Abstract
OBJECTIVE:
The transfusion of older packed red blood cells (PRBC) may be harmful in critically ill patients. We sought to determine the association between PRBC age and mortality among trauma patients requiring massive PRBC transfusion.
METHODS:
We analyzed data from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial. Subjects in the parent trial included critically injured adult patients admitted to one of 12 North American Level I trauma centers who received at least one unit PRBCs and were predicted to require massive blood transfusion. The primary exposure was volume of PRBC units transfused during the first 24 hours of hospitalization, stratified by PRBC age category: 0–7 days, 8–14 days, 15–21 days, and 22+ days. The primary outcome was 24-hour mortality. We evaluated the association between transfused volume of each PRBC age category and 24-hour survival using random effects logistic regression, adjusting for total PRBC volume, patient age, sex, race, mechanism of injury, Injury Severity Score, Revised Trauma Score, clinical site, and trial treatment group.
RESULTS:
The 678 patients included in the analysis received a total of 8,830 PRBC units. One hundred (14.8%) patients died within the first 24 hours. On multivariable analysis, the number of 22+ days old PRBCs was independently associated with increased 24-hour mortality (adjusted odds ratio [OR] 1.05 per PRBC unit; 95% confidence interval [CI]: 1.01–1.08); 0–7 days old OR 0.97 (CI 0.88–1.08), 8–14 days OR 1.04 (CI 0.99–1.09), 15–21 days OR 1.02 (CI 0.98–1.06). Results of sensitivity analyses were similar only among those who received ≥10 PRBC units.
CONCLUSIONS:
Increasing quantities of older PRBCs are associated with increased likelihood of 24-hour mortality in trauma patients receiving massive PRBC transfusion (≥10 units), but not in those who receive <10 units.
Keywords: trauma, erythrocytes, blood component transfusion/methods, shock, wounds and injuries/mortality, wounds and injuries/complications
INTRODUCTION
Background
Trauma is a leading cause of death among adults. The primary cause of death in the first 48 hours after injury is hemorrhage.1 The transfusion of blood components (packed red blood cells [PRBCs], platelets, and plasma) is a standard resuscitation intervention in patients with hemorrhagic shock.
The age of transfused PRBCs may be important in the outcomes of patients transfused due to traumatic hemorrhagic shock. Although current standards permit PRBC storage for up to 42 days, important changes occur to erythrocytes as the blood ages, including alteration and breakdown of cellular structure, release of oxygen, free hemoglobin, iron, and other pro-inflammatory microparticles, and lactic acid accumulation from anaerobic metabolism.2–4 These changes begin almost immediately once donated PRBCs are collected, with effects worsening over time.
Animal models of trauma-hemorrhage and resuscitation demonstrate significant consequences related to the use of stored blood, such as: increased levels of pro-inflammatory mediators,5 adherence of red blood cells to the microvasculature and limited tissue oxygenation,6 and increased mortality in the first four hours following transfusion of stored PRBCs.7 In humans, clinical signs of stored blood toxicity include thrombosis, infection, multiple organ failure, and death.8, 9
Importance
Recent prospective randomized clinical research studies (Informing Fresh versus Old Red cell Management [INFORM], Standard Issue Transfusion versus Fresher Red-Cell Use in Intensive Care [TRANSFUSE], Age of Blood Experiment [ABlE], and Red Cell Storage Study [RECESS]) found no association between transfused PRBC age and outcomes.10–13 However, these studies occurred in cardiac surgery and general critical care patients.14 The studies did not include patients with major trauma or those who often require large volume transfusions administered over short time periods (≥10 PRBC units in 24 hours).
Patients with traumatic hemorrhagic shock may be particularly susceptible to stored blood toxicity, as they require large volumes of PRBCs administered within a very short period of time, and experience widespread tissue damage and inflammation that animal studies suggest may render these patients vulnerable to stored blood toxicity. In such animal models, while increased end organ injury, nosocomial infection and mortality result when injured animals in hemorrhagic shock receive older PRBCs, similar adverse events do not occur in the setting of hemorrhagic shock without injury.7, 23–28These observations may help to explain why prior studies have not observed harm from older PRBC transfusion in non-trauma populations. We suspect that the rapid timing and intensity of massive PRBC transfusion may exacerbate the inflammation of traumatic hemorrhagic shock and the potential for stored blood toxicity. Understanding the association between PRBC age and outcomes is important to help optimize the outcomes after traumatic hemorrhagic shock. A conceptual model of the interplay between volume and age of PRBC units transfused to critically ill patients is shown in Figure 1A.
Figure 1.
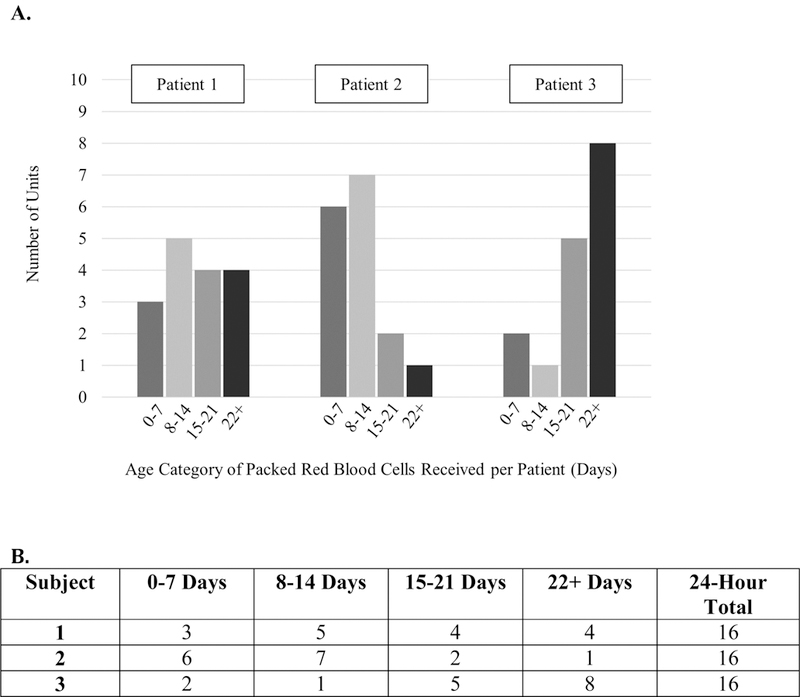
Conceptual model of dynamic relationship between packed red blood cell age and volume, and 24-hour mortality status.
A) This graph depicts three examples of patients who have similar injury severities and receive the same volume of packed red blood cells, but different volumes of packed red blood cells in each age category. We hypothesize that severely injured patients who receive more older packed red blood cell units experience a greater likelihood of mortality.
B) Example of analysis with categorization of packed red blood cell volume (units) per age group transfused to each patient.
Goals of this Investigation
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial is one of the only large-scale clinical trials of massive transfusion in trauma patients.15 We sought to examine the association among transfused PRBC age and patient outcomes in the PROPPR trial.
MATERIALS AND METHODS
Theoretical Model of the Problem
In this study we hypothesized that patient outcomes are influenced by exposure to aged PRCs. Exposure to aged blood includes two factors: 1) the age of the PRBC unit (0–7d, 8–14d, 15–22d, and 22-days), and 2) the total number of units of each PRBC age group. See Figure 1B for an example of this categorization. Confounders in this relationship include patient factors (age sex race), total blood transfused, and injury severity (ISS, RTS). We therefore conceptualized fitting a multivariable model with 24-hour death as the primary outcome, and multiple variables accounting for exposure to different blood age categories.
Study Design and Setting
We performed a secondary analysis of data from the PROPPR trial.16 This study was approved by the Institutional Review Board of the University of Alabama at Birmingham. The PROPPR trial was a pragmatic, phase 3, multisite, randomized trial completed in 2015. The trial compared the use of a 1:1:1 to a 1:1:2 plasma:platelet:PRBC ratio in relation to mortality among severely injured trauma patients predicted to receive massive transfusion. Twelve level I trauma centers in North America participated in the study. The complete methods of the PROPPR intervention have been previously described.15
Selection of Participants
Patients enrolled in the PROPPR trial were adults aged ≥15 years, admitted to the study site directly from the scene of injury, who met the criteria for highest level trauma activation, received at least one unit of PRBCs in the first hour of hospitalization, and who were predicted to require massive transfusion (≥10 PRBC unit in the first 24 hours) indicated by an Assessment of Blood Consumption score of ≥2. We included all patients enrolled in the PROPPR trial in the current analysis.
Measurements
The primary exposures were the age and volume of transfused PRBC units, as the trial investigators recorded the shelf age (days) of all transfused PRBC units. We categorized PRBC age based on four storage time frames: 0–7 days, 8–14 days, 15–21 days and 22+ days. These time frames were chosen based on documented changes noted as beginning within the first seven days of storage,6 to be consistent with prior blood age studies,10–12, 17 and to capture the impact of cellular breakdown that occurs in PRBCs during the first four weeks of storage and worsens with prolonged storage. We defined PRBC volume in terms of PRBC units. We characterized the number of PRBC units using both continuous (number of units) and categorical (0 units, 1–10, 11–20, 21+ units) schemes. Massive transfusion has traditionally been defined as ≥10 PRBC units in 24 hours, with an alternative definition of ≥3 PRBC units transfused in one hour.18 Four transfusion volume categories were selected to reflect the variation in patient transfusion requirements, as PROPPR criteria specified that enrolled patients were those “anticipated to require massive transfusion” though not all patient s were, in fact, massively transfused.
Outcome Measures
Consistent with the original PROPPR trial, we studied 24-hour mortality as the primary outcome;15 secondary outcomes were 30-day mortality and the composite variable of [24-hour death or the development of at least two major adverse events]. Adverse events relevant to the analysis included acute lung injury, acute kidney injury, acute respiratory distress syndrome, cardiac arrest, deep vein thrombosis, infection, multiple organ failure, myocardial infarction, pulmonary embolism, sepsis, stroke, Systemic Inflammatory Response Syndrome, transfusion-associated circulatory overload, and transfusion-related metabolic complication such as hyperkalemia or hypocalcemia.
Statistical Analysis
We determined the volume and age distribution of PRBC units transfused in the study. We evaluated the association between the number of transfused PRBC units in each PRBC age category and 24-hour survival using random effects logistic regression. We accounted for clustering by clinical site and adjusted for age, sex, race, mechanism of injury (penetrating, blunt, burn), Injury Severity Score, Revised Trauma Score, and PROPPR trial treatment group. We repeated the analysis characterizing PRBC volume on a categorical basis (0–7 days, 8–14 days, 15–21 days, and 22+ days). We also adjusted for total volume PRBC units transfused in the first 24 hours after hospital admission (1–10 units, 11–20 units, and 21+ units). In addition, we also stratified the analysis by those who received <10 or ≥10 PRBC units in the first 24 hours after hospital admission. Summary measures, such as averages, were not used as this would assume an equal mixing of effects of each blood age (e.g., the deleterious effects of the stored blood negated by the protective effects of the fresh blood).
We determined the number of patients experiencing ≥2 adverse events. Because 24-hour death is a competing risk for adverse events, we assessed the association between PRBC age-volume and the composite variable [≥2 adverse events or 24-hour death]. We also assessed the association between PRBC age-volume and 30-day mortality.
Revised trauma score (RTS) was missing for 73 patients (59 had missing respiratory rate, 22 had missing systolic blood pressure, and one missing both parameters); patients with missing RTS values were omitted from the primary analyses, all others were included. In a sensitivity analysis, we repeated the analysis using multiply imputed RTS values. Because RTS was not normally distributed, we conducted the imputation using predictive mean matching. We added study site as an indicator variable to account for clustering within study site. We carried out the multiple imputation with 20 iterations, combining the estimates using Rubin’s rules. 19 We conducted all analyses using Stata v.14.2 (Stata, Inc., College Station, Texas).
RESULTS
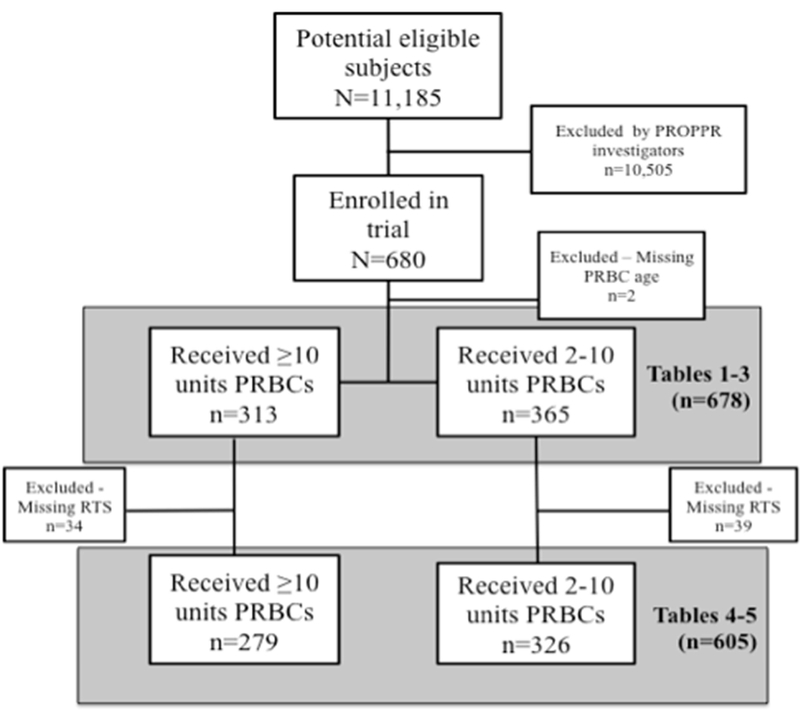
The PROPPR trial enrolled a total of 680 patients; we excluded two patients due to missing PRBC age data, leaving 678 in the analysis (see Figure 2). Patients were primarily male and White, with a median age of 34 years (IQR 25–51) (Table 1). Study patients received a total of 8,830 units PRBCs in the first 24 hours of treatment. Individual patients received a median of nine units (IQR 5.5–15) PRBCs in the first 24 hours of presentation to the hospital. Patients who received ≥10 PRBC units received a median of 17 units (IQR 12–25) PRBCs, and patients who received <10 PRBC units received a median of six units (IQR 4–7) PRBCs in the first 24 hours. Figure 3 depicts the median number of units received by patients in each PRBC age group (0–7 days, 8–14 days, 15–21 days, and 22+ days), stratified by 24-hour mortality status. In other words, this graph shows the median number of PRBC units of each age group that was received for people who survived compared with those who did not. Therefore, each patient may be represented in more than one PRBC age category depending on their 24-hour mortality status and the ages of PRBC units they received. The median PRBC unit age was 19 days (IQR 13–27); the distribution of PRBC unit age varied widely across study sites, from 12 days (IQR 8–14) in site 1 to 28 days (IQR 24–31) in site 12. The distribution of ages for PRBCs transfused by study site is shown in Figure 4. Upon further analysis, no associations were found among study site and patient mortality.
Figure 2.
Flow chart of patients included in the analysis.
PROPPR = Pragmatic, Randomized Optimal Platelet and Plasma Ratios trial; PRBC = packed red blood cells; RTS = revised trauma score.
TABLE 1.
Patient and transfusiona characteristics (N = 678 subjects)
| Characteristic | All Patients | Massively Transfused (n=313) |
Non-Massively Transfused (n=365) |
|---|---|---|---|
| Age (years), median (IQR) | 34 (25–51) | 33 (24–52) | 35 (25–50) |
| Male sex, No. (%) | 541 (80.5) | 251 (80.2) | 293 (80.3) |
| Race, No. (%) | |||
| White | 432 (63.7) | 200 (63.9) | 232 (63.6) |
| Black | 186 (27.4) | 86 (27.5) | 100 (27.4) |
| Other | 60 (8.9) | 27 (8.6) | 33 (9.0) |
| Mechanism of injury, No. (%) | |||
| Blunt | 350 (51.6) | 174 (55.6) | 176 (48.2) |
| Penetrating | 320 (47.2) | 135 (43.1) | 185 (50.7) |
| Both | 8 (1.2) | 4 (1.3) | 4 (1.1) |
| Injury Severity Score, median (IQR) | 26 (17–41) | 33 (22–42) | 24 (14–34) |
| Revised Trauma Score, median (IQR) - n=605 | 6.8 (4.1–7.8) | 6.4 (4.1–7.6) | 6.9 (4.1–7.8) |
| Glasgow Coma Scale score, median (IQR) | 14 (3–15) | 13 (3–15) | 14 (3–15) |
| Systolic blood pressure (mm Hg), median (IQR) - n=656 | 102 (80–126) | 100 (80–126) | 104 (82–126) |
| Diastolic blood pressure (mm Hg), median (IQR) - n=561 | 70 (51–91) | 70 (50–91) | 70 (53–91) |
| Heart rate (beats per minute), median (IQR) – n=675 | 114 (94–133) | 121 (98–139) | 110 (93–127) |
| Respiratory rate (breaths per minute), median (IQR) – n=619 | 20 (17–26) | 20 (17–27) | 20 (18–26) |
| Total PRBCs transfused for all patients (n) | 8,830 units | 6,776 units | 2,054 units |
| PRBC unit age (days), median (IQR) | 19 (13–27) | 20 (16–26) | 19 (14–26) |
| Total PRBC units per subject (n), median (IQR) | 9 (5–15); min 1, max 115 |
17 (12–25); min 5, max 115 |
6 (4–7); min 1, max 9 |
| Total 0–7 day old PRBC units per subject (n), median (IQR) | 3 (2–5); min 1, max 38 | 0 (0–2); min 0, max 38 |
0 (0–0); min 0, max 8 |
| Total 8–14 day old PRBC units per subject (n), median (IQR) | 4 (2–7); min 1, max 58 | 3 (0–7); min 0, max 58 |
1 (0–1); min 0, max 9 |
| Total 15–21 day old PRBC units per subject (n), median (IQR) | 3 (2–6); min 1, max 35 | 3 (0–8); min 0, max 56 |
1 (0–2); min 0, max 8 |
| Total 22+ day old PRBC units per subject (n), median (IQR) | 5 (3–10); min 1, max 69 |
6 (2–12); min 0, max 69 |
1 (0–3); min 0, max 9 |
| Total PRBC units per study site (n), median (IQR) | 647.5 (499.5–795.0); min 230, max 2017 |
469.5 (350.5–583.0); min 150, max 1682 |
142 (98.5–260.0); min 76, max 335 |
Data reflect PRBCs transfused in the first 24 hours of treatment. All PRBCs given in the PROPPR trial were stored in one of three additive solutions. All solutions available in the United States and Canada have equivalent storage durations [29]. PRBC = packed red blood cells. IQR = interquartile range. Numbers may not equal 100% due to missing data.
Figure 3.
Volume of packed red blood cells transfused in the first 24 hours, 24-hour mortality, and the proportion of packed red blood cell per storage age group. For each graph, squares demonstrates the mortality rate and confidence interval (center) for the patients (top right) who received that specific combination of the total packed red blood cell units and the proportion of packed red blood cell units stored for more than 7 (3A.), 14 (3B.), and 21 (3C.) days.
Figure 4.

Proportion of PRBC ages for transfused among PROPPR study sites.
PRBC = packed red blood cells; PROPPR = Pragmatic, Optimal Platelet and Plasma Ratios trial.
One hundred (14.8%) patients died within the first 24 hours of hospitalization (Table 2). The most common major adverse events were infection (30.1%), sepsis (28.0%) and acute kidney injury (23.5%). At least two serious adverse events occurred in 43.5% of subjects. The composite outcome [24-hour death or ≥2 adverse events] occurred in 57.1%. The 30-day mortality rate was 24.2%.
TABLE 2.
Outcomes and major adverse events, n=678 patients
| Adverse Event | N (%) |
|---|---|
| Major Adverse Events | |
| Acute lung injury | 104 (15.3) |
| Acute kidney injury | 159 (23.5) |
| Acute respiratory distress syndrome | 94 (13.9) |
| Deep vein thrombosis | 49 (7.2) |
| Infectiona | 204 (30.1) |
| Multiple organ failure | 35 (5.2) |
| Myocardial infarction | 2 (0.3) |
| Pulmonary embolism, asymptomatic | 22 (3.2) |
| Pulmonary embolism, symptomatic | 27 (4.0) |
| Sepsis | 190 (28.0) |
| Stroke | 19 (2.8) |
| Ventilator-associated pneumonia | 120 (17.7) |
| Transfusion-associated circulatory overload | 1 (0.2) |
| Transfusion-related metabolic complicationb | 112 (16.5) |
| Rhabdomyolysis | 18 (2.7) |
| 24-Hour Death | 100 (14.8) |
| ≥2 Adverse Events | 195 (43.5) |
| 24-Hour Death or ≥2 Adverse Events | 387 (57.1) |
| 30-Day Death | 164 (24.2)c |
Includes urinary tract infection, wound infection, line infection, etc.
Includes hypocalcemia, hyperkalemia
30-day status not known for 4 subjects
On multivariable analysis, the number of PRBCs ≥22 days old was independently associated with increased 24-hour death (adjusted odds ratio [OR] of 1.05 per PRBC unit; 95% CI: 1.01–1.08) (Table 3). In other words, transfusion of each additional unit of PRBCs aged ≥22 days was associated with a 5% increase in mortality risk. However, this association was noted only for patients who received ≥10 PRBC units; when PRBC volume was modeled as a categorical variable (1–10 units, 11–20 units, 21+ units), increased 24-hour death was limited to patients receiving over 21 units of 22+ day old PRBC units (Table 4).
TABLE 3.
Associations between PRBC age and 24-hour mortality. Data in cells reflect odds ratios (OR) for death per additional PRBC unit received. Odds ratios estimated from random effects models, accounting for clustering by study site. Multivariable models adjusted for patient age, sex, race, mechanism of injury, Injury Severity Score, Revised Trauma Score and PROPPR trial treatment group. Model for All Patients additionally adjusted for total PRBC units transfused in the first 24 hours after admission. PRBC = Packed red blood cell.
| Variable | Unadjusted OR (95% CI) |
Multivariable Adjusted – All Patients OR (95% CI) |
Multivariable Adjusted – Patients receiving ≥10 units PRBCs (n=313) OR (95% CI) |
Multivariable Adjusted - Patients receiving <10 units PRBCs (n=361) OR (95% CI) |
|---|---|---|---|---|
| PRBC Age Category | ||||
| 0–7 days | 0.96 (0.89–1.06) | 0.97 (0.88–1.08) | 0.96 (0.86–1.08) | 0.95 (0.68–1.32) |
| 8–14 | 1.06 (1.03–1.10) | 1.04 (0.99–1.09) | 1.04 (0.99–1.09) | 1.00 (0.79–1.27) |
| 15–21 | 1.02 (0.98–1.05) | 1.02 (0.98–1.06) | 1.02 (0.98–1.06) | 0.86 (0.64–1.14) |
| 22+ | 1.04 (1.02–1.07) | 1.05 (1.01–1.08) | 1.05 (1.01–1.08) | 1.05 (0.83–1.32) |
| Total PRBC Units | ||||
| 1–10 | Referent | |||
| 11–20 | 1.43 (0.77–2.65) | |||
| 21+ | 1.09 (0.40–2.98) | |||
| Patient age | 1.01 (1.00–1.03) | 1.02 (1.00–1.03) | 1.02 (0.99–1.04) | |
| Gender (male vs female) | 1.23 (0.68–2.21) | 1.92 (0.89–4.11) | 0.62 (0.22–1.75) | |
| Race | ||||
| White | Referent | Referent | Referent | |
| Black | 1.52 (0.84–2.79) | 1.46 (0.68–3.16) | 1.36 (0.46–4.06) | |
| Other | 0.55 (0.20–1.56) | 0.71 (0.21–2.47) | 0.27 (0.03–2.29) | |
| Mechanism of injury | ||||
| Blunt vs. penetrating | 1.41 (0.71–2.78) | 2.08 (0.97–4.46) | 0.86 (0.30–2.44) | |
| Burn vs. penetrating | 6.01 (0.98–37.0) | N/A | N/A | |
| Injury Severity Score | 1.03 (1.01–1.05) | 1.55 (1.11–2.17) | 1.32 (0.80–2.17) | |
| Revised Trauma Score | 0.66 (0.56–0.77) | 0.52 (0.37–0.72) | 0.35 (0.22–0.55) | |
| PROPPR trial treatment group | 1.45 (0.85–2.50) | 1.03 (0.55–1.94) | 2.24 (0.97–5.17) | |
TABLE 4.
Associations between PRBC age and 24-hour mortality, stratified by PRBC age and volume.
| Total Volume (Units) of Each PRBC Age Category | |||
|---|---|---|---|
| PRBC Age Category | 1–10 | 11–20 | 21+ |
| 0–7 days | 0.82 (0.44–1.51) (n=196) |
N/A (n=4) |
N/A (n=6) |
| 8–14 | 1.62 (0.88–2.99) (n=377) |
0.92 (0.23–3.65) (n=32) |
6.41 (0.88–46.5) (n=13) |
| 15–21 | 0.72 (0.40–1.30) (n=379) |
1.20 (0.36–3.93) (n=26) |
1.85 (0.52–6.53) (n=23) |
| 22+ | 2.56 (1.19–5.48) (n=384) |
7.80 (2.99–20.34) (n=64) |
4.18 (1.24–14.04) (n=36) |
Data in cells reflect adjusted odds ratios for death per additional unit of PRBC received. Odds ratios estimated from a multivariable random effects model adjusted for patient age, sex, race, mechanism of injury, Injury Severity Score, Revised Trauma Score, total PRBC units transfused in the first 24 hours after admission, PROPPR trial treatment group, and accounting for clustering by study site. Patients may be represented in more PRBC = Packed red blood cell.
Increased numbers of 8–14, 15–21 and 22+ day old PRBC units were associated with increased odds of the composite outcome of [≥2 adverse events or 24-hour death] (Table 5). When PRBC volume was characterized on a categorical basis, these associations persisted for 8–14 and 22+ day old PRBC units. The number of 15–21 and 22+ day old PRBCs were associated with increased 30-day mortality.
TABLE 5.
Associations between PRBC age, major transfusion-related adverse events and 30-day death
| PRBC Age Category | ≥2 Adverse Events or 24-Hour Death OR (95% CI) |
≥2 Adverse Events or 24-Hour Death OR (95% CI) |
30-Day Death OR (95% CI) |
30-Day Death OR (95% CI) |
|---|---|---|---|---|
|
PRBC Volume (Units) as Continuous Variable | ||||
| 0–7 days | 1.08 (0.995–1.17) | 1.00 (0.91–1.11) | ||
| 8–14 | 1.13 (1.06–1.19) | 1.03 (0.98–1.08) | ||
| 15–21 | 1.08 (1.03–1.14) | 1.06 (1.02–1.10) | ||
| 22+ | 1.11 (1.07–1.16) | 1.09 (1.04–1.12) | ||
|
PRBC Volume (Units) as Categorical Variable | ||||
| 0–7 days | ||||
| 0 units | Referent | Referent | ||
| 1–10 | 0.87 (0.57–1.33) | 0.95 (0.56–1.63) | ||
| 11–20 | N/A | N/A | ||
| 21+ | 1.02 (0.13–8.32) | 0.44 (0.01–21.29) | ||
| 8–14 days | ||||
| 0 units | Referent | Referent | ||
| 1–10 | 1.80 (1.20–2.69) | 1.42 (0.81–2.50) | ||
| 11–20 | 2.87 (1.07–7.73) | 1.98 (0.70–5.59) | ||
| 21+ | 3.90 (0.64–23.84) | 2.20 (0.19–25.47) | ||
| 15–21 days | ||||
| 0 units | Referent | Referent | ||
| 1–10 | 0.94 (0.64–1.37) | 1.15 (0.67–1.96) | ||
| 11–20 | 3.35 (0.90–12.52) | 1.66 (0.49–5.59) | ||
| 21+ | 2.23 (0.58–8.51) | 4.49 (1.38–14.65) | ||
| 22+ days | ||||
| 0 units | Referent | Referent | ||
| 1–10 | 1.62 (1.05–2.52) | 1.90 (1.003–3.58) | ||
| 11–20 | 4.48 (2.08–9.68) | 12.46 (5.09–30.51) | ||
| 21+ | 6.83 (2.24–20.81) | 5.11 (1.59–16.41) | ||
Odds ratios (OR) estimated from random effects models, adjusted for patient age, sex, race, mechanism of injury, Injury Severity Score, Revised Trauma Score, total PRBC units transfused in the first 24 hours after admission, PROPPR trial treatment group, and accounting for clustering by study site. PRBC = packed red blood cell.
When repeating the analysis using imputed RTS values for the 73 patients with missing respiratory rate or systolic blood pressure or both, we observed slightly different results (see Table, Supplemental Digital Content 1). With PRBC volume characterized as a continuous variable, the number of 22+ day and 8–14 day PRBC units were significantly associated with 24-hour death. When PRBC volume was modeled on a categorical basis, the association between the number of 22+ day old PRBC units and 24-hour death persisted. In addition, the number of 8–14 day old PRBC units was associated with increased odds of death.
When characterized on a continuous basis, the number of PRBC units was associated with increased odds of 24-hour death or ≥2 adverse events, regardless of PRBC unit age (see Table, Supplemental Digital Content 2). However, when examining PRBC age on a categorical basis, this association seemed to be limited to 8–14 day and 22+ day old PRBC. There was also evidence of increased 30-day mortality with the number of 8–14 day, 15–21 day and 22+ day PRBC units.
LIMITATIONS
This analysis has important limitations; prospective validation of these results is mandatory prior to changes in clinical practice. For example, total transfused PRBC volume may serve as a proxy of the degree of physiologic damage or injury. Although we accounted for site clustering using random effects regression, we observed notable variation in the median PRBC age across the study sites suggesting that practice variation may influence these results. In terms of allocating PRBC units based on age, this aspect of care was not incorporated as part of the treatment protocol for patients enrolled in the PROPPR trial. Furthermore, no standard protocols exist to guide blood banks or emergency medical services in the choice of PRBC units based on storage age. Thus, these factors may have also influenced outcomes on both the patient and study site level.
While our sensitivity analyses supported the primary findings, some of the additional findings exhibited uncertain patterns and wide confidence intervals. These results may indeed be attributed to large number of clinical and demographic variables included in the analysis to properly control for confounding factors; in fact, it is possible that these findings could be random. As such, caution should be used when interpreting these findings; application to clinical settings is not recommended. A randomized controlled trial is the only way to overcome these limitations and to clarify the effect of PRBC age on traumatic hemorrhagic shock. Additional efforts must also be taken to identify those patient factors associated with vulnerability to stored blood toxicity.
The purpose of the PROPPR trial was to compare blood product ratios, not PRBC age. While the trial applied strict inclusion and exclusion criteria and employed standardized protocols at all study sites, variations in patient-mix and clinical protocols used over the course of hospitalization may have influenced the results. Other factors of influence to consider include: time from injury to initiation of care, diagnosed and undiagnosed comorbidities, and variation in care outside of transfusion protocols, both within and among study sites. While we used panel regression techniques to account for clustering by study center, we limited further inference to protect site confidentiality. Finally, we must acknowledge the limitations associated with a secondary analysis of a randomized trial. Our findings may suggest a possible risk of adverse outcomes related to the transfusion of stored PRBCs in the trauma population, but require validation with a Level I prospective study specifically designed to address the issue of blood age in the trauma setting.
Although we focused on the effect of transfused PRBCs, platelets are also associated with cellular breakdown during storage and increased risk of complications after trauma.20, 21 In addition, practitioners and blood banks may have selected specific aged PRBCs for massive transfusion. However, given the tempo of PRBC transfusions in PROPPR, we do not expect systematic bias. Finally, the nature of the PROPPR trial did not allow for definitive diagnosis of all injuries prior to study enrollment. As such, patients with traumatic brain injury associated with coagulopathy and complications after trauma were included in the study sample.22
DISCUSSION
In the current analysis, the transfusion of 22+ day old PRBCs was associated with increased 24-hour death in patients who received ≥10 units in the first 24 hours after hospital admission. The magnitude of associated harm was related to the number of such units transfused. Thus, critically injured patients who required transfusion of ≥10 PRBC units experienced a 5% increase in their risk of mortality with transfusion of each additional PRBC unit aged 22 days or more. The prevalence of adverse events and 30-day mortality was similarly higher for those receiving older PRBC units, with hints of these harmful effects with PRBC units as young as 8 days old. These observations highlight the potentially toxic effects of transfusion with older PRBC units in traumatic hemorrhagic shock. It must be noted that these risks will not necessarily increase in a linear fashion as suggested by the results. In fact, with transfusion of each additional PRBC unit, the patient’s status will change and their risk of mortality altered based on their current physiologic condition. Therefore, caution must be used in the interpretation of these findings. Application to clinical practice is not warranted based on these results; rather, additional randomized studies are needed to confirm these findings among trauma patients who receive ≥10 units PRBCs.
Our analysis has important contrasts with prior trial of transfused PRBC age.13 The INFORM trial randomized 20,858 patients requiring PRBC transfusion either treatment with freshest or oldest-available PRBC units, finding no difference in mortality.17 However, the trial excluded patients requiring uncrossmatched or massive quantity blood transfusion. Similarly, the TRANSFUSE, ABlE, and RECESS studies compared the effect of the freshest available PRBCs with the standard-issue or oldest available PRBCs in intensive care patients, critically ill patients, and elective cardiac surgery patients, respectively.10,11,12 These studies found no difference in mortality, length of stay, or complications among those who received fresh PRBCs versus standard-issue PRBCs. However, only 15% of patients were injured, and the subjects received a limited number of PRBC units (median 4 units or less).10–12, 17 Our use of the PROPPR data offered perspectives from a large, multicenter series of very high acuity traumatic hemorrhagic shock patients receive large volumes of blood products in rapid fashion.14 We observed that the transfusion of 22+ day old blood was most consistently associated with poor outcomes among patients who were massively transfused. However, the odds of 30-day death appeared to also be associated with the number of 15–21 day old PRBC units. In addition, the odds of negative outcomes (e.g., the composite outcome of [≥2 adverse events or 24-hour death]) also appeared to be associated with the number of 8–14 day and 15–21 day old PRBC units.
Therefore, while our results most clearly suggest avoiding PRBCs greater than 22 days old in those who are massively transfused, refraining from or minimizing 8–14 and 15–21 day old PRBC in this population may have merit. Though we observed that this association, transfusion strategies used in clinical practice must be selected prospectively in response to anticipated transfusion need; post hoc classification of massive transfusion is not clinically useful. Randomized controlled trials that examine the effect of stored PRBC units of all ages are, therefore, still needed in this patient population. Thus, it follows that the natural next step includes a trial that enrolls only adult patients with major trauma who are anticipated to require massive transfusion, and who are randomized to receive all fresh or all standard-issue stored PRBCs. Finally, our findings suggest that development of a “stored blood index” may be useful to clinicians and investigators. Such an index may quantify the risk of adverse outcomes associated with transfusion of each unit of PRBCs based on storage age.
An important limitation of our analysis is the absence of PRBC age randomization. Multiple factors may influence the patterns of RBC age given to a patient, including confounding by indication and the PRBC storage practices of an institution. A randomized trial assigning patients in traumatic hemorrhagic shock to younger versus older PRBC units may help to clarify this association. An alternate strategy may entail defining the harmful mechanisms of stored blood toxicity, potentially leading to pharmacologic interventions to block the harmful effects of stored PRBCs.
In summary, the transfusion of older PRBCs in patients with traumatic hemorrhagic shock who received ≥10 PRBC units was associated with worsened outcomes. Injured patients requiring massive blood transfusion may benefit from receipt of fresh PRBC units.
Supplementary Material
Financial support and acknowledgements:
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863), the U.S. Department of Defense, as well as Defence Research and Development Canada in partnership with the Canadian Institutes of Health Research (CIHR), Institute of Circulatory and Respiratory Health (CRR-120612). The opinions or conclusions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of any sponsor. This manuscript has been reviewed by the PROPPR Publication Committee for scientific content and consistency of data interpretation with previous PROPPR publications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS
Ethics approval and consent to participate:
This study was approved by the Institutional Review Board of the University of Alabama at Birmingham. As this study involved secondary analysis of existing data, no participant consent was needed.
Consent for publication:
Not applicable
Availability of data and material:
The datasets generated and/or analysed during the current study are available in the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center, available on request at: https://biolincc.nhlbi.nih.gov/studies/proppr/
Competing interests:
JRH receives patent royalties from the United States Army and the University of Maryland for improved red blood cell storage solutions. The rest of the authors declare that they have no competing interests.
Trial registration: Clinicaltrials.gov NCT01545232. First submitted 29 February 2012.
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006;60:S3–11. [DOI] [PubMed] [Google Scholar]
- 2.Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia 2015;70 Suppl 1:29–e12. [DOI] [PubMed] [Google Scholar]
- 3.Oh JY, Stapley R, Harper V, Marques MB, Patel RP. Predicting storage-dependent damage to red blood cells using nitrite oxidation kinetics, peroxiredoxin-2 oxidation, and hemoglobin and free heme measurements. Transfusion 2015;55:2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess JR. Measures of stored red blood cell quality. Vox Sang 2014;107:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Jackman RP, Utter GH, Muench MO, et al. Distinct roles of trauma and transfusion in induction of immune modulation after injury. Transfusion 2012;52:2533–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin-Yee IH, Gray-Statchuk L, Milkovich S, Ellis CG. Transfusion of stored red blood cells adhere in the rat microvasculature. Transfusion 2009;49:2304–2310. [DOI] [PubMed] [Google Scholar]
- 7.Stapley R, Rodriguez C, Oh JY, et al. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med 2015;85:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion 2012;52:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu L, Triulzi DJ. Clinical effects of red blood cell storage. Cancer Control 2015;22:26–37. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DJ, McQuilten ZK, Nichol A, et al. Age of Red Cells for Transfusion and Outcomes in Critically Ill Adults. N Engl J Med 2017. [DOI] [PubMed]
- 11.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410–1418. [DOI] [PubMed] [Google Scholar]
- 12.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015;372:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med 2016;375:1937–1945. [DOI] [PubMed] [Google Scholar]
- 14.Sparrow RL. Red blood cell storage duration and trauma. Transfus Med Rev 2015;29:120–126. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcomb JB, Tilley BC, Baraniuk S, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial. 2015. National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center
- 17.Cook RJ, Heddle NM, Lee KA, et al. Red blood cell storage and in-hospital mortality: a secondary analysis of the INFORM randomised controlled trial. Lancet Haematol 2017. [DOI] [PubMed]
- 18.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg 2013;74:396–400; discussion 400–392. [DOI] [PubMed] [Google Scholar]
- 19.Rubin D Multiple Imputation for Nonresponse in Surveys. Wiley Series in Probability and Mathematical Statistics: Applied Probability and Statistics New York, NY: Wiley & Sons; 1987. [Google Scholar]
- 20.Perales Villarroel JP, Figueredo R, Guan Y, et al. Increased platelet storage time is associated with mitochondrial dysfunction and impaired platelet function. J Surg Res 2013;184:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Branco BC, Rhee P, et al. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma 2011;71:1766–1773; discussion 1773–1764. [DOI] [PubMed] [Google Scholar]
- 22.Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: a systematic review and meta-analysis. Injury 2014;45:819–824. [DOI] [PubMed] [Google Scholar]
- 23.Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg 2012;73:S128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangalmurti NS, Xiong Z, Hulver M, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood 2009;113:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson SE, Johnson RA, Craig T, et al. Transfusion-related acute lung injury in a rat model of trauma-hemorrhage. J Trauma 2011;70:466–471. [DOI] [PubMed] [Google Scholar]
- 26.Cortes-Puch I, Wang D, Sun J, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood 2014;123:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology 2012;117:1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron DM, Beloiartsev A, Nakagawa A, et al. Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs*. Crit Care Med 2013;41:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.