Abstract
Background
Approximately 800 million women and children have anaemia, a condition thought to cause almost 9% of the global burden of years lived with disability. Around half this burden could be amenable to interventions that involve the provision of iron. Maize (corn) is one of the world's most important cereal grains and is cultivated across most of the globe. Several programmes around the world have fortified maize flour and other maize‐derived foodstuffs with iron and other vitamins and minerals to combat anaemia and iron deficiency.
Objectives
To assess the effects of iron fortification of maize flour, corn meal and fortified maize flour products for anaemia and iron status in the general population.
Search methods
We searched the following international and regional sources in December 2017 and January 2018: Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; MEDLINE (R) In Process; Embase; Web of Science (both the Social Science Citation Index and the Science Citation Index); CINAHL Ebsco; POPLINE; AGRICOLA (agricola.nal.usda.gov); BIOSIS (ISI); Bibliomap and TRoPHI; IBECS; Scielo; Global Index Medicus ‐ AFRO (includes African Index Medicus); EMRO (includes Index Medicus for the Eastern Mediterranean Region); LILACS; PAHO (Pan American Health Library); WHOLIS (WHO Library); WPRO (includes Western Pacific Region Index Medicus); IMSEAR, Index Medicus for the South‐East Asian Region; IndMED, Indian medical journals; and the Native Health Research Database. We searched clinicaltrials.gov and the International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned studies on 17 January 2018 and contacted authors of such studies to obtain further information or eligible data if available.
For assistance in identifying ongoing or unpublished studies, we also contacted relevant international organisations and agencies working in food fortification on 9 August 2016.
Selection criteria
We included cluster‐ or individually randomised controlled trials and observational studies. Interventions included (central/industrial) fortification of maize flour or corn meal with iron alone or with other vitamins and minerals and provided to individuals over 2 years of age (including pregnant and lactating women) from any country.
Data collection and analysis
Two review authors independently assessed the eligibility of studies for inclusion, extracted data from included studies and assessed the risk of bias of the included studies. Trial designs with a comparison group were included to assess the effects of interventions. Trial designs without a control or comparison group (uncontrolled before‐and‐after studies) were included for completeness but were not considered in assessments of the overall effectiveness of interventions or used to draw conclusions regarding the effects of interventions in the review.
Main results
Our search yielded 4529 records. After initial screening of titles and abstracts, we reviewed the full text of 75 studies (80 records). We included 5 studies and excluded 70. All the included studies assessed the effects of providing maize products fortified with iron plus other vitamins and minerals versus unfortified maize flour. No studies compared this intervention to no intervention or looked at the relative effect of flour and products fortified with iron alone (without other vitamins and minerals). Three were randomised trials involving 2610 participants, and two were uncontrolled before‐and‐after studies involving 849 participants.
Only three studies contributed data for the meta‐analysis and included children aged 2 to 11.9 years and women. Compared to unfortified maize flour, it is uncertain whether fortifying maize flour or corn meal with iron and other vitamins and minerals has any effect on anaemia (risk ratio (RR) 0.90, 95% confidence interval (CI) 0.58 to 1.40; 2 studies; 1027 participants; very low‐certainty evidence), or on the risk of iron deficiency (RR 0.75, 95% CI 0.49 to 1.15; 2 studies; 1102 participants; very low‐certainty evidence), haemoglobin concentration (mean difference (MD) 1.25 g/L, 95% CI −2.36 to 4.86 g/L; 3 studies; 1144 participants; very low‐certainty evidence) or ferritin concentrations (MD 0.48 µg/L, 95% CI −0.37 to 1.33 µg/L; 1 study; 584 participants; very low‐certainty evidence).
None of the studies reported on any adverse effects. We judged the certainty of the evidence to be very low based on GRADE, so we are uncertain whether the results reflect the true effect of the intervention. We downgraded evidence due to high risk of selection bias and unclear risk of performance bias in one of two included studies, high heterogeneity and wide CIs crossing the line of no effect for anaemia prevalence and haemoglobin concentration.
Authors' conclusions
It is uncertain whether fortifying maize flour with iron and other vitamins and minerals reduces the risk of anaemia or iron deficiency in children aged over 2 years or in adults. Moreover, the evidence is too uncertain to conclude whether iron‐fortified maize flour, corn meal or fortified maize flour products have any effect on reducing the risk of anaemia or on improving haemoglobin concentration in the population.
We are uncertain whether fortification of maize flour with iron reduces anaemia among the general population, as the certainty of the evidence is very low. No studies reported on any adverse effects.
Public organisations funded three of the five included studies, while the private sector gave grants to universities to perform the other two. The presence of industry funding for some of these trials did not appear to positively influence results from these studies.
The reduced number of studies, including only two age groups (children and women of reproductive age), as well as the limited number of comparisons (only one out of the four planned) constitute the main limitations of this review.
Plain language summary
Fortification of maize flour with iron for preventing anaemia and iron deficiency
What is the aim of this review?
The aim of this Cochrane Review was to determine the effects of fortifying maize flour, corn meal and fortified‐maize flour products with iron for anaemia and iron status in the general population. We searched for relevant published studies to answer this question and analysed all relevant information.
Key messages
It is uncertain whether fortifying maize flour with iron and other vitamins and minerals reduces the risk of iron deficiency. The evidence is also too uncertain to conclude whether iron‐fortified maize flour, corn meal or fortified maize flour products have any effect on reducing the risk of anaemia or on improving haemoglobin concentration in the population. We do not know whether fortifying maize flour with iron reduces anaemia in the general population, as the evidence was very unreliable. No studies reported on any harmful effects.
What was studied in the review?
Approximately 496 million non‐pregnant women, 32 million pregnant women, and 273 million children were thought to be anaemic as of 2011. Iron deficiency is considered to be the single most prevalent nutrient deficiency worldwide, but at least half this burden is considered responsive to interventions that involve providing people with iron. Fortification means adding vitamins and minerals to foods to increase their nutritional value. In public health, fortifying staple foods is considered one way of reducing micronutrient deficiencies without changing usual and culturally acceptable diets.
Maize (corn) is one of the world's most important cereal grains. In sub‐Saharan Africa, some parts of Southeast Asia and Latin America, where iron deficiency is endemic, maize is a dietary staple for more than 200 million people. Fortification of maize flour with iron (and in some cases, other nutrients) is mandatory in Brazil, Costa Rica, El Salvador, Kenya, Mexico, Nigeria, Rwanda, South Africa, Tanzania, Uganda, the USA and Venezuela.
What are the main results of the review?
We found five relevant studies, but none looked at the effects of maize flour or maize flour products fortified with iron alone. Five studies compared the effects of maize flour or maize‐flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize‐flour products (not containing iron or any other vitamin and minerals). Three of these five studies randomly divided a collective total of 2302 children aged 2 to 12 years and 130 indigenous women into groups receiving fortified versus unfortified maize flour or corn flour. One study was from Kenya and the other two took place in Mexico. The remaining two studies did not provide data to assess the effects of fortification relative to a comparison group. Three of five of the included studies were funded by public organisations and two by funds granted by private sector to universities. The presence of industry funding for some of these trials did not appear to positively influence results from these studies.
It is uncertain whether fortifying maize flour with iron and other vitamins and minerals reduces the risk of iron deficiency in the general population. The evidence is also too uncertain to conclude whether iron‐fortified maize flour, corn meal or fortified maize flour products have any effect on reducing the risk of anaemia or on improving haemoglobin concentration in the population. We are uncertain whether fortification of maize flour with iron reduces anaemia among the general population.
How up‐to‐date is this review?
We searched for studies that had been published up to 17 January 2018.
Summary of findings
Summary of findings for the main comparison. Provision of maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products.
| Provision of maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products | ||||||
| Patient or population: general population older than 2 years of age (including pregnant women), in Kenya and Mexico Setting: population‐based intervention Intervention: maize flour or maize flour products fortified with iron plus other vitamins and minerals Comparison: unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unfortified maize flours or maize flour products | Risk with maize flour or products fortified with iron plus other vitamins and minerals | |||||
| Anaemia (defined as haemoglobin (Hb) below WHO cut‐off, adjusted for altitude and smoking, as appropriate) | Study population | RR 0.90 (0.58 to 1.40) | 1027 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d,e | Included studies: Andang'o 2007; Villalpando 2002 (C); data for Villalpando 2002 (C) is adjusted for the clustering effect. | |
| 300 per 1000 | 270 per 1000 (174 to 420) | |||||
| Iron deficiency (as defined by trialists, based on a biomarker of iron status) | Study population | RR 0.75 (0.49 to 1.15) | 1102 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d,e | Included studies: Andang'o 2007; Villalpando 2002 (C) | |
| 220 per 1000 | 116 per 1000 (88 to 151) | |||||
| Haemoglobin concentration (in g/L) | MD 1.25 g/L higher (2.36 lower to 4.86 higher) | — | 1144 (3 RCTs) | ⊕⊝⊝⊝ Very lowe,f,g,h | Included studies: Andang'o 2007; Carrasco 2011 (C); Villalpando 2002 (C). Carrasco 2011 (C) reported a change in haemoglobin concentration from 131 g/L to 133 g/L in the fortified group and from 131 g/L to 132 g/L in the control group (no reported measures of dispersion of data) after 10 months of receiving fortified maize flour. Data for Villalpando 2002 (C) is adjusted for the clustering effect. We include some imputed SDs and adjustment for clustering. | |
| Ferritin concentration (in µg/L) | MD 0.48 µg/L (0.37 lower to 1.33 higher) | — | 584 (1 RCT) | ⊕⊝⊝⊝ Very lowd,e,i,j | Included study: Villalpando 2002 (C) | |
| Any adverse effects (including constipation, nausea, vomiting, heartburn or diarrhoea, as measured by trialists) | No studies reported on any adverse effects. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe risk of bias was high in three domains in Andang'o 2007 and high or unclear in all other domains in Villalpando 2002 (C). bThere is inconsistency in the results of the two included studies, one suggesting a benefit, and another suggesting the opposite effect. cDowngraded for indirectness, as both studies were in schoolchildren in rural settings in Mexico (6‐11 years of age) and Kenya (aged 3‐8 years), that provided products (tortillas and porridge) prepared with maize flour following different preparations. dThere is imprecision, as the confidence intervals are wide. dOne study is unpublished in the scientific literature and available only with a link to a draft in the agency site (Villalpando 2002 (C)). fThe risk of bias was high in three domains in all included trials. gThere is inconsistency in the results of the three included studies, two suggesting a benefit (Carrasco 2011 (C); Villalpando 2002 (C)), and one suggesting the opposite effect (Andang'o 2007). hDowngraded for indirectness, as the studies were conducted in rural settings in Mexico (Carrasco 2011 (C) among women 14‐65 years of age; Villalpando 2002 (C) among schoolchildren 6‐11 years of age; and Kenya (Andang'o 2007: among schoolchildren aged 3‐8 years) that provided products (nixtamalised‐maize flour tortillas (Carrasco 2011 (C); Villalpando 2002 (C)) and porridge (Andang'o 2007)) prepared with maize flour following different preparations. iThe risk of bias was high or unclear in all domains in Villalpando 2002 (C). jDowngraded for indirectness, as there is only one study contributing data for this outcome, and the trial was conducted in rural settings in Mexico in schoolchildren 6‐11 years of age (Villalpando 2002 (C)) .
Background
Description of the condition
Approximately 496 million non‐pregnant women, 32 million pregnant women, and 273 million children were thought to be anaemic as of 2011 (WHO 2015), with only a modest reduction in the prevalence over the preceding two decades (Stevens 2013). Anaemia may contribute almost 9% of the global burden of years lived with disability (Kassebaum 2014). About half this burden is considered responsive to interventions that involve provision of iron (WHO 2015), confirming the importance of iron deficiency to this problem. Iron deficiency is thus considered to be the single most prevalent nutrient deficiency worldwide.
Iron deficiency is associated with considerable morbidity across the life cycle. In preschoolers, iron‐deficiency anaemia appears to be associated with potentially irreversible impairments in cognitive development, and in school‐aged children iron‐deficiency anaemia is associated with learning problems and poor educational performance (Beard 2007), while restitution of iron deficiency in school‐aged children improves cognitive outcomes (Low 2013). In adolescents and non‐pregnant women, iron deficiency is associated with impaired physical capacity (Pasricha 2014), as well as reduced work performance. Prevention of iron‐deficiency anaemia during pregnancy improves birth weight (Peña‐Rosas 2015), a condition which in turn is associated with reduced infant iron endowment and a subsequent increased risk of iron‐deficiency anaemia (Wharton 1999). Maternal iron deficiency is a global problem that may contribute to high rates of maternal depression and non‐responsive caregiving (Black 2011).
The principal causes of iron deficiency include inadequate dietary iron intake due to a low‐iron diet or one that contains inhibitors of iron absorption (Nair 2009), along with increased loss of iron because of chronic blood loss, in some settings due to intestinal hookworm infection (Stoltzfus 1996). Poor dietary intake and limited bioavailability (the quantity or fraction of the iron consumed that is absorbed and utilised) is considered a major contributor to the global burden of iron deficiency. Populations consuming diets that chiefly comprise cereals such as maize, wheat and rice, with inadequate intake of iron‐rich foods (in particular meat, but also legumes, nuts and other vegetables), are at high risk of iron deficiency. Cereals (including maize) contain phytates, which bind to iron and prevent its absorption in the intestine (Sharpe 1950).
Iron deficiency is most likely to occur during times of increased iron requirements. Most commonly, iron deficiency occurs in toddlers when rapid growth results in expansion of the blood volume and an escalation in iron requirements for production of red blood cells, during adolescence when growth and red cell production escalates again (Wharton 1999), in adolescent girls due to the onset of menstruation and its associated blood loss, and in pregnant women who must supply iron to the developing foetus and are undergoing expansion in blood volume and vigorous erythropoiesis (Scholl 2000).
Strategies to improve iron intake include improving overall dietary diversity, supplementation, point‐of‐use fortification of foods with micronutrient powders and fortification of staple foods with iron (Pasricha 2013). Increasing the availability and consumption of a nutritionally adequate diet is the most sustainable long‐term solution, not just for overcoming iron deficiency and anaemia, but for overcoming other micronutrient deficiencies as well (FAO 2011). Food‐based approaches include increasing overall food intake; increasing consumption of micronutrient‐rich foods; modifying intake of dietary iron inhibitors and enhancers; using improved processing, preservation and preparation techniques; educating consumers for behaviour change, improving food quality, safety and public health; and fortifying foods (FAO 2011).
Wheat, maize and rice represent the most important cereal crops for human consumption, accounting for 94% of the total cereal consumption (Ranum 2014). With an ability to grow in diverse climates, maize – the world's primary coarse grain – is cultivated in most parts of the world, although the vast quantity of production is concentrated in the Americas, especially the USA. In that country, transgenic (genetically modified) maize accounts for 85% of plantings. The major export markets have shifted increasingly to low‐ and middle‐income countries. In 2012, the total world production of maize was over 875 million tonnes, with the USA, China and Brazil producing the vast majority of the total volume (Ranum 2014). Currently, about 55% of world consumption of coarse grains is used for animal feed, but in many countries (mainly in sub‐Saharan Africa and Latin America), they are also directly used for human consumption. At the global level, about 17% of aggregate consumption of coarse grains is devoted to food, but the share rises to as much as 80% in sub‐Saharan Africa (FAO 2012).
Description of the intervention
Fortification is the addition of micronutrients to foods and is usually applied centrally, at the point of food production. Due to the relatively low cost and potential for wide distribution, food fortification has been proposed as one of the most cost‐effective of all health interventions. The success of fortification depends on several factors. The food 'vehicle' to which the fortificant is added must be consumed in adequate quantities by the population at risk of the micronutrient deficiency. Additionally the fortificants used must be effectively absorbed and should not diminish the taste, colour or smell of the food (WHO/FAO 2006). A variety of iron fortificants are suitable for use in flours, including ferrous sulphate, elemental iron powders, ferrous fumarate and sodium iron ethylenediaminetetraacetate (EDTA) (Hurrell 2002; Hurrell 2010).
For its part, maize (corn) is one of the world's most important cereal grains. It is a dietary staple for more than 200 million people and provides approximately 20% of the world's calories (Nuss 2010). Maize products include corn flour, porridges, breakfast cereals, tortillas, tamales and arepas. Maize has comparable energy density to other cereal crops and is a relatively good source of vitamin A, but it is also rich in phytate, a compound that potently inhibits iron availability for absorption (McKevith 2004). In sub‐Saharan Africa, some parts of Southeast Asia and Latin America, where iron deficiency is endemic, maize is a dietary staple (Nuss 2010). For example, consumption of maize in the World Health Organization (WHO) African Region ranges from 52 g to 328 g per person per day, while in the Americas it ranges from 50 g to 267 g/person/day; consumption is much lower in the Western Pacific Region and elsewhere in Asia (Ranum 2014).
Maize processing and products
A maize kernel comprises the outer covering (pericarp and aleurone), the endosperm (the largest fraction of the kernel), the germ (the embryo and scutellum), and the tip cap (Gwirtz 2014). Genetic background, variety, environmental conditions, plant age and geographic location can impact kernel composition within and between maize varieties (Nuss 2010). Maize contains about 72% starch, mainly found in the endosperm. The predominant source of fibre is the pericarp, although smaller amounts of fibre are also found in the endosperm. Maize contains about 10% protein, chiefly in the endosperm and germ: importantly, the essential amino acids lysine and tryptophan are only present in maize in small and inadequate quantities (FAO 1992). Quality protein maize varieties have been developed to contain high levels of lysine and tryptophan (Prasanna 2001). Fat and lipid account for 3% to 6% of maize.
In general, maize is deficient in vitamin B12 and contains niacin in an inaccessible form, placing populations that consume high quantities of maize without sufficient dietary diversity at risk of pellagra (FAO 1992). Maize contains only modest amounts of zinc and negligible amounts of iron, absorption of which is further diminished by the presence of phytates: the bioavailability of iron from corn is thus estimated to be less than 5% (Beiseigel 2007). Phytases, genetically modified low‐phytate maize variants, and some pre‐processing methods such as addition of ascorbic acid, may improve iron availability from maize (Beiseigel 2007; Hurrell 2002; Troesch 2011).
Following harvest, maize undergoes several processing steps prior to preparation of an end product. Cobs are dried, hulled and shelled to remove the kernels prior to milling (ILO 1984). The two chief forms of milling are wet and dry milling (Gwirtz 2014): wet milling is used to obtain starch, edible corn oil, sweeteners and syrups, and animal feed products; dry milling is used to obtain flour, meal and grits. Some maize products use whole maize, while others use degerminated kernels (Codex 1985a; Codex 1985b). This is an important consideration as it may impact the overall nutritional contents. Maize meal/flour derived from dry milling is used for corn bread; polenta in Italy; angu in Brazil, mamaliga in Romania; mush in the USA; mealie pap in South Africa; and sadza, nshima and ugali in African countries (Herbst 2001). Corn flakes are also derived from corn meal that has undergone extrusion (Nuss 2010).
In many settings, maize grains undergo pre‐processing prior to milling (Peña‐Rosas 2014a). The process of nixtamalisation refers to cooking maize grains in a diluted alkali solution (traditionally, limewater – sodium and calcium hydroxide, ash or lye) (Gwirtz 2014). Following washing, the pericarp is removed (hulling), leaving the endosperm and germ (Katz 1974). The softened grain may then be wet‐milled to produce dough (masa), which can be used to make tortillas, tamales and arepas. Alternatively, the nixtamalised grain may be dried in ovens, ground and then prepared as nixtamalised corn flour (masa harina) that can be reconstituted at the time of use to produce the full range of corn masa products. This flour is commercially available for purchase and consumption, expediting the maize preparation process for regular consumers (Bressani 1997). While nixtamalisation was common for many centuries in local and home settings, it has now been adapted for large‐scale corn masa flour production.
Nixtamalisation gives the final product a characteristic flavour, changing the nutritional properties of the maize. Nixtamalised maize flour contains niacin in a bioavailable form, and populations consuming maize produced in this way do not develop pellagra (Bressani 1997). Due to calcium absorbed from the lime, nixtamalised maize flour has a high calcium content and can provide most of the daily calcium requirement to populations consuming this product as a staple (Wyatt 1994). Phytic acid content is reduced in nixtamalised maize products. Iron content may be slightly increased by the process; variable changes in bioavailability have been reported, with some suggesting increased availability due to reduced phytate content and others suggesting impaired bioavailability, perhaps due to inhibition by calcium (Bressani 1997). Limewater‐treated corn remains deficient in other B‐complex vitamins and essential amino acids including lysine and tryptophan (Bressani 1990).
Precooking is another procedure applied to dehulled and degermed corn products after milling and is common in some South American countries. Precooked maize flour is the product obtained from white or yellow corn, composed mainly of endosperm. By this process, the maize kernel is sequentially dehulled, degermed, precooked, dried, flaked and reduced into a fine powder (Covenin 1996; Vielma 1998).
The definitions of corn flour and cornmeal vary widely. The US Food and Drug Administration (FDA) defines corn flour and meal as products obtained from the grinding of dried corn grains (yellow or white). These regulations define the size, the moisture content of each product, and the amount of fibre and fat that is retained in the product. Corn flour (white or yellow) must be able to pass through the narrowest sieves, while corn meal must be able to pass through wider sieves, but not through sieves that would permit passage of corn grits; corn meal must contain at least 1.2% fibre (FDA 2011). Maize meal and flour may also be included as part of a composite flour, in combination with other products. Composite flours are mixtures of flours from tubers rich in starch (Popper 2006). In most countries, the extraction rate for maize varies from 60% to 100% depending on the product. The range for yellow maize goods is 60–65% in the United States. Higher extraction rate levels are found in other countries (Ranum 2014).
Fortification of maize flour and other sub‐products produced from maize has been implemented in several settings around the world. Mass fortification of maize flour with iron has been a reality for many years in several countries in Africa and the Americas (Dary 2002a; Garcia‐Casal 2002). Voluntary fortification of maize with iron (and in some cases, other nutrients) has been introduced in Ghana, Malawi and Mauritania. Mandatory fortification with at least iron has been implemented in Brazil, Costa Rica, El Salvador, Kenya, Mexico, Nigeria, Rwanda, South Africa, Tanzania, Uganda, the USA and Venezuela (Peña‐Rosas 2014a). Fortification programmes require support by legislative frameworks that include regulations and standards to ensure the appropriate micronutrient contents are added to the foods and appropriately labelled (Makhumula 2014). Fortification of maize with iron is likely to be relatively cheap (for example, USD 3.19 per metric tonne) and would add an estimated 0.03% to 0.2% to household expenditure among average families in sub‐Saharan contexts (0.07% to 0.8% to the overall household expenditure among the poorest families in these settings), assuming the costs of fortification were fully passed on to the consumer (Fiedler 2014).
How the intervention might work
Iron fortification aims to improve the nutritional status of populations at risk of iron deficiency and anaemia by increasing dietary content and thus iron intake. Iron added to fortify maize is generally retained during production, distribution and cooking processes (unlike, for example, other minerals such as B group vitamins) (Dunn 2014). Several fortificants are available for maize flour; selecting one implies a trade‐off between bioavailability, maximal concentration that can be added without affecting sensory aspects, cost and availability. The bioavailability, stability and sensory effects of different iron fortificants have been described (Dary 2002a). Ferrous sulphate has high bioavailability and has been used to fortify bread, pasta and infant formula. Although it is effective when added to flour, it may adversely affect flavours, especially following storage. Ferrous fumarate is also well absorbed, has a bioavailability similar to ferrous sulphate and overcomes many of the problems associated with adverse effects on taste. Electrolytic iron compounds added to cereals have poor bioavailability, especially related to high particle size, and they also produce adverse effects on taste at the higher concentrations required to achieve optimal dietary iron intake (Cook 1983; Hallberg 1982). Other iron compounds such as sodium iron EDTA (NaFeEDTA), ferrous bis‐glycinate and tris‐glycinate (Bovell‐Benjamin 2000; Hertptramp 2004; Mendoza 2001), which protect iron from dietary inhibitors of absorption (i.e. phytates), have superior bioavailability and do not impact product taste compared to other compounds, but their higher costs may be limiting; colour and rancidity has been associated with the latter compound following storage of wheat flour. Finally, encapsulated ferrous sulphate and ferrous fumarate, in which iron is encapsulated in an oil layer, has minimal reactivity with the food matrix but offers high bioavailability; this approach is limited by the relatively high cost of the fortificant (Dary 2002b; Hurrell 2002).
The amount and final concentration of additional iron added to the food vehicle depends on the daily intake of that food by the population as well as the characteristics of the fortificant as described. Based on the expected intake of the vehicle and the bioavailability of the iron, the concentration of fortificant added can then be adjusted to achieve an appropriate daily absorption of iron (˜1 mg to 2 mg per day) (WHO 2009). Formal testing of the absorption of iron added to the vehicle can also be performed using isotopic testing. Iron absorption may be further improved by adding an enhancer such as ascorbic acid and by reducing the level of phytic acid (inhibitor) using one of a variety of techniques (Hurrell 2002). A revised model of the appropriate content of fortificant when several vehicles are being considered simultaneously has been developed, based on the prevalence of micronutrient deficiency in the target community, the optimal intakes of the vehicle (accounting for its macro nutritional value), and the expected maximal potential intake (to avoid excess consumption of the fortificant) (Guamuch 2014).
Thus, there are varying approaches to fortifying staple products (including maize flour and food products containing it) with iron, with different specific vehicles employed, heterogenous types and concentrations of fortificants, and diverse complementary strategies for enhancing iron absorption.
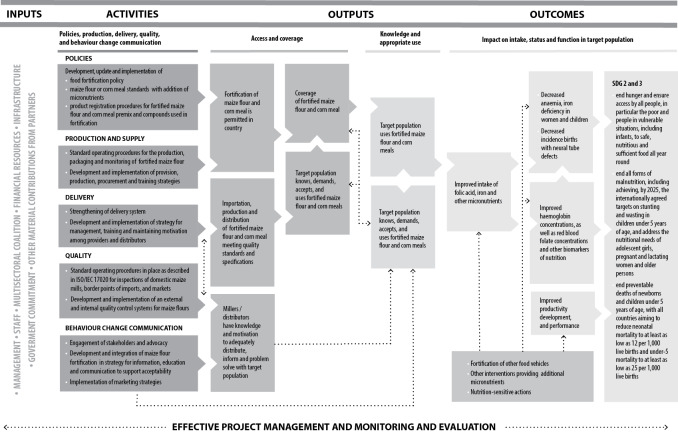
This review evaluated, based on existing research, the effects of maize flour fortification with iron as a public health intervention. The World Health Organization/Centers for Disease Control and Prevention (WHO/CDC) logic model for micronutrient interventions in public health depicts the programme theory and plausible relationships between inputs and expected improvement in Sustainable Development Goals (SDGs), which can be adapted to different contexts (WHO/CDC 2011). The effectiveness of maize flour fortification with iron in public health depends on several factors related to policies and legislation regulations; production and supply of the fortified maize flour; the development of delivery systems for the fortified maize flour; the development and implementation of external and internal food quality control systems; and the development and implementation of strategies for information, education and communication for behaviour change among consumers. Figure 1 presents a generic logic model for micronutrient interventions that depicts these processes and outcomes.
1.
Logic model for maize flour and corn meal fortification with iron and other micronutrients in public health (adapted from WHO/CDC 2011)
Risks of flour fortification with iron
Studies have pointed to several potential adverse effects of flour fortification. Iron overload, associated with long‐term excessive iron absorption, is usually associated with hereditary disorders such as thalassaemia, pyruvate kinase deficiency, congenital dyserythropoietic anaemia, some cases of glucose‐6‐phosphate dehydrogenase (G6PD) deficiency, hereditary spherocytosis and sideroblastic anaemia, or with acquired conditions such as sideroblastic and other dyserythropoietic anaemias. Individuals with hereditary or acquired anaemia requiring multiple long‐term transfusions will also develop iron overload. Such individuals may be at risk of excess iron absorption and/or exacerbation of iron overload if dietary iron content is inadvertently high (Pasricha 2013). Although men and postmenopausal women do not have a mechanism for losing iron and therefore may be at greater risk of accumulating iron in the long term, their consumption of iron‐fortified foods does not appear to increase their risk of iron overload (Ballot 1989; Brittenham 2004; Pouraram 2012). Hereditary haemochromatosis is characterised by an accelerated rate of intestinal iron absorption and progressive iron deposition in various tissues that typically begins to be expressed in the third to fifth decades of life but may also occur in children. Hereditary haemochromatosis due to mutations of the HFE gene is chiefly found in populations of European descent but is less common among other ethnicities (Adams 2005). Some authors have speculated that fortification of staple foods with iron would restore (or partially restore) iron intake to 'recommended' levels, which could pose a risk to individuals predisposed to iron overload; however, this risk would remain lower than in high‐income countries (Brittenham 2004).
There is evidence that iron supplementation may exacerbate the risk of malaria in children, perhaps via inducing a reticulocytosis during recovery from anaemia (Pasricha 2018). Whether iron fortification of staple foods likewise causes this risk is uncertain.
Why it is important to do this review
Iron deficiency and anaemia remain important public health problems worldwide. One of the major limitations for development of iron fortification guidelines is a lack of a strong evidence base for this intervention (Dary 2002b). Although maize flour fortification with iron alone, or in combination with other micronutrients, has been implemented in many countries, to date there has been no systematic assessment of the safety and effectiveness of this intervention to inform policy making. This systematic review complements the results of another Cochrane Review investigating the effects of fortification of wheat and maize flour with folic acid for population health outcomes (De‐Regil 2016). The effects and safety of fortification of wheat flour with iron for reducing anaemia and improving iron status in populations is being assessed in another review (Peña‐Rosas 2014b).
Objectives
To assess the effects of iron fortification of maize flour, corn meal and fortified maize flour products on anaemia and iron status in the general population.
For the purpose of this review, we consider fortified maize flour products to include any food prepared from fortified corn meal or maize flour.
Methods
Criteria for considering studies for this review
Types of studies
Fortification of maize flour is an intervention that aims to reach the entire population of a country or large sections of the population and is frequently delivered through the market system. Thus, we considered that we would not be able to assess the benefits and harms of food fortification if we only included randomised controlled trials (RCTs) and also examined data from other study designs.
We included:
RCTs, with randomisation at either individual or cluster level;
quasi‐randomised trials (where allocation of treatment has been made, for example, by alternate allocation, date of birth, alphabetical order);
non‐randomised controlled studies;
-
observational studies that are prospective and have a control group;
cohort studies (prospective and retrospective);
controlled before‐and‐after studies;
interrupted time series (ITS) with at least three measurement points both before‐and‐after intervention.
In addition to the above‐mentioned study designs, we also considered uncontrolled before‐and‐after studies for inclusion in this review for completeness, as we anticipated many studies would include measures of impact at a regional or national level and use uncontrolled trial designs. Such trials, however, were not considered in assessments of the overall effectiveness of interventions or used to draw conclusions regarding the effects of interventions in the review.
Types of participants
General population older than 2 years of age (including pregnant women), from any country. We excluded studies of interventions targeted toward participants with a critical illness or severe co‐morbidities. We excluded children under 2 years of age since they are not the intended beneficiaries of maize flour and corn meal fortification.
Types of interventions
Interventions included in this review are those in which maize flours and/or maize flour sub‐products have been fortified with iron alone or iron plus other vitamins/minerals. Maize flour is defined as white or yellow maize (corn) flour or maize (corn) meal that is produced by grinding dried maize grains (FDA 2011), including nixtamalised dehydrated corn flour, also known as 'masa flour', as well as precooked corn flour. We included composite flours containing more than 50% maize within the definition of flour in this review. Maize flour products include all products derived from fortified corn meal and flour (e.g. breads, cereals, polenta, porridges, grits, arepas). Fortification of the maize flour or corn meal must have occurred during flour production for the study to have been included.
Comparisons included:
maize flour or maize flour products fortified with iron alone versus no intervention;
maize flour or maize flour products fortified with iron plus other vitamins and minerals versus no intervention;
maize flour or maize flour products fortified with iron alone versus unfortified maize flours or maize flour products (not containing iron or any other vitamin and minerals);
maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals).
Studies with co‐interventions (e.g. fortified maize flour plus education) were eligible only if the comparison group also received the co‐intervention (e.g. education alone).
We excluded studies evaluating products derived from wet milling of maize, including corn starch (which is often called 'corn flour' in the UK and Australia) and products that are fortified after recomposition of the flour. For example, if a study used maize flour to prepare a bread product, and fortification occurred at the level of bread production, we excluded this study.
We did not include comparisons of maize (corn) fortification versus other forms of micronutrient interventions comprising iron supplementation, point‐of‐use fortification of maize products with micronutrients powders or lipid‐based spreads, biofortified crops with micronutrients, or fortification of wheat flour with iron or folic acid, as other Cochrane Reviews are currently investigating these interventions.
Types of outcome measures
Primary outcomes
The primary outcomes across all populations in this review were as follows.
Anaemia (defined as haemoglobin (Hb) below WHO cut‐off, adjusted for altitude and smoking as appropriate).
Iron deficiency (as defined by trialists, based on a biomarker of iron status).
Haemoglobin concentration (in g/L).
Iron status (as reported: ferritin, transferrin saturation, soluble transferrin receptor, soluble transferrin receptor‐ferritin index, total iron binding capacity, serum iron).
Any adverse effects (as defined by trialists).
Secondary outcomes
We looked at the following secondary outcomes in all population groups.
Iron‐deficiency anaemia (as defined by trialists).
Malaria severity (only for malaria settings).
Malaria incidence (only for malaria settings).
We also looked at secondary outcomes of interest by population group.
Children (2 to 11.9 years of age)
Cognitive development (as defined by trialists)
Motor skill development (as defined by trialists)
Growth: height‐for‐age (Z‐scores)
Growth: weight‐for‐height (Z‐scores)
Pregnant women (any age)
Premature birth (less than 37 weeks)
Very premature birth (less than 34 weeks)
Low birth weight (less than 2500 g)
Congenital anomalies (including neural tube defect, cleft lip, cleft palate, congenital cardiovascular defects and other birth defects as reported by trialists)
Adults (men and women 19 years of age and older)
Work capacity (as defined by trialists)
High ferritin concentrations (defined as more than 150 mg/L)
All groups
Where the reports presented figures with combined data for all populations, we also included them.
Search methods for identification of studies
Electronic searches
We searched the following international and regional sources.
International databases
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 13 December 2017)
MEDLINE Ovid (1946 to 13 December 2017)
MEDLINE (R) In Process Ovid (1946 to 13 December 2017)
Embase Ovid (1980 to 13 December 2017 week 50)
Web of Science (both the Social Science Citation Index and the Science Citation Index (ISI); up to 12 December 2017)
CINAHL Ebsco (up to 13 December 2017)
AGRICOLA (agricola.nal.usda.gov) (up to 20 December 2017)
BIOSIS ISI (up to 13 December 2017)
Regional databases
IBECS (ibecs.isciii.es) (searched 20 December 2017)
SciELO (www.scielo.br) (searched 20 December 2017)
Global Index Medicus ‐ AFRO (includes African Index Medicus) (searched 20 December 2017)
EMRO (includes Index Medicus for the Eastern Mediterranean Region) (searched 20 December 2017)
LILACS searched 20 December 2017)
PAHO (Pan American Health Library) (searched 20 December 2017)
WHOLIS (WHO Library) (searched 20 December 2017)
WPRO (includes Western Pacific Region Index Medicus) (searched 22 January 2018)
IMSEAR, Index Medicus for the South‐East Asian Region (searched 20 December 2017)
IndMED, Indian medical journals (indmed.nic.in) (searched 20 December 2017)
Native Health Research Database (hscssl.unm.edu/nhd) (searched 20 December 2017)
For theses we searched WorldCat, Networked Digital Library of Theses and Dissertations, DART‐Europe E‐theses Portal, Australasian Digital Theses Program, Theses Canada Portal and ProQuest‐Desertations and Theses (2012 to 18 August 2016). We conducted the searches in 2012 initially and updated them in 2015, 2016 and 2017.
We also contacted the Trials Search Co‐ordinator of the Cochrane Public Health Group to search the Cochrane Public Health Group Specialised Register.
We searched clinicaltrials.gov and the International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned studies (searched 17 January 2018) and contacted authors of such studies to obtain further information or eligible data if available. Additionally we searched the United Nations Children's Fund (UNICEF) Evaluation and Research Database (ERD) to identify abstracts and full‐text reports of evaluations, studies and surveys related to programmes on maize flour and corn meal fortification.
The search used keyword and controlled vocabulary (when available), using the search terms set out in Appendix 1. We adapted these terms as appropriate for each database. We did not apply any language or date restrictions.
We handsearched the five journals with the highest number of included studies in the last 12 months to capture any article that may not have been indexed in the databases at the time of the search. As maize fortification technologies are not novel, we did not apply time restrictions. We contacted authors of included studies and checked reference lists of included papers to identify additional records.
If we identified articles written in a language other than English, we commissioned their translations. If this was not possible, we sought advice from the Cochrane Public Health Group. We stored such articles in the 'Awaiting assessment' section of the review, pending translation.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we contacted the Department of Nutrition for Health and Development and the regional offices of WHO, as well as the CDC nutrition section, UNICEF, the United Nations' World Food Programme (WFP), Nutrition International, Global Alliance for Improved Nutrition (GAIN), Hellen Keller International (HKI), World Vision, Sight and Life, PATH, premix producers DSM and BASF and the Food Fortification Initiative (FFI) (contacted 9 August 2016).
Data collection and analysis
Selection of studies
Three review authors (MNGC, SP, JPPR) independently screened the titles and abstracts of articles retrieved by each search to assess eligibility, as determined by the inclusion and exclusion criteria listed above. We retrieved full copies of all eligible papers. When it was not possible to rule out a title or abstract with certainty, we tried to obtain the full text of the article for further evaluation. If full articles were not available, we attempted to contact the authors to obtain further details of the study. Failing this, we classified studies as 'awaiting assessment' pending the availability of further information. We resolved disagreements at any stage of the eligibility assessment process through discussion and consultation with a third author (JPPR) where necessary.
Data extraction and management
Two review authors (MNGC, JPPR) extracted data independently using data extraction forms based on those from the Cochrane Effective Practice and Organisation of Care (EPOC) Group and the Cochrane Public Health Group (Cochrane PHG 2011).
All review authors were involved in piloting the form using a subset of articles to enhance consistency amongst review authors and, based on this, we modified some sections of the form. We collected information on study design, study setting and participants (number and characteristics), and provided a full description of the interventions examined. We extracted details of outcomes measured (including a description of how and when outcomes were measured) and results.
We designed the form so that we were able to record results for our prespecified outcomes and for other (non‐prespecified) outcomes (although such outcomes did not underpin any of our conclusions). We extracted additional items relating to study recruitment and the implementation of the intervention, including number of sites for an intervention, similarity of recruitment procedures at different sites, presence of protocol deviations, levels of compliance/use of flours in different sites within studies, resources required for implementation, and findings from any process evaluations.
We used the PROGRESS (place of residence, race/ethnicity, occupation, gender, religion/culture, education, socioeconomic status, social capital) checklist to measure disadvantage across categories of social differentiation and recorded whether or not study authors reported data by sociodemographic characteristics known to be important from an equity perspective (Ueffing 2011). We also recorded whether or not studies included specific strategies to address diversity or disadvantage. We attempted to identify and categorise factors that determine the differential availability, accessibility, acceptability and effective usage of fortified maize flour and corn meal among population groups. We also defined key areas of monitoring and suggested appropriate policy action used to promote equity in access to these products.
We highlighted especially the importance of a significant difference in iron status by fortifying maize flours or cornmeal for the health and nutritional implications in countries where maize is a staple food.
For eligible studies, two review authors (MNGC, JPPR) independently extracted data using the agreed form. Two authors (MNGC, JPPR) entered data into Review Manager 5 (RevMan 5) software (RevMan 2014), and two authors (SPR, LMDR) carried out checks for accuracy. We resolved any discrepancies through discussion.
When information regarding any aspect of study design or results was unclear, we contacted authors of the original reports, asking them to provide further details.
Assessment of risk of bias in included studies
Two review authors (MNGC, JPPR) independently assessed the risk of bias in the included studies using the Cochrane EPOC 'Risk of bias' tool (EPOC 2017). Quality domains assessed included: allocation sequence generation, allocation concealment, similarity of baseline characteristics and outcome measurements, blinding (personnel and outcome assessors), handling of incomplete outcome data, protection against contamination, reporting of outcomes and other sources of bias (including source of study funding). We classified findings into three categories: low (low risk of bias for key quality domains, i.e. allocation sequence generation and concealment), high (high risk of bias for one or more of the key domains) and unclear (unclear risk of bias for one or more key domains). We resolved any disagreement by discussion or by involving an additional review team member (LMDR). In a future update of the review the use of ROBINS‐I tool to assess risk of bias in non‐randomized studies of interventions should be used (Sterne 2016).
Measures of treatment effect
Dichotomous data
For dichotomous data, we present proportions, and for two‐group comparisons, we report results as average risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
We report results for continuous outcomes as the mean difference (MD) with 95% CIs if outcomes were measured in the same way between trials. Where some studies have reported endpoint data and others have reported change from baseline data (with errors), we combined these in the meta‐analysis if the outcomes had been reported using the same scale.
If we did not find enough studies or could not pool the studies, we summarised the results in a narrative form.
Unit of analysis issues
Cluster‐randomised trials
We planned to combine results from both cluster‐ and individually randomised studies if there was little heterogeneity between the studies. We labelled cluster‐randomised trials with a '(C)'. If the authors of cluster‐randomised trials had conducted their analyses at a different level than that of allocation and had not appropriately accounted for the cluster design in their analyses, we calculated trials' effective sample size to account for the effect of clustering in data. We utilised the intra cluster correlation coefficient (ICC) derived from the trial (if available), or from another source (e.g. using the ICCs derived from other, similar trials) (Gulliford 1999) and then calculated the design effect with the formula provided in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We reported this approach when we used it and undertook sensitivity analysis to investigate the effect of variations in ICC.
We made an adjustment for design effect for both the continuous outcome of haemoglobin concentrations and dichotomous outcome of anaemia in one included study, Villalpando 2002 (C), and for haemoglobin concentrations in Carrasco 2011 (C).
Studies with more than two treatment groups
If we identified studies with more than two intervention groups (multi‐arm studies), where possible we combined groups to create a single pair‐wise comparison or used the methods set out in Higgins 2011a to avoid double‐counting study participants. For the subgroup analyses, when two or more study arms shared the control group, we divided the control group (events and total population) over the number of relevant subgroups to avoid double‐counting the participants.
Cross‐over trials
We did not find any cross‐over trials.
Dealing with missing data
We tried to contact the authors if missing outcome data were unclear or had not been fully reported. We captured the missing data in the data extraction form and reported it in the 'Risk of bias' tables.
For all outcomes, we carried out analysis, as far as possible, on an intention‐to‐treat basis, so for randomised trials, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised, minus any participants whose outcomes were known to be missing. For non‐randomised studies, where possible, we analysed data according to initial group allocation irrespective of whether or not participants received or adhered to the planned intervention.
Assessment of heterogeneity
We examined the forest plots from meta‐analysis to visually assess the level of heterogeneity (in terms of the size or direction of treatment effect) among studies. We used the I2 and Tau2 statistics, and the Chi2 test to quantify the level of heterogeneity among the trials in each analysis. If we identified moderate or substantial heterogeneity, we explored it by pre‐specified subgroup effects analysis.
Heterogeneity may be a particular concern in non‐randomised studies, and where there was evidence of heterogeneity, we summarised findings using a forest plot but do not present the pooled estimate.
We exercise caution in the interpretation of those results with high levels of unexplained heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and we thought the missing data introduced serious bias, we explored the impact of including such studies in the overall assessment of results through a sensitivity analysis.
We plan that in future updates, if more than 10 studies reporting the same outcome of interest are available, we will generate funnel plots in RevMan 2014 and visually examine them for asymmetry. Where we pool studies in meta‐analysis, we will order studies in terms of weight, so that a visual examination of forest plots allows us to assess whether the results from smaller and larger studies are similar, or if there are any apparent differences (i.e. we checked that the effect size is similar in smaller and larger studies).
Data synthesis
We carried out meta‐analyses to provide an overall estimate of treatment effect when more that one study examined the same intervention, provided that studies used similar methods and measured the same outcome in similar ways in similar populations. We did not combine results from randomised and non‐randomised studies together in meta‐analysis, nor did we present pooled estimates for non‐randomised studies with different types of study designs. Evidence on different outcomes may be available from different types of studies (for example, it is likely that data on less common adverse effects are reported in larger non‐randomised studies). Where there was evidence on a particular outcome from both randomised trials and non‐randomised studies, we used the evidence from trials at lower risk of bias to estimate treatment effect.
Where there was evidence from several randomised trials, or non‐randomised studies at low risk of bias, we carried out statistical analysis using RevMan 5 (RevMan 2014). We used a random‐effects meta‐analysis for combining data, as we anticipated that there would be natural heterogeneity between studies attributable to the different doses, durations, populations and implementation/delivery strategies. For continuous variables we used the inverse variance method, while for dichotomous variables we used the one proposed by Mantel‐Haenszel (Higgins 2011a).
We explored heterogeneity according to the subgroups identified in the following section. In addition, we used narrative synthesis, guided by the data extraction form in terms of how to group and summarise studies, to explore intervention implementation (using information about resource use and findings from process evaluations), and we described the impact of interventions by sociodemographic characteristics known to be important from an equity perspective based on the PROGRESS framework, where this information was available. Religion was not available from any study, so we do not present it in the table.
Subgroup analysis and investigation of heterogeneity
Where data were available, we planned to conduct subgroup analyses according to the following variables.
Prevalence of anaemia among trial participants at baseline: less than 20%; 20% to 39%; 40% or higher.
Sex: males; females; mixed/unknown.
Type of processing: whole maize milled meal; degermed maize milled meal; nixtamalised flour, precooked or refined, others including mixtures of cereal flours.
Type of iron compound: high relative bioavailability (e.g. iron EDTA) versus ferrous sulphate and comparable relative bioavailability (e.g. fumarate) versus low relative bioavailability (e.g. reduced iron, electrolytic iron, others).
Corn meal fibre/phytate content.
Malaria endemicity at the time that the trial was conducted: malaria setting versus non/unknown malaria setting.
Length of the intervention: less than six months; six months to one year; more than one year.
Dose of elemental iron per 100 g of product.
We did not formally carry out a subgroup analysis given the scarcity of included studies.
Sensitivity analysis
As there were only three RCTs included in meta‐analysis, we did not carry out sensitivity analyses to examine the effects of removing studies at high risk of bias (those with high or unclear risk of bias for allocation concealment, lack of similarity of baseline outcome measurements, or incomplete outcome data). We assessed the effects of the pooled estimated when doing imputations (Table 2) on the haemoglobin concentrations SD for Carrasco 2011 (C) and included it in the meta‐analysis. There was no change in the direction or significance of the effect.
1. Standard deviation computation for haemoglobin concentrations. Scenarios with imputed SD values and results of the overall meta‐analysis for the outcome.
| Scenario | Source (P value or literature) | P | Standard deviation (g/L) | Results in the pooled estimate (meta‐analysis) (RR) |
| Scenario 1 | Shamah‐Levy 2003 | 26.77 | 1.29 (95% CI −2.75 to 5.33) | |
| Scenario 2 | Martinez 1995 | 16 | 1.25 (95% CI −2.36 to 4.86) | |
| Scenario 3a | P value 1 Carrasco 2011 (C) | 0.05 | 8.92 | 1.19 (95% CI −1.82 to 4.19) |
| Scenario 4a | P value 2 Carrasco 2011 (C) | 0.025 | 7.8 | 1.17 (95% CI −1.69 to 4.04) |
| Scenario 5a | P value 3 Carrasco 2011 (C) | 0.01 | 6.78 | 1.16 (95% CI −1.57 to 3.89) |
aThe third to fifth scenarios were obtained by computing the corresponding t‐values, standard errors, and standard deviations for the change in haemoglobin concentration from 131 g/L to 133 g/L for the fortified corn flour group, which was a significant difference (P < 0.05). RR: relative risk
'Summary of findings' table
To assess the certainty of evidence for each important outcome, we used the GRADE approach (Langendam 2013; Guyatt 2008), and the GRADE profiler allowed us to import data fromRevMan 5 to create a 'Summary of findings' table (GRADEpro GDT 2015; RevMan 2014). These tables provide outcome‐specific information concerning the overall certainty of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered. We included only primary outcomes from RCTs in the 'Summary of findings' tables. For each individual outcome, two review authors independently assessed the certainty of the evidence using the GRADE approach (Balshem 2011), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias. We expressed the results as being at one of four levels of certainty (high, moderate, low or very low).
Results
Description of studies
Results of the search
We initially carried out a single search for this review in 2012 and updated it in 2015, 2016 and 2017. The study flow is depicted in Figure 2. We identified 6363 records through database searching and through the additional search strategy. After removing duplicates, we screened 4529 records and assessed the full text of 80 records (75 studies) and excluded 70 studies with reasons. We did not find any ongoing studies.
2.
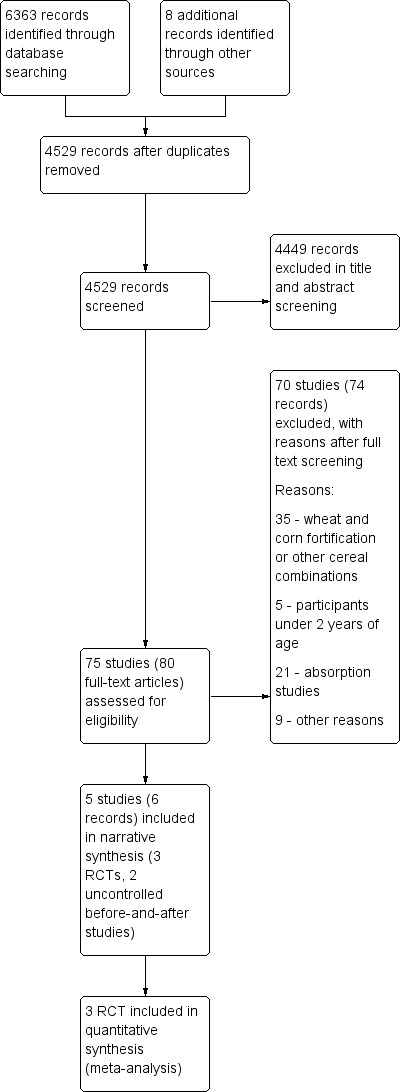
Study flow diagram.
Included studies
The details of included studies, including participants, intervention, outcomes and source of funding are presented in the Characteristics of included studies tables.
Study designs
We included five studies in this review (Andang'o 2007; Carrasco 2011 (C); Miglioranza 2009; Seal 2007; Villalpando 2002 (C)), involving 3459 participants. Table 3 shows the summary characteristics of the included studies. Three studies were RCTs (Andang'o 2007; Villalpando 2002 (C), Carrasco 2011 (C)). Villalpando 2002 (C) and Carrasco 2011 (C) were cluster‐randomised trials and are thus marked as such with a '(C)'. The other two studies were uncontrolled before‐and‐after studies (Miglioranza 2009; Seal 2007); authors described the latter as an RCT in one report but as a before‐and‐after study elsewhere. Three studies contributed data to the meta‐analysis (Andang'o 2007; Carrasco 2011 (C); Villalpando 2002 (C)).
2. Summary of characteristics of included studies.
| Study name and year | Location | Intervention | Duration intervention | Age and size of sample | Outcomes | Overall risk of bias | Study design |
| Andang'o 2007 | 4 schools in Marafa, in the hinterland of Malindi district, in the semiarid coastal lowlands of Kenya | Participants were randomly assigned to 1 of 4 groups: group 1 (n = 121) received uji prepared with whole maize flour fortified with 56 mg elemental iron per kg flour (as NaFeEDTA) and vitamin A, thiamine, riboflavin, and niacin; group 2 (n = 139) received uji prepared with whole maize flour fortified with 28 mg elemental iron per kg of flour (as NaFeEDTA) and the same vitamins and minerals; group 3 (n = 127) received uji with 56 mg elemental iron per kg of flour (as electrolytic iron) and the same vitamins and minerals; group 4 (n = 128) received uji prepared from unfortified whole maize flour. | 5 months | Children 3‐8 years of age: 260 girls and 256 boys | Iron‐deficiency anaemia, iron deficiency, anaemia, haemoglobin concentration, plasma ferritin concentrations and soluble transferrin receptor, weight, height, C‐reactive protein, any adverse effects | Low | Randomised control trial with 4 arms |
| Carrasco 2011 (C) | Huejutla de Reyes (Hidalgo), Atlacomulco (Estado de México) and Huatusco (Veracruz) counties in Mexico | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 155): received 20 kg monthly of fortified maize flour containing 42.4 mg iron (as ferrous fumarate per 100 g, vitamin A, folic acid, zinc and niacin; group 2 (n = 153) received 20 kg of unfortified flour monthly | 10 months | 308 indigenous and non‐indigenous women 14‐64 years of age | Weight, waist, hip, body mass index, and waist circumference, haemoglobin concentrations at 4 and 6 months | High | Double‐blind randomised trial with 2‐arms |
| Miglioranza 2009 | 9 peripheral city centres in Londrina, Parana (southern Brazil) | Administration of corn flour‐derived products fortified with elemental iron. Each participant ingested 100 g/d of products | 6 months | 162 children and adolescents 7‐14 years of age: 76 girls and 86 boys | Haemoglobin concentration, serum iron, total iron binding capacity, percentage transferrin saturation and serum ferritin concentration | High | Uncontrolled before‐and‐after study |
| Seal 2007 | Nangweshi refugee camp, located in the Western Province of Zambia | Each household received a 400 g ration of 97% extraction, fortified maize meal per registered refugee. The fortificant formulation contained vitamin A, thiamine, riboflavin, nicotinamide, vitamin B6, vitamin B12, folic acid, 35 mg elemental iron, zinc | 8 months | 69 boys and 86 girls; 109 adolescent boys and 104 adolescent girls; and 118 adult women | Haemoglobin, transferrin receptor, serum retinol, dietary assessment, anthropometry, malaria blood film, haematuria (proxy for schistosomiasis) | High | Uncontrolled before‐and‐after study |
| Villalpando 2002 (C) | 34 boarding houses Oaxaca, Mexico | Shelters were randomised to receive 1 of 3 types of flour (not fortified, fortified with reduced or fortified with iron NaFeEDTA. All flours contained thiamine, riboflavin, niacin, folic acid and zinc. The amount of added iron was 30 mg/kg as reduced iron or as NaFeEDTA. | 9 months | 1786 children 6‐11 years of age | Socioeconomic variables, anthropometry, food intake, haemoglobin, protoporphyrin, serum iron, TIBC, UIBC, ferritin, folates, niacin, thiamine riboflavin, diet composition and fortification mix composition | High | Cluster‐randomised trial with 3 arms |
NaFeEDTA: ferric sodium ethylenediaminetetraacetate;TIBC: total iron binding capacity; UIBC: unsaturated iron binding capacity
We describe the results of the other two studies narratively (Miglioranza 2009; Seal 2007). They did not contribute data to meta‐analysis and did not directly inform the conclusions of the review.
Participants
Age
Andang'o 2007 involved children aged 3 to 8 years; Villalpando 2002 (C), children aged 6 to 11 years; Miglioranza 2009, children and adolescents aged 7 to 14 years; Seal 2007 in young children aged 6 to 59 months, adolescents aged 10 to 19 years and women aged 20 to 49 years; and Carrasco 2011 (C) in adult women. We also included Seal 2007 trials as the average age was 32 months in the sub‐study for children.
Gender
One study included only women (Carrasco 2011 (C)), while the other four studies included participants of both sexes (Andang'o 2007; Miglioranza 2009; Seal 2007; Villalpando 2002 (C)). Andang'o 2007 included 260 girls and 256 boys; Miglioranza 2009, 76 girls and 86 boys; and Seal 2007, 69 boys and 86 girls, 109 adolescent boys and 104 adolescent girls, and 118 adult women. Villalpando 2002 (C) did not report sex distribution.
Prevalence of anaemia among trial participants at baseline
The prevalence of anaemia was less than 20% in two studies (Miglioranza 2009; Villalpando 2002 (C)); 40% or higher in Andang'o 2007 and not reported in Carrasco 2011 (C). In Seal 2007, the baseline prevalence of anaemia was less than 20% in two study arms: adolescents and adult women, while in the group of children the prevalence of anaemia was higher than 40%.
Settings
Andang'o 2007 took place in Marafa, a semi‐arid region of coastal Kenya; Carrasco 2011 (C) was conducted in women living in the Huejutla de Reyes (Hidalgo), Atlacomulco (Estado de México) and Huatusco (Veracruz) counties of Mexico; and Villalpando 2002 (C) among children living in shelters in Oaxaca, Mexico. As for the uncontrolled before‐and‐after studies, Miglioranza 2009 was conducted in Londrina, Parana (southern Brazil), and Seal 2007 among a Nangweshi refugee population camp in Zambia.
Table 4 compares the PROGRESS‐Plus parameters of equity in the included studies. All studies were performed in disadvantageous social and economic settings with high prevalence of micronutrient deficiencies, especially iron. None of the five studies mentioned or explored cultural or religious factors that may have contributed to social determinants of anaemia. Moreover, none of the studies explored or included analyses of social inequities and the determinants at play. However, such considerations may have been beyond the scope and focus of the five studies, which were of a more clinical and medical nature.
3. PROGRESS PLUS Equity checklist of included studies.
| Study | Place | Race/ethnicity | Occupation | Gender | Education | Socioeconomic status | Social status |
Others: Disability Age Sexual orientation |
Overall Progress+ |
| Andang'o 2007 | 4 schools in Marafa, in the hinterland of Malindi district, in the semiarid coastal lowlands of Kenya | Mijikenda tribal groups | Children who were enrolled in nursery | 260 girls and 256 boys | First year of primary school | Poor socioeconomic status. Children receive a daily meal through government‐funded feeding programmes. | Local families are mostly poor subsistence farmers. | Children 3‐8 years of age. No details on sexual orientation. Children with mental disabilities were excluded. | The study enrolled children 3‐8 years of age from poor subsistence farmer families attending 4 schools in a semiarid coastal lowlands of Kenya. |
| Carrasco 2011 (C) | Huejutla de Reyes (Hidalgo), Atlacomulco (Estado de México) and Huatusco (Veracruz) counties in Mexico | Rural indigenous (speaking a language other than Spanish) and non‐indigenous (speaking only Spanish) Mexican women 14‐64 years of age living in highly marginalised communities | Not reported. | Women | Not reported | Communities are classified as highly marginalised and rural (less than 2500 inhabitants) by the Consejo Nacional de Poblacion | Highly marginalised | Women 14‐64 years of age. No details provided on sexual orientation or disability | The study enrolled 308 women from highly marginalised communities |
| Miglioranza 2009 | 9 peripheral city centres in Londrina, Parana (southern Brazil) | Not reported | Participants from public educational centres providing recreation, pedagogic assistance, cultural activities and meals for children under 14 years of age | 76 girls and 86 boys | 85% of the children's parents have no more than 3‐4 years of schooling. School levels of child participants not reported |
Annual income is less than USD 2000 per capita. | Families living under precarious socioeconomic
condition. Annual income is less than USD 2000 per capita. Most meals provided at the public education centres are this population's only access to food. |
Children and adolescents 7‐14 years of age. No details provided on sexual orientation or disability. | 162 children and adolescents attending 9 public educational centres in peripheral Londrina southern Brazil. Precarious socioeconomic conditions |
| Seal 2007 | Nangweshi refugee camp, opened in 2000 in response to the influx of refugees fleeing the Angolan civil war. Located in the Western Province of Zambia, about 180 km from the border with Angola. The population in June 2003 was 26,061 with 8404 households | The population of the camp are mainly from southern and eastern Angola | Not reported | 69 boys and 86 girls; 109 adolescent boys and 104 adolescent girls; and 118 adult women | Not reported | All refugees fleeing war in Angola | Inhabitants of a large refugee camp | Not reported | The study was undertaken among Angolan adolescents, children and women belonging to the same households in a refugee camp in Zambia. |
| Villalpando 2002 (C) | 34 boarding houses for 1786 schoolchildren in the State of Oaxaca, Mexico | Not reported | Primary school students | The study states that distribution of participants was balanced by age and sex. Details not reported | Not reported | Participants from shelters although socioeconomic status not described per se | Participants from shelters although social status not described per se | Children 6‐11 years of age | This study was conducted among children 6‐11 years of age living in public boarding houses in Oaxaca, Mexico |
Malaria endemicity
Two studies took place in malaria settings (Andang'o 2007; Seal 2007). In Seal 2007, malaria parasitaemia was present in less than 10% of participants and in positive cases, the median levels were low at 175 and 210 parasites/mL for children and adolescents, respectively. Andang'o 2007 took place during the months of highest malaria transmission, mainly due to Plasmodium falciparum, with a prevalence of malaria antigenaemia in all groups ranging from 46.4% to 51.2%. The other three studies do not report on malaria endemicity in the study setting (Carrasco 2011 (C); Miglioranza 2009; Villalpando 2002 (C)) .
Interventions
No studies assessed our first three comparisons, that is:
maize flour or maize flour products fortified with iron alone versus no intervention;
maize flour or maize flour products fortified with iron plus other vitamins and minerals versus no intervention; or
maize flour or maize flour products fortified with iron alone versus unfortified maize flours or maize flour products (not containing iron or any other vitamin and minerals).
This made it impossible to isolate the effects of iron in the fortification of maize flour.
All included studies compared maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals). Andang'o 2007 provided porridge (uji) prepared with whole maize flour and sweetened with sugar. The fortification group received elemental iron per kg flour (as NaFeEDTA or electrolytic iron) and vitamin A (2500 μg/kg), thiamin (3.5 mg/kg), riboflavin (4.0 mg/kg), and niacin (45.0 mg/kg). Carrasco 2011 (C) provided 20 kg monthly of maize flour; the fortified flour contained (per 100 g): 1.5 g soy protein, 42.4 mg elemental iron (as ferrous fumarate), 120 µg vitamin A, 548 µg (0.54 mg) folic acid, 33.3 mg zinc and 6.5 mg niacin. Villalpando 2002 (C) also provided nixtamalised corn flour; in the fortification group, this contained elemental iron (as reduced iron or NaFeEDTA) and 5 mg/kg thiamin, 3 mg/kg riboflavin, 35 mg/kg niacin, 2000 µg (2 mg)/kg folic acid and 40 mg/kg zinc.
With regard to the uncontrolled before‐and‐after studies, in Brazil Miglioranza 2009 provided corn flour‐derived sweet and savoury products (biscuits, cakes and pies), fortified with elemental iron powder (as reduced iron) and 350 µg (0.35 mg) folic acid in children and adolescents, while in the Zambian refugee camp, Seal 2007 provided 97% extraction fortified maize meal containing 2100 µg retinol equivalents, 4.4 mg thiamin, 2.6 mg riboflavin, 35 mg nicotinamide, 2.5 mg vitamin B6, 10 mg vitamin B12, 1500 µg (1.5 mg) folic acid, 35 mg elemental iron and 20 mg zinc in addition to a food ration containing 120 g pulses (beans or peas), 20 g vegetable oil and 10 g salt. The maize grain included in the pre‐intervention ration was replaced by the fortified maize meal during the period of the intervention.
Type of flour processing
Two studies evaluated the effects on nixtamalised maize flour (Carrasco 2011 (C); Villalpando 2002 (C)); two studies used whole maize milled meal (Andang'o 2007; Seal 2007); and another used degermed maize milled meal (Miglioranza 2009).
Duration of the intervention
The duration of the studies varied. Two studies lasted six months (Andang'o 2007; Miglioranza 2009); Carrasco 2011 (C) lasted 10 months; Seal 2007 lasted 7 months; and Villalpando 2002 (C)) had a duration of 9 months.
Iron compounds and dose of elemental iron per 100 g of product
The iron compounds and doses used in the fortification of the maize flours in the included studies varied. Andang'o 2007 used 2.8 mg and 5.6 mg per 100 g of maize flour; Seal 2007, 3.5 mg elemental iron per 100 g of flour; Villalpando 2002 (C), 3 mg elemental iron (as reduced iron or NaFeEDTA) per 100 g of flour; Miglioranza 2009, 9.8 mg reduced iron per 100 g flour; and Carrasco 2011 (C), 42.4 mg ferrous fumarate per 100 g maize flour.
Extraction rate
One study reported the extraction rate to be 97% (Seal 2007). The extraction rate was unknown/mixed/unreported in all other included studies.
Corn meal fibre/phytate content
The included studies did not report on fibre/phytate content.
Outcomes
Most of the outcomes reported were of haematological nature. Three studies reported on anaemia, defined as haemoglobin (Hb) below 110 g/L or 115 g/L and adjusted for altitude as appropriate (Andang'o 2007; Seal 2007; Villalpando 2002 (C)), and four studies reported iron deficiency (as defined by trialists, based on a biomarker of iron status) (Andang'o 2007; Miglioranza 2009; Seal 2007; Villalpando 2002 (C)), but the data were not extractable in all of them. All five studies reported haemoglobin concentration (in g/L) (Andang'o 2007; Carrasco 2011 (C); Miglioranza 2009; Seal 2007; Villalpando 2002 (C)).
Three studies reported iron status as iron deficiency and mean ferritin (Andang'o 2007; Miglioranza 2009; Villalpando 2002 (C)). Two studies reported on iron‐deficiency anaemia (Andang'o 2007; Miglioranza 2009), two on serum transferrin receptor (Andang'o 2007; Seal 2007), and one on transferrin saturation (Miglioranza 2009).
No studies reported any adverse effects.
Funding
Studies received funding from research grants, non‐government organisations and the private sector, sometimes in combination. Andang'o 2007 received funding from Unilever Food and Research Institute and Akzo Nobel Chemicals, and Unilever also participated in the study design and data interpretation. A state company, DICONSA, supported Carrasco 2011 (C). Nutrimilho Ind. & Com. de Alimentos, Ltd provided the fortified foods for Miglioranza 2009. The Micronutrient Initiative (now called Nutrition International), Canada and the Institute of Child Health in collaboration with African Humanitarian Action and CARE International supported Seal 2007 with additional support from the WPF and UNICEF. The National Institute of Public Health, a governmental institution in Mexico, supported Villalpando 2002 (C).
Excluded studies
Altogether we excluded 70 studies, some for more than one reason. The main reason for exclusion was the type of study, as most of them were absorption studies without an intervention and measured iron absorption from iron isotopes (stable or radioisotopes). The age group was another common criteria for exclusion since some studies were in infants and young children under the age of 24 months. Figure 2 summarises the details for exclusions.
We encountered limitations in the available studies due to the simultaneous presence of other fortification programmes. That was the case of the studies from ongoing programmes in Brazil and Venezuela, where wheat and maize fortification programmes started almost at the same time, and although both programmes reported data on haemoglobin, anaemia prevalence, ferritin and iron deficiency prevalence. The studies could not isolate the effects coming from maize flour fortification alone. In the case of the fortification programme in Brazil, at least eight studies report results on the effect of the fortification programme (both wheat and maize flours) on haematological outcomes in different population groups (Abreu 2009; Assuncao 2007a; Assuncao 2007b; Assuncao 2012; Da Silva 2012; De Souza 2011; Fujimori 2011; Sato 2008; Sato 2011). The reported results are varied for the same age group or physiological condition. Two studies in preschool children showed no change in haemoglobin concentration (113 g/L) and a change in anaemia prevalence (from 28.5% to 31.3%) two years after fortification was initiated (Assuncao 2007a; Assuncao 2012). Studies in pregnant women showed no change in anaemia prevalence or haemoglobin concentration (Sato 2008; Sato 2011), or they reported considerable reductions, from 40.3% to 28.8% in Da Silva 2012 and from 27.2% to 11.5% in De Souza 2011, without major changes in haemoglobin concentrations. The Venezuelan fortification programme showed an important impact reducing the prevalence of anaemia and iron deficiency, after initiating the inclusion of iron and vitamins in wheat and maize flours (Garcia‐Casal 2002; Layrisse 1996; Layrisse 2002). The prevalence of anaemia and iron deficiency in a sub‐sample of children and adolescents from urban areas of Venezuela decreased from 18.1% to 9.3% and from 37.2% to 15.8%, respectively, after 2 years of initiating the programme (Table 5).
4. Simultaneous maize flour and wheat flour fortification.
| Reference | Country | Design | Population | Pre‐fortification | Postfortification | ||||
| N | Hb concentration (g/L) |
Anaemia prevalence (%) |
N | Hb concentration (g/L) |
Anaemia prevalence (%) |
||||
| Abreu 2009 | Brazil | Uncontrolled before‐and‐after study Based on records |
Pregnant women | 384 | 124.6 ± 14.3 | 14.3 | 384 | 125.3 ±11.7 | 8.07 |
| Assuncao 2007a | Brazil | Uncontrolled before‐and‐after study Based on records |
Preschool children | 453 | 113 ± 28 | — | 923 | 113 ± 25 | — |
| Assuncao 2012 | Brazil | Uncontrolled before‐and‐after study Based on records |
Preschool children | 507 | — | 28.5 | 799 | — | 31.3 |
| Da Silva 2012 | Brazil | Retrospective cross‐sectional analysis from secondary data | Pregnant women | 391 | 116.6 ± 10.0 | 40.3 | 387 | 119.7 ± 10.5 | 28.8 |
| De Souza 2011 | Brazil | Retrospective cross‐sectional analysis from secondary data | Pregnant women | 427 | 117 ± 12 | — | 427 | 124 ± 13 | — |
| Fujimori 2011 | Brazil | Retrospective cross‐sectional analysis from secondary data | Pregnant women | 6062 | 118 ± 13 | — | 6057 | 129 ± 12 | — |
| Layrisse 1996 | Venezuela | Uncontrolled before‐and‐after study | Children and adolescents | 282 | — | 19 | 317 | — | 9.3 |
| Sato 2008 | Brazil | Retrospective cross‐sectional analysis from secondary data | Pregnant women | 390 | 123.5 ± 10 | — | 360 | 123.9 ± 11 | — |
| Sato 2011 | Brazil | Retrospective cross‐sectional analysis from secondary data | Pregnant women | 12119 | — | 25 | 12119 | — | 20 |
Hb: haemoglobin.
Risk of bias in included studies
The Characteristics of included studies section presents the risk of bias for each included study, and Figure 3 and Figure 4 show the summary judgements and an overall summary of the risk of bias. We judged the overall risk of bias to be low in one trial, Andang'o 2007, and high in the remaining studies.
3.
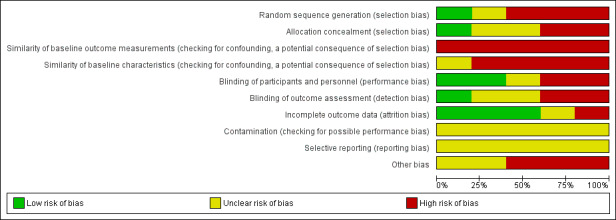
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation, allocation concealment and similarity of baseline characteristics and outcomes
Only three studies were RCTs that could have adopted allocation concealment and deployed appropriate blinding. Only one of these, Andang'o 2007, adequately described sequence generation and allocation concealment. Carrasco 2011 (C) was at unclear risk of bias for random sequence generation and allocation concealment. Villalpando 2002 (C) was at high risk of bias from sequence generation and allocation concealment, whilst the remaining two studies were not RCTs (Miglioranza 2009; Seal 2007). Baseline characteristics including baseline measures of the outcomes were dissimilar between groups in the trials, so we considered all studies to be at high risk of bias for baseline dissimilarity, a potential indicator of selection bias.
Blinding
Two studies described adequate blinding of participants and outcome assessors and were therefore at low risk of performance and detection bias (Andang'o 2007; Carrasco 2011 (C)). One randomised trial did not provide sufficient information to assess blinding (Villalpando 2002 (C)), whilst the remaining two studies were not RCTs and are thus at high risk of bias from inadequate blinding (Miglioranza 2009; Seal 2007).
Incomplete outcome data
Three studies (two RCTs and one before‐and‐after study without control) had low levels of loss to follow‐up, so we considered them to be at low risk of attrition bias (Andang'o 2007; Carrasco 2011 (C); Seal 2007). One study appeared to obtain endpoint assessments from less than 50% of the baseline recruited population (Miglioranza 2009). One RCT did not provide sufficient information to assess attrition bias (Villalpando 2002 (C)), so we judged it to be at unclear risk.
Contamination
Two studies used the same participants, followed longitudinally (Miglioranza 2009; Seal 2007), and It is unclear if there was contamination from the other intervention. For the other thee studies (Andang'o 2007; Carrasco 2011 (C); Villalpando 2002 (C)), there was no adequate information available to assess contamination of the trial from other sources.
Selective reporting
None of the studies provided adequate information for assessing risk of bias from selective reporting, and we did not have access to the study protocols.
Other potential sources of bias
Andang'o 2007 only included participants if they consumed at least 50% of the fortified food during the run in phase – this may have led to a form of selection bias. One study included only participants from very high risk communities, which may not be representative of the general population (Carrasco 2011 (C)).
Effects of interventions
See: Table 1
We were able to include only two RCTs in the meta‐analysis (Andang'o 2007; Villalpando 2002 (C)). No data were available for three of the four comparisons (i.e. maize fortified with iron versus no intervention; maize fortified with iron and other vitamins or minerals versus no intervention; maize fortified with iron alone versus unfortified maize). Data were available for the comparison: maize fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals). Data for many of the primary outcomes were not reported in the included trials. We sought to account for potential clinical heterogeneity by adopting random‐effects meta‐analysis. We also present results in the Table 1.
Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products
Anaemia
Randomised controlled trials
Two RCTs involving 1027 participants contributed data for this outcome (Andang'o 2007; Villalpando 2002 (C)). It is uncertain whether fortification reduces the risk of anaemia (risk ratio (RR) 0.90, 95% CI 0.58 to 1.40, P = 0.65, very low‐certainty evidence). Heterogeneity was high (Tau² = 0.08; Chi² = 4.11, df = 1 (P = 0.04); I² = 76%), so results should be interpreted with caution.
Other study designs
One uncontrolled before‐and‐after study reported a decrease in the prevalence of anaemia from 47.7% to 24.3% after distributing a fortified maize meal to children aged 6 to 59 months of age in a refugee camp in Zambia for 12 months (Seal 2007).
Seal 2007 also reported an increase in the prevalence of anaemia, from 19.2% (95% CI 14.2% to 25.2%) to 24.4% (95% CI 18.3% to 31.5%), in 212 adolescents aged 10 to 19 years, after receiving a fortified maize meal for one year. Prevalence of anaemia also increased in 118 women aged 20 to 49 years old, from 16.5% (95% CI 9.8% to 26.1%) to 27.6% (95% CI 17.1% 41.1%).
Iron deficiency
Randomised controlled trials
Two RCTs recruiting 1277 children (after adjustment the effective sample size changed to 1102 participants) reported on prevalence of iron deficiency at study end (Andang'o 2007; Villalpando 2002 (C)). Iron deficiency was defined by ferritin concentrations of less than 20 µg/L. The intervention reduced the risk of iron deficiency (RR 0.75, 95% CI 0.49 to 1.15, P < 0.001). However, after adjustment for design effect we are uncertain whether fortification reduces the risk of iron deficiency (RR 0.75, 95% CI 0.49 to 1.15, P < 0.001), as the certainty of the evidence was very low. Heterogeneity was low (Tau² = 0.04; Chi² = 1.77, df = 1 (P = 0.18); I² = 43%).
Other study designs
One uncontrolled before‐and‐after study in 162 children aged 7 to 14 years showed a reduction in the prevalence of iron deficiency from 14.9% to 1.2% six months after initiating the fortification programme (Miglioranza 2009).
We could not attempt formal comparison of effect sizes between subgroups. Among children receiving fortification with high iron bioavailability compounds, there was a bigger reduction in the prevalence of iron deficiency (RR 0.30, 95% CI 0.17 to 0.53) compared with children receiving lower bioavailability compounds (RR 0.62, 95% CI 0.17 to 2.30).
Haemoglobin concentration
Randomised controlled trials
Three RCTs recruiting 1144 participants reported on haemoglobin concentrations at endpoint (Andang'o 2007; Carrasco 2011 (C); Villalpando 2002 (C)). Fortification did not have an effect on haemoglobin concentrations (mean difference (MD) 1.25 g/L, 95% CI −2.36 to 4.86; 3 studies; 1144 participants; very low‐certainty evidence). Heterogeneity was high (Tau² = 7.26; Chi² = 8.07, df = 2 (P = 0.02); I² = 75%), probably because studies used different maize flour and food production processes.
Other study designs
One uncontrolled before‐and‐after study (Seal 2007) reported haemoglobin concentration in women, showing a decrease from 131 g/L (95% CI 127 to 135) to 126 g/L (95% CI 121 to 132).
Two uncontrolled before‐and‐after studies likewise showed no effect of fortification on haemoglobin concentration at endpoint. One of the studies showed no change from 122 g/L after the fortification period (Miglioranza 2009), while in the other, haemoglobin concentration increased from 109 g/L to 118 g/L in children (Seal 2007).
With only two eligible studies, we could not attempt a formal comparison of effect sizes between subgroups. Neither of the studies found a significant beneficial effect from fortification on haemoglobin concentrations.
In adolescents, Seal 2007 showed an increase in haemoglobin concentration from 129 g/L (95% CI 127 to 132) to 131 g/L (95% CI 128 to 133).
Iron status (ferritin)
Randomised controlled trials
One RCT recruiting 758 children (effective sample size after adjustment is 584 participants) reported on ferritin concentrations at endpoint (Villalpando 2002 (C)). Fortification did not have an effect on ferritin concentrations (MD 0.48 µg/L, 95% CI −0.37 to 1.33, P = 1.00).
Other study designs
One uncontrolled before‐and‐after study reported a change in ferritin concentration from 27.3 µg/L to 35.5 µg/L (Miglioranza 2009).
Any adverse effects
No studies reported on any adverse effects.
There were no included trials in pregnant women or in adult men.
Iron‐deficiency anaemia
Randomised controlled trials
Andang'o 2007 reported on the effects of fortification on iron‐deficiency anaemia and did not find any effect (RR 1.04, 95% CI 0.58, 1.88, P = 0.89).
Other study designs
In one uncontrolled before‐and‐after study in 86 boys and 76 girls aged 7 to 14 years (Miglioranza 2009), iron‐deficiency anaemia decreased from 14.9% to 1.4% six months after receiving corn‐flour fortified products.
Discussion
Summary of main results
This review was undertaken to determine the effect of maize flour and corn meal fortified with iron for controlling anaemia and iron deficiency. We included five studies, involving 3459 participants. Three studies were RCTs (Andang'o 2007; Villalpando 2002 (C), Carrasco 2011 (C)), and the other two were uncontrolled before‐and‐after studies (Miglioranza 2009; Seal 2007). Two studies were in children: aged 3 to 8 years in Andang'o 2007 and aged 6 to 11 years in Villalpando 2002 (C). Miglioranza 2009 involved children and adolescents aged 7 to 14 years, while Seal 2007 included young children (6 to 59 months of age), adolescents (10 to 19 years of age) and women (20 to 49 years of age). Carrasco 2011 (C) recruited adolescents and women aged 14 to 64 years.
Only three studies, involving children aged 2 to 11.9 years, could contribute data to the meta‐analysis (Andang'o 2007; Carrasco 2011 (C)Villalpando 2002 (C)). Compared with unfortified maize flour, it is uncertain whether maize flour or corn meal fortified with iron and other vitamins and minerals has any effect on anaemia, iron deficiency, haemoglobin concentration or ferritin concentrations, as the certainty of the evidence was very low.
Although mass fortification of maize flour with iron has been a reality for many years in several countries in the Americas (Dary 2002a; Garcia‐Casal 2002), as well as in Africa (Fiedler 2014), this review shows the very limited amount of evidence for any effect of fortification of maize flour with iron on the prevalence of anaemia and iron deficiency in the general population.
Another important limitation of the review relates to the assessment of potential adverse effects of fortification, an issue that can be attributable to the study designs and the duration of the studies. The studies do not aim to assess potential adverse events such as the effects of maize flour fortification with micronutrients on the absorption of other nutrients present in the usual diet or the displacement of some foods for the fortified maize flour in the medium and long term. It is important that policymakers consider existing interventions providing vitamins and minerals and assure that coherent, effective and responsible fortification practices and policies are in place in the public sector (Dwyer 2015). Other potential unforeseen consequences include not reaching the intended target populations or excessive intake of micronutrients in some groups when other staple foods are already being fortified (Garcia‐Casal 2018).
We found rather limited data for this review. In a future update, if there are additional data available, more sophisticated syntheses and methodological approaches may be appropriate.
Overall completeness and applicability of evidence
None of the included studies focused on the effect of maize flour fortified exclusively with iron on anaemia and iron deficiency. Rather, they compared maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals), which made it impossible to isolate the effects of iron fortification in this food vehicle. All the included studies dealt with the effect of iron and other vitamins and minerals on anaemia and iron deficiency compared to unfortified maize flour. No studies assessed maize flour fortified with iron alone versus no intervention; maize flour fortified with iron plus other vitamins and minerals versus no intervention; or maize flour fortified with iron alone versus unfortified maize flour (not containing iron nor any other vitamin and minerals).
From the two uncontrolled before‐and‐after studies we found that fortification of maize flour or flour products with iron and other vitamins and minerals reduced anaemia, iron deficiency and iron‐deficiency anaemia prevalence and increased ferritin concentration in children, although the intervention had no effect on haemoglobin concentration (Miglioranza 2009; Seal 2007). In adolescents in one uncontrolled before‐and‐after study, anaemia prevalence increased from 19.2% to 24.4%, and haemoglobin concentration from 129 g/L to 131 g/L (Seal 2007). In adult women, an RCT reported a change in haemoglobin concentration from 131 g/L to 133 g/L in the fortification group and from 131 g/L to 132 g/L in the control group (Carrasco 2011 (C)), while an uncontrolled before‐and‐after study reported a decrease from 131 g/L to 126 g/L (Seal 2007).
Quality of the evidence
The certainty of the evidence in the five included studies was generally very low, as the risk of bias was high in many domains for the included trials, so we are uncertain whether the results reflect the true effect of the intervention. For the three studies included in the meta‐analysis (Andang'o 2007; Carrasco 2011 (C); Villalpando 2002 (C)), the certainty of the evidence was very low for all primary outcomes (Table 1). Villalpando 2002 (C) was at high risk of bias due to its randomisation method and allocation concealment and at unclear risk of bias for blinding. Moreover, both Andang'o 2007 and Villalpando 2002 (C) showed wide confidence intervals crossing the line of no effect for the outcomes of anaemia, iron deficiency and iron‐deficiency anaemia.
Potential biases in the review process
There were a number of potential biases in the review process, which we attempted to minimise in several ways: two review authors independently assessed eligibility for inclusion, and two authors checked data extraction, assessments of risk of bias and data entry. However, carrying out reviews is not an exact science and can require a number of subjective judgements; it is possible that a different review team may have reached different decisions regarding eligibility and risk of bias. There was insufficient information to describe the risk of bias assessments in various domains in studies randomised at the individual versus cluster level.
We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
In addition to this, it was impossible to ascertain whether the included studies were conducted in populations already reached by mandatory staple foods fortification programmes.
Agreements and disagreements with other studies or reviews
A systematic review of food fortification with iron or biofortified crops with increased iron content in the general population assessed the effect of iron fortification on haemoglobin and serum ferritin concentrations and the prevalence of iron deficiency and anaemia (Gera 2012). The review included 60 RCTs, 36 of which were on cereals and 19 in wheat and rice, with no mention of maize flour. The review showed that iron fortification of foods resulted in a significant increase in haemoglobin and serum ferritin and a reduced risk of anaemia and iron deficiency. This review included only iron as fortificant and excluded studies evaluating point‐of‐use fortification.
Another systematic review aimed to identify all available evidence for the impact of fortification interventions (Das 2013). Selected studies included all foods fortified with a single, dual or multiple micronutrients, and review authors analysed the impact of fortification on health outcomes and relevant biochemical indicators in women and children. For iron status, the studied biomarkers were serum ferritin, serum transferrin, haemoglobin and anaemia. Of 201 included studies, 121 studies were in infants and children, 79 were in women and 1 was in both women and children. In children, the pooled analyses from the RCTs demonstrated a significant increase in haemoglobin concentration, serum ferritin levels and reduction in anaemia. Also, iron fortification led to a significant increase in serum ferritin and haemoglobin levels in women of reproductive age and pregnant women. This review included all foods grouped as processed foods, staples (no mention of maize flour) or condiments.
Additionally, another systematic review addressed the impact of multiple micronutrients provided via fortification on micronutrient status, growth, health, and cognitive development in school children (Best 2011). This review included food interventions providing fortified foods or beverages as well as fortificants that were added to foods or drinks on‐site or at home, comparing the effects of multiple micronutrients provided via fortified versus unfortified food. The review identified 12 eligible studies, concluding that multi‐micronutrient food fortification (containing at least iron and vitamin A or beta‐carotene, iodine, zinc, B‐vitamins and vitamin C) consistently improved micronutrient status and reduced the prevalence of anaemia. Besides this effect on anaemia prevalence, other differences with the present review reside in the food vehicles used for fortification, the predominance of included studies using beverages for fortification, and the addition of fortificants to foods or drinks on‐site or at home.
The previously mentioned interventions in Brazil and Venezuela (Assuncao 2012; Da Silva 2012; De Souza 2011; Layrisse 2002), fortifying simultaneously wheat and maize flours, had a positive impact on anaemia in Venezuela, while in Brazil they showed contradictory results depending on the age group. It is possible that in countries with diets based in cereals with cereal‐based diets where iron deficiency and anaemia are prevalent problems, fortification of more than one staple cereal food could help to improve the impact of interventions on iron status.
In interventions in different age groups and all countries that used fortified maize flour alone or in combination with other foods, most showed improvements in iron status and anaemia (Aguirre Arenas 2013; Chilenje Infant Growth 2010; Faber 2005; Macharia‐Mutie 2012), while others showed no effect (Assuncao 2012; Bisimwa 2012), and one systematic review suggested a publication bias favouring a positive effect of food fortification on anaemia in children (Assuncao 2007a).
Authors' conclusions
Implications for practice.
Due to the limited information in the literature, the analysis was limited to a few studies, and we do not have sufficient evidence to determine an effect. It is uncertain whether fortification of maize flour with iron and other vitamins and minerals reduces the risk of iron deficiency in populations aged 2 years or older. The evidence is also too uncertain to conclude whether iron‐fortified maize flour, corn meal or fortified maize flour products have any effect on reducing the risk of anaemia or in improving haemoglobin concentration in the general population.
No studies reported on any adverse effects. The presence of industry funding for some of these trials did not appear to positively influence results from these studies. There was very limited information that could be included in this review, and more studies are needed in maize flour and products to determine the effect of maize flour fortification as an intervention for preventing anaemia.
Implications for research.
More well‐designed RCTs designed to evaluate the effect of iron alone or with iron plus other vitamins and minerals on maize fortification are needed, since both interventions are currently being implemented as public health strategies worldwide. The effect of iron alone or with other vitamins and minerals could have a different impact on anaemia and iron deficiency in different settings.
Also, studies on the effect of including iron compounds of different bioavailability and costs in fortification trials on anaemia and iron deficiency, especially in low‐socioeconomic settings, are also needed. Studies are required in all age groups (especially pregnant women, infants and in general groups vulnerable to iron deficiency) and physiological conditions.
Since fortification programmes on maize and cornmeal are frequently initiated in combination with fortification of other food items (especially wheat flour) it would be interesting to consider broadening the review question to consider at least fortification of both maize and wheat flours together.
Acknowledgements
We would like to thank Cochrane Public Health for their editorial support in the preparation of the review, and Ms Evelyn Boy‐Mena for her support in the interpretation of the PROGRESS‐Plus framework in the included studies.
We thank Dr Lucero Lopez and Dr Ricardo Martinez Martinez for their support in the adjustments for the intra cluster correlations in the included studies and the imputations of standard deviations in Carrasco 2011 (C) and Villalpando 2002 (C). We are also grateful to Newton Opiyo, associate editor from the Editorial & Methods Department, Cochrane Central Executive for the useful technical input and revisions of this review.
We kindly acknowledge Joanne Abbott, information specialist (www.abbottinformation.co.uk), for her support in the search strategy and updated searches.
The World Health Organization (WHO) and Luz Maria De‐Regil, Jeffrey A Gwirtz, Sant‐Rayn Pasricha retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
Appendices
Appendix 1. Database search strategy
Fortification of maize flour with iron for preventing anaemia and iron deficiency in populations
Database search strategy updated 20 December 2017
1. Cochrane Central Database of Controlled Trials (CENTRAL)
ID Search
#1 MeSH descriptor: [Zea mays] this term only
#2 MeSH descriptor: [Flour] this term only
#3 z* next mays
#4 zeamays
#5 corn or cornmeal or cornflour*
#6 maize or mielies or mealies
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Food, Fortified] this term only
#9 MeSH descriptor: [Iron] this term only
#10 MeSH descriptor: [Iron Compounds] 1 tree(s) exploded
#11 Fe or ferrous or ferric
#12 NaFeEDTA
#13 (iron near/5 (enhanc* or enrich* or fortif*))
#14 (iron near/5 (cereal* or diet* or flour* or food* or intake*))
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #7 and #15
2. Ovid MEDLINE(R)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Flour/
2 Zea mays/
3 (z$ mays or zeamays).tw.
4 (corn or cornmeal or cornflour$).tw.
5 (maize or mielies or mealies).tw.
6 or/1‐5
7 Food, Fortified/
8 iron/
9 exp Iron Compounds/
10 (Fe or ferrous or ferric).tw.
11 NaFeEDTA.tw.
12 (iron adj5 (cereal$ or diet$ or flour$ or food$ or intake$)).tw.
13 (iron adj5 (enhanc$ or enrich$ or fortif$)).tw.
14 or/7‐13
15 6 and 14
16 exp animals/ not humans/
17 15 not 16
3. Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations
1 Flour.tw.
2 (z$ mays or zeamays).tw.
3 (corn or cornmeal or cornflour$).tw.
4 (maize or mielies or mealies).tw.
5 or/1‐4
6 iron.tw.
7 (Fe or ferrous or ferric).tw.
8 NaFeEDTA.tw.
9 or/6‐8
10 5 and 9
4. Embase
1 flour/
2 exp maize/
3 (z$ mays or zeamays).tw.
4 (corn or cornmeal or cornflour$).tw.
5 (maize or mielies or mealies).tw.
6 or/1‐5
7 diet supplementation/ and (fortif$ or enrich$ or enhanc$).tw.
8 iron/
9 iron derivative/
10 ferrous ion/
11 (Fe or ferrous or ferric).tw.
12 NaFeEDTA.tw.
13 (iron adj5 (enhanc$ or enrich$ or fortif$)).tw.
14 (iron adj5 (cereal$ or diet$ or flour$ or food$ or intake$)).tw.
15 or/7‐14
16 6 and 15
17 animal/ not human/
18 16 not 17
5. CINAHL Plus (EBSCO Host)
S16 S8 and S15 Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 369 Edit S16
S15 S9 or S10 or S11 or S12 or S13 or S14 Search modes ‐ Boolean/Phrase
Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 11983 Edit S15
S14 iron* Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 8628 Edit S14
S13 NaFeEDTA Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 17 Edit S13
S12 Fe or ferrous* or ferric* Search modes ‐ Boolean/Phrase Interface ‐
EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 1657 Edit S12
S11 (MH "Iron Compounds+") Search modes ‐ Boolean/Phrase Interface ‐
EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 2454 Edit S11
S10 (MH "Iron") Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 3848 Edit S10
S9 (MH "Food, Fortified") Search modes ‐ Boolean/Phrase Interface ‐
EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 1969 Edit S9
S8 S1 or S2 or S3 or S4 or S5 or S6 or S7 Search modes ‐ Boolean/Phrase
Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 3710 Edit S8
S7 maize or mielies or mealies Search modes ‐ Boolean/Phrase Interface ‐
EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 168 Edit S7
S6 corn or corn meal or cornflour* Search modes ‐ Boolean/Phrase Interface
‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 800 Edit S6
S5 zeamays Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 2 Edit S5
S4 z* mays Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 30 Edit S4
S3 flour* Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 962 Edit S3
S2 (MH "Corn") Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 384 Edit S2
S1 (MH "Cereals") Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 1986 Edit S1
6. Science Citation Index Expanded (SCI‐Exp) via Web of Science
## 5 #4 AND #3
# 4 TS=(( food OR maize or mieles or mealies or "z* mays" or zeamays or cereal* or corn or cornmeal or cornflour* or flour*) NEAR (fortif* OR enrich* or enhance*))
# 3 #2 OR #1
# 2 TS=(NaFeEDTA)
# 1 TS=(iron* or Fe or ferrous* or ferric*)
7. Social Science Citation Index (SSCI) via Web of Science
## 5 #4 AND #3
# 4 TS=(( food OR maize or mieles or mealies or "z* mays" or zeamays or cereal* or corn or cornmeal or cornflour* or flour*) NEAR (fortif* OR enrich* or enhance*))
# 3 #2 OR #1
# 2 TS=(NaFeEDTA)
# 1 TS=(iron* or Fe or ferrous* or ferric*)
8. POPLINE http://www.popline.org
Searched in the Global field for:
( maize OR mielies OR mealies OR "zea mays" OR zeamays OR cereal* OR corn OR cornmeal OR cornflour* OR flour* ) AND
(iron* OR Fe OR ferrous* OR ferric* OR NaFeEDTA) AND
(fortif* OR enhanc* OR enrich*)
9. AGRICOLA (http://agricola.nal.usda.gov/)
Search Request: Search = (maize OR corn? OR mealies OR mielies OR zea OR mays OR zmays)[in Keyword Anywhere] AND (enhanc? OR enrich? OR fortif?)[in Keyword Anywhere] AND (iron? OR Fe OR ferrous OR ferric OR NaFeEDTA)[in Keyword Anywhere]
10 (a)African Index Medicus (AIM) (http://www.who.int/library/databases/afro/en/) and AFRO Library
10 (b) AFRO Library http://afrolib.afro.who.int/cgi‐bin/wxis.exe/iah/
Zea or maize or corn or cornmeal or cornflour or mealies or mielies or flour
11. IndMED http://indmed.nic.in/indmed.html
Search for
(maize or corn$ or flour or zea ) and (iron$ or Fe or ferric$ or ferrous$ or NaFeEDTA )
12. Western Pacific Region Index Medicus ( http://www.wprim.org/)
15 12 #14 and #7
14 2846 #13 or #12 or #11 or #10 or #9 or #8
13 1688 Default:iron
12 3 Default: NaFeEDTA
11 770 Default: (Fe or ferrous or ferric)
10 129 MeSH Heading:Iron Compounds/MeSH Tree 1/All Subheadings
9 859 MeSH:iron
8 5 MeSH:Food, Fortified
7 373 #6 or #5 or #4 or #3 or #2 or #1
6 61 Default:(maize or mielies or mealies)
5 298 Default:corn or cornmeal or cornflour%
4 40 Default:zea mays
3 0 Default:zeamays
2 8 MeSH:Flour
1 29 MeSH:zea mays
13. IMEMR WHO‐ EMRO (http://applications.emro.who.int/library/Databases/wxis.exe/Library/Databases/iah/?IsisScript=iah/iah.xis&lang=I&base=imemr)
Search on : "ZEA MAYS" or "corn" or maize or corn$ or flour$ or mealies or mielies [KeyWords] and "IRON" or "IRON COMPOUNDS" or iron$ or fe or ferrous$ or ferric$ [KeyWords]
14. IMSEAR (http://imsear.hellis.org/)
(((maize OR mielies OR mealies OR "zea mays" OR zeamays OR cereal* OR corn OR cornmeal OR cornflour* OR flour*)) AND ((iron* OR Fe OR ferrous* OR ferric* OR NaFeEDTA)))
15. SciELO (http://www.scielo.br/)
Database : article
Search on : ZEA MAYS or maize or corn or cornmeal$ OR cornflour$ or flour$ or mealies or mielies [All indexes] and iron$ or fe or ferrous$ or ferric$ or NaFeEDTA [All indexes]
16. WHOLIS ( http://regional.bvsalud.org/php/index.php)
Search > (MH: "Zea Mays" or MH:"Flour" or (zea mays) or zeamays or cereal or corn or cornmeal or cornflour or maize or mielies or mealies ) AND (MH:"iron" or MH:"iron compounds" or iron* or Fe or ferrous* or ferric* or NaFeEDTA)
17. LILACS (http://regional.bvsalud.org/php/index.php)
Search > (MH: "Zea Mays" or MH:"Flour" or (zea mays) or zeamays or cereal or corn or cornmeal or cornflour or maize or mielies or mealies ) AND (MH:"iron" or MH:"iron compounds" or iron* or Fe or ferrous* or ferric* or NaFeEDTA)
18. IBECS (http://regional.bvsalud.org/php/index.php)
Search > (MH: "Zea Mays" or MH:"Flour" or (zea mays) or zeamays or cereal or corn or cornmeal or cornflour or maize or mielies or mealies ) AND (MH:"iron" or MH:"iron compounds" or iron* or Fe or ferrous* or ferric* or NaFeEDTA)
19. PAHO (http://regional.bvsalud.org/php/index.php)
Search > (MH: "Zea Mays" or MH:"Flour" or (zea mays) or zeamays or cereal or corn or cornmeal or cornflour or maize or mielies or mealies ) AND (MH:"iron" or MH:"iron compounds" or iron* or Fe or ferrous* or ferric* or NaFeEDTA)
20. Native Health Database (https://hscssl.unm.edu/nhd/)
KEYWORDS for: maize or corn or cornflour or cornmeal or mealies or mielies or "zea mays"
We modified search terms as necessary when searching other databases. We did not apply any date or language restrictions, and obtained translations of relevant data where necessary.
21. Clinicaltrials.gov (https://clinicaltrials.gov/)
fortified maize flour OR enriched maize flour OR fortified corn flour OR enriched corn flour
22. International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/)
fortified maize flour OR enriched maize flour OR fortified corn flour OR enriched corn flour
Data and analyses
Comparison 1. Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (defined as haemoglobin (Hb) below WHO cut‐off, adjusted for altitude and smoking, as appropriate) | 2 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.40] |
| 2 Iron deficiency (as defined by trialists, based on a biomarker of iron status) | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.49, 1.15] |
| 3 Haemoglobin concentration (in g/L) | 3 | 1144 | Mean Difference (IV, Random, 95% CI) | 1.25 [‐2.36, 4.86] |
| 4 Ferritin concentrations (in µg/L) | 1 | 584 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.37, 1.33] |
| 5 Iron‐deficiency anaemia (as defined by trialists) | 1 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.58, 1.88] |
1.1. Analysis.
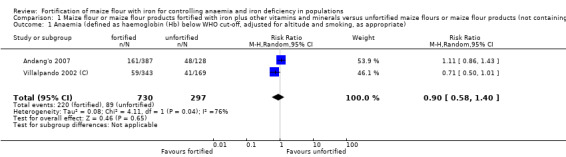
Comparison 1 Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals), Outcome 1 Anaemia (defined as haemoglobin (Hb) below WHO cut‐off, adjusted for altitude and smoking, as appropriate).
1.2. Analysis.
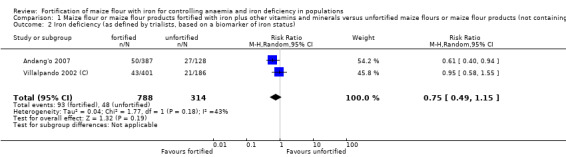
Comparison 1 Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals), Outcome 2 Iron deficiency (as defined by trialists, based on a biomarker of iron status).
1.3. Analysis.
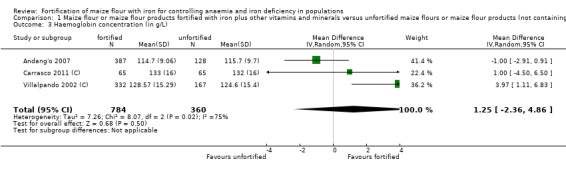
Comparison 1 Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals), Outcome 3 Haemoglobin concentration (in g/L).
1.4. Analysis.
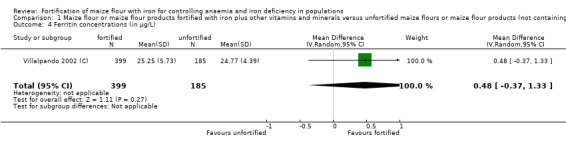
Comparison 1 Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals), Outcome 4 Ferritin concentrations (in µg/L).
1.5. Analysis.
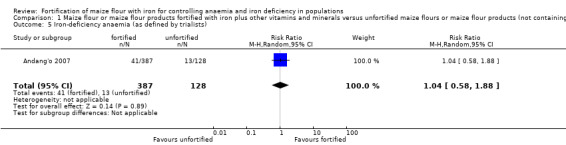
Comparison 1 Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals), Outcome 5 Iron‐deficiency anaemia (as defined by trialists).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andang'o 2007.
| Methods | Randomised controlled trial with 4 arms | |
| Participants | 516 children (260 girls and 256 boys) 3‐8 years of age from 4 schools in Marafa, a semi‐arid region in coastal Kenya, who consumed at least 50% of the pre‐specified amount of cooked maize flour and had no overt disease, no severe malnutrition, no mental disability and haemoglobin concentrations of 70 g/L or higher
|
|
| Interventions | Participants were randomly assigned to 1 of 4 groups: group 1 (n = 121) received porridge (uji) prepared with whole maize flour and sweetened with sugar, fortified with 56 mg elemental iron per kg flour (as NaFeEDTA) and vitamin A (2500 μg/kg), thiamin (3·5 mg/kg), riboflavin (4.0 mg/kg), and niacin (45.0 mg/kg); group 2 (n = 139) received uji prepared with whole maize flour and sweetened with sugar fortified with 28 mg elemental iron per Kg of flour (as NaFeEDTA) and vitamin A (2500 μg/kg), thiamin (3.5 mg/kg), riboflavin (4.0 mg/kg), and niacin (45.0 mg/kg); group 3 (n = 127) received uji prepared with whole maize flour and sweetened with sugar fortified with 56 mg elemental iron per Kg of flour (as electrolytic iron) and vitamin A (2500 μg/kg), thiamin (3.5 mg/kg), riboflavin (4.0 mg/kg), and niacin (45.0 mg/kg); group 4 (n = 128) received uji prepared with unfortified whole maize flour and sweetened with sugar. Children 3‐5 years of age received 700 mL uji (corresponding to 100 g of whole maize flour), and children 6‐8 years of age received 1000 mL uji (corresponding to 150 mg whole maize flour). Children received these amounts of uji in two equally divided portions five days a week during 5 months. The premix was supplied by Roche (Basel, Switzerland). Participants who had fever or other signs of malaria and who tested positive for malaria were given sulphadoxine‐pyrimethamine before the start of the intervention. Fieldwork took place between May and November 2004, to reduce non‐compliance due to holidays in April and August, and from December to early January. Because of this constraint, the trial period coincided with the two rainy seasons (March‐May and October‐December), in which malaria transmission is highest. The median particle size of electrolytic iron was 34 µm.
|
|
| Outcomes | Iron‐deficiency anaemia, iron deficiency, anaemia, haemoglobin concentration, plasma ferritin concentrations and soluble transferrin receptor, weight, height, C‐reactive protein, any adverse events | |
| Notes | Source of funding: Private sector (Unilever Food and Research Institute, Vlaardingen, and Akzo Nobel Chemicals, Arnhem, Netherlands). Unilever Food and Research Institute participated in study design and data interpretation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation code was generated by simple randomisation with a table of random numbers by an investigator who did not participate in the screening or enrolment of children. |
| Allocation concealment (selection bias) | Low risk | Each flour type was labelled with colour‐coded packaging. This code was withheld from participants and investigators. Eligible children were randomly allocated to 1 of 4 treatment groups, which were colour coded to correspond to the flour packaging. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | "Haemoglobin concentrations were highest in the placebo group." |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | High risk | 49% of the children had current or recent malaria infection. Additionally 290 children (56%) had anaemia, 78 children (15%) iron deficiency, and 54 children (11%) had iron‐deficiency anaemia at baseline. A total of 71 (58.7%) participants in the high‐dose NaFeEDTA, 84 (60.4%) in the low‐dose NaFeEDTA, 75 (59.1%) participants in the electrolytic iron and 60 (46.9%) participants in the placebo group were anaemic at baseline. Also, 8 out of 121 (6.6%) participants in the high‐dose NaFeEDTA, 13 out of 140 (9.3%) in the low‐dose NaFeEDTA, 6 out of 127 in the electrolytic iron (4.7%) and 9 out of 128 (7.0%) in the placebo group had inflammation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded (personnel remained blind until all data was collected and analysis started). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by intention‐to‐treat, except that retrospectively they excluded one child with abnormal iron status and sickle‐cell anaemia. Missing data were imputed blindly, before starting primary analysis, as the mean or median values from children without missing values in the same group. |
| Contamination (checking for possible performance bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | All children were given a target daily amount of cooked unfortified flour to assess the acceptability of the fortification vehicle and adherence with consumption of the target quantity 4 weeks before beginning the intervention. Only children who consumed at least 50% of the cooked unfortified flour were considered for eligibility. |
Carrasco 2011 (C).
| Methods | Double‐blind randomised trial with 2 arms. Units of randomisation were households living in the chosen counties. | |
| Participants | 308 rural indigenous (non‐Spanish speaking) and non‐indigenous (speaking only Spanish) Mexican women, 14‐64 years of age living in the Huejutla de Reyes (Hidalgo), Atlacomulco (Estado de México) and Huatusco (Veracruz) counties In a separate reference, the results for the 395 infants and young children (7 to 18 months old) received fortified (n = 195) or unfortified (n = 200) corn flour for 10 months. The indicators of impact were: nutritional status, mental and psychomotor development and blood haemoglobin levels. There were no differences between the experimental group and the control group for those parameters. These data are not considered in this review as they pertain to a study group under 2 years of age.
|
|
| Interventions | Families were randomly assigned to 1 of 2 groups: group 1 (n = 155): received 20 kg monthly of fortified maize flour containing per 100 g: 1.5 g soy protein, 42.4 mg iron (as ferrous fumarate), 120 µg vitamin A, 548 µg (0.54 mg) folic acid, 33.3 mg zinc and 6.5 mg niacin; group 2 (n = 153) received 20 kg of unfortified maize flour monthly. The intervention was provided for 10 months. Families with more than 10 members received an additional 5 kg of maize flour. Only the data for the women are included in the analysis.
|
|
| Outcomes | Weight, waist, hip, body mass index, and waist circumference, haemoglobin concentrations at 4 and 6 months | |
| Notes | In order to adjust for design effect in the cluster‐randomised trial, we computed the effective sample size of fortified corn flour (3 clusters) versus the non‐fortified corn flour (3 clusters) for the haemoglobin measure outcome. The mean size of each cluster was 51.33, and the intra cluster correlation coefficient was *ICC = 0.02723, corresponding to a design effect of 2.37. Thus, the fortified corn flour sample size is 65 (from N = 155), and non‐fortified corn flour sample size is 65 (from N = 153). The ICC 0.02723 for continuous haemoglobin outcome for the 3 rural communities was taken as equivalent to the ICC for postal code reported from one reference (Gulliford 1999). There were no changes in Hb concentrations among women receiving fortified or unfortified maize flours (133 g/L versus 131 g/L) but there was no SD presented. We carried out imputation of standard deviations from other studies in women. In order to compute the SD of the haemoglobin continuous outcome for the fortified corn flour group (change in haemoglobin concentration from 131 g/L to 133 g/L) versus the non‐fortified corn flour control group (change 131 g/L to 132 g/L), we imputed the corresponding SD value computing potential scenarios with the information given by the authors, and making the assumption that the SD value of fortified corn flour and non‐fortified corn flour control groups are equal using similar populations in Mexico (Martinez 1995; Shamah‐Levy 2003). The data can be seen in Table 2. Source of funding: DICONSA, previously CONASUPO (Compañía Nacional de Subsidios Populares) C/COL/2907/2010. Diconsa is a mayor state participation company that belongs to the Social Development Sector. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | 88.6% of the participants were indigenous and 59.1% had low height (less than 149.9 cm). The baseline characteristics were similar for Hb concentrations, weight, body mass index, waist and hip measurements. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | High risk | There were some differences at baseline in the characteristics of the indigenous populations in comparison to non‐indigenous, as well as in the three different locations: Huejutla, Huatusco and Atlacomulco |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded to the intervention groups. The study is reported as double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to the intervention groups. Quote: "Neither women nor the researchers knew whether the provided maize flour was fortified or unfortified." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are presented for all the participants randomised. |
| Contamination (checking for possible performance bias) | Unclear risk | There is insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The marginalised communities where the studies were carried out were those classified as high risk of malnutrition and anaemia and are rural (less than 2500 inhabitants according to the National Council of Population in Mexico (short CONAPO, in Spanish). The index of marginalisation seeks to establish an analytical parameter to understand when a sector of society is in a situation where opportunities for development or the capacity to find them are not present. |
Miglioranza 2009.
| Methods | Uncontrolled before‐and‐after study | |
| Participants | 362 children and adolescents 7‐14 years of age from the 9 peripheral city centres in Londrina, Parana (southern Brazil). Only 162 individuals (86 boys and 76 girls, aged 7‐14 years) completed the study.
|
|
| Interventions | Fortification was accomplished by administering corn flour‐derived sweet and savoury products (biscuits, cakes and pies), fortified with elemental iron powder in the form of reduced iron (H2‐reduced Fe < 45 mm or 325 mesh) for 6 months. The corn flour‐derived products had been previously evaluated and found to have an excellent acceptance. Each participant ingested daily 100 g of the corn flour‐derived products, containing 76 g carbohydrates, 2 g fat, 8 g protein, 9.8 mg elemental iron and 350 µg (0.35 mg) folic acid.
|
|
| Outcomes | Haemoglobin concentration, serum iron, total iron binding capacity, percentage transferrin saturation and serum ferritin concentration. IDA was defined when Hb level lower than 120 g/L and serum ferritin level lower than 20 µg/L were found, and ID when serum ferritin had fallen to less than 20 µg/L. |
|
| Notes | Source of funding: Foods were provided by Nutrimilho Ind. & Com. de Alimentos, Ltd (Maringa, Parana, Brazil). "Universidade Estadual de Londrina, which maintains reagents and facilities for laboratory training of students, and those obtained through the Official Agreements between the University and the company Nutrimilho, which obtains rebate of income taxes for supporting the institution in developing research." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial. Uncontrolled before‐and‐after study |
| Allocation concealment (selection bias) | High risk | All participants were exposed to the fortified flour. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | The study is an uncontrolled before‐and‐after intervention study |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | High risk | The study is a pre and postintervention study without a control group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All children received the fortified flour products. The study was open. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was open. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 162 individuals out of 362 participants (86 boys and 76 girls, aged 7‐14 years) completed the study. |
| Contamination (checking for possible performance bias) | Unclear risk | The study was done on the same children followed longitudinally. It is unclear if there was contamination from another intervention. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | There is insufficient information to permit judgement. |
Seal 2007.
| Methods | Uncontrolled before‐and‐after study using a longitudinal cohort | |
| Participants | 155 children (6‐59 months; 69 boys, 86 girls), 213 adolescents (10‐19 years; 109 boys, 104 girls), and 91 women (20‐49 years) of Nangweshi refugee camp in Zambia
|
|
| Interventions | Each household received a 400 g ration of 97% extraction, fortified maize meal per registered refugee, irrespective of age, throughout the duration of the intervention period, which lasted from November 2003 until June 2004. The ration supplied by the World Food Programme also included, per person per day, 120 g pulses (beans or peas), 20 g vegetable oil and 10 g salt. The maize grain included in the pre‐intervention ration was replaced by the fortified maize meal during the period of the intervention. The fortificant formulation contained 2100 mg RE vitamin A, 4.4 mg thiamin, 2.6 mg riboflavin, 35 mg nicotinamide, 2.5 mg vitamin B6, 10 mg vitamin B12, 1500 µg (1.5 mg) folic acid, 35 mg elemental iron and 20 mg zinc. It was added at flour stage to the maize meal, which was then blended, bagged and stored for a maximum of 4 weeks prior to distribution.
|
|
| Outcomes | Haemoglobin, transferrin receptor, serum retinol, dietary assessment, anthropometry, malaria blood film, haematuria (proxy for schistosomiasis) | |
| Notes | Source of funding: the Micronutrient Initiative, Canada and the Institute of Child Health in collaboration with African Humanitarian Action and CARE International. Additional assistance was provided by the World Food Programme, United Nations High Commissioner for Refugees and CORD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not an RCT. 150 households were selected using a systematic random sampling method with houses as the sampling unit; this was for recruitment to the study, not allocation to treatment. |
| Allocation concealment (selection bias) | High risk | Not an RCT. A random number was drawn between 1 and the sampling interval. This determined the first household to survey. Subsequent households were selected by adding the sampling interval to the current house number. This ensured equal probability of selection of households and individuals. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | Uncontrolled before‐and‐after study using a longitudinal cohort |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | High risk | Uncontrolled before‐and‐after study using a longitudinal cohort |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were minimal losses to follow‐up in the study. |
| Contamination (checking for possible performance bias) | Unclear risk | The study was done on the same participants followed longitudinally. It is unclear if there was contamination from another intervention. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | There is insufficient information to permit judgement. |
Villalpando 2002 (C).
| Methods | Cluster‐randomised trial with 3 arms. Randomisation at the shelter level | |
| Participants | 1786 children 6‐11 years of age (sex not reported) from 34 shelters in Mexico; mean of 34 children/shelter
|
|
| Interventions | The total number of children participating in the study was 1786 from the 34 selected shelters. Each hostel housed on average 42 children, who were there to receive primary education. Shelters were randomised to receive 1 of 3 types of flour (not fortified, fortified with reduced iron or fortified with iron NaFeEDTA. Each shelter was randomly assigned to receive 1 of 3 types of nixtamalised corn flour (not fortified, fortified with reduced iron, and fortified with NaFeEDTA). All flours contained 5 mg/kg thiamin, 3 mg/kg riboflavin, 35 mg/kg niacin, 2 mg/kg folic acid and 40 mg/kg zinc. The amount of added elemental iron was 30 mg/kg (as reduced iron or NaFeEDTA). Each shelter received enough flour to prepare tortillas or other typical preparations for 9 months. Social, anthropometrical and blood samples were collected at baseline, 4 and 9 months of the intervention.
|
|
| Outcomes | Socioeconomic variables, anthropometry, food intake, haemoglobin, protoporphyrin, serum iron, TIBC, UIBC, ferritin, folates, niacin, thiamine riboflavin, diet composition and fortification mix composition | |
| Notes | This study was separated as 2 studies: 1 comparing flour fortification with iron EDTA and another comparing flour fortification with reduced iron in comparison to unfortified flour. An adjustment for design effect was made for both (continuous) haemoglobin and (dichotomous) anaemia outcomes. The ICC 0.02723 for outcome type for both haemoglobin and anaemia was estimated from the ICC for postal code reported in the literature (Gulliford 1999). In presenting the results for haemoglobin concentration outcome, we adjusted for design effect in the cluster‐randomised trial, and we computed the effective sample size of unfortified corn flour (12 clusters) versus the fortified combined group corn flour reduced iron and NaFeEDTA (22 clusters) for the haemoglobin measure outcome. The mean size of each cluster was 23.82, and the intra cluster correlation coefficient was *ICC 0.02723 corresponding to a design effect of 1.6214. Thus, for the reduced iron and NaFeEDTA sample size we made an adjustment to N = 332 (from N = 539), and for the unfortified corn flour the adjusted sample size would be N = 167 (from N = 271). In presenting the results for anaemia, we adjusted for design effect in the cluster‐randomised trial and computed the effective sample size of unfortified corn flour (12 clusters) versus the fortified combined group corn flour with reduced iron and NaFeEDTA (22 clusters). The mean size of each cluster was 24.82, and the intra cluster correlation coefficient was *ICC 0.02723 corresponding to a design effect of 1.6487. Thus, the total adjusted sample size for both fortified flour with reduced iron and with NaFeEDTA sample size was adjusted to N = 343 (from N = 565), and the unfortified corn flour sample size was adjusted to N = 169 (from N = 279). For the serum ferritin concentrations, the mean size of each cluster was 22.29, and the intra cluster correlation coefficient was *ICC 0.01393 corresponding to a design effect of 1.2966. Thus, Fe‐ and NaFeEDTA sample size was adjusted to N = 399 (from N = 518), and non‐fortified corn flour sample size to N =185 (from N = 240). For the outcome iron deficiency adjustments, we computed the effective sample size of non‐fortified corn flour (12 clusters) versus the fortified combined group corn fluor Fe‐ and NaFeEDTA (22 clusters) for the ID measure outcome. The mean size of each cluster was 22.41, and the intra cluster correlation coefficient was *ICC 0.01393, corresponding to a design effect of 1.2982. Thus, Fe‐ and NaFeEDTA sample size N = 401 (from N = 521), and non‐fortified corn flour sample size N = 186 (from N = 241). Taking into account the ID group prevalence (corn flour Fe‐ = 11.1%, NaFeEDTA = 10.4%, and non‐fortified = 11.2%), and the design effect, the number of ID fortified events were 43 from N = 401, and 21 from N = 186 in the non‐fortified group. In order to obtain the mean value and standard deviation for the non‐fortified corn fluor group and the combined group corn flour (reduced iron and NaFeEDTA), we used the formula suggested in Higgins 2011b. The unfortified corn flour group was composed of 4 groups: iron deficient and anaemic; non‐iron deficient and anaemic; iron deficient and not anaemic; non‐iron deficient and not anaemic. Similarly for the fortified corn flour group (reduced iron) was composed of similar groups. The fortified corn flour (with NaFeEDTA) was also composed of 4< similar groups that where combined to form the fortified corn flour group. The corresponding effective sample sizes for the unfortified and fortified corn flour group were computed used the design effect as explained above. Source of funding: National Institute of Public Health, Cuernavaca, Mexico |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation not reported. The study is reported as randomised but in another report it appears as pre‐post with control group. |
| Allocation concealment (selection bias) | Unclear risk | The shelters were assigned a type of flour. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | The prevalence of anaemia at baseline was 13.6%, 10.6% and 11.1% in the 3 different groups. Similarly the prevalence of low ferritin concentrations in the 3 groups differed among the groups. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if the participants and personnel were blinded to the intervention groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if the participants and personnel were blinded to the intervention groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is insufficient information to permit judgement. |
| Contamination (checking for possible performance bias) | Unclear risk | There is insufficient information to permit judgement and the report is a draft from which no sufficient information is available. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The draft version of the results is unpublished and only available through the link to the agency in Mexico. |
Hb: haemoglobin; ICC: intra cluster correlation coefficient; ID: iron deficiency; IDA: iron‐deficiency anaemia; NaFeEDTA: ferric sodium ethylenediaminetetraacetate; SD: standard deviation; TIBC: total iron binding capacity; UIBC: unsaturated iron binding capacity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abreu 2009 | 768 women, 384 in fortification group and 384 in unfortified group. A pre‐ post study without control group to compare the impact of the national fortification programme that started in Brazil in 2004. This particular thesis is related to haemoglobin changes in 786 pregnant women from San Bernardo do Campo, Brazil. The programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Aguirre Arenas 2013 | Changes in anaemia status in a sample of 98 girls and 96 boys, aged 6 to 24 months old, from 3 indigenous rural areas of Mexico (mountain, altiplano and coast) after provision for 9 months of soy (3%) and iron‐fortified corn flour, were evaluated in a non‐experimental pre‐post evaluation study. Demographic, anthropometric and biochemical variables were analysed. The decreased prevalence of anaemia suggests that the provision of soy (3%) and iron fortified corn flour is a viable alternative for combating childhood anaemia. Children 6 to 24 months old. Our review is for individuals older than 2 years. |
| Ashworth 1973 | 42 healthy Jamaican infants, aged 5 months to 2 years. Protocol indicates older than 2 years. Maize meal was prepared from radioactive grains and no extra iron was added. Our protocol states the use of fortified maize flour as inclusion criteria for studies. This is an absorption study. The type of intervention is outside the scope of this review. |
| Assuncao 2007a | A systematic review was conducted to identify studies assessing the effect of food fortification with iron on childhood anaemia. Of 21 studies reviewed, only 1 failed to report a positive, favourable effect of iron fortification, indicating the possibility of publication bias. The studies showed important methodological limitations. The 2 studies with the best methodological scores showed opposite results. The authors did not perform any numerical analyses of data. From the 2 studies that had potential for use, 1 (Layrisse) was individually included in our review and the other from Brazil (Vitolo) used a blend of cereals where maize was less than 50%. We decided to exclude this reference. |
| Assuncao 2007b | Probabilistic sample of 453 children (0‐5 years) at baseline, 923 for 12 months and 863 for 24 months. Samples were taken between May and July 2004, prior to compulsory iron fortification of flour, and then at 12 and 24 months postfortification implementation (2005 and 2006). No control group. The programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Assuncao 2012 | Series of population‐based surveys conducted in 2004 (baseline study), 2005, 2006 and 2008, in children under 6 years of age residing in the urban area of the city of Pelotas, Southern Brazil (N = 507 in 2004; N = 960 in 2005; N = 893 in 2006; N = 799 in 2008). In all included children, haemoglobin was determined by finger puncture. In 2008, a sub‐sample of children (n = 114) provided venous blood samples to measure body Fe reserve parameters (ferritin and transferrin saturation). The programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Beiseigel 2007 | 2 randomised, 2 × 2 factorial experiments compared women's iron absorption from 2 maize varieties (ACR and TZB; n = 26) and 2 bean varieties (great northern and pinto; n = 13), each fed with and without ascorbic acid (AA) from orange juice. Nonhaem iron bioavailability was determined from 2‐week retention of extrinsic radio iron tracers and was compared with Caco‐2 cell and algorithm results from identical meals. Excluded because it is an isotopic Fe absorption study. There is no control group or fortification with iron. |
| Berg 1978 | 295 healthy, mature infants who were fed various dietary regimens of iron‐fortified products, including cereal, whole milk and corn syrup. Milk and cereals were fortified. No indication of the cereals administered (wheat, rice, maize???). The ingredient coming from maize was corn syrup. Study of infants 0‐27 months old. The age of the children participants is outside the scope of this review. |
| Bisimwa 2012 | Infants were randomly assigned at 6 months of age to receive either ready‐to‐use complementary food (RUCF) (n = 691) or UNIMIX (n = 692) for 6 months. The objective was to assess the effectiveness of a fortified soybean‐maize‐sorghum ready‐to‐use paste compared with a fortified corn soy blend (UNIMIX) porridge on the prevalence of underweight and stunting among infants in South Kivu Province, Democratic Republic of Congo. Study in infants 6 months old. Our review is for individuals older than 2 years. Although ready‐to‐use paste contains only 29% whole maize, UNIMIX contains 70% to 80% maize. Corn content in flours should be at least 50% to be considered for review. No haemoglobin determination |
| Bjorn‐Rasmussen 1972 | 25 male students, 21 to 27 years old, volunteered for this study. It was a first step in testing the hypothesis that food iron can be regarded as a 2‐pool system (haem and nonhaem) with respect to absorption. Radioiron absorption test from intrinsically labelled corn, wheat and eggs. It is a punctual value of iron absorption from radioactive maize, without an intervention or administration of corn for a certain period or comparison with a control group. No fortification of corn flour. Maize meal was prepared from radioactive grains and no extra iron was added. Our protocol states the use of fortified maize flour as inclusion criteria for studies. No results on Hb or anaemia. Measures of individual haemoglobin concentration to determine iron status and not compared to control group |
| Bovell‐Benjamin 1999a | Paper on sensory quality and storage stability of iron fortified maize |
| Bovell‐Benjamin 2000 | 10 men (19‐30 years old) and 21 women (18 to 48 years old) participated in a study to compare iron absorption from ferrous sulfate, ferrous bisglycinate, and ferric trisglycinate in whole‐maize meal; to determine whether iron from ferrous bisglycinate and ferrous sulfate exchanges in the intestinal pool; and to assess iron absorption from ferrous bisglycinate and ferric trisglycinate over a range of iron statuses. Excluded because maize meal was prepared from regular maize and a radioactive tag was added. Our protocol states the use of fortified maize flour as inclusion criteria for studies. It is an isolated study reporting iron absorption from extrinsically tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Chang 2012 | Millet porridge with cabbage, tofu and pork‐fillet wheat dumplings were fortified with 2 mg iron either as ferrous sulfate or NaFeEDTA. Excluded because there is no corn flour fortification |
| Cook 1997 | 6 studies were performed in groups containing 8‐12 volunteers. Absorption was measured from 4 separate test meals in each participant. The 57 volunteers who participated included 30 men and 27 women ranging in age from 21 to 37 years (mean: 25 years). The participants denied any history of disorders or current use of medications that might affect the gastrointestinal absorption of iron, and all stated that they were in good general health. 10 participants (1 man, 9 women) were iron deficient as defined by a serum ferritin concentration < 12 µg/L, but only one was anaemic. Excluded because it is a radioiron absorption test from extrinsically labelled corn. The fortification of corn flour at laboratory level. Maize meal was prepared from regular maize, and a radioactive tag was added. Our protocol states the use of fortified maize flour as inclusion criteria for studies. Measures of individual haemoglobin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from extrinsically tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Da Silva 2012 | Socioeconomic, demographic, obstetric and haemoglobin concentration data were collected in 778 pregnant women attending prenatal care. 2 study groups were created: the first referred to the period before fortification (G1, n = 391), including women whose parturition happened before June 2004; and the second referred to the period after fortification (G2, n = 387), including women whose last menstrual cycle happened after June 2005. Excluded because the programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of effect or if it is due to wheat or corn flour. |
| Davidsson 2002 | Iron bioavailability was measured in 33 Guatemalan girls aged 12‐13 years by a stable‐isotope technique based on erythrocyte incorporation 14 d after intake. The objective was to evaluate to evaluate the bioavailability of iron from meals based on corn tortillas and black bean paste that were fortified with ferrous fumarate, ferrous sulfate, or NaFeEDTA and to investigate the potential of Na2EDTA to increase the bioavailability of iron from ferrous fumarate. Excluded because fortification of corn flour was at laboratory level, using stable isotopes of iron. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Davidsson 2003 | Iron absorption study using stable isotopes with the objective of evaluating the influence of retinyl palmitate added to Fe‐fortified maize porridge on erythrocyte incorporation of Fe in children with vitamin A deficiency, before and after vitamin A supplementation. The study included 13 children (7‐13 years old) with low plasma retinol concentrations (9 boys, 4 girls). Retinyl palmitate added to the labelled test meals significantly decreased erythrocyte incorporation of Fe in children with vitamin A deficiency at baseline but had no statistically significant effect 3 weeks after vitamin A supplementation. Excluded because the fortification of corn with iron at laboratory level, using stable isotopes of iron and vitamin A deficient children. Measures of individual haemoglobin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| De Souza 2011 | The data were obtained from 854 medical records of the women distributed into 2 groups: no fortification (427 women who would deliver before June 2004) and fortification (427 women whose last menstruation was after June 2005). Women with a haemoglobin level < 11.0g/dL were considered anaemic. The programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Del Real 2002 | 196 children (4‐6 years old) with sociodemographic, anthropometric, anaemia, VA deficiency and food intake data. 13% had anaemia, 9% had VA deficiency according to CIC, and 0.5% according to serum retinol (< 0.70 µmol/L), 30% were at risk of VA deficiency (0.70 mmol/L to 1.05 mmol/L). 17%, 37%, and 5% of the sample had an insufficient intake (< 80% of recommended daily amount) of energy, iron, and VA, respectively. When excluding from the analysis the amount of iron and VA from corn flour enrichment, an additional 38% and 10% of the sample showed deficient intakes of each nutrient, respectively. Excluded because it is a descriptive investigation based on food consumption from a cross‐sectional study. Blood sample taken to determine prevalence of anaemia. There was no intervention or comparisons with a control group. |
| Derman 1977 | Iron absorption from radio‐iron added to a maize‐weal porridge, was measured in 116 volunteer multiparous Indian women using the radio‐Fe erythrocyte utilisation method. The meals were fed with and without tea or coffee and with and without varying amounts of ascorbic acid. Ascorbic acid was capable of improving Fe absorption from a cereal source. It partially overcame the inhibitory effect of tea and could facilitate the absorption of at least some forms of Fe that may contaminate food. Excluded because there was no fortification of corn with iron at laboratory level, using radioactive isotopes of iron to determine absorption. Fortification was performed in sugar, but with ascorbic acid and no iron. Measures of individual haemoglobin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Derman 1982 | The study compared the absorption from ferritin and ferric hydroxide in 35 multiparous women when fed in water, in maize porridge and in maize porridge containing 100 mg ascorbic acid. The fraction of iron in ferritin and ferric hydroxide that enters the 'common pool' of nonhaem dietary iron is profoundly influenced by the nature of the diet. The greater the concentration of enhancing ligands, the closer the absorption of iron from these compounds approximates that of the nonhaem dietary iron pool. Excluded for use of unfortified corn meal with radioactive iron, ferritin or ferric hydroxide at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Diaz 2002 | To determine the prevalence of iron deficiency of 96 children (2 to 5 years old) with sociodemographic, anthropometric, food intake data and a blood sample for determinations of haemoglobin concentration, hematocrit, mean corpuscular volume, free erythrocyte protoporphyrin, zinc protoporphyrin and serum ferritin. 99% and 69% of the children consumed daily bread and iron fortified milk (Leche Purita Cereal), respectively. It is highly likely that the iron fortification of wheat flour and the milk distributed by the National Complementary Food Program has improved iron nutrition status of children. No important iron intake from corn. No data on corn flour fortification or consumption. Excluded because it is a descriptive investigation based on food consumption from a cross‐sectional study. Blood sample taken to determine prevalence of anaemia. There was no intervention or comparisons with a control group. |
| El Hamdouch 2010 | 4 surveys in children 2 to 5 years old from rural and urban areas: 2006 (N = 1258), Dec 2006‐Jan 2007 (N = 1256), Dec 2007‐Jan 2008 (N = 1237) and May‐Jun 2008 (N = 1122). Samples of preschool children taken to perform haemoglobin test. Excluded because it is a descriptive investigation based on food consumption from a cross‐sectional study. Blood sample taken to determine prevalence of anaemia. There was no intervention or comparisons with a control group. Consumption of wheat flour, no corn |
| Faber 2005 | Infants aged 6‐12 months (N = 361) were randomly assigned to receive either the fortified or unfortified porridge for 6 months. Primary outcomes were haemoglobin and serum retinol, zinc, and ferritin concentrations and motor development. Growth was assessed as a secondary outcome. Primary and secondary outcomes were assessed at baseline and 6 months. 292 infants completed the study. Excluded because it was performed in infants. |
| Figueroa Cardenas 2001 | Exluded because it is a study on the effect of the addition of vitamins and soy protein on the quality characteristics of nixtamal tortillas and the loss of nutrients during the nixtamalisation process. |
| Filteau 2010 | A randomised double‐blind trial in Lusaka, Zambia of 2 locally made infant foods: porridges made of flour composed of maize (65%), beans (15%), bambaranuts (5%) and groundnuts (15%). One flour contained a basal and the other a rich level of micronutrient fortification. Infants (N = 743) aged 6 months were randomised to receive either regime for 12 months. The primary outcome was stunting (length‐for‐age z score) at age 18 months. No significant differences were seen between trial arms overall in proportion stunted at 18 months, mean length‐for‐age z score, or rate of hospital referral or death. Among children of HIV‐infected mothers who breastfed, 6 months (53% of HIV‐infected mothers), the richly‐fortified porridge increased length‐for‐age and reduced stunting. Excluded because it was performed in infants 6‐18 months of age |
| Fujimori 2011 | 12,119 women, 6,062 in the pre‐fortification group and 6,057 in the postfortification group. A repeated cross‐sectional panel study of public health care centres of municipalities in the 5 Brazilian regions was conducted. Retrospective data were obtained from 12,119 medical records of pregnant women distributed in 2 groups: before fortification (delivery prior to June 2004) and after fortification (date of last period after June 2005). The programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Garcia‐Casal 1998 | 104 adults were fed 3 cereal‐based diets, labelled with either 59Fe or 55Fe in 6 studies. Each diet contained different concentrations of vitamin A (from 0.37 mmol/100 g to 2.78 mmol/100 g cereal) or beta‐carotene (from 0.58 mmol/100 g to 2.06 mmol/100 g cereal). Vitamin A and beta‐carotene may form a complex with iron, keeping it soluble in the intestinal lumen and preventing the inhibitory effect of phytates and polyphenols on iron absorption. Excluded for use of unfortified corn meal that is added with ferrous fumarate and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Garcia‐Casal 2002 | Results from 3 surveys carried out in 1997, 1998, and 1999 on the same age and socioeconomic group that had been evaluated in 1990, 1992, and 1994. Data from blood samples taken from the children during surveys from 1992 to 1999 (2173 children and adolescents in the low socioeconomic strata of the Caracas population). The comparison between the 1992 and 1994 surveys of the Caracas population showed a significant reduction in the prevalence of iron deficiency and anaemia, which dropped from 37% and 19% in 1992 to 16% and 9% in 1994, respectively, after only 1 year of fortification. Then, there were no significant differences for anaemia or iron deficiency in the last 3 surveys. Prevalence results from the last 7 years seem to indicate that after a dramatic reduction in 1994, iron deficiency tended to stabilise. Prevalence of anaemia also diminished dramatically from 1992 to 1994, but for the last 3 surveys it reached the same levels of prevalence as were reported before the fortification programme was started. The median ferritin concentration showed a clear tendency to increase, even though the increase was small in the last 3 surveys. This index increased significantly in 1997, 1998, and 1999 compared with the 1992 survey, when the iron fortification programme had not yet been started. Excluded because Venezuelan fortification programme started in 1993 enriching precooked corn flour with iron, vitamin A, thiamine, niacin, and riboflavin. White wheat flour was enriched with the same nutrients, except for vitamin A, and fortification of wheat started only a few months later. It is not possible to separate the effect of each fortified item on anaemia prevalence. |
| Garcia‐Casal 2003 | Adult participants (n = 87; 7 men and 80 women) from Valencia (Carabobo State, Venezuela) voluntarily participated in this study to determine relative iron absorption from reduced iron‐fortified corn flakes and the role of vitamins A and C improving absorption. Iron absorption was measured calculating radioactive iron incorporation into participants' blood. There was a significant 3.6‐times increase in iron absorption when both vitamins were administered together. In vitro solubility tests with ferric chloride, electrolytic and reduced irons showed an important role of vitamins A and C enhancing iron solubility at pH 6 to the values found at pH 2. Addition of vitamins A and C to a ready‐to‐eat cereal significantly improves iron absorption by a process at least partially due, to the solubilising effect of these vitamins on reduced iron. Addition of both vitamins in the same meal produced an increment in absorption corresponding to the additive factor of each vitamin. Excluded because the study was on breakfast cereal. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Garcia‐Casal 2006 | 128 adults received wheat‐based or corn‐based breakfasts containing ferrous fumarate, radioactive iron, and lycopene, lutein, or zeaxanthin at different concentrations. The effect of coffee was also evaluated. Iron absorption was measured by calculating radioactive iron incorporation into blood 15 days after the administration of the radioactive meal. Excluded because the study used unfortified corn meal combined with ferrous fumarate and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Hallberg 1984 | A study in 49 adults of both sexes (24 to 46 years of age) compared different methods for increasing the absorption of iron from a simple Latin American‐type meal composed of maize, rice, and black beans. Excluded because corn flour was used, but was not fortified, using stable isotopes of iron. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from complete meals, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Heijblom 2007 | A cross‐sectional survey was conducted in a representative sample of 424 randomly selected first graders (ages 6 to 11 years) from public schools located in the Northern Public Health Region of Brasília. The study objectives were to: determine the prevalence of anaemia; compare the results obtained in 2004 to those of a similar survey conducted in the same area in 1998. Excluded because reports haemoglobin levels from 2 surveys 1998 and 2004, without intervention. Reports prevalence of anaemia in children from a community. A cross‐sectional survey in a representative sample of 424 randomly selected first graders. The programme is on wheat and corn flour fortification started in 2002. It is not possible to determine the magnitude of the effect or if it is due to wheat or corn. |
| Hurrell 2003 | Iron absorption was measured in 78 participants aged 21‐38 years. The composite group included 34 men and 44 women to measure the influence of phytic acid degradation on iron absorption from cereal porridges. An exogenous phytase was used to fully degrade phytic acid during the manufacture of 9 roller‐dried complementary foods based on rice, wheat, maize, oat, sorghum, and a wheat‐soy blend. Iron absorption from the phytate‐free and native phytate porridges prepared with water or milk (wheat only) was measured in adult humans with an extrinsic‐label radio‐iron technique. Excluded because fortification of corn flour at laboratory level, using stable isotopes of iron. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Janmohamed 2016 (C) | 457 pregnant women at least 18 years old, in the first trimester of pregnancy, and planning to stay in their home village throughout their pregnancy from 75 village clusters in 2 districts (Boribo and Rolea Phear) of Kampong Chhnang Province in central Cambodia, during the study period from August 2011 to June 2012. All 75 village clusters located in the geographic catchment area of 4 health centres were randomly allocated to 1 of 2 groups: group 1 (n = 37 villages, 333 participants) received Corn Soya Blend Plus (a maize and soybean flour fortified with vitamin and minerals) supplements from the first trimester to delivery; group 2 (n = 38 villages, 214 participants) did not receive the intervention. Each woman in the intervention group was provided a 6.75‐kg bag of Corn Soya Blend Plus, containing 0.75 kg pre added sugar for palatability, on a monthly basis along with 300 mL supply of vitamin A‐ and vitamin D‐fortified palmolein oil, provided each month to be added during the cooking process (w10 mL per ration). The daily Corn Soya Blend Plus ration (200 g of dry flour) provided about 760 kcal, 27 g protein (14% of total kcal), and 5 g fat (6% of total kcal). The 10 mL daily ration of oil provided about 90 kcal of additional energy. Women in both groups received tablets containing 60 mg elemental iron and 400 µg (0.4 mg) folic acid provided by nurse midwives during antenatal visits as per standard care, haemoglobin testing and treatment of anaemia and prenatal counselling in the participants homes. Excluded because the intervention group received fortified maize flour plus fortified oil. The type of intervention is outside the scope of this review. |
| Jyväkorpi 2006 | 751 children: 353 girls and 398 boys 3‐14 years old. The objectives were to determine the iron status of children as well as to identify and quantify the sources of iron intake and the presence of relevant enhancers and inhibitors of iron absorption in the diet. Haemoglobin, serum ferritin, and transferrin saturation percentages were used to evaluate the iron status in a school‐based sample of 762 children. Excluded because reported haemoglobin levels from a cross sectional survey. No intervention, no comparisons |
| Krebs 2012 | Cluster‐randomised efficacy trial in the Democratic Republic of Congo, Zambia, Guatemala, and Pakistan, comparing provision of meat to multiple micronutrient fortified cereals to 532 infants and young children 6‐18 months of age. Excluded because intervention was not maize based, and multiple micronutrients prevented identification of effect of iron alone. |
| Layrisse 1996 | Before‐and‐after studies of populations exposed to mass fortification of corn and wheat flour in Venezuela. Precooked yellow and white maize and wheat flours were enriched with 20 mg and 50 mg Fe (as ferrous fumarate)/kg flour, respectively. The corn flour was also enriched with vitamin A, thiamine, riboflavin, and niacin, whereas the wheat flour did not include vitamin A. This study reports results of preliminary survey undertaken following introduction of intervention (N = 317) and comparison to 2 earlier surveys undertaken prior to intervention (N = 499, N = 282). The study was excluded because the groups were not reported separately by intervention, and because maize was fortified with multiple micronutrients and not iron alone. |
| Layrisse 1997a | 94 volunteers, 76 women and 18 men were studied to find out the interaction of vitamin A with the inhibitors of iron absorption, from a basal breakfast containing bread from either 100 g of fortified or unfortified precooked corn flour or 100 g of white wheat flour, 50 g of cheese and 10 g of margarine. Bread was labelled with either 55Fe or 59Fe. This bread was made from commercial flours fortified with iron as ferrous fumarate and vitamins and some experiments with unfortified flour, adding iron at the laboratory. Excluded because it is an absorption study. Used corn flour with ferrous fumarate and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Layrisse 1997b | Adult participants (N = 94; 17 men and 77 women) were fed breads prepared from fortified corn or wheat based diets, labelled with either 59Fe or 55Fe. Each diet contained different concentrations of vitamin A and/or coffee. Excluded for use of fortified corn flour and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Layrisse 2000a | A total of 74 participants, 56 women and 18 men, ages 15‐50 years, were studied in 5 experiments to determine the bioavailability of iron amino acid chelate (ferrochel) added to fortify breads prepared from either precooked corn flour or white wheat flour, cheese and margarine compared with the same basal breakfast enriched with either ferrous sulfate or iron‐EDTA. The inhibitory effect of phytate and polyphenols on iron absorption from ferrochel was also tested. Studies 1 and 2 were performed to determine iron absorption from ferrous sulfate, ferrochel and Fe‐EDTA; study 3 to measure iron absorption from ferrous sulfate and ferrochel given in the same meal and in different meals; study 4 to evaluate the effect of polyphenols on iron absorption from ferrochel and study 5 to assess the effect of polyphenols on iron absorption from ferrochel. Excluded because it is an absorption study. Used corn flour with ferrous fumarate and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Layrisse 2000b | 174 participants were studied to find out the interaction of vitamin A or beta‐carotene with the inhibitors of iron absorption, from a bread prepared from either 100 g of precooked corn flour or 100 g of white wheat flour, 50 g of cheese and 10 g of margarine. Bread was labelled with either 55Fe or 59Fe. This bread was made from commercial flours fortified with iron as ferrous fumarate and vitamins. Excluded because it is an absorption study Used corn flour with ferrous fumarate and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Layrisse 2002 | Data from blood samples taken from the children during surveys from 1992 to 1999 (2173 children and adolescents in the low socioeconomic strata of the Caracas population). The comparison between the 1992 and 1994 surveys of the Caracas population showed a significant reduction in the prevalence of iron deficiency and anaemia, which dropped from 37% and 19% in 1992 to 16% and 9% in 1994, respectively, after only 1 year of fortification. Then, there were no significant differences for anaemia or iron deficiency in the last 3 surveys. Prevalence results from the last 7 years seem to indicate that after a dramatic reduction in 1994, iron deficiency tended to stabilise. Prevalence of anaemia also diminished dramatically from 1992 to 1994, but for the last 3 surveys it reached the same levels of prevalence as were reported before the fortification programme was started. The median ferritin concentration showed a clear tendency to increase, even though the increase was small in the last 3 surveys. This index increased significantly in 1997, 1998, and 1999 compared with the 1992 survey, when the iron fortification programme had not yet been started. Excluded because Venezuelan fortification programme started in 1993 enriching precooked corn flour with iron, vitamin A, thiamine, niacin, and riboflavin.White wheat flour was enriched with the same nutrients, except for vitamin A, and fortification of wheat started only a few months later. It is not possible to separate the effect of each fortified item on anaemia prevalence. |
| Li 1995 | A 3‐month investigation of cereal fortification with ferric ammonium citrate significantly decreased the prevalence of anaemia among Beijing infants 1‐13 months of age compared with controls. Excluded because it is a study performed in infants. It is unclear if the study was done in maize. |
| Macharia‐Mutie 2012 | In a 16‐week intervention trial, children (N = 279; 12‐59 months) were randomly assigned to: unrefined maize porridge (control; 4.1 mg of iron/meal; phytate:iron molar ratio 5:1); unrefined maize (30%) and amaranth grain (70%) porridge (amaranth group; 23 mg of iron/meal; phytate:iron molar ratio 3:1); or unrefined maize porridge with multiple micronutrient powders (MNP group; 6.6 mg iron/ meal; phytate:iron molar ratio 2.6:1; 2.5 mg iron as NaFeEDTA). Primary outcomes were anaemia and iron status with treatment effects estimated relative to control. Excluded because age group include 12 to 56 months, without age separation. Protocol includes infants and young children younger than 24 months of age. Study groups: maize alone group with no added iron; amaranth group: flour only contains 30% maize, the protocol indicates at least 50% should be maize; and MNP group. Part of exclusion criteria from protocol |
| MacPhail 1981 | Iron absorption from Fe(III)EDTA by agents known to promote or inhibit absorption was examined in 101 volunteer multiparous Indian women. Fe absorption from Fe(III)EDTA was compared with absorption of intrinsic food Fe in other set of 28 participants. Also the urinary excretion of radio‐Fe after oral administration of 59Fe(III)EDTA was studied in 24 participants, and evidence of intraluminal exchange of Fe was examined. Excluded for use of corn meal that is added with Fe(III)EDTA and radioactive iron at laboratory level, using radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Manary 2002 | This study describes a community‐based method used in rural Malawi to remove dietary phytate, reporting an improvement in the iron status of 10 children (5 boys, 5 girls aged 2‐5 years) that participated in the trial for 40 days. Phytate was removed by soaking maize flour in excess water with phytase and decanting the water before cooking the flour. Iron status in 10 adults, as measured by soluble transferrin receptor and zinc protoporphyrin, was improved but not normal. Excluded because evaluated a maize alone group without added iron. No fortification and the flour was modified. |
| Martinez‐Torres 1991 | Precooked maize flour was used as food vehicle to test the absorption of several iron compounds (ferrous sulfate, electrolytic iron, monoferric EDTA, and ferrous fumarate. With the aim of choosing the most adequate for food fortification programmes. 73 volunteers participated in an absorption study that resulted in the higher absorption from EDTA, followed by ferrous sulfate, fumarate and electrolytic iron. Excluded because it is an absorption study that use radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin, plasma iron, plasma ferritin and the unsaturated iron binding capacity were performed to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from radio‐iron, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Mendoza 1998 | Iron absorption from tortillas was evaluated by using the extrinsic tag method and was measured as the incorporation of radiolabeled iron into the red blood cells of 13 nonanaemic men, aged 19 to 35 years, 2 weeks after intake. Excluded for use of radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from modified maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Mendoza 2001 | Iron absorption from porridges prepared from the same low phytate maize (LPM) (lpa‐1‐1 mutant) and unmodified wild‐type maize (WTM), both of which were fortified with either ferrous sulfate or sodium iron EDTA. 14 healthy non‐anaemic women aged 19‐42 years participate in an absorption test to measure the amount of radio‐iron incorporated into red blood cells (extrinsic tag method) 12 d after consumption of the study diets. Excluded for use of radioactive isotopes of iron to determine absorption. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from modified maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Nesamvuni 2005 | A randomised parallel intervention study was used in which 21 experimental children and their families received maize meal fortified with vitamin A, thiamine, riboflavin and pyridoxine, while 23 control children and their families received unfortified maize meal. The maize meal was provided for 12 months to replace the maize meal habitually consumed by these households. Excluded because there was no iron fortification. Children 1‐3 years, no subgroup by age |
| Nieman 2011 | 73 children (N = 42 boys, N = 31 girls) ranging in age from 7 to 13 years (mean ± SD age, 9.9 ± 1.7 years), and 65 completed all phases of the study. Participants were randomised to 1 of 3 groups – low, moderate, or high fortification – breakfast cereals administered daily for 2 months in double‐blinded fashion. The 'medium' fortified cereal contained B‐complex vitamins, vitamins A and C, iron, zinc, and calcium, with the addition of vitamin E and higher amounts of vitamins A and C, and zinc in the 'high' group. Immune measures included delayed‐typed hypersensitivity, global IgG antibody response over 4 weeks to pneumococcal vaccination, salivary IgA concentration, natural killer cell activity, and granulocyte phagocytosis and oxidative burst activity. Excluded because it is a study in breakfast cereal. Fortification was during product preparation and not at a flour level. Same dough, added fortification while preparing the cereal lots. There is not an unfortified cereal or no‐intervention control group. Only immunological outcomes. No measure of haemoglobin |
| Patel 2005 | The study was a controlled, comparative clinical effectiveness trial of 2 supplementary feeding regimens in children at risk of malnutrition from seven centres in rural Malawi. A stepped‐wedge design with systematic allocation was used for assigning children to receive either ready‐to‐use therapeutic food (RUTF) (N = 331) or micronutrient‐fortified corn/soy‐blend (N = 41) for up to 8 weeks. The primary outcomes were recovery, defined as weight‐for‐height > 90%, and the rate of weight gain. Excluded because included children 10 to 60 months old. No iron‐related outcomes. The use of ready to use lipid‐based formulas in an exclusion criteria from protocol |
| Pouraram 2002 | Before‐and‐after intervention included 78 non‐anaemic apparently healthy 40‐ to 65‐year‐old men, randomly selected from Semnan, in the northeast of Iran. Fortification of wheat flour with 30 mg of iron as ferrous sulfate/kg. Data were collected at 3 time points. Evaluation of oxidative stress biomarkers as well as the assessment of iron status was performed in all 3 stages. After baseline data collection, the flour fortification programme was started with 30 mg/kg iron as ferrous sulfate. Excluded because was performed inwheat flour in 0% anaemic adult men |
| Rosado 2012 | To compare zinc absorption from corn tortilla fortified with zinc oxide versus zinc sulfate and to determine the effect of simultaneous addition of 2 doses of iron on zinc bioavailability. A randomised, double‐blind, cross‐over design was carried out in 2 phases. In the first phase, 10 adult women received corn tortillas with either 20 mg/kg of zinc oxide added, 20 mg/kg of zinc sulfate added, or no zinc added. In the second phase, 10 adult women received corn tortilla with 20 mg/kg of zinc oxide added and either with no iron added or with iron added at 1 of 2 different levels. Zinc absorption was measured by the stable isotope method. Excluded because it is anabsorption study, with stable isotopes determining absorption of zinc from nixtamalised tortillas. No iron intervention, no iron outcomes. Fortification of corn flour at laboratory level, using stable isotopes of zinc. |
| Sadighi 2008 | Fortification ofwheat flour in Iran |
| Sato 2008 | This study evaluated the impact of iron‐fortified flours in the prevalence of anaemia and haemoglobin levels of pregnant women. Cross‐sectional study at a Health Center School in São Paulo, Brazil. Data obtained from 750 pregnant women's medical records and discriminated into 2 groups, before and after fortification: non‐fortified and fortified. Excluded because the programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Sato 2011 | Pregnant women were divided into 2 groups: before‐fortification (delivery before June 2004) and after‐fortification (last menstrual period after June 2005). The sample included 12 119 records from public healthcare services located in the 5 Brazilian regions. Retrospective data were collected from medical records. Excluded because the programme is on wheat and corn flour fortification. It is not possible to determine the magnitude of the change or if the effect is due to wheat or corn. |
| Sichert‐Hellert 2001 | To assess long‐term data on changes in fortified food supply or consumption patterns, nutrient intake, and time trends in the DONALD study (Dortmund Nutritional and Anthropometric Longitudinally Designed Study). Evaluation of nutrient intake between 1985 and 2000 (total and from fortified foods) of 2‐14 year‐old boys (n = 383) and girls (n = 404) enrolled in the DONALD study. Food products were defined as fortified if enriched with at least one of the following nutrients: vitamin A or provitamin A carotenoids (summarised as Vitamin A), vitamins E, B1, B2, B6, C, niacin, folate, calcium or iron. Excluded because there is no intervention. No administration of iron |
| Sinisterra 2015 | A prospective, double‐blind, cluster‐randomised trial in a rural and indigenous area of Panama. During a 6‐month period, 36 rural community soup kitchens were divided into 2 groups randomly assigned to receive either: group‐A: 90 g of cereal with 10 mg of iron as ferrous gluconate stabilised with glycine; or group‐B (n = 129): 90 g of cereal with 10 mg of iron as ferrous bisglycinate chelate. A total of 393 children aged 24‐59 months of both sexes were recruited. Excluded because fortification occurred on a precooked product (i.e. not fortification of the flour). |
| SUSTAIN 2000 | Apparently healthy adolescent Central American girls (12‐13 years, max. body weight approximally. 40 kg; 11 girls per study, total 33 girls) were recruited at public high schools in Guatemala City. To evaluate Fe bioavailability from test meals based on corn masa flour tortillas and black bean paste fortified with ferrous sulfate, ferrous fumarate (with and without added Na2EDTA) and NaFeEDTA. Corn masa flour was fortified with ferrous fumarate with and without Na2EDTA, ferrous sulfate, or NaFeEDTA (including fortification iron). Excluded because it is an absorption study with stable isotopes of iron. No intervention |
| Troesch 2011 | Iron absorption profiles from FeSO4 with ascorbic acid and from NaFeEDTA, as well as the serum hepcidin and NTBI were measured using stable isotope appearance curves (SIAC) in response to meals. Healthy women (n = 16) were given 6 mg oral iron as labelled FeSO4 and NaFeEDTA with a maize porridge using a cross‐over design. Stable isotope appearance curves, non‐transferrin bound iron, and serum hepcidin were measured over 8 h after the meal. Excluded because fortification of corn flour is at laboratory level, using stable isotopes of iron. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Van den Briel 2007 | This paper outlines 3 approaches by World Food Programme (WFP) to fortifying cereals in Afghanistan, Angola, and Zambia. It examines the challenges faced and the outcomes achieved. In Afghanistan, attempts to mill and fortify wheat flour using small‐scale chakki mills were successful, but much larger‐scale efforts would be needed to promote demand and reach the level of consumption required to address serious iron deficiencies across the country. In Angola, maize has been fortified to combat the persistent occurrence of pellagra, a micronutrient deficiency disease found among people whose diets are dominated by maize. By providing fortification equipment to a commercial mill at the port of Lobito and using a vitamin and mineral pre‐mix provided by UNICEF, this project has overcome many of the difficulties common in countries emerging from conflict to provide monthly fortified maize rations to some 115,000 beneficiaries. In Zambia, iron deficiency anaemia was a serious problem among camp‐restricted refugees. WFP and its partners imported, installed, and trained workers in the use of 2 containerised milling and fortification units, halved iron‐deficiency anaemia, and reduced vitamin A deficiency among camp residents. In addition, the World Food Programme dramatically reduced waiting times for refugees who used to have their whole grain maize rations milled at small local facilities with insufficient milling capacity. Excluded because the article relates experiences of WFP fortifying cereals in Afghanistan, Angola and Zambia. No administration of iron, no iron related outcomes |
| Vasconcelos 2008 | A time‐series study that compared 228 paired pregnant women through 2 cross‐sectional assessments: in 2004, before flour fortification, and a year later. Pregnancy, socioeconomic and demographic data, body mass index and food consumption patterns were collected. The latter was determined by applying the Semiquantitative Food Frequency Questionnaire and included foods containing wheat and corn flours. Excluded because it is a retrospective cross‐sectional analysis of food consumption patterns from secondary data. Paired data means that in 2005 they looked for data on women with the same characteristics (trimester of pregnancy, mother age, obstetric risk) as the ones interviewed in 2004. No biochemical, iron related outcomes. |
| Vitolo 1998 | 54 children (29 girls and 25 boys) in 2 intervention groups. Group I children from nursery with a mean age of 2 years and 2 months were dependent on nurses for feeding. Group II children from maternal with a mean age of 3 years and 6 months that could feed by themselves. Both groups were fed twice a day with a porridge containing a mixture of cereals (43% de corn starch, 40% corn flour, 10% oat flour, 4% rice flour, 2% rye and 7.5% sugar) for 2 months. The preparation of each portion has 15 g of product in 200 mL whole milk. After 2 months of intervention weight, height and haemoglobin determinations were repeated. Excluded because there is no iron fortification. The flour administered contained less than 50% corn flour |
| Walczyk 2003 | Iron absorption from corn bread with or without added vitamin A (retinyl palmitate) was determined in 5 studies in 42 young adult human participants by using either a stable‐isotope method (2 studies) or a radioisotope technique (3 studies). Iron absorption was measured by erythrocyte incorporation of the isotopic labels and by whole‐body retention of 59Fe. Corn bread was served with water (studies 1 and 3) or coffee (studies 2, 4, and 5). The studies differed in the amounts and chemical forms of added tracer and fortification iron. No effect of vitamin A on iron absorption from the test meals was identified in the individual studies. Excluded because it is an absorption study. Fortification of corn flour at laboratory level, using radioactive and stable isotopes of iron. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Walter 2003 | To determine the most suitable fortificant that would improve iron bioavailability. In tortillas prepared with commercial precooked, lime‐treated, corn‐masa flour, the in vitro solubility of the following forms of iron were examined: native iron with and without Na2EDTA, elemental reduced iron plus Na2EDTA, ferrous fumarate with and without Na2EDTA, bisglycine iron, ferrous sulfate and NaFeEDTA. In vivo bioavailability in humans with double radio‐iron erythrocyte incorporation of ferrous fumarate with and without Na2EDTA, bisglycine iron, NaFeEDTA and native iron plus Na2EDTA, beans and rice were also evaluated in vitro. Excluded because it is an in vitro solubility study. Fortification of corn flour at laboratory level, using radioactive isotopes of iron. Iron was added to the dough, not to the flour. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| Walter 2004 | To measure the bioavailability of elemental iron in Mexican style corn masa flour tortillas and to evaluate the effects of Na2EDTA. A stable isotope of H2‐reduced iron powder was used, with and without Na2EDTA in tortillas prepared with corn masa flour. 2 groups of 5‐ to 7‐year‐old children (12/group) were fed tortillas fortified with 3 mg/100 g of H2‐reduced 58Fe with a mean particle size of 15 um. In 1 group, Na2EDTA was incorporated at a ratio of 1:2 mol/mol. The next day, 57Fe ascorbate was given as a reference dose. After 14 d, blood samples were analysed for isotopic enrichment. Excluded because it is an absorption study. Fortification of corn flour at laboratory level, using stable isotopes of iron. Iron was added to the dough, not to the flour. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
| World Food Programme 2000 | This is a manual that provides technical specification for the manufacture of Super cereal soy blend, used in adults and children over 6 months. No intervention |
| Zimmermann 2010 | This is an in vitro solubility study. Test meals based on degermed maize flour and milk powder and fortified with [57Fe]ferrous fumarate or [58Fe] ferrous sulfate, given to healthy Mexican preschool children (N = 18; aged 3.6 ± 1.0 years) and their mothers (N = 18; aged: 28.0 ± 5.2 years). Iron absorption was calculated on the basis of incorporation of isotopes into erythrocytes after 14 d and was adjusted for differences in iron status. Measures of individual haemoglobin and ferritin concentration to determine iron status and not compared to control group. It is an isolated study reporting iron absorption from tagged maize, without an intervention or administration of corn for a certain period or comparison with a control group. |
Differences between protocol and review
There are some differences between the protocol and the review.
We included study designs different from RCTs since we anticipated that many studies would include measures of impact at regional or national level. These reports are usually performed as quasi‐experimental research designs, like before‐and‐after studies.
For the assessment of risk of bias for observational studies we also used the domains of the EPOC tool and considered other aspects such as the included number of sites for an intervention, the similarity of recruitment methods at different sites, the independence of intervention to other changes, the presence of protocol deviations, levels of compliance/use of flours in different sites within studies, and the effects of the intervention on data collection.
We planned to use a combination of risk of bias for within studies and across studies using two of three domains to summarise the overall risk of bias. In the review, we summarised the overall risk of bias by primary outcome within each study using GRADE methodology.
Methods section: we did not calculate the standardised mean difference (SMD) with 95% confidence intervals to combine trials that measured the same outcome (e.g. haemoglobin) using different methods, since all included studies used the same methods.
Although we planned to evaluate the primary outcomes by subgroup analysis, we did not formally carry out a subgroup analysis given the scarcity of included studies.
We removed extraction rate for subgroup analysis. Nonetheless, we describe the maize flour extraction rate when this information was available, but did not use it for any analysis as only one study (Seal 2007) provided this information.
We have changed the term 'any adverse side effects', to 'any adverse effects' to include any adverse effect, expected or unexpected by the trialists.
Contributions of authors
Maria Nieves Garcia‐Casal, Sant‐Rayn Pasricha and Juan Pablo Peña‐Rosas screened the references. Maria Nieves Garcia‐Casal and Juan Pablo Peña‐Rosas extracted the data from the included studies and reported on the excluded studies. Luz Maria De‐Regil and Juan Pablo Peña‐Rosas prepared the GRADE 'Summary of findings' table and contributed to the discussion. All authors provided input and contributed to conclusions, implications for practice and research.
Disclaimer: Juan‐Pablo Peña‐Rosas and Maria Nieves Garcia‐Casal are full‐time WHO staff members. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy or views of the WHO.
Sources of support
Internal sources
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
External sources
-
Global Alliance for Improved Nutrition (GAIN), Switzerland.
WHO acknowledges the Global Alliance for Improved Nutrition (GAIN) for their financial support to the Evidence and Programme Guidance Unit for conducting systematic reviews on micronutrients interventions.
-
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Dr Maria Nieves Garcia‐Casal, Dr Jeff Gwirtz and Dr Belinda J Burford received partial financial support from the Department of Nutrition for Health and Development for this work.
-
The Bill & Melinda Gates Foundation, USA.
WHO acknowledges the financial support from The Bill & Melinda Gates Foundation for the development of systematic reviews of the evidence on the effects of nutrition interventions
-
Centers for Disease Control and Prevention (CDC), USA.
WHO thanks the International Micronutrient Malnutrition Prevention and Control Programme (IMMPaCt) from the National Center on Chronic Disease Prevention and Health Promotion for their financial and technical support to the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development for the conduct of systematic reviews of the evidence in nutrition interventions.
Declarations of interest
Sant‐Rayn S Pasricha ‐ has received an unrestricted research grant as a co‐investigator from Vifor Pharma Ltd and has served as a consultant to the Meat & Livestock Authority Australia.
Luz Maria De‐Regil ‐ works for Nutrition International, an international non‐governmental organisation that supports, among other interventions, fortification programmes and implementation research of maize and wheat flours, rice and condiments. None of these research studies met the inclusion criteria of this review. She is currently a member of the executive management committee of Food Fortification Initiative. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy or views of FFI or Nutrition International.
Maria Nieves Garcia‐Casal is an author of some studies on iron fortification of maize flour, none of which were included in the review.
Juan Pablo Peña‐Rosas ‐ none known.
Jeff Gwirtz is currently president at JAG Service Inc, a consulting firm that provides technical advising, research, training and trouble‐shooting in the US and international grain‐based foods sectors. He was not involved in the screening or selection of the studies and provided technical support for the preparation of the Background section and overall interpretation of the results and conclusions.
New
References
References to studies included in this review
Andang'o 2007 {published data only}
- Andang'o PE, Osendarp SJ, Ayah R, West CE, Mwaniki DL. Efficacy of iron‐fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet 2007;369(9575):1799‐1806. [DOI] [PubMed] [Google Scholar]
Carrasco 2011 (C) {published data only}
- Carrasco Quintero M, Ortiz Hernandez L, Chavez Villasana A, Roldin Amaro JA, Guarneros Soto N, Aguirre Arenas J, et al. Impact of consumption of corn flour with low level enrichment in children of rural zones. Nutricion Hospitalaria 2011;26(5):1097‐1104. [DOI] [PubMed] [Google Scholar]
- Carrasco Quintero Mdel R, Ortiz Hernández L, Roldán Amaro JA, Chávez Villasana A, Aguirre Arenas J, Aguilar Carrasco FR. Effect of consumption of corn flour enriched with soja on nutrition status of indigenous women of Mexico [Efecto del consumo de una harina de maíz enriquecida con soja en el estado de nutrición de mujeres indígenas de México]. Revista Española de Salud Publica 2013;87(3):293‐302. [DOI] [PubMed] [Google Scholar]
Miglioranza 2009 {published data only}
- Miglioranza LH, Breganó JW, Dichi I, Matsuo T, Dichi JB, Barbosa DS. Effectiveness of fortification of corn flour‐derived products with hydrogen‐reduced elemental iron on iron‐deficiency anaemia in children and adolescents in southern Brazil. Public Health Nutrition 2009;12(2):244‐8. [DOI] [PubMed] [Google Scholar]
Seal 2007 {published data only}
- Seal A, Kafwembe E, Kassim I, Hong M, Wesley A, Wood J, et al. Maize meal fortification is associated with improved vitamin A and iron status in adolescents and reduced childhood anaemia in a food aid‐dependent refugee population. Public Health Nutrition 2007;11(7):720‐728. [DOI] [PubMed] [Google Scholar]
Villalpando 2002 (C) {unpublished data only}
- Villalpando S [per comm]. Impact of fortification of corn meal with micronutrients on the nutritional status of iron, zinc, folate in rural school children: evaluation of effectiveness [Impacto de la fortificación con micronutrimentos de la harina de maíz, sobre el estado nutricio de hierro, zinc y ácido fólico en niños escolares rurales: evaluación de eficacia]. (personal communication) 2014.
References to studies excluded from this review
Abreu 2009 {published data only}
- Abreu L. Impacto da Fortificação das Farinhas com Ferro no Controle da Anemia em Gestantes: Estudo em um Serviço Público de Saúde do Município de São Bernardo do Campo [Master's thesis]. Sao Paulo: Faculdade de Saude Publica, Universidade de Sao Paulo, 2009. [Google Scholar]
Aguirre Arenas 2013 {published data only}
- Aguirre Arenas J, Chávez Villasana A, Medina Carranza BE, García Villegas EA, Carrasco MR, Guarneros Soto N. Impact of the provision of fortified cornmeal on anemia in preschoolers in the indigenous areas of Mexico [Impacto del suministro de harina de maíz fortificada en la anemia de preescolares de zonas indígenas de México]. Gaceta Sanitaria 2013;27(6):541‐544. [DOI] [PubMed] [Google Scholar]
Ashworth 1973 {published data only}
- Ashworth A, Milner PF, Waterlow JC, Walker RB. Absorption of iron from maize (Zea mays L.) and soya beans (Glycine hispida Max.) in Jamaican infants. British Journal of Nutrition 1973;29(2):269‐78. [DOI] [PubMed] [Google Scholar]
Assuncao 2007a {published data only}
- Assuncao MC, Santos IS. Effect of food fortification with iron on childhood anemia: a review study. Cadernos De Saude Publica 2007;23(2):269‐81. [DOI] [PubMed] [Google Scholar]
Assuncao 2007b {published data only}
- Assuncao MC, Santos IS, Barros AJ, Gigante DP, Victora CG. Effect of iron fortification of flour on anemia in preschool children in Pelotas, Brazil. Revista de Saude Publica/Journal of Public Health 2007;41(4):539‐48. [DOI] [PubMed] [Google Scholar]
Assuncao 2012 {published data only}
- Assuncao MC, Santos IS, Barros AJ, Gigante DP, Victora CG. Flour fortification with iron has no impact on anaemia in urban Brazilian children. Public Health Nutrition 2012;15(10):1796‐801. [DOI] [PubMed] [Google Scholar]
Beiseigel 2007 {published data only}
- Beiseigel JM, Hunt JR, Glahn RP, Welch RM, Menkir A, Maziya‐Dixon BB. Iron bioavailability from maize and beans: a comparison of human measurements with Caco‐2 cell and algorithm predictions. American Journal of Clinical Nutrition 2007;86(2):388‐96. [DOI] [PubMed] [Google Scholar]
Berg 1978 {published data only}
- Berg RB, Pelt W. Screening and prevention of nutritional anemia during infancy. A prospective study of food fortification. JAMA 1978;240(13):1362‐5. [PubMed] [Google Scholar]
Bisimwa 2012 {published data only}
- Bisimwa G, Owino VO, Bahwere P, Dramaix M, Donnen P, Dibari F, Collins S. Randomized controlled trial of the effectiveness of a soybean‐maize‐sorghum‐based ready‐to‐use complementary food paste on infant growth in South Kivu, Democratic Republic of Congo. American Journal of Clinical Nutrition 2012;95(5):1157‐64. [DOI] [PubMed] [Google Scholar]
Bjorn‐Rasmussen 1972 {published data only}
- Bjorn‐Rasmussen E, Hallberg L, Walker RB. Food iron absorption in man. I. Isotopic exchange between food iron and inorganic iron salt added to food: studies on maize, wheat, and eggs. American Journal of Clinical Nutrition 1972;25(3):317‐23. [DOI] [PubMed] [Google Scholar]
Bovell‐Benjamin 1999a {published data only}
- Bovell‐Benjamin AC, Allen LH, Frankel EN, Guinard JX. Sensory quality and lipid oxidation of maize porridge as affected by iron amino acid chelates and EDTA. Journal of Food Science 1999;64(2):371‐6. [Google Scholar]
Bovell‐Benjamin 2000 {published data only}
- Bovell‐Benjamin AC, Viteri FE, Allen LH. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. American Journal of Clinical Nutrition 2000;71(6):1563‐9. [DOI] [PubMed] [Google Scholar]
Chang 2012 {published data only}
- Chang S, Huang Z, Ma Y, Piao J, Yang X, Zeder C, Hurrell RF, Egli I. Mixture of ferric sodium ethylenediaminetetraacetate (NaFeEDTA) and ferrous sulfate: an effective iron fortificant for complementary foods for young Chinese children. Food and Nutrition Bulletin 2012;33(2):111‐6. [DOI] [PubMed] [Google Scholar]
Cook 1997 {published data only}
- Cook JD, Reddy MB, Burri J, Juillerat MA, Hurrell RF. The influence of different cereal grains on iron absorption from infant cereal foods. American Journal of Clinical Nutrition 1997;65(4):964‐9. [DOI] [PubMed] [Google Scholar]
Da Silva 2012 {published data only}
- Silva CL, Saunders C, Szarfarc SC, Fujimori E, Veiga GV. Anaemia in pregnant women before and after the mandatory fortification of wheat and corn flours with iron. Public Health Nutrition 2012;15(10):1802‐9. [DOI] [PubMed] [Google Scholar]
Davidsson 2002 {published data only}
Davidsson 2003 {published data only}
Del Real 2002 {published data only}
- Real S, Páez MC, Solano L, Fajardo Z. Pre‐cooked corn flour intake and its contribution of iron and vitamin A in low income preschoolers [Consumo de harina de maíz precocida y su aporte de hierro y vitamina A en preescolares de bajos recursos económicos]. Archivos Latinoamericanos de Nutricion 2002;52(3):274‐81. [PubMed] [Google Scholar]
Derman 1977 {published data only}
Derman 1982 {published data only}
De Souza 2011 {published data only}
- Souza MD, Damasceno CVX, Szarfarc SC, Fujimori E, Araujo MAD, Moreira‐Araujo RSD. Fortification of flours with iron and control of anemia in pregnant women in Teresina, Piaui, Brazil. Revista de Nutricao‐Brazilian Journal of Nutrition 2011;24(5):679‐88. [Google Scholar]
Diaz 2002 {published data only}
- Díaz MS, Guerra P, Campos MS, Letelier MA, Olivares M. Prevalence of iron deficiency in preschool children from la Pintana County [Prevalencia de deficiencia de hierro en preescolares de la comuna La Pintana]. Revista Chilena de Nutricion 2002;29(1):10‐13. [Google Scholar]
El Hamdouch 2010 {published data only}
- Hamdouchi A, Kari K, Rjimati L, Haloui N, Mzibri M, Aguenaou H, Mokhtar N. Impact of flour fortification with elemental iron on the prevalence of anaemia among preschool children in Morocco [Impact de l'enrichissement de la farine en fer élémentaire sur la prévalence de l'anémie chez les enfants en âge préscolaire au Maroc]. Eastern Mediterranean Health Journal 2010;6(11):1148‐52. [PubMed] [Google Scholar]
Faber 2005 {published data only}
Figueroa Cardenas 2001 {published data only}
- Figueroa Cárdenas JD, Acero Godinez MG, Vasco NL Méndez, Lozano Guzmán A, Flores Acosta LM, González‐Hernández J. Fortification and evaluation of the nixtamal tortillas [Fortificacion y evaluacion de tortillas de nixtamal]. Archivos Latinoamericanos de Nutricion 2001;51(3):293‐302. [PubMed] [Google Scholar]
Filteau 2010 {published data only}
- Filteau S, Kasonka L, Gibson R, Gompels UA, Jaffar S, Kafwembe E, et al. Micronutrient fortification to improve growth and health of maternally HIV‐unexposed and exposed Zambian infants: a randomised controlled trial. PLOS ONE 2010;5(6):e11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno D, Kowa PK, Bwalya HK, Siame J, Grantham‐McGregor S, Baisley K, et al. Rich micronutrient fortification of locally produced infant food does not improve mental and motor development of Zambian infants: a randomised controlled trial. British Journal of Nutrition 2012;107(4):556‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujimori 2011 {published data only}
- Fujimori E, Sato APS, Szarfarc SC, Veiga GV, Oliveira VA, Colli C, et al. Anemia in Brazilian pregnant women before and after flour fortification with iron. Revista de Saude Publica 2011; Vol. 45, issue 6. [DOI] [PubMed]
- Fujimori EE, Szarfarc SC, Sato AP, Sayuri A. Impact of flours fortification with iron in anemia control of pregnant women attended at Brazilian public health services (Abstract). Annals of Nutrition & Metabolism 2009;55(Suppl 1):298. [Google Scholar]
Garcia‐Casal 1998 {published data only}
- García‐Casal MN, Layrisse M, Solano L, Barón MA, Arguello F, Llovera D, et al. Vitamin A and beta‐carotene can improve nonheme iron absorption from rice, wheat and corn by humans. Journal of Nutrition 1998;128(3):646‐50. [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2002 {published data only}
- Garcia‐Casal MN, Layrisse M. Iron fortification of flour in Venezuela. Nutrition Reviews 2002;60(7 part 2):S26‐S29. [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2003 {published data only}
- Garcia‐Casal MN, Layrisse M, Pena‐Rosas JP, Ramirez J, Leets I, Matus P. Iron absorption from elemental iron‐fortified corn flakes In humans. Role of vitamins A and C. Nutrition Research 2003;23(4):451‐63. [Google Scholar]
Garcia‐Casal 2006 {published data only}
- Garcia‐Casal MN. Carotenoids increase iron absorption from cereal‐based food in the human. Nutrition Research 2006; Vol. 26, issue 7:340‐4.
Hallberg 1984 {published data only}
- Hallberg L, Rossander L. Improvement of iron nutrition in developing countries: comparison of adding meat, soy protein, ascorbic acid, citric acid, and ferrous sulphate on iron absorption from a simple Latin American‐type of meal. American Journal of Clinical Nutrition 1984; Vol. 39, issue 4:577‐83. [DOI] [PubMed]
Heijblom 2007 {published data only}
- Heijblom GS, Santos LM. Iron deficiency anemia in first grade students from public schools in a region of Brasília, DF [Anemia ferropriva em escolares da primeira serie do ensino fundamental da rede publica de educacao de uma regiao de Brasilia, DF]. Revista Brasileira de Epidemiologia 2007;10(2):258‐66. [Google Scholar]
Hurrell 2003 {published data only}
Janmohamed 2016 (C) {published data only}
- Janmohamed A, Karakochuk CD, Boungnasiri S, Chapman GE, Janssen PA, Brant R, et al. Prenatal supplementation with Corn Soya Blend Plus reduces the risk of maternal anemia in late gestation and lowers the rate of preterm birth but does not significantly improve maternal weight gain and birth anthropometric measurements in rural Cambodian women: a randomized trial. American Journal of Clinical Nutrition 2016;103(2):559‐66. [DOI] [PubMed] [Google Scholar]
Jyväkorpi 2006 {published data only}
- Jyväkorpi SK, Martínez H, Pineda A, Pizarro S, Monárrez‐Espino J. Iron nutrition in schoolchildren of Western Mexico: the effect of iron fortification. Ecology of Food and Nutrition 2006;45(6):431‐47. [Google Scholar]
Krebs 2012 {published data only}
- Krebs NF, Mazariegos M, Chomba E, Sami N, Pasha O, Tshefu A, et al. Randomized controlled trial of meat compared with multimicronutrient‐fortified cereal in infants and toddlers with high stunting rates in diverse settings. American Journal of Clinical Nutrition 2012;96(4):840‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Layrisse 1996 {published data only}
- García‐Casal MN, Layrisse M. Iron fortification of flours in Venezuela. Nutrition Reviews 2002;60(7 Pt 2):S26‐9. [DOI] [PubMed] [Google Scholar]
- Layrisse M, Chaves JF, Mendez‐Castellano, Bosch V, Tropper E, Bastardo B, et al. Early response to the effect of iron fortification in the Venezuelan population. American Journal of Clinical Nutrition 1996;64(6):903‐7. [DOI] [PubMed] [Google Scholar]
Layrisse 1997a {published data only}
- Layrisse M, Garcia‐Casal MN, Solano L, Baron MA, Arguello F, Llovera D, et al. The role of vitamin A on the inhibitors of nonheme iron absorption: preliminary results. Journal of Nutritional Biochemistry 1997;8(2):61‐7. [Google Scholar]
Layrisse 1997b {published data only}
- Layrisse M, Garcia Casal M, Solano L, Baron M, Arguello F, et al. Fortification of the corn flour and the wheat with iron and vitamins in the Venezuelan population derived: experiments of the obtainables results [Fortificación de las harinas de maíz y de trigo con hierro y vitaminas en la población Venezolana: experimentos derivados de los resultados obtenidos]. Anales Venezolanos de Nutricion 1997;10(1):58‐61. [Google Scholar]
Layrisse 2000a {published data only}
Layrisse 2000b {published data only}
- Layrisse M, Garcia Casal M, Solano L, Baron M, Arguello F, Llovera D, et al. New property of vitamin A and b‐carotene on human iron absorption: effect on phytate and polyphenols as inhibitors of iron absorption. Archivos Latinoamericanos de Nutricion 2000;50(3):243‐8. [PubMed] [Google Scholar]
Layrisse 2002 {published data only}
- Layrisse M, Garcia‐Casal MN, Mendez‐Castellano H, Jimenez M, Olavarria H, Chavez JF, Gonzalez E. Impact of fortification of flours with iron to reduce the prevalence of anemia and iron deficiency among schoolage children in Caracas, Venezuela: a follow‐up. Food and Nutrition Bulletin 2002;23(4):384‐9. [DOI] [PubMed] [Google Scholar]
Li 1995 {published data only}
- Li T. Iron deficiency in infancy and young children in China. Opportunities for Micronutrient Interventions. Arlington: John Snow JSI, 1995.
Macharia‐Mutie 2012 {published data only}
- Macharia‐Mutie CW, Moretti D, Omusundi AM, Mwangi AM, Kok FJ, Zimmermann MB, et al. Maize porridge enriched with a micronutrient powder containing low‐dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. Journal of Nutrition 2012;142(9):1756‐63. [DOI] [PubMed] [Google Scholar]
MacPhail 1981 {published data only}
- MacPhail AP, Bothwell TH, Torrance JD, Derman DP, Bezwoda WR, Charlton RW, et al. Factors affecting the absorption of iron from Fe(III)EDTA. British Journal of Nutrition 1981;45(2):215‐27. [DOI] [PubMed] [Google Scholar]
Manary 2002 {published data only}
- Manary MJ, Krebs NF, Gibson RS, Broadhead RL, Hambidge KM. Community‐based dietary phytate reduction and its effect on iron status in Malawian children. Annals of Tropical Paediatrics 2002;22(2):133‐6. [DOI] [PubMed] [Google Scholar]
Martinez‐Torres 1991 {published data only}
- Martinez‐Torres C, Racca E, Rivero F, Cano M, Leets I, Tropper E, et al. Iron fortification of precooked maize flour. Interciencia 1991;16(5):254‐60. [Google Scholar]
Mendoza 1998 {published data only}
- Mendoza C, Viteri FE, Lonnerdal B, Young KA, Raboy V, Brown KH. Effect of genetically modified, low‐phytic acid maize on absorption of iron from tortillas. American Journal of Clinical Nutrition 1998;68(5):1123‐7. [DOI] [PubMed] [Google Scholar]
Mendoza 2001 {published data only}
- Mendoza C, Viteri FE, Lonnerdal B, Raboy V, Young KA, Brown KH. Absorption of iron from unmodified maize and genetically altered, low‐phytate maize fortified with ferrous sulfate or sodium iron EDTA. American Journal of Clinical Nutrition 2001;73(1):80‐5. [DOI] [PubMed] [Google Scholar]
Nesamvuni 2005 {published data only}
Nieman 2011 {published data only}
- Nieman DC, Henson DA, Sha W. Ingestion of micronutrient fortified breakfast cereal has no influence on immune function in healthy children: a randomized controlled trial. Nutrition Journal 2011;10(36):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2005 {published data only}
- Patel MP, Sandige HL, Ndekha MJ, Briend A, Ashorn P, Manary MJ. Supplemental feeding with ready‐to‐use therapeutic food in Malawian children at risk of malnutrition. Journal of Health, Population and Nutrition 2005;23(4):351‐7. [PubMed] [Google Scholar]
Pouraram 2002 {published data only}
- Pouraram H, Elmadfa I, Dorosty AR, Abtahi M, Neyestani TR, Sadeghian S. Long‐term consequences of iron‐fortified flour consumption in nonanemic men. Annals of Nutrition & Metabolism 2012;60(2):115‐21. [DOI] [PubMed] [Google Scholar]
Rosado 2012 {published data only}
- Rosado J, Díaz M, Muñoz E, Westcott J, González K, Krebs NF, et al. Bioavailability of zinc oxide added to corn tortilla is similar to that of zinc sulfate and is not affected by simultaneous addition of iron. Food and Nutrition Bulletin 2012;33(4):261‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadighi 2008 {published data only}
- Sadighi J, Sheikholeslam R, Mohammad K, Pouraram H, Abdollahi Z, Samadpour K, et al. Flour fortification with iron: a mid‐term evaluation. Public Health 2008;122(3):313‐21. [DOI] [PubMed] [Google Scholar]
Sato 2008 {published data only}
- Sato A, Fujimori E, Szarfarc S, Sato J, Bonadio I. Prevalence of anemia in pregnant and iron fortification of flours. Texto & Contexto Enfermagem 2008; Vol. 17, issue 3:474‐81.
Sato 2011 {published data only}
- Sato A, Fujimori E, Szarfarc SC, Araujo CRMA, Oliveira Queiroz VA, Moreira‐Araújo RSR, et al. Anaemia in pregnant women assisted by public health services of the five Brazilian regions before and after the policy of fortification of flours with iron (SP1‐8). Journal of Epidemiology and Community Health 2011;65(1):A376. [Google Scholar]
Sichert‐Hellert 2001 {published data only}
- Sichert‐Hellert W, Kersting M, Manz F. Changes in time‐trends of nutrient intake from fortified and non‐fortified food in German children and adolescents –15 year results of the DONALD Study. European Journal of Nutrition 2001;40(2):49‐55. [DOI] [PubMed] [Google Scholar]
Sinisterra 2015 {published data only}
- Sinisterra O, Fontes F, Carrasco Y, Pons E, Ulloa D, et al. Efficacy of ferrous gluconate and ferrous bisglycinate as fortificants in maize‐based complementary food on iron status in children 25‐59 months (PO 2442). Annals of Nutrition & Metabolism 2013;63(suppl 1):1‐1960. [Google Scholar]
- Sinisterra O, Fontes F, Carrasco Y, Pons E, Ulloa D, Lay LF, et al. Effectiveness of a precooked maize‐based cereal fortified with ferrous gluconate stabilized with glycine or ferrous bisglycinate on iron status of children 24‐59 months of age [Efectividad de un cereal de maíz precocido fortificado con gluconato ferroso estabilizado con glicina o bisglicinato ferroso sobre el estatus de hierro de niños de 24 a 59 meses]. Revista Chilena de Nutricion 2013;40(4):369‐75. [Google Scholar]
SUSTAIN 2000 {published data only}
- SUSTAIN. Storage, Sensory and Bioavailability Evaluation of Iron‐fortified Corn Masa Flour: Final Report. Washington DC: USAID, 2000. [Google Scholar]
Troesch 2011 {published data only}
- Troesch B, Egli I, Zeder C, Hurrell RF, Zimmermann MB. Fortification iron as ferrous sulfate plus ascorbic acid is more rapidly absorbed than as sodium iron EDTA but neither increases serum nontransferrin‐bound iron in women. Journal of Nutrition 2011;141(5):822‐7. [DOI] [PubMed] [Google Scholar]
Van den Briel 2007 {published data only}
- Briel T, Cheung E, Zewari J, Khan R. Fortifying food in the field to boost nutrition: case studies from Afghanistan, Angola, and Zambia. Food and Nutrition Bulletin 2008;28(3):353‐63. [DOI] [PubMed] [Google Scholar]
Vasconcelos 2008 {published data only}
- Vasconcelos IAL, Cortes MH, Coitinho DC. Foods subject to mandatory fortification with iron: a study with pregnant women [Alimentos sujeitos à fortificação compulsóriacom ferro: um estudo com gestantes]. Brazilian Journal of Nutrition 2008;21(2):149‐60. [Google Scholar]
Vitolo 1998 {published data only}
- Vitolo MR, Aguirre AN, Kondo MR, Giuliano Y, Ferreira N, Lopez FA. Effect of the use of iron‐enriched cereal on the serum hemoglobin levels and anthropometric measurements of preschool children. Revista de Nutricao/Brazilian Journal of Nutrition 1998;11(2):163‐7. [Google Scholar]
Walczyk 2003 {published data only}
- Walczyk T, Davidson L, Rossander‐Hulthen L, Hallberg L, Hurrell RF. No enhancing effect of vitamin A on iron absorption in humans. American Journal of Clinical Nutrition 2003;77(1):144‐9. [DOI] [PubMed] [Google Scholar]
Walter 2003 {published data only}
- Walter T, Pizarro F, Olivares M. Iron bioavailability in corn‐masa tortillas is improved by the addition of disodium EDTA. Journal of Nutrition 2003;133(10):3158‐61. [DOI] [PubMed] [Google Scholar]
Walter 2004 {published data only}
World Food Programme 2000 {published data only}
- World Food Programme. Sustain Report: Technical Specifications for the Manufacture of: Super Cereal Corn Soya Blend. Vol. 1, Rome: World Food Programme, 2000:1‐8. [Google Scholar]
Zimmermann 2010 {published data only}
- Zimmermann MB, Harrington M, Villalpando S, Hurrell RF. Nonheme‐iron absorption in first‐degree relatives is highly correlated: a stable‐isotope study in mother‐child pairs. American Journal of Clinical Nutrition 2010;91(3):802‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2005
- Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, et al. Hemochromatosis and iron‐overload screening in a racially diverse population. New England Journal of Medicine 2005;28(352):1769‐78. [DOI] [PubMed] [Google Scholar]
Ballot 1989
- Ballot DE, MacPhail AP, Bothwell TH, Gillooly M, Mayet FG. Fortification of curry powder with NaFe(111)EDTA in an iron‐deficient population: report of a controlled iron‐fortification trial. American Journal of Clinical Nutrition 1989;49(1):162‐9. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Beard 2007
- Beard J. Recent evidence from human and animal studies regarding iron status and infant development. Journal of Nutrition 2007;137(2):524S‐30S. [DOI] [PubMed] [Google Scholar]
Best 2011
- Best C, Neufingerl N, Miller Del Rosso J, Transler C, Briel T, Osendarp S. Can multi‐micronutrient food fortification improve the micronutrient status, growth, health, and cognition of school children? A systematic review. Nutrition Reviews 2011;69(4):186‐204. [DOI] [PubMed] [Google Scholar]
Black 2011
- Black MM, Quigg AM, Hurley KM, Pepper MR. Iron deficiency and iron‐deficiency anemia in the first two years of life: strategies to prevent loss of developmental potential. Nutrition Reviews 2011;69(Suppl 1):S64‐S70. [DOI] [PubMed] [Google Scholar]
Bressani 1990
- Bressani R, Benavides V, Acevedo E, Ortiz MA. Changes in selected nutrient contents and in protein quality of common and quality‐protein maize during rural tortilla preparation. Cereal Chemistry 1990;67:515‐8. [Google Scholar]
Bressani 1997
- Bressani R, Rooney L, Saldivar SOS. Fortification of Corn Masa Flour with Iron and/or Other Nutrients: a Literature and Industry Experience Review. Washington DC: SUSTAIN, 1997. [Google Scholar]
Brittenham 2004
- Brittenham G. Safety of flour fortification with iron. Wheat flour fortification: current knowledge and practical applications: workshop proceedings. Flour Fortification Initiative; 2004,December 1‐3; Cuernavaca, Mexico. http://web1.sph.emory.edu/users/hpacho2/PartnershipsMaize/FFI_2004.pdf. Atlanta: Flour Fortification Initiative, 2004. [www.ffinetwork.org/why_fortify/documents/IronSafety2004.pdf] [Google Scholar]
Chilenje Infant Growth 2010
- Chilenje Infant Growth, Nutrition and Infection (CIGNIS) Study Team. Micronutrient fortification to improve growth and health of maternally HIV‐unexposed and exposed Zambian infants: a randomised controlled trial. PLOS ONE 2010;5(6):e11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane PHG 2011
- Cochrane PHG. Guide for developing a Cochrane Protocol. Cochrane Public Health Group, The Cochrane Collaboration 2011.
Codex 1985a
- Codex Alimentarius. CODEX standard for whole maize (corn) meal (CODEX STAN 154‐1985). 1985. www.codexalimentarius.org (accessed 26 July 2012).
Codex 1985b
- Codex Alimentarius. CODEX standard for degermed maize (corn) meal and maize (corn) grits (CODEX STAN 155‐1985). 1985. www.codexalimentarius.org (accessed 26 July 2012).
Cook 1983
- Cook J, Reusser M. Iron fortification: an update. American Journal of Clinical Nutrition 1983;38(4):648‐59. [DOI] [PubMed] [Google Scholar]
Covenin 1996
- COVENIN. Norma Venezolana. Norm 2135: Precooked corn flour [Norma 2135: Harina de maíz precocida]. 1996. Available from: www.sencamer.gob.ve/sencamer/normas/2135‐96.pdf. 3rd. Caracas: COVENIN.
Dary 2002a
- Dary O. Staple food fortification with iron: a multifactorial decision. Nutrition Reviews 2002;60(7 Pt 2):S34‐41; discussion S46‐9. [DOI] [PubMed] [Google Scholar]
Dary 2002b
- Dary O, Freire W, Kim S. Iron compounds for food fortification: guidelines for Latin America and the Caribbean 2002. Nutrition Reviews 2002;60(7 Pt 2):S50‐61. [DOI] [PubMed] [Google Scholar]
Das 2013
- Das J, Salam R, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review. SystematicReviews 2013;2(67):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2016
- De‐Regil LM, Finkelstein JL, Sæterdal I, Gaitán D, Peña‐Rosas JP. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2014
- Dunn ML, Jain V, Klein BP. Stability of key micronutrients added to fortified maize flours and corn meal. Annals of the New York Academy of Sciences April 2014;1312:15‐25. [DOI] [PubMed] [Google Scholar]
Dwyer 2015
- Dwyer JT, Wiemer KL, Dary O, Keen CL, King JC, et al. Fortification and health: challenges and opportunities. Advances in Nutrition 2015;6(1):124‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2017
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews, 2017. EPOC resources for review authors. Available at epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
FAO 1992
- Food, Agriculture Organization of the United Nations. Maize in Human Nutrition. Rome: Food and Agriculture Organization of the United Nations, 1992. [Google Scholar]
FAO 2011
- Food, Agriculture Organization of the United Nations. Combating iron deficiency: food‐based approaches, 2011. Available from www.fao.org/docrep/013/am027e/am027e00.pdf. Rome: Food and Agriculture Organization of the United Nations and CAB International.
FAO 2012
- Food, Agriculture Organization of the United Nations. FAO Statistical Yearbook. World Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations, 2012. [www.fao.org/docrep/015/i2490e/i2490e00.htm] [Google Scholar]
FDA 2011
- US Food, Drug Administration. Code of Federal Regulations (CFR) Title 21 Food and Drugs. Part 137 ‐ Cereal flours and related products. Subpart B‐‐Requirements for Specific Standardized Cereal Flours and Related Products. Washington, DC: FDA, 2011. [Google Scholar]
Fiedler 2014
- Fiedler JL, Afidra R, Mugambi G, Tehinse J, Kabaghe G, Zulu R, Lividini K, Smitz MF, Jallier V, Guyondet C, Bermudez O. Maize flour fortification in Africa: markets, feasibility, coverage, and costs. Annals of the New York Academy of Sciences 2014;1312:26‐39. [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2018
- Garcia‐Casal MN, Mowson R, Rogers LM, Grajeda R, consultation working groups. Risk of excessive intake of vitamins and minerals delivered through public health interventions: objectives, results, conclusions of the meeting, and the way forward. Annals of the New York Academy of Sciences 2018;13975:1‐16. [DOI] [PubMed] [Google Scholar]
Gera 2012
- Gera T, Sachdev H, Boy E. Effect of iron‐fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials. American Journal of Clinical Nutrition 2012;96:309‐24. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed prior to 2 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guamuch 2014
- Guamuch M, Dary O, Rambelson Z, Cruz V, Villalpando S, Tom C, et al. Model for estimating nutrient addition contents to staple foods fortified simultaneously: Mexico and Kampala data. Annals of the New York Academy of Sciences 2014;1312:76‐90. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwirtz 2014
- Gwirtz JA, Garcia‐Casal MN. Processing maize flour and corn meal food products. Annals of the New York Academy of Sciences 2014;1312:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallberg 1982
- Hallberg L. Iron nutrition and food iron fortification. Seminars in Hematology 1982;19(1):31‐41. [PubMed] [Google Scholar]
Herbst 2001
- Herbst ST. The Food Lovers Companion. 3rd Edition. New York: The Barron Education Series, 2001. [Google Scholar]
Hertptramp 2004
- Hertrampf E, Olivares M. Iron amino acid chelates. International Journal for Vitamin and Nutrition Research 2004;74(6):435‐43. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hurrell 2002
- Hurrell R. How to ensure adequate iron absorption from iron‐fortified food. Nutrition Reviews 2002;60(7 Pt 2):S7‐15; discussion S43. [DOI] [PubMed] [Google Scholar]
Hurrell 2010
- Hurrell R, Ranum P, Pee S, Biebinger R, Hulthen L, Johnson Q, et al. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food and Nutrition Bulletin 2010;31(1 Suppl):S7‐S21. [DOI] [PubMed] [Google Scholar]
ILO 1984
- International Labor Organisation. Small Scale Maize Milling. Geneva: ILO, 1984. [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123(5):614‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1974
- Katz SH, Hediger ML, Valleroy LA. Traditional maize processing techniques in the New World. Science 1974;184(4138):765‐73. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2013
- Low M, Farrell A, Biggs BA, Pasricha SR. Effects of daily iron supplementation in primary‐school‐aged children: systematic review and meta‐analysis of randomized controlled trials. CMAJ 2013 Nov 19;185(17):E791‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makhumula 2014
- Makhumula P, Dary O, Guamuch M, Tom C, Afidra R, Rambeloson X. Legislative frameworks for corn flour and maize meal fortification. Annals of the New York Academy of Sciences 2014;1312:91‐104. [DOI] [PubMed] [Google Scholar]
Martinez 1995
- Martínez H, González‐Cossío T, Flores M, Rivera‐Dommarco J, Lezana MA, Sepúlveda‐Amor J. Anemia in women of reproductive age. The results of a national probability survey [Anemia en mujeres de edad reproductiva: resultados de una encuesta probabilística nacional]. Salud Publica de Mexico 1995;37(2):108‐19. [PubMed] [Google Scholar]
McKevith 2004
- McKevith B. Nutritional aspects of cereals. Nutrition Bulletin 2004;29(2):111‐42. [Google Scholar]
Nair 2009
- Nair KM, Iyengar V. Iron content, bioavailability & factors affecting iron status of Indians. Indian Journal of Medical Research 2009;130(5):634‐45. [PubMed] [Google Scholar]
Nuss 2010
- Nuss ET, Tanumihardjo SA. Maize: a paramount staple crop in the context of global nutrition. Comprehensive Reviews in Food Science and Food Safety 2010;9:417‐36. [DOI] [PubMed] [Google Scholar]
Pasricha 2013
- Pasricha SR, Drakesmith AH, Black JF, Hipgrave D, Biggs BA. Control of iron deficiency anemia in low and middle‐income countries. Blood 2013;121(14):2607‐17. [DOI] [PubMed] [Google Scholar]
Pasricha 2014
- Pasricha SR, Low M, Thompson J, Farrell A, De‐Regil LM. Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta‐analysis. Journal of Nutrition 2014;144(6):906‐914. [DOI] [PubMed] [Google Scholar]
Pasricha 2018
- Pasricha SR, Armitage AE, Prentice AM, Drakesmith H. Reducing anaemia in low income countries: control of infection is essential.. British Medical Journal 2018;362:k3165. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2014a
- Peña‐Rosas JP, Garcia‐Casal MN, Pachón H, Mclean MS, Arabi M. Technical considerations for maize flour and corn meal fortification in public health: consultation rationale and summary. Annals of the New York Academy of Sciences 2014;1312:1‐7. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2014b
- Peña‐Rosas JP, Field MS, Burford BJ, De‐Regil LM. Wheat flour fortification with iron for reducing anaemia and improving iron status in populations. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011302] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015
- Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Popper 2006
- Popper L. Composite flours. Future of Flour ‐ a Compendium of Flour Improvement. Verlag Agrimedia, 2006. [Google Scholar]
Pouraram 2012
- Pouraram H, Elmadfa I, Dorosty AR, Abtahi M, Neyestani TR, Sadeghian S. Long‐term consequences of iron‐fortified flour consumption in nonanemic men. Annals of Nutrition and Metabolism 2012;60(2):115‐21. [DOI] [PubMed] [Google Scholar]
Prasanna 2001
- Prasanna BN, Vasal SK, Kassahun B, Singh NN. Quality protein maize. Current Science 2001;81(10):1308‐19. [Google Scholar]
Ranum 2014
- Ranum P, Peña‐Rosas JP, Garcia‐Casal MN. Global maize production, utilization, and consumption. Annals of the New York Academy of Sciencies 2014;1312:105‐12. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scholl 2000
- Scholl TO, Reilly T. Anemia, iron and pregnancy outcome. Journal of Nutrition 2000;130(2S Suppl):443S‐7S. [DOI] [PubMed] [Google Scholar]
Shamah‐Levy 2003
- Shamah‐Levy T, Villalpando S, Rivera JA, Mejía‐Rodríguez F, Camacho‐Cisneros M, Monterrubio EA. Anemia in Mexican women: a public health problem. Salud Publica de Mexico 2003;45(Suppl 4):S499‐507. [DOI] [PubMed] [Google Scholar]
Sharpe 1950
- Sharpe LM, Peacock WC, Cooke R, Harris RS. The effect of phytate and food factors on iron absorption. Journal of Nutrition 1950;41(3):433‐46. [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ (Clinical Research Ed.) 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995‐2011: a systematic analysis of population ‐ representative data. Lancet Global Health 2013;1(1):e16‐25. [10.1016/S2214‐109X(13)70001‐9; PMC4547326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoltzfus 1996
- Stoltzfus RJ, Albonico M, Chwaya HM, Savioli L, Tielsch J, Schulze K, et al. Hemoquant determination of hookworm‐related blood loss and its role in iron deficiency in African children. American Journal of Tropical Medicine and Hygiene 1996;55(4):399‐404. [DOI] [PubMed] [Google Scholar]
Ueffing 2011
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E for the Campbell and Cochrane Equity Methods Group. Equity Checklist for Systematic Review Authors. Version 2011‐11‐08. www.equity.cochrane.org/files/equitychecklist2011.pdf (accessed 13 September 2012).
Vielma 1998
- Vielma M. Characterization of the agroindustry of precooked corn flour in Venezuela [Caracterización de la agroindustria de harina precocida de maíz en Venezuela]. Revista de la Facultad de Agronomia, Universidad del Zulia 1998;15:472‐85. [Google Scholar]
Wharton 1999
- Wharton BA. Iron deficiency in children: detection and prevention. British Journal of Haematology 1999;106(2):270‐80. [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. Recommendations on Wheat and Maize Flour Fortification Meeting Report: Interim Consensus Statement (WHO/NMH/NHD/MNM/09.1). Geneva: World Health Organization, 2009. [www.who.int/nutrition/publications/micronutrients/wheat_maize_fort.pdf] [PubMed] [Google Scholar]
WHO 2015
- WHO. The global prevalence of anaemia in 2011. The Global Prevalence of Anaemia in 2011. Geneva: World Health Organization, 2015. [Google Scholar]
WHO/CDC 2011
- World Health Organization, Centers for Disease Control and Prevention. Logic model for micronutrient interventions in public health. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.5). Geneva: World Health Organization, 2011. [www.who.int/vmnis/toolkit/WHO‐CDC‐english_colour.pdf] [Google Scholar]
WHO/FAO 2006
- Allen L, Benoist B, Dary O, Hurrell R (eds), World Health Organization, Food, Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization, 2006. [Google Scholar]
Wyatt 1994
- Wyatt JC, Triana‐Trejos A. Soluble and insoluble Fe, Zn, Ca and phytates in foods commonly consumed in Northern Mexico. Journal of Agricultural and Food Chemistry 1994;42:2204‐9. [Google Scholar]
References to other published versions of this review
Pasricha 2012
- Pasricha SR, De‐Regil LM, Garcia‐Casal MN, Burford BJ, Gwirtz JA, Peña‐Rosas JP. Fortification of maize flour with iron for preventing anaemia and iron deficiency in populations (protocol). Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010187] [DOI] [PMC free article] [PubMed] [Google Scholar]