Abstract
Background
The use of etomidate for emergency airway interventions in critically ill patients is very common. In one large registry trial, etomidate was the most commonly used agent for this indication. Etomidate is known to suppress adrenal gland function, but it remains unclear whether or not this adrenal gland dysfunction affects mortality.
Objectives
The primary objective was to assess, in populations of critically ill patients, whether a single induction dose of etomidate for emergency airway intervention affects mortality.
The secondary objectives were to address, in populations of critically ill patients, whether a single induction dose of etomidate for emergency airway intervention affects adrenal gland function, organ dysfunction, or health services utilization (as measured by intensive care unit (ICU) length of stay (LOS), duration of mechanical ventilation, or vasopressor requirements).
We repeated analyses within subgroups defined by the aetiologies of critical illness, timing of adrenal gland function measurement, and the type of comparator drug used.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; CINAHL; EMBASE; LILACS; International Pharmaceutical Abstracts; Web of Science; the Database of Abstracts of Reviews of Effects (DARE); and ISI BIOSIS Citation indexSM on 8 February 2013. We reran the searches in August 2014. We will deal with any studies of interest when we update the review.
We also searched the Scopus database of dissertations and conference proceedings and the US Food and Drug Administration Database. We handsearched major emergency medicine, critical care, and anaesthesiology journals.
We handsearched the conference proceedings of major emergency medicine, anaesthesia, and critical care conferences from 1990 to current, and performed a grey literature search of the following: Current Controlled Trials; National Health Service – The National Research Register; ClinicalTrials.gov; NEAR website.
Selection criteria
We included randomized controlled trials in patients undergoing emergency endotracheal intubation for critical illness, including but not limited to trauma, stroke, myocardial infarction, arrhythmia, septic shock, hypovolaemic or haemorrhagic shock, and undifferentiated shock states. We included single (bolus) dose etomidate for emergency airway intervention compared to any other rapid‐acting intravenous bolus single‐dose induction agent.
Data collection and analysis
Refinement of our initial search results by title review, and then by abstract review was carried out by three review authors. Full‐text review of potential studies was based on their adherence to our inclusion and exclusion criteria. This was decided by three independent review authors. We reported the decisions regarding inclusion and exclusion in accordance with the PRISMA statement.
Electronic database searching yielded 1635 potential titles, and our grey literature search yielded an additional 31 potential titles. Duplicate titles were filtered leaving 1395 titles which underwent review of their titles and abstracts by three review authors. Sixty seven titles were judged to be relevant to our review, however only eight met our inclusion criteria and seven were included in our analysis.
Main results
We included eight studies in the review and seven in the meta‐analysis. Of those seven studies, only two were judged to be at low risk of bias. Overall, no strong evidence exists that etomidate increases mortality in critically ill patients when compared to other bolus dose induction agents (odds ratio (OR) 1.17; 95% confidence interval (CI) 0.86 to 1.60, 6 studies, 772 participants, moderate quality evidence). Due to a large number of participants lost to follow‐up, we performed a post hoc sensitivity analysis. This gave a similar result (OR 1.15; 95% CI 0.86 to 1.53). There was evidence that the use of etomidate in critically ill patients was associated with a positive adrenocorticotropic hormone (ACTH) stimulation test, and this difference was more pronounced at between 4 to 6 hours (OR 19.98; 95% CI 3.95 to 101.11) than after 12 hours (OR 2.37; 95% CI 1.61 to 3.47) post‐dosing. Etomidate's use in critically ill patients was associated with a small increase in SOFA score, indicating a higher risk of multisystem organ failure (mean difference (MD) 0.70; 95% CI 0.01 to 1.39, 2 studies, 591 participants, high quality evidence), but this difference was not clinically meaningful. Etomidate use did not have an effect on ICU LOS (MD 1.70 days; 95% CI ‐2.00 to 5.40, 4 studies, 621 participants, moderate quality evidence), hospital LOS (MD 2.41 days; 95% CI ‐7.08 to 11.91, 3 studies, 152 participants, moderate quality evidence), duration of mechanical ventilation (MD 2.14 days; 95% CI ‐1.67 to 5.95, 3 studies, 621 participants, moderate quality evidence), or duration of vasopressor use (MD 1.00 day; 95% CI ‐0.53 to 2.53, 1 study, 469 participants).
Authors' conclusions
Although we have not found conclusive evidence that etomidate increases mortality or healthcare resource utilization in critically ill patients, it does seem to increase the risk of adrenal gland dysfunction and multi‐organ system dysfunction by a small amount. The clinical significance of this finding is unknown. This evidence is judged to be of moderate quality, owing mainly to significant attrition bias in some of the smaller studies, and new research may influence the outcomes of our review. The applicability of these data may be limited by the fact that 42% of the patients in our review were intubated for "being comatose", a population less likely to benefit from the haemodynamic stability inherent in etomidate use, and less at risk from its potential negative downstream effects of adrenal suppression.
Plain language summary
Etomidate for sedating critically ill people during emergency endotracheal intubation
Review question
Does a single dose of etomidate increase mortality or complications in people who are critically ill and undergoing emergency endotracheal intubation?
Background
People who are critically ill often need help breathing. One way to do this is called endotracheal intubation. This involves placing a tube into the windpipe (trachea) and having a ventilator (breathing machine) help the patient breathe.
People are often given sedative agents during endotracheal intubation to make them unaware of the procedure. Many sedative agents cause a potentially harmful drop in blood pressure.
Etomidate is commonly used to sedate patients before endotracheal intubation because it has minimal effects on blood pressure. However, when someone is given etomidate their adrenal glands do not function as well. This may be harmful to them.
Study characteristics
We looked at the evidence up to February 2013 and found 1666 studies. We included eight studies in our review and seven studies (involving 772 patients) in our meta‐analysis. The studies involved people who were in an unstable condition and critically ill. They were given one dose of etomidate or another sedative agent for endotracheal intubation. We reran the search in August 2014. We will deal with any studies of interest when we update the review.
Results
No strong evidence exists to suggest that etomidate, when compared to other bolus dose induction agents, increases mortality in critically ill patients. We must be careful in interpreting this finding because only large studies would be able to show a difference in mortality. So far, no such study has been completed.
Etomidate does seem to impair adrenal gland functioning. Functioning is impaired most between four and six hours after etomidate is given.
Sequential Organ Failure Assessment (SOFA) scores are used to find out how badly someone’s organs are failing. Using etomidate results in worse SOFA scores but this difference is small and not clinically meaningful.
The effects of impaired adrenal gland functioning and higher SOFA scores on people’s health is unknown. Using etomidate does not seem to increase the length of time someone is in hospital (including an intensive care unit), the length of time a person is connected to a mechanical ventilator (a machine to assist with breathing), or the use of vasopressors (medicines to increase blood pressure).
Quality of the evidence
Most of the evidence was moderate quality. This is mainly because some small studies we looked at did not check up on people adequately after they were intubated.
Most people that were involved in one study were intubated because they were in a coma. These people comprise 42% of those involved in the studies we looked at. People in a coma are unlike other critically ill people because they may not benefit to the same extent from having stable blood pressure during endotracheal intubation, which etomidate provides, nor are they at high risk from impaired adrenal gland function compared to other critically ill patients, for example those with severe infection.
Summary of findings
Summary of findings for the main comparison. Etomidate versus all other induction agents for endotracheal intubation in critically ill patients.
| Etomidate versus all other induction agents for endotracheal intubation in critically ill patients | ||||||
| Patient or population: patients with endotracheal intubation in critically ill patients Settings: Intervention: etomidate versus all other induction agents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Etomidate versus all other induction agents | |||||
| Mortality | Study population | OR 1.17 (0.86 to 1.6) | 772 (6 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 296 per 1000 | 330 per 1000 (265 to 402) | |||||
| Moderate | ||||||
| 241 per 1000 | 271 per 1000 (214 to 337) | |||||
| ACTH stimulation test ACTH stimulation test is considered positive if the change in serum cortisol level was less than 9 μg/dL (248 nmol/L) after the administration of 250 μg of cosyntropin Follow‐up: 24 hours | Study population | OR 2.37 (1.61 to 3.47) | 519 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 228 per 1000 | 411 per 1000 (322 to 506) | |||||
| Moderate | ||||||
| 167 per 1000 | 322 per 1000 (244 to 410) | |||||
| Random serum cortisol levels (μg/dL) after receiving intervention | The mean random serum cortisol levels (μg/dl) after receiving intervention in the control groups was 21 to 28 µg/dL | The mean random serum cortisol levels (μg/dl) after receiving intervention in the intervention groups was 4.96 lower (8.06 to 1.86 lower) | 105 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| Organ system dysfunction Sequential Organ Failure Assessment (SOFA) Score. Scale from: 1 to 24 | The mean organ system dysfunction in the control groups was 9.6 | The mean organ system dysfunction in the intervention groups was 0.7 higher (0.01 to 1.39 higher) | 469 (1 study) | ⊕⊕⊕⊕ high | ||
| ICU length of stay (days) | The mean ICU length of stay (days) in the control groups was 3 to 22 days | The mean ICU length of stay (days) in the intervention groups was 1.7 higher (2 lower to 5.4 higher) | 621 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| Hospital length of stay (days) | The mean hospital length of stay (days) in the control groups was 6.4 to 10 days | The mean hospital length of stay (days) in the intervention groups was 2.41 higher (7.08 lower to 11.91 higher) | 152 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| Duration of mechanical ventilation (days) | The mean duration of mechanical ventilation (days) in the control groups was 1.5 to 13 days | The mean duration of mechanical ventilation (days) in the intervention groups was 2.14 higher (1.67 lower to 5.95 higher) | 621 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 A significant number of patients were lost to follow‐up
Background
More than 60% of all emergency airway interventions in the United States (US) use etomidate as the bolus induction agent (Sivilotti 2003) owing to its favourable haemodynamic properties and ease of dosing. Data from the National Emergency Airway Registry (NEAR) show that etomidate is the most commonly used induction agent for emergency airway intervention (Sivilotti 2003). The NEAR registry currently collects data on emergency department airway interventions from 25 hospitals in five countries. At the time of Sivilotti's publication in 2003, the database included 20 hospitals in the US, one in Canada, and one in Asia. Etomidate was used in 62% of all rapid sequence intubations (RSI) (1468 of 2380 patients) (Sivilotti 2003). Benzodiazepines were used 18% of the time and were the next most common agents used. In the NEAR database, 63% of emergency physicians and 26% of anaesthesiologists used etomidate (Sivilotti 2003). In the Corticosteroid Therapy of Septic Shock (CORTICUS) trial examining steroid use in septic intensive care unit (ICU) patients, 19% of these critically ill patients received etomidate (Cuthbertson 2009).
Etomidate suppresses the normal cortisol production of the adrenal glands through inhibition of 11‐beta‐hydroxylase. In one trial, 94% of patients receiving etomidate failed to respond to a corticotropin stimulation test (Absalom 1999), but whether this suppression leads to clinically relevant outcomes is uncertain. Specifically, it is unknown if a single dose of etomidate affects the mortality of critically ill patients. Given its widespread popularity, there is potential for harm if mortality is increased through its use. Should there not be a negative effect on mortality, then many clinicians can be reassured that this medication is safe for use in the sickest patients. Either outcome of this review would be practice changing for the large number of physicians caring for critically ill patients.
Description of the condition
Many critically ill patients require airway control, where an endotracheal tube is placed within the trachea. This procedure is painful and difficult to tolerate when awake, so most clinicians sedate patients for the procedure. Indications for airway control in critical illness are numerous but broadly include airway protection when airway patency is threatened by distorted anatomy or the patient's level of consciousness. This therapy is also used to support respiratory failure and to allow mechanical ventilation in patients with haemodynamic instability, including patients with septic shock and other critical illnesses.
Patients requiring this therapy typically have abnormal vital signs including hypotension, tachycardia, hypoxia, or an altered level of consciousness. Insults to the patient's central nervous system (CNS) and other vital organs may be exacerbated if the induction agents worsen hypotension. Rapidly acting induction agents decrease critically ill patients' blood pressure further through vasodilatory effects or direct myocardial suppression, or both.
Description of the intervention
When faced with the decision to obtain airway control in critically ill patients, clinicians must weigh the benefits and potential harms of a multitude of pharmacological agents. They must then apply this decision to a complex and dynamic physiological state in patients sensitive to further physiological insults. Several classes of agents are used to sedate critically ill patients, each with their own benefits and weaknesses.
Etomidate is a short‐acting intravenous (IV) medication used for anaesthesia induction and sedation. Single‐dose etomidate is commonly used to facilitate endotracheal intubation in critically ill patients because etomidate is less likely to cause a harmful drop in blood pressure than other induction agents, after an induction dose of 0.3 mg/kg IV. After this dose, there are minimal changes in heart rate, stroke volume, or cardiac output; and mean arterial blood pressure may decrease up to 15% because of decreases in systemic vascular resistance. Etomidate (1‐(1‐phenylethyl)‐1H‐imidazole‐5‐carboxylic acid ethyl ester) is a carboxylated imidazole derivative used for the induction of general anaesthesia. Following a standard dose (0.3 mg/kg), hypnosis occurs in less than one minute and is maintained for 4 to 10 minutes by producing gamma‐aminobutyric acid (GABA)‐like effects on the CNS.
Benzodiazepines (midazolam, lorazepam, diazepam, etc.) induce CNS depression through GABA effects. Midazolam is a commonly used rapid‐acting benzodiazepine for RSI. Midazolam, when administered as an IV bolus (0.05 to 0.15 mg/kg) for induction of anaesthesia has an onset of action of one to two minutes. Duration of action after an induction dose of 0.15 mg/kg IV to young healthy volunteers was 17 minutes to awakening. The clinical effects of midazolam can be prolonged in elderly patients or patients with impaired renal or hepatic function.
Propofol (2,6‐diisopropylphenol) is presumed to exert its sedative‐hypnotic effects through a GABA receptor interaction. At a standard dose of 1.5 to 2.5 mg/kg IV, anaesthesia is induced in less than one minute (10 to 50 seconds) and is maintained for five minutes. Propofol produces decreases in systemic blood pressure and has a negative inotropic effect. Bradycardia and asystole have been observed after induction of anaesthesia with propofol, potentially owing to a decrease in sympathetic nervous system activity that results in a predominance of parasympathetic activity.
Opiate derivatives (morphine, fentanyl, remifentanil, etc.) in large doses have been used as the sole anaesthetic in critically ill patients. Morphine and hydromorphone can cause histamine release, which causes hypotension owing to peripheral vasodilation. Fentanyl and remifentanil do not cause release of histamine. Dose, metabolism, elimination, and side effects vary depending on the opioid administered. Morphine, hydromorphone, and remifentanil may produce mild decreases in systemic blood pressure and heart rate. Fentanyl may produce bradycardia.
Ketamine is another induction agent with favourable haemodynamic and kinetic profiles. At IV bolus doses of 1 to 2 mg/kg for induction, dissociation occurs within 30 to 60 seconds with a duration of action of 10 to 20 minutes. Ketamine produces cardiovascular effects that resemble sympathetic nervous system stimulation. The mechanisms for these ketamine‐induced cardiovascular effects are complex. Direct stimulation of the CNS leading to increased sympathetic nervous system outflow seems to the most important mechanism for cardiovascular stimulation (Wong 1974). This may result in an increase in systemic and pulmonary arterial blood pressure, heart rate, cardiac output, cardiac workload, and therefore myocardial oxygen demand. Ketamine also has a direct negative cardiac inotropic effect. This effect is usually overshadowed by central sympathetic stimulation but occasionally critically ill patients respond to ketamine with decreases in systemic blood pressure and cardiac output, which may reflect depletion of endogenous catecholamine stores and exhaustion of sympathetic nervous system compensatory mechanisms. It is not known to inhibit the adrenal axis (Stoelting 2006).
How the intervention might work
The use of etomidate infusions in the ICU setting has been largely abandoned secondary to adrenal suppression and increased mortality. This is thought to be mediated by etomidate's transient, reversible suppression of 11‐beta‐hydroxylase, an enzyme responsible for the production of active steroids from the adrenal glands (de Jong 1984). There is a growing body of literature supporting the hypothesis that even single‐dose etomidate causes adrenal suppression (de Jong 1984; Hildreth 2008; Jabre 2009; Zed 2006). The association of adrenal dysfunction in septic shock patients, and the possible survival benefit from exogenous steroids, has been debated and investigated in the critical care literature (Annane 2002; Annane 2004; Cronin 1995; Sprung 2008).
Whether the adrenal dysfunction has a causal role in mortality, or is simply another indicator of organ dysfunction, remains unclear.
Why it is important to do this review
Debate remains regarding the clinical effects of single dose etomidate in critically ill patients. Systematic reviews on the subject have led to conflicting results.
Hohl et al pooled data from seven studies examining the effects of etomidate in critically ill patients. None of the individual studies were powered to detect a mortality difference, and a pooled odds ratio (OR) estimate of mortality showed no statistical difference (Hohl 2010).
Albert et al published a systematic review of 19 etomidate trials (Albert 2011). The authors concluded that strong evidence exists for an increased relative risk for etomidate‐induced adrenal suppression. They also stated that weak evidence exists for any association between etomidate and mortality. The authors very correctly assert that the mortality conclusions are weak based on: "a preponderance of non‐randomized trials and heterogeneity of studies". In their review, Albert et al combined clinically heterogeneous data from 15 retrospective, observational, and non‐randomized trials with four prospective randomized trials. The conclusion that etomidate use is associated with greater mortality in critically ill patients must be interpreted with caution (Albert 2011).
Chan and colleagues also published a meta‐analysis of randomized controlled trials and observational studies examining the effects of etomidate on adrenal insufficiency and all‐cause mortality in septic patients. In this meta‐analysis, they report a pooled relative risk of 1.20 (95% confidence interval (CI) 1.02 to 1.42) for mortality, and a pooled relative risk of 1.33 (95% CI 1.22 to 1.46) for the development of adrenal insufficiency (Chan 2012). The practice of combining observational and randomized data is methodologically questionable, and the results must be interpreted with caution.
While it is clear that etomidate is associated with transient adrenal suppression, the literature has yet to answer whether or not this effect is clinically meaningful. It is also unclear whether the immediate haemodynamic safety benefits of etomidate outweigh its potential harm from transient adrenal suppression.
Given the vast number of doses administered on an annual basis to critically ill patients, there exists a risk of harm if mortality is affected by etomidate‐induced adrenal suppression. Should no harm be identified, then physicians can be reassured that the use of an otherwise favourable drug is safe.
Objectives
The primary objective was to assess, in populations of critically ill patients, whether a single induction dose of etomidate for emergency airway intervention affects mortality.
The secondary objectives were to address, in populations of critically ill patients, whether a single induction dose of etomidate for emergency airway intervention affects adrenal gland function, organ dysfunction, or health services utilization (as measured by ICU length of stay (LOS), duration of mechanical ventilation, or vasopressor requirements).
We repeated analyses within subgroups defined by the aetiologies of critical illness, timing of adrenal gland function measurement, and the type of comparator drug used.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs).
We excluded non‐randomized or quasi‐randomized trials.
Types of participants
We included adult and paediatric patients undergoing emergency endotracheal intubation (defined as endotracheal intubation for an unstable clinical condition) for critical illness, including but not limited to: trauma, stroke, myocardial infarction, arrhythmia, septic shock, hypovolaemic or haemorrhagic shock, and undifferentiated shock states.
We excluded elective anaesthesia induction in stable patients.
Types of interventions
We included a single (bolus) dose of etomidate for emergency airway intervention compared to any other rapid‐acting IV bolus single‐dose induction agent (ketamine, midazolam, propofol, thiopental, etc.).
We excluded etomidate infusions and etomidate use for indications other than airway intervention (for example procedural sedation).
Types of outcome measures
Primary outcomes
All‐cause mortality. Mortality data at 30 days (including sensitivity analysis of death before 24 hours, up to 7 days, and 28 days) will be reported if available.
Secondary outcomes
Mortality at 30 days within groups of patients with adrenal gland dysfunction, as available in published reports.
-
Adrenal gland dysfunction (at times < 4 hours, between 4 to 6 hours, between 6 to 12 hours, and > 12 hours from etomidate dose) as described by Marik 2008, defined as:
random cortisol level < 10 μg/dL (276 nmol/L);
failed adrenocorticotropic hormone (ACTH) stimulation tests where the delta cortisol is < 9 μg/dL (248 nmol/L) after a 250 μg cosyntropin administration (or body surface‐area appropriate dose in the paediatric population).
-
Organ dysfunction:
Sequential Organ Failure Assessment (SOFA) score;
other validated systems for reporting organ dysfunction.
ICU LOS (sensitivity analysis stratified by patients who died before 24 hours, within 7 days, and within 28 days).
Duration of mechanical ventilation (sensitivity analysis stratified by patients who died before 24 hours, within 7 days, and within 28 days).
Vasopressor requirements (duration in days of any vasoactive medication infusion).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 12); MEDLINE via OvidSP (1950 to 8 February 2013); CINAHL (EBSCOhost) (1982 to 8 February 2013); EMBASE via OvidSP (1980 to 8 February 2013); LILACS (BIREME) (1982 to 8 February 2013); International Pharmaceutical Abstracts (1970 to 8 February 2013); Web of Science (1980 to 8 February 2013); the Database of Abstracts of Reviews of Effects (DARE) (8 February 2013); and ISI BIOSIS Citation indexSM (1969 to 8 February 2013).
We also searched the Scopus database of dissertations and conference proceedings (1980 to 8 February 2013) and the US Food and Drug Administration (FDA) Database (1980 to 8 February 2013).
We did not limit the selection of studies by region of publication or by language.
We adopted the MEDLINE search strategy for searching all other databases (see Appendix 1 for detailed search strategies).
We reran the search in August, 2014. We will deal with any studies of interest when we update the review.
Searching other resources
We handsearched the following medical journals from 2000 to February 2013:
Annals of Emergency Medicine;
Academic Emergency Medicine;
Canadian Journal of Emergency Medicine;
Emergency Medicine Clinics of North America;
Journal of Emergency Medicine;
Anesthesiology;
Canadian Journal of Anesthesia;
Anesthesia and Analgesia;
British Journal of Anaesthesia;
Journal of Trauma;
Intensive Care Medicine;
Critical Care Medicine;
Chest;
American Journal of Respiratory and Critical Care Medicine.
We searched the conference proceedings of major emergency medicine, anaesthesia, and critical care conferences from 1990 to February 2013 to identify data published in abstract form only.
A grey literature search included electronic searches of the following clinical trial registry websites:
Current Controlled Trials;
National Health Service – The National Research Register;
ClinicalTrials.gov;
NEAR website.
We contacted authors of all ongoing trials for unreported data. We contacted drug manufacturers and asked them to provide any published or unpublished data, however these publications were not provided to us, citing that, "Clinical studies not already published are proprietary information. Regretfully, for this reason, this data cannot be provided" (Lloyd 2013 [pers comm]).
The bibliographies of all relevant retrieved articles identified in the search above were handsearched for any missed studies.
Data collection and analysis
Selection of studies
Refinement of our initial search results by title review and then by abstract review was carried out by three review authors (EB, IB, SR). We decided the final inclusion for full‐text review by majority vote (two out of three).
Full‐text review of potential studies was based on their adherence to our inclusion and exclusion criteria. This was decided by three independent review authors (EB, IB, SR). We resolved disagreements by open discussion and consensus agreement, with the principle review author (EB) making the final decision. If required, we contacted the authors of studies to clarify their eligibility for inclusion and whether their publication was a duplicate report of a single study. If any doubt remained, the default was inclusion for data extraction.
We reported the decisions regarding inclusion and exclusion in accordance with the PRISMA statement (Figure 1). We analysed multiple reports of a single study as a single study.
1.
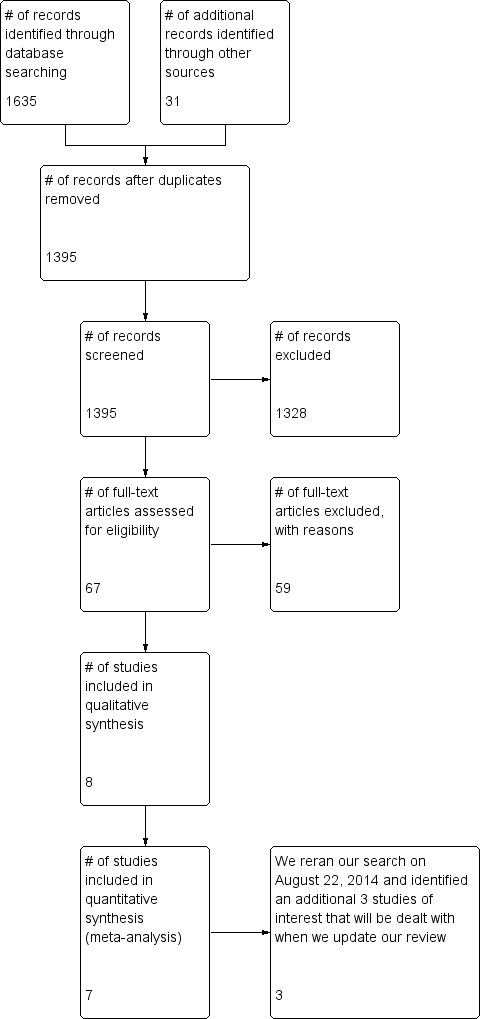
Study flow diagram.
We were not blinded to the authors or journals of publication.
Data extraction and management
Three review authors (EB, IB, SR) independently extracted the data from studies using a data extraction form (see Appendix 2). We (EB, IB, SR) pilot tested the data extraction form on 10 articles selected at random from our initial search strategy and applied it to studies that both met, and did not meet, our inclusion criteria. We did not modify the data extraction methodology and form after this pilot test.
We extracted the following data, according to outcome:
all‐cause mortality;
-
Adrenal gland dysfunction:
random serum cortisol levels,
positive ACTH stimulation tests;
-
Health services utilization:
hospital LOS in days (mean, standard deviation (SD), numbers (n); as well as median and interquartile range (IQR) as reported),
ICU LOS in days (mean, SD, n; as well as median and IQR as reported),
duration of mechanical ventilation in days (mean, SD, n; as well as median and IQR as reported),
-
vasopressor requirements,
duration of any vasopressor requirement in days (mean, SD, n).
Assessment of risk of bias in included studies
We performed risk of bias assessments using the 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and any other potential threats to validity. We judged each criterion regarding its risk of bias and recorded an assessment of the magnitude and direction of each source of bias. Data points for the risk of bias assessment are part of our data extraction form, attached as Appendix 2.
We considered a trial to have a low risk of bias if all domains were assessed as adequate. We considered a trial to have a high risk of bias if one or more domain was assessed as inadequate or unclear.
We report the 'Risk of bias' table (Figure 2) as part of the table 'Characteristics of included studies' and present a 'Risk of bias summary' figure (Figure 3), which details all of the judgements made for all included studies in the review.
2.
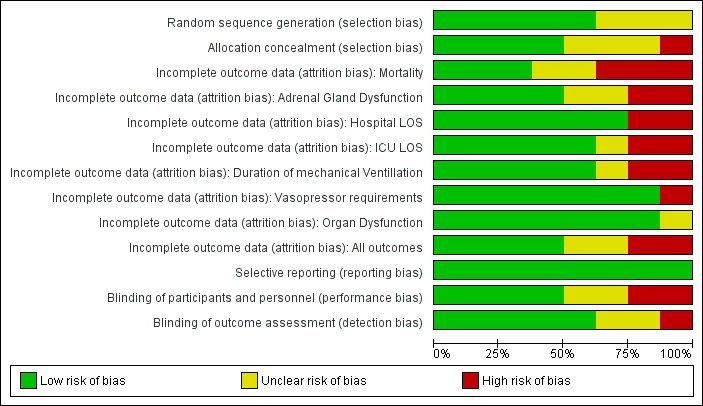
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
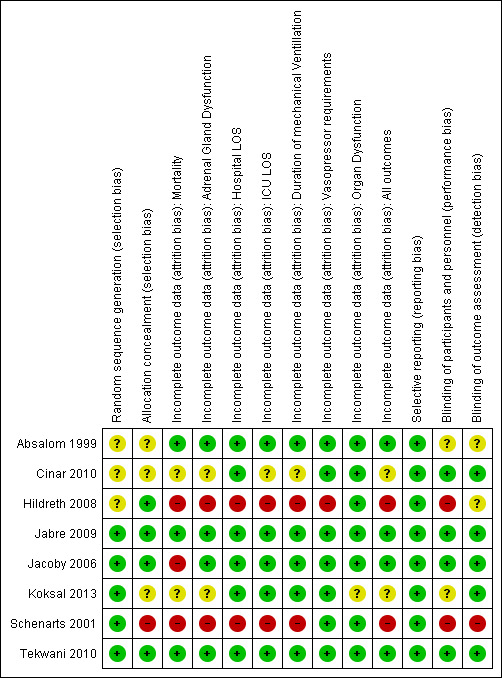
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We assessed the risk of bias for each outcome independently. Studies were not weighted by risk of bias for our analysis.
Measures of treatment effect
We reported the odds ratio (OR) for dichotomous data (mortality, ACTH stimulation tests). We reported the mean difference (MD) and SD for continuous data with the same unit of measure, and the standardized mean difference (SMD) for continuous data that were reported using different units of measure (hospital and ICU LOS, duration of mechanical ventilation, duration of vasopressor requirements, serum cortisol levels). Serum cortisol levels reported in nmol/L were converted to μg/dL, where possible, using the 'SIU Conversion Calculator' embedded within the Micromedex 2.0 system by Truven Health Analytics Inc (Truven 2013).
Variables with non‐normal distributions, reported as medians with IQRs, were assumed to be normally distributed for the purposes of this analysis. The median was assumed to be an acceptable estimation of the mean, and the IQR was considered to be 1.35 times the SD. This process is described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and has been used by other review authors in the past (Zacharias 2013).
Unit of analysis issues
The unit of analysis was the patient.
In studies with multiple treatment arms, only interventions relevant to our review were included for analysis, and a description of these studies is included in the 'Characteristics of included studies' table. We combined all control groups (for example propofol, ketamine, and benzodiazepines) for comparison with the intervention (etomidate). We analysed each pair‐wise comparison separately as subgroup analyses for mortality. For dichotomous outcomes, such as mortality, we divided the number of events and the total number of patients in proportions similar to the proportions of the total number of participants per experimental group.
The nature of the intervention precluded case cross‐over trial designs. Therefore, we did not encounter any of these study designs. Cluster‐randomized trials were included in our analysis if they reported the outcome data ignoring the cluster design for the total number of individuals.
Dealing with missing data
Whenever possible, we contacted the authors of studies and asked for primary data (Cinar 2010; Hildreth 2008; Koksal 2013).
Where this was not possible, we performed an intention‐to‐treat (ITT) analysis on mortality data where we assumed that all missing data represented patient deaths in both the intervention and control groups.
Assessment of heterogeneity
We only pooled data meta‐analytically for clinically homogenous data reporting the same outcome measures. For all pooled data, we used the Chi2 statistic, degrees of freedom, and the I2 statistic to assess the degree of statistical heterogeneity across the studies. If the I2 was < 40%, we reported the results of pooled data using meta‐analytic techniques. If the I2 was > 40%, we used sensitivity analysis to explore potential causes for the heterogeneity.
Assessment of reporting biases
We did not employ a funnel plot of included studies to ensure that reporting bias did not significantly affect our findings as fewer than 10 trials were included (Egger 1997) in this review.
Data synthesis
We conducted a meta‐analysis, using a random‐effects model, for studies with similar design and interventions.
We report ORs with 95% confidence intervals (CIs) for dichotomous variables such as mortality, and MDs with 95% CIs for continuous variables. When different scales or units were used to report continuous data, we calculated and reported the SMDs.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis on the following:
comparator drug;
different aetiologies of critical illness (i.e. septic, cardiogenic, trauma, undifferentiated).
We had planned to perform a subgroup analysis on paediatric patients, but no reports of paediatric patients were identified.
Sensitivity analysis
We planned to conduct sensitivity analyses on the following data:
mortality timing, less than 24 hours versus 7 days, 28 days, and all;
ICU LOS stratified by timing of mortality;
duration of mechanical ventilation stratified by timing of mortality;
sensitivity analysis where studies assessed as being at high risk of bias were excluded.
We were unable to conduct these sensitivity analyses due to unavailable data.
We conducted sensitivity analysis to assess the impact of excluding poor quality studies, assessed as being at high risk of bias. Only two studies were judged to be at low risk of bias (Jabre 2009; Tekwani 2010).
Summary of findings tables
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (mortality, adrenal gland dysfunction, organ dysfunction, ICU LOS) in our review and constructed a summary of findings (SoF) table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
See the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables below.
Results of the search
Electronic database searching yielded 1635 potential titles, and our grey literature search yielded an additional 31 potential tiles. Duplicate titles were filtered leaving 1395 titles which underwent review of their titles and abstracts by three review authors (EB, IB, SR). We judged 68 titles to be relevant to our review, however only eight met our inclusion criteria. One title (Cinar 2010) met our inclusion criteria but could not be included in our meta‐analysis because it was a poster at a research meeting that did not present its numerical data. This paper presented results like "Groups’ mean serum cortisol and 11b‐deoxycortisol concentrations were similar before and five minutes after intubation (P < 0.05). The groups were not significantly different with regards to intubation conditions, length of ICU stay, duration of mechanical ventilation, and mortality (P < 0.05)" (Cinar 2010). Multiple attempts were made to contact both the primary and supervising authors, but no reply was received. As a result we included seven studies in our meta‐analysis (Figure 1).
We reran our search on 22 August 2014 and identified an additional three studies of interest (Driver 2014; Freund 2014; Punt 2014). We will deal with these studies of interest when we update the review.
Included studies
We included eight studies in our review and seven studies in our meta‐analysis. All seven were RCTs of critically ill patients requiring emergency airway intervention, with a combined census of 772 patients. The studies were conducted in North American (Hildreth 2008; Schenarts 2001; Tekwani 2010) or European (Absalom 1999; Jabre 2009; Koksal 2013) tertiary care centres, or in pre‐hospital settings in the United States (Jacoby 2006). All studies randomized patients to receive etomidate or other single‐dose induction agents including thiopentone (Absalom 1999), fentanyl and midazolam (Hildreth 2008), ketamine (Jabre 2009), or midazolam alone (Jacoby 2006; Koksal 2013; Schenarts 2001; Tekwani 2010).
There were differences in the overall mortality rates reported within the studies, which may suggest clinical heterogeneity. Most studies reported an overall mortality rate of 23% to 38% (Absalom 1999; Jabre 2009; Jacoby 2006; Tekwani 2010), however two studies reported overall mortality rates of 3% (Schenarts 2001) and 7% (Hildreth 2008).
Excluded studies
We excluded 17 studies. The majority of these studies were excluded because of their observational design or they were retrospective reviews of charts or databases. Please refer to 'Characteristics of excluded studies'. Three studies warranted specific discussion (Asehnoune 2012; Cherfan 2011; Cuthbertson 2009).
Asehnoune reported a substudy of the HYPOLYTE trial, which intended to report the impact of etomidate on the rate of hospital acquired pneumonia in trauma patients intubated for more than 48 hours. They also performed ACTH stimulation tests. While the use of etomidate was prospectively collected, the participants in this trial were randomized, in a double blind, placebo controlled manner, to receive either hydrocortisone or placebo. These patients were not randomized to receive etomidate or other induction agents (Asehnoune 2012).
Cherfan evaluated the effect of low‐dose steroids on septic patients intubated with etomidate. This was a nested cohort study within a RCT, which evaluated the use of low‐dose hydrocortisone in septic cirrhotic patients. Mortality was compared between groups who did, and did not, receive etomidate (Cherfan 2011).
Cuthbertson's study received a lot of attention when published. It concluded that etomidate use was associated with a statistically significant increase in mortality (61.0% versus 44.6%) and the authors cautioned against using etomidate. This study was an a priori substudy of the CORTICUS trial, a multi‐centre, double blind, placebo controlled RCT comparing patients who received either hydrocortisone or placebo. Cuthbertson's report compared mortality in patients who received etomidate within 72 hours of enrolment to those who did not. It may stand to reason that the higher mortality rate seen in the etomidate group was due to their more severe underlying illness and was not an effect of the drug. Using multivariate analysis, the authors controlled for the severity of illness and still showed an increase in mortality (42.7% versus 30.5%) in the patients who received etomidate. This study was excluded from our analysis because individual patients were randomized to receive either placebo or hydrocortisone, and the data relating to the exposure to etomidate was observational (Cuthbertson 2009).
Studies awaiting classification
Three studies (Driver 2014; Freund 2014; Punt 2014) are awaiting classification (see 'Characteristics of studies awaiting classification'). We will deal with these studies of interest when we update the review.
Risk of bias in included studies
Please refer to our 'Characteristics of included studies', risk of bias graph (Figure 2), and risk of bias summary (Figure 3) for our judgements regarding each study's risk of bias. Overall, only two studies (Jabre 2009; Tekwani 2010) were judged to be at low risk of bias.
Allocation
In only three of the eight trials (Cinar 2010; Jacoby 2006; Tekwani 2010) were the healthcare worker(s) administering the experimental treatment blinded. In two trials (Absalom 1999; Koksal 2013) it is unclear, whereas in one trial (Jabre 2009), despite there being an appropriate randomization process in place, the physicians administering the study medication were unblinded. This raised significant concerns as unblinded healthcare workers with preconceived beliefs about the safety and efficacy of the study drugs may have altered the randomization sequence. The concealment methodology utilized would make it unlikely that the allocation sequence of the participants could have been altered. Cinar 2010 described their methodology as a "prospective, randomized, double blinded study", but did not elaborate on their methods of sequence generation or concealment. This was likely adequate, but the risk of bias remained unclear in the absence of this description.
Blinding
In three of the eight trials (Cinar 2010; Jacoby 2006; Tekwani 2010) the healthcare worker(s) administering the experimental treatment were blinded. In one trial (Jabre 2009), although the workers administering the study drug were unblinded, the teams providing all subsequent care were blinded to treatment allocation. It was unclear to what degree knowledge of treatment allocation may have altered patient care. An obvious possibility was the administration of steroids to patients who received etomidate. Differences in fluid resuscitation, administration of vasoactive medications, or other critical care unit treatments were unlikely but cannot be ruled out.
Virtually all eight included trials' outcomes were objective (ACTH stimulation test results, serum cortisol levels, mortality, etc) and the trials (Cinar 2010; Jacoby 2006) that did use more subjective outcomes (ease of intubation, vocal cord visualization, etc.) blinded the assessors. On this basis, detection bias was not a concern when interpreting the conclusions from this review.
Incomplete outcome data
One of the advantages of critical care research is the ability to obtain excellent patient follow‐up (as patients are intubated and ventilated). Yet, many of these trials are threatened by the significant proportions of patients lost to follow‐up. In one unblinded trial (Hildreth 2008) over 50% of eligible patients were not enrolled (a significant proportion of which were because of "protocol violations"), and no additional explanation was provided. In one trial (Jacoby 2006), close to 20% of enrolled patients were lost to follow‐up. In another trial (Schenarts 2001), 9/31 patients were excluded.
In two of the larger trials (Jabre 2009; Tekwani 2010), follow‐up was excellent with only two patients lost to follow‐up. One of the smaller trials (Absalom 1999) also had very good follow‐up, with only one patient lost to follow‐up.
In six of the eight trials, follow‐up was either poor or not described. Fortunately the large trials did demonstrate excellent followup, thereby reducing concern about attrition bias.
Selective reporting
In the current controversy regarding the haemodynamic effect of etomidate, the adrenal suppression, and ultimately any effect it may or may not have on mortality, any finding is noteworthy and publishable. It was unlikely that negative studies would not be submitted for publication (and we did not identify any examples of this during our handsearch of conference abstracts), or that negative findings within a study (so‐called within study reporting bias) would not be described.
Other potential sources of bias
Designing a trial to compare single‐dose etomidate to another agent(s) is fraught with challenges. The heterogeneity of the patient populations that require emergency intubation, managing informed consent for an emergent treatment, and blinding the healthcare team to medications that require emergent dose titration are impediments to bias free studies.
Another important source of bias is the characteristics of the study population. The concern of negative consequences from adrenal suppression is widely believed to be most important in critically ill patients in shock, particularly those with sepsis. In one recent trial (Jabre 2009), the majority of patients were intubated for airway protection due to neurologic compromise. It was not surprising that etomidate‐induced adrenal suppression did not affect SOFA score as these patients were neither septic nor in shock.
Effects of interventions
See: Table 1
Primary outcome: mortality
None of the included studies reported mortality as their primary outcome, and none were powered to detect a mortality difference. No individual trial showed a significant difference in mortality. There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 2.80, df = 5 (P = 0.73); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 390 patients receiving etomidate, compared to 382 patients receiving other induction agents, showed no difference in mortality (OR 1.17; 95% CI 0.86 to 1.60) (see Analysis 1.1).
1.1. Analysis.
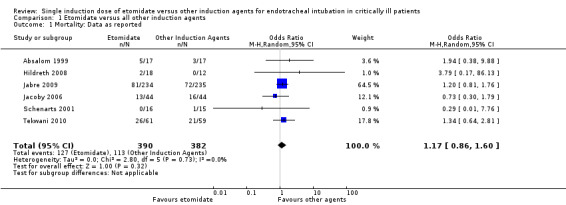
Comparison 1 Etomidate versus all other induction agents, Outcome 1 Mortality: Data as reported.
We performed a subgroup analysis comparing etomidate to individual comparator agents. Only one study (Absalom 1999) compared etomidate to thiopentone. We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. When 17 patients receiving etomidate were compared to 17 patients receiving thiopentone, there was no significant difference in mortality (OR 1.94; 95% CI 0.38 to 9.88). Four studies compared etomidate to midazolam (with or without fentanyl) (Hildreth 2008; Jacoby 2006; Schenarts 2001, Tekwani 2010). There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 2.28, df = 3 (P = 0.52); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 139 patients receiving etomidate, compared to 130 patients receiving midazolam, showed no difference in mortality (OR 1.06; 95% CI 0.61 to 1.83). Only one study (Jabre 2009) compared etomidate to ketamine. We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. When 234 patients receiving etomidate were compared to 235 patients receiving ketamine, there was no statistically significant difference in mortality (OR 1.20; 95% CI 0.81 to 1.76) (see Analysis 1.2).
1.2. Analysis.
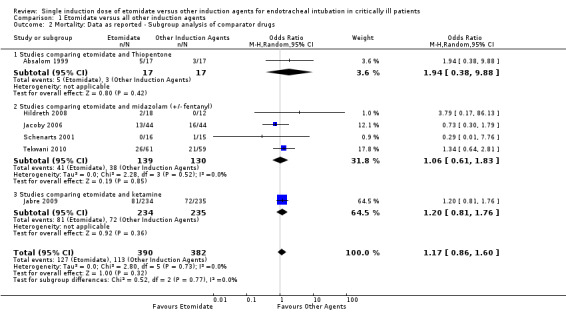
Comparison 1 Etomidate versus all other induction agents, Outcome 2 Mortality: Data as reported ‐ Subgroup analysis of comparator drugs.
We performed a subgroup analysis comparing the effects of etomidate on different etiologies of critical illness. Only one study (Tekwani 2010) studied etomidate specifically in patients with septic shock. We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. When 61 septic patients receiving etomidate were compared to 59 septic patients receiving other agents, there was no significant difference in mortality (OR 1.34; 95% CI 0.64 to 2.81). Only one study (Hildreth 2008) studied etomidate specifically in traumatized patients. We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. When 18 trauma patients receiving etomidate were compared to 12 trauma patients receiving other agents, there was no statistically significant difference in mortality (OR 3.79; 95% CI 0.17 to 86.13). No studies involving patients in cardiogenic shock were identified. Four studies reported results for patients with undifferentiated, or unreported, etiologies of critical illness (Absalom 1999; Jabre 2009; Jacoby 2006; Schenarts 2001). There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 2.07, df = 3 (P = 0.56); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 311 undifferentiated patients receiving etomidate, compared to 311 undifferentiated patients receiving other induction agents, showed no difference in mortality (OR 1.12; 95% CI 0.79 to 1.58) (see Analysis 1.3).
1.3. Analysis.
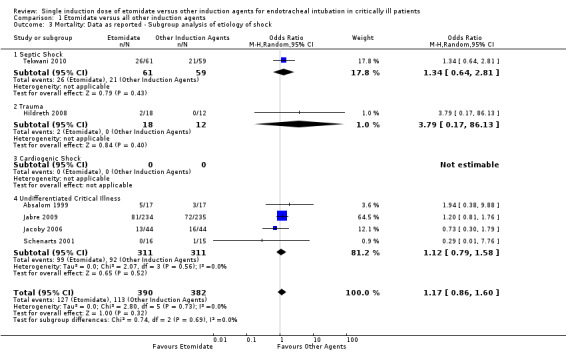
Comparison 1 Etomidate versus all other induction agents, Outcome 3 Mortality: Data as reported ‐ Subgroup analysis of etiology of shock.
Only two studies were judged to be at low risk of bias (Jabre 2009; Tekwani 2010). We analysed mortality when only these two studies were included. There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 0.07, df = 1 (P = 0.79); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 295 patients receiving etomidate, compared to 294 patients receiving other induction agents, showed no difference in mortality (OR 1.23; 95% CI 0.87 to 1.73) (see Analysis 1.4).
1.4. Analysis.
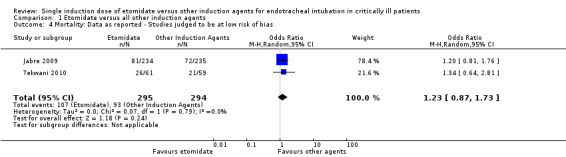
Comparison 1 Etomidate versus all other induction agents, Outcome 4 Mortality: Data as reported ‐ Studies judged to be at low risk of bias.
Due to a large proportion of patients lost to follow‐up, we performed a post hoc sensitivity analysis where all missing patients were assumed to have died. There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 2.64, df = 5 (P = 0.76); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 425 patients receiving etomidate, compared to 408 patients receiving other induction agents, showed no difference in mortality (OR 1.15; 95% CI 0.86 to 1.53) (see Analysis 1.5).
1.5. Analysis.
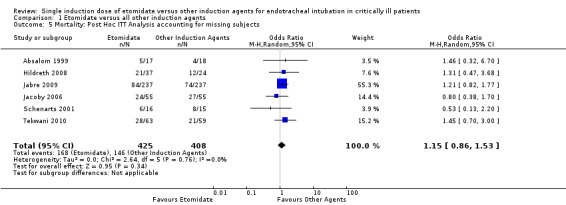
Comparison 1 Etomidate versus all other induction agents, Outcome 5 Mortality: Post Hoc ITT Analysis accounting for missing subjects.
The 30 day mortality data were not available. We reported mortality data at the individual study conclusion.
Secondary outcomes
ACTH stimulation test
Four studies reported dichotomous data regarding ACTH stimulation tests (Absalom 1999; Hildreth 2008; Jabre 2009; Schenarts 2001). ACTH stimulation tests were considered to be positive or negative according to standardized criteria (Marik 2008). There was some statistical heterogeneity in this comparison (Tau² = 0.42; Chi² = 6.55, df = 4 (P = 0.16); I² = 39%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. No studies reported the results of ACTH stimulation tests performed less than four hours after receiving etomidate, or the comparator. Two studies (Hildreth 2008; Schenarts 2001) reported the results of ACTH stimulation tests performed between four and six hours after induction. There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 0.20, df = 1 (P = 0.66); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 28 patients receiving etomidate, compared to 20 patients receiving other induction agents, showed a significant difference in the proportion of positive ACTH stimulation tests (OR 19.98; 95% CI 3.95 to 101.11). Only one study (Schenarts 2001) reported ACTH stimulation test results performed between 6 and 12 hours post‐induction. None of the patients in either treatment arm demonstrated a positive ACTH stimulation test (n etomidate = 7, n other = 7), so the treatment effect could not be estimated. Three studies (Absalom 1999; Jabre 2009; Schenarts 2001) reported results of ACTH stimulation tests performed more than 12 hours after induction. There was no statistical heterogeneity in this comparison (Tau² = 0.00; Chi² = 0.03, df = 2 (P = 0.99); I² = 0%). We employed a random‐effects model for meta‐analysis, describing the OR and 95% CI. The pooled result of 260 patients receiving etomidate, compared to 259 patients receiving other induction agents, showed a significant difference in the proportion of positive ACTH stimulation tests (OR 2.37; 95% CI 1.61 to 3.47) (see Analysis 1.6).
1.6. Analysis.
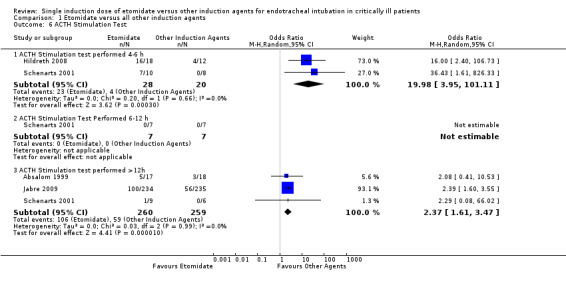
Comparison 1 Etomidate versus all other induction agents, Outcome 6 ACTH Stimulation Test.
Random serum cortisol levels
Three studies (Absalom 1999; Hildreth 2008; Koksal 2013) reported continuous data regarding random serum cortisol levels. There was statistical heterogeneity in this comparison (Tau² = 2.38; Chi² = 4.46, df = 3 (P = 0.22); I² = 33%). We employed a random‐effects model for meta‐analysis, describing the MD and 95% CI. The pooled result of 75 patients receiving etomidate, compared to 70 patients receiving other induction agents, showed a significant difference in the random serum cortisol level (MD ‐4.96; 95% CI ‐8.06 to ‐1.86) (see Analysis 1.7). For Analysis 1.7.2 and Analysis 1.7.4, the sample sizes in the Koksal 2013 paper were adjusted from 20 to 10 in each of the treatment and control groups in order to avoid double counting the sample size in the overall estimate of effect. The mean and SD values were left unchanged.
1.7. Analysis.
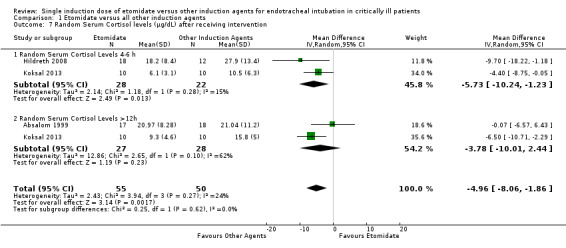
Comparison 1 Etomidate versus all other induction agents, Outcome 7 Random Serum Cortisol levels (μg/dL) after receiving intervention.
SOFA score
One study (Jabre 2009) reported the effects of etomidate on SOFA score. Statistical heterogeneity was not calculated. We employed a random‐effects model for meta‐analysis, describing the MD and 95% CI. The pooled result of 234 patients receiving etomidate, compared to 235 patients receiving other induction agents, showed a significant difference in the SOFA score (MD 0.70; 95% CI 0.01 to 1.39) favouring other induction agents over etomidate (see Analysis 1.8).
1.8. Analysis.
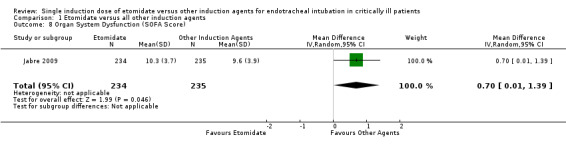
Comparison 1 Etomidate versus all other induction agents, Outcome 8 Organ System Dysfunction (SOFA Score).
ICU length of stay (LOS)
Three studies (Hildreth 2008; Jabre 2009; Tekwani 2010) reported the effects of etomidate on ICU LOS. There was significant statistical heterogeneity in this comparison (Tau² = 8.82; Chi² = 12.89, df = 2 (P = 0.002); I² = 84%). We employed a random‐effects model for meta‐analysis, describing the MD and 95% CI. The pooled result of 315 patients receiving etomidate, compared to 306 patients receiving other induction agents, showed no significant difference in ICU LOS (MD 1.70; 95% CI ‐2.00 to 5.40) (see Analysis 1.9).
1.9. Analysis.
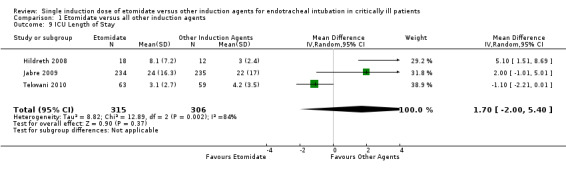
Comparison 1 Etomidate versus all other induction agents, Outcome 9 ICU Length of Stay.
Hosptial length of stay (LOS)
Two studies (Hildreth 2008; Tekwani 2010) reported the effects of etomidate on hospital LOS. There was significant statistical heterogeneity in this comparison (Tau² = 42.71; Chi² = 10.85, df = 1 (P = 0.0010); I² = 91%). We employed a random‐effects model for meta‐analysis, describing the MD and 95% CI. The pooled result of 81 patients receiving etomidate, compared to 71 patients receiving other induction agents, showed no statistically significant difference in hospital LOS (MD 2.41; 95% CI ‐7.08 to 11.91) (see Analysis 1.10).
1.10. Analysis.
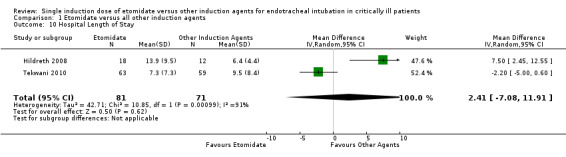
Comparison 1 Etomidate versus all other induction agents, Outcome 10 Hospital Length of Stay.
Duration of mechanical ventilation
Three studies (Hildreth 2008; Jabre 2009; Tekwani 2010) reported the effects of etomidate on duration of mechanical ventilation. There was significant statistical heterogeneity in this comparison (Tau² = 9.59; Chi² = 14.75, df = 2 (P = 0.0006); I² = 86%). We employed a random‐effects model for meta‐analysis, describing the MD and 95% CI. The pooled result of 315 patients receiving etomidate, compared to 306 patients receiving other induction agents, showed no significant difference in the duration of mechanical ventilation (MD 2.14; 95% CI ‐1.67 to 5.95) (see Analysis 1.11).
1.11. Analysis.
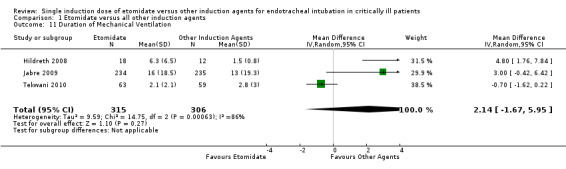
Comparison 1 Etomidate versus all other induction agents, Outcome 11 Duration of Mechanical Ventilation.
Duration of vasopressor support
Only one study (Jabre 2009} reported the effects of etomidate on the duration of vasopressor support. We employed a random‐effects model for meta‐analysis, describing the MD and 95% CI. The pooled result of 234 patients receiving etomidate, compared to 235 patients receiving other induction agents, showed no significant difference in the duration of vasopressor support (MD 1.00; 95% CI ‐0.53 to 2.53) (see Analysis 1.12).
1.12. Analysis.
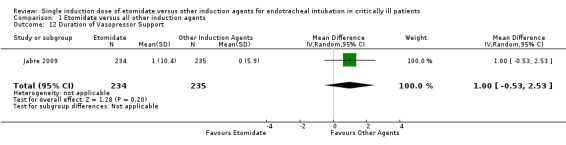
Comparison 1 Etomidate versus all other induction agents, Outcome 12 Duration of Vasopressor Support.
Discussion
Summary of main results
There are insufficient data to conclude that etomidate use is associated with an increased risk of harm, or to conclude that it is safe.
Mortality
A non‐significant trend towards increased mortality was observed. Overall, no strong evidence exists to suggest that etomidate, when compared to other bolus dose induction agents, increases mortality in critically ill patients (OR 1.17; 95% CI 0.86 to 1.60).
No strong evidence exists to suggest that etomidate increases mortality when compared to thiopentone (OR 1.94; 95% CI 0.38 to 9.88), midazolam with or without fentanyl (OR 1.06; 95% CI 0.61 to 1.83), or ketamine (OR 1.20; 95% CI 0.81 to 1.76).
No strong evidence exists to suggest that etomidate increases mortality in the subset of critically ill patients with septic shock (OR 1.34; 95% CI 0.64 to 2.81), trauma (OR 3.79; 95% CI 0.17 to 86.13), cardiogenic shock (no data), or undifferentiated critical illness (OR 1.12; 95% CI 0.79 to 1.58).
This lack of strong evidence for increased mortality due to the use of etomidate remains when only studies judged to be at low risk of bias are included in the analysis (OR 1.23; 95% CI 0.87 to 1.73). Due to a large number of participants lost to follow‐up, we performed a post hoc sensitivity analysis where all patients lost to follow up were assumed to have died. Also, in this scenario no strong evidence of increased mortality associated with etomidate use was identified (OR 1.15; 95% CI 0.86 to 1.53).
Therefore, with the existing evidence to date, despite a trend towards harm, etomidate use does not seem to cause an increase in mortality when used for emergency endotracheal intubation in patients with critical illness. This evidence is of moderate quality.
Adrenal function
Overall, strong evidence of moderate quality suggests that the use of etomidate in critically ill patients increases the likelihood of a positive ACTH stimulation test, and this difference is greater at four to six hours (OR 19.98; 95% CI 3.95 to 101.11) than after 12 hours post‐induction (OR 2.37; 95% CI 1.61 to 3.47). Likewise, the use of etomidate in critically ill patients appears to be associated with statistically significant reductions in random serum cortisol levels (MD ‐4.96; 95% CI ‐8.06 to ‐1.86). Again, this difference is more pronounced at four to six hours (MD ‐5.73; 95% CI ‐10.24 to ‐1.23) after induction compared to greater than 12 hours (MD ‐3.78; 95% CI ‐10.01 to 2.44) when it is no longer statistically significant. This evidence is of moderate quality.
Organ system dysfunction (SOFA score)
The use of etomidate in critically ill patients is associated with a statistically significant increase in SOFA score, indicating a higher risk of multi‐system organ failure when compared to other induction agents (MD 0.70; 95% CI 0.01 to 1.39). This evidence is of high quality. It should be noted that Jabre (Jabre 2009) concluded that no difference in SOFA score could be attributed to the use of etomidate, while we have concluded that a difference does exist. This is due to the fact that Jabre 2009 utilized one decimal place in their data analysis and concluded that the mean difference in SOFA score of 0·7 (95% CI 0·0 to 1·4) was insignificant because the confidence interval included 0.0. When the raw data were entered into RevMan for analysis, data were calculated to the second decimal place, which reached statistical significance because the lower confidence interval was 0.01. The SOFA score is a numerical surrogate score for organ system dysfunction that ranges from 0 (good organ function) to 24 (worse organ function). The difference in the maximum SOFA score in the etomidate group was 10.3 compared to 9.6 in the ketamine group, with a difference of 0.7 between groups (Jabre 2009). Regardless of whether this difference was statistically significant it is not clinically meaningful.
Health services utilization
Non‐significant trends towards increased health services utilization were observed. However, when compared to other induction agents, no strong evidence exists to suggest that etomidate increases ICU length of stay (MD 1.70; 95% CI ‐2.00 to 5.40), hospital length of stay (MD 2.41; 95% CI ‐7.08 to 11.91), duration of mechanical ventilation (MD 2.14; 95% CI ‐1.67 to 5.95), or duration of vasopressor use (MD 1.00; 95% CI ‐0.53 to 2.53). This evidence is of moderate quality.
Overall completeness and applicability of evidence
We searched for randomized trials that compared etomidate to another induction agent or a combination of agents for endotracheal intubation of critically ill patients. We identified only seven published papers and one unpublished paper. For an agent as commonly used as etomidate this is a very small number of studies. Despite this small number of studies we are confident that all eligible reports were identified. The total number of patients from all studies was only 772.
In the largest trial included in our analysis (Jabre 2009) 69% of patients in both arms were intubated for being comatose, while the minority were intubated for 'shock', 'acute respiratory failure', or 'other' reasons. These obtunded patients (n = 324) account for 42% of all patients in our review. Although it is accepted that patients obtunded secondary to intracranial catastrophes and drug intoxications are critically ill, this is not the group most likely to benefit from the haemodynamic stability inherent in etomidate use, nor are they the patients most at risk from its potential negative downstream effects of adrenal suppression. Applying conclusions from a small trial with a high preponderance of obtunded patients to patients in shock should be done with caution. Larger studies of patients in shock are still required to assess the appropriateness of etomidate's use.
Each of the individual trials included in our review had point estimates of effect suggesting increased mortality with the use of etomidate, but none were powered sufficiently to detect a difference. All confidence intervals crossed the line of no effect. The fact that all of the studies, and our pooled estimate, cross the line of no effect is likely because all studies were underpowered. It is possible that with more studies the 95% confidence intervals will no longer cross the line of no effect.
Quality of the evidence
Using the principals of the GRADE system, the quality of the data from randomized controlled trials (with the exception of SOFA score) were downgraded to 'moderate'. This is due mainly to methodological limitations and the high proportions of patients lost to follow‐up in the smaller studies (Hildreth 2008; Jacoby 2006; Schenarts 2001). Other methodological limitations involving randomization and allocation concealment supported this downgrade as well. In particular, the authors of one study did not describe their sequence generation or concealment (Absalom 1999); another unblinded study (Hildreth 2008) only enrolled half of the eligible patients, with almost half of the excluded patients excluded due to 'protocol violations'. A third study (Schenarts 2001) was unblinded and did not describe their allocation concealment procedures. In addition, one third of the patients in this unblinded study were excluded from the analysis, raising a significant concern in relation to bias (Schenarts 2001).
We did not downgrade our quality of evidence based on directness as all trials directly compared etomidate to a comparator agent in directly generalizable populations.
We did not downgrade our quality of evidence based on heterogeneity or inconsistency as we demonstrated very little statistical heterogeneity, and all studies reported similar results. There were differences in the overall mortality rates reported within the studies, which may suggest clinical heterogeneity. Most studies reported an overall mortality rate of 23% to 38% (Absalom 1999; Jabre 2009; Jacoby 2006; Tekwani 2010), however two studies reported overall mortality rates of 3% (Schenarts 2001) and 7% (Hildreth 2008).
We did not downgrade our quality of evidence based on imprecision as the reported confidence intervals were sufficiently narrow.
We did not downgrade our quality of evidence based on the potential for publication bias. While we did not employ a funnel plot (we did not identify 10 studies for inclusion), all studies reported similar findings and the risk of publication bias was judged to be low.
Potential biases in the review process
We believe that our search methodology was sufficient to minimize the chances that any study was missed. We did not employ a funnel plot because fewer than 10 studies were included in our analysis. We believe that our three investigator, data abstraction process also minimized the chances for individual beliefs to influence our results. Some of our statistical analysis has led to transformed data. Specifically, we were unable to enter non‐normally distributed data into RevMan as they were reported as medians and IQRs. Instead, we replicated the data transformation process of other investigators (Zacharias 2013) and roughly transformed these data into means and standard deviations. While all data were transformed in the same manner, the exact precision of the results may be altered by this process. If future editions of RevMan allow median and IQR data to be analysed primarily, we will report the data as such in future review updates. This data transformation affects data regarding hospital and ICU length of stay, duration of ventilator support, and duration of vasopressor support. It does not affect data on adrenal gland dysfunction or mortality.
Agreements and disagreements with other studies or reviews
Three other systematic reviews have been published on the topic of etomidate use in critically ill patients. Our results were similar to Hohl et al, where the pooled data were from seven studies examining the effects of etomidate in critically ill patients. None of the individual studies were powered to detect a mortality difference, and a pooled odds ratio estimate of mortality showed no statistical difference (Hohl 2010).
Albert et al published a systematic review of 19 etomidate trials (Albert 2011). The authors concluded that strong evidence exists for an increased relative risk for etomidate‐induced adrenal suppression. They also stated that weak evidence exists for any association between etomidate and mortality. The authors very correctly assert that the mortality conclusions are weak based on "a preponderance of non‐randomized trials and heterogeneity of studies". In their review, Albert et al combined clinically heterogeneous data from 15 retrospective, observational, and non‐randomized trials with four prospective randomized trials. Conclusions drawn from combining such heterogeneous trials should be interpreted with caution.
Chan and colleagues also published a meta‐analysis of randomized controlled trials and observational studies examining the effects of etomidate on adrenal insufficiency and all‐cause mortality in septic patients. In this meta‐analysis, they reported a pooled relative risk of 1.20 (95% CI 1.02 to 1.42) for mortality, and a pooled relative risk of 1.33 (95% CI 1.22 to 1.46) for the development of adrenal insufficiency (Chan 2012). The combination of observational and randomized data may be subject to bias and the results must be interpreted appropriately.
Authors' conclusions
Implications for practice.
Based on the currently available evidence, the use of etomidate in critically ill patients does not seem to increase mortality, organ system dysfunction, or healthcare resource utilization. This observation must be interpreted with caution owing to the moderate quality of evidence, the patient population described above, and the fact that no randomized trial to date has been adequately powered to detect a mortality difference. As in non‐critically ill patients, it appears that etomidate's use does negatively affect adrenal gland function but it is unclear whether or not this adrenal gland dysfunction influences patient outcomes in the first four to six hours (more so than after 12 hours). Again, this must be interpreted with caution, acknowledging the moderate quality of evidence. The use of etomidate is associated with an increase in SOFA score indicating an increased risk of organ system dysfunction. This increase in SOFA score is not clinically meaningful. With respect to healthcare resource utilization, no strong evidence exists to suggest that etomidate increases ICU length of stay, hospital length of stay, or duration of mechanical ventilation.
Implications for research.
Additional randomized trials of high quality are still required to confirm the safety of etomidate's use in critically ill patients. Due to the moderate quality of the existing evidence, new research may influence the outcomes of our review upon updating the review.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Stephan Kettner (content editor), Nathan Pace (statistical editor), Djillali Annane, Michael Murray (peer reviewers) and Anne Lyddiatt (consumer) for their help and editorial advice during the preparation of the protocol and the systematic review.
Appendices
Appendix 1. Search strategies
CENTRAL (The Cochrane Library)
#1MeSH descriptor: [Imidazoles] this term only #2MeSH descriptor: [Benzyl Compounds] explode all trees #3MeSH descriptor: [Etomidate] explode all trees #4hypnomidate or amidate or et?omidat* or r?26?490 or R?16659 or radenar?on #5#1 or #2 or #3 or #4 #6MeSH descriptor: [Anesthesia, Intravenous] this term only #7MeSH descriptor: [Anesthesia] this term only #8MeSH descriptor: [Intubation] explode all trees #9MeSH descriptor: [Intubation, Intratracheal] explode all trees #10MeSH descriptor: [Anesthesia, General] explode all trees #11MeSH descriptor: [Anesthetics] explode all trees #12(airway near protect*) or laryngoscop* or sedat*:ti,ab or hypnotic or (intubat* or an?esthe*):ti,ab #13MeSH descriptor: [Laryngoscopy] explode all trees #14MeSH descriptor: [Hypnotics and Sedatives] explode all trees #15(#6 or #7 or #8 o #9 or #10 or #11 or #12 or #13) and #14 #16MeSH descriptor: [Deep Sedation] explode all trees #17MeSH descriptor: [Conscious Sedation] explode all trees #18MeSH descriptor: [Intensive Care Units] explode all trees #19MeSH descriptor: [Burn Units] explode all trees #20MeSH descriptor: [Coronary Care Units] explode all trees #21MeSH descriptor: [Recovery Room] explode all trees #22MeSH descriptor: [Respiratory Care Units] explode all trees #23MeSH descriptor: [Intensive Care] explode all trees #24MeSH descriptor: [Critical Care] explode all trees #25MeSH descriptor: [Emergencies] explode all trees #26MeSH descriptor: [Emergency Treatment] explode all trees #27MeSH descriptor: [Emergency Service, Hospital] explode all trees #28MeSH descriptor: [Trauma Centers] explode all trees #29MeSH descriptor: [Emergency Medical Services] explode all trees #30MeSH descriptor: [Critical Illness] explode all trees #31(single bolus dose or induction or emergenc* or ambulanc* or trauma* or ((intensive or critical* or serious*) near (ill* or care or sick*)) or ICU):ti,ab or shock:ti,ab #32#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 #33#5 and #32
MEDLINE (OvidSP)
1. Imidazoles/ or Benzyl Compounds/ or hypnomidate.mp. or amidate.af. or exp Etomidate/ or et?omidat$.af. or (r?26?490 or R?16659).mp. or radenar?on.mp. 2. ((Intubation, Intratracheal/ or Intubation/ or intubat$.ti,ab. or anesthesia/ or anesthesia, intravenous/ or an?esthesia.ti,ab. or anesthesia, general/ or anesthetics/ or an?esthetic$.ti,ab. or (airway adj5 protect$).mp. or Laryngoscopy/ or laryngoscop$.mp. or sedat$.mp. or Hypnotics.mp.) and Sedatives/) or Deep Sedation/ or single bolus dose.mp. or induction.mp. or Conscious Sedation/ or intensive care units/ or burn units/ or coronary care units/ or recovery room/ or respiratory care units/ or Intensive Care/ or Critical Care/ or Emergencies/ or emergenc$.mp. or Emergency Treatment/ or ambulanc$.mp. or emergency service, hospital/ or trauma centers/ or Emergency medical services/ or trauma.ti,ab. or Critical Illness/ or ((intensive or critical$ or serious$) adj5 (ill$ or care or sick$)).mp. or ICU.mp. or shock$.ti,ab. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
EMBASE (OvidSP)
1. imidazole derivative/ or benzyl derivative/ or amidate.af. or et?omidat$.af. or (r?26?490 or R?16659).mp. or radenar?on.mp. or hypnomidate.mp. 2. ((endotracheal intubation/ or intubation/ or anesthesia/ or intravenous anesthesia/ or general anesthesia/ or anesthetic agent/ or intubat$.ti,ab. or an?esthe$.ti,ab. or (airway adj3 protect$).ti,ab. or laryngoscopy/ or laryngoscop$.ti,ab. or sedat$.ti,ab. or hypnotics.ti,ab.) and sedative agent/) or deep sedation/ or conscious sedation/ or intensive care unit/ or burn/ or coronary care unit/ or recovery room/ or intensive care unit/ or intensive care/ or emergency/ or emergency treatment/ or emergency health service/ or critical illness/ or (emergenc$ or single bolus doseor inductionor or ambulanc$ or trauma or ((intensive or critical$ or serious$) adj3 (ill$ or care or sick$)) or ICU or shock$).ti,ab. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
CINAHL (EBSCOhost)
S1 ((MM "Imidazoles") OR (MM "Benzyl Compounds") OR (MM "Etomidate")) OR (etomidate or radenaron or hypnomidate) S2 ((MH "Intubation, Intratracheal") OR (MH "Anesthesia") OR (MH "Anesthetics")) OR ( intubat* or an?esthe* or (airway and protect*) or laryngoscop* or sedat* or hypnotic*) S3 (MM "Hypnotics and Sedatives") S4 S2 AND S3 S5 ((MH "Sedation") OR (MH "Intensive Care Units") OR (MH "Burn Units") OR (MH "Coronary Care Units") OR (MH "Respiratory Care Units") OR (MH "Critical Care") OR (MH "Emergencies") OR (MH "Emergency Treatment (Non‐Cinahl)") OR (MH "Trauma Centers") OR (MH "Emergency Medical Services") OR (MH "Critical Illness")) OR (emergenc* or ambulanc* or trauma ) OR (((intensive or critical* or serious*) and (ill* or care or sick*))) OR ( ICU or shock*) S6 S4 OR S5 S7 random* or (trial* and (clinical or control*)) or placebo* or multicenter or prospective or ((blind* or mask*) and (single or double or triple or treble)) S8 S1 AND S6 AND S7
ISI Web of Science
#1 TS=(imidazoles or benzyl compounds or hypnomidate or amidate or etomidate or (r?26?490 or r?16659) or radenaron) #2 TS=((intubation, intratracheal or intubation or intubat* or anesthesia or anesthesia, intravenous or an?esthesia or anesthesia, general or anesthetics or an?esthetic* or (airway same protect*) or laryngoscopy or laryngoscop* or sedat* or hypnotics) and sedatives) or TS=(deep sedation or single bolus dose or induction or conscious sedation or intensive care units or burn units or coronary care units or recovery room or respiratory care units or intensive care or critical care or emergencies or emergenc* or emergency treatment or ambulanc* or emergency service, hospital or trauma centers or emergency medical services or trauma or critical illness or ((intensive or critical* or serious*) same (ill* or care or sick*)) or icu or shock*) #3 #1 and #2
LILACS (BIREME)
(hypnomidate or amidate or etomidate or radenaron)
BIOSIS Citation IndexSM
#1 TS=(hypnomidate or amidate or etomidate or radenaron) #2 TS=((intubation, intratracheal or intubation or intubat* or anesthesia or anesthesia, intravenous or an?esthesia or anesthesia, general or anesthetics or an?esthetic* or (airway same protect*) or laryngoscopy or laryngoscop* or sedat* or hypnotics) and sedatives) or TS=(deep sedation or single bolus dose or induction or conscious sedation or intensive care units or burn units or coronary care units or recovery room or respiratory care units or intensive care or critical care or emergencies or emergenc* or emergency treatment or ambulanc* or emergency service, hospital or trauma centers or emergency medical services or trauma or critical illness or ((intensive or critical* or serious*) same (ill* or care or sick*)) or icu or shock*) #3 TS=(random* or (trial* SAME (clinical or control*)) or placebo* or multicenter or prospective or ((blind* or mask*) SAME (single or double or triple or treble))) #4 #1 and #2 and #3
Appendix 2. Data extraction form
Data Extraction Form Reviewer: Bruder, Ball, Ridi
| Study Identifier | Author | Year |
| Citation: Authors. Title. Journal. Year; Volume (Issue): pages | ||
Eligibility for review
|
Inclusion criteria p Comparison of a single bolus dose of etomidate to any other rapid‐acting, IV bolus dose induction agent p RCT p Human data p Emergency airway intervention p Critical illness |
Exclusion criteria p Patients were extubated within 24 h p Etomidate infusion p Non‐randomized p Exogenous steroid replacement p Etomidate use for indications other than airway intervention p Elective anaesthesia induction |
Methods
| Objectives |
Primary |
Secondary |
| Participants |
Inclusion criteria |
Exclusion criteria |
| Indication for Intubation | ||
| Aetiology of Shock (circle) | Sepsis, Trauma, Cardiac, Undifferentiated, Unknown | |
| Experimental intervention | Etomidate, Dose, Route | |
| Control intervention | Drug, Dose, Route | |
| Co‐interventions | ||
| Study duration | ||
| Study location | ||
| Sample size calculation | Yes, No | |
| Funding source | ||
| Analysis | Intention‐to‐treat? Appropriate statistics? Other | |
Outcomes
| Measured | Definition, Units & Timeframe | |
| Mortality | Y/N | |
| Cortisol levels | Y/N | |
| ACTH stimulation test | Y/N | |
| Hospital LOS | Y/N | |
| ICU LOS | Y/N | |
| Post‐intubation haemodynamics | Y/N | |
| Ventilator days | Y/N | |
| Vasopressor requirements | Y/N |
Risk of bias assessment
| Domain | Description | Judgment | |
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Was the allocation sequence adequately generated? Low risk/High risk/Unclear |
|
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Was allocation adequately concealed? Low risk/High risk/Unclear |
|
| Blinding | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective Assess blinding for each outcome |
Was knowledge of the allocated intervention adequately prevented during the study? Low risk/High risk/Unclear |
|
| Incomplete outcome data | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors | Were incomplete outcome data adequately addressed? Low risk/High risk/Unclear |
|
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found | Are reports of the study free of suggestion of selective outcome reporting? Low risk/High risk/Unclear |
|
| Other bias | Selection bias (differences in comparison groups) | |
Was the study apparently free of other problems that could put it at a high risk of bias? Low risk/High risk/Unclear |
| Performance bias (differences in the care provided other than the experimental intervention) | |||
| Attrition bias (differences in withdrawals, loss to follow‐up, or protocol deviations) | |||
| Detection bias (differences in outcome assessment between groups) | |||
| Other | |
Results
| Baseline characteristics | Etomidate | Comparator | P value |
| Participants (n) | |||
| Age (mean, SD) | |||
| % Male (mean, SD) | |||
| Lost (n) | |||
| Disease severity | |||
| Comorbidities | |||
| Baseline heart rate (bpm) | |||
| Baseline blood pressure (MAP) | |||
| Main results | |||
| Dichotomous | |||
| Mortality (numbers, 2 x 2 table) Yes No |
|||
| ACTH Stim (numbers, 2 x 2 table) Pos Neg |
|||
| Continuous | |||
| Cortisol levels (#, mean, SD) | |||
| Hospital LOS (days) (#, mean, SD) | |||
| ICU LOS (days) (#, mean, SD) | |||
| Post‐intubation haemodynamics | |||
| Ventilator days (days) (#, mean, SD) | |||
| Vasopressor requirements | |||
| Other | |||
NOTES
Data and analyses
Comparison 1. Etomidate versus all other induction agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality: Data as reported | 6 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.60] |
| 2 Mortality: Data as reported ‐ Subgroup analysis of comparator drugs | 6 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.60] |
| 2.1 Studies comparing etomidate and Thiopentone | 1 | 34 | Odds Ratio (M‐H, Random, 95% CI) | 1.94 [0.38, 9.88] |
| 2.2 Studies comparing etomidate and midazolam (+/‐ fentanyl) | 4 | 269 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.61, 1.83] |
| 2.3 Studies comparing etomidate and ketamine | 1 | 469 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.81, 1.76] |
| 3 Mortality: Data as reported ‐ Subgroup analysis of etiology of shock | 6 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.60] |
| 3.1 Septic Shock | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.64, 2.81] |
| 3.2 Trauma | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 3.79 [0.17, 86.13] |
| 3.3 Cardiogenic Shock | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Undifferentiated Critical Illness | 4 | 622 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.79, 1.58] |
| 4 Mortality: Data as reported ‐ Studies judged to be at low risk of bias | 2 | 589 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.73] |
| 5 Mortality: Post Hoc ITT Analysis accounting for missing subjects | 6 | 833 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.86, 1.53] |
| 6 ACTH Stimulation Test | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 ACTH Stimulation test performed 4‐6 h | 2 | 48 | Odds Ratio (M‐H, Random, 95% CI) | 19.98 [3.95, 101.11] |
| 6.2 ACTH Stimulation Test Performed 6‐12 h | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 ACTH Stimulation test performed >12h | 3 | 519 | Odds Ratio (M‐H, Random, 95% CI) | 2.37 [1.61, 3.47] |
| 7 Random Serum Cortisol levels (μg/dL) after receiving intervention | 3 | 105 | Mean Difference (IV, Random, 95% CI) | ‐4.96 [‐8.06, ‐1.86] |
| 7.1 Random Serum Cortisol Levels 4‐6 h | 2 | 50 | Mean Difference (IV, Random, 95% CI) | ‐5.73 [‐10.24, ‐1.23] |
| 7.2 Random Serum Cortisol Levels >12h | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐3.78 [‐10.01, 2.44] |
| 8 Organ System Dysfunction (SOFA Score) | 1 | 469 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.01, 1.39] |
| 9 ICU Length of Stay | 3 | 621 | Mean Difference (IV, Random, 95% CI) | 1.70 [0.00, 5.40] |
| 10 Hospital Length of Stay | 2 | 152 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐7.08, 11.91] |
| 11 Duration of Mechanical Ventilation | 3 | 621 | Mean Difference (IV, Random, 95% CI) | 2.14 [‐1.67, 5.95] |
| 12 Duration of Vasopressor Support | 1 | 469 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.53, 2.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Absalom 1999.
| Methods | RCT comparing etomidate (n = 17) to thiopentone (n = 18) | |
| Participants | 35 patients: ASA1 ≥ III, with 2 or more organ systems failure, and requiring admission to ICU | |
| Interventions | Induction with etomidate or thiopentone (unreported doses) | |
| Outcomes | Adrenal gland function using ACTH stimulation test 24 hours post‐intervention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly allocated to receive either etomidate or thiopentone" (p861). Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Sequence generation and concealment not described |
| Incomplete outcome data (attrition bias) Mortality | Low risk | One patient was lost from the thiopentone group. This is unlikely to affect results |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | Low risk | One patient was lost from the thiopentone group. This is unlikely to affect results |
| Incomplete outcome data (attrition bias) Hospital LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) ICU LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | Low risk | N/A |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | N/A |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient was lost from the thiopentone group. This is unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | All relevant data reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of blinding reported, however the outcome is entirely objective |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding reported, however the outcome is entirely objective |
Cinar 2010.
| Methods | RCT comparing etomidate (n = 12) and ketamine (n = 10) | |
| Participants | 22 ICU patients | |
| Interventions | Etomidate 0.3 mg/kg versus ketamine 2 mg/kg | |
| Outcomes | Intubating conditions, haemodynamic response to intubation, total serum cortisol 5 min post‐induction, duration of mechanical ventilation, ICU length of stay, mortality | |
| Notes | Need more information. This was conference poster and no actual data is presented. Primary author and supervising author contacted by e‐mail. Waiting on response. Unable to include in analysis due to lack of data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospective, randomized, double blinded study" reported, but sequence generation and concealment not described |
| Allocation concealment (selection bias) | Unclear risk | "prospective, randomized, double blinded study" reported, but sequence generation and concealment not described |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | No description of incomplete data. Impacts on risk estimates not possible to describe, but bias possible |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | Unclear risk | No description of incomplete data. Impacts on risk estimates not possible to describe, but bias possible |
| Incomplete outcome data (attrition bias) Hospital LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) ICU LOS | Unclear risk | No description of incomplete data. Impacts on risk estimates not possible to describe, but bias possible |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | Unclear risk | No description of incomplete data. Impacts on risk estimates not possible to describe, but bias possible |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | N/A |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of incomplete data. Impacts on risk estimates not possible to describe, but bias possible |
| Selective reporting (reporting bias) | Low risk | all relevant outcomes addressed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "prospective, randomized, double blinded study" reported, but no further description |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "prospective, randomized, double blinded study" reported. Exact nature of double blinding not fully specified, so effects on measurement remain unknown |
Hildreth 2008.
| Methods | RCT comparing etomidate (n = 18) to fentanyl and midazolam combination (n = 12) | |
| Participants | Adult trauma patients requiring intubation < 48 hours post‐injury | |
| Interventions | Etomidate 0.3 mg/kg versus fentanyl 100 µg and midazolam 5 mg | |
| Outcomes | Primary: Adrenal function (ACTH Stimulation test and random serum cortisol levels at 4 to 6 h after intervention). Secondary: vasopressor requirements, post‐intubation haemodynamics, transfusion requirements, mortality, hospital LOS2, ICU LOS2, ventilator days | |
| Notes | Clarification about sequence generation, allocation concealment, and details about the protocol violations have been sought from the author. Raw data for ACTH stimulation tests were provided by the author. Risk of bias judgments may change once this information is received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports "randomization packets" but no description of sequence generation. "Medical personnel who participated in randomization procedures were trained". Effects on randomization unclear |
| Allocation concealment (selection bias) | Low risk | "Envelopes were half [etomidate] and half [Fentanyul/Midazolam] and sealed. Opened in ED or helicopter or ICU by person intubating. No way it could have been predicted prior to enrolment. Envelopes were identical." (Hildreth 2013 [pers comm]) |
| Incomplete outcome data (attrition bias) Mortality | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Incomplete outcome data (attrition bias) Hospital LOS | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Incomplete outcome data (attrition bias) ICU LOS | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Incomplete outcome data (attrition bias) Vasopressor requirements | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This study reported 61 eligible patients, but only enrolled 30. Of the 31 patients who were excluded, 14 (45%) were excluded for protocol violations. In an unblinded study, this raises significant concerns for the reviewers, and this study is judged at high risk of bias for attrition bias |
| Selective reporting (reporting bias) | Low risk | All relevant data reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study. Unlikely to affect empiric outcomes relevant to this review, however, when considered in the context of the high rate of patient exclusion for protocol violation, the risk of bias due to unblinded assessors and personnel is high |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Non‐blinded study. Unlikely to affect empiric outcomes relevant to this review |
Jabre 2009.
| Methods | RCT comparing etomidate (n = 234) and ketamine (n = 235) | |
| Participants | Adult pre‐hospital, emergency department, or ICU patients requiring intubation for being comatose, shock, acute respiratory failure, or 'other' | |
| Interventions | Etomidate 0.3 mg/kg versus ketamine 2 mg/kg | |
| Outcomes | Primary: maximum Sequential Organ Failure Assessment (SOFA) score. Secondary: change in SOFA score, 28 day mortality, ICU LOS2, ventilation duration, vasopressor use, ACTH stimulation test performed 0 to 48 h after intervention | |
| Notes | ICU length of stay reported as "ICU free days at day 28", Vasopressor use reported as "Catecholamine free days until day 28", ventilation duration reported as "Mechanical ventilation free days at day 28". These variables were transformed by subtracting these results from 28 days to estimate the duration of each of these variables. This analysis assumes a single ICU visit, ventilation treatment, vasopressor use; 69% of patients in both arms were intubated for being comatose, while the minority were intubated for shock, acute respiratory failure, or 'other' reasons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Utilized a computerized random number generator |
| Allocation concealment (selection bias) | Low risk | Study drugs were sealed in sequentially numbered, identical boxes containing the entire treatment for each patient. "The emergency physician enrolling patients was aware of study group assignment. However nurses and intensivists in the intensive care unit were masked to the treatment assigned" (Jabre 2009. p294). While this single blind methodology raises concern, the allocation concealment was adequate to prevent enrolling physicians from altering the allocation sequence |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Incomplete outcome data (attrition bias) Hospital LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) ICU LOS | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data on 2 patients from each group, and one patient in the etomidate withdrew consent. Unlikely to affect results, given the small proportion of the sample size. Effect on risk estimates likely to be negligible |
| Selective reporting (reporting bias) | Low risk | All relevant data reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Emergency physicians giving the drugs were not blinded, but nurses and ICU physicians providing care for the duration of the study were blinded. Unlikely to affect the performance of participants and personnel, hence the effects of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Emergency physicians giving the drugs were not blinded, but nurses and ICU physicians providing care for the duration of the study were blinded. Unlikely to affect the outcome assessment, and hence opportunity for information bias minimized |
Jacoby 2006.
| Methods | RCT comparing etomidate (n = 55) and midazolam (n = 55) | |
| Participants | Pre‐hospital (EMS) intubation for respiratory distress or altered level of consciousness | |
| Interventions | Etomidate (20mg) versus midazolam (7 mg) | |
| Outcomes | Primary: intubation success. Secondary: vocal cord visualization, number of attempts or intubation difficulty, post‐intubation hypotension, vomiting, fasciculations, survival to discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random number table |
| Allocation concealment (selection bias) | Low risk | Numbered syringes, identical to each other, volume corrected. |
| Incomplete outcome data (attrition bias) Mortality | High risk | 115 patients enrolled in the study and 110 (55 per group) patients completed. Mortality data available for 88 (44 per group). Equal proportion in each group of lost patients (11 per group), but a description of lost patients with regards to mortality was not reported. Effects on risk estimates unclear due to this loss to follow‐up. Selection bias possible, but direction of bias not predictable |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) Hospital LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) ICU LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | Low risk | N/A |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | N/A |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 115 patients enrolled in the study, and 110 patients completed. Patients accounted for, and small proportion of lost patients unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | All relevant data reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded. Master allocation lists were never accessed so blinding was not compromised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Master allocation lists were never accessed so blinding was not compromised |
Koksal 2013.
| Methods | RCT comparing etomidate (n = 20), etomidate + methylprednisolone (n = 20), and midazolam (n = 20) | |
| Participants | Adult patients, ASA III‐IV, requiring intubation in the ED or ICU | |
| Interventions | Group 1: etomidate 0.3 mg/kg, Group 2: etomidate 0.3 mg/kg AND methylprednisolone 2 mg/kg, Group 3: midazolam 0.5 mg/kg | |
| Outcomes | Primary: Random serum cortisol levels at 4 and 24 h. Secondary: post‐intubation haemodynamics | |
| Notes | Group 2 was excluded from our analysis because methylprednisolone was given. All results from this study exclude the outcomes for the 20 patients in group 2. This is an unpublished study identified in our grey literature search. The author has kindly provided us with a copy of the manuscript for inclusion. The study is to be submitted for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Awaiting details from author |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | Awaiting details from author |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | Unclear risk | Awaiting details from author |
| Incomplete outcome data (attrition bias) Hospital LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) ICU LOS | Low risk | N/A |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | Low risk | N/A |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | N/A |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Awaiting details from author |
| Selective reporting (reporting bias) | Low risk | All outcomes from trial registration were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | single blind study. Only outcome assessor was blind. Awaiting more details from author regarding allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | single blind study. Only outcome assessor was blind |
Schenarts 2001.
| Methods | RCT comparing etomidate (n = 16) and midazolam (n = 15) | |
| Participants | Adult patients requiring intubation in a tertiary care emergency department | |
| Interventions | Etomidate 0.3 mg/kg versus midazolam 0.05 to 0.1 mg/kg | |
| Outcomes | Primary: ACTH Stimulation tests at 4, 12 and 24 hours after intervention. Secondary: mortality, random serum cortisol levels (at 4, 12, and 24 h), hospital LOS2, ICU LOS2, ventilator duration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization table |
| Allocation concealment (selection bias) | High risk | No description of allocation concealment reported. However, with the lack of blinding, in the setting of significant incomplete data (9 of 31 patients), the reviewers have considerable concern regarding the allocation concealment |
| Incomplete outcome data (attrition bias) Mortality | High risk | 9 of 31 enrolled patients have incomplete data, and were excluded from analysis. Effects on risk estimates unclear, although could be substantial; risk of bias high |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | High risk | 9 of 31 enrolled patients have incomplete data, and were excluded from analysis. Effects on risk estimates unclear, although could be substantial; risk of bias high |
| Incomplete outcome data (attrition bias) Hospital LOS | High risk | 9 of 31 enrolled patients have incomplete data, and were excluded from analysis. Effects on risk estimates unclear, although could be substantial; risk of bias high |
| Incomplete outcome data (attrition bias) ICU LOS | High risk | 9 of 31 enrolled patients have incomplete data, and were excluded from analysis. Effects on risk estimates unclear, although could be substantial; risk of bias high |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | High risk | 9 of 31 enrolled patients have incomplete data, and were excluded from analysis. Effects on risk estimates unclear, although could be substantial; risk of bias high |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | N/A |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 of 31 enrolled patients have incomplete data, and were excluded from analysis. Effects on risk estimates unclear, although could be substantial; risk of bias high |
| Selective reporting (reporting bias) | Low risk | All relevant data reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded, and in the setting of a significant proportion of patients excluded due to incomplete data, the reviewers have considerable concern that the unblinded nature of this study has led to bias in risk estimates |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded, and in the setting of a significant proportion of patients excluded due to incomplete data, the reviewers have considerable concern that the unblinded nature of this study has led to bias in risk estimates |
Tekwani 2010.
| Methods | RCT comparing Etomidate (n=63) and midazolam (n = 59) | |
| Participants | Septic adults requiring intubation in a tertiary care emergency department | |
| Interventions | Etomidate 0.3 mg/kg versus midazolam 0.1 mg/kg | |
| Outcomes | Primary: Hospital LOS2. Secondary: inhospital mortality, ICL LOS2, ventilator duration, post‐intubation haemodynamics, vasopressor requirements | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Kits numbered and dispensed by an automated medication dispensing cabinet. No description of the blocking factor were provided, but given the computer generated sequence generation and automated medication dispensing, we believe that participant anticipation of subsequent allocation would be difficult, and that the subsequent risk of bias is low |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Only 2 patients had incomplete data. Likely to have minimal effects on the strength and direction of risk estimates |
| Incomplete outcome data (attrition bias) Adrenal Gland Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) Hospital LOS | Low risk | Only 2 patients had incomplete data. Likely to have minimal effects on the strength and direction of risk estimates |
| Incomplete outcome data (attrition bias) ICU LOS | Low risk | Only 2 patients had incomplete data. Likely to have minimal effects on the strength and direction of risk estimates |
| Incomplete outcome data (attrition bias) Duration of mechanical Ventillation | Low risk | Only 2 patients had incomplete data. Likely to have minimal effects on the strength and direction of risk estimates |
| Incomplete outcome data (attrition bias) Vasopressor requirements | Low risk | Only 2 patients had incomplete data. Likely to have minimal effects on the strength and direction of risk estimates |
| Incomplete outcome data (attrition bias) Organ Dysfunction | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 patients had incomplete data. Likely to have minimal effects on the strength and direction of risk estimates |
| Selective reporting (reporting bias) | Low risk | All relevant data reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical vials with volume‐equivalent concentrations. Double blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identical vials with volume‐equivalent concentrations. Data collectors were blinded |
1. American Society of Anesthesiologists (ASA) physical status
2. LOS = Length of stay
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asehnoune 2012 | This is a substudy of the HYPOLYTE (Roquilly 2011) multi‐centred double blind RCT in which trauma patients were randomized to receive hydrocortisone or placebo |
| Baird 2009 | Observational study |
| Borner 1985 | Elective anaesthesia induction |
| Bramwell 2002 | Not RCT |
| Cherfan 2011 | This was a nested cohort study within an RCT evaluating hydrocortisone use in septic cirrhotic patients |
| Cotton 2008 | Retrospective registry study |
| Cuthbertson 2009 | This was an a priori substudy of the CORTICUS trial (Sprung 2008). In this double blind RCT, patients were randomized to receive hydrocortisone or placebo |
| den Brinker 2008 | Retrospective review |
| Dmello 2010 | Retrospecitve study |
| Ehrman 2010 | Retrospective cohort study |
| McPhee 2013 | Retrospective cohort study |
| Mohammad 2006 | Retrospective study |
| Morel 2011 | Elective cardiac surgery induction |
| Ray 2007 | Retrospective chart review |
| Vinclair 2008 | Observational cohort study |
| Warner Kier 2009 | Not RCT |
| Zed 2006 | Observational study |
Characteristics of studies awaiting assessment [ordered by study ID]
Driver 2014.
| Methods | Randomized clinical trial. Single blind where ICU physicians but not ER physicians were blinded |
| Participants | n = 98. All patients receiving RSI |
| Interventions | Ketamine 2 mg/kg versus etomidate 0.3 mg/kg |
| Outcomes | Primary: 30 day mortality, or discharge, whichever occurred first. Secondary: first pass intubation success, number of intubation attempts, number of post‐intubation sedative boluses within 6 hours, post‐intubation hypotension within 6 hours, post‐intubation hypoxaemia within 2 hours |
| Notes | This is an interim report of their study. 98 patients have been enrolled. This reports the outcomes of 58 patients with available data |
Freund 2014.
| Methods | Ancillary study. Off‐shoot of KETASED study which was an RCT comparing ketamine and etomidate |
| Participants | n = 310. Patients who underwent pre‐hospital RSI, and who had ACTH stimulation test within 24 h or ICU admission |
| Interventions | Patients in the KETASED study were randomized to ketamine or etomidate. This study groups patients as having RAI or not, and then identifying risk factors based on this grouping, not on whether they received etomidate or ketamine |
| Outcomes | The objective of this study was to assess the effect of relative adrenal insufficiency (RAI) on prognostic outcomes after RSI, and factors associated with the onset of RAI |
| Notes | This was the same population as the Jabre 2009 study. This sample size is larger because this report includes the patients who were excluded by Jabre who had died before arrival to hospital, or who were discharged from the ICU before the 3 day mark |
Punt 2014.
| Methods | Single centre prospective open label study. Block randomized by ICU. Each ICU delivered the same agent for 10 months, and they switched to the other agent for an additional 10 months |
| Participants | n = 322. ICU patients requiring tracheal intubation |
| Interventions | Etomidate 0.2 to 0.3 mg/kg versus (S‐ketamine 0.5 mg/kg + midazolam 2.5 mg) |
| Outcomes | Primary: 28 day mortality. Secondary: length of stay, usage of norepinephrine, and cortisol concentrations |
| Notes |
Differences between protocol and review
The secondary outcome, duration of vasopressor support, was proposed to be measured in hours in the protocol (Bruder 2012). The data were reported in the literature in days, so we also reported these data in days.
Our protocol (Bruder 2012) stated that we would report both the OR and RR. This review has only reported the OR. We chose to do this in order to simplify our results and minimize information clutter.
Our protocol (Bruder 2012) stated, "We will exclude non‐randomized or quasi‐randomized trials". We chose to include one study that block randomized patients in blocks of four (Jabre 2009). While this deviates from our protocol, we chose to include this study because it was the single largest study on the topic with otherwise sound methodology and low risk of bias. This study also had a very low rate of attrition. We judged the small blocks of four in conjunction with computer generated randomization and identically sealed and sequentially numbered drug boxes to mitigate the shortfalls of block randomization. We feel that this deviation from our protocol did not introduce bias into our review.
Our review included a post hoc sensitivity analysis of our mortality data (Analysis 1.5) that was not described in our protocol. In designing our protocol, we did not anticipate the large number of patients lost to follow‐up. This sensitivity analysis was added to ensure our mortality outcome results were true. We feel that this deviation from our protocol did not introduce bias into our review.
Contributions of authors
Conceiving the review: Eric A Bruder (EB), Ian Ball (IB), Corinne Hohl (CH)
Co‐ordinating the review: EB
Undertaking manual searches: EB, IB, Stacy Ridi (SR)
Screening search results: EB, IB, SR
Organizing retrieval of papers: EB
Screening retrieved papers against inclusion criteria: EB, IB, SR
Appraising quality of papers: EB, IB, SR
Abstracting data from papers: EB, IB, SR
Writing to authors of papers for additional information: EB
Providing additional data about papers: EB
Obtaining and screening data on unpublished studies: EB
Data management for the review: EB, William Pickett (WP)
Entering data into Review Manager (RevMan 5.2): EB, IB, SR
RevMan statistical data: EB, IB, WP
Other statistical analysis not using RevMan: WP
Double entry of data: (data entered by person one: EB; data entered by person two: IB)
Interpretation of data: EB, IB, WP
Statistical inferences: WP
Writing the review: EB, IB
Securing funding for the review: N/A
Performing previous work that was the foundation of the present study: CH
Guarantor for the review (one author): EB
Person responsible for reading and checking review before submission: EB
Declarations of interest
Eric Bruder: none known.
Ian Ball: none known.
Stacy Ridi: none known.
William Pickett: none known.
Corinne Hohl: Dr Hohl is a full‐time faculty member with the Department of Emergency Medicine at the University of British Columbia. She has received reimbursement for travel expenses by the Canadian Association of Emergency Physicians to present on this topic in Montreal, 2010.
Edited (no change to conclusions)
References
References to studies included in this review
Absalom 1999 {published data only}
- Absalom A, Pledger D, Kong A. Adrenocortical function in critically ill patients 24 h after a single dose of etomidate. Anaesthesia 1999;54(9):861‐7. [PUBMED: 10460557] [DOI] [PubMed] [Google Scholar]
- Absalom AR, Pledger DR, Kong A. Effects of a single dose of etomidate on adreno‐cortical function in the critically ill. British Journal of Anaesthesia 1997;79 (5):679P. [Google Scholar]
Cinar 2010 {published data only}
- Cinar O, Pirat A, Zeyneloglu P, Bayraktar N, Arslan G. Hemodynamic and metabolic responses to ketamine and etomidate sedations during endotracheal intubation in critically ill patients. Critical Care Medicine. Conference: 40th Critical Care Congress of the Society of Critical Care Medicine San Diego, CA United States. San Diego, CA United States, 2010; Vol. 38 (12 Suppl).
Hildreth 2008 {published data only}
- Hildreth AN, Mejia VA, Maxwell RA, Smith PW, Dart BW, Barker DE. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. Journal of Trauma Injury, Infection, and Critical Care 2008;65(3):573‐8. [PUBMED: 18784570] [DOI] [PubMed] [Google Scholar]
Jabre 2009 {published data only}
- Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard‐Hibon A, Vivien B, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet 2009;374(9686):293‐300. [PUBMED: 19573904] [DOI] [PubMed] [Google Scholar]
Jacoby 2006 {published data only}
- Jacoby J, Heller M, Nicholas J, Patel N, Cesta M, Smith G, et al. Etomidate Versus Midazolam for Out‐of‐Hospital Intubation: A Prospective, Randomized Trial. Annals of Emergency Medicine 2006;47(6):525‐30. [PUBMED: 16713778] [DOI] [PubMed] [Google Scholar]
- Jacoby JL, Cesta M, McGee J, Heller MB, Reed J. Etomidate versus midazolam for out‐of hospital Intubation: A prospective randomized trial ‐ Abstract 175 ACEP Research Forum 2003. Annals Of Emergency Medicine 2003;42(4):S48. [DOI] [PubMed] [Google Scholar]
Koksal 2013 {unpublished data only}
Schenarts 2001 {published data only}
- Schenarts CL, Burton JH, Riker RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Academic Emergency Medicine 2001;8(1):1‐7. [PUBMED: 11136139] [DOI] [PubMed] [Google Scholar]
- Schenarts CL, Riker RR. 1999 SAEM Annual Meeting abstracts. Abstract 449: Adrenocortical dysfunction following etomidate induction in emergency department. Academic Emergency Medicine 1999;6(5):520. [DOI] [PubMed] [Google Scholar]
Tekwani 2010 {published data only}
- Tekwani K, Watts H, Sweis R, Rzechula K, Kulstad E. The effect of etomidate on hospital length of stay of patients with sepsis: A prospective, randomized study: Conference: American College of Emergency Physicians, ACEP 2009 Research Forum. Annals of Emergency Medicine 2009;54(3):S4. [Google Scholar]
- Tekwani KL, Watts HF, Sweis RT, Rzechula KH, Kulstad EB. A comparison of the effects of etomidate and midazolam on hospital length of stay in patients with suspected sepsis: a prospective, randomized study. Annals of Emergency Medicine 2010;56(5):481‐9. [PUBMED: 20828877] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asehnoune 2012 {published data only}
- Asehnoune K, Mahe PJ, Seguin P, Jaber S, Jung B, Guitton C, et al. Etomidate increases susceptibility to pneumonia in trauma patients. Intensive Care Medicine 2012;38(10):1673‐82. [PUBMED: 22777514] [DOI] [PubMed] [Google Scholar]
Baird 2009 {published data only}
- Baird CR, Hay AW, McKeown DW, Ray DC. Rapid sequence induction in the emergency department: induction drug and outcome of patients admitted to the intensive care unit. Emergency Medicine Journal 2009;26(8):576‐9. [PUBMED: 19625554] [DOI] [PubMed] [Google Scholar]
Borner 1985 {published data only}
- Borner U, Gips H, Boldt J, Hoge R, Bormann B, Hempelmann G. [Effect of an introductory dose of etomidate, methohexital and midazolam on adrenal cortex function before and after ACTH‐stimulation]. Deutsche Medizinische Wochenschrift 1985;10(19):750‐2. [DOI] [PubMed] [Google Scholar]
Bramwell 2002 {published data only}
- Bramwell KJ, Haizlip J, Pribble C, VanDerHeyden TC, Witte M. The effect of etomidate on intracranial pressure, mean arterial pressure, and cerebral perfusion pressure in pediatric patients with severe traumatic brain injury. Annals of Emergency Medicine 2002;40(4):S22. [DOI] [PubMed] [Google Scholar]
Cherfan 2011 {published data only}
- Cherfan AJ, Tamim HM, AlJumah A, Rishu AH, Al‐Abdulkareem A, Al Knawy BA, et al. Etomidate and mortality in cirrhotic patients with septic shock. BMC Clinical Pharmacology 2011;11:22. [PUBMED: 22208901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 2008 {published data only}
- Cotton BA, Guillamondegui OD, Fleming SB, Carpenter RO, Patel SH, Morris JA Jr, et al. Increased risk of adrenal insufficiency following etomidate exposure in critically injured patients. Archives of Surgery 2008;143(1):62‐7. [PUBMED: 18209154] [DOI] [PubMed] [Google Scholar]
Cuthbertson 2009 {published data only}
- Cuthbertson BH, Sprung CL, Annane D, Chevret S, Garfield M, Goodman S, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Medicine 2009;35(11):1868‐76. [PUBMED: 19652948] [DOI] [PubMed] [Google Scholar]
den Brinker 2008 {published data only}
- Brinker M, Hokken‐Koelega ACS, Hazelzet JA, Jong FH, Hop WCJ, Joosten KFM. One single dose of etomidate negatively influences adrenocortical performance for at least 24h in children with meningococcal sepsis. Intensive Care Medicine 2008;34(1):163‐8. [PUBMED: 17710382] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dmello 2010 {published data only}
- Dmello D, Taylor S, O'Brien J, Matuschak GM. Outcomes of etomidate in severe sepsis and septic shock. Chest 2010;138(6):1327‐32. [PUBMED: 20651024] [DOI] [PubMed] [Google Scholar]
Ehrman 2010 {published data only}
- Ehrman R, Wira C, Hayward A, Lomax A, Mullen M. Etomidate use In sepsis does not increase mortality. Annals of Emergency Medicine 2010;56(3):S117. [Google Scholar]
McPhee 2013 {published data only}
- McPhee LC, Badawi O, Fraser GL, Lerwick PA, Riker RR, Zuckerman IH, et al. Single‐dose etomidate is not associated with increased mortality in ICU patients with sepsis: analysis of a large electronic ICU database. Critical Care Medicine 2013;41(3):774‐83. [PUBMED: 23318491] [DOI] [PubMed] [Google Scholar]
Mohammad 2006 {published data only}
- Mohammad Z, Afessa B, Finkielman JD. The incidence of relative adrenal insufficiency in patients with septic shock after the administration of etomidate. Critical Care 2006;10(4):R105. [PUBMED: 16859529] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morel 2011 {published data only}
- Morel J, Salard M, Castelain C, Bayon MC, Lambert P, Vola M, et al. Haemodynamic consequences of etomidate administration in elective cardiac surgery: a randomized double‐blinded study. British Journal of Anaesthesia 2011;107(4):503‐9. [PUBMED: 21685487 ] [DOI] [PubMed] [Google Scholar]
Ray 2007 {published data only}
- Ray DC, McKeown DW. Effect of induction agent on vasopressor and steroid use, and outcome in patients with septic shock. Critical Care 2007;11(3):R56. [PUBMED: 17506873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinclair 2008 {published data only}
- Vinclair M, Broux C, Faure P, Brun J, Genty C, Jacquot C, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Medicine 2008;34(4):714‐9. [PUBMED: 18092151] [DOI] [PubMed] [Google Scholar]
Warner Kier 2009 {published data only}
- Warner KJ, Cuschieri J, Jurkovich GJ, Bulger EM. Single‐dose etomidate for rapid sequence intubation may impact outcome after severe injury. Journal of Trauma Injury, Infection, and Critical Care 2009;67(1):45‐50. [PUBMED: 19590307] [DOI] [PubMed] [Google Scholar]
Zed 2006 {published data only}
- Zed PJ, Abu‐Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Academic Emergency Medicine 2006;13(4):378‐83. [PUBMED: 16531603] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Driver 2014 {published data only}
- Driver B, Moore J, Reardon R, Steinberg L, Antolick A, Usher S, Miner J. Ketamine versus etomidate for ED rapid sequence intubation. Academic Emergency Medicine 2014;21: 5 Suppl 1:S117. [Google Scholar]
Freund 2014 {published data only}
- Freund Y, Jabre P, Mourad J, Lapostolle F, Reuter PG, Woimant M, et al. Relative adrenal insufficiency in critically ill patient after rapid sequence intubation: KETASED ancillary study. Journal of Critical Care 2014;29(3):386‐9. [DOI] [PubMed] [Google Scholar]
Punt 2014 {published data only}
- Punt CD, Dormans TPJ, Oosterhuis WP, Boer W, Depoorter B, Linden CJ, et al. Etomidate and S‐ketamine for the intubation of patients on the intensive care unit: A prospective, open‐label study. Netherlands Journal of Critical Care 2014;18(2):4‐7. [Google Scholar]
Additional references
Albert 2011
- Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Medicine 2011;37(6):901‐10. [PUBMED: 21373823] [DOI] [PubMed] [Google Scholar]
Annane 2002
- Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Annane 2004
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis. BMJ 2004;480:329‐480. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2012
- Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta‐analysis. Critical Care Medicine 2012;40(11):2945‐53. [PUBMED: 22971586] [DOI] [PubMed] [Google Scholar]
Cronin 1995
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature. Critical Care Medicine 1995;23:1430‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jong 1984
- Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta‐hydroxylation. Journal of Clinical Endocrinology and Metabolism 2084;59:1143‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Flack‐Ytter Y, Schunemann HJ. GRADE Working Group. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hildreth 2013 [pers comm]
- Hildreth A. Re: stim test results [personal communication]. E‐mail to: E Bruder 13 November, 2013.
Hohl 2010
- Hohl CM, Kelly‐Smith CH, Yeung TC, Sweet DD, Doyle‐Waters MM, Schulzer M. The effect of a bolus dose of etomidate on cortisol levels, mortality, and health services utilization: a systematic review. Annals of Emergency Medicine 2010;56:105‐13. [PUBMED: 20346542] [DOI] [PubMed] [Google Scholar]
Lloyd 2013 [pers comm]
- Lloyd J. Inquiry Response for Hospira US2013‐11982 [personal communication]. E‐mail to: E Bruder 10 July, 2013.
Marik 2008
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. American College of Critical Care Medicine. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine 2008;36(6):1937‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roquilly 2011
- Roquilly A, Mahe PJ, Seguin P, Guitton C, Floch H, Tellier AC, et al. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA 2011;305(12):1201‐9. [PUBMED: 21427372] [DOI] [PubMed] [Google Scholar]
Sivilotti 2003
- Sivilotti MLA, Filbin MR, Murray HE, Slasor P, Walls RM, NEAR Investigators. Does the sedative agent facilitate emergency rapid sequence intubation?. Academic Emergency Medicine 2003;10(6):612‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sprung 2008
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. New England Journal of Medicine 2008;8:111‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stoelting 2006
- Stoelting R, Hiller S. Pharmacology & Physiology in Anesthetic Practice. Fourth. Philadelphia: Lippincott Williams & Wilkins, 2006. [Google Scholar]
Truven 2013
- Truven Health Ac. Micromedex 2.0 SIU Conversion Calculator. http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/02664B/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/61BF2F/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.Calculators# Accessed July, 2013.
Wong 1974
- Wong DHW, Jenkins LC. Experimental study of mechanism of action of ketamine on central nervous system. Canadian Anaesthetists Society Journal 1974;21(1):57‐67. [DOI] [PubMed] [Google Scholar]
Zacharias 2013
- Zacharias M, Mugawar M, Herbison GP, Walker RJ, Hovhannisyan K, Sivalingam P, Conlon NP. Interventions for protecting renal function in the perioperative period. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD003590.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bruder 2012
- Bruder EA, Ball I, Ridi S, Pickett W, Hohl C. Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010225] [DOI] [PMC free article] [PubMed] [Google Scholar]


