Abstract
Background
Evidence on the health effects of total polyunsaturated fatty acids (PUFA) is equivocal. Fish oils are rich in omega‐3 PUFA and plant oils in omega‐6 PUFA. Evidence suggests that increasing PUFA‐rich foods, supplements or supplemented foods can reduce serum cholesterol, but may increase body weight, so overall cardiovascular effects are unclear.
Objectives
To assess effects of increasing total PUFA intake on cardiovascular disease and all‐cause mortality, lipids and adiposity in adults.
Search methods
We searched CENTRAL, MEDLINE and Embase to April 2017 and clinicaltrials.gov and the World Health Organization International Clinical Trials Registry Platform to September 2016, without language restrictions. We checked trials included in relevant systematic reviews.
Selection criteria
We included randomised controlled trials (RCTs) comparing higher with lower PUFA intakes in adults with or without cardiovascular disease that assessed effects over 12 months or longer. We included full texts, abstracts, trials registry entries and unpublished data. Outcomes were all‐cause mortality, cardiovascular disease mortality and events, risk factors (blood lipids, adiposity, blood pressure), and adverse events. We excluded trials where we could not separate effects of PUFA intake from other dietary, lifestyle or medication interventions.
Data collection and analysis
Two review authors independently screened titles and abstracts, assessed trials for inclusion, extracted data, and assessed risk of bias. We wrote to authors of included trials for further data. Meta‐analyses used random‐effects analysis, sensitivity analyses included fixed‐effects and limiting to low summary risk of bias. We assessed GRADE quality of evidence.
Main results
We included 49 RCTs randomising 24,272 participants, with duration of one to eight years. Eleven included trials were at low summary risk of bias, 33 recruited participants without cardiovascular disease. Baseline PUFA intake was unclear in most trials, but 3.9% to 8% of total energy intake where reported. Most trials gave supplemental capsules, but eight gave dietary advice, eight gave supplemental foods such as nuts or margarine, and three used a combination of methods to increase PUFA.
Increasing PUFA intake probably has little or no effect on all‐cause mortality (risk 7.8% vs 7.6%, risk ratio (RR) 0.98, 95% confidence interval (CI) 0.89 to 1.07, 19,290 participants in 24 trials), but probably slightly reduces risk of coronary heart disease events from 14.2% to 12.3% (RR 0.87, 95% CI 0.72 to 1.06, 15 trials, 10,076 participants) and cardiovascular disease events from 14.6% to 13.0% (RR 0.89, 95% CI 0.79 to 1.01, 17,799 participants in 21 trials), all moderate‐quality evidence. Increasing PUFA may slightly reduce risk of coronary heart disease death (6.6% to 6.1%, RR 0.91, 95% CI 0.78 to 1.06, 9 trials, 8810 participants) andstroke (1.2% to 1.1%, RR 0.91, 95% CI 0.58 to 1.44, 11 trials, 14,742 participants, though confidence intervals include important harms), but has little or no effect on cardiovascular mortality (RR 1.02, 95% CI 0.82 to 1.26, 16 trials, 15,107 participants) all low‐quality evidence. Effects of increasing PUFA on major adverse cardiac and cerebrovascular events and atrial fibrillation are unclear as evidence is of very low quality.
Increasing PUFA intake probably slightly decreases triglycerides (by 15%, MD ‐0.12 mmol/L, 95% CI ‐0.20 to ‐0.04, 20 trials, 3905 participants), but has little or no effect on total cholesterol (mean difference (MD) ‐0.12 mmol/L, 95% CI ‐0.23 to ‐0.02, 26 trials, 8072 participants), high‐density lipoprotein (HDL) (MD ‐0.01 mmol/L, 95% CI ‐0.02 to 0.01, 18 trials, 4674 participants) or low‐density lipoprotein (LDL) (MD ‐0.01 mmol/L, 95% CI ‐0.09 to 0.06, 15 trials, 3362 participants). Increasing PUFA probably has little or no effect on adiposity (body weight MD 0.76 kg, 95% CI 0.34 to 1.19, 12 trials, 7100 participants).
Effects of increasing PUFA on serious adverse events such as pulmonary embolism and bleeding are unclear as the evidence is of very low quality.
Authors' conclusions
This is the most extensive systematic review of RCTs conducted to date to assess effects of increasing PUFA on cardiovascular disease, mortality, lipids or adiposity. Increasing PUFA intake probably slightly reduces risk of coronary heart disease and cardiovascular disease events, may slightly reduce risk of coronary heart disease mortality and stroke (though not ruling out harms), but has little or no effect on all‐cause or cardiovascular disease mortality. The mechanism may be via TG reduction.
Plain language summary
Polyunsaturated fatty acids for prevention and treatment of diseases of the heart and circulation
Review question
We reviewed randomised trials (participants have an equal chance to be assigned to either treatment) examining effects of increasing intake of polyunsaturated fatty acids (PUFA) on deaths and diseases of the heart and circulation (cardiovascular diseases), including heart attacks and stroke.
Background
We eat PUFA in our usual food, but quantities of PUFA eaten vary. There is some evidence that increasing the amount of PUFA we eat can reduce our blood cholesterol and make us less likely to develop cardiovascular disease, particularly if PUFAs are eaten instead of saturated fats (fats from animal sources such as meat and cheese). But eating more PUFA may increase our body weight, and omega‐6 fats (one component of PUFA) may worsen cardiovascular risk by increasing inflammation. Evidence on the benefits or harms of increasing PUFA intake on diseases of the heart and circulation, or on other health outcomes, is inconclusive.
Trial characteristics
Evidence in this Cochrane Review is current to 27 April 2017. We included 49 trials randomising 24,272 participants, for one to eight years. These trials assessed effects of eating more, compared to less PUFA, on diseases of the heart and circulation, and deaths. Twelve trials were very trustworthy (had low risk of bias overall). Participants were men and women, some with existing illnesses and some not. Trials took place in North America, Asia, Europe and Australia, and sixteen were funded only by national or charitable agencies.
Key results
Increasing PUFA probably makes little or no difference (neither benefit nor harm) to our risk of death (moderate‐quality evidence), and may make little or no difference to our risk of dying from cardiovascular disease (low‐quality evidence). However, increasing PUFA probably slightly reduces our risk of heart disease events and of combined heart and stroke events (moderate‐quality evidence). Fifty three people would need to eat more PUFA to prevent one person experiencing a heart disease event, and 63 people to prevent one person experiencing a heart or stroke event. Increasing PUFA may very slightly reduce risk of death due to heart disease, as well as stroke, but harm is possible (low‐quality evidence). PUFA probably slightly reduces fats circulating in the blood (triglycerides, moderate‐quality evidence but without effects on other lipids or adiposity). The evidence mainly comes from dietary‐advice trials of men living in high‐income countries.
Summary of findings
Background
Description of the condition
The World Health Organization (WHO) reports cardiovascular diseases as the primary cause of death in the world (WHO 2016). In 2012 they estimated that 17.5 million people died from cardiovascular diseases, three‐quarters of whom were in low‐ to middle‐income countries. Cardiovascular diseases are disorders of the heart and blood vessels and include a range of conditions. Some are diseases of blood vessels supplying the heart (coronary heart disease), brain (cerebrovascular disease), or arms or legs (peripheral arterial disease). Others are due to infection (rheumatic heart disease, where damage to the heart muscle and valves is due to rheumatic fever), are present at birth (congenital heart disease), or are due to blood clots (deep vein thrombosis and pulmonary embolism) (WHO 2016). This review is concerned with the forms of cardiovascular disease that are potentially modifiable by dietary means, particularly coronary heart disease and cerebrovascular disease.
Description of the intervention
Polyunsaturated fatty acids (PUFAs) are fats that include at least two double carbon‐to‐carbon bonds (unsaturated carbon bonds) in their long hydrocarbon chain. This makes the fats pack less well, so they tend to be liquid at room temperature, rather than solid like many saturated fats. PUFAs can be omega‐3 (where the first double bond is three carbons away from the methyl‐carbon end of the molecule), omega‐6 or omega‐9 (although most omega‐9 fats do not have at least two double bonds, so are not included). Fish and plant oils are often rich in PUFAs, with fish being rich in omega‐3 and plant oils rich in omega‐6. Two PUFAs, alpha‐linolenic acid (omega‐3) and linoleic acid (omega‐6), are essential nutrients in humans.
Dietary fats have been implicated in cardiovascular health since Keys published his groundbreaking study linking plasma cholesterol and dietary saturated fat (Keys 1950), and Oliver reported higher levels of low density lipoprotein (LDL) in those surviving myocardial infarction compared to controls without myocardial infarction (Oliver 1953). In 1965 Hegsted published an equation that quantified the relationship between dietary fat and serum total cholesterol, suggesting that increasing saturated fats increased serum cholesterol, while increasing PUFA reduced serum cholesterol (Hegsted 1965). More recently there has been debate about what type of PUFA may be protective, with interest in omega‐3 PUFAs following randomised controlled trials (RCTs) with dietary fish and fish oil supplementation interventions in the 1980s and 1990s (Burr 1989; GISSI‐P 1999), although subsequent trials have been equivocal (Abdelhamid 2018; Hooper 2006). Similarly, while there are good theoretical grounds for suggesting that omega‐6 fats may be protective against cardiovascular diseases, the RCT evidence is limited (Hooper 2018). However, there is evidence that replacing saturated fats with PUFAs does protect against cardiovascular disease, and that PUFAs appear to be more protective than reducing saturated fats and replacing them with carbohydrates (Hooper 2015a). On the other hand, reducing dietary fat (including PUFAs) appears to result in lower weight in adults, suggesting that lower PUFA intake would tend to protect against cardiovascular disease (Hooper 2015b).
How the intervention might work
PUFAs are generally thought to work by producing a reduction in serum total cholesterol and LDL, which slows the progress of atherosclerosis (a complex syndrome in which plaque builds up inside the arteries over time, reducing blood flow and leading to an increased risk of blood clots), and so delays or prevents the onset of cardiovascular and cerebrovascular disease. This theory is reinforced by evidence that replacing saturated fats with polyunsaturated fats is associated with greater reductions in cardiovascular events and with greater reduction of serum total cholesterol (Hooper 2015a). Additional modes of action have been proposed for omega‐3 PUFAs (particularly EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) both fish‐based omega‐3 polyunsaturated fatty acids. These modes of action include: lowering of blood pressure; reducing thrombotic tendency; anti‐inflammatory and antiarrhythmic effects; improving vascular endothelial function; increasing plaque stability (through increased plaque calcification); and improving insulin sensitivity (Calder 2012; Ohwada 2016). Omega‐6 PUFAs may reflect the general lipid‐lowering effects of PUFAs, but there has been concern that high levels of omega‐6 intake can increase production of 2‐series prostaglandins and 4‐series leukotrienes compared with the 3‐series prostaglandins and 5‐series leukotrienes associated with omega‐3 intake. As the 2‐series prostaglandins and 4‐series leukotrienes exert a more potent pro‐inflammatory effect, omega‐6 could increase the risk of cardiovascular disease by promoting inflammation (Russo 2009).
Why it is important to do this review
The evidence on the health effects of total PUFA intake, which is the combination of omega‐3 and omega‐6 fats, is equivocal. As cardiovascular diseases are important determinants of health, that particularly burden the poorest people (WHO 2016), we need to understand the role of PUFAs to provide the best advice for individuals and populations about how to eat to reduce the risk of ill health. This assessment of health effects of total PUFA intake is needed alongside updated assessment of the effects of omega‐3 and omega‐6 fats (Hooper 2018; Abdelhamid 2018).
The World Health Organization (WHO) is currently updating its guidance on polyunsaturated fatty acid intake in adults and children. This new review was commissioned by WHO Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health in order to inform and contribute to the development of updated WHO recommendations. The results of this review including GRADE assessments were discussed and reviewed by the WHO NUGAG Subgroup on Diet and Health as part of their guideline development process. This is a new review and forms a set with Abdelhamid 2018 (assessing effects of omega‐3 fats), Hooper 2018 (assessing effects of omega‐6 fats), reviews of diabetes and glucose tolerance (Brown 2017), inflammatory bowel disease (IBD) (Thorpe 2017), cognition (Jimoh 2017), depression (Hanson 2017a), bone and muscle health (Abdelhamid 2017), and cancers (Hanson 2017b).
Objectives
To assess effects of increasing total PUFA intake on cardiovascular disease and all‐cause mortality, lipids and adiposity in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared higher with lower polyunsaturated fatty acid intakes and assessed effects over at least 12 months (12 months' continuous involvement). We included trials reported as full text, those published as abstracts only, as trials registry entries and unpublished data. We did not include cross‐over trials (unless we could use data from the first part of the cross‐over only), as this design is inappropriate for outcomes such as cardiovascular disease events or mortality, but included cluster‐randomised trials, as long as there were at least six clusters (to facilitate equivalence of the arms at baseline).
Types of participants
We included trials of adults (18 years of age and above). Included participants could be adults who were well, or with increased risk of cancer, those undergoing ‐ or who had undergone ‐ coronary artery bypass grafting or angioplasty, and those with current or previous cardiovascular disease, diabetes mellitus, rheumatoid arthritis, depression, cognitive impairment, or multiple sclerosis. We were interested in both primary and secondary prevention, so included people with or without a history of cardiovascular disease.
We excluded participants who were pregnant or acutely ill, and defined acute illness as including people with diagnosed current cancer, undergoing heart or renal transplantation, with HIV or AIDS, on haemodialysis, with immunoglobulin A (IgA) glomerulonephritis, or any other renal problem except diabetic nephropathy. Our reasoning was to exclude people with conditions that may affect the relationship between polyunsaturated fatty acids and cardiovascular disease events.
Where trials included some adults and some people under 18 years of age, then we included the trial if at least 90% of participants were aged 18 years or over at baseline, or where outcomes for adults could be separated from those for younger people.
Types of interventions
Eligible trials compared higher with lower total polyunsaturated fatty acid (PUFA) intakes. The intervention had to be dietary supplementation, or a provided diet, or advice on diet. The advice, foodstuffs or supplements had to aim to increase or decrease total PUFA intake, or a dietary component high in total PUFA intake such as vegetable oil, or, if no clear aim was stated (but implied, such as aiming to provide a 'heart health', 'reduced fat' or 'Mediterranean' diet), then the intervention had to achieve an increase or decrease of at least 10% of the baseline total PUFA level.
Supplementation had to be in oil or capsule form, or as foodstuffs provided, to be consumed by mouth (we excluded enteral and parenteral feeds, and enemas). Trials were included if they compared the effect of this intervention with usual diet, no advice, no supplementation or placebo (as appropriate) or with a lower PUFA intake.
We did not include trials if they included multiple risk factor intervention on lifestyle factors such as weight reduction, smoking or physical activity goals, or differential dietary interventions not involving dietary fats (such as advice to eat more fruit and vegetables, increase fibre, or take a vitamin supplement), except where that other intervention was a direct replacement for polyunsaturated fatty acids or the effect of the fat intervention could be separated out from the other interventions. Where a single intervention that increased PUFA intake (such as increasing walnuts, sunflower oil or a margarine) included additional nutrients (they all do) we included it, regardless of what nutrients were displaced. We interpreted this consistently across the review.
We made decisions on inclusion using the following decision tree:
Include if the trial aimed to increase total PUFA regardless of dose (or aimed to increase a combination of omega‐3 and omega‐6). If not then assess point 2.
Include if the trial provided within‐trial intervention and control group total PUFA intake data, and the difference was 10% or more of the control group total PUFA intake OR the difference was 10% or more of baseline total PUFA intake or an assumed baseline intake of 6% of energy (6% E) from total PUFA. The assumed baseline intake of 6% E from total PUFA was an average from the trials for which there were data, so we included trials that provided 0.6% E or above (or ≥ 1.33 g/d) more or less total PUFA to the intervention arm compared to control. If not then assess point 3.
Include if the trial provided within‐trial intervention and control group total PUFA intake aims, and the difference was 10% or more of the control group total PUFA intake OR the difference was 10% or more of baseline total PUFA intake or an assumed baseline intake of 6% E from total PUFA. Where intake information came from trial aims we looked for corroboration that there was a higher total PUFA intake in one arm than the other, using information on control group supplements or advice, body fat markers of total PUFA or serum total cholesterol. Where a suggested higher intake of PUFA in one arm by trial aims was contradicted by biomarker or total cholesterol data (assuming lower total cholesterol with higher PUFA) we excluded. We included trials that provided an additional total PUFA of 0.6% E or more, or 1.33 g/d or more to the intervention arm compared to control (taking into account PUFA content of placebo and excluding if placebo content was unclear). If no inclusion from point 3 then we excluded the trial.
We documented our reasoning over inclusion decisions in Characteristics of included studies (see 'Inclusion basis') and reasons for exclusion in Characteristics of excluded studies. We also ran sensitivity analyses on risk of bias from compliance (see Sensitivity analysis).
Types of outcome measures
Primary outcomes
Primary outcomes were:
all‐cause mortality;
coronary heart disease events: number of participants experiencing at least one myocardial infarction (fatal or non‐fatal) or angina;
stroke (number of participants experiencing an ischaemic and/or haemorrhagic stroke); and
major adverse cardiac and cerebrovascular events (MACCEs, used where we could assess the numbers of participants experiencing fatal or non‐fatal myocardial infarction, unstable angina or stroke).
Secondary outcomes
Secondary outcomes were all systematically reviewed. If any trial fulfilled the other inclusion criteria and reported a secondary outcome (even if no primary outcomes were reported) we included it. Secondary outcomes included:
cardiovascular mortality (deaths due to cardiovascular causes including myocardial infarction and stroke)
cardiovascular events (all available data on number of participants experiencing any of fatal and non‐fatal myocardial infarction, angina and/or stroke);
coronary heart disease mortality;
myocardial infarction;
sudden cardiac death;
atrial fibrillation (arrhythmias including atrial fibrillation, ventricular fibrillation and ventricular tachycardia);
angina;
heart failure;
Peripheral arterial disease (PAD);
revascularisation (participants experiencing angioplasty or coronary artery bypass grafting);
measures of adiposity (including body weight, body mass index (BMI), waist circumference, percentage body fat);
serum lipids (including total cholesterol, fasting triglycerides, high‐density lipoprotein (HDL) and low density lipoprotein (LDL)).
Tertiary outcomes
Tertiary outcomes (not formally systematically reviewed) included:
blood pressure (systolic and diastolic);
quality‐of‐life measures (such as feelings of health and time off work);
economic costs;
serious adverse events (all serious adverse events presented were collated but cancers, inflammatory bowel disease, neurocognitive outcomes such as dementia, diabetes, functional outcomes and depression are not reported here);
dropouts.
We included trials where data on any primary or secondary outcome were available in published reports or based on contact with trial authors. We collated data on tertiary outcomes where they were present in included trials. Data on cancers (Hanson 2017b), inflammatory bowel disease (Thorpe 2017), neurocognitive outcomes including dementia (Jimoh 2017), diabetes (Brown 2017), bone and muscle outcomes (Abdelhamid 2017) and depression (Hanson 2017a) are reported fully and systematically in associated reviews within this series, rather than a subset being presented within this review.
Where it was clear that no participants experienced a particular primary or secondary outcome (and the study had not collected data on other primary or secondary outcomes) we excluded the trial. For example, on exploration, a number of trial authors confirmed that no participants had died or experienced heart attacks in their trials; in the absence of other primary or secondary outcomes being recorded we excluded these from this review and noted them in the exclusion list. Their inclusion into the review would have swollen the size of the review without adding any useful data.
Key outcomes
When the WHO NUGAG Subgroup on Diet and Health requested this review they named the following as key outcomes to inform their planned dietary guidance:
all‐cause mortality;
cardiovascular disease mortality;
cardiovascular disease events
coronary heart disease mortality
coronary heart disease events
stroke
atrial fibrillation (arrhythmia)
serum lipids including total cholesterol, fasting triglycerides, HDL and LDL; and
measures of adiposity (body weight and BMI)
We were not able make all of these primary outcomes. However, because WHO NUGAG Subgroup on Diet and Health will use these outcomes to underpin guidance, we carried out sensitivity analyses, subgroup analyses and GRADE assessment of quality of evidence for them, even when they were not primary outcomes. All of these outcomes were formally systematically reviewed.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 27 April 2017 to identify reports of relevant randomised controlled trials:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library;
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 27 April 2017);
Embase Classic and Embase (Ovid, 1947 to 27 April 2017).
We adapted the search strategy for MEDLINE (Ovid) from the search strategy in Hooper 2018 and also used it to locate trials to update Hooper 2018. This complex strategy was adapted for use in the other databases (Appendix 1). We applied the Cochrane sensitivity and precision‐maximising RCT filter to MEDLINE (Ovid), and for Embase, we applied terms recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
As we were also running searches for, updating and extending, another existing Cochrane Review of the effects of omega‐3 fats on health outcomes (Abdelhamid 2018), and there was a great deal of overlap between the searches, the omega‐3 searches were also run to May 2017, using the same RCT filters (Appendix 2). The results of these searches were de‐duplicated with the results from the searches for this review and all the titles and abstracts assessed as a single set. We created a dataset of RCTs that compared higher versus lower omega‐6 fats, omega‐3 fats or total PUFA in adults with a duration of at least 6 months. We used this dataset as the wider trial pool from which to select included trials for all the systematic reviews in this series (Abdelhamid 2016; Abdelhamid 2017; Abdelhamid 2018; Brown 2017; Hanson 2017a; Hanson 2017b; Jimoh 2017; Hooper 2018; Thorpe 2017).
We searched two clinical trials registers, ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP, www.who.int/ictrp/en/) during September 2016 for registry entries for relevant completed and ongoing trials.
Searching other resources
We checked included trials of relevant systematic reviews, and wrote to authors of included studies for additional trials and trial data (including unpublished outcome data).
We attempted to obtain full‐text translations or evaluations of all relevant non‐English articles. Where these were not available we translated papers ourselves using our existing language skills and language translation software.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts identified by the searches and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. All review authors carried out screening. All articles coded for retrieval by either reviewer were collected in full text. We retrieved full‐text study reports/publications and two review authors independently screened the full text, assessed studies for inclusion, and identified and recorded reasons for exclusion of ineligible trials (LH and AA). We resolved any disagreement through discussion. Where a trial met our inclusion criteria with the exception that they did not report any relevant outcome, we wrote to the trial author to ask whether any relevant outcomes occurred. We excluded trials when no relevant primary or secondary outcome events had occurred and the trial had not collected any data on our primary or secondary continuous outcomes.
We identified and collated multiple reports of the same trial (as each trial, rather than each report, was the unit of interest in the review). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We developed a draft data collection form for collating study characteristics and outcome data, then all review authors piloted the form on a single included trial to standardise data extraction and improve the data extraction form. All review authors took part in data extraction. Two review authors each extracted the following characteristics from included trials, independently in duplicate:
bibliographic details;
trial registration database and number;
methods: trial design, total trial duration, details of any 'run in' period, number of study centres and location, trial setting, withdrawals, and trial dates;
participants: number randomised in each arm, number analysed in each arm, mean age, age range, gender, health status, cardiovascular disease risk and a brief description of participants. We categorised baseline cardiovascular risk as primary prevention (participants not included on the basis of having existing cardiovascular disease) and secondary prevention (participants included on the basis of existing cardiovascular disease, such as angina or a previous stroke or myocardial infarction);
interventions: intervention (including composition and dose of PUFA intake advised or supplement used), comparison, concomitant medications, and excluded medications;
outcomes: primary, secondary and tertiary outcomes specified in trial registry, data on outcomes reported in publications and by contact with authors, time points reported. We assessed dichotomous outcomes at the latest point of available follow‐up within the trial, while we assessed continuous outcomes at the latest point available in the trial (and after at least 12 months);
process data: intake data (mean and standard deviation (SD) of total PUFA, omega‐3, omega‐6, total fat, saturated fat, monounsaturated fat (MUFA), carbohydrate, protein, energy, alcohol and trans fat intake), biomarker data (erythrocyte, serum or adipose tissue fatty acid status data) and serum total cholesterol in intervention and control groups at latest point available during RCT;
study funding and notable conflicts of interest of trial authors.
We resolved disagreements between data extractions by consensus or by involving a third person (LH or AA). One review author (AA or LH) transferred data into the Review Manager 5 (RevMan 5) file (RevMan 2014). We double‐checked that data had been entered correctly from the agreed data extraction by comparing the data presented in the systematic review with data extraction (AA, JB, TJB or LH).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each trial, alongside data extraction, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). All review authors carried out data extraction and assessment of risk of bias. We resolved disagreements by discussion or by involving another author (LH or AA). We assessed the risk of bias according to the following domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias);
attention bias (another aspect of performance bias, where the intervention or control groups receive more time and/or attention from trial or health personnel during the trial); and
compliance (to be assessed as at low risk of bias regarding compliance, the higher PUFA arm had to demonstrate an increase in PUFA over control in a body biomarker (total PUFA had to be assessed by at least linoleic acid plus one or more further components of PUFA), or greater reduction in total cholesterol in the higher PUFA arm. Where lipid biomarker and total cholesterol contradicted each other we chose unclear.
other risk of bias
These are the domains of the Cochrane 'Risk of bias' tool, with the exceptions of attention bias and compliance, which were specific to our review and added after discussion with the WHO NUGAG Subgroup on Diet and Health. We followed recommendations in Higgins 2011a, recording funding data in the Characteristics of included studies but not using them as a separate issue for assessing risk of bias.
We graded each potential source of bias as high, low or unclear risk and provided trial details, a quote from the trial report, or both, together with a justification for our judgment in the 'Risk of bias' tables. We assessed summary risk of bias for each trial. Where information on risk of bias related to unpublished data or correspondence with a trial author, we noted it in the 'Risk of bias' tables. Further details of how we interpreted the risk of bias elements across trials are found in Table 3.
1. Risk of bias assessment ‐ detailed assessment methods.
| Risk of bias element | Criteria for low risk of bias | Criteria for unclear | Criteria for high risk of bias |
| Selection bias: random sequence generation | The trial authors needed to have described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. For example “the randomisation sequence was computer‐generated”. We allowed that a good method of randomisation was strongly implied if the trial authors discussed stratification and/or blocking. Therefore, if they were not explicit about their randomisation method but did describe stratification or blocking we assessed this as low risk. | The trial authors have not described their method in sufficient detail for the assessment of whether it would produce comparable groups. For example, the trial authors state “the trial was randomised” and provide no further information. | The randomisation method was assessed as not truly random, and may not produce comparable groups. |
| Selection bias: allocation concealment | The trial authors needed to have described the method used to conceal allocation sequence in sufficient detail to determine whether the allocations could have been foreseen in advance of, or during, enrolment. Good methods included putting allocation codes in opaque, sealed envelopes (ideally prepared by someone outside the treatment or assessment teams and sequentially numbered), using a telephone allocation system after the participants had consented to participate or providing a random number that links to a specific set of capsules prepared and distributed centrally or by an arms‐length pharmacist. | The authors gave insufficient detail as to method. | The allocation was known in advance of participants consenting to take part in the trial. |
| Performance bias: blinding of participants and personnel | The trial authors needed to have described all measures used, if any, to blind trial participants and personnel from knowledge of which intervention a participant received. Ideally, they should also have provided information relating to whether the intended blinding was effective. For example, the authors could say “both the intervention and placebo capsules looked and tasted the same.” However if the trial authors did not provide information on whether the blinding was effective, but sufficient detail was given on a good method of blinding, then it was assumed that the blinding was effective and the risk of bias was low. | Insufficient methodological details were provided e.g. “the trial was blinded.” | The trial was unblinded or where blinding was broken, e.g. “the capsules were visually identical but the participants reported a strong fishy flavour in the intervention group only.” |
| Detection bias: blinding of outcome assessment | Trial authors needed to have described measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Ideally, they should also have provided information relating to whether the intended blinding was effective. For example, the authors could say “the outcome assessors had no knowledge of the group allocation, and both the intervention and placebo capsules looked and tasted the same so the self‐assessment scales were also blinded.” However if the trial authors did not provide information on whether the blinding was effective, but sufficient detail was given on a good method of blinding of the assessors, then it was assumed that the blinding was effective and the risk of bias is low. All biochemical assessment (lipids, glucose, CRP, insulin, PSA etc.) were considered at low risk of detection bias if outcome assessor blinding or double blinding was stated. | Insufficient methodological details were provided e.g. “the trial was blinded.” | The trial was unblinded or blinding was broken, e.g. for a self‐assessment measure “the capsules were visually identical but the participants reported a strong fishy flavour in the intervention group only.” (Because the level of blinding could vary by outcome assessment of risk of bias was based on blinding of the review's primary outcome(s). Where primary outcomes had different assessments we opted for the higher risk of bias but noted that risk of bias was lower for other outcomes. |
| Attrition bias: incomplete outcome data | The trial authors needed to describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. They needed to report the number of attrition/exclusions, the numbers in each group at each time point, reasons for attrition/exclusion and any re‐inclusions in analyses. Ideally, they would report how they imputed any missing data e.g. last observation carried forward. There needed to be a reasonable balance of attrition/exclusions between trial arms and ≤ 20% of the sample should be lost over a year. | The trial authors didn't state reasons for attrition/exclusion, or were unclear about the numbers lost to attrition/exclusion in each trial arm. | The trial authors demonstrated a substantial difference in the rates of attrition/ exclusions between the trial arms and/or > 20% of the baseline sample was lost over a year (> 10% over 6 months). |
| Reporting bias: selective outcome reporting | The trial authors needed to have published their trial protocol or trials registry entry before the end of the trial’s recruitment period i.e. prospectively. They needed to have reported on all of the primary and secondary outcomes listed in the protocol/registry entry. Reporting additional secondary outcomes in the results paper(s), although not ideal, was deemed to still be low risk. | No trial protocol or trials registry entry was found, it was registered retrospectively, or the dates of registration and participant recruitment were unclear. | The trial authors did not report at least one primary or secondary outcome listed in the protocol/registry entry OR the results paper(s) reported a primary outcome that was not listed at all in the protocol or not listed as a primary outcome in the protocol. |
| Other sources of bias: Attention bias | The trial authors needed to have reported that participants in all trial arms received the same amount of attention and time from researchers and clinical teams. For example, “All participants attended the clinic for a baseline assessment which took 2 hours. They were then followed with monthly telephone calls, and finally attended for a 6 month assessment at the clinic which took 1 hour.” If the trial only differed by the content of the capsules, and the assessment schedule was not stated to differ between the two arms, it was assumed to be at low risk. | The trial authors did not state the attention each arm received. | Participants in different arms received different amounts of attention. For example, “The intervention group only attended for additional assessments at months 2, 4, and 6” or “the rates of relapse differed substantially between the groups which led to differing amounts of treatment time and attention,” or “the intervention group received a 40 minute dietary education session.” |
| Other sources of bias: limited compliance | The higher PUFA arm had to demonstrate an increase in PUFA fats over control in a body biomarker (total PUFA had to be assessed by at least LA plus one or more further components of PUFA), or greater reduction in TC in the higher PUFA arm. | Biomarker data not reported or not in a way that could be interpreted. Where lipid biomarker and TC contradicted each other we chose unclear. | Measures of compliance were reported but did not suggest higher total PUFA in the appropriate arm. |
| Other sources of bias: other | In the absence of any additional issues this item was coded "low risk of bias" | If fraud concerns had been raised and the paper had been withdrawn, or the trial author had been found guilty of fraud by a legal or medical entity the paper was excluded from the review. However if fraud concerns were raised, but the journal had not withdrawn the paper, and the trial author had not been formally sanctioned; then the trial was included in the review, but concerns were raised here, and the risk of bias for this item was high. |
LA: linoleic acid; PUFA: polyunsaturated fatty acids; TC: total cholesterol
Summary risk of bias
Schultz 1995 found that poorly concealed allocation was associated with a 40% greater effect size and so randomisation and allocation concealment are core issues for all trials. Lack of blinding is associated with bias, though smaller levels of bias than lack of allocation concealment (Savovic 2012), especially in trials with objectively measured outcomes (Wood 2008). Most of our outcomes were objectively measured. Although we originally planned to assess summary risk of bias in the same way across all trials in this Cochrane Review, the omega‐3 Cochrane Review and the omega‐6 Cochrane Review (Abdelhamid 2016; Abdelhamid 2018; Hooper 2018) we adopted a different approach after discussing the different nature of supplement trials compared to dietary advice or food provision trials with the NUGAG Subgroup on Diet and Health.
We considered a supplement or capsule‐type trial to be at low summary risk of bias, where we judged randomisation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessors adequate. We considered all other trials at moderate or high risk of bias (a single category).
We considered a dietary‐advice or all‐food‐provided‐type trial to be at low summary risk of bias, where we judged randomisation, allocation concealment, and blinding of outcome assessors adequate. We considered all other trials at moderate or high risk of bias (a single category).
Assessment of bias in conducting the systematic review
We conducted this Cochrane Review according to the published Cochrane protocol and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous data as mean difference (MD) with 95% CIs. We presented continuous data with a consistent direction of effect (as a smaller reading is generally positive), with the exception of HDL, where an increase is positive.
We used change data (change from baseline to latest point in trial in each arm) for continuous data where available with appropriate variance data. When change data were not available we used absolute data from the latest point in each trial arm, unless baseline data were too different between arms. (We considered baseline data too different to use when the change in both arms, from baseline to end data, was smaller than the baseline difference between arms). Where continuous data were too different to use this we noted it in the outcome section of Characteristics of included studies but we did not add data to meta‐analyses.
We intended narrative description of skewed data reported as medians (without variance data or with interquartile ranges). We added these data to forest plots so that there could be visual comparison of findings (though we did not include these data in meta‐analyses). We intended to use standardised mean differences (SMD) to combine data where included trials had used different scales to measure the same factor (such as quality of life). We did not find any such data, so did not use SMD. We converted data on different scales to the same scale, such as mg/dL and mmol/L for lipids.
Unit of analysis issues
Trials with multiple intervention groups
Where trials included more than two arms we assessed all arms for inclusion. Where there were more than one intervention arm and a single control arm we combined dichotomous and continuous data for the intervention arms and compared them to the single control arm. This meant there were no problems with trial participants appearing more than once in any forest plot.
Cluster‐RCTs
Where cluster‐RCTs were included we planned to account for unit of analysis issues by data extracting a direct estimate of the required effect measure (for example, a RR with its CI) from an analysis that accounted for the cluster design properly (for example, an analysis based on a ‘multilevel model’, a ‘variance components analysis’ or that used ‘generalised estimating equations (GEEs)’). Where these data were available we planned to use them in meta‐analysis using the generic inverse‐variance method (Deeks 2011). Where no such correct analysis of the cluster‐randomised data were available, we planned to use approximate analyses using intra‐cluster correlation co‐efficient (ICC) analysis as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b, section 16.3.4). We did not identify any such cluster‐randomised trials, so we did not need this methodology.
Dealing with missing data
We contacted (or attempted to contact) the authors of all potentially included RCTs to better assess inclusion. We contacted authors of all included trials that had randomised at least 100 participants (and some smaller trials) to request available data on all of the trial outcomes relevant to our set of reviews and key information on risk of bias. Due to limited resources, we focused on contacting authors of larger trials, who we thought were most likely to provide substantial quantities of useful data. We sent an email and a posted letter to the corresponding author at the latest address we were able to obtain (tracking latest publications in Medline). Where data on at least one review outcome were available (and at least one person had experienced a relevant outcome), we included the RCT, and asked the authors to provide any additional data about trial methodology or risk of bias.
Where papers reported continuous results as change from baseline we used these data, otherwise we used data at the latest point available. We did not impute change data.
Assessment of heterogeneity
We used the I2 statistic (Higgins 2003) to measure heterogeneity among the trials in each analysis. Where we identified substantial heterogeneity (assumed when I2 was greater than 50%, as 30% to 60% represents moderate heterogeneity and we were allowing for the varied dietary interventions included as well as potential dose effects) we reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
Where we were able to pool at least 10 trials, we created and examined a funnel plot to explore possible reporting biases for the primary outcomes (Sterne 2011).
We noted where we were aware of missing data. This occurred where trial methods noted that an outcome had been measured but those data had not been presented or had been presented but not by trial arm, where continuous data were unbalanced at baseline, or presented as medians or as means but without variance information.
Data synthesis
We undertook meta‐analyses only where we considered it to be meaningful, that is, where the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We carried out statistical analysis using RevMan 5 (RevMan 2014). We used a random‐effects model, as dietary interventions are complex and somewhat heterogeneous by their nature (more so than most medical treatments), but we compared the results of random‐effects and fixed‐effect meta‐analysis in sensitivity analyses. As the random‐effects model assigns more weight to smaller trials, it is more conservative and may lead to imprecise estimates of effect. We also carried out sensitivity analyses to assess the effects of methodological rigour (see Sensitivity analysis).
'Summary of findings' table
We created a 'Summary of findings' table for the primary outcomes:
all‐cause mortality;
coronary heart disease events;
stroke; and
MACCEs.
As WHO NUGAG Subgroup on Diet and Health required a specific set of key outcomes for their guidance, we created a second 'Summary of findings' table for the key outcomes not represented in the main 'Summary of findings' table:
cardiovascular mortality;
cardiovascular events;
coronary heart disease mortality;
atrial fibrillation;
measures of adiposity ‐ body weight;
measures of adiposity ‐ BMI; and
serum lipids (including total cholesterol, fasting triglycerides, HDL and LDL).
We used the five GRADE considerations (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the trials that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 (Higgins 2011a) and Chapter 12 (Schünemann 2011) of the Cochrane Handbook for Systematic Reviews of Interventions, and used GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of trials using footnotes and made comments to aid reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We explored the effects of PUFA intake on primary outcomes and key outcomes by performing exploratory subgroup analyses on:
total PUFA dose (and dose response: total PUFA dose < 1% E, 1% E to < 2% E; 2% E to < 5% E and ≥ 5% E where dose is the difference in total PUFA intake between intervention and control arms);
trial duration: trials with medium follow‐up (12 to 23 months), medium to long follow‐up (24 to 47 months), and long follow‐up (48 months or more);
baseline risk of cardiovascular disease (primary prevention, or secondary prevention);
baseline total PUFA intake (< 6% E from total PUFA, 6% E to < 11% E, and ≥ 11% E from total PUFA);
replacement of saturated fat, MUFA, carbohydrate and protein with total PUFA;
participants' sex (> 70% of the control group were men, > 70% of the control group were women, and mixed men and women);
participants' age (mean age in control group < 50 years, 50 to < 65 years and ≥ 65 years);
statin use (at least 50% of control group on statins versus fewer than 50% on statins); and
intervention type (dietary advice, supplements (capsules), supplemental foods and all foods provided, or any combination)
We also planned to subgroup by change in the omega‐3/omega‐6 fat ratio (assessing whether the intervention primarily increased omega‐3 fats (putting up the ratio) or omega‐6 fats (lowering the ratio)). However, in almost no trials did we have information allowing us to calculate the omega‐3/omega‐6 fat ratio, so we did not carry out this subgrouping.
The 6% E and 11% E cut‐offs for total PUFA were prespecified by WHO NUGAG Subgroup on Diet and Health, as their existing recommendations for PUFA intake were 6% E to 11% E in adults (WHO/FAO 2008).
We have not discussed differential effects of omega‐3 and omega‐6 PUFAs in this review, as separate reviews address the effects of omega‐3 and omega‐6 fats on cardiovascular disease in more detail (Hooper 2018; Abdelhamid 2018).
We used the formal test for subgroup interactions in RevMan 5 (RevMan 2014). These subgroupings were requested by WHO NUGAG Subgroup on Diet and Health to better help them understand the data. The danger of having so many subgroup analyses is that they may be over‐interpreted, increasing the risk of a type one error.
Meta‐regression
We planned meta‐regression to further explore effects of total PUFA dose (looking for evidence of dose response), baseline total PUFA intake and duration on dichotomous primary and secondary outcomes with at least seven included trials and for which subgrouping was undertaken. However baseline total PUFA intake was only clear in a handful of trials, so we did not run meta‐regression by baseline PUFA intake. Random‐effects meta‐regression (Berkley 1995) was performed using the STATA command metareg (Sharp 1998): log(e) relative risk versus [dose or primary/secondary prevention or type of intervention or risk of bias or duration], weighted by the standard error of the log(e) relative risk. Where there were no events in one arm we added 0.1 to the numbers for both groups (so a trial with 10 people experiencing stroke in one arm but none in the other arm would be entered as 10.1 and 0.1).
Sensitivity analysis
We planned to carry out the following sensitivity analyses on all primary outcomes, and key outcomes:
only including trials with a low risk of bias for allocation concealment;
only including trials with a low risk of attention bias;
only including trials with a low risk of bias from compliance;
only including trials at low summary risk of bias;
only including all trials up to 2010, plus trials post‐2010 that were registered in a trials register (Roberts 2015, regardless of the date of registration);
only including trials with no industry funding reported (trials with funding or support from partial bodies such as government boards to support specific foods or where funding was not mentioned were also excluded);
only including trials with less than 10% difference in intake of trans fats between trial arms during the intervention;
only including trials that randomised at least 100 participants;
only including trials that randomised at least 250 participants;
using fixed‐effect meta‐analysis.
Unfortunately almost no data on trans fats were available, so we did not carry out sensitivity analysis around trans fats.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included trials for this review. Outcome data were interpreted as follows:
Is there an effect? (Options were ‘increased risk’, ‘decreased risk’, or ‘little or no effect’). Our main outcome measures were RR and MD so we decided on existence of an effect using RR. RR >8% (RR <0.92 or >1.08) for the highest quality evidence suggested increased or decreased risk (otherwise little or no effect). The presence or not of an effect was decided on the RR for the main analysis and sensitivity analyses.
For continuous outcomes increasing PUFA was considered to have little or no effect unless effect sizes were at least 5% of baseline (or 2% in the case of cumulative outcomes such as adiposity).
Quality of evidence was assessed using GRADE assessment (GRADE Working Group 2004) for key outcomes. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to the trials that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), plus GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of trials using footnotes and made comments to aid reader's understanding of the review.
Where there was a suggested effect the size of effect was assessed using the MD, NNT or ARR.
We avoided making recommendations for practice and our implications for research suggest priorities for future research and outline the remaining uncertainties in the area.
Results
Description of studies
Results of the search
The electronic searches for the full set of reviews (populating the dataset of all trials that assessed effects of higher versus lower omega‐6, omega‐3 or PUFA over at least 6 months) generated 37,810 titles and abstracts, which we de‐duplicated to 19,772 hits. We assessed these along with 53 studies previously included from Hooper 2018 and Abdelhamid 2018, to reassess for inclusion; 986 potentially relevant trials registry entries; and 35 new references gained from systematic review reference lists. In total, we assessed 20,846 titles and abstracts in duplicate to decide whether to retrieve full texts. We ultimately assessed 2155 full‐text reports, of which 226 were systematic reviews. Two review authors independently assessed the remaining 1929 papers for inclusion and grouped them into studies. Of these, we included 364 RCTs in a wider database of trials that underpinned the full set of reviews (this review and several others including Abdelhamid 2018; Abdelhamid 2017; Hooper 2018; Brown 2017; Hanson 2017a; Hanson 2017b; Jimoh 2017; Thorpe 2017). This wider set of trials included RCTs of omega‐3, omega‐6 or total polyunsaturated fatty acids (PUFA) interventions with a duration of at least six months (Figure 1) and comprised 1020 reports (papers, abstracts and trials registry entries), plus additional data from 121 authors.
1.
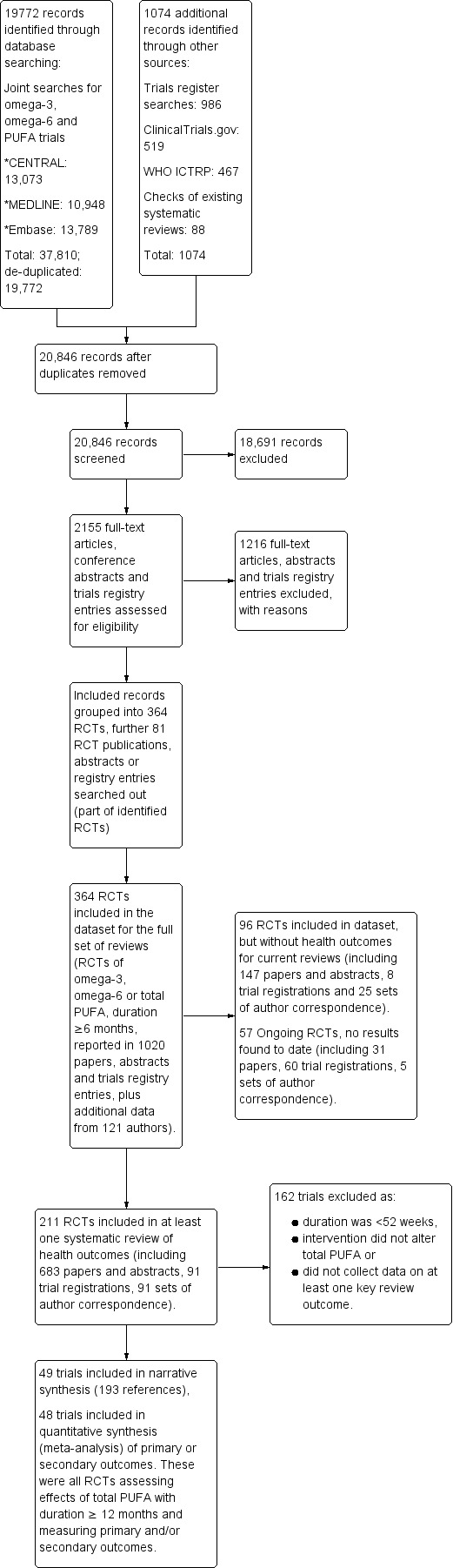
Study flow diagram.
Of these 364 RCTs:
22 RCTs (26 documents) assessed effects of PUFA over at least one year but were ongoing (without published outcome data);
293 RCTs (811 documents) did not assess effects of a high enough dose of PUFA, did not assess relevant outcomes or had a duration of less than one year, so we excluded them from this review; and
49 RCTs (183 documents) were eligible for inclusion in this review.
Of these 49 included RCTs, 48 were included in meta‐analyses.
Details of the flow of trials are in Figure 1.
The 22 potential ongoing trials are described in the table of Characteristics of ongoing studies. These trials are very difficult to assess for inclusion in terms of total PUFA dose until further details are published. We will formally assess these trials for inclusion when we update this review.
Included studies
The details of the methods, participants, intervention, comparison group, and outcome measures for each of the included trials are shown in the Characteristics of included studies table. Forty‐nine trials, including 24,272 randomised participants, met the inclusion criteria. Trials ranged in size from 36 randomised participants (Rossing 1996) to 4997 randomised participants (PREDIMED 2013), with 15 trials randomising at least 250 participants (AlphaOmega ‐ ALA; Bates 1989; DART fat 1989; EPIC‐1 2008; EPIC‐2 2008; EPOCH 2011; FAAT ‐ Leaf 2005; MRC 1968; NDHS Open 1st 1968; ORL 2013; PREDIMED 2013; Sydney Diet‐Heart 1978; Veterans Admin 1969; WAHA ‐ Ros 2016; WINS 2006).
Twenty‐two trials recruited mostly men (at least 70% men in the control group, Ahn 2016; AlphaOmega ‐ ALA; DART fat 1989; DIPP‐Tokudome 2015; Doi 2014; Dullaart 1992; FAAT ‐ Leaf 2005; GLAMT 1993; HARP‐ Sacks 1995; Kumar 2012; Ley 2004; Mendis 2001; MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; Nodari 2011 HF; Nye 1990; ORL 2013; Raitt 2005; Sydney Diet‐Heart 1978; Veterans Admin 1969; Vijayakumar 2014), six trials recruited mostly women (at least 70% women in the control group, Bassey 2000‐Post; Bassey 2000‐Pre; Dodin 2005; Proudman 2015; Simon 1997; WINS 2006), 16 recruited similar numbers of men and women while five trials did not state the sex or participants (Bates 1977; EPOCH 2011; HERO‐Tapsell 2009; McIllmurray 1987; Rose 1965).
Almost half of the trials (24 trials) recruited participants with a mean age between 50 and 65 years, but 12 trials recruited younger participants (mean age < 50 years, Bassey 2000‐Pre; Bates 1978; Bates 1989; Dullaart 1992; EPIC‐1 2008; EPIC‐2 2008; NDHS Faribault 1968; NDHS Open 1st 1968; Puri 2005; Rossing 1996; Simon 1997; Sydney Diet‐Heart 1978), eight trials recruited older participants (mean age 65 years or more, AlphaOmega ‐ ALA; Doi 2014; FAAT ‐ Leaf 2005; Kumar 2013; Nodari 2011 AF; PREDIMED 2013; Veterans Admin 1969; WAHA ‐ Ros 2016), and five trials did not give a mean age or range that could be classified (Ahn 2016; Bates 1977; EPOCH 2011; Houtsmuller 1979; Mendis 2001).
Twenty trials were conducted in Europe (AlphaOmega ‐ ALA; Bassey 2000‐Post; Bassey 2000‐Pre; Bates 1977; Bates 1978; Bates 1989; Brox 2001; DART fat 1989; Dullaart 1992; GLAMT 1993; Houtsmuller 1979; MARINA ‐ Sanders 2011; McIllmurray 1987; MRC 1968; Nodari 2011 AF; Nodari 2011 HF; PREDIMED 2013; Rose 1965; Rossing 1996; WELCOME 2015), 10 in North America (Black 1994; Dodin 2005; FAAT ‐ Leaf 2005; HARP‐ Sacks 1995; NDHS Faribault 1968; NDHS Open 1st 1968; Raitt 2005; Simon 1997; Veterans Admin 1969; WINS 2006), seven in Asia (Ahn 2016; DIPP‐Tokudome 2015; Doi 2014; Mendis 2001; Mita 2007; ORL 2013; Vijayakumar 2014), eight in Australia or New Zealand (EPOCH 2011; HERO‐Tapsell 2009; Kumar 2012; Kumar 2013; Ley 2004; Nye 1990; Proudman 2015; Sydney Diet‐Heart 1978), while four trials were conducted across several continents (EPIC‐1 2008; EPIC‐2 2008; Puri 2005; WAHA ‐ Ros 2016).
The trials varied in the types of participants recruited and their level of cardiovascular risk. Most trials recruited participants without a personal history of cardiovascular disease (primary prevention), but 16 recruited participants with existing cardiovascular disease of some sort (secondary prevention of cardiovascular disease, Ahn 2016; AlphaOmega ‐ ALA; DART fat 1989; Doi 2014; FAAT ‐ Leaf 2005; HARP‐ Sacks 1995; Kumar 2012; Kumar 2013; MRC 1968; Nodari 2011 AF; Nodari 2011 HF; Nye 1990; Raitt 2005; Rose 1965; Sydney Diet‐Heart 1978; Vijayakumar 2014).
Total PUFA dose (the difference in total PUFA between intervention and control arms) was between 0.6% E and less than 1% E for 13 trials (Doi 2014; EPOCH 2011; FAAT ‐ Leaf 2005; Kumar 2012; Kumar 2013; Ley 2004; MARINA ‐ Sanders 2011; Mita 2007; Nodari 2011 AF; Nodari 2011 HF; ORL 2013; Puri 2005; Raitt 2005), 1% E to less than 2% E total PUFA in 17 trials (Ahn 2016; AlphaOmega ‐ ALA; Bassey 2000‐Post; Bassey 2000‐Pre; Bates 1977; Bates 1978; Bates 1989; Brox 2001; DIPP‐Tokudome 2015; Dodin 2005; EPIC‐1 2008; EPIC‐2 2008; Nye 1990; PREDIMED 2013; Proudman 2015; WELCOME 2015; WINS 2006), 2% E to less than 5% E in eight trials (Black 1994; DART fat 1989; Dullaart 1992; GLAMT 1993; HARP‐ Sacks 1995; McIllmurray 1987; Mendis 2001; Rossing 1996), and at least 5% E from total PUFA in 11 trials (HERO‐Tapsell 2009; Houtsmuller 1979; MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; Rose 1965; Simon 1997; Sydney Diet‐Heart 1978; Veterans Admin 1969; Vijayakumar 2014; WAHA ‐ Ros 2016).
Increases in total PUFA were delivered to participants in various ways. Most trials gave supplemental capsules or foods taken as supplements (supplemental oil drunk with meals in Rose 1965, seal or cod liver oil drunk in Brox 2001 and flax seed incorporated into foods in Dodin 2005), while eight trials gave dietary advice resulting in increased PUFA (Black 1994; DART fat 1989; Dullaart 1992; Houtsmuller 1979; Ley 2004; Simon 1997; Sydney Diet‐Heart 1978; WINS 2006), eight trials gave supplemental foods such as margarines or nuts (AlphaOmega ‐ ALA; HERO‐Tapsell 2009; NDHS Faribault 1968; NDHS Open 1st 1968; PREDIMED 2013; Veterans Admin 1969; Vijayakumar 2014; WAHA ‐ Ros 2016), and three trials used a combination of methods (DIPP‐Tokudome 2015; Mendis 2001; MRC 1968).
Baseline total PUFA intake was unclear in most trials, but where information was provided it ranged from 3.9% E (NDHS Open 1st 1968) to 8% E (Black 1994) in control groups. Seven trials had baseline total PUFA intake less than 6% E (Dodin 2005; HERO‐Tapsell 2009; Ley 2004; NDHS Faribault 1968; NDHS Open 1st 1968; Veterans Admin 1969; WINS 2006), while nine had baselines of at least 6% E PUFA (Black 1994; DART fat 1989; DIPP‐Tokudome 2015; Dullaart 1992; MARINA ‐ Sanders 2011; PREDIMED 2013; Simon 1997; Sydney Diet‐Heart 1978; WAHA ‐ Ros 2016). PUFA replaced saturated fat at least partially in nine trials (DART fat 1989; Dullaart 1992; HARP‐ Sacks 1995; MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; Sydney Diet‐Heart 1978; Veterans Admin 1969; Vijayakumar 2014), replaced monounsaturated fats in 21 trials (AlphaOmega ‐ ALA; Bates 1977; Bates 1978; Bates 1989; EPOCH 2011; FAAT ‐ Leaf 2005; HARP‐ Sacks 1995; MARINA ‐ Sanders 2011; NDHS Faribault 1968; NDHS Open 1st 1968; Nodari 2011 AF; Nodari 2011 HF; Nye 1990; PREDIMED 2013; Proudman 2015; Raitt 2005; Rose 1965; Rossing 1996; Sydney Diet‐Heart 1978; Veterans Admin 1969; WELCOME 2015), replaced carbohydrate in 11 trials (Black 1994; DIPP‐Tokudome 2015; Dodin 2005; Houtsmuller 1979; Ley 2004; MARINA ‐ Sanders 2011; Mendis 2001; Rose 1965; Simon 1997; WAHA ‐ Ros 2016; WINS 2006), and replaced protein at least partially in four trials (HERO‐Tapsell 2009; Ley 2004; MRC 1968; WAHA ‐ Ros 2016). In some trials PUFA replaced several dietary components, in others there was one main replacement, but replacements were unclear for 14 trials (Ahn 2016; Bassey 2000‐Post; Bassey 2000‐Pre; Brox 2001; Doi 2014; GLAMT 1993; Kumar 2012; Kumar 2013; EPIC‐1 2008; EPIC‐2 2008; McIllmurray 1987; Mita 2007; ORL 2013; Puri 2005).
In most trials fewer than 50% of participants in the control group were taking statins (assumed in trials published before 1994 when the 4S Trial 1994 was published showing overall benefits from statins in higher‐risk populations and statin use began to rise, and in populations not at particular cardiovascular disease risk), but in seven trials at least 50% of participants were taking statins (Ahn 2016; AlphaOmega ‐ ALA; Doi 2014; HERO‐Tapsell 2009; Kumar 2013; Vijayakumar 2014; WELCOME 2015), and three trials were unclear (FAAT ‐ Leaf 2005; Ley 2004; WAHA ‐ Ros 2016).
The duration of the intervention was one to less than two years in most trials, but was two to less than four years in 16 trials (AlphaOmega ‐ ALA; Bates 1977; Bates 1978; Bates 1989; Black 1994; DART fat 1989; DIPP‐Tokudome 2015; Dullaart 1992; HARP‐ Sacks 1995; McIllmurray 1987; Mita 2007; Raitt 2005; Rose 1965; Simon 1997; Vijayakumar 2014; WAHA ‐ Ros 2016), and four years or more in duration in six trials (Houtsmuller 1979; MRC 1968; PREDIMED 2013; Sydney Diet‐Heart 1978; Veterans Admin 1969; WINS 2006).
Included trials were published over half a century between the 1960s (Rose 1965; MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; Veterans Admin 1969) and the 2010s (Ahn 2016; AlphaOmega ‐ ALA; DIPP‐Tokudome 2015; Doi 2014; EPOCH 2011; Kumar 2012; Kumar 2013; MARINA ‐ Sanders 2011; Nodari 2011 AF; Nodari 2011 HF; ORL 2013; PREDIMED 2013; Proudman 2015; Vijayakumar 2014; WAHA ‐ Ros 2016; WELCOME 2015), with some trials published in each decade.
Funding sources were reported and appeared to be purely from national or charitable agencies in 17 trials (Ahn 2016; Black 1994; Brox 2001; DIPP‐Tokudome 2015; Dullaart 1992; FAAT ‐ Leaf 2005; Houtsmuller 1979; Ley 2004; MARINA ‐ Sanders 2011; Mendis 2001; MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; Nodari 2011 AF; Sydney Diet‐Heart 1978; Vijayakumar 2014; WINS 2006). Seven trials appeared to be directly funded by industrial sources (Bassey 2000‐Post; Bassey 2000‐Pre; EPIC‐1 2008; EPIC‐2 2008; GLAMT 1993; ORL 2013; Puri 2005), two funded by bodies set up to promote specific foods (HERO‐Tapsell 2009; WAHA ‐ Ros 2016), 16 trials funded by some governmental or charity sources with additional funding or support from commercial sources (AlphaOmega ‐ ALA; Bates 1977; Bates 1978; Bates 1989; DART fat 1989; EPOCH 2011; HARP‐ Sacks 1995; Kumar 2012; Nye 1990; PREDIMED 2013; Proudman 2015; Raitt 2005; Rossing 1996; Simon 1997; Veterans Admin 1969; WELCOME 2015), two trials that included authors on industry honoraria (Doi 2014; Nodari 2011 HF), and five trials where funding was not reported (Dodin 2005; Kumar 2013; McIllmurray 1987; Mita 2007; Rose 1965).
Most included trials had a single intervention arm and a single control arm, but some trials were more complex.
Bates 1977 had four arms, two intervention arms each had their own control arm, so were dealt with as separate trials. Both were included, as deaths appear to have occurred, but it is no longer clear how many or which arms they occurred in.
Bates 1978 also had two intervention arms each with their own control arm, but comparison C versus D did not have any relevant outcome data so we excluded it. We only included A versus B.
Brox 2001 had two intervention arms and one control arm. For all outcomes, we combined the two intervention groups and compared to the single control group.
DART fat 1989 was a factorial trial, but we have included only one of the three factorial interventions in this review, so all participants have been included only once.
MARINA ‐ Sanders 2011 had three intervention arms of different doses and one control arm. Only one intervention arm was included in this review (D2) and compared to the control arm.
NDHS Faribault 1968 and NDHS Open 1st 1968 each had three intervention arms and a single control. We combined data for the three arms and compared them to the single control arm in each trial.
Nye 1990 had three arms, but one was irrelevant to this review so not included.
ORL 2013 had three arms, but we only included two arms (higher vs lower dose omega‐3)
PREDIMED 2013 had three arms, a Mediterranean diet with nuts, a Mediterranean diet with olive oil and a low‐fat arm. For this review we compared the Mediterranean diet with nuts (high PUFA) with the Mediterranean diet with olive oil (low PUFA) as these two arms were very similar but with different PUFA intakes. For many outcomes data were reported in publications by trial centre (or combination of trial centres), so we checked for overlap of participants then reported the outcome centre by centre where we were sure that no participants were included more than once.
Excluded studies
We have presented details and reasons for exclusion of the trials that most closely missed the inclusion criteria in the Characteristics of excluded studies table.
Risk of bias in included studies
Our assessment of risk of bias of included trials is summarised in Figure 2 and detailed by trial in Figure 3. We assessed eleven of the 49 included trials as being at low summary risk of bias; eight trials as being at low risk of bias from randomisation, allocation concealment, performance and detection biases (AlphaOmega ‐ ALA; EPOCH 2011; MARINA ‐ Sanders 2011; NDHS Faribault 1968; NDHS Open 1st 1968; Proudman 2015; Puri 2005; WELCOME 2015), and three trials, which were dietary advice or provision trials, as being at low risk of bias from randomisation, allocation concealment and detection bias (Ley 2004; Sydney Diet‐Heart 1978; WINS 2006). We assessed the remaining 37 trials as being at moderate or high risk of bias.
2.
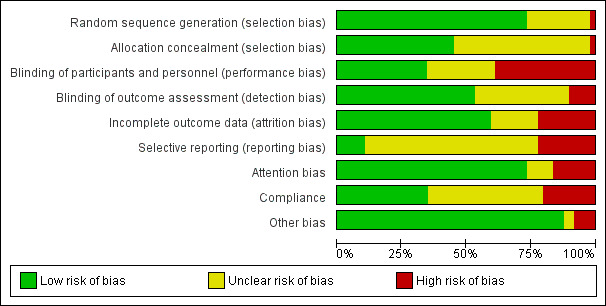
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
3.
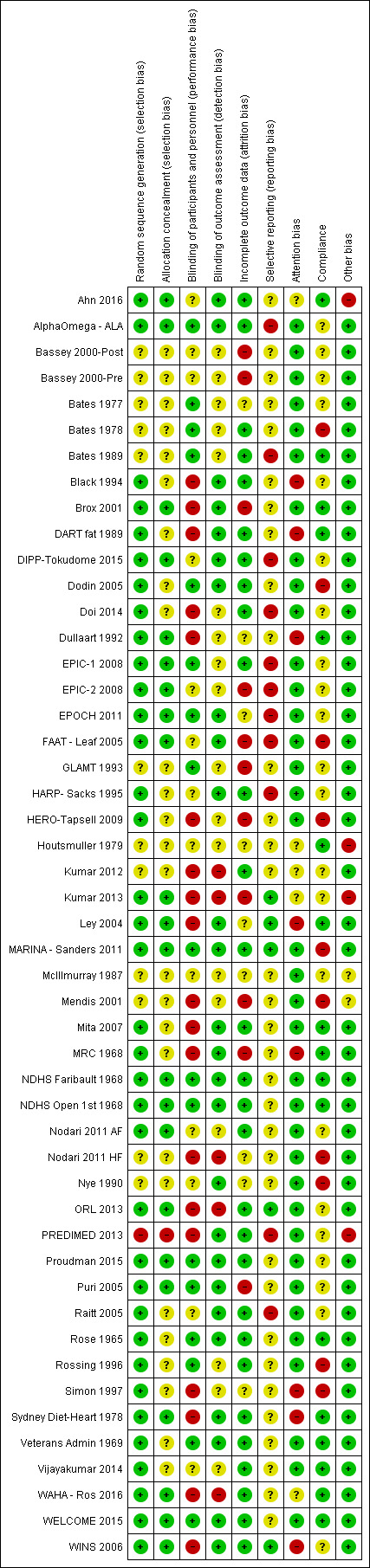
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
Allocation
Randomisation was adequate in 36 of the 49 trials, not well described in 12 trials and at high risk in one trial (PREDIMED 2013). Allocation concealment was appropriate in 22 included trials, unclear in 26, and at high risk of bias in one (PREDIMED 2013). Twenty‐two trials were at low risk of selection bias, with low risk of bias from both randomisation and allocation concealment (see Figure 3).
Blinding
Blinding of participants and personnel appeared at low risk of bias for 17 trials, unclear for 13 and at high risk of bias for the remaining 19 trials. Blinding of outcome assessors was at low risk of bias in 26 trials, unclear in 18 and at high risk of bias in five trials. Eleven trials were well blinded, at low risk of bias from both blinding of participants, personnel and outcome assessors.
Incomplete outcome data
Twenty‐nine trials appeared to be at low risk of attrition bias, 11 were at high risk and the remainder unclear.
Selective reporting
We found five trials that had a trials registry entry or protocol published before data collection was completed, and reported all outcomes suggested in the entry or protocol. Thirty‐three were unclear, generally because no trials registry entry or protocol was identified, or because they were published after the end of data collection. We found 11 trials were at high risk of selective reporting, as at least one outcome suggested in the trials registry entry or protocol was not reported in full.
We attempted to access additional outcome data as well as methodological data from most included trials. We established contact with most trial authors, and received data on outcomes that had not been fully published from many (noted in Characteristics of included studies for relevant trials), although some trial authors were unable to provide additional information or repeated phrases from their published papers. We tried to contact, but did not receive any reply from, authors of 10 trials (Ahn 2016; Doi 2014; GLAMT 1993; Houtsmuller 1979; Kumar 2012; Kumar 2013; Mendis 2001; Nodari 2011 AF; ORL 2013; Raitt 2005). We did not attempt to contact authors of some of the oldest trials, as the trials were conducted in the 1960s and their authors were unlikely to be accessible (NDHS Faribault 1968; NDHS Open 1st 1968; Rose 1965; Veterans Admin 1969), although we had made contact with the retired statistician of another older trial when including that trial in an earlier systematic review (MRC 1968). We did not attempt to contact authors of five trials (Bassey 2000‐Post; Bassey 2000‐Pre; HERO‐Tapsell 2009; Mita 2007; Nye 1990).
Other potential sources of bias
We assessed attention bias, where intervention participants appeared to receive more time or attention from health professionals than those in the control group. Thirty‐six trials appeared to be at low risk of attention bias, eight were at high risk, and the remaining five were unclear.
We assessed compliance, to ensure that PUFA truly appeared to have been higher in one arm than the other, by looking for evidence of changes or differences in a body biomarker (total PUFA had to be assessed by at least linoleic acid (LA) plus one or more further components of PUFA), or greater reduction in total cholesterol in the higher PUFA arm. Where lipid biomarker and total cholesterol contradicted each other we chose unclear. We found that 17 trials demonstrated appropriate compliance, 10 suggested poor compliance while 22 trials were unclear.
Four trials were found to be at high risk from other potential bias. Ahn 2016 was unclear about whether the control arm received a placebo or not, and some SDs appeared to be incorrectly reported. When we looked for additional data on Houtsmuller 1979 we found that concerns had been raised over potential research fraud of the first author in later trials (assessing effects of diet on cancer). While no concerns were found about the included research we felt that this did potentially reflect a risk of fraud in the included trial. In Kumar 2013, 21 of the 39 participants randomised to the intervention were inexplicably crossed over to the control condition at six months, so that 12‐month outcomes were only reported for 17 of the 39 randomised participants. The main publication of PREDIMED 2013 was retracted and republished in 2018 due to randomisation and allocation concealment problems not mentioned in the initial publication that resulted in a distribution of baseline variables inconsistent with randomisation (Carlisle 2017).
We found McIllmurray 1987 and Mendis 2001 to be at unclear risk of other bias, as neither described their control group interventions. The remaining trials were considered to be at low risk of other potential bias.
Effects of interventions
Summary of findings for the main comparison. Higher polyunsaturated fatty acid (PUFA) compared to lower PUFA for cardiovascular disease ‐ primary outcomes.
| Higher PUFA compared to lower PUFA for CVD | ||||||
|
Patient or population: people with or without existing CVD, men and women
Setting: includes free‐living participants and those living in institutions. Includes participants from all continents but most events occurred in trials carried out in Europe or North America.
Intervention: higher PUFA intake
Comparison: lower PUFA intake Eligible trials compared higher with lower total PUFA intakes. The intervention had to be dietary supplementation, or a provided diet, or advice on diet. The advice, foodstuffs or supplements had to aim to increase or decrease total PUFA intake, or a dietary component high in total PUFA intake such as vegetable oil, or, if no clear aim was stated (but implied, such as aiming to provide a 'heart health', 'reduced fat' or 'Mediterranean' diet) then the intervention had to achieve an increase or decrease of at least 10% of the baseline total PUFA level. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lower PUFA | Risk with higher PUFA | |||||
| All‐cause mortality Follow‐up: range 12 months to 96 months | No CVD at baseline (primary prevention) | RR 0.98 (0.89 to 1.07) | 19,290 (24 RCTs) | ⊕⊕⊕⊝ Moderatea | Increasing PUFA intake probably has little or no effect on all‐cause mortality (risk alters from 7.8% to 7.6% in the overall study population), moderate‐quality evidence | |
| 34 per 1000 | 33 per 1000 (27 to 41) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 117 per 1000 | 115 per 1000 (101 to 131) | |||||
|
Coronary heart disease events Follow‐up: range 12 months to 96 months |
No CVD at baseline (primary prevention) | RR 0.87 (0.72 to 1.06) | 10,076 (15 RCTs) | ⊕⊕⊕⊝ Moderateb | Increasing PUFA intake may reduce risk of CHD events (from 14.2% to 12.3% in the study population, NNT = 53), moderate‐quality evidence. | |
| 134 per 1000 | 71 per 1000 (34 to 149) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 143 per 1000 | 137 per 1000 (122 to 156) | |||||
| Stroke Follow‐up: range 12 months to 96 months | No CVD at baseline (primary prevention) | RR 0.91 (0.58 to 1.44) | 14,742 (11 RCTs) | ⊕⊕⊝⊝ Lowc | Increasing PUFA intake may reduce risk of stroke (from 1.2% to 1.1% in the study population, NNT= 1000), low‐quality evidence. However, the 95% confidence intervals include important harms as well as benefit. | |
| 21 per 1000 | 15 per 1000 (10 to 24) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 5 per 1000 | 6 per 1000 (3 to 13) | |||||
| Major adverse cardiac and cerebrovascular events Follow‐up: range 24 months to 96 months | No CVD at baseline (primary prevention) | RR 0.84 (0.59 to 1.20) | 2879 (2 RCTs) | ⊕⊝⊝⊝ Very lowd | Effects of increasing PUFA on MACCEs are unclear as the evidence is of very low quality. | |
| 206 per 1000 | 142 per 1000 (105 to 192) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 332 per 1000 | 329 per 1000 (289 to 372) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CVD: cardiovascular disease; OR: odds ratio; PUFA: polyunsaturated fatty acids; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAll‐cause mortality
- Risk of bias: effect size did not alter when restricted to trials at low summary risk of bias, low risk of bias from allocation, attention or compliance. Not downgraded.
- Inconsistency: consistent effects, I2 statistic less than 50%. Not downgraded.
- Indirectness: most data came from trials of men, but some were from trials of women or men and women combined. Most events occurred in older participants, but events also occurred in younger and middle‐aged participants. Included trials were from all continents but most events occurred in trials carried out in Europe or North America. Not downgraded.
- Imprecision: over 1400 events occurred in trials including over 19,000 participants over at least 12 months. However, 95% CI included important benefits. Downgraded once.
- Publication bias: funnel plot did not suggest small study bias, we are aware of few events that could not be added to the meta‐analysis. Not downgraded.
bCoronary heart disease events
- Risk of bias: sensitivity analyses restricting trials to low risk of bias for attention and compliance give similar results to the main analysis, as do restricting to trials without industry funding or pre‐2010 and trials on trials registries, and larger trials all confirmed a small beneficial effect on coronary heart disease (CHD) events. However, limiting to trials at low risk of bias from allocation concealment and to trials of low summary risk of bias suggest increased CHD risk with more PUFA, making us less certain of the effect of increasing PUFA on this outcome. It was further noted by the WHO NUGAG Subgroup on Diet and Health that although limiting to trials at low risk of bias from allocation concealment and to trials of low summary risk of bias suggest increased CHD risk with more PUFA, results of the most heavily weighted trial are consistent with results of the main analysis, while the next largest trial differs from the main result; therefore, confidence in the results of these analyses is low and the outcome was not downgraded. Not downgraded, but part of the downgrading for imprecision was for risk of bias.
- Inconsistency: consistent effects, I2 statistic less than 50%. Not downgraded.
- Indirectness: most events occurred in men, and in high‐income countries. Not downgraded.
- Imprecision: the 95% confidence intervals did not exclude harm from increased PUFA. Downgraded once (with risk of bias).
- Publication bias: funnel plot did not suggest small study bias, we are aware of few events that could not be added to the meta‐analysis. Not downgraded.
cStroke
- Risk of bias: some sensitivity analyses suggested benefit of increased PUFA, some suggested harm or little effect. It was further noted by the WHO NUGAG Subgroup on Diet and Health that in most analyses, the most heavily weighted trials were consistent with the main results, and the outcome was therefore not downgraded. Not downgraded, but part of the downgrading for imprecision was for risk of bias.
- Inconsistency: consistent effects, I2 statistic less than 50%. Not downgraded.
- Indirectness: most events occurred in men, and in high‐income countries. Not downgraded.
- Imprecision: with only 166 participants experiencing a stroke imprecision was high, the 95% confidence intervals did not exclude important harm from increased PUFA. Downgraded twice (with risk of bias).
- Publication bias: funnel plot did not suggest small study bias, we are aware of few events that could not be added to the meta‐analysis. Not downgraded.
dMajor adverse cardiac and cerebrovascular events (MACCEs)
- Risk of bias: neither of the included trials were at low risk from allocation concealment, or at low summary risk of bias. Downgraded once.
- Inconsistency: I2 statistic = 79%. Downgraded once.
- Indirectness: all participants of the included trials were men, and trials were conducted in Europe and North America. Not downgraded.
- Imprecision: 817 people experienced MACCEs, although harm was not excluded by the 95% CI. Downgraded once.
- Publication bias: not possible to assess with only 2 trials. Not downgraded.
Summary of findings 2. Higher polyunsaturated fatty acid (PUFA) compared to lower PUFA for cardiovascular disease ‐ additional key outcomes.
| Higher PUFA compared to lower PUFA ‐ dichotomous secondary outcomes for prevention of cardiovascular disease | ||||||
|
Patient or population: people with or without existing cardiovascular disease, men and women
Setting: includes free‐living participants and those living in institutions. Includes participants from all continents but most events and assessments occurred in trials carried out in Europe or North America.
Intervention: higher PUFA intake
Comparison: lower PUFA intake Eligible trials compared higher with lower total PUFA intakes. The intervention had to be dietary supplementation, or a provided diet, or advice on diet. The advice, foodstuffs or supplements had to aim to increase or decrease total PUFA intake, or a dietary component high in total PUFA intake such as vegetable oil, or, if no clear aim was stated (but implied, such as aiming to provide a 'heart health', 'reduced fat' or 'Mediterranean' diet) then the intervention had to achieve an increase or decrease of at least 10% of the baseline total PUFA level. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lower PUFA | Risk with higher PUFA | |||||
| Cardiovascular mortality Follow‐up: range 12 months to 96 months | No CVD at baseline (primary prevention) | RR 1.02 (0.82 to 1.26) | 15,107 (16 RCTs) | ⊕⊕⊝⊝ Lowa | Increasing PUFA intake may have little or no effect on cardiovascular mortality (risk alters from 4.8% to 4.9% in the study population), low‐quality evidence. | |
| 36 per 1000 | 31 per 1000 (19 to 50) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 57 per 1000 | 64 per 1000 (52 to 77) | |||||
|
Cardiovascular events Follow‐up: range 12 months to 96 months |
No CVD at baseline (primary prevention) | RR 0.89 (0.79 to 1.01) | 17,799 (21 RCTs) | ⊕⊕⊕⊝ Moderateb | Increasing PUFA intake probably reduces risk of CVD events (from 14.6% to 13.0% in the study population, NNT = 63), moderate‐quality evidence. | |
| 54 per 1000 | 46 per 1000 (39 to 54) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 233 per 1000 | 208 per 1000 (175 to 245) | |||||
| Coronary heart disease mortality Follow‐up: range 12 months to 96 months | No CVD at baseline (primary prevention) | RR 0.91 (0.78 to 1.06) | 8810 (9 RCTs) | ⊕⊕⊝⊝ Lowc | Increasing PUFA intake may reduce risk of CHD death (from 6.6% to 6.1% in the study population, NNT = 200), low‐quality evidence. | |
| 52 per 1000 | 44 per 1000 (16 to 122) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 68 per 1000 | 61 per 1000 (53 to 72) | |||||
| Atrial fibrillation and arrhythmias Follow‐up: range 12 months to 60 months | No CVD at baseline (primary prevention) | RR 0.87 (0.72 to 1.06) | 11692 (11 RCTs) | ⊕⊝⊝⊝ Very lowd | The effect of increasing PUFA intake on atrial fibrillation is unclear as the evidence is of very low quality. | |
| 26 per 1000 | 34 per 1000 (25 to 46) | |||||
| CVD at baseline (secondary prevention) | ||||||
| 119 per 1000 | 95 per 1000 (80 to 114) | |||||
| Adiposity ‐ body weight, kg Follow‐up: range 12 months to 60 months | Mean body weight was 81.0 kg | MD 0.76 higher (0.34 higher to 1.19 higher) | ‐ | 7100 (13 RCTs) | ⊕⊕⊕⊝ Moderatee | Higher PUFA intake probably has little or no effect on body weight. |
| Adiposity ‐ BMI, kg/m2 follow‐up: range 12 months to 60 months | Mean BMI was 26.9 kg/m2 | MD 0.17 higher (0.08 lower to 0.42 higher) | ‐ | 4798 (8 RCTs) | ⊕⊕⊝⊝ Lowf | Higher PUFA intake may have little or no effect on BMI. |
| Serum total cholesterol (TC, mmol/L) Follow‐up: range 12 months to 96 months | Mean serum TC was 5.46 mmol/L | MD 0.12 lower (0.23 lower to 0.02 lower) | ‐ | 8072 (27 RCTs) | ⊕⊕⊕⊕ Highg | Higher PUFA intake has little or no effect on TC. |
| Serum fasting triglyceride (TG, mmol/L) Follow‐up: range 12 months to 72 months | Mean serum TG was 1.57 mmol/L | MD 0.12 lower (0.2 lower to 0.04 lower) | ‐ | 3905 (20 RCTs) | ⊕⊕⊕⊝ Moderateh | Higher PUFA intake probably reduces TG levels. |
| Serum high‐density lipoprotein (HDL, mmol/L) Follow‐up: range 12 months to 60 months | Mean serum HDL 1.31 mmol/L | MD 0.01 lower (0.02 lower to 0.01 higher) | ‐ | 4674 (18 RCTs) | ⊕⊕⊕⊝ Moderatei | Higher PUFA intake probably has little or no effect on HDL. |
| Serum low‐density lipoprotein (LDL, mmol/L) Follow‐up: range 12 months to 60 months | Mean serum LDL 2.86 mmol/L | MD 0.01 lower (0.09 lower to 0.06 higher) | ‐ | 3362 (15 RCTs) | ⊕⊕⊕⊝ Moderatej | Higher PUFA intake probably has little or no effect on LDL. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: Body Mass Index; CI: confidence interval; CVD: cardiovascular disease; MD: mean difference; OR: odds ratio; PUFA: polyunsaturated fatty acids; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aCardiovascular mortality
- Risk of bias: limiting trials to those at low summary risk of bias, low risk from allocation concealment, from attention bias, from compliance, by trial funding and trial size suggests small benefits and harms from increasing PUFA intake. Tends to confirm lack of important effect. Not downgraded.
- Inconsistency: I2 statistic less than 50%, not downgraded.
- Indirectness: most events occurred in men, and in trials carried out in high‐income nations. Not downgraded.
- Imprecision: 95% confidence intervals range from important benefit to important harm. Downgraded twice.
- Publication bias: some suggestion that one or two small trials may be missing. If added in they would tend to increase the RR. Not a large effect, not downgraded.
bCardiovascular events
- Risk of bias: sensitivity analyses suggested reduced risk of CVD events with more PUFA, lack of effect, and some harm. Downgraded once.
- Inconsistency: I2 statistic less than 50%, not downgraded.
- Indirectness: most events occurred in men, and in trials carried out in high‐income nations. Not downgraded.
- Imprecision: over 17,000 participants randomised, of whom more than 2400 experienced CVD events. 95% confidence intervals excluded important harms. Not downgraded.
- Publication bias: no suggestion of missing trials in the funnel plot. Not downgraded.
cCoronary heart disease mortality (CHD)
- Risk of bias: all sensitivity analyses concurred that increased PUFA reduced risk of CHD deaths. Not downgraded.
- Inconsistency: I2 statistic less than 50%, not downgraded.
- Indirectness: most events occurred in men, and in trials carried out in high‐income nations. Not downgraded.
- Imprecision: over 8800 participants randomised and over 500 CHD deaths. However, the 95% confidence intervals didn't exclude important harm. Downgraded once.
- Publication bias: some suggestion of publication bias. If present replacing missing trials would tend to raise the risk ratio towards 1.0 (no effect). Downgraded once.
dAtrial fibrillation and arrhythmias
- Risk of bias: no included trials were at low risk of compliance problems, all other sensitivity analyses suggested reduced risk of AF with increased PUFA. However there was no dose response, a suggestion of benefit in short trials, and harm in longer trials supported by meta‐regression. Downgraded once.
- Inconsistency: I2 statistic greater than 50%. Downgraded once.
- Indirectness: most events occurred in men, and in trials carried out in high‐income nations. Not downgraded.
- Imprecision: 95% confidence intervals exclude serious harm, but included the null. Downgraded once.
- Publication bias: no suggestion of missing trials in the funnel plot. Not downgraded.
eAdiposity ‐ body weight
- Risk of bias: sensitivity analyses assessing effects of different biases all suggested greater weight gain in those taking higher total PUFA. Not downgraded.
- Inconsistency: I2 statistic greater than 50% but partially explained by type of intervention and duration of intervention. Not downgraded.
- Indirectness: weight was assessed in both men and women, but all trials were conducted in high‐income countries. Not downgraded.
- Imprecision: 95% confidence intervals only included increased weight with increased PUFA intake. Not downgraded.
- Publication bias: the funnel plot suggests that some trials with less weight gain in the higher PUFA arm may be missing. Two trials with weight data could not be included in meta‐analysis, but they also suggested greater weight gain in the higher PUFA arm. Other missing trials, if due to publication bias, are likely to have not been published because they suggested increased weight in the higher PUFA arm, so are likely to support the main analysis. Downgraded once.
fAdiposity ‐ Body Mass Index (BMI)
- Risk of bias: sensitivity analyses assessing effects of different biases all suggested greater weight gain in those taking higher total PUFA. Not downgraded.
- Inconsistency: I2 statistic greater than 50%, and not explained by subgrouping. Downgraded once.
- Indirectness: weight was assessed in both men and women, but all trials were conducted in high‐income countries. Not downgraded.
- Imprecision: 95% confidence intervals did not include important benefits, but did include the null. Downgraded once.
- Publication bias: no suggestion of missing data. Not downgraded.
gSerum total cholesterol (TC)
- Risk of bias: sensitivity analyses all suggested greater lipid reduction with higher PUFA intake. Not downgraded.
- Inconsistency: I2 statistic greater than 50%, and while no single factor explains this there were greater TC reductions with low statin use, higher PUFA dose, lower baseline PUFA, and replacement of saturated fats and monounsaturated fats. Not downgraded.
- Indirectness: data provided by men and women, and comes from high‐income and low‐ to middle‐income countries. Not downgraded.
- Imprecision: data came from thousands of participants and 95% confidence intervals did not include harm. Not downgraded.
- Publication bias: funnel plot not interpretable, known missing data are consistent with data used in meta‐analysis. Not downgraded.
hSerum triglycerides (TG)
- Risk of bias: sensitivity analyses all suggested greater lipid reduction with higher PUFA intake. Not downgraded.
- Inconsistency: I2 statistic = 50%, without any clear explanation from subgrouping. Downgraded once.
- Indirectness: data provided by men and women, and comes from high‐income and industrialising countries. Not downgraded.
- Imprecision: data came from thousands of participants and 95% confidence intervals did not include harm. Not downgraded.
- Publication bias: no suggestion of missing data. Not downgraded.
iSerum HDL
- Risk of bias: consistent lack of effect of PUFA in all sensitivity analyses. Not downgraded.
- Inconsistency: I2 statistic less than 50%. Not downgraded.
- Indirectness: data provided by men and women, and comes from high‐income and industrialising countries. Not downgraded.
- Imprecision: data came from thousands of participants and confidence interval excludes important effects. Not downgraded.
- Publication bias: some trials with lower HDL appear to be missing. Downgraded once.
jSerum LDL
- Risk of bias: consistent lack of effect of PUFA in all sensitivity analyses. Not downgraded.
- Inconsistency: I2 statistic less than 50%. Not downgraded.
- Indirectness: data provided by men and women, and comes from high‐income and industrialising countries. Not downgraded.
- Imprecision: data came from thousands of participants and confidence interval excludes important effects. Not downgraded.
- Publication bias: some trials with lower LDL appear to be missing. Downgraded once.
Primary outcomes
For 'Summary of findings' table on primary outcomes see Table 1.
All‐cause mortality
PUFA intake probably has little or no effect on all‐cause mortality (moderate‐quality evidence).
Twenty‐four trials including 19,290 participants reported at least one death and could be added to the meta‐analysis. There was no clear effect of more PUFA compared to less PUFA intake on all‐cause mortality (RR 0.98, 95% CI 0.89 to 1.07, I2 = 0%, 1443 deaths; Analysis 1.1). This lack of effect did not differ in fixed‐effect analysis (RR 0.98, 95% CI 0.89 to 1.07; Analysis 1.3), or sensitivity analysis restricting to trials at low risk of bias for allocation concealment (RR 1.03, 95% CI 0.87 to 1.22), low risk of attention bias (RR 0.96, 95% CI 0.87 to 1.07), compliance bias (RR 1.01, 95% CI 0.89 to 1.14), low summary risk of bias (RR 1.04, 95% CI 0.87 to 1.26), trials registry or pre‐2010 publication (RR 0.99, 95% CI 0.90 to 1.08), trials without any industry funding (RR 1.09, 95% CI 0.84 to 1.42), that randomised at least 100 participants (RR 0.98, 95% CI 0.89 to 1.08) or at least 250 participants (RR 1.00, 95% CI 0.91 to 1.10; Analysis 1.2). The funnel plot did not suggest any publication bias, though we are aware of two trials with deaths that we were not able to add to the analyses (Bates 1977; Simon 1997).
1.1. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 1 ALL‐CAUSE MORTALITY.
1.3. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 3 All‐cause mortality ‐ SA fixed‐effect.
1.2. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 2 All‐cause mortality ‐ SA.
Subgrouping did not suggest differential effects by total PUFA dose (Analysis 1.4), duration (Analysis 1.5), primary or secondary prevention (Analysis 1.6), baseline PUFA intake (Analysis 1.7), dietary component displaced by the increase in PUFA (Analysis 1.8), participant sex (Analysis 1.9), participant age (Analysis 1.10), statin use (Analysis 1.11), or type of intervention (Analysis 1.12). There was no suggestion of important effects in any of the four trials each taking more than 10% of the weight in meta‐analysis (AlphaOmega ‐ ALA; DART fat 1989; PREDIMED 2013; Veterans Admin 1969).
1.4. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 4 All‐cause mortality ‐ subgroup by PUFA dose.
1.5. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 5 All‐cause mortality ‐ subgroup by duration.
1.6. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 6 All‐cause mortality ‐ subgroup by primary or secondary prevention.
1.7. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 7 All‐cause mortality ‐ subgroup by baseline PUFA dose.
1.8. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 8 All‐cause mortality ‐ subgroup by replacement.
1.9. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 9 All‐cause mortality ‐ subgroup by sex.
1.10. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 10 All‐cause mortality ‐ subgroup by age.
1.11. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 11 All‐cause mortality ‐ subgroup by statin use.
1.12. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 12 All‐cause mortality ‐ subgroup by intervention type.
Meta‐regression did not suggest any relationships between total PUFA dose (P = 0.94) or trial duration (P = 0.81) and all‐cause mortality. We did not run meta‐regression of baseline PUFA dose and all‐cause mortality as few trials provided this information.
We downgraded the GRADE evidence level for imprecision as the 95% CI included important benefits (moderate‐quality evidence), Table 1.
Coronary heart disease events
PUFA intake probably reduces risk of coronary heart disease events slightly (NNT 53, moderate‐quality evidence).
Fifteen trials including 10,076 participants were included and 1351 participants reported at least one coronary heart disease event. Meta‐analysis suggested that higher PUFA intake resulted in 13% fewer participants having coronary heart disease events (RR 0.87, 95% CI 0.72 to 1.06, I2 = 45%; Analysis 1.13). None of the four trials that carried at least 10% of the weight of the meta‐analyses suggested statistically significant effects in their own right (AlphaOmega ‐ ALA; DART fat 1989; MRC 1968; Veterans Admin 1969). The funnel plot did not suggest any serious publication bias (not shown).
1.13. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 13 CORONARY HEART DISEASE (CHD) EVENTS: myocardial infarction (fatal or non‐fatal) or angina.
Sensitivity analyses using fixed‐effects analysis suggested a 10% reduction in risk of coronary heart disease with increased PUFA (RR 0.90, 95% CI 0.82 to 0.99; Analysis 1.15), but other sensitivity analyses suggested varying results both sides of no effect (RR 1.00). These included restricting to trials at low risk of bias for allocation concealment (RR 1.14, 95% CI 0.73 to 1.78), low risk of attention bias (RR 0.86, 95% CI 0.72 to 1.02), compliance bias (RR 0.87, 95% CI 0.65 to 1.17), low summary risk of bias (RR 1.18, 95% CI 0.76 to 1.81), trials registry or pre‐2010 publication (RR 0.87, 95% CI 0.72 to 1.06), trials without any industry funding (RR 0.72, 95% CI 0.31 to 1.63), that randomised at least 100 participants (RR 0.87, 95% CI 0.70 to 1.08) or at least 250 participants (RR 0.94, 95% CI 0.82 to 1.09; Analysis 1.14).
1.15. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 15 CHD events ‐ SA fixed‐effect.
1.14. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 14 CHD events ‐ SA.
Subgrouping by PUFA dose and trial duration did not suggest important differences between subgroups, or dose or duration effects. There were only six events in trials with doses of less than 1% E (Analysis 1.16) and 21 events in trials of less than two years (Analysis 1.17). Meta‐regression did not suggest any relationship between PUFA dose (P = 0.69) or trial duration (P = 0.51) and coronary heart disease events.
1.16. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 16 CHD events ‐ subgroup by PUFA dose.
1.17. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 17 CHD events ‐ subgroup by duration.
Subgrouping did not suggest differential effects by primary or secondary prevention (P = 0.12; Analysis 1.18), baseline PUFA intake (Analysis 1.19), replacement of saturated fat or MUFA with PUFA (Analysis 1.20), age (Analysis 1.22), statin use or intervention type (Analysis 1.23; Analysis 1.24). Most coronary heart disease events occurred in trials of men, there is insufficient information to understand effects in other subgroups, though rather surprisingly there was a significant difference between subgroups of men and women combined and trials of mostly men or mostly women (Analysis 1.21).
1.18. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 18 CHD events ‐ subgroup by primary or secondary prevention.
1.19. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 19 CHD events ‐ subgroup by baseline PUFA dose.
1.20. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 20 CHD events ‐ subgroup by replacement.
1.22. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 22 CHD events ‐ subgroup by age.
1.23. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 23 CHD events ‐ subgroup by statin use.
1.24. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 24 CHD events ‐ subgroup by intervention type.
1.21. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 21 CHD events ‐ subgroup by sex.
We downgraded the GRADE evidence level for imprecision and risk of bias combined (as despite over 10,000 participants the 95% confidence intervals included harm from increasing PUFA as well as benefit). PUFA intake probably reduces risk of coronary heart disease events, from 14.2% to 12.3% in the study populations, NNT 53 (moderate‐quality evidence), Table 1.
Stroke
PUFA intake may very slightly reduce risk of stroke (NNT 1000, low‐quality evidence). However, the 95% confidence intervals include important harms as well as benefit.
Eleven trials including 14,742 participants of whom 166 experienced at least one fatal or non‐fatal stroke. Meta‐analysis suggested some reduction in risk of stroke with increased PUFA, but confidence intervals were wide (RR 0.91, 95% CI 0.58 to 1.44, I2 = 24%; Analysis 1.25). The funnel plot did not suggest any small study bias (not shown).
1.25. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 25 STROKE ‐ fatal & non fatal.
This suggestion of benefit from PUFA was also seen in the fixed‐effect sensitivity analysis (RR 0.82, 95% CI 0.61 to 1.11, Analysis 1.27). While sensitivity analyses retaining only trials at low risk of bias from allocation concealment, attention and low summary risk of bias all suggested reduced stroke risk with increased PUFA, as did those on trials registers or pre‐2010, and trials of at least 100 participants, this was not the case for sensitivity analyses of trials at low risk of bias from compliance (RR 1.36, 95% CI 0.45 to 4.11, I2 = 56%), trials without industry funding or of at least 250 participants (RR 0.98, 95% CI 0.60 to 1.60, I2 = 33%), Analysis 1.26.
1.27. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 27 Stroke ‐ SA fixed‐effect.
1.26. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 26 Stroke ‐ SA.
Subgrouping did not suggest greater effects with higher doses of PUFA (Analysis 1.28), or longer duration (Analysis 1.29), without significant differences between subgroups. Meta‐regression did not suggest relationships between PUFA dose and stroke (P = 0.69), but there was limited non‐statistically significant suggestion of greater benefit in longer trials (P = 0.11).
1.28. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 28 Stroke ‐ subgroup by PUFA dose.
1.29. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 29 Stroke ‐ subgroup by duration.
There were no significant differences between subgroups by primary or secondary prevention (P = 0.20; Analysis 1.30), baseline PUFA dose (Analysis 1.31), sex (Analysis 1.33), statin use (Analysis 1.35), fatal or non‐fatal stroke (Analysis 1.37), replacement (Analysis 1.32), or intervention type (Analysis 1.36). There were differences when subgrouping was by age (Analysis 1.34), but greater protection at older age was balanced by harm in mid‐life ‐ a confusing picture. Where data on ischaemic or haemorrhagic stroke could be separated out, both subgroups suggested harm from increased PUFA, while data on combined ischaemic and haemorrhagic events suggested benefit from increased PUFA, again a non‐intuitive pattern (Analysis 1.38).
1.30. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 30 Stroke ‐ subgroup by primary or secondary prevention.
1.31. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 31 Stroke ‐ subgroup by baseline PUFA dose.
1.33. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 33 Stroke ‐ subgroup by sex.
1.35. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 35 Stroke ‐ subgroup by statin use.
1.37. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 37 Stroke ‐ subgroup by fatal & non fatal.
1.32. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 32 Stroke ‐ subgroup by replacement.
1.36. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 36 Stroke ‐ subgroup by intervention type.
1.34. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 34 Stroke ‐ subgroup by age.
1.38. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 38 Stroke ‐ subgroup by ischaemic & haemorrhagic.
We downgraded the GRADE quality assessment twice for imprecision (even though over 14,000 participants were included only 166 people experienced stroke so we were underpowered to assess effects). PUFA intake may reduce risk of stroke, from 1.2% to 1.1% in the study populations, NNT 1000 (low‐quality evidence), but harms are not ruled out, Table 1.
Major adverse cardiac and cardiovascular events (MACCEs)
Effects of PUFA on risk of MACCEs are unclear as data are of very low quality.
Two trials recruited 1879 participants, and 817 people experienced at least one MACCE. The trials suggested a 16% decrease in MACCE risk with increased PUFA, but were highly heterogeneous (RR 0.84, 95% CI 0.59 to 1.20, I2 = 79%; Analysis 1.39). With only two included trials assessment of small study bias was not possible, and fixed‐effect analysis also suggested some benefit of PUFA (RR 0.92, 95% CI 0.82 to 1.04, I2 = 79%; Analysis 1.41). Most sensitivity analyses preserved the suggested reduction of risk of MACCEs with increased PUFA but no trials were at low summary risk of bias and none were at low risk of bias from allocation concealment (Analysis 1.40).
1.39. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 39 MAJOR ADVERSE CARDIAC & CEREBROVASCULAR EVENTS (MACCEs).
1.41. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 41 MACCEs ‐ SA fixed‐effect.
1.40. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 40 MACCEs ‐ SA.
With only two trials, subgrouping was generally uninformative (Analysis 1.42; Analysis 1.43; Analysis 1.44; Analysis 1.45; Analysis 1.46; Analysis 1.47; Analysis 1.48; Analysis 1.49; Analysis 1.50). Whenever the two trials were in separate subgroups there was a statistically significant difference between subgroups. We did not attempt meta‐regression.
1.42. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 42 MACCEs ‐ subgroup by PUFA dose.
1.43. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 43 MACCEs ‐ subgroup by duration.
1.44. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 44 MACCEs ‐ subgroup by primary or secondary prevention.
1.45. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 45 MACCEs ‐ subgroup by baseline PUFA dose.
1.46. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 46 MACCEs ‐ subgroup by replacement.
1.47. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 47 MACCEs ‐ subgroup by sex.
1.48. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 48 MACCEs ‐ subgroup by age.
1.49. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 49 MACCEs ‐ subgroup by statin use.
1.50. Analysis.
Comparison 1 Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes, Outcome 50 MACCEs ‐ subgroup by intervention type.
We downgraded GRADE assessment for risk of bias, inconsistency and imprecision. Effects of PUFA on risk of MACCEs was unclear as data are of very low quality, Table 1.
Secondary outcomes
We formally systematically reviews secondary outcomes, in that we included all relevant trials that collected data on any of these outcomes. Table 2 displays GRADE assessments for the key outcomes not included in this review's primary outcomes.
Cardiovascular mortality
Increasing PUFA intake may have little or no effect on cardiovascular mortality (low‐quality evidence).
Sixteen trials randomising 15,107 participants of whom 729 died of cardiovascular causes were included. Meta‐analysis suggested little effect of PUFA intake on cardiovascular disease deaths (RR 1.02, 95% CI 0.82 to 1.26, I2 = 31%; Analysis 2.1). Sensitivity analyses suggested small non‐significant benefits (limiting to trials at low risk of bias for attention) or non‐significant harms (limiting to trials at low risk of bias for allocation concealment, compliance, summary risk of bias, trials registry entry or pre‐2010, no industry funding, and larger trials; Analysis 2.2), and fixed‐effect analysis suggested no effect (RR 1.01, 95% CI 0.88 to 1.16; Analysis 2.3). The funnel plot suggested that one or two smaller trials with RRs greater than 1.00 might be missing ‐ replacing these would tend to raise the RR, suggesting slight harm.
2.1. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 1 CARDIOVASCULAR MORTALITY.
2.2. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 2 Cardiovascular mortality ‐ SA.
2.3. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 3 Cardiovascular mortality ‐ SA fixed‐effect.
Subgrouping by PUFA dose suggested no statistically significant subgroup differences (Analysis 2.4). Meta‐regression did not suggest any relationship with dose (P = 0.54). Subgrouping by duration showed no important differences between subgroups (P = 0.72; Analysis 2.5). Meta‐regression on duration was not statistically significant (P = 0.11).
2.4. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 4 Cardiovascular mortality ‐ subgroup by PUFA dose.
2.5. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 5 Cardiovascular mortality ‐ subgroup by duration.
Subgrouping by primary or secondary prevention, replacement, sex, statin use, and intervention type did not explain any of the heterogeneity and subgroups did not differ significantly (Analysis 2.6; Analysis 2.8; Analysis 2.9; Analysis 2.11; Analysis 2.12). Subgrouping by baseline PUFA intake included six trials and suggested benefit of increasing PUFA intake in groups with baseline total PUFA intake less than 6% E (RR 0.71, 95% CI 0.52 to 0.97, I2 = 0%, 141 cardiovascular disease deaths), but harm in groups with higher baseline PUFA intake (RR 1.32, 95% CI 1.07 to 1.62, I2 = 0%, 326 cardiovascular disease deaths), removing heterogeneity and suggesting a statistically significant test for subgroup differences (P = 0.003; Analysis 2.7). Subgrouping by participant age also reduced heterogeneity and suggested significant subgroup differences (P = 0.02; Analysis 2.10), suggesting harm from additional PUFA in adults aged under 50 years, more modest harm in those aged 50 to 65 years, and benefit in those aged at least 65 years. These data could suggest greater utility of increasing total PUFA when baseline intake is low, and in older adults, but given the small number of trials caution is appropriate.
2.6. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 6 Cardiovascular mortality ‐ subgroup by primary or secondary prevention.
2.8. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 8 Cardiovascular mortality ‐ subgroup by replacement.
2.9. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 9 Cardiovascular mortality ‐ subgroup by sex.
2.11. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 11 Cardiovascular mortality ‐ subgroup by statin use.
2.12. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 12 Cardiovascular mortality ‐ subgroup by intervention type.
2.7. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 7 Cardiovascular mortality ‐ subgroup by baseline PUFA dose.
2.10. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 10 Cardiovascular mortality ‐ subgroup by age.
We downgraded the GRADE assessment twice for imprecision (as important benefits and harms were included in the 95% confidence intervals). Increasing PUFA intake may have little or no effect on cardiovascular mortality (low‐quality evidence).
Cardiovascular events
Increasing PUFA intake probably reduces risk of cardiovascular events a little (NNT 59, moderate‐quality evidence).
Twenty trials randomising 17,799 participants reported at least one cardiovascular event in 2442 participants. Meta‐analysis suggested that increasing total PUFA intake reduced the risk of cardiovascular disease events by 11%, with little heterogeneity (RR 0.89, 95% CI 0.79 to 1.01, I2 = 30%; Analysis 2.13), as did fixed‐effect analysis (RR 0.92, 95% CI 0.86 to 0.98; Analysis 2.15). Sensitivity analyses limiting to trials with low risk of bias from attention bias, trials registry entry or pre‐2010, trials with at least 100 or at least 250 participants all retained suggestion of benefit from increased PUFA, while sensitivity analyses for allocation concealment, compliance, and industry funding suggested no important effects, and limiting to studies with low summary risk of bias suggested increased risk (Analysis 2.14). The funnel plot did not suggest small study bias (not shown).
2.13. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 13 CARDIOVASCULAR EVENTS.
2.15. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 15 CVD events ‐ SA fixed‐effect.
2.14. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 14 CVD events ‐ SA.
Subgrouping by PUFA dose and trial duration did not show statistically significant differences between subgroups (P = 0.17 and 0.18 respectively; Analysis 2.16; Analysis 2.17). Meta‐regression did not suggest relationships between cardiovascular disease events and PUFA dose (P = 0.78) or trial duration (P = 0.70).
2.16. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 16 CVD events ‐ subgroup by PUFA dose.
2.17. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 17 CVD events ‐ subgroup by duration.
Subgrouping by primary or secondary prevention, baseline PUFA dose, replacement, sex, statin use, and intervention type did not reduce heterogeneity and did not suggest significant differences between subgroups (Analysis 2.18; Analysis 2.19; Analysis 2.20; Analysis 2.21; Analysis 2.23; Analysis 2.24). Subgrouping by participant age suggested harm in younger participants (RR 1.66, 95% CI 1.05 to 2.61, I2 = 0%), but benefit in middle‐aged and older participants (RR 0.86, 95% CI 0.78 to 0.96, I2 = 0%), with statistically significant differences between subgroups (P = 0.03; Analysis 2.22).
2.18. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 18 CVD events ‐ subgroup by primary or secondary prevention.
2.19. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 19 CVD events ‐ subgroup by baseline PUFA dose.
2.20. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 20 CVD events ‐ subgroup by replacement.
2.21. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 21 CVD events ‐ subgroup by sex.
2.23. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 23 CVD events ‐ subgroup by statin use.
2.24. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 24 CVD events ‐ subgroup by intervention type.
2.22. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 22 CVD events ‐ subgroup by age.
We downgraded the GRADE assessment for risk of bias (as sensitivity analyses suggested benefit, no effect and some harm from increased PUFA). Increasing PUFA intake probably reduces risk of cardiovascular events from 14.6% to 13.0% in study populations, NNT 63 (moderate‐quality evidence).
Coronary heart disease mortality
Increasing PUFA intake may reduce risk of coronary heart disease death by a small amount (NNT 200, low‐quality evidence).
Nine trials randomised 8810 participants of whom 556 died of coronary heart disease. Meta‐analysis suggested that increasing PUFA intake reduced risk of coronary heart disease death, without heterogeneity (RR 0.91, 95% CI 0.78 to 1.06, I2 = 0%; Analysis 2.25). Results from the fixed‐effect analysis were very similar (RR 0.90, 95% CI 0.77 to 1.05, I2 = 0%; Analysis 2.27). Although nine trials provided data, of the 556 deaths, 340 occurred in DART fat 1989, which carried 65% of the weight of the meta‐analysis, and 138 occurred in AlphaOmega ‐ ALA, which carried 23% of the weight. Results of all the sensitivity analyses were similar, all suggesting modest protection from increased PUFA (Analysis 2.26), although no subgroups were statistically significant. The funnel plot suggested that some small trials with RR over 1.0 may be missing, and if these trials were added back in they would tend to raise the RR towards 1.0 (Figure 4).
2.25. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 25 CORONARY HEART DISEASE (CHD) MORTALITY.
2.27. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 27 CHD mortality ‐ SA fixed‐effect.
2.26. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 26 CHD mortality ‐ SA.
4.
Funnel plot of comparison 2. Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, outcome: 2.25 CORONARY HEART DISEASE (CHD) MORTALITY
Subgrouping by dose and duration did not suggest subgroup differences (P = 0.92 and 0.90 respectively), though there was a counter‐intuitive suggestion that lower doses and shorter durations produced greater benefits (Analysis 2.28; Analysis 2.29). Meta‐regression did not suggest strong relationships between PUFA dose (P = 0.62) or trial duration (P = 0.71) and risk of coronary heart disease death.
2.28. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 28 CHD mortality ‐ subgroup by PUFA dose.
2.29. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 29 CHD mortality ‐ subgroup by duration.
Subgrouping by primary or secondary cardiovascular disease prevention, baseline PUFA dose, replacement, sex, age, statin use, or intervention type did not suggest important differences between subgroups (Analysis 2.30; Analysis 2.31; Analysis 2.32; Analysis 2.33; Analysis 2.34; Analysis 2.35; Analysis 2.36).
2.30. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 30 CHD mortality ‐ subgroup by primary or secondary prevention.
2.31. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 31 CHD mortality ‐ subgroup by baseline PUFA dose.
2.32. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 32 CHD mortality ‐ subgroup by replacement.
2.33. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 33 CHD mortality ‐ subgroup by sex.
2.34. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 34 CHD mortality ‐ subgroup by age.
2.35. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 35 CHD mortality ‐ subgroup by statin use.
2.36. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 36 CHD mortality ‐ subgroup by intervention type.
We downgraded the GRADE assessment for imprecision and publication bias. Increasing PUFA intake may reduce risk of coronary heart disease death a little from 6.6% to 6.1% in the study populations, NNT 200 (low‐quality evidence).
Myocardial infarction
Increasing PUFA may reduce risk of myocardial infarction.
Fifteen trials randomising 15,609 participants recorded 880 myocardial infarctions. Meta‐analysis suggested that increasing PUFA reduced the risk of myocardial infarction by 12% without heterogeneity (RR 0.88, 95% CI 0.78 to 0.99, I2 = 0%; Analysis 2.37). We did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regression for this outcome.
2.37. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 37 MYOCARDIAL INFARCTION (MI) ‐ fatal and non fatal.
Sudden cardiac death
The effect of increasing PUFA on sudden cardiac death is unclear.
Five trials recruited 1731 participants of whom 69 experienced sudden cardiac death. Meta‐analysis suggested some benefit from increasing PUFA (RR 0.80, 95% CI 0.50 to 1.29, I2 = 0%; Analysis 2.38), but the effect was not statistically significant, and did not exclude important harms. There were insufficient trials to assess the funnel plot. We did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regression for this outcome.
2.38. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 38 SUDDEN CARDIAC DEATH (SCD).
Atrial fibrillation
The effect of increasing PUFA intake on atrial fibrillation is unclear as the evidence is of very low quality.
Eleven trials recruited 11,692 participants of whom 811 experienced new or recurrent atrial fibrillation. Meta‐analysis suggested that increasing PUFA reduced the risk of atrial fibrillation by 13% with substantial heterogeneity (RR 0.87, 95% CI 0.72 to 1.06, I2 = 57%, Analysis 2.39). Fixed‐effect analysis suggested marginal statistical significance (RR 0.87, 95% CI 0.72 to 1.06, I2 = 57%; Analysis 2.39). Sensitivity analyses generally suggested a non‐significant protective effect of the trials at lower risk of bias, but trials without industry funding and those at low risk from allocation concealment suggested a statistically significant reduction(Analysis 2.40). No trials were at low risk of bias from compliance problems.
2.39. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 39 ATRIAL FIBRILLATION (AF) & ARRHYTHMIAS (including AF, ventricular tachycardia (VT), ventricular fibrillation(VF).
2.40. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 40 AF ‐ SA.
Subgrouping by new or recurrent atrial fibrillation suggested no important differences between subgroups (P = 0.31; Analysis 2.39). Subgrouping by PUFA dose did not suggest important differences between subgroups (Analysis 2.42), but subgrouping by duration suggested greater protection in shorter trials (P = 0.001; Analysis 2.43). Meta‐regression suggested that there was no relationship between PUFA dose and atrial fibrillation (P = 0.91), but there was a marginally significant relationship between duration and risk of atrial fibrillation (with benefit in shorter trials and harm in longer trials, P = 0.056).
2.42. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 42 AF ‐ subgroup by PUFA dose.
2.43. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 43 AF ‐ subgroup by duration.
Subgrouping suggested no important effects by replacement, sex, age, intervention type or statin use (no subgroup differences; Analysis 2.46; Analysis 2.47; Analysis 2.48; Analysis 2.49; Analysis 2.50). Subgrouping suggested that PUFA was harmful in primary prevention (RR 1.33, 95% CI 0.99 to 1.79, I2 = 0%) and beneficial in secondary prevention of cardiovascular disease (RR 0.80, 95% CI 0.67 to 0.96, I2 = 58%), with significant subgroup differences (P = 0.004; Analysis 2.44). Only one trial had a known baseline PUFA intake so subgrouping was unhelpful (Analysis 2.45).
2.46. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 46 AF ‐ subgroup by replacement.
2.47. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 47 Atrial fibrillation ‐ subgroup by sex.
2.48. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 48 AF ‐ subgroup by age.
2.49. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 49 AF ‐ subgroup by statin use.
2.50. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 50 AF ‐ subgroup by intervention type.
2.44. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 44 AF ‐ subgroup by primary or secondary prevention.
2.45. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 45 Atrial fibrillation ‐ subgroup by baseline PUFA dose.
We downgraded the GRADE assessment for risk of bias, inconsistency and imprecision. The effect of increasing PUFA intake on atrial fibrillation is unclear as the evidence is of very low quality.
Angina
The effect of increasing PUFA intake on angina is unclear.
Seven trials including 2070 participants reported 100 participants experiencing new or worsening angina. Meta‐analysis suggested that increasing PUFA reduced risk of angina (RR 0.64, 95% CI 0.35 to 1.16, I2 = 46%; Analysis 2.51). There were insufficient trials to assess the funnel plot and we did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regression for this outcome. One included trial had adequate allocation concealment and none were at low summary risk of bias.
2.51. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 51 ANGINA.
Heart failure
The effect of increasing PUFA intake on heart failure is unclear.
Seven trials including 25,257 participants reported 137 participants experiencing new or worsening heart failure. Meta‐analysis suggested that increasing PUFA reduced risk of heart failure but results were heterogeneous and important harms were not excluded (RR 0.74, 95% CI 0.40 to 1.36, I2 = 54%; Analysis 2.52). There were insufficient trials to assess the funnel plot and we did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regression for this outcome. Two included trials had adequate allocation concealment and one was at low summary risk of bias.
2.52. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 52 HEART FAILURE.
Peripheral arterial disease
Increasing PUFA intake may increase the risk of peripheral arterial disease.
Four trials including 8937 participants reported 97 participants experiencing new or worsening peripheral arterial disease. Meta‐analysis suggested that increasing PUFA increased risk of peripheral arterial disease but important benefits were not excluded (RR 1.20, 95% CI 0.81 to 1.77, I2 = 0%; Analysis 2.53). There were insufficient trials to assess the funnel plot and we did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regression for this outcome. Two included trials had adequate allocation concealment and two were at low summary risk of bias.
2.53. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 53 PERIPHERAL ARTERIAL DISEASE (PAD).
Revascularisation
The effect of increasing PUFA intake on revascularisation is unclear.
Six trials including 1182 participants reported 46 participants undergoing revascularisation. Meta‐analysis suggested that increasing PUFA reduced risk of revascularisation but important harms were not excluded (RR 0.70, 95% CI 0.40 to 1.24, I2 = 0%; Analysis 2.54). There were insufficient trials to assess the funnel plot and we did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regression for this outcome. One included trial had adequate allocation concealment and one was at low summary risk of bias.
2.54. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 54 REVASCULARISATION ‐ angioplasty and/or coronary artery bypass grafting.
Adiposity ‐ body weight
Higher PUFA intake probably has little or no effect on body weight (moderate‐quality evidence).
Twelve trials presenting 15 comparisons, of which 13 could be included in meta‐analyses, included 7100 participants with data on body weight. Meta‐analyses suggested that weight increased <2% with increased PUFA intake, although trials were heterogeneous (MD 0.76 kg, 95% CI 0.34 to 1.19, I2 = 59%; Analysis 3.1). The funnel plot suggested that some trials with smaller weight increases or reductions in the increased PUFA group may be missing. If replaced, these trials would tend to reduce the weight increase in the higher PUFA participants. Two trials (both also suggesting increased weight in the higher PUFA arm) did not provide variance data so could not be included in the meta‐analysis, though they are shown in the forest plot (Analysis 3.1). A further five trials collected data on weight but did not provide those data in a way that could be included in meta‐analysis (MARINA ‐ Sanders 2011; NDHS Faribault 1968; NDHS Open 1st 1968; Simon 1997; Vijayakumar 2014).
3.1. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 1 ADIPOSITY ‐ BODY WEIGHT, kg.
The effect was larger, but still <2%, when we used fixed‐effect analysis (MD 1.08 kg, 95% CI 0.96 to 1.21; Analysis 3.3). Sensitivity analyses all suggested increased body weight with increased PUFA intake (although not statistically significantly when we limited to trials at low risk of compliance bias, Analysis 3.2).
3.3. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 3 Body weight, kg ‐ SA fixed‐effect.
3.2. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 2 Body weight, kg ‐ SA.
Subgrouping by PUFA dose, duration, primary or secondary prevention, replacement, sex, age and statin use did not differ significantly by subgroups (Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.8; Analysis 3.9; Analysis 3.10; Analysis 3.11). There were important differences between subgroups when grouping by baseline PUFA dose, with greater weight increases for those with lower baseline PUFA intake (Analysis 3.7). Subgrouping by intervention type suggested differences between subgroups (P = 0.01; Analysis 3.12), suggesting greater weight increases with increased PUFA intake by dietary advice (MD 2.37 kg, 95% CI 1.19 to 3.55, I2 = 0%) than in trials of supplemental foods or diet provided (MD 0.71 kg, 95% CI 0.18 to 1.25, I2 = 73%), or in supplemental trials (MD 0.37 kg, 95% CI ‐0.18 to 0.91, I2 = 0%).
3.4. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 4 Body weight, kg ‐ subgroup by PUFA dose.
3.5. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 5 Body weight, kg ‐ subgroup by duration.
3.6. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 6 Body weight, kg ‐ subgroup by primary or secondary prevention.
3.8. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 8 Body weight, kg ‐ subgroup by replacement.
3.9. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 9 Body weight, kg ‐ subgroup by sex.
3.10. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 10 Body weight, kg ‐ subgroup by age.
3.11. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 11 Body weight, kg ‐ subgroup by statin use.
3.7. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 7 Body weight, kg ‐ subgroup by baseline PUFA dose.
3.12. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 12 Body weight, kg ‐ subgroup by intervention type.
We downgraded the GRADE assessment of evidence for publication bias, leading to a moderate quality of evidence. Higher PUFA intake probably has little or no effect on body weight.
Adiposity ‐ BMI
Higher PUFA intake may have little or no effect on BMI (low‐quality evidence).
Eight trials reported 11 comparisons including 4798 participants with BMI reported. Meta‐analysis suggested that increasing PUFA intake results in <2% change in BMI, but effects were heterogeneous (MD 0.17 kg/m2, 95% CI ‐0.08 to 0.42, I2 = 80%, Analysis 3.13). Fixed‐effect analysis was statistically significant (MD 0.27 kg/m2, 95% CI 0.20 to 0.35, I2 = 80%; Analysis 3.15) but represented only a 1% change. The funnel plot did not suggest any small study bias, and we are aware of two trials that assessed BMI but did not provide data that could be used in meta‐analysis (Simon 1997; Vijayakumar 2014). Sensitivity analyses all confirmed slightly increased BMI with increased PUFA intake (Analysis 3.14).
3.13. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 13 ADIPOSITY ‐ Body Mass Index (BMI), kg/m2.
3.15. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 15 BMI, kg/m2 ‐ SA fixed‐effect.
3.14. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 14 BMI, kg/m2 ‐ SA.
Subgrouping by PUFA dose, duration, primary or secondary prevention, baseline PUFA intake, replacement, sex, age, statin use and intervention type did not suggest important differences between subgroups (Analysis 3.16; Analysis 3.17; Analysis 3.18; Analysis 3.19; Analysis 3.20; Analysis 3.21; Analysis 3.22; Analysis 3.23; Analysis 3.24), and did not reduce heterogeneity.
3.16. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 16 BMI, kg/m2 ‐ subgroup by PUFA dose.
3.17. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 17 BMI, kg/m2 ‐ subgroup by duration.
3.18. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 18 BMI, kg/m2 ‐ subgroup by primary or secondary prevention.
3.19. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 19 BMI, kg/m2 ‐ subgroup by baseline PUFA dose.
3.20. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 20 BMI, kg/m2 ‐ subgroup by replacement.
3.21. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 21 BMI, kg/m2 ‐ subgroup by sex.
3.22. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 22 BMI, kg/m2 ‐ subgroup by age.
3.23. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 23 BMI, kg/m2 ‐ subgroup by statin use.
3.24. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 24 BMI, kg/m2 ‐ subgroup by intervention type.
We downgraded the GRADE assessment for inconsistency and imprecision, leading to low‐quality evidence. Higher PUFA intake may have little or no effect on BMI.
Adiposity ‐ other measures
Several trials reported waist circumference (1298 participants in two trials; Analysis 3.25), percentage body fat (309 participants in two trials; Analysis 3.26) and body fat in kg (214 participants in a single trial; Analysis 3.27). Meta‐analyses on waist circumference and percentage body fat both suggested slightly greater weight gain in those on higher PUFA intake, while the single trial with data on body fat in kg suggested no difference in body fat regardless of PUFA intake. We are aware of several trials that assessed adiposity but did not provide data in a format that could be included in meta‐analysis. HERO‐Tapsell 2009 and Simon 1997 assessed percentage of body fat, and WAHA ‐ Ros 2016 assessed waist circumference (shown in the meta‐analysis but without variance data).
3.25. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 25 Adiposity ‐ waist circumference, cm.
3.26. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 26 Adiposity ‐ % body fat.
3.27. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 27 Adiposity ‐ body fat, kg.
Lipids ‐ serum total cholesterol
Higher PUFA intake has little or no effect on total cholesterol (high‐quality evidence).
Twenty six trials, incorporating data from 8072 participants (and 28 trial arms), provided data on serum total cholesterol. Meta‐analysis suggested that increasing PUFA intake reduced total cholesterol by <5%, although data were heterogeneous (MD ‐0.12 mmol/L, 95% CI ‐0.23 to ‐0.02, I2 = 79%; Analysis 3.28). The funnel plot was difficult to interpret, but we were aware of one trial (MRC 1968) that provided total cholesterol data without variance information, so could not be included in meta‐analysis. This trial also suggested reduced total cholesterol in the higher PUFA arm (Analysis 3.28). Total cholesterol data from five trials (Dullaart 1992; EPOCH 2011; ORL 2013; Veterans Admin 1969; WINS 2006) could not be included in meta‐analysis, so are missing.
3.28. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 28 Serum TOTAL CHOLESTEROL (TC, mmoL/L).
Sensitivity analyses, including fixed‐effect analysis, all suggested greater total cholesterol reduction with higher PUFA intake, although some were not statistically significant (Analysis 3.29; Analysis 3.30).
3.29. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 29 TC, mmoL/L ‐ SA.
3.30. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 30 TC, mmoL/L ‐ SA fixed‐effect.
Subgrouping by PUFA dose and duration did not suggest important differences between subgroups (Analysis 3.31; Analysis 3.32). We did not plan to run meta‐regressions for continuous outcomes. Subgrouping by primary or secondary prevention, baseline PUFA intake, replacement, age, sex, statin use and intervention type did not suggest important differences between subgroups (Analysis 3.33; Analysis 3.34; Analysis 3.35; Analysis 3.36; Analysis 3.37; Analysis 3.38; Analysis 3.39).
3.31. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 31 TC, mmoL/L ‐ subgroup by PUFA dose.
3.32. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 32 TC, mmoL/L ‐ subgroup by duration.
3.33. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 33 TC, mmoL/L ‐ subgroup by primary or secondary prevention.
3.34. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 34 TC, mmoL/L ‐ subgroup by baseline PUFA dose.
3.35. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 35 TC, mmoL/L ‐ subgroup by replacement.
3.36. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 36 TC, mmoL/L ‐ subgroup by sex.
3.37. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 37 TC, mmoL/L ‐ subgroup by age.
3.38. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 38 TC, mmoL/L ‐ subgroup by statin use.
3.39. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 39 TC, mmoL/L ‐ subgroup by intervention type.
We did not downgrade the GRADE assessment of evidence. Higher PUFA intake has little or no effect on total cholesterol (high‐quality evidence).
Lipids ‐ serum fasting triglyceride
Higher PUFA intake probably leads to lower triglyceride levels (moderate‐quality evidence).
Twenty trials incorporating data from 3905 participants (and 22 trial arms) provided data on serum triglycerides. Meta‐analysis suggested that increasing PUFA intake reduced triglycerides, although data were heterogeneous (MD ‐0.12 mmol/L, 95% CI ‐0.20 to ‐0.04, I2 = 50%; Analysis 3.40). The funnel plot did not suggest small study bias, but we are aware of a further eight trials that did not report triglycerides in a way that could be incorporated into meta‐analysis (Ahn 2016; EPOCH 2011; NDHS Faribault 1968; NDHS Open 1st 1968; ORL 2013; Rossing 1996; WAHA ‐ Ros 2016; WINS 2006).
3.40. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 40 Serum fasting TRIGLYCERIDE (TG, mmoL/L).
Sensitivity analyses, including fixed‐effect analysis, all suggested greater triglyceride reduction with higher PUFA intake, although some were not statistically significant (Analysis 3.41; Analysis 3.42).
3.41. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 41 TG, mmoL/L ‐ SA.
3.42. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 42 TG, mmoL/L ‐ SA fixed‐effect.
Subgroup analyses did not suggest differential effects by dose, duration, baseline PUFA intake, replacement, statin use, intervention type, primary or secondary prevention, sex, or age (Analysis 3.43; Analysis 3.44; Analysis 3.45; Analysis 3.46; Analysis 3.47; Analysis 3.48; Analysis 3.49; Analysis 3.50).
3.43. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 43 TG, mmoL/L ‐ subgroup by PUFA dose.
3.44. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 44 TG, mmoL/L ‐ subgroup by duration.
3.45. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 45 TG, mmoL/L ‐ subgroup by primary or secondary prevention.
3.46. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 46 TG, mmoL/L ‐ subgroup by baseline PUFA dose.
3.47. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 47 TG, mmoL/L ‐ subgroup by replacement.
3.48. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 48 TG, mmoL/L ‐ subgroup by sex.
3.49. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 49 TG, mmoL/L ‐ subgroup by age.
3.50. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 50 TG, mmoL/L ‐ subgroup by statin use.
We downgraded the GRADE evidence once for inconsistency. Higher PUFA intake probably leads to lower triglyceride levels (moderate‐quality evidence).
Lipids ‐ high density lipoprotein (HDL)
Higher PUFA intake probably has litle or no effect on HDL (moderate‐quality evidence).
Eighteen trials incorporating data from 4674 participants (and 20 trial arms) provided data on HDL. Meta‐analysis suggested that increasing PUFA intake had little or no effect on HDL, without heterogeneity (MD ‐0.01 mmol/L, 95% CI ‐0.02 to 0.01, I2 = 0%; Analysis 3.52). The funnel plot suggested that some trials with lower HDL in the higher PUFA arms may be missing, and adding any such trials into the meta‐analysis would tend to lead to lower HDL with higher PUFA. We are aware of five trials that measured HDL but did not report the data in a way that could be incorporated into meta‐analysis (EPOCH 2011; ORL 2013; Rossing 1996; WAHA ‐ Ros 2016; WINS 2006).
3.52. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 52 Serum HIGH DENSITY LIPOPROTEIN (HDL, mmoL/L).
Sensitivity analyses, including fixed‐effect analysis, all confirmed lack of an important effect (Analysis 3.53; Analysis 3.54).
3.53. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 53 HDL, mmoL/L ‐ SA.
3.54. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 54 HDL, mmoL/L ‐ SA fixed‐effect.
Subgrouping did not suggest differential effects of PUFA dose, duration, primary or secondary prevention, baseline PUFA intake, replacement, sex, age, statin use or intervention type (Analysis 3.55; Analysis 3.56; Analysis 3.57; Analysis 3.58; Analysis 3.59; Analysis 3.60; Analysis 3.61; Analysis 3.62; Analysis 3.63).
3.55. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 55 HDL, mmoL/L ‐ subgroup by PUFA dose.
3.56. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 56 HDL, mmoL/L ‐ subgroup by duration.
3.57. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 57 HDL, mmoL/L ‐ subgroup by primary or secondary prevention.
3.58. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 58 HDL, mmoL/L ‐ subgroup by baseline PUFA dose.
3.59. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 59 HDL, mmoL/L ‐ subgroup by replacement.
3.60. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 60 HDL, mmoL/L ‐ subgroup by sex.
3.61. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 61 HDL, mmoL/L ‐ subgroup by age.
3.62. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 62 HDL, mmoL/L ‐ subgroup by statin use.
3.63. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 63 HDL, mmoL/L ‐ subgroup by intervention type.
We downgraded the GRADE assessment for publication bias. Higher PUFA intake probably has little or no effect on HDL (moderate‐quality evidence).
Lipids ‐ low density lipoprotein (LDL)
Higher PUFA intake probably has little or no effect on LDL (moderate‐quality evidence).
Fifteen trials incorporating data from 3362 participants (and 17 trial arms) provided data on LDL. Meta‐analysis suggested that increasing PUFA intake had little or no effect on LDL, without major heterogeneity (MD ‐0.01 mmol/L, 95% CI ‐0.09 to 0.06, I2 = 44%; Analysis 3.64). The funnel plot suggested that some trials with lower LDL associated with higher PUFA may be missing, adding such trials in would tend to suggest that increasing PUFA reduces LDL. We are aware of three trials that measured LDL but did not report it in a way that could be included in meta‐analysis (Dullaart 1992; EPOCH 2011; ORL 2013).
3.64. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 64 Serum LOW DENSITY LIPOPROTEIN (LDL, mmoL/L).
Sensitivity analyses, including fixed‐effect analysis, all confirmed this lack of effect (Analysis 3.65; Analysis 3.66).
3.65. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 65 LDL, mmoL/L ‐ SA.
3.66. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 66 LDL, mmoL/L ‐ SA fixed‐effect.
Subgrouping did not suggest differential effects of PUFA dose, duration, primary or secondary prevention, baseline PUFA intake, replacement, sex, age, statin use or intervention type (Analysis 3.68; Analysis 3.69; Analysis 3.70; Analysis 3.71; Analysis 3.72; Analysis 3.73; Analysis 3.74; Analysis 3.75).
3.68. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 68 LDL, mmoL/L ‐ subgroup by duration.
3.69. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 69 LDL, mmoL/L ‐ subgroup by primary or secondary prevention.
3.70. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 70 LDL, mmoL/L ‐ subgroup by baseline PUFA dose.
3.71. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 71 LDL, mmoL/L ‐ subgroup by replacement.
3.72. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 72 LDL, mmoL/L ‐ subgroup by sex.
3.73. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 73 LDL, mmoL/L ‐ subgroup by age.
3.74. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 74 LDL, mmoL/L ‐ subgroup by statin use.
3.75. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 75 LDL, mmoL/L ‐ subgroup by intervention type.
We downgraded the GRADE assessment for publication bias. Higher PUFA intake probably has little or no effect on LDL (moderate‐quality evidence).
Tertiary outcomes
We did not formally systematically review tertiary outcomes. Where the included trials reported these outcomes, we collated and analysed them.
Blood pressure, systolic and diastolic
Nine trials reported systolic blood pressure from 7356 participants, and eight trials reported diastolic blood pressure from 7327 participants. There was no suggestion of an effect of increased PUFA on systolic (MD ‐0.47 mmHg, 95% CI ‐2.20 to 1.26, I2 = 47%; Analysis 4.1) or diastolic blood pressure (MD 0.24 mmHg, 95% CI ‐0.55 to 1.02, I2 = 31%; Analysis 4.2). There were insufficient trials to assess the funnel plots, but we are aware of four trials that assessed blood pressure and did not report it fully (EPOCH 2011; MRC 1968; NDHS Open 1st 1968; Rossing 1996), though the data from MRC 1968 are displayed in the forest plot. We did not plan to carry out sensitivity analyses, subgroup analyses or meta‐regressions for these outcomes. Six of the trials in each analysis had low risk of bias from allocation concealment, and six were at low summary risk of bias. Lack of reporting of this commonly collected outcome may suggest publication bias, and the four trials with missing data would tend to confirm this.
4.1. Analysis.
Comparison 4 Higher PUFA vs lower PUFA intake ‐ tertiary outcomes, Outcome 1 SYSTOLIC BLOOD PRESSURE (sBP, mmHg).
4.2. Analysis.
Comparison 4 Higher PUFA vs lower PUFA intake ‐ tertiary outcomes, Outcome 2 DIASTOLIC BLOOD PRESSURE (dBP, mmHg).
Quality of life
One trial (Dodin 2005) assessed the effect of their flaxseed intervention on quality of life, using the MENQOL scale. MENQOL assesses the impact of four domains (vasomotor, psychosocial, physical and sexual) of menopausal symptoms over the previous month with scores ranging from 0 (no impact, high quality of life) to 32 (very poor quality of life in all domains). They found that over 12 months the MENQOL score fell slightly in both groups (intervention group ‐0.23, SD 0.62, N = 85, control group ‐0.14, SD 0.58, N = 94). This suggested little effect of the intervention on quality of life related to menopausal symptoms. We found no further data on quality of life in the included trials, though dropouts may provide some information on how willing to continue the interventions participants were.
Economic costs
We did not find any data on economic costs in the included trials.
Serious adverse events
Adverse events reported in one or two trials each included the following, with no clear effects for any outcomes (Analysis 4.3).
4.3. Analysis.
Comparison 4 Higher PUFA vs lower PUFA intake ‐ tertiary outcomes, Outcome 3 SERIOUS ADVERSE EVENTS (SAEs).
Pulmonary embolism (RR 2.15, 95% CI 0.48 to 9.57, I2 = 0%, 2 trials, 2087 participants, 7 events)
Mutliple sclerosis worsened or acute attack (RR 1.11, 95% CI 0.95 to 1.30, I2 = 0%, 2 trials, 268 participants, 142 events)
Bleeding (RR 0.80, 95% CI 0.34 to 1.85, I2 = 0%, 2 trials, 748 participants, 21 events)
Gastrointestinal hospitalisation (RR 1.75, 95% CI 0.53 to 5.79, 1 trial, 200 participants, 11 events)
Retinopathy diagnosis (RR 1.02, 95% CI 0.56 to 1.86, 1 trial, 2424 participants, 42 events)
Effects of increased PUFA intake on dementia and neurocognitive outcomes (Jimoh 2017), type 2 diabetes and measures of glucose metabolism (Brown 2017), inflammatory bowel disease and inflammatory markers (Thorpe 2017), cancers (Hanson 2017b), depression and anxiety (Hanson 2017a) and functional outcomes (Abdelhamid 2017) are systematically reviewed elsewhere, so we have not reported results of effects seen in trials included in this review, as they are a potentially misleading subset. The systematic reviews on these health outcomes are not yet published, so we have provided references to their protocols so that the systematic reviews can be located.
Effects of increasing PUFA on pulmonary embolism and bleeding are unclear as the evidence is of very low quality.
Dropouts
Twenty‐seven trials reported 1675 dropouts, suggesting that being in the higher or lower PUFA arm did not make much difference to the likelihood of dropping out (RR 0.99, 95% CI 0.87 to 1.13, I2 = 41%; Analysis 4.4). This may suggest that increasing PUFA is an acceptable intervention.
4.4. Analysis.
Comparison 4 Higher PUFA vs lower PUFA intake ‐ tertiary outcomes, Outcome 4 DROPOUTS.
Discussion
Summary of main results
This Cochrane Review included 49 RCTs randomising 24,272 participants, for one to eight years. We identified 22 potential ongoing trials. Total PUFA dose (the difference in total PUFA between intervention and control arms) was 0.6% E to less than 1% E for 13 trials, 1% E to less than 2% E in 17 trials, 2% E to less than 5% E in eight trials, and 5% E or more from total PUFA in 11 trials. We assessed 11 of the 49 included trials as being at low summary risk of bias.
Increasing PUFA intake probably has little or no effect on all‐cause mortality (risk changes from 7.8% to 7.6%, RR 0.98, 95% CI 0.89 to 1.07, I2 = 0%, 1443 deaths, 24 trials, moderate‐quality evidence, downgraded for imprecision). Increasing PUFA probably reduces the risk of coronary heart disease events (from 14.2% to 12.3%, RR 0.87, 95% CI 0.72 to 1.06, I2 = 45%, 1351 people with coronary heart disease events, 15 trials, moderate quality evidence, downgraded for imprecision and risk of bias combined) and stroke (from 1.2% to 1.1%, RR 0.91, 95% CI 0.58 to 1.44, I2 = 24%, 166 strokes, 11 trials, however the confindence intervals included important harm, low‐quality evidence downgraded once for imprecision and once for risk of bias and imprecision combined). Effects on MACCEs (RR 0.84, 95% CI 0.59 to 1.20, I2 = 79%, 817 events, 2 trials) are unclear as evidence is of very low quality (downgraded for risk of bias, imprecision and inconsistency).
For secondary outcomes we found that increasing PUFA intake probably reduces risk of cardiovascular disease events (from 14.6% to 13.0%, RR 0.89, 95% CI 0.79 to 1.01, I2 = 30%, 2442 events, 21 trials, moderate‐quality evidence). Increasing PUFA intake may slightly reduce risk of coronary heart disease death from 6.6% to 6.1% (RR 0.91, 95% CI 0.78 to 1.06, I2 = 0%, 556 coronary heart disease deaths, 9 trials) and myocardial infarction (RR 0.88, 95% CI 0.78 to 0.99, I2 = 0%, 880 myocardial infarctions, 15 trials) but may increase the risk of peripheral arterial disease (RR 1.20, 95% CI 0.81 to 1.77, I2 = 0%, 97 events, 4 trials) and have little or no effect on cardiovascular mortality (4.8% to 4.9%, RR 1.02, 95% CI 0.82 to 1.26, I2 = 31%, 729 cardiovascular disease deaths, 16 trials), all low‐quality evidence. The effect of increasing PUFA on sudden cardiac death, angina, atrial fibrillation, heart failure and revascularisation is unclear as the evidence is of very low quality.
High‐quality evidence suggests that increasing PUFA intake has little or no effect on total serum cholesterol over at least one year (˜2% change, MD ‐0.12 mmol/L, 95% CI ‐0.23 to ‐0.02, I2 = 79%, 8072 participants, 26 trials), but moderate quality evidence suggests PUFA decreases triglycerides by ˜15% (MD ‐0.12 mmol/L, 95% CI ‐0.20 to ‐0.04, I2 = 50%, 3905 participants, 20 trials) though has little effect on HDL (MD ‐0.01 mmol/L, 95% CI ‐0.02 to 0.01, I2 = 0%, 4674 participants, 18 trials) or LDL (MD ‐0.01 mmol/L, 95% CI ‐0.09 to 0.06, I2 = 44%, 3362 participants, 15 trials) (all moderate‐quality evidence). Increasing PUFA intake probably has little or no effect on body weight (MD 0.76 kg, 95% CI 0.34 to 1.19, I2 = 59%, 7100 participants, 12 trials) or other measures of adiposity. .
There was limited information on blood pressure, quality of life, economic outcomes or adverse health effects. Effects of increasing PUFA on pulmonary embolism and bleeding are unclear as the evidence is of very low quality. Effects of PUFA intake on other serious adverse health effects (cancers, inflammatory bowel disease, depression or anxiety, neurocognitive outcomes, functional outcomes and diabetes) are systematically reviewed and reported elsewhere.
We looked for dose and duration effects using subgrouping and meta‐regression, finding none except a duration effect in atrial fibrillation, with protective effects in shorter trials (up to two years), little or no effect in trials of two to less than four years, and harm in longer trials (Analysis 2.43). We found no evidence of no linear dose effects, though assessment of PUFA doses actually delivered by trials were difficult to ascertain, often due to missing control information. Baseline PUFA intake (or PUFA intake in the control group as a proxy) were poorly reported, reducing our ability to see subgroup differences ‐ there was a suggestion of greater benefit of PUFA with lower baseline PUFA intake for cardiovascular disease mortality, but not for other cardiovascular outcomes.
There were no clear patterns of differential effects across outcomes by primary or secondary prevention, replacement, sex, age, statin use or intervention type. Subgrouping did not suggest differences between effects in primary or secondary prevention, except for atrial fibrillation, where increasing PUFA in primary prevention was harmful and increasing PUFA in secondary prevention was beneficial (Analysis 2.44). For cardiovascular disease mortality there was a suggestion of harm from increasing PUFA intake in younger adults, smaller levels of harm in middle‐aged adults and benefit in those aged at least 65 years (Analysis 2.10). This pattern was repeated for cardiovascular disease events, except that some benefit was seen in the middle‐aged group (Analysis 2.22), although this pattern was not seen for other outcomes. Dietary advice had little or no effect on adiposity (Analysis 3.12; Analysis 3.24), while dietary advice and supplements to increase PUFA appeared to reduce triglyceride to a greater extent than supplemental foods or diet provided (Analysis 3.51).
3.51. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 51 TG, mmoL/L ‐ subgroup by intervention type.
Overall completeness and applicability of evidence
Included trials randomised 24,272 participants over periods of at least a year. Participants were men and women aged from their 20s into their 80s but most trials recruited participants with a mean age of 50 to 65 years. Most coronary heart disease events occurred in these 'middle‐aged' trials, but most deaths occurred in trials of older adults. Twenty‐two trials included 70% or more men, and many of these were exclusively in men, six included 70% or more women, and sixteen included a balanced proportion of men and women. Despite this, most coronary heart disease events (1289 of 1351) and deaths (1134 of 1443) occurred in trials mainly of men, so while women are included it is not clear whether any effects are generalisable to them. Similarly, while younger adults are included, most events occurred in older adults, which partly explains the lack of appearance of some trials of younger adults in many of the analyses on health events. We included these trials as they reported data on lipids or adiposity, or both, and sometimes one or two health events.
Two included trials were from countries with developing economies (Mendis 2001 from Sri Lanka and Vijayakumar 2014 from India) but while both provided lipid data, the only events were two deaths in Vijayakumar 2014. This means that the bulk of the information in this review is from countries with developed economies. Some trials were from areas with non‐western dietary practices, including South Korea (Ahn 2016), Japan (DIPP‐Tokudome 2015; Doi 2014; Mita 2007; ORL 2013), Sri Lanka (Mendis 2001), and India (Vijayakumar 2014), however often the dietary intakes of these populations at baseline and during the trial were not well described.
Our data spring from trials conducted from the 1960s (MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; Rose 1965; Veterans Admin 1969) to the present, and during this sixty year period cardiovascular disease incidence has altered. For example, in 2010 one in four deaths worldwide was from ischaemic heart disease or stroke, up from one in five in 1990 (Lozano 2012). But this worldwide increase hides more complex trends, with different rates and trends in different parts of the world. Death rates from CHD in men aged 35 to 74 were 839/100,000 in Ukraine in 2000, but ˜200/100,000 in the USA and UK, and only 54/100,000 in Japan. Rates in women were lower but followed the same trends by country (WHO 2004). In the UK as in most high‐income countries age‐standardised death rates from coronary heart disease in adults of all ages fell by 72% between 1979 and 2013, and stroke mortality fell by 71% over the same period (Bhatnagar 2016). Globally age‐adjusted annual incidence of stroke in men and women of all ages has increased slightly from 1990 to 2010, but this masks falls in high‐income countries and rises in low and middle‐income countries (Feigin 2014; Carandang 2006). While we assess effects using risk ratios in this review so that we can see relative effects regardless of baseline incidence, baseline incidence affects absolute effects including numbers needed to treat. Our results suggest that we need to increase total PUFA intake in ˜53 people to prevent one person experiencing a CHD event, in ˜63 people to prevent a CVD event, and even more for CHD death and stroke. But in populations at greater risk NNTs will be lower (fewer people needing to increase their PUFA to prevent one person experiencing an event), and in lower risk populations NNTs will be higher. The greatest import of dietary increases in total PUFA intake is likely to be in low‐ and middle‐income countries where rates of CVD are higher (and rising).
Results relate to both primary and secondary prevention of cardiovascular disease. However, as would be expected, most events occurred in those with existing cardiovascular disease. For example, 1130 of 1443 deaths (78%) were in participants with cardiovascular disease at baseline (Analysis 1.6). Effects in the secondary prevention group (risk barely altering from 11.7% in the lower PUFA arm to 11.5% (95% CI 10.1 to 13.1%) in the higher PUFA arm) were similar to those without cardiovascular disease at baseline (primary prevention, risk barely altering from 3.4% in the lower PUFA arm to 3.3% (95% CI 2.7 to 4.1%) in the higher PUFA arm). For cardiovascular disease events 2013 of 2435 people (83%) experiencing cardiovascular disease events had existing cardiovascular disease at baseline (Analysis 2.18). Risk of a cardiovascular disease event fell by 2.5% from 23.3% to 20.8% (95% CI 17.5% to 24.5%) in secondary prevention, and fell by 0.9% from 5.8% to 4.9% (95% CI 4.2% to 5.9%) in primary prevention when increasing PUFA intake.
We are aware of missing trials. We were unable to access data for AFORRD; NCT00309439; NCT00410020; Chandrakala 2010 or ACTRN12610000594022, which all appeared likely to be eligible. They were all registered before the end of 2010 or had planned finish dates up to the end of 2015, hence appear to be completed but unpublished (see Characteristics of ongoing studies). We are also aware of some missing data within included trials ‐ for example there were deaths in Bates 1977 but they were reported combined with dropouts and the trial author no longer has the data, and two deaths in Simon 1997 not reported by intervention arm. Houtsmuller 1979 reported coronary heart disease events and mortality, but not all‐cause deaths or cardiovascular disease events. Sixteen trials (Ahn 2016; Black 1994; Dullaart 1992; EPOCH 2011; HERO‐Tapsell 2009; MARINA ‐ Sanders 2011; MRC 1968; NDHS Faribault 1968; NDHS Open 1st 1968; ORL 2013; Rossing 1996; Simon 1997; Veterans Admin 1969; Vijayakumar 2014; WAHA ‐ Ros 2016; WINS 2006) reported at least one continuous outcome without variance data or without change data and with baseline data too different to allow us to use end data (so we missed at least six sets of data on total cholesterol, eight on triglyceride, seven on body weight and four sets on blood pressure). On the other hand, we were provided the full dataset on events for DART fat 1989, so were able to include data for almost all of our outcomes, data for Sydney Diet‐Heart 1978 were well reported in recent re‐analyses and the trial authors kindly augmented these data, and outcome data in Veterans Admin 1969 were very well reported, so data are probably almost complete for these large trials. Authors of many other trials provided some additional data on outcomes and/or confirmed that no participants experienced specific outcomes.
We identified 22 potential ongoing trials (Characteristics of ongoing studies), but these trials are very difficult to assess for inclusion in terms of total PUFA dose, until further details are published. We will formally assess these trials for inclusion when we update this review. Two of these trials specifically include women, who are underrepresented in trials already included in this review (NCT01784042; NCT02295059). Other ongoing trials appear generally to be in both men and women, which will increase the proportion of data provided by women. Two trials appear to be planned for developing economies (India Chandrakala 2010 and China n‐3 on plasma lipid), but the majority appear to be carried out in Europe, North America and Australia. It is not possible to assess whether any of these trials will document trans fat intake or status, or indeed intake or status of other key fats and nutrients. There is no suggestion that any of these trials are targeting participants with low baseline total PUFA intakes. Overall, they may begin to address information about women more thoroughly, but not deficiencies in the database of information on participants from lower‐income countries, and they are not clearly of higher quality when it comes to assessment of dietary intakes and nutritional status before and during the trials.
We all consume PUFA already (it is essential in our diets). It would be useful to understand whether increasing PUFA in people who eat very little has the same effect as increasing PUFA in people already consuming large amounts. Unfortunately few trials assessed overall dietary intake of participants at baseline or through the trial. Only 16 of the 49 included trials provide information on baseline or control‐arm PUFA intake (we used control‐arm PUFA intake in lieu of baseline PUFA intake where no baseline intake was given and the control arm were on 'usual intake'). Of these 16 trials, participants in seven consumed less than 6% E from PUFA and nine 6% E and above. Despite these limited data there is a pattern across the review that effects in participants with less than 6% E PUFA intake at baseline are positive, but effects in those with higher baseline PUFA intake are negative or neutral ‐ though we do not see statistically significant differences between subgroups and data are very limited. The pattern is evident for coronary heart disease events (Analysis 1.19), stroke (Analysis 1.31), MACCEs (Analysis 1.45), cardiovascular disease mortality (Analysis 2.7), and cardiovascular disease events (Analysis 2.19), but not in all‐cause mortality where no effects are seen in any group (Analysis 1.7) or coronary heart disease mortality (Analysis 2.31), and we lack data for atrial fibrillation. This relationship needs to be checked in future trials, but suggests that increasing total PUFA intake to at least 6% E may be appropriate.
Other subgrouping and meta‐regression effects that would tend to support true effects of increasing total PUFA on some cardiovascular outcomes include seeing greater effects with higher PUFA doses or with longer duration (for dichotomous outcomes). We consistently do not see dose or duration effects within the review, and this weakens our findings of health effects arising from increasing PUFA.
Total PUFA is the sum of omega‐3, omega‐6 and some omega‐9 fats, which may have their own specific effects on our outcomes. We have assessed specific effects of omega‐3 (Abdelhamid 2018) and omega‐6 (Hooper 2018) in separate reviews, but this review aims to assess whether there is a group effect of PUFAs. It would be useful to assess effects of omega‐3/omega‐6 ratio in this review ‐ but these data are not available. Similarly data on trans fats would be useful, as it is possible that some trials increased trans fats when providing PUFA (through use of partially hydrogenated fats). There is evidence that trans fats may be harmful (de Souza 2015), and so may confound our understanding of the PUFA trials. Unfortunately almost no information on trans fat intake was found, so we could not assess this issue.
Despite systematic review evidence that omega‐3 fats do not influence cardiovascular disease risk (Abdelhamid 2018) there is a theory that the ratio of omega‐3 to omega‐6 fats is important for cardiovascular health and body weight (Simopoulos 2016). We planned to subgroup by change in the omega‐3/omega‐6 fat ratio, assessing whether the intervention primarily increased omega‐3 fats (putting up the ratio) or omega‐6 fats (lowering the ratio). However, only three trials (DIPP‐Tokudome 2015; PREDIMED 2013; WAHA ‐ Ros 2016) reported both omega‐3 and omega‐6 intakes (understanding supplemental intakes only would not be adequate). This means that we cannot use this review to assess health effects of altering the omega‐3/omega‐6 ratio.
There were no clear dose or duration effects in the review. While we would expect that replacing saturated fat, MUFA or carbohydrate with PUFA would give different health effects, we do see greatest reduction in total cholesterol with replacement of saturated fat (Analysis 3.35), and greatest reduction of triglyceride with replacement of MUFA (Analysis 3.47). However, there are no statistically significant differences between subgroups for these outcomes or any other health outcomes. There are no clear replacement effects. It is also surprising to see increased PUFA intake reducing total cholesterol and triglyceride (Analysis 3.28; Analysis 3.40), with no change in LDL (Analysis 3.64). The Friedewald equation (Friedewald 1972) states that 'total cholesterol = LDL + HDL + triglyceride/2.19' (all components in mmol/L), so for the changes of total cholesterol and triglyceride we see, we would expect similar falls in LDL, but this is not seen. Reasons for this are not clear, but it is possible that changes in very low density lipoprotein (VLDL) added to triglyceride reductions and very small changes in HDL and LDL could add up to the overall total cholesterol reduction.
Overall, included data are applicable, but not entirely complete. While further trials of increasing PUFA intake in women and in developing economies are needed, they should include participants with low PUFA intakes at baseline, as well as those with higher intakes. Dietary advice needs to ensure that trans fat intake is kept low as PUFA increases, and intakes of all fat fractions, including trans fat intakes should be assessed and checked using reliable biomarkers.
Quality of the evidence
GRADE assessment includes consideration of risk of bias, inconsistency, indirectness, publication bias and imprecision (Table 1 and Table 2).
We assessed risk of bias by assessing whether effect sizes and directions altered when limited to trials at low risk of bias from allocation concealment, from attention bias, from compliance, trials at low summary risk of bias, with trials registry registration (or pre‐2010), without industry funding, and that randomised at least 100 or 250 participants. Sensitivity analyses generally supported the primary analysis for all‐cause mortality, coronary heart disease mortality, cardiovascular disease mortality, weight and lipid outcomes (Analysis 1.2; Analysis 2.2; Analysis 2.14; Analysis 2.26; Analysis 3.2; Analysis 3.14; Analysis 3.29; Analysis 3.41; Analysis 3.53; Analysis 3.65), so we did not downgrade these for risk of bias. Either sensitivity analyses contradicted the primary analyses (for coronary heart disease events and stroke; Analysis 1.14; Analysis 1.26) or there were no trials at low summary risk of bias, or low risk of compliance problems (MACCEs and atrial fibrillation; Analysis 1.40; Analysis 2.40), so we downgraded these outcomes for risk of bias.
We judged imprecision by whether the 95% CI included the null, and whether it included important benefits and harms. Where both important benefits and harms were included within the confidence interval we downgraded twice, where it only included the null we downgraded once unless there was a very small overlap. We downgraded the evidence on all primary and some secondary outcomes for imprecision, suggesting that included trials may still be underpowered to determine effectiveness on these outcomes. There was no evidence of under‐powering for lipid outcomes.
We judged inconsistency using the I2 statistic for each primary and secondary outcome. We considered an I2 statistic greater than 50% to be a problem and led to us downgrading for inconsistency unless we found an element that explained that inconsistency (through subgrouping or meta‐regression). We downgraded the primary outcome, MACCEs for inconsistency, and also secondary outcomes, atrial fibrillation, BMI and triglyceride.
We judged indirectness according to whether data on an outcome related to both women and men, those with and without cardiovascular disease at baseline, and whether low‐ and middle‐income, and high‐income countries were represented. While indirectness is important, we suspect that the mechanisms of action of PUFA are similar in all these populations so we did not downgrade for indirectness.
We judged publication bias according to whether there was any suggestion of publication or small study bias in the funnel plot, or where we knew that data were missing that differed from the summary assessment. We downgraded the secondary outcomes, coronary heart disease mortality, body weight, HDL and LDL for publication bias.
Trial funding can be an important indicator of study bias but is not included in 'Risk of bias' assessment. Sixteen trials reported funding sources, which appeared to be purely from national or charitable agencies, seven trials appeared to be directly funded by industrial sources, two funded by bodies set up to promote specific foods, 16 by some governmental or charity sources with additional funding or support from commercial sources, two trials included authors on industry honoraria, and five trials did not report funding.
Trial pre‐registration or early publication of a trial protocol is helpful in understanding potential biases in data presentation (including outcome selection bias). We ran sensitivity analyses assessing whether trials that were pre‐registered or had a published protocol suggested different effects than trials without such documentation. We found trials registry entries for most included trials published after 2010. Making datasets of all outcomes available via trials registers would also help systematic reviewers to gather all appropriate data, and minimise publication bias.
Applying the GRADE criteria suggests that we have high‐quality evidence on effects of PUFA on serum total cholesterol (not downgraded), moderate‐quality evidence on all‐cause mortality, coronary heart disease events, cardiovascular disease events, body weight, triglyceride, HDL and LDL (each downgraded once), and low‐quality evidence for stroke, cardiovascular disease mortality, coronary heart disease mortality and BMI (each downgraded twice). All other evidence was of very low quality. Reasons for grading, and statements of findings based on these levels of evidence are found in Table 1 and Table 2.
Potential biases in the review process
We conducted a large number of sensitivity analyses and subgroup analyses for each primary outcome, as well as some secondary outcomes (key outcomes). The danger in these is that subgroups may be spuriously statistically significant, but we used them to check the stability of our primary analyses, as well as to try to explain heterogeneity, assessing for dose effects, duration effects and differential effects by what PUFA replaces in the diet. We have tried not to over‐interpret any of these analyses.
We only considered trials with interventions or follow‐up periods of 12 months or more, making the review relevant for public health interventions. We considered including shorter trials, but were concerned that if we found no effect then this might be due to including trials too short to reflect health effects of increasing or decreasing PUFA intake. The decision on duration depended on assumed mechanism of action of PUFA. If we assumed a cholesterol‐led atherosclerotic mechanism then we could justify deciding only to include trials of at least two years' duration. However another mechanism discussed for omega‐3 and omega‐6 fats includes inflammation ‐ likely to work more quickly than atherosclerosis, so allowing six months for equilibration of body tissues with the new dietary intake, and a further six months to allow for reflection of this new status in health outcomes, appears most appropriate to us. We ran subgroup analyses to assess whether trial duration made an important difference to our primary outcomes. We did not find any suggestion of greater effects in longer trials (those of at least four years) compared to shorter trials (one to less than two years, or two to less than four years) for all‐cause mortality (Analysis 1.5), coronary heart disease events (Analysis 1.17), or stroke (Analysis 1.29). Only two trials provided data on MACCEs, but these two trials did suggest a protective effect in the longer trial (Analysis 1.43). Meta‐regression did not suggest duration effects for any primary outcome. Similarly there were no duration effects in subgrouping or meta‐regression for cardiovascular disease mortality (Analysis 2.5), cardiovascular disease events (Analysis 2.17), or coronary heart disease mortality (Analysis 2.29), though visual inspection tended to suggest greater protection in the shortest trials, despite them reporting few events. There was a suggestion of a duration effect for atrial fibrillation, but the suggestion was for greater effect in shorter trials, and no effect in longer trials (Analysis 2.43). Conversely participants taking more PUFA gained more weight and their BMI rose more in longer trials (Analysis 3.5; Analysis 3.17).
Our inclusion criteria could potentially cause some bias. Few trials directly aimed to assess effects of increasing PUFA with usual or lower PUFA intake, so included trials are a combination of trials that aimed to increase PUFA, trials that aimed to increase omega‐3 or omega‐6 fats and resulted in an increase of at least 10% of baseline PUFA intake, and trials that aimed to reduce total fat intake and resulted in a decrease of at least 10% of baseline PUFA intake (while not aiming to alter dietary components other than fat or replacements for the change in PUFA). This allowed assessment of effects of altering PUFA intake, but we had to exclude trials that may have been relevant but did not report aims for or effects on total PUFA, so we may be missing other trials that would be relevant to this review. It is also possible that we included trials that aimed to increase or decrease total PUFA but did not achieve the planned changes in PUFA intake. To help guard against this we also conducted sensitivity analyses around compliance, removing trials where we did not have biomarker confirmation of a difference in PUFA status between trial arms.
Even though we excluded clearly multifactorial trials, when we alter one dietary component, other components inevitably alter too. For example, when PUFA intake is increased we need to reduce energy intake elsewhere, so saturated fat or carbohydrate intake may fall to compensate. The danger is that we may see a health effect from increasing PUFA that is actually due to a reduction in saturated fat. However, in this review some trials that increased PUFA reduced saturated fat, and in other trials PUFA and saturated fat were both reduced in the intervention arm. Regardless of which arm the trial considered to be the intervention arm we compared the arm with higher PUFA against the arm with lower PUFA to look for consistent effects of higher PUFA intake. Because saturated fat (and other dietary components) sometimes moved with PUFA and sometimes moved in the opposite direction the only consistent difference between arms was in PUFA intake. This means that health effects noted are unlikely to be spurious effects of other dietary components. Combining higher versus lower PUFA intake across different types of trials may balance out effects of other dietary (fat and non‐fat) components while providing power to assess health effects of changing PUFA.
Agreements and disagreements with other studies or reviews
We recently published a Cochrane Review of long‐term RCTs that assessed effects of reducing saturated fats, replacing them with a variety of other energy sources (Hooper 2015a). This review found no effect of reducing saturated fats on all‐cause mortality or cardiovascular disease mortality, but the evidence suggested that reducing saturated fats reduced the risk of cardiovascular disease events (RR 0.83, 95% CI 0.72 to 0.96, I2 = 65%, including 4377 events in over 53,000 randomised participants). Subgrouping, assessing whether the saturated fats were being replaced by PUFA, MUFA, carbohydrate and/or protein found that there were no statistically significant effects in these subgroups except where saturated fat was being replaced by PUFA (RR 0.73, 95% CI 0.58 to 0.92, I2 = 69%, 884 events in over 3000 participants). Hooper 2015a confirmed results expected from the Friedewald equation (Friedewald 1972). The trials included in the saturated fat review and this one are distinct due to rather different inclusion criteria (for example, the saturated fat review only included trials of at least two years duration, and included trials with dietary interventions decreasing saturated fat plus altering other dietary variables). The implications of the reviews are similar ‐ Hooper 2015a suggests that reducing saturated fat and replacement by polyunsaturated fats reduces the risk of cardiovascular disease events, while this review also suggests that increasing PUFA may reduce the risk of cardiovascular disease events, as well as coronary heart disease mortality (as well as reducing triglyceride, but not total cholesterol).
Two previous systematic reviews of RCTs assessed effects of PUFA replacing saturated fat: Ramsden 2010 and Mozaffarian 2010. Ramsden 2010 included seven trials that compared increasing mixed omega‐3 and omega‐6 PUFA or omega‐6 alone and replacing dietary saturated fat with usual dietary intake. Their data suggested no effect on all‐cause mortality (RR 0.99, 95% CI 0.89 to 1.11), but likely reductions in coronary heart disease mortality (RR 0.91, 95% CI 0.74 to 1.10), and myocardial infarction and cardiac death combined (RR 0.85, 95% CI 0.73 to 0.99). These are similar results to this review (no effect on all‐cause mortality, reductions in coronary heart disease mortality and myocardial infarction). Ramsden 2010 included fewer trials than this review, four trials that we included (MRC 1968; Rose 1965; Sydney Diet‐Heart 1978; Veterans Admin 1969), and three we excluded. We excluded two for being multifactorial (Oslo Diet‐Heart 1966; STARS 1992) and one for having inconsistent enrolment so that many participants were included for less than 12 months continuously (Minnesota Coronary 1989). The other systematic review, Mozaffarian 2010, also included seven trials replacing saturated fat with PUFA, three that we included (DART fat 1989; MRC 1968; Veterans Admin 1969), and four that we excluded. One we excluded due to lack of randomisation (Finnish Mental Hosp 1972), one for inconsistent enrolment (Minnesota Coronary 1989), and two because the intervention was multifactorial (Oslo Diet‐Heart 1966; STARS 1992). Mozaffarian 2010 found that increasing PUFA by replacing saturated fat reduced coronary heart disease events by 19% (RR 0.81, 95% CI 0.70 to 0.95), unlike this review, where we found that the evidence was of very low quality, so could not assess effects on this outcome.
Recent observational data of more than 30,000 adults aged over 30 years from the National Health and Nutrition Examination Survey (NHANES) was not entirely consistent with our results. They suggested that the tertile of adults with highest PUFA intake were at lowest risk of all‐cause mortality (HR 0.94, 95% CI 0.90 to 0.98 compared to the tertile with lowest intake) and cardiovascular disease mortality (HR 0.93, 95% CI 0.89 to 0.97), when adjusted for ethnicity, BMI, alcohol intake, smoking, education, physical activity, fibre intake and blood pressure (Ricci 2018).
We found that increased PUFA intake had little effect on body weight, but other systematic reviews have found that reducing dietary fat (including PUFAs) appears to result in lower weight in adults. As weight gain may increase cardiovascular risk, this may work against more positive lowering of total cholesterol and triglycerides when assessing overall effects of increasing PUFA on cardiovascular disease (Hooper 2015b).
We interpreted the total cholesterol and weight results using QRisk 2‐2017 (QRISK 2‐2017). In a Pakistani non‐smoking male aged 64 years without existing cardiovascular disease or diabetes, height 173 cm, weighing 81 kg with systolic blood pressure of 145 mmHg and total cholesterol 5.46 mmol/L, HDL 1.31 (total cholesterol/HDL 4.17) at baseline (typical values for the trials in this review) their 10‐year QRISK 2‐2017 score would be 23.5%. A reduction of total cholesterol by 0.12 mmol/L, HDL by 0.01 mmol/L and weight rise of 0.76 kg (the changes indicated by this review) reduces the QRISK 2‐2017 score slightly to 23.2%. QRISK 2‐2017 suggests that in 1000 people with the same risk factors, 235 are likely to have a heart attack or stroke within the next 10 years at baseline, falling to 232 having a heart attack or stroke following increased PUFA intake. Three people of the 1000 would be prevented from experiencing a heart attack or stroke by the increased PUFA. This is a smaller effect than the estimated reduction from 58 per 1000 to 49 (95% CI 42 to 59) per 1000 predicted for primary prevention of cardiovascular disease events within this review (Table 2).
Authors' conclusions
Implications for practice.
Increasing polyunsaturated fatty acid (PUFA) intake probably makes little or no difference (neither benefit nor harm) to all‐cause mortality and probably slightly reduces the risk of coronary heart disease events and cardiovascular disease events (all moderate‐quality evidence). Increased PUFA intake may slightly reduce risk of coronary heart disease mortality and stroke (although for stroke the confidence intervals include important harm), but may have little or no effect on cardiovascular disease mortality (all low‐quality evidence). Increasing PUFA probably reduces triglyceride, but has little or no effect on total cholesterol and probably has little or no effect on high‐density lipoprotein (HDL) or low‐density lipoprotein (LDL) and with little change in body weight.
This suggests that increasing PUFA intake may have beneficial effects on risk of cardiovascular disease events, coronary heart disease mortality, coronary heart disease events and stroke. The mechanism may be via reduction of triglyceride.
Implications for research.
Further trials assessing cardiovascular effects of increasing PUFA intake in women and people living in developing economies are needed. Given the low power for assessing effects by baseline PUFA, more research in populations with a low baseline intake of < 6% E is needed to understand whether there is greater benefit from increasing PUFA intake in these groups. Further trials should include participants with low PUFA intakes at baseline, as well as those with higher intakes. Dietary advice needs to ensure that trans fat intake is kept low as PUFA increases. Intake and status of all fat fractions, including trans fat, should be assessed and checked using reliable biomarkers.
What's new
| Date | Event | Description |
|---|---|---|
| 26 November 2018 | New citation required but conclusions have not changed | Corrected interpretation of effect sizes on TC and adiposity. There was little or no effect of increased PUFA on TC, body weight and BMI (as effect size represented < 5% change from baseline for TC and < 2% change from baseline for adiposity measures). Relevant methodology clarified. |
| 26 November 2018 | Amended | Amendments made in abstract, plain language summary, results, discussion, conclusions and study flow. |
Acknowledgements
Thank you, to all of the authors of primary trials who kindly provided us with the best set of data available, including: D Kromhout, Wageningen University (AlphaOmega ‐ ALA; AlphaOmega ‐ EPA+DHA); HS Black, Veterans Affairs Medical Center (Black 1994); J Brox, University hospital of North Norway (Brox 2001); ML Burr, University of Wales and A Ness, University of Bristol (DART fat 1989); S Tokudome, National Institue of Health and Nutrition, Japan (DIPP‐Tokudome 2015); S Dodin, Universite Laval (Dodin 2005); RPF Dullaart, University of Groningen, Netherlands (Dullaart 1992); D Schoenfeld, Harvard Medical School (FAAT ‐ Leaf 2005); London, P Metcalf, University of Auckland, New Zeland (Ley 2004); T Sanders, King's College, London, UK (MARINA ‐ Sanders 2011); T Sheldon, University of York, UK (MRC 1968); M James, Royal Adelaide Hospital, Australia (Proudman 2015); MS Simon, Wayne State University, Simon 1997; C Ramsden, National Institutes of Health, USA, D Zamora, University of North Carolina and Boonseng Leelarthaepin, retired (Sydney Diet‐Heart 1978); M Vijayakumar, Amrita Institute of Medical Sciences, India (Vijayakumar 2014); J Sabaté, Loma Linda University, California (WAHA ‐ Ros 2016); E Scorletti, University of Southampton, UK (WELCOME 2015).
Thanks also to the authors who replied but were not able to provide further details or confirmed no relevant outcomes, including: D Bates, Royal Victoria Infirmary, Newcastle on Tyne, R Dworkin, University of Rochester, UK, (Bates 1977; Bates 1978; Bates 1989); MB McIllmurray, Cancer Care, UK (McIllmurray 1987); B Puri, Imperial College London (Puri 2005); M Raitt, Oregon Health & Science University, USA (Raitt 2005); DP Rose, American Health Foundation (WINS 2006).
Thank you to Juan‐Pablo Casas for discussions on the protocol, and to all Cochrane Heart staff and editors for fast and helpful comments and support. Thank you also to the World Health Organization for commissioning and funding the review.
This review was carried out by the Polyunsaturated Fats and Health (PUFAH) Group, and written by the authors on behalf of the PUFAH group. The Polyunsaturated Fats and Health (PUFAH) group includes Asmaa Abdelhamid1, Zoya Ahmed1, Sarah MA Ajabnoor1,6, Fai K AlAbdulghafoor1, Lena Al‐Khudairy2, Priti Biswas3, Julii Suzanne Brainard1, Charlene Bridges4, Tracey J Brown1, Katherine HO Deane3, Daisy H Donaldson1, Sarah Hanson3, Oluseyi F Jimoh1, Nicole Martin4, Katie Maas1, Helen J Moore5, Alex T O’Brien1, Karen Rees2, Ruksana Sivakaran1, Fujian Song1, Carolyn D Summerbell5,, Gabrielle C Thorpe3, Xia Wang1 , Ailsa Welch1, Lauren Winstanley1, Helen V Worthington7 and Lee Hooper1 (1Norwich Medical School, University of East Anglia, 2Warwick Medical School, University of Warwick, 3School of Health Sciences, University of East Anglia, 4Cochrane Heart, University College London, 5Durham University, 6Clinical Nutrition Department, Faculty of Applied Medical Sciences, King Abdulaziz University, KSA, 7School of Dentistry, University of Manchester).
Appendices
Appendix 1. Searches run for this review, to 27 April 2017
These searches have each been run from database inception, then de‐duplicated with each other. The RCT filter for MEDLINE is the Cochrane sensitivity and precision‐maximising RCT filter, and for Embase, terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions have been applied (Lefebvre 2011).
CENTRAL
#1 MeSH descriptor: [Fatty Acids, Essential] explode all trees
#2 MeSH descriptor: [Fatty Acids, Unsaturated] this term only
#3 ((polyunsaturat* or poly‐unsaturat*) near/3 fat*)
#4 (poly* adj4 unsat* near/4 fatty acid*)
#5 PUFA
#6 MeSH descriptor: [Fatty Acids, Omega‐6] explode all trees
#7 omega‐6
#8 (n‐6 near/4 acid*) or ("n 6" near/4 acid*)
#9 linoleic acid*
#10 MeSH descriptor: [Corn Oil] this term only
#11 MeSH descriptor: [Cottonseed Oil] this term only
#12 MeSH descriptor: [Olive Oil] this term only
#13 MeSH descriptor: [Safflower Oil] this term only
#14 MeSH descriptor: [Sesame Oil] this term only
#15 MeSH descriptor: [Soybean Oil] this term only
#16 ((corn or maize or mazola) near/4 oil*)
#17 (cottonseed* or (cotton next seed*))
#18 (olive near/4 oil*)
#19 (safflower near/4 oil*)
#20 (sesame near/4 oil*)
#21 ((soy bean or soybean) near/4 (oil* or fat*))
#22 (so?a near/4 oil*)
#23 so?aoil*
#24 (soy near/4 oil*)
#25 (sunflower near/4 oil*)
#26 helianth*
#27 (grapeseed near/4 oil*)
#28 (canola near/4 oil*)
#29 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
MEDLINE Ovid
1. exp fatty acids, essential/
2. fatty acids, unsaturated/
3. ((polyunsaturat* or poly‐unsaturat*) adj3 fat*).ti,ab.
4. (poly* adj4 unsat* adj4 fatty acid*).ti,ab.
5. PUFA.ti,ab.
6. exp fatty acids, omega‐6/
7. omega‐6.ti,ab.
8. (n‐6 adj4 acid*).ti,ab.
9. linoleic acid*.ti,ab.
10. corn oil/ or cottonseed oil/ or olive oil/ or safflower oil/ or sesame oil/ or soybean oil/
11. ((corn or maize or mazola) adj4 oil*).ti,ab.
12. (cottonseed* or (cotton adj seed*)).ti,ab.
13. (olive adj4 oil*).ti,ab.
14. (safflower adj4 oil*).ti,ab.
15. (sesame adj4 oil*).ti,ab.
16. ((soy bean or soybean) adj4 (oil* or fat*)).ti,ab.
17. (so?a adj4 oil*).ti,ab.
18. so?aoil*.ti,ab.
19. (soy adj4 oil*).ti,ab.
20. (sunflower adj4 oil*).ti,ab.
21. helianth*.ti,ab.
22. (grapeseed adj4 oil*).ti,ab.
23. (canola adj4 oil*).ti,ab.
24. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25. randomized controlled trial.pt.
26. controlled clinical trial.pt.
27. randomized.ab.
28. placebo.ab.
29. clinical trials as topic.sh.
30. randomly.ab.
31. trial.ti.
32. 25 or 26 or 27 or 28 or 29 or 30 or 31
33. exp animals/ not humans.sh.
34. 32 not 33
35. 24 and 34
Embase Ovid
1. exp essential fatty acid/
2. unsaturated fatty acid/ or docosapentaenoic acid/ or omega 6 fatty acid/ or polyunsaturated fatty acid/
3. ((polyunsaturat* or poly‐unsaturat*) adj3 fat*).ti,ab.
4. (poly* adj4 unsat* adj4 fatty acid*).ti,ab.
5. PUFA.ti,ab.
6. omega‐6.ti,ab.
7. (n‐6 adj4 acid*).ti,ab.
8. linoleic acid*.ti,ab.
9. edible oil/ or canola oil/ or corn oil/ or cotton seed oil/ or olive oil/ or safflower oil/ or safflower oil plus soybean oil/ or sesame seed oil/ or soybean oil/ or sunflower oil/
10. ((corn or maize or mazola) adj4 oil*).ti,ab.
11. (cottonseed* or (cotton adj seed*)).ti,ab.
12. (olive adj4 oil*).ti,ab.
13. (safflower adj4 oil*).ti,ab.
14. (sesame adj4 oil*).ti,ab.
15. ((soy bean or soybean) adj4 (oil* or fat*)).ti,ab.
16. (so?a adj4 oil*).ti,ab.
17. so?aoil*.ti,ab.
18. (soy adj4 oil*).ti,ab.
19. (sunflower adj4 oil*).ti,ab.
20. helianth*.ti,ab.
21. (grapeseed adj4 oil*).ti,ab.
22. (canola adj4 oil*).ti,ab.
23. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. double blind procedure/
25. single blind procedure/
26. randomized controlled trial/
27. ((double* or single*) adj blind*).ti,ab.
28. (random* or placebo*).ti,ab.
29. 24 or 25 or 26 or 27 or 28
30. (animal/ or nonhuman/) not human/
31. 29 not 30
32. 23 and 31
Appendix 2. Searches run for the allied review, to 27 April 2017
The searches for the omega‐3 review (Abdelhamid 2018) were last run in 20 February 2002. We have updated the search strategies and have now re‐run the searches to identify any records added to the databases since the last search. We applied date limits to the terms from the original strategies so that only new records would be found, but have not applied date limits to the newly added terms. The RCT filter for MEDLINE is the Cochrane sensitivity and precision‐maximising RCT filter, and for Embase, we have applied terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
CENTRAL
#1 MeSH descriptor: [Fish Oils] explode all trees
#2 MeSH descriptor: [Linseed Oil] this term only
#3 MeSH descriptor: [Linolenic Acids] this term only
#4 MeSH descriptor: [Fatty Acids, Omega‐3] explode all trees
#5 (fish near/3 oil*)
#6 (oil* near/3 (cod* or marin*))
#7 (omega‐3 or omega3 or (omega* near/5 fat*))
#8 eicosapentaen*
#9 docosahexaen*
#10 (oil* near/3 (flax* or rapeseed* or canola*))
#11 (Linolen* or alpha‐linolen* or alphalinolen*)
#12 (perilla* or linseed* or maxepa*)
#13 (oil* near/3 (rape or colza))
#14 (marin* near/3 lipid*)
#15 (naudicelle* or herring* or sild)
#16 (clupe* near/3 hareng*)
#17 (whitebait or sardine* or sardina* or pilchard* or sprat* or brisling*)
#18 (salmo* near/3 trut*)
#19 (trout or bloater or kipper* or salmon or mackerel* or scomb* or conger* or tuna or tunny or tunafish or tuna‐fish)
#20 (thunnus* or swordfish* or xiphias* or dogfish or scyliorrhinus*)
#21 (crab or crabs or (cancer pagarus))
#22 (DHA or EPA)
#23 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 Publication Year from 2002 to 2016
#24 MeSH descriptor: [Salmoniformes] explode all trees
#25 MeSH descriptor: [Tuna] this term only
#26 MeSH descriptor: [alpha‐Linolenic Acid] this term only
#27 MeSH descriptor: [Flax] this term only
#28 (fish near/3 (diet* or capsul* or nutrit* or supplement*))
#29 (icosapentaen* or docosapentaen*)
#30 (oil* near/3 (purslane or mustard* or candlenut* or stillingia or walnut*))
#31 (laks or lax)
#32 (ALA or DPA)
#33 (algal near oil*)
#34 #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33
#35 #23 or #34
MEDLINE Ovid
1. exp Fish Oils/
2. Linseed Oil/
3. linolenic acids/ or alpha‐linolenic acid/
4. Flax/
5. exp Fatty Acids, Omega‐3/
6. (fish adj3 (diet* or nutrit* or oil* or supplement*)).ti,ab.
7. (oil* adj3 (cod* or marin*)).ti,ab.
8. (omega‐3 or omega3 or (omega* adj5 fat*)).ti,ab.
9. eicosapentaen*.ti,ab.
10. docosahexaen*.ti,ab.
11. (oil* adj3 (flax* or rapeseed* or canola*)).ti,ab.
12. (Linolen* or alpha‐linolen* or alphalinolen*).ti,ab.
13. (perilla* or linseed* or maxepa*).ti,ab.
14. (oil* adj3 (rape or colza)).ti,ab.
15. (marin* adj3 lipid*).ti,ab.
16. (naudicelle* or herring* or sild).ti,ab.
17. (clupe* adj3 hareng*).ti,ab.
18. (whitebait or sardine* or sardina* or pilchard* or sprat* or brisling*).ti,ab.
19. (salmo* adj3 trut*).ti,ab.
20. (trout or bloater or kipper* or salmon or mackerel* or scomb* or conger* or tuna or tunny or tunafish or tuna‐fish).ti,ab.
21. (thunnus* or swordfish* or xiphias* or dogfish or scyliorrhinus* or laks or lax).ti,ab.
22. (crab or crabs or cancer pagarus).ti,ab.
23. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. randomized controlled trial.pt.
25. controlled clinical trial.pt.
26. randomized.ab.
27. placebo.ab.
28. clinical trials as topic.sh.
29. randomly.ab.
30. trial.ti.
31. 24 or 25 or 26 or 27 or 28 or 29 or 30
32. exp animals/ not humans.sh.
33. 31 not 32
34. 23 and 33
35. limit 34 to ed=20020201‐20160721
36. exp salmoniformes/ or tuna/
37. (fish adj3 capsul*).ti,ab.
38. icosapentaen*.ti,ab.
39. docosapentaen*.ti,ab.
40. (oil* adj3 (purslane or mustard* or candlenut* or stillingia or walnut*)).ti,ab.
41. 36 or 37 or 38 or 39 or 40
42. 33 and 41
43. 35 or 42
Embase Ovid
1. exp salmoniformes/ or tuna/
2. fish oil/
3. linseed oil/
4. linolenic acid/
5. Flax/
6. omega 3 fatty acid/
7. (fish adj3 (diet* or nutrit* or oil* or supplement*)).ti,ab.
8. (oil* adj3 (cod* or marin*)).ti,ab.
9. (omega‐3 or omega3 or (omega* adj5 fat*)).ti,ab.
10. (eicosapentaen* or icosapentaen*).ti,ab.
11. docosahexaen*.ti,ab.
12. (oil* adj3 (flax* or rapeseed* or canola*)).ti,ab.
13. (Linolen* or alpha‐linolen* or alphalinolen*).ti,ab.
14. (perilla* or linseed* or maxepa*).ti,ab.
15. (marin* adj3 lipid*).ti,ab.
16. (naudicelle* or herring* or sild).ti,ab.
17. (clupe* adj3 hareng*).ti,ab.
18. (whitebait or sardine* or sardina* or pilchard* or sprat* or brisling*).ti,ab.
19. (salmo* adj3 trut*).ti,ab.
20. (trout or bloater or kipper* or salmon or mackerel* or scomb* or conger* or tuna or tunny or tunafish or tuna‐fish).ti,ab.
21. (thunnus* or swordfish* or xiphias* or dogfish or scyliorrhinus* or laks or lax).ti,ab.
22. (crab or crabs or (cancer adj3 pagarus)).ti,ab.
23. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. random$.tw.
25. placebo$.tw.
26. (doubl$ adj blind$).tw.
27. (singl$ adj blind$).tw.
28. double blind procedure/
29. randomized controlled trial/
30. single blind procedure/
31. 24 or 25 or 26 or 27 or 28 or 29 or 30
32. (animal/ or nonhuman/) not human/
33. 31 not 32
34. 23 and 33
35. (2002* or 2003* or 2004* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016*).dd,em.
36. 34 and 35
37. exp salmonine/
38. (fish adj3 capsul*).ti,ab.
39. docosapentaen*.ti,ab.
40. (ALA or DHA or DPA or EPA).ti,ab.
41. (algal adj oil*).ti,ab.
42. 37 or 38 or 39 or 40 or 41
43. 33 and 42
44. 36 or 43
Data and analyses
Comparison 1. Higher polyunsaturated fatty acids (PUFA) vs lower PUFA intake ‐ primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ALL‐CAUSE MORTALITY | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 2 All‐cause mortality ‐ SA | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias for allocation concealment | 11 | 9639 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.22] |
| 2.2 Low risk of bias for attention | 17 | 13622 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.07] |
| 2.3 Low risk of bias for compliance | 10 | 4776 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.14] |
| 2.4 Low summary risk of bias | 5 | 8092 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.26] |
| 2.5 Trials registry or pre‐2010 | 22 | 18852 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.08] |
| 2.6 No industry funding | 9 | 4508 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.84, 1.42] |
| 2.7 Randomised 100+ participants | 20 | 19029 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.08] |
| 2.8 Randomised 250+ participants | 11 | 17457 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.10] |
| 3 All‐cause mortality ‐ SA fixed‐effect | 24 | 19290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.07] |
| 4 All‐cause mortality ‐ subgroup by PUFA dose | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 4.1 total PUFA < 1.0% E | 5 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.33, 1.34] |
| 4.2 total PUFA 1.0 to < 2.0% E | 9 | 13766 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.13] |
| 4.3 total PUFA 2.0 to < 5.0% E | 4 | 2295 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.20] |
| 4.4 total PUFA ≥ 5.0% E | 6 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.26] |
| 5 All‐cause mortality ‐ subgroup by duration | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 5.1 Medium duration 1 to < 2 years | 8 | 1940 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.55] |
| 5.2 Medium‐long duration 2 to < 4 years | 11 | 8219 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.10] |
| 5.3 Long duration 4+ years | 5 | 9131 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.14] |
| 6 All‐cause mortality ‐ subgroup by primary or secondary prevention | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 6.1 Primary prevention of CVD | 13 | 9549 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.78, 1.20] |
| 6.2 Secondary prevention of CVD | 11 | 9741 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.12] |
| 7 All‐cause mortality ‐ subgroup by baseline PUFA dose | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 7.1 Baseline total PUFA < 6% E | 4 | 3643 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.84, 1.14] |
| 7.2 Baseline total PUFA 6 to < 11% E | 5 | 7826 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.24] |
| 7.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Baseline total PUFA unclear | 15 | 7821 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 8 All‐cause mortality ‐ subgroup by replacement | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 PUFA replaced SFA | 6 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.15] |
| 8.2 PUFA replaced monounsaturated fats | 11 | 12526 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.12] |
| 8.3 PUFA replaced carbohydrate | 5 | 2965 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.58, 1.70] |
| 8.4 PUFA replaced protein | 2 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.44] |
| 8.5 PUFA replaced unclear | 6 | 1227 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.14] |
| 9 All‐cause mortality ‐ subgroup by sex | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 9.1 > 70% men | 13 | 10252 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.10] |
| 9.2 > 70% women | 1 | 2437 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.43, 1.65] |
| 9.3 men & women | 8 | 6498 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.79, 1.29] |
| 9.4 sex not reported | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.51, 1.59] |
| 10 All‐cause mortality ‐ subgroup by age | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 10.1 Mean age < 50 years | 6 | 1852 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.95, 2.27] |
| 10.2 Mean age 50 to < 65 years | 12 | 6040 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 10.3 Mean age 65+ years | 6 | 11398 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 11 All‐cause mortality ‐ subgroup by statin use | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 11.1 < 50% on statins | 18 | 13399 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.10] |
| 11.2 50+% on statins | 4 | 5353 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.26, 1.51] |
| 11.3 Percentage on statins unclear | 2 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.56, 2.37] |
| 12 All‐cause mortality ‐ subgroup by intervention type | 24 | 19290 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 12.1 Dietary advice | 4 | 4739 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.23] |
| 12.2 Supplemental foods & diet provided | 5 | 11104 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.10] |
| 12.3 Supplements (capsules & unusual foods) | 12 | 2391 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.52, 1.11] |
| 12.4 Any combination | 3 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.72, 1.74] |
| 13 CORONARY HEART DISEASE (CHD) EVENTS: myocardial infarction (fatal or non‐fatal) or angina | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 14 CHD events ‐ SA | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Low risk of bias for allocation concealment | 5 | 5946 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.73, 1.78] |
| 14.2 Low risk of bias for attention | 11 | 7090 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.02] |
| 14.3 Low risk of bias for compliance | 7 | 4006 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.17] |
| 14.4 Low summary risk of bias | 4 | 5826 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.76, 1.81] |
| 14.5 Trials registry or pre‐2010 | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 14.6 No industry funding | 4 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.31, 1.63] |
| 14.7 Randomised 100+ participants | 12 | 9869 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 14.8 Randomised 250+ participants | 6 | 8958 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 15 CHD events ‐ SA fixed‐effect | 15 | 10076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 16 CHD events ‐ subgroup by PUFA dose | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 16.1 total PUFA < 1.0% E | 3 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.20, 4.89] |
| 16.2 total PUFA 1.0 to < 2.0% E | 4 | 5170 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.13] |
| 16.3 total PUFA 2.0 to < 5.0% E | 3 | 2224 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.04] |
| 16.4 total PUFA > 5.0% E | 5 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.54, 1.36] |
| 17 CHD events ‐ subgroup by duration | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 17.1 Medium duration 1 to < 2 years | 6 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.27, 1.30] |
| 17.2 Medium‐long duration 2 to < 4 years | 5 | 7204 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 17.3 Long duration 4+ years | 4 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.46, 1.35] |
| 18 CHD events ‐ subgroup by primary or secondary prevention | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 18.1 Primary prevention of CVD | 6 | 1710 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.25, 1.11] |
| 18.2 Secondary prevention of CVD | 9 | 8366 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.09] |
| 19 CHD events ‐ subgroup by baseline PUFA dose | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 19.1 Baseline total PUFA < 6% E | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.04] |
| 19.2 Baseline total PUFA 6 to < 11% E | 2 | 2491 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.68, 2.01] |
| 19.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Baseline total PUFA unclear | 12 | 6739 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.06] |
| 20 CHD events ‐ subgroup by replacement | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 PUFA replaced saturated fats | 4 | 3730 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.78, 1.19] |
| 20.2 PUFA replaced monounsaturated fats | 9 | 7079 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.20] |
| 20.3 PUFA replaced carbohydrate | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.12, 2.65] |
| 20.4 PUFA replaced protein | 1 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.69, 1.37] |
| 20.5 PUFA replaced unclear | 3 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.52] |
| 21 CHD events ‐ subgroup by sex | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 21.1 > 70% men | 10 | 9269 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.05] |
| 21.2 > 70% women | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.08, 44.38] |
| 21.3 men & women | 2 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.51] |
| 21.4 sex not reported | 2 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.66, 2.50] |
| 22 CHD events ‐ subgroup by age | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 22.1 Mean age < 50 years | 1 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.00, 2.67] |
| 22.2 Mean age 50 to < 65 years | 9 | 3204 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.03] |
| 22.3 Mean age 65+ years | 3 | 5921 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.04] |
| 22.4 Mean age unclear | 2 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.06, 4.64] |
| 23 CHD events ‐ subgroup by statin use | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 23.1 < 50% on statins | 13 | 5001 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.66, 1.09] |
| 23.2 50+% on statins | 2 | 5075 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.17] |
| 24 CHD events ‐ subgroup by intervention type | 15 | 10076 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 24.1 Dietary advice | 2 | 2135 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.15, 1.77] |
| 24.2 Supplemental foods & diet provided | 2 | 5683 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.04] |
| 24.3 Supplements (capsules & unusual foods) | 9 | 1407 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.37] |
| 24.4 Any combination | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.74, 2.02] |
| 25 STROKE ‐ fatal & non fatal | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 26 Stroke ‐ SA | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 Low risk of bias for allocation concealment | 4 | 6022 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.55, 2.38] |
| 26.2 Low risk of bias for attention | 8 | 11858 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.98] |
| 26.3 Low risk of bias for compliance | 4 | 3730 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.45, 4.11] |
| 26.4 Low summary risk of bias | 3 | 5686 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.23] |
| 26.5 Trials registry or pre‐2010 | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 26.6 No industry funding | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.32, 8.62] |
| 26.7 Randomised 100+ participants | 10 | 14662 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.45] |
| 26.8 Randomised 250+ participants | 8 | 14291 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.60] |
| 27 Stroke ‐ SA fixed‐effect | 11 | 14742 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
| 28 Stroke ‐ subgroup by PUFA dose | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 28.1 total PUFA < 1.0% E | 4 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.14, 6.55] |
| 28.2 total PUFA 1.0 to < 2.0% E | 2 | 9834 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.07] |
| 28.3 total PUFA 2.0 to < 5.0% E | 2 | 2113 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.99, 10.72] |
| 28.4 total PUFA > 5.0% E | 3 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.33] |
| 29 Stroke ‐ subgroup by duration | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 29.1 Medium duration 1 to < 2 years | 4 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.14, 6.55] |
| 29.2 Medium‐long duration 2 to < 4 years | 3 | 6950 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.61, 4.16] |
| 29.3 Long duration 4+ years | 4 | 6694 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 0.97] |
| 30 Stroke ‐ subgroup by primary or secondary prevention | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 30.1 Primary prevention of cardiovascular disease (CVD) | 4 | 6570 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.45, 1.11] |
| 30.2 Secondary prevention of CVD | 7 | 8172 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.59, 2.62] |
| 31 Stroke ‐ subgroup by baseline PUFA dose | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 31.1 Baseline total PUFA < 6% E | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.15] |
| 31.2 Baseline total PUFA 6 to < 11% E | 3 | 7488 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.41, 3.59] |
| 31.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.4 Baseline total PUFA unclear | 7 | 6408 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.41] |
| 32 Stroke ‐ subgroup by replacement | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 32.1 PUFA replaced saturated fats | 4 | 3730 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.45, 4.11] |
| 32.2 PUFA replaced monounsaturated fats | 7 | 11742 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.52, 0.99] |
| 32.3 PUFA replaced carbohydrates | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.4 PUFA replaced protein | 1 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 4.88 [0.24, 100.89] |
| 32.5 PUFA replaced unclear | 2 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.02, 29.08] |
| 33 Stroke ‐ subgroup by sex | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 33.1 > 70% men | 9 | 9354 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.56, 1.93] |
| 33.2 > 70% women | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 men & women | 1 | 4997 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.05] |
| 33.4 sex not reported | 1 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 5.03 [0.24, 104.01] |
| 34 Stroke ‐ subgroup by age | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 34.1 Mean age < 50 years | 1 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.15, 7.55] |
| 34.2 Mean age 50 to < 65 years | 5 | 2975 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [1.05, 7.64] |
| 34.3 Mean age 65+ years | 4 | 10918 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.94] |
| 34.4 Mean age unclear | 1 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 5.03 [0.24, 104.01] |
| 35 Stroke ‐ subgroup by statin use | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 35.1 < 50% on statins | 9 | 9667 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.78] |
| 35.2 50+% on statins | 2 | 5075 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.07, 3.40] |
| 36 Stroke ‐ subgroup by intervention type | 11 | 14742 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.44] |
| 36.1 Dietary advice | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 3.32 [0.92, 12.04] |
| 36.2 Supplemental foods & diet provided | 3 | 10680 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.96] |
| 36.3 Supplements (capsules & unusual foods) | 5 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.25, 5.62] |
| 36.4 Any combination | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.32, 8.62] |
| 37 Stroke ‐ subgroup by fatal & non fatal | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 37.1 Fatal stroke | 4 | 6534 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.38, 1.60] |
| 37.2 Non‐fatal stroke | 2 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.09, 2.51] |
| 37.3 Only combined fatal & non fatal data provided | 6 | 7970 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.56, 4.07] |
| 38 Stroke ‐ subgroup by ischaemic & haemorrhagic | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 38.1 Ischaemic stroke | 3 | 2762 | Risk Ratio (M‐H, Random, 95% CI) | 4.66 [1.00, 21.63] |
| 38.2 Haemorrhagic stroke | 3 | 2762 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.48, 7.85] |
| 38.3 Only combined ischaemic and haemorrhagic data provided | 8 | 11980 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.50, 0.97] |
| 39 MAJOR ADVERSE CARDIAC & CEREBROVASCULAR EVENTS (MACCEs) | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 40 MACCEs ‐ SA | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 40.1 Low risk of bias for allocation concealment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Low risk of bias for attention | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 40.3 Low risk of bias for compliance | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 40.4 Low summary risk of bias | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.5 Trials registry or pre‐2010 | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 40.6 No industry funding | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.7 Randomised 100+ participants | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 40.8 Randomised 250+ participants | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 41 MACCEs ‐ SA fixed‐effect | 2 | 2879 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.04] |
| 42 MACCEs ‐ subgroup by PUFA dose | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 42.1 total PUFA < 1.0% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 total PUFA 1.0 to < 2.0% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.3 total PUFA 2.0 to < 5.0% E | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 42.4 total PUFA > 5.0% E | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 43 MACCEs ‐ subgroup by duration | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 43.1 Medium duration 1 to < 2 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 43.2 Medium‐long duration 2 to < 4 years | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 43.3 Long duration 4+ years | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 44 MACCEs ‐ subgroup by primary or secondary prevention | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 44.1 Primary prevention of CVD | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 44.2 Secondary prevention of CVD | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 45 MACCEs ‐ subgroup by baseline PUFA dose | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 45.1 Baseline total PUFA < 6% E | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 45.2 Baseline total PUFA 6 to < 11% E | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 45.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45.4 Baseline total PUFA unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46 MACCEs ‐ subgroup by replacement | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 46.1 PUFA replaced saturated fats | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 46.2 PUFA replaced monounsaturated fats | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 46.3 PUFA replaced carbohydrates | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.4 PUFA replaced protein | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.5 PUFA replaced unclear | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 47 MACCEs ‐ subgroup by sex | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 47.1 > 70% men | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 47.2 > 70% women | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 47.3 men & women | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 47.4 sex not reported | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 48 MACCEs ‐ subgroup by age | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 48.1 Mean age < 50 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 48.2 Mean age 50 to < 65 years | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 48.3 Mean age 65+ years | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 49 MACCEs ‐ subgroup by statin use | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 49.1 < 50% on statins | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 49.2 50+% on statins | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 50 MACCEs ‐ subgroup by intervention type | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
| 50.1 Dietary advice | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 50.2 Supplemental foods & diet provided | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 50.3 Supplements (capsules & unusual foods) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 50.4 Any combination | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CARDIOVASCULAR MORTALITY | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 2 Cardiovascular mortality ‐ SA | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias for allocation concealment | 6 | 6031 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.85, 1.38] |
| 2.2 Low risk of bias for attention | 9 | 11774 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 2.3 Low risk of bias for compliance | 8 | 4142 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.49] |
| 2.4 Low summary risk of bias | 3 | 5431 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.77, 1.83] |
| 2.5 Trials registry or pre‐2010 | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 2.6 No industry funding | 7 | 1744 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.79, 1.79] |
| 2.7 Randomised 100+ participants | 13 | 14895 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.28] |
| 2.8 Randomised 250+ participants | 7 | 13966 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 3 Cardiovascular mortality ‐ SA fixed‐effect | 16 | 15107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.16] |
| 4 Cardiovascular mortality ‐ subgroup by PUFA dose | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 4.1 total PUFA < 1.0% E | 5 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.38, 1.51] |
| 4.2 total PUFA 1.0 to < 2.0% E | 3 | 9954 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.30] |
| 4.3 total PUFA 2.0 to < 5.0% E | 3 | 2246 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.96, 1.62] |
| 4.4 total PUFA > 5.0% E | 5 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.62, 1.63] |
| 5 Cardiovascular mortality ‐ subgroup by duration | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 5.1 Medium duration 1 to <2 years | 5 | 974 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.67] |
| 5.2 Medium‐long duration 2 to < 4 years | 6 | 7337 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.88, 1.36] |
| 5.3 Long duration 4+ years | 5 | 6796 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.67, 1.55] |
| 6 Cardiovascular mortality ‐ subgroup by primary or secondary prevention | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 6.1 Primary prevention of cardiovascular disease (CVD) | 7 | 6412 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.54, 1.41] |
| 6.2 Secondary prevention of CVD | 9 | 8695 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.36] |
| 7 Cardiovascular mortality ‐ subgroup by baseline PUFA dose | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 7.1 Baseline total PUFA < 6% E | 2 | 982 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.97] |
| 7.2 Baseline total PUFA 6 to < 11% E | 4 | 7621 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.07, 1.62] |
| 7.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Baseline total PUFA unclear | 10 | 6504 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.16] |
| 8 Cardiovascular mortality ‐ subgroup by replacement | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 PUFA replaced saturated fats | 4 | 3730 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.76, 1.54] |
| 8.2 PUFA replaced monounsaturated fats | 8 | 11874 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.30] |
| 8.3 PUFA replaced carbohydrates | 4 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.30, 4.71] |
| 8.4 PUFA replaced protein | 2 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.66, 1.77] |
| 8.5 PUFA replaced unclear | 3 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.07, 1.37] |
| 9 Cardiovascular mortality ‐ subgroup by sex | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 9.1 > 70% men | 10 | 9623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.78, 1.27] |
| 9.2 > 70% women | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 men & women | 5 | 5430 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.47] |
| 9.4 sex not reported | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.41, 5.84] |
| 10 Cardiovascular mortality ‐ subgroup by age | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 10.1 Mean age < 50 years | 1 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.99, 2.55] |
| 10.2 Mean age 50 to < 65 years | 8 | 3149 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.48] |
| 10.3 Mean age 65+ years | 6 | 11398 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 10.4 Mean age unclear | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.60] |
| 11 Cardiovascular mortality ‐ subgroup by statin use | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 11.1 < 50% on statins | 11 | 9416 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.40] |
| 11.2 50+% on statins | 3 | 5153 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.67, 1.22] |
| 11.3 Percentage on statins unclear | 2 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.47, 2.54] |
| 12 Cardiovascular mortality ‐ subgroup by intervention type | 16 | 15107 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.82, 1.26] |
| 12.1 Dietary advice | 4 | 2404 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.42, 3.12] |
| 12.2 Supplemental foods & diet provided | 3 | 10680 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.19] |
| 12.3 Supplements (capsules & unusual foods) | 7 | 1172 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.42, 1.40] |
| 12.4 Any combination | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.87, 1.95] |
| 13 CARDIOVASCULAR EVENTS | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 14 CVD events ‐ SA | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Low risk of bias for allocation concealment | 11 | 8714 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 14.2 Low risk of bias for attention | 16 | 14111 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.97] |
| 14.3 Low risk of bias for compliance | 8 | 5697 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.14] |
| 14.4 Low summary risk of bias | 6 | 7014 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.83, 1.67] |
| 14.5 Trials registry or pre‐2010 | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 14.6 No industry funding | 5 | 2440 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.44] |
| 14.7 Randomised 100+ participants | 18 | 17587 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.00] |
| 14.8 Randomised 250+ participants | 11 | 16524 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.85, 1.02] |
| 15 CVD events ‐ SA fixed‐effect | 21 | 17799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.86, 0.98] |
| 16 CVD events ‐ subgroup by PUFA dose | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 16.1 total PUFA < 1.0% E | 7 | 1563 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.42, 0.96] |
| 16.2 total PUFA 1.0 to < 2.0% E | 5 | 10468 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
| 16.3 total PUFA 2.0 to < 5.0% E | 3 | 2224 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 16.4 total PUFA > 5.0% E | 6 | 3544 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.29] |
| 17 CVD events ‐ subgroup by duration | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 17.1 Medium duration 1 to < 2 years | 11 | 3175 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 0.99] |
| 17.2 Medium‐long duration 2 to < 4 years | 6 | 7930 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 17.3 Long duration 4+ years | 4 | 6694 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 18 CVD events ‐ subgroup by primary or secondary prevention | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 18.1 Primary prevention of CVD | 10 | 8893 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 18.2 Secondary prevention of CVD | 11 | 8906 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.75, 1.05] |
| 19 CVD events ‐ subgroup by baseline PUFA dose | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 19.1 Baseline total PUFA < 6% E | 2 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 1.01] |
| 19.2 Baseline total PUFA 6 to < 11% E | 4 | 8214 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.85, 1.22] |
| 19.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Baseline total PUFA unclear | 15 | 7672 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.69, 0.98] |
| 20 CVD events ‐ subgroup by replacement | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 PUFA replaced saturated fats | 6 | 5523 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.14] |
| 20.2 PUFA replaced monounsaturated fats | 12 | 13605 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.76, 1.08] |
| 20.3 PUFA replaced carbohydrates | 2 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.70, 2.01] |
| 20.4 PUFA replaced protein | 2 | 1119 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.07] |
| 20.5 PUFA replaced unclear | 6 | 1042 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.95] |
| 21 CVD events ‐ subgroup by sex | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 21.1 > 70% men | 12 | 10798 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.74, 1.00] |
| 21.2 > 70% women | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.08, 44.38] |
| 21.3 men & women | 6 | 6416 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.68, 1.18] |
| 21.4 sex not reported | 2 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.80, 2.20] |
| 22 CVD events ‐ subgroup by age | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 22.1 Mean age < 50 years | 4 | 2020 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.05, 2.61] |
| 22.2 Mean age 50 to < 65 years | 9 | 3264 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.08] |
| 22.3 Mean age 65+ years | 7 | 12124 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.96] |
| 22.4 Mean age unclear | 1 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.54, 4.83] |
| 23 CVD events ‐ subgroup by statin use | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 23.1 < 50% on statins | 16 | 11518 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 23.2 50+% on statins | 3 | 5153 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.25] |
| 23.3 Percentage on statins unclear | 2 | 1128 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.53, 1.21] |
| 24 CVD events ‐ subgroup by intervention type | 21 | 17799 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 24.1 Dietary advice | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.09] |
| 24.2 Supplemental foods & diet provided | 5 | 12473 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.99] |
| 24.3 Supplements (capsules & unusual foods) | 13 | 2442 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.04] |
| 24.4 Any combination | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.57, 2.13] |
| 25 CORONARY HEART DISEASE (CHD) MORTALITY | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 26 CHD mortality ‐ SA | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 Low risk of bias for allocation concealment | 3 | 5359 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.25] |
| 26.2 Low risk of bias for attention | 8 | 6777 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.18] |
| 26.3 Low risk of bias for compliance | 4 | 3053 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.10] |
| 26.4 Low summary risk of bias | 1 | 4837 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.28] |
| 26.5 Trials registry or pre‐2010 | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 26.6 No industry funding | 2 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.25, 2.58] |
| 26.7 Randomised 100+ participants | 7 | 8676 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.77, 1.06] |
| 26.8 Randomised 250+ participants | 4 | 8118 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.08] |
| 27 CHD mortality ‐ SA fixed‐effect | 9 | 8810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 28 CHD mortality ‐ subgroup by PUFA dose | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 28.1 total PUFA < 1.0% E | 3 | 840 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.35, 1.59] |
| 28.2 total PUFA 1.0 to < 2.0% E | 2 | 4957 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.41, 1.76] |
| 28.3 total PUFA 2.0 to < 5.0% E | 2 | 2113 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.74, 1.10] |
| 28.4 total PUFA > 5.0% E | 2 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.78] |
| 29 CHD mortality ‐ subgroup by duration | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 29.1 Medium duration 1 to < 2 years | 3 | 760 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.34, 1.83] |
| 29.2 Medium‐long duration 2 to < 4 years | 5 | 7204 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 29.3 Long duration 4+ years | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.57, 1.75] |
| 30 CHD mortality ‐ subgroup by primary or secondary prevention | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 30.1 Primary prevention of CVD | 2 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.34] |
| 30.2 Secondary prevention of CVD | 7 | 7844 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.06] |
| 31 CHD mortality ‐ subgroup by baseline PUFA dose | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 31.1 Baseline total PUFA < 6% E | 1 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.57, 1.75] |
| 31.2 Baseline total PUFA 6 to < 11% E | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 31.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.4 Baseline total PUFA unclear | 7 | 5931 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.66, 1.19] |
| 32 CHD mortality ‐ subgroup by replacement | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 32.1 PUFA replaced saturated fats | 2 | 2879 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.10] |
| 32.2 PUFA replaced monounsaturated fats | 6 | 6419 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 32.3 PUFA replaced carbohydrates | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.18, 19.29] |
| 32.4 PUFA replaced protein | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.5 PUFA replaced unclear | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.65] |
| 33 CHD mortality ‐ subgroup by sex | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 33.1 > 70% men | 7 | 8636 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 33.2 > 70% women | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 men & women | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.05] |
| 33.4 sex not reported | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.18, 19.29] |
| 34 CHD mortality ‐ subgroup by age | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 34.1 Mean age < 50 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 Mean age 50 to < 65 years | 5 | 2487 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.08] |
| 34.3 Mean age 65+ years | 4 | 6323 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.23] |
| 35 CHD mortality ‐ subgroup by statin use | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 35.1 < 50% on statins | 6 | 3333 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.75, 1.08] |
| 35.2 50+% on statins | 2 | 5075 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 35.3 Percentage on statins unclear | 1 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.41, 2.49] |
| 36 CHD mortality ‐ subgroup by intervention type | 9 | 8810 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 36.1 Dietary advice | 1 | 2033 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 36.2 Supplemental foods & diet provided | 2 | 5683 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
| 36.3 Supplements (capsules & unusual foods) | 6 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.43] |
| 36.4 Any combination | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 MYOCARDIAL INFARCTION (MI) ‐ fatal and non fatal | 15 | 15609 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 38 SUDDEN CARDIAC DEATH (SCD) | 5 | 1731 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.29] |
| 39 ATRIAL FIBRILLATION (AF) & ARRHYTHMIAS (including AF, ventricular tachycardia (VT), ventricular fibrillation(VF) | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 39.1 Recurrent arrhythmia | 4 | 979 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.01] |
| 39.2 New arrhythmia | 7 | 10713 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.70, 1.46] |
| 40 AF ‐ SA | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 40.1 Low risk of bias for allocation concealment | 7 | 6679 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.88] |
| 40.2 Low risk of bias for attention | 10 | 11514 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.72, 1.13] |
| 40.3 Low risk of bias for compliance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.4 Low summary risk of bias | 3 | 5368 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.59, 1.12] |
| 40.5 Trials registry or pre‐2010 | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 40.6 No industry funding | 2 | 601 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.88] |
| 40.7 Randomised 100+ participants | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 40.8 Randomised 250+ participants | 6 | 10842 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.28] |
| 41 AF ‐ SA fixed‐effect | 11 | 11692 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 41.1 Recurrent arrhythmia | 4 | 979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 41.2 New arrhythmia | 7 | 10713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.28] |
| 42 AF ‐ subgroup by PUFA dose | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 42.1 total PUFA < 1.0% E | 7 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.99] |
| 42.2 total PUFA 1.0 to < 2.0% E | 4 | 9853 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.70, 1.60] |
| 42.3 total PUFA 2.0 to < 5.0% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.4 total PUFA 5.0+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 43 AF ‐ subgroup by duration | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 43.1 Medium duration 1 to < 2 years | 8 | 2153 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.65, 0.83] |
| 43.2 Medium‐long duration 2 to < 4 years | 2 | 5037 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.36] |
| 43.3 Long duration 4+ years | 1 | 4502 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.98, 1.79] |
| 44 AF ‐ subgroup by primary or secondary prevention | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 44.1 Primary prevention of CVD | 5 | 5743 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.99, 1.79] |
| 44.2 Secondary prevention of CVD | 6 | 5949 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.96] |
| 45 Atrial fibrillation ‐ subgroup by baseline PUFA dose | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 45.1 Baseline total PUFA < 6% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45.2 Baseline total PUFA 6 to < 11% E | 1 | 4502 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.98, 1.79] |
| 45.3 Baseline total PUFA 11+% E | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45.4 Baseline total PUFA unclear | 10 | 7190 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.69, 0.95] |
| 46 AF ‐ subgroup by replacement | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 46.1 PUFA replaced saturated fats | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.2 PUFA replaced monounsaturated fats | 8 | 10804 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 46.3 PUFA replaced carbohydrates | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.4 PUFA replaced protein | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.5 PUFA replaced unclear | 3 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.64, 0.88] |
| 47 Atrial fibrillation ‐ subgroup by sex | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 47.1 > 70% men | 6 | 6086 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 1.01] |
| 47.2 > 70% women | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.08, 44.38] |
| 47.3 men & women | 3 | 5075 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.50, 1.93] |
| 47.4 sex not reported | 1 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.18, 21.99] |
| 48 AF ‐ subgroup by age | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 48.1 Mean age < 50 years | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 72.40] |
| 48.2 Mean age 50 to < 65 years | 5 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.23] |
| 48.3 Mean age 65+ years | 4 | 9940 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.15] |
| 48.4 Mean age unclear | 1 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.18, 21.99] |
| 49 AF ‐ subgroup by statin use | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 49.1 < 50% on statins | 9 | 6453 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.21] |
| 49.2 50+% on statins | 1 | 4837 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.10] |
| 49.3 Percentage on statins unclear | 1 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.01] |
| 50 AF ‐ subgroup by intervention type | 11 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 50.1 Dietary advice | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 50.2 Supplemental foods & diet provided | 2 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.62, 1.70] |
| 50.3 Supplements (capsules & unusual foods) | 9 | 2353 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.98] |
| 50.4 Any combination | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 51 ANGINA | 7 | 2070 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.35, 1.16] |
| 52 HEART FAILURE | 7 | 25257 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.40, 1.36] |
| 53 PERIPHERAL ARTERIAL DISEASE (PAD) | 4 | 8937 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.81, 1.77] |
| 54 REVASCULARISATION ‐ angioplasty and/or coronary artery bypass grafting | 6 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.24] |
2.41. Analysis.
Comparison 2 Higher PUFA vs lower PUFA ‐ dichotomous secondary outcomes, Outcome 41 AF ‐ SA fixed‐effect.
Comparison 3. Higher PUFA vs lower PUFA ‐ continuous secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADIPOSITY ‐ BODY WEIGHT, kg | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 2 Body weight, kg ‐ SA | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias for allocation concealment | 5 | 2586 | Mean Difference (IV, Random, 95% CI) | 1.72 [0.29, 3.15] |
| 2.2 Low risk of bias for attention | 7 | 4156 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.08, 1.06] |
| 2.3 Low risk of bias for compliance | 5 | 756 | Mean Difference (IV, Random, 95% CI) | 1.59 [‐0.11, 3.28] |
| 2.4 Low summary risk of bias | 4 | 2550 | Mean Difference (IV, Random, 95% CI) | 1.81 [0.23, 3.38] |
| 2.5 Trials registry or pre‐2010 | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 2.6 No industry funding | 6 | 2783 | Mean Difference (IV, Random, 95% CI) | 1.62 [0.11, 3.14] |
| 2.7 Randomised 100+ participants | 8 | 6885 | Mean Difference (IV, Random, 95% CI) | 0.89 [0.41, 1.36] |
| 2.8 Randomised 250+ participants | 5 | 6539 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.34, 1.28] |
| 3 Body weight, kg ‐ SA fixed‐effect | 13 | 7100 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [0.96, 1.21] |
| 4 Body weight, kg ‐ subgroup by PUFA dose | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 4.1 total PUFA < 1.0% E | 2 | 287 | Mean Difference (IV, Random, 95% CI) | 1.78 [‐1.46, 5.01] |
| 4.2 total PUFA 1.0 to < 2.0% E | 5 | 6079 | Mean Difference (IV, Random, 95% CI) | 0.74 [0.18, 1.30] |
| 4.3 total PUFA 2.0 to < 5.0% E | 3 | 210 | Mean Difference (IV, Random, 95% CI) | 1.47 [‐3.60, 6.53] |
| 4.4 total PUFA 5.0+% E | 3 | 524 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐0.10, 1.60] |
| 5 Body weight, kg ‐ subgroup by duration | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 5.1 Medium duration 1 to < 2 years | 6 | 502 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.20, 1.14] |
| 5.2 Medium‐long duration 2 to < 4 years | 4 | 522 | Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.06, 1.62] |
| 5.3 Long duration 4+ years | 3 | 6076 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.27, 1.54] |
| 6 Body weight, kg ‐ subgroup by primary or secondary prevention | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 6.1 Primary prevention of CVD | 11 | 6864 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.33, 1.19] |
| 6.2 Secondary prevention of CVD | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐5.43, 9.43] |
| 7 Body weight, kg ‐ subgroup by baseline PUFA dose | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 7.1 Baseline total PUFA < 6% E | 3 | 2339 | Mean Difference (IV, Random, 95% CI) | 2.37 [1.18, 3.56] |
| 7.2 Baseline total PUFA 6 to < 11% E | 5 | 4345 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.21, 1.15] |
| 7.3 Baseline total PUFA 11+% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Baseline total PUFA unclear | 5 | 416 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.68, 1.03] |
| 8 Body weight, kg ‐ subgroup by replacement | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 PUFA replaced saturated fats | 3 | 248 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐5.15, 6.34] |
| 8.2 PUFA replaced monounsaturated fats | 4 | 4036 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.15, 1.23] |
| 8.3 PUFA replaced carbohydrates | 5 | 2882 | Mean Difference (IV, Random, 95% CI) | 1.23 [0.27, 2.20] |
| 8.4 PUFA replaced protein | 4 | 660 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐0.64, 3.75] |
| 8.5 unclear | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.80, 0.95] |
| 9 Body weight, kg ‐ subgroup by sex | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 9.1 > 70% men | 4 | 408 | Mean Difference (IV, Random, 95% CI) | 3.14 [0.31, 5.98] |
| 9.2 > 70% women | 3 | 2253 | Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.60, 2.17] |
| 9.3 men & women | 5 | 4404 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.22, 1.18] |
| 9.4 sex not reported | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐10.57, 9.97] |
| 10 Body weight, kg ‐ subgroup by age | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 10.1 Mean age < 50 years | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐1.12, 1.54] |
| 10.2 Mean age 50 to < 65 years | 9 | 2978 | Mean Difference (IV, Random, 95% CI) | 1.15 [0.12, 2.18] |
| 10.3 Mean age 65+ years | 2 | 4043 | Mean Difference (IV, Random, 95% CI) | 0.71 [0.16, 1.26] |
| 11 Body weight, kg ‐ subgroup by statin use | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 11.1 < 50% on statins | 9 | 6522 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.21, 1.17] |
| 11.2 50+% on statins | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐2.43, 7.83] |
| 11.3 Percentage on statins unclear | 2 | 448 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐1.08, 4.84] |
| 12 Body weight, kg ‐ subgroup by intervention type | 13 | 7100 | Mean Difference (IV, Random, 95% CI) | 0.76 [0.34, 1.19] |
| 12.1 Dietary advice | 4 | 2455 | Mean Difference (IV, Random, 95% CI) | 2.37 [1.19, 3.55] |
| 12.2 Supplemental foods & diet provided | 3 | 4078 | Mean Difference (IV, Random, 95% CI) | 0.71 [0.18, 1.25] |
| 12.3 Supplements (capsules & unusual foods) | 5 | 390 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.18, 0.91] |
| 12.4 Any combination | 1 | 177 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 ADIPOSITY ‐ Body Mass Index (BMI), kg/m2 | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 14 BMI, kg/m2 ‐ SA | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Low risk of bias for allocation concealment | 4 | 3894 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.15, 0.88] |
| 14.2 Low risk of bias for attention | 6 | 2259 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.42] |
| 14.3 Low risk of bias for compliance | 3 | 526 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐0.86, 2.78] |
| 14.4 Low summary risk of bias | 4 | 3894 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.15, 0.88] |
| 14.5 Trials registry or pre‐2010 | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 14.6 No industry funding | 2 | 2539 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.70, 1.26] |
| 14.7 Randomised 100+ participants | 7 | 4738 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.09, 0.41] |
| 14.8 Randomised 250+ participants | 4 | 4331 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.46] |
| 15 BMI, kg/m2 ‐ SA fixed‐effect | 8 | 4798 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [0.20, 0.35] |
| 16 BMI, kg/m2 ‐ subgroup by PUFA dose | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 16.1 total PUFA < 1.0% E | 2 | 193 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.17, 0.18] |
| 16.2 total PUFA 1.0 to < 2.0% E | 5 | 4234 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.03, 0.55] |
| 16.3 total PUFA 2.0 to < 5.0% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 total PUFA 5.0+% E | 1 | 371 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 17 BMI, kg/m2 ‐ subgroup by duration | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 17.1 Medium duration 1 to < 2 years | 3 | 407 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐1.40, 1.81] |
| 17.2 Medium‐long duration 2 to < 4 years | 2 | 1320 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.03, 0.34] |
| 17.3 Long duration 4+ years | 3 | 3071 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.12, 0.55] |
| 18 BMI, kg/m2 ‐ subgroup by primary or secondary prevention | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 18.1 Primary prevention of CVD | 5 | 3034 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.09, 0.69] |
| 18.2 Secondary prevention of CVD | 3 | 1764 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.13, 0.19] |
| 19 BMI, kg/m2 ‐ subgroup by baseline PUFA dose | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 19.1 Baseline total PUFA < 6% E | 2 | 2347 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐2.51, 1.99] |
| 19.2 Baseline total PUFA 6 to < 11% E | 2 | 903 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| 19.3 Baseline total PUFA 11+% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Baseline total PUFA unclear | 4 | 1548 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.16, 0.48] |
| 20 BMI, kg/m2 ‐ subgroup by replacement | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 PUFA replaced saturated fats | 1 | 371 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 20.2 PUFA replaced monounsaturated fats | 5 | 2391 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.11, 0.39] |
| 20.3 PUFA replaced carbohydrates | 2 | 2347 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐2.51, 1.99] |
| 20.4 PUFA replaced protein | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 PUFA replaced unclear | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.18, 3.18] |
| 21 BMI, kg/m2 ‐ subgroup by sex | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 21.1 > 70% men | 3 | 1764 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.13, 0.19] |
| 21.2 > 70% women | 2 | 2347 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐2.51, 1.99] |
| 21.3 men & women | 3 | 687 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.08, 0.71] |
| 22 BMI, kg/m2 ‐ subgroup by age | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 22.1 Mean age < 50 years | 1 | 371 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 22.2 Mean age 50 to < 65 years | 5 | 2635 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.42, 1.18] |
| 22.3 Mean age 65+ years | 2 | 1792 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 23 BMI, kg/m2 ‐ subgroup by statin use | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 23.1 < 50% on statins | 6 | 3443 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.17, 0.42] |
| 23.2 50+% on statins | 2 | 1355 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐1.19, 3.56] |
| 23.3 Percentage on statins unclear | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 BMI, kg/m2 ‐ subgroup by intervention type | 8 | 4798 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.08, 0.42] |
| 24.1 Dietary advice | 1 | 2168 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.30, 1.30] |
| 24.2 Supplemental foods & diet provided | 2 | 1792 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 24.3 Supplements (capsules & unusual foods) | 4 | 467 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.99, 1.64] |
| 24.4 Any combination | 1 | 371 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.16] |
| 25 Adiposity ‐ waist circumference, cm | 3 | 1298 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.32, 0.83] |
| 26 Adiposity ‐ % body fat | 2 | 309 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.41, 5.21] |
| 27 Adiposity ‐ body fat, kg | 1 | 214 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.12, 1.12] |
| 28 Serum TOTAL CHOLESTEROL (TC, mmoL/L) | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 29 TC, mmoL/L ‐ SA | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 29.1 Low risk of bias for allocation concealment | 10 | 3548 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.36, 0.03] |
| 29.2 Low risk of bias for attention | 19 | 4830 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.23, 0.04] |
| 29.3 Low risk of bias for compliance | 15 | 5642 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.39, ‐0.14] |
| 29.4 Low summary risk of bias | 7 | 3204 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.46, 0.01] |
| 29.5 Trials registry or pre‐2010 | 25 | 7808 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.24, ‐0.03] |
| 29.6 No industry funding | 11 | 2570 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.39, 0.01] |
| 29.7 Randomised 100+ participants | 19 | 7711 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.27, ‐0.05] |
| 29.8 Randomised 250+ participants | 9 | 6348 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.05] |
| 30 TC, mmoL/L ‐ SA fixed‐effect | 27 | 8072 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.26, ‐0.18] |
| 31 TC, mmoL/L ‐ subgroup by PUFA dose | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 31.1 total PUFA < 1.0% E | 4 | 480 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.15, 0.13] |
| 31.2 total PUFA 1.0 to < 2.0% E | 8 | 2170 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.16, 0.04] |
| 31.3 total PUFA 2.0 to < 5.0% E | 4 | 1857 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.31, 0.25] |
| 31.4 total PUFA 5.0+% E | 11 | 3565 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.45, ‐0.10] |
| 32 TC, mmoL/L ‐ subgroup by duration | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 32.1 Medium duration 1 to < 2 years | 13 | 2168 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 32.2 Medium‐long duration 2 to < 4 years | 9 | 4012 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.19, 0.05] |
| 32.3 Long duration 4+ years | 5 | 1892 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.40, ‐0.06] |
| 33 TC, mmoL/L ‐ subgroup by primary or secondary prevention | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 33.1 Primary prevention of CVD | 17 | 4006 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.26, 0.02] |
| 33.2 Secondary prevention of CVD | 10 | 4066 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.24, ‐0.00] |
| 34 TC, mmoL/L ‐ subgroup by baseline PUFA dose | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 34.1 Baseline total PUFA < 6% E | 6 | 2347 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.09] |
| 34.2 Baseline total PUFA 6 to < 11% E | 7 | 3394 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.21, 0.04] |
| 34.3 Baseline total PUFA 11+% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.4 Baseline total PUFA unclear | 14 | 2331 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.16, 0.04] |
| 35 TC, mmoL/L ‐ subgroup by replacement | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 35.1 PUFA replaced saturated fats | 8 | 4572 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.50, ‐0.14] |
| 35.2 PUFA replaced monounsaturated fats | 13 | 4500 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.33, ‐0.00] |
| 35.3 PUFA replaced carbohydrates | 9 | 1394 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.10] |
| 35.4 PUFA replaced protein | 4 | 862 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.30, 0.24] |
| 35.5 PUFA replaced unclear | 3 | 238 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.29, 0.12] |
| 36 TC, mmoL/L ‐ subgroup by sex | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 36.1 > 70% men | 15 | 6393 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, ‐0.01] |
| 36.2 > 70% women | 2 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.64, 0.61] |
| 36.3 men & women | 8 | 1367 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.22, 0.01] |
| 36.4 sex not reported | 2 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.80, 0.73] |
| 37 TC, mmoL/L ‐ subgroup by age | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 37.1 Mean age < 50 years | 5 | 1713 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.59, ‐0.02] |
| 37.2 Mean age 50 to < 65 years | 15 | 3250 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.17, 0.06] |
| 37.3 Mean age 65+ years | 4 | 2885 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.00] |
| 37.4 Mean age unclear | 3 | 224 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.52, 0.20] |
| 38 TC, mmoL/L ‐ subgroup by statin use | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 38.1 < 50% on statins | 20 | 5818 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.28, ‐0.03] |
| 38.2 50+% on statins | 5 | 1604 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.08] |
| 38.3 Percentage on statins unclear | 2 | 650 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.35, 0.15] |
| 39 TC, mmoL/L ‐ subgroup by intervention type | 27 | 8072 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 39.1 Dietary advice | 4 | 2019 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 39.2 Supplemental foods & diet provided | 8 | 4264 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.37, ‐0.01] |
| 39.3 Supplements (capsules & unusual foods) | 11 | 934 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.02] |
| 39.4 Any combination | 4 | 855 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.34, 0.29] |
| 40 Serum fasting TRIGLYCERIDE (TG, mmoL/L) | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 41 TG, mmoL/L ‐ SA | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 41.1 Low risk of bias for allocation concealment | 9 | 2686 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.28, ‐0.06] |
| 41.2 Low risk of bias for attention | 15 | 3108 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.20, ‐0.01] |
| 41.3 Low risk of bias for compliance | 8 | 1175 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 41.4 Low summary risk of bias | 5 | 2050 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.26, ‐0.03] |
| 41.5 Trials registry or pre‐2010 | 19 | 3715 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 41.6 No industry funding | 8 | 1196 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.25, 0.09] |
| 41.7 Randomised 100+ participants | 14 | 3637 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.19, ‐0.06] |
| 41.8 Randomised 250+ participants | 5 | 2472 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.27, ‐0.07] |
| 42 TG, mmoL/L ‐ SA fixed‐effect | 20 | 3905 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.16, ‐0.06] |
| 43 TG, mmoL/L ‐ subgroup by PUFA dose | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 43.1 total PUFA < 1.0% E | 5 | 815 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.37, ‐0.02] |
| 43.2 total PUFA 1.0 to < 2.0% E | 7 | 2091 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.15, ‐0.01] |
| 43.3 total PUFA 2.0 to < 5.0% E | 3 | 149 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.91, 0.75] |
| 43.4 total PUFA 5.0+% E | 5 | 850 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.20, 0.06] |
| 44 TG, mmoL/L ‐ subgroup by duration | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 44.1 Medium duration 1 to < 2 years | 10 | 1246 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.28, 0.04] |
| 44.2 Medium‐long duration 2 to < 4 years | 7 | 1787 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.18, 0.07] |
| 44.3 Long duration 4+ years | 3 | 872 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.23, ‐0.03] |
| 45 TG, mmoL/L ‐ subgroup by primary or secondary prevention | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 45.1 Primary prevention of CVD | 14 | 1831 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.01] |
| 45.2 Secondary prevention of CVD | 6 | 2074 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.22, 0.00] |
| 46 TG, mmoL/L ‐ subgroup by baseline PUFA dose | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 46.1 Baseline total PUFA < 6% E | 3 | 350 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.16, 0.17] |
| 46.2 Baseline total PUFA 6 to < 11% E | 6 | 1195 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.23, ‐0.06] |
| 46.3 Baseline total PUFA 11+% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.4 Baseline total PUFA unclear | 11 | 2360 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.27, 0.01] |
| 47 TG, mmoL/L ‐ subgroup by replacement | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 47.1 PUFA replaced saturated fats | 4 | 719 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 47.2 PUFA replaced monounsaturated fats | 8 | 2448 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.24, ‐0.08] |
| 47.3 PUFA replaced carbohydrates | 7 | 848 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.23, 0.14] |
| 47.4 PUFA replaced protein | 2 | 171 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.26, 0.51] |
| 47.5 PUFA replaced unclear | 3 | 499 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.21] |
| 48 TG, mmoL/L ‐ subgroup by sex | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 48.1 > 70% men | 11 | 2796 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.23, 0.03] |
| 48.2 > 70% women | 2 | 250 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.13] |
| 48.3 men & women | 6 | 824 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.28, ‐0.09] |
| 48.4 sex not reported | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.39, 0.99] |
| 49 TG, mmoL/L ‐ subgroup by age | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 49.1 Mean age < 50 years | 3 | 565 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.26, 0.04] |
| 49.2 Mean age 50 to < 65 years | 13 | 1662 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.03] |
| 49.3 Mean age 65+ years | 2 | 1528 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.17, 0.01] |
| 49.4 Mean age unclear | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.68, 0.96] |
| 50 TG, mmoL/L ‐ subgroup by statin use | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 50.1 < 50% on statins | 15 | 2239 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.24, ‐0.04] |
| 50.2 50+% on statins | 4 | 1530 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.15, 0.08] |
| 50.3 Percentage on statins unclear | 1 | 136 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.42, 0.50] |
| 51 TG, mmoL/L ‐ subgroup by intervention type | 20 | 3905 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 51.1 Dietary advice | 4 | 339 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.00] |
| 51.2 Supplemental foods & diet provided | 4 | 1753 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.12, 0.03] |
| 51.3 Supplements (capsules & unusual foods) | 9 | 1140 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.35, ‐0.10] |
| 51.4 Any combination | 3 | 673 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.22, 0.46] |
| 52 Serum HIGH DENSITY LIPOPROTEIN (HDL, mmoL/L) | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 53 HDL, mmoL/L ‐ SA | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 53.1 Low risk of bias for allocation concealment | 8 | 1968 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.01] |
| 53.2 Low risk of bias for attention | 13 | 2641 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 53.3 Low risk of bias for compliance | 8 | 2410 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 53.4 Low summary risk of bias | 4 | 1592 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.01] |
| 53.5 Trials registry or pre‐2010 | 16 | 4410 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 53.6 No industry funding | 7 | 717 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 53.7 Randomised 100+ participants | 11 | 4332 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 53.8 Randomised 250+ participants | 4 | 3394 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 54 HDL, mmoL/L ‐ SA fixed‐effect | 18 | 4674 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 55 HDL, mmoL/L ‐ subgroup by PUFA dose | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 55.1 total PUFA < 1.0% E | 3 | 347 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 55.2 total PUFA 1.0 to < 2.0% E | 8 | 2166 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 55.3 total PUFA 2.0 to < 5.0% E | 4 | 1864 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 55.4 total PUFA 5.0+% E | 3 | 297 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.04, 0.14] |
| 56 HDL, mmoL/L ‐ subgroup by duration | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 56.1 Medium duration 1 to < 2 years | 9 | 852 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 56.2 Medium‐long duration 2 to < 4 years | 8 | 3504 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 56.3 Long duration 4+ years | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.09] |
| 57 HDL, mmoL/L ‐ subgroup by primary or secondary prevention | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 57.1 Primary prevention of CVD | 12 | 1402 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 57.2 Secondary prevention of CVD | 6 | 3272 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 58 HDL, mmoL/L ‐ subgroup by baseline PUFA dose | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 58.1 Baseline total PUFA < 6% E | 3 | 350 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 58.2 Baseline total PUFA 6 to < 11% E | 6 | 2454 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 58.3 Baseline total PUFA 11+% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 58.4 Baseline total PUFA unclear | 9 | 1870 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 59 HDL, mmoL/L ‐ subgroup by replacement | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 59.1 PUFA replaced saturated fats | 4 | 1976 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 59.2 PUFA replaced monounsaturated fats | 6 | 1857 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 59.3 PUFA replaced carbohydrates | 6 | 754 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 59.4 PUFA replaced protein | 2 | 171 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 59.5 PUFA replaced unclear | 3 | 238 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.04, 0.14] |
| 60 HDL, mmoL/L ‐ subgroup by sex | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 60.1 > 70% men | 10 | 3660 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 60.2 > 70% women | 2 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.21, 0.17] |
| 60.3 men & women | 5 | 728 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 60.4 sex not reported | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.17, 0.37] |
| 61 HDL, mmoL/L ‐ subgroup by age | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 61.1 Mean age < 50 years | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.13, 0.23] |
| 61.2 Mean age 50 to < 65 years | 12 | 2910 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 61.3 Mean age 65+ years | 2 | 1528 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.04, 0.03] |
| 61.4 Mean age unclear | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 62 HDL, mmoL/L ‐ subgroup by statin use | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 62.1 < 50% on statins | 12 | 2934 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 62.2 50+% on statins | 5 | 1604 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.01] |
| 62.3 Percentage on statins unclear | 1 | 136 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.08, 0.14] |
| 63 HDL, mmoL/L ‐ subgroup by intervention type | 18 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 63.1 Dietary advice | 4 | 1959 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 63.2 Supplemental foods & diet provided | 4 | 1753 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 63.3 Supplements (capsules & unusual foods) | 8 | 746 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 63.4 Any combination | 2 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 64 Serum LOW DENSITY LIPOPROTEIN (LDL, mmoL/L) | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 65 LDL, mmoL/L ‐ SA | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 65.1 Low risk of bias for allocation concealment | 6 | 1915 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.10] |
| 65.2 Low risk of bias for attention | 11 | 2566 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 65.3 Low risk of bias for compliance | 5 | 1009 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.17, 0.06] |
| 65.4 Low summary risk of bias | 4 | 1506 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.06, 0.09] |
| 65.5 Trials registry or pre‐2010 | 13 | 3098 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.07] |
| 65.6 No industry funding | 4 | 415 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.11, 0.21] |
| 65.7 Randomised 100+ participants | 10 | 3114 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 65.8 Randomised 250+ participants | 5 | 2442 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.15, 0.08] |
| 66 LDL, mmoL/L ‐ SA fixed‐effect | 15 | 3362 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 67 LDL, mmoL/L ‐ subgroup by PUFA dose | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 67.1 total PUFA < 1.0% E | 3 | 622 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.03, 0.19] |
| 67.2 total PUFA 1.0 to < 2.0% E | 5 | 1790 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| 67.3 total PUFA 2.0 to < 5.0% E | 3 | 142 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.13, 0.38] |
| 67.4 total PUFA 5.0+% E | 4 | 808 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.21, 0.09] |
| 68 LDL, mmoL/L ‐ subgroup by duration | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 68.1 Medium duration 1 to < 2 years | 9 | 1085 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.07, 0.10] |
| 68.2 Medium‐long duration 2 to < 4 years | 5 | 1959 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.13, 0.12] |
| 68.3 Long duration 4+ years | 1 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.49, 0.28] |
| 69 LDL, mmoL/L ‐ subgroup by primary or secondary prevention | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 69.1 Primary prevention of CVD | 11 | 1915 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.14, 0.07] |
| 69.2 Secondary prevention of CVD | 4 | 1447 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 70 LDL, mmoL/L ‐ subgroup by baseline PUFA dose | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 70.1 Baseline total PUFA < 6% E | 3 | 347 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.30, 0.15] |
| 70.2 Baseline total PUFA 6 to < 11%E | 4 | 1055 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.22, 0.12] |
| 70.3 Baseline total PUFA 11+% E | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 70.4 Baseline total PUFA unclear | 8 | 1960 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.03, 0.10] |
| 71 LDL, mmoL/L ‐ subgroup by replacement | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 71.1 PUFA replaced saturated fats | 2 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.21, 0.14] |
| 71.2 PUFA replaced monounsaturated fats | 6 | 1776 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.12, 0.12] |
| 71.3 PUFA replaced carbohydrates | 6 | 1106 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 71.4 PUFA replaced protein | 3 | 682 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.26, 0.10] |
| 71.5 PUFA replaced unclear | 2 | 409 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.03, 0.23] |
| 72 LDL, mmoL/L ‐ subgroup by sex | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 72.1 > 70% men | 7 | 1972 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.10] |
| 72.2 > 70% women | 2 | 251 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.46, 0.49] |
| 72.3 men & women | 5 | 1107 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.21, 0.06] |
| 72.4 sex not reported | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.59, 0.39] |
| 73 LDL, mmoL/L ‐ subgroup by age | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 73.1 Mean age < 50 years | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.11, 0.61] |
| 73.2 Mean age 50 to < 65 years | 8 | 1177 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 73.3 Mean age 65+ years | 3 | 1956 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.07] |
| 73.4 Mean age unclear | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.26, 0.36] |
| 74 LDL, mmoL/L ‐ subgroup by statin use | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 74.1 < 50% on statins | 8 | 1197 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 74.2 50+% on statins | 5 | 1515 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.07, 0.08] |
| 74.3 Percentage on statins unclear | 2 | 650 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.33, 0.25] |
| 75 LDL, mmoL/L ‐ subgroup by intervention type | 15 | 3362 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 75.1 Dietary advice | 2 | 208 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.05, 0.48] |
| 75.2 Supplemental foods & diet provided | 5 | 2178 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.05] |
| 75.3 Supplements (capsules & unusual foods) | 7 | 922 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 75.4 Any combination | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.51, 0.37] |
3.67. Analysis.
Comparison 3 Higher PUFA vs lower PUFA ‐ continuous secondary outcomes, Outcome 67 LDL, mmoL/L ‐ subgroup by PUFA dose.
Comparison 4. Higher PUFA vs lower PUFA intake ‐ tertiary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SYSTOLIC BLOOD PRESSURE (sBP, mmHg) | 10 | 7356 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.20, 1.26] |
| 2 DIASTOLIC BLOOD PRESSURE (dBP, mmHg) | 9 | 7327 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.55, 1.02] |
| 3 SERIOUS ADVERSE EVENTS (SAEs) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Pulmonary embolism | 2 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.48, 9.57] |
| 3.2 Multiple Sclerosis worsened or had acute attack ‐ GLA supplement | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.95, 1.30] |
| 3.3 Bleeding | 2 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.34, 1.85] |
| 3.4 GI hospitalisation | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.53, 5.79] |
| 3.5 Retinopathy | 1 | 2424 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.56, 1.86] |
| 4 DROPOUTS | 27 | 8574 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 2016.
| Methods | RCT, parallel, (n3 EPA + DHA versus nil, both with statins), 12 months Summary risk of bias: moderate or high |
|
| Participants | Statin‐treated CAD patients undergoing PCI N: 38 intervention, 36 control Level of risk for CVD: high Male: 63.2% intervention, 72.2% control Mean age (SD): 59.6 (9.1) intervention, 60.7 (0.8) [sic] control Age range: unclear Smokers: 36.8% intervention, 58.3% control Hypertension: 50% in both groups Medications taken by ≥ 50% of those in the control group: aspirin, clopidogrel, ACEi/ARB, beta blockers, atorvastatin Medications taken by 20%‐49% of those in the control group: cilostazol Medications taken by some, but < 20% of the control group: rosuvastatin, nitrates, calcium antagonists Location: South Korea Ethinicity: not reported |
|
| Interventions | Type: supplement (capsule) Comparison: EPA + DHA vs unclear (nil) Intervention: 3 g of ω‐3 PUFA containing 1395 mg of EPA and 1125 mg of DHA/d. No further details Control: unclear whether control group were given placebo or only statins Dose aim: increase 2.5 g/d EPA + DHA, 1% E n‐3 Baseline PUFA unclear Compliance by biomarkers: no tissue fatty acids reported, but TC was reduced by 31.5% in intervention and by 20.9% in the control group, supporting greater PUFA intake in the intervention arm. Compliance by dietary intake: not reported
Compliance, other methods: unclear how it was measured but reported good compliance with no numbers Inclusion basis: planned dose suggested total PUFA intake 2.5 g/d higher in intervention, or 1.13% E PUFA dose. There were no biomarker or dietary intake data to confirm this, but greater reductions in TC in the intervention arm supports. > 10% increase from assumed baseline of 6% E PUFA PUFA dose: 1.13% E Length of intervention: 12 months |
|
| Outcomes | Main trial outcome: change in atherosclerotic burden Dropouts: none Available outcomes: lipids (TG reported as median , IQR so not used), atheroma volume, neointimal volume index Response to contact: contact attempted but no response to date. |
|
| Notes | Trial funding: the trial was supported by clinical research grant from Pusan National University Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation was carried out using random number tables to assign each participant to the intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned randomisation numbers sequentially on recruitment to the trial, and the randomisation codes were retained by the clinical research co‐ordinator. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The personnel responsible for randomisation as well as those performing laboratory measurements were blinded to the randomisation assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial register entry found |
| Attention bias | Unclear risk | No details |
| Compliance | Low risk | No fatty acid levels reported, but TC lower in higher PUFA arm. |
| Other bias | High risk | It's unclear whether the trial was placebo controlled or the control group had no intervention. Also, some of the SDs appear to be incorrectly reported. |
AlphaOmega ‐ ALA.
| Methods | RCT, 2 x 2 (n3 ALA vs MUFA), 40 months Summary risk of bias: low |
|
| Participants | 60‐80 year olds with previous MI N: intervention 2409 (1197 ALA, 1212 ALA + EPA + DHA), control 2428 (1236 MUFA, 1192 EPA + DHA). All analysed in ITT analysis Level of risk for CVD: high Male: 77.9% intervention, 78.7% control Mean age (SD): 69.0 (5.6) intervention, 68.9 (5.6) control Age range: 60‐80 years Smokers: 17.4% intervention, 18% control Hypertension: unclear Medications taken by ≥ 50% of those in the control group: lipid‐lowering medication, antihypertensives, antithrombotics Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: antiarrythmic drugs, antidiabetic drugs Location: Netherlands Ethinicty: not reported |
|
| Interventions | Type: supplementary margarine Comparsion: ALA vs MUFA Intervention: 20 g/d of enriched margarine incorporating: 2 g ALA. 8 x 250 g margarine tubs delivered every 12 weeks Control: 20 g/d of margarine. No additional n‐3 PUFAs. Identical margarine (oleic acid) placebo Dose aim: increase 2 g/d, 1% E n‐3 Baseline PUFA unclear Compliance by biomarkers: plasma cholesteryl esters had clearly higher ALA in the two ALA arms, no data for total PUFA, no serum TC reported post‐baseline Compliance by dietary intake: margarine composition data ‐ summing LA, ALA, EPA, DPA and DHA total PUFA dose in ALA margarine (compared to placebo) was +14.4% E. As planned intake was 20 g/d, intake was 2.88 g/d total PUFA, or 1.3% E from total PUFA. Total PUFA in ALA + EPA + DHA (compared to EPA + DHA margarine) was 11.3% E, or 2.26 g/d total PUFA, 1.02% E total PUFA.
Compliance, other methods: unused margarine tubs were returned‐ daily intakes of margarine and n‐3 fatty acids were calculated on the basis of the amount unused. Adherence was measured by levels of fatty acids in plasma cholesteryl esters, margarine and questionnaires. 90.5% of participants adhered to the protocol and consumed 20.6 (2.8) g/d of margarine. Inclusion basis: planned total PUFA intake 1.02 and 1.30% E higher in control than intervention, > 10% higher than assumed 6% E from total PUFA at baseline. PUFA dose: 1.02% E in ALA + EPA + DHA vs EPA + DHA, 1.3% E in ALA margarine vs placebo margarine Duration of intervention: 40 months |
|
| Outcomes | Main trial outcome: CVD events Dropouts: 91 died, 98 discontinued intervention, 93 died, 93 discontinued control Available outcomes: deaths, MI, CVD events, VF/VT, incident CVD Response to contact: yes (data provided) | |
| Notes | This is a 2 x 2 trial, using ALA margarine vs MUFA margarine (this part) and EPA/DHA margarine vs MUFA margarine (the next trial). The 4 arms were ALA margarine, EPA/DHA margarine, mixture of the 2 interventions and MUFA margarine. This table represents the AL‐ only intervention. Where possible data represent the full trial population for each comparison (ALA margarine plus combined intervention vs MUFA margarine plus EPA/DHA margarine). As this review assesses effects of total PUFA, and doses of total PUFA were higher in the ALA arms we have omitted the EPA/DHA data when pooling would otherwise have meant that each participant was represented twice in meta‐analysis. Trial funding: Netherlands Heart Foundation, National Institutes of Health and Unilever R&D (latter provided unrestricted grant for distribution of trial margarines). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On the computer by a random‐number generator before the start of the trial |
| Allocation concealment (selection bias) | Low risk | Trial author confirmed allocation was concealed from clinicians/ researchers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The 4 types of margarine were “similar in taste, texture and colour". A trained test panel did not perceive a fishy taste or odour. Randomisation tables were stored safely under supervision |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation tables were stored safely under supervision. There was an independent statistician for data analysis. Quote: "Events were coded by three members of the end‐point adjudication committee who were unaware of the identity of the patient, the identity of the treating physician and the patients assigned study group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up for events. Computerised linkage with municipal registries. 2531 participants were only followed up for baseline anthropometric and medical measurements. |
| Selective reporting (reporting bias) | High risk | Sudden cardiac death endpoint omitted. Registered in August 2005, recruitment was from 2002‐2006. Outcomes papers published in 2010 |
| Attention bias | Low risk | All participants appear to have had similar frequency and quantity of attention and follow‐up |
| Compliance | Unclear risk | Only plasma cholesteryl esters of ALA were reported and were higher in intervention arms (unclear regarding total PUFA), no TC reported. |
| Other bias | Low risk | None noted |
Bassey 2000‐Post.
| Methods | RCT, (high PUFA GLA+DHA+EPA vs low PUFA, both with Ca), 12 months Summary risk of bias: moderate or high |
|
| Participants | Healthy postmenopausal women N: 21 intervention, 24 control (total randomised 57) Level of risk for CVD: low Male: 0% intervention, 0% control Mean age (SD): 58 (4.6) intervention, 55 (4.6) control Age range: 50‐65 years (inclusion) Smokers: 20.8% intervention, 19% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported (Women on confounding drug therapy were excluded.) Location: UK Ethinicty: not reported |
|
| Interventions | Type: capsules Comparsion: evening primrose oil + fish oil vs nil Intervention 10 large capsules/d of efacal (Ca 1.0 g, evening primrose oil 4.0 g (85% or 3.4 g/d PUFA) and marine fish oil 440 mg), divided in doses with meals Control: large capsules of 1 g Ca Dose aim: increase ˜3.5 g/d PUFA, 1.6% E PUFA Baseline PUFA unclear Compliance by biomarkers: neither biomarkers nor TC data reported Compliance by dietary intake: not reported
Compliance, other methods: assessed by counting returned capsules at each visit, reported compliance > 90% Inclusion basis: no intention to increase total PUFA, planned dose ˜3.5 g/d PUFA, 1.6% E PUFA, > 10% higher than assumed 6% E from total PUFA at baseline PUFA dose: 1.6% E PUFA Length of intervention: 12 months |
|
| Outcomes | Main trial outcome: BMD Dropouts: 23% (unclear by arm) Available outcomes: weight Response to contact: not attempted | |
| Notes | Trial funding: Scotia Pharmaceuticals Plc, Guildford, UK Mortality reported (1 death but unclear in which arm) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomized by staff at Scotia Pharmaceuticals Plc" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind stated but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors were blinded for the BMD measurements but unclear for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23% were lost to follow‐up, unclear by arm and not all were accounted for |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry record |
| Attention bias | Low risk | No difference was noted for intervention/control groups |
| Compliance | Unclear risk | Neither biomarkers nor TC data reported |
| Other bias | Low risk | None noted |
Bassey 2000‐Pre.
| Methods | RCT, (high PUFA GLA+DHA+EPA vs low PUFA, both with Ca), 12 months Summary risk of bias: moderate or high |
|
| Participants | Healthy pre‐menopausal women N: 19 intervention, 24 control (total randomised 64) Level of risk for CVD: low Male: 0% intervention, 0% control Mean age (SD): 34 (4.4) intervention, 35 (4.9) control Age range: 25‐40 years (inclusion) Smokers: 0% intervention, 0% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported (Women on confounding drug therapy were excluded) Location: UK Ethinicty: not reported |
|
| Interventions | Type: capsules Comparsion: evening primrose oil + fish oil vs nil Intervention 10 large capsules/d of efacal (Ca 1.0 g, evening primrose oil 4.0 g and marine fish oil 440 mg), divided in doses with meals Control: large capsules of 1 g Ca Dose aim: increase ˜3.5 g/d PUFA, 1.6% E PUFA Baseline PUFA unclear Compliance by biomarkers: neither biomarkers nor TC data reported Compliance by dietary intake: not reported
Compliance, other methods: assessed by counting returned capsules at each visit, reported compliance > 90% (median > 9 capsules/d in both treatment and control groups) Inclusion basis: no intention to increase total PUFA, planned dose ˜3.5 g/d PUFA, 1.6% E PUFA, > 10% higher than assumed 6% E from total PUFA at baseline PUFA dose: 1.6% E PUFA Length of intervention: 12 months |
|
| Outcomes | Main trial outcome: BMD Dropouts: 31% (unclear by arm) Available outcomes: weight Response to contact: not attempted | |
| Notes | Trial funding: Scotia Pharmaceuticals Plc, Guildford, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomized by staff at Scotia Pharmaceuticals Plc" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind stated but no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors were blinded for the BMD measurements but unclear for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31% were lost to follow‐up, unclear by arm and not all were accounted for |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry record |
| Attention bias | Low risk | No difference was noted for intervention/control groups |
| Compliance | Unclear risk | Neither biomarkers nor TC data reported |
| Other bias | Low risk | None noted |
Bates 1977.
| Methods | RCT, parallel, 4 arms (n6 GLA+LA vs MUFA), 2 years Summary risk of bias: moderate to high |
|
| Participants | People with chronic progressive multiple sclerosis
CVD risk: low
N; intervention A, C: 38 per arm; control B, D: 38 per arm
Mean years in trial: 2
% male: unclear (no statistically significant difference between groups)
Age: unclear (no statistically significant difference between groups) Age range: unclear Smokers: unclear Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: UK Ethenicty: not reported |
|
| Interventions | Type: supplement Comparison: GLA + linoleic (n6) vs oleic (MUFA) Intervention aims A: increase PUFAs with addition of 8 x 0.6 mL/d of Naudicelle oil in capsules (360 mg/d GLA plus 3.42 g/d linoleic acid plus < 1% ALA) Control aims B: increase MUFAs with addition of 8 x 0.6 mL/d of oleic acid in capsules (4.8 g oleic acid/d) A vs B dose aim: increase 0.34 g/d GLA, 3.78 g/d or 34 kcal or 1.7% E n‐6 Intervention aims C: increase linoleic acid with addition of 11.5 g/d in a spread Control aims D: increase oleic acid with addition of 4 g/d in a spread C vs D dose aim: increase 11.5 g/d or 104 kcal or 5% E n‐6 Baseline PUFA: unclear Compliance by biomarkers: unclear, no serum TC reported, no tissue fatty acids reported Compliance by dietary intake assessment: unclear, not reported
Compliance, other methods: not reported Inclusion basis: aimed to increase total PUFA intake PUFA dose: A vs B 1.7% E PUFA, C vs D 5% E PUFA Duration of intervention: 2 years |
|
| Outcomes | Main trial outcome: progression or regression of multiple sclerosis
Dropouts: unclear in all arms (deaths and dropouts reported together)
Available outcomes: multiple sclerosis progression (deaths occurred but reported with dropouts, so numbers and arms unclear) Response to contact: yes, Professor Bates stated that data on mortality are no longer available. |
|
| Notes | Trial funding: Multiple Sclerosis Society, Van den Berghs provided intervention and control spreads free | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper states "double blind", capsules of "identical appearance" and "similar spread" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Paper states "double blind" with no further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Deaths and dropouts combined, no reasons for dropping out provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry located |
| Attention bias | Low risk | Capsules and spreads provided to all participants, no suggestion of attention bias |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | Low risk | None found |
Bates 1978.
| Methods | RCT, parallel, 2 arms (n6 GLA+LA vs MUFA), using supplements (further 2 arms of n6 LA vs MUFA using supplementary foods not included as no outcome data), 2 years Summary risk of bias: moderate to high |
|
| Participants | People with acute remitting multiple sclerosis
CVD risk: low
N; intervention A, C: 29 per arm; control B, D: 29 per arm
Mean years in trial: 2
% male: intervention A 34.48%; intervention C 17.24%; control B 34.48%; control D 37.93% Age (SD) years: intervention A 35 (9); intervention C 34 (8); control B 32 (7); control D 33 (5) Age range: unclear Smokers: unclear Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: UK Ethenicty: not reported |
|
| Interventions | Type: supplement Comparison: GLA and linoleic (n6) vs oleic (MUFA) Intervention aims A: 8 x Naudicelle capsules/d, 2.92 g/d LA plus 0.34 g/d GLA Control aims B: 8 x capsules/d (4 g/d oleic acid), 4 g/d MUFA A vs B dose aim: increase 0.34 g/d GLA, 3.26 g/d or 29 kcal or 1.5% E n‐6 Intervention aims C: linoleic acid spread (23 g/d linoleic acid) Control aims D: oleic acid spread (16 g/d oleic acid) C vs D dose aim: increase 23 g/d LA or 207 kcal or 10.4% E n‐6 Baseline PUFA: unclear Compliance by biomarkers: good for C vs D, poor for A vs B, no serum TC reported, "estimations of total fatty acids in patients before and after 12‐24 months' treatment showed that the percentage of linoleic and arachidonic acids increased significantly only in those patients taking the linoleic acid spread (group C)". Compliance by dietary intake: unclear, not reported
Compliance, other methods: not reported Inclusion basis: aimed to increase PUFA intake, but C vs D had no outcome data so was excluded. PUFA dose: A vs B 1.5% E PUFA, C vs D 10.4% E PUFA (assumed from omega‐6 doses) Duration of intervention: 2 years |
|
| Outcomes | Main trial outcome: progression or regression of multiple sclerosis
Dropouts: A 0, B 1, C 3, D 6
Available outcomes: multiple sclerosis progression, deaths (nil in arms A, C and D) Response to contact: contact with Dr Bates |
|
| Notes | Trial funding: Multiple Sclerosis Society, Van den Berghs provided intervention and control spreads free | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated” |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly allocated” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper states "double blind", capsules of "identical appearance" and "similar spread" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Paper states "double blind" with no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fairly well described, from 0‐6 dropouts per arm over 2 years (each 29 randomised) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry located |
| Attention bias | Low risk | Appears equivalent |
| Compliance | High risk | No serum TC reported. Paper reports Quote: "estimations of total fatty acids in patients before and after 12‐24 months' treatment showed that the percentage of linoleic and arachidonic acids increased significantly only in those patients taking the linoleic acid spread (group C)". Only A vs B had outcomes for this review, data suggests poor compliance in this group. |
| Other bias | Low risk | None found |
Bates 1989.
| Methods | RCT, parallel, (n3 EPA + DHA vs MUFA), 24 months Summary risk of bias: moderate or high |
|
| Participants | People with multiple sclerosis N: 155 intervention, 157 control (analysed, int: 145 cont: 147) Level of risk for CVD: low Male: 34.2% intervention, 30.6% control Mean age (SD): 34.0 (6.6) intervention, 33.7 (6.3) control Age range: not reported but 16‐45 years inclusion criteria Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49%: not reported Medications taken by some, but < 20%: not reported Location: UK Ethnicity: not reported |
|
| Interventions | Type: supplement (fish oil capsule) Comparison: EPA + DHA vs MUFA Intervention: 20 x 0.5 g/d capsules MaxEPA fish body oil (10 g/d fish oil providing 1.71 g/d EPA + 1.14 g/d DHA + 10 IU/d vitamin E), plus all advised to reduce animal fat and ensure plentiful omega‐6 fats. EPA + DHA 2.85 g/d Control: 20 x 0.5 g/d capsules olive oil (10 g/d olive oil), plus all advised to reduce animal fat and ensure plentiful omega‐6 fats. All capsules contained 0.5 IU vitamin E and 100 ppm dodecylgallate to minimise peroxide formation. Dose aim: intervention increase 2.85 g/d EPA + DHA, 1.3% E n‐3, omega‐6 dose unclear. Control assumed to have similar PUFA content to intervention, apart from EPA + DHA, dose 1.3% E PUFA Baseline PUFA not reported Compliance by biomarkers: adding serum EPA, DHA, LA and AA intervention 51.5% PUFA, control 47.6% PUFA. TC not reported Compliance using dietary assessment: not reported
Compliance, other measures: not reported Inclusion basis: intended doses suggested total PUFA intake 1.3% E higher in intervention than control > 10% more than assumed 6% E PUFA at baseline PUFA dose: 1.3% E Duration of intervention: 24 months (5 years mentioned but outcomes not reported) |
|
| Outcomes | Main trial outcome: multiple sclerosis progress Dropouts: 10 intervention, 10 control Available outcomes: all‐cause mortality, progress of multiple sclerosis, rate of multiple sclerosis relapse Response to contact: yes (no data provided) |
|
| Notes | Trial funding: Multiple Sclerosis Society of Great Britain and Northern Ireland but Marfleet Refining provided fish oil and placebo capsules | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | No further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper states research was "double blind" and control capsules Quote: "had the same appearance and flavour as the fish oil capsules and were packed and dispensed in identical fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk at reported time points |
| Selective reporting (reporting bias) | High risk | No protocol or trials registration entries found. Trial was intended to run for 5 years, but outcomes only appear to be reported for the first 2 years. |
| Attention bias | Low risk | Unlikely as each had capsules |
| Compliance | Low risk | Adding serum EPA, DHA, LA and AA intervention 51.5% PUFA, control 47.6% PUFA. TC not reported |
| Other bias | Low risk | Not noted |
Black 1994.
| Methods | RCT, parallel, (low fat diet vs usual diet), 24 months Summary risk of bias: moderate or high |
|
| Participants | People with non‐melanoma skin cancer
N: 66 intervention, 67 control (analysed, 57 int, 58 cont) Level of risk for CVD: low Male: 54% intervention, 67% control Mean age (SD): 50.6 (9.7) intervention, 52.3 (13.2) control Age range: not reported Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: USA Ethnicity: white 100% (excluded from trial if of Asian, Black, Hispanic or American Indian ancestry) |
|
| Interventions | Type: dietary advice Comparison: reduced fat (lower omega‐6 and total PUFA) vs usual diet Intervention: aims total fat 20% E, protein 15% E, CHO 65% E; methods 8 x weekly classes plus monthly follow‐up sessions, with behavioural techniques being taught following individual approach (not clear if in a group or individual). 4‐month intervals clinic examination by dermatologist. Intervention delivered face to face by a dietitian Control: aims usual diet; methods no dietary change, 4‐month intervals clinic examination by dermatologist Dose aim: reduce total fat to 20% E, 15% E protein, 65% E CHO, particularly complex CHO (fat reduction included reducing omega‐6 and total PUFA, no aim provided) Baseline PUFA 8% E Compliance by biomarkers: unclear, no serum TC reported, no tissue fatty acids Compliance by dietary intake: all assessed "during study", months 4‐24, using 7‐day food records verified by a dietitian
Inclusion basis: dietary intake data suggested total PUFA intake 3.3% E higher in control than intervention PUFA dose: ‐3.3% E Duration of intervention: 24 months (mean 1.9 years in trial) |
|
| Outcomes | Main trial outcome: incidence of actinic keratosis and non‐melanoma skin cancer Dropouts: unclear intervention, unclear control Available outcomes: deaths, CVD deaths, cancer deaths (none), (weight data provided but without variance) Response to contact: Prof Black provided data on mortality |
|
| Notes | Trial funding: National Cancer Institute NOTE: for this trial the higher PUFA arm is the control, and lower PUFA arm is the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "list of randomly generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Dietary advice provided, so participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "examined .... by dermatologists unaware of their treatment assignments". Deaths (all‐cause and CVD) not considered relevant to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For mortality. Unclear for other outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry found |
| Attention bias | High risk | Weekly classes and monthly follow‐up in intervention group, 4‐monthly check‐ups only in control |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | Low risk | None noted |
Brox 2001.
| Methods | RCT, parallel, 3 arms (n3 EPA + DHA from cod liver oil vs n3 EPA + DHA from seal oil vs nil), 14 months Summary risk of bias: moderate or high | |
| Participants | People with moderate hypercholesterolaemia N: 40 seal oil (SO), 40 cod liver oil, 40 control (numbers analysed vary by outcome) Level of risk for CVD: moderate (dyslipidaemia) Male: 53% seal oil, 50% cod liver oil, 48% control Mean age, SD: 53.2 seal oil, 55.0 cod liver oil, 55.8 control Age range: 43‐66 Smokers: unclear Hypertension: unclear Medications taken by ≥ 50% of those in the control group: none allowed Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Norway Ethnicity: not reported |
|
| Interventions | Type: supplement (oil) Comparison: EPA + DHA vs nil Intervention: intervention: seal oil, 15 mL/d (2.6 g, 1.1 g/d EPA + 1.5 g/d DHA) (total n‐3 3.9 g/d, total PUFA 4.2 g/d): EPA + DHA 2.6 g/d Cod liver oil, 15 mL/d (3.3 g, 1.5 g/d EPA + 1.8 g/d DHA) (total n‐3 4.1 g/d, total PUFA 4.35 g/d): EPA + DHA 3.3 g/d Control: nil, no supplement PUFA dose seal oil aim: (intended) increase 2.6 g/d EPA + DHA, 1.2% E n‐3, 1.9% E PUFA PUFA dose cod liver oil aim: (intended) increase 3.3 g/d EPA + DHA, 1.5% E n‐3, 2.0% E PUFA Baseline PUFA unclear Compliance by biomarkers: serum omega‐3 fatty acids, rose from around 1 mmol/L to 2.4 (seal oil), 2.1 (cod liver oil) and 1.2 mmol/L (control). Latest total PUFA in serum was 10.3 mmol/L seal oil, 9.9 mmol/L cod liver oil, 7.3 mmol/L control. Serum TC reported in intervention arms but not control, fell from 8.2 mmol/L at baseline to 7.8 mmol/L at 14 months in seal oil, 8.3 to 8.0 in cod liver oil (further data provided by trial authors) Compliance by dietary intake: not reported
Compliance, other measures: no other data Inclusion basis: intended dose appeared to be 1.9% or 2.0% increase in intervention arms compared to control, > 10% greater intake than the assumed 6% E from PUFA at baseline. Supported by serum fatty acid composition being higher in both intervention arms at 14 months than the control arm. PUFA dose: 1.9% E SO, 2.0% E CLO Length of intervention: 14 months |
|
| Outcomes | Main trial outcome: serum lipids Dropouts: 8 seal oil, 2 cod liver oil, 1 control Available outcomes: total and CV deaths, MI, combined CV events, TC, TG and HDL, adverse events (no stroke or SCD occurred, weight reported but too different at baseline and only reported to 6 months, data also provided by trial authors on apolipoproteins A1 and B, and Lp(a), but not used) Response to contact: yes (trial author provided methodological details and outcome data) | |
| Notes | Data of 2 intervention groups combined for dichotomous outcomes and cod liver oil vs control data used for continuous outcomes. Trial funding: the trial was supported by the program Medical Research in Finnmark County, University of Tromsø |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | J Brox stated (personal communication, January 2017) Quote: "The randomisation of the 120 participants was done by first generating 3 groups (seal oil, cod liver oil, control), then giving each participant a number (1‐120), putting all the numbers into the same hat and blindly drawing one number at the time from the hat. The first 40 numbers (1‐40) were allocated to the seal oil group, the next 40 numbers (41‐80) to the cod liver oil group and the rest (81‐120) were allocated to the control group." |
| Allocation concealment (selection bias) | Low risk | J Brox stated (personal communication, January 2017) Quote: "The researcher/clinician who invited the participants had no knowledge of to which group the participants would be allocated" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "controls were aware ‐ not given a supplement" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | J Brox stated (personal communication, 2003) Quote: "All the persons involved in the drawing & analysing of blood were unaware of treatment. The technicians analysing the blood did not have any personal contact with the participants except K. Olaussen who did the FA analysis… she only had access to the sample numbers not names and code. The participants did not know their number” [says elsewhere that K Olaussen did not know allocations]. "The only assessor was J Brox who did not have any personal contact with the participants, had nothing to do with the randomising or analysing process, or the collecting of results." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seal oil group 10 dropouts, cod liver oil 3 dropouts, control group 3 dropouts. So substantial differences in rates of dropouts between the groups. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol or trials register entry was found. |
| Attention bias | Low risk | No suggestion of differential attention |
| Compliance | Low risk | Latest total PUFA in serum was 10.3 mmol/L seal oil, 9.9 mmol/L cod liver oil, 7.3 mmol/L control. Serum TC reported in intervention arms but not control |
| Other bias | Low risk | No further bias noted |
DART fat 1989.
| Methods | Diet And Reinfarction Trial (DART) RCT, 2 x 2 x 2 factorial (n6 LA vs mixed fats), also increased fish and increased fibre arms, 2 years Summary risk of bias: moderate to high |
|
| Participants | Men recovering from an MI
CVD risk: high
N: intervention: randomised 1018, analysed unclear; control: randomised 1015, analysed unclear
Mean years in trial: control 1.9, randomised 1.9
% male: 100%
Age: mean control 56.8, intervention 56.4 years Age range: all < 70 years Smokers: control 62.7%, intervention 61.2% Hypertension: intervention 24%, control 23.3% Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: beta‐blockers, other anti‐hypertensives, anti‐anginals Medications taken by some, but < 20% of the control group: anti‐coagulant, aspirin, other anti‐platelet, digoxin, other cardiac drugs Location: UK Ethenicty: not reported |
|
| Interventions | Type: dietary advice Comparison: ↑ polyunsaturated oil and margarines (n6) vs usual dietary fats (SFA) Intervention aims: reduce fat intake to 30% E, increase polyunsaturated to saturated ratio (P/S) to 1.0 (using polyunsaturated oils and margarines), weight‐reducing advice if BMI > 30 (dietitians provided the participants and their wives with initial individual advice and a diet information sheet, participants were revisited for further advice, recipes, encouragement at 1, 3, 6, 9, 12, 15, 18 and 21 months) Control aims: no dietary advice on fat, weight‐reducing advice if BMI > 30 (dietitians provided 'sensible eating' advice without specific information on fats) Dose aim: unclear Baseline n‐6: unclear, but control PUFA intake 6.8% E Compliance by biomarkers: good, serum TC significantly reduced in intervention compared to control (‐0.26 mmol/L, 95% CI ‐0.37 to ‐0.15) Compliance by dietary intake: assessed using a 7‐day weighted food diary, of a 25% random subsample
Compliance, other measures: no other data Inclusion basis: intended to increase PUFA/SFA ratio, as well as reduce total fat. TC was lower in intervention than control, and intake data suggest PUFA intake higher by 2.8% E in intervention than control, > 10% greater than baseline of 6.8% E. PUFA dose: 2.8% E Duration of intervention: 2 years |
|
| Outcomes | Main trial outcomes: mortality, reinfarction
Dropouts: all followed for events regardless of compliance (ITT)
Available outcomes: CV events (CV deaths plus non‐fatal MI), cancer deaths, total MI, non‐fatal MI, TC, HDL Response to contact: yes, Professor Burr provided additional data and information on methodology |
|
| Notes | Note: this was a 2 x 2 x 2 factorial trial, and so some in each group were randomised to increased fatty fish and/or increased cereal fibre. Trial funding: Welsh Scheme for Development of Health and Social Research, Welsh Heart Research Foundation, Flora Project (commercial), Health Promotion Research Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Unclear if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Very difficult to blind trials where participants need to make their own dietary changes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessors were not aware of study allocation" (Prof Burr, personal communication). Method of blinding not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | GPs contacted for information on mortality and morbidity when participants did not attend, data collected from mortality register |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry located |
| Attention bias | High risk | Those given dietary advice almost certainly given more time and attention than those in the control group (with no dietary advice) |
| Compliance | Low risk | TC significantly reduced in intervention compared to control (‐0.26 mmol/L, 95% CI ‐0.37 to ‐0.15) |
| Other bias | Low risk | None found |
DIPP‐Tokudome 2015.
| Methods | Dietary Intervention for Patients Polypectomized for tumours of the colorectum (DIPP) RCT, parallel, 2 arms (n3 EPA + DHA + ALA vs nil), 24 months Summary risk of bias: moderate or high |
|
| Participants | Poeple previously polypectomised for colorectal tumours N: 104 intervention, 101 control Level of risk for CVD: low Male: 73.1% intervention, 74.3% control Mean age (SD): 58.3 (9.5) intervention, 59.7 (8.9) control Age range: 35‐75 Smokers: 65.4% intervention, 61.4% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: supplements Medications taken by 20%‐49% of those in the control group: none Medications taken by some, but < 20% of the control group: oral contraceptive pills Location: Japan Ethnicity: not reported |
|
| Interventions | Type: advice plus supplement (fish oil capsules) Comparison: n3 EPA + DHA + ALA vs nil Intervention: advice to reduce total fat intake, decrease consumption of n‐6 PUFAs, increase intake of n‐3 PUFAs from fish/marine foods, increase intake of n‐3 PUFAs from perilla oil rich in ALA, and take 8 capsules of fish oil/day (equivalent to 96 mg/day of EPA and 360 mg/day of DHA) Control: advice to decrease intake of fats/oils as a whole Dose aim: increase 0.46 g/d EPA + DHA plus EPA + DHA from fish plus ALA from perilla, unclearn3, unclear PUFA Baseline PUFA: unclear but control 6.3% E PUFA Compliance by biomarkers: plasma fatty acid concentrations, fatty acid compositions in the membranes of red blood cells and the sigmoid colon. Plasma fatty acids suggested higher total PUFA intakes in intervention group (at 24 months 4.91 mmol/L, SD 1.23 in intervention group, 4.59 mmol/L, SD 0.76 in control). But TC higher in intervention (5.52 mmol/L, SD 0.9) than control (5.40 mmol/L, SD 0.79) at 24 months. Compliance by dietary intake: assessed using semi‐quantitative food frequency questionnaire
Compliance, other measures: none Inclusion basis: no intention to increase total PUFA. Intention was to increase omega‐3 but dose unclear. Total PUFA intakes were higher in intervention than control by 1.1%E, > 10% more than control PUFA dose: 1.1% E Length of intervention: 24 months |
|
| Outcomes | Main trial outcome: number and size of colorectal tumours Dropouts: 3 intervention, 5 control Available outcomes: all‐cause mortality, dietary intake, plasma fatty acids, lipids, side effects, glucose Response to contact: yes (methodological details provided) |
|
| Notes | Trial funding: all were either government or charity grants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated using random digit number for allocation of participants. |
| Allocation concealment (selection bias) | Low risk | Trial author confirmed "Allocation information was blinded to clinicians and researchers" but no methodology provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | From the 2015 paper, "The attending physicians as well as the participants were blinded to the assignment information". However in the discussion section they say "complete participant blinding could not have been achieved because free‐living participants might have exchanged information on their dietary intervention, say in the hospital waiting room". Trial author confirmed blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "physicians, including colonoscopists, a scientist who conducted blood and specimen analyses, and pathologists were blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were accounted for. |
| Selective reporting (reporting bias) | High risk | The researchers chose not to report data on the number, size and pathological type of the colorectal tumours as they said they would in the trials register. They reported more outcomes in the paper than initially stated. UMIN000000461 Registered 03 August 2006, recruitment completed 01 March 2007 |
| Attention bias | Low risk | Participants were given equal follow‐up. |
| Compliance | Unclear risk | Plasma fatty acids suggested higher total PUFA intakes in intervention group (at 24 months 4.91 mmol/L, SD 1.23 in intervention group, 4.59 mmol/L, SD 0.76 in control). But TC higher in intervention (5.52 mmol/L, SD 0.9) than control (5.40 mmol/L, SD 0.79) at 24 months. |
| Other bias | Low risk | None noted |
Dodin 2005.
| Methods | RCT, parallel, (n3 ALA vs n6 LA), 12 months Summary risk of bias: moderate or high |
|
| Participants | Healthy menopausal women N: 101 intervention, 98 control (analysed, intervention: 85 control: 94) Level of risk for CVD: low Male: 0% intervention, 0% control Mean age (SD): 54.0 (4.0) intervention, 55.4 (4.5) control Age range: 49‐65 Smokers: 8% intervention, 6% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Canada Ethnicity: French Canadian |
|
| Interventions | Type: food supplement (flaxseed) Comparison: more ALA vs less ALA Intervention: 40 g/d flaxseed incorporated into diets (providing 21,071 g total lignans, 180 calories, 16 g lipids (57% ALA), and 11 g total dietary fibre): 9.1 g/d ALA Control: 40 g/d wheat germ incorporated into diets (providing 196 g total lignans, 144 calories, 4 g lipids (6.9% ALA), and 6 g total dietary fibre: 0.26 g/d PUFA Dose aim: increase 8.8 g/d PUFA, 4.0% E PUFA Baseline PUFA 5.4% E Compliance by biomarkers: plasma fatty acid total PUFA (summing LA, GLA, AA, EPA, DHA, DPA, ALA) increased 3.02% from baseline to 12 months in control, increased 1.99% in intervention. Compliance by dietary intake: assessed by 3‐day food diary at baseline and 12 months
Compliance, other methods: first morning urine collection was performed at randomisation and at month 12 to measure urinary lignin levels. In addition, trial participants recorded their daily intake of seeds on diary cards and were asked to return unused bread and packages of seeds at each visit. Good compliance reported Inclusion basis: no intention to increase total PUFA, planned dose ˜4.0% E PUFA, dietary intake data suggested 1.5% E PUFA, biomarkers suggested greater PUFA intake in control, TC rose in control and fell in intervention. Using dietary intake dose of 1.5% E PUFA higher in intervention, > 10% higher than 5.6% E from total PUFA at baseline. PUFA dose: 1.5% E PUFA Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: BMD Dropouts: 26 intervention, 17 control (but 13/17 had an endpoint evaluation) Available outcomes: weight, BMI, QoL, BP, lipids, glucose, adverse events, dietary intake, plasma fatty acids Response to contact: yes, trial author confirmed that no CV events or deaths occurred during the trial |
|
| Notes | Trial authors replied to tell us that there were no deaths or CV events during the trial Trial funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was prepared by the clinical unit of the research centre using computer‐generated randomisation in blocks of 4‐8 |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, staff, and statisticians were blinded to dietary assignments for the duration of the trial. Quote: “a local baker prepared loaves of bread. Each week, the loaves of bread were delivered in sealed, opaque unmarked wrappers to the Department of Food and Nutrition Sciences at Laval University. The seeds were ground up and vacuum‐packed in the same laboratory. The Department of Food and Nutrition Sciences was responsible for labelling the bags of bread and packages of seeds with the subject’s randomization number. Bread and packages of seeds were provided on a 3‐month basis. The foods that both groups received was similar in appearance and packaging and was kept frozen until consumption to avoid essential fatty acid." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, staff, and statisticians were blinded to dietary assignments for the duration of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT. Loss to follow‐up 10%, reasons given. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registry entry found |
| Attention bias | Low risk | All participants had same number of visits |
| Compliance | High risk | Plasma fatty acid total PUFA (summing LA, GLA, AA, EPA, DHA, DPA, ALA) increased 3.02% from baseline to 12 months in control, increased 1.99% in intervention. |
| Other bias | Low risk | None noted |
Doi 2014.
| Methods | RCT, parallel, (n3 EPA vs nil, both with statins), 12 months Summary risk of bias: moderate or high |
|
| Participants | Patients having PCI after acute MI N: 119 intervention, 119 control analysed Level of risk for CVD: high Male: 77% intervention, 76% control Mean age (SD): 70 (11) intervention, 71 (12) control Age range: unclear Smokers: 28% intervention, 32% control Hypertension: 71% intervention, 69% control Medications taken by ≥ 50% of those in the control group: aspirin, ticlopidine, ß‐blockers, statins (as part of treatment) Medications taken by 20%‐49% of those in the control group: ARB/ ACE inhibitors Medications taken by some, but < 20% of the control group: none Location: Japan Ethnicity: not reported |
|
| Interventions | Type: supplement (EPA) Comparison: EPA vs nil Intervention: purified EPA ethyl esters (> 98%) 1.8 g/d EPA within 24 h after PCI plus statins Control: statins with no EPA Dose aim: increase 1.8 g/d EPA + DHA, 0.8% E n‐3, 0.8 %E PUFA Baseline PUFA: unclear Compliance by biomarkers: plasma EPA reported at 6‐8 months, higher in intervention (162.8 mg/L) than control (65.5 mg/L). No further biomarker or TC data reported Compliance by dietary intake: not reported
Compliance, other measures: not reported Inclusion basis: no intention to increase total PUFA. Intention was to increase omega‐3 by 0.8% E. Total PUFA appear to be 0.8% E higher in intervention, > 10% more than assumed 6% E baseline PUFA dose: 0.8% E Length of intervention: 12 months |
|
| Outcomes | Main trial outcome: CV events Dropouts: 1 intervention, 2 control Available outcomes: mortality, stroke, MI, sudden death, CV death, revascularisation Response to contact: contact attempted but no response to date. |
|
| Notes | Trial funding: trial registry states "self‐funded". The trial authors received honoraria from Mochida Pharmaceutical Co. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation plan, which included stratification by age and sex. |
| Allocation concealment (selection bias) | Unclear risk | Carried out by research technician but unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label but blind endpoint |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data on outcomes were collected from clinical charts. Unclear if blinded. Diagnoses were confirmed by investigator blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 dropouts, similar rates between the groups and reasons given |
| Selective reporting (reporting bias) | High risk | Data collection completed before trial registry entry. Only 1% dropout |
| Attention bias | Low risk | Timing of follow‐ups similar |
| Compliance | Unclear risk | Plasma EPA reported at 6‐8 months, higher in intervention (162.8 mg/L) than control (65.5 mg/L). No further biomarker or TC data reported |
| Other bias | Low risk | None observed |
Dullaart 1992.
| Methods | RCT, parallel, 2 arms (n6 LA vs mixed fats), 2 years Summary risk of bias: moderate to high |
|
| Participants | People with type I diabetes with elevated urinary albumin
CVD risk: moderate
Intervention: randomised 18, analysed 16 Control: randomised 20, analysed 20 % male: 81% intervention, 75% control Age: mean (SD) intervention 44 (12), control 41 (14) Age range: unclear (21‐65 inclusion) Smokers: intervention 50%, control 55% Hypertension: intervention 6%, control 10% Medications taken by ≥ 50% of those in the control group: insulin Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: anti‐hypertensives Location: Netherlands Ethnicty: not reported |
|
| Interventions | Type: dietary advice Comparison: LA (n6) vs usual diet Intervention: diet advice given at every visit throughout the 2‐year period to increase linoleic acid achieving a polyunsaturated: saturated fatty acid ratio close to 1.0. Advice to replace butter or saturated margarines by polyunsaturated margarines and to restrict the intake of SFA from meat and milk products Control: to continue their usual diet. All participants were urged not to alter total fat and protein content. Dose: aim unclear Baseline PUFA: 6.6% E PUFA Compliance: TC fell more in intervention (‐0.45 mmol/L) than control (0.10 mmol/L) from baseline to 2 years. Significant difference between plasma cholesteryl ester LA in intervention and control at 2 years Plasma cholesteryl esters at 2 years
Dietary assessment using 1 week dietary recall, reported at 2 years.
Compliance, other methods: not reported Inclusion basis: aimed to increase LA rather than total PUFA intake. Intake data suggests 3.5% E PUFA dose, > 10% increase from control 9% E intake Supported by plasma cholesteryl ester LA and TC PUFA dose: 3.5% E PUFA Duration of intervention: 2 years |
|
| Outcomes | Main trial outcomes: albuminuria and lipids
Dropouts: intervention 2 of 20, control 4 of 20
Available outcomes: weight, HDL , TGs, HbA1c (TC, glucose, insulin reported but too different at baseline to use, LDL not reported in control group, renal outcomes such as glomerular filtration rate, albuminuria, mean arterial pressure not used) Response to contact: yes, trial author confirmed no MI or other CVD events occurred during trial |
|
| Notes | Most outcomes are estimated from figures. Trial funding: Dutch Diabetes Research Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were stratified according to sex and randomised in blocks of ten men and six women" |
| Allocation concealment (selection bias) | Low risk | Assigned using opaque sealed envelopes by independent statistical investigator with no contact with participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding. Participants could not be blinded as they received dietary advice. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details on dropouts apart from the exclusion of 2 intervention participants from the trial due to pregnancy and decision not to participate. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration located |
| Attention bias | High risk | Likely that diet‐advice group had more time and attention |
| Compliance | Low risk | TC fell more in intervention (‐0.45 mmol/L) than control (0.10 mmol/L) from baseline to 2 years. Significant difference between plasma cholesteryl ester LA in intervention and control at 2 years |
| Other bias | Low risk | None noted |
EPIC‐1 2008.
| Methods | EPANOVA in Crohn's disease, trial 1 (EPIC‐1) RCT, parallel, 2 arms (n3 EPA + DHA vs mixed fats), 52 weeks Summary risk of bias: moderate or high |
|
| Participants | Adults with quiescent Crohn’s Disease Activity Index score < 150 N: 188 intervention, 186 control Level of risk for CVD: low Male: 48.1% intervention, 41.1% control Mean age (SD): 40.5 (15.2) intervention, 38.2 (13.1) control Age range: 18‐70 years Smokers: 30.6% intervention, 34.4% control Hypertension: unclear Medications taken by ≥ 50% of those in the control group: oral 5‐ASA therapy, systemic corticosteroids – prednisolone, budesonide Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: antibiotic therapy, topical rectal therapy, immune‐modifying agents, immune modifiers/biologics Location: Canada, Europe, Israel, USA Ethnicity: not reported |
|
| Interventions | Type: supplement (capsule) Comparison: EPA + DHA vs MCT Intervention: 2 x 2 1 g gelatine capsules omega‐3‐free fatty acids (Epanova‐ 2.2 g EPA, 0.8 g DHA) Control: 4 x1 g capsules medium‐chain triglycerides Dose aim: increase 3.0 g/d EPA + DHA, 1.4% E n‐3, 1.4% E PUFA Baseline PUFA: unclear Compliance by biomarkers: not reported, neither fatty acids nor TC Compliance by dietary intake: not reported
Compliance, other measures: pill counts, 79.2% adhered intervention, 75.6% adhered control Inclusion basis: no intention to increase total PUFA. Intention was to increase 3.0 g/d EPA + DHA, 1.4% E n‐3, 1.4% E PUFA, > 10% greater than assumed baseline of 6% E. PUFA dose: 1.4% E Length of intervention: mean 52 weeks |
|
| Outcomes | Main trial outcome: Crohns relapse‐free time
Dropouts: 80 intervention, 91 control
Available outcomes: total deaths, non‐fatal arrhythmias, cancer diagnoses, cancer deaths, adverse events Response to contact: yes (data provided) |
|
| Notes | Trial funding: Tillotts Pharma, trial authors had extensive financial disclosures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by number generator. Used a centralised randomisation procedure via interactive voice‐recognition system. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation (see above) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding stated, identical capsule (slow‐release capsules). Neither investigator nor participant knew the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial states double‐blind but does not state that outcome assessors were blinded or provide a mechanism for this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of dropouts and reasons provided. 171 of 187 in intervention group and 174 of 184 in control group provided data for primary outcome, (7% dropout), though 80 in the intervention group and 91 in the control group terminated early. |
| Selective reporting (reporting bias) | High risk | Trials registration (NCT00613197) first received in 2008, but trial started in 2003, and was published in 2008. |
| Attention bias | Low risk | As investigators were blinded attention bias was not possible. |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | Low risk | No further bias noted |
EPIC‐2 2008.
| Methods | EPANOVA in Crohn's disease, trial 2 (EPIC‐2) RCT, parallel, 2 arms (n3 EPA + DHA vs mixed fats), 58 weeks Summary risk of bias: moderate or high |
|
| Participants | Adults with a confirmed diagnosis of Crohn’s disease and a Crohn’s Disease Activity Index score < 150 who are responding to steroid induction therapy N: intervention, 189, control 190 (187 intervention, 188 control analysed) Level of risk for CVD: low (people with quiescent Crohn’s disease) Male: 48.1% intervention, 41.1% control Mean age (SD): 38.5 (13.8) intervention, 40.0 (13.6) years control Age range: > 16 years Smokers: 25.1% intervention, 37.2% control Hypertension: unclear Medications taken by ≥ 50% of those in the control group: systemic corticosteroids – prednisolone, budesonide (but tapered and discontinued during the trial) Medications taken by 20%‐49% of those in the control group: only reported for prior 12 months Medications taken by some, but < 20% of the control group: only reported for prior 12 months Location: Canada, Europe, Israel, USA Ethnicity: not reported |
|
| Interventions | Type: supplement (capsule) Comparison: EPA + DHA vs MCT Intervention: 2 x 2 1 g gelatine capsules omega‐3‐free fatty acids (Epanova) providing total dose ˜2.2 g/d EPA, 0.8 g/d DHA: EPA + DHA ˜3.0 g/d Control: 2 x 2 1 g capsules medium‐chain triglyceride oil Dose aim: increase 3.0 g/d EPA + DHA, 1.4% E n‐3, 1.4% E PUFA Baseline PUFA: unclear Compliance by biomarkers: not reported, neither fatty acids nor TC Compliance by dietary intake: not reported
Compliance, other measures: measured by participant interviews and pill counts, 75.4% adhered intervention, 81.4% adhered control Inclusion basis: no intention to increase total PUFA. Intention was to increase 3.0 g/d EPA + DHA, 1.4% E n‐3, 1.4% E PUFA, > 10% greater than assumed baseline of 6% E PUFA dose: 1.4% E Length of intervention: mean 58 weeks |
|
| Outcomes | Main trial outcome: maintain Crohns symptomatic remission Dropouts: 114 intervention, 112 control Available outcomes: mortality, CV events (nil), cancer diagnoses, adverse events Response to contact: yes (data provided) | |
| Notes | Trial funding: Tillotts Pharma, trial authors had extensive financial disclosures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by number generator. Used a centralised randomisation procedure via interactive voice‐recognition system. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation (see above) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinding stated, identical capsule (slow‐release capsules). Neither investigator nor participant knew the allocation. However no information provided on capsules taste or smell. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial states double‐blind but does not state that outcome assessors were blinded or provide a mechanism for this |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of dropouts and reasons provided, however 114 of 189 in intervention group and 112 of 190 in control group terminated early. |
| Selective reporting (reporting bias) | High risk | NCT00074542. First received 2003, trial start 2002. Published 2008. Some outcomes, such as quality of life, stated in trials registry but not in published papers. |
| Attention bias | Low risk | As investigators were blinded attention bias was not possible. |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | Low risk | No further bias noted |
EPOCH 2011.
| Methods | Older People, Omega‐3 and Cognitive Health (EPOCH) RCT, parallel (n3 EPA + DHA vs MUFA), 18 months Summary risk of bias: low |
|
| Participants | Healthy older adults with no cognitive impairment N: 195 intervention, 196 control (reported by trial author) Level of risk for CVD: low Male: not reported Mean age (SD): not reported Age range: not reported, but 65‐90 recruited Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Australia Ethnicity: not reported |
|
| Interventions | Type: supplement (fish oil capsules) Comparison: high EPA + DHA vs MUFA and low EPA + DHA Intervention: 4 capsules/d (1.72 g/d DHA and 0.60 g/d EPA): EPA + DHA 2.32 g/d Control: 4 capsules/d (3.960 g/d olive oil and 40 mg/d fish oil), 0.8 g/d PUFA (assuming 20% of olive oil is PUFA) Dose aim: increase 2.28 g/d EPA + DHA, or 1.52 g/d PUFA (subtracting control data), 0.68% E PUFA Baseline PUFA unclear Compliance by biomarkers: erythrocyte membrane n‐3 LC PUFA status assessed but no useful data reported, no TC data Compliance by dietary intake: not reported
Compliance, other methods: count of all unused supplements returned at 3‐monthly intervals, plus self‐report calendars, mailed back on a monthly basis. If compliance fell below 85% (re calendars), they were contacted by a researcher who noted the reasons. Inclusion basis: no intention to increase total PUFA intake. Dose aim 1.52 g/d PUFA or 0.68% E total PUFA, > 10% increase from assumed 6% E PUFA baseline. No data on biomarkers, intake or TC PUFA dose: 0.68% E Length of intervention: 18 months |
|
| Outcomes | Main trial outcome: change in cognitive performance Dropouts: not reported Available outcomes: mortality (nil), MI, stroke, revascularisation, atrial fibrillation, CV events. Planned outcomes, not reported in publications, included: cognitive outcomes, functional outcomes, glucose, lipids, plasma fatty acids, BP, inflammation and oxidative stress. Response to contact: yes (data provided) |
|
| Notes | Trial authors reported some events, but don't appear to be published. Trial funding: EPAX donated the Omega‐3 concentrate and Blackmores Pty Ltd donated the placebo and packaging of the Omega‐3 concentrate. The trial was supported by the Brailsford Robertson Award 2007‐2008 (University of Adelaide and CSIRO Food and Nutritional Sciences), and is funded by a National Health and Medical Research Project Grant (#578800). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Age‐stratified, permuted‐block randomisation, with mixed block‐sizes (2‐8, size unknown to trial investigators), 1:1 allocation. Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | An independent researcher prepared allocation to treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The researchers, project staff, and participants remained blinded to treatment allocation until the trial was completed and the database locked. No information provided on capsules' appearance, taste or smell, but fish oil added to control to make taste similar. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data for each group presented, and no attrition data presented |
| Selective reporting (reporting bias) | High risk | Registered at ACTRN12607000278437. Only cognitive functions reported for whole population (not by arm). No secondary outcomes reported (Mini Mental State Examination; perceived health status, depressive symptoms, positive and negative affect, life satisfaction, self‐reported cognitive functioning, and functional capacity; BP; biomarkers of glucose, glycated haemoglobin, TGs, TC, HDL, LDL, homocysteine, CRP, Malondialdehyde (MDA), and telomere length). |
| Attention bias | Low risk | All had the same contact and attention |
| Compliance | Unclear risk | Compliance assessed by erythrocyte membrane n‐3 long‐chain PUFA status but results not reported, no TC or biomarker data on total PUFA |
| Other bias | Low risk | None noted |
FAAT ‐ Leaf 2005.
| Methods | Fatty Acid Antiarrhythmia Trial ‐ FAAT Randomisation: RCT, parallel, 2 arms, (n3 EPA + DHA vs MUFA), 12 months Summary risk of bias: moderate or high |
|
| Participants | People with implanted cardioverter defibrillators (ICDs) N: intervention 200, control 202 Level of risk for CVD: high (participants with ICDs). Male: intervention 84.5%, control 81.7% Mean age (SD): intervention 65.7 (11.6), control 65.3 (11.7) years Age range: unclear Smokers: intervention 15%, control 11.4% Hypertension: unclear Medications taken by ≥ 50% of those in the control group: ACEi, beta‐blockers Medications taken by 20%‐49%: diuretics Medications taken by some, but < 20%: Ca channel blockers, amiodarone, sotalol, type 1 antiarrhythmics Location: USA Ethnicity: intervention 95.5% white, control 96.5% white |
|
| Interventions | Type: supplement/capsule Comparison: EPA + DHA vs MUFA Intervention: 4 x 1 g/d fish oil gelatin capsules, 2.6 g EPA + DHA/d (Pronova Biocare, quantities of EPA + DHA unclear): EPA + DHA 2.6 g/d Control: 4 x 1 g/d olive oil capsules, 4 g/d (in identical gelatin capsules, < 0.06 g/d EPA + < 0.06 g/d DHA) All were advised to use olive oil rather than the common plant seed oils for cooking, dressings, and sauces PUFA Dose: (intended) Dose aim: intervention 2.6 g/d EPA + DHA, 1.2% E n3, 1.2% E PUFA, control 4 g olive oil, 20% LA, 0.8 g/d PUFA, 0.36% E PUFA. Difference 0.84% E PUFA Baseline PUFA: unclear Compliance by biomarkers: platelet phospholipid EPA + DHA higher in intervention group than control, no data on total PUFA or TC Compliance by dietary intake: not reported
Compliance, other measures: pill counts suggested greater omega‐3 intake in intervention participants. 35% were non‐compliers (36.5% intervention, 34.2% control) Inclusion basis: no intention to increase total PUFA. Intention was to increase omega‐3, difference between arms was 0.84% E PUFA, > 10% more than control PUFA dose: 0.84% E Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: fatal VF/VT Dropouts: intervention 13 deaths, unclear number of dropouts; control 12 deaths, dropouts unclear Available outcomes: deaths, CV deaths, deaths from heart failure, fatal arrhythmias, MI, angina Response to contact: yes (data provided) | |
| Notes | Trial funding: the trial was supported in part by a grant from the NHLBI, NIH (HL62154) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation tables for each collaborating site, stratified by site |
| Allocation concealment (selection bias) | Low risk | Trial author confirmed allocation was concealed from investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial referred to as "double blind" and gelatin capsules (verum and placebo) were stated as being of identical appearance but no discussion of taste or smell. Trial author confirmed that investigators and participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | VT and VF events were assessed blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large numbers dropped out so some deaths etc. may have been missed, 35% discontinued early due to non‐compliance but were assessed at trial end, data censored for some participants |
| Selective reporting (reporting bias) | High risk | Trials registry data received September 2005, paper published November 2005 |
| Attention bias | Low risk | Time and attention appeared similar between the 2 arms |
| Compliance | High risk | Platelet phospholipid EPA + DHA higher in intervention group than control, no data on total PUFA or TC |
| Other bias | Low risk | None noted |
GLAMT 1993.
| Methods | Gamma Linolenic Acid Multicentre Trial (GLAMT) RCT, 2‐arm, parallel (n6 GLA vs non‐fat), 1 year Summary risk of bias: moderate to high |
|
| Participants | People with mild diabetic neuropathy
CVD risk: moderate
Control: randomised 57, analysed 48 (with ≥ 1 evaluation)
Intervention: randomised 54, analysed 52
Mean years in trial: control 1.0, randomised 1.0
% male: intervention 67%, control 79%,
Age, mean (SD) years: intervention 53.3 (11.1), control 52.9 (11.4) Age range: unclear Smokers: unclear Hypertension: unclear Medications taken by ≥ 50% of those in the control group: insulin Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: UK and Finland Ethenicty: not reported |
|
| Interventions | Type: supplement Comparison: GLA (n‐6) vs placebo (paraffin) Control aims: 12 capsules/d paraffin Intervention aims: 12 capsules/d evening primrose oil (EP4, equivalent to Epogam): 0.48 g/d GLA plus LA (stated as the major constituent, dose not given, if assume 0.7 g/capsule then 8.4 g/d*) Dose aim: increase 0.48 g/d GLA or 4 kcal or 0.2% E GLA, increase ˜8.4 g/d LA or 76 kcal or 3.8% E LA, total 4% E n6 Baseline PUFA: unclear Compliance by biomarkers: unclear, no serum TC or tissue fatty acid levels reported Compliance by dietary intake: unclear
Compliance, other methods: not reported Inclusion basis: aimed to increase GLA intake rather than total PUFA. Dose aim appeared to be ˜4% E PUFA (from omega‐6 data), >10% more than assumed baseline of 6% E PUFA. No confirmatory biomarker or intake data PUFA dose: 4% E PUFA (estimated from aim) Duration of intervention: 1 year |
|
| Outcomes | Main trial outcome: measures of diabetic neuropathy
Dropouts: intervention 10, control 17
Available outcomes: MI, cancer (no deaths) Response to contact: contact attempted but no response to date. |
|
| Notes | Trial funding: Scotia Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind, and Quote: "Active and placebo capsules were indistinguishable in taste or appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear, though trial described as double‐blind no methods or statement of blinding of outcome assessors was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for withdrawal usually given, but high and dissimilar |
| Selective reporting (reporting bias) | Unclear risk | No clear protocol or trials registry entry found |
| Attention bias | Low risk | Appeared similar |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | Low risk | None identified |
HARP‐ Sacks 1995.
| Methods | Harvard Atherosclerosis Reversibility Project (HARP) RCT, (n3 EPA + DHA vs MUFA), 24 months Summary risk of bias: moderate or high |
|
| Participants | People with coronary heart disease N: 41 intervention, 39 control (99.9% follow‐up at trial end) Level of risk for CVD: high Male: 93.5% intervention, 92.9 % control Mean age (SD): 62 (7) intervention, 62 (7) years control Age range: 30‐75 Smokers: 0% (exclusion criteria) Hypertension: 48% intervention, 36% control Medications taken by ≥ 50% of those in the control group: beta blockers, antiplatelet agents Medications taken by 20%‐49% of those in the control group: Ca channel blockers, nitrates Medications taken by some, but < 20% of the control group: ACE inhibitors, oral hypoglycaemic drugs Location: USA Ethenicity: not reported |
|
| Interventions | Type: supplement (capsule) Comparison: n3 vs MUFA Intervention: 12 fish oil capsules/d (Promega, Parke‐Davis) in divided doses, preferably after meals. Each fish oil capsule contained 500 mg of n‐3 PUFAs composed of EPA (240 mg), DHA (160 mg) and other (100 mg) (mainly DPA) providing total daily dose of 6 g/d of n‐3 fatty acids. Control: olive oil capsules identical in appearance to the fish oil capsules, 6 g/d olive oil, 1.2 g/d LA Dose aim: increase 4.8 g/d PUFA, 2.2% E PUFA Baseline PUFA: unclear Compliance by biomarkers: adipose fatty acids (sum of LCn3 fats, AA & LA) were 21.2% in intervention group, 20.4% in control group. TC was slightly higher in intervention (5.02 mmol/L, SD 0.96) than control (4.99 mmol/L, SD 0.62) at 28 months Compliance by dietary intake: not reported.
Compliance, other measures: capsule counts, adherence averaged 80% intervention, and 90% control Inclusion basis: no intention to increase total PUFA. Intention was to increase omega‐3, difference between arms was 4.8 g/d PUFA, 2.2% E PUFA, > 10% increase from assumed baseline of 6% E PUFA PUFA dose: 2.2% E PUFA Duration of intervention: average 28 months |
|
| Outcomes | Main trial outcome: regression of coronary artery lesions Dropouts: 10 intervention, 11 control Available outcomes: total and CV deaths, fatal and non‐fatal MI, stroke, angioplasty or coronary artery bypass graft, unstable angina, CHD, cancer diagnosis, combined CV events, side effects Response to contact: yes | |
| Notes | Trial funding: National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, Bethesda, Maryland, Warner Lambert‐Parke Davis (pharmaceutical company), East Hanover, New Jersey; and by an Established Investigator Award to Dr. Sacks from the American Heart Association, Dallas, Texas | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomization” stratified by clinical management regime and TC/HDL ratio |
| Allocation concealment (selection bias) | Unclear risk | No further details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “patients and personnel responsible for lab measurements, cardiac catheterization, and analysis of angiography films were blinded to the treatment assignment”. Although capsules were identical in appearance, no information on their taste and smell |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “patients and personnel responsible for lab measurements, cardiac catheterization, and analysis of angiography films were blinded to the treatment assignment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate over 28 months and all reasons are well‐documented. |
| Selective reporting (reporting bias) | High risk | Trial registered retrospectively after publication |
| Attention bias | Low risk | Nothing in description implies the arms were treated differently |
| Compliance | Unclear risk | Adipose fatty acids (sum of LCn3 fats, AA & LA) were 21.2% in intervention group, 20.4% in control group. TC was slightly higher in intervention (5.02 mmol/L, SD 0.96) than control (4.99 mmol/L, SD 0.62) at 28 months. |
| Other bias | Low risk | None noted |
HERO‐Tapsell 2009.
| Methods | Healthy Eating to Reduce Overweight in people with type 2 diabetes (HERO) RCT, parallel, (n3 ALA vs low n3), 12 months Summary risk of bias: moderate or high |
|
| Participants | Overweight adults with non‐insulin treated diabetes N: 26 intervention, 24 control (analysed, int: 18 cont: 17) Level of risk for CVD: moderate Male %: not reported Mean age (SD): 54 (8.7), not reported by arm Age range: 33‐70 Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: lipid‐lowering drugs, oral hypoglycemics Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Australia Ethnicity: not reported |
|
| Interventions | Type: food supplement (walnuts) Comparison: ALA vs nil Intervention: 30 g/d snack portions of walnuts, aim 30% E fat (10% SFA, 10% MUFA, 10% PUFA), 20% E protein, 50% E CHO, P/S ratio of 1.0. Advised not to take fish oil supplements, ALA dose unclear Control: no supplements, aim 30% E fat (10% SFA, 15% MUFA, 5% PUFA), 20% E protein, 50% E CHO Both groups were given low‐fat isocaloric dietary advice plus advice to brisk walk 30 min 3 times/week Dose aim: increase 5% E PUFA Baseline PUFA: unclear but control 5.5% E PUFA Compliance by biomarkers: omega‐3 fats measured by erythrocyte membrane fatty acid levels which were similar in both groups, no other PUFAs reported. TC fell by 0.3 mmol/L from baseline to 12 months in control, and fell by 0.1 mmol/L in the intervention. Compliance by dietary intake: all assessed at 12 months using validated diet history interview and 3‐day food records
Compliance, other measures: not reported Inclusion basis: no intention to increase total PUFA. Intention was to increase walnuts, which included increasing PUFA in place of MUFA. Dietary intake data suggested an increase of 6.5% E from PUFA compared to control, > 10% increase from control group baseline of 5.1% E from PUFA PUFA dose: 6.5% E PUFA Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: change in body weight and % body fat Dropouts: 8 intervention, 5 control Available outcomes: all‐cause mortality (nil deaths), weight, visceral adipose tissue, lipids, glucose, insulin, HbA1c (body fat % and subcutaneous adipose tissue measured but too different at baseline to use) Response to contact: not yet attempted |
|
| Notes | Body fat % was too different between groups at baseline hence data not used. Trial funding: California Walnuts Commission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using a computerised random‐number generator by a researcher independent of the subject interface |
| Allocation concealment (selection bias) | Unclear risk | No further details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Subjects, but not dietitians, were blinded to the type of overall diet (a prepackaged 30 g snack portion of walnuts was given to the walnut group unbeknown to the controls)". However, there was no placebo supplement so blinding not truly feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Paper states “code was concealed from the researchers collecting data, as well as from subjects.” However as participants could not be blinded outcome assessors may not have been (problem for measures of adiposity, not for biochemical measures). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, 35 of 50 analysed (30% attrition rate) |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered but post‐analysis |
| Attention bias | Low risk | Both groups appear to have had same level of attention |
| Compliance | High risk | Omega‐3 fats measured by erythrocyte membrane fatty acid levels which were similar in both groups, no other PUFAs reported. TC fell by 0.3 mmol/L from baseline to 12 months in control, and fell by 0.1 mmol/L in the intervention. |
| Other bias | Low risk | None noted |
Houtsmuller 1979.
| Methods | RCT, parallel, (increase LA vs usual diet), 72 months maximum Summary risk of bias: moderate or high |
|
| Participants | Adults with newly diagnosed diabetes N: 51 intervention, 51 control (analysed unclear intervention, unclear control) Level of risk for CVD: moderate Male: 56% overall (not stated by intervention arm) Mean age (SD): not reported intervention, not reported control Age range: not reported Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: statins (probably) Location: Netherlands Ethenicity: not reported |
|
| Interventions | Type: dietary advice Comparison: omega‐6 vs SFA and CHO Intervention: aims total fat 40% E, 1/3 LA, CHO 45% E, protein 15% E; methods unclear, surveyed by dietitian. Intervention appears to have been delivered by dietitian but no details on format or frequency. Control: aims SFA 35% E, CHO 50% E, protein 15% E; methods unclear, surveyed by dietitian Dose aims: increase ˜9% E LA (aims imply no LA in control, but paper states LA was 4 x higher in intervention than control, est 3% E control, 12% E int, so increase of ˜9% E) Baseline PUFA: unclear Compliance by biomarkers: good, serum TC significantly reduced in intervention compared to control (‐0.47 mmol/L, 95% CI ‐0.76 to ‐0.18), no significant differences in men, but significant improvements in women from 3 years. Compliance by dietary intake: unclear (not reported)
Compliance, other measures: not reported Inclusion basis: aimed to increase LA, not total PUFA. Appears to have increased LA by ˜9% E so assume increase in total PUFA also ˜9% E, > 10% increase from control group baseline of ˜3% E from PUFA PUFA dose: 9% E PUFA Duration of intervention: 72 months |
|
| Outcomes | Main trial outcome: progression of diabetic retinopathy Dropouts: unclear intervention, unclear control Available outcomes: CV events (total MI and angina), TC, TGs (data read off graph), CHD mortality (fatal MI), CHD events (MI, angina), progression of retinopathy Response to contact: contact attempted but no response to date. |
|
| Notes | Trial funding: Dutch Heart Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants matched in pairs then randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear, though unlikely as dietary advice provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, deaths, cancer and CV events are dropouts, trialists asked for data ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry found |
| Attention bias | Unclear risk | No details provided |
| Compliance | Low risk | TC significantly reduced in intervention compared to control (‐0.47 mmol/L, 95% CI ‐0.76 to ‐0.18) |
| Other bias | High risk | Some concerns around fraud in the first author's later research on diet in cancer. No allegations found regarding his research in diabetes (but much information is in Dutch). |
Kumar 2012.
| Methods | RCT, parallel, (n3 EPA + DHA vs nil), 12 months Summary risk of bias: moderate or high |
|
| Participants | People with persistent AF on warfarin N: 92 intervention, 90 control (91 and 87 analysed ITT) Level of risk for CVD: high Male %: 82.4 intervention, 72.4 control Mean age (SD): 63 (10) intervention, 61 (13) control Age range: 18‐85 (inclusion criteria) Smokers: 22.2% intervention, 11.5% control Hypertension: 45.6% intervention, 58.6% control Medications taken by ≥ 50% of those in the control group: anti‐arrhythmic drugs, renin‐angiotensin system inhibitors Medications taken by 20%‐49% of those in the control group: statins Medications taken by some, but < 20% of the control group: not reported Location: Australia Ethnicity: not reported |
|
| Interventions | Type: fish oil capsule Comparison: EPA + DHA vs nil Intervention: 6 capsules/d of a fish oil preparation containing a total dose of 1.02 g of EPA and 0.72 g DHA. Participants in the omega‐3 group were asked to continue fish oils till a maximum of 1 year or till return of persistent AF. Control: no supplements. Participants were advised not to take any fish oil supplements All participants underwent cardioversion following randomisation. Dose aim: increase 1.74 g/d EPA + DHA, 0.8% E n‐3, 0.8% E PUFA Baseline PUFA: unclear Compliance by biomarkers: phospholipid fatty acid status measured at cardioversion, DHA and EPA higher in intervention (EPA 2.5% fat, DHA 6.3% fat) than control (EPA 1.2% fat, DHA 3.4% fat), both P < 0.001. No other PUFAs, or TC, reported Compliance by dietary intake: not reported
Compliance, other measures: monitored on a weekly basis via telephone and during follow‐up by using a pill count, results not reported Inclusion basis: no intention to increase total PUFA. Intention was to increase 1.74 g/d EPA + DHA, 0.8% E PUFA > 10% greater than assumed baseline of 6% E. No biomarker, TC or intake data to confirm PUFA dose: 0.8% E Duration of intervention: 1 year (or AF recurrence) |
|
| Outcomes | Main trial outcome: AF recurrence Dropouts: 4 intervention, 0 control Available outcomes: all‐cause mortality (nil death), AF recurrence, time to AF recurrence, adverse events. Response to contact: written but no answer yet |
|
| Notes | Trial funding: the trial was funded in part by the National Heart Foundation of Australia and the Pfizer Cardiovascular Lipid Research Grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised to a control or an omega‐3 group in a 1:1 fashion (no methodological details) |
| Allocation concealment (selection bias) | Unclear risk | No further details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label with no placebo control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT conducted |
| Selective reporting (reporting bias) | Unclear risk | Trial registered 2005 but data collection started 2003 |
| Attention bias | Unclear risk | Intervention group had capsules, while control group did not. Potential for greater contact and checking with intervention group, otherwise groups seem to have had the same care. |
| Compliance | Unclear risk | Phospholipid fatty acid status measured at cardioversion, DHA and EPA higher in intervention (EPA 2.5% fat, DHA 6.3% fat) than control (EPA 1.2% fat, DHA 3.4% fat), both P < 0.001. No other PUFAs, or TC, reported |
| Other bias | Low risk | None noted |
Kumar 2013.
| Methods | RCT, parallel, (n3 EPA + DHA vs nil), 12 months Summary risk of bias: moderate or high |
|
| Participants | Adults > 60 years with sinoatrial node disease and dual chamber pacemakers N: 39 intervention, 39 control (only 18 vs 39 for 12‐month analyses) Level of risk for CVD: moderate/high Male %: 46% intervention, 56% control Mean age (SD): 78 (7) intervention, 77 (8) control Age range: not reported Smokers: not reported Hypertension: 72% Medications taken by ≥ 50% of those in the control group: statins, renin‐angiotensin system inhibitors Medications taken by 20%‐49% of those in the control group: anti‐arrhythmic drugs Medications taken by some, but < 20% of the control group: not reported Location: Australia Ethnicity: not reported |
|
| Interventions | Type: omega‐3 capsule Comparison: EPA + DHA vs nil Intervention: a triglyceride preparation containing a total of 6 g/day of omega‐3 PUFAs of which 1.8 g/day were n‐3 (1.02 g EPA and 0.72 g DHA) Control: no supplements Dose aim: increase 1.74 g/d EPA + DHA, 0.8% E n‐3, 0.8% E PUFA Baseline PUFA: unclear Compliance by biomarkers: phospholipid fatty acid status measured at randomisation and at 1‐3 months, DHA and EPA increased in intervention, not in control. No other PUFAs, or TC, reported Compliance by dietary intake: measured via weekly diet history, but no results reported
Compliance, other measures: measured by weekly pill count, results not reported Inclusion basis: no intention to increase total PUFA. Intention was to increase 1.74 g/d EPA + DHA, 0.8% E PUFA >10% greater than assumed baseline of 6% E. No biomarker, TC or intake data to confirm PUFA dose: 0.8% E Duration of intervention: median 378 days |
|
| Outcomes | Main trial outcome: AF burden Dropouts: 1 intervention, 0 control Available outcomes: all‐cause mortality, CV mortality, AF (frequency and duration but not recurrence so not used), adverse events Response to contact: written, no reply to date |
|
| Notes | Trial funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using sequentially numbered, opaque, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “At each visit, stored AT/AF diagnostic data were retrieved in an un‐blinded fashion” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 1 lost, and reason explained. But group baseline size to cross‐over is huge. Doesn’t report just the 17 or 18 metrics at baseline, no idea why the 21 were the ones switched and mixed with the control |
| Selective reporting (reporting bias) | Low risk | Trial prospectively registered and outcomes stated were reported |
| Attention bias | Unclear risk | Only difference would be handing out the capsules, rest seems the same. However, one group is getting supplements and the other nil |
| Compliance | Unclear risk | Phospholipid fatty acid status measured at randomisation and at 1‐3 months, DHA and EPA increased in intervention, not in control. No other PUFAs, or TC, reported |
| Other bias | High risk | 21 of the 39 randomised to the intervention were crossed over to control at six months so 12‐month outcomes are reported for 17/18 intervention group while baseline characteristics are reported for all 39 participants. |
Ley 2004.
| Methods | RCT, parallel, (reduced total fat vs usual diet), 12 months Summary risk of bias: low (dietary advice trial) |
|
| Participants | Adults with impaired glucose intolerance or high normal blood glucose N: 85 intervention, 90 control (176 between both groups) (analysed 66 intervention: 70 control at 1 year, 112 between both groups at 5 years) Level of risk for CVD: moderate Male: 80% intervention, 68% control Mean age (SD): 52.5 (SE 0.8) intervention, 52.0 (SE 0.8) control Age range: not reported Smokers: 23% intervention, 9% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: BP medication taken by 27% intervention, 18% control Location: New Zealand Ethnicity: European 67% intervention, 77% control, Maori 11% intervention, 7% control, Pacific islander 20% intervention, 13% control, other 3% intervention, 4% control (outcomes not provided by ethnicity) |
|
| Interventions | Type: diet advice Comparison: reduced fat vs usual diet Intervention: aim reduced fat diet (no specific goal stated); methods monthly small group meetings to follow a 1‐year structured programme aimed at reducing dietary fat, includes education, personal goal setting, self‐monitoring Control: aim usual diet; methods usual intake plus general advice on healthy eating consistent with the New Zealand guidelines and standard dietary information for people with nutrition‐related problems upon entering the trial. Dose aim: no goal stated Baseline PUFA: unclear but lower PUFA arm 4% E PUFA Compliance by biomarkers: erythrocyte ALA increased by 28% in control, reduced by 17% in intervention (in a subsample of participants, % of total fatty acids in red blood cells also increased in control group compared to intervention), no other erythrocyte fatty acids reported. TC fell by 0.15 mmol/L (SE 0.09) in control, and by 0.05 mmol/L (SE 0.17) in intervention to 1 year Compliance by dietary intake: mean of five, 24‐h diet recalls over 2 years of trial
Compliance, other methods: not reported Inclusion basis: aimed to reduce total fat, not to alter total PUFA. Resulted in fall of 0.8% E total PUFA in intervention, > 10% increase from 5.3% E PUFA at baseline PUFA dose: 0.8% E PUFA (from dietary intake data) Duration of intervention: 12 months (later data reported, but intervention only lasted 1 year) |
|
| Outcomes | Main trial outcome: lipids, glucose, BP Dropouts: unclear intervention, unclear control Available outcomes: mortality, CVD mortality, combined CV events (including MI, angina, stroke, heart failure), diabetes diagnosis, total MI, stroke, cancer diagnoses, cancer deaths, CHD events (MI or angina), weight, total, LDL and HDL, TGs, BP Author contact: Dr Metcalf provided additional methodology and outcome data |
|
| Notes | Trial funding: National Heart Foundation of New Zealand, Aukland Medical Research Foundation, Lotteries Medical Board and the Health Research Council of New Zealand NOTE: total PUFA intake lower in intervention than control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unmarked opaque envelopes were opened by the person recruiting, unable to alter allocation later (trial author stated in their reply to us that randomisation and preparation of the envelopes was by people not involved in recruitment). |
| Allocation concealment (selection bias) | Low risk | Unmarked opaque envelopes were opened by the person recruiting, unable to alter allocation later |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Dietary advice, not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial authors stated that those assessing lipids were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, deaths, cancer and CV events are dropouts, trialists asked for data but they were unable to provide any ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Low risk | No protocol or trials registry entry found |
| Attention bias | High risk | Regular meetings in intervention group, not in control |
| Compliance | Low risk | Erythrocyte ALA increased by 28% in control, reduced by 17% in intervention (in a subsample of participants, % of total fatty acids in red blood cells also increased in control group compared to intervention), no other erythrocyte fatty acids reported. TC fell by 0.15 mmol/L (SE 0.09) in control (the arm higher in PUFA), and by 0.05 mmol/L (SE 0.17) in intervention to 1 year (control group should have been higher in total PUFA in this trial). |
| Other bias | Low risk | None noted |
MARINA ‐ Sanders 2011.
| Methods | Modulation of Atherosclerosis Risk by Increasing dose of N‐3 fatty Acids (MARINA) RCT, parallel, 4 arms (n3 EPA + DHA at 3 doses vs MUFA), G2 vs control included, 12 months Summary risk of bias: low |
|
| Participants | Non‐smoking men and women aged 45‐70 years N: intervention 279 in 3 groups (G1 0.45 g/d N = 94, G2 0.9 g/d N = 93, G3 1.8 g/d N = 92), control: 88 (analysed G1 0.45 g/d N = 81, G2 0.9 g/d N = 80, G3 1.8 g/d N = 80, control 71) Level of risk for CVD: low Male: 38.7% intervention, 38.6% control Mean age (CI): G1:55 (53, 56), G2:55 (54, 56), G3: 55 (54, 57) intervention 55 (54,57) control Age range: 45‐70 Smokers: 0% intervention, 0% control Hypertension: 5.4% intervention, 5% control Medications taken by ≥ 50% of those in the control group: none Medications taken by 20%‐49% of those in the control group: none Medications taken by some, but < 20% of the control group: statins, antihypertensives, hormone replacement therapy, thyroxine Location: UK Ethnicity: G1: white 80.9%, black 4.3%, Asian 6.4%, Far Eastern 4.3%, other 4.3% G2: white 78.5%, black 6.5%, Asian 10.8%, Far Eastern 0%, other 4.3% G3: white 85.9%, black 1.1%, Asian 2.2%, Far Eastern 4.3%, other 6.5% Control: white 77.3%, black 10.2%, Asian 6.8%, Far Eastern 2.3%, other 3.4% |
|
| Interventions | Type: supplement (fish oil capsules) Comparison 1: EPA + DHA vs MUFA Comparison 2: high EPA + DHA vs low EPA + DHA Intervention: 3 x 1 g oil gelatin capsule/day consisting of blend of EPA concentrate, DHA concentrate, refined olive oil and 0.1 wt% peppermint oil Providing a daily dose of; 0.45 g, 0.9 g, or 1.8 g/d (all with EPA/DHA ratio of 1.51) Control: 3 gelatin capsules/d containing refined olive oil + 0.1% peppermint oil Dose aim: (intended) increase 0.45 g/d EPA + DHA, 0.2% E n‐3 or increase 0.9 g/d EPA + DHA, 0.4% E n‐3 or increase 1.8 g/d EPA + DHA, 0.8% E n‐3 Baseline PUFA 6.2% E Compliance by biomarkers: EPA and DHA in erythrocyte lipids increased in dose‐dependent manner compared with placebo, indicating long‐term compliance with intervention. TC rose by 0.1 mmol/L in both the control and intervention (G2, 0.9 g/d group) from baseline to end. No other biomarkers reported Compliance by dietary intake: all assessed after treatment (assumed at 12 months), using food frequency questionnaire (checked for completeness). Intervention group refers to G2 (0.9 g/d):
Compliance by other measures: measured by capsule counting, 88.5% of participants consumed > 90% of capsules provided Inclusion basis: dietary intake data suggested total PUFA intake 0.7% E higher in control than intervention (> 10% increase from baseline of 6.2% E from PUFA) PUFA dose: 0.7% E Length of intervention: 12 months |
|
| Outcomes | Main trial outcome: endothelial function, arterial stiffness Dropouts: 38 intervention (13,13,12), 17 control Available outcomes (for G2 vs control used): lipids, dietary intake, CRP, BP (supine and ambulatory ‐ numeric data not provided, but trial states that there were no significant differences between arms). Weight data not used, as baseline is different between groups (FMD, arterials stiffness, carotid intima media thickness, heart rate variability, heart rate, endothelial progenitor cells reported but not used) Contact with authors: yes (many outcomes above provided in end of trial report from authors) |
|
| Notes | NOTE: outcome data used G2 (0.9 g/d EPA + DHA) vs placebo for continuous outcomes, as this was the comparison where dietary data suggested that total PUFA increased by > 10% compared with placebo. Trial funding: Food Standards Agency |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the random allocation sequence was generated with a computer program by using the process of minimisation to balance age, sex and ethnicity between treatment groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "We enrolled eligible participants and the trial database program allocated a series of capsules to the participant" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "blends of the test fat with 0.1wt% peppermint oil to disguise the fish taste of the EPA and DHA" Peppermint oil in both intervention and control capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The treatments associated with the capsule codes were concealed from all investigators and associated clinical staff until the data analysis was complete. The code breaker was an employee of MedSciNet who constructed the trial database." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15% withdrawal, reasons for attrition reported |
| Selective reporting (reporting bias) | Low risk | Outcomes published match trials register. Registered September 2008, trial started June 2008, ended December 2010, main publication 2011 |
| Attention bias | Low risk | No difference between groups |
| Compliance | High risk | EPA and DHA in erythrocyte lipids increased in dose‐dependent manner compared with placebo, indicating long‐term compliance with intervention. TC rose by 0.1 mmol/L in both the control and intervention (G2, 0.9 g/d group) from baseline to end. No other biomarkers reported |
| Other bias | Low risk | None noted |
McIllmurray 1987.
| Methods | RCT, parallel, 2 arms (GLA vs "inert placebo"), 40 months Summary risk of bias: moderate to high |
|
| Participants | People within 1 month following operation to remove Dukes's C colorectal cancer N: intervention 25 (plus some dropouts), control: 24 (plus some dropouts (analysed intervention 25, control 24). 5 dropped out, but arms unclear Level of risk for CVD: low Male: not reported Mean age (SD) years: intervention 62.1 (not reported), control 64.8 (not reported) Age range: intervention 48‐81, control 45‐77 Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: UK Ethnicity: not reported |
|
| Interventions | Type: supplement (Efamol) Comparison: GLA vs "inert placebo" (unclear what) Intervention: 6 capsules/d containing 500 mg GLA plus 10 mg natural vitamin E (Efamol). GLA 0.5 g/d, 60 mg/d vitamin E. Plus vitamin supplements including vitamin C, zinc sulphate and pyridoxine. Control: 6 capsules/d containing an inert placebo, identical in appearance (not specified what). Plus vitamin supplements including vitamin C, zinc sulphate and pyridoxine. Dose aim: (assuming placebo contains no PUFA) increase 0.5 g/d GLA, 5 kcal or 0.2% E GLA, assume 70% LA*, 4.2 g/d or 37.8 kcal/d or 1.9% E LA, 2.1% E n6 Baseline PUFA: unclear Compliance by biomarkers: unclear, no serum TC or tissue fatty acid levels reported. Compliance by dietary intake: unclear, states that one participant stopped taking the supplements at 12 months
Compliance, other methods: not reported Inclusion basis: aimed to increase GLA rather than total PUFA. Aimed to increase omega‐6 by 2.1% E, assume 2.2% E increase for PUFA, > 10% of assumed 6% E PUFA baseline. No confirmatory biomarker, TC or intake data. PUFA dose: 2.2% E PUFA Duration of intervention: 40 months |
|
| Outcomes | Main trial outcome: unclear, "survival", probably mortality
Dropouts: 5 (unclear from which groups)
Available outcomes: mortality, cancer mortality (face flushing reported as a side effect, but no numbers provided and assumed due to concomitant pyridoxine) Response to contact: Professor McIllmurray replied, "I don't have the records...so I have nothing more than what appears in the publication. I do not recall there being any cardiovascular events." |
|
| Notes | Trial funding: not stated, Efamol Ltd provided the Efamol capsules and inert capsules. *EPO described as being ˜70% LA in some publications, this and a 1 g capsule size have been assumed where no other details are provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned at random" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details apart from the placebo was identical in appearance to the Efamol capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 dropouts, unclear from which arms |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials register entry found |
| Attention bias | Low risk | Supplement provided, no suggestion of attention bias |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | Unclear risk | None noted, but contents of placebo capsules unclear |
Mendis 2001.
| Methods | RCT, 2 arms, parallel (n6 LA vs non‐fat) dietary advice, 1 year Summary risk of bias: moderate to high |
|
| Participants | Healthy volunteers responding to survey. Some had hyperlipidaemia.
CVD risk: low
N: 30 intervention, 30 control (analysed 26 intervention, 28 control)
% male: 78% (total)
Mean age: not reported Age range: 20‐65 years Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported *lipid‐lowering medications as well as many others were not allowed. Location: Sri Lanka Ethnicty: 100% Sri Lanakan |
|
| Interventions | Type: diet advice plus test fat supplement Comparison: n‐6 vs non‐fat (unclear if CHO, protein or both) Intervention: group B received a diet containing 20% E as fat (4.7% coconut fat) plus 7.5 g/d test fat containing soybean fat‐sesame fat (3:1, v/v containing PUFA:MUFA ratio 2). Fat intake in group B was, therefore, 24% energy intake. (test fat provided additional 5 g/d PUFA mainly LA) Control: Group A received a diet containing 20% E as fat (4.7% E coconut fat). Dose aim: increase 5 g/d PUFA, 2.2% E PUFA Baseline PUFA: unclear Compliance by biomarkers: poor, serum TC was not significantly reduced in intervention compared to control (0.16 mmol/L, 95% CI ‐0.18 to 0.50). The intervention group were stated as having higher dietary PUFA:SFA ratio than controls, but no blood levels of fatty acids were reported. Compliance by dietary intake: unclear, measured by field workers' visits and using food diaries.
Compliance, other methods: not reported Inclusion basis: did not aim to increase PUFA (but replace SFA with unsaturated fats). Did appear to increase unsaturated fat by 4.4% E, and test fat reported as mainly LA. Aim was to increase PUFA by 2.2% E, assume this achieved though no biomarker or dietary intake data and TC was not reduced in intervention. PUFA dose: 2.2% E PUFA Duration of intervention: 1 year |
|
| Outcomes | Main trial outcome: serum lipids
Dropouts: intervention 4, control 2
Available outcomes: lipids Response to contact: contact attempted but no response to date. |
|
| Notes | Trial funding: funded by the National Science Foundation of Sri Lanka | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised to 2 groups (groups A and B). This was done in such a way that the 38 hyperlipidaemic participants were equally divided between the two groups. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The groups had different diets with test fat added to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six participants dropped out at 6 months but their data are not included in the analysis at all |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial register entry found |
| Attention bias | Low risk | Appeared similar |
| Compliance | High risk | TC was higher in intervention than control (0.16 mmol/L, 95% CI ‐0.18 to 0.50). The intervention group were stated as having higher dietary PUFA:SFA ratio than controls, but no blood levels of FAs were reported. |
| Other bias | Unclear risk | No details provided on the form or method of supply of diet or test fat |
Mita 2007.
| Methods | RCT, parallel, (n3 EPA vs nil), 2 years Summary risk of bias: moderate or high |
|
| Participants | Japanese people with type 2 diabetes N: intervention 40, control 41 (analysed 30, 30) Level of risk for CVD: moderate Male: 53% intervention, 67% control Mean age (SD): 59 (11.2) intervention 61.2 (8.4) control Age range: not reported Smokers: 40% intervention, 43% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: oral hypoglycemics Medications taken by 20%‐49% of those in the control group: insulin, lipid‐lowering drugs, antihypertensives Medications taken by some, but < 20% of the control group: antithrombotics Location: Japan Ethnicity: 100% Japanese |
|
| Interventions | Type: supplement (EPA oil capsules) Comparison: EPA vs nil Intervention: 1.8 g/d EPA as EPADEL capsules (Mochida Pharmaceutical Co Ltd Japan) 98% pure ethyl‐ester EPA (unclear how many capsules) Control: no intervention Dose aim: increase 1.8 g/d EPA + DHA, 0.8% E n‐3, assumed 0.8% E from total PUFA as no control Baseline PUFA not reported Compliance by biomarkers: no tissue fatty acids reported, but TC lower in intervention arm (5.37 mmol/L SD 0.74 at baseline, 5.15 mmol/L SD 0.83 at 2 years), than control (5.37 mmol/L SD 1.03 at baseline, 5.27 mmol/L SD 0.99 at 2 years) Compliance by dietary intake: not reported
Compliance, other methods: checked during 3‐month reviews throughout trial and 5 participants were excluded for poor compliance but no details on method or results. Inclusion basis: planned dose suggested in increase in total PUFA (by 0.8% E, > 10% increase from an assumed baseline of 6% E), and higher PUFA in the intervention is backed up by TC data PUFA dose: 0.8% E Length of intervention: mean 2.1 (0.2) years |
|
| Outcomes | Main trial outcome: progression of diabetic macroangiopathy measured by carotid intima‐media thickness and brachial‐ankle pulse wave velocity. Dropouts: 10 intervention, 11 control Available outcomes: BMI, lipids, BP, HbA1c, cancer diagnosis (BP data not used as groups very different at baseline) Response to contact: not yet attempted |
|
| Notes | Trial funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly divided into 2 groups matched for age and gender |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors of main trial outcomes were blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate (26%) over 2 years. All dropouts explained, however, 5 were excluded for poor compliance but no clear predefined protocol for exclusion. |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Attention bias | Low risk | All participants had the same contact |
| Compliance | Low risk | No tissue fatty acids reported, but TC lower in intervention arm (5.37 mmol/L SD 0.74 at baseline, 5.15 mmol/L SD 0.83 at 2 years), than control (5.37 mmol/L SD 1.03 at baseline, 5.27 mmol/L SD 0.99 at 2 years) |
| Other bias | Low risk | None noted |
MRC 1968.
| Methods | Medical Research Council (MRC) RCT, 2 arm, parallel (n6 LA vs mixed fats), 4 years Summary risk of bias: moderate to high |
|
| Participants | Free‐living men who have survived a first MI (UK)
CVD risk: high
Control: randomised 194, analysed 181 at 2 years
Intervention: randomised 199, analysed 172 at 2 years
Mean years in trial: control 3.7, intervention 3.8
% male: 100
Age: unclear Age range: all < 60 years Smokers: control 84%, intervention 81% Hypertension: control 12%, intervention 8% Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: UK Ethnicty: not reported |
|
| Interventions | Type: diet advice plus supplement Comparison: ↑ soya oil (n‐6) vs usual diet (some SFA replacement, otherwise unclear) Control aims: usual diet Intervention aims: reduce dietary fat to 35 g/d fat, add 84 g/d soya oil Dose aim: increase 84 g/d soya oil or 756 kcal or 37.8% E soya (assume 50% LA, so 18.9% E LA, assume 58% PUFA so21.9% E PUFA) Baseline PUFA: unclear Compliance by biomarkers: serum TC reported but without variance info, but TC lower in intervention than control consistently post‐baseline. Report stated that, "tissue fat of the men on the soya‐bean oil diet was less saturated than that of the controls" and that further information would be published elsewhere. No statistical significance or variance data mentioned. Compliance by dietary intake: unclear
Compliance, other methods: not reported Inclusion basis: aimed to replace SFA with PUFA. PUFA dose: 21.9% E PUFA (aim) Duration of intervention: 4 years |
|
| Outcomes | Main trial outcomes: MI or sudden death
Dropouts: intervention 199 randomised, 181 at 2 years, 91 at 4 years. Control: 194 randomised, 172 at 2 years, 85 at 4 years Available outcomes: mortality, CV mortality (CV deaths plus non‐fatal MI), total MI, non‐fatal MI (data for weight, TC and BP, but no variance info) Response to contact: reply from trial statistician, JA Heady, in 1999 |
|
| Notes | Some data not usable due to lack of variance. For all, data at 4 years, control N = 89, intervention N = 88 Weight change: intervention 0 kg, control ‐3 kg TC change: intervention ‐1.11 mmol/L, control ‐0.47 mmol/L Systolic BP change: intervention +2 mmHg, control 0 mmHg Diastolic BP change: intervention ‐1 mmHg, control +3 mmHg Trial funding: Medical Research Council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using random numbers, by blocks within hospitals" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Big changes to fat intake in intervention group while control group ate their usual diet |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Suspected relapses were assessed at regular intervals by a review committee unaware of the patients diet group" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data collection was thorough, but some participants dropped out and contact was lost. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry located |
| Attention bias | High risk | Dietary intervention, control ate usual diet, so likely that intervention group received more time and support, though this is not clear from paper |
| Compliance | Low risk | TC lower in intervention than control consistently post‐baseline. Report stated that "tissue fat of the men on the soya‐bean oil diet was less saturated than that of the controls" and that further information would be published elsewhere. |
| Other bias | Low risk | None noted |
NDHS Faribault 1968.
| Methods | National Diet‐Heart Study (NDHS) ‐ Faribault site RCT, several arms, parallel (n6 LA vs SFA), 1 year Summary risk of bias: low |
|
| Participants | Men living in a mental health institute CVD risk: low N: interventions B, C, E combined: randomised 167, analysed 143; control: randomised 57, analysed 52 Mean years in trial: interventions 0.9, control 1.0, % male: 100 Age: unclear Age range: all 45‐54 years Smokers: 55%‐59% current smokers in each arm Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: USA Ethnicty: not reported |
|
| Interventions | Type: diet provided (residential institution) Comparison: ↑ PUFA (n‐6) vs usual institutional diet (SFA and MUFA) Control aims: total fat 40% E, SFA 16%‐18% E, dietary cholesterol 650‐750 mg/d, P/S 0.4 (so PUFA 6.8% E) (whole diet provided) Intervention aims: B (C, E) total fat 30% E (40% E, 40% E), SFA < 9% E (< 9% E, not stated), dietary cholesterol 350‐450 mg/d (350‐450 mg/d, not stated), PUFA 15% E (18‐20% E, not stated), P/S 1.5 (2.0, 4.4) (equivalent to Minnesota Coronary Trial diet) (whole diet provided) Dose aim: increase B 8.2% E, C 12.2% E, E unclear n‐6 Baseline n‐6 (table IX2): 4.4% E LA, 4.8% E PUFA Compliance by biomarkers: serum TC significantly reduced in intervention compared to control (‐0.91 mmol/L, 95% CI ‐1.17 to ‐0.65). Fatty acid composition of red blood cells suggests that LA was higher in intervention arms (table X6: LA rose by 4 in control, by 5‐7 in other arms, at the expense of MUFA, which rose by 1 in control, fell by 4 or 5 in other arms. Palmitic acid fell by 5 in control, and fell by 4 in intervention arms, stearic did not alter in control, rose by 1 or 2 in intervention arms ‐ no statistical significance or variance info provided, units unclear, probably % of LA+oleic+palmitic+stearic) Compliance by dietary intake: good. Assessed from 7‐day food records after 28 and 44 weeks combined (tables IX8&9)
Compliance, other methods: 3.6% of days were lost (diet not eaten) Inclusion basis: aimed to increase PUFA intake as well as increase PUFA/SFA, reduce SFA slightly and reduce dietary cholesterol. PUFA dose: B 7.5% E, C 13.2% E, E 17.7% E PUFA Duration of intervention: 1 year |
|
| Outcomes | Main trial outcomes: lipid levels and dietary assessment Dropouts: B 7, C 10, E 7, D (control) 5 Available outcomes: mortality, TC (weight and TG data available but without SDs) Response to contact: not attempted as trial completed in 1967 |
|
| Notes | Data entered as all interventions combined (B+C+E) vs control (D) Dose calculations Interventions: B PUFA 15% E, ↑8.2% E Control: 17% E SFA, P/S 0.4 so PUFA 6.8% E C PUFA 19% E, ↑12.2% E D unclear ↑% E? Mean for all interventions ↑10.2% E Trial funding: National Heart Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Institution so all participants and trial staff blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were reported as blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Institution so able to follow‐up all participants through trial. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry found |
| Attention bias | Low risk | Equivalent, diet provided to both groups |
| Compliance | Low risk | TC significantly reduced in intervention compared to control (‐0.91 mmol/L, 95% CI ‐1.17 to ‐0.65). Fatty acid composition of red blood cells suggests LA was higher in intervention arms |
| Other bias | Low risk | None found |
NDHS Open 1st 1968.
| Methods | National Diet‐Heart Study (NDHS) ‐ open first phase RCT, several arms, parallel (n6 LA vs SFA), 1 year Summary risk of bias: low |
|
| Participants | Free‐living men aged 45‐54 years CVD risk: low Interventions B, C, X combined: randomised 829, analysed 726 Control: randomised 382, analysed 341 Mean years in trial: control 0.95, Interventions 0.93 % male: 100 Age: unclear Age range: all 45‐54 years Smokers: 39%‐40% current smokers in each arm Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: USA Ethnicty: white 98.2%, non‐white 1.8% (not reported by intervention arm) |
|
| Interventions | Type: diet provided (bought from a trial shop) Comparison: ↑ PUFA (n‐6) vs usual diet (replacement of SFA and MUFA) Control aims: total fat 40% E, dietary cholesterol 650‐750 mg/d, P/S 0.4 (assume PUFA 6.8% E as at Faribault) (foods bought from a trial shop ‐ normal foods) Intervention aims: B (C, X) total fat 30% E (40% E, 30% E), SFA < 9% E (< 9% E, < 9% E), dietary cholesterol 350‐450 mg/d (350‐450 mg/d, 350‐450 mg/d), PUFA 15% E (18% E‐20% E, 15% E), P/S 1.5 (2.0, 1.5) (foods bought from a trial shop ‐ SFAs removed and replaced by polyunsaturated oils and fats) Dose aim: increase B 8.2% E, C 12.2% E, X 8.2% E n‐6 Baseline n‐6 (tables IX 1&3): 3.7% LA, 3.9% PUFA Compliance by biomarkers: serum TC significantly reduced in intervention compared to control (‐0.45 mmol/L, 95% CI ‐0.55 to ‐0.35). Data on fatty acid composition of red blood cells provided in chapter 10 (table X6: LA rose by 1 in control, by 2‐3 in other arms, at the expense of MUFA which did not alter in control, fell by 2‐3 in other arms. Palmitic acid remained constant in control and remained constant or fell by 1 in intervention arms, stearic did not alter in control and remained constant or rose by 1 in intervention arms ‐ no statistical significance or variance info provided, units unclear, probably % of LA+oleic+palmitic+stearic). Compliance by dietary intake: good. Nutritionists' subjective adherence ratings of excellent or good (as compared to fair or poor) intervention B 58%, intervention C 60%, control D 55%. Dietary intake computed from 7‐day food records at 28 weeks (table IX3, no later data found):
Compliance, other methods: also assessed adherence ratings by nutritionists, subjectively, by recall and by food records. Poor adherence by 17%‐29%, others were fair, good or excellent. Inclusion basis: aimed to increase PUFA intake as well as increase PUFA/SFA, reduce SFA slightly and reduce dietary cholesterol. PUFA dose: achieved B 5.0% E, C 8.3% E, X 1.6 PUFA Duration of intervention: 1 year |
|
| Outcomes | Main trial outcomes: lipid levels and dietary assessment Dropouts: intervention B 42, C 34, X 5, control D 36 Available outcomes: CV events (MI and PAD events), cancer diagnoses, TC (weight, diastolic BP and TG data available but without SDs) Response to contact: not attempted as trial completed in 1967 |
|
| Notes | All intervention arms combined for data analysis Aim was to replace saturates with polyunsaturates, but oils used were omega‐6 fats Dose calculations Control: assume from Faribault 17% E SFA, P/S 0.4 so PUFA 6.8% E Interventions: B PUFA 15% E, ↑8.2% E C PUFA 19% E, ↑12.2% E X PUFA 15% E, ↑8.2% E Mean for all interventions ↑10% E Trial funding: National Heart Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Allocation concealment (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and trial personnel (aside from the store manager) were blinded to allocation. Blinding of participants was checked using a questionnaire, which found no difference between intervention and control participants in guesses at dietary composition. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were reported as blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% dropouts, well described |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry entry found |
| Attention bias | Low risk | Equivalent, both groups bought special foods from trial shop |
| Compliance | Low risk | TC significantly reduced in intervention compared to control (‐0.45 mmol/L, 95% CI ‐0.55 to ‐0.35). Data on fatty acid composition of red blood cells shows LA rose by 1 in control, by 2‐3 in other arms, at the expense of MUFA, which did not alter in control, fell by 2 or 3 in other arms. |
| Other bias | Low risk | None noted |
Nodari 2011 AF.
| Methods | RCT, parallel, (n3 DHA + EPA vs MUFA), 12 months Summary risk of bias: moderate or high |
|
| Participants | Adults with persistent AF with ≥ 1 relapse after cardioversion N: 102 intervention, 103 control (analysed, intervention: 94 control: 94) Level of risk for CVD: high Male: 70% intervention, 63% control Mean age (SD): 70 (6) intervention, 69 (9) control Age range: not reported (18‐80 inclusion criteria) Smokers: 10% intervention, 9.1% control Hypertension: 47% intervention, 40% control Medications taken by ≥ 50% of those in the control group: beta‐blockers, ACE inhibitors, anticoagulant therapy, amiodarone Medications taken by 20%‐49% of those in the control group: diuretics, antiplatelet, statins Medications taken by some, but < 20% of the control group: Ca channel blockers Location: Italy Ethnicity: not reported |
|
| Interventions | Type: supplement (Omacor) Comparison: EPA and DH+A vs MUFA Intervention: 2 x1 g/d Omacor (total 1.7 g/d EPA + DHA at a ratio of 0.9‐1.5) Control: 2 x1 g/d olive oil (gelatin capsules identical in appearance to Omacor) Dose aim: increase 1.7 g/d EPA + DHA, 0.8% E n‐3, 0.8% E PUFA Baseline PUFA not reported Compliance by biomarkers: unclear, no biomarkers, no TC reported. Compliance by dietary intake: not reported
Compliance, other measures: none reported Inclusion basis: intended dose was an increase 1.7 g/d EPA + DHA without differences in other PUFAs, so assumed dose 0.8% E PUFA, > 10% increase in total PUFA from assumed baseline of 6% E. No biomarker, TC or dietary intake data to support this. PUFA dose: 0.8% E Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: probability of maintenance of sinus rhythm Dropouts: 6 intervention, 5 control Available outcomes: adverse events, AF recurrence (nil death) Response to contact: no (contact established with trial author but no data received in this trial) |
|
| Notes | Trial funding: ‘Centro per lo Studio ed il Trattamento dello Scompenso Cardiaco’ of the University of Brescia, Brescia, Italy. The work of Dr Campia was supported by National Institutes of Health grant K12 HL083790‐01a1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment followed a computer‐generated randomisation list obtained using blocks of size 4 |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was kept in the research pharmacy area and was available only to unblinded pharmacy personnel until after the database was locked. At that time, the unblinded patient treatment information was made available to the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo gelatin capsules identical in appearance to Omacor. However no information provided as to their smell and taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised were accounted for. ITT for main outcomes |
| Selective reporting (reporting bias) | Unclear risk | NCT01198275. Registered retrospectively in September 2010, trial started January 2006, completed May 2008, main publication 2011 |
| Attention bias | Low risk | No difference between groups |
| Compliance | Unclear risk | No biomarkers, no TC reported |
| Other bias | Low risk | None noted |
Nodari 2011 HF.
| Methods | RCT, parallel, (n3 DHA + EPA vs MUFA), 12 months Summary risk of bias: moderate or high |
|
| Participants | People with heart failure (non‐ischaemic dilated cardiomyopathy) N: 67 intervention, 66 control (analysed, intervention: 67 control: 66) Level of risk for CVD: high Male: 95.5% intervention, 84.9% control Mean age (SD): 61 (11) intervention, 64 (9) control Age range: not reported (18‐75 inclusion criteria) Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: beta‐blockers, ACEi, furosemide, amiodarone, aldosterone blockers Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: statins, ARB Location: Italy Ethnicity: not reported |
|
| Interventions | Type: supplement (Omacor) Comparison: EPA + DHA vs MUFA Intervention: 2 x1 g/d Omacor (1.7 g/d EPA + DHA at a ratio of 0.9:1.5) Control: 2 x1 g/d olive oil (gelatin capsules identical in appearance to Omacor) Dose aim: increase 1.7 g/d EPA + DHA, 0.8% E n‐3, 0.8% E PUFA Baseline PUFA not reported Compliance by biomarkers: circulating free fatty acid EPA + DHA 0.83% of circulating FFAs in intervention group, 0.41% in control group, but no omega‐6 or total PUFA reported. TC equivalent at baseline (187 mg/dL) and similar at 1 year (4.8 mmol/L, SD 0.62 intervention, 4.9 mmol/L, SD 0.62 control) Compliance by dietary intake: not reported
Compliance, other measures: pill counts ‐ participants were withdrawn if < 80% capsules taken (none were withdrawn) Inclusion basis: intended dose was an increase 1.7 g/d EPA + DHA without differences in other PUFAs, so assumed dose 0.8% E PUFA, > 10% increase in total PUFA from assumed baseline of 6% E. No biomarker or dietary intake data but supported by TC PUFA dose: 0.8% E Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: left ventricular function and functional capacity Dropouts: 0 intervention, 0 control Available outcomes: mortality (nil death), combined CVD events, AF, BMI, hospitalisation for CV reasons, hospitalisation for worsening heart failure, lipids, blood glucose (but too different at baseline to use), serum cytokine Response to contact: yes, additional data and methodological data provided |
|
| Notes | Trial funding: Centro per lo Studio ed il Trattamento dello Scompenso Cardiaco, one author was a consultant for 8 pharmaceutical companies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Paper states that placebo and verum were identical and that the trial was double‐blind, but blinding of participants not checked. Trial author confirmed investigators not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial author confirmed assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether all participants were assessed for all outcomes (e.g. hospitalisation), but some outcomes report no attrition |
| Selective reporting (reporting bias) | Unclear risk | NCT01223703 ‐ trial registration Octpber 2010, recruitment November 2007‐June 2009. Retrospective |
| Attention bias | Low risk | No suggestion of this, and investigators appeared blinded (so could not differ in attention provided by allocation) |
| Compliance | High risk | Circulating free fatty acid EPA + DHA 0.83% of circulating FFAs in intervention group, 0.41% in control group, but no omega‐6 or total PUFA reported. TC equivalent at baseline (187 mg/dL) and similar at 1 year (4.8 mmol/L, SD 0.62 intervention, 4.9 mmol/L, SD 0.62 control) |
| Other bias | Low risk | None noted |
Nye 1990.
| Methods | Randomisation: parallel, 3 groups (n3 EPA vs MUFA vs aspirin and dipyridamole), 1 year Risk of bias: moderate or high |
|
| Participants | People undergoing percutaneous transluminal coronary angioplasty N: 36 intervention, 37 control (also 35 allocated to arm 3, aspirin and dipyridamole) Level of risk for CVD: high (people undergoing angioplasty) Male: 78% intervention, 76% control Mean age (SD): 54 (8) intervention, 55 (8) control years Age range: unclear Smokers: unclear Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: New Zealand Ethnicity: unclear |
|
| Interventions | Type: supplement (capsules) Comparison: EPA vs MUFA Intervention: maxEPA capsules 12/d (2.2 g EPA) Control: olive oil capsules, 12/d, identical to MaxEPA. Both capsules had vit E Dose aim: increase 2.2 g/d EPA + DHA, 1.0% E n‐3, 1.0% E PUFA Baseline PUFA not reported Compliance by biomarkers: plasma EPA increased in the intervention group by 0.49 mmol/L (95% CI 0.34‐0.64), while were "unchanged" in the control group, but no other PUFA data were presented. However, TC appeared higher in the intervention group (6.55 mmol/L, SD 1.09 in intervention, 6.07 mmol/L, SD 1.33 in control, presumably at the end of the intervention). Compliance by dietary intake: not reported
Compliance, other measures: none reported Inclusion basis: intended dose was an increase 2.2 g/d EPA + DHA. With no suggestion of differences in other PUFAs assumed dose was 1.0% E PUFA, > 10% increase in total PUFA from assumed baseline of 6%E. No biomarker or dietary intake data but challenged by TC PUFA dose: 1.0% E Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: angina, restenosis Dropouts: none Available outcomes: angina, interventions, lipids (nil death) Response to contact: not attempted | |
| Notes | Trial funding: Medical Rsearch Council of New Zealand and Scherer Ltd (who supplied MaxEPA and the olive oil capsules) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided without exclusions into 3 groups" |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no further info |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States that placebo capsules were identical to the MaxEPA, and Quote: "neither the patient nor the attending cardiologist knew which capsules were being used" But no masking of taste was reported, and participant guesses as to allocation were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the patient, nor the attending cardiologist knew which capsules were being used” ... “Angioplasty was repeated electively at one year or before where symptoms recurred, and assessed without knowledge of the patient’s treatment group.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some participants were lost to follow‐up and reasons for this were unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registration found |
| Attention bias | Low risk | No suggestion of attention bias, symptomatic participants were reviewed between scheduled visits, otherwise all on the same schedule |
| Compliance | High risk | Plasma EPA increased in the intervention group by 0.49 mmol/L (95% CI 0.34‐0.64), while were "unchanged" in the control group, but no other PUFA data were presented. However, TC appeared higher in the intervention group (6.07 mmol/L, SD 1.33 in control, 6.55 mmol/L, SD 1.09 in intervention, presumably at the end of the intervention). |
| Other bias | Low risk | No further bias noted |
ORL 2013.
| Methods | RCT‐ parallel, 3 arms (n3 EPA + DHA high dose vs n3 EPA + DHA low dose vs n3 EPA), 12 months Summary risk of bias: moderate or high |
|
| Participants | Population: Japanese adults with hypertriglyceridaemia N: 171 intervention (4 g TAK), 165 control (2 g TAK) Level of risk for CVD: moderate Male: 70.8% intervention, 71.5% control Mean age (SD): 55.9 (10.12) intervention, 56 (10.95) control Age range: 20‐74 Smokers (current): 27.5% intervention, 31.5% control Hypertension: 66.7% intervention, 67.3% control Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49%: statin Medications taken by some, but < 20%: not reported Location: Japan Ethnicity: unclear |
|
| Interventions | Type: supplement (TAK‐085 capsules) Comparison: EPA + DHA higher vs lower dose Intervention: 1 x2 /d capsule each containing 2 g of TAK‐085 (1 g of fatty acid in TAK‐085 capsules contains approximately 465 mg of EPA‐E plus 375 mg of DHA‐E). Total dose of 1.86 g/d EPA & 1.5 g/d DHA Control: 1 capsule/d containing 2 g of TAK‐085 (1 g of fatty acid in TAK‐085 capsules contains approximately 465 mg of EPA‐E plus 375 mg of DHA‐E) Total dose of 0.93 g/d EPA and 0.75 g/d DHA Dose aim high TAK vs low TAK: increase 1.68 g/d EPA + DHA, 0.8% E n3, 0.8% E PUFA assumed (no details of other capsule components provided) Baseline PUFA not reported Compliance by biomarkers: plasma free fatty acids did not differ between high and low TAK for AA, while EPA and DHA were higher in high TAK by 52 weeks. There was a small difference in change in TC between high and low TAK, statistical significance unclear Compliance by dietary intake: not reported
Compliance by pill count or equivalent: monitored every 4 weeks, mean rate of compliance reported as > 96% in each group. Inclusion basis: intended omega‐3 increase in high TAK was 0.8% E greater than low TAK, and no suggestion of different intakes of other PUFAs between arms PUFA dose: 0.8% E Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: safety outcomes and adverse events Dropouts: 8 G1, 14 G2, 21 G3 Available outcomes: adverse events (including CVD events, cancers), CRP, waist circumference, weight, BP (nil death), lipids provided as % change from baseline, but no baseline data available, so not used in meta‐analyses Response to contact: contact attempted but no response to date |
|
| Notes | A third arm of EPA‐E 1.8 g supplementation is not used here. Outcome data used TAK‐4 vs TAK‐2 Trial funding: funded by Takeda Pharmaceutical Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified according to statin use and performed by an independent registration centre |
| Allocation concealment (selection bias) | Low risk | Randomisation was stratified according to statin use and performed by an independent registration centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐ label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for and analysed for main outcomes |
| Selective reporting (reporting bias) | Low risk | Trials registry entry May 2011, trial start date November 2009, completion November 2011, so partially retrospective. However, entry appears to reflect reported outcomes. |
| Attention bias | Low risk | Capsules, appeared equivalent |
| Compliance | Unclear risk | Plasma free fatty acids did not differ between high and low TAK for AA, while EPA and DHA were higher in high TAK by 52 weeks. There was a 1% difference in change in TC between high and low TAK, statistical significance unclear. |
| Other bias | Low risk | None noted |
PREDIMED 2013.
| Methods | PREvención con Dieta MEDiterránea (PREDIMED) RCT, parallel, 3 arms (high PUFA vs low PUFA, Mediterranean diet with nuts or olive oil), also low‐fat arm, 60 months Summary risk of bias: moderate to high |
|
| Participants | Men aged 55‐80 years and women aged 60‐80 years, free of CVD but with diabetes or ≥ 3 CVD risk factors N: intervention (Med with nuts) 2454, control (Med with olive oil) 2543 ‐ also low‐fat arm, not discussed here, 2450 Level of risk for CVD: moderate Male: intervention 46%, control 41.3% Mean age (SD): intervention 67 (6), control 67 (6) years Age range: 55‐80 years Smokers: intervention 14.5%, control 13.9% (current smokers) Hypertension: intervention 82.4%, control 82.1% Medications taken by ≥ 50% of those in the control group: nil Medications taken by 20%‐49% of those in the control group: ACEi, diuretics, other antihypertensives, statins, oral hypoglycaemics, antiplatelet therapy Medications taken by some, but < 20% of the control group: insulin, non‐statin lipid‐lowering, hormone replacement therapy Location: Spain Ethinicty: white from Europe 97%, Hispanic from Central or South America 1%‐2%, other 1.5% |
|
| Interventions | Type: dietary advice and food supplement Comparison: PUFA vs MUFA Intervention: Mediterranean dietary advice plus 30 g/d mixed nuts (15 g walnuts, 7.5 g hazelnuts, 7.5 g almonds, provided, rich in ALA and linoleic) ‐ intensive education on diet with individual and up to 20 group sessions with dietitian. Control: Mediterranean dietary advice plus 1 L/week extra‐virgin olive oil (provided) ‐ intensive education on diet with individual and up to 20 group sessions with dietitian. Dose aim: unclear, food rather than nutrient goals provided, nuts (PUFA) vs olive oil (MUFA) Baseline PUFA 6.4% E in intervention, 6.1% E in control Compliance by biomarkers: unclear, no serum TC reported, no tissue fatty acids Compliance by dietary intake: all assessed at end of trial using a 137‐item food frequency questionnaire
Compliance by other methods: scores on the 14‐item Mediterranean‐diet screener increased for the participants in both Mediterranean diet groups. Participants assigned to a Mediterranean diet with extra‐virgin olive oil and those assigned to a Mediterranean diet with nuts significantly increased their consumption of extra virgin olive oil (to 50 g/d and 32 g/d, respectively) and nuts (to 0.9 and 6 servings/week, respectively). Inclusion basis: dietary intake data suggested total PUFA intake 1.6% E higher in intervention than control PUFA dose: 1.6% E Duration of intervention: 56 months median |
|
| Outcomes | Main trial outcome: CVD events
Dropouts: intervention 6.3% lost to follow‐up for ≥ 2 years, control 3.6% lost to follow‐up for ≥ 2 years
Available outcomes: deaths, CV mortality, stroke, MI, CV events. Outcome data not altered in the republication of the main paper (Estruch 2018). Response to contact: contact established but no additional data provided |
|
| Notes | All data used were for the Mediterranean diet with nuts vs Mediterranean diet with olive oil, which is higher vs lower PUFA. As nuts were mixed it is not clear whether they were high in ALA or not (probably varied). Trial funding: mainly governmental funding, but olive oil and nuts were provided by companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Tables of random allocation were centrally elaborated. However the main paper (Estruch 2013) was retracted and republished (as Estruch 2018) following a statistical analysis suggesting that baseline variables did not appear consistent with randomisation (Carlisle 2017). The republication states that partners were included in the trial without randomisation (in the same arms as family members) and that some clinics allocated by clinic rather than applying the protocol specified individual randomisation. This puts allocation concealment of some participants at high risk. |
| Allocation concealment (selection bias) | High risk | Trial nurses in charge of the random allocation were independent of the nursing staff, allocation was performed centrally. However, see note on random sequence generation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Olive oil and nuts arms could not be blinded to participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All medical records related to end points were examined by the end‐point adjudication committee, whose members were unaware of the trial‐group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We used four sources of information to identify end points: repeated contacts with participants, contacts with family physicians, a yearly review of medical records, and consultation of the National Death Index." Attrition was < 10% per year, explained and balanced. |
| Selective reporting (reporting bias) | High risk | Many outcomes in the trials registry entry are not reported by allocated group for the full set of trial participants (for example, cognition) |
| Attention bias | Low risk | These appear very similar between the two Mediterranean diet groups |
| Compliance | Unclear risk | Neither tissue PUFA biomarkers nor TC data reported |
| Other bias | High risk | Retraction and republication in 2018 due to randomisation problems not reported in the initial publication. However, new outcome data not provided. |
Proudman 2015.
| Methods | RCT, parallel, (n3 EPA + DHA high dose vs n3 EPA + DHA low dose), 12 months Summary risk of bias: low |
|
| Participants | People with rheumatoid arthritis < 12 months' duration, disease‐modifying anti‐rheumatic drugs (DMARD)‐naive N: 87 intervention, 53 control (analysed, intervention: 75 control: 47) Level of risk for CVD: low Male: 29% intervention, 25% control Mean age (SD): 56.1 (15.9) intervention, 55.5 (14.1) control Age range: unclear Smokers: 65.1% intervention, 54.7% control (includes current & previous smokers) Hypertension: not reported Medications taken by ≥ 50% of those in the control group: triple DMARD therapy (sulfasalazine 0.5 g/d, hydroxychloroquine 200 mg twice/day and methotrexate 10 mg once/week) Medications taken by 20%‐49% of those in the control group: NSAIDS Medications taken by some, but < 20% of the control group: oral or parenteral steroids Location: Australia Ethnicity: not reported |
|
| Interventions | Type: supplement (fish oil) Comparison: high EPA + DHA vs low EPA + DHA + MUFA Intervention: 10 mL/d fish oil concentrate (BLT Incromega TG3525) providing 5.5 g/d (3.2 EPA + 2.3 DHA) Control: 10 mL/d Sunola oil:capelin oil (2:1) providing 0·21 g EPA + 0·19 g/d DHA as TG (0.40 g/d EPA + DHA). Sunola oil was stated to be a monounsaturated oil. Dose aim: increase 5.1 g/d EPA + DHA, 2.3% E n‐3, 2.3% E PUFA Baseline PUFA not reported Compliance by biomarkers: unclear, no serum TC reported, plasma phospholipid EPA and DHA reported, but not by intervention group, no other tissue fatty acids reported Compliance by dietary intake: not reported
Compliance by other methods: consumption checked at each visit. 100% compliance would be consumption of 3650 mL oil at 12 months. The fish oil group was less compliant than the control group with median intakes of 2482 mL and 3248 mL, respectively (P = 0.015, Mann‐Whitney U test). This provided an average daily intake of EPA + DHA of 3.7 g and 0.36 g in the fish oil and control groups, respectively. Inclusion basis: compliance data suggested that omega‐3 fats increased by 3.3 g/d EPA + DHA, or 29.7 kcal/d, or 1.5% E. This is > 10% increase of assumed 6% E total PUFA intake at baseline, assuming no or minor PUFA in control (described as MUFA oil). PUFA dose: 1.5% E total PUFA Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: DMARD failure and remission Dropouts: 11 intervention, 6 control Available outcomes: mortality (nil death), adverse events including CVD, Disease Activity Score, diabetes, BMI change Response to contact: yes, trial authors supplied methodology data plus BMI change. |
|
| Notes | DAS scores are reported as median and IQR in Proudman 2012 abstract (see Proudman 2015) Trial funding: the trial was supported by ‘the National Health Medical Research Council of Australia and Royal Adelaide Hospital Research Committee. Melrose Health has provided support for ongoing studies.’ The oil used in the trial was made by the Royal Adelaide Hospital Pharmacy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was prepared using an online random number generator and involved randomly permuted blocks of size six." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by the RAH pharmacy, which also prepared and provided the study oils in 500 mL identical dark brown bottles labelled with consecutive study numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both participants and investigators/assessors were blinded to the group allocation. Although the control oil was paler in colour than the fish oil, this was not evident in the brown bottles. The ‘fishy’ odour of each oil was similar." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both participants and investigators/assessors were blinded to the group allocation’" Quote: "Investigators and subjects remained blinded for all withdrawals." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The flow of all trial participants shown in FIGURE 2 |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported in trial register matched with the outcomes reported in publications. However, the trial was retrospectively registered ‐ registered in 2013, recruitment began in 2001. |
| Attention bias | Low risk | No difference between groups |
| Compliance | Unclear risk | No TC reported, plasma phospholipid EPA and DHA reported, but not by intervention group, no other tissue fatty acids reported |
| Other bias | Low risk | None noted |
Puri 2005.
| Methods | RCT, parallel (n3 EPA vs non‐fat), 2 arms, 12 months Summary risk of bias: low |
|
| Participants | People with Huntington's disease N: 67 intervention, 68 control (analysed, intervention: 39 control: 44) Level of risk for CVD: low Male: 57% intervention, 44% control Mean age (SD): 50 (9.3) intervention, 49 (9.0) control Age range: not reported Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: antidepressants Medications taken by some, but < 20%: neuroleptics Location: Australia, Canada, UK, USA Ethnicity: white (black, Asian) 94% (4%, 1%) intervention, 97% (3%, 0%) control |
|
| Interventions | Type: supplement (ethyl‐EPA) Comparison: EPA vs paraffin (non‐fat) Intervention: 2 x 2 x 500 mg capsules/d, total dose of 2 g/d ethyl‐EPA (code name LAX‐101, purity 95%) Control: 2 x 2 x 500 mg capsules/d liquid paraffin Dose aim: increase 1.9 g/d EPA + DHA, 0.86% E n‐3, 0.86% E PUFA Baseline PUFA not reported Compliance by biomarkers: no serum TC reported, no tissue fatty acids reported Compliance by dietary intake: not reported
Compliance by other methods: 38 were excluded for protocol violations, 4 intervention and 16 control were non‐compliant with capsules Inclusion basis: intended that omega‐3 fats increased by 1.9 g/d EPA + DHA, or 0.86% E from omega‐3 fats. This was compared to paraffin (no fat), so dose of total PUFA was 0.86% E. This is > 10% increase of assumed 6% E total PUFA intake at baseline PUFA dose: 0.86% E total PUFA Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: functional status in Huntington's disease Dropouts: 7 intervention, 7 control Available outcomes: measures of functional capacity, CV events, cancers (no deaths) Response to contact: yes (replied to say that no CV mortality or fatal MI occurred) |
|
| Notes | Trial funding: "Amarin Neuroscience Ltd. (formerly known as Laxdale Ltd.) was responsible for organizing and funding this clinical trial" as well as paying the salaries of several investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After screening and acceptance... patients were assigned to treatment by receiving a numbered pack supplied by a clinical trials packaging organization ... independent of all other aspects of the trial. Randomization was stratified in a block size of four, with the appropriate number of blocks allocated to each centre. PCI Clinical Services held the randomization code until the database had been closed and all patients had been assigned" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo and ethyl‐EPA capsules were of identical appearance" (though taste and smell not reported). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation described as "double‐blind", "neither the participants nor the participating medical staff had access to this code during the course of the study" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clearly reported and complete, however > 20% attrition |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry identified |
| Attention bias | Low risk | Unlikely |
| Compliance | Unclear risk | No TC or tissue fatty acids reported |
| Other bias | Low risk | None noted |
Raitt 2005.
| Methods | RCT, parallel, (n3 EPA + DHA vs MUFA), 24 months Summary risk of bias: moderate or high |
|
| Participants | People with implantable cardioverter defibrillators and recent sustained VT/VF N: 100 intervention, 100 control Level of risk for CVD: high Male: 86% intervention, 86% control Mean age (SD): 63 (13) intervention, 62 (13) control Age range: not reported but 18‐75 inclusion criteria Smokers: not reported Hypertension: 46% intervention, 55% control Medications taken by ≥ 50% of those in the control group: diuretic, beta blockers, ACEi Medications taken by 20%‐49% of those in the control group: digoxin, statins Medications taken by some, but < 20% of the control group: Ca channel blocker Location: USA Ethnicity: white 94% intervention, 97% control |
|
| Interventions | Type: supplement (fish oil capsules vs olive oil capsules) Comparison: EPA + DHA vs MUFA Intervention: 1.8 g/d fish oil capsules (Hoffman LaRoche, including ethyl esters of EPA and DHA, 0.76 g/d EPA, 0.54 g/d DHA) Control: 1.8 g/d olive oil capsules (Hoffman LaRoche, 73% oleic acid) Dose aim: increase 1.3 g/d EPA + DHA, 0.6% E n‐3, 0.6% E PUFA Baseline PUFA not reported Compliance by biomarkers: while control group plasma and platelet DHA and EPA did not change, there were increases of 2%‐8.3% in the intervention group. Plasma and red blood cell omega‐3 fats were higher in intervention than control participants at all time points (P < 0.001). No data on total PUFA or LA plasma or red blood cell fats, and no TC reported. Compliance by dietary intake: not reported
Compliance by other methods: no others reported Inclusion basis: aims suggested total PUFA intake 0.6% E higher in intervention than control, a 10% increase on assumed 6% E from PUFA at baseline PUFA dose: 0.6% E Duration of intervention: 24 months (median 718 days) |
|
| Outcomes | Main trial outcome: time to first episode of VT/VF Dropouts: 17 intervention, 26 control Available outcomes: deaths, CV death, MI, angina, revascularisation, atrial fibrillation, sudden cardiac death, cancer Response to contact: contact attempted but no response to date. |
|
| Notes | Trial funding: NIH and Hoffman LaRoche | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated block randomisation scheme" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ICD traces were viewed by researchers blinded to allocation, "double blind placebo‐controlled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost all participants were included in outcome assessment, well described |
| Selective reporting (reporting bias) | High risk | NCT registered in February 2000, trial carried out from February 1999 to January 2004. Most outcomes stated in registry entry reported, but quality of life missing. |
| Attention bias | Low risk | Capsules were the only different interventions between arms, little opportunity for attention bias |
| Compliance | Unclear risk | No data on total PUFA or LA plasma or red blood cell fats, and no TC reported |
| Other bias | Low risk | None noted |
Rose 1965.
| Methods | RCT, 2 arms, parallel (n6 LA vs MUFA), 24 months Summary risk of bias: moderate to high |
|
| Participants | People with ischaemic heart disease
CVD risk: high
N: 28 intervention, 26 control (analysed 15 intervention, 12 control)
% male: not reported
Mean age: 52.6 intervention, 55 control (no SDs) Age range: not reported Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: UK Ethnicty: not reported |
|
| Interventions | Type: test oil provided (equivalent advice to both arms) Comparison: n‐6 vs MUFA Intervention: 80 g/day corn oil to be taken in 3 equal doses at meal‐times plus participants were instructed to avoid fried foods. Fatty meat, sausages, pastry, ice‐cream, cheese, cakes, milk, eggs, butter were restricted: assuming 80% LA in corn oil, 64 g/d LA or 576 kcal/d or 28.8% E from LA Control: 80 g/day olive oil plus participants were instructed to avoid fried foods, fatty meat, sausages, pastry, ice‐cream, cheese, cakes, milk, eggs, butter were restricted. assuming 12% LA and 69% MUFA in olive oil, 9.6 g/d LA or 4.3% E LA and 55.2 g/d MUFA or 24.8% E Dose aim: +24.5% E from LA, ‐24.8% E MUFA Baseline PUFA: unclear Compliance using biomarkers: serum TC reduced, but not statistically significantly reduced in intervention compared to control (‐0.49 mmol/L, 95% CI ‐1.34 to 0.36). No fatty acid biomarkers reported. Compliance using dietary assessment: poor. Measured using questionnaire. Mean intake of oil in intervention was 595 kcal/d or 476 kcal/d LA or 23.8% E, in control 540 kcal/d or 3.2% E LA and 18.6% E MUFA, achieved: +20.6% E from LA, ‐18.6% E MUFA within the oils, unclear how diet altered
Compliance by other methods: no others reported Inclusion basis: aim was to increase omega‐6 fats, not total PUFA. Total PUFA not reported but LA dose so big that total PUFA must have been increased in intervention compared to control. Best estimate 20.6% E total PUFA dose, > 10% increase from baseline PUFA dose: according to questionnaire 20.6% E from LA, assume equivalent to 20.6% E from total PUFA Duration of intervention: 2 years |
|
| Outcomes | Main trial outcome: occurrence of infraction
Dropouts: 6 intervention, 11 control?, details provided in table but unclear how many dropped out.
Available outcomes: major CVD events, MI (fatal and non‐fatal), sudden death, serum cholesterol Response to contact: not attempted as published in the 1960s |
|
| Notes | Trial funding: no details The trial had a 3rd control arm (no intervention), which has not been used here. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | When a new participant was accepted for the trial a sealed envelope was opened containing the allocation instructions. In the case of participants allocated to an oil group the instructions referred only to a code number. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The physicians in charge knew which participants were receiving oil, but they did not know until the end of the trial the kind of oil that they were receiving. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The electrocardiograms were assessed without the knowledge of the participant's treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52% intervention, and 57% control remained in the trial after 24 months. However, the list of reasons and complications is provided. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry record or protocol found |
| Attention bias | Low risk | Oil provided to both groups, appeared similar |
| Compliance | Low risk | TC somewhat reduced in intervention compared to control (‐0.49 mmol/L, 95% CI ‐1.34 to 0.36). No fatty acid biomarkers reported |
| Other bias | Low risk | None noted |
Rossing 1996.
| Methods | RCT, parallel, (n3 EPA + DHA vs MUFA), 12 months Summary risk of bias: moderate or high |
|
| Participants | Adults with insulin‐dependant diabetes mellitus, diabetic nephropathy and normal BP N: 18 intervention, 18 control (analysed, 17 intervention, 15 control) Level of risk for CVD: moderate Male: 64% intervention, 67% control Mean age (SD) years: 32 (7) intervention, 34 (10) control Age range: 18‐55 years Smokers: 50% intervention, 47% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: insulin Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Denmark Ethnicity: not reported |
|
| Interventions | Type: supplement Comparison: fish oil vs olive oil Intervention: cod‐liver oil emulsion (Pharma‐Vinci A/S Denmark). EPA 2 g/d, DHA 2.6 g/d, plus 24.1% SFA, 45.6% MUFA, 23.6% EPA + DHA, 6.7% other fats. Assumed total PUFA 4.6 g/d Control: olive oil emulsion (Pharma‐Vinci A/S Denmark). 15.1% SFA, 76.9% MUFA, 8.0% other fats. Assumed total PUFA 0 g/d Dose aim: increase 4.6 g/d EPA + DHA, 2.1% E n‐3, 2.1% E PUFA Baseline PUFA: unclear Compliance using biomarkers: assessed through omega‐3 incorporation in platelets, and the paper reports significantly higher omega‐3 levels in platelets at 12 months. EPA % was 0.59 (SE 0.07) in control, 2.70 (SE 0.29) in intervention arm latest reading. DHA % was 1.99 (SE0.13) control, 3.57 (SE 0.18) intervention (P < 0.001 between intervention and control for both). Total PUFA not reported. HOWEVER serum TC rose more in the intervention arm (+ 0.46 mmol/L) than control (+ 0.13 mmol/L) during the trial. Compliance using dietary assessment: poor. Unclear how measured, only protein reported
Compliance by other methods: no others reported Inclusion basis: aim was to increase omega‐3 fats, not total PUFA. Total PUFA not reported but omega‐3 dose rose by 2.1% E, so assume total PUFA did also as compared to MUFA. Best estimate 2.1% E total PUFA dose, more than 10% increase from assumed baseline of 6% E PUFA dose: intended dose only, 2.1% E Duration of intervention: 12 months |
|
| Outcomes | Main trial outcome: diabetic nephropathy Dropouts: 1 intervention, 3 control (though 3 further intervention participants are not included in all data) Available outcomes: mortality (nil), breast cancer, TC, LDL, systolic BP (TGs reported as medians so not used, albuminuria, fractional albumin clearance, transcapillary escape rate of albumin, prothrombin fragment reported as geometric means or medians, HbA1c, HDL and diastolic BP too different at baseline to include, glomerular filtration rate (GFR), plasminogen activator inhibitor‐1 (PAI1), tissue plasminogen activator (TPA), fibrinogen etc. not relevant) Trial author reply: yes |
|
| Notes | Trial funding: supported by The Danish Heart Association. Eskisol Fish oil and placebo oil emulsions were provided by Pharma‐Vinci A/S, Frederiksvaerk, Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using "concealed randomization to receive either fish oil or olive oil in blocks of 4 according to their glomerular filtration rate.” |
| Allocation concealment (selection bias) | Unclear risk | No further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Active and placebo (olive oil) were given as emulsions with orange flavour. At the end patients were allowed to guess about treatment and ˜50% were right” (from trial author response) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts similar between groups although relatively high for small sample size. 3 dropouts from fish oil and 1 from control due to side effects. ITT appears to have been given for albuminuria only. |
| Selective reporting (reporting bias) | Unclear risk | No trials registry entry or protocol found |
| Attention bias | Low risk | Time and attention appear to be the same. All participants were given dietary advice. |
| Compliance | High risk | Total PUFA in body fractions not reported. However, serum TC rose more in the intervention arm (+0.46 mmol/L) than control (+0.13 mmol/L) during the trial. |
| Other bias | Low risk | None noted |
Simon 1997.
| Methods | RCT, parallel, (low fat with low PUFA vs usual diet), 24 months Summary risk of bias: moderate or high |
|
| Participants | Women with a high risk of breast cancer N: 98 intervention, 96 control (analysed 72 intervention: 75 control) Level of risk for CVD: low Male: 0% intervention, 0% control Mean age (SD): 46 (not reported) intervention, 46 (not reported) control Age range: not reported Smokers: not reported Hypertension: not reported Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported (those on statins excluded) Location: USA Ethenicity: white 89%, African American 9%, Hispanic 2% |
|
| Interventions | Type: dietary advice Comparison: reduced fat including PUFA (intervention) vs usual diet Intervention: aims total fat 15% E; methods biweekly individual dietetic appointments over 3 months followed by monthly individual or group appointments, including education, goal setting, evaluation, feedback and self‐monitoring. Intervention delivered face to face by a dietitian Control: aim usual diet, no stated intervention(s) Dose aim: unclear PUFA Baseline 7.7% E PUFA Compliance by biomarkers: no fatty acid biomarkers reported, TC reported in a subgroup and fell by 0.34 mmol/L in intervention and fell by 0.08 mmol/L in control over 1 year Compliance by dietary intake: assessed using 3‐day 24‐h recalls every 3 months, 1 year data reported
Compliance, other methods: not reported Inclusion basis: no intention to increase total PUFA stated. Acheived total PUFA reduction of 6.7% E in intervention compared to control at 1 year, > 10% higher than baseline 7.7% E from total PUFA PUFA dose: ‐6.7% E PUFA Compliance: dietary assessment Duration of intervention: 24 months (mean years in trial: control 1.8, intervention 1.7) |
|
| Outcomes | Main trial outcome: intervention feasibility Dropouts: unclear intervention, unclear control Available outcomes: TC, TG, LDL and HDL (2 deaths, but unclear in which arms, 8 cancer diagnosis but not clear in which arms), (weight, BMI, % body fat and waist‐hip ratio reported but all too unbalanced at baseline to use) Trial author contact: Dr Simon confirmed that some deaths occurred (but not in which arms) and sent a further reference. |
|
| Notes | Trial funding: Marilyn J Smith Fund, Harper‐Grace Hospitals, the Wesley Foundation, National Cancer Institute, Karmanos Cancer Institute Core Grant, the United Foundation of Detroit Trialaim was to reduce total fat to 15% E (SFA not mentioned), but PUFA fat intake in the intervention group was significantly lower than in the control group. Note: PUFA lower in intervention arm, so higher PUFA arm is the control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age and randomised (block size 2) |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not clearly enough described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded (as given dietary advice or not), personnel unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, deaths, cancer and CV events are dropouts ‐ unclear if any data missing |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry found |
| Attention bias | High risk | Time and attention in the intervention group not mirrored in control |
| Compliance | High risk | No fatty acid biomarkers reported, TC reported in a subgroup and fell by 0.34 mmol/L in intervention and fell by 0.08 mmol/L in control over 1 year (but control group should have been higher in PUFA in this trial) |
| Other bias | Low risk | None noted |
Sydney Diet‐Heart 1978.
| Methods | Sydney Diet‐Heart Study RCT, 2 arm, parallel (n6 LA vs SFA), 4.3 years Summary risk of bias: low (as diet advice trial) |
|
| Participants | Men with previous MI
CVD risk: high
Control: randomised 237, analysed 221 at 2 years
Intervention: randomised 221, analysed 205 at 2 years
Mean years in trial: control 4.3, intervention 4.3
% male: 100
Age: mean intervention 48.7 (SD 6.8), control 49.1 (SD 6.5) Age range: 30‐59 years Smokers: intervention 71.5%, control 68.8% Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Australia Ethenicty: not reported |
|
| Interventions | Type: diet advice and supplemental foods Comparison: ↑ safflower oil and safflower oil‐based margarine (n‐6) vs usual diet (reduced SFA and MUFA) Control aims: reduction in energy if overweight, no other specific dietary advice, allowed to use PUFA margarine instead of butter (no specific dietary instruction, except re weight) Intervention aims: SFA 10% E, PUFA 15% E, reduction in energy if overweight, dietary cholesterol < 300 mg/day through provision of safflower oil and safflower margarine (advised and tutored individually, diet assessed 3 times in first year, twice annually thereafter) Dose aim: increase 6.6% E PUFA, most of which n6 Baseline n‐6: unclear, 6.1% E PUFA, mostly n6 Compliance by biomarkers: serum TC significantly reduced in intervention compared to control (‐0.30 mmol/L, 95% CI ‐0.51 to ‐0.09). No body fatty acid markers reported Compliance by dietary intake: good. From diet records, medians provided
Compliance, other methods: not reported Inclusion basis: aimed to increase total PUFA intake as well as reduce SFA PUFA dose: 7.1% E PUFA (from dietary intake data) Duration of intervention: 2‐7 years |
|
| Outcomes | Main trial outcomes: CV mortality and morbidity Dropouts: unclear, probably 16 dropouts in each arm, but participants were included from 2‐7 years Available outcomes: mortality, TC, TG Response to contact: yes, further data provided |
|
| Notes | Trial funding: Life Insurance Medical Research Fund of Australia and New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers ... generated by a research assistant and was concealed until after medical evaluations and testing at baseline were completed" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Very difficult to blind trials where participants need to make their own dietary changes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Initially masked to group assignment (though success of blinding not checked) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Survival analysis used |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry located |
| Attention bias | High risk | Different levels of dietary support (non‐dietary aspects were equivalent) |
| Compliance | Low risk | TC significantly reduced in intervention compared to control (‐0.30 mmol/L, 95% CI ‐0.51 to ‐0.09). No body fatty acid markers reported. |
| Other bias | Low risk | None noted |
Veterans Admin 1969.
| Methods | Veterans Administration Trial RCT, 2 arms, parallel (n6 LA vs SFA), up to 8 years Summary risk of bias: moderate to high |
|
| Participants | Men living at the Veterans Administration Centre
CVD risk: low
Control: randomised 422, analysed 422
Intervention: randomised 424, analysed 424
Mean years in trial: control 3.7, intervention 3.7
% male: 100
Age: mean control 65.6, intervention 65.4 Age range: all 54‐88 years Smokers: intervention 283, control 279 (unknown intervention 41, control 58) Hypertension: unclear Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: digitalis, diuretics, oestrogens, corticoids, androgens, coumarins, nicotinic acid Location: USA Ethnicity: white 90%, black 7%, Asian 1%, Hispanic 1%, other 1% |
|
| Interventions | Type: diet provided (residential institution) Comparison: ↑ corn, soybean, safflower and cottonseed oils (n‐6) vs usual institutional diet Control aims: provided, total fat 40% E (whole diet provided) Intervention aims: total fat 40% E, 2/3 of SFA replaced by unsaturated fats (from corn, soybean, safflower and cottonseed oils), dietary cholesterol reduced (whole diet provided) Dose aim: 2/3 of baseline SFA is increase of ˜12%E PUFA Baseline n‐6: 4% E LA, control arm 4.8% E PUFA Compliance by biomarkers: subcutaneous 18:2 + 18:3 11.7% fat at baseline, rising to 12.8% fat in control and 34.8% fat in intervention (after "prolonged" adherence to diet). Serum TC reduced, but not statistically significantly in intervention compared to control (‐0.37 mmol/L, 95% CI ‐0.77 to 0.03). Compliance by dietary intake: unclear,checked using coloured tickets to assess dining room attendance ‐ described as 49% in intervention and 56% in controls. Laboratory analysis of the mean of over 400 weekly collections of diet provided:
Compliance by other methods: no others reported Inclusion basis: aim was to increase unsaturated fats, not total PUFA. Total PUFA not reported but LA dose 11.7% E (best estimate), > 10% increase from baseline of ˜5% E PUFA dose: 11.7% E from total PUFA (best estimate from food composition data) Duration of intervention: up to 8‐9 years |
|
| Outcomes | Main trial outcomes: mortality, heart disease Dropouts: intervention 117, control 58 withdrawals over whole trial, a few participants were involved for up to 8‐9 years Available outcomes: mortality, CV mortality (sudden death, definite MI, definite stroke, angina, PAD events), cancer deaths, cancer diagnoses, stroke, non‐fatal MI, total MI, CHD deaths (fatal MI and sudden death due to CHD), CHD events (any MI or sudden death due to CHD), some data on TC, but no variance info Response to contact: attempted but no author contact established (trial published in 1969) |
|
| Notes | Trial dates: recruitment 1959‐1967 Trial funding: mainly US Public Health Service, Los Angeles County Heart Assoc, Arthur Dodd Fuller Assoc, but Corn Products Co (provided Corn oil and margarine), National Soybean Processors Assoc (provided soybean oil), Pitman‐Moore Co (provided margarine), Frozen Desserts Co (imitation ice cream). All trial authors worked for academic or health institutions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers used" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Institution provided diet in a masked fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physician knowledge of allocation was assessed and found not much better than random |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All followed up via Veterans Admin system |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trials registry entry located |
| Attention bias | Low risk | Appeared equivalent, diet provided to both arms |
| Compliance | Low risk | Subcutaneous 18:2 + 18:3 11.7% fat at baseline, rising to 12.8% fat in control and 34.8% fat in intervention (after "prolonged" adherence to diet). TC reduced, but not statistically significantly in intervention compared to control (‐0.37 mmol/L, 95% CI ‐0.77 to 0.03). |
| Other bias | Low risk | None found |
Vijayakumar 2014.
| Methods | RCT, 2 arms, parallel (n6 LA vs SFA), 2 years Summary risk of bias: moderate to high |
|
| Participants | People with stable coronary artery disease CVD risk: high N: intervention (sunflower oil): 100 randomised, analysed at 2 years 94; control (coconut oil): 100 randomised, analysed at 2 years 96 Mean years in trial: 2 % male: intervention 92.9%, control 93.9% Age, mean (SD) years: intervention 59.0 (8.9), control 59.0 (8.4) Age range: unclear Smokers, ex: intervention 57.1%, control 54.1% Hypertension: intervention 55.1%, control 58.2% Medications taken by ≥ 50% of those in the control group: statins Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: fibrates, nicotinic acid Location: India Ethnicity: not reported |
|
| Interventions | Type: food (cooking oil) provided Comparison: sunflower oil (n6) vs coconut oil (SFA) Intervention aims: whole family to use branded sunflower oil for cooking (15% E provided in form of sunflower oil, ˜66% PUFA) Control aims: whole family to use branded coconut oil for cooking (15% E provided in form of coconut oil, ˜5% PUFA) Dose aim: increase 9.2% E PUFA Baseline PUFA: unclear Compliance by biomarkers: Serum TC reduced but not significantly reduced in intervention compared to control (‐0.06 mmol/L, 95% CI ‐0.22 to 0.34) though rose slightly in control, fell slightly in intervention. No biomarker data reported Compliance by dietary intake: unclear. Reports that 7‐day recall and diet diaries were used to monitor intake, but results not provided.
Compliance, other methods: oils were provided for family members to encourage compliance Inclusion basis: did not aim to increase total PUFA intake. Quantity and standard compositions suggest dose ˜9.2% E total PUFA, > 10% more than assumed baseline of 6% E PUFA PUFA dose: 9.2% E PUFA Duration of intervention: 2 years |
|
| Outcomes | Main trial outcome: CV risk factors
Dropouts: intervention 6 lost, control 4 lost
Available outcomes: lipids, death, revascularisation, (glycaemic control, weight, BMI available but unbalanced at baseline) Response to contact: author replied and provided additional outcome data |
|
| Notes | Trial funding: coconut development board, Amrita Institute of Medical Science and Research. Sponsors had no role in trial design or analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with 5 blocks of 40 |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unlikely as participants and their families used branded oils |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% withdrawals. Clear, with reasons |
| Selective reporting (reporting bias) | Unclear risk | Unclear, no protocol or trials register entry found |
| Attention bias | Low risk | Appeared equivalent |
| Compliance | Low risk | TC reduced in intervention compared to control (‐0.06 mmol/L, 95% CI ‐0.22 to 0.34, rose slightly in control, fell slightly in intervention). No biomarker data reported |
| Other bias | Low risk | None noted |
WAHA ‐ Ros 2016.
| Methods | The Walnut and Healthy Aging Study (WAHA) 2 arms, parallel RCT (n3 ALA vs mixed fats, ALA provided as walnuts), 2 years Summary risk of bias: moderate to high |
|
| Participants | Middle‐aged, healthy adults N: 362 intervention, 346 control (only preliminary data on 260 intervention, and 254 control available) Level of risk for CVD: low Male: 32.6% intervention, 31.5% control Mean age (SD): 69.4 (3.8) intervention, 68.9 (3.5) control Age range: 63‐79 (inclusion criteria) Smokers: 4.4% intervention, 1.2% control Hypertension: 52.8% intervention, 52.9% control Medications taken by ≥ 50% of those in the control group: not reported Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: Spain and USA Ethnicity: not reported |
|
| Interventions | Type: supplement (food) Comparison: ALA vs nil Intervention: 15% of daily energy intake as walnuts. The estimated amount of walnuts ranged from 1‐2 oz/d (˜30–60 g/day). Sachets for daily consumption containing 30, 45, or 60 g of raw, pieced walnuts were provided as 8‐week allotments to be eaten daily, preferably as the raw product, either as a snack or by incorporating them into shakes, yogurts, cereals, or salads. To improve participants’ compliance, 1‐ kg extra walnut allowances were provided every 2 months to take into account family needs. Control: usual diet without walnuts Compliance: assessed by dietitians through Food Frequency Questionnaires, recount of empty packages, and changes in fatty acids concentrations. 95% consumed ≥ 1 oz./d. The proportion of α‐linolenic acid in red blood cell counts increased in the walnut group by 0.162% (95% CI 0.143 to 0.181) and in the control group by 0.015% (95% CI −0.005 to 0.035) (P < 0.001). Dose aim: increase (assuming 10% E in walnuts is ALA) 1.5% E n3 ALA. 45 g walnut gives ˜65% or 29.3 g oil, of which ˜68% PUFA, 19.9 g/d oil, 9% E PUFA. Baseline PUFA: unclear, control 7.9% E PUFA Compliance by biomarkers: erythrocyte ALA increased by 28% in intervention, reduced by 17% in control (in a subsample of participants, percentage of total fatty acids in red blood cells also increased in intervention group compared to control, no other erythrocyte fatty acids reported. TC fell by 0.19 mmol/L (SD 0.04) in intervention, and by 0.01 mmol/L (SD 0.04) in control to 1 year. Compliance by dietary intake: mean of five, 24‐h diet recalls over 2 years of trial
Compliance, other methods: assessed by dietitians through Food Frequency Questionnaires and recount of empty packages, 95% consumed ≥ 28g/d Inclusion basis: aimed to increase walnuts, not total PUFA. Resulted in increase of 7.4% E total PUFA PUFA dose: 7.4% E PUFA (from dietary intake data) Duration of intervention: 2 years |
|
| Outcomes | Main trial outcome: change in cognitive decline (results not yet published)
Dropouts: 36 intervention, 21 control (after 1 year) Available outcomes: CVD events, cancers, lipids (for TG and HDL only data states "no between diet differences were observed"), weight (waist circumference was provided but without variance, abstract stated that "there were no significant changes in body fat and waist‐to‐hip ratio over time and between the two groups"). Cognitive, ophthalmological, inflammatory markers, glycaemic status and other outcomes are not yet available. Response to contact: author replied and provided additional outcome and methodological data |
|
| Notes | Trial funding: funding was provided by the Calfornia Walnut Commission. The 2‐year results as well the full 1‐year results are yet to be published. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized to either the control or walnut group using a computerized random number table with stratification by center, sex, and age range. Couples entering the trial were treated as one number and were randomized into the same group". |
| Allocation concealment (selection bias) | Low risk | Author reply stated "Baseline subject data was collected before randomization. Randomization was done by the clinician, pressing the key on the computer" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind. Quote: "An unavoidable limitation of the study is not being able to blind participants to the intervention since it consists of a whole food” Rajaram 2017 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blind. Author reply stated "Study personnel not in contact with the subjects were blind to the treatment assignment. So (lab technicians, ophthalmology technician, neuro cognitive testers) were not aware of the treatment assignment. Of course clinicians who were visited by subjects every two months, knew the treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38/362 dropouts in intervention group = 10.5%. 34/346 dropouts in control group = 9.8%. Similar dropout in groups over 2 years. |
| Selective reporting (reporting bias) | Unclear risk | Although prospectively registered, no full results paper published – results from conference abstracts and papers only report some secondary outcomes and dietary data. |
| Attention bias | Unclear risk | Not enough detail to assess |
| Compliance | Low risk | Erythrocyte ALA increased by 28% in intervention, reduced by 17% in control (in a subsample of participants), percentage of total fatty acids in red blood cells also increased in intervention group compared to control, no other erythrocyte fatty acids reported. TC fell by 0.19 mmol/L (SD 0.04) in intervention, and by 0.01 mmol/L (SD 0.04) in control to 1 year. |
| Other bias | Low risk | None noted |
WELCOME 2015.
| Methods | RCT, parallel, (n3 EPA + DHA vs MUFA), 15‐18 months Summary risk of bias: low |
|
| Participants | Patients with NAFLD N: 51 intervention, 52 control (analysed, 47 intervention, 48 control) Level of risk for CVD: moderate Male: 49% intervention, 67% control Mean age (SD): 48.6 (11.1) intervention, 54 (9.6) control Age range: not reported (18‐75 inclusion criteria) Smokers: 14.3% intervention, 11.8% control Hypertension: not reported Medications taken by ≥ 50% of those in the control group: lipid‐lowering drugs Medications taken by 20%‐49% of those in the control group: antihypertensives, metformin (data not provided by group) Medications taken by some, but < 20% of the control group: none reported Location: UK Ethenicity: not reported |
|
| Interventions | Type: supplement (Omacor capsules) Comparison: DHA + EPA vs MUFA Intervention: 4 g Omacor/d (providing 1.84 g EPA, 1.52 g DHA as ethyl esters)), 3.36 g/d EPA + DHA Control: 4 g olive oil capsules/d (providing; ALA 1%, oleic acid 67%, palmitic acid 15%, stearic acid 2%, n‐6 fat: 15%), 0.64 g/d PUFA Dose aim: increase 2.72 g/d PUFA, 1.22% E PUFA Baseline PUFA unclear Compliance by biomarkers: erythrocyte EPA + DHA both increased in intervention, not in control (EPA% 1.0%, SD 0.2% in control vs 2.4% SD 1.8% in intervention at latest point, DHA% 5.0 SD 1.0 in control, 7.1% SD 1.3% in intervention), no other fatty acids reported. TC remained 4.8 mmol/L in control but fell by 0.2 mmol/L to 4.7 mmol/L in intervention at 15‐18 months. Compliance by dietary intake: not reported
Compliance, other methods: assessed by recording the returned unused capsules, but results not reported Inclusion basis: no intention to increase total PUFA stated. Planned total PUFA increase 2.72 g/d PUFA, 1.22% E PUFA, > 10% higher than assumed 6% E from total PUFA at baseline. Confirmed by TC fall in intervention, no other biomarker or intake data PUFA dose: 1.22%E PUFA Duration of intervention: 15‐18 months |
|
| Outcomes | Main trial outcome: changes in mean liver fat %, changes in 2 liver fibrosis scores, change in serum biomarkers Dropouts: 4 intervention, 4 control Available outcomes: weight, BMI, lipids, BP, glucose, insulin sensitivity, body fat measures, liver enzymes, HbA1c, serum n‐3 fatty acids, trial authors provided details of diabetes diagnoses, % body fat, BP and carotid intima media thickness. Response to contact: yes |
|
| Notes | Trial funding: Omacor and placebo were provided by Pronova Biopharma through Abbott Laboratories, Southampton, UK. This work was supported by a National Institute for Health Research (NIHR) Southampton Biomedical Research Unit grant and by a Diabetes UK Allied Health Research training fellowship awarded to KGM (Diabetes UK. BDA 09/ 0003937). CDB, PCC and ES were supported in part by the NIHR Southampton Biomedical Research Centre (McCormick‐2015, p9; see WELCOME 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were block randomised by an independent clinical trials pharmacist ....randomised according to standardised procedures (computerised block randomisation) by a research pharmacist at University Hospital Southampton NHS Foundation Trust. Simple randomisation in blocks of 4... |
| Allocation concealment (selection bias) | Low risk | Only the clinical trials pharmacist was unblinded, and randomisation group allocation was concealed from all trial members throughout the trial. (McCormick‐2015, p2). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper states that only the clinical trials pharmacist was unblinded, and randomisation group allocation was concealed from all trial members throughout the trial. However, the trial register record states "single blind (investigator)". Although the capsules were identical, no information provided as to their smell and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The ITT included all participants randomised who had complete data (baseline and end‐of‐trial measurements), regardless of whether they were later found to be ineligible, a protocol violator, given the wrong treatment allocation, or never treated). (Scorletti 2014, p4; see WELCOME 2015) |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered September 2008, trial start September 2009, end February 2017. Outcome data for cardiac function not yet published (may be ongoing as trial only recently completed), though other CV measures reported |
| Attention bias | Low risk | Both groups had the same attention |
| Compliance | Low risk | Erythrocyte EPA + DHA both increased in intervention, not in control (EPA% 1.0%, SD 0.2% in control vs 2.4% SD 1.8% in intervention at latest point, DHA% 5.0 SD 1.0 in control, 7.1% SD 1.3% in intervention), no other fatty acids reported. TC remained 4.8 mmol/L in control but fell by 0.2 mmol/L to 4.7 mmol/L in intervention at 15‐18 months. |
| Other bias | Low risk | None noted |
WINS 2006.
| Methods | Women's Intervention Nutrition Study (WINS) RCT, parallel, (reduced fat with reduced PUFA vs usual diet), 60 months Summary risk of bias: low (as diet advice trial) |
|
| Participants | Women with localised resected breast cancer N: 975 intervention, 1462 control (analysed 975 int, 1462 cont) Level of risk for CVD: low Male: 0% intervention, 0% control Mean age (95% CI): 58.6 (44.4‐72.8) intervention, 58.5 (43.6‐73.4) control Age range: not reported, all postmenopausal Smokers: 49.9% intervention, 48.7% control never smokers Hypertension: not reported Medications taken by ≥ 50% of those in the control group: menopausal hormone therapy (65.3% intervention, 64.0% control), tamoxifen (47.7% tamoxifen alone, 38.5% tamoxifen plus chemotherapy in intervention, 47.4% and 38.0% respectively in control), all were on chemotherapy, most on radiotherapy Medications taken by 20%‐49% of those in the control group: not reported Medications taken by some, but < 20% of the control group: not reported Location: USA Ethnicity: 85% white, 5% black, 4% Hispanic, 5% Asian or Pacific Islander, < 1% American Indian or unknown (no outcome data based on ethnicity) |
|
| Interventions | Type: dietary advice Comparison: reduced fat intake (with reduced PUFA) vs usual diet Intervention: aims total fat 15%‐20% E; methods 8 biweekly individual dietetic sessions plus 3‐monthly contact and optional monthly group sessions, incorporating individual fat gram goals, social cognitive theory, self‐monitoring, goal setting, modelling, social support and relapse prevention and management. Intervention was delivered face to face individually by trained dietitian Control: aims minimal nutritional counselling focused on nutritional adequacy; methods one baseline dietetic session plus 3‐monthly sessions Dose aim: unclear PUFA Baseline 5.4% E PUFA Compliance by biomarkers: no fatty acid biomarkers reported, TC reported but only in a subgroup (N = 18 at 2 years) and unbalanced at baseline so not used in analyses, little change but TC fell by 6 mg/dL in intervention and increased by 0.8 mg/dL in control over 2 years Compliance by dietary intake: assessed using unannounced phone calls over several days, 1‐year data reported apart from protein and carbohydrate which were 6‐month data
Compliance, other methods: not reported Inclusion basis: no intention to increase total PUFA stated. Acheived total PUFA reduction of 1.9% E in intervention compared to control at 1 year, > 10% higher than baseline 5.4% E from total PUFA PUFA dose: ‐1.9% E PUFA Duration of intervention: 60 months |
|
| Outcomes | Main trial outcome: dietary fat intake, TC, weight and waist Dropouts: 45 lost to follow‐up, 170 discontinued intervention, 66 lost and 106 discontinued control Available outcomes: all‐cause mortality, cancer diagnoses (including recurrences), new breast cancer diagnoses, weight, BMI (TC, TG, HDL, insulin provided in tiny subgroup ‐ 9 participants in each group at 2 years ‐ and unbalanced at baseline, not useable) Author contact: limited information received |
|
| Notes | Trial funding: National Cancer Institute, Breast Cancer Research Foundation, American Institute for Cancer Research *SDs appear incorrect, probably SEs? NOTE: control arm is the arm higher in PUFA, intervention arm lower in PUFA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random stratified permuted block design, carried out at the statistical co‐ordinating centre of WINS |
| Allocation concealment (selection bias) | Low risk | Random stratified permuted block design, carried out at the statistical co‐ordinating centre of WINS |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not for dietary advice and participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes assessed by the blinded outcome committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All assessed |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in protocol all appear to have been published |
| Attention bias | High risk | Intervention group appear to have received more time and attention |
| Compliance | Unclear risk | No fatty acid biomarkers reported, TC reported but only in a subgroup (n = 18 at 2 years) and unbalanced at baseline so not used in analyses, little change but TC fell by 6 mg/dL in intervention and increased by 0.8 mg/dL in control over 2 years (note, control group should be higher in PUFA in this trial). Overall changes not reported |
| Other bias | Low risk | None noted |
AA: arachidonic acid; ACEi: angiotensin‐converting‐enzyme inhibitor; AF: atrial fibrillation; ALA: alpha‐linolenic acid (a plant‐based omega‐3 fat); ARB: Angiotensin II receptor blockers; BMD: bone mineral density; BMI: body mass index (weight in kg divided by height in m squared); BP: blood pressure; Ca: calcium; CAD: coronary artery disease; CHO: carbohydrate; CLO: cod‐liver oil; CRP: C‐reactive protein; CV: cardiovascular; CVD: cardiovascular diseases; DHA: docosahexaenoic acid (a fish‐based omega‐3 polyunsaturated fatty acid); DPA: docosapentaenoic acid (a fish‐based omega‐3 polyunsaturated fatty acid); E: energy; EPA: eicosapentaenoic acid (a fish‐based omega‐3 polyunsaturated fatty acid); FMD: fibromuscular dysplasia; GLA: gamma linolenic acid (an omega‐6 polyunsaturated fatty acid); HDL: high density lipoprotein (a fraction of TC, measured in human blood); ICD: implanted cardioverter defibrillator; ITT: intention to treat analysis; IQR: interquartile range; kcal: calories; LDL: low density lipoprotein (a fraction of TC, measured in human blood); LA: linoleic acid (an omega‐6 polyunsaturated fatty acid); MD: mean difference; MI: myocardial infarction; MUFA: monounsaturated fatty acid or monounsaturated fat; IQR: interquartile range; N: number or participants; NAFLD: non‐alcoholic fatty liver disease; NSAIDs: nonsteroidal antiflammatory drugs; P: P value; PCI: percutaneous coronary intervention; PUFA: polyunsaturated fatty acid; P/S: polyunsaturated to saturated fatty acid ratio; PAD: peripheral arterial disease; QoL: quality of life; RCT: randomised controlled trial; SCD: sudden cardiac death; SD: standard deviation; SE: standard error; SFA: saturated fatty acid or saturated fat; SO: seal oil; TC: total cholesterol (measured in human blood); TG: triglycerides (measured in human blood); VF: ventricular fibrillation; VT: ventricular tachycardia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADCS‐Quinn 2010 | Compared DHA vs omega‐6, no intention to increase total PUFA. Intervention 1.02 g/d algal‐derived DHA compared to 2 g of soy or corn oil. Biggest difference would be 1 g/d total PUFA, 0.45% E, < 10% change from assumed 6% E baseline PUFA |
| AFFORD 2014 | Aim was to assess effects of high‐dose fish oils, compared EPA + DHA (1.6 g/d EPA + 0.8 g/d DHA) vs omega‐6 safflower oil (4 g/d, ˜80% LA). Assumed 2.4 g/d or 1.08% E omega‐3 in intervention, 3.2 g/d or 1.44% E omega‐6 fats in control, difference 0.8 g/d or 0.36% E total PUFA. This was < 10% increase from assumed baseline of 6% E total PUFA. No biomarker, lipid or dietary intake data to support. |
| AlphaOmega ‐ EPA+DHA | Aim was to increase omega‐3 fats. Margarine composition data ‐ summing LA, ALA, EPA, DPA and DHA total PUFA suggested dose in EPA + DHA margarine (compared to placebo) was 3.8% E. As planned intake was 20 g/d, intake was 0.76 g/d total PUFA, or 0.3% E from total PUFA. Total PUFA in ALA + EPA + DHA (compared to ALA margarine) was 0.7% E, or 0.14 g/d total PUFA, 0.06%E total PUFA. These were both < 10% higher than assumed 6% E from PUFA at baseline. TC levels did not alter by intervention. |
| AREDS2 2014 | Aimed to increase omega‐3 fats, compared EPA + DHA (350 mg/d DHA plus 650 mg/d EPA) vs nil. Intended increase 1.0 g/d, 0.5% E n‐3, assume 0.5% E PUFA (< 10% increase from assumed 6% E PUFA at baseline). No biomarker, lipid or dietary intake data to support or refute |
| ASCEND | Ongoing trial. Intervention omega‐3 (1 g/d: 0.41 g EPA, 0.34 g DHA) vs olive oil placebo (plus or minus aspirin). Dose appears < 1.33 g/d total PUFA, < 0.6% E PUFA, so excluded |
| Azadbakht 2007 | Weight reduction goals as well as fat modification, multifactorial |
| Baldassarre 2006 | Aim to increase omega‐3. Compared LCn3 (1.8 g/d EPA + DHA, 0.12 g LA, 1.92 g/d PUFA) vs MUFA (˜20% LA or 1.2 g/d PUFA). Dose 0.72 g/d PUFA, 0.3% E total PUFA, < 10% of baseline assumed 6% E PUFA. No biomarker data except on EPA + DHA, no dietary intake data presented, no postbaseline TC data but LDL increased in intervention arm and remained static in control. |
| Berson 2004 | DHA vs omega‐6, there appeared to be roughly the same amount of PUFA in both intervention and control supplements, but exact composition unclear (1.2g/d DHA plus 1.8 g vegetable oil vs 3 g/d mixed soy and corn oils (half each). Appeared to be < 10% difference in total PUFA between arms. |
| Caldwell 2011 | Compared EPA + DHA vs omega‐6, did not report intention to increase total PUFA. Intervention 2.1 g/d n3 (1050 mg EPA, 750 mg DHA and 300 mg other n3), control 3 g/d soybean oil (approx 60% PUFA plus 8% fish oil, 2.04 g/d), PUFA Dose 0.06 g/d, 0.03% E PUFA, < 10% increase from assumed 6% E baseline. Only erythrocyte fatty acid ratio reported, no TC or dietary intake data reported |
| DART 2 ‐ Burr 2003 | Aimed to increase oily fish intake or update of fish oil capsules. No PUFA aim, no PUFA biomarkers (though plasma EPA rose 1.23 mg/dl in intervention, fell 0.16 mg/dL in control over 6 months) or intake data reported. Aim for those on capsules was increase 0.5 g/d EPA + DHA, 0.2% E n‐3, 0.2% E PUFA. < 10% increase from assumed 6% E from PUFA baseline |
| DART fish Burr 1989 | EPA + DHA vs nil, aimed to increase omega‐3 intake by increasing fatty fish intake. No total PUFA goals or data on intake, serum fatty acids or serum cholesterol. Dose aim increase 0.5 g/d EPA + DHA, 0.2% E n‐3, 0.2% E PUFA. < 10% increase over assumed 6% E PUFA at baseline |
| Derosa 2016 | Compared EPA + DHA vs filler (non‐fat), no intention to increase total PUFA. Omega‐3 dose unclear, states intention in intervention of 3 x 1 g capsule/d n‐3 PUFAs (ethylic esters, each 1‐g capsule of n‐3 PUFAs contains highly concentrated ethyl esters of omega‐3 fatty acids, primarily EPA, and DHA in the proportion of 0.9–1.5), compared to placebo of "sucrose, mannitol and mineral salts magnesium stearate and silicon dioxide, used as anti‐caking agents". Both groups were given diet and exercise advice. No biomarker or intake data provided on omega‐3 or total PUFAs, TC not significantly different between arms. If omega‐3 dose was 1 g/d, or 0.45% E this would be < 10% E increase from an assumed baseline of 6% E |
| Deslypere 1992 | Compared LCN3 vs MUFA, no intention to increase total PUFA. Intended dose appeared relevant for 6 and 6 capsule arms (increase 1.12 g/d EPA + DHA, 0.5% E n‐3, 0.5% E PUFA or 2.24g/d EPA + DHA, 1.0% E n‐3, 1.0% E PUFA or 3.4 g/d EPA + DHA, 1.5% E n‐3, 1.5% E PUFA) but total PUFA intake appeared equal in all arms (subtracting SFA and MUFA from total fat), and erythrocyte membrane fatty acids similar in all arms (summing EPA, DHA, DPA, LA and AA, 30.6% fatty acids for 9‐capsule arm, 30.5% 6 capsules, 29.9% 3 capsules and 29.1% fatty acids in control arm. Did not appear to be > 10% increase in total PUFA between intervention and control arms |
| DISAF ‐ Harrison 2005 | Compared EPA + DHA vs nil, did not aim to increase total PUFA. Aimed to increase 1.4 g/d EPA + DHA, 0.6% E n‐3, this equates to 0.6% E PUFA in intervention arm, no change in control. While red cell membrane EPA and DHA increased in the intervention group, not in control, AA was reported as falling in intervention. PUFA (summed EPA + DHA and AA was 17.8% in intervention, 17.6% in control. Other PUFAs and TC not reported. Difference in total PUFA between intervention and control < 10% control |
| DO Health | Ongoing trial. Intervention omega‐3 (1 g/d, ratio EPA:DHA = 1:2) vs placebo capsules (plus or minus vitamin D3 and strength home exercise). Dose of total PUFA appears < 1.33 g/d, < 0.6% E PUFA, so excluded |
| DO IT ‐ Einvik 2010 | Compared EPA + DHA vs omega‐6, no aim to increase total PUFA. Intervention aim 2.4 g/d of omega‐3 PUFA (EPA + DHA 1.32 g/d, assume 1.08 g/d ALA or other omega‐3) vs corn oil (2.24 g/d LA). 2.4 g/d omega‐3, 1.1% E n3 vs 2.24 g/d LA or 1.0% E LA, PUFA dose 0.1% E. < 10% increase from assumed 6% E baseline. Serum fatty acids suggest < 10% more total PUFA in both intervention arms than controls, no difference in TC between arms. |
| DO IT 2006 | Dietary advice arm provided multifactorial dietary advice, while the supplementary arm was a specifically omega‐3 intervention (so included in the omega‐3 review). |
| EPE‐A study 2014 | Compared: high EPA vs low EPA vs placebo (contents not reported). PUFA content of placebo unknown. High EPA (increase 2.7 g/d EPA + DHA, 1.2% E omega‐3, 1.2% E PUFA) vs low EPA (increase 1.8 g/d EPA + DHA, 0.8% E omega‐3, 0.8% E PUFA), PUFA dose 0.4% E, < 10% increase from assumed 6% E at baseline. Serum EPA to AA (0.57 in high dose, 0.40 in low dose, 0.09 in control), TC rose by 8 mg/dL in control, by 4 mg/dL in high dose and by 3 mg/dL in moderate dose). |
| Erdogan 2007 | Intervention and control group contents unclear, so unclear if more PUFA vs less |
| Finnish Mental Hosp 1972 | Not randomised (cluster‐randomised, but < 6 clusters) |
| FLAX‐PAD 2013 | Compared ALA (in milled flaxseed) vs mixed dietary oils (composition unclear). No intention to increase total PUFA. Quantity of ALA and other PUFA unclear in both arms. Plasma levels of enterolignans and ALA rose in ALA arm, no details for control. No suggestion that total PUFA intake was higher in either arm, exclude |
| FORWARD 2013 | Compared EPA + DHA vs MUFA, no aim to increase total PUFA. Intervention provided 0.86 g/d EPA + DHA, 0.4% E n‐3, 0.4% E PUFA, control provided 1 g/d olive oil, or 0.2 g/d LA. Total PUFA dose 0.66 g/d PUFA, 0.3% E, < 10% higher than assumed 6% E PUFA baseline. |
| FOSTAR 2016 | Compared high EPA + DHA vs low EPA + DHA plus ALA. Intervention fruit juice mixed with fish oil supplement (18% EPA, 12% DHA, 4.5 g/day total omega‐3), control 15 mL Sunola oil/d (fish oil 2 mL plus 13 mL canola oil, omega‐3 ≤ 0.45 g EPA + DHA plus 3.9 g/d PUFA in canola, 4.4 g/d PUFA). ˜0.1 g/d PUFA more in intervention, < 10% more than assumed 6% E PUFA at baseline. |
| Franzen 1993 | Compared EPA + DHA vs MUFA. No intention to increase total PUFA stated but increased omega‐3 (20% EPA, 15% DHA, 3.15 g/day total omega‐3) vs increased olive oil (6.3 g/day MUFA, 1.35 g/day SFA, 1.35 g/d total omega‐6 fat). This suggests increase 1.8 g/d PUFA, 0.8% E PUFA, but serum fatty acids (summing EPA, DHA, ALA, LA, AA, DPA) suggested higher total PUFA in control (182 mg/dL PUFA in intervention, 195 mg/dL in control). However, TC rose more in control than intervention. Change in total PUFA unclear, exclude |
| Gill 2012 | Compared omega‐3 with placebo (unclear what), no aim to increase total PUFA. Control group contents unclear, so unclear if more PUFA vs less, no biomarker or intake data, TC reported only as "no significant change". Change in total PUFA unclear, exclude |
| GISSI‐HF 2008 | Compared EPA + DHA vs MUFA, no aim to increase total PUFA. Intervention increased 1 g/d omega‐3, 1 g/d olive oil, or 0.2 g/d LA in control, dose 0.8 g/d total PUFA, 0.36% E PUFA, < 10% increase from assumed 6% E PUFA. Fatty acid status did not provide total PUFA or any omega‐6 PUFAs, TC data provided for intervention but not control. |
| GISSI‐P 1999 | Compared EPA + DHA vs nil, no aim to increase total PUFA. Intervention dose 0.86 g/d EPA + DHA, 0.4% E n‐3, 0.4% E PUFA, < 10% increase from assumed 6% E PUFA. No biomarker or intake data, TC appeared to rise slightly more in intervention than control arms to 6 months |
| JELIS 2007 | Compared EPA fats with nil, no intention to increase total PUFA. Intended omega‐3 dose was 1.8 g/d EPA, compared to nil, and both groups received "appropriate" dietary advice (not described further). This suggests increases in total PUFA (0.8% E n‐3, 0.8% E PUFA), but increase in plasma PUFAs (sum of omega‐3 and omega‐6 fats, including EPA, DHA, DPA, ALA, LA, GLA, AA), was higher in control (+26.2 mg/mL) than intervention (+ 20 mg/mL). TC not reported, LDL change was equivalent (but all on statins). Difference in total PUFA appears < 10% of baseline PUFA intake assumed to be 6% E |
| Lorenz‐Meyer 1996 | Compared EPA + DHA vs omega‐6, no intention to increase total PUFA. Intervention increased EPA + DHA 5.1 g/d vs 6 g/d LA, 0.9 g/d or 0.45% E difference, < 10% increase over assumed 6% E PUFA. No biomarker or TC or intake data reported |
| Mansel 1990 | Did not aim to alter total PUFA, aimed to increase 0.48 g/d GLA or 4 kcal or 0.2% EGLA, increase ˜8.4 g/d LA or 76 kcal or 3.8% ELA, total 4% En6, estimated total PUFA dose 4% E. No serum TC or tissue fatty acid levels reported, no dietary intake data. No deaths or cardiovascular events occurred, only breast cancer diagnoses reported. |
| MAPT 2017 | Compared EPA + DHA vs paraffin oil (non fat). Intervention 1.025 g/d DHA + EPA compared to flavoured paraffin oil. (Also aims 3 and 4 as above plus multi‐domain intervention (nutrition, physical exercise, cognitive stimulation, social activities). Intended increase 1.03 g/d EPA + DHA, 0.5% E n3, 0.5% E PUFA, < 10% more than assumed 6% E PUFA baseline |
| MARGARIN Bemelmans 2002 | Omega‐3 vs omega‐6. Compared omega‐3 (ALA‐rich margarine, 80% fat of which 15% was ALA and 46% LA) with omega‐6 (LA‐rich margarine, 80% fat of which 0.3% was ALA and 58% LA). Margarines eaten as desired, so doses unclear. Serum cholesterol ester fatty acid changes suggest rises in ALA in omega‐3 arm and rises in LA in the LA arm, with rough equivalence in total PUFA between arms. TC fell slightly more in LA arms than ALA arms, but fell in all arms. Arms appear equivalent in total PUFA intake. |
| MENU ‐ Rock 2016 | Compared walnut‐rich moderate fat diet (ALA) vs moderate fat diet (MUFA), did not aim to increase total PUFA. Intervention was advice to follow walnut‐rich higher fat diet (35% E fat with limited SFA, MUFA encouraged, including 42 g/d walnuts, 45% E CHO, 20% E protein) vs exactly as intervention goals without walnuts. Unclear how total PUFA altered in each arm, mean LDL at 1 year was 2.97 mmol/L in both arms, TC not reported. Red blood cell fatty acid ALA and LA reported at 1 year (summed 12.5% in intervention, 12.2% in control) but other fatty acids not reported. PUFA dose unclear, excluded. |
| Michalsen 2006 | Multifactorial ‐ combination of diet (focusing on ALA and oily fish as well as Mediterranean diet more generally), exercise and stress‐reduction programme and advice in intervention, general written dietary and stress advice in control |
| Middleton 2002 | Intervention and control descriptions unclear. Compared EPA + DHA + GLA vs LA, but unclear which arm was higher in PUFA, or quantity of PUFA in either arm. |
| Minnesota Coronary 1989 | While participants were involved in this trial for over 1 year on average they could move in and out of the institution in which the trial took place, and therefore in and out of the trial over the duration of the trial. Most participants were not involved in the trial continuously for ≥ 1 year |
| Moy 2001 | Aim was to reduce dietary fat (total and saturated fat reductions appear to have been achieved) but effects on PUFAs unclear (total PUFA, omega‐6 and omega‐3 intakes not reported) |
| NAT2 2015 | No aim to increase total PUFA, aimed to increase omega‐3 fats. Intervention was 1110 mg/d n‐3 FAs (EPA: 270 mg/day DHA: 840 mg/day) vs olive oil capsules (containing 0.2 g total PUFA). Total PUFA dose would be 0.91 g/d, or 0.4% E PUFA. Red blood cell lipid EPA and DHA presented, but not total PUFA. Dietary intake data suggest 0.5% E difference in total PUFA between arms (< 10% increase from assumed 6% E from PUFA at baseline). |
| Norouzi 2014 | Compared LCn3 with placebo (no details). Intervention 1.056 g/d LCn3 plus 0.056 g/d omega‐6, 1.112 g/d PUFA in intervention, control group contents unclear, so unclear whether more PUFA vs less. No biomarker, TC or dietary intake data to help. No intention to increase total PUFA and no information on whether PUFA was increased substantially in one arm compared to the other, exclude |
| Norwegian ‐ Natvig 1968 | Aim was to increase vegetable oil intake, comparing ALA (linseed oil) with omega‐6 (sunflower oil). Intervention was linseed oil, 10 mL/d (55% ALA), 5.5 g/d ALA, 1.5g/d LA (7.04 g total PUFA), control was sunflower oil, 10 mL/d (1.4% ALA), 0.14 g/d ALA, 6.3 g/d LA or 6.42 g/d omega‐6 (6.56 g/d total PUFA). Intended total PUFA dose was 0.48 g/d lower total PUFA or 0.22% E from PUFA lower in intervention (< 10% change from assumed 6% E baseline). No biomarker or dietary intake data, except slightly lower TC at 6 months in intervention arm. |
| NutriStroke 2009 | Compared LCn3 with unclear placebo. No intention to increase total PUFA. Intervention 0.5 g/d LCn3, assume 0.5 g/d PUFA. Control group contents unclear, but state no PUFA. PUFA dose 0.5 g/d or 0.23% E PUFA, < 10% increase from assumed 6% E PUFA baseline. No biomarker, TC or dietary intake data to confirm. |
| OFAMI ‐ Nilsen 2001 | Omega‐3 vs omega‐6 comparison, aim to assess effects of omega‐3 increase, total PUFA doses in each arm unclear, no dietary intake data provided. |
| OMEGA 2014 | Did not aim to alter total PUFA. Aimed to increase omega‐3 fats, vs MUFA control, but only increased omega‐3 fats by 0.4% E (< 10% of assumed baseline of 6% E from PUFA). No dietary intake data provided |
| OPAL ‐ Dangour 2010 | Aimed to increase omega‐3 fats, not total PUFA, compared omega‐3 supplement with olive oil, omega‐3 dose 0.7 g/d or 0.3% E (< 10% of assumed baseline of 6% E from PUFA). No dietary intake data provided. |
| ORIGIN 2012 | Aimed to increase omega‐3 fats, not total PUFA. Compared omega‐3 supplement with olive oil placebo, EPA + DHA vs MUFA. Aimed to increase 0.84 g/d EPA + DHA, 0.4% E n‐3, 0.4% E PUFA (< 10% of assumed baseline of 6% E from PUFA). No dietary intake data provided |
| Oslo Diet‐Heart 1966 | Multifactorial dietary intervention (cannot separate out the effects of PUFAs from other dietary interventions). |
| Oxford Retinopathy 1978 | Multifactorial dietary intervention (cannot separate out the effects of PUFAs from other dietary interventions). |
| POUNDS Lost Sacks 2009 | Manipulation of total fat intake, but no details of fat types aimed for or achieved in any arms. |
| Ramirez‐Ramirez 2013 | Omega‐3 vs omega‐6 (DHA + EPA vs sunflower oil). Quantities of total PUFA in each arm unclear, but likely to have been similar (< 10% of assumed baseline of 6% E from PUFA). Aimed to assess omega‐3 effects, no dietary intake data provided |
| Reed 2014 | Omega‐3 vs omega‐6 (EPA + DHA vs GLA + sunflower oil). Doses of total PUFA in each arm unclear but likely to have been similar (< 10% of assumed baseline of 6% E from PUFA). Aimed to assess omega‐3 effects and omega‐6 effects, not total PUFA. Paper states that there were no differences between arms for TC or dietary intake. |
| Risk and Prevention | Omega‐3 vs MUFA, but small PUFA dose (intended to increase 0.86 g/d EPA + DHA, 0.4% E n3, 0.4% E PUFA). Aimed to assess effects of omega‐3 fats, not total PUFA, intended dose too small (< 10% of assumed baseline of 6% E from PUFA). No difference between arms for change in TC from baseline to 5 years (P = 0.52) |
| Sandhu 2016 | Aimed to increase omega‐3 fats. Intended dose suggested higher omega‐3 fats in Lovaza and Lovaza & Raloxifee compared to control and Raloxifene 30 mg (as no placebo was provided. However, plasma fatty acid concentration suggested that total PUFA was not higher in these arms. Mean summed plasma fatty acid omega‐3 fats higher in Lovaza and Lovaza & Raloxifee arms compared to control and Raloxifene 30 mg at 2 years. However omega‐6 fats were equivalently lower, mean total PUFA (summing omega‐3 and omega‐6) similar in both arms. |
| Schirmer 2007 | Compared n‐6 (GLA) vs MUFA, did not aim to increase total PUFA. Intervention included 0.89 g/d GLA plus ˜0.9 g/d LA or 0.8% E n6. control included 1 g/d LA, 0.45% E LA. Difference 0.35% E omega‐6, assume same for PUFA, < 10% more than assumed 6% E baseline total PUFA. No biomarker, TC or dietary intake data. |
| SCIMO ‐ von Schacky 1999 | Aimed to increase omega‐3 fats. Intended omega‐3 dose was 1.03 g/d EPA + DHA, 0.5% E n‐3. This would translate to 0.5% E PUFA, but the placebo was probably fairly rich in total PUFA. Excluded as probably < 10% increase in total PUFA in intervention compared to control. Erythrocyte phospholipid fatty acid composition confirmed rise in EPA and DHA but didn't report further PUFAs. Serum total cholesterol dropped very slightly more in intervention than control (TC ‐0.1mmol/L in int, ‐0.05mmol/L in cont from baseline to 24 months). |
| Shinto 2014 | Compared EPA + DHA vs n‐6, did not intend to increase total PUFA. Intervention 1.650 g/d LCn3, 1.65 g/d PUFA vs 3 g/d soybean oil (˜60% PUFA), 1.8 g/d PUFA. Dose is 0.15 g/d PUFA, 0.07% E PUFA, < 10% change from assumed 6% E PUFA baseline. No biomarker (except red blood cell EPA + DHA), dietary intake or TC data |
| SHOT ‐ Eritsland 1996 | Aim was to increase omega‐3 fats. Intervenion was omega‐3 vs nil, and provided 3.3 g/d EPA + DHA, or 1.5% E from omega‐3 fats. This suggests increase of 1.5% E from PUFA, but serum fatty acid PUFA assessments were 645 mg/L in the control (up 43 mg/L from 603 at baseline), and 621 mg/L (up 28 mg/L from 593) in the intervention group at 9 months, suggesting lower or equivalent total PUFA intake in the intervention compared with control. Serum TC remained constant over the trial in both arms. |
| Sianni 2013 | Control group contents unclear, so unclear if more PUFA vs less. Aimed to increase omega‐3 fats, intervention group received 4 g/d omega‐3 fats, placebo not described. As only an abstract could be found, and contact could not be established with the authors we excluded this trial. |
| SMART Tapsell 2013 | Compared fish + fish oil supplements vs fish + olive oil supplements vs olive oil supplements. Did not aim to increase total PUFA. Comparisons with olive oil supplement arm are multifactorial so excluded. Fish + fish oil supplements (capsules including 420 mg/d EPA + 210 mg/d DHA, 0.63 g/d EPA + DHA) vs fish plus olive oil supplements (1 g olive oil/d, assume 0.2 g/d PUFA) has equivalent diets with differing supplements between arms. Dose 0.43 g/d PUFA, 0.2% E from PUFA, < 10% increase from assumed 6% E PUFA at baseline. |
| SOFA 2006 | Aimed to increase omega‐3 fats. Comparison was EPA + DHA (961 mg n‐3 PUFAS) vs MUFA + omega‐6 (2 g/d high‐oleic acid sunflower oil). Omega‐3 dose was only 0.96 g/d, or 0.4% E from omega‐3. As there was some PUFA in the placebo it was unlikely that total PUFA was increased more than 10% of baseline. No biomarker data found to confirm or refute this. |
| Sofi 2010 | Aimed to increase omega‐3 fats. Comparison was EPA + DHA (6.5 mL/d olive oil enriched with n‐3 plus dietary recommendations, 0.83 g n‐3/d of which 0.47 g/d EPA & 0.24 g/d DHA) vs MUFA (6.5 mL/d olive oil plus dietary recommendations). Omega‐3 dose was 0.71 g/d EPA + DHA, 0.3% E n‐3, equivalent to 0.3% E PUFA (< 10% increase from assumed 6% E PUFA baseline). No fatty acid biomarker data, TC fell more in control than intervention. |
| STARS 1992 | Intervention encouraged to increase plant‐derived soluble fibre as well as alter dietary fats, multifactorial |
| Stoll 2001 | Ongoing trial. NCT00010868. The PI, Andrew Stoll, appears to have been struck off the medical register in Massachusetts in 2011 (Commonwealth of Massachusetts Board of Registration in Medicine, Adjudicatory Case number 2011‐026) so it has not been possible to contact him and no publication of results has been found. |
| STRENGTH | Ongoing trial. Intervention omega‐3 carboxylic acid capsule (Epanova, not less than 800 mg/g) and statin vs corn oil placebo capsule and statin. Omega‐3 vs omega‐6, unlikely to reach PUFA dose of > 1.33 g/d or 0.6% E. |
| SU.FOL.OM3 Galan 2010 | Compared EPA + DHA vs non‐fat placebo, no intention to increase total PUFA. Intervention 400 mg/d EPA and 200 mg/d DHA compared to liquid paraffin with fish flavour. Intended dose 0.6 g/d EPA + DHA, 0.3% E PUFA, < 10% change from assumed 6% E PUFA baseline. No biomarker (aside from plasma EPA + DHA), TC (apart from baseline) or dietary intake data provided. |
| Søndergaard 2003 | Multifactorial dietary intervention (cannot separate out the effects of PUFAs from other dietary interventions). |
| Tande 2016 | Compared EPA + DHA vs MUFA, did not intend to increase total PUFA. Intervention 2 g/d calanus oil (85% wax ester with a sum of neutral lipids > 90%, 11% oil is EPA + DHA, or 0.22 g/d EPA + DHA), control 2 g/d olive oil (analysis indicated this olive oil was primarily oleic acid (76.9%), palmitic acid (10.2%), and linoleic acid (7.7%), assumed 0.14 g/d LA), overall dose 0.08 g/d PUFA, 0.04% PUFA. < 10% increase from assumed 6% E PUFA. TC increased by 0.02 mmoL/L in intervention to 1 year, fell 0.08 mmoL/L in control, no further biomarker or intake data |
| Tay 2015 | Multifactorial dietary intervention (cannot separate out the effects of PUFAs from other dietary interventions). |
| THIS DIET ‐ Tuttle 2008 | Aim was to achieve a Mediterranean‐style diet, and compare it to a low‐fat diet. All intervention and control participants were advised to reduce SFA and dietary cholesterol, increase fruits and vegetables and whole grains. In addition intervention participants were encouraged to increase cold‐water fish and oils from olives, canola and soybeans. Plasma fatty acid composition suggested that omega‐3 increased in the intervention arm compared to control (rising 0.1% in control, rising 0.6% in intervention) while omega‐6 fats reduced in the intervention (rising 0.7% in control, falling 0.1% in intervention). This confirms dietary intake data suggesting that total PUFA increased by 0.9% E in control, and increased by only 0.1% E in intervention, to equivalence at 24 months (total PUFA intake at 24 months 5.7% E, SD 3.1 in control, 5.7% E, SD 2.4 in intervention). No total PUFA difference between arms during trial, so excluded |
| VITAL | Ongoing trial. Intervention omega‐3 (Omacor fish oil, EPA + DHA 1 g/d: 465 mg EPA; 375 mg DHA) vs placebo (plus or minus vitamin D3). Placebo unclear but very unlikely to attain a dose of > 1.33 g/d PUFA or 0.6% E |
| Weinstock‐Guttman 2005 | Aim was to compare low fat diet (15% E from fat) plus EPA + DHA supplements (3.3 g/d EPA + DHA, 1.5% E n3) with low‐fat diet (30% E from fat) plus olive oil capsules. Total PUFA in each arm (aimed or achieved) is not clear. Serum fatty acids were assessed, data reported on MUFA, EPA, DHA, DPA, combined omega‐3 fats and SFA, but not total fat intake or total PUFAs. TC was not reported and LDL rose slightly in both groups, more in the control (30% E fat) than intervention (15% E fat). Dietary intake not reported |
| WHI 2006 | Dietary intervention was of dietary fat and also fruit and vegetables, multifactorial |
| Zhang 2016 | Compared DHA vs corn oil (n6). No aim to increase total PUFA, intervention 1.0 g/d DHA, 0.45% E n3, control 1.1 g/d PUFA, 0.5% E PUFA, dose 0.05% E PUFA, < 10% increase from assumed 6% E PUFA baseline. No fatty acid (except very small increase in serum DHA in intervention, unclear if statistically significant), TC or dietary intake data |
| Özaydin 2011 | Compared omega‐3 supplement with nil (no placebo). Intended omega‐3 dose was increase 0.6 g/d EPA + DHA, 0.3% E n‐3, 0.3% E PUFA. Baseline total PUFA not reported, nor intake or body marker data. Assume baseline 6% E PUFA, dose < 10% increase |
AA: arachidonic acid; ALA: alpha‐linolenic acid (a plant‐based omega‐3 fat); CHO: carbohydrate; DHA: docosahexaenoic acid (a fish‐based omega‐3 polyunsaturated fat); DPA: docosapentaenoic acid (a fish‐based omega‐3 polyunsaturated fat); EPA: eicosapentaenoic acid (a fish‐based omega‐3 polyunsaturated fat); GLA: gamma linolenic acid (an omega‐6 polyunsaturated fat); LA: linoleic acid (an omega‐6 polyunsaturated fat); LDL: low density lipoprotein (a fraction of TC, measured in human blood); MUFA: monounsaturated fatty acid or monounsaturated fat; PUFA: polyunsaturated fatty acid or polyunsaturated fat; SFA: saturated fatty acid or saturated fat; TC: total cholesterol (measured in human blood)
Characteristics of ongoing studies [ordered by study ID]
AC Omega3.
| Trial name or title | The Aboriginal cardiovascular omega‐3 randomised controlled trial (AC Omega3) |
| Methods | RCT |
| Participants | Indigenous Australian adults with stable coronary artery disease |
| Interventions | Each for 12 months: Arm 1: omega‐3 (1800 mg/d AlaskOmega: 3 capsules/d: 400 mg EPA and 200 mg DHA) Arm 2: placebo mixed oil capsules (1000 mg/d: 3 capsules/d containing palm oil, gelatin, glycerol, sunflower oil, rapeseed oil, mixed tocopherols, and a "small amount" of fish oil ((or taste) to aid blinding) |
| Outcomes | Primary: serum non‐HDL cholesterol Secondary: triglycerides, total cholesterol, LDL, HDL, lipid functionality by cholesterol efflux and CETP, heart rate variability, platelet function and thrombosis markers, inflammation markers, cumulative combined rate of major adverse cardiac events (including death, non‐fatal MI, unstable angina, non‐fatal stroke, revascularisation and cardiac‐related hospital admissions) |
| Starting date | Registered on Trials Registry: 10 July 2014 Trial start date: 1 October 2014 Trial completion date est: unclear |
| Contact information | Alex Brown (PI), Wardliparingga Aboriginal Unit, Adelaide, Australia, alex.brown@sahmri.com |
| Notes | ACTRN12614000732684 Alex Brown contacted in 2016: confirmed trial is actively recruiting |
ACTRN12610000594022.
| Trial name or title | Clinical efficacy of fish oil as adjunct therapy for patients with chronic periodontitis |
| Methods | RCT |
| Participants | Adults (25‐80 years, non‐smokers) with newly diagnosed severe but non aggressive periodontitis |
| Interventions | Each for 13 months: Arm 1: fish oil rich in EPA (6 x 500 mg capsules/d: 277 mg EPA; 27 mg DHA) and standard periodontal treatment (scaling and debridement) Arm 2: fish oil rich in DHA (6 x 500 mg capsules/d: 66 mg EPA; 258 mg DHA) and standard periodontal treatment Arm 3: soya oil placebo (6 x 500 mg capsules/d) and standard periodontal treatment |
| Outcomes | Primary: probing pocket depth, clinical attachment level (CAL) Secondary: inflammatory biomarkers in gingival crevicular fluid, erythrocyte omega‐3, C‐reactive protein |
| Starting date | Registered on Trials Registry: 23 July 2010 Trial start date: July 2010 Trial completion date est: unclear |
| Contact information | Mark Bartold, University of Adelaide, mark.bartold@adelaide.edu.au |
| Notes | ACTRN12610000594022 PhD, Boram Park, available giving 4‐month outcome data for pilot trial n = 33 participants Mark Bartold written to in 2016. Confirmed preparing full publications for submission |
ACTRN12613000034730.
| Trial name or title | Intervention of testosterone & fish oil for the prevention of Alzheimer's disease: InTrePad |
| Methods | RCT |
| Participants | PiB‐PET (Pittsburgh compound B)‐positive men aged ≥ 60 years with subjective memory complaints |
| Interventions | Each for 56 weeks: Arm 1: DHA capsules (1720 mg/d) and testosterone undecanoate (intramuscular injection 1000 mg/4 mL every 8 weeks) Arm 2: placebo DHA and testosterone undecanoate (intramuscular injection 1000 mg/4 mL every 8 weeks) Arm 3: placebo DHA and placebo testosterone |
| Outcomes | Primary: PiB score Secondary: neuropsychological, mood and daily functioning questionnaires, beta amyloid levels, fluorodeoxyglucose to assess brain glucose metabolism, inflammatory and oxidative biomarkers, hippocampal volume, quality of life, safety and tolerability of treatment |
| Starting date | Registered on Trials Registry: 14 January 2013 Trial start date: 28 February 2013 Trial completion date est: |
| Contact information | Ralph Martins (PI), Sir James McCusker Alzheimer's Disease Research Unit, Hollywood Medical Centre, Nedlands, Australia, r.martins@ecu.edu.au |
| Notes | ACTRN12613000034730 Ralph Martins written to in 2016‐ no response |
AFORRD.
| Trial name or title | Atorvastatin in factorial with omega‐3 fatty acid risk reduction in diabetes (AFORRD) |
| Methods | RCT |
| Participants | People with type 2 diabetes with no known CVD and not taking lipid‐lowering therapy, adults (> 18 years) N: intervention 397, control 403 (analysed intervention 371, control 361) |
| Interventions | Each for 12 months: Arm 1: atorvastatin (Lipitor 20 mg/d) and olive oil placebo (2 g/d) Arm 2: omega‐3 (Omacor 2 g/d: 46% EPA, 38% DHA) and placebo tablets for atorvastatin Arm 3: atorvastatin (Lipitor 20 mg/d) and Omega‐3 (Omacor 2 g/d: 46% EPA, 38% DHA) Arm 4: placebo tablets for atorvastatin and olive oil placebo (2 g/d) |
| Outcomes | Primary: lipid profiles Secondary: phytosterol changes, HbA1c ,estimated CVD risk using the UK Prospective Diabetes Study risk engine |
| Starting date | Registered on Trials Registry: 4 April 2004 Trial start date: 1 November 2004 Trial completion date est: 31 July 2006 |
| Contact information | Rury Holman, Oxford Centre for Diabetes |
| Notes | ISRCTN76737502 Rury Holman contacted in 2016: confirmed results are not yet published, but planned |
Beyond Aging Project.
| Trial name or title | The Beyond Ageing Project phase 2: a selective prevention trial using novel pharmacotherapies in an older age cohort at risk for depression |
| Methods | RCT |
| Participants | Older adults (≥ 60 years) at risk of depression (K‐10 score ranging from 16‐29) who initially participated in the first Beyond Ageing Project |
| Interventions | Each for 12 months: Arm 1: omega‐3 (4 capsules, total 2 g/d: 1200 mg EPA and 800 mg DHA) and placebo microcrystalline cellulose (1 capsule) Arm 2: paraffin oil placebo (4 capsules) and sertraline hydrochloride (1 capsule, 50 mg) Arm 3: paraffin oil placebo (4 capsules) and placebo microcrystalline cellulose (1 capsule) |
| Outcomes | Primary: depressive symptoms (PHQ‐9) Secondary: cognitive decline, Mini Mental State Exam, brain metabolism, hippocampal volume, anxiety (Generalized Anxiety Disorder‐7 (GAD‐7)), disability (World Health Organziation Disability Assessment Schedule‐II (WHODAS‐II)), sleeping problems (Pittsburgh Sleep Quality Index (PSQI)), exercise (Active Australian Survey) |
| Starting date | Registered on Trials Registry: 12 January 2010 Trial start date: June 2011 Trial completion date est: Main results expected in 2017 |
| Contact information | Ian Hickie (PI), Brain and Mind Centre, University of Sydney, ian.hickie@sydney.adu.au |
| Notes | ACTRN12610000032055 |
Chandrakala 2010.
| Trial name or title | Long‐term effects of a reduced fat diet intervention in pre‐diabetes |
| Methods | RCT |
| Participants | Participants with pre‐diabetes (IFG/IGT), 201 participants discussed in 1 abstract, 134 in a later abstract |
| Interventions | Each for 3 years: Arm 1: reduced‐fat diet (fat content ≤ 20% total energy, ratio of PUFA/SFA 0.8 to 1.0) Arm 2: normal/control diet |
| Outcomes | Incidence of diabetes, BMI, lipids, insulin, plasma glucose, HbA1c, BP, nutritional intake |
| Starting date | Registered on Trials Registry: no registration found Trial start date: not stated Trial completion date est: not stated |
| Contact information | Chandrakala Galla, chandrakala.galla@gmail.com; Arpana Gaddam, dr.arpanag@gmail.com |
| Notes | We wrote to trial authors in 2016: Dr Gaddam confirmed work submitted as a PhD but not published in full. Requested copy of PhD thesis, but no reply to date. Funding: DiabetOmics India |
n‐3 for Vascular Cognitive Aging.
| Trial name or title | n‐3 PUFA for vascular cognitive aging |
| Methods | RCT |
| Participants | Older adults (≥ 80 years) at high risk for cognitive decline and dementia of Alzheimer's type |
| Interventions | Each for 3 years: Arm 1: omega‐3 fish oil (1.65 g/d EPA + DHA) Arm 2: soybean oil placebo (1.65 g/d) |
| Outcomes | Primary: total cerebral white matter volume Secondary: biomarkers of endothelial health, total brain atrophy, medial temporal lobe atrophy, ventricular expansion, Trail Making Test part B, digit symbol Wechsler Adult Intelligence Scale‐Revised (WAIS‐R), cerebral blood flow, fractional anisotropy within frontal gyri |
| Starting date | Registered on Trials Registry: 24 September 2013 Trial start date: May 2014 Trial completion date est: March 2019 |
| Contact information | Alena Borgatti, borgatti@ohsu.edu; James Dursch, dursch@ohsu.edu; Gene Bowman and Lynne Shinto (PIs), Oregon Health and Science University |
| Notes | NCT01953705 |
n‐3 on plasma lipid.
| Trial name or title | Influence of different sources of n‐3 fatty acid on plasma lipid in moderately hypercholesterolaemic subjects |
| Methods | RCT |
| Participants | Adults (40‐65 years) with mild to moderate hypercholesterolaemia |
| Interventions | Arm 1: EPA/DHA 1.8 g/d Arm 2: EPA/DHA 3.6 g/d Arm 3: ALA 4 g/d Arm 4: placebo |
| Outcomes | Fatty acids, lipids, cytokines (IL‐6, IL‐1a) |
| Starting date | Registered on Trials Registry: 13 March 2012 Trial start date: unclear Trial completion date est: unclear |
| Contact information | Su Yixiang, Sun‐Yat Sen University, China, suyx@mail.sysu.edu.cn; Zhou Quan, Guangzhou Medical University, joan_zq@126.com |
| Notes | ChiCTR‐TRC‐12002014 Su Yixiang and Zhou Quan contacted in 2016: no response |
NCT00309439.
| Trial name or title | Studies of serum PSA to help resolve the current implication of alpha‐linolenic acid and prostate cancer |
| Methods | RCT |
| Participants | Adults 18‐77 years |
| Interventions | Arm 1: ALA‐rich diet Arm 2: control (not detailed) |
| Outcomes | PSA, atrial fibrillation |
| Starting date | Registered on Trials Registry: 29 March 2006 Trial start date: unclear Trial completion date est: unclear |
| Contact information | David Jenkins, University of Toronto, nutritionproject@smh.toronto.on.ca |
| Notes |
NCT00309439 David Jenkins written to in 2016: confirmed not published in full and data incomplete |
NCT00410020.
| Trial name or title | Arrhythmia prevention with an alpha‐linolenic enriched diet |
| Methods | RCT, parallel, 2 arms, 12 months |
| Participants | 98 people with successful atrial fibrillation electrical cardioversion |
| Interventions | Canola margarine and oil, rich in ALA, versus a conventional diet (control), for 1 year |
| Outcomes | Length of time to first recurrence of atrial fibrillation |
| Starting date | June 1999, expected finish date June 2003, registered December 2006 so appears to have been carried out |
| Contact information | Principal Investigator: Jean‐Paul Broustet, MD, PhD, Universitary Hospital Haut‐Lévêque Bordeaux France |
| Notes | NCT00410020, registered Dec 2006, no publication found |
NCT01047449.
| Trial name or title | Improving the results of heart bypass surgery using new approaches to surgery and medication (SUPERIORSVG) |
| Methods | RCT |
| Participants | Adults having coronary artery bypass graft (CABG) using saphenous vein graft (SVG) |
| Interventions | Each for 12 months: Arm 1: fish oil supplements (2 x 1 g/d Ocean Nutrition capsules: 55% fish oils EPA:DHA 33%:22%) and SVG conventionally harvested Arm 2: placebo and SVG conventionally harvested Arm 3: fish oil supplements (2 x 1 g/d Ocean Nutrition capsules: 55% fish oils EPA:DHA 33%:22%) and SVG no‐touch harvest Arm 4: placebo and SVG no‐touch harvest |
| Outcomes | Primary: proportion of grafts occluded Secondary: significant stenosis, adverse SVG harvesting events, composite outcome of all‐cause mortality, non‐fatal MI and repeat revascularisation |
| Starting date | Registered on Trials Registry: 12 Jan 2010 Trial start date: July 2011 Trial completion date est: Dec 2016 |
| Contact information | Stephen Fremes, Sunnybrook Health Sciences Centre (PI) |
| Notes | NCT01047449 |
NCT01513252.
| Trial name or title | Long‐term effects of interventional strategies to prevent cognitive decline in elderly (MAPT PLUS) |
| Methods | RCT ‐ extension of MAPT trial |
| Participants | Participants of MAPT trial |
| Interventions | Follow‐up, 2‐year extension of participants in MAPT, after completion of MAPT interventions |
| Outcomes | Primary: cognitive and functional status (Grober and Buschke test) Secondary: markers of cerebral atrophy, cost effectiveness |
| Starting date | Registered on Trials Registry: 30 December 2011 Trial start date: December 2011 Trial completion date est: November 2016 |
| Contact information | Bruno Vellas (PI), University Hospital, Toulouse, vellas.b@chu‐toulouse.fr |
| Notes |
NCT01513252 Bruno Vellas written to in 2016‐ no response |
NCT01784042.
| Trial name or title | Dietary energy restriction and omega‐3 fatty acids on mammary tissue |
| Methods | RCT |
| Participants | Overweight women (30‐55 years) with increased breast cancer risk |
| Interventions | For 1 year: Arm 1: Lovaza (omega‐3‐acid ethyl esters) Arm 2: Lovaza and dietary energy restriction Arm 3: placebo Arm 4: placebo and dietary energy restriction |
| Outcomes | Ki67 expression at 1 year |
| Starting date | Registered on Trials Registry: 31 January 2013 Trial start date: March 2013 Trial completion date est: March 2018 |
| Contact information | Andrea Manni, Hershey Medical Centre, amanni@hmc.psu.edu (PI) or Cynthia DuBrock, cdubrock@hmc.psu.edu |
| Notes | NCT01784042 |
NCT02128763.
| Trial name or title | Dry eye assessment and management trial (DREAM) |
| Methods | RCT |
| Participants | Adults with dry eye |
| Interventions | Each for 2 years Arm 1: omega‐3 supplements (2000 mg EPA + 1000 mg DHA/d as 5 gelcaps) Arm 2: olive oil supplements (5 gelcaps) |
| Outcomes | Primary: Ocular Surface Disease Index (OSDI) score Secondary: other eye health measures, SF‐36, healthcare utilisation costs, cost effectiveness |
| Starting date | Registered on Trials Registry 28 April 2014 Trial start date: November 2014 Trial completion date est: July 2017 |
| Contact information | Penny Asbell, Mount Sinai Icahn School of Medicine (Trial Chair), Maureen Maguire, University of Pennsylvania (PI) |
| Notes | NCT02128763 |
NCT02211560.
| Trial name or title | Investigating a phosphatidylserine based dietary approach for the management of mild cognitive impairment |
| Methods | RCT |
| Participants | People with mild cognitive impairment (MCI) aged 65‐85 years |
| Interventions | Each for 24 months: Arm 1: phosphatidylserine omega‐3 (DHA enriched) Arm 2: placebo cellulose capsules |
| Outcomes | Primary: selective reminding test (SRT) Secondary: MMSE, neurological battery test (NBT), dementia (DSM‐4 criteria), mini sleep questionnaire (MSQ), Hamilton Anxiety rating scale (HAM‐A), safety and adverse events |
| Starting date | Registered on Trials Registry: 6 August 2014 Trial start date: September 2014 Trial completion date est: September 2019 |
| Contact information | Nadia Niemerzyanski, nadiaN@enzymotec.com; Yael Richter, yaelr@enzymotec.com |
| Notes | NCT02211560 |
NCT02295059.
| Trial name or title | Omega‐3 fatty acids and ERPR(‐)HER2(+/‐) breast cancer prevention |
| Methods | RCT |
| Participants | Women at risk for recurrent breast cancer‐ with prior diagnosis of stage 0‐III breast cancer and completion of surgery, chemotherapy or trastuzumab or radiation therapy |
| Interventions | Each for 12 months: Arm 1: omega‐3 high‐dose capsules (5 g/d EPA + DHA) Arm 2: omega‐3 low‐dose capsules (0.9 g/d EPA + DHA) |
| Outcomes | Primary: breast adipose tissue metabolites Secondary: cytomorphology or cell proliferation of mammary epithelial cells, DNA promoter methylation and pro‐inflammatory gene expression in mammary epithelial and adipose tissue |
| Starting date | Registered on Trials Registry: 14 October 2014 Trial start date: August 2014 Trial completion date est: January 2019 |
| Contact information | Anitra Sumbry, anitra.sumbry@osumc.edu; Lisa Yee (PI), Ohio State University |
| Notes | NCT02295059 |
NCT02676466.
| Trial name or title | Enabling reduction of low‐grade inflammation in seniors (ENRGISE) |
| Methods | RCT |
| Participants | People aged 70+ years with self‐reported walking or stair‐climbing difficulty |
| Interventions | Each for 1 year Arm 1: omega‐3 fish oil (1.4 g/d for 6 months, possibly increasing to 2.8 g/d) Arm 2: losartan 25 mg/d Arm 3: placebo corn oil (for omega‐3) plus placebo cellulose (for losartan) Arm 4: omega‐3 plus losartan Arm 5: placebo corn oil (for omega‐3) Arm 6: placebo cellulose (for losartan) |
| Outcomes | Primary: IL6, 400‐meter walk test Secondary: short physical performance battery, frailty, hand grip strength, knee dynamometry, Short Form (SF)‐36 |
| Starting date | Registered on Trials Registry 3 February 2016 Trial start date: February 2016 Trial completion date est: March 2018 |
| Contact information | Jane Lu janelu@ufl.edu Michael Stancil mstancil@ufl.edu |
| Notes | NCT02676466 |
NCT02719327.
| Trial name or title | Impact of icosapent ethyl on Alzheimer's disease (AD) biomarkers in preclinical adults |
| Methods | RCT |
| Participants | Cognitively healthy adults aged 50‐70 years whose parents had AD |
| Interventions | Each for 18 months: Arm 1: icosapent ethyl EPA (Vascepa) 4 g/d gel cap Arm 2: matching gel cap placebo |
| Outcomes | Primary: cerebral blood flow by magnetic resonance imaging Secondary: cerebrospinal fluid biomarkers of Alzheimer's disease, cognitive performance (preclinical Alzheimer's cognitive composite, PACC) |
| Starting date | Registered on Trials Registry: 21 March 2016 Trial start date: December 2016 Trial completion date est: November 2021 |
| Contact information | Cynthia Carlsson, cynthia.carlsson@va.gov; Elena Beckman, elena.beckman@va.gov |
| Notes | NCT02719327 |
OMEMI.
| Trial name or title | Omega‐3 fatty acids in elderly patients with myocardial infarction trial (OMEMI) |
| Methods | RCT |
| Participants | Elderly patients (70‐82 years) with acute MI |
| Interventions | Each for 24 months: Arm 1: omega‐3 capsules, 3/d (Pikasol, total of 1.8 g/d EPA + DHA) and standard therapy Arm 2: corn oil placebo, 3/d and standard therapy |
| Outcomes | Primary: composite of total mortality, first non‐fatal recurring acute MI, stroke and revascularisation Secondary: new onset atrial fibrillation, adipose tissue, serum fatty acids, makers of endothelial function, inflammation, coagulation and fibrinolytic activity, genes associated with atherothrombosis |
| Starting date | Registered on Trials Registry: 16 April 2013 Trial start date: November 2012 Trial completion date est: November 2019 |
| Contact information | Svein Solheim, Center for Clinical Heart Research, Oslo University Hospital, arnljot.tveit@vestreviken.no |
| Notes | NCT01841944 |
REDUCE‐IT.
| Trial name or title | Reduction of cardiovascular events with EPA‐intervention trial (REDUCE‐IT) |
| Methods | RCT |
| Participants | Patients (45 years or over) with hypertriglyceridaemia, with cardiovascular disease or at high risk for cardiovascular disease, and on statin |
| Interventions | Each for 4‐6 years: Arm 1: EPA ethyl ester (AMR101 4 g/d) Arm 2: placebo |
| Outcomes | Primary: composite of CV death, MI, stroke, coronary revascularisation and hospitalisation for unstable angina Secondary: incidence of additional cardiovascular events, lipid and lipoprotein levels |
| Starting date | Registered on Trials Registry: 13 December 2011 Trial start date: November 2011 Trial completion date est: December 2017 |
| Contact information | Deepak Bhatt (PI), Brigham and Women's Hospital |
| Notes | NCT01492361 |
seAFOOD.
| Trial name or title | The seafood (systematic evaluation of aspirin and fish oil) polyp prevention trial |
| Methods | RCT |
| Participants | NHS Bowel Cancer Screening Programme patients (55‐73 years) identified as "high risk" (≥ 5 small adenomas; or ≥ 3 adenomas with at least one being ≥ 10 mm in diameter) after their 1st screening colonoscopy |
| Interventions | Each for 12 months: Arm 1: EPA (ALFA capsules: 2 x 500 mg/d = 2 g/d) and aspirin placebo (1/d) Arm 2: EPA placebo (capric and caprylic acid triglycerides: 2/d) and aspirin EC (1/d = 300 mg/d) Arm 3: EPA (ALFA capsules: 2 x 500 mg/d = 2 g/d) and aspirin EC (1/d = 300 mg/d) Arm 4: EPA placebo (capric and caprylic acid triglycerides: 2/d) and aspirin placebo (1/d) |
| Outcomes | Primary: number of participants with ≥ 1 adenomas at 12 months Secondary: adverse events, number of "advanced" adenomas per participant, number of "high risk" participants re‐classified as "intermediate risk", number participants with ≥ 1 advanced adenomas, adenoma region in the colorectum, total number of adenomas per participant, number of participants with colorectal cancer, levels of bioactive lipid mediators e.g. omega‐3 |
| Starting date | Trial Registration entry: 6 May 2011 Trial start date: 30 May 2011 Estimated trial completion: 31 July 2017 |
| Contact information | Mark Hull, Leeds Institute of Molecular Medicine, m.a.hull@leeds.ac.uk |
| Notes | ISRCTN05926847 EudraCT 2010‐020943‐10 www.seafood‐trial.co.uk |
UMIN000012825.
| Trial name or title | Effect of PUFA on vascular healing process in hypercholesterolemic patients with ACS |
| Methods | RCT |
| Participants | Hypercholesterolemic patients (20‐80 years) with acute coronary syndrome who have received successful optical coherence tomography (OCT)‐guided percutaneous coronary intervention (PCI) |
| Interventions | Each for 12 months: Arm 1: intensive lipid‐lowering therapy with both statin and EPA + DHA Arm 2: intensive lipid‐lowering therapy with both statin and EPA Arm 3: standard lipid‐lowering therapy with statins |
| Outcomes | Primary: changes in OCT parameter Secondary: lipids, serum plasma profile, inflammatory parameters, adverse cardiovascular events |
| Starting date | Registered on Trials Registry: 1 February 2014 Trial start date: 1 February 2014 Trial completion date est: 30 June 2019 |
| Contact information | Shiro Uemura (PI), Nara Medical University, Japan, suemura@naramed‐u.ac.jp |
| Notes | UMIN000012825 |
BMI: Body Mass Index; CETP: cholesteryl ester transfer protein; CVD: cardiovascular disease; DHA: docosahexaenoic acid;EPA: eicosapentaenoic acid;HDL: high density lipoprotein: LDL: low density lipoprotein:MI: myocardial infarction; MMSE: Mini Mental State Examination; PSA: prostate‐specific antigen; RCT: randomised controlled trial;
Differences between protocol and review
In the protocol we planned to omit small trials (that randomised fewer than 100 participants) due to concerns over small study bias and the consequent potential for random error to result in false positive conclusions (Roberts 2015).
To ensure that the largest body of RCT evidence was considered in the formulation of recommendations, the WHO NUGAG Subgroup on Diet and Health requested that trials of all sizes be included as long as they fit the other inclusion criteria.
To do this we re‐assessed all titles and abstracts in duplicate to ensure that we collected all smaller as well as larger trials, and carried out a sensitivity analysis omitting trials that had randomised fewer than 100 participants, as well as omitting trials that randomised fewer than 250 participants (this sensitivity analysis was already agreed).
We were also requested by WHO NUGAG Subgroup on Diet and Health to add the following sensitivity analyses:
only including trials with a low risk of bias from compliance, and
only including trials at low summary risk of bias.
We intended to assess causality (another aspect of performance bias, where a trial intervention included changes other than the change in PUFA intake, when there would be high risk of bias) but as we limited inclusion to trials where the dietary changes were limited to dietary fats this was not needed and so omitted. We also planned to assess whether a trial was pre‐registered on a trials register (registration date is before outcome data collection begins; Roberts 2015) but we incorporated the issue of pre‐registration into selective outcome reporting and did not use a separate form of assessment. We recorded funding data in the Characteristics of included studies and did not use them as a separate issue for assessing risk of bias, as recommended (Higgins 2011a).
Contributions of authors
LH conceived this review and wrote the first draft of the protocol; LH drafted the searches, which were developed, refined, run and de‐duplicated by CB. ASA, NM, CB, XW, JSB, TJB, SH, OFJ, SMAA, FS, KHOD and LH screened titles and abstracts; ASA, JSB, SMAA, SH, NM, XW and LH assessed full‐text papers for inclusion; LH, SH and JSB searched trials registers and assessed entries for inclusion; XW, NM, LH and ASA located full texts, LH and ASA managed assessment and collection of titles, abstracts and full texts, data extraction and 'Risk of bias' assessment; all authors carried out data extraction and assessed risk of bias. LH, KHOD and JSB designed 'Risk of bias' assessment; JSB, KHOD, SMAA, SH, TJB, ASA and LH wrote to trial authors; LH, KHOD, JSB, TJB and ASA carried out data checks; JSB, TJB, SMAA, XW, LH and ASA tabulated intake and status data. NM, CB, FS, KHOD, JSB and LH provided methodological support. ASA and LH entered data into Review Manager 5 and ran meta‐analyses, carried out sensitivity analyses and subgrouping. LH carried out the meta‐regression, wrote the first draft of the review, and wrote the World Health Organization report. LH and ASA carried out GRADE assessment and interpretation. All review authors critically read and commented on the final draft, and agreed it for submission. LH is the guarantor.
Sources of support
Internal sources
University of East Anglia, UK.
-
Cochrane Heart Group, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK
External sources
-
World Health Organization nutrition guidance expert advisory group (NUGAG), Switzerland.
WHO NUGAG Subgroup on Diet and Health requested and funded this systematic review
Declarations of interest
ASA: This review was funded by a grant from the World Health Organization. NM: None known CB: None known XW: This review was funded by a grant from the World Health Organization. JSB: This review was funded by a grant from the World Health Organization. TJB: This review was funded by a grant from the World Health Organization. SH: This review was funded by a grant from the World Health Organization. OFJ: This review was funded by a grant from the World Health Organization. SMAA: This review was funded by a grant from the World Health Organization. FS: This review was funded by a grant from the World Health Organization. KHOD: This review was funded by a grant from the World Health Organization. LH: This review was funded by a grant from the World Health Organization.
Edited (no change to conclusions)
References
References to studies included in this review
Ahn 2016 {published data only (unpublished sought but not used)}
- Ahn J, Park SK, Park TS, Kim JH, Yun E, Kim SP, et al. Effect of n‐3 polyunsaturated fatty acids on regression of coronary atherosclerosis in statin treated patients undergoing percutaneous coronary intervention. Korean Circulation Journal 2016;46(4):481‐9. [PUBMED: 27482256] [DOI] [PMC free article] [PubMed] [Google Scholar]
AlphaOmega ‐ ALA {published and unpublished data}
- Brouwer IA, Geleijnse JM, Klaasen VM, Smit LA, Giltay EJ, Goede J, et al. Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the Alpha Omega Trial. PloS One 2013;8(12):e81519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n‐3 fatty acids on major cardiovascular events in statin users and non‐users with a history of myocardial infarction. European Heart Journal 2012;33(13):1582‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleijnse J, Giltay E, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2011;1:S512. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized, double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2012;8(4):278‐87. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Schouten EG, Goede J, Oude Griep LM, Teitsma‐Jansen AM, et al. Effect of low doses of n‐3 fatty acids on cardiovascular diseases in 4,837 post‐myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. American Heart Journal 2010;159(4):539‐46. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Heijboer AC, Goede J, Oude Griep LM, Blankenstein MA, et al. No effects of n‐3 fatty acid supplementation on serum total testosterone levels in older men: the Alpha Omega Trial. International Journal of Andrology 2012;35(5):680‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Kromhout D. Effects of n‐3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. American Journal of Clinical Nutrition 2011;94(6):1442‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen E, Gemen E, Geleijnse M, Kusters R, Kromhout D, Giltay E. Effects of n‐3 fatty acids on decline of kidney function after myocardial infarction: Alpha Omega Trial. Nephrology, Dialysis, Transplantation 2012;27:ii64. [Google Scholar]
- Hoogeveen EK, Geleijnse JM, Kromhout D, Giltay EJ. No effect of n‐3 fatty acids on high‐sensitivity C‐reactive protein after myocardial infarction: the Alpha Omega Trial. European Journal of Preventive Cardiology 2014;21(11):1429‐36. [DOI] [PubMed] [Google Scholar]
- Hoogeveen EK, Geleijnse JM, Kromhout D, Stijnen T, Gemen EF, Kusters R, et al. Effect of omega‐3 fatty acids on kidney function after myocardial infarction: the Alpha Omega Trial. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(10):1676‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Geleijnse JM, Goede J, Oude Griep LM, Mulder BJ, Boer MJ, et al. N‐3 fatty acids, ventricular arrhythmia‐related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care 2011;34(12):2515‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Giltay EJ, Geleijnse JM. N‐3 fatty acids and cardiovascular events after myocardial infarction. New England Journal of Medicine 2010;363(18):2015‐26. [DOI] [PubMed] [Google Scholar]
- NCT00127452. Alpha Omega Trial: study of omega‐3 fatty acids and coronary mortality. clinicaltrials.gov/ct2/show/NCT00127452 (first posted 5 August 2005).
Bassey 2000‐Post {published data only}
- Bassey EJ, Littlewood JJ, Rothwell MC, Pye DW. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre‐ and postmenopausal women: two randomized controlled trials of Efacal v. calcium alone. British Journal of Nutrition 2000;83(6):629‐35. [PUBMED: 10911771] [DOI] [PubMed] [Google Scholar]
Bassey 2000‐Pre {published data only}
- Bassey EJ, Littlewood JJ, Rothwell MC, Pye DW. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre‐ and postmenopausal women: two randomized controlled trials of Efacal v. calcium alone. British Journal of Nutrition 2000;83(6):629‐35. [PUBMED: 10911771] [DOI] [PubMed] [Google Scholar]
Bates 1977 {published data only (unpublished sought but not used)}
- Bates D, Fawcett PR, Shaw DA, Weightman D. Trial of polyunsaturated fatty acids in non‐relapsing multiple sclerosis. British Medical Journal 1977;2(6092):932‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Bates D, Millar JH, Paty DW. Linoleic acid and multiple sclerosis: a reanalysis of three double‐blind trials. Neurology 1984;34(11):1441‐5. [DOI] [PubMed] [Google Scholar]
Bates 1978 {published data only (unpublished sought but not used)}
- Bates D, Fawcett PR, Shaw DA, Weightman D. Polyunsaturated fatty acids in treatment of acute remitting multiple sclerosis. British Medical Journal 1978;2(6149):1390‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Bates D, Millar JH, Paty DW. Linoleic acid and multiple sclerosis: a reanalysis of three double‐blind trials. Neurology 1984;34(11):1441‐5. [DOI] [PubMed] [Google Scholar]
Bates 1989 {published data only (unpublished sought but not used)}
- Bates D, Cartlidge NE, French JM, Jackson MJ, Nightingale S, Shaw DA, et al. A double‐blind controlled trial of long chain n‐3 polyunsaturated fatty acids in the treatment of multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 1989;52(1):18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 1994 {published and unpublished data}
- Black HS, Herd JA, Goldberg LH, Wolf‐JE J, Thornby JI, Rosen T, et al. Effect of a low‐fat diet on the incidence of actinic keratosis. New England Journal of Medicine 1994;330(18):1272‐5. [DOI] [PubMed] [Google Scholar]
- Black HS, Thornby JI, Wolf‐JE J, Goldberg LH, Herd JA, Rosen T, et al. Evidence that a low‐fat diet reduces the occurrence of non‐melanoma skin cancer. International Journal of Cancer 1995;62(2):165‐9. [DOI] [PubMed] [Google Scholar]
- Jaax S, Scott LW, Wolf‐JE J, Thornby JI, Black HS. General guidelines for a low‐fat diet effective in the management and prevention of nonmelanoma skin cancer. Nutrition and Cancer 1997;27(2):150‐6. [DOI] [PubMed] [Google Scholar]
Brox 2001 {published and unpublished data}
- Brox J, Olaussen K, Osterud B, Elvevoll EO, Bjornstad E, Brattebog G, et al. A long‐term seal‐ and cod‐liver‐oil supplementation in hypercholesterolemic subjects. Lipids 2001;36(1):7‐13. [DOI] [PubMed] [Google Scholar]
DART fat 1989 {published and unpublished data}
- Burr ML, Fehily AM. Fish and the heart. Lancet 1989;ii:1450‐2. [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2(8666):757‐61. [DOI] [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Rogers S, Welsby E, King S, Sandham S. Diet and reinfarction trial (DART): design, recruitment, and compliance. European Heart Journal 1989;10(6):558‐67. [DOI] [PubMed] [Google Scholar]
- Burr ML, Holliday RM, Fehily AM, Whitehead PJ. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART). European Heart Journal 1992;13(2):166‐70. [DOI] [PubMed] [Google Scholar]
- Burr ML, Sweetham PM, Fehily AM. Diet and reinfarction [letter]. European Heart Journal 1994;15(8):1152‐3. [DOI] [PubMed] [Google Scholar]
- Fehily AM, Vaughan‐Williams E, Shiels K, Williams AH, Horner M, Bingham G, et al. The effect of dietary advice on nutrient intakes: evidence from the diet and reinfarction trial (DART). Journal of Human Nutrition & Dietetics 1989;2(4):225‐5. [Google Scholar]
DIPP‐Tokudome 2015 {published and unpublished data}
- Tokudome S, Kuriki K, Yokoyama Y, Sasaki M, Joh T, Kamiya T, et al. Dietary n‐3/long‐chain n‐3 polyunsaturated fatty acids for prevention of sporadic colorectal tumors: a randomized controlled trial in polypectomized participants. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2015;94:1‐11. [PUBMED: 25451556] [DOI] [PubMed] [Google Scholar]
- Tokudome S, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Kuriki K, et al. Rationale and study design of dietary intervention in patients polypectomized for tumors of the colorectum. Japanese Journal of Clinical Oncology 2002;32(12):550‐3. [PUBMED: 12578906] [DOI] [PubMed] [Google Scholar]
- UMIN000000461. Dietary intervention in patients polypectomized for tumors of the colorectum. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000000534 (date of disclosure 3 August 2006).
Dodin 2005 {published and unpublished data}
- Dodin S, Cunnane SC, Masse B, Lemay A, Jacques H, Asselin G, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: a randomized, double‐blind, placebo‐controlled trial. Nutrition (Burbank, Los Angeles County, Calif.) 2008;24(1):23‐30. [PUBMED: 17981439] [DOI] [PubMed] [Google Scholar]
- Dodin S, Lemay A, Jacques H, Legare F, Forest JC, Masse B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: a randomized, double‐blind, wheat germ placebo‐controlled clinical trial. Journal of Clinical Endocrinology and Metabolism 2005;90(3):1390‐7. [PUBMED: 15613422] [DOI] [PubMed] [Google Scholar]
Doi 2014 {published data only (unpublished sought but not used)}
- Doi M, Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, et al. Clinical outcomes of early initiation of pure eicosapentaenoic acid supplement after percutaneous coronary intervention in patients with acute coronary syndrome. European Heart Journal 2014;35(Abstract Supplement):1156. [Google Scholar]
- Doi M, Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, et al. Early eicosapentaenoic acid treatment after percutaneous coronary intervention reduces acute inflammatory responses and ventricular arrhythmias in patients with acute myocardial infarction: a randomized, controlled study. International Journal of Cardiology 2014;176(3):577‐82. [PUBMED: 25305703] [DOI] [PubMed] [Google Scholar]
- Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, Tsukuda S, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1‐year outcomes of a randomized controlled study. International Journal of Cardiology 2017;228:173‐9. [DOI: 10.1016/j.ijcard.2016.11.105] [DOI] [PubMed] [Google Scholar]
- Nosaka K, Miyoshi T, Okawa K, Tsukuda S, Sogo M, Nishibe T, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1‐year outcomes of a randomized controlled study. Journal of the American College of Cardiology 2016;67:573. [DOI] [PubMed] [Google Scholar]
- UMIN000016723. Clinical effect of early loading of eicosepentaenoic acid after percutaneous coronary intervention in patients with acute coronary syndrome: a prospective, open‐labeled, randomized controlled clinical trial. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000019385 (date of disclosure 1 April 2015).
Dullaart 1992 {published and unpublished data}
- Dullaart RP, Beusekamp BJ, Meijer S, Hoogenberg K, Doormaal JJ, Sluiter WJ. Long‐term effects of linoleic‐acid‐enriched diet on albuminuria and lipid levels in type 1 (insulin‐dependent) diabetic patients with elevated urinary albumin excretion. Diabetologia 1992;35(2):165‐72. [PUBMED: 1547922] [DOI] [PubMed] [Google Scholar]
EPIC‐1 2008 {published and unpublished data}
- Feagan BG, Sandborn WJ, Mittmann U, Bar‐Meir S, D'Haens G, Bradette M, et al. Omega‐3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA 2008;299(14):1690‐7. [DOI] [PubMed] [Google Scholar]
- NCT00613197. EPANOVA in Crohn's disease, study 1 (EPIC‐1). clinicaltrials.gov/ct2/show/NCT00613197 (first posted 12 February 2008).
EPIC‐2 2008 {published and unpublished data}
- Feagan BG, Sandborn WJ, Mittmann U, Bar‐Meir S, D'Haens G, Bradette M, et al. Omega‐3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA 2008;229(14):1690‐7. [DOI] [PubMed] [Google Scholar]
- NCT00074542. An efficacy and safety study of omega‐3 free fatty acids (Epanova) for the maintenance of symptomatic remission in subjects with Crohn's disease. clinicaltrials.gov/ct2/show/NCT00074542 (first posted 16 December 2003).
EPOCH 2011 {published and unpublished data}
- ACTRN12607000278437. Older people, omega‐3, and cognitive health [An 18 month study investigating the effects of long chain omega‐3 polyunsaturated fatty acids supplementation on cognition and wellbeing in older people]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12607000278437 (date registered 24 May 2007).
- Danthiir V, Burns NR, Nettelbeck T, Wilson C, Wittert G. The older people, omega‐3, and cognitive health (EPOCH) trial design and methodology: a randomised, double‐blind, controlled trial investigating the effect of long‐chain omega‐3 fatty acids on cognitive ageing and wellbeing in cognitively healthy older adults. Nutrition Journal 2011;10:117. [PUBMED: 22011460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthiir V, Hosking D, Burns NR, Wilson C, Nettelbeck T, Calvaresi E, et al. Cognitive performance in older adults is inversely associated with fish consumption but not erythrocyte membrane n‐3 fatty acids. Journal of Nutrition 2014;144(3):311‐20. [PUBMED: 24353345] [DOI] [PubMed] [Google Scholar]
FAAT ‐ Leaf 2005 {published and unpublished data}
- Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, et al. Prevention of fatal arrhythmias in high‐risk subjects by fish oil n‐3 fatty acid intake. Circulation 2005;112(18):2762‐8. [DOI] [PubMed] [Google Scholar]
- NCT00004559. Fatty Acid Antiarrhythmia Trial (FAAT). clinicaltrials.gov/ct2/show/NCT00004559 (first posted 10 February 2000).
GLAMT 1993 {published data only (unpublished sought but not used)}
- Keen H, Payan J, Allawi J, Walker J, Jamal GA, Weir AI, et al. Treatment of diabetic neuropathy with gamma‐linolenic acid. Diabetes Care 1993;16(1):8‐15. [DOI] [PubMed] [Google Scholar]
HARP‐ Sacks 1995 {published and unpublished data}
- NCT00000461. Harvard Atherosclerosis Reversibility Project (HARP). clinicaltrials.gov/ct2/show/NCT00000461 (first posted 28 October 1999).
- Pasternak RC, Brown LE, Stone PH, Silverman DI, Gibson CM, Sacks FM. Effect of combination therapy with lipid‐reducing drugs in patients with coronary heart disease and "normal" cholesterol levels. A randomized, placebo‐controlled trial. Annals of Internal Medicine 1996;125(7):529‐40. [PUBMED: 8815751] [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pasternak RC, Gibson CM, Rosner B, Stone PH. Effect on coronary atherosclerosis of decrease in plasma cholesterol concentrations in normocholesterolaemic patients. Lancet 1994;344(8931):1182‐6. [PUBMED: 7934538] [DOI] [PubMed] [Google Scholar]
- Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC. Controlled trial of fish oil for regression of human coronary atherosclerosis. Journal of the American College of Cardiology 1995;25(7):1492‐8. [DOI] [PubMed] [Google Scholar]
HERO‐Tapsell 2009 {published and unpublished data}
- ACTRN12607000600448. Healthy eating to reduce overweight in people with type 2 diabetes [The role of walnuts in satiety and energy balance in overweight individuals with type 2 diabetes mellitus]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82353 (date registered 21 November 2007).
- Tan SY. Dietary Manipulation and Weight Management [thesis]. Wollongong (Australia): School of Health Sciences, University of Wollongong, 2010. [Google Scholar]
- Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, et al. Long‐term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. European Journal of Clinical Nutrition 2009;63(8):1008‐15. [PUBMED: 19352378] [DOI] [PubMed] [Google Scholar]
Houtsmuller 1979 {published data only (unpublished sought but not used)}
- Houtsmuller AJ, Hal‐Ferwerda J, Zahn KJ, Henkes HE. Favourable influences of linoleic acid on the progression of diabetic micro‐ and macroangiopathy. Nutrition and Metabolism 1980;24(Suppl 1):105‐18. [DOI] [PubMed] [Google Scholar]
- Houtsmuller AJ, Hal‐Ferwerda J, Zahn KJ, Henkes HE. Influence of different diets on the progression of diabetic retinopathy. Progress in Food Nutritition and Science 1980;4(5):41‐6. [PubMed] [Google Scholar]
- Houtsmuller AJ, Zahn KJ, Henkes HE. Unsaturated fats and progression of diabetic retinopathy. Documenta Ophthalmologia 1979;48(2):363‐71. [DOI] [PubMed] [Google Scholar]
Kumar 2012 {published data only (unpublished sought but not used)}
- Kumar S, Sutherland F, Morton JB, Lee G, Morgan J, Wong J, et al. Long‐term omega‐3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm 2012;9(4):483‐91. [DOI: 10.1016/j.hrthm.2011.11.034] [DOI] [PubMed] [Google Scholar]
- NCT00232219. Use of fish oils to reduce recurrence of atrial fibrillation following DC cardioversion. clinicaltrials.gov/ct2/show/NCT00232219 (first posted 4 October 2005).
Kumar 2013 {published data only}
- Kumar S, Sutherland F, Stevenson I, Lee JM, Garg ML, Sparks PB. Effects of long‐term omega‐3 polyunsaturated fatty acid supplementation on paroxysmal atrial tachyarrhythmia burden in patients with implanted pacemakers: results from a prospective randomised study. International Journal of Cardiology 2013;168(4):3812‐7. [PUBMED: 23890856] [DOI] [PubMed] [Google Scholar]
- NCT00232245. Use of fish oils to reduce the frequency and duration of episodes of atrial fibrillation in patients with paroxysmal atrial fibrillation. clinicaltrials.gov/ct2/show/NCT00232245 (first posted 4 October 2005).
Ley 2004 {published and unpublished data}
- Ley SJ, Metcalf PA, Scragg RKR, Swinburn BA. Long‐term effects of a reduced fat diet intervention on cardiovascular disease risk factors in individuals with glucose intolerance. Diabetes Research and Clinical Practice 2004;63:103‐12. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Metcalf PA, Ley SJ. Long‐term (5‐year) effects of a reduced‐fat diet intervention in individuals with glucose intolerance. Diabetes Care 2001;24(4):619‐24. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Woollard GA, Chang EC, Wilson MR. Effects of reduced‐fat diets consumed ad libitum on intake of nutrients particularly antioxidant vitamins. Journal of the American Dietetic Association 1999;99(11):1400‐5. [DOI] [PubMed] [Google Scholar]
MARINA ‐ Sanders 2011 {published and unpublished data}
- Al‐Hilal M, Alsaleh A, Maniou Z, Lewis FJ, Hall WL, Sanders TA, et al. Genetic variation at the FADS1‐FADS2 gene locus influences delta‐5 desaturase activity and LC‐PUFA proportions after fish oil supplement. Journal of Lipid Research 2013;54(2):542‐51. [PUBMED: 23160180] [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSaleh A, Maniou Z, Lewis FJ, Hall WL, Sanders TA, O'Dell SD. Interaction between a CSK gene variant and fish oil intake influences blood pressure in healthy adults. Journal of Nutrition 2014;144(3):267‐72. [PUBMED: 24401815] [DOI] [PubMed] [Google Scholar]
- Alsaleh A, Crepostnaia D, Maniou Z, Lewis FJ, Hall WL, Sanders TA, et al. Adiponectin gene variant interacts with fish oil supplementation to influence serum adiponectin in older individuals. Journal of Nutrition 2013;143(7):1021‐7. [PUBMED: 23658423] [DOI] [PubMed] [Google Scholar]
- Hall WL, Hay G, Maniou Z, Seed PT, Chowienczyk PJ, Sanders TA. Effect of low doses of long chain n‐3 polyunsaturated fatty acids on sleep‐time heart rate variability: a randomized, controlled trial. International Journal of Cardiology 2013;168:4439‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN66664610. Vascular effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA): the MARINA study. www.isrctn.com/ISRCTN66664610 (date assigned 17 December 2008).
- Pinto AM, Hall WL, Sanders TAB. Effect of low doses of long chain n‐3 PUFA intake on daytime heart rate variability: results from the MARINA study. European Journal of Preventive Cardiology 2015;1:S146. [Google Scholar]
- Proudman S, Spargo L, Hall C, McWilliams L, Lee A, Maureen R, et al. Fish oil in rheumatoid arthritis: a randomised, double blind trial comparing high dose with low dose. Internal Medicine Journal 2012;42:2‐3. [Google Scholar]
- Sanders TA, Hall WL, Maniou Z, Lewis F, Seed PT, Chowienczyk PJ. Effect of low doses of long‐chain n‐3 PUFAs on endothelial function and arterial stiffness: a randomized controlled trial. American Journal of Clinical Nutrition 2011;94(4):973‐80. [PUBMED: 21865334] [DOI] [PubMed] [Google Scholar]
- Sanders TAB, Chowienczyk PJ, Hall W, Lewis F, Seed P, Maniou Z, et al. The influences of increasing intakes of EPA and DHA on vascular function and risk factors for cardiovascular disease. Food Standards Agency Project N02041. Final Report 2011.
McIllmurray 1987 {published data only (unpublished sought but not used)}
- McIllmurray MB, Turkie W. Controlled trial of gamma linolenic acid in Duke's C colorectal cancer. BMJ (Clinical Research Ed.) 1987;294(6582):1260 (Erratum in: BMJ 1987;295(6596):475). [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendis 2001 {published data only (unpublished sought but not used)}
- Mendis S, Samarajeewa U, Thattil RO. Coconut fat and serum lipoproteins: effects of partial replacement with unsaturated fats. British Journal of Nutrition 2001;85(5):583‐9. [DOI] [PubMed] [Google Scholar]
Mita 2007 {published data only}
- Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, et al. Eicosapentaenoic acid reduces the progression of carotid intima‐media thickness in patients with type 2 diabetes. Atherosclerosis 2007;191(1):162‐7. [DOI] [PubMed] [Google Scholar]
MRC 1968 {published and unpublished data}
- Ederer F, Leren P, Turpeinen O, Frantz ID Jr. Cancer among men on cholesterol lowering diets: experience of five clinical trials. Lancet 1971;2:203‐6. [DOI] [PubMed] [Google Scholar]
- Heady JA. Are PUFA harmful?. British Medical Journal 1974;1(898):115‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC. Controlled trial of soya‐bean oil in myocardial infarction; report of a medical research committee to the Medical Research Council. Lancet 1968;2(570):693‐9. [PubMed] [Google Scholar]
NDHS Faribault 1968 {published data only}
- The National Diet‐Heart Study. Nutrition Reviews 1968; Vol. 26, issue 5:133‐6. [DOI] [PubMed]
- The National Diet‐Heart Study final report. Circulation 1968; Vol. 37, issue II:1‐428. [PubMed]
- Baker BM, Frantz ID Jr, Keys A, Kinsell LW, Page IH, Stamler J, et al. The National Diet‐Heart Study: an initial report. JAMA 1963;185:105‐6. [DOI] [PubMed] [Google Scholar]
- Brown HB. The National Diet Heart Study ‐ implications for dietitians and nutritionists. Journal of the American Dietetic Association 1968;52:279‐87. [PubMed] [Google Scholar]
- Ederer F, Leren P, Turpeinen O, Frantz ID Jr. Cancer among men on cholesterol‐lowering diets: experience from five clinical trials. Lancet 1971;298(7717):203‐6. [DOI] [PubMed] [Google Scholar]
- Page IH, Brown HB. Some observations on the National Diet‐Heart Study. Circulation 1968;37:313‐5. [DOI] [PubMed] [Google Scholar]
NDHS Open 1st 1968 {published data only}
- The National Diet‐Heart Study. Nutrition Reviews 1968; Vol. 26, issue 5:133‐6. [DOI] [PubMed]
- The National Diet‐Heart Study final report. Circulation 1968; Vol. 37, issue II:1‐428. [PubMed]
- Baker BM, Frantz ID Jr, Keys A, Kinsell LW, Page IH, Stamler J, et al. The National Diet‐Heart Study: an initial report. JAMA 1963;185:105‐6. [DOI] [PubMed] [Google Scholar]
- Brown HB. The National Diet Heart Study ‐ implications for dietitians and nutritionists. Journal of the American Dietetic Association 1968;52:279‐87. [PubMed] [Google Scholar]
- Ederer F, Leren P, Turpeinen O, Frantz ID Jr. Cancer among men on cholesterol‐lowering diets: experience from five clinical trials. Lancet 1971;298(7717):203‐6. [DOI] [PubMed] [Google Scholar]
- Page IH, Brown HB. Some observations on the National Diet‐Heart Study. Circulation 1968;37:313‐5. [DOI] [PubMed] [Google Scholar]
Nodari 2011 AF {published data only (unpublished sought but not used)}
- NCT01198275. N‐3 polyunsaturated fatty acids (PUFAs) in the prevention of atrial fibrillation. clinicaltrials.gov/ct2/show/NCT01198275 (first posted 21 July 2011).
- Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, et al. N‐3 polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation 2011;124(10):1100‐6. [PUBMED: 21844082] [DOI] [PubMed] [Google Scholar]
Nodari 2011 HF {published and unpublished data}
- NCT01223703. PUFAs and left ventricular function in heart failure (CS‐PUFA‐02). clinicaltrials.gov/ct2/show/NCT01223703 (first posted 19 October 2010).
- NCT01223703. PUFAs and left ventricular function in heart failure (CS‐PUFA‐02). clinicaltrials.gov/ct2/show/results/NCT01223703 (first posted 19 October 2010).
- Nodari S, Triggiani M, Berlinghieri N, Milesi G, Foresti A, Gheorghiade M, et al. Effects of n‐3 polyunsaturated fatty acids on left ventricular function and functional capacity in heart failure patients. European Heart Journal 2010;31:850. [Google Scholar]
- Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, et al. Effects of n‐3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. Journal of the American College of Cardiology 2011;57(7):870‐9. [DOI] [PubMed] [Google Scholar]
- Nodari S, Triggiani M, Campia U, Zhao L, Manerba A, Milesi G, et al. Plasma levels of n‐3 polyunsaturated fatty acids and risk of hospitalization in patients with non‐ischemic cardiomyopathy. Circulation 2012;126(21 SUPPL 1):17431. [Google Scholar]
Nye 1990 {published data only}
- Ilsey CDJ, Nye ER, Sutherland W, Ram J, Ablett MB. Randomised placebo controlled trial of MAXEPA and aspirin/persantin after successful coronary angioplasty. Australian & New Zealand Journal of Medicine 1987;17:559. [Google Scholar]
- Nye ER, Ablett MB, Robertson MC, Ilsley CD, Sutherland WH. Effect of eicosapentaenoic acid on restenosis rate, clinical course and blood lipids in patients after percutaneous transluminal coronary angioplasty. Australian and New Zealand Journal of Medicine 1990;20(4):549‐52. [DOI] [PubMed] [Google Scholar]
ORL 2013 {published data only (unpublished sought but not used)}
- NCT01350999. Long‐term efficacy and safety study of TAK‐085 in participants with hypertriglyceridemia. clinicaltrials.gov/ct2/show/NCT01350999 (first posted 10 May 2011).
- Tatsuno I, Saito Y, Kudou K, Ootake J. Long‐term safety and efficacy of TAK‐085 in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega‐3 fatty acids randomized long‐term (ORL) study. Journal of Clinical Lipidology 2013;7(6):615‐25. [PUBMED: 24314359] [DOI] [PubMed] [Google Scholar]
PREDIMED 2013 {published data only (unpublished sought but not used)}
- PREDIMED study ‐ Mediteranian diet in the primary prevention of cardiovascular disease: research protocol, version 1. www.predimed.es/uploads/8/0/5/1/8051451/_1estr_protocol_olf.pdf (accessed prior to 11 April 2018).
- Alvarez‐Perez J, Sanchez‐Villegas A, Diaz‐Benitez EM, Ruano‐Rodriguez C, Corella D, Martinez‐Gonzalez MA, et al. Influence of a Mediterranean dietary pattern on body fat distribution: results of the PREDIMED‐Canarias intervention randomized trial. Journal of the American College of Nutrition 2016;35(6):1‐13. [DOI] [PubMed] [Google Scholar]
- Babio N, Toledo E, Estruch R, Ros E, Martinez‐Gonzalez MA, Castaner O, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ Canadian Medical Association Journal 2014;186(17):E649‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo F, Perona JS, Prades J, Funari SS, Gomez‐Gracia E, Conde M, et al. Mediterranean‐style diet effect on the structural properties of the erythrocyte cell membrane of hypertensive patients: the Prevencion con Dieta Mediterranea Study. Hypertension 2009;54(5):1143‐50. [DOI] [PubMed] [Google Scholar]
- Bullo M, Amigo‐Correig P, Marquez‐Sandoval F, Babio N, Martinez‐Gonzalez MA, Estruch R, et al. Mediterranean diet and high dietary acid load associated with mixed nuts: effect on bone metabolism in elderly subjects. Journal of the American Geriatrics Society 2009;57(10):1789‐98. [DOI] [PubMed] [Google Scholar]
- Casas R, Sacanella E, Urpi‐Sarda M, Chiva‐Blanch G, Ros E, Martinez‐Gonzalez MA, et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PloS One 2014;9(6):e100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas RM, Urpi‐Sarda M, Chiva‐Blanch G, Valderas‐Martinez P, Arranz S, Roth I, et al. Inhibition of circulating immune cells related to atherogenesis intervention after 1, 3 and 5 years with Mediterranean diet. Annals of Nutrition and Metabolism 2013;62:43‐4. [Google Scholar]
- Cohen J, Rimm EB, Martinez‐Gonzalez MA, Salas‐Salvado J, Covas MI, Corella D, et al. The association between obesity status and long‐term adherence to Mediterranean diet in the PREDIMED trial. Circulation 2013;127(12 Meeting Abstracts):AP098. [Google Scholar]
- Damasceno NR, Sala‐Vila A, Cofan M, Perez‐Heras AM, Fito M, Ruiz‐Gutierrez V, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013;230(2):347‐53. [DOI] [PubMed] [Google Scholar]
- Domenech M, Estruch R, Ros E, Coca A. Effect of the Mediterranean diet on blood pressure: the ambulatory blood pressure substudy (Predimed‐ABPM). Journal of Hypertension 2010;28:e373. [Google Scholar]
- Domenech M, Roman P, Lapetra J, Garcia de la Corte FJ, Sala‐Vila A, Torre R, et al. Mediterranean diet reduces 24‐hour ambulatory blood pressure, blood glucose, and lipids: one‐year randomized, clinical trial. Hypertension 2014;64(1):69‐76. [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. New England Journal of Medicine 2013;368(14):1279‐90 (Erratum in: New England Journal of Medicine 2014;370(9):886). [DOI: 10.1056/NEJMoa1200303] [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas‐Salvadó J, Covas M, Corella D, Arós F, et al. Retraction and republication: Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368:1279‐90. NEJM 2018;378:25. [DOI: 10.1056/NEJMc1805501] [DOI] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts [this is a republication of primary paper Estruch 2013]. New England Journal of Medicine 2018;378:e34. [DOI: 10.1056/NEJMoa1800389] [DOI] [PubMed] [Google Scholar]
- ISRCTN35739639. Effects of Mediterranean diet on the primary prevention of cardiovascular disease. www.isrctn.com/ISRCTN35739639 (date assigned 5 October 2005).
- Martínez‐González MÁ, Corella D, Salas‐Salvadó J, Ros E, Covas MI, Fiol M, et al. Cohort profile: design and methods of the PREDIMED study. International Journal of Epidemiology 2012;41(2):377‐85. [DOI: 10.1093/ije/dyq250] [DOI] [PubMed] [Google Scholar]
- Papadaki A, Martínez‐González MÁ, Alonso‐Gómez A, Rekondo J, Salas‐Salvadó J, Corella D, et al. Mediterranean diet and risk of heart failure: results from the PREDIMED randomized controlled trial. European Journal of Heart Failure 2017;19(9):1179‐85. [DOI: 10.1002/ejhf.750] [DOI] [PubMed] [Google Scholar]
- Razquin C, Martinez JA, Martinez‐Gonzalez MA, Mitjavila MT, Estruch R, Marti A. A 3 years follow‐up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. European Journal of Clinical Nutrition 2009;63(12):1387‐93. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Canela M, Estruch R, Corella D, Salas‐Salvado J, Martinez‐Gonzalez MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA 2014;311(4):415‐7. [DOI] [PubMed] [Google Scholar]
- Sala‐Vila A, Romero‐Mamani ES, Gilabert R, Nunez I, Torre R, Corella D, et al. Changes in ultrasound‐assessed carotid intima‐media thickness and plaque with a Mediterranean diet: a substudy of the PREDIMED trial. Arteriosclerosis, Thrombosis & Vascular Biology 2014;34(2):439‐45. [DOI] [PubMed] [Google Scholar]
- Salas‐Salvado J, Bullo M, Babio N, Martinez‐Gonzalez MA, Ibarrola‐Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED‐Reus nutrition intervention randomized trial. Diabetes Care 2011;34(1):14‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas‐Salvado J, Bullo M, Estruch R, Ros E, Covas MI, Ibarrola‐Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Annals of Internal Medicine 2014;160(1):1‐10. [DOI] [PubMed] [Google Scholar]
- Salas‐Salvado J, Fernandez‐Ballart J, Ros E, Martinez‐Gonzalez MA, Fito M, Estruch R, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one‐year results of the PREDIMED randomized trial. Archives of Internal Medicine 2008;168(22):2449‐58. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Villegas A, Martinez‐Gonzalez MA, Estruch R, Salas‐Salvado J, Corella D, Covas MI, et al. Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Medicine 2013;11:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola R, Fito M, Estruch R, Salas‐Salvado J, Corella D, Torre R, et al. Effect of a traditional Mediterranean diet on apolipoproteins B, A‐I, and their ratio: a randomized, controlled trial. Atherosclerosis 2011;218(1):174‐80. [DOI] [PubMed] [Google Scholar]
- Toledo E, Hu FB, Estruch R, Buil‐Cosiales P, Corella D, Salas‐Salvado J, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Medicine 2013;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo E, Salas‐Salvado J, Donat‐Vargas C, Buil‐Cosiales P, Estruch R, Ros E, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED Trial: a randomized clinical trial. JAMA Internal Medicine 2015;175(11):1752‐60. [DOI] [PubMed] [Google Scholar]
- Valls‐Pedret C, Sala‐Vila A, Serra‐Mir M, Corella D, Torre R, Martinez‐Gonzalez MA, et al. Mediterranean diet and age‐related cognitive decline: a randomized clinical trial. JAMA Internal Medicine 2015;175(7):1094‐103. [DOI] [PubMed] [Google Scholar]
Proudman 2015 {published and unpublished data}
- ACTRN12613000579796. Fish oil in recent onset rheumatoid arthritis: High versus low dose fish oil on a background of dose‐responsive combination disease‐modifying anti‐rheumatic drugs [Fish oil in anti‐inflammatory doses in recent onset rheumatoid arthritis: a randomized, double‐blind controlled trial within algorithm‐based drug use with DMARD use as the primary outcome]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364265 (date registered 23 May 2013).
- Proudman S, Spargo L, Hall C, McWilliams L, Lee A, Maureen R, et al. Fish oil in rheumatoid arthritis: a randomised, double blind trial comparing high dose with low dose. Internal Medicine Journal 2012;42(Suppl 1):2‐3. [Google Scholar]
- Proudman SM, Cleland LG, Metcalf RG, Sullivan TR, Spargo LD, James MJ. Plasma n‐3 fatty acids and clinical outcomes in recent‐onset rheumatoid arthritis. British Journal of Nutrition 2015;114(6):885‐90. [PUBMED: 26283657] [DOI] [PubMed] [Google Scholar]
- Proudman SM, James MJ, Spargo LD, Metcalf RG, Sullivan TR, Rischmueller M, et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double‐blind controlled trial within algorithm‐based drug use. Annals of the Rheumatic Diseases 2015;74(1):89‐95. [PUBMED: 24081439] [DOI] [PubMed] [Google Scholar]
Puri 2005 {published and unpublished data}
- ISRCTN79170611. A multicentre, multinational, double blind, randomised, parallel group, placebo‐controlled study of ethyl‐eicosapentaenoate (EPA) in patients with Huntington's disease (HD). doi.org/10.1186/ISRCTN79170611 (date assigned 3 February 2003).
- Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, et al. Ethyl‐EPA in Huntington disease: a double‐blind, randomized, placebo‐controlled trial. Neurology 2005;65(2):286‐92. [DOI] [PubMed] [Google Scholar]
Raitt 2005 {published data only (unpublished sought but not used)}
- NCT00004558. Antiarrhythmic effects of n‐3 fatty acids. clinicaltrials.gov/ct2/show/NCT00004558 (first posted 10 February 2000).
- Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA 2005;293(23):2884‐91. [DOI] [PubMed] [Google Scholar]
Rose 1965 {published data only}
- Rose GA, Thomson WB, Williams RT. Corn oil in treatment of ischaemic heart diseases. British Medical Journal 1965;1(5449):1531‐3. [PUBMED: 14288105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossing 1996 {published and unpublished data}
- Myrup B, Rossing P, Jensen T, Parving HH, Holmer G, Gram J, et al. Lack of effect of fish oil supplementation on coagulation and transcapillary escape rate of albumin in insulin‐dependent diabetic patients with diabetic nephropathy. Scandinavian Journal of Clinical & Laboratory Investigation 2001;61(5):349‐56. [DOI] [PubMed] [Google Scholar]
- Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH. Fish oil in diabetic nephropathy. Diabetes Care 1996;19(11):1214‐9. [DOI] [PubMed] [Google Scholar]
Simon 1997 {published and unpublished data}
- Djuric Z, Heilbrun LK, Reading BA, Boomer A, Valeriote FA, Martino S. Effects of a low fat diet on levels of oxidative damage to DNA in human peripheral nucleated blood cells. Journal of the National Cancer Institute 1991;83(11):766‐9. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Martino S, Heilbrun LK, Hart RW. Dietary modulation of oxidative DNA damage. Advances In Experimental Medicine and Biology 1994;354:71‐83. [DOI] [PubMed] [Google Scholar]
- Kasim SE, Martino S, Kim P‐N, Khilnani S, Boomer A, Depper J, et al. Dietary and anthropometric determinants of plasma lipoproteins during a long‐term low‐fat diet in healthy women. American Journal of Clinical Nutrition 1993;57:146‐53. [DOI] [PubMed] [Google Scholar]
- Simon MS, Heilbrun LK, Boomer A, Kresge C, Depper J, Kim PN, et al. A randomised trial of a low‐fat dietary intervention in women at high risk for breast cancer. Nutrition and Cancer 1997;27(2):136‐42. [DOI] [PubMed] [Google Scholar]
Sydney Diet‐Heart 1978 {published and unpublished data}
- Blacket RB, Leelarthaepin B, McGilchrist C, Palmer AJ, Woodhill JM. The synergistic effect of weight loss and changes in dietary lipids on the serum cholesterol of obese men with hypercholesterolaemia: implications for prevention of coronary heart disease. Australian and New Zealand Journal of Medicine 1979;9:521‐9. [DOI] [PubMed] [Google Scholar]
- Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak‐Hong SF, Faurot KR, Suchindran CM, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta‐analysis. BMJ 2013;346:e8707 (Erratum in: BMJ 2013;346:f903). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhill JM, Palmer AJ, Leelarthaepin B, McGilchrist C, Blacket RB. Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Advances in Experimental Medicine and Biology 1978;109:317‐30. [DOI] [PubMed] [Google Scholar]
Veterans Admin 1969 {published data only}
- Dayton S, Hashimoto S, Dixon W, Pearce ML. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. Journal of Lipid Research 1966;7:103‐11. [PubMed] [Google Scholar]
- Dayton S, Hashimoto S, Pearce ML. Adipose tissue linoleic acid as a criterion of adherence to a modified diet. Journal of Lipid Research 1967;8:508‐10. [PubMed] [Google Scholar]
- Dayton S, Hashimoto S, Pearce ML. Influence of a diet high in unsaturated fat upon composition of arterial tissue and atheromata in man. Circulation 1965;32:911‐24. [DOI] [PubMed] [Google Scholar]
- Dayton S, Hashimoto S, Rosenblum D, Pearce M. Vitamin E status of humans during prolonged feeding of unsaturated fats. Journal of Laboratory and Clinical Medicine 1965;65(5):739‐47. [PubMed] [Google Scholar]
- Dayton S, Pearce ML. Diet and atherosclerosis. Lancet 1970;1(644):473‐4. [DOI] [PubMed] [Google Scholar]
- Dayton S, Pearce ML. Diet and cardiovascular diseases. Lancet 1969;1(584):51‐2. [DOI] [PubMed] [Google Scholar]
- Dayton S, Pearce ML. Diet high in unsaturated fat: a controlled clinical trial. Minnesota Medicine 1969;1969:1237‐42. [DOI] [PubMed] [Google Scholar]
- Dayton S, Pearce ML. Prevention of coronary heart disease and other complications of atherosclerosis by modified diet. American Journal of Medicine 1969;46:751‐62. [DOI] [PubMed] [Google Scholar]
- Dayton S, Pearce ML. Trial of unsaturated‐fat diet. Lancet 1968;2(581):1296‐7. [DOI] [PubMed] [Google Scholar]
- Dayton S, Pearce ML, Goldman H, Harnish A, Plotkin D, Shickman M, et al. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet 1968;2(577):1060‐2. [DOI] [PubMed] [Google Scholar]
- Dayton S, Pearce ML, Hashimoto S, Dixon WJ, Tomayasu U. A controlled clinical trial of a diet high in unsaturated fat in preventing complications of atherosclerosis. Circulation 1969;15(1, Suppl 2):II‐1‐63. [Google Scholar]
- Dayton S, Pearce ML, Hashimoto S, Fakler LJ, Hiscock E, Dixon WJ. A controlled clinical trial of a diet high in unsaturated fat. New England Journal of Medicine 1962;266:1017‐23. [DOI] [PubMed] [Google Scholar]
- Ederer F, Leren P, Turpeinen O, Frantz ID Jr. Cancer among men on cholesterol‐lowering diets: experience from five clinical trials. Lancet 1971;298(7717):203‐6. [DOI] [PubMed] [Google Scholar]
- Hiscock E, Dayton S, Pearce ML, Hashimoto S. A palatable diet high in unsaturated fat. Journal of the American Dietetic Association 1962;40:427‐31. [PubMed] [Google Scholar]
- Pearce ML, Dayton S. Incidence of cancer in men on a diet high in polyunsaturated fat. Lancet 1971;1(697):464‐7. [DOI] [PubMed] [Google Scholar]
- Sturdevant RA, Pearce ML, Dayton S. Increased prevalence of cholelithiasis in men ingesting a serum‐cholesterol‐lowering diet. New England Journal of Medicine 1973;288(1):24‐7. [DOI] [PubMed] [Google Scholar]
- Tompkins MJ, Dayton S, Pearce ML. Effect of long‐term feeding of various fats on whole blood clotting times in men. Journal of Laboratory and Clinical Medicine 1964;64(5):763‐72. [PubMed] [Google Scholar]
Vijayakumar 2014 {published and unpublished data}
- Nandakumar S, Vijayakumar M, Krishnan S. Coconut oil vs sunflower oil in atherosclerosis: challenges in dietary intervention. Indian Heart Journal 2014;66:S11. [Google Scholar]
- Vijayakumar M, Krishnaan S, Sundram KR, Vasudevan DM, Nandakumar S. What oil in patients with established coronary artery disease: outcomes of two year dietary intervention with coconut oil & sunflower oil. Indian Heart Journal 2014;66:S12. [Google Scholar]
- Vijayakumar M, Vasudevan DM, Sundaram KR, Krishnan S, Vaidyanathan K, Nandakumar S, et al. A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart Journal 2016;68:498‐506. [DOI: 10.1016/j.ihj.2015.10.384] [DOI] [PMC free article] [PubMed] [Google Scholar]
WAHA ‐ Ros 2016 {published and unpublished data}
- Bitok E, Jaceldo‐Siegl K, Rajaram S, Serra‐Mir M, Roth I, Feitas‐Simoes T, et al. Favourable nutrient intake and displacement with long‐term walnut supplementation among elderly: results of a randomised trial. British Journal of Nutrition 2017;118(3):201‐9. [DOI: 10.1017/S0007114517001957] [DOI] [PubMed] [Google Scholar]
- Bitok E, Rajaram S, Ros E, Kazzi N, Huey L, Jaceldo K, et al. Does a daily walnut supplement given for a year result in body weight gain?. FASEB Journal 2016;30(1):1157. [Google Scholar]
- Huey L, Bitok E, Kazzi N, Sirirat R, Haddad Tabrizi S, Ros E, et al. Dietary compliance of walnut or no walnut intake in a 1‐year randomized intervention trial among free‐living elderly in the Walnuts and Healthy Aging Study (WAHA). FASEB Journal 2016; Vol. 30:1157.10.
- NCT01634841. Walnuts and Healthy Aging (WAHA). clinicaltrials.gov/ct2/show/NCT01634841 (first posted 6 July 2012).
- Rajaram S, Valls‐Pedret C, Cofan M, Sabate J, Serra‐Mir M, Perez‐Heras AM, et al. The Walnuts and Healthy Aging Study (WAHA): protocol for a nutritional intervention trial with walnuts on brain aging. Frontiers in Aging Neuroscience 2016;8:333. [PUBMED: 28119602] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros E, Rajaram S, Sala‐Vila A, Serra‐Mir M, Valls‐Pedret C, Cofan M, et al. Effect of a 1‐year walnut supplementation on blood lipids among older individuals: findings from the walnuts and healthy aging (WAHA) study. FASEB Journal 2016;30(1 Suppl):293‐4. [Google Scholar]
WELCOME 2015 {published and unpublished data}
- Bhatia L, Scorletti E, Curzen N, Clough GF, Calder PC, Byrne CD. Improvement in non‐alcoholic fatty liver disease severity is associated with a reduction in carotid intima‐media thickness progression. Atherosclerosis 2016;246:13‐20. [PUBMED: 26748347] [DOI] [PubMed] [Google Scholar]
- Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2014;34(6):1155‐61. [PUBMED: 24743428] [DOI] [PubMed] [Google Scholar]
- Byrne CD, Targher G. Time to replace assessment of liver histology with MR‐based imaging tests to assess efficacy of interventions for nonalcoholic fatty liver disease. Gastroenterology 2016; Vol. 150, issue 1:7‐10. [PUBMED: 26602219] [DOI] [PubMed]
- Clough GF, McCormick KG, Scorletti E, Bhatia L, Calder PC, Griffin MJ, et al. Higher body fat percentage is associated with enhanced temperature perception in NAFLD: results from the randomised Wessex Evaluation of fatty Liver and Cardiovascular markers in NAFLD with OMacor thErapy trial (WELCOME) trial. Diabetologia 2016;59(7):1422‐9. [PUBMED: 27106721] [DOI] [PubMed] [Google Scholar]
- McCormick KG, Scorletti E, Bhatia L, Calder PC, Griffin MJ, Clough GF, et al. Impact of high dose n‐3 polyunsaturated fatty acid treatment on measures of microvascular function and vibration perception in non‐alcoholic fatty liver disease: results from the randomised WELCOME trial. Diabetologia 2015;58(8):1916‐25. [PUBMED: 26021488] [DOI] [PubMed] [Google Scholar]
- NCT00760513. Treatment of non alcoholic fatty liver disease with n‐3 fatty acids. clinicaltrials.gov/ct2/show/NCT00760513 (first posted 26 September 2008).
- Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Calder PC, et al. Design and rationale of the WELCOME trial: a randomised, placebo controlled study to test the efficacy of purified long chainomega‐3 fatty acid treatment in non‐alcoholic fatty liver disease. Contemporary Clinical Trials 2014;37(2):301‐11 (Erratum in: Contemporary Clinical Trials 2014 May;38(1):156). [PUBMED: 24556343] [DOI] [PubMed] [Google Scholar]
- Scorletti E, West AL, Bhatia L, Hoile SP, McCormick KG, Burdge GC, et al. Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: results from the WELCOME trial. Journal of Hepatology 2015;63(6):1476‐83. [PUBMED: 26272871] [DOI] [PubMed] [Google Scholar]
- Targher G, Byrne CD. Clinical review: nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. Journal of Clinical Endocrinology and Metabolism 2013;98(2):483‐95. [PUBMED: 23293330] [DOI] [PubMed] [Google Scholar]
WINS 2006 {published and unpublished data}
- Chlebowski RT, Blackburn GL, Buzzard IM, Rose DP, Martino S, Khandekar JD, et al. Adherence to a dietary fat intake reduction program in postmenopausal women receiving therapy for early breast cancer. The Women's Intervention Nutrition Study. Journal of Clinical Oncology 1993;11(11):2072‐80. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the women's intervention nutrition study. JNCI Journal of the National Cancer Institute 2006;98(24):1767‐76. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Rose DP, Buzzard IM, Blackburn GL, York M, Insull W, et al. Dietary fat reduction in adjuvant breast cancer therapy: current rationale and feasibility issues. Adjuvant. The Cancer Journal 1990;6:357‐63. [Google Scholar]
- Hoy MK, Winters BL, Chlebowski RT, Papoutsakis C, Shapiro A, Lubin MP, et al. Implementing a low‐fat eating plan in the Women's Intervention Nutrition Study. Journal of the American Dietetic Association 2009;109(4):688‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP, Chlebowski RT, Connolly JM, Jones LA, Wynder EL. Effects of tamoxifen adjuvant therapy and a low‐fat diet on serum binding proteins and estradiol bioavailability in postmenopausal breast cancer patients. Cancer Research 1992;52:5386‐90. [PubMed] [Google Scholar]
- Rose DP, Connolly JM, Chlebowski RT, Buzzard IM, Wynder EL. The effects of a low‐fat dietary intervention and tamoxifen adjuvant therapy on the serum estrogen and sex hormone‐binding globulin concentrations of postmenopausal breast cancer patients. Breast Cancer Research & Treatment 1993;27(3):253‐62. [DOI] [PubMed] [Google Scholar]
- Wynder EL, Cohen LA, Winters BL. The challenges of assessing fat intake in cancer research investigations. Journal of the American Dietetic Association 1997;97(7 Suppl):S5‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ADCS‐Quinn 2010 {published data only}
- Quinn JF, Raman R, Thomas RG, Yurko‐Mauro K, Nelson EB, Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304(17):1903‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
AFFORD 2014 {published data only}
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. Journal of the American College of Cardiology 2014;64(14):1441‐8. [DOI] [PubMed] [Google Scholar]
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Multicentre trial of fish oil for the reduction of atrial fibrillation recurrence, inflammation and oxidative stress: the Atrial Fibrillation Fish Oil Research Study. Canadian Journal of Cardiology 2013;1:S383. [DOI] [PubMed] [Google Scholar]
AlphaOmega ‐ EPA+DHA {published and unpublished data}
- Brouwer IA, Geleijnse JM, Klaasen VM, Smit LA, Giltay EJ, Goede J, et al. Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the alpha omega trial. PLoS One 2013;8(12):e81519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n‐3 fatty acids on major cardiovascular events in statin users and non‐users with a history of myocardial infarction. European Heart Journal 2012;33(13):1582‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleijnse J, Giltay E, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2011;1:S512. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized, double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2012;8(4):278‐87. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Giltay EJ, Schouten EG, Goede J, Oude Griep LM, Teitsma‐Jansen AM, et al. Effect of low doses of n‐3 fatty acids on cardiovascular diseases in 4,837 post‐myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. American Heart Journal 2010;159(4):539‐46. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Heijboer AC, Goede J, Oude Griep LM, Blankenstein MA, et al. No effects of n‐3 fatty acid supplementation on serum total testosterone levels in older men: the Alpha Omega Trial. International Journal of Andrology 2012;35(5):680‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Kromhout D. Effects of n‐3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. American Journal of Clinical Nutrition 2011;94(6):1442‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen E, Gemen E, Geleijnse M, Kusters R, Kromhout D, Giltay E. Effects of n‐3 fatty acids on decline of kidney function after myocardial infarction: Alpha Omega trial. Nephrology Dialysis Transplantation 2012;27:ii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen EK, Geleijnse JM, Kromhout D, Giltay EJ. No effect of n‐3 fatty acids on high‐sensitivity C‐reactive protein after myocardial infarction: the Alpha Omega trial. European Journal of Preventive Cardiology 2014;21(11):1429‐36. [DOI] [PubMed] [Google Scholar]
- Hoogeveen EK, Geleijnse JM, Kromhout D, Stijnen T, Gemen EF, Kusters R, et al. Effect of omega‐3 fatty acids on kidney function after myocardial infarction: the Alpha Omega trial. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(10):1676‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Geleijnse JM, Goede J, Oude Griep LM, Mulder BJ, Boer MJ, et al. N‐3 fatty acids, ventricular arrhythmia‐related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care 2011;34(12):2515‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Giltay EJ, Geleijnse JM. N‐3 fatty acids and cardiovascular events after myocardial infarction. New England Journal of Medicine 2010;363(18):2015‐26. [DOI] [PubMed] [Google Scholar]
AREDS2 2014 {published and unpublished data}
- Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, et al. Effect of long‐chain omega‐3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age‐Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Internal Medicine 2014;174(5):763‐71. [DOI] [PubMed] [Google Scholar]
- Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, et al. The Age‐Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119(11):2282‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE. In reply: making sense of the evidence from the age‐related eye disease study 2 randomized clinical trial. JAMA Ophthalmology 2014;132(8):1031‐2. [DOI] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Launer LJ, Grodstein F, Bernstein PS, et al. Effect of omega‐3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA 2015;314(8):791‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL III, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age‐related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmology 2014;132(2):142‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, SanGiovanni JP, Ferris FL, Wong WT, Agron E, Clemons TE, et al. Lutein/zeaxanthin for the treatment of age‐related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmology 2013;131(7):843‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh N, Nicholson BP, Agron E, Clemons TE, Bressler SB, Rosenfeld PJ, et al. Visual acuity after cataract surgery in patients with age‐related macular degeneration: age‐related eye disease study 2 report number 5. Ophthalmology 2014;121(6):1229‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
ASCEND {unpublished data only}
- Bowman L, Aung T, Haynes R, Armitage J. ASCEND: design and baseline characteristics of a large randomised trial in diabetes. Diabetes 2012;61:A556‐7. [Google Scholar]
Azadbakht 2007 {published and unpublished data}
- Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Better dietary adherence and weight maintenance achieved by a long‐term moderate fat diet. British Journal of Nutrition 2007;97:399‐404. [DOI] [PubMed] [Google Scholar]
Baldassarre 2006 {published data only}
- Baldassarre D, Amato M, Eligini S, Barbieri SS, Mussoni L, Frigerio B, et al. Effect of n‐3 fatty acids on carotid atherosclerosis and haemostasis in patients with combined hyperlipoproteinemia: a double‐blind pilot study in primary prevention. Annals of Medicine 2006;38(5):367‐75. [DOI: 10.1080/07853890600852880] [DOI] [PubMed] [Google Scholar]
Berson 2004 {published data only}
- Berson EL, Rosner B, Sandberg MA, Weigel‐DiFranco C, Moser A, Brockhurst RJ, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Archives of Ophthalmology 2004;122(9):1297‐305. [DOI] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Weigel‐DiFranco C, Moser A, Brockhurst RJ, et al. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: subgroup analyses. Archives of Ophthalmology 2004;122(9):1306‐14. [DOI] [PubMed] [Google Scholar]
Caldwell 2011 {published data only}
- Argo CK, Patrie JT, Lackner C, Henry TD, Lange EE, Weltman AL, et al. Effects of n‐3 fish oil on metabolic and histological parameters in NASH: a double‐blind, randomized, placebo‐controlled trial. Journal of Hepatology 2015;62(1):190‐7. [PUBMED: 25195547] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell SH, Argo CK, Henry TD, Lackner C, Pramoonjago P, Weltman AL, et al. Dissociated histological and metabolic effects of omega‐e (3000 mg/d) versus placebo with both exercise and diet in a double‐blind randomized controlled trial of NASH. Journal of Hepatology 2011; Vol. 54:S8.
DART 2 ‐ Burr 2003 {published and unpublished data}
- Burr ML, Ashfield‐Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. European Journal of Clinical Nutrition 2003;57(2):193‐200. [DOI] [PubMed] [Google Scholar]
- Ness AR, Ashfield‐Watt PAL, Whiting JM, Smith GD, Hughes J, Burr ML. The long‐term effect of dietary advice on the diet of men with angina: the diet and angina randomized trial. Journal of Human Nutrition and Dietetics 2004;17:1‐3. [DOI] [PubMed] [Google Scholar]
- Ness AR, Gallacher JE, Bennett PD, Gunnell DJ, Rogers PJ, Kessler D, et al. Advice to eat fish and mood: a randomised controlled trial in men with angina. Nutritional Neuroscience 2003;6(1):63‐5. [DOI] [PubMed] [Google Scholar]
DART fish Burr 1989 {published and unpublished data}
- Burr ML, Fehily AM. Fatty fish and heart disease: a randomized controlled trial. World Review of Nutrition and Dietetics 1991;66:306‐12. [DOI] [PubMed] [Google Scholar]
- Burr ML, Fehily AM. Fish and the heart [letter]. Lancet 1989;ii:1451‐2. [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2(8666):757‐61. [DOI] [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Rogers S, Welsby E, King S, Sandham S. Diet and reinfarction trial (DART): design, recruitment, and compliance. European Heart Journal 1989;10(6):558‐67. [DOI] [PubMed] [Google Scholar]
- Burr ML, Holliday RM, Fehily AM, Whitehead PJ. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART). European Heart Journal 1992;13(2):166‐70. [DOI] [PubMed] [Google Scholar]
- Burr ML, Sweetham PM, Fehily AM. Diet and reinfarction [letter]. European Heart Journal 1994;15(8):1152‐3. [DOI] [PubMed] [Google Scholar]
- Fehily AM, Vaughan‐Williams E, Shiels K, Williams AH, Horner M, Bingham G, et al. Factors influencing compliance with dietary advice: the Diet and Reinfarction Trial (DART). Journal of Human Nutrition and Dietetics 1991;4:33‐42. [Google Scholar]
- Fehily AM, Vaughan‐Williams E, Shiels K, Williams AH, Horner M, Bingham G, et al. The effect of dietary advice on nutrient intakes: evidence from the diet and reinfarction trial (DART). Journal of Human Nutrition & Dietetics 1989;2:4235. [Google Scholar]
- Ness AR, Hughes J, Elwood PC, Whitley E, Smith GD, Burr ML. The long‐term effect of dietary advice in men with coronary disease: follow‐up of the Diet and Reinfarction Trial (DART). European Journal of Clinical Nutrition 2002;56(6):512‐8. [DOI] [PubMed] [Google Scholar]
- Ness AR, Whitley E, Burr ML, Elwood PC, Smith GD, Ebrahim S. The long‐term effect of advice to eat more fish on blood pressure in men with coronary disease: results from the diet and reinfarction trial. Journal of Human Hypertension 1999;13(11):729‐33. [DOI] [PubMed] [Google Scholar]
Derosa 2016 {published and unpublished data}
- Derosa G, Cicero AF, D'Angelo A, Borghi C, Maffioli P. Effects of n‐3 PUFAs on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. BioFactors (Oxford, England) 2016;42(3):316‐22. [PUBMED: 27040503] [DOI] [PubMed] [Google Scholar]
Deslypere 1992 {published and unpublished data}
- Blok WL, Deslypere JP, Demacker PN, Ven‐Jongekrijg J, Hectors MP, Meer JW, et al. Pro‐ and anti‐inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. European Journal of Clinical Investigation 1997;27(12):1003‐8. [PUBMED: 9466128] [DOI] [PubMed] [Google Scholar]
- Deslypere JP. Influence of supplementation with n‐3 fatty acids on different coronary risk factors in men: a placebo controlled study. Verhandelingen ‐ Koninklijke Vlaamse Academie voor Geneeskunde van Belgie 1992;54(3):189‐216. [PUBMED: 1413984] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18‐month controlled study. Journal of Lipid Research 1997;38(10):2012‐22. [PUBMED: 9374124] [PubMed] [Google Scholar]
DISAF ‐ Harrison 2005 {published and unpublished data}
- Harrison RA, Elton P. From pies to pilchards: dietary assistants increase consumption of oil rich fish. Journal of Epidemiology and Community Health 2000;Suppl:6. [Google Scholar]
- Harrison RA, Elton PJ. Can an oil‐rich fish diet improve treatment outcomes following cardioversion for atrial fibrillation? A randomised controlled trial. Study design and compliance [poster]. International Society for the Study of Fatty Acids and Lipids (ISSFAL); 2003; Montreal, Canada. 2003.
- Harrison RA, Elton PJ. Is there a role for long‐chain omega3 or oil‐rich fish in the treatment of atrial fibrillation?. Medical Hypotheses 2005;64(1):59‐63. [PUBMED: 15533612] [DOI] [PubMed] [Google Scholar]
- Harrison RA, Purnell P, Elton PJ. Using community‐based dietary assistants to increase the intake of oil‐rich fish among older people. European Journal of Public Health 2003;13(Suppl 1):105. [Google Scholar]
DO Health {published data only}
- NCT01745263. DO‐HEALTH / vitamin D3 ‐ omega3 ‐ home exercise ‐ healthy ageing and longevity trial. clinicaltrials.gov/ct2/show/NCT01745263 (first posted 10 December 2012).
DO IT 2006 {published and unpublished data}
- Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI, et al. Supplementation with fish oil affects the association between very long‐chain n‐3 polyunsaturated fatty acids in serum non‐esterified fatty acids and soluble vascular cell adhesion molecule‐1. Clinical Science 2003;105(1):13‐20. [DOI] [PubMed] [Google Scholar]
- Ellingsen I, Hjerkinn EM, Seljeflot I, Arnesen H, Tonstad S, Ellingsen I, et al. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. British Journal of Nutrition 2008;99(3):674‐81 (Erratum in: British Journal of Nutrition 2008;99(3):697). [DOI] [PubMed] [Google Scholar]
- Ellingsen I, Seljeflot I, Arnesen H, Tonstad S, et al. Vitamin C consumption is associated with less progression in carotid intima media thickness in elderly men: a 3‐year intervention study. Nutrition Metabolism & Cardiovascular Diseases 2009;19(1):8‐14. [DOI] [PubMed] [Google Scholar]
- Furenes EB, Seljeflot I, Solheim S, Hjerkinn EM, Arnesen H, Furenes EB, et al. Long‐term influence of diet and/or omega‐3 fatty acids on matrix metalloproteinase‐9 and pregnancy‐associated plasma protein A in men at high risk of coronary heart disease. Scandinavian Journal of Clinical & Laboratory Investigation 2008;68(3):177‐84. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, et al. Effect of diet or very long chain omega‐3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima‐media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13(3):325‐33. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, et al. Influence of long‐term intervention with dietary counselling, long‐chain n‐3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long‐standing hyperlipidemia. American Journal of Clinical Nutrition 2005;81(3):583‐9. [DOI] [PubMed] [Google Scholar]
- Lindman AS, Pedersen JI, Hjerkinn EM, Arnesen H, Veierod MB, Ellingsen I, et al. The effects of long‐term diet and omega‐3 fatty acid supplementation on coagulation factor VII and serum phospholipids with special emphasis on the R353Q polymorphism of the FVII gene. Thrombosis & Haemostasis 2004;91(6):1097‐104. [DOI] [PubMed] [Google Scholar]
- Troseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin‐18 are reduced by diet and n‐3 fatty acid intervention in elderly high‐risk men. Metabolism: Clinical & Experimental 2009;58(11):1543‐9. [DOI] [PubMed] [Google Scholar]
- Troseid M, Seljeflot I, Hjerkinn EM, Arnesen H. Interleukin‐18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: synergistic effect of inflammation and hyperglycemia. Diabetes Care 2009;32(3):486‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
DO IT ‐ Einvik 2010 {published and unpublished data}
- Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI. Supplementation with fish oil affects the association between very long‐chain n‐3 polyunsaturated fatty acids in serum non‐esterified fatty acids and soluble vascular cell adhesion molecule‐1. Clinical Science 2003;105(1):13‐20. [DOI] [PubMed] [Google Scholar]
- Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Ellingsen I, Seljeflot I. Effect of diet and omega‐3 fatty acid intervention on asymmetric dimethylarginine. Nutrition & Metabolism 2006;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid HMA, Arnesen H, Hjerkinn EM, Lyberg T, Ellingsen I, Seljeflot I. Effect of diet and omega‐3 fatty acid intervention on asymmetric dimethylarginine. Nutrition and Metabolism 2006;3:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einvik G, Ekeberg O, Lavik JG, Ellingsen I, Klemsdal TO, Hjerkinn EM. The influence of long‐term awareness of hyperlipidemia and of 3 years of dietary counseling on depression, anxiety, and quality of life. Journal of Psychosomatic Research 2010;68(6):567‐72. [DOI] [PubMed] [Google Scholar]
- Einvik G, Klemsdal TO, Sandvik L, Hjerkinn EM. A randomized clinical trial on n‐3 polyunsaturated fatty acids supplementation and all‐cause mortality in elderly men at high cardiovascular risk. European Journal of Cardiovascular Prevention & Rehabilitation 2010;17(5):588‐92. [DOI] [PubMed] [Google Scholar]
- Ellingsen I, Hjerkinn EM, Arnesen H, Seljeflot I, Hjermann I, Tonstad S. Follow‐up of diet and cardiovascular risk factors 20 years after cessation of intervention in the Oslo Diet and Antismoking study. European Journal of Clinical Nutrition 2006;60(3):378‐85. [DOI] [PubMed] [Google Scholar]
- Furenes EB, Seljeflot I, Solheim S, Hjerkinn EM, Arnesen H, Furenes EB. Long‐term influence of diet and/or omega‐3 fatty acids on matrix metalloproteinase‐9 and pregnancy‐associated plasma protein A in men at high risk of coronary heart disease. Scandinavian Journal of Clinical and Laboratory Investigation 2008;68(3):177‐84. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, et al. Effect of diet or very long chain omega‐3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima‐media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13(3):325‐33. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, et al. Influence of long‐term intervention with dietary counseling, long‐chain n‐3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long‐standing hyperlipidemia. American Journal of Clinical Nutrition 2005;81(3):583‐9. [DOI] [PubMed] [Google Scholar]
- Lindman AS, Pedersen JI, Hjerkinn EM, Arnesen H, Veierod MB, Ellingsen I, et al. The effects of long‐term diet and omega‐3 fatty acid supplementation on coagulation factor VII and serum phospholipids with special emphasis on the R353Q polymorphism of the FVII gene. Thrombosis & Haemostasis 2004;91(6):1097‐104. [DOI] [PubMed] [Google Scholar]
- Troseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin‐18 are reduced by diet and n‐3 fatty acid intervention in elderly high‐risk men. Metabolism: Clinical and Experimental 2009;58(11):1543‐9. [DOI] [PubMed] [Google Scholar]
- Troseid M, Seljeflot I, Weiss TW, Klemsdal TO, Hjerkinn EM, Arnesen H. Arterial stiffness is independently associated with interleukin‐18 and components of the metabolic syndrome. Atherosclerosis 2010;209(2):337‐9. [DOI] [PubMed] [Google Scholar]
EPE‐A study 2014 {published and unpublished data}
- Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl‐eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014;147(2):377‐84.e1. [PUBMED: 24818764] [DOI] [PubMed] [Google Scholar]
Erdogan 2007 {published data only}
- Erdogan A, Bayer M, Kollath D, Greiss H, Voss R, Neumann T, et al. Omega AF study: polyunsaturated fatty acids (PUFA) for prevention of atrial fibrillation relapse after successful external cardioversion. Heart Rhythm 2007;4(5):S185‐6. [Google Scholar]
- Heidt MC, Vician M, Stracke SKH, Stadlbauer T, Grebe MT, Boening A, et al. Beneficial effects of intravenously administered n‐3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thoracic and Cardiovascular Surgeon 2009;57:276‐80. [DOI: 10.1055/s-0029-1185301] [DOI] [PubMed] [Google Scholar]
- Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, et al. N‐3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta‐analysis of randomized controlled trials. Journal of the American Heart Association 2013;2(1):e005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finnish Mental Hosp 1972 {published data only}
- Miettinen M, Turpeinen O, Karvonen MJ, Elosuo R, Paavilainen E. Effect of cholesterol‐lowering diet on mortality from coronary heart‐disease and other causes: a twelve‐year clinical trial in men and women. Lancet 1972;2(782):835‐8. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Turpeinen O, Karvonen MJ, Pekkarinen M, Paavilainen E, Elosuo R. Dietary prevention of coronary heart disease in women: the Finnish mental hospital study. International Journal of Epidemiology 1983;12(1):17‐25. [DOI] [PubMed] [Google Scholar]
- Turpeinen O, Miettinen M, Karvonen M, Roine P, Pekkarinen M, Lehtosuo EJ, et al. Dietary prevention of coronary heart disease: long‐term experiment. I. Observations on male subjects. American Journal of Clinical Nutrition 1968;21(4):255‐76. [DOI] [PubMed] [Google Scholar]
FLAX‐PAD 2013 {published data only}
- Caligiuri SP, Aukema HM, Ravandi A, Guzman R, Dibrov E, Pierce GN. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an alpha‐linolenic acid‐induced inhibition of soluble epoxide hydrolase. Hypertension 2014;64(1):53‐9. [PUBMED: 24777981] [DOI] [PubMed] [Google Scholar]
- Caligiuri SP, Rodriguez‐Leyva D, Aukema HM, Ravandi A, Weighell W, Guzman R, et al. Dietary flaxseed reduces central aortic blood pressure without cardiac involvement but through changes in plasma oxylipins. Hypertension 2016;68(4):1031‐8. [PUBMED: 27528063] [DOI] [PubMed] [Google Scholar]
- Edel A, Rodriguez‐Leyva D, Weighell W, Vallee R, Aliani M, Guzman R, et al. Flaxseed lignan metabolites elicit antihypertensive effects in pad patients in the flax‐pad trial. Annals of Nutrition and Metabolism 2013;63:1339. [Google Scholar]
- Edel AL, Rodriguez‐Leyva D, Maddaford TG, Caligiuri SP, Austria JA, Weighell W, et al. Dietary flaxseed independently lowers circulating cholesterol and lowers it beyond the effects of cholesterol‐lowering medications alone in patients with peripheral artery disease. Journal of Nutrition 2015;145(4):749‐57. [PUBMED: 25694068] [DOI] [PubMed] [Google Scholar]
- Leyva DR, Zahradka P, Ramjiawan B, Guzman R, Aliani M, Pierce GN. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral artery disease: rationale and design of the FLAX‐PAD randomized controlled trial. Contemporary Clinical Trials 2011;32(5):724‐30. [PUBMED: 21616170] [DOI] [PubMed] [Google Scholar]
- Pierce GN, Edel AL, LaVallee R, Caligiuri S, Aukema H, Ravandi A, et al. The use of dietary flaxseed to promote cardiovascular health. Acta Physiologica 2014;211:15. [Google Scholar]
- Pierce GN, Rodriguez‐Leyva D, Edel A, Guzman R, Aliani M. The clinical use of flaxseed as a powerful nutritional intervention to treat cardiovascular disease. Cardiology (Switzerland) 2013;126:201. [Google Scholar]
- Rodriguez‐Leyva D, Weighell W, Edel AL, LaVallee R, Dibrov E, Pinneker R, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension 2013;62(6):1081‐9. [PUBMED: 24126178] [DOI] [PubMed] [Google Scholar]
FORWARD 2013 {published and unpublished data}
- Macchia A, Grancelli H, Varini S, Nul D, Laffaye N, Mariani J, et al. Omega‐3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the FORWARD (randomized trial to assess efficacy of PUFA for the maintenance of sinus rhythm in persistent atrial fibrillation) trial. Journal of the American College of Cardiology 2013;61(4):463‐8. [PUBMED: 23265344] [DOI] [PubMed] [Google Scholar]
- Macchia A, Varini S, Grancelli H, Nul D, Laffaye N, Ferrante D, et al. The rationale and design of the FORomegaARD trial: a randomized, double‐blind, placebo‐controlled, independent study to test the efficacy of n‐3 PUFA for the maintenance of normal sinus rhythm in patients with previous atrial fibrillation. American Heart Journal 2009;157(3):423‐7. [PUBMED: 19249410] [DOI] [PubMed] [Google Scholar]
FOSTAR 2016 {published and unpublished data}
- Chen JS, Hill CL, Lester S, Ruediger CD, Battersby R, Jones G, et al. Supplementation with omega‐3 fish oil has no effect on bone mineral density in adults with knee osteoarthritis: a 2‐year randomized controlled trial. Osteoporosis International 2016;27(5):1897‐905. [PUBMED: 26694596] [DOI] [PubMed] [Google Scholar]
- Hill C, Lester SE, Jones G. Response to 'Low‐dose versus high‐dose fish oil for pain reduction and function improvement in patients with knee osteoarthritis' by Chen et al. Annals of the Rheumatic Diseases 2016; Vol. 75, issue 1:e8. [PUBMED: 26662278] [DOI] [PubMed]
- Hill CL, March LM, Aitken D, Lester SE, Battersby R, Hynes K, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Annals of the Rheumatic Diseases 2016;75(1):23‐9. [PUBMED: 26353789] [DOI] [PubMed] [Google Scholar]
Franzen 1993 {published and unpublished data}
- Franzen D. Unknown. Catheterization and Cardiovascular Diagnosis 1993;28:301‐10. [DOI] [PubMed] [Google Scholar]
- Franzen D, Geisel J, Hopp HW, Oette K, Hilger HH. Long‐term effects of low dosage fish oil on serum lipids and lipoproteins [Langzeiteffekte von niedrigdosiertem fischol auf serumlipide und lipoproteine]. Medizinische Klinik 1993;88(3):134‐8. [PubMed] [Google Scholar]
Gill 2012 {published data only}
- Gill EA, Chen MA, Paramsothy P, Fish B, Isquith D, Thirumalai A, et al. Abstract 12697: Omega‐3 fatty acids effects on carotid IMT in metabolic syndrome. Circulation 2014;130:A1269. [Google Scholar]
- Gill EA, Chen MA, Thirumalai A, Fish B, Paramsothy P. Omega‐3 fatty acids improve dyslipidemia but not inflammatory markers in metabolic syndrome. Journal of Clinical Lipidology 2012;6:278‐9. [Google Scholar]
GISSI‐HF 2008 {published data only}
- Aleksova A, Masson S, Maggioni AP, Lucci D, Fabbri G, Beretta L, et al. N‐3 polyunsaturated fatty acids and atrial fibrillation in patients with chronic heart failure: the GISSI‐HF trial. European Journal of Heart Failure 2013;15(11):1289‐95. [DOI] [PubMed] [Google Scholar]
- Canepa M, Temporelli PL, Rossi A, Gonzini L, Nicolosi GL, Staszewsky L, et al. Prevalence and prognostic impact of chronic obstructive pulmonary disease in patients with chronic heart failure. Data from the GISSI‐Heart Failure trial. European Journal of Heart Failure 2016;18:442‐3. [DOI] [PubMed] [Google Scholar]
- Cowie MR, Cure S, Bianic F, McGuire A, Goodall G, Tavazzi L. Cost‐effectiveness of highly purified omega‐3 polyunsaturated fatty acid ethyl esters in the treatment of chronic heart failure: results of Markov modelling in a UK setting. European Journal of Heart Failure 2011;13(6):681‐9. [DOI: 10.1093/eurjhf/hfr023] [DOI] [PubMed] [Google Scholar]
- Finzi A, Barlera S, Serra DM, Rossi MG, Ruggeri A, Mezzani A, et al. Antiarrhythmic effects of n‐3 PUFA in patients with heart failure and an implantable cardioverter defibrillator in the GISSI‐HF trial. European Heart Journal 2009;30:279. [Google Scholar]
- Finzi AA, Latini R, Barlera S, Rossi MG, Ruggeri A, Mezzani A, et al. Effects of n‐3 polyunsaturated fatty acids on malignant ventricular arrhythmias in patients with chronic heart failure and implantable cardioverter‐defibrillators: a substudy of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca (GISSI‐HF) trial. American Heart Journal 2011;161(2):338‐43. [DOI: 10.1016/j.ahj.2010.10.032] [DOI] [PubMed] [Google Scholar]
- Ghio S, Scelsi L, Latini R, Masson S, Eleuteri E, Palvarini M, et al. Effects of n‐3 polyunsaturated fatty acids and of rosuvastatin on left ventricular function in chronic heart failure: a substudy of GISSI‐HF trial. European Journal of Heart Failure 2010;12(12):1345‐53. [DOI] [PubMed] [Google Scholar]
- Rovere MT, Barlera S, Staszewsky L, Mezzani A, Midi P, Marchioli R, et al. Effect of n‐3PUFA on heart rate variability. Data from the GISSI‐HF holter substudy. Circulation 2011;124(21 SUPPL 1):14829. [Google Scholar]
- Rovere MT, Pinna GD, Maestri R, Barlera S, Bernardinangeli M, Veniani M, et al. Autonomic markers and cardiovascular and arrhythmic events in heart failure patients: still a place in prognostication? Data from the GISSI‐HF trial. European Journal of Heart Failure 2012;14(12):1410‐9. [DOI] [PubMed] [Google Scholar]
- Rovere MT, Staszewsky L, Barlera S, Maestri R, Mezzani A, Midi P, et al. N‐3PUFA and holter‐derived autonomic variables in patients with heart failure: data from the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca (GISSI‐HF) Holter substudy. Heart Rhythm 2013;10(2):226‐32. [DOI] [PubMed] [Google Scholar]
- Latini R, Masson S, Tacconi M, Bernasconi R, Dragani L, Milani V, et al. Circulating levels of n‐3 polyunsaturated fatty acids in patients with chronic heart failure. Data from the GISSI‐HF trial. European Heart Journal 2011;32:919. [Google Scholar]
- Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, et al. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI‐HF trial. Eupropean Heart Journal 2009;30(19):232736. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Aldegheri MP, Borghese L, Franzosi MG, Latini R, Marfisi RM, et al. Time course analysis of the effect of n‐3 PUFA on fatal and non fatal arrhythmias in heart failure: secondary results of the GISSI‐HF trial. European Heart Journal 2009;30:165. [Google Scholar]
- Marchioli R, Cucchi G, Gualco A, Franzosi MG, Levantesi G, Maggioni AP, et al. Time course analysis of the effect of n‐3 PUFA on fatal and non fatal heart failure: Secondary results of the GISSI‐HF trial. European Heart Journal 2009;30:432. [Google Scholar]
- Marchioli R, Franzosi MG, Latini R, Maggioni AP, Marfisi RM, Minneci C, et al. Prognostic ability of a Mediterranean dietary score in heart failure: preliminary analysis of the GISSI‐Heart failure trial. European Heart Journal 2009;30:1026. [Google Scholar]
- Marchioli R, Franzosi MG, Latini R, Maggioni AP, Marfisi RM, Nicolosi GL, et al. Effect of n‐3 PUFA in heart failure patients with different dietary habits: preliminary results of the GISSI‐heart failure trial. European Heart Journal 2009;30:426. [Google Scholar]
- Marchioli R, Franzosi MG, Levantesi G, Marfisi RM, Maggioni AP, Nicolosi GL, et al. Effect of n‐3 PUFA according to fish intake: preliminary results of GISSI‐Heart Failure. European Heart Journal 2009;30:707. [Google Scholar]
- Marchioli R, Levantesi G, Silletta MG, Barlera S, Bernardinangeli M, Carbonieri E, et al. Effect of n‐3 polyunsaturated fatty acids and rosuvastatin in patients with heart failure: results of the GISSI‐HF trial. Expert Review of Cardiovascular Therapy 2009;7(7):735‐48. [DOI] [PubMed] [Google Scholar]
- Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI‐Heart Failure trial. Circulation: Heart Failure 2010;3(1):65‐72. [DOI] [PubMed] [Google Scholar]
- Masson S, Marchioli R, Mozaffarian D, Bernasconi R, Milani V, Dragani L, et al. Plasma n‐3 polyunsaturated fatty acids in chronic heart failure in the GISSI‐Heart Failure trial: relation with fish intake, circulating biomarkers, and mortality. American Heart Journal 2013;165(2):208‐15. [DOI] [PubMed] [Google Scholar]
- Røysland R, Masson S, Omland T, Milani V, Bjerre M, Flyvbjerg A, et al. Prognostic value of osteoprotegerin in chronic heart failure: the GISSI‐HF trial. American Heart Journal 2010;160(2):286‐93. [DOI: 10.1016/j.ahj.2010.05.015] [DOI] [PubMed] [Google Scholar]
- Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of n‐3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;372(9645):1223‐30. [DOI: 10.1016/S01406736(08)61239-8] [DOI] [PubMed] [Google Scholar]
- Tavazzi L, Tognoni G, Franzosi MG, Latini R, Maggioni AP, Marchioli R, et al. Rationale and design of the GISSI heart failure trial: a large trial to assess the effects of n‐3 polyunsaturated fatty acids and rosuvastatin in symptomatic congestive heart failure. European Journal of Heart Failure 2004;6(5):635‐41. [DOI: 10.1016/j.ejheart.2004.03.001] [DOI] [PubMed] [Google Scholar]
GISSI‐P 1999 {published data only}
- Franzosi MG, Brunetti M, Marchioli R, Marfisi RM, Tognoni G, Valagussa F, et al. Cost‐effectiveness analysis of n‐3 polyunsaturated fatty acids (PUFA) after myocardial infarction: results from Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto (GISSI)‐Prevenzione trial. Pharmacoeconomics 2001;19(4):411‐20. [DOI] [PubMed] [Google Scholar]
- GISSI‐Prevenzione Investigators. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Lancet 1999;354:447‐55. [PubMed] [Google Scholar]
- Marchioli R. Treatment with n‐3 polyunsaturated fatty acids after myocardial infarction: results of GISSI‐Prevenzione trial. European Heart Journal Supplements 2001;3(Supplement D):D85‐D97. [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Gregorio DDMR, Franzosi MG, et al. Early protection against sudden death by n‐3 polyunsaturated fatty acids after myocardial infarction: time course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)‐Prevenzione. Circulation 2002;105:1897‐903. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Pasquale A. The biochemical, pharmacological and epidemiological reference picture of the GISSI‐Prevention. Giornale Italiano di Cardiologia 1993;23(9):933‐64. [PubMed] [Google Scholar]
- Marchioli R, Valagussa F. The results of the GISSI‐Prevenzione trial in the general framework of secondary prevention. European Heart Journal 2000;21(12):949‐52. [DOI] [PubMed] [Google Scholar]
JELIS 2007 {published and unpublished data}
- Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR‐AMI, ASTAMI, JELIS, MEGA, REVIVE‐II, SURVIVE, and PROACTIVE. The European Journal of Heart Failure 2006;8(1):105‐10. [DOI: 10.1016/j.ejheart.2005.12.003] [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Yokoyama M, Saito Y, Matsuzaki M, Origasa H, Oikawa S, et al. Preventive effects of eicosapentaenoic acid on coronary artery disease in patients with peripheral artery disease. Circulation Journal 2010;74(7):1451‐7. [DOI: 10.1253/circj.CJ-09-0520] [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin‐treated patients with coronary artery disease. Secondary prevention analysis from JELIS. Circulation Journal 2009;73(7):1283‐90. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, et al. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: sub‐analysis of the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2009;206(2):535‐9. [DOI: 10.1016/j.atherosclerosis.2009.03.029] [DOI] [PubMed] [Google Scholar]
- Origasa H, Yokoyama M, Matsuzaki M, Saito Y, Matsuzawa Y, JELIS Investigators. Clinical importance of adherence to treatment with eicosapentaenoic acid by patients with hypercholesterolemia. Circulation Journal 2010;74(3):510‐7. [DOI: 10.1253/circj.CJ-09-0746] [DOI] [PubMed] [Google Scholar]
- Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub‐analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008;200(1):135‐40. [DOI: 10.1016/j.atherosclerosis.2008.06.003] [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke 2008;39(8):2052‐8. [DOI: 10.1161/STROKEAHA.107.509455] [DOI] [PubMed] [Google Scholar]
- Yamanouchi D, Komori K. Eicosapentaenoic acid as the gold standard for patients with peripheral artery disease? Subanalysis of the JELIS trial. Circulation Journal 2010;74(7):1298‐9. [DOI: 10.1253/circj.CJ-10-0449] [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet 2007;369(9567):1090‐8. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, for the JELIS Investigators. Effects of eicosapentaenoic acid on cardiovascular events in Japanese patients with hypercholesterolaemia: rationale, design, and baseline characteristics of the Japan EPA Lipid Intervention Study (JELIS). American Heart Journal 2003;146:613‐20. [DOI] [PubMed] [Google Scholar]
Lorenz‐Meyer 1996 {published and unpublished data}
- Lorenz‐Meyer H, Bauer P, Nicolay C, Schulz B, Purrmann J, Fleig WE, et al. Omega‐3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Study Group Members (German Crohn's Disease Study Group). Scandinavian Journal of Gastroenterology 1996;31(8):778‐85. [DOI] [PubMed] [Google Scholar]
Mansel 1990 {published data only}
- Mansel RE, Gateley CA, Harrison BJ, Melhuish J, Sheridan W, Pye JK, et al. Effects and tolerability of n‐6 essential fatty acid supplementation in patients with recurrent breast cysts: a randomized double‐blind placebo‐controlled trial. Journal of Nutritional Medicine 1990;1(3):195. [Google Scholar]
- Mansel RE, Harrison BJ, Melhuish J, Sheridan W, Pye JK, Pritchard G, et al. A randomized trial of dietary intervention with essential fatty acids in patients with categorized cysts. Annals of the New York Academy of Sciences 1990;586:288‐94. [DOI] [PubMed] [Google Scholar]
- Mansel RE, Pye JK, Hughes LE. Effects of essential fatty acids on cyclical mastalgia and noncyclical breat disorders. In: Horrobin DE editor(s). Omega‐6 Essential Fatty Acids: Pathophysiology and Roles in Clinical Medicine. 1st Edition. New York: Wiley‐Liss, 1990:557‐66. [ISBN 0‐471‐SAh93‐4] [Google Scholar]
MAPT 2017 {published data only}
- Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurology 2017;16:377‐89. [DOI: 10.1016/S1474-4422(17)30040-6] [DOI] [PubMed] [Google Scholar]
- Carrie I, Kan GA, Gillette‐Guyonnet S, Andrieu S, Dartigues JF, Touchon J, et al. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial). Journal of Nutrition, Health & Aging 2012;16(4):355‐9. [DOI] [PubMed] [Google Scholar]
- Delrieu J, Andrieu S, Pahor M, Cantet C, Cesari M, Ousset PJ, et al. Neuropsychological profile of “cognitive frailty” subjects in MAPT study. Journal of Prevention of Alzheimer's Disease 2016;3(3):151‐9. [DOI: 10.14283/jpad.2016.94] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrieu J, Payoux P, Hitzel A, Peiffer S, Abellan Van Kan G, Gillette S, et al. Multidomain Alzheimer's disease preventive trial: Florbetapir ancillary study. Alzheimer's & Dementia 2011;1:S419. [Google Scholar]
- Fougere B, Barreto PD, Goisser S, Soriano G, Guyonnet S, Andrieu S, et al. Red blood cell membrane omega‐3 fatty acid levels and physical performance: cross‐sectional data from the MAPT study. Clinical Nutrition 2017;pii(S0261‐5614(17)30128‐0):1‐4. [DOI: 10.1016/j.clnu.2017.04.005] [DOI] [PubMed] [Google Scholar]
- Gillette S. The multidomain Alzheimer preventive trial (MAPT): a new approach for the prevention of Alzheimer's disease. Alzheimer's & Dementia 2009;5(4):145. [DOI] [PubMed] [Google Scholar]
- Gillette‐Guyonnet S, Andrieu S, Dantoine T, Dartigues JF, Touchon J, Vellas B, et al. Commentary on "A roadmap for the prevention of dementia II. Leon Thal Symposium 2008." The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer's disease. Alzheimer's & Dementia 2009;5(2):114‐21. [DOI] [PubMed] [Google Scholar]
- Gillette‐Guyonnet S, Vellas B, Andrieu S, Dupuy C, Carrié I. MAPT study: a 3‐year randomized trial of omega 3 and/or multidomain intervention for the prevention of cognitive decline in frail elderly subjects‐rationale, design and baseline data. Alzheimer's & Dementia 2011;1:S97‐8. [Google Scholar]
- Vellas B, Carrie I, Gillette‐Guyonnet S, Touchon J, Dantoine T, Dartigues JF, et al. MAPT study: a multidomain approach for preventing ALzheimer's disease: design and baseline data. Journal of Prevention of Alzheimers Disease 2014;1(1):13‐22. [PMC free article] [PubMed] [Google Scholar]
- Vellas B, Carrie I, Guyonnet S, Touchon J, Dantoine T, Dartigues JF, et al. MAPT (multi‐domain Alzheimer's prevention trial): results at 36 months. Alzheimer's & Dementia 2015;1:331. [Google Scholar]
- Vellas B, Touchon J, Weiner M. MAPT (multidomain Alzheimer preventive trial) imaging (MRI, FDG‐PET, amyloid‐PET) data. Journal of Nutrition, Health and Aging 2012;16 (9):812‐5. [DOI] [PubMed] [Google Scholar]
MARGARIN Bemelmans 2002 {published data only (unpublished sought but not used)}
- Bemelmans WJ, Broer J, Vries JH, Hulshof KF, May JF, Meyboom‐De Jong B. Impact of Mediterranean diet education versus posted leaflet on dietary habits and serum cholesterol in a high risk population for cardiovascular disease. Public Health Nutrition 2000;3(3):273‐83. [DOI] [PubMed] [Google Scholar]
- Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet AJ, Lefrandt JD, et al. Effect of an increased intake of alpha‐linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean alpha‐linolenic enriched Groningen dietary intervention (MARGARIN) study. American Journal of Clinical Nutrition 2002;75:221‐7. [DOI] [PubMed] [Google Scholar]
- Bemelmans WJ, Lefrandt JD, Feskens EJ, Broer J, Tervaert JW, May JF, et al. Change in saturated fat intake is associated with progression of carotid and femoral intima‐media thickness, and with levels of soluble intercellular adhesion molecule‐1. Atherosclerosis 2002;163(1):113‐20. [DOI] [PubMed] [Google Scholar]
- Bemelmans WJ, Lefrandt JD, Feskens EJ, Haelst PL, Broer J, Meyboom‐de Jong B, et al. Increased alpha‐linolenic acid intake lowers C‐reactive protein, but has no effect on markers of atherosclerosis. European Journal of Clinical Nutrition 2004;58(7):1083‐9. [DOI] [PubMed] [Google Scholar]
- Bemelmans WJ, Muskiet FA, Feskens EJ, Vries JH, Broer J, May JF, et al. Associations of alpha‐linolenic acid and linoleic acid with risk factors for coronary heart disease. European Journal of Clinical Nutrition 2000;54(12):865‐71. [DOI] [PubMed] [Google Scholar]
- Siero FW, Broer J, Bemelmans WJ, Meyboom‐de Jong BM. Impact of group nutrition education and surplus value of Prochaska based stage‐matched information on health‐related cognitions and on Mediterranean nutrition behaviour. Health Education Research 2000;15(5):635‐47. [DOI] [PubMed] [Google Scholar]
MENU ‐ Rock 2016 {published data only}
- Le T, Flatt SW, Natarajan L, Pakiz B, Quintana EL, Heath DD, et al. Effects of diet composition and insulin resistance status on plasma lipid levels in a weight loss intervention in women. Journal of the American Heart Association 2016;5(1):e002771. [DOI: 10.1161/JAHA.115.002771] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Pakiz B, Quintana EL, Heath DD, Rana BK, et al. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1‐year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism 2016;65(11):1605‐13. [DOI: 10.1016/j.metabol.2016.07.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michalsen 2006 {published data only}
- Michalsen A, Lehmann N, Pithan C, Knoblauch NT, Moebus S, Kannenberg F, et al. Mediterranean diet has no effect on markers of inflammation and metabolic risk factors in patients with coronary artery disease. European Journal of Clinical Nutrition 2006;60(4):478‐85. [DOI] [PubMed] [Google Scholar]
Middleton 2002 {published data only}
- Middleton SJ, Naylor S, Woolner J, Hunter JO. A double‐blind, randomized, placebo‐controlled trial of essential fatty acid supplementation in the maintenance of remission of ulcerative colitis. Alimentary Pharmacology & Therapeutics 2002;16(6):1131‐5. [PUBMED: 12030955] [DOI] [PubMed] [Google Scholar]
Minnesota Coronary 1989 {published data only}
- Brewer ER, Ashman PL, Kuba K. The Minnesota Coronary Survey: composition of diets, adherence and serum lipid response. Circulation 1975;51 and 52(Suppl II):269. [Google Scholar]
- Dawson EA, Gatewood LC. The Minnesota Coronary Survey: methodology and characteristics of the population. Circulation 1975;51 and 52(Suppl II):271. [Google Scholar]
- Frantz ID Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, et al. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis 1989;9(1):129‐35. [DOI] [PubMed] [Google Scholar]
- Frantz ID, Dawson EA, Kuba K, et al. The Minnesota Coronary Survey: effect of diet on cardiovascular events and deaths. Circulation 1975;51 and 52(Suppl II):4. [Google Scholar]
- Ramsden CE, Zamora D, Majchrzak‐Hong S, Faurot KR, Broste SK, Frantz RP, et al. Re‐evaluation of the traditional diet‐heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968‐73). BMJ 2016;353:i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moy 2001 {published and unpublished data}
- Moy TF, Yanek LR, Raqueno JV, Bezirdjian PJ, Blumenthal RS, Wilder LB, et al. Dietary counseling for high blood cholesterol in families at risk of coronary disease. Preventive Cardiology 2001;4(4):158‐64. [DOI] [PubMed] [Google Scholar]
NAT2 2015 {published data only}
- Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH. Circulating omega‐3 fatty acids and neovascular age‐related macular degeneration. Investigative Ophthalmology & Visual Science 2014;55(3):2010‐9. [PUBMED: 24557349] [DOI] [PubMed] [Google Scholar]
- Merle BM, Richard F, Benlian P, Puche N, Delcourt C, Souied EH. CFH Y402H and ARMS2 A69S polymorphisms and oral supplementation with docosahexaenoic acid in neovascular age‐related macular degeneration patients: the NAT2 study. PloS One 2015;10(7):e0130816. [PUBMED: 26132079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querques G, Merle BM, Pumariega NM, Benlian P, Delcourt C, Zourdani A, et al. Dynamic drusen remodelling in participants of the Nutritional AMD Treatment‐2 (NAT‐2) randomized trial. PloS One 2016;11(2):e0149219. [PUBMED: 26901353] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, et al. Oral docosahexaenoic acid in the prevention of exudative age‐related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 2013;120(8):1619‐31. [PUBMED: 23395546] [DOI] [PubMed] [Google Scholar]
Norouzi 2014 {published data only}
- Norouzi Javidan A, Sabour H, Latifi S, Abrishamkar M, Soltani Z, Shidfar F, et al. Does consumption of polyunsaturated fatty acids influence on neurorehabilitation in traumatic spinal cord‐injured individuals? A double‐blinded clinical trial. Spinal Cord 2014;52(5):378‐82. [PUBMED: 24637568] [DOI] [PubMed] [Google Scholar]
- Sabour H, Norouzi Javidan A, Latifi S, Shidfar F, Heshmat R, Emami Razavi SH, et al. Omega‐3 fatty acids' effect on leptin and adiponectin concentrations in patients with spinal cord injury: a double‐blinded randomized clinical trial. Journal of Spinal Cord Medicine 2015;38(5):599‐606. [PUBMED: 25096818] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norwegian ‐ Natvig 1968 {published data only}
- Natvig H. The effect of unsaturated fatty acids on the incidence of coronary infarction, etc. Tidsskrift for Den Norske Laegeforening 1967;87(11):1033‐41. [PubMed] [Google Scholar]
- Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K. A controlled trial of the effect of linolenic acid on incidence of coronary heart disease. The Norwegian vegetable oil experiment of 1965‐66. Scandinavian Journal of Clinical & Laboratory Investigation ‐ Supplement 1968;105:1‐20. [PubMed] [Google Scholar]
NutriStroke 2009 {published data only}
- Garbagnati F, Cairella G, Martino A, Multari M, Scognamiglio U, Venturiero V, et al. Is antioxidant and n‐3 supplementation able to improve functional status in post‐stroke patients? Results from the Nutristroke Trial. Cerebrovascular Diseases (Basel, Switzerland) 2009;27(4):375‐83. [DOI: 10.1159/000207441] [DOI] [PubMed] [Google Scholar]
OFAMI ‐ Nilsen 2001 {published and unpublished data}
- Aarsetoy H, Brugger‐Andersen T, Hetland O, Grundt H, Nilsen DW. Long term influence of regular intake of high dose n‐3 fatty acids on CD40‐ligand, pregnancy‐associated plasma protein A and matrix metalloproteinase‐9 following acute myocardial infarction. Thrombosis & Haemostasis 2006;95(2):329‐36. [DOI] [PubMed] [Google Scholar]
- Grundt H, Hetland O, Nilsen DW. Changes in tissue factor and activated factor XII following an acute myocardial infarction were uninfluenced by high doses of n‐3 polyunsaturated fatty acids. Thrombosis & Haemostasis 2003;89(4):752‐9. [PubMed] [Google Scholar]
- Grundt H, Nilsen DW, Hetland O, Mansoor MA. Clinical outcome and atherothrombogenic risk profile after prolonged wash‐out following long‐term treatment with high doses of n‐3 PUFAs in patients with an acute myocardial infarction. Clinical Nutrition 2004;23(4):491‐500. [DOI] [PubMed] [Google Scholar]
- Grundt H, Nilsen DW, Mansoor MA, Hetland O, Nordoy A. Reduction in homocysteine by n‐3 polyunsaturated fatty acids after 1 year in a randomised double‐blind study following an acute myocardial infarction: no effect on endothelial adhesion properties. Pathophysiology of Haemostasis & Thrombosis 2003;33(2):88‐95. [DOI] [PubMed] [Google Scholar]
- Grundt H, Nilsen DW, Mansoor MA, Nordoy A. Increased lipid peroxidation during long‐term intervention with high doses of n‐3 fatty acids (PUFAs) following an acute myocardial infarction. European Journal of Clinical Nutrition 2003;57(6):793‐800. [DOI] [PubMed] [Google Scholar]
- Naesgaard PA, Grundt H, Brede C, Nilsen DW. The effect on vitamin D levels of long‐term high‐dose treatment with a concentrated omega‐3 compound (Omacor/lovaza) in patients hospitalized with a myocardial infarction. Circulation 2014;130(Suppl 2):A17245. [Google Scholar]
- Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high‐dose concentrate of n‐3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. American Journal of Clinical Nutrition 2001;74(1):50‐6. [DOI] [PubMed] [Google Scholar]
- Poenitz V, Grundt H, Bottazzi B, Cuccovillo I, Mantovani A, Nilsen DW. Pentraxin 3 is uninfluenced by high doses of concentrated omega‐3 fatty acids administered for 12 months following an acute myocardial infarction. Circulation 2012;126(21 Suppl 1):A13464. [Google Scholar]
OMEGA 2014 {published and unpublished data}
- Rauch B, Riemer T, Schwaab B, Schneider S, Diller F, Gohlke H, et al. Short‐term comprehensive cardiac rehabilitation after AMI is associated with reduced 1‐year mortality: results from the OMEGA study. European Journal of Preventive Cardiology 2014;21(9):1060‐9. [DOI] [PubMed] [Google Scholar]
- Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation 2010;122(21):2152‐9. [DOI: 10.1161/CIRCULATIONAHA.110.948562] [DOI] [PubMed] [Google Scholar]
- Rauch B, Schiele R, Schneider S, Gohlke H, Diller F, Gottwik M, et al. Highly purified omega‐3 fatty acids for secondary prevention of sudden cardiac death after myocardial infarction‐aims and methods of the OMEGA‐study. Cardiovascular Drugs and Therapy / Sponsored by the International Society of Cardiovascular Pharmacotherapy 2006;20(5):365‐75. [DOI: 10.1007/s10557-006-0495-6] [DOI] [PubMed] [Google Scholar]
OPAL ‐ Dangour 2010 {published and unpublished data}
- Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. Effect of 2‐y n‐3 long‐chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double‐blind, controlled trial. American Journal of Clinical Nutrition 2010;91(6):1725‐32. [DOI] [PubMed] [Google Scholar]
- Dangour AD, Allen E, Elbourne D, Fletcher A, Richards M, Uauy R. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. Journal of Nutrition, Health & Aging 2009;13(3):198‐202. [DOI] [PubMed] [Google Scholar]
- Dangour AD, Allen E, Elbourne D, Fletcher AE, Neveu MM, Uauy R, et al. N‐3 fatty acids and retinal function. Ophthalmology 2013;120(3):643. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Dangour AD, Clemens F, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. A randomised controlled trial investigating the effect of n‐3 long‐chain polyunsaturated fatty acid supplementation on cognitive and retinal function in cognitively healthy older people: the Older People And n‐3 Long‐chain polyunsaturated fatty acids (OPAL) study protocol [ISRCTN72331636]. Nutrition Journal 2006;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
ORIGIN 2012 {published data only}
- Bordeleau L, Yakubovich N, Dagenais G, Rosenstock J, Ryden LE, Spinas G, et al. Cancer outcomes in patients with dysglycemia on basal insulin: results of the ORIGIN trial. Diabetes 2013;62:A72. [Google Scholar]
- Bordeleau L, Yakubovich N, Dagenais GR, Rosenstock J, Probstfield J, Chang Yu P, et al. The association of basal insulin glargine and/or n‐3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 2014;37(5):1360‐6. [DOI] [PubMed] [Google Scholar]
- Lonn EM, Bosch J, Diaz R, Lopez‐Jaramillo P, Ramachandran A, Hancu N, et al. Effect of insulin glargine and n‐3FA on carotid intima‐media thickness in people with dysglycemia at high risk for cardiovascular events: the glucose reduction and atherosclerosis continuing evaluation study (ORIGIN‐GRACE). Diabetes Care 2013;36(9):2466‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioni AP, Fabbri G, Bosch J, Dyal L, Ryden LE, Gerstein HC, et al. Effects of n‐3 fatty acids on long‐term outcomes of high risk patients with type 2 diabetes mellitus or IGF/IGT with a recent myocardial infarction. European Heart Journal 2013;34:352. [Google Scholar]
- Origin Trial Investigators, Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, et al. N‐3 fatty acids and cardiovascular outcomes in patients with dysglycemia. New England Journal of Medicine 2012;367(4):309‐18. [DOI] [PubMed] [Google Scholar]
- Origin Trial Investigators, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN trial (Outcome Reduction with an Initial Glargine Intervention). American Heart Journal 2008;155(1):26‐32, 32. [DOI] [PubMed] [Google Scholar]
- Origin Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. New England Journal of Medicine 2012;367(4):319‐28. [DOI] [PubMed] [Google Scholar]
- Punthakee Z, Gerstein HC, Bosch J, Tyrwhitt J, Jung H, Lee SF, et al. Cardiovascular and other outcomes postintervention with insulin glargine and omega‐3 fatty acids (ORIGINALE). Diabetes Care 2016;39(5):709‐16. [DOI] [PubMed] [Google Scholar]
Oslo Diet‐Heart 1966 {published and unpublished data}
- Leren P. Prevention of coronary heart disease, some results from the Oslo secondary and primary intervention studies. Journal of the American College of Nutrition 1989;8:407‐10. [DOI] [PubMed] [Google Scholar]
- Leren P. The Oslo diet‐heart study. Eleven year report. Circulation 1970;42:935‐42. [DOI] [PubMed] [Google Scholar]
- Leren P. The effect of a cholesterol lowering diet in male survivors of myocardial infarction (a controlled clinical trial) [Virkningen av cholesterolsenkende diett hos menn som har gjennomgatt hjerteinfarkt. Et kontrollert klinisk fors/ok]. Nordisk Medicin 1967;77(21):658‐61. [PubMed] [Google Scholar]
- Leren P. The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Medica Scandinavica. Supplementum 1966;466:1‐92. [PubMed] [Google Scholar]
- Leren P. The effect of plasma‐cholesterol‐lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Bulletin of the New York Academy of Medicine 1968;44:1012‐20. [PMC free article] [PubMed] [Google Scholar]
Oxford Retinopathy 1978 {published and unpublished data}
- Coppack SW, Doll HA, Pim B, Hockaday TDR. Intravenous glucose tolerance and mortality in non‐insulin‐dependant diabetes mellitus. Quarterly Journal of Medicine 1990;75:451‐60. [PubMed] [Google Scholar]
- Hillson RM, Hockaday TDR, Mann JI, Newton DJ. Hyperinsulinaemia is associated with development of ECG abnormalities in diabetics. Diabetes Research 1984;1:143‐9. [PubMed] [Google Scholar]
- Hockaday TD, Hockaday JM, Mann JI, Turner RC. Prospective comparison of modified fat‐high‐carbohydrate with standard low‐carbohydrate dietary advice in the treatment of diabetes: one year follow‐up study. British Journal of Nutrition 1978;39(2):357‐62. [DOI] [PubMed] [Google Scholar]
- Howard‐Williams J, Patel P, Jelfs R, Carter RD, Awdry P, Bron A, et al. Polyunsaturated fatty acids and diabetic retinopathy. British Journal of Ophthalmology 1985;69(1):15‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Espinoza I, Howard WJ, Mann JI, Carter RD, Hockaday TD. Fatty acid composition of platelet phospholipids in non‐insulin‐dependent diabetics randomized for dietary advice. British Journal of Nutrition 1984;52(1):41‐7. [DOI] [PubMed] [Google Scholar]
Özaydin 2011 {published data only}
- Özaydin M, Erdoğan D, Tayyar S, Uysal BA, Doğan A, Içli A, et al. N‐3 polyunsaturated fatty acids administration does not reduce the recurrence rates of atrial fibrillation and inflammation after electrical cardioversion: a prospective randomized study. Anadolu Kardiyoloji Dergisi 2011;11(4):305‐9. [DOI: 10.5152/akd.2011.080] [DOI] [PubMed] [Google Scholar]
POUNDS Lost Sacks 2009 {published and unpublished data}
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight‐loss diets with different compositions of fat, protein, and carbohydrates. New England Journal of Medicine 2009;360(9):859‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, et al. Effects of 4 weight‐loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. American Journal of Clinical Nutrition 2012;95(3):614‐25. [DOI: 10.3945/ajcn.111.026328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez‐Ramirez 2013 {published data only}
- Ramirez‐Ramirez V, Macias‐Islas MA, Ortiz GG, Pacheco‐Moises F, Torres‐Sanchez ED, Sorto‐Gomez TE, et al. Efficacy of fish oil on serum of TNF α , IL‐1 β , and IL‐6 oxidative stress markers in multiple sclerosis treated with interferon beta‐1b. Oxidative Medicine and Cellular Longevity 2013;2013:709493. [DOI: 10.1155/2013/709493] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorto‐Gomez TE, Ortiz GG, Pacheco‐Moises FP, Torres‐Sanchez ED, Ramirez‐Ramirez V, Macias‐Islas MA, et al. Effect of fish oil on glutathione redox system in multiple sclerosis. American Journal of Neurodegenerative Disease 2016;5(2):145‐51. [PMC free article] [PubMed] [Google Scholar]
Reed 2014 {published and unpublished data}
- Olendzki BC, Leung K, Buskirk S, Reed G, Zurier RB. Treatment of rheumatoid arthritis with marine and botanical oils: influence on serum lipids. Evidence‐Based Complementary & Alternative Medicine: eCAM 2011;2011:827286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GW, Leung K, Rossetti RG, Vanbuskirk S, Sharp JT, Zurier RB. Treatment of rheumatoid arthritis with marine and botanical oils: an 18‐month, randomized, and double‐blind trial. Evidence‐Based Complementary & Alternative Medicine: eCAM 2014;2014:857456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Risk and Prevention {published and unpublished data}
- Rischio and Prevenzione Investigators. Efficacy of n‐3 polyunsaturated fatty acids and feasibility of optimizing preventive strategies in patients at high cardiovascular risk: rationale, design and baseline characteristics of the Rischio and Prevenzione study, a large randomised trial in general practice. Trials 2010;11(1):68. [DOI: 10.1186/1745-6215-11-68] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, et al. N‐3 fatty acids in patients with multiple cardiovascular risk factors. New England Journal of Medicine 2013;368(19):1800‐8. [DOI] [PubMed] [Google Scholar]
- Visentin G, Risk & Prevention Study Group. Towards evidence‐based practice via practice‐based evidence: the Italian experience. Family Practice 2008;25 Suppl 1:i71‐4. [DOI] [PubMed] [Google Scholar]
Sandhu 2016 {published data only}
- Sandhu N, Schetter SE, Liao J, Hartman TJ, Richie JP, McGinley J, et al. Influence of obesity on breast density reduction by omega‐3 fatty acids: evidence from a randomized clinical trial. Cancer Prevention Research (Philadelphia, Pa.) 2016;9(4):275‐82. [PUBMED: 26714774] [DOI] [PubMed] [Google Scholar]
- Signori C, DuBrock C, Richie JP, Prokopczyk B, Demers LM, Hamilton C, et al. Administration of omega‐3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. European Journal of Clinical Nutrition 2012;66(8):878‐84. [PUBMED: 22669332] [DOI] [PubMed] [Google Scholar]
Schirmer 2007 {published data only}
- Schirmer MA, Phinney SD. Gamma‐linolenate reduces weight regain in formerly obese humans. Journal of Nutrition 2007;137(6):1430‐5. [PUBMED: 17513402] [DOI] [PubMed] [Google Scholar]
SCIMO ‐ von Schacky 1999 {published and unpublished data}
- Angerer P, Kothny W, Stork S, Schacky C. Effect of dietary supplementation with omega‐3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovascular Research 2002;54(1):183‐90. [DOI] [PubMed] [Google Scholar]
- Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega‐3 fatty acids on coronary atherosclerosis. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1999;130(7):554‐62. [DOI] [PubMed] [Google Scholar]
- Schacky C, Baumann K, Angerer P. The effect of n‐3 fatty acids on coronary atherosclerosis: results from SCIMO, an angiographic study, background and implications. Lipids 2001;36 Suppl:S99‐102. [DOI] [PubMed] [Google Scholar]
Shinto 2014 {published data only}
- NCT00090402. Fish oil and alpha lipoic acid in treating Alzheimer's Disease. clinicaltrials.gov/ct2/show/NCT00090402 (first posted 26 August 2014).
- Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf‐Wagner S, et al. A randomized placebo‐controlled pilot trial of omega‐3 fatty acids and alpha lipoic acid in Alzheimer's disease. Journal of Alzheimer's Disease 2014;38(1):111‐20. [PUBMED: 24077434] [DOI] [PMC free article] [PubMed] [Google Scholar]
SHOT ‐ Eritsland 1996 {published and unpublished data}
- Eritsland J, Arnesen H, Berg K, Seljeflot I, Abdelnoor M. Serum Lp(a) lipoprotein levels in patients with coronary artery disease and the influence of long‐term n‐3 fatty acid supplementation. Scandinavian Journal of Clinical and Laboratory Investigation 1995;55(4):295‐300. [DOI] [PubMed] [Google Scholar]
- Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of dietary supplementation with n‐3 fatty acids on coronary artery bypass graft patency. American Journal of Cardiology 1996;77(1):31‐6. [DOI] [PubMed] [Google Scholar]
- Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of supplementation with n‐3 fatty acids on graft patency in patients undergoing coronary artery bypass operation. Results from SHOT study. European Heart Journal 1994;15:29. [Google Scholar]
- Eritsland J, Arnesen H, Seljeflot I, Hostmark AT. Long‐term metabolic effects of n‐3 polyunsaturated fatty acids in patients with coronary artery disease. American Journal of Clinical Nutrition 1995;61(4):831‐6. [DOI] [PubMed] [Google Scholar]
- Eritsland J, Arnesen H, Seljeflot I, Kierulf P. Long‐term effects of n‐3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagulation & Fibrinolysis 1995;6(1):17‐22. [DOI] [PubMed] [Google Scholar]
- Eritsland J, Seljeflot I, Abdelnoor M, Arnesen H. Long‐term influence of omega‐3 fatty acids on fibrinolysis, fibrinogen, and serum lipids. Fibrinolysis 1994;8(2):120‐5. [Google Scholar]
- Eritsland J, Seljeflot I, Abdelnoor M, Arnesen H, Torjesen PA. Long‐term effects of n‐3 fatty acids on serum lipids and glycaemic control. Scandinavian Journal of Clinical and Laboratory Investigation 1994;54(4):273‐80. [DOI] [PubMed] [Google Scholar]
- Eritsland J, Seljeflot I, Arnesen H, Abdelnoor M. Long‐term effects of fish oil supplementation in patients with coronary artery disease: influence on lipoproteins, coagulation and fibrinolysis [abstract]. Thrombosis Research 1992;65:75. [Google Scholar]
- Eritsland J, Seljeflot I, Arnesen H, Abdelnoor M. Long‐term influence of omega‐3 fatty acids on fibrinolysis, fibrinogen, and serum lipids. Thrombosis and Haemostasis 1993;69:1065. [Google Scholar]
- Eritsland J, Seljeflot I, Arnesen H, Westvik AB, Kierulf P. Effect of long‐term, moderate‐dose supplementation with omega‐3 fatty acids on monocyte procoagulant activity and release of interleukin‐6 in patients with coronary artery disease. Thrombosis Research 1995;77(4):337‐46. [DOI] [PubMed] [Google Scholar]
Sianni 2013 {published data only}
- Sianni A, Matsoukis I, Ganotopoulou A, Paraskevas P, Asimis A, Tsivilis N, et al. PP128‐sun effect of omega 3 fatty acids in patients with hypertension and atrial fibrillation. Clinical Nutrition 2013;32(Suppl 1):S70‐1. [Google Scholar]
SMART Tapsell 2013 {published data only}
- Anil S, Charlton KE, Tapsell LC, Probst Y, Ndanuko R, Batterham MJ. Identification of dietary patterns associated with blood pressure in a sample of overweight Australians. Journal of Human Hypertension 2016;30(11):672‐8. [DOI: 10.1038/jhh.2016.10] [DOI] [PubMed] [Google Scholar]
- Tapsell LC, Batterham MJ, Charlton KE. Effect of dietary restriction and n‐3 PUFA supplementation on insulin resistance in obese adults. FASEB Journal 2010;24:733.9. [Google Scholar]
- Tapsell LC, Batterham MJ, Charlton KE, Neale EP, Probst YC, O'Shea JE, et al. Foods, nutrients or whole diets: effects of targeting fish and LCn3PUFA consumption in a 12mo weight loss trial. BMC Public Health 2013;13:1231. [PUBMED: 24369765] [DOI] [PMC free article] [PubMed] [Google Scholar]
SOFA 2006 {published and unpublished data}
- Brouwer IA, Katan MB, Schouten EG, Camm AJ, Hauer RNW, Wever EFD, et al. Rationale and design of a clinical trial on n‐3 fatty acids and cardiac arrhythmia (SOFA). Annals of Nutrition & Metabolism 2001;45(Suppl 1):79. [Google Scholar]
- Brouwer IA, Raitt MH, Dullemeijer C, Kraemer DF, Zock PL, Morris C, et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. European Heart Journal 2009;30(7):820‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, Wever EF, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega‐3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA 2006;295(22):2613‐9. [DOI] [PubMed] [Google Scholar]
- Brouwer IA, Zock PL, Wever EFD, Hauer RNW, Camm AJ, Bocker D, et al. Rationale and design of a randomised controlled clinical trial on supplemental intake of n‐3 fatty acids and incidence of cardiac arrhythmia: SOFA. European Journal of Clinical Nutrition 2003;57:1323‐30. [DOI] [PubMed] [Google Scholar]
Sofi 2010 {published data only}
- Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF, et al. Effects of a 1‐year dietary intervention with n‐3 polyunsaturated fatty acid‐enriched olive oil on non‐alcoholic fatty liver disease patients: a preliminary study. International Journal of food Sciences and Nutrition 2010;61(8):792‐802. [PUBMED: 20465434] [DOI] [PubMed] [Google Scholar]
STARS 1992 {published and unpublished data}
- Blann AD, Jackson P, Bath PM, Watts GF. Von Willebrand factor, a possible indicator of endothelial cell damage, decreases during long‐term compliance with a lipid‐lowering diet. Journal of Internal Medicine 1995;237:557‐61. [DOI] [PubMed] [Google Scholar]
- Watts GF. Nutritional, metabolic, and genetic determinants of the progression of coronary heart disease. STARS Group. Journal of Cardiovascular Pharmacology 1995;25(Suppl 4):S11‐9. [PubMed] [Google Scholar]
- Watts GF, Brunt JNH, Coltart DJ, Lewis B. The St. Thomas Atherosclerosis Regression Study (STARS). Atherosclerosis 1992;97:231. [Google Scholar]
- Watts GF, Jackson P, Burke V, Lewis B. Dietary fatty acids and progression of coronary artery disease in men. American Journal of Clinical Nutrition 1996;64:202‐9. [DOI] [PubMed] [Google Scholar]
- Watts GF, Jackson P, Mandalia S, Brunt JN, Lewis ES, Coltart DJ, et al. Nutrient intake and progression of coronary artery disease. American Journal of Cardiology 1994;73(5):328‐32. [DOI] [PubMed] [Google Scholar]
- Watts GF, Lewis B, Brunt JN, Lewis ES, Coltart DJ, Smith LD, et al. Effects on coronary artery disease of lipid‐lowering diet, or diet plus cholestyramine, in the St Thomas' Atherosclerosis Regression Study (STARS). Lancet 1992;339(8793):563‐9. [DOI] [PubMed] [Google Scholar]
- Watts GF, Lewis B, Brunt JNH, Swan AV. Coronary atheroma regression trials. Lancet 1992;339(i):1241‐3. [PubMed] [Google Scholar]
- Watts GF, Lewis B, Jackson P, Burke V, Lewis ES, Brunt JN, et al. Relationships between nutrient intake and progression/regression of coronary atherosclerosis as assessed by serial quantitative angiography. Canadian Journal of Cardiology 1995;11(Suppl G):110G‐4G. [PubMed] [Google Scholar]
- Watts GF, Mandalia S, Brunt JN, Slavin BM, Coltart DJ, Lewis B. Independent associations between plasma lipoprotein subfraction levels and the course of coronary artery disease in the St. Thomas' Atherosclerosis Regression Study (STARS). Metabolism: Clinical and Experimental 1993;42:1461‐7. [DOI] [PubMed] [Google Scholar]
- Watts GF, Mandalia S, Slavin BM, Brunt JN, Coltart DJ, Lewis B. Metabolic determinants of the course of coronary artery disease in men. Clinical Chemistry 1994;40(12):2240‐6. [PubMed] [Google Scholar]
Stoll 2001 {published data only}
- NCT00010868. Omega‐3 fatty acids in bipolar disorder. clinicaltrials.gov/ct2/show/NCT00010868 (first posted 5 February 2001).
STRENGTH {published data only}
- NCT02104817. Outcomes study to assess statin residual risk reduction with EpaNova in high CV risk patients with hypertriglyceridemia (STRENGTH). clinicaltrials.gov/ct2/show/NCT02104817 2014.
SU.FOL.OM3 Galan 2010 {published and unpublished data}
- Ahluwalia N, Blacher J, Szabo De Edelenyi F, Faure P, Julia C, Hercberg S, et al. Prognostic value of multiple emerging biomarkers in cardiovascular risk prediction in patients with stable cardiovascular disease. Atherosclerosis 2013;228(2):478‐84. [DOI] [PubMed] [Google Scholar]
- Andreeva VA, Galan P, Torres M, Julia C, Hercberg S, Kesse‐Guyot E. Supplementation with B vitamins or n‐3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B‐6 and B‐12 and/or OMega‐3 fatty acids (SU.FOL.OM3) randomized trial. American Journal of Clinical Nutrition 2012;96(1):208‐14. [DOI] [PubMed] [Google Scholar]
- Andreeva VA, Kesse‐Guyot E, Barberger‐Gateau P, Fezeu L, Hercberg S, Galan P. Cognitive function after supplementation with B vitamins and long‐chain omega‐3 fatty acids: ancillary findings from the SU.FOL.OM3 randomized trial. American Journal of Clinical Nutrition 2011;94(1):278‐86. [DOI] [PubMed] [Google Scholar]
- Andreeva VA, Latarche C, Hercberg S, Briancon S, Galan P, Kesse‐Guyot E. B vitamin and/or n‐3 fatty acid supplementation and health‐related quality of life: ancillary findings from the SU.FOL.OM3 randomized trial. PLoS One 2014;9(1):e84844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva VA, Touvier M, Kesse‐Guyot E, Julia C, Galan P, Hercberg S. B vitamin and/or omega‐3 fatty acid supplementation and cancer: ancillary findings from the supplementation with folate, vitamins B6 and B12, and/or omega‐3 fatty acids (SU.FOL.OM3) randomized trial. Archives of Internal Medicine 2012;172(7):540‐7. [DOI] [PubMed] [Google Scholar]
- Arnaud J, Bost M, Vitoux D, Labarere J, Galan P, Faure H, et al. Effect of low dose antioxidant vitamin and trace element supplementation on the urinary concentrations of thromboxane and prostacyclin metabolites. Journal of the American College of Nutrition 2007;26(5):405‐11. [DOI] [PubMed] [Google Scholar]
- Astorg P, Bertrais S, Alessandri JM, Guesnet P, Kesse‐Guyot E, Linard A, et al. Long‐chain n‐3 fatty acid levels in baseline serum phospholipids do not predict later occurrence of depressive episodes: a nested case‐control study within a cohort of middle‐aged French men and women. Prostaglandins Leukotrienes & Essential Fatty Acids 2009;81(4):265‐71. [DOI] [PubMed] [Google Scholar]
- Astorg P, Couthouis A, Bertrais S, Arnault N, Meneton P, Guesnet P, et al. Association of fish and long‐chain n‐3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle‐aged French men and women. Prostaglandins Leukotrienes & Essential Fatty Acids 2008;78(3):171‐82. [DOI] [PubMed] [Google Scholar]
- Blacher J, Czernichow S, Paillard F, Ducimetiere P, Hercberg S, Galan P, et al. Cardiovascular effects of B‐vitamins and/or N‐3 fatty acids: the SU.FOL.OM3 trial. International Journal of Cardiology 2013;167(2):508‐13. [DOI] [PubMed] [Google Scholar]
- Blacher J, Safar ME, Ly C, Szabo De Edelenyi F, Hercberg S, Galan P. Blood pressure variability: cardiovascular risk integrator or independent risk factor. Journal of Human Hypertension 2015;29(2):122‐6. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Bruckert E, Oppert JM, Bertrais S, Paillard F, Astorg P, et al. Intake of added oils and fats among middle‐aged French adults: relationships with educational level and region of residence. Journal of the American Dietetic Association 2005;105(12):1889‐94. [DOI] [PubMed] [Google Scholar]
- Fezeu LK, Laporte F, Kesse‐Guyot E, Andreeva VA, Blacher J, Hercberg S, et al. Baseline plasma fatty acids profile and incident cardiovascular events in the SU.FOL.OM3 trial: the evidence revisited. PLoS One 2014;9(4):e92548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P, Briancon S, Blacher J, Czernichow S, Hercberg S. The SU.FOL.OM3 study: a secondary prevention trial testing the impact of supplementation with folate and B‐vitamins and/or Omega‐3 PUFA on fatal and non fatal cardiovascular events, design, methods and participants characteristics. Trials 2008;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P, Briancon S, Blacher J, Czernichow S, Hercberg S. The SU.FOL.OM3 study: a secondary prevention trial testing the impact of supplementation with folate and B‐vitamins and/or omega‐3 PUFA on fatal and non fatal cardiovascular events, design, methods and participants characteristics. Trials 2008;9:35. [DOI: 10.1186/1745-6215-9-35] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P, Briancon S, Blacher J, Czernichow S, Hercberg S. The scientific basis of the SU.FOL.OM3 study: a secondary intervention trial of folate, B6 and B12 vitamins and/or omega3 fatty acid supplements in the prevention of recurrent ischemic events. Sang Thrombose Vaisseaux 2009;21(4):207‐13. [Google Scholar]
- Galan P, Briançon S, Blacher J, Czernichow S, Hercberg S. The scientific basis of the SU.FOL.OM3 study: a secondary intervention trial of folate, B6 and B12 vitamins and/or omega3 fatty acid supplements in the prevention of recurrent ischemic events [Bases scientifiques de l'étude SUFOLOM3: essai de prévention secondaire visant à tester l'impact d'une supplémentation en folates, vitamines B6 et B12 et/ ou acides gras oméga‐3 dans la prévention de la récidive de pathologies ischémiques]. Sang Thrombose Vaisseaux 2009;21(4):207‐13. [Google Scholar]
- Galan P, Kesse‐Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. [DOI: 10.1136/bmj.c6273] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P, Bree A, Mennen L, Potier de Courcy G, Preziozi P, Bertrais S, et al. Background and rationale of the SU.FOL.OM3 study: double‐blind randomized placebo‐controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega‐3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries. Journal of Nutrition, Health & Aging 2003;7(6):428‐35. [PubMed] [Google Scholar]
- Julia C, Meunier N, Touvier M, Ahluwalia N, Sapin V, Papet I, et al. Dietary patterns and risk of elevated C‐reactive protein concentrations 12 years later. British Journal of Nutrition 2013;110(4):747‐54. [DOI] [PubMed] [Google Scholar]
- Julia C, Touvier M, Meunier N, Papet I, Galan P, Hercberg S, et al. Intakes of PUFAs were inversely associated with plasma C‐reactive protein 12 years later in a middle‐aged population with vitamin E intake as an effect modifier. Journal of Nutrition 2013;143(11):1760‐6. [DOI] [PubMed] [Google Scholar]
- Kesse‐Guyot E, Peneau S, Hercberg S, Galan P, Vogt L, Escande M, et al. Thirteen‐year prospective study between fish consumption, long‐chain n‐3 fatty acids intakes and cognitive function. Journal of Nutrition, Health and Aging 2011;15(2):115‐20. [DOI] [PubMed] [Google Scholar]
- Latreille J, Kesse‐Guyot E, Malvy D, Andreeva V, Galan P, Tschachler E, et al. Association between dietary intake of n‐3 polyunsaturated fatty acids and severity of skin photoaging in a middle‐aged Caucasian population. Journal of Dermatological Science 2013;72(3):233‐9. [DOI] [PubMed] [Google Scholar]
- Latreille J, Kesse‐Guyot E, Malvy D, Andreeva V, Galan P, Tschachler E, et al. Dietary monounsaturated fatty acids intake and risk of skin photoaging. PLoS One 2012;7(9):e44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouchieu C, Chajes V, Laporte F, Kesse‐Guyot E, Galan P, Hercberg S, et al. Prospective associations between plasma saturated, monounsaturated and polyunsaturated fatty acids and overall and breast cancer risk ‐ modulation by antioxidants: a nested case‐control study. PLoS One 2014;9(2):e90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo De Edelenyi F, Vergnaud AC, Ahluwalia N, Julia C, Hercberg S, Blacher J, et al. Effect of B‐vitamins and n‐3 PUFA supplementation for 5 years on blood pressure in patients with CVD. British Journal of Nutrition 2012;107(6):921‐7. [DOI] [PubMed] [Google Scholar]
- Touvier M, Kesse‐Guyot E, Andreeva VA, Fezeu L, Charnaux N, Sutton A, et al. Modulation of the association between plasma intercellular adhesion molecule‐1 and cancer risk by n‐3 PUFA intake: a nested case‐control study. American Journal of Clinical Nutrition 2012;95(4):944‐50. [DOI] [PubMed] [Google Scholar]
- Vesin C, Galan P, Gautier B, Czernichow S, Hercberg S, Blacher J. Control of baseline cardiovascular risk factors in the SU‐FOL‐OM3 study cohort: does the localization of the arterial event matter?. European Journal of Cardiovascular Prevention & Rehabilitation 2010;17(5):541‐8. [DOI] [PubMed] [Google Scholar]
- Bree A, Mennen LI, Hercberg S, Galan P. Evidence for a protective (synergistic?) effect of B‐vitamins and omega‐3 fatty acids on cardiovascular diseases. European Journal of Clinical Nutrition 2004;58(5):732‐44. [DOI] [PubMed] [Google Scholar]
Søndergaard 2003 {published and unpublished data}
- Sondergaard E, Moller JE, Egstrup K. Effect of dietary intervention and lipid‐lowering treatment on brachial vasoreactivity in patients with ischemic heart disease and hypercholesterolemia. American Heart Journal 2003;145(5):E19. [DOI] [PubMed] [Google Scholar]
Tande 2016 {published data only}
- Tande KS, Vo TD, Lynch BS. Clinical safety evaluation of marine oil derived from Calanus finmarchicus. Regulatory Toxicology and Pharmacology : RTP 2016;80:25‐31. [PUBMED: 27233921] [DOI] [PubMed] [Google Scholar]
Tay 2015 {published data only}
- Tay J, Luscombe‐Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. Comparison of low‐ and high‐carbohydrate diets for type 2 diabetes management: a randomized trial. American Journal of Clinical Nutrition 2015;102(4):780‐90. [DOI] [PubMed] [Google Scholar]
- Tay J, Thompson CH, Luscombe‐Marsh ND, Noakes M, Buckley JD, Wittert GA, et al. Long‐term effects of a very low carbohydrate compared with a high carbohydrate diet on renal function in individuals with type 2 diabetes: a randomized trial. Medicine 2015;94(47):e2181. [DOI: 10.1097/MD.0000000000002181] [DOI] [PMC free article] [PubMed] [Google Scholar]
THIS DIET ‐ Tuttle 2008 {published and unpublished data}
- Tuttle KR, Shuler LA, Packard DP, Milton JE, Daratha KB, Bibus DM, et al. Comparison of low‐fat versus Mediterranean‐style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). American Journal of Cardiology 2008;101(11):1523‐30. [PUBMED: 18489927] [DOI] [PubMed] [Google Scholar]
VITAL {published data only}
- Bassuk SS, Manson JE, Lee IM, Cook NR, Christen WG, Bubes VY, et al. Baseline characteristics of participants in the VITamin D and OmegA‐3 TriaL (VITAL). Contemporary Clinical Trials 2016;47:235‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Litonjua AA, Carey VJ, Manson JE, Buring JE, Lee IM, et al. Lung VITAL: rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega‐3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults. Contemporary Clinical Trials 2016;47:185‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Luttmann‐Gibson H, Litonjua AA, Friedenberg G, Gordon D, Lee IM, et al. Baseline chronic obstructive pulmonary disease in the lung vitamin D and omega‐3 trial. American Journal of Respiratory and Critical Care Medicine 2014;189:D44 COPD. [Google Scholar]
- Kang JH, Grodstein F, Manson JAE. Cognitive substudy of the vitamin d and omega‐3 trial (VITAL‐Cog): design of a large randomized trial of omega‐3 and vitamin d supplements in relation to cognitive change. Alzheimer's & Dementia 2015;1:608. [Google Scholar]
- LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL‐bone health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega‐3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA‐3 TriaL (VITAL). Contemporary Clinical Trials 2015;41:259‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JAE. Vitamin D and cancer and cardiovascular disease: ready for prime time?. Menopause 2010;17(6):1215. [Google Scholar]
- Manson JE. Vitamin D and the heart: why we need large‐scale clinical trials. Cleveland Clinic Journal of Medicine 2010;77(12):903‐10. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and omegA‐3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega‐3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemporary Clinical Trials 2012;33(1):159‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE. Update on the Vitamin D and OmegA‐3 trial (VITAL). Journal of Steroid Biochemistry & Molecular Biology 2016;155(Pt B):252‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinstock‐Guttman 2005 {published data only}
- Weinstock‐Guttman B, Baier M, Park Y, Feichter J, Lee‐Kwen P, Gallagher E, et al. Low fat dietary intervention with n‐3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins, Leukotrienes and Essential Fatty Acids 2005;73:397‐404. [DOI: 10.1016/j.plefa.2005.05.024] [DOI] [PubMed] [Google Scholar]
WHI 2006 {published data only}
- Anderson G, Cummings S, Freedman LS, Furberg C, Henderson M, Johnson SR, et al. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Annals of Epidemiology 2003;13(9 Suppl):S5‐17. [DOI] [PubMed] [Google Scholar]
- Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low‐fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):643‐54. [DOI] [PubMed] [Google Scholar]
- Bowen D, Ehret C, Pedersen M, Snetselaar L, Johnson M, Tinker L, et al. Results of an adjunct dietary intervention program in the Women's Health Initiative. Journal of the American Dietetic Association 2002;102(11):1631‐7. [DOI] [PubMed] [Google Scholar]
- Carty CL, Kooperberg C, Neuhouser ML, Tinker L, Howard B, Wactawski‐Wende J, et al. Low‐fat dietary pattern and change in body‐composition traits in the Women's Health Initiative Dietary Modification Trial. American Journal of Clinical Nutrition 2011;93:516‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Annals of Epidemiology 2003;13(9 Suppl):S122‐8. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Annals of Epidemiology 2003;13(9 Suppl):S18‐77. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Patterson RE, Gorfine M, Ebbeling CB, Jeor ST, Chlebowski RT, et al. Differences between estimated caloric requirements and self‐reported caloric intake in the Women's Health Initiative. Annals of Epidemiology 2003;13(9):629‐37. [DOI] [PubMed] [Google Scholar]
- Howard BV. Dietary fat and cardiovascular disease: putting the Women's Health Initiative in perspective. Nutrition Metabolism & Cardiovascular Diseases 2007;17(3):171‐4. [DOI] [PubMed] [Google Scholar]
- Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, et al. Low‐fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA 2006;295(1):39‐49. [DOI] [PubMed] [Google Scholar]
- Howard BV, Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil‐Smoller S, et al. Low‐fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):655‐66. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, et al. Use of recovery biomarkers to calibrate nutrient consumption self‐reports in the Women's Health Initiative. American Journal of Epidemiology 2008;167(10):1247‐59. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal A, Rodabough R, Caan B, Lillington L, Mossavar‐Rahmani Y, et al. Changes in food sources of dietary fat in response to an intensive low‐fat dietary intervention: early results from the Women's Health Initiative. Journal of the American Dietetic Association 2003;103(4):454‐60. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, gurs‐Collins T, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Annals of Epidemiology 1999;9(3):178‐87. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low‐fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):629‐42. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low‐fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. Journal of the National Cancer Institute 2007;99(20):1534‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels‐Tinker L, Howard B, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13(9 Suppl):S87‐97. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Wallace R, Safford MM, Pettinger M, Cochrane B, Ko MG, et al. Another treatment gap: restarting secondary prevention medications: the Women's Health Initiative. Journal of Clinical Lipidology 2010;4:36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Finnegan LP, Harlan WR, Pinn VW, Clifford C, McGowan JA. The evolution of the Women's Health Initiative: perspectives from the NIH. Journal of the American Medical Women's Association 1995;50(2):50‐5. [PubMed] [Google Scholar]
- The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Bonds DE, Margolis KL, Manson JE, Howard BV, Larson J, et al. Low‐fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women's Health Initiative randomized controlled dietary modification trial. Archives of Internal Medicine 2008;168(14):1500‐11. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Perri MG, Patterson RE, Bowen DJ, McIntosh M, Parker LM, et al. The effects of physical and emotional status on adherence to a low‐fat dietary pattern in the Women's Health Initiative. Journal of the American Dietetic Association 2002;102(6):789‐800. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Rosal MC, Young AF, Perri MG, Patterson RE, Horn L, et al. Predictors of dietary change and maintenance in the Women's Health Initiative Dietary Modification Trial. Journal of the American Dietetic Association 2007;107(7):1155‐66. [DOI] [PubMed] [Google Scholar]
- Women's Health Initiative Study Group. Dietary adherence in the Women's Health Initiative Dietary Modification Trial. Journal of the American Dietetic Association 2004;104(4):654‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang YP, Miao R, Li Q, Wu T, Ma F. Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: A 12‐month randomized, double‐blind, placebo‐controlled trial. Journal of Alzheimer's Disease 2016;55(2):497‐507. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
AC Omega3 {published data only}
- ACTRN12614000732684. The Aboriginal cardiovascular omega‐3 randomised controlled trial [The effect of omega‐3 supplementation on adverse cardiovascular (CV) events among Indigenous Australians with stable coronary artery disease: a randomized controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366337 (date registered 10 July 2014).
ACTRN12610000594022 {published data only}
- ACTRN12610000594022. Fish oil as adjunct therapy for periodontitis [Clinical efficacy of fish oil as adjunct therapy for patients with chronic periodontitis]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335470 (date registered 23 July 2010).
ACTRN12613000034730 {published data only}
- ACTRN12613000034730. Intervention of testosterone & fish oil as a possible strategy for the prevention of Alzheimer’s Disease [A 56 week, double‐blind, randomised, placebo‐controlled trial to determine the efficacy of testosterone, with and without DHA supplementation in PiB positive men with subjective memory complaints as a strategy to prevent the development of Alzheimer’s disease]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363372 (date registered 14 January 2013).
AFORRD {published and unpublished data}
- Holman RR, Paul S, Farmer A, Tucker L, Stratton IM, Neil HA, et al. Atorvastatin in factorial with omega‐3 EE90 risk reduction in diabetes (AFORRD): a randomised controlled trial. Diabetologia 2009;52(1):50‐9. [DOI] [PubMed] [Google Scholar]
- Neil HA, Ceglarek U, Thiery J, Paul S, Farmer A, Holman RR. Impact of atorvastatin and omega‐3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: a randomised placebo controlled factorial trial. Atherosclerosis 2010;213(2):512‐7. [DOI] [PubMed] [Google Scholar]
Beyond Aging Project {published data only}
- Cockayne NL, Duffy SL, Bonomally R, English A, Amminger PG, Mackinnon A, et al. The Beyond Ageing Project Phase 2‐‐a double‐blind, selective prevention, randomised, placebo‐controlled trial of omega‐3 fatty acids and sertraline in an older age cohort at risk for depression: study protocol for a randomized controlled trial. Trials 2015;16:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandrakala 2010 {published data only (unpublished sought but not used)}
- Chandrakala G, Arpana G, Rao PV. Long‐term effects of a reduced fat diet intervention in pre‐diabetes. professional.diabetes.org/meeting/scientific‐sessions/70th‐scientific‐sessions‐2010 (accessed before 11 April 2018).
- Chandrakala G, Arpana G, Sreenivas T, Rao PV. Low‐fat (<20%) diets prevent type 2 diabetes mellitus. Diabetes 2012;61:A190. [Google Scholar]
n‐3 for Vascular Cognitive Aging {published data only}
- Shinto L, Silbert LC, Dodge HH, Quinn JF, Howieson D, Kaye J, et al. Omega 3 fatty acids for the prevention of vascular cognitive aging: methods and rationale for a phase II trial. Alzheimer's & Dementia 2015:P2‐305.
n‐3 on plasma lipid {published data only}
- ChiCTR‐TRC‐12002014. Influence of different source of n‐3 fatty acid on plasma lipid in moderately hypercholesterolemia subject and the valid dosage. www.chictr.org.cn/hvshowproject.aspx?id=2374 (date last refreshed on 3 May 2015).
NCT00309439 {published data only}
- NCT00309439. ALA and prostate cancer. clinicaltrials.gov/ct2/show/NCT00309439 (first posted 31 March 2006).
NCT00410020 {published data only}
- NCT00410020. Arrhythmia prevention with an alpha‐linolenic enriched diet. clinicaltrials.gov/ct2/show/NCT00410020 (first posted 12 December 2006).
NCT01047449 {published data only}
- NCT01047449. Improving the results of heart bypass surgery using new approaches to surgery and medication (SUPERIORSVG). clinicaltrials.gov/ct2/show/NCT01047449 (first posted 13 January 2010).
NCT01513252 {published data only}
- NCT01513252. Long‐term effects of interventional strategies to prevent cognitive decline in elderly (MAPT‐PLUS). clinicaltrials.gov/ct2/show/NCT01513252 (first posted 20 January 2012).
NCT01784042 {published data only}
- NCT01784042. Dietary energy restriction and omega‐3 fatty acids on mammary tissue. clinicaltrials.gov/ct2/show/NCT01784042 (first posted 5 February 2013).
NCT02128763 {published data only}
- NCT02128763. Dry eye assessment and management study (DREAM). clinicaltrials.gov/ct2/show/NCT02128763 (first posted 1 May 2014).
NCT02211560 {published data only}
- NCT02211560. Investigating a phosphatidylserine based dietary approach for the management of mild cognitive impairment. clinicaltrials.gov/ct2/show/NCT02211560 (first posted 7 August 2014).
NCT02295059 {published data only}
- NCT02295059. Omega 3 fatty acids and ERPR(‐)HER2(+/‐) breast cancer prevention. clinicaltrials.gov/ct2/show/NCT02295059 (first posted 20 November 2014).
NCT02676466 {published data only}
- NCT02676466. The ENRGISE pilot study (ENRGISE). clinicaltrials.gov/ct2/show/NCT02676466 (first posted 8 February 2016).
NCT02719327 {published data only}
- NCT02719327. Brain amyloid and vascular effects of eicosapentaenoic acid (BRAVE‐EPA). clinicaltrials.gov/ct2/show/NCT02719327 (first posted 25 March 2016).
OMEMI {published data only}
- Laake K, Myhre P, Nordby LM, Seljeflot I, Abdelnoor M, Smith P, et al. Effects of omega 3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study. BMC Geriatrics 2014;14:74. [http://www.biomedcentral.com/1471‐2318/14/74] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laake K, Seljeflot I, Schmidt EB, Myhre P, Tveit A, Arnesen H, et al. Serum fatty acids, traditional risk factors, and comorbidity as related to myocardial injury in an elderly population with acute myocardial infarction. Journal of Lipids 2016;2016:4945720. [http://dx.doi.org/10.1155/2016/4945720] [DOI] [PMC free article] [PubMed] [Google Scholar]
REDUCE‐IT {published data only}
- Bhatt DL, Steg G, Brinton EA, Jacobson TA, Miller M, Tardif J‐C, et al. Rationale and design of REDUCE‐IT: Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. Clinical Cardiology 2017;40:138‐48. [DOI: 10.1002/clc.22692] [DOI] [PMC free article] [PubMed] [Google Scholar]
seAFOOD {published data only}
- Hull MA, Sandell AC, Montgomery AA, Logan RF, Clifford GM, Rees CJ, et al. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized controlled trial. Trials 2013;14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN05926847. The seAFOod (Systematic Evaluation of Aspirin and Fish Oil) polyp prevention trial. www.isrctn.com/ISRCTN05926847 (data assigned 6 May 2011).
UMIN000012825 {published data only}
- UMIN000012825. Effect of polysaturated fatty acids on vascular healing process in hyper‐cholesterolemic patients with acute coronary syndrome. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000014981 (first posted 1 February 2014).
Additional references
4S Trial 1994
- 4S trial authors. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383‐9. [PubMed] [Google Scholar]
Abdelhamid 2017
- Abdelhamid A, Hooper L, Welch A. Polyunsaturated fatty acids for musculoskeletal health and functional status in older adults. PROSPERO 2017; Vol. www.crd.york.ac.uk/prospero/display_record.php?RecordID=79211:CRD42017079211.
Abdelhamid 2018
- Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, et al. Omega‐3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD003177.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkley 1995
- Berkley CS, Hoaglin DC, Mosteller F, Colditz GA. A random‐effects regression model for meta‐analysis. Statistics in Medicine 1995;14(4):395‐411. [DOI] [PubMed] [Google Scholar]
Bhatnagar 2016
- Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016;102:1945‐1952. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017
- Brown T, Song F, Wang X, Brainard J, Hooper L. Dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus. PROSPERO 2017:CRD42017064110. [DOI] [PMC free article] [PubMed]
Burr 1989
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2(8666):757‐61. [DOI] [PubMed] [Google Scholar]
Calder 2012
- Calder PC. Mechanisms of action of (n‐3) fatty acids. Journal of Nutrition 2012;142(3):592S‐9S. [DOI: 10.3945/%E2%80%8Bjn.111.155259] [DOI] [PubMed] [Google Scholar]
Carandang 2006
- Carandang R, Seshadri S, Beiser A, Kelly‐Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30‐day mortality of stroke over the past 50 years. JAMA 2006;296(24):2939‐46. [DOI: 10.1001/jama.296.24.2939] [DOI] [PubMed] [Google Scholar]
Carlisle 2017
- Carlisle JB. Data fabrication and other reasons for non‐random sampling in5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944‐952. [DOI: 10.1111/anae.13938] [DOI] [PubMed] [Google Scholar]
de Souza 2015
- Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta‐analysis of observational studies. BMJ 2015;351:h3978. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Feigin 2014
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245‐55. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedewald 1972
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499‐502. [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 26 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hanson 2017a
- Hanson S, Biswas P, Jimoh OF, O'Brien A, Hooper L, Abdelhamid A, et al. Dietary polyunsaturated fat for prevention and treatment of depression and anxiety. PROSPERO 2017:CRD42017056092.
Hanson 2017b
- Hanson S, Thorpe G, Winstanley L, Abdelhamid A, Hooper L. Effects of supplementary dietary polyunsaturated fat on cancer incidence. PROSPERO 2017:CRD42017056109.
Hegsted 1965
- Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. American Journal of Clinical Nutrition 1965;17(5):281‐95. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hooper 2006
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 2006;322:752. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2015a
- Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011737] [DOI] [PubMed] [Google Scholar]
Hooper 2015b
- Hooper L, Abdelhamid A, Bunn D, Brown T, Summerbell CD, Skeaff DM. Effects of total fat intake on body weight. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011834] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2018
- Hooper L, Al‐Khudairy L, Abdelhamid AS, Rees K, Brainard JS, Brown TJ, et al. Omega‐6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD011094.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jimoh 2017
- Jimoh OF, Brainard J, Deane KA, Biswas P, Donaldson D, Hooper L. Dietary polyunsaturated fat for prevention and treatment of neurocognitive disorders. PROSPERO 2017:CRD42017019049.
Keys 1950
- Keys A, Mickelsen O, Miller EVO, Carleton B. The relation in man between cholesterol levels in the diet and in the blood. Science 1950;112:79‐81. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095‐128. [DOI: 10.1016/S0140-6736(12)61728-0.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLoS 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozaffarian 2010
- Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta‐analysis of randomized controlled trials. PLoS Medicine 2010;7(3):e1000252. [DOI: 10.1371/journal.pmed.1000252.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohwada 2016
- Ohwada T, Yokokawa T, Kanno Y, Hotsuki Y, Sakamoto T, Watanabe K, et al. Vascular composition data supporting the role of N‐3 polyunsaturated fatty acids in the prevention of cardiovascular disease events. Data Brief 2016;7:1237‐47. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 1953
- Oliver MF, Boyd GS. The plasma lipids in coronary artery disease. British Heart Journal 1953;15:387‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
QRISK 2‐2017
- ClinRisk Ltd. QRISK®2‐2017 risk calculator. qrisk.org/2017/index.php (accessed before 11 April 2018).
Ramsden 2010
- Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. N‐6 fatty acid‐specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta‐analysis of randomised controlled trials. British Journal of Nutrition 2010;104(11):1586‐600. [DOI: 10.1017/S0007114510004010] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Ricci 2018
- Ricci C, Baumgartner J, Zec M, Kruger HS, Smuts CM. Type of dietary fat intakes in relation to all‐cause and cause‐specific mortality in US adults: an iso‐energetic substitution analysis from the American National Health and Nutrition Examination Survey linked to the US mortality registry. British Journal of Nutrition 2018;119(4):456‐63. [DOI: 10.1017/S0007114517003889] [DOI] [PubMed] [Google Scholar]
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI: ] [DOI] [PubMed] [Google Scholar]
Russo 2009
- Russo GL. Dietary n‐6 and n‐3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochemical Pharmacology 2009;77(6):937‐46. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16:1‐82. [DOI] [PubMed] [Google Scholar]
Schultz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sharp 1998
- Sharp S. Meta‐analysis regression. Stata Technical Bulletin 1998;42:16‐22. [Google Scholar]
Simopoulos 2016
- Simopoulos AP. An increase in the omega‐6/omega‐3 fatty acid ratio increases the risk for obesity. Nutrients 2016;8(3):128. [DOI: 10.3390/nu8030128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thorpe 2017
- Thorpe G, Ajabnoor S, Ahmed Z, Abdelhamid A, Hooper L. Dietary polyunsaturated fat for prevention and treatment of inflammatory bowel disease. PROSPERO 2017:CRD42017068704.
WHO 2004
- World Health Organization. World Health Statistics. www3.who.int/whosis 2004.
WHO 2016
- World Health Organization. Cardiovascular diseases (CVDs). Fact sheet number 317. www.who.int/mediacentre/factsheets/fs317/en/ reviewed June 2016:(accessed 26 July 2016).
WHO/FAO 2008
- Joint FAO/WHO Expert Consultation on Fats, Fatty Acids in Human Nutrition. Interim summary of conclusions and dietary recommendations on total fat & fatty acids. www.who.int/nutrition/topics/FFA_interim_recommendations/en/ 10 ‐ 14 November 2008; Vol. WHO, Geneva.
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz K, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Abdelhamid 2016
- Abdelhamid A, Martin N, Bridges C, Song F, Deane KHO, Hooper L. Polyunsaturated fat intake for prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012345] [DOI] [Google Scholar]


