Abstract
Background
Infant formulas containing hydrolysed proteins have been widely advocated for preventing allergic disease in infants, in place of standard cow’s milk formula (CMF). However, it is unclear whether the clinical trial evidence supports this.
Objectives
To compare effects on allergic disease when infants are fed a hydrolysed formula versus CMF or human breast milk. If hydrolysed formulas are effective, to determine what type of hydrolysed formula is most effective, including extensively or partially hydrolysed formula (EHF/PHF). To determine whether infants at low or high risk of allergic disease, and whether infants receiving early short‐term (first few days after birth) or prolonged formula feeding benefit from hydrolysed formulas.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 11), MEDLINE (1948 to 3 November 2017), and Embase (1974 to 3 November 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles and previous reviews for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We searched for randomised and quasi‐randomised trials that compared use of a hydrolysed formula versus human milk or CMF. Outcomes with ≥ 80% follow‐up of participants from eligible trials were eligible for inclusion.
Data collection and analysis
Two review authors independently selected trials, assessed trial quality and extracted data from the included studies. Fixed‐effect analyses were performed. The treatment effects were expressed as risk ratio (RR) and risk difference (RD) with 95% confidence intervals and quality of evidence using the GRADE quality of evidence approach. The primary outcome was all allergic disease (including asthma, atopic dermatitis, allergic rhinitis and food allergy).
Main results
A total of 16 studies were included.
Two studies assessed the effect of three to four days infant supplementation with an EHF while in hospital after birth versus pasteurised human milk feed. A single study enrolling 90 infants reported no difference in all allergic disease (RR 1.43, 95% CI 0.38 to 5.37) or any specific allergic disease up to childhood including cow's milk allergy (CMA) (RR 7.11, 95% CI 0.35 to 143.84). A single study reported no difference in infant CMA (RR 0.87, 95% CI 0.52 to 1.46; participants = 3559). Quality of evidence was assessed as very low for all outcomes.
No eligible trials compared prolonged hydrolysed formula versus human milk feeding.
Two studies assessed the effect of three to four days infant supplementation with an EHF versus a CMF. A single study enrolling 90 infants reported no difference in all allergic disease (RR 1.37, 95% CI 0.33 to 5.71; participants = 77) or any specific allergic disease including CMA up to childhood. A single study reported a reduction in infant CMA of borderline significance (RR 0.62, 95% CI 0.38 to 1.00; participants = 3473). Quality of evidence was assessed as very low for all outcomes.
Twelve studies assessed the effect of prolonged infant feeding with a hydrolysed formula compared with a CMF. The data showed no difference in all allergic disease in infants (typical RR 0.88, 95% CI 0.76 to 1.01; participants = 2852; studies = 8) and children (typical RR 0.85, 95% CI 0.69 to 1.05; participants = 950; studies = 2), and no difference in any specific allergic disease including infant asthma (typical RR 0.57, 95% CI 0.31 to 1.04; participants = 318; studies = 4), eczema (typical RR 0.93, 95% CI 0.79 to 1.09; participants = 2896; studies = 9), rhinitis (typical RR 0.52, 95% CI 0.14 to 1.85; participants = 256; studies = 3), food allergy (typical RR 1.42, 95% CI 0.87 to 2.33; participants = 479; studies = 2), and CMA (RR 2.31, 95% CI 0.24 to 21.97; participants = 338; studies = 1). Quality of evidence was assessed as very low for all outcomes.
Authors' conclusions
We found no evidence to support short‐term or prolonged feeding with a hydrolysed formula compared with exclusive breast feeding for prevention of allergic disease. Very low‐quality evidence indicates that short‐term use of an EHF compared with a CMF may prevent infant CMA. Further trials are recommended before implementation of this practice.
We found no evidence to support prolonged feeding with a hydrolysed formula compared with a CMF for prevention of allergic disease in infants unable to be exclusively breast fed.
Plain language summary
Formulas containing hydrolysed protein for prevention of allergic disease in infants
Review question
Does feeding infants with a formula containing hydrolysed protein result in decreased risk of developing allergic disease such as asthma, dermatitis/eczema, hay fever and food allergy during infancy and childhood?
Background
Allergic disease is responsible for a substantial health burden among infants, children and adults. Early dietary intake may influence the development of allergic disease. When babies are not exclusively breast fed, use of hydrolysed formula instead of ordinary cow's milk formula may reduce allergic disease among babies and children, although additional studies are needed to confirm this. Infant formulas have been designed to lower the chance of infants developing allergic disease. These include hydrolysed cow's milk and soy milk formulas. Hydrolysed formulas break down milk proteins into smaller, potentially less allergy‐producing proteins.
Results
This review of trials found no evidence to support feeding with a hydrolysed formula to prevent allergic disease in preference to exclusive breast feeding. This review also found that for infants who are unable to be exclusively breast fed, there is no evidence that prolonged infant feeding with a hydrolysed formula compared with a cow's milk is associated with any difference in allergic disease, asthma, eczema, rhinitis, food allergy or cow's milk formula at any time point. However, limited data in infants who are exclusively formula fed suggest that feeding with a hydrolysed formula instead of a cow's milk formula may reduce infant allergic disease. Concerns regarding quality of the evidence and consistency of the results indicate that continued study is needed. The evidence in this review comes from literature searches updated until November 2017.
Conclusions
We found no substantial evidence to support short‐term or prolonged feeding with a hydrolysed formula compared with a cow's milk formula for prevention of allergic disease in infants unable to be exclusively breast fed.
Summary of findings
Background
Description of the condition
Food allergy and other allergic disease are prevalent and represent a substantial health problem that may be increasing in developed countries (Burr 1989; Halken 2004; Prescott 2005; Schultz Larsen 1996). Although less than half of those who develop childhood allergic disease have a first‐degree relative with a history of allergic disease, the risk of allergic disease increases substantially with a positive family history (Bergmann 1994; Sears 1996; Tariq 1998). Approximately 10% of children without an allergic first‐degree relative develop allergic disease compared with 20% to 30% with an allergic first‐degree relative (parent or sibling) and 40% to 50% with two affected relatives (Arshad 2005; Bergmann 1997; Hansen 1993; Kjellman 1977). The predictive value of family history is increased with the addition of cord blood immunoglobulin (Ig) E antibody testing, although its accuracy may not be adequate for population screening (Bergmann 1997; Bergmann 1998; Tariq 1998).
Manifestations of allergic disease are age dependent. Infants commonly present with symptoms and signs of atopic eczema, gastrointestinal symptoms and recurrent wheezing. Asthma and rhino conjunctivitis become prevalent in later childhood. Sensitisation to allergens tends to follow a characteristic pattern (Halken 2004), with sensitisation to food allergens in the first two to three years of life, followed by indoor allergens (e.g. house dust mite, pets) and subsequently outdoor allergens (e.g. rye, timothy grass). The cumulative prevalence of allergic disease during childhood is high, with up to 7% to 8% developing a food allergy, 15% to 20% atopic eczema and 31% to 34% asthma or recurrent wheezing (Halken 2004). Of these, 7% to 10% will continue to have asthma symptoms beyond five years of age (Halken 2004). Food hypersensitivities affect approximately 6% of infants younger than three years, and prevalence decreases over the first decade (Osterballe 2005; Sampson 2004).
Allergic disease may be diagnosed by questionnaire or clinician assessment, and the diagnosis may be confirmed by specific skin or serological testing, or by allergen challenge. Diagnostic criteria for different allergic diseases are not uniform, and the mode of ascertainment of allergic disease is variable. Although tests of bronchial hyper‐responsiveness, challenge tests and classical tests of IgE‐mediated allergy have an imperfect correlation with allergy symptoms and clinical signs (Darsow 2000; Peat 2000), they are associated with an increased likelihood of allergic disease (Ronmark 2001; Sears 1998; Sly 1999; Strachan 1996). In addition, some evidence suggests that questionnaires, although compromised by selection and recall bias (Peat 2001), are suitable for allergic disease screening (Kilpeläinen 2001; Ravault 2001). This review includes trials that diagnosed allergic disease by questionnaire or by clinician assessment, with or without confirmation by laboratory testing. Criteria for diagnosis of allergic disease should include typical symptoms and/or signs, with evidence of precipitants, persistence or recurrence typical of allergic disease, or with test evidence confirming atopy or bronchial hyperreactivity.
The World Allergy Organization 2003 consensus (Johansson 2004) recommended that the term 'hypersensitivity' should be used to describe objectively reproducible symptoms or signs initiated by exposure to a defined stimulus at a dose tolerated by normal persons. 'Allergy' is a hypersensitivity reaction initiated by specific immunological mechanisms. The term 'food allergy' is used when immunological mechanisms have been demonstrated. Food‐specific IgG antibodies in serum are not of clinical importance but merely indicate previous exposure to a specific food. If IgE is involved in the reaction, the term 'IgE‐mediated food allergy' is appropriate. Food hypersensitivity is diagnosed by resolution of typical symptoms with elimination from the diet, with confirmation by blinded challenge. Around 2% to 3% of babies develop hypersensitivity to a particular food. Principal symptoms among infants with proven cow's milk protein hypersensitivity (CMPH) are gastrointestinal (˜ 50%), dermatological (˜ 31%) and respiratory (˜ 19%) in nature (Høst 1994; Høst 1995; Schrander 1993). Two of every three infants with CMPH have a family history of atopy (Schrander 1993). CMPH is strongly associated with feeding of cow's milk formula (CMF) to infants during the first month of life (Høst 1991). Many infants with CMPH become tolerant over time, with approximately 30% at one year, 50% at two years and 70% at three years tolerant to cow's milk challenge. The risk of persisting hypersensitivity is increased with evidence of atopy (Høst 1995).
Description of the intervention
Measures to prevent allergic disease including food allergy have included maternal allergen avoidance during pregnancy (Custovic 2000; Custovic 2001; Kramer 2012; Zeiger 1989) and/or lactation (Custovic 2000; Custovic 2001; Zeiger 1989), periods of exclusive breast feeding (Custovic 2000; Custovic 2001; Gruskay 1982; Oddy 1999; Saarinen 1995; Saarinen 2000), and avoidance of potential allergens including food and environmental antigens during the first year of life and beyond (Custovic 2000). Formulas prescribed for infants with the intention of preventing allergic disease including food allergy include hydrolysed cow's milk and elemental formula, as well as soy or hydrolysed soy formula. These formulas may be produced from cow's milk or soy milk, may be derived predominantly from whey or casein proteins and may be partially or extensively hydrolysed. Protein modification is performed through a variety of physiochemical processes including ultra heating and enzymatic cleavage, most often with trypsin and chymotrypsin.
The American Academy of Pediatrics (AAP) recommends that hypoallergenic formulas should be tested in trials in which investigators examine human infants for toxicity and suitability to maintain a positive nitrogen balance, while attempting to predict whether infants allergic to cow’s milk will react adversely to these formulas (AAP 2000). These formulas are studied in infants with cow’s milk or cow’s milk‐based formula allergic reactions verified by double‐blind placebo‐controlled challenge (DBPCC) (Bock 1988). At a minimum, these tests should ensure with 95% confidence that 90% of infants with documented cow’s milk allergy respond to treatment and do not react during challenge (Kleinman 1991). Protein particle size does not appear to be a prerequisite for defining a formula as hypoallergenic, although amino acid‐based formulas and those with more extensive hydrolysis are less likely to produce reactions among infants with cow's milk allergy (CMA) (Hill 2007). Although universal agreement has not been reached on the definition (Chaffen 2010; Høst 1999), for the purposes of this review an extensively hydrolysed formula (EHF) will be regarded as one meeting the AAP definition for hypoallergenic formula (AAP 2000), and those with less extensive hydrolysis will be regarded as partially hydrolysed.
How the intervention might work
Infants' immune systems become sensitised or tolerant to allergens in the order in which they are exposed. Early life exposure to allergens occurs frequently through ingested protein, particularly cow's milk protein in formula (Muraro 2004). Amino acid‐based and extensively hydrolysed protein formulas are produced so as to substantially reduce the antigenicity of the protein and prevent sensitisation of infants to commonly ingested antigens, including cow's milk protein. However, low concentrations of food allergens, especially cow's milk proteins, are present in human milk. It has been suggested that the low incidence of cow's milk protein allergy among exclusively breast fed infants ‐ at 0.5% in unselected infants and 1.3% in high‐risk infants ‐ in prospective birth cohort studies was due to low‐level exposure‐induced tolerance rather than to disease (Halken 2004). It has been proposed that prolonged exposure to allergenic proteins or to proteins with reduced but not absent allergenicity may induce tolerance over time (Allen 2009). Although most infants with cow’s milk hypersensitivity exhibit this in the first year of life, more than 80% subsequently develop clinical tolerance (Katz 2011; Sampson 2004). The concern is that early avoidance of cow's milk protein may reduce the likelihood that infants will subsequently develop tolerance to the allergen (Katz 2010).
Why it is important to do this review
The aim of this review is to gather evidence on the use of hydrolysed formulas for prevention of allergic disease including food allergy. This review does not include treatment of infants with clinically recognised allergic disease.
Objectives
To compare effects on allergic disease including food allergy when infants are fed a hydrolysed formula versus cow's milk formula (CMF) or human breast milk. If hydrolysed formulas are effective, to determine what type of hydrolysed formula is most effective, including extensively or partially hydrolysed formula (EHF/PHF). To determine which infants at low or high risk of allergic disease and which infants receiving early, short‐term or prolonged formula feeding may benefit from hydrolysed formulas.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised and quasi‐randomised trials that compared the use of a hydrolysed formula versus human milk or cow's milk formula (CMF). Randomised and quasi‐randomised (e.g. using alternation) trials with ≥ 80% follow‐up of participants were eligible for inclusion.
Types of participants
Infants in the first six months of life without clinical evidence of allergic disease.
Types of interventions
Hydrolysed formulas included:
hydrolysed cow's milk and soy formulas; and
extensively and partially hydrolysed formulas (EHF/PHF).
Hydrolysed formulas may be used for:
early, short‐term supplementary or sole formula feeding of infants unable to be exclusively breast fed in the first days of life;
prolonged supplementation of breast fed infants or infants fed solely with formula in the first months of life; and
weaning from the breast with infant formula.
The control group may include infants who receive:
exclusive human milk (breast fed or expressed); and
cow's milk formula (CMF).
Study authors had to pre specify the following comparisons.
Early short‐term hydrolysed formula versus human milk.
Prolonged use of a hydrolysed formula versus human milk.
Early short‐term hydrolysed formula versus CMF.
Prolonged use of a hydrolysed formula versus CMF.
Prespecified subgroup analyses included the following (see Subgroup analysis and investigation of heterogeneity for definitions).
Infant risk of allergic disease.
Low‐risk infants (no family history of allergic disease among first‐degree relatives).
High‐risk infants (family history of allergic disease among first‐degree relatives or high cord IgE level).
Extent of protein hydrolysis.
EHF versus CMF.
PHF versus CMF.
EHF versus PHF.
Indication for use.
Prolonged sole formula feeding.
Supplemental feeding or weaning from the breast using infant formula.
Method of ascertainment of allergic disease.
Allergic disease confirmed by test.
Blinded measurement for allergic disease.
Type of protein hydrolysate used.
Partially hydrolysed whey formula versus cow's milk formula.
Partially hydrolysed casein formula versus cow's milk formula.
Extensively hydrolysed whey formula versus cow's milk formula.
Extensively hydrolysed casein formula versus cow's milk formula.
Hydrolysed soy formula versus cow's milk formula.
We excluded studies that included other allergy prevention interventions (e.g. maternal dietary avoidance measures, environmental allergy reduction measures) in the treatment group and not in the control group. Studies that provided other allergy prevention interventions for both treatment and control groups were eligible.
Types of outcome measures
Primary outcomes
All allergic diseases, including asthma, atopic dermatitis, allergic rhinitis and food allergy
Secondary outcomes
Asthma
Atopic dermatitis/eczema
Allergic rhinitis
Cow's milk or soy protein allergy
Food allergy
Urticaria
Anaphylaxis
In the 2017 review update, we no longer reported previously reported food hypersensitivity and potential harms, including growth parameters, cost and infant feed refusal. Definitions of allergic disease must be compatible with the World Allergy Organization 2003 consensus (Johansson 2004).
Researchers may have diagnosed a specific allergic disease on the basis of:
history of recurrent and persistent symptoms typical of the allergic disease;
clinician diagnosis of allergic disease; or
clinical allergy confirmed by testing including detection of allergen sensitisation by skin testing or by serological testing for specific IgE (e.g. radioallergosorbent (RAST), enzyme‐allergosorbent (EAST)), or asthma confirmed by respiratory function testing for the presence of bronchial hyper‐responsiveness confirmed by elimination/challenge.
Investigators used the following definitions of age of allergic disease.
Infant allergic disease incidence: allergic disease occurring up to two years of age.
Childhood allergic disease incidence: allergic disease occurring up to 10 years of age (or up to age of latest report between two and 10 years).
Childhood allergic disease prevalence: reported allergic disease that was present between two and 10 years of age.
Adolescent allergic disease: allergic disease present from 10 to 18 years of age.
Adult allergic disease: allergic disease present after 18 years of age.
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 11) in the Cochrane Library; MEDLINE (1948 to 3 November 2017) and Embase (1974 to 3 November 2017). We also searched citations of authors of included studies and citation lists of articles and reviews. We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
We documented the search strategies in Appendix 1,Appendix 2, and Appendix 3.
Searching other resources
We performed a search of previous reviews including cross references (all articles referenced), abstracts, conferences (Paediatric Academic Societies 2003 to 2016; Perinatal Society of Australia and New Zealand 2003 to 2017), recent review citations and expert informants.
We also updated in November 2017 our search of clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; who.int/ictrp). Search strategies are detailed in Appendix 4 and Appendix 5.
Data collection and analysis
This review updates previous versions (Osborn 2003; Osborn 2006a; Osborn 2006b; Osborn 2017a; Osborn 2017b).
Each review author independently assessed eligibility of studies for inclusion. We included only studies with ≥ 80% reporting of randomised infants. We used the criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of included trials regarding adequacy of randomisation and allocation concealment, blinding of parents or caregivers and assessors to intervention and completeness of assessment of all randomised individuals. We used a data collection form to aid extraction of relevant information and data from each included study. Each review author extracted the data separately, and review authors compared data and resolved differences by consensus. We used the standard methods of the Cochrane Neonatal Review Group to synthesise the data and expressed effects as risk ratio (RR), risk difference (RD) with 95% confidence intervals (CIs) for categorical data, and planned to use mean difference (MD) and 95% CIs for continuous data. We used the Chi² test to examine data for heterogeneity, and we quantified heterogeneity using the I² statistic. We used the fixed‐effect model for meta‐analysis when enrolled infants and interventions were similar and no significant heterogeneity was found. We explored sources of heterogeneity by performing subgroup analysis.
The term 'hydrolysed formula' used without a reference to type refers to both extensively and partially hydrolysed formulas (EHF/PHF). We did not pool studies that used hydrolysed formula for early (first few days of life) supplemental or sole infant feeding with studies that used hydrolysed formula for prolonged feeding. We performed all comparisons by including only studies with no different co‐interventions prescribed for prevention of allergic disease in either study arm (e.g. in treatment group but not in control group). Allergic disease‐preventing co‐interventions included modifications to mother's diet when pregnant or breast feeding and environmental modifications such as avoidance of pet hair and host dust mite reduction measures. The protocol did not originally pre specify that we should restrict analyses to studies with no differential co‐interventions.
Selection of studies
We included all randomised and quasi‐randomised controlled trials fulfilling the selection criteria described in the previous section. Each review author reviewed the search results and separately selected studies for inclusion. Review authors resolved disagreements by discussion.
Data extraction and management
Each review author extracted the data separately. Review authors compared data and resolved differences by consensus.
We obtained additional method details and data from the authors of two studies (Hill 2000; von Berg 2003). For one study (Lam 1992), we extracted methods and data from a thesis.
For the 2016 update, we performed all analyses using Review Manager software (Review Manager 2014).
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Review Group to assess the methodological quality of included trials. We evaluated the quality of included trials in terms of adequacy of randomisation and allocation concealment, blinding of parents or caregivers and assessors to intervention and completeness of assessment in all randomised individuals.
For the 2016 and 2017 updates, we incorporated previous assessments into RevMan 5 'Risk of bias' tables. We assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017) by examining the following.
Sequence generation (checking for possible selection bias)
Adequate (any truly random process, e.g. random number table; computer random number generator)
Inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number)
Unclear
Allocation concealment (checking for possible selection bias)
Adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes)
Inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth)
Unclear
Blinding (checking for possible performance bias)
Adequate, inadequate or unclear for participants
Adequate, inadequate or unclear for personnel
Adequate, inadequate or unclear for outcome assessors
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Adequate (< 20% missing data)
Inadequate
Unclear
Selective reporting bias
Adequate (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported)
Inadequate (when not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported)
Unclear
Other sources of bias
We assessed the possibility of other sources of bias (e.g. early termination of trial due to data‐dependent process, extreme baseline imbalance) as follows.
Yes.
No.
Unclear.
Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). With reference to the criteria above, we assessed the likely magnitude and direction of bias, and whether it was likely to have an impact on study findings. We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis section).
Measures of treatment effect
We used the standard methods of the Cochrane Neonatal Review Group to synthesise data and expressed effects as risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs) for categorical data, and planned to use mean difference (MD) with 95% CIs for continuous data.
Unit of analysis issues
The unit of analysis was the individual participant (infant).
Dealing with missing data
We recorded missing data in 'Risk of bias' tables and assessed the effect of missing data by performing sensitivity analysis.
We performed all analyses by 'intention to treat' when data were available. When intention‐to‐treat data were not available, we reported data as infants assessed by group of assignment as well as losses after randomisation.
Assessment of heterogeneity
We used the two formal statistics described here.
Chi2 test: to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. This test has low power when the number of studies included in the meta‐analysis is small, so we planned to set the probability at the 10% level of significance.
I2 statistic, to ensure that pooling of data is valid. We graded the degree of heterogeneity as follows: none (< 25%); low (25% to 49%); moderate (50% to 74%); or high (≥ 75%).
When we found evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity by conducting sensitivity and subgroup analyses to look for evidence of bias or methodological differences between trials.
Assessment of reporting biases
We documented in the Characteristics of excluded studies table all studies that reported use of a prebiotic in a potentially eligible infant population but did not report allergic disease‐related outcomes. We assessed reporting and publication bias by examining the degree of asymmetry of a funnel plot.
Data synthesis
We used the fixed‐effect model and Mantel‐Haenszel methods for meta‐analysis.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: all allergic disease; specific allergies including asthma, atopic dermatitis/eczema, allergic rhinitis, cow's milk or soy protein allergy, food allergy, urticaria and anaphylaxis.
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create ‘Summary of findings’ tables to report the quality of the evidence.
The GRADE approach yields an assessment of the quality of a body of evidence by one of four grades.
High: we are very confident that the true effect lies close to the estimate of effect.
Moderate: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of effect.
Very low: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses according to the following.
Infant risk of allergic disease: low‐risk infants (no family history of allergic disease in first‐degree relatives); high‐risk infants (family history of allergic disease in first‐degree relatives or high cord blood IgE level).
Extent of protein hydrolysis: EHF versus CMF; PHF versus CMF; EHF versus PHF. An EHF should meet the definition provided by the AAP Committee on Nutrition (AAP 2000) ‐ extensively hydrolysed proteins derived from cow's milk in which most of the nitrogen is present in the form of free amino acids and peptides ≤ 1500 kDaltons ‐ and should, at a minimum, ensure with 95% confidence that 90% of infants with documented cow's milk allergy (CMA) will not react with defined symptoms to the formula under double‐blind, placebo‐controlled conditions.
Indication for use: prolonged sole formula feeding; supplemental formula feeding; weaning from the breast with infant formula.
Method of ascertainment of allergic disease: clinical allergic disease confirmed by challenge testing or by testing for atopy (e.g. skin testing or serological testing for specific IgE, asthma confirmed by testing for presence of bronchial hyper‐responsiveness, food allergy confirmed by elimination/challenge). Included in this definition is clinical allergic disease in a patient for whom atopy has been confirmed by testing (e.g. asthma when atopy has been confirmed by skin prick testing or RAST for specific IgE); blinded measurement for allergic disease ‐ when measurement of outcome was blinded to treatment allocation (this analysis was not prespecified).
Type of protein hydrolysate used: partially hydrolysed whey formula versus CMF; partially hydrolysed casein formula versus CMF; extensively hydrolysed whey formula versus CMF; extensively hydrolysed casein formula versus CMF; hydrolysed soy formula versus CMF.
Sensitivity analysis
We prespecified a sensitivity analysis to determine whether review findings were affected by including only studies at low risk of bias, defined as those with adequate randomisation and allocation concealment and < 10% loss to follow‐up.
Results
Description of studies
This report updates a previous publication that was withdrawn (Osborn 2017a; Osborn 2017b). A data entry error in the review was identified that had potential to impact the review conclusions. This review corrects the data error and updates the search.
Results of the search
We performed searches on 22 January 2016 (see Figure 1 for study flow diagram). We searched MEDLINE using OVID and retrieved 198 reports (Appendix 1); CENTRAL and retrieved 242 reports (Appendix 3); and Embase and retrieved 199 reports (Appendix 2). We identified eight ongoing or unpublished studies (NCT01987154; NCT00936637; NCT01156493; NCT01735123; NCT01700205; NCT01210391; NCT01036243; Yin 2015) (see Appendix 4 and Appendix 5). We updated the search on 3 November 2017 and found one additional excluded study (Boyle 2016) and an additional 15‐year follow‐up report from a previously included study (von Berg 2003).
1.

Study flow diagram: review update.
The search of MEDLINE, CENTRAL and Embase was updated from January 2016 to November 2017. After de‐duplication, 32 additional reports were reviewed. No additional reports of included studies were found. Five additional reports were found that were added to excluded studies (Boyle 2016; Knip 2010; Knip 2011; Scalabrin 2009; Xinias 2017). An expert informant identified an additional unpublished study, the author was unable to get sponsor approval for data release for (Arshad 1990) (see Characteristics of studies awaiting classification).
Included studies
We assessed 16 studies as eligible for inclusion ‐ see Characteristics of included studies table. A previously included study (Sorensen 2007) has been moved to excluded studies as it did not reported an allergic disease outcome. The search revealed an additional study that we assessed as an included study (Kwinta 2009). We found an additional publication (Hill 2000) of a study that we had previously assessed as an excluded study as it had reported a per‐protocol analysis with excess losses. We combined a new report of an 'intention‐to‐treat' analysis with trial data obtained from study authors and have assessed this trial as an included study. Data from sequential publications of the GINI study (von Berg 2003) beyond three years remain ineligible for inclusion in this review owing to excess losses to follow‐up. For the GINI study, data from a single centre (Wessel) has been used in this review as other centres had excess losses (> 20%) at all time points. We have moved to the excluded studies list five previously included studies (Maggio 2005; Picaud 2001; Sorensen 2007; Szajewska 2001; Vandenplas 1993) that have reported no outcome data able to be used in this review.
Types of infants enrolled
High risk of allergic disease: a total of 13 studies (Chirico 1997; de Seta 1994; Halken 2000; Hill 2000; Lam 1992; Mallet 1992; Marini 1996; Nentwich 2001; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) enrolled infants at high risk of allergic disease on the basis of a history of allergic disease in a first‐degree relative and/or a high cord IgE level, although Lam 1992 did not report 'high‐risk' criteria.
Risk of allergic disease not specified: three studies did not enrol infants on the basis of risk of allergic disease; Juvonen 1996 enrolled healthy term infants, although 62% had a family history of allergic disease; Kwinta 2009 enrolled very low birth weight infants (≤ 1500 g); and Saarinen 1999 enrolled healthy term infants requiring supplemental feeding in hospital.
Low risk of allergic disease: no study reporting allergic disease outcomes enrolled infants at low risk of allergic disease.
Types of interventions
See Characteristics of included studies for types of formula used in each study.
Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants
Two studies (Juvonen 1996; Saarinen 1999) compared a hydrolysed formula versus pasteurised donor human milk used for early short‐term infant feeding. Juvonen 1996 gave sole bottle feeds for three days, then all infants were exclusively breast fed. Saarinen 1999 gave supplemental feeds when required while infants were in hospital (average four days). Mothers were then encouraged to breast feed.
Early short‐term feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ low‐risk infants
Two studies (Juvonen 1996; Saarinen 1999) compared a hydrolysed formula versus CMF for early short‐term infant feeding. Juvonen 1996 gave sole bottle feeds for three days, then all infants were exclusively breast fed. Saarinen 1999 gave supplemental feeds when required while infants were in hospital (average four days). Mothers were then encouraged to breast feed.
Prolonged feeding: hydrolysed formula versus human milk feeding
We found no studies for this comparison.
Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF)
Twelve studies compared prolonged supplemental or sole feeding with a hydrolysed formula versus CMF without differential co‐interventions (Chirico 1997; de Seta 1994; Hill 2000; Kwinta 2009; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993). Three studies (Chirico 1997; Marini 1996; Oldaeus 1997) reported additional allergy avoidance measures in both hydrolysed formula and CMF groups.
Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ low‐risk infants
Kwinta 2009 compared prolonged feeding with a hydrolysed formula versus CMF in low‐risk infants.
Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ high‐risk infants
Eleven studies (Chirico 1997; de Seta 1994; Hill 2000; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) compared prolonged feeding with a hydrolysed formula versus CMF in high‐risk infants.
Prolonged feeding: partially hydrolysed formula (PHF) versus cow's milk formula (CMF)
Eleven studies (Chirico 1997; de Seta 1994; Kwinta 2009; Hill 2000; Lam 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) compared prolonged feeding with a PHF versus CMF.
Prolonged feeding: extensively hydrolysed formula (EHF) versus cow's milk formula (CMF)
Four studies (Kwinta 2009; Mallet 1992; Oldaeus 1997; von Berg 2003) compared prolonged feeding with an EHF versus CMF.
Prolonged feeding: extensively hydrolysed formula (EHF) versus partially hydrolysed formula (PHF)
Four studies (Halken 2000; Nentwich 2001; Oldaeus 1997; von Berg 2003) compared prolonged feeding with an EHF versus a PHF.
Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula (CMF)
Seven studies reported prolonged exclusive hydrolysed formula versus CMF (Chirico 1997; de Seta 1994; Kwinta 2009; Lam 1992; Marini 1996; Vandenplas 1992; Willems 1993).
Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ studies with blinded measurement
Four studies assessed allergic disease without knowledge of participant allocation (Kwinta 2009; Oldaeus 1997; Vandenplas 1992; von Berg 2003).
Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ studies at low risk of bias
We assessed no studies as eligible for inclusion in the sensitivity analysis of adequate study methods (adequate randomisation, allocation concealment and < 10% losses to follow‐up).
Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula (CMF)
Nine studies (Chirico 1997; de Seta 1994; Hill 2000; Lam 1992; Marini 1996; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) compared a partially hydrolysed whey formula versus CMF.
Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula (CMF)
Two studies (Kwinta 2009; Oldaeus 1997) compared a PHF containing casein versus CMF.
Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula (CMF)
One study (von Berg 2003) compared an extensively hydrolysed whey formula versus CMF.
Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula
Two studies (Halken 2000; von Berg 2003) compared an extensively hydrolysed casein formula versus CMF.
No study compared a hydrolysed soy formula versus CMF. No study compared a hydrolysed soy formula versus hydrolysed CMF.
Types of outcomes
Definitions for allergic disease varied between studies, but usually required persistent or recurring symptoms and signs in the absence of another obvious clinical explanation. For definitions of 'all allergic disease' and type of allergic disease for each study, see Characteristics of included studies.
Studies (with timing) reporting clinician‐diagnosed allergic disease included Chirico 1997 (six months); de Seta 1994 (six and 24 months); Halken 2000 (six, 12 and 18 months); Hill 2000 (two and six to seven years); Juvonen 1996 (three years); Kwinta 2009 (five to seven years); Lam 1992 (six months); Mallet 1992 (one, two and four years); Marini 1996 (six months, one and three years); Nentwich 2001 (six and 12 months); Oldaeus 1997 (nurse examination at three, six, nine, 12 and 18 months and doctor visit at 18 months); Saarinen 1999 (mean age at follow‐up of 27 months, range 18 to 34 months); Tsai 1991 (one, two, four, six and 12 months); Vandenplas 1992 (12 months); and von Berg 2003 (12 months and three, six, 10 and 15 years ‐ note excess losses from three years of age).
Three studies reported using questionnaire‐diagnosed allergic disease: Hill 2000 (six to seven years); Kwinta 2009 (five to seven years); and Willems 1993 (three months and one year).
Seven studies reported specific food allergy (Halken 2000; Hill 2000; Juvonen 1996; Oldaeus 1997; Saarinen 1999; Vandenplas 1992; von Berg 2003).
Excluded studies
We excluded 86 studies and documented reasons for exclusion in the Characteristics of excluded studies table. Here, we document controlled trials of hydrolysed formula according to types of participants and reasons for exclusion.
Preterm or low birth weight infants
The following studies did not report allergic disease: Agosti 2003; Baldassarre 2017; Florendo 2009; Maggio 2005; Mihatsch 1999 (cross‐over trial); Mihatsch 2002 (excess losses); Pauls 1996 (abstract format only); Picaud 2001; Raupp 1995; Riezzo 2001; Rigo 1994b (unclear allocation); Szajewska 2001; and Yu 2014 (likely non‐random, cross‐over).
Term healthy infants
Akerblom 2005 enrolled infants at high risk of diabetes and did not report allergic disease; Akimoto 1997 reported allergic disease (non‐random); NCT00936637 did not report allergic disease; Berseth 2009 (multiple formula differences) did not report allergic disease; Borschel 2013 did not report allergic disease; Borschel 2014a (multiple formula differences) did not report allergic disease; Borschel 2014b (multiple formula differences) did not report allergic disease; Burks 2008 (excess losses) did not report allergic disease; de Jong 1998 used protein‐free formula; Decsi 1992 did not report allergic disease; Decsi 1998 did not report allergic disease; Exl 1998 reported allergic disease (non‐random); Fukushima 1997 reported allergic disease (excess losses); Hartman 1994 reported intolerance not allergic disease and reported unclear losses (abstract format only); Hernell 2003 (unclear allocation) did not report allergic disease; Keller 1996 (unclear allocation) reported allergic disease; Knip 2010 enrolled infants at high risk of diabetes and did not report allergic disease; Lasekan 2006 did not report allergic disease; Medjad‐Guillou 1992 (cross‐over trial) did not report allergic disease; Mennella 2011a did not report allergic disease; Moran 1992 reported excess losses; Paronen 2000 enrolled infants at risk of diabetes and did not report allergic disease; Rigo 1994a (unclear allocation) did not report allergic disease; Scalabrin 2009 (excess losses) reported adverse events including allergic disease; Schmelzle 2003 (excess losses) did not report allergic disease; Schmitz 1992 did not report allergic disease (excess losses); Schrander 1993 was observational; Silva Rey 1996 (unclear allocation; excess losses) reported in a thesis only; Staelens 2008 did not report allergic disease; Tariq 1998 was an observational trial; Knip 2011 enrolled infants at high risk of diabetes and reported adverse events at 10 years including asthma and other allergic disease (excess losses); Vaarala 1995 did not report allergic disease; Vaarala 2012 (enrolled infants at risk of diabetes) did not report allergic disease; and Vandenplas 1993 did not report allergic disease.
Term infants at high risk of allergic disease
Arshad 1990 was unable to get approval from the study sponsor to release data so remains unpublished. Arshad 1992a reported multiple differential allergic disease‐reducing co‐interventions; Barberi 1993 reported unclear allocation, excess losses, and allergic disease (abstract format only); Bergmann 1996a reported allergic disease and non‐random allocation; Boyle 2016 reported multiple differential allergic disease‐reducing co‐interventions (prebiotic and hydrolysed formula) and reported allergic disease; Chan 2002 reported allergic disease and excess losses; Chan‐Yeung 2000 reported multiple differential allergic disease‐reducing co‐interventions; Chandra 1989a reported data that could not be verified after allegations of fraud; Chandra 1989b reported data that could not be verified after allegations of fraud; D'Agata 1996 reported unclear allocation, imbalances between groups and allergic disease; Giovannini 1994 reported excess losses and did not report allergic disease; Halken 1992 reported allergic disease and excess losses; Hattevig 1989 trial of maternal allergen avoidance only; Kuo 2011 was observational; Iikura 1995 reported unclear allocation and imbalances between groups (abstract format only); Martinez‐Valverde 1993 unclear allocation reported in thesis only; Moran 1992 reported excess losses and allergic disease; Nentwich 2003 was an observational trial; Odelram 1996 reported excess losses and allergic disease; Porch 1998 reported excess losses; Schmidt 1995 was an observational trial; Shao 2006 reported multiple differential allergic disease‐reducing co‐interventions; Sorensen 2007 has not reported allergic disease data to date; Szajewska 2004 reported excess losses and allergic disease; Vandenplas 1988 reported unclear allocation and losses; Wopereis 2014 has not reported allergic disease to date (abstract format only); and Zeiger 1989 reported multiple co‐interventions and excess losses.
Infants with allergic disease, infantile colic, gastro‐oesophageal reflux symptoms or feed intolerance
Arikan 2008 did not report allergic disease; NCT01987154 enrolled infants with CMPI; Campbell 1989 did not report allergic disease (non‐random); Corvaglia 2013 did not report allergic disease; Hill 1995b did not report allergic disease; Høst 1991 challenge test in infants with reactions ‐ observational study; Lucassen 2000 did not report allergic disease; Nocerino 2012 did not report allergic disease (abstract format only); Savino 2003 was observational; Savino 2006 reported multiple formula differences; Taubman 1988 did not report allergic disease; Xinias 2017 did not report allergic disease; and Zeiger 1989 reported allergic disease and excess losses.
Risk of bias in included studies
We have summarised risk of bias in included studies in Figure 2; and Figure 3. Overall, we considered no studies to be at 'low risk' of bias.
2.
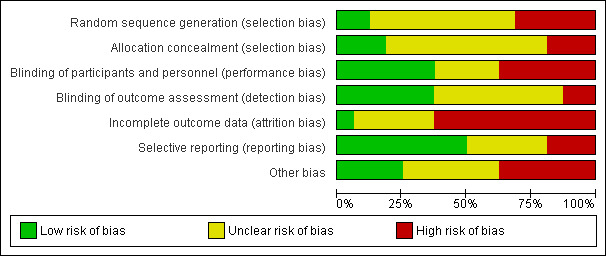
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
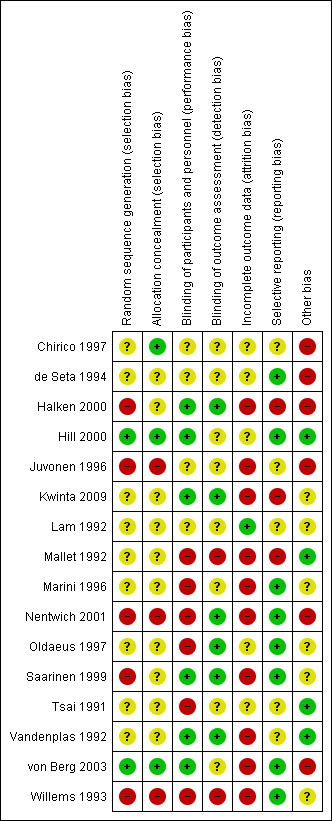
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation
Two studies reported an adequate method of randomisation (Hill 2000; von Berg 2003). Nine studies reported random allocation of infants but not the method of randomisation used (Chirico 1997; de Seta 1994; Kwinta 2009; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992), so we assessed their risk as 'unclear'. Five studies reported quasi‐random methods of participant allocation, including Halken 2000 (by date of birth), Juvonen 1996 (by day of month), Nentwich 2001 (odd and even numbers), Saarinen 1999 and Willems 1993 (month of birth), so were assessed as at 'high risk' of bias.
Allocation concealment
Three studies are at 'low risk' of bias due to allocation concealment (Chirico 1997; Hill 2000; von Berg 2003). Nine studies were at 'unclear' risk including de Seta 1994 did not report method of allocation, Halken 2000 used quasi‐random allocation method but blinded intervention, Kwinta 2009 used unclear allocation method, Lam 1992 did not report method, Mallet 1992 did not report method, Marini 1996 did not report method, Oldaeus 1997 did not report method, Saarinen 1999 used quasi‐random allocation method but blinded intervention, Tsai 1991 did not report method, and Vandenplas 1992 (used unclear allocation method). We assessed three studies as having 'high risk' for allocation concealment ‐ Juvonen 1996 quasi‐random allocation, unblinded study, Nentwich 2001 quasi‐random allocation, unblinded prescribing and Willems 1993 quasi‐random allocation, unblinded study.
Blinding
Six studies reported blinding of participants and personnel (Halken 2000; Hill 2000; Kwinta 2009; Saarinen 1999; Vandenplas 1992; von Berg 2003); we assessed four studies as having 'unclear' risk, as they did not report details (Chirico 1997; de Seta 1994; Juvonen 1996; Lam 1992) and six studies as 'high risk as they were unblinded (Mallet 1992; Marini 1996; Nentwich 2001; Oldaeus 1997; Tsai 1991; Willems 1993).
Six studies reported blinding of measurement (Halken 2000; Kwinta 2009; Nentwich 2001; Oldaeus 1997; Saarinen 1999; Vandenplas 1992). Eight studies did not report blinding of measurement of clinical allergic disease (Chirico 1997; de Seta 1994; Hill 2000; Juvonen 1996; Lam 1992; Marini 1996; Tsai 1991; von Berg 2003). Mallet 1992 and Willems 1993 performed unblinded measurements.
Incomplete outcome data
We included in this review only studies that reported < 20% loss to follow‐up. Studies reported the following losses to follow‐up: Chirico 1997 ‐ unclear; de Seta 1994 ‐ none reported; Halken 2000 ‐ 20% at 18 months; Hill 2000 ‐ 7.3% at two years and 20% at six to seven years; Juvonen 1996 ‐ 10% at three years; Kwinta 2009 ‐ 16% at five to seven years; Lam 1992 ‐ 8% at six months; Mallet 1992 ‐ 5% to 8% at four months but > 20% at one to four years; Marini 1996 ‐ 13% at two years and 19% at three years; Nentwich 2001 ‐ 19% at 12 months; Oldaeus 1997 ‐ 9% at 18 months; Saarinen 1999 ‐ unclear, although all infants were reported to be seen routinely in well‐baby clinics; Tsai 1991 ‐ 9% at 12 months; Vandenplas 1992 ‐ 11% at 12 months and > 20% at three and five years; von Berg 2003 ‐ for infants born in Wesel: 14.5% at one year and 19% at three years (all other analyses and time points > 20%); and Willems 1993 ‐ 13% at one year.
Studies with < 10% loss to follow‐up included de Seta 1994 ‐ none reported, Hill 2000 ‐ 7.3% at two years, Lam 1992 ‐ 8% at six months, Mallet 1992 ‐ 5% to 8% at four months, Oldaeus 1997 ‐ 9% at 18 months and Tsai 1991 ‐ 9% at 12 months.
Selective reporting
Eight studies were at 'low risk' of reporting bias with prespecified primary outcomes reported (de Seta 1994; Hill 2000; Marini 1996; Nentwich 2001; Oldaeus 1997; Saarinen 1999; von Berg 2003; Willems 1993). We assessed five studies as being at 'unclear' risk of reporting bias (Chirico 1997; Juvonen 1996; Lam 1992; Tsai 1991; Vandenplas 1992), and three as being at 'high risk' (Halken 2000; Kwinta 2009; Mallet 1992).
Other potential sources of bias
Six studies had imbalances between groups after randomisation, so we considered them to be at 'high risk' of bias (Chirico 1997; de Seta 1994; Halken 2000; Juvonen 1996; Nentwich 2001; von Berg 2003). Six studies did not report sufficient details of baseline characteristics or described differences of 'unclear' importance (Kwinta 2009; Lam 1992; Marini 1996; Oldaeus 1997; Saarinen 1999; Willems 1993). We considered four studies to have well‐balanced groups after randomisation with no other identified source of bias (Hill 2000; Mallet 1992; Tsai 1991; Vandenplas 1992).
We assessed no studies as having 'low risk' of bias overall ('low risk' of selection bias, performance and measurement bias and attrition bias with < 10% loss to follow‐up).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants for prevention of allergic disease.
| Early short‐term feeding of hydrolysed formula versus human milk for prevention of allergic disease | ||||||
| Patient or population: infants not selected for allergic disease risk. Settings: hospitals. Intervention: early short‐term feeding: hydrolysed formula versus human milk feeding for prevention of allergic disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early short‐term feeding: hydrolysed formula vs human milk ‐ low‐risk infants | |||||
| All allergic disease ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.43 (0.38 to 5.37) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 75 per 1000 | 108 per 1000 (29 to 405) | |||||
| Asthma ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 0.48 (0.05 to 4.41) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 57 per 1000 | 27 per 1000 (3 to 250) | |||||
| Eczema ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 0.48 (0.05 to 4.41) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 57 per 1000 | 27 per 1000 (3 to 250) | |||||
| Food allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.43 (0.38 to 5.37) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 75 per 1000 | 108 per 1000 (29 to 405) | |||||
| Cow's milk allergy ‐ infancy (incidence) Follow‐up: mean 27 months | Study population | RR 0.87 (0.52 to 1.46) | 3559 (1 study) | ⊕⊝⊝⊝ very lowc,d,e | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 17 per 1000 | 15 per 1000 (9 to 25) | |||||
| Cow's milk allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 7.11 (0.35 to 143.84) | 90 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMethodological concerns including quasi‐random sequence allocation, incomplete outcome data and imbalances at baseline bImprecision of estimate ‐ single small study cReported by only a single study dMethodological concerns including quasi‐random sequence allocation and incomplete outcome data eImpression of estimate ‐ low incidence of outcome
Summary of findings 2. Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants for prevention of allergic disease and food allergy.
| Early short‐term feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease | ||||||
| Patient or population: infants not selected for allergic disease risk. Settings: hospitals. Intervention: early short‐term feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early short‐term feeding: hydrolysed formula vs cow's milk formula ‐ low‐risk infants | |||||
| All allergic disease ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.37 (0.33 to 5.71) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 77 per 1000 | 105 per 1000 (25 to 439) | |||||
| Asthma ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 3.08 (0.13 to 73.26) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Eczema ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 0.34 (0.04 to 3.15) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 77 per 1000 | 26 per 1000 (3 to 242) | |||||
| Food allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 1.37 (0.33 to 5.71) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 77 per 1000 | 105 per 1000 (25 to 439) | |||||
| Cow's milk allergy ‐ infancy (incidence) Follow‐up: mean 3 years | Study population | RR 0.62 (0.38 to 1) | 3473 (1 study) | ⊕⊝⊝⊝ very lowa,c,d | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 24 per 1000 | 15 per 1000 (9 to 24) | |||||
| Cow's milk allergy ‐ childhood (incidence) Follow‐up: mean 3 years | Study population | RR 5.13 (0.25 to 103.43) | 77 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | Quality of evidence downgraded due to risk of bias, imprecision and potential for reporting or publication bias. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aMethodological concerns including quasi‐random sequence allocation, incomplete outcome data and imbalances at baseline bImprecision of estimate ‐ single small study cReported only by a single study dImprecision of estimate ‐ low incidence of outcome
Summary of findings 3. Prolonged feeding: hydrolysed formula versus cow's milk formula for prevention of allergic disease.
| Prolonged feeding: hydrolysed formula versus cow's milk formula for prevention of allergic disease | ||||||
| Patient or population: infants. Settings: home. Intervention: prolonged feeding of hydrolysed formula versus cow's milk formula for prevention of allergic disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prolonged feeding: hydrolysed formula versus cow's milk formula | |||||
| All allergic disease ‐ Infancy (incidence) Follow‐up: 2 years | Study population | RR 0.88 (0.76 to 1.01) | 2852 (8 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: low‐risk or high‐risk infants; infants fed a partially hydrolysed formula versus cow's milk formula; infants fed extensively hydrolysed formula versus cow's milk formula; or infants fed partially versus extensively hydrolysed formula. A reduction in infant allergy was found in subgroup analysis of studies that enrolled infants receiving prolonged exclusive formula feeding (typical RR 0.61, 95% CI 0.46 to 0.80; participants = 425; studies = 5; I2 = 0%). |
|
| 274 per 1000 | 241 per 1000 (208 to 277) | |||||
| All allergic disease ‐ Childhood (incidence) Follow‐up: 3 years | Study population | RR 0.85 (0.69 to 1.05) | 950 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: high‐risk infants; infants fed a partially hydrolysed formula versus cow's milk formula; infants fed extensively hydrolysed formula versus cow's milk formula; or infants fed partially versus extensively hydrolysed formula. A single small study that enrolled infants receiving prolonged exclusive formula feeding reported a reduction in childhood allergy incidence (RR 0.42, 95% CI 0.19 to 0.90; participants = 78). |
|
| 353 per 1000 | 300 per 1000 (244 to 371) | |||||
| Asthma ‐ Infancy (incidence) Follow‐up: 2 years | Study population | RR 0.57 (0.31 to 1.04) | 318 (4 studies) | ⊕⊝⊝⊝ very low1,3,4 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: high‐risk infants; infants fed a partially hydrolysed formula versus cow's milk formula; infants fed extensively hydrolysed formula versus cow's milk formula; infants fed partially versus extensively hydrolysed formula; or infants receiving prolonged exclusive formula feeding. |
|
| 175 per 1000 | 100 per 1000 (54 to 182) | |||||
| Eczema ‐ Infancy (incidence) Follow‐up: 2 years | Study population | RR 0.93 (0.79 to 1.09) | 2896 (9 studies) | ⊕⊝⊝⊝ very low1,3,4,5 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: high‐risk infants; infants fed a partially hydrolysed formula versus cow's milk formula; infants fed extensively hydrolysed formula versus cow's milk formula; infants fed partially versus extensively hydrolysed formula; or infants receiving prolonged exclusive formula feeding. |
|
| 202 per 1000 | 187 per 1000 (159 to 220) | |||||
| Rhinitis ‐ Infancy (incidence) Follow‐up: 2 years | Study population | RR 0.52 (0.14 to 1.85) | 256 (3 studies) | ⊕⊝⊝⊝ very low1,3,4 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: high‐risk infants; infants fed extensively hydrolysed formula versus cow's milk formula; infants fed partially versus extensively hydrolysed formula; or infants receiving prolonged exclusive formula feeding. |
|
| 58 per 1000 | 30 per 1000 (8 to 107) | |||||
| Food allergy ‐ Infancy (incidence) Follow‐up: 2 years | Study population | RR 1.42 (0.87 to 2.33) | 479 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: high‐risk infants; infants fed a partially hydrolysed formula versus cow's milk formula; infants fed extensively hydrolysed formula versus cow's milk formula; or infants fed partially versus extensively hydrolysed formula. |
|
| 109 per 1000 | 155 per 1000 (95 to 254) | |||||
| Cow's milk allergy ‐ Infancy (incidence) Follow‐up: 2 years | Study population | RR 2.31 (0.24 to 21.97) | 338 (1 study) | ⊕⊝⊝⊝ very low3,6 | Quality of evidence downgraded due to risk of bias, imprecision and risk of reporting or publication bias. Subgroup analyses found no differences in: high‐risk infants; or infants fed a partially hydrolysed formula versus cow's milk formula. |
|
| 7 per 1000 | 16 per 1000 (2 to 149) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Majority of studies had substantial methodological concerns. 2 Moderate heterogeneity between studies found. 3 Wide confidence intervals. 4 Substantial number of studies did not report outcome. 5 Funnel plot appears asymmetric. 6 Reported by a single study.
Analyses
Comparison 1. Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants
We included two studies (Juvonen 1996; Saarinen 1999) that compared a short duration (three to four days whilst in hospital) of early supplemental or sole hydrolysed formula versus donor human milk feeds in infants who were subsequently encouraged to breast feed.
Juvonen 1996 (90 infants) reported no difference in childhood incidence of allergic disease (risk ratio (RR) 1.43, 95% confidence interval (CI) 0.38 to 5.37), asthma (RR 0.48, 95% CI 0.05 to 4.41), eczema (RR 0.48, 95% CI 0.05 to 4.41), no difference in food allergy (RR 1.43, 95% CI 0.38 to 5.37) and no difference in cow's milk allergy (CMA) (RR 7.11, 95% CI 0.35 to 143.84) at three years. Saarinen 1999 reported no difference in childhood incidence of CMA (3559 infants; RR 0.87, 95% CI 0.52 to 1.46) up to a mean age of 27 months. Quality of evidence was assessed as 'very low' for all outcomes (see Table 1).
We considered the following subgroup analyses, but as no significant benefits were reported, we did not wish to duplicate the results.
Both studies enrolled infants irrespective of family history of allergic disease or food allergy in first‐degree relatives.
Extent of protein hydrolysis: Juvonen 1996 and Saarinen 1999 compared an extensively hydrolysed formula (EHF) versus pasteurised donor human milk.
Indication for use: both studies used formula for early short‐term infant formula feeding.
Method of ascertainment of allergic disease: Saarinen 1999 reported outcomes of an unblinded elimination/challenge for CMA. Juvonen 1996 did not report criteria for diagnosis of allergic disease.
Type of protein hydrolysate used: Juvonen 1996 compared an extensively hydrolysed casein formula versus pasteurised donor human milk. Saarinen 1999 compared an extensively hydrolysed whey formula versus pasteurised donor human milk.
Sensitivity analysis
We considered neither study to be at 'low risk' of bias.
Comparison 2. Early short‐term feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ low‐risk infants
Two studies (Juvonen 1996; Saarinen 1999) compared a short duration (three to four days whilst in hospital) of early supplemental or sole feeding with a hydrolysed formula versus CMF. Both trials subsequently encouraged all mothers to breast feed.
Juvonen 1996 (77 infants) reported no difference in childhood allergic disease incidence (RR 1.37, 95% CI 0.33 to 5.71), no difference in childhood asthma incidence (RR 3.08, 95% CI 0.13 to 73.26), no difference in childhood eczema incidence (RR 0.34, 95% CI 0.04 to 3.15), no difference in childhood food allergy (RR 1.37, 95% CI 0.33 to 5.71) and no difference in childhood CMA (RR 5.13, 95% CI 0.25 to 103.43). Saarinen 1999 reported a reduction in infant CMA incidence of borderline significance (3473 infants; RR 0.62, 95% CI 0.38 to 1.00; risk difference ‐0.01, 95% CI ‐0.02 to 0.00; P = 0.05). Quality of evidence was assessed as 'very low' for all outcomes (see Table 2).
We considered the following subgroup analyses, but as no significant benefits were reported, we did not wish to duplicate the results.
Both studies enrolled infants irrespective of family history allergic disease in first‐degree relatives.
Extent of protein hydrolysis: Juvonen 1996 and Saarinen 1999 compared an EHF versus CMF.
Indication for use: both studies used formula for early short‐term infant formula feeding.
Method of ascertainment of allergic disease: Saarinen 1999 reported outcomes of an unblinded elimination/challenge for CMA. Juvonen 1996 did not report criteria for diagnosis of allergic disease.
Type of protein hydrolysate used: Juvonen 1996 compared an extensively hydrolysed casein formula versus CMF. Saarinen 1999 compared an extensively hydrolysed whey formula versus CMF.
Sensitivity analysis
We considered both studies to be at high risk of bias.
Comparison 3. Prolonged feeding: hydrolysed formula versus human milk feeding
We found no studies that compared prolonged feeding with hydrolysed formula versus human milk feeding.
Comparison 4. Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF)
Twelve studies (Chirico 1997; de Seta 1994; Hill 2000; Kwinta 2009; Lam 1992; Mallet 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) reported outcomes comparing prolonged hydrolysed formula versus CMF feeding.
Meta‐analysis found no difference in infant allergic disease (typical RR 0.88, 95% CI 0.76 to 1.01; participants = 2852; studies = 8; I2 = 48% [heterogeneity low]). Meta‐analysis found no difference in childhood allergic disease incidence (typical RR 0.85, 95% CI 0.69 to 1.05; participants = 950; studies = 2; I2 = 73% [heterogeneity moderate]). One study (Kwinta 2009) reported no difference in childhood allergic disease prevalence (RR 1.76, 95% CI 0.76 to 4.09; participants = 62). See Figure 4 for funnel plot.
4.
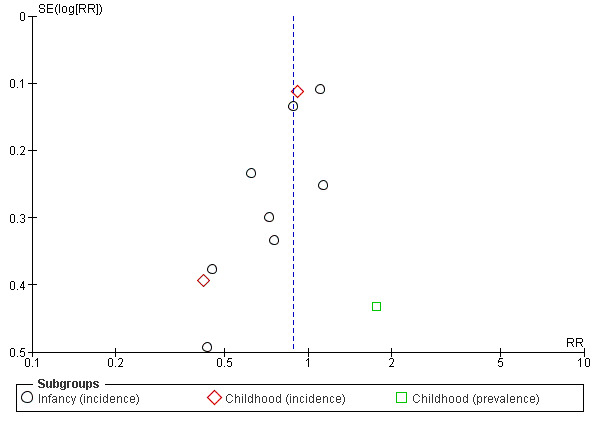
Funnel plot of comparison: 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, outcome: 4.1 All allergic disease.
Meta‐analysis found no difference in infant asthma (typical RR 0.57, 95% CI 0.31 to 1.04; participants = 318; studies = 4; I2 = 0%). Marini 1996 reported no difference in childhood asthma incidence (RR 0.38, 95% CI 0.08 to 1.84; participants = 78). Meta‐analysis found no difference in childhood asthma prevalence (typical RR 1.03, 95% CI 0.79 to 1.33; participants = 1229; studies = 3; I2 = 26% [heterogeneity low]).
Meta‐analysis found no difference in infant eczema (typical RR 0.93, 95% CI 0.79 to 1.09; participants = 2896; studies = 9; I2 = 9%). Meta‐analysis found no difference in childhood eczema incidence (typical RR 0.83, 95% CI 0.63 to 1.10; participants = 950; studies = 2; I2 = 40% [heterogeneity low]). Meta‐analysis found no difference in childhood eczema prevalence (typical RR 0.86, 95% CI 0.66 to 1.12; participants = 1228; studies = 3; I2 = 53% [heterogeneity moderate]). (See Figure 5 for funnel plot.)
5.
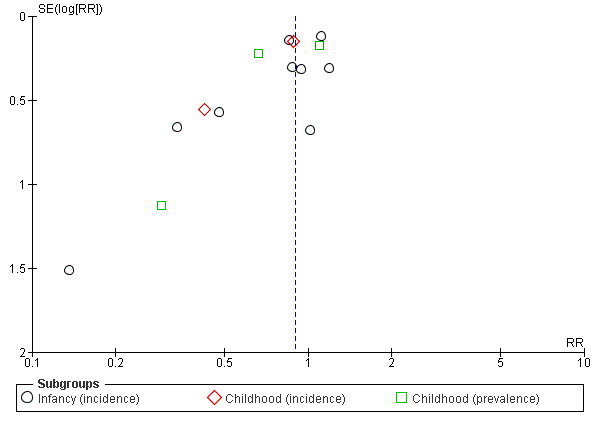
Funnel plot of comparison: 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, outcome: 4.3 Eczema.
Meta‐analysis found no difference in infant rhinitis (typical RR 0.52, 95% CI 0.14 to 1.85; participants = 256; studies = 3; I2 = 0%). Marini 1996 reported no difference in childhood rhinitis incidence (RR 0.47, 95% CI 0.04 to 5.03; participants = 78). Meta‐analysis found no difference in childhood rhinitis prevalence (typical RR 0.97, 95% CI 0.66 to 1.41; participants = 357; studies = 2; I2 = 0%).
Meta‐analysis found no difference in infant food allergy (typical RR 1.42, 95% CI 0.87 to 2.33; participants = 479; studies = 2; I2 = 0%). Hill 2000 reported no difference in infant CMA (RR 2.31, 95% CI 0.24 to 21.97; participants = 338). Another study (Vandenplas 1992) previously included in this analysis is no longer included as it did not report IgE‐mediated CMA consistent with WHO criteria (Johansson 2004).
Quality of evidence was assessed as 'very low' for all outcomes (see Table 3).
Subgroup analyses (Comparisons 5 to 10)
Comparison 5. Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ low‐risk infants
Kwinta 2009 compared prolonged feeding with a hydrolysed formula versus CMF in low‐risk infants. Kwinta 2009 reported no difference in childhood prevalence of allergic disease (RR 1.76, 95% CI 0.76 to 4.09; participants = 62), asthma (RR 2.20, 95% CI 0.77 to 6.26; participants = 62), eczema (RR 0.29, 95% CI 0.03 to 2.66; participants = 62) and rhinitis (RR 1.32, 95% CI 0.53 to 3.26; participants = 62).
Comparison 6. Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ high‐risk infants
Eight studies (de Seta 1994; Hill 2000; Lam 1992; Marini 1996; Oldaeus 1997; Vandenplas 1992; von Berg 2003; Willems 1993) compared prolonged hydrolysed formula feeding versus CMF feeding in high‐risk infants.
Meta‐analysis found no difference in infant allergic disease incidence (typical RR 0.88, 95% CI 0.76 to 1.01; participants = 2852; studies = 8; I2 = 48% [heterogeneity low]). Meta‐analysis found no difference in childhood allergic disease incidence (typical RR 0.85, 95% CI 0.69 to 1.05; participants = 950; studies = 2; I2 = 73% [heterogeneity moderate]).
Meta‐analysis found no difference in infant asthma incidence (typical RR 0.57, 95% CI 0.31 to 1.04; participants = 318; studies = 4; I2 = 0%). Marini 1996 reported no difference in childhood asthma incidence (RR 0.38, 95% CI 0.08 to 1.84; participants = 78). Meta‐analysis found no difference in childhood asthma prevalence (typical RR 0.97, 95% CI 0.74 to 1.27; participants = 1167; studies = 2; I2 = 0%).
Meta‐analysis found no difference in infant eczema incidence (typical RR 0.93, 95% CI 0.79 to 1.09; participants = 2896; studies = 9; I2 = 9%), no difference in childhood eczema incidence (typical RR 0.83, 95% CI 0.63 to 1.10; participants = 950; studies = 2; I2 = 40% [heterogeneity low]) and no difference in childhood eczema prevalence (typical RR 0.89, 95% CI 0.68 to 1.15; participants = 1166; studies = 2; I2 = 69% [heterogeneity moderate]).
Meta‐analysis found no difference in infant rhinitis incidence (typical RR 0.52, 95% CI 0.14 to 1.85; participants = 256; studies = 3; I2 = 0%). Marini 1996 reported no difference in childhood rhinitis incidence (RR 0.47, 95% CI 0.04 to 5.03; participants = 78). Hill 2000 reported no difference in childhood rhinitis prevalence (RR 0.90, 95% CI 0.59 to 1.37; participants = 295).
Meta‐analysis found no difference in infant food allergic disease incidence (typical RR 1.42, 95% CI 0.87 to 2.33; participants = 479; studies = 2; I2 = 0%).
Hill 2000 reported no difference in infant CMA incidence (RR 2.31, 95% CI 0.24 to 21.97; participants = 338).
Comparison 7. Prolonged feeding: partially hydrolysed formula (PHF) versus cow's milk formula (CMF)
Eleven studies (Chirico 1997; de Seta 1994; Kwinta 2009; Hill 2000; Lam 1992; Marini 1996; Oldaeus 1997; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993) reported outcomes that compared prolonged feeding with a PHF versus a CMF.
Meta‐analysis found no difference in infant allergic disease incidence (typical RR 0.89, 95% CI 0.77 to 1.04; participants = 1820; studies = 8; I2 = 53% [heterogeneity moderate]) and no difference in childhood allergic disease incidence (typical RR 0.86, 95% CI 0.67 to 1.10; participants = 510; studies = 2; I2 = 75% [heterogeneity high]). Marini 1996 reported a reduction in childhood allergic disease incidence (78 infants; RR 0.42, 95% CI 0.19 to 0.90), whereas von Berg 2003 reported no difference (432 infants; RR 0.95, 95% CI 0.73 to 1.25).
Meta‐analysis found no difference in infant asthma (typical RR 0.54, 95% CI 0.28 to 1.04; participants = 268; studies = 4; I2 = 0%). Marini 1996 reported no difference in childhood asthma incidence (RR 0.38, 95% CI 0.08 to 1.84; participants = 78). Meta‐analysis found no difference in childhood asthma prevalence (typical RR 1.05, 95% CI 0.80 to 1.38; participants = 789; studies = 3; I2 = 31% [heterogeneity low]).
Meta‐analysis found no difference in infant eczema (typical RR 0.98, 95% CI 0.82 to 1.16; participants = 1699; studies = 8; I2 = 0%), no difference in childhood eczema incidence (typical RR 0.85, 95% CI 0.61 to 1.19; participants = 510; studies = 2; I2 = 46% [heterogeneity low]) and no difference in childhood eczema prevalence (typical RR 0.92, 95% CI 0.69 to 1.22; participants = 788; studies = 3; I2 = 32%).
Meta‐analysis found no difference in infant rhinitis (typical RR 0.40, 95% CI 0.09 to 1.70; participants = 206; studies = 3; I2 = 0%). Marini 1996 reported no difference in childhood rhinitis incidence (RR 0.47, 95% CI 0.04 to 5.03; participants = 78). Meta‐analysis found no difference in childhood rhinitis prevalence (typical RR 0.97, 95% CI 0.66 to 1.41; participants = 357; studies = 2; I2 = 0%).
Meta‐analysis found no difference in infant food allergy (typical RR 1.53, 95% CI 0.93 to 2.49; participants = 429; studies = 2; I2 = 13%). Neither study reported a difference.
Hill 2000 reported no difference in infant CMA incidence (RR 2.31, 95% CI 0.24 to 21.97; participants = 338).
Comparison 8. Prolonged feeding: extensively hydrolysed formula (EHF) versus cow's milk formula (CMF)
Four studies (Kwinta 2009; Mallet 1992; Oldaeus 1997; von Berg 2003) compared prolonged feeding with an EHF versus CMF.
Meta‐analysis found no difference in infant allergic disease (typical RR 0.87, 95% CI 0.68 to 1.13; participants = 1561; studies = 2; I2 = 0%). von Berg 2003 reported no difference in childhood allergic disease incidence (RR 0.89, 95% CI 0.71 to 1.13; participants = 651). Kwinta 2009 reported no difference in childhood allergic disease prevalence (RR 1.76, 95% CI 0.76 to 4.09; participants = 62).
Oldaeus 1997 reported no difference in infant asthma (RR 0.61, 95% CI 0.18 to 2.04; participants = 96). Meta‐analysis found no difference in childhood asthma prevalence (typical RR 1.15, 95% CI 0.76 to 1.72; participants = 713; studies = 2; I2 = 43% [heterogeneity low]).
Meta‐analysis found no difference in infant eczema (typical RR 0.83, 95% CI 0.63 to 1.08; participants = 1726; studies = 3; I2 = 19%). von Berg 2003 reported no difference in childhood eczema incidence (RR 0.86, 95% CI 0.63 to 1.17; participants = 651). Meta‐analysis found a reduction in childhood eczema prevalence (typical RR 0.61, 95% CI 0.39 to 0.97; participants = 713; studies = 2; I2 = 0%).
Oldaeus 1997 reported no difference in infant rhinitis incidence (RR 2.76, 95% CI 0.12 to 66.22; participants = 96). Kwinta 2009 found no difference in childhood rhinitis prevalence (RR 1.32, 95% CI 0.53 to 3.26; participants = 62).
Oldaeus 1997 reported no difference in food allergy (RR 1.15, 95% CI 0.33 to 4.02; participants = 96).
Comparison 9. Prolonged feeding: extensively hydrolysed formula (EHF) versus partially hydrolysed formula (PHF)
Four studies (Halken 2000; Nentwich 2001; Oldaeus 1997; von Berg 2003) compared prolonged feeding with an EHF versus a PHF.
Meta‐analysis found no difference in infant allergic disease (three studies, 1806 infants; typical RR 0.93, 95% CI 0.75 to 1.16; I² = 0%). von Berg 2003 reported no difference in childhood allergic disease incidence (RR 0.93, 95% CI 0.74 to 1.18; participants = 661).
Meta‐analysis found no difference in infant asthma incidence (typical RR 1.72, 95% CI 0.74 to 3.96; participants = 341; studies = 2; I2 = 0%). von Berg 2003 reported no difference in childhood asthma prevalence (RR 0.89, 95% CI 0.58 to 1.35; participants = 661).
Meta‐analysis found no difference in infant eczema (typical RR 0.89, 95% CI 0.73 to 1.10; participants = 1865; studies = 4; I2 = 0%). von Berg 2003 reported no difference in childhood eczema incidence (RR 0.92, 95% CI 0.67 to 1.26; participants = 661) and no difference in childhood eczema prevalence (RR 0.90, 95% CI 0.54 to 1.52; participants = 661).
Meta‐analysis found no difference in infant rhinitis (typical RR 1.25, 95% CI 0.36 to 4.29; participants = 341; studies = 2; I2 = 0%).
Meta‐analysis found a reduction in infant food allergy (typical RR 0.43, 95% CI 0.19 to 0.99; participants = 341; studies = 2; I2 = 0%).
Halken 2000 reported no difference in infant CMA (RR 0.13, 95% CI 0.01 to 1.16; participants = 246) and no difference in infant urticaria (RR 1.32, 95% CI 0.26 to 6.66; participants = 246).
Comparison 10. Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula (CMF)
Seven studies reported prolonged exclusive hydrolysed formula versus CMF (Chirico 1997; de Seta 1994; Kwinta 2009; Lam 1992; Marini 1996; Vandenplas 1992; Willems 1993).
Meta‐analysis found a reduction in infant allergic disease (typical RR 0.61, 95% CI 0.46 to 0.80; participants = 425; studies = 5; I2 = 0%). Marini 1996 reported a reduction in childhood allergic disease incidence (RR 0.42, 95% CI 0.19 to 0.90; participants = 78). Kwinta 2009 reported no difference in childhood allergic disease prevalence (RR 1.76, 95% CI 0.76 to 4.09; participants = 62).
Meta‐analysis found no difference in infant asthma (typical RR 0.57, 95% CI 0.25 to 1.31; participants = 144; studies = 2; I2 = 0%). Marini 1996 reported no difference in childhood asthma incidence (RR 0.38, 95% CI 0.08 to 1.84; participants = 78). Kwinta 2009 reported no difference in childhood asthma prevalence (RR 2.20, 95% CI 0.77 to 6.26; participants = 62).
Meta‐analysis found no difference in infant eczema (typical RR 0.74, 95% CI 0.45 to 1.21; participants = 271; studies = 4; I2 = 0%). Marini 1996 reported no difference in childhood eczema incidence (RR 0.42, 95% CI 0.14 to 1.26; participants = 78). Kwinta 2009 reported no difference in childhood eczema prevalence (RR 0.29, 95% CI 0.03 to 2.66; participants = 62).
Marini 1996 reported that no infants had rhinitis and found no difference in childhood rhinitis incidence (RR 0.47, 95% CI 0.04 to 5.03; participants = 78). Kwinta 2009 reported no difference in childhood rhinitis prevalence (RR 1.32, 95% CI 0.53 to 3.26; participants = 62).
Sensitivity analyses (Comparisons 11 and 12)
Comparison 11. Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ studies with blinded measurement
Four studies reported assessment for allergic disease without knowledge of participant allocation (Kwinta 2009; Oldaeus 1997; Vandenplas 1992; von Berg 2003).
Meta‐analysis found no difference in infant allergic disease (typical RR 0.87, 95% CI 0.69 to 1.08; participants = 2156; studies = 3; I2 = 52%). von Berg 2003 reported no difference in childhood allergic disease incidence (RR 0.91, 95% CI 0.73 to 1.14; participants = 872). Kwinta 2009 reported no difference in childhood allergic disease prevalence (RR 1.76, 95% CI 0.76 to 4.09; participants = 62).
Oldaeus 1997 reported no difference in infant asthma (RR 0.48, 95% CI 0.17 to 1.42; participants = 141). Meta‐analysis found no difference in childhood asthma prevalence (typical RR 1.17, 95% CI 0.80 to 1.73; participants = 934; studies = 2; I2 = 38% [heterogeneity low]).
Meta‐analysis found no difference in infant eczema (typical RR 0.90, 95% CI 0.70 to 1.16; participants = 2089; studies = 2; I2 = 0%). von Berg 2003 reported no difference in childhood eczema incidence (RR 0.88, 95% CI 0.66 to 1.18; participants = 872). Meta‐analysis found a reduction in childhood eczema prevalence (typical RR 0.64, 95% CI 0.42 to 0.97; participants = 934; studies = 2; I2 = 0%).
Oldaeus 1997 reported no difference in infant rhinitis (RR 1.47, 95% CI 0.06 to 35.37; participants = 141). Kwinta 2009 (62 infants) reported no difference in childhood rhinitis prevalence (RR 1.32, 95% CI 0.53 to 3.26; participants = 62)
Oldaeus 1997 reported no difference in infant food allergy (RR 1.82, 95% CI 0.64 to 5.16; participants = 141).
Comparison 12. Prolonged feeding: hydrolysed formula versus cow's milk formula (CMF) ‐ studies at low risk of bias
We assessed no studies as eligible for inclusion in the sensitivity analysis of studies at low risk of bias (adequate randomisation, allocation concealment and < 10% loss to follow‐up).
Additional analyses (Comparisons 13 to 17)
Comparison 13. Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula (CMF)
Nine studies compared a partially hydrolysed whey formula versus CMF (Chirico 1997; de Seta 1994; Hill 2000; Lam 1992; Marini 1996; Tsai 1991; Vandenplas 1992; von Berg 2003; Willems 1993).
Meta‐analysis found a reduction in infant allergic disease (typical RR 0.77, 95% CI 0.59 to 1.00; participants = 1729; studies = 7; I2 = 53% [heterogeneity moderate]) and no difference in childhood allergic disease incidence (typical RR 0.68, 95% CI 0.31 to 1.52; participants = 510; studies = 2; I2 = 75% [heterogeneity high).
Meta‐analysis found no difference in infant asthma (typical RR 0.61, 95% CI 0.29 to 1.28; participants = 177; studies = 3; I2 = 0%). Marini 1996 reported no difference in childhood asthma incidence (RR 0.38, 95% CI 0.08 to 1.84; participants = 78). Meta‐analysis found no difference in childhood asthma prevalence (typical RR 0.98, 95% CI 0.73 to 1.31; participants = 727; studies = 2; I2 = 0%).
Meta‐analysis found no difference in infant eczema (typical RR 0.96, 95% CI 0.80 to 1.14; participants = 1608; studies = 7; I2 = 0%), no difference in childhood eczema incidence (typical RR 0.85, 95% CI 0.61 to 1.19; participants = 510; studies = 2; I2 = 46% [heterogeneity low]) and no difference in childhood eczema prevalence (typical RR 0.94, 95% CI 0.71 to 1.26; participants = 726; studies = 2; I2 = 44% [heterogeneity low]).
Meta‐analysis found no difference in infant rhinitis (typical RR 0.40, 95% CI 0.09 to 1.70; participants = 115; studies = 2; I2 = 0%). Marini 1996 reported no difference in childhood rhinitis incidence (RR 0.47, 95% CI 0.04 to 5.03; participants = 78). Hill 2000 reported no difference in childhood rhinitis prevalence (RR 0.90, 95% CI 0.59 to 1.37; participants = 295).
Hill 2000 reported no difference in infant food allergy (RR 1.31, 95% CI 0.75 to 2.30; participants = 338).
Hill 2000 reported no difference in infant CMA (RR 2.31, 95% CI 0.24 to 21.97; participants = 338).
Comparison 14. Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula (CMF)
Two studies compared a PHF containing casein versus CMF (Kwinta 2009; Oldaeus 1997).
Oldaeus 1997 reported no difference in infant allergic disease incidence (RR 1.36, 95% CI 0.80 to 2.31; participants = 91). Kwinta 2009 reported no difference in childhood allergic disease prevalence (RR 1.76, 95% CI 0.76 to 4.09; participants = 62).
Oldaeus 1997 reported no difference in infant asthma (RR 0.34, 95% CI 0.07 to 1.60; participants = 91). Kwinta 2009 reported no difference in childhood asthma prevalence (RR 2.20, 95% CI 0.77 to 6.26; participants = 62).
Oldaeus 1997 reported no difference in infant eczema (RR 1.30, 95% CI 0.66 to 2.55; participants = 91). Kwinta 2009 reported no difference in childhood eczema prevalence (RR 0.29, 95% CI 0.03 to 2.66; participants = 62).
Oldaeus 1997 reported that no infant had rhinitis. Kwinta 2009 reported no difference in childhood rhinitis prevalence (RR 1.32, 95% CI 0.53 to 3.26; participants = 62).
Oldaeus 1997 reported no difference in infant food allergy (RR 2.56, 95% CI 0.86 to 7.56; participants = 91).
Comparison 15. Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula (CMF)
von Berg 2003 compared an extensively hydrolysed whey formula versus CMF.
von Berg 2003 reported no difference in infant allergic disease (RR 0.97, 95% CI 0.71 to 1.34; participants = 972) and no difference in childhood allergic disease incidence (RR 1.07, 95% CI 0.82 to 1.38; participants = 431).
von Berg 2003 reported no difference in childhood asthma prevalence (RR 1.19, 95% CI 0.73 to 1.94; participants = 431).
von Berg 2003 reported no difference in infant eczema (RR 1.00, 95% CI 0.72 to 1.40; participants = 972), no difference in childhood eczema incidence (RR 1.06, 95% CI 0.75 to 1.49; participants = 431) and no difference in childhood eczema prevalence (RR 0.78, 95% CI 0.46 to 1.33; participants = 431).
Comparison 16. Prolonged feeding: extensively hydrolysed casein formula versus cow's milk formula (CMF)
von Berg 2003 compared an extensively hydrolysed casein formula versus CMF.
von Berg 2003 reported no difference in infant allergic disease (RR 0.76, 95% CI 0.54 to 1.07; participants = 976) and a reduction in childhood allergic disease incidence (RR 0.72, 95% CI 0.53 to 0.97; participants = 431).
von Berg 2003 reported no difference in childhood asthma prevalence (RR 0.84, 95% CI 0.49 to 1.45; participants = 431).
von Berg 2003 reported no difference in infant eczema (RR 0.69, 95% CI 0.47 to 1.00; participants = 976), a reduction in childhood eczema incidence (RR 0.66, 95% CI 0.44 to 0.98; participants = 431) and a reduction in childhood eczema prevalence (RR 0.50, 95% CI 0.27 to 0.92; participants = 431).
Comparison 17. Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula
Two studies compared an extensively hydrolysed casein formula versus an extensively hydrolysed whey formula (Halken 2000; von Berg 2003).
Meta‐analysis found no difference in infant allergic disease (typical RR 0.96, 95% CI 0.73 to 1.26; participants = 1143; studies = 2; I2 = 82% [heterogeneity high]). Neither study reported a difference. von Berg 2003 reported a reduction in childhood allergic disease incidence (RR 0.68, 95% CI 0.50 to 0.90; participants = 440).
Halken 2000 reported no difference in infant asthma (RR 2.28, 95% CI 0.83 to 6.28; participants = 161) von Berg 2003 reported no difference in childhood asthma prevalence (RR 0.71, 95% CI 0.42 to 1.19; participants = 440).
Meta‐analysis found no difference in infant eczema (typical RR 0.81, 95% CI 0.59 to 1.10; participants = 1143; studies = 2; I2 = 70% [heterogeneity moderate]). von Berg 2003 reported a reduction in childhood eczema incidence (RR 0.62, 95% CI 0.42 to 0.92; participants = 440) and no difference in childhood eczema prevalence (RR 0.64, 95% CI 0.33 to 1.21; participants = 440).
Halken 2000 reported no difference in infant rhinitis (RR 2.08, 95% CI 0.39 to 11.02; participants = 161), no difference in infant food allergy (RR 1.45, 95% CI 0.48 to 4.39; participants = 161), no difference in infant CMA (RR 5.19, 95% CI 0.25 to 106.38; participants = 161) and no difference in urticaria (RR 4.15, 95% CI 0.47 to 36.34; participants = 161).
Discussion
Summary of main results
Early short‐term infant feeding
Two studies (Juvonen 1996; Saarinen 1999) assessed the effect of three to four days' supplementation with a hydrolysed formula versus cow's milk formula (CMF) versus pasteurised human milk feeds whilst in hospital after birth.
Hydrolysed formula versus pasteurised human milk:Juvonen 1996 assessed outcomes in 90 infants and reported no difference in rates of allergic disease, asthma, eczema, food allergy and cow's milk allergy (CMA) up to three years of age (GRADE quality of evidence very low ‐ see Table 1). Quality of evidence was downgraded due to risk of bias (quasi‐random sequence allocation, incomplete outcome data and imbalances at baseline), imprecision and potential for reporting or publication bias. Saarinen 1999 assessed outcomes in 3559 infants and reported no difference in the rate of CMA at a mean age of 27 months (GRADE quality of evidence very low ‐ see Table 1). Quality of evidence was downgraded due to risk of bias (quasi‐random sequence allocation, incomplete outcome data), imprecision and potential for reporting or publication bias.
Hydrolysed formula versus CMF:Juvonen 1996 reporting outcomes in 77 infants reported no difference in rates of allergic disease, asthma, eczema, food allergy and CMA up to three years of age (GRADE quality of evidence very low ‐ see Table 2). Quality of evidence was downgraded due to risk of bias (quasi‐random sequence allocation, incomplete outcome data and imbalances at baseline), imprecision and potential for reporting or publication bias. Saarinen 1999 reporting outcomes in 3473 infants reported a reduction in CMA at a mean age of 27 months (GRADE quality of evidence very low ‐ see Table 2). Quality of evidence was downgraded due to risk of bias (quasi‐random sequence allocation, incomplete outcome data), imprecision and potential for reporting or publication bias.
Prolonged infant feeding
The most commonly reported outcomes were infant allergic disease incidence (8 studies, 2852 infants), childhood asthma prevalence (3 studies, 1229 infants), infant eczema incidence (9 studies, 2896 infants), childhood rhinitis prevalence (2 studies, 357 infants), food allergy (2 studies 479 infants), and CMA (1 study, 338 infants). Prolonged infant feeding with a hydrolysed formula compared with a CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis. The GRADE quality of evidence was very low for all outcomes with downgrading due to risk of bias, imprecision, and risk of reporting or publication bias (see Table 3). Prolonged infant feeding with a hydrolysed formula compared with a CMF was also not associated with any difference in infant food allergy or CMA. The GRADE quality of evidence was downgraded due to risk of bias, imprecision, and risk of reporting or publication bias. Childhood food allergy or CMA were not reported.
Subgroup analyses
Infant risk of allergic disease
Infants at low risk of allergic disease: a single study (Kwinta 2009) assessing outcomes for 62 infants reported no difference in childhood prevalence of allergic disease, asthma or eczema.
Infants at high risk of allergic disease: prolonged infant feeding with a hydrolysed formula compared with CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant food allergy or CMA.
Type of hydrolysed formula
Prolonged feeding with a partially hydrolysed formula (PHF) versus a CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant food allergy or CMA.
Prolonged feeding with an extensively hydrolysed formula (EHF) versus a CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant food allergy.
Prolonged feeding with an EHF versus a PHF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant CMA or infant urticaria. Prolonged feeding with an EHF versus a PHF was associated with a reduction in infant food allergy of borderline statistical significance (typical RR 0.43, 95% CI 0.19 to 0.99; participants = 341; studies = 2; I2 = 0%; typical risk difference (RD) ‐0.06, 95% CI ‐0.11, ‐0.00).
Prolonged exclusive formula feeding
Prolonged exclusive feeding with a hydrolysed formula versus CMF was associated with a reduction in infant allergic disease (typical RR 0.61, 95% CI 0.46 to 0.80; participants = 425; studies = 5; I2 = 0%; typical RD ‐0.16, 95% CI ‐0.25, ‐0.08; number needed to treat for an additional beneficial outcome (NNTB) 6, 95% CI 4, 12.5). Single small studies also reported a reduction in childhood allergic disease incidence but not childhood allergic disease prevalence. Prolonged exclusive feeding with a hydrolysed formula versus CMF was not associated with any differences in infant or childhood asthma or eczema, or childhood rhinitis.
Specific type of hydrolysed formula (not prespecified analyses)
Prolonged feeding with a partially hydrolysed whey formula versus CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant food allergy or CMA.
Prolonged feeding with a partially hydrolysed casein‐containing formula versus CMF was not associated with any differences in infant or childhood allergy, asthma, eczema or rhinitis; or infant food allergy.
Prolonged feeding with an extensively hydrolysed whey formula versus CMF was not associated with any differences in infant or childhood allergic disease, infant asthma, or infant or childhood eczema.
Prolonged feeding with an extensively hydrolysed casein formula versus CMF was not associated with any differences in infant allergic disease, childhood asthma, or infant eczema. There was a reduction in childhood allergic disease (RR 0.72, 95% CI 0.53 to 0.97; participants = 431; studies = 1; RD ‐0.10, 95% CI ‐0.18, ‐0.01), childhood eczema incidence (RR 0.66, 95% CI 0.44 to 0.98; participants = 431; studies = 1; RD ‐0.08, 95% ‐0.15, ‐0.00) and childhood eczema prevalence (RR 0.50, 95% CI 0.27 to 0.92; participants = 431; studies = 1; RD ‐0.06, 95%CI ‐0.12, ‐0.01).
Prolonged feeding with an extensively hydrolysed casein formula versus extensively hydrolysed whey formula was associated with no difference in infant allergic disease but a reduction in childhood allergic disease .
Overall completeness and applicability of evidence
For short‐term feeding (e.g. for three to four days whilst in hospital), a single small study (Juvonen 1996) is underpowered to determine an effect of hydrolysed formula compared with human milk or CMF on childhood allergic disease, asthma, eczema or rhinitis. A much larger study (Saarinen 1999) enrolling 5317 infants reported no difference in CMA upon comparing infants receiving short‐term feeding with an extensively hydrolysed whey formula versus human milk, and a reduction in CMA of borderline statistical significance when comparing infants fed an extensively hydrolysed whey formula versus CMF. Additional studies are needed to determine if infants benefit from short‐term feeding with a hydrolysed formula in hospital when human milk is unavailable for prevention of allergic disease and CMA. No studies have reported allergic disease outcomes resulting from short‐term use of a PHF for infants in hospital. Additional studies are needed to determine if infants derive benefit from using a PHF for short‐term feeding in hospital when human milk is unavailable for prevention of allergic disease and CMA.
For women unable to exclusively breast feed, no studies have compared prolonged use of a hydrolysed formula versus human milk (e.g. from a donor milk bank) for prevention of allergic disease. This review has not compared the effect of human milk versus CMF feeding so cannot provide a conclusion about potential benefit derived from use of a human milk bank for prevention of allergic disease.
The overall analyses found there was no benefit from prolonged use of a hydrolysed formula versus CMF for prevention of infant or childhood allergic disease, asthma, eczema, rhinitis, food allergy or CMA for infants not exclusively breast fed. The largest analyses were for infant allergic disease incidence (8 studies, 2852 infants) and infant eczema incidence (9 studies, 2896 infants). These analyses were considered imprecise so further studies are needed to determine of infants derive benefit from using a hydrolysed formula prolonged feeding when unable to be exclusively breast fed for prevention of allergic disease. Analyses of other allergic disease outcomes including for asthma, rhinitis, food allergy and CMA are even more underpowered.
Subgroup analysis found some evidence of benefit from prolonged exclusive feeding (infants not breast fed) with a hydrolysed formula versus a CMF which was associated with a reduction in infant allergic disease, but not asthma, eczema or rhinitis. However, relatively few trials assessed the effect of exclusive formula feeding so analyses are largely underpowered. The largest analysis was infant allergic disease incidence which included five studies reporting outcomes of only 425 infants. No data were available to assess the effect of exclusive hydrolysed formula feeding on the incidence of food allergy or CMA.
Subgroup analyses comparing prolonged use of a PHF versus CMF, or an EHF versus CMF, found no evidence of benefit from use of a hydrolysed formula for prevention of allergic disease or any type of allergic disease. Subgroup analyses of specific types of hydrolysed formulas are inconsistent, so findings should be treated with caution. Positive findings included: 1. Prolonged feeding with an EHF versus a PHF was associated with a reduction in infant food allergy (two studies (Halken 2000; Oldaeus 1997) reporting outcomes for 341 infants); and 2. Prolonged feeding with an extensively hydrolysed casein formula versus CMF was associated with a reduction in childhood allergic disease, childhood eczema incidence and childhood eczema prevalence. However, these findings are limited to the single centre findings of a multicentre study (von Berg 2003). A large trial (Knip 2011) comparing an extensively hydrolysed casein formula to an adapted CMF supplemented with 20% of the casein hydrolysate in infants at risk of type 1 diabetes mellitis was excluded from the review due to excess losses (2997 of 5156 randomised infants not included in analysis = 58%). The study reported no significant difference in adverse events including asthma and other forms of allergic disease at 10 years of age (extensively hydrolysed casein formula 339 / 1081 versus CMF 363 / 1078; RR 0.93, 95% CI 0.82, 1.05). If the Knip 2011 is included in meta‐analysis, the finding of reduced childhood allergic disease incidence is of borderline significance with moderate heterogeneity (typical RR 0.90, 95% CI 0.80 to 1.00; participants = 2590; studies = 2; I2 = 60%). Additional studies are required to determine if prolonged feeding with an extensively hydrolysed casein formula versus CMF reduces allergic disease in infants at high risk of allergic disease unable to be exclusively breast fed.
Quality of the evidence
Early short‐term infant feeding: two studies reported the effect of short‐term infant feeding of a hydrolysed formula versus human milk and cow's milk. Both studies (Juvonen 1996; Saarinen 1999) had substantial methodological concerns (Figure 3). Analyses of the larger study (Saarinen 1999), which enrolled 5317 infants, were restricted to reporting of CMA. The other study of 90 infants (Juvonen 1996) was underpowered to detect important differences in other allergic disease outcomes. We downgraded the GRADE quality of evidence for an effect on allergic disease and CMA to 'very low' due to risk of bias, imprecision and potential for reporting or publication bias (Table 1; Table 2).
Prolonged infant feeding with a hydrolysed formula compared with CMF: we downgraded the GRADE quality of evidence for all outcomes due to risk of bias, imprecision and potential for reporting or publication bias for all outcomes (Table 3). We had substantial methodological concerns regarding these studies (see Figure 2; Figure 3) and considered none to be at 'low risk' of bias. We had substantial concerns regarding the potential for publication bias or reporting bias in that substantial numbers of studies, including those in high‐risk infants, have not comprehensively reported allergic disease outcomes (see Characteristics of excluded studies). In addition, three studies (Giovannini 1994; Sorensen 2007; Wopereis 2014) enrolling infants at high risk of allergic disease have not reported outcomes to date. All analyses were considered to lack precision, so benefits from use of a hydrolysed formula are not excluded.
Potential biases in the review process
This review had strict prespecified inclusion criteria that included reporting data only when outcomes for ≥ 80% of allocated infants were noted. Ten studies (Barberi 1993; Chan 2002; Giovannini 1994; Halken 1992; Moran 1992; Odelram 1996; Porch 1998; Szajewska 2004; Vandenplas 1988; Zeiger 1989) had excess losses to follow‐up, so a substantial quantity of data are not included in this review. Losses to follow‐up do not necessarily bias a study. However, when losses do bias a study, it is not possible to predict the direction of effect. The review authors have consistently applied this inclusion criterion through all updates of this review. Although the excess losses criteria is objective and not determined by the estimate of effect from each of the studies, the exclusion of these studies has the potential to bias the review and reduce the power of the review to find an effect.
This review includes allergic disease outcomes measured using a variety of methods including physician assessment, interview and questionnaire, with and without additional clinical challenge or testing for sensitisation. This was done as the review's objective was to assess the effect on clinical allergic disease and not sensitisation. Different methods of ascertainment of clinical allergy are likely to have different levels of accuracy and objectivity.
This review includes unpublished data to reduce concern regarding publication bias. Not all studies have been reported in peer‐reviewed journals. Some studies were provided as university theses or by formula companies. Only one formula company to date has provided additional studies (Nestle). We excluded two studies after allegations of fraud were made and the authors of these published articles did not respond to requests for data (Chandra 1989a; Chandra 1989b). In addition, a substantial number of studies have failed to report or comprehensively report outcomes. We consider the analyses reported in this review to be at risk of publication bias and/or reporting bias.
This review includes studies that use random and quasi‐random methods of participant allocation. See Quality of the evidence.
We obtained data from study authors for several analyses to overcome reporting problems with publications and losses. One study (Hill 2000) reported data for infants who were not contemporaneously randomised, and study authors kindly provided data for contemporaneously randomised infants, which the review authors believe overcame our concern regarding selection bias. For a second study (von Berg 2003) that published outcomes to 15 years, to overcome problems related to excess losses, study authors kindly provided additional data from one centre (Wessel) that reported an intention‐to‐treat analysis up to three years with < 20% losses; we have included these data in this review. Losses to follow‐up after three years were excessive (> 20%), so we have excluded from this review follow‐up data from this study beyond three years.
Agreements and disagreements with other studies or reviews
The European Academy of Allergy and Clinical Immunology (EAACI) has published systematic reviews and guidelines for primary prevention of food allergy and anaphylaxis (de Silva 2014; Muraro 2014). For prevention of food allergy, two systematic reviews and four randomised trials found benefit from extensively hydrolysed whey or casein formula, although one study found no benefit. Two systematic reviews, two randomised trials and two non‐randomised comparisons noted benefit when partially hydrolysed formula was compared with CMF. One randomised trial and one non‐randomised study found no effect (de Silva 2014).
Recommendations that have been provided for primary prevention of food allergy include the following.
"Recommendations for all infants:
no special diet during pregnancy or for the lactating mother;
exclusive breast feeding for four to six months.
Further recommendations for high‐risk infants:
if supplement is needed during the first four months, a documented hypoallergenic formula is recommended.
Introduction of complementary foods after the age of four months according to normal standard weaning practices and nutrition recommendations, for all children irrespective of atopic heredity" (Muraro 2014).
Five published systematic reviews have examined hydrolysed formula for prevention of allergic disease (Alexander 2010; Boyle 2016a; de Silva 2014; Iskedjian 2010; Szajewska 2010). Only the report of Boyle 2016a had no potential conflicts of interest. A systematic review (Alexander 2010) studied 100% whey protein partially hydrolysed formula (PHF‐W) compared with intact protein CMF in healthy infants. A meta‐analysis revealed a reduction in atopic dermatitis (11 studies; typical RR 0.56, 95% CI 0.40 to 0.76). A published funnel plot appears asymmetrical, suggesting lack of small negative studies. Meta‐analysis limited to 'top‐tier studies' showed a similar reduction in atopic dermatitis (four studies; typical RR 0.45, 95% CI 0.30 to 0.70). Review authors concluded, .."exclusive breast‐feeding should be encouraged as the standard for infant nutrition in the first months of life. For infants who are not exclusively breast‐fed, feeding with PHF‐W instead of CMF reduces the risk of AD in infants, particularly in infants with a family history of allergic disease." This work was partially funded by Nestle.
A recent systematic review (Boyle 2016a) found 37 eligible trials of hydrolysed formula including more than 19,000 participants. Review authors found evidence of conflict of interest and high or unclear risk of bias in most studies of allergic outcomes and evidence of publication bias in studies of eczema and wheeze. Overall, no consistent evidence indicated that PHFs or EHFs reduce the risk of allergic or autoimmune outcomes among infants at high pre‐existing risk for these outcomes. Odds ratios for eczema from birth to four years of age were 0.84 (95% CI 0.67 to 1.07) for PHF; 0.55 (95% CI 0.28 to 1.09) for extensively hydrolysed casein‐based formula; and 1.12 (95% CI 0.88 to 1.42) for extensively hydrolysed whey‐based formula compared with CMF. Review authors concluded that findings do not support current guidelines recommending the use of hydrolysed formula to prevent allergic disease in high‐risk infants. Methodologically, the Boyle 2016a review differs from this review in that it included studies with > 20% loss to follow‐up and reported infant allergic disease assessed up to four years of age.
Another systematic review (de Silva 2014) reported mixed findings regarding the preventive benefits of breast feeding for infants at high or normal risk, but evidence suggested that recommendations should include avoiding cow's milk and substituting extensively or partially hydrolysed whey or casein formulas for infants at high risk for the first four months.
Another systematic review (Szajewska 2010) assessed the effect of using a partially hydrolysed 100% whey formula (pHF) in reducing risk of allergic disease among healthy infants at high risk for allergic disease. The summary of findings reports that "meta‐analysis showed that pHF compared to SF (standard formula) reduced the risk of all allergic diseases, particularly atopic dermatitis/eczema, at some time points among children at high risk for allergic disease. Limited data suggest that the use of pHF compared with SF reduced the risk of gastrointestinal symptoms and food allergy. The pooled results did not provide evidence of a difference in the effect of pHF versus SF on the incidence of either wheezing/asthma or rhinitis. Few significant differences in outcomes were found between children who received pHF versus an extensively hydrolyzed whey formula. No significant differences in outcomes were found between children who received pHF versus an extensively hydrolyzed casein formula. These results should be interpreted with caution due to a lack of methodological rigor in many trials." A grant from Nestle Nutrition Institute supported this review.
Another review (Iskedjian 2010) compared a partially hydrolysed 100% whey‐based infant formula (PHF‐W) versus extensively hydrolysed whey‐ (EHF‐Whey) or casein‐based (EHF‐Casein) infant formula for prevention of atopic dermatitis (AD) among infants who cannot be breast fed exclusively. Meta‐analysis revealed no difference in AD when PHF‐W was compared with EHF‐Whey (typical RR 0.75, 95% CI 0.54 to 1.05 at 0 to 12 months; and typical RR 0.80, 95% CI 0.63 to 1.02 at 0 to 36 months); and no difference when PHF‐W was compared with EHF‐Casein (typical RR 1.06, 95% CI 0.74 to 1.53 at 0 to 12 months; and typical RR 1.13, 95% CI 0.87 to 1.47 at 0 to 36 months). Review authors concluded that the efficacy of PHF‐W falls within the range of efficacy of both EHF formulas (whey and casein). The Nestle Nutrition Institute supported this review.
In a report (Chung 2012) on the FDA Health Claim Review on whey‐protein partially hydrolysed infant formula and atopic dermatitis, "the FDA evaluated human intervention studies that evaluated the role of W‐PHF in reducing the risk of AD. The FDA concluded there is little to very little evidence, respectively, to support a qualified health claim concerning the relationship between intake of W‐PHF and a reduced risk of AD in partially breast fed and exclusively formula‐fed infants throughout the first year after birth and up to 3 years of age. In addition, the FDA required a warning statement be displayed along with the health claim to indicate to consumers that partially hydrolyzed infant formulas are not hypoallergenic and should not be fed to infants who are allergic to milk or to infants with existing milk allergy symptoms."
Evidence from this review for hydrolysed formula versus CMF
This systematic review found prolonged feeding with a hydrolysed formula compared with a CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis, or infant food allergy or CMA. The GRADE quality of evidence was downgraded due to risk of bias, imprecision, and risk of reporting or publication bias. However, a subgroup analysis of infants exclusively formula fed found a reduction in infant allergic disease (typical RR 0.61, 95% CI 0.46 to 0.80; participants = 425; studies = 5; I2 = 0%; typical RD ‐0.16, 95% CI ‐0.25, ‐0.08; NNTB 6, 95% CI 4, 12.5), but no differences in infant or childhood asthma or eczema, or childhood rhinitis.
Evidence from this review for PHF versus CMF
This systematic review found prolonged feeding with a PHF versus a CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant food allergy or CMA.
Evidence from this review for EHF versus PHF
This systematic review found prolonged feeding with an EHF versus a CMF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant food allergy. This systematic review found prolonged feeding with an EHF versus a PHF was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis; or infant CMA or infant urticaria. Prolonged feeding with an EHF versus a PHF was associated with a reduction in infant food allergy (typical RR 0.43, 95% CI 0.19 to 0.99; participants = 341; studies = 2; I2 = 0%; typical RD ‐0.06, 95% CI ‐0.11, ‐0.00).
Authors' conclusions
Implications for practice.
We found no evidence to support feeding with a hydrolysed formula compared with exclusive breast feeding for prevention of allergic disease. Until high‐quality trials compare prolonged hydrolysed formula feeding versus breast or expressed human milk feeding, hydrolysed formula should not be routinely offered to infants for prevention of allergic disease in preference to breast milk.
We found no evidence of benefit from use of a hydrolysed formula in preference to human milk for early, short‐term feeding of low‐risk infants. When an infant requires a formula for short‐term feeding (e.g. three to four days in hospital), very low‐quality evidence indicates that use of an extensively hydrolysed formula (EHF) may prevent cow’s milk allergy (CMA). No included studies assessed the effect of short‐term feeding with a partially hydrolysed formula (PHF). On current evidence, a hydrolysed formula cannot be recommended for early short‐term feeding of infants unable to be exclusively breast fed.
For infants (irrespective of risk of allergic disease) who cannot be exclusively breast fed, very low‐quality evidence suggests that prolonged feeding with a hydrolysed formula compared with a cow’s milk formula (CMF) was not associated with any differences in infant or childhood allergic disease, asthma, eczema or rhinitis, or infant food allergy or CMA.
Implications for research.
Additional studies are needed to determine if infants benefit from short‐term feeding with a hydrolysed formula in hospital when human milk is unavailable for prevention of allergic disease and CMA.
For infants unable to be exclusively breast fed, the quality of evidence for the effect of prolonged use of a hydrolysed formula versus a CMF for prevention of allergic disease or CMA was graded as very low. Although overall there were no significant effects found, subgroup analysis of infants exclusively formula fed found a reduction in infant allergic disease from use of a hydrolysed formula compared to a CMF. The analysis was limited to five trials reporting outcomes for 425 infants. An additional subgroup analysis of prolonged feeding with an extensively hydrolysed casein formula versus a CMF also found a reduction in childhood allergic disease incidence, and eczema incidence and prevalence, although the findings are limited to a single study (von Berg 2003). Further studies are needed to determine if infants derive benefit from using a hydrolysed formula for prolonged feeding when unable to be exclusively breast fed for prevention of allergic disease. Considerations in trial design include enrolling infants at high risk of allergic disease; stratifying patient allocation according to whether the infant receives exclusive or partial formula feeding; and the specific type of hydrolysed formula. Trials should perform intention‐to‐treat analyses by reporting all infants randomised in allocated group.
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2018 | New citation required and conclusions have changed | The conclusions of the review have changed. |
| 17 October 2018 | New search has been performed | To address a data entry error, the review was updated. The error was corrected and a new search was performed. No new studies for inclusion were found, but one follow‐up report of an included study was located. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 25 May 2017 | Amended | A data entry error in the review has been identified that may impact the review conclusions. The review will be re‐published following revision and peer review. |
| 31 October 2016 | New search has been performed | See above |
| 31 October 2016 | New citation required and conclusions have changed | We identified 8 new ongoing or unpublished studies; we found 1 additional excluded study and an additional 15‐year follow‐up report from a previously included study; for 1 previously excluded study, trial authors provided additional data now included in the review. We added the GRADE method and SoF tables. We downgraded review conclusions. |
| 18 September 2008 | Amended | We converted this review to new review format |
| 27 July 2006 | New search has been performed | We reviewed the eligibility of all trials. We included several new studies and updated reports. We partially redid comparisons to better meet the objectives and methods specified in the protocol. Additionally, we performed previously specified subgroup analyses according to whether studies had blinded measurement for allergy and whether enrolled infants who were solely formula fed were fed 100% whey formula or casein‐containing formula (according to degree of hydrolysis) Exclusion of 2 previously included trials and inclusion of a new large trial resulted in substantial changes to the review and to the review conclusions |
| 27 July 2006 | New citation required and conclusions have changed | We made substantive amendments |
Acknowledgements
We acknowledge the authors of studies who kindly responded to requests for additional methodological information and unpublished data that have been included in this review (Hill 2000; von Berg 2003). We acknowledge Nestle, which provided copies of unpublished studies, theses and publications. We acknowledge Robert Boyle who provided data checking and constructive feedback on a previous version of this review (Osborn 2017a; Osborn 2017b).
Appendices
Appendix 1. MEDLINE search strategy
Ovid MEDLINE 1946 to November 2017
1 hydrolyzed.mp.
2 hydrolysed.mp.
3 protein hydrolysate.mp. or exp protein hydrolysate/
4 formula.mp. or exp artificial milk/
5 1 or 2 or 3
6 4 and 5
7 limit 6 to (humans and clinical trial, all)
Appendix 2. Embase search strategy
Embase Classic 1947 to 1973, Embase 1974 to 03 November 2017
1 hydrolyzed.mp.
2 hydrolysed.mp.
3 exp protein hydrolysis/
4 formula.mp. or exp artificial milk/
5 1 or 2 or 3
6 4 and 5
7 limit 6 to ( human and (clinical trial or randomized controlled trial or controlled clinical trial))
Appendix 3. CENTRAL search strategy
Cochrane Central Register of Controlled Trials November 2017
1 hydrolyzed.mp.
2 hydrolysed.mp.
3 protein hydrolysate.mp. or exp protein hydrolysate/
4 formula.mp. or exp artificial milk/
5 1 or 2 or 3
6 4 and 5
Appendix 4. Clinicaltrials.gov search strategy
Searched November 2017:
(hydrolysed OR hydrolyzed) AND formula limited to recruiting studies and child.
Appendix 5. EU Clinical Trials Register search strategy
Searched 3 November 2017;
Separate searches for 'hydrolysed'; hydrolyzed.
Data and analyses
Comparison 1. Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.38, 5.37] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.41] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.41] |
| 4 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.38, 5.37] |
| 5 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 3559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.52, 1.46] |
| 5.2 Childhood (incidence) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.35, 143.84] |
1.1. Analysis.
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 1 All allergic disease.
1.2. Analysis.
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 2 Asthma.
1.3. Analysis.
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 3 Eczema.
1.4. Analysis.
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 4 Food allergy.
1.5. Analysis.
Comparison 1 Early short‐term feeding: hydrolysed formula versus human milk feeding ‐ low‐risk infants, Outcome 5 Cow's milk allergy.
Comparison 2. Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.33, 5.71] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 73.26] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.15] |
| 4 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.33, 5.71] |
| 5 Cow's milk allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 3473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.00] |
| 5.2 Childhood (incidence) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.43] |
2.1. Analysis.
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 1 All allergic disease.
2.2. Analysis.
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 2 Asthma.
2.3. Analysis.
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 3 Eczema.
2.4. Analysis.
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 4 Food allergy.
2.5. Analysis.
Comparison 2 Early short‐term feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 5 Cow's milk allergy.
Comparison 4. Prolonged feeding: hydrolysed formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 8 | 2852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.01] |
| 1.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.05] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.04] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 3 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.33] |
| 3 Eczema | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 9 | 2896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 3.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 3.3 Childhood (prevalence) | 3 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.12] |
| 4 Rhinitis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.14, 1.85] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.41] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.87, 2.33] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.24, 21.97] |
4.1. Analysis.
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 1 All allergic disease.
4.2. Analysis.
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
4.3. Analysis.
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
4.4. Analysis.
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
4.5. Analysis.
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 5 Food allergy.
4.6. Analysis.
Comparison 4 Prolonged feeding: hydrolysed formula versus cow's milk formula, Outcome 6 Cow's milk allergy.
Comparison 5. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.77, 6.26] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.66] |
| 4 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
5.1. Analysis.
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 1 All allergic disease.
5.2. Analysis.
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 2 Asthma.
5.3. Analysis.
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 3 Eczema.
5.4. Analysis.
Comparison 5 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ low‐risk infants, Outcome 4 Rhinitis.
Comparison 6. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 8 | 2852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.01] |
| 1.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.69, 1.05] |
| 2 Asthma | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.04] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 3 Eczema | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 9 | 2896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 3.2 Childhood (incidence) | 2 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 3.3 Childhood (prevalence) | 2 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.15] |
| 4 Rhinitis | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.14, 1.85] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.87, 2.33] |
| 5.2 Childhood (incidence) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Childhood (prevalence) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.24, 21.97] |
| 6.2 Childhood (prevalence) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 1 All allergic disease.
6.2. Analysis.
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 2 Asthma.
6.3. Analysis.
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 3 Eczema.
6.4. Analysis.
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 4 Rhinitis.
6.5. Analysis.
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 5 Food allergy.
6.6. Analysis.
Comparison 6 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ high‐risk infants, Outcome 6 Cow's milk allergy.
Comparison 7. Prolonged feeding: partially hydrolysed formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 8 | 1820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| 1.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 2 Asthma | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 4 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.04] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 3 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 3 Eczema | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 8 | 1699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.16] |
| 3.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 3.3 Childhood (prevalence) | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.22] |
| 4 Rhinitis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 3 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.70] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.41] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.93, 2.49] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.24, 21.97] |
7.1. Analysis.
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 1 All allergic disease.
7.2. Analysis.
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
7.3. Analysis.
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
7.4. Analysis.
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
7.5. Analysis.
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 5 Food allergy.
7.6. Analysis.
Comparison 7 Prolonged feeding: partially hydrolysed formula versus cow's milk formula, Outcome 6 Cow's milk allergy.
Comparison 8. Prolonged feeding: extensively hydrolysed formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 2 | 1561 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.13] |
| 1.2 Childhood (incidence) | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.13] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.04] |
| 2.2 Childhood (prevalence) | 2 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.72] |
| 3 Eczema | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 3 | 1726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.08] |
| 3.2 Childhood (incidence) | 1 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.17] |
| 3.3 Childhood (prevalence) | 2 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.97] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.12, 66.22] |
| 4.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.33, 4.02] |
8.1. Analysis.
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 1 All allergic disease.
8.2. Analysis.
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
8.3. Analysis.
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
8.4. Analysis.
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
8.5. Analysis.
Comparison 8 Prolonged feeding: extensively hydrolysed formula versus cow's milk formula, Outcome 5 Food allergy.
Comparison 9. Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 3 | 1806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
| 1.2 Childhood (incidence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.74, 1.18] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.74, 3.96] |
| 2.2 Childhood (prevalence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.35] |
| 3 Eczema | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 4 | 1865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.10] |
| 3.2 Childhood (incidence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 3.3 Childhood (prevalence) | 1 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.52] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.36, 4.29] |
| 5 Food allergy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.99] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 1.16] |
| 7 Urticaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Infancy (incidence) | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.26, 6.66] |
9.1. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 1 All allergic disease.
9.2. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 2 Asthma.
9.3. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 3 Eczema.
9.4. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 4 Rhinitis.
9.5. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 5 Food allergy.
9.6. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 6 Cow's milk allergy.
9.7. Analysis.
Comparison 9 Prolonged feeding: extensively hydrolysed formula versus partially hydrolysed formula, Outcome 7 Urticaria.
Comparison 10. Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 5 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| 1.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.90] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.31] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.77, 6.26] |
| 3 Eczema | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 4 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.21] |
| 3.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.26] |
| 3.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.66] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
10.1. Analysis.
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 1 All allergic disease.
10.2. Analysis.
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 2 Asthma.
10.3. Analysis.
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 3 Eczema.
10.4. Analysis.
Comparison 10 Prolonged exclusive feeding: hydrolysed formula versus cow's milk formula, Outcome 4 Rhinitis.
Comparison 11. Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 3 | 2156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.69, 1.08] |
| 1.2 Childhood (incidence) | 1 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| 1.3 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.17, 1.42] |
| 2.2 Childhood (prevalence) | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.80, 1.73] |
| 3 Eczema | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 2 | 2089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.16] |
| 3.2 Childhood (incidence) | 1 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.18] |
| 3.3 Childhood (prevalence) | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.42, 0.97] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.06, 35.37] |
| 4.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.64, 5.16] |
11.1. Analysis.
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 1 All allergic disease.
11.2. Analysis.
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 2 Asthma.
11.3. Analysis.
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 3 Eczema.
11.4. Analysis.
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 4 Rhinitis.
11.5. Analysis.
Comparison 11 Prolonged feeding: hydrolysed formula versus cow's milk formula ‐ studies with blinded measurement, Outcome 5 Food allergy.
Comparison 13. Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 7 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.59, 1.00] |
| 1.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.52] |
| 2 Asthma | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 3 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.28] |
| 2.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.84] |
| 2.3 Childhood (prevalence) | 2 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.73, 1.31] |
| 3 Eczema | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 7 | 1608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.14] |
| 3.2 Childhood (incidence) | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 3.3 Childhood (prevalence) | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.26] |
| 4 Rhinitis | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 2 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.70] |
| 4.2 Childhood (incidence) | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.03] |
| 4.3 Childhood (prevalence) | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.75, 2.30] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.24, 21.97] |
13.1. Analysis.
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 1 All allergic disease.
13.2. Analysis.
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 2 Asthma.
13.3. Analysis.
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 3 Eczema.
13.4. Analysis.
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 4 Rhinitis.
13.5. Analysis.
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 5 Food allergy.
13.6. Analysis.
Comparison 13 Prolonged feeding: partially hydrolysed whey formula versus cow's milk formula, Outcome 6 Cow's milk allergy.
Comparison 14. Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.80, 2.31] |
| 1.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2 Asthma | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.60] |
| 2.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.77, 6.26] |
| 3 Eczema | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.66, 2.55] |
| 3.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.66] |
| 4 Rhinitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Childhood (prevalence) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.53, 3.26] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.86, 7.56] |
14.1. Analysis.
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 1 All allergic disease.
14.2. Analysis.
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 2 Asthma.
14.3. Analysis.
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 3 Eczema.
14.4. Analysis.
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 4 Rhinitis.
14.5. Analysis.
Comparison 14 Prolonged feeding: partially hydrolysed casein‐containing formula versus cow's milk formula, Outcome 5 Food allergy.
Comparison 15. Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 1 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.34] |
| 1.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.38] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.94] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 1 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.40] |
| 3.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.49] |
| 3.3 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.33] |
15.1. Analysis.
Comparison 15 Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula, Outcome 1 All allergic disease.
15.2. Analysis.
Comparison 15 Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula, Outcome 2 Asthma.
15.3. Analysis.
Comparison 15 Prolonged feeding: extensively hydrolysed whey formula versus cow's milk formula, Outcome 3 Eczema.
Comparison 16. Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
| 1.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.97] |
| 2 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.45] |
| 3 Eczema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.47, 1.00] |
| 3.2 Childhood (incidence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.98] |
| 3.3 Childhood (prevalence) | 1 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.27, 0.92] |
16.1. Analysis.
Comparison 16 Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula, Outcome 1 All allergic disease.
16.2. Analysis.
Comparison 16 Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula, Outcome 2 Asthma.
16.3. Analysis.
Comparison 16 Prolonged feeding: extensively hydrolysed casein formula versus cow' milk formula, Outcome 3 Eczema.
Comparison 17. Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All allergic disease | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infancy (incidence) | 2 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 1.2 Childhood (incidence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.90] |
| 2 Asthma | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.83, 6.28] |
| 2.2 Childhood (prevalence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.19] |
| 3 Eczema | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infancy (incidence) | 2 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.10] |
| 3.2 Childhood (incidence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.92] |
| 3.3 Childhood (prevalence) | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.21] |
| 4 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.39, 11.02] |
| 5 Food allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.48, 4.39] |
| 6 Cow's milk allergy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.19 [0.25, 106.38] |
| 7 Urticaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Infancy (incidence) | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [0.47, 36.34] |
17.1. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 1 All allergic disease.
17.2. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 2 Asthma.
17.3. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 3 Eczema.
17.4. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 4 Rhinitis.
17.5. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 5 Food allergy.
17.6. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 6 Cow's milk allergy.
17.7. Analysis.
Comparison 17 Prolonged feeding: extensively hydrolysed casein formula versus extensively hydrolysed whey formula, Outcome 7 Urticaria.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chirico 1997.
| Methods | Single‐centre randomised controlled trial in Italy. | |
| Participants | Inclusion criteria: infants of mothers with atopy (rhinitis, asthma, eczema or food intolerance) who "could not breast feed". | |
| Interventions | Treatment (n = 21): partially hydrolysed cow's milk whey formula (Vivena HA‐Primigiorni HA). Control (n = 14): CMF (brand not reported). Co‐interventions (in all 'at‐risk infants'): avoidance of passive smoking, exposure to pets and mites, avoidance of nurseries, delayed weaning to 6 months of age. | |
| Outcomes | Primary outcome(s): immunogenicity and antigenicity of pHWF in at‐risk infants, including RAST for milk and egg proteins, total and specific IgE and specific IgG and IgG4 subclass antibodies. Other outcomes: eczema: defined as a pruritic, chronic or chronically relapsing dermatitis with typical features and distribution. Follow‐up to 6 months (infant eczema incidence). | |
| Notes | Trial of sole prolonged partially hydrolysed whey CMF and environmental allergen avoidance vs CMF. Conflict of interest: none reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | After informed consent was obtained from parents, infants considered at risk of atopy and who could not be breast fed were randomised (by the sealed‐envelope method) to treatment on the first day of life. Only infants who were exclusively breast fed, special formula fed or traditional formula fed and who were not exposed to passive smoking during the first 6 months of life were included in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for clinical outcomes. Reported for measurement of sensitisation (specific IgE). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of enrolled infants not reported. Only infants who received 6 months of allocated formula analysed. |
| Selective reporting (reporting bias) | Unclear risk | Specific clinical outcomes not reported to be prespecified. |
| Other bias | High risk | Unequal numbers in groups reported. |
de Seta 1994.
| Methods | Single‐centre randomised controlled trial in Italy. | |
| Participants | Inclusion criteria: infants with at least 1 first‐degree relative with allergic disease. When history in doubt, SPTs or RAST performed. | |
| Interventions | Treatment (n = 23): pHWF (Nidina‐HA, Nestle). Control (n = 39): CMF (Nidina, Nestle). Co‐interventions: none reported. Formula only to 6 months, then 'normal' diet. | |
| Outcomes | Primary outcome(s): allergic disease at 6 and 24 months (infant allergic disease). Other outcomes: physician clinical examination and/or telephone contact to determine incidence of allergic disease. CMPI, eczema and asthma diagnosed clinically according to standard criteria. | |
| Notes | Trial of sole prolonged pHWF vs CMF. Conflict of interest: none reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported. |
| Selective reporting (reporting bias) | Low risk | Prespecified allergic disease and timing of reporting. |
| Other bias | High risk | Group characteristics not reported. Group sizes unequal. |
Halken 2000.
| Methods | Multi‐centre quasi‐randomised (alternation) controlled trial in Denmark. | |
| Participants | Inclusion criteria: infants with bi‐parental atopy or uniparental atopy and cord IgE ≥ 0.3 kU/L. | |
| Interventions | Supplemental or sole formula feeding with: Treatment 1 (n = 79): extensively hydrolysed casein formula (Nutramigen). Treatment 2 (n = 82): extensively hydrolysed whey formula (Profylac). Control (n = 85): pHWF (NAN‐HA). Recommended duration of feeding: 4 months. Co‐interventions (all infants): delay solids and cow's milk to 4 months; avoid smoke, pets, damp housing. | |
| Outcomes | Primary outcome(s): allergic disease. Other outcomes: physician examination at 6, 12 and 18 months (infant allergic disease). Definitions Any atopy: symptoms of asthma, atopic dermatitis, allergic rhino conjunctivitis or at least 2 episodes of allergic urticaria. Asthma: clinician diagnosed, ≥ 3 episodes of recurrent wheezing needing bronchodilators. Atopic dermatitis: physical examination, ≥ 3 months' duration. Allergic rhino conjunctivitis: ≥ 1 month or recurrent symptoms. Food allergy: confirmed by unblinded elimination/challenge. CMA/CMPI: confirmed by unblinded elimination/challenge and exclusion of lactose intolerance and infection. | |
| Notes | Trial of supplemental or sole pHWF vs extensively hydrolysed casein formula vs extensively hydrolysed whey formula in high‐risk infants. Control group of non‐randomly allocated breast fed infants not included in analysis. Conflict of interest: funded by the Danish Dairy Foundation. Companies provided formula and funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random. Postnatal allocation by date of birth. |
| Allocation concealment (selection bias) | Unclear risk | Allocation predictable but intervention blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used formula tins labelled 'A, B or C'. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ‘The blinded coding of the products was not revealed until all the data registration was complete’. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear as to exact numbers in each group not completing the study. Of initial population of 595 infants, 92% were included in study and 80% completed follow‐up. Reasons for losses included parental refusal (19), received other formula in first days (23), 'dropped out' (36), not seen at 18 months, did not fulfil inclusion criteria (4) and non‐compliance (32). |
| Selective reporting (reporting bias) | High risk | Not intention‐to‐treat analysis. |
| Other bias | High risk | Some baseline differences between study groups (bi‐parental atopy: Nutramigen 38%, Profylac 22%, Nan HA 39%). |
Hill 2000.
| Methods | Randomised controlled trial in Australia. | |
| Participants | Inclusion criteria: Mother‐baby pairs were enrolled if the unborn child had a first‐degree relative with a history of eczema, asthma, allergic rhinitis or food allergy. | |
| Interventions |
Treatment 1 (n = 206): pHWF (NAN HA; Nestle, Biessenhoffen, Germany). Treatment 2 (n = 208): soy‐based formula (ProSobee; Mead Johnson Nutrition/Bristol Myers, Melbourne, Australia). Control CMF (n = 206): CMF (NAN; Nestle, Tongala, Australia). Mothers were encouraged to initiate and maintain breast feeding for at least 6 months. Study formulas introduced at cessation or partial cessation of breast feeding or as a breast milk substitute if breast feeding was not intended. Approximately 50% of infants received some allocated formula by 4 months of age; 16.5% never received allocated formula because of continuing breast feeding (13.6%; n = 78/575) or use of a non‐allocated formula (2.9%; n = 17/575). |
|
| Outcomes | Childhood outcomes based on parent report during telephone interviews up to age 2 (every 4 weeks until 64 weeks, then at 78 and 104 weeks), and at 6 or 7 years. SPTs were performed at 6, 12 and 24 months according to a standard technique by 1 of 3 allergy‐trained research nurses. Primary aim: to determine incidence of allergic manifestations (eczema and food reactions) up to 2 years of age in high‐risk infants. Definitions Eczema: doctor‐diagnosed eczema or any rash that was treated with topical steroid preparation (excluding rash that affected only the scalp or nappy region). Food reaction: within 2 hours of ingesting that food, child developed an acute skin rash (urticaria, angioedema, erythematous or morbilliform), a flare of pre‐existing eczema, signs of anaphylaxis or vomiting. Any allergic manifestation: presence of eczema or food reaction within first 2 years of life. Positive SPT: a wheal of at least 3 mm (mean) diameter with a positive (histamine) control. Childhood outcomes based on parent report during telephone interviews conducted when children were 6 or 7 years of age were defined as follows.
|
|
| Notes |
Sponsor: Nestec Ltd, a subsidiary of Nestle Australia, provided the study formula and staff funding for the first 6 years of the study. A.J. Lowe has received research support from Dairy Australia. K.J. Allen has received research support from Wyeth and Nutricia. M.J. Abramson has received research support from the National Health and Medical Research Council. D.J. Hill has received research support from Nestle Australia, SHS International and Nutricia Initial study reports were not intention‐to‐treat. Latest report is an intention‐to‐treat analysis so is now considered eligible for inclusion. Data obtained from study authors excluded enrolments in the first 9 to 10 months, when infants were not randomised to a hydrolysed formula. Executives from Nestlé Australia wrote to the editors of JACI on 12 May 2010 to raise scientific concerns about the study, including alleged discrepancies between the interim report and the publication accepted by JACI Before publication, and with full information on concerns raised by Nestlé, the following organisations reviewed these issues.
Study authors documented that internal audits of study data included re‐entry of infant feeding data for all infants and a random audit of outcome data for 100/620 infants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician created each of the computer‐generated allocation schedules. |
| Allocation concealment (selection bias) | Low risk | Mother‐baby pairs were allocated to the next sequential number as they were enrolled in the study and were assigned to the formula code allocated to that number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Staff were blind to allocation codes and to group of allocation at the time of outcome assessment. Cans of formula were labelled at an independent location. Parents of participants were informed of the identity of the assigned formula only after the child’s second birthday. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff were blind to allocation codes and to group of allocation at the time of outcome assessment. However, random allocation list was available to research staff. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 years: 45/620 (7.3%) excluded; 6 to 7 years: 125/620 (20%) excluded. Excluded as lost contact or parent refused further follow‐up. Low risk 2 years, high risk 6 to 7 years owing to excess losses. |
| Selective reporting (reporting bias) | Low risk | Prospective protocol registration (ACTRN12609000734268). Primary endpoints stated in the objectives reported in the results. |
| Other bias | Low risk | Infants allocated to CMF and pHWF groups similar on baseline risk factors. No differences between groups in terms of duration of exclusive breast feeding or age of introduction of solids Quote: "The first 97 infants were randomized to either the CMF or soy study groups. When the pHWFbecame available, a new random allocation series was generated with a higher proportion allocated to the pHWF to obtain equal numbers in each formula group." This review incorporates only infants allocated to the 3 groups contemporaneously. |
Juvonen 1996.
| Methods | Population derived (city of Malmo) quasi‐randomised (alternation) controlled trial in Sweden. | |
| Participants | Inclusion criteria: 144 healthy term infants of pregnant mother volunteers. 62% had family history of atopy. | |
| Interventions | Early sole feeding for 3 days. Subsequently, all infants exclusively breast fed. Treatment 1 (n = 53): pasteurised human milk feeds from milk bank. Treatment 2 (n = 38): casein EHF (Nutramigen). Control (n = 39): CMF (Baby Semp). | |
| Outcomes | Primary outcome(s): macromolecular absorption, antibody production and allergic symptoms. Other outcomes: serum IgE at 4 days, 8 months, 1 and 2 years; SPT at 1 and 2 years; clinical allergic disease to 3 years (child allergic disease incidence). Criteria for allergic disease diagnosis not reported. | |
| Notes | Trial of early (first 3 days) sole human milk vs CMF vs extensively hydrolysed casein formula. Use of volunteers meant possible selection of high‐risk infants. Conflict of interest: unclear; work supported by several foundations; affiliations not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random, infants allocated according to day of the month. |
| Allocation concealment (selection bias) | High risk | Predictable allocation; method of concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Group numbers unequal. 15/144 (10%) lost to 3 years. |
| Selective reporting (reporting bias) | Unclear risk | Aim to measure 'allergic symptoms'. |
| Other bias | High risk | Potentially clinically important imbalances between groups (maternal smoking, bi‐parental atopy). |
Kwinta 2009.
| Methods | Single‐centre randomised controlled trial in Poland. | |
| Participants |
Inclusion criteria: birth weight ≤ 1500 g, age on admission < 72 hours, negative blood culture on admission. Exclusion criteria: GI tract anomalies, early‐onset gram‐negative sepsis, necrotising enterocolitis before the beginning of enteral feeding, Apgar score < 3 at 5 minutes, intraventricular haemorrhage grade IV. |
|
| Interventions | Infants fed for 1 month. Treatment (n = 40): extensively hydrolysed protein‐based formula (casein hydrolysate formula; Pregestimil, Mead and Johnson). Control (n = 40): cow's milk‐based formula (Bebilon Neonatal, Nutricia). |
|
| Outcomes |
Primary outcome: at 5 to 7 years of age, presence of atopic disease according to the following categories: obvious atopic disease, possible atopic disease, no atopic disease.
Secondary outcomes: sensitisation status (atopic status) determined by IgE, by SPT or by CD4 + CCR4 + CD4 + CXCR3 + lymphocyte ratio. Definitions Parents asked to complete ISAAC (International Study of Asthma and Allergies in Childhood) questionnaire assessing past and current health status of the child. All questions verified by physicians during face‐to‐face discussion. All children examined by an investigator for the presence of atopic eczema, (rhino)conjunctivitis, wheezing and other clinical signs of allergic disease. |
|
| Notes |
Sponsor: supported by unrestricted grant from Nutricia Research Foundation. This is a follow‐on trial of a randomised controlled trial of feeding during first month of life with a formula containing lactose (Bebilon Nenatal) vs a lactose‐free formula (Pregestimil) (Kwinta 2002). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | 89 infants enrolled, 80 included ‐ unclear as to timing of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study formulas were prepared by the hospital pharmacy in the blind manner". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All children were examined by an investigator in the blinded fashion for the presence of atopic eczema, (rhino)conjunctivitis, wheezing, and other clinical signs of allergy". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/89 excluded after enrolment. 6 infants died before discharge. Hydrolysed group 8/37 and CMF group 4/37 lost before follow‐up. Overall, 62/74 (84%) survivors reported. |
| Selective reporting (reporting bias) | High risk | Study originally set up to evaluate the influence of different enteral feeding protocols on early morbidity of VLBW infants. |
| Other bias | Unclear risk | Some clinically but not statistically significant differences in baseline family history of atopy: CMF 5/29 (17%); HF 3/33 (9%). |
Lam 1992.
| Methods | Single‐centre randomised controlled trial in Hong Kong. | |
| Participants | Inclusion criteria: infants not breast fed or who stopped breast feeding in first 2 weeks. 'High‐risk infants' but criteria not reported. | |
| Interventions | Allocated to: Treatment (n = 50): pHWF (Nan HA, Nestle). Control (n = 50): CMF (Nan, Nestle). Co‐interventions: none reported. Solids withheld for 6 months. | |
| Outcomes | Primary outcome(s): allergic manifestations in first 6 months. Other outcomes: growth parameters in first 6 months. Definitions Atopic symptoms included colic, respiratory atopy (wheeze and rhinitis) and skin atopy (eczema and urticaria). Eczema not defined. | |
| Notes | Trial of prolonged feeding in infants at high risk of allergic disease with pHWF vs CMF. Numbers of infants with atopic manifestations at 6 months converted from percentages. Conflict of interest: internal report of Nestle. Data not published. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported 'double‐blind randomisation'; method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/100 (8%) ‐ 6 in HF group and 2 in CMF group. |
| Selective reporting (reporting bias) | Unclear risk | Allergic disease definitions not reported. |
| Other bias | Unclear risk | Group characteristics not reported. |
Mallet 1992.
| Methods | Single‐centre randomised controlled trial in France. | |
| Participants | Inclusion criteria: 177 infants with immediate family history of allergic disease. Allergic disease score used. | |
| Interventions | Sole or supplementary formula feeding for at least 4 months Treatment (n = 92): extensively hydrolysed casein formula (Pregestemil, Mead Johnson). Control (n = 85): CMF (Galliazyme, Gallia, France). No co‐interventions. | |
| Outcomes | Primary outcome(s): allergic disease. Other outcomes: clinician assessment for allergic disease. Eczema and IgE assessed at 4 months; eczema, asthma and CMA assessed at 1, 2 and 4 years. Definitions Atopic eczema: graded as mild (< 4 patches), moderate or severe. Asthmatic bronchitis: grade 1 (2 to 4 occurrences per year) and grade 2 (> 4 per year). CMA: confirmed by type 1 reagin allergy (specific IgE RAST) or malabsorption. | |
| Notes | Excess losses at all time periods except 4 months (infant allergic disease incidence). Trial of supplemental or sole extensively hydrolysed casein formula vs CMF. Results for only 4 months included (infant allergic disease incidence). Conflict of interest: Mead Johnson and Gallia supplied formula. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants randomised postnatally; method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not blinded.... and formulas were easily distinguishable by taste and smell. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both parents and paediatricians knew which formula was fed to the infant. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HF group (n = 92): 5 (5%) at 4 months, 21 (23%) at 1 year, 14 (15%) at 2 years, 22 (24%) at 4 years.
CMF group (n = 85): 7 (8%) at 4 months, 32 (38%) at 1 year, 24 (28%) at 2 years, 31 (36%) at 4 years. Three children failed to follow diet prescriptions and were excluded. No withdrawal seemed to be motivated by any abnormality linked to cow's milk intolerance. |
| Selective reporting (reporting bias) | High risk | Quote: "Aim of assessing the allergy prevention effects". Reported multiple allergic disease outcomes, grades of severity and time points. |
| Other bias | Low risk | Well‐balanced groups after allocation. |
Marini 1996.
| Methods | Three‐centre randomised controlled trial in Italy. | |
| Participants | Inclusion criteria: maternal questionnaire used to identify infants with well‐defined family history of allergic disease in either parent. | |
| Interventions | Infants randomised were those whose mothers did not wish to breast feed or had insufficient milk. Treatment (n = 48): 'moderately' HF (Nidina HA, Nestle). Control (n = 47): CMF (Nan, Nestle). Formula feeding advised to 5 months. Co‐interventions (both groups): maternal cow's milk and food avoidance measures for breast feeding mothers. For infants, cow's milk and allergenic foods avoided to 1 year. Advice given to modify environmental exposure (smoking, pets, carpets, avoiding infant community care to 2 years). | |
| Outcomes | Primary outcome(s): allergic manifestations and nutritional adequacy of formula. Other outcomes: weight, length and head circumference at 6 months, 1 and 3 years. Physician‐diagnosed allergic disease. Definitions Atopic dermatitis: typical rash in at least 2 areas. Recurrent wheezing: ≥ 3 episodes and physician diagnosed. Recurrent urticaria: ≥ 2 episodes after exposure to particular antigen. GI symptoms: vomiting and/or diarrhoea after exclusion of infection and lactose intolerance, not confirmed by blinded elimination/challenge. Allergic rhinitis: ≥ 3 weeks rhinorrhoea. RAST and SPTs also performed in affected individuals. Follow‐up performed at 1, 2 (infant allergic disease) and 3 years (child allergic disease). | |
| Notes | Trial of prolonged supplemental or sole 'moderately' hydrolysed whey formula vs CMF feeding in high‐risk infants. Co‐interventions in both groups. Conflict of interest: none reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Postnatal 'random' allocation of infants; method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | quote: "Where artificial feeding was required... babies... randomly allocated to formula (a) or formula (b) ... (but the mothers were not blinded to the allocation)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Physicians "unaware of the dietary regimen", but insufficient information reported on blinding of personnel to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Hydrolysed group losses 5 (10%) at 1 year, 6 (13%) at 2 years, 8 (17%) at 3 years. Cow's milk group losses 6 (13%) at 1 year, 7 (15%) at 2 years, 9 (19%) at 3 years. |
| Selective reporting (reporting bias) | Low risk | Prespecified allergic disease outcomes. Standardised definitions. |
| Other bias | Unclear risk | Insufficient data reported at baseline for allergic disease risk factors between study groups. |
Nentwich 2001.
| Methods | Single‐centre quasi‐randomised (alternation) controlled trial in Czech Republic. | |
| Participants | Inclusion criteria: pregnant women who themselves, husbands or children attended an allergic disease or dermatology outpatient clinic (i.e. family history of atopy in first‐degree relative). | |
| Interventions | Mothers encouraged to breast feed for at least 6 months to avoid cow's milk and highly allergenic foods. Allocated sole or supplemental formula if unable to solely breast feed according to prenatal treatment allocation. Treatment 1 (n = 37): partially hydrolysed whey CMF (Beba HA, Nestle, Denmark). Treatment 2 (n = 35): extensively hydrolysed whey CMF (Hipp HA, Hipp GnbH, Gmunden, Austria). Co‐interventions: all mothers encouraged to breast feed for 6 months, avoid cow's milk for first 6 months, introduce solids after 6 months and delay allergenic foods to after 12 months. At 6 months: 24/37 fed PHF and 21/35 fed EHF. At 12 months: 31/37 fed PHF and 28/35 EHF. | |
| Outcomes | Primary outcome(s): antigen‐specific reactivity of mononuclear cells to cow's milk protein; cow's milk‐specific IgE and IgG; atopic skin symptoms. Other outcomes: symptom diaries kept. Blinded paediatrician assessment for atopic dermatitis. Reported weights up to 12 months (data not given). Definitions Atopic dermatitis: typical rash in at least 2 locations relapsing for at least 3 months' duration. Standardised score used (SCORAD). Allergic disease reported at 6 and 12 months (infant allergic disease). | |
| Notes | Trial of sole or supplemental feeding pHWF vs extensively hydrolysed whey formula in high‐risk infants unable to be completely breast fed in first 6 months. Conflict of interest: supported by research grants. The "study done independently of infant food companies". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random ‐ prenatal randomisation by odd and even numbers. |
| Allocation concealment (selection bias) | High risk | Prenatal randomisation by odd and even numbers. Postnatal allocation to formula if unable to fully breast feed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Paediatrician prescribing treatment aware of allocation. Formula not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Second paediatrician unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/73 (1%) post randomisation loss. 13/72 (18%) not fed HF and reported in separate group. |
| Selective reporting (reporting bias) | Low risk | Prespecified atopic skin symptoms in first 12 months of life. Standardised definitions. |
| Other bias | High risk | Groups appeared well balanced after allocation. However, not intention‐to‐treat analysis. |
Oldaeus 1997.
| Methods | Population derived (well‐baby clinics in 3 towns) randomised controlled trial in Sweden. | |
| Participants | Inclusion criteria: term newborn infants with 2 allergic family members or 1 allergic family member and cord IgE ≥ 0.5 U/L. Mean age of weaning between 3 and 4 months. | |
| Interventions | In infants weaning from the breast: Treatment 1 (n = 55): extensively hydrolysed casein formula (Nutramigen, Mead Johnson). Treatment 2 (n = 51): partially hydrolysed formula whey:casein ratio 60:40 (Mead Johnson). Control (n = 49): CMF (Enfamil, Mead Johnson). Co‐interventions: both groups advised to not smoke and avoid pets. Solids introduced after 4 months. Avoidance of cow's milk, eggs, fish and citrus until after 9 months. | |
| Outcomes | Primary outcome(s): atopic and allergic disease at 18 months (infant allergic disease incidence). Other outcomes: nurse examination at 3, 6, 9, 12, 18 months and doctor visit at 18 months. SPTS at each visit and specific IgE RAST at 9, 12, 18 months. Definitions Atopic dermatitis: standard scoring system used. Food reactions: double‐blind placebo‐controlled challenges for formula milk reactions. Asthma: recurrent wheeze with doctor confirmation. Allergic rhinitis: doctor verified and allergen sensitisation proved. Gastrointestinal allergic disease: positive unblinded oral challenge to food to which infant was sensitised. | |
| Notes | Trial of extensively hydrolysed vs partially hydrolysed vs CMF for weaning high‐risk infants. Conflict of interest: co‐investigator from formula company. Formula supplied by Mead Johnson. Study supported by Bristol‐Myers Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by age at weaning. Infants randomised when weaning commenced. Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Identical coded tins used for extensively and partially hydrolysed formulas; no blinding of CMF tin. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Nurses and investigators blinded throughout the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14/155 post‐randomisation losses (9%) at 18 months. Feeding problems resulted in 11 losses (EHF 3, PHF 6, CMF 2). |
| Selective reporting (reporting bias) | Low risk | Primary endpoints reported in results. |
| Other bias | Unclear risk | Incomplete reporting of potential confounders in groups. Potential imbalance (furry animals at home: EHF 22%, PHF 6%, CMF 16%) between groups after allocation, although reported to be not statistically significant. |
Saarinen 1999.
| Methods | Three‐hospital quasi‐random (allocation by month) study in Finland. | |
| Participants | Inclusion criteria: healthy full‐term infants requiring supplemental feeding in hospital. | |
| Interventions | Early supplementary feeding in hospital with: Control 1 (n = 1758): CMF (Tutteli, Vali, Finland). Control 2 (n = 1844): pasteurised donor human milk. Treatment (n = 1715): extensively hydrolysed whey formula (Pepti‐Junior, Nutricia, the Netherlands). Average duration hospital stay 4 days. Mothers encouraged to breast feed. Supplemental CMF used after discharge when required. Solids introduced at 4 to 6 months. No co‐interventions. | |
| Outcomes |
Primary outcome(s): CMA.
Other outcomes: CMA ‐ mothers contacted researchers if symptoms suggestive of CMA appeared. Well baby clinics also informed of study (all infants seen average 8 times in first 12 months). Definitions CMA: unblinded elimination/challenge performed. Mean age follow‐up 27 months (range 18 to 34 months) (infant allergic disease). |
|
| Notes | Trial of early supplemental human milk vs extensively hydrolysed whey formula vs CMF. Potential ascertainment bias as compliance with reporting not assessed. Conflict of interest: supported by Nutricia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random allocation by month of birth and hospital born. |
| Allocation concealment (selection bias) | Unclear risk | Possible if blinding maintained. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Colour‐coded bottles used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding maintained until last follow‐up assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6209/6267 (99%) eligible mothers returned baseline questionnaire. Mothers were asked to call study author if symptoms of CMA appeared. Compliance not assessed.
Diary of infant feeding regimen returned by 118/118 mothers of infants subsequently found to be hypersensitive to CM and 76% CM‐tolerant infants. Well‐baby clinics of the area, in which every infant is seen an average of 8 times during the first 12 months of life, were informed of the study. |
| Selective reporting (reporting bias) | Low risk | Primary endpoint was an adverse reaction to challenge with CM. |
| Other bias | Unclear risk | Baseline characteristics not reported. |
Tsai 1991.
| Methods | Single‐centre randomised controlled trial in Taiwan. | |
| Participants | Inclusion criteria: healthy term infants. Family history of allergic disease score used. Infants with score > 3 enrolled. | |
| Interventions | Treatment (n = 15): infants breast fed for 1 to 2 months, then fed PHF for subsequent 4 months (Nan HA, Nestle). All except 2 infants received formula. Control (n = 18): regular formula from birth. No co‐interventions reported. | |
| Outcomes | Primary outcome(s): allergic diseases. Other outcomes: seen at 1, 2, 4, 6, 12 months (infant allergic disease incidence) in well baby clinic. Total and specific IgE at 2, 6, 12 months. SPTs in cases of suspected allergic disease. Growth in weight and height up to 12 months. Definitions Atopic dermatitis: grading score used (mild: faint lesions on forehead or cheek without treatment; moderate and severe: lesions required treatment). Allergic rhinitis: typical symptoms in early morning. Wheezing: any. | |
| Notes | Trial of prolonged supplementary or sole pHWF vs CMF. Data not reported in group of allocation for clinical allergic disease confirmed by skin prick testing, and possibly for growth. Conflict of interest: financial support and formula provided by ANPING Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Qquote: "The newborns were randomly allocated to two groups". Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely. Infants fed CMF from birth. Those fed PHF breast fed for 1 to 2 months. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cross‐over of 3 infants from hydrolysed to CMF group (unclear which reported outcomes this affected). |
| Selective reporting (reporting bias) | Unclear risk | Did not report prespecified definition of specific allergic disease or time of reporting. |
| Other bias | Low risk | No reported differences between study groups for cord blood IgE, family history allergic disease score or duration of follow‐up. |
Vandenplas 1992.
| Methods | Single‐centre randomised controlled trial in Belgium. | |
| Participants | Inclusion criteria: infants with at least 2 first‐degree relatives with allergic disease, whose mothers intended not to breast feed. | |
| Interventions | Exclusive formula feeding for 6 months with: Treatment (n = 32): pHWF (Nan HA, Nestle). Control (n = 35): CMF (Nan, Nestle). Co‐interventions (both groups): grated apple from 4 months. 'Normal' diet after 6 months. | |
| Outcomes | Primary outcome(s): atopic disease. Other outcomes: blinded physician assessment for allergic disease monthly for first year of life. Total IgE, specific RAST, IgG4 antibodies, SPTs. Definitions Atopic dermatitis: at least 3 of 4 criteria including typical rash, recurrence or chronicity and specific IgE. Urticaria: no definition given. Allergic wheezing: cough without infection ≥ 24 hours. Chronic rhinitis: clear nasal discharge. CMPI: confirmed by unblinded elimination/challenge. Allergic diarrhoea: infection excluded. Infants with diarrhoea had jejunal biopsy performed. Follow‐up to 12 months (infant allergic disease incidence). | |
| Notes | Trial of prolonged sole pHWF vs CMF in high‐risk infants 3‐ and 5‐year results excluded owing to excess losses.
Data for cumulative specific allergic disease manifestations up to 12 months not extractable separately. Data for CMA and sensitisation not able to be extracted separately. Data included in previous version of review now considered to include infants with CMPI and infants without symptoms and with sensitisation. Conflict of interest: Nestle provided formula and performed statistical analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants postnatally allocated; method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Infants were given 1 of 2 coded formulas... or an adapted formula with native cow's milk proteins, delivered in an unlabelled package. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Follow‐up was blinded as much as possible: neither the parents nor the physician(s) involved in the follow‐up were informed about the nature of the formula". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 months: 8/75 (11%) post‐randomisation losses. At 3 and 5 years: 17/75 (23%) lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Reported prespecified definitions of specific allergic disease but not time of reporting. |
| Other bias | Low risk | Groups similar at baseline for risk factors for allergic disease. |
von Berg 2003.
| Methods | Multi‐centre randomised controlled trial in Germany. | |
| Participants | Inclusion criteria: high risk of allergic disease in healthy infants with at least one first‐degree family member with allergic disease Exclusion criteria: severe acquired or congenital diseases, gestation < 37 weeks, birth weight < 2500 g, > 14 days, intake cow's milk‐based formula before inclusion, incapability of parent to comply with study protocol. | |
| Interventions | Mothers encouraged to breast feed for at least 4 months. Study formula provided for when sole breast feeding no longer continued and provided until infant 6 months of age. Infants (all centres: N = 2252; Wessel: n = 1087) randomised to: Treatment 1 (all centres: n = 557; Wessel: n = 273): partially hydrolysed 100% whey formula (Beba HA, Nestle, Vevey, Switzerland) Treatment 2 (all centres: n = 559; Wessel: n = 265): extensively hydrolysed 100% whey formula (Hipp HA, Hipp, Pfaffenhofen, Germany) Treatment 3 (all centres: n = 580; Wessel: n = 281): lactose‐free, extensively hydrolysed 100% casein formula (Nutramigen, Mead Johnson, Diezenbach, Germany) Control (all centres: n = 556; Wessel: n = 268): CMF with casein:whey ratio 40:60 (Nutrilon Premium, Nutricia/Numico, Zoetermeer, the Netherlands) Co‐interventions: all groups received advice about breast feeding for at least 4 months, preferably 6; no dietary restrictions during lactation; not to feed solids during study period, and thereafter to add 1 food a week and avoid common allergenic foods in first year. 58.4% of infants received study formula | |
| Outcomes |
Primary outcome(s): allergic disease.
Other outcomes: allergic disease (atopic manifestations), asthma and eczema.
Definitions
Allergic disease (atopic manifestations) diagnosed at 12 months (infant allergic disease) as atopic dermatitis, allergic urticaria or gastrointestinal food allergy.
Atopic dermatitis: typical morphology and distribution of skin lesions; pruritus; chronicity (duration ≥ 14 days, chronically relapsing or both); confirmed on skin examination by a second specially trained allergist; severity rated using the SCORAD method.
Allergic urticaria: at least 2 episodes of itching eruptions or swelling with typical appearance, observed by parents or physician, caused by the same allergen. In case of a single episode, immunological evidence (specific SPT or allergen‐specific IgE level ≥ 0.35 KU/L or positive provocation response).
Gastrointestinal food allergy: suspected if GI symptoms not explained by any other condition, and if unblinded elimination challenge reproduced symptoms. GI allergic disease definite if a positive standardised elimination/challenge procedure. Double‐blind, placebo‐controlled food challenge performed in cases of uncertain reactions.
At 3 years, childhood allergic disease included atopic dermatitis, urticaria, food allergy with manifestation in the gastrointestinal tract and asthma.
Allergic asthma: diagnosed from parental report of relevant symptoms (wheeze and/or cough without infection) or regular use of asthma medication in the child's third year of life. Asthma symptoms included wheezing or cough for at least 2 weeks (acute laryngotracheitis excluded); exercise‐induced wheeze or cough at any time (with crying, laughing or activity); and episodes of wheezing or dry night‐time cough. At 6 years, reported physician‐diagnosed allergic diseases (atopic dermatitis, food allergy, allergic urticaria, asthma and hay fever/allergic rhinitis). At 10 years, reported physician‐diagnosed asthma, allergic or atopic eczema/dermatitis, hay fever/allergic rhinitis, urticaria and food allergy. Also, if present, at 10 years, parents reported a physician’s diagnosis during past 4 years, treatment in past 12 months or both. At 15 years, parent‐reported physician‐diagnosed asthma, allergic rhinitis (AR) and eczema, spirometric indices and sensitisation. |
|
| Notes | Data for intention‐to‐treat analyses of all infants (including breast fed infants) according to study centre provided by study authors for all allergic disease, asthma and eczema. Intention‐to‐treat 3‐year data for food allergy not provided.
Analyses meeting inclusion criteria for the review are intention‐to‐treat analyses including breast fed infants for all study centres at 1 year and infants enrolled in Wesel for 3‐year outcomes. Excess losses beyond 3 years, so data not included in analyses. Trial of prolonged breast feeding with supplemental or sole formula feeding when required comparing use of CMF, pHWF, extensively hydrolysed whey formula and lactose‐free extensively hydrolysed casein formula. Sponsor: study supported by Federal Ministry for Education, Science, Research and Technology and the Child Health Research Foundation. Formulas provided by Nestle, Hipp, Milupa, Numico and Mead Johnson. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list stratified for single or double parental atopy and study region. |
| Allocation concealment (selection bias) | Low risk | Random sequence generation and blinded intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinding of parents and the study team was guaranteed by using identically labelled tins for the study formula coded with 4 different letters for each of the 4 formulas". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In intention‐to‐treat analyses for study centres (Munich and Wesel) that included breast fed infants: 1 year: 304/2252 (13.5% lost to follow‐up). 3 years: 692/2254 (31% lost to follow‐up). 6 years: 572/2252 (25% lost to follow‐up). 10 years: 1115 to 1145/2522 (44% to 45% lost to follow‐up). 15 years: Response rates were 61.1% (1377/2252) ‐ lost to follow‐up 875/2252 (39%) Breast fed infants randomised to interventions who did not receive interventions were followed up only in Wesel. In intention‐to‐treat analyses for Wesel only: 1 year: 158/1087 (14.5% lost to follow‐up). 3 years: 206/1087 (19% lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | Primary endpoints and specific timings stated in the methods were reported in the results in the original study report (von Berg 2003). |
| Other bias | High risk | Quote: ".. significant bias in the eHF‐C group because disproportionally more children had to be excluded as a result of noncompliance with the study formula (18% [47/257] in the eHF‐C group vs 10% to 12% in the other study formula groups, P = 0.02)". |
Willems 1993.
| Methods | Single‐centre quasi‐randomised (allocated by month) controlled trial in Belgium. | |
| Participants | Inclusion criteria: infants not breast fed with a family history of allergic disease and cord IgE ≥ 0.5 IU/L. | |
| Interventions | Prolonged sole formula feeding with: Control: (n = 55): CMF. Treatment (n = 67): pHWF (Nan HA). Formula used for first 3 months, then unrestricted diet. No co‐interventions. | |
| Outcomes | Primary outcome(s): allergic disease. Other outcomes: paediatrician‐administered questionnaire at 3 months and 1 year for allergic disease (infant allergic disease incidence). Definitions Allergic disease included eczema, asthma, recurrent episodes of bronchitis, persistent rhinitis, persistent gastrointestinal symptoms (excluding infection) and sleeping difficulties. No specific definitions given. | |
| Notes | High rate (45%) of non‐compliance with formula. Trial of prolonged sole pHWF vs CMF in high‐risk infants. Conflict of interest: unclear; co‐investigator from FNRS, Brussels. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random ‐ infants postnatally allocated by even or odd month of birth. |
| Allocation concealment (selection bias) | High risk | Quasi‐random and unblinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Formulas not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/135 (13%) high‐risk infants did not complete the study. |
| Selective reporting (reporting bias) | Low risk | Prespecified allergic disease. |
| Other bias | Unclear risk | Did not report baseline characteristics of groups. |
CM: cow’s milk CMA: cow’s milk allergy CMF: cow’s milk formula CMPI: cow’s milk protein intolerance EHF: extensively hydrolysed formula GI: gastrointestinal HF: hydrolysed formula Ig: immunoglobulin ISAAC: International Study of Asthma and Allergies in Childhood PHF: partially hydrolysed formula pHWF: partially hydrolysed whey formula RAST: radio‐allergosorbent SPT: skin prick test VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agosti 2003 | AGA preterm infants allocated to low hydrolysed protein formula vs intact protein formula. Method of allocation not reported. Did not measure allergy. n = 20. |
| Akerblom 2005 | 242 newborn infants who had a first‐degree relative with type 1 diabetes and carried risk‐associated HLA‐DQB1 alleles. After exclusive breast feeding, the infants underwent a double‐blind, randomised pilot trial of either casein hydrolysate or conventional cow's milk‐based formula until the age of 6‐8 months. Did not report allergic disease or food allergy. |
| Akimoto 1997 | Cohort study. |
| Arikan 2008 | Randomised families with colicky infants to 4 different intervention groups ‐ massage, sucrose solution, herbal tea and hydrolysed formula and control group. Did not report allergy. n = 35 per group. |
| Arshad 1990 | Randomised controlled trial in infants at high risk of allergy (family history of allergy in two or more of mother/father/sibling) receiving formula (n = 60 in each group). Cow’s milk formula versus hydrolysed formula (extensively hydrolysed casein formula Aptamil HA, Milupa) for the prevention of allergy. Reported allergy at 12 months. Completed, however author unable to get approval from sponsor to release data. |
| Arshad 1992a | Had multiple allergies preventing co‐interventions in treatment group and not in control group |
| Baldassarre 2017 | RCT of infants 28‐33 weeks' gestational age; birth weight of 700 g to 1750 g and AGA within 24 hours of first enteral feeding (n = 65). Cow's milk protein versus EHF feeding. Did not report allergy. Reported feeding advancement and markers of feeding tolerance. |
| Barberi 1993 | Infants at high risk of atopy randomised to HF vs CMF. 440 received allocated formula. Method of allocation not reported. Excess post‐randomisation losses ‐ 278/815 (34%). No intention‐to‐treat analysis. n = 815. |
| Bergmann 1996a | Infants at risk of atopy were assigned to 1 of 3 nutritional groups and were followed up through the first 6 months of life: 20 infants were breast fed, 28 received a predominantly whey protein‐based infant formula, 53 received an ultrafiltrated partially hydrolysed whey protein formula. Non‐random allocation. n = 101. |
| Berseth 2009 | Double‐blind randomised controlled trial in healthy formula‐fed term infants with no family history of allergy of 70% lactose, partially hydrolyzed cow's milk protein formula supplemented with docosahexaenoic acid and arachidonic acid vs full lactose intact cow milk protein formula supplemented with docosahexaenoic acid and arachidonic acid. Did not report allergy. Measured adverse effects and tolerance. n = 335. |
| Borschel 2013 | Healthy term infants randomised to an amino acid–based formula or an extensively hydrolysed casein‐based formula. Did not report allergy. n = 213. |
| Borschel 2014a | RCT in healthy term infants fed whey‐based, palm olein oil–free PHF vs whey‐based, palm olein oil‐containing PHF (not eligible comparison). Did not report allergy. n = 209. |
| Borschel 2014b | RCT in healthy term infants fed ready‐to‐feed or powdered form of an extensively hydrolysed casein‐based formula (not eligible comparison). Did not report allergy. n = 195. |
| Boyle 2016 | RCT in infants at high risk of allergy fed PHF supplemented with prebiotic vs standard CMF without prebiotic. Not an eligible comparison. Reported allergy. n = 863. |
| Burks 2008 | Randomised healthy term infants to amino acid‐based formula vs EHF. Excess post‐randomisation losses (33%). Reported adverse events. Did not report allergy. n = 165. |
| Campbell 1989 | Enrolled infants with colic. Not randomised. |
| Chan 2002 | Trial of sole prolonged partially hydrolysed CMF vs CMF in infants at high risk of atopy. Excess losses (28%). n = 110. |
| Chan‐Yeung 2000 | Infants at high risk of allergy. Had multiple allergy‐preventing co‐interventions in treatment group and not in control group. n = 545. |
| Chandra 1989a | Original data unable to be verified. |
| Chandra 1989b | Original data unable to be verified. |
| Corvaglia 2013 | Randomised preterm infants with symptomatic gastroesophageal reflux to extensively hydrolysed preterm formula vs standard preterm formula. Did not report allergy. n = 40. |
| D'Agata 1996 | Infants at high risk of allergy. 30 exclusively breast fed, 50 hypoallergenic milk‐fed, 30 soy milk‐fed and 15 fed with conventional milk formula. Method of allocation unspecified. Substantial imbalances in numbers. Reported allergy. |
| de Jong 1998 | Trial of early supplementation of CMF vs a protein‐free placebo formula (not hydrolysed protein) in breast fed infants |
| Decsi 1992 | Healthy term infants who were breast fed or received conventional formula (Mildibe, EGIS; Pre‐Aptamil, Milupa), or a formula containing hydrolysed proteins (Aptamil H.A., Milupa). Did not report allergy. n = 10 per group. |
| Decsi 1998 | Term infants fed conventional formula or PHF. Excess losses (27% hydrolysed formula group and 21% CMF group). Did not report allergy. n = 11 per group. |
| Exl 1998 | Allocated to intervention (breast feeding, hydrolysed formula and delayed weaning) according to geographical area. |
| Florendo 2009 | Preterm infants randomised to feeds with a standard non‐hydrolysed whey–casein vs partially hydrolysed whey preterm infant formula. Reported feed intolerance. Did not report allergy. n = 80. |
| Fukushima 1997 | Trial of maternal allergen avoidance and infant supplemental or sole hydrolysed or CMF feeding when required. Differential losses with excess losses in maternal and infant hydrolysed formula group (27%) and in maternal hydrolysed formula and infant CMF group (23%). |
| Giovannini 1994 | Study in infants at high risk of atopy given human milk vs soy formula vs whey‐based low‐degree hydrolysate vs casein‐based high‐degree hydrolysate vs soy plus collagen‐based high‐degree hydrolysate formula. Did not report allergy. Excess post allocation losses (56/138) not analysed in group of assignment (solely breast fed infants reported separately). n = 138. |
| Halken 1992 | Trial of prolonged supplementary or sole extensively vs partially hydrolysed ultrafiltrated formula in high‐risk infants. Only infants who received hydrolysed formula included in analysis. Excess losses after allocation (24%). n = 158. |
| Hartman 1994 | Randomised trial in healthy infants allocated to soy formula, CMF or PHF. Abstract only. Losses unclear. Reported intolerance and allergy but data not extractable from abstract. n = 510. |
| Hattevig 1989 | Trial of maternal allergen avoidance. The diet for infants was the same in both groups. During first 6 months, only breast milk and or formula based on hydrolysed casein (Nutramigen) given. |
| Hernell 2003 | Breast fed infants, infants fed hydrolysate formulas, infants fed milk formula. Method of allocation not reported. Allergy not reported. n = 55. |
| Hill 1995b | Enrolled infants with 'colic'. Randomised to casein hydroIysate or CMF. Did not report allergy. n = 151. |
| Høst 1991 | Cohort study. Casein hydrolysate (Nutramigen) used in elimination/challenge. |
| Iikura 1995 | Abstract form only. Method of allocation unclear. Substantial differences in group sizes. |
| Keller 1996 | Allocation to various feeding regimens including hydrolysed formula performed by nurses 'at random'. "Maternal decision respected". Unlikely to be random allocation. Allergy reported. |
| Knip 2010 | Randomised trial 230 infants with HLA‐conferred susceptibility to type 1 diabetes and at least one family member with type 1 diabetes to receive either a casein hydrolysate formula or a conventional cow’s‐milk–based formula (control) whenever breast milk was not available during the first 6 to 8 months of life. Did not report allergy outcomes. |
| Knip 2011 | Enrolled infants at risk of type 1 diabetes mellitus. Intervention commenced at weaning. Excess losses reported: 2997 / 5156 randomised infants not included in analysis = 58%. Reported adverse events including asthma, other forms of allergy at 10 years of age. |
| Kuo 2011 | Prospective, observational, uncontrolled cohort study in newborns who had at least 1 first‐degree family member with a history of atopy and could not breast feed. Fed with HF or CM for at least 6 months and monitored prospectively at 6, 18 and 36 months of age. |
| Lasekan 2006 | Assessed growth efficacy, gastrointestinal tolerance and plasma biochemical measurements of healthy infants receiving a partially hydrolysed rice protein‐based formula vs CMF for the first 16 weeks after birth. Did not report allergy. n = 65. |
| Lucassen 2000 | Enrolled infants with excessive crying. Randomised healthy, thriving, formula‐fed infants, < 6 months old, crying > 3 hours per day on at least 3 days per week to whey hydrolysate formula or standard formula. Did not report allergy. n = 43. |
| Maggio 2005 | Randomised controlled trial of preterm formula with hydrolysed cow’s milk proteins reporting short‐term growth and urinary and plasma amino acid levels. Did not report allergy. n = 21. |
| Martinez‐Valverde 1993 | No definition of allergic symptoms reported in first 4 months. In Spanish version of thesis, method of treatment allocation not extractable independently. |
| Medjad‐Guillou 1992 | Cross‐over trial of CMF vs hydrolysed formula in healthy term infants. Did not report allergy. |
| Mennella 2011a | Healthy infants randomly assigned to CMF or PHF between 0.5 and 7.5 months of age. Did not report allergy. n = 79. |
| Mihatsch 1999 | Cross‐over trial examining effects of hydrolysed formula on plasma amino acids and gastrointestinal transit time in preterm infants. Did not report allergy. |
| Mihatsch 2002 | Randomised trial of partially hydrolysed preterm infant formula vs CMF in VLBW infants at low risk of atopy establishing enteral feeds. Excess post‐randomisation exclusions 48/135 (36%). Did not report allergy. n = 135. |
| Mitchell 1977 | Trial of lactose hydrolysed milk, not protein hydrolysed. Enrolled slightly undernourished Aboriginal children < 3 years of age. |
| Moran 1992 | Trial of supplementary or sole hydrolysed formula vs CMF in low‐risk infants. Excessive losses > 20% in both groups. n = 205. |
| NCT00936637 | Enrolled health term infants assigned to one of three nutritional groups: 20 infants were breast fed, 28 received an adapted predominantly whey protein based infant formula, 53 received an ultrafiltrated, partially hydrolysed whey protein formula. Did not report allergy. |
| NCT01987154 | Enrolled infants with presumptive cow’s milk allergic colitis. Randomly assigned to EHCF with LGG (Nutramigen LGG) or without (Nutramigen) (EHCF LGG). |
| Nentwich 2003 | Observational study of infants fed a whey hydrolysate formula compared with exclusively breast fed controls. |
| Nocerino 2012 | Randomised trial of standard formula vs partially hydrolysed whey formula in infants with diagnosed infant colic. Did not report allergy. Published as conference abstract only. n = unclear (52 reported). |
| Odelram 1996 | Trial of extensively hydrolysed vs CMF for weaning of high‐risk infants. Excluded trial, as 13 losses in addition to 9 post‐randomisation exclusions (total 27%). n = 91. |
| Paronen 2000 | Enrolled infants at high genetic risk for diabetes. Did not report allergy. n = 119. |
| Pauls 1996 | Trial of formulas with hydrolysed vs non‐hydrolysed protein for starting enteral feedings in preterm infants < 1500 g. Did not report allergy. Only outcomes to day 6 reported. Reported in abstract format only. |
| Picaud 2001 | Preterm infants randomly assigned to PHF or conventional formula. Did not report allergy. n = 16. |
| Porch 1998 | Infants at high risk of allergy randomly assigned extensively hydrolysed casein formula (Nutramigen); partially hydrolysed whey formula (Good Start); and soy formula. Excess losses 51/181 (28%). n = 181. |
| Raupp 1995 | Trial of sole hydrolysed formula vs CMF in low birth weight infants. Excess post‐randomisation losses. Allergy not reported. n = 39 |
| Riezzo 2001 | Randomised trial of standard and hydrolysate formulas in preterm infants. Allergy not reported. n = 36. |
| Rigo 1994a | Method of treatment allocation unclear. Allocated successive infants to formulas. Trial of 5 different types of hydrolysed formula in healthy term infants. Extent of hydrolysis not reported. Allergy not reported. n = 74. |
| Rigo 1994b | Trial of hydrolysate formula in preterm infants. Method of allocation not stated. Allergy not reported. n = 19. |
| Savino 2003 | Observational study of whey hydrolysate formula enrolling infants with "minor feeding problems". |
| Savino 2006 | Randomised to receive formula containing partially hydrolysed whey proteins, prebiotic oligosaccharides, with a high beta‐palmitic acid content, or standard formula and simethicone (multiple formula differences). |
| Scalabrin 2009 | RCT of healthy term infants who received extensively hydrolysed casein formula, the same formula supplemented with Lactobacillus rhamnosus GG or partially hydrolysed whey:casein (60:40) formula supplemented with LGG (PHFLGG). Excess losses at all time points. Reported adverse events including allergy. n = 289. |
| Schmelzle 2003 | Randomised healthy formula‐fed term infant trial of partially hydrolysed whey infant formula vs standard infant CMF. Excess losses ‐ 52 (34%). Allergy not reported. n = 154. |
| Schmidt 1995 | Observational study in high‐risk infants (infants allocated formula at parents' discretion). |
| Schmitz 1992 | Trial in normal breast fed infants of early supplementary hydrolysed formula vs CMF. Did not report allergy. Excess losses at 1 year. n = 256. |
| Schrander 1993 | Cohort study of newborn infants to determine incidence of CMPI and response to Pregomin (Milupa) protein hydrolysate formula. |
| Shao 2006 | Multiple dietary interventions including hydrolysed formula in treatment group for infants at high risk of atopy who were not able to be breast fed. |
| Silva Rey 1996 | Trial of partially hydrolysed milk formula. Excess losses ‐ 124/276 (45%) ‐ 42 losses by 6 months and further 82 excluded post allocation. Method of allocation not reported. n = 276. |
| Sorensen 2007 | Infants (n = 242) with a family history of allergy assigned to one of 3 randomised groups: weaned to either CMF, EHW or PHW formula. All infants were breast fed for 3 months, then study formulas was were given for 1 month. Published as abstract only. Allergy not reported to date. |
| Staelens 2008 | Double‐blind randomised cross‐over study in healthy newborns fed CMF vs PHF vs EHF. Allergy not reported. n = 17. |
| Szajewska 2001 | Low birth weight infants were assigned randomly to receive extensive protein hydrolysate preterm formula, partial protein hydrolysate preterm formula and standard preterm formula. Allergy not reported. n = 61. |
| Szajewska 2004 | Randomised trial of extensively hydrolysed preterm formula vs partially hydrolysed preterm formula vs CMF in high risk for atopy preterm infants. Excess post‐randomisation losses at all times ‐ 22/90 (24%) at 4 to 5 months. n = 90. |
| Tariq 1998 | Cohort study. |
| Taubman 1988 | Enrolled infants with excessive crying ('colic'). Randomised to hydrolysed casein formula or counselling. Allergy not reported. n = 20. |
| Vaarala 1995 | Double‐blind trial; 10 infants received cow's milk‐based formula and 10 received a casein hydrolysate formula until the age of 9 months. Allergy not reported. n = 20. |
| Vaarala 2012 | Infants with HLA‐conferred susceptibility to type 1 diabetes randomly assigned to receive CMF (n = 389), whey‐based hydrolysed formula (WHF) (n = 350) or whey‐based FINDIA formula essentially free of bovine insulin (n = 365). Allergy not reported. n = 1104. |
| Vandenplas 1988 | Retrospective study. Embedded intervention study. Trial of formula, breast milk or a hypoallergenic formula. Method of allocation reported to be "chronological". Losses unclear. n = 75 |
| Vandenplas 1993 | Randomised healthy term infants to whey intermediate hydrolysed formula vs CMF. Allergy not reported. n = 45. |
| Wopereis 2014 | Infants at high risk of allergy randomised to PHF containing a specific mixture of oligosaccharides or to standard CMF for the first 6 months of life. Allergy not reported to date. Published abstract only. n = 108. |
| Xinias 2017 | Enrolled infants with colic allocated to formula containing partially hydrolyzed whey protein, reduced lactose, Bifidobacterium lactis (B lactis) and galacto‐oligosaccharides. Allergy not reported. |
| Yu 2014 | Likely non‐random allocation. Cross‐over design. Enrolled preterm infants. Did not report allergy. |
| Zeiger 1989 | Trial in mothers and infants at high risk of atopy of maternal dietary avoidance in pregnancy and lactation, and infant allergen avoidance through encouragement of breast feeding with supplemental or weaning formula use and subsequent dietary restriction vs usual maternal diet and infant feeding with use of supplementary or weaning CMF. Excluded as multiple co‐interventions and excess losses. |
AGA: appropriate for gestational age CM: cow’s milk CMF: cow’s milk formula CMPI: cow’s milk protein intolerance EHF: extensively hydrolysed formula HF: hydrolysed formula HLA: human leukocyte antigen PHF: partially hydrolysed formula PHFLGG: partially hydrolysed formula supplemented with LGG RCT: randomised controlled trial VLBW: very low birth weight WHF: whey‐based hydrolysed formula
Characteristics of ongoing studies [ordered by study ID]
NCT01036243.
| Trial name or title | Digestive tolerance of slightly hydrolyzed starter infant formula with probiotics |
| Methods | Randomised controlled trial. |
| Participants | n = 480 Inclusion criteria
Exclusion criteria
|
| Interventions | Hydrolysed formula with probiotics. Acidified hydrolysed formula. Hydrolysed formula without probiotics. Standard infant formula. |
| Outcomes | Incidence of crying/fussing from 1 to 3 months. Growth and night sleep. |
| Starting date | December 2009; study completion date: April 2012. |
| Contact information | A/Prof. Boosba Vivatvakin |
| Notes | ClinicalTrials.gov Identifier:NCT01036243 |
NCT01156493.
| Trial name or title | Hydrolized protein formula for premature infants |
| Methods | Randomised controlled trial. |
| Participants | n = 118 Inclusion criteria
Exclusion criteria
|
| Interventions | Protein hydrolysed formula. Standard premature formula. |
| Outcomes | Primary outcome measure: time to achieve full feeds. Secondary outcome measures: postnatal days to achieve full feeds. |
| Starting date | July 2010; study completion date: September 2015. |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT01156493. No report to date found. |
NCT01210391.
| Trial name or title | Growth of infants fed an extensively hydrolyzed infant formula |
| Methods | Randomised controlled trial. |
| Participants | n = 282 Inclusion criteria
Exclusion criteria
|
| Interventions | Extensively hydrolysed whey infant formula vs extensively hydrolysed casein infant formula. |
| Outcomes | Weight gain to 4 months. Mean weight gain (grams/d) from enrolment to 4 months of age. |
| Starting date | November 2010; study completion date: July 2013. |
| Contact information | Ricardo Sorensen, MD, Louisiana State University Health Sciences Center in New Orleans. |
| Notes | ClinicalTrials.gov Identifier: NCT01210391 |
NCT01700205.
| Trial name or title | "Watch Your Baby Grow" Study (GRO) |
| Methods | Randomised controlled trial. |
| Participants | n = 144 Inclusion criteria
Exclusion criteria
|
| Interventions | Extensively hydrolysed infant formula. Standard CMF during first year of life. |
| Outcomes | Primary outcome measures: growth and energy balance. Secondary outcome measures: Intake and feeding behaviours, genotype. |
| Starting date | October 2012. Estimated completion date: December 2017. |
| Contact information | Julie A. Mennella |
| Notes | ClinicalTrials.gov Identifier: NCT01700205 |
NCT01735123.
| Trial name or title | Early Dietary Intervention and later signs of beta‐cell Autoimmunity (EDIA) |
| Methods | Randomised controlled trial. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: extensively hydrolysed casein formula. Experimental: cow's milk‐based infant formula. |
| Outcomes | Primary outcome measure: intestinal permeability determined at the age of 3, 6, 9 and 12 months with the lactulose/mannitol test. Secondary outcome measure: serum metabolic profile. Serum metabolic profile will be analysed with metabolomics at the age of 3, 6, 9 and 12 months. Other outcome measures: intestinal microbiome. |
| Starting date | January 2013. Completion date August 2016. |
| Contact information | Mikael Knip, MD; mikael.knip@helsinki.fi |
| Notes | ClinicalTrials.gov Identifier: NCT01735123. |
Yin 2015.
| Trial name or title | Application effect of extensively hydrolyzed milk protein formula and follow‐up in preterm children with a gestational age of less than 34 weeks |
| Methods | Randomised single‐blind and controlled clinical trial. |
| Participants | Preterm children of gestational age less than 34 weeks who cannot be breast fed. |
| Interventions | Extensively hydrolysed milk protein (100% whey protein) formula vs preterm children’s formula until discharge from neonatal intensive care unit. |
| Outcomes | First endpoint: food intolerance in preterm children. Second endpoint variables include (1) time to achieve full enteral nutrition; (2) time of parenteral nutrition; (3) time of NICU stay; (4) meconium drainage time; (5) daily spontaneous faecal discharge conditions; (6) growth; (7) total protein, albumin, calcium, phosphorus and alkaline phosphatase; and (8) neonatal necrotising enterocolitis, cholestasis, parenteral nutrition associated with cholestasis and congenital hypothyroidism. |
| Starting date | 2014 |
| Contact information | Li‐Xing Qia; qiao_lixing@163.com |
| Notes | Chinese Clinical Trial Registry (http://www.chictr.org.cn/); ChiCTR‐IOR‐14005696 |
CMF: cow’s milk formula GA: gestational age GI: gastrointestinal HLA: human leukocyte antigen IV: intravenous NICU: neonatal intensive care unit
Differences between protocol and review
Review authors have deleted outcomes related to sensitisation in infants with clinical allergic disease that were incorporated in previous versions of the review to ensure that this review was focused appropriately on clinical allergic disease ‐ not on surrogate testing.
Contributions of authors
DO and JS independently performed the literature search, extracted data and checked the accuracy of the review. Both review authors independently performed the literature search, extracted data and checked the accuracy of the updated reviews. LJ reviewed risk of bias and GRADE summary of findings assessments.
Sources of support
Internal sources
No sources of support supplied
External sources
Australian Satellite of the Cochrane Neonatal Review Group, Australia.
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
Review authors DO and JS have been invited speakers at industry‐organised scientific meetings. Neither has accepted an honorarium. LJ has no conflicts of interest to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Chirico 1997 {published data only}
- Chirico G, Gasparoni A, Ciardelli L, Amici M, Colombo A, Rondini G. Immunogenicity and antigenicity of a partially hydrolyzed cow's milk infant formula. Allergy 1997;52(1):82‐8. [PUBMED: 9062633] [DOI] [PubMed] [Google Scholar]
de Seta 1994 {published data only}
- Seta L, Siani P, Cirillo G, Gruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow‐up at 24 months. The preliminary results. [Italian]. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 1994;16(3):251‐4. [PUBMED: 7971447] [PubMed] [Google Scholar]
Halken 2000 {published data only}
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatric Allergy and Immunology 2000;11(3):149‐61. [PUBMED: 10981524] [DOI] [PubMed] [Google Scholar]
Hill 2000 {published and unpublished data}
- Hill D. A report on the analysis of the Melbourne Atopy Cohort Study. A study designed to test the effectiveness of different formula types on the development of atopic symptoms and signs on a cohort of atopy‐at‐risk infants. Report at year 2. Nestec Internal Report.
- Hill DJ, Sporik R, Thorburn J, Hosking CS. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. Journal of Pediatrics 2000;137(4):475‐9. [DOI: 10.1067/mpd.2000.108207; PUBMED: 11035824] [DOI] [PubMed] [Google Scholar]
- Koplin J, Dharmage SC, Gurrin L, Osborne N, Tang ML, Lowe AJ, et al. Soy consumption is not a risk factor for peanut sensitization. Journal of Allergy and Clinical Immunology 2008;121(6):1455‐9. [DOI: 10.1016/j.jaci.2008.03.017; PUBMED: 18436294] [DOI] [PubMed] [Google Scholar]
- Lowe A, Hosking C, Bennett C, Allen K, Carlin J, Dharmage S, et al. The Melbourne Atopy Cohort Study: a RCT of a partially hydrolysed whey formula for the prevention of allergic disease. Internal Medicine Journal 2008;38:A158. [Google Scholar]
- Lowe AJ, Abramson MJ, Hosking CS, Carlin JB, Bennett CM, Dharmage SC, et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clinical & Experimental Allergy 2007;37(4):536‐42; Erratum in: Clinical & Experimental Allergy 2007; 37(12);1895. [DOI: 10.1111/j.1365-2222.2007.02691.x; PUBMED: 17430350] [DOI] [PubMed] [Google Scholar]
- Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Allen KJ, Robertson CF, et al. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ 2010;341:c4616. [PUBMED: 20843914] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AJ, Hosking CS, Bennett CM, Allen KJ, Axelrad C, Carlin JB, et al. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high‐risk children: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2011;128(2):360‐5.e4. [DOI: 10.1016/j.jaci.2010.05.006; PUBMED: 21696814] [DOI] [PubMed] [Google Scholar]
- Stoney RM, Woods RK, Hosking CS, Hill DJ, Abramson MJ, Thien FC. Maternal breast milk long‐chain n‐3 fatty acids are associated with increased risk of atopy in breastfed infants. Clinical and Experimental Allergy 2004;34(2):194‐200; Erratum in: Clinical & Experimental Allergy 2007; 37(12);1895. [PUBMED: 14987297] [DOI] [PubMed] [Google Scholar]
- Thomson JA, Widjaja C, Darmaputra AA, Lowe A, Matheson MC, Bennett CM, et al. Early childhood infections and immunisation and the development of allergic disease in particular asthma in a high‐risk cohort: a prospective study of allergy‐prone children from birth to six years. Pediatric Allergy and Immunology 2010;21(7):1076‐85. [DOI: 10.1111/j.1399-3038.2010.01018.x; PUBMED: 20337970] [DOI] [PubMed] [Google Scholar]
Juvonen 1996 {published data only}
- Juvonen P, Mansson M, Andersson C, Jakobsson I. Allergy development and macromolecular absorption in infants with different feeding regimens during the first three days of life. A three‐year prospective follow‐up. Acta Paediatrica 1996;85(9):1047‐52. [PUBMED: 8888916] [DOI] [PubMed] [Google Scholar]
- Juvonen P, Mansson M, Jakobsson I. Does early diet have an effect on subsequent macromolecular absorption and serum IgE?. Journal of Pediatric Gastroenterology and Nutrition 1994;18(3):344‐9. [PUBMED: 8057219] [DOI] [PubMed] [Google Scholar]
- Juvonen P, Mansson M, Kjellman NI, Bjorksten B, Jakobsson I. Development of immunoglobulin G and immunoglobulin E antibodies to cow's milk proteins and ovalbumin after a temporary neonatal exposure to hydrolyzed and whole cow's milk proteins. Pediatric Allergy and Immunology 1999;10(3):191‐8. [PUBMED: 10565560] [DOI] [PubMed] [Google Scholar]
Kwinta 2009 {published data only}
- Kwinta P, Sawiec P, Klimek M, Lis G, Cichocka‐Jarosz E, Pietrzyk JJ. Correlation between early neonatal diet and atopic symptoms up to 5‐7 years of age in very low birth weight infants: follow‐up of randomized, double‐blind study. Pediatric Allergy and Immunology 2009;20(5):458‐66. [DOI: 10.1111/j.1399-3038.2008.00814.x; PUBMED: 19490477] [DOI] [PubMed] [Google Scholar]
Lam 1992 {unpublished data only}
- Lam BC, Yeung CY. The effect of breast milk, infant formula and hypoallergenic formula on incidence of atopic manifestation in high risk infants. Nestle Internal Report 1992.
Mallet 1992 {published data only}
- Mallet E, Henocq A. Long‐term prevention of allergic diseases by using protein hydrolysate formula in at‐risk infants. Journal of Pediatrics 1992;121(5 Pt 2):S95‐100. [PUBMED: 1447641] [DOI] [PubMed] [Google Scholar]
Marini 1996 {published data only}
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years' follow‐up. Acta Paediatrica Supplement 1996;414:1‐21. [PUBMED: 8831855] [DOI] [PubMed] [Google Scholar]
Nentwich 2001 {published data only}
- Nentwich I, Michkova E, Nevoral J, Urbanek R, Szepfalusi Z. Cow's milk‐specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy 2001;56(12):1144‐56. [PUBMED: 11736743] [DOI] [PubMed] [Google Scholar]
Oldaeus 1997 {published data only}
- Oldaeus G, Anjou K, Bjorksten B, Moran JR, Kjellman NI. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Archives of Disease in Childhood 1997;77(1):4‐10. [PUBMED: 9279143] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldaeus G, Bjorksten B, Jenmalm MC, Kjellman NI. Cow's milk IgE and IgG antibody responses to cow's milk formulas. Allergy 1999;54(4):352‐7. [PUBMED: 10371094] [DOI] [PubMed] [Google Scholar]
Saarinen 1999 {published data only}
- Saarinen KM, Juntunen‐Backman K, Jarvenpaa AL, Klemetti P, Kuitunen P, Lope L, et al. Breast‐feeding and the development of cows' milk protein allergy. Advances in Experimental Medicine and Biology 2000;478:121‐30. [DOI: 10.1007/0-306-46830-1_10; PUBMED: 11065065] [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Juntunen‐Backman K, Jarvenpaa AL, Kuitunen P, Lope L, Renlund M, et al. Supplementary feeding in maternity hospitals and the risk of cow's milk allergy: a prospective study of 6209 infants. Journal of Allergy and Clinical Immunology 1999;104(2 Pt 1):457‐61. [PUBMED: 10452771] [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Savilahti E. Infant feeding patterns affect the subsequent immunological features in cow's milk allergy. Clinical and Experimental Allergy 2000;30(3):400‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Suomalainen H, Savilahti E. Diagnostic value of skin‐prick and patch tests and serum eosinophil cationic protein and cow's milk‐specific IgE in infants with cow's milk allergy. Clinical and Experimental Allergy 2001;31(3):423‐9. [PUBMED: 11260154] [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Vaarala O, Klemetti P, Savilahti E. Transforming growth factor‐beta1 in mothers' colostrum and immune responses to cows' milk proteins in infants with cows' milk allergy. Journal of Allergy and Clinical Immunology 1999;104(5):1093‐8. [PUBMED: 10550758] [DOI] [PubMed] [Google Scholar]
- Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. European Journal of Nutrition 2009;48(4):243‐9. [DOI: 10.1007/s00394-009-0008-z; PUBMED: 19263185] [DOI] [PubMed] [Google Scholar]
- Savilahti EM, Saarinen KM, Savilahti E. Specific antibodies to cow's milk proteins in infants: effect of early feeding and diagnosis of cow's milk allergy. European Journal of Nutrition 2010;49(8):501‐4. [DOI: 10.1007/s00394-010-0109-8; PUBMED: 20405136] [DOI] [PubMed] [Google Scholar]
- Siltanen M, Kajosaari M, Poussa T, Saarinen KM, Savilahti E. A dual long‐term effect of breastfeeding on atopy in relation to heredity in children at 4 years of age. Allergy 2003;58(6):524‐30. [PUBMED: 12757455] [DOI] [PubMed] [Google Scholar]
Tsai 1991 {published data only}
- Tsai YT, Chou CC, Hsieh KH. The effect of hypoallergenic formula on the occurrence of allergic diseases in high risk infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1991;32(3):137‐44. [PUBMED: 1776437] [PubMed] [Google Scholar]
Vandenplas 1992 {published data only}
- Vandenplas Y. Atopy at 3 years in high‐risk infants fed whey hydrolysate or conventional formula. Lancet 1992;339(8801):1118. [PUBMED: 1349137] [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Hauser B, Borre C, Clybouw C, Mahler T, Hachimi‐Idrissi S, et al. The long‐term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. European Journal of Pediatrics 1995;154(6):488‐94. [PUBMED: 7671948] [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Hauser B, Borre C, Sacre L, Dab I. Effect of a whey hydrolysate prophylaxis of atopic disease. Annals of Allergy 1992;68(5):419‐24. [PUBMED: 1586005] [PubMed] [Google Scholar]
von Berg 2003 {published and unpublished data}
- Berg A, Kramer U, Link E, Bollrath C, Heinrich J, Brockow I, et al. GINIplus study group. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course ‐ the GINIplus study up to the age of 6 years. Clinical and Experimental Allergy 2010;40(4):627‐36. [DOI: 10.1111/j.1365-2222.2009.03444.x; PUBMED: 20082618] [DOI] [PubMed] [Google Scholar]
- Brockow I, Zutavern A, Hoffmann U, Grubl A, Berg A, Koletzko S, et al. GINIplus Study Group. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. Journal of Investigational Allergology & Clinical Immunology 2009;19(3):180‐7. [DOI: ] [PubMed] [Google Scholar]
- Laubereau B, Brockow I, Zirngibl A, Koletzko S, Gruebl A, Berg A, et al. GINI Study Group. Effect of breast‐feeding on the development of atopic dermatitis during the first 3 years of life ‐ results from the GINI‐birth cohort study. Journal of Pediatrics 2004;144(5):602‐7. [DOI: 10.1016/j.jpeds.2003.12.029; PUBMED: 15126993] [DOI] [PubMed] [Google Scholar]
- Laubereau B, Filipiak‐Pittroff B, Berg A, Grubl A, Reinhardt D, Wichmann HE, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Archives of Disease in Childhood 2004;89(11):993‐7. [DOI: 10.1136/adc.2003.043265; PUBMED: 15499049] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzehak P, Sausenthaler S, Koletzko S, Bauer CP, Schaaf B, Berg A, et al. Period‐specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. European Journal of Epidemiology 2009;24(8):449‐67. [DOI: 10.1007/s10654-009-9356-5; PUBMED: 19521784] [DOI] [PubMed] [Google Scholar]
- Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, Berg A, Kramer U, et al. GINI‐plus Study Group. Long‐term effects of hydrolyzed protein infant formulas on growth ‐ extended follow‐up to 10 y of age: results from the German Infant Nutritional Intervention (GINI) study. American Journal of Clinical Nutrition 2011;94(6 Suppl):1803S‐7S. [DOI: 10.3945/ajcn.110.000679; PUBMED: 21849601] [DOI] [PubMed] [Google Scholar]
- Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, Berg A, Kramer U, et al. German Infant Nutritional Intervention Study Group. Short‐ and long‐term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: results from the German Infant Nutritional Intervention Study. American Journal of Clinical Nutrition 2009;89(6):1846‐56. [DOI: 10.3945/ajcn.2008.27373; PUBMED: 19369380] [DOI] [PubMed] [Google Scholar]
- Sausenthaler S, Koletzko S, Koletzko B, Reinhardt D, Kramer U, Berg A, et al. GINIplus study group. Effect of hydrolysed formula feeding on taste preferences at 10 years. Data from the German Infant Nutritional Intervention Program Plus Study. Clinical Nutrition 2010;29(3):304‐6. [DOI: 10.1016/j.clnu.2010.01.007; PUBMED: 20110140] [DOI] [PubMed] [Google Scholar]
- Schoetzau A, Filipiak‐Pittroff B, Franke K, Koletzko S, Berg A, Gruebl A, et al. German Infant Nutritional Intervention Study Group. Effect of exclusive breast‐feeding and early solid food avoidance on the incidence of atopic dermatitis in high‐risk infants at 1 year of age. Pediatric Allergy and Immunology 2002;13(4):234‐42. [PUBMED: 12390439] [DOI] [PubMed] [Google Scholar]
- Schoetzau A, Gehring U, Franke K, Grubl A, Koletzko S, Berg A, et al. Gini Study Group. Maternal compliance with nutritional recommendations in an allergy preventive programme. Archives of Disease in Childhood 2002;86(3):180‐4. [PUBMED: 11861235] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A, Filipiak‐Pittroff B, Krämer U, Link E, Heinrich J, Koletzko S, et al. The German Infant Nutritional Intervention Study (GINI) for the preventive effect of hydrolysed infant formulas in infants at high risk for allergic diseases. Design and selected results. Allergologie 2012;35(1):32‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A, Filipiak‐Pittroff B, Schulz H, Hoffmann U, Link E, Sussmann M, et al. GINIplus study group. Allergic manifestation 15 years after early intervention with hydrolyzed formulas ‐ the GINI study. Allergy: European Journal of Allergy and Clinical Immunology 2016;71(2):210‐9. [DOI: 10.1111/all.12790; PUBMED: 26465137] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A, Filipiak B, Koletzko S, Krämer U, Hoffmann B, Link E, et al. Long‐term effect of cow milk protein hydrolysates in high‐risk children. Results from the 10‐years follow‐up of the GINI study. Allergy: European Journal of Allergy and Clinical Immunology 2013;68:295‐6. [Google Scholar]
- Berg A, Filipiak‐Pittroff B, Kramer U, Hoffmann B, Link E, Beckmann C, et al. GINIplus study group. Allergies in high‐risk schoolchildren after early intervention with cow's milk protein hydrolysates: 10‐year results from the German Infant Nutritional Intervention (GINI) study. Journal of Allergy and Clinical Immunology 2013;131(6):1565‐73. [DOI: 10.1016/j.jaci.2013.01.006; PUBMED: 23506844] [DOI] [PubMed] [Google Scholar]
- Berg A, Filipiak‐Pittroff B, Kramer U, Link E, Bollrath C, Brockow I, et al. GINIplus study group. Preventive effect of hydrolyzed infant formulas persists until age 6 years: long‐term results from the German Infant Nutritional Intervention Study (GINI). Journal of Allergy and Clinical Immunology 2008;121(6):1442‐7. [DOI: 10.1016/j.jaci.2008.04.021; PUBMED: 18539195] [DOI] [PubMed] [Google Scholar]
- Berg A, Koletzko S, Filipiak‐Pittroff B, Laubereau B, Grubl A, Wichmann HE, et al. German Infant Nutritional Intervention Study Group. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three‐year results of the German Infant Nutritional Intervention Study. Journal of Allergy and Clinical Immunology 2007;119(3):718‐25. [DOI: 10.1016/j.jaci.2006.11.017; PUBMED: 17240440] [DOI] [PubMed] [Google Scholar]
- Berg A, Koletzko S, Grubl A, Filipiak‐Pittroff B, Wichmann HE, Bauer CP, et al. German Infant Nutritional Intervention Study Group. The effect of hydrolyzed cow's milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double‐blind trial. Journal of Allergy and Clinical Immunology 2003;111(3):533‐40. [PUBMED: 12642834] [DOI] [PubMed] [Google Scholar]
Willems 1993 {published data only}
- Willems R, Duchateau J, Magrez P, Denis R, Casimir G. Influence of hypoallergenic milk formula on the incidence of early allergic manifestations in infants predisposed to atopic diseases. Annals of Allergy 1993;71(2):147‐50. [PUBMED: 8346868] [PubMed] [Google Scholar]
References to studies excluded from this review
Agosti 2003 {published data only}
- Agosti M, Pugni L, Ramenghi LA, Mosca F, Marini A. Hydrolysed proteins in preterm formula: influence on plasma aminoacids, blood fatty acids and insulinaemia. Acta Paediatrica Supplement 2003;91(441):34‐8. [14599039] [DOI] [PubMed] [Google Scholar]
Akerblom 2005 {published data only}
- Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, et al. National TRIGR Study Groups. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: A pilot study. Diabetologia 2005;48(5):829‐37; Erratum in: Diabetologia 2005; Aug;48(8):1676. [DOI: 10.1007/s00125-005-1733-3; PUBMED: 15838685] [DOI] [PubMed] [Google Scholar]
- Virtanen SM, Barlund S, Salonen M, Savilahti E, Reunanen A, Paronen J, et al. Finnish TRIGR Study Group. Feasibility and compliance in a nutritional primary prevention trial in infants at increased risk for type 1 diabetes. Acta Paediatrica 2011;100(4):557‐64. [DOI: 10.1111/j.1651-2227.2010.02107.x; PUBMED: 21114527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akimoto 1997 {published data only}
- Akimoto K, Saito H, Akasawa A, Iikura Y. [Preventative effect of a whey hydrolyzed formula (Nestle, NAN H.A.) on the development of allergic symptoms in infants]. Arerugi [Allergy] 1997;46(10):1044‐51. [PUBMED: 9404092] [PubMed] [Google Scholar]
Arikan 2008 {published data only}
- Arikan D, Alp H, Gozum S, Orbak Z, Cifci EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17(13):1754‐61. [PUBMED: 18592627] [DOI] [PubMed] [Google Scholar]
Arshad 1990 {unpublished data only}
- Arshad SH. Randomised controlled trial of cow’s milk formula versus hydrolysed formula for the prevention of allergy. Author email communication November 2017.
Arshad 1992a {published data only}
- Arshad SH, Bateman B, Matthews SM. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: a randomised controlled study. Thorax 2003;58(6):489‐93. [PUBMED: 12775858] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992;339(8808):1493‐7. [PUBMED: 1351183] [DOI] [PubMed] [Google Scholar]
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. Journal of Allergy and Clinical Immunology 1994;93(5):842‐6. [PUBMED: 8182225] [DOI] [PubMed] [Google Scholar]
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51(2):89‐93. [PUBMED: 8738513] [PubMed] [Google Scholar]
- Scott M, Kurukulaaratchy R, Roberts G, Clayton B, Matthews S, Arshad S. Primary prevention of asthma, outcomes at 17 year follow up. Allergy 2010;65(S92):315. [Google Scholar]
- Scott M, Roberts G, Kurukulaaratchy R, Clayton B, Matthews S, Arshad S. Primary prevention of eczema, outcomes at 17 year follow up. Allergy 2010;65(S92):314. [Google Scholar]
- Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax 2012;67(12):1046‐51. [DOI: 10.1136/thoraxjnl-2012-202150; PUBMED: 22858926] [DOI] [PubMed] [Google Scholar]
Baldassarre 2017 {published data only}
- Baldassarre ME, Mauro A, Fanelli M, Montagna O, Wampler J, Cooper T, et al. Shorter time to full enteral feedings among infants fed an intact protein (IP) vs an extensively hydrolyzed (EH) formula does not appear to be related to differences in gastric emptying. Journal of Pediatric Gastroenterology and Nutrition 2017;64(Suppl 1):920‐1. [Google Scholar]
Barberi 1993 {unpublished data only}
- Barberi I, Salpietro DC, Catalioto G, Fulia F. Clinical trial of a new hypoallergenic formula for risk infants. 2nd World Congress of Perinatal Medicine; 1993 Sep 19‐24; Rome (Italy). 1993.
Bergmann 1996a {published data only}
- Bergmann RL, Dannemann A, Edenharter G, Bergmann KE, Monch E, Dudenhausen JW, et al. Influence of ultrafiltrated, partially hydrolysed formula on growth and health of young infants. Monatsschrift fur Kinderheilkunde 1996;144(2):152‐8. [Google Scholar]
Berseth 2009 {published data only}
- Berseth CL, Mitmesser SH, Ziegler EE, Marunycz JD, Vanderhoof J. Tolerance of a standard intact protein formula versus a partially hydrolyzed formula in healthy, term infants. Nutrition Journal 2009;8:27. [DOI: 10.1186/1475-2891-8-27; PUBMED: 19545360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borschel 2013 {published data only}
- Borschel MW, Ziegler EE, Wedig RT, Oliver JS. Growth of healthy term infants fed an extensively hydrolyzed casein‐based or free amino acid‐based infant formula: a randomized, double‐blind, controlled trial. Clinical Pediatrics 2013;52(10):910‐7. [DOI: 10.1177/0009922813492883; PUBMED: 23820000] [DOI] [PubMed] [Google Scholar]
Borschel 2014a {published data only}
- Borschel MW, Choe YS, Kajzer JA. Growth of healthy term infants fed partially hydrolyzed whey‐based infant formula: a randomized, blinded, controlled trial. Clinical Pediatrics 2014;53(14):1375‐82. [DOI: 10.1177/0009922814541804; PUBMED: 25009115] [DOI] [PubMed] [Google Scholar]
Borschel 2014b {published data only}
- Borschel MW, Baggs GE, Barrett‐Reis B. Growth of healthy term infants fed ready‐to‐feed and powdered forms of an extensively hydrolyzed casein‐based infant formula: a randomized, blinded, controlled trial. Clinical Pediatrics 2014;53(6):585‐92. [DOI: 10.1177/0009922814528036; PUBMED: 24662422] [DOI] [PubMed] [Google Scholar]
Boyle 2016 {published data only}
- Boyle RJ, Tang MLK, Chiang WC, Chua MC, Ismail I, Nauta A, et al. PATCH study investigators. Prebiotic‐supplemented partially hydrolysed cow's milk formula for the prevention of eczema in high‐risk infants: a randomized controlled trial. Allergy 2016;71(5):701‐10. [DOI: 10.1111/all.12848; PUBMED: 27111273] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Rijnierse A, Nauta A, Boyle R, Hourihane J, Chiang W, et al. Influence of early feeding patterns on eczema development in high‐risk infants. Allergy 2017;72:15. [Google Scholar]
Burks 2008 {published data only}
- Burks W, Jones SM, Berseth CL, Harris C, Sampson HA, Scalabrin DM. Hypoallergenicity and effects on growth and tolerance of a new amino acid‐based formula with docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 2008;153(2):266‐71. [DOI: 10.1016/j.jpeds.2008.02.043; PUBMED: 18534230] [DOI] [PubMed] [Google Scholar]
Campbell 1989 {published data only}
- Campbell JP. Dietary therapy of infant colic: a double‐blind study. Ceskoslovenska Pediatrie 1993;48(4):199‐202. [PUBMED: 8495532] [PubMed] [Google Scholar]
- Campbell JP. Dietary treatment of infant colic: a double‐blind study. Journal of the Royal College of General Practitioners 1989;39(318):11‐4. [PUBMED: 2553940] [PMC free article] [PubMed] [Google Scholar]
Chan 2002 {published data only}
- Chan YH, Shek LP, Aw M, Quak SH, Lee BW. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. Journal of Paediatrics and Child Health 2002;38(1):84‐8. [PUBMED: 11869407] [DOI] [PubMed] [Google Scholar]
Chandra 1989a {published data only}
- Chandra RK, Puri S, Hamed A. Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. BMJ 1989;299(6693):228‐30; Erratum in: BMJ 1989;299(6704):896; Retraction in: BMJ 2015; 351(h5682). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Chandra 1989b {published data only}
- Chandra RK. Five‐year follow‐up of high‐risk infants with family history of allergy who were exclusively breast‐fed or fed partial whey hydrolysate, soy, and conventional cow's milk formulas. Journal of Pediatric Gastroenterology and Nutrition 1997;24(4):380‐8; Expression of concern in: Journal of Pediatric Gastroenterology and Nutrition 2016; 63(2):307. [PUBMED: 9144119] [DOI] [PubMed] [Google Scholar]
- Chandra RK, Hamed A. Cumulative incidence of atopic disorders in high risk infants fed whey hydrolysate, soy, and conventional cow milk formulas. Annals of Allergy 1991;67(2 Pt 1):129‐32. [PUBMED: 1867449] [PubMed] [Google Scholar]
- Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Annals of Allergy 1989;63(2):102‐6. [PUBMED: 2669565] [PubMed] [Google Scholar]
Chan‐Yeung 2000 {published data only}
- Becker A, Watson W, Ferguson A, Dimich‐Ward H, Chan‐Yeung M. The Canadian asthma primary prevention study: outcomes at 2 years of age. Journal of Allergy and Clinical Immunology 2004;113(4):650‐6. [DOI: 10.1016/j.jaci.2004.01.754; PUBMED: 15100668] [DOI] [PubMed] [Google Scholar]
- Chan‐Yeung M, Manfreda J, Dimich‐Ward H, Ferguson A, Watson W, Becker A. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high‐risk infants. Archives of Pediatrics & Adolescent Medicine 2000;154(7):657‐63. [PUBMED: 10891016] [DOI] [PubMed] [Google Scholar]
Corvaglia 2013 {published data only}
- Corvaglia L, Mariani E, Aceti A, Galletti S, Faldella G. Extensively hydrolyzed protein formula reduces acid gastro‐esophageal reflux in symptomatic preterm infants. Early Human Development 2013;89(7):453‐5. [DOI: 10.1016/j.earlhumdev.2013.04.003; PUBMED: 23642476] [DOI] [PubMed] [Google Scholar]
D'Agata 1996 {published data only}
- D'Agata A, Betta P, Sciacca P, Morano C, Pratico G, Curreri R, et al. Role of dietary prevention in newborns at risk for atopy. Results of a follow‐up study. Pediatria Medica e Chirurgica 1996;18(5):469‐72. [PUBMED: 9053884] [PubMed] [Google Scholar]
Decsi 1992 {published data only}
- Decsi T, Volker V, Szasz M, Ezer E, Mehes K. [Comparison of human milk with 3 different types of infant food in the nutrition of full‐term neonates]. Orvosi Hetilap 1992;133(33):2087‐91. [PUBMED: 1501859] [PubMed] [Google Scholar]
Decsi 1998 {published data only}
- Decsi T, Veitl V, Burus I. Plasma amino acid concentrations, indexes of protein metabolism and growth in healthy, full‐term infants fed partially hydrolyzed infant formula. Journal of Pediatric Gastroenterology and Nutrition 1998;27(1):12‐6. [PUBMED: 9669720] [DOI] [PubMed] [Google Scholar]
de Jong 1998 {published data only}
- Jong MH, Scharp‐Van Der Linden VT, Aalberse R, Heymans HS, Brunekreef B. The effect of brief neonatal exposure to cows' milk on atopic symptoms up to age 5. Archives of Disease in Childhood 2002;86(5):365‐9. [PUBMED: 11970933] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong MH, Scharp‐van der Linden VT, Aalberse RC, Oosting J, Tijssen JG, Groot CJ. Randomised controlled trial of brief neonatal exposure to cows' milk on the development of atopy. Archives of Disease in Childhood 1998;79(2):126‐30. [PUBMED: 9797592] [DOI] [PMC free article] [PubMed] [Google Scholar]
Exl 1998 {published data only}
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen reduced dietary intervention programme. The ZUFF‐study‐programme. Part I: study design and 6‐month nutritional behaviour. European Journal of Nutrition 2000;39(3):89‐102. [PUBMED: 10918990] [DOI] [PubMed] [Google Scholar]
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen‐reduced dietary intervention programme: the ZUFF‐STUDY‐PROGRAMME. Part II: infant growth and health status to age 6 months. European Journal of Nutrition 2000;39(4):145‐56. [PUBMED: 11079734] [DOI] [PubMed] [Google Scholar]
- Exl BM, Deland U, Wall M, Preysch U, Secretin MC, Shmerling DH. Zug‐Frauenfeld Nutritional Survey ('Zuff Study'): allergen‐reduced nutrition in a normal infant population and its health‐related effects. Results at the age of six months. Nutrition Research 1998;18(8):1443‐62. [DOI: 10.1016/S0271-5317(98)00121-3] [DOI] [Google Scholar]
Florendo 2009 {published data only}
- Florendo KN, Bellflower B, Zwol A, Cooke RJ. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. Journal of Perinatology 2009;29(2):106‐11. [DOI: 10.1038/jp.2008.124; PUBMED: 18716627] [DOI] [PubMed] [Google Scholar]
Fukushima 1997 {published data only}
- Fukushima Y, Iwamoto K, Takeuchi‐Nakashima A, Akamatsu N, Fujino‐Numata N, Yoshikoshi M, et al. Preventive effect of whey hydrolysate formulas for mothers and infants against allergy development in infants for the first 2 years. Journal of Nutritional Science and Vitaminology 1997;43(3):397‐411. [PUBMED: 9268927] [DOI] [PubMed] [Google Scholar]
Giovannini 1994 {published data only}
- Giovannini M, Agostoni C, Fiocchi A, Bellu R, Trojan S, Riva E. Antigen‐reduced infant formulas versus human milk: growth and metabolic parameters in the first 6 months of life. Journal of the American College of Nutrition 1994;13(4):357‐63. [PUBMED: 7963141] [DOI] [PubMed] [Google Scholar]
Halken 1992 {published data only}
- Halken S, Host A, Hansen LG, Osterballe O. Effect of an allergy prevention programme on incidence of atopic symptoms in infancy. A prospective study of 159 "high‐risk" infants. Allergy 1992;47(5):545‐53. [PUBMED: 1485660] [DOI] [PubMed] [Google Scholar]
- Halken S, Host A, Hansen LG, Osterballe O. Prevention of allergy in infants. A prospective study of 159 high‐risk children. Ugeskrift for Laeger 1994;156(3):308‐12. [PUBMED: 8296423] [PubMed] [Google Scholar]
- Halken S, Host A, Hansen LG, Osterballe O. Preventive effect of feeding high‐risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatric Allergy and Immunology 1993;4(4):173‐81. [PUBMED: 8298708] [DOI] [PubMed] [Google Scholar]
Hartman 1994 {unpublished data only}
- Hartman CT, Fredericks GL, Katz ES, Brown CA. Prevalence of symptomatic formula intolerance and allergy in a general infant population. Journal of Allergy and Clinical Immunology 1994;93(1):210. [(A286)] [Google Scholar]
Hattevig 1989 {published data only}
- Hattevig G, Kjellman B, Sigurs N, Bjorksten B, Kjellman NI. Effect of maternal avoidance of eggs, cow's milk and fish during lactation upon allergic manifestations in infants. Clinical and Experimental Allergy 1989;19(1):27‐32. [PUBMED: 2702510] [DOI] [PubMed] [Google Scholar]
- Hattevig G, Kjellman B, Sigurs N, Grodzinsky E, Hed J, Bjorksten B. The effect of maternal avoidance of eggs, cow's milk, and fish during lactation on the development of IgE, IgG, and IgA antibodies in infants. Journal of Allergy and Clinical Immunology 1990;85(1 Pt 1):108‐15. [PUBMED: 2299096] [DOI] [PubMed] [Google Scholar]
- Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatrica 1999;88(1):7‐12. [PUBMED: 10090539] [PubMed] [Google Scholar]
- Paronen J, Bjorksten B, Hattevig G, Akerblom HK, Vaarala O. Effect of maternal diet during lactation on development of bovine insulin‐binding antibodies in children at risk for allergy. Journal of Allergy and Clinical Immunology 2000;106(2):302‐6. [DOI: 10.1067/mai.2000.108110; PUBMED: 10932074] [DOI] [PubMed] [Google Scholar]
- Sigurs N, Hattevig G, Kjellman B. Maternal avoidance of eggs, cow's milk, and fish during lactation: effect on allergic manifestations, skin‐prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics 1992;89(4 Pt 2):735‐9. [PUBMED: 1557270] [PubMed] [Google Scholar]
Hernell 2003 {published data only}
- Hernell O, Lonnerdal B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. American Journal of Clinical Nutrition 2003;78(2):296‐301. [DOI: 10.1093/ajcn/78.2.296; PUBMED: 12885712] [DOI] [PubMed] [Google Scholar]
Hill 1995b {published data only}
- Hill DJ, Hudson IL, Sheffield LJ, Shelton MJ, Menahem S, Hosking CS. A low allergen diet is a significant intervention in infantile colic: results of a community‐based study. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):886‐92. [PUBMED: 8543745] [DOI] [PubMed] [Google Scholar]
Høst 1991 {published data only}
- Høst A. Importance of the first meal on the development of cow's milk allergy and intolerance. Allergy Proceedings 1991;12(4):227‐32. [PUBMED: 1936970] [DOI] [PubMed] [Google Scholar]
Iikura 1995 {unpublished data only}
- Iikura Y, Akimoto K, Ebisawa M, Onda T, Akazawa A, Saito H, et al. Effect of hydrolyzed whey protein formula for babies on development of allergic symptoms during infancy. Nestle Nutrition Workshop Series. Vol. 34, Baltimore, Maryland: Raven Press, 1995:269‐75. [Google Scholar]
Keller 1996 {published data only}
- Keller KM, Burgin‐Wolff A, Lippold R, Wirth S, Lentze MJ. The diagnostic significance of IgG cow's milk protein antibodies re‐evaluated. European Journal of Pediatrics 1996;155(4):331‐7. [PUBMED: 8777930] [DOI] [PubMed] [Google Scholar]
Knip 2010 {published data only}
- Hyytinen M, Savilahti E, Virtanen SM, Harkonen T, Ilonen J, Luopajarvi K, et al. Finnish TRIGR Pilot Study Group. Avoidance of cow's milk‐based formula for at‐risk infants does not reduce development of celiac disease: a randomized controlled trial. Gastroenterology 2017;153(4):961‐70.e3. [DOI] [PubMed] [Google Scholar]
- Knip M, Virtanen SM, Seppa K, Ilonen J, Savilahti E, Vaarala O, et al. Finnish TRIGR Study Group. Dietary intervention in infancy and later signs of beta‐cell autoimmunity. New England Journal of Medicine 2010;363(20):1900‐8. [DOI: 10.1056/NEJMoa1004809; PUBMED: 21067382] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knip 2011 {published data only}
- Knip M, Akerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, et al. TRIGR Study Group. Hydrolyzed infant formula and early beta‐cell autoimmunity: a randomized clinical trial. JAMA 2014;311(22):2279‐87. [DOI: 10.1001/jama.2014.5610; PUBMED: 24915259] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knip M, Virtanen SM, Becker D, Dupre J, Krischer JP, Akerblom HK, TRIGR Study Group. Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin‐dependent diabetes mellitus in the Genetically at Risk (TRIGR). American Journal of Clinical Nutrition 2011;94(6 Suppl):1814S‐20S. [DOI: 10.3945/ajcn.110.000711; PUBMED: 21653795] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischer JP. Hydrolyzed infant formula and early beta‐cell autoimmunity. Diabetes 2014;63:A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00179777. TRIGR ‐ Primary prevention study for Type 1 Diabetes in children at risk [TRIGR ‐ Trial to Reduce IDDM in the Genetically at Risk]. clinicaltrials.gov/show/NCT00179777 (first received 16 September 2005).
- TRIGR Study Group, Akerblom HK, Krischer J, Virtanen SM, Berseth C, Becker D, Dupre J, et al. The trial to reduce IDDM in the genetically at risk (TRIGR) study: recruitment, intervention and follow‐up. Diabetologia 2011;54(3):627‐33; Erratum in: Diabetologia. 2011;54(8):2210. [DOI: 10.1007/s00125-010-1964-9; NCT00179777; PUBMED: 21153533] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group for the TRIGR Study Group, Knip M, Åkerblom HK, Al Taji E, Becker D, Bruining J, Castano L, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of Type 1 diabetes: The TRIGR randomized clinical trial. JAMA 2018;319(1):38‐48. [DOI: 10.1001/jama.2017.19826; NCT00179777; PUBMED: 29297078] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuo 2011 {published data only}
- Kuo HC, Liu CA, Ou CY, Hsu TY, Wang CL, Huang HC, et al. Partial protein‐hydrolyzed infant formula decreased food sensitization but not allergic diseases in a prospective birth cohort study. International Archives of Allergy and Immunology 2011;154(4):310‐7. [DOI: 10.1159/000321823; PUBMED: 20975282] [DOI] [PubMed] [Google Scholar]
Lasekan 2006 {published data only}
- Lasekan JB, Koo WW, Walters J, Neylan M, Luebbers S. Growth, tolerance and biochemical measures in healthy infants fed a partially hydrolyzed rice protein‐based formula: a randomized, blinded, prospective trial. Journal of the American College of Nutrition 2006;25(1):12‐9. [PUBMED: 16522927] [DOI] [PubMed] [Google Scholar]
Lucassen 2000 {published data only}
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate. A double‐blind, randomized, placebo‐controlled trial. Pediatrics 2000;106(6):1349‐54. [PUBMED: 11099588] [DOI] [PubMed] [Google Scholar]
Maggio 2005 {published data only}
- Maggio L, Zuppa AA, Sawatzki G, Valsasina R, Schubert W, Tortorolo G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatrica 2005;94(1):75‐84. [PUBMED: 15858965] [DOI] [PubMed] [Google Scholar]
- Zuppa AA, Girlando P, Scapillati ME, Maggio L, Romagnoli C, Tortorolo G. [Effects on growth, tolerability and biochemical parameters of two different human milk fortifiers in very low birth‐weight newborns]. Pediatria Medica e Chirurgica 2004;26(1):45‐9. [PUBMED: 15529811] [PubMed] [Google Scholar]
Martinez‐Valverde 1993 {unpublished data only}
- Martinenez‐Valverde A, Aljma‐Garcia JM. Study and evaluation of serum IgE and specific total IgE against alpha‐lactalbumin, beta‐lactoglobulin and casein with hypoallergenic formulas (HA) and other types of feeding [Thesis]. Spain: Universidad de Malaga, 1993. [Google Scholar]
Medjad‐Guillou 1992 {published data only}
- Medjad‐Guillou N, Henocq A, Arnaud‐Battandier F. Does the hydrolysis of proteins change the acceptability and the digestive tolerance of milk for infants? The results of a comparative and randomized prospective study. Annales de Pediatrie 1992;39(3):202‐6. [PUBMED: 1570949] [PubMed] [Google Scholar]
Mennella 2011a {published data only}
- Mennella JA, Castor SM. Sensitive period in flavor learning: effects of duration of exposure to formula flavors on food likes during infancy. Clinical Nutrition 2012;31(6):1022‐5. [DOI: 10.1016/j.clnu.2012.05.005; PUBMED: 22652361] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. American Journal of Clinical Nutrition 2009;90(3):780S‐8S. [DOI: 10.3945/ajcn.2009.27462O; PUBMED: 19605570] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Lukasewycz LD, Castor SM, Beauchamp GK. The timing and duration of a sensitive period in human flavor learning: a randomized trial. American Journal of Clinical Nutrition 2011;93(5):1019‐24. [DOI: 10.3945/ajcn.110.003541; 21310829; NCT00994747] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Trabulsi JC, Papas MA. Effects of cow milk versus extensive protein hydrolysate formulas on infant cognitive development. Amino Acids 2016;48(3):697‐705. [DOI: 10.1007/s00726-015-2118-7; NCT00994747; PUBMED: 26497857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mihatsch 1999 {published data only}
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90(2):196‐8. [PUBMED: 11236051] [DOI] [PubMed] [Google Scholar]
- Mihatsch WA, Pohlandt F. Protein hydrolysate formula maintains homeostasis of plasma amino acids in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 1999;29(4):406‐10. [PUBMED: 10512399] [DOI] [PubMed] [Google Scholar]
Mihatsch 2002 {published data only}
- Mihatsch WA, Franz AR, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002;110(6):1199‐203. [PUBMED: 12456919] [DOI] [PubMed] [Google Scholar]
Mitchell 1977 {published data only}
- Mitchell JD, Brand J, Halbisch J. Weight‐gain inhibition by lactose in Australian Aboriginal children. A controlled trial of normal and lactose hydrolysed milk. Lancet 1977;1(8010):500‐2. [PUBMED: 65606] [DOI] [PubMed] [Google Scholar]
Moran 1992 {published data only}
- Moran JR. Effects of prolonged exposure to partially hydrolyzed milk protein. Journal of Pediatrics 1992;121(5 Pt 2):S90‐4. [PUBMED: 1447640] [DOI] [PubMed] [Google Scholar]
NCT00936637 {published data only}
- NCT00936637. Effects on growth of an extensively hydrolyzed formula fed to term [A 16‐week growth study of an extensively hydrolyzed infant formula, 3 Months treatment and 1 month follow‐up for a duration of 4 months]. clinicaltrials.gov/show/NCT00936637 (first received 10 July 2009).
NCT01987154 {published data only}
- NCT01987154. Tolerance of an extensively hydrolyzed protein infant formula versus a premature infant formula. clinicaltrials.gov/show/NCT01987154 (first received 19 November 2013).
Nentwich 2003 {published data only}
- Nentwich I, Pazdirkova A, Koberska I, Pokojska E, Szepfalusi Z, Lokaj J. Cow milk‐specific humoral and cellular immune response in infants with high risk of atopy under feeding a whey hydrolysate infant formula. Klinische Padiatrie 2003;215(5):275‐9. [DOI: 10.1055/s-2003-42664; PUBMED: 14520590] [DOI] [PubMed] [Google Scholar]
Nocerino 2012 {published data only}
- Nocerino R, Marco G, Cosenza L, Pezzella V, Passariello A, Terrin G, et al. Efficacy of a new partially hydrolyzed whey formula on infant colic: a randomized controlled trial. Digestive and Liver Disease 2012;44:S272. [Google Scholar]
Odelram 1996 {published data only}
- Odelram H, Vanto T, Jacobsen L, Kjellman NI. Whey hydrolysate compared with cow's milk‐based formula for weaning at about 6 months of age in high allergy‐risk infants: effects on atopic disease and sensitization. Allergy 1996;51(3):192‐5. [PUBMED: 8781676] [PubMed] [Google Scholar]
Paronen 2000 {published data only}
- Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HK, et al. Effect of cow's milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Finnish Trial to Reduce IDDM in the Genetically at Risk Study Group. Diabetes 2000;49(10):1657‐65. [PUBMED: 11016449] [DOI] [PubMed] [Google Scholar]
Pauls 1996 {published data only}
- Pauls J, Bauer K, Versmold H. Randomized controlled trial of formulas with hydrolyzed versus non‐hydrolyzed protein for starting enteral feedings in preterm infants <1500g body weight. Journal of Pediatric Gastroenterology and Nutrition 1996;22:450 (abstract 164). [Google Scholar]
Picaud 2001 {published data only}
- Picaud JC, Lapillonne A, Rigo J, Normand S, Reygrobellet B, Claris O, et al. Nitrogen utilization and bone mineralization in very low birth weight infants fed partially hydrolyzed preterm formula. Seminars in Perinatology 2002;26(6):439‐46; Retraction in: Seminars in Perinatology 2013; 37(2):138. [PUBMED: 12537316] [DOI] [PubMed] [Google Scholar]
- Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, et al. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2001;32(5):555‐61. [PUBMED: 11429516] [DOI] [PubMed] [Google Scholar]
Porch 1998 {published data only}
- Porch MC, Shahane AD, Leiva LE, Elston RC, Sorensen RU. Influence of breast milk, soy or two hydrolyzed formulas on the development of allergic manifestations in infants at risk. Nutrition Research 1998;18(8):1413‐24. [Google Scholar]
Raupp 1995 {published data only}
- Raupp P, Kries R, Gobel C, Fox A, Lemburg P, Schmidt E, et al. Bone density and biochemical parameters of bone mineral metabolism and acid load in preterm infants. Cow's milk formula versus hydrolysed protein preparation. Monatsschr Kinderheilkd 1995;143(7 (Suppl 2)):S140‐6. [Google Scholar]
Riezzo 2001 {published data only}
- Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. Gastric electrical activity and gastric emptying in preterm newborns fed standard and hydrolysate formulas. Journal of Pediatric Gastroenterology and Nutrition 2001;33(3):290‐5. [PUBMED: 11593124] [DOI] [PubMed] [Google Scholar]
Rigo 1994a {published data only}
- Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. European Journal of Clinical Nutrition 1995;49(Suppl 1):S26‐38. [PUBMED: 8647061] [PubMed] [Google Scholar]
- Rigo J, Salle BL, Putet G, Senterre J. Nutritional evaluation of various protein hydrolysate formulae in term infants during the first month of life. Acta Paediatrica Supplement 1994;402:100‐4. [PUBMED: 7841611] [DOI] [PubMed] [Google Scholar]
Rigo 1994b {published data only}
- Rigo J, Senterre J. Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatrica Supplement 1994;405:98‐104. [PUBMED: 7734800] [DOI] [PubMed] [Google Scholar]
Savino 2003 {published data only}
- Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo‐ and galacto‐oligosaccharides. Acta Paediatrica Supplement 2003;91(441):86‐90. [PUBMED: 14599049] [DOI] [PubMed] [Google Scholar]
Savino 2006 {published data only}
- Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. European Journal of Clinical Nutrition 2006;60(11):1304‐10. [DOI: 10.1038/sj.ejcn.1602457; PUBMED: 16736065] [DOI] [PubMed] [Google Scholar]
Scalabrin 2009 {published data only}
- Scalabrin D, Berseth C, Marunycz J, Mitmesser S. Long term safety, growth, and health effects of lactobacillus GG (LGG) added to partially and extensively hydrolyzed infant formulas. Allergy: European Journal of Allergy and Clinical Immunology 2009;64(1171):447. [Google Scholar]
- Scalabrin D, Harris C, Johnston WH, Berseth CL. Long‐term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: a 5‐year follow‐up. European Journal of Pediatrics 2017;176(2):217‐24. [DOI: 10.1007/s00431-016-2825-4; PUBMED: 27975116] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrin DM, Johnston WH, Hoffman DR, P'Pool VL, Harris CL, Mitmesser SH. Growth and tolerance of healthy term infants receiving hydrolyzed infant formulas supplemented with Lactobacillus rhamnosus GG: randomized, double‐blind, controlled trial. Clinical Pediatrics 2009;48(7):734‐44. [DOI: 10.1177/0009922809332682; NCT00655720; PUBMED: 19264721] [DOI] [PubMed] [Google Scholar]
- Scalabrin DMF, Harris C, Strong PV, Liu B, Berseth CL. Infant supplementation with Lactobacillus rhamnosus GG (LGG) and long‐term growth and health: a 5‐year follow‐up. Allergy: European Journal of Allergy and Clinical Immunology 2014;69(451):196‐7. [Google Scholar]
Schmelzle 2003 {published data only}
- Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Bockler HM, et al. Randomized double‐blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta‐palmitic acid level, and nondigestible oligosaccharides. Journal of Pediatric Gastroenterology and Nutrition 2003;36(3):343‐51. [PUBMED: 12604972] [DOI] [PubMed] [Google Scholar]
Schmidt 1995 {published data only}
- Schmidt E, Eden‐Kohler J, Tonkaboni F, Tolle J. Alimentary allergy prevention in infants with increased allergic risk: the effect of different feeding regimens in the first 6 months on atopic manifestations during the first year of life. A large scale feeding trial. Nestle Nutritional Workshop Series. Vol. 34, Baltimore, Maryland: Raven Press, 1995:231‐48. [Google Scholar]
Schmitz 1992 {published data only}
- Schmitz J, Digeon B, Chastang C, Dupouy D, Leroux B, Robillard P, et al. Effects of brief early exposure to partially hydrolyzed and whole cow milk proteins. Journal of Pediatrics 1992;121(5 Pt 2):S85‐9. [PUBMED: 1447639] [DOI] [PubMed] [Google Scholar]
Schrander 1993 {published data only}
- Schrander JJ, Bogart JP, Forget PP, Schrander‐Stumpel CT, Kuijten RH, Kester AD. Cow's milk protein intolerance in infants under 1 year of age: a prospective epidemiological study. European Journal of Pediatrics 1993;152(8):640‐4. [PUBMED: 8404966] [DOI] [PubMed] [Google Scholar]
Shao 2006 {published data only}
- Shao J, Sheng J, Dong W, Li YZ, Yu SC. Effects of feeding intervention on development of eczema in atopy high‐risk infants: an 18‐month follow‐up study. Zhonghua Er Ke Za Zhi 2006;44(9):684‐7. [PUBMED: 17217664] [PubMed] [Google Scholar]
Silva Rey 1996 {unpublished data only}
- Silva Rey AL, Garcia G, Nogales A. Preventatibve Effect of a Partially Hydrolyzed Milk Formula [Thesis]. Madrid, Spain, 1996. [Google Scholar]
Sorensen 2007 {published data only}
- Sorensen RU, Inostroza J, Hebal E, Vinet AM, Fierro J. Weaning to a partially hydrolyzed whey formula at three months of age protects against the later development of anti‐cow milk lgE antibodies in comparison to whole cows milk or extensively hydrolyzed whey formulas. Journal of Allergy and Clinical Immunology 2007;119(1):S120. [Google Scholar]
Staelens 2008 {published data only}
- Staelens S, Driessche M, Barclay D, Carrie‐Faessler AL, Haschke F, Verbeke K, et al. Gastric emptying in healthy newborns fed an intact protein formula, a partially and an extensively hydrolysed formula. Clinical Nutrition 2008;27(2):264‐8. [DOI: 10.1016/j.clnu.2007.12.009; PUBMED: 18280619] [DOI] [PubMed] [Google Scholar]
Szajewska 2001 {published data only}
- Szajewska H, Albrecht P, Stoinska B, Prochowska A, Gawecka A, Laskowska‐Klita T. Extensive and partial protein hydrolysate preterm formulas: the effect on growth rate, protein metabolism indices, and plasma amino acid concentrations. Journal of Pediatric Gastroenterology and Nutrition 2001;32(3):303‐9. [PUBMED: 11345180] [DOI] [PubMed] [Google Scholar]
Szajewska 2004 {published data only}
- Szajewska H, Mrukowicz JZ, Stoinska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double‐blind trial. Acta Paediatrica 2004;93(9):1159‐65. [PUBMED: 15384877] [PubMed] [Google Scholar]
Tariq 1998 {published data only}
- Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. Journal of Allergy and Clinical Immunology 1998;101(5):587‐93. [DOI: 10.1016/S0091-6749(98)70164-2; PUBMED: 9600493] [DOI] [PubMed] [Google Scholar]
Taubman 1988 {published data only}
- Taubman B. Parental counseling compared with elimination of cow's milk or soy milk protein for the treatment of infant colic syndrome: a randomized trial. Pediatrics 1988;81(6):756‐61. [PUBMED: 3285312] [PubMed] [Google Scholar]
Vaarala 1995 {published data only}
- Paronen J, Vaarala O, Savilahti E, Saukkonen T, Akerblom HK. Soluble adhesion molecules and oral antigen feeding in infants. Pediatric Research 1996;40(2):276‐9. [DOI: 10.1203/00006450-199608000-00014; PUBMED: 8827777] [DOI] [PubMed] [Google Scholar]
- Vaarala O, Saukkonen T, Savilahti E, Klemola T, Akerblom HK. Development of immune response to cow's milk proteins in infants receiving cow's milk or hydrolyzed formula. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):917‐23. [PUBMED: 8543750] [DOI] [PubMed] [Google Scholar]
Vaarala 2012 {published data only}
- Vaarala O, Ilonen J, Ruohtula T, Pesola J, Virtanen SM, Harkonen T, et al. Removal of bovine insulin from cow's milk formula and early initiation of beta‐cell autoimmunity in the FINDIA pilot study. Archives of Pediatrics & Adolescent Medicine 2012;166(7):608‐14. [DOI: 10.1001/archpediatrics.2011.1559; PUBMED: 22393174] [DOI] [PubMed] [Google Scholar]
Vandenplas 1988 {published data only}
- Vandenplas Y, Deneyer M, Sacre L, Loeb H. Preliminary data on a field study with a new hypo‐allergenic formula. European Journal of Pediatrics 1988;148(3):274‐7. [PUBMED: 3215202] [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Malfroot A, Dab I. Short‐term prevention of cow's milk protein allergy in infants. Immunology and Allergy Practice 1989;11:430‐7. [Google Scholar]
Vandenplas 1993 {published data only}
- Hauser B, Blecker U, Keymolen K, Suys B, Gerlo E, Vandenplas Y. Plasma amino acid concentrations in term‐born infants fed a whey predominant or a whey hydrolysate formula. JPEN. Journal of Parenteral and Enteral Nutrition 1997;21(1):27‐30. [DOI: 10.1177/014860719702100127; PUBMED: 9002081] [DOI] [PubMed] [Google Scholar]
- Hauser B, Keymolen K, Blecker U, Suys B, Bougatef A, Loeb H, et al. A comparative evaluation of whey hydrolysate and whey‐predominant formulas. How well do infants accept and tolerate them?. Clinical Pediatrics 1993;62:433‐7. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Hauser B, Blecker U, Suys B, Peeters S, Keymolen K, et al. The nutritional value of a whey hydrolysate formula compared with a whey‐predominant formula in healthy infants. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):92‐6. [PUBMED: 8350218] [DOI] [PubMed] [Google Scholar]
Wopereis 2014 {published data only}
- Wopereis H, Sim K, Shaw A, Martin R, Oozeer R, Warner JO, et al. The developmental gut microbiota is modulated towards a pattern closer to breastfed infants by a partially hydrolyzed cow's milk formula supplemented with specific prebiotic oligosaccharides. Allergy: European Journal of Allergy and Clinical Immunology 2014;69:315. [Google Scholar]
Xinias 2017 {published data only}
- Xinias I, Analitis A, Vandenplas Y. A synbiotic partial whey hydrolysate with reduced lactose decreases infantile colic and improves quality of life; A randomized open pilot study. Journal of Pediatric Gastroenterology and Nutrition 2017;64:143. [Google Scholar]
Yu 2014 {published data only}
- Yu MX, Zhuang SQ, Wang DH, Zhou XY, Liu XH, Shi LP, et al. Effects of extensively hydrolyzed protein formula on feeding and growth in preterm infants: a multicenter controlled clinical study. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(7):684‐90. [PUBMED: 25008873] [PubMed] [Google Scholar]
Zeiger 1989 {published data only}
- Zeiger RS, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. Journal of Allergy and Clinical Immunology 1993;91(3):723‐34. [PUBMED: 8454794] [DOI] [PubMed] [Google Scholar]
- Zeiger RS, Heller S. The development and prediction of atopy in high‐risk children: follow‐up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. Journal of Allergy and Clinical Immunology 1995;95(6):1179‐90. [PUBMED: 7797786] [DOI] [PubMed] [Google Scholar]
- Zeiger RS, Heller S, Mellon MH, Forsythe AB, O'Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food‐allergen avoidance on development of atopy in early infancy: a randomized study. Journal of Allergy and Clinical Immunology 1989;84:72‐89. [DOI] [PubMed] [Google Scholar]
- Zeiger RS, Heller S, Mellon MH, Halsey JF, Hamburger RN, Sampson HA. Genetic and environmental factors affecting the development of atopy through age 4 in children of atopic parents: a prospective randomized study of food allergen avoidance. Pediatric Allergy and Immunology 1992;3(3):110‐27. [Google Scholar]
References to ongoing studies
NCT01036243 {published data only}
- NCT01036243. Digestive tolerance of slightly hydrolyzed starter infant formula with probiotics [Tolerance of a slightly hydrolyzed starter formula containing probiotics]. clinicaltrials.gov/show/NCT01036243 (first received 21 December 2009).
NCT01156493 {published data only}
- NCT01156493. Hydrolized protein formula for premature infants [Randomized trial of hydrolyzed protein premature formula]. clinicaltrials.gov/show/ NCT01156493 (first received 02 July 2010).
NCT01210391 {unpublished data only}
- NCT01210391. Growth of infants fed an extensively hydrolyzed infant formula [Assessment of growth of infants fed a 100% whey extensively hydrolyzed formula]. clinicaltrials.gov/show/NCT01210391 (first received 28 September 2010).
NCT01700205 {published data only}
- NCT01700205. "Watch your baby grow" study (GRO) [Impact of diet composition on energy balance and satiety during infancy]. clinicaltrials.gov/show/NCT01700205 (first received 4 October 2012).
NCT01735123 {unpublished data only}
- NCT01735123. Early dietary intervention and later signs of beta‐cell autoimmunity (EDIA). clinicaltrials.gov/show/NCT01735123 (first received 28 November 2012).
Yin 2015 {published data only}
- Yin LP, Qian LJ, Zhu H, Chen Y, Li H, Han JN, et al. Application effect of extensively hydrolyzed milk protein formula and follow‐up in preterm children with a gestational age of less than 34 weeks: study protocol for a randomized controlled trial. Trials 2015;16:498. [DOI: 10.1186/s13063-015-1030-5; PUBMED: 26537897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAP 2000
- Americal Academy of Pediatrics: Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106(2 Pt 1):346‐9. [PUBMED: 10920165] [PubMed] [Google Scholar]
Alexander 2010
- Alexander DD, Cabana MD. Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a meta‐analysis. Journal of Pediatric Gastroenterology and Nutrition 2010;50(4):422‐30. [DOI: 10.1097/MPG.0b013e3181cea52b; PUBMED: 20216095] [DOI] [PubMed] [Google Scholar]
Allen 2009
- Allen CW, Campbell DE, Kemp AS. Food allergy: is strict avoidance the only answer?. Pediatric Allergy and Immunology 2009;20(5):415‐22. [DOI: 10.1111/j.1399-3038.2008.00811.x; PUBMED: 18798797] [DOI] [PubMed] [Google Scholar]
Arshad 2005
- Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest 2005;127(2):502‐8. [DOI: 10.1378/chest.127.2.502; PUBMED: 15705988] [DOI] [PubMed] [Google Scholar]
Bergmann 1994
- Bergmann RL, Bergmann KE, Lau‐Schadensdorf S, Luck W, Dannemann A, Bauer CP, et al. Atopic diseases in infancy. The German multicenter atopy study (MAS‐90). Pediatric Allergy and Immunology 1994;5(6 Suppl):19‐25. [PUBMED: 7728224] [DOI] [PubMed] [Google Scholar]
Bergmann 1997
- Bergmann RL, Edenharter G, Bergmann KE, Guggenmoos‐Holzmann I, Forster J, Bauer CP, et al. Predictability of early atopy by cord blood‐IgE and parental history. Clinical and Experimental Allergy 1997;27(7):752‐60. [PUBMED: 9249267] [PubMed] [Google Scholar]
Bergmann 1998
- Bergmann R, Woodcock A. Whole population or high‐risk group? Childhood asthma. European Respiratory Journal. Supplement 1998;27:9S‐12S. [PUBMED: 9699777] [PubMed] [Google Scholar]
Bock 1988
- Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double‐blind, placebo‐controlled food challenge (DBPCFC) as an office procedure: a manual. Journal of Allergy and Clinical Immunology 1988;82(6):986‐97. [PUBMED: 3060514] [DOI] [PubMed] [Google Scholar]
Boyle 2016a
- Boyle RJ, Ierodiakonou D, Khan T, Chivinge J, Robinson Z, Geoghegan N, et al. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta‐analysis. BMJ 2016;352:i974. [PUBMED: 26956579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burr 1989
- Burr ML, Butland BK, King S, Vaughan‐Williams E. Changes in asthma prevalence: two surveys 15 years apart. Archives of Disease in Childhood 1989;64(10):1452‐6. [PUBMED: 2817930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaffen 2010
- Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA 2010;303(18):1848‐56. [DOI: 10.1001/jama.2010.582; PUBMED: 20460624] [DOI] [PubMed] [Google Scholar]
Chung 2012
- Chung CS, Yamini S, Trumbo PR. FDA's health claim review: whey‐protein partially hydrolyzed infant formula and atopic dermatitis. Pediatrics 2012;130(2):e408‐14. [DOI: 10.1542/peds.2012-0333; PUBMED: 22778306] [DOI] [PubMed] [Google Scholar]
Custovic 2000
- Custovic A, Simpson BM, Simpson A, Hallam C, Craven M, Brutsche M, et al. Manchester Asthma and Allergy Study: low‐allergen environment can be achieved and maintained during pregnancy and in early life. Journal of Allergy and Clinical Immunology 2000;105(2 Pt 1):252‐8. [PUBMED: 10669844] [DOI] [PubMed] [Google Scholar]
Custovic 2001
- Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. NAC Manchester Asthma and Allergy Study Group. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet 2001;358(9277):188‐93. [DOI: 10.1016/S0140-6736(01)05406-X; PUBMED: 11476835] [DOI] [PubMed] [Google Scholar]
Darsow 2000
- Darsow U, Ring J. Airborne and dietary allergens in atopic eczema: a comprehensive review of diagnostic tests. Clinical and Experimental Dermatology 2000;25(7):544‐51. [PUBMED: 11122226] [DOI] [PubMed] [Google Scholar]
de Silva 2014
- Silva D, Geromi M, Halken S, Host A, Panesar SS, Muraro A, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. Primary prevention of food allergy in children and adults: systematic review. Allergy 2014;69(5):581‐9. [DOI: 10.1111/all.12334; PUBMED: 24433563] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 22 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gruskay 1982
- Gruskay FL. Comparison of breast, cow, and soy feedings in the prevention of onset of allergic disease: a 15‐year prospective study. Clinical Pediatrics 1982;21(8):486‐91. [DOI: 10.1177/000992288202100807; PUBMED: 7200847] [DOI] [PubMed] [Google Scholar]
Halken 2004
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatric Allergy and Immunology 2004;15(Suppl 16):4‐5. [DOI: 10.1111/j.1399-3038.2004.0148b.x; PUBMED: 15125698] [DOI] [PubMed] [Google Scholar]
Hansen 1993
- Hansen LG, Halken S, Host A, Moller K, Osterballe O. Prediction of allergy from family history and cord blood IgE levels. A follow‐up at the age of 5 years. Cord blood IgE. IV. Pediatric Allergy and Immunology 1993;4(1):34‐40. [PUBMED: 8348254] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Hill 2007
- Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ. The efficacy of amino acid‐based formulas in relieving the symptoms of cow's milk allergy: a systematic review. Clinical and Experimental Allergy 2007;37(6):808‐22. [DOI: 10.1111/j.1365-2222.2007.02724.x; PUBMED: 17517094] [DOI] [PubMed] [Google Scholar]
Høst 1994
- Høst A. Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatric Allergy and Immunology 1994;5(5 Suppl):1‐36. [PubMed] [Google Scholar]
Høst 1995
- Høst A, Jacobsen HP, Halken S, Holmenlund D. The natural history of cow's milk protein allergy/intolerance. European Journal of Clinical Nutrition 1995;49(Suppl 1):S13‐8. [PUBMED: 8647059] [PubMed] [Google Scholar]
Høst 1999
- Høst A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Archives of Disease in Childhood 1999;81(1):80‐4. [PUBMED: 10373144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iskedjian 2010
- Iskedjian M, Szajewska H, Spieldenner J, Farah B, Berbari J. Meta‐analysis of a partially hydrolysed 100%‐whey infant formula vs. extensively hydrolysed infant formulas in the prevention of atopic dermatitis. Current Medical Research and Opinion 2010;26(11):2599‐606. [DOI: 10.1185/03007995.2010.525475; PUBMED: 20925453] [DOI] [PubMed] [Google Scholar]
Johansson 2004
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use. Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832‐6. [DOI: 10.1016/j.jaci.2003.12.591; PUBMED: 15131563] [DOI] [PubMed] [Google Scholar]
Katz 2010
- Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow's milk protein is protective against IgE‐mediated cow's milk protein allergy. Journal of Allergy and Clinical Immunology 2010;126(1):77‐82 e1. [DOI: 10.1016/j.jaci.2010.04.020; PUBMED: 20541249] [DOI] [PubMed] [Google Scholar]
Katz 2011
- Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein‐induced enterocolitis syndrome to cow's milk: a large‐scale, prospective population‐based study. Journal of Allergy and Clinical Immunology 2011;127(3):647‐53 e1‐3. [DOI: 10.1016/j.jaci.2010.12.1105; PUBMED: 21377033] [DOI] [PubMed] [Google Scholar]
Kilpeläinen 2001
- Kilpeläinen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy 2001;56(5):377‐84. [PUBMED: 11350300] [DOI] [PubMed] [Google Scholar]
Kjellman 1977
- Kjellman NI. Atopic disease in seven‐year‐old children. Incidence in relation to family history. Acta Paediatrica Scandinavica 1997;66(4):465‐71. [PUBMED: 899762] [DOI] [PubMed] [Google Scholar]
Kleinman 1991
- Kleinman RE, Bahna S, Powell GF, Sampson HA. Use of infant formulas in infants with cow milk allergy. A review and recommendations. Pediatric Allergy and Immunology 1991;2(4):146‐55. [Google Scholar]
Kramer 2012
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000133.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwinta 2002
- Kwinta P, Mitkowska Z, Kruczek P, Tomasik T, Pietrzyk JJ. Influence of the lactose free and lactose containing diet on prevalence of gram‐negative sepsis and feeding intolerance in very low birth weight infants: double‐blind randomized trial. Przeglad Lekarski 2002;59(Suppl 1):63–6. [PUBMED: 12108078] [PubMed] [Google Scholar]
Muraro 2004
- Muraro A, Dreborg S, Halken S, Host A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part II. Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria of allergic diseases. Pediatric Allergy and Immunology 2004;15(3):196‐205. [DOI: 10.1111/j.1399-3038.2004.00128.x; PUBMED: 15209950] [DOI] [PubMed] [Google Scholar]
Muraro 2014
- Muraro A, Halken S, Arshad SH, Beyer K, Dubois AE, Du Toit G, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014;69(5):590‐601. [DOI] [PubMed] [Google Scholar]
Oddy 1999
- Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, et al. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ 1999;319(7213):815‐9. [PUBMED: 10496824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osterballe 2005
- Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev‐Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatric Allergy and Immunology 2005;16(7):567‐73. [DOI: 10.1111/j.1399-3038.2005.00251.x; PUBMED: 16238581] [DOI] [PubMed] [Google Scholar]
Peat 2000
- Peat JK, Toelle BG, Mellis CM. Problems and possibilities in understanding the natural history of asthma. Journal of Allergy and Clinical Immunology 2000;106(3 Suppl):S144‐52. [PUBMED: 10984395] [DOI] [PubMed] [Google Scholar]
Peat 2001
- Peat JK, Toelle BG, Marks GB, Mellis CM. Continuing the debate about measuring asthma in population studies. Thorax 2001;56(5):406‐11. [PUBMED: 11312411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prescott 2005
- Prescott SL, Tang ML, Australasian Society of Clinical Immunology and Allergy. The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children. Medical Journal of Australia 2005;182(9):464‐7. [PUBMED: 15865590] [DOI] [PubMed] [Google Scholar]
Ravault 2001
- Ravault C, Kauffmann F. Validity of the IUATLD (1986) questionnaire in the EGEA study. International Union Against Tuberculosis and Lung Disease. Epidemiological study on the genetics and environment of asthma, bronchial hyperresponsiveness and atopy. Journal of Tuberculosis and Lung Disease 2001;5(2):191‐6. [PUBMED: 11258514] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronmark 2001
- Ronmark E, Jonsson E, Platts‐Mills T, Lundback B. Incidence and remission of asthma in schoolchildren: report from the obstructive lung disease in northern Sweden studies. Pediatrics 2001;107(3):E37. [PUBMED: 11230618] [DOI] [PubMed] [Google Scholar]
Saarinen 1995
- Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow‐up study until 17 years old. Lancet 1995;346(8982):1065‐9. [PUBMED: 7564787] [DOI] [PubMed] [Google Scholar]
Saarinen 2000
- Saarinen KM, Savilahti E. Infant feeding patterns affect the subsequent immunological features in cow's milk allergy. Clinical & Experimental Allergy 2000;30(3):400‐6. [PUBMED: 10691899] [DOI] [PubMed] [Google Scholar]
Sampson 2004
- Sampson HA. Update on food allergy. Journal of Allergy and Clinical Immunology 2004;113(5):805‐19. [DOI: 10.1016/j.jaci.2004.03.014; PUBMED: 15131561] [DOI] [PubMed] [Google Scholar]
Schultz Larsen 1996
- Schultz Larsen F. Atopic dermatitis: an increasing problem. Pediatric Allergy and Immunology 1996;7(9 Suppl):51‐3. [PUBMED: 9156729] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sears 1996
- Sears MR, Holdaway MD, Flannery EM, Herbison GP, Silva PA. Parental and neonatal risk factors for atopy, airway hyper‐responsiveness, and asthma. Archives of Disease in Childhood 1996;75(5):392‐8. [PUBMED: 8957951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sears 1998
- Sears MR. Evolution of asthma through childhood. Clinical & Experimental Allergy 1998;28(Suppl 5):82‐91. [PUBMED: 9988452] [DOI] [PubMed] [Google Scholar]
Sly 1999
- Sly RM. Changing prevalence of allergic rhinitis and asthma. Annals of Allergy, Asthma and Immunology 1999;82(3):233‐48. [DOI: 10.1016/S1081-1206(10)62603-8; PUBMED: 10094214] [DOI] [PubMed] [Google Scholar]
Strachan 1996
- Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996;312(7040):1195‐9. [PUBMED: 8634562] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szajewska 2010
- Szajewska H, Horvath A. Meta‐analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Current Medical Research and Opinion 2010;26:423‐37. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Osborn 2003
- Osborn DA, Sinn JK. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003664] [DOI] [PubMed] [Google Scholar]
Osborn 2006a
- Osborn DA, Sinn JK. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003664.pub2] [DOI] [PubMed] [Google Scholar]
Osborn 2006b
- Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003664.pub3] [DOI] [PubMed] [Google Scholar]
Osborn 2017a
- Osborn DA, Sinn JK, Jones LJ. Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD003664.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2017b
- Osborn DA, Sinn JK, Jones LJ. Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD003664.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


