Abstract
Background
Chronic limb‐threatening ischaemia (CLTI) is a manifestation of peripheral arterial disease (PAD) that includes chronic ischaemic rest pain or ischaemic skin lesions, ulcers, or gangrene for longer than two weeks. The severity of the disease depends on the extent of arterial stenosis and the availability of collateral circulation. Treatment for CLTI aims to relieve ischaemic pain, heal ischaemic ulcers, prevent limb loss, improve quality of life, and prolong survival. CLTI due to occlusive disease in the infrapopliteal arterial circulation (below‐knee circulation) can be treated via an endovascular technique by a balloon opening the narrowed vessel, so called angioplasty, with or without the additional deployment of a scaffold made of metal alloy or other material, so called stenting. Endovascular interventions in the infrapopliteal vasculature may improve symptoms in patients with CLTI by re‐establishing in‐line blood flow to the foot. Controversy remains as to whether a balloon should be used alone to open the vessel, or whether a stent should also be deployed.
Objectives
To determine the efficacy and safety of percutaneous transluminal angioplasty (PTA) alone versus PTA with stenting of infrapopliteal arterial lesions (anterior tibial artery, posterior tibial artery, fibular artery (formerly known as peroneal artery), and common tibioperoneal trunk) for patients with chronic limb‐threatening ischaemia (CLTI).
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, and AMED databases, as well as World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 25 June 2018. We applied no language restrictions.
Selection criteria
We planned to include randomised or quasi‐randomised controlled trials comparing PTA versus PTA with a stent and including patients aged 18 years or over with CLTI. We defined CLTI as Fontaine stage III (ischaemic rest pain) and IV (ischaemic ulcers or gangrene) or consistent with Rutherford category 4 (ischaemic rest pain), 5 (minor tissue loss), and 6 (major tissue loss), with stenotic (> 50% luminal loss) or occluded infrapopliteal artery, including tibiofibular trunk, anterior tibial artery, posterior tibial artery, and fibular artery. We included all types of stents irrespective of design (e.g. bare‐metal, drug‐eluting, bio‐absorbable).
Data collection and analysis
Two review authors (CC‐TH and GNCK) independently selected suitable trials, assessed trial quality, and extracted data. An additional third review author (MLvD) assessed trial quality and, when necessary, acted as arbiter for study selection and data extraction. Outcomes included technical success of the procedure, procedural complications, patency, major amputation, and mortality. We assessed the quality of evidence using the GRADE approach.
Main results
We included in the review seven trials with 542 participants. One trial randomised limbs to undergo PTA alone or PTA with stent placement, and the remaining studies randomised participants. Five trials with 476 participants show that the technical success rate was greater in the stent group than in the angioplasty group (odds ratio (OR) 3.00, 95% confidence interval (CI) 1.14 to 7.93; 476 lesions; 5 studies; I² = 23%). Meta‐analysis of three eligible trials with 456 participants did not show a clear difference in short‐term (within six months) patency between infrapopliteal arterial lesions treated with PTA and those treated with PTA and stenting (OR 0.88, 95% CI 0.37 to 2.11; 456 lesions; 3 studies; I² = 77%). Results also did not show clear differences between treatment groups in procedure complication rate (OR 0.87, 95% CI 0.01 to 53.60; 360 participants; 5 studies; I² = 85%), rate of major amputations at 12 months (OR 1.34, 95% CI 0.56 to 3.22; 306 participants; 4 studies; I² = 0%), and rate of mortality at 12 months (OR 0.71, 95% CI 0.43 to 1.17; 497 participants; 6 studies; I² = 0%). Heterogeneity between studies was high for the outcomes procedure complications and primary patency. The overall methodological quality of the trials included in this review was moderate due to selection and performance bias. Studies used different regimens for pretreatment and post‐treatment antiplatelet/anticoagulant medication. We downgraded the certainty of the overall evidence for all outcomes by one level to moderate due to inconsistency of results across studies and large confidence intervals (small numbers of trials and participants).
Authors' conclusions
Trials show that the immediate technical success rate of restoring luminal patency is higher in the stent group but reveal no clear differences in short‐term patency at six months between infrapopliteal arterial lesions treated with PTA with stenting versus those treated with PTA without stenting. We ascertained no clear differences between groups in periprocedural complications, major amputation, and mortality. However, use of different regimens for pretreatment and post‐treatment antiplatelet/anticoagulant medication and the duration of its use within and between trials may have influenced the outcomes. Limited currently available data suggest that high‐quality evidence is insufficient to show that PTA with stent insertion is superior to use of standard PTA alone without stenting for treatment of infrapopliteal arterial lesions. Further studies should standardise the use of antiplatelets/anticoagulants before and after the intervention to improve the comparability of the two treatments.
Plain language summary
Angioplasty versus stenting for below‐knee arterial disease in people with chronic limb‐threatening ischaemia
Background
Chronic limb‐threatening ischaemia (CLTI) is a manifestation of peripheral arterial disease that occurs as chronic ischaemic rest pain or ischaemic skin lesions, ulcers, or gangrene with symptoms present for longer than two weeks. The symptoms are a result of impaired blood flow to the leg and the foot due to narrowing of the arteries by atherosclerosis. Atherosclerosis is a disease of the arteries caused by a buildup of plaque composed of fat, cholesterol, calcium, and other substances in the blood; over time, the plaque narrows the artery. Patients can have narrowing of the artery in the thigh or below the knee. This review focusses on a subgroup of patients with below‐knee arterial disease (infrapopliteal arterial disease) who might benefit from an intervention that re‐establishes blood flow by inserting and inflating a balloon to re‐open the narrowed artery (percutaneous transluminal angioplasty). This can be performed with or without additional placement of a stent (a scaffold made of metal alloy or other material). The types of stents used in this procedure vary from a simple bare‐metal stent to a stent coated with medication. However, it is not clear whether deploying stents after ballooning in narrowed below‐knee arteries (infrapopliteal arteries) provides any additional benefit for the patient.
Study characteristics and key results
We identified seven trials with a combined total of 542 participants comparing percutaneous transluminal angioplasty (PTA) alone versus PTA with stent placement (current until June 2018). One trial randomised limbs to PTA alone or PTA with stent placement, and the remaining studies randomised participants. Full analysis of five trials shows that the technical success rate of re‐opening the narrowed artery was higher in the stent group than in the PTA group. However, we noted no clear differences in patency (opened vessel remaining open) of the treated vessel at six months. The complication rate of the procedure, the number of major amputations at 12 months, and the number of deaths at 12 months also did not differ greatly between treatment groups.
Certainty of the evidence
The overall certainty of evidence provided by the trials included in this review was moderate. Trials differed in their methods. Two studies reported poorly on the methods used to generate random numbers and to allocate participants to different groups. All studies were unblinded. All included studies were rated as direct in their relevance to the review question. Overall, we downgraded the certainty of evidence for all outcomes by one level to moderate due to inconsistency of results across studies and the small numbers of studies and participants.
Conclusion
PTA with stent placement is better than PTA alone for restoring vessel patency immediately; however we found no clear difference in short‐term patency at six months between the two groups. Trials show no clear differences between groups in complications at or around the time of the procedure, major amputation, and death. Currently available data suggest that high‐certainty evidence is insufficient to show that PTA with stent placement is superior to PTA alone for treatment of infrapopliteal arterial lesions. Further studies should standardise the use of blood‐thinning drugs (antiplatelets/anticoagulants) before and after both interventions to improve the comparability of the two treatments.
Summary of findings
Summary of findings for the main comparison. PTA compared with stent for infrapopliteal arterial lesions in chronic limb‐threatening ischaemia.
| PTA compared with stent for infrapopliteal arterial lesions in chronic limb‐threatening ischaemia | ||||||
| Patient or population: people with infrapopliteal arterial lesions in chronic limb‐threatening ischaemia Setting: hospital and outpatient follow‐up Intervention: stent Comparison: PTA | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | Comments | ||
| PTA | Stent | Difference | ||||
| Technical success ITT No. of limbs: 476 (5 RCTs) | OR 3.00 (1.14 to 7.93) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 93.3% | 97.6% (94.0 to 99.1) | 4.4% more (0.8 more to 5.8 more) | ||||
| Technical success TA No. of limbs: 474 (5 RCTs) | OR 2.78 (1.04 to 7.41) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 93.7% | 97.6% (93.9 to 99.1) | 4.0% more (0.2 more to 5.4 more) | ||||
| Procedural complications ITT No. of participants: 360 (5 RCTs) | OR 0.87 (0.01 to 53.60) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 7.4% | 6.5% (0.1 to 81.1) | 0.9% fewer (7.3 fewer to 73.7 more) | ||||
| Procedural complications TA No. of participants: 359 (5 RCTs) | OR 0.84 (0.01 to 47.70) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 7.4% | 6.3% (0.1 to 79.3) | 1.1% fewer (7.4 fewer to 71.9 more) | ||||
| Primary patency < 6 months ITT No. of lesions: 456 (3 RCTs) | OR 0.88 (0.37 to 2.11) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 33.3% | 30.6% (15.6 to 51.3) | 2.8% fewer (17.7 fewer to 18 more) | ||||
| Primary patency < 6 months TA No. of lesions: 309 (3 RCTs) | OR 0.97 (0.32 to 3.00) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 45.9% | 45.2% (21.4 to 71.8) | 0.8% fewer (24.6 fewer to 25.9 more) | ||||
| Mortality TA No. of participants: 487 (6 RCTs) | OR 0.70 (0.42 to 1.15) | Study population | ⊕⊕⊕⊝ MODERATEa | |||
| 19.3% | 14.3% (9.1 to 21.5) | 5% fewer (10.2 fewer to 2.3 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; OR: odds ratio; PTA: percutaneous transluminal angioplasty; RCT: randomised controlled trial; TA: treatment analysis. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level due to inconsistency of results across different studies and imprecision (small numbers and wide confidence intervals).
Background
Description of the condition
Atherosclerosis is the most common cause of peripheral arterial disease (PAD) of the lower extremities. Chronic limb‐threatening ischaemia (CLTI), also known as critical limb ischaemia (CLI), is a manifestation of PAD that refers to the presence of chronic ischaemic rest pain or ischaemic skin lesions, ulcers, or gangrene, with symptoms present for longer than two weeks (Hirsch 2006; Norgren 2007). The severity of the disease depends on the extent of arterial stenosis and the availability of collateral circulation. Objective tests that support the diagnosis of CLTI include ankle‐brachial index (ABI), toe systolic pressure, and transcutaneous oxygen tension (Hirsch 2006; Norgren 2007). CLTI is listed as stage III and IV in the Fontaine classification and as categories 4, 5, and 6 in the Rutherford classification (Table 2). The incidence of CLTI is between 500 and 1000 new cases every year in a European or North American population of one million (Norgren 2007). The diagnosis of CLTI is associated with a poor prognosis for both amputation‐free survival and overall survival (Norgren 2007). The prognosis of a patient with CLTI one year after diagnosis is death in 20%, and the major amputation rate varies from around 10% to 40% (Dormandy 1999). Observational studies of patients with CLTI who are not candidates for revascularisation suggest that only about half of these patients will be alive without a major amputation a year after the onset of CLTI (Holdsworth 1997; Norgren 2007); some of them may still have rest pain, gangrene, or ulcers. Approximately 25% will have died, and 25% will have required a major amputation (Norgren 2007; Wolfe 1986).
1. Classification of peripheral arterial disease: Fontaine stages and Rutherford categories.
| Fontaine | Rutherford (adapted from table from Norgren 2007) | |||
| Stage | Clinical | Grade | Category | Clinical |
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate to severe claudication | I | 2 | Moderate claudication |
| I | 3 | Severe claudication | ||
| III | Ischaemic rest pain | II | 4 | Ischaemic pain at rest |
| IV | Ulceration or gangrene | III | 5 | Minor tissue loss |
| III | 6 | Major tissue loss | ||
Description of the intervention
Treatment for CLTI aims to relieve ischaemic pain, heal ischaemic ulcers, prevent limb loss, improve quality of life, and prolong survival (Norgren 2007). Interventions for CLTI may include conservative therapy, revascularisation, or amputation. Progressive gangrene, rapidly enlarging wounds, and continuous ischaemic rest pain often mandate the need for intervention. Although infrainguinal bypass surgery remains the cornerstone of CLTI treatment, not all patients are suitable candidates. Patients may lack a conduit or target, may be non‐ambulatory, or may have an extensive soft tissue infection overlying a bypass target. Most commonly, patients have medical comorbidities that make them unacceptable surgical candidates, given that the associated mortality rate is approximately 2% (Conte 2001).
How the intervention might work
Endovascular interventions in the infrapopliteal vasculature involve additional challenges of small‐calibre vessels and more diffuse atherosclerotic disease. Potential obstacles include early thrombosis and late luminal loss due to intimal hyperplasia formation, as well as complications of acute vessel occlusion, embolism, and vessel perforation during the procedure. A meta‐analysis of infrapopliteal percutaneous transluminal angioplasty (PTA) compared to popliteal‐to‐distal vein bypass surgery shows that the bypass graft had better primary and secondary patency, but that limb salvage was comparable for the two treatments, suggesting the potential of PTA for treating CLTI (Romiti 2008). Trials are providing increasing evidence to support a recommendation for morbid PTA patients with CLTI as a result of infrapopliteal artery lesions, provided that in‐line flow to the foot can be re‐established (Norgren 2007). Controversy remains as to whether primary stenting of infrapopliteal arteries should be performed in patients with CLTI to improve outflow or to increase patency of proximal endovascular interventions or bypass surgery. Currently, stenting is often reserved as a bailout option in cases of flow‐limiting dissection, residual stenosis, or elastic recoil.
Why it is important to do this review
Recent advancements in stent design and growing expertise of interventionalists have made it possible to treat complex lesions that were previously known to have inferior outcomes, including long‐segment lesions, those with eccentric calcification, unstable lesions, and occlusions. A variety of novel stent designs are available, ranging from bare‐metal, metal‐absorbable, carbofilm‐coated, bio‐absorbable stents to drug‐eluting stents. In particular, the drug‐eluting stent has demonstrated efficacy for inhibiting neo‐intimal hyperplasia in the coronary arteries, thereby reducing repeat revascularisation procedures, as compared with the standard bare‐metal coronary stent (Morice 2002; Moses 2003; Schofer 2003). Whether the efficacy of coronary technology can be translated to the infrapopliteal vasculature remains to be determined. We are interested to learn whether PTA with primary stenting offers advantages in improving outcomes compared with PTA alone. If sufficient data are available, this systematic review will also compare different stent designs.
Objectives
To determine the efficacy and safety of percutaneous transluminal angioplasty (PTA) alone versus PTA with stenting of infrapopliteal arterial lesions (anterior tibial artery, posterior tibial artery, fibular artery (formerly known as peroneal artery), and common tibioperoneal trunk) for patients with chronic limb‐threatening ischaemia (CLTI).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised controlled trials comparing PTA devices versus PTA with a stent. We made the distinction between PTA with the primary intention of stent placement versus PTA with stent placement as a secondary intention. Quasi‐randomised controlled trials use a method of allocating participants that is not truly random, for example, odd or even hospital number or date of birth, or they use alternation techniques to allocate treatment groups.
Types of participants
We included adults (aged 18 years or older) with chronic limb‐threatening ischaemia (CLTI). We defined CLTI as Fontaine stage III (ischaemic rest pain) and IV (ischaemic ulcers or gangrene) or consistent with Rutherford categories 4 (ischaemic rest pain), 5 (minor tissue loss), and 6 (major tissue loss), with stenotic (> 50% luminal loss) or occluded infrapopliteal artery, including tibiofibular trunk, anterior tibial artery, posterior tibial artery, and fibular artery. This review includes participants with type 1 and type 2 diabetes.
Types of interventions
Intervention: PTA with stenting
Comparison: PTA alone (with bailout stenting after suboptimal or complicated PTA)
We included all types of stents, irrespective of design (e.g. bare‐metal, drug‐eluting, bio‐absorbable).
Atherectomy was not permitted in either group.
Types of outcome measures
Primary outcomes
Technical success defined as absence of residual stenosis < 30% and absence of flow‐limiting dissection on final catheter angiogram
Procedural complications, including death as a direct result of the procedure, vascular injury requiring vascular repair by surgical or non‐surgical techniques, arterial dissection, major bleeding, stroke, myocardial infarction (MI), renal failure, retroperitoneal bleed, embolisation resulting in partial or total arterial occlusion, unplanned tibial or pedal bypass, major infection, compartment syndrome, acute renal failure, access site infection, groin haematoma, pseudoaneurysm, and arteriovenous fistula
Primary patency defined as < 50% loss of luminal diameter at the treated site (determined by computerised tomography (CT) angiogram, magnetic resonance (MR) angiogram, or Doppler ultrasound) without re‐intervention in the interim
Secondary patency reflecting the fate of initial and subsequent PTA procedures combined, and determined by CT/MR angiogram or Doppler ultrasound as either the absence of a haemodynamically significant re‐stenosis or > 50% re‐stenosis
Secondary outcomes
Major amputation
Mortality
Clinical outcome of the treated ischaemic leg based on Rutherford or Fontaine classification
Healed or persistent ulcers
Ankle‐brachial index (ABI) or toe‐brachial index (TBI)
Quality of life assessment
Search methods for identification of studies
We applied no language restrictions to the search.
Electronic searches
The Cochrane Vascular Information Specialist first searched the following databases for relevant trials on 22 March 2017.
Cochrane Vascular Specialised Register.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2), in the Cochrane Library, via the Cochrane Register of Studies (http://www.metaxis.com/CRSWeb/Index.asp).
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Information Specialist also searched the following trials registries on 22 March 2017 for details of ongoing and unpublished studies, using the terms 'popliteal' and 'stent'.
ClinicalTrials.gov (clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
International Standard Randomized Controlled Trials Number (ISRCTN) Register (http://www.isrctn.com/).
The Cochrane Vascular Information Specialist subsequently conducted systematic top‐up searches of the following databases.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched from 1 January 2017 to 25 June 2018).
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, via the Cochrane Register of Studies Online (CRSO; 2018, Issue 5).
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily, and Ovid MEDLINE®) (searched from 1 January 2017 to 25 June 2018).
Embase Ovid (searched from 1 January 2017 to 25 June 2018).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (searched from 1 January 2017 to 25 June 2018).
Allied and Complementary Medicine Database (AMED) Ovid (searched from 1 January 2017 to 25 June 2018).
The Information Specialist modelled search strategies for the listed databases on the search strategy designed for CENTRAL. When appropriate, review authors combined these strategies with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials (RCTs) and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6; Lefevbre 2011). We have provided search strategies for the major databases in Appendix 2.
The Information Specialist also performed top‐up searches of the following trials registries on 25 June 2018.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We also searched citations within identified studies.
Data collection and analysis
We identified all randomised or quasi‐randomised trials that compare PTA devices versus PTA with stenting of infrapopliteal arterial lesions (anterior tibial artery, posterior tibial artery, fibular artery, and common tibioperoneal trunk) for patients with chronic limb‐threatening ischaemia. We assessed outcome measures as follows: outcomes concerning technical success, long‐term occlusions, and adverse events. We assessed the primary outcome measures technical success and procedural complications within 30 days of the index intervention. We assessed the remaining outcome measures at intervals up to three months, up to six months, up to one year, and annually thereafter, when data were available. If researchers reported different time points, we also considered these.
Selection of studies
Two review authors (CC‐TH and GNCK) independently assessed studies identified for inclusion in this review using the criteria stated above. In the case of disagreement, a third review author (MLvD) acted as arbiter.
Data extraction and management
Two review authors (CC‐TH and GNCK) independently extracted data from the studies included in this review using a standard data extraction form. In cases of disagreement, a third review author (MLvD) acted as arbiter.
Assessment of risk of bias in included studies
Three review authors (CC‐TH, GNCK, and MLvD) assessed the risk of bias for each study as described in the Cochrane Handbook for Systematic Reviews of Interventions for each of the following domains (Higgins 2011).
Random sequence generation.
Allocation concealment.
Blinding (of participants, personnel, and outcome assessors).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
We expressed judgements for each ’Risk of bias’ domain as low, high, or unclear risk of bias. If researchers described and used appropriate and adequate methods, we assessed the risk as 'low'. We assessed the risk of bias as 'high' when available information described or suggested inadequate methods (e.g. non‐random methods of allocation). An 'unclear' risk of bias indicates that study authors provided insufficient information.
Measures of treatment effect
When dealing with dichotomous outcome measures, we calculated a pooled estimate of the treatment effect for each outcome across trials using the odds ratio (OR) (the odds of an outcome among treatment‐allocated participants to the corresponding odds among control participants) and the 95% confidence interval (CI). For continuous outcomes, we recorded either mean change from baseline for each group or mean post‐intervention values and standard deviations for each group. When appropriate, we then calculated a pooled estimate of treatment effect by calculating the mean difference and the 95% CI.
Unit of analysis issues
We did not include cross‐over trials in this review because researchers designated only a single treatment to each group. If treatment by percutaneous transluminal angioplasty (PTA) is successful, it is inappropriate to expose study participants to other forms of intervention (i.e. stenting). We considered cluster‐randomised trials, but, as the unit of analysis is the patient, we planned to make adjustments for clustering in the final analysis according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
In case of randomisation at the level of the limb, we considered outcome data for each limb separately. In case of randomisation per patient, we adjusted for clustering when considering outcome data per limb. As per guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we conducted the analysis at the same level as the allocation, using a summary measurement from each cluster when individual participant data were available.
Dealing with missing data
To enable an intention‐to‐treat (ITT) analysis, we sought data on the number of participants with each outcome event by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or was otherwise excluded from treatment or follow‐up. Review authors requested missing data from the original investigators, when necessary.
Assessment of heterogeneity
We assessed statistical heterogeneity in the meta‐analysis using the I² statistic and explored reasons for heterogeneity (Higgins 2011). Thresholds for interpretation of I² can be misleading because the importance of inconsistency depends on several factors. We used the rough guide to interpretation as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered a level of heterogeneity of 50% or greater as significant.
We planned to assess clinical heterogeneity by conducting subgroup analyses to stratify available data (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to investigate publication bias (referring to the phenomenon that studies with a positive outcome are more likely to be published) by using funnel plots if we identified 10 or more studies for inclusion in the review (Higgins 2011). We captured selective reporting of outcomes under Assessment of risk of bias in included studies.
Data synthesis
We planned to use a fixed‐effect model in our analysis. In cases of significant heterogeneity (I² > 50%), we pooled the data using a random‐effects model (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses with participants stratified by the following factors, if we had included five or more studies in the meta‐analysis.
Age 18 to 65 years and 65 years or older.
Gender.
Type 1 or type 2 diabetes.
Different stent designs: stents can be classified by the four parameters proposed by Nelken 2004 according to method of deployment, geometry, construction materials, and treated stents (coated stents and drug‐eluting stents) (Table 3).
Severity and extent of disease based on the TransAtlantic Inter‐Society Consensus II classification.
2. A proposed classification of stents by individual parameters.
| A proposed classification of stents by individual parameters (table from Nelken 2004) | |
| Deployment method | Balloon expandable/angioplasty or self‐expanding |
| Geometry | Closed cell, open cell, modified connectors; weave‐braided, knitted; spiral coil, helix |
| Construction materials | Stainless steel, 316L, full hard stainless; tantalum; platinum; nitinol; cobalt alloys; bio‐absorbable |
| Treated stents | Coated stents and drug‐eluting stents: metals, bound drugs (passivation), ceramics, polymers, drug‐eluting stents |
Sensitivity analysis
We planned to undertake sensitivity analysis to explore the impact of risk of bias on meta‐analysis of the overall estimate of effect by first entering only trials with adequate allocation concealment and blinding, and then gradually adding trials with high(er) risk of bias.
'Summary of findings'
We presented the main findings of the review concerning certainty of evidence, magnitude of effect of the interventions examined, and sum of available data for the outcomes technical success intention‐to‐treat analysis (ITT) and treatment analysis (TA), procedural complications ITT and TA, primary patency less than six months ITT and TA, and mortality TA in a 'Summary of findings' table, according to the GRADE principles, as described by Higgins 2011 and Atkins 2004. We evaluated evidence based on risk of bias of the included studies, inconsistency, indirectness and imprecision of the data, and publication bias. We used GRADEprofiler (GRADEpro) software to assist in preparation of the 'Summary of findings' table (www.gradepro.org).
Results
Description of studies
Results of the search
See Figure 1.
1.
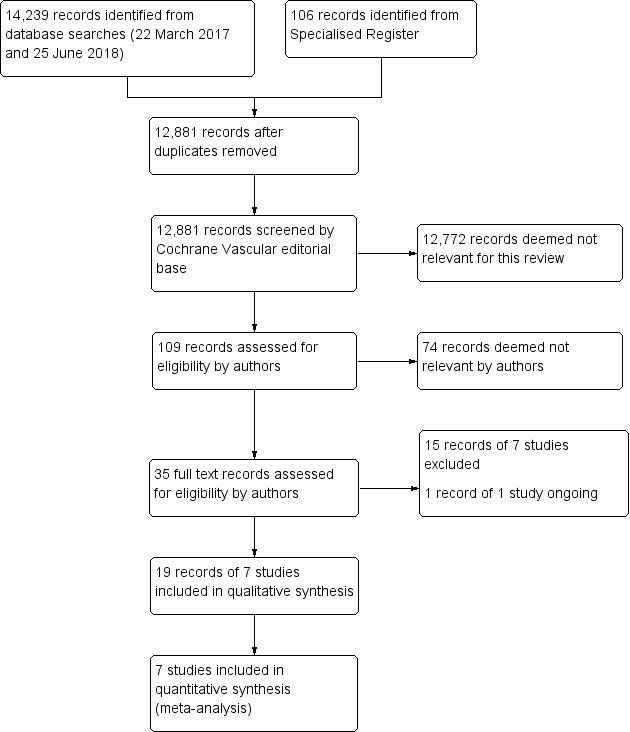
Study flow diagram.
Included studies
Seven randomised controlled trials met the criteria for inclusion (Bosiers 2009; Brodmann 2011; Rand 2006; Rand 2011; Randon 2010; Spreen 2016; Tepe 2010). All studies were performed at tertiary hospitals or through multi‐centre collaboration. The study population consisted of patients with symptomatic chronic limb ischaemia Fontaine stage III and IV (Rand 2006), Rutherford stage 4 to 5 (Bosiers 2009; Rand 2011), Rutherford stage 4 to 6 (Brodmann 2011; Randon 2010; Spreen 2016), and Rutherford stage 5 to 6 (Tepe 2010). Rand 2011 randomised limbs for treatment, and all remaining trials randomised participants but reported event rates at the level of arterial lesions or limbs. Age, gender, and risk factors of participants in the included trials were comparable. The stent material used in the stenting group was variable between studies but can be separated into drug‐eluting stents ‐ Spreen 2016 and Tepe 2010 ‐ versus non‐drug‐eluting stents ‐ Bosiers 2009,Brodmann 2011,Rand 2006,Rand 2011, and Randon 2010. Types of non‐drug‐eluting stents used in these trials also varied in terms of stent design and material: absorbable metal stent (Bosiers 2009), silicon‐carbide coating stent (Brodmann 2011), carbostent (Rand 2006; Rand 2011), and self‐expandable stent (Randon 2010). Also, use of dual antiplatelet therapy varied between control and experimental groups in individual trials and between trials. See Characteristics of included studies for further details.
Excluded studies
We excluded seven trials (Bosiers 2012; Bradbury 2010; Rastan 2011; Scheinert 2012; Schulte 2015; Siablis 2007; Siablis 2014).
Bosiers 2012 and Rastan 2011 compared two different stents. Bradbury 2010 presented a description of severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter‐Society Consensus II classification in the BASIL trial. Scheinert 2012, Schulte 2015, and Siablis 2014 included patients with symptomatic peripheral arterial disease and Rutherford stage 3 to 5 manifested in the infrapopliteal arterial territory, whereas in this review, we planned to include only patients with stage 4 disease and above. These studies did not provide data on the subgroup of patients with stage 4 or above disease; therefore we excluded them from the review. Siablis 2007 performed stenting as a bailout procedure for suboptimal angioplasty, and outcomes reflected a comparison between two different types of stents, which is not within the scope of this review.
See Characteristics of excluded studies.
Ongoing studies
We identified one ongoing study (NCT01644487).
Risk of bias in included studies
2.
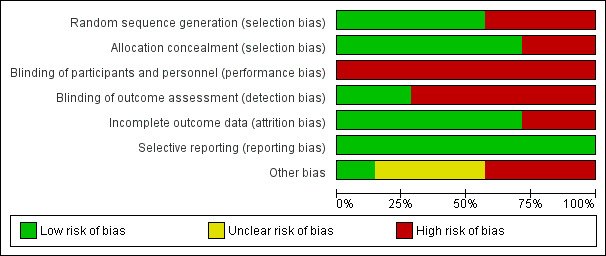
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
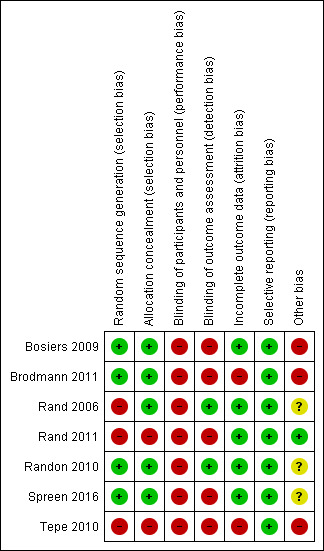
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies used computer‐generated randomisation procedures (Bosiers 2009; Brodmann 2011; Randon 2010; Spreen 2016). The others did not describe the generation process, and we classified them as having high risk of selection bias (Rand 2006; Rand 2011; Tepe 2010).
Five studies described concealment of allocation as using sealed envelopes (Bosiers 2009; Brodmann 2011; Rand 2006; Randon 2010; Spreen 2016). Two studies did not describe the allocation concealment process; we therefore classified them as having high risk of bias for this criterion (Rand 2011; Tepe 2010).
Blinding
None of the included studies described blinding of participants or doctors performing the intervention. Although blinding of the treating doctor is not possible in this context, blinding of participants could have been considered. Given the potential impact on the overall effect estimate associated with non‐blinding, even if this is not feasible in a given setting, we classified all studies as having high risk of bias in this domain.
Blinding of outcome assessment is feasible in the context of the studied interventions; however only two studies described blinding of the person assessing outcomes (Rand 2006; Randon 2010). We classified the remaining studies as having high risk of bias.
Incomplete outcome data
All included studies, except two (Brodmann 2011; Tepe 2010), accounted for all participants randomised in the study. We therefore classed these as having high risk of bias. Three studies performed ITT analysis (Bosiers 2009; Rand 2011; Spreen 2016), one study performed survival analysis (Rand 2006), and one study reported no losses to follow‐up (Randon 2010).
Selective reporting
All included studies reported on the outcomes they intended to measure; we therefore classed them as having low risk of reporting bias.
Other potential sources of bias
Conflicts of interest and funding
Bosiers 2009 was funded totally by BIOTRONIK AG, which was responsible for administration and monitoring of the study. Therefore, we classed this study as having high risk in this domain. Rand 2011 also received funding from the manufacturer of stents, but study authors described that they were completely in control of data analysis and publication; therefore, we classified this study as having low risk in this domain. Randon 2010 does not mention any support or conflict of interest; we therefore classified it as having unclear risk. Tepe 2010 mentioned that the study was supported by Eli Lilly but did not explicitly mention the independence of the research team; we therefore classified this study as having high risk.
Comparability of participants in groups
In Brodmann 2011, the two intervention groups were not comparable. This study was originally conceived as a multi‐centre trial, but it included participants from only one centre. Brodmann 2011 reported an imbalance in cardiovascular risk factors, with a higher percentage of baseline cardiovascular risk factors in the stent group than in the PTA group. This imbalance could reflect high risk of bias. Rand 2006 described administration of clopidogrel only to participants who received stents ‐ not to participants who underwent PTA alone. Study authors stated: "We also observed a higher incidence of PTAs than stent applications per patient. This might be due to a certain degree of investigator bias, as potentially one balloon can be used for several lesions in contrast to the necessity of one stent per lesion." We therefore judged Rand 2006 to be at unclear risk of other bias.
Effects of interventions
See: Table 1
Primary outcomes
Technical success
Technical success: ITT
The event rate for technical success is defined by success of treated infrapopliteal arterial lesions, with the exception of two trials (Brodmann 2011; Spreen 2016), which counted limbs ‐ Spreen 2016 ‐ and numbers of participants ‐ Brodmann 2011 ‐ respectively. Although Spreen 2016 provided data on participants, limbs, and lesions, Spreen 2016 excluded several participants post randomisation before they received the allocated treatment. Therefore, we report technical success in the ITT analysis with the limb as the unit of analysis. We included in the meta‐analysis five trials with a total of 476 lesions (Bosiers 2009; Rand 2006; Rand 2011; Randon 2010; Tepe 2010). The primary success rate was higher among stented lesions (odds ratio (OR) 3.00, 95% confidence interval (CI) 1.14 to 7.93; P = 0.03; 5 studies; I² = 23%; moderate‐certainty evidence). Research results are heavily weighted by one trial in which seven PTA without stenting group participants (7/57) with 11 lesions (11/75) crossed over to the PTA with stenting arm due to dissection in at least one of the lesions and, in the case of one participant, due to significant residual stenosis (Bosiers 2009). See Analysis 1.1.
1.1. Analysis.
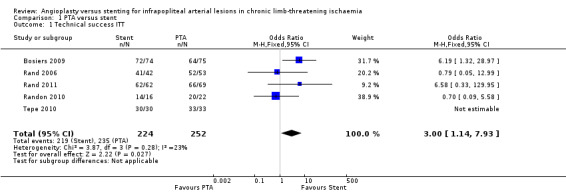
Comparison 1 PTA versus stent, Outcome 1 Technical success ITT.
In Brodmann 2011, the procedure was successful in 94% (31/33) of participants treated with PTA alone and in 100% (21/21) of those treated with PTA with stents. Two participants in the PTA alone group encountered extended dissections in the treated vessels requiring secondary stent placement.
Spreen 2016 provided data on participants, limbs, and numbers of lesions; however investigators excluded several participants from the study post randomisation. Therefore, we report technical success in the ITT analysis with limb as the unit of analysis. Spreen 2016 randomised 69 limbs to the PTA alone group and 75 limbs to the PTA with stent group. This study excluded three participants/three limbs post randomisation to the PTA alone group and one participant/one limb post randomisation to the PTA with stent group. Spreen 2016 treated 14 limbs in the PTA alone group with a bailout stent. Seven participants in the PTA alone group had > 50% stenosis or occlusion, and six in the stent group had > 50% stenosis or occlusion. Hence the success rate was 65% (45/69) in the PTA alone group and 91% (68/75) in the PTA with stent group.
Technical success: TA
The event rate for technical success is defined by success of treated infrapopliteal arterial lesions, with the exception of two trials (Brodmann 2011; Spreen 2016), which counted limbs ‐ Spreen 2016 ‐ and numbers of participants ‐ Brodmann 2011 ‐ respectively. Although Spreen 2016 provided data on participants, limbs, and lesions, study authors did not specify the number of lesions in the PTA alone group requiring bailout stenting. Therefore, we report technical success in the treatment analysis with the limb as the unit of analysis.
We included five trials with a total of 474 lesions reporting this outcome (Bosiers 2009; Rand 2006; Rand 2011; Randon 2010; Tepe 2010). The primary success rate was higher among stented lesions (OR 2.78, 95% CI 1.04 to 7.41; P = 0.04; 5 studies; I² = 15%; moderate‐certainty evidence). See Analysis 1.2.
1.2. Analysis.
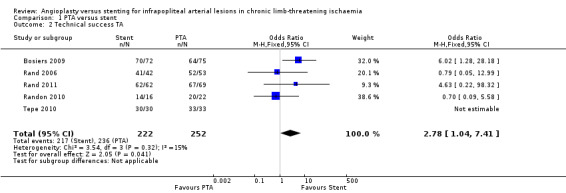
Comparison 1 PTA versus stent, Outcome 2 Technical success TA.
Brodmann 2011 reported a technical success rate of 94% (31/33) in the PTA alone group and 100% (21/21) in the PTA with stenting group. Two participants from the PTA alone group crossed over to the stent group due to arterial dissection of the treated vessels requiring stent placement.
Spreen 2016 randomised 69 limbs to the PTA alone group and 75 limbs to the PTA with stent group. Investigators excluded three participants/three limbs post randomisation to the PTA alone group and one participant/one limb post randomisation to the PTA with stent group. They treated 14 limbs in the PTA group with a bailout stent. Seven participants in the PTA alone group had > 50% stenosis or occlusion, and six in the PTA plus stent group had > 50% stenosis or occlusion. Hence the success rate was 65% (45/69) in the PTA alone group and 90% (68/75) in the PTA with stent group.
Procedural complications
Procedural complications: ITT
Procedural complications are reported per individual participant. We analysed five trials with 360 participants (Bosiers 2009; Brodmann 2011; Rand 2011; Randon 2010; Tepe 2010). Bosiers 2009, Rand 2011, and Tepe 2010 reported no procedural complications and found no clear differences between participants in PTA alone and PTA with stenting groups (OR 0.87, 95% CI 0.01 to 53.60; 360 participants; 5 studies; I² = 85%; moderate‐certainty evidence). See Analysis 1.3.
1.3. Analysis.
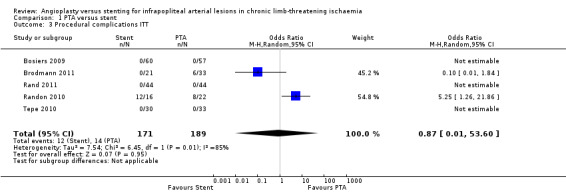
Comparison 1 PTA versus stent, Outcome 3 Procedural complications ITT.
Spreen 2016 reported procedural complications per limb: 22% (15/69 limbs) in the PTA group and 27% (20/75 limbs) in the stenting group. These complications included haematoma, material dysfunction, acute thrombosis, distal embolus, and pseudoaneurysm. Serious adverse events occurred in 22% (15/69 limbs) in the PTA alone group and in 20% (15/75 limbs) in the PTA with stenting group. These included gastrointestinal bleeding, ischaemic cerebral event and cerebral haemorrhage, pneumonia, cardiac disease, renal failure, and non‐CLTI‐related infection. Spreen 2016 reported that overall, the incidence of periprocedural complications and serious adverse events did not differ significantly between the two groups.
Rand 2006 reported one puncture site haematoma and one case of post‐procedural sepsis but did not specify in which group these complications occurred.
Procedural complications: TA
Procedural complications are reported per individual participant. We analysed five trials with 359 participants (Bosiers 2009; Brodmann 2011; Rand 2011; Randon 2010; Tepe 2010). We found no clear difference between participants in PTA alone and PTA with stenting groups (OR 0.84, 95% CI 0.01 to 47.70; moderate‐certainty evidence). Heterogeneity was significant (I² = 84%). Bosiers 2009, Rand 2011, and Tepe 2010 reported no cases of procedural complications. See Analysis 1.4.
1.4. Analysis.
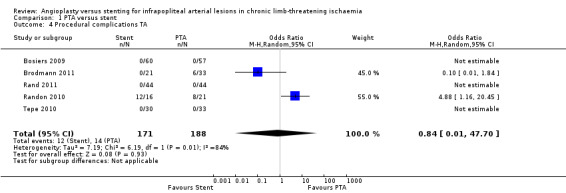
Comparison 1 PTA versus stent, Outcome 4 Procedural complications TA.
Spreen 2016 reported procedural complications per limb: 23% (15/66 limbs) in the PTA group and 27% (20/74 limbs) in the stenting group. Serious adverse events occurred in 23% (15/66 limbs) in the PTA group and in 20% (15/74 limbs) in the stenting group. We have reported details of these complications and serious adverse events in the section above.
Primary patency at six months
Primary patency at six months: ITT
We included three trials with a total of 456 lesions (Bosiers 2009; Rand 2006; Spreen 2016). We found no clear differences between PTA alone and PTA with stenting groups (OR 0.88, 95% CI 0.37 to 2.11; moderate‐certainty evidence). Heterogeneity was significant (I² = 77%). See Analysis 1.5.
1.5. Analysis.
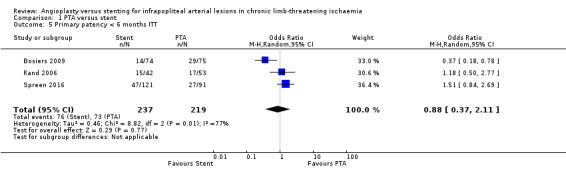
Comparison 1 PTA versus stent, Outcome 5 Primary patency < 6 months ITT.
Randon 2010 reported patency outcomes with the participant as the unit of measurement, and we did not include this study in the meta‐analysis. Cumulative primary and secondary patency rates were 76% and 85% at six months for the PTA alone group, and 80% and 91% at six months for the PTA with stenting group. Randon 2010 reported no significant differences in primary or secondary patency between the two treatment groups.
Primary patency at six months: TA
We included three trials with a total of 309 lesions (Bosiers 2009; Rand 2006; Spreen 2016). We found no clear differences between participants in PTA alone and PTA with stenting groups (OR 0.97, 95% CI 0.32 to 3.00; moderate‐certainty evidence). Heterogeneity was significant (I² = 82%). See Analysis 1.6.
1.6. Analysis.
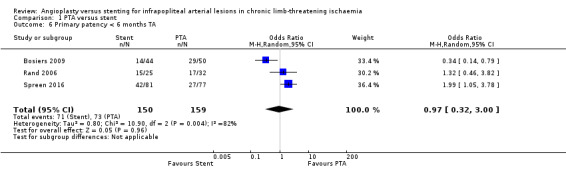
Comparison 1 PTA versus stent, Outcome 6 Primary patency < 6 months TA.
Brodmann 2011 reported primary patency per participant at six months, with 60.7% patency in the PTA alone group and 52.6% in the PTA with stent group.
Primary patency at 12 months: ITT
Most trials did not report data on patency of treated lesions beyond six months.
Brodmann 2011 reported primary patency per participant at 12 months, with 48.1% patency in the PTA alone group and 35.3% in the PTA with stent group.
Rand 2011 reported patency results at nine months: the minimal lumen diameter after nine months was not significantly different between the PTA alone group and the PTA with stent group. The percentage of residual diameter stenosis also was not significantly different: 43% in the PTA alone group versus 39% in the PTA with stent group. In addition, binary re‐stenosis for a 50% and a 70% threshold was not significantly different: 34.6% in the PTA alone group versus 23.8% in the PTA with stent group, and 15.4% in the PTA alone group versus 9.5% in the PTA with stent group, respectively.
Randon 2010 defined primary patency as clinical primary patency: this means freedom from re‐stenosis; occlusion with recurrence of ischaemic rest pain or recurrence of ulceration, leading to redo angioplasty; bypass surgery; or major amputation. Randon 2010 defined secondary patency as freedom from redo angioplasty until recurrence of symptoms. Randon 2010 reported that cumulative primary and secondary patency rates for the PTA alone group were 66% and 79.5% at 12 months, and primary and secondary patency rates for the PTA with stenting group were 56% and 64% at 12 months. Results show no clear differences in primary or secondary patency between the two groups.
Secondary patency
Two trials reported secondary patency after repeat angioplasty for re‐stenosis or recurrence of symptoms (Randon 2010; Brodmann 2011). In Randon 2010, cumulative secondary patency rates for the PTA alone group were 85% at six months and 79.5% at 12 months. Secondary patency rates for the PTA with stenting group were 91% at six months and 64% at 12 months. Results show no clear differences in primary or secondary patency between the two treatment groups. Brodmann 2011 also reported on secondary patency, with no reported differences between PTA alone and PTA with stenting groups at six months, but at 12 months, patency rates were 70.4% in the PTA alone group and 52.9% in the PTA with stent group.
Secondary outcomes
Major amputations < 12 months after the index intervention
Major amputations < 12 months after the index intervention: ITT
We analysed four trials with 306 participants (Bosiers 2009; Rand 2011; Randon 2010; Tepe 2010); we found no clear differences in major amputations between PTA alone and PTA with stenting groups (OR 1.34, 95% CI 0.56 to 3.22).
Spreen 2016 reported a major amputation rate of 38% (13/69 limbs) in the PTA alone group and 20% (8/75 limbs) in the PTA with stenting group. Brodmann 2011 reported minor amputations only. Rand 2006 reported one major amputation in a participant undergoing stent application.
Major amputations < 12 months after the index intervention: TA
We analysed four trials with 252 participants (Bosiers 2009; Rand 2011; Randon 2010; Tepe 2010); we found no clear differences in major amputations between PTA alone and PTA with stenting groups (OR 1.41, 95% CI 0.59 to 3.40).
Mortality within 12 months after the index intervention
Mortality within 12 months after the index intervention: ITT
We analysed six trials with 497 participants (Bosiers 2009; Brodmann 2011; Rand 2011; Randon 2010; Spreen 2016; Tepe 2010); we noted no clear differences in mortality between PTA alone and PTA with stenting groups (OR 0.71, 95% CI 0.43 to 1.17; moderate‐certainty evidence).
Rand 2006 reported one death but did not specify the treatment group in which this occurred.
Mortality within 12 months after the index intervention: TA
We analysed six trials with 487 participants (Bosiers 2009; Brodmann 2011; Rand 2011; Randon 2010; Spreen 2016; Tepe 2010); we found no clear differences between participants in PTA alone and PTA with stenting groups (OR 0.7, 95% CI 0.42 to 1.15).
Clinical outcome of the treated ischaemic leg using the Rutherford or Fontaine classification at < six months and at < 12 months
Brodmann 2011 reported improvement by at least one Rutherford category in a total of 33 (75.0%) participants at 12 months: 22 (81.5%) in the PTA alone group and 11 (64.7%) in the PTA with stent group (P value as reported by study authors = NS).
Bosiers 2009 reported six‐month clinical status of participants by the evolution of the Rutherford category. Investigators reported improvement by at least one Rutherford category in 65.9% (27/41) in the PTA alone group and in 69.2% (27/39) in the PTA with stent group with no statistically significant differences between groups by either ITT or treatment analysis.
In Rand 2011, clinical results based on the American Heart Association Clinical Improvement Score show clinical improvement at three months in 20 of 32 participants (62.5%) in the PTA alone group. Twelve of the 32 participants (37.5%) had clinical worsening or remained stable. The PTA with stent group shows clinical improvement in 27 of 33 participants (81.8%) and clinically worsening or stable disease in six participants (18.2%). At nine months' follow‐up, the PTA alone group included 24 participants and the PTA with stent group included 19 participants. At nine months, 14 of 24 participants (58.3%) in the PTA alone group show improved clinical status, and the remaining 10 participants (41.7%) show clinical worsening or remain stable. The PTA with stent group shows nine of 19 participants (47.4%) with clinical improvement and 10 of 19 participants (52.6%) with clinically worsening or stable disease.
The remaining included studies did not report on the clinical outcome of the treated ischaemic leg using the Rutherford or Fontaine classification at < six months and at < 12 months.
Healed or persistent ulcers at < six months' and at < 12 months' follow‐up
Brodmann 2011 reported that complete ulcer healing at 12 months was evident in 21 (63.6%) participants: 16 (80.0%) treated with PTA alone and five (38.5%) treated with PTA with stenting became ulcer free (P as reported by study authors = 0.006).
Tepe 2010 described general reduction in mean ulcer size (cm²) in both PTA alone and PTA with stent groups without performing statistical analysis. In the PTA alone group (PTA with or without abciximab), mean ulcer sizes were 8.4 cm² and 15 cm², respectively, at baseline; 2.9 cm² and 13 cm² at two months; and 0.63 cm² and 1 cm² at nine months. In the PTA with stent group (bare‐metal stent and drug‐eluting stent), mean ulcer sizes were 48.7 cm² and 11.6 cm², respectively, at baseline; 39.1 cm² and 5.3 cm² at two months; and 32.1 cm² and 2.9 cm² at nine months.
The remaining included studies did not report on healed or persistent ulcers at < six months' and < 12 months' follow‐up.
Ankle‐brachial index (ABI) or toe‐brachial index (TBI) at < six months' and < 12 months' follow‐up
Rand 2011 reported that ABIs at three months were 0.7 ± 0.3 for the PTA alone group and 0.9 ± 0.1 for the PTA with stent group (no significant difference). At nine months, they were 0.8 ± 0.3 for the PTA alone group and 0.8 ± 0.1 for the PTA with stent group (no significant differences).
Spreen 2016 reported significant improvement in mean ABIs and toe pressure after six months and after 12 months among survivors of both treatment groups compared with baseline (P ≤ 0.005). Spreen 2016 also reported that these improvements were comparable in both treatment groups.
Bosiers 2009 reported that ABIs at baseline were 0.7 ± 0.3 for the PTA alone group and 0.8 ± 0.5 for the PTA with stent group. At 24 hours after endovascular treatment, ABIs increased significantly to 1.0 ± 0.2 in the PTA alone group and 1.0 ± 0.4 in the PTA with stent group. At six months, they were 0.9 ± 0.3 for the PTA alone group and 0.9 ± 0.4 for the PTA with stent group (no significant differences).
Quality of life assessment
Included trials did not perform this assessment.
Long‐term follow‐up
Very limited follow‐up data are available beyond the 12‐month period. Spreen 2017 published long‐term clinical outcomes of the PADI trial (Spreen 2016). Unfortunately, Spreen 2017 did not separate out participants who had only PTA and those who had PTA with bailout bare‐metal stent. Nevertheless, limited available results show higher primary patency rates after the drug‐eluting stent compared with PTA with or without a bailout bare‐metal stent at one, three, and four years' follow‐up. The five‐year major amputation rate was lower in the drug‐eluting stent group than in the PTA with or without bailout bare‐metal stent group (19.3% vs 34.0%; P = 0.091). Overall survival rates were comparable (Spreen 2017).
Subgroup analyses, sensitivity analyses, and assessment of publication bias
We did not perform subgroup analyses, as included trials did not provide data specific for age, gender, diabetes status, or TransAtlantic Inter‐Society Consensus II classification. However, we did perform sensitivity analysis by removing studies deemed at high risk of bias, such as Rand 2011 and Tepe 2010. However, this did not change the conclusion for relevant outcomes.
We did not perform subgroup analysis for different types of stents. Although stents used in the trial can be broadly separated into drug‐eluting stents ‐ Spreen 2016 and Tepe 2010 ‐ and non‐drug‐eluting stents ‐ Bosiers 2009, Brodmann 2011, Rand 2006, Rand 2011, and Randon 2010 ‐ the types of non‐drug‐eluting stents used vary significantly in material and design, and included absorbable metal stents (Bosiers 2009), silicon‐carbide coating stents (Brodmann 2011), carbostents (Rand 2006; Rand 2011), and self‐expandable stents (Randon 2010). Pooling these varied types of non‐drug‐eluting stents into a single group was not considered appropriate. Last, the small number of participants included in these trials limits the ability of researchers to detect subgroup effects.
Discussion
Summary of main results
Our review shows that technical success is significantly greater in the percutaneous transluminal angioplasty (PTA) with stent group than in the PTA alone group. This may due in part to the use of stenting as a bailout solution to arterial dissection, which is a complication of PTA, as demonstrated in the heavily weighted Bosiers 2012 trial. Overall, we found no clear differences in complication rates between PTA with stent and PTA alone groups, but heterogeneity between studies was significant. Similarly, we observed no clear differences in short‐term patency at six months between the two treatment groups. Very few trials reported longer‐term follow‐up (up to 12 months), and only Brodmann 2011, Rand 2011, and Randon 2010 provide data on patency at 12 months. These studies do not show a clear difference in patency between the two treatment groups. Rates of major amputation and mortality were not significantly different between the two treatment groups. We performed sensitivity analysis by removing studies at high risk of bias, such as Rand 2011 and Tepe 2010, but this analysis did not alter our overall conclusions.
Overall completeness and applicability of evidence
Most of the trials included in this review provided only short‐term follow‐up of up to six months. Only three trials provided follow‐up data on long‐term patency extending to 12 months. Although long‐term durability of the stent and long‐term patency of the treated lesion remain unknown due to the inherent high morbidity and mortality of cardiovascular risk factors associated with chronic limb‐threatening ischaemia, the benefit of achieving short‐term vessel patency may still be clinically relevant. Last, trials show inconsistency in the use of periprocedural anticoagulation and the use of oral anticoagulation or antiplatelet medications post treatment. The PTA with stenting group was more likely to receive antiplatelet medications post treatment, as clinicians have the added burden of preventing stent re‐stenosis in these patients compared to those treated with PTA alone. These confounding variables could influence the outcome of patency.
Not all studies reported on all outcomes, and pooled analysis was not always possible, for example, for secondary patency.
Quality of the evidence
The overall methodological quality of the included studies was moderate. Studies generally reported poorly on methods used to allocate participants to different study groups. All studies were unblinded, but this can be justified by the nature of the intervention, as it is not possible to ensure blinding of doctors performing the angioplasty or placing the stent. Theoretically, it could be possible to blind participants or outcome assessors to the intervention performed; however, none of the included studies described blinding of participants and/or outcome assessors. Most trials poorly reported conflicts of interest and details of financial support.
Outcomes of the included studies are relevant and are generalisable to the clinical population, hence we found no serious indirectness. We considered moderate to severe heterogeneity as inconsistency of results, and we found serious inconsistency for outcomes concerning technical success rate, complications, and six‐month patency. We therefore downgraded the certainty of evidence by one level to moderate for all outcomes due to inconsistency of results across different studies and imprecision (small numbers and wide confidence intervals).
A major confounder is inconsistency in the use of anticoagulation and antiplatelet medications between PTA alone and PTA with stenting groups, as well as between trials. Established evidence suggests that patients with peripheral vascular disease treated by angioplasty or stenting would benefit from receiving aspirin at a dose of 50 mg to 300 mg daily, started before angioplasty or stenting and continued for at least two years or given lifelong (Robertson 2012). On the other hand, proven benefit of clopidogrel or dual antiplatelet or anticoagulant use in patients undergoing peripheral vascular interventions has not been definitively established.
Last, only Rand 2011 randomised limbs; all other included trials randomised at the participant level but reported outcomes at the level of arterial lesions or limbs. Such trials do not adjust for the non‐independence between arterial lesions within the same patient (e.g. by applying cluster analysis). In our meta‐analysis, we did not use generalised estimated equations, as individual participant data were not available to us. Therefore, we may have overestimated the estimated effects of treatments. However, given that the number of participants with bilateral lesions is small, we assume that the unit of analysis error does not have a major impact on the overall result. Please refer to Characteristics of included studies for details on bilateral lesions.
Potential biases in the review process
The Cochrane Vascular Information Specialisist searched multiple databases and trials registers to identify trials for this review. The review authors also independently searched references lists in other studies and reviews, and it is likely that we have included in this review all major trials on this subject matter. However, it is possible that despite extensive searches in multiple databases, we may have missed relevant studies for inclusion.
Two review authors independently performed all data selection and extraction with consultation from a third review author to ensure completeness and to exclude bias and error.
Different trials reported outcomes at different time intervals, and to allow meta‐analysis, we have pooled data from various time frames within the prespecified periods of six months and 12 months. For example, researchers assessed primary vessel patency at two, three, six, nine, and 12 months. We pooled the data into two separate time frames ‐ six months and 12 months. Similarily, we assessed cumulative mortality and major amputation for all trials at 12 months. This may have created bias in favour of studies reporting outcomes at earlier time points, as it is possible that adverse or unintended outcomes might have occurred later and would not have been captured.
Agreements and disagreements with other studies or reviews
We identified an existing systematic review and meta‐analysis that compared the role of drug‐eluting stents versus angioplasty or bare‐metal stents in infrapopliteal arterial disease (Fusaro 2013). This review identified five trials, but four of these trials compared drug‐eluting stents versus bare‐metal stents, and this comparison is not relevant to the objective of our review. The only trial that is relevant to our objective is Tepe 2010, which we included in our analysis.
The Yang 2014 review included 16 studies, nine of which were retrospective studies, four prospective non‐randomised studies, and three randomised controlled trials (RCTs). Two of the included RCTs are not relevant to our topic, as investigators compared drug‐eluting stents versus bare‐metal stents (Bosiers 2012; Rastan 2011). We identified one of the included RCTs through our search, but we excluded it from our analysis (Scheinert 2012). This trial included Rutherford stage 3 to 5 infrapopliteal arterial disease without providing data for stages 2, 4, and 5 separately. In our review, we planned to include only patients with stage 4 disease and above.
Another systematic review and meta‐analysis shows close resemblance to our review (Wu 2014). Wu 2014 included six prospective RCTs (Bosiers 2009; Brodmann 2011; Rand 2006; Rand 2011; Randon 2010; Scheinert 2012). Our review includes these trials and has added two other trials (Spreen 2016; Tepe 2010), although we excluded Scheinert 2012 for the reasons mentioned above. Outcomes assessed in the Wu 2014 review include immediate technical success, primary and secondary patency, limb salvage, and patient survival (assessed at six‐month and 12‐month intervals). However, Wu 2014 did not include procedural complications in its analysis and found that immediate technical success was greater in the PTA with stent group (96.2%) than in the PTA alone group (93.3%), but this finding was not statistically significant (odds ratio (OR) 0.59, 95% confidence interval (CI) 0.24 to 1.47). In contrast, we found a difference in the immediate technical success rate. The difference in conclusions between these reviews is likely to be influenced by differences among included trials. In relation to patency at six months, Wu 2014 analysed four studies and showed no significant differences between the PTA alone group of 73.4% and the PTA with stent group of 75.9% (OR 0.94, 95% CI 0.48 to 1.8) (Bosiers 2009; Rand 2006; Rand 2011; Randon 2010). We analysed Bosiers 2009, Rand 2006, and Spreen 2016 and revealed a similar result of no clear differences in primary patency at six months. In our review, we analysed cumulative mortality at 12 months, whereas Wu 2014 analysed patient survival at six months and 12 months; both reviews found no clear differences between the two treatment groups.
Authors' conclusions
Implications for practice.
Our meta‐analysis of five trials (four trials with estimable data) including participants with chronic limb‐threatening ischaemia (CLTI) shows a greater technical success rate in the PTA with stent group than in the PTA alone group but no clear differences in short‐term patency (at six months) between infrapopliteal arterial lesions treated with PTA alone and those treated with PTA in combination with stenting. We found no clear differences in complication rates between PTA and PTA with stent groups. Overall, the 12‐month major amputation rate and the mortality rate are not clearly different between PTA and PTA with stent groups. However, the use of different regimens for pretreatment and post‐treatment antiplatelet medication, such as clopidogrel, and the duration of its use within and between trials may have influenced the outcome. Based on limited currently available data and on the results of this meta‐analysis, high‐quality evidence is insufficient to suggest that stent insertion is superior to standard PTA alone without stenting for treatment of infrapopliteal arterial lesions. Stent insertion could be reserved for use as a 'bailout' procedure when arterial dissection is encountered.
Implications for research.
More consistent trial reporting is needed on both randomisation of limbs as the unit of analysis and use of antiplatelet and anticoagulant treatment before and after the intervention. Future trials should use limbs as the unit of allocation and reporting, and use of antiplatelet and anticoagulant treatment before and after the intervention should be standardised in upcoming trials. Future trials also must implement standardised reporting of outcomes and time intervals for re‐assessment; this will allow better comparison of data between trials.
Acknowledgements
We thank the Cochrane Vascular editorial staff for assistance and guidance.
Appendices
Appendix 1. CENTRAL search strategy, 22 March 2017
| Search run on Wed Mar 22 2017 | ||
| #1 | MESH DESCRIPTOR Arteriosclerosis | 869 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 72 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 645 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 737 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 726 |
| #7 | MESH DESCRIPTOR Ischemia | 803 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2236 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9508 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 8384 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3533 |
| #12 | (claudic* or IC):TI,AB,KY | 3229 |
| #13 | (isch* or CLI):TI,AB,KY | 24787 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 11 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 99 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 158 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 82 |
| #19 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1113 |
| #20 | MESH DESCRIPTOR Popliteal Artery | 282 |
| #21 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #22 | (((poplite* or fempop* or infrapopliteal or tibial or tibiofibular or peroneal) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 244 |
| #23 | (below knee):TI,AB,KY | 253 |
| #24 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 | 45012 |
| #25 | MESH DESCRIPTOR Angioplasty EXPLODE ALL TREES | 4177 |
| #26 | (angioplas* or percutan* or PTA or venoplasty):TI,AB,KY | 14283 |
| #27 | (recanali* or revascular*):TI,AB,KY | 7840 |
| #28 | dilat*:TI,AB,KY | 7797 |
| #29 | (balloon or baloon):TI,AB,KY | 7197 |
| #30 | MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES | 6721 |
| #31 | endovascular:TI,AB,KY | 1653 |
| #32 | MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES | 412 |
| #33 | MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES | 408 |
| #34 | MESH DESCRIPTOR Stents EXPLODE ALL TREES | 3323 |
| #35 | (stent* or graft* or endograft* or endoprosthe*):TI,AB,KY | 25857 |
| #36 | powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex | 591 |
| #37 | #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 | 48585 |
| #38 | #24 AND #37 | 10184 |
| #39 | coronary:TI | 17747 |
| #40 | renal:TI | 12744 |
| #41 | myocardial:TI | 11080 |
| #42 | heart:TI | 16995 |
| #43 | (carotid OR cerebral OR stroke):TI | 26794 |
| #44 | #39 OR #40 OR #41 OR #42 OR #43 | 79250 |
| #45 | #38 NOT #44 | 4490 |
Appendix 2. Database searches, 25 June 2018
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Arteriosclerosis 946 #2 MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 78 #4 MESH DESCRIPTOR Atherosclerosis 1057 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 818 #6 MESH DESCRIPTOR Intermittent Claudication 823 #7 MESH DESCRIPTOR Ischemia 1529 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2772 #9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY 12059 #10 ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 10524 #11 (peripheral near3 dis*):TI,AB,KY 4805 #12 (claudic* or IC):TI,AB,KY 4059 #13 (isch* or CLI):TI,AB,KY 31792 #14 arteriopathic:TI,AB,KY 7 #15 dysvascular*:TI,AB,KY 20 #16 (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 130 #17 (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 218 #18 ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 106 #19 MESH DESCRIPTOR Leg EXPLODE ALL TREES 2795 #20 MESH DESCRIPTOR Popliteal Artery 301 #21 MESH DESCRIPTOR Tibial Arteries 37 #22 ((poplite* or fempop* or infrapopliteal or tibial or tibiofibular or peroneal) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 340 #23 (below knee):TI,AB,KY 299 #24 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 58387 #25 MESH DESCRIPTOR Angioplasty EXPLODE ALL TREES 4285 #26 (angioplas* or percutan* or PTA or venoplasty):TI,AB,KY 17853 #27 (recanali* or revascular*):TI,AB,KY 9723 #28 dilat*:TI,AB,KY 9421 #29 (balloon or baloon):TI,AB,KY 8392 #30 MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES 7420 #31 endovascular:TI,AB,KY 2483 #32 MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES 430 #33 MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES 432 #34 MESH DESCRIPTOR Stents EXPLODE ALL TREES 3725 #35 (stent* or graft* or endograft* or endoprosthe*):TI,AB,KY 32859 #36 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex ):TI,AB,KY 791 #37 #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 61169 #38 #24 AND #37 13389 #39 coronary:TI 20963 #40 renal:TI 15210 #41 myocardial:TI 12539 #42 heart:TI 20907 #43 (carotid OR cerebral OR stroke):TI 32775 #44 #39 OR #40 OR #41 OR #42 OR #43 95337 #45 #38 NOT #44 6176 #46 01/01/2017 TO 25/06/2018:CD 292648 #47 #45 AND #46 1911 |
1911 |
| Clinicaltrials.gov | peripheral artery disease OR pvd | Angioplasty OR stent OR stenting OR Endovascular Procedures | Start date on or after 01/01/2017 | Last update posted on or before 06/26/2018 | 42 |
| ICTRP Search Portal | peripheral artery disease OR pvd | Angioplasty OR stent OR stenting OR Endovascular Procedures | 16 |
| MEDLINE | 1 ARTERIOSCLEROSIS/ 56443 2 exp ARTERIOLOSCLEROSIS/ 149 3 Arteriosclerosis Obliterans/ 3974 4 ATHEROSCLEROSIS/ 30942 5 Arterial Occlusive Diseases/ 26481 6 Intermittent Claudication/ 7594 7 ISCHEMIA/ 47483 8 exp Peripheral Vascular Diseases/ 50011 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 170972 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 142839 11 (peripheral adj3 dis*).ti,ab. 37713 12 (claudic* or IC).ti,ab. 61912 13 (isch* or CLI).ti,ab. 345304 14 arteriopathic.ti,ab. 162 15 dysvascular*.ti,ab. 216 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 707 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1808 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1478 19 exp LEG/bs [Blood Supply] 25021 20 Popliteal Artery/ 8971 21 Tibial Arteries/ 1482 22 ((poplite* or fempop* or infrapopliteal or tibial or tibiofibular or peroneal) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2222 23 below knee.ti,ab. 2705 24 or/1‐23 771207 25 exp ANGIOPLASTY/ 59069 26 (angioplas* or percutan* or PTA or venoplasty).ti,ab. 158270 27 (recanali* or revascular*).ti,ab. 63853 28 dilat*.ti,ab. 132027 29 (balloon or baloon).ti,ab. 58152 30 exp Endovascular Procedures/ 106227 31 endovascular.ti,ab. 41162 32 exp Blood Vessel Prosthesis/ 27389 33 exp Blood Vessel Prosthesis Implantation/ 20589 34 exp STENTS/ 68512 35 (stent* or graft* or endograft* or endoprosthe*).ti,ab. 379475 36 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex).ti,ab. 118081 37 or/25‐36 858143 38 24 and 37 118933 39 coronary.ti. 187093 40 renal.ti. 255164 41 myocardial.ti. 143393 42 heart.ti. 279300 43 (carotid or cerebral or stroke).ti,ab. 565188 44 or/39‐43 1350042 45 38 not 44 65413 46 randomized controlled trial.pt. 462606 47 controlled clinical trial.pt. 92454 48 randomized.ab. 414104 49 placebo.ab. 189646 50 drug therapy.fs. 2024675 51 randomly.ab. 292381 52 trial.ab. 430649 53 groups.ab. 1805468 54 or/46‐53 4223684 55 exp animals/ not humans.sh. 4466015 56 54 not 55 3651071 57 45 and 56 12116 58 (2017* or 2018*).ed. 1396008 59 57 and 58 931 |
931 |
| Embase | 1 arteriosclerosis/ 33965 2 exp arteriolosclerosis/ 598 3 peripheral occlusive artery disease/ 33214 4 atherosclerosis/ 136080 5 peripheral occlusive artery disease/ 33214 6 intermittent claudication/ 9762 7 ischemia/ 76632 8 exp peripheral vascular disease/ 1659635 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 236192 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 197030 11 (peripheral adj3 dis*).ti,ab. 54293 12 (claudic* or IC).ti,ab. 62619 13 (isch* or CLI).ti,ab. 501091 14 arteriopathic.ti,ab. 206 15 dysvascular*.ti,ab. 239 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 987 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2663 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2085 19 popliteal artery/ 8511 20 tibial artery/ 2626 21 ((poplite* or fempop* or infrapopliteal or tibial or tibiofibular or peroneal) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 3292 22 below knee.ti,ab. 3440 23 or/1‐22 2005601 24 exp angioplasty/ 82655 25 angioplas*.ti,ab. 56519 26 (recanali* or revascular*).ti,ab. 98197 27 (balloon or baloon).ti,ab. 89610 28 exp endovascular surgery/ 30698 29 endovascular.ti,ab. 60580 30 exp blood vessel prosthesis/ 13367 31 exp stent/ 152155 32 (stent* or graft* or endograft* or endoprosthe*).ti,ab. 525915 33 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex).ti,ab. 77069 34 or/24‐33 819330 35 23 and 34 268004 36 coronary.ti. 248958 37 renal.ti. 321018 38 myocardial.ti. 187858 39 heart.ti. 353011 40 (carotid or cerebral or stroke).ti. 355251 41 or/36‐40 1382539 42 35 not 41 155933 43 randomized controlled trial/ 506719 44 controlled clinical trial/ 460124 45 random$.ti,ab. 1312412 46 randomization/ 78443 47 intermethod comparison/ 236277 48 placebo.ti,ab. 273953 49 (compare or compared or comparison).ti. 470472 50 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1757754 51 (open adj label).ti,ab. 64655 52 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 209368 53 double blind procedure/ 151022 54 parallel group$1.ti,ab. 21843 55 (crossover or cross over).ti,ab. 93145 56 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 283623 57 (assigned or allocated).ti,ab. 332735 58 (controlled adj7 (study or design or trial)).ti,ab. 295615 59 (volunteer or volunteers).ti,ab. 224740 60 trial.ti. 251607 61 or/43‐60 4046836 62 42 and 61 33396 63 (2017* or 2018*).em. 3617606 64 62 and 63 6301 |
6301 |
| CINAHL | S55 S53 AND S54 416 S54 EM 2017 OR EM 2018 367,971 S53 S40 AND S52 3,559 S52 S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 341,911 S51 MH "Random Assignment" 38,635 S50 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 32,720 S49 MH "Crossover Design" 11,198 S48 MH "Factorial Design" 919 S47 MH "Placebos" 8,351 S46 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,480 S45 TX crossover OR "cross‐over" 14,551 S44 AB placebo* 28,287 S43 TX random* 218,886 S42 TX trial* 250,284 S41 TX "latin square" 142 S40 33 NOT 39 15,078 S39 S34 OR S35 OR S36 OR S37 OR S38 136,026 S38 TI carotid OR cerebral OR stroke 50,135 S37 TI heart 45,069 S36 TI myocardial 15,858 S35 TI coronary 27,524 S34 S23 AND S33 12,767 S33 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 72,689 S32 TX powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex 4,750 S31 TX stent* or graft* or endograft* or endoprosthe* 34,651 S30 (MH "Stents+") 10,010 S29 (MH "Blood Vessel Prosthesis") 1,016 S28 TX balloon or baloon 6,736 S27 TX dilat* 10,252 S26 TX recanali* or revascular* 8,067 S25 TX angioplas* or percutan* or PTA or venoplasty 23,625 S24 (MH "Angioplasty+") 8,925 S23 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 90,560 S22 TX below knee 1,665 S21 TX (poplite* or fempop* or infrapopliteal or tibial or tibiofibular or peroneal) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 303 S20 (MH "Tibial Arteries") 146 S19 (MH "Popliteal Artery") 363 S18 (MH "Leg/SU") 258 S17 TX (lower n3 extrem*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 122 S16 TX limb n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 278 S15 TX (leg n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 124 S14 TX dysvascular* 172 S13 TX arteriopathic 10 S12 TX isch* or CLI 39,424 S11 TX claudic* or IC 5,857 S10 TX peripheral n3 dis* 9,254 S9 TX (arter* or vascular or vein* or veno* or peripher*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 12,664 S8 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,395 S7 (MH "Peripheral Vascular Diseases+") 10,402 S6 (MH "Ischemia") 3,371 S5 (MH "Intermittent Claudication") 852 S4 (MH "Arterial Occlusive Diseases") 1,608 S3 (MH "Atherosclerosis") 3,336 S2 (MH "Arteriosclerosis") 4,829 S1 (MH "Arteriosclerosis") 4,829 |
416 |
| AMED | 1 ARTERIOSCLEROSIS/ 78 2 ATHEROSCLEROSIS/ 221 3 Intermittent Claudication/ 73 4 ISCHEMIA/ 263 5 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 802 6 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 458 7 (peripheral adj3 dis*).ti,ab. 435 8 (claudic* or IC).ti,ab. 1024 9 (isch* or CLI).ti,ab. 1666 10 arteriopathic.ti,ab. 1 11 dysvascular*.ti,ab. 57 12 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 21 13 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 32 14 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 15 ((poplite* or fempop* or infrapopliteal or tibial or tibiofibular or peroneal) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 87 16 below knee.ti,ab. 359 17 or/1‐16 4609 18 (angioplas* or percutan* or PTA or venoplasty).mp. [mp=abstract, heading words, title] 792 19 (recanali* or revascular*).ti,ab. 136 20 dilat*.ti,ab. 220 21 (balloon or baloon).ti,ab. 90 22 endovascular.ti,ab. 28 23 exp Stents/ 189 24 (stent* or graft* or endograft* or endoprosthe*).ti,ab. 1604 25 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex).ti,ab. 217 26 or/18‐25 2896 27 17 and 26 177 28 exp CLINICAL TRIALS/ 3749 29 RANDOM ALLOCATION/ 314 30 DOUBLE BLIND METHOD/ 657 31 Clinical trial.pt. 1211 32 (clinic* adj trial*).tw. 5381 33 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2833 34 PLACEBOS/ 586 35 placebo*.tw. 3102 36 random*.tw. 17520 37 PROSPECTIVE STUDIES/ 1097 38 or/28‐37 22515 39 27 and 38 24 40 ("2017" or "2018").yr. 2075 41 39 and 40 0 |
0 |
Data and analyses
Comparison 1. PTA versus stent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Technical success ITT | 5 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.00 [1.14, 7.93] |
| 2 Technical success TA | 5 | 474 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.04, 7.41] |
| 3 Procedural complications ITT | 5 | 360 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.01, 53.60] |
| 4 Procedural complications TA | 5 | 359 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.01, 47.70] |
| 5 Primary patency < 6 months ITT | 3 | 456 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.37, 2.11] |
| 6 Primary patency < 6 months TA | 3 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.32, 3.00] |
| 7 Amputation ITT | 4 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.56, 3.22] |
| 8 Amputation TA | 4 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.59, 3.40] |
| 9 Mortality ITT | 6 | 497 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.17] |
| 10 Mortality TA | 6 | 487 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.15] |
1.7. Analysis.
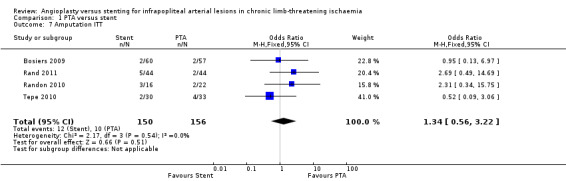
Comparison 1 PTA versus stent, Outcome 7 Amputation ITT.
1.8. Analysis.
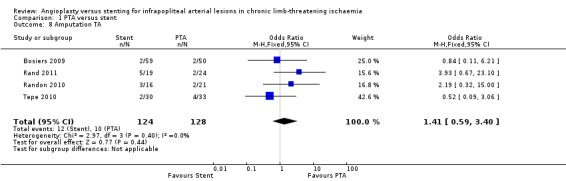
Comparison 1 PTA versus stent, Outcome 8 Amputation TA.
1.9. Analysis.
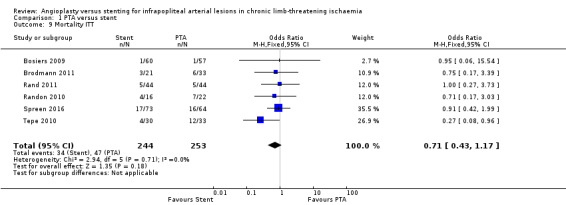
Comparison 1 PTA versus stent, Outcome 9 Mortality ITT.
1.10. Analysis.
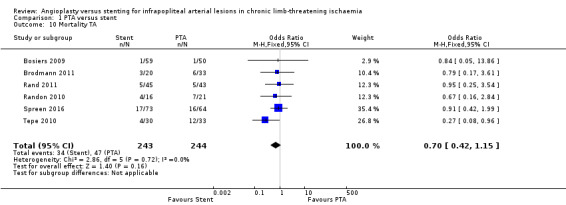
Comparison 1 PTA versus stent, Outcome 10 Mortality TA.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bosiers 2009.
| Methods |
Country: Absorbable Metal Stents (AMS) INSIGHT investigators (13 clinical sites in Austria, Belgium, Germany, and The Netherlands)
Setting: multi‐centre tertiary hospital
Study design: RCT Level of randomisation: participant |
|
| Participants | No. of participants randomised: 57 were randomised to the percutaneous transluminal angioplasty (PTA) group, and 60 to the AMS group. In total, 149 lesions were treated in 117 participants, which resulted in a total of 74 lesions in the AMS arm and 75 lesions in the PTA control arm Exclusions post randomisation: none Shifted to another treatment arm: if stenosis persisted to be > 50% or a flow‐limiting dissection occurred, the participant underwent implantation of the AMS study stent and ended up in the cross‐over group. Seven PTA group participants (7/57) with 11 lesions (11/75) crossed over to the other treatment arm due to dissections in at least 1 of the lesions and, in the case of 1 participant, due to significant residual stenosis Number of participants evaluated: 7 PTA group participants (7/57) with 11 lesions (11/75) crossed over to the other treatment arm due to dissection in at least 1 of the lesions and, in the case of 1 patient, due to significant residual stenosis. These participants were included in the PTA + AMS group, which was not considered in the on‐treatment data analysis performed by study authors. One participant randomised for stenting (1/60) with a double lesion (2/74) underwent implantation of a non‐study stent (self‐expanding) due to severe tortuosity of the iliac artery. Therefore, this participant was not considered in the on‐treatment analysis performed by study authors. The final on‐treatment cohort consisted of 50 participants with 64 lesions treated with PTA only and 59 participants with 72 lesions who underwent implantation of the study stent. Therefore, according to the study authors, ITT technical success, which was based on visual assessment, was achieved in 60 of 60 participants in the AMS group (100%), and in 55 of 57 participants in the PTA group (96.4%). For one PTA lesion, data on technical success were not provided by the investigator, and this participant's treatment was considered a non‐success Age (mean), years: PTA only 73.1, AMS 74.7 Gender: PTA group: 41 male/16 female, AMS stent group: 31 male/29 female Inclusion criteria: stenotic (> 50%) or occlusive atherosclerotic disease of the infrapopliteal arteries, length of lesion < 15 mm (< 1 stent length), reference vessel diameter 3.0 mm to 3.5 mm, maximum of 2 lesions in 1 infrapopliteal vessel treated in the study or in 2 vessels of 2 different legs, symptomatic critical limb ischaemia (Rutherford 4 and 5), patient ≥ 50 years of age, life expectancy > 6 months, no child‐bearing potential or negative serum pregnancy test within 7 days of the index procedure, participant willing and able to return at appropriate follow‐up times for the duration of the study, patient provision of written patient informed consent that is approved by the ethics committee Exclusion criteria: patient refusal of treatment; reference segment diameter not suitable for available stent design; length of lesion requiring more than 1 stent implantation; previously implanted stent(s) or PTA at the same lesion site; lesion lying within or adjacent to an aneurysm; inflow‐limiting arterial lesions left untreated; known allergy to heparin, aspirin, or other anticoagulant/antiplatelet therapies or bleeding diatheses, or unable or unwilling to tolerate such therapies; taking phenprocoumon (Marcumar); history of prior life‐threatening contrast medium reaction; currently enrolled in another investigational device or drug trial; currently breastfeeding, pregnant, or intending to become pregnant; mentally ill or retarded; liable for military or civilian service | |
| Interventions |
AMS stenting group:
PTA group (control):
Medication:
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to either PTA or AMS implantation. The randomization list was generated using PROC PLAN of SAS (Statistical Analysis Software)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered sealed envelopes contained information on the treatment to be applied. The sealed envelopes were opened only after the lesion was successfully crossed with the guidewire, and then patients were allocated either to stent or to PTA alone" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary safety endpoint at 1 month reported for 57/57 in the PTA group and for 59/60 in the AMS group Primary efficacy endpoint at 6 months reported for 40/57 in the PTA group and for 37/60 in the AMS group 7 participants in the PTA group crossed over to other treatment (included in PTA + AMS group for on‐treatment analysis) 1 participant in the AMS group underwent implantation of a non‐study stent (not included in on‐treatment analysis) Sudy authors provide on‐treatment and ITT analyses |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Quote: "The devices used in the study were the first‐generation AMS and the Pleon Explorer angioplasty balloon catheter, both developed by BIOTRONIK AG (Switzerland). The sponsor, BIOTRONIK AG, funded the total study costs and was responsible for the study administration and monitoring of the study" |
Brodmann 2011.
| Methods |
Country: Austria
Setting: monocentre university hospital
Study design: RCT Level of randomisation: participant |
|
| Participants |
No. of participants randomised: 54 patients were randomised to primary stenting (balloon expandable stent) or PTA alone Exclusions post randomisation: not mentioned; assumed none Shifted to another arm: 2 participants in PTA group with dissection in treated vessels required secondary stent, and total of 3 stents were placed Number of participants evaluated: overall 54 participants were included, with 33 assigned to the PTA group and 21 to the stent group Age (mean), years: 74.9 PTA, 68.9 stent Gender: 13/33 males PTA, 12/21 males stent Inclusion criteria: critical limb ischaemia, with Rutherford classification 4 to 6; lesion criteria characterised as the following: isolated stenoses > 70%, sequential stenoses up to cumulative length 12 cm, or total occlusion of crural arteries with maximum length 12 cm; target vessel must be a distal runoff vessel; written informed consent; life expectancy ≥ 12 months Exclusion criteria: endovascular procedure at the target vessel within the last 3 months; refused informed consent; known allergy against clopidogrel or aspirin; indication for oral anticoagulation (atrial fibrillation); concomitant participation in another clinical trial. Lesions in the inflow arteries needing to be treated were submitted to standardised treatment of femoropopliteal arteries |
|
| Interventions | For all patients eligible for the trial, antegrade access was chosen. After a 6 F sheath (Brite Tip, Cordis, Johnson & Johnson, New Brunswick, NJ, USA) was introduced, a diagnostic angiogram was obtained. 3000 units of unfractionated heparin was administered The target lesion in the infrapopliteal arteries was selected, and in case of successful passage of the target lesion with a hydrophilic‐coated guidewire (Standard Glide Wire, 0.035 inch, Terumo Medical Corporation, Somerset, NJ, USA; V‐18, Control™ Wire, Boston Scientific, Marlborough, MA, USA; or Hi‐Torque Sparta Core 14 Guide Wire, Abbott Vascular, Abbott Park, IL, USA) and the help of a support catheter, the randomisation process was done as described above If the lesion could not be passed with a guidewire, the patient was not included. To secure standardised documentation of the target lesion, a measuring tape was applied, leading down from the popliteal fossa to the foot of the patient. Before and after the procedure, angiography was performed in 2 planes, with a difference in angle of at least 30 degrees. In case of lesions in the inflow arteries, these were treated before the revascularisation procedure of the infrapopliteal arteries was performed PTA group:
Stenting group:
Medication:
|
|
| Outcomes | The main study endpoint was 1‐year clinical benefit, defined by improvement of at least 1 Rutherford category compared to baseline Quote: "Follow‐up examinations for all patients in the trial were performed the day aft er the successful procedure, at month 3, 6 and 12 thereafter. At each date a clinical evaluation referring to the Rutherford classification was done. In case of Rutherford classification 5 – 6 the wound was measured geometrically and compared in size to the prior visits. For rest pain evaluation, a standardised pain scale (NRS, numeric rating scale) was used. Walking distance was evaluated by treadmill testing. Ankle brachial index (ABI) and colour coded duplex sonography of the target lesion were done at each visit. The target lesion was targeted by duplex sonography referring to the measurement during the intervention. A measuring tape was once again applied leading down from the popliteal fossa to the foot of the patient to refind the formerly treated lesion. Colour coded duplex sonography was performed by two experienced study technicians and included an evaluation of the whole artery treated. The definition of 70% re‐stenosis was based on a proximal PVR > 3,4 calculated on duplex ultrasound. PVR was defined as peak systolic flow velocity in the lesion divided by the peak systolic flow velocity ˜1 cm proximal to the lesion. If a relevant re‐obstruction was suspected digital subtraction angiography (DSA) was performed, and at month 12 all patients underwent magnetic angiography (MRA). In all patients at each time of evaluation concomitant medication and medical events (especially cardiovascular events) were taken" Secondary endpoints were 3‐month and 6‐month primary patency rate; 3‐, 6‐, and 12‐month secondary patency; and 12‐month target lesion revascularisation rate The second primary endpoint was 12‐month primary patency, defined as freedom from re‐stenosis > 70% detected with duplex ultrasound Quote: "The definition of 70% restenosis was based on a proximal PVR > 3,4 calculated on duplex ultrasound. PVR was defined as peak systolic flow velocity in the lesion divided by the peak systolic flow velocity ˜1 cm proximal the lesion" Major adverse events were any amputation, the need for acute surgical revascularisation, and death related to the procedure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process was conducted with a computer‐generated list (blocked randomisation)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were listed for primary stent implantation or PTA alone with the usage of sequential numerated closed envelopes, which contained information about the planned procedure" Quote: "The blinding was warranted due to sealed envelopes and randomisation was done in the catheter lab immediately after successful passing of the target lesion with the guide wire. The randomisation was performed per patient" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The whole follow up period of 12 months was completed by 44 patients. 9 patients (16.7%) had died, 8 due to cardiovascular death, one had developed lung cancer. One patient refused to show up for the follow up visits after 3 months" No ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Quote: "Originally the trial was planned as a multicenter trial with the appropriate randomisation therefore, but at the end it was performed as a monocentric study, because the other participating centers had difficulties including appropriate patients. They did not include any patient over the whole study period. This explains the different numbers of patients in the two treatment groups" Quote: "At baseline evaluation all cardiovascular risk factors were more pronounced in the stent group than in the PTA group, with the biggest and statistically significant difference in hyperlipidemia in spite of lipidemic treatment (n = 14 (66.7%) versus 6 (18.2%); P < 0.0001). For diabetes mellitus, concerning the fact of insulin therapy there was a difference between the stent and PTA group, too (47.6% versus 36.4%)" |
Rand 2006.
| Methods |
Country: Austria
Setting: hospital
Study design: RCT Level of randomisation: participant |
|
| Participants |
No. of participants randomised: The study population consisted of 51 patients who were treated for critical chronic limb ischaemia (Fontaine stages III and IV), defined as rest pain, ischaemic ulcer, and gangrene. Patients with only claudication were not included in this study
Exclusions post randomisation: none Number of participants evaluated: 51 participants with 95 lesions: PTA group: 27 participants (53 lesions), stent group: 24 participants (42 lesions) Shifted to another arm: none Number of participants evaluated: 51; 44 were consecutively investigated and randomised at 1 centre to treatment of lesions by either PTA or stent application; 7 from 2 other centres were enrolled Age (mean), years: 72; mean age for the individual group not specified Gender: did not specify Inclusion criteria: chronic critical limb ischaemia stages III and IV of the Fontaine classification; isolated stenosis > 70% or occlusion of the tibial arteries; up to 3 lesions; lesions that were up to 3 cm with cumulative lesion length ≤ 9 cm, including the tibiofibular trunk, anterior and posterior tibial arteries, and fibular artery. There was no further limitation regarding lesion position. Patients with a significant inflow obstruction at the pelvic or superficial femoral artery level were not included Exclusion criteria: evidence of a systemic coagulopathy with anticoagulant and antiplatelet treatment contraindicated; previously implanted stents in the target lesion; total occlusion in the target vessel following the target lesion; without distal runoff; inflammatory vascular disease; peptic ulcer or gastric/intestinal bleeding in the previous 6 months; clinically assessed intolerance to contrast medium |
|
| Interventions |
PTA group:
Stenting group:
Medication:
|
|
| Outcomes |
Primary endpoint:
Secondary endpoints:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number generation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Numbered envelopes were prepared for one‐to‐one randomization to either PTA or primary stent placement. The randomization was performed per patient. Therefore, all lesions in a particular patient had to be treated by either PTA or primary stent placement" Type of envelope not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data evaluation performed by the 2 readers in a double‐blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 51 patients, 2 patients died, 3 patients underwent amputation, 1 patient underwent major heart surgery, which did not allow further follow‐up, and 8 patients were lost to follow‐up" Survival analysis performed |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Unclear risk | Quote: "Due to its main effect on early restenosis clopidogrel is given only to patients who have received stents and not to patients who underwent PTA, as early restenosis is not regarded a major problem in this patient group" Quote: "We also observed a higher incidence of PTAs than stent applications per patient. This might be due to a certain degree of investigator bias, as potentially one balloon can be used for several lesions in contrast to the necessity of one stent per lesion" Quote: "The study was supported by the Ludwig Boltzmann Institute for Radiologic Tumor Diagnosis and the Ludwig Boltzmann Institute of Interdisciplinary Vascular Research" |
Rand 2011.
| Methods |
Country: InPeria II study at 6 centres in Europe (Austria, Italy, and Germany)
Setting: tertiary and university hospitals
Study design: RCT Level of randomisation: limb |
|
| Participants |
No. of participants: 88 consecutive patients: PTA group: 44 participants; stent group: 44 participants
Age (mean), years: PTA group 62.2, stent group 68.2
Gender: PTA group 28 male/17 female, stent group 30 male/11 female
Exclusions post randomisation: none
Shifted to another arm: during the intervention in the PTA group, if residual stenosis (> 30%) and flow‐limiting dissection were present, the participant was treated with stent; thus the participant crossed over into the stent group
Number of participants evaluated: 88; 44 in the PTA group (45 treated limbs) and 44 in the stent group (44 treated limbs). Total: 131 treated lesions (PTA group: 69 lesions; stent group: 62 lesions) Losses to follow‐up: 3 in the PTA group and 5 in the stent group died within the first 3 months after treatment. Two additional participants in the PTA group died between 3 and 6 months after treatment. Fifteen participants were not available for follow‐up investigations at 3 months and an additional 20 participants were not available for investigations at 9 months owing to death or non‐compliance Inclusion criteria: symptomatic CLTI (stage 4 or 5 according to the Rutherford classification) due to a de novo lesion of an infrapopliteal artery. Lesions with stenosis ≥ 50% of their diameter were considered for the trial. Patients with substantial inflow stenosis were eligible for inclusion if the stenosis had been successfully treated without complications. The target infrapopliteal artery was eligible provided that in‐line circulation to the foot distal to the lesion was present Exclusion criteria: previous treatment; total occlusion in the target vessel; no distal arterial runoff; underlying disease (e.g. renal failure, bleeding disorders) |
|
| Interventions |
PTA group (control):
Stenting group:
Medication:
|
|
| Outcomes |
Primary endpoints assessed at 3 months and 9 months:
Secondary endpoints assessed at 9 months:
|
|
| Notes | Data analysis performed in this review used limbs as unit of measurement instead of treated lesion(s) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization (stent vs PTA) was performed in a 1:1 ratio, so that all patients enrolled in this study were randomized to undergo either stent placement or PTA alone for each leg separately. This means each leg in each patient was treated individually (stent vs PTA), but all lesions in the same leg would have been treated with the same procedure allocated to that leg" Method of allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for; ITT analysis reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported; flow chart provided |
| Other bias | Low risk | Quote: "This study was pursued with support from Sorin Biomedica Cardio (Saluggia, Italy), which provided the investigational devices. The authors had complete control of the data and information submitted for publication, which was firmly in the hands of the Medical University of Vienna and the Karl Landsteiner Society, St Poelten, Austria, and was unbiased by industry" |
Randon 2010.
| Methods |
Country: Belgium
Setting: Ghent University Hospital
Study design: single‐centre RCT Level of randomisation: participant |
|
| Participants |
No. of participants randomised: total of 38 limbs in 35 participants with critical limb ischaemia were randomised to angioplasty (n = 22) or primary stenting (n = 16)
Exclusions post randomisation: 1 participant in the PTA group did not receive intervention (n = 1); reason: stenosis < 70%
Shifted to another arm: none
Number of participants evaluated: 22 in the PTA group and 16 in the stenting group
Age (mean), years: 72 years old in both groups
Gender: PTA group: 14 male/8 female; stenting group: 6 male/10 female
Inclusion criteria: All patients with CLTI (Rutherford 4 to 6, Fontaine III and IV) hospitalised at the Department of Vascular Surgery, Ghent University Hospital, for primary angioplasty of 1 or more crural vessels were randomised to primary stenting or angioplasty alone. For most patients, this was a last attempt before major amputation because of intractable pain or tissue loss. Some had no adequate venous conduit or no surgical target vessel, and the level of comorbidity was generally too high for general anaesthesia (ASA scores III and IV). All patients with stenosis of 70% or occlusions of the crural arteries were considered suitable for endovascular therapy. The length of the lesion was not an exclusion criterion, as even stenoses or occlusions > 10 cm were accepted (an exclusion criterion in most angioplasty studies and registries) Exclusion criteria: acute limb ischaemia; multi‐segmental inflow lesions (longer than 3 cm) above the knee; sepsis; myocardial infarction during previous 14 days; blue toe syndrome (microembolisation); inability to ambulate. Patients who needed bypass surgery for popliteal or superficial femoral occlusions and those who needed simultaneous angioplasty of the crural and more then one proximal vessel were excluded Nine patients needed concomitant proximal angioplasty for stenosis: 6 patients at the level of the popliteal artery, 2 at the level of the superficial femoral artery, and 1 at the level of the common iliac artery. There were 20 men and 18 women. The mean age of all patients did not diverge statistically from the mean age of the subgroups (72 ± 9.8 years; range 50 to 88 years). In 15 limbs, 2 arteries were treated, and in 1 patient, all 3 crural arteries: only 1 of these vessels was included in the study |
|
| Interventions |
Stenting group:
PTA group (control):
Additional intervention:
Medication:
|
|
| Outcomes |
Quote: "Patients were examined every 3 – 6 months after discharge till the end of the trial. Standard duplex scanning was performed every 6 months by one independent experienced investigator to exclude bias. The PSV was measured over the stent if possible. In the case of angioplasty alone the PSV was measured over the whole length of the treated artery. When the PSV was > 400 cm/s or when the treated artery was re‐occluded, and the patients showed recurrence of rest pain, cessation of ulcer healing, or a new ulcer, a new angiography was performed. In most of the patients ABPI measurements were not possible or not reliable due to calcifications of the vessels. We preferred duplex over angiography for follow up because of the renal comorbidity of our patients and the fact that the most important outcome for these patients is not patency of the vessel but relief of rest pain and healing of their ulcers" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by computer‐generated randomization sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed by means of sealed, consecutively numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Standard duplex scanning was performed every 6 months by one independent experienced investigator to exclude bias" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Unclear risk | Declaration of conflict of interest not stated in the paper |
Spreen 2016.
| Methods |
Country: The Netherlands
Setting: 3 major vascular centres in the Netherlands
Study design: multi‐centre RCT Level of randomisation: participant |
|
| Participants |
No. of participants randomised: A total of 144 limbs in 137 patients with critical limb ischaemia were randomised to angioplasty (69 limbs in 67 participants) or primary stenting (75 limbs in 74 participants) (4 participants included for 2 limbs, with 1 limb in each arm)
Exclusions post randomisation: 3 participants (3 limbs) in the PTA group did not receive intervention; reasons: intermittent claudication, renal failure without dialysis, and coagulation disorder. One participant (1 limb) in the stent group did not receive intervention; reason: vessel too small. Overall, 64 participants (66 limbs) received the allocated PTA intervention, and 73 participants (74 limbs) received the allocated stent treatment Shifted to another arm: none Age (mean), years: PTA group 73, stent group 74 Gender: PTA group 47 male/17 female, stent group 49 male/24 female Gender: PTA group: 14 male/8 female, stent group 6 male/10 female Inclusion criteria: age > 18 years; if female patient with child‐bearing potential, may not be pregnant at study entry and must utilise reliable birth control for the duration of participation in the study; must be willing and able to comply with the specified follow‐up evaluation; critical limb ischaemia, defined as Rutherford category 4 (ischaemic rest pain), 5 (minor tissue loss), or 6 (major tissue loss); stenosis (> 50% luminal loss) or occlusion of an infrapopliteal artery, including the tibiofibular trunk, the anterior tibial artery, the posterior tibial artery, and the fibular artery; target lesion length ≤ 90 mm; artery to be treated with diameter ≥ 2 mm and ≤ 6 mm; patent common iliac, external iliac, superficial femoral, and popliteal artery on the ipsilateral side before randomisation, possibly after treatment during the same session. At least 1 patent crural (anterior tibial, posterior tibial, or fibular) artery with expected unobstructed runoff to ankle level after treatment Exclusion criteria: acute limb ischaemia; previous amputation of affected limb at or above ankle level; subacute limb ischaemia, which requires thrombolysis as first treatment modality; active bleeding or bleeding diathesis; recent (≤ 3 months) haemorrhagic stroke or any other CNS abnormality with increased risk of haemorrhage, such as intracranial neoplasm, arteriovenous malformation, intracranial aneurysm, or aneurysm repair; gastrointestinal or genitourinary bleeding of clinical significance within the previous 6 weeks before treatment; aneurysm in common femoral, superficial femoral, or popliteal artery on the ipsilateral side; surgical revascularisation involving the same limb within 30 days before the index procedure or planned surgical revascularisation of the same limb within 30 days of the index procedure; previous implanted stent at the index site; life expectancy < 6 months or other factors making clinical follow‐up difficult; known allergy to acetylsalicylic acid (aspirin), clopidogrel, heparin, or paclitaxel; known allergy to contrast media; known heparin‐induced thrombocytopaenia (HIT type 2); unable or unwilling to tolerate anticoagulant, antiplatelet therapy, or contrast media; creatinine clearance 20 mL/min (as derived from Cockcroft‐Gault formula); severely calcified lesions with expected resistance to stenting; poor inflow due to ipsilateral stenosis or occlusions of the iliac or femoropopliteal arteries that cannot be treated during the same session; significant vessel tortuosity or other parameters prohibiting access to the lesions and/or delivery of the stent; without (expected) distal runoff to the index site |
|
| Interventions |
Stenting group:
PTA group (control):
Medication:
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence on a 1:1 basis. Randomisation per limb and stratified in blocks per centre. Block size (n = 4) known only to the statistician |
| Allocation concealment (selection bias) | Low risk | Sealed and opaque envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, operators, and investigators not blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data accounted for. ITT analysis reported |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Unclear risk | One study author has received speakers’ fees from Cordis Corporation, Fremont, CA, USA; Cook Medical, Bloomington, IN, USA; and AngioDynamics, Latham, NY, USA |
Tepe 2010.
| Methods |
Country: Germany
Setting: hospital
Study design: RCT Level of randomisation: participant |
|
| Participants | No. of participants randomised: 60 with current ulcers randomised Exclusions post randomisation: not mentioned Shifted to another arm: 3 participants received additional stent placement after primary endpoint: 2 in the PTA group and 1 in the stenting group Number of participants evaluated: 60 (63 limbs) Age (mean), years: stenting group 72.8, PTA group 72.2 Gender: 42 males, 21 females Inclusion criteria: Rutherford stage 5 or 6 with current ulcers on the basis of arterial disease; patent vessel to the distal lower leg; index lesion maximum 5 cm in length Exclusion criteria: not mentioned | |
| Interventions |
Sixty participants with current ulcers were randomly assigned to receive:
Medication:
Concurrent stenoses of the inflow or outflow tract were treated in the same session by PTA |
|
| Outcomes | Angiographic endpoints consisted of primary re‐stenosis at 2 months and 6 months and overall patency. Re‐stenosis was defined as re‐narrowing of the index lesion by ≥ 50% Clinical endpoints were healing of ulceration, amputation rate, and overall survival Technical endpoints included technical success rate, subacute re‐occlusions, and re‐stenosis Technical success was defined as < 30% residual stenosis after the intervention |
|
| Notes | In the forest plot, the 4 groups were re‐classified into 2 broad groups: stenting and PTA Stenting group: sirolimus‐coated stent with abciximab (n = 14) and bare stent with abciximab (n = 16) PTA group: PTA with abciximab (n = 14) and PTA alone (n = 19) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number generation not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total of 63 limbs in 60 participants randomised Quote: "In total, 44 patients were available for follow up after two months and 37 patients after six months, respectively" |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Study supported by Eli Lilly; no explicit mention of the independence of the research team |
ABI: ankle‐brachial index. ABPI: ankle‐brachial pressure index. AMS: absorbable metal stent. ASA: acetylsalicylic acid. ASA: American Society of Anesthesiologists. BW: body weight. CFDU: colour flow Doppler ultrasound. CLTI: chronic limb‐threatening ischaemia. CNS: central nervous system. CTA: computed tomography angiography. DS: diameter stenosis. DSA: digital subtraction angiography. HIT: heparin‐induced thrombocytopaenia. ITT: intention‐to‐treat. LLL: late lumen loss. MLD: minimal lumen diameter. MRA: magnetic resonance angiography. PSV: peak systolic velocity. PTA: percutaneous transluminal angioplasty. PVR: peak velocity ratio. QVA: quantitative vascular angiography. RCT: randomised controlled trial. RVD: reference vessel diameter. TLR: target lesion revascularisation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bosiers 2012 | Comparison of 2 different stents |
| Bradbury 2010 | Description of severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter‐Society Consensus II classification in the BASIL trial |
| Rastan 2011 | Comparison of 2 different stents |
| Scheinert 2012 | ACHILLES trial: RCT of stent vs angioplasty for treatment of infrapopliteal arterial disease. Rutherford stages 3 to 5 were included. As outlined in our protocol, we intended to include in our analysis only patients with Rutherford stages 4 to 6. Further, the study did not provide subgroup data specific to stage 4 and 5 patients and thus is excluded from the review |
| Schulte 2015 | EXPAND trial: RCT of stent vs angioplasty for treatment of infrapopliteal arterial disease. Rutherford stages 3 to 5 were included. As outlined in our protocol, we intended to include in our analysis only patients with Rutherford stages 4 to 6. Further, the study did not provide subgroup data specific to stage 4 and 5 patients and thus is excluded from the review |
| Siablis 2007 | Prospective, non‐randomised, single‐centre, controlled, double‐arm study. Stenting was performed as a bailout procedure for suboptimal angioplasty results (flow‐limiting dissection, elastic recoil, or post‐angioplasty residual stenosis > 30%). In the first 29 participants, infrapopliteal stenting was performed with bare‐metal stents (group B), and in the other 29 participants, sirolimus‐eluting stents were used (group S) |
| Siablis 2014 | IDEA trial: RCT comparing paclitaxel‐coated balloon angioplasty vs drug‐eluting stents in long infrapopliteal lesions. Inclusion criteria were Rutherford classes 3 to 6 and angiographically documented infrapopliteal disease with minimum lesion length of 70 mm. As outlined in our protocol, we intended to include in our analysis only patients with Rutherford stages 4 to 6. Further, the study did not provide subgroup data specific to stage 4 and 5 patients and thus is excluded from the review |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01644487.
| Trial name or title | Self Expanding Nitinol Stent Versus Balloon Angioplasty Alone for the Below The Knee Arteries (SENSBTK) |
| Methods | Study type: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Enrolment: 50 Age eligible for study: 20 to 80 years Genders eligible for study: both Accepts healthy volunteers: no Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental group: a group of patients who will undergo subsequent primary stenting following successful conventional balloon angioplasty Active comparator: a group of patients who will undergo routine conventional balloon angioplasty alone without stenting |
| Outcomes | Primary outcome measures: angiographic binary re‐stenosis rate (time frame: 12 months) |
| Starting date | Study start date: July 2012 Estimated study completion date: July 2018 Estimated primary completion date: July 2017 (final data collection date for primary outcome measure) |
| Contact information | Principal investigator contact: Seung Woon Rha, MD, PhD; 82226263020; swrha617@yahoo.co.kr Contact: Yun Hyeong Cho, MD, PhD; 82318106776; princette@hanmail.net |
| Notes |
PTA: percutaneous transluminal angioplasty.
Differences between protocol and review
We have added a paragraph to the methods section to explain how we addressed unit of analysis issues regarding participant or limb randomisation in this review.
We have added 'technical success' as a primary outcome and have upgraded the outcome 'procedural complication' to a primary outcome.
We have removed target lesion revascularisation (TLR) and minor amputation as outcome measures. TLR, defined as repeat percutaneous or surgical revascularisation of a lesion anywhere within the stent or within the 5‐mm borders proximal or distal to the stent, is dependent on the willingness of the operator to intervene, regardless of the patency of the treated target. We therefore decided that this was not an appropriate outcome. Minor amputation of devascularised tissue/gangrene is considered part of the treatment for CLTI, and although revascularisation is aimed at preventing further tissue loss, it does not affect the outcome of tissue that is not viable and hence is considered not relevant as an outcome measure.
Contributions of authors
CC‐TH: selected studies, extracted data, assessed risk of bias, wrote the review. DS: commented on the review. GNCK: selected studies, extracted data, assessed risk of bias, wrote the review. JAR: commented on the review. CA: commented on the review. MLvD: acted as arbiter for study selection and data extraction, assessed risk of bias, wrote the review. provided support with methodological aspects of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular (10/4001/14). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
CC‐TH: none known. DS: none known. GNCK: none known. JAR: none known. CA: none known. MLvD: none known.
New
References
References to studies included in this review
Bosiers 2009 {published data only}
- Bosiers M, Peeters P, D'Archambeau O, Hendriks J, Pilger E, Duber C, et al. AMS insight: absorbable metal stent implantation for treatment of "below the knee" critical limb ischaemia ‐ 6 month analysis. Cardiovascular and Interventional Radiological Society of Europe Annual Meeting Abstracts; 2008 Sep 13‐17; Copenhagen, Denmark 2008:296. [DOI] [PMC free article] [PubMed]
- Bosiers M, Peeters P, D'Archambeau O, Hendriks J, Pilger E, Düber C, et al. AMS INSIGHT ‐ absorbable metal stent implantation for treatment of below‐the‐knee critical limb ischemia: 6‐month analysis. Cardiovascular and Interventional Radiology 2009;32(3):424‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00572494. AMS INSIGHT 1 Study ‐ Bioabsorbable Metal Stent Investigation in Chronic Limb Ischemia Treatment (AMS‐INSIGHT1). https://clinicaltrials.gov/ct2/show/results/NCT00572494 (date first received: 12 December 2007).
Brodmann 2011 {published data only}
- Brodmann M, Froehlich H, Dorr A, Gary T, Hafner F, Deutschmann H, et al. Percutaneous transluminal angioplasty versus primary stenting in infrapopliteal arteries in critical limb ischemia. Zeitschrift fur Gefassmedizin 2012;9(2):13. [DOI] [PubMed] [Google Scholar]
- Brodmann M, Froehlich H, Dorr A, Gary T, Portugaller RH, Deutschmann H, et al. Percutaneous transluminal angioplasty versus primary stenting in infrapopliteal arteries in critical limb ischemia. Vasa 2011;40(6):482‐90. [DOI] [PubMed] [Google Scholar]
Rand 2006 {published data only}
- Rand T, Basile A, Cejna M, Fleischmann D, Funovics M, Gschwendtner M, et al. PTA versus carbofilm‐coated stents in infrapopliteal arteries: pilot study. Cardiovascular and Interventional Radiology 2006;29(1):29‐38. [DOI] [PubMed] [Google Scholar]
Rand 2011 {published data only}
- Rand T, Jahnke T, Lammer J, Manninen H, Maynar M, Mueller‐Huelsbeck S, et al. Stents vs PTA: infra‐popliteal randomized trial. Cardiovascular and Interventional Radiological Society of Europe annual meeting abstracts; 2008 sep 13‐17; Copenhagen, Denmark. 2008:296.
- Rand T, Lammer J, Rabbia C, Maynar M, Zander T, Jahnke T, et al. Percutaneous transluminal angioplasty versus turbostatic carbon‐coated stents in infrapopliteal arteries: InPeria II trial. Radiology 2011;261(2):634‐42. [DOI] [PubMed] [Google Scholar]
Randon 2010 {published data only}
- Randon C, Jacobs B, Ryck F, Vermassen F. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovascular and Interventional Radiology 2010;33(2):260‐9. [DOI] [PubMed] [Google Scholar]
- Randon C, Vermassen F. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovascular and Interventional Radiology 2010; Vol. 33, issue Suppl 2:192. [DOI] [PubMed]
Spreen 2016 {published data only}
- Martens JM, Knippenberg B, Vos JA, Vries JP, Hansen BE, Overhagen H, et al. Update on PADI trial: percutaneous transluminal angioplasty and drug‐eluting stents for infrapopliteal lesions in critical limb ischemia. Journal of Vascular Surgery 2009;50(3):687‐9. [DOI] [PubMed] [Google Scholar]
- NCT00471289. PTA and drug eluting stents for infrapopliteal lesions in critical limb ischemia (PADI). https://clinicaltrials.gov/ct2/show/NCT00471289?term=NCT00471289&rank=1 (date first received: 7 May 2007).
- Spreen M, Mali WPTM, Martens J, Overhagen H. Morphological results of PADI trial: percutaneous transluminal angioplasty versus drug‐eluting stents for infrapopliteal lesions in critical limb ischemia. Cardiovascular and Interventional Radiology 2015;38(3 Suppl 1):S165. [Google Scholar]
- Spreen M, Martens J, Mali WPTM, Overhagen H. Clinical results of PADI trial: percutaneous transluminal angioplasty versus drug‐eluting stents for infrapopliteal lesions in critical limb ischaemia. Cardiovascular and interventional radiology 2015;38(3 Suppl 1):S292‐3. [Google Scholar]
- Spreen MI, Martens JM, Hansen BE, Knippenberg B, Verhey E, Dijk LC, et al. Percutaneous transluminal angioplasty and drug‐eluting stents for infrapopliteal lesions in critical limb ischemia (PADI) trial. Circulation. Cardiovascular Interventions 2016;9(2):e002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen MI, Martens JM, Knippenberg B, Dijk LC, Vries JPM, Vos JA, et al. Long‐term follow‐up of the PADI trial: percutaneous transluminal angioplasty versus drug‐eluting stents for infrapopliteal lesions in critical limb ischemia. Journal of American Heart Association 2017;6(4):pii: e004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tepe 2010 {published data only}
- NCT00163254. Below study ‐ Balloon angioplasty or stents with ReoPro for prevention of subacute reocclusion in arteries below the knee. https://clinicaltrials.gov/ct2/show/NCT00163254 (date first received: 8 September 2005).
- Tepe G, Heller S, Wiskirchen J, Stauder N, Claussen CD, Duda SH. Drug‐eluting stents versus percutaneous transluminal angioplasty with glycoprotein IIb/IIIa blockade below the knee in patients with current ulcers ‐ the BELOW study. Cardiovascular and Interventional Radiological Society of Europe, CIRSE 2006.
- Tepe G, Schmehl J, Heller S, Brechtel K, Heuschmid M, Fenchel M, et al. Drug eluting stents versus PTA with GP IIb/IIIa blockade below the knee in patients with current ulcers ‐ the BELOW study. Journal of Cardiovascular Surgery 2010;51(2):203‐12. [PubMed] [Google Scholar]
References to studies excluded from this review
Bosiers 2012 {published data only}
- Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, et al. Randomized comparison of everolimus‐eluting versus bare‐metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. Journal of Vascular Surgery 2012;55(2):390‐8. [DOI] [PubMed] [Google Scholar]
Bradbury 2010 {published data only}
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a description of the severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter‐Society Consensus IIclassification. Journal of Vascular Surgery 2010;51:32S‐42S. [DOI] [PubMed] [Google Scholar]
Rastan 2011 {published data only}
- Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, et al. Sirolimus‐eluting stents vs. bare‐metal stents for treatment of focal lesions in infrapopliteal arteries: a double‐blind, multi‐centre, randomized clinical trial. European Heart Journal 2011;32(18):2274‐81. [DOI] [PubMed] [Google Scholar]
Scheinert 2012 {published data only}
- Katsanos K, Spiliopoulos S, Diamantopoulos A, Siablis D, Karnabatidis D, Scheinert D. Wound healing outcomes and health‐related quality‐of‐life changes in the ACHILLES trial: 1‐year results from a prospective randomized controlled trial of infrapopliteal balloon angioplasty versus sirolimus‐eluting stenting in patients with Ischemic peripheral arterial disease. JACC Cardiovascular Interventions 2016;9(3):259‐67. [DOI] [PubMed] [Google Scholar]
- Katsanos KNS, Krankenberg H, Baumgartner I, Peregrin JHL, Scheinert D. The ACHILLES study, a prospective, randomized, multicenter comparison of balloon angioplasty and CYPHER SELECT plus stent implantation in the treatment of patients with ischemic infrapopliteal arterial disease. Cardiovascular and Interventional Radiology 2011;34:505. [Google Scholar]
- NCT00640770. Comparing Angioplasty and DES in the Treatment of Subjects With Ischemic Infrapopliteal Arterial Disease (ACHILLES). https://clinicaltrials.gov/ct2/show/NCT00640770 (date first received: 14 March 2008).
- Scheinert D. A prospective, randomized, multicenter comparison of balloon angioplasty and the CYPHER SELECT© Plus coronary and infrapopliteal stent in the treatment of subjects with ischemic infrapopliteal arterial disease. Leipzig Interventional Course 2011.
- Scheinert D, Katsanos K, Zeller T, Koppensteiner R, Commeau P, Bosiers M, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus‐eluting stent in patients with ischemic peripheral arterial disease: 1‐year results from the ACHILLES trial. Journal of the American College of Cardiology 2012;60(22):2290‐5. [DOI] [PubMed] [Google Scholar]
Schulte 2015 {published data only}
- Langhoff R. EXPAND trial shows no benefit for primary bare metal stenting over angioplasty below the knee. Interventional News 2013.
- NCT00906022. Self Expanding Nitinol Stent Versus Percutaneous Transluminal Arterial Angioplasty (PTA) With Optional Bailout Stenting in Case of PTA Failure in Patients With Symptomatic Critical Limb Ischemia or Severe Intermittent Claudication (EXPAND). http://clinicaltrials.gov/ct2/show/NCT00906022?term=expand&rank=1 (date first received: 20 May 2009).
- Schulte KL, Brodmann M, Schellong S, Tan K, Langhoff R, Torsello G, et al. EXPAND: percutaneous transluminal angioplasty vs primary stenting in patients with severe claudication and critical limb ischaemia with infrapopliteal vascular lesions. Eurointervention. EuroPCR Abstracts. 2013; Vol. 9.
- Schulte KL, Pilger E, Schellong S, Tan KT, Baumann F, Langhoff R, et al. Primary Self‐EXPANDing nitinol stenting vs balloon angioplasty with optional bailout stenting for the treatment of infrapopliteal artery disease in patients with severe intermittent claudication or critical limb ischemia (EXPAND study). Journal of Endovascular Therapy 2015;22(5):690‐7. [DOI] [PubMed] [Google Scholar]
Siablis 2007 {published data only}
- Siablis D, Karnabatidis D, Katsanos K, Kagadis GC, Kraniotis P, Diamantopoulos A, et al. Sirolimus‐eluting versus bare stents after suboptimal infrapopliteal angioplasty for critical limb ischemia: enduring 1‐year angiographic and clinical benefit. Journal of Endovascular Therapy 2007;14(2):241‐50. [DOI] [PubMed] [Google Scholar]
Siablis 2014 {published data only}
- NCT01517997. Infrapopliteal drug eluting angioplasty versus stenting (IDEAS‐I). http://clinicaltrials.gov/ct2/show/NCT01517997?term=angioplasty+and+stent+and+infrapopliteal&rank=2 (date first received: 2 December 2011).
- Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovascular interventions 2014;7(9):1048‐56. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01644487 {published data only}
- NCT01644487. Self‐Expanding Nitinol Stent Versus Balloon Angioplasty Alone for the Below the Knee Arteries (SENS‐BTK). https://clinicaltrials.gov/ct2/show/NCT01644487?term=NCT01644487&rank=1 (date first received: 17 July 2012).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conte 2001
- Conte MS, Belkin M, Upchurch GR, Mannick JA, Whittemore AD, Donaldson MC. Impact of increasing comorbidity on infrainguinal reconstruction: a 20 year perspective. Annals of Surgery 2001;233(3):445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dormandy 1999
- Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Seminars in Vascular Surgery 1999;12(2):142‐7. [PubMed] [Google Scholar]
Fusaro 2013
- Fusaro M, Cassese S, Ndrepepa G, Tepe G, King L, Ott I, et al. Drug‐eluting stents for revascularization of infrapopliteal arteries: updated meta‐analysis of randomized trials. JACC Cardiovascular Interventions 2013;6(12):1284‐93. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hirsch 2006
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation 2006;113(11):e463‐654. [DOI] [PubMed] [Google Scholar]
Holdsworth 1997
- Holdsworth J. District hospital management and outcome of critical lower limb ischaemia: comparison with national figures. European Journal of Vascular and Endovascular Surgery 1997;13(2):159‐63. [DOI] [PubMed] [Google Scholar]
Lefevbre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Morice 2002
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. New England Journal of Medicine 2002;346(23):1773‐80. [DOI] [PubMed] [Google Scholar]
Moses 2003
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. New England Journal of Medicine 2003;349(14):1315‐23. [DOI] [PubMed] [Google Scholar]
Nelken 2004
- Nelken N, Schneider PA. Advances in stent technology and drug‐eluting stents. Surgical Clinics of North America 2004;84(5):1203‐36. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter‐Society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45 Suppl:5‐67. [DOI] [PubMed] [Google Scholar]
Robertson 2012
- Robertson L, Ghouri MA, Kovacs F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD002071.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romiti 2008
- Romiti M, Albers M, Brochado‐Neto FC, Durazzo AE, Pereira CA, Luccia N. Meta‐analysis of infrapopliteal angioplasty for chronic critical limb ischemia. Journal of Vascular Surgery 2008;47(5):975‐81. [DOI] [PubMed] [Google Scholar]
Schofer 2003
- Schofer J, Schluter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al. Sirolimus‐eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double‐blind, randomised controlled trial (E‐SIRIUS). Lancet 2003;362(9390):1093‐9. [DOI] [PubMed] [Google Scholar]
Spreen 2017
- Spreen MI, Martens JM, Knippenberg B, Dijk LC, Vries JPM, Vos JA, et al. Long‐term follow‐up of the PADI trial: percutaneous transluminal angioplasty versus drug‐eluting stents for infrapopliteal lesions in critical limb ischemia. Journal of American Heart Association 2017;6(4):pii: e004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1986
- Wolfe JHN. Defining the outcome of critical ischaemia: a one year prospective study. British Journal of Surgery 1986;73(6):321. [Google Scholar]
Wu 2014
- Wu R, Yao C, Wang S, Xu X, Wang M, Li Z, et al. Percutaneous transluminal angioplasty versus primary stenting in infrapopliteal arterial disease: a meta‐analysis of randomized trials. Journal of Vascular Surgery 2014;59:1711‐20. [DOI] [PubMed] [Google Scholar]
Yang 2014
- Yang X, Lu X, Ye K, Li X, Qin J, Jiang M. Systematic review and meta‐analysis of balloon angioplasty versus primary stenting in the infrapopliteal disease. Vascular and Endovascular Surgery 2014;48(1):18‐26. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hsu 2011
- Hsu CCT, Rophael JA, Mofidi R, Kwan GNC, Driel ML. Percutaneous transluminal arterial angioplasty versus stenting for infrapopliteal arterial lesions in critical limb ischaemia. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009195] [DOI] [Google Scholar]


