Abstract
Background
Non‐selective beta‐blockers are recommended for the prevention of bleeding in people with cirrhosis, portal hypertension and gastroesophageal varices. Carvedilol is a non‐selective beta‐blocker with additional intrinsic alpha1‐blocking effects, which may be superior to traditional, non‐selective beta‐blockers in reducing portal pressure and, therefore, in reducing the risk of upper gastrointestinal bleeding.
Objectives
To assess the beneficial and harmful effects of carvedilol compared with traditional, non‐selective beta‐blockers for adults with cirrhosis and gastroesophageal varices.
Search methods
We combined searches in the Cochrane Hepato‐Biliary's Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, and Science Citation Index with manual searches. The last search update was 08 May 2018.
Selection criteria
We included randomised clinical trials comparing carvedilol versus traditional, non‐selective beta‐blockers, irrespective of publication status, blinding, or language. We included trials evaluating both primary and secondary prevention of upper gastrointestinal bleeding in adults with cirrhosis and verified gastroesophageal varices.
Data collection and analysis
Three review authors (AZ, RJ and LH), independently extracted data. The primary outcome measures were mortality, upper gastrointestinal bleeding and serious adverse events. We undertook meta‐analyses and presented results using risk ratios (RR) or mean differences (MD), both with 95% confidence intervals (CIs), and I2 values as a marker of heterogeneity. We assessed bias control using the Cochrane Hepato‐Biliary domains and the quality of the evidence with GRADE.
Main results
Eleven trials fulfilled our inclusion criteria. One trial did not report clinical outcomes. We included the remaining 10 randomised clinical trials, involving 810 participants with cirrhosis and oesophageal varices, in our analyses. The intervention comparisons were carvedilol versus propranolol (nine trials), or nadolol (one trial). Six trials were of short duration (mean 6 (range 1 to 12) weeks), while four were of longer duration (13.5 (6 to 30) months). Three trials evaluated primary prevention; three evaluated secondary prevention; while four evaluated both primary and secondary prevention. We classified all trials as at 'high risk of bias'. We gathered mortality data from seven trials involving 507 participants; no events occurred in four of these. Sixteen of 254 participants receiving carvedilol and 19 of 253 participants receiving propranolol or nadolol died (RR 0.86, 95% CI 0.48 to 1.53; I2 = 0%, low‐quality evidence). There appeared to be no differences between carvedilol versus traditional, non‐selective beta‐blockers and the risks of upper gastrointestinal bleeding (RR 0.77, 95% CI 0.43 to 1.37; 810 participants; 10 trials; I2 = 45%, very low‐quality evidence) and serious adverse events (RR 0.97, 95% CI 0.67 to 1.42; 810 participants; 10 trials; I2 = 14%, low‐quality evidence). Significantly more deaths, episodes of upper gastrointestinal bleeding and serious adverse events occurred in the long‐term trials but there was not enough information to determine whether there were differences between carvedilol and traditional, non‐selective beta‐blockers, by trial duration. There was also insufficient information to detect differences in the effects of these interventions in trials evaluating primary or secondary prevention. There appeared to be no differences in the risk of non‐serious adverse events between carvedilol versus its comparators (RR 0.55, 95% CI 0.23 to 1.29; 596 participants; 6 trials; I2 = 88%; very low‐quality evidence). Use of carvedilol was associated with a greater reduction in hepatic venous pressure gradient than traditional, non‐selective beta‐blockers both in absolute (MD ‐1.75 mmHg, 95% CI ‐2.60 to ‐0.89; 368 participants; 6 trials; I2 = 0%; low‐quality evidence) and percentage terms (MD ‐8.02%, 95% CI ‐11.49% to ‐4.55%; 368 participants; 6 trials; I2 = 0%; low‐quality evidence). However, we did not observe a concomitant reduction in the number of participants who failed to achieve a sufficient haemodynamic response (RR 0.76, 95% CI 0.57 to 1.02; 368 participants; 6 trials; I2 = 42%; very low‐quality evidence) or in clinical outcomes.
Authors' conclusions
We found no clear beneficial or harmful effects of carvedilol versus traditional, non‐selective beta‐blockers on mortality, upper gastrointestinal bleeding, serious or non‐serious adverse events despite the fact that carvedilol was more effective at reducing the hepatic venous pressure gradient. However, the evidence was of low or very low quality, and hence the findings are uncertain. Additional evidence is required from adequately powered, long‐term, double‐blind, randomised clinical trials, which evaluate both clinical and haemodynamic outcomes.
Plain language summary
Is carvedilol more effective or safer than traditional, non‐selective beta‐blockers for people with cirrhosis and gastroesophageal varices?
Background
Cirrhosis is a chronic disorder of the liver that results in an increase in its stiffness. As a result of the increased stiffness, the pressure in the blood vessels draining into the liver ‐ the portal system ‐ is increased. The increased portal blood pressure can result in the development of abnormally dilated blood vessels or varicose veins in the stomach and oesophagus (gastroesophageal varices). These varices can burst and the bleeding that follows can be life‐threatening. Drugs that reduce the portal blood pressure can help deflate the gastroesophageal varices and hence reduce the risk of bleeding. The drugs most commonly used are called non‐selective beta‐blockers. A newer drug, carvedilol, is also a beta blocker but has additional actions and may be more effective at reducing the portal pressure and hence the risk of variceal bleeding.
Review question
We investigated the effects and safety of carvedilol in people with cirrhosis and oesophageal varices by reviewing clinical trials in which people were randomly allocated to treatment with carvedilol or to a traditional beta‐blocker.
Search date
May 2018
Trial funding sources
Two of the 11 randomised clinical trials included in the review received no funding or other support from pharmaceutical companies. Two did receive financial support from pharmaceutical companies while a further three received free supplies of the trial drugs. Four trials did not provide funding information.
Trial characteristics
We included 11 randomised clinical trials, but were only able to gather information for our analyses from 10 trials involving 810 participants. The length of treatment ranged from one week to 30 months.
Key results
Our analyses found no differences in the effects of carvedilol on the rates of death, bleeding or serious and non‐serious complications compared with traditional, non‐selective beta‐blockers. Carvedilol lowered the portal pressure more effectively than the traditional, non‐selective beta‐blockers, but did not increase the number of participants in whom the pressure was reduced enough to reduce the risk of bleeding.
Quality of the evidence
We classified the evidence as of low or very low quality, so further trials are needed.
Summary of findings
Summary of findings for the main comparison. Carvedilol compared to traditional, non‐selective beta‐blockers for adults with cirrhosis, portal hypertension and gastroesophageal varices.
| Carvedilol compared to traditional, non‐selective beta‐blockers for adults with cirrhosis and gastroesophageal varices | ||||||
| Patient or population: adults with cirrhosis and gastroesophageal varices Setting: outpatient Intervention: carvedilol Comparison: traditional, non‐selective beta‐blockers: propranolol (9 trials); nadolol (1 trial) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐selective beta‐blockers | Risk with carvedilol | |||||
| Mortality | Trial population | RR 0.86 (0.48 to 1.53) | 507 (7 RCTs) | ⊕⊕⊝⊝ Low1,2 | Downgraded due to bias risk (one level) and imprecision (one level) | |
| 75 per 1000 | 65 per 1000 (36 to 115) | |||||
| Upper gastrointestinal bleeding | Trial population | RR 0.77 (0.43 to 1.37) | 810 (10 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | Downgraded due to bias risk (one level), inconsistency (one level), and imprecision (one level) | |
| 180 per 1000 | 139 per 1000 (77 to 247) | |||||
| Serious adverse events | Trial population | RR 0.97 (0.67 to 1.42) | 810 (10 RCTs) | ⊕⊕⊝⊝ Low1,2 | Downgraded due to bias risk (one level) and imprecision (one level) | |
| 198 per 1000 | 191 per 1000 (123 to 289) | |||||
| Non‐serious adverse events | Trial population | RR 0.55 (0.23 to 1.29) | 596 (6 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | Downgraded due to bias risk (one level), inconsistency (one level), and imprecision (one level) | |
| 298 per 1000 | 164 per 1000 (69 to 384) | |||||
| Hepatic venous pressure gradient at end of treatment (mmHg) | The mean hepatic venous pressure gradient at end of treatment (mmHg) ranged from 10.01 to 15.20 mmHg | MD 1.75 lower (2.6 lower to 0.89 lower) | ‐ | 368 (6 RCTs) | ⊕⊕⊝⊝ Low1,2,4 | Downgraded due to bias risk (one level) and imprecision (one level) |
| Reduction in hepatic venous pressure gradient (%) | The mean reduction in hepatic venous pressure gradient (%) ranged from 19.2 to 28.3 mmHg | MD 8.02 lower (11.49 lower to 4.55 lower) | ‐ | 368 (6 RCTs) | ⊕⊕⊝⊝ Low1,2,4 | Downgraded due to bias risk (one level) and imprecision (one level) |
| Haemodynamic treatment failure | Trial population | RR 0.76 (0.57 to 1.02) | 368 (6 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | Downgraded due to bias risk (one level), inconsistency (one level), and imprecision (one level) | |
| 591 per 1000 | 449 per 1000 (337 to 603) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1None of the included trials were at 'low risk of bias' in the overall assessment based on the Cochrane Hepato‐Biliary domains (hbg.cochrane.org/information‐authors). 2The number of events, participants and trials were small and the confidence intervals very wide. 3Heterogeneity between studies was significant. 4The hepatic venous pressure gradient is a validated surrogate outcome reflecting the risk of bleeding and mortality.
Background
Description of the condition
Portal hypertension is a very common and serious complication of cirrhosis. It develops as a result of increased vascular resistance to portal flow (D'Amico 1999). In people with cirrhosis this resistance develops as a result of an increase in liver stiffness secondary to the development of scar tissue and regenerating nodules within the hepatic parenchyma (Moreau 2006). In addition, changes occurring in the liver sinusoids also play a role; here activation of hepatic stellate cells results in the deposition of extracellular matrix proteins and collagen, while the development of microthrombi in the small hepatic arteries results in further parenchyma loss. These mechanical factors account for approximately 70% of the increase in hepatic resistance to portal blood flow. The remaining 30% is due to two factors: firstly, active contraction of sinusoidal stellate cells, myofibroblasts in the portal tract and vascular smooth muscle cells in the hepatic vasculature; and, secondly, sinusoidal endothelial cell dysfunction characterised by impaired release of vasodilatory agents, mainly nitric oxide, by the endothelial nitric oxide synthase (e‐NOS); the resulting lack of nitric oxide results in further increase in hepatic resistance and worsening portal hypertension as the general increase in vasoconstrictor drive, in this setting, is insufficiently opposed (Bosch 2015; Brunner 2017; Iwakiri 2014).
The increased pressure within the portal system causes blood to be redirected through vessels with less vascular resistance, in particular anastomoses or shunts between the portal and systemic vasculature. These 'portal‐systemic collaterals' can develop in several sites within the body; the most important being the lower end of the oesophagus and the upper part of the stomach where they appear, on endoscopy, as dilated tortuous submucosal veins or 'varices' protruding into the lumen. Other factors also promote the formation of collateral vessels including the process of active angiogenesis driven by vascular endothelial growth factor (Bosch 2015; Brunner 2017).
Portal hypertension develops as a result of an increase in the pressure gradient between the portal vein and the inferior vena cava. This can be measured indirectly via hepatic vein catheterisation, which involves inferring the pressure in the portal vein by measuring and calculating the difference between the wedge pressure in a hepatic vein and the free hepatic venous pressure. This, so called, 'hepatic venous pressure gradient' strongly correlates with the true pressure in the portal vein (Thalheimer 2005). Portal hypertension is defined by a hepatic venous pressure gradient of more than 5 mm Hg, but the risk of developing gastroesophageal varices does not increase until the pressure reaches 10 mm Hg (Ripoll 2007). Thus, a hepatic venous pressure gradient of 10 mm Hg or higher is termed 'clinically significant portal hypertension'.
The development of more advanced portal hypertension is accompanied by splanchnic vasodilation mediated by a variety of vasodilators including nitric oxide, carbon monoxide, endogenous cannabinoids and glucagon; this increases the blood flow in the collateral blood vessels further worsening the portal hypertension; this in turn accelerates the development of collateral vessels resulting in further splanchnic vasodilatation, hence creating a vicious cycle (Bosch 2015; Brunner 2017; Iwakiri 2014).
The development of gastroesophageal varices is one of the most significant consequences of portal hypertension, as these vessels are prone to rupture, resulting in catastrophic gastrointestinal bleeding with a high associated morbidity and mortality. Varices are more common in people with severe liver disease; thus, they are found in approximately one‐third of people with well‐compensated cirrhosis but in around 90% of people with severely decompensated disease (Kovalak 2007). The incidence of varices in people with compensated cirrhosis is around 7% (Groszmann 2005); they develop at a rate of 5% to 9% per year in people without varices at presentation (Groszmann 2005; Merli 2003); the rate of progression from small to large varices is about 10% per year (Merli 2003).
The incidence of variceal haemorrhage in people with gastroesophageal varices is approximately 10% to 15 % per year (Groszmann 2005; NIEC 1988). A number of risk factors for bleeding have been identified, including: (i) the severity of liver disease; (ii) the size of the varices and their endoscopic appearance; large and pellucid varices with red whale markings (areas of thinning of the variceal wall), are more likely to bleed than small varices (D'Amico 1999; NIEC 1988); and, (iii) the degree of portal hypertension ‐ bleeding is more likely to occur when the hepatic venous pressure gradient is more than 12 mmHg (Groszmann 1990). Without some form of intervention bleeding usually recurs within one to two years after an incident event (Bosch 2003).
The pressure gradient across the portal system is determined by the product of the blood flow in the portal vein and the vascular resistance opposing the flow. Thus, drugs that reduce the portal flow or the hepatic vascular resistance, or both, will reduce the portal pressure. Traditional, non‐selective beta‐blockers, such as propranolol and nadolol, block the beta1 adrenergic receptors in the heart and the beta2 adrenergic receptors in the periphery. Beta1 blockade of cardiac receptors reduces heart rate and cardiac output and subsequently decreases flow into the splanchnic circulation. Beta2 blockade leads to unopposed alpha1 adrenergic activity, which causes splanchnic vasoconstriction and a further reduction of portal inflow (Calés 1999; Groszmann 2005). Traditional, non‐selective beta‐blockers have been shown to effectively prevent variceal bleeding and to reduce bleeding‐associated mortality (Lebrec 1981; Poynard 1991). Bleeding is significantly less likely to occur if, as a result of treatment, the hepatic venous pressure gradient is reduced to 12 mm Hg or less (optimal response), or by at least 20% of its baseline value (good haemodynamic response) (Albillos 2007; D'Amico 2006; Turnes 2006). However, a large proportion of people who do not achieve this degree of pressure reduction, so called haemodynamic non‐responders, also seem to experience a protective effect from treatment. This is either because of a decrease of collateral and thus variceal blood flow, even without a marked decrease in the hepatic venous pressure gradient and/or because of a reduction of bacterial translocation and bacterial infections that may trigger bleeding per se (Thalheimer 2007).
Approximately 15% of people with cirrhosis may have absolute or relative contraindications to the use of traditional, non‐selective beta‐blockers, for example, peripheral vascular diseases, diabetes mellitus, chronic obstructive pulmonary disease and asthma. Adverse effects such as fatigue, weakness, and shortness of breath are common and may result in the need to reduce the dose or even to discontinuation the drug in a further 15% (Longacre 2008). In addition, a long‐term satisfactory haemodynamic response is only obtained in 33% to 50% of treated patients (Albillos 2007; Bosch 2003; García‐Pagán 1990; Reiberger 2013). Use of low doses of a vasodilator, such as isosorbide mononitrate, may result in an additional decrease in portal pressure in about a third of non‐responders. However, isosorbide mononitrate is ineffective when used alone for the prevention of upper gastrointestinal bleeding in this setting (García‐Pagán 2001), and there is little evidence that it confers additional haemodynamic benefit in people who are already responsive to a beta‐blocker. Use of a combination of isosorbide mononitrate and a beta‐blocker is associated with an increase in non‐serious adverse events compared to use of a beta‐blocker alone (García‐Pagán 2003).
Carvedilol is a non‐selective beta‐blocker with intrinsic anti‐alpha‐adrenergic activity and a mild vasodilating effect (Hemstreet 2004; Lo 2012; Tripathi 2002). It has been reported to more effectively lower portal pressure than propranolol or nadolol after both acute and chronic administration (Sinagra 2014). In addition, it has been reported that approximately 50% of people who do not achieve a good haemodynamic response with traditional, non‐selective beta‐blockers will do so with carvedilol (Reiberger 2013).
Description of the intervention
The traditional, non‐selective beta‐blockers, propranolol and nadolol, are used to treat angina, systemic hypertension, certain cardiac rhythm disorders, other heart or circulatory conditions, tremors, and migraine. Carvedilol is a non‐selective beta‐blocker but additionally displays intrinsic alpha1‐blocking effects and the potential to stimulate release of vasodilatory nitric oxide; it is used to treat systemic hypertension, chronic heart failure, and chronic stable angina. All three drugs are used to treat portal hypertension in patients with cirrhosis; all three are administered orally.
How the intervention might work
The presence of cirrhosis is complicated by the development of portal hypertension and gastroesophageal varices. The pressure in the portal system can be reduced by decreasing portal flow and/or hepatic vascular resistance. Traditional, non‐selective beta‐blockers, such as propranolol and nadolol, have an affinity for both beta1‐ and beta2‐adrenoceptors. Beta1 blockade of the cardiac receptors reduces heart rate and cardiac output and subsequently decreases blood flow into the splanchnic circulation. Beta2 blockade leads to unopposed alpha1 adrenergic activity and causes splanchnic vasoconstriction and a further reduction in portal inflow (Calés 1999; Groszmann 2005). The non‐selective beta‐blocker carvedilol has additional alpha1‐adrenoceptor blocking effects that may further reduce intrahepatic vascular resistance, augmenting the effect on the portal pressure (Brunner 2017).
Why it is important to do this review
The annual risk of people with cirrhosis developing varices, in European countries, is 7% to 8%, and the annual risk of bleeding from these varices is 5% to 15% (Asrani 2013). A number of pharmacological and endoscopic interventions have improved prognosis in patients with variceal haemorrhage but six‐week mortality rates remain high at 15% to 20% (Carbonell 2004; Chalasani 2003; D'Amico 2003; Hobolth 2010). The management of people with variceal bleeding is expensive, hence it is important to identify interventions that are both clinically and cost‐effective (Thabut 2007).
Five previous trials have found that carvedilol is more effective at reducing portal pressure than traditional, non‐selective beta blockers (Bañares 1999; Bañares 2002; De 2002; Hobolth 2012; Lin 2004). Previous meta‐analyses have combined trials investigating the immediate haemodynamic effects of these agents with those evaluating their clinical effects (Aguilar‐Olivos 2014; Chen 2015; Li 2016; Sinagra 2014). Thus, the comparative clinical benefits and harms of these agents remain unclear. We performed a systematic review with meta‐analyses of the beneficial and harmful effects of carvedilol versus traditional, non‐selective beta‐blockers, administered for at least one week, in people with cirrhosis and gastroesophageal varices.
Objectives
To assess the beneficial and harmful effects of carvedilol compared with traditional, non‐selective beta‐blockers for adults with cirrhosis and gastroesophageal varices.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials regardless of their publication status, language or blinding in our primary analyses. If, during the selection of trials, we identified observational studies (i.e. quasi‐randomised studies, cohort studies, or patient reports), which reported adverse events caused by, or associated with, the interventions under review, we included them for that purpose. We did not specifically search for observational studies for inclusion in this review, which is a known limitation.
Types of participants
The participants were adults (> 18 years) with cirrhosis and endoscopically/radiologically verified gastroesophageal varices.
Types of interventions
We compared carvedilol versus the traditional, non‐selective beta‐blockers propranolol or nadolol. We allowed effective co‐interventions if administered equally to the intervention and control groups and only included trials with a follow‐up period of at least one week.
Types of outcome measures
We assessed all outcomes at the maximum duration of follow‐up.
Primary outcomes
Mortality (all‐cause)
Upper gastrointestinal bleeding
Serious adverse events. We defined adverse events as any untoward medical occurrence (ICH GCP 1997), and considered adverse events as serious if they resulted in death, were life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability or incapacity. In this review serious adverse event included mortality and upper gastrointestinal bleeding and we analysed them as a composite outcome (hbg.cochrane.org/information‐authors)
Secondary outcomes
Non‐serious adverse events. All adverse events that did not fulfil the criteria for serious adverse events (as described above) (ICH GCP 1997).
Health‐related quality of life
Hepatic venous pressure gradient assessed as an absolute value at the end of the trial period or as a percentage change from baseline.
Treatment failure defined as failure to achieve a reduction in hepatic venous pressure gradient to less than 12 mmHg or by at least 20% from baseline.
Search methods for identification of studies
We combined electronic and manual searches.
Electronic searches
We searched:
Cochrane Hepato‐Biliary's Controlled Trials Register (hbg.cochrane.org/specialised‐register)
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4) in the Cochrane Library;
MEDLINE Ovid (1946 to May 2018);
Embase Ovid (1974 to May 2018);
LILACS (Bireme; 1982 to May 2018);
Science Citation Index Expanded (Web of Science; 1900 to May 2018); and
Conference Proceedings Citation Index – Science (Web of Science; 1990 to May 2018; Royle 2003), using the strategies described in Appendix 1.
We did not have access to Chinese, Russian, or Japanese databases. We plan to search these additional databases in future updates, should they become available via the Cochrane Hepato‐Biliary Group.
Searching other resources
We searched the reference lists of papers identified in the electronic searches and wrote to authors of the identified clinical trials and relevant pharmaceutical companies for additional data, if required. We searched the conference proceedings of the British Society of Gastroenterology (BSG), the European Association for the Study of the Liver (EASL), the United European Gastroenterology Week (UEGW), the American Gastroenterological Association (AGA), and the American Association for the Study of Liver Diseases (AASLD) (2000 to 2018). We also searched the online trial registries ClinicalTrial.gov (clinicaltrials.gov/); the European Medicines Agency (EMA) (www.ema.europa.eu/ema/); the WHO International Clinical Trial Registry Platform (www.who.int/ictrp); Google Scholar; the Food and Drug Administration (FDA) (www.fda.gov), and pharmaceutical company sources, for ongoing or unpublished trials.
Data collection and analysis
We performed the review following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the Cochrane Hepato‐Biliary Group (hbg.cochrane.org/), and the Methodological Expectations of Cochrane Intervention Reviews (MECIR) guidelines (MECIR 2014).
Selection of studies
All review authors participated in the literature searches, identified trials eligible for inclusion, and participated in the decisions regarding the eligibility of trials for consideration. We listed the excluded trials with the reason for their omission. If trial data were reported in more than one publication, we selected the report with the largest number of participants and the longest duration of follow‐up as our primary reference.
Data extraction and management
Four review authors (AZ, RJ, LH and LG) independently extracted data and evaluated bias. If data on patient trial characteristics, bias or outcomes were not described in the published reports, we wrote to the authors to obtain missing information.
Assessment of risk of bias in included studies
We followed Cochrane Hepato‐Biliary recommendations for assessing the risk of bias in the included trials, based on the definitions described below (hbg.cochrane.org/information‐authors). We assessed each domain separately as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and combined the domains into an overall score. We classified trials as low risk of bias only if none of the domains was designated as being at unclear or high risk of bias.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: blinding of participants and personnel performed adequately using a placebo. We defined lack of blinding as not likely to affect the evaluation of mortality (Savović 2012a; Savović 2012b).
Unclear risk of bias: insufficient information to assess blinding.
High risk of bias: no blinding or incomplete blinding.
Blinding of outcome assessors
Low risk of bias: blinding of outcome assessors performed adequately using a placebo. We defined lack of blinding as not likely to affect the evaluation of mortality (Savović 2012a; Savović 2012b).
Unclear risk of bias: there was insufficient information to blinding.
High risk of bias: no blinding or incomplete blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk: the trial reported the following pre‐defined outcomes: mortality, upper gastrointestinal bleeding, and adverse events. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought were those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk: not all pre‐defined were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk: one or more pre‐defined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support.
Uncertain risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support (Lundh 2018).
Other bias
Low risk of bias: the trial appeared to be free of other bias domains including: vested interests and medicinal dosing problems (as defined below) that could put it at risk of bias. We will also assess for‐profit bias using the definitions listed below.
Uncertain risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (funding from a for‐profit organisation or the administration of inappropriate treatment being given to the controls such as an inappropriate dose).
Overall bias assessment
Low risk of bias: all domains were low risk of bias using the definitions described above.
High risk of bias: one or more of the bias domains were of unclear or high risk of bias.
Measures of treatment effect
We analysed dichotomous data using risk ratios (RR) and continuous outcomes using mean differences (MD), both with 95% confidence intervals (CI).
Unit of analysis issues
We did not identify any cross‐over trials. However if such trials were to be identified in future updates we will only use data from the first treatment period.
Dealing with missing data
We planned to undertake analyses to evaluate the influence of missing data (Higgins 2008), including, worst‐case scenario analysis, and extreme worst‐case and best‐case scenario analyses (hbg.cochrane.org/information‐authors). However, we did not identify any randomised clinical trials with missing outcome data.
Assessment of heterogeneity
We expressed heterogeneity as I2 values using the following thresholds: 0% to 40% (unimportant), 40% to 60% (moderate), 60% to 80% (substantial), and more than 80% (considerable). We used this information in the interpretation and description of our analyses, and included the information in a 'Summary of findings' table.
Assessment of reporting biases
We planned to use visual inspection of funnel plots and regression analyses to evaluate reporting biases if our analysis included at least 10 trials with reported events (Egger 1997; Harbord 2006), However, our review did not reach this number threshold.
Data synthesis
Meta‐analysis
We performed our meta‐analyses and regression analyses using Review Manager 5 (Review Manager 2014) and STATA version 15 (STATA). We performed random‐effects and fixed‐effect meta‐analyses. The estimates of the random‐effects and fixed‐effect meta‐analyses were similar for all analyses. Thus, we assumed that any small‐trial effects had little influence on the intervention effect estimates. For random‐effects models, precision decreased with increasing heterogeneity and confidence intervals widened correspondingly. Accordingly, the random‐effects model provided the most conservative (and more correct) estimate of the intervention effect. Thus, we only report the results of the random‐effects meta‐analyses.
Trial Sequential Analysis
We planned to perform a Trial Sequential Analysis of our primary outcomes to evaluate the risk of random error associated with sparse data and cumulative testing, and to evaluate futility (Higgins 2008; Wetterslev 2008). We planned to undertake the analyses with alpha 3%, power 90% and the results of the random‐effects meta‐analyses (upper 95% CI), to determine the relative risk reduction and control group event. However, the number of events, participants, and trials were clearly insufficient and none of the primary outcomes showed beneficial or harmful effects of carvedilol or traditional, non‐selective beta‐blockers. Thus, we did not undertake any Trial Sequential Analyses.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to analyse the influence of:
risk of bias;
trial duration of three months or less compared to more than three months;
primary or secondary prevention of upper gastrointestinal bleeding;
proportion of people with ascites;
compliance with recommended standards for hepatic venous pressure gradient measurement (Suk 2014).
We were not able to undertake subgroup analyses for risk of bias as we classified all the included trials as being at high risk for all outcomes. We were able to undertake subgroup analyses of trial duration for the outcomes mortality, upper gastrointestinal bleeding, serious and non‐serious adverse events but not for haemodynamic outcomes, as none of the longer‐term trials undertook these measurements. We were able to undertake subgroup analyses for primary and secondary prevention for all outcomes but only in trials where all the included participants were treated for either primary or secondary prevention; none of the trials including mixed populations for primary and secondary prevention provided separate analyses for the two groups. We were unable to undertake subgroup analyses to assess the potential influence of including participants with ascites as the data available were insufficient. Finally, we were not able to undertake subgroup analyses for the measurement of the hepatic venous pressure gradient as insufficient details of compliance with recommended standards were provided in the published reports.
Sensitivity analysis
We planned to undertake worst‐case scenario analyses, as described in Dealing with missing data. However, outcome date sets were complete in the intervention or control groups in all of the included trials.
Quality of the evidence: GRADE
We used the GRADE system to assess the quality of the evidence, for outcomes reported in the review, considering the within‐trial risk of bias, directness of evidence, heterogeneity, precision of effect estimate, and risk of publication bias (Schünemann 2013).
'Summary of findings' table
We used GRADEproGDT 2015 to generate a 'Summary of findings' table with information about outcomes, risk of bias and the results of the meta‐analyses (Table 1).
Results
Description of studies
We included 11 randomised clinical trials (Characteristics of included studies) and excluded six randomised trials and observational studies (Characteristics of excluded studies).
Results of the search
We identified 553 records in the electronic searches and nine records in the manual searches. After excluding duplicates and records that were clearly irrelevant, we retrieved 18 articles for detailed assessment. We excluded two randomised clinical trials evaluating acute (less than one week) haemodynamic effects (Bañares 1999; Lin 2004); one randomised clinical trial comparing carvedilol versus a cardioselective beta‐blocker (Silkauskaite 2013); one randomised clinical trial evaluating the effects of beta‐blockers on the risk of decompensation in participants without oesophageal varices (NCT01059396); one quasi‐randomised trial (Bonaccorso 2017); and one observational study (Reiberger 2013). We also identified an ongoing trial, which may be eligible for inclusion in future updates of this review (NCT02385422). We displayed the results of the search in a flow diagram (Figure 1) as recommended (PRISMA 2009).
1.
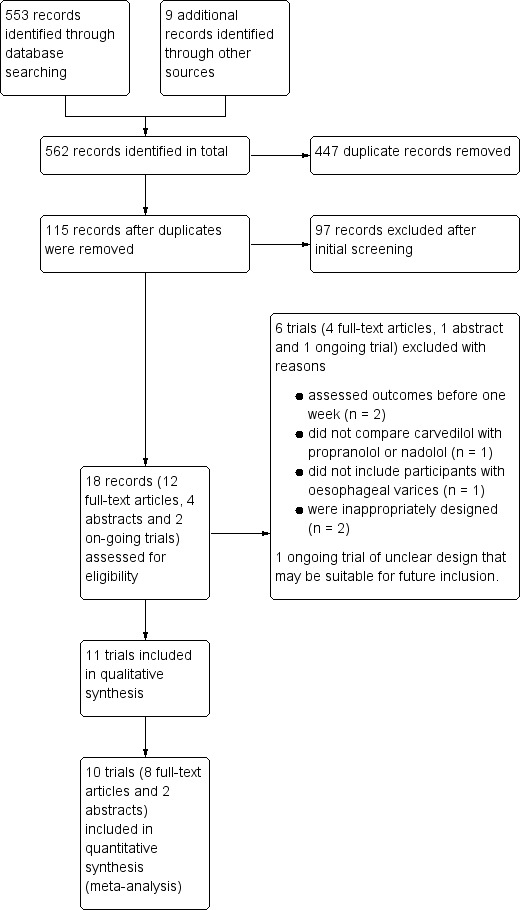
Study flow diagram for the identification and selection of randomised clinical trials
We included 11 randomised clinical trials, eight published as full papers (Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014) and three as abstracts (Agarwala 2011; Hanno 2016; Wei 2018). We were not able to gather appropriate outcome data from one trial published in abstract form (Hanno 2016); this trial compared carvedilol versus propranolol but also included two groups allocated to band ligation alone or band ligation and carvedilol; the primary outcome variable was the size of varices at endoscopy. Accordingly, we were only able to gather data outcome data from 10 trials (Agarwala 2011; Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014; Wei 2018).
Included studies
Nine randomised clinical trials were single‐centre (Agarwala 2011; Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hanno 2016; Lo 2012; Mo 2014; Wei 2018) while two were multi‐centre (Hobolth 2012; Kim 2016). The countries of origin were China (Mo 2014; Wei 2018), Denmark (Hobolth 2012), Egypt ( ElRahim 2018; Hanno 2016), India (Agarwala 2011; De 2002; Gupta 2016), Korea (Kim 2016), Spain (Bañares 2002), and Taiwan (Lo 2012).
Participants
The trials included 810 participants with cirrhosis and oesophageal varices. The majority of participants had cirrhosis secondary to alcohol misuse or chronic viral hepatitis. The diagnosis of portal hypertension was based on the presence of oesophageal varices in all 11 included trials together with an elevated hepatic venous pressure gradient in the seven trials in which it was measured (Bañares 2002; De 2002; Gupta 2016; Hanno 2016; Hobolth 2012; Kim 2016; Mo 2014) (Table 2.
1. Included participants, definition of portal hypertension, grading of varices and type of intervention.
| Trial | Definition of portal hypertension | Size of oesophageal varices | Primary prevention | Secondary prevention | Proportion of participants for primary/secondary prevention |
| Agarwala 2011 | Endoscopically verified oesophageal varices | Not defined | Yes | Yes | Unclear |
| Bañares 2002 | Endoscopically verified oesophageal varices and a basal hepatic venous pressure gradient > 12 mmHg | Small or large (classification not defined) | Yes | No | 100%/0% |
| De 2002 | Endoscopically verified oesophageal varices and a basal hepatic venous pressure gradient ≥ 12 mmHg | Varices < grade 2 according to Japanese Research Society for Portal Hypertension | Yes | Yes | 61%/39% |
| ElRahim 2018 | Endoscopically verified oesophageal varices | Medium or large (classification not defined) | Yes | No | 100%/0% |
| Gupta 2016 | Endoscopically verified oesophageal varices and a basal hepatic venous pressure gradient > 12 mmHg | Grade 2‐4 as described in Paquet 1982 | No | Yes | 0%/100% |
| Hanno 2016 | Endoscopically verified oesophageal varices | Grade 3 or 4 | Not described | Not described | Not described |
| Hobolth 2012 | Endoscopically verified oesophageal varicesa and a basal hepatic venous pressure gradient ≥ 12 mmHg | Grade 1 to 3 as described in Baveno III | Yes | Yes | 74%/26% |
| Kim 2016 | Endoscopically verified oesophageal varices and a basal hepatic venous pressure gradient > 12 mmHg | Grade 2 or 3 according to Beppu 1981 | Yes | No | 100%/0% |
| Lo 2012 | Endoscopically verified oesophageal varices | Grade 1‐3 according to Baveno III | No | Yes | 0%/100% |
| Mo 2014 | Endoscopically or CT scan‐verified oesophageal varices and a basal hepatic venous pressure gradient > 5 mmHg | Not defined (no endoscopic classification used) | Yes | Yes | 48%/52% |
| Wei 2018 | Endoscopically verified oesophageal varices | Not defined (classification not defined) | No | Yes | 0%/100% |
aA subgroup of 12 participants had a HVPG > 12 mmHg but did not have oesophageal varices; we excluded this subgroups from our analyses
Three trials evaluated primary prevention (Bañares 2002; ElRahim 2018; Kim 2016); three evaluated secondary prevention (Gupta 2016; Lo 2012; Wei 2018); four evaluated primary and secondary prevention (Agarwala 2011; De 2002; Hobolth 2012; Mo 2014); while the remaining trial did not specify the type of prevention investigated (Hanno 2016). One trial included some participants who did not have oesophageal varices (Hobolth 2012); we excluded this subset of participants from our analyses. Six trials were classified as short‐term (≤ 3 months' duration) (Bañares 2002; De 2002; Gupta 2016; Hobolth 2012; Kim 2016; Mo 2014) and five as long‐term (> 3 months' duration) (Agarwala 2011; ElRahim 2018; Hanno 2016; Lo 2012; Wei 2018).
Intervention
All trials evaluated carvedilol; the mean (range) dose, in the nine trials providing the information, was 13.4 ( 6.25 to 31.0) mg per day (Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hanno 2016; Hobolth 2012; Kim 2016; Lo 2012; Wei 2018). One trial did not provide information on the dose of drug used nor on how it was administered (Agarwala 2011). Three trials used a fixed dose of carvedilol (De 2002; Hanno 2016; Kim 2016), while in the remaining trials the dose was titrated to achieve a 25% reduction in heart rate or a reduction to 55 to 60 beats per minute (Bañares 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Lo 2012; Mo 2014; Wei 2018).
Comparators
Participants in the control groups received traditional, non‐selective beta‐blockers; either propranolol (Agarwala 2011; Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hanno 2016; Hobolth 2012; Mo 2014; Wei 2018), or nadolol (Lo 2012). The mean daily dose of propranolol, in the trials that provided the information, was 73.5 (17.7 to 152.6) mg per day (Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hanno 2016; Hobolth 2012; Mo 2014; Wei 2018); the mean daily dose of nadolol was 45 (20 to 80) mg per day, (Lo 2012). One trial did not provide information on the dose of drug used nor on how it was administered (Agarwala 2011). Two trials used a fixed dose of propranolol (De 2002; Hanno 2016), while in the remaining trials the dose was titrated to achieve a 25% reduction in heart rate or a reduction to 55 to 60 beats per minute (Bañares 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014; Wei 2018).
Cointerventions
In one of the included trials, participants in the control group received isosorbide mononitrate (Lo 2012); this medication has not been shown to significantly affect outcomes in people with cirrhosis and oesophageal varices receiving beta‐blockers for prevention of upper gastrointestinal bleeding (García‐Pagán 2001; García‐Pagán 2003), although use of the combination has been associated with an increase in non‐serious adverse events (García‐Pagán 2003). In a further trial participants in both the intervention and control groups underwent endoscopic band ligation (Gupta 2016).
Outcomes
We were able to gather clinical outcome data from 10 of the 11 trials (Agarwala 2011; Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014; Wei 2018). Two trials did not report mortality data (Agarwala 2011; Wei 2018), whilst a further trial reported mortality but not by allocation group (ElRahim 2018). All 10 trials provided data on upper gastrointestinal/variceal bleeding. Data on adverse events were available from 10 trials (Agarwala 2011; Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014; Wei 2018). Information on the hepatic venous pressure gradient and the proportion of participants who failed to achieved a satisfactory reduction was available from six trials (Bañares 2002; De 2002; Gupta 2016; Hobolth 2012; Kim 2016; Mo 2014).
Excluded studies
We excluded four randomised clinical trials, one quasi‐randomised trial and one observational study (Characteristics of excluded studies). One further trial, currently reported as ongoing, was excluded because of insufficient information but it might be eligible for inclusion in future updates of this review (NCT02385422)
Two randomised clinical trials evaluated the acute haemodynamic effects of carvedilol versus propranolol (Bañares 1999; Lin 2004). The trials followed participants for less than one week and were excluded on this basis. The first trial included 35 participants and measured acute haemodynamic changes over a maximum period of two hours (Bañares 1999); carvedilol 25 mg was found to be superior to propranolol in reducing the hepatic venous pressure gradient. The second trial compared the acute haemodynamic effects of carvedilol versus propranolol 40 mg plus isosorbide mononitrate 20 mg in 22 participants with cirrhosis (Lin 2004). There was no difference in the systemic haemodynamic response between groups at 90 minutes, but carvedilol produced a greater reduction in the hepatic venous pressure gradient.
One randomised clinical trial evaluated the haemodynamic responses to carvedilol versus nebivolol in 20 participants with cirrhosis and oesophageal varices with no history of variceal bleeding (Silkauskaite 2013). Nebivolol is not a traditional, non‐selective beta‐blocker but a beta1‐selective adrenergic receptor antagonist with nitric oxide‐mediating vasodilatory properties; the trial was excluded on this basis (Broeders 2000). Both drugs reduced the hepatic venous pressure gradient; the effect of carvedilol was more pronounced, especially after 14 days of treatment.
The final randomised clinical trial was excluded because its primary aim was to determine the effectiveness of beta‐blockers in preventing hepatic decompensation in participants with cirrhosis with no or minimal oesophageal varices (NCT01059396). Participants were randomised to propranolol (or carvedilol in non‐responders) or to placebo; thus, there was no direct comparison of the effects of carvedilol versus propranolol.
In the quasi‐randomised trial, investigators allocated treatment based on inclusion date (Bonaccorso 2017); participants enrolled in the first half of the recruiting period received propranolol while those enrolled in the second half received carvedilol. The trial included 75 participants and evaluated primary prevention. In total, 16.3% in the carvedilol group and 40.6% in the propranolol group had ascites, indicating that the allocation was probably skewed. Nineteen participants died (all liver ‐related); 11 of 43 participants in the carvedilol group and 8 of 42 participants in the propranolol group (RR 1.34; 95% CI 0.60 to 3.01). There were three 'occurrences' of variceal bleeding in each group. Fifteen participants (34.9%), in the carvedilol and 23 (71.9%), in the propranolol group had inadequate haemodynamic responses.
In the observational study (Reiberger 2013), 104 participants with cirrhosis and oesophageal varices with no history of variceal bleeding were given propranolol; those with an inadequate reduction in their hepatic venous pressure gradient were given carvedilol; participants who were unresponsive to both medications underwent endoscopic band ligation. Responders to carvedilol showed a significantly greater hepatic venous pressure gradient reduction than propranolol responders.
Risk of bias in included studies
We carried out the risk of bias assessment based on the information retrieved from the publications and from investigators . We identified potential bias in all of the included trials (Figure 2).
2.
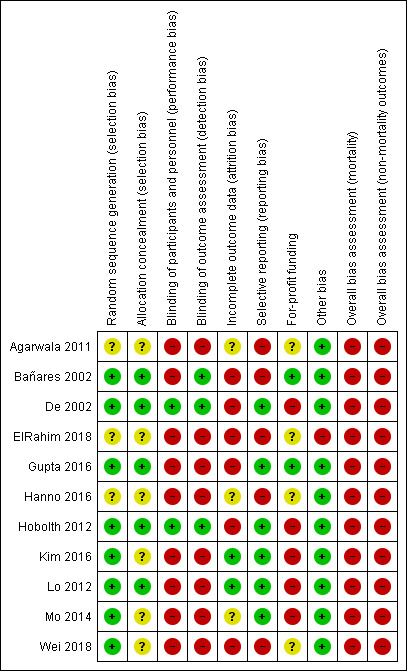
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
Allocation
In five trials the allocation sequence generation and allocation concealment were adequate, so we classified them at low risk for selection bias (Bañares 2002; De 2002; Gupta 2016; Hobolth 2012; Lo 2012); in the remaining six trials the risk of selection bias was unclear (Agarwala 2011; ElRahim 2018; Hanno 2016; Kim 2016; Mo 2014; Wei 2018).
Blinding
Two trials were conducted double‐blind (De 2002; Hobolth 2012), so we classified them at low risk of performance and detection bias; we classified one single‐blinded trial at high risk of performance bias but at low risk of detection bias (Bañares 2002). The remaining eight trials were open without blinding and hence were at high risk of bias for this domain (Agarwala 2011; ElRahim 2018; Gupta 2016; Hanno 2016; Kim 2016; Lo 2012; Mo 2014; Wei 2018).
Incomplete outcome data
Two trials provided full outcome data and included all participants in their analyses (Kim 2016; Lo 2012). We classified these trials at low risk of attrition bias. We classified three trials at unclear risk of attrition bias (Agarwala 2011; Hanno 2016; Mo 2014), and the remaining six trials at high risk of bias for this domain (Bañares 2002; De 2002; ElRahim 2018; Gupta 2016; Hobolth 2012; Wei 2018).
Selective reporting
We classified six trials at low risk of reporting bias (De 2002; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014). Five trials did not report fully on mortality or serious adverse events and so we classified them as being at high risk of bias for this domain (Agarwala 2011; Bañares 2002; ElRahim 2018; Hanno 2016; Wei 2018).
For‐profit funding
Two trials did not receive industry funding or other support and so we classified them as being at low risk of bias (Bañares 2002; Gupta 2016). Five trials received funding or other support from pharmaceutical companies (De 2002; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014), and were classified as at high risk of bias. The remaining four trials did not describe funding (Agarwala 2011; ElRahim 2018; Hanno 2016; Wei 2018), and so we classified them as at unclear risk of bias for this domain.
Other potential sources of bias
One trial reallocated participants initially randomised to carvedilol or propranolol to band ligation if they had contraindications to medical interventions (ElRahim 2018); we classified this trial as at high risk for other sources of bias. We did not identify any other potential sources of bias in the remaining included trials.
Overall risk of bias
We classified all trials at high risk of bias for all outcomes.
Effects of interventions
See: Table 1
Primary outcomes
Mortality
We were able to gather mortality data from seven trials involving 507 participants. No events occurred in four of these seven trials. Random‐effects meta‐analysis found no difference between carvedilol versus traditional, non‐selective beta‐blockers for risk of death (RR 0.86, 95% CI 0.48 to 1.53; I2 = 0%; Analysis 1.1). Significantly more deaths occurred In the single, long‐term trial that reported on this outcome than in the six short‐term trials that did so (32 (26.4%) compared to 3 (0.8%); P = 1.3 x 10‐18); subgroup analysis showed no difference in the effect of carvedilol on mortality, by trial duration (test for subgroup differences: Chi² = 0.01; P = 0.94; Analysis 1.2). We were only able to gather mortality data from two trials evaluating primary prevention and two evaluating secondary prevention. None of the participants in the primary prevention trials died, so we were unable to assess subgroup differences, by prevention type (Analysis 1.3).
1.1. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 1 Mortality (overall).
1.2. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 2 Mortality (duration).
1.3. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 3 Mortality (prevention type).
Upper gastrointestinal bleeding
We were able to gather data on upper gastrointestinal bleeding from 10 trials involving 810 participants. Random‐effects meta‐analysis found no difference between carvedilol versus traditional, non‐selective beta‐blockers on the risk of upper gastrointestinal bleeding (RR 0.77, 95% CI 0.43 to 1.37; 10 trials; I2 = 45%; Analysis 1.4). Significantly more upper gastrointestinal bleeding episodes were reported in the four long‐term trials than in the six short‐term trials (124 (29.2%), compared to 9 (2.3%); P = 2.6 x 10‐27); subgroup analysis showed no difference between carvedilol versus traditional, non‐selective beta‐blockers for this outcome, by trial duration (test for subgroup differences: Chi² = 0.10; P = 0.75; Analysis 1.5). Subgroup analyses showed no difference in the effect of carvedilol in trials evaluating primary or secondary prevention (test for subgroup differences: Chi² = 1.06; P = 0.30; Analysis 1.6).
1.4. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 4 Upper gastrointestinal bleeding (overall).
1.5. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 5 Upper gastrointestinal bleeding (duration).
1.6. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 6 Upper gastrointestinal bleeding (prevention type).
Serious adverse events
We were able to gather information on serious adverse events from 10 trials involving 810 participants. Random‐effect meta‐analysis showed no difference between carvedilol versus traditional, non‐selective beta‐blockers on the risk of serious adverse events (RR 0.97, 95% CI 0.67 to 1.42; I2 = 14%; Analysis 1.7) (Table 3). Significantly more serious adverse events were reported in the long‐term compared to the short‐term trials (138 (32.5%), compared to 17 (4.4%); P = 6.5 x 10‐26); subgroup analysis showed no difference between carvedilol and traditional, non‐selective beta‐blockers and the risk of serious adverse events, by trial duration (test for subgroup differences: Chi² = 1.08; P = 0.30; Analysis 1.8). Subgroup analyses showed no difference in the effect of carvedilol in trials evaluating primary or secondary prevention (test for subgroup differences: Chi² = 0.89; P = 0.35; Analysis 1.9).
1.7. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 7 Serious adverse events (overall).
2. Serious adverse events.
| Trial | Carvedilol | Control group | ||
| Number of participants | Serious adverse events | Number of participants | Serious adverse events | |
| Agarwala 2011 | 54 |
|
48 |
|
| Bañares 2002 | 26 |
|
25 |
|
| De 2002 | 18 |
|
18 |
|
| ElRahim 2018 | 84 |
|
92 |
|
| Gupta 2016 | 30 |
|
29 |
|
| Hobolth 2012 | 16 |
|
18 |
|
| Kim 2016 | 55 |
|
55 |
|
| Lo 2012 | 60 |
|
60 |
|
| Mo 2014 | 48 |
|
48 |
|
| Wei 2018 | 13 |
|
12 |
|
1.8. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 8 Serious adverse events (duration).
1.9. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 9 Serious adverse events (prevention type).
Secondary outcomes
Non‐serious adverse events
Seven trials reported non‐serious adverse events but only six, involving 596 participants, reported these data on a per‐participant basis. The remaining trial (Bañares 2002), reported on the number of participants experiencing individual non‐serious adverse events, so that its inclusion in the overall assessment would have risked errors from double counting. There was no clear difference in the overall occurrence of non‐serious adverse events between intervention groups (RR 0.55, 95% CI 0.23 to 1.29; I2 = 88%; Analysis 1.10). There was no significant difference in the overall incidence of these events in the short‐ and long‐term trials (64 (21.4%), compared to 72 (24.2%); P = 0.44; test for subgroup differences: Chi² = 2.90; P = 0.09; Analysis 1.11). The non‐serious adverse events recorded included: hypotension, minor worsening of ascites and hepatic encephalopathy, shortness of breath, impotence, insomnia, fatigue, vertigo, bradycardia, and gastrointestinal discomfort; there were no significant differences in the incidences of the individual events between intervention groups (test for subgroup differences: Chi² = 4.39; P = 0.88; Analysis 1.12). Likewise there was no significant difference in the incidence or types of events in subgroups stratified by primary or secondary prevention (test for subgroup differences: Chi² = 0.00; P = 0.98; Analysis 1.13).
1.10. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 10 Non‐serious adverse events (overall).
1.11. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 11 Non‐serious adverse events (duration).
1.12. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 12 Non‐serious adverse events (event type).
1.13. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 13 Non‐serious adverse events (prevention type).
Health‐related quality of life
None of the trials evaluated health‐related quality of life.
Haemodynamic responses
Haemodynamic responses were reported in six trials involving 368 participants before and after a mean (range), of 5.8 (1 to 12) weeks of treatment. In comparison with traditional, non‐selective beta‐blockers, use of carvedilol was associated with a significantly greater reduction in the absolute hepatic venous pressure gradient at the end of treatment (MD ‐1.75 mmHg, 95% CI ‐2.60 to ‐0.89; I2 = 0%; P < 0.001; Analysis 1.14) and in the end percentage change in hepatic venous pressure gradient over baseline (MD ‐8.02%, 95% CI ‐11.49 to ‐4.55; I2 = 0%; P < 0.0001; Analysis 1.15). However, use of carvedilol was not associated with a reduction in the number of participants who failed to achieve a satisfactory haemodynamic response (RR 0.76, 95% CI 0.57 to 1.02; I2 = 42%; P = 0.07; Analysis 1.16). None of the long‐term trials measured haemodynamic responses, so we were not able to assess subgroup differences, by trial duration. There were no significant differences in haemodynamic responses in subgroups stratified by type of prevention (Analysis 1.17; Analysis 1.18; Analysis 1.19).
1.14. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 14 Hepatic venous pressure gradient, end of treatment (mmHg) (overall).
1.15. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 15 Reduction in hepatic venous pressure gradient (%) (overall).
1.16. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 16 Haemodynamic treatment failure (overall).
1.17. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 17 Hepatic venous pressure gradient, end of treatment (mmHg) (prevention type).
1.18. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 18 Reduction in hepatic venous pressure gradient (%) (prevention type).
1.19. Analysis.
Comparison 1 Carvedilol versus non‐selective beta‐blockers, Outcome 19 Haemodynamic treatment failure (prevention type).
'Summary of findings' table
We downgraded the quality of the evidence to low for four outcomes (mortality; serious adverse events; end of treatment, and percentage reduction in hepatic venous pressure gradient), based on the within‐trial risk of bias (one level), and imprecision (one level). We downgraded three further outcomes by an additional level to very low quality (upper gastrointestinal bleeding; non‐serious adverse events; haemodynamic treatment failure), based on inconsistency between trials within the analyses (Table 1).
Discussion
Summary of main results
This review found no differences in the clinical effects of carvedilol compared with the traditional, non‐selective beta‐blockers, propranolol or nadolol, in people with cirrhosis. Rates of mortality, upper gastrointestinal bleeding, and adverse events were comparable between intervention groups. The quality of the evidence was low, mainly due to the small numbers of both events and participants in the included trials. Thus, we cannot make any definite conclusions about clinical efficacy. Carvedilol was associated with an 8% greater decrease in the hepatic venous pressure gradient during the treatment period, although there was no clear difference between carvedilol versus traditional, non‐selective beta‐blockers in the number of participants who did not achieve the target reduction in hepatic venous pressure gradient. Thus, the findings in relation to the haemodynamic responses, and whether or not they are clinically meaningful, is also inconclusive.
Overall completeness and applicability of evidence
This review included 11 randomised clinical trials. We were able to extract outcome data from 10 trials involving 810 adult participants with cirrhosis and oesophageal varices. The trials were generally small; the mean (range), number of participants was 81 (25‐176). Only four of the included trials conducted a sample size calculation for assessment of statistical power (Bañares 2002; Hobolth 2012; Kim 2016; Lo 2012); of these, only two (Hobolth 2012; Lo 2012), met their target sample size after withdrawal or loss of participants. Thus, several of the trials were likely to be underpowered to detect a difference in the effectiveness and safety of the interventions. In addition, all 11 trials were classified as at high risk of bias for all outcomes.
Although all the included trials compared carvedilol with a traditional non‐selective beta blocker, the dosages of the interventions varied widely between trials, as did the dosing schedules and the duration of treatment. Thus, in trials that provided the information, the average daily dose of carvedilol ranged from 6.25 mg (Gupta 2016), to 31 mg (Bañares 2002); the average daily dose of propranolol, the comparator used in nine of the trials, varied from 17.7 mg (Wei 2018), to 152.6 mg (Kim 2016). Four trials used a fixed dose of carvedilol (De 2002; Hanno 2016; Kim 2016; Lo 2012), while two used a fixed dose of propranolol (De 2002; Hanno 2016); the remaining trials titrated the dosage to achieve a 25% reduction in heart rate or a reduction to 55 to 60 beats per minute. The follow‐up periods were relatively short with a mean of 27 weeks and a range of one week (De 2002; Mo 2014), to 30 months (Lo 2012). Three of the 10 trials evaluated primary prevention (Bañares 2002; ElRahim 2018; Kim 2016), while three evaluated secondary prevention (Gupta 2016; Lo 2012; Wei 2018). The remaining four trials evaluated both primary and secondary prevention (Agarwala 2011; De 2002; Hobolth 2012; Mo 2014); these four trials did not provide any analyses of the effect of treatments by prevention type, and we were not able to obtain these data from the trial authors. Thus, the subgroup analyses by prevention type involved a maximum of six trials. Six of the 10 trials measured haemodynamic responses (Bañares 2002; De 2002; Gupta 2016; Hobolth 2012; Kim 2016; Mo 2014). The degree of variation in a number of important aspects relating to the conduct of these trials is reflected in the inter‐trial variation in outcomes.
The use of carvedilol was associated with significantly greater absolute and relative reductions in hepatic venous pressure gradient but not in the number of participants who showed a satisfactory haemodynamic response; this additional reduction in the pressure gradient was not associated with better clinical outcomes. However, the small number of included trials, relatively short follow‐up periods, and the low quality of the evidence across all outcomes means that the result of this review is inconclusive.
The analysis of mortality included events from only three trials (De 2002; Hobolth 2012; Lo 2012). One trial, which compared carvedilol with nadolol (Lo 2012), included 121 participants followed for 30 months; and recorded a total of 32 deaths, with similar mortality rates between carvedilol and traditional, non‐selective beta‐blockers. Lo 2012 did not assess haemodynamic responses invasively, so there was no direct measure of the effectiveness or otherwise of the medication on portal pressure. The remaining two trials compared carvedilol versus propranolol (De 2002; Hobolth 2012); one, involving 36 participants, followed over seven days, reported two events (De 2002), while the other, involving 34 participants, followed over 90 days, reported one event (Hobolth 2012). In both, the haemodynamic responses favoured carvedilol but only marginally in one (De 2002). Thus, we cannot draw any conclusion, based on these three trials, on the effects on mortality.
The analysis of upper gastrointestinal bleeding included 10 trials; the occurrence of upper gastrointestinal bleeding was significantly greater in the long‐term trials but we found no significant difference in the risk between interventions. All of the short‐term trials assessed haemodynamic responses directly; we found no association between the haemodynamic response and the risk of bleeding. None of the long‐term trials measured haemodynamic responses, although in one (ElRahim 2018), the varices were reassessed endoscopically after one year of treatment and no differences were found in variceal grade reduction between carvedilol and propranolol.
The analysis of serious adverse events included 10 trials; again the number of serious adverse event was significantly greater in the long‐term trials but there was no difference in the risk of serious adverse events in participants receiving carvedilol or a comparator. Similarly, we found no association between the haemodynamic responses and the risk of serious adverse event in trials in which they were measured. There was no difference in the risk of non‐serious adverse events between the short‐ and long‐term trials and no relationship to haemodynamic responses.
We planned to undertake a series of subgroup analyses. However, few of these were possible. We were not able to draw conclusions about whether carvedilol might be more efficacious if used for primary or secondary prevention because of the limited number of studies available; We were unable to include four trials that did not provide separate results for participants who had previously had a variceal bleed and those who had not (Agarwala 2011; De 2002; Hobolth 2012; Mo 2014). Likewise, we were not able to look at the possible effects of treatment on hepatic function as none of the trials in this review included the incidence of hepatic decompensation as an outcome. Ripoll and colleagues (Ripoll 2007) showed that for every mmHg increase in hepatic venous pressure gradient, the risk of hepatic decompensation increases by 11%. One of the included trials reported that the treatment‐related reduction in the hepatic venous pressure gradient was greater in participants with more severely decompensated cirrhosis, irrespective of the intervention used (Bañares 2002). However, a further included trial found no relationship between the degree of hepatic decompensation and the magnitude of haemodynamic benefit (De 2002). Thus, the association between the degree of functional hepatic impairment and the haemodynamic response remains unclear. It has been suggested that carvedilol could worsen fluid retention in people with cirrhosis via activation of the renin‐angiotensin‐aldosterone system (Hobolth 2012). Four of the included trials reported worsening of ascites, as a non‐serious adverse event (Bañares 2002; Gupta 2016; Hobolth 2012; Kim 2016); the average incidence was around 15% in both the carvedilol and propranolol groups.
We were able to undertake subgroup analyses based on the duration of treatment. For the purposes of these analyses we classified the included trials as either short‐term (≤ 3 months; mean 6 (1 to 12) weeks), or long‐term (> 3 months; mean 13.5 (6 to 30) months). Significantly more deaths, bleeding episodes and serious adverse events occurred in the trials of longer duration (Agarwala 2011; ElRahim 2018; Lo 2012; Wei 2018), although with no difference in frequency between carvedilol and its comparators. The fact that more events were observed in the long‐term trials may simply be because there were more opportunities for events to occur. However, the possibility that treatment in these trials may have been sub‐optimal over time should also be considered. Five of the six short‐term trials (Bañares 2002; Gupta 2016; Hobolth 2012; Kim 2016; Mo 2014), adjusted drug dosages to achieve a 25% reduction in heart rate or a reduction to 55 to 60 beats per minute; in addition, all six directly measured haemodynamic responses (Bañares 2002; De 2002; Gupta 2016; Hobolth 2012; Kim 2016; Mo 2014). In contrast, only one of the long‐term trials (ElRahim 2018), titrated drug dosages in relation to heart rate reduction; one used a fixed dose of carvedilol but titrated the dose of nadolol in response to heart rate (Lo 2012); one stipulated that they titrated the drug dosages but did not provide information on how this was done (Wei 2018); the final long‐term trial did not provide any information on drug dosages or drug schedules (Agarwala 2011). One long‐term trial assessed the effect of treatment on portal pressure indirectly by repeat endoscopy and grading of the varices (ElRahim 2018); the remaining three trials did not assess the effect of treatment on portal pressure (Agarwala 2011; Lo 2012; Wei 2018). We found no association between haemodynamic responses to treatment and subsequent clinical events; however, these findings were based on the results of the short‐term trials only and it can not be assumed that this would pertain in the longer term (Tripathi 2002).
Consequently, further adequately powered, long‐term trials are needed, which measure both clinical and haemodynamic outcomes. Measurement of the hepatic venous pressure gradient is the reference method for the assessment of portal pressure. However, it is invasive, expensive and requires dedicated hospital resources and experienced staff. Consequently, it is not widely available. Advances in the non‐invasive evaluation of portal hypertension, including measurement of stiffness in the liver and stiffness/congestion of the spleen together with contrast enhanced ultrasound, could be used in the context of a clinical trial (Bolognesi 2017). Future studies should also examine outcomes in relation to the severity of the liver disease and whether prevention is primary or secondary.
This review included participants with portal hypertension secondary to chronic liver disease. In consequence, the results may not pertain to people with portal hypertension associated with schistosomiasis, portal/splenic vein thrombosis, Budd‐Chiari syndrome and other rarer conditions of pre‐ or post‐sinusoidal block.
Overall, carvedilol appears to be as efficacious and safe as propranolol and nadolol for the treatment of portal hypertension in patients with cirrhosis and oesophageal varices. We found no evidence that it was more efficacious than traditional non‐selective beta‐blockers and no evidence that it was a safer to use, but with all the caveats listed above.
Quality of the evidence
The main reasons for downgrading the evidence in this review are bias, imprecision and inconsistency.
Bias
As recommended, we combined the individual bias domains in an overall assessment (hbg.cochrane.org/information‐authors). We identified potential biases in all of the included trials. We defined mortality, but not serious adverse events, as an outcome that is robust to performance and detection bias (Savović 2012a; Savović 2012b). This decision can be questioned, as lack of blinding is not likely to influence the assessment of events such as upper gastrointestinal bleeding. Only two trials were conducted double‐blind (De 2002; Hobolth 2012); we classified one single‐blinded trial as at high risk of performance bias but at low risk of detection bias (Bañares 2002), while the remaining eight trials were open without blinding and hence were at high risk of bias for this domain. Only two trials provided full outcome data and included all participants in their analyses (Kim 2016; Lo 2012); we classified the remaining trials as at unclear or high risk of attrition bias. Six trials reported outcome data on all of the primary outcome measures (De 2002; Gupta 2016; Hobolth 2012; Kim 2016; Lo 2012; Mo 2014); the remaining trials did not so we classified them at high risk of reporting bias. We classified any type of for‐profit funding, including the gratuitous supply of interventions or placebo, as introducing a high risk of for‐profit bias (hbg.cochrane.org/information‐authors); we classified only two trials at low risk of bias for this domain (Bañares 2002; Gupta 2016). The decision to include this domain is debatable (Higgins 2017). We classified all of the included trials at high risk in the overall assessments of mortality and non‐mortality outcomes.
Imprecision
Only 11 randomised clinical trials were available for inclusion; the sample sizes in the included trials were generally small and the number of events were limited. The effect estimates had very wide confidence intervals.
Inconsistency
There was considerable between‐trial inconsistency for the outcome non‐serious adverse events (I2 = 88%), and moderate inconsistency for the outcomes upper gastrointestinal bleeding (I2 = 45%), and haemodynamic treatment failure (I2 = 42%).
Based on the assessment of bias control combined with inconsistency, we classified the quality of the evidence as low for the assessment of mortality, serious adverse events and the absolute and relative reductions in hepatic venous pressure gradient, and further downgraded the outcomes upper gastrointestinal bleeding, non‐serious adverse events, and haemodynamic treatment failure to very low, based on inconsistency between trials within the analyses.
Potential biases in the review process
We undertook the review based on current recommendations for bias control ((hbg.cochrane.org/information‐authors; Higgins 2017). We attempted to minimise possible selection bias (Page 2014), by using a comprehensive search strategy. Thus, we combined searches in electronic databases with hand searches of the biographies of identified studies and the conference proceedings and abstract books from relevant national and International society meetings. We consider it unlikely that we have failed to identify any published trials.
Agreements and disagreements with other studies or reviews
A number of systematic reviews with meta‐analyses of carvedilol have been undertaken. Aguilar‐Olivos 2014 included four randomised clinical trials (Bañares 1999; Bañares 2002; De 2002; Hobolth 2012), one of which (Bañares 1999), we excluded from the present review because the follow‐up period, after drug administration, was only two hours. The findings reported by Aguilar‐Olivos 2014 mirror those of the present review in relation to mortality, gastrointestinal bleeding, serious and non‐serious adverse events, and the reduction in hepatic venous portal pressure. However, they reported fewer treatment failures in participants receiving carvedilol. Three subsequent systematic reviews with meta‐analyses, focusing specifically on the haemodynamic effects of carvedilol versus propranolol, also reported outcomes in favour of carvedilol (Chen 2015; Li 2016; Sinagra 2014). The first of these (Sinagra 2014) included five trials (Bañares 1999; Bañares 2002; De 2002; Hobolth 2012; Lin 2004), two of which were short‐term haemodynamic studies which we chose not to include in our review (Bañares 1999; Lin 2004). In the three long‐term studies, the mean difference in the percentage reduction in the hepatic venous pressure gradient after treatment was ‐6.61%, favouring carvedilol. The second of these reviews (Chen 2015), included five published studies (Bañares 1999; Bañares 2002; De 2002; Hobolth 2012; Lin 2004), and data from two possible doctoral theses (Qu 2012; Ren 2012); we included three of these studies in our review (Bañares 2002; De 2002; Hobolth 2012). The authors found that the mean difference in the percentage reduction in hepatic venous pressure gradient after treatment in the three long‐term trials was −6.80% (CI −11.53 to −2.07), favouring carvedilol. In addition, the number of participants showing a favourable haemodynamic response was significantly higher in the those receiving carvedilol. The final review (Li 2016), looked at the haemodynamic effects of carvedilol in comparison with traditional, non‐selective beta blockers and endoscopic variceal ligation. Seven of the included trials compared the haemodynamic effects of carvedilol and propranolol (Bañares 1999; Bañares 2002; De 2002; Hobolth 2012; Kim 2016; Lin 2004; Mo 2014). Of these, we excluded two from our review, as they reported very short‐term effects only (Bañares 2002; Lin 2004), while one abstract, by Sohn and colleagues, published in 2013, is included in our review as a full paper (Kim 2016). Li 2016 reported that use of carvedilol was associated with a greater percentage reduction in hepatic venous pressure gradient within six months (MD −8.49%, 95% CI −12.36 to −4.63). These systematic reviews combined short‐ and long‐term studies in their meta‐analyses and made no attempt to determine if the reported reductions in percentage hepatic venous pressure gradient reduction were clinically meaningful.
Medication dosages varied widely across the few trials included in our review, hence we were not able to establish an optimal dose for the greatest portal haemodynamic benefits. However, one discussion article (Tsochatzis 2009), described an inverse relationship between the dose of carvedilol and the magnitude of the hepatic venous pressure gradient reduction, based on four trials, one of which we included in our review (Bañares 2002; Bruha 2006; Stanley 1999;Tripathi 2002). Additionally, another narrative review (Tripathi 2010), which included 10 trials (Bañares 1999; Bañares 2002; Bruha 2006; De 2002; Forrest 1996; Lin 2004; Sekiyama 1997; Silkauskaite 2013; Stanley 1999; Tripathi 2002), concluded that a dose of 12.5 mg of carvedilol provides the best compromise between haemodynamic benefit and minimising adverse events, particularly in patients with ascites. Further exploration of the relationships between the dose of medication, the way in which it is introduced, and the subsequent haemodynamic and clinical outcomes, is needed.
Authors' conclusions
Implications for practice.
The analyses provided low‐quality evidence that carvedilol is more effective than traditional, non‐selective beta‐blockers in reducing the hepatic venous pressure gradient although its use was not associated with an increase in the number of people achieving a significant haemodynamic response, nor did the enhanced haemodynamic response translate into clinical benefits on outcomes such as mortality and adverse events including upper gastrointestinal bleeding. Participants seemed to tolerate carvedilol just as well as traditional, non‐selective beta‐blockers, even at higher doses, provided titration was slow.
Implications for research.
We employed the EPICOT format to formulate research recommendations (Brown 2006). Overall, the use of carvedilol in clinical practice in patients with cirrhosis and oesophageal varices remains unclear, and additional evidence from future randomised clinical trials is required to evaluate this further.
Evidence (what is the current state of evidence?): this review of 10 randomised controlled trials with extractable data, showed that carvedilol produces a significantly greater reduction in the hepatic venous pressure gradient compared with traditional, non‐selective beta blockers, but that its use is not associated with an increase in the number of participants with an adequate haemodynamic response or in any additional clinical benefit. However, the quality of this evidence is low; only two of the 10 trials were double‐blind, and there is a paucity of large, long‐term trials that directly compare the two interventions and provide relevant outcome data. Further exploration of the relationship between the dose of medication, the way it is administered and the subsequent haemodynamic and clinical outcomes is needed for carvedilol and traditional non‐selective beta blockers. Further evidence is also required on both the clinical and haemodynamic outcomes in relation to the type of prevention (primary versus secondary) and the degree of hepatic decompensation. No data are available, at present, on health‐related quality of life outcomes.
Participants (what is the population of interest?): adults with cirrhosis and gastroesophageal varices, with or without a history of previous variceal bleeding
Inverventions (what are the interventions of interest?): carvedilol
Comparisons (what are the comparisons of interest?): traditional, non‐selective beta‐blockers such as propranolol or nadolol
Outcomes (what are the outcomes of interest?): all‐cause mortality; upper gastrointestinal bleeding, serious and non‐serious adverse events; haemodynamic responses including absolute and relative changes in hepatic venous pressure gradient and the adequacy of the treatment response, defined as attainment of reduction in the hepatic venous pressure gradient to less than 12 mmHg, or more than 20% reduction from baseline; health‐related quality of life
Time stamp (date of literature search): May 2018
Acknowledgements
Peer reviewers: Ulrich Thalheimer, UK; Gautam Mehta, UK Contact editor: Christian Gluud, Denmark Sign‐off editor: Christian Gluud, Denmark
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Electronic search strategy
| Database | Time span | Search strategy |
| Cochrane Hepato‐Biliary Controlled Trials Register | May 2018 | (carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis) AND (beta‐blocker* or beta‐adren* or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol*) AND (portal hypertension) |
| Cochrane Central Register of Controlled Trials (CENTRAL) | 2018, Issue 4 | #1 (carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis) #2 MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees #3 (beta‐blocker* or beta‐adren* or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol*) #4 #2 or #3 #5 MeSH descriptor: [Hypertension, Portal] explode all trees #6 portal hypertension #7 #5 or #6 #8 #1 and #4 and #7 |
| MEDLINE Ovid | 1946 to May 2018 | 1. (carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 2. exp Adrenergic beta‐Antagonists/ 3. (beta‐blocker* or beta‐adren* or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 4. 2 or 3 5. exp Hypertension, Portal/ 6. portal hypertension.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 7. 5 or 6 8. 1 and 4 and 7 |
| Embase Ovid | 1974 to May 2018 | 1. (carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 2. exp Adrenergic beta‐Antagonists/ 3. (beta‐blocker* or beta‐adren* or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 4. 2 or 3 5. exp Hypertension, Portal/ 6. portal hypertension.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 7. 5 or 6 8. 1 and 4 and 7 |
| LILACS (Bireme) | 1982 to May 2018 | (carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis) [Words] and (beta‐blocker$ or beta‐adren$ or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol$) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to May 2018 | #4 #3 AND #2 AND #1 #3 TS=(portal hypertension) #2 TS=(beta‐blocker* or beta‐adren* or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol*) #1 TS=(carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to May 2018 | #4 #3 AND #2 AND #1 #3 TS=(portal hypertension) #2 TS=(beta‐blocker* or beta‐adren* or beta antagonists or betaspace or blocadren or corgard or indernal or innopran or levatol or nadolol or penbutolol or propranolol or sorine or solatol or timol*) #1 TS=(carvedilol or KRKA or hexal or carvil or coreg or dilatrend or eucardic or carloc or actavis) |
Data and analyses
Comparison 1. Carvedilol versus non‐selective beta‐blockers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (overall) | 7 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 2 Mortality (duration) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Long‐term | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.48, 1.57] |
| 2.2 Short‐term | 6 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.05, 12.43] |
| 3 Mortality (prevention type) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Primary prevention | 2 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Secondary prevention | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.48, 1.57] |
| 4 Upper gastrointestinal bleeding (overall) | 10 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.43, 1.37] |
| 5 Upper gastrointestinal bleeding (duration) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Long‐term | 4 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.55] |
| 5.2 Short‐term | 6 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.24, 3.48] |
| 6 Upper gastrointestinal bleeding (prevention type) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Primary prevention | 3 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.71, 3.06] |
| 6.2 Secondary prevention | 3 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.29] |
| 7 Serious adverse events (overall) | 10 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.67, 1.42] |
| 8 Serious adverse events (duration) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Long‐term | 4 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.46, 1.52] |
| 8.2 Short‐term | 6 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.55, 4.68] |
| 9 Serious adverse events (prevention type) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Primary prevention | 3 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.60, 3.75] |
| 9.2 Secondary prevention | 3 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.13] |
| 10 Non‐serious adverse events (overall) | 6 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.23, 1.29] |
| 11 Non‐serious adverse events (duration) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Long‐term | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.17, 0.60] |
| 11.2 Short‐term | 4 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.34, 2.02] |
| 12 Non‐serious adverse events (event type) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Hypotension | 4 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.26, 1.79] |
| 12.2 Non‐serious hepatic encephalopathy | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.21, 4.32] |
| 12.3 Shortness of breath | 6 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.42, 2.03] |
| 12.4 Worsening of ascites | 4 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.72, 4.14] |
| 12.5 Fatigue | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.49] |
| 12.6 Bradycardia | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.17, 1.54] |
| 12.7 Vertigo | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 4.53] |
| 12.8 Insomnia | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.29, 2.47] |
| 12.9 Gastrointestinal discomfort | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.13, 3.22] |
| 12.10 Impotence | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.08, 5.09] |
| 13 Non‐serious adverse events (prevention type) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Primary prevention | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.25, 0.68] |
| 13.2 Secondary prevention | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.40] |
| 14 Hepatic venous pressure gradient, end of treatment (mmHg) (overall) | 6 | 368 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.60, ‐0.89] |
| 15 Reduction in hepatic venous pressure gradient (%) (overall) | 6 | 368 | Mean Difference (IV, Random, 95% CI) | ‐8.02 [‐11.49, ‐4.55] |
| 16 Haemodynamic treatment failure (overall) | 6 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.02] |
| 17 Hepatic venous pressure gradient, end of treatment (mmHg) (prevention type) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Primary prevention | 2 | 156 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐3.65, ‐1.03] |
| 17.2 Secondary prevention | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.45, 1.25] |
| 18 Reduction in hepatic venous pressure gradient (%) (prevention type) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Primary prevention | 2 | 156 | Mean Difference (IV, Random, 95% CI) | ‐7.71 [‐12.49, ‐2.93] |
| 18.2 Secondary prevention | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐12.13, 2.73] |
| 19 Haemodynamic treatment failure (prevention type) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Primary prevention | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.59, 1.00] |
| 19.2 Secondary prevention | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwala 2011.
| Methods | Single‐centre, open, randomised clinical trial evaluating primary and secondary prevention | |
| Participants | 102 participants with cirrhosis and endoscopically proven oesophageal varices, with or without previous bleeding Proportion of men: not reported Mean age: not reported Proportion for primary prevention: unclear Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: not reported Dose of propranolol: not reported Treatment duration: 6 months |
|
| Outcomes | Outcomes included in meta‐analysis: bleeding after at least 6 months | |
| Country of origin | India | |
| Publication status | Abstract | |
| Inclusion period | Not reported | |
| Notes | The trial report describes a statistically insignificant association between carvedilol and systemic hypotension, but does not include any quantification. Outcome data were not available by type of prevention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors describe intension to treat analyses, but the number of participants lost to follow‐up and the methods used to undertake the analyses are unclear. |
| Selective reporting (reporting bias) | High risk | The trial does not describe mortality and only reports the statistical significance of differences in adverse events. We did not have access to the trial protocol. |
| For‐profit funding | Unclear risk | Not described |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Bañares 2002.
| Methods | Single‐blind, parallel‐arm, single‐centre randomised clinical trial evaluating primary prevention | |
| Participants | 51 participants with cirrhosis and endoscopically proven oesophageal varices without previous bleeding Proportion of men: carvedilol 73%; propranolol 60% Mean age: carvedilol 57.9 years; propranolol 58.4 years Proportion for primary prevention: carvedilol 100%; propranolol 100% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: 6.25 mg once daily titrated to a mean of 31 mg to achieve a 25% reduction in heart rate Dose of propranolol: 20 mg once daily titrated to a mean of 73 mg to achieve a 25% reduction in heart rate Treatment duration: approximately 3 months |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, upper gastrointestinal bleeding, serious adverse events, non‐serious adverse events; reduction in hepatic venous pressure gradient and haemodynamic response (gradient reduction by ≥ 20% from baseline or to ≤ 12 mmHg) after a mean period of 11 weeks. | |
| Country of origin | Spain | |
| Publication status | Full‐paper | |
| Inclusion period | Not reported | |
| Notes | The publication does not describe the type of serious adverse events. The discussion section gives the impression that serious adverse events were associated with systemic hypotension, although this is not stated specifically. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The trial only describes per protocol analyses in the haemodynamic assessments. |
| Selective reporting (reporting bias) | High risk | The trial report does not describe the types of serious adverse events. We did not have access to the trial protocol. |
| For‐profit funding | Low risk | No for‐profit funding |
| Other bias | Low risk | No other biases |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
De 2002.
| Methods | Double‐blind, parallel‐arm, single‐centre, randomised clinical trial evaluating primary and secondary prevention | |
| Participants | 36 participants with cirrhosis and endoscopically verified varices with 1 previous bleeding episode in the 7 to 10 days before inclusion, or without previous bleeding Proportion of men: carvedilol 83%; propranolol 94% Mean age: carvedilol 42.3 years; propranolol 47.3 years Proportion for primary prevention: carvedilol 61.1%; propranolol 61.1% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: 12.5 mg once daily Dose of propranolol: 80 mg once daily Treatment duration: 7 days |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, variceal bleeding, adverse events, reduction in hepatic venous pressure gradient, treatment response (gradient reduction by ≥ 20% from baseline or to ≤ 12 mmHg) assessed, after 7 days | |
| Country of origin | India | |
| Publication status | Full‐paper | |
| Inclusion period | Not reported | |
| Notes | Outcome data were not available by type of prevention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots (independent researcher not otherwise involved in the trial) |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The investigators only report per protocol analyses of haemodynamic changes. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes are described. We did not have access to the trial protocol. |
| For‐profit funding | High risk | Support from ICI Pharmaceuticals India Ltd, India (supplied propranolol) and Sun Pharmaceutical Industries Ltd, India (supplied carvedilol). |
| Other bias | Low risk | No other biases |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
ElRahim 2018.
| Methods | Single‐centre, open, randomised clinical trial evaluating primary prevention | |
| Participants | 330 participants with cirrhosis and endoscopically‐proven medium to large varices, without previous bleeding Proportion of men: carvedilol 34%; propranolol 44% Mean age: carvedilol 51.2 years; propranolol 51.8 years Proportion for primary prevention: carvedilol 100%; propranolol 100% Proportion with:
|
|
| Interventions |
Intervention comparison: band ligation vs propranolol vs carvedilol Dose of carvedilol: initially 6.25 mg daily, titrated to reach 12.5 to 50 mg, to achieve a 25% reduction in heart rate while remaining > 55 beats per minute; mean 12.5 mg ± standard deviation 6.3 mg Dose of propranolol: initially 40 mg daily, titrated to achieve a 25% reduction in heart rate while remaining > 55 beats per minute; mean 43.01 mg ± standard deviation 7.30 mg Treatment duration: 12 months |
|
| Outcomes | Outcomes included in meta‐analysis: variceal bleeding and adverse events after 1 year | |
| Country of origin | Egypt | |
| Publication status | Full‐paper | |
| Inclusion period | May 2015‐June 2016 | |
| Notes | This trial did not include portal haemodynamic measures. The trial report did not describe the allocation group of participants who died or participants with serious adverse events. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | 'Envelope technique'. The trial report does not describe if the envelopes were opaque, serially numbered or sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The analysis excluded participants who dropped out, bled or died. |
| Selective reporting (reporting bias) | High risk | The allocation group of participants who died is not described. We did not have access to the trial protocol. |
| For‐profit funding | Unclear risk | Not described |
| Other bias | High risk | Investigators reallocated participants initially randomised to carvedilol or propranolol to banding ligation if they had contraindications to medical interventions. |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Gupta 2016.
| Methods | Open‐label, parallel‐arm, single‐centre randomised clinical trial evaluating secondary prevention | |
| Participants | 59 participants with cirrhosis and portal hypertension (hepatic venous pressure gradient > 12 mmHg) with a previous bleeding episode Proportion of men: carvedilol 96.7%; propranolol 89.7% Mean age: carvedilol 41.7 years; propranolol 45.0 years Proportion for primary prevention: carvedilol 0%; propranolol 0% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: initially 3.125 mg twice daily; increased in 3.125 mg increments until heart rate was between 55 to 60 beats per minute, or to a total daily dose of 25 mg, or to intolerance; median 6.25 mg Dose of propranolol: initially 40 mg once daily, increased in 20 to 40 mg increments until the heart rate was between 55 to 60 beats per minute, to a total daily dose of 320 mg, or to intolerance; median 40 mg Co‐intervention: all participants underwent endoscopic banding ligation Treatment duration: 1 month |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, variceal bleeding, adverse events, reduction in hepatic venous pressure gradient, treatment response (gradient reduction by ≥ 20% from baseline or to < 12 mmHg), after 1 month | |
| Country of origin | India | |
| Publication status | Full‐paper | |
| Inclusion period | 1 June 2013‐31 December 2013 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The analyses exclude participants who dropped out |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes are reported. We did not have access to the trial protocol. |
| For‐profit funding | Low risk | No for‐profit funding |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Hanno 2016.
| Methods | Open‐label, parallel‐arm, single‐centre randomised clinical trial. The trial report does not specify if primary or secondary prevention was assessed | |
| Participants | 40 participants with cirrhosis and grade 3 or 4 oesophageal varices allocated to carvedilol vs propranolol Proportion of men: not reported Mean age: not reported Proportion for primary prevention: not reported Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: 6.25 mg once daily increased to 6.25 mg twice daily after 1 week Dose of propranolol: 20 mg 3 times daily Treatment duration: 12 months |
|
| Outcomes | Outcomes included in meta‐analyses: none | |
| Country of origin | Egypt | |
| Publication status | Abstract | |
| Inclusion period | Not described | |
| Notes | The trial report does not describe any of the outcomes assessed in our review. Thus, we could not include this trial in our quantitative analyses (meta‐analyses). The trial also includes 2 groups allocated to banding ligation alone (n = 20) or banding ligation and carvedilol (n = 20). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up and withdrawals are not described |
| Selective reporting (reporting bias) | High risk | Mortality and variceal bleeding are not reported. We did not have access to the trial protocol. |
| For‐profit funding | Unclear risk | Not described |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Hobolth 2012.
| Methods | Double‐blind, parallel‐arm, multi‐centre, randomised clinical trial evaluating pre‐prevention, primary, and secondary prevention | |
| Participants | 46 participants with portal hypertension (hepatic venous pressure gradient ≥ 12 mmHg). We excluded 12 participants without varices and included 34 participants with oesophageal varices. We combined all participants in our analyses (as primary or secondary prevention) because we did not have information on previous bleeding episodes (see notes). Proportion of men: carvedilol 56.3%; propranolol 72.2% Proportion for primary prevention: carvedilol 76.2%; propranolol 70.6% Mean age: carvedilol 58.1 years; propranolol 54.4 years Proportion with:
|
|
| Interventions |
Type of beta‐blockers: carvedilol vs propranolol Dose of carvedilol: initially 6.25 mg, the dose was titrated to achieve a 25% reduction in heart rate; mean 14 mg ± standard deviation 7 mg Dose of propranolol: initially 80 mg, the dose was titrated to achieve a 25% reduction in heart rate; mean 122 mg ± standard deviation 64 mg Treatment duration: 3 months |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, variceal bleeding, adverse events, reduction in hepatic venous pressure gradient, treatment response (gradient reduction by ≥ 20% from baseline or to < 12 mmHg), after a mean duration of 92.7 days | |
| Country of origin | Denmark | |
| Publication status | Full‐paper | |
| Inclusion period | September 2003‐August 2009 | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers (block randomisation) |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We did not have access to information about 1 participant. The published trial reports per protocol analyses. We were only able to undertake per protocol analyses for continuous outcomes |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes are described. We had access to the trial protocol. |
| For‐profit funding | High risk | Funding from Roche; propranolol supplied by Nycomed, Denmark and carvedilol by Roche, Switzerland. |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Kim 2016.
| Methods | Parallel‐arm, open‐label, multi‐centre, randomised clinical trial evaluating primary prevention | |
| Participants | 110 participants with cirrhosis, endoscopically verified grade 2 or 3 varices, and no previous bleeding Proportion of men: carvedilol 74.5%; propranolol 76.4% Mean age: carvedilol 51.7 years; propranolol 70.6 years Proportion for primary prevention: carvedilol 100%; propranolol 100% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: initially 6.25 mg once daily, increased to 12.5 mg; mean 11.6 mg/dL (range 6.25‐12.5 mg/dL) Dose of propranolol: initially 20 mg twice daily, titrated until heart rate decreased by 25%, or to 55 beats per minute; mean 152.6 mg/dL (range 40‐320 mg/dL) Treatment duration: 6 weeks |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, variceal bleeding, adverse events, reduction in hepatic venous pressure gradient, treatment response (gradient reduction by ≥ 20% from baseline or to < 12 mmHg), after 6 weeks | |
| Country of origin | Korea | |
| Publication status | Full‐paper | |
| Inclusion period | August 2012‐December 2014 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, random number table, block size of 2 and stratified block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding or participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All participants are accounted for and included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes are reported. We did not have access to the trial protocol. |
| For‐profit funding | High risk | Received funding from ChongKunDang Pharmaceutical |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Lo 2012.
| Methods | Open, parallel‐arm, single‐centre randomised clinical trial evaluating secondary prevention | |
| Participants | 121 participants with cirrhosis and previous variceal bleeding. Proportion of men: carvedilol 11.9%; nadolol 20% Mean age: carvedilol 53.0 years; nadolol 49.8 years Proportion for primary prevention: carvedilol 0%; nadolol 0% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs nadolol Initial dose of carvedilol: 6.25 mg once daily, increased to 6.25 mg twice daily if systolic blood pressure > 100 mmHg. Dose tapered if systolic blood pressure < 90 mmHg; mean 10.4 mg ± standard deviation 2.2 mg (range 6.25‐12.5 mg) Initial dose of nadolol: 20 mg once daily, titrated to achieve a 25% reduction in heart rate, or 55 beats per minute; mean 45 mg ± standard 13 mg (range 20‐80 mg) Co‐intervention: participants in the control group also received isosorbide mononitrate Treatment duration: the trial was terminated 6 months after enrolment of the last participant. |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, variceal bleeding, and adverse events after a median duration of 30 months (21 days‐4 years) | |
| Country of origin | Taiwan | |
| Publication status | Full‐paper | |
| Inclusion period | March 2005‐July 2009 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding or participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessment except endoscopists determining site of variceal bleed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in each group was lost to follow‐up; 4 participants in the carvedilol group and 5 participants in the nadolol group did not comply with the trial protocol. All participants accounted for; intention to treat analyses employed using last observed response carried forward |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcome measures are reported. We did not have accesses to the trial protocol. |
| For‐profit funding | High risk | Carvedilol supplied by Roche, Italy; nadolol supplied by E.R. SQUIBB SONS, Taiwan |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Mo 2014.
| Methods | Open, parallel‐arm, single‐centre randomised clinical trial evaluating primary and secondary prevention | |
| Participants | 96 participants with cirrhosis and oesophageal varices Proportion of men: carvedilol 75.0%; propranolol 68.8% Mean age: carvedilol 51.6 years; propranolol 52.8 years Proportion for primary prevention: carvedilol 52.1%; propranolol 43.8% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: initially 12.5 mg once daily, titrated within first 3 days until 20% to 25% reduction in heart rate, or to 55 beats per minute and blood pressure > 90/60 mmHg Dose of propranolol: initially 10.0 mg three times a day, titrated within first 3 days until 20% to 25% reduction in heart rate, or 55 beats per minute and blood pressure > 90/60 mmHg The investigators reduced the dose or stopped treatment if the mean arterial pressure or heart rate was too low Treatment duration: 7 days |
|
| Outcomes | Outcomes included in meta‐analysis: mortality, variceal bleeding, adverse events, reduction in hepatic venous pressure gradient, treatment response (gradient reduction by ≥ 20% from baseline or to ≤ 12 mmHg), after 1 week | |
| Country of origin | China | |
| Publication status | Full‐paper | |
| Inclusion period | March 2013‐March 2014 | |
| Notes | Outcome data were not available by type of prevention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial without blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial without blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up and withdrawals are not described. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcome measures are reported. We did not have accesses to the trial protocol. |
| For‐profit funding | High risk | Carvedilol supplied by Qilu Tianhe Pharmaceutical Co., Ltd; propranolol supplied by Sinopharm Shantou Jinshi Pharmaceutical Co., Ltd |
| Other bias | Low risk | No other biases identified |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Wei 2018.
| Methods | Open‐label, parallel‐arm, single‐centre randomised clinical trial evaluating secondary prevention | |
| Participants | 25 participants with cirrhosis and oesophageal varices Proportion of men: not reported Mean age: not reported Proportion for primary prevention: carvedilol 0%; propranolol 0% Proportion with:
|
|
| Interventions |
Intervention comparison: carvedilol vs propranolol Dose of carvedilol: titrated to a mean dose of 10 mg Dose of propranolol: titrated to a mean dose of 17.73 mg ± standard deviation 9.32 mg Treatment duration: 6 months |
|
| Outcomes | Outcomes included in meta‐analysis: variceal bleeding after a duration of 6 months | |
| Country of origin | China | |
| Publication status | Abstract | |
| Inclusion period | 1 March 2015‐31 August 2015 | |
| Notes | The trial also includes a control group of participants treated with traditional, non‐selective beta‐blockers before inclusion in the trial. We did not include these participants in our analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label for participants and researchers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded, but doctors performing endoscopy were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The trial report describes that 9 participants (4 allocated to carvedilol and 5 to non‐selective beta‐blockers) dropped out or were withdrawn due to "intolerance or reluctance to follow the protocol". Some of these participants are excluded from the analyses. The abstract includes these participants in a second control group. |
| Selective reporting (reporting bias) | High risk | The trial does not describe mortality. We did not have access to the trial protocol. |
| For‐profit funding | Unclear risk | Not reported |
| Other bias | Low risk | No other bias |
| Overall bias assessment (mortality) | High risk | High risk of bias |
| Overall bias assessment (non‐mortality outcomes) | High risk | High risk of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bañares 1999 | Spanish, randomised clinical trial comparing the acute haemodynamic effects of carvedilol or propranolol vs placebo over 2 hour; involved 35 people with cirrhosis and endoscopically proven varices with or without a history of previous bleeding. Ineligible trial period; our review only included trials with a follow‐up period of at least 1 week. |
| Bonaccorso 2017 | Italian, quasi‐randomised trial published in abstract form comparing carvedilol with propranolol for the primary prevention of variceal bleeding; involved 75 people with cirrhosis and endoscopically‐proven varices. Inappropriate trial design ‐ participants enrolled during the first half of the recruiting period were allocated to propranolol arm; those in the second half of the recruiting period to the carvedilol arm. |
| Lin 2004 | Taiwanese, randomised clinical trial comparing the acute haemodynamic effects of carvedilol versus propranolol plus isosorbide‐5‐mononitrate over 90 minutes; involved 35 people with cirrhosis and portal hypertension Ineligible trial period; our review only included trials with a follow‐up period of at least one week. |
| NCT01059396 | Ongoing Spanish, multicentre, randomised clinical trial of the effectiveness of treatment with beta‐blockers to prevent hepatic decompensation Participants had cirrhosis but did not have oesophageal varices. There is no direct comparison of carvedilol and propranolol; the trial compares propranolol with placebo; non‐responders are given carvedilol. |
| Reiberger 2013 | Austrian, non‐randomised, cohort study involving104 participants with cirrhosis and endoscopically‐proven varices with no history of bleeding Inappropriate trial design ‐ all participants received propranolol, and only non‐responders received carvedilol. |
| Silkauskaite 2013 | Lithuanian, open‐label, randomised clinical trial comparing the acute and chronic haemodynamic effects of carvedilol versus nebivolol for primary prevention of variceal bleeding; involved 20 people with cirrhosis and endoscopically‐proven varices This review sought to compare carvedilol with traditional, non‐selective beta‐blockers only; nebivolol is a selective β1adrenergic receptor blocker that has nitric oxide‐potentiating vasodilatory effects. |
Characteristics of ongoing studies [ordered by study ID]
NCT02385422.
| Trial name or title | The effect of carvedilol vs propranolol in cirrhotic participants with variceal bleeding |
| Methods | To compare the efficacy and safety of carvedilol and propranolol in participants with cirrhosis‐related oesophagogastric varices after multiple endoscopic treatments for secondary prophylaxis Participants will receive an endoscopic examination after they have been followed up on trial drug for 6 months, and if they have recurrence of varices or deterioration of varices, they are considered to be in need of endoscopic re‐treatment |
| Participants | Cirrhotic participants with gastroesophageal varices confirmed by endoscopy sub classified at the time of recruitment as having 1) mild oesophageal varices; 2) gastric varices with a diameter < 5 mm; or 3) variceal eradication at the time of recruiting; history of variceal bleeding treated on at least 3 occasions by variceal banding |
| Interventions |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | March 2015 |
| Contact information | chen.shiyao@zs‐hospital.sh.cn |
| Notes | We contacted trial authors for unpublished data in May 2017 but they have yet to respond |
Differences between protocol and review
We updated the methods according to the current recommendations of the Cochrane Hepato‐Biliary Group. The updates include changes to the wording of the bias assessment; obligatory inclusion of observational studies for the assessment of adverse events; and searching the LILACS database. In the results section we have reported the absolute and relative changes in hepatic venous pressure gradient at the end of the trial period, and the proportion of participants who were treatment failures under the generic heading 'Haemodynamnic responses'.
Contributions of authors
AZ, RJ and LH: extracted data LLG and MYM cross‐checked the extracted data AZ, RJ, and LLG: undertook the statistical analyses AZ and RJ: drafted the review LLG and MYM critically reviewed the initial drafts
All the review authors participated in the selection of randomised clinical trials; interpretation of the results and approved the final version of the review before submission
MYM is guarantor of the review.
Sources of support
Internal sources
No funding received, Other.
External sources
No funding received, Other.
Declarations of interest
AZ: no conflicts of interest RJ: no conflicts of interest LH: is an investigator on one of the included trials (Hobolth 2012), which received support from Roche. FB: is an investigator on one of the included trials (Hobolth 2012), which received support from Roche. LLG: has been an investigator in studies funded by Norgine, Abbvie, Intercept, and Merck; has received funding for travel expenses from Novo Nordisk; has received funding for lectures from Eli Lilly and Norgine, and funding for research from Alexion. MYM: no conflicts of interest
New
References
References to studies included in this review
Agarwala 2011 {published data only}
- Agarwala V, Prakash G, Singh R, Paliwal P, Tiwari S, Juber S, et al. Evaluation of treatment with carvedilol in comparison to propranolol in primary/secondary prophylaxis of gastroesophageal variceal bleeding. Indian Journal of Gastroenterology 2011;30(Suppl 1):A46. [DOI: 10.1007/s12664-011-0142-4] [DOI] [Google Scholar]
Bañares 2002 {published data only}
- Bañares R, Moitinho E, Matilla A, Garcia‐Pagan JC, Lampreave JL, Piera C, et al. Randomized comparison of long‐term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology (Baltimore, Md.) 2002;36(6):1367‐73. [PUBMED: 12447861] [DOI] [PubMed] [Google Scholar]
De 2002 {published data only}
- De BK, Das D, Sen S, Biswas PK, Mandal SK, Majumdar D, et al. Acute and 7‐day portal pressure response to carvedilol and propranolol in cirrhotics. Journal of Gastroenterology and Hepatology 2002;17(2):183‐9. [PUBMED: 11966949] [DOI] [PubMed] [Google Scholar]
ElRahim 2018 {published data only}
- Abd ElRahim AY, Fouad R, Khairy M, Elsharkawy A, Fathalah W, Khatamish H, et al. Efficacy of carvedilol versus propranolol versus variceal band ligation for primary prevention of variceal bleeding. Hepatology International 2018;12(1):75‐82. [PUBMED: 29185106 ] [DOI] [PubMed] [Google Scholar]
Gupta 2016 {published and unpublished data}
- Gupta V, Rawat R, Shalimar, Saraya A. Carvedilol versus propranolol effect on hepatic venous pressure gradient at 1 month in patients with index variceal bleed: RCT. Hepatology International 2016;11(2):181‐7. [PUBMED: 27624505] [DOI] [PubMed] [Google Scholar]
Hanno 2016 {published data only (unpublished sought but not used)}
- Hanno AE, Kheir AH, Wazzan DA, Mohamed MI. Medical versus endoscopic treatment of oesophageal varices in liver cirrhosis. United European Gastroenterology Journal 2016;5(Suppl 1):A307. [Google Scholar]
Hobolth 2012 {published and unpublished data}
- Hobolth L, Bendtsen F, Hansen EF, Møller S. Effects of carvedilol and propranolol on circulatory regulation and oxygenation in cirrhosis: a randomised study. Digestive and Liver Diseases 2014;46(3):251‐6. [PUBMED: 24290869] [DOI] [PubMed] [Google Scholar]
- Hobolth L, Møller S, Grønbæk H, Roelsgaard K, Bendtsen F, Feldager Hansen E. Carvedilol or propranolol in portal hypertension? A randomized comparison. Scandinavian Journal of Gastroenterology 2012;47(2):467‐74. [PUBMED: 22401315] [DOI] [PubMed] [Google Scholar]
Kim 2016 {published and unpublished data}
- Kim SG, Kim TY, Sohn JH, Um SH, Seo YS, Baik SK, et al. A randomized, multi‐center, open‐label study to evaluate the efficacy of carvedilol vs. propranolol to reduce portal pressure in patients with liver cirrhosis. American Journal of Gastroenterology 2016;111(11):1582‐90. [PUBMED: 27575713] [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim TY, Sohn JH, Um SH, Seo YS, Baik SK, et al. A randomized, multi‐center, open‐label study to evaluate the long‐term effect of carvedilol versus propranolol on reduction in portal pressure in patients with cirrhosis: CARPE study. Hepatology (Baltimore, Md.) 2015;62(Suppl 1):576A. [Google Scholar]
- Sohn JH, Kim TY, Um SH, Seo YS, Baik SK, Kim MY, et al. A randomized, multi‐center, phase IV open‐label study to evaluate and compare the effect of carvedilol versus propranolol on reduction in portal pressure inpatients with cirrhosis: an interim analysis. Hepatology (Baltimore, Md.) 2013;58(Suppl 1):990A. [DOI: 10.1002/hep26874] [DOI] [Google Scholar]
Lo 2012 {published data only}
- Lo GH, Chen WC, Wang HM, Yu HC. Randomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleeding. Journal of Gastroenterology and Hepatology 2012;27(11):1681‐7. [PUBMED: 22849337] [DOI] [PubMed] [Google Scholar]
Mo 2014 {published data only}
- Mo CY, Li SL. Short‐term effect of carvedilol vs propranolol in reduction of hepatic venous pressure gradient in patients with cirrhotic portal hypertension. World Chinese Journal of Digestology 2014;22(27):4146‐50. [DOI: 10.11569/wcjd.v22.i27.4146] [DOI] [Google Scholar]
Wei 2018 {published data only}
- Wei Y. The effect of carvedilol vs propranolol in patients with cirrhosis related gastroesophageal varices for secondary prophylaxis: a randomized controlled trial. Hepatology International 2018;12(2 Suppl):S571. [Google Scholar]
References to studies excluded from this review
Bañares 1999 {published data only}
- Bañares R, Moitinho E, Piqueras B, Casado M, Garcia‐Pagan JC, Diego A, et al. Carvedilol, a new nonselective beta‐blocker with intrinsic anti‐ alpha1‐adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology (Baltimore, Md.) 1999;30(1):79‐83. [PUBMED: 10385642] [DOI] [PubMed] [Google Scholar]
Bonaccorso 2017 {published and unpublished data}
- Bonaccorso A, Calvaruso V, Simone F, Mogavero G, Bronte F, Licata M, et al. Effectiveness of beta‐blockers (BB) in primary prevention of esophageal varices (EV) bleeding in patients with cirrhosis: a prospective evaluation by hepatic venous pressure gradient (HVPG) measurement. Hepatology (Baltimore, Md.) 2017;66(S1):252‐3A. [PUBMED: 28965360]28965360 [Google Scholar]
- Butera G, Simone F, Calvaruso V, Orlando R, Bonaccorso A, Cicco M, et al. Effectiveness of beta‐blocker in primary prevention of variceal bleeding in patients with cirrhosis: a prospective evaluation by hepatic venous pressure gradient measurement. Digestive and Liver Disease 2014;46(4):e141. [DOI: 10.1016/j.dld.2014.08.029] [DOI] [Google Scholar]
- Calvaruso V, Bonaccorso A, Simone F, Butera G, Marco V, Craxì A, et al. Effectiveness of beta‐blockers in primary prevention of bleeding: a comparison of propranolol vs. carvedilol by HVPG. Journal of Hepatology 2016;64(2 Suppl):S290. [DOI: 10.1016/S0168-8278(16)00361-5] [DOI] [Google Scholar]
Lin 2004 {published data only}
- Lin HC, Yang YY, Hou MC, Huang YT, Lee FY, Lee SD. Acute administration of carvedilol is more effective than propranolol plus isosorbide‐5‐mononitrate in the reduction of portal pressure in patients with viral cirrhosis. American Journal of Gastroenterology 2004;99(10):1953‐8. [PUBMED: 15447755] [DOI] [PubMed] [Google Scholar]
NCT01059396 {published and unpublished data}
- NCT01059396. Study on ß‐blockers to prevent decompensation of cirrhosis with HTPortal (PREDESCI) [Multicenter, randomized, double‐blind, placebo‐controlled study on the effectiveness of treatment with beta‐blockers to prevent decompensation of cirrhosis with portal hypertension]. clinicaltrials.gov/ct2/show/NCT01059396 (date first received 29 January 2010).
Reiberger 2013 {published data only}
- Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non‐response to propranolol. Gut 2013;62(11):1634‐41. [PUBMED: 23250049] [DOI] [PubMed] [Google Scholar]
Silkauskaite 2013 {published data only}
- Silkauskaite V, Kupcinskas J, Pranculis A, Jonaitis L, Petrenkiene V, Kupcinskas L. Acute and 14‐day hepatic venous pressure gradient response to carvedilol and nebivolol in patients with liver cirrhosis. Medicina 2013;49(11):467‐73. [PUBMED: 24823927] [PubMed] [Google Scholar]
References to ongoing studies
NCT02385422 {published data only}
- Chen S. The effect of carvedilol vs propranolol in cirrhotic patients with variceal bleeding. ClinicalTrials.gov Identifier: NCT02385422.
Additional references
Aguilar‐Olivos 2014
- Aguilar‐Olivos N, Motola‐Kuba M, Candia R, Arrese M, Mendez‐Sanchez N, Uribe M, et al. Hemodynamic effect of carvedilol vs. propranolol in cirrhotic patients: systematic review and meta‐analysis. Annals of Hepatology 2014;13(4):420‐8. [PUBMED: 24927613] [PubMed] [Google Scholar]
Albillos 2007
- Albillos A, Banares R, Gonzalez M, Ripoll C, Gonzalez R, Catalina MV, et al. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: a meta‐analysis. American Journal of Gastroenterology 2007;102(5):1116‐26. [PUBMED: 17391317] [DOI] [PubMed] [Google Scholar]
Asrani 2013
- Asrani SK, Kamath PS. Natural history of cirrhosis. Current Gastroenterology Reports 2013;15(2):308. [PUBMED: 23314828] [DOI] [PubMed] [Google Scholar]
Baveno III
- Sarin SK, Primignani M, Agarwal SR. Gastric varices. In: Franchis R editor(s). Portal Hypertension III. Proceedings of the Third Baveno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies. Oxford: Blackwell, Science, 2001:76‐96. [Google Scholar]
Beppu 1981
- Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointestinal Endoscopy 1981;27(4):213‐8. [PUBMED: 6975734] [DOI] [PubMed] [Google Scholar]
Bolognesi 2017
- Bolognesi M, Pascoli M, Sacerdoti D. Clinical role of non‐invasive assessment of portal hypertension. World Journal of Gastroenterology 2017;23(1):1‐10. [DOI: 10.3748/wjg.v23.i1.1; PMC5221; PUBMED: 28104976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosch 2003
- Bosch J, Garcia‐Pagan JC. Prevention of variceal rebleeding. Lancet 2003;361(9361):952‐4. [PUBMED: 12648985] [DOI] [PubMed] [Google Scholar]
Bosch 2015
- Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. Journal of Hepatology 2015;62(S1):S121‐30. [DOI: 10.1016/j.jhep.2015.01.003; PMC4519833; PUBMED: 25920081] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broeders 2000
- Broeders MA, Doevendans PA, Bekkers BC, Bronsaer R, Gorsel E, Heemskerk JW, et al. Nebivolol: a third‐generation beta‐blocker that augments vascular nitric oxide release: endothelial beta(2)‐adrenergic receptor‐mediated nitric oxide production. Circulation 2000;102(6):677‐84. [PUBMED: 10931809] [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ (Clinical Research Ed.) 2006; Vol. 333, issue 7572:804‐6. [PUBMED: PMC1602035] [DOI] [PMC free article] [PubMed]
Bruha 2006
- Bruha R, Vitek L, Petrtyl J, Lenicek M, Urbanek P, Zelenka J, et al. Effect of carvedilol on portal hypertension depends on the degree of endothelial activation and inflammatory changes. Scandinavian Journal of Gastroenterology 2006;41(12):1454‐63. [PUBMED: 17101577] [DOI] [PubMed] [Google Scholar]
Brunner 2017
- Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver International 2017;37(Suppl 1):104–15. [DOI: 10.1111/liv.13277] [DOI] [PubMed] [Google Scholar]
Calés 1999
- Calés P, Oberti F, Payen JL, Naveau S, Guyader D, Blanc P, et al. Lack of effect of propranolol in the prevention of large oesophageal varices in patients with cirrhosis: a randomized trial. French‐Speaking Club for the Study of Portal Hypertension. European Journal of Gastroenterology and Hepatology 1999;11(7):741‐5. [PUBMED: 10445794] [DOI] [PubMed] [Google Scholar]
Carbonell 2004
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology (Baltimore, Md.) 2004;40(3):652‐9. [PUBMED: 15349904] [DOI] [PubMed] [Google Scholar]
Chalasani 2003
- Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. American Journal of Gastroenterology 2003;98(3):653‐9. [PUBMED: 12650802] [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen S, Wang JJ, Wang QQ, Hu JW, Dong S, Hu LJ, et al. The effect of carvedilol and propranolol on portal hypertension in patients with cirrhosis: a meta‐analysis. Patient Preference and Adherence 2015;14(9):961‐70. [PUBMED: 26203230] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 1999
- D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence‐based approach. Seminars in Liver Disease 1999;19(4):475‐505. [PUBMED: 10643630] [DOI] [PubMed] [Google Scholar]
D'Amico 2003
- D'Amico G, Franchis R. Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology (Baltimore, Md.) 2003;38(3):599‐612. [PUBMED: 12939586] [DOI] [PubMed] [Google Scholar]
D'Amico 2006
- D'Amico G, Garcia‐Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131(5):1611‐24. [PUBMED: 17101332] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forrest 1996
- Forrest EH, Bouchier IA, Hayes PC. Acute haemodynamic changes after oral carvedilol, a vasodilating beta‐blocker, in patients with cirrhosis. Journal of Hepatology 1996;25(6):909‐15. [9007720] [DOI] [PubMed] [Google Scholar]
García‐Pagán 1990
- García‐Pagán JC, Navasa M, Bosch J, Bru C, Pizcueta P, Rodés J. Enhancement of portal pressure reduction by the association of isosorbide‐5‐mononitrate to propranolol administration in patients with cirrhosis. Hepatology (Baltimore, Md.) 1990;11(2):230‐8. [PUBMED: 2307401] [DOI] [PubMed] [Google Scholar]
García‐Pagán 2001
- García‐Pagán JC, Villanueva C, Vila MC, Albillos A, Genescà J, Ruiz‐Del‐Arbol L, et al. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive ß‐blockers. Gastroenterology 2001;121(4):908‐14. [PUBMED: 11606504 ] [PubMed] [Google Scholar]
García‐Pagán 2003
- García‐Pagán JC, Morillas R, Bañares R, Albillos A, Villanueva C, Vila C, et al. Propranolol plus placebo versus propranolol plus isosorbide‐5‐mononitratein the prevention of a first variceal bleed: a double‐blind RCT. Hepatology (Baltimore, Md.) 2003;37(6):1260‐6. [PUBMED: 12774003] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Groszmann 1990
- Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia‐Tsao G, Navasa M, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990;99(5):1401‐7. [PUBMED: 2210246] [DOI] [PubMed] [Google Scholar]
Groszmann 2005
- Groszmann RJ, Garcia‐Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta‐blockers to prevent gastroesophageal varices in patients with cirrhosis. New England Journal of Medicine 2005;353(21):2254–61. [PUBMED: 16306522 ] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] [DOI] [PubMed] [Google Scholar]
Hemstreet 2004
- Hemstreet BA. Evaluation of carvedilol for the treatment of portal hypertension. Pharmacotherapy 2004;24(1):94‐104. [PUBMED: 14740791] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins J, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials. Clinical Trials 2008;5:225‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from: www.training.cochrane.org/handbook.
Hobolth 2010
- Hobolth L, Krag A, Bendtsen F. The recent reduction in mortality from bleeding oesophageal varices is primarily observed from days 1 to 5. Liver International 2010;30(3):455‐62. [PUBMED: 19968778] [DOI] [PubMed] [Google Scholar]
ICH GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Iwakiri 2014
- Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis ‐ current status and future directions. Journal of Hepatology 2014; Vol. 61, issue 4:912‐24. [PUBMED: 24911462] [DOI] [PMC free article] [PubMed]
Japanese Research Society for Portal Hypertension
- Japanese Research Society for Portal Hypertension. The general rules for recording endoscopic findings on esophageal varices. Japanese Journal of Surgery 1980;10(1):84‐7. [PUBMED: 7373958] [DOI] [PubMed] [Google Scholar]
Kovalak 2007
- Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointestinal Endoscopy 2007;65(1):82‐8. [DOI] [PubMed] [Google Scholar]
Lebrec 1981
- Lebrec D, Nouel O, Bernuau J, Bouygues M, Rueff B, Benhamou JP. Propranolol in prevention of recurrent gastrointestinal bleeding in cirrhotic patients. Lancet 1981;1(8226):920‐1. [PUBMED: 6112329] [DOI] [PubMed] [Google Scholar]
Li 2016
- Li T, Ke W, Sun P, Chen X, Belgaumkar A, Huang Y, et al. Carvedilol for portal hypertension in cirrhosis: systematic review with meta‐analysis. BMJ Open 2016;6(5):e010902. [DOI: 10.1136/bmjopen-2015-010902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Longacre 2008
- Longacre AV, Imaeda A, Garcia‐Tsao G, Fraenkel L. A pilot project examining the predicted preferences of patients and physicians in the primary prophylaxis of variceal hemorrhage. Hepatology (Baltimore, Md.) 2008;47(1):169‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2018
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome: systematic review with meta‐analysis. Intensive Care Medicine 2018;44(10):1603‐12. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PubMed] [Google Scholar]
MECIR 2014
- Methodological Expectations of Cochrane Intervention Reviews (MECIR). www.editorial‐unit.cochrane.org/mecir (accessed 3 February 2015).
Merli 2003
- Merli M, Nicolini G, Angeloni S, Rinaldi V, Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. Journal of Hepatology 2003;38(3):266‐72. [PUBMED: 12586291] [DOI] [PubMed] [Google Scholar]
Moreau 2006
- Moreau R, Lebrec D. Molecular and structural basis of portal hypertension. Clinics in Liver Disease 2006;10(3):445‐57. [PUBMED: 17162222] [DOI] [PubMed] [Google Scholar]
NIEC 1988
- The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. New England Journal of Medicine 1988;319(15):983‐9. [PUBMED: 3262200] [DOI] [PubMed] [Google Scholar]
Page 2014
- Page MJ, McKenzie JE, Kirkham J, Dwan K, Kramer S, Green S, et al. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.MR000035.pub2; MR000035] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paquet 1982
- Paquet KJ. Prophylactic endoscopic sclerosing treatment of the esophageal wall in varices ‐‐ a prospective controlled randomized trial. Endoscopy 1982;14(1):4‐5. [PUBMED: 7035153] [DOI] [PubMed] [Google Scholar]
Poynard 1991
- Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, et al. Beta‐adrenergic‐antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco‐Italian Multicenter Study Group. New England Journal of Medicine 1991;324(22):1532‐8. [PUBMED: 1674104] [DOI] [PubMed] [Google Scholar]
PRISMA 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. British Medical Journal 2009;339:b2535. [DOI: 10.1136/bmj.b2535; PMC2714657; PUBMED: 19622551] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qu 2012
- Qu CX. The preliminary research of the short‐term reduction of hepatic vein pressure gradient to predict the long‐term curative effect of beta‐blockers. Shandong University 2012.
Ren 2012
- Ren RR. The comparison of recent curative effect on the reduction of hepatic vein pressure gradient in patients with cirrhosis. Shandong University 2012.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripoll 2007
- Ripoll C, Groszmann R, Garcia‐Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007;133(2):481‐8. [PUBMED: 17681169] [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sekiyama 1997
- Sekiyama T, Komeichi H, Nagano T, Ohsuga M, Terada H, Katsuta Y, et al. Effects of the alpha‐/beta‐blocking agent carvedilol on hepatic and systemic hemodynamics in patients with cirrhosis and portal hypertension. Arzneimittelforschung 1997;47(4):353‐5. [PUBMED: 9150854] [PubMed] [Google Scholar]
Sinagra 2014
- Sinagra E, Perricone G, D'Amico M, Tine F, D'Amico G. Systematic review with meta‐analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Alimentary Pharmacology & Therapeutics 2014;39(6):557‐68. [PUBMED: 24461301] [DOI] [PubMed] [Google Scholar]
Stanley 1999
- Stanley AJ, Therapondos G, Helmy A, Hayes PC. Acute and chronic haemodynamic and renal effects of carvedilol in patients with cirrhosis. Journal of Hepatology 1999;30(3):479‐84. [DOI] [PubMed] [Google Scholar]
STATA [Computer program]
- Stata Corp, Texas, USA. STATA 13. Stata Corp, Texas, USA, 2007.
Suk 2014
- Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clinical and Molecular Hepatology 2014;20(1):6‐14. [CENTRAL: 3992331; DOI: 10.3350/cmh.2014.20.1.6.; PUBMED: 24757653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thabut 2007
- Thabut D, Hammer M, Cai Y, Carbonell N. Cost of treatment of oesophageal variceal bleeding in patients with cirrhosis in France: results of a French survey. European Journal of Gastroenterology & Hepatology 2007;19(8):679‐86. [PUBMED: 17625438] [DOI] [PubMed] [Google Scholar]
Thalheimer 2005
- Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Patch D, Burroughs AK. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Digestive and Liver Diseases 2005;37(8):601‐8. [PUBMED: 15908290] [DOI] [PubMed] [Google Scholar]
Thalheimer 2007
- Thalheimer U, Bosch J, Burroughs AK. How to prevent varices from bleeding: shades of grey‐‐the case for nonselective beta blockers. Gastroenterology 2007;133(6):2029‐36. [DOI: 10.1053/j.gastro.2007.10.028; PUBMED: 18054573] [DOI] [PubMed] [Google Scholar]
Tripathi 2002
- Tripathi D, Therapondos G, Lui HF, Stanley AJ, Hayes PC. Haemodynamic effects of acute and chronic administration of low‐dose carvedilol, a vasodilating beta‐blocker, in patients with cirrhosis and portal hypertension. Alimentary Pharmacology & Therapeutics 2002;16(3):373‐80. [PUBMED: 11876689] [DOI] [PubMed] [Google Scholar]
Tripathi 2010
- Tripathi D, Hayes PC. The role of carvedilol in the management of portal hypertension. European Journal of Gastroenterology & Hepatology 2010;22(8):905‐11. [PUBMED: 20093937] [DOI] [PubMed] [Google Scholar]
Tsochatzis 2009
- Tsochatzis EA, Triantos CK, Burroughs AK. Gastrointestinal bleeding: carvedilol‐the best beta‐blocker for primary prophylaxis?. Nature Reviews Gastroenterology and Hepatology 2009;6(12):692‐4. [PUBMED: 19946301] [DOI] [PubMed] [Google Scholar]
Turnes 2006
- Turnes J, Garcia‐Pagan JC, Abraldes JG, Hernandez‐Guerra M, Dell'Era A, Bosch J. Pharmacological reduction of portal pressure and long‐term risk of first variceal bleeding in patients with cirrhosis. American Journal of Gastroenterology 2006;101(3):506‐12. [PUBMED: 16542287] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(8):64‐75. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hobolth 2015
- Hobolth L, Bendtsen F, Gluud LL. Carvedilol versus non‐selective beta‐blockers for portal hypertension in cirrhosis. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011510] [DOI] [PMC free article] [PubMed] [Google Scholar]


