Abstract
Background
There has been renewal of interest in the use of prophylactic antibiotics to reduce the frequency of exacerbations and improve quality of life in chronic obstructive pulmonary disease (COPD).
Objectives
To determine whether or not regular (continuous, intermittent or pulsed) treatment of COPD patients with prophylactic antibiotics reduces exacerbations or affects quality of life.
Search methods
We searched the Cochrane Airways Group Trials Register and bibliographies of relevant studies. The latest literature search was performed on 27 July 2018.
Selection criteria
Randomised controlled trials (RCTs) that compared prophylactic antibiotics with placebo in patients with COPD.
Data collection and analysis
We used the standard Cochrane methods. Two independent review authors selected studies for inclusion, extracted data, and assessed risk of bias. We resolved discrepancies by involving a third review author.
Main results
We included 14 studies involving 3932 participants in this review. We identified two further studies meeting inclusion criteria but both were terminated early without providing results. All studies were published between 2001 and 2015. Nine studies were of continuous macrolide antibiotics, two studies were of intermittent antibiotic prophylaxis (three times per week) and two were of pulsed antibiotic regimens (e.g. five days every eight weeks). The final study included one continuous, one intermittent and one pulsed arm. The antibiotics investigated were azithromycin, erythromycin, clarithromycin, doxycyline, roxithromycin and moxifloxacin. The study duration varied from three months to 36 months and all used intention‐to‐treat analysis. Most of the pooled results were of moderate quality. The risk of bias of the included studies was generally low.
The studies recruited participants with a mean age between 65 and 72 years and mostly at least moderate‐severity COPD. Five studies only included participants with frequent exacerbations and two studies recruited participants requiring systemic steroids or antibiotics or both, or who were at the end stage of their disease and required oxygen. One study recruited participants with pulmonary hypertension secondary to COPD and a further study was specifically designed to asses whether eradication of Chlamydia pneumoniae reduced exacerbation rates.
The co‐primary outcomes for this review were the number of exacerbations and quality of life.
With use of prophylactic antibiotics, the number of participants experiencing one or more exacerbations was reduced (odds ratio (OR) 0.57, 95% CI 0.42 to 0.78; participants = 2716; studies = 8; moderate‐quality evidence). This represented a reduction from 61% of participants in the control group compared to 47% in the treatment group (95% CI 39% to 55%). The number needed to treat for an additional beneficial outcome with prophylactic antibiotics given for three to 12 months to prevent one person from experiencing an exacerbation (NNTB) was 8 (95% CI 5 to 17). The test for subgroup difference suggested that continuous and intermittent antibiotics may be more effective than pulsed antibiotics (P = 0.02, I² = 73.3%).
The frequency of exacerbations per patient per year was also reduced with prophylactic antibiotic treatment (rate ratio 0.67; 95% CI 0.54 to 0.83; participants = 1384; studies = 5; moderate‐quality evidence). Although we were unable to pool the result, six of the seven studies reporting time to first exacerbation identified an increase (i.e. benefit) with antibiotics, which was reported as statistically significant in four studies.
There was a statistically significant improvement in quality of life as measured by the St George's Respiratory Questionnaire (SGRQ) with prophylactic antibiotic treatment, but this was smaller than the four unit improvement that is regarded as being clinically significant (mean difference (MD) ‐1.94, 95% CI ‐3.13 to ‐0.75; participants = 2237; studies = 7, high‐quality evidence).
Prophylactic antibiotics showed no significant effect on the secondary outcomes of frequency of hospital admissions, change in forced expiratory volume in one second (FEV1), serious adverse events or all‐cause mortality (moderate‐quality evidence). There was some evidence of benefit in exercise tolerance, but this was driven by a single study of lower methodological quality.
The adverse events that were recorded varied among the studies depending on the antibiotics used. Azithromycin was associated with significant hearing loss in the treatment group, which was in many cases reversible or partially reversible. The moxifloxacin pulsed study reported a significantly higher number of adverse events in the treatment arm due to the marked increase in gastrointestinal adverse events (P < 0.001). Some adverse events that led to drug discontinuation, such as development of long QTc or tinnitus, were not significantly more frequent in the treatment group than the placebo group but pose important considerations in clinical practice.
The development of antibiotic resistance in the community is of major concern. Six studies reported on this, but we were unable to combine results. One study found newly colonised participants to have higher rates of antibiotic resistance. Participants colonised with moxifloxacin‐sensitive pseudomonas at initiation of therapy rapidly became resistant with the quinolone treatment. A further study with three active treatment arms found an increase in the degree of antibiotic resistance of isolates in all three arms after 13 weeks treatment.
Authors' conclusions
Use of continuous and intermittent prophylactic antibiotics results in a clinically significant benefit in reducing exacerbations in COPD patients. All studies of continuous and intermittent antibiotics used macrolides, hence the noted benefit applies only to the use of macrolide antibiotics prescribed at least three times per week. The impact of pulsed antibiotics remains uncertain and requires further research.
The studies in this review included mostly participants who were frequent exacerbators with at least moderate‐severity COPD. There were also older individuals with a mean age over 65 years. The results of these studies apply only to the group of participants who were studied in these studies and may not be generalisable to other groups.
Because of concerns about antibiotic resistance and specific adverse effects, consideration of prophylactic antibiotic use should be mindful of the balance between benefits to individual patients and the potential harms to society created by antibiotic overuse. Monitoring of significant side effects including hearing loss, tinnitus, and long QTc in the community in this elderly patient group may require extra health resources.
Plain language summary
Preventative antibiotic therapy for people with COPD
What is COPD?
COPD is a common chronic respiratory disease mainly affecting people who smoke now or have done so previously. It could become the third leading cause of death worldwide by 2020. People with COPD experience gradually worsening shortness of breath and cough with sputum (phlegm) because of permanent damage to their airways and lungs. Those with COPD may have flare‐ups (or exacerbations) most commonly with respiratory infections. Exacerbations may lead to further irreversible loss of lung function, as well as days off work, hospital admission, reduction in quality of life, or even death.
Why did we do this review?
We wanted to find out if giving antibiotics to prevent a flare‐up ('prophylactic' antibiotics) would reduce the frequency of flare‐ups and improve quality of life. Studies that were taken into consideration used either continuous prophylactic antibiotics (every day), or antibiotics that were used intermittently (three times per week) or pulsed (e.g. for five days every eight weeks)
What evidence did we find?
We carried out the latest search for studies in July 2018. We found 14 randomised controlled trials (RCTs) involving 3932 participants. All studies were published between 2001 and 2015. Nine studies were of continuous antibiotics, two studies were of intermittent antibiotic prophylaxis and two were of pulsed antibiotics. The final study included one continuous, one intermittent, one pulsed and one placebo arm. The antibiotics investigated were azithromycin, erythromycin, clarithromycin, roxithromycin, doxycycline and moxifloxacin. On average, the people involved in the studies were 65 to 72 years old and had moderate or severe COPD. Three studies included participants with frequent exacerbations and two of the studies recruited participants requiring steroid tablets or antibiotics or both, or who were at the end stage of their disease and required oxygen. One study only included people with a particular complication of COPD, involving the heart and blood vessels in the lungs (known as pulmonary hypertension).
Results and conclusions
We found that, with the use of antibiotics, the number of participants who developed an exacerbation reduced markedly. For every eight participants treated, one person would be prevented from suffering an exacerbation. However, not all the antibiotic regimens had the same impact on exacerbations. The results suggested that antibiotics given at least three times per week may be more effective than antibiotics given daily for a few days followed by a break of several weeks. We also found there may have been a benefit on patient‐reported quality of life with the antibiotics. On the other hand, use of antibiotics did not significantly affect the number of deaths due to any cause, the frequency of hospitalisation, or the loss of lung function during the study period.
Even though there may be fewer exacerbations with antibiotics, there are considerable drawbacks of taking antibiotics. First, there were specific adverse events associated with the antibiotics, which differed according to the antibiotic used; second, patients have to take antibiotics regularly for months or years; finally, the resulting increase in antibiotic resistance will have implications for both individual patients and the wider community through reducing the effectiveness of currently available antibiotics.
Because of concerns about antibiotic resistance and specific adverse effects, consideration of prophylactic antibiotic use should be mindful of the balance between benefits to individual patients and the potential harms to society created by antibiotic overuse.
Summary of findings
Summary of findings for the main comparison. Antibiotics versus placebo for COPD.
| Antibiotics versus placebo for COPD (data from pulsed and continuous courses of antibiotics presented in the same table) | ||||||
|
Patient or population: Adults (aged 40 or over) with COPD presenting with 1 or more exacerbations in the previous year. The two larger studies (Albert 2011; Sethi 2010) recruited participants who required systemic steroids or antibiotics for exacerbations or participants on supplemental oxygen Settings: Outpatients presenting to hospital clinics Intervention: Administration of an oral prophylactic antibiotic continuously or intermittently Comparison: Administration of a placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics versus placebo | |||||
|
Number of people with one or more exacerbations WMD of follow‐up of 49 weeks |
606 per 1,000 |
468 per 1,000 (393 to 546) |
OR 0.57 (0.42 to 0.78) |
2716 (8 RCTs) | ⊕⊕⊕⊝ Moderate1 | Subgroup analysis of continuous versus intermittent versus pulsed antibiotics suggested that pulsed antibiotics were less effective at reducing exacerbations (P = 0.01 for subgroup difference; I2 = 77.3%) |
|
Rate of exacerbation per patient/year WMD of follow‐up 54 weeks |
Rate ratio 0.67 (0.54 to 0.83) |
1384 (5 RCTs) |
⊕⊕⊕⊝ Moderate2 |
Test for subgroup difference between continuous and intermittent antibiotics not significant (P = 0.38; I2 = 0%) | ||
| HRQoL, SGRQ (total score) Scale from: 0 to 100. SGRQ comprises responses to 50 items, 0 being the best possible score and 100 the worst. WMD of follow‐up 48 weeks | The mean change in SGRQ ranged across control groups from a 0.9 unit increase to a 5.7 unit decrease | The mean HRQoL (SGRQ total score) in the intervention group was 1.94 lower (3.13 lower to 0.75 lower) | 2237 (7 RCTs) | ⊕⊕⊕⊕ High | The minimally clinically important response to treatment is described as 4 points Test for subgroup differences between continuous, intermittent and pulsed antibiotics not significant (P = 0.35; I² = 5.2%) |
|
| All‐cause mortality WMD of follow‐up 70 weeks | 78 per 1000 |
68 per 1,000 (53 to 88) |
OR 0.87 (0.66 to 1.15) |
3309 (6 RCTs) | ⊕⊕⊕⊝ Moderate3 | Test for subgroup differences between continuous, intermittent and pulsed antibiotics not significant (P = 0.60; I² = 0%) |
| Serious adverse events WMD of follow‐up 51 weeks | 253 per 1000 |
229 per 1,000 (200 to 262) |
OR 0.88 (0.74 to 1.05) |
2978 (9 RCTs) | ⊕⊕⊕⊝ Moderate3 | See Effects of interventions for specific adverse events related to the individual antibiotics. Test for subgroup differences between continuous, intermittent and pulsed antibiotics not significant (P = 0.60; I² = 0%) |
|
Any adverse event WMD of follow‐up 47 weeks |
640 per 1,000 | 655 per 1,000 (551 to 748) | OR 1.07 (0.69 to 1.67) | 512 (4 RCTs) | ⊕⊕⊕⊝ Moderate3 | Test for subgroup differences between continuous, intermittent and pulsed antibiotics not significant (P = 0.28), I² = 21.9%) |
|
FEV1 (mL) WMD of follow‐up 26 weeks |
The mean FEV1 in the control group ranged from 1,000 to 2,320 mL | The mean FEV1 in the intervention group was 20.21 mL higher (26.19 lower to 66.61 higher) | ‐ | 658 (6 RCTs) | ⊕⊕⊕⊝ Moderate4 | MCID for this outcomes was approximately 100 mLs. Mean difference and confidence interval lied within this MCID. Test for subgroup differences between continuous, intermittent and pulsed antibiotics not significant (P = 0.37; I² = 0.6%) |
| *The basis for the assumed risk was the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FEV1: forced expiratory volume in 1 second; HRQoL: health‐related quality of life; MCID: minimum clinically important difference;OR: Odds ratio; SGRQ: St George's respiratory questionnaireWMD: weight mean duration | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Clinical and statistical heterogeneity between studies (I2 = 58%), partly explained by antibiotic regimen. Downgraded once for inconsistency
2 Clinical and statistical heterogeneity between trials (I2 = 52%). Downgraded once for inconsistency 3 Confidence intervals included the possibility that prophylactic antibiotics may increase or decrease mortality or adverse events. Downgraded once for imprecision
4Confidence interval included both a decrease or increase in FEV1 associated with the intervention. However, the mean difference and confidence interval lay within the MCID. No downgrade
5Studies contributing majority of weight in analysis reported outcome at approximately 3 months. Duration may be too short to detect a difference in lung function between groups. Downgraded once for indirectness
Background
Description of the condition
The Global Initiative for Chronic Obstructive Lung Diseases (GOLD) defines chronic obstructive pulmonary disease (COPD) as "a common, preventable and treatable disease, that is characterised by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar (small air sacs within the lungs where gas exchange takes place) abnormalities usually caused by significant exposure to noxious particles or gases" (GOLD 2018). It has become a leading cause of morbidity and mortality worldwide, with latest figures suggesting it was responsible for approximately 3.2 million deaths globally in 2015, making it the fourth leading cause of death that year (WHO). Projections estimate it will become the third leading cause of death worldwide by 2020, due to an aging global population with prolonged exposure to COPD risk factors (GOLD 2018). COPD in high‐income countries is almost exclusively a disease of tobacco smoking, although a small proportion of nonsmokers have COPD secondary to passive smoking or genetic diseases, including alpha1 antitrypsin deficiency (nonsmokers with COPD are usually excluded from clinical trials). In lower‐income countries the major risk factor is indoor pollution (burning wood for heating or biomass fuels for cooking) which contributes more than smoking to the disease burden (WHO). Most reported deaths due to COPD are from high‐ and middle‐income countries; however, it is estimated that 90% of COPD‐related deaths occur in low‐middle‐income countries, where population‐based prevention strategies are either inaccessible or not implemented (WHO).
COPD is diagnosed by spirometry (a type of breathing assessment) and clinical symptoms of dyspnoea (difficulty breathing), chronic cough, or sputum production and a history of exposure to known risk factors, e.g. smoking. To make the diagnosis, a post‐bronchodilator cut‐off of a ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) less than 0.7 is used as an objective measure of airflow limitation. To individualise the management of COPD for each patient, GOLD has developed staging systems to classify severity. Patients are graded from stage one to stage four according to spirometric criteria, with stage one representing mild airflow obstruction (FEV1 ≥ 80% predicted), stage two moderate (FEV1 < 80% but ≥ 50% predicted), stage three severe (FEV1 < 50% but ≥ 30% predicted) and stage four very severe (FEV1 < 30%) (GOLD 2018). However, the most recent report de‐emphasises FEV1 as a useful prognostic tool at an individual level and instead focuses on functional limitation and symptoms to guide therapy for stable COPD (Group A: 0 to 1 exacerbations not leading to hospital admission and COPD assessment test (CAT) score < 10 or Modified Medical Research Council Dyspnea Scale (MMRC) 0 to 1; group B: 0 to 1 exacerbations not leading to hospital admission and CAT ≥ 10 or MMRC ≥ 2; group C: ≥ 2 exacerbations or ≥ 1 exacerbation leading to hospital admission and CAT < 10 or MMRC 0 to 1; group D: ≥ 2 exacerbations or ≥ 1 exacerbation leading to hospital admission and CAT ≥ 10 or MMRC ≥ 2)).
Many people with COPD experience acute exacerbations, which are defined as "an acute worsening of respiratory symptoms that result in additional therapy" (GOLD 2018). Exacerbations of COPD range in severity between individuals, have a substantial impact on quality of life and contribute to overall disease progression (GOLD 2018). Therefore, preventing and treating exacerbations is an important part of COPD management in order to improve quality of life and prognostic outcomes. Exacerbations of COPD are a common cause of days off work and hospital admissions (TSANZ 2004), and so have a significant socioeconomic impact globally. Furthermore the long‐term prognosis following hospitalisation for an exacerbation of COPD is poor, with a five‐year mortality rate of approximately 50% (GOLD 2018).
Respiratory infections are known triggers for COPD exacerbations, with current evidence suggesting that viral infections account for the majority of exacerbations (GOLD 2018; Woodhead 2011). However, bacterial respiratory infections and changes in the local environment, such as an increase in air pollution, are also recognised as exacerbation triggers (Papi 2006). The role of bacteria in exacerbations is an area that has been greatly studied, yet remains controversial. It has been demonstrated that respiratory bacterial loads are greater in patients with stable COPD compared to healthy individuals, and that the bacterial loads increase with disease severity (Beasley 2012). However, the high bacterial isolation rates in stable COPD makes it difficult to identify a causative role of bacteria in exacerbations. Despite this, studies do suggest an increase in bacterial infection rates amongst participants with acute COPD exacerbations (Beasley 2012). Wilkinson 2006 found that the prevalence of potentially pathogenic microorganisms rose from 48.2% at baseline in stable COPD participants to 69.6% in the same group of participants at the time of an exacerbation. The most commonly isolated bacterial organisms in COPD exacerbations include "Haemophilus influenzae (11% of all exacerbating participants), Streptococcus pneumoniae (10%), Moraxella catarrhalis (10%) and Pseudomonas aeruginosa (4%), with Gram‐negative bacteria occurring more rarely" (Sapey 2006).
There are numerous evidence‐based approaches that aim to reduce the number of COPD exacerbations. An essential first step is the avoidance of cigarette smoke and air pollution, wherever possible. Furthermore, vaccination against influenza is a universally accepted measure to prevent COPD exacerbations. Vaccination for pneumococcal disease may also reduce pneumonia and COPD exacerbations (Lee 2007). Inhaled medications shown to reduce exacerbation frequency include tiotropium, a long‐acting muscarinic antagonist (LAMA, UPLIFT 2008), long‐acting beta agonists (LABA, Wang 2012) and corticosteroids (ICS, TORCH 2007). Oral medications shown to reduce exacerbations include phosphodiesterase 4 (PDE4) inhibitors (Chong 2017) and mucolytic agents (drugs that help break down sputum making it easier to cough up) (Poole 2012).
Description of the intervention
One approach to reduce exacerbation frequency has been to use prophylactic antibiotics. The word prophylactic comes from the Greek for 'an advance guard', an apt term for a measure taken to fend off a disease or another unwanted consequence. A prophylactic intervention is a medication or treatment designed and used to prevent a disease from occurring. Thirty years ago, the use of prophylactic antibiotics was common for chronic bronchitis in both the United Kingdom and elsewhere, but concerns over effectiveness and antibiotic resistance led to a decline in this approach.
How the intervention might work
COPD is characterised by persistent airways inflammation due to chronic bacterial colonisation of the damaged respiratory epithelium (the layer of cells lining the airways) leading to the continuing release of bacterial and host‐mediated pro‐inflammatory factors and additional epithelial damage (Matkovic 2013; Sethi 2008). In an exacerbation, there is superimposed acute inflammation (Hurst 2006). By reducing bacterial colonisation, chronic antibiotic therapy could help in reducing progression of the disease by breaking the above vicious cycle. In addition, some antibiotics have intrinsic anti‐inflammatory properties (Martinez 2008).
Why it is important to do this review
This review incorporates and builds upon earlier Cochrane reviews.The most recent review concluded that the "use of continuous prophylactic macrolide antibiotics for a period of up to 12 months is likely to reduce the number of patients with exacerbations and exacerbation frequency, increase the median time to first exacerbation and possibly health‐related quality of life" (Herath 2013). However, adverse effects and the potential for the development of antibiotic resistance remain a concern. Since the 2013 review, a number of new studies into prophylactic antibiotic use in COPD have been published. Given the fine balance between the need to reduce exacerbation frequency in COPD, with the threat of widespread antibiotic resistance, it is important that the most up‐to‐date research is incorporated into this review, so that physicians and patients can make well informed decisions before embarking on long‐term treatment. This updated review also expands on the analysis of specific prophylactic antibiotic regimens including continuous, intermittent, and pulsed regimens to determine their relative efficacy and safety. Furthermore, many of the new studies have included more comprehensive assessments of quality of life indicators, which were not previously explored in great detail.
Objectives
To determine whether or not regular (continuous, intermittent or pulsed) treatment of COPD patients with prophylactic antibiotics reduces exacerbations or affects quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of antibiotic versus placebo. Trials comparing different antibiotics head‐to‐head will form the basis of another review. We planned to include cluster‐randomised trials and crossover trials, if found.
Types of participants
We included studies of adults (older than 18 years of age) with a diagnosis of COPD, as defined by the American Thoracic Society, European Respiratory Society or GOLD, with airflow obstruction evident by spirometry (post‐bronchodilator FEV1 of less than 80% of the predicted value and an FEV1/FVC of 0.7 or less). The review included studies only if they confirmed diagnosis with lung function testing (spirometry).
We excluded studies of participants with bronchiectasis, asthma, or genetic diseases, such as cystic fibrosis or primary ciliary dyskinesia (which may also lead to chronic airflow limitation as part of a secondary process). Where we encountered trials that included participants with these diseases in addition to participants with COPD, we only extracted the data for the participants with COPD, where the data were presented separately. However, although the studies excluded participants with clinical presentation of bronchiectasis, computed tomography (CT) screening to confirm radiological evidence of bronchiectasis was performed only in two studies (Albert 2011 and Uzun 2014) prior to study entry.
Types of interventions
We included studies of oral antibiotics, including penicillin (amoxycillin, amoxicillin, clavulanic acid), tetracycline (doxycycline, tetracycline), quinolones (ciprofloxacin, moxifloxacin), macrolides (clarithromycin, erythromycin, roxithromycin, azithromycin) and sulphonamides (co‐trimoxazole), administered in appropriate doses for a period of at least three months.
Types of outcome measures
Primary outcomes
Number of exacerbations, using an accepted definition. This included total numbers of participants with one or more exacerbation as well as the frequency of exacerbations in the study period and time to first exacerbation.
Health‐related quality of life, using an accepted measure such as the St George's Respiratory Questionnaire (SGRQ) (Jones 2009) or Chronic Respiratory Diseases Questionnaire (CRQ) (Guyatt 1987).
Secondary outcomes
Duration and severity (using an accepted definition) of exacerbations;
Days of disability (defined as days where the participant was unable to undertake normal activities);
Frequency and duration of hospital admissions;
Reduction in lung function from baseline, as measured by FEV1 and FVC;
Drug resistance as measured by microbial sensitivity;
Death due to all‐cause mortality, as well as due to respiratory causes;
Adverse effects.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways Trials Register up to 27 July 2018 with no restrictions on language or type of publication. The Cochrane Airways Trials Register is maintained by the information specialist for Cochrane Airways and contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies (CRS);
Weekly searches of MEDLINE Ovid SP;
Weekly searches of Embase Ovid SP;
Monthly searches of PsycINFO Ovid SP;
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature);
Monthly searches of AMED EBSCO (Allied and Complementary Medicine);
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We conducted a search of ClinicalTrials.gov with the search strategy in Appendix 3 up to 27 July 2018. For the 2018 update, we managed references using Rayyan (Ouzzani 2016).
Searching other resources
We checked the reference lists of all eligible primary studies and review articles for additional references. For the original review, we contacted authors of Mygind 2010 and asked them to supply the data from their unpublished study. For the 2018 update, we contacted the authors of all newly included studies and we are grateful for the responses received from the authors of Berkhof 2013; Shafuddin 2015; Simpson 2014 and Uzun 2014. We have checked the references of the included and excluded studies from the previous review on chronic bronchitis for possible studies (Staykova 2003).
Data collection and analysis
Selection of studies
For this update, two review authors (SH and RN) independently screened the abstracts of studies identified by the search as to whether or not they met our inclusion criteria. We obtained the full texts of publications for those that were considered definite or possible for inclusion. These were then reviewed independently by two review authors (SH and RN) to assess eligibility. We resolved any disagreement by discussion and consensus followed by an independent opinion from the third investigator (PP).
Data extraction and management
Both review authors independently extracted the data from the eligible studies.
We extracted the following data.
Methods: study design, duration of follow‐up.
Participants: age, gender, smoking status, study setting, inclusion and exclusion criteria.
Intervention: drug name, dose, duration of treatment, control or standard therapy.
Information on outcome measures.
Where appropriate, we have combined the data from studies using RevMan 5 2008.
Assessment of risk of bias in included studies
Two investigators independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear risk.
Measures of treatment effect
Results for continuous variables were expressed using a random‐effects model mean difference (MD) with 95% confidence interval (CI). Results for pooled outcomes with dichotomous variables were expressed using a random‐effects model odds ratio (OR) with 95% CI. We regarded a P value of less than 0.05 as statistically significant. We combined rate data (e.g. number of exacerbations per participant per year) using generic inverse variance (GIV) and expressed the outcome as a rate ratio.
For ease of communication and clarity, the number needed to treat for an additional beneficial outcome (NNTB) was derived from the OR and mean control group event rate using Visual Rx.
Unit of analysis issues
We did not find any crossover trials or cluster‐randomised trials that met our inclusion criteria. However, if we had encountered them, we planned to evaluate the cluster‐randomised trials for trial quality and, if the design and analysis were of poor quality, exclude them. We planned to analyse any eligible cluster‐randomised trials with the help of a statistician.
Dealing with missing data
We contacted the investigators from Mygind 2010 in writing in order to verify key study characteristics and to obtain missing numerical outcome data. We were unable to get more details.
Assessment of heterogeneity
From the forest plot, we tested for heterogeneity where the CIs did not overlap with each other. We used the I2 statistic to measure heterogeneity among the studies in each analysis. Where we identified heterogeneity (I2 ≥ 40%), we explored this using a prespecified subgroup analysis. We used the following overlapping cut‐off to define heterogeneity (Higgins 2011).
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity
Assessment of reporting biases
Where we suspected reporting bias, we attempted to contact the study authors to ask them to provide the missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, the impact of including such studies in the overall assessment of results was explored by a sensitivity analysis.
Data synthesis
For the 2018 update, we subgrouped all meta‐analyses by regimen, grouping interventions into continuous (i.e. daily) antibiotic use, intermittent (e.g. two or three times per week) antibiotic use and pulsed (e.g. daily for five days every four weeks) antibiotic use. We performed meta‐analysis only where the study populations were sufficiently similar for pooling to make sense.
We created a 'Summary of findings' table using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and GRADEpro software for the following outcomes.
Number of exacerbations, using an accepted definition.
Days of disability (defined as days where the participant was unable to undertake normal activities).
Frequency and duration of hospital admissions.
Health‐related quality of life, using an accepted measure such as SGRQ or CRQ.
Death.
Drug resistance.
Other adverse effects of treatment.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for the primary outcome (number of exacerbations).
Severity of COPD according to FEV1 and the GOLD criteria.
Type of antibiotic.
Duration of antibiotic use ( ≥ 3 months to < 6 months, ≥ 6 months to < 12 months and ≥ 12 months).
Year of conduct of study (2005 to 2009, 2010 to 2014 and 2014 to 2019)
Whether the antibiotic was used primarily as an antimicrobial or as an anti‐inflammatory agent.
Treatment regimen including dose, frequency, route of administration.
History of exacerbations (studies in which participants were only included in they had experienced at least one exacerbation in the preceding year versus those in which exacerbation history was not an inclusion criteria).
Sensitivity analysis
We conducted a sensitivity analysis on our primary outcome (people with one or more exacerbations) by removing studies judged to be at high or unclear risk of bias for the domains of sequence generation, allocation concealment, or blinding.
Results
Description of studies
Results of the search
We included seven studies in the 2013 version of this review. For the 2018 update, we identified 202 records through database searching and a further 76 additional records through other sources. We screened 265 records after removing duplicates. We excluded 226 records on the basis of the title and abstracts, leaving 39 full‐text articles which we assessed for eligibility. Of these, we excluded 10 studies (12 full‐text articles) and identified one as awaiting classification (Characteristics of studies awaiting classification). We identified four new ongoing studies, four new references to an included study (Albert 2011) and one ongoing study was moved to the included studies section (Uzun 2014). We moved one study from the excluded studies section to be a subreference of an included study (Banerjee 2005).
We identified nine new studies that were eligible for inclusion in this systematic review, taking the total number of eligible studies to 16 (Figure 1).
1.
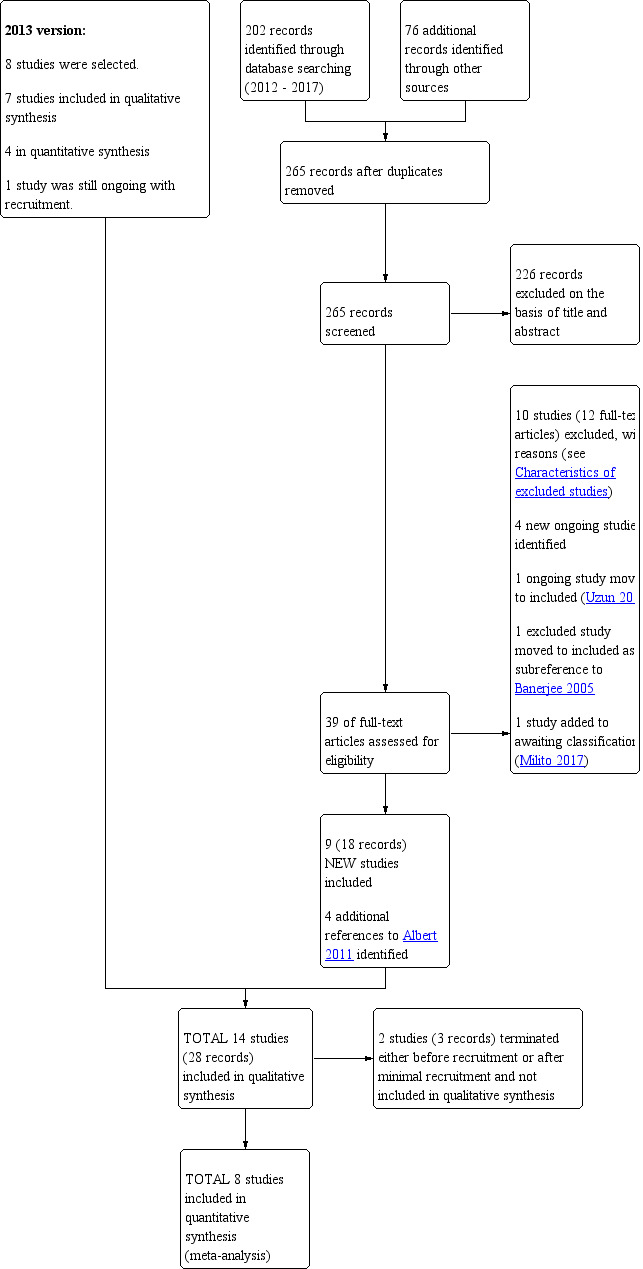
Study flow diagram: review update
Included studies
We identified 16 studies as eligible for the systematic review (Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; He 2010; Mygind 2010; NCT00524095; NCT02628769; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Suzuki 2001; Tan 2016; Uzun 2014; Wang 2017). The study durations varied from three to 36 months. For reasons not given, one study was terminated before the treatment phase (NCT00524095) and another was terminated after enrolment of five participants due to hepatotoxicity of the study drug, solithromycin (NCT02628769). See Characteristics of included studies and Table 2 for further details.
1. Summary of study characteristics.
| Study | Country | No. of patients | Age (range unless otherwise stated) | % predicted FEV1 (range ) unless otherwise stated | Intervention | Comparator | Duration of treatment |
| Albert 2011 | United States of America | 1142 | 65 ‐ 66 | 39 ‐ 40 | Azithromycin 250 mg daily | Placebo | 12 months |
| Banerjee 2005 | United Kingdom | 67 | 65.1 ‐ 68.1 | 42.5 ‐ 43.9 | Clarithromycin ‐ long‐acting Klaricid XL 500 mg daily | Placebo | 3 months |
| Berkhof 2013 | Netherlands | 84 | 67 ‐ 68 | 47.4 ‐ 49.8 | Azithromycin 250 mg 3 times a week | Placebo | 12 weeks |
| Brill 2015 | United Kingdom | 99 | 67.9 ‐ 70.4 | 44 ‐ 53 | Moxifloxacin 400 mg/day for 5 days every 4 weeks | Placebo | 13 weeks |
| Doxycycline 100 mg daily | |||||||
| Azithromycin 250 mg 3 times a week | |||||||
| He 2010 | China | 36 | 68.8 ‐ 69.3 | 42.1 ‐ 44.3 | Erythromycin 125 mg 3 times a day | Placebo | 6 months |
| Mygind 2010 | Denmark | 575 | 71 (median) | 38.4 (median) | Azithromycin 500 mg 3 days a month | Placebo | 36 months |
| NCT00524095 (terminated; details given represent proposal) | Italy | 210 | 45 ‐ 85 | N/A | Azithromycin 500 mg 3 times a week for 6 months, then fluticasone 500 μg twice a day for 6 months | Usual care | 1 year |
| Fluticasone 500 μg twice a day for 6 months, then azithromycin 500 mg 3 times a week for 6 months | |||||||
| NCT02628769 (terminated; details given represent proposal) | United Kingdom | 5 | N/A | N/A | Solithromycin 400 mg daily | Placebo | 28 days |
| Seemungal 2008 | United Kingdom | 109 | 66 ‐ 68 | 49.25 ‐ 50.55 | Erythromycin 250 mg twice a day | Placebo | 12 months |
| Sethi 2010 | International | 1157 | 66.1 ‐ 66.6 | 40.6 ‐ 42.2 | Moxifloxacin 400 mg daily for 5 days every 8 weeks | Placebo | 48 weeks |
| Shafuddin 2015 | Australia & New Zealand | 292 | 65.8 ‐ 67.6 | 32.53 ‐ 35.8 | Roxithromycin 300 mg daily and Doxycycline 100 mg daily | Placebo | 12 weeks |
| Roxithromycin 300 mg daily | |||||||
| Simpson 2014 | Australia | 30 | 69.9 ‐ 71.1 | 51.1 ‐ 56.5 | Azithromycin 250 mg daily | Placebo | 12 weeks |
| Suzuki 2001 | Japan | 109 | 69.1 ‐ 71.7 | 1.3 ‐ 1.47 L | Erythromycin 200 ‐ 400 mg daily | Riboflavin 10 mg daily | Unclear |
| Tan 2016 | China | 54 | 67.3 ‐ 69.3 | 42.1 ‐ 46.5 | Erythromycin 125 mg 3 times a day for 12 months | Placebo | 12 months |
| Erythromcyin 125 mg 3 times a day for 6 months | |||||||
| Uzun 2014 | Netherlands | 92 | 64.7 ‐ 64.9 | 44.2 ‐ 45 | Azithromycin 500 mg three times a week | Placebo | 12 months |
| Wang 2017 | China | 86 | 70.54 ‐ 72.43 | Unclear | Azithromycin 250 mg daily | Simvastatin 20 mg daily | 6 months |
FEV1: forced expiratory volume in one second.
From this point forward, we will describe only the 14 completed studies, involving 3932 participants.
Nine studies involving 1925 participants investigated continuous macrolide antibiotics administered on at least a daily basis. These included azithromycin (Albert 2011; Simpson 2014; Wang 2017), erythromycin (He 2010; Seemungal 2008; Suzuki 2001; Tan 2016), roxithromycin (Shafuddin 2015), and clarithromycin (Banerjee 2005). Shafuddin 2015 compared the combination of a macrolide and tetracycline (roxithromycin and doxycycline) with roxithromycin alone, and included a placebo arm.
Two studies involving 176 participants investigated intermittent antibiotics which were administered three times a week for 12 weeks and 12 months respectively (Berkhof 2013; Uzun 2014).
Two studies involving 1732 participants investigated pulsed antibiotic prophylaxis (Mygind 2010; Sethi 2010). In Mygind 2010, azithromycin was given for three days every month for 36 months and in Sethi 2010, moxifloxacin was given for five days every eight weeks for a total of six antibiotic courses.
One study that involved 99 participants compared three treatment arms with placebo for a duration of 13 weeks. One arm involved a continuous regimen (doxycycline 100 mg daily), one arm involved an intermittent regimen (azithromycin 250 mg for 3 times a week) and one arm involved a pulsed regimen (moxifloxacin daily for five days every four weeks) (Brill 2015). As such, Brill 2015 was included in the subgroup analyses for all three regimen groups, with the control group split three ways.
All except one study (Wang 2017) were randomised, placebo‐controlled, parallel group trials. Ten studies were double‐blinded. One was single‐blinded (Brill 2015), one was not blinded (Suzuki 2001), and there were no comments regarding blinding methods in two of the studies (Tan 2016; Wang 2017). All studies were published in journals except Mygind 2010, which was an oral presentation at the European Respiratory Society Conference in 2010. The studies were published or presented between 2001 and 2017.
All studies, except Tan 2016 and Wang 2017, listed exacerbation frequency and/or health‐related quality of life as primary, co‐primary or secondary outcomes. Twelve studies were analysed using intention‐to‐treat analysis. For two studies, it was unclear if intention‐to‐treat analysis was used (Tan 2016; Wang 2017). Sethi 2010 reported both a per protocol analysis as well as an intention‐to‐treat analysis, but, for the review, we have included only the intention‐to‐treat analysis results.
Of note, the Shafuddin 2015 study was originally designed "to test the hypothesis that Chlamydia pneumoniae (now Chlamydophilia pneumoniae) was a pathogenic factor in the aetiology of COPD and that eradication of C. pneumoniae infections could reduce exacerbation rates". In view of this original hypothesis, the study design was such that all included participants tested positive for C. pneumoniae, and the antibiotic regimens were chosen with the aim of eradicating C. pneumoniae infection specifically. This included a combined treatment arm of roxithromycin and doxycycline, which was thought to be more successful at eradicating C. pneumoniae compared to roxithromycin alone. In their background text, the authors explained that "this hypothesis is now considered unsubstantiated and is no longer believed to be clinically relevant". However, they have used their collected data to examine the effect of prophylactic antibiotic therapy on COPD exacerbations, reporting that their data may reasonably be applied to the general COPD population with frequent exacerbations, as differences between their included participants with C. pneumoniae are unlikely to have an effect on efficacy endpoints or the interpretation of results (Shafuddin 2015).
Study funding
Albert 2011 was supported by grants from the National Institutes of Health, Banerjee 2005 received a grant from Abbott, Berkhof 2013 received financial support from Stichting Astma Bestrijding, Brill 2015 was funded by the National Institute for Health Research, He 2010 was supported by grants from the National Nature Science Foundation of China, Seemungal 2008 was supported by the British Lung foundation, Sethi 2010 was supported by a research grant from Bayer HealthCare AB, Shafuddin 2015 was supported by Sanofi‐Aventis Australia Pty Ltd, Simpson 2014 was funded by the National Health and Medical Research Council of Australia, Tan 2016 was funded by the National Nature Science Foundation of China and the Guangxi Natural Science Foundation, and Uzun 2014 was funded by a trust called SoLong, which is associated with the department of Respiratory Medicine of the Amphia Hospital in the Netherlands. From the material available to us, the funding for Mygind 2010 and Suzuki 2001 was unclear. Wang 2017 reported that they had no grant support or financial disclosures.
Excluded studies
Excluded studies are listed in the Characteristics of excluded studies table, along with the reasons for exclusion.
Risk of bias in included studies
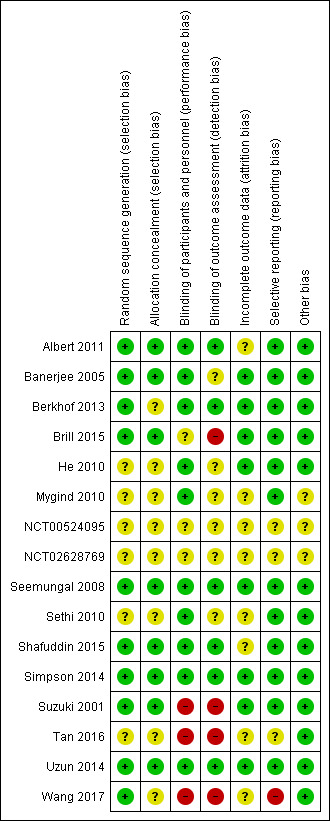
Judgements and reasons for the judgements can be found in Characteristics of included studies and an overview of our judgements can be found in Figure 2.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was well described in ten of the studies, which we judged to be at low risk of bias in this domain (Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; Seemungal 2008; Shafuddin 2015; Simpson 2014; Suzuki 2001; Uzun 2014; Wang 2017). Four studies did not describe random sequence generation clearly and we judged them to be at unclear risk (He 2010; Mygind 2010; Sethi 2010; Tan 2016).
Allocation concealment was well described in eight studies, which we judged to be at low risk of bias in this domain (Albert 2011; Banerjee 2005; Brill 2015; Seemungal 2008; Shafuddin 2015; Simpson 2014; Suzuki 2001; Uzun 2014). Six studies did not describe allocation concealment clearly and we judged them to be at unclear risk in this domain (Berkhof 2013; He 2010; Mygind 2010; Sethi 2010; Tan 2016; Wang 2017).
Mygind 2010 is a conference presentation, and, as such, we had access to limited data. We were not successful in obtaining further information from authors despite multiple attempts by email and post.
Blinding
Blinding of the participants and personnel (performance bias) was described in ten of the included studies (Albert 2011; Banerjee 2005; Berkhof 2013; He 2010; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Uzun 2014), which we rated as low risk. Suzuki 2001 was not blinded and therefore judged to be at high risk of bias. Brill 2015 was a single‐blinded study with only the participants being blinded to treatment allocation, so we judged this to be at unclear risk of bias. Blinding of participants and personnel was not described in Tan 2016 or Wang 2017 and despite multiple attempts by email to obtain further information from the corresponding authors, no responses have been received. We therefore judged these studies to be at high risk of bias.
Blinding of the outcome assessment (detection bias) was well described in six of the included studies (Albert 2011; Berkhof 2013; Seemungal 2008; Shafuddin 2015; Simpson 2014; Uzun 2014), while four were judged to be at a high risk of bias (Brill 2015; Suzuki 2001; Tan 2016; Wang 2017), and the remaining four were unclear.
Incomplete outcome data
Outcomes of the study participants were well described using either a CONSORT diagram (Albert 2011; Berkhof 2013; Brill 2015; He 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Uzun 2014) or by a dedicated paragraph or table (Banerjee 2005; Suzuki 2001; Tan 2016). Overall, withdrawal rates were similar between both studies and treatments and we judged these studies to be at low risk of attrition bias, with the exception of four studies (Albert 2011; Sethi 2010; Shafuddin 2015; Tan 2016).
In both Albert 2011 and Sethi 2010, we noted the reason for missing health‐related quality of life (HRQoL) data was not given, and we therefore rated the studies at unclear risk. We also judged Shafuddin 2015 to be at unclear risk because more participants dropped out of the combined antibiotic treatment arm compared to the single antibiotic and placebo arms (21 versus 13 versus 10), although all randomised participants were included in the intention‐to‐treat analysis. We judged Tan 2016 to be at unclear risk because the authors did not describe how many participants were analysed at each time point.
Mygind 2010 was a conference presentation of unpublished data and thus we had limited information on which to judge the attrition bias and we therefore rated the study to be at unclear risk. Wang 2017 also did not include any information on the outcomes of study participants and we judged it to be at unclear risk.
Selective reporting
Twelve of the included studies reported all prespecified primary and secondary outcomes and these were judged to be at low risk of bias. For Tan 2016, we were unable to identify a prospective trial registration or protocol so it was not clear if outcomes of interest for this review may have been collected but not reported (e.g. serious adverse events, exacerbations, and quality of life). We identified one study as being at high risk for selective reporting bias (Wang 2017). See Characteristics of included studies to view bias tables for more details.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
An overview of the results together with a summary of the our confidence in the evidence per outcome is presented in Table 1.
Primary outcome: number of participants with one or more exacerbations
We included eight studies in the meta‐analysis of the number of participants experiencing one or more exacerbations of COPD (Albert 2011; Berkhof 2013; Brill 2015; He 2010; Seemungal 2008; Sethi 2010; Simpson 2014; Uzun 2014). Suzuki 2001 was not included in the meta‐analysis as this study was not blinded.
We found that prophylactic antibiotics reduce the overall odds of having one or more exacerbations over the treatment period compared to placebo (OR 0.57, 95% CI 0.42 to 0.78; participants = 2716; studies = 8; I2 = 42%; moderate‐quality evidence, Analysis 1.1; Figure 3). This equates to a 13.9 percentage‐point reduction in absolute risk. In the control group, 61 people out of 100 had one or more exacerbations compared to 47 (95% CI 39 to 55) out of 100 in the antibiotic group (Figure 4). The number needed to treat for an additional beneficial outcome (NNTB) was 8 (95% CI 5 to 17).
1.1. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 1 Number of people with one or more exacerbations.
3.
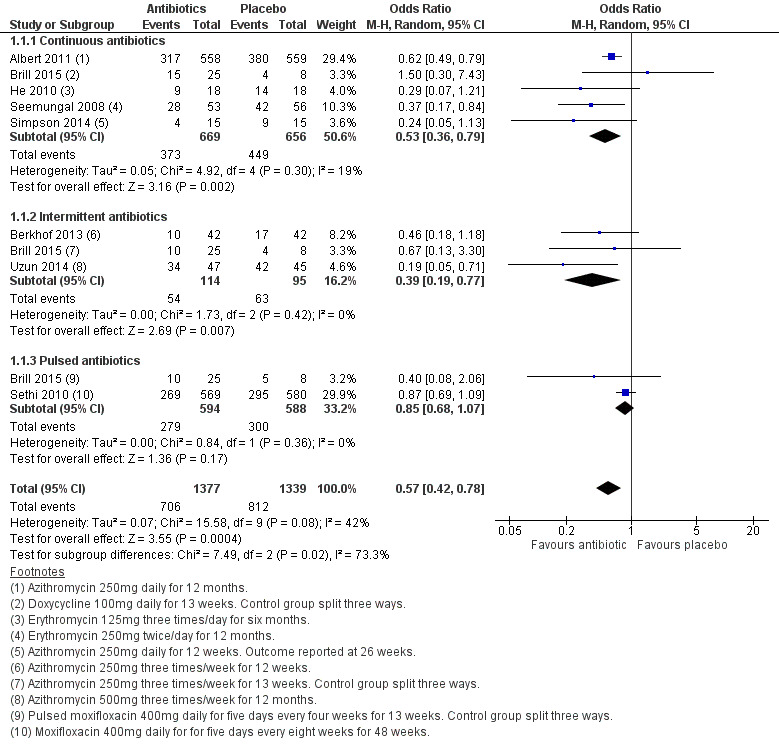
Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.1 Number of people with one or more exacerbations.
4.
In the control group, 61 people out of 100 had one or more exacerbations over 12 weeks to 12 months, compared to 47 (95% CI 39 to 55) out of 100 for the antibiotic treatment group (Analysis 1.1)
The heterogeneity of the eight studies analysed for this outcome was moderate (I2 = 42%), which has been explored using pre‐planned subgroup analyses.
Of these studies, five (including one arm of Brill 2015), were of continuous antibiotic prophylaxis. Compared to placebo, continuous antibiotics reduced the number of participants experiencing one or more exacerbations (OR 0.53, 95% CI 0.36 to 0.79; participants = 1325; studies = 5; I2 = 19%; Analysis 1.1). This equated to a number needed to treat for an additional beneficial outcome of 7 (95% CI 5 to 19).
Similarly, the analysis of three studies investigating intermittent antibiotic regimens (including one arm of Brill 2015) suggested a benefit in favour of antibiotics compared to placebo in reducing the number of participants experiencing one or more exacerbations (OR 0.39, 95% CI 0.19 to 0.77; participants = 209; studies = 3; I2 = 0%; Analysis 1.1).The number needed to treat to for an additional beneficial outcome was 5 (95% CI 3 to 17).
Pulsed antibiotic regimens did not significantly reduce the number of people with at least one exacerbation (OR 0.85, 95% CI 0.68 to 1.07; participants = 1182; studies = 2; I2 = 0% Analysis 1.1).
The test for subgroup differences between continuous, intermittent, and pulsed regimens suggested a statistically significant difference between the groups (Chi² = 7.49, df = 2 (P = 0.02), I² = 73.3%). However, this was largely driven by the pulsed antibiotic subgroup, as when this subgroup was removed, the test for subgroup differences between the continuous and intermittent antibiotic groups was not significant (Chi² = 0.62, df = 1 (P = 0.43), I² = 0%).
One study of 84 participants, that investigated continuous azithromycin versus placebo, reported the number of exacerbations of COPD that required hospitalisation (Berkhof 2013). The number of events were too infrequent to draw any conclusion on the impact of prophylactic antibiotics in this situation.
Primary outcome: rate of exacerbations per patient per year
The exacerbation rate was expressed as a rate ratio, which was calculated using the generic inverse variance (GIV) method in RevMan software.
Five studies contributed data to this analysis, four investigating continuous regimens (Albert 2011; He 2010; Seemungal 2008; Simpson 2014) and one investigating an intermittent regimen (Uzun 2014). Compared to placebo, prophylactic antibiotics reduced the rate of exacerbations per patient per year (rate ratio 0.67, 95% CI 0.54 to 0.83; participants = 1384; studies = 5; moderate‐quality evidence; Analysis 1.4; Figure 5). Considering the different regimens separately, use of continuous prophylactic antibiotics was also associated with a reduction (rate ratio 0.69, 95% CI 0.54 to 0.89; participants = 1292; studies = 4) as was use of intermittent antibiotics (rate ratio 0.58, 95% CI 0.42 to 0.80; participants = 92; studies = 1). There was a moderate level of heterogeneity among the included studies (I2 = 52%).
1.4. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 4 Rate of exacerbation per patient per year.
5.
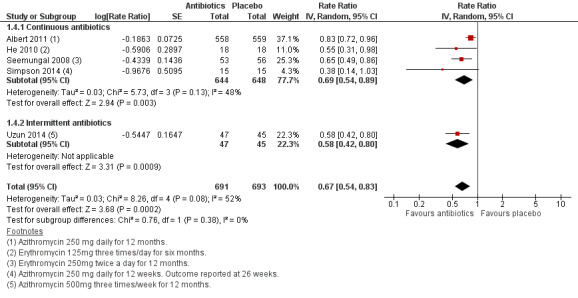
Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.4 Rate of exacerbation per patient per year.
A subgroup analysis performed in two studies (Albert 2011; Sethi 2010), according to the severity of COPD as defined by the GOLD criteria which were current at that time (2011), did not show a difference between the subgroups in the effect of antibiotics on exacerbation frequency (Analysis 1.8).
1.8. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 8 COPD exacerbations according to severity of COPD ‐ pulsed antibiotics.
| COPD exacerbations according to severity of COPD ‐ pulsed antibiotics | |||
|---|---|---|---|
| Study | Gold stage | Odds ratio for exacerbations, moxifloxacin vs placebo (95% CI) | P value |
| Sethi 2010 | 2 : FEV1 (80% ‐ 50%) | 0.65 (0.39 to 1.06) | 0.091 |
| Sethi 2010 | 3 : FEV1 (50% ‐ 30%) | 0.81 (0.58 to 1.10) | 0.192 |
| Sethi 2010 | 4 : FEV1 (< 30%) | 0.83 (0.54 to 1.28) | 0.459 |
Time to first exacerbation
The median time to first exacerbation was analysed using a Kaplan‐Meier survival curve and log‐rank test. We did not perform a meta‐analysis for this outcome and findings from individual studies are tabulated in Analysis 1.5. Data were available for six studies involving 2620 participants (Albert 2011; Berkhof 2013; He 2010; Seemungal 2008; Sethi 2010; Uzun 2014).
1.5. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 5 Time to the first exacerbation.
| Time to the first exacerbation | |||||
|---|---|---|---|---|---|
| Study | MEDIAN Time to 1st exacerbation (days) treatment | MEDIAN Time to 1st exacerbation (days) placebo | P value | Test used | Hazard ratio |
| Continuous antibiotic | |||||
| Albert 2011 | 266 (227 to 313) | 174 (143 to 215) | P < 0.001 | log‐rank test | 0.73 (0.63, 0.84) |
| He 2010 | 155 | 86 | P = 0.032 | Kaplan‐Meier survival analysis | not given |
| Seemungal 2008 | 271 | 89 | P = 0.02 | log‐rank test | not given |
| Intermittent antibiotics | |||||
| Berkhof 2013 | 20th percentile time to the first exacerbation 105 (30) | 20th percentile time to the first exacerbation 66 (21) | P = 0.13 | log‐rank test | not given |
| Uzun 2014 | 130 (95% CI 28 to 232) | 59 (95% CI 31 to 87) | P = 0.001 | log‐rank test | not given |
| Pulsed antibiotics | |||||
| Sethi 2010 | 364 | 336 | P = 0.062 | Kaplan‐Meier survival analysis | not given |
Three studies used continuous prophylactic antibiotics, involving 1287 participants. Use of a continuous prophylactic antibiotic lengthened the time to first exacerbation in all three studies compared with placebo, and this was a statistically significant difference in all three. In Albert 2011, this was 266 days (antibiotic) versus 174 days (placebo) (P < 0.001); in He 2010,155 days versus 86 days (P = 0.032) and in Seemungal 2008, 271 days versus 89 days (P = 0.02).
Two studies, involving a total of 176 participants, investigated intermittent antibiotics and similarly found the time to first exacerbation was lengthened in both studies. In Uzun 2014, this was 130 days versus 59 days (P = 0.001). In Berkhof 2013, the 20th percentile time to first exacerbation was 105 days (antibiotic) versus 66 days (placebo), but this was not statistically significantly different (P = 0.13).
The median time to the first exacerbation in Sethi 2010 was increased by the use of pulsed antibiotics, but the difference was not statistically significant: 364 days versus 336 days (P = 0.062).
One study, Shafuddin 2015, that involved 292 participants allocated to receive either continuous roxithromycin and doxycyline, continuous roxithromycin alone or placebo, reported the mean time (in days) to first exacerbation of COPD. Their results were inconclusive for this outcome and there was no significant difference between the treatment arms (MD ‐17 days, 95% CI ‐46 to 13; participants = 266; I2 = 0%)
In Albert 2011, which used continuous azithromycin, a predefined subgroup analysis in 22 subgroups found that prophylactic antibiotics were associated with greater treatment effects in terms of lengthening time to first exacerbation in participants who had given up smoking (test for interaction P = 0.012), were not on steroid inhaler treatment at enrolment (P = 0.032), or older than 65 years (P = 0.012).
Primary outcome: health‐related quality of life
Health‐related quality of life was explored in nine studies (Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; He 2010; Mygind 2010; Sethi 2010; Simpson 2014; Uzun 2014). The quality of life assessment tools used in these studies included the St George's Respiratory Questionnaire (SGRQ) (Jones 2009), the Leicester Cough Questionnaire (LCQ) (Birring 2003), the Short Form Health Survey‐36 (SF‐36), the Short Form Health Survey‐12 (SF‐12), the Chronic Respiratory Disease Questionnaire (CRQ) and the Clinical COPD Questionnaire (CCQ). We were not able to include data from Banerjee 2005 and Mygind 2010 in the meta‐analyses.
Seven studies assessed quality of life using the SGRQ. The meta‐analysis demonstrated a benefit of prophylactic antibiotics compared to placebo (MD ‐1.94, 95% CI ‐3.13 to ‐0.75; participants = 2237; studies = 7; high‐quality evidence; Analysis 1.9; Figure 6). However, the mean difference did not reach the level of clinical significance according to the conventional cut‐off of at least a four‐unit reduction (Jones 2009).
1.9. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 9 HRQoL, SGRQ (total score).
6.
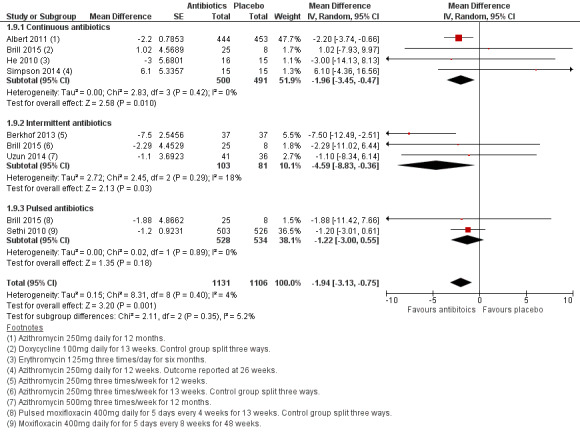
Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.9 HRQoL, SGRQ (total score).
When we grouped the studies by antibiotic regimen, we noted a similar improvement with continuous prophylactic antibiotic use (MD ‐1.96, 95% CI ‐3.45 to ‐0.47; participants = 991; studies = 4). While the pooled mean difference did not exceed the MCID of four units, it should be noted that Albert 2011 (1142 participants) reported a responder analysis, which demonstrated that more participants in the continuous azithromycin group (43%) than the placebo group (36%) had at least a four‐unit reduction in the SGRQ (P = 0.03). We found a larger benefit with intermittent antibiotic use (MD ‐4.59, 95% CI ‐8.83 to ‐0.36; participants = 184; studies = 3). There was no statistically or clinically significant improvement in SGRQ total scores with pulsed antibiotics (MD ‐1.22, 95% CI ‐3.00 to 0.55; participants = 1062; studies = 2; Analysis 1.9). However, the formal test for subgroup difference did not identify a significant difference between continuous, intermittent, and pulsed antibiotics.
The SGRQ comprises three subcomponents; symptom score, impact score, and activity score. Four studies presented data for these subcomponents which were analysed separately (Albert 2011; Berkhof 2013: Sethi 2010; Uzun 2014). All three domains improved with antibiotics compared to placebo, although the improvement in the activity score was more uncertain (symptom score: MD ‐4.07, 95% CI ‐5.72 to ‐2.41; impact score: MD ‐2.56, 95% CI ‐5.02 to ‐0.10; activity score: MD ‐0.99, 95% CI ‐2.62 to 0.65; Analysis 1.10). In terms of clinically significant improvements with antibiotic use versus placebo in the subgroup analysis, the symptom score showed the greatest difference, with three studies having greater than a four‐unit mean difference (Berkhof 2013 9.3 units, Uzun 2014 5.7 units and Sethi 2010 4.4 units).
1.10. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 10 HRQoL, SGRQ (domains).
The authors of Banerjee 2005, Mygind 2010 and Simpson 2014 reported no statistically significant difference in the total SGRQ scores. However, Banerjee 2005 did report a significant improvement only in the symptom domain score in the participants treated with continuous prophylactic clarithromycin over a three‐month period (MD ‐10.2; 95% CI ‐18.7 to ‐1.6).
In addition to the SGRQ, Berkhof 2013 used the LCQ and SF‐36 as quality of life assessment tools. The LCQ is a 19‐point questionnaire designed to assess cough‐related quality of life that is divided into three domains (physical, psychological, and social). The domain scores range from one to seven and therefore the total score range is three to 21; higher scores are indicative of a better quality of life. The authors of Berkhof 2013 reported an improvement in total LCQ score with intermittent azithromycin use (MD 1.30, 95% CI 0.32 to 2.28; Analysis 1.11). The only domain that did not show a clear improvement with the antibiotics was the social domain (MD 0.40, 95% CI ‐0.15 to 0.95; Analysis 1.14).
1.11. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 11 HRQoL, LCQ (total).
1.14. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 14 HRQoL, LCQ (domains).
Four studies used the SF‐36 (Albert 2011; Banerjee 2005; Berkhof 2013; He 2010). The SF‐36 is a 36‐item nonspecific health‐related quality of life questionnaire. It is divided into eight domains that are each scored on a 100‐point scale where 100 is equivalent to no disability. The domains include general health, physical functioning, bodily pain, vitality, role emotional, social functioning, mental health, and role physical. Three of the four studies (Albert 2011; Berkhof 2013; He 2010) that used SF‐36 to assess quality of life, involving 1262 participants, presented data that we were able to extract for meta‐analysis. Albert 2011 used continuous azithromycin for 12 months, Berkhof 2013 used intermittent azithromycin for three months and He 2010 used continuous erythromycin for six months. Given the spread of treatment duration and the different time points of data available for us to extract, we chose to extract data as close to six months as possible, which involved outcomes at six months for Albert 2011 and He 2010, and three months for Berkhof 2013. Only the general health domain showed a clear benefit of antibiotics over placebo (MD 4.06, 95% CI 0.70 to 7.42; participants = 1071; studies = 3; I2 = 18%; Analysis 1.13) but the confidence intervals for each domain effect estimate were highly overlapping. Banerjee 2005 also used the SF‐36 but the raw data were not available for extraction for inclusion in the meta‐analysis; however, in their text; they reported a significant improvement in the physical functioning score in the group that used prophylactic clarithromycin for three months (MD 12.9; 95% CI 3.1 to 22.6).
1.13. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 13 HRQoL SF‐36 (domains).
One study used SF‐12 in addition to the SGRQ, and found no difference in the mental (MD 0.90, 95% CI ‐4.68 to 6.48) nor the physical (MD ‐0.40, 95% CI ‐5.10 to 4.30) health domains after 12 months of treatment with prophylactic antibiotics (intermittent azithromycin) (overall; MD 0.14, 95% CI ‐3.45 to 3.73; Analysis 1.12) (Uzun 2014) . They did, however, report "a significant difference in mean change in the mental component score at three months in favour of azithromycin (MD 6.6; CI 1.4 to 11.8; P = 0.013)" (Uzun 2014).
1.12. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 12 HRQoL, SF‐12 (domains).
Simpson 2014 similarly found no significant improvement in quality of life as assessed by the CCQ with continuous antibiotic (azithromycin) use at the end of their treatment period (MD 1.80, 95% CI ‐5.11 to 8.71; Analysis 1.15). Finally, Shafuddin 2015 used the CRQ (which assesses four domains: dyspnoea, fatigue, emotional function, and mastery) and found no significant improvement in any of these domains with continuous antibiotic use (roxithromycin/doxycycline or doxycycline alone) (Analysis 1.16).
1.15. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 15 HRQoL, CCQ (total).
1.16. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 16 HRQoL, CRQ (domains).
Secondary outcome: frequency of hospitalisation
The frequency of hospitalisation was assessed using data from four studies involving 2958 participants (Albert 2011; Mygind 2010; Sethi 2010; Suzuki 2001). In this update, none of the new studies presented data on the frequency of hospitalisation and as such, our data remained unchanged from the previous update (Herath 2013).
The study by Sethi 2010, involving pulsed moxifloxacin in 1157 participants, did not show any improvement in the hospitalisation frequency (131/569 treatment arm versus 136/580 placebo arm; P = 0.46; Analysis 1.18).
1.18. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 18 Frequency of hospital admissions ‐ pulsed antibiotics.
| Frequency of hospital admissions ‐ pulsed antibiotics | |||
|---|---|---|---|
| Study | Frequency of hospitalisation (%) on moxifloxacin | Frequency of hospitalisation (%) on placebo | P value |
| Sethi 2010 | 131 (23.02%) | 136 (23.45%) | 0.46 |
The study by Albert 2011, involving continuous azithromycin in 1142 participants, calculated the rate of exacerbations requiring hospitalisation per patient per year according to the severity of COPD by the GOLD criteria (Analysis 1.18). The rate ratio was 0.77 (GOLD stage 2), 0.89 (GOLD stage 3) and 0.72 (GOLD stage 4). There were not adequate data to calculate the statistical significance of this outcome but there did not appear to be a trend.
The other two studies had inadequate data to calculate the mean event rate per year. Of these, one study involving 109 participants found a statistically significant reduction (P < 0.001) in hospitalisation while using erythromycin 200 to 400 mg daily for a 12‐month period (Suzuki 2001). The other study (Mygind 2010) did not show a statistically significant difference in the frequency of hospitalisations.
Secondary outcome: duration of exacerbations
The duration of exacerbations was addressed by only two studies involving 684 participants (Mygind 2010; Seemungal 2008), again already included in the previous version of this review (Herath 2013). None of the new studies in this update presented data on the impact of prophylactic antibiotics on the duration of exacerbations. Seemungal 2008 showed that antibiotic use was associated with a lower median number of exacerbation days: 9 days (interquartile range (IQR) 6 to 13 days) compared to 13 days on placebo (IQR 6 to 24 days) (P = 0.036). Similar findings were reported by Mygind 2010. This study had 575 participants and used pulsed azithromycin over a 36‐month period. The median number of exacerbation days (at home or in hospital) was 93 in the azithromycin group compared to 111 in the placebo group (P = 0.04). Prophylactic pulsed antibiotic use (Mygind 2010) reduced the number of days with severe exacerbations managed at home: a median of 31 days versus 42.5 days for the placebo group (P = 0.01). A meta‐analysis was not carried out for this comparison due to paucity of data.
Furthermore, Mygind 2010 reported data on hospitalisation due to COPD exacerbations. The study showed no difference in the number of hospitalisations between the treatment and placebo arms; however, there was a median reduction in hospital stay from 18 days in the placebo group to 15.5 days in the treatment group. No P value was stated for this comparison.
Secondary outcome: days of disability
Only one study reported on the number of days the participant was unable to undertake normal activity (Mygind 2010). The median number of days spent at home due to a mild exacerbation was no different between the treatment and placebo arms (42 days in each arm). However, there was a reduction in the median number of days spent at home due to a moderate to severe exacerbation from 42.5 days in the placebo group to 31 days in the azithromycin group (P = 0.01).
Secondary outcome: change in lung function
Change in lung function was addressed in nine studies (Berkhof 2013; Brill 2015; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Tan 2016; Uzun 2014). Six analysed changes in FEV1 in a total of 658 participants. The meta‐analysis showed no significant difference in FEV1 (MD 20 mL, 95% CI ‐26 to 67; participants = 658; studies = 9; moderate‐quality evidence; Analysis 1.20) with prophylactic antibiotics (continuous, intermittent, or pulsed) compared to placebo. Similarly, there was no significant difference in FEV1 % predicted values (MD 0.33, 95% CI ‐1.56 to 2.22; participants = 1737; studies = 6; Analysis 1.22). However, there appeared to be an improvement in FVC with antibiotic use (combining available data for continuous and intermittent regimens) (MD 0.12 L, 95% CI 0.01 to 0.23; participants = 514; studies = 4; Analysis 1.21). We did not detect any statistically significant differences between the antibiotic regimen subgroups.
1.20. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 20 FEV1 (mL).
1.22. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 22 FEV1 % predicted.
1.21. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 21 FVC (L).
Secondary outcome: functional capacity
Two studies (Tan 2016 and Uzun 2014) assessed functional exercise capacity. Both studies measured the six‐minute walk test (6MWT) at baseline, three, six, nine, and 12 months. The meta‐analysis demonstrated a significant difference in favour of antibiotics in improving performance at 12 months, but with a high level of heterogeneity (MD 68 m, 95% CI 16 to 119; participants = 126; studies = 2; I2 = 64%; Analysis 1.23). Tan 2016, an unblinded study considered to be at high risk of bias, investigated continuous antibiotics and had two treatment arms (group A: erythromycin 125 mg three times a day for 12 months and group B: erythromycin 125 mg three times a day for six months) and a placebo arm. Their data suggested an improvement in exercise capacity at six months with erythromycin use compared to placebo, with similar results seen for both treatment groups, as would be expected (group A: mean distance 388 m ± 62 , n = 17; group B: mean distance 389 m ± 61, n = 17; placebo mean distance 326 m ± 79, n = 15). Uzun 2014 investigated the use of intermittent azithromycin and, when isolated from Tan 2016, this study did not show an improvement in 6MWT results with antibiotic use (MD 36, 95% CI ‐16 to 88; participants = 77).
1.23. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 23 Exercise capacity (6MWT).
Secondary outcome: death (all‐cause and respiratory aetiology)
Mortality data were reported in six studies involving 3309 participants and were combined in a meta‐analysis (Albert 2011; Berkhof 2013; Mygind 2010; Sethi 2010; Shafuddin 2015; Uzun 2014). There was no significant difference between the treatment and placebo arms in all‐cause mortality (OR 0.87, 95% CI 0.66 to 1.15; participants = 3309; studies = 6; I2 = 0%; moderate‐quality evidence; Analysis 1.24), but confidence intervals were not sufficiently narrow to exclude a clinically important difference. Data on mortality secondary to a respiratory cause were available in the two larger studies (Albert 2011; Sethi 2010), which again showed no significant difference between groups (OR 1.17, 95% CI 0.63 to 2.19; Analysis 1.25), but the estimate was imprecise.
1.24. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 24 All‐cause mortality.
1.25. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 25 Respiratory‐related mortality.
Secondary outcome: serious adverse events
Adverse events were well explained in ten studies (Albert 2011; Berkhof 2013; Brill 2015; He 2010; Seemungal 2008; Sethi 2010; Simpson 2014; Shafuddin 2015; Tan 2016; Uzun 2014), but there was no uniform system for reporting them.
There was a reduction in serious adverse events as defined by the trialists, with prophylactic antibiotics, but the confidence interval included no difference (OR 0.88, 95% CI 0.74 to 1.05; participants = 2978; studies = 9; I2 = 0%; moderate‐quality evidence; Analysis 1.26). Similarly, there was no significant difference in the total number of any adverse event, as defined by the trialists, between antibiotic prophylaxis and placebo arms (OR 1.07, 95% CI 0.69 to 1.67; participants = 512; studies = 4; I2 = 0%; moderate‐quality evidence; Analysis 1.27), but the confidence interval was wide.
1.26. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 26 Serious adverse events.
1.27. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 27 Any adverse event.
Looking at specific adverse events, there were no significant differences in the number of adverse events between the treatment and placebo arms related to the respiratory system (Analysis 1.28.1), gastrointestinal system (Analysis 1.28.2), QTc prolongation (Analysis 1.28.3), musculoskeletal system (Analysis 1.28.5), hypersensitivity (Analysis 1.28.6), nervous system (Analysis 1.28.7) or the cardiovascular system (Analysis 1.28.8), but all estimates lacked precision.
1.28. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 28 Adverse events (specific).
The adverse event most frequently recorded across the studies (Albert 2011; Berkhof 2013; He 2010; Seemungal 2008; Sethi 2010; Simpson 2014) was gastrointestinal in origin (OR 1.16, 95% CI 0.43 to 3.11; participants = 2522; studies = 6; I2 = 72%, Analysis 1.28). There was significant heterogeneity among these studies which suggested differences among the antibiotics and their adverse events for each study.
Individual studies did show some differences which may have clinical relevance.
Sethi 2010 reported significantly higher numbers of adverse events in the treatment arm with moxifloxacin (P < 0.001) secondary to increased gastrointestinal adverse events including diarrhoea, nausea, and vomiting (OR 7.17; 95% CI 2.49 to 20.63; Analysis 1.28), representing a number needed to treat for an additional harmful outcome (NNTH) of 25 (95% CI 98 to 9). The intervention group in this study received moxifloxacin 400 mg daily for 5 days, every 8 weeks for 48 weeks. A single case of diarrhoea was reported secondary to Clostridium difficile in the placebo group. Sethi 2010 stated that the adverse events were drug‐related.
Albert 2011 reported that azithromycin 250 mg daily for a 12‐month period was associated with a significant increase in hearing impairment (OR 1.39; 95% CI 1.05 to 1.85) representing a NNTH of 18 (95% CI 128 to 9). The authors reported that the majority of the drug discontinuations due to a drug‐related adverse events were due to hearing impairment (treatment group, N = 142 (25%) versus placebo group, N = 110 (20%) by three months). It should be noted that all participants in this study had baseline audiometry, with participants with hearing impairment below the 95% percentile excluded from the study. Since there were a large number of participants in both the treatment and placebo arms that had drug discontinuation secondary to hearing loss, the authors commented that this could be due to a measurement error.
In Albert 2011, while there were no statistically significant differences observed in cardiovascular disease or QTc prolongation, six participants in the treatment group had to discontinue the medication due to development of prolonged QTc compared to four participants in the placebo group (P = 0.55). This study excluded participants with tachycardia, long QTc, and participants taking medications that could prolong the QTc.
In the non‐blinded study of Suzuki 2001, it was reported that participants in the treatment group did not have any apparent adverse effects from erythromycin therapy during the study period.
Shafuddin 2015 reported one case of an abnormal electrocardiogram (ECG) deemed to be related to the combined roxithromycin/doxycyline medication.
Brill 2015 found that 40% of adverse events, although reported as minor, were in the moxifloxacin group, with half of those being gastrointestinal. In four cases, therapy was withdrawn in this group.
Secondary outcome: antibiotic resistance
The development of antibiotic resistance was assessed in six studies involving 2610 participants (Albert 2011; Banerjee 2005; Brill 2015; He 2010; Seemungal 2008; Sethi 2010). Because of the variety of ways in which resistance was evaluated and reported, it has proved impossible to combine these results in a meta‐analysis.
In Brill 2015, both sputum bacterial load and antibiotic resistance was assessed pre‐ and post‐ 13 weeks antibiotic treatment. Bacterial load was reduced by all three antibiotic treatments, and most substantially (by 62%) in the pulsed moxifloxacin arm, but there was not a statistically significant difference when compared to placebo in any of the three treatment arms. The most common isolate both pre and post‐treatment was non‐Pneumoniae streptococcus species. There were increases in the degree of antibiotic resistance of isolates in all three antibiotic arms after 13 weeks treatment. Compared to placebo, moxifloxacin was associated with a factor increase in mean inhibitory concentration (MIC) of 4.82 (95% CI 1.44 to 16.19, P = 0.01), doxycycline 3.74 (95% CI 1.46 to 9.58, P = 0.01) and azithromycin 6.23 (95% CI 1.66 to 23.35, P = 0.01). Furthermore, isolates from participants in the doxycycline group were more likely to be resistant to doxycycline than in the placebo group (OR 5.77, 95% CI 1.40 to 23.74, P = 0.02). ORs for the moxifloxacin and azithromycin were also greater than 2, but not statistically significant.
Albert 2011 used sputum from participants who could expectorate as well as nasopharyngeal swabs. They found only 15% of participants were able to expectorate at the end of the three‐month treatment period. The commonest organisms identified in the treatment versus placebo groups were: Staphylococcus aureus (N = 60 (10.7%) versus N = 71 (12.7%)); Moraxella spp (N=13 (2.3%) versus N = 6 (1%)); and S. pneumoniae (N = 6 (1.1%) versus N = 6 (1.1%)). The predominance of S. aureus in this COPD population was out of keeping with the usual pathogens anticipated and was thought to be due to the nasopharyngeal sampling. During the study period, the participants in the placebo group without bacterial colonisation (N = 172) became colonised at a significantly higher rate than those treated with 250 mg of daily azithromycin (N = 66) (P < 0.001). However, in the group that became newly colonised throughout the study period, the resistance to macrolide was higher in the treatment group: 81% compared to 41% in the placebo group (P < 0.001).
In Sethi 2010, which used pulsed moxifloxacin over a 48‐week period, the sampling for organisms was carried out using sputum sampling and rectal sampling. Only 24% of all participants could produce sputum. The commonest organisms isolated were: H. influenzae (8.3%), Haemophilus parainfluenzae (6.6%) and S. pneumoniae (4.3%); S. aureus was isolated in 2.6%. The MIC for moxifloxacin for H. influenzae, H. parainfluenzae, S. pneumoniae, M. catarrhalis and S. aureus did not change during the study period. A single moxifloxacin‐resistant S. pneumoniae isolate was identified at the end of the study period (MIC 4 mg/L). There were one to three moxifloxacin‐resistant isolates at different points of the study that were not persistent. Participants who had produced cultures of moxifloxacin‐resistant pseudomonas were excluded from the study. However, participants with moxifloxacin‐sensitive pseudomonas were included. During the 24th week of the study, the median MIC of moxifloxacin had increased to 4 mg/L, which returned to baseline at the end of the treatment period. The median MIC of the placebo group with pseudomonas‐sensitive moxifloxacin increased from 0.5 mg/L to 2 mg/L at the end of the treatment period. The study authors recommended not using moxifloxacin in patients with known pseudomonas colonisation owing to the possibility of developing rapid resistance.
Seemungal 2008 investigated 109 participants with twice daily erythromycin 250 mg over a 12‐month period. They encountered only one participant who developed resistance to S. pneumoniae at the end of the treatment period. All H. influenzae isolated (22/109) were found to be resistant to erythromycin. The microorganism milieu was as expected for the COPD population: H. influenzae (N = 22/36), S. pneumoniae (N = 6/36) and M. catarrhalis (N = 3/36).
The other two studies, (Banerjee 2005 using long‐acting clarithromycin 500 mg daily (Klaricid XL 500 mg) and He 2010 using erythromycin 125 mg every eight hours), found a similar milieu of respiratory pathogens. They did not observe significant differences in the colonisation rate of the organisms or emergence of resistance. However, these two studies were of a shorter duration than those mentioned above, six months and three months, respectively.
Subgroup and sensitivity analyses
Subgroup analyses
We performed subgroup analysis on our primary outcome only: number of people with one or more exacerbations. We subgrouped studies according to mean baseline FEV1 % predicted but only one study was included in the > 50% predicted subgroup and thus we cannot draw any conclusions from this analysis (Analysis 2.1). We did not find any statistically significant impact of study duration (three to six months, six to 12 months and over 12 months) on this outcome (Analysis 2.2). We subgrouped studies according to their date of publication in five‐year groups from 2005 to 2009, 2010 to 2014 and 2015 onwards (Analysis 2.3). Six out of the eight studies appearing in this analysis were published between 2010 and 2014, so again, this analysis was inconclusive. When subgrouped according to frequency of antibiotic administration (once daily, 2 to 3 times daily, 2 to 3 times per week and pulsed), there was some evidence that pulsed antibiotics were less effective than regimens that require more frequent dosing, in keeping with our other findings (Analysis 2.4; test for subgroup differences: Chi² = 9.51, df = 3 (P = 0.02), I² = 68.4%). Finally, we did not find a statistically significant subgroup difference when we grouped studies according to exacerbation history as an inclusion criterion (studies in which participants were required to have had at least one exacerbation in the preceding year versus those in which exacerbation history was not an inclusion criterion) (Analysis 2.5). However, it should be noted that many of the studies included in the second subgroup recruited participants who had a positive exacerbation history, despite it not being a requirement for study entry.
2.1. Analysis.
Comparison 2 Subgroup analyses, Outcome 1 Subgroup analysis: number of people with one or more exacerbations by mean % predicted FEV1.
2.2. Analysis.
Comparison 2 Subgroup analyses, Outcome 2 Subgroup analysis: number of people with one or more exacerbations by treatment duration.
2.3. Analysis.
Comparison 2 Subgroup analyses, Outcome 3 Subgroup analysis: number of people with one or more exacerbations by year carried out.
2.4. Analysis.
Comparison 2 Subgroup analyses, Outcome 4 Subgroup analysis: number of people with one or more exacerbations by regimen.
2.5. Analysis.
Comparison 2 Subgroup analyses, Outcome 5 Subgroup analysis: number of people with one or more exacerbations by exacerbation history.
Sensitivity analyses
We applied our sensitivity analysis to our primary outcome only: number of people with one or more exacerbations. This resulted in the removal of all three arms of the single‐blind study Brill 2015. This had a minimal impact on the effect estimate (0.54, 95% CI 0.38 to 0.77 versus OR 0.57, 95% CI 0.42 to 0.78).
Discussion
Summary of main results
In this review, we analysed a total of 14 completed studies, involving 3932 participants, that investigated the use of prophylactic antibiotics for the prevention of COPD exacerbations. All participants were adult (over the age of 40 years), with the mean age between 65 and 72 years. Most participants had at least moderate‐severity COPD and we only included studies if they confirmed this diagnosis with spirometry. The antibiotics investigated were azithromycin, erythromycin, clarithromycin, doxycyline, roxithromycin, and moxifloxacin. Nine studies involving 1925 participants investigated continuous macrolide antibiotic regimens, two studies involving 176 participants investigated intermittent azithromycin antibiotic regimens (administered three times a week), two studies involving 1732 participants investigated different pulsed antibiotic regimens (one macrolide and one quinolone) and one study involving 99 participants compared three treatment arms with placebo; one continuous doxycycline regimen, one intermittent azithromycin regimen and one pulsed moxifloxacin regimen. The study duration varied from three months to 36 months and all used intention‐to‐treat analysis. Most of the pooled results were of moderate quality. The risk of bias of the included studies was generally low, and we did not downgrade the quality of evidence for risk of bias.
Primary outcomes
Eight studies including 2716 participants were included in the meta‐analysis of the number of people with one or more exacerbations (Figure 3). The results of this analysis indicated that the number of participants experiencing one or more exacerbations was significantly reduced with the use of prophylactic antibiotics (OR 0.57, 95% CI 0.42 to 0.78; participants = 2716; studies = 10; I2 = 42%) .The subgroup analysis for this outcome suggested that there was a statistically significant difference between continuous and intermittent regimens and pulsed regimens for the number of people with one or more exacerbations, with pulsed antibiotic regimens having a smaller treatment effect. Similarly, the rate of exacerbations per patient per year with continuous and intermittent antibiotic use were significantly reduced (Analysis 1.4; moderate‐quality evidence). The median time to first exacerbation was lengthened with continuous and intermittent antibiotic prophylaxis (Analysis 1.5), however, only the continuous antibiotic regimens and one intermittent antibiotic regimen were associated with statistically significant delays. Pulsed antibiotic prophylaxis did not appear to be associated with the same benefits.
Health‐related quality of life was explored in nine studies using different assessment tools, as detailed in the above results. The results were mixed, with some studies finding no or uncertain improvement in quality of life indicators with antibiotic prophylaxis whilst others found improvement in some quality of life domains. The most commonly used quality of life scale was the SGRQ. It was used in seven studies involving 2237 participants and our meta‐analysis suggested a statistically significant benefit in quality of life total score with antibiotic prophylaxis compared to placebo (MD ‐1.94, 95% CI ‐3.13 to ‐0.75; participants = 2237; high‐quality evidence) (Analysis 1.9). While this mean difference did not reach the accepted level of clinical significance (Jones 2009), a responder analysis carried out in Albert 2011 demonstrated that more participants in the continuous azithromycin group (43%) than the placebo group (36%) had at least a four‐unit reduction in the SGRQ (P = 0.03). Again, with subgroup analysis, where an improvement in quality of life was identified, it appeared to be with continuous and intermittent antibiotic prophylaxis. No clear improvement was found with pulsed antibiotic regimens.
Secondary outcomes
One study, Suzuki 2001, found a statistically significant reduction in hospital admissions with prophylactic erythromycin use, whilst three other studies found no significant reduction in hospital admissions (Albert 2011; Mygind 2010; Sethi 2010). There was no statistically significant difference between antibiotic prophylaxis and placebo in FEV1, all‐cause mortality, or adverse events (moderate‐quality evidence) but, for both mortality and adverse events, confidence intervals were too wide to rule out an effect. However, specific adverse events reported in some of the studies may have clinical relevance, given their severity.
The 6MWT was used to measure the functional capacity in two studies in the meta‐analysis involving 126 participants (Analysis 1.23). One study, considered to be at high risk of bias (Tan 2016) investigated continuous antibiotics and demonstrated a statistically significant benefit in the 6MWT (MD 84.5 m (45.7 to 123.29) with use of antibiotics whilst the result was uncertain in a study using intermittent antibiotics (Uzun 2014).
The development of antibiotic resistance was addressed in six studies involving 2486 participants. Due to the multitude of methodologies used to detect and report resistance, the data were not able to be pooled in a meta‐analysis. Of concern, four of these six studies identified evidence of antibiotic resistance. However, there was not sufficient evidence for us to predict how the use of prophylactic antibiotics would affect the resistance patterns in the community and studies with a longer duration of follow‐up will be required to assess this further.
Overall completeness and applicability of evidence
The participants in the studies in this review were aged 40 years or over (mean age, 67 years) and had at least moderate‐severity COPD (mean FEV1 1.2 L). Three studies (Albert 2011; Mygind 2010; Sethi 2010) included participants who experienced one to two exacerbations during the previous year. Uzun 2014 included participants who had experienced three exacerbations within the last year. Albert 2011 and Sethi 2010 included participants who were on long‐term supplemental oxygen or systemic steroids, or both (see Characteristics of included studies, Table 1). Hence, the results can be generalised only to this group of participants who are at the more severe end of the COPD spectrum. Such participants may also be more likely to be receiving high‐dose ICS, which have been associated with an increased risk of pneumonia (Kew 2014). This may further decrease generalisability to broader COPD populations.
Data from Albert 2011 suggested that prophylactic antibiotics may be most useful in patients who have given up smoking, who are not on any inhaler treatment (long‐acting beta agonist (LABA), long‐acting muscarinic antagonist (LAMA), or inhaled corticosteroid (ICS) in any combination), on oxygen therapy, or older than 65 years.
Although these results were obtained from prespecified subgroup analyses, the sample sizes were much smaller than the randomised sample size. There were 22 analyses carried out giving a 62% chance of one analysis yielding a statistically significant result. For this reason, the authors are aware that there may be false positives in these results and recommend additional studies that are adequately powered to explore these subgroups.
It is also important to note that the study participants had undergone strict exclusion criteria. Participants with tachycardia, arrhythmia, long QTc, as well as being on medication that could potentially increase the QTc (a long list), or baseline hearing deficit based on audiometry were excluded. Participants will likely have been excluded from the trial if they were currently prescribed an interacting medication that could not be stopped or substituted and this may have further limited the applicability of the evidence to the wider COPD population. There was regular monitoring throughout the study period and drugs were discontinued in the event of a significant adverse event. Furthermore, only two studies (Albert 2011 and Uzun 2014) performed CT chest to screen for participants with underlying bronchiectasis. This is a drawback as, although this review had a strict criteria for COPD diagnosis, many people with COPD (estimates range from 4% to 72%), especially those with recurrent infections, have a degree of underlying bronchiectasis (Martinez‐Garcia 2017). Therefore, identifying the group with bronchiectasis and analysing this group as a subgroup would have given us valuable information on whether the group would benefit more from prophylactic antibiotics. Furthermore, it is not clear from the studies the extent to which participants had other treatments for their COPD optimised (for example, smoking cessation programmes, pulmonary rehabilitation, vaccination).
In current clinical practice, prophylactic antibiotics tend to be used as a last resort because of concerns about antibiotic resistance in the community. If an informed decision is made to start prophylactic antibiotics in a particular patient, there needs to be baseline checks to confirm the identity of the infection (for example, sputum cultures), ECG, and consideration of audiometry, depending on the planned antibiotic, as well as ongoing monitoring of the same.
Although all the included studies were conducted in the last 20 years, even over this time period, the care of people with COPD has changed markedly, as evidenced by regular guideline updates (GOLD 2018). The threshold for both admission and duration of hospital stay once admitted has changed and more care is carried out at home in many settings. For example, a retrospective analysis of data in the UK suggested that the mean duration of hospital stay for an emergency admission with COPD fell by approximately one day between 2006 and 2010, in keeping with international trends (Harries 2015).
A further limitation of the evidence presented is that most of the included studies measured outcomes at or soon after discontinuation of antibiotics. We therefore cannot comment on any possible prolonged benefits or harms which might extend beyond the treatment period. Furthermore, drug interactions may also require consideration, particularly as macrolide antibiotics may interact with other commonly prescribed drugs in the COPD population, such as statins and theophylline (BNF 2018).
Finally, the analyses of continuous and intermittent antibiotics were dominated by macrolide antibiotics (e.g. azithromycin and erythromycin). The majority of the evidence for pulsed antibiotics was contributed by studies of moxifloxacin, a quinolone. Therefore, care should be taken when drawing conclusions about the relative efficacy of continuous, intermittent and pulsed regimens as this comparison is confounded by the different classes of antibiotic used in the studies.
Quality of the evidence
Overall, we graded the quality of the evidence to be moderate, meaning "further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate". We presented our grade ratings in Table 1.
We downgraded three outcomes (mortality, serious adverse events, and all adverse events) for imprecision; the confidence intervals were not sufficiently narrow enough to rule out a potentially important effect. We downgraded two outcomes (number of participants with one or more exacerbations and exacerbation rate) for inconsistency; the I2 was greater than 50% in both analyses. We downgraded FEV1 (mL) for indirectness as the studies contributing the majority of the weight in this analysis were of short duration. We were not able to create a funnel plot as no analyses contained 10 unique studies, so publication bias could not be formally assessed. Finally, while we rated four studies to be at high risk of bias in at least one domain, the overall methodological quality of the studies contributing data to the meta‐analyses was good, and therefore we did not downgrade any outcome for risk of bias.
Potential biases in the review process
We attempted to minimise bias during the review process by completing a comprehensive electronic search of all published and unpublished data, as well as handsearching the bibliographies from selected studies. The data were extracted and full‐text articles reviewed by three authors with disagreement being resolved by discussion.
However, not all included studies clearly described the criteria used to diagnose an acute exacerbation of COPD. This may have led to us combining results from studies in which different criteria were used, which may impact the interpretability of these analyses. Furthermore, exacerbation rates in COPD vary markedly between seasons; shorter studies may therefore fail to accurately reflect the true year‐round exacerbation burden. In addition, our categorisation of continuous, intermittent, and pulsed regimens was a post hoc decision for the 2018 update, although taken before data analysis. We recognise that, due to sustained tissue concentrations of azithromycin, a three times a week regimen could be considered essentially continuous (Matzneller 2013). Despite this, we suggest that intermittent regimens have different implications for patient adherence and costs and therefore maintained these subgroups for analysis.
Agreements and disagreements with other studies or reviews
Our findings are consistent with an earlier Cochrane review of prophylactic antibiotics in chronic bronchitis (Staykova 2003), even though the definitions of cases are tighter in the present review. The study findings further strengthen and are in line with the findings of the previous version of this Cochrane review, published in 2013 (Herath 2013).
A case‐based review article in the New England Journal of Medicine (NEJM) on antibiotic prevention of acute exacerbations of COPD recommends the use of azithromycin 250 mg three times a week to reduce exacerbation frequency in patients on maximal COPD treatment who were still having two or more exacerbations per year (Wenzel 2012). Careful selection, prior investigations, and proper follow‐up were emphasised, which is concordant with our findings and recommendations.
Authors' conclusions
Implications for practice.
Use of prophylactic macrolide antibiotics for a period of up to 12 months is likely to reduce the number of patients with one or more exacerbations, exacerbation frequency, increase the median time to first exacerbation and improve health‐related quality of life. Benefits appear to be driven by continuous and intermittent macrolide regimens, with pulsed regimens being less effective. However, the benefits need to be balanced against the risk of harm, notably antibiotic resistance, and the cost and adherence implications for the patient and the health care system, as well as potential costs of monitoring for adverse effects.
Reducing the frequency of exacerbations would reduce healthcare costs and might be expected to preserve lung function and quality of life, as well as lower the risk of mortality, although we did not find evidence of any of the latter in this review. In part, this is due to the dearth of studies that have addressed the frequency and duration of hospital admissions and the relatively small numbers of participants in most of the studies; that is, the studies were underpowered to measure these outcomes.The more recent studies did not address the days of disability or hospital admissions and therefore there was no additional contribution in this area since the 2013 review.
The benefit in prevention of exacerbations was seen in the type of participants that were included in the studies. These participants had at least moderately severe COPD and were mostly frequent exacerbators. Evidence available from a single study suggests that individuals over 65 years benefited more than younger individuals. Hence, carefully identifying the patient group that would benefit most from the use of prophylactic antibiotics is of paramount importance.
Although the selection of patients for prophylactic antibiotics is critical, the evidence base for making statements about patient selection is poor since not all studies have used the same selection criteria, only three studies used frequent exacerbations as an inclusion criterion, and only two studies screened for bronchiectasis with CT imaging.
There are some potentially serious adverse effects with prophylactic antibiotics. Furthermore, development of antibiotic resistance remains of major concern. This is particularly so for those patients colonised with pseudomonas. More broadly, there are calls worldwide to reduce the total amount of antibiotics prescribed. Hence, even though the NNTs to prevent one person having an exacerbation were relatively small, this has to be balanced with the risks of harm either to that individual or indirectly to others via antibiotic resistance. So far, the evidence suggests that, with the use of prophylactic antibiotics, the sputum bacterial load reduces. However, included studies reported that, in participants with ongoing bacterial isolation, the isolates had increased resistance to the given antibiotic, sometimes demonstrating a MIC with a four‐fold rise at the end of the study period. One study that had follow‐up to 36 months had demonstrated that the rise in MIC may be transient and may return to baseline with cessation of antibiotics during the follow‐up period. Therefore, there remains uncertainty about the long‐term effects of the use of prophylactic antibiotics in the bacterial milieu in the community in terms of developing persistent resistance in this era of constant fear of developing 'super‐bugs'.
Implications for research.
While there is a growing body of evidence to support the use of prophylactic antibiotics in people with COPD, better identification of the subsets of patients who would benefit most would be of great value in future research, so as to deliver targeted treatment to the right patient. Future trialists should ensure that the study population is well characterised, with a particular focus on the potential effect modifiers of prophylactic antibiotic therapy, including underlying bronchiectasis, exacerbation frequency, bacterial colonisation, and baseline FEV1. Future studies that incorporate potential biomarkers might be useful for patient selection. Importantly, participants in all arms of clinical studies of prophylactic antibiotics should receive the full package of evidence‐based interventions in COPD, as well as the study interventions.
At present, there is a larger body of data available for the use of continuous antibiotic use and their benefit in comparison to intermittent or pulsed antibiotics. It would be worthwhile exploring the benefit of intermittent or pulsed antibiotics further as administering them less frequently may improve patient adherence, reduce adverse effects, and costs to both the patient and healthcare systems. Head‐to‐head comparisons of pulsed versus intermittent versus continuous antibiotics would be useful in this context. Furthermore, shorter placebo‐controlled studies through winter months, when exacerbations are more common, might be useful to determine if this is a pragmatic strategy. Regimens which focus on winter months only might also improve adherence and reduce costs, but this needs to be tested in a trial setting.
Duration of hospital admissions and days of disability due to an exacerbation were not well addressed in the studies. Future studies should document these outcomes. Additionally, a cost‐effectiveness analysis would be useful. Most of the costs in severe COPD are related to hospitalisation. On the face of it, antibiotics may be cheaper than some inhalers, but there are hidden costs, such as the cost of screening for adverse effects and the direct and indirect costs of antimicrobial resistance.
A challenge is how to balance the benefits of antibiotic therapy to the individual over the potential harms to the community through antibiotic resistance. The maximal duration of continuous prophylactic antibiotics was 12 months, and for pulsed antibiotics it was 36 months. Hence, there are no data on the impact of very long‐term antibiotic use on antibiotic resistance patterns in the community. The latter will require local surveillance and the correlation of resistance and prescribing patterns. This will help us understand if the higher MIC noted during the use of prophylactic antibiotics would return to baseline when antibiotics were discontinued and after how long, which would enable us to determine the need for 'breaks' from treatment, as well as determine the duration of these 'breaks'. If persistent resistance patterns are identified despite these measures, this would increase the need for caution in the use of prophylactic antibiotics. Furthermore, modelling of resistance detected in trials at a population level might be a useful approach.
Yet to be determined is whether prophylactic antibiotics are able to alter the rate of deterioration of lung function in COPD and, if they do, whether this is due to antibiotic or anti‐inflammatory effects. This would be a helpful advance in the understanding of the pathophysiology of this common and debilitating disease. Long‐term follow‐up studies of participants who have been in prophylactic antibiotic studies may help to answer these questions. Identifing biomarkers of inflammation in these patients would also help if antibiotics with anti‐inflammatory effects work better in this group.
Future studies directed at the use of inhaled antibiotics, which may result in fewer systemic effects and higher local concentrations, would be useful in reducing potential side effects of oral prophylactic antibiotic therapy. Such studies were excluded from this review.
Studies looking into continuous or intermittent antibiotic therapy in clear relation to other interventions that are useful for frequent exacerbators would help determine the advantages and disadvantages of antibiotic therapy over the already established measures used to reduce exacerbations. The subgroup analysis conducted of one of the included studies (Albert 2011) suggests that further exploration of the modifying effect of baseline treatments such as LAMA, LABA, and ICS is warranted.
Feedback
Feedback, 30 June 2014
Summary
1) One of the conclusions in the review was that there was no significant difference in total serious adverse events (SAE) between treatment and placebo. The definition of a serious adverse event includes any untoward medical occurrence that results in death, is life‐threatening, requires hospitalisation or prolongation of hospitalisation, or results in persistent or significant disability (1).
According to this definition, a moderate to severe COPD exacerbation would be considered a SAE. It is unclear whether the four analysed studies (Albert, He, Seemungal, Sethi) included COPD exacerbation in their SAE data. The following concerns only exist if trial authors included moderate to severe COPD exacerbations as SAEs. If the total numbers of SAEs are approximately equal between the treatment arms and COPD exacerbations were decreased in the antibiotic group, one can expect another type of SAE to have increased in the antibiotic group. Our fear is that there may be an unidentified SAE occurring in the antibiotic arm that is not present in the placebo arm.
We noted your documentation of attempting to contact the Mygind authors for more information and were curious as to whether Banerjee or Suzuki could be reached to determine more about SAE reporting in their trials. We also emailed the authors of Albert 2011 to inquire about how SAEs were classified and documented in that trial.
Furthermore, the three studies for which SAE data were not available (Banerjee, Suzuki, Mygind) have 751 participants, which equates to 23.5% of the total review participants. The missing SAE data from these three studies could potentially change the conclusion of this review on SAEs. Based on the two concerns we expressed above, additional information will be needed to confirm any difference in SAEs between treatment and placebo.
In addition, these further conclusions about the possible SAEs associated with prophylactic antibiotics are required before patients can make an informed decision about whether the benefits of therapy justify the risks.
2) In the review, the authors discussed that a cost‐effectiveness analysis would be useful in deciding the value of antibiotics in prophylaxis of COPD exacerbation. Upon reviewing the Albert 2011 trial, we noted that cost‐effectiveness was a secondary outcome that was included in the study protocol but not reported in the final data. The protocol suggested that this would have been calculated as the ratio of incremental costs to the ratio of incremental quality‐adjusted life years. If this missing data can be obtained from the authors and included into the review, it will be an added piece of valuable information for the readers when considering practice changes.
3) In the analysis on frequency of hospitalisation, the authors report the rate ratios of exacerbation requiring hospitalisation (/patient/year), but stated it as the rate ratios of exacerbation (/patient/year). Since the Albert 2011 trial reported both the rates of exacerbation (/patient/year) and the rates of exacerbation requiring hospitalisation (/patient/year), we feel it would be in the interest of clarity to state “rate of exacerbations requiring hospitalisation per patient per year according to the severity of COPD by the GOLD criteria.”
References
1. Therapeutics Initiative. Serious adverse event analysis: lipid‐lowering therapy revisited. Therapeutics Letter. 2001 Aug‐Oct; Issue 42. Available from: www.ti.ubc.ca/pages/letter42.htm [cited 2014 Jun 22]
Reply
Question 1
We agree that elucidating all antibiotic‐related SAEs is of paramount importance for patients and physicians prescribing these prophylactic antibiotics in order to make informed choices.
Albert and colleagues included COPD as a cause of fatal SAE causing death in 10 patients in the antibiotic group and 7 patients in the placebo group. However, given that there were 317 exacerbations in 558 patients in the antibiotic group and 380 exacerbations in 559 patients in the placebo group, it does not seem possible that COPD exacerbations were looked at and included as SAEs. However, we agree that this needs to be clarified with the authors and we have written to Albert and colleagues requesting clarification as to whether they included moderate to severe COPD exacerbations as a SAE.
In the Seemungal and colleagues study, the only listed adverse events are upper GI, lower GI, rash, and "other". Relooking at the raw data, 28 out of the 53 patients in the treatment group and 42 out of the 56 patients in the placebo group had experienced COPD exacerbations; hence they did not include COPD exacerbations as SAEs.
In the He study, listed adverse events were abdominal pain and heart failure in two patients in the antibiotic group and respiratory insuffiencey in two patients in the placebo group. Again, the COPD exacerbations were not included as SAEs.
In the Sethi and colleagues study, COPD exacerbations were not reported as SAEs. Looking closely at the adverse event table from the paper, only four patients reported dyspnoea in the treatment group and no patients reported dyspnoea in the placebo group. Given the much larger number of COPD exacerbations that had occurred in both groups, it seems that exacerbations were not included as SAEs.
The Mygind study did not report SAEs in detail. However, they categorised adverse events as upper GI, lower GI, infection and "other" and did not include COPD exacerbations as adverse events. However, these data were from an oral presentation slide and, as mentioned in the paper, we did not receive a response from the authors.
The Banerjee study had indicated in the paper that one participant withdrew in the treatment arm due to GI disturbance.
The Suzuki study had listed that one patient was excluded from the treatment group due to diarrhoea, with no other antibiotic‐related adverse event listed.
In summary, all four papers with detailed data on adverse events (Albert, He, Seemungal, Sethi) did not include COPD exacerbations as SAEs according to the raw data we have. Albert had included COPD as a cause of death and we have written to the authors to clarify this point. The three studies with limited data (Banerjee, Suzuki, and Mygind) had not listed COPD exacerbations as SAEs.
While moderate to severe COPD exacerbations, by definition, qualify as SAEs, the most likely rationale for reporting these as separate entities was that exacerbations were the primary outcomes assessed by these studies. Separating exacerbations from adverse events will give a better idea of whether there are other SAEs that would occur in the treatment group (other than the assessed primary outcome of exacerbations).
Question 2
We agree with this point. Assessing cost‐effectiveness of this intervention is of fundamental importance and we have written to the Albert group requesting that the group supply these data. This certainly is a factor that we will explore during the future updates.
Question 3
We agree that the sentence you have suggested clarifies the meaning better and have made the suggested change.
At the time of publication of this feedback, we await a response from Albert and colleagues.
Contributors
Debbie Au and Caitlin Lang, Lower Mainland Pharmacy Services, Pharmacy Residents, UBC
Dr. Aaron Tejani, Lower Mainland Pharmacy Services, Medication Use Evaluation Coordinator
What's new
| Date | Event | Description |
|---|---|---|
| 27 July 2018 | New citation required and conclusions have changed | Conclusions strengthened and evidence now suggests that continuous and intermittent regimens may be more effective than pulsed regimens. Additonal information about antibiotic resistance added. Text updated throughout review. Two new authors added to author team for 2018 update. Seven new studies added to qualitative synthesis (Berkhof 2013; Brill 2015; Shafuddin 2015; Simpson 2014; Tan 2016; Uzun 2014; Wang 2017). Fourteen studies now included in qualitative synthesis and eight studies in quantitative synthesis. Outcomes subgrouped by antibiotic regimen (continuous, intermittent, and pulsed). |
| 27 July 2018 | New search has been performed | Literature search updated. |
History
Protocol first published: Issue 4, 2012 Review first published: Issue 11, 2013
| Date | Event | Description |
|---|---|---|
| 26 August 2014 | Feedback has been incorporated | Feedback and author response added to the review. |
Acknowledgements
We would like to acknowledge the contribution made by Dr Peter Black to the earlier review, as well as his lasting influence as a teacher, mentor, friend, and colleague.
We would like to acknowledge the statistical support given by Ms Anne Sophie Vallerd (statistician), University of Sydney, Australia, in an earlier version of this review.
We acknowledge the support of staff at the Cochrane Airways Group, especially Dr Emma Dennett and Dr Chris Cates.
Milo Puhan was the editor for this review and commented critically on the review.
We would like to thank the following authors for providing addition information about their studies for the 2018 update: Dr J.W.K. Van den Berg (Berkhof 2013); Dr E. Shafuddin (Shafuddin 2015); Professor J. Simpson (Simpson 2014) and Dr S. Uzun (Uzun 2014).
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| Embase (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD/chronic bronchitis search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
[The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases]
Appendix 2. Search strategy to identify relevant records from the Cochrane Airways Trials Register
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All #2 MeSH DESCRIPTOR Bronchitis, Chronic #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) #4 COPD:MISC1 #5 (COPD OR COAD OR COBD):TI,AB,KW #6 #1 OR #2 OR #3 OR #4 OR #5 #7 MeSH DESCRIPTOR Anti‐Bacterial Agents Explode 1 #8 chemoprophylaxis #9 antibiotic* NEAR prophyla* #10 continuous NEAR antibiotic* #11 antibiotic* #12 penicillin #13 phenoxymethylpenicillin #14 phenethicillin #15 amoxicillin #16 amoxycillin #17 clavulanic acid #18 tetracycline #19 oxytetracycline #20 doxycycline #21 quinolone #22 ciprofloxacin #23 moxifloxacin #24 macrolide #25 erythromycin #26 roxithromycin #27 azithromycin #28 sulphonamide #29 co‐trimoxazole #30 sulphaphenazole #31 trimethoprim #32 sigmamycin #33 tetracycline AND oleandomycin #34 sulfamethoxazole #35 sulfaphenazole #36 sulfonamide #37 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 #38 #6 and #37
Appendix 3. Search strategy for ClinicalTrials.gov
| Intervention | antibiotic OR *cillin OR *mycin OR *cycline OR *floxacin OR *azole OR macrolide OR clavulanic OR sulfonamide OR quinolone OR trimethoprim |
| Condition | COPD |
| Study type | Interventional |
Data and analyses
Comparison 1. Antibiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with one or more exacerbations | 8 | 2716 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 1.1 Continuous antibiotics | 5 | 1325 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.36, 0.79] |
| 1.2 Intermittent antibiotics | 3 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.19, 0.77] |
| 1.3 Pulsed antibiotics | 2 | 1182 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.07] |
| 2 Number of people with one or more exacerbations requiring hospitalisation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Intermittent antibiotics | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Annualised exacerbation rate per patient per year during 12‐week active treatment | Other data | No numeric data | ||
| 3.1 Continuous antibiotics | Other data | No numeric data | ||
| 4 Rate of exacerbation per patient per year | 5 | 1384 | Rate Ratio (Random, 95% CI) | 0.67 [0.54, 0.83] |
| 4.1 Continuous antibiotics | 4 | 1292 | Rate Ratio (Random, 95% CI) | 0.69 [0.54, 0.89] |
| 4.2 Intermittent antibiotics | 1 | 92 | Rate Ratio (Random, 95% CI) | 0.58 [0.42, 0.80] |
| 5 Time to the first exacerbation | Other data | No numeric data | ||
| 5.1 Continuous antibiotic | Other data | No numeric data | ||
| 5.2 Intermittent antibiotics | Other data | No numeric data | ||
| 5.3 Pulsed antibiotics | Other data | No numeric data | ||
| 6 Mean time to first exacerbation (days) | 1 | 266 | Mean Difference (IV, Random, 95% CI) | ‐16.59 [‐46.05, 12.86] |
| 6.1 Continuous antibiotics | 1 | 266 | Mean Difference (IV, Random, 95% CI) | ‐16.59 [‐46.05, 12.86] |
| 7 COPD exacerbations according to severity of COPD ‐ continuous antibiotics | Other data | No numeric data | ||
| 8 COPD exacerbations according to severity of COPD ‐ pulsed antibiotics | Other data | No numeric data | ||
| 9 HRQoL, SGRQ (total score) | 7 | 2237 | Mean Difference (Random, 95% CI) | ‐1.94 [‐3.13, ‐0.75] |
| 9.1 Continuous antibiotics | 4 | 991 | Mean Difference (Random, 95% CI) | ‐1.96 [‐3.45, ‐0.47] |
| 9.2 Intermittent antibiotics | 3 | 184 | Mean Difference (Random, 95% CI) | ‐4.59 [‐8.83, ‐0.36] |
| 9.3 Pulsed antibiotics | 2 | 1062 | Mean Difference (Random, 95% CI) | ‐1.22 [‐3.00, 0.55] |
| 10 HRQoL, SGRQ (domains) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 SGRQ activity | 4 | 2077 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐2.62, 0.65] |
| 10.2 SGRQ symptoms | 4 | 2077 | Mean Difference (IV, Random, 95% CI) | ‐4.07 [‐5.72, ‐2.41] |
| 10.3 SGRQ impact | 4 | 2077 | Mean Difference (IV, Random, 95% CI) | ‐2.56 [‐5.02, ‐0.10] |
| 11 HRQoL, LCQ (total) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Intermittent antibiotics | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 HRQoL, SF‐12 (domains) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 SF‐12 physical | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 SF‐12 mental | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 HRQoL SF‐36 (domains) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 SF‐36 general health | 3 | 1071 | Mean Difference (IV, Random, 95% CI) | 4.06 [0.70, 7.42] |
| 13.2 SF‐36 physical functioning | 3 | 1071 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐1.01, 4.77] |
| 13.3 SF‐36 bodily pain | 3 | 1072 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐2.47, 3.53] |
| 13.4 SF‐36 vitality | 3 | 1070 | Mean Difference (IV, Random, 95% CI) | 2.03 [‐0.38, 4.43] |
| 13.5 SF‐36 role emotional | 3 | 1072 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐5.55, 4.04] |
| 13.6 SF‐36 social functioning | 3 | 1072 | Mean Difference (IV, Random, 95% CI) | 7.19 [‐2.40, 16.78] |
| 13.7 SF‐36 mental health | 3 | 1070 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐1.13, 5.86] |
| 13.8 SF‐36 role physical | 3 | 1072 | Mean Difference (IV, Random, 95% CI) | 4.63 [‐9.82, 19.09] |
| 14 HRQoL, LCQ (domains) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 LCQ physical | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.12, 0.68] |
| 14.2 LCQ psychological | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.22, 0.78] |
| 14.3 LCQ social | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 0.4 [‐0.15, 0.95] |
| 15 HRQoL, CCQ (total) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Continuous antibiotics | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 HRQoL, CRQ (domains) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 CRQ dyspnoea | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.79, 0.94] |
| 16.2 CRQ fatigue | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.74, 0.62] |
| 16.3 CRQ emotional function | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.94, 0.62] |
| 16.4 CRQ mastery | 1 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐1.29, 0.87] |
| 17 Frequency of hospital admissions ‐ continuous antibiotics | Other data | No numeric data | ||
| 18 Frequency of hospital admissions ‐ pulsed antibiotics | Other data | No numeric data | ||
| 19 Duration of exacerbation | Other data | No numeric data | ||
| 19.1 Continuous antibiotics | Other data | No numeric data | ||
| 19.2 Pulsed antibiotics | Other data | No numeric data | ||
| 20 FEV1 (mL) | 6 | 658 | Mean Difference (Random, 95% CI) | 20.21 [‐26.19, 66.61] |
| 20.1 Continuous antibiotics | 4 | 441 | Mean Difference (Random, 95% CI) | ‐12.69 [‐77.66, 52.28] |
| 20.2 Intermittent antibiotics | 3 | 184 | Mean Difference (Random, 95% CI) | 53.95 [‐16.90, 124.81] |
| 20.3 Pulsed antibiotics | 1 | 33 | Mean Difference (Random, 95% CI) | 58.0 [‐129.63, 245.63] |
| 21 FVC (L) | 4 | 514 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.01, 0.23] |
| 21.1 Continuous antibiotics | 2 | 363 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.30] |
| 21.2 Intermittent antibiotics | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.01, 0.36] |
| 22 FEV1 % predicted | 6 | 1737 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐1.56, 2.22] |
| 22.1 Continuous antibiotics | 3 | 437 | Mean Difference (IV, Random, 95% CI) | 1.43 [‐1.97, 4.83] |
| 22.2 Intermittent antibiotics | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 1.67 [‐1.33, 4.68] |
| 22.3 Pulsed antibiotics | 1 | 1149 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐3.28, 0.58] |
| 23 Exercise capacity (6MWT) | 2 | 126 | Mean Difference (IV, Random, 95% CI) | 67.67 [16.20, 119.14] |
| 23.1 Continuous antibiotics | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 84.20 [13.38, 155.03] |
| 23.2 Intermittent antibiotics | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 36.0 [‐15.53, 87.53] |
| 24 All‐cause mortality | 6 | 3309 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.15] |
| 24.1 Continuous antibiotics | 2 | 1409 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.51] |
| 24.2 Intermittent antibiotics | 2 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.92] |
| 24.3 Pulsed antibiotics | 2 | 1724 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.23] |
| 25 Respiratory‐related mortality | 2 | 2266 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.63, 2.19] |
| 25.1 Continuous antibiotics | 1 | 1117 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.54, 3.81] |
| 25.2 Pulsed antibiotics | 1 | 1149 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.45, 2.29] |
| 26 Serious adverse events | 9 | 2978 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.05] |
| 26.1 Continuous antibiotics | 7 | 1671 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.05] |
| 26.2 Intermittent antibiotics | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.12, 2.43] |
| 26.3 Pulsed antibiotics | 2 | 1182 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.34] |
| 27 Any adverse event | 4 | 512 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.69, 1.67] |
| 27.1 Continuous antibiotics | 3 | 355 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.62, 1.73] |
| 27.2 Intermittent antibiotics | 2 | 124 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.37, 2.41] |
| 27.3 Pulsed antibiotics | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 11.52 [0.60, 221.75] |
| 28 Adverse events (specific) | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 28.1 Respiratory disorders | 3 | 2350 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.33, 3.41] |
| 28.2 Gastrointestinal disorders | 6 | 2522 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 28.3 QTc prolongation | 1 | 1117 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.39, 3.51] |
| 28.4 Hearing impairment | 1 | 1117 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [1.05, 1.85] |
| 28.5 Musculoskeletal disorders | 1 | 1149 | Odds Ratio (M‐H, Random, 95% CI) | 3.07 [0.32, 29.59] |
| 28.6 Hypersensitivity/skin rash | 2 | 1258 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.63, 4.26] |
| 28.7 Nervous system disorders | 2 | 1179 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.38, 4.08] |
| 28.8 Cardiovascular | 1 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.18, 23.51] |
1.2. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 2 Number of people with one or more exacerbations requiring hospitalisation.
1.3. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 3 Annualised exacerbation rate per patient per year during 12‐week active treatment.
| Annualised exacerbation rate per patient per year during 12‐week active treatment | |||
|---|---|---|---|
| Study | Intervention arm | Rate per patient per year | P value compared to control |
| Continuous antibiotics | |||
| Shafuddin 2015 | Roxithromycin | 1.74 | 0.2545 |
| Shafuddin 2015 | Roxithromycin+doxycyline | 1.64 | 0.1709 |
| Shafuddin 2015 | Placebo | 2.25 | N/A |
1.6. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 6 Mean time to first exacerbation (days).
1.7. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 7 COPD exacerbations according to severity of COPD ‐ continuous antibiotics.
| COPD exacerbations according to severity of COPD ‐ continuous antibiotics | |||
|---|---|---|---|
| Study | Gold stage | Rate of exacerbations per patient year on azithromycin (Mean +/‐ SD) | Rate of exacerbations per patient year on placebo (Mean +/‐ SD) |
| Albert 2011 | 2 : FEV1 (80% ‐ 50%) | 1.02 (+/‐ 0.15) | 1.68 (+/‐ 0.16) |
| Albert 2011 | 3 : FEV1 (50% ‐ 30%) | 1.53 (+/‐ 0.13) | 1.75 (+/‐ 0.13) |
| Albert 2011 | 4 : FEV1 < 30% | 1.75 (+/‐ 0.12) | 2.05 (+/‐ 0.28) |
1.17. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 17 Frequency of hospital admissions ‐ continuous antibiotics.
| Frequency of hospital admissions ‐ continuous antibiotics | |||
|---|---|---|---|
| Study | GOLD stage | Rate of hospitalisations per patient year on moxifloxacin (Mean +/‐SD) | Rate of hospitalisations per patient year on placebo (Mean +/‐SD) |
| Albert 2011 | 2 : FEV1 (80% ‐ 50%) | 0.50 +/‐0.12 | 0.65 +/‐ 0.11 |
| Albert 2011 | 3 : FEV1 (50% ‐ 30%) | 0.85 +/‐ 0.12 | 0.96 +/‐ 0.12 |
| Albert 2011 | 4 : FEV1 < 30% | 0.74 +/‐ 0.12 | 1.03 +/‐ 0.27 |
1.19. Analysis.
Comparison 1 Antibiotics versus placebo, Outcome 19 Duration of exacerbation.
| Duration of exacerbation | |||
|---|---|---|---|
| Study | Median days of exacerbation, treatment arm | Median days of exacerbation, placebo arm | P value |
| Continuous antibiotics | |||
| Seemungal 2008 | 9 (6 to14) | 13 (6 to 24) | 0.036 Mann‐Whitney test |
| Pulsed antibiotics | |||
| Mygind 2010 | 93 (total exacerbation days at home or hospitalised) | 111 (total exacerbations days at home or hospitalised) | 0.04 |
Comparison 2. Subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subgroup analysis: number of people with one or more exacerbations by mean % predicted FEV1 | 8 | 2716 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 1.1 Mean FEV1 % ≥ 50 | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.13] |
| 1.2 Mean FEV1 % < 50 | 7 | 2686 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.81] |
| 2 Subgroup analysis: number of people with one or more exacerbations by treatment duration | 8 | 2716 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 2.1 ≥ 3 months to < 6 months | 3 | 213 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.94] |
| 2.2 ≥ 6 months to < 12 months | 2 | 1185 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.24, 1.69] |
| 2.3 ≥ 12 months | 3 | 1318 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.25, 0.81] |
| 3 Subgroup analysis: number of people with one or more exacerbations by year carried out | 8 | 2716 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 3.1 2005 to 2009 | 1 | 109 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.84] |
| 3.2 2010 to 2014 | 6 | 2508 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.83] |
| 3.3 2015 to 2019 | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.29, 1.89] |
| 4 Subgroup analysis: number of people with one or more exacerbations by regimen | 8 | 2716 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 4.1 Once daily antibiotic | 3 | 1180 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.34, 1.09] |
| 4.2 Twice or three times daily antibiotic | 2 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.17, 0.71] |
| 4.3 Three times a week antibiotic | 3 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.19, 0.77] |
| 4.4 Pulsed antibiotic | 2 | 1182 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.07] |
| 5 Subgroup analysis: number of people with one or more exacerbations by exacerbation history | 8 | 2716 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 5.1 Inclusion criteria of ≥ 1 exacerbation in preceding year | 3 | 2358 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 0.99] |
| 5.2 Exacerbation history not an inclusion criteria | 5 | 358 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albert 2011.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled clinical trial with 12‐month treatment duration Intention‐to‐treat analysis |
|
| Participants | N = 1142. Aged 40 years or over. Mean age (years): 65 (azithromycin) and 66 (placebo) 41% female Severity of COPD: moderate or worse as defined by GOLD criteria Mean FEV1 (L): 1.10 (SD 0.50) (azithromycin) and 1.12 (SD 0.52) (placebo) Presence of either a) using continuous supplemental oxygen, or b) received systemic glucocorticoids within the previous year/had gone to an emergency room/hospitalisation for an acute exacerbation No acute exacerbation of COPD for at least 4 weeks Exclusions: asthma, resting heart rate > 100/min, prolonged QT interval > 450 ms, using medications that prolong QTc, hearing impairment documented by audiometry |
|
| Interventions | Prophylaxis: 1. Azithromycin 250 mg daily 2. Placebo |
|
| Outcomes | Primary: 1. Time to the first acute exacerbation of COPD Secondary: 1. Quality of life 2. Nasopharyngeal colonisation of selected respiratory pathogens 3. Compliance to the treatment 4. Adverse events |
|
| Notes | Funding: Grants listed from National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The stratified random sequence generation was well described in the journal article under "protocol": "Randomization will be carried out by linking to the Data Coordinating Center through a website, (http://www.copdcrn.org)". |
| Allocation concealment (selection bias) | Low risk | Well explained. Central allocation was pharmacy controlled: "Only the pharmacist and the staff of the Data Coordinating Center knew this schedule and the pharmacists could not know the actual assignment until after the DCC specified an accession number. Treatment assignment was only disclosed in cases of emergency." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active drug and placebo will be identical in appearance. Both participants and treating medical staff were blinded: "The actual assignment will only be revealed in cases of emergencies where caregivers need to know what drugs the person was taking to provide treatment, or to avoid prescribing other medications that might adversely interact with the study drug. Active‐drug and placebo capsules will be identical in appearance." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial staff were unaware of the randomisation: "Clinic staff (who undertook outcome assessment) will make no attempt to determine the content of any capsules except in cases of emergency". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data accounted for in a consort diagram for the entire study. However, data on the secondary outcome (HRQoL) had reported loss to follow‐up of 20% in the prophylactic antibiotic arm and 18% on the placebo arm. The reasons for the missing data pertaining to HRQoL were not given. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported |
| Other bias | Low risk | No other bias identified |
Banerjee 2005.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled clinical trial. Treatment duration of 3 months. Intention‐to‐treat analysis | |
| Participants | N = 67 Mean age (years): 65.1 (clarithromycin) and 68.1 (placebo) Mean FEV1 (L): 1.12 (clarithromycin) and 1.13 (placebo) Severity of COPD: moderate or worse according to BTS guidelines. All participants were taking ICS Patients enrolled from hospital clinics No acute exacerbations of COPD over the last 6 weeks Exclusions: Previous documented allergies to macrolides; a clinical history of lung cancer, asthma or bronchiectasis |
|
| Interventions | Prophylaxis: 1. Clarithromycin (long‐acting Klaricid XL 500 mg/daily) 2. Placebo |
|
| Outcomes | Primary: 1. Health‐related quality of life Secondary: 1. Infective exacerbation rate 2. Shuttle walk test 3. Serum CRP level 4. Sputum bacterial quantities load |
|
| Notes | Funding: Received grant support from Abbott Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was carried out: "Subjects were then block randomised into a prospective, double‐blind controlled study... Patients were randomised by the Birmingham Heartlands Hospital pharmacy department, independent of trial staff". |
| Allocation concealment (selection bias) | Low risk | Participant randomisation was not known to the trial staff. Randomisation carried out by the Birmingham Hospital pharmacy department: "Patients were randomised by the Birmingham Heartlands Hospital pharmacy department, independent of trial staff". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial staff were unaware of the allocation, but blinding of outcome assessors not clearly described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data described |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | No other bias identified |
Berkhof 2013.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled clinical trial. Treatment duration of 12 weeks; 6‐week post‐treatment follow‐up Intention‐to‐treat analysis |
|
| Participants | N = 84. Aged 40 years or over. Mean age (years): 67 (azithromycin) and 68 (placebo) Female: 26% (azithromycin) and 24% (placebo) Mean FEV1 % predicted: 49.8 (SD 16.4) (azithromycin) and 47.4 (SD 12.9) (placebo) Clinical diagnosis of COPD: GOLD stage ≥ 2 (defined as a post bronchodilator of FEV1 < 80% and a ratio of FEV1/FVC < 70%), and were suffering from chronic productive cough, defined as cough for at least the last 12 weeks, in two subsequent years Exclusions: prior history of asthma; use of intravenous or OCS and/or antibiotics for an exacerbation three weeks before inclusion; other relevant lung or liver diseases at the discretion of the treating physician; pregnancy or lactation; use of macrolides in the last six weeks prior to inclusion; allergy or intolerance to macrolides; or use of other investigational medication started two months prior to inclusion |
|
| Interventions | Prophylaxis: 1. Azithromycin 250 mg 3 times a week 2. Placebo |
|
| Outcomes | Primary: 1. mean LCQ total and domain scores Seondary: 1. SGRQ total score 2. SF‐36 score 3. Post‐bronchodilator spirometry 4. Blood values 5. Microbiology 6. Time to first exacerbation of COPD 7. Exacerbations 8. Hospitalizations for COPD 9. Adverse events |
|
| Notes | Funding: "We want to thank Stichting Astma Bestrijding (SAB) for financial support." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation codes were generated using a computer allocation program, with a 1:1 ratio and a permutated block size of 4." |
| Allocation concealment (selection bias) | Unclear risk | Not specifically described, but probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators, research nurses, and participants were masked to treatment allocation until final analyses of the data were performed". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators, research nurses, and participants were masked to treatment allocation until final analyses of the data were performed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout low and balanced. All participants accounted for in flow diagram |
| Selective reporting (reporting bias) | Low risk | FEV1 measured but not reported in a way allowing inclusion in meta‐analysis in the published paper, but authors supplied additional data on request |
| Other bias | Low risk | No other bias identified |
Brill 2015.
| Methods | Prospective, randomised, single‐blind, placebo‐controlled clinical trial. Treatment duration of 13 weeks Intention‐to‐treat analysis |
|
| Participants | N = 99. Aged 45 to 80 years. Mean age (years) 70.0 (moxifloxacin), 70.4 (doxycyline), 67.9 (azithromycin) and 68.7 (placebo) Female: 32% (moxifloxacin), 28% (doxycyline), 36% (azithromycin), and 25% (placebo) Mean FEV1 % predicted: 52 (SD 13) (moxifloxacin), 53 (SD 14) (doxycyline), 44 (SD 17), (azithromycin), and 53 (SD 13) (placebo) Stable patients with chronic bronchitis (self‐reported sputum expectoration on most days when clinically stable) and spirometrically‐confirmed COPD (defined by FEV1 < 80% predicted, FEV1 to FVC ratio < 0.7, and a history of smoking) Exclusions: patients who reported either treatment for an exacerbation, an episode of symptoms worsening in the 4 weeks prior to screening, or were unable to enrol for safety reasons (significant hepatic/renal impairment, QT prolongation, pre‐existing long‐term antibiotic use, and hypersensitivity to the treatments under investigation) |
|
| Interventions | Prophylaxis: 1. Moxifloxacin 400 mg daily for 5 days every 4 weeks 2. Doxycyline 100 mg daily 3. Azithromycin 250 mg 3 times a week 4. Placebo |
|
| Outcomes | Primary: 1. Change in sputum bacterial load, as assessed by quantitative culture. Secondary: 1. Changes in resistance to the three tested antibiotics 2. Changes in FEV1 3. Adherence to therapy 4, Health status as measured by total SGRQ scores 5. Adverse events Exploratory: 1. Changes in sputum bacterial load as assessed by 16S rRNA gene‐targeted qPCR 2. Changes in sputum inflammation |
|
| Notes | Funding: funded by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research programme (RP‐PG‐0109‐10056) and the NIHR Royal Brompton Respiratory Biomedical Research Unit. The moxifloxacin for the study was provided by Bayer Pharma AG, Berlin, Germany and the study sponsor was University College, London, UK. Neither Bayer, the funder, nor the Sponsor had any influence in the study design, collection, analysis and interpretation of the data, the writing of the report, or the decision to submit for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Internet randomisation into groups of 1:1:1:1 was performed using a computer‐generated permuted block system of variable sizes (Sealed Envelope, UK)". |
| Allocation concealment (selection bias) | Low risk | "Internet randomisation into groups of 1:1:1:1 was performed using a computer‐generated permuted block system of variable sizes (Sealed Envelope, UK). Participants remained blinded to treatment allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients remained blinded to treatment allocation". However, not clear if study personnel were blinded. Described as single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description of outcome assessor blinding, although blinded participants assessed outcomes such as quality of life |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout low and balanced. All participants accounted for in flow diagram |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review reported |
| Other bias | Low risk | No other bias identified |
He 2010.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled clinical trial. Treatment duration was 6 months. Intention‐to‐treat analysis | |
| Participants | N = 36. Participants were 40 years or older. Mean age (years): 68.8 (erythromycin) and 69.3 (placebo) Females: 17% (erythromycin) versus 10% (placebo) FEV1 between 30% to 70% predicted. Mean FEV1 (L): 1.12 (erythromycin) versus 1.02 (placebo) At least 10 pack/year smoking history No acute exacerbations during the previous 1 month Exclusions: patients with significant other respiratory disorders other than COPD; history of unstable cardiovascular disease; hypersensitivity to macrolides |
|
| Interventions | Prophylaxis: 1. Erythromycin 125 mg 3 times a day 2. Placebo |
|
| Outcomes | Primary: 1. Number of acute COPD exacerbations 2. Neutrophil count in sputum Secondary: 1. Quality of life 2. Spirometry |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation done but not clearly explained: "Eligible participants were randomly assigned to receive oral erythromycin....or placebo for 6 months." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as a double‐blind trial: "Eligible participants were randomly assigned to receive oral erythromycin....or placebo for 6 months." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcomes assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data described using a CONSORT diagram |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | No other bias identified |
Mygind 2010.
| Methods | Prospective, randomised, placebo‐controlled double‐blind study. Treatment duration was 36 months. Intention‐to‐treat analysis | |
| Participants | N = 575. Aged > 50 years Severity of COPD was moderate or severe with FEV1 < 60% predicted. Mean FEV1 (L) was 0.9 (both treatment and placebo arms) At least one admission to hospital with exacerbation of COPD Ex or current smokers Exclusions: end stage COPD patients (if not expected to survive over 3 years), or bedridden patients; patients with a history of asthma, bronchiectasis, or other significant respiratory disease; history of azithromycin allergy; patients with heart, liver or renal insufficiency; already receiving prophylactic antibiotic |
|
| Interventions | Intermittent prophylaxis: 1. Azithromycin 500 mg/daily for 3 days every months, for 36 months 2. Placebo daily for 3 days every month, for 36 months |
|
| Outcomes | Primary: 1. Rate of decline in lung function (FEV1) Secondary: 1. Frequency of exacerbation 2. Health‐related quality of life (SGRQ) 3. Adverse events 4. Mortality 5. Duration of exacerbations 6. Number of days of hospitalisation 7. Frequency of hospitalisation |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as a "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcomes assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data were presented. Only 55% completed 3 years |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | This was a conference presentation and not a full publication. Attempts to contact the authors were not successful. Only limited data are available for evaluation of the risk of bias |
NCT00524095.
| Methods | Prospective, randomised, controlled cross‐over trial. Open‐label. Planned 52 weeks treatment duration | |
| Participants | Planned recruitment of 210 participants aged 45 to 85 years Smokers or former smokers of at least 10 pack‐years, COPD demonstrated by forced spirometry with FEV1 > 0.7 L, FEV1 post‐bronchodilator < 60% and FEV1/FVC < 70%, bronchodilator test performed at inclusion or no more than 6 months before inclusion should have been negative (increase in FEV1 < 200 mL and 12%, 10 minutes after administration of 2 puffs of salbutamol). Stable phase defined by clinical criteria of the attending investigator, but at least 6 weeks from the last exacerbation Exclusions: receiving OCS at any dose or another immunosuppressor, formal contraindication for sputum collection, or impossibility to obtain a sample of sputum valid for analysis, allergy to steroids or macrolides |
|
| Interventions | Prophylaxis: 1. Azithromycin 500 mg 3 times a week for 6 months and then inhaled steroids (fluticasone 500 μg twice a day) for 6 months 2. Inhaled steroids (fluticasone 500 μg twice a day) for 6 months and then azithromycin 500 mg 3 times a week for 6 months 3. Usual care |
|
| Outcomes | Primary 1. Effects of treatments on bronchial inflammation parameters Secondary: 2. Effects of treatments on exacerbations frequency (time frame: six months) 3. Effects of treatments on pulmonary function (time frame: six months) |
|
| Notes | NB: study terminated before treatment phase. Reason not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not assessed as study terminated before treatment phase |
| Allocation concealment (selection bias) | Unclear risk | Not assessed as study terminated before treatment phase |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not assessed as study terminated before treatment phase |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not assessed as study terminated before treatment phase |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not assessed as study terminated before treatment phase |
| Selective reporting (reporting bias) | Unclear risk | Not assessed as study terminated before treatment phase |
| Other bias | Unclear risk | Not assessed as study terminated before treatment phase |
NCT02628769.
| Methods | Prospective, randomised, controlled double‐blind cross‐over trial. Planned 12 weeks treatment duration | |
| Participants | N = 5. Aged 45 years and older History of cigarette smoking > 10 pack‐years, post‐bronchodilator FEV1/FVC of < 0.70 and FEV1 of 30% to 79% of predicted normal value, nonpregnant females, willing and able to comply with all study visits and procedures, a suitable candidate for oral therapy and be able to swallow capsules intact, no evidence of active bacterial infection in sputum by qPCR evaluation Exclusions: acute exacerbation of COPD within the previous 60 days or during the washout period of the study, any condition that could possibly affect oral drug absorption, currently taking medication for HIV, chronic hepatitis B, or hepatitis C virus infection, currently taking theophylline or other xanthine medication, currently taking warfarin, known concomitant infection, QTc greater than 450 msec for males or females as corrected by the Fridericia formula, current use of drugs known to prolong the QT interval, concomitant use of drugs, foods, or herbal products known to be moderate to potent inhibitors of CYP3A4 isozymes, any use within the prior 7 days of drugs or herbal products known to be moderate to potent inducers of CYP3A4 isozymes, required current use of drugs with narrow therapeutic indices that are principally metabolised by CYP3A4 or transported by P‐glycoprotein, for which a drug interaction with solithromycin could result in higher and possibly unsafe exposures to these drugs, history of organ transplant, cytotoxic chemotherapy or radiation therapy within the previous 3 months, known neuromuscular disorder from clinical history, known significant renal, hepatic, or haematologic impairment, any investigational drugs taken or investigational devices used within 4 weeks before administration of the first dose of the study drug, history of intolerance or hypersensitivity to macrolide antibiotics, any concomitant condition that, in the opinion of the Investigator, would preclude an evaluation of a response or make it unlikely that the contemplated course of therapy and follow‐up could be completed (e.g. life expectancy < 30 days) |
|
| Interventions | Prophylaxis: 1. Solithromycin 400 mg daily 2. Placebo |
|
| Outcomes | Primary:
1. Number of sputum neutrophils per mL
Secondary:
1. Sputum chemokines 2. Concentrations of CXCL8 in nasal lining fluid 3. FEV1 4. R5 to R20 (assessed by impulse oscillometry) 5. CAT scores 6. Adverse events Exploratory: 1. Activity of HDAC2 in sputum macrophages from participants 2. Activity of PI3K in sputum macrophages from participants 3. Activity of NF‐κB in sputum macrophages from participants 4. Levels of the serum CRP 5. Levels of serum biomarkers fibrinogen |
|
| Notes | NB: study terminated after enrolment of 5 participants due to hepatotoxicity of the study drug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
| Allocation concealment (selection bias) | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
| Selective reporting (reporting bias) | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
| Other bias | Unclear risk | Not assessed as study terminated after enrolment of 5 participants |
Seemungal 2008.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled clinical trial with 12 month follow‐up | |
| Participants | N = 109. Participants recruited from outpatient chest clinic from a single centre Mean age (years): 66 (erythromycin) and 68 (placebo) Females: 38% (erythromycin) and 36% (placebo) Severity of COPD was moderate to severe. FEV1 between 30% to 70% predicted. Mean FEV1 (L): 1.27 (erythromycin) and 1.36 (placebo) Exclusions: history of asthma, bronchiectasis, neoplasia, unstable cardiac status (including prolonged QTc and arrhythmias), macrolide allergy, or history of abnormal liver functions |
|
| Interventions | Prophylaxis: 1. Erythromycin 250 mg twice daily 2. Placebo |
|
| Outcomes | Primary: 1. Exacerbation frequency 2. Airway inflammation |
|
| Notes | Calculated sample size was 115 for 90% power and P value 0.05. However only 109 participants were recruited Funding: British Lung Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted block random sequence generation carried out: "Computer‐generated randomization...Randomization was taken in blocks of 10 (5 placebo, 5 erythromycin)". |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomization numbers were stored in sealed envelopes. Medication was randomized before commencement of the study by the hospital pharmacy, independently of trial staff, and patients were automatically dispensed the next allocated treatment". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo and erythromycin (250 mg) were concealed in identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Unblinding occurred after data entry" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes/dropouts explained in a CONSORT diagram |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | No other bias identified |
Sethi 2010.
| Methods | Prospective double‐blind randomised placebo‐controlled clinical trial. Total treatment period was 48 weeks 1. Analysis was done using intention‐to‐treat and per protocol. For this review, only the results of the intention‐to‐treat analysis were taken 2. Exacerbation of COPD was defined by two definitions. A primary definition (any confirmed acute exacerbation of COPD, unconfirmed pneumonia, or any other lower respiratory tract infections) and a secondary definition (only confirmed exacerbations of COPD, excluding confirmed/unconfirmed pneumonia and any other lower respiratory tract infection) For this review, only the primary definition was used as it was an extended definition and hence was the more conservative definition |
|
| Participants | N = 1157. Aged 45 years or over. Severity of COPD was GOLD stage 2 or worse. Had at least 2 exacerbations requiring treatment with antibiotics and/or oral steroids in the 12 months prior to enrolment. Total follow‐up period was 72 weeks. Total treatment period was 48 weeks |
|
| Interventions | Pulsed prophylaxis: 1. Moxifloxacin 400 mg/daily for 5 days. Treatment repeated every 8 weeks for a total of 6 courses 2. Placebo daily for 5 days. Treatment repeated every 8 weeks for a total of 6 courses |
|
| Outcomes | Primary: 1. Frequency of exacerbations Secondary: 1. HRQoL (assessed using SGRQ) 2. Hospitalisations 3. Mortality 4. Changes in lung function 5. Adverse events |
|
| Notes | Funding: received grant support from Bayer HealthCare AG | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double‐blind, placebo‐controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcomes assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data were described using a CONSORT diagram for the entire study. However, data on the secondary outcome, HRQoL, had reported loss to follow‐up of 12% in the prophylactic antibiotic arm and 10% in the placebo arm. The reasons for the missing data pertaining to HRQoL outcome were not given |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were well described |
| Other bias | Low risk | Data were analysed as intention‐to‐treat as well as per protocol analysis. Both analyses were published |
Shafuddin 2015.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled trial. Duration of treatment 13 weeks with 48 week post‐treatment follow up Intention‐to‐treat analysis Originally designed to investigate the role antibiotics in eradicating C. pneumoniae in patients with COPD |
|
| Participants | N = 292. Aged 45 years and above. Mean age (years): 68.5 (roxithromycin/doxycyline), 67.6 (roxithromycin), and 66.7 (placebo) Female: 36.6% (roxithromycin/doxycyline), 14.4% (doxycyline), 28.7% (placebo) Mean FEV1 % predicted, mean: 32.53 (SD 13.55) (roxithromycin/doxycyline), 33.93 (SD 15.3) (doxycyline), 35.8 (SD 15.2) (placebo) Meeting spirometric criteria for COPD (FEV1 ≤ 70 % predicted, FEV1/FVC ≤ 60 %, reversibility of ≤ 10 % of predicted FEV1 or ≤ 200 mL if predicted FEV1 ≤ 2 L); smoking history ≥ 20 pack years; and at least three confirmed moderate or severe COPD exacerbations in the past two years (i.e. requiring treatment with antibiotics and/or OCS and/or hospitalisation), positive serology for C. pneumoniae (IgG antibody titre ≥ 1:64). Exclusions: pulmonary disease other than COPD; treatment with antibiotics, exacerbation or an investigational drug in the four weeks before randomisation; pregnancy (serum pregnancy test) or breast feeding; history of hypersensitivity to macrolides, tetracyclines, beta‐lactams or sulfamethoxazole:trimethoprim; serious cardiovascular, hepatic, renal or other systemic diseases; known long QT syndrome or corrected QT interval (QTc) > 450 ms, sick sinus syndrome, bradycardia (< 50 beats per minute) or severe hypokalaemia; epilepsy; treatment with medicine known to have important interaction with macrolides or tetracyclines; impaired hepatic function (aspartate aminotransferase or alanine aminotransferase ≥ 2 times of the upper limit of normal (ULN), alkaline phosphatase ≥ 1.25 times the ULN, bilirubin > 2 times the ULN and albumin < 30 g/L); or unlikely to comply |
|
| Interventions | Prophylaxis: 1. Roxithromycin 300 mg daily plus doxycycline 100 mg daily 2. Roxithromycin 100 mg daily 3. Placebo |
|
| Outcomes | Primary: 1. COPD exacerbations over 48‐week post‐treatment period Secondary 1. COPD exacerbations over the 12‐week treatment period and the first and last 24‐week post‐treatment periods 2. FEV1 and FVC over 60‐week period 3. CRQ scores over 60‐week period 4. Adverse events |
|
| Notes | Funding: supported by Sanofi‐Aventis Australia Pty Ltd (formally Hoechst Marion Roussel Pty Ltd). Sanofi‐Aventis had no role in the preparation of this manuscript for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each eligible patient was assigned a sequential subject number followed by randomisation number provided by Hoechst Marion Roussel, Australia. Subjects were supplied with one of the three treatments according to their randomisation number". Clinical trials registry clarified: "computer sequence generation used for randomisation of subjects into treatment arms with 1:1:1 ratio". |
| Allocation concealment (selection bias) | Low risk | "Each eligible patient was assigned a sequential subject number followed by randomisation number provided by Hoechst Marion Roussel, Australia. Subjects were supplied with one of the three treatments according to their randomisation number." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study medication was packed by Hoechst Marion Roussel in bottles labelled with the randomisation and batch numbers. The investigators, pharmacists and subjects were blinded to the study medication in these bottles." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Correspondence with trialists confirmed that all participants, personnel, and outcome assessors remained blinded until data had been analysed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More participants dropped out of combined antibiotics treatment arm (21 versus 13 in single antibiotic arm and 10 in placebo arm), although, according to trialists, reasons were not related to study medication. All participants included in ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review reported |
| Other bias | Low risk | No other bias identified |
Simpson 2014.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled trial. Duration of treatment 12 weeks with 12 week post‐treatment follow up Intention‐to‐treat analysis |
|
| Participants | N = 30. Aged 55 years and above. Mean age (years): 71.7 (azithromycin) and 69.9 (placebo) Female: 40% (azithromycin) and 33.3% (placebo) FEV1% predicted, mean: 56.5 (SD 13.7) (azithromycin) and 51.1 (SD 13.7) (placebo) Adults (males and nonpregnant females) with a doctor's diagnosis of symptomatic COPD, post‐bronchodilator FEV1/FVC < 70% and FEV1 < 80% and persistent neutrophilic bronchitis defined as sputum neutrophil proportion of more than 61% or more than 162 x 104/mL sputum neutrophils demonstrated on two occasions Exclusions: no reported exacerbations or alterations in respiratory medications in the previous 4 weeks, inability to produce an adequate sputum sample, a FEV1 < 0.5 L, current smoking or having ceased smoking in the past 6 months, a known hypersensitivity to macrolides, an ECG assessment showing a prolonged QTc interval or an impairment of liver function |
|
| Interventions | Prophylaxis: 1. Azithromycin 250 mg daily 2. Placebo |
|
| Outcomes | Primary: 1. Reduction in sputum CXCL8 Secondary: 1. Change in sputum neutrophil proportion 2. Total bacterial load in sputum 3. Health care utilisation 4. Quality of life (SGRQ) 5. Severe exacerbations 6. Pulmonary function tests 7. Chest computed tomography to measure airway thickness 8. Adverse events |
|
| Notes | Funding: funded by the National Health and Medical Research Council of Australia through a project grant, ID 455508 2007–2009. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Concealed random allocation was undertaken by a blinded staff member who took no further part in the study...A random numbers table was computer generated (www.randomization.com) for treatment allocation using permuted blocks of six and participants were stratified according to smoking history (never or previous smokers)." |
| Allocation concealment (selection bias) | Low risk | "Concealed random allocation was undertaken by a blinded staff member who took no further part in the study..The active medication and placebo were prepared and packaged identically by a compounding chemist and dispensed by the John Hunter Hospital pharmacy according to the random number table." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both participants and study staff were blinded to the assignment of intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The people assessing the outcomes are described as blinded in the trial registration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low and balanced dropout. Reasons for discontinuation unrelated to study medication |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review reported |
| Other bias | Low risk | No other bias identified |
Suzuki 2001.
| Methods | Prospective, randomised, placebo‐controlled clinical trial. Not blinded | |
| Participants | N = 109 Mean age (years): 69 (erythromycin) and 72 (placebo) Mean FEV1 (L): 1.47 (erythromycin) and 1.30 (placebo) Females: 13% in erythromycin group versus 18% in placebo group All study participants were treated with sustained‐release theophylline and inhaled anticholinergic agents Exclusions: patients diagnosed with bronchiectasis or diffuse pan bronchiolitis |
|
| Interventions | Prophylaxis: 1. Erythromycin 200 mg to 400 mg/daily 2. Placebo |
|
| Outcomes | 1. Acute exacerbations of COPD 2. Adverse events |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by a random‐number table, and the list was held independently of the investigators". |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by a random‐number table, and the list was held independently of the investigators". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This study was not blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This study was not blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant excluded due to adverse events of erythromycin, all participants clearly accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | No other bias identified |
Tan 2016.
| Methods | Prospective, randomised controlled trial. Blinding not stated in main trial report. Treatment duration 52 weeks | |
| Participants | N = 54. Age range: 49 to 70 years. Mean age (years): 68.8 (erythromycin, 12 months), 67.3 (erythromycin, 6 months) and 69.3 (control) Female: 16.7% (erythromycin, 12 months), 5.6% (erythromycin, 6 months) and 11.1% (control) Mean FEV1 % predicted: 44.8 (SD 13.9) (erythromycin, 12 months), 46.5 (SD 8.9) (erythromycin, 6 months), and 42.1 (SD 18.6) (control) Stable COPD outpatients (GOLD stages II–IV of 2006 guidelines: FEV 1 < 80% predicted and FEV1/FVC < 70% after bronchial relaxation); no acute exacerbation; no change in therapeutic schedule; and no treatment with any antibiotics or glucocorticoids in the previous 4 weeks Exclusions: patients with bronchial asthma, primary bronchiectasis, diffuse panbronchiolitis, active tuberculosis, lung cancer, pneumoconiosis, or other lung diseases with restrictive ventilatory impairment; patients with other serious systemic illnesses such as cardiovascular, nervous, or endocrine system illnesses, blood, hepatic, or kidney diseases, and malignant tumours; patients who were not cooperative or were completely unable to communicate; and patients who experienced serious adverse reactions to erythromycin |
|
| Interventions | Prophylaxis: 1. Erythromycin 125 mg 3 times a day for 12 months 2. Erythromycin 125 mg 3 times a day for 6 months 3. Control group (no antibiotic treatment) |
|
| Outcomes | 1. Concentrations of IL‐17 and IL‐23 in peripheral blood and induced sputum 2. Six‐minute walk distance (Primary and secondary outcomes not specified) |
|
| Notes | Funding: funded by the National Nature Science Foundation of China (81460009) and the Guangxi Natural Science Foundation (2015GXNSFAA139189, Z2012077, and Z2012081). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly divided" but method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel described. Assumed open‐label (although abstract stated double‐blind). Authors contacted but no response received to date |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors described. Assumed open‐label (although abstract stated double‐blind). Authors contacted but no response received to date |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low and balanced dropout but details not given of how many people were analysed at each time point |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration or protocol identified so not clear if outcomes of interest for this review may have been collected but not reported (e.g. serious adverse events, exacerbations, quality of life) |
| Other bias | Low risk | No additional bias identified |
Uzun 2014.
| Methods | Prospective, randomised double‐blind placebo‐controlled trial. Treatment duration 52 weeks Intention‐to‐treat analysis |
|
| Participants | N = 92. Aged 18 years and above. Mean age (years): 64.7 (azithromycin) and 64.9 (placebo) Female: 53% (azithromycin) and 60% (placebo) Mean FEV1 % predicted: 44.2 (SD 19.3) (azithromycin) and 45.0 (SD 19.5) (placebo) Diagnosis of COPD according to the GOLD guidelines, had received treatment for three or more exacerbations of COPD in the previous year for which they received steroids or antibiotic treatment, clinically stable, and could not have had a COPD exacerbation or respiratory‐tract infection in the month before involvement in the study Exclusions: history of other clinically significant respiratory diseases (e.g. asthma, cystic fibrosis); presence of bronchiectasis, as assessed by CT scan; maintenance antibiotic treatment; use of more than 10 mg prednisolone a day; allergy to macrolides; pregnancy or lactation in women; liver disease (alanine transaminase or aspartate transaminase concentrations that were two or more times the upper limit of normal); malignant disease of any kind for which the patient received treatment or was being monitored as part of follow‐up after treatment; heart failure; and the use of drugs that could adversely interact with macrolides and for which therapeutic monitoring could not be undertaken. |
|
| Interventions | Prophylaxis: 1. Azithromycin 500 mg 3 times per week 2. Placebo |
|
| Outcomes | Primary: 1. Rate of exacerbations of COPD Secondary: 1. Time to first exacerbation 2. Hospital admission for acute exacerbations 3. Change in proportion of exacerbations needing admission to hospital versus treatment in an outpatient department compared with the previous year 4. Treatment for an acute exacerbation of COPD 5. FEV1 after bronchodilation 6. FVC after bronchodilation 7. Six‐minute walk test 8. Quality of life, as assessed by the SF‐12 and the SGRQ 9. Acquisition of macrolide resistant microorganisms in sputum 10. Adverse events |
|
| Notes | Funding: SoLong Trust. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent pharmacy randomly assigned patients (1:1), via a computer‐generated randomisation sequence with permuted blocks of ten." |
| Allocation concealment (selection bias) | Low risk | "Patients were automatically given the next allocated treatment by clinical trials staff at the hospital pharmacy. Participants and investigators were masked to treatment allocation throughout the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants and investigators were masked to treatment allocation throughout the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After data collection and data cleaning were completed, and after final database lock, investigators were unmasked and could assess outcomes and complete the data analysis". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher drop out in placebo arm, but results from the unadjusted and adjusted per‐protocol analyses were almost identical to those from the intention‐to‐treat analysis and all participants included in safety analysis. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review reported |
| Other bias | Low risk | No additional bias identified |
Wang 2017.
| Methods | Prospective, parallel, randomised controlled trial. Blinding not reported. Duration of treatment 26 weeks | |
| Participants | N = 86. Age range: 61 to 83 years. Mean age (years): 70.5 (azithromycin) and 72.4 (placebo) Female: 44.2% (azithromycin) and 37.2 (placebo) 10 cases of cardiac functional grade II, 27 cases of grade III and 6 cases of grade IV (azithromycin) and 11 cases of cardiac functional grade II, 23 cases of grade III and 9 cases of grade IV (placebo) Patients with pulmonary hypertension secondary to COPD. Patients whose mean arterial pressure was detected as not less than 25 mmHg by right cardiac catheterisation in a quiescent condition or as no less than 30 mmHg in a motion state, and patients who had not suffered from acute attack of COPD or acute lung infection. Exclusions: severe cardiac, hepatic, and liver function abnormality, pulmonary thromboembolism, allergic rhinitis, asthma or primary pulmonary hypertension, or were allergic to the drugs used in the study |
|
| Interventions | Prophylaxis: 1. Azithromycin 250 mg daily 2. Control group (no antibiotic treatment) |
|
| Outcomes | 1. PaO2 2. PaCO2 3. Blood pH 4. FEV1 5. FVC 6. Six minutes walking distance 7. Pulmonary arterial pressure |
|
| Notes | Funding: "Grant Support & Financial Disclosures: None". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly divided into an observation group and a control group using random number table, 43 in each group". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel described. Assumed open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors described. Assumed open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | No prospective trial registration or protocol identified. Dyspnea grade reported as measured in the abstract and not reported. Not clear currently if FEV1 and FVC variance were SDs or SEs. |
| Other bias | Low risk | No additional bias identified |
BTS: British Thoracic Society; CAT: COPD assessment test; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; CRQ: chronic respiratory disease questionnaire; CYP: cytochrome P450; CXCL8: C‐X‐C motif ligand 8 (interleukin 8); ECG: electrocardiogram; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HDAC2: histone deacetylase 2; HIV: human immunodeficiency virus; HRQoL: health related quality of life; ICS: inhaled corticosteroid; IgG: immunoglobulin G; IL: interleukin; ITT: intention‐to‐treat; L: litres; LCQ: Leicester Cough Questionnaire; NF‐κB: nuclear factor kappa‐light‐chain‐enhancer of activated B cells; OCS: oral corticosteroids; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; pH: potential of hydrogen; PI3K: phosphoinositide 3‐kinase; qPCR: quantitative polymerase chain reaction; QTc: Q‐T Corrected (corrected Q‐T interval); QT: Q‐T interval; rRNA: ribosomal ribonucleic acid; R5‐R20: total respiratory system resistance, measured at 5 to 20 Hz; SD: standard deviation; SF‐12/36: Short‐Form 12/36; SGRQ: St George's Respiratory Questionnaire; ULN: upper limit of normal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beeh 2016 | Comparison: ELOM‐080 (a distillate of essential oils) versus placebo Problem: drug under investigation not a conventional antibiotic |
| Bier 1971 | Comparison: doxycyclin versus placebo Problem: spirometric criteria were not used in diagnosing COPD |
| Blasi 2010 | Comparison: azithromycin 500 mg three day a week for 6 months versus placebo Problem: pilot study, uncontrolled Study done on tracheostomy patients |
| Bruninx 1973 | Comparison: bactrim versus ledermycin over 1070 months Problem: 1) heterogeneous participant population including bronchiectasis, anthracosilicosis, and bronchitis; 2) no placebo arm |
| Buchanan 1958 | Comparison: tetracycline 250 mg twice daily versus placebo for 12 months duration Problems: single‐blinded (only participants were blinded); spirometric criteria were not used to diagnose COPD |
| Bussi 1980 | Comparison: intermittent tetracyclines 200 mg weekly for 3 years versus placebo Problem: spirometry criteria not used for diagnosis of COPD. Heterogenic group of participants |
| Davies 1961 | Comparison: tetracycline for 2 days each week versus placebo Problem: spirometric criteria were not used in diagnosing COPD; blinding not known |
| Douglas 1957 | Not a randomised controlled trial Heterogeneous group of participants including large proportion with bronchiectasis Intial treatment with intramuscular penicillin Participants who failed penicillin were allocated to either chloramphenicol 0.5 g 6‐hourly or oxytetracycline 0.5 g 6‐hourly |
| Edwards 1958 | Comparison: oxytetracycline or sulfonamide versus placebo Problems: H. influenzae vaccination co‐administered; no suitable outcome measures |
| Elmes 1957 | Comparison: oxytetracycline versus placebo Problem: not prophylactic, antibiotic versus placebo at the onset of symptoms |
| Fletcher 1966 | Comparison: treatment for 7 months/year over 5‐year period. 1) oxytetracycline 0.5 g daily for 7 months over years 1 to 3; 2) oxytetracycline 0.5 g twice daily over 7 months in year 4; 3) oxytetacyline 1 g twice daily over 7 months in year 5; versus placebo Problem: spirometric criteria not used to diagnose COPD |
| Frances 1964 | Problem: spirometric criteria were not used to diagnose COPD |
| Francis 1960 | Comparison: 3 groups: 1) tetracycline 250 mg twice daily for 3 months; 2) penicillin V 312 mg twice daily for 3 months; 3) placebo for 3 months Problems: spirometric criteria were not used in diagnosing COPD |
| Goslings 1967 | Comparison: 1) sulfaphenazole 500 mg twice daily; 2) tetracycline 500 mg twice daily; 3) saccharum 500 mg twice daily (placebo) over 5‐month period Problem: spirometric criteria were not used to diagnose COPD |
| Grossman 1998 | Comparison: ciprofloxacin 500 mg twice daily versus placebo for acute exacerbations of chronic bronchitis, treatment given during acute exacerbations during 12‐month period versus usual care during an acute exacerbation Problem: ciprofloxacin was given during an exacerbation of chronic bronchitis. Not prophylaxis |
| Hahn 1972 | Comparison: tetracycline or ampicillin versus placebo Problems: not a true long‐term prophylaxis. Prophylaxis is defined as antibiotics instituted by the participants at the first sign of a cold and were continued only for 5 days |
| Haidl 2013 | Comparison: inhaled tobramycin versus placebo Problem: antibiotic given via inhalation, not orally |
| Hallett 1959 | Comparison: erythromycin 250 mg 4 times a day versus placebo for 12 week duration Problem: not a randomised controlled trial; participants were matched in pairs (treatment and placebo groups) on the basis of similar clinical characteristics |
| Helm 1956 | Not a randomised controlled trial |
| Johnston 1961 | Comparison: Four treatment arms 1.Tetracycline 500 mg twice daily for 6 months treatment per year for 5 years 2. Placebo for 6 months treatment per year for 5 years 3. Tetracycline for the first 2 winters and placebo for the next three 4. Placebo for 2 winters and tetracycline for the next three Problem: Partial crossover due to re‐randomisation after two years Spirometric criteria were not used to diagnose COPD |
| Johnston 1961 | Comparison: phenethicillin versus placebo Problems: spirometric criteria were not used to diagnose COPD |
| Kilpatrick 1954 | Comparison: sulphadimidine 0.5 g three times daily versus placebo for 3 to 6 months Problem: spirometric criteria were not used when diagnosing COPD |
| Legler 1977 | Problem: not randomised Spirometric criteria were not used for diagnosing COPD |
| Liippo 1987 | Comparison: trimethoprim 300 mg per day versus placebo. Treatment for 6 months duration Problem: heterogeneous group of participants. Participants with bronchiectasis and asthma included. Spirometry criteria for COPD not used |
| Maraffi 2010 | Review article on 13 previous randomised controlled trials from 1957 to 2010 |
| Matthys 2015 | Wrong intervention: drug being trialled is not an antibiotic |
| May 1956 | Comparison: oxytetracycline or tetracycline versus 'controlled group' who were observed and antibiotic prophylaxis was not given Problem: not a true randomised controlled trial. The 'controlled group' consisted of 14 participants who were observed without any prophylactic therapy. They were not randomly selected |
| Miravitlles 2009 | Comparison: moxifloxacin 400 mg daily versus placebo Problem: short duration of study with only 5 days of treatment |
| Moyes 1959 | Comparison: four groups: 1) erythromycin 1g daily for 7 days ,then a course of 1 g daily for five days taken at the sign of first infection; 2) erythromycin 1 g daily for 7 days, then a regular course of 1 g daily for five days every 4 weeks; 3) tetracycline 1 g daily for 7 days , then a course of 1 g daily for five days taken at the sign of first infection 4) tetracycline 1 g daily for 7 days , then 750 mg/daily for 4 months Problems: no placebo group |
| Murdoch 1959 | Comparison: sigamycin (167 mg of tetracycline and 83 mg of oleandomycin) versus placebo for 3 months Problem: spirometric criteria not used in diagnosing COPD |
| Murray 1964 | Comparison: ampicillin 250 mg 4 times daily versus placebo over 17 months Problem: spirometric criteria were not used to diagnose COPD. Unclear whether randomisation took place |
| Nicholson 2016 | Problem: not a randomised controlled trial |
| Norman 1962 | Comparison: tetracycline 1 g daily or placebo for 3 months and then the groups were crossed over with continuation of treatment for further 3 months Problem: randomised cross‐over trial. Spirometry criteria not used when diagnosing COPD |
| Pines 1967 | Comparison: sulphormethoxine 2 g weekly for 10 weeks versus placebo Problems: spirometric criteria were not used in diagnosing COPD patients |
| Pridie 1960 | Comparison: penicillin‐sulphonamide, oxytetracycline versus placebo Problem: spirometric criteria were not taken into account when diagnosing COPD |
| Prins 2016 | Duration of intervention too short: 3 weeks of doxycycline |
| Ras 1984 | Comparison: 1) erythromycin 1500 mg/day for 2 weeks followed by 100 mg/day for 12 weeks; 2) amoxycillin 1500 mg/day for 2 weeks followed by 100 mg/day for 12 weeks; 3) placebo Problem: randomisation not well explained. Spirometric criteria not used when diagnosing COPD |
| Segal 2017 | Comparison: azithromycin versus placebo Problem: study of effect on microbiome; duration too short (8 weeks) |
| Siva 2014 | Duration of intervention too short: 7 days of levofloxacin |
| Stass 2013 | Problem: trial of one‐off dose of inhaled ciprofloxacin to assess lung deposition patterns |
| Vandenbergh 1970 | Comparison: sulfonamide 2 g once a week versus placebo for 6 months Problem: none of the primary outcomes were measured (frequency of exacerbations or quality of life). Spirometric criteria were not used in diagnosing COPD |
| Velzen 2016 | Comparison: long‐term effects of antibiotics given for acute exacerbations of COPD Problem: antibiotics given for acute COPD, not as prophylaxis |
| Vermeersch 2016 | Comparison: azithromycin versus placebo for acute exacerbations of COPD Problem: antibiotics given for acute COPD, not as prophylaxis |
| Watanabe 1991 | Comparison; 1) ofloxacin 200 mg daily for 6 months; 2) ofloxacin 200 mg three times daily for 2 weeks followed by 2 weeks without treatment for 6 months Problem: prophylaxis was given to participants with any chronic respiratory tract infection, including bronchiectasis and pulmonary tuberculosis. No placebo arm |
| Watanabe 1994 | Comparison: ciprofloxacin 200 mg/daily versus erythromycin 200 mg/daily versus combined ciprofloxacin 200 mg/d + erythromycin 200 mg/d Problem: no placebo. Participants with bronchiectasis included |
| Watanabe 1995 | Duplicate study of Watanabe 1991 with addition of 7 participants |
| Webster 1971 | Comparison: trimethoprim‐sulfamethoxazole versus sulfamethoxazole Problem: no placebo group. Treatment duration was only 10 days |
COPD: chronic obstructive pulmonary disease
Characteristics of studies awaiting assessment [ordered by study ID]
Milito 2017.
| Methods | Multicentre randomised placebo‐controlled double‐blind trial |
| Participants | 89 participants with primary antibody deficiency and chronic obstructive pulmonary disease with exacerbations |
| Interventions | Azithomycin 250 mg three times per week on consecutive days for 24 months versus placebo |
| Outcomes | Exacerbations, no use of additional antibiotics, an increase of respiratory volumes, an improvement of the Health‐Related Quality of Life measures |
| Notes | Conference abstract describing study protocol and participant dropouts only. Study was due to complete in December 2016, but we have been unable to identify a full‐text publication to confirm eligibility and extract outcomes. |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IOR‐16008820.
| Trial name or title | Effect of low‐dose erythromycin on the treatment of COPD |
| Methods | Randomised parallel group controlled trial. Planned recruitment 160 participants. |
| Participants | 1. Participants meeting the GOLD 2015 diagnostic criteria for COPD (a ratio of post‐bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity of < 70%,and a post‐bronchodilator FEV1 of < 80% of the predicted value); 2. Participants aged 40 years and more; 3. Participants were past or present cigarette smokers with at least a 10 pack‐year smoking history; 4. Participants were studied when clinically stable for at least 4 weeks following an exacerbation; no change in any therapy; no received systemic glucocorticoids |
| Interventions | Intervention: erythromycin Control: placebo |
| Outcomes | Exacerbations of COPD, lung function |
| Starting date | July 2016 |
| Contact information | Zhong Xiaoning (xiaoningzhong@sina.com) Department of Respiratory Medicine, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China |
| Notes | Study due to complete July 2019 |
NCT02205242.
| Trial name or title | BACE trial ‐ Physical activity as a crucial patient‐reported outcome in COPD |
| Methods | Randomised parallel group controlled trial. Planned recruitment 500 participants (planned to measure physical activity outcomes for a subset of 60 participants) |
| Participants | 1. Established diagnosis of COPD by medical doctor (based on clinical history OR pulmonary function test) 2. Smoking history of at least 10 pack‐years (10 pack‐years were defined as 20 cigarettes a day for 10 years, or 10 cigarettes a day for 20 years, etc.) 3. Current hospitalisation for potential infectious AECOPD treated with standard therapy 4. History of at least one exacerbation during the last year (prior to the current hospital admission) for which systemic steroids and/or antibiotics were taken 5. ECG at admission |
| Interventions | Intervention: from day 1 up to and including day 3: 500 mg azithromycin PO once a day, from day 4 up to and including day 90: 250 mg azithromycin PO once every 2 days Control: from day 1 up to and including day 3: 500 mg placebo PO once a day, from day 4 up to and including day 90: 250 mg placebo PO once every 2 days |
| Outcomes | Objective physical activity levels measured by an activity monitor |
| Starting date | September 2014 |
| Contact information | Wim Janssens (wim.janssens@kuleuven.be) Katholieke Universiteit Leuven, O&N I Herestraat 49 ‐ box 706, 3000 Leuven, Belgium |
| Notes | Study due to complete April 2018 |
NCT02205255.
| Trial name or title | BACE trial ‐ the pharmaco‐economic impact of the azithromycin intervention |
| Methods | Randomised parallel group controlled trial. Planned recruitment 350 participants (a second subanalysis of the BACE trial including a detailed cost‐effectiveness study) |
| Participants | 1. Established diagnosis of COPD by medical doctor (based on clinical history OR pulmonary function test) 2. Smoking history of at least 10 pack‐years (10 pack‐years were defined as 20 cigarettes a day for 10 years, or 10 cigarettes a day for 20 years, etc.) 3. Current hospitalisation for potential infectious AECOPD treated with standard therapy 4. History of at least one exacerbation during the last year (prior to the current hospital admission) for which systemic steroids and/or antibiotics were taken 5. ECG at admission |
| Interventions | Intervention: from day 1 up to and including day 3: 500 mg azithromycin PO once a day, from day 4 up to and including day 90: 250 mg azithromycin PO once every 2 days Control: from day 1 up to and including day 3: 500 mg placebo PO once a day, from day 4 up to and including day 90: 250 mg placebo PO once every 2 days |
| Outcomes | Total costs, direct costs, indirect costs |
| Starting date | August 2014 |
| Contact information | Wim Janssens (wim.janssens@kuleuven.be) Katholieke Universiteit Leuven, O&N I Herestraat 49 ‐ box 706, 3000 Leuven, Belgium |
| Notes | April 2018 |
NCT02305940.
| Trial name or title | A phase III double‐blind, randomised, placebo controlled trial of long term therapy on exacerbation rate in patients with stable COPD using doxycycline |
| Methods | Randomised parallel group controlled trial |
| Participants | Aged 45 years and over Confirmed COPD diagnosis Severity of disease: participants with a measured FEV1 < 80% of predicted normal values At least one treated exacerbation (participant recalled an episode of symptomatic worsening which was treated and was consistent with a COPD exacerbation) in the previous year |
| Interventions | Intervention: doxycycline 100 mg once daily, for a total duration of 52 weeks Control: placebo |
| Outcomes | Primary: rate of exacerbations (per person/year) Secondary: lung function (FEV1, FVC, FEV1/FVC ratio, FEV1 as % predicted), SGRQ (total and individual components) respiratory health status (using diary cards), airway bacteria numbers from sputum samples, changes in C‐reactive protein (CRP) levels from baseline, hospital admissions, time to 1st exacerbation, rate of exacerbations treated with steroids and antibiotics, adherence, antibiotic resistance |
| Starting date | July 2014 |
| Contact information | Wisia Wedzicha (j.wedzicha@imperial.ac.uk) Emmanuel Kaye Building, Royal Brompton Campus, Imperial College, London, UK |
| Notes | July 2017 |
AECOPD: acute exacerbation of chronic obstructive pulmonary disease; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; ECG: electrocardiogram; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; PO: per os (orally)
Differences between protocol and review
The original protocol stated that studies using antibiotics at least three times a week for a minimum period of three months would be included. We have amended this to include a regular schedule of pulsed antibiotics for a period of at least three months in order to include more studies using pulsed antibiotics. Furthermore, in the 2018 update, we chose to group analyses into continuous antibiotic regimens (at least daily), intermittent (i.e. two to three times per week) and pulsed (e.g. five days of antibiotics every eight weeks).
In 2018, we also extracted and included data on functional capacity (i.e. six‐minute walk test). Before seeing the results, it was agreed amongst the author team that this was a patient‐important outcome and if data were available, they should be extracted and presented.
For the 2018 update we used a random‐effects rather than fixed‐effect model in the meta‐analyses because we judged a random‐effects model to more accurately reflect the underlying clinical heterogeneity of the included studies. Furthermore, we added cut‐offs for identifying statistical heterogeneity according to Higgins 2011.
Some additional text has been added to the background and discussion for the 2018 update.
In a post‐hoc decision, we excluded data from Suzuki 2001 from the primary analysis as the study was not blinded.
Contributions of authors
For the 2013 version of the review, equal contributions were made from both authors (SH and PP) to the protocol, data extraction, analysis, write‐up, and response to reviewers comments.
For the 2018 update, SH and RN screened included studies, with input from PP. SH, SM, and RN performed data extraction and entry and carried out the analysis, with advice and input from PP. SM updated the background text, methods, and results. All four authors contributed to interpreting the analyses, writing up results, and discussion and all approved the final version of the review.
Sources of support
Internal sources
-
Rebecca Normansell, UK.
St George's, University of London
External sources
-
National Institute for Health Research, UK.
Cochrane Programme Grant 16/114/21: NHS priorities in the management of chronic respiratory disease.
Declarations of interest
RN is joint Coordinating Editor of Cochrane Airways and supported by an National Institute of Health Research grant.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Albert 2011 {published data only}
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. New England Journal of Medicine 2011;365:689‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1503‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FJ, Connett J, Voelker H, Criner GJ, Han MK, Make BJ, et al. Chronic azithromycin therapy decreases the risk of re‐hospitalization in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A4383. [Google Scholar]
- O'Reilly PJ, Jackson PL, Wells JM, Dransfield MT, Scanlon PD, Blalock, JE. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open 2013;3(12):e004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PG, Chatila W, Connett JE, Criner GJ, Curtis JL, Dransfield MT, et al. Tumour necrosis factor receptor‐75 and risk of COPD exacerbation in the azithromycin trial. European Respiratory Journal 2014;43:295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2005 {published data only}
- Banerjee D, Honeybourne D, Khair OA. The effect of oral Clarithromycin on bronchial airway inflammation in moderate‐to‐severe stable COPD: a randomised controlled trial. Treatments in Respiratory Medicine 2004;3:59‐65. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Khair O, Honeybourne D. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respiratory Medicine 2005;99:208‐15. [DOI] [PubMed] [Google Scholar]
Berkhof 2013 {published data only}
- Berkhof FF, Doornewaard‐ten Hertog NE, Uil SM, Kerstjens HAM, Berg JWK. Azithromycin and cough‐specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respiratory Research 2013;14(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhof FF, Hertog NE, Uil SM, Kerstjens HAM, Berg JK, CACTUS study group. Randomized controlled trial of prophylactic azithromycin on cough‐specific health status in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187:A2449. [Google Scholar]
Brill 2015 {published data only}
- Brill S, James P, Cuthbertson L, Cookson W, Moffatt M, Wedzicha J. Haemophilus dominance of the stable COPD microbiome is associated with greater bacterial load and inflammation and is modulated by prophylactic antibiotic therapy. European Respiratory Journal 2015;46:OA4746. [Google Scholar]
- Brill S, Law M, Allinson J, El‐Emir E, McHugh T, Donaldson G, et al. Bacterial resistance induction with prophylactic antibiotics in COPD. European Respiratory Journal 2014;44:P4731. [Google Scholar]
- Brill SE, Law M, El‐Emir E, Allinson JP, James P, Maddox V, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax 2015;70(10):930‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill SE, Law M, El‐Emir E, Allinson P, Nazareth I, Donaldson GC, et al. Effect of antibiotics on airway bacteria in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;198:A2874. [Google Scholar]
He 2010 {published data only}
- He ZY, Ou LM, Zhang JQ, Bai J, Liu GN, Li MH, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration 2010;80:445‐52. [DOI] [PubMed] [Google Scholar]
Mygind 2010 {published data only}
- Mygind LH, Pedersen C, Vestbo J, Christensen JJ, Frimodt‐Moller N, Kristiansen IS, et al. A randomised, placebo‐controlled 3 years study of prophylactic azithromycin in 575 patients with chronic obstructive pulmonary disease. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010.
NCT00524095 {published data only}
- NCT00524095. Bronchiectasis in chronic obstructive pulmonary disease (COPD) patients: role of prophylaxis. clinicaltrials.gov/ct2/show/NCT00524095 (first received 3 September 2007).
NCT02628769 {published data only}
- Batista CM. A bench to bedside investigation into defective innate immunity in chronic obstructive pulmonary disease. National Heart & Lung Institute, Imperial College London, UK; 2018.
- NCT02628769. A study to evaluate the anti‐inflammatory effects of solithromycin in chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02628769 (first received 11 December 2015).
Seemungal 2008 {published data only}
- Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long‐term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2008;178:1139‐47. [DOI] [PubMed] [Google Scholar]
Sethi 2010 {published data only}
- Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized control trial. Respiratory Research 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shafuddin 2015 {published data only}
- Shafuddin E, Mills GD, Holmes MD, Poole PJ, Mullins PR, Black PN. A double‐blind, randomised, placebo‐controlled study of roxithromycin and doxycycline combination, roxithromycin alone, or matching placebo for 12 weeks in adults with frequent exacerbations of chronic obstructive pulmonary disease. Journal of Negative Results in Biomedicine 2015;14:15. [DOI: 10.1186/s12952-015-0034-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2014 {published data only}
- Simpson JL, Powell H, Baines KJ, Milne D, Coxson HO, Hansbro PM, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLOS One 2014;9(8):e105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2001 {published data only}
- Suzuki T, Yani M, Yamaya M, Satoh Nakagawa T, Sekizawa K, Ishida S, et al. Erythromycin and common cold in COPD. Chest 2001;120:730‐3. [DOI] [PubMed] [Google Scholar]
Tan 2016 {published data only}
- Tan C, Huang H, Zhang J, He Z, Zhong X, Bai J. Effects of low‐dose and long‐term treatment with erythromycin on IL‐17 and IL‐23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Chest 2016;149:387A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Huang H, Zhang J, He Z, Zhong X, Bai J. Effects of low‐dose and long‐term treatment with erythromycin on interleukin‐17 and interleukin‐23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Mediators of Inflammation 2016:4173962. [DOI: 10.1155/2016/4173962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uzun 2014 {published data only}
- Djamin RS, Uzun S, Ermens AAM, Kerstens R, Hoogsteden HC, Aerts JGJV, et al. Which predictors in COPD patients with the frequent exacerbator phenotype predict the treatment response to maintenance therapy with azithromycin?. European Respiratory Journal 2016;48:PA3713. [Google Scholar]
- Uzun S, Djamin RS, Aerts JGJV, Eerden MM. Patients with COPD Gold C & D: the effect of long‐term treatment with azithromycin on exacerbation risk assessed by the Gold Framework. American Journal of Respiratory and Critical Care Medicine 2014;189:A5967. [Google Scholar]
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, Van't Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double‐blind,placebo‐controlled trial. Lancet Respiratory Medicine 2014;2(5):361‐8. [DOI] [PubMed] [Google Scholar]
- Uzun S, Djamin RS, Mulder PGH, Kluytmans JAJW, Pelle AJ, Van't Veer NE, et al. Effect of azithromycin maintenance treatment in patients with frequent exacerbations of COPD (columbus): a randomized, double‐blind, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A2884. [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang P, Yang J, Yang Y, Ding Z. Effect of azithromycin in combination with simvastatin in the treatment of chronic obstructive pulmonary disease complicated by pulmonary arterial hypertension. Pakistan Journal of Medicine Science 2017;33(2):260‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Beeh 2016 {published data only}
- Beeh K‐M, Beier J, Candler H, Wittig T. Effect of ELOM‐080 on exacerbations and symptoms in COPD patients with a chronic bronchitis phenotype ‐ a post‐hoc analysis of a randomized, double‐blind, placebo‐controlled clinical trial. International Journal of COPD 2016;11(1):2877‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bier 1971 {published data only}
- Beier VA. Trial of preventive treatment of patients with chronic bronchitis [Versuch einer prophylaktischen Behandlung chronischer Bronchitiker]. Wiener Medizinische Wochenschrift 1971;37:642‐3. [PubMed] [Google Scholar]
Blasi 2010 {published data only}
- Blasi F, Bonardi D, Aliberti S. Long term azithromycin use in patients with chronic obstructive pulmonary disease and tracheostomy. Pulmonary Pharmacology and Theraputics 2010;23:200‐7. [DOI] [PubMed] [Google Scholar]
Bruninx 1973 {published data only}
- Bruninx M, Koster J, Golard P, Libert P, Minette A, Mottard L, et al. Prophylactic administration of Bactrim in chronic bronchitis. Acta Tuberculosea et Pneumologica Belgica 1973;5‐6:483‐502. [PubMed] [Google Scholar]
Buchanan 1958 {published data only}
- Buchanan J, Buchanan W, Melrose A, McGuinness J, Price A. Long term prophylactic administration of tetracycline to chronic bronchitis. Lancet 1958;2(7049):719‐22. [DOI] [PubMed] [Google Scholar]
Bussi 1980 {published data only}
- Bussi S, Murciano D, Botto MJ, Pariente R. Assessment of chemoprophylaxis with intermittent tetracycline in chronic‐bronchitis ‐ a functional follow‐up for 3 years. Revue Francaise des Maladies Respiratoires 1980;8(5):351‐6. [PubMed] [Google Scholar]
Davies 1961 {published data only}
- Davies A, Grobow E, Tompsett R, McClement J. Bacterial Infection and some effects of chemoprophylaxis in chronic pulmonary emphysema. American Journal of Medicine 1961;31:365‐81. [DOI] [PubMed] [Google Scholar]
Douglas 1957 {published data only}
- Douglas A, Somner A, Marks B, Grant I. Effect of antibiotics on purulent sputum. Lancet 1957;273(6988):214‐8. [DOI] [PubMed] [Google Scholar]
Edwards 1958 {published data only}
- Edwards G, Fear E. Adult chronic bronchitis ‐ continuous antibiotic therapy. British Medical Journal 1958;2(5103):1010‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmes 1957 {published data only}
- Elmes P, Fletcher C, Dutton A. Prophylactic use of oxytetracycline for exacerbations of chronic bronchitis. British Medical Journal 1957;2(5056):1272‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 1966 {published data only}
- Calder M, Lutz W, Schonell ME. A five year study of bacteriology and prophylactic chemotherapy in patients with chronic bronchitis. British Journal of Diseases of the Chest 1968;62:93‐9. [DOI] [PubMed] [Google Scholar]
- Fletcher DM, Ball JD, Carstairs LW, Cooch AHC, Crofton JM, Edge JR, et al. Value of chemoprophylaxis and chemotherapy in early chronic bronchitis. A report to the Medical Research Council by their working party on trials of chemotherapy in early chronic bronchitis. British Medical Journal 1966;1:1317‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frances 1964 {published data only}
- Frances R, May J, Spicer C. Influence of daily penicillin, tetracycline, erythromycin and sulphamethoxypyridazine on exacerbation of bronchitis. British Medical Journal 1964;1:728‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 1960 {published data only}
- Francis R, Spicer C. Chemotherapy in chronic bronchitis. Influence of daily penicillin and tetracycline on exacerbations and their cost. British Medical Journal 1960;30(1):297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goslings 1967 {published data only}
- Goslings W, Djajadiningrat R, Bergstein P, Holle P. Continous suppressive antimicrobial treatment in chronic infected bronchitis during winter months. Disease of the Chest 1967;52(3):376‐80. [DOI] [PubMed] [Google Scholar]
Grossman 1998 {published data only}
- Grossman R, Mukherjee J, Vaughan D, Cook R, LaForge J, Lampron N, et al. A 1‐year community based health economic study of ciprofloxacin treatment in acute exacerbations of chronic bronchitis: the Canadian Ciprofloxacin Health Economics Study Group. Chest 1998;113:131‐41. [DOI] [PubMed] [Google Scholar]
- Torrance G, Walker V, Grossman R, Mukherjee J, Vaughan D, Forge J, et al. Economic evaluation of ciprofloxacin compared with usual anti‐bacterial care for the treatment of acute exacerbations of chronic bronchitis in patients followed for 1 year. Pharmacoeconomics 1999;16:499‐520. [DOI] [PubMed] [Google Scholar]
Hahn 1972 {published data only}
- Hahn HH, MacGregor RR, Counts CK, Smith HE, Beaty HN. Ampicillin and tetracyclin in the treatment and prophylaxis of chronic bronchitis. Antimicrobial Agents and Chemotherapy 1972;2(1):45‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haidl 2013 {published data only}
- Haidl P, Bargon J, Gessler T, Pfeifer M, Randerath W, Voshaar T, et al. Effect of inhalation of tobramycin for 12 months on reduction of hospitalisation rate in severe COPD. Pneumologie 2013;67(9):514‐9. [DOI] [PubMed] [Google Scholar]
Hallett 1959 {published data only}
- Hallet WY, Beali GN, Kirby WMM. Chemoprophylaxis in chronic obstructive pulmonary emphysema. Chemoprophylaxis in Emphysema 1959;80:716‐23. [DOI] [PubMed] [Google Scholar]
Helm 1956 {published data only}
- Helm W, May JR, Livingstone JL. Long‐term oxytetracycline (Terramycin) therapy in advanced chronic respiratory infections. Lancet 1956;267(6839):775‐7. [DOI] [PubMed] [Google Scholar]
Johnston 1961 {published data only}
- Johnston R, Lockhart W, Smith D, Cadman D. A trial of phenethicillin in chronic bronchitis. British Medical Journal 1961;2(5258):985‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 1961 {published data only}
- Johnston R, McNeil R, Smith D, Dempster M, Nairn J, Purvis M, et al. Five year winter prophylaxis for chronic bronchitis. British Medical Journal 1969;4:265‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilpatrick 1954 {published data only}
- Kilpatrick G, Oldham P. Sulphonmide prophylaxis in chronic bronchitis. British Medical Journal 1954;2(4884):385‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legler 1977 {published data only}
- Legler F, Jansen W. Double blind long term study on a combination of tetracycline, theophylline, doxylamine succinate , etafedrine, phenylephedrine and guaifenesine in chronic bronchitis. Arzneimittel‐Forschung 1977;27:883‐8. [PubMed] [Google Scholar]
Liippo 1987 {published data only}
- Liippo K, Pelliniemi T, Lehto H. Trimethoprim prophylaxis of acute exacerbations in chronic obstructive pulmonary disease. Acta Medica Scandinavica 1987;221:455‐9. [DOI] [PubMed] [Google Scholar]
Maraffi 2010 {published data only}
- Maraffi T, Piffer F, Cosentini R. Prophylactic antibiotic therapy in chronic obstructive pulmonary disease. Theraputic Advances in Respiratory Disease 2010;4:135‐7. [DOI] [PubMed] [Google Scholar]
Matthys 2015 {published data only}
- Matthys H, Malek FA. Antibiotic use in patients with COPD receiving EPs 7630 as an add‐on treatment. Atemwegs‐und Lungenkrankheiten 2016;41(1):27‐34. [Google Scholar]
May 1956 {published data only}
- May RJ. Long term chemotherapy in chronic bronchitis. Lancet 1956;271(6947):814‐9. [DOI] [PubMed] [Google Scholar]
Miravitlles 2009 {published data only}
- Miravitlles M, Marin A, Monso E, Vila S, Roza C, Hervas R, et al. Efficacy of moxifloxacin in the treatment of bronchial colonisation in COPD. European Respiratory Journal 2009;34:1066‐71. [DOI] [PubMed] [Google Scholar]
Moyes 1959 {published data only}
- Moyes EN, Kalinowski SZ. Prophylactic chemotherapy in chronic bronchitis. Tubercle 1959;40:112‐8. [DOI] [PubMed] [Google Scholar]
Murdoch 1959 {published data only}
- Murdoch J, Leckie W, Downie J, Swain R, Gould J. An evaluation of continuous antibiotic therapy in chronic bronchitis. British Medical Journal 1959;2(5162):1277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1964 {published data only}
- Murray EA. A trial of ampicillin in chronic bronchitis. Journal of the College of General Practitioners 1964;7:244‐52. [PMC free article] [PubMed] [Google Scholar]
Nicholson 2016 {published data only}
- Nicholson TT, Franciosi A, Landers S, Butler MW. Assessing potential risks of treatment with long‐term azithromycin in COPD patients: long‐term oxygen users beware?. Irish Journal of Medical Science 2016;185(4):993‐7. [DOI] [PubMed] [Google Scholar]
Norman 1962 {published data only}
- Norman PS, Hook EW, Petersdorf RG, Cluff LE, Godfrey MP, Levy AH. Long term tetracycline treatment of chronic bronchitis. JAMA 1962;179(11):833‐7. [DOI] [PubMed] [Google Scholar]
Pines 1967 {published data only}
- Pines A. Controlled trials of a sulphonamide given weekly to prevent exacerbations of chronic bronchitis. British Medical Journal 1967;3:202‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pridie 1960 {published data only}
- Pridie RB, Datta N, Massey DG, Poole GW, Schneeweiss J, Stradling P, et al. A trial of continuous winter chemotherapy in chronic bronchitis. Lancet 1960;2(7153):723‐7. [DOI] [PubMed] [Google Scholar]
Prins 2016 {published data only}
- Prins HJ, Daniels JM, Lindeman JH, Lutter R, Boersma WG. Effects of doxycycline on local and systemic inflammation in stable COPD patients, a randomized clinical trial. Respiratory Medicine 2016;110:46‐52. [DOI] [PubMed] [Google Scholar]
Ras 1984 {published data only}
- Ras J, Anderson R, Eftychis H, Koch U, Theron A, Vanwyk H, et al. Chemoprophylaxis with erythromycin stearate or amoxacillin in patients with chronic bronchitis ‐ effects on cellular and humoral immune functions. South African Medical Journal 1984;66:955‐8. [PubMed] [Google Scholar]
Segal 2017 {published data only}
- Segal LN, Clemente JC, Wu BG, Wikoff WR, Gao Z, Li Y, et al. Randomised, double‐blind, placebo‐controlled trial with azithromycin selects for anti‐inflammatory microbial metabolites in the emphysematous lung. Thorax 2017;72(1):13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal LN, Wu B, Clemente J, Wikof W, Alekseyenko A, Berger KI, et al. Effects of azithromycin on lung microbiome, metabolome and immune phenotype of early emphysema subjects: a randomized controlled pilot study (Abstract). American Journal of Respiratory and Critical Care Medicine 2017;189:A2475. [Google Scholar]
Siva 2014 {published data only}
- Siva R, Bafadhel M, Monteiro W, Brightling CE, Pavord ID. Effect of levofloxacin on neutrophilic airway inflammation in stable COPD: a randomized, double‐blind, placebo‐controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:179‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stass 2013 {published data only}
- Stass H, Nagelschmitz J, Kappeler D, Weimann B. Lung deposition of ciprofloxacin dry powder for inhalation in healthy subjects and patients suffering from chronic obstructive pulmonary disease or non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2013;187:A1507. [Google Scholar]
Vandenbergh 1970 {published data only}
- Vandenbergh E, Clement J, Woestijne K. Prevention of exacerbations of bronchitis: trial of a long acting sulphonamide. British Journal of Diseases of the Chest 1970;64:58‐62. [DOI] [PubMed] [Google Scholar]
Velzen 2016 {published data only}
- Velzen P, Ter Riet G, Bresser P, Berg BTJ, Berg WK, Daniels MA. Long‐term effects of antibiotics in COPD exacerbations: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2016;193:A1021. [Google Scholar]
Vermeersch 2016 {published data only}
- Vermeersch K, Everaerts S, Ninane V, Gabrovska M, Aumann J, Deslypere G, et al. Time‐to‐treatment failure in the Belgian randomized controlled trial with azithromycin for acute COPD exacerbations requiring hospitalization. European Respiratory Journal 2016;48:OA1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeersch K, Gabrovska M, Deslypere G, Demedts IK, Slabbynck H, Aumann J, et al. The Belgian trial with azithromycin for acute COPD exacerbations requiring hospitalization: an investigator‐initiated study protocol for a multicenter, randomized, double‐blind, placebo‐controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:687‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 1991 {published data only}
- Watanabe A. Once daily versus every two week multidose ofloxacin in patients with acute exacerbations of chronic respiratory disease. Infection 1991;19:S384‐7. [DOI] [PubMed] [Google Scholar]
Watanabe 1994 {published data only}
- Watanabe A, Motomiya M, Nukiwa T, Nakai Y, Honda Y, Konno K. A well‐controlled comparative clinical study of the combination regimen of ciprofloxacin plus erythromycin for the treatment of repeated acute exacerbations of chronic respiratory tract infections. Chemotherapy 1994;42:1194‐201. [Google Scholar]
Watanabe 1995 {published data only}
- Watanabe A, Oizumi K, Motomiya M, Nukiwa T. Daily single‐dose regimen and alternate two‐week triple dose/day regimen of oral ofloxacin for the prophylaxis and control of exacerbations of chronic respiratory tract infections. Tohoku Journal of Experimental Medicine 1995;176:25‐33. [DOI] [PubMed] [Google Scholar]
Webster 1971 {published data only}
- Webster I. A double blind cross‐over trail of trimethoprim and sulphamethoxazole in chronic bronchitis. Thorax 1971;26:319‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Milito 2017 {published data only}
- Milito C, Pulvirenti F, Tabolli S, Carello R. Antibiotic prophylaxis in primary antibody deficiency patients: study design PT. Journal of Clinical Immunology 2017;37:240‐1. [Google Scholar]
References to ongoing studies
ChiCTR‐IOR‐16008820 {published data only}
- ChiCTR‐IOR‐16008820. Effect of low‐dose erythromycin on the treatment of COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐IOR‐16008820 (first received 11 July 2016).
NCT02205242 {published data only}
- NCT02205242. BACE trial substudy 1 ‐ PROactive substudy (PROactive). clinicaltrials.gov/show/NCT02205242 (first received 31 July 2014).
NCT02205255 {published data only}
- NCT02205255. BACE trial substudy 2 ‐ FarmEc substudy (FarmEc). clinicaltrials.gov/ct2/show/NCT02205255 (first received 31 July 2014).
NCT02305940 {published data only}
- NCT02305940. Effects of long term antibiotic therapy on exacerbation rate in stable COPD patients. clinicaltrials.gov/show/NCT02305940 (first received 3 December 2014).
Additional references
Beasley 2012
- Beasley V, Joshi P, Singanayagam A, Molyneaux P, Johnston SL, Mallia P. Lung microbiology and exacerbations in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:555‐69. [DOI: 10.2147/COPD.S28286; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3437812/] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birring 2003
- Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58(4):339‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2018
- Joint Formulary Committee. British National Formulary. London: BMJ Group and Pharmaceutical Press, 2018. [http://www.medicinescomplete.com] [Google Scholar]
Chong 2017
- Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD002309.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2018
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2018 report). goldcopd.org/wp‐content/uploads/2017/11/GOLD‐2018‐v6.0‐FINAL‐revised‐20‐Nov_WMS.pdf (accessed prior to 20 July 2018).
Guyatt 1987
- Guyatt G, Berman L, Townsend M, Pugsley S, Chambers L. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42:773‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harries 2015
- Harries TH, Thornton HV, Crichton S, Schofield P, Gilkes A, White PT. Length of stay of COPD hospital admissions between 2006 and 2010: a retrospective longitudinal study. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:603‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hurst 2006
- Hurst JR, Perera WR, Wilkinson TMA, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2006;173:71‐8. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones P. St George's Respiratory Questionnaire Manual Version 2.3. www.healthstatus.sgul.ac.uk/SGRQ_download/SGRQ%20Manual%20June%202009.pdf (accessed prior to 20 July 2018).
Kew 2014
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010115.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2007
- Lee TA, Pharm D, Weaver FM. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. Journal of General Internal Medicine 2007;22(1):62‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2008
- Martinez FJ, Curtis JL, Albery R. Role of macrolide therapy in chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2008;3:331‐50. [PUBMED: 18990961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Garcia 2017
- Martinez‐Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity?. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:1401‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matkovic 2013
- Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype?. Respiratory Medicine 2013;107:10‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matzneller 2013
- Matzneller P, Krasniqi S, Kinzig M, Sörgel F, Hüttner S, Lackner E, et al. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrobial Agents and Chemotherapy 2013;57(4):1736‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI: 10.1186/s13643-016-0384-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papi 2006
- Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2006;3:3. [http://www.atsjournals.org/doi/full/10.1513/pats.200512‐125SF] [DOI] [PubMed] [Google Scholar]
Poole 2012
- Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001287.pub4] [DOI] [PubMed] [Google Scholar]
RevMan 5 2008 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3.5. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sapey 2006
- Sapey E, Stockley RA. COPD exacerbations 2: aetiology. Thorax 2006;61(3):250‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sethi 2008
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359:2355‐65. [DOI] [PubMed] [Google Scholar]
Staykova 2003
- Staykova T, Black PN, Chacko EE, Poole P. Prophylactic antibiotic therapy for chronic bronchitis. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004105] [DOI] [PubMed] [Google Scholar]
TORCH 2007
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2007;175(144):149. [DOI] [PubMed] [Google Scholar]
TSANZ 2004
- Thoracic Society of Australia and New Zealand (New Zealand Branch). Standards for adult respiratory and sleep services in New Zealand. www.health.govt.nz/system/.../standardsforadultrespiratoryandsleepservices.doc (accessed prior to 20 July 2018).
UPLIFT 2008
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. 4‐year trial of tiotropium in chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359:1543‐54. [DOI] [PubMed] [Google Scholar]
Visual Rx [Computer program]
- Visual Rx. www.nntonline.net/visualrx/ (accessed October 2013).
Wang 2012
- Wang J, Nie B, Xiong W, Xu Y. Effect of long‐acting beta‐agonists on the frequency of COPD exacerbations: a meta‐analysis. Journal of Clinical Pharmacy and Therapeutics 2012;37:204‐11. [DOI] [PubMed] [Google Scholar]
Wenzel 2012
- Wenzel RP, Fowler AA, Edmond MB. Antibiotic prevention of acute exacerbations of COPD. New England Journal of Medicine 2012;367(4):340‐7. [DOI] [PubMed] [Google Scholar]
WHO
- World Health Organization. Chronic obstructive pulmonary disease. www.who.int/respiratory/copd/en/ (accessed 18 November 2011).
Wilkinson 2006
- Wilkinson T, Hurst J, Perera W, Wilks M, Donaldson G, Wedzicha J. Effects of interactions between lower airway bacterial and rhinoviral infections in exacerbations of COPD. Chest 2006;129(2):317‐24. [http://www.sciencedirect.com/science/article/pii/S0012369215387523?via%3Dihub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodhead 2011
- Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Leven M, et al. Guidelines for the management of adult lower respiratory tract infections. Clinical Microbiology and Infection 2011;17(6):E1‐E59. [https://doi.org/10.1111/j.1469‐0691.2011.03672.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Herath 2013
- Herath S, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD009764.pub2] [DOI] [PubMed] [Google Scholar]


