randomly).tw. 50 (crossover$ or cross‐over$).tw. 51 placebo.ab. 52 (doubl$ adj blind$).tw. 53 assign$.ab. 54 allocat$.ab. 55 or/46‐54 56 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 57 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 58 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. 59 55 not (56 or 57 or 58) 60 16 and 45 and 59 and (20 or 26 or 30 or 35 or 41) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 20 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other Terms: randomised Study Type: Interventional Studies Condition / Disease: Hypertension Intervention: Angiotensin‐Converting Enzyme Inhibitors OR Angiotensin Receptor Antagonists OR aliskiren OR remikiren ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: WHO International Clinical Trials Registry Platform Search Date: 22 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition: hypertens* Interventions: Angiotensin‐Converting Enzyme Inhibitor* OR ace inhibitor* OR alacepril OR altiopril OR benazepril OR captopril OR ceronapril OR cilazapril OR delapril OR derapril OR enalapril OR enalaprilat OR fosinopril OR idapril OR imidapril OR lisinopril OR moexipril OR moveltipril OR pentopril OR perindopril OR quinapril OR ramipril OR spirapril OR temocapril OR trandolapril OR zofenopril OR Angiotensin Receptor Antagonist* OR arb OR arbs OR candesartan OR eprosartan OR irbesartan OR losartan OR olmesartan OR tasosartan OR telmisartan OR valsartan OR KT3‐671 OR pratosartan OR renin inhibitor* OR aliskiren OR remikiren Recruitment status: ALL
Data and analyses
Comparison 1. RAS inhibitors vs CCBs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause death | 5 | 35226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.09] |
| 2 Total CV events | 6 | 35223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.02] |
| 3 Total HF | 5 | 35143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 4 Total MI | 5 | 35043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 5 Total stroke | 4 | 34673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.32] |
| 6 ESRF | 4 | 19551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.05] |
| 7 SBP | 20 | 36437 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [0.90, 1.56] |
| 8 DBP | 20 | 36437 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [0.79, 1.18] |
| 9 HR | 5 | 540 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.63, 2.22] |
Comparison 2. RAS inhibitors vs thiazides.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause death | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 2 Total CV events | 2 | 24379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.00, 1.11] |
| 3 Total HF | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.07, 1.31] |
| 4 Total MI | 2 | 24379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 5 Total stroke | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 6 ESRF | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.37] |
| 7 SBP | 10 | 26382 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.20, 1.99] |
| 8 DBP | 9 | 26335 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.36, 0.13] |
| 9 HR | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐2.87, 4.19] |
Comparison 3. RAS inhibitors vs beta‐blockers (β‐blockers).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause death | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 2 Total CV events | 2 | 9239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.98] |
| 3 Total HF | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 4 Total MI | 2 | 9239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.27] |
| 5 Total stroke | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.63, 0.88] |
| 6 ESRF | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| 7 SBP | 16 | 10905 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐1.22, 0.11] |
| 8 DBP | 16 | 10905 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.14, 0.83] |
| 9 HR | 10 | 9979 | Mean Difference (IV, Fixed, 95% CI) | 6.05 [5.59, 6.50] |
Comparison 4. RAS inhibitors vs alpha‐blockers (α‐blockers).
Comparison 5. RAS inhibitors vs CNS active drug.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ALLHAT 2002.
| Methods | Allocation: computer‐generated randomization Blinding: double‐blinded, and blinded assessment Duration: 4.9 ± 1.4 years Funding: National Heart, Lung and Blood Institute and financial support from Pfizer |
|
| Participants | Diagnosis: the risk factors included previous (> 6 months) MI or stroke, LVH demonstrated by ECG or echocardiography, history of T2DM, current cigarette smoking, HDL of < 35 mg/dL (< 0.91 mmol/L), or documentation of other atherosclerotic CVD N = 33357 Age: 55 years or older Sex: 47% women, 53% men History: not reported Inclusion criteria: stage 1 or 2 hypertension, 55 years or older, 1 additional risk factor for CHD events Excluded: individual with a history of hospitalized or treated symptomatic HF and/or left ventricular ejection fraction of < 35% |
|
| Interventions | RAS inhibitor: lisinopril; CCB: amlodipine; thiazide: chlorthalidone Step 1: 12.5 mg/day, 12.5 mg/day (sham titration), 25 mg/day for chlorthalidone; 2.5 mg/day, 5 mg/day, 10 mg/day for amlodipine; 10 mg/day, 20 mg/day, 40 mg/day for lisinopril Step 2: add‐on, atenolol 25 mg/day‐100 mg/day; 0.05 mg/day‐0.2 mg/day of reserpine; clonidine 0.1 mg‐0.3 mg twice daily Step 3: 25 mg‐100 mg twice daily of hydralazine. Other drugs, including low doses of open‐label step 1 drug classes, were permitted if clinically indicated |
|
| Outcomes | Primary outcomes: fatal CHD or non‐fatal MI combined Secondary outcomes:
Other secondary outcomes: cancer, incident ECG LVH, ESRF (dialysis, renal transplant, or death), slope of the reciprocal of longitudinal serum creatinine measurements |
|
| Notes | Participants assigned to lisinopril were less likely to be black and more likely to be women, had untreated hypertension, evidence of CHD or atherosclerotic CVD, and a lower mean serum glucose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Clinical trials center was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical trials center judged by clinic investigator reports, copies of death certificates, and hospital discharge summaries |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Although the study was supported by the government and industry, insufficient information was found to evaluate the risk as high or low |
Ariff 2006.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: this study was supported by a grant from AstraZeneca |
|
| Participants | Diagnosis: uncontrolled essential hypertension: 160/100 mmHg in untreated participants or 140/90 mmHg in treated participants plus evidence of target‐organ damage; accelerated hypertension: 220/120 mmHg N = 88 Median age (range): candesartan group 56 (37‐73) years; atenolol group 54 (39‐76) years Sex: 37.5% women, 62.5% men History: median duration of hypertension (range): candesartan 4 (1‐36) years; atenolol 3 (1‐36) years Inclusion criteria: uncontrolled essential hypertension Exclusion criteria: evidence of accelerated hypertension, MI or stroke within previous 6 months, DM, or any other condition that precluded participation |
|
| Interventions | RAS inhibitor: candesartan; beta‐blocker: atenolol Candesartan 8 mg or 16 mg daily Atenolol 50 mg or 100 mg daily Add‐ons HCTZ, felodipine doxazosin |
|
| Outcomes | SBP and DBP were measured in the right arm with an Omron HEM‐705‐CP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no patient withdrawals |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
BENEDICT 2004 (formerly Ruggenenti 2004).
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 48 months Funding: supported in part by Abbott (Ludwigshafen, Germany) |
|
| Participants | Diagnosis: arterial hypertension, defined as an untreated SBP ≥ 130 mmHg or a DBP ≥ 85 mmHg, or as the need for antihypertensive therapy to attain a SBP or DBP under these levels T2DM diagnosed according to the criteria of the WHO N = 604: trandolapril group 301; verapamil group 303 Age: trandolapril group 61.6 ± 8.1 years; verapamil group 62.5 ± 8.2 years Sex: 47% women, 53% men History: duration of diabetes (SD): trandolapril group mean 7.7 (6.7) years; verapamil group 8.2 (6.4) years Inclusion criteria: people aged 40 years or older with hypertension and a known history of T2DM not exceeding 25 years, a urinary albumin excretion rate > 20 μg/min in at least 2 of 3 consecutive, sterile, overnight samples, and a serum creatinine concentration of ≤ 1.5 mg/dL (133 μmol/L) Exclusion criteria: HbA1c > 11%, nondiabetic renal disease, and a specific indication for or contraindication to ACE‐inhibitor therapy or non‐dihydropyridine CCB therapy |
|
| Interventions | RAS inhibitor: trandolapril; CCB: verapamil Verapamil 240 mg/day Trandolapril 2 mg/day Add‐ons step 1, HCTZ or furosemide; step 2, doxazosin, prazosin, clonidine, methyldopa, or beta‐blockers (allowed on the basis of specific indications, such as cardiac ischemic disease, but only if not contraindicated on the basis of ECG findings, such as bradyarrhythmias and delayed atrioventricular conduction); and step 3, minoxidil or long‐acting dihydropyridine CCBs. The use of potassium‐sparing diuretics, inhibitors of the renin–angiotensin system, and non‐dihydropyridine CCBs different from the study drugs was not allowed |
|
| Outcomes | Trough SBP and DBP (Korotkoff phases 1 and 5, respectively) recorded as the mean of 3 morning measurements (to the nearest 2 mmHg) taken before the administration of a study drug CV death |
|
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes in the Methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Buus 2004.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: this work was supported by grants from Institut de Recherches Internationales Servier and the Danish Heart Foundation. MJM had support from the Danish Medical Research Council |
|
| Participants | Diagnosis: sitting DBP was 100 mmHg‐120 mmHg, measured 3 times with a mercury sphygmomanometer N = 30 Age: perindopril group 49 ± 2 years; atenolol group 51 ± 2 years Sex: 27% women, 73% men History: not reported Inclusion criteria: people with sitting DBP of 100mmHg‐120 mmHg. People suspected of secondary hypertension underwent isotope renography, spiral computed tomographic scan of the renal arteries, or urinary sampling of catecholamines, but none showed signs of secondary hypertension, and all were included Excluded: not reported |
|
| Interventions | RAS inhibitor: perindopril; beta‐blocker: atenolol Perindopril 4 mg or 8 mg daily Atenolol 50 mg or 100 mg daily Add‐on, bendroflumethiazide |
|
| Outcomes | HR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Buus 2007.
| Methods | Allocation: randomization was balanced to ensure an equal gender and age distribution in the 2 groups Blinding: double‐blinded Duration: 1 year Funding: this work was supported in part by grants from Institute de Recherces Internationales Servier and the Danish Heart Foundation |
|
| Participants | Diagnosis: not reported N = 31 Age: perindopril group 49 ± 7.7 years, atenolol group 51 ± 7.7 years Sex: 26% women, 74% men History: not reported Inclusion criteria: sitting DBP of 100 mmHg–120 mmHg Exclusion criteria: symptoms or signs of ischemic heart disease or secondary hypertension |
|
| Interventions | RAS inhibitor: perindopril; beta‐blocker: atenolol Perindopril 4 mg or 8 mg daily Atenolol 50 mg or 100 mg daily Add‐ons, bendroflumethiazide |
|
| Outcomes | BPs measured 3 times with a mercury sphygmomanometer, on 2 or 3 occasions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Low risk | Government funded trial |
Dahlöf 1993.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 6 months Funding: Gothenburg Medical Society and Merck Sharp and Dohme (Sweden) AB |
|
| Participants | Diagnosis: non‐malignant essential hypertension: DBP > 95 mmHg at least 3 times on placebo N = 28 Age: 22‐64 years Sex: 100% men History: not reported Inclusion criterion: previously untreated men with non‐malignant essential hypertension Exclusion criteria: secondary hypertension or signs of CV end‐organ damage (except LVH and hypertensive retinopathy) |
|
| Interventions | RAS inhibitor: enalapril; thiazide: HCTZ Enalapril average daily doses 34.9 mg HCTZ average daily doses 53.5 mg |
|
| Outcomes | Supine BP: mercury sphygmomanometer, adequate cuff size, Korotkoff sounds 1 and 5 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "enalapril and hydrochlorothiazide were compared in a double‐blinded, randomised, parallel group design." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quoted, "the groups were well balanced regarding demographic variables, cardiac hypertrophy and retinopathy." "All patients were still on randomised monotherapy after 6 months and were included in the analysis irrespective of blood pressure response." |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Dahlöf 2002.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 36 weeks Funding: Merck Co Inc |
|
| Participants | Diagnosis: not reported N = 210 Age: 21‐80 years Sex: 39% women, 61% men History: not reported Inclusion criteria: men and women, aged 21–80 years, with mild to moderate essential hypertension and ECG‐documented LVH assessed up to 30 days before enrolment. Eligible participants had trough sitting DBP of 95–115 mmHg or sitting SBP of 160 mmHg–200 mmHg, or both, while receiving placebo for 2–4 weeks, and a left ventricular mass index (LVMI) > 120 g/m² for men and > 105 g/m² for women Exclusion criteria: a LV end‐diastolic dimension > 60 mm, irrespective of the LVMI, systolic dysfunction or significant valvular disease |
|
| Interventions | RAS inhibitor: losartan; beta‐blocker: atenolol Losartan 50 mg or 100 mg daily Atenolol 50 mg or 100 mg daily Add‐on, HCTZ |
|
| Outcomes | Clinical DBP and SBP were measured at trough (22–26 hours after the previous study medication) with a standard mercury sphygmomanometer, with the participant in the sitting position after 5 min of rest, at every clinic visit (baseline and treatment). The trialists used means of 3 consecutive measurements at 2–3 min intervals | |
| Notes | The patient population included in this study differed from those included in the LIFE echocardiographic sub‐study, although the treatment regimens compared were the same | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as high or low |
Dalla 2004.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: not reported |
|
| Participants | Diagnosis: persistent microalbuminuria: AER 20 g/min‐200 g/min during the last 3 months; mild to moderate hypertension: mean DBP between 85 mmHg‐109 mmHg and SBP < 180 mmHg; T2DM: diagnosed according to the criteria of the WHO N = 180 Age: 40‐70 years Sex: 27% women, 73% men History: time since diabetes diagnosed (years): lercanidipine group 10 ± 6.4; atenolol group 11 ± 7.9 (means ± SD) Inclusion criteria: mild to moderate hypertensive people aged from 40‐70 years, affected by T2DM with the presence of persistent microalbuminuria Exclusion criteria: arterial hypertension outside the range specified above; secondary arterial hypertension; orthostatic hypotension (SBP decrease > 20 mmHg after standing for 2 min); AER < 20 μg/min, ≥ 20 μg/min not persistent, > 200 μg/min; HbA1c > 10%; cardiac insufficiency (classes NYHA III‐IV); arrhythmias; valvular disease; CHDs; unstable angina pectoris; complete left bundle branch block; HR < 50 or > 100 bpm; acute MI or cerebrovascular accident 3 months prior to recruitment; transaminases > 2 times the normal limit; serum creatinine > 141.4 μmol/L; anemia (hemoglobin <10 g/dL); hypertensive retinopathy grade III‐IV; obesity (body mass index > 35 kg/m2); known hypersensitivity to dihydropyridine derivates or to ACE‐inhibitors |
|
| Interventions | RAS inhibitor: ramipril; CCB: lercanidipine Lercanidipine 10 mg or 20 mg/day Ramipril 5 mg or 10 mg/day Add‐ons HCTZ, atenolol |
|
| Outcomes | BP measured using a mercury sphygmomanometer (Korotkoff phase 1 and 5) with participants in a sitting position after at least 10 min of rest. 2 blood pressure recordings, taken 3 min apart, were obtained. If the 2 DBP values differed by more than 5 mmHg, an additional measurement was taken and included in the calculated average HF Stroke |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Derosa 2004.
| Methods | Allocation: randomization accomplished by the drawing of envelopes containing randomization codes prepared by a statistician Blinding: double‐blinded. All study staff were blinded to treatment assignment Duration: 12 months Funding: not reported |
|
| Participants | Diagnosis: nonsmoking people with T2DM for ≥ 2 years (HbA1c < 7.0%); mild hypertension (DBP 90mmHg‐99 mmHg) N = 116 Age: telmisartan group 52 ± 5 years, nifedipine group 53 ± 4 years Sex: 50% women, 50% men History: known DM for > 2 years Inclusion criteria: mild hypertension with T2DM Exclusion criteria: secondary hypertension; malignant hypertension; unstable angina; MI within the preceding 6 months; abnormalities of liver or renal function; or contraindications to or current use of ARBs or ACE inhibitors |
|
| Interventions | RAS inhibitor: telmisartan; CCB: nifedipine Telmisartan 40 mg/day Nifedipine Gastro‐Intestinal Therapeutic System (GITS) 20 mg/day |
|
| Outcomes | BP was measured using a standard mercury sphygmomanometer (Korotkoff 1 and 5) in the seated position | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was accomplished by the drawing of envelopes containing randomization codes prepared by a statistician." Whether the envelopes were opaque was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "this 12‐months, randomised, double‐blind trial was conducted at the Department of Internal Medicine and Therapeutics of the University of Pavia in Italy." "All study staff were blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "a copy of the code was provided only to the individual responsible for performing the statistical analysis." Reviewer comment: possible high risk of bias because the statistical analysis was not blinded, but it would not result in detection bias; the method of blinding of outcome assessment was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew |
| Selective reporting (reporting bias) | High risk | Quote: "at each clinical visit, heart rate was measured after the patient had been seated for >=10 minutes." Reviewer comment: high risk due to failure to report HR |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Derosa 2005.
| Methods | Allocation: randomization performed by the drawing of envelopes containing randomization codes prepared by a statistician Blinding: blinding of the investigators and participants was maintained by using identical numbered bottles prepared by the hospital pharmacy Duration: 12 months Funding: not reported |
|
| Participants | Diagnosis: not reported N = 96 Age: doxazosin group 53 ± 9 years, irbesartan group 52 ± 10 years Sex: 51% women, 49% men History: not reported Inclusion criteria: T2DM; mild hypertension (DBP > 90 mmHg and < 105 mmHg) Exclusion criteria: secondary hypertension; malignant hypertension; unstable angina; MI; and/or liver/renal function abnormalities |
|
| Interventions | RAS inhibitor: irbesartan; alpha‐blocker: doxazosin Doxazosin 4 mg daily Irbesartan 300 mg daily |
|
| Outcomes | SBP (Korotkoff 1) and DBP (Korotkoff 4) measurements were obtained from participants in the seated position using a standard mercury sphygmomanometer (Erkameter 3000, ERKA, Bad Tolz, Germany) with a cuff of appropriate size | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was performed by the drawing of envelopes containing randomization codes prepared by a statistician." Whether the envelopes were opaque was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "ninety‐six patients with type 2 diabetes … were enrolled in this randomised, double‐blind trial." "Blinding of investigators and patients was maintained using identical numbered bottles prepared by the hospital pharmacy." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "… no subject experienced adverse effects serious enough to warrant discontinuing either drug … " Despite the absence of numbers for participants reported in the data tables, the statement above was sufficient |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Derosa 2014.
| Methods | Allocation: randomized Blindness: double blind. Interventions were supplied as identical, opaque, white capsules in coded bottles to ensure the blind status of the study Duration: 24 months Funding: not reported |
|
| Participants | Diagnosis: essential hypertension [DBP >90 and <110 mmHg and/or SBP>140 mmHg and <180 mmHg] N= 222 Age: < 65 years old Sex: 51.8% women, 48.2% men History: no reported Inclusion criteria: outpatients of both sex, aged < 65 years, with a first diagnosis of essential hypertension and naïve to antihypertensive treatment Excluded: secondary hypertension, severe hypertension (SBP >180 mmHg or DBP >110 mmHg), hypertrophic cardiomyopathies due to aetiologies other than hypertension, history of heart failure or a left ventricular ejection fraction (LVEF) ≤50%, history of angina, stroke, transient ischaemic cerebral attack, coronary artery bypass surgery or myocardial infarction any time prior to visit 1, concurrent symptomatic arrhythmia, liver dysfunction (AST or ALT values exceeding 2‐fold the upper limit), creatinine >1.5 mg/dL and known hypersensitivity to the study drugs. Pregnant women as well as women of childbearing potential were excluded. Patients with endocrine, infective or inflammatory disorders were excluded, as well as were those taking anti‐inflammatory medications |
|
| Interventions | RAS inhibitor: enalapril; CCB: lercanidipine Enalapril 20mg daily Lercanidipine 10mg daily |
|
| Outcomes | Blood pressure measurements were obtained from each patient (using the right arm) in the seated position, using a standard mercury sphygmomanometer (Erkameter 3000; ERKA, Bad Tolz, Germany) (Korotkoff I and V) with a cuff of appropriate size. BP was measured by the same investigator at each visit, in the morning before daily drug intake and after the patient had rested for ≥10 min in a quiet room. Three successive BPreadings were obtained at 1‐min intervals, and the mean of the 3 readings was calculated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was unlikely to have an impact on the results of the trial. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported. |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low. |
Devereux 2001.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 48 weeks Funding: this study was supported by grant CDSP 964‐0A from Merck & Co, Whitehouse Station, NJ |
|
| Participants | Diagnosis: not reported N = 303; enalapril group 148; nifedipine group 155 Age: nalapril group 3.5 ± 9.0 years; nifedipine group 63.0 ± 8.6 years Sex : 34.3% women, 65.7% men History: not reported Inclusion criteria: seated SBP of 140 mmHg and/or DBP of 90 mmHg (Korotkoff phase 5) for previous 4 weeks if taking antihypertensive medications or 150 mmHg and/or 90 mmHg, respectively, if unmedicated Exclusion criteria: people with left ventricular ejection fraction ,40%, severe valvular disease, or coexisting cardiomyopathy on screening ECG. Initially, people receiving treatment with ACE inhibitors or CCBs were excluded |
|
| Interventions | RAS inhibitor: enalapril; CCB: nifedipine Enalapril 10 mg or 20 mg/day Nifedipine 30 mg or 60 mg/day Add‐ons HCTZ, atenolol |
|
| Outcomes | Reduction of SBP, DBP, and HR | |
| Notes | When the frequent use of ACE inhibitors or CCBs by participants with LVH became evident, participants were enrolled with stratified randomization to assure balanced representation in both treatment arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Although the pre‐specified outcomes were not available in the methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Esnault 2008.
| Methods | Allocation: the randomization schedule was generated by a statistical analysis system Blinding: double‐blinded Duration: 3 years Funding: Pfizer Inc. The biostatistics department of Pfizer was responsible for entering data, quality controls, and blinded statistical analysis; Pfizer had no other role in the study performance, analysis, and reporting |
|
| Participants | Diagnosis: malignant hypertension i.e. DBP > 120 mmHg; congestive heart disease according to New York Heart Association class II–IV N = 263 Age: 18‐80 years Sex: 40.7% women, 59.3% men History: not reported Inclusion criteria: nondiabetic adults, aged 18‐80 years, non‐nephrotic adult hypertensive patients with creatinine clearance of 20 mL‐ 60 mL/min·1.73 m² (Cockcroft‐Gault) Exclusion criteria: nephrotic proteinuria; secondary or malignant hypertension; a major CV event within previous 3 months; angina pectoris; CHD; uncontrolled arrhythmias; II–III degree atrioventricular block; need for steroids, nonsteroidal anti‐inflammatory or cytotoxic drugs; women of childbearing potential not using appropriate contraception; or any disease that could limit the ability of the patient to comply with the protocol requirements |
|
| Interventions | RAS inhibitor: enalapril; CCB: amlodipine Amlodipine 5 mg or 10 mg/day Enalapril 5 mg or 10 mg/day Add‐ons: atenolol (50 mg/day‐100 mg/day), loop diuretics (furosemide, 20 mg/day‐500 mg/day or torsemide 5 mg/day‐200 mg/day), alpha‐blockers (prazosin, 2.5 mg/day‐5 mg/day or doxazosin 1 mg/day‐16 mg/day), and centrally acting drugs (rilmenidine, 1 mg/day‐2 mg/day or methyldopa 250 mg/day‐500 mg/day) |
|
| Outcomes | All‐cause death, renal failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization schedule was generated by a statistical analysis system |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Although the role of the funding company was unlikely to have an impact on the study, no other information was found to evaluate the risk as either high or low |
Estacio 1998.
| Methods | Allocation: randomly assigned Blinding: double‐blinded Duration: 67 months Funding: Bayer Pharmaceutical Company, a grant (DK50298‐02) from the National Institute of Diabetes and Digestive and Kidney Diseases; Dr Hiatt was the recipient of an Academic Award in Vascular Disease from the National Institutes of Health |
|
| Participants | Diagnosis: NIDDM, mean base‐line DBP ≥ 90 mmHg N = 470 (all in analysis) Age: 40‐74 years Sex: 32.6% women, 67.4% men History: not reported, probably outpatients Inclusion criteria: participants enrolled in the ABCD Trial were between the ages of 40 and 74 years at the time of recruitment and were identified according to diagnosis‐related groups from the pharmacy and billing lists of participating healthcare systems in the Denver metropolitan area. All participants in the ABCD Trial had NIDDM, diagnosed according to criteria based on those of the WHO report of 1985. All enrolled subjects had DBP of 80 mmHg or higher and were receiving no antihypertensive medications at the time of randomization Exclusion criteria: a known allergy to dihydropyridine CCBs or ACE inhibitors; MI or cerebrovascular accident within the previous 6 months; coronary‐artery bypass surgery within the previous 3 months; unstable angina pectoris within the previous 6 months; New York Heart Association class III or IV CHF; an absolute need for therapy with ACE inhibitors or CCBs; were receiving hemodialysis or peritoneal dialysis; or serum creatinine concentration > 3 mg/dL (265 μmol/L) |
|
| Interventions | RAS inhibitor: enalapril; CCB: nisoldipine Nisoldipine 10 mg/day, with increases to 20 mg/day, 40 mg/day, and 60 mg/day, plus placebo in place of enalapril (Sular, Zeneca, Wilmington, Del) Enalapril 5 mg/day, with increases to 10 mg/day, 20 mg/day, and 40 mg/day, plus placebo in place of nisoldipine (Vasotec, Merck, Whitehouse Station, NJ) Open‐label, step‐wise, additional medication: metoprolol and HCTZ when participants did not achieve the target BP Notes: 99 participants in the enalapril group took a beta‐blocker, compared with 89 in the nisoldipine group (P value 0.035). 119 participants assigned to enalapril took a diuretic agent, as did 93 assigned to nisoldipine (P value 0.02) |
|
| Outcomes | Binary data: fatal MI, non‐fatal MI, cerebrovascular accident, congestive HF, death from CV causes, death from any cause | |
| Notes | Significantly more participants discontinued nisoldipine than enalapril because of headaches (P value 0.009). Significantly more discontinued enalapril because of malaise or fatigue (P value 0.005) or uncontrolled hypertension (P value 0.04) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The drugs and placebos were administered in a double‐blinded manner |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All CV events were reviewed by an independent endpoints committee whose members were blinded to the patients' assigned treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | High risk | Add‐on medication was not balanced between groups. Quote: "Ninety‐nine patients in the enalapril group took a beta‐blocker, as compared with 89 patients in the nisoldipine group (P=0.035). One hundred nineteen patients assigned to enalapril took a diuretic agent, as compared to 93 assigned to nisoldipine (P=0.02)." |
Fogari 2012.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: no funding source reported |
|
| Participants | Diagnosis: stage I hypertension: SBP ≥ 140 mmHg and < 160 mmHg and/or DBP ≥ 90 mmHg and < 100 mmHg N = 378 Age: 68 ± 8 years old Sex: 54.8% women, 45.2% men History: 49.7% participants had enlarged atrial size Inclusion criteria: consecutive outpatients of either sex, age 40–80 years, with stage I hypertension; in sinus rhythm, but with ≥ 2 ECG‐documented episodes of symptomatic AF in the previous 6 months, each lasting > 60 min but < 7 days and terminating spontaneously Exclusion criteria: ECG evidence of (LVH; treatment with ARBs, ACE inhibitors, or antiarrhythmic agents; cardioversion within the previous 3 months; secondary hypertension; MI or stroke in the preceding 6 months; CHF; coronary heart disease; valvular disease; cardiac surgery during the previous 6 months; significant thyroid, pulmonary, renal, or hepatic disease;pregnancy or fertile woman; and known hypersensitivity or contraindications to the study medications |
|
| Interventions | RAS inhibitor: telmisartan; CCB: amlodipine Telmisartan 80 mg per day for the previous 4 weeks, 120 mg per day for 5th to 8th week, 160 mg per day until the end of the study Amlodipine 5 mg per day for the previous 4 weeks, 7.5 mg per day for 5th to 8th week, 10 mg per day until the end of the study |
|
| Outcomes | BP measured in the seated position using a standard mercury sphygmomanometer (Korotkoff I and V) with a cuff of appropriate size. Measurements always taken in the morning before daily drug intake (i.e. 24 hours after dosing, at trough) and after the subject had rested for 10 min in a quiet room. 3 successive BP readings taken at 1‐min intervals and averaged | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Statement as "randomised, controlled, double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data flow was clearly stated, and missing data had little influence on results |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Gerritsen 1998.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: Bayer |
|
| Participants | Diagnosis: "The criteria for hypertension were a sitting DBP in the range 90–115 mmHg and a SBP < 200 mmHg, in patients who had not been administered blood pressure lowering drugs during the previous weeks." N = 80 Age: nitrendipine group 66.9 ± 6.2 years, enalapril group 58.8 ± 9.5 years Sex: 38.8% women, 61.2% men History: not reported Inclusion criteria: people with NIDDM and hypertension who were being treated by general practitioners in the Rotterdam area; DM diagnosed by general practitioner. Participants were being treated with diet or drugs (either an oral hypoglycemic or insulin); metabolic control had to be acceptable and was defined as an HbA1c level < 11.5% Exclusion criteria: class III or IV CHF, uncontrolled arrhythmias or severe or unstable angina pectoris; MI or stroke during the previous 3 months; history of other major illnesses or known intolerance to dihydropyridines or ACE inhibitors |
|
| Interventions | RAS inhibitor: enalapril; CCB: nitrendipine Nitrendipine 20 mg twice a day for previous 4 weeks, 40 mg twice a day until the end of the study Enalapril 20 mg once a day for previous 4 weeks, 40 mg once a day until the end of the study Acebutolol added when needed. |
|
| Outcomes | Changes in DBP, and SBP measured using an automated device (Dinamap, Arlington, Texas, USA) MI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Gottdiener 1998.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: the Cooperative Studies Program of the Department of Veterans Affairs Research and Development Service |
|
| Participants | Diagnosis: not reported N = 1105 Age: captopril group 57.4 ± 10 years, atenolol group 60.4 ± 9.4 years, diltiazem group 59.5 ± 9.2 years, prazosin group 60.1 ± 8.1 years, HCTZ group 58.1 ± 11.5 years, clonidine group 58.3 ± 9.8 years Sex: 100% men History: not reported Inclusion criteria: DBP 95 mmHg‐109 mmHg Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: captopril; CCB: diltiazem; thiazide: HCTZ; beta‐blocker: atenolol; alpha‐blocker: prazosin; CNS active drug: clonidine Atenolol 25 mg, 50 mg, 100 mg daily for 8‐week titration and 100mg daily for maintenance Captopril 12.5 mg, 25 mg, 50 mg twice daily for 8‐week titration and 50mg daily for maintenance Clonidine 0.1 mg, 0.2 mg, 0.3 mg twice daily for 8‐week titration and 0.3mg daily for maintenance Diltiazem‐SR 60 mg, 120 mg, 180 mg twice daily for 8‐week titration and 180mg daily for maintenance HCTZ 12.5 mg, 25 mg, 50 mg daily for 8‐week titration and 50mg daily for maintenance Prazosin 2 mg, 5 mg, 10 mg twice daily for 8‐week titration and 10mg daily for maintenance |
|
| Outcomes | BP was measured with a cuff sphygmomanometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… were randomly allocated to double‐blind treatment with 1 of 6 drugs." Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "… were randomly allocated to double‐blind treatment with 1 of 6 drugs." Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "… were randomly allocated to double‐blind treatment with 1 of 6 drugs." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants accounted for in analysis of each group in Table 1 in the original article was far fewer than those included in the study |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Hajjar 2013.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 12 months Funding: NIA and NIH |
|
| Participants | Diagnosis: hypertension i.e. SBP > 140 mmHg or DBP < 90 mmHg or receiving antihypertensive medications N = 47 Age: 60 years and above Sex: 57.4% women, 42.6% men History: Coronary artery disease: lisinopril group 35%; candesartan group 56%; HCTZ group 46% Hyperlipidemia: lisinopril group 35%; candesartan group 56%; HCTZ group 38% Inclusion criteria: 60 years or older, hypertension, executive dysfunction based on a score < 10 on the executive clock draw test (CLOX1) Exclusion criteria: individuals with possible dementia; intolerance to the study medications; SBP > 200 mmHg, DBP > 110 mmHg; serum creatinine > 2.0 mg/dL or serum potassium > 5.3 mEq/dL at baseline; receiving > 2 antihypertensive medications; presence of CHF, DM, or stroke; and inability to perform the study procedures or unwilling to stop currently used antihypertensive medications |
|
| Interventions | RAS inhibitors: lisinopril, candesartan; thiazide: HCTZ Lisinopril: 10 mg increased to 20 mg then 40 mg if needed Candesartan: 8 mg increased to 16 mg then 32 mg if needed HCTZ: 12.5 mg increased to 25 mg if needed Long‐acting nifedipine (30 mg increased to 60 mg and 90 mg) was added, followed by long‐acting metoprolol (12.5 mg increased to 25 mg and 50 mg) if needed. |
|
| Outcomes | BP: 2 seated blood pressure readings were performed and averaged at each visit | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a computer‐generated random allocation sequence occurred after baseline data collection |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The drugs were administered in a double‐blinded manner |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data flow was clearly stated |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Hauf‐Zachariou 1993.
| Methods | Allocation: randomized Blinding: double‐blinded. All drugs were given as capsules of identical appearance Duration: 26 weeks Funding: not reported |
|
| Participants | Diagnosis: not reported N = 220 Age: range:30‐77 years; mean age: carvedilol group 57 years, captopril group 58 years Sex: 40% women, 60% men History: not reported Inclusion criteria: essential hypertension with a DBP of 95 mmHg‐114 mmHg and dyslipidemia Exclusion criteria: secondary hypertension; unstable angina; gross hepatic or renal impairment; insulin‐dependent or unstable DM; or other major diseases |
|
| Interventions | RAS inhibitor: captopril; beta‐blocker: carvedilol Carvedilol 25 mg‐50 mg daily Captopril 25 mg‐50 mg daily |
|
| Outcomes | BP was measured with a calibrated mercury sphygmomanometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to fixed oral doses of …" Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was a multicenter, double‐blind, randomised (block size of 4), parallel group trial,…" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "233 were randomised to treatment (carvedilol 116, captopril 117)… 13 patents prematurely terminated the study after randomization, of whom 7 (carvedilol 1, captopril 6) were withdrawn because of protocol violation…The others who withdrew prematurely (carvedilol 5, captopril 1) were regarded as being eligible for the efficacy analysis until their last day in the trial." |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Hayoz 2012.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 42 weeks Funding: Novartis Pharmaceuticals |
|
| Participants | Diagnosis: moderate hypertension i.e. SBP ≥ 140 mmHg, DBP < 110 mmHg, and pulse pressure ≥ 50 mmHg N = 109 Age: 50‐75 years Sex: 100% women History: duration of hypertension ± SD; taking valsartan for 6.8 ± 7 years; taking amlodipine for 8.3 ± 6.4 years Inclusion criteria: postmenopausal women; moderate hypertension Exclusion criteria: BP above the safety limit of SBP ≥ 180 mmHg and ⁄ or DBP ≥ 110 mmHg before or at any point during the study; people with a history of type 1 or T2DM; Raynaud disease; AF or other arrhythmia; evidence of secondary form of hypertension; cerebrovascular accidents; transient ischemic cerebral attack or MI; CHF; clinically significant valvular heart disease; history of malignancy including leukemia and lymphoma; life‐threatening disease; known hypersensitivity or contraindications to valsartan, other ARBs, thiazide diuretics, amlodipine or other CCBs, and glycerin trinitrite |
|
| Interventions | RAS inhibitor: valsartan; CCB: amlodipine Valsartan 160 mg per day for previous 4 weeks, force‐titrated to 320 mg until the end of the study Amlodipine 5 mgper day for previous 4 weeks, force‐titrated to , 10 mg until the end of the study Open label HCTZ added if needed for week 12 onwards |
|
| Outcomes | BP measured using a standard sphygmomanometer with the appropriate cuff size in accordance with the American Heart Association Committee Report on BP determination. All BPs were measured 3 times at 1‐min intervals while the participant was sitting for a minimum of 5 min |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "randomised, controlled, double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants in each group in figure 1 and table 2 did not match |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Himmelmann 1996.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: not reported |
|
| Participants | Diagnosis: not reported N = 149 Age: cilazapril group 65 ± 6.9 years, atenolol group 67 ± 6.2 years Sex: 52.3% women, 47.7% men History: not reported Inclusion criteria: DBP 95 mmHg‐115 mmHg Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: cilazapril; beta‐blocker: atenolol Cilazapril 2.5 mg or 5 mg/day Atenolol 50 mg or 100 mg/day |
|
| Outcomes | BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a prospective, randomised, double blind trial …" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Hughes 2008.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: Pfizer International |
|
| Participants | Diagnosis: hypertension defined as a sitting BP not taking drugs for hypertension > 140/90 mmHg. N = 25 Age: 24‐71 years Sex: 32% women, 68% men History: duration of hypertension: lisinopril group: 11 ± 3.60 years; amlodipine group: 54 ± 1.84 years Inclusion criteria: untreated hypertension (previously untreated or antihypertensive treatment discontinued for at least 1 year) Exclusion criteria: accelerated hypertension; secondary hypertension; DM; familial hypercholesterolemia; HF or any other significant concomitant disease |
|
| Interventions | RAS inhibitor: lisinopril; CCB: amlodipine Amlodipine 5 mg‐10 mg daily Lisinopril 5 mg‐20 mg daily Open‐label add‐on medication, doxazosin and bendroflumethiazide |
|
| Outcomes | Clinical SBP and DBP were measured in the right arm of the individual seated using a validated semiautomated sphygmomanometer (Sentron, Bard Biochemical, Illinois, USA) HR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
IDNT 2001.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: Bristol‐Myers Squibb Institute for Medical Research and Sanofi‐Synthelabo. Additionally, Dr Berl has received research grants from Pfizer |
|
| Participants | Diagnosis: hypertension: SBP of > 135 mmHg while sitting, DBP of > 85 mmHg while sitting, or documented treatment with antihypertensive agents ESRF: as indicated by the initiation of dialysis, renal transplantation, or a serum creatinine concentration of at least 6.0 mg/dL (530 μmol/L) N = 1146 Age: irbesartan group 59.3 ± 7.1 years; amlodipine group 59.1 ± 7.9 years Sex: 35.7% women, 64.3% men History: CVD: irbesartan group 158 (27%); amlodipine group 171 (30%) Inclusion criteria: aged 30‐70 years, a documented diagnosis of T2DM, hypertension, and proteinuria, with urinary protein excretion of at least 900 mg/24 hours; serum creatinine concentration 1.0 mg/dL‐3.0 mg/dL (88 μmol/L and 265 μmol/L) in women and 1.2 mg/dL‐3.0 mg/dL (106 μmol/L and 265 μmol/L) in men Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: irbesartan; CCB: amlodipine Irbesartan 75 mg to 300 mg/day Amlodipine 2.5 mg to 10 mg/day Add‐ons, other antihypertensive agents except ACE inhibitors, ARBs, and CCB |
|
| Outcomes | ESRF, death from any cause | |
| Notes | Randomization was performed by central office. However, generation of randomization sequence was not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | High risk | Many authors had received research grants form Bristol‐Myers Squibb |
LIFE 2002.
| Methods | Allocation: used a computer‐generated allocation schedule Blinding: participants, clinicians, and assessment blinded. "LIFE is an investigator‐initiated, double‐masked, double‐dummy, randomised comparison", "An endpoint classification committee of two masked clinicians reviewed clinical records of all CV events reported by clinical centers to determine whether they met endpoint criteria." Duration: at least 4 years, mean 4.8 ± 0.9 years Funding: study data was in Merck database. Merck provided steering committee for this review free access to all data |
|
| Participants | Diagnosis: not reported N = 9193 Age: 55‐80 years Sex: 54.0%, 46.0% men History: Any vascular disease: losartan group1203 (26%); atenolol group1104 (24%); all participants 2307 (25%) Coronary heart disease: losartan group 771 (17%); atenolol group 698 (15%); all participants 1469 (16%) Cerebrovascular disease: losartan group 369 (8%); atenolol group 359 (8%); all participants 728 (8%) Peripheral vascular disease: losartan group 276 (6%); atenolol group 244 (5%); all participants 520 (6%) AF: losartan group 150 (3%); atenolol group 174 (4%); all participants 324 (4%) Isolated systolic hypertension: losartan group 660 (14%); atenolol group 666 (15%); all participants 1326 (14%) DM: losartan group 586 (13%); atenolol group 609 (13%); all participants 1195 (13%) Inclusion criteria: previously treated or untreated hypertension and ECG signs of LVH Trough sitting SBP 160 mmHg–200 mmHg, DBP 95 mmHg‐115 mmHg, or both Exclusion criteria: secondary hypertension; MI or stroke within the previous 6 months; angina pectoris requiring treatment with beta‐blockers or calcium‐antagonists; HF or LV ejection fraction of ≤ 40%; or a disorder that, in the treating physician's opinion, required treatment with losartan or another angiotensin–II type 1‐receptor antagonist, atenolol or another beta‐blocker, HCTZ, or ACE inhibitors |
|
| Interventions | RAS inhibitor: losartan; beta‐blocker: atenolol. Losartan: mean 82 ± 24 mg Atenolol: mean 79 ± 26 mg HCTZ added when needed |
|
| Outcomes | Change in SBP, change in sitting SBP, sitting DBP, HR. Primary endpoint: CV morbidity, death and a composite endpoint (CV death, MI, stroke) An independent endpoint classification committee reviewed all the events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked endpoint classification committee was responsible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the previous published paper were all reported |
| Other bias | High risk | Funded and conducted by Merck |
Malmqvist 2002.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 48 weeks Funding: not reported |
|
| Participants | Diagnosis: not reported N = 92 Age: irbesartan group 55 ± 9 years; atenolol group 54 ± 9 years Sex: 37.0% women, 63.0% men History: not reported Inclusion criteria: hypertensive people with ECG‐diagnosed LVH Exclusion criteria: known secondary hypertension; renal failure; LV dysfunction (ejection fraction 45%); coronary and valvular heart disease; stroke, and other serious concomitant diseases. No participant had a prior MI or AF |
|
| Interventions | RAS inhibitor: irbesartan; beta‐blocker: atenolol Irbesartan 150 mg or 300 mg daily Atenolol 50 mg or 100 mg daily Add‐ons HCTZ, felodipine |
|
| Outcomes | SBP and DBP at rest measured using a mercury sphygmomanometer after at least 10 min of rest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported. |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low. |
NESTOR 2015.
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 12 months Funding: An unrestricted grant from the Institut de Recherches Internationales Servier |
|
| Participants | Diagnosis: hypertensive Type 2 diabetic people with microalbuminuria N = 565 Age: Indapamide SR 60.7 ± 9.9 years, Enalapril 59.2 ± 10.0 years Sex: 37.5% women, 62.5% men History: Diabetes was required to be controlled by diet with or without 1 or more oral antidiabetic treatments, unchanged for at least 3 months Inclusion criteria: age 35 to 80 years, type 2 diabetes, essential hypertension (systolic BP 140 – 180 mmHg and diastolic BP < 110 mmHg), and persistent microalbuminuria (albumin excretion rate between 20 and 200 mg/min in 2 of 3 overnight urine samples collected during the placebo run–in period) Exclusion criteria: severe hypertension (systolic BP > 180 mmHg and/or diastolic BP > 110 mmHg), obesity (body mass index [BMI] > 40 kg/m2), hematuria or leucocyturia, and urinary tract infection |
|
| Interventions | RAS inhibitor: Enalapril; thiazide: Indapamide SR Indapamide SR 1.5 mg daily n = 282 Enalapril 10 mg daily n = 283 from week 6, additional open–label antihypertensive treatment could be added in a stepwise manner to achieve target BP levels, with all steps separated by a 6–week interval Step 1: amlodipine 5 mg once daily Step 2: amlodipine 10 mg once daily Step 3: amlodipine 10 mg plus atenolol 50 mg once daily Step 4: amlodipine 10 mg plus atenolol 100 mg once daily |
|
| Outcomes | 1. BMI: calculated as body weight (in kg) divided by body height (in meters) squared 2. Systolic and diastolic BP levels: with a mercury sphygmomanometer, in the morning before drug intake, after at least a 10–minute rest, in the supine position. 3 consecutive BP measurements were taken at 3–minute intervals, and averaged at W6, W12, W18, W24, W36, and W52 3. Medical history: reviewing the participants’ medical records 4. Fasting plasma sodium, potassium, creatinine, glucose, triglycerides, total cholesterol, high–density lipoprotein (HDL) cholesterol, low–density lipoprotein (LDL) cholesterol: using standard methods, in the central laboratory of each center, before randomization and at the end of the study 5. Creatinine clearance rate: calculated using the Modification of Diet in Renal Disease formula |
|
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Although the prespecified outcomes were not available in the Methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Ostman 1998.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 6 months Funding: supported by the Parke Davis Company |
|
| Participants | Diagnosis: hypertension i.e. supine BP 95 mmHg‐109 mmHg (Korotkoff phase 5) N = 60 Age: 35‐75 years old Sex: 38.3% women, 61.7% men History: Quinapril group median duration of DM 4.6 years; median duration of treated hypertension 11.7 years Metoprolol group median duration of DM 3.7 years; median duration of treated hypertension 8.8 years Inclusion criteria: NIDDM in stable blood glucose control, essential hypertension Exclusion criteria: CHF; MI; angina pectoris treated with drugs other than nitrates; hemodynamically serious valvular heart disease and secondary or malignant hypertension; treatment with thiazides or lipid‐lowering agents, or both, in the preceding 12 months; treatments with loop‐diuretics in the preceding 3 months; chronic therapy with non‐steroidal anti‐inflammatories. Serum levels of AST or ALT > 2 μkat/L(umol/(s*L); hyperlipoproteinemia; cholesterol > 8 mM; triglycerides > 4 mM; proteinuria (> 0.5 g/L) |
|
| Interventions | RAS inhibitor: quinapril; beta‐blocker: metoprolol Quinapril 20 mg daily Metoprolol 100 mg daily Felodipine added when needed |
|
| Outcomes | Supine BP measured by a sphygmomanometer | |
| Notes | "The doses were chosen to give equipotency in the antihypertensive effect." No differences between the reductions in standing SBP and DBP were found, however, standing SBP and DBP were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Although the pre‐specified outcomes were not available in the methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Parrinello 2009.
| Methods | Allocation: double‐blinded randomization using a computer algorithm designed prior to commencement of the study Blinding: each participant was identified with an allocation number that was associated with treatment groups according to a computer‐generated allocation schedule; physicians were blinded to the treatment‐associated allocation number Duration: 12 months Funding: no funding sources reported |
|
| Participants | Diagnosis: not reported N = 72 Age: range: 29‐63 years; mean age ± SD: 52 ± 12 years Sex: 44.4% women, 55.6% men History: not reported Inclusion criteria: a diagnosis of essential hypertension (ESH stage 1 or 2 hypertension) established by history and physical examination, together with the absence of clinical findings suggestive of a secondary hypertension, according to ESH guidelines Exclusion criteria: other CV diseases (defined as MI or angina pectoris, heart block, valvular disease, HF and claudication); concomitant LVH (defined according to ECG criteria); other target organ damage (including hypertensive retinopathy); micro‐ or macroalbuminuria or renal diseases; insulin‐dependent or NIDDM; electrolyte imbalances; alcoholism or psychiatric problems, or both; taking antihypertensive drugs; or contraindications to beta‐blockers |
|
| Interventions | RAS inhibitor: losartan; beta‐blocker: bisoprolol Bisoprolol 5 mg daily Losartan 50 mg daily HCTZ was added when needed |
|
| Outcomes | SBP and DBP measured in triplicate with a mercury sphygmomanometer after 5 min in a supine position. The Korotkoff phase V sound was used to determine DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double‐blind randomization performed using a computer algorithm designed prior to commencement of the study |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each participant was identified with an allocation number that was associated with treatment groups according to a computer‐generated allocation schedule; physicians were blinded to the treatment‐associated allocation number |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Pedersen 1997.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 24 months Funding: Danish Medical Research Council and Astra Cardiovascular, Denmark |
|
| Participants | Diagnosis: not reported N = 14 Age: 25‐63 years Sex: 28.6% women, 71.4% men History: not reported Inclusion criteria: men and women aged 18‐65 years; chronic glomerulonephritis verified by renal biopsy; creatinine clearance 15 ml/min‐130 ml/min; and arterial hypertension with a DBP between 90 mmHg‐110 mmHg, calculated as the mean value of measurement on 3 different days after discontinuation of antihypertensive treatment for 2 weeks. Exclusion criteria: nephrotic syndrome; extracapillary glomerulonephritis; systemic disease with glomerulonephritis; liver disease; DM; HF; pregnancy; and unwillingness to participate Withdrawal criteria during the study were: development of exclusion criteria; progression to end‐stage renal disease; DBP > 110 mmHg at 3 consecutive visits in the outpatient clinic; and side effects |
|
| Interventions | RAS inhibitor: ramipril; CCB: felodipine Three dose levels, low dose (LD), medium dose (MD), and high dose (HD) were used in each group. The dose of medicine was gradually increased(LD to MD to HD) in order to obtain a diastolic blood pressure of 90 mmHg or less: Ramipril 1.25 mg (LD), 2.5 mg (MD), 5.0 mg (HD) daily Felodipine 5 mg (LD), 10 mg (MD), 20 mg (HD) daily |
|
| Outcomes | Blood pressures were means of 3 determinations measured after 1 hour's rest in the supine position with an interval of a few min between the determinations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was prospective, double‐blind and placebo‐controlled." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 of 33 included subjects withdrew before the end of the study. The proportion of the participants dropping out of the trial was too much. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | High risk | Table 1 and Table 2 differ in the baseline BP data |
Petersen 2001.
| Methods | Allocation: randomized: "The study was a randomised and double blind comparison" Blinding: double‐blinded Duration: 6 months before randomization, 21 months after randomization or until need of dialysis Funding: isradipine and spirapril were supplied by Novartis and the study was supported by Novartis. Statistical assistance was supported by a grant from the Danish Medical Research Council |
|
| Participants | Diagnosis: chronic renal failure (serum creatinine between 150 μmol/L‐600 μmol/L) and hypertension (BP > 140/95 mmHg) N = 36 Age: 18‐75 years Sex: 36% women, 64% men History: previously treated and untreated people with hypertension Inclusion criteria: chronic, inactive renal disease and serum creatinine between 150 μmol/L‐600 μmol/L, (DBP > 95 mmHg, or SBP > 140 mmHg without treatment) Exclusion criteria: renal artery stenosis or severe CHF |
|
| Interventions | RAS inhibitor: spirapril; CCB: isradipine Isradipine 5 mg daily Spirapril 6 mg daily Loop diuretics and labetolol were accepted add‐ons when target BP was not sufficient |
|
| Outcomes | ESRF SBP, mercury sphygmomanometer and Korotkoff Phase 1, sitting DBP, mercury sphygmomanometer and Korotkoff Phase 5, sitting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The data flow was not mentioned |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Roman 1998.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 6 months Funding: supported in part by a grant from Hoescht Marion Roussel, Inc |
|
| Participants | Diagnosis: not reported N = 50 Age: ramipril group 52.7 ± 6.9 years; HCTZ group 50.1 ± 7.7 years Sex: 27% women; 73% men History: not reported Inclusion criteria: seated DBP of 95 mmHg‐114 mmHg Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: Ramipril; Thiazide: HCTZ Ramipril 5 mg, 10 mg, 20 mg daily HCTZ 12.5 mg, 25 mg, 50mg daily |
|
| Outcomes | BP | |
| Notes | SD were not reported in the original article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of sequence generation was not described. Some baseline characteristics (gender, height, body surface area, sleep blood pressure) differed between two active treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Fifty essential hypertensives participated in a double‐blind study for 6 months and were randomised to either ramipril or hydrochlorothiazide (HCTZ)." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Probably low, as other unrelated outcomes use blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | High risk | Some baseline characteristics (gender, height, body surface area, sleep blood pressure) differ between two active treatment groups |
Schiffrin 1994.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: Hoffmann‐LaRoche Canada, Medical Research Council of Canada to the Multidisciplinary Research Group on Hypertension |
|
| Participants | Diagnosis: hypertension, i.e. on more than 2 occasions recumbent SBP > 140 mmHg or DBP > 90 mmHg. The diagnosis of essential hypertension was established by absence of clinical evidence of secondary hypertension N = 17 Age: Cilazapril group 39.1 ± 2.3 years; Atenolol group 42.4 ± 1.6 years Sex: 100% men History: not reported Inclusion criteria: hypertensive men who were untreated or had not received antihypertensive medication for at least 6 months; 25‐50 years old Exclusion criteria: people who smoked > 5 cigarettes/day; abnormal fasting blood glucose level; serum creatinine concentration > 150 μmol/L; or any other systemic disease |
|
| Interventions | RAS inhibitor: cilazapril; beta‐blocker: atenolol Atenolol identical 50 mg and 100 mg tablets Cilazapril 2.5 mg and 5 mg tablets Long‐acting nifedipine was added if needed |
|
| Outcomes | SBP and DBP measured by standard mercury sphygmomanometer in sitting position after 15 min rest | |
| Notes | Men only included as participants due to the potential teratogenicity of nifedipine, which would be used if goal BP was not achieved with the drugs being studied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECG was read by a cardiologist unaware of the protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Schmieder 2009.
| Methods | Allocation: randomized, "Randomization by center was performed by the interactive voice response system provider with the use of a validated system that automates the random assignment of patients to randomization numbers. Randomization data were kept strictly confidential until the time of unblinding." Blinding: double‐blinded Duration: 1 year Funding: Novartis Pharmaceuticals Corporation, East Hanover, NJ |
|
| Participants | Diagnosis: hypertension, mean sitting DBP > 90 mmHg and < 110 mmHg at the single‐blind placebo run‐in visit N = 962 Age: Aliskiren group 56.1 ± 10.9 years; HCTZ group 55.7 ± 10.9 years Sex: 36% women; 64% men History: mean duration of hypertension was 7.1 years. 35.2% of participants were classified as obese (body mass index 30 kg/m2), and 10.9% had DM (according to medical history) Inclusion criteria: outpatients aged 18 years or over with essential hypertension Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: aliskiren; thiazide: HCTZ Aliskiren 150 mg 300 mg daily HCTZ 12.5 mg 25 mg daily Amlodipine was added when needed |
|
| Outcomes | Mean sitting DBP and SBP were measured by a mercury sphygmomanometer. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization by center was performed by the interactive voice response system provider with the use of a validated system that automates the random assignment of patients to randomization numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization data were kept strictly confidential until the time of unblinding." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Schneider 2004.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 18 months Funding: sponsored in part by Bristol‐Myers Squibb and Sanofi Synthelabo, Germany |
|
| Participants | Diagnosis: not reported N = 237 Age: irbesartan group 54.2 ± 8.0 years; atenolol group 55.5 ± 7.9 years Sex: 45% women; 55% men History: duration of hypertension: irbesartan group 5.3 ± 6.0 years; atenolol group 5.2 ± 6.7 years Inclusion criteria: men and women aged between 25‐65 years;SBP of 150 mmHg‐200 mmHg or a DBP of 95 mmHg‐115 mmHg and mild target organ damage defined as intima media thickness of the common carotid artery on the leading side ≥ 0.8 mm and ≤1.5 mm Exclusion criteria: known or suspected secondary hypertension; coronary heart disease; cerebrovascular disease; peripheral vascular disease; renovascular disease; insulin‐dependent DM; uncontrolled non‐insulin‐dependent DM; history of intolerance to atenolol, irbesartan, other angiotensin receptor blockers, HCTZ, or amlodipine; and pretreatment with an ACE inhibitor or an angiotensin receptor blocker within the last 6 months |
|
| Interventions | RAS inhibitor: irbesartan; beta‐blocker: atenolol Irbesartan 150 mg/day or 300 mg/day Atenolol 50 mg/day or 100 mg/day Add‐on HCTZ, amlodipine |
|
| Outcomes | BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | ECG measurement and assessment was blinded, but BP measurement was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Schram 2005.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 12 months Funding: "AstraZeneca provided funding for this clinical trial (to CDAS), but had no influence on the data analyses or manuscript preparation." |
|
| Participants | Diagnosis: not reported N = 60 Age: Lisinopril group 62 ± 8 years; Candesartan group 60 ± 7 years; HCTZ group 63 ± 6 years Sex: 45% women; 55% men History: not reported Inclusion criteria: for the run‐in period were: T2DM ≥ 6 months (WHO criteria 1985); age 35‐70 years; "Caucasian ethnicity"; and urinary albumin excretion < 100 mg/24 hours. Patients with a sitting BP > 140/90 mmHg and < 190/120 mmHg after the run‐in period had an ECG. Participants were included if LVMI 490 g/m² in men or 470 g/m² in women Exclusion criteria: pregnancy or planning a pregnancy; a history of MI, angina pectoris, coronary artery bypass surgery, angioplasty, stroke, CHF, malignancy or other serious illnesses; serum creatinine > 140 mmol/L; body mass index 435 kg/m²; alcohol or drug abuse, or both; or participation in other clinical trials |
|
| Interventions | RAS inhibitor: lisinopril, candesartan; thiazide: HCTZ HCTZ 12.5 mg daily Candesartan 8 mg daily Lisinopril 10 mg daily Add‐on: consecutively, 12.5 mg HCTZ, doubling study medication; 5 mg felodipine, 50 mg metoprolol, 2 mg doxazosin, 5mg felodipine; 50 mg metoprolol, 2 mg doxazosin, 5 mg felodipine, 100 mg metoprolol, and 4 mg doxazosin |
|
| Outcomes | BP after 5 min of seated rest (mean of 3 consecutive measurements) | |
| Notes | Participants were limited to people of "Caucasian ethnicity". The reason was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Although the role of company was unlikely to have an impact on the study, no other information was found to evaluate the risk as either high or low |
Seedat 1998.
| Methods | Allocation: alternative allocation Blinding: double‐blinded Duration: 1 year Funding: this study was supported by Institut de Recherches Internationales, (IRIS) France |
|
| Participants | Diagnosis: DBP 95 mmHg‐115 mmHg. N = 100 Age: Perindopril group 54.3 ± 7.3 years; Atenolol group 56.5 ± 6.9 years Sex: 88% women; 12% men History: duration of hypertension was 8.2 ± 6.2 years and 99% of participants were on previous treatment.Duration of diabetes was 6.6 ± 5.4 years. Proteinuria was present in 40% of participants. Fundal changes consisting of hypertensive and diabetic retinopathy were present in 60% of participants Inclusion criteria: T2DM with hypertension and DBP 95 mmHg‐115 mmHg Exclusion criteria: albuminuria < 200 mg/min (300 mg in 24 hours) or macroalbuminuria > 3.5 g/24 hours; severe complications of hypertension such as stroke, HF, renal failure; severe diabetic retinopathy (neovascularization, vitreous hemorrhages or retinal detachment); contraindications to beta‐blockers or ACE inhibitors; people with poor metabolic control; and women with childbearing potential |
|
| Interventions | RAS inhibitor: perindopril; beta‐blocker: atenolol Perindopril 4 mg, 8 mg daily Atenolol 50 mg, 100 mg daily HCTZ, nifedipine were added when needed |
|
| Outcomes | Pulse rate and sitting and standing BP evaluated within 12 hours post administration at each review visit. BP determined by taking a mean of 3 readings with the Dinamap (Criticon, Johnson and Johnson) apparatus with the participant seated after 5 min rest | |
| Notes | There were no participants of European origin as the hospital serves only black and Indian people. Black people were excluded because they do not respond well to ACE inhibitors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | High risk | Alternative allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
SILVHIA 2001.
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 48 weeks Funding: Karolinska Institutet, Stockholm, Sweden, the Swedish Heart‐Lung Foundation,Stiftelsen Serafimerlasarettet, Stockholm, Sweden, Bristol‐Myers Squibb Pharmaceutical Research Institute, Princeton, NJ, USA, and Sanofi‐Synthelabo, Paris, France |
|
| Participants | Diagnosis: Mild‐to‐moderate hypertension and left ventricular hypertrophy N = 101 Age: Irbesartan group 54 ± 8 years; Atenolol group 54 ± 10 years Sex: 32% women; 68% men History: Mild‐to‐moderate hypertension and LV hypertrophy Inclusion criteria: Women with mild‐to‐moderate hypertension and LV hypertrophy. All antihypertensive agents were withdrawn appropriately before the start of a 4‐ to 6‐week single‐blind placebo lead‐in period. At the end of the placebo period, participants were determined eligible for the double‐blind part of the study if the mean of 3 seated diastolic blood pressures (SeDBP) taken 1 min apart was 90 ‐ 115 mmHg on 2 consecutive visits, with values differing no more than 8 mmHg Exclusion criteria: If patients had an LV ejection fraction < 45%, any significant concomitant diseases, or were taking any other medications that might interfere with the efficacy assessments or that would present safety hazards |
|
| Interventions | RAS inhibitor: Irbesartan; beta‐blocker: atenolol Irbesartan 150 mg/d Atenolol 50 mg/d If SeDBP was > 90 mmHg after 6 weeks,irbesartan 300 mg/d or atenolol 100 mg/d If SeDBP remained > 90 mmHg at week 12, open‐label hydrochlorothiazide (HCTZ) 12.5 mg/d (titrated to 25 mg if necessary) At week 24, open‐label felodipine 5 ‐ 10 mg/d if required At the end of the study, 40% of participants in the irbesartan group and 49% in the atenolol group remained on the monotherapy. |
|
| Outcomes | 1. Blood pressure: At all clinic visits, trough (24 ± 3 h after the last dose) SeSBP and SeDBP were measured using a mercury sphygmomanometer. After resting for at least 10 mins in the seated position, blood pressure was determined as the average of 3 replicate measurements taken 1 min apart 2. Heart rate: Heart rate was then recorded in the seated position 3. Total peripheral resistance: Mean arterial pressure was calculated as SeDBP + (SeSBP ‐ SeDBP)/3. Total peripheral was calculated by dividing mean arterial pressure by cardiac output (i.e. stroke volume 3 heart rate), and expressed as peripheral resistance units (PRU) 4. Echocardiography (LVMI, left ventricular mass index; IVS, intraventricular septum; PWT, posterior wall thickness; LVEDD, left ventricular end‐diastolic diameter; RWT, relative wall thickness; EF, ejection fraction.): Echocardiography was performed with the woman in the left semilateral position. The ultrasound devices used were the Acuson 128 X P/10 (Mountain View, California, USA), Vingmed CFM 750 (Vingmed Sound,Horten, Norway) and HP SONOS 2500 (Andover,Massachusetts, USA). Measurements were performed on 3 ‐ 5 consecutive beats, from which the mean values were calculated. Basic measurements of LV dimensions in diastole (LVEDD) and systole (LVESD), and intra‐ventricular septum (IVS) thickness and posterior wall thickness in diastole (PWT) were made by M‐mode technique The ejection fraction was measured according to the recommendations of the American Society of Echocardiography |
|
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | High risk | Number of participants reported in different outcomes are not consistent |
Sørensen 1998.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: this study was supported by a grant from Bayer AG; lisinopril tablets were supplied by Zeneca |
|
| Participants | Diagnosis: not reported N = 48; nisoldipine group 25, lisinopril group 23 Age: nisoldipine group 41 ± 9 years; lisinopril group 34 ± 7 years Sex: 33% women; 67% men History: duration of DM, nisoldipine group 25 ± 6 years; lisinopril group 24 ± 6 years (means ± SD) Inclusion criteria: type I DM with hypertension and diabetic nephropathy Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: lisinopril; CCB: nisoldipine Nisoldipine coat core 20 mg‐40 mg daily Lisinopril 10 mg‐20 mg daily |
|
| Outcomes | BP measured with a standard clinical sphygmomanometer in the upper arm at heart level. DBP obtained as Korotkoff Phase 5 | |
| Notes | Coat core: a dosage form of Nisoldipine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We performed a 1‐year double‐blind, double‐dummy randomised controlled study …" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Tarnow 1999.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: supported by Bayer AG; lisinipril was supplied by Zeneca |
|
| Participants | Diagnosis: hypertension: DBP > 90 mmHg or ongoing treatment with antihypertensive medication N = 40 Age: Nisoldipine group 40 ± 9 years; Lisinopril group 34 ± 7 years Sex: 35% women; 65% men History: duration of DM: nisoldipine group 40 ± 9 years; lisinopril group 34 ± 7 years Inclusion criteria: hypertensive people between the ages of 18‐55 years with a GFR > 40 ml/min·1.73m², and had developed diabetes before the age of 41 years Exclusion criteria: not reported |
|
| Interventions | RAS inhibitor: lisinopril; CCB: nisoldipine Nisoldipine coat core 20 mg or 40 mg daily Lisinopril 10 mg or 20 mg daily Add‐on, diuretic (mainly furosemide). 1 participant in the lisinopril group was prescribed a cardioselective beta‐blocker after 6 months |
|
| Outcomes | BP was measured after 15 min rest in the supine position | |
| Notes | All patients were white, had been insulin‐dependent from the time of diagnosis, and received at least 2 daily injections of human insulin In 14 participants (6 in the nisoldipine group), diuretic treatment was continued because of edema. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 of 50 withdrew before the end of the study |
| Selective reporting (reporting bias) | Low risk | Although the pre‐specified outcomes were not available in the methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Tedesco 1999.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 26 months Funding: not reported |
|
| Participants | Diagnosis: not reported N = 69 Age: 30‐73 years Sex: 48% women; 52% men History: not reported Inclusion criteria: DBP between 90 mmHg‐114 mmHg Exclusion criteria: recent MI or stroke; renal diseases; and CHF |
|
| Interventions | RAS inhibitor: losartan; thiazide: HCTZ Losartan 50 mg daily HCTZ 25 mg daily |
|
| Outcomes | Supine BP measurements using a mercury sphygmomanometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "After 2 weeks, in a double‐blind study, the subjects were randomly allocated to either treatment with…" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Questions in the Quality of life questionnaire were posed by a trained investigator blinded to clinical and active treatment The way in which BP assessments were made was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Terpstra 2004.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: Pfizer, Netherlands |
|
| Participants | Diagnosis: not reported N = 149 Age: amlodipine group 67 ± 4 years; lisinopril group 67 ± 4 years. Sex: 50% women, 50% men History: not reported Inclusion criteria: people with previously untreated mild to moderate hypertension Exclusion criteria: office BP > 220/115 mmHg; unstable BP after placebo treatment period, defined as the differences in DBP or SBP before placebo treatment of 10 mmHg or 20 mmHg, respectively; secondary hypertension of any etiology; angina pectoris; manifest coronary artery disease; current or recent history of CHF; hemodynamically significant valvular heart disease; cardiac arrhythmias; renal insufficiency; and insulin‐dependent DM |
|
| Interventions | RAS inhibitor: lisinopril; CCB: amlodipine Amlodipine 5 mg, 10 mg Lisinopril 10 mg, 20 mg |
|
| Outcomes | BP: Korotkoff phase 1 and 5, sitting position HR |
|
| Notes | Participant numbers at 1 year and 2 year were not reported for BP; instead, "end of trial" was used in the tables of BP results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "we performed a double‐blind, randomised study in a Dutch rural population, …" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "ECG examinations were performed by the same observer, who was unaware of the identity of patients or BP measurements at baseline and after 1 and 2 years of active treatment." Statistical analysis was performed by an independent agency |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | High risk | HR was listed in the "Methods", but no detailed data were reported in "Results", though statements like "heart rate did not significantly change during treatment, …" were evident Participant numbers in Table 2 do not match those stated in the article |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
TOHMS 1993.
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 4.4 years Funding: this study was supported by grant NIH‐R01‐HL34767 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md; and Pfizer Inc, New York, NY, and Merck Sharp & Dohme Research Laboratories, Rahway, NJ |
|
| Participants | Diagnosis: not reported N = 597 Age: 45‐69 years Sex: 62% women, 38% men History: not reported Inclusion criteria: men and women aged 45–69 years, DBP 90 mmHg‐99 mmHg at both of the first 2 eligibility visits and averaged 90 mmHg‐99 mmHg over the 3 eligibility visits Exclusion criteria: use of > 1 type of antihypertensive drug; inability to obtain a technically satisfactory baseline ECG; angina; at least 50% of meals eaten away from home; unwillingness or inability to make nutritional changes; CV disease; life threatening illness; LVH |
|
| Interventions | RAS inhibitor: enalapril; CCB: amlodipine; thiazide: chlorthalidone; beta‐blocker: acebutolol; alpha‐blocker: doxazosin Nutritional‐hygienic intervention plus one of the following 6 treatments:
|
|
| Outcomes | BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
VALUE 2004.
| Methods | Allocation: randomized. Computer‐generated random sequence centrally prepared by sponsor Blinding: double‐blinded (patients and clinicians) Duration: 4 ‐ 6 years, 4.2 ± 1.2 years (means ± SD) Funding: Novartis for design (interactively), data management, data analysis |
|
| Participants | Diagnosis: hypertension defined as a mean sitting SBP between 160 mmHg ‐ 210 mmHg (inclusive), and a mean sitting DBP of < 115 mmHg N = 15,245: valsartan group 7649; amlodipine group 7596 Age: 50 years or older Sex: 42% women, 58% men Antihypertensive medication taken at time of randomization: Previously treated for hypertension: valsartan group 7088 (92.7%); amlodipine group 6989 (92.0%) ACE inhibitor: valsartan group 3148 (41.3%); amlodipine group 3135 (41.4%) Angiotensin‐receptor blocker: valsartan group 812 (10.7%); amlodipine group 800 (10.6%) Alpha‐blockers: valsartan group 540 (7.1%); amlodipine group 495 (6.5%) Beta‐blockers: valsartan group 2496 (32.7%); amlodipine group 2551 (33.7%) Calcium‐channel antagonist: valsartan group 3181 (41.7%); amlodipine group 3048 (40.2%) Diuretics as monotherapy: valsartan group 2047 (26.9%); amlodipine group 2020 (26.7%) Fixed‐dose diuretic combinations: valsartan group 686 (9.0%); amlodipine group 634 (8.4%) Qualifying disease factors: Coronary heart disease: valsartan group 3490 (45.6%); amlodipine group 3491 (46.0%) Peripheral arterial disease: valsartan group 1052 (13.8%); amlodipine group 1062 (14.0%) Stroke or TIA: valsartan group 1513 (19.8%); amlodipine group 1501 (19.8%) LVH with strain pattern: valsartan group 454 (5.9%); amlodipine group 462 (6.1%) Inclusion criteria: men or women of any racial background, 50 years of age and older, with CV risk factors or disease according to an algorithm based on age and sex Exclusion criteria: renal artery stenosis; pregnancy; acute MI; percutaneous trans luminal coronary angioplasty or coronary artery bypass graft within the past 3 months; clinically relevant valvular disease; cerebrovascular accident in the past 3 months; severe hepatic disease; severe chronic renal failure; CHF requiring ACE inhibitor therapy; monotherapy with beta‐blockers for both coronary artery disease and hypertension |
|
| Interventions | RAS inhibitor: valsartan; CCB: amlodipine Valsartan 80 mg, median 151.7 mg, range 83.2 mg‐158.5 mg Amlodipine 5 mg, median 8.5 mg, range 5.0 mg‐9.9 mg |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random sequence centrally prepared by sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quoted: "A statistician on the executive committee independently analyzed data to validate and further explore the analyses done by statisticians employed by the sponsor." And "An endpoint committee, blinded to therapy allocation, reviewed the clinical records of all CV events reported by clinical centers and adjudicated according to the protocol criteria." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | High risk | Quoted: "The proportion of patients receiving valsartan monotherapy as the last recorded study medication was significantly smaller than that of patients receiving amlodipine monotherapy, and a larger proportion of patients in the valsartan group received the highest dose of study drug plus hydrochlorothiazide or plus other antihypertensive drugs than in the amlodipine group." Reviewer comment: the proportion of monotherapy and highest dose (include HCTZ and other non‐study add‐on drugs) was not balanced between the 2 groups |
Xiao 2016.
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 36 months Funding: Clinical project of Third Military Medical University (2010XLC04) and 3 grants from the Natural Science Foundation of China (Nos 81172773, 81202286 and 81473068) |
|
| Participants | Diagnosis: essential hypertension combined with i‐IFG N = 227 Age: mean 45.02 in Losartan potassium group, 46.59 in Levamlodipine besylate Sex: 46% women, 54% men History: patients with EH combined with i‐IFG Inclusion criteria: (1) age between 18 and 70 years (2) i‐IFG criteria: participants received at least 2 fasting glucose (FG) examinations on different days in the Clinical Laboratory of the Southwest Hospital, and the results showed 5.6 mmol l‐1 < FPG < 7 mmol l‐1 and postprandial 2‐hr plasma glucose (2hPG) < 7.8 mmol l‐1 (3) hypertension criteria: the BP was the average of 3 measurements of BP in the right arm after sitting still for 5 minutes using a cuff sphygmomanometer, which conformed to the standards formulated in the 2010 Chinese guidelines for the management of hypertension: systolic BP (SBP) ≥140 mmHg and diastolic BP (DBP) ≥ 90 mmHg (4) participants had not used antihypertensive drugs within the previous 2 weeks (5) participants who would like to come back for follow‐up in the next 3 years Exclusion criteria: (1) women who were incapable or unwilling to provide written informed consent (2) evidence of liver disease (alanine aminotransferase or aspartate aminotransferase greater than twice the normal upper limit) or kidney disease (serum creatinine > 95 μmol l‐1) (3) secondary hypertension, urinary tract infection, renal artery stenosis, hyperkalemia, pregnancy, lactation, recent cerebral hemorrhage or cerebral infarction, or severe heart failure (4) use of hypoglycemic medication or insulin in the previous 5 years (5) allergy to the drugs in this study (6) participants who refused to come back to the hospital for follow‐up |
|
| Interventions | RAS inhibitor: losartan; CCB: levamlodipine Losartan potassium at 50 or 100 mg Levamlodipine besylate at 2.5 or 5 mg |
|
| Outcomes | SBP, DBP: monitored in the long term and re‐examined every 12 months in the Southwest Hospital Fasting insulin (FINS): tested in the Department of Nuclear Medicine in Southwest Hospital by radioimmunoassay Insulin sensitivity index (ISI): ISI = In (1/(FPG× FINS)) FPG: tested in Clinical Laboratory in Southwest Hospital by the glucose oxidase method 2‐hr insulin (2hINS): tested in the Department of Nuclear Medicine in Southwest Hospital by radioimmunoassay 2Hpg: tested in Clinical Laboratory in Southwest Hospital by the glucose oxidase method Glycohemoglobin (HbA1C): tested in the Clinical Laboratory in Southwest Hospital by enzymatic methods Body mass index (BMI) : BMI ≥ 24 kg m2 was overweight and BMI ≥ 28 kg m2 was obesity Total cholesterol, total triglycerides (TGs), low‐density lipoprotein cholesterol(LDL‐C), high‐density lipoprotein cholesterol (HDL‐C): tested in the Clinical Laboratory in Southwest Hospital by enzymatic methods Dyslipidemia: diagnosed according to the dyslipidemia indicators in the diagnostic standards of metabolic syndrome proposed by the International Diabetes Federation in 2005: TG ≥ 1.7 mmol l1 and HDL‐C < 1.29 mmol l−1 (females) |
|
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the Methods were all reported |
| Other bias | Unclear risk | Insufficient information found to evaluate the risk as either high or low |
Zeltner 2008.
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 3 years Funding: Astra‐Zeneca provided the study medication |
|
| Participants | Diagnosis: autosomal dominant polycystic kidney disease (ADPKD) defined by ultrasonographic criteria as described by Ravine et al (Ravine 1994) and a positive family history Hypertension: casual BP 140/90 mmHg or presence of an antihypertensive medication, or both N = 37; ramipril group 17; metoprolol group 20 Age: 18 ‐ 65 years Sex: 54% women, 46% men (only per‐protocol subjects available) Inclusion criteria: confirmed diagnosis of ADPKD; aged 18–65 years; evidence for hypertension; serum creatinine ≤ 4.0 mg/dL. Exclusion criteria: serum creatinine > 4.0 mg/dL; MI or cerebrovascular accident in the past 12 months; known intolerance to study medication; pregnancy or women not using contraception; evidence for severe hepatic disease; use of immunosuppressants or non‐steroidal anti‐inflammatory drugs; CHF; alcohol abuse or consumption of narcotics; the presence of a malignant disease or non‐compliance of the participants |
|
| Interventions | RAS inhibitor: ramipril; beta‐blocker: metoprolol Ramipril 2.5 mg or 5 mg daily Metoprolol 50 mg or 100 mg daily Add‐on medication, open‐label, felodipine, doxazosin, furosemide |
|
| Outcomes | Casual SBP and DBP measured with a standard mercury sphygmomanometer at each clinical visit. BP readings were taken with the participant seated after 5 min of rest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Abbreviations
ACE: angiotensin converting enzyme ADPKD: autosomal dominant polycystic kidney disease AER: albumin excretion rate AF: atrial fibrillation ALT: alanine transaminase ARB: angiotensin II receptor agonist AST: aspartate transaminase bpm: beats per minute BP: blood pressure CCB: calcium channel blocker CHD: coronary heart disease CHF: congestive heart failure CV: cardiovascular CVD: cardiovascular disease(s) DBP: diastolic blood pressure DM: diabetes mellitus ECG: electrocardiograph ESH: European Society of Hypertension ESRF: end stage renal failure GFR: glomerular filtration rate HbA1c: glycosylated hemoglobin HCTZ: hydrochlorothiazide HDL: high‐density lipoprotein HF: heart failure HR: heart rate LV: left ventricular LVH: left ventricular hypertrophy LVMI: left ventricular mass index MI: myocardial infarction min: minute(s) NIA: national institutes of aging NIDDM: non‐insulin‐dependent diabetes mellitus NIH: national institute on health SBP: systolic blood pressure SD: standard deviation SEM: standard error of mean SR: slow release TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AASK 2002 | Mortality and morbidity data were not reported in a form that could be extracted and entered |
| ANBP2 2003 | Not double‐blinded trial; study used PROBE (prospective, randomized, open‐label design, with blinded assessments of end points) design |
| Materson 1993 | The outcome of this study was BP control rate, so we could not extract relevant data |
| Okin 2012 | Outcomes were grouped and analyzed according to blood potassium. We have no available data to extract associated with different medications |
| Peng 2015 | This study included participants with high risk of hypertension but excluded people diagnosed as hypertensive |
| Preston 1998 | The study focused on choice of an initial antihypertensive agent by using renin profile methods versus age‐race methods. There were no available data for this review |
Differences between protocol and review
In the protocol, we identified non‐fatal serious adverse events (SAEs) as a primary outcome. However, when we extracted the data from included studies, none of them reported total SAEs in a manner that we could use in the review.
In the process of data extraction, we found that quite a few trials reported heart failure (HF) as a primary outcome, which was not specified in the protocol. Since HF is an important clinical endpoint, we added it to the primary outcomes in the review.
In this review, we replaced cardiovascular (CV) mortality with total CV events, to best reflect the overall effect, and because the cause of death was often not easy to identify due to few autopsies being performed.
We changed the author list to reflect the actual contributions of each author to this updated review.
Contributions of authors
James Wright formulated the idea for the review, developed the basis for the protocol and contributed to the interpretation of the finding and writing of the review. Wen Lu Tang took the lead role in searching, identifying and assessing studies, in data extraction and analysis, and in writing up the review. Yu Jie Chen took the executive role in identifying and assessing studies, in data extraction and analysis, and in writing up the updated review.
Hao Xue took the executive role in identifying and assessing studies, in data extraction and analysis, and in writing up the review (in the earlier version).
Liang Jin Li, Jia Yang Song, Ru Qiu, Qian Li and Hao Xue took part in identifying studies, and also checked data, and modified the draft In this updated review.
Zhuang Lu, Lu Wei Pang and Gan Mi Wang took part in identifying studies with the aid of Gavin Wong, and also checked data, and contributed to writing the review (in the earlier version); Zhuang Lu in particular spent a lot of time and energy on the above work.
Sources of support
Internal sources
University of British Columbia, Department of Anesthesiology, Pharmacology & Therapeutics, Canada.
External sources
Shanghai Municipal Commission of Health and Family Planning, 2016, China.
Declarations of interest
Yu Jie Chen: nothing to declare
Liang Jin Li: nothing to declare
Wen Lu Tang: nothing to declare
Jia Yang Song: nothing to declare
Ru Qiu: nothing to declare
Qian Li: nothing to declare
Hao Xue: nothing to declare
James M Wright: nothing to declare
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ALLHAT 2002 {published data only}
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981‐97. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2006;166(20):2191‐2201. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Jones CL, Davis BR, Basile JN, Goff DC, Ciocon JO, et al. Baseline characteristics of the diabetic participants in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Diabetes Care 2001;24(4):654‐8. [DOI] [PubMed] [Google Scholar]
- Black HR, Davis B, Barzilay J, Nwachuku C, Baimbridge C, Marginean H, et al. Metabolic and clinical outcomes in nondiabetic individuals with the metabolic syndrome assigned to chlorthalidone, amlodipine, or lisinopril as initial treatment for hypertension: a report from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Diabetes Care 2008;31(2):353‐60. [DOI] [PubMed] [Google Scholar]
- Cushman WC, Ford CE, Einhorn P, Wright JT, Preston RA, Davis BR, Basile JN. Blood pressure control by randomized drug group in ALLHAT. American Journal of Hypertension 2004;17(5):30A. [Google Scholar]
- Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. Circulation 2008;118(22):2259‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, et al. Role of diuretics in the prevention of heart failure: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Circulation 2006;113(18):2201‐10. [DOI] [PubMed] [Google Scholar]
- Grimm RH, Davis BR, Cutler JA, Piller LB, Margolis K, Barzilay J, et al. Did blood pressure medication withdrawal prior to randomization influence subsequent rates of heart failure in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial?. Journal of the American College of Cardiology 2007;49(9 Suppl A):350a. [Google Scholar]
- Grimm RH, Davis BR, Piller LB, Cutler JA, Margolis KL, Barzilay J, et al. Heart failure in ALLHAT: did blood pressure medication at study entry influence outcome?. Journal of Clinical Hypertension 2009;11(9):466‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, et al. Atrial fibrillation at baseline and during follow‐up in ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial). Journal of the American College of Cardiology 2009;54(22):2023‐31. [DOI] [PubMed] [Google Scholar]
- Leenen FH, Nwachuku CE, Black HR, Cushman WC, Davis BR, Simpson LM, et al. Clinical events in high‐risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin‐converting enzyme inhibitor in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2006;48(3):374‐84. [DOI] [PubMed] [Google Scholar]
- Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker‐Foster C, et al. Antihypertensive pharmacogenetic effect of fibrinogen‐beta variant ‐455G>A on cardiovascular disease, end‐stage renal disease, and mortality: the GenHAT study. Pharmacogenetics and Genomics 2009;19(6):415‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker‐Foster C, et al. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. [Erratum appears in JAMA. 2009 Sep 9;302(10):1057‐8]. JAMA 2008;299(3):296‐307. [DOI] [PubMed] [Google Scholar]
- Probstfield JL, Cushman WC, Davis BR, Pressel S, Cutler JA, Einhorn P, et al. Mortality and morbidity during and after the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). European Society of Cardiology, ESC Congress; 2010 Aug 28‐Sep 1; Stockholm, Sweden. 2010; Vol. 31:321.
- Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT Jr, Whelton PK, et al. Renal outcomes in high‐risk hypertensive patients treated with an angiotensin‐converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2005;165(8):936‐46. [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005;293(13):1595‐608. [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Harris‐Haywood S, Pressel S, Barzilay J, Baimbridge C, Bareis CJ, et al. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2008;168(2):207‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lynch AI, Davis BR, Ford CE, Boerwinkle E, Eckfeldt JH, et al. Pharmacogenetic association of NOS3 variants with cardiovascular disease in patients with hypertension: the GenHAT study. PLoS ONE [Electronic Resource] 2012;7(3):e34217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ariff 2006 {published data only}
- Ariff B, Zambanini A, Vamadeva S, Barratt D, Xu Y, Sever P, et al. Candesartan‐ and atenolol‐based treatments induce different patterns of carotid artery and left ventricular remodeling in hypertension. Stroke 2006;37(9):2381‐4. [DOI] [PubMed] [Google Scholar]
BENEDICT 2004 (formerly Ruggenenti 2004) {published data only}
- Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, et al. Preventing microalbuminuria in type 2 diabetes. New England Journal of Medicine 2004;351(19):1941‐51. [DOI] [PubMed] [Google Scholar]
Buus 2004 {published data only}
- Buus NH, Bottcher M, Jorgensen CG, Christensen KL, Thygesen K, Nielsen TT, et al. Myocardial perfusion during long‐term angiotensin‐converting enzyme inhibition or beta‐blockade in patients with essential hypertension. Hypertension 2004;44(4):465‐70. [DOI] [PubMed] [Google Scholar]
Buus 2007 {published data only}
- Buus NH, Jorgensen CG, Mulvany MJ, Sorensen KE. Large and small artery endothelial function in patients with essential hypertension‐‐effect of ACE inhibition and beta‐blockade. Blood Pressure 2007;16(2):106‐13. [DOI] [PubMed] [Google Scholar]
Dahlöf 1993 {published data only}
- Dahlöf B, Hansson L. The influence of antihypertensive therapy on the structural arteriolar changes in essential hypertension: different effects of enalapril and hydrochlorothiazide. Journal of Internal Medicine 1993;234(3):271‐9. [DOI] [PubMed] [Google Scholar]
Dahlöf 2002 {published data only}
- Dahlöf B, Zanchetti A, Diez J, Nicholls MG, Yu CM, Barrios V, et al. Effects of losartan and atenolol on left ventricular mass and neurohormonal profile in patients with essential hypertension and left ventricular hypertrophy. Journal of Hypertension 2002;20(9):1855‐64. [DOI] [PubMed] [Google Scholar]
Dalla 2004 {published data only}
- Dalla Vestra M, Pozza G, Mosca A, Grazioli V, Lapolla A, Fioretto P, et al. Effect of lercanidipine compared with ramipril on albumin excretion rate in hypertensive Type 2 diabetic patients with microalbuminuria: DIAL study (diabete, ipertensione, albuminuria, lercanidipina). Diabetes Nutrition & Metabolism 2004;17(5):259‐66. [PubMed] [Google Scholar]
Derosa 2004 {published data only}
- Derosa G, Cicero AF, Bertone G, Piccinni MN, Fogari E, Ciccarelli L, et al. Comparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12‐month, randomized, double‐blind study. Clinical Therapeutics 2004;26(8):1228‐36. [DOI] [PubMed] [Google Scholar]
Derosa 2005 {published data only}
- Derosa G, Cicero AF, Gaddi A, Mugellini A, Ciccarelli L, Fogari R. Effects of doxazosin and irbesartan on blood pressure and metabolic control in patients with type 2 diabetes and hypertension. Journal of Cardiovascular Pharmacology 2005;45(6):599‐604. [DOI] [PubMed] [Google Scholar]
Derosa 2014 {published data only}
- Derosa G, Bonaventura A, Bianchi DRL, Fogari E, Angelo AD, Maffioli P. Effects of enalapril/lercanidipine combination on some emerging biomarkers in cardiovascular risk stratification in hypertensive patients. Journal of Clinical Pharmacy and Therapeutics 2014;39:277–85. [DOI] [PubMed] [Google Scholar]
Devereux 2001 {published data only}
- Devereux RB, Palmieri V, Sharpe N, Quattro V, Bella JN, Simone G, et al. Effects of once‐daily angiotensin‐converting enzyme inhibition and calcium channel blockade‐based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (preserve) trial. Circulation 2001;104(11):1248‐54. [DOI] [PubMed] [Google Scholar]
Esnault 2008 {published data only}
- Esnault VL, Brown EA, Apetrei E, Bagon J, Calvo C, DeChatel R, et al. The effects of amlodipine and enalapril on renal function in adults with hypertension and nondiabetic nephropathies: a 3‐year, randomized, multicenter, double‐blind, placebo‐controlled study. Clinical Therapeutics 2008;30(3):482‐98. [DOI] [PubMed] [Google Scholar]
Estacio 1998 {published data only}
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23:B54‐64. [PubMed] [Google Scholar]
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645‐52. [DOI] [PubMed] [Google Scholar]
- Estacio RO, Schrier RW. Antihypertensive therapy in type 2 diabetes: implications of the appropriate blood pressure control in diabetes (ABCD) trial. American Journal of Cardiology 1998;82(9b):9r‐14r. [DOI] [PubMed] [Google Scholar]
Fogari 2012 {published data only}
- Fogari RZ. Effect of telmisartan on paroxysmal atrial fibrillation recurrence in hypertensive patients with normal or increased left atrial size. Clinical Cardiology 2012;35:359‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerritsen 1998 {published data only}
- Gerritsen TA, Bak AA, Stolk RP, Jonker JJ, Grobbee DE. Effects of nitrendipine and enalapril on left ventricular mass in patients with non‐insulin‐dependent diabetes mellitus and hypertension. Journal of Hypertension 1998;16(5):689‐96. [DOI] [PubMed] [Google Scholar]
Gottdiener 1998 {published data only}
- Gottdiener JS, Reda DJ, Williams DW, Materson BJ, Cushman W, Anderson RJ. Effect of single‐drug therapy on reduction of left atrial size in mild to moderate hypertension. Circulation 1998;98(2):140‐8. [DOI] [PubMed] [Google Scholar]
Hajjar 2013 {published data only}
- Hajjar I, Hart M, Chen YL, Mack W, Milberg W, Chui H, et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double‐blind randomized clinical trial. Archives of Internal Medicine 2012;172(5):442‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Hart M, Chen YL, Mack W, Novak V, Chui C, et al. Antihypertensive therapy and cerebral hemodynamics in executive mild cognitive impairment: results of a pilot randomized clinical trial. Journal of the American Geriatrics Society 2013;61(2):194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauf‐Zachariou 1993 {published data only}
- Hauf‐Zachariou U, Widmann L, Zülsdorf B, Hennig M, Lang PD. A double‐blind comparison of the effects of carvedilol and captopril on serum lipid concentrations in patients with mild to moderate essential hypertension and dyslipidaemia. European Journal of Clinical Pharmacology 1993;45(2):95‐100. [DOI] [PubMed] [Google Scholar]
Hayoz 2012 {published data only}
- Hayoz D, Zappe DH, Meyer MA, Baek I, Kandra A, Joly MP, et al. Changes in aortic pulse wave velocity in hypertensive postmenopausal women: comparison between a calcium channel blocker vs angiotensin receptor blocker regimen. Journal of Clinical Hypertension 2012;14(11):773‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Himmelmann 1996 {published data only}
- Himmelmann A, Hansson L, Hansson BG, Hedstrand H, Skogström K, Ohrvik J, et al. Long‐term renal preservation in essential hypertension. Angiotensin converting enzyme inhibition is superior to beta‐blockade. American Journal of Hypertension 1996;9(9):850‐3. [DOI] [PubMed] [Google Scholar]
Hughes 2008 {published data only}
- Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez‐Perez ME, McG Thom SA. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. Journal of Hypertension 2008;26(8):1703‐7. [DOI] [PubMed] [Google Scholar]
IDNT 2001 {published data only}
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Annals of Internal Medicine 2003;138(7):542‐9. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine 2001;345(12):851‐60. [DOI] [PubMed] [Google Scholar]
LIFE 2002 {published data only}
- Dahlöf B, Devereux R, Faire U, Fyhrquist F, Hedner T, Ibsen H, et al. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: rationale, design, and methods. The LIFE Study Group. American Journal of Hypertension 1997;10(7 Pt 1):705‐13. [PubMed] [Google Scholar]
- Dahlöf B, Devereux RB, Julius S, Kjeldsen SE, Beevers G, Faire U, et al. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint Reduction in Hypertension. Hypertension 1998;32(6):989‐97. [DOI] [PubMed] [Google Scholar]
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359(9311):995‐1003. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Dahlöf B, Kjeldsen SE, Julius S, Aurup P, Beevers G, et al. Effects of losartan or atenolol in hypertensive patients without clinically evident vascular disease: a substudy of the LIFE randomized trial. Annals of Internal Medicine 2003;139(3):169‐77. [DOI] [PubMed] [Google Scholar]
- Fossum E, Moan A, Kjeldsen SE, Devereux RB, Julius S, Snapinn SM, et al. The effect of losartan versus atenolol on cardiovascular morbidity and mortality in patients with hypertension taking aspirin: the Losartan Intervention for Endpoint Reduction in hypertension (LIFE) study. Journal of the American College of Cardiology 2005;46(5):770‐5. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Wachtell K, Papademetriou V, Olsen MH, Devereux RB, Fyhrquist F, et al. Cardiovascular morbidity and mortality in hypertensive patients with lower versus higher risk: a LIFE substudy. Hypertension 2005;46(3):492‐9. [DOI] [PubMed] [Google Scholar]
- Gerdts E, Franklin S, Rieck A, Papademetriou V, Wachtell K, Nieminen M, et al. Pulse pressure, left ventricular function and cardiovascular events during antihypertensive treatment (the LIFE study). Blood Pressure 2009;18(4):180‐6. [DOI] [PubMed] [Google Scholar]
- Julius S, Alderman MH, Beevers G, Dahlöf B, Devereux RB, Douglas JG, et al. Cardiovascular risk reduction in hypertensive black patients with left ventricular hypertrophy: the LIFE study. Journal of the American College of Cardiology 2004;43(6):1047‐55. [DOI] [PubMed] [Google Scholar]
- Kizer JR, Dahlof B, Kjeldsen SE, Julius S, Beevers G, Faire U, et al. Stroke reduction in hypertensive adults with cardiac hypertrophy randomized to losartan versus atenolol: the Losartan Intervention For Endpoint reduction in hypertension study. Hypertension 2005;45(1):46‐52. [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Lyle PA, Kizer JR, Dahlof B, Devereux RB, Julius S, et al. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. Journal of Clinical Hypertension 2005;7(3):152‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359(9311):1004‐10. [DOI] [PubMed] [Google Scholar]
- Okin PM. Serial evaluation of electrocardiographic left ventricular hypertrophy for prediction of risk in hypertensive patients. Journal of Electrocardiology 2009;42(6):584‐8. [DOI] [PubMed] [Google Scholar]
- Okin PM, Bang CN, Wachtell K, Hille DA, Kjeldsen SE, Dahlof B, et al. Relationship of sudden cardiac death to new‐onset atrial fibrillation in hypertensive patients with left ventricular hypertrophy. Circulation: Arrhythmia and Electrophysiology 2013;6(2):243‐51. [DOI] [PubMed] [Google Scholar]
- Okin PM, Hille DA, Kjeldsen SE, Lindholm LH, Edelman JM, Dahlof B, et al. Greater regression of electrocardiographic left ventricular hypertrophy during hydrochlorothiazide therapy in hypertensive patients. American Journal of Hypertension 2010;23(7):786‐93. [DOI] [PubMed] [Google Scholar]
- Olsen MH, Wachtell K, Beevers G, Dahlof B, Simone G, Devereux RB, et al. Effects of losartan compared with atenolol on lipids in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention For Endpoint reduction in hypertension study. Journal of Hypertension 2009;27(3):567‐74. [DOI] [PubMed] [Google Scholar]
- Os I, Franco V, Kjeldsen SE, Manhem K, Devereux RB, Gerdts E, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2008;51(4):1103‐8. [DOI] [PubMed] [Google Scholar]
Malmqvist 2002 {published data only}
- Malmqvist K, Kahan T, Edner M, Bergfeldt L. Comparison of actions of irbesartan versus atenolol on cardiac repolarization in hypertensive left ventricular hypertrophy: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation Versus Atenolol (SILVHIA). American Journal of Cardiology 2002;90(10):1107‐12. [DOI] [PubMed] [Google Scholar]
- Malmqvist K, Öhman KP, Lind L, Nyström F, Kahan T. Long‐term effects of irbesartan and atenolol on the renin‐angiotensin‐aldosterone system in human primary hypertension: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). Journal of Cardiovascular Pharmacology 2003;42:719‐26. [DOI] [PubMed] [Google Scholar]
- Mortsell D, Malmqvist K, Held C, Kahan T. Irbesartan reduces common carotid artery intima‐media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study. Journal of Internal Medicine 2007;261(5):472‐9. [DOI] [PubMed] [Google Scholar]
NESTOR 2015 {published data only}
- Marre M, Puig JG, Kokot F, Fernandez M, Jermendy G, Opie L, et al. Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: The NESTOR* study. Journal of Hypertension 2004;22(8):1613‐22. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Agnoletti D, Wang JG, Xu Y, Safar ME. Natriuresis and blood pressure reduction in hypertensive patients with diabetes mellitus: the NESTOR study. Journal of the American Society of Hypertension 2015;9(1):21‐8. [DOI] [PubMed] [Google Scholar]
Ostman 1998 {published data only}
- Ostman J, Asplund K, Bystedt T, Dahlöf B, Jern S, Kjellström T, et al. Comparison of effects of quinapril and metoprolol on glycaemic control, serum lipids, blood pressure, albuminuria and quality of life in non‐insulin‐dependent diabetes mellitus patients with hypertension. Swedish Quinapril Group. Journal of Internal Medicine 1998;244(2):95‐107. [DOI] [PubMed] [Google Scholar]
Parrinello 2009 {published data only}
- Parrinello G, Paterna S, Torres D, Pasquale P, Mezzero M, Rocca G, et al. One‐year renal and cardiac effects of bisoprolol versus losartan in recently diagnosed hypertensive patients: a randomized, double‐blind study. Clinical Drug Investigation 2009;29(9):591‐600. [DOI] [PubMed] [Google Scholar]
Pedersen 1997 {published data only}
- Pedersen EB, Bech JN, Nielsen CB, Kornerup HJ, Hansen HE, Spencer ES, et al. A comparison of the effect of ramipril, felodipine and placebo on glomerular filtration rate, albuminuria, blood pressure and vasoactive hormones in chronic glomerulonephritis. A randomized, prospective, double‐blind, placebo‐controlled study over two years. Scandinavian Journal of Clinical & Laboratory Investigation 1997;57(8):673‐81. [DOI] [PubMed] [Google Scholar]
Petersen 2001 {published data only}
- Petersen LJ, Petersen JR, Talleruphuus U, Moller ML, Ladefoged SD, Mehlsen J. A randomized and double‐blind comparison of isradipine and spirapril as monotherapy and in combination on the decline in renal function in patients with chronic renal failure and hypertension. Clinical Nephrology 2001;55:375‐83. [PubMed] [Google Scholar]
Roman 1998 {published data only}
- Roman MJ, Alderman MH, Pickering TG, Pini R, Keating JO, Sealey JE, et al. Differential effects of angiotensin converting enzyme inhibition and diuretic therapy on reductions in ambulatory blood pressure, left ventricular mass, and vascular hypertrophy. American Journal of Hypertension 1998;11(4 Pt 1):387‐96. [DOI] [PubMed] [Google Scholar]
Schiffrin 1994 {published data only}
- Schiffrin EL, Deng LY, Larochelle P. Effects of a beta‐blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension 1994;23(1):83‐91. [DOI] [PubMed] [Google Scholar]
Schmieder 2009 {published data only}
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, et al. Long‐term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12‐month randomized, double‐blind comparator trial with hydrochlorothiazide. Circulation 2009;119(3):417‐25. [DOI] [PubMed] [Google Scholar]
Schneider 2004 {published data only}
- Schneider MP, Klingbeil AU, Delles C, Ludwig M, Kolloch RE, Krekler M, et al. Effect of irbesartan versus atenolol on left ventricular mass and voltage: results of the CardioVascular Irbesartan Project. Hypertension 2004;44(1):61‐6. [DOI] [PubMed] [Google Scholar]
Schram 2005 {published data only}
- Schram MT, Ittersum FJ, Spoelstra‐de Man A, Dijk RA, Schalkwijk CG, Ijzerman RG, et al. Aggressive antihypertensive therapy based on hydrochlorothiazide, candesartan or lisinopril as initial choice in hypertensive type II diabetic individuals: effects on albumin excretion, endothelial function and inflammation in a double‐blind, randomized clinical trial. Journal of Human Hypertension 2005;19(6):429‐37. [DOI] [PubMed] [Google Scholar]
Seedat 1998 {published data only}
- Seedat YK, Randeree IG. Antihypertensive effect and tolerability of perindopril in Indian hypertensive and type 2 diabetic patients: 1‐year randomised, double‐blind, parallel study vs atenolol. Clinical Drug Investigation 1998;16(3):229‐240. [DOI] [PubMed] [Google Scholar]
SILVHIA 2001 {published data only}
- Malmqvist K, Kahan T, Edner M, Hagg A, Lind L, Muller‐Brunotte R, et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. Journal of Hypertension 2001;19(6):1167‐76. [DOI] [PubMed] [Google Scholar]
Sørensen 1998 {published data only}
- Sørensen VB, Rossing P, Tarnow L, Parving H, Nørgaard T, Kastrup J. Effects of nisoldipine and lisinopril on microvascular dysfunction in hypertensive type I diabetes patients with nephropathy. Clinical Science 1998;95(6):709‐17. [DOI] [PubMed] [Google Scholar]
Tarnow 1999 {published data only}
- Tarnow L, Sato A, Ali S, Rossing P, Nielsen FS, Parving HH. Effects of nisoldipine and lisinopril on left ventricular mass and function in diabetic nephropathy. Diabetes Care 1999;22(3):491‐4. [DOI] [PubMed] [Google Scholar]
Tedesco 1999 {published data only}
- Tedesco MA, Ratti G, Mennella S, Manzo G, Grieco M, Rainone AC, et al. Comparison of losartan and hydrochlorothiazide on cognitive function and quality of life in hypertensive patients. American Journal of Hypertension 1999;12(11 Pt 1):1130‐4. [DOI] [PubMed] [Google Scholar]
Terpstra 2004 {published data only}
- Terpstra WF, May JF, Smit AJ, Graeff PA, Meyboom‐de Jong B, Crijns HJ. Effects of amlodipine and lisinopril on intima‐media thickness in previously untreated, elderly hypertensive patients (the ELVERA trial). Journal of Hypertension 2004;22(7):1309‐16. [DOI] [PubMed] [Google Scholar]
TOHMS 1993 {published data only}
- Neaton JD, Grimm RH Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA 1993;270(6):713‐24. [PubMed] [Google Scholar]
VALUE 2004 {published data only}
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363(9426):2022‐31. [DOI] [PubMed] [Google Scholar]
- Julius S, Weber MA, Kjeldsen SE, McInnes GT, Zanchetti A, Brunner HR, et al. The Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension 2006;48(3):385‐91. [DOI] [PubMed] [Google Scholar]
- Mann J, Julius S. The Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) trial of cardiovascular events in hypertension. Rationale and design. Blood Pressure 1998;7(3):176‐83. [DOI] [PubMed] [Google Scholar]
- Sandset E, Krarup LH, Berge E, Kjeldsen SE, Julius S, Holzhauer B. Predictors of stroke recurrence in high‐risk, hypertensive patients. Journal of Hypertension 2010;28(A):E33. [Google Scholar]
- Zanchetti A, Julius S, Kjeldsen S, McInnes GT, Hua T, Weber M, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: an analysis of findings from the VALUE trial. Journal of Hypertension 2006;24(11):2163‐8. [DOI] [PubMed] [Google Scholar]
Xiao 2016 {published data only}
- Xiao WY, Ning N, Tan MH, Jiang XS, Zhou L, Liu L, et al. Effects of antihypertensive drugs losartan and levamlodipine besylate on insulin resistance in patients with essential hypertension combined with isolated impaired fasting glucose. Hypertension Research 2016;39(8):321‐6. [DOI] [PubMed] [Google Scholar]
Zeltner 2008 {published data only}
- Zeltner R, Poliak R, Stiasny B, Schmieder RE, Schulze BD. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2008;23(2):573‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AASK 2002 {published data only}
- Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. JAMA 2002;288(19):2421‐31. [DOI] [PubMed] [Google Scholar]
ANBP2 2003 {published data only}
- Chowdhury EK, Owen A, Ademi Z, Krum H, Johnston CI, Wing LMH, et al. Short‐ and long‐term survival in treated elderly hypertensive patients with or without diabetes: findings from the Second Australian National Blood Pressure study. American Journal of Hypertension 2014;27(2):199‐206. [DOI] [PubMed] [Google Scholar]
- Wing LMH, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, et al. A comparison of outcomes with angiotensin‐converting‐‐enzyme inhibitors and diuretics for hypertension in the elderly. New England Journal of Medicine 2003;348:583‐92. [DOI] [PubMed] [Google Scholar]
Materson 1993 {published data only}
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single‐drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. New England Journal of Medicine 1993;328(13):914‐21. [DOI] [PubMed] [Google Scholar]
Okin 2012 {published data only}
- Okin PM, Kjeldsen SE, Lindholm LH, Dahlof B, Devereux RB. The relationship of electrocardiographic left ventricular hypertrophy to decreased serum potassium. Blood Pressure 2012;21:146‐52. [DOI] [PubMed] [Google Scholar]
Peng 2015 {published data only}
- Peng J, Zhao Y, Zhang H, Liu Z, Wang Z, Tang M, et al. Prevention of metabolic disorders with telmisartan and indapamide in a Chinese population with high‐normal blood pressure. Hypertension Research 2015;38(2):123‐31. [DOI] [PubMed] [Google Scholar]
Preston 1998 {published data only}
- Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, et al. Age‐race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. JAMA 1998;280(13):1168‐72. [DOI] [PubMed] [Google Scholar]
Additional references
ABCD‐HT 2000
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(suppl 2):B54‐64. [PubMed] [Google Scholar]
ADA 2013
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2013;36(suppl 1):S11‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aurell 1992
- Aurell M, Bjorck S. Determinants of progressive renal disease in diabetes mellitus. Kidney Int Suppl 1992;41(suppl 36):S38‐42. [PubMed] [Google Scholar]
CAPPP 2001
- Niskanen L, Hedner T, Hansson L, Lanke J, Niklason A, CAPPP Study Group. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first‐line therapy with an ACE inhibitor compared with a diuretic/beta‐blocker‐based treatment regimen: a subanalysis of the Captopril Prevention Project (CAPPP). Diabetes Care 2001;24:2091‐6. [DOI] [PubMed] [Google Scholar]
Casas 2005
- Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, et al. Effect of inhibitors of the renin‐angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta‐analysis. Lancet 2005;366(9502):2026‐33. [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD003654.pub4] [DOI] [PubMed] [Google Scholar]
CPG 2013
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada ‐ Treatment of Hypertension. Canadian Journal of Diabetes 2013;37(Suppl 1):S117‐8. [DOI] [PubMed] [Google Scholar]
DeStefano 1993
- DeStefano F, Ford ES, Newman J, Stevenson JM, Wetterhall SF, Anda RF, et al. Risk factors for coronary heart disease mortality among persons with diabetes. Annals of Epidemiology 1993;3:27‐34. [DOI] [PubMed] [Google Scholar]
Duprez 2006
- Duprez, D. Role of the renin‐angiotensin‐aldosterone system in vascular remodeling and inflammation: a clinical review. Journal of Hypertension 2006;24:983‐91. [DOI] [PubMed] [Google Scholar]
Dzau 2001
- Dzau VJ. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension 2001;37:1047‐52. [DOI] [PubMed] [Google Scholar]
ESH/ESC 2013
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of Hypertension 2013;31(7):1281‐357. [DOI] [PubMed] [Google Scholar]
Geiss 2002
- Geiss LS, Rolka DB, Engelgau MM. Elevated blood pressure among U.S. adults with diabetes, 1988‐1994. American Journal of Preventive Medicine 2002;22:42‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HOPE 2000
- Heart Outcomes Prevention Evaluation (HOPE) Study investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICROHOPE substudy. Lancet 2000;355:253‐9. [PubMed] [Google Scholar]
Inzucchi 2005
- Inzucchi SE, Sherwin RS. The prevention of type 2 diabetes mellitus. Endocrinology and Metabolism Clinics of North America 2005;34(1):199‐219. [DOI] [PubMed] [Google Scholar]
JNC‐8 2014
- James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507‐20. [DOI] [PubMed] [Google Scholar]
Kasiske 1993
- Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF. Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta regression analysis. Annals of Internal Medicine 1993;118(2):129‐38. [DOI] [PubMed] [Google Scholar]
Kocks 2002
- Kocks MJ, Zeeuw D, Navis GJ. Optimal blood pressure control and antihypertensive regimens in hypertensive renal disease. Current Opinion in Nephrology and Hypertension 2002;11:135‐40. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li ECK, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009096.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MARVAL 2002
- MARVAL Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus. Circulation 2002;106:672‐8. [DOI] [PubMed] [Google Scholar]
Pahor 2000
- Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Furberg CD. Therapeutic benefits of ACE inhibitors and other antihypertensive drugs in patients with type 2 diabetes. Diabetes Care 2000;23:888‐92. [DOI] [PubMed] [Google Scholar]
PROGRESS 2001
- PROGRESS Collaborative Study Group. Randomised trial of perindopril based blood pressure‐lowering regimen among 6108 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033‐41. [DOI] [PubMed] [Google Scholar]
Ravine 1994
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid‐Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994;343(8901):824‐7. [DOI] [PubMed] [Google Scholar]
RENAAL 2001
- RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 2001;345:861‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saha 2008
- Saha SA, Molnar J, Arora RR. Tissue angiotensin‐converting enzyme inhibitors for the prevention of cardiovascular disease in patients with diabetes mellitus without left ventricular systolic dysfunction or clinical evidence of heart failure: a pooled meta‐analysis of randomized placebo‐controlled clinical trials. Diabetes Obesity and Metabolism 2008;10(1):41‐52. [DOI] [PubMed] [Google Scholar]
Siebenhofer 2004
- Siebenhofer A, Plank J, Horvath K, Berghold A, Sutton AJ, Sommer R, et al. Angiotensin receptor blockers as anti‐hypertensive treatment for patients with diabetes mellitus: meta‐analysis of controlled double‐blind randomized trials. Diabetic Medicine 2004;21(1):18‐25. [DOI] [PubMed] [Google Scholar]
Strippoli 2005
- Strippoli GFM, Craig ME, Craig JC. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004136.pub2] [DOI] [PubMed] [Google Scholar]
Strippoli 2006
- Strippoli GFM, Bonifati C, Craig ME, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006257] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]
Wu 2013
- Wu HY, Huang JW, Lin HJ, Liao WC, Peng YS, Hung KY, et al. Comparative effectiveness of renin‐angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and bayesian network meta‐analysis. BMJ 2013;347(7933):f6008. [DOI: 10.1136/bmj.f6008] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Xue 2015
- Xue H, Lu Z, Tang WL, Pang LW, Wang GM, Wong GWK, Wright JM. First‐line drugs inhibiting the renin angiotensin system versus other first‐line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008170.pub2] [DOI] [PubMed] [Google Scholar]