Abstract
Background
Stillbirth affects at least 2.6 million families worldwide every year and has enduring consequences for parents and health services. Parents entering a subsequent pregnancy following stillbirth face a risk of stillbirth recurrence, alongside increased risks of other adverse pregnancy outcomes and psychosocial challenges. These parents may benefit from a range of interventions to optimise their short‐ and longer‐term medical health and psychosocial well‐being.
Objectives
To assess the effects of different interventions or models of care prior to and during subsequent pregnancies following stillbirth on maternal, fetal, neonatal and family health outcomes, and health service utilisation.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (6 June 2018), along with ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (18 June 2018).
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐randomised controlled trials (qRCTs). Trials using a cluster‐randomised design were eligible for inclusion, but we found no such reports. We included trials published as abstract only, provided sufficient information was available to allow assessment of trial eligibility and risk of bias. We excluded cross‐over trials.
Data collection and analysis
Two review authors independently assessed trials for eligibility and undertook data extraction and 'Risk of bias' assessments. We extracted data from published reports, or sourced data directly from trialists. We checked the data for accuracy and resolved discrepancies by discussion or correspondence with trialists, or both. We conducted an assessment of the quality of the evidence using the GRADE approach.
Main results
We included nine RCTs and one qRCT, and judged them to be at low to moderate risk of bias. Trials were carried out between the years 1964 and 2015 and took place predominantly in high‐income countries in Europe. All trials assessed medical interventions; no trials assessed psychosocial interventions or incorporated psychosocial aspects of care. Trials evaluated the use of antiplatelet agents (low‐dose aspirin (LDA) or low‐molecular‐weight heparin (LMWH), or both), third‐party leukocyte immunisation, intravenous immunoglobulin, and progestogen. Trial participants were women who were either pregnant or attempting to conceive following a pregnancy loss, fetal death, or adverse outcome in a previous pregnancy.
We extracted data for 222 women who had experienced a previous stillbirth of 20 weeks' gestation or more from the broader trial data sets, and included them in this review. Our GRADE assessments of the quality of evidence ranged from very low to low, due largely to serious imprecision in effect estimates as a result of small sample sizes, low numbers of events, and wide confidence intervals (CIs) crossing the line of no effect. Most of the analyses in this review were not sufficiently powered to detect differences in the outcomes assessed. The results presented are therefore largely uncertain.
Main comparisons
LMWH versus no treatment/standard care (three RCTs, 123 women, depending on the outcome)
It was uncertain whether LMWH reduced the risk of stillbirth (risk ratio (RR) 2.58, 95% CI 0.40 to 16.62; 3 trials; 122 participants; low‐quality evidence), adverse perinatal outcome (RR 0.81, 95% CI 0.20 to 3.32; 2 trials; 77 participants; low‐quality evidence), adverse maternal psychological effects (RR 1.00, 95% CI 0.07 to 14.90; 1 trial; 40 participants; very low‐quality evidence), perinatal mortality (RR 2.58, 95% CI 0.40 to 16.62; 3 trials; 122 participants; low‐quality evidence), or any preterm birth (< 37 weeks) (RR 1.01, 0.58 to 1.74; 3 trials; 114 participants; low‐quality evidence). No neonatal deaths were reported in the trials assessed and no data were available for maternal‐infant attachment. There was no clear evidence of a difference between the groups among the remaining secondary outcomes.
LDA versus placebo (one RCT, 24 women)
It was uncertain whether LDA reduced the risk of stillbirth (RR 0.85, 95% CI 0.06 to 12.01), neonatal death (RR 0.29, 95% CI 0.01 to 6.38), adverse perinatal outcome (RR 0.28, 95% CI 0.03 to 2.34), perinatal mortality, or any preterm birth (< 37 weeks) (both of the latter RR 0.42, 95% CI 0.04 to 4.06; all very low‐quality evidence). No data were available for adverse maternal psychological effects or maternal‐infant attachment. LDA appeared to be associated with an increase in birthweight (mean difference (MD) 790.00 g, 95% CI 295.03 to 1284.97 g) when compared to placebo, but this result was very unstable due to the extremely small sample size. Whether LDA has any effect on the remaining secondary outcomes was also uncertain.
Other comparisons
LDA appeared to be associated with an increase in birthweight when compared to LDA + LMWH (MD −650.00 g, 95% CI −1210.33 to −89.67 g; 1 trial; 29 infants), as did third‐party leukocyte immunisation when compared to placebo (MD 1195.00 g, 95% CI 273.35 to 2116.65 g; 1 trial, 4 infants), but these results were again very unstable due to extremely small sample sizes. The effects of the interventions on the remaining outcomes were also uncertain.
Authors' conclusions
There is insufficient evidence in this review to inform clinical practice about the effectiveness of interventions to improve care prior to and during subsequent pregnancies following a stillbirth. There is a clear and urgent need for well‐designed trials addressing this research question. The evaluation of medical interventions such as LDA, in the specific context of stillbirth prevention (and recurrent stillbirth prevention), is warranted. However, appropriate methodologies to evaluate such therapies need to be determined, particularly where clinical equipoise may be lacking. Careful trial design and multicentre collaboration is necessary to carry out trials that would be sufficiently large to detect differences in statistically rare outcomes such as stillbirth and neonatal death. The evaluation of psychosocial interventions addressing maternal‐fetal attachment and parental anxiety and depression is also an urgent priority. In a randomised‐trial context, such trials may allocate parents to different forms of support, to determine which have the greatest benefit with the least financial cost. Importantly, consistency in nomenclature and in data collection across all future trials (randomised and non‐randomised) may be facilitated by a core outcomes data set for stillbirth research. All future trials should assess short‐ and longer‐term psychosocial outcomes for parents and families, alongside economic costs of interventions.
Plain language summary
Interventions for improving outcomes in pregnancies that follow stillbirth
We aimed to compare the effectiveness of different interventions or models of care in improving pregnancy outcomes for parents who have had a previous stillbirth at 20 weeks' gestation or more. The care could be initiated before pregnancy, or during pregnancy, labour, or birth.
What is the issue?
Every year at least 2.6 million families experience the tragedy of stillbirth. This is a devastating event that can have long‐term consequences and change parents' attitudes to future pregnancies. Many different causes can lead to stillbirth, and sometimes multiple causes occur together. Causes such as long‐term health problems in the mother are still present in subsequent pregnancies. The parents may therefore benefit from special care before becoming pregnant again. Such care may be highly diverse, addressing a range of risk factors, conditions, and other considerations. This care can take the form of counselling or social support programmes to assist with dealing with grief, anxiety and depression; better managing a mother's health before conception to address health issues; and assisting with high‐risk behaviours or risk factors such as being overweight, smoking, or alcohol use. Once pregnant, the mother can be closely watched, possibly with extra antenatal visits or by attending special antenatal clinics. A planned early birth may also be considered.
Why is this important?
Parents who have had a stillborn baby are more likely to have another stillbirth than parents who have not had a stillborn baby before. In their next pregnancy, parents often experience anxiety and depression, and ongoing worry about whether their baby will survive. It is important to be able to work out from high‐quality clinical studies which interventions are helpful in preventing stillbirth from happening again, and in improving the health and well‐being of these parents and families.
What evidence did we find?
We searched for evidence from randomised controlled trials published up to June 2018. We included 10 studies at low to moderate risk of bias. All but one study were from high‐income countries, mainly in developed areas of Europe. The women in the studies were either pregnant or attempting to conceive after having a miscarriage, a stillborn baby, or a serious complication in a previous pregnancy. The interventions included two types of drugs (low‐dose aspirin and low‐molecular‐weight heparin) that reduce blood clotting and may help the placenta to function (six trials), pre‐conception injection of blood cells (third‐party leukocyte immunisation) to help mothers' immune systems to cope with pregnancy (one trial), a special type of antibody (intravenous immunoglobulin) given into a vein to improve the functioning of the pregnant woman's immune system (two trials), and injections of a medication (progestogen) that acts like the pregnancy hormone progesterone (one trial). We evaluated data from 222 women who had previously had a stillborn baby at 20 weeks' gestation or more.
We were unable to determine whether any of these interventions reduced the chance of having another stillborn baby in the subsequent pregnancy; or whether the interventions reduced the chances of babies dying or having serious complications in the first month of life, because the studies not large enough for us to have confidence in the findings. Largely because of this, we judged the quality of evidence in this review to be very low to low. Two interventions (low‐dose aspirin and third‐party leukocyte immunisation) appeared to increase the birthweight of babies, but these findings are not reliable due to the small numbers of babies included.
The included studies provided very little information about psychological outcomes of parents or longer‐term outcomes of children and families.
What does this mean?
There is insufficient evidence from the studies included in this review to know which interventions are helpful in preventing subsequent stillbirths and improving the health and well‐being of parents and families in pregnancies that follow a stillbirth. More targeted studies are needed, which include larger numbers of women/parents who have previously experienced a stillbirth. We urgently need studies testing what forms of psychological support are most helpful in reducing anxiety and depression for these parents. Any studies carried out in future should measure the financial costs of interventions, and longer‐term health outcomes of families and children.
Summary of findings
Background
Description of the condition
Stillbirth is a devastating outcome of pregnancy, with enduring psychosocial consequences for parents, including anxiety and depression, guilt, complicated grief, social isolation, and relationship breakdown (Heazell 2016). Stillbirth also has profound economic impacts on parents, families, and the wider community (Heazell 2016; Ogwulu 2015). The definition of stillbirth in terms of gestational age varies across geographical settings. For international comparisons, the World Health Organization recommends reporting of stillbirths of 28 weeks' gestation or more, although most high‐income countries (HICs) adopt a lower gestational age cut‐off point (Flenady 2015). In this review, we define stillbirth as the death of an unborn baby at 20 weeks' gestation or more.
Globally, at least 2.6 million babies are stillborn in the third trimester each year (Lawn 2016). While data from many parts of the world are incomplete, it is known that the vast majority of these deaths (98%) occur in low‐ and middle‐income countries (LMICs), and that over 40% occur in the intrapartum period, often associated with obstetric emergencies (Lawn 2016; Reinebrant 2018). Wide variation exists across and within countries, with stillbirth rates estimated to be below five per 1000 births in HICs (Flenady 2016), compared with approximately 32 per 1000 in sub‐Saharan Africa and South Asia (Lawn 2016).
There are many maternal and fetal conditions associated with stillbirth. These conditions often co‐exist, and include maternal infections, non‐communicable diseases, nutrition and lifestyle factors, malaria, fetal growth restriction, and advanced maternal age (Lawn 2016). In LMICs, limited access to skilled birth attendants and low rates of caesarean section are also believed to be important. Maternal undernutrition is prevalent in many low‐income countries and contributes to various adverse pregnancy outcomes including fetal growth restriction (Black 2008a), which is an important risk factor for stillbirth. In HICs, common risk factors for stillbirth include maternal overweight and obesity, advanced maternal age, primiparity, and smoking (Flenady 2011).
A systematic review of stillbirth recurrence in HICs, including over three million women, reported an almost five‐fold increase in the risk of stillbirth in the pregnancy following stillbirth from all causes (Lamont 2015). However, predicting recurrence risk in a specific pregnancy is difficult, as the risk depends on a variety of factors, such as the aetiology of the index stillbirth. For example, while there is little evidence to draw upon, it is possible that deaths related to placental insufficiency or a pre‐existing maternal condition have a greater recurrence risk. Conversely, recurrence is less likely for isolated events such as maternal injury leading to placental abruption (Robson 2001).
When the cause of stillbirth is unexplained, the risk of recurrence is unclear (Lamont 2015). It is possible that recurrence following truly unexplained stillbirth is no higher than that of the general population (Onwude 2006; Robson 2001). While this may be reassuring for some women and their families, a history of stillbirth has been shown to be associated with higher frequencies of other complications in the next pregnancy, including increased rates of induced labour, elective and emergency caesarean birth, instrumental birth and other adverse outcomes, such as preterm birth, low birthweight, placental abruption, pre‐eclampsia, gestational diabetes (Black 2008b; Heinonen 2000; Robson 2001), chorioamnionitis, and neonatal death (Getahun 2009). Some of these outcomes may be in part due to care providers' and women's hypervigilance, rather than inherent biological risk (Robson 2006).
Previous stillbirth is also commonly associated with intense anxiety and fear in the next pregnancy, with some women feeling a lack of confidence in their capacity to maintain a healthy pregnancy (Gravensteen 2018; Meaney 2017; Mills 2014). The fear of experiencing another loss may further increase risk, as stress during pregnancy has also been associated with adverse pregnancy outcomes, such as preterm birth (Dunkel Schetter 2011; Van den Bergh 2005) and low birthweight (Baibazarova 2013; Dunkel Schetter 2011; Su 2015; Van den Bergh 2005), possibly mediated by placental function (O'Donnell 2009). Anxiety and fear may also prompt some parents to refrain from attachment to their baby (Lee 2017; Mills 2014). Disorganised attachment has been observed in infants born subsequent to stillbirth, which may in turn increase these infants' risk of psychological and behavioural problems in childhood (Hughes 2001).
The global reduction in stillbirth rates has not matched that for maternal or neonatal mortality (Lawn 2016). A persisting issue facing providers of maternity care is therefore how to manage the next and subsequent pregnancies. International data have shown that parents who experienced a stillbirth in a previous pregnancy are commonly offered additional antenatal visits and additional ultrasound scans in their next pregnancy, particularly following later‐gestation stillbirths (defined as >30 weeks' gestation (Wojcieszek 2018). Indeed, surveys and interviews have found that women themselves wanted high levels of surveillance and early birth in pregnancies following stillbirth or perinatal death (Mills 2016; Robson 2009; Simmons 2011). Similarly, a survey of Australian obstetricians found that many health professionals were likely to recommend close surveillance and early birth in pregnancies after stillbirth (Robson 2006). However, while early birth has some potential to reduce the rate of stillbirth, it may also be associated with iatrogenic complications (caused by treatment or diagnostic procedures) as alluded to earlier, including prematurity and its associated adverse outcomes, failed induction, instrumental birth, emergency caesarean birth, and postpartum haemorrhage (Paull 2013).
Description of the intervention
The care and management of women in the next and subsequent pregnancies following stillbirth may be different from the care of women who have never been pregnant, or who have never had a complicated pregnancy. It is possible that a number of management decisions will be required, some guided by causes, circumstances, or risk factors associated with the prior stillbirth (Monari 2010; Paull 2013; Reddy 2007; Robson 2010; Saade 2011). Therefore, while discrete interventions may be assessed to care for women in the next and subsequent pregnancies, care might also involve different management algorithms, protocols, guidelines, or models of care, combining multiple interventions in order to optimise outcomes for families.
Care prior to subsequent pregnancies might first focus on counselling on stillbirth recurrence risk for parents considering a subsequent pregnancy after stillbirth, to provide information and decision‐making support on:
interpregnancy interval; pre‐conception health.
Alternatively, or in addition, care prior to or during subsequent pregnancies might focus on managing/addressing specific defined causes or circumstances of the index stillbirth, such as interventions to treat, manage or address:
diabetes; hypertensive disorders; thyroid disorders; acquired or inherited thrombophilia; systemic lupus erythematosus; blood group antibodies; maternal cardiac disease; other medical conditions; chronic infectious conditions (HIV, hepatitis, syphilis); periodontal disease; preterm labour; and cervical insufficiency.
Care could also be focused on addressing the presence of modifiable high‐risk behaviours or risk factors, such as interventions to reduce:
obesity; smoking; alcohol use.
In the case of unexplained stillbirth and also where causes, circumstances or risk factors have been identified, care may focus on fetal surveillance and timing and mode of birth, such as:
maternal assessment of fetal movements; fetal heart rate monitoring or cardiotocography; early and/or regular ultrasound surveillance (to assess fetal growth, placental size or structure amniotic fluid index, Doppler assessment of uterine or umbilical blood flow); and/or
elective induction of labour; elective caesarean birth; early birth; intrapartum monitoring.
Care prior to or during subsequent pregnancies might also focus on specific psychosocial needs, such as:
specialised antenatal classes for bereaved parents; peer‐support programmes and grief counselling; and additional antenatal visits or therapies to address anxiety, depression, and maternal‐infant attachment.
How the intervention might work
Care prior to and during subsequent pregnancies following stillbirth has the potential to be highly diverse, addressing a range of risk factors, conditions, and other considerations. First, counselling on stillbirth recurrence risk may facilitate informed decision‐making for parents considering a pregnancy subsequent to stillbirth (Fockler 2017; Paull 2013). Such counselling may include information on interpregnancy interval, preconception health, and the risks and benefits of delaying a subsequent pregnancy in each unique case. For women who become pregnant, understanding the cause of the index stillbirth (if known) will facilitate the development of an individualised management plan in the subsequent pregnancy to address the cause directly, and therefore reduce the likelihood of recurrence. For pre‐existing maternal conditions that are likely to recur (e.g. diabetes), stabilisation of the condition may reduce stillbirth recurrence risk. Cessation of smoking and pre‐conception interventions addressing maternal overweight and obesity may also reduce risk (Monari 2010). Where no cause of death for the index stillbirth has been identified, frequent monitoring may enable early detection of developing complications and may prompt expedited birth where appropriate (Fockler 2017; Robson 2010). Interventions designed to improve maternal mental health may reduce stress in pregnancy, lessening the likelihood of adverse effects such as low birthweight and preterm birth, while also enhancing maternal‐fetal attachment. Additional antenatal visits, for example, may provide parents with more opportunities for reassurance, and have been welcomed by parents in pregnancies subsequent to stillbirth or neonatal death (Meaney 2017; Mills 2014).
Interventions might be provided in isolation or in combination; for example, in the form of a specialised model of care or a dedicated clinic for families entering a pregnancy after loss (e.g. Meredith 2017).
Why it is important to do this review
Despite the known risk of stillbirth recurrence and the far‐reaching impacts of stillbirth on subsequent pregnancies and beyond, there is a paucity of information on care prior to and during these pregnancies to improve health outcomes. Women who are pregnant after having a previous stillbirth, and their partners, comprise a small but unique group who may benefit from specialised and individualised care both medically and psychosocially, but there are currently little data to inform clinical practice.
Objectives
To assess the effects of different interventions or models of care prior to and during subsequent pregnancies following stillbirth on maternal, fetal, neonatal and family health outcomes, and health service utilisation.
Methods
Criteria for considering studies for this review
Types of studies
Eligible trial designs included randomised controlled trials (RCTs), quasi‐randomised controlled trials (qRCTs), and cluster‐randomised trials. We excluded cross‐over trials. We included trials published as abstract only, provided sufficient information was available to allow us to assess trial eligibility and risk of bias.
Types of participants
Parents who had experienced a stillbirth of 20 weeks' gestation or more who were pregnant or considering a subsequent pregnancy. We included trials with parents who had experienced a pregnancy loss prior to 20 weeks' gestation, or a neonatal death, alongside parents who had experienced a stillbirth according to our definition (i.e. a death of 20 weeks' gestation or more) only if participant data relating to previous stillbirths of 20 weeks' gestation or more could be disaggregated from the broader trial populations.
Types of interventions
We included any single intervention, combination of interventions or tailored model of care/algorithm/guideline/protocol for improving health outcomes in subsequent pregnancies following stillbirth, compared with no intervention or standard care.
We also included studies where one intervention/combination of interventions/tailored model of care was compared with another.
For the trials captured in this review, all interventions began prior to or during pregnancy. For future updates of this review, we will also include studies in which the intervention/model of care began during labour and birth or shortly after birth, if we find such trials.
Eligible interventions for this review could include, for example, targeted management to address previous causes or circumstances of prior stillbirth (e.g. diabetes, hypertensive disorders); care to address high‐risk behaviours/risk factors (e.g. obesity, smoking); care focused on fetal surveillance and timing and mode of birth; and care to address specific psychosocial needs (See above Description of the intervention for further details).
Types of outcome measures
We assessed the following outcomes.
Primary outcomes
Stillbirth;
Neonatal death;
Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity such as hypoxic‐ischaemic encephalopathy; intracranial haemorrhage; retinopathy of prematurity; necrotising enterocolitis);
Adverse maternal psychological effects (anxiety, depression or complicated grief).
Secondary outcomes
Fetal, neonatal and childhood outcomes
Perinatal mortality
Preterm birth (any preterm birth; very preterm birth; late preterm birth)
Birthweight, low birthweight, small‐for‐gestational age
Apgar score less than seven at five minutes
Respiratory distress syndrome
Neonatal jaundice
Psychological and behavioural problems in childhood
Anxiety or depression or both in childhood
Long‐term neurodevelopmental and educational outcomes
Quality of life
Maternal outcomes
Adherence to the intervention (process outcomes) (i.e. smoking cessation; lifestyle changes – changes in diet, physical activity, weight loss) (pre‐pregnancy and during pregnancy)
Caesarean birth (elective; emergency)
Induction of labour
Instrumental vaginal birth
Placental abruption
Pre‐eclampsia
Gestational diabetes
Chorioamnionitis
Postpartum haemorrhage
Satisfaction with care
Serious maternal outcome (composite outcome including death, cardiac arrest, respiratory arrest, admission to intensive care)
Breastfeeding
Maternal‐infant attachment
Quality of life
Health service utilisation
Antenatal care attendance
Maternal antenatal admission
Duration of maternal hospital stay (days)
Duration of neonatal hospital stay (days)
Admission to the neonatal intensive care unit
Duration of neonatal intensive care unit stay (days)
Antenatal ultrasound scans
Cost
Family outcomes
Partner anxiety, depression or complicated grief
Partner quality of life
Relationship breakdown/disharmony
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth's Trials Register by contacting their Information Specialist (6 June 2018).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth's Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (18 June 2018) for unpublished, planned, and ongoing trial reports using the terms given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
We used the following methods for assessing studies identified by the search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved disagreements through discussion or, where required, by consulting a third review author.
We prepared a PRISMA study flow diagram to map out the number of records identified, included and excluded (Liberati 2009); see Figure 1.
Data extraction and management
We designed purpose‐built electronic forms to manage data extraction. For eligible studies, two review authors extracted the data using the agreed form(s), or sourced the required data directly from trialists. We resolved discrepancies through discussion or, where required, by referral to a third review author. We entered the data into Review Manager 5 software (RevMan 2014) and checked them for accuracy. When information about any of the above was absent or unclear, we attempted to contact trialists to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook) (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
We assessed the following domains.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random‐number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
We note 'Partial' blinding, if identified.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes; in this review as 'objective' and 'subjective' outcomes (a subjective outcome being one that requires some level of human judgement).
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or could be supplied by trialists, we included missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
We found no cluster‐randomised controlled trials for inclusion in this review. If we identify cluster‐randomised controlled trials in future updates, we will assess risks of bias according to the criteria given in the Handbook (Higgins 2011).
Assessment of the quality of the evidence using the GRADE approach
We evaluated the quality of the evidence using the GRADE approach as outlined in the GRADE handbook. The GRADE approach uses five considerations (trial limitations (risk of bias); consistency of effect; imprecision; indirectness; and publication bias) to assess the quality of the body of evidence for specific outcomes. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations. In this review we used the GRADE approach to assess the following outcomes.
Stillbirth.
Neonatal death.
Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity such as hypoxic‐ischaemic encephalopathy; intracranial haemorrhage; retinopathy of prematurity; necrotising enterocolitis).
Adverse maternal psychological effects (anxiety, depression or complicated grief).
Perinatal mortality.
Any preterm birth (birth < 37 weeks).
Maternal‐infant attachment.
We conducted assessments of the quality of evidence for two comparisons: (1) low‐molecular‐weight heparin (LMWH) versus no treatment/standard care (main comparison); and (2) low‐dose aspirin (LDA) versus placebo. We selected the main comparison for assessment based on its having the highest number of included studies. We selected the second comparison based on its relevance to modern clinical practice, given the already widespread use of aspirin for the prevention of placenta‐mediated complications (Askie 2007; Bujold 2010; Roberge 2013; Roberge 2016), which has been extended to the prevention of recurrent stillbirth (Fockler 2017).
We used GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create a 'Summary of findings' table. We present a summary of the intervention effect and a measure of quality according to the GRADE approach in a 'Summary of findings' table for each of the above outcomes.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with a 95% confidence interval.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but we may include trials of this type in future updates. If cluster‐randomised trials are included, we will carry out analyses alongside individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Higgins 2011), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and if the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
We also plan to include multi‐armed trials, ensuring analyses are independent. If we include multi‐armed trials, we will split the 'shared' group into two or more groups with smaller sample size, and include two or more (reasonably independent) comparisons. Alternatively, we will combine groups to create a single pair‐wise comparison.
Cross‐over trials
We exclude cross‐over designs as these are unlikely to be a valid study design for Pregnancy and Childbirth reviews.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis, i.e. we attempted to include all participants randomised to each group in the analyses, regardless of whether they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
For some of the outcomes in this review (e.g. caesarean section, induction of labour, and various fetal and neonatal outcomes), some participants were known to be ineligible for inclusion (e.g. due to miscarriage or other pregnancy loss). To account for this, we conducted analyses both using the as‐randomised denominators and, where provided by trialists, the revised denominators, i.e. removing from the denominators any participants who could not have contributed data. We performed sensitivity analyses to assess the impact of the choice of denominators. Whether the data were analysed using the as‐randomised or revised denominators did not influence any of the overall results. We therefore present the data using the revised denominators.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if an I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. Heterogeneity statistics are reported where data were available for more than one trial in the meta‐analysis. Where there were no events or where events were reported in only one trial within a meta‐analysis (e.g. in only one trial was there any reported pre‐eclampsia), we state 'heterogeneity: not applicable'.
Assessment of reporting biases
The meta‐analyses in the current review included a maximum of three trials. In future updates of this review, if there are 10 or more trials in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials' populations and methods to be sufficiently similar.
In future updates of this review, if there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if we detect substantial statistical heterogeneity, we will use random‐effects meta‐analysis to produce an overall summary, if we consider an average treatment effect across trials to be clinically meaningful. We will treat the random‐effects summary as the average of the range of possible treatment effects, and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and to consider whether an overall summary was meaningful, and if so, to use random‐effects analysis to produce it.
We planned the following subgroup analyses for the review's primary outcomes:
cause(s) of previous stillbirth: known recurrent cause(s) versus known non‐recurrent cause(s) versus unexplained stillbirth;
setting: low‐ or middle‐income country versus high‐income country;
psychosocial support: included in intervention versus not included (for interventions not primarily focused on psychosocial support); and
timing of start or duration of the intervention: pre‐pregnancy versus during pregnancy versus during delivery.
For this version of the review, we were unable to conduct the above subgroup analyses due to lack of variation in these factors across the comparison, unavailability of data, or due to there being no reported events across the primary outcomes.
In future updates of this review, we will conduct the following additional subgroup analyses:
subsequent pregnancy order: immediate subsequent pregnancy versus any subsequent pregnancy;
target of intervention: mother versus partner.
We will explore subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
Sensitivity analysis
We did not conduct any planned sensitivity analyses in this review due to the low number of included trials. In future updates of this review we will carry out sensitivity analyses to explore the effects of high attrition rates with trials showing attrition greater than 20% excluded from the analyses, to assess whether this makes any difference to the overall result. We will also carry out sensitivity analyses to explore the effect of bias (including for quasi‐randomised trials), assessed by random‐sequence generation and concealment of allocation, with trials assessed as high or unknown risk of bias for these domains being excluded from the analyses. Where ICCs are used, we will carry out sensitivity analyses to explore the effects of variation in ICC values and in the randomisation unit (i.e. individual versus cluster). We will limit all sensitivity analyses to the primary outcomes.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Trials Register retrieved 272 reports, equating to 177 unique trials (as some trials were published in multiple reports). A further 90 and 75 records were retrieved from our searches of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP), respectively. We removed duplicate records and clearly ineligible trials at the screening stage. Where there was insufficient information to assess eligibility as part of abstract screening, we progressed the trials to full‐text review. See: Figure 1.
1.
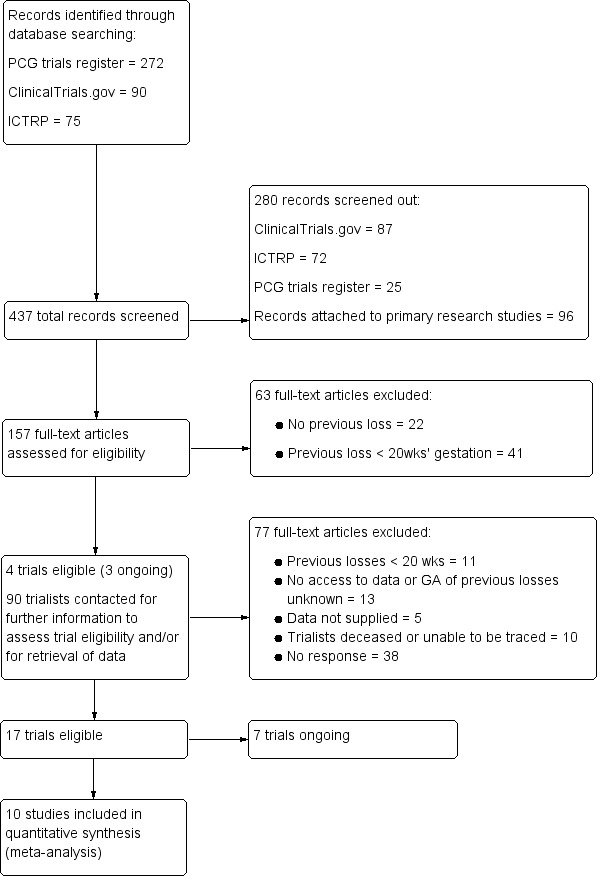
PRISMA study flow diagram.
GA: gestational age; ICTRP: WHO International Clinical Trials Registry Platform; PCG: Pregnancy and Childbirth Group
We reviewed a total of 157 trials in full text. As reports on many of the trials used terms such as previous 'pregnancy loss', 'miscarriage', 'abortion', and 'fetal death' without defining these by gestational age, or as they included women with previous pregnancy losses at various gestational ages, it was unclear whether these trials would be eligible for inclusion. We progressed such trials to a second phase of full‐text review, whereby we contacted the associated trialists for further information about the trial populations, and/or to determine whether the data from parents who experienced a previous stillbirth of 20 weeks' gestation or more could be disaggregated from those of the broader trial populations. We set a deadline for responses from trialists and, if we did not receive a response by this time, we excluded the trial. See: Figure 1.
We attempted to contact the primary trialist (or an identified colleague or co‐author of the primary trialist) at least twice before excluding the trial on the basis on non‐response. Given that the potentially eligible trials were carried out from the year 1964 onwards, we took care to identify up‐to‐date contact details, where such details were not readily available through the published reports. We sought trialists' current institution and email address through Google searches or searches in PubMed, or both, for recent publications from the same author, co‐author, or colleague.
We ultimately included 10 trials in the review (Ahmed 2014; Christiansen 1994; Christiansen 1995; Christiansen 2002; Gris 2004; Levine 1964; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015) and describe them in detail below (See Included studies and Characteristics of included studies).
We identified a further seven ongoing trials. These trials were being undertaken in Australia (McLindon 2011); Brazil (Alves 2014); Canada (Rodger 2017); Egypt (El‐refaie 2016); Netherlands (De Jong 2015); and UK (Hezelgrave 2016 and Schreiber 2017). The interventions assessed in these ongoing trials were:
twice daily magnesium citrate capsule starting from 12 to 20 weeks' gestation and continuing until birth, compared to placebo (Alves 2014);
daily 40 mg LMWH subcutaneous injection starting immediately after randomisation upon confirmation of pregnancy, compared to standard pregnancy surveillance (alone) (De Jong 2015);
daily 400 mg vaginal progesterone suppository compared to cervical cerclage (El‐refaie 2016);
cervical cerclage compared to daily 200 mg vaginal progesterone compared to cervical pessary (three‐armed trial) (Hezelgrave 2016);
nightly 400 mg progesterone pessary from seven to 12 weeks' gestation compared to placebo (McLindon 2011);
daily 81 mg LDA from randomisation until birth compared to LMWH and daily LDA (LMWH dosages at discretion of attending physician) until 37 weeks' gestation (Rodger 2017);
oral hydroxychloroquine compared to placebo (Schreiber 2017).
The Rodger 2017 trial is a feasibility study, aiming to determine the feasibility of a future multicentre trial of LDA prophylaxis for recurrent pregnancy loss. For further details in all ongoing trials, see Characteristics of ongoing studies.
Included studies
Design
Of the 10 included trials, nine were RCTs and one trial (Levine 1964) was a quasi‐RCT using alternate allocation. One trial (Ahmed 2014) adopted a three‐arm design, comparing LDA to combined LDA and LMWH, and placebo.
Sample sizes
Sample sizes for the individual trials ranged from 34 (Christiansen 1995) to 449 women (Schleussner 2015). After extraction of data exclusively from women who had experienced a previous stillbirth of 20 weeks' gestation or more, sample sizes for the individual trials ranged from four (Christiansen 1994) to 45 women (Rey 2009).
Setting
The trials were undertaken in hospitals and obstetric referral clinics in Canada (Levine 1964; Rey 2009), Denmark (Christiansen 1994; Christiansen 1995; Christiansen 2002), France (Gris 2004), Israel (Salim 2016), Italy (Martinelli 2012), and Pakistan (Ahmed 2014). One trial was undertaken across both Austria and Germany (Schleussner 2015).
Dates of trials, funding and declarations of interest
The trials were carried out between the years 1964 and 2015. With the exception of Levine 1964, all trials were carried out from 1994 onwards.
Funding sources were reported in seven trials (Christiansen 1994; Christiansen 1995; Christiansen 2002; Gris 2004; Martinelli 2012; Rey 2009; Schleussner 2015). Sources of funding included pharmaceutical companies (Martinelli 2012; Rey 2009; Schleussner 2015), community charities (Christiansen 1994; Christiansen 1995; Christiansen 2002), and research grants/institutional funding (Gris 2004). All trials that reported pharmaceutical company funding stated that the funding body was not involved the study's design, analysis, interpretation, or reporting of data. One trial (Levine 1964) did not report funding sources. Salim 2016 was supported by Emek Medical Centre and the Ahmed 2014 trial reported that no funding was received (information obtained upon correspondence with trialists).
For six trials (Ahmed 2014; Christiansen 1994; Christiansen 1995; Christiansen 2002; Gris 2004; Levine 1964), the published reports did not state whether the trialists had any declarations of interest. With the exception of Levine 1964, these trialists confirmed through correspondence that there were no declarations of interest. Declarations of interest were included in the published reports for two trials (Rey 2009; Schleussner 2015), including speakers' honoraria, research grants, and personal fees from pharmaceutical companies such as Pfizer (see Characteristics of included studies). The authors of Salim 2016 declared no conflicts of interest and the authors of Martinelli 2012 declared 'no competing financial interests'.
Participants
All participants were women who were either pregnant or attempting to conceive following pregnancy loss, fetal death, or an adverse outcome in a previous pregnancy. Most of the trials were focused on recurrent idiopathic/unexplained pregnancy loss (including 'miscarriage' and 'abortion'). Recurrent pregnancy loss was variably defined, in terms of the number of previous deaths, their gestational age cut‐off points, and whether the deaths were consecutive (see Characteristics of included studies).
Of the six trials (Ahmed 2014; Gris 2004; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015) assessing LDA or LMWH or both, Ahmed 2014 included women with two or more previous consecutive, unexplained pregnancy losses prior to 24 weeks' gestation. Gris 2004 targeted women with a prior unexplained fetal death and a constitutional thrombophilic disorder, and included women with one single unexplained pregnancy loss of at least 10 weeks' gestation. Martinelli 2012, Rey 2009, and Salim 2016 targeted women with previous placenta‐mediated complications including pre‐eclampsia, fetal growth restriction (FGR), low‐birthweight, placental abruption, and fetal death (with varying definitions). Rey 2009 excluded women with current thrombophilic disorders, whereas Gris 2004 included these women, provided that such disorders were not associated with previous pregnancy losses.
Schleussner 2015 included women who had at least two consecutive pregnancy losses prior to 12 weeks' gestation or one pregnancy loss at 12 weeks' gestation or more. Salim 2016 included women who had three losses prior to 13 weeks' gestation, two losses between 14 and 22 weeks' gestation, or any pregnancy loss after 23 weeks' gestation. Gris 2004, Martinelli 2012, Rey 2009, Salim 2016, and Schleussner 2015 excluded women for whom previous pregnancy losses could be explained by specific maternal conditions or clinical findings. Such factors varied between the trials, but included infectious diseases, endocrinological and immunological disorders, chromosomal abnormalities, and alcohol or illicit drug use. Women with an absolute need for heparin were excluded from Martinelli 2012, Rey 2009, Salim 2016, and Schleussner 2015. Rey 2009, Salim 2016, and Schleussner 2015 further excluded women with allergies or other contra‐indications to the given interventions. Exclusion criteria for Ahmed 2014 were not stated.
Levine 1964 assessed progestogen and included women with three consecutive unexplained pregnancy losses (from six weeks to full‐term of pregnancy) and no symptoms of threatened pregnancy loss at the time of study enrolment.
Christiansen 1994, which assessed third‐party leukocyte immunisation, included women who had three consecutive unexplained pregnancy losses and a maximum of one pregnancy loss after 14 weeks' gestation. Women with antiphospholipid syndrome (APS), including lupus anticoagulant and anticardiolipin antibodies, were excluded.
Christiansen 1995 and Christiansen 2002 assessed intravenous immunoglobulin G (IgG). Both trials included women who had a history of recurrent unexplained miscarriages (with varying definitions) and no existing immunoglobulin A (IgA) deficiency. Additional inclusion and exclusion criteria are detailed in Characteristics of included studies.
Interventions and comparisons
Anticoagulant and antiplatelet agents
LDA was assessed in two trials (Ahmed 2014; Gris 2004). LDA was administered orally at 75 mg (Ahmed 2014) and 100 mg (Gris 2004) daily. LMWH was assessed in six trials (Ahmed 2014; Gris 2004; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015). The LMWH agents administered included enoxaparin (Ahmed 2014; Gris 2004; Salim 2016), nadroparin (Martinelli 2012), and dalteparin (Rey 2009; Schleussner 2015). All were self‐administered by subcutaneous injection. LMWH dosages ranged from 3800 IU to 6000 IU, depending on pre‐pregnancy bodyweight (with the exception of Salim 2016 ‐ see below). Where stated, injections were started in early pregnancy and ceased from 24 weeks' gestation (Schleussner 2015) to 36 weeks' gestation or birth (whichever came first) (Ahmed 2014; Rey 2009; Salim 2016).
Gris 2004 compared LDA to LMWH, while Martinelli 2012; Rey 2009; and Schleussner 2015 compared LMWH to no treatment/standard care. For Martinelli 2012, standard care included LDA intake and medical surveillance through monthly visits. Women in the control group in Schleussner 2015 received multivitamins containing folic acid. Salim 2016 compared an adjusted dose of LMWH according to anti‐factor Xa levels (a measurement of plasma LMWH), to a fixed dose of 40 mg LMWH a day. All women in Salim 2016 who had anti‐phospholipid antibodies were also given LDA.
In the three‐armed trial reported in Ahmed 2014, LDA and LMWH were administered as described above. Women in the placebo arm received intensive pregnancy surveillance alongside a matching schedule of placebo tablets. No such placebo injections were described for the LMWH arm.
Third‐party leukocyte immunisation
Christiansen 1994 assessed immunisations with 150 mL leukocyte‐enriched blood from erythrocyte‐compatible third‐party blood donors. Immunisations were administered on an outpatient basis and started pre‐conception and continued until conception. The control group received a matching schedule of placebo injections.
Intravenous IgG
Christiansen 1995 and Christiansen 2002 assessed the use of Nordimmun, a human IgG preparation administered on an outpatient basis. Doses varied according to pre‐pregnancy bodyweight or gestational age, or both. Infusions were started early in pregnancy on a weekly‐basis and progressed to a fortnightly basis from eight weeks' gestation in Christiansen 1995 and from 10 weeks' gestation in Christiansen 2002. Infusions ceased at 26 weeks' gestation in Christiansen 2002 and at 34 weeks' gestation in Christiansen 1995. In both trials, the control group received a matching schedule of placebo injections.
Progestogen
Levine 1964 assessed progestogen (Delalutin). Women received a weekly 500 mg injection from their first visit until the 36th week of pregnancy or until termination of pregnancy/pregnancy loss. The control group received a weekly placebo injection.
Outcomes
With the exception of Levine 1964, we obtained all data presented in our meta‐analyses through contacting trialists. We supplied each trialist with a tailored data‐request form seeking the required, prespecified outcome data, and any information on trial methods and procedures that could not be gleaned from the published reports, or that required clarification. Where they were measured, data for most of the prespecified review outcomes were made available and are included in the current analyses.
Data on stillbirths were available for all 10 trials. Data on neonatal deaths were available for nine of the 10 trials, and data on adverse perinatal outcomes were available for eight of the 10 trials. Data on adverse maternal psychological effects were only available from Martinelli 2012. Most trials measured important secondary outcomes, including preterm birth, induction of labour, and serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care). No trials measured maternal‐fetal attachment, or longer‐term outcomes of children, such as psychological and behavioural problems, anxiety, depression, or neurodevelopmental problems. No trials measured economic costs of interventions, nor quality of life for mothers, partners, or children.
Levine 1964 reported individual participant data (from which we included only data from women who had a previous stillbirth of 20 weeks' gestation or more). We were unable to retrieve further information about the trial methodology or about outcomes that were not reported in the study report. In addition, data from this trial are reported descriptively only, due to ambiguity in the data for two of the primary outcomes (stillbirth; adverse perinatal outcome). Specifically, it was reported that one woman in the control group had an abortion, but the gestational age at which the death occurred was unclear (the death occurred after the 10th weekly progestogen injection, but the time at which injections began was not reported).
Excluded studies
We excluded 140 trials following full‐text review, or after contacting trialists for further information to determine eligibility. Trials were most commonly excluded based on the women's previous pregnancy losses occurring at less than 20 weeks' gestation (52 trials; 37%). Twenty‐two trials (16%) recruited women who had not experienced a previous pregnancy loss. Of the 90 trialists contacted for further information to determine eligibility, 38 (42%) did not respond. For 13 trials (14%), the trialists no longer had access to the trial data and/or did not collect data on the gestational age of women's previous pregnancy losses. Ten trialists (11%) were deceased or could not be traced, and five trialists (6%) declined to provide data for this review. Reasons for declining to provide data were: lack of funding/resources to carry out the required subgroup analyses; trialist illness; concern over the validity of the review methodology; having already provided data for other reviews; and reasons unknown.
Risk of bias in included studies
We judged the risk of bias in the trials for methodology and reporting to be low to moderate. For a summary of the risks of bias across the included trials, see Figure 2 and Figure 3.
2.
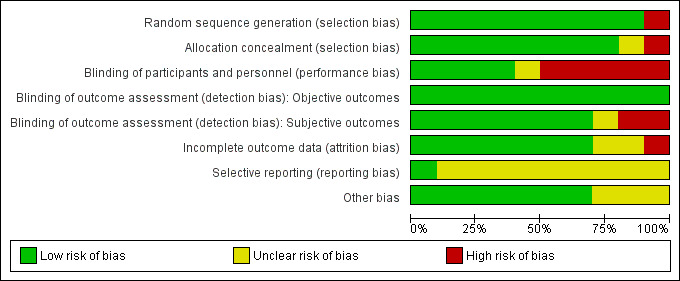
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
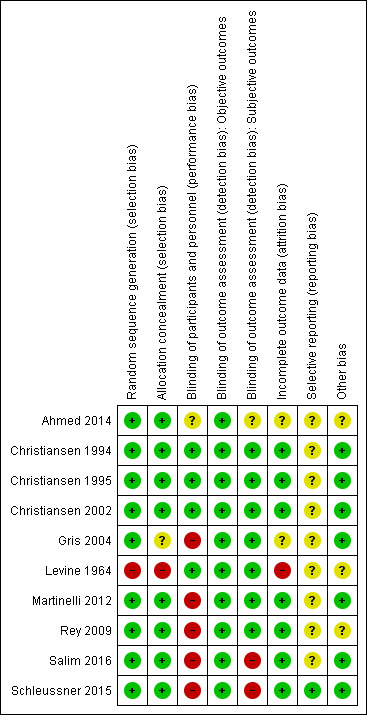
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
With the exception of Levine 1964, which used an alternate allocation procedure, all trials described an adequate process of random sequence generation, using computer‐generated programmes. We therefore assessed Levine 1964 to be at high risk for both domains of selection bias. Insufficient details were available to assess the adequacy of allocation concealment for Gris 2004. The remaining trials described adequate methods for allocation concealment, including central allocation (Martinelli 2012; Schleussner 2015) and sealed, opaque, consecutively‐labelled envelopes (Ahmed 2014; Christiansen 1995; Rey 2009).
Blinding
We rated Christiansen 1994, Christiansen 1995, Christiansen 2002, and Levine 1964, all placebo‐controlled trials, at low risk of performance and detection bias. The parallel trials assessing LMWH therapies (Gris 2004; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015) were all unblinded, and we therefore judged them to be at high risk of performance bias. Adequacy of blinding procedures for Ahmed 2014 was unclear. Specifically, while women in the control arm of this trial received placebo tablets matching those provided in the LDA arm, there did not appear to be a schedule of placebo injections matching those provided in the combined LDA and LMWH arm. When considering objective outcomes only (e.g. stillbirth, neonatal death), we assessed all trials to be at low risk of performance and detection bias.
Incomplete outcome data
We considered seven of the 10 trials (Christiansen 1994; Christiansen 1995; Christiansen 2002; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015) to be at low risk of attrition bias. Risk of attrition bias in Ahmed 2014 and Gris 2004 was unclear. Loss of follow‐up and reasons for loss of follow‐up were reported in both trials, but the groups to which participants had been allocated was unclear. We rated Levine 1964 at high risk of attrition bias, due to the high proportion of women (46%) who were excluded post‐randomisation.
Selective reporting
With the exception of Schleussner 2015, we judged the risk of reporting bias in all trials to be unclear. No trial protocols were available for Ahmed 2014, Christiansen 1994, Christiansen 1995, Christiansen 2002, Gris 2004, or Levine 1964, while we noted only retrospective trial registration for Rey 2009 and Salim 2016. We judged Martinelli 2012 to have unclear risk of reporting bias, due to conflicting information about the primary outcome reported in the published report compared to that given in conference reports. Limited details were available in the trial protocol for clarification, and one outcome (changes in platelet count (PLT) or aspartate aminotransferase (AST)/alanine aminotransferase (ALT)) appeared to be missing from the published report. All outcomes were reported as prespecified in the trial protocol for Schleussner 2015, which we considered to be at low risk of reporting bias.
Other potential sources of bias
We did not identify other sources of bias for seven of the 10 trials (Christiansen 1994; Christiansen 1995; Christiansen 2002; Gris 2004; Martinelli 2012; Salim 2016; Schleussner 2015). Risk of other bias was unclear in Rey 2009, where there appeared to be some baseline imbalance for LDA use and ethnicity. This trial was stopped early following slow recruitment and favourable interim analyses. The trialists noted "We are aware that stopping the study may have led to exaggerated effect sizes" (study report p. 63). The Martinelli 2012 trial was stopped early due to futility and safety concerns, following review by the trial Data and Safety Monitoring Board. However, in accordance with the Handbook (Higgins 2011), we considered the influence of early trial cessation as part of our GRADE assessments of the quality of evidence, and not as part of our 'Risk of bias' assessments.
Ahmed 2014 and Levine 1964 provided insufficient methodological detail to allow us to determine other potential sources of bias.
Effects of interventions
for the main comparison.
| Low‐molecular‐weight heparin compared to no treatment/standard care for improving outcomes | ||||||
| Patient or population: women with a previous stillbirth of ≥ 20 weeks' gestation who are pregnant or considering a subsequent pregnancy Setting: obstetric units and outpatient clinics in Germany, Austria, Canada, and Italy Intervention: low‐molecular‐weight heparin Comparison: no treatment/standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment/standard care | Risk with low‐molecular‐weight heparin | |||||
| Stillbirth | Study population | RR 2.58 (0.40 to 16.62) | 122 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | ‐ | |
| 17 per 1000 | 43 per 1000 (7 to 277) | |||||
| Neonatal death | Study population | ‐ | 122 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b,d | No neonatal deaths reported | |
| see comment | see comment | |||||
| Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | Study population | RR 0.81 (0.20 to 3.32) | 77 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b,c,e | ‐ | |
| 100 per 1000 | 85 per 1000 (13 to 577) | |||||
| Adverse maternal psychological effects (anxiety, depression or complicated grief) | Study population | RR 1.00 (0.07 to 14.90) | 40 (1 RCT) | ⊕⊝⊝⊝ VERY LOWf,g,h | ‐ | |
| 50 per 1000 | 50 per 1000 (4 to 745) | |||||
| Perinatal mortality | Study population | RR 2.58 (0.40 to 16.62) | 122 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | ‐ | |
| 17 per 1000 | 43 per 1000 (7 to 277) | |||||
| Any preterm birth (birth < 37 weeks) | Study population | RR 1.01 (0.58 to 1.74) | 114 (3 RCTs) | ⊕⊕⊝⊝ LOWa,c,i | ‐ | |
| 304 per 1000 | 310 per 1000 (152 to 638) | |||||
| Maternal‐infant attachment ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome was not measured in the trials included |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially differentj is low Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differentj is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differentj is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differentj is very high. | ||||||
aUnblinded trials (trial limitations), although not downgraded for this as outcome is objective. bWomen who investigators judged would have poor adherence were also excluded from one trial (trial limitations), although not downgraded for this. c(‐2) Downgraded for 'very serious' imprecision: small number of participants, small number of trials, wide confidence intervals crossing the line of no effect, few and/or no events, early cessation of trial(s). d(‐2) Downgraded for 'very serious' imprecision: small number of participants, small number of trials, no events, early cessation of trial(s). eOpposite directions of effect between trials (inconsistency), although not downgraded for this as confidence intervals overlap and cross the line of no effect; 12 = 31%. fOnly one trial included, therefore inconsistency cannot be assessed. g(‐1) Downgraded for 'serious' trial limitations: unblinded trial, subjective outcome. h(‐2) Downgraded for 'very serious' imprecision: single, small trial with wide confidence interval crossing the line of no effect, few events, early cessation of trial. iOpposite directions of effect between trials (inconsistency), although not downgraded for this as confidence intervals overlap and cross the line of no effect; 12 = 35%. jSubstantially different = a large enough difference that it might affect a decision.
2.
| Low‐dose aspirin compared to placebo for improving outcomes in subsequent pregnancies following stillbirth | ||||||
| Patient or population: women with a previous stillbirth of > 20 weeks' gestation who are pregnant or considering a subsequent pregnancy Setting: tertiary referral obstetric hospital, Pakistan Intervention: low‐dose aspirin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with low‐dose aspirin | |||||
| Stillbirth | Study population | RR 0.85 (0.06 to 12.01) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 91 per 1000 | 77 per 1000 (5 to 1000) | |||||
| Neonatal death | Study population | RR 0.29 (0.01 to 6.38) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 91 per 1000 | 26 per 1000 (1 to 580) | |||||
| Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | Study population | RR 0.28 (0.03 to 2.34) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 273 per 1000 | 76 per 1000 (8 to 638) | |||||
| Adverse maternal psychological effects (anxiety, depression or complicated grief) ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome was not measured in this trial |
| Perinatal mortality | Study population | RR 0.42 (0.04 to 4.06) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 182 per 1000 | 76 per 1000 (7 to 738) | |||||
| Any preterm birth (birth < 37 weeks) | Study population | RR 0.42 (0.04 to 4.06) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ‐ | |
| 182 per 1000 | 76 per 1000 (7 to 738) | |||||
| Maternal‐infant attachment ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome was not measured in this trial |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially differentd is low Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially differentd is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially differentd is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially differentd is very high. | ||||||
a(‐1) Downgraded for 'serious' trial limitations: unclear attrition bias and selective reporting. Details on blinding of participants and personnel unclear for the enoxaparin arm (three‐armed trial ‐ not downgraded for this as possible lack of blinding for enoxaparin does not affect the current comparison; outcome is objective). bOnly one trial included, so inconsistency cannot be assessed. c(‐2) Downgraded for 'very serious' imprecision: single, small trial with wide CIs crossing the line of no effect, and few events. dSubstantially different = a large enough difference that it might affect a decision.
We undertook analyses as follows.
Comparison 1: LMWH versus no treatment/standard care (Martinelli 2012; Rey 2009; Schleussner 2015).
Comparison 2: LDA versus placebo (Ahmed 2014).
Comparison 3: LDA + LMWH versus LDA alone (Ahmed 2014).
Comparison 4: LDA + LMWH versus placebo (Ahmed 2014).
Comparison 5: LMWH versus LDA (Gris 2004).
Comparison 6: LMWH (dose adjusted according to anti‐factor Xa levels) versus LMWH (fixed dose) (Salim 2016).
Comparison 7: Third‐party leukocyte immunisation versus placebo (Christiansen 1994).
Comparison 8: Intravenous IgG versus placebo (Christiansen 1995; Christiansen 2002).
Comparison 9: Progestogen versus placebo (Levine 1964).
We conducted assessment of the quality of evidence for comparisons 1 and 2, and judged them to be very low to low, in accordance with the GRADE approach (see Table 1 and Table 2). All but one of the comparisons (comparison 1) were based on data from only one or two trials, all with extremely small sample sizes. Very serious imprecision in the data was evident for all comparisons, including comparison 1. It was therefore largely uncertain whether there was any benefit or harm across the interventions assessed in this review (regardless of GRADE assessments).
Comparison 1: LMWH versus no treatment/standard care
Three trials contributed to the comparison of LMWH versus no treatment/standard care (Martinelli 2012; Rey 2009; Schleussner 2015).
A total of 123 women and their infants were included, depending on the outcome. The numbers of trials and participants by outcome are provided below.
Primary outcomes
Due largely to the imprecision in the data available, it was uncertain whether LMWH reduced the risk of stillbirth (risk ratio (RR) 2.58, 95% confidence interval (CI) 0.40 to 16.62; 3 trials; 122 participants; Analysis 1.1; low‐quality evidence), adverse perinatal outcome (RR 0.81, 95% CI 0.20 to 3.32; 2 trials; 77 participants; Analysis 1.3; low‐quality evidence), or adverse maternal psychological effects (RR 1.00, 95% CI 0.07 to 14.90; 1 trial; 40 participants; Analysis 1.4; very low‐quality evidence), when compared to no treatment/standard care. No neonatal deaths were reported.
1.1. Analysis.
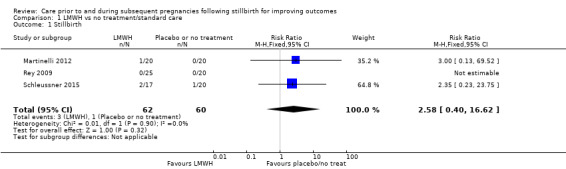
Comparison 1 LMWH vs no treatment/standard care, Outcome 1 Stillbirth.
1.3. Analysis.
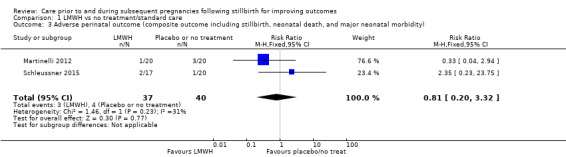
Comparison 1 LMWH vs no treatment/standard care, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
1.4. Analysis.
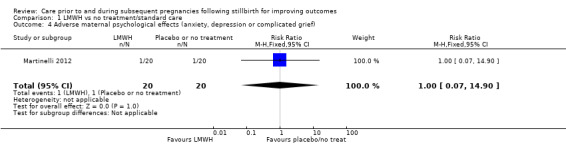
Comparison 1 LMWH vs no treatment/standard care, Outcome 4 Adverse maternal psychological effects (anxiety, depression or complicated grief).
Secondary outcomes
The effect of LMWH on the risk of perinatal mortality (RR 2.58, 95% CI 0.40 to 16.62; 3 trials; 122 participants; Analysis 1.5; low‐quality evidence) and any preterm birth < 37 weeks (RR 1.01, 0.58 to 1.74; 3 trials; 114 participants; Analysis 1.8; low‐quality evidence) was also uncertain.
1.5. Analysis.
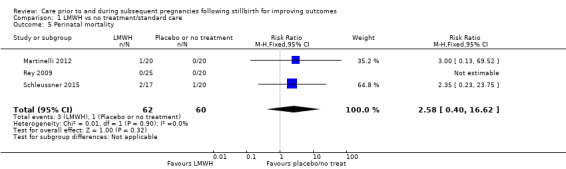
Comparison 1 LMWH vs no treatment/standard care, Outcome 5 Perinatal mortality.
1.8. Analysis.
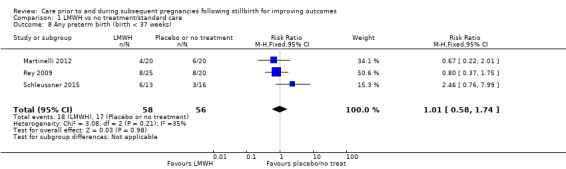
Comparison 1 LMWH vs no treatment/standard care, Outcome 8 Any preterm birth (birth < 37 weeks).
There was no clear evidence of a difference between the groups on the remaining secondary outcomes: very preterm birth (28 to < 32 weeks) (RR 0.94, 95% CI 0.31 to 2.82; 3 trials; 114 participants; Analysis 1.6); late preterm birth (32 to < 37 weeks) (RR 0.78, 95% CI 0.36 to 1.66; 3 trials; 114 participants; Analysis 1.7); birthweight (mean difference (MD) −225.26 g, 95% CI −546.36 to 95.84 g; 3 trials; 109 participants; Analysis 1.9); low birthweight (RR 1.00, 95% CI 0.45 to 2.21; 2 trials; 85 participants; Analysis 1.10); small‐for‐gestational age (RR 1.32, 95% CI 0.57 to 3.08; 3 trials; 115 participants; Analysis 1.11); Apgar score less than seven at five minutes (RR 3.33, 95% CI 0.58 to 19.29; 2 trials; 69 participants; Analysis 1.12); adherence to the intervention (RR 1.03, 95% CI 0.88 to 1.22; 2 trials; 85 participants; Analysis 1.15); caesarean birth (elective) (RR 2.05, 95% CI 0.83 to 5.07; 3 trials; 115 participants; Analysis 1.16); caesarean birth (emergency) (RR 0.88, 95% CI 0.39 to 1.99; 3 trials; 115 participants; Analysis 1.17); induction of labour (RR 0.64, 95% CI 0.33 to 1.22; 2 trials; 85 participants; Analysis 1.18); Instrumental vaginal birth (RR 3.00, 95% CI 0.13 to 69.52; 1 trial; 40 participants; Analysis 1.19); placental abruption (RR 0.32, 95% CI 0.04 to 2.91; 3 trials; 115 participants; Analysis 1.20); pre‐eclampsia (RR 0.40, 95% CI 0.04 to 4.10; 3 trials; 115 participants; Analysis 1.21); gestational diabetes (RR 1.28, 95% CI 0.50 to 3.25; 2 trials; 85 participants; Analysis 1.22); chorioamnionitis (RR 1.60, 95% CI 0.33 to 7.86; 2 trials; 85 participants; Analysis 1.23); postpartum haemorrhage (RR 1.59, 95% CI 0.27 to 9.45; 2 trials; 70 participants; Analysis 1.24); serious maternal outcome (RR 0.27, 95% CI 0.01 to 6.27; 3 trials; 123 participants; Analysis 1.25); maternal antenatal admission (RR 0.60, 95% CI 0.15 to 2.38; 1 trial; 45 participants; Analysis 1.26); duration of maternal hospital stay (MD −0.02 days, 95% CI −2.01 to 1.97 days; 1 trial; 45 participants; Analysis 1.27); duration of neonatal hospital stay (MD 1.70 days, 95% CI −8.70 to 12.10 days; 1 trial; 45 participants; Analysis 1.28); admission to the neonatal intensive care unit (RR 0.80, 95% CI 0.27 to 2.38; 1 trial; 45 participants; Analysis 1.29); or duration of neonatal intensive care unit stay (MD 4.20 days, 95% CI −25.13 to 33.53 days; 1 trial; 10 participants; Analysis 1.30).
1.6. Analysis.
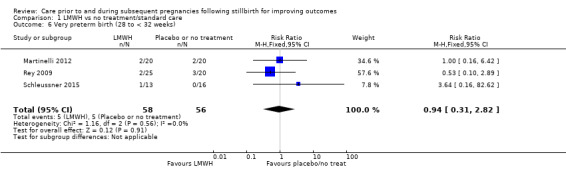
Comparison 1 LMWH vs no treatment/standard care, Outcome 6 Very preterm birth (28 to < 32 weeks).
1.7. Analysis.
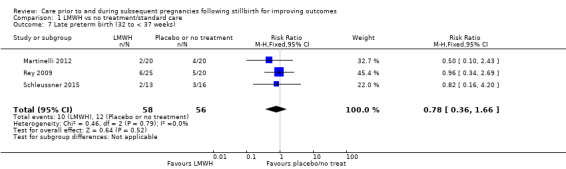
Comparison 1 LMWH vs no treatment/standard care, Outcome 7 Late preterm birth (32 to < 37 weeks).
1.9. Analysis.
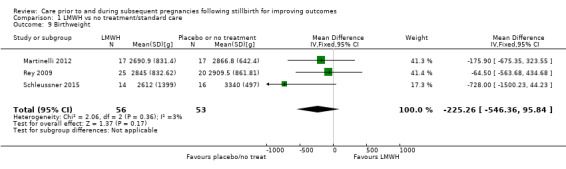
Comparison 1 LMWH vs no treatment/standard care, Outcome 9 Birthweight.
1.10. Analysis.
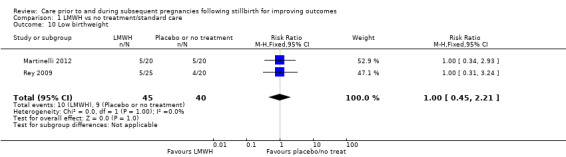
Comparison 1 LMWH vs no treatment/standard care, Outcome 10 Low birthweight.
1.11. Analysis.
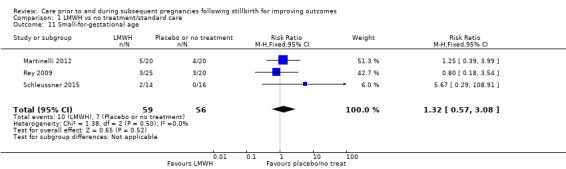
Comparison 1 LMWH vs no treatment/standard care, Outcome 11 Small‐for‐gestational age.
1.12. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 12 Apgar score less than seven at five minutes.
1.15. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 15 Adherence to the intervention.
1.16. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 16 Caesarean birth (elective).
1.17. Analysis.
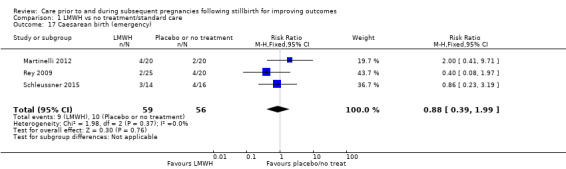
Comparison 1 LMWH vs no treatment/standard care, Outcome 17 Caesarean birth (emergency).
1.18. Analysis.
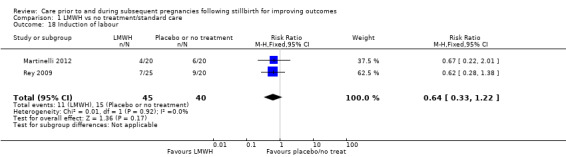
Comparison 1 LMWH vs no treatment/standard care, Outcome 18 Induction of labour.
1.19. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 19 Instrumental vaginal birth.
1.20. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 20 Placental abruption.
1.21. Analysis.
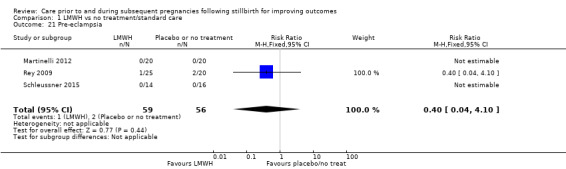
Comparison 1 LMWH vs no treatment/standard care, Outcome 21 Pre‐eclampsia.
1.22. Analysis.
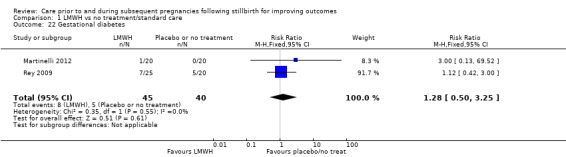
Comparison 1 LMWH vs no treatment/standard care, Outcome 22 Gestational diabetes.
1.23. Analysis.
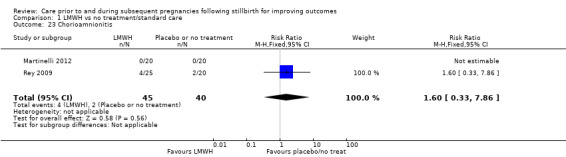
Comparison 1 LMWH vs no treatment/standard care, Outcome 23 Chorioamnionitis.
1.24. Analysis.
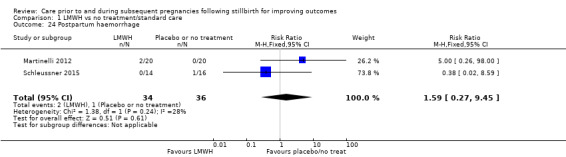
Comparison 1 LMWH vs no treatment/standard care, Outcome 24 Postpartum haemorrhage.
1.25. Analysis.
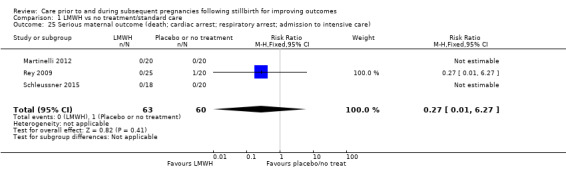
Comparison 1 LMWH vs no treatment/standard care, Outcome 25 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
1.26. Analysis.
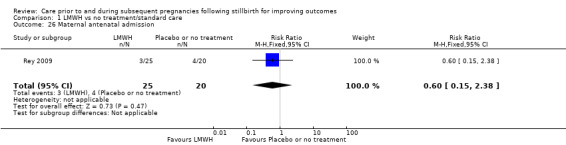
Comparison 1 LMWH vs no treatment/standard care, Outcome 26 Maternal antenatal admission.
1.27. Analysis.
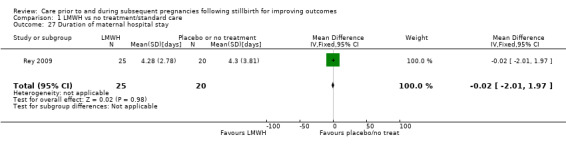
Comparison 1 LMWH vs no treatment/standard care, Outcome 27 Duration of maternal hospital stay.
1.28. Analysis.
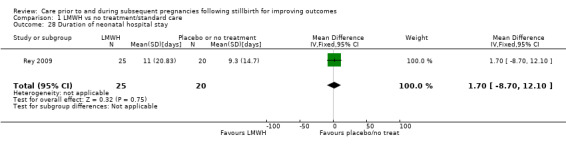
Comparison 1 LMWH vs no treatment/standard care, Outcome 28 Duration of neonatal hospital stay.
1.29. Analysis.
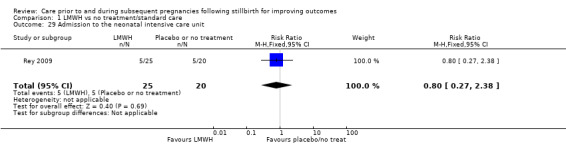
Comparison 1 LMWH vs no treatment/standard care, Outcome 29 Admission to the neonatal intensive care unit.
1.30. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 30 Duration of neonatal intensive care unit stay.
There were no reported instances of respiratory distress syndrome (1 trial; 40 participants) or neonatal jaundice (1 trial; 40 participants).
Heterogeneity
Adverse perinatal outcome: We chose to use a fixed‐effect model for this analysis. In our Methods, we stated that we would use a random‐effects model if heterogeneity was evident. We specified that we would regard heterogeneity to be present if the I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. However, in this analysis (Analysis 1.3), although the I2 value was just above 30%, (31%), there were no differences in the results between a fixed‐ or a random‐effects model. The P value was also high (P = 0.23). We therefore retained a fixed‐effect model.
Any preterm birth < 37 weeks: We chose to use a fixed‐effect model for this analysis. In our Methods, we stated that we would use a random‐effects model if heterogeneity was evident. We specified that we would regard heterogeneity to be present if the I2 was greater than 30% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. However, in this analysis (Analysis 1.8), although the I2 value was just above 30%, (35%), there were no differences in the results between a fixed‐ or a random‐effects model. The P value was also high (P = 0.21). We therefore retained a fixed‐effect model.
Comparison 2: LDA versus placebo
One trial (Ahmed 2014) contributed to the comparison of LDA versus placebo. The trial also assessed combined LDA and LMWH as part of a three‐armed design, including a total of 40 women. We included all arms of the trial as separate, independent comparisons (comparisons 2, 3, and 4). The comparison of LDA versus placebo included 24 women and 24 infants.
In all outcomes presented below, there was one trial and 24 participants, unless otherwise stated.
Primary outcomes
It was uncertain whether LDA reduced the risk of stillbirth (RR 0.85, 95% CI 0.06 to 12.01; Analysis 2.1), neonatal death (RR 0.29, 95% CI 0.01 to 6.38; Analysis 2.2), or adverse perinatal outcome (RR 0.28, 95% CI 0.03 to 2.34; Analysis 2.3) (all very low‐quality evidence), when compared to placebo. No data were available for adverse maternal psychological effects.
2.1. Analysis.
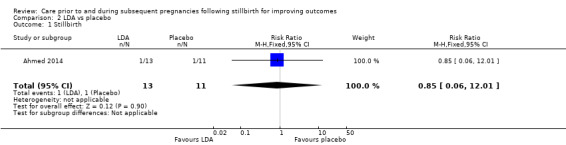
Comparison 2 LDA vs placebo, Outcome 1 Stillbirth.
2.2. Analysis.
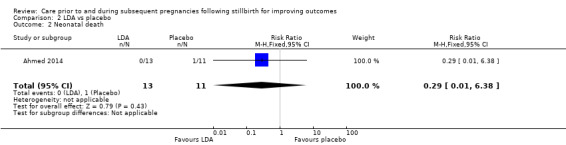
Comparison 2 LDA vs placebo, Outcome 2 Neonatal death.
2.3. Analysis.
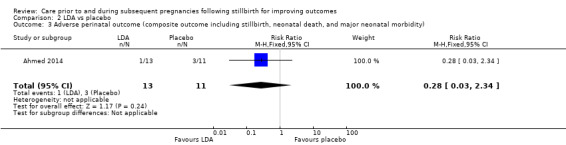
Comparison 2 LDA vs placebo, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
Secondary outcomes
LDA appeared to be associated with an increase in birthweight when compared to placebo (MD 790.00 g, 95% CI 295.03 to 1284.97 g; Analysis 2.8). Whether LDA had any effect on the following secondary outcomes was uncertain: perinatal mortality (RR 0.42, 95% CI 0.04 to 4.06; Analysis 2.4) and any preterm birth < 37 weeks (RR 0.42, 95% CI 0.04 to 4.06; Analysis 2.7), both very low‐quality evidence; very preterm birth (28 to < 32 weeks) (RR 0.29, 95% CI 0.01 to 6.38; Analysis 2.5); late preterm birth (32 to < 37 weeks) (RR 0.85, 95% CI 0.06 to 12.01; Analysis 2.6); low birthweight (RR 0.28, 95% CI 0.03 to 2.34; Analysis 2.9); small‐for‐gestational age (RR 0.29, 95% CI 0.01 to 6.38; Analysis 2.10); respiratory distress syndrome (RR 0.29, 95% CI 0.01 to 6.38; Analysis 2.11); adherence to the intervention (RR 1.00, 95% CI 0.86 to 1.17; Analysis 2.12); caesarean birth (elective) (RR 0.63, 95% CI 0.18 to 2.24; Analysis 2.13); caesarean birth (emergency) (RR 1.69, 95% CI 0.38 to 7.55; Analysis 2.14); induction of labour (RR 1.69, 95% CI 0.38 to 7.55; Analysis 2.15); instrumental vaginal birth (RR 2.57, 95% CI 0.12 to 57.44; Analysis 2.16); placental abruption (RR 2.57, 95% CI 0.12 to 57.44; Analysis 2.17); pre‐eclampsia (RR 0.85, 95% CI 0.14 to 5.06; Analysis 2.18); gestational diabetes (RR 0.42, 95% CI 0.04 to 4.06; Analysis 2.19); postpartum haemorrhage (RR 0.29, 95% CI 0.01 to 6.38; Analysis 2.20); antenatal care attendance (RR 1.00, 95% CI 0.86 to 1.17; Analysis 2.22); duration of maternal hospital stay (MD 0.00 days, 95% CI −2.41 to 2.41 days; Analysis 2.23); duration of neonatal hospital stay (MD −2.00 days, 95% CI −4.41 to 0.41 days; Analysis 2.24); admission to the neonatal intensive care unit (RR 1.48, 95% CI 0.58 to 3.75; Analysis 2.25); and duration of neonatal intensive care unit stay (MD −2.00 days, 95% CI −5.29 to 1.29 days; 1 trial; 11 participants; Analysis 2.26).
2.8. Analysis.
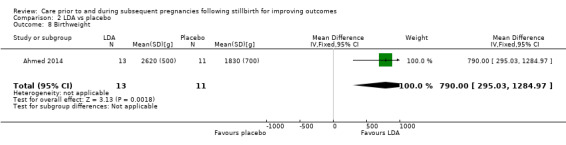
Comparison 2 LDA vs placebo, Outcome 8 Birthweight.
2.4. Analysis.
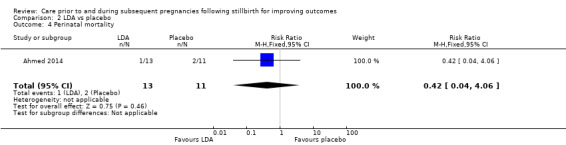
Comparison 2 LDA vs placebo, Outcome 4 Perinatal mortality.
2.7. Analysis.
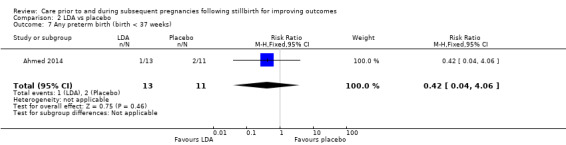
Comparison 2 LDA vs placebo, Outcome 7 Any preterm birth (birth < 37 weeks).
2.5. Analysis.
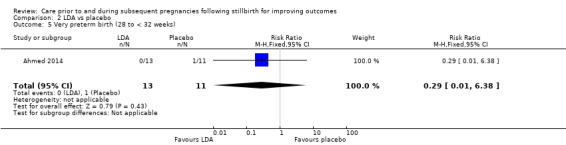
Comparison 2 LDA vs placebo, Outcome 5 Very preterm birth (28 to < 32 weeks).
2.6. Analysis.
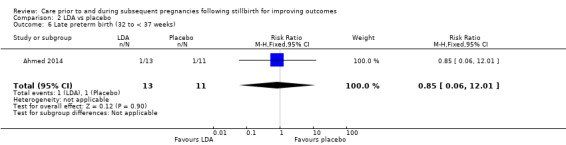
Comparison 2 LDA vs placebo, Outcome 6 Late preterm birth (32 to < 37 weeks).
2.9. Analysis.
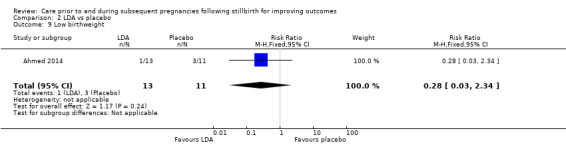
Comparison 2 LDA vs placebo, Outcome 9 Low birthweight.
2.10. Analysis.
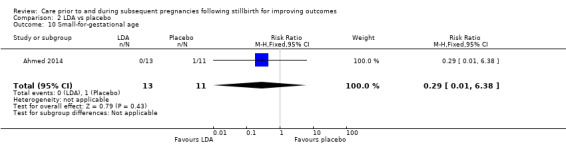
Comparison 2 LDA vs placebo, Outcome 10 Small‐for‐gestational age.
2.11. Analysis.
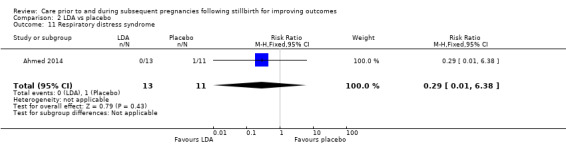
Comparison 2 LDA vs placebo, Outcome 11 Respiratory distress syndrome.
2.12. Analysis.
Comparison 2 LDA vs placebo, Outcome 12 Adherence to the intervention.
2.13. Analysis.
Comparison 2 LDA vs placebo, Outcome 13 Caesarean birth (elective).
2.14. Analysis.
Comparison 2 LDA vs placebo, Outcome 14 Caesarean birth (emergency).
2.15. Analysis.
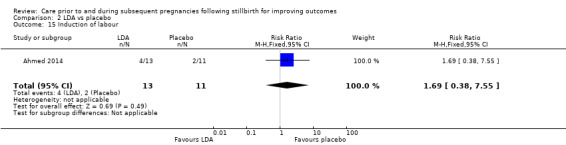
Comparison 2 LDA vs placebo, Outcome 15 Induction of labour.
2.16. Analysis.
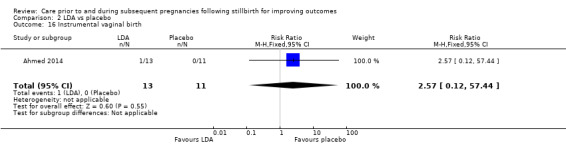
Comparison 2 LDA vs placebo, Outcome 16 Instrumental vaginal birth.
2.17. Analysis.
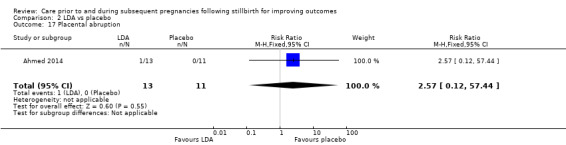
Comparison 2 LDA vs placebo, Outcome 17 Placental abruption.
2.18. Analysis.
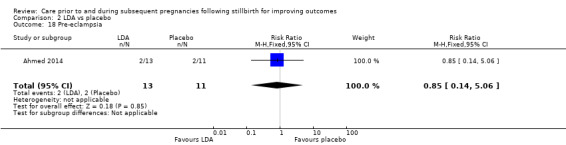
Comparison 2 LDA vs placebo, Outcome 18 Pre‐eclampsia.
2.19. Analysis.
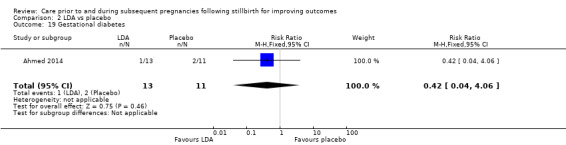
Comparison 2 LDA vs placebo, Outcome 19 Gestational diabetes.
2.20. Analysis.
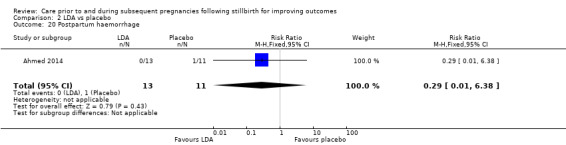
Comparison 2 LDA vs placebo, Outcome 20 Postpartum haemorrhage.
2.22. Analysis.
Comparison 2 LDA vs placebo, Outcome 22 Antenatal care attendance.
2.23. Analysis.
Comparison 2 LDA vs placebo, Outcome 23 Duration of maternal hospital stay.
2.24. Analysis.
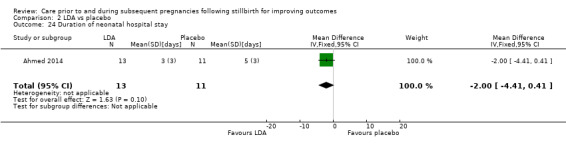
Comparison 2 LDA vs placebo, Outcome 24 Duration of neonatal hospital stay.
2.25. Analysis.
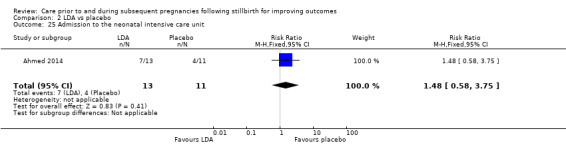
Comparison 2 LDA vs placebo, Outcome 25 Admission to the neonatal intensive care unit.
2.26. Analysis.
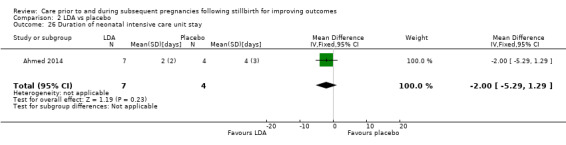
Comparison 2 LDA vs placebo, Outcome 26 Duration of neonatal intensive care unit stay.
There were no reported instances of serious maternal outcome.
Comparison 3: LDA + LMWH versus LDA alone
Arm two of the Ahmed 2014 trial assessed LDA + LMWH versus LDA alone among 29 women and 29 infants.
In all outcomes presented below, there was one trial and 29 participants, unless otherwise stated.
Primary outcome
It was uncertain whether LDA + LMWH reduced the risk of stillbirth (RR 0.27, 95% CI 0.01 to 6.23; Analysis 3.1) or adverse perinatal outcome (RR 2.44, 95% CI 0.29 to 20.75; Analysis 3.3), when compared to LDA alone. No neonatal deaths were reported and no data were available for adverse maternal psychological effects.
3.1. Analysis.
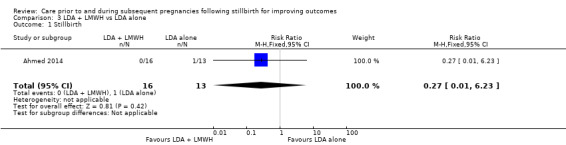
Comparison 3 LDA + LMWH vs LDA alone, Outcome 1 Stillbirth.
3.3. Analysis.
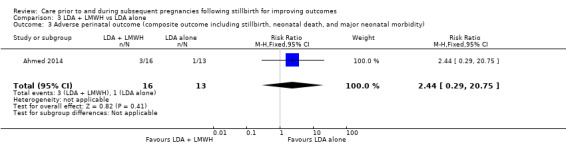
Comparison 3 LDA + LMWH vs LDA alone, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
Secondary outcomes
LDA appeared to be associated with an increase in birthweight when compared to LDA + LMWH (MD −650.00 g, 95% CI −1210.33 to −89.67 g; Analysis 3.8). Whether there were any differences between the groups on the following secondary outcomes was uncertain: perinatal mortality (RR 0.27, 95% CI 0.01 to 6.23; Analysis 3.4); very preterm birth (28 to < 32 weeks) (RR 2.47, 95% CI 0.11 to 56.03; Analysis 3.5); late preterm birth (32 to < 37 weeks) (RR 0.81, 95% CI 0.06 to 11.77; Analysis 3.6); any preterm birth (< 27 weeks) (RR 3.25, 95% CI 0.41 to 25.64; Analysis 3.7); low birthweight (RR 1.63, 95% CI 0.17 to 15.99; Analysis 3.9); small‐for‐gestational age (RR 2.47, 95% CI 0.11 to 56.03; Analysis 3.10); respiratory distress syndrome (RR 2.47, 95% CI 0.11 to 56.03; Analysis 3.11); adherence to the intervention (RR 1.00, 95% CI 0.88 to 1.14; Analysis 3.12); caesarean birth (elective) (RR 1.35, 95% CI 0.40 to 4.63; Analysis 3.13); caesarean birth (emergency) (RR 0.20, 95% CI 0.03 to 1.60; Analysis 3.14); induction of labour (RR 0.61, 95% CI 0.17 to 2.25; Analysis 3.15); instrumental vaginal birth (RR 0.81, 95% CI 0.06 to 11.77; Analysis 3.16); placental abruption (RR 0.81, 95% CI 0.06 to 11.77; Analysis 3.17); pre‐eclampsia (RR 0.41, 95% CI 0.04 to 4.00; Analysis 3.18); gestational diabetes (RR 0.27, 95% CI 0.01 to 6.23; Analysis 3.19); postpartum haemorrhage (RR 2.47, 95% CI 0.11 to 56.03; Analysis 3.20); antenatal care attendance (RR 1.00, 95% CI 0.88 to 1.14; Analysis 3.22); duration of maternal hospital stay (MD 0.00 days, 95% CI −2.55 to 2.55 days; Analysis 3.23); duration of neonatal hospital stay (MD 2.00 days, 95% CI −0.55 to 4.55 days; Analysis 3.24); admission to the neonatal intensive care unit (RR 1.16, 95% CI 0.62 to 2.18; Analysis 3.25); and duration of neonatal intensive care unit stay (MD 2.00 days, 95% CI −0.38 to 4.38 days; 1 trial; 17 participants; Analysis 3.26).
3.8. Analysis.
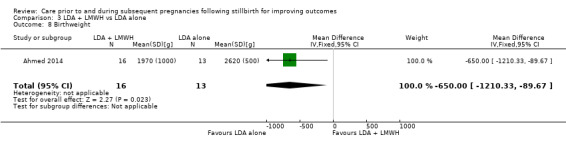
Comparison 3 LDA + LMWH vs LDA alone, Outcome 8 Birthweight.
3.4. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 4 Perinatal mortality.
3.5. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 5 Very preterm birth (28 to < 32 weeks).
3.6. Analysis.
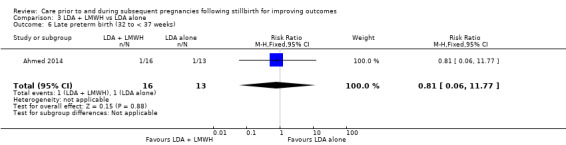
Comparison 3 LDA + LMWH vs LDA alone, Outcome 6 Late preterm birth (32 to < 37 weeks).
3.7. Analysis.
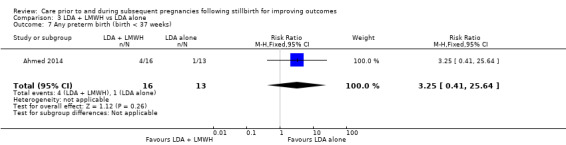
Comparison 3 LDA + LMWH vs LDA alone, Outcome 7 Any preterm birth (birth < 37 weeks).
3.9. Analysis.
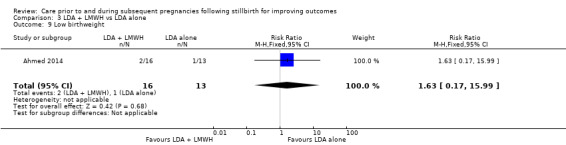
Comparison 3 LDA + LMWH vs LDA alone, Outcome 9 Low birthweight.
3.10. Analysis.
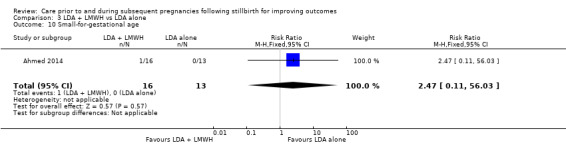
Comparison 3 LDA + LMWH vs LDA alone, Outcome 10 Small‐for‐gestational age.
3.11. Analysis.
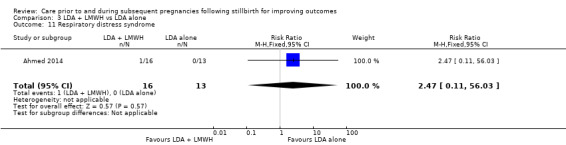
Comparison 3 LDA + LMWH vs LDA alone, Outcome 11 Respiratory distress syndrome.
3.12. Analysis.
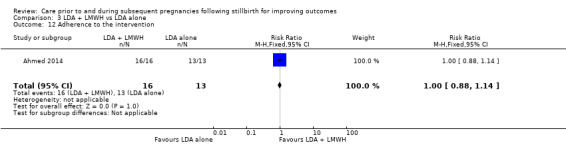
Comparison 3 LDA + LMWH vs LDA alone, Outcome 12 Adherence to the intervention.
3.13. Analysis.
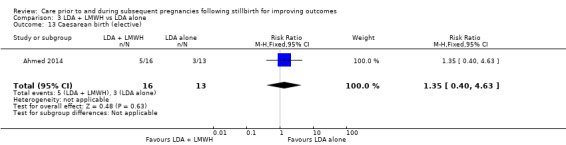
Comparison 3 LDA + LMWH vs LDA alone, Outcome 13 Caesarean birth (elective).
3.14. Analysis.
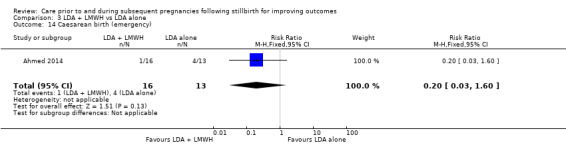
Comparison 3 LDA + LMWH vs LDA alone, Outcome 14 Caesarean birth (emergency).
3.15. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 15 Induction of labour.
3.16. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 16 Instrumental vaginal birth.
3.17. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 17 Placental abruption.
3.18. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 18 Pre‐eclampsia.
3.19. Analysis.
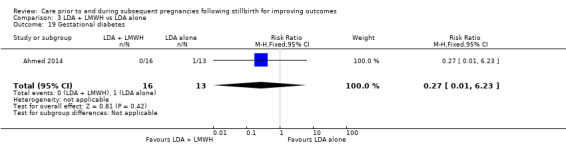
Comparison 3 LDA + LMWH vs LDA alone, Outcome 19 Gestational diabetes.
3.20. Analysis.
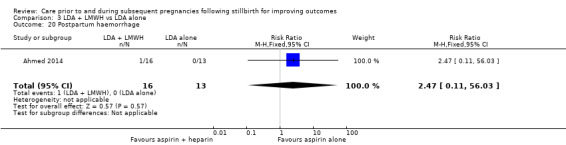
Comparison 3 LDA + LMWH vs LDA alone, Outcome 20 Postpartum haemorrhage.
3.22. Analysis.
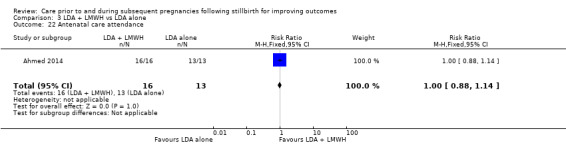
Comparison 3 LDA + LMWH vs LDA alone, Outcome 22 Antenatal care attendance.
3.23. Analysis.
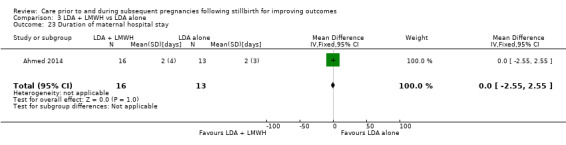
Comparison 3 LDA + LMWH vs LDA alone, Outcome 23 Duration of maternal hospital stay.
3.24. Analysis.
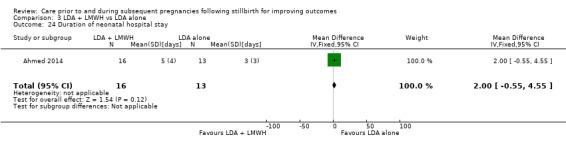
Comparison 3 LDA + LMWH vs LDA alone, Outcome 24 Duration of neonatal hospital stay.
3.25. Analysis.
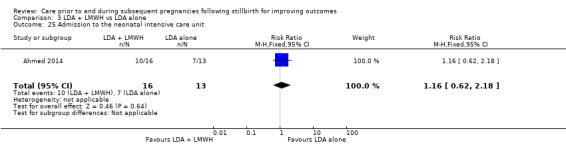
Comparison 3 LDA + LMWH vs LDA alone, Outcome 25 Admission to the neonatal intensive care unit.
3.26. Analysis.
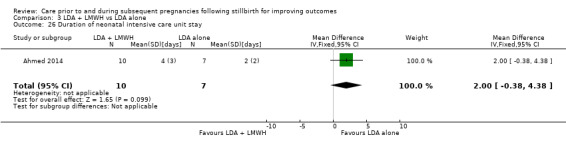
Comparison 3 LDA + LMWH vs LDA alone, Outcome 26 Duration of neonatal intensive care unit stay.
There were no reported instances of serious maternal outcome.
Comparison 4: LDA + LMWH versus placebo
Arm three of the Ahmed 2014 trial assessed LDA + LMWH versus placebo among 27 women and 27 infants.
In all outcomes presented below, there was one trial and 27 participants, unless otherwise stated.
Primary outcomes
It was uncertain whether LDA + LMWH reduced the risk of stillbirth or neonatal death (both RR 0.24, 95% CI 0.01 to 5.30; Analysis 4.1 and Analysis 4.2), or adverse perinatal outcome (RR 0.69, 95% CI 0.17 to 2.80; Analysis 4.3), when compared to placebo. No data were available for adverse maternal psychological effects.
4.1. Analysis.
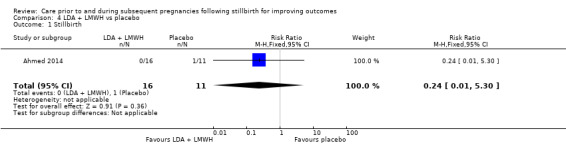
Comparison 4 LDA + LMWH vs placebo, Outcome 1 Stillbirth.
4.2. Analysis.
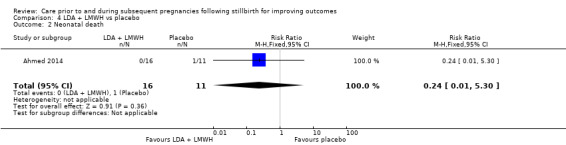
Comparison 4 LDA + LMWH vs placebo, Outcome 2 Neonatal death.
4.3. Analysis.
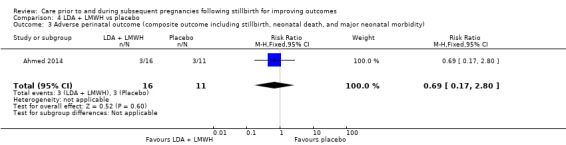
Comparison 4 LDA + LMWH vs placebo, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
Secondary outcomes
Whether LDA + LMWH had any effect on the following secondary outcomes was also uncertain: perinatal mortality (RR 0.14, 95% CI 0.01 to 2.68; Analysis 4.4); very preterm birth (28 to < 32 weeks) and late preterm birth (32 to < 37 weeks) (both RR 0.69, 95% CI 0.05 to 9.86; Analysis 4.5 and Analysis 4.6); any preterm birth (< 37 weeks) (RR 1.38, 95% CI 0.30 to 6.25; Analysis 4.7); birthweight (MD 140.00 g, 95% CI −501.26 to 781.26 g; Analysis 4.8); low birthweight (RR 0.46, 95% CI 0.09 to 2.31; Analysis 4.9); small‐for‐gestational age and respiratory distress syndrome (both RR 0.69, 95% CI 0.05 to 9.86; Analysis 4.10 and Analysis 4.11); adherence to the intervention (RR 1.00, 95% CI 0.87 to 1.16; Analysis 4.12); caesarean birth (elective) (RR 0.86, 95% CI 0.30 to 2.50; Analysis 4.13); caesarean birth (emergency) (RR 0.34, 95% CI 0.04 to 3.34; Analysis 4.14); induction of labour (RR 1.03, 95% CI 0.20 to 5.19; Analysis 4.15); instrumental vaginal birth (RR 2.12, 95% CI 0.09 to 47.68; Analysis 4.16); placental abruption (RR 2.12, 95% CI 0.09 to 47.68; Analysis 4.17); pre‐eclampsia (RR 0.34, 95% CI 0.04 to 3.34; Analysis 4.18); gestational diabetes (RR 0.14, 95% CI 0.01 to 2.68; Analysis 4.19); postpartum haemorrhage (RR 0.69, 95% CI 0.05 to 9.86; Analysis 4.20); antenatal care attendance (RR 1.00, 95% CI 0.87 to 1.16; Analysis 4.22); duration of maternal hospital stay (MD 0.00 days, 95% CI −2.64 to 2.64 days; Analysis 4.23); duration of neonatal hospital stay (MD 0.00 days, 95% CI −2.64 to 2.64 days; Analysis 4.24); admission to the neonatal intensive care unit (RR 1.72, 95% CI 0.72 to 4.10; Analysis 4.25); and duration of neonatal intensive care unit stay (MD 0.00 days, 95% CI −3.48 to 3.48 days; 1 trial; 14 participants; Analysis 4.26).
4.4. Analysis.
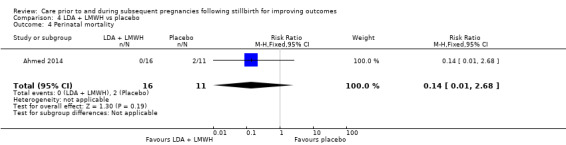
Comparison 4 LDA + LMWH vs placebo, Outcome 4 Perinatal mortality.
4.5. Analysis.
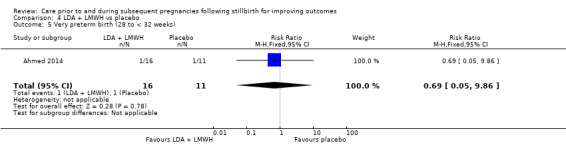
Comparison 4 LDA + LMWH vs placebo, Outcome 5 Very preterm birth (28 to < 32 weeks).
4.6. Analysis.
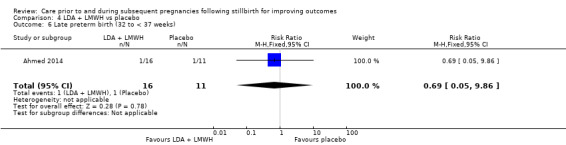
Comparison 4 LDA + LMWH vs placebo, Outcome 6 Late preterm birth (32 to < 37 weeks).
4.7. Analysis.
Comparison 4 LDA + LMWH vs placebo, Outcome 7 Any preterm birth (birth < 37 weeks).
4.8. Analysis.
Comparison 4 LDA + LMWH vs placebo, Outcome 8 Birthweight.
4.9. Analysis.
Comparison 4 LDA + LMWH vs placebo, Outcome 9 Low birthweight.
4.10. Analysis.
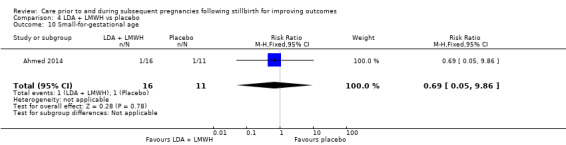
Comparison 4 LDA + LMWH vs placebo, Outcome 10 Small‐for‐gestational age.
4.11. Analysis.
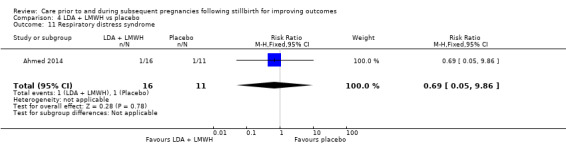
Comparison 4 LDA + LMWH vs placebo, Outcome 11 Respiratory distress syndrome.
4.12. Analysis.
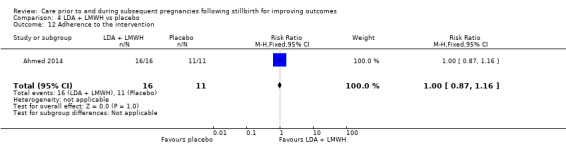
Comparison 4 LDA + LMWH vs placebo, Outcome 12 Adherence to the intervention.
4.13. Analysis.
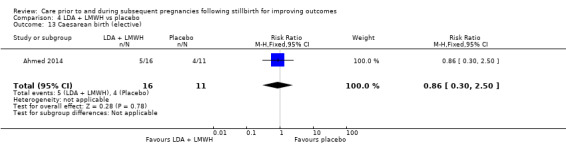
Comparison 4 LDA + LMWH vs placebo, Outcome 13 Caesarean birth (elective).
4.14. Analysis.
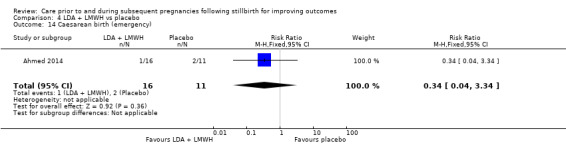
Comparison 4 LDA + LMWH vs placebo, Outcome 14 Caesarean birth (emergency).
4.15. Analysis.
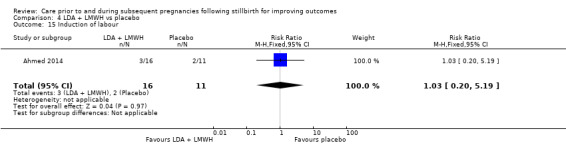
Comparison 4 LDA + LMWH vs placebo, Outcome 15 Induction of labour.
4.16. Analysis.
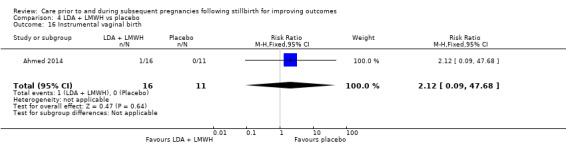
Comparison 4 LDA + LMWH vs placebo, Outcome 16 Instrumental vaginal birth.
4.17. Analysis.
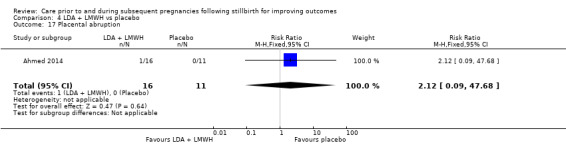
Comparison 4 LDA + LMWH vs placebo, Outcome 17 Placental abruption.
4.18. Analysis.
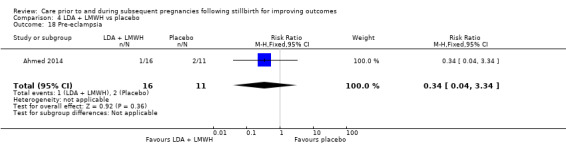
Comparison 4 LDA + LMWH vs placebo, Outcome 18 Pre‐eclampsia.
4.19. Analysis.
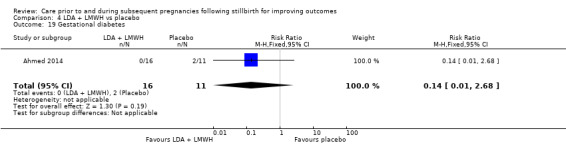
Comparison 4 LDA + LMWH vs placebo, Outcome 19 Gestational diabetes.
4.20. Analysis.
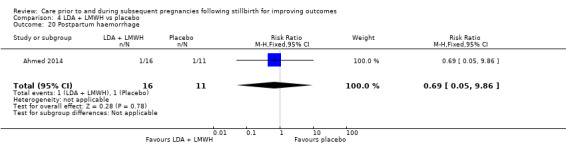
Comparison 4 LDA + LMWH vs placebo, Outcome 20 Postpartum haemorrhage.
4.22. Analysis.
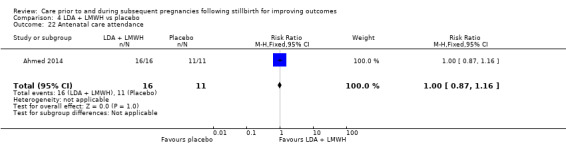
Comparison 4 LDA + LMWH vs placebo, Outcome 22 Antenatal care attendance.
4.23. Analysis.
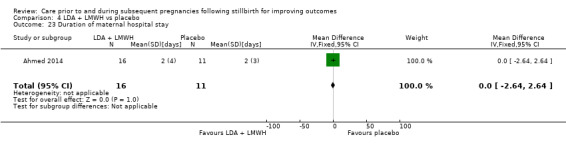
Comparison 4 LDA + LMWH vs placebo, Outcome 23 Duration of maternal hospital stay.
4.24. Analysis.
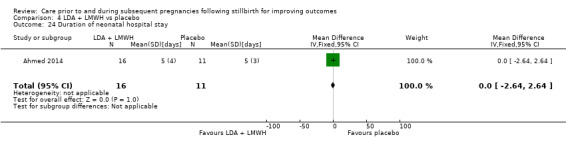
Comparison 4 LDA + LMWH vs placebo, Outcome 24 Duration of neonatal hospital stay.
4.25. Analysis.
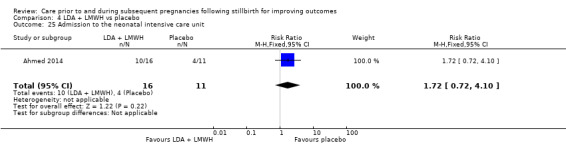
Comparison 4 LDA + LMWH vs placebo, Outcome 25 Admission to the neonatal intensive care unit.
4.26. Analysis.
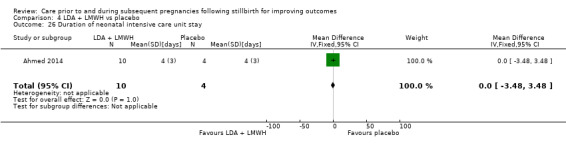
Comparison 4 LDA + LMWH vs placebo, Outcome 26 Duration of neonatal intensive care unit stay.
There were no reported instances of serious maternal outcome.
Comparison 5: LMWH versus LDA
One trial (Gris 2004), including 22 women and 22 infants, contributed to the comparison of LMWH versus LDA.
In all outcomes presented below, there was one trial and 22 participants, unless otherwise stated.
Primary outcomes
It was uncertain whether LMWH reduced the risk of stillbirth or adverse perinatal outcome (both RR 3.55, 95% CI 0.16 to 78.56; Analysis 5.1 and Analysis 5.3), when compared to LDA. No neonatal deaths occurred. No data were available for adverse maternal psychological effects.
5.1. Analysis.
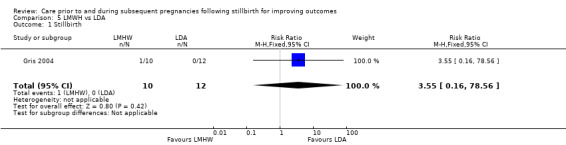
Comparison 5 LMWH vs LDA, Outcome 1 Stillbirth.
5.3. Analysis.
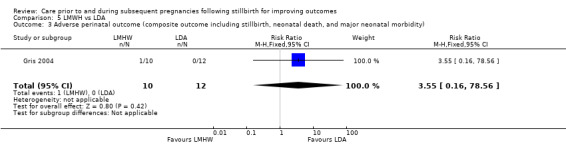
Comparison 5 LMWH vs LDA, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
Secondary outcomes
Whether there were any differences between the groups on the following secondary outcomes was also uncertain: perinatal mortality (RR 3.55, 95% CI 0.16 to 78.56; Analysis 5.4); late preterm birth (32 to < 27 weeks) (RR 1.20, 95% CI 0.09 to 16.84; Analysis 5.6); any preterm birth (< 37 weeks) (RR 0.60, 95% CI 0.06 to 5.69; Analysis 5.7); birthweight (MD 75.00 g, 95% CI ‐151.69 to 301.69 g; Analysis 5.8); low birthweight (RR 0.60, 95% CI 0.06 to 5.69; Analysis 5.9); small‐for‐gestational age (RR 0.60, 95% CI 0.06 to 5.69; Analysis 5.10); Apgar score less than seven at five minutes (RR 0.80, 95% CI 0.16 to 3.88; Analysis 5.11); respiratory distress syndrome (RR 1.20, 95% CI 0.09 to 16.84; Analysis 5.12); neonatal jaundice (RR 0.90, 95% CI 0.47 to 1.72; Analysis 5.13); caesarean birth (elective) (RR 1.20, 95% CI 0.20 to 7.05; Analysis 5.14); caesarean birth (emergency) (RR 0.60, 95% CI 0.06 to 5.69; Analysis 5.15); induction of labour (RR 8.27, 95% CI 0.48 to 143.35; Analysis 5.16); pre‐eclampsia (RR 0.60, 95% CI 0.06 to 5.69; Analysis 5.19); breastfeeding (RR 0.96, 95% CI 0.35 to 2.64; Analysis 5.23); maternal antenatal admission (RR 0.60, 95% CI 0.06 to 5.69; Analysis 5.24); duration of maternal hospital stay (MD 0.00 days, 95% CI −0.19 to 0.19 days; Analysis 5.25); duration of neonatal hospital stay (MD 0.00 days, 95% CI −0.30 to 0.30 days; Analysis 5.26); admission to the neonatal intensive care unit (RR 1.20, 95% CI 0.09 to 16.84; Analysis 5.27); and duration of neonatal intensive care unit stay (MD 0.22 days, 95% CI −8.52 to 8.96 days; 1 trial; 2 participants; Analysis 5.28).
5.4. Analysis.
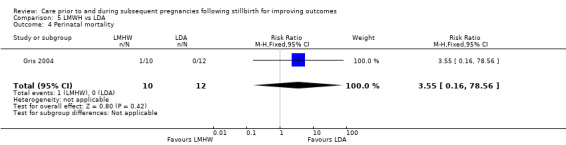
Comparison 5 LMWH vs LDA, Outcome 4 Perinatal mortality.
5.6. Analysis.
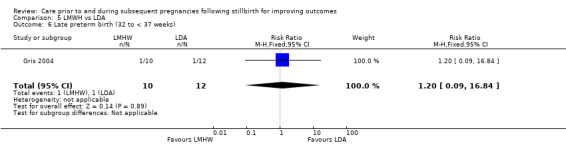
Comparison 5 LMWH vs LDA, Outcome 6 Late preterm birth (32 to < 37 weeks).
5.7. Analysis.
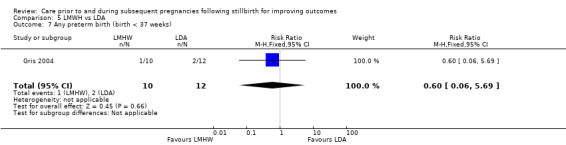
Comparison 5 LMWH vs LDA, Outcome 7 Any preterm birth (birth < 37 weeks).
5.8. Analysis.
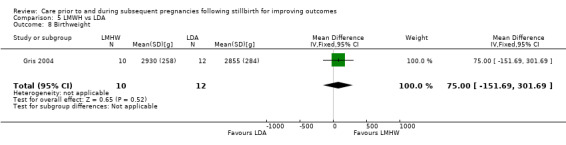
Comparison 5 LMWH vs LDA, Outcome 8 Birthweight.
5.9. Analysis.
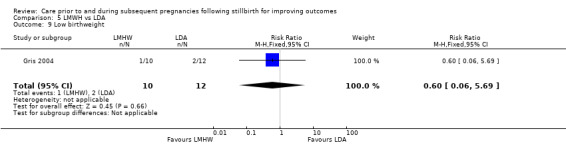
Comparison 5 LMWH vs LDA, Outcome 9 Low birthweight.
5.10. Analysis.
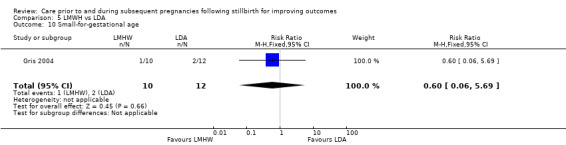
Comparison 5 LMWH vs LDA, Outcome 10 Small‐for‐gestational age.
5.11. Analysis.
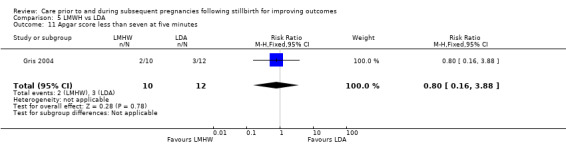
Comparison 5 LMWH vs LDA, Outcome 11 Apgar score less than seven at five minutes.
5.12. Analysis.
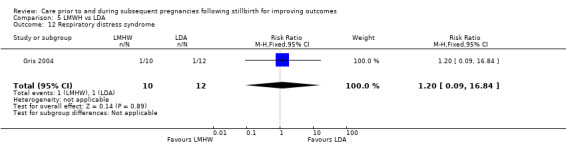
Comparison 5 LMWH vs LDA, Outcome 12 Respiratory distress syndrome.
5.13. Analysis.
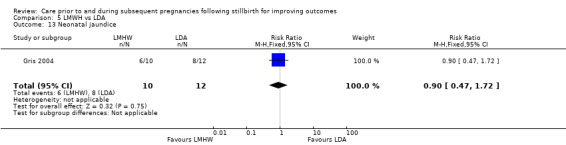
Comparison 5 LMWH vs LDA, Outcome 13 Neonatal jaundice.
5.14. Analysis.
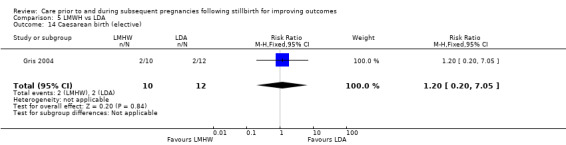
Comparison 5 LMWH vs LDA, Outcome 14 Caesarean birth (elective).
5.15. Analysis.
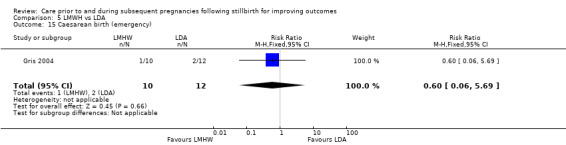
Comparison 5 LMWH vs LDA, Outcome 15 Caesarean birth (emergency).
5.16. Analysis.
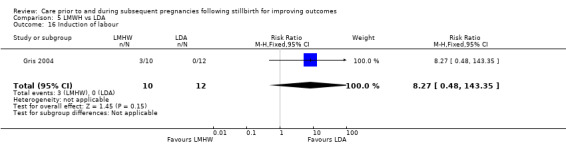
Comparison 5 LMWH vs LDA, Outcome 16 Induction of labour.
5.19. Analysis.
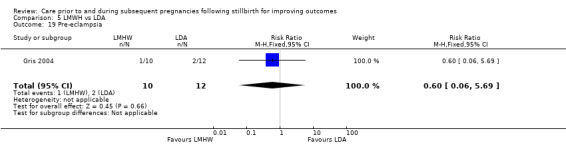
Comparison 5 LMWH vs LDA, Outcome 19 Pre‐eclampsia.
5.23. Analysis.
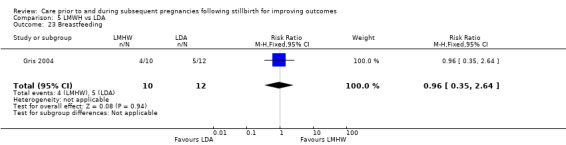
Comparison 5 LMWH vs LDA, Outcome 23 Breastfeeding.
5.24. Analysis.
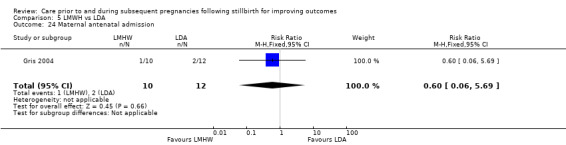
Comparison 5 LMWH vs LDA, Outcome 24 Maternal antenatal admission.
5.25. Analysis.
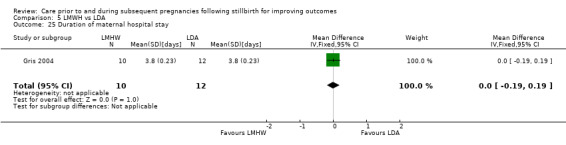
Comparison 5 LMWH vs LDA, Outcome 25 Duration of maternal hospital stay.
5.26. Analysis.
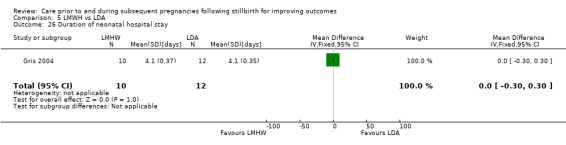
Comparison 5 LMWH vs LDA, Outcome 26 Duration of neonatal hospital stay.
5.27. Analysis.
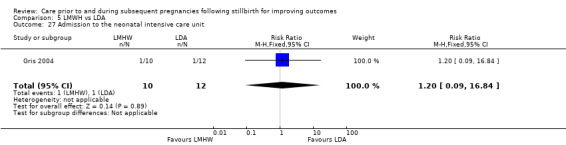
Comparison 5 LMWH vs LDA, Outcome 27 Admission to the neonatal intensive care unit.
5.28. Analysis.
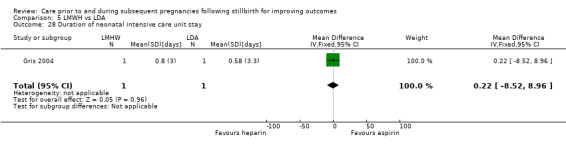
Comparison 5 LMWH vs LDA, Outcome 28 Duration of neonatal intensive care unit stay.
There were no reported instances of very preterm birth; instrumental vaginal birth; placental abruption; gestational diabetes; postpartum haemorrhage; or serious maternal outcome.
Comparison 6: LMWH (dose adjusted according to anti‐factor Xa levels) versus LMWH (fixed dose)
One trial (Salim 2016) assessed LMWH with doses adjusted according to anti‐factor Xa levels against a fixed dose of LMWH. Data from 13 women and 13 infants are included.
In all outcomes presented below, there was one trial and 13 participants.
Primary outcomes
There were no reported stillbirths. No data were available for neonatal deaths, adverse perinatal outcome, or adverse maternal psychological effects.
Secondary outcomes
It was uncertain whether an adjusted dose of LMWH compared to a fixed dose had any effect on very preterm birth (28 to < 32 weeks) (RR 0.58, 95% CI 0.07 to 4.95; Analysis 6.2); late preterm birth (32 to < 37 weeks) (RR 0.23, 95% CI 0.01 to 4.00; Analysis 6.3); any preterm birth (< 37 weeks) (RR 0.29, 95% CI 0.04 to 1.95; Analysis 6.4); birthweight (MD 812.00 g, 95% CI −257.81 to 1881.81 g; Analysis 6.5); low birthweight (RR 0.39, 95% CI 0.05 to 2.83; Analysis 6.6); small‐for‐gestational age (RR 1.17, 95% CI 0.09 to 14.92; Analysis 6.7); adherence to the intervention (RR 1.00, 95% CI 0.76 to 1.31; Analysis 6.9); caesarean birth (elective) (RR 1.17, 95% CI 0.09 to 14.92; Analysis 6.10); caesarean birth (emergency) (RR 0.58, 95% CI 0.07 to 4.95; Analysis 6.11); induction of labour (RR 0.70, 95% CI 0.28 to 1.77; Analysis 6.12); placental abruption (RR 0.38, 95% CI 0.02 to 7.93; Analysis 6.14); pre‐eclampsia (RR 0.38, 95% CI 0.02 to 7.93; Analysis 6.15); gestational diabetes (RR 0.38, 95% CI 0.02 to 7.93; Analysis 6.16); postpartum haemorrhage (RR 0.16, 95% CI 0.01 to 2.64; Analysis 6.18); and admission to neonatal intensive care unit (RR 0.58, 95% CI 0.07 to 4.95; Analysis 6.20).
6.2. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 2 Very preterm birth (28 to < 32 weeks).
6.3. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 3 Late preterm birth (32 to < 37 weeks).
6.4. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 4 Any preterm birth (birth < 37weeks).
6.5. Analysis.
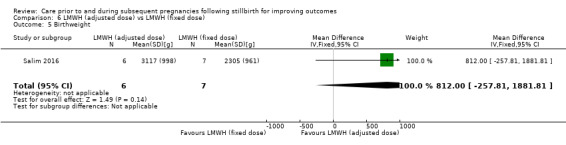
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 5 Birthweight.
6.6. Analysis.
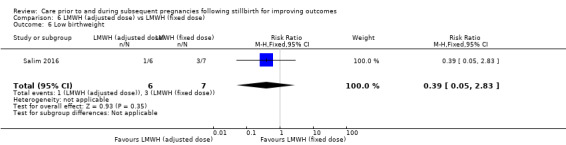
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 6 Low birthweight.
6.7. Analysis.
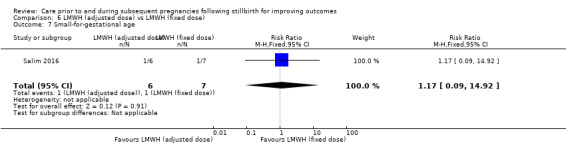
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 7 Small‐for‐gestational age.
6.9. Analysis.
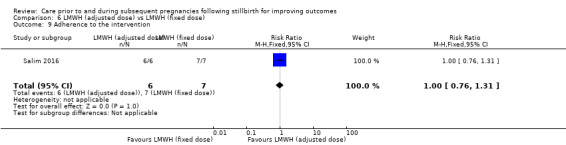
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 9 Adherence to the intervention.
6.10. Analysis.
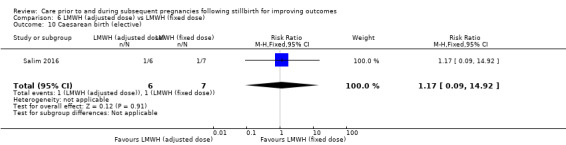
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 10 Caesarean birth (elective).
6.11. Analysis.
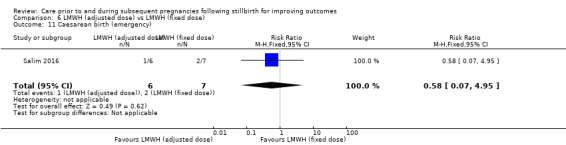
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 11 Caesarean birth (emergency).
6.12. Analysis.
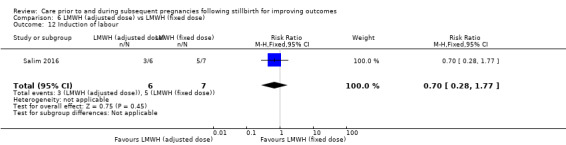
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 12 Induction of labour.
6.14. Analysis.
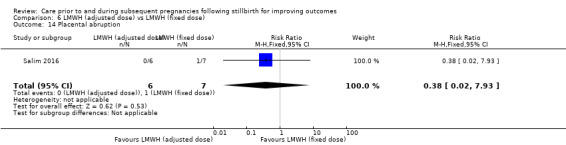
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 14 Placental abruption.
6.15. Analysis.
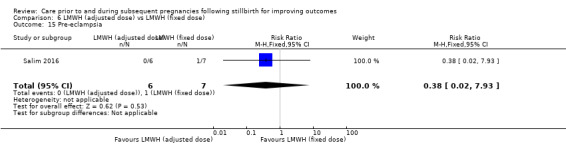
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 15 Pre‐eclampsia.
6.16. Analysis.
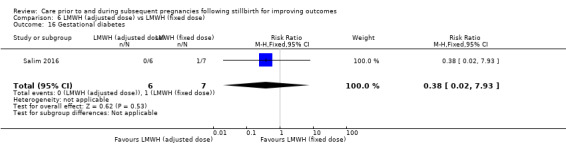
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 16 Gestational diabetes.
6.18. Analysis.
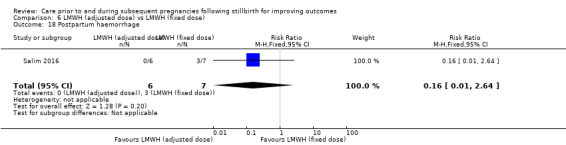
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 18 Postpartum haemorrhage.
6.20. Analysis.
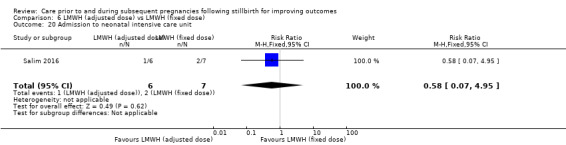
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 20 Admission to neonatal intensive care unit.
There were no reported instances of Apgar score less than seven at five minutes; instrumental vaginal birth; chorioamnionitis; or serious maternal outcome.
Comparison 7: Leukocyte immunisation versus placebo
One trial (Christiansen 1994) assessed third‐party leukocyte immunisation against placebo. Data from four women and four infants are included.
In all outcomes presented below, there was one trial and four participants.
Primary outcomes
There were no instances of stillbirth, neonatal death, or adverse perinatal outcome. No data were available for adverse maternal psychological effects.
Secondary outcomes
Leukocyte immunisation appeared to be associated with an increase in infant birthweight among the two women who received this intervention when compared to placebo (MD 1195.00 g, 95% CI 273.35 to 2116.65 g; Analysis 7.8). Whether leukocyte immunisation had any effect on the following secondary outcomes was uncertain: late preterm birth (32 to < 37 weeks) (RR 0.33, 95% CI 0.02 to 5.33; Analysis 7.6); any preterm birth (< 37 weeks) (RR 0.33, 95% CI 0.02 to 5.33; Analysis 7.7); low birthweight (RR 0.33, 95% CI 0.02 to 5.33; Analysis 7.9); adherence to the intervention (RR 1.00, 95% CI 0.49 to 2.05; Analysis 7.12); and caesarean birth (emergency) (RR 0.33, 95% CI 0.02 to 5.33; Analysis 7.14).
7.8. Analysis.
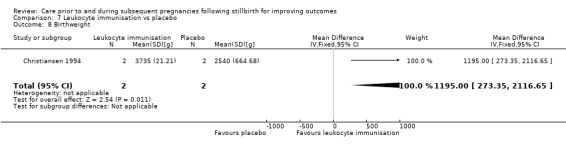
Comparison 7 Leukocyte immunisation vs placebo, Outcome 8 Birthweight.
7.6. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 6 Late preterm birth (32 to < 37 weeks).
7.7. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 7 Any preterm birth (birth < 37 weeks).
7.9. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 9 Low birthweight.
7.12. Analysis.
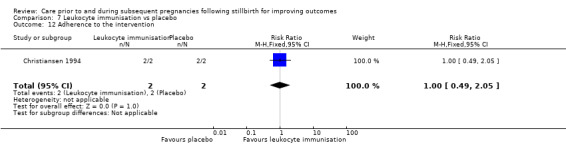
Comparison 7 Leukocyte immunisation vs placebo, Outcome 12 Adherence to the intervention.
7.14. Analysis.
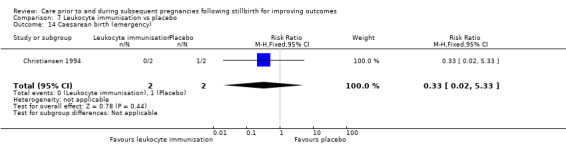
Comparison 7 Leukocyte immunisation vs placebo, Outcome 14 Caesarean birth (emergency).
There were no reported instances of perinatal mortality; very preterm birth; small‐for‐gestational age; Apgar score less than seven at five minutes; caesarean birth (elective); induction of labour; instrumental vaginal birth; placental abruption; pre‐eclampsia; gestational diabetes; chorioamnionitis; or serious maternal outcome.
Comparison 8: Intravenous IgG versus placebo
Two trials (Christiansen 1995; Christiansen 2002) assessed intravenous IgG against placebo.
A total of 13 women and their infants were included, depending on the outcome. The numbers of trials and participants for each outcome are provided below.
Primary outcomes
It was uncertain whether intravenous IgG reduced the risk of stillbirth (RR 0.74, 95% CI 0.16 to 3.40; 2 trials; 7 participants; Analysis 8.1) or adverse perinatal outcome (RR 0.74, 95% CI 0.16 to 3.40; 2 trials; 7 participants; Analysis 8.3). No neonatal deaths were reported and no data were available for adverse maternal psychological effects.
8.1. Analysis.
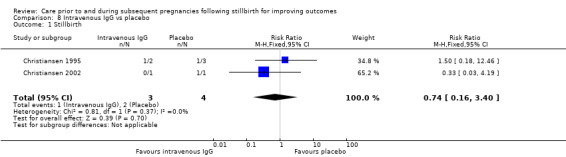
Comparison 8 Intravenous IgG vs placebo, Outcome 1 Stillbirth.
8.3. Analysis.
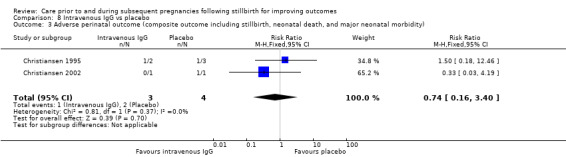
Comparison 8 Intravenous IgG vs placebo, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
Secondary outcomes
Both trials reported individual birthweights among the neonates assessed. For Christiansen 1995, birthweight data were available for one liveborn neonate in the intervention group (2390 g) and two liveborn neonates in the placebo group (2650 and 2530 g). For Christiansen 2002, one liveborn infant in the intervention group weighed 2950 g.
Whether intravenous IgG had any effect on the following secondary outcomes was uncertain: perinatal mortality (RR 0.74, 95% CI 0.16 to 3.40; 2 trials; 7 participants; Analysis 8.4); late preterm birth (32 to < 37 weeks) (RR 0.44, 95% CI 0.03 to 7.52; 2 trials; 7 participants; Analysis 8.6); any preterm birth (< 37 weeks) (RR 0.44, 95% CI 0.03 to 7.52; 2 trials; 7 participants; Analysis 8.7); low birthweight (RR 4.00, 95% CI 0.24 to 67.71; 2 trials; 7 participants; Analysis 8.8); adherence to the intervention (RR 1.00, 95% CI 0.69 to 1.44; 2 trials; 13 participants; Analysis 8.13); caesarean birth (elective) (RR 1.50, 95% CI 0.18 to 12.46; 2 trials; 7 participants; Analysis 8.14); induction of labour (RR 3.00, 95% CI 0.24 to 37.67; 2 trials; 7 participants; Analysis 8.16); and instrumental vaginal birth (RR 1.16, 95% CI 0.21 to 6.35; 2 trials; 7 participants; Analysis 8.17).
8.4. Analysis.
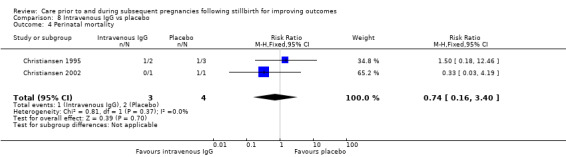
Comparison 8 Intravenous IgG vs placebo, Outcome 4 Perinatal mortality.
8.6. Analysis.
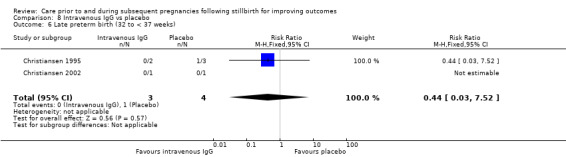
Comparison 8 Intravenous IgG vs placebo, Outcome 6 Late preterm birth (32 to < 37 weeks).
8.7. Analysis.
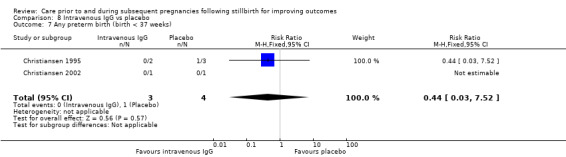
Comparison 8 Intravenous IgG vs placebo, Outcome 7 Any preterm birth (birth < 37 weeks).
8.8. Analysis.
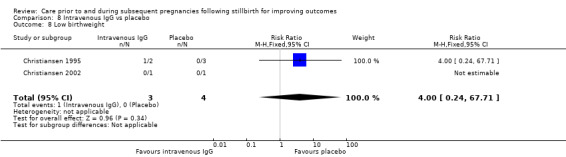
Comparison 8 Intravenous IgG vs placebo, Outcome 8 Low birthweight.
8.13. Analysis.
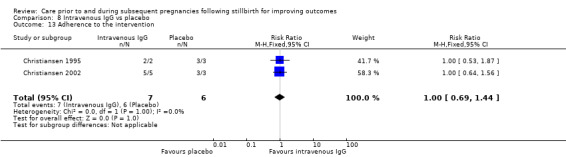
Comparison 8 Intravenous IgG vs placebo, Outcome 13 Adherence to the intervention.
8.14. Analysis.
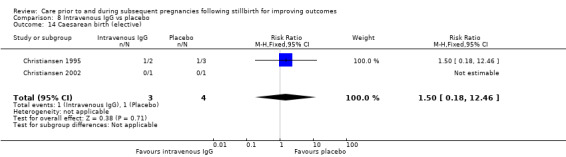
Comparison 8 Intravenous IgG vs placebo, Outcome 14 Caesarean birth (elective).
8.16. Analysis.
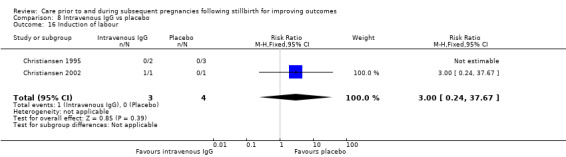
Comparison 8 Intravenous IgG vs placebo, Outcome 16 Induction of labour.
8.17. Analysis.
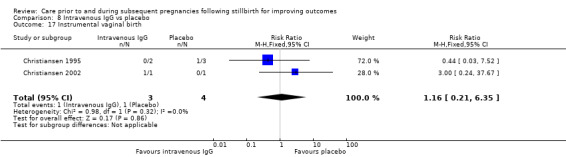
Comparison 8 Intravenous IgG vs placebo, Outcome 17 Instrumental vaginal birth.
There were no reported instances of very preterm birth (2 trials; 7 participants); small‐for‐gestational age (1 trial; 2 participants); Apgar score less than seven at five minutes (2 trials; 7 participants); respiratory distress syndrome (1 trial; 2 participants); neonatal jaundice (1 trial; 2 participants); caesarean birth (emergency) (2 trials; 7 participants); placental abruption (2 trials; 7 participants); pre‐eclampsia (2 trials; 7 participants); gestational diabetes (2 trials; 7 participants); chorioamnionitis (2 trials; 7 participants); postpartum haemorrhage (1 trial; 2 participants); serious maternal outcome (2 trials; 7 participants); admission to the neonatal intensive care unit (1 trial; 2 participants).
Comparison 9: Progestogen versus placebo
One trial (Levine 1964) assessed progestogen against placebo. We include data from a total of seven women and five infants in this review. We report the data descriptively only, due to ambiguity in the data for two of the primary outcomes (see above).
Primary outcomes
There were no stillbirths, neonatal deaths, or adverse perinatal outcomes in the intervention group among the four women included. Three women were assessed in the control group, of which one woman miscarried. Another woman experienced an 'abortion' but the gestational age at which the death occurred was unclear. It is possible that this death was a stillbirth according to our definition. Adverse maternal psychological effects were not reported.
Secondary outcomes
Two women in the intervention group and one woman in the control group experienced a late preterm birth (32 to < 37 weeks). There were no reported instances of very preterm birth (28 to < 32 weeks). Birthweights were reported for all four infants in the intervention group. Birthweights were provided in pounds and ounces, which we converted to kilograms before calculating a mean figure, equalling 1978 g. Birthweight was available for one infant in the control group, reported as 4 lb 11 oz (2126 g). Data pertaining to low birthweight was not provided in the study report. Applying the WHO definition (< 2500 g), all infants in the trial were of low birthweight.
There appeared to be 100% adherence to the intervention, according to the study report: "The injections were discontinued only when patients proved not to be pregnant or failed to return regularly for their weekly administration" (study report p. 31). As reported, "No untoward reactions were encountered" (study report p. 31).
Discussion
Summary of main results
This review set out to assess the effects of different interventions or models of care prior to and during subsequent pregnancies following stillbirth on maternal, fetal, neonatal and family health outcomes, and health service utilisation. We included 10 trials at low to moderate risk of bias, assessing low‐dose aspirin (LDA) or low‐molecular‐weight heparin (LMWH), or both (Ahmed 2014; Gris 2004; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015); third‐party leukocyte immunisation (Christiansen 1994); intravenous IgG (Christiansen 1995; Christiansen 2002); and progestogen (Levine 1964). The review includes data from 222 women and their infants. We assessed the trials under nine comparisons. All but one of the comparisons were based on data from only one or two trials, all with extremely small sample sizes. As a result, the analyses were not sufficiently powered to detect differences in most of the outcomes assessed. Based on the GRADE approach, the quality of the evidence assessed in this review was very low to low.
Among the primary outcomes, it was uncertain whether the interventions assessed had any effect on stillbirth, neonatal death, or adverse perinatal outcome. There appeared to be a minor trend across these outcomes in favour of LDA when compared to placebo, although serious imprecision in the data limits any meaningful interpretation of these findings. Data on adverse maternal psychological effects were available from one trial only, assessing LMWH against no treatment (Martinelli 2012), and results were uncertain: among the 40 women included, there were two reported events, one in each trial arm (very low‐quality evidence).
For secondary outcomes, we observed a possible increase in birthweight associated with LDA and third‐party leukocyte immunisation. However, the reliability of these findings is limited substantially due to the extremely low number of participants included in the associated analyses, each of which included only one trial. The clinical significance of these findings is also limited, given the lack of accompanying differences in the outcomes of low birthweight or small‐for‐gestational age.
For the remaining secondary outcomes, there was no clear evidence of benefit or harm across the interventions, although these results were largely uncertain due to insufficient data.
Overall completeness and applicability of evidence
The evidence around interventions to improve outcomes in subsequent pregnancies following stillbirth is sparse. Our review set out to capture a broad range of medical and psychosocial interventions addressing this research question, including outcomes of mothers and partners, and longer‐term outcomes of children. Our review also set out to capture interventions spanning pre‐conception through to birth. However, the 10 eligible trials were focused on medical interventions among mothers (most commonly LDA and LMWH), administered largely during pregnancy. Other potentially beneficial interventions were not assessed, including information and decision‐making support on interpregnancy interval and pre‐conception health, early or regular ultrasound surveillance, elective induction of labour or elective early caesarean birth, and intrapartum monitoring. None of the trials assessed partner or (longer‐term) childhood outcomes. Psychosocial outcomes were rarely measured, and in no trials was psychosocial well‐being a component of, or adjunctive to, the given medical interventions. It should be acknowledged, however, that non‐randomised, qualitative methodologies are generally more likely to be adopted for such psychosocial interventions, and therefore by design would not have been captured in this review.
This review is also unable to offer information on the relative cost effectiveness of the interventions assessed, due to unavailability of data. Further, the trials included in this review typically focused on women who had a history of unexplained pregnancy loss. With the exception of thrombophilia disorders, no trials targeted women with (other) specific risk factors for stillbirth that may have explained or contributed to their previous pregnancy losses; such as diabetes, hypertensive disorders, obesity, smoking, or alcohol use.
With regard to the data that were available for this review, we found only a small number of trials, many of which had relatively few participants (only Schleussner 2015 included more than 200 women). Sample sizes for our meta‐analyses were further reduced by restricting our data extraction to women who had experienced a previous stillbirth of 20 weeks' gestation or more, in accordance with the review's inclusion criteria. As a result, the analyses were not sufficiently powered to detect differences in most of the outcomes assessed. We are therefore unable to draw firm conclusions about the potential effectiveness of the specified interventions for improving outcomes in subsequent pregnancies following stillbirth.
The overall completeness and applicability of evidence was also limited by variation in the characteristics of the women included in the trials. Most of the trials focused on recurrent pregnancy loss, but recurrent pregnancy loss was variably defined (e.g. the number of previous deaths, their gestational age cut‐off points, and whether the deaths were consecutive). Although we extracted outcome data exclusively from women who had a previous stillbirth of 20 weeks' gestation or more, as stated in our review protocol, the broader trial populations from which these women were recruited remained somewhat heterogeneous. Variation in the primary objectives of trials, and therefore their inclusion and exclusion criteria, was also evident. Most trials focused on the prevention of recurrent pregnancy loss and reported livebirth rates as primary outcomes, but three of the trials (Martinelli 2012; Rey 2009; Salim 2016) focused directly on the prevention of recurrent placenta‐mediated complications such as pre‐eclampsia, and measured stillbirth as part of a composite outcome. The impact of these variations has been mitigated somewhat by the separation of comparisons according to interventions and comparators, but should be highlighted nonetheless.
The trials in this review recruited women who had a history of pregnancy loss more broadly, rather than a history specifically of stillbirth. The women had therefore experienced previous pregnancy losses at varying stages of pregnancy. Again, while we only extracted data from the population of women relevant to this review (women who had a previous stillbirth of 20 weeks' gestation or more), it is important to consider the broader context in which the trials were conducted, given the widely varying pathophysiology of deaths according to gestational age (Silver 2011). This emphasises the urgent need for trials that specifically address therapeutic or management strategies in pregnancies after stillbirth, as opposed to other forms of pregnancy loss. Importantly, future trials should adequately describe and consider the cause of the previous stillbirth in the study population, as this will affect both the choice of intervention and its potential effect size. For example, LDA may be beneficial in women with maternal vascular malperfusion, but it would not be expected to have a beneficial effect in women who had stillbirth secondary to preterm prelabour rupture of membranes.
Finally, all but one of the trials (Ahmed 2014) were conducted in high‐income countries. Of the interventions considered in this review, while aspirin is a simple and relatively inexpensive medical therapy that is feasible to implement in low‐resource settings, it would be considerably more difficult to implement expensive immunotherapies such as third‐party leukocyte immunisation and intravenous IgG in these settings (Wong 2014). Additionally, such interventions may have limited relevance in LMICs, where stillbirths appear to be caused far more often by infections, antepartum haemorrhage, pre‐eclampsia/eclampsia, and intrapartum complications (Goldenberg 2016; McClure 2018), than by maternal immunological disturbances that affect largely the earlier developmental stages of pregnancy.
Quality of the evidence
The risk of bias in the trials in terms of their methodology and reporting was low to moderate. Most of the trials described adequate methods of random sequence generation, allocation concealment, and blinding of outcome assessment. For most trials there appeared to be low risk of attrition bias. However, the trials assessing LMWH (Ahmed 2014; Gris 2004; Martinelli 2012; Rey 2009; Salim 2016; Schleussner 2015) were at high or unclear risk of performance bias, due to a lack (or possible lack) of blinding of participants. The risk of reporting bias was unclear in most trials, due to unavailability of study protocols. The trials of the highest quality were those assessing third‐party leukocyte immunisation (Christiansen 1994) and intravenous IgG (Christiansen 1995; Christiansen 2002). Limited methodological details were available for the trial assessing progestogen (Levine 1964), which we judged to be of the lowest quality.
We used the GRADE methodology to assess the quality of evidence provided for the two main comparisons, on the outcomes of stillbirth, neonatal death, adverse perinatal outcome, adverse maternal psychological effects, perinatal mortality, preterm birth (< 37 weeks), and maternal‐infant attachment. The 'Summary of findings' tables (see Table 1; Table 2) show the quality of evidence across these critical outcomes to be very low to low. As outlined already, we downgraded evidence predominantly due to serious imprecision in the effect estimates; specifically, wide confidence intervals crossing the line of no effect, small sample sizes, and low event rates. We also downgraded some outcomes due to design shortcomings in the associated trials.
Potential biases in the review process
We conducted our review in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We aimed to reduce bias wherever possible by having two review authors independently assess trial eligibility, perform data extraction, and carry out 'Risk of bias' evaluations and GRADE assessments of evidence.
We aimed to reduce bias in trial selection by comprehensive searches of available data. We conducted the original search for trials in this area using Cochrane Pregnancy and Childbirth's Trials Register, and included trials directly addressing stillbirth/late fetal loss. We subsequently determined that, given varying nomenclature in the literature and varying definitions of stillbirth in terms of gestational age, it was necessary to expand this search. We liaised with the Cochrane Pregnancy and Childbirth Group's Information Specialist to carry out a second search, ensuring the capture of trials dealing with pregnancy loss/miscarriage/abortion in addition to stillbirth/fetal death, as these trials potentially included women who had experienced a previous stillbirth according to our definition (a death of 20 weeks' gestation or more). Our additional searches for ongoing trials within ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) also followed this principle.
While our searches were exhaustive, we have not included data from a number of potentially relevant trials. Specifically, our assessments of the eligibility of trials necessitated contacting the authors of 90 trials, as many of the yielded search results used terms such as previous 'pregnancy loss', 'miscarriage', 'abortion', and 'fetal death' without defining these by gestational age, rendering it unclear whether these trials would be eligible for inclusion. In addition, some of the trials captured in our searches included women who had experienced a previous stillbirth of 20 weeks' gestation or more alongside women who had experienced a previous earlier pregnancy loss, necessitating a disaggregation of data in order for the trial to be eligible for inclusion. Of the 90 potentially eligible trials, 11 were confirmed to include only women who had a previous pregnancy loss of less than 20 weeks' gestation, and we can therefore be confident of the non‐applicability of these trials to the current review. However, we excluded 66 trials for other reasons (see Study Flow Diagram:Figure 1). The evidence presented in this review is therefore potentially biased towards trials for which the trialists were contactable, and willing and able to assist with the necessary data extraction.
We were unable to explore the potential for publication bias statistically, due to insufficient numbers of trials within each meta‐analysis.
Agreements and disagreements with other studies or reviews
It is difficult to compare the results of this review against those of existing reviews, due to inconsistencies in review characteristics and objectives and, particularly, in trial populations. The limited data available for this review, alongside variation across existing reviews in adopted nomenclature and the definitions of terms such as 'miscarriage', 'abortion', and 'fetal death', limit our capacity to make meaningful contrasts in this regard. There are no existing Cochrane Reviews of interventions specific to the population of parents who have experienced, or are at risk of, a recurrent stillbirth of 20 weeks' gestation or more.
Nonetheless, a Cochrane Review of immunotherapy for recurrent miscarriage (Wong 2014), including three trials assessing third‐party leukocyte immunisations and eight assessing intravenous IgG, found that neither intervention increased rates of livebirth when compared to placebo (the same was shown for the other immunotherapies assessed; i.e. paternal white cell immunisation and trophoblast membrane immunisation). The review methods did not define recurrent miscarriage in terms of gestational age, but women who had a previous stillbirth of 20 weeks' gestation or more appeared to be among the trials included (and their data analysed together with women who had previous pregnancy losses at earlier gestational ages). The review concluded that such immunotherapies were ineffective in improving rates of livebirth among women with unexplained recurrent miscarriage and should therefore be abandoned, particularly given the financial cost and potentially harmful side effects of these therapies (as noted in the Christiansen 1994 trial).
A Cochrane Review of progestogens for preventing recurrent miscarriage has also been conducted (Haas 2018). The review evaluated the effects of progestogen treatment during the first trimester of pregnancy, among women who had a history of miscarriage, which was usually defined as pregnancy loss at less than 20 weeks' gestation, and was typically of unknown aetiology. Data from 11 trials assessing progestogens against placebo or no treatment among 2359 women showed a probable reduction in miscarriage for the intervention group, which appeared more pronounced for women who had a history of three or more miscarriages, compared to women who had a history of two or more miscarriages. Meta‐analyses of three included trials and 1199 women showed a possible reduction in stillbirth (measured as a secondary outcome and defined in accordance with this review), although the authors urged a high degree of caution in interpreting this finding, as the outcome was driven largely by one trial with a potentially disproportionate rate of miscarriage (Haas 2018). Elsewhere, a meta‐analysis of 10 RCTs, including 1586 women with unexplained recurrent pregnancy loss (up to 24 weeks' gestation) found that progestogens initiated in the first trimester were associated with higher livebirth rates when compared to placebo or no treatment (Saccone 2017). Importantly, the beneficial effects of therapy were evident only among the eight trials assessing progestins (synthetic progesterone), not in those assessing natural progesterone. The authors called for further research to identify the most effective preparations, doses, and routes of administration of such progestins for preventing recurrent unexplained pregnancy loss (Saccone 2017).
A growing body of evidence for the use of anticoagulants (aspirin or heparin, or both) during pregnancy is available. A Cochrane Review of nine trials including 1228 women with unexplained recurrent miscarriage, with or without inherited thrombophilia, found that evidence of a beneficial effect of anticoagulants on rates of livebirth among this population was lacking, regardless of the type and combinations of anticoagulants administered (De Jong 2014). The review, which included women who had two or more previous miscarriages up to 24 weeks' gestation, also showed no evidence of benefit of anticoagulants for secondary outcomes, including preterm delivery, pre‐eclampsia, and intrauterine growth restriction. Side effects including bleeding and skin irritation were not consistently reported across the trials, but appeared more frequent among the women receiving LMWH in one study. Given the low number of included trials and their varying methodological quality, the authors called for further trials addressing this research question. A Cochrane Review of aspirin or heparin (or both) for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss is currently underway (Scheres 2017).
More recent evidence for the use of heparin varies. An individual‐patient‐data (IPD) meta‐analysis (Rodger 2016) of 963 women across eight trials found no benefit of LMWH on placenta‐mediated complications, except among women who had a previous placental abruption. It is possible that unfractionated heparin has a therapeutic effect on placenta‐mediated complications within the sub‐population of women exhibiting antiphospholipid antibodies, but further research is required (Duffett 2015). A meta‐analysis of eight trials including 483 women who had inherited thrombophilia (Skeith 2016) found no benefit of LMWH for preventing recurrent late pregnancy loss (> 10 weeks) in this sub‐population.
The largest body of evidence promulgating the use of LDA during pregnancy centres on the prevention of pre‐eclampsia and its sequelae. A Cochrane Review of 37,560 women at risk of pre‐eclampsia across 59 trials found antiplatelet agents (predominantly LDA) reduced the risk of pre‐eclampsia by 17% when compared to placebo or no treatment, alongside reductions in preterm birth and small‐for‐gestational age (Duley 2007). No reduction specifically in stillbirth was shown. However, when analysed as a composite outcome together with neonatal and infant death, antiplatelet agents reduced the risk of death by 14%. Analogous results were shown in an IPD meta‐analysis of 32,217 women across 31 trials (Askie 2007). Of note, subgroup analyses in the Askie 2007 report suggested antiplatelet agents (mainly LDA) reduced the risk of stillbirth among women in their second or subsequent pregnancy who had a history of hypertensive disorder, when compared to women in their second or subsequent pregnancy with no such history of hypertensive disorder, although the authors advised discretion in interpreting this finding due to the role of chance when performing multiple comparisons.
More recent systematic reviews have further reinforced the benefits of LDA among populations of women at risk of pre‐eclampsia, particularly when therapy is started early in pregnancy. As shown by Bujold 2010, among the included trials in which LDA was started within the first 16 weeks of pregnancy, a reduction in pre‐eclampsia and intrauterine growth restriction was evident. LDA also reduced the risk of severe pre‐eclampsia, gestational hypertension, and preterm birth within this sub‐population of trials. No such benefits of LDA were shown among the trials in which LDA was started after 16 weeks. A further systematic review including 27,222 women across 42 trials found that LDA started within the first 16 weeks of pregnancy reduced the risk of perinatal death when compared to control (Roberge 2013), suggesting that interventions to improve placental perfusion need to begin early in pregnancy to positively affect placental development.
Our review is unable to offer any information about psychosocial support interventions. However, a recent systematic review of RCTs (San Lazaro 2017) assessing interventions to reduce stress, anxiety, or depression among women in pregnancies after miscarriage (defined as pregnancy loss from conception until 24 weeks' gestation), found no eligible trials.
We have not addressed other interventions in this review, including information and decision‐making support on interpregnancy interval and pre‐conception health, early or regular ultrasound surveillance, elective induction of labour, elective early caesarean birth, and intrapartum monitoring, due to a lack of eligible trials. To our knowledge, there are no systematic reviews or individual RCTs assessing these interventions in the context of prevention of recurrent stillbirth.
Authors' conclusions
Implications for practice.
There is insufficient evidence in this review to inform clinical practice about the effectiveness of interventions to improve care prior to and during subsequent pregnancies following stillbirth. The scarcity of eligible trials, the limited amount of data available, and the imprecision in the data presented in this review do not permit the formulation of any clear conclusions or implications for practice.
Implications for research.
This review highlights the urgent need for well‐designed trials evaluating the effect of interventions for improving outcomes among parents entering or considering a subsequent pregnancy following stillbirth. Such trials should target this specific population of parents, and define clear and consistent objectives, inclusion criteria, and outcomes related to the intervention assessed. Such trials also need to be sufficiently powered to detect differences in statistically rare but important outcomes, such as stillbirth and neonatal death. To detect a 30% reduction in stillbirth in a subsequent pregnancy (from 2.5% to 1.75%) with 80% power requires almost 6000 participants in each arm. Achieving adequate statistical power is therefore unlikely to be feasible in single‐centre RCTs, but may be feasible by conducting pragmatic, multicentre, possibly international RCTs, that enable data collection from the requisite large sample sizes.
Future trials of LDA specifically for the prevention of stillbirth and recurrent stillbirth appear warranted. Given the simplicity and low financial costs of this medical therapy, such trials would be feasible to conduct across diverse settings, including LMICs. However, we must acknowledge the challenges in undertaking trials adopting a randomised design in this context. There are scarce data on the contemporary feasibility of RCTs comparing a specific intervention to placebo in this population. As one potential barrier, women who have had a previous stillbirth secondary to placental malperfusion, which has a considerable recurrence risk (Monari 2016), may not be willing to be randomised to a placebo arm in a subsequent pregnancy. Furthermore, their treating clinicians may not endorse such a trial, given its potential lack of clinical equipoise. Consequently, data are needed to determine the most appropriate methodologies to evaluate such therapies. Stepped‐wedge cluster‐RCTs provide (all) participants with the given intervention in a stepwise fashion, and therefore may overcome some of the ethical and logistical difficulties associated with traditional RCTs in this context (Hussey 2007; Mdege 2011). The APPLE feasibility trial (Rodger 2017), estimated for completion in 2020, may shed light on the specific reasons for non‐consent into RCTs addressing recurrent pregnancy loss.
This review has also highlighted many challenges in compiling evidence addressing the current and related review questions within the pregnancy and childbirth context. Consistency in the definition of key outcomes, such as stillbirth, alongside consistency in data collection and presentation, will considerably aid the meaningful interpretation of evidence within this space. Future trials (including those that report data from non‐randomised/observational studies) may benefit from a core outcomes data set for stillbirth research, which may promote consistency in nomenclature, and facilitate the collection of data across well‐defined populations and outcome measures. Such a core outcomes data set should include short‐ and longer‐term psychosocial outcomes for families, as well as economic outcomes, both of which were largely absent from measurement in the trials in this review.
As the bulk of evidence available to inform clinical practice for care in subsequent pregnancies following a stillbirth, at least in RCTs, appears focused heavily on medical aspects of care, psychosocial interventions addressing maternal‐fetal attachment and parental anxiety and depression are particularly called for. Ensuring clinical equipoise, these trials could randomise parents to different forms of support to determine which interventions yield the greatest benefit for parents and families, with the least economic cost.
Acknowledgements
We thank the Cochrane Pregnancy and Childbirth Group for support with title registration and protocol development.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team) and the Group's Statistical Adviser.
We thank the following trialists, and their research teams, for completing the required analyses and data request forms that enabled each trial to be included in this review. We appreciate that some trialists consulted primary medical files without any financial support in order to supply the required data.
Dr Farhat Ahmed, Fatima Memorial College of Medicine & Dentistry, Lahore, Pakistan;
Professor Ole Christiansen, Aalborg University Hospital and Copenhagen University Hospital, Denmark;
Drs Gali Garmi and Raed Salim, Emek Medical Center and Rappaport Faculty of Medicine, Haifa, Israel;
Professor Jean‐Christophe Gris, Groupe Hospitalo‐Universitaire Caremeau, Nîmes cédex, France;
Drs Ida Martinelli and Annalisa Perna, IRCCS Foundation Ca 'Granda‐Major Hospital Policlinico, Milan, Italy;
Dr Evelyne Rey, CHU Sainte Justine, Montréal, Québec, Canada; and
Dr David Petroff and Professor Ekkehard Schleussner, Universitaet Leipzig Haertelstr, Leipzig, Germany.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
Search terms were: 'stillbirth AND previous'; 'miscarriage AND previous'; 'abortion AND previous'; and 'pregnancy loss AND previous'.
NB: The terms 'miscarriage', 'pregnancy loss', and 'abortion' were included to account for varying definitions of stillbirth in terms of gestational age (e.g. some reports may define 'miscarriage' as a fetal death < 24 weeks, which would consequently include some stillbirths, according to our definition of 20 weeks' gestation or more).
Data and analyses
Comparison 1. LMWH vs no treatment/standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.40, 16.62] |
| 2 Neonatal death | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.20, 3.32] |
| 4 Adverse maternal psychological effects (anxiety, depression or complicated grief) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 5 Perinatal mortality | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.40, 16.62] |
| 6 Very preterm birth (28 to < 32 weeks) | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.82] |
| 7 Late preterm birth (32 to < 37 weeks) | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.66] |
| 8 Any preterm birth (birth < 37 weeks) | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.58, 1.74] |
| 9 Birthweight | 3 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐225.26 [‐546.36, 95.84] |
| 10 Low birthweight | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.45, 2.21] |
| 11 Small‐for‐gestational age | 3 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.57, 3.08] |
| 12 Apgar score less than seven at five minutes | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.58, 19.29] |
| 13 Respiratory distress syndrome | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal jaundice | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adherence to the intervention | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.22] |
| 16 Caesarean birth (elective) | 3 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.83, 5.07] |
| 17 Caesarean birth (emergency) | 3 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.39, 1.99] |
| 18 Induction of labour | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.22] |
| 19 Instrumental vaginal birth | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 20 Placental abruption | 3 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04, 2.91] |
| 21 Pre‐eclampsia | 3 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.04, 4.10] |
| 22 Gestational diabetes | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.50, 3.25] |
| 23 Chorioamnionitis | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.33, 7.86] |
| 24 Postpartum haemorrhage | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.27, 9.45] |
| 25 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 3 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 6.27] |
| 26 Maternal antenatal admission | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
| 27 Duration of maternal hospital stay | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐2.01, 1.97] |
| 28 Duration of neonatal hospital stay | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐8.70, 12.10] |
| 29 Admission to the neonatal intensive care unit | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.27, 2.38] |
| 30 Duration of neonatal intensive care unit stay | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐25.13, 33.53] |
1.2. Analysis.
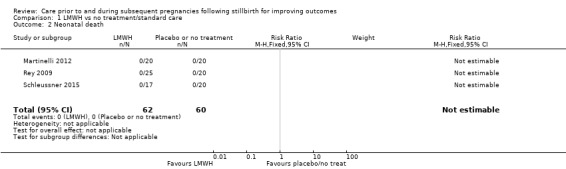
Comparison 1 LMWH vs no treatment/standard care, Outcome 2 Neonatal death.
1.13. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 13 Respiratory distress syndrome.
1.14. Analysis.
Comparison 1 LMWH vs no treatment/standard care, Outcome 14 Neonatal jaundice.
Comparison 2. LDA vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 2 Neonatal death | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.38] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.34] |
| 4 Perinatal mortality | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.04, 4.06] |
| 5 Very preterm birth (28 to < 32 weeks) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.38] |
| 6 Late preterm birth (32 to < 37 weeks) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 7 Any preterm birth (birth < 37 weeks) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.04, 4.06] |
| 8 Birthweight | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 790.0 [295.03, 1284.97] |
| 9 Low birthweight | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.34] |
| 10 Small‐for‐gestational age | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.38] |
| 11 Respiratory distress syndrome | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.38] |
| 12 Adherence to the intervention | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.86, 1.17] |
| 13 Caesarean birth (elective) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.18, 2.24] |
| 14 Caesarean birth (emergency) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.38, 7.55] |
| 15 Induction of labour | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.38, 7.55] |
| 16 Instrumental vaginal birth | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.12, 57.44] |
| 17 Placental abruption | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.12, 57.44] |
| 18 Pre‐eclampsia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.14, 5.06] |
| 19 Gestational diabetes | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.04, 4.06] |
| 20 Postpartum haemorrhage | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.38] |
| 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Antenatal care attendance | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.86, 1.17] |
| 23 Duration of maternal hospital stay | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.41, 2.41] |
| 24 Duration of neonatal hospital stay | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐4.41, 0.41] |
| 25 Admission to the neonatal intensive care unit | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.58, 3.75] |
| 26 Duration of neonatal intensive care unit stay | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.29, 1.29] |
2.21. Analysis.
Comparison 2 LDA vs placebo, Outcome 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
Comparison 3. LDA + LMWH vs LDA alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 6.23] |
| 2 Neonatal death | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.29, 20.75] |
| 4 Perinatal mortality | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 6.23] |
| 5 Very preterm birth (28 to < 32 weeks) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.11, 56.03] |
| 6 Late preterm birth (32 to < 37 weeks) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.06, 11.77] |
| 7 Any preterm birth (birth < 37 weeks) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.41, 25.64] |
| 8 Birthweight | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐650.0 [‐1210.33, ‐89.67] |
| 9 Low birthweight | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.17, 15.99] |
| 10 Small‐for‐gestational age | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.11, 56.03] |
| 11 Respiratory distress syndrome | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.11, 56.03] |
| 12 Adherence to the intervention | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.88, 1.14] |
| 13 Caesarean birth (elective) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.40, 4.63] |
| 14 Caesarean birth (emergency) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.03, 1.60] |
| 15 Induction of labour | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.17, 2.25] |
| 16 Instrumental vaginal birth | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.06, 11.77] |
| 17 Placental abruption | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.06, 11.77] |
| 18 Pre‐eclampsia | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 4.00] |
| 19 Gestational diabetes | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 6.23] |
| 20 Postpartum haemorrhage | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.11, 56.03] |
| 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Antenatal care attendance | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.88, 1.14] |
| 23 Duration of maternal hospital stay | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.55, 2.55] |
| 24 Duration of neonatal hospital stay | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.55, 4.55] |
| 25 Admission to the neonatal intensive care unit | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 26 Duration of neonatal intensive care unit stay | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.38, 4.38] |
3.2. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 2 Neonatal death.
3.21. Analysis.
Comparison 3 LDA + LMWH vs LDA alone, Outcome 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
Comparison 4. LDA + LMWH vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.30] |
| 2 Neonatal death | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.30] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.17, 2.80] |
| 4 Perinatal mortality | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 5 Very preterm birth (28 to < 32 weeks) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.05, 9.86] |
| 6 Late preterm birth (32 to < 37 weeks) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.05, 9.86] |
| 7 Any preterm birth (birth < 37 weeks) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.30, 6.25] |
| 8 Birthweight | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 140.0 [‐501.26, 781.26] |
| 9 Low birthweight | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 10 Small‐for‐gestational age | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.05, 9.86] |
| 11 Respiratory distress syndrome | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.05, 9.86] |
| 12 Adherence to the intervention | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.87, 1.16] |
| 13 Caesarean birth (elective) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.50] |
| 14 Caesarean birth (emergency) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.34] |
| 15 Induction of labour | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.20, 5.19] |
| 16 Instrumental vaginal birth | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.09, 47.68] |
| 17 Placental abruption | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.09, 47.68] |
| 18 Pre‐eclampsia | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.34] |
| 19 Gestational diabetes | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 20 Postpartum haemorrhage | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.05, 9.86] |
| 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Antenatal care attendance | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.87, 1.16] |
| 23 Duration of maternal hospital stay | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.64, 2.64] |
| 24 Duration of neonatal hospital stay | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.64, 2.64] |
| 25 Admission to the neonatal intensive care unit | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.72, 4.10] |
| 26 Duration of neonatal intensive care unit stay | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.48, 3.48] |
4.21. Analysis.
Comparison 4 LDA + LMWH vs placebo, Outcome 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
Comparison 5. LMWH vs LDA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.16, 78.56] |
| 2 Neonatal death | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.16, 78.56] |
| 4 Perinatal mortality | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.16, 78.56] |
| 5 Very preterm birth (28 to < 32 weeks) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Late preterm birth (32 to < 37 weeks) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.09, 16.84] |
| 7 Any preterm birth (birth < 37 weeks) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.06, 5.69] |
| 8 Birthweight | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 75.0 [‐151.69, 301.69] |
| 9 Low birthweight | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.06, 5.69] |
| 10 Small‐for‐gestational age | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.06, 5.69] |
| 11 Apgar score less than seven at five minutes | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.16, 3.88] |
| 12 Respiratory distress syndrome | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.09, 16.84] |
| 13 Neonatal jaundice | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.72] |
| 14 Caesarean birth (elective) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.20, 7.05] |
| 15 Caesarean birth (emergency) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.06, 5.69] |
| 16 Induction of labour | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.27 [0.48, 143.35] |
| 17 Instrumental vaginal birth | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Placental abruption | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Pre‐eclampsia | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.06, 5.69] |
| 20 Gestational diabetes | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postpartum haemorrhage | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Breastfeeding | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.35, 2.64] |
| 24 Maternal antenatal admission | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.06, 5.69] |
| 25 Duration of maternal hospital stay | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.19, 0.19] |
| 26 Duration of neonatal hospital stay | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.30, 0.30] |
| 27 Admission to the neonatal intensive care unit | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.09, 16.84] |
| 28 Duration of neonatal intensive care unit stay | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐8.52, 8.96] |
5.2. Analysis.
Comparison 5 LMWH vs LDA, Outcome 2 Neonatal death.
5.5. Analysis.
Comparison 5 LMWH vs LDA, Outcome 5 Very preterm birth (28 to < 32 weeks).
5.17. Analysis.
Comparison 5 LMWH vs LDA, Outcome 17 Instrumental vaginal birth.
5.18. Analysis.
Comparison 5 LMWH vs LDA, Outcome 18 Placental abruption.
5.20. Analysis.
Comparison 5 LMWH vs LDA, Outcome 20 Gestational diabetes.
5.21. Analysis.
Comparison 5 LMWH vs LDA, Outcome 21 Postpartum haemorrhage.
5.22. Analysis.
Comparison 5 LMWH vs LDA, Outcome 22 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
Comparison 6. LMWH (adjusted dose) vs LMWH (fixed dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Very preterm birth (28 to < 32 weeks) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.07, 4.95] |
| 3 Late preterm birth (32 to < 37 weeks) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.00] |
| 4 Any preterm birth (birth < 37weeks) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 1.95] |
| 5 Birthweight | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 812.0 [‐257.81, 1881.81] |
| 6 Low birthweight | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 7 Small‐for‐gestational age | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 8 Apgar score less than seven at five minutes | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adherence to the intervention | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.76, 1.31] |
| 10 Caesarean birth (elective) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 11 Caesarean birth (emergency) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.07, 4.95] |
| 12 Induction of labour | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.28, 1.77] |
| 13 Instrumental vaginal birth | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Placental abruption | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 7.93] |
| 15 Pre‐eclampsia | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 7.93] |
| 16 Gestational diabetes | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 7.93] |
| 17 Chorioamnionitis | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Postpartum haemorrhage | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.64] |
| 19 Serious maternal outcome (death, cardiac arrest, respiratory arrest, admission to intensive care) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Admission to neonatal intensive care unit | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.07, 4.95] |
6.1. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 1 Stillbirth.
6.8. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 8 Apgar score less than seven at five minutes.
6.13. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 13 Instrumental vaginal birth.
6.17. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 17 Chorioamnionitis.
6.19. Analysis.
Comparison 6 LMWH (adjusted dose) vs LMWH (fixed dose), Outcome 19 Serious maternal outcome (death, cardiac arrest, respiratory arrest, admission to intensive care).
Comparison 7. Leukocyte immunisation vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Neonatal death | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perinatal mortality | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Very preterm birth (28 to < 32 weeks) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Late preterm birth (32 to < 37 weeks) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 5.33] |
| 7 Any preterm birth (birth < 37 weeks) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 5.33] |
| 8 Birthweight | 1 | 4 | Mean Difference (IV, Fixed, 95% CI) | 1195.0 [273.35, 2116.65] |
| 9 Low birthweight | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 5.33] |
| 10 Small‐for‐gestational age | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Apgar score less than seven at five minutes | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Adherence to the intervention | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.49, 2.05] |
| 13 Caesarean birth (elective) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Caesarean birth (emergency) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 5.33] |
| 15 Induction of labour | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Instrumental vaginal birth | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Placental abruption | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Pre‐eclampsia | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Gestational diabetes | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Chorioamnionitis | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 1 Stillbirth.
7.2. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 2 Neonatal death.
7.3. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity).
7.4. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 4 Perinatal mortality.
7.5. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 5 Very preterm birth (28 to < 32 weeks).
7.10. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 10 Small‐for‐gestational age.
7.11. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 11 Apgar score less than seven at five minutes.
7.13. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 13 Caesarean birth (elective).
7.15. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 15 Induction of labour.
7.16. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 16 Instrumental vaginal birth.
7.17. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 17 Placental abruption.
7.18. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 18 Pre‐eclampsia.
7.19. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 19 Gestational diabetes.
7.20. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 20 Chorioamnionitis.
7.21. Analysis.
Comparison 7 Leukocyte immunisation vs placebo, Outcome 21 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
Comparison 8. Intravenous IgG vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.40] |
| 2 Neonatal death | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.40] |
| 4 Perinatal mortality | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.40] |
| 5 Very preterm birth (28 to < 32 weeks) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Late preterm birth (32 to < 37 weeks) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.03, 7.52] |
| 7 Any preterm birth (birth < 37 weeks) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.03, 7.52] |
| 8 Low birthweight | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.24, 67.71] |
| 9 Small‐for‐gestational age | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Apgar score less than seven at five minutes | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Respiratory distress syndrome | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Neonatal jaundice | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Adherence to the intervention | 2 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.69, 1.44] |
| 14 Caesarean birth (elective) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.18, 12.46] |
| 15 Caesarean birth (emergency) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Induction of labour | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.24, 37.67] |
| 17 Instrumental vaginal birth | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.21, 6.35] |
| 18 Placental abruption | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Pre‐eclampsia | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Gestational diabetes | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Chorioamnionitis | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postpartum haemorrhage | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care) | 2 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Admission to the neonatal intensive care unit | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.2. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 2 Neonatal death.
8.5. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 5 Very preterm birth (28 to < 32 weeks).
8.9. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 9 Small‐for‐gestational age.
8.10. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 10 Apgar score less than seven at five minutes.
8.11. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 11 Respiratory distress syndrome.
8.12. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 12 Neonatal jaundice.
8.15. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 15 Caesarean birth (emergency).
8.18. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 18 Placental abruption.
8.19. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 19 Pre‐eclampsia.
8.20. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 20 Gestational diabetes.
8.21. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 21 Chorioamnionitis.
8.22. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 22 Postpartum haemorrhage.
8.23. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 23 Serious maternal outcome (death; cardiac arrest; respiratory arrest; admission to intensive care).
8.24. Analysis.
Comparison 8 Intravenous IgG vs placebo, Outcome 24 Admission to the neonatal intensive care unit.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2014.
| Methods | Randomised controlled trial (3‐armed) | |
| Participants | 172 women were randomised. Data from 40 eligible women were included in this review Setting: Fatima Memorial Hospital, Pakistan, tertiary referral obstetric hospital with advanced obstetric and gynaecology care and neonatology care Women with a diagnosis of idiopathic recurrent miscarriage (defined as 2 consecutive miscarriages, loss < 24 weeks' gestation, with urine for pregnancy test positive along with clinical manifestation of miscarriage or ultrasonographic evidence of histopathological evidence of products of conception) Dates of recruitment: not stated Inclusion criteria: previous idiopathic recurrent miscarriage (2 or more consecutive losses < 24 weeks' gestation after exclusion of all known causes of recurrent miscarriage) Exclusion criteria: not stated |
|
| Interventions |
Aspirin: 75 mg oral aspirin daily from 6 weeks onward (fetal cardiac activity positive) until 36 weeks. Number randomised unclear (see notes), data from 54 women were analysed Combined aspirin and enoxaparin (heparin): 40 mg enoxaparin (LMWH) subcutaneous injection daily from 6 weeks onward (fetal cardiac activity positive) until start of labour. Aspirin as above. Number randomised unclear (see notes), data from 56 women were analysed Placebo: intensive pregnancy surveillance with placebo in similar packs to aspirin, orally daily from 6 weeks onward until 36 weeks. Number randomised unclear (see notes), data from 50 women were analysed |
|
| Outcomes | Primary outcome: livebirth rate. Secondary outcomes: serious adverse events during pregnancy (miscarriage, intrauterine fetal death (fetal death > 24 weeks)), SGA, preterm birth, APH and PPH. | |
| Funding source(s) | Not reported in abstract; trialists confirmed there was no funding for the trial | |
| Declarations of interest | Not reported in abstract; trialists confirmed there were no conflicts of interests | |
| Information source(s) | Primary publication (published as abstract only) and completed data request form | |
| Notes | Assessment of trial methodology and risk of bias was carried out based on the trial abstract only (and additional information provided by the trialists). 12 women were excluded post‐randomisation, though unclear to which group these women had been assigned (2 participants did not want to continue Clexane after 24 weeks' gestation. A further 7 participants were lost to follow‐up and 3 did not adhere to treatment) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An online computer program was used to generate the random sequence. We assumed the number of patients to be booked during the study period (2007‐2013) to be 400 (though the actual number enrolled was 172) and created 3 arms, placebo, aspirin alone and aspirin with Clexane [heparin]. We followed the random sequence as generated by the program as the patients were booked/enrolled in the study" (information provided by trialists) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, consecutively‐labelled envelopes which were taken in order as the women were booked/enrolled in the study (information provided by trialists) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | While it was reported that the aspirin and placebo tablets were provided in similar packs, there did not appear to be a placebo for the heparin (enoxaparin) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Lack of blinding unlikely to influence objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No detail was provided as to whether outcome assessors were blinded for subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants did not want to continue Clexane after 24 weeks' gestation. A further 7 participants were lost to follow‐up and 3 did not adhere to treatment, but it is not clear to which group these participants were assigned |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting with no access to published trial protocol; trial published in abstract form only |
| Other bias | Unclear risk | Insufficient methodological detail provided in abstract to determine other potential sources of bias |
Christiansen 1994.
| Methods | Randomised controlled trial | |
| Participants | 75 women were randomised. Data from 4 eligible women were included in this review Setting: Aalborg Hospital, Denmark Dates of recruitment: appeared to commence from October 1987, end date not stated Inclusion criteria: women with recurrent unexplained miscarriage who had suffered at least 3 consecutive miscarriages; with no abnormalities in the non‐immunological investigation programme; with a maximum of 1 pregnancy loss after 14 weeks' gestation; who were not consanguineous with spouse; who were negative for lymphocytotoxic antibodies; who were negative for lupus anticoagulant; who did not have antinuclear antibodies titres > 80 or anti‐DNA levels > 8.0 mg/L Exclusion criteria: auto‐antibodies including lupus anticoagulant, anticardiolipin antibodies, antinuclear antibodies, anti‐double‐stranded deoxyribonucleic‐acid (NB: anticardiolipin antibodies in the absence of lupus anticoagulant were not excluded) |
|
| Interventions |
Third‐party leukocytes: women were immunised with intravenous infusions with buffy coat (leukocyte enriched blood) from erythrocyte‐compatible third‐party blood donors. Women received the initial immunisations twice prior to conception 1 month apart. Before the immunisations, 200 mL of blood was drawn from each woman; after 1 hour women were infused intravenously with 150 mL of buffy coat from 2 third‐party donors. Women received 1 repeated infusion of buffy coat from 2 third‐party donors every 5th month until conception had occurred. Infusions were carried out on an outpatient basis. Total number randomised: n = 49 (43 analysed) Placebo: women were immunised with intravenous infusions of their own autologous blood, with the regimen as described above. Total number randomised: n = 26 (23 analysed) |
|
| Outcomes | Study report: livebirth; miscarriage; ectopic pregnancy; time from last immunisation to pregnancy; birthweight; preterm birth; Apgar score ≤ 7 at 5 minutes; malformations; admission to hospital in first year (and causes for admission); immunological parameters (lymphocyte subsets: levels of CD2+, CD4+, CD8+ and CD16+ cells (%) lymphocyte antibodies: positive (%)) | |
| Funding source(s) | Research Fund of Aalborg Voluntary Blood Donors, the Research Fund of the County of North Jutland and Aalborg Stifts Julelotteri (local diocese Christmas lottery) | |
| Declarations of interest | Not in study report; trialists confirmed there were no conflicts of interests | |
| Information source(s) | Primary publication and completed data request form | |
| Notes | There were no significant different in livebirths between the groups, but subgroup analyses of women with primary recurrent miscarriages (no previous livebirths) suggested benefit of immunisation, as did subgroup analyses of women with primary recurrent miscarriage and no pregnancies progressing beyond 14 weeks' gestation. A 2:1 allocation ratio was adopted to make participation more appealing and because a third allocation group involving immunisation with husbands' lymphocytes had been planned, but was later abandoned due to unacceptable side‐effects, including dizziness and formation of red cell antibodies. Severe reactions were noted in both women and children that could have been attributed to treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The leading consultant at the Department allocated participants by drawing lots |
| Allocation concealment (selection bias) | Low risk | The leading consultant at the Department performed allocation by drawing lots each time a new patient had signed the informed consent for participation. The consultant had not been in contact or known the reproductive history of the women. When allocation was done before pregnancy, a message was given to the blood bank to prepare allogenous or autologous buffy coat for the first transfusion within a few days |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the couple nor the women's obstetricians knew whether active treatment or placebo was provided" (study report p. 262) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Trialists confirmed that doctors involved in outcome assessment were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Women (who completed questionnaires 1 year after birth) and doctors were reported to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75 women were randomised; 9 (12%) were excluded from analyses, 6/49 (12%) from the intervention group, and 3/26 (12%) from the control group; all except one of the exclusions were due to women not being pregnant during the trial period |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting with no access to published trial protocol |
| Other bias | Low risk | No other obvious sources of bias identified |
Christiansen 1995.
| Methods | Randomised controlled trial | |
| Participants | 34 women were randomised. Data from 5 eligible women were included in this review Setting: Aalborg Hospital, Denmark. Dates of recruitment: not stated Inclusion criteria: women with 3 or more consecutive pregnancy losses (defined as spontaneous abortion or the unexplained intrauterine death of the fetus before 28 weeks' gestation), who either 1) had a history of secondary recurrent spontaneous abortion (3 or more spontaneous abortions subsequent to a birth) or 2) had at least 1 pregnancy loss after the 14th gestational week; for all women these previous pregnancy losses were unexplained (following hysterosalpingography or hysteroscopy, karyotyping and measurement of the mid‐luteal serum progesterone concentration). Women were identified preconception, and randomised once pregnancy was confirmed Exclusion criteria: systemic lupus erythematosus or IgA deficiency |
|
| Interventions |
Intravenous IgG: women received Nordimmun, a human IgG, given in a solution also containing human albumin and saccharose. Doses ranged from 25 ‐ 35 g depending on pre‐pregnancy bodyweight. Doses were administered weekly from 5 ‐ 8 weeks' gestation, then fortnightly from 8 ‐ 34 weeks' gestation. Total number randomised: n = 17 (17 analysed) Placebo: women receive a placebo – a solution containing only human albumin and saccharose. Women received the placebo according to the same regimen detailed above. Total number randomised: n = 17 (17 analysed) |
|
| Outcomes | Study report: successful pregnancies; surviving infants; birthweight; preterm birth; Apgar 10 after 5 minutes; congenital malformations; pregnancy complications; immunological parameters (APTT concentrations; antinuclear factor, anti‐ds‐DNA, rheumatoid factor, anticardiolipin, all autoantibodies; plasma C3 neodeterminants; total serum IgG; main lymphocyte subpopulations; ALAT concentrations); side effects/symptoms | |
| Funding source(s) | Novo‐Nordisk A/S, Denmark (commercial global health company), and Aalborg Stiftstidendes Julelotteri (local newspaper lottery) | |
| Declarations of interest | Not in study report; trialists confirmed there were no conflicts of interests | |
| Information source(s) | Primary publication and completed data request form | |
| Notes | The study report notes that no significant differences between the groups were evident at baseline on clinical and demographic characteristics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Quote: "The sealed envelope method" was used (study report p. 2,691) Envelopes were stored at the Department of Clinical Immunology, Aalborg Hospital and at the Nordisk Gentofte company, Copenhagen and reported to be opaque by trialists Quote: "The list assigned the pregnant patient the next free consecutive allocation number" (additional information provided by trialists) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. Quote: "The packages of Nordimmun/placebo could not be distinguished between, and the codes were blinded for both the patients and hospital staff, including the authors" (study report p. 2,691). The study treatments were packaged in identical bottles at the Nordisk Gentofte pharmaceutical company; the bottles could only be distinguished by allocation number (additional information provided by trialists) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Doctors involved in outcome assessment were reported to be blind by trialists |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Doctors involved in outcome assessment were reported to be blind by trialists |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes assessed using ITT, 100% compliance with treatment and no exclusions from the analyses |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting with no access to published trial protocol |
| Other bias | Low risk | No other obvious sources of bias identified, although the number of previous pregnancy losses "was accidentally biased at allocation" (study report p. 2,692) with women in the intervention group having a higher number (4.6 vs. 3.9; P < 0.10 "NS") |
Christiansen 2002.
| Methods | Randomised controlled trial | |
| Participants | 58 women were randomised. Data from 8 eligible women were included in this review Setting: Aalborg Hospital, Aalborg, Denmark Dates of recruitment: June 1994 to June 1999 Inclusion criteria: women with (i) a history of 4 or more confirmed unexplained miscarriages before the end of the 26th gestational week, of which the last 3 had been consecutive; (ii) no uterine or parental chromosomal abnormality; (iii) regular menstruations with cycle length between 21 and 35 days; (iv) written informed consent; and (v) a positive pregnancy test carried out at the hospital All miscarriages stated by the women were confirmed by searching hospital records or by contacting the practitioners. All women had normal findings by hysterosalpingography or hysteroscopy, and all couples had normal chromosomes by ordinary G‐band technique Exclusion criteria: (i) total IgA deficiency; (ii) autoimmune rheumatic disease; (iii) insulin‐dependent diabetes mellitus; (iv) pregnancy obtained by IVF or controlled ovarian stimulation; and (v) application to participate in the trial later than 7 days after the expected menstruation |
|
| Interventions |
Intravenous IgG: Nordimmun human IgG preparation, containing 4.6% human IgG, 1.5% human albumin, 4.6% sucrose and 0.15 mol/l sodium. Women received their first infusion immediately after randomisation; at each intravenous infusion until 20 weeks' gestation, 0.8 g of study drug per kg bodyweight was administered; from 20 to 26 weeks' gestation 1.0 g of study drug per kg bodyweight was given. Women received infusions weekly from gestational weeks 5 ‐ 10, then fortnightly until 26 weeks (total 14 infusions in successful pregnancies). All infusions were given on an outpatient basis Total number randomised: n = 29 (29 analysed) Placebo: women received a placebo drug containing 1.5% human albumin, 4.6% sucrose and 0.15 mol/l sodium Placebo given according to regimen described above Total number randomised: n = 29 (29 analysed) |
|
| Outcomes | Study report: live births; pregnancy losses/miscarriages; GA at birth; birthweight; Apgar score ≤ 7 at 5 minutes; caesarean section; neonatal disorders; maternal safety parameters (clinical symptoms; hepatitis and HIV transmission; signs of hepatic and renal affection); fetal safety parameter (health and development of child at 3 and 12 months after birth) | |
| Funding source(s) | HemaSure AS of Gentofte, Denmark (pharmaceutical company) | |
| Declarations of interest | Not in study report; trialists confirmed there were no conflicts of interest | |
| Information source(s) | Primary publication and completed data request form | |
| Notes | The study report notes that no significant differences between groups were evident at baseline on clinical and demographic characteristics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. Quote: "Allocation to the treatment arms was made according to a computer‐generated randomization list which was retained by HemaSure A/S, Copenhagen, during conduct of the trial" (study report p. 810) |
| Allocation concealment (selection bias) | Low risk | As above and
Quote: "The randomization code was blinded to the patients and hospital staff (including the authors) until after the last included patient had given birth and all data had been entered into a computer database in April 2000 by an independent clinical research organization (Ecron Wiedey GmbH, Konstanz, Germany)" (study report p. 810) Trialists confirmed patients were allocated to the next free consecutive number of randomisation from a list kept at the Department of Clinical Immunology, which gave no information on treatment group (the computer‐generated randomisation list, linking allocation numbers to treatment group was retained by HemaSure A/S during the trial); the drugs were packaged in identical bottles at HemaSure A/S, and were only distinguished by the allocation number (additional information provided by trialists) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial Quote: "The placebo drug could not be distinguished visually from the active drug. Bottles containing either Nordimmun or placebo were marked with their allocation numbers, but otherwise were identical" (study report p. 810) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Trialists confirmed that all doctors and nurses treating the patients involved in outcome assessment were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Trialists confirmed that all doctors and nurses treating the patients involved in outcome assessment were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes assessed using ITT, no participants were lost to follow‐up or discontinued infusions |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting with no access to published trial protocol |
| Other bias | Low risk | No other obvious sources of bias identified |
Gris 2004.
| Methods | Randomised controlled trial | |
| Participants | 174 women were randomised. Data from 22 eligible women were included in this review Setting: Mediterranean Abnormal Pregnancy Study Program; patients referred across the Southern French Region Languedoc‐Roussillon. Dates of recruitment: not stated Inclusion criteria: women with 1 single unexplained pregnancy loss from the 10th week of amenorrhoea with no unexplained pregnancy losses before the beginning of the 10th week of amenorrhoea and no explained pregnancy losses associated with a factor V Leiden mutation, a factor II G20210A mutation (all heterozygous), or a protein S deficiency Exclusion criteria: presumptive aetiologic factor (hysterosalpingogram, karyotype in both parents, glucose tolerance test, toxoplasmosis serology, thyroid function, serum prolactin levels, normal luteal phase of at least 12 days and plasma progesterone above 25 ng/mL, absence of antinuclear factor, or antiphospholipid/antiprotein antibodies (lupus anticoagulant, anticardiolipin, anti–beta2‐glycoprotein I, anti–annexinV, anti‐phosphatidylethanolamine, IgG, and IgM), absence of antithrombin or protein C deficiency, fasting plasma total homocysteine lower than 15 μM/L; any antecedent of venous or arterial thrombosis; any pregnancy loss before the beginning of the 10th week of amenorrhoea; any lethal fetal defect; fetal haemorrhage; pregnancy‐induced hypertension with its complications; any infectious disease during pregnancy; known erythroblastosis fetalis, immune thrombocytopenic purpura, or fetomaternal alloimmune thrombocytopenia; trauma during pregnancy; diabetes mellitus; tobacco consumption at least equal to 10 cigarettes a days |
|
| Interventions |
Enoxaparin (heparin): 40 mg LMWH enoxaparin, daily subcutaneous injection self‐administered percutaneously in the abdomen at 8 pm. Treatment began at the 8th week of amenorrhoea after a positive pregnancy test Number randomised unclear (see notes), data from 80 women were analysed Aspirin: 100 mg low‐dose aspirin, taken daily at 8 pm Number randomised unclear (see notes), data from 80 women were analysed |
|
| Outcomes | Outcomes reported in study report: live births; pregnancy losses from the beginning of the 8th week; preterm birth; pre‐eclampsia; caesarean birth; vaginal birth; birthweight; SGA; heparin‐induced thrombocytopenia, abnormal skin reactions, clinical manifestation of spontaneous bone pain; digestive intolerance; haemorrhages | |
| Funding source(s) | "Supported by grants from Diagnostica Stago, Biopep S.A., and Baxter Healthcare Corporation" (study report p 3,695). Institutional funding also received | |
| Declarations of interest | Not reported in study report; trialists confirmed there were no conflicts of interest | |
| Information source(s) | Primary publication and completed data request form | |
| Notes | 14 women were excluded post‐randomisation, though unclear to which group these women had been assigned All women took 5 mg daily folic acid from 1 month preconception and throughout pregnancy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was performed blindly and at random by an independent statistician using a computer random‐number generator to ensure equal distribution of women with specific thrombophilia disorders among the groups (e.g. half of women with Protein S deficiency allocated to each arm, half with Factor V Leiden mutation allocated to each arm) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed opaque envelopes sent by an independent statistician" (additional information provided by trialists). Whether envelopes were consecutively numbered was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and physicians were aware of the treatment being taken" (study report p. 3698) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Trialists confirmed outcome assessment was blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Trialists confirmed outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appeared that 174 women were randomised, and 14 (8%) were excluded from analyses with 160 analysed. 12 women had an early pregnancy loss and were excluded; unclear why additional 2 women were excluded. Not clearly reported from which groups these women were excluded |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting with no access to published trial protocol |
| Other bias | Low risk | No other obvious sources of bias identified |
Levine 1964.
| Methods | Quasi‐randomised controlled trial | |
| Participants | 56 women were randomised. Data from 7 eligible women were included in this review Setting: Chicago Board of Health Prenatal Clinics, Canada Dates of recruitment: not stated Inclusion criteria: women with 3 consecutive spontaneous abortions prior to the present pregnancy, 16 weeks' gestation or less at study enrolment, with no symptoms of threatened abortion, with voluntary agreement to the conditions of the study, with no obvious physical defects. Exclusion criteria: as above; no other exclusions reported |
|
| Interventions |
Progestogen/progestational agent (Delalutin): 500 mg injection of 17a‐hydroxyprogesterone caproate weekly, started at first visit, continued until the 36th week of pregnancy or termination, whichever occurred first Number randomised unclear (see notes), data from 15 women were analysed Placebo: weekly placebo injection, as above Number randomised unclear (see notes), data from 15 women were analysed |
|
| Outcomes | "Salvaged" pregnancies (live babies); abortions; complications of treatment; "significant undesirable manifestations" for women; discontinuation of injections; "hormonal effects" for infants. | |
| Funding source(s) | "Delalutin and placebo were supplied through the generosity of Dr. E. C. Reinfenstin, Jr., Squibb Institute for Medical Research, New Brunswick, New Jersey" (study report p. 31) | |
| Declarations of interest | Not reported in study report | |
| Information source(s) | Primary publication only | |
| Notes | 26 women were excluded post‐randomisation, though unclear to which group these women had been assigned Trialist now deceased; no further information sought. Results are reported descriptively only due to problems with data reporting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Trial was quasi‐randomised: Quote: "As patients were accepted into the study they were alternately placed into Group A or Group B" (study report p. 31) |
| Allocation concealment (selection bias) | High risk | As above, trial was quasi‐randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigation was designed as a controlled double‐blind study – i.e., neither physician nor patient knew if actual drug or placebo was given" (study report p. 31) Specific detail regarding similarity of intervention and placebo was not provided, but considered likely that this could have been achieved successfully |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Lack of blinding unlikely to influence objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No detail provided as to whether outcome assessors were blinded for subjective outcomes, but complications of treatment were likely assessed by physicians/patients (who were blinded) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 56 women were randomised; 26 (46%) were excluded post‐randomisation (16: found not to be pregnant; 10: did not return for injections, and thus not followed up) |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting with no access to published trial protocol |
| Other bias | Unclear risk | Insufficient methodological detail provided to determine other potential sources of bias |
Martinelli 2012.
| Methods | Randomised controlled trial | |
| Participants | 135 women were randomised. Data from 40 eligible women were included in this review Setting: 8 obstetric units in Italy Dates of recruitment: April 2007 to April 2010 Inclusion criteria: pregnant women referred to a participating centre at a GA < 12 weeks, who provided written informed consent, with any of the following events complicating previous pregnancies: (1) mild pre‐eclampsia, defined by blood pressure higher than 140/90 mmHg on 2 or more occasions after the 20th gestational week plus proteinuria ≥ 0.3 g/24 hours or > 2+ on dipstick testing; severe pre‐eclampsia, defined by blood pressure higher than 160/100 mmHg plus proteinuria more than or equal to 0.5 g/24 hours or 3+ on dipstick testing, or concomitant placental abruption, FGR, or fetal loss; eclampsia, defined by the occurrence of new‐onset seizures in a pre‐eclamptic woman; (2) HELLP syndrome, defined by the concomitant presence of signs of haemolysis (lactate dehydrogenase > 600 IU/L or serum bilirubin > 1.2 mg/dL or presence of schistocytes in the peripheral blood), serum aspartate transaminase more than 70 IU/L and thrombocytopenia (platelet count < 100,000/mm3); (3) spontaneous fetal loss after the 15th gestational week; (4) FGR, defined by birthweight below the 10th percentile for GA together with a percentile reduction from the growth curve of the abdominal circumference more than 40% by ultrasound; and (5) placental abruption, defined by vaginal bleeding with or without uterine tenderness and fetal distress followed by emergency delivery after 24 gestational weeks. Exclusion criteria: women with the above previous pregnancy complications most likely explained by anatomic, chromosomal, endocrine, immunologic abnormalities or intercurrent traumatic or infectious events or who, at the time of screening evaluation, reported previous venous or arterial thrombotic events or were found to have a multiple pregnancy, diabetes mellitus, immunologic disorders, abnormal placental insertion, alcohol or drug abuse, < 50,000 platelets/mm3, renal impairment, or any medical condition requiring continued anticoagulant or antiplatelet treatment, including low‐dose aspirin, during pregnancy. "Antiphospholipid antibodies were tested in fresh unfrozen plasma within 24 hours since blood sampling, and their results were promptly communicated to the participating centers; women who tested positive were excluded from the study" (study report p. 3270) |
|
| Interventions |
Nadroparin (heparin): 3800 IU/40 mg LMWH (nadroparin), daily subcutaneous injection combined with medical surveillance (monthly visits and controls of maternal weight, blood pressure, aspirin intake, abdominal growth, and ultrasound evaluation of fetal biometry) and were actively followed up to delivery or to complete resolution of any intercurrent adverse event, or both. Mean GA at randomisation was 11 weeks Total number randomised: n = 67 (63 analysed) No treatment: medical surveillance alone (monthly visits and controls of maternal weight, blood pressure, aspirin intake, abdominal growth, and ultrasound evaluation of fetal biometry) Total number randomised: n = 68 (65 analysed) |
|
| Outcomes | Composite endpoint of pre‐eclampsia, eclampsia, HELLP syndrome, intrauterine fetal death, FGR, or placental abruption; Pregnancy complications: miscarriage; termination; gestational diabetes; gestational hypertension; cholestasis; premature rupture of membranes; oligohydramnios; placental praevia; risk of preterm delivery; abnormal uterine artery velocimetry; others; Other maternal adverse events: bleeding; thrombocytopenia; others; Fetal/neonatal adverse events: chromosomal or congenital abnormalities; abnormal cardiotocography; others;Other outcomes: abnormal uterine artery velocimetry; GA at birth; delivery after 38th week; delivery at or before 38th week (before 35th week; before 31st week); caesarean section; birthweight < 10th centile; birthweight 10th to 49th centile; birthweight 50th to 89th centile; birthweight > 90th centile; birthweight; Apgar score < 7 | |
| Funding source(s) | Trial supported by the Italian Drug Agency (Agenzia Italiana del Farmaco) of the Ministry of Health (grant for independent research; trial registration: EudraCT 2006‐004205‐26). The study report notes "No pharmaceutical company was involved in any phases of the trial, including protocol design, study conduction, co‐ordination and monitoring, data handling and analysis, and manuscript writing" (study report p. 3270) | |
| Declarations of interest | "The authors declare no competing financial interests" (study report p. 3275) | |
| Information source(s) | Primary publication; Abstract/interim analyses: Martinelli I. LMWH in pregnant women with previous obstetrical complications. A multicenter, randomised trial. Pathophysiology of Haemostasis and Thrombosis 2010;37 Suppl 1:A3; HAPPY study protocol CRF (supplied by trialists) and completed data request form The trial was registered at ricerca‐clinica.agenziafarmaco.it as EudraCT 2006‐004205‐26 |
|
| Notes | Study ceased after first planned interim analyses due to futility and for safety considerations. The trial Data and Safety Monitoring Board concluded at this time that LMWH prophylaxis was ineffective in the prevention of late pregnancy complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer randomization list was generated by the Laboratory of Biostatistics of the Mario Negri Institute (Ranica, Italy)" (study report p. 3270) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patient randomisation numbers and study arm were requested by phone or fax and centrally assigned by the treatment secretariat at the Mario Negri Institute" (study report p. 3270) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants and care providers were not blind to study treatments" (study report p. 3270) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The primary outcome was a composite end point of pre‐eclampsia, eclampsia, HELLP syndrome, intrauterine fetal death, FGR, or placental abruption. These outcomes were allocated on the basis of the same criteria used at screening evaluation by an independent adjudicator (P.R.) who was blinded to treatment allocation" (study report p. 3270); further, lack of blinding not considered likely to impact objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Assessed by an independent adjudicator (P.R.) who was blinded to treatment allocation" (study report p. 3270) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses employed. Of 135 women randomised, 7 (5%) were excluded from primary outcome analyses (4 in heparin group, 3 in medical surveillance group) |
| Selective reporting (reporting bias) | Unclear risk | Published study reports: "The primary outcome was a composite end‐point of pre‐eclampsia, eclampsia, HELLP syndrome, intrauterine fetal death, FGR, or placental abruption" (study report p. 3270), although published conference abstract reports: "The primary outcome was the live‐birth rate." Trial protocol was available, but limited detail provided in protocol regarding nature of prespecified outcomes; 1 such outcome "changes in platelet count (PLT) or aspartate aminotransferase (AST)/alanine aminotransferase (ALT)" was not reported in study report |
| Other bias | Low risk | No other obvious sources of bias identified |
Rey 2009.
| Methods | Randomised controlled trial | |
| Participants | 114 women were randomised. Data from 45 eligible women were included in this review Setting: 6 high‐risk pregnancy referral centres in Canada Dates of recruitment: August 2000 to June 2007 Inclusion criteria: GA < 17 weeks at randomisation; normal thrombophilia screen (absence of heterozygous or homozygous factor (F)V Leiden or prothrombin 20210A mutations; negative testing for lupus anticoagulant and absence of moderate or high levels of anticardiolipin antibodies; normal levels of antithrombin, protein C, protein S and normal level of homocysteine or absence of the homozygous MTHFR C677T mutation) and; 1 or more of the following complications in the immediate previous pregnancy: severe PET resulting in delivery before 34 6/7 weeks; unexplained newborn weight less than the 5th percentile; abruptio placenta resulting in delivery before 34 6/7 weeks or in fetal death after 19 6/7 weeks; 1 or more episodes of unexplained intrauterine death after 19 6/7 weeks or 2 episodes of unexplained fetal death between 12 and 19 6/7 weeks Exclusion criteria: any of the following in the previous or the current pregnancy: multiple gestation, alcohol or illicit drug use, underlying metabolic disease other than hypertension which could promote SGA or stillbirth (such as diabetes, hyperthyroidism and renal disease), uterine malformation, placental or cord pathologies, cytomegalovirus or toxoplasmosis infection, known fetal malformation or chromosomal anomaly at randomisation; a previous venous or arterial thrombotic event, known allergy to heparin or LMWH, contraindication to dalteparin or an absolute indication for anticoagulant therapy |
|
| Interventions |
Dalteparin (heparin): subcutaneous self‐injection of dalteparin administered daily at 4000 IU daily for women weighing < 60 kg, 5000 IU for women weighing 60 – 90 kg and 6000 IU for women weighing > 90 kg (weights as at randomisation). Injections were stopped at 36 weeks' gestation or birth (whichever came first). A complete blood count was performed twice in the first 2 weeks and thereafter, at the discretion of the participating centre Total number randomised: n = 57 (55 analysed) No treatment Total number randomised: n = 57 (55 analysed) |
|
| Outcomes |
Primary outcome: composite of severe pre‐eclampsia, birthweight > 5th percentile, major abruptio placentae resulting in delivery < 34 weeks' gestation or fetal death > 20 weeks' gestation Secondary outcomes: non‐severe pre‐eclampsia; newborn weight between the 6th and 10th percentile; GA at delivery. Other outcomes reported: fetal loss < 20 weeks; fetal loss > 20 weeks; use of antihypertensive agents; gestational diabetes; haemoglobin at birth; antenatal hospitalisation for hypertension/fetal indications; preterm prelabour rupture of membranes; preterm labour; induction of labour; induction for hypertension/fetal indications; vaginal birth; magnesium sulphate; preterm birth (< 37 weeks, < 34 weeks, < 30 weeks); delivery for pre‐eclampsia/fetal indications (< 37 weeks, < 34 weeks, < 30 weeks); birthweight; birthweight percentile; birthweight < 2500 g; newborn intensive care unit admission; safety: skin reaction; heparin‐induced thrombocytopenia or haemorrhage (other than local bruising at injection site) |
|
| Funding source(s) | Trial financially supported by the Canadian Foundation for Women's Health, Pharmacia & Upjohn Inc and Pfizer Canada. The study report notes the funders were not involved in the study design, data collection, analyses, interpretation or writing of the manuscript. | |
| Declarations of interest | "S.R. Kahn and M. Rodger have received speaker's honoraria and investigator‐initiated grants‐in‐aid from various manufacturers of LMWH. The other authors state that they have no conflict of interest" (study report p. 63) | |
| Information source(s) | Primary publication and completed data request form. Trial registration at www.isrctn.com/ISRCTN78732833 (retrospectively registered in 2008) | |
| Notes | Clinical trials identifier: ISRCTN78732833 Study was stopped early due to slow recruitment and following interim analyses demonstrating a decrease in the primary outcome at P < 0.005. Trialists noted: "We are aware that stopping the study may have led to exaggerated effect sizes" (study report p. 63) Most of the participants (78.4%) were recruited at 1 hospital (CHU Sainte‐Justine) Women with spontaneous abortion < 12 weeks' gestation were censored a posteriori from the analyses as they could not develop primary or secondary outcomes Low birthweight defined as weight < 2500 g SGA defined by percentile < 10th percentile Prematurity and neonatal intensive care stay was taken as the proxy for neonatal morbidity Adherence defined by observance of treatment. 1 woman switched from control to intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women were randomized in a 1:1 ratio to dalteparin vs. no dalteparin (open‐label control) groups, using a computer generated random numbers table (blocks of six)" (study report p. 59) |
| Allocation concealment (selection bias) | Low risk | Quote: "Each generated number was linked to a sealed, opaque envelope containing the name of the group to which the woman was randomized" (study report p. 59). Envelopes were sequentially numbered and taken in order (information provided by trialist) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded trial |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Two adjudicators blinded to treatment assignment and not involved in the study reviewed all the patient report forms" (study report p. 59) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Two adjudicators blinded to treatment assignment and not involved in the study reviewed all the patient report forms" (study report p. 59) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses employed. Minimal and equal attrition between groups |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective outcome reporting. Retrospective trial registration |
| Other bias | Unclear risk | Although the statistical significance of differences was not reported, there appeared to be some baseline imbalance for aspirin use and ethnicity; trialists report results of adjusted analyses |
Salim 2016.
| Methods | Randomised controlled trial | |
| Participants | 144 women were randomised. Data from 13 eligible women were included in this review Setting: University teaching hospital and 3 specialised community clinics focusing on high‐risk pregnancies in Israel Dates of recruitment: October 2009 to January 2015 Inclusion criteria: singleton pregnancy at 14 weeks' gestation or less at enrolment and prior placenta‐mediated pregnancy complications or a lower‐leg thrombotic event alongside any diagnosed thrombophilia (details provided in study report). Prior placenta‐mediated pregnancy complications included prior severe pre‐eclampsia, prior SGA with placenta‐related antepartum signs, prior placental abruption, or prior unexplained pregnancy loss (3 losses before 13 weeks, 2 losses between 14 and 22 weeks' gestation, or any loss after 23 weeks' gestation) Exclusion criteria: women with pre‐gestational diabetes or women with prior need for therapeutic dosages of LMWH or contraindication to LMWH, plus women who had previous pregnancy complications that could be attributed to multiple gestations, major congenital or chromosomal abnormalities, fetal infection, or hydrops fetalis |
|
| Interventions |
Enoxaparin (heparin; adjusted dose): daily by subcutaneous self‐injection of 40 mg initially, then adjusted by fractions of 20 mg according to anti‐factor Xa levels. Injections continued from enrolment to birth. Targeted prophylactic level was determined at 0.2 IU/mL or more 3½ to 4 hours post‐injection by blood sample. Results were computerised and dose adjusted accordingly in the next visit. Women attended follow‐up visits every 3 – 4 weeks. Women with anti‐phospholipid antibodies were also given LDA
From birth to 42 days, all women were prescribed daily enoxaparin 40 mg by subcutaneous injection. Otherwise, standard antepartum and peripartum care given Total number randomised: n = 74 (74 analysed) Enoxaparin (heparin; fixed dose): daily by subcutaneous self‐injection of 40 mg regardless of anti‐factor Xa levels. Injections continued from enrolment to birth. Anti‐factor Xa levels were examined approximately every 8 – 10 weeks for all women. Women with anti‐phospholipid antibodies were also given LDA Total number randomised: n = 70 (66 analysed) From birth to 42 days, all women were prescribed daily enoxaparin 40 mg by subcutaneous injection. Otherwise, standard antepartum and peripartum care given |
|
| Outcomes | Primary outcome: composite of any pregnancy loss after randomisation, pre‐eclampsia, birth of a SGA infant, placental abruption, or objectively documented VTE Secondary outcomes: GA at birth, preterm birth, mode of delivery, and maternal complications related to enoxaparin use (thrombocytopenia, antepartum bleeding, and symptomatic fracture) | |
| Funding source(s) | Not stated in article. Trialists advised that the trial was supported by Emek Medical Center | |
| Declarations of interest | None declared | |
| Information source(s) | Primary publication and completed data request. Trial registration at www.clinicaltrials.gov/ct2/show/NCT01068795 (registered during the trial in 2010) | |
| Notes | Cross‐over of 1 woman from the fixed‐ to the adjusted‐dose group (ITT analyses carried out) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation sequence generation programme with 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation sequence results were kept in the delivery ward in a closed study box. The site investigator enrolled participants after confirming eligibility. The sequence was concealed until intervention was assigned (and after obtaining a signed informed consent)" (study report p. 3) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded trial |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Lack of blinding unlikely to influence objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Assessments were undertaken by the research team and the participating women, none of whom were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses employed. 4 women excluded post‐randomisation from the control group with reasons given (2 women delivered at another institution; 1 discontinued intervention due side‐effects and 1 discontinued due to ineligibility) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes specified in Methods are reported. Retrospective trial registration |
| Other bias | Low risk | No other obvious sources of bias identified |
Schleussner 2015.
| Methods | Randomised controlled trial | |
| Participants | 449 women were randomised. Data from 38 eligible women were included in this review Setting: 14 outpatient clinics specialising in recurrent pregnancy loss in Austria and Germany; 9 were university hospitals, 3 were general women's hospitals, and 2 were centres for reproductive medicine Dates of recruitment: December 2006 to August 2012 Inclusion criteria: women aged over 18 years with at least 2 consecutive early miscarriages (< 12 weeks' gestation) or 1 late miscarriage (≥ 12 weeks' gestation) and a viable singleton pregnancy of 5 to 8 weeks' gestation as detected by ultrasonography. No other trial participation in 30 days prior to enrolment Exclusion criteria: previous miscarriages due to chromosomal, uterine, or fetal structural anomalies or infection; current diabetes mellitus; known nicotine, drug, or alcohol use; HIV infection; anticipated poor adherence (as judged by investigators); clinical need for heparin therapy according to the criteria from the ETHIG I trial or any contraindication to LMWH; homozygous factor V Leiden mutations, homozygous prothrombin mutations, antiphospholipid antibody syndrome |
|
| Interventions |
Dalteparin (heparin): 5000 IU dalteparin–sodium self‐administered daily as a subcutaneous injection until 24 weeks' gestation. Women received 30 syringes at each study visit and self‐administered daily. Total number randomised: n = 226 (220 analysed) No treatment: no placebo injections. Both groups received multivitamins containing folic acid. Total number randomised: n = 223 (214 analysed) |
|
| Outcomes |
Primary outcome: ongoing pregnancy rate up to and including the 24th week of gestation as recorded in the final study visit Secondary outcomes: live‐birth rate/stillbirth; mean duration of gestation at miscarriage; preterm birth (34 to 36 weeks, 28 to 33 weeks, < 38 weeks); intrauterine growth restriction < 5th percentile; pre‐eclampsia or HELLP syndrome; placental abruption; safety outcomes: maternal death; withdrawals due to serious adverse events; serious adverse events (vaginal haemorrhage; other haemorrhage; cervical incompetence/preterm labour without birth; GI problems; infection; other); adverse events that were not serious adverse events (vaginal haemorrhage; other haemorrhage; cervical incompetence/preterm labour without birth; GI problems; infection; other); complications during birth; fetal structural abnormalities; thrombocytopenia, osteoporosis Other: use of concomitant medication; adherence |
|
| Funding source(s) | "The ETHIG II trial received an unrestricted grant from Pfizer Pharma and was given the multivitamin supplements free of charge from Merck Selbstmedikation. The funding sources played no role in the design, conduct, analysis, reporting, or interpretation of results or the decision to submit the manuscript for publication" (study report p. 603) | |
| Declarations of interest | Conflicts provided at: www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14‐2062 Dr Bauersachs reports personal fees from Leo, personal fees from Pfizer, during the conduct of the study; personal fees from LEO, personal fees from Pfizer, personal fees from Sanofi‐Aventis, outside the submitted work. Dr Schleussner reports grants from Pfizer Pharma GmbH Germany, non‐financial support from Merck Selbstmedikation GmbH Germany, during the conduct of the study Dr Toth reports other from Pfizer (company), during the conduct of the study Dr Bohlmann reports other from Pfizer, during the conduct of the study All other authors report nothing to disclose |
|
| Information source(s) | Primary publication and its web appendices; Abstracts: Schleussner E, Bohlmann M. Kamin G. et al. Low‐molecular‐weight heparin for the prevention of habitual abortion ‐ introduction of the multicentre study ETHIG 2 and discussion of the current data. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S220 and Schleussner E, Kamin G. Seeliger G. Rogenhofer N. Toth B. Low‐molecular‐weight heparin in recurrent pregnancy loss‐Results of the ETHIG II study. Thrombosis Research 2013;131(Suppl 1):S73 clinicaltrials.gov/ct2/show/NCT00400387 Completed data request form | |
| Notes | Trial registration number: NCT00400387 Women from both groups received multivitamins containing folic acid (Femibion 800 Metafolin [Merck]) from allocation up to 24 weeks' gestation. Women returned for study visits at 9, 12, 16, 20, and 24 weeks' gestation. The trial protocol stipulated that acetylsalicylic acid (ASA) may not be used The primary focus of the study was prevention of miscarriage. Therefore, while trial protocol did not foresee the use of LMWH after 24 weeks' gestation, use of LMWH beyond this was permitted Number analysed for the primary outcome was further reduced from 220 to 215 in the intervention group and from 214 to 211 in the control group; only these women were included in some secondary outcomes At least 39 women in the intervention group continued LMWH after 24 weeks and at least 15 women in the control group began use of LMWH after 24 weeks. These women continued LMWH to 35 weeks on average Women who investigators judged would have poor adherence were also excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by week of gestation, trial site, and inherited thrombophilia using the minimization method described by Pocock and Simon (14). Using weights of 1 for the stratification factors, 0.01 for the overall group balance (1:1 ratio), and a random component, we chose the group that would minimize differences in the stratification factors between the groups with 80% probability. The trial statistician prepared the allocation algorithm, which was implemented at the clinical trial center. Physicians provided the patient information to the center, where a data manager generated the group allocation" (study report p. 602) |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Placebo injections were not used, and neither trial staff nor patients were blinded" (study report p. 601) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Lack of blinding unlikely to influence objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Assessed by local gynaecologists who were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 449 women were randomised; 15 (3%) were excluded post‐randomisation from primary outcome analysis, and a total of 23 (5%) from birth (secondary) outcome analyses. Numbers and reasons for exclusion similar across groups |
| Selective reporting (reporting bias) | Low risk | Trial protocol provided with published trial report; outcomes reported as prespecified;
Quote: "No important changes were made to the outcomes after commencement of the trial" (study report p. 602) We noted the protocol listed a composite outcome of late pregnancy complications which was not reported as such (reported instead as individual outcomes only), but judged this as low risk of bias |
| Other bias | Low risk | No other obvious sources of bias identified |
ALAT: alanine aminotransferase; Apgar: appearance, pulse, grimace, activity, respiration; APH: antepartum haemorrhage; APTT: activated partial thromboplastin time; DNA: deoxyribonucleic acid; FGR: fetal growth restriction; GA: gestational age; HELLP: haemolysis, elevated liver enzymes, low platelet count; HIV: human immunodeficiency virus; IgA: immunoglobulin A; IgG: Immunoglobulin G; ITT: intention‐to‐treat; IU: international units; IVF: in vitro fertilisation; kg: kilogram; LMWH: low‐molecular‐weight heparin; mg: milligram; MTHFR: methylene‐tetrahydrofolate reductase; NICU: neonatal intensive care unit; NS: non‐significant; PET: pre‐eclamptic toxaemia; PPH: postpartum haemorrhage; SGA: small‐for‐gestational age; VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelhafez 2014 | No response from trialists |
| Ahmadi 2017 | No response from trialists |
| Alalaf 2012 | Previous loss < 20 weeks' gestation |
| Aoki 1993 | Previous loss < 20 weeks' gestation |
| Baber 1988 | No previous loss |
| Badawy 2008 | Previous loss < 20 weeks' gestation |
| Bao 2017 | No response from trialists |
| Berle 1980 | Authors deceased or unable to be located |
| Blomqvist 2017 | Previous loss < 20 weeks' gestation |
| Blumenfeld 1992 | Previous loss < 20 weeks' gestation |
| Branch 2000 | No access to data or GA of previous losses unknown |
| Brenner 2005 | No response from trialists |
| Carta 2005 | Previous loss < 20 weeks' gestation |
| Cauchi 1991 | Previous loss < 20 weeks' gestation |
| Chakravarty 2012 | No response from trialists |
| Check 1995 | No response from trialists |
| Christiansen 1992 | No access to data or GA of previous losses unknown |
| Christiansen 2015 | Previous loss < 20 weeks' gestation |
| Clark 2010 | No access to data or GA of previous losses unknown |
| Cohen 1996 | No response from trialists |
| Coomarasamy 2015 | Previous loss < 20 weeks' gestation |
| Cote‐Arsenault 2014 | No response from trialists |
| Coulam 1995 | Previous loss < 20 weeks' gestation |
| Cowchock 1992 | No access to data or GA of previous losses unknown |
| Cowchock 1995 | Previous loss < 20 weeks' gestation |
| Cowchock 1997 | No access to data or GA of previous losses unknown |
| Dal Canto 2012 | No response from trialists |
| Dendrinos 2007 | Previous loss < 20 weeks' gestation |
| DeVeciana 2001 | No previous loss |
| Dolitzky 2006 | Data not supplied |
| El‐Zibdeh 2005 | Previous loss < 20 weeks' gestation |
| Elmahashi 2014 | Previous loss < 20 weeks' gestation |
| Epperson 2011 | No response from trialists |
| Famina 2015 | No previous loss |
| Farquharson 2002 | No access to data or GA of previous losses unknown |
| Fawzy 2008 | No response from trialists |
| Fuchs 1966 | Authors deceased or unable to be located |
| Gao 2015 | No response from trialists |
| Gatenby 1993 | Previous loss < 20 weeks' gestation |
| Gerhard 1987 | No previous loss |
| German RSA/IVIG Group 1994 | Previous loss < 20 weeks' gestation |
| Geva 1998 | No previous loss |
| Giancotti 2012 | No response from trialists |
| Goel 2006 | No response from trialists |
| Goldzieher 1964 | Authors deceased or unable to be located |
| Gomaa 2014 | Previous loss < 20 weeks' gestation |
| Gris 1995 | Previous loss < 20 weeks' gestation |
| Gris 2010 | No previous loss |
| Gris 2011 | No previous loss |
| Harrison 1992 | Previous loss < 20 weeks' gestation |
| Ho 1991 | Previous loss < 20 weeks' gestation |
| Illeni 1994 | Previous loss < 20 weeks' gestation |
| Ismail 2016 | Data not supplied |
| Ismail 2018 | No response from trialists |
| Jablonowska 1999 | Previous loss < 20 weeks' gestation |
| Johnson 1975 | Authors deceased or unable to be located |
| Johnson 1991 | Previous loss < 20 weeks' gestation |
| Kaaja 1993 | No previous loss |
| Kaandorp 2010 | Previous loss < 20 weeks' gestation |
| Kayatas 2013 | No response from trialists |
| Khan 2017 | Previous loss < 20 weeks' gestation |
| Kilpatrick 1993 | No access to data or GA of previous losses unknown |
| Kim 1997 | No response from trialists |
| Kim 2012 | No response from trialists |
| Klopper 1965 | Authors deceased or unable to be located |
| Kumar 2014 | Previous loss < 20 weeks' gestation |
| Kutteh 1996 | Data not supplied |
| Kwon 2012 | No previous loss |
| Laskin 1997 | No access to data or GA of previous losses unknown |
| Laskin 2009 | No access to data or GA of previous losses unknown |
| Lazzarin 2009 | Previous loss < 20 weeks' gestation |
| Li 1998 | No response from trialists |
| MacDonald 1972 | Authors deceased or unable to be located |
| Maged 2016 | Previous loss < 20 weeks' gestation |
| Mahmoud 2004 | No response from trialists |
| Malathi 2011 | No response from trialists |
| Malinowski 2003 | No response from trialists |
| Mankuta 1999 | No access to data or GA of previous losses unknown |
| Meng 2016 | Previous loss < 20 weeks' gestation |
| Mohamed 2014 | No response from trialists |
| Moller 1965 | No previous loss |
| Mowbray 1985 | Authors deceased or unable to be located |
| Nagpal 2001 | Authors deceased or unable to be located |
| Navidian 2018 | No previous loss |
| Nazari 2015 | No response from trialists |
| Noble 2005 | Previous loss < 20 weeks' gestation |
| Norman 2006 | No previous loss |
| Norman 2016 | Data not supplied |
| Ober 1999 | No response from trialists |
| Pandey 2004 | Previous loss < 20 weeks' gestation |
| Pasquier 2015 | Previous loss < 20 weeks' gestation |
| Pattison 2000 | No access to data or GA of previous losses unknown |
| Perino 1997 | Previous loss < 20 weeks' gestation |
| Prietl 1992 | No previous loss |
| Quenby 1992 | Previous loss < 20 weeks' gestation |
| Quenby 1994 | Previous loss < 20 weeks' gestation |
| Quenby 2007 | Previous loss < 20 weeks' gestation |
| Qureshi 2005 | No previous loss |
| Raddatz 2005 | Previous loss < 20 weeks' gestation |
| Rafiee 2015 | Previous loss < 20 weeks' gestation |
| Rai 1997 | No response from trialists |
| Rai 2005 | No response from trialists |
| Rajan 1993 | No access to data or GA of previous losses unknown |
| Reijnders 1988 | No response from trialists |
| Reznikoff‐Etievant 1994 | Authors deceased or unable to be located |
| Rodger 2014 | Data not supplied |
| Saad 2014 | No response from trialists |
| Salman 2012 | No response from trialists |
| Samantha 2013 | No previous loss |
| Scarpellini 2009 | Previous loss < 20 weeks' gestation |
| Scarpellini 2017 | No response from trialists |
| Schisterman 2014 | Previous loss < 20 weeks' gestation |
| Scott 1996 | No access to data or GA of previous losses unknown |
| Shaaban 2017 | Previous loss < 20 weeks' gestation |
| Sharifi Saki 2015 | No response from trialists |
| Shearman 1963 | Authors deceased or unable to be located |
| Shefras 1995 | No previous loss |
| Shu 2002 | Previous loss < 20 weeks' gestation |
| Silver 1993 | No response from trialists |
| Smitz 1992 | No previous loss |
| Sondergaard 1985 | No previous loss |
| Stephenson 2004 | No previous loss |
| Stephenson 2010 | Previous loss < 20 weeks' gestation |
| Stray‐Pedersen 1996 | Previous loss < 20 weeks' gestation |
| Sun 2010 | No response from trialists |
| Svigos 1982 | Previous loss < 20 weeks' gestation |
| Swyer 1953 | Previous loss < 20 weeks' gestation |
| Tang 2013 | Previous loss < 20 weeks' gestation |
| Tognoni 1980 | No previous loss |
| Triolo 2003 | Previous loss < 20 weeks' gestation |
| Tulppala 1997 | No response from trialists |
| Turner 1966 | No previous loss |
| Vahid Dastjerdi 1999 | No response from trialists |
| Van Hoorn 2016 | No previous loss |
| Vaquero 2001 | Previous loss < 20 weeks' gestation |
| Visser 2011 | No response from trialists |
| Walch 2005 | Previous loss < 20 weeks' gestation |
| Xiao 2013 | Previous loss < 20 weeks' gestation |
| Zafardoust 2017 | Previous loss < 20 weeks' gestation |
| Zolghadri 2010 | No response from trialists |
GA: gestational age
Characteristics of ongoing studies [ordered by study ID]
Alves 2014.
| Trial name or title | The BRAzil MAGnesium (BRAMAG) trial: a randomised clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria: women aged 18 ‐ 45 years, 12 weeks 1 day to 20 weeks 6 days gestation, with accurate estimated date of confinement (based on last menstrual period among women with a regular menstrual cycle, or by a first trimester pregnancy dating ultrasound), with a singleton pregnancy, currently residing within Recife or Petrolina, with 1 or more of the following risk factors, related to:
Exclusion criteria: women with known uncontrolled hyperthyroidism, known active parathyroid disease of any kind, chronic kidney disease (defined by an estimated glomerular filtration rate < 60 mg/min/1.73 m2, with chronic diarrhoeal disease, with high serum magnesium concentration > 9.5 mmol/dL |
| Interventions |
Oral magnesium: magnesium citrate capsules (150 mg elemental Mg2+ citrate per capsule), manufactured by IMIP’s Department of Pharmacology; 1 capsule twice daily starting at 12 to 20 weeks' gestation and continued until birth Note: discrepancy between trial registration (160 mg) and published trial protocol (150 mg) Placebo: matched placebo capsules, manufactured by IMIP's Department of Pharmacology, and identical in colour and shape; 1 capsule twice daily |
| Outcomes |
Composite perinatal outcome: preterm birth before 37 weeks' gestation; stillbirth after 20 weeks' gestation; neonatal death before 28 days after birth; or small‐for‐gestational‐age birthweight under the 3rd percentile Composite maternal outcome: pre‐eclampsia or eclampsia arising before 37 weeks' gestation; severe non‐proteinuric hypertension arising before 37 weeks' gestation; placental abruption; maternal stroke during pregnancy or ≤ 7 days after delivery; or maternal death during pregnancy or ≤ 7 days after delivery Note: trial registration reports preterm birth as the primary outcome of interest, and gestational diabetes mellitus, pre‐eclampsia, and low birthweight as secondary outcomes of interest |
| Starting date | March 2014 |
| Contact information | Dr Joao G Alves: joaoguilherme@imip.org.br Instituto de Medicina Integral Prof Fernando Figueira, Recife, Pernambuco, Brazil |
| Information source(s) | Published protocol: Alves JG, et al. The BRAzil MAGnesium (BRAMAG) trial: a randomised clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity. BMC Pregnancy and Childbirth 2014;14:222 Trial registration:clinicaltrials.gov/show/NCT02032186 |
| Notes |
Clinical trials identifier: NCT02032186 Estimated enrolment: 3000 Recruitment status at submission of this review: the official recruitment status of this trial is unknown. Correspondence with the trialists indicated that the trial was disrupted by the Zika virus. The trial had been completed but analyses of the data have yet to begin |
De Jong 2015.
| Trial name or title | ALIFE2 study: low‐molecular‐weight heparin for women with recurrent miscarriage and inherited thrombophilia |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria: women aged 18 to 42 years, with recurrent miscarriage and/or intra‐uterine fetal deaths (that is ≥ 2 miscarriages or intra‐uterine fetal deaths, irrespective of gestational age) (not necessarily consecutive), with confirmed inherited thrombophilia (factor V Leiden mutation; prothrombin gene mutation (G20210A); protein S deficiency; protein C deficiency; antithrombin deficiency), with pregnancy confirmed by urine pregnancy test, willing and able to give informed consent Exclusion criteria: duration of current pregnancy ≥ 7 weeks, based on first day of last menstruation; indication for anticoagulant treatment during pregnancy (e.g. prosthetic heart valves, a history of venous thromboembolism or antiphospholipid syndrome); contraindications to LMWH (previous heparin‐induced thrombocytopenia, active bleeding or renal insufficiency with creatinine clearance of < 30 mL/minute); known allergy to at least 3 different LMWH preparations; previous inclusion in the ALIFE2 study (for another pregnancy) |
| Interventions |
Low‐molecular‐weight heparin: LMWH in addition to standard pregnancy surveillance. Women will immediately begin injecting themselves once daily in either the upper leg or abdomen with Clexane (enoxaparin, Sanofi‐Aventis Netherlands B.V., Kampenringweg 45 E, 2803 PE GOUDA, the Netherlands) 40 mg LMWH, 100 mg/mL, 0.4 mL syringe. If the recommended intervention is unavailable another type of LMWH in a dosage equivalent to enoxaparin 40 mg may be chosen (see published protocol). Women will continue the treatment until the first signs of labour, or in the case of planned delivery, according to local policy (at least 12 hours prior to caesarean section/neuraxial anaesthesia). Women will be randomised upon confirmation of pregnancy and treatment will continue until start of labour. Standard care: women in the control group will receive standard pregnancy surveillance |
| Outcomes |
Primary: live birth Secondary: Efficacy: ongoing pregnancy beyond 12 weeks' gestation; pre‐eclampsia; HELLP syndrome; intrauterine growth restriction; placental abruption; premature birth; intra‐uterine fetal death; major congenital anomalies; composite of confirmed deep vein thrombosis and confirmed pulmonary embolism; Safety: clinically‐relevant bleeding; postpartum bleeding and severe postpartum bleeding; major bleeding; clinically relevant non‐major bleeding; minor bleeding, including increased tendency to bruising not fulfilling the criteria for clinically relevant non‐major bleeding; heparin‐induced thrombocytopenia (defined according to ACCP criteria); allergic reactions (redness or itching) localised at the injection site of LMWH; type 1 allergy: e.g. generalised symptoms including anaphylaxis |
| Starting date | Planned starting date: 1 September 2012 Start of recruitment: December 2012 |
| Contact information | Department of Vascular Medicine, Academic Medical Center, University of Amsterdam, The Netherlands Scientific queries: Dr S Middeldorp Phone: +31 20 5665976 Email: alife@amc.uva.nl General queries: Dr Luuk Scheres, MD Phone: +31 20 5667516 Email: l.j.scheres@amc.uva.nl |
| Information source(s) | Published protocol: De Jong PG et al. ALIFE2 study: low‐molecular‐weight heparin for women with recurrent miscarriage and inherited thrombophilia‐study protocol for a randomised controlled trial. Trials 2015;16:208. Trial website: www.studies‐obsgyn.nl/ALIFE2/page.asp?page_id=1344 |
| Notes |
Clinical trials identifier: NTR3361 Target sample size: 399 Recruitment status at submission of this review: recruiting |
El‐refaie 2016.
| Trial name or title | Vaginal progesterone versus cervical cerclage for pregnant women with sonographic short cervix and history of preterm labour and/or midtrimester miscarriage |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria: women aged 20 ‐ 35 years, with a singleton pregnancy, a history of preterm labour and/or mid‐trimester miscarriage in a previous pregnancy, and cervical length of 15 ‐ 25 mm by transvaginal sonography at 16 ‐ 25 weeks' gestation Exclusion criteria: women aged < 20 or > 35 years, with congenital uterine malformations, multi‐fetal pregnancies, known major fetal structural or chromosomal abnormality, known allergy or contraindication (relative or absolute) to progesterone therapy, presence of contraindication to cervical cerclage, medical conditions complicating pregnancy, vaginal bleeding |
| Interventions |
Progesterone: vaginal progesterone suppositories (Cyclogest®, Actavis, Barnstaple, EX32 8NS, United Kingdom) 400 mg/day Cervical cerclage: cervical cerclage by transvaginal placement of purse‐string stitch suture at the cervicovaginal junction, without mobilisation of the urinary bladder (McDonald cervical cerclage) |
| Outcomes | Primary: preterm labour before 35 weeks Secondary: delivery before 37 weeks; low birthweight (< 2500 g); neonatal respiratory distress syndrome; early neonatal death |
| Starting date | February 2016 |
| Contact information | Dr Waleed El‐refaie: wrefaie@yahoo.com Obstetrics and Gynecology Department in Mansoura University Hospital Recruiting, Mansoura, Dakahlia, Egypt, 35111 |
| Information source(s) | Trial registration: clinicaltrials.gov/ct2/show/NCT02673359 Correspondence with investigators |
| Notes |
Clinical trials identifier: NCT02673359 Estimated enrolment: 220 Estimated study completion date: June 2019 Final data collection date for primary outcome measure: May 2019 Recruitment status at submission of this review: recruiting |
Hezelgrave 2016.
| Trial name or title | Rationale and design of SuPPoRT: a multi‐centre randomised controlled trial to compare 3 treatments: cervical cerclage, cervical pessary and vaginal progesterone, for the prevention of preterm birth in women who develop a short cervix |
| Methods | Randomised controlled trial (3‐armed) |
| Participants | Women aged 18 ‐ 50 with a singleton pregnancy and a short cervix (< 25 mm on transvaginal ultrasound), between 14 and 23 + 6 weeks' gestation and 1 or more of the following risk factors: previous preterm premature rupture of the fetal membranes; previous preterm birth/second trimester loss; any cervical procedure to treat abnormal smears; or Incidental finding of a short cervix on ultrasound scan |
| Interventions |
Cervical cerclage: performed within 7 days of recruitment Vaginal progesterone: 200 mg pessary self‐inserted once daily from recruitment until 34 weeks' gestation or delivery (whichever occurs first) Cervical pessary: pessary inserted by clinician within 7 days of recruitment and removed by clinician at 37 weeks' gestation (or in the event of established labour). Rescue pessary inserted if cervix shortens and membranes become visible prior to 24 weeks' gestation |
| Outcomes | Primary: preterm birth prior to 37 weeks Secondary: adverse perinatal outcome (composite of stillbirth and neonatal death prior to discharge or 1 (or more) of intraventricular haemorrhage, periventricular leukomalacia, hypoxic ischaemic encephalopathy, necrotising enterocolitis, bronchopulmonary dysplasia and sepsis); delivery < 30 and 34 completed weeks' gestation; gestation at delivery; time between intervention and delivery; requirement for rescue cerclage; other maternal and fetal outcomes: clinical course, therapies administered, maternal and fetal morbidity and mortality data until discharge or 28 days postnatal (whichever soonest); participant and clinician's perceptions of treatment; health costs at 28 days post‐natal; biochemical endpoints |
| Starting date | July 2015 |
| Contact information | Dr Natasha Hezelgrave natasha.hezelgrave@kcl.ac.uk King's College London & St Thomas's Hospital, London, United Kingdom |
| Information source(s) | Published protocol: Hezelgrave NL, Watson HA, Ridout A, Diab F, Seed PT, Chin‐Smith E, et al. Rationale and design of SuPPoRT: a multi‐centre randomised controlled trial to compare 3 treatments: cervical cerclage, cervical pessary and vaginal progesterone, for the prevention of preterm birth in women who develop a short cervix. BMC Pregnancy and Childbirth 2016;16(1):358 Trial registration: doi.org/10.1186/ISRCTN13364447 |
| Notes |
EudraCT identifier: 2015‐000456‐15 Estimated enrolment: 540 Estimated study completion date: July 2018 Recruitment status at submission of this review: no longer recruiting. Reporting of results anticipated April ‐ July 2019 |
McLindon 2011.
| Trial name or title |
Public title: Does using progesterone reduce the miscarriage rate in high risk pregnancies? Scientific title: In pregnant women with previous subfertility, does progesterone supplementation decrease the likelihood of miscarriage? |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: women aged > 18 years with previous diagnosis of subfertility (no pregnancy after 12 months random unprotected intercourse, a history of 3 or more miscarriages, failing to achieve an ongoing pregnancy after 12 months of random unprotected intercourse); pregnancy less than 7 weeks + 0 days Exclusion criteria: pregnancy following Assisted Reproductive Technologies |
| Interventions |
Progesterone: 400 mg progesterone (pessary), nightly from 7 ‐ 12 weeks Placebo: placebo pessary, carrier compound of the intervention pessary, identical in appearance |
| Outcomes | Primary: miscarriage (pregnancy loss < 20 weeks' gestation) Secondary: antepartum haemorrhage; presentation with bleeding after 20 weeks' gestation and volume of blood lost; birthweight; congenital anomaly; gestation at birth; livebirth; threatened miscarriage; presentation with bleeding or pain or both prior to 20 weeks |
| Starting date | January 2012 |
| Contact information | Dr Luke McLindon: lucas.mclindon@mater.org.au Mater Mothers' Hospital, Raymond Terrace, South Brisbane, Queensland 4101 Australia |
| Information source(s) | Trial registrations: apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611000401954 www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000401954 Correspondence with investigators |
| Notes |
Clinical trials identifier: ACTRN12611000401954 Anticipated sample size: 344 Estimated study completion date: December 2017 Recruitment status at submission of this review: recruiting |
Rodger 2017.
| Trial name or title | Antiphospholipid syndrome low‐molecular‐weight heparin pregnancy loss evaluation: The pilot study (APPLE). |
| Methods | Randomised controlled trial (feasibility trial) |
| Participants | Women aged 18 years or more with confirmed pregnancy and antiphospholipid syndrome, plus 2 or more unexplained pregnancy losses before 10 weeks' gestation, and/or 1 or more unexplained pregnancy losses at or after 10 weeks' gestation |
| Interventions |
Low‐dose aspirin: daily 81 mg oral LDA from randomisation until delivery Low‐molecular‐weight heparin: and low‐dose aspirin: LMWH and daily LDA (LMWH dosages at discretion of attending physician) until 37 weeks' gestation. Suggested regimen: daily 4500 IU tinzaparin by subcutaneous injection until 20 weeks' gestation, followed by 4500 IU by subcutaneous injection twice daily until 37 weeks' gestation |
| Outcomes | Primary: feasibility (mean recruitment rate per centre per month) Secondary: essential documents (proportion of sites requiring > 18 months to obtain all approvals/authorisations; eligibility (proportion among those screened); consent (among those eligible); withdrawals/loss to follow‐up; cross‐over rate; drug compliance; non‐consent and reasons |
| Starting date | November 2017 |
| Contact information | Marc Rodger, MD mrodger@toh.ca Ottawa Hospital Research Institute, Canada |
| Information source(s) | Trial registration: clinicaltrials.gov/ct2/show/NCT03100123 |
| Notes |
Clinical trials identifier: NCT03100123 Estimated enrolment: 24 (feasibility trial) Estimated primary completion date: November 2019 Estimated study completion date: January 2020 Recruitment status at submission of this review: recruiting |
Schreiber 2017.
| Trial name or title | HYPATIA: A study of HYdroxychloroquine to improve Pregnancy outcome in women with AnTIphospholipid Antibodies. |
| Methods | Randomised controlled trial |
| Participants | Women with known persistent antiphospholipid antibodies who are planning pregnancy |
| Interventions |
Hydroxychloroquine (HCQ): 200 mg film‐coated tablet taken orally Placebo: placebo tablet taken orally |
| Outcomes | Primary: composite of 3 principal aPL‐related adverse pregnancy outcomes: 1 or more pregnancy loss(es) (either < 10 weeks' gestation or beyond 10 weeks' gestation of a morphologically normal fetus documented by ultrasound or by direct examination of the fetus) and premature birth of a morphologically normal neonate before 34 weeks due to any of: pre‐eclampsia, eclampsia, recognised features of placental insufficiency Secondary: individual components of the composite primary outcomes, plus: gestational age at delivery; birthweight; caesarean birth; Apgar score < 7 at 5 minutes; neonatal morbidity (bleeding or thrombotic complications, infections, congenital abnormalities); days to hospital discharge following delivery (mother and child); thrombotic events in the mother during pregnancy and 6 weeks postpartum; days of neonate in special care; safety and tolerability of hydroxychloroquine in the mother and neonate |
| Starting date | March 2018 |
| Contact information | Prof Beverley J Hunt beverley.hunt@gstt.nhs.uk Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom |
| Information source(s) | Published protocol: Schreiber K, Breen K, Robinson SE, Hunt BJ, Jacobsen S, Cohen H, et al. Hydroxychloroquine to improve pregnancy outcome in women with antiphospholipid antibodies (HYPATIA) protocol: a multinational randomized controlled trial of hydroxychloroquine versus placebo in addition to standard treatment in pregnant women with antiphospholipid syndrome or antibodies. Seminars in Thrombosis and Hemostasis 2017;43(6):562‐571 Trial registration: www.clinicaltrialsregister.eu/ctr‐search/search?query=2016‐002256‐25 |
| Notes |
EudraCT Number: 2016‐002256‐25 Target sample size: 328 Recruitment status at submission of this review: ongoing |
ACCP: American College of Chest Physicians; APL: antiphospholipid antibodies; BMI: body mass index; g: gram; HELLP: haemolysis, elevated liver enzymes, low platelet count; IU: international units; kg: kilogram; LDA: low‐dose aspirin; LMWH: low‐molecular‐weight heparin; mg: milligram; mL: millilitre; mm: millimetre
Differences between protocol and review
Our protocol (Wojcieszek 2016) stated that we would carry out searches of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) using the term 'stillbirth'. We subsequently noted that, given varying nomenclature in the literature and varying definitions of stillbirth by gestational age, additional search terms were warranted (e.g. some reports may define 'miscarriage' as a fetal death < 24 weeks' gestation, which would consequently include some stillbirths, according to our definition of 20 weeks' gestation or more). We added the terms 'pregnancy loss', 'abortion', and 'miscarriage' to ensure full capture of potentially relevant populations of parents.
In the original protocol we had not planned subgroup analyses for the timing of the start or duration of the intervention (pre‐pregnancy versus during pregnancy versus during delivery) or target of intervention (mother versus partner). Although data in this review did not permit completion of these analyses, we will consider them in future updates if new trials are added.
In our original protocol, we included the 'Adverse perinatal outcome (composite outcome including stillbirth, neonatal death, and major neonatal morbidity)'. In the review, we expand this to include examples of major neonatal morbidity (hypoxic‐ischaemic encephalopathy; intracranial haemorrhage; retinopathy of prematurity; necrotising enterocolitis).
In the original protocol we had stated that we would include each of the preterm birth outcomes (any preterm birth, very preterm birth, and late preterm birth) in the GRADE assessment of the quality of evidence. As a maximum of seven outcomes can be included in GRADE assessments, we selected any preterm birth exclusively for inclusion in these assessments.
Finally, in the original protocol we had stated that types of participants would include 'women'. So that fathers and partners were not excluded, we subsequently expanded the population of interest to 'parents', and liaised with the Cochrane Pregnancy and Childbirth's Information Specialist to ensure that relevant trials would be captured by the outlined search strategy.
Contributions of authors
Aleena M Wojcieszek, Vicki Flenady, and Philippa Middleton designed the review with contribution from all authors. Aleena M Wojcieszek led the drafting of the review, data analyses, interpretation, and reporting of results with Vicki Flenady, Philippa Middleton and Emily Shepherd. Aleena M Wojcieszek and Emily Shepherd screened search records from the initial searches (carried out in 2016) and completed all data extractions and 'Risk of bias' assessments. Aleena M Wojcieszek, Zohra Lassi, and Margaret Murphy screened search records from the updated searches (carried out in 2018) and Aleena M Wojcieszek and Zohra Lassi completed the data extractions and 'Risk of bias' assessments. Aleena M Wojcieszek contacted trialists for further information and developed and compiled data requests. Aleena M Wojcieszek and Vicki Flenady undertook GRADE assessment of the quality of evidence. All authors contributed to the interpretation of results and formulation of conclusions. Philippa Middleton, Vicki Flenady, Emily Shepherd and Zohra Lassi provided a methodological perspective; Robert M Silver, David Ellwood and Alexander Heazell provided a clinical perspective (obstetrics), and Trish Wilson and Margaret Murphy provided a clinical perspective (midwifery).
Sources of support
Internal sources
Mater Research Institute, The University of Queensland, Australia.
Robinson Research Institute, The University of Adelaide, Australia.
Women's & Children's Health Research Institute, The University of Adelaide, Australia.
National Institute for Health Research: Alexander Heazell: National Institute of Health Research (NIHR) Clinician Scientist Award (CS‐2013‐13‐009), UK.
External sources
National Health and Medical Research Council (NHMRC), Australia.
Declarations of interest
Aleena M Wojcieszek: none known.
Emily Shepherd: none known.
Philippa Middleton: none known.
Zohra S Lassi: none known.
Trish Wilson: none known.
Margaret M Murphy: none known.
Alexander EP Heazell: Alexander EP Heazell's salary is funded by his National Institute of Health Research (NIHR) Clinician Scientist Award (CS‐2013‐13‐009) although this review is not directly funded by this award. He also receives salary support from Tommy's Charity as Director of the Tommy's Stillbirth Research Centre, University of Manchester. This review is part of this programme of work into improving care in pregnancies after stillbirth. Alexander E P Heazell is the Clinical Lead for a specialist antenatal service for women who have experienced a stillbirth in previous pregnancy.
David A Ellwood: David Ellwood has received sitting fees from the Australian Medical Council but this work is not related to this Cochrane Review. He has received payment for providing expert witness reviews for medico‐legal cases – these cases are in no way related to the topic under review. I am the co‐Director of an NHMRC Centre for Research Excellence ‐ the centre is related to stillbirth and will cover all aspects of research on this topic.
Robert M Silver: Robert M Silver has been awarded NIH grants unrelated to this work. He is a member of the International stillbirth Alliance Scientific Research Committee. He has carried out paid consultancy for Gestavision (a company developing a diagnostic for pre‐eclampsia) and has received payment for grand rounds at several universities.
Vicki Flenady: none known
New
References
References to studies included in this review
Ahmed 2014 {published and unpublished data}
- Ahmed F, Faryad N, Naeem N. Use of aspirin alone or heparin and aspirin in idiopathic recurrent miscarriages: empirical or evidence based management. Sri Lanka Journal of Obstetrics & Gynaecology 2014;36(Suppl 1):29. [Google Scholar]
Christiansen 1994 {published and unpublished data}
- Christiansen OB, Mathiesen O, Husth M, Lauritsen JG, Grunnet N. Placebo‐controlled trial of active immunization with third party leukocytes in recurrent miscarriage. Acta Obstetricia et Gynecologica Scandinavica 1994;73(3):261‐8. [DOI] [PubMed] [Google Scholar]
Christiansen 1995 {published and unpublished data}
- Christiansen OB, Mathiesen O, Husth M, Rasmussen KL, Ingerslev HJ, Lauritsen JG, et al. Placebo‐controlled trial of treatment of unexplained secondary recurrent spontaneous abortions and recurrent late spontaneous abortions with IV immunoglobulin. Human Reproduction 1995;10(10):2690‐5. [DOI] [PubMed] [Google Scholar]
Christiansen 2002 {published and unpublished data}
- Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double‐blind, placebo‐controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Human Reproduction 2002;17(3):809‐16. [DOI] [PubMed] [Google Scholar]
Gris 2004 {published and unpublished data}
- Gris JC, Mercier E, Quéré I, Lavigne‐Lissalde G, Cochery‐Nouvellon E, Hoffet M, et al. Low‐molecular‐weight heparin versus low‐dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004;103:3695‐9. [DOI] [PubMed] [Google Scholar]
Levine 1964 {published data only}
- LeVine L. Habitual abortion: a controlled clinical study of progestational therapy. Western Journal of Surgery 1964;72:30‐6. [PubMed] [Google Scholar]
Martinelli 2012 {published and unpublished data}
- Martinelli I, Ruggenenti P, Cetin I, Pardi G, Perna A, Vergani P, et al. Heparin in pregnant women with previous placenta‐mediated pregnancy complications: A prospective, randomized, multicenter, controlled clinical trial. Blood 2012;119(14):3269‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rey 2009 {published and unpublished data}
- Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental‐mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7(1):58–64. [DOI] [PubMed] [Google Scholar]
Salim 2016 {published data only}
- Salim R, Nachum Z, Gavish I, Romano S, Braverman M, Garmi G. Adjusting enoxaparin dosage according to anti‐FXa levels and pregnancy outcome in thrombophilic women. A randomised controlled trial. Thrombosis and Haemostasis 2016;116(4):687‐95. [DOI] [PubMed] [Google Scholar]
Schleussner 2015 {published and unpublished data}
- Schleussner E, Kamin G, Seliger G, Rogenhofer N, Ebner S, Toth B, et al. Low‐molecular‐weight heparin for women with unexplained recurrent pregnancy loss: a multicenter trial with a minimization randomization scheme. Annals of Internal Medicine 2015;162(9):601‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelhafez 2014 {published data only}
- NCT02303171. Use of warfarin after the first trimester in pregnant women with antiphospholipid syndrome. clinicaltrials.gov/ct2/show/record/NCT02303171 (first received 27 November 2014).
Ahmadi 2017 {published data only}
- Ahmadi M. Immunomodulatory effects of IVIg on pregnancy rate of patient with recurrent pregnancy loss. clinicaltrials.gov/ct2/show/NCT03174951 (first received 5 June 2017).
Alalaf 2012 {published data only}
- Alalaf S. Bemiparin versus low dose aspirin for management of recurrent early pregnancy losses due to antiphospholipid antibody syndrome. Archives of Gynecology and Obstetrics 2012;285(3):641‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoki 1993 {published data only}
- Aoki K, Kajiura S, Matsumoto Y, Yagami Y. Clinical evaluation of immunotherapy in early pregnancy with x‐irradiated paternal mononuclear cells for primary recurrent aborters. American Journal of Obstetrics and Gynecology 1993;169(3):649‐53. [DOI] [PubMed] [Google Scholar]
Baber 1988 {published data only}
- Baber R, Kuan R, Porter R, Saunders D. Early pregnancy support in an in‐vitro fertilization program: does human chorionic gonadotropin reduce the miscarriage rate?. Asia‐Oceania Journal of Obstetrics & Gynaecology 1988;14(4):453‐5. [DOI] [PubMed] [Google Scholar]
Badawy 2008 {published data only}
- Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I. Low‐molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. Journal of Obstetrics and Gynaecology 2008;28(3):280‐4. [DOI] [PubMed] [Google Scholar]
Bao 2017 {published data only}
- Bao SH, Zhou Q, Frempong ST, Tu WY, Sheng SL, Liao H. Use of d‐dimer measurement to guide anticoagulant treatment in recurrent pregnancy loss associated with antiphospholipid syndrome. American Journal of Reproductive Immunology 2017;78(6):e12770. [DOI] [PubMed] [Google Scholar]
Berle 1980 {published data only}
- Berle P, Budenz M, Michaelis J. Is hormonal therapy still justified in imminent abortion?. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184(5):353‐8. [PubMed] [Google Scholar]
Blomqvist 2017 {published data only}
- Blomqvist L, Hellgren M, Strandell A. Acetylsalicylic acid for prevention of pregnancy loss: a randomized trial. Human Reproduction 2017;32:i11‐2. [Google Scholar]
Blumenfeld 1992 {published data only}
- Blumenfeld Z, Ruach M. Early pregnancy wastage: the role of repetitive human chorionic gonadotropin supplementation during the first 8 weeks of gestation. Fertility and Sterility 1992;58(1):19‐23. [DOI] [PubMed] [Google Scholar]
Branch 2000 {published data only}
- Branch DW, Peaceman AM, Druzin M, Silver RK, El‐Sayed Y, Silver RM, et al. A multicenter, placebo‐controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy; The Pregnancy Loss Study Group. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 1):122‐7. [DOI] [PubMed] [Google Scholar]
Brenner 2005 {published data only}
- Brenner B, Bar J, Ellis M, Yarom I, Yohai D, Samueloff A, for the Live‐Enox Investigators. Effects of enoxaparin on late pregnancy complications and neonatal outcome in women with recurrent pregnancy loss and thrombophilia: results from the live‐enox study. Fertility and Sterility 2005;84(3):770‐3. [DOI] [PubMed] [Google Scholar]
Carta 2005 {published data only}
- Carta G, Iovenitti P, Falciglia K. Recurrent miscarriage associated with antiphospholipid antibodies: prophylactic treatment with low‐dose aspirin and fish oil derivates. Clinical and Experimental Obstetrics & Gynecology 2005;32(1):49‐51. [PubMed] [Google Scholar]
Cauchi 1991 {published data only}
- Cauchi MN, Lim D, Young DE, Kloss M, Pepperell RJ. Treatment of recurrent aborters by immunization with paternal cells ‐‐ controlled trial. American Journal of Reproductive Immunology 1991;25(1):16‐7. [DOI] [PubMed] [Google Scholar]
Chakravarty 2012 {published data only}
- Chakravarty BN, Ganesh A, Chowdhuri K, Shyam T, Ghosh S, Chattopadhyay R. Assessment of endometrial vascularity following dydrogesterone and micronized progesterone administration in idiopathic recurrent miscarriage‐a preliminary study. Human Reproduction 2012;27(Suppl 2):P089. [Google Scholar]
Check 1995 {published data only}
- Check JH, Tarquini P, Gandy P, Lauer C. A randomized study comparing the efficacy of reducing the spontaneous abortion rate following lymphocyte immunotherapy and progesterone treatment vs progesterone alone in primary habitual aborters. Gynecologic and Obstetric Investigation 1995;39(4):257‐61. [DOI] [PubMed] [Google Scholar]
Christiansen 1992 {published data only}
- Christiansen OB, Christiansen BS, Husth M, Mathiesen O, Lauritsen J, Grunnet N. Prospective study of anticardiolipin antibodies in immunized and untreated women with recurrent spontaneous abortions. Fertility and Sterility 1992;58(2):328‐34. [DOI] [PubMed] [Google Scholar]
Christiansen 2015 {published data only}
- Christiansen O, Larsen E, Egerup P, Lunoee L, Egestad L, Nielsen H. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double‐blind, placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(4):500‐8. [DOI] [PubMed] [Google Scholar]
Clark 2010 {published data only}
- Clark P, Walker ID, Langhorne P, Crichton L, Thomson A, Greaves M, et al. on behalf of the Scottish Pregnancy Intervention Study collaborators. SPIN (Scottish Pregnancy Intervention) study: a multicenter, randomized controlled trial of low‐molecular‐weight heparin and low‐dose aspirin in women with recurrent miscarriage. Blood 2010;115(21):4162‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1996 {published data only}
- Cohen H. Randomized trial of aspirin versus aspirin and heparin in pregnant women with the antiphospholipid syndrome. Annales de Medecine Interne 1996;147(Suppl 1):44. [PubMed] [Google Scholar]
Coomarasamy 2015 {published data only}
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141‐8. [DOI] [PubMed] [Google Scholar]
Cote‐Arsenault 2014 {published data only}
- Cote‐Arsenault D, Krowchuk H, Schwartz K, McCoy P. Evidence‐based intervention with women pregnant after perinatal loss. MCN: The American Journal of Maternal Child Nursing 2014;39(3):177‐86. [DOI] [PubMed] [Google Scholar]
Coulam 1995 {published data only}
- Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. American Journal of Reproductive Immunology 1995;34(6):333‐7. [DOI] [PubMed] [Google Scholar]
Cowchock 1992 {published data only}
- Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low‐dose heparin treatment. American Journal of Obstetrics and Gynecology 1992;166(5):1318‐23. [DOI] [PubMed] [Google Scholar]
Cowchock 1995 {published data only}
- Cowchock FS, Smith JB. Fertility among women with recurrent spontaneous abortions ‐ the effect of paternal cell immunization treatment. American Journal of Reproductive Immunology 1995;33(2):176‐81. [DOI] [PubMed] [Google Scholar]
Cowchock 1997 {published data only}
- Cowchock S, Reece EA. Do low‐risk pregnant women with antiphospholipid antibodies need to be treated? Organizing group of the antiphospholipid antibody treatment trial. American Journal of Obstetrics and Gynecology 1997;176(5):1099‐100. [DOI] [PubMed] [Google Scholar]
Dal Canto 2012 {published data only}
- Dal Canto M. Prospective randomized controlled trial to assess the efficacy of embryogen culture medium to improve ongoing pregnancy and implantation rates in ivf treatments of patients with a previous history of pregnancy loss. clinicaltrials.gov/ct2/show/NCT01689428 (first received 21 September 2012).
Dendrinos 2007 {published data only}
- Dendrinos S, Kalogirou I, Makrakis E, Theodoridis T, Mahmound EA, Christopoulou‐Cokkinou V, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clinical and Experimental Obstetrics & Gynecology 2007;34(3):143‐5. [PubMed] [Google Scholar]
DeVeciana 2001 {published data only}
- Veciana M, Trail P, Dattel B, Slotnick RN, Abuhamad A. Dalteparin versus unfractionated heparin for prophylactic anticoagulation during pregnancy. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S182. [Google Scholar]
Dolitzky 2006 {published data only}
- Dolitzky M, Inbal A, Segal Y, Weiss A, Brenner B, Carp H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertility and Sterility 2006;86(2):362‐6. [DOI] [PubMed] [Google Scholar]
Elmahashi 2014 {published data only}
- Elmahashi MO, Elbareg AM, Essadi FM, Ashur BM, Adam I. Low dose aspirin and low‐molecular‐weight heparin in the treatment of pregnant Libyan women with recurrent miscarriage. BMC Research Notes 2014;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Zibdeh 2005 {published data only}
- El‐Zibdeh, MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry and Molecular Biology 2005;97(5):431‐4. [DOI] [PubMed] [Google Scholar]
Epperson 2011 {published data only}
- Epperson CN. Effectiveness of cognitive processing therapy in pregnant women with a history of pregnancy loss/complication. clinicaltrials.gov/ct2/show/results/NCT01277354 (first received 14 January 2011).
Famina 2015 {published data only}
- Famina M. The effect of dydrogesterone on placental angiogenesis and pregnancy outcomes in women with threatened miscarriages. Giornale Italiano di Ostetricia e Ginecologia 2015;37(4):155‐8. [Google Scholar]
Farquharson 2002 {published data only}
- Farquharson RG, Quenby S. Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstetrics and Gynecology 2002;100(3):408‐13. [DOI] [PubMed] [Google Scholar]
Fawzy 2008 {published data only}
- Fawzy M, Shokeir T, El‐Tatongy M, Warda O, El‐Refaiey AA, Mosbah A. Treatment options and pregnancy outcome in women with idiopathic recurrent miscarriage: a randomized placebo‐controlled study. Archives of Gynecology and Obstetrics 2008;278(1):33‐8. [DOI] [PubMed] [Google Scholar]
Fuchs 1966 {published data only}
- Fuchs F, Olsen P. An attempted double‐blind controlled trial of progesterone therapy in habitual abortion. Ugeskrift for Laeger 1966;128:1461‐2. [PubMed] [Google Scholar]
Gao 2015 {published data only}
- Gao HY, Tao EX, Wang Y, Yue QA, Ren CE, Yan LF. Immunomodulatory and clinical effects of the "tiaomian iii decoction" in patients with blood blocking antibody deficiency and recurrent spontaneous abortion. Genetics and Molecular Research 2015;14(2):3421‐5. [DOI] [PubMed] [Google Scholar]
Gatenby 1993 {published data only}
- Gatenby PA, Cameron K, Simes J, Adelstein S, Bennett MJ, Jansen RP, et al. Treatment of recurrent spontaneous abortion by immunization with paternal lymphocytes: results of a controlled trial. American Journal of Reproductive Immunology 1993;29(2):88‐94. [DOI] [PubMed] [Google Scholar]
Gerhard 1987 {published data only}
- Gerhard I, Gwinner B, Eggert‐Kruse W, Runnebaum B. Double‐blind controlled trial of progesterone substitution in threatened abortion. Biological Research in Pregnancy and Perinatology 1987;8(1):26‐34. [PubMed] [Google Scholar]
German RSA/IVIG Group 1994 {published data only}
- The German RSA/IVIG Group. Intravenous immunoglobulin in the prevention of recurrent miscarriage: The German RSA/IVIG Group. British Journal of Obstetrics and Gynaecology 1994;101(12):1072‐7. [DOI] [PubMed] [Google Scholar]
Geva 1998 {published data only}
- Geva E, Amit A, Lerner‐Geva L, Lessing JB. Prevention of early pregnancy loss in autoantibody seropositive women. Lancet 1998;351(9095):34‐5. [DOI] [PubMed] [Google Scholar]
Giancotti 2012 {published data only}
- Giancotti A, Torre RL, Spagnuolo A, D'Ambrosio V, Cerekja A, Piazze J, et al. Efficacy of three different antithrombotic regimens on pregnancy outcome in pregnant women affected by recurrent pregnancy loss. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(7):1191‐4. [DOI] [PubMed] [Google Scholar]
Goel 2006 {published data only}
- Goel N, Tuli A, Choudhry R. The role of aspirin versus aspirin and heparin in cases of recurrent abortions with raised anticardiolipin antibodies. Medical Science Monitor 2006;12(3):132‐6. [PubMed] [Google Scholar]
Goldzieher 1964 {published data only}
- Goldzieher JW. Double‐blind trial of a progestin in habitual abortion. JAMA 1964;188(7):651‐4. [DOI] [PubMed] [Google Scholar]
Gomaa 2014 {published data only}
- Gomaa MF, Elkholy AG, El‐Said MM, Abdel‐Salam NE. Combined oral prednisolone and heparin versus heparin: the effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double‐blind placebo randomized controlled trial. Archives of Gynecology and Obstetrics 2014;290(4):757‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gris 1995 {published data only}
- Gris J, Neveu S, Tailland M, Courtieu C, Mares P, Schved J. Use of a low‐molecular weight heparin (enoxaparin) or of a phenformin‐like substance (moroxydine chloride) in primary early recurrent aborters with an impaired fibrinolytic capacity. Thrombosis and Haemostasis 1995;73(3):362‐7. [PubMed] [Google Scholar]
Gris 2010 {published data only}
- Gris JC, Chauleur C, Faillie JL, Baer G, Mares P, Fabbro‐Peray P, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae: the pilot randomised controlled NOH‐AP trial. Thrombosis and Haemostasis 2010;104(4):771‐9. [DOI] [PubMed] [Google Scholar]
Gris 2011 {published data only}
- Gris JC, Chauleur C, Molinari N, Mares P, Fabbro‐Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre‐eclampsia: The pilot randomised controlled NOH‐PE trial. Thrombosis and Haemostasis 2011;106(6):1053‐61. [DOI] [PubMed] [Google Scholar]
Harrison 1992 {published data only}
- Harrison, RF. Human chorionic gonadotrophin (hCG) in the management of recurrent abortion; results of a multi‐centre placebo‐controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1992;47(3):175‐9. [DOI] [PubMed] [Google Scholar]
Ho 1991 {published data only}
- Ho HN, Gill TJ, Hsieh HJ, Jiang JJ, Lee TY, Hsieh CY. Immunotherapy for recurrent spontaneous abortions in a Chinese population. American Journal of Reproductive Immunology 1991;25(1):10‐5. [DOI] [PubMed] [Google Scholar]
Illeni 1994 {published data only}
- Illeni MT, Marelli G, Parazzini F, Acaia B, Bocciolone L. Bontempelli M, et al. Immunotherapy and recurrent abortion: a randomized clinical trial. Human Reproduction 1994;9(7):1247‐9. [DOI] [PubMed] [Google Scholar]
Ismail 2016 {published data only}
- Ismail AM, Hamed AH, Saso S, Abu‐Elhasan AM, Abu‐Elghar MM, Abdelmeged AN. Randomized controlled study of pre‐conception thromboprophylaxis among patients with recurrent spontaneous abortion related to antiphospholipid syndrome. International Journal of Gynecology and Obstetrics 2016;132(2):219‐23. [DOI] [PubMed] [Google Scholar]
Ismail 2018 {published data only}
- Ismail AM, Abbas AM, Ali MK, Amin AF. Peri‐conceptional progesterone treatment in women with unexplained recurrent miscarriage: a randomized double‐blind placebo‐controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2018;31(3):388‐94. [DOI] [PubMed] [Google Scholar]
Jablonowska 1999 {published data only}
- Jablonowska B, Selbing A, Palfi M, Ernerudh J, Kjellberg S, Lindton B. Prevention of recurrent spontaneous abortion by intravenous immunoglobulin: a double‐blind placebo‐controlled study. Human Reproduction 1999;14(3):838‐41. [DOI] [PubMed] [Google Scholar]
Johnson 1975 {published data only}
- Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha‐hydroxyprogesterone caproate in the prevention of premature labor. New England Journal of Medicine 1975;293(14):675‐80. [DOI] [PubMed] [Google Scholar]
Johnson 1991 {published data only}
- Johnson PM, Ramsden GH, Chia KV, Hart CA, Farquharson RG, Francis WJA. A combined randomised double‐blind and open study of trophoblast membrane infusion (TMI) in unexplained recurrent miscarriage. Cellular and Molecular Biology of the Materno‐Fetal Relationship. Vol. 212, John Libbey Eurotext Ltd, 1991:277‐84. [Google Scholar]
Kaaja 1993 {published data only}
- Kaaja R, Julkunen H, Viinikka L, Ylikorkala O. Production of prostacylin and thromboxane in lupus pregnancies: effect of small dose of aspirin. Obstetrics and Gynecology 1993;81(3):327‐31. [PubMed] [Google Scholar]
Kaandorp 2010 {published data only}
- Kaandorp SP, Goddijn M, Post JA, Hutten BA, Verhoeve HR, Hamulyák K, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. New England Journal of Medicine 2010;362(17):1586‐96. [DOI] [PubMed] [Google Scholar]
Kayatas 2013 {published data only}
- Kayatas S, Yuksel H, Ertekin A, Cam C. Comparison of tinzaparin with enoxaparin in unexplained recurrent miscarriage. Fertility and Sterility 2013;100(3 Suppl 1):S319. [Google Scholar]
Khan 2017 {published data only}
- Khan ES, Basharat A, Jamil M, Ayub S, Khan MA. Preventive role of low‐molecular‐weight heparin in unexplained recurrent pregnancy loss. South African Journal of Obstetrics and Gynaecology 2017;23(1):17‐9. [Google Scholar]
Kilpatrick 1993 {published data only}
- Kilpatrick DC, Kitchin AJ, Liston WA. Humoral immune response to lymphocyte antigens in early pregnancy and after leucocyte immunotherapy. Journal of Obstetrics and Gynaecology 1993;13:77‐81. [Google Scholar]
Kim 1997 {published data only}
- Kim CH, Chae HD, Koo JN, Kim NY, Kang BM, Chi HS. Comparison of pregnancy outcome between low dose aspirin alone and aspirin plus prednisolone treatment in recurrent spontaneous abortion associated with antiphospholipid antibodies. Korean Journal of Obstetrics and Gynecology 1997;40(7):1404‐11. [Google Scholar]
Kim 2012 {published data only}
- Kim CH, Lee KH, Kim SH, Chae HD, Kang BM, Jung KS. Effect of etanercept treatment in women with unexplained primary recurrent pregnancy loss. Human Reproduction 2012;27(Suppl 2):P098. [Google Scholar]
Klopper 1965 {published data only}
- Klopper AI, MacNaughton MC. Hormones in recurrent abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1022‐8. [Google Scholar]
Kumar 2014 {published data only}
- Kumar A, Begum N, Prasad S, Aggarwal S, Sharma S. Oral dydrogesterone treatment during early pregnancy to prevent recurrent pregnancy loss and its role in modulation of cytokine production: a double‐blind, randomized, parallel, placebo‐controlled trial. Fertility and Sterility 2014;102(5):1357‐63.e3. [DOI] [PubMed] [Google Scholar]
Kutteh 1996 {published data only}
- Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody‐associated recurrent pregnancy loss with lower dose heparin and aspirin. American Journal of Reproductive Immunology 1996;35(4):402‐7. [DOI] [PubMed] [Google Scholar]
Kwon 2012 {published data only}
- Kwon SK, Kim CH, Ahn JW, Lee KH, Chae HD, Kang BM. Effect of intravenous immunoglobulin on pregnancy outcome following IVF/ICSI in infertile patients with endometriosis. Fertility and Sterility 2012;98 Suppl 1(3):S263 Abstract no:O‐511. [Google Scholar]
Laskin 1997 {published data only}
- Laskin CA, Bombardier C, Hannah ME, Mandel FP, Ritchie JW, Farewell V, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. New England Journal of Medicine 1997;337(3):148‐53. [DOI] [PubMed] [Google Scholar]
Laskin 2009 {published data only}
- Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker G, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. Journal of Rheumatology 2009;36(2):279‐87. [DOI] [PubMed] [Google Scholar]
Lazzarin 2009 {published data only}
- Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, Arduini D. Low‐dose aspirin and omega‐3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertility and Sterility 2009;92(1):296‐300. [DOI] [PubMed] [Google Scholar]
Li 1998 {published data only}
- Li D, Li C, Zhu Y. Comparative study of the third party and paternal leukocyte immunization in recurrent spontaneous abortion of lowered maternal‐fetal immuno‐recognition. Zhonghua Fu Chan Ke Za Zhi [Chinese Journal of Obstetrics & Gynecology] 1998;33(10):597‐600. [PubMed] [Google Scholar]
MacDonald 1972 {published data only}
- MacDonald RR, Goulden R, Oakey RE. Cervical mucus, vaginal cytology and steroid excretion in recurrent abortion. Obstetrics and Gynecology 1972;40(3):394‐402. [PubMed] [Google Scholar]
Maged 2016 {published data only}
- Maged AM, Abdelhafiz A, Mostafa WA, El‐Nassery N, Fouad M, Salah E, et al. The role of prophylactic use of low dose aspirin and calheparin in patients with unexplained recurrent abortion. Gynecological Endocrinology 2016;32(12):970‐2. [DOI] [PubMed] [Google Scholar]
Mahmoud 2004 {published data only}
- Mahmoud F, Diejomaoh M, Omu A, Abul H, Haines D. Effect of IgG therapy on lymphocyte subpopulations in the peripheral blood of Kuwaiti women experiencing recurrent pregnancy loss. Gynecologic and Obstetric Investigation 2004;58(2):77‐83. [DOI] [PubMed] [Google Scholar]
Malathi 2011 {published data only}
- Malathi V. Treatment outcome in women suffering from recurrent miscarriages and antiphospholipid syndrome. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:184.
Malinowski 2003 {published data only}
- Malinowski A, Dynski MA, Maciolek‐Blewniewska G, Glowacka E, Pawlowski T, Babula G. Treatment outcome in women suffering from recurrent miscarriages and antiphospholipid syndrome. Ginekologia Polska 2003;74(10):1213‐22. [PubMed] [Google Scholar]
Mankuta 1999 {published data only}
- Mankuta D, Spitzer KA, Seaward G, Farine D, Ryan G, Clark‐Soloninka CA, et al. Prednisone does not affect the biophysical score in pregnant women with autoantibodies. American Journal of Obstetrics and Gynecology 1999;180:S163. [Google Scholar]
Meng 2016 {published data only}
- Meng L, Lin J, Chen L, Wang Z, Liu M, Liu Y, et al. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Archives of Gynecology and Obstetrics 2016;294(1):29‐39. [DOI] [PubMed] [Google Scholar]
Mohamed 2014 {published data only}
- Mohamed KA, Saad AS. Enoxaparin and aspirin therapy for recurrent pregnancy loss due to anti‐phospholipid syndrome (APS). Middle East Fertility Society Journal 2014;19(3):176‐82. [Google Scholar]
Moller 1965 {published data only}
- Moller KJ, Fuchs F. Double blind controlled trial of 6‐methyl‐17‐acetoxyprogesterone in threatened abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1042‐4. [Google Scholar]
Mowbray 1985 {published data only}
- Mowbray JF, Gibbings C, Liddell H, Reginald PW, Underwood JL, Beard RW. Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet 1985;1(8435):941‐3. [DOI] [PubMed] [Google Scholar]
Nagpal 2001 {published data only}
- Nagpal M, Malhotra R. Should human chorionic gonadotropin supplementation be used as a routine prophylaxis in high risk pregnancies?. Journal of Obstetrics and Gynecology of India 2001;51(4):65‐7. [Google Scholar]
Navidian 2018 {published data only}
- Navidian A, Saravani Z. Impact of cognitive behavioral‐based counseling on grief symptoms severity in mothers after stillbirth. Iranian Journal of Psychiatry and Behavioral Sciences 2018;12(1):e9275. [Google Scholar]
Nazari 2015 {published data only}
- Nazari Z, Ghaffari J, Ebadi A. Comparison of the effect of aspirin and heparin with or without intravenous immunoglobulin in treatment of recurrent abortion with unknown etiology: a clinical study. Journal of Natural Science, Biology, and Medicine 2015;6(Suppl 1):S17‐S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noble 2005 {published data only}
- Noble LS, Kutteh WH, Lashey N, Franklin RD, Herrada J. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low‐molecular‐weight heparin versus unfractionated heparin. Fertility and Sterility 2005;83(3):684‐90. [DOI] [PubMed] [Google Scholar]
Norman 2006 {published data only}
- Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double‐blind, placebo‐controlled study and meta‐analysis. Lancet 2009;373(9680):2034‐40. [DOI] [PubMed] [Google Scholar]
Norman 2016 {published data only}
- Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM Study): a multicentre, randomised, double‐blind trial. Lancet 2016;387(10033):2106‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ober 1999 {published data only}
- Ober C, Karrison T, Odem RR, Barnes RB, Branch DW, Stephenson MD, et al. Mononuclear‐cell immunisation in prevention of recurrent miscarriages: a randomised trial. Lancet 1999;354(9176):365‐9. [DOI] [PubMed] [Google Scholar]
Pandey 2004 {published data only}
- Pandey MK, Agrawal S. Induction of MLR‐Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. International Immunopharmacology 2004;4(2):289‐98. [DOI] [PubMed] [Google Scholar]
Pasquier 2015 {published data only}
- Pasquier E, Bohec C, Chauleur C, Bretelle F, Gal G, Debarge V, et al. Heparin for prevention of unexplained recurrent miscarriage in non‐thrombophilic women: A multicenter randomized double‐blind placebo‐controlled trial. Thrombosis Research 2015;135(Suppl 1):S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pattison 2000 {published data only}
- Pattison NS, Chamley LW, Birdsall M, Zanderigo AM, Liddell HS, McDougall J. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2000;183(4):1008‐12. [DOI] [PubMed] [Google Scholar]
Perino 1997 {published data only}
- Perino A, Vassiliadis A, Vucetich A, Colacurci N, Menato G, Cignitti M, et al. Short‐term therapy for recurrent abortion using intravenous immunoglobulins: results of a double‐blind placebo‐controlled Italian study. Human Reproduction 1997;12(11):2388‐92. [DOI] [PubMed] [Google Scholar]
Prietl 1992 {published data only}
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17alpha‐hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction 1992;7(1):1‐5. [DOI] [PubMed] [Google Scholar]
Quenby 1992 {published data only}
- Quenby S, Farquharson R, Ramsden G. The obstetric outcome of patients with positive anticardiolipin antibodies: aspirin vs no treatment. 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:443.
Quenby 1994 {published data only}
- Quenby S, Farquharson RG. Human chorionic gonadotropin supplementation in recurring pregnancy loss: a controlled trial. Fertility and Sterility 1994;62(4):708‐10. [DOI] [PubMed] [Google Scholar]
Quenby 2007 {published data only}
- Quenby S. A randomised controlled trial of prednisolone for women with recurrent miscarriage and high levels of uterine natural killer cells in the endometrium. isrctn.com/ISRCTN28090716 (first received 5 July 2007).
Qureshi 2005 {published data only}
- Qureshi NS, Edi‐Osagie EC, Ogbo V, Ray S, Hopkins R. First trimester threatened miscarriage treatment with human chorionic gonadotrophins: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112(11):1536‐41. [DOI] [PubMed] [Google Scholar]
Raddatz 2005 {published data only}
- Raddatz G. Habitual abortion study: oral dydrogesterone treatment during pregnancy in women with recurrent miscarriage. clinicaltrials.gov/ct2/show/NCT00193674 (first received 19 September 2005).
Rafiee 2015 {published data only}
- Rafiee M, Gharagozloo M, Ghahiri A, Mehrabian F, Maracy MR, Kouhpayeh S, et al. Altered th17/treg ratio in recurrent miscarriage after treatment with paternal lymphocytes and vitamin d3: a double‐blind placebo‐controlled study. Iranian Journal of Immunology 2015;12(4):252‐62. [PubMed] [Google Scholar]
Rai 1997 {published data only}
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997;314(7076):253‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rai 2005 {published data only}
- Rai R. Steroids and antiphospholipid syndrome ‐ related pregnancy loss. clinicaltrials.gov/ct2/show/NCT00180778 (first received 16 September 2005).
Rajan 1993 {published data only}
- Rajan L, Oakley A. No pills for heartache: the importance of social support for women who suffer pregnancy loss. Journal of Reproductive and Infant Psychology 1993;11(2):75‐87. [Google Scholar]
Reijnders 1988 {published data only}
- Reijnders FJ, Thomas CM, Doesburg WH, Rolland R, Eskes T. Endocrine effects of 17 alpha‐hydroxyprogesterone caproate during early pregnancy: a double‐blind clinical trial. British Journal of Obstetrics and Gynaecology 1988;95(5):462‐8. [DOI] [PubMed] [Google Scholar]
Reznikoff‐Etievant 1994 {published data only}
- Reznikoff‐Etievant MF. Abstracts of contributors' individual data submitted to the worldwide prospective observation study on immunotherapy for treatment of recurrent spontaneous abortion. American Journal of Reproductive Immunology 1994;32:266‐7. [Google Scholar]
Rodger 2014 {published data only}
- Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open‐label randomised trial. Lancet 2014;384(9955):1673‐83. [DOI] [PubMed] [Google Scholar]
Saad 2014 {published data only}
- Saad A, Mohamed K. Pregnancy outcomes in women with recurrent miscarriage treated with low dose aspirin and unfractionated heparin. clinicaltrials.gov/ct2/show/NCT02144064 (first received 21 May 2014).
Salman 2012 {published data only}
- Salman SA, Shaaban OM, Zahran KM, Fathalla MM, Anan MA. Low molecular weight heparin (LMWH) for treatment of recurrent miscarriage negatively tested for anti phospholipid antibodies: A randomized controlled trial. Fertility and Sterility 2012;98(3 Suppl 1):S191. [Google Scholar]
Samantha 2013 {published data only}
- Samantha P, Barillari G, Venturelli U, Turello M. Thromboprophylaxis with two different dosages of LMWH (calcic nadroparin) in pregnant women with thrombophilia: a single center experience. Journal of Thrombosis and Haemostasis 2013;11:96‐7. [Google Scholar]
Scarpellini 2009 {published data only}
- Scarpellini F, Sbracia M. Use of granulocyte colony‐stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Human Reproduction 2009;24(11):2703‐8. [DOI] [PubMed] [Google Scholar]
Scarpellini 2017 {published data only}
- Scarpellini F, Balili A, Sbracia M. G‐CSF treatment in unexplained recurrent pregnancy loss: Results of a controlled trial on 120 women and total data on ten years experience. Journal of Perinatal Medicine 2017;45(Suppl 1):28. [Google Scholar]
Schisterman 2014 {published data only}
- Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski‐Wende J, Townsend JM, et al. Preconception low‐dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014;384(9937):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1996 {published data only}
- Scott JR, Branch WD, Dudley D. [personal communication]. Letter to: Cochrane Pregnancy and Childbirth Group 23 August 1996.
Shaaban 2017 {published data only}
- Shaaban OM, Abbas AM, Zahran KM, Fathalla MM, Anan MA, Salman SA. Low‐molecular‐weight heparin for the treatment of unexplained recurrent miscarriage with negative antiphospholipid antibodies: a randomized controlled trial. Clinical and Applied Thrombosis/Hemostasis 2017;23(6):567‐72. [DOI] [PubMed] [Google Scholar]
Sharifi Saki 2015 {published data only}
- Sharifi Saki S. Effectiveness of mindfulness‐based cognitive therapy group training in reducing anxiety and meta‐worry of women having repeated spontaneous abortion. en.irct.ir/trial/18498 (first received 17 July 2015).
Shearman 1963 {published data only}
- Shearman RP, Garrett WJ. Double‐blind study of effect of 17‐hydroxyprogesterone caproate on abortion rate. British Medical Journal 1963;1(5326):292‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shefras 1995 {published data only}
- Shefras J, Farquharson RG. Heparin therapy, bone density and pregnancy. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:93.
Shu 2002 {published data only}
- Shu J, Miao P, Wang RJ. Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive early recurrent spontaneous abortion. Chinese Journal of Integrated Traditional and Western Medicine 2002;22(6):414‐6. [PubMed] [Google Scholar]
Silver 1993 {published data only}
- Silver RK, MacGregor SN, Sholl JS, Hobart JM, Neerhof MG, Ragin A. Comparative trial of prednisone plus aspirin vs aspirin alone in the treatment of anticardiolipin antibody‐positive obstetric patients. American Journal of Obstetrics and Gynecology 1993;169(6):1411‐7. [DOI] [PubMed] [Google Scholar]
Smitz 1992 {published data only}
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. Randomized prospective trial comparing supplementation of the luteal phase and of early pregnancy by natural progesterone given by intramuscular or vaginal administration. Revue Francaise de Gynecologie et d Obstetrique 1992;10(87):507‐16. [PubMed] [Google Scholar]
Sondergaard 1985 {published data only}
- Sondergaard F, Ottesen B, Detlefsen GU, Schierup L, Pederson SC, Lebech PE. Progesterone treatment of cases of threatened pre‐term delivery in women with a low level of plasma progesterone. Contraception, Fertilité et Sexualité 1985;13:1227‐31. [Google Scholar]
Stephenson 2004 {published data only}
- Stephenson MD, Ballem PJ, Tsang P, Purkiss S, Ensworth S, Houlihan E, et al. Treatment of antiphospholipid antibody syndrome (APS) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. Journal of Obstetrics and Gynaecology Canada: JOGC 2004;26(8):729‐34. [DOI] [PubMed] [Google Scholar]
Stephenson 2010 {published data only}
- Stephenson MD, Kutteh WH, Purkiss S, Librach C, Schultz P, Houlihan E, et al. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo‐controlled trial. Human Reproduction 2010;25(9):2203‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stray‐Pedersen 1996 {published data only}
- Stray‐Pedersen S. [personal communication]. Letter to: Cochrane Pregnancy and Childbirth Group 23 August 1996.
Sun 2010 {published data only}
- Sun X‐G, Liu XY, Zhu R, Fan GS, Zhang Y, Chen FL. Effectiveness of intravenous immunoglobulin therapy in treating unexplained recurrent spontaneous abortion and its effect on the level of serum soluble human leucocyte antigen G. Acta Academiae Medicinae Sinicae 2010;32(5):483‐7. [DOI] [PubMed] [Google Scholar]
Svigos 1982 {published data only}
- Svigos J. Preliminary experience with the use of human chorionic gonadotrophin therapy in women with repeated abortion. Clinical Reproduction and Fertility 1982;1(2):131‐5. [PubMed] [Google Scholar]
Swyer 1953 {published data only}
- Swyer GI, Daley D. Progesterone implantation in habitual abortion. British Medical Journal 1953;1(4819):1073–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang AW, Alfirevic Z, Turner MA, Drury JA, Small R, Quenby S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Human Reproduction 2013;28(7):1743‐52. [DOI] [PubMed] [Google Scholar]
Tognoni 1980 {published data only}
- Tognoni G, Ferrario L, Inzalaco M, Crosignani PG. Progestagens in threatened abortion. Lancet 1980;2(8206):1242‐3. [DOI] [PubMed] [Google Scholar]
Triolo 2003 {published data only}
- Triolo G, Ferrante A, Ciccia F, Accardo‐Palumbo A, Perino A. Castelli A, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis and Rheumatism 2003;48(3):728‐31. [DOI] [PubMed] [Google Scholar]
Tulppala 1997 {published data only}
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Ailus K, Palosuo T, Ylikorkala O. Low dose aspirin in the prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(1):191. [DOI] [PubMed] [Google Scholar]
Turner 1966 {published data only}
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95(2):222‐7. [DOI] [PubMed] [Google Scholar]
Vahid Dastjerdi 1999 {published data only}
- Vahid Dastjerdi M, Moini A, Aleyasin A, Kashaf H, Marsoosi V, Aghahosscini A. The effect of acetyl salicylic acid and prednisolone before and during pregnancy in reducing unexplained recurrent abortions. Fertility and Sterility 1999;72:S203. [Google Scholar]
Van Hoorn 2016 {published data only}
- Hoorn ME, Hague WM, Pampus MG, Bezemer D, Vries JI. Low‐molecular‐weight heparin and aspirin in the prevention of recurrent early‐onset pre‐eclampsia in women with antiphospholipid antibodies: the FRUIT‐RCT. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;197:168‐73. [DOI] [PubMed] [Google Scholar]
Vaquero 2001 {published data only}
- Vaquero E, Lazzarin N, Valensise H, Menghini S, Pierro G, Cesa F, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: a comparative study of intravenous immunoglobulin versus prednisone plus low‐dose aspirin. American Journal of Reproductive Immunology 2001;45(3):174‐9. [DOI] [PubMed] [Google Scholar]
Visser 2011 {published data only}
- Visser J, Ulander VM, Helmerhorst FM, Lampinen K, Morin‐Papunen L, Bloemenkamp KW, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia ‐ HABENOX*: a randomised multicentre trial. Thrombosis and Haemostasis 2011;105(2):295‐301. [DOI] [PubMed] [Google Scholar]
Walch 2005 {published data only}
- Walch K, Hefler L, Nagele F. Oral dydrogesterone treatment during the first trimester of pregnancy: the prevention of miscarriage study (PROMIS). A double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. Journal of Maternal‐fetal & Neonatal Medicine 2005;18(4):265‐9. [DOI] [PubMed] [Google Scholar]
Xiao 2013 {published data only}
- Xiao J, Xiong J, Zhu F, He L. Effect of prednisone, aspirin, low molecular weight heparin and intravenous immunoglobulin on outcome of pregnancy in women with antiphospholipid syndrome. Experimental and Therapeutic Medicine 2013;5(1):287‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zafardoust 2017 {published data only}
- Zafardoust S, Mehdi Akhondi M, Sadeghi Mohammad R, Mohammadzadeh A, Karimi A, Jouhari S, et al. Efficacy of intrauterine injection of granulocyte colony stimulating factor (g‐csf) on treatment of unexplained recurrent miscarriage: a pilot rct study. Journal of Reproduction & Infertility 2017;18(4):379‐85. [PMC free article] [PubMed] [Google Scholar]
Zolghadri 2010 {published data only}
- Zolghadri J, Ahmadpour F, Momtahan M, Tavana Z, Foroughinia L. Evaluation of the efficacy of aspirin and low molecular weight heparin in patients with unexplained recurrent spontaneous abortionsIranian. Red Crescent Medical Journal 2010;12(5):548‐52. [Google Scholar]
References to ongoing studies
Alves 2014 {published data only}
- Alves JG, Araujo CA, Pontes IE, Guimaraes AC, Ray JG. The BRAzil MAGnesium (BRAMAG) trial: a randomized clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity. BMC Pregnancy and Childbirth 2014;14(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jong 2015 {published data only}
- Jong PG, Quenby S, Bloemenkamp KWM, Braams‐Lisman BAM, Bruin JP, Coomarasamy A, et al. ALIFE2 study: low‐molecular‐weight heparin for women with recurrent miscarriage and inherited thrombophilia ‐ study protocol for a randomized controlled trial. Trials 2015;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐refaie 2016 {published data only}
- El‐refaie W. Vaginal progesterone versus cervical cerclage for pregnant women with short cervix and history of PTL and/or MTM. clinicaltrials.gov/ct2/show/NCT02673359 (first received 3 February 2016).
Hezelgrave 2016 {published data only}
- Hezelgrave NL, Watson HA, Ridout A, Diab F, Seed PT, Chin‐Smith E, et al. Rationale and design of SuPPoRT: a multi‐centre randomised controlled trial to compare three treatments: cervical cerclage, cervical pessary and vaginal progesterone, for the prevention of preterm birth in women who develop a short cervix. BMC Pregnancy and Childbirth 2016;16(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLindon 2011 {published data only}
- McLindon L. In pregnant women with previous subfertility, does progesterone supplementation decrease the likelihood of miscarriage?. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336812 (first received 18 April 2011).
Rodger 2017 {published data only}
- Rodger M. A pilot study assessing the feasibility of a randomized controlled trial evaluating aspirin versus low‐molecular‐weight heparin (LMWH) and aspirin in women with antiphospholipid syndrome and pregnancy loss. clinicaltrials.gov/ct2/show/NCT03100123 (4 April 2017).
Schreiber 2017 {published data only}
- Schreiber K, Breen K, Robinson SE, Hunt BJ, Jacobsen S, Cohen H, et al. Hydroxychloroquine to improve pregnancy outcome in women with antiphospholipid antibodies (HYPATIA) protocol: a multinational randomized controlled trial of hydroxychloroquine versus placebo in addition to standard treatment in pregnant women with antiphospholipid syndrome or antibodies. Seminars in Thrombosis and Hemostasis 2017;43(6):562‐71. [DOI] [PubMed] [Google Scholar]
Additional references
Askie 2007
- Askie LM, Duley L, Henderson‐Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre‐eclampsia: a meta‐analysis of individual patient data. Lancet 2007;369(9575):1791‐8. [DOI] [PubMed] [Google Scholar]
Baibazarova 2013
- Baibazarova E, Beek C, Cohen‐Kettenis PT, Buitelaar J, Shelton KH, Goozen SH. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology 2013;38(6):907‐15. [DOI] [PubMed] [Google Scholar]
Black 2008a
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI] [PubMed] [Google Scholar]
Black 2008b
- Black M, Shetty A, Bhattacharya S. Obstetric outcomes subsequent to intrauterine death in the first pregnancy. BJOG: an international journal of obstetrics and gynaecology 2008;115(2):269‐74. [DOI] [PubMed] [Google Scholar]
Bujold 2010
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta‐analysis. Obstetrics and Gynecology 2010;116(2):402‐14. [DOI] [PubMed] [Google Scholar]
De Jong 2014
- Jong PG, Kaandorp S, Nisio M, Goddijn M, Middeldorp S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD004734.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffett 2015
- Duffett L, Rodger M. LMWH to prevent placenta‐mediated pregnancy complications: an update. British Journal of Haematology 2015;168(5):619‐38. [DOI] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Dunkel Schetter 2011
- Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology 2011;62:531‐58. [DOI] [PubMed] [Google Scholar]
Flenady 2011
- Flenady V, Koopmans L, Middleton P, Froen JF, Smith, GC, Gibbons K, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet 2011;377(9774):1331–40. [DOI] [PubMed] [Google Scholar]
Flenady 2015
- Flenady V. Epidemiology of fetal and neonatal pathology. In: Khong TY, Malcomson RDG editor(s). Keeling's Fetal and Neonatal Pathology. 1. Springer, Cham, 2015. [Google Scholar]
Flenady 2016
- Flenady V, Wojcieszek AM, Middleton P, Ellwood D, Erwich, J, Coory M, et al. Lancet Ending Preventable Stillbirths series Study Group. Stillbirths: recall to action in high‐income countries. Lancet 2016;387(10019):691‐702. [DOI] [PubMed] [Google Scholar]
Fockler 2017
- Fockler ME, Ladhani NN, Watson J, Barrett JF. Pregnancy subsequent to stillbirth: medical and psychosocial aspects of care. Seminars in Fetal and Neonatal Medicine 2017;22(3):186‐92. [DOI] [PubMed] [Google Scholar]
Getahun 2009
- Getahun D, Lawrence JM, Fassett MJ, Strickland D, Koebnick C, Chen W, et al. The association between stillbirth in the first pregnancy and subsequent adverse perinatal outcomes. American Journal of Obstetrics and Gynecology 2009;201(4):378. [DOI] [PubMed] [Google Scholar]
Goldenberg 2016
- Goldenberg RL, Saleem S, Pasha O, Harrison MS, McClure EM. Reducing stillbirths in low‐income countries. Acta Obstetricia et Gynecologica Scandinavica 2016;95(2):135‐43. [DOI] [PubMed] [Google Scholar]
Gravensteen 2018
- Gravensteen IK, Jacobsen EM, Sandset PM, Helgadottir LB, Radestad I, Sandvik L, et al. Anxiety, depression and relationship satisfaction in the pregnancy following stillbirth and after the birth of a live‐born baby: a prospective study. BMC Pregnancy and Childbirth 2018;18(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2018
- Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD003511.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heazell 2016
- Heazell AE, Siassakos D, Blencowe H, Bhutta ZA, Cacciatore J, Dang N, et al. Lancet Ending Preventable Stillbirths series Study Group. Stillbirths: economic and psychosocial consequences. Lancet 2016;387(10018):604‐16. [DOI] [PubMed] [Google Scholar]
Heinonen 2000
- Heinonen S, Kirkinen P. Pregnancy outcome after previous stillbirth resulting from causes other than maternal conditions and fetal abnormalities. Birth 2000;27(1):33‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 2001
- Hughes P, Turton P, Hopper E, McGauley GA, Fonagy P. Disorganised attachment behaviour among infants born subsequent to stillbirth. Journal of Child Psychology and Psychiatry and Allied Disciplines 2001;42(6):791‐801. [PubMed] [Google Scholar]
Hussey 2007
- Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemporary Clinical Trials 2007;28(2):182‐91. [DOI] [PubMed] [Google Scholar]
Lamont 2015
- Lamont K, Scott NW, Jones GT, Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta‐analysis. BMJ 2015;350:h3080. [DOI] [PubMed] [Google Scholar]
Lawn 2016
- Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors and potential for progress towards 2030. Lancet 2016;387(10018):587‐603. [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee L, McKenzie‐McHarg K, Horsch A. The impact of miscarriage and stillbirth on maternal–fetal relationships: an integrative review. Journal of Reproductive and Infant Psychology 2017;35(1):32‐52. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research ed.) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2018
- McClure EM, Garces A, Saleem S, Moore JL, Bose CL, Esamai F, et al. Global Network for Women's and Children's Health Research: probable causes of stillbirth in low‐ and middle‐income countries using a prospectively defined classification system. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):131‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mdege 2011
- Mdege ND, Man M‐S, Taylor CA, Torgerson DJ. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. Journal of Clinical Epidemiology 2011;64(9):936‐48. [DOI] [PubMed] [Google Scholar]
Meaney 2017
- Meaney S, Everard CM, Gallagher S, O'Donoghue K. Parents' concerns about future pregnancy after stillbirth: a qualitative study. Health Expectations 2017;20(4):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meredith 2017
- Meredith P, Wilson T, Branjerdporn G, Strong J, Desha L. 'Not just a normal mum': a qualitative investigation of a support service for women who are pregnant subsequent to perinatal loss. BMC Pregnancy and Childbirth 2017;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2014
- Mills TA, Ricklesford C, Cooke A, Heazell AE, Whitworth M, Lavender T. Parents' experiences and expectations of care in pregnancy after stillbirth or neonatal death: a metasynthesis. BJOG: an international journal of obstetrics and gynaecology 2014;121(8):943‐50. [DOI] [PubMed] [Google Scholar]
Mills 2016
- Mills TA, Ricklesford C, Heazell AEP, Cooke A, Lavender T. Marvellous to mediocre: findings of national survey of UK practice and provision of care in pregnancies after stillbirth or neonatal death. BMC Pregnancy and Childbirth 2016;16(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monari 2010
- Monari F, Facchinetti F. Management of subsequent pregnancy after antepartum stillbirth. A review. Journal of Maternal‐fetal & Neonatal Medicine 2010;23(10):1073‐84. [DOI] [PubMed] [Google Scholar]
Monari 2016
- Monari F, Pedrielli G, Vergani P, Pozzi E, Mecacci F, Serena C, et al. Adverse perinatal outcome in subsequent pregnancy after stillbirth by placental vascular disorders. PloS One 2016;11(5):e0155761. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2009
- O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Developmental Neuroscience 2009;31(4):285‐92. [DOI] [PubMed] [Google Scholar]
Ogwulu 2015
- Ogwulu CB, Jackson LJ, Heazell AE, Roberts TE. Exploring the intangible economic costs of stillbirth. BMC Pregnancy and Childbirth 2015;15:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onwude 2006
- Onwude JL, Eisman V, Selo‐Ojeme DO. Recurrent stillbirths: a matched case‐control study of unexplained stillbirths at term. Journal of Obstetrics and Gynaecology 2006;26(3):205‐7. [DOI] [PubMed] [Google Scholar]
Paull 2013
- Paull C, Robson S. After stillbirth, what next?. O&G Magazine 2013; Vol. 15, issue 4:579.
Reddy 2007
- Reddy UM. Prediction and prevention of recurrent stillbirth. Obstetrics and Gynecology 2007;110(5):1151‐64. [DOI] [PubMed] [Google Scholar]
Reinebrant 2018
- Reinebrant HE, Leisher SH, Coory M, Henry S, Wojcieszek AM, Gardener G, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth [2018]. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):212‐24. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberge 2013
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low‐dose aspirin: a meta‐analysis. Ultrasound in Obstetrics & Gynecology 2013;41(5):491‐9. [DOI] [PubMed] [Google Scholar]
Roberge 2016
- Roberge S, Odibo AO, Bujold E. Aspirin for the prevention of preeclampsia and Intrauterine growth restriction. Clinics in Laboratory Medicine 2016;36(2):319‐29. [DOI] [PubMed] [Google Scholar]
Robson 2001
- Robson S, Chan A, Keane RJ, Luke CG. Subsequent birth outcomes after an unexplained stillbirth: preliminary population‐based retrospective cohort study. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(1):29‐35. [DOI] [PubMed] [Google Scholar]
Robson 2006
- Robson S, Thompson J, Ellwood D. Obstetric management of the next pregnancy after an unexplained stillbirth: an anonymous postal survey of Australian obstetricians. Australian & New Zealand Journal of Obstetrics & Gynaecology 2006;46(4):278‐81. [DOI] [PubMed] [Google Scholar]
Robson 2009
- Robson SJ, Leader LR, Dear KBG, Bennett MJ. Women’s expectations of management in their next pregnancy after an unexplained stillbirth: an Internet‐based empirical study. Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(6):642‐6. [DOI] [PubMed] [Google Scholar]
Robson 2010
- Robson SJ, Leader LR. Management of subsequent pregnancy after an unexplained stillbirth. Journal of Perinatology 2010;30(5):305‐10. [DOI] [PubMed] [Google Scholar]
Rodger 2016
- Rodger MA, Gris J‐C, Vries JI, Martinelli I, Rey É, Schleussner E, et al. Low‐molecular‐weight heparin and recurrent placenta‐mediated pregnancy complications: a meta‐analysis of individual patient data from randomised controlled trials. Lancet 2016;388(10060):2629‐41. [DOI] [PubMed] [Google Scholar]
Saade 2011
- Saade G. Management of the subsequent pregnancy. Stillbirth: Prediction, Prevention and Management. 1st Edition. Wiley‐Blackwell, 2011. [Google Scholar]
Saccone 2017
- Saccone G, Schoen C, Franasiak JM, Scott RT Jr, Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta‐analysis of randomized, controlled trials. Fertility and Sterility 2017;107(2):430‐8.e3. [DOI] [PubMed] [Google Scholar]
San Lazaro 2017
- San Lazaro Campillo I, Meaney S, McNamara K, O'Donoghue K. Psychological and support interventions to reduce levels of stress, anxiety or depression on women's subsequent pregnancy with a history of miscarriage: an empty systematic review. BMJ Open 2017;7(9):e017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheres 2017
- Scheres LJ, Marijnen MC, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silver 2011
- Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA. Nomenclature for pregnancy outcomes: time for a change. Obstetrics and Gynecology 2011;118(6):1402‐8. [DOI] [PubMed] [Google Scholar]
Simmons 2011
- Simmons HA, Goldberg LS. 'High‐risk' pregnancy after perinatal loss: understanding the label. Midwifery 2011;27(4):452‐7. [DOI] [PubMed] [Google Scholar]
Skeith 2016
- Skeith L, Carrier M, Kaaja R, Martinelli I, Petroff D, Schleussner E, et al. A meta‐analysis of low‐molecular‐weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood 2016;127(13):1650‐5. [DOI] [PubMed] [Google Scholar]
Su 2015
- Su Q, Zhang H, Zhang Y, Zhang H, Ding D, Zeng J, et al. Maternal stress in gestation: Birth outcomes and stress‐related hormone response of the neonates. Pediatrics and Neonatology 2015;56(6):376–81. [DOI] [PubMed] [Google Scholar]
Van den Bergh 2005
- Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neuroscience and Biobehavioral Reviews 2005;29(2):237‐58. [DOI] [PubMed] [Google Scholar]
Wojcieszek 2018
- Wojcieszek AM, Boyle FM, Belizán JM, Cassidy J, Cassidy P, Erwich J, et al. Care in subsequent pregnancies following stillbirth: an international survey of parents. BJOG: an international journal of obstetrics and gynaecology 2018;125(2):193‐201. [DOI] [PubMed] [Google Scholar]
Wong 2014
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD000112.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wojcieszek 2016
- Wojcieszek AM, Shepherd E, Middleton P, Lassi ZS, Wilson T, Heazell AEP, et al. Care prior to and during subsequent pregnancies following stillbirth for improving outcomes. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012203] [DOI] [PMC free article] [PubMed] [Google Scholar]


