Abstract
Background
Antihypertensive drugs are often used in the belief that lowering blood pressure will prevent progression to more severe disease, and thereby improve pregnancy outcome. This Cochrane Review is an updated review, first published in 2001 and subsequently updated in 2007 and 2014.
Objectives
To assess the effects of antihypertensive drug treatments for women with mild to moderate hypertension during pregnancy.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (13 September 2017), and reference lists of retrieved studies.
Selection criteria
All randomised trials evaluating any antihypertensive drug treatment for mild to moderate hypertension during pregnancy, defined as systolic blood pressure 140 to 169 mmHg and/or diastolic blood pressure 90 to 109 mmHg. Comparisons were of one or more antihypertensive drug(s) with placebo, with no antihypertensive drug, or with another antihypertensive drug, and where treatment was planned to continue for at least seven days.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
For this update, we included 63 trials (data from 58 trials, 5909 women), with moderate to high risk of bias overall.
We carried out GRADE assessments for the main 'antihypertensive drug versus placebo/no antihypertensive drug' comparison only. Evidence was graded from very low to moderate certainty, with downgrading mainly due to design limitations and imprecision.For many outcomes, trials contributing data evaluated different hypertensive drugs; while we did not downgrade for this indirectness, results should be interpreted with caution.
Antihypertensive drug versus placebo/no antihypertensive drug (31 trials, 3485 women)
Primary outcomes: moderate‐certainty evidence suggests that use of antihypertensive drug(s) probably halves the risk of developing severe hypertension (risk ratio (RR) 0.49; 95% confidence interval (CI) 0.40 to 0.60; 20 trials, 2558 women), but may have little or no effect on the risk of proteinuria/pre‐eclampsia (average risk ratio (aRR) 0.92; 95% CI 0.75 to 1.14; 23 trials, 2851 women; low‐certainty evidence). Moderate‐certainty evidence also shows that antihypertensive drug(s) probably have little or no effect in the risk of total reported fetal or neonatal death (including miscarriage) (aRR 0.72; 95% CI 0.50 to 1.04; 29 trials, 3365 women), small‐for‐gestational‐age babies (aRR 0.96; 95% CI 0.78 to 1.18; 21 trials, 2686 babies) or preterm birth less than 37 weeks (aRR 0.96; 95% CI 0.83 to 1.12; 15 trials, 2141 women).
Secondary outcomes: we are uncertain of the effect of antihypertensive drug(s) on the risk of maternal death, severe pre‐eclampsia, or eclampsia, orimpaired long‐term growth and development of the baby in infancy and childhood, because the certainty of this evidence is very low. There may be little or no effect on the risk of changed/stopped drugs due to maternal side‐effects, or admission to neonatal or intensive care nursery (low‐certainty evidence). There is probably little or no difference in the risk of elective delivery (moderate‐certainty evidence).
Antihypertensive drug versus another antihypertensive drug (29 trials, 2774 women)
Primary outcomes: beta blockers and calcium channel blockers together in the meta‐analysis appear to be more effective than methyldopa in avoiding an episode of severe hypertension (RR 0.70; 95% CI 0.56 to 0.88; 11 trials, 638 women). There was also an increase in this risk when other antihypertensive drugs were compared with calcium channel blockers (RR 1.86; 95% CI 1.09 to 3.15; 5 trials, 223 women), but no evidence of a difference when methyldopa and calcium channel blockers together were compared with beta blockers (RR1.18, 95% CI 0.95 to 1.48; 10 trials, 692 women). No evidence of a difference in the risk of proteinuria/pre‐eclampsia was found when alternative drugs were compared with methyldopa (aRR 0.78; 95% CI 0.58 to 1.06; 11 trials, 997 women), with calcium channel blockers (aRR: 1.24, 95% CI 0.70 to 2.19; 5 trials, 375 women), or with beta blockers (aRR 1.21, 95% CI 0.88 to 1.67; 12 trials, 1107 women).
For the babies, we found no evidence of a difference in the risk oftotal reported fetal or neonatal death (including miscarriage) when comparing other antihypertensive drugs with methyldopa (aRR 0.77, 95% CI 0.52 to 1.14; 22 trials, 1791 babies), with calcium channel blockers (aRR 0.90, 95% CI 0.52 to 1.57; nine trials, 700 babies), or with beta blockers (aRR: 1.23, 95% CI 0.81 to 1.88; 19 trials, 1652 babies); nor in the risk for small‐for‐gestational age in the comparison with methyldopa (aRR 0.79, 95% CI 0.52 to 1.20; seven trials, 597 babies), with calcium channel blockers (aRR 1.05, 95% CI 0.64 to 1.73; four trials, 200 babies), or with beta blockers (average RR 1.13, 95% CI 0.80 to 1.60; 7 trials, 680 babies). No evidence of an overall difference among groups in the risk of preterm birth (less than 37 weeks) was found in the comparison with methyldopa (aRR: 0.91; 95% CI 0.68 to 1.22; 11 trials, 835 women), with calcium channel blockers (aRR 0.85, 95% CI 0.59 to 1.23; six trials, 330 women), or with beta blockers (aRR 1.22, 95% CI 0.90 to 1.66; 9 trials, 806 women).
Secondary outcomes: There were no cases of maternal death andeclampsia. There is no evidence of a difference in the risk of severe pre‐eclampsia, changed/stopped drug due to maternal side‐effects, elective delivery, admission to neonatal or intensive care nursery when other antihypertensive drugs are compared with methyldopa, calcium channel blockers or beta blockers. Impaired long‐term growth and development in infancy and childhood was not reported for these comparisons.
Authors' conclusions
Antihypertensive drug therapy for mild to moderate hypertension during pregnancy reduces the risk of severe hypertension. The effect on other clinically important outcomes remains unclear. If antihypertensive drugs are used, beta blockers and calcium channel blockers appear to be more effective than the alternatives for preventing severe hypertension. High‐quality large sample‐sized randomised controlled trials are required in order to provide reliable estimates of the benefits and adverse effects of antihypertensive treatment for mild to moderate hypertension for both mother and baby, as well as costs to the health services, women and their families.
Plain language summary
Antihypertensive drug therapy for mild to moderate hypertension during pregnancy
What is the issue?
The aim of this review was to determine the benefits and adverse effects of blood pressure‐lowering drugs (antihypertensive drugs) for pregnant women with mild to moderate hypertension (high blood pressure). The other aim was to assess the benefits and adverse effects of these drugs for their babies.
Why is this important?
During pregnancy, up to one in 10 women have blood pressure readings that are above normal. For some women, their blood pressure remains slightly high (termed ‘mild to moderate high blood pressure’), with no apparent complications. Some of these women go on to develop very high blood pressure. Very high blood pressure can result in a medical emergency if it affects the woman’s organs (such as her liver, or brain in the form of a stroke). Also, it can seriously affect the growth and health of her baby.
Drugs that lower blood pressure are used to treat mild to moderate high blood pressure, in the belief that this treatment will prevent the blood pressure from continuing to rise. Over the years, information from good quality research studies has been contradictory, so we cannot be sure if this drug treatment is worthwhile.
What evidence did we find?
This Cochrane Review is an update of a review that was first published in 2001 and updated in 2007 and 2014. We searched for randomised controlled trials in September 2017, and this review now includes data from 58 trials involving more than 5900 women. A total of 31 trials with 3485 women compared a number of different blood pressure‐lowering drugs to a placebo or no treatment. There were a further 29 trials involving 2774 women which compared one blood pressure‐lowering drug with another one.
The evidence showed that treating pregnant women who had moderately raised blood pressure with blood pressure‐lowering drugs probably halved the number of the women developing severe high blood pressure (20 trials, 2558 women). However, blood pressure‐lowering drugs probably had little or no effect on the risk of the baby dying (29 trials, 3365 women), and there is insufficient data on maternal deaths to make a judgement on whether this risk is lowered (five trials, 525 women).
The use of blood pressure‐lowering drugs may have little or no effect on the number of the women developing pre‐eclampsia (23 trials, 2851 women), or the number of women who had to change drugs because of side‐effects (16 trials, 1503 women).
We found no difference in the number of babies born preterm, that is before 37 weeks (15 trials, 2141 women). There was also no difference in the number of babies born small for their gestational age (21 trials, 2686 babies).
The quality of the evidence was mostly moderate (but for pre‐eclampsia it was low). This was due to a number of small studies, and problems with the way the studies were undertaken.
The available evidence is still insufficient to demonstrate if there is any antihypertensive drug that is better than another. However, beta blockers and calcium channel blockers seem to be better than the alternative drugs for blood pressure control.
What does this mean?
More research is needed in order to confirm the true effect of antihypertensive drugs in mothers and in their babies, and to identify the drug which would be best.
Summary of findings
Background
Description of the condition
Hypertension during pregnancy is common. One in 10 women will have high blood pressure at some time before delivery, and pre‐eclampsia complicates between 2% to 8% of all pregnancies worldwide (Abalos 2013). Hypertensive disorders of pregnancy, particularly pre‐eclampsia and eclampsia, constitute important causes of severe acute morbidity, long‐term disability and death among mothers and babies (Abalos 2014a; Khan 2006). Pre‐eclampsia is discussed in more detail in the generic protocol of interventions for prevention of pre‐eclampsia (Meher 2005).
There is a general consensus about the classification of hypertensive disorders during pregnancy (NHBPEP 2000) considering four broad categories: (a) gestational hypertension or pregnancy‐induced hypertension, which is hypertension newly diagnosed after 20 weeks of gestation without proteinuria; (b) pre‐eclampsia, which is hypertension developed after 20 weeks of gestation with proteinuria; (c) chronic hypertension, or essential hypertension, which is pre‐existing hypertension; and (d) chronic hypertension with superimposed pre‐eclampsia. Recently, the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy extended the diagnosis of pre‐eclampsia to those cases in which hypertension, in the absence of significant proteinuria, is associated with other systemic findings such as thrombocytopenia, worsening liver or renal function, pulmonary oedema or new‐onset cerebral or visual disturbances (ACOG Task Force 2013).
Moderate hypertension is defined as systolic blood pressure of 140 mmHg or more, and/or diastolic blood pressure of 90 mmHg or above on two consecutive occasions at least four hours apart. Severe hypertension is defined as systolic blood pressure of 160 mmHg or 170 mmHg and/or diastolic blood pressure of 110 mmHg or more two consecutive occasions up to 15 minutes apart (ACOG Task Force 2013; Canadian HDP Working Group 2014; NHBPEP 2000). Korotkoff phase V (disappearance of sounds) is now widely recommended as more reliable than phase IV (muffling) for measuring diastolic blood pressure (Canadian HDP Working Group 2014).
For this review we have accepted broad and pragmatic criteria for identifying women with mild to moderate hypertension during pregnancy. This reflects clinical practice, and is justifiable in the context of randomised trials as within each study the same criteria will have been used for women in both groups.
Description of the intervention
A wide variety of drugs have been advocated for lowering blood pressure in pregnant women with hypertension, and each group has different potential side‐effects and adverse events. In this review we evaluate individual agents within the class or family to which they belong, as each class has a similar mechanism of action.
How the intervention might work
Alpha agonists: inhibit vasoconstriction via a centrally mediated effect (Ingenito 1970). Methyldopa is the most widely used alpha agonist, and became available in 1963. Clonidine is also an alpha agonist, although it has the disadvantage that sudden withdrawal may cause a hypertensive crisis (Isaac 1980). Common side‐effects of methyldopa include dizziness, lightheadedness, drowsiness, headache, stuffy nose, and weakness, especially when starting this medication and when dosage is increased. Other side‐effects include swelling, muscle pain, dry mouth, swollen or "black" tongue, gastrointestinal symptoms and depression (depressed mood, unusual thoughts, and nightmares).
Beta blockers: beta‐adrenoceptor blocking drugs block adrenoceptors in the heart, peripheral blood vessels, airways, pancreas and liver (Frishman 1979). Labetalol has an additional arteriolar vasodilating action that lowers peripheral resistance, but is usually classified with the beta blockers. Side‐effects of beta blockers include oedema, postural hypotension, bradycardia, cold or cyanotic extremities, rashes, masking of the normal response to hypoglycaemia (sweating and tachycardia), nausea, dyspepsia, vomiting, difficulty in micturition (including acute urinary bladder retention, dizziness, headache, taste distortion, vertigo, and paraesthesia.
Calcium channel blockers: include amlodipine, isradipine, nifedipine, nicardipine, nimodipine and verapamil. These drugs inhibit influx of calcium ions to vascular smooth muscle resulting in arterial vasodilatation (Robinson 1980). Common side‐effects of calcium channel blockers include dizziness, giddiness, lightheadedness, headaches, heat sensation, weakness, flushing, palpitations, transient hypotension, heartburn, nausea, dyspnoea, nasal congestion, and muscle cramps.
Peripheral vasodilators: hydralazine is a vasodilator with a direct relaxing effect on smooth muscle in the blood vessels, predominantly in the arterioles (Stunkard 1954). The most frequently reported side‐effects are palpitations and tachycardia. Other side‐effects include flushing, hypotension, nausea, vomiting, diarrhoea, gastrointestinal disturbances, headache, arthralgia, joint swelling, myalgia, and anorexia.
Serotonin receptor antagonists: ketanserin is a selective serotonin receptor antagonist with weak adrenergic receptor blocking properties (Frishman 1995). The drug is effective in lowering blood pressure in essential hypertension. It also inhibits platelet aggregation. Side‐effects of ketanserin includes dizziness, headache, drowsiness, fatigue, dry mouth, sedation, lightheadedness, lack of concentration, gastrointestinal disturbances. Rare but serious side‐effects includes ventricular tachycardia.
Nitric‐oxid donors: glyceryl trinitrate is a nitric oxide donor with vasodilator effect in perivascular smooth‐muscle cells (Seligman 1994). Side‐effects include chest pain, hypoxaemia, difficulty breathing, cyanosis, tachycardia, throbbing headache, spinning sensation, postural hypotension, dizziness, drowsiness, and weakness.
Phosphodiesterase inhibitors: sildenafil (usually associated with treatment of erectile dysfunction in men) has been attracting the attention of clinicians and researchers. This is a phosphodiesterase type 5 inhibitor that increases intracellular cyclic guanosine monophosphate (cGMP) in the vascular smooth muscle, resulting in vasodilatation (Wareing 2004). The most common adverse reactions reported in clinical trials are headache, flushing, dyspepsia, abnormal vision, nasal congestion, back pain, myalgia, nausea, dizziness, and rash.
Why it is important to do this review
The role of antihypertensive therapy for pregnant women with mild to moderate hypertension is unclear. As there is no immediate need to lower mild to moderate rises in blood pressure, the rationale for treatment is that it will prevent or delay progression to more severe disease, thereby benefiting the woman or her baby, or both, and reducing consumption of health service resources. As well as reducing blood pressure, the belief has been that these drugs reduce the risk of preterm delivery and placental abruption and improve fetal growth. The aim of this review is to assess the potential benefits and hazards, to the woman and baby, of antihypertensive drugs for the treatment of mild to moderate hypertension during pregnancy. If antihypertensive agents are overall beneficial, a secondary aim will be to assess the comparative effects of alternative agents.
There are other types of interventions for women with mild to moderate hypertension during pregnancy that are not considered in this review. Interventions covered by other reviews include salt restriction (Duley 1999), antiplatelet agents (Duley 2007), abdominal decompression (Hofmeyr 2012), and bed rest, with or without hospitalisation (Meher 2005a). The role of diuretics for women with hypertension during pregnancy is covered by a separate Cochrane review (Churchill 2007), as is prevention and treatment of postpartum hypertension (Magee 2013).
For women with severe hypertension, usually defined as 170 mmHg or more systolic blood pressure or 110 mmHg or more diastolic blood pressure, there is a risk of direct arterial damage and so antihypertensive drugs are used to lower blood pressure (Gifford 1990; Redman 1993). The question of which drug is best in this situation is considered in another Cochrane review and not discussed further here (Duley 2013).
A separate review assessing the effect of alternative oral beta blocker regimens in mild to moderate hypertension during pregnancy is underway. Beta blockers are included in this review as part of all the spectrum of antihypertensive drugs.
Objectives
To determine the possible benefits, risks and side‐effects of antihypertensive drug treatments for women with mild to moderate hypertension during pregnancy (defined whenever possible as a systolic blood pressure of 140 to 169 mmHg or diastolic blood pressure of 90 to 109 mmHg, or both). Also, to compare the differential effects of alternative drug regimens.
The comparisons are of:
any antihypertensive drug with either no drug or placebo;
one antihypertensive drug compared with another. For this review, the commonly used group of drugs are regarded as controls and compared with all other group of drugs (for example, other antihypertensives versus methyldopa, other antihypertensives versus calcium channel blockers, and other antihypertensives versus beta blockers).
Methods
Criteria for considering studies for this review
Types of studies
All cluster‐ and‐ individually‐randomised trials evaluating any antihypertensive drug treatment for mild to moderate hypertension during pregnancy. Quasi‐randomised trials were excluded. Cross‐over trials were not eligible for inclusion.
Types of participants
The review includes women with mild to moderate hypertension during pregnancy, defined whenever possible as those with systolic blood pressure 140 to 169 mmHg and/or diastolic blood pressure 90 to 109 mmHg. Studies in which participants were described as having 'mild to moderate' hypertension but the range of blood pressures was not clearly specified were also included. Women were included regardless of whether they had proteinuria or not, and irrespective of previous antihypertensive treatment or whether the pregnancy was singleton or multiple.
Women who had given birth before trial entry were excluded, as were women with severe hypertension (defined whenever possible as either systolic blood pressure of 170 mmHg or more, or diastolic blood pressure 110 mmHg or more). Studies that included a substantial proportion of women who did not have mild to moderate hypertension were excluded, unless data were available on outcomes for those with mild to moderate hypertension only.
Types of interventions
Any comparison of one or more antihypertensive drug with either placebo or no antihypertensive drug was included, as were comparisons of one antihypertensive drug with another. Studies were excluded if the intention was to treat for less than seven days, as a longer period of treatment would be necessary for any substantive clinical effect. Comparisons of two drugs of the same class were also excluded, although these may be included in future updates if clinically relevant. Drugs that aimed to reduce the risk of pregnancy‐induced hypertension progressing to pre‐eclampsia but are not antihypertensive agents were also excluded.
Types of outcome measures
Primary outcomes
Main outcomes were pre‐specified as follows.
Severe hypertension: defined whenever possible as either systolic blood pressure 170 mmHg or more, or diastolic blood pressure 110 mmHg or more. Trials where the definition of severe hypertension was not clear, or where the cut‐off was up to 10 mmHg lower were also included and were clearly documented.
Proteinuria/pre‐eclampsia: defined whenever possible as new proteinuria (1+ or more or 300 mg/24 hours).
Total reported fetal or neonatal death (including miscarriage): fetal deaths included miscarriage (fetal losses before viability, usually taken as 20 or 24 weeks) and stillbirths (after 24 weeks, or however defined). Perinatal deaths are stillbirths plus deaths in the first week of life. Neonatal deaths are deaths in the first 28 days.
Small‐for‐gestational age: low birthweight for gestational age, below the third, fifth or 10th percentile, using the most severe reported.
Preterm birth: all births before 37 completed weeks.
Secondary outcomes
For the woman
Maternal death.
Severe pre‐eclampsia: defined whenever possible as severe hypertension with proteinuria 2+ or more, or 2 g or more/24 hours, with or without other signs of symptoms, or as moderate hypertension with proteinuria 3+ or more. Haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome is a form of severe pre‐eclampsia and was therefore included here as well as a separate measure. Trials reporting imminent eclampsia, or where the definition of severe pre‐eclampsia was not clear were also included.
Eclampsia.
HELLP syndrome.
Severe maternal morbidity: such as liver or renal failure, disseminated intravascular coagulation and cerebrovascular accident (stroke).
Need for additional antihypertensive drug/s to control blood pressure.
Miscarriage (fetal losses before viability, usually taken as before 20 or 24 weeks).
Elective delivery: combines elective caesarean sections and elective induction of labour at term or before term.
Caesarean section.
Antenatal hospital admission and length of stay more than seven days: hospital and day care units were to be reported separately.
Placental abruption.
Side‐effects: any reported side‐effects or severe adverse events.
Changed/stopped drug due to maternal side‐effects.
For the baby
Very low, less than four, five‐minute Apgar score.
Severe prematurity, all births less than 32 or less than 34 weeks' gestation.
Admission to neonatal or intensive care nursery.
Respiratory distress syndrome.
Other morbidity possibly related to maternal drug therapy, such as hypo‐ or hypertension, hypoglycaemia and bradycardia (with beta blockers).
Impaired long‐term growth and development in infancy and childhood.
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (13 September 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (13 September 2017) for unpublished, planned and ongoing trial reports (See: Appendix 1)
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeAbalos 2014.
For this update, the following methods were used for assessing the 45 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison ‐ any hypertensive drug versus no antihypertensive drugs/placebo.
Primary outcomes
Severe hypertension
Proteinuria/pre‐eclampsia
Total reported fetal or neonatal (including miscarriage)
Small‐for‐gestational age
Preterm birth
Secondary outcomes
Maternal
Maternal death
Severe pre‐eclampsia
Eclampsia
Elective delivery
Changed/stopped drug due to maternal side‐effects
Fetal/infant outcomes
Admission to neonatal or intensive care nursery
Follow‐up of the children at one year: cerebral palsy
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Although there were no cluster‐randomised trials identified to date for inclusion, we will include them in future updates in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook [Section 16.3.4] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
Dealing with more than two intervention groups
For multi‐arm studies, all intervention arms were mentioned and described in the Characteristics of included studies table, including the number of women randomised to each arm. Appropriate pair‐wise comparisons were selected for the meta‐analysis in order to avoid double‐counting of one of the arms. For example, when two different antihypertensive drugs were compared with placebo, the active drug groups were combined into one arm for the comparison antihypertensive drugs versus no antihypertensive drugs/placebo. These two arms, were included separately in the meta‐analysis of alternative regimens.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
For the comparison of antihypertensive drug/s with placebo or no treatment the following subgroup analyses were pre‐specified:
by class of drug (such as alpha agonists, beta blockers and calcium channel blockers);
by type of hypertensive disorder at trial entry: mild to moderate hypertension alone; mild to moderate hypertension with proteinuria; chronic hypertension; unspecified;
by gestational age at trial entry: less than about 32 weeks' gestation; about 32 weeks or more gestation; or unclassified/mixed;
by whether a placebo was used: placebo, no placebo.
The subgroup analysis by type of drug is presented for all outcomes. The remaining subgroups are presented for the pre‐specified primary outcomes only.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not conducted sensitivity analyses. In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies (high and unclear risk of bias) being excluded from the analyses in order to assess whether this makes any difference to the overall result. If future updates include cluster‐randomised trials, we will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
Results
Description of studies
Results of the search
See: Figure 1.
1.
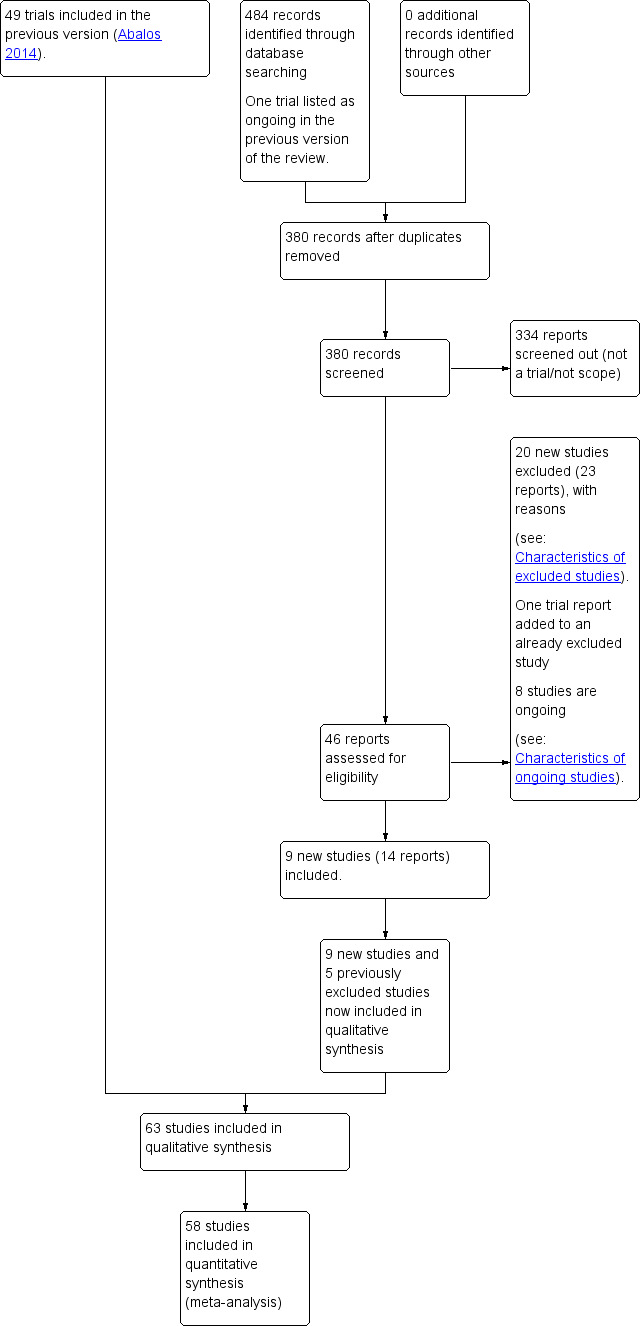
Study flow diagram.
We retrieved 45 trial reports to assess from the updated search in September 2017 and we also reassessed one ongoing trial listed in the previous version of the review. We included nine new trials (14 reports), and five previously excluded studies in the qualitative synthesis and excluded 20 (23 reports) and added an additional reference to an already excluded study. Eight trials are ongoing.
Included studies
In total, 63 studies were included in this review. From these, 58 trials (5909 women) contributed data: 36 trials (3629 women) were conducted in high‐income countries (Australia, France, Hong Kong, Ireland, Israel, Italy, Sweden, UK and USA), and 22 trials (2280 women) in middle‐ or low‐income countries (Argentina, Brazil, Caribbean Islands, India, Pakistan, Panama, South Africa, Sudan and Venezuela).
Methods and trial dates
Three trials (405 women) were published in the 1960s and 1970s, 22 (1854 women) in the 1980s, 17 (1718 women) in the 1990s, and 16 (1927 women) after the year 2000. All included trials are small. The largest study recruited 314 women. Only six studies had comparison arms containing more than 100 women. Two three‐arm trials of methyldopa versus labetalol versus no treatment (India 2012; USA 1990a) were included in the comparison of any antihypertensive with placebo/no antihypertensive, and in the comparison of one drug with another. One three‐arm trial of nifedipine versus methyldopa versus labetalol (India 2015a), was included in the comparison one drug versus another. Another three‐arm trial of furosemide versus amlodipine versus aspirin (Panama 2014), was only included in the comparison one drug versus another (furosemide versus amlodipine), as aspirin is not an antihypertensive and was only prescribed in the third arm.
Funding sources
Six of the included trials reported to be funded by universities or non commercial organisations; 14 trials were funded by industry and two trials stated to have no funding source. The remaining trials provided no information about funding.
Trials authors' declaration of interest
Authors of UK 2017 declared that "C. Nelson‐Piercy reports personal fees from Alliance Pharmaceuticals, UCB Pharmaceuticals, LEO Pharmaceuticals, Sanofi Aventis, and Warner Chilcott outside the submitted work. J.K. Cruickshank is current President of the Artery Society which has had donations from Servier Pharmaceuticals" and that "the other authors report no conflicts". Authors of Brazil 2016, India 2012, India 2013c, India 2015a, India 2015b, Panama 2014 and UK 2009 declared that they had no conflicts of interest. Of the remaining studies, none included any declarations of interest.
Interventions and comparisons
The antihypertensive drugs used in these trials include: alpha agonists (methyldopa), beta blockers (acebutolol, atenolol, labetalol, mepindolol, metoprolol, pindolol, oxprenolol and propranolol), calcium channel blockers (amlodipine, isradipine, nicardipine, nifedipine, nimodipine and verapamil), vasodilators (hydralazine and prazozin), ketanserin, glyceryl trinitrate (GTN), furosemide and sildenafil. All drugs were given orally, except glyceryl trinitrate, which was given transdermally. The dose for several agents varied considerably between studies, in both amount and duration of therapy.
The antihypertensive drug was compared with placebo, or no antihypertensive drug, in 31 trials (3480 women). Of these trials, 17 evaluated beta blockers (1902 women), seven using a placebo for the control group and 10 comparing with no treatment. Methyldopa was evaluated in eight trials (986 women), one comparison with placebo, and seven with no antihypertensive treatment. One trial (118 women) compared isradipine with placebo, another trial (199 women) compared verapamil with placebo, and three studies (583 women) compared nifedipine with no drug treatment. Prazozin was compared with placebo in one trial (32 women), and GTN was compared with placebo patches in another (16 women). Two newly included trials (Brazil 2016; UK 2009) compared sildenafil with placebo in 130 women.
Alternative drug regimens were compared in 30 trials (3093 women). Twenty‐five of these studies compared methyldopa with another agent. In 19 trials (2041 women) the comparison was with beta blockers, in four it was nifedipine (389 women), in one (111 women) it was nimodipine, and in another ketanserin (20 women). Two trials (354 women) compared labetalol versus nifedipine. One small trial (36 women) compared nifedipine with GTN. In one study (100 women), metoprolol was compared with nicardipine and in another (42 women) furosemide was compared with amlodipine (the aspirin arm of this study was not considered for this review).
Gestation at trial entry
Twenty of the 58 included studies contributing to data recruited women during the second and third trimester of pregnancy, and 21 recruited only during the third trimester. Eight studies recruited women during the first and second trimester. Gestational age at trial entry was not reported in nine studies.
Severity and type of hypertension disease at trial entry
Mild to moderate hypertension was defined as a diastolic blood pressure of 90 mmHg or more in 42 studies. In five trials, the definition was 95 mmHg or more and in four it was 100 mmHg or more. In two trials, the cut‐off was 85 mmHg, In the remaining five studies, authors merely stated 'mild to moderate hypertension', or 'pregnancy‐induced hypertension', or 'diagnosed hypertension'. Women with proteinuria were excluded from trial entry in 19 studies, whilst in eight trials all women had proteinuria at recruitment. Fourteen trials included women regardless of whether or not they had proteinuria, and the proportion of women with proteinuria ranged from 4% to 47%. In the remaining 17 trials the presence of proteinuria at trial entry was not reported.
Ten studies only recruited women with chronic hypertension. Women with chronic hypertension were excluded from 22 trials. Ten trials included women regardless of whether or not they had chronic hypertension, although outcomes were often not reported separately. In the remaining 16 trials, chronic hypertension at trial entry was not mentioned.
Methods for measuring blood pressure
Only four trials masked the assessment of blood pressure by using a random zero sphygmomanometer (Australia 1983; UK 1976; UK 1983a; UK 1983b). For assessment of diastolic blood pressure, Korotkoff phase IV sound was used in 16 trials and Korotkoff phase V was used for 10 studies. Criteria for blood pressure measurement were not mentioned in 32 trials.
Definition of small‐for‐gestational age
Small‐for‐gestational age was defined in a variety of ways in the 34 trials reporting this outcome. Five studies used birthweight below the fifth centile and 14 below the 10th centile. Four trials used other definitions, and in the remaining 11 trials, small‐for‐gestational age was not defined.
One outcome specified in our protocol, very low (less than four) five‐minute Apgar score, is not included in this review as it was not reported by any trial.
When pooling the data, and in the absence of statistical heterogeneity, we used fixed‐effect meta‐analysis only for the outcome severe hypertension with the assumption that all drugs are expected to lower blood pressure. For the other outcomes, we used random‐effects meta‐analysis as different drugs' effects might vary due to their different mechanisms of action, dosages, length of treatment, etc.
Excluded studies
Ninety‐six studies were excluded from the review. Of these, 41 were conducted in high‐income countries (Australia, Belgium, Denmark, Finland, France, Germany, Israel, Italy, Japan, Netherlands, Sweden, UK and USA), and 55 in low‐ and middle‐income countries (Argentina, Brazil, China, Cuba, Czech Republic, Dominican Republic, Egypt, Hungary, India, Iran, Kuwait, Mexico, Pakistan, Panama, Philippines, Russia, Singapore, Slovakia, South Africa, Sri Lanka, Uganda and Venezuela). The oldest excluded study was published 1957, one was published in 1978, 54 were published in the 1980s and 1990s, and 42 have been published since the millennium.
The language of publication was English (for 78 papers), Chinese (six), Spanish (five), Portuguese (three), Czech (two), French (one), and Russian (one). Language was not a reason for exclusion. Twenty‐six papers (21/101) were published only as congress abstracts, and 13 are protocols registered in ClinicalTrials.Gov or similar pages. They were excluded as the intervention is not eligible (i.e. severe hypertension, prevention trial in high‐risk women, etc). Three studies were excluded after personal communication with authors. Authors of 14 papers provided additional information about methods and/or clinical outcomes. The reasons for exclusions were as follows.
Methodological problems (25 studies): either they were not randomised trials (12 studies) or they used quasi‐random methods for treatment allocation (nine studies), or more than 20% of women were excluded after randomisation (four studies).
Participants were not eligible for the review (18 studies): either some or all of the women had severe hypertension (nine studies), or some women had normal blood pressure, and data were not presented separately for the women with mild to moderate hypertension, or all women were at high risk of developing hypertension in a prophylactic trial (eight studies), or the intervention was administered postpartum (one).
Intervention was not eligible for the review (45 studies): either the comparison was of drugs within the same class (eight studies), or the allocated intervention was for less than seven days (15 trials), or it was not an antihypertensive drug (21) or the study had a mixed control group (one).
No clinical outcomes measured (eight studies): there are no data on clinical outcomes (three congress abstracts, of which authors were contacted but with no responses to date). Three reports were personal communications of planned randomised controlled trials, but no information could be found about their completion.
Risk of bias in included studies
Overall, the quality of the studies included in this review is moderate to poor (Figure 2; Figure 3).
2.
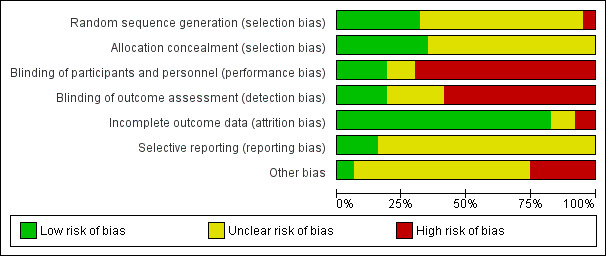
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
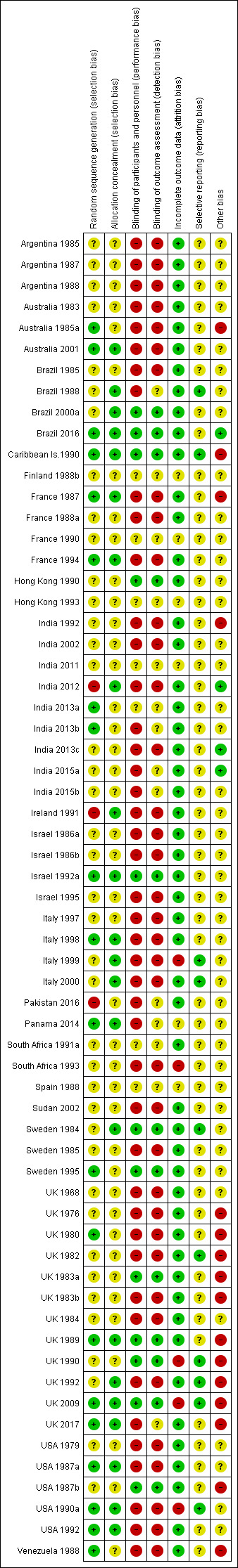
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Methods for generating the random sequence were described in 23 trials out of 63 (36.5%).
We assessed these trials as being at a low risk of bias ‐ methods included computer generation (Australia 2001, France 1994; India 2013a; Italy 1998; Panama 2014; UK 2009; UK 2017; USA 1987a; USA 1990a; USA 1992), random‐number tables (UK 1980; UK 1989; Venezuela 1988), series of random numbers (Australia 1985a; India 2013b; Israel 1992a). Although three studies did not describe the method used for allocation, the authors mentioned that it was in blocks of five (Brazil 2016), six (Sweden 1995), 10 (Caribbean Is.1990), or nine at each centre (France 1987).
We assessed three trials as being at a high risk of bias due to inadequate methods, namely "mixed‐up opaque envelopes" (India 2012), "lottery method" (Pakistan 2016) and "cards shuffled into a random order and numbered in sequence" (Ireland 1991). See Figure 2, Figure 3.
Methods for generating the random sequence were not described in the remaining 40 trials.
Allocation concealment
Concealment of allocation was adequate for only 22 of the 63 trials (34.9%), being unclear whether concealment was adequate in the remaining 41 trials. Methods for concealing the allocation included on‐line computer system (UK 2017), telephone randomisation (Australia 2001; Italy 1998; Sweden 1984; UK 2009), blinded treatment packs (Brazil 1988; Brazil 2000a; Caribbean Is.1990; Israel 1992a; Italy 2000; UK 1989), consecutive, sealed identical envelopes (UK 1992) or blinded or sealed envelopes (Brazil 2016; France 1987; France 1994; India 2012; Ireland 1991; Italy 1999; Panama 2014; USA 1987a; USA 1990a; USA 1992), or just "envelopes" (South Africa 1991a, Sweden 1985; UK 1982). Most trials with unclear concealment of allocation were described as 'randomised' with no details on how this was achieved. Some of these studies were stated to be double blind, but with no information about how this was achieved. Three trials with unclear concealment used random‐number tables without mentioning any attempt to conceal the allocation (Sweden 1995; UK 1980; Venezuela 1988). See Figure 2, Figure 3.
Blinding
Performance bias
From 31 trials evaluating whether or not hypertension should be treated with antihypertensives, only 14 (45%) compared the active drug with placebo, 12 of them were described by authors as double‐blind (Brazil 2000a; Brazil 2016; Caribbean Is.1990; Hong Kong 1990; Israel 1992a; Sweden 1984; Sweden 1995; UK 1983a; UK 1989; UK 1990; UK 2009; USA 1987b). One placebo‐controlled trial was reported as single‐blind (Australia 2001), and another, even though placebo‐controlled, did not mention whether it was single‐ or double‐blinded (South Africa 1991a). See Figure 2, Figure 3
Detection bias
All trials evaluating alternative treatments for mild/moderate hypertension during pregnancy were open‐label trials, and no attempts were made to blind outcome assessment in order to prevent detection bias. See Figure 2, Figure 3
Incomplete outcome data
As per protocol, trials were excluded from the review (or a particular outcome was excluded from the analysis) if it was not possible to enter data on an intention‐to‐treat basis, or lf 20% or more participants were excluded or withdrawn. We also downgraded the score of a trial to a high risk of bias status if more than 10% of randomised women were withdrawn from the analysis. Most trials reported the outcomes for all (or nearly all) women randomised (low risk of bias), although only six trials described the women who met the study eligibility criteria, but were not randomised (Sweden 1984; Sweden 1985; UK 1976; UK 1983a; UK 2009; UK 2017). Five trials reported losses greater than 10%: Italy 1999 (17%), South Africa 1993 (10.3%), UK 1990 (12%), UK 2009 (10.3%) and USA 1990a (12%), with high risk of bias. See Figure 2, Figure 3.
Selective reporting
Thirty‐three trials (52%) reported on the risk of a woman for developing severe hypertension (one of the primary outcomes of this review). However, most trials evaluated the effect that the antihypertensive under scrutiny may have on blood pressure (mean blood pressure after treatment, % fall in blood pressure, etc.). Proteinuria (either new proteinuria or the aggravation of previously existing) was reported in 46 trials. Total reported fetal or neonatal deaths were evaluated in 48 trials, preterm birth in 25 and small‐for‐gestational‐age babies in 28. Most analyses were carried out using data from published reports. Authors were contacted for unpublished information related to the methods and the main outcomes; we obtained data from Australia 2001; Brazil 1988; Brazil 2000a; Caribbean Is.1990; Ireland 1991; Italy 1999; Italy 2000; Sweden 1984; Sweden 1995; UK 1982; UK 1990; UK 1992; USA 1990a. See Figure 2, Figure 3,
Funnel plots were assessed, see Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12; Figure 13; Figure 14; Figure 15; Figure 16; Figure 17; Figure 18; Figure 19; Figure 20; Figure 21; Figure 22; Figure 23; Figure 24; Figure 25.
4.
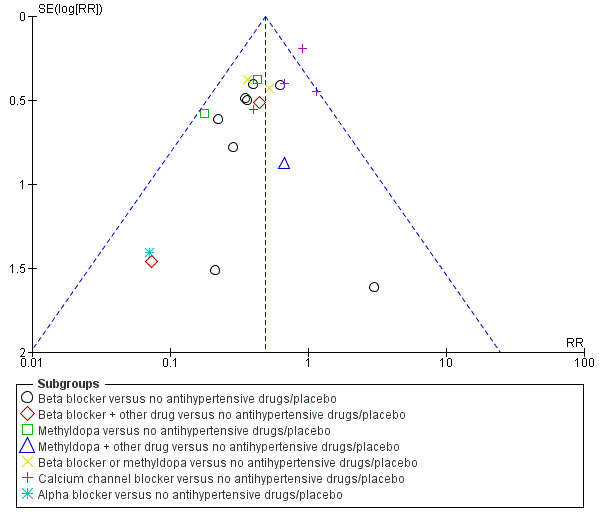
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.1 Severe hypertension.
5.
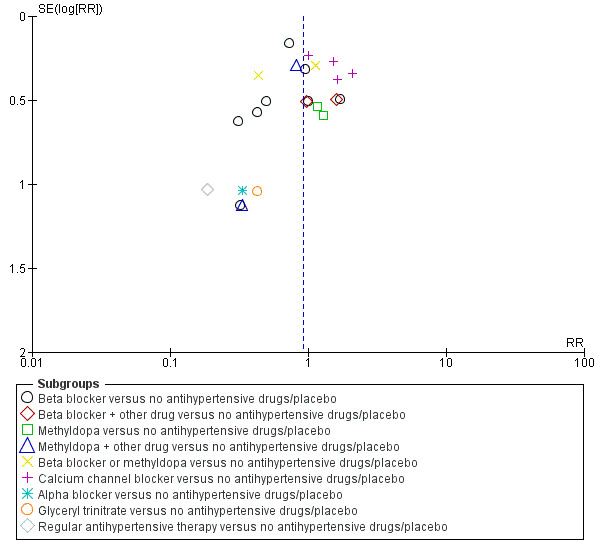
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.2 Proteinuria/pre‐eclampsia.
6.
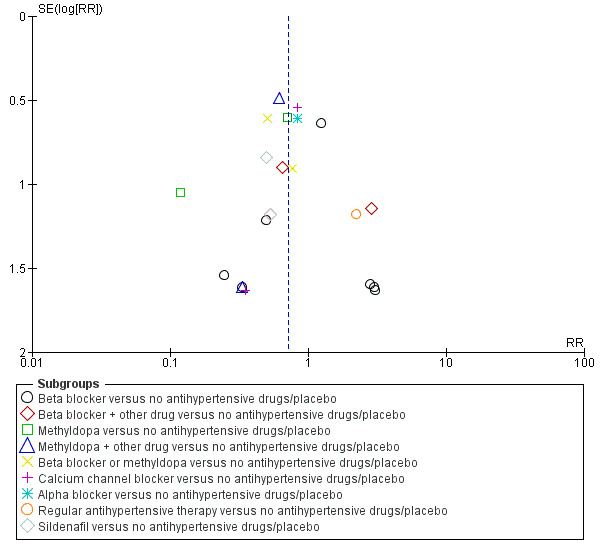
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.3 Total reported fetal or neonatal death (including miscarriage).
7.
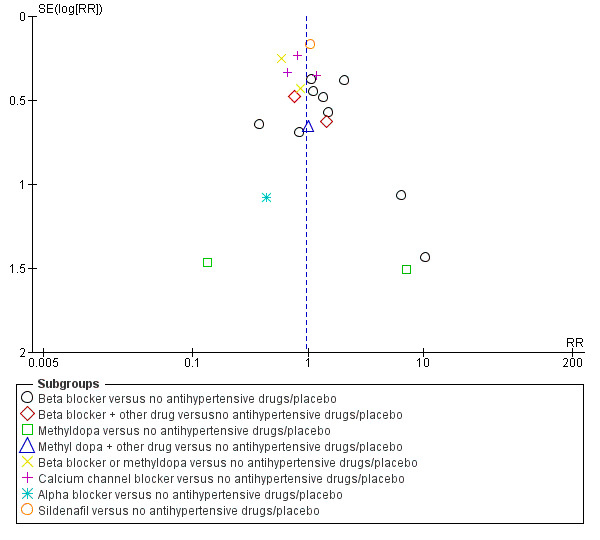
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.5 Small‐for‐gestational age.
8.
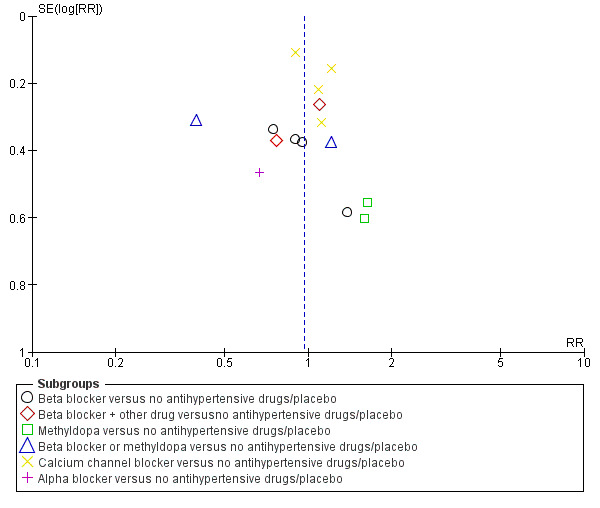
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.7 Preterm birth (< 37 weeks).
9.
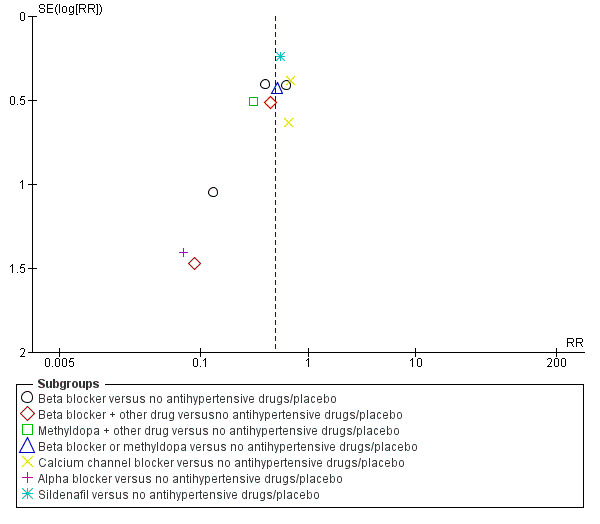
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.14 Need for additional antihypertensive drug/s.
10.
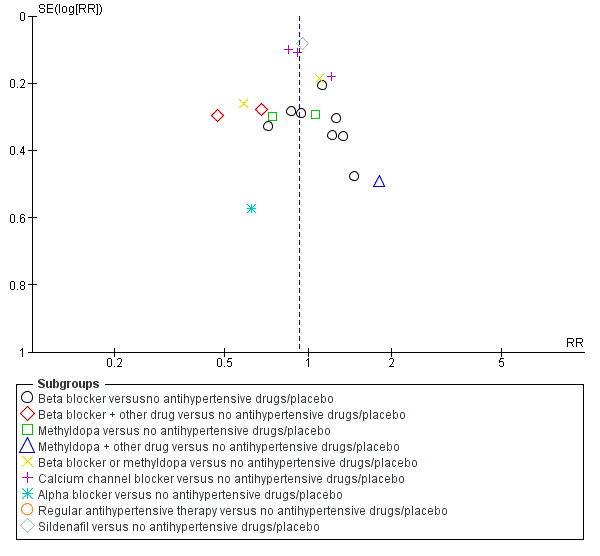
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.16 Caesarean section.
11.
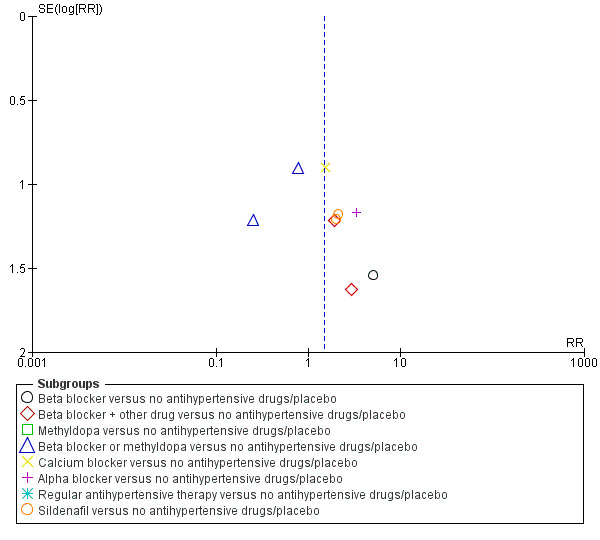
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.19 Placental abruption.
12.
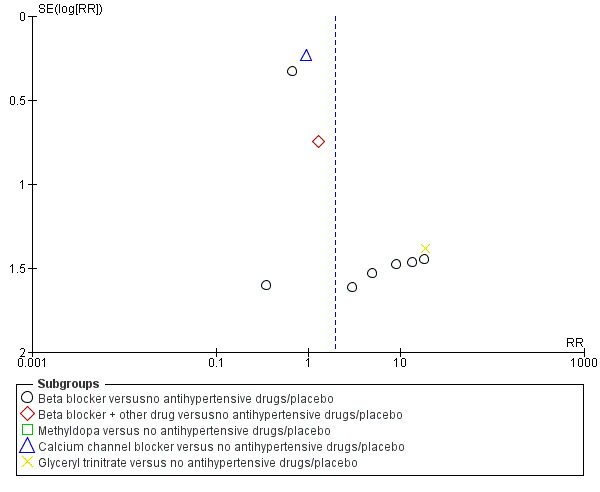
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.20 Maternal side‐effects.
13.
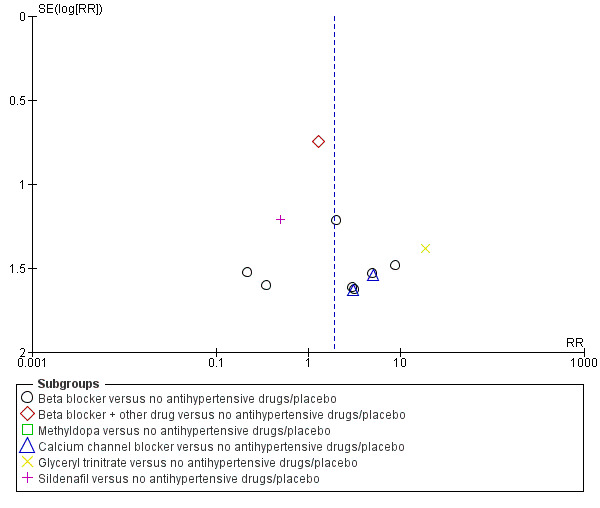
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.21 Changed/stopped drugs due to maternal side‐effects.
14.
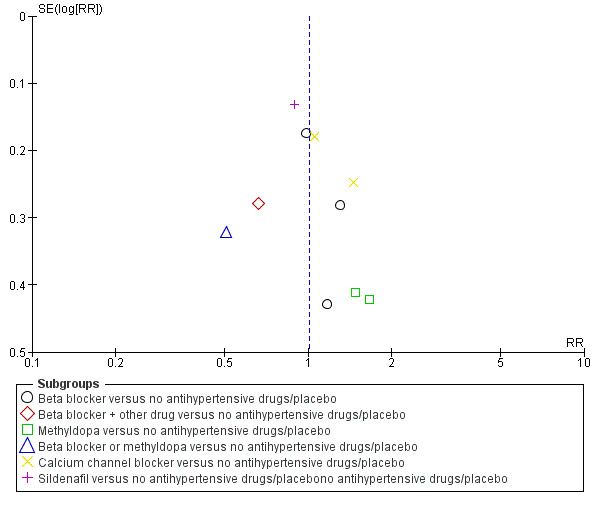
Funnel plot of comparison: 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), outcome: 1.22 Admission to neonatal or intensive care nursery.
15.
Funnel plot of comparison: 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), outcome: 5.1 Severe hypertension.
16.
Funnel plot of comparison: 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), outcome: 5.2 Proteinuria/pre‐eclampsia.
17.
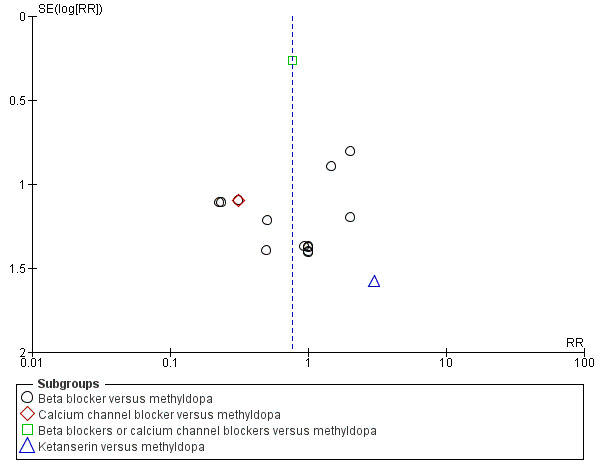
Funnel plot of comparison: 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), outcome: 5.3 Total reported fetal or neonatal death (including miscarriage).
18.
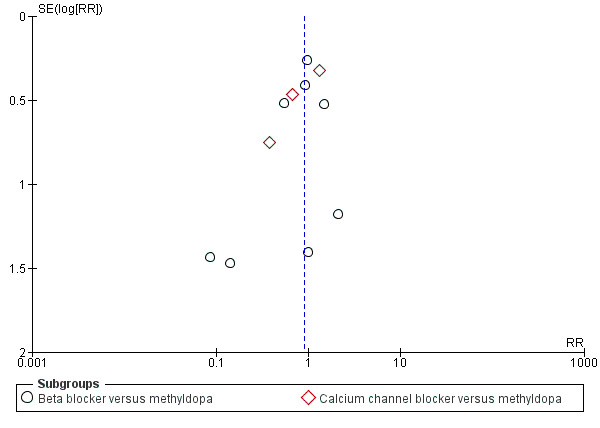
Funnel plot of comparison: 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), outcome: 5.5 Preterm birth (< 37 weeks).
19.
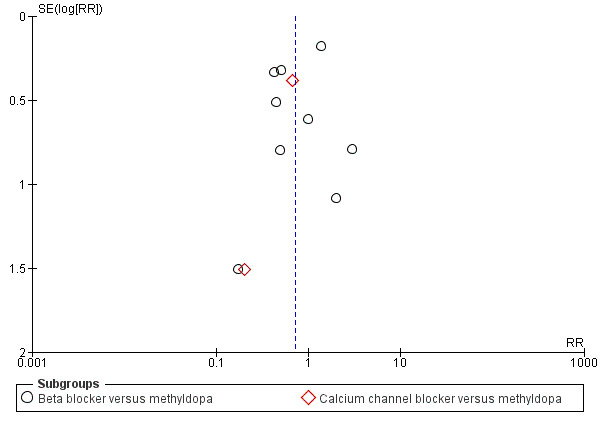
Funnel plot of comparison: 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), outcome: 5.8 Need for additional antihypertensive drug/s.
20.
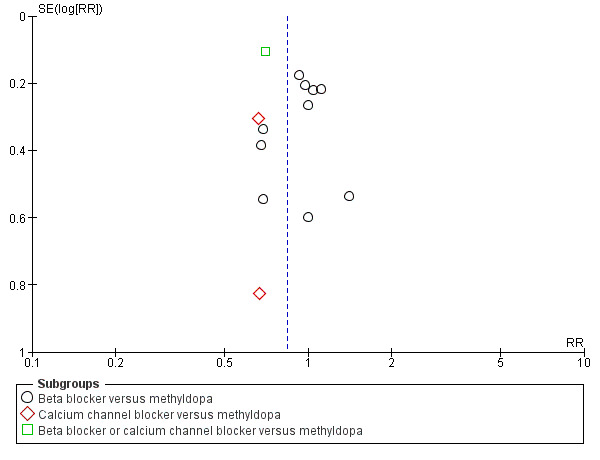
Funnel plot of comparison: 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), outcome: 5.10 Caesarean section.
21.
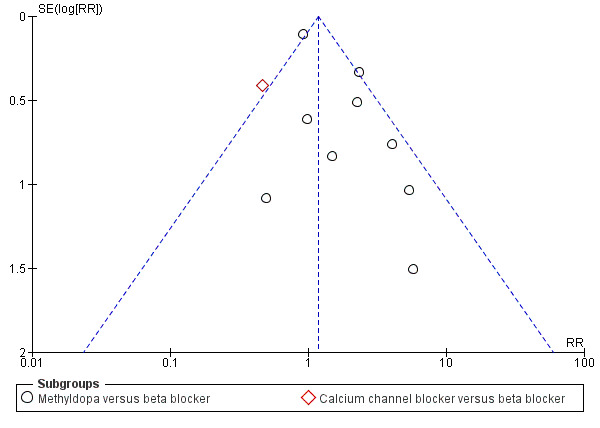
Funnel plot of comparison: 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), outcome: 7.1 Severe hypertension.
22.
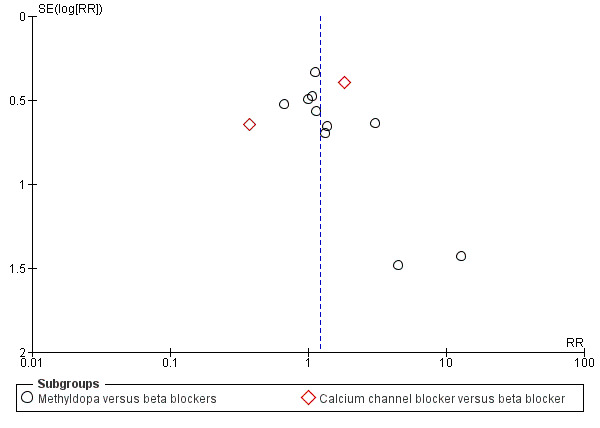
Funnel plot of comparison: 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), outcome: 7.2 Proteinuria/pre‐eclampsia.
23.
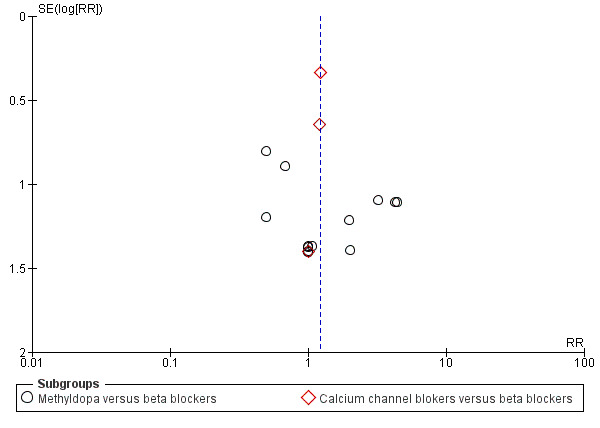
Funnel plot of comparison: 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), outcome: 7.3 Total reported fetal or neonatal death (including miscarriage).
24.
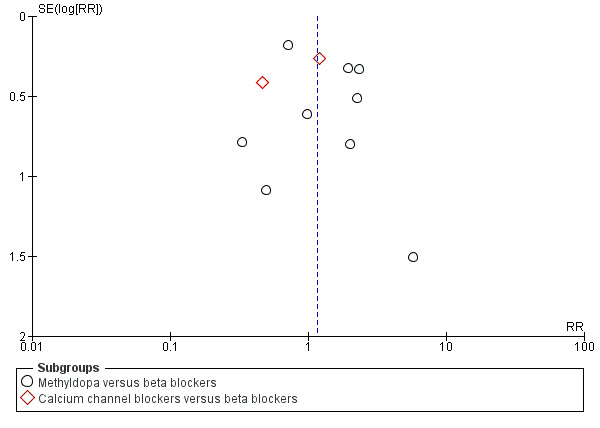
Funnel plot of comparison: 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), outcome: 7.11 Need for additional antihypertensive drug/s.
25.
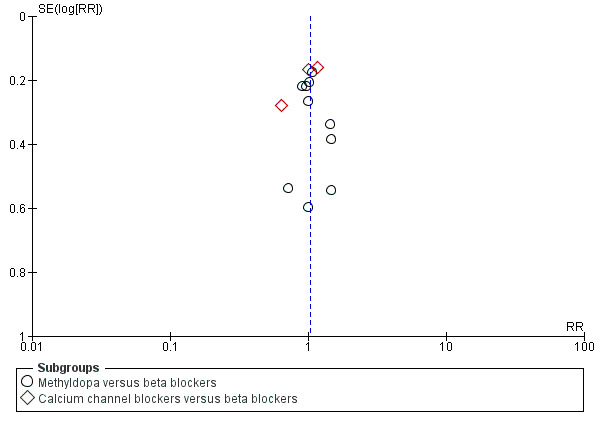
Funnel plot of comparison: 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), outcome: 7.13 Caesarean section.
Other potential sources of bias
Baseline characteristics were similar between groups in most trials, thus in the absence of other potential sources of bias they were scored as at low risk. In one trial (Australia 1985a) co‐interventions were unevenly distributed: 48% of oxprenolol group and 35% of methyldopa group received a second or third antihypertensive. In another study (Caribbean Is.1990), treatment was unblinded in 23 women (15%) and other treatment started, 16 for uncontrolled BP (five experimental, 11 control) and seven for poor compliance/side‐effects (four experimental, three control). In another trial (UK 2017) some baseline characteristics (such as smoking or chronic hypertension) were not balanced. So these three studies were assessed as high risk. In 13 trials, funding source included the Industry totally or partially (France 1987, India 1992UK 1976; UK 1980; UK 1982; UK 1983a; UK 1983b; UK 1989; UK 1990; UK 1992; UK 2009; USA 1987b; Venezuela 1988), so they were assessed as high risk of bias. In 22 studies, the funding sources was not declared and theywere classified as unclear risk of bias.
Informed consent was mentioned in the majority of trials. In one study, informed consent was obtained only from women allocated to the treatment arm (Ireland 1991).
Sample size and power calculations were reported for nine trials (Brazil 2016; Caribbean Is.1990; France 1987; Ireland 1991; Italy 1998; Pakistan 2016; UK 1989; UK 2009; UK 2017).
Effects of interventions
Summary of findings for the main comparison. Any antihypertensive drug compared to no antihypertensive drugs/placebo for mild to moderate hypertension during pregnancy (primary outcomes).
| Any antihypertensive drug compared to no antihypertensive drugs/placebo for mild to moderate hypertension during pregnancy | ||||||
| Patient or population: women with mild to moderate hypertension during pregnancy Setting: hospital settings in high‐ low‐ and middle‐income countries Intervention: any antihypertensive drug (beta blockers, methyldopa, calcium channel blockers, alpha blockers, glyceryl trinitrate, sildenafil) alone or in combination Comparison: placebo or no antihypertensive drug | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no drugs/placebo | Risk with any antihypertensive drug | |||||
| Maternal | ||||||
| Severe hypertension | Study population | RR 0.49 (0.40 to 0.60) | 2558 (20 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 198 per 1000 | 97 per 1000 (79 to 119) | |||||
| Proteinuria/pre‐eclampsia | Study population | RR 0.92 (0.75 to 1.14) | 2851 (23 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 4 | ||
| 185 per 1000 | 171 per 1000 (139 to 211) | |||||
| Fetal/neonatal/infant | ||||||
| Total reported fetal or neonatal deaths (including miscarriage) | Study population | RR 0.72 (0.50 to 1.04) | 3365 (29 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 41 per 1000 | 28 per 1000 (20 to 40) | |||||
| Small‐for‐gestational age | Study population | RR 0.96 (0.78 to 1.18) | 2686 (21 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 152 per 1000 | 149 per 1000 (125 to 176) | |||||
| Preterm birth (< 37 weeks) | Study population | RR 0.96 (0.83 to 1.12) | 2141 (15 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 277 per 1000 | 266 per 1000 (236 to 305) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Studies contributing data had design limitations
2 We have not downgraded for indirectness although trials examined a range of different antihypertensive drugs
3 Fairly wide 95% CI but not downgraded for imprecision as the CIs touched but did not cross .75 or 1.25
4 The funnel plot does suggest some asymmetry which may indicate small study effect and possible publication bias
Summary of findings 2. Any antihypertensive drug compared to no antihypertensive drugs/placebo for mild to moderate hypertension during pregnancy (secondary maternal and fetal/neonatal/infant outcomes).
| Any antihypertensive drug compared to no antihypertensive drugs/placebo for mild to moderate hypertension during pregnancy | ||||||
|
Patient or population: women with mild to moderate hypertension during pregnancy
Setting: hospital settings in high‐ low‐ and middle‐income countries Intervention: any antihypertensive drug (beta blockers, methyldopa, calcium channel blockers, alpha blockers, glyceryl trinitrate, sildenafil) alone or in combination Comparison: placebo or no antihypertensive drug | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no antihypertensive drugs/placebo | Risk with any antihypertensive drug | |||||
| Maternal | ||||||
| Maternal death | Study population | RR 1.11 (0.18 to 7.02) | 525 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 4 per 1000 | 5 per 1000 (1 to 20) | |||||
| Severe pre‐eclampsia | Study population | RR 0.56 (0.15 to 2.02) | 416 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 76 per 1000 | 42 per 1000 (11 to 153) | |||||
| Eclampsia | Study population | RR 0.52 (0.13 to 2.06) | 713 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 14 per 1000 | 7 per 1000 (2 to 28) | |||||
| Changed/stopped drugs due to maternal side‐effects | Study population | RR 1.93 (0.92 to 4.06) | 1503 (16 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 12 per 1000 | 27 per 1000 (15 to 51) | |||||
| Elective delivery (induction of labour + elective caesarean section) | Study population | RR 0.93 (0.84 to 1.04) | 710 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 716 per 1000 | 651 per 1000 (594 to 716) | |||||
| Fetal/neonatal/infant | ||||||
| Admission to neonatal or intensive care nursery | Study population | RR 1.01 (0.83 to 1.22) | 1570 (10 RCTs) | ⊕⊕⊝⊝ LOW 1 2 5 | ||
| 284 per 1000 | 287 per 1000 (236 to 347) | |||||
| Follow‐up of the children at 1 year: cerebral palsy | Study population | RR 0.33 (0.01 to 8.01) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 18 per 1000 | 6 per 1000 (0 to 146) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Studies contributing data had design limitations
2 We have not downgraded for indirectness although trials examined a range of different antihypertensive drugs
3 Wide 95% CI crossing the line of no effect and low event rate
4 Trials contributing data examined a range of different hypertensive drugs
5 The funnel plot does suggest some asymmetry which may indicate small study effect and possible publication bias
(1) Any antihypertensive drug versus no antihypertensive drugs/placebo
Overall, 31 trials with a total of 3485 women compared an antihypertensive drug with placebo or no antihypertensive drug.
Primary outcomes
Severe hypertension
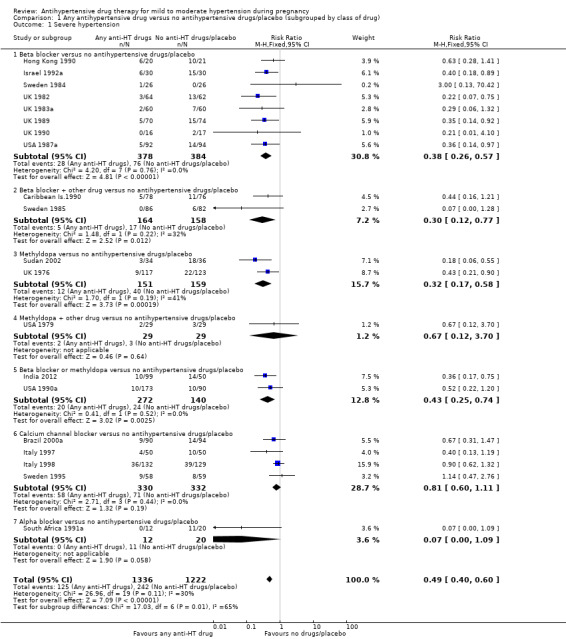
There is probably a halving in the risk of developing severe hypertension associated with the use of antihypertensive drug/s (20 trials, 2558 women; risk ratio (RR) 0.49; (95% confidence interval (CI) 0.40 to 0.60; moderate‐certainty evidence), Analysis 1.1.
1.1. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 1 Severe hypertension.
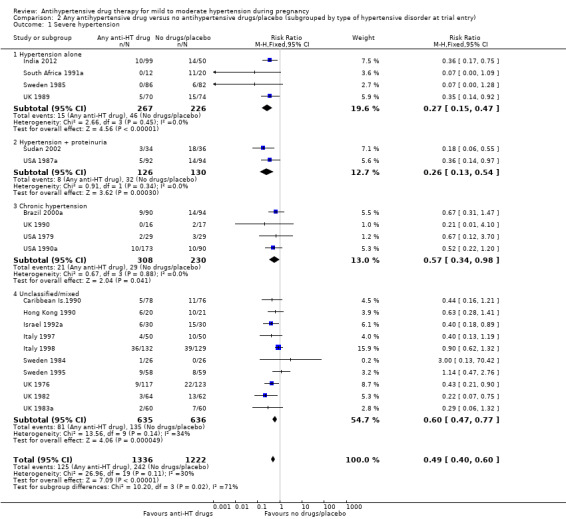
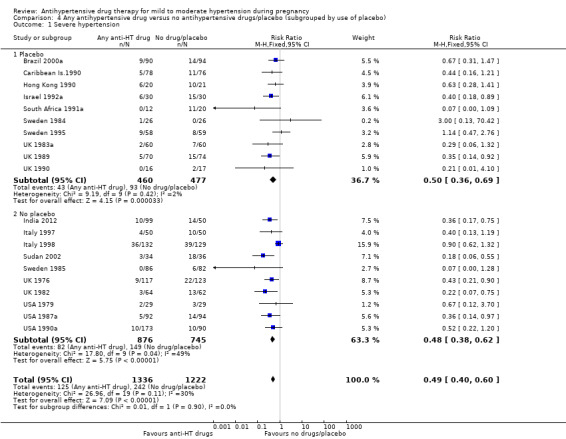
Subgroup analyses Although differences were observed between the classes of drugs subgroups (test for subgroup differences: Chi² = 17.03, df = 6 (P = 0.009), I² = 64.8%), this effect was strikingly consistent regardless of the hypertensive disorder at trial entry hypertension alone: four trials, (493 women) (RR 0.27; 95% CI 0.15 to 0.47), Analysis 2.1.1; hypertension + proteinuria: two trials, (256 women) (RR 0.26; 95% CI 0.13 to 0.54), Analysis 2.1.2; chronic hypertension: four trials, (538 women) (RR 0.57; 95 % CI 0.34 to 0.98), Analysis 2.1.3; unclassified/mixed: 10 trials, (1271 women) (RR 0.60; 95% CI 0.47 to 0.77), Analysis 2.1.4 (test for subgroup differences: Chi² = 10.20, df = 3 (P = 0.02), I² = 70.6%). The same effect was observed when a placebo or no antihypertensive drugs were used for the control group (with placebo: 10 trials (937 women) (RR 0.50, 95 % CI 0.36 to 0.69), Analysis 4.1.1; with no antihypertensive treatment: 10 trials (1621 women) (RR: 0.48, 95 % CI 0.38 to 0.62) Analysis 4.1.2 (test for subgroup differences: Chi² = 0.01, df = 1 (P = 0.90, I² = 0%); or when gestational age at trial entry was less than 32 weeks, seven trials (1071 women) (RR 0.63; 95% CI 0.47 to 0.83) Analysis 3.1,1, or was unclassified/mixed:12 trials (1367 women) (RR 0.40; 95% CI 0.30 to 0.53) Analysis 3.1,3, but not in the only trial recruiting women with gestational age over 32 weeks, (120 women) (RR 0.29; 95% CI 0.06 to 1.32) Analysis 3.1,2 (test for subgroup differences: Chi² = 5.49, df = 2 (P = 0.06), I² = 63.6%).
2.1. Analysis.
Comparison 2 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by type of hypertensive disorder at trial entry), Outcome 1 Severe hypertension.
4.1. Analysis.
Comparison 4 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by use of placebo), Outcome 1 Severe hypertension.
3.1. Analysis.
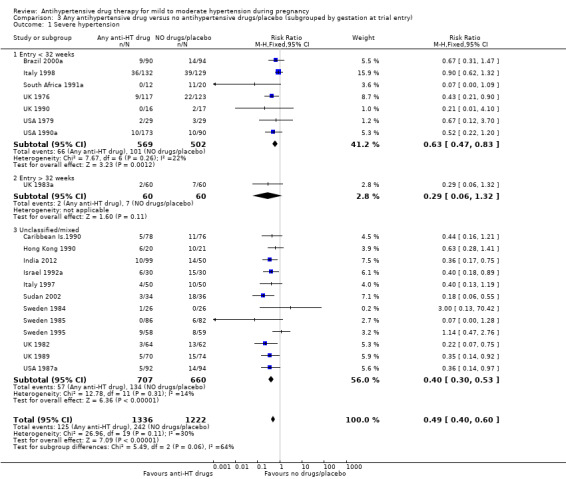
Comparison 3 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by gestation at trial entry), Outcome 1 Severe hypertension.
Proteinuria/pre‐eclampsia
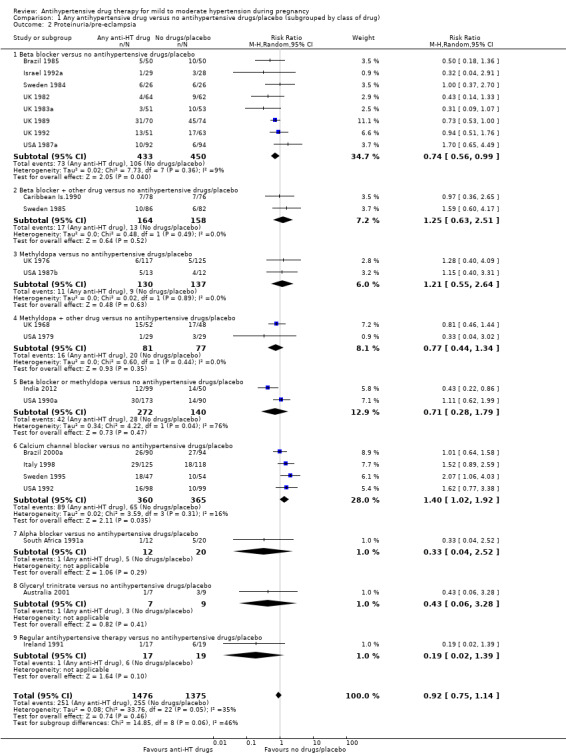
Low‐certainty evidence suggests that there may be little or no difference in the risk of developing proteinuria/pre‐eclampsia in the 23 trials (2851 women) reporting this outcome (average risk ratio (aRR) 0.92, 95% CI 0.75 to 1.14), Analysis 1.2.
1.2. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 2 Proteinuria/pre‐eclampsia.
Subgroup analyses These results are consistent regardless of the use of placebo (with placebo: aRR 0.90; 95% CI: 0.65 to 1.24; without placebo: aRR 0.94 ; 95% CI 0.69 to 1.26), test for subgroup differences: Chi² = 0.04, df = 1 (P = 0.85), I² = 0%). Analysis 4.2. However, when other subgroups are considered, there is evidence of a difference between subgroups. There is a reduction in the risk of developing proteinuria/pre‐eclampsia in women treated with beta blockers versus no antihypertensive drugs/placebo (eight trials, 883 women; aRR 0.74; 95% CI 0.56 to 0.99) Analysis 1.2.1. However, in trials evaluating calcium channel blockers versus no antihypertensive drugs/placebo (four trials, 725 women), the risk of pre‐eclampsia appears to increase (aRR 1.40; 95% CI 1.02 to 1.92), Analysis 1.2.6. For the other class of drugs, no evidence of an overall difference was found. Test for subgroup differences: Chi² = 14.85, df = 8 (P = 0.06), I² = 46.1%). A reduction in the risk of developing proteinuria/pre‐eclampsia was also seen in those women with hypertension alone, when subgrouped by type of hypertensive disorder at trial entry (eight trials, 684 women; aRR 0.74; 95% CI 0.53 to 1.02), but not in the other subgroups (Test for subgroup differences: Chi² = 5.73, df = 3 (P = 0.13), I² = 47.6%) Analysis 2.2. When subgrouped by gestational age at trial entry, a reduction in the risk was only seen in two trials of 120 women recruited after 32 weeks' gestation (aRR 0.34; 95% CI 0.12 to 0.97), but not in the other subgroups (Test for subgroup differences: Chi² = 4.43, df = 2 (P = 0.11), I² = 54.9%). Analysis 3.2.
4.2. Analysis.
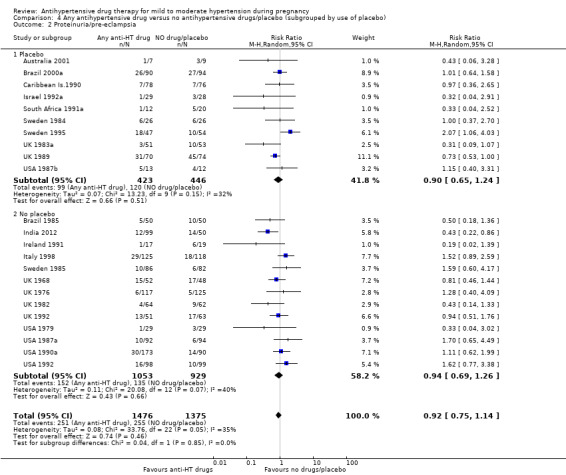
Comparison 4 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by use of placebo), Outcome 2 Proteinuria/pre‐eclampsia.
2.2. Analysis.
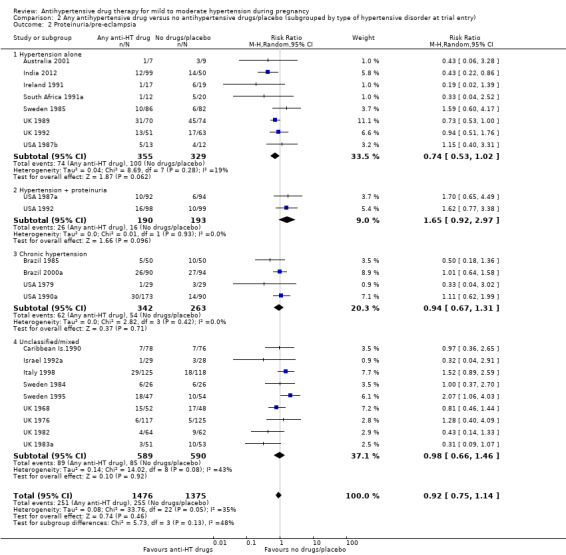
Comparison 2 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by type of hypertensive disorder at trial entry), Outcome 2 Proteinuria/pre‐eclampsia.
3.2. Analysis.
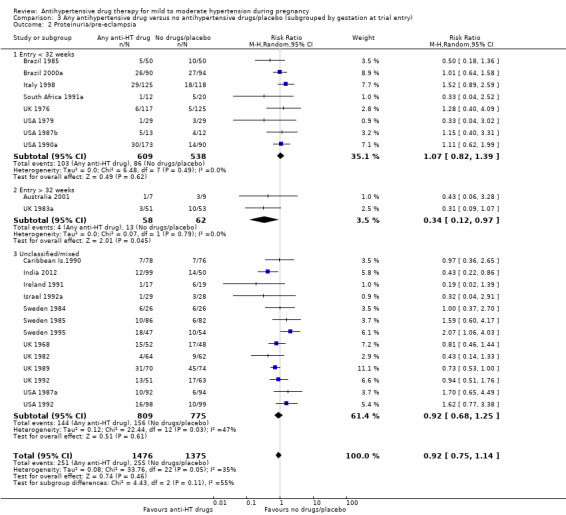
Comparison 3 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by gestation at trial entry), Outcome 2 Proteinuria/pre‐eclampsia.
Total reported fetal or neonatal deaths (including miscarriage)
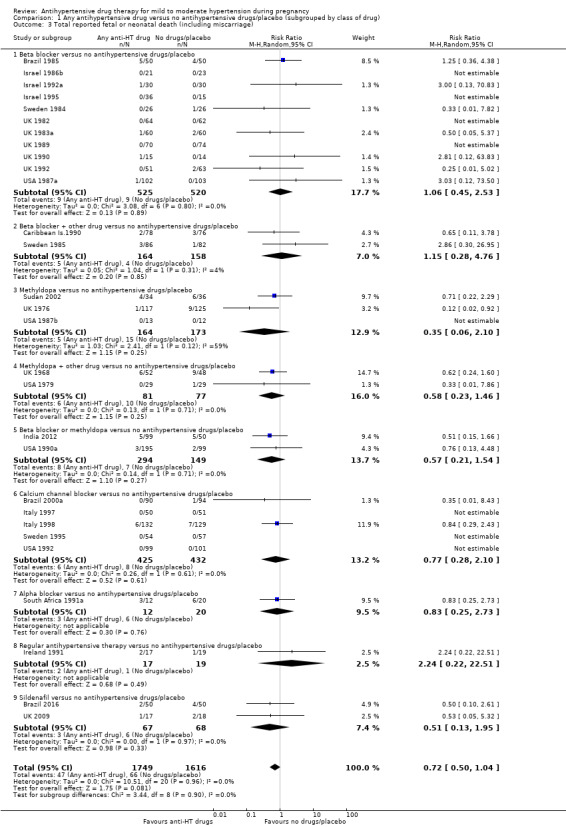
Moderate‐certainty evidence suggests that antihypertensive drug(s) probably have little or no effect on the overall risk of the fetus or baby dying (including miscarriage). The confidence intervals show that the effect includes everything from a 50% reduction to a 4% increase (29 trials, 3365 women; aRR 0.72, 95% CI 0.50 to 1.04; moderate‐certainty evidence), Analysis 1.3.
1.3. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
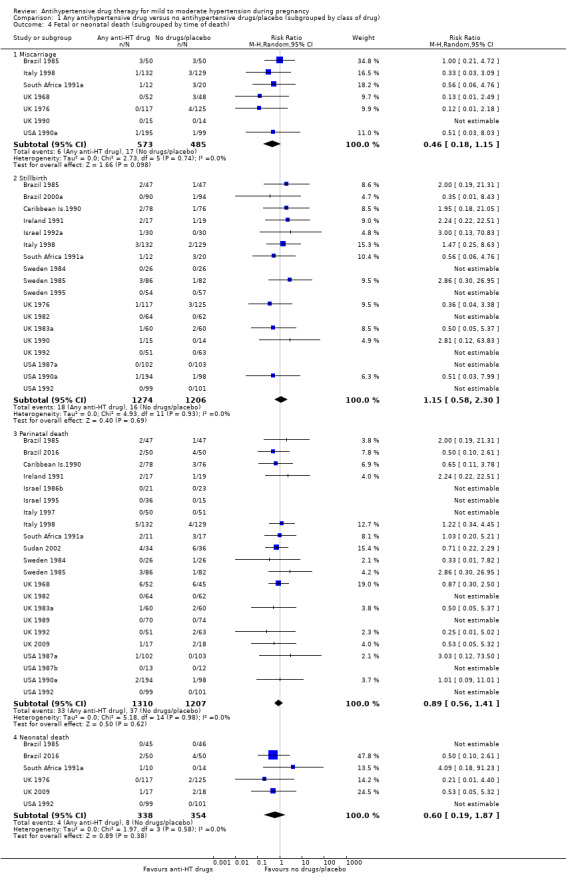
Subgroup analyses No evidence of an overall difference was found between any of the pre‐specified subgroups (type of drugs (Analysis 1.3), time of death (Analysis 1.4), of hypertensive disorder at trial entry (Analysis 2.3), gestational age at trial entry Analysis 3.3), or the use of placebo (Analysis 4.3).
1.4. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 4 Fetal or neonatal death (subgrouped by time of death).
2.3. Analysis.
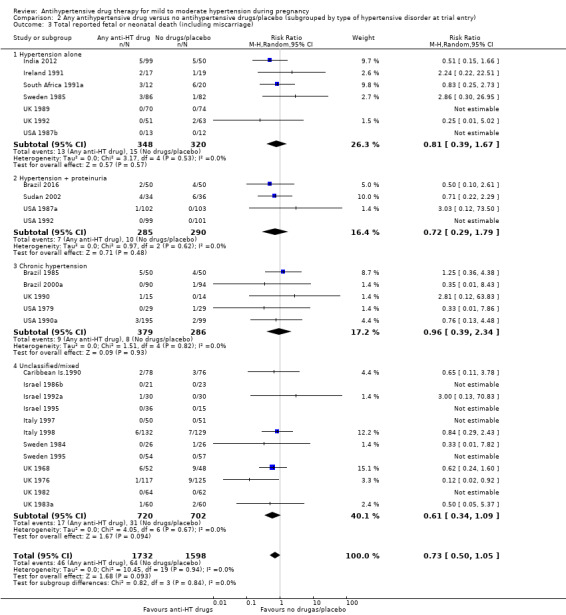
Comparison 2 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by type of hypertensive disorder at trial entry), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
3.3. Analysis.
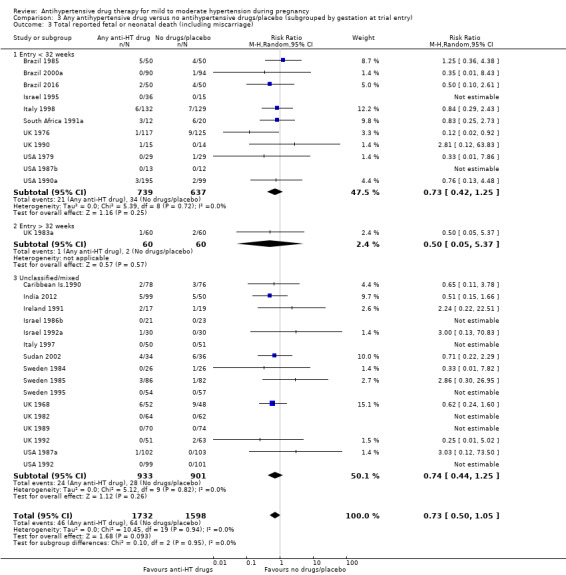
Comparison 3 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by gestation at trial entry), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
4.3. Analysis.
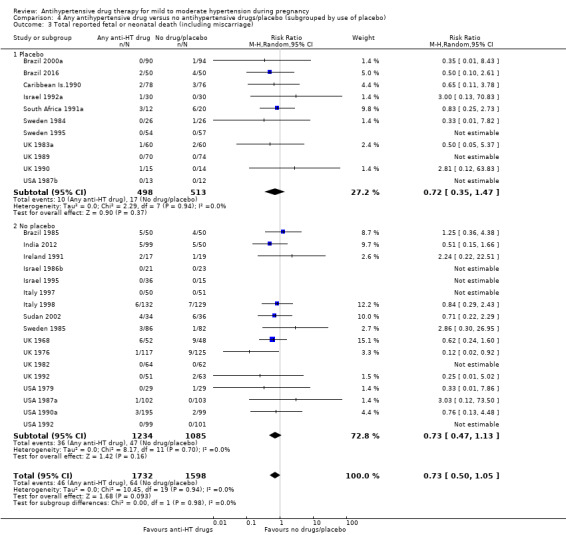
Comparison 4 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by use of placebo), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
Small‐for‐gestational age
There is probably little or no difference in the risk of having small‐for‐gestational age babies (aRR 0.96; 95% CI 0.78 to 1.18; moderate‐certainty evidence), Analysis 1.5 in the 21 trials (2686 women) reporting this outcome.Figure 9
1.5. Analysis.
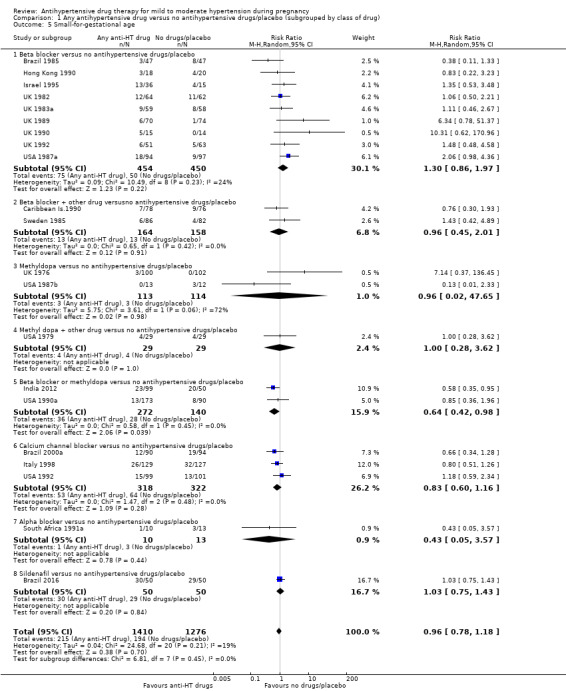
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 5 Small‐for‐gestational age.
Subgroup analyses No evidence of differences were found between any of the pre‐specified subgroups (severity of intrauterine growth restriction (IUGR) (Analysis 1.6), type of hypertensive disorder at trial entry (Analysis 2.4), gestational age at trial entry (Analysis 3.4), use of placebo (Analysis 4.4)). When the subgroup "class of drugs" is considered, a reduction in the risk of having small‐for‐gestational age babies was seen in two small trials (412 women) comparing together beta blocker or methyldopa versus no antihypertensive drugs/placebo (aRR: 0.64; 95% CI 0.42 to 0.98 (Analysis 1.5.5). However, this effect was not seen in the nine trials (904 women) comparing beta blockers versus no antihypertensive drugs/placebo (aRR 1.30; 95% CI 0.86 to 1.97) (Analysis 1.5.1), nor in the two trials (227 women) comparing methyldopa versus no antihypertensive drugs/placebo (aRR 0.96; 95% CI 0.02 to 47.65) (Analysis 1.5.3), nor in the other classes of drugs evaluated (Test for subgroup differences: Chi² = 6.81, df = 7 (P = 0.45), I² = 0%) Analysis 1.5.
1.6. Analysis.
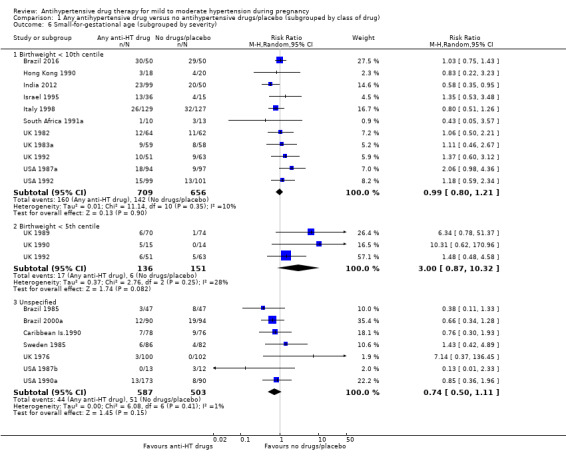
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 6 Small‐for‐gestational age (subgrouped by severity).
2.4. Analysis.
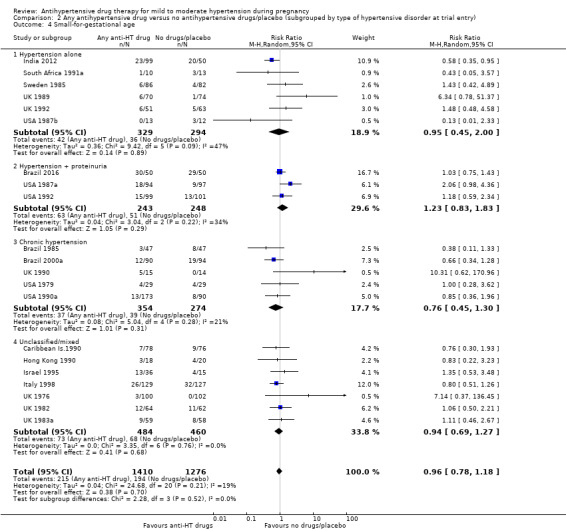
Comparison 2 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by type of hypertensive disorder at trial entry), Outcome 4 Small‐for‐gestational age.
3.4. Analysis.
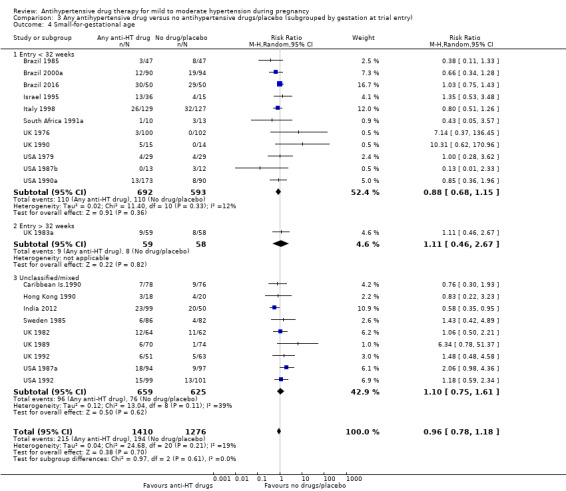
Comparison 3 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by gestation at trial entry), Outcome 4 Small‐for‐gestational age.
4.4. Analysis.
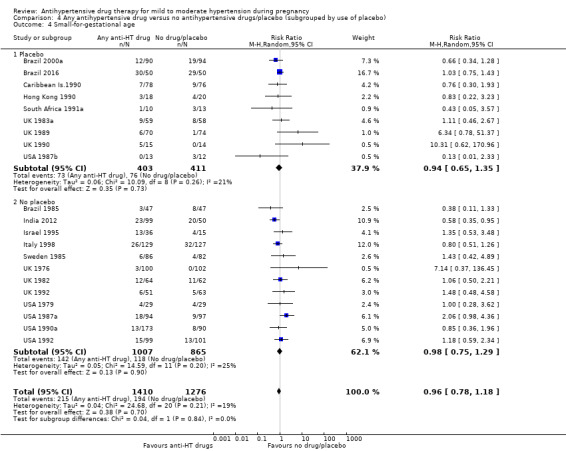
Comparison 4 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by use of placebo), Outcome 4 Small‐for‐gestational age.
Preterm birth (less than 37 weeks)
Moderate‐certainty evidence suggests that there is probably little or no difference among groups in the 15 trials (2141 women) reporting this outcome (aRR 0.96; 95% CI 0.83 to 1.12;), Analysis 1.7.
1.7. Analysis.
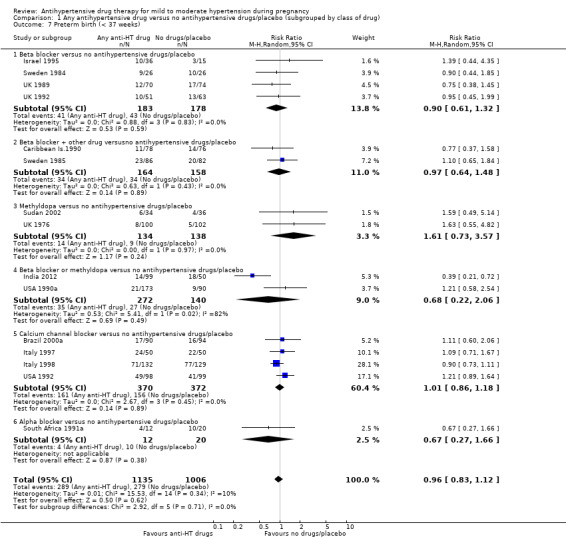
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 7 Preterm birth (< 37 weeks).
Subgroup analyses No evidence of an overall difference was found between any of the pre‐specified subgroups (type of drugs (Analysis 1.7.), gestational age at delivery (Analysis 1.8), hypertensive disorder at trial entry (Analysis 2.5), gestational age at trial entry Analysis 3.5), or use of placebo (Analysis 4.5)).
1.8. Analysis.
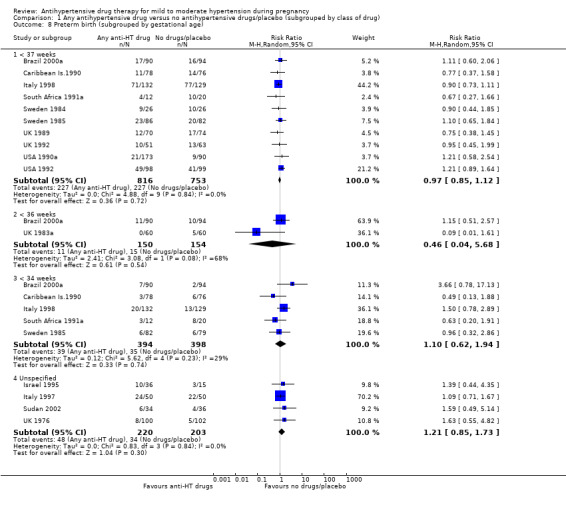
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 8 Preterm birth (subgrouped by gestational age).
2.5. Analysis.
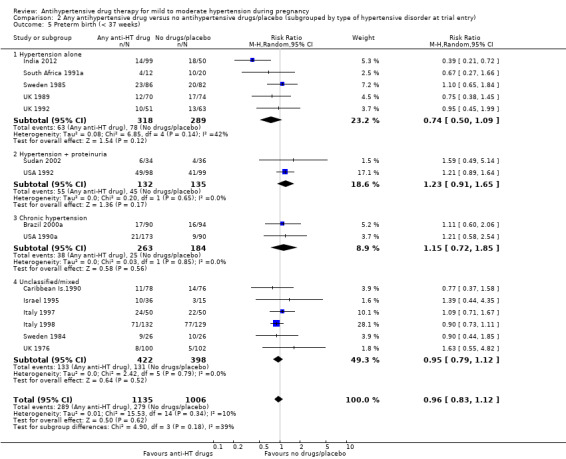
Comparison 2 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by type of hypertensive disorder at trial entry), Outcome 5 Preterm birth (< 37 weeks).
3.5. Analysis.
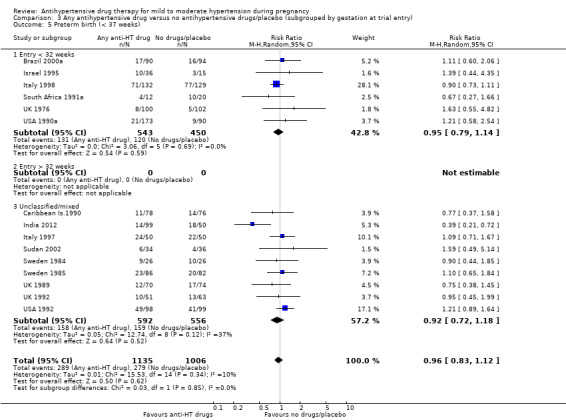
Comparison 3 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by gestation at trial entry), Outcome 5 Preterm birth (< 37 weeks).
4.5. Analysis.
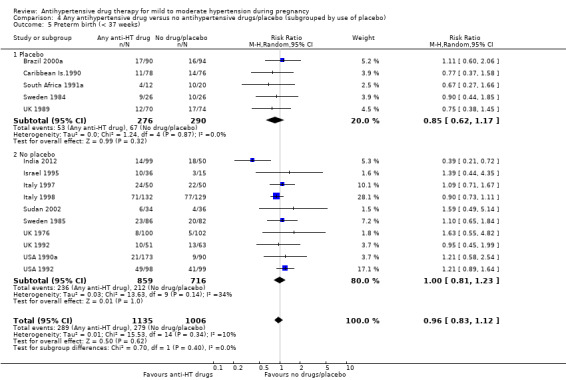
Comparison 4 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by use of placebo), Outcome 5 Preterm birth (< 37 weeks).
Secondary outcomes
Maternal
We are uncertain of the effect of antihypertensive drug(s) on the risk ofmaternal death (aRR 1.11; 95% CI 0.18 to 7.02; 5 trials, 525 women; Analysis 1.9), severe pre‐eclampsia (aRR 0.56; 95% CI 0.15 to 2.02; 3 trials, 416 women; Analysis 1.10), eclampsia (aRR 0.52; 95% CI 0.13 to 2.06; 7 trials, 713 women; Analysis 1.11), because the certainty of the evidence was very low.
1.9. Analysis.
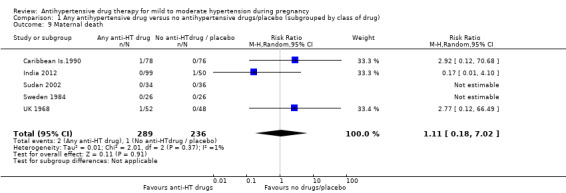
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 9 Maternal death.
1.10. Analysis.
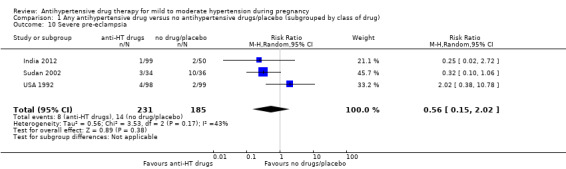
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 10 Severe pre‐eclampsia.
1.11. Analysis.
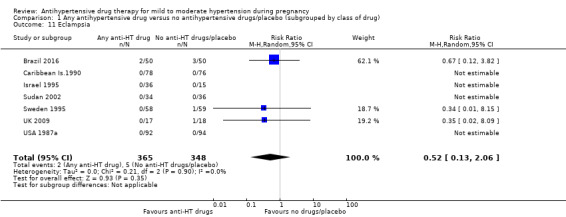
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 11 Eclampsia.
Three trials (332 women) evaluated HELLP syndrome (haemolysis, elevated liver enzymes and low platelets) with no evidence of an overall difference (aRR 1.06; 95% CI 0.32 to 3.50; Analysis 1.12). For the outcome severe maternal morbidity, only pulmonary oedema was reported, with no evidence of an overall difference (aRR 1.22; 95% CI 0.13 ‐11.75; 2 trials, 325 women; Analysis 1.13). There was a reduction in the need for additional antihypertensive drug(s) associated with the use of antihypertensive drug(s), reported in 11 trials with 1385 women (aRR 0.49; 95% CI 0.38 to 0.65; Analysis 1.14). There was no evidence of an overall difference in the risk of miscarriage (aRR 0.46; 95% CI 0.18 to 1.15; 7 trials, 1058 women; Analysis 1.4).
1.12. Analysis.
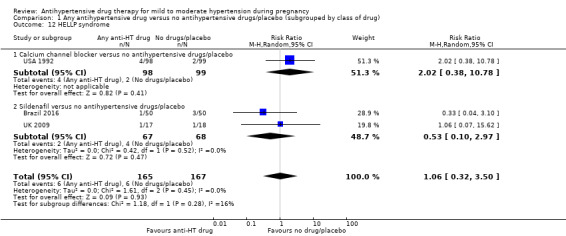
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 12 HELLP syndrome.
1.13. Analysis.
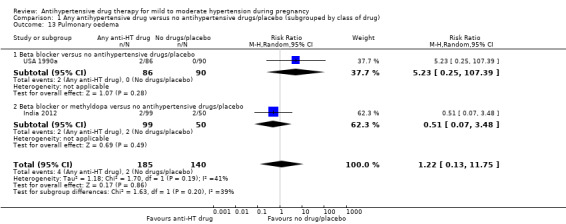
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 13 Pulmonary oedema.
1.14. Analysis.
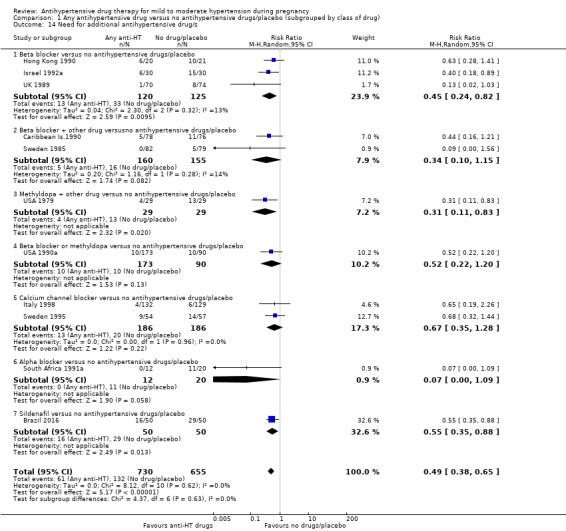
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 14 Need for additional antihypertensive drug/s.
There is probably little or no difference in the risk ofelective delivery between the antihypertensive drug(s) and placebo/no antihypertensive drug(s) groups based on evidence from four trials, 710 women (aRR 0.93; 95% CI 0.84 to 1.04; moderate‐certainty evidence; Analysis 1.15).
1.15. Analysis.
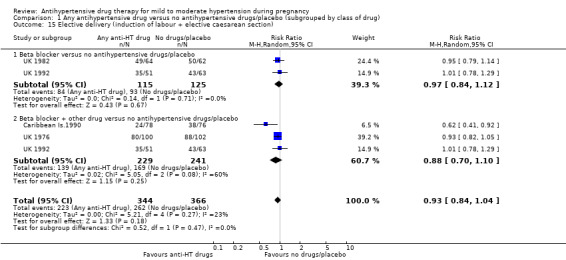
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 15 Elective delivery (induction of labour + elective caesarean section).
There is no evidence of an overall difference in the following maternal secondary outcomes: caesarean section (aRR 0.93; 95% CI 0.84 to 1.03; 21 trials, 2724 women; Analysis 1.16), and induction of labour (aRR 0.93; 95% CI 0.75‐1.15; 5 trials, 563 women; Analysis 1.17), antenatal hospital admission (aRR 0.87; 96% CI 0.71 to 1.08; 4 trials with 455 women; Analysis 1.18), placental abruption (aRR 1.49; 95% CI 0.70 to 3.17; 13 trials,1568 women; Analysis 1.19), and maternal side‐effects (aRR 1.99; 95% CI 0.89 to 4.43; 11 trials, 934 women; Analysis 1.20).
1.16. Analysis.
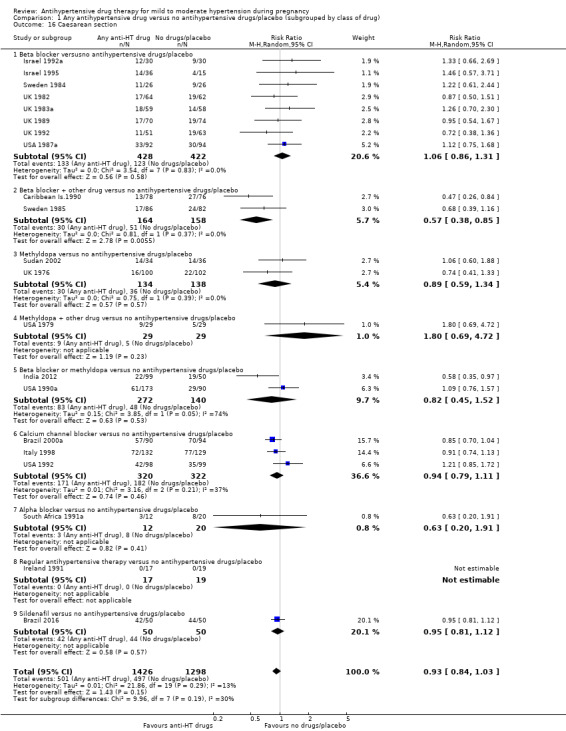
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 16 Caesarean section.
1.17. Analysis.
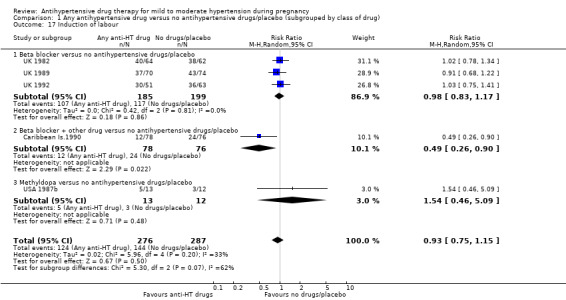
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 17 Induction of labour.
1.18. Analysis.
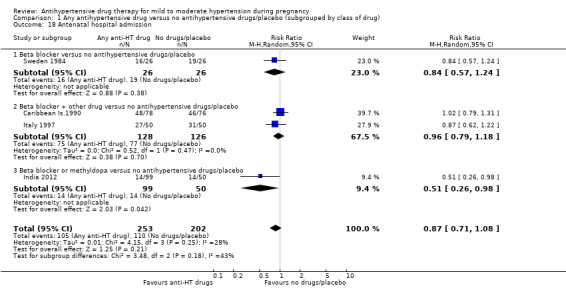
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 18 Antenatal hospital admission.
1.19. Analysis.
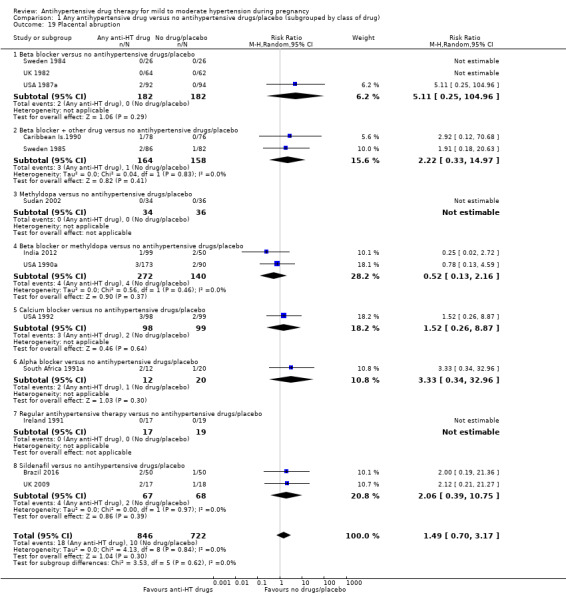
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 19 Placental abruption.
1.20. Analysis.
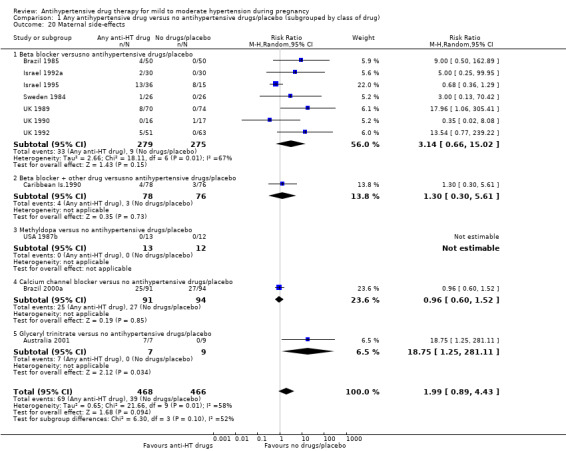
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 20 Maternal side‐effects.
There may be little or no effect on the risk of changed/stopped drugs due to maternal side‐effects in 16 trials, 1503 women (aRR 1.93; 95% CI 0.92 to 4.26; low‐certainty evidence; Analysis 1.21).
1.21. Analysis.
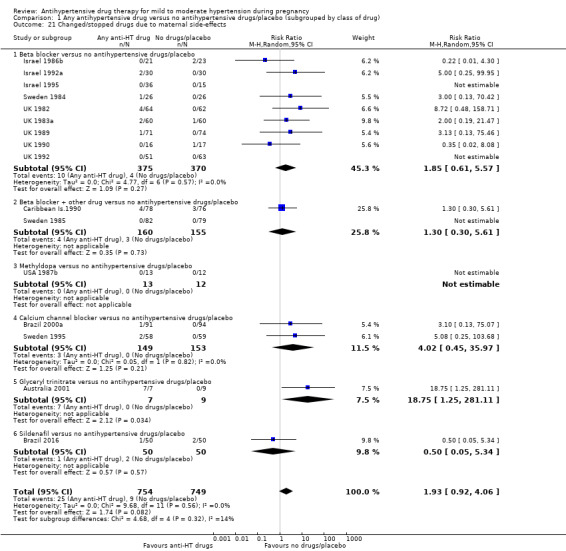
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 21 Changed/stopped drugs due to maternal side‐effects.
Fetal/neonatal/infant
Low‐certainty evidence suggests that treatment with antihypertensive drugs may make little or no difference in the risk of admission to neonatal or intensive care nursery when compared with no antihypertensive treatment or placebo in the in 10 trials (1570 babies) reporting this outcome (aRR 1.01; 95% CI 0.83 to 1.22; Heterogeneity: Tau² = 0.03; Chi² = 13.48, df = 9 (P = 0.14); I² = 33%, Analysis 1.22.
1.22. Analysis.
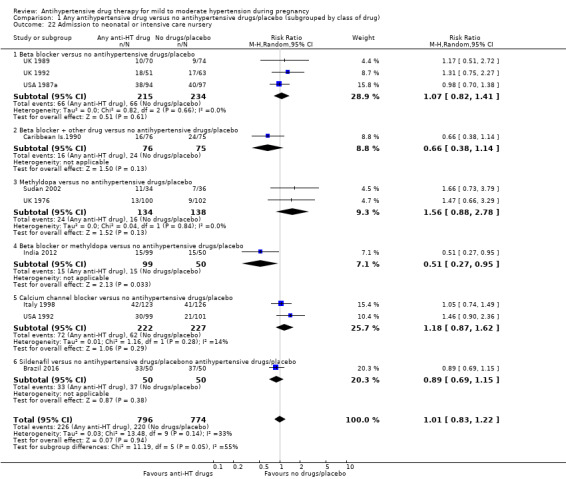
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 22 Admission to neonatal or intensive care nursery.
There appears to be a halving in the risk of developing respiratory distress syndrome associated with the use of antihypertensive drug/s in six trials evaluating 925 babies (aRR 0.53; 95% CI 0.29 to 0.99; Analysis 1.23). This effect is seen in the subgroup of beta blocker versus no antihypertensive drugs/placebo (3 trials, 412 babies; aRR 0.32; 95% CI 0.13 to 0.83; Analysis 1.23.1), with no evidence of an overall difference in the other subgroups of drugs: (Test for subgroup differences: Chi² = 4.11, df = 3 (P = 0.25), I² = 27.0%; Analysis 1.23).
1.23. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 23 Respiratory distress syndrome.
There is no evidence of an overall difference in the remaining neonatal outcomes: neonatal hypoglycaemia (aRR 0.77; 95% CI 0.51 to 1.15; 6 trials, 962 babies; Analysis 1.24), neonatal bradycardia (aRR 1.28; 95% CI 0.31 to 5.24; 3 trials, 418 babies; Analysis 1.25), and neonatal jaundice (aRR 0.78, 95% CI 0.53 to 1.15; 3 trials, 529 babies; Analysis 1.26).
1.24. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 24 Neonatal hypoglycaemia.
1.25. Analysis.
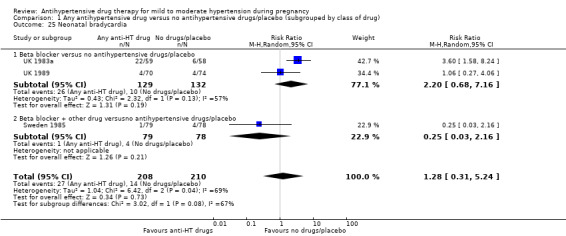
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 25 Neonatal bradycardia.
1.26. Analysis.
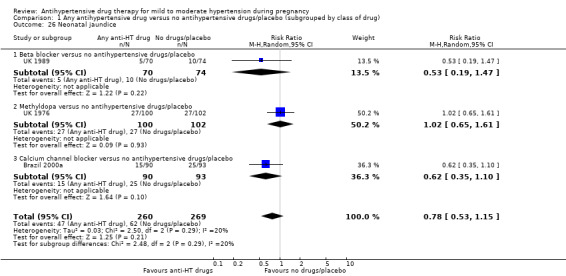
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 26 Neonatal jaundice.
We are uncertain of the effect of antihypertensive drug(s) on the risk of impaired long‐term growth and development in infancy and childhood – one trial (110 infants) reported on the risk of cerebral palsy at one‐year follow‐up (aRR 0.33; 95% CI 0.01 to 8.01; Analysis 1.27). There was no evidence of an overall difference in one trial (190 children) following a subset of surviving children at seven and a half years of age and measuring chronic ill health or impaired hearing/vision (Analysis 1.28). Very low, less than four, five‐minute Apgar score outcome was not reported.
1.27. Analysis.
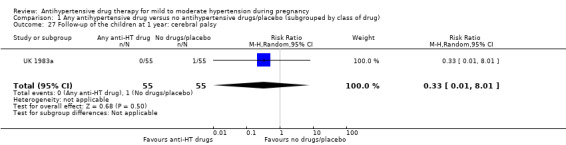
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 27 Follow‐up of the children at 1 year: cerebral palsy.
1.28. Analysis.
Comparison 1 Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug), Outcome 28 Follow‐up of the children at 7 1/2 years.
(2) One hypertensive drug versus another
Overall, 29 trials with a total of 2774 women compared one antihypertensive drug with another. For this review, the commonly used group of drugs are regarded as control and compared with all other group of drugs (for example, other antihypertensives versus methyldopa, other antihypertensives versus calcium channel blockers, and other antihypertensives versus beta blockers)
Primary outcomes
Severe hypertension
Any antihypertensive drug versus methyldopa: beta blockers and calcium channel blockers together in the meta‐analysis appear to be more effective than methyldopa in avoiding an episode of severe hypertension (RR 0.70; 95% CI 0.56 to 0.88, 11 trials, 638 women; Analysis 5.1).
5.1. Analysis.
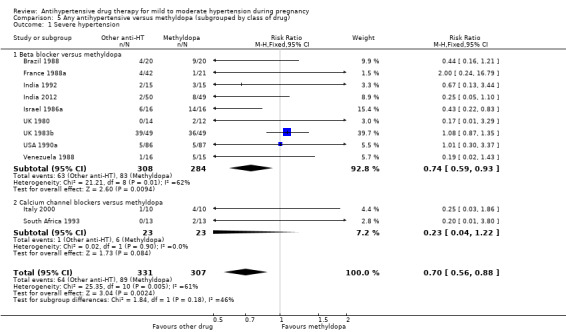
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 1 Severe hypertension.
Subgroup analysis This effect was seen for the subgroup beta blockers versus methyldopa (9 trials, 592 women); RR 0.74; 95% CI 0.59 to 0.93, Analysis 5.1.1), but not in calcium channel blockers versus methyldopa (2 trials, 46 women; RR 0.23; 95% CI 0.04 to 1.22, Analysis 5.1.2). Test for subgroup differences: Chi² = 1.84, df = 1 (P = 0.18), I² = 45.6%.
Any antihypertensive drug versus calcium channel blockers: there is an increase in the risk of developing severe hypertension when other antihypertensive drugs were compared together with calcium channel blockers (RR 1.86; 95% CI 1.09 to 3.15, 5 trials, 223 women). Analysis 6.1.
6.1. Analysis.
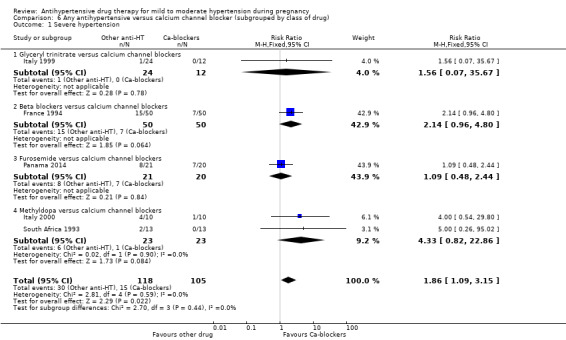
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 1 Severe hypertension.
Subgroup analysis Metroprolol versus nicardipine was reported by one trial (100 women) (RR 2.14; 95% CI 0.96 to 4.80, Analysis 6.1.2); glyceryl trinitrate versus nifedipine by one trial, (36 women),(RR 1.56; 95% CI 1.07 to 35.67, Analysis 6.1.1); amlodipine versus furosemide by another trial, (41 women)., (RR 1.09; 95% CI 0.48 to 2.44. Analysis 6.1.3); and methyldopa versus calcium channel blockers in two trials (46 women) (RR 4.33, 95% CI 0.82 to 22.86.Analysis 6.1.4). Test for subgroup differences: Chi² = 2.70, df = 3 (P = 0.44), I² = 0%.
Any antihypertensive drug versus beta blockers: there is no evidence of an overall difference in the risk of developing severe hypertension when methyldopa and calcium channel blockers together are compared with beta blockers (RR: 1.18, 95% CI 0.95 to 1.48; 10 trials, 692 women; Analysis 7.1).
7.1. Analysis.
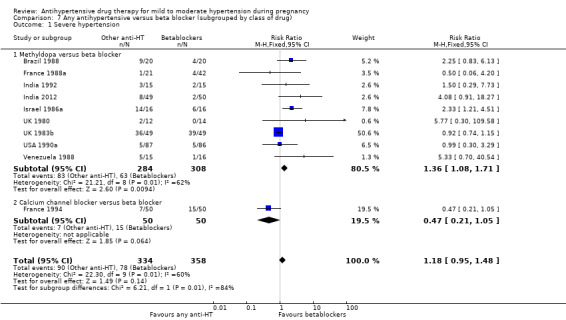
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 1 Severe hypertension.
Subgroup analysis As stated above, this effect was seen for the subgroup of methyldopa versus beta blockers, nine trials (592 women) (RR 1.36, 95% CI 1.08 to 1.71; Analysis 7.1.1), but not in the comparison of calcium channel blockers versus beta blockers, one trial, (100 women) (RR: 0.47, 95% CI 0.21 to 1.05; Analysis 7.1.2). Test for subgroup differences: Chi² = 6.21, df = 1 (P = 0.01), I² = 83.9%.
Proteinuria/pre‐eclampsia
Any antihypertensive drug versus methyldopa: there is no evidence of an overall difference in the risk of developing proteinuria/pre‐eclampsia when either beta blockers or calcium channel blockers are compared with methyldopa (aRR 0.78; 95% CI 0.58 to 1.06; 11 trials, 997 women; Analysis 5.2).
5.2. Analysis.
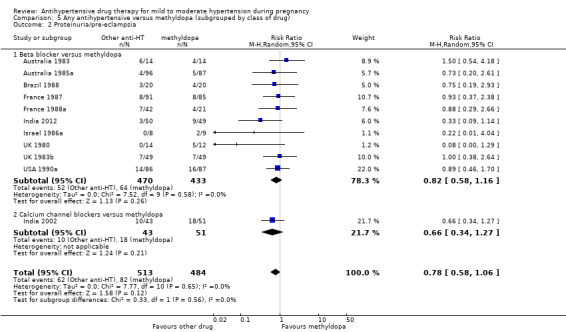
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 2 Proteinuria/pre‐eclampsia.
Subgroup analysis Beta blockers versus methyldopa (10 trials, 903 women; aRR 0.82; 95% CI 0.58 to 1.16; Analysis 5.2.1), calcium channel blockers versus methyldopa (1 trial, 94 women; aRR 0.66; 95% CI 0.34 to 1.27; Analysis 5.2.2). Test for subgroup differences: Chi² = 0.33, df = 1 (P = 0.56), I² = 0%.
Any antihypertensive drug versus calcium channel blockers: there is no evidence of an overall difference in the risk of developing proteinuria/pre‐eclampsia when alternative drugs are compared with calcium channel blockers (aRR: 1.24, 95% CI 0.70 to 2.19; 5 trials, 375 women; Analysis 6.2).
6.2. Analysis.
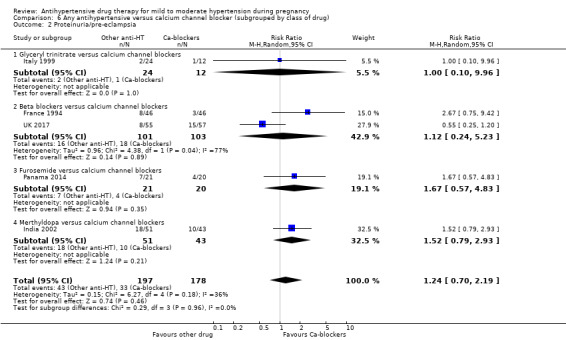
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 2 Proteinuria/pre‐eclampsia.
Subgroup analysis Two trials (204 women) compared beta blockers with calcium channel blockers (aRR 1.12; 95% CI 0.24 to 5.23; Analysis 6.2.2). One trial (36 women) compared glyceryl trinitrate with nifedipine, (aRR: 1.00; 95% CI 0.10 to 9.96; Analysis 6.2.1), one trial (41 women) compared furosemide versus amlodipine (aRR: 1.67; 95% CI 0.57 to 4.83; Analysis 6.2.3)., and one trial (94 women) compared methyldopa with calcium channel blockers (aRR: 1.52, 95% CI 0.79 to 2.93; Analysis 6.2.4). Test for subgroup differences: Chi² = 0.29, df = 3 (P = 0.96), I² = 0%.
Any antihypertensive drug versus beta blockers: there is no evidence of an overall difference in the risk of developing proteinuria/pre‐eclampsia when methyldopa and calcium channel blockers together are compared with beta blockers (aRR: 1.21, 95% CI 0.88 to 1.67; 12 trials, 1107 women; Analysis 7.2).
7.2. Analysis.
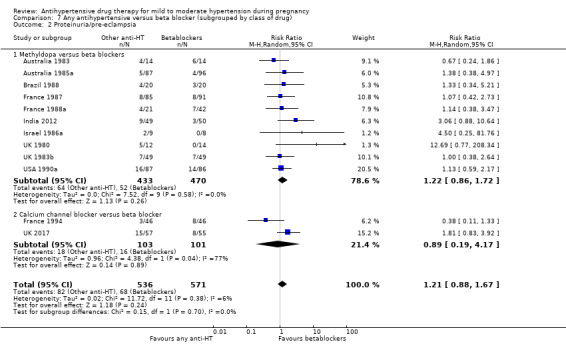
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 2 Proteinuria/pre‐eclampsia.
Subgroup analysis Methyldopa versus beta blockers was evaluated in 10 trials, 903 women; (aRR: 1.22, 95% CI 0.86 to 1.72; Analysis 7.2.1); calcium channel blockers versus beta blockers in two trials, 204 women; (aRR: 0.89, 95% CI 0.19 to 4.17; Analysis 7.2.2). Test for subgroup differences: Chi² = 0.15, df = 1 (P = 0.70), I² = 0%.
Total reported fetal or neonatal deaths (including miscarriage)
Any antihypertensive drug versus methyldopa: there is no evidence of an overall difference in the risk of the baby dying (aRR: 0.77, 95% CI 0.52 to 1.14; 22 trials, 1711 babies; Analysis 5.3), when any antihypertensive drug was compared with methyldopa.
5.3. Analysis.
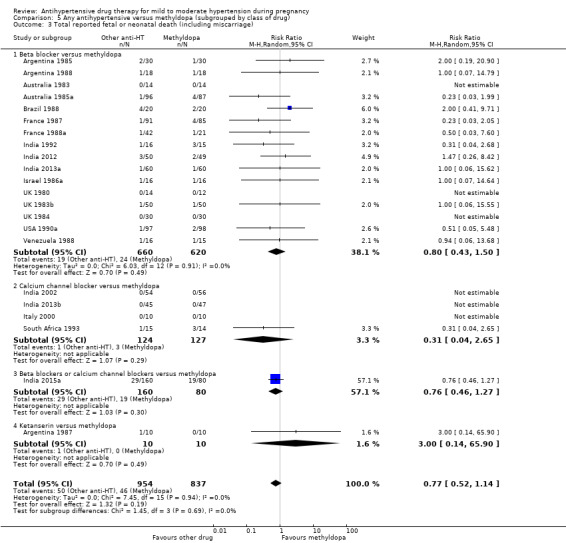
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
Subgroup analysis
Beta blockers were compared with methyldopa in 16 trials (1280 women; aRR 0.80, 95% CI 0.43 to 1.50; Analysis 5.3.1), calcium channel blockers were compared with methyldopa in 4 trials (251 women), (aRR 0.31; 95% CI 0.04 to 2.65; Analysis 5.3.2); beta blockers or calcium channel blockers versus methyldopa in one trial (240 women), (aRR 0.76; 95% CI 0.46 to 1.27; Analysis 5.3.3); and ketanserin versus methyldopa in one trial (20 women), (aRR 3.00, 95% CI 0.14 to 65.90; Analysis 5.3.4). Test for subgroup differences: Chi² = 1.45, df = 3 (P = 0.69), I² = 0%.
Any antihypertensive drug versus calcium channel blockers: no evidence of an overall difference was found when beta blockers (metoprolol or labetalol), glyceryl trinitrate, furosemide or methyldopa were compared with calcium channel blockers (aRR: 0.90, 95% CI 0.52 to 1.57; 9 trials, 700 babies; Analysis 6.3).
6.3. Analysis.
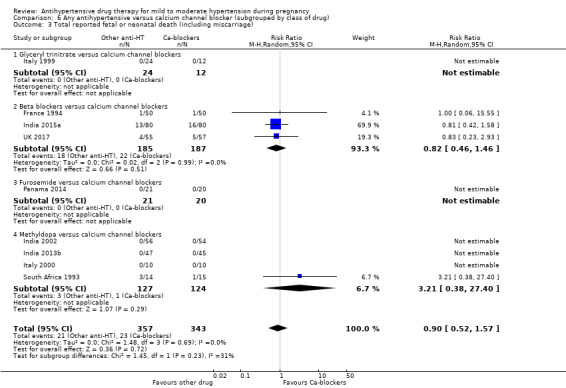
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
Subgroup analysis Glyceryl trinitrate versus calcium channel blockers was compared in one trial (36 babies) with no events, Analysis 6.3.1; beta blockers versus calcium channel blockers in three trials (372 women), (aRR 0.82, 95% CI 0.46 to 1.46; Analysis 6.3.2); furosemide versus calcium channel blockers were compared in one small trial (41 babies) with no events Analysis 6.3.3.; and methyldopa versus calcium channel blockers in four (251 babies), (aRR: 3.21, 95% CI 0.38 to 27.40; Analysis 6.3.4). Test for subgroup differences: Chi² = 1.45, df = 1 (P = 0.23), I² = 30.9%
Any antihypertensive drug versus beta blockers: there is no evidence of an overall difference in the risk of baby dying when methyldopa and calcium channel blockers together are compared with beta blockers (aRR: 1.23, 95% CI 0.81 to 1.88; 19 trials, 1652 babies; Analysis 7.3).
7.3. Analysis.
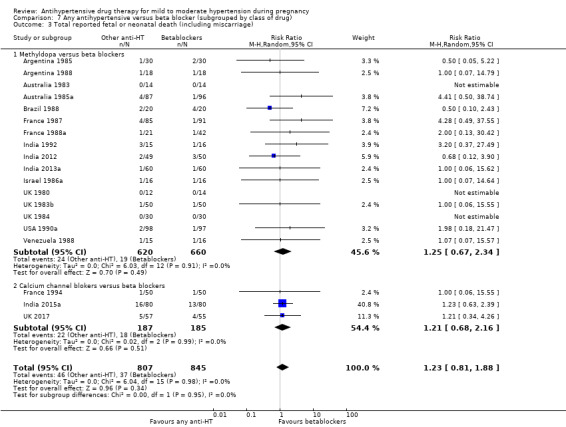
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 3 Total reported fetal or neonatal death (including miscarriage).
Subgroup analysis Methyldopa versus beta blockers was evaluated in 16 trials with 1280 babies (aRR: 1.25, 95% CI 0.67 to 2.34; Analysis 7.3.1); calcium channel blockers versus beta blockers in three trials with 372 babies (aRR: 1.21, 95% CI 0.68 to 2.16; Analysis 7.3.2). Test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.95), I² = 0%.
Small‐for‐gestational age
Any antihypertensive drug versus methyldopa: only seven small trials evaluating 597 babies reported this outcome. There is no evidence of an overall difference in the risk of having a small‐for‐gestational age baby (aRR: 0.79, 95% CI 0.52 to 1.20;, Analysis 5.4), when other antihypertensive drugs were compared with methyldopa.
5.4. Analysis.
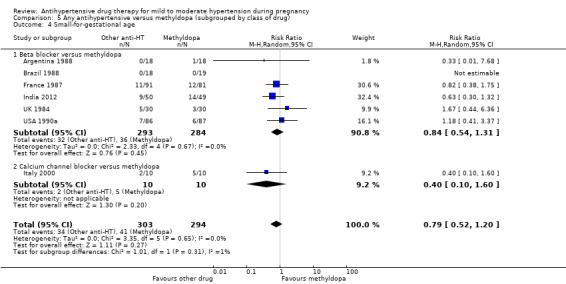
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 4 Small‐for‐gestational age.
Subgroup analysis Six trials (577 babies) compared beta blockers with methyldopa (RR 0.84; 95% CI 0.54 to 1.31;, Analysis 5.4.1), and one trial (20 babies) compared nifedipine versus methyldopa (RR 0.40; 95% CI 0.10 to 1.60; Analysis 5.4.2). Test for subgroup differences: Chi² = 1.01, df = 1 (P = 0.31), I² = 1.0%.
Any antihypertensive drug versus calcium channel blockers: four trials (200 women) reported this outcome. There is no evidence of an overall difference in the risk of having a small‐for‐gestational age baby (aRR: 1.05, 95% CI 0.64 to 1.73; Analysis 6.4), when other antihypertensive drugs were compared with calcium channel blockers.
6.4. Analysis.
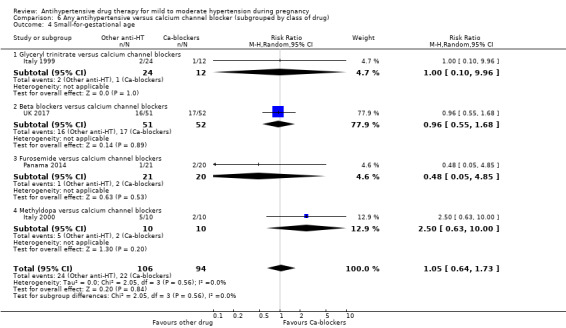
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 4 Small‐for‐gestational age.
Subgroup analysis One trial (36 babies) compared glyceryl trinitrate with nifedipine (RR 1.00; 95% CI 0.10 to 9.96; Analysis 6.4.1), another study (103 babies) compared labetalol with nifedipine (RR 0.96; 95% CI 0.55 to 1.68; Analysis 6.4.2), another trial (41 babies) compared furosemide with amlodipine (RR 0.48; 95% CI 0.05 to 4.85; Analysis 6.4.3), and one small trial (20 babies) compared methyldopa versus nifedipine (RR 2.50, 95% CI 0.63 to 10.00; Analysis 6.4.4). Test for subgroup differences: Chi² = 2.05, df = 3 (P = 0.56), I² = 0%
Any antihypertensive drug versus beta blockers: there is no evidence of an overall difference in the risk of having a small‐for‐gestational age baby when methyldopa and calcium channel blockers together are compared with beta blockers (aRR: 1.13, 95% CI 0.80 to 1.60; 7 trials, 680 babies; Analysis 7.4).
7.4. Analysis.
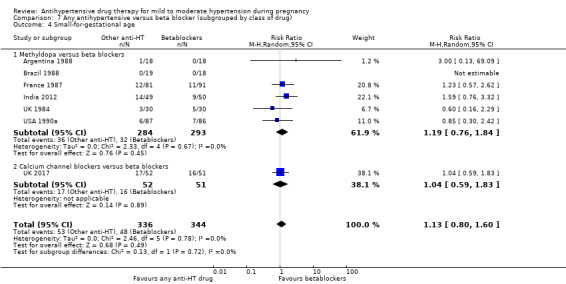
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 4 Small‐for‐gestational age.
Subgroup analysis Methyldopa versus beta blockers was evaluated in six trials with 577 babies (aRR: 1.19, 95% CI 0.76 to 1.84; Analysis 7.4.1); calcium channel blockers versus beta blockers in one trial with 103 babies (RR: 1.04, 95% CI 0.59 to 1.83; Analysis 7.4.2). Test for subgroup differences: Chi² = 0.13, df = 1 (P = 0.72), I² = 0%.
Preterm birth (less than 37 weeks)
Any antihypertensive drug versus methyldopa: only 11 trials (835 women) comparing other antihypertensives with methyldopa reported this outcome, with no evidence of an overall difference among groups (aRR: 0.91; 95% CI 0.68 to 1.22; Analysis 5.5).
5.5. Analysis.
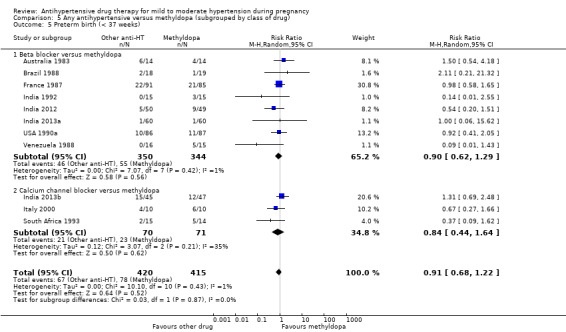
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 5 Preterm birth (< 37 weeks).
Subgroup analysis Beta blockers were compared with methyldopa in eight trials (694 women), aRR: 0.90, 95% CI 0.62 to 1.29, Analysis 5.5.1. Calcium channel blockers were compared with methyldopa in three trials (141 women), aRR: 0.84, 95% CI 0.44 to 1.64, Analysis 5.5.2. Test for subgroup differences: Chi² = 0.03, df = 1 (P = 0.87), I² = 0%.
Any antihypertensive drug versus calcium channel blockers: preterm birth was reported by six small trials (330 women), with no evidence of an overall difference among groups (aRR: 0.85, 95% CI 0.59 to 1.23; Analysis 6.5).
6.5. Analysis.
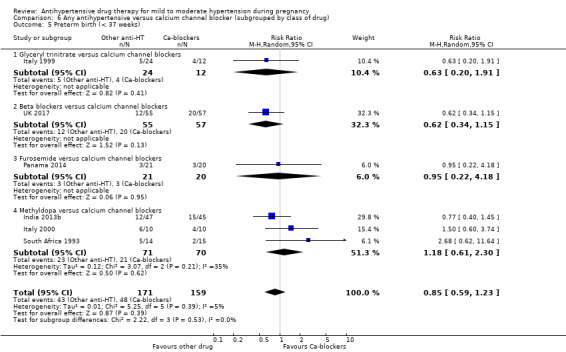
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 5 Preterm birth (< 37 weeks).
Subgroup analysis Glyceryl trinitrate versus nifedipine (1 trial, 136 women; RR 0.63; 95% CI 0.20 to 1.91; Analysis 6.5.1), furosemide versus amlodipine (1 trial, 41 women; RR 0.95; 95% CI 0.22 to 4.18; Analysis 6.5.3), labetalol versus nifedipine (1 trial, 112 women; RR 0.62; 95% CI 0.34 to 1.15; Analysis 6.5.2), and methyldopa versus calcium channel blockers (3 trials, 141 women, aRR: 1.18, 95% CI 0.61 to 2.30; Analysis 6.5.4). Test for subgroup differences: Chi² = 2.22, df = 3 (P = 0.53), I² = 0%.
Any antihypertensive drug versus beta blockers: there is no evidence of an overall difference in the risk of having a preterm birth when methyldopa and calcium channel blockers together are compared with beta blockers (aRR: 1.22, 95% CI 0.90 to 1.66; 9 trials, 806 women; Analysis 7.5).
7.5. Analysis.
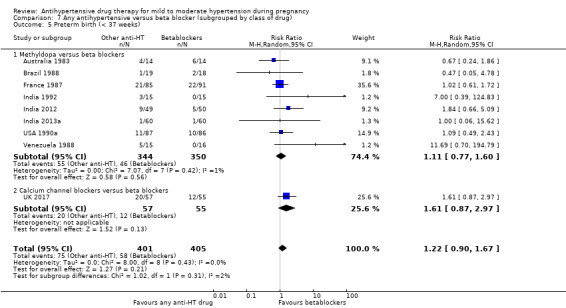
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 5 Preterm birth (< 37 weeks).
Subgroup analysis Methyldopa versus beta blockers was evaluated in eight trials with 694 women (aRR: 1.11, 95% CI 0.77 to 1.60; Analysis 7.5.1); calcium channel blockers versus beta blockers in one trial with 112 women (RR: 1.61, 95% CI 0.87 to 2.97; Analysis 7.4.2). Test for subgroup differences: Chi² = 1.02, df = 1 (P = 0.31), I² = 2.2%.
Secondary outcomes
Any antihypertensive drug versus methyldopa: maternal
Meta‐analysis shows a reduction in the risk ofcaesarean section, reported by 13 trials involving 1330 women, when alternative drugs are compared with methyldopa (aRR 0.84, 95% CI 0.74 to 0.95; Analysis 5.10). There is no evidence of an overall difference for the rest of the pre‐specified maternal secondary outcomes. Severe pre‐eclampsia was reported by one trial (311 women) of labetalol versus methyldopa (RR 1.15; 95% CI 0.56 to 2.33; Analysis 5.6). There were no cases of eclampsia in one trial (92 women) of nifedipine versus methyldopa, Analysis 5.7. Need for additional antihypertensive drug(s) was reported in 13 trials with 1081 women (aRR 0.73; 95% CI 0.47 to 1.13; Analysis 5.8). Elective delivery was reported in seven trials with 655 women (aRR: 0.99, 95% CI 0.90 to 1.09; Analysis 5.9) and induction of labour (aRR 1.14, 95% CI 0.89 to 1.45; 3 trials, 452 women; Analysis 5.11). Antenatal hospital admission was reported by two trials (275 women) of beta blockers versus methyldopa (aRR 0.56; 95% CI 0.17 to 1.86; Analysis 5.12). Placental abruption was reported by one trial (173 women) (RR 2.02; 95% CI 0.19 to 21.90; Analysis 5.13). Maternal side‐effects were reported by six trials (412 women) of beta blockers versus methyldopa (aRR 0.22; 95% CI 0.02 to 2.09; Analysis 5.14) and changed/stopped drug due to maternal side‐effects was reported by four trials (272 women) of beta blockers versus methyldopa (aRR 2.80; 95% CI 0.12 to 67.91; Analysis 5.15). Maternal deaths, HELLP syndrome, severe maternal morbidity, elective delivery andmiscarriage were not reported for this comparison.
5.10. Analysis.
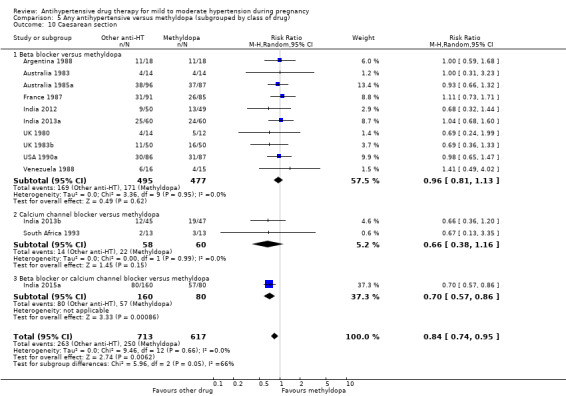
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 10 Caesarean section.
5.6. Analysis.
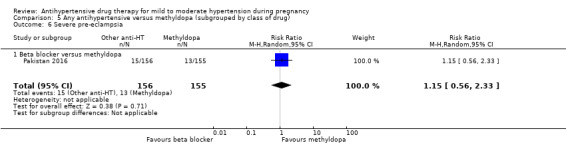
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 6 Severe pre‐eclampsia.
5.7. Analysis.
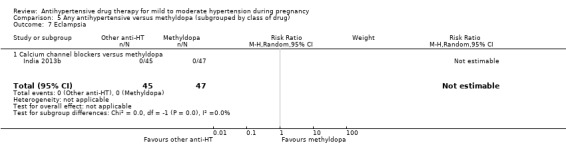
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 7 Eclampsia.
5.8. Analysis.
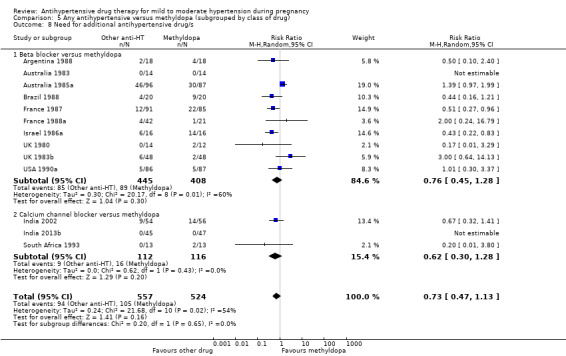
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 8 Need for additional antihypertensive drug/s.
5.9. Analysis.
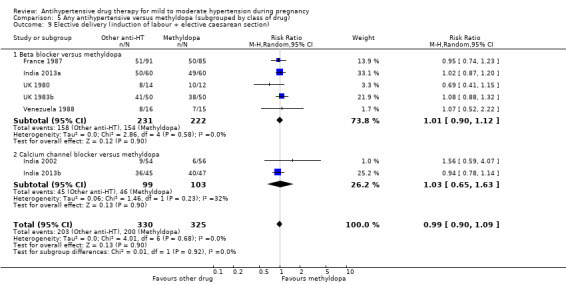
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 9 Elective delivery (induction of labour + elective caesarean section).
5.11. Analysis.
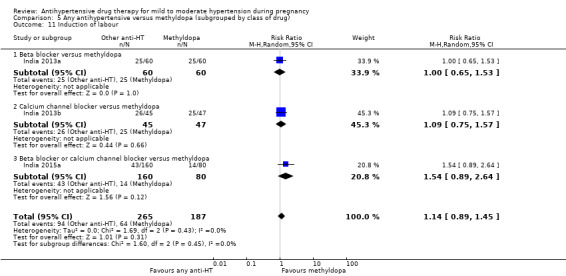
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 11 Induction of labour.
5.12. Analysis.
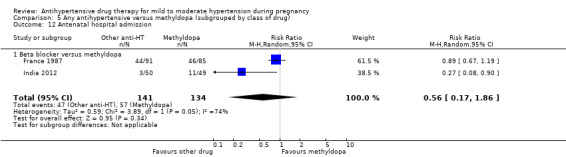
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 12 Antenatal hospital admission.
5.13. Analysis.
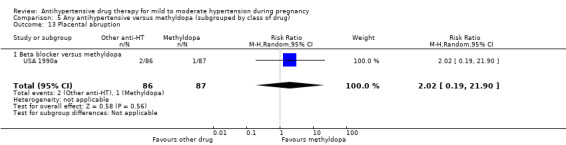
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 13 Placental abruption.
5.14. Analysis.
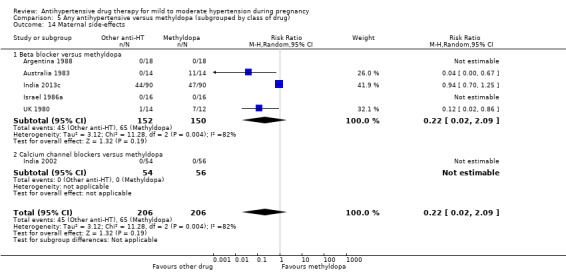
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 14 Maternal side‐effects.
5.15. Analysis.
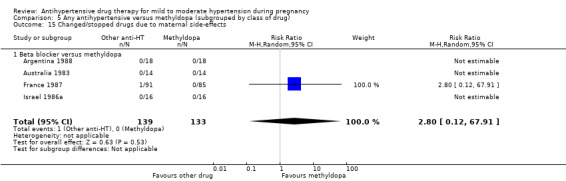
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 15 Changed/stopped drugs due to maternal side‐effects.
Any antihypertensive drug versus methyldopa: fetal/neonatal/infant
There is no evidence of an overall difference for all the pre‐specified neonatal secondary outcomes in this comparison. Admission to neonatal or intensive care nursery was reported by six trials (688 babies) (aRR 0.95, 95% CI 0.72 to 1.27; Analysis 5.16), respiratory distress syndrome by one trial (118 babies) (RR: 0.86, 95% CI 0.31 to 2.40; Analysis 5.17), and neonatal hypoglycaemia by five trials (439 babies) (aRR: 0.99, 95% CI 0.47 to 2.05; Analysis 5.18). Neonatal bradycardia was reported by two trials (146 babies) (aRR 3.00, 95% CI 0.12 to 72.18; Analysis 5.19), and neonatal jaundice by the same two trials (146 babies) (aRR 1.05, 95% CI 0.48 to 2.29; Analysis 5.20). Very low ‐ less than four‐ five‐minute Apgar score, severe prematurity, (less than 32 or less than 34 weeks' gestation), andimpaired long‐term growth and development in infancy and childhood were not reported for this comparison.
5.16. Analysis.
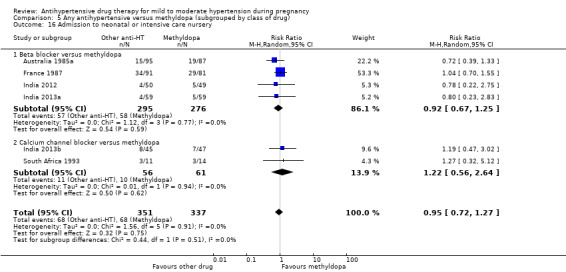
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 16 Admission to neonatal or intensive care nursery.
5.17. Analysis.
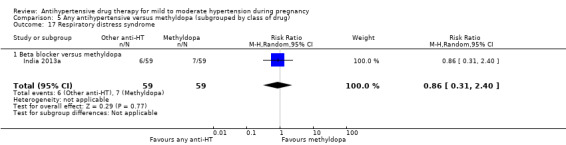
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 17 Respiratory distress syndrome.
5.18. Analysis.
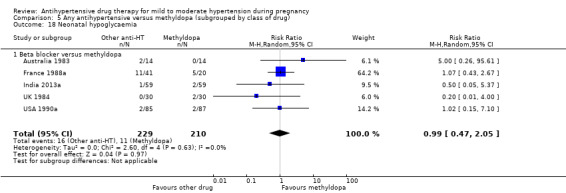
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 18 Neonatal hypoglycaemia.
5.19. Analysis.
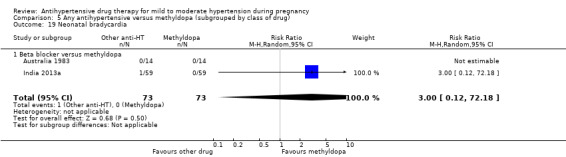
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 19 Neonatal bradycardia.
5.20. Analysis.
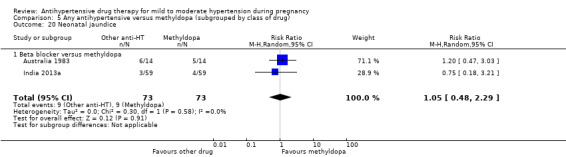
Comparison 5 Any antihypertensive versus methyldopa (subgrouped by class of drug), Outcome 20 Neonatal jaundice.
Any antihypertensive drug versus calcium channel blockers: maternal
There is no evidence of an overall difference for all the pre‐specified maternal secondary outcomes in this comparison. Maternal death (Analysis 6.6), eclampsia (Analysis 6.7) and pulmonary oedema (Analysis 6.9) were reported only by one trial (112 women) of labetalol versus nifedipine with no cases in either groups. HELLP syndrome was reported by two trials (212 women) of beta blockers versus calcium channel blockers (aRR 1.78; 95% CI 0.38 to 8.20; Analysis 6.8). Need for additional antihypertensive drug(s) was reported by five trials (440 women) (aRR 1.36; 95% CI 0.78 to 2.36; Analysis 6.10). Elective delivery was reported in three trials with 302 women (aRR 0.98, 95% CI 0.82 to 1.17; Analysis 6.11); caesarean section (six trials, 531 women, aRR: 1.05, 95% CI 0.86 to 1.29; Analysis 6.12, and induction of labour (two trials, 252 women, aRR 0.99, 95% CI 0.74 to 1.34; Analysis 6.12). Placental abruption was reported by three trials (253 women) (aRR 1.05; 95% CI 0.16 to 6.86; Analysis 6.14). Maternal side‐effects were reported by three trials (322 women) (aRR 1.40; 95% CI 0.85 to 2.29; Analysis 6.15), and changed/stopped drug due to maternal side‐effects was reported by three trials (248 women) (aRR 1.55; 95% CI 0.56 to 4.31; Analysis 6.16). Severe pre‐eclampsia, severe maternal morbidity, antenatal hospital admission andmiscarriage were not reported for this comparison.
6.6. Analysis.
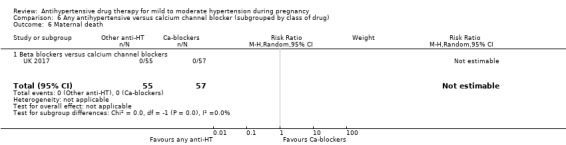
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 6 Maternal death.
6.7. Analysis.
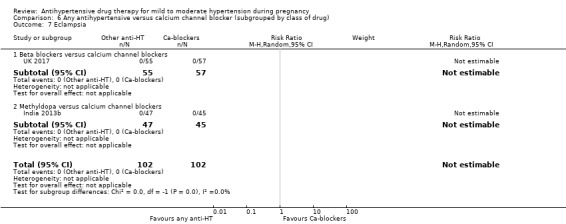
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 7 Eclampsia.
6.9. Analysis.
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 9 Pulmonary oedema.
6.8. Analysis.
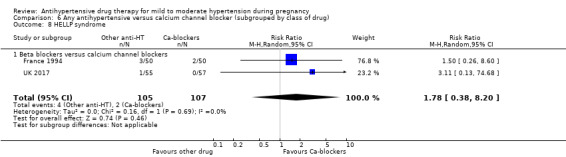
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 8 HELLP syndrome.
6.10. Analysis.
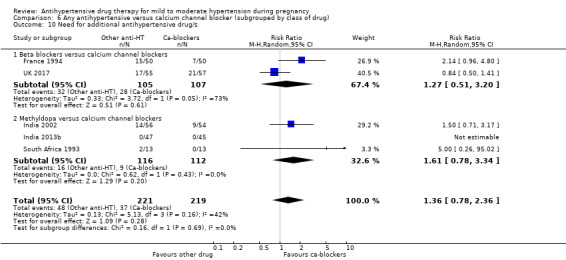
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 10 Need for additional antihypertensive drug/s.
6.11. Analysis.
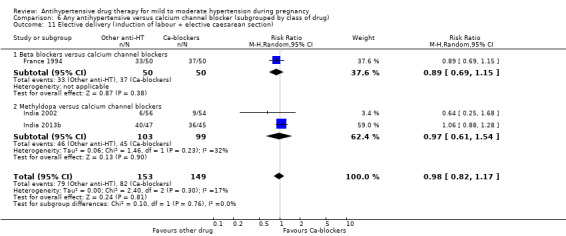
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 11 Elective delivery (induction of labour + elective caesarean section).
6.12. Analysis.
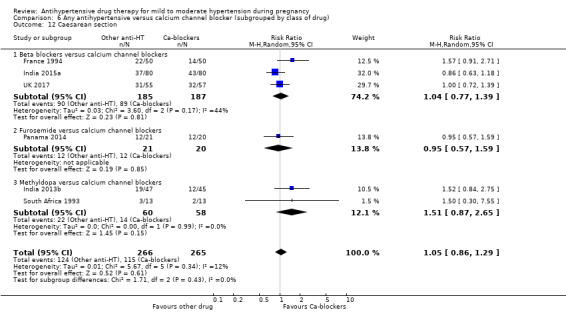
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 12 Caesarean section.
6.14. Analysis.
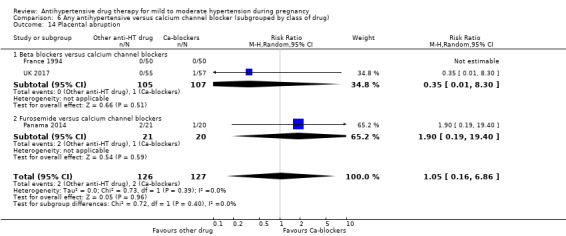
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 14 Placental abruption.
6.15. Analysis.
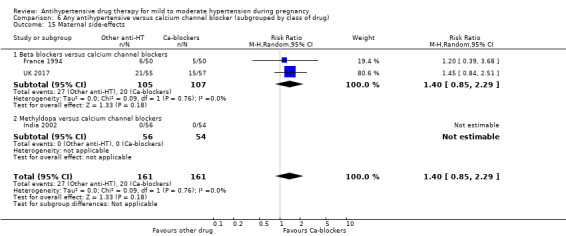
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 15 Maternal side‐effects.
6.16. Analysis.
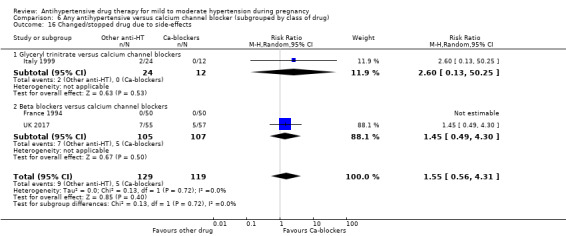
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 16 Changed/stopped drug due to side‐effects.
Any antihypertensive drug versus calcium channel blockers: fetal/neonatal/infant
There is no evidence of an overall difference for all the pre‐specified neonatal secondary outcomes in this comparison. Admission to neonatal or intensive care nursery (aRR 0.86, 95% CI 0.54 to 1.7; 4 trials, 319 babies; Analysis 6.17), respiratory distress syndrome (RR: 0.65, 95% CI 0.27 to 1.54; 1 trial, 103 babies; Analysis 6.18), neonatal hypoglycaemia by the same trial (103 babies) (RR 0.76, 95% CI 0.29 to 2.05; Analysis 6.19.Neonatal bradycardia, neonatal jaundice, very low ‐ less than four‐ five‐minute Apgar score, severe prematurity, (less than 32 or less than 34 weeks' gestation), andimpaired long‐term growth and development in infancy and childhood were not reported for this comparison.
6.17. Analysis.
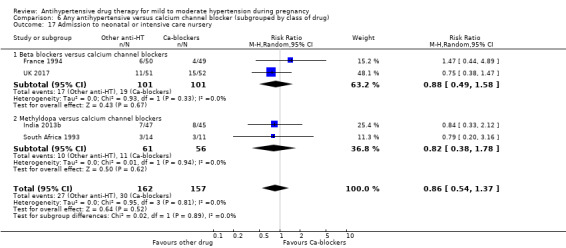
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 17 Admission to neonatal or intensive care nursery.
6.18. Analysis.
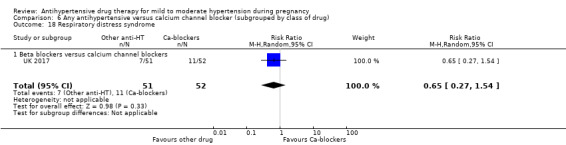
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 18 Respiratory distress syndrome.
6.19. Analysis.
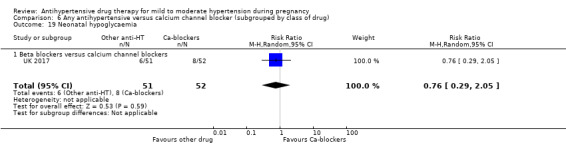
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 19 Neonatal hypoglycaemia.
Any antihypertensive drug versus beta blockers: maternal
There is no evidence of an overall difference for all the pre‐specified maternal secondary outcomes in this comparison. Maternal death (Analysis 7.6), eclampsia (Analysis 7.8) and pulmonary oedema (Analysis 7.10) were reported only by one trial (112 women) of nifedipine versus labetalol already described in Comparison 6 with no cases in both groups. Severe pre‐eclampsia was reported by one trial (311 women) of methyldopa versus labetalol (RR 0.87; 95% CI 0.43 to 1.77; Analysis 7.7). HELLP syndrome was reported by two trials (212 women) of calcium channel blocker versus beta blockers (aRR: 0.56; 95% CI 0.12 to 2.60; Analysis 7.9). Need for additional antihypertensive drug(s) was reported in 12 trials with 1065 women (aRR 1.15; 95% CI 0.76 to 1.75; Analysis 7.11), elective delivery in six trials with 553 women (aRR 1.01, 95% CI 0.91 to 1.12; Analysis 7.12), caesarean section (aRR: 1.03, 95% CI 0.90 to 1.17; 13 trials, 1344 women; Analysis 7.13), induction of labour (aRR 0.94, 95% CI 0.68 to 1.31; 2 trials, 280 women; Analysis 7.14). Antenatal hospital admission was reported by two trials (275 women) of methyldopa versus beta blockers (aRR 1.78; 95% CI 0.54 to 5.90; Analysis 7.15. Placental abruption was reported by three trials with 385 women (aRR 0.93; 95% CI 0.14 to 6.28; Analysis 7.16). Maternal side‐effects were reported by seven trials with 514 women (aRR 1.27; 95% CI 0.64 to 2.53; Analysis 7.17), and changed/stopped drug due to maternal side‐effects was reported by six trials with 484 women (aRR 0.64; 95% CI 0.23 to 1.80; Analysis 7.18). Severe maternal morbidity andmiscarriage were not reported for this comparison.
7.6. Analysis.
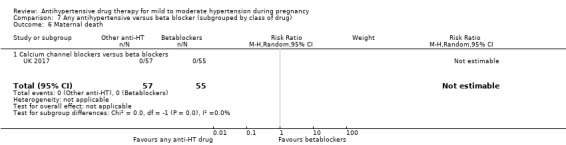
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 6 Maternal death.
7.8. Analysis.
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 8 Eclampsia.
7.10. Analysis.
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 10 Pulmonary oedema.
7.7. Analysis.
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 7 Severe pre‐eclampsia.
7.9. Analysis.
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 9 HELLP syndrome.
7.11. Analysis.
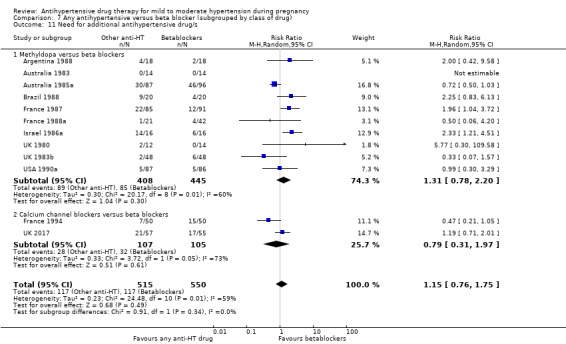
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 11 Need for additional antihypertensive drug/s.
7.12. Analysis.
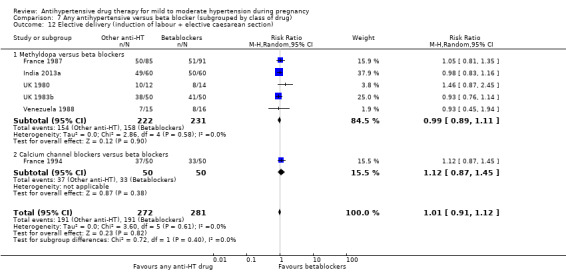
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 12 Elective delivery (induction of labour + elective caesarean section).
7.13. Analysis.
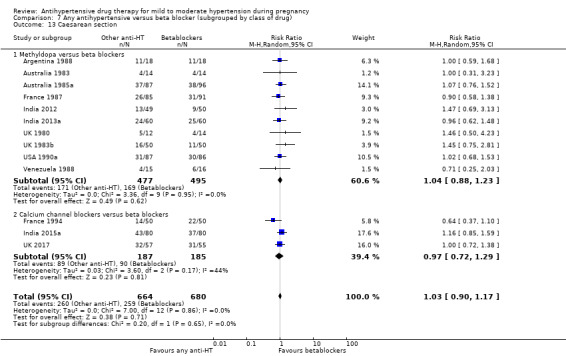
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 13 Caesarean section.
7.14. Analysis.
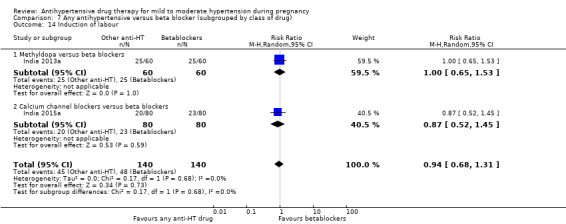
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 14 Induction of labour.
7.15. Analysis.
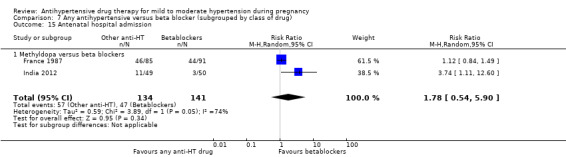
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 15 Antenatal hospital admission.
7.16. Analysis.
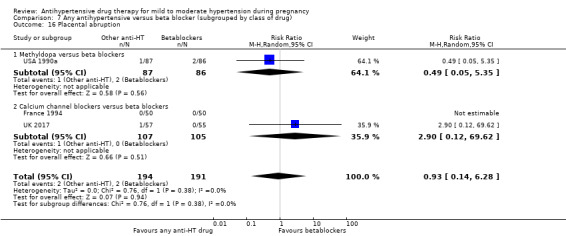
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 16 Placental abruption.
7.17. Analysis.
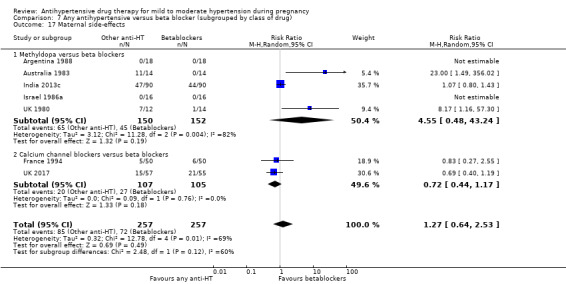
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 17 Maternal side‐effects.
7.18. Analysis.
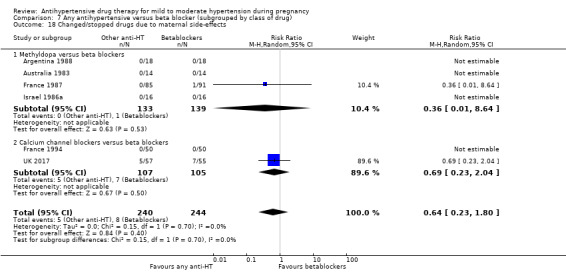
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 18 Changed/stopped drugs due to maternal side‐effects.
Any antihypertensive drug versus beta blockers: fetal/neonatal/infant
There is no evidence of an overall difference for all the pre‐specified neonatal secondary outcomes in this comparison. Admission to neonatal or intensive care nursery was reported by six trials (773 babies) (aRR 1.10, 95% CI 0.84 to 1.45; Analysis 7.19); respiratory distress syndrome by two trials (221 babies) (RR 1.37, 95% CI 0.71 to 2.66; Analysis 7.20); and neonatal hypoglycaemia by six trials (542 babies) (aRR 1.11, 95% CI 0.62 to 2.00; Analysis 7.21). Neonatal bradycardia was reported by two trials (146 babies) (aRR 0.33, 95% CI 0.01 to 8.02; Analysis 7.22), and neonatal jaundice by the same two trials (146 babies) (aRR 0.95, 95% CI 0.44 to 2.09; Analysis 7.23.Very low ‐ less than four‐ five‐minute Apgar score, severe prematurity, (less than 32 or less than 34 weeks' gestation), andimpaired long‐term growth and development in infancy and childhood were not reported for this comparison.
7.19. Analysis.
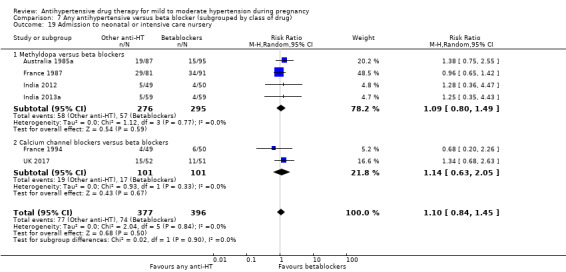
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 19 Admission to neonatal or intensive care nursery.
7.20. Analysis.
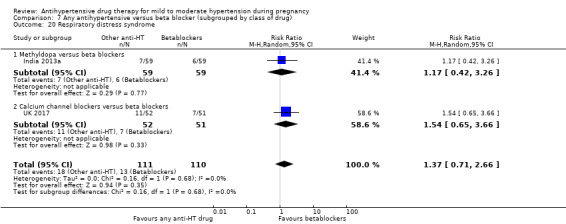
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 20 Respiratory distress syndrome.
7.21. Analysis.
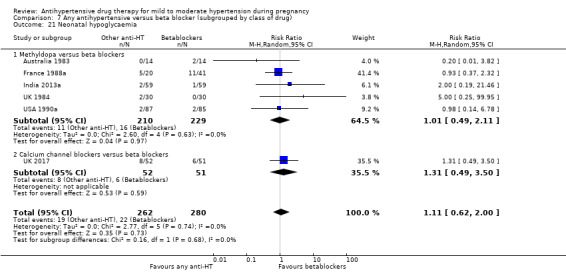
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 21 Neonatal hypoglycaemia.
7.22. Analysis.
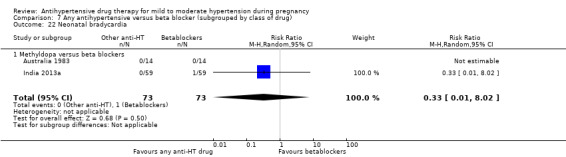
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 22 Neonatal bradycardia.
7.23. Analysis.
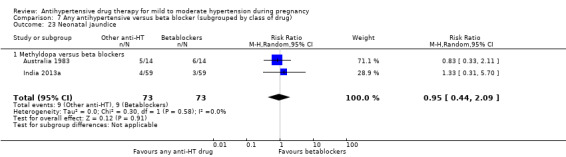
Comparison 7 Any antihypertensive versus beta blocker (subgrouped by class of drug), Outcome 23 Neonatal jaundice.
Discussion
Summary of main results
Moderate‐certainty evidence suggests that antihypertensive drugs, rather than placebo or no treatment, probably half the risk that a pregnant woman with mild or moderate hypertension will have one or more episodes of severe hypertension. This is unsurprising, as the antihypertensive effects of these agents have been well demonstrated in the non‐pregnant population. Also unsurprising is that women allocated an antihypertensive were less likely to need another agent for blood pressure control than those allocated placebo or no antihypertensive treatment. The clinical importance of this reduction in risk depends on factors such as duration and severity of the hypertension, and the impact on the consequences of severe hypertension, such as antenatal hospital admission or stroke. As few trials reported on antenatal hospital admission, this risk remains unclear. One of the main objectives in treating women with mild to moderate hypertension with antihypertensive drugs is to prevent or delay progression to pre‐eclampsia. Low‐certainty evidence suggests that the effect on this risk may be anywhere between a 25% reduction and a 14% increase associated with antihypertensive therapy. The review is also underpowered to assess rare serious adverse events such as stroke.
For this review update, moderate‐certainty evidence shows that there is probably little or no effect on the overall risk of the fetus or baby dying (including miscarriage). Although there is a trend towards a reduction in the overall risk associated with the use of antihypertensive drugs, confidence intervals show that the effect includes everything from a 50% reduction to a 4% increase.
Moderate certainty of the evidence also shows that there is probably little or no effect in the risk of having small‐for‐gestational‐age babies. The confidence intervals include everything from a 14% reduction to a 18% increase on this risk. It has been argued that lowering maternal blood pressure may cause fetal growth restriction (von Dadelszen 2000). Although in this review we have not found such an effect. Being small‐for‐gestational age is an important marker for neurodevelopmental delay. The ideal timing of delivery for such babies is unclear, regardless of whether the woman has raised blood pressure or not (Thornton 2004).
In relation to the risk of elective delivery and preterm birth of less that 37 weeks' gestation, moderate‐certainty evidence found that there is probably little or no difference among women treated and untreated with antihypertensives during pregnancy.
Few children exposed to antihypertensive drugs in utero have been followed up beyond the perinatal period. Two trials (Italy 1998; UK 1983a) have reported follow‐up of a total of 110 children at age one year, and of 190 children at age 18 months, respectively. Another (UK 1976) reported follow‐up at seven and a half years of age for children randomised before 28 weeks' gestation. All studies were too small to provide reliable estimates of the benefits and adverse effects for surviving children.
If an antihypertensive drug is used for women with mild to moderate hypertension, methyldopa seems to be less effective than an alternative drug (in our comparisons beta blockers and calcium channel blockers) for preventing severe hypertension, and probably women receiving methyldopa have an increased risk of caesarean section than those receiving other antihypertensives. There is insufficient evidence for conclusions about comparative side‐effects of the alternative drugs being studied, although there is potential for reporting or detection bias as this outcome was only reported by half the trials, all of them open‐label studies. The comparative effects on other outcomes for both mother and babies are unclear.
Overall completeness and applicability of evidence
We believe that this is a comprehensive review that covers the most up‐to‐date evidence from randomised controlled trials conducted around the world. We did not apply language restrictions for selecting studies.
Nine eligible trials (1186 women) have been published since this review, first available in 2001, was subsequently updated in 2007 and 2014. However, still 25 (out of 58) trials included in this review were published before 1990 ‐ almost 30 years ago. Evidence from these trials accounts for 40% of the total number of women recruited. Maternal and perinatal survival, availability of resources, technologies for diagnosis and treatment of complications, as well as indications for termination of pregnancy for maternal or perinatal conditions could have changed substantially since then. On the other hand, only a quarter of women considered in this review were recruited in low‐ and middle‐income countries, where keeping the woman in a stable condition aiming at prolonging pregnancy may have major impact on neonatal survival.
These considerations, amongst others, may lead to a different response to the uncertainties presented in this review in different settings.
Currently, there are eight ongoing trials expecting to recruit over 6000 women, seven of them comparing one or more antihypertensive agents versus no treatment or placebo, and the other comparing alternative antihypertensive drugs. Thus, it is expected that this review may double in size when ongoing trials are concluded.
Quality of the evidence
Overall, the quality of the studies included in this review is moderate to poor. Concealment of allocation was adequate for only 32 of the 58 trials (57%) contributing with data. Most trials with unclear concealment of allocation were described as 'randomised' with no further details. Some of these studies were stated to be double‐blind, but with no information about how this was achieved. Only 14 of the studies evaluating a single agent used a placebo for the control group. All trials comparing alternative antihypertensive drugs were open‐label trials. None of the trials comparing a single drug against no treatment, or those comparing one agent with another, mentioned blinding in the assessment of outcome. The primary outcome reported by trials usually was mean blood pressure, or a fall in blood pressure, thus sample size to test the hypothesis was in general small for other clinically important outcomes, such as maternal or perinatal morbidity. The largest trial in this review recruited only 314 women, and only six studies had comparison arms of more than 100 women. Nine trials (out of 64) reported sample size and power calculations.
Evidence for individual outcomes was graded from very low to moderate certainty; downgrading was mainly due to study design limitations and imprecision of effect estimates. For many outcomes, trials contributing data examined a range of different hypertensive drugs with different doses and mechanisms of action. While we did not downgrade for indirectness, we conservatively used random‐effects model in meta‐analyses for these outcomes, thus overall results should be interpreted with caution.
Potential biases in the review process
A large number of outcomes are reported in these trials, and for many, data are only available from a small number of studies. There is therefore considerable potential to be misled by reporting bias. For example, in the comparison of any antihypertensive with no antihypertensive drugs/placebo, only six of the 31 trials reported respiratory distress syndrome, and all had results favouring antihypertensive treatment. Without further information, it is impossible to know whether the other 25 trials did not collect these data, or whether they did not report it because it did not favour antihypertensive therapy.
We also report data from a large number of subgroups. Although these subgroups were all pre‐specified, the numbers in many cases are small, and for one in 20 the difference will be statistically significant purely by chance. Results from these subgroups should therefore be interpreted with caution, as there is considerable potential to be misled by random errors.
Agreements and disagreements with other studies or reviews
In the National Institute for Health and Clinical Excellence (NICE) guideline CG107Hypertension in pregnancy: The management of hypertensive disorders during pregnancy, the authors do not recommend antihypertensive drugs in mild pregnancy‐induced hypertension or pre‐eclampsia. Only when blood pressure is greater than 150/100 mmHg, labetalol, methyldopa or nifedipine are advised as the drugs of choice (NICE 2011). On the other hand, the Canadian Hypertensive Disorders of Pregnancy Working Group updated their 2008 Guidelines in 2014 and recommends to keep systolic blood pressure (SBP) at 130 to 155 mmHg and diastolic blood pressure (DBP) at 80 to 105 mmHg with antihypertensive drugs in pregnant women with non‐severe hypertension without comorbid conditions, and recommends pharmacological treatment in all women with mild to moderate hypertension with comorbid conditions to keep SBP at < 140 mmHg and DBP at < 90 mmHg (Canadian HDP Working Group 2014).
Authors' conclusions
Implications for practice.
Antihypertensive drug therapy for mild to moderate hypertension during pregnancy reduces the risk of severe hypertension and the need for additional antihypertensive drugs. The effect on total reported fetal and neonatal death (including miscarriage) remains unclear.
If antihypertensive drugs are used, beta blockers and calcium channel blockers appear to be more effective than the alternatives for preventing severe hypertension. Reporting of side‐effects are scarce, so the previous experience of the clinician and the woman's preference may guide the choice of agent.
Implications for research.
High‐quality, large‐sized randomised controlled trials are required in order to provide reliable estimates of the true benefits and adverse effects of antihypertensive treatment for mild to moderate hypertension. We need to know the effects for both mother and baby, as well as the costs to the health services, to women and to their families. Other outcomes relevant to women could be included in such studies, such as antenatal hospital admission, clinic visits, and disruption to their family and working life. The development of a Core Outcome Set (COS) for studies reporting on hypertensive disorders prevention and treatment is currently underway. Long‐term follow‐up of children entered into such trials as fetuses is needed in order to assess whether there are any effects on infant and child development. Trials could also assess the level of blood pressure at which antihypertensive treatment becomes worthwhile.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2018 | New citation required and conclusions have changed | New co‐author joined the team: Dr Celina Gialdini. Nine new studies included (two of sildenafil versus placebo, three of labetalol versus methyldopa, one of nifedipine versus methyldopa, one of nifedipine versus labetalol, one of amlodipine versus furosemide and a three‐arm trial of nifedipine versus labetalol versus methyldopa). Twenty new studies were excluded with reasons and there are eight new ongoing trials. Five studies Finland 1988b; France 1990; Hong Kong 1993; India 2011; Spain 1988, excluded in previous versions of this review for not reporting clinical outcomes, are now included, specifying that they do not contribute data. Figure 1: study flow diagram was added. The introduction was updated in line with new definitions for pre‐eclampsia, and the inclusion of new agents (amlodipine and sildenafil). Common side‐effects of each family of drugs were added in the introduction section "How the intervention might work". Conclusions changed: Implications for practice now reflect the benefits of avoiding hypertensive crises and use of additional antihypertensives, and the possible benefits of beta‐blockers and calcium channel blockers on avoiding a hypertensive crisis. |
| 13 September 2017 | New search has been performed | Search updated. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 14 June 2013 | New citation required but conclusions have not changed | Three new trials were added for this update: one of beta blockers versus methyldopa; one of calcium channel blockers versus methyldopa; and a three‐arm trial of labetalol versus methyldopa versus no treatment. |
| 31 May 2013 | New search has been performed | Review authors updated. April 2013: search updated. Nineteen trials added to excluded studies (not all are new studies as, for some, new information has become available leading them to be reclassified as excluded). Four figures added: 'Risk of bias' graph, 'Risk of bias' summary, and two funnel plots. |
| 6 August 2012 | Amended | Search updated. Nineteen new reports added to Studies awaiting classification. |
| 8 May 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New search has been performed | March 2006: Search updated. Six new trials were added to included studies. Twenty‐seven new trials added to excluded studies (not all are new studies as, for some, new information has become available leading them to be reclassified as excluded). Changes in the outcome tree (calcium channel blockers versus beta blockers are no longer referred to the beta blockers review (Magee 2000) as this comparison is now part of the group 'any antihypertensive versus calcium channel blockers'). Changes in the text to reflect new data. An entire section describing the general characteristics of the excluded trials has been added in the text. Acknowledgements to authors for unpublished data. |
Acknowledgements
Thanks to Rory Collins and David Henderson Smart for their contribution to earlier versions of this review.
Thanks to Therese Dowswell for her invaluable support and help for the 2018 update, including preparation of GRADE tables.
Thanks to Marta Roqué i Figuls from the Iberoamerican Cochrane Centre, Barcelona, (Spain), and to Zulma Ortiz from the Centro de Investigaciones Epidemiologicas, Academia Nacional de Medicina, Buenos Aires, (Argentina) for their help in locating original papers in Spanish and Portuguese.
Special thanks to Greg Davis (Australia 2001), Salvio Freire (Brazil 1988), Soubhi Kahhale and Silvana Darcie (Brazil 2000b), Denis J Nascimento (Brazil 2000a), Pierre‐Francois Plouin (Caribbean Is.1990), Elba Gomez Sosa (Cuba 1994), Fakhrolmolouk Yassaee (Iran 2000), Sean Blake and Dermot MacDonald (Ireland 1991), Laura Pello (Italy 1988), Isabella Neri and Fabio Facchinetti (Italy 1999), Claudio Borghi (Italy 2000), Klas Wichman and Orvar Finnström (Sweden 1984), Stellan Högstedt (Sweden 1985), James Walker (UK 1982), Peter C Rubin (UK 1990), DJ Cruickshank (UK 1992), Baha Sibai (USA 1990a), and Silvia Sanchez (Venezuela 1985), who kindly provided unpublished data for this review.
The Centre for Perinatal Health Services Research, University of Sydney, supported previous versions of this review.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
ICTRP
hypertension AND pregnancy
pre‐eclampsia AND pregnancy
preeclampsia AND pregnancy
ClinicalTrials.gov
Advanced search
hypertension, pregnancy induced (in Condition) AND Intervention studies
pre‐eclampsia (in condition) AND Intervention studies
Data and analyses
Comparison 1. Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by class of drug).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 20 | 2558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.40, 0.60] |
| 1.1 Beta blocker versus no antihypertensive drugs/placebo | 8 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.26, 0.57] |
| 1.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.77] |
| 1.3 Methyldopa versus no antihypertensive drugs/placebo | 2 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.58] |
| 1.4 Methyldopa + other drug versus no antihypertensive drugs/placebo | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.70] |
| 1.5 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.25, 0.74] |
| 1.6 Calcium channel blocker versus no antihypertensive drugs/placebo | 4 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.11] |
| 1.7 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.09] |
| 2 Proteinuria/pre‐eclampsia | 23 | 2851 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2.1 Beta blocker versus no antihypertensive drugs/placebo | 8 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.56, 0.99] |
| 2.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.63, 2.51] |
| 2.3 Methyldopa versus no antihypertensive drugs/placebo | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.55, 2.64] |
| 2.4 Methyldopa + other drug versus no antihypertensive drugs/placebo | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.44, 1.34] |
| 2.5 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.28, 1.79] |
| 2.6 Calcium channel blocker versus no antihypertensive drugs/placebo | 4 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.02, 1.92] |
| 2.7 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.52] |
| 2.8 Glyceryl trinitrate versus no antihypertensive drugs/placebo | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.28] |
| 2.9 Regular antihypertensive therapy versus no antihypertensive drugs/placebo | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.39] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 29 | 3365 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 3.1 Beta blocker versus no antihypertensive drugs/placebo | 11 | 1045 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.45, 2.53] |
| 3.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.28, 4.76] |
| 3.3 Methyldopa versus no antihypertensive drugs/placebo | 3 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 2.10] |
| 3.4 Methyldopa + other drug versus no antihypertensive drugs/placebo | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.46] |
| 3.5 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.54] |
| 3.6 Calcium channel blocker versus no antihypertensive drugs/placebo | 5 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.28, 2.10] |
| 3.7 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.25, 2.73] |
| 3.8 Regular antihypertensive therapy versus no antihypertensive drugs/placebo | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.22, 22.51] |
| 3.9 Sildenafil versus no antihypertensive drugs/placebo | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.13, 1.95] |
| 4 Fetal or neonatal death (subgrouped by time of death) | 27 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Miscarriage | 7 | 1058 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.15] |
| 4.2 Stillbirth | 18 | 2480 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.58, 2.30] |
| 4.3 Perinatal death | 22 | 2517 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.41] |
| 4.4 Neonatal death | 6 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.87] |
| 5 Small‐for‐gestational age | 21 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 5.1 Beta blocker versus no antihypertensive drugs/placebo | 9 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.86, 1.97] |
| 5.2 Beta blocker + other drug versusno antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.01] |
| 5.3 Methyldopa versus no antihypertensive drugs/placebo | 2 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.02, 47.65] |
| 5.4 Methyl dopa + other drug versus no antihypertensive drugs/placebo | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.62] |
| 5.5 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.98] |
| 5.6 Calcium channel blocker versus no antihypertensive drugs/placebo | 3 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.60, 1.16] |
| 5.7 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.57] |
| 5.8 Sildenafil versus no antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.43] |
| 6 Small‐for‐gestational age (subgrouped by severity) | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Birthweight < 10th centile | 11 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.21] |
| 6.2 Birthweight < 5th centile | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.87, 10.32] |
| 6.3 Unspecified | 7 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.11] |
| 7 Preterm birth (< 37 weeks) | 15 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 7.1 Beta blocker versus no antihypertensive drugs/placebo | 4 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.61, 1.32] |
| 7.2 Beta blocker + other drug versusno antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.48] |
| 7.3 Methyldopa versus no antihypertensive drugs/placebo | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.73, 3.57] |
| 7.4 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.22, 2.06] |
| 7.5 Calcium channel blocker versus no antihypertensive drugs/placebo | 4 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.86, 1.18] |
| 7.6 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.66] |
| 8 Preterm birth (subgrouped by gestational age) | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 < 37 weeks | 10 | 1569 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.12] |
| 8.2 < 36 weeks | 2 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.04, 5.68] |
| 8.3 < 34 weeks | 5 | 792 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.62, 1.94] |
| 8.4 Unspecified | 4 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.73] |
| 9 Maternal death | 5 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.18, 7.02] |
| 10 Severe pre‐eclampsia | 3 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.02] |
| 11 Eclampsia | 7 | 713 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.13, 2.06] |
| 12 HELLP syndrome | 3 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.32, 3.50] |
| 12.1 Calcium channel blocker versus no antihypertensive drugs/placebo | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.38, 10.78] |
| 12.2 Sildenafil versus no antihypertensive drugs/placebo | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.10, 2.97] |
| 13 Pulmonary oedema | 2 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.13, 11.75] |
| 13.1 Beta blocker versus no antihypertensive drugs/placebo | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 5.23 [0.25, 107.39] |
| 13.2 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.07, 3.48] |
| 14 Need for additional antihypertensive drug/s | 11 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.38, 0.65] |
| 14.1 Beta blocker versus no antihypertensive drugs/placebo | 3 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.82] |
| 14.2 Beta blocker + other drug versusno antihypertensive drugs/placebo | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.10, 1.15] |
| 14.3 Methyldopa + other drug versus no antihypertensive drugs/placebo | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.83] |
| 14.4 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.22, 1.20] |
| 14.5 Calcium channel blocker versus no antihypertensive drugs/placebo | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.35, 1.28] |
| 14.6 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.09] |
| 14.7 Sildenafil versus no antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.35, 0.88] |
| 15 Elective delivery (induction of labour + elective caesarean section) | 4 | 710 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.04] |
| 15.1 Beta blocker versus no antihypertensive drugs/placebo | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.12] |
| 15.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.10] |
| 16 Caesarean section | 21 | 2724 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 16.1 Beta blocker versusno antihypertensive drugs/placebo | 8 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.86, 1.31] |
| 16.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.85] |
| 16.3 Methyldopa versus no antihypertensive drugs/placebo | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 16.4 Methyldopa + other drug versus no antihypertensive drugs/placebo | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.8 [0.69, 4.72] |
| 16.5 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.45, 1.52] |
| 16.6 Calcium channel blocker versus no antihypertensive drugs/placebo | 3 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.11] |
| 16.7 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.20, 1.91] |
| 16.8 Regular antihypertensive therapy versus no antihypertensive drugs/placebo | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.9 Sildenafil versus no antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.12] |
| 17 Induction of labour | 5 | 563 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 17.1 Beta blocker versus no antihypertensive drugs/placebo | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.17] |
| 17.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.26, 0.90] |
| 17.3 Methyldopa versus no antihypertensive drugs/placebo | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.46, 5.09] |
| 18 Antenatal hospital admission | 4 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.08] |
| 18.1 Beta blocker versus no antihypertensive drugs/placebo | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.24] |
| 18.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.18] |
| 18.3 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.26, 0.98] |
| 19 Placental abruption | 13 | 1568 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.70, 3.17] |
| 19.1 Beta blocker versus no antihypertensive drugs/placebo | 3 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 5.11 [0.25, 104.96] |
| 19.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [0.33, 14.97] |
| 19.3 Methyldopa versus no antihypertensive drugs/placebo | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 2 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.13, 2.16] |
| 19.5 Calcium blocker versus no antihypertensive drugs/placebo | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.26, 8.87] |
| 19.6 Alpha blocker versus no antihypertensive drugs/placebo | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 3.33 [0.34, 32.96] |
| 19.7 Regular antihypertensive therapy versus no antihypertensive drugs/placebo | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.8 Sildenafil versus no antihypertensive drugs/placebo | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.39, 10.75] |
| 20 Maternal side‐effects | 11 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.89, 4.43] |
| 20.1 Beta blocker versusno antihypertensive drugs/placebo | 7 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.66, 15.02] |
| 20.2 Beta blocker + other drug versusno antihypertensive drugs/placebo | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.30, 5.61] |
| 20.3 Methyldopa versus no antihypertensive drugs/placebo | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Calcium channel blocker versus no antihypertensive drugs/placebo | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.60, 1.52] |
| 20.5 Glyceryl trinitrate versus no antihypertensive drugs/placebo | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 18.75 [1.25, 281.11] |
| 21 Changed/stopped drugs due to maternal side‐effects | 16 | 1503 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.92, 4.06] |
| 21.1 Beta blocker versus no antihypertensive drugs/placebo | 9 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.61, 5.57] |
| 21.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.30, 5.61] |
| 21.3 Methyldopa versus no antihypertensive drugs/placebo | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Calcium channel blocker versus no antihypertensive drugs/placebo | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 4.02 [0.45, 35.97] |
| 21.5 Glyceryl trinitrate versus no antihypertensive drugs/placebo | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 18.75 [1.25, 281.11] |
| 21.6 Sildenafil versus no antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.34] |
| 22 Admission to neonatal or intensive care nursery | 10 | 1570 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.83, 1.22] |
| 22.1 Beta blocker versus no antihypertensive drugs/placebo | 3 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.82, 1.41] |
| 22.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.14] |
| 22.3 Methyldopa versus no antihypertensive drugs/placebo | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.88, 2.78] |
| 22.4 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.95] |
| 22.5 Calcium channel blocker versus no antihypertensive drugs/placebo | 2 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.87, 1.62] |
| 22.6 Sildenafil versus no antihypertensive drugs/placebono antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 23 Respiratory distress syndrome | 6 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.99] |
| 23.1 Beta blocker versus no antihypertensive drugs/placebo | 3 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.13, 0.83] |
| 23.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.10] |
| 23.3 Calcium channel blocker versus no antihypertensive drugs/placebo | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.06] |
| 23.4 Sildenafil versus no antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.42] |
| 24 Neonatal hypoglycaemia | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.15] |
| 24.1 Beta blocker versus no antihypertensive drugs/placebo | 2 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.13, 3.83] |
| 24.2 Beta blocker + other drug versus no antihypertensive drugs/placebo | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.35, 1.84] |
| 24.3 Beta blocker or methyldopa versus no antihypertensive drugs/placebo | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.23, 18.24] |
| 24.4 Calcium channel blocker versus no antihypertensive drugs/placebo | 1 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.39, 1.21] |
| 24.5 Sildenafil versus no antihypertensive drugs/placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.23, 2.81] |
| 25 Neonatal bradycardia | 3 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.31, 5.24] |
| 25.1 Beta blocker versus no antihypertensive drugs/placebo | 2 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.68, 7.16] |
| 25.2 Beta blocker + other drug versusno antihypertensive drugs/placebo | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.16] |
| 26 Neonatal jaundice | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.15] |
| 26.1 Beta blocker versus no antihypertensive drugs/placebo | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.19, 1.47] |
| 26.2 Methyldopa versus no antihypertensive drugs/placebo | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.61] |
| 26.3 Calcium channel blocker versus no antihypertensive drugs/placebo | 1 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.10] |
| 27 Follow‐up of the children at 1 year: cerebral palsy | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 28 Follow‐up of the children at 7 1/2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 28.1 Chronic ill health | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.16, 3.06] |
| 28.2 Impaired hearing | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.38, 3.14] |
| 28.3 Impaired vision | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
Comparison 2. Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by type of hypertensive disorder at trial entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 20 | 2558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.40, 0.60] |
| 1.1 Hypertension alone | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.15, 0.47] |
| 1.2 Hypertension + proteinuria | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 1.3 Chronic hypertension | 4 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.34, 0.98] |
| 1.4 Unclassified/mixed | 10 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.47, 0.77] |
| 2 Proteinuria/pre‐eclampsia | 23 | 2851 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2.1 Hypertension alone | 8 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| 2.2 Hypertension + proteinuria | 2 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.92, 2.97] |
| 2.3 Chronic hypertension | 4 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.31] |
| 2.4 Unclassified/mixed | 9 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.46] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 28 | 3330 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.05] |
| 3.1 Hypertension alone | 7 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.39, 1.67] |
| 3.2 Hypertension + proteinuria | 4 | 575 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.29, 1.79] |
| 3.3 Chronic hypertension | 5 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.39, 2.34] |
| 3.4 Unclassified/mixed | 12 | 1422 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.34, 1.09] |
| 4 Small‐for‐gestational age | 21 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 4.1 Hypertension alone | 6 | 623 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.45, 2.00] |
| 4.2 Hypertension + proteinuria | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.83, 1.83] |
| 4.3 Chronic hypertension | 5 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.30] |
| 4.4 Unclassified/mixed | 7 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 5 Preterm birth (< 37 weeks) | 15 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 5.1 Hypertension alone | 5 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.09] |
| 5.2 Hypertension + proteinuria | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.91, 1.65] |
| 5.3 Chronic hypertension | 2 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.72, 1.85] |
| 5.4 Unclassified/mixed | 6 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.12] |
Comparison 3. Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by gestation at trial entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 20 | 2558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.40, 0.60] |
| 1.1 Entry < 32 weeks | 7 | 1071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.83] |
| 1.2 Entry > 32 weeks | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.32] |
| 1.3 Unclassified/mixed | 12 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.30, 0.53] |
| 2 Proteinuria/pre‐eclampsia | 23 | 2851 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2.1 Entry < 32 weeks | 8 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.82, 1.39] |
| 2.2 Entry > 32 weeks | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.12, 0.97] |
| 2.3 Unclassified/mixed | 13 | 1584 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.25] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 28 | 3330 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.05] |
| 3.1 Entry < 32 weeks | 11 | 1376 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.42, 1.25] |
| 3.2 Entry > 32 weeks | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.37] |
| 3.3 Unclassified/mixed | 16 | 1834 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.44, 1.25] |
| 4 Small‐for‐gestational age | 21 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 4.1 Entry < 32 weeks | 11 | 1285 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.68, 1.15] |
| 4.2 Entry > 32 weeks | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.46, 2.67] |
| 4.3 Unclassified/mixed | 9 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.75, 1.61] |
| 5 Preterm birth (< 37 weeks) | 15 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 5.1 Entry < 32 weeks | 6 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.14] |
| 5.2 Entry > 32 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Unclassified/mixed | 9 | 1148 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.18] |
Comparison 4. Any antihypertensive drug versus no antihypertensive drugs/placebo (subgrouped by use of placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 20 | 2558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.40, 0.60] |
| 1.1 Placebo | 10 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 1.2 No placebo | 10 | 1621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.62] |
| 2 Proteinuria/pre‐eclampsia | 23 | 2851 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2.1 Placebo | 10 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.65, 1.24] |
| 2.2 No placebo | 13 | 1982 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.26] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 28 | 3330 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.05] |
| 3.1 Placebo | 11 | 1011 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.35, 1.47] |
| 3.2 No placebo | 17 | 2319 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.47, 1.13] |
| 4 Small‐for‐gestational age | 21 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 4.1 Placebo | 9 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.35] |
| 4.2 No placebo | 12 | 1872 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.29] |
| 5 Preterm birth (< 37 weeks) | 15 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 5.1 Placebo | 5 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 5.2 No placebo | 10 | 1575 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
Comparison 5. Any antihypertensive versus methyldopa (subgrouped by class of drug).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 11 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.88] |
| 1.1 Beta blocker versus methyldopa | 9 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 1.2 Calcium channel blockers versus methyldopa | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.22] |
| 2 Proteinuria/pre‐eclampsia | 11 | 997 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.58, 1.06] |
| 2.1 Beta blocker versus methyldopa | 10 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 2.2 Calcium channel blockers versus methyldopa | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.27] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 22 | 1791 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.14] |
| 3.1 Beta blocker versus methyldopa | 16 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.43, 1.50] |
| 3.2 Calcium channel blocker versus methyldopa | 4 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.65] |
| 3.3 Beta blockers or calcium channel blockers versus methyldopa | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.27] |
| 3.4 Ketanserin versus methyldopa | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 4 Small‐for‐gestational age | 7 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.52, 1.20] |
| 4.1 Beta blocker versus methyldopa | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 4.2 Calcium channel blocker versus methyldopa | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.10, 1.60] |
| 5 Preterm birth (< 37 weeks) | 11 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.22] |
| 5.1 Beta blocker versus methyldopa | 8 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.62, 1.29] |
| 5.2 Calcium channel blocker versus methyldopa | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.44, 1.64] |
| 6 Severe pre‐eclampsia | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.56, 2.33] |
| 6.1 Beta blocker versus methyldopa | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.56, 2.33] |
| 7 Eclampsia | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Calcium channel blockers versus methyldopa | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Need for additional antihypertensive drug/s | 13 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.47, 1.13] |
| 8.1 Beta blocker versus methyldopa | 10 | 853 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.28] |
| 8.2 Calcium channel blocker versus methyldopa | 3 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.30, 1.28] |
| 9 Elective delivery (induction of labour + elective caesarean section) | 7 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.90, 1.09] |
| 9.1 Beta blocker versus methyldopa | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.12] |
| 9.2 Calcium channel blocker versus methyldopa | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.65, 1.63] |
| 10 Caesarean section | 13 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.74, 0.95] |
| 10.1 Beta blocker versus methyldopa | 10 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.13] |
| 10.2 Calcium channel blocker versus methyldopa | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.16] |
| 10.3 Beta blocker or calcium channel blocker versus methyldopa | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.57, 0.86] |
| 11 Induction of labour | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.89, 1.45] |
| 11.1 Beta blocker versus methyldopa | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.65, 1.53] |
| 11.2 Calcium channel blocker versus methyldopa | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.75, 1.57] |
| 11.3 Beta blocker or calcium channel blocker versus methyldopa | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.89, 2.64] |
| 12 Antenatal hospital admission | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.17, 1.86] |
| 12.1 Beta blocker versus methyldopa | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.17, 1.86] |
| 13 Placental abruption | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.19, 21.90] |
| 13.1 Beta blocker versus methyldopa | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.19, 21.90] |
| 14 Maternal side‐effects | 6 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 2.09] |
| 14.1 Beta blocker versus methyldopa | 5 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 2.09] |
| 14.2 Calcium channel blockers versus methyldopa | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Changed/stopped drugs due to maternal side‐effects | 4 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.12, 67.91] |
| 15.1 Beta blocker versus methyldopa | 4 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.12, 67.91] |
| 16 Admission to neonatal or intensive care nursery | 6 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.27] |
| 16.1 Beta blocker versus methyldopa | 4 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.67, 1.25] |
| 16.2 Calcium channel blocker versus methyldopa | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.56, 2.64] |
| 17 Respiratory distress syndrome | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.31, 2.40] |
| 17.1 Beta blocker versus methyldopa | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.31, 2.40] |
| 18 Neonatal hypoglycaemia | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.05] |
| 18.1 Beta blocker versus methyldopa | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.05] |
| 19 Neonatal bradycardia | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.18] |
| 19.1 Beta blocker versus methyldopa | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.18] |
| 20 Neonatal jaundice | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.48, 2.29] |
| 20.1 Beta blocker versus methyldopa | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.48, 2.29] |
Comparison 6. Any antihypertensive versus calcium channel blocker (subgrouped by class of drug).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 5 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.09, 3.15] |
| 1.1 Glyceryl trinitrate versus calcium channel blockers | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.07, 35.67] |
| 1.2 Beta blockers versus calcium channel blockers | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.96, 4.80] |
| 1.3 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.48, 2.44] |
| 1.4 Methyldopa versus calcium channel blockers | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.82, 22.86] |
| 2 Proteinuria/pre‐eclampsia | 5 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.70, 2.19] |
| 2.1 Glyceryl trinitrate versus calcium channel blockers | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.10, 9.96] |
| 2.2 Beta blockers versus calcium channel blockers | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.24, 5.23] |
| 2.3 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.57, 4.83] |
| 2.4 Merthyldopa versus calcium channel blockers | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.79, 2.93] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 9 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.57] |
| 3.1 Glyceryl trinitrate versus calcium channel blockers | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Beta blockers versus calcium channel blockers | 3 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.46] |
| 3.3 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Methyldopa versus calcium channel blockers | 4 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.38, 27.40] |
| 4 Small‐for‐gestational age | 4 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.73] |
| 4.1 Glyceryl trinitrate versus calcium channel blockers | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.10, 9.96] |
| 4.2 Beta blockers versus calcium channel blockers | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.68] |
| 4.3 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.05, 4.85] |
| 4.4 Methyldopa versus calcium channel blockers | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.63, 10.00] |
| 5 Preterm birth (< 37 weeks) | 6 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.23] |
| 5.1 Glyceryl trinitrate versus calcium channel blockers | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.20, 1.91] |
| 5.2 Beta blockers versus calcium channel blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.34, 1.15] |
| 5.3 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.22, 4.18] |
| 5.4 Methyldopa versus calcium channel blockers | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.61, 2.30] |
| 6 Maternal death | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Beta blockers versus calcium channel blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Eclampsia | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Beta blockers versus calcium channel blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Methyldopa versus calcium channel blockers | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 HELLP syndrome | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.38, 8.20] |
| 8.1 Beta blockers versus calcium channel blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.38, 8.20] |
| 9 Pulmonary oedema | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Beta blockers versus calcium channel blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Need for additional antihypertensive drug/s | 5 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.78, 2.36] |
| 10.1 Beta blockers versus calcium channel blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.51, 3.20] |
| 10.2 Methyldopa versus calcium channel blockers | 3 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.78, 3.34] |
| 11 Elective delivery (induction of labour + elective caesarean section) | 3 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 11.1 Beta blockers versus calcium channel blockers | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 11.2 Methyldopa versus calcium channel blockers | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.61, 1.54] |
| 12 Caesarean section | 6 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.29] |
| 12.1 Beta blockers versus calcium channel blockers | 3 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.77, 1.39] |
| 12.2 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.57, 1.59] |
| 12.3 Methyldopa versus calcium channel blockers | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.87, 2.65] |
| 13 Induction of labour | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.34] |
| 13.1 Beta blockers versus calcium channel blockers | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.69, 1.92] |
| 13.2 Methyldopa versus calcium channel blockers | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.64, 1.33] |
| 14 Placental abruption | 3 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.86] |
| 14.1 Beta blockers versus calcium channel blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.30] |
| 14.2 Furosemide versus calcium channel blockers | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.19, 19.40] |
| 15 Maternal side‐effects | 3 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.85, 2.29] |
| 15.1 Beta blockers versus calcium channel blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.85, 2.29] |
| 15.2 Methyldopa versus calcium channel blockers | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Changed/stopped drug due to side‐effects | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.56, 4.31] |
| 16.1 Glyceryl trinitrate versus calcium channel blockers | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.6 [0.13, 50.25] |
| 16.2 Beta blockers versus calcium channel blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.49, 4.30] |
| 17 Admission to neonatal or intensive care nursery | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.54, 1.37] |
| 17.1 Beta blockers versus calcium channel blockers | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.58] |
| 17.2 Methyldopa versus calcium channel blockers | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.38, 1.78] |
| 18 Respiratory distress syndrome | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.27, 1.54] |
| 18.1 Beta blockers versus calcium channel blockers | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.27, 1.54] |
| 19 Neonatal hypoglycaemia | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.29, 2.05] |
| 19.1 Beta blockers versus calcium channel blockers | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.29, 2.05] |
6.13. Analysis.
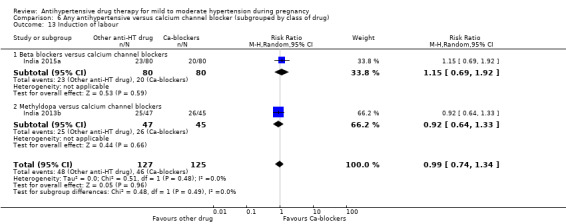
Comparison 6 Any antihypertensive versus calcium channel blocker (subgrouped by class of drug), Outcome 13 Induction of labour.
Comparison 7. Any antihypertensive versus beta blocker (subgrouped by class of drug).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypertension | 10 | 692 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.48] |
| 1.1 Methyldopa versus beta blocker | 9 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.08, 1.71] |
| 1.2 Calcium channel blocker versus beta blocker | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.05] |
| 2 Proteinuria/pre‐eclampsia | 12 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.88, 1.67] |
| 2.1 Methyldopa versus beta blockers | 10 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.72] |
| 2.2 Calcium channel blocker versus beta blocker | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.19, 4.17] |
| 3 Total reported fetal or neonatal death (including miscarriage) | 19 | 1652 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.81, 1.88] |
| 3.1 Methyldopa versus beta blockers | 16 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.67, 2.34] |
| 3.2 Calcium channel blokers versus beta blockers | 3 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.68, 2.16] |
| 4 Small‐for‐gestational age | 7 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.80, 1.60] |
| 4.1 Methyldopa versus beta blockers | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.84] |
| 4.2 Calcium channel blockers versus beta blockers | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.59, 1.83] |
| 5 Preterm birth (< 37 weeks) | 9 | 806 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.90, 1.67] |
| 5.1 Methyldopa versus beta blockers | 8 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.77, 1.60] |
| 5.2 Calcium channel blockers versus beta blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.87, 2.97] |
| 6 Maternal death | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Calcium channel blockers versus beta blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Severe pre‐eclampsia | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 7.1 Methyldopa versus beta blockers | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 8 Eclampsia | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Calcium channel blockers versus beta blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 HELLP syndrome | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.12, 2.60] |
| 9.1 Calcium channel blockers versus beta blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.12, 2.60] |
| 10 Pulmonary oedema | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Calcium channel blockers versus beta blockers | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Need for additional antihypertensive drug/s | 12 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.76, 1.75] |
| 11.1 Methyldopa versus beta blockers | 10 | 853 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.78, 2.20] |
| 11.2 Calcium channel blockers versus beta blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.31, 1.97] |
| 12 Elective delivery (induction of labour + elective caesarean section) | 6 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| 12.1 Methyldopa versus beta blockers | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.11] |
| 12.2 Calcium channel blockers versus beta blockers | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.87, 1.45] |
| 13 Caesarean section | 13 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 13.1 Methyldopa versus beta blockers | 10 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.88, 1.23] |
| 13.2 Calcium channel blockers versus beta blockers | 3 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.29] |
| 14 Induction of labour | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.31] |
| 14.1 Methyldopa versus beta blockers | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.65, 1.53] |
| 14.2 Calcium channel blockers versus beta blockers | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.45] |
| 15 Antenatal hospital admission | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.54, 5.90] |
| 15.1 Methyldopa versus beta blockers | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.54, 5.90] |
| 16 Placental abruption | 3 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.14, 6.28] |
| 16.1 Methyldopa versus beta blockers | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.05, 5.35] |
| 16.2 Calcium channel blockers versus beta blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.12, 69.62] |
| 17 Maternal side‐effects | 7 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.64, 2.53] |
| 17.1 Methyldopa versus beta blockers | 5 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 4.55 [0.48, 43.24] |
| 17.2 Calcium channel blockers versus beta blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 18 Changed/stopped drugs due to maternal side‐effects | 6 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.23, 1.80] |
| 18.1 Methyldopa versus beta blockers | 4 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.64] |
| 18.2 Calcium channel blockers versus beta blockers | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.23, 2.04] |
| 19 Admission to neonatal or intensive care nursery | 6 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.84, 1.45] |
| 19.1 Methyldopa versus beta blockers | 4 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.80, 1.49] |
| 19.2 Calcium channel blockers versus beta blockers | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.63, 2.05] |
| 20 Respiratory distress syndrome | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.71, 2.66] |
| 20.1 Methyldopa versus beta blockers | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.42, 3.26] |
| 20.2 Calcium channel blockers versus beta blockers | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.65, 3.66] |
| 21 Neonatal hypoglycaemia | 6 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.62, 2.00] |
| 21.1 Methyldopa versus beta blockers | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.49, 2.11] |
| 21.2 Calcium channel blockers versus beta blockers | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.49, 3.50] |
| 22 Neonatal bradycardia | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 22.1 Methyldopa versus beta blockers | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 23 Neonatal jaundice | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.44, 2.09] |
| 23.1 Methyldopa versus beta blockers | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.44, 2.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Argentina 1985.
| Methods | Allocation concealment: not stated. Authors state"...randomly divided into two groups...".Two arms. | |
| Participants | 60 women with SBP >/= 160 mmHg and/or DBP >/= 100 mmHg x 2, 24 hrs apart, with or without proteinuria at trial entry. Excluded: > 1 drug to control BP, or contraindication for beta blockers. | |
| Interventions | Exp: oral atenolol 50 mg/day to 250 mg/day (30 women) Control: oral methyldopa 750 mg/day to 2000 mg/day (30 women) | |
| Outcomes | Women: BP (mean). Babies: gestational age, birthweight, Apgar score, stillbirth, neonatal deaths. | |
| Notes | Main paper in Spanish. Methods for measuring BP not mentioned. Funding: no information about funding source. Declaration of interests not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: "...randomly divided into two groups..." no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Argentina 1987.
| Methods | Allocation concealment: not stated. Authors state "open randomised study". Two arms. | |
| Participants | 20 women with SBP > 159 mmHg and/or DBP > 99 mmHg x 2, 24 hrs apart, +/‐ proteinuria. Excluded: > 1 drug to control BP, or hypertensive emergency. | |
| Interventions | Exp: oral ketanserin 20 mg/day to 80 mg/day (10 women) Control: oral methyldopa 500 mg/day to 2000 mg/day (10 women) | |
| Outcomes | Women: none reported. Babies: stillbirth, neonatal death, birthweight (mean), gestation at delivery (mean). | |
| Notes | Interim report of study ongoing in 1987. Methods for measuring BP not mentioned. Funding: no information about funding source. Declaration of interests not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: quote: "...open randomised study..." no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Argentina 1988.
| Methods | Allocation concealment: not stated. Authors state "randomised' 'divided into 2 equal groups". Two arms | |
| Participants | 36 women > 14 weeks' gestation with BP >/= 140/90 mmHg and </= 170/110 mmHg. | |
| Interventions | Exp: oral mepindolol, increasing weekly doses, from 5 mg/day to 10 mg/day. (18 women) Control: oral methyldopa, increasing weekly doses from 500 mg/day to 2000 mg/day. (18 women) | |
| Outcomes | Women: additional antihypertensive, caesarean section, side‐effects, maternal complications. Babies: stillbirth, SGA (undefined). | |
| Notes | Methods for measuring BP not mentioned. Available only as an abstract. Funding: no information about funding source. Declaration of interests not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: quote: "...randomised study..." no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study abstract. |
| Other bias | Unclear risk | No information about funding source.Declaration of interests not described. |
Australia 1983.
| Methods | Allocation concealment: not stated. Authors state "randomly allocated". Two arms | |
| Participants | 28 women in antenatal clinics with mild‐moderate PIH (BP >/= 140/90 mmHg x 2 at least 24 hrs apart). Excluded: impaired renal function. | |
| Interventions | Exp: oral propranolol 30 mg/day 160 mg/day. (14 women) Control: oral methyldopa 500 mg/day to1000 mg/day. (14 women) | |
| Outcomes | Women: severe HT, proteinuria (undefined), additional antihypertensive, changed drugs due to side‐effects, caesarean section. Babies: perinatal death, preterm delivery, jaundice, bradycardia, hypoglycaemia, birthweight (mean). | |
| Notes | London School of Hygiene sphygmomanometer (random zero) used. No mention of which Korotkoff sound used for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: quote "...randomly allocated..." no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described |
Australia 1985a.
| Methods | Allocation concealment: not stated. Authors state "allocated by series of random numbers". Two arms | |
| Participants | 183 women with singleton pregnancy and mild HT (DBP >/= 90 mmHg x 2, 24 hrs apart, or DBP >/= 95 mmHg x 2, 12 hrs apart, or DBP >/= 100 mmHg x 2, 8 hrs apart). | |
| Interventions | Exp: oral oxprenolol 40 mg x 2/day to 320 mg x 2/day. (96 women)
Control: oral methyldopa 250 mg x 2/day to 1000 mg x 3/day. (87 women) If BP not controlled, hydralazine in both groups. |
|
| Outcomes | Women: severe HT, proteinuria ('heavy and increasing requiring delivery'), additional antihypertensive, induction of labour, caesarean section, Babies: stillbirth, neonatal death, admission to SCBU, days in SCBU, RDS, birthweight. (mean), Apgar (mean). | |
| Notes | Korotkoff phase IV used for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...were allocated by a series of random numbers to...", no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Co‐interventions: 48% of oxprenolol group and 35% of methyldopa group received a second or third antihypertensive. |
Australia 2001.
| Methods | Allocation concealment: central telephone randomisation Although authors stated it was a placebo‐controlled trial, data provided by authors suggest that they may have used a patch for the control, but not a matching placebo. Two arms | |
| Participants | 16 women with gestational HT, defined as quote: "de novo" HT after 20 weeks' gestation of > 140 and/or 90 mmHg on 2 readings, 6 hrs apart; or a rise in systolic pressure of > 25 mmHg or a diastolic of 15 mmHg from a BP pre‐pregnancy or in the first trimester. | |
| Interventions | Exp: transdermal GTN patches 10 mg. (7 women) Control: patch for the control, but not a matching placebo. (9 women) | |
| Outcomes | Women: PE, side‐effects. Babies: not reported. | |
| Notes | Trial planned to recruit 220 women and stopped early due to side‐effects (headache) in the treatment group. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation. |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as quote: "...single blind, placebo controlled trial...". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study letter to editor. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described |
Brazil 1985.
| Methods | Allocation concealment: not stated. Authors state: "...patients were randomly divided into two groups...". Two arms. | |
| Participants | 100 women with chronic HT diagnosed before 20th week, BP >/= 140/90 mmHg x 2, 5 mins apart. With no proteinuria and no contraindication to beta blockers. | |
| Interventions | Exp: oral pindolol 10 mg/day to 30 mg/day. (50 women) Control: no treatment. (50 women) | |
| Outcomes | Women: MAP, severe PE, side‐effects. Babies: abortions, fetal deaths, neonatal deaths, gestational age, birthweight, IUGR, Apgar score, congenital malformations, hypoglycaemia. | |
| Notes | Methods for measuring BP not mentioned. Main paper in Portuguese. Funding: no information about funding source. Declaration of interests not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: "...patients were randomly divided into two groups...", no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Brazil 1988.
| Methods | Allocation concealment: consecutive numbered treatment boxes. Two arms | |
| Participants | 40 pregnant women with chronic HT with DBP =/> 95 mmHg, without proteinuria. | |
| Interventions | Exp: oral pindolol 10 mg/day to 30 mg/day. (20 women) Control: oral methyldopa 500 mg/day to 2000 mg/day. (20 women) | |
| Outcomes | Women: BP, need for additional antihypertensives, severe HT, superimposed PE. Babies: birthweight, Apgar score, fetal and neonatal death, preterm birth, SGA (undefined). | |
| Notes | Main paper in Portuguese. Methods for measuring BP not mentioned. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Consecutive numbered treatment boxes (personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Low risk | Published and unpublished data provided by author. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Brazil 2000a.
| Methods | Allocation concealment: trial drug supplied by pharmacy in packs with serial numbers. Withdrawals: 15 women (7.5%) excluded from the analysis (5 delivered in other hospitals, 9 dropped out of the study or failed to comply with treatment, 1 due to side‐effects). Two arms | |
| Participants | 199 singleton pregnant women with mild/moderate chronic HT (DBP > 90 mmHg and =/< 110 mmHg before 20 weeks' gestation, or with history of chronic HT), before 25 weeks' gestation and giving informed consent. Excluded: renal, cardiac or hepatic disease, IUGR diagnosed before trial entry, alcohol/drug abuse. | |
| Interventions | Exp: oral verapamil 240 mg x 3/day. (90 women) Control: oral placebo. (94 women) | |
| Outcomes | Women: BP, heart rate, severe HT, superimposed PE, side‐effects, mode of delivery. Babies: birthweight, gestational age, SGA, Apgar score, jaundice, hypoglycaemia, mortality. | |
| Notes | Korotkoff phase IV used for DBP. Main report in Portuguese, presented as a Doctoral Thesis. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Consecutive numbered treatment packs containing the study drug or placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, only pharmacist providing treatment packs aware of the codes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, only pharmacist providing treatment packs aware of the codes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 withdrawals (7.5%): 5 women delivered in other hospitals, 10 withdrew consent (1 due to side‐effects). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study thesis. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Brazil 2016.
| Methods | Allocation concealment: sealed and numbered opaque envelopes. Two arms | |
| Participants | 100 singleton pregnant women with PE, defined as development of HT after 20 weeks of gestation (DBP blood pressure 90 mmHg or greater, SBP 140 mmHg or greater, or both) with associated proteinuria (greater than 300 mg in 24 hours), at 24 to 33 weeks' gestation. Excluded: history of HT, chronic disorders such as diabetes, fetal malformations, or maternal or fetal comorbidities or both, use of erythromycin, ketoconazole, itraconazole, antiretroviral agents, or any other medication that could interact with sildenafil, patients using antihypertensive medications other than a‐methyldopa, not giving consent. | |
| Interventions | Exp: oral sildenafil citrate at 50 mg every 8 hours. (50 women) Control: identical placebo capsules. (50 women) |
|
| Outcomes | Women: time interval between randomisation and delivery, MAP, Doppler velocimetry of the uterine, umbilical, and middle cerebral arteries, need for an additional antihypertensive drug, caesarean section, eclampsia, placental abruption, maternal cardiac failure, HELLP syndrome, PPH, maternal side‐effects. Babies: IUGR (< 10th centile), birthweight, Apgar score, NICU admission, days in NICU, neonatal infection, IVH, thrombocytopenia, hypotension, necrotising enterocolitis, hypoglycaemia, pneumothorax, convulsions, neonatal demise, neonatal side‐effects. |
|
| Notes | Korotkoff phase IV used for DBP. Funding: funded by university or non commercial organisation. The authors reported no potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation (blocks of 5). |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study investigators, attending physicians, ultrasonographers, neonatologists, and patients were blinded regarding allocation to placebo or sildenafil groups until the end of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study investigators, attending physicians, ultrasonographers, neonatologists, and patients were blinded regarding allocation to placebo or sildenafil groups until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 women (7%) discontinued treatment due to concerns about the possibility of being allocated to a placebo (2 in each group), or due to side‐effects (1 in treatment group, 2 in placebo group). However, authors stated that women consent in the use of their data and presented results in 100 women randomised. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups appear comparable at baseline. Funded by university or non commercial organisation. Authors stated not potential conflict of interests. |
Caribbean Is.1990.
| Methods | Allocation concealment: women given number corresponding to sealed envelope and treatment batch. Envelope contained unblinding, kept by investigator and only opened when necessary. Envelopes collected at end of study. 2 centres.
Withdrawals: 1 woman, from placebo group. Two arms |
|
| Participants | 155 women with singleton pregnancy at 20 to 36 weeks' gestation, DBP < 85 mmHg x 2 before 20 weeks and > 84 mmHg after 20 weeks. Excluded: type I diabetes, congestive heart failure, cardiac block, asthma, pre‐pregnancy HT, antihypertensive treatment during current pregnancy. | |
| Interventions | Exp: oral oxprenolol 160 mg x 2/day to 320 mg x 2/day. Hydralazine 50 mg to 100 mg added if necessary to keep DBP < 86 mmHg. (78 women) Control: oral placebo, identical appearance. (77 women) |
|
| Outcomes | Women: death, mean BP, severe HT, proteinuria (> 1+ or 0.25 g/L), additional antihypertensive, eclampsia, changed drugs due to side‐effects, elective delivery, caesarean section, hospital admission, days in hospital, placental abruption. Babies: perinatal death, preterm delivery (< 37 weeks), birthweight (mean), SGA (undefined, excludes stillbirth), 5 mins Apgar < 7, admission to SCBU, RDS. | |
| Notes | Korotkoff phase IV used for DBP. For 23 women (15%), treatment unblinded and other treatment started. 16 for uncontrolled BP (5 exp, 11 control) and 7 for poor compliance/side‐effects (4 exp, 3 control). Additional data provided by authors. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was in stratified blocks of ten..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...sealed envelopes and individual batch of drugs containing either the maximum amount of active drug that could be used in 20 weeks of treatment or placebos of identical appearance." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 women (0.6%) lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Published and unpublished data provided by author. |
| Other bias | High risk | For 23 women (15%), treatment unblinded and other treatment started. 16 for uncontrolled BP (5 exp, 11 control) and 7 for poor compliance/side‐effects (4 exp, 3 control). Funded by industry. |
Finland 1988b.
| Methods | Quote: "randomised pilot trial". No further information. | |
| Participants | 25 women with PIH. | |
| Interventions | Exp: oral nifedipine, 30 mg to 60 mg. daily (13 women) Control: no treatment (12 women) | |
| Outcomes | mean DBP, birthweight (mean). | |
| Notes | Available as abstract only. Not possible to extract data. Reported as an ongoing study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | Funding source and declaration of interests not described. |
France 1987.
| Methods | Allocation concealment: quote "blinded envelopes". Stratified in blocks of 10 at each clinic. Multicentre, 12 hospitals. Withdrawals: 12 women (6%). 5 labetalol (3 lost to follow‐up and 2 given methyldopa) and 7 methyldopa (all lost to follow‐up). Two arms | |
| Participants | 188 women with singleton pregnancy at 12 to 34 weeks' gestation, booked < 20 weeks and DBP >/= 90 mmHg. Excluded: previous antihypertensive treatment this pregnancy, diabetes, depression, contraindication to beta blockers. | |
| Interventions | Exp: oral labetalol 200 mg x 2/day to 600 mg x 2/day. (96 women) Control: oral methyldopa 250 mg x 2/day to 750 mg x 2/day. (92 women) | |
| Outcomes | Women: proteinuria (> 2+ or 0.5 g/L), admission to hospital, caesarean section, elective delivery, additional antihypertensive, side‐effects, changed drugs due to side‐effects. Babies: stillbirth, neonatal death, admission to SCBU, SGA (< 5th centile, excludes stillbirths), preterm delivery (< 37 weeks), 5 mins Apgar < 8. | |
| Notes | Korotkoff phase IV used for DBP. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...stratified blocks of ten at each clinic..." |
| Allocation concealment (selection bias) | Low risk | Blinded envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 women (6.4%) excluded from analysis: 10 lost to follow‐up (3 from labetalol and 7 from methyldopa group), and 2 quote: "protocol violation". |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Groups appear comparable at baseline. Funded by industry. |
France 1988a.
| Methods | Allocation concealment: not stated. Authors state "random order". 3‐arm study, the two beta blocker arms were combined for the analysis. | |
| Participants | 63 women at 7‐36 weeks' gestation with DBP > 90 mmHg x 2, 8 days apart). | |
| Interventions | Exp: (1) oral acebutolol 400 mg to 1200 mg (21 women); (2) oral labetalol 400 mg to 1200 mg (21 women). Control: oral methyldopa 500 mg to 1500 mg (21 women). | |
| Outcomes | Women: PE, caesarean section. Babies: perinatal death, preterm delivery, birthweight (mean), Apgar, admission to SCBU, hypoglycaemia. | |
| Notes | No mention about Korotkoff sound considered for DBP. Main paper in French. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Apres tirage au sort, effectué par groupe de 9 patientes..." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
France 1990.
| Methods | Quote "randomised protocol". No further details. | |
| Participants | 21 women with moderate HT (SBP 140‐180 mmHg and DBP 90‐120 mmHg). | |
| Interventions | EXP: oral atenolol (n = 12) (no doses reported) CONT: oral nifedipine (n = 9) (no doses reported) |
|
| Outcomes | BP, Doppler measures, birthweight and length, Apgar score, admission to SCBU. | |
| Notes | Not possible to extract data. Available as congress abstracts only (1 in English, 3 in French) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | Funding source and declaration of interests not described. |
France 1994.
| Methods | Allocation concealment: sealed envelopes drawn by physician. Ordered using list of computer‐generated random numbers. Two arms | |
| Participants | 100 women with singleton pregnancy at > 20 weeks' gestation and mild‐moderate HT (BP >/= 140/90 mmHg x 2). No other antihypertensive medication at trial entry. | |
| Interventions | Exp: oral nicardipine 20 mg x 3/day.(50 women) Control: oral metoprolol (slow release) 200 mg/day.(50 women) | |
| Outcomes | Women: severe HT, proteinuria (undefined), HELLP syndrome, additional antihypertensive, changed drug due to side‐effects, induction of labour, caesarean section. Babies: perinatal death, umbilical Doppler, admission to SCBU. | |
| Notes | Korotkoff phase IV used for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Hong Kong 1990.
| Methods | Allocation concealment: not stated. Authors state: "randomised double‐blind". Two arms | |
| Participants | 41 healthy nulliparous women admitted for PE (BP >/= 140/90 mmHg x 2 within 24 hours). | |
| Interventions | Exp: oral labetalol 200 mg x 3/day. (20 women) Control: oral placebo (character not stated). (21 women) | |
| Outcomes | Women: mean BP, severe HT, additional antihypertensive. Babies: birthweight (mean), SGA (< 10th centile), gestation at delivery (mean). | |
| Notes | Trial reported as in progress in 1990. Missing data for some babies. No description of how BP measured. Available only as an abstract. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: authors only state: "randomised double‐blind controlled study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study abstract. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Hong Kong 1993.
| Methods | Allocated in quote"randomised double manner". No further information. 4 women (6.2%) excluded after randomisation. No further details. | |
| Participants | 65 primigravid women with a singleton pregnancy at > 20 weeks' gestation and BP 140‐165/90‐105 mmHg x 2, 6 hrs apart but no proteinuria. | |
| Interventions | Exp: oral labetalol. No dose specified. Number of women not specified. Control: oral placebo. No dose specified. Number of women not specified | |
| Outcomes | BP, need for additional antihypertensives, induction of labour, proteinuria, gestational age, mode of delivery, birthweight, Apgar score | |
| Notes | Available as abstract only. Not possible to extract data. Declaration of interests not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | Funding source and declaration of interests not described. |
India 1992.
| Methods | Allocation concealment: not stated. Authors state "randomly allocated". Two arms | |
| Participants | 30 primigravid women at 24‐37 weeks' gestation with mild‐moderate PIH (BP >/ = 140/90 mmHg x 2, 6 hrs apart). Excluded: UTI, heart disease or other cause of HT. | |
| Interventions | Exp: oral metoprolol 50‐150 mg x 2/day. (15 women) Control: oral methyldopa 250 mg x 3/day, increased to 2000 mg/day. (15 women) | |
| Outcomes | Women: severe HT. Babies: perinatal death, preterm delivery, gestation at delivery, birthweight, Apgar at 1 min and 5 mins (mean). | |
| Notes | Method for measuring BP not mentioned. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: quote: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Funded by industry. |
India 2002.
| Methods | Methods not stated, authors only state "...randomised by number..." Two arms | |
| Participants | 118 primigravid women with HT (BP > 140/90 x 2, 60 hours apart) during pregnancy with and without proteinuria at > 20 weeks' gestation. Excluded: 2nd or more pregnancies, history of chronic HT, use of anti HT drugs. |
|
| Interventions | Exp: oral Nimodipine 30 mg every 6 hrs. (54 women) Control: oral methyldopa 250 mg every 6 hrs. (56 women) |
|
| Outcomes | MAP, additional antihypertensives, serum creatinine and liver enzymes, induction of labour, serious maternal side‐effects, perinatal deaths. | |
| Notes | Method for measuring BP not mentioned. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: quote: "...randomised by number...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 dropouts (6%), not specified in which group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
India 2011.
| Methods | Prospective randomised comparative study. No further details | |
| Participants | 200 women with singleton pregnancy, 28 to 40 weeks' gestation with PIH. | |
| Interventions | Exp: oral labetalol. No dose specified. Number of women not specified. Control: oral methyldopa. No dose specified. Number of women not specified. | |
| Outcomes | BP, control of proteinuria, mode of delivery, neonatal Apgar score, NICU admission, neonatal complications, perinatal deaths. | |
| Notes | Available as abstract only. Not possible to extract data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Funding source and declaration of interests not described |
India 2012.
| Methods | Mixed sealed envelopes containing the assigned intervention. 3‐arm trial. | |
| Participants | 149 women with PIH (140‐159/90 to 109 mmHg x 2, 6 hrs apart, without proteinuria) at 20‐38 weeks' gestation. Excluded: chronic HT, previous anti HT medication, secondary HT, UTI, diabetes, known medical/psychiatric disorders, multiple pregnancy, placenta previa, Rh isoimmunisation, congenital abnormality. |
|
| Interventions | Exp 1: oral labetalol 100 mg x 2/day (up to 2500 mg). (50 women) Exp 2: oral methyldopa 250 mg x 2/day (up to 2000 mg). (49 women) Control: no treatment. (50 women) |
|
| Outcomes | BP, laboratory parameters, gestational age at delivery, birthweight, 5‐min Apgar score, maternal adverse events, maternal death, major morbidity, severe HT, proteinuria, severe PE, antenatal hospital admission, caesarean section, perinatal death, SGA, preterm babies, Admission to NICU. | |
| Notes | Korotkoff phase V for DBP. Funding: funded by university or non commercial organisation. Authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Allocation cards labelled L (labetalol), M (methyldopa) or N (no antihypertensive) were prepared by a staff clerk in equal numbers of 50 each and put into opaque envelopes that were subsequently sealed, mixed up and numbered 1‐150." |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman withdrew consent in methyldopa group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups appear comparable at baseline. Funded by university or non commercial organisation. Authors declared no conflict of interest. |
India 2013a.
| Methods | Allocation concealment: not described, quote: "...prospective randomized study,.." no further details. Two arms | |
| Participants | 120 pre‐eclamptic women after 20 weeks of gestation with BP more than 140/90 mmHg on 2 separate occasion 6 hrs apart, proteinuria +1 dipstick in 2 midstream samples collected 4 hrs apart. Singleton with vertex presentation. Excluded: diabetes, Rh isoimmunisation, multi‐fetal pregnancy, thyrotoxicosis, cardiac disease, chronic HT, renal disease, bronchial asthma, antepartum haemorrhage, malnutrition, poli‐hydramnios, already receiving antihypertensive drugs. |
|
| Interventions | Exp: oral labetalol 100 mg 3 times a day. (90 women) Control: oral methyldopa 250 mg 3 times a day. (90 women) |
|
| Outcomes | Women: response in lowering BP over a period of 7 days, blood urea and serum creatinine, platelet count, mode of onset of labour, gestational age at delivery, mode of delivery, side‐effects. Babies: birthweight, stillbirth, preterm delivery, Apgar score, RDS, jaundice, bradycardia, hypoglycaemia, need for NICU. |
|
| Notes | Method for measuring BP not mentioned. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote:"...prospective randomized study,.." no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
India 2013b.
| Methods | Allocation concealment: not described. Authors state "randomized, double‐blind, comparative trial". Two arms | |
| Participants | 100 singleton pregnant women with sitting SBP of 150 to 169 mmHg or DBP (disappearance of sounds phase V, Korotkoff sounds) of 100 mmHg to 109 mmHg on 2 occasions at least 4 hrs apart. All patients in the study were more than 32 weeks pregnant and were normotensive before 20 weeks of pregnancy. Excluded: past history of HT, diabetes, renal disease, asthma, congestive heart failure, known atrioventricular block, other illness, or fetal congenital abnormality including intrauterine growth retardation |
|
| Interventions | Exp: 10 mg oral nifedipine four times a day. (50 women) Control: 250 mg oral methyldopa 3 times a day. (50 women) |
|
| Outcomes | Women: successful control of SBP < 150 mmHg and DBP < 100 mmHg without using additional drugs, maternal side‐effects such as hypotension, maternal tachycardia, headache, flushing, nausea and vomiting, dizziness, abnormal cardiotocogram or cardiovascular accidents after starting the antihypertensive medication. Babies: mean birth weight (kg), Apgar score at 5 minutes, Abnormal CTG, NICU admission, stillbirths. |
|
| Notes | Korotkoff phase V for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by a series of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patient and the investigator were blinded regarding treatment". No further details. However, the dosage of comparative treatments was different (nifedipine four times a day versus methyldopa 3 times a day). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 women (8%) excluded from analysis for failure to comply regular follow‐up, 3 in methyldopa and 5 in nifedipine group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study reports. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
India 2013c.
| Methods | Quote: "...patients were divided into two groups randomly..." Two arms | |
| Participants | 180 pregnant women at > 20 weeks' gestation with BP > 140/90 mmHg x 2, 6 hours apart and proteinuria 1+ on dipstick x 2, 4 hours apart. | |
| Interventions | Exp: oral Labetalol 100 mg three times daily (doubled every 48 hours until BP control). (90 women) Control: oral Methyldopa 250 mg three times daily (doubled every 48 hours until BP control). (90 women) |
|
| Outcomes | Fall in BP after 7 days treatment, average dose of drug used, induction of labour, maternal side‐effects. | |
| Notes | Method for measuring BP not mentioned. Funding: no funding source. Authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: quote: "...patients were divided into two groups randomly...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups appear comparable at baseline. No funding source. Authors declared no conflict of interests. |
India 2015a.
| Methods | Allocation concealment: Not described. Quote "...Patients (80 each) were randomised to each of the three treatment arms..." No further details. Three arms | |
| Participants | 240 pregnant women with SBP of ≥140 mmHg and a DBP of ≥ 90 mmHg requiring medication and gestational age more than 20 weeks of pregnancy. Excluded: bronchial asthma, any pre‐existing cardiovascular disorder, diabetes, Rh isoimmunisation, patients at risk of major obstetric complications ‐ antepartum haemorrhage, malnutrition, multifetal gestation and hydramnios during the current pregnancy and patients who had already received antihypertensive drugs. |
|
| Interventions | Group 1: oral nifedipine 10 mg twice daily (80 women) Group 2: oral methyldopa 250 mg three times daily (80 women) Group 3: oral labetalol 100 mg twice daily (80 women) |
|
| Outcomes | Women: control of BP over a period of 7 days, time required to control BP, average dose of drugs required to control BP, onset of labour‐spontaneous/induced or caesarean section, adverse effects of drugs Babies: Stillbirth |
|
| Notes | Korotkoff phase IV for DBP. Funding: no funding source. Authors declared no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Quote: "...patients randomly divided in three groups..." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ope label trial (different dosages). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups appear comparable at baseline. No funding source. Authors declared no conflict of interests. |
India 2015b.
| Methods | Allocation concealment: not described.Quote: "...prospective randomized controlled parallel group study...". No further details. Two arms | |
| Participants | 100 pregnant women with SBP ≥160 mmHg and DBP ≥110 mmHg after 20 weeks of gestation. Excluded: history of pre‐existing HT, renal diseases, immunological disorders, liver diseases, diabetes mellitus, epilepsy, thyroid diseases, eclampsia, unwilling or unable to comply with the study proceedings. |
|
| Interventions | Exp: oral labetalol 100 mg twice a day (up to 800 mg). (50 women) Control: oral methyldopa 250 mg 3 times a day (up to 3 g). (50 women) |
|
| Outcomes | Women: the mean SBP, mean DBP, and MAP at time of delivery, adverse events. | |
| Notes | Korotkoff phase V for DBP. Funding: no funding source. Authors declared no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Quote: "...prospective randomized controlled parallel group study...". No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial (different dosages) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Baseline characteristics at study entry (apart from BP) not reported. No funding source. Authors declared no conflict of interests. |
Ireland 1991.
| Methods | Allocation concealment: cards with 'test' or 'control' sealed in envelopes, shuffled and then numbered in sequence. Consecutive envelopes opened.Two arms | |
| Participants | 36 women < 38 weeks' gestation with BP >/= 140/90 mmHg on 2 separate days, without proteinuria. Excluded: if lived too far from the hospital to attend for frequent examinations. | |
| Interventions | Exp: choice between oral atenolol 50 mg/day to 100 mg/day and oral methyldopa 750 mg/day to 2250 mg/day. If monotherapy inadequate, 2 drugs combined. Oral bendrofluazide 2.5 mg to 5.0 mg added as a third agent when necessary. (17 women) Control: no antihypertensive. (19 women) | |
| Outcomes | Women: MAP, proteinuria. Babies: perinatal death, Apgar, gestation age at delivery, birthweight, birthweight < 50th centile. | |
| Notes | Korotkoff phase V used for DBP. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...shuffled into random order..." |
| Allocation concealment (selection bias) | Low risk | Sealed, identical numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Israel 1986a.
| Methods | Allocation concealment: not stated. Authors state "randomly allocated". Two arms | |
| Participants | 32 women with singleton pregnancy at 27‐33 weeks' gestation with PIH (DBP >/= 95 mmHg x 2 at least 6 hrs apart). Excluded: history of chronic renal disease or essential HT. | |
| Interventions | Exp: oral pindolol 15 mg/day. (16 women) Control: oral methyldopa up to 2000 mg/day (no other details). (16 women) | |
| Outcomes | Women: severe HT, new proteinuria (> 2+ or 0.5 g/L), eclampsia, side‐effects, additional antihypertensive, changed drugs due to side‐effects. Babies: neonatal death, birthweight (mean), abnormal antenatal fetal heart rate, gestation at delivery (mean). | |
| Notes | Methods for BP measurement not mentioned. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Israel 1986b.
| Methods | Allocation concealment: not stated. Authors state "randomly allocated". 2 women with side‐effects on hydralazine crossed over to pindolol + hydralazine, and reported in this group. Data only included if available as intention‐to‐treat. Two arms | |
| Participants | 44 women at < 37 weeks with BP >/= 150/90 mmHg x 2 at least 24 hrs apart. Excluded: insulin‐dependent diabetes, obstructive lung disease, contraindication to pindolol or hydralazine. | |
| Interventions | Exp: oral hydralazine 50 mg/day to 100 mg/day + oral pindolol 10 mg/day to 25 mg/day (in 2 daily doses). (21 women) Control: oral hydralazine 50 mg/day to 100 mg/day (in 2 daily doses). (23 women) | |
| Outcomes | Women: severe HT, proteinuria (> 1 g in 24 hr), side‐effects, changed drug due to side‐effects, caesarean section. Babies: preterm delivery, SGA (< 250 on Usher's curve), hypoglycaemia, hypothermia, low Apgar score. | |
| Notes | No mention of which Korotkoff sound used. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. 2 women with side‐effects on hydralazine crossed over to pindolol + hydralazine, and reported in this group. Data only included if available as intention‐to‐treat. No information about funding source. Declaration of interests not described. |
Israel 1992a.
| Methods | Allocation concealment: trial drug supplied by pharmacy in packs with serial numbers, in blocks of 6. Two arms | |
| Participants | 60 women < 35 weeks' gestation with DBP 85‐99 mmHg x 2, 12 hrs apart, and no treatment for HT during this pregnancy. Excluded: multiple pregnancy, contraindication to beta blockers or insulin‐dependent diabetes. | |
| Interventions | Exp: oral pindolol 5 mg x 2/day. If DBP still >/= 85 mmHg on day 3, increased to 5 mg x 3/day, if no response next day, increased to 10 mg x 2/day. (30 women)
Control: oral identical placebo. (30 women) If DBP 100‐109 mmHg x2 or > 110 mmHg x1, hydralazine added for pindolol group. In placebo group, pindolol given first, followed by hydralazine if DBP > 100 mmHg. |
|
| Outcomes | Women: additional antihypertensive, days in hospital, proteinuria > 2+ or > 0.5 g/L, treatment stopped due to side‐effects, caesarean section. Babies: perinatal death, gestation at delivery (mean), birthweight, 5‐min Apgar > 7, SGA (< 10th centile), hypoglycaemia, jaundice. | |
| Notes | Korotkoff IV used for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "...in a random fashion, in blocks of six, using serial numbers..." |
| Allocation concealment (selection bias) | Low risk | Pindolol and placebo tablets supplied by pharmacy in numbered packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Quote:"...placebo of identical appearance...". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Codes were broken only in the presence of severe side‐effects. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Israel 1995.
| Methods | Allocation concealment: not stated. Authors state "randomly allocated". 3‐arm trial. The two beta blocker arms were combined for analysis. | |
| Participants | 51 women with BP 140‐160/95‐110 mmHg. Excluded: proteinuria > 2+, contraindication to beta blockers, or any other disease. | |
| Interventions | Exp: (1) oral hydralazine 60 mg/day to 200 mg/day + oral propranolol 40 mg/day to 120 mg/day (17 women); (2) oral hydralazine 60 mg/day to 200 mg/day + pindolol 5 mg/day to 15 mg/day. (19 women) Control: oral hydralazine 60 mg/day to 200 mg/day. (15 women) | |
| Outcomes | Women: eclampsia, severe maternal morbidity, side‐effects, caesarean section. Babies: perinatal death, preterm delivery, SGA (< 10th centile), birthweight (mean). | |
| Notes | Korotkoff phase V used for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not describe. Quote:: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Italy 1997.
| Methods | Allocation concealment: not stated. Authors state: "randomly allocated". Two arms | |
| Participants | 100 primigravid women at 26 to 36 weeks' gestation with SBP 140 to 160 mmHg, and DBP 90 to 110 mmHg in first 24 hrs after admission and proteinuria < 300 mg/24 hr. Excluded: if other medical maternal or fetal pathology (IUGR or altered biophysical profile). | |
| Interventions | Exp: oral nifedipine 40 mg/day to 120 mg/day orally and bed rest. (50 women) Control: bed rest alone. (50 women) | |
| Outcomes | Women: severe HT, proteinuria, days in hospital before delivery. Babies: stillbirth, neonatal death, gestation at delivery (mean), birthweight, placental weight, SGA (undefined). | |
| Notes | Methods for measuring BP not stated. Article in Italian. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Italy 1998.
| Methods | Allocation concealment: central telephone randomisation, stratified by centre and type of HT (chronic, gestational or unclassified). Multicentre, 33 hospitals.
Withdrawals: 22 women (8%), 13 exp and 9 control lost to follow‐up.
Follow‐up of children at 18 months: 190/252 (77%) responded to postal survey. Two arms |
|
| Participants | 283 women at 12‐34 weeks' gestation, with mild‐moderate HT (DBP 90‐110 mmHg x 2, 4 hrs apart). Excluded: chronic diseases (such as diabetes or renal disease), fetal malformations, previous antihypertensive treatment or contraindications to nifedipine. | |
| Interventions | Exp: oral slow‐release nifedipine 20 mg to 80 mg x 2/day orally. (145 women) Control: no antihypertensive. (138 women) | |
| Outcomes | Women: severe HT, proteinuria, caesarean section, admission to intensive care. Babies: perinatal death, birthweight, SGA (< 10th centile), preterm delivery (< 34 and < 37 weeks), admission to SCBU, hyperglycaemia, jaundice, RDS, other serious neonatal problems. | |
| Notes | Classification of hypertensive disorders using Davey and MacGillivray system. Methods for measuring BP not mentioned.
Data from follow‐up excluded as > 20% lost. Funding: funded by university or non commercial organisation. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers balanced by centre and stratified by type of HT. |
| Allocation concealment (selection bias) | Low risk | Allocation by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 women (7.8%) lost to follow‐up.13 in nifedipine group and 9 in control group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. Funded by university or non commercial organisation Declaration of interests not described. |
Italy 1999.
| Methods | Allocation concealment: consecutive‐numbered, opaque, sealed envelopes. 3‐arm study. 6 women (17%) left the study due to side‐effects (2 women) or mother's or baby's worsening conditions (4). Three arms | |
| Participants | 36 women with singleton pregnancy, gestation > 24 weeks and PIH or PE (BP 140/90 mmHg or more, PE if proteinuria > 300 mg/24 hr). Excluded: fetal abnormalities or chromosomal disorders, renal or hepatic disease, chronic HT. | |
| Interventions | Exp (1): transdermal GTN 10 mg continuously 24 hr/day (12 women). Exp (2): transdermal GTN 10 mg intermittently for 16 hrs/day.(12 women) Control: oral nifedipine 40 mg/day orally. (12 women) | |
| Outcomes | Women: caesarean section, BP (mean), stopped drug due to side‐effects, severe HT, proteinuria/PE. Babies: birthweight, fetal/neonatal deaths, preterm birth, IUGR, gestation at birth (mean). | |
| Notes | Korotkoff phase IV used for DBP. In the analysis the 2 GTN arms have been combined. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Consecutive opaque sealed envelopes (personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 withdrawals (16.6%). 3 in group 1, 1 in group 2 and 2 in group 3. |
| Selective reporting (reporting bias) | Low risk | Additional data provided by authors. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Italy 2000.
| Methods | Allocation concealment: consecutive‐numbered treatment boxes.Two arms | |
| Participants | 20 women with PE (no further details). | |
| Interventions | Exp: oral nifedipine GITS 30 mg/day to 60 mg/day. (10 women) Control: oral methyldopa 500 mg/day to 1000 mg/day. (10 women) | |
| Outcomes | Women: BP, PE, Doppler abnormalities, need for drug adjustment, severe HT. Babies: fetal and neonatal death, preterm birth, SGA (undefined), Apgar score. | |
| Notes | Published as an abstract only. Method for measuring BP not stated. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Consecutive numbered treatment boxes provided by pharmacy (personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind (only for participants). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Low risk | Additional data provided by authors. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Pakistan 2016.
| Methods | Allocation concealment: not described. Quote: "...Randomized control trial..." No further details. Two arms | |
| Participants | 314 pregnant women after 20th week of gestation with SBP 150 mm to 160 mm of Hg and DBP 100 mm to 110 mm of Hg. Excluded: history of chronic/essential HT, diabetes mellitus, cerebrovascular disease, chronic renal failure, multiple pregnancy or collagen disease. |
|
| Interventions | Exp: oral labetalol 100 mg 3 or 4 times a day (up to 1200 mg a day). (157 women) Control: oral methyldopa 250 mg per day 3 to 4 divided doses (up to 500 mg). (157 women) |
|
| Outcomes | Women: successful lowering of BP (achieving desired BP 140/90), progress to severe HT, side‐effects. Babies: not reported. |
|
| Notes | Methods for measuring BP not stated. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...Patient were randomly assigned to either group A or Group B by lottery method..." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐ label trial (different dosages). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women (1.3%) lost to follow‐up (2 in each group). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Baseline characteristics at admission not reported. No information about funding source. Declaration of interests not described. |
Panama 2014.
| Methods | Allocation concealment quote: "...concealed through the use of sealed envelopes." | |
| Participants | 63 pregnant women with singleton or twin pregnancy and mild/moderate chronic HT at 20 weeks of gestation.
Excluded: chronic HT with BP 160 mmHg systolic or 110 mmHg diastolic; renal failure or pre‐existing renal disease; diabetes mellitus; autoimmune disease; major fetal abnormalities; oligohydramnios; fetal death. Three arms |
|
| Interventions | Exp 1: 20 mg of oral furosemide a day. (21 women) Exp 2: 5 mg of oral amlodipine a day. (21 women) Control: 75 mg of orally administered acetylsalicylic acid a day.(21 women) |
|
| Outcomes | Women: gestational age at delivery, indication for delivery, hospitalisation during pregnancy and mode of delivery, placental abruption, severe HT, aggregate PE, pulmonary oedema, HELLP syndrome, renal insufficiency, eclampsia, disseminated intravascular coagulation. Babies: fetal or neonatal death, birthweight, Apgar scores, RDS, intraventricular haemorrhage grade III and IV, necrotising enterocolitis, neonatal sepsis. | |
| Notes | Methods for measuring BP not stated. Funding: no information about funding source. Authors declared no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code with block size of 6. The other investigators did not have access to the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "...concealed through the use of sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 women (5%) refused to continue in the study (one in the amlodipine group and 2 in the AAS group). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Authors declared no conflict of interests. |
South Africa 1991a.
| Methods | Allocation concealment: cards labelled R and Q picked blindly from a box, these identified drug container. Two arms | |
| Participants | 32 women at 12 to 30 weeks' gestation with a singleton pregnancy and BP >/= 140/90 mmHg x 2 at least 6 hrs apart, no proteinuria, no antihypertensive therapy and no other drug treatment. | |
| Interventions | Exp: oral prazosin 1 mg to 5 mg x 3/day. (12 women) Control: oral identical placebo. (20 women) | |
| Outcomes | Women: severe HT, proteinuria, duration of treatment, placental abruption, caesarean section. Babies: perinatal death, gestation at delivery (mean), birthweight, SGA (< 10th centile) preterm delivery (< 37 weeks). | |
| Notes | Method for measuring BP not mentioned. The trial stopped early. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...in a randomised way, participants either received...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although identical placebo tablets were used, there is no information about whether it was a double‐blind or a single‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
South Africa 1993.
| Methods | Allocation concealment: not stated. Authors state: "randomised open study". Withdrawals: 3 women (10%) lost to follow‐up, but outcome for babies reported. Two arms | |
| Participants | 29 women at 29 to 36 weeks' gestation with mild‐moderate HT (DBP 90‐110 mmHg). | |
| Interventions | Exp: oral nifedipine started at 30 mg/day. (13 women)
Control: oral methyldopa started at 750 mg/day. ( 13 women) Stated that "'dose adjustments were made, when necessary, every second day until control of BP was obtained". |
|
| Outcomes | Women: additional antihypertensive, caesarean section, induction of labour, side‐effects. Babies: stillbirth, preterm delivery, gestation at delivery (mean), admission to SCBU. | |
| Notes | Method for measuring BP not mentioned. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described: "...randomised, prospective open study...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 women (10.3%) did not complete the trial; 2 from 1 group and 1 from the other, not clear from the report in which group they were. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
Spain 1988.
| Methods | Quote: "double‐blind, placebo‐controlled trial", no further information. | |
| Participants | 31 women with mild HT (BP 140/90 to 160/90 110 mmHg) despite bed rest in hospital. | |
| Interventions | EXP: oral labetalol 200 mg to 600 mg a day. Number of women not specified. CONT: oral placebo tablets. Number of women not specified. |
|
| Outcomes | severe HT, need for additional antihypertensives, MAP, caesarean section, perinatal deaths, fetal distress. | |
| Notes | Not possible to extract data. Available as abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Funding source and declaration of interests not described |
Sudan 2002.
| Methods | Allocation concealment: not stated. Authors state::"...patients were randomly allocated..." Two arms | |
| Participants | 70 primigravid women with PE (BP =/> 90/109 mmHg x 2, 6 hrs apart plus 2+ proteinuria in dipsticks) at 28 to 36 weeks' gestation. Singleton pregnancy. | |
| Interventions | Exp: oral methyldopa 750 mg/day to 4000 mg/day (34 women)
Control: oral no drug treatment. (36 women) All women in both groups were admitted to hospital for bed rest. |
|
| Outcomes | Women: BP, abruptio, imminent eclampsia, eclampsia, preterm delivery, caesarean section, maternal death. Babies: birthweight, IUGR, admission to SCBU (reported as 'referral of baby'), perinatal deaths, Apgar score. | |
| Notes | Korotkoff IV sound used for DBP. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote "...patients were randomly allocated...". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Sweden 1984.
| Methods | Allocation concealment: telephone randomisation, no further details. Two arms | |
| Participants | 52 women in antenatal clinic at < 37 weeks' gestation with singleton pregnancy, BP >/= 140/90 mmHg or an increase of at least 30 mmHg SBP or 15 mmHg DBP x 2 within 24 hrs. Excluded: imminent eclampsia, serious fetal distress, severe HT (> 170/110 mmHg), Rh disease, diabetes, contraindication to beta blockers, 'social or psychological handicaps'. | |
| Interventions | Exp: oral metoprolol 100 mg x 2/day to 200 mg x 2/day. (26 women) Control: oral identical placebo x 2/day.(26 women) | |
| Outcomes | Women: proteinuria (>/= 2+), severe HT, changed drugs due to side‐effects, hospital admission, placental abruption, caesarean section. Babies: perinatal death, gestation at delivery (mean) Apgar (mean). | |
| Notes | Korotkoff phase V used for DBP. Additional data provided by authors. Funding: funded by charity or foundation. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation (personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Placebos of identical appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Low risk | Unpublished data provided by author. |
| Other bias | Unclear risk | Declaration of interests not described. Funded by charity or foundation. |
Sweden 1985.
| Methods | Allocation concealment quote:"'envelope randomisation". No further information. Withdrawals: 7 women (4%) dropped out (4 exp, 3 control). Multicentre, not stated how many hospitals. Two arms | |
| Participants | 168 women in antenatal ward with singleton pregnancy at < 37 weeks, DBP >/= 90 mmHg x 2, no proteinuria. Excluded: diabetes, asthma, heart disease, psychiatric or psychological disorders. | |
| Interventions | Exp: oral metoprolol 50 mg/day to 200 mg/day + oral hydralazine 50 mg/day to 300 mg/day. (86 women) Control: no antihypertensive. (82 women) | |
| Outcomes | Women: severe HT, proteinuria (> 1+ or 0.25 g/L), changed drugs due to side‐effects, placental abruption, caesarean section. Babies: stillbirth, neonatal death, preterm delivery (< 37 and < 34 weeks), SGA (undefined), bradycardia, hypoglycaemia, Apgar < 7 at 1 min and 5 mins, RDS. | |
| Notes | Korotkoff phase V used for DBP. Additional data provided by authors. Funding: no information about funding. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. '...envelope randomisation...'. No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 7 women (4%) dropped out (4 exp, 3 control). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Sweden 1995.
| Methods | Allocation concealment: authors state: "randomised by numbers to treatment with capsules". Randomisation in blocks of 6. Information about allocation kept in sealed envelopes, opened if severe complications or side‐effects. 5 centres in Sweden, 1 in Denmark.
Withdrawals: 7 women (6%), 1 dropout, 6 not re‐evaluated after 3 days (4 exp, 2 control). Two arms |
|
| Participants | 118 women at 26 to 37 weeks, with singleton pregnancy and DBP 95 mmHg to 110 mmHg. Excluded: if delivery expected within a week, history of alcohol or drug abuse, or other medication known to be toxic. | |
| Interventions | Exp:oral isradipine (slow release) 5 mg x 2/day. (58 women) Control: oral placebo x 2/day. (59 women) | |
| Outcomes | Women: eclampsia, severe HT (DBP >/= 110 mmHg), proteinuria >/= 2+, need for additional antihypertensive, MAP, caesarean section, induction of labour, side‐effects. Babies: perinatal death, gestation at delivery (mean), admission to SCBU, birthweight (mean), placental weight. | |
| Notes | Korotkoff phase IV used for DBP. Description of BP measurements technique, and of criteria used to define HT and proteinuria. Funding: no information about funding. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by numbers in blocks of 6..." |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote:"...parallel double blind study...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 withdrawals (6%). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
UK 1968.
| Methods | Allocation concealment: not stated. Authors state: "allocated at random". Two arms | |
| Participants | 100 pregnant women with DBP >/= 90 mmHg or more x 2, 48 hrs apart. | |
| Interventions | Exp: oral methyldopa 250 mg x 2/day to 1000 mg x 2/day + oral bendrofluazide 5 mg/day to 10 mg/day. (52 women) Control: no treatment. (48 women) | |
| Outcomes | Women: mean BP, proteinuria, residual HT, length of gestation. Babies: birthweight (mean), perinatal death. | |
| Notes | Methods for measuring BP not mentioned. According with BP at entry, women were divided in 2 groups: 'moderate' for those with DBP = or > 90 mmHg at entry (n = 42), and 'severe' for those with DBP = or > 100 mmHg (n = 58). For the main outcomes, results are presented together. Funding: funded by university or non commercial organisation. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...allocated at random...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Funded by university or non commercial organisation. Declaration of interests not described. |
UK 1976.
| Methods | Allocation concealment: not stated. Authors state: "randomly allocated".
Withdrawals: 5 women (2%) withdrawn from exp group.
Follow‐up of 202 live born children. At 4 years, 34 (17%) lost to follow‐up. At 7, years 7 (3%) lost to follow‐up. Two arms |
|
| Participants | 247 women with BP >/= 140/90 mmHg if < 28 weeks' gestation, or >/= 150/95 mmHg if > 28 weeks' gestation x 2, 24 hrs apart. Excluded: diabetes, multiple pregnancy, Rh immunisation. Women > 36 weeks' gestation excluded during first year of the trial, thereafter excluded if > 32 weeks' gestation. | |
| Interventions | Exp: oral methyldopa 750 mg/day to 4000 mg/day. (122 women)
Control: no antihypertensive. (125 women) Hydralazine if severe HT. |
|
| Outcomes | Women: severe HT, proteinuria, caesarean section, elective delivery, side‐effects, changed drug due to side‐effects. Babies: perinatal death, birthweight (mean), gestation at delivery (mean), SGA (< 2 SD below mean), babies nursed in an incubator, neurodevelopment at 4 and 7 years. | |
| Notes | Korotkoff phase IV used for DBP. Random zero sphygmomanometer. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "Treatment was allocated randomly...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals (2%). |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Groups appear comparable at baseline. Funded by industry. |
UK 1980.
| Methods | Allocation concealment: randomly allocated using random‐number table. Two arms | |
| Participants | 26 women < 38 weeks' gestation with PIH and no contraindication to beta blockers. | |
| Interventions | Exp: oral labetalol 400 mg/day to 800 mg/day. (14 women) Control: oral methyldopa 750 mg/day to 1500 mg/day. (12 women) | |
| Outcomes | Women: proteinuria, severe HT, caesarean section, induction of labour, side‐effects. Babies: stillbirth, birthweight (mean), gestation at delivery (mean), 1‐min Apgar, admission to SCBU, jaundice. | |
| Notes | Korotkoff phase IV used for DBP. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Groups appear comparable at baseline.Funded by industry. |
UK 1982.
| Methods | Allocation concealment: envelope randomisation, no further information. Two arms | |
| Participants | 126 women with either chronic HT or PIH, and DBP > 95 mmHg if < 20 weeks or 95 mmHg to 109 mmHg if > 20 weeks. | |
| Interventions | Exp: oral labetalol 100 mg x 2/day, increased to maximum of 1200 mg/day. (62 women)
Control: no antihypertensive.(64 women) If BP not controlled, hydralazine 25 mg x 3/day, increased to maximum of 200 mg/day. |
|
| Outcomes | Women: severe HT, proteinuria (undefined), caesarean section, placental abruption. Babies: perinatal death, SGA (< 10th centile). | |
| Notes | Methods for measuring BP not mentioned. Additional data provided by authors. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Envelope randomisation (personal communication). No details on whether they were opaque, sealed and consecutively numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Low risk | Additional data provided by author. |
| Other bias | High risk | Funded by industry. |
UK 1983a.
| Methods | Allocation concealment: authors state "allocated in double‐blind and randomised manner'"
Withdrawals: some data missing for 35 women (29%). Data for each outcome only included if < 20% excluded.
Follow‐up: 110 children (92%) seen at 1 year. Two arms |
|
| Participants | 120 women with PIH in third trimester admitted for bed rest, SBP 140 mmHg to 170 mmHg and DBP 90 mmHg to 110 mmHg x 2, 24 hrs apart. Excluded: women with contraindication to beta blockers. | |
| Interventions | Exp: oral atenolol 100 mg/day. to 200 mg/day. (60 women) Control: oral placebo. (60 women) | |
| Outcomes | Women: proteinuria (> 0.5 g/24 hrs), severe HT, additional antihypertensive, changed treatment due to side‐effects, side‐effects, admission to hospital prior to delivery, caesarean section. Babies: perinatal death, SGA (< 10th centile), bradycardia, hypoglycaemia, jaundice, RDS. At 1 year: cerebral palsy, IQ < 1 SD below mean, weight. | |
| Notes | Korotkoff phase IV used for DBP. Random zero sphygmomanometer used for measuring BP. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...allocated in double‐blind, randomised manner...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: some data missing for 35 women (29%). Data for each outcome only included if < 20% excluded. Follow‐up: 110 children (92%) seen at 1 year. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Funded by industry. |
UK 1983b.
| Methods | Allocation concealment: not stated. Authors state: "allocated at random". Stratified by gestational age. Two arms | |
| Participants | 100 women with singleton pregnancy and DBP >/= 95 mmHg x 2 at least 24 hrs apart, or > 105 mmHg x 1. Excluded: asthma, heart failure, or heart block, diabetes, renal disease, or taking other hypertensive medication. | |
| Interventions | Exp: oral oxprenolol 80 mg x 2/day to 320 mg x 2/day. (50 women)
Control: oral methyldopa 250 mg x 3/day to 1000 mg x 3/day. (50 women) If BP not controlled, hydralazine added to both groups. |
|
| Outcomes | Women: severe HT, proteinuria (> trace on dipstick), induction of labour, caesarean section, additional antihypertensive, hospital admission. Babies: perinatal death, birthweight (mean), 5‐min Apgar < 7, antenatal fetal heart rate. | |
| Notes | Korotkoff phase IV used for DBP. Random zero sphygmomanometer. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described "...allocated at random...". Stratified by gestational age. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women (4%) excluded, 2 lost to follow‐up, 2 abortions. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Funding: funded by industry. |
UK 1984.
| Methods | Allocation concealment: not stated. Authors state: "'randomised trial". Two arms | |
| Participants | 60 women at 18 to 36 weeks' gestation with undefined HT. | |
| Interventions | Exp: oral atenolol 100 mg/day. (30 women) Control: oral methyldopa 250 mg x 3/day. (30 women) | |
| Outcomes | Women: proteinuria (undefined). Babies: stillbirth, birthweight, SGA (< 10th centile) bradycardia, hypoglycaemia. | |
| Notes | Korotkoff phase V used for DBP. Funding: no information. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...randomised trial...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
UK 1989.
| Methods | Allocation concealment: drug and placebo sent by manufacturer to hospital pharmacy with list of random numbers. Then dispensed by pharmacists. 5 centres.
Withdrawals: 8 (5%), 6 exp, 2 control. 2 women withdrew, 1 treated with ward stock labetalol, 1 developed rash, and 4 did not fulfil entry criteria. Two arms |
|
| Participants | 152 women from antenatal wards at 20 to 38 weeks' gestation with SBP 140 mmHg to 160 mmHg and DBP 90 mmHg to 105 mmHg x 2, 24 hrs apart, and no proteinuria. Excluded: history of HT, renal, metabolic, cardiovascular, respiratory or collagen disease. | |
| Interventions | Exp: oral labetalol 100 mg x 3/day to 200 mg x 3/day. Control: oral identical placebo. | |
| Outcomes | Women: mean BP, severe HT, proteinuria (undefined), induction of labour, caesarean section, days in hospital (mean), side‐effects. Babies: perinatal death, preterm delivery (< 37 weeks), SGA (< 5th centile), admission to SCBU, RDS. | |
| Notes | Korotkoff phase IV used for DBP. Conventional sphygmomanometers used to measure BP. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers. |
| Allocation concealment (selection bias) | Low risk | Drug and placebo sent by manufacturer to hospital pharmacy with list of random numbers. Then dispensed by pharmacists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 women excluded after randomisation (5.3%), without specification of the group they belonged. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Funded by industry. |
UK 1990.
| Methods | Allocation concealment: not stated. Authors state "randomised" but no other information. Withdrawals: 4 (12%), 1 exp (changed her mind), 3 control (2 severe HT, 1 breathlessness). Two arms | |
| Participants | 33 women 12 to 24 weeks' gestation with SBP 140 mmHg to 170 mmHg and DBP 90 mmHg to 110 mmHg x 2, 24 hrs apart. Excluded: if 'usual' contraindications to beta blockers. | |
| Interventions | Exp: oral atenolol 50 mg/day to 200 mg/day. (16 women) Control: oral placebo (character not stated). (17 women) | |
| Outcomes | Women: mean BP, severe HT, stopped drug due to side‐effects. Babies: stillbirth, birthweight, SGA (< 5th centile), placental weight, gestation at delivery (mean). | |
| Notes | Korotkoff phase V used for DBP. The trial was stopped early when the principal investigator left Glasgow. Additional data provided by authors. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote:"...randomised, placebo‐controlled, double blind study...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 withdrawals (12.1%). |
| Selective reporting (reporting bias) | Low risk | Additional data provided by authors. |
| Other bias | High risk | Funded by industry. |
UK 1992.
| Methods | Allocation concealment: numbered, sealed opaque envelopes. Stratified by parity. Two arms | |
| Participants | 114 women with singleton pregnancy at 24 to 39 weeks' gestation with DBP > 90 mmHg for > 24 hrs and no proteinuria. Excluded: psychoneurosis, cardiac abnormality, diabetes, asthma, contraindication to beta blockers, antenatal antihypertensive treatment. | |
| Interventions | Exp: oral labetalol 100 mg x 2/day, increased up to 400 mg x 3/day. (51 women) Control: no antihypertensive. (63 women) | |
| Outcomes | Women: proteinuria (> 1+ or 0.25 g/L), duration of stay in hospital (mean), side‐effects, changed drug due to side‐effects, elective delivery, caesarean section. Babies: perinatal death, gestation at delivery (mean), preterm delivery (< 37 weeks), SGA (< 5th centile), admission to SCBU, length of stay in hospital (mean). | |
| Notes | Korotkoff phase IV used for DBP. Additional data provided by authors. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed opaque envelopes. Stratified by parity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Low risk | Additional data provided by authors. |
| Other bias | High risk | Groups appear comparable at baseline. Funded by industry. |
UK 2009.
| Methods | Allocation concealment: centralised automated interactive voice response system. Two arms | |
| Participants | 39 singleton pregnant women with PE, defined as DBP ≥ 90 mmHg on 2 separate occasions, at least 4 hrs apart occurring after 20 weeks' gestation and associated with significant proteinuria (≥ 2 + protein on urinalysis), and women who developed superimposed PE on background chronic HT if they developed new significant proteinuria. Excluded: delivery indicated within 24 hrs, uncontrolled HT (BP > 170/110 mmHg), renal or collagen vascular disease, relative contraindications to sildenafil or taking medications that interact with sildenafil. |
|
| Interventions | Exp: oral sildenafil 20 mg three times daily. (up to 80 mg a day). (19 women) Control: oral placebo of identical appearance three times daily (20 women) |
|
| Outcomes | Women: prolongation of pregnancy from randomisation to delivery (days),umbilical artery pulsatility index, maternal serum urate, maternal BP, adverse events, dosing and pharmacokinetics of sildenafil in women with PE. Babies: Apgar scores, umbilical arterial and venous cord gases at delivery and placental weight, fetal adverse events. |
|
| Notes | Methods for measuring BP not mentioned. Funding: funded by industry. Conflict of interests declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 ratio using a centralised automated interactive voice response system. Randomisation stratified by centre, gestational age (24 to 28 weeks, ≥ 28 to 34 weeks) and disease status (PE alone or PE with IUGR). |
| Allocation concealment (selection bias) | Low risk | Centralised automated interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Placebo of identical appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 women (10.3%) withdrew consent after randomisation (2 in each group). |
| Selective reporting (reporting bias) | Low risk | Protocol registered in advance. ClinicalTrials.gov Identifier: NCT00141310. |
| Other bias | High risk | Small numbers. Funded by industry. Conflict of interests declared. |
UK 2017.
| Methods | Allocation concealment: computer web‐based system. Two arms | |
| Participants | 114 pregnant women with a prenatal diagnosis of chronic HT (treated or untreated) or BP readings ≥ 140 mmHg systolic or ≥/90 mmHg diastolic before 20 weeks’ gestation requiring antihypertensive treatment before 27 + 6, gestation between 12 + 0 and 27 + 6 weeks, singleton pregnancies, aged > 18 years, and the ability to provide written informed consent. Excluded: contraindication (relative or absolute) to either antihypertensive agent. |
|
| Interventions | Exp: oral nifedipine modified release 20 mg (10 mg twice per day) and maximum 80 mg (40 mg twice per day). (58 women) Control: oral labetalol 200 mg (100 mg twice per day) and maximum 1800 mg (600 mg 3 times per day). (56 women) |
|
| Outcomes | Women: maximum SBP post‐randomisation until delivery (mean highest systolic and corresponding diastolic in each treatment group), mean systolic and mean DBP, days with SBP >= 160 mmHg, >= 150 mmHg, or DBP < 80 mmHg, superimposed PE, gestation at delivery, additional antihypertensive agents, mode of delivery, estimated blood loss, other adverse maternal outcomes. Babies: birthweight, admission to NICU, condition of the fetus at birth (including Apgar score and umbilical cord gas analysis), customised birthweight centiles, and other adverse neonatal outcomes. |
|
| Notes | BP readings using manual and automated devices validated for use in pregnancy. Korotkoff sound considered for DBP not reported. Funding: funded by university or non commercial organisation. Conflict of interests declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | MedSciNet online minimisation protocol with stratification for: gestation at randomisation, maternity centre, SBP at randomisation, and ethnicity. |
| Allocation concealment (selection bias) | Low risk | Online system. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 women lost to follow‐up and 1 women withdrew consent (one in each group) (1.8%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered in advance. ISRCTN40973936 |
| Other bias | High risk | Imbalance in some baseline characteristics. Conflict of interests declared. |
USA 1979.
| Methods | Allocation concealment: not stated. Authors state: "allocated randomly to treatment or no treatment". Two arms | |
| Participants | 58 women with HT before pregnancy or BP >/= 140/90 mmHg x 2 more than 24 hrs apart before 20 weeks' gestation. Excluded: DBP > 100 mmHg, nulliparous, other major medical or obstetric problem. | |
| Interventions | Exp: oral methyldopa 750 mg/day to 2000 mg/day, oral hydrochlorothiazide 50 mg/day, oral hydralazine 75 mg/day to 250 mg/day. (29 women) Control: no antihypertensive. (29 women) | |
| Outcomes | Women: severe HT, proteinuria (> 1+ or > 300 mg/L in 24 hrs), caesarean section. Babies: perinatal death, gestation at delivery, birthweight < 2500 g, fetal distress, SGA (undefined). | |
| Notes | No information about how BP measured. In exp group, 11 women had methyldopa + hydrochlorothiazide, 10 hydralazine + hydrochlorothiazide, 8 had all 3 drugs. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...allocated randomly to treatment or no treatment...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
USA 1987a.
| Methods | Allocation concealment: physician drew a sealed envelope containing assignment. Withdrawals: 14 women (7%), 8 exp and 6 control refused hospitalisation, but data reported for perinatal death. Two arms | |
| Participants | 200 primigravid women in hospital at 26 to 35 weeks' gestation with SBP 140 mmHg to 160 mmHg and DBP 90 mmHg to 110 mmHg, proteinuria > 0.3 g/L and uric acid > 4.6 mg/dL. Excluded: associated medical and obstetrical complications, other antihypertensive medication. | |
| Interventions | Exp: hospitalisation + oral labetalol 300 mg/day, increased every few days to max 2400 mg/day. (100 women) Control: hospitalisation alone. (100 women) | |
| Outcomes | Women: severe HT, increased proteinuria, eclampsia, placental abruption, caesarean section, renal function, days gained during management. Babies: perinatal death, gestation at delivery (mean), birthweight (mean), placental weight, admission to SCBU, SGA (< 10th centile). | |
| Notes | No mention of how BP measured. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 withdrawals (7%). 8 in treatment group and 6 in control group refused hospitalisation, but data reported for perinatal death. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | No information about funding source. Declaration of interests not described. |
USA 1987b.
| Methods | Allocation concealment: not stated. Authors state: "randomly allocated", no further information. Two arms | |
| Participants | 25 women at < 34 weeks' gestation, singleton pregnancy with BP 140/90 mmHg x 2 at least 6 hrs apart and no proteinuria. Presumed chronic HT. | |
| Interventions | Exp: oral methyldopa 750 mg x 3/day to 2000 mg x 4/day. (13 women) Control: oral placebo, in the same way. (12 women) If severe PE, hydralazine or MgSO4 added. | |
| Outcomes | Women: MAP, new proteinuria (2+ or greater on urine dipsticks), PE (defined as a sudden rise of 30 mmHg SBP or 15 mmHg DBP and weight gain > 2 lbs/week, or proteinuria > 2+), elective delivery, side‐effects. Babies: perinatal death, gestation at delivery (mean), birthweight (mean and < 50th centile). | |
| Notes | No information about how BP measured. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Quote: "...randomly allocated...". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Funded by industry. |
USA 1990a.
| Methods | Allocation concealment: envelope randomisation, using computer‐generated random numbers. 3‐arm study. | |
| Participants | 300 women in antenatal ward with chronic mild‐moderate HT at 6‐13 weeks' gestation. All had chronic HT before pregnancy and no associated medical complications. | |
| Interventions | Exp: (1) methyldopa 750 mg/day to 4000 mg/day (no other details) (100 women). (2) labetalol 300 mg/day to 2400 mg/day (no other details) (100 women). Control: no antihypertensive (100 women). | |
| Outcomes | Women: PE (defined as HT, proteinuria, and hyperuricaemia), additional antihypertensive, days in hospital, placental abruption, congestive heart failure, serum creatinine, uric acid. Babies: perinatal death, gestation at delivery, birthweight < 2.5 kg, preterm delivery (< 37 weeks), SGA (undefined), admission to SCBU, hypoglycaemia, 5‐min Apgar < 7. | |
| Notes | Korotkoff phase IV used for DBP. 36% of women were taking an antihypertensive at the time of trial entry. Additional data provided by authors. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes (personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusions: 37 women (12%): 13 in methyldopa arm (10 excluded due to poor compliance, 1 twin, 1 abortion and 1 lost to follow‐up); 14 in the labetalol arm (11 excluded due to poor compliance, 2 twin, and 1 lost to follow‐up) and 10 in the control arm (8 due to poor compliance, 1 twin and 1 spontaneous abortion). |
| Selective reporting (reporting bias) | Low risk | Additional data provided by authors. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
USA 1992.
| Methods | Allocation concealment: physician drew sealed envelope containing assignment. Computer‐generated random numbers. Withdrawals: 3 women (1.5%) lost to follow‐up (2 exp, 1 control). Two arms | |
| Participants | 200 primigravid women at 26 to 36 weeks' gestation with SBP 140 mmHg to 160 mmHg and/or DBP 90 mmHg to 110 mmHg 24 hrs after hospitalisation, proteinuria > 300 mg/24 hrs, and/or uric acid > 6 mg/dL. Excluded: associated medical or obstetric complications, or fetal compromise (suspected abnormal fetal growth by US, abnormal fetal testing). | |
| Interventions | Exp: oral nifedipine 40 mg/day to 120 mg/day. (100 women) Control: bed rest alone. (100 women) | |
| Outcomes | Women: MAP, severe proteinuria (> 5 g/24 hrs), antenatal hospital stay (mean), days gained during management, caesarean section, placental abruption, HELLP syndrome. Babies: stillbirth, neonatal death, birthweight, preterm delivery (< 37 weeks), SGA (< 10th centile), admission to SCBU, days in SCBU (mean). | |
| Notes | Method of measuring BP not mentioned. Funding: no information about funding source. Declaration of interests not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women lost to follow‐up (1.5%). 2 in treatment group and 1 in control group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appear comparable at baseline. No information about funding source. Declaration of interests not described. |
Venezuela 1988.
| Methods | Allocation concealment: not stated. Treatment assigned using random‐number tables. Two arms | |
| Participants | 31 women > 14 weeks' gestation with either chronic HT or mild‐moderate PIH (BP 140 to 169 mmHg/90 to 109 mmHg x 2 after 5‐min rest). Excluded: contraindication to beta blockers, Rh or haemorrhagic disorders. | |
| Interventions | Exp: oral mepindolol 5 mg/day, increased weekly to 10 mg/day. (16 women) Control: oral methyldopa 250 mg x 2/day increased weekly to 250 mg x 4/day. (15 women) | |
| Outcomes | Women: severe HT, caesarean section, induction of labour. Babies: perinatal death, gestation at delivery, birthweight, Apgar score. | |
| Notes | Main paper in Spanish.
Method of measuring BP not mentioned. Funding: funded by industry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described: [Estudio prospectivo randomizado] "randomised, prospective trial". No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | High risk | Funded by industry. |
AAA: acetylsalicylic acid BP: blood pressure CTG: cardiotocography DBP: diastolic blood pressure exp: experimental GITS: gastrointestinal therapeutic system GTN: glyceryl trinitrate HELLP: syndrome of haemolysis, elevated liver enzymes and low platelets hr(s): hour(s) HT: hypertension IUGR: intrauterine growth restriction IV: intravenous IVH: intraventricular haemorrhage MAP: mean arterial pressure mg: milligram (s) MgSO4: magnesium sulphate min(s): minute(s) NICU: neonatal intensive care unit PE: pre‐eclampsia PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage RDS: respiratory distress syndrome Rh: Rhesus SBP: systolic blood pressure SCBU: special care baby unit SD: standard deviation SGA: small‐for‐gestational age US: ultrasound UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Argentina 1990 | Most women with severe chronic HT. Women with mild‐moderate HT not analysed separately. Methods: open, prospective, randomised, comparative 3‐arm trial. Participants: 90 women with severe chronic HT. Intervention: Atenolol (50 mg to 200 mg daily), vs methyldopa (500 mg to 2000 mg daily) vs ketanserin (80 mg to 120 mg daily). Outcomes: BP, gestational age at delivery, birthweight, 1‐min Apgar score, fetal and neonatal mortality. |
| Argentina 1994 | Not clearly randomised. Available as abstract only. Methods: 'divided into two groups'. No further information. Participants: 187 women with chronic HT (n = 66) or gestational HT (n = 121). Interventions: atenolol 40 mg/day to 100 mg/day vs methyldopa 250 mg/day to 2000 mg/day. Outcomes: superimposed PE, maternal BP, birthweight. |
| Australia 1985b | Comparison of 2 alpha agonists. Methods: quote "prospective, double blinded". Women entered in a numerical sequence. No numbers missed or used a second time. Participants: 100 women with BP > 130/85 mmHg or a rise of 30/15 mmHg from previous values. Intervention: clonidine 150 mcg/day to 1200 mcg/day vs methyldopa 250 mg/day to 2000 mg/day. If additional treatment needed, hydralazine. Outcomes: severe HT, need for additional drug, stopped treatment due to side‐effects, stillbirth, neonatal death, preterm delivery, birthweight (mean), SGA, 5‐min Apgar. |
| Australia 1991 | Entry criteria was DBP greater than 1 SD above the reported mean for gestational age. Mean BP of recruited women was 129/84 mmHg at entry to the trial (122 to 136 mmHg/79 to 89 mmHg) for the placebo group, and 126/82 mmHg (118‐134/79‐85) for the treatment group. Participants: 52 nulliparous with singleton pregnancies between 28 and 34 weeks of gestation, without proteinuria. Intervention: clonidine from 200 mcg to 800 mcg a day plus hydralazine from 50 mg to 200 mg a day, and placebo. Outcomes: severe HT, imminent eclampsia, eclampsia, severe proteinuria, antepartum haemorrhage, HELLP syndrome, fetal distress, fetal death, IUGR. |
| Belgium 1988 | Comparison of 2 beta blockers. Available as abstract only. Methods: 'randomised', no further information. Participants: 23 women with BP at least 140/90 mmHg x 2 and no proteinuria. Intervention: atenolol 100 mg a day vs pindolol 15 mg a day. Outcomes: umbilical PI, maternal BP, birthweight, Apgar score. |
| Brazil 1997 | Single‐dose intervention. No clinical outcomes studied (effect of nifedipine in placental blood flow). Article in Portuguese. Only abstract translated into English. Methods: double‐blind, placebo‐controlled, RCT. Participants: 65 women with PE. Intervention: nifedipine 20 mg orally (only dose) vs placebo. Outcomes: placental blood flow prior and 30 mins after the intervention. |
| Brazil 2000b | Quasi‐random design. Main paper in Portuguese. Methods: alternated allocation (data extracted from original thesis). 11 women (10.5%) excluded after trial entry. Participants: 105 women with singleton pregnancies diagnosed with PE, chronic HT, and PE superimposed to chronic HT. Intervention: isradipine (slow release), 5 mg every 12 hrs vs atenolol 50 mg every 12 hrs. Outcomes: BP, maternal heart rate, proteinuria, maternal side‐effects, mode of delivery, gestational age, birthweight, SGA babies, Apgar score. |
| Brazil 2000c | 40 women (24%) excluded after randomisation. Reasons for exclusion were: missed appointment for Doppler (70%), non‐compliance (20%), side‐effects (7.5%), preterm delivery (2.5%). Data were not presented by treatment arm. Main paper in Portuguese. Methods: randomised, double‐blind, placebo‐controlled trial. Participants: 123 pregnant women with chronic hypertension. Intervention: verapamil 240 mg/day vs placebo during 30 days. Outcomes: Doppler PI, RI and S/D ratio, incidence of PE, birthweight, gestational age at delivery, SGA. |
| China 1991 | Herbal medicine vs magnesium sulphate. No clinical data available. Article in Chinese. Only abstract translated into English. Methods: not reported. Authors state: "...randomly designed to...". Participants: 75 women with 'hypertension syndrome of pregnancy'. Intervention: Magnesium sulphate 20 g/day to 25 g/day vs ligustrazine 120 mg/day. to 160 mg/day. Outcomes: MAP, proteinuria, haematocrit, side‐effects, positive rate of NST, Apgar score. |
| China 1993 | Only dose intervention. Sublingual nifedipine previous to caesarean section. Article in Chinese. Only abstract translated into English. Methods: not reported. Indexed as publication type: RCT. Participants: 33 women with PE undergoing emergent caesarean section. Intervention: sublingual nifedipine, 16 mg (only dose). Control group not reported in abstract. Outcomes: MAP, systolic and DBP, maternal heart rate, postoperative haematocrit, side‐effects. |
| China 1998 | Single‐dose intervention. No clinical outcomes studied (effect of nimodipine in retinal blood flow). Article in Chinese. Only abstract translated into English. Methods: not stated. Indexed as publication type: RCT. Participants: 28 women with PIH. Intervention: nimodipine 30 mg orally (only dose) vs IV magnesium sulphate. Outcomes: retinal PI. |
| China 1999 | Herbal medicine + nifedipine vs nifedipine. No clinical outcomes studied. Article in Chinese. Only abstract translated into English. Methods: not stated. Indexed as publication type: RCT. Participants: 95 women with PIH. Intervention: prepared rhubarb + nifedipine vs nifedipine. Outcomes: serum lipids, and other blood tests. |
| China 2000 | Less than 7 days treatment. Treatment was given only during labour. Article in Chinese. Only abstract translated into English. Methods: quote "64 cases of PIH were randomly divided into...". Participants: 64 women with PIH. Interventions: Nifedipine orally given every 6 hrs during labour vs no treatment. Outcomes: postpartum haemorrhage. |
| China 2014 | Not mild‐moderate HT. Only women with pulmonary HT. Article in Chinese. Only abstract translated into English. Methods: "...randomly divided into group and control group..." Participants: 64 pregnant women with pulmonary arterial HT. Interventions: Sildenafil 25 mg three times daily vs low‐flow oxygen and low‐salt diet. Outcomes: variation of the blood oxygen saturation, pulmonary artery systolic pressure, haemodynamic parameters and pregnancy outcomes, including delivery modes, neonatal weight, morbidity of mother and fetus. |
| China 2017 | Intervention (Celastrol, traditional Chinese herbal medicine) is not an antihypertensive. Severe PE. Methods: stratified permuted‐block randomisation method with DBP as a factor. Participants: 522 pregnant women with severe HT and no history of use of antihypertensive drugs; no history of heart failure during the course of the pregnancy; no history of HELLP syndrome or chronic HT; and no history of smoking or diabetes. Interventions: oral nifedipine (10‐mg capsule, up to 5 doses) and celastrol (10‐mg capsule, up to 5 doses) every 15 mins until BP was no higher than 150/100 mmHg vs oral nifedipine (10‐mg capsule, up to 5 doses) plus glucose (10‐mg capsule, up to 5 doses) as placebo every 15 mins until BP was no higher than 150/100 mmHg. Outcomes: time required to control HT; time before another hypertensive crisis, number of dosages required to control HT; maternal and neonatal adverse effects. |
| Cuba 1994 | Quasi‐random design. Article in Spanish. Methods: alternate allocation (data provided by author). Participants: 90 pregnant women with chronic HT. Intervention: methyldopa (1 g/day to 2 g/day) or hydralazine (100 mg/day to 200 mg/day) vs no treatment. Outcomes: BP, superimposed PE, abruption, preterm delivery, LBW, Apgar score, RDS, hypoxia, fetal death. |
| Czech Republic 1993 | Comparison of 2 beta blockers. Article in Czech. Only abstract translated into English. Methods: 'divided at random'. No further information. Participants: 40 women with DBP 95 mmHg to 105 mmHg. Intervention: atenolol 50 mg/day to 100 mg/day vs bisoprolol 5 mg/day to 10 mg/day. Outcomes: BP, maternal heart rate, side‐effects. |
| Denmark 1991 | Intervention is not an antihypertensive: magnesium vs placebo. Methods: quote "...patients were allocated in a double‐blind and randomised manner, based in a computer‐generated list of numbers...". Participants: 61 women with PIH. Chronic HT excluded. Withdrawals: 3 women (2 from intervention group, 1 from control group) excluded after randomisation. Intervention: 48‐hrs of either IV magnesium or placebo infusion followed by daily oral magnesium or placebo tablets. Outcomes: MAP, caesarean section, induction of labour, side‐effects, gestational age, birthweight, Apgar score, admission to SCBU and days of stay. |
| Denmark 2000 | Intervention is not an antihypertensive: magnesium vs methyldopa. Methods: RCT. Allocation concealment by numbered sealed opaque envelopes. Participants: 33 women with PIH. Chronic HT excluded. Intervention: magnesium, 48‐hrs IV infusion followed by daily oral magnesium vs methyldopa 250 mg x 4/day. Outcomes: BP, gestational age, birthweight, admission to SCBU and length of stay, serum magnesium. |
| Dominican Republic 1992a | Not a RCT. Article in Spanish. Only abstract translated into English. Methods: not stated. Authors only state "...divided into 2 groups...". Women known as given the drugs under study were also included. Participants: 50 pregnant women with chronic HT + superimposed PE. Intervention: slow‐release nifedipine 20 mg every 8 hrs vs methyldopa 500 mg every 12 hrs. Outcomes: BP, Apgar score. |
| Dominican Republic 1992b | Not a RCT. Article in Spanish. Only abstract translated into English. Methods: not stated. Authors only state"...divided into 2 groups...". Participants: 30 pregnant women with severe PE. Intervention: methyldopa 250 mg to 500 mg every 5 hrs vs hydralazine 20 mg to 50 mg every 8 hrs. Outcomes: BP, maternal side‐effects. |
| Egypt 1988 | No relevant clinical outcomes studied. Available as abstract only. Methods: quote "patients were randomly allocated to three treatment groups". No further information. Participants: 50 primigravidae with pre‐eclamptic toxaemia and 20 multigravidae with essential HT in their late pregnancy. Interventions: 3‐arm trial: bromocriptine 5 mg, methyldopa 1 g, and placebo, in different combinations. No further information. Outcomes: serum prolactin and serum placental lactogen, BP. 1‐year follow‐up reported. |
| Egypt 1993 | 1‐week intervention. Outcomes measured at 30 mins, 3 and 7 days. Methods: 'randomly allocated'. Participants: 30 women with PE in the third trimester. 25 women had mild PE with DBP 100 mmHg to 109 mmHg and 5 had severe PE with DBP >/= 110 mmHg. Intervention: nifedipine 20 mg every 8 hrs for 7 days or placebo in the same time and duration. Outcomes: BP and fetal heart rate measured at 30 mins, 3 and 7 days. Renal function tests and Doppler scans of umbilical cord. |
| Egypt 1997 | The intervention is not an antihypertensive. Naltrexone vs placebo. Available as an abstract only. Methods: quote "...were randomly allocated to either naltrexone (...) or placebo". Participants: 20 women with PIH at 30 to 36 weeks' gestation. Intervention: naltrexone (opioid receptor antagonist), 50 mg every 12 hrs vs placebo. Outcomes: BP, proteinuria, oedema, prolactin levels, gestational age, status of the baby at birth. |
| Egypt 2009 | The intervention is not an antihypertensive. Ozone therapy (rectal insufflations). Methods: quote "...were randomly assigned into two groups in equal numbers as follow...". Participants: 30 hypertensive pregnant women at 24 weeks' gestation. Intervention: ozone therapy by rectal Insufflations 3 sessions/week for 7 weeks + methyldopa vs methyldopa. Outcomes: BP, resistance and PI, dose of methyldopa required. |
| Egypt 2017 | The intervention is not an antihypertensive. Ezomeprazole vs placebo. Protocol registration of an ongoing study. ClinicalTrials.gov: NCT03213639 Methods: RCT. Double‐blind, placebo‐controlled. Participants: target 390 pregnant women with early onset PE at 28 to 31 weeks' gestation. Intervention: esomeprazole single dose of 40 mg orally once a day vs identical placebo. Outcomes: HELLP syndrome, change in serum level of sFlt‐1 and endoglin, prolongation of gestation, side‐effects. |
| Finland 1988a | Comparison of 2 beta blockers. Methods: quote "according to randomisation table". No further information. Participants: 51 women with BP > 149/94 mmHg x 2 in sitting position after 2 days bed rest in hospital. Intervention: atenolol 50 mg/day to 100 mg/day vs pindolol 10 mg/day to 20 mg/day. If needed, hydralazine 150 mg/day added. Outcomes: stillbirths, side‐effects, need for additional drug, caesarean section, gestation at delivery (mean), birthweight (mean), 5‐min Apgar. |
| Finland 1995 | Comparison of 2 beta blockers. Less than 7 days treatment, single‐dose study. Women with mild HT not reported separately from severe HT. Methods: 'randomly chosen'. No further information. Participants: 24 women with a singleton pregnancy at 28 to 40 weeks, and either mild or severe PE (BP > 160/110 mmHg plus proteinuria > 5 g/24 hrs, or BP 140/90 mmHg to 160/110 mmHg plus proteinuria < 5 g/24 hrs). Intervention: atenolol 0.15 mg/kg IV vs pindolol 0.006 mg/kg IV in 100 mL of Ringer's solution. Infusion time 15 to 20 mins. Outcomes: utero and umbilico‐placental vascular impedance, fetal haemodynamics and cardiac function. |
| Finland 1999 | Main outcomes were assessed only at 5‐7 days of inclusion. 29% of women were excluded from the analysis. Methods: randomised, double‐blind, double‐dummy study. Participants: 24 women with singleton pregnancies between 29 and 39 weeks with BP > 140/90 mmHg x2, 6 hrs apart, and proteinuria > 0.3 g in 24‐hr urine collection. Intervention: isradipine 2.5 mg twice daily or placebo vs metoprolol 50 mg twice daily or placebo (double‐dummy study). Outcomes: insulin sensitivity, uric acid, degree of proteinuria, lipids and lipoproteins, BP, umbilical artery RI, birthweight, placental weight, caesarean section, Apgar scores. |
| France 1986 | Severe HT in hospitalised patients. Methods: 'Randomised'. No further details Participants: 35 women with severe HT induced by pregnancy. Intervention: labetalol vs clonidine orally Outcomes: BP, need for additional treatment, blood uric acid, duration of pregnancy, birthweight, neonatal Apgar score, neonatal hypoglycaemia, neonatal hypocalaemia. |
| France 1988b | No clinical outcomes studied. Outcomes assessed at 4 weeks after trial entry. Available as abstract only. Methods: 'randomised' no further information. 3‐arm study. Participants: 29 women with isolated HT after quote: "a mean period of 18 weeks of pregnancy". Intervention: pindolol vs atenolol vs methyldopa. Outcomes: BP, maternal heart rate, serum sodium, potasium, uric acid, creatinine, plasma renin activity and aldosterone. |
| Germany 2012 | Not mild‐moderate HT. Prevention study. Methods: randomised, placebo‐controlled, double‐blind study. Participants: 111 pregnant women with abnormal placental perfusion at 19 to 24 weeks' gestation. Intervention: NO‐donor penterythriltetranitrat (n = 54) vs placebo (n = 57). Outcomes: utero‐placental perfusion, preterm birth, IUGR, PE, fetal deaths. |
| Hungary 1999 | 28% of women excluded after randomisation (7 because of treatment duration not exceeding 10 days and 2 dropped out). Methods: allocation 'according to randomisation list'. No further information. Participants: 32 healthy primigravidae with BP at least 140/90 mmHg x 2 at least 6 hrs apart. Interventions: calcium dobesilate 2 g a day vs placebo. Outcomes: new proteinuria, caesarean section, placental abruption, preterm delivery. |
| India 1999 | Intervention is an antiplatelet agent. Available as abstract only. Methods: randomised, placebo‐controlled trial. Participants: 163 women with PIH of 20 to 32 weeks' gestation. Intervention: aspirin 60 mg a day vs placebo from 22 until 38 weeks of gestation. Outcomes: prevention of PIH grade B (BP 160/110 mmHg x 2, 4 hrs apart), proteinuria 2+ or more, perinatal mortality, maternal mortality, eclampsia, SGA (< 10th centile). |
| India 2012a | Includes women with severe HT. IV labetalol given to this subgroup, not analysed separately. Methods: prospective, randomised controlled parallel‐group study. Participants: 90 women with singleton, vertex pregnancies at 20 to 40 weeks' gestation with BP >/= 140/90, with and without proteinuria. Intervention: oral or IV Labetalol vs oral methyldopa. Outcomes: serum urea and creatinine, significant proteinuria, severe maternal complications, induction of labour, caesarean section, lactation, gestational age at delivery, Apgar score, admission to NICU, RDS, bradycardia, jaundice, hypoglycaemia. |
| India 2012b | Intervention is not an antihypertensive: magnesium sulphate vs placebo. No clinical outcomes studied. 27% women (18/66) excluded after randomisation. Methods: women were randomly allocated by sealed envelopes containing computer‐generated random numbers. Double‐blind, placebo‐controlled. Participants: 66 women with mild PE or PIH after 34 weeks' gestation. Intervention: 24‐hrs of either IV magnesium sulphate or placebo infusion. Outcomes: umbilical artery and fetal middle cerebral artery PI. |
| India 2013d | Severe HT. Protocol registration of an ongoing study. ClinicalTrials.gov: NCT01912677. CTRI/2013/08/003866 Methods: open‐label RCT. Participants: target 671 pregnant women with severe HT. Intervention: 3‐arm trial. Oral nifedipine vs oral labetalol vs oral methyldopa. Outcomes: number of hourly BP's in severe range. No other outcomes listed. |
| India 2015c | Severe HT. Pilot feasibility study of Protocol registered as India 2013b. ClinicalTrials.gov: NCT01912677. CTRI/2013/08/003866 Methods: open‐label RCT. Participants: 30 pregnant women in India with a sustained SBP P160 mmHg or DBP P110 mmHg. Intervention: oral nifedipine (10 mg up to 3 total doses in 6 hrs) vs oral labetalol (200 mg up to 3 total doses in 6 hrs) vs a single dose of oral methyldopa 1000 mg. Outcomes: achievement of the targeted BP within 6 hrs, use of additional antihypertensives, adverse maternal and neonatal effects, and side‐effect profile. |
| India 2015d | Severe HT. No clinical outcomes measured. Available as abstract only. Methods: RCT Participants: 30 women with severe PE having acute HT (more than or equal to 160/105 mmHg) Intervention: IV labetalol vs oral nifedipine Outcomes: Doppler vascular PI, RI, S/D ratio of umbilical (UA), middle cerebral artery (MCA), maternal uterine and renal artery flow. |
| India 2016 | No clinical outcomes measured. Methods: single‐blind RCT. Participants: 60 primiparous pregnant women with PE (BP > 140/90 mmHg and proteinuria or oedema). Intervention: 4‐arm trial. Methyldopa vs Methyldopa + vit C vs nifedipine vs nifedipine + vit C. Outcomes: serum levels of malondialdehyde (MDA) Superoxide Dismutase (SOD) before and after treatment in the 4 groups. No other clinical outcomes reported. |
| Iran 2000 | Quasi‐random design (data from personal communication). Available as abstract only. Methods: quote: "patients were sequentially assigned to one of two randomised group"'. Alternate allocation (data obtained from personal communication). Participants: 37 pregnant women over 26 weeks' gestation with BP over 140/90 mmHg (after 24 to 48 hrs resting) + proteinuria or generalised oedema. Intervention: nifedipine 10 mg three times daily. vs hydralazine 10 mg three times daily. Outcomes: BP, termination of pregnancy, side‐effects. |
| Iran 2005 | Intervention is not an antihypertensive: IV magnesium sulphate vs oral magnesium chloride. No clinical outcomes studied. Methods: randomised controlled open clinical trial. Participants: 68 women with mild PE. Intervention: IV magnesium sulphate (2 g/hrs) or oral magnesium chloride (4 g/2hrs). Outcomes: serum Mg levels at 3, 6 and 12 hrs after administration. |
| Iran 2012 | 7‐day treatment. No clinical outcomes proposed. Protocol registration of an ongoing study. ClinicalTrials.gov: NCT01674127 Methods: placebo‐controlled RCT. Participants: 50 women with mild PE (no further details). Intervention: methyldopa 500 mg day vs placebo. Outcomes: uterine artery diameter, uterine artery blood flow, umbilical artery and fetal middle‐cerebral artery by Doppler ultrasound. |
| Iran 2016 | Not a RCT. Protocol for an observational single‐arm study. Irct registration number: IRCT201602067676N5 Methods: observational. Participants: target 80 pregnant women with chronic HT. Intervention: sequential use of methyldopa 250 mg twice daily 4g/day, Hydralazine 5 mg to 10 mg IV every 20 mins 30 mg, Nifidipine 10 mg twice daily and diltiazem 120 mg to 180 mg every day. Outcomes: superimposed PE, acute kidney injury, placental abruption, fetal death. |
| Israel 1988 | Comparison of 2 beta blockers. Published as abstract only. Methods: quote: "allocated in blind and randomised manner". No further information. Participants: 30 women with SBP 140 mmHg to 170 mmHg and DBP 90 mmHg to 110 mmHg x 2, 6 hrs apart. Intervention: Atenolol 100 mg plus 2 placebo tablets vs pindolol 5 mg x 3/day. Outcomes: gestation at delivery (mean). |
| Israel 1992b | Comparison of 2 beta blockers. Methods: quote: "randomly allocated to double blind treatment". No further information. Participants: 20 women with mild PE, BP >/= 140/90 mmHg. Interventions: propranolol 40 mg x 3/day vs pindolol 5 mg x 3/day, for 7 days. Outcomes: BP, umbilical artery Doppler. |
| Israel 1999 | Single‐dose intervention. Methods: double‐blind, placebo‐controlled RCT. Participants: 23 women with PIH. Intervention: sublingual tablet of Isosorbide dinitrate (5 mg) or placebo (single dose). Outcomes: maternal BP and heart rate, umbilical artery Doppler. |
| Italy 1986 | Not an RCT (matched controls). Available as abstract only. Methods: 'randomised protocol', no further information, for group A (nifedipine or atenolol), control group (B) was matched by age and parity with group A. Results in group A were not presented separately. Participants: 10 women with mild‐moderate HT in the third trimester (group A). Interventions: atenolol 100 mg a day or slow‐release nifedipine 20 mg x 2/day (group A) vs diuretics or bed rest (group B). Outcomes: BP, gestational age, birthweight, Apgar score, serum bilirubin, preterm delivery, RDS, side‐effects. |
| Italy 1988 | Quasi random design. Study abandoned (5 women recruited) Personal communication. Methods: quasi‐random design. Participats: pregnant women with mild‐moderate HT (BP = or > 140/90). Intervention: pharmacological treatment vs bed rest. Outcomes: pregnancy outcomes (no further details). |
| Italy 1990a | Quasi‐randomised design. 2 trials with same methods reported in 1 paper (1) 44 women (2) 50 women. Methods: allocation by quote: "order of attendance at clinic or department". Participants: women with BP =/> 140/90 mmHg x 2 over 8 hrs, normal BP before pregnancy. Intervention: (1) slow‐release verapamil 360 mcg/day to 480 mcg/day vs pindolol 15 mg/day to 20 mg/day. (2) slow‐release verapamil 360 mcg/day to 480 mcg/day vs atenolol 100 mg/day to 150 mg/day. Outcomes: caesarean section, baby death, Apgar (mean), gestation at delivery (mean). |
| Italy 1990b | Intervention is an antiplatelet agent. No clinical outcomes reported. Available as abstract only. Methods: quote: "...using a random selection...". No further information. Participants: 20 women with PIH before 36 weeks' gestation. Intervention: picotamide (no dose reported) vs no treatment. Outcomes: platelet aggregation, ADP‐threshold values, collagen concentration thresholds. |
| Italy 2000a | Women had chronic HT or history of HT or IUGR (results were not presented separately). Methods: quote "...patients were randomly allocated to two treatments...". Participants: 68 women with either chronic HT or with previous history of PE or IUGR. Intervention: glyceryl trinitrate transdermal patch (5 mg/24 hrs) for 14 to 16 hrs/day from 16 to 38 weeks' gestation vs observation. Outcomes: hypertensive syndrome, preterm delivery, abruptio, birthweight, IUGR, Apgar score, admission to SCBU, RDS, neonatal death, umbilical and cerebral artery PI. |
| Italy 2001 | Not clearly randomised. No clinical data studied. Available as congress abstract only. Methods: not stated. Participants: 24 women with PIH. Intervention: isosorbide dinitrate sublingual every 6 hrs (n = 12) vs nifedipine 20 mg daily (n = 12). Outcomes: apoptosis in placental tissues. |
| Italy 2004 | Intervention is not an antihypertensive: acupuncture + methyldopa vs. methyldopa. Protocol for a future RCT. Available as abstract only. Methods: planned RCT Participants: 60 women with chronic HT at 16 to 20 weeks' gestation. Intervention: acupuncture + methyldopa vs. methyldopa. Outcomes:need for antihypertensives. |
| Italy 2006 | Intervention is not an antihypertensive. Single‐dose treatment. Methods: double‐blind, randomised, cross‐over design. Participants: 15 pregnant women at 30 to 34 weeks' gestation with mild/moderate PIH. Intervention: L‐Arginine 20 g/500 mL vs placebo infusion. Outcomes: systolic and DBP, fetal heart rate and fetal movements. |
| Italy 2008 | Intervention is not an antihypertensive. Available only as an abstract. Methods: double‐blind, randomised, placebo‐controlled trial. Participants: 20 pregnant women with PE. Intervention: L‐Arginine 1.66g 3 times a day vs placebo. Outcomes: Serum L‐arginine levels, adverse maternal and fetal outcomes. |
| Italy 2010 | Intervention is not an antihypertensive. Methods: double‐blind, randomised, placebo‐controlled trial. Participants: 80 pregnant women with mild chronic HT. Intervention: L‐Arginine vs placebo. Outcomes: BP, need for additional antihypertensives, superimposed PE, adverse maternal and fetal outcomes. |
| Italy 2012 | Not a RCT. Sequential allocation. Methods: sequential allocation. Participants: 400 pregnant women with early‐onset (20 to 27 weeks’ gestation) mild GH (systolic and DBP < 170/110 mmHg). Intervention: 4‐arm trial. nifedipine (Group A) vs nifedipine and NO donors vs nifedipine and oral fluids vs nifedipine, NO donors and oral fluids. Outcomes: total peripheral vascular resistance, severe maternal and fetal complications (placental abruption, HELLP syndrome, fetal growth restriction, severe RDS; and perinatal death. |
| Japan 1997 | Single‐dose intervention. No clinical outcomes studied. Methods: quote "...randomly allocated into two groups using sealed envelopes...". Participants: 18 pregnant women with SBP = or > 140 mmHg and DBP = or > 90 mmHg, with or without proteinuria and oedema. Intervention: isosorbide dinitrate patches (40 mg, only dose) and bed rest vs bed rest alone. Outcomes: systolic and DBP, uterine and umbilical Doppler velocimetry. |
| Japan 2016b | Intervention is not an antihypertensive (statin therapy for PE). Protocol for an ongoing study. UMIN‐CTR: UMIN000020686 Methods: open‐‐label RCT. Participants: target 50 pregnant women with PE with hyperlipaemia. Intervention: pravastatin 10 mg/day vs no treatment. Outcomes: maternal BP, body weight, oedema, liver function, kidney function, serum lipids, urinary protein, postpartum BP, extend the period of pregnancy, maternal serum soluble fms‐like tyrosine kinase 1, maternal serum tumour necrosis factor alfa, maternal urine F2‐Isoprostanes. |
| Japan 2017 | Not mild‐moderate HT. Prevention trial. Protocol of an ongoing trial. UMIN ID: UMIN000027270 Methods: open‐RCT. Participants: target 20 pregnant women with history of severe hypertensive disorders of pregnancy that required delivery prior to 34 weeks' gestation at 12‐16 weeks' gestation. Intervention: tadalafil (20 mg/day) vs conventional management. Outcomes: recurrence rate of hypertensive disorders of pregnancy, completion rate of the treatment regimen, Efficacy monitoring, US estimated fetal weight (g), fetal head circumference (cm), Doppler imaging of umbilical arterial, middle cerebral arterial, uterine arterial blood flow, deep est amniotic fluid pocket (cm), prolongation of gestational age at birth (days), birthweight (g), gestational age, Apgar score, umbilical artery pH and base excess values, incidence of PE, neonatal morbidity, rate of obstetric complications, perinatal mortality, neonatal mortality. |
| Kuwait 1995 | Not clearly randomised. Methods: 'randomly allocated in sequence'. No further information. Participants: 120 primigravid women > 26 weeks' gestation, with SBP 120 mmHg to 140 mmHg and DBP 95 mmHg to 105 mmHg persisting for 3 days. Intervention: labetalol 100 mg to 300 mg x 3/day vs methyldopa 250 mg to 750 mg x 3/day. Outcomes: maternal MAP, proteinuria (undefined), placental abruption, caesarean section, elective delivery, side‐effects, 1‐min Apgar score < 5, days on SCBU, birthweight (mean). |
| Mexico 2008 | Intervention is not an antihypertensive. Available only as an abstract. Methods: randomised, placebo‐controlled trial. Participants: 100 pregnant women with PE. Intervention: L‐Arginine vs placebo. Outcomes: birthweight, SGA babies. |
| Netherlands 2014 | Post‐partum HT. Protocol of an ongoing trial. EudraCT Number: 2014‐002524‐27. Methods: double‐blind RCT. Participants: target 30 women with postpartum HT after a pre‐eclamptic pregnancy. Intervention: 4‐arm trial. Placebo vs losartan vs low sodium diet vs moxonidine. Outcomes: mean 24‐hr BP, RAAS‐activity, SNS‐activity, endothelial function, arterial stiffness, lipid metabolism, insulin sensitivity, oxidative stress and systemic inflammation. |
| Pakistan 1994 | Intervention is an antiplatelet agent. Methods: 'randomly divided into two groups'. No further information. Participants: 200 women, 1 group with previous history of PIH (100 women) and other with mild essential HT or those developing BP 140/90 mmHg x 2 at least 15 days apart (100 women). Intervention: aspirin 75 mg twice daily vs routine antihypertensive treatment with beta blockers or calcium channel blockers when DBP exceeded 100 mmHg. Outcomes: development of PE. No other relevant outcomes reported. |
| Panama 2012 | Severe HT. Ongoing trial. Methods: randomised, open‐label trial. Participants: 284 (estimated) women with at > 24 weeks' gestation with severe HT (SBP > 160 mmHg/DBP > 110 mmHg). Intervention: 5 mg IV hydralazine every 15 min until BP controlled vs. IV labetalol ant increasing doses until BP controlled. Outcomes: BP control. |
| Philippines 2000 | 3 days treatment. No relevant clinical outcomes studied. Available as abstract only. Methods: randomised, double‐blind, placebo‐controlled trial. Participants: 16 pre‐eclamptics (no further details). Intervention: nitrol patch 5 mg for 16 hrs for 3 consecutive days vs the same regimen using a gauze only. Outcomes: uterine and umbilical Doppler velocimetry. |
| Russia 1993 | Possibly not a RCT. Full text awaiting translation from Russian. Abstract only in English. Participants: 92 women with slight and medium‐severe HT at 24 to 39 weeks' gestation. Interventions: venodilators, prazosin and cordafen are all mentioned. Not clear how the groups were constructed. |
| Singapore 1996 | More than 20% of women excluded, 6 women (22%) excluded because delivered in the week after trial entry. Methods: 'by opening a sealed envelope'. Participants: 27 women with singleton pregnancies, DBP 90 mmHg or above and proteinuria. Interventions: isradipine (slow release) 5 mg a day vs methyldopa 750 mg a day. Outcomes: MAP, side‐effects, caesarean section, perinatal mortality, birthweight, admission to SCBU, Apgar score, maternal and fetal haemodynamics (by Doppler). |
| Singapore 1998 | No relevant clinical outcomes studied. Methods: 'randomised', no further information. Participants: 30 women with PE, DBP >/= 90 mmHg and proteinuria >/= 300 mg/24 hrs. Interventions: methyldopa 250 mg x 3/day to 500 mg x 3/day vs isradipine 5 mg to 10 mg once/day. Outcomes: haemostatic parameters only (thrombelastography, fibrinogen, antithrombin III, thrombin‐antithrombin‐complex, beta‐thromboglobulin, plasminogen activators, plasminogen activators inhibitors, and plasminogen). |
| Slovakia 2002 | Not a RCT. Methods: cohort study. Participants: women with HT during pregnancy. Interventions: acebutolol vs other antihypertensives. Outcomes: maternal and perinatal adverse events. |
| South Africa 1988 | Quasi‐random design. Less than 7 days treatment, single‐dose study. No clinical outcomes reported. Methods: quasi‐random design, using last digit of the hospital number. Participants: 18 women in the last trimester of pregnancy with HT +/‐ proteinuria. Interventions: nifedipine 5 mg vs placebo (single dose). Outcomes: measures of uteroplacental blood flow. |
| South Africa 1990 | Included women with severe HT (DBP 100 mmHg to 120 mmHg). Methods: 'randomly allocated', no further information. Participants: 60 women at 28 to 36 weeks' gestation with mean 24‐hr DBP 100 mmHg to 120 mmHg +/‐ proteinuria. Intervention: indoramin 50 mg twice daily vs methyldopa 1 g twice daily vs placebo 1 tablet daily. Outcomes: MAP, need for additional antihypertensive. |
| South Africa 1991b | Quasi‐random design. Single‐dose intervention. Methods: allocation quote: "by virtue of the last digit of their folder number". Participants: 19 women at > 28 weeks' gestation, singleton pregnancy and HT (defined as mean DBP >/= 90 mmHg). Intervention: sublingual nifedipine 5 mg vs placebo (single dose). Outcomes: DBP (mean), maternal and fetal heart rate, gestational age, side‐effects. |
| South Africa 1997 | Most women did not have HT. Eligibility criteria DBP >/= 80 mmHg, before 20 weeks' gestation. Of 138 recruited women, less than half had DBP >/= 90 mmHg. Results for this group were not presented separately. Methods: sequentially‐numbered sealed boxes containing drug or placebo. Participants: 138 women between 12 to 20 weeks' gestation with DBP 80 mmHg to 109 mmHg, without antihypertensive therapy. Intervention: ketanserin 40 mg to 80 mg a day vs placebo. Outcomes: severe HT, proteinuria, placental abruption, other drugs needed, perinatal deaths, SGA (< 10th centile), birthweight. |
| South Africa 2004 | Less than 7 days treatment. Available as abstract only. Methods: 'randomised'. No further details. Participants: preliminary data of 177 pre‐eclamptic women being delivered. Intervention: IV magnesium sulphate vs labetalol (20 mg IV then 200 mg orally every 6 hours). Outcomes: incidence of eclampsia, additional anti‐ HT therapy, side‐ effects (flushing), intrapartum complications, neonatal outcomes. |
| South Africa 2015 | Intervention (ezomeprazole) is not an antihypertensive. Protocol of an ongoing trial. Pan African Clinical Trial Registry: PACTR201504000771349. Methods: double‐blind, placebo‐controlled RCT. Participants: target 120 pregnant women with early onset PE at a gestational age between 26 + 0 and 31 + 6 weeks. Intervention: 40 mg of esomeprazole vs identical placebo tablet orally once a day. Outcomes: prolongation of pregnancy, maternal, fetal and neonatal mortality and morbidity, maternal serum biomarkers (including sFlt, sEng and endothelin 1) and biomarkers in placental samples. |
| South Africa 2016 | Intervention (metformin) is not an antihypertensive. Protocol of an ongoing trial. Pan African Clinical Trial Registry: PACTR201608001752102. Methods: double‐blind RCT. Participants: target 120 pregnant women with early onset PE at a gestational age between 26 + 0 and 31 + 6 weeks. Intervention: metformin 3g/day vs identical placebo tablets. Outcomes: prolongation of pregnancy, maternal, fetal and neonatal mortality and morbidity, maternal serum biomarkers (including sFlt, sEng and endothelin 1). |
| Sri Lanka 1994 | Quasi‐random design. Methods: quote: "patients were alternately allocated". Participants: 126 women with PIH. Interventions: nifedipine 30 mg/day to 90 mg/day vs methyldopa 750 mg/day to 2000 mg/day. Outcomes: severe HT, gestation at delivery (mean), birthweight (mean). |
| Sweden 1992 | Comparison of 2 beta blockers. Methods:quote: "'randomly allocated" using "double‐blind dummy technique". No further information. Participants: 32 women admitted to hospital with PIH in the third trimester (BP >/= 140/90 mmHg x 2 at least 4 hrs apart) and normotensive in the first trimester. Intervention: atenolol 50 mg x 2/day vs pindolol 5 mg x 2/day, for at least 1 week. Outcomes: side‐effects, caesarean section, maternal haemodynamics, fetal haemodynamics, admission to SCBU, birthweight (mean), 5‐min Apgar score. |
| Sweden 1993 | It is not clear from papers whether reported data represent only a subgroup of women. Methods: not stated. Authors state "allocated at random". Participants: 20 women at 26 to 37 weeks' gestation with 'persistent' DBP >/= 100 mmHg and proteinuria. Intervention: labetalol 300 mg/day to 1000 mg/day orally (if necessary, IV 25 mg bolus followed by 25 mg/hr to 65 mg/hr infusion), vs hydralazine 75 mg/day to 400 mg/day orally (if necessary, 1.5 mg/hr to 6.0 mg/hr infusion). Outcomes: severe HT, additional antihypertensive, caesarean section, neonatal death, birthweight (mean), gestation at delivery (mean), SGA (2 SD below mean), bradycardia, hypotension, hypoglycaemia, 5‐min Apgar < 7, RDS, cord pH (< 7.20). |
| Uganda 1992 | Status unknown. Personal communication of a planned RCT of aspirin and methyldopa in moderate HT during pregnancy. No further data. |
| UK 1978 | Included women with severe HT. Methods: 'randomly allocated'. No further information. Participants: 74 women with singleton pregnancy with DBP > or = to 170/100 mmHg x 2 at up to 36 weeks' gestation. Intervention: labetalol 100 mg (max 1200 mg daily) vs methyldopa 250 mg (up to 4000 mg daily). Outcomes: severe HT, proteinuria ('greater than trace'), additional antihypertensive therapy, changed drugs due to maternal side‐effects, caesarean section, perinatal mortality, SGA infants (< 10th centile), intubated, umbilical cord pH. |
| UK 1991 | Less than 7 days treatment, single‐dose study. Methods: sequentially‐numbered, sealed envelopes. Participants: 30 women with singleton pregnancy and HT, defined as BP >/= 140/90 mmHg. Intervention: 10 mg hydralazine IV vs or 100 mg labetalol IV, as single dose. Outcomes: MAP, maternal and fetal heart rate, side‐effects, umbilical artery PI. |
| UK 2015 | Not mild‐moderate HT. Protocol of an ongoing prevention trial in high‐risk women. Intervention is not an antihypertensive (statins). EUCTR2016‐005206‐19‐BE. ISRCTN17787139. Methods: double‐blind, placebo‐controlled RCT. Participants: target 1684 pregnant women at high risk for preterm‐PE at 11 to 13 weeks by the algorithm combining maternal history and characteristics, biophysical findings (MAP and uterine artery Dopplers) and biochemical factors (placental growth factor). Intervention: pravastatin 20 mg vs placebo, orally once a day. Outcomes: incidence of preterm PE < 37 weeks, < 34 weeks, birthweight, fetal and neonatal weight, NICU admission, composite measure of neonatal mortality and morbidity, placental abruption, spontaneous preterm delivery < 34 weeks and < 37 weeks. |
| USA 1957 | Not randomised. Although a group of women received placebo, results are presented together with a group of matched controls. Included women with severe HT. Methods: not stated. Participants: 106 pregnant women with chronic HT and 28 women with severe PE. In addition, 671 women with chronic HT were included as controls. Intervention: oral reserpine 0.25 mg/day to 3 mg/day (n = 80) vs placebo (n = 26). 28 women received IV reserpine. Outcomes: status at birth, birthweight. |
| USA 1981 | Study included 63 women, but only 21 randomised. Outcomes not reported separately for randomised women. Methods: 'randomly and blindly assigned'. No further information. Participants: 21 women with BP 140/90 mmHg or above in a seated position or at rest, x 2, 6 or more hrs apart. Intervention: hydralazine 25 mg x 3/day vs methyldopa 250 mg x 3/day vs placebo x 3/day. Outcomes: MAP, caesarean section, induction of labour, birthweight. |
| USA 1990b | Status unknown. Personal communication of a planned RCT of oral hypotensive agents in early onset PE. No further data. |
| USA 1991 | Not a randomised trial. No clinical outcomes reported. Methods: placebo group were matched as controls. Participants: 16 women at 17 to 22 weeks' gestation. Intervention: 10 mg sublingual nifedipine vs placebo. Outcomes: S/D ratio of the uterine artery, maternal BP, maternal heart rate. |
| USA 2005 | Intervention is not an antihypertensive. Protocol of an ongoing trial. Study NCT00194974. Methods: RCT. Sequentially numbered opaque envelopes. Participants: 50 pregnant women with chronic HT. Intervention: target BP of 120 to 130/80‐85 mmHg vs target BP 140 to 150/90‐100 mmHg. Outcomes: BP, superimposed PE, worsening HT, HELLP syndrome, gestational age, birthweight, serious perinatal complications. |
| USA 2006 | Study abandoned (2 women recruited). Personal communication. ClinicalTrials.gov Identifier: NCT00329511 Methods: open‐label RCT. Participants: pregnant women between 14 to 28 weeks of gestation with chronic HT requiring antihypertensive therapy (BP < 180/110). Intervention: methyldopa 250 mg to 750 mg in 4 doses vs clonidine patch 0.1 mg to 0.4 mg once a week. Outcomes: patient compliance, change in mean arterial BP at weeks 1,2,3,4 in comparison to baseline BP at the initial visit, side‐effects to each medication as reported by the patients. |
| USA 2016b | Not mild‐moderate HT. Prevention trial in high‐risk women. Intervention is not an antihypertensive (statins). Methods: pilot, multicentre, double‐blind, placebo‐controlled, RCT. Participants: 20 pregnant women at high risk of PE at 12.0 to 16.6 weeks. Intervention: pravastatin 10 mg vs placebo, orally once a day. Outcomes: number and type of maternal and fetal/neonatal adverse events, pharmacokinetic parameters of the drug. |
| Venezuela 1985 | Not randomised. Included women with severe HT. Article in Spanish. Methods: alternated allocation (personal communication). Participants: 32 pregnant women at > 25 weeks' gestation with severe PE (defined as BP 160/110 or 140/90) and symptoms as headache, epigastric pain, blurred vision or hyperreflexia. Intervention: labetalol 200 mg/day to 800 mg/day vs methyldopa 750 mg/day to 2000 mg/day. Outcomes: maternal MAP, maternal pulse rate, gestational age at delivery, birthweight, 1‐min Apgar, fetal and neonatal death. |
| Venezuela 1997 | Not a RCT. Matched controls. Article in Spanish. Methods: controls were women treated with methyldopa in the same study period, with the same characteristics than the study group. Participants: 20 women with PIH. Intervention: labetalol 200 mg to 300 mg orally given every 12 hrs vs methyldopa from 500 mg/day to 1500 mg/day Outcomes: BP, severe HT, gestational age, induction of labour, caesarean section, birthweight. |
| Venezuela 2001 | No relevant clinical outcomes studied. Less than 7 days of treatment. Available as abstract only. Methods: Authors state"...were randomly assigned to..." No further information. Participants: 30 pre‐eclamptic. No further information. Intervention: transdermal nitroglycerin (7 mg for 12 hrs for 2 consecutive days) vs placebo. Outcomes: umbilical S/D ratio, PI and RI by Doppler ultrasound. |
| Venezuela 2007 | Mixed control group. Available only as an abstract. Method: quote: "...were randomly assigned to two study groups...". Participants: 30 women with pre‐eclampsia and gestational HT. Intervention: carvedilol vs traditional antihypertensive drugs (methyldopa and/or nifedipine). Outcomes: BP control, additional antihypertensives, maternal and fetal complications, Apgar score, fetal heart rate. |
ADP: adenosine diphosphate BP: blood pressure DBP: diastolic blood pressure HELLP: syndrome of haemolysis, elevated liver enzymes and low platelets hr(s): hour(s) HT: hypertension IUGR: intrauterine growth retardation IV: intravenous LBW: low birthweight min(s): minute(s) MAP: mean arterial pressure mg: magnesium NICU: neonatal intensive care unit NO‐donor: nitric oxide donor NST: non‐stress test PIH: pregnancy‐induced hypertension PI: pulsatility index PE: pre‐eclampsia RAAS: Renin‐Angiotensin‐Aldosterone System RCT: randomised controlled trial RDS: respiratory distress syndrome RI: resistance index SBP: systolic blood pressure SCBU: special care baby unit SD: standard deviation S/D ratio: ratio between peak systolic to end‐diastolic flow velocity SGA: small‐for‐gestational age SNS: sympathetic nervous system US: ultrasound vs: versus
Characteristics of ongoing studies [ordered by study ID]
Canada 2006.
| Trial name or title | Use of a nitric oxide (ISMN) for the prevention and management of pre‐eclampsia (pilot study). ISRCTN45790835. |
| Methods | Randomised, multicentre, blinded, placebo‐controlled trial. |
| Participants | Target: 80 women. All women who have been diagnosed with pre‐eclampsia, are being followed clinically and who provide informed consent. For a diagnosis of pre‐eclampsia a patient must meet all 3 criteria: 1. SBP greater than 140 mmHg or an increase of 30 mmHg from the participant’s baseline (with that increase present at 2 measurements taken 6 hours apart). 2. DBP greater than 90 mmHg or an increase of 15 mmHg from the participant’s baseline (with that increase present at 2 measurements taken 6 hours apart). 3. Proteinuria greater than 0.3 g in 24‐hour urine or 2+ on dipstick. |
| Interventions | Treatment arm: experimental intervention: daily dose of low‐dose Isosorbide‐5‐mononitrate (ISMN) (30 mg) following diagnosis of pre‐eclampsia after 24 weeks' gestation until delivery. Control intervention: matching placebo containing lactose. Patients randomly assigned to either receive low dose ISMN (30 mg) as stated above or placebo. |
| Outcomes | Primary outcome: randomisation‐to‐delivery interval between ISMN/placebo groups, measured at delivery. Secondary outcomes: serial change in biochemical markers in treatment/no treatment groups, measured at routine obstetrical visits until delivery (generally every 2 weeks), incidence of any side‐effects (major or minor), measured at routine obstetrical visits until delivery (generally every 2 weeks), neonatal outcomes (composite of neonatal morbidity), measured at delivery. |
| Starting date | 01/01/2007 |
| Contact information | Clinical Research Centre Queen's University Kingston General Hospital 76 Stuart Street Angada 4 Room 5‐415 Kingston K7L 2V7 Canada +1 613 549 6666 ext. 3936 gns@post.queensu.ca [mail to:gns@post.queensu.ca] |
| Notes | Ongoing trial. Author contacted. |
Egypt 2017a.
| Trial name or title | Labetalol for chronic hypertension. Pan African Clinical Trials Registry: PACTR201707002463243. |
| Methods | Double‐blind RCT. |
| Participants | Target: 480 women. Pregnant women diagnosed with mild to moderate chronic hypertension, with SBP of 140 mmHg to 159 mmHg or DBP of 90 mmHg to 109 mmHg, at the beginning of pregnancy. |
| Interventions | Treatment arm 1: Methyldopa 1000 ‐ 2000 mg per day in divided doses. Treatment arm 2: Labetalol 100 mg to 300 mg per day in divided doses. Control arm: placebo 1 to 2 tablets daily. |
| Outcomes | Maternal outcomes (no further details) and neonatal outcomes (no further details). |
| Starting date | 01/08/2017. |
| Contact information | Dr Mohamed Rezk (00201006237186) m_rezk9207@yahoo.com 25 Yasin Abdelghafar street, Shibin Elkom, (002048) Egypt. |
| Notes | Ongoing. |
Egypt 2017b.
| Trial name or title | Use of sildenafil citrate in management of mild pre‐eclampsia. ClinicalTrials.gov Identifier: NCT03262961. |
| Methods | Double‐blinded, randomised, placebo‐controlled trial. |
| Participants | Target: 80 women Uncomplicated mild pre‐eclampsia at 28 to 36 weeks' gestation with singleton viable pregnancy (age: 18 to 35 years). |
| Interventions | Treatment arm: sildenafil citrate (Respatio® 20 mg tablets manufactured by Pharma Right Group, Egypt) divided into 3 doses per day (every 8 hours) till termination of pregnancy. Control arm: a placebo drug that has the same shape, size and colour but without the active ingredient and it would also be taken in a similar way. The placebo tablet will be manufactured at the faculty of pharmacy, Assiut University. |
| Outcomes | Primary outcomes: gestational age at time of termination and progression to severe pre‐eclampsia. Secondary outcomes: neonatal survival, birthweight, Apgar score at 1 and 5 minutes and direct postnatal need to NICU, maternal BP, method of termination of pregnancy, side‐effects (headache, flushing and dyspepsia), evaluation of the effect of sildenafil citrate on the feto‐maternal circulation through the Doppler ultrasound. |
| Starting date | September 15, 2016 |
| Contact information | Contact: Fady Abdallah (00201002837042) fady.nasif@yahoo.com
Contact: Hisham Abou‐Taleb (00201003332139) hishamaboutaleb1@yahoo.com Assiut University. |
| Notes | Ongoing. |
Japan 2016a.
| Trial name or title | A multicenter phase II trial of the efficacy and safety of tadalafil with pre‐eclampsia. UMIN ID: UMIN000024042 |
| Methods | Not stated. |
| Participants | Target: 160 women. Women with pregnancy‐induced hypertension, age = or > 20; SBP = or > 140 or DBP = or > 90 or proteinuria 0 or > 300 mg/day; gestational age between 20 + 0 and 33 + 6 weeks; singleton pregnancy; written informed consent. |
| Interventions | Treatment arm: oral administration of tadalafil (20 mg/day) added to the conventional management until delivery. Control arm: the conventional management of FGR consisted of evaluation of fetal well‐being by ultrasonography including Doppler imaging and fetal heart rate monitoring to evaluate possible pregnancy termination. |
| Outcomes | Primary outcome: enrolment‐to‐delivery interval (days). Secondary outcomes: completion rate of the treatment regimen, efficacy monitoring, severe pre‐eclampsia, liver dysfunction, eclampsia, pulmonary edema, FGR, birthweight (g), gestational age at birth, Apgar score, umbilical artery pH and base excess values, neonatal morbidity, offspring outcome until 1.5 years of age, safety monitoring, obstetric complications, perinatal mortality, neonatal mortality. |
| Starting date | 17/10/2016 |
| Contact information | Mie University Hospital, Department of Obstetrics and Gynecology. 2‐174 Edobashi Tsu‐city; Mie; Japan 059‐232‐1111 tadafer.study@gmail.com |
| Notes | Ongoing. |
Netherlands 2015.
| Trial name or title | Early Vvascular Adjustments during hypertensive pregnancy (EVA). ClinicalTrials.gov Identifier: NCT02531490. |
| Methods | Open‐label RCT. |
| Participants | Target: 368 Pregnant women ages 18 years or older, before 37 weeks of gestational age, diagnosed with mild to moderate gestational hypertension. |
| Interventions | Intervention arm 1: women with a hyperdynamic vasodilated profile, characterised by a mean arterial pressure/heart rate (Hr) ratio ≤ 1.1 are prescribed a beta blocker. Intervention arm 2: women with a hypodynamic vasoconstrictive profile (MAPhr ratio ≥ 1.4) are prescribed nifedipine. Intervention arm 3: women with normodynamic profile (MAP/Hr ratio in between 1.1 and 1.4) are prescribed methyldopa. Control arm: no antihypertensive. |
| Outcomes | Primary outcomes: severe hypertension, pre‐eclampsia. Secondary outcomes: pattern of change of the hemodynamic profile, measured by the ratio of mean arterial pressure and heart rate; diameter aortic outflow tract and left ventricular outflow tract, left ventricular volume after diastole and systole measured by transthoracic echocardiography; number of patients with concentric left ventricular remodeling or concentric hypertrophy; Apgar score; SGA; PTB; Stillbirth, perinatal mortality, morbidity: chronic lung disease, neonatal sepsis, severe IVH > grade II, periventricular leucomalacia > grade I, and necrotizing enterocolitis. Days on ventilation support, length of admission to neonatal or intensive care nursery, and total days in hospital until 3 months corrected age: maternal and neonatal side‐effects; composite of maternal complications including: mortality, stroke, eclampsia, blindness, uncontrolled hypertension, respiratory failure, birth related variables, needed level of care. |
| Starting date | December 2015. |
| Contact information | Maastricht University Medical Center, Netherlands. Contact: Eva Mulder, MD (0031650504243) eva.mulder@mumc.nl Contact: Marc Spaanderman, professor (0031433874774) marc.spaanderman@mumc.nl |
| Notes | Ongoing. |
USA 2014.
| Trial name or title | Chronic Hypertension and Pregnancy (CHAP) Project (CHAP). ClinicalTrials.gov Identifier: NCT02299414 |
| Methods | Pragmatic multicentre, open‐label RCT. |
| Participants | Target: 4700 women. Women with chronic hypertension in pregnancy with new or untreated chronic hypertension, blood pressure 140‐159 SBP or 90‐104 DBP OR known chronic hypertension on monotherapy and taking any antihypertensive and blood pressure ≤ 159/104 (including those with BP < 140/90); singleton; and viable pregnancy < 23 weeks of gestation. |
| Interventions | Treatment arm: 1st line anti‐hypertensive (Labetalol or Nifedipine ER) started; escalate to maximum dose and a preferred 2nd line medication if needed (nifedipine ER or labetalol). Control arm: no anti‐hypertensive therapy (unless BP is severe). |
| Outcomes | Primary outcomes: composite adverse perinatal outcome (total reported fetal or neonatal death up to 2 weeks; pre‐eclampsia with severe features hypertension and proteinuria or hypertension and severe features per ACOG, placental abruption, or indicated PTB < 35 weeks not due to spontaneous preterm labour or membrane rupture).SGA (birthweight less than 10th percentile for gestational age at birth according to accepted national standard). Secondary outcomes: maternal death, heart failure, stroke, encephalopathy, myocardial infarction or ischaemia, pulmonary oedema, admission to neonatal or intensive care nursery, or renal failure; perinatal death, IVH III or IV, BPD or chronic lung disease, NEC, ROP, seizures, proven sepsis; adherence to antihypertensive therapy after delivery. |
| Starting date | 09/2016. |
| Contact information | University of Alabama at Birmingham ‐ USA Clinical Coordinating Center Contact: Alan Tita, MD, PhD (205‐934‐5612) atita@uabmc.edu Contact: Michelle Feese, MPH (205‐975‐8633) mfeese@uab.edu |
| Notes | Ongoing. |
USA 2016a.
| Trial name or title | Efficacy of Sildenafil in preterm preeclampsia (SIL). ClinicalTrials.gov Identifier: NCT02782559 |
| Methods | Double‐blind, placebo‐controlled RCT. |
| Participants | Target: 44. Hospitalised patients of gestational age of ≥ 24 0/7 weeks to ≤ 32 0/7 weeks; dating of pregnancy by ultrasound < or equal to 22 weeks or IVF conception and diagnosis of preterm pre‐eclampsia or superimposed pre‐eclampsia. |
| Interventions | Treatment arm: sildenafil 40 mg oral tablet 3 times a day from randomisation until delivery. Control arm: matched to oral capsule of active treatment 3 times a day from randomisation until delivery. |
| Outcomes | Primary outcomes: duration of pregnancy from diagnosis/randomisation until delivery. Secondary outcomes: not reported. |
| Starting date | July 2016 |
| Contact information | The University of Texas Health Science Center, Houston ‐ USA Contact: Robyn P Roberts, MD (734‐934‐4227) Robyn.P.Roberts@uth.tmc.edu Contact: Maria Hutchinson (713‐500‐0510) Maria.S.keefer@uth.tmc.edu |
| Notes | Ongoing. |
USA 2017.
| Trial name or title | Impedance cardiography to decrease the risk of preeclampsia. ClinicalTrials.gov Identifier: NCT03245970 |
| Methods | Open‐label RCT. |
| Participants | Target: 400 women Pregnant patients 18 to 51 years old Less than 20 weeks' gestation with mild chronic hypertension ‐ not on antihypertensive medications. |
| Interventions | Treatment arm 1: labetalol hydrocholoride 200 mg orally every 12 hours prescribed for increased cardiac output as determined by impedance cardiography. Treatment arm 2: nifedipine 60 mg orally daily prescribed for increased systemic vascular resistance as determined by impedance cardiography. Treatment arm 3: atenolol 25 mg daily prescribed for increased cardiac output with tachycardia or maternal pulse rate 110 or greater. Control arm: no pharmacological treatment. |
| Outcomes | Primary outcomes: rates of pre‐eclampsia in chronically hypertensive pregnant women. Secondary outcomes: not reported. |
| Starting date | 24/04/2017 |
| Contact information | HIgh‐risk obstetrical consultants
Knoxville, Tennessee, United States, 37920 Contact: Craig V. Towers, MD (865‐305‐8888) ctowers@utmck.edu Contact: Beth W. Weitz, APN (865‐305‐8888 ext 3275) bweitz@utmck.edu |
| Notes |
BP: blood pressure DBP: diastolic blood pressure FGR: fetal growth restriction IV: intravenous IVF: in vitro ferilisation IVH: intraventricular haemorrhage MAP: mean arterial pressure NEC: necrotising enterocolitis NICU: neonatal intensive care unit PPH: postpartum haemorrhage PTB: preterm birth RCT: randomised controlled trial ROP: retinopathy of prematurity, SBP: systolic blood pressure SGA: small‐for‐gestational age
Differences between protocol and review
Methods section updated.
For the 2018 update, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
New co‐author joined the team: Dr Celina Gialdini.
A new outcome (maternal death) was added as a secondary outcome, as it was prioritised in WHO recommendations for prevention and treatment of pre‐eclampsia and eclampsia (WHO 2011).
A new subgroup (methyldopa versus calcium channel blockers) was added for all reporting outcomes in comparison 6: "Any antihypertensive drug versus calcium channel blockers" (analysis) as new included studies allows to incorporate them in meta‐analysis.
A new comparison was added: "Any antihypertensive drug versus beta blockers" (Analysis 7) as new included studies allows meta‐analysis.
As recommended by the statistical editor, when pooling the data, and in the absence of statistical heterogeneity, we used fixed‐effect meta‐analysis only for the outcome severe hypertension with the assumption that all drugs are expected to lower blood pressure. For the other outcomes, we used random‐effects meta‐analysis as different drug effects might vary due to their different mechanisms of action, dosages, length of treatment, etc. This has an impact on results for some outcomes, contrasting with previous versions of this review.
Contributions of authors
For this update, all changes on the text of the review were drafted by E Abalos with input by L Duley and C Gialdini. E Abalos and C Gialdini performed the methodological assessment of all new studies, extracted the data and populated all the tables. E Abalos, C Gialdini, L Duley and W Steyn have commented on and agreed the final version.
E Abalos is the guarantor of the review.
Sources of support
Internal sources
Centro Rosarino de Estudios Perinatales. Rosario, Argentina.
Nottingham Clinical Trials Unit, University of Nottingham, UK.
External sources
No sources of support supplied
Declarations of interest
E Abalos: none known.
L Duley has been awarded an NIHR research grant for a programme of work on care at very preterm birth. She has no conflict of interest.
W Steyn is the author of one excluded trial (South Africa 1997). He has no other conflict of interest.
C Gialdini: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Argentina 1985 {published data only}
- Voto L, Lapidus A, Neira J, Margulies M. Atenolol vs alpha methyldopa in the treatment of hypertension in pregnancy. 5th International Congress for the International Society for the study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, UK. 1986:138.
- Voto LS, Lapidus AM, Neira J, Margulies M. Treatment of hypertension during pregnancy: atenolol versus alpha‐methyldopa [Tratamiento de la hipertensión en el embarazo: Atenolol versus Alfa Metildopa]. Obstetricia y Ginecología Latino‐Americanas 1985;43(9‐10):335‐41. [Google Scholar]
Argentina 1987 {published data only}
- Voto LS, Zin C, Neira J, Lapidus AM, Margulies M. Ketanserin versus alpha methyldopa in the treatment of hypertension during pregnancy: a preliminary report. Journal of Cardiovascular Pharmacology 1987;10(Suppl 3):S101‐S103. [PubMed] [Google Scholar]
Argentina 1988 {published data only}
- Casavilla F, Vega HR. Prospective and randomized study on mepindolol and alpha‐methydopa efficacy in arterial hypertension (AH) treatment during pregnancy. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:182.
Australia 1983 {published data only}
- Livingstone I, Craswell PW, Bevan EB, Smith MT, Eadie MJ. Propranolol in pregnancy ‐ three‐year prospective study. Clinical and Experimental Hypertension 1983;2:341‐50. [DOI] [PubMed] [Google Scholar]
Australia 1985a {published data only}
- Gallery ED, Gyory AZ. Antihypertensive therapy in pregnancy [abstract]. Kidney International 1984;26(2):232. [Google Scholar]
- Gallery ED, Hunyor SN, Saunders DM, Gyory AZ. A randomized trial of oxyprenolol and a‐methyl dopa in therapy of hypertension in pregnancy. 1st Congress of the International Society of Hypertension in Pregnancy; 1978 September 27‐29; Dublin, Ireland. 1978:88.
- Gallery ED, Ross MR, Gyory AZ. Antihypertensive treatment in pregnancy: analysis of different responses to oxprenolol and methyldopa. BMJ 1985;291:563‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallery ED, Saunders DM, Hunyor SN, Gyory AZ. Randomised comparison of methyldopa and oxprenolol for treatment of hypertension in pregnancy. BMJ 1979;1:1591‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Australia 2001 {published and unpublished data}
Brazil 1985 {published data only}
- Kahhale S, Carrara W, Barros AC, Zugaib M, Neme B. A comparative study between treated (beta‐blocker pindolol) and untreated chronic hypertension. 4th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:56.
- Kahhale S, Zugaib M, Carrara W, Paula FJ, Sabbaga E, Neme B. Comparative study of chronic hypertensive pregnant women treated and non‐treated with pindolol [Estudio comparativo de gestantes hipertensas crônicas tratadas e näo tratadas com betabloqueador pindolol]. Ginecologia e Obstetrícia Brasileiras 1985;8(2):85‐9. [Google Scholar]
Brazil 1988 {published and unpublished data}
- Freire S, França LA, Rau de Almeida Callou M, Alves de Oliveira JE, Barbosa Filho J. Comparative study with pindolol and methyldopa in pregnant women with chronic hypertension [Estudo comparativo com pindolol e metildopa em gestantes com hipertensão arterial crônica]. Jornal Brasileiro de Ginecologia 1988;98(3):157‐60. [Google Scholar]
Brazil 2000a {published and unpublished data}
- Nascimento DJ. [Avaliaçäo do uso do verapamil em gestantes com formas näo graves de doença hipertensiva vascular crônica] Evaluation of the use of Verapamil with Non‐serious Form of Chronic Vascular Hypertension Disease During Pregnancy [Doctoral thesis]. Brazil: Facultade Evangélica de Medicina do Paraná, 2000. [Google Scholar]
Brazil 2016 {published data only}
- Trapani A Jr, Goncalves LF, Trapani TF, Vieira S, Pires M, Pires MM. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: A randomized controlled trial. Obstetrics and Gynecology 2016;128(2):253‐9. [DOI] [PubMed] [Google Scholar]
Caribbean Is.1990 {published and unpublished data}
- Plouin PF, Breart G, Llado J, Dalle M, Keller ME, Goujon H, et al. A randomized comparison of early with conservative use of antihypertensive drugs in the management of pregnancy‐induced hypertension. British Journal of Obstetrics and Gynaecology 1990;97:134‐41. [DOI] [PubMed] [Google Scholar]
Finland 1988b {published data only}
- Yla‐Outinen A, Tuimala R. Management of pregnancy induced hypertension by using nifedipine ‐ a prospective randomized trial. 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:133.
France 1987 {published data only}
- Plouin PF, Breart G, Maillard F, Papiernik E, Relier JP, Labetolol Methyldopa Study Group. Antihypertensive efficacy and perinatal safety of labetalol in the treatment of hypertension in pregnancy: a randomised comparison to methyldopa [Effets maternels et securite perinatale du labetalol dans le traitement des hypertensions de la grossesse. Comparaison a la methyldopa par un essai cooperatif randomise]. Archives des Maladies du Coeur 1987;80:952‐5. [PubMed] [Google Scholar]
- Plouin PF, Breart G, Maillard F, Papiernik E, Relier JP, the Labetolol Methyldopa Study Group. Comparison of antihypertensive efficacy and perinatal safety of labetalol and methyldopa in the treatment of hypertension in pregnancy: a randomized controlled trial. British Journal of Obstetrics and Gynaecology 1988;95:868‐76. [DOI] [PubMed] [Google Scholar]
France 1988a {published data only}
- Blazquez, Lardoux H, Leperlier E. Randomized and comparative study of methyl dopa (MD), acebutolol (ACE) and labetalol for the treatment of moderate hypertension during pregnancy (HDP). 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:100.
- Lardoux H, Blazquez G, Leperlier E, Gerard J. Randomized and comparative study of methyldopa (MD), acebutolol (ACE) and labetalol for the treatment of moderate hypertension during pregnancy (HDP) [Essai ouvert, comparatif, avec tirage au sort pour le traitement de l'HTA gravidique moderee: methyldopa, acetbutolol, labetalol]. Archives des Maladies du Coeur 1988;91:137‐40. [PubMed] [Google Scholar]
France 1990 {published data only}
- Bonnin P, Mintz P, Kedra AW, Pruna A, Ciraru‐Vigneron N, Savin E, et al. Effects of nifedipine and atenolol on the fetal‐maternal circulation in moderate hypertension in pregnancy [Effets de la nifedipine et de l'atenolol sur les circulations foeto‐maternelles en cas d'hypertension arterielle gravidique moderee]. Therapie 1990;45(6):525. [Google Scholar]
- Ciraru‐Vigneron N, Pruna A, Akposso K, Bonnin P, Kedra W, Mintz P, et al. Comparison of the effects of nefedipine and atenolol in the treatment of uncomplicated hypertension in pregnancy [Comparaison des effets de la nifedipine et de l'atenolol dans le traitement de l'hypertension arterielle gravidique non compliquée]. Therapie 1992;47(3):221. [Google Scholar]
- Ciraru‐Vigneron N, Pruna A, Mintz P, Bonnin P, Lefevre V, Beaufils M, et al. Comparison of nifedipine and atenolol in treatment of moderate pregnancy hypertension. Proceedings of the 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:340.
- Mintz P, Ciraru‐Vigneron N, Ravina JH, Pruna A, Idatte JH, Bonnin P, et al. Comparison of the effects of nifedipine and atenolol in the treatment of non‐complicated hypertension in pregnancy [Comparaison des effets de la nifedipine et de l'atenolol dans le traitement de l'hypertension arterielle gravidique non compliquée]. Therapie 1989;44(6):461. [Google Scholar]
France 1994 {published data only}
- Jannet D, Carbonne B, Sebban E, Milliez J. Nicardipine vs metoprolol in the treatment of hypertension during pregnancy: a randomized comparative trial. International Journal of Gynecology & Obstetrics 1995;49:225. [PubMed] [Google Scholar]
- Jannet D, Carbonne B, Sebban E, Milliez J. Nicardipine vs metoprolol in the treatment of hypertension during pregnancy: a randomized comparative trial. Obstetrics & Gynecology 1994;84:354‐9. [PubMed] [Google Scholar]
Hong Kong 1990 {published data only}
- Li CY, Lao TT, Yu KM, Wong SP, Leung CF. The effect of labetalol on mild pre‐eclampsia. Proceedings of the 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:191.
Hong Kong 1993 {published data only}
- Yuen FT, Mo YP, Yin LC, Terence L. A double‐blind randomised placebo controlled trial of labetalol in the treatment of mild pregnancy induced hypertension. Proceedings of the 2nd International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1993; Hong Kong, Singapore. 1993.
India 1992 {published data only}
- Oumachigui A, Verghese M, Balachander J. A comparative evaluation of metoprolol and methyldopa in the management of pregnancy induced hypertension. Indian Heart Journal 1992;44:39‐41. [PubMed] [Google Scholar]
India 2002 {published data only}
- Banerjee G, Chatterjee D, Chatterjee A, Mukherjee P, Das A. A randomized controlled trial on use of nimodipine in mild P.I.H. Journal of Obstetrics and Gynecology of India 2002;52(4):44‐6. [Google Scholar]
India 2011 {published data only}
- Pandey K, Gupta N, Gupta R, Gupta S. Comparing obstetric outcome using labetalol/methyldopa in pregnancy induced hypertension. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:46.
India 2012 {published data only}
- Molvi SN, Mir S, Rana VS, Jabeen F, Rauoof A. Role of antihypertensive therapy in mild to moderate pregnancy‐induced hypertension: A prospective randomized study comparing labetalol with alpha methyldopa. Archives of Gynecology and Obstetrics 2012;285(6):1553‐62. [DOI] [PubMed] [Google Scholar]
India 2013a {published data only}
- Srivastava B, Usha R, Bhardwaj R, Gaur S, Joshi G, Nag P. Comparative study of efficacy and adverse effect of labetalol and methyldopa in patients with pregnancy ‐induced hypertension. Indian Journal of Pharmacology 2013; Vol. 45.
India 2013b {published data only}
- Aparna J. A randomized, double‐blind, comparative trial of nifedipine and methyldopa in moderate pregnancy induced hypertension. Der Pharmacia Lettre 2013;5(4):274‐7. [Google Scholar]
India 2013c {published data only}
- Subhedar V, Inamdar S, Hariharan C, Subhedar S. Comparison of efficacy of labetalol and methyldopa in patients with pregnancy‐induced hypertension. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(1):27‐34. [Google Scholar]
India 2015a {published data only}
- Babbar K, Armo M, Bhanja RL. A comparative study of efficacy of antihypertensive drugs and feto‐maternal outcome in the treatment of pregnancy induced hypertension. Journal of Reproduction, Contraception, Obstetrics and Gynecology 2015;4(6):1846‐52. [Google Scholar]
India 2015b {published data only}
- Singhal S, Gupta AK. A comparative randomized controlled parallel group study of efficacy and tolerability of labetalol versus methyldopa in the treatment of mild pre‐eclampsia. Internatiional Journal of Basic and Clinical Pharmacology 2015; Vol. 4, issue 3:442‐5.
Ireland 1991 {published and unpublished data}
- Blake S, MacDonald D. The prevention of the maternal manifestations of pre‐eclampsia by intensive antihypertensive treatment. British Journal of Obstetrics and Gynaecology 1991;98:244‐8. [DOI] [PubMed] [Google Scholar]
Israel 1986a {published data only}
- Ellenbogen A, Jaschevatzky O, Davidson A, Anderman S, Grunstein S. Management of pregnancy‐induced hypertension with pindolol ‐ comparative study with methyldopa. International Journal of Gynecology & Obstetrics 1986;24:3‐7. [DOI] [PubMed] [Google Scholar]
- Jaschevatzky OE, Davidson A, Ellenbogen A, Anderman S, Grunstein S. Comparative study of pindolol and methyldopa and transplacental cross of pindolol in PIH. 5th International Congress of the International Society for the study of Hypertension in Pregnancy; 1986 7‐10 July; Nottingham, England. 1986:137.
Israel 1986b {published data only}
- Rosenfeld J, Bott‐Kanner G, Boner G, Nissenkorn A, Friedman S, Ovadia J, et al. Treatment of hypertension during pregnancy with hydralazine monotherapy or with combined therapy with hydralazine and pindolol. European Journal of Obstetrics & Gynecology and Reproductive Biology 1986;22:197‐204. [DOI] [PubMed] [Google Scholar]
Israel 1992a {published data only}
- Bar J, Hirsch M, Friedman S, Fuchs J, Ovadia J, Bott‐Kanner G. The effect of beta‐adrenergic blocker pindolol on platelet function in chronic hypertensive pregnancies. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:155.
- Bott‐Kanner G, Hirsch M, Boner G, Ovadia J, Modan M, Friedmann S, et al. Evaluation of antihypertensive therapy (AHT) in the management of hypertension in pregnancy (HP). 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:11.
- Bott‐Kanner G, Hirsch M, Friedman S, Boner G, Ovadia J, Merlob P, et al. Antihypertensive therapy in the management of hypertension in pregnancy ‐ a clinical double‐blind study of pindolol. Clinical and Experimental Hypertension 1992;B11:207‐20. [Google Scholar]
- Hirsch M, Bar J, Bott‐Kanner G, Kaplan B, Fuchs J, Ovadia J. Effect of the beta‐adrenergic blocker pindolol on platelet function in chronic hypertensive pregnancy. Hypertension in Pregnancy 1996;15:193‐202. [Google Scholar]
Israel 1995 {published data only}
- Paran E, Baile Y, Holzberg G, Masor M, Zmora E, Insler V. Treatment of hypertension in pregnancy: different regime, different outcome. 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:105.
- Paran E, Holzberg G, Mazor M, Zmora E, Insler V. Beta‐adrenergic blocking agents in the treatment of pregnancy‐induced hypertension. International Journal of Clinical Pharmacology and Therapeutics 1995;33:119‐23. [PubMed] [Google Scholar]
- Paran E, Maisner I, Holzberg G, Mazor M, Zmora E, Biale Y, et al. Beta‐blocker treatment of PIH correlated with uteroplacental blood flow measurement. 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:109.
Italy 1997 {published data only}
- Catalano D, Ercolano S, Pollio F, Ascione L, Russo C, Santi B, et al. Evaluation of nifedipine monotherapy in the management of pregnancy hypertension [Valuazione della monoterapia con nifedipina nel management della gestosi EPH]. Giornale Italiano Di Ostetricia e Ginecologia 1997;6:373‐5. [Google Scholar]
Italy 1998 {published data only}
- Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. BJOG: an international journal of obstetrics and gynaecology 2000;107:792‐4. [DOI] [PubMed] [Google Scholar]
- Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. British Journal of Obstetrics and Gynaecology 1998;105:718‐22. [DOI] [PubMed] [Google Scholar]
- Luchini L, Bortolus R, Parazzini F. Multicentric, randomized, clinical trial on the efficacy of long‐acting nifedipine in improving the prognosis of pregnancy in women with mild or moderate chronic or pregnancy‐induced hypertension. Journal of Nephrology 1991;6:51‐4. [Google Scholar]
Italy 1999 {published and unpublished data}
- Neri I, Ternelli G, Facchinetti F, Volpe A. Effectiveness of oral nifedipine and transdermal glyceryltrinitrate on 24‐hours blood pressure values in pregnancy‐induced hypertension. Hypertension in Pregnancy 2000;19(Suppl 1):O65. [Google Scholar]
- Neri I, Valensise H, Fachinetti F, Menghini S, Romanini C, Volpe A. 24‐hour ambulatory blood pressure monitoring: a comparison between transdermal glyceryl‐trinitrate and oral nifedipine. Hypertension in Pregnancy 1999;18:107‐13. [DOI] [PubMed] [Google Scholar]
Italy 2000 {published and unpublished data}
- Borghi C, Degli Esposti D, Cassani A, Immordino V, Bovicelli L, Ambrosioni E. The treatment of hypertension in pregnancy. Journal of Hypertension 2002;20(Suppl 2):S52‐S56. [PubMed] [Google Scholar]
- Borghi C, Immordino V, Degli Esposti D, Boschi S, Cassani A, Bentivenga C, et al. Comparison between nifedipine‐gits and methyldopa on blood pressure control, utero‐placental hemodynamic and fetal outcome in patients with pre‐eclampsia. Hypertension in Pregnancy 2000;19(Suppl 1):P8. [Google Scholar]
Pakistan 2016 {published data only}
- Sharif N, Usman I, Azhar T. Pregnancy induced hypertension; to compare efficacy of methyldopa and labetalol in management. Professional Medical Journal 2016; Vol. 23, issue 10:1187‐93.
Panama 2014 {published data only}
- Vigil‐De Gracia P, Dominguez L, Solis A. Management of chronic hypertension during pregnancy with furosemide, amlodipine or aspirin: a pilot clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(13):1291‐4. [DOI] [PubMed] [Google Scholar]
South Africa 1991a {published data only}
- Odendaal HJ, Schabort I, Pattinson RC. Prazosin for the treatment of hypertension in pregnancy: a randomized control trial. In: Oxford Database of Perinatal Trials, Version 1.2, Disk Issue 6, Autumn. Chalmers I (ed). Oxford: Oxford University Press.1991.
South Africa 1993 {published data only}
- Eloff WJ. The use of nifedipine vs methyldopa in mild to moderate pregnancy associated hypertension. Proceedings of the 12th Conference on Priorities in Perinatal Care; 1993; South Africa. 1993:130‐3.
Spain 1988 {published data only}
- Torres Pons PJ, Roca M, Codina C, Cobo E, Cararach V, Gonzalez‐Merlo J. Labetalol in the treatment of mild hypertension in pregnancy. A double blind controlled trial. 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:102.
Sudan 2002 {published data only}
- Elhassan EM, Mirghani OA, Habour AB, Adam I. Methyldopa versus no drug treatment in the management of mild pre‐eclampsia. East African Medical Journal 2002;71(4):172‐5. [DOI] [PubMed] [Google Scholar]
Sweden 1984 {published and unpublished data}
- Bjorksten B, Finnstrom O, Wichman K. Intrauterine exposure to the beta‐adrenergic receptor‐blocking agent metoprolol and allergy. International Archives of Allergy and Immunology 1988;87:59‐62. [DOI] [PubMed] [Google Scholar]
- Finnstrom O, Ezitis J, Ryden G, Wichman K. Neonatal effects of beta‐blocking drugs in pregnancy. Acta Obstetricia et Gynecologica Scandinavica Supplement 1984;118:91‐3. [DOI] [PubMed] [Google Scholar]
- Ryden G, Karlberg BE, Wichman K. Metoprolol in the treatment of hypertension in pregnancy ‐ effect on the mother and foetus. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 Jun 18‐21; Amsterdam, The Netherlands. 1984:38.
- Wichman K. Metoprolol in the treatment of mild to moderate hypertension in pregnancy ‐ effects on fetal heart activity. Clinical and Experimental Hypertension 1986;5:195‐202. [Google Scholar]
- Wichman K, Ezitis J, Finnstrom O, Ryden G. Metoprolol in the treatment of hypertension in pregnancy ‐ effect on the newborn baby. Clinical and Experimental Hypertension 1984;B3:153. [Google Scholar]
- Wichman K, Ezitis J, Finnstrom O, Ryden G. Metoprolol in the treatment of hypertension in pregnancy ‐ effects on the newborn baby. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:40.
- Wichman K, Karlberg BE, Ryden G. Metoprolol in the treatment of mild to moderate hypertension in pregnancy ‐ effects on the mother. Clinical and Experimental Hypertension 1985;B4:141‐56. [Google Scholar]
- Wichman K, Ryden G, Karlberg BE. A placebo controlled trial of metoprolol in the treatment of hypertension in pregnancy. Scandinavian Journal of Clinical and Laboratory Investigation 1984;106:90‐5. [DOI] [PubMed] [Google Scholar]
Sweden 1985 {published and unpublished data}
- Hogstedt S, Lindberg B, Lindeberg S, Ludviksson K, Sandstrom B. Effect of metoprolol on fetal heart rate patterns during pregnancy and delivery. Clinical and Experimental Hypertension 1984;B3:152. [Google Scholar]
- Högstedt S, Lindberg B, Lindberg S, Ludviksson K, Sandström B. Effect of metoprolol on fetal heart rate patterns during pregnancy and delivery. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:39.
- Högstedt S, Lindeberg S, Axelsson O, Lindmark G, Rane A, Sandström B, et al. A prospective controlled trial of metoprolol‐hydralazine treatment in hypertension during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1985;64:505‐10. [DOI] [PubMed] [Google Scholar]
- Lindeberg S, Axelsson O, Lindeberg BS, Lindmark G, Ludviksson K, Sandström B. Effect of metoprolol in combination with hydralazine on fetal heart rate patterns during hypertensive pregnancy and delivery. Clinical and Experimental Hypertension 1985;4(1):87‐94. [Google Scholar]
Sweden 1995 {published data only}
- Wide‐Swensson D, Ingemarsson I, Lunell NO, Lindberg B, Lindeberg S, Marsal K, et al. Calcium channel blockade (isradipine) in treatment of hypertension in pregnancy: a randomized placebo‐controlled study. International Journal of Obstetrics & Gynecology 1994;46:3. [DOI] [PubMed] [Google Scholar]
- Wide‐Swensson DH, Ingemarsson I, Lunell NO, Forman A, Skajaa K, Lindberg B. Calcium channel blockade (isradipine) in treatment of hypertension in pregnancy: a randomized placebo‐controlled study. American Journal of Obstetrics and Gynecology 1995;173:872‐8. [DOI] [PubMed] [Google Scholar]
UK 1968 {published data only}
- Leather HM, Humphreys DM, Baker P, Chadd MA. A controlled trial of hypotensive agents in hypertension in pregnancy. Lancet 1968;2:488‐90. [DOI] [PubMed] [Google Scholar]
UK 1976 {published data only}
- Cockburn J, Moar VA, Ounsted MK, Redman CW. Final report on study of hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet 1982;1:647‐9. [DOI] [PubMed] [Google Scholar]
- Moar VA, Jefferies MA, Mutch LM, Ounsted MK, Redman CW. Neonatal head circumference and the treatment of maternal hypertension. British Journal of Obstetrics and Gynaecology 1978;85:933‐7. [DOI] [PubMed] [Google Scholar]
- Mutch LM, Moar VA, Ounsted MK, Redman CW. Hypertension during pregnancy, with and without specific hypotensive treatment. I. Perinatal factors and neonatal morbidity. Early Human Development 1977;1:47‐57. [DOI] [PubMed] [Google Scholar]
- Mutch LM, Moar VA, Ounsted MK, Redman CW. Hypertension during pregnancy, with and without specific hypotensive treatment. II The growth and development of the infant in the first year of life. Early Human Development 1977;1:59‐67. [DOI] [PubMed] [Google Scholar]
- Ounsted M. The children of women who had hypertension during pregnancy. In: Rubin PC editor(s). Hypertension in Pregnancy. Amsterdam: Elsevier Science Publishers B.V, 1988:341‐62. [Google Scholar]
- Ounsted MK, Moar VA, Good FJ, Redman CW. Hypertension during pregnancy with and without specific treatment: the development of the children at the age of four years. British Journal of Obstetrics and Gynaecology 1980;87:19‐24. [DOI] [PubMed] [Google Scholar]
- Redman CW, Beilin LJ, Bonnar J. Treatment of hypertension in pregnancy with methyldopa: blood pressure control and side effects. British Journal of Obstetrics and Gynaecology 1977;84:419‐26. [DOI] [PubMed] [Google Scholar]
- Redman CW, Beilin LJ, Bonnar J, Ounsted MK. Fetal outcome in trial of antihypertensive treatment in pregnancy. Lancet 1976;2:753‐6. [DOI] [PubMed] [Google Scholar]
UK 1980 {published data only}
- Lamming GD, Pipkin FB, Symonds EM. Comparison of the alpha and beta blocking drug, labetalol, and methyl dopa in the treatment of moderate and severe pregnancy‐induced hypertension. Clinical and Experimental Hypertension 1980;2:865‐95. [DOI] [PubMed] [Google Scholar]
- Lamming GD, Symonds EM. Comparison of the alpha and beta blocking drug labetalol and aldomet in the treatment of pregnancy induced hypertension. Proceedings of the 2nd Congress of the International Society for the Study of Hypertension in Pregnancy; 1980 December 1‐4; Cairo, Egypt. 1980:9.
- Lamming GD, Symonds EM. Use of labetalol and methyldopa in pregnancy‐induced hypertension. British Journal of Clinical Pharmacology 1979;8:217S‐222S. [PMC free article] [PubMed] [Google Scholar]
UK 1982 {published and unpublished data}
- Cameron AD, Walker JJ, Bonduelle M, Calder AA. A randomised trial of the antihypertensive agent, labetalol, against bed rest in pregnancy hypertension. Archives of Gynecology 1985;237(Suppl 1):295. [Google Scholar]
- Lang GD, Lowe GD, Walker JJ, Forbes CD, Calder AA. Increased blood viscosity in pre‐eclampsia: effect of treatment with labetalol. Thrombosis and Haemostasis 1983;50:156. [Google Scholar]
- Lang GD, Lowe GD, Walker JJ, Forbes CD, Prentice CR, Calder AA. Blood rheology in pre‐eclampsia and intrauterine growth retardation: effects of blood pressure reduction with labetalol. British Journal of Obstetrics and Gynaecology 1984;91:438‐43. [DOI] [PubMed] [Google Scholar]
- Walker J. Intrauterine Growth Retardation and Maternal Labetalol Treatment in a Random Allocation Controlled Study [MD thesis]. Scotland: University of Glasgow, 1992:141‐55. [Google Scholar]
- Walker JJ, Crooks A, Erwin L, Calder AA. Labetalol in pregnancy‐induced hypertension: fetal and maternal effects. In: Reilly A, Symonds EM editor(s). International Congress Series 591. Amsterdam: Excerpta Medica, 1982:148‐60. [Google Scholar]
UK 1983a {published data only}
- Reynolds B, Butters L, Evans J, Adams T, Rubin PC. First year of life after the use of atenolol in pregnancy associated hypertension. Archives of Disease in Childhood 1984;59:1061‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin PC, Butters L, Clark D, Sumner D, Belfield A, Pledger D, et al. Obstetric aspects of the use in pregnancy‐associated hypertension of the beta‐adrenoceptor antagonist atenolol. American Journal of Obstetrics and Gynecology 1984;150:389‐92. [DOI] [PubMed] [Google Scholar]
- Rubin PC, Butters L, Clark DM, Low RA, Reid JL. Atenolol in the management of hypertension during pregnancy. Drugs 1983;25:212‐4. [Google Scholar]
- Rubin PC, Butters L, Clark DM, Reynolds B, Sumner DJ, Steedman D. Placebo‐controlled trial of atenolol in treatment of pregnancy‐associated hypertension. Lancet 1983;1:431‐4. [PubMed] [Google Scholar]
- Rubin PC, Butters L, Reynolds B, Low RAL. Atenolol in the management of pregnancy associated hypertension: obstetric and paediatric aspects. 4th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:41.
UK 1983b {published data only}
- Fidler J, Smith V, Fayers P, Swiet M. Randomised controlled comparative study of methyldopa and oxprenolol in treatment of hypertension in pregnancy. British Medical Journal 1983;286:1927‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler J, Smith V, Fayers P, Swiet M. Randomised controlled comparative study of methyldopa and oxprenolol in treatment of hypertension in pregnancy. Obstetrical and Gynecological Survey 1983;286:202‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK 1984 {published data only}
- Thorley KJ. Atenolol: side effects in the newborn infant. British Medical Journal 1982;285:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley KJ. Randomised trial of atenolol and methyl dopa in pregnancy related hypertension. 4th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:53.
- Thorley KJ. Randomised trial of atenolol and methyl dopa in pregnancy related hypertension. Clinical and Experimental Hypertension 1984;133:168. [Google Scholar]
UK 1989 {published data only}
- Pickles C, Symonds E, Brinkman C. Effects of labetalol (L) vs placebo (P) on arterial pressure (AP) and forearm venous distensibility (FVD) in women with pregnancy induced hypertension (PIH). 5th International Congress of the International Society for the study of Hypertension in Pregnancy; 1986 7‐10 July; Nottingham, England. 1986:92.
- Pickles CJ, Broughton Pipkin F, Symonds EM. A randomised placebo controlled trial of labetalol in the treatment of mild to moderate pregnancy induced hypertension. Britisth Journal of Obstetrics and Gynaecology 1992;99:964‐8. [DOI] [PubMed] [Google Scholar]
- Pickles CJ, Symonds EM, Broughton Pipkin F. The fetal outcome in a randomized double‐blind controlled trial of labetalol vs placebo in pregnancy‐induced hypertension. British Journal of Obstetrics and Gynaecology 1989;96:38‐43. [DOI] [PubMed] [Google Scholar]
- Pickles CJ, Symonds EM, Broughton Pipkin F. The fetal outcome of early intervention therapy in pregnancy induced hypertension. A randomised, double‐blind controlled trial of labetalol vs placebo. 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:107.
UK 1990 {published and unpublished data}
- Butters L, Kennedy S, Rubin PC. Atenolol in essential hypertension during pregnancy. BMJ 1990;301:587‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK 1992 {published and unpublished data}
- Cruickshank DJ, Campbell D, Robertson AA, MacGillivray I. Intra‐uterine growth retardation and maternal labetalol treatment in a random allocation controlled study. Journal of Obstetrics and Gynaecology 1992;12:223‐7. [Google Scholar]
- Cruickshank DJ, Campbell DM. Atenolol in essential hypertension during pregnancy. BMJ 1990;301:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank DJ, Robertson AA, Campbell DM, MacGillivray I. Does labetalol influence the development of proteinuria in pregnancy hypertension? A randomised controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;45:47‐51. [DOI] [PubMed] [Google Scholar]
- Cruickshank DJ, Robertson AA, Campbell DM, MacGillivray I. Maternal obstetric outcome measures in a randomised controlled study of labetalol in the treatment of hypertension in pregnancy. Clinical and Experimental Hypertension 1991;B10:333‐4. [Google Scholar]
UK 2009 {published data only}
- NCT00141310. A randomised, double‐blind, placebo‐controlled, flexible‐dose, parallel‐group study to evaluate the efficacy, safety and toleration of oral sildenafil citrate administered in the dose range of 20 ‐ 80 mg TID for the treatment of pre‐eclampsia. (PET). clinicaltrials.gov/ct2/show/record/NCT00141310 (first received: 30 August 2005).
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double‐blinded, placebo‐controlled trial of the phosphodiesterase type 5 inhibitor sildenafil in the treatment of preeclampsia. Hypertension in Pregnancy 2009;28:369‐82. [DOI] [PubMed] [Google Scholar]
- Samangaya RA, Wareing M, Skillern L, Baker PN. Phosphodiesterase inhibitor effect on small artery function in preeclampsia. Hypertension in Pregnancy 2011;30(2):144‐52. [DOI] [PubMed] [Google Scholar]
UK 2017 {published data only}
- EUCTR2013‐003144‐23‐GB. Pregnancy and chronic hypertension: nifedipine or labetalol as antihypertensive treatment ‐ PANDA. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2013‐003144‐23 (first received 30 October 2013).
- Webster L, Gill C, Seed P, Bramham K, Wiesender C, Nelson‐Piercy C, et al. Effect of antihypertensive treatment on placental, renal and endothelial biomarkers in pregnancy complicated by chronic hypertension. BJOG: an international journal of obstetrics and gynaecology 2017;124:12. [Google Scholar]
- Webster L, Myers J, Nelson‐Piercy C, Watt‐Coote I, Wiesender C, Seed PT, et al. Pregnancy and chronic hypertension: Nifedipine vs labetalol as antihypertensive treatment (the PANDA study). Pregnancy Hypertension 2016;6:141. [Google Scholar]
- Webster LM, Myers JE, Nelson‐Piercy C, Harding K, Cruickshank JK, Watt‐Coote I, et al. Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension 2017;70(5):915‐22. [DOI] [PubMed] [Google Scholar]
- Webster LM, Myers JE, Nelson‐Piercy C, Watt‐Coote I, Wiesender C, Seed PT, Chappell LC. Pregnancy and chronic hypertension: Nifedipine vs labetalol as antihypertensive treatment (the Panda study). Ophthalmology 2016;6(3):141. [Google Scholar]
USA 1979 {published data only}
- Arias F, Zamora J. Antihypertensive treatment and pregnancy outcome in patients with mild chronic hypertension. Obstetrics & Gynecology 1979;53:489‐94. [PubMed] [Google Scholar]
USA 1987a {published data only}
- Sibai BM, Gonzalez AR, Mabie WC, Moretti M. A comparison of labetalol plus hospitalization vs hospitalization alone in the management of preeclampsia remote from term. Obstetrics & Gynecology 1987;70:323‐7. [PubMed] [Google Scholar]
USA 1987b {published data only}
- Weitz C, Khouzami V, Maxwell K, Johnson JW. Treatment of hypertension in pregnancy with methyldopa: a randomized double blind study. International Journal of Gynecology & Obstetrics 1987;25:35‐40. [DOI] [PubMed] [Google Scholar]
USA 1990a {published and unpublished data}
- Sibai BM. The need of hypertensive drugs in management of mild to moderate pregnant hypertension. Proceedings of the 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:133.
- Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication vs methyldopa or labetalol in chronic hypertension during pregnancy. American Journal of Obstetrics and Gynecology 1990;162:960‐7. [DOI] [PubMed] [Google Scholar]
USA 1992 {published data only}
- Barton JR, Mercer BM, Sibai BM. The effect of nifedipine on urinary excretion of calcium in preeclampsia. American Journal of Perinatology 1997;14:609‐12. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Barton JR, Akl S, Sarinoglu C, Mercer BM. A randomized prospective comparison of nifedipine and bed rest vs bed rest alone in the management of preeclampsia remote from term. Amercian Journal of Obstetrics and Gynecology 1992;167:879‐84. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Barton JR, Akl S, Sarinoglu C, Mercer BM. A randomized prospective comparison of nifedipine and bed rest vs bed rest alone in the management of preeclampsia remote from term. American Journal of Obstetrics and Gynecology 1992;166:280. [DOI] [PubMed] [Google Scholar]
Venezuela 1988 {published data only}
- Faneite PJ, Gonzalez X, Salazar G. Evaluation of antihypertensives in pregnancy: prospective randomized study of mepindolol and alpha methyldopa [Evaluación de antihipertensivos en embarazadas: Mepindolol y alfametildopa. Estudio prospectivo y randomizado]. Revista de Obstetricia y Ginecologia de Venezuela 1988;48:139‐43. [Google Scholar]
References to studies excluded from this review
Argentina 1990 {published data only}
- Voto LS, Quiroga CA, Lapidus AM, Catuzzi P, Uranga IF, Margulies M. Effectiveness of antihypertensive drugs in the treatment of hypertension in pregnancy. Clinical and Experimental Hypertension ‐ Part B Hypertension in Pregnancy 1990;9(3):339‐48. [Google Scholar]
Argentina 1994 {published data only}
- Fabregues G, Sure P, Knaudt S, Gonzalez Pena J, Esper R. The use of atenolol to prevent preeclampsia and superimposed preeclampsia. International Journal of Gynecology & Obstetrics 1994;46:95. [Google Scholar]
Australia 1985b {published data only}
- Henderson‐Smart DJ, Horvath JS, Phippard A, Korda A, Child A, Duggin GG, et al. Effect of antihypertensive drugs on neonatal blood pressure. Clinical and Experimental Pharmacology and Physiology 1984;11(4):351‐4. [DOI] [PubMed] [Google Scholar]
- Horvath JS, Henderson‐Smart DJ, Phippard A, Korda A, Child A, Duggin GG, et al. The effect of antihypertensive drugs on neonatal blood pressure. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:42.
- Horvath JS, Phippard A, Korda A, Henderson‐Smart D, Child A, Duggin GG, et al. A controlled trial comparing clonidine hydrochloride and alpha methyl dopa in pregnant women with hypertension. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:246.
- Horvath JS, Phippard A, Korda A, Henderson‐Smart DJ, Child A, Tiller DJ. Clonidine hydrochloride ‐ a safe and effective antihypertensive agent in pregnancy. Obstetrics & Gynecology 1985;66:634‐8. [PubMed] [Google Scholar]
- Horvath JS, Phipparel A, Korda A, Henderson‐Smart D, Child A, Duggin GG, et al. A controlled trial comparing clonidine hydrochloride and alpha methyl dopa in pregnant women [abstract]. Kidney International 1984;26(2):233. [Google Scholar]
Australia 1991 {published and unpublished data}
- Phippard A, Fisher W, Child A, Henderson‐Smart D, Horvarth J, Korda A, et al. A randomized, placebo‐controlled study on the effect of therapy for mild to moderate hypertension in primigravidae. 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:112.
- Phippard AF, Fischer WE, Horvath JS, Child AG, Korda AR, Henderson‐Smart DJ, et al. Early blood pressure control improves pregnancy outcome in primigravid women with mild hypertension. Medical Journal of Australia 1991;154:378‐82. [DOI] [PubMed] [Google Scholar]
Belgium 1988 {published data only}
- Loquet P, Buytaert P, Renier M. The influence of pindolol and atenolol on serial feto‐placental blood flow measurements in non‐proteinuric hypertension in pregnancy. 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:111.
- Renier M, Loquet P, Buytaert P. The influence of pindolol and atenolol on serial feto‐placental blood flow measurements in non‐proteinuric hypertension in pregnancy. Proceedings of the 11th European Congress of Perinatal Medicine;1988 April 10‐13; Rome, Italy. 1988.
Brazil 1997 {published data only}
- Pereira TS, Pereira CI, Junior JA, Netto HC. Influence of nifedipine on the fetal placental flow. Jornal Brasileiro de Ginecologia 1997;107(10):371‐80. [Google Scholar]
Brazil 2000b {published data only}
- Alves EA. Estudo prospectivo, comparativo da Isradipina e Atenolol no tratamento de gestantes hipertensas [Prospective, comparative study of isradipine and atenolol for the treatment of hypertensive pregnant women] [Doctoral Thesis]. Faculty of Medicine, University of São Paulo, 1998. [Google Scholar]
- Darcie S, Leone CR, Calil VM, Prescinotti EP, Kahhale S, Zugaib M. Glycemia in newborns of hypertensive mothers according to maternal treatment [Glicemia no recém‐nascido de mäe hipertensas de acordo com a terapêutica materna]. Revista do Hospital das Clínicas 2004;59(5):244‐0. [DOI] [PubMed] [Google Scholar]
- Kahhale S, Alves EA, Takiuti NH, Zugaib M. Efficacy and safety of isradipina and atenolol in hypertensive disorders in pregnancy [abstract]. Hypertension in Pregnancy 2000;19(1):98. [Google Scholar]
- Leone CR, Calil VM, Darcie S, Prescinotti EP, Kahhale S, Zugaib M. Growth of newborn of the mothers with hypertension during the first year of life: therapeutics in pregnancy on the newborn [Crescimento de recém‐nascidos de mäes hipertensas durante o primeiro ano de vida: influência da terapêutica materna]. Revista Paulista de Pediatría 2003;21(1):19‐26. [Google Scholar]
Brazil 2000c {published data only}
- Vasconcellos MJ, Chaves Netto H, Kahhale S. Incidence of small fetuses for the gestational age among chronic hypertensive women in use of verapamil (chronic oral use). Hypertension in Pregnancy 2000;19(Suppl 1):P166. [Google Scholar]
- Vasconcellos MJ, Chaves Netto H, Kahhale S. Verapamil: its effect on placental circulation in mild to moderate chronic hypertensive pregnant women. Hypertension in Pregnancy 2000;19(Suppl 1):P163. [Google Scholar]
- Vasconcellos MJ, Chaves Netto H, Kahhale S, Almeida PJ. Use of verapamil in chronic hypertensive pregnant women: flow analysis of uterine and umbilical artery [Uso do verapamil em gestantes hipertensas crônicas: análise do fluxo das artérias uterinas e umbilical]. Revista Brasileira de Ginecologia é Obstetrícia 2000;22(5):265‐74. [Google Scholar]
- Vasconcellos MJ, Chaves Netto H, Kahhale S, Almeida PJ. Verapamil use in chronic hypertension pregnant women. XVI FIGO World Congress of Obstetrics and Gynaecology; 2000 Sept 3‐8; Washington DC. Book 2. 2000:46.
China 1991 {published data only}
- Qian XH, Huang YL, Wu SP. Treatment of hypertension syndrome of pregnancy with ligustrazine. Chung Hsi I Chieh Ho Tsa Chih 1991;11(9):533‐4, 516. [PubMed] [Google Scholar]
China 1993 {published data only}
- Hong YJ, Lin CF, Chen JC, Pan P, Wong KL, Wei TT. Nifedipine in preeclampsia for cesarean section. Ma Tsui Hsueh Tsa Chi (Acta Anaesthesiologica Sinica) 1993;31(1):43‐8. [PubMed] [Google Scholar]
China 1998 {published data only}
- Li M, Yang M, Qiu X. The effect of nimodipine on retinal blood flow in pregnancy induced hypertension. Chung‐Hua Fu Chan Ko Tsa Chih 1998;33(7):397‐9. [PubMed] [Google Scholar]
China 1999 {published data only}
- Wang Z, Song H. Clinical observation on therapeutical effect of prepared rhubarb in treating pregnancy induced hypertension. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1999;19:725‐7. [PubMed] [Google Scholar]
China 2000 {published data only}
- Yang X, Liu Y. The effect of nifedipine on postpartum blood loss in patients with pregnancy induced hypertension. Zhonghua Fu Chan Ke Za Zhi 2000;35(3):151‐2. [PubMed] [Google Scholar]
China 2014 {published data only}
- Sun X, Wang K, Wang W, Li B. Clinical study on sildenafil in treatment of pregnant women with pulmonary arterial hypertension [Article in Chinese]. Zhonghua Fu Chan Ke Za Zhi 2014;49(6):414‐8. [PubMed] [Google Scholar]
China 2017 {published data only}
- Xiao S, Zhang M, Liang Y, Wang D. Celastrol synergizes with oral nifedipine to attenuate hypertension in preeclampsia: a randomized, placebo‐controlled, and double blinded trial. Journal of the American Society of Hypertension 2017;11(9):598‐603. [DOI] [PubMed] [Google Scholar]
Cuba 1994 {published and unpublished data}
- Gómez Sosa E, Miró Maceo M, Alcade MT. Mild chronic hypertension: antihypertensive medication and maternal and perinatal results [Hipertensión crónica moderada: medicación antihipertensiva y resultados maternos y perinatales]. Revista Cubana de Medicina General Integral 1994;10(4):340‐3. [Google Scholar]
Czech Republic 1993 {published data only}
- Soucek M, Prasek J, Spinarova L. Atenolol and bisoprolol in the treatment of mild and moderately severe hypertension [Atenolol a bisoprolol v lécbe mírné a stredne tezké hypertenze]. Vnitrni Lekarstvi 1993;39(6):541‐8. [PubMed] [Google Scholar]
Denmark 1991 {published data only}
- Rudnicki M, Frölich A, Fischer Rasmussen W, McNair P. The effect of magnesium on maternal blood pressure in pregnancy‐induced hypertension. Acta Obstetricia et Gynecologica Scandinavica 1991;70:445‐50. [DOI] [PubMed] [Google Scholar]
- Rudnicki M, Frölich A, Fischer‐Rasmussen W. Magnesium supplement in pregnancy‐induced hypertension: effects on maternal and neonatal magnesium and calcium homeostasis. Mineral and Electrolyte Metabolism 1991;17(6):399‐403. [PubMed] [Google Scholar]
- Rudnicki M, Junge J, Frölich A, Ornvold K, Fischer‐Rasmussen W. Magnesium supplement in pregnancy‐induced hypertension. A clinicopathological study. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 1990;98(12):1123‐7. [DOI] [PubMed] [Google Scholar]
Denmark 2000 {published data only}
- Rudnicki M, Frølich A, Pilsgaard K, Nyrnberg L, Møller M, Sanchez M, et al. Comparison of magnesium and methyldopa for the control of blood pressure in pregnancies complicated with hypertension. Gynecologic and Obstetric Investigation 2000;49:231‐5. [DOI] [PubMed] [Google Scholar]
Dominican Republic 1992a {published data only}
- Cid‐Troncoso J, Pérez‐Pérez M, Jiménez JA, Núñez‐Diplan AM, Balbi M, Santana E. Nifedipine retard vs methyldopa in chronic hypertension with superimposed toxemia [Nifedipina retard vs. alfa‐metil‐dopa en la hipertensión arterial crónica mas toxemia agregada]. Revista Médica Dominicana 1992;53(2/3):76‐8. [Google Scholar]
Dominican Republic 1992b {published data only}
- Deschamps C, Guzman P, Mejia M, Sanchez Ramirez PB, Sanchez Beltre JE, Gomez Hernandez J. Comparison of hydralazine and alpha methyldopa in hypertension in pregnancy [Comparacion de hidralazina y alfametildopa en la hipertension durante el embarazo]. Acta Medica Dominicana 1992;14(6):222‐4. [Google Scholar]
Egypt 1988 {published data only}
- Salem HT, Ghanemah S, Seleem S, Sayed EH, Abdel‐Latif A, Chard T. Bromocriptine therapy in pre‐eclamptic toxaemia of pregnancy (PET). World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:184.
Egypt 1993 {published data only}
- Ismail AA, Medhat I, Tawfic TAS, Kholeif A. Evaluation of calcium‐antagonist (Nifedipine) in the treatment of pre‐eclampsia. International Journal of Gynecology & Obstetrics 1993;40:39‐43. [DOI] [PubMed] [Google Scholar]
Egypt 1997 {published data only}
- Salem HT, Salah M, Kotb HI, Makarem MH, Mostafa SA, Mohamed SA. The use of specific opioid receptor antagonist (Naltrexone) in hypertensive pregnancy. Research Activities on Reproductive Health. Assiut: Assiut University, Faculty of Medicine, 1997:74. [Google Scholar]
Egypt 2009 {published data only}
- Tanbouli T, Mawsouf MN, Re L, Martinez‐Sanchez G, Saaed G, El SM, et al. Effect of ozone therapy on foetoplacental blood flow in hypertensive pregnant women. International Journal of Ozone Therapy 2009;8(2):211‐6. [Google Scholar]
Egypt 2017 {published data only}
- Abbas AM, NCT03213639. Use of esomeprazole in treatment of early onset preeclampsia:a double blind randomized, placebo‐controlled trial. clinicaltrials.gov/show/NCT03213639 (first received 21 Augsut 2017).
Finland 1988a {published data only}
- Tuimala R, Hartikainen‐Sorri AL. Randomised comparison of atenolol and pindolol for treatment of hypertension in pregnancy. Current Therapeutic Research 1988;44(4):579‐84. [Google Scholar]
- Tuimala R, Hartikainen‐Sorri AL. Treatment of hypertension during pregnancy by beta blocking agents. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:52.
Finland 1995 {published data only}
- Räsänen J, Joupilla P. Uterine and fetal hemodynamics and fetal cardiac function after atenolol and pindolol infusion. A randomized study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;62:195‐201. [DOI] [PubMed] [Google Scholar]
Finland 1999 {published data only}
- Laivuori HM, Laakso M, Tikkanen MJ, Cacciatore B, Ylikorkala RO, Kaaja Rj. Short‐term metabolic effects of isradipine and metoprolol in pre‐eclampsia. Journal of Hypertension 1999;17(8):1189‐94. [DOI] [PubMed] [Google Scholar]
France 1986 {published data only}
- Fievet P, Esper N, Gueroult JF, Gueroult JC, Fournier A. Comparative study of clonidine and labetalol in severe hypertension induced by pregnancy. Congress of the International Society for the Study of Hypertension in Pregnancy. 7‐10 July 1986; Vol. Nottingham, England:136.
France 1988b {published data only}
- Madonna O, M'Pio I, Labeeuw M, Pozet N, Zech P. Evaluation of renal function in pregnant hypertensives under treatment. A comparison between methyldopa, pindolol and atenolol. 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:260.
Germany 2012 {published data only}
- Groten T, Fitzgerald J, Lehmann T, Schneider U, Kahler C, Schleussner E. Reduction of preeclampsia related complications with the NO‐donor penterythriltetranitrat (petn) in risk pregnancies ‐ A prospective randomized double‐blind placebo pilot study. Pregnancy Hypertension 2012;2(3):181. [DOI] [PubMed] [Google Scholar]
Hungary 1999 {published data only}
- Tamás P, Csermely T, Ertl T, Vizer M, Szabó I, Prievara FT. Calcium dobesilate lowers the blood pressure in mild to moderate midtrimester hypertension: a pilot study. Gynecologic and Obstetric Investigation 1999;47:210‐3. [DOI] [PubMed] [Google Scholar]
- Tamás P, Csermely T, Szabó EI. Doxium ‐ a novel drug in the management of gestational hypertension?. 16th European Congress of Perinatal Medicine; 1998 June 10‐13; Zagreb. 1998.
- Tamás P, Szabó I, Szekely J, Csermely T, Prievara FT, Nemeth L, et al. Effects of doxium 500(tm) in gestational hypertension [A Doxium 500 (R) hatasanak vizgalata terhessegi hypertoniaban (kettos vak, placebo‐kontrollalt tanulmany)]. Magyar Noorvosok Lapja 1997;60:181‐7. [Google Scholar]
India 1999 {published data only}
- Shenoy S, Chandrika D, Pisharody R. RCT of low dose aspirin to prevent the progression of pregnancy induced hypertension grade A to B. Journal of Clinical Epidemiology 1999;52(Suppl 1):28S. [Google Scholar]
India 2012a {published data only}
- Verma R, Lahon K, TonpaY SD, Kale VJ. A comparative randomised controlled parallel group study of maternal, fetal and neonatal outcomes of labetalol versus methyldopa in the treatment of new onset hypertension during pregnancy. International Journal of Pharma & Bio Sciences 2012;3(1):201‐11. [Google Scholar]
- Verma R, Lahon K, Tonpay SD, Kale VJ, Jaim DK. A comparative randomised controlled parallel group study of efficacy and tolerability outcomes of labetalol versus methyldopa in the treatment of new onset hypertension during pregnancy. International Journal of Life Science & Pharma Research 2012;2(1):L23‐31. [Google Scholar]
India 2012b {published data only}
- Dasgupta S. Does prophylactic magnesium sulphate in mild preeclampsia reduce umbilical artery pulsatility index? A randomized placebo controlled trial. Clinical Trials Registry ‐ India (http://www.ctri.nic.in) (accessed 8 July 2011) 2011.
- Dasgupta S, Ghosh D, Seal SL, Kamilya G, Karmakar M, Saha D. Randomized controlled study comparing effect of magnesium sulfate with placebo on fetal umbilical artery and middle cerebral artery blood flow in mild preeclampsia at >= 34 weeks gestational age. Journal of Obstetrics and Gynaecology Research 2012;38(5):763‐71. [DOI] [PubMed] [Google Scholar]
India 2013d {published data only}
- Bracken H. Oral antihypertensive regimens for management of hypertension in pregnancy. clinicaltrials.gov/ct2/show/NCT01912677 (first received 29 July 2013).
- Mundle S, CTRI/2013/08/003866. Oral antihypertensive regimens for management of hypertension in pregnancy. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=7167 (first received 2 August 2013).
India 2015c {published data only}
- Easterling T, Bracken H, Mundle S, Magee L, vonDadelszen P, Winikoff B. Oral antihypertensive regimens for management of severe hypertension in pregnancy: Results from a pilot study in India. Pregnancy Hypertension 2015;5(1):120. [Google Scholar]
India 2015d {published data only}
- Gainder S, Thakur M, Saha SC, Prakash M. To study the changes in maternal and fetal hemodynamics with intravenous labetalol or nifedipine in acute severe hypertension. Pregnancy Hypertension 2015;5(1):30. [DOI] [PubMed] [Google Scholar]
India 2016 {published data only}
- Mujawar JR, Patel SS. Circulating biomarkers of oxidative stress in preeclampsia and efficacy of antioxidant vitamin C supplementation. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2016;7(1):1498‐506. [Google Scholar]
Iran 2000 {published and unpublished data}
- Yassae F. A comparison of the effect of nifedipine and hydralazine in treatment of pregnancy induced hypertension. XVI FIGO World Congress of Obstetric and Gynecology (Book 3); 2000 Sept 3‐8; Washington DC, USA. 2000:93.
Iran 2005 {published data only}
- Ghahiri A, Berjis K. A comparison between intravenous magnesium sulfate and oral magnesium chloride in mild preeclampsia. Journal of Research in Medical Sciences 2005;10(1):6‐9. [Google Scholar]
Iran 2012 {published data only}
- NCT01674127. Effect of methyldopa on uterine artery diameter in pregnant women with mild preeclampsia. clinicaltrials.gov/ct2/show/record/NCT01674127 (first received 24 August 2012).
Iran 2016 {published data only}
- IRCT201602067676N5. Pregnancy outcomes with chronic hypertension on different antihypertensive therapy. http://en.search.irct.ir/view/28468 (first received 23 February 2016).
Israel 1988 {published data only}
- Davidson A, Ellenbogen A, Jaschevatzky O, Attias Y, Anderman S, Grunstein S. Randomized comparison of pindolol and atenolol for treatment of hypertension during pregnancy. 6th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Quebec, Canada. 1988:99.
Israel 1992b {published data only}
- Meizner I, Paran E, Katz M, Holcberg G, Insler V. Flow velocity analysis of umbilical and uterine artery flow in pre‐eclampsia treated with propranolol or pindolol. Journal of Clinical Ultrasound 1992;20:115‐9. [DOI] [PubMed] [Google Scholar]
Israel 1999 {published data only}
- Thaler I, Amit A, Kamil D, Itskovitz‐Eldor J. The effect of isosorbide dinitrate on placental blood flow and maternal blood pressure in women with pregnancy induced hypertension. American Journal of Hypertension 1999;12(4):341‐7. [DOI] [PubMed] [Google Scholar]
Italy 1986 {published data only}
- Martinelli P, Ferrara LA, Pasanisi F, Arpaia F, Paladini D, Mancini M. Nifedipine and atenolol in the treatment of pregnancy associated hypertension. 5th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, England. 1986:128.
Italy 1988 {unpublished data only}
- Pello L. Multicentric trial on the treatment of the hypertension in pregnancy [Ricerca multicéntrica sul trattamento del'ipertenzione in gravidanza]. Personal communication 1988.
Italy 1990a {published data only}
- Marlettini MG, Crippa S, Morselli‐Labate AM, Contarini A, Orlandi C. Randomised comparison of calcium antagonists and beta‐blockers in the treatment of pregnancy‐induced hypertension. Current Therapeutic Research, Clinical and Experimental 1990;48(4):684‐94. [Google Scholar]
- Orlandi C, Marlettini MG, Cassani A, Crippa S. Antihypertensive therapy in pregnancy. Proceedings of the 11th European Congress of Perinatal Medicine; 1988; Rome, Italy. 1988:271.
Italy 1990b {published data only}
- Iorio R, Horvath S, Marinoni E, Manzari G, Martinico E, Bresadola M. Use of a thromboxane receptor inhibitor in pregnancy‐induced hypertension. Proceedings of the 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990.
Italy 2000a {published data only}
- Picciolo C, Roncaglia N, Neri I, Pasta F, Arreghini A, Facchinetti F. Nitric oxide in the prevention of pre‐eclampsia. Prenatal and Neonatal Medicine 2000;5:212‐5. [Google Scholar]
Italy 2001 {published data only}
- Scarpellini F, Sbracia M, Lecchini S, Bolis P. Nitric oxide donor treatment reduces apoptosis in placental vessels of pregnancy induced hypertension [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S177. [Google Scholar]
Italy 2004 {published data only}
- Neri I, Venturini P, Battista Allais G, Facchinetti F. Acupuncture plus alfa‐metildopa in the treatment of chronic hypertension: a protocol design [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):54. [Google Scholar]
Italy 2006 {published data only}
- Facchinetti F, Piccinini F, Pizzi C, Bukowski R, Volpe A, Saade G. Effect of arginine supplementation in patients with gestational hypertension. American Journal of Obstetrics and Gynecology 2002;187(6):S213. [Google Scholar]
- Neri I, Blasi I, Facchinetti F. Effects of acute L‐arginine infusion on non‐stress test in hypertensive pregnant women. Journal of Maternal‐Fetal & Neonatal Medicine 2004;16(1):23‐6. [DOI] [PubMed] [Google Scholar]
- Neri I, Jasonni VM, Gori GF, Blasi I, Facchinetti F. Effect of L‐arginine on blood pressure in pregnancy‐induced hypertension: a randomized placebo‐controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(5):277‐81. [DOI] [PubMed] [Google Scholar]
Italy 2008 {published data only}
- Noris M, Fratelli N, Rampello S, Gherardi G, Platto C, Todeschini M, et al. A double‐blind, randomized, pilot study on L‐arginine oral supplementation in pre‐eclamptic pregnancies. Hypertension in Pregnancy 2008;27(4):505. [Google Scholar]
Italy 2010 {published data only}
- Neri I, Monari F, Sgarbi L, Berardi A, Masellis G, Facchinetti F. L‐Arginine supplementation in women with chronic hypertension: Impact on blood pressure and maternal and neonatal complications. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(12):1456‐60. [DOI] [PubMed] [Google Scholar]
Italy 2012 {published data only}
- Vasapollo B, Novelli GP, Gagliardi G, Tiralongo GM, Pisani I, Manfellotto D, et al. Medical treatment of early‐onset mild gestational hypertension reduces total peripheral vascular resistance and influences maternal and fetal complications. Ultrasound in Obstetrics & Gynecology 2012;40(3):325‐31. [DOI] [PubMed] [Google Scholar]
Japan 1997 {published data only}
- Makino Y, Izumi H, Makino I, Shirakawa K. The effect of nitric oxide on uterine and umbilical artery flow velocity waveform in pre‐eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;73(2):139‐43. [DOI] [PubMed] [Google Scholar]
Japan 2016b {published data only}
- Koneoh E, JPRN‐UMIN000020686. Statin therapy for preeclampsia. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000023870 (date received 22 January 2016).
Japan 2017 {published data only}
- JPRN‐UMIN000027270. A study on prevention of hypertensive disorders of pregnancy who have a history of severe hypertensive disorders of pregnancy ‐pre‐test for multi center trial. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000031037 (first received 15 May 2017).
Kuwait 1995 {published data only}
- El‐Qarmalawi AM, Morsy AH, Al‐Fadly A, Obeid A, Hashem M. Labetalol vs methyldopa in the treatment of pregnancy‐induced hypertension. International Journal of Gynecology & Obstetrics 1995;49:125‐30. [DOI] [PubMed] [Google Scholar]
Mexico 2008 {published data only}
- Lopez‐Molina K, Gonzalez‐Altamirano JC, Valdivia‐Silva JE. Early L‐arginine therapy improves notably the fetal growth in preeclamptic women. A randomized controlled trial. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA 2008:Abstract no: 801.
Netherlands 2014 {published data only}
- EUCTR2014‐002524‐27‐NL. A randomized, placebo‐controlled, double blind, 4‐period, cross‐over trial, to study blood pressure lowering effects of losartan, Moxonidine and Low sodium diet in former pre‐eclamptic women ‐ PALM study. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2014‐002524‐27 (first received 18 December 2014).
Pakistan 1994 {published data only}
- Gilani A, Khan Z. Role of aspirin in management of pregnancy induced hypertension. A study in Pakistani population. Specialist 1994;10(4):323‐5. [Google Scholar]
Panama 2012 {published data only}
- Reyes OA, NCT01538875. Hydralazine vs. labetalol for the management of hypertensive crisis in patients with hypertensive disorders of pregnancy. a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01538875 (first received 24 February 2012).
Philippines 2000 {published data only}
- Decano MB, Cabrera LT. The effects of transdermal nitroglicerin (nitrol patch) on the uterine and umbilical artery blood flow in preeclampsia: a randomized double blind placebo controlled study. XVI Figo World Congress of Obstetric and Gynecology (Book 1); 2000 Sept 3‐8; Washington DC, USA. 2000:26.
Russia 1993 {published data only}
- Osadchaia OV, Nazarenko LG, Bobritskaia VV. The clinical and hemodynamic aspects of using peripheral vasodilators in hypertension in pregnant women [Klinicheskie i gemodinamicheskie aspekty primeneniia perifericheskikh vazodilatorov pri gipertenzii beremennykh]. Akusherstvo i Ginekologia 1993;1993(2):16‐20. [PubMed] [Google Scholar]
Singapore 1996 {published data only}
- Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Randomised controlled trial of methyldopa and isradipine in preeclampsia ‐ effects on uteroplacental and fetal hemodynamics. Journal of Perinatal Medicine 1996;24:177‐84. [DOI] [PubMed] [Google Scholar]
- Wide‐Swensson D, Montan S, Arulkumaran S, Ingemarsson I, Ratnam SS. Effect of methyldopa and isradipine on fetal heart rate pattern assessed by computerized cardiotocography in human pregnancy. American Journal of Obstetrics and Gynecology 1993;169:1581‐5. [DOI] [PubMed] [Google Scholar]
Singapore 1998 {published data only}
- Yin KH, Koh SC, Malcus P, Montan S, Biswas A, Arulkumaran S, et al. Preeclampsia: haemostatic status and the short‐term effects of methyldopa and isradipine therapy. Journal of Obstetrics and Gynaecology Research 1998;24(3):231‐8. [DOI] [PubMed] [Google Scholar]
Slovakia 2002 {published data only}
- Sirotiaková J, Mlyncek M, Kriska M, Letkovicová M. Acebutolol in the treatment of arterial hypertension in pregnancy‐‐comparison with commonly used hypertensive agents [Acebutolol v liecbe arteriovej hypertenzie v gravidite‐‐porovnanie s bezne uzivanymi antihypertenzivami]. Ceska Gynekologie 2002;67(1):8‐15. [PubMed] [Google Scholar]
South Africa 1988 {published data only}
- Lindow SW, Davies N, Davey DA, Smith JA. The effect of sublingual nifedipine on uteroplacental blood flow in hypertensive pregnancy. British Journal of Obstetrics and Gynaecology 1988;95:1276‐81. [DOI] [PubMed] [Google Scholar]
South Africa 1990 {published data only}
- Anthony J, Rees AE, Davey DA. Indoramin in the treatment of pregnancy hypertension. South African Medical Journal 1990;78:458‐61. [PubMed] [Google Scholar]
South Africa 1991b {published data only}
- Puzey MS, Ackovic KL, Lindow SW, Gonin R. The effect of nifedipine on fetal umbilical artery Doppler waveforms in pregnancies complicated by hypertension. South African Medical Journal 1991;79:192‐4. [PubMed] [Google Scholar]
South Africa 1997 {published data only}
- Steyn DW, Odendaal HJ. Blood pressure patterns in pregnant patients on oral ketanserin. Cardiovascular Journal of Southern Africa 2001;12(2):82‐7. [PubMed] [Google Scholar]
- Steyn DW, Odendaal HJ. Fetal heart baseline variability in patients on oral ketanserin. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy;1996 August 4‐8; Seattle, Washington, USA. 1996:171.
- Steyn DW, Odendaal HJ. Maternal outcome six years after presenting with mild to moderate mid‐trimester hypertension during pregnancy. 21st Conference on Priorities in Perinatal Care in South Africa; 2002 March 5‐8; Eastern Cape, South Africa. 2002.
- Steyn DW, Odendaal HJ. Maternal outcome six years after presenting with mild to moderate mid‐trimester hypertension during pregnancy. Hypertension in Pregnancy 2002;21(Suppl 1):9. [Google Scholar]
- Steyn DW, Odendaal HJ. Oral ketanserin in mild midtrimester hypertension ‐ a randomised controlled trial. Proceedings of the 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:88‐9.
- Steyn DW, Odendaal HJ. Oral ketanserin plus aspirin decrease the incidence of proteinuric hypertension and perinatal mortality in patients with mild midtrimester hypertension. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy;1996 August 4‐8; Seattle, Washington, USA. 1996:131.
- Steyn DW, Odendaal HJ. Randomised controlled trial of ketanserin and aspirin in prevention of pre‐eclampsia. Lancet 1997;350:1267‐71. [DOI] [PubMed] [Google Scholar]
- Steyn DW, Odendaal HJ. The effect of oral ketanserin on fetal heart rate parameters. Journal of Maternal Fetal Investigation 1998;8:126‐9. [PubMed] [Google Scholar]
- Steyn DW, Odendaal HJ, Kirsten GF. Mental development in children six years after in utero exposure to ketanserin ‐ a follow‐up study of a randomized controlled trial. Hypertension in Pregnancy 2002;21(Suppl 1):131. [Google Scholar]
South Africa 2004 {published data only}
- Warren J, Lacoursiere Y, Varner M, Silver R, Anthony J, Belfort M. First interim report on the Labetalol versus Magnesium Sulfate for the prevention of Eclampsia Trial (LAMPET). Hypertension in Pregnancy 2004;23(Suppl 1):9. [Google Scholar]
South Africa 2015 {published data only}
- Cluver CA, Walker SP, Mol BW, Theron GB, Hall DR, Hiscock R, et al. Double blind, randomised, placebo‐controlled trial to evaluate the efficacy of esomeprazole to treat early onset pre‐eclampsia (PIE Trial): a study protocol. BMJ Open 2015;5(10):e008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
South Africa 2016 {published data only}
- Cluver C, PACTR201608001752102. Pre‐eclampsia Intervention 2 (PI2) trial: a double blind randomised control trial of metformin for the treatment of early onset pre‐eclampsia. http://www.pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201608001752102 (first received 23 August 2016).
Sri Lanka 1994 {published data only}
- Jayawardana J, Lekamge N. A comparison of nifedipine with methyldopa in pregnancy induced hypertension. Ceylon Medical Journal 1994;39:87‐90. [PubMed] [Google Scholar]
Sweden 1992 {published data only}
- Montan S, Ingemarsson I, Marsál K, Sjöberg NO. Negative influence of atenolol compared with pindolol on fetal hemodynamics in pregnancies with hypertension ‐ a controlled study. Proceedings of 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:192.
- Montan S, Ingemarsson I, Marsál K, Sjöberg NO. Randomised controlled trial of atenolol and pindolol in human pregnancy: effects on fetal haemodynamics. BMJ 1992;304:946‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweden 1993 {published data only}
- Hjertberg R, Belfrage P, Faxelius G, Lagercrantz H. Neonatal outcome in infants <1500g, a randomized study of labetalol vs dihydralazine‐treated hypertensive pregnant women. Proceedings of the 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:361.
- Hjertberg R, Faxelius G, Belfrage P. Comparison of outcome of labetalol or hydralazine therapy during hypertension in pregnancy in very low birth weight infants. Acta Obstetricia et Gynecologica Scandinavica 1993;72:611‐5. [DOI] [PubMed] [Google Scholar]
- Hjertberg R, Faxelius G, Lagercrantz H. Neonatal adaptation in hypertensive pregnancy ‐ a study of labetalol vs hydralazine treatment. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:18. [DOI] [PubMed]
- Hjertberg R, Faxelius G, Lagercrantz H. Neonatal adaption in hypertensive pregnancy ‐ a study of labetalol vs hydralazine treatment. Journal of Perinatal Medicine 1993;21:69‐75. [DOI] [PubMed] [Google Scholar]
Uganda 1992 {unpublished data only}
- Lule J. Aspirin and methyldopa for moderate hypertension in pregnancy. Personal communication June 6 1992.
UK 1978 {published data only}
- Redman CW. A controlled trial of the treatment of hypertension in pregnancy: labetalol compared with methyldopa. The investigation of labetalol in the management of hypertension in pregnancy. International Congress Series 591. Amsterdam: Excerpta Medica, 1978:101‐10. [Google Scholar]
- Redman CW. Controlled trials of treatment of hypertension during pregnancy. Proceedings of the 8th International Congress of Nephrology; 1981; Athens, Greece. 1981:523‐4.
UK 1991 {published data only}
- Harper A, Murnaghan GA. Maternal and fetal haemodynamics in hypertensive pregnancies during maternal treatment with intravenous hydralazine or labetalol. British Journal of Obstetrics and Gynaecology 1991;98:453‐9. [DOI] [PubMed] [Google Scholar]
- Harper A, Murnaghan GA, Erskine L, Ritchie JK. Response of fetal umbilical artery time velocity waveforms to antihypertensive drugs. Proceedings of the 24th British Congress of Obstetrics and Gynaecology;1986 April 15‐18; Cardiff, UK. 1986:33.
UK 2015 {published data only}
- EUCTR2016‐005206‐19‐BE. Randomised controlled trial with pravastatin versus placebo for prevention of preeclampsia ‐ STATIN. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016‐005206‐19‐BE (first received 7 June 2017).
- Poon L, ISRCTN17787139. Randomised controlled trial with pravastatin versus placebo for prevention of preeclampsia. isrctn.com/ISRCTN17787139 (first received 11 November 2015).
USA 1957 {published data only}
- Landesman R, McLarn WD, Ollstein RN, Mendelsohn B. Reserpine in toxemia of pregnancy. Obstetrics & Gynecology 1957;9:377‐83. [PubMed] [Google Scholar]
USA 1981 {published data only}
- Welt SI, Dorminy JH, Jelovsek FR, Crenshaw MC, Gall SA. The effect of prophylactic management and therapeutics on hypertensive disease in pregnancy: preliminary studies. Obstetrics & Gynecology 1981;57:557‐65. [PubMed] [Google Scholar]
USA 1990b {unpublished data only}
- Weiner CP. Trial to evaluate oral hypotensive agents in early onset preeclampsia. Personal communication November 1990.
USA 1991 {published data only}
- Thaler I, Wiener Z, Manor D, Itskovitz J. Effect of calcium channel blocker nifedipine on uterine artery flow velocity waveforms. Journal of Ultrasound in Medicine 1991;10(6):301‐4. [DOI] [PubMed] [Google Scholar]
USA 2005 {published data only}
- August P. Treatment targets for chronic hypertension in pregnancy. http://clinicaltrials.gov/ct2/show/record/NCT00194974 (accessed 17 May 2013) 2005.
USA 2006 {published data only}
- Hameed HB, NCT00329511. A comparison of compliance between clonidine patch and methyldopa for the treatment of chronic hypertension in pregnancy. clinicaltrials.gov/show/NCT00329511 (first received 22 May 2006).
USA 2016b {published data only}
- Caritis S. Pravastatin for the prevention of preeclampsia in high‐risk women. clinicaltrials.gov/ct2/show/NCT01717586 (first received 3 January 2012).
- Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high‐risk pregnant women: a pilot randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(6):720.e1‐720.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venezuela 1985 {published and unpublished data}
- Sánchez S, Zschaeck D, Alfonso C, González I, Kalbakdij B. Labetalol in the treatment of hypertension associated with pregnancy [Labetalol en el tratamiento de la hipertensión arterial asociada al embarazo]. Centro Médico 1985;24(81):101‐9. [Google Scholar]
Venezuela 1997 {published data only}
- González JC, Andolcetti R. Labetalol vs. methyldopa in the treatment of hypertension in pregnancy [Labetalol vs metildopa en el tratamiento de la hipertensión arterial asociada con el embarazo]. Boletín Médico de Postgrado 1997;13(1):3‐8. [Google Scholar]
Venezuela 2001 {published data only}
- Reyna‐Villasmil E, Prieto‐Franchi M, Guerra‐Velázquez M, Torres‐Montilla M. Effect of transdermal nitroglycerin on umbilical artery blood flow in preeclampsia (abstract). Journal of Perinatal Medicine 2001;29(Suppl 1):486. [Google Scholar]
Venezuela 2007 {published data only}
- Centeno DM, Urbina JC, Las Salas M, Fuenmayor AJ. Effect of carvedilol in the treatment of hypertension in pregnant women. XLIV ERA‐EDTA Congress; 2007 June 21‐24; Barcelona, Spain. 2007:Poster no: FP078.
References to ongoing studies
Canada 2006 {published data only}
- Smith G, ISRCTN45790835. Use of a nitric oxide (ISMN) for the prevention and management of pre‐eclampsia (pilot study). isrctn.com/ISRCTN45790835 (first received 29 November 2006).
Egypt 2017a {published data only}
- Rezk M, PACTR201707002463243. Methyldopa versus labetalol or no medication for treatment of chronic hypertension during pregnancy: a double blinded randomized clinical trial. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201707002463243 (first received 24 July 2017).
Egypt 2017b {published data only}
- Abdallah F, NCT03262961. Use of sildenafil citrate in management of mild pre‐eclampsia: a randomized controlled trial. clinicaltrials.gov/show/NCT03262961 (first received 19 August 2017).
Japan 2016a {published data only}
- Kubo M, JPRN‐UMIN000024042. A multicenter phase II trial of the efficacy and safety of tadalafil with pre‐eclampsia. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000027686 (first received 15 September 2016).
Netherlands 2015 {published data only}
- Spaandermann M, NCT02531490. Personalized hemodynamically guided antihypertensive treatment in pregnant women with mild to moderate hypertension: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02531490 (first received 5 August 2015).
USA 2014 {published data only}
- Tita A, NCT02299414. A pragmatic multicenter randomized clinical trial (RCT) of antihypertensive therapy for mild chronic hypertension during pregnancy: chronic hypertension and pregnancy (CHAP) project. clinicaltrials.gov/ct2/show/record/NCT02299414 (first received 28 October 2014).
USA 2016a {published data only}
- Roberts R, NCT02782559. Randomized controlled trial to assess efficacy of Sildenafil in addition to expectant management for the treatment of preterm preeclampsia. clinicaltrials.gov/ct2/show/NCT02782559 (first received 11 February 2016).
USA 2017 {published data only}
- Towers CV, NCT03245970. Use of impedance cardiography to decrease the risk of preeclampsia. https://clinicaltrials.gov/ct2/show/NCT03245970 (first received 8 August 2017).
Additional references
Abalos 2013
- Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;170(1):1‐7. [DOI] [PubMed] [Google Scholar]
Abalos 2014a
- Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, et al. on behalf of the WHO Multicountry Survey on Maternaland Newborn Health Research Network. Pre‐eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World HealthOrganization Multicountry Survey on Maternal and Newborn Health. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 1):14‐24. [DOI] [PubMed] [Google Scholar]
ACOG Task Force 2013
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and Gynecology 2013;122(5):1122‐31. [DOI] [PubMed] [Google Scholar]
Canadian HDP Working Group 2014
- Magee LA, Pels A, Helewa M, Rey E, Dadelszen P, Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertension 2014;4(2):105‐45. [DOI] [PubMed] [Google Scholar]
Churchill 2007
- Churchill D, Beevers GD, Meher S, Rhodes C. Diuretics for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 1999
- Duley L, Henderson‐Smart DJ. Reduced salt intake compared to normal dietary salt, or high intake, in pregnancy. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2013
- Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD001449.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frishman 1979
- Frishman W. Clinical pharmacology of the new beta‐adrenergic drugs. Part 1. Pharmacodynamic and pharmacokinetic properties. American Heart Journal 1979;97:663‐70. [DOI] [PubMed] [Google Scholar]
Frishman 1995
- Frishman WH, Huberfeld S, Okin S, Wang Y‐H, Kumar A, Shareef B. Serotonin and serotonin antagonism in cardiovascular and non‐cardiovascular disease. Journal of Clinical Pharmacology 1995;35:541‐72. [DOI] [PubMed] [Google Scholar]
Gifford 1990
- Gifford RW, August P, Chesley LC, Cunningham G, Ferris TF, Lindheimer MD, et al. National high blood pressure education program working group report on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 1990;163:1691‐712. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2012
- Hofmeyr GJ. Abdominal decompression for suspected fetal compromise/pre‐eclampsia. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000004.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingenito 1970
- Ingenito AJ, Barrett JP, Procita L. A centrally mediated peripheral hypotensive effect of methyldopa. Journal of Pharmacology and Experimental Therapeutics 1970;175:593‐9. [PubMed] [Google Scholar]
Isaac 1980
- Isaac L. Clonidine in the central nervous system: site of mechanism of hypotensive action. Journal of Cardiovascular Pharmacology 1980;2(1):S5‐S19. [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066–74. [DOI] [PubMed] [Google Scholar]
Magee 2013
- Magee L, Dadelszen P. Prevention and treatment of postpartum hypertension. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD004351.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, on behalf of the Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Meher 2005a
- Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003514.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHBPEP 2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183(Suppl 1):S1‐S22. [PubMed] [Google Scholar]
NICE 2011
- NICE. Hypertension in pregnancy: The management of hypertensive disorders during pregnancy. NICE Clinical Guideline 107. Manchester, UK: National Institute for Health and Clinical Excellence, 2011:1‐53. [Google Scholar]
Redman 1993
- Redman CWG, Roberts JM. Management of pre‐eclampsia. Lancet 1993;341:1451‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1980
- Robinson BF, Dobbs RJ, Kelsey CR. Effects of nifedipine on resistance vessels, arteries and veins in man. British Journal of Clinical Pharmacology 1980;10:433‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seligman 1994
- Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. American Journal of Obstetrics and Gynecology 1994;171(4):944‐8. [DOI] [PubMed] [Google Scholar]
Stunkard 1954
- Stunkard A, Wertheimer L, Redisch W. Studies on hydralazine; evidences for a peripheral site of action. Journal of Clinical Investigation 1954;33:1047‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornton 2004
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M, GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004;364(9433):513‐20. [DOI] [PubMed] [Google Scholar]
von Dadelszen 2000
- Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta‐analysis. Lancet 2000;355:87‐92. [DOI] [PubMed] [Google Scholar]
Wareing 2004
- Wareing M, Myers JE, O'Hara M, Kenny LC, Warren AY, Taggart MJ, et al. Effects of a phosphodiesterase‐5 (PDE5) inhibitor on endothelium‐dependent relaxation of myometrial small arteries. American Journal of Obstetrics and Gynecology 2004;190(5):1283‐90. [DOI] [PubMed] [Google Scholar]
WHO 2011
- WHO. WHO recommendations for the prevention and treatment of pre‐eclampsia and eclampsia. WHO recommendations for the prevention and treatment of pre‐eclampsia and eclampsia. Geneva: WHO press, World Health Organization, October 2011. [PubMed] [Google Scholar]
References to other published versions of this review
Abalos 2001
- Abalos E, Duley L, Steyn DW, Henderson‐Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002252] [DOI] [PubMed] [Google Scholar]
Abalos 2007
- Abalos E, Duley L, Steyn DW, Henderson‐Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002252.pub2] [DOI] [PubMed] [Google Scholar]
Abalos 2014
- Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD002252.pub3] [DOI] [PubMed] [Google Scholar]


