Abstract
Purpose:
To describe four cases of presumably immunocompetent patients with herpes simplex virus (HSV) keratitis unresponsive (n=3) or allergic (n=1) to conventional antiviral therapy that improved with oral valganciclovir treatment.
Methods:
Retrospective case series of four patients with HSV keratitis treated with oral valganciclovir between March 2016 and June 2018.
Results:
We reviewed the records of four patients with recurrent epithelial HSV keratitis. Three patients were on antiviral prophylaxis due to a history of HSV keratitis. All patients were on oral acyclovir, valacyclovir and/or famciclovir treatment with/without topical antiviral therapy for 4 to 6 months for prophylaxis and/or recurrent dendriform epithelial keratitis. While 3 patients had recurrent episodes during their active prophylaxis with oral antiviral therapies, one patient had a recurrence after she discontinued her oral prophylactic antiviral therapy due to recurrent self-reported allergic reactions. The patients presented with recurrent dendriform epithelial keratitis despite conventional antiviral therapy. We initiated oral valganciclovir 900 mg twice a day for 10 days as a treatment dose, followed by 900 mg daily for prophylaxis. The corneal epithelium subsequently healed within the first two weeks in all patients. The mean follow-up time for patients on valganciclovir prophylaxis was 8 months (range: 6–12 months), and none of the patients presented with any further recurrences.
Conclusion:
In case of treatment-related side effects or failure with conventional antiviral therapies, oral valganciclovir may present an alternative for the treatment and prophylaxis of HSV keratitis.
Keywords: herpes simplex, keratitis, valganciclovir, prophylaxis, antiviral
INTRODUCTION
Herpes simplex virus (HSV) keratitis is the leading cause of corneal blindness in developed countries.1 The recurrent nature of the HSV keratitis may result in corneal nerve damage, which could lead to neurotrophic keratopathy with persistent epithelial defects, and also contribute to the development of corneal scarring, corneal melting, and potentially corneal perforation and permanent vision loss.2 Adequate prophylaxis and treatment in a timely manner are crucial to prevent disease and recurrences and to preserve vision.
Treatment options include a variety of nucleoside analogues, including oral acyclovir, valacyclovir, penciclovir, famciclovir and topical ganciclovir, as well as DNA synthesis inhibitors such as foscarnet and cidofovir.3 However, failure with conventional therapies in HSV keratitis can be challenging and result in permanent vision loss. Valganciclovir is a pro-drug of ganciclovir with higher bioavailability, which is a synthetic analog of 2′-deoxy-guanosine and a competitive inhibitor of deoxyguanosine triphosphate (dGTP), showing activity against the herpes virus family.4,5 Herein we present a case series of 4 patients, demonstrating the efficacy of oral valganciclovir in the treatment and prophylaxis of HSV keratitis refractory or intolerant to current antiviral therapies.
CASE PRESENTATIONS
This retrospective chart review of patients seen at the Cornea Service of the New England Eye Center, Tufts Medical Center, Boston, MA was approved by the Institutional Board Review of Tufts Medical Center/Tufts University Health Sciences. The protocol conformed to the Declaration of Helsinki and adhered to the Health Insurance Portability and Accountability Act (HIPAA).
Case 1
A 75-year-old Asian male presented with periorbital erythema and edema of the left eye, accompanied by blurred vision, 2 weeks post cataract surgery. He had a history of left eye 7th cranial nerve palsy, and subsequent paralytic ectropion of the lower lid, causing exposure that was corrected 5 years ago with adequate gain of lid closure. His best-corrected visual acuity (BCVA) on presentation was 20/25 OD and 20/50 OS. Slit-lamp examination showed a 4.5 mm × 0.8 mm vertical corneal ulcer inferiorly OS, with surrounding diffuse superficial punctate epithelial erosions (SPEE) after his cataract surgery. On his follow-up visit 3 days later, the vertical ulcer that stained for fluorescein staining had enlarged to 4.6 mm × 2.5 mm, with terminal endbulbs staining for rose Bengal. The patient was on prednisolone acetate 1% drops three times daily after surgery OS. Oral acyclovir 400 mg five times a day was initiated, along with topical moxifloxacin 0.5% three times a day OS and prednisolone was stopped. On follow-up examination a week later, while the previous lesion had become significantly smaller and turned into a linear defect, a new epithelial dendriform lesion (3.4 mm horizontally × 3.3 mm vertically) was observed inferior to the visual axis concurrent with decreased vision of counting fingers (CF) at 5 meters. Thus, topical ganciclovir 0.15% five times a day OS was added for 15 days in conjunction with oral acyclovir 400 mg three times a day. For the duration of 2 months that the patient was on oral acyclovir, the patient presented with 2 additional episodes of dendriform epithelial recurrences. The first epithelial recurrence resolved within 2 weeks with topical ganciclovir 0.15% five times a day and acyclovir 400 mg five times a day, while the second epithelial episode persisted for 20 days despite topical ganciclovir 0.15% gel five times a day and an increased dose of oral acyclovir 800 mg five times a day. Hence, oral acyclovir treatment was replaced with famciclovir 500 mg three times a day and a lateral tarsorrhaphy was performed to limit corneal exposure. However, the patient presented with 2 additional recurrences after complete epithelial healing over the following 2 months after the while on oral famciclovir 500 mg three times a day and topical ganciclovir 0.15% gel therapy three times a day, increasing to five times a day with each new recurrence. Therefore he was assessed for a possible immune deficiency by the infectious disease department. His blood work was within normal limits, except for a mild thrombocytopenia [134 K/uL (N:150–400 K/uL)]. During these 2 months, the first episode resolved within 3 weeks with ganciclovir 0.15% gel five times a day and famciclovir 500 mg three times a day. Topical therapy of ganciclovir 0.15% was reduced to three times a day. Once the second episode occured, valganciclovir 900 mg twice a day was initiated given the multiple recurrences as a replacement therapy for oral famciclovir. After the initial treatment period of 10 days, slit-lamp examination showed total epithelial healing and valganciclovir dose was decreased to 900 mg once daily prophylactic dose. The patient was then followed-up for 8 months on valganciclovir prophylaxis. His BCVA improved to 20/50 OS, his corneal epithelium remained intact, the stromal haze decreased and he demonstrated no additional episodes of recurrence.
Case 2
An 80-year-old Caucasian female was referred with recurrent epithelial HSV keratitis after her second Descemet’s stripping automated endothelial keratoplasty (DSAEK) in her left eye. Her first DSAEK was performed for corneal decompensation after cataract surgery associated with Fuchs’ endothelial corneal dystrophy (FECD). The patient underwent a second DSAEK 6 months after her first transplant failed, which was noted to have presented with epithelial HSV keratitis that recurred three times by her previous physician with last episode presenting before her initial visit at our center. The last recurrence was treated with trifluridine 1% five times a day and valacyclovir 1000 mg three times a day. A week prior to her initial presentation to us, trifluridine 1% drops were decreased to twice a day due to complete epithelial healing, while keeping her on the same prophylactic valacyclovir dose. Upon presentation, her BCVA was 20/70 OD and 20/100–1 OS and her left eye was notable for a 1 mm × 2 mm active dendriform lesion with underlying corneal edema, despite oral antiviral prophylaxis with valacyclovir 1000mg three times a day. The decreased vision OD was attributed a combination of grade 2 nuclear sclerosis cataract and Fuchs’ endothelial corneal dystrophy causing corneal haze. Laser in vivo confocal microscopy (IVCM) images showed complete absence of corneal nerves, supporting the diagnosis of neurotrophic keratopathy and a potential viral etiology, which may present with asymmetrical nerve density, presence of dendriform and inflammatory cells, and severely diminished endothelial cells in the left eye, with an endothelial cell count of 1586±26 cells/mm2 OD and 628±24 cells/mm2 OS. Considering the second DSEAK was complicated with increased posterior pressure and vitreous loss during surgery, ending with low endothelial cell count and decompensation, the patient was deemed a very poor surgical candidate. Given our recent observations of failure with famciclovir therapy in other patients that had failed acyclovir and valacyclovir, famciclovir was not considered, to prevent potential permanent vision loss, should the patient not respond to this therapy. Valacyclovir was replaced with oral valganciclovir 900 mg twice a day for 10 days and ganciclovir 0.15% gel five times a day, and prednisolone acetate 1% drops twice a day OS was prescribed. On her follow-up examination 2 weeks later, her corrected vision had improved to 20/300 OS, and the dendriform epithelial lesion had cleared. After a month of therapy, topical ganciclovir treatment was discontinued; prednisolone acetate drops were decreased to once a day, and valganciclovir was decreased to 900 mg once a day prophylactic dose. After 3 months, prednisolone acetate was replaced with loteprednol etabonate once a day. The patient’s vision improved within the first month of treatment and reached to 20/100 after 4 months and remained stable for a follow up of 10 months on valganciclovir prophylaxis with a clear corneal graft and no sign of rejection or further recurrence.
Case 3
A 62-year-old Caucasian female with a history of Sjögren’s disease was followed for dry eye disease for the past 2 years. The patient had a history of herpes zoster ophthalmicus (HZO) OD in 2014, and was on oral acyclovir prophylaxis (800 mg twice a day) as a result of consequent epithelial HSV keratitis that presented with corneal ulceration 2 years after her HZO episode. During a routine follow-up visit, her slit-lamp examination revealed 4 raised epithelial dendriform lesions with underlying haze in the right eye ad without any epithelial defect, while she was asymptomatic. Her BCVA was 20/30 OD and 20/20 OS. Acyclovir was subsequently increased to 800 mg three times a day. On her follow-up visit a week later, the epithelial dendriform lesions were covering an area of 2 clock hours of the temporal cornea, with central epithelial defects and terminal endbulbs of the lesions staining with rose Bengal. Therefore, oral acyclovir was increased to 800 mg five times a day and topical ganciclovir five times a day was added to the right eye. After a month of therapy, the epithelial dendriform lesion had cleared and her prophylactic acyclovir dose was adjusted to 800 mg three times a day and topical antiviral therapy was discontinued. During her follow-up, the patient presented with a new dendriform lesion centrally (Fig. 1). Thus, topical ganciclovir 0.15% gel five times a day was initiated and acyclovir therapy was increased to 800 mg five times a day. Three days later the lesion had resolved, hence ganciclovir 0.15% gel was stopped after a total of 2 more weeks and her oral antiviral therapy was replaced with famciclovir 250 mg three times a day, considering she had a flare-up with a higher prophylactic acyclovir dose. On her 3-week follow-up, the patient complained of headaches and tremors, which had resolved with discontinuation of famciclovir 2 days prior to her visit. Her slit-lamp examination showed no recurrence and topical ganciclovir 0.15% gel was therefore decreased to once a day for a two days and then discontinued, with oral acyclovir 800 mg three times a day being restarted. One month later, the patient presented with foggy vision and increased light sensitivity and had initiated ganciclovir 0.15% gel herself prior to the emergent visit. Slit-lamp examination demonstrated diffuse focal central SPEE without epithelial dendriform lesions, which could have been due to resolution with topical antiviral therapy. At this point, acyclovir was replaced with oral valganciclovir 900 mg twice a day for 10 days, in conjunction with topical ganciclovir 0.15% gel, with the corneal SPEE clearing within 2 weeks. Valganciclovir was then decreased to the prophylactic dose of 900 mg once daily. During the following 6 months on valganciclovir prophylaxis, the patients corrected vision improved to 20/25 OD and no further recurrences were observed.
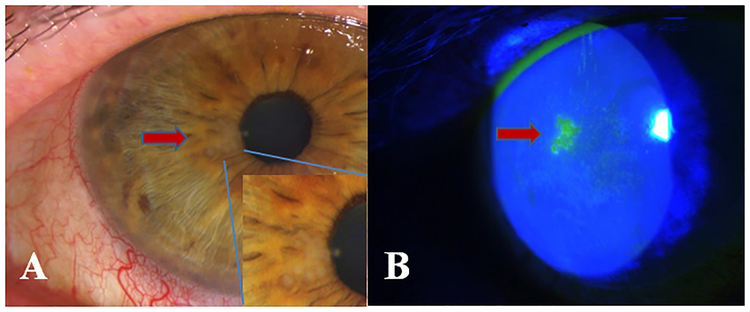
Figure.1.
A- Paracentral epithelial keratitis and conjunctival injection with magnified image of the vesicules visible in the epithelium B- Fluorescein staining of the herpetic dendriform lesion visible under cobalt blue light.
Case 4
An 89-year-old Caucasian female was referred in 2016for decreased visual acuity in her right eye for the past month. The patient had a history of bilateral cataract surgery and selective laser trabeculoplasty (SLT) in 2014 OU due to pseudoexfoliation glaucoma. On presentation, her visual acuity had decreased from 20/100 to 20/400 OD and was stable at 20/400 OS. Prior to this visit, her vision was compromised in both eyes due to advanced glaucoma and a macular scar in her left eye. On slit-lamp examination, the right eye showed large central scarring with overlying irregular corneal epithelium and an inferior pannus, diffuse lissamine green staining that was attributed to severe blepharitis, keratic precipitates, and anterior chamber reaction of +1 cell and +1 flare. IVCM showed complete absence of nerves and significant increased epithelial and stromal immune and inflammatory cells, supporting the diagnosis of neurotrophic keratopathy and a potential viral etiology. The presentation of keratouveitis and lack of corneal nerves was highly suggestive of herpetic disease; hence prophylactic oral valacyclovir 500 mg three times a day, topical loteprednol etobante 0.5% drops four times a day, and ocular surface lubrication were initiated. Two months after the initiation of the therapy, the patient underwent gastric surgery and discontinued valacyclovir due to a skin rash during her hospitalization. Within 3 months, her vision deteriorated to counting fingers at 3 feet and she presented with an approximately 4 mm epithelial dendriform ulcer inferiorly with surrounding dense diffuse SPEE (Fig 2). Topical ganciclovir 0.15% gel five times a day was initiated, together with oral famciclovir 500 mg three times a day. Even though the dendriform lesions resolved, the patient discontinued famciclovir after 2 weeks due to a skin rash. Thus weight-adjusted oral valganciclovir prophylaxis of 450 mg once a day was initiated due to allergic reactions to the alternative drugs. During the subsequent 7 months on valganciclovir prophylaxis no additional recurrences were observed.
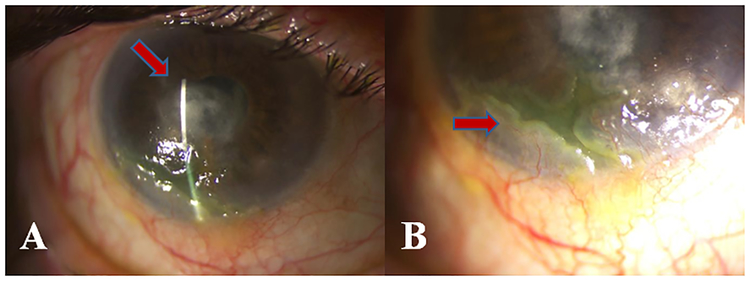
Figure.2.
A- Inferotemporal corneal ulceration with central stromal scarring B- Magnified image of the corneal ulceration with peripheral neovascularization and pannus formation
DISCUSSION
HSV is highly associated with ocular pathologies including epithelial keratitis1 and the DNA can be found in the trigeminal ganglia in at least 90% of the world’s population.6 The diagnosis of epithelial HSV keratitis requires obtaining a good ocular history and a thorough slit-lamp examination. Vital dye (fluorescein and rose Bengal) staining can aid in visualization of epithelial dendriform lesions with stippling of terminal endbulbs typical to HSV keratitis6 Moreover, significantly decreased or complete absence of subbasal corneal nerves are associated with HSV keratitis as previously shown.7 Thus, in challenging cases without pathognomonic clinical signs, IVCM may provide supporting information.
The first effective drug used for HSV keratitis was idoxuridine, a pyrimidine analogue, in 1962.8 Since then, the current treatment options include a variety of nucleoside analogues, including acyclovir, valacyclovir, penciclovir and ganciclovir, as well as DNA synthesis inhibitors such as foscarnet and cidofovir.3 Currently, there are 3 oral antiviral agents available in the United States for the treatment and prophylaxis of HSV keratitis; acyclovir, valacyclovir and famciclovir.6 The mechanism of action for nucleoside analogues is through thymidine kinase (TK).9 When exposed to herpes virus-infected cells, these nucleoside analogues turn into their active triphosphate form via TK.9,10 After activation, the triphosphate form of acyclovir gets incorporated into viral DNA chain in place of guanosine triphosphate11,12 Mutations in the TK gene have been associated with acyclovir resistance (AR).12 The prevalence of acyclovir resistant HSV infections in immune-deficient patients is considered to be between 3.5%−10%.9 While the overall prevalence of AR infections has not been high in immunocompetent patients (0.3%),4 it can be as high as 6.4% for AR HSV keratitis.3
Ganciclovir is a guanosine analogue primarily used orally in cytomegalovirus (CMV) infections, but also has in vitro activity against all herpes viruses.13 CMV or HSV infected cells induce the phosphorylation of ganciclovir, activating the drug to inhibit viral DNA polymerization.14 Phosphorylated ganciclovir inhibits the viral DNA synthesis by competing deoxyguanosine that can be differentiated from acyclovir that competes with guanosine.15 As such, topical ganciclovir, which shows a higher corneal permeability compared with topical acyclovir, has recently been used for herpetic epithelial dendriform lesions.16
The efficacy of topical ganciclovir has been demonstrated in a number of randomized clinical trials.16,17 While the comparison between outcomes of ganciclovir to acyclovir have been limited by patient heterogeneity, there are some publications suggesting better results with topical ganciclovir.18 When compared to idoxuridine, ganciclovir also presented to be significantly more effective for HSV keratitis (RR 1.11; 95% CI 1.05 to 1.19).18 While topical ganciclovir has been widely used in Europe since 1995 and in the US since 2009 for herpetic epithelial keratitis,10 the primary use of oral ganciclovir/valganciclovir in ophthalmology is currently limited to CMV retinitis, especially in immune-deficient and transplant patients.15,19
Aside from being the preferred treatment for CMV infections, a recent prospective, randomized, multi-center, controlled clinical trial conducted by Wang et. al.,20 also showed the efficacy of oral ganciclovir by assessing 173 eyes of 173 patients with HSV keratitis. The study compared topical antiviral therapy with 0.15% GCV ophthalmic gel and 0.1% fluorometholone drops to oral ganciclovir therapy and oral acyclovir therapy. Patients were followed up for a mean period of 32.1±12.3 months and evaluated at first and second week of therapy, and every other week until recovery. The GCV treated group showed a shorter healing time when compared to other groups (p=0.00). The recurrence within the follow up time was also significantly lower (17.2%), however the recurrence rate was similar to that of ACV treated group (p=0.358). GCV also proved to be a safe choice with only one patient noted to have an adverse reaction (neutropenia). The authors concluded that the short-term use of ganciclovir reduces the healing time, as well as the recurrence rate of herpetic keratitis.
The mechanism of actions for acyclovir and ganciclovir are similar and thus; theoretically, there may be a cross-resistance to treatment. While the majority of ACV resistance is attributed to TK gene mutations, viral DNA polymerase gene mutations have also been shown albeit less frequent.3 In contrast, ganciclovir resistance has been associated with viral protein kinase and viral DNA polymerase mutations.14,21 Therefore, in cases of ACV resistance, GCV could potentially still be effective. In fact, a recent in vitro study by Wang et. al. has shown that GCV was therapeutically effective in DNA polymerase associated AR HSV-1 clone treatment.22
Valganciclovir is the pro-drug of ganciclovir, which has better intestinal absorption quality and higher biocompatibility.23 At a dose of 900 mg, valganciclovir reaches the same blood levels of 5 mg/body weight of intravenous (IV) ganciclovir due to its high absolute bioavailability of 60 percent, with similar rates of adverse events to IV ganciclovir, such as neutropenia.5 It is an FDA approved oral antiviral commonly used to treat CMV infections in immunosuppressed patients.15 Jahnji et al.24 presented a case with persistent anterior chamber inflammation unresponsive to oral acyclovir. After an anterior chamber tap, the patient was diagnosed with CMV endotheliitis and was treated successfully with oral valganciclovir. Further, Kasetsuwan et al.25 presented a case with concomitant infections of CMV and HSV that presented with disciform endotheliitis that was refractory to topical steroids and oral acyclovir. Anterior chamber PCR yielded the presence of CMV in addition to HSV, and therefore, oral valganciclovir 900 mg twice a day was combined with oral acyclovir for 6 weeks and then decreased down to 450 mg twice a day as maintenance with successful resolution of anterior chamber inflammation. Follow up PCR was also negative for both HSV and CMV.
Herein, we present a case series of four presumed immuno-competent patients with recurrent epithelial herpetic keratitis episodes while on conventional antiviral prophylaxis. Even though it is difficult to exclude potential, but unlikely other viral etiologies, all cases presented with clinical signs typical for epithelial HSV keratitis. Valganciclovir therapy was initiated in three of these patients due to lack of response and/or frequent recurrences. The last patient received valganciclovir therapy as a result of her previous allergic reactions to conventional antivirals. A limitation of our study is that we were not able to perform antiviral susceptibility testing to verify/rule out antiviral resistance. However, recurrences and failure to treatment with conventional antiviral drugs, as well as positive response to both topical ganciclovir and oral valganciclovir are suggestive of possible drug resistance. Hence all of the patients’ systemic antiviral therapies and prophylaxis were replaced with valganciclovir. All patients showed resolution of HSV keratitis within 2 weeks and none demonstrated further recurrences while on valganciclovir prophylaxis. To our knowledge this is the first case series of recurrent epithelial herpetic keratitis patients, unresponsive/intolerant to conventional antiviral agents, successfully treated with oral valganciclovir. We demonstrate that oral valganciclovir therapy can present a feasible alternative to current antiviral options for treatment and prophylaxis of recurrent herpetic keratitis.
Acknowledgments
Financial Support: Funding was provided through grants from NIH R01-EY022695 (PH) and Massachusetts Lions Eye Foundation.
Footnotes
Meeting Presentations: This study was presented, in part, at the Ocular Microbiology and Immunology Group Meeting, Chicago, IL, November 10, 2017
Conflict of Interest: The authors don’t have any financial/conflicting interests to disclose
REFERENCES
- 1.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 2012; 57:448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci 2015; 56:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan R, de Vries RD, Osterhaus AD, Remeijer L et al. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis 2008; 198:659–663. [DOI] [PubMed] [Google Scholar]
- 4.Bacon TH, Levin MJ, Leary JJ et al. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev 2003; 16:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin DF, Sierra-Madero J, Walmsley S et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med 2002; 346:1119–1126. [DOI] [PubMed] [Google Scholar]
- 6.White ML, Chodosh J. American Academy of Ophthalmology, clinical statements. Herpes simplex virus keratitis: a treatment guideline 2014. (https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline) Accessed May 5, 2018
- 7.Hamrah P, Cruzat A, Dastjerdi MH et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology 2010; 117:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman H, Martola EL, Dohlman C. Use of 5-iodo-2’-deoxyuridine (IDU) in treatment of herpes simplex keratitis. Arch Ophthalmol 1962; 68:235–239. [DOI] [PubMed] [Google Scholar]
- 9.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 2011; 55:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsatsos M, MacGregor C, Athanasiadis I et al. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol 2016; 44:824–837. [DOI] [PubMed] [Google Scholar]
- 11.Birkmann A, Zimmermann H. HSV antivirals - current and future treatment options. Curr Opin Virol 2016; 18:9–13. [DOI] [PubMed] [Google Scholar]
- 12.Pan D, Kaye SB, Hopkins M et al. Common and new acyclovir resistant herpes simplex virus-1 mutants causing bilateral recurrent herpetic keratitis in an immunocompetent patient. J Infect Dis 2014; 209:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faulds D, Heel RC. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 1990; 39:597–638. [DOI] [PubMed] [Google Scholar]
- 14.Sahin A, Hamrah P. Acute herpetic keratitis: What is the role for ganciclovir ophthalmic gel? Ophthalmol Eye Dis 2012; 4:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil AJ, Sharma A, Kenney MC et al. Valganciclovir in the treatment of cytomegalovirus retinitis in HIV-infected patients. Clin Ophthalmol 2010; 4:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colin J, Hoh HB, Easty DL et al. Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea 1997; 16:393–399. [PubMed] [Google Scholar]
- 17.Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: safety and efficacy of a new treatment for herpes simplex keratitis. Curr Eye Res 2012; 37:654–660. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev 2015; 1: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabardi S, Asipenko N, Fleming J et al. Evaluation of low-versus high-dose valganciclovir for prevention of cytomegalovirus disease in high-risk renal transplant recipients. Transplantation 2015; 99:1499–1505. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Wang L, Wu N et al. Clinical efficacy of oral ganciclovir for prophylaxis and treatment of recurrent herpes simplex keratitis. Chin Med J (Engl) 2015; 128:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roche Laboratories—Cytovene IV/Cytovene Complete Product Information, Feb 2010:3. Available at: http://www.gene.com/gene/products/information/cytovene/pdf/pi.pdf. Accessed December 13, 2018
- 22.Wang LX, Takayama-Ito M, Kinoshita-Yamaguchi H et al. Characterization of DNA polymerase-associated acyclovir-resistant herpes simplex virus type 1: mutations, sensitivity to antiviral compounds, neurovirulence, and in-vivo sensitivity to treatment. Japanese journal of infectious diseases 2013; 66:404–410. [DOI] [PubMed] [Google Scholar]
- 23.Perrottet N, Csajka C, Pascual M et al. Population pharmacokinetics of ganciclovir in solid-organ transplant recipients receiving oral valganciclovir. Antimicrob Agents Chemother 2009; 53:3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jhanji V, Kwok R, Young AL. Eighteen months of anterior chamber inflammation. BMJ Case Rep 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasetsuwan N, Tangmonkongvoragul C. Concomitant herpes simplex virus and cytomegalovirus endotheliitis in immunocompetent patient. BMJ Case Rep 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]