Abstract
Background:
Heavy metal contamination is widespread in Bangladesh. Previous studies have observed lead increases blood pressure over time. However, the role of other metal contaminants and essential micronutrients, which could also adversely affect blood pressure or act as protective factors, is understudied.
Objectives:
We therefore evaluated the associations of lead, manganese, and selenium with blood and pulse pressure trajectories.
Methods:
We prospectively followed placebo-assigned participants nested within a randomized trial for the prevention of arsenic-related skin cancer (n=255). Blood lead, manganese, and selenium were measured at baseline; blood pressure was measured at baseline and at 3 biennial follow-up examinations. Mixed-effect linear regression models were used to estimate associations with average annual changes in systolic, diastolic, and pulse pressure.
Results:
In models simultaneously adjusted for baseline blood lead, manganese, and selenium concentrations in addition to other potential confounders, lead was linearly associated with increases in systolic blood pressure, but not with diastolic blood pressure or pulse pressure. A non-linear association was observed for manganese, such that mid-range concentrations were associated with decreases in systolic, diastolic, and pulse pressure. Baseline selenium concentrations in the highest quartile were also associated with longitudinal decreases in both systolic and diastolic blood pressure, while null associations were observed with pulse pressure. In exploratory analyses, the combination of mid-range manganese and high selenium concentrations completely offset lead-associated increases in blood and pulse pressure.
Conclusions:
The results indicate a direct, linear association of lead exposure with systolic blood pressure, and manganese and selenium exposures within certain ranges may have a blood pressure-lowering effect in this population.
Keywords: Toxic metals, essential metals, blood pressure, pulse pressure, Bangladesh
Capsule:
The findings of this study suggest toxic lead exposures remain a significant public health problem in Bangladesh where high levels are associated with high blood pressure; however, the nutritionally essential micronutrients manganese and selenium appear to confer protection from the hypertensive effects of lead.
Introduction
Lead contamination is a major public health problem in Bangladesh where unsafe levels have been recorded in drinking water supplies and in ambient air (Frisbie et al. 2002; Salam et al. 2008). Leaded gasoline was banned in 1999, but background contamination persists (Begum et al. 2013). In addition, leaded paint and industrial emissions are widespread (Mitra et al. 2009). Toxic metal exposures are increasingly being recognized as potentially important risk factors for the development of cardiovascular disease (Lamas et al. 2016). For lead in particular, increases in blood pressure and hypertension are among the most widely studied clinical manifestations (Navas-Acien et al. 2007). The evidence suggests that even low-level exposures to lead (i.e., blood lead concentrations below 10 μg/dL) have a hypertensive effect (Gambelunghe et al. 2016).
In contrast to toxic metals, other metals are needed at trace amounts for normal physiologic functioning. Manganese is one such essential micronutrient that can be a toxicant when exposures are excessive. Recent studies have observed associations of manganese body burden (as measured in blood and toenails) and recent exposure (as measured from dietary recalls) with blood pressure (Choi and Bae 2013; Lee and Kim 2011; Mordukhovich et al. 2012). However, these studies have been limited by cross-sectional designs and have found inconsistent patterns. Like lead, elevated concentrations of manganese have been documented in Bangladesh’s groundwater (Frisbie et al. 2002). In general though, the most common sources of manganese are dietary, with grains and vegetables primary contributors to dietary intakes (Institute of Medicine 2001). Selenium is another antioxidant trace element that may play a role in the development of cardiovascular disease (Institute of Medicine 2000). A cross-sectional analysis of serum selenium concentrations observed a positive relationship with hypertension, but the opposite was found in a prospective study evaluating selenium in whole blood (Laclaustra et al. 2009; Nawrot et al. 2007).
Given both the essentiality and potential toxicity of manganese and selenium, previous studies may have observed conflicting results by assuming linear relationships with blood pressure, or because different study populations may be at differing points of dose-response curves. In addition, there is a lack of prospective data for the manganese-blood pressure relationship. To date, it is unclear how these micronutrients affect blood pressure over time, or if joint exposures with lead could modify any effects. To that end, we evaluated the longitudinal associations of lead, manganese, and selenium with blood pressure in a prospective cohort of Bangladeshi adults.
Materials and Methods
Study Population
The Bangladesh Vitamin E and Selenium Trial (BEST) participants are residents of rural communities in Bangladesh enrolled in a 2×2 factorial, randomized controlled trial of 7,000 adults having manifest arsenical skin lesions, designed to evaluate the efficacy of vitamin E and selenium supplementation as chemopreventive agents. Enrollment procedures are described elsewhere (Argos et al. 2013). Briefly, individuals were permanent residents in the arsenic-endemic study area (Araihazar districts of Narayanganj, Comilla, Noakhali, and the Matlab district of Chandpur), and provided signed informed consent. Individuals who were pregnant, unwilling to discontinue current vitamin use, had a prior history of cancer, were too ill to participate, or were unwilling to provide blood and urine samples were excluded. Participants were randomized between April 2006 and August 2009 and underwent biennial follow-up clinical examinations for a 6-year period. For the purposes of this study, we restricted analyses to participants randomized to the placebo arm (n=1,753).
Biomarkers of Exposure
Of placebo-assigned participants enrolled in the study, 255 were randomly selected for blood measurements of lead, manganese, and selenium at the baseline examination. Non-fasting venous blood samples were collected in 10 mL vacutainer tubes, stored in portable 4°C coolers immediately after collection, and processed within 2 to 8 hours of collection in the field laboratory. Upon receipt, samples were stored at −80°C until analysis. Samples were thawed, thoroughly mixed, and diluted 50 times. Concentrations of lead, manganese, and selenium were measured in whole blood using ICP Mass Spectrophotometer, model ELAN DRC II, manufactured by Perkin Elmer. Detection limits were 0.02 μg/dL for lead, 0.4 μg/L for manganese, and 0.4 μg/L for selenium. Inter-assay coefficients of variation were 2.4 to 4.7 for lead, 5.1 to 7.8 for manganese, and 4.3 to 4.6 for selenium. Intra-assay coefficients of variation were 1.9 to 2.0 for lead, 2.7 to 2.8 for manganese, and 1.4 to 2.4 for selenium. Since BEST was designed as a clinical trial for the prevention of non-melanoma skin cancer among individuals with visible arsenic toxicity, urinary arsenic concentrations were additionally measured. After enrollment to the trial, participants whose primary drinking water source contained unsafe levels of arsenic were provided with a filter at enrollment to reduce their exposure.(Argos et al. 2013) We therefore considered arsenic exposure to be a potential confounder, rather than a main exposure of interest.
Blood Pressure, Other Clinical Parameters, and Sociodemographic Factors
General clinical examinations of participants were conducted at baseline, 2-, 4-, and 6-years follow-up by trained study physicians. Blood pressure and anthropomorphic measurements were collected following standard protocols (Argos et al. 2013). At each examination, after a 5 minute period of rest, the study physician obtained two seated blood pressure measurements using an automated sphygmomanometer. The average systolic and diastolic value at each examination was calculated for use in subsequent analyses; pulse pressure was calculated as the difference between the average systolic and diastolic blood pressure measurements at each visit. Body weight was measured to the nearest kilogram at baseline and every biennial clinical evaluation. Height was measured at baseline and recorded to the nearest centimeter.
A health and lifestyle questionnaire that included demographic characteristics (age, sex, educational duration, among others), medication use, and health history, was administered by the study physician in Bengali. Participants were specifically asked about all prescription and over-the-counter medications regularly taken and were asked to show the medications or prescriptions to the study physician. Medications were standardized to generic names then sorted into categories allowing for the identification of antihypertensive and antidiabetes medication. At the last follow-up visit, blood specimens from all participants were measured for glycated hemoglobin (HbA1c) in the field laboratory on a Lambda UV/ViS spectrophotometer (Perkin-Elmer, Waltham, MA). Using these sources of information, we defined diabetes as either self-report of a physician diagnosis, antidiabetes medication use, or HbA1c at or above 6.1%. The 6.1% cutpoint, which is lower than the 6.5% value recommended by the American Diabetes Association, was selected based on prior studies of HbA1c within South Asian populations (Bhowmik et al. 2016; Mohan et al. 2010).
Statistical Analyses
Analyses were confined to the random subset of placebo-assigned participants with measured concentrations of lead, manganese, and selenium at baseline (255 of 1,753 individuals). Mixed-effects regression models of systolic, diastolic, and pulse pressure were performed separately. The general model forms were as follows:
where BPij is the blood (or pulse) pressure at time j for the ith individual. Xi0 is a row vector of the baseline biomarker(s), which were modeled using dummy variables for quartiles of exposure with the lowest serving as the referent category. Time is the duration between the baseline examination and the blood pressure measurement (i.e., 0, 2, 4, or 6 years). The β coefficients for Xi0(Timeij) are the estimated annual changes in blood or pulse pressure corresponding to the respective biomarker quartile. Zij is a matrix of potential confounders, representing either covariates measured at baseline or at each examination (i.e., time-varying). μi0 is a random intercept for each individual used to account for the within-subject correlation of the repeated blood pressure measures, and eij is the error term.
Potential confounders were selected based on a priori knowledge of factors associated with exposures to metals and blood pressure. All models were sex- and age-adjusted. Single biomarker models were performed to evaluate the individual effect of each biomarker on changes in blood pressure. Mutually adjusted models included baseline blood concentrations of lead, manganese, and selenium to control for confounding by co-exposures. A third multivariable model was constructed to further adjust for study site (Araihazar or Matlab), smoking status (current, former, or never), educational duration (years), creatinine-corrected urinary arsenic concentration (μg/g). Fully-adjusted models included all previously mentioned covariates with the addition of time-varying body mass index, time-varying diabetes status, and time-varying antihypertensive medication use, which were conceptualized as potential intermediates of metal exposures-blood pressure trajectory associations. Indicator variables were used to model categorical variables, while continuous variables (i.e., age, educational duration, urinary arsenic concentrations, and body mass index) were modeled as restricted cubic splines with knots at the 10th, 50th, and 90th percentiles to allow for non-linearity. Tests for linear trends in the longitudinal associations between the biomarkers and blood pressure were conducted by modeling concentration quartiles as ordinal variables. All analyses were conducted in Stata version 14.2 (College Station, TX).
Joint Associations
We performed an exploratory analysis to evaluate joint associations of lead, manganese, and selenium with blood and pulse pressure trajectories. For each metal, we identified the quartile associated with the greatest change in blood or pulse pressure over time based on regression coefficients from our primary analyses, including both the single-biomarker and multiple-biomarker models. Participants were then classified as having baseline metal concentrations within or outside of these ranges and divided participants into mutually exclusive groups reflecting combinations of exposure levels. Mixed-effects regression models were used to estimate annual changes in systolic blood pressure, diastolic blood pressure, and pulse pressure and 95% confidence interval (95% CI) associated with each group, accounting for covariates.
Sensitivity Analyses
Because antihypertensive medications can lower blood pressure, we conducted sensitivity analyses to evaluate the robustness of our results. First, we repeated analyses excluding individuals who reported antihypertensive medication use at baseline or at any follow-up visit. Second, we added a constant value (10 mmHg for systolic, 5 mmHg for diastolic) to blood pressure among those using antihypertensive medications, and reanalyzed the corrected blood and pulse pressure measurements (Tobin et al. 2005).
In addition to analyzing blood pressure as a continuous variable, we also evaluated hypertensive status in order to gain insights into the clinical relevance of lead, manganese, and selenium exposures. We defined hypertension as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, antihypertensive medication use, or self-reported physician diagnosed hypertension. Participants classified as hypertensive at the baseline examination were excluded from these analyses (n=77). Among the remaining 178 participants, exposure biomarker quartiles were redefined. Discrete-time hazard models were used to estimate hazard ratios with 95% confidence intervals for hypertension incidence (Chizmar 2000). The models were based on the probability of becoming hypertensive during each two-year follow-up period, conditional on being normotensive during the prior follow-up interval. Participants who were lost to follow-up were censored; participants who remained normotensive were additionally censored at the end of study participation. The proportional-hazards assumption was checked by testing interaction terms with time. The confounders included in the discrete-time hazard models were the same as those in our main analyses.
Results
Of the study participants, 234 (91.8%) had all four blood pressure measurements (baseline and 3 follow-ups), 16 had three measurements (baseline and 2 follow-ups), and 5 individuals had two measurements (baseline and 1 follow-up). On average, systolic and diastolic blood pressures were relatively stable over the study period, while antihypertensive medication use increased (Table 1). All participants were found to have detectable levels of arsenic in their urine, and lead, manganese, and selenium in their blood. Overall, median blood concentrations at baseline were 8.5 μg/dL for lead, 10.0 μg/L for manganese, and 122.0 μg/L for selenium. Lead concentrations were weakly positively correlated with manganese (Pearson correlation = 0.13, p-value = 0.04) and selenium concentrations (Pearson correlation = 0.21, p-value < 0.001). Blood concentrations of selenium and manganese concentrations were not correlated (Pearson correlation = 0.03, p-value = 0.67). Table 2 describes how concentrations differed by sociodemographic and clinical characteristics. Levels of blood lead were greater among individuals from Araihazar, males, and current and former smokers. Participants with greater concentrations of manganese in their blood at baseline tended to be women, ages 25–37 years, and former or never smokers. Selenium concentrations at baseline were higher among males and were positively associated with body mass index.
Table 1.
Blood pressure, pulse pressure, and antihypertensive medication use over time
| N | Systolic blood pressure (mmHg) Mean ± SD | Diastolic blood pressure (mmHg) Mean ± SD | Pulse pressure (mmHg) Mean ± SD | Antihypertensive medication use n (%) | |
|---|---|---|---|---|---|
| Baseline | 255 | 119 ± 16 | 78 ± 10 | 41 ± 11 | 19 (7.5) |
| Visit 1 | 255 | 115 ± 17 | 76 ± 10 | 40 ± 11 | 22 (8.6) |
| Visit 2 | 248 | 113 ± 17 | 74 ± 11 | 39 ± 11 | 27 (10.9) |
| Visit 3 | 236 | 118 ± 18 | 77 ± 10 | 40 ± 12 | 36 (15.3) |
Table 2.
Blood concentrations by participant characteristics at baseline
| N | Lead (μg/dL) | Manganese (μg/L) | Selenium (μg/L) | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR)a | p-value | Median (IQR)a | p-value | Median (IQR)a | p-value | ||
| Overall | 255 | 8.5 (4.2–13.8) | 10.0 (8.2–12.4) | 122.0 (111.0–136.0) | |||
| Study site | <0.01 | 0.19 | 0.45 | ||||
| Araihazar | 155 | 11.9 (8. 9–15.4) | 9.7 (8.2–12.3) | 123.0 (111.0–139.0) | |||
| Matlab | 100 | 3.5 (2.6–5.1) | 10.3 (8.4–13.1) | 122.0 (110.0–136.0) | |||
| Gender | <0.01 | <0.01 | 0.02 | ||||
| Female | 140 | 5.8 (3.3–10.5) | 10.8 (8. 6–13.8) | 121.0 (107.0–134.5) | |||
| Male | 115 | 11.6 (5.9–15.1) | 9.1 (7.9–11.0) | 125.0 (114.0–140.0) | |||
| Age (years) | 0.50 | 0.01 | 0.33 | ||||
| 25–37 | 88 | 8.0 (3.6–13.5) | 10.7 (8. 7–13.8) | 121.5 (111.5–132.5) | |||
| 38–46 | 82 | 8.1 (4.3–14.4) | 9.4 (8.2–11.7) | 127.5 (109.0–140.0) | |||
| 47–64 | 85 | 9.2 (5.0–12.8) | 9.4 (7.8–12.0) | 120.0 (110.0–130.0) | |||
| Smoking status | <0.01 | 0.01 | 0.59 | ||||
| Current | 60 | 11.9 (6. 1–15.1) | 9.2 (7.8–10.6) | 125.0 (112.5–140.0) | |||
| Former | 24 | 10.9 (5.4–16.7) | 10.2 (8.3–12.5) | 120.0 (112.5–140.5) | |||
| Never | 171 | 7.1 (3.5–11.8) | 10.3 (8.4–13.1) | 122.0 (110.0–136.0) | |||
| Education duration (years) | 0.21 | 0.86 | 0.85 | ||||
| 0 | 101 | 9.9 (5.0–14.4) | 9.8 (8.2–12.4) | 122.0 (110.0–139.0) | |||
| 1–5 | 83 | 7.8 (3.9–12.8) | 10.0 (8.6–12.3) | 122.0 (110.0–138.0) | |||
| 6–15 | 71 | 7.4 (3.7–13.4) | 10.1 (8.1–12.3) | 124.0 (116.0–134.0) | |||
| Urinary arsenic (μg/g creatinine) | <0.01 | 0.02 | <0.01 | ||||
| 22–99 | 66 | 10.4 (7.1–15.3) | 8.7 (7.7–11.5) | 128.0 (121.0–140.0) | |||
| 100–399 | 90 | 10.0 (5.0–14.8) | 10.0 (8.4–12.9) | 125.5 (113.0–141.0) | |||
| 400–2240 | 99 | 5.4 (2.8–10.3) | 10.4 (8.6–12.5) | 117.0 (104.0–128.0) | |||
| Body mass index (kg/m2) | 0.07 | 0.32 | <0.01 | ||||
| 17.0–18.6 | 101 | 7.6 (3.5–11.9) | 10.1 (8. 7–12.3) | 117.0 (107.0–128.0) | |||
| 18.7–21.5 | 83 | 7.4 (4.2–14.4) | 10.1 (8.1–12.9) | 125.0 (118.0–143.0) | |||
| 21.6–32.9 | 71 | 9.7 (5.6–14.9) | 9.4 (8.0–11.8) | 129.0 (118.0–143.0) | |||
| Diabetes | 0.69 | 0.05 | 0.13 | ||||
| No | 251 | 8.5 (4.2–13.8) | 10.1 (8. 3–12.4) | 122.0 (110.0–136.0) | |||
| Yes | 4 | 9.9 (7.2–12.3) | 8.2 (7.9–8.2) | 135.0 (127.5–141.5) | |||
| Antihypertensive medication use | 0.31 | 0.27 | 0.87 | ||||
| No | 236 | 8.7 (4.3–13.9) | 10.1 (8. 3–12.4) | 122.0 (111.0–136.0) | |||
| Yes | 19 | 6.4 (4.2–10.0) | 9.0 (8.1–11.4) | 122.0 (110.0–141.0) | |||
IQR = interquartile range (25th −75th percentiles)
Single biomarker sex- and age-adjusted point estimates differed substantially (<10%) from the models with mutual adjustment for all three biomarkers, suggesting lead, manganese, and selenium confound one another (see Supplemental Material; Tables S1–3). For example, lead concentrations in the highest quartile were more strongly associated with longitudinal increases in systolic blood pressure after accounting for manganese and selenium. Estimates from mutually adjusted models, however, were similar when comparing only sex- and age-adjustment to models with further adjustment for site, smoking status, educational duration, creatinine-corrected urinary arsenic concentration (see Supplemental Material; Tables S1–3, Models 2 and 3).
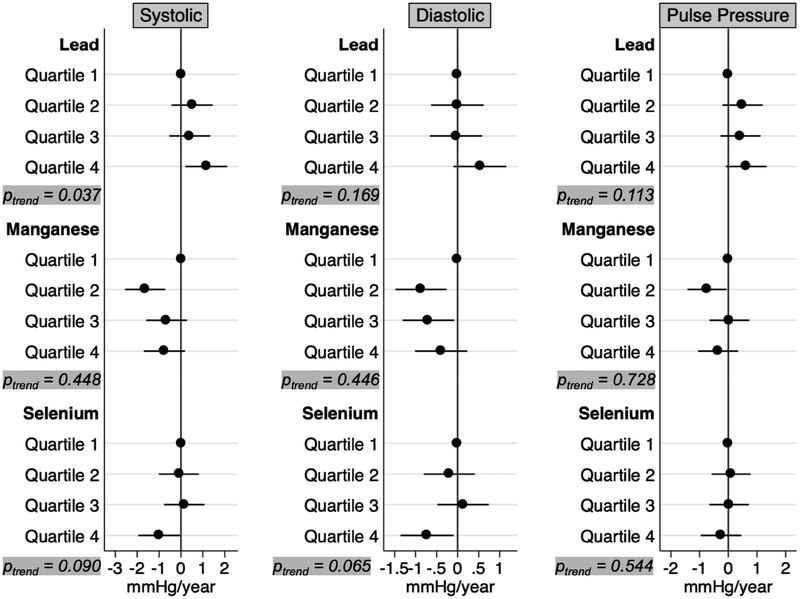
In fully adjusted models, blood lead concentrations in the highest quartile were associated with longitudinal increases of 1.16 mmHg per year (95% CI: 0.21, 2.11) in systolic blood pressure, 0.53 mmHg per year (95% CI: −0.10, 1.16) in diastolic blood pressure, and 0.63 mmHg per year (−0.08, 1.34) in pulse pressure even after accounting for body mass index, diabetes, and antihypertensive use (Figure 1). The association between lead exposure and systolic blood pressure appeared to be monotonic (Ptrend = 0.037). Mid-range manganese concentrations were associated with declines in blood pressure; systolic blood pressure decreased −1.64 (95% CI: −2.56, −0. 72) mmHg annually among individuals in the second quartile, diastolic blood pressures decreased −0.88 (95% CI: −1.48, −0.27) and −0.70 (95% CI: −1.31, −0.08) mmHg annually among individuals in the second and third quartiles, respectively, and pulse pressure decreased by 0.74 (95% CI: −1.42, −0.06) mmHg annually among individuals in the second quartile. Yearly decreases of 0.99 (95% CI: −1.95, −0.04) mmHg in systolic blood pressure and 0.73 (95% CI: −1.36, −0.09) mmHg in diastolic blood pressure were observed among those in the highest quartile of selenium exposure, whereas associations with pulse pressure were mostly null.
Figure 1.
Annual changes in blood and pulse pressure, mutually adjusted for baseline blood lead, manganese and selenium, in addition to age, sex, site, smoking status, educational duration, creatinine-corrected urinary arsenic concentration, diabetes, body mass index, and antihypertensive use.
Joint Associations
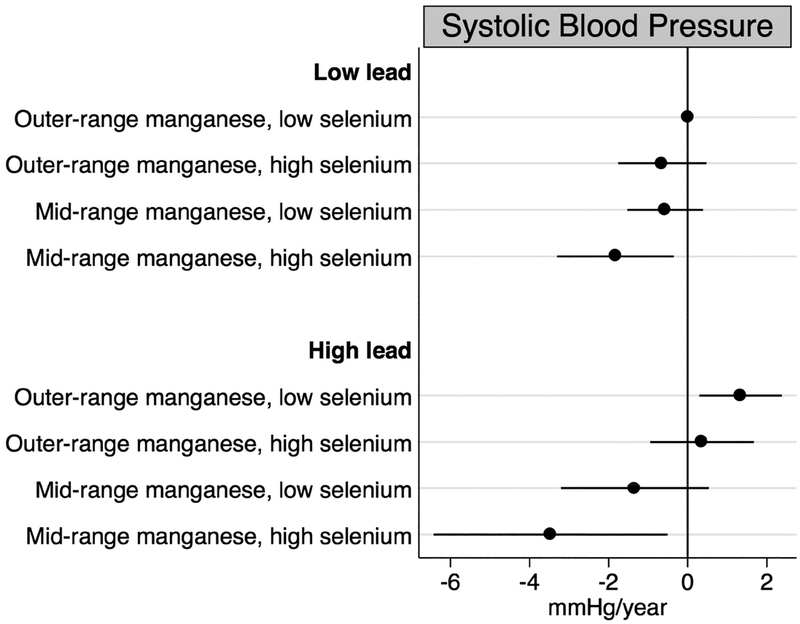
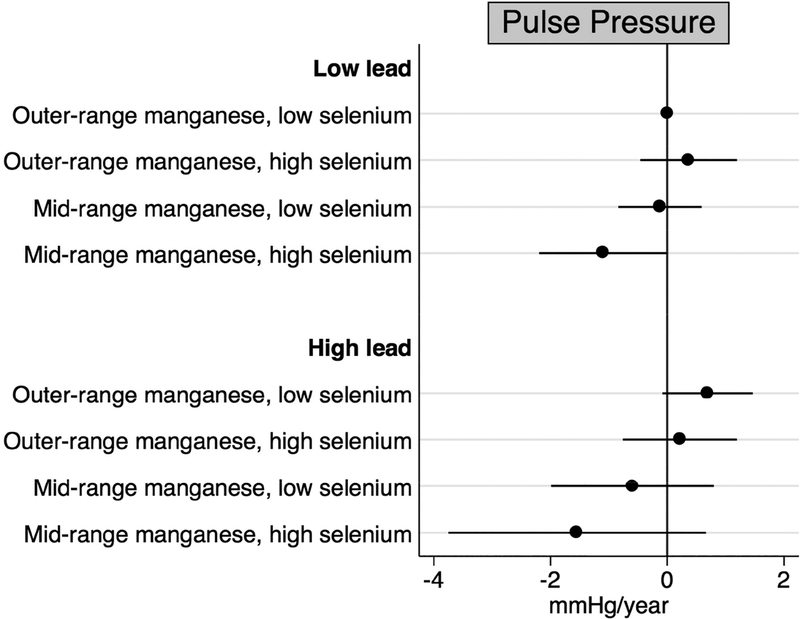
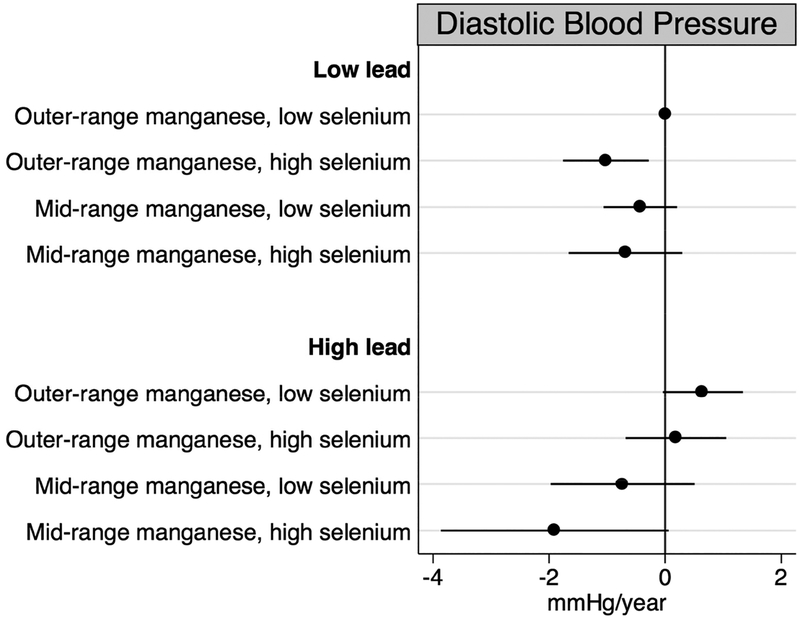
Results from our main analyses indicated the highest quartile of baseline lead, the second quartile of baseline manganese, and the highest quartile of baseline selenium concentrations were consistently associated with the largest changes in blood and pulse pressure over time. Thus, we characterized concentrations as follows: lead concentration below (<136.0 μg/L) compared to at or above the 75th percentile (≥136.0 μg/L); manganese concentration outside the 25th to 50th percentiles (<8.2 or >10.0 μg/L) compared to the 25th to 50th percentile interquartile range (8.2 to 10.0 μg/L); and selenium concentration below (<137.0 μg/L) compared to at or above the 75th percentile (≥137.0 μg/L). In fully adjusted models, we observed mid-range manganese concentrations and high selenium concentrations to be associated with longitudinal declines in blood and pulse pressure (Figures 2–4). These associations were most pronounced for individuals who were also highly exposed to lead. Specifically, individuals whose blood had high levels of lead coupled with mid-range manganese and high selenium concentrations experienced decreases of 3.47 (95% CI: −6.43, −0.51) mmHg/year in systolic blood pressure, 1.90 (95% CI: −3.86, 0.06) mmHg/year in diastolic blood pressure, and 1.54 (95% CI: −3.75, 0.66) mmHg/year in pulse pressure representing departures from additivity. For diastolic blood pressure only, we also observed the combination of low lead, outer-range manganese, and high selenium concentrations to be associated with annual declines of 1.02 (95% CI: −1.76, −0.29) mmHg relative to those with low lead, outer-range manganese, and low selenium concentrations.
Figure 2.
Annual changes in systolic blood pressure by combinations of baseline blood lead, manganese, and selenium concentrations, adjusted for age, sex, site, smoking status, educational duration, creatinine-corrected urinary arsenic concentration, diabetes, body mass index, and antihypertensive use.
Figure 4.
Annual changes in pulse pressure by combinations of baseline blood lead, manganese, and selenium concentrations, adjusted for age, sex, site, smoking status, educational duration, creatinine-corrected urinary arsenic concentration, diabetes, body mass index, and antihypertensive use.
Sensitivity Analyses
Sensitivity analyses to address biases from antihypertensive medication use yielded similar results to our main analyses (see Supplemental Material; see Figures S1 and S2). Between 2006 and 2015, 46 incident hypertension cases were identified among the 178 participants analyzed – 19 cases at the first follow-up, 9 at the second, and 18 at the third (see Supplemental Material; see Figure S3). Median (25th, 75th percentile) values of the biomarkers were as follows: 7.8 (4.0–13.8) μg/dL for lead, 10.1 (8.3–13.0) μg/L for manganese, and 121.0 (110.0–135.0 μg/L for selenium. Only manganese was significantly associated with hypertension risk; baseline manganese concentrations in the second quartile were associated with a 79% decrease in the incidence of hypertension in the fully adjusted model relative to the lowest quartile (see Supplemental Material; see Table S4, model 3, HR: 0.21, 95% CI: 0.06, 0.81). The hazard ratio estimates for manganese concentrations in the third and fourth quartiles, as well as for lead and selenium concentrations were largely null.
Discussion
Within this population of rural Bangladeshi adults, the median blood lead concentration was 8.5 μg/dL blood lead. For adults, concentrations above 5.0 μg/dL are considered elevated, thus this population has exposure levels of clinical and public health concern (Alarcon et al. 2016). Without a formal exposure risk assessment, the sources of lead exposure are unclear but may be attributed to industrial (e.g., lead acid battery manufacturing, leaded paint, ceramics) and agricultural (e.g., pesticides) applications of lead, which are not currently regulated in Bangladesh (Ahmad et al. 2014; Biswas et al. 2003). Concentrations of manganese were generally normal (4–15 μg/L) in the study sample (Agency for Toxic Substances and Disease Registry (ATSDR) 2012; Association of Occupational and Environmental Clinics 2013). Whole blood selenium does not have established reference levels, but the observed concentrations were similar to those reported in previous studies (Agency for Toxic Substances and Disease Registry (ATSDR) 2003; Chen et al. 2007). The results of our analyses suggest that both manganese and selenium have the potential to lower blood pressure when exposures are within certain ranges. For manganese, blood concentrations within the second and third quartiles (8.2–12.4 μg/L) were consistently associated with significant annual declines in blood pressure and pulse pressure, suggesting a U- or J-shaped dose-response. For selenium, blood concentrations in the highest quartile (137–214 μg/L) were the only range of exposure to have a significant blood pressure-lowering effect. Without evidence of a linear trend, these results indicate the possibility of a threshold effect for selenium exposure. In contrast, lead exposures raised blood pressures annually in a dose-dependent manner. Moreover, the findings from our exploratory analysis suggest that narrow ranges of manganese coupled with greater selenium could counteract accelerations in blood and pulse pressure associated with high lead exposures.
This study is the first to our knowledge to prospectively evaluate the collective role of essential micronutrients and toxic metals with blood pressure. We found no statistical evidence of effect modification but did observe manganese and selenium exerted lowering effects on blood pressure in contrast to the increases associated with greater lead exposure. These results are consistent with prior longitudinal studies of lead, and somewhat consistent with a longitudinal study of selenium that observed a significant inverse association with hypertension, but only among men (Glenn et al. 2003; Glenn et al. 2006; Nawrot et al. 2007). Our finding of non-linear associations for manganese with blood and pulse pressure is novel, and may have been missed by other studies if linearity was assumed or if the exposure distribution of the study population was different. The precise mechanisms underlying these relationships have not yet been entirely elucidated. Estimates from our models that included and did not include body mass index and diabetes were similar, suggesting metals may affect blood pressure through mechanisms other than weight gain or alterations of glucose metabolism. In the literature, it has been hypothesized that lead exposure triggers acute responses from the autonomic nervous system, or stimulates more chronic oxidative stress and inflammation pathways (Chang et al. 2005; Cosselman et al. 2015; Kim et al. 2015). In contrast, manganese and selenium function through enzymes (manganese metalloenzymes and selenoproteins, respectively), of which several possess antioxidant properties; thus, these essential micronutrients may confer protection from oxidative stress (Institute of Medicine 2000, 2001).
There are several limitations to this study. For one, the biomarkers used to evaluate exposure status may be suboptimal. Blood concentrations of lead are routinely used in environmental epidemiologic studies as an indicator of recent exposure, but the half-life of lead in blood is only 36 days. Bone concentrations of lead are preferable as they are indicative of cumulative exposures, however, testing requires specialized imaging equipment (Agency for Toxic Substances and Disease Registry (ATSDR) 2007). Blood may be a particularly poor biological matrix for the measurement of manganese, with a half-life of only 2 hours reported in rats, whereas the half-life for selenium has been estimated to be 100 days (Agency for Toxic Substances and Disease Registry (ATSDR) 2012; Reilly 1996). Non-conventional and non-invasive matrices such as hair, saliva, and nails may provide more accurate measures of long-term exposures, but have not yet been comprehensively evaluated with regards to lead, manganese, or selenium (Gil et al. 2015). Nevertheless, we found significant associations between blood concentrations of these elements and changes in blood pressure over time, for which there is biologic plausibility. Furthermore, since manganese and selenium are essential micronutrients that are consumed daily rather than episodically, we do not expect large variations in the amounts consumed from dietary sources. Blood concentrations were only measured at baseline; thus, we could not evaluate changes in exposure levels over time, or how increases or reductions in exposure levels could affect blood pressure trajectories. However, we expect changes in exposure levels to be minimal for two reasons: (1) metals are persistent environmental toxicants, and (2) food consumption patterns, while broadly shifting in Bangladesh in recent years to incorporate more non-starch foods, were likely relatively stable at the individual-level during the 6-year follow-up period (Waid et al. 2017). We did not evaluate other micronutrients, such as iron, zinc, or copper that may protect against the toxicity of lead by reducing absorption or retention (Agency for Toxic Substances and Disease Registry (ATSDR) 2007). Similarly, we did not collect dietary information from participants at baseline. Certain dietary patterns, particularly those with a high sodium content, are widely recognized as risk factors for hypertension (Forman et al. 2012). If a high sodium dietary pattern is also related to micronutrient intakes and/or exposures to toxic metals from contaminated foods, our results may be subject to residual confounding. Without dietary data, we were additionally unable to characterize population-specific sources of manganese and selenium that may be utilized in future interventions. However, there are some data to suggest that rice and fish are the primary sources of selenium whereas drinking water and leafy vegetables contribute to manganese intakes in the Bangladeshi population (Al-Rmalli et al. 2016; Rahman et al. 2012). Lastly, this study was conducted within a sample of adults highly exposed to arsenic, so the results may not be generalizable to other populations.
Conclusions
In a relatively short study period, we observed opposing changes in blood pressure associated with lead, manganese, and selenium exposures. These changes were relatively small (<2 mmHg/year) but would be clinically meaningful over the course of many years. With Bangladesh currently in the midst of transitioning from a public health burden of communicable to non-communicable disease, an estimated 20% of the adult population is already suffering from hypertension.(Islam and Majumder 2012). This number is only expected to grow, thus, if confirmed, our results could be of great public health importance. The toxic effects of lead are well-documented, but our findings suggest that many Bangladeshis have elevated levels of exposure (Agency for Toxic Substances and Disease Registry (ATSDR) 2007; Alarcon et al. 2016). In addition to hypertension, kidney damage and neurocognitive effects are of concern at levels these high, particularly for vulnerable populations like pregnant women and children. Ongoing efforts to reduce arsenic exposure in the area may also want to consider remediation of lead from water sources. More research is necessary to determine the major sources of current lead exposure. If exposures are predominantly from workplace exposures or cigarette smoking, regulatory measures targeted at reducing lead in occupational settings or in tobacco products may prove more effective. Our findings additionally indicated that optimizing intakes of nutritionally essential micronutrients could promote the lowering of blood pressures. Future studies should assess the efficacy of dietary or supplemental interventions on micronutrient intakes for hypertension prevention.
Supplementary Material
Figure 3.
Annual changes in diastolic blood pressure by combinations of baseline blood lead, manganese, and selenium concentrations, adjusted for age, sex, site, smoking status, educational duration, creatinine-corrected urinary arsenic concentration, diabetes, body mass index, and antihypertensive use.
Heavy metal contamination is endemic in Bangladesh where high-level exposures to certain toxic metals can result in hypertension
Epidemiologic research on other metals, including essential nutrients, in relation to blood pressure has been sparse
In a longitudinal study of Bangladeshi adults, we observed positive associations of lead with increases in blood pressure over a 6-year period; in contrast, manganese and selenium exposures within certain ranges were associated with blood pressure declines
This study highlights the potential for essential nutrients manganese and selenium as anti-hypertensive agents in a lead-exposed population
Acknowledgements:
This work was supported by the National Institutes of Health grant numbers R01CA107431, P42ES010349, R01ES024423, and T32HL125294.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: No disclosures to report.
References
- Agency for Toxic Substances and Disease Registry (ATSDR). (2003). Toxicological profile for selenium. Retrieved from https://www.atsdr.cdc.gov/toxprofiles/tp92.pdf. [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). (2007). Toxicological profile for lead. Retrieved from https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). (2012). Toxicological profile for manganese. Retrieved from https://www.atsdr.cdc.gov/toxprofiles/tp151.pdf. [PubMed]
- Ahmad SA, Khan MH, Khandker S, Sarwar AFM, Yasmin N, Faruquee MH, & Yasmin R (2014). Blood Lead Levels and Health Problems of Lead Acid Battery Workers in Bangladesh. Scientific World Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rmalli SW, Jenkins RO, & Haris PI (2016). Intake of arsenic and selenium in a Bangladeshi population investigated using inductively coupled plasma mass spectrometry. Biomedical Spetroscopy and Imaging, 5(4), 373–391. [Google Scholar]
- Alarcon WA, State Adult Blood Lead, E., Surveillance Program, I., Davidson S, Dufour B, Roach M, … Melia S (2016). Elevated Blood Lead Levels Among Employed Adults - United States, 1994–2013. MMWR Morbidity and mortality weekly report, 63(55), 59–65. [DOI] [PubMed] [Google Scholar]
- Argos M, Rahman M, Parvez F, Dignam J, Islam T, Quasem I, … Ahsan H (2013). Baseline comorbidities in a skin cancer prevention trial in Bangladesh. Eur J Clin Invest, 43(6), 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Occupational and Environmental Clinics. (2013). Medical management guidelines for lead-exposed adults. Retrieved from http://www.aoec.org/documents/positions/mmg_revision_with_cste_2013.pdf
- Begum BA, Hopke PK, & Markwitz A (2013). Air pollution by fine particulate matter in Bangladesh. Atmospheric Pollution Research, 4(1), 75–86. doi: 10.5094/Apr.2013.008 [DOI] [Google Scholar]
- Bhowmik B, Diep LM, Munir SB, Rahman M, Wright E, & Mahmood S (2016). HbA1c as a diagnostic tool for diabetes and pre-diabetes: the Bangladesh experience (vol 30, pg e70, 2013). Diabetic Medicine, 33(2), 271–271. doi: 10.1111/dme.13018 [DOI] [PubMed] [Google Scholar]
- Biswas SK, Tarafdar SA, Islam A, Khaliquzzaman M, Tervahattu H, & Kupiainen K (2003). Impact of unleaded gasoline introduction on the concentration of lead in the air of Dhaka, Bangladesh. Journal of the Air & Waste Management Association, 53(11), 1355–1362. [DOI] [PubMed] [Google Scholar]
- Chang HR, Tsao DA, Yu HS, & Ho CK (2005). The change of beta-adrenergic system after cessation of lead exposure. Toxicology, 207(1), 73–80. doi: 10.1016/j.tox.2004.08.018 [DOI] [PubMed] [Google Scholar]
- Chen Y, Hall M, Graziano JH, Slavkovich V, van Geen A, Parvez F, & Ahsan H (2007). A prospective study of blood selenium levels and the risk of arsenic-related premalignant skin lesions. Cancer Epidemiol Biomarkers Prev, 16(2), 207–213. doi: 10.1158/1055-9965.EPI-06-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizmar JF (2000). A discrete-time hazard analysis of the role of gender in persistence in the economics major. Journal of Economic Education, 31(2), 107–118. doi:Doi 10.2307/1183183 [DOI] [Google Scholar]
- Choi MK, & Bae YJ (2013). Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in Korean adults: the Korea National Health and Nutrition Examination Survey (2007–2008). Biol Trace Elem Res, 156(1–3), 56–66. doi: 10.1007/s12011-013-9852-z [DOI] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A, & Kaufman JD (2015). Environmental factors in cardiovascular disease. Nat Rev Cardiol, 12(11), 627–642. doi: 10.1038/nrcardio.2015.152 [DOI] [PubMed] [Google Scholar]
- Frisbie SH, Ortega R, Maynard DM, & Sarkar B (2002). The concentrations of arsenic and other toxic elements in Bangladesh’s drinking water. Environ Health Perspect, 110(11), 1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman JP, Scheven L, de Jong PE, Bakker SJL, Curhan GC, & Gansevoort RT (2012). Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation, 125(25), 3108–3116. doi: 10.1161/CIRCULATIONAHA.112.096115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambelunghe A, Sallsten G, Borne Y, Forsgard N, Hedblad B, Nilsson P, … Barregard L (2016). Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ Res, 149, 157–163. doi: 10.1016/j.envres.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Glenn BS, Bandeen-Roche K, Lee BK, Weaver VM, Todd AC, & Schwartz BS (2006). Changes in systolic blood pressure associated with lead in blood and bone. Epidemiology, 17(5), 538–544. doi: 10.1097/01.ede.0000231284.19078.4b [DOI] [PubMed] [Google Scholar]
- Glenn BS, Stewart WF, Links JM, Todd AC, & Schwartz BS (2003). The longitudinal association of lead with blood pressure. Epidemiology, 14(1), 30–36. doi: 10.1097/01.EDE.0000032429.13232.9C [DOI] [PubMed] [Google Scholar]
- Gil F, & Hernández AF (2015). Toxicological importance of human biomonitoring of metallic and metalloid elements in different biological samples. Food Chem Toxicol, 80, 287–297. doi: 10.1016/j.fct.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2000). In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC). [PubMed] [Google Scholar]
- Institute of Medicine. (2001). In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC). [Google Scholar]
- Islam AK, & Majumder AA (2012). Hypertension in Bangladesh: a review. Indian Heart J, 64(3), 319–323. doi: 10.1016/S0019-4832(12)60096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Ye BJ, … Hong YS (2015). Evaluation and management of lead exposure. Ann Occup Environ Med, 27, 30. doi: 10.1186/s40557-015-0085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, & Guallar E (2009). Serum selenium concentrations and hypertension in the US Population. Circ Cardiovasc Qual Outcomes, 2(4), 369–376. doi: 10.1161/CIRCOUTCOMES.108.831552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Navas-Acien A, Mark DB, & Lee KL (2016). Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J Am Coll Cardiol, 67(20), 2411–2418. doi: 10.1016/j.jacc.2016.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, & Kim Y (2011). Relationship between blood manganese and blood pressure in the Korean general population according to KNHANES 2008. Environ Res, 111(6), 797–803. doi: 10.1016/j.envres.2011.05.005 [DOI] [PubMed] [Google Scholar]
- Mitra AK, Haque A, Islam M, & Bashar SA (2009). Lead poisoning: an alarming public health problem in Bangladesh. Int J Environ Res Public Health, 6(1), 84–95. doi: 10.3390/ijerph6010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, & Narayan KM (2010). A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care, 33(3), 515–519. doi: 10.2337/dc09-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, … Schwartz J (2012). Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect, 120(1), 98–104. doi: 10.1289/ehp.1002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, & Rothenberg SJ (2007). Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect, 115(3), 472–482. doi: 10.1289/ehp.9785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Roels HA, Den Hond E, Thijs L, Fagard RH, … Struijker-Boudier HA (2007). Blood pressure and blood selenium: a cross-sectional and longitudinal population study. Eur Heart J, 28(5), 628–633. doi: 10.1093/eurheartj/ehl479 [DOI] [PubMed] [Google Scholar]
- Rahman MM, Asaduzzaman M, & Naidu R (2013). Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J Hazard Mater, 262, 1056–63. doi: 10.1016/j.jhazmat.2012.06.045. [DOI] [PubMed] [Google Scholar]
- Reilly C (1996). Selenium in food and health. London ; New York: Blackie Academic & Professional. [Google Scholar]
- Salam A, Hossain T, Siddique MNA, & Alam AMS (2008). Characteristics of atmospheric trace gases, particulate matter, and heavy metal pollution in Dhaka, Bangladesh. Air Quality Atmosphere and Health, 1(2), 101–109. doi: 10.1007/s11869-008-0017-8 [DOI] [Google Scholar]
- Tobin MD, Sheehan NA, Scurrah KJ, & Burton PR (2005). Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med, 24(19), 2911–2935. doi: 10.1002/sim.2165 [DOI] [PubMed] [Google Scholar]
- Waid JL, Ali M, Thilstedc SH, & Gabryscha S (2017). Dietary change in Bangladesh from 1985 to 2010. Global Food Security. doi: 10.1016/j.gfs.2017.09.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.