Abstract
Background
This is an update of a previous Cochrane Review published in 2012, Issue 9.
Surgery for endometrial cancer (hysterectomy with removal of both fallopian tubes and ovaries) is performed through laparotomy. It has been suggested that the laparoscopic approach is associated with a reduction in operative morbidity. Over the last two decades there has been a steady increase of the use of laparoscopy for endometrial cancer. This review investigated the evidence of benefits and harms of laparoscopic surgery compared with laparotomy for presumed early stage endometrial cancer.
Objectives
To compare overall survival (OS) and disease free survival (DFS) for laparoscopic surgery versus laparotomy in women with presumed early stage endometrial cancer.
Search methods
For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 5) in the Cochrane Library, MEDLINE via Ovid (April 2012 to June 2018) and Embase via Ovid (April 2012 to June 2018). We also searched registers of clinical trials, abstracts of scientific meetings and reference lists of included studies. The trial registers included NHMRC Clinical Trials Register, UKCCCR Register of Cancer Trials, Meta‐Register and Physician Data Query Protocol.
Selection criteria
Randomised controlled trials (RCTs) comparing laparoscopy and laparotomy for early stage endometrial cancer.
Data collection and analysis
We independently abstracted data and assessed risk of bias. We used hazard ratios (HRs) for OS and recurrence free survival (RFS), risk ratios (RR) for severe adverse events and mean differences (MD) for continuous outcomes in women who received laparoscopy or laparotomy with 9% confidence intervals (CI). These were pooled in random‐effects meta‐analyses.
Main results
We identified one new study in this update of the review. The review contains nine RCTs comparing laparoscopy with laparotomy for the surgical management of early stage endometrial cancer.
All nine studies met the inclusion criteria and assessed 4389 women at the end of the studies. Six studies assessing 3993 participants with early stage endometrial cancer found no significant difference in the risk of death between women who underwent laparoscopy and women who underwent laparotomy (HR 1.04, 95% 0.86 to 1.25; moderate‐certainty evidence) and five studies assessing 3710 participants found no significant difference in the risk of recurrence between the laparoscopy and laparotomy groups (HR 1.14, 95% CI 0.90 to 1.43; moderate‐certainty evidence). There was no significant difference in the rate of perioperative death; women requiring a blood transfusion; and bladder, ureteric, bowel and vascular injury. However, one meta‐analysis of three studies found that women in the laparoscopy group lost significantly less blood than women in the laparotomy group (MD –106.82 mL, 95% CI –141.59 to –72.06; low‐certainty evidence). A further meta‐analysis of two studies, which assessed 3344 women and included one very large trial of over 2500 participants, found that there was no clinical difference in the risk of severe postoperative complications in women in the laparoscopy and laparotomy groups (RR 0.78, 95% CI 0.44 to 1.38). Most studies were at moderate risk of bias. All nine studies reported hospital stay and results showed that on average, laparoscopy was associated with a significantly shorter hospital stay.
Authors' conclusions
This review found low to moderate‐certainty evidence to support the role of laparoscopy for the management of early endometrial cancer. For presumed early stage primary endometrioid adenocarcinoma of the endometrium, laparoscopy is associated with similar OS and DFS. Furthermore, laparoscopy is associated with reduced operative morbidity and hospital stay. There is no significant difference in severe postoperative morbidity between the two modalities.
The certainty of evidence for OS and RFS was moderate and was downgraded for unclear risk of bias profiles and imprecision in effect estimates. However, most studies used adequate methods of sequence generation and concealment of allocation so studies were not prone to selection bias. Adverse event outcomes were downgraded for the same reasons and additionally for low event rates and low power thus these outcomes provided low‐certainty evidence.
Keywords: Female; Humans; Blood Loss, Surgical; Blood Loss, Surgical/statistics & numerical data; Disease‐Free Survival; Endometrial Neoplasms; Endometrial Neoplasms/mortality; Endometrial Neoplasms/pathology; Endometrial Neoplasms/surgery; Hysterectomy; Hysterectomy/adverse effects; Hysterectomy/methods; Hysterectomy/mortality; Laparoscopy; Laparoscopy/adverse effects; Laparoscopy/methods; Laparoscopy/mortality; Laparotomy; Laparotomy/adverse effects; Laparotomy/methods; Laparotomy/mortality; Length of Stay; Neoplasm Recurrence, Local; Postoperative Complications; Randomized Controlled Trials as Topic
Plain language summary
Laparoscopy versus laparotomy for the management of presumed early stage endometrial cancer
Background Worldwide, cancer of the womb or 'endometrial cancer' is the fifth most common cancer among women up to 65 years of age and has a higher incidence in high income countries than in low and middle income countries. For women with cancer of the womb, removal of the womb (hysterectomy) and removal of both fallopian tubes (tubes along which eggs travel from the ovaries to the womb) and ovaries (which produce eggs) is considered current standard treatment. Other treatments include radiotherapy and chemotherapy. Traditionally, surgery for cancer of the womb is performed through a laparotomy (open cut in the abdomen).
Review question This review compared overall survival (length of time that the woman remained alive) and disease free survival (length of time that the women remained disease‐free) for laparoscopic (keyhole) surgery with laparotomy in women with presumed early endometrial cancer.
Key results Results from six trials where women were randomly put into one of two treatment groups showed no difference in the risk of death between women who had laparoscopy and women who had laparotomy. In addition, results from five randomised trials confirmed no difference in the risk of cancer recurrence between women who had laparoscopy and women who had laparotomy. Notably, laparoscopy was associated with less blood loss and earlier discharge from hospital.
Certainty of the evidence The certainty of the evidence for overall and recurrence free survival was moderate. Certainty for side effects was low.
What were the conclusions? This review update confirms the findings of the previous review that laparoscopy (keyhole) is an effective and viable alternative to laparotomy (open surgery) in the treatment of early stage endometrial cancer. With regards to long term survival outcomes, treatment by laparoscopy is comparable to laparotomy.
Summary of findings
Summary of findings for the main comparison. Laparoscopy versus laparotomy for early stage endometrial cancer.
| Laparoscopy versus laparotomy for early stage endometrial cancer | ||||||
|
Patient or population: adult women diagnosed with early stage (I to IIa) endometrial cancer undergoing surgery as primary treatment. Settings: randomised controlled trials (RCTs) Intervention: laparotomy, total abdominal hysterectomy (TAH) Comparison: laparoscopy; laparoscopically assisted vaginal hysterectomy (LAVH) or total laparoscopic hysterectomy (TLH) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopy | Laparotomy | |||||
|
Overall survival (survival until death from all causes) |
Not estimable due to reporting of HR and heterogeneous length of follow‐up across trials. We did not arbitrarily choose a snap shot in time in which to use as basis to calculate the assumed and corresponding risks as this may be misleading. |
HR 1.04 (0.86 to 1.25) |
3993 participants (6 studies) |
⊕⊕⊕⊝ Moderatea,b | The overall certainty of evidence for this outcome was moderate and was downgraded for unclear risk of bias profiles and imprecision (although most trials used adequate methods of sequence generation and concealment of allocation). | |
| Recurrence free survival | Not estimable due to reporting of HR and heterogeneous length of follow‐up across trials. We did not arbitrarily choose a snap shot in time in which to use as basis to calculate the assumed and corresponding risks as this may be misleading. |
HR 1.14 (0.90 to 1.43) |
3710 participants (5 studies) |
⊕⊕⊕⊝ Moderatea,b | The overall certainty of evidence for this outcome was moderate and was downgraded for unclear risk of bias profiles and imprecision (although most trials used adequate methods of sequence generation and concealment of allocation). | |
| Serious adverse events (range of outcomes) | Generally low proportion of event rates so assumed risks were not computed. | RRs were not statistically significant for any of the adverse event outcomes. Estimated blood loss was statistically significant on a continuous scale but the difference was not clinically important (MD –108.6 mL (95% CI –141.59 to –72.06). | Range 313 to 3894 (2 to 8 studies) |
⊕⊕⊝⊝ Lowa,b,c | The overall certainty of evidence for this outcome was low and was downgraded for unclear risk of bias profiles, imprecision and low event rates (although most trials used adequate methods of sequence generation and concealment of allocation). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded (by half a point) for uncertainty in a number of potential biases in included trials due to their unclear risk of bias profiles. bDowngraded (by half a point) for imprecision in effect estimates. cDowngraded for low event rates and low power in the adverse event analyses.
Background
Description of the condition
Endometrial cancer is a cancer of the lining of the uterus and worldwide it is the sixth most commonly diagnosed cancer and the 14th leading cause of cancer death in women, with 320,000 estimated new cases and 76,000 deaths in 2012 (Ferlay 2015). A woman's risk of developing endometrial cancer by 65 years of age ranges from 0.46% in low and middle income countries to 0.92% in high income countries (GLOBOCAN 2008). Endometrial cancer is predominantly a disease of postmenopausal women and most cases occur in women aged 50 years and older. The majority of women (71%) present with stage I disease (Creasman 2006). Overall survival (OS) in stage I disease is high with more than 90% of women being disease‐free five years after surgery (Creasman 2006).
The incidence of endometrial cancer is steadily rising and risk factors include an ageing population, obesity, diabetes mellitus, nulliparity, late menopause, unopposed oestrogen intake or oestrogen‐producing tumours, history of breast cancer and use of tamoxifen (Berek 2010). Of the risk factors obesity seems to be by far the most consistent factor with some studies reporting up to 81% of women being obese, and 19% to 36% being morbidly obese (Smits 2013).
Endometrial carcinoma is usually limited to the uterus at the time of diagnosis and essentially carries a good overall prognosis. The prognosis depends on various factors, which include histological grading, depth of invasion into the myometrium, lymph node involvement, tumour size, lymphovascular space invasion (LVSI), stage of disease and treatment received, including radiotherapy and chemotherapy (Berek 2010).
For women with endometrial cancer, removal of the uterus (hysterectomy) and removal of both fallopian tubes and ovaries is considered current standard treatment; other treatments include adjuvant radiotherapy and chemotherapy. Pelvic/para‐aortic lymph node dissection with or without omental biopsy has been suggested for high grade tumours, tumours with unfavourable histological types and tumours invading the myometrium (Eltabbakh 2002). Traditionally, surgery for endometrial cancer is performed using a laparotomy (Marana 1999).
The laparoscopic approach results in a reduction in operative morbidity, including wound infection, blood loss and ileus (obstruction of the bowel) in overweight and elderly women (Eltabbakh 2000; Holub 2000; Obermair 2005; Scribner 2001; Smits 2013). In addition, it has been suggested that OS and disease free survival (DFS) is comparable to laparotomy (Eltabbakh 2002; Obermair 2004; Tozzi 2005).
The International Federation of Gynaecology and Obstetrics (FIGO) staging in endometrial cancer is surgical and hysterectomy is required to determine the depth of myometrial invasion and cervical involvement (Shepherd 1989). FIGO staging for endometrial cancer was updated in 2009, but all studies included started recruitment prior to the introduction of FIGO (2009) staging, therefore, we used 1989 FIGO staging. In this staging system, the presence of cancer cells in the peritoneal washing equates to stage IIIA disease, while in contrast, the presence of positive washings does not alter the updated FIGO staging (FIGO Staging 2009).
Description of the intervention
The standard treatment for endometrial cancer remains total hysterectomy and bilateral salpingo‐oophorectomy, which can be performed either through laparotomy or minimal access laparoscopic approach.
Why it is important to do this review
Evidence suggests that laparoscopic surgery is an acceptable alternative to the conventional laparotomy for the treatment of endometrial cancer. Staging in endometrial cancer is surgical and hysterectomy is required to determine the depth of myometrial invasion and cervical involvement (Shepherd 1989). The laparoscopic approach may result in a reduction in operative morbidity, including wound infection in overweight and elderly women (Eltabbakh 2000; Hauspy 2010; Obermair 2005; Scribner 2001). In addition, it has been suggested that the OS and DFS is comparable to laparotomy (Eltabbakh 2002; Obermair 2004; Seracchioli 2005). There have been a few reports of port‐site recurrence and vaginal recurrence after laparoscopy for endometrial cancer (Muntz 1999; Sanjuan 2005). The risk of port‐site metastases may be reduced by closure of the port site in layers (Tjalma 2003), and the risk of vaginal recurrence reduced by avoiding uterine manipulation during laparoscopy. There is mounting evidence to suggest that laparoscopy is an acceptable alternative to laparotomy in the surgical treatment of endometrial cancer. In this review, we aimed to establish whether laparoscopy is as good as, or better than, the conventional laparotomy for the treatment of endometrial cancer.
Objectives
To compare the overall survival (OS) and disease free survival (DFS) for laparoscopic surgery versus laparotomy in women with presumed early endometrial cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing laparoscopy with laparotomy for presumed early stage endometrial cancer.
Types of participants
Inclusion
Adult women diagnosed with endometrial cancer undergoing surgery as primary treatment. Since staging of endometrial cancer is surgical, cases included were identified on the basis of no evidence of extrauterine disease preoperatively.
We included studies with at least 70% of women with stage I to IIA disease, as it was expected that some studies would have small percentages of women with more advanced (stage IIb, III and IV) disease.
We excluded women without a preoperative diagnosis of endometrial cancer (e.g. diagnosed with endometrial hyperplasia).
Types of interventions
Laparotomy, total abdominal hysterectomy (TAH)
Laparoscopy; laparoscopically assisted vaginal hysterectomy (LAVH) or total laparoscopic hysterectomy (TLH)
Types of outcome measures
Primary outcomes
Overall survival (OS): survival until death from all causes. Survival was assessed from the time when women were enrolled in the trial.
Recurrence free survival (RFS): length of time after treatment during which a woman survived with no sign of disease recurrence.
Secondary outcomes
Local recurrence (port site, vaginal vault at laparoscopy and abdominal incision at laparotomy).
Distant recurrence.
-
Severe adverse events CTCAE 2006:
perioperative death within 30 days;
injuries (urinary tract, vascular, bowel);
lymphoedema;
venous thromboembolism;
grade III or IV early and late complications.
Blood loss including need for transfusion.
Length of hospital stay/delayed discharge.
Quality of life (QoL) after six months or more post operation, measured using a scale that was validated through reporting of norms in a peer‐reviewed publication.
Search methods for identification of studies
There were no languages restrictions and we carried out translations when necessary.
Electronic searches
We search the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 5) in the Cochrane Library;
MEDLINE via Ovid April 2012 to June week 2 2018;
Embase via Ovid April 2012 to 2018 week 24;
The MEDLINE, Embase, and CENTRAL search strategies are presented in Appendix 3; Appendix 4; and Appendix 5.
All relevant articles found were identified on PubMed and using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and grey literature
We searched metaRegister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing trials. We also searched the NHMRC Clinical Trials Register and UKCCCR Register of Cancer Trials.
Handsearching
We handsearched the citation list of relevant publications, abstracts of scientific meetings and list of included studies and contacted experts in the field to identify further reports. Reports of conferences were handsearched in the following sources:
Gynecologic Oncology;
International Journal of Gynecological Cancer;
British Journal of Cancer;
British Gynaecological Cancer Society (BGCS);
Journal of Clinical Oncology (JCO);
British Cancer Research Meeting;
Annual Meeting of the International Gynecologic Cancer Society;
Annual Meeting of the American Society of Gynecologic Oncologist;
Annual Meeting of The European Society of Medical Oncology (ESMO);
Annual Meeting of the American Society of Clinical Oncology (ASCO);
BioMed (open text publisher); AACR conferences;
ESGO conference;
ASGO conference.
Reference lists
We handsearched the reference lists of all relevant studies for further studies.
Correspondence
We contacted authors of relevant studies to ask if they knew of further data which may or may not have been published.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database Endnote, removed duplicates and three review authors (KG, HD, AL) independently examined the remaining references. These review authors screened titles and abstracts of references identified by the search and eliminated articles that were obviously not relevant to the search question. When all review authors determined that the trial was not eligible for inclusion no further action was taken. When one or more of the review authors determined that the article may have been eligible for inclusion, we obtained the full text article. Each review author then independently determined if these studies were eligible for inclusion. We resolved disagreements about inclusions by discussion. We sought further information from the authors when papers contained insufficient information to make a decision about eligibility. The review authors were not blinded to article authors or journals.
For the update, we downloaded all titles and abstracts retrieved by electronic searching to Endnote and removed duplicates. At least two review authors (KG, AL) independently examined the remaining references. We excluded studies that clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors independently assessed the eligibility of retrieved papers and, when necessary, requested additional information from study authors. These two review authors resolved disagreements by discussion. We documented reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
For included trials, we abstracted data as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (KG, HD) independently extracted the following data:
Author, year of publication and journal citation (including language).
Country.
Setting.
Inclusion and exclusion criteria.
Study design, methodology.
Study population (participant characteristics, age, stage and postoperative residual disease).
Number of participants in each arm of the trial.
Total number of intervention groups.
Endometrial carcinoma details (FIGO stage, histology, tumour grade).
Intervention details; LAVH, TLH/laparotomy.
Type of surgeon: gynaecological oncologist, general gynaecologist.
Experience of surgeon: consultant, trainee.
Operative time.
Variations in technique, conversion to laparotomy rates.
Pelvic or para‐aortic lymphadenectomy, or both.
Lymph node yield.
Length of follow‐up.
Withdrawals from treatment protocol.
Risk of bias in study (see below).
-
Outcomes: OS, PFS, QoL and adverse events:
for each outcome: outcome definition;
unit of measurement (if relevant);
for scales: upper and lower limits, and whether high or low score was good;
results: number of participants allocated to each intervention group;
for each outcome of interest: sample size; missing participants.
We extracted outcome data as below.
For time to event (OS and RFS) data, we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each group who experienced the outcome of interest and the number of participants assessed at endpoint, to estimate a risk ratio (RR).
We extracted both unadjusted and adjusted statistics, if reported.
Where possible, all data extracted were those relevant to an intention to treat (ITT) analysis, in which participants were analysed in the groups to which they were assigned.
We noted the time points at which outcomes were collected and reported.
Two review authors (KG, AB) independently abstracted data onto a data abstraction form in accordance with Cochrane guidelines (Higgins 2011). We resolved differences between review authors by discussion or by appeal to a third review author (ADL) when necessary. Where appropriate, we contacted trial authors for further information and updated data.
Assessment of risk of bias in included studies
Two review authors (KG, AB) independently used the Cochrane 'Risk of bias' tool to assess risk of bias of the included studies (Higgins 2011). They resolved differences by discussion or by appeal to a third review author (AL) and presented results in a 'Risk of bias' graph and a 'Risk of bias' summary (Figure 1). Results were interpreted in light of the findings with respect to risk of bias.
1.
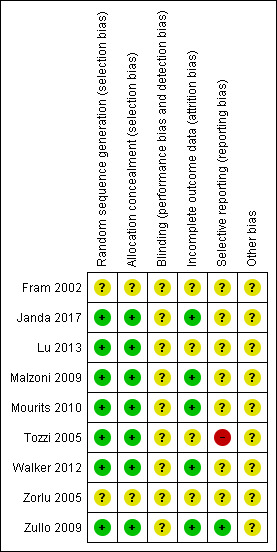
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The 'Risk of bias' tool included assessment of the following domains.
Sequence generation.
Allocation concealment.
Blinding (assessment of blinding was restricted to blinding of outcome assessors, since it is generally not possible to blind participants and treatment providers to surgical interventions).
-
Incomplete outcome data: we coded a satisfactory level of:
yes, if less than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
no, if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms;
unclear if loss to follow‐up was not reported.
Selective reporting of outcomes.
Other possible sources of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time to event data, we used the hazard ratio (HR) and 95% confidence intervals (CI).
For dichotomous outcomes, we used the RR and 95% CIs.
For continuous outcomes, we used the mean difference (MD) and 95% CIs between treatment arms (if studies measured outcomes on the same scale). If studies had measured outcomes on different scales, we would have used the standardised mean difference (SMD).
Dealing with missing data
Missing outcome data were not imputed for the primary outcome. If data were missing or only imputed data were reported, we contacted trial authors to request data on the outcomes but only for participants who were assessed.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between studies which could not be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and, where possible, by subgroup analyses (see below). When there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Assessment of reporting biases
We examined funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects. When there was evidence of small‐study effects, we considered publication bias as only one of several possible explanations. Where these plots suggested that treatment effects may not have be sampled from a symmetric distribution, as assumed by the random‐effects model, we performed sensitivity analyses using fixed‐effect models.
Data synthesis
We pooled the results of clinically similar studies in meta‐analyses.
For time‐to‐event data, we pooled HR using the generic inverse variance facility of Review Manager 5 (Review Manager 2014).
For dichotomous outcomes, we calculated the RR for each study and pooled these.
For continuous outcomes, we pooled the MDs between the treatment arms at the end of follow‐up as studies measured the outcome on the same scale. In future updates of this review, if studies include outcomes measured on different scales, we will use SMDs.
All meta‐analyses used random‐effects models with inverse variance weighting (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
There were no a priori subgroup analyses.
We considered factors such as age, stage, grade, length of follow‐up and adjusted/unadjusted analysis in interpretation of heterogeneity.
Sensitivity analysis
Sensitivity analyses excluded studies that did not report adequate:
concealment of allocation;
blinding the outcome assessor.
Results
Description of studies
See: Characteristics of included studies table.
Results of the search
The search strategy identified 1760 unique references. Screening of titles and abstracts identified 25 references which were potentially eligible for review. Screening of the full text of these references excluded nine (two references reported the results of the same systematic review and the references were nested so there were eight unique references) for the reasons described in the Characteristics of excluded studies table. The remaining 16 references described eight RCTs which met our inclusion criteria and are described in the Characteristics of included studies table.
The new search from April 2012 to June 2018 yielded 1321 additional references (CENTRAL 239 references, MEDLINE 293 references, Embase 789 references). We excluded 964 articles that obviously did not meet the inclusion criteria. We retrieved 15 articles in full and subjected them to full‐text screening. We subsequently excluded 12 of these. One additional RCT met the inclusion criteria, and two articles provided additional data from a previously included RCT.
Included studies
All nine studies were randomised comparisons of laparoscopy versus laparotomy for apparent early endometrial cancer. The nine included studies randomised eligible women; 2449 women to laparoscopic surgery and 1495 to laparotomy (Fram 2002; Janda 2017; Lu 2013; Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zorlu 2005; Zullo 2009).
Design
Four of the studies were multicentre trials (Janda 2017; Mourits 2010; Walker 2012; Zullo 2009), with three having 2:1 intervention to control randomisation (Janda 2017; Mourits 2010; Walker 2012), and one trial randomising an equal number of women in each group (Zullo 2009). The remaining five studies were all set in single centres with approximately 1:1 randomisation. These studies were set in Italy (Malzoni 2009), Australia (Fram 2002), Germany (Tozzi 2005), Turkey (Zorlu 2005), and China (Lu 2013).
Participants
The studies varied in size and ranged from small (Fram 2002; Zorlu 2005; Zullo 2009), where fewer than 100 women were randomised in the trial, to very large (Walker 2012), where 2591 women were randomised in the trial. The remaining five studies included between 122 and 332 women (Janda 2017; Lu 2013; Malzoni 2009; Mourits 2010; Tozzi 2005).
Fram 2002: 61 women were randomised: 32 women in the laparotomy group, mean age 60.6 years, body mass index (BMI) 26.2; 29 women in the laparoscopy group, mean age 61.2 years, BMI 25.7.
Janda 2017: overall, 332 women were included in the QoL component: 142 women in the TAH group, mean age 62.7 years (standard deviation (SD) 9.7), 49 (34.5%) had BMI of 35 and over, and all had performance status (PS) 0 to 1; 190 women in the TLH group, mean age 62.8 years (SD 10), 72 (38%) had BMI 35 or greater and all had PS 0 to 1.
Lu 2013: 151 women in the laparoscopy group, median age 56.6 years (range 27 to 82); 121 women in the laparotomy group, median age 57.2 (range 29 to 79).
Malzoni 2009: 159 women were randomised: 78 women in the laparotomy group, mean age 63 years (SD 14; 95% CI 43 to 84), mean BMI 29 (SD 7.3; 95% CI 17 to 39), stage I/IIA disease 68/78 (87%); 81 women in the laparoscopy group, mean age 60 years (SD 11; 95% CI 39 to 81), mean BMI 28 (SD 6.9; 95% CI 19 to 37), stage I/IIA disease 75/81 (92.5%).
Mourits 2010: 283 women were randomised: 96 women in the laparotomy group, mean age 63 years (range 39 to 86), mean BMI 28 (range 19 to 48), stage I disease 75/185 (87%); 187 women in the laparoscopy group, mean age 62 years (range 40 to 89), mean BMI 29 (range 17 to 55), stage I disease 130/185 (87%).
Tozzi 2005: 122 women were randomised: 63 women in the laparoscopy group, mean age 67 years (range 35 to 88), mean BMI 31.3 (20.2 to 43.6), stage I disease "similar in 2 groups", endometrioid histology 52 (82.5%); 59 women in the laparotomy group, mean age 66 years (range 36 to 89), mean BMI 32.1 (range 20 to 51.3), stage I disease "similar in 2 groups", endometrioid histology 52 (88.1%).
Walker 2012: 1682 women in the laparoscopy group, median age 63 years (interquartile range (IQR) 55 to 72), median BMI 28 (IQR 24 to 34), stage I/IIA disease 1287/1630 (76.5%); 909 women in the laparotomy group, median age 63 years (IQR 55 to 71), median BMI 29 (IQR 24 to 34), stage I/IIA disease 700/886 (77%).
Zorlu 2005: 52 women were randomised: 26 women in the laparoscopy group, mean age 56.6 years (range 40 to 72), BMI 24.4; 26 in the laparotomy group, mean age 54.9 years (range 36 to 77), BMI 26.2.
Zullo 2009: 84 women were randomised; 42 women in the laparoscopy group, mean age 62.1 years (SD 14.5), BMI 29.9 (SD 7.5); 42 women to the laparotomy group, mean age 61.5 years (SD 13.3), BMI 31.8 (SD 8.5).
Interventions
Three studies described LAVH, bilateral salpingo‐oophorectomy, peritoneal washings, with or without pelvic lymph node dissection versus laparotomy, TAH, bilateral salpingo‐oophorectomy, peritoneal washings, with or without pelvic lymph node dissection (Fram 2002; Tozzi 2005; Zullo 2009).
Two studies compared TLH, bilateral salpingo‐oophorectomy, peritoneal washings, with or without pelvic lymph node dissection with or without para‐aortic lymph node dissection versus laparotomy, vertical midline skin incision, TAH, bilateral salpingo‐oophorectomy, peritoneal washings, with or without pelvic lymph node dissection with or without para‐aortic lymph node dissection (Janda 2017; Malzoni 2009).
One trial compared TLH, bilateral salpingo‐oophorectomy, peritoneal washings versus laparotomy, TAH, bilateral salpingo‐oophorectomy and peritoneal washings (Mourits 2010).
One trial compared laparoscopic hysterectomy including laparoscopic assisted techniques, total laparoscopic approaches, and rarely robotics with pelvic lymph node sampling and para‐aortic lymph node sampling versus laparotomy, washings, extrafascial hysterectomy and bilateral salpingo‐oophorectomy with pelvic lymph node sampling and para‐aortic lymph node sampling (Walker 2012).
One trial described the surgical procedure as traditional laparotomy surgery versus laparoscopic surgery; no further details of the intervention and control were given (Zorlu 2005).
One trial did not describe the surgical procedures, but noted that all women received pelvic lymphadenectomy and para‐aortic lymph nodes sampling. Women with positive pelvic lymph node discovered at frozen section evaluation and non‐endometrioid carcinomas received para‐aortic lymphadenectomy. In histologically confirmed tumour infiltration of the endocervix, radical hysterectomy with pelvic and para‐aortic lymph node dissection was performed. Infracolic omentectomy was performed in serous and clear cell endometrial carcinomas (Lu 2013).
Outcomes
Six studies reported OS and RFS and used appropriate statistical techniques (HRs to correctly allow for censoring) (Janda 2017; Lu 2013; Malzoni 2009; Tozzi 2005; Walker 2012; Zullo 2009). Zullo 2009 explicitly reported an HR with corresponding 95% CI for both survival outcomes. Three studies reported the number of women in each group who died and experienced disease recurrence (Janda 2017; Malzoni 2009; Tozzi 2005); they gave the exact log rank P value from the Kaplan Meier survival plots so it was possible to estimate the HR using this information (Parmar 1998). Lu 2013 trial also reported the number of women in each group who died and gave the exact log rank P value from the Kaplan Meier survival plots so it was possible to estimate the HR; however, it was not possible to report a relative effect for recurrence survival.
Five studies either explicitly reported or gave sufficient information to deduce perioperative death within 30 days or six weeks using the Kaplan Meier plots or other information in the text (Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zullo 2009).
Seven studies incompletely reported severe adverse events (Fram 2002; Janda 2017; Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zullo 2009).
Four studies reported estimated blood loss (continuous) (Lu 2013; Malzoni 2009; Tozzi 2005; Zullo 2009). Eight studies reported the dichotomous outcome of needing a blood transfusion (Fram 2002; Janda 2017; Lu 2013; Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zullo 2009).
All studies reported operative time.
All studies reported hospital stay.
Three studies reported QoL data (Janda 2017; Mourits 2010; Zullo 2009). Two studies used the 36‐item Short‐Form Healthy Survey (SF‐36) score (Mourits 2010; Zullo 2009). Janda 2017 measured QoL with a variety of validated subscales including physical, functional and body image scores as well as social and emotional components of QoL. This trial used Functional Assessment of Cancer Therapy – General (FACT‐G) summary and EuroQoL‐Visual Analog Scale scores.
Excluded studies
We excluded eight studies/references after obtaining the full text for the following reasons (de la Orden 2008; Ghezzi 2006; Ghezzi 2010; Imesch 2009; Ju 2009; Lin 2007; Liu 2009; Palomba 2009):
one study, there was no laparotomy comparison arm. This study compared two laparoscopic procedures (LAVH versus TLH) (Ghezzi 2006);
five studies were not RCTs (de la Orden 2008; Ghezzi 2010; Ju 2009; Lin 2007; Liu 2009);
two references were systematic review articles, which yielded no further included studies (Imesch 2009; Palomba 2009).
For further details of all the excluded studies see the Characteristics of excluded studies table.
Risk of bias in included studies
Five studies were at moderate risk of bias. They satisfied three of the criteria that we used to assess risk of bias (Janda 2017; Malzoni 2009; Mourits 2010; Walker 2012; Zullo 2009). Two studies were at moderate to high risk of bias as they satisfied two of the criteria (Lu 2013; Tozzi 2005), and two studies were at high risk of bias as they did not satisfy any of the criteria (Fram 2002; Zorlu 2005).
Seven studies reported the method of generation of the sequence of random numbers used to allocate women to treatment arms and concealment of this allocation sequence from participants and healthcare professionals involved in the trial (low risk of bias; Janda 2017; Lu 2013; Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zullo 2009). Two studies did not report this (unclear risk of bias; Fram 2002; Zorlu 2005). None of the studies reported blinding of the outcome assessor (unclear risk of bias). Five studies reported that at least 80% of women who were enrolled were assessed at endpoint (low risk of bias; Janda 2017; Malzoni 2009; Mourits 2010; Walker 2012; Zullo 2009); this was unclear in four studies (Fram 2002; Lu 2013; Tozzi 2005; Zorlu 2005). It was unclear whether any additional form of bias may have been present in any of the nine included studies, but we suspected that outcomes may have been selectively reported in Tozzi 2005 because outcome definitions varied between the three different publications of the same trial (high risk of bias). It was unclear whether outcomes were selectively reported in any of the other studies.
Effects of interventions
See: Table 1
For dichotomous outcomes, we were unable to estimate an RR for comparisons of treatments, if one or both treatment groups experienced no events, as in the comparison of laparoscopy versus laparotomy for the severe early and late complication outcomes. We applied the default Review Manager 5 continuity correction (where a small increment is added to the zero) in cases of one zero event field for studies that were included in a meta‐analysis (Review Manager 2014).
Laparoscopy versus laparotomy
Primary outcomes
Overall survival
Meta‐analysis of six RCTs, assessing 3993 participants, found no significant difference in the risk of death between women who underwent laparoscopy and women who underwent laparotomy (HR 1.04, 95% CI 0.86 to 1.25; Analysis 1.1; Figure 2) (Janda 2017; Lu 2013; Malzoni 2009; Tozzi 2005; Walker 2012; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) might not have been important (I2 = 0%).
1.1. Analysis.
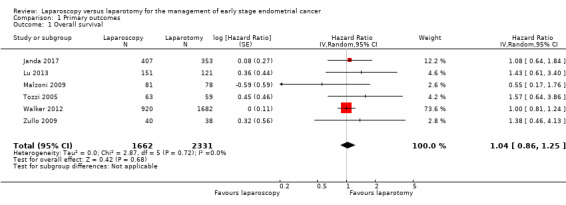
Comparison 1 Primary outcomes, Outcome 1 Overall survival.
2.
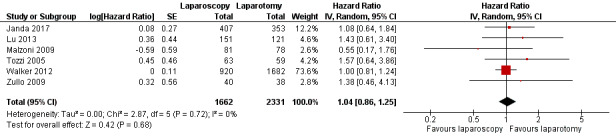
Forest plot of comparison: 1 Primary outcomes, outcome: 1.1 Overall survival.
After a median duration of follow‐up of 38.5 months (range 2 to 81), Malzoni 2009 reported 12 deaths (7.5%); five (6%) assigned to laparoscopy and seven (9%) assigned to laparotomy. Three of these 10 participants died from intercurrent disease; one in the laparoscopy group and two in the laparotomy group. Nine participants (4.4%) died from endometrial cancer; four (5%) in the laparoscopy group and five (6.4%) in the laparotomy group.
At the end of the follow‐up period of seven years, Zullo 2009 reported 13 deaths; 7/40 (17.5%) participants died in the laparoscopy group and 6/38 (16%) participants died in the laparotomy group. Specifically, six (15%) participants died in the laparoscopy group and five (13%) participants died in the laparotomy group died of endometrial cancer.
After a median follow‐up of 44 months (range 5 to 96 months), Tozzi 2005 reported OS in the laparoscopy group of 83% and in the laparotomy group of 86.5%. In participants with stage I disease, OS was 86.5% in the laparoscopy group versus 90% in the laparotomy group.
After a median duration of follow‐up of 68 months (range 2 to 153 months), Lu 2013 reported 21 (7.7%) deaths, 9 (6%) in the laparoscopy group and 12 (9.9%) in the laparotomy group. Fifteen of these 21 participants died from intercurrent disease, six in the laparoscopy group and nine in the laparotomy group. Six participants died from endometrial cancer, three in each group. OS rates were 94% in the laparoscopy group and 90.1% in the laparotomy group. Five year survival rates were 96% in the laparoscopy group and 91% in the laparotomy group (P > 0.05).
Janda 2017 had a median follow‐up of 4.5 years, in which 24 (6.8%) participants in the laparotomy group and 30 (7.4%) participants in the laparoscopy group died. The 4.5‐year OS rate was 92.0% in the laparoscopy group versus 92.4% in the laparotomy group.
After a median follow‐up of 59.3 months (range 38 to 62.9 months) for participants in the laparoscopy group and 59.3 months (range 37.9 to 63.0 months) for the participants in the laparotomy group, Walker 2012 reported 350 deaths (13.5%): 229 (13.6%) assigned to the laparoscopy group and 121 (13.3%) assigned to the laparotomy group of which 224 deaths resulted from disease (152 in the laparoscopy group; 72 in the laparotomy group). The estimated five year OS was almost identical in both arms at 89.8% (Analysis 1.1).
Recurrence free survival
Meta‐analysis of five RCTs, assessing 3710 participants, found no statistically significant difference in the risk of disease recurrence between women who underwent laparoscopy and those who underwent laparotomy (HR 1.14, 95% CI 0.90 to 1.43; Analysis 1.2; Figure 3) (Janda 2017; Malzoni 2009; Tozzi 2005; Walker 2012; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance might not have been important (I2 = 0%).
1.2. Analysis.
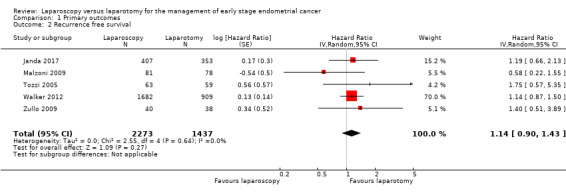
Comparison 1 Primary outcomes, Outcome 2 Recurrence free survival.
3.
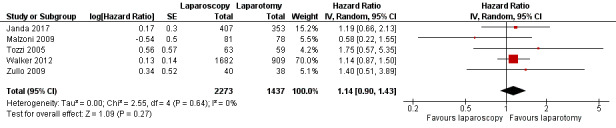
Forest plot of comparison: 1 Primary outcomes, outcome: 1.2 Recurrence free survival.
After a median duration of follow‐up of 4.5 years, Janda 2017 reported 12 (3%) participants with primary site recurrence in the laparotomy group and 14 (3%) participants in the laparoscopy group. Two percent or less of participants experienced a relapse in the pelvis, abdomen, at distant or at multiple sites in both groups. There were two participants with port‐site metastases in the laparotomy group and two participants in the laparoscopy group.
After a median duration of follow‐up of 38.5 months (range 2 to 81 months), Malzoni 2009 reported the total recurrence rate of the entire population to be 10% (16 participants) consisting of 7/81 (8.6%) participants in the laparoscopic group versus 9/78 (11.5%) participants in the laparotomy group. Nine (5.7%) participants had distant recurrence (four in the laparoscopic group and five in the laparotomy group), while seven (4.4%) participants experienced local recurrence (three in the laparoscopic group and four in the laparotomy group).
After median follow‐up of 44 months (range 5 to 96 months), Tozzi 2005 reported 8/63 (12.6%) participants in the laparoscopy group had recurrence versus 5/59 (8.5%) participants in the laparotomy group. Ten (8.2%) participants had distant recurrence (six in the laparoscopy group and four in the laparotomy group), while only three (2.4%) participants experienced local recurrence (two in the laparoscopy group and one in the laparotomy group).
After a median follow‐up of 59 months, Walker 2012 reported 309 recurrences (210 (12.5%) in the laparoscopy group; 99 (10.9%) in the laparotomy group). The actual recurrence rates were substantially lower than anticipated, resulting in an estimated three‐year recurrence rate of 11.4% in the laparoscopy group and 10.2% in the laparotomy group, giving a difference of 1.14% (90% lower bound, 1.28; 95% upper bound, 4.0).
Secondary outcomes
Local and distant recurrence
After seven years of follow‐up, Zullo 2009 reported 8/40 (20%) participants in the laparoscopy group and 7/38 (18.4%) participants in the laparotomy group had disease recurrence. Specifically, three (7.5%) participants in the laparoscopy group and no participants in the laparotomy group had a vaginal cuff recurrence. Only one (2.5%) participant in the laparoscopic group had a port‐site recurrence. There were pelvic recurrences in 2/40 (5%) participants in the laparoscopy group and 6/38 (16%) participants in the laparotomy group. Two of 40 (5%) participants in the laparoscopy group and 1/38 (2.6%) participants in the laparotomy group had distant metastases.
After a median duration of follow‐up of 68 months (range 2 to 153 months), Lu 2013 reported seven (4.6%) participants in the laparoscopy group versus six (5.0%) participants in the laparotomy group had a recurrence. There were four recurrences in the laparoscopic group at peritoneal and liver sites and three recurrences at the vaginal vault, but none were in the laparoscopic port sites. There were three recurrences in the laparotomy group at abdominal incision, and another three recurrences at peritoneal and liver sites. There was no difference in the rate of recurrence or survival between the different approaches.
Severe adverse events
Perioperative death within 30 days
Walker 2012 found no statistically significant difference in perioperative death rate within 30 days between women in the laparoscopy and laparotomy groups (RR 0.68, 95% CI 0.27 to 1.71). Mourits 2010 found no statistically significant difference in perioperative death rate within six weeks between women in the laparoscopy and laparotomy groups (RR 1.54, 95% CI 0.16 to 14.61). Three studies did not observe any woman in either group dying within 30 days or six weeks of their surgery, but perioperative mortality was not reported (or could not be deduced) in any of the other studies (Analysis 2.1; Figure 4) (Malzoni 2009; Tozzi 2005; Zullo 2009). Overall, there was no statistically significant difference in perioperative death rate within one month to six weeks between women in the laparoscopy and laparotomy groups (RR 0.76, 95% CI 0.32 to 1.79; I2 = 0%). In total, there were 22 cases of perioperative mortality in all five studies (13/2053 in the laparoscopic group and 9/1180 in the laparotomy group).
2.1. Analysis.
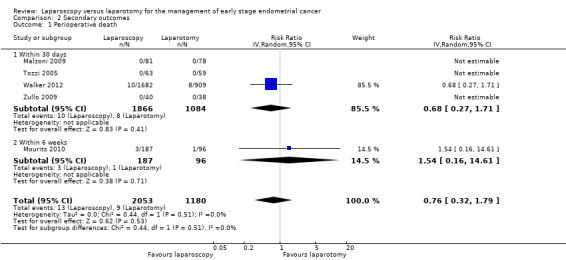
Comparison 2 Secondary outcomes, Outcome 1 Perioperative death.
4.
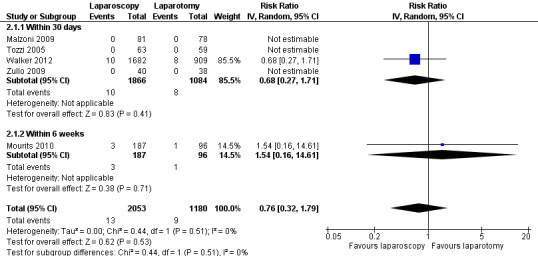
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.1 Perioperative death.
Bladder injury
Meta‐analysis of seven RCTs, assessing 3342 participants, found no statistically significant difference in the risk of bladder injury in women in the laparoscopy and laparotomy groups (RR 1.12, 95% CI 0.54 to 2.32; Analysis 2.2) (Fram 2002; Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zorlu 2005; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%). There were 36 reported cases of bladder injury in the seven studies (23/2106 in the laparoscopic group and 13/1236 in the laparotomy group).
2.2. Analysis.
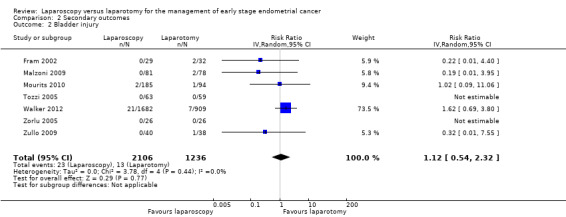
Comparison 2 Secondary outcomes, Outcome 2 Bladder injury.
Ureteric injury
Meta‐analysis of three RCTs, assessing 3463 participants, found no statistically significant difference in the risk of ureteric injury in women in the laparoscopy and laparotomy groups (RR 1.41, 95% CI 0.60 to 3.28; Analysis 2.3) (Mourits 2010; Walker 2012; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than change was not important (I2 = 0%). There were 25 reported cases of ureteric injury in the three studies (18/2189 in the laparoscopic group and 7/1274 in the laparotomy group).
2.3. Analysis.
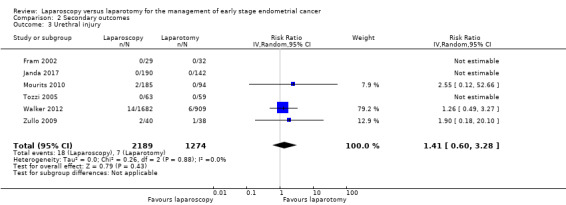
Comparison 2 Secondary outcomes, Outcome 3 Urethral injury.
Vascular injury
Meta‐analysis of five RCTs, assessing 3401 participants, found no statistically significant difference in the risk of vascular injury in women in the laparoscopy and laparotomy groups (RR 0.83, 95% CI 0.23 to 2.94; Analysis 2.4) (Janda 2017; Lu 2013; Tozzi 2005; Walker 2012; Zullo 2009).
2.4. Analysis.
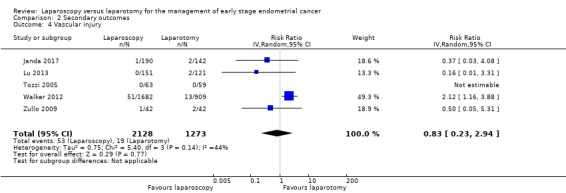
Comparison 2 Secondary outcomes, Outcome 4 Vascular injury.
The percentage of the variability in effect estimates that was due to heterogeneity rather than chance may have represented moderate heterogeneity (I2 = 45%). There were 72 reported cases of vascular injury in the five studies (53/2126 in the laparoscopy group and 19/1269 in the laparotomy group).
Bowel injury
Meta‐analysis of four RCTs, assessing 3070 participants, found no statistically significant difference in the risk of bowel injury in women in the laparoscopy and laparotomy groups (RR 1.28, 95% CI 0.75 to 2.18; Analysis 2.5) (Mourits 2010; Tozzi 2005; Walker 2012; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%). There were 61 reported cases of bowel injury in the four studies (43/1970 in the laparoscopic group and 18/1100 in the laparotomy group).
2.5. Analysis.
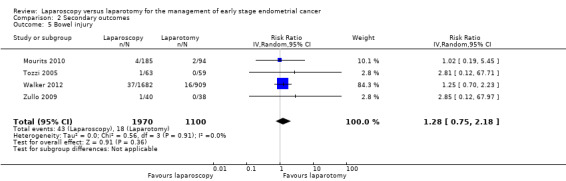
Comparison 2 Secondary outcomes, Outcome 5 Bowel injury.
Severe early complications
Tozzi 2005 reported that two women experienced severe early complications and both these women were in the laparotomy group (0/63 in the laparoscopy group and 2/59 in the laparotomy group).
Severe postoperative complications
Serious adverse postoperative complications were defined as any event that resulted in death, was immediately life threatening, required hospitalisation or prolongation of an existing hospitalisation, or that resulted in persistent or significant disability/incapacity.
Meta‐analysis of two RCTs, assessing 3344 participants, found no statistically significant difference in the risk of severe postoperative complications in women in the laparoscopy and laparotomy groups (RR 0.78, 95% CI 0.44 to 1.38; Analysis 2.6) (Janda 2017; Walker 2012). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance may have represented substantial heterogeneity (I2 = 85%).
2.6. Analysis.
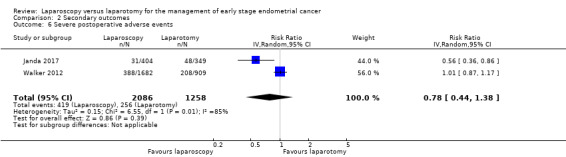
Comparison 2 Secondary outcomes, Outcome 6 Severe postoperative adverse events.
Severe late complications
Tozzi 2005 reported that seven women experienced severe late complications and all of these women were in the laparotomy group (0/63 in the laparoscopy group and 7/59 in the laparotomy group).
Blood loss including need for transfusion
Estimated blood loss
Meta‐analysis of three RCTs, assessing 313 participants, found that laparoscopy was associated with a large and statistically significant reduction in blood loss compared with laparotomy (MD –106.82 mL, 95% CI –141.59 to –72.06; Analysis 2.7) (Malzoni 2009; Tozzi 2005; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance may have represented substantial heterogeneity (I2 = 68%). All three individual studies showed a statistically significant reduction in blood loss in favour of laparoscopy and the substantial heterogeneity indicated by the high I2 statistic was due to the magnitude of estimated blood loss being considerably greater in Tozzi 2005.
2.7. Analysis.
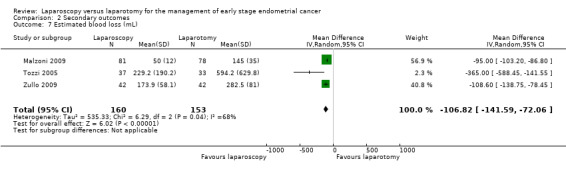
Comparison 2 Secondary outcomes, Outcome 7 Estimated blood loss (mL).
In Fram 2002, there was a statistically significant reduction in blood loss with laparoscopy compared with laparotomy (mean blood loss: 145.5 mL in the laparoscopy group versus 501.6 mL in the laparotomy group; P < 0.05).
In Lu 2013, there was a statistically significant reduction in blood loss with laparoscopy compared with laparotomy (median blood loss: 86 mL in the laparoscopy group versus 419 mL in the laparotomy group; P = 0.01).
Blood transfusion required
Meta‐analysis of eight RCTs, assessing 3894 participants, found no statistically significant difference in the risk of requiring a blood transfusion in women in the laparoscopy and laparotomy groups (RR 0.53, 95% CI 0.22 to 1.27; Analysis 2.8) (Fram 2002; Janda 2017; Lu 2013; Malzoni 2009; Mourits 2010; Tozzi 2005; Walker 2012; Zullo 2009). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance may have represented moderate heterogeneity (I2 = 51%). The majority of events were observed in the largest trial (Walker 2012), where 209 women needed a blood transfusion (143/1682 in the laparoscopy group and 66/909 in the laparotomy group). The difference in the number of women needing transfusions in the two groups in this trial was also not statistically significant (RR 1.17, 95% CI 0.88 to 1.55).
2.8. Analysis.
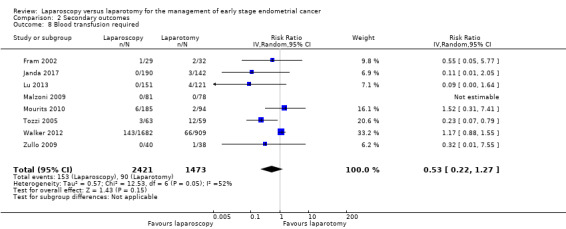
Comparison 2 Secondary outcomes, Outcome 8 Blood transfusion required.
Length of hospital stay/delayed discharge
All studies reported hospital stay and showed that on average laparoscopy had a significantly shorter hospital stay.
Fram 2002: there was a significant difference (P < 0.05) in days of hospitalisation between groups (2.3 days in the laparoscopy group versus 5.5 days in the laparotomy group).
Janda 2017: a significantly higher proportion of participants who were assigned to laparotomy stayed in hospital for longer than two days (139/142 in the laparotomy group versus 72/190 in the laparoscopy group; P < 0.0001).
Lu 2013: the median operating time was 211 minutes (range 100 to 460) in the laparoscopy group and 261 minutes (range 90 to 570) in the laparotomy group (P < 0.01). The median length of hospital stay was three days in the laparoscopy group and six days in the laparotomy group (P < 0.01).
Malzoni 2009: the mean length of hospital stay was 5.1 days (SD 1.2; 95% CI 1 to 7) in the laparotomy group and 2.1 days (SD 0.5; 95% CI 1 to 5) in the laparoscopy group (P < 0.01).
Mourits 2010: there was shorter hospital stay (P < 0.01) and a faster recovery (P < 0.01) in the laparoscopy group, but the procedure took longer than in the laparotomy group (P < 0.0001).
Tozzi 2005: duration of hospital stay was 8.6 days (SD 2.7) in the laparoscopy group and 11.7 days (SD 3.8) in the laparotomy group (P < 0.001). This is the only study with a mean hospital stay for laparoscopy of about eight days.
Walker 2012: hospitalisation of more than two days was significantly lower with laparoscopy (52% in the laparoscopy group versus 94% in the laparotomy group; P< 0.01).
Zorlu 2005: the laparoscopic group had a significantly shorter hospital stay than the laparotomy group (4.1 days in the laparoscopy group versus 8.2 days in the laparotomy group; z = 1.96, P < 0.05).
Zullo 2009: compared with the laparotomy group, the laparoscopy group had a significantly lower mean hospital stay (3.0 days (SD 1.4) in the laparoscopy group versus 6.9 days (SD 2.6) in the laparotomy group) and length of time needed to return to full activity or work (28.2 days (SD 12.8) in the laparoscopy group versus 47.8 days (SD 24.7) in the laparotomy group; P < 0.05 for both).
Quality of life
Three studies reported QoL data (Janda 2017; Mourits 2010; Zullo 2009).
At baseline, Zullo 2009 reported no difference in total SF‐36 score between the two surgical treatment groups. During the first three years from surgery, the total SF‐36 scores were significantly higher in the laparoscopic surgery group than in the laparotomy group (P < 0.05). Throughout the trial, there was no significant change in the laparoscopic group, whereas there was a significant improvement in total SF‐36 score in the laparotomy group at the four‐year follow‐up visit (P < 0.05). At the four‐year follow‐up visit and at the next visits, there were no significant differences between groups. The different domains of the SF‐36 are discussed in the Included studies section.
Janda 2017 reported QoL improvements from baseline during early and later phases of recovery favoured laparoscopy compared with laparotomy for treatment of stage I endometrial cancer. The study measured QoL with a variety of validated subscales. Compared with laparotomy, participants who had laparoscopy had significantly greater improvements in QoL from pre surgery at both early (up to four weeks) and late (up to six months) postoperative recovery. Differences in sub scale scores reflected better physical, functional and overall QoL, as well as body image in the laparoscopic group, whereas social and emotional components of QoL remained largely stable across postsurgery time points and between groups.
Mourits 2010 reported QoL (SF‐36), Sexual Activity Questionnaire (SAQ), Body Image Scale (BIS) and visual analogue scale (VAS) at baseline, six weeks, three months and six months postsurgery. Women who had been sexually active in the month before receiving the questionnaire completed the SAQ. Overall response rate was 90.1%, compliance did not differ significantly between groups, neither did the median scores at baseline for all QoL scales. Participants who had laparoscopy scored significantly higher on the physical functioning sub scale of the SF‐36 at six weeks and on the role‐physical sub scale at three months after the procedure. Participants who had laparotomy scored significantly higher on the vitality sub scale of the mental dimension three months after surgery. There were no differences between groups for the other subscales. There were no differences between groups over time in the sums of the mental and physical dimensions.
The TLH and TAH groups did not differ significantly at baseline or over time in the VAS, BIS or SAQ.
Discussion
Summary of main results
We found nine studies that met our inclusion criteria, these studies randomised 3944 women. Six studies assessing 3993 women reported OS. Meta‐analysis of these six RCTs found no statistically significant difference in the risk of death between women who underwent laparoscopy and those who underwent laparotomy (HR 1.04, 95% CI 0.86 to 1.25; Figure 2) (Janda 2017; Lu 2013; Malzoni 2009; Tozzi 2005; Walker 2012; Zullo 2009).
Five studies assessing 3710 women reported on disease recurrence (Janda 2017; Malzoni 2009; Tozzi 2005; Walker 2012; Zullo 2009). The meta‐analysis of these studies found no significant difference in the risk of disease recurrence between women who underwent laparoscopy and women who underwent laparotomy (HR 1.14, 95% CI 0.90 to 1.43; Figure 3). After a median follow‐up of 4.5 years, Janda 2017 reported 24 deaths (6.8%) and 30 deaths (7.4%) and 14 recurrences (3%) in the laparoscopy group and 12 recurrences (3%) in the laparotomy group. The 4.5‐year OS rate was 92.0% in the laparoscopy group and 92.4% in the laparotomy group.
Malzoni 2009 found no significant difference between the two groups in DFS (P = 0.28). After a median follow‐up of 38.5 months (range 2 to 81 months) 91% of participants were free of disease in the laparoscopy group versus 88.5% in the laparotomy group. After seven years of follow‐up, Zullo 2009 reported 8/40 (20%) participants in the laparoscopy group and 7/38 (18%) participants in the laparotomy group had disease recurrence. There was no difference between group in the cumulative recurrence rates (8/40 (20%) participants in the laparoscopy group and 7/38 (18%) participants in the laparotomy group) and deaths (7/40 (17.5%) participants in the laparoscopy group and 6/38 (16%) participants in the laparotomy group).
After 44 months of follow‐up, Tozzi 2005 reported 19/122 (15.5%) participants died, 11 (17%) in the laparoscopy group and eight (13%) in the laparotomy group. Ten (52.6%) of these 19 participants died from intercurrent disease, five in each group. After 59 months of follow‐up, Walker 2012 reported 350 deaths, 121 (13.1%) in the laparotomy group and 229 (13.5%) in the laparoscopy group. After 68 months of follow‐up, Lu 2013 reported 21 deaths (7.7%), nine (6%) in the laparoscopy group and 12 (9.9%) in the laparotomy group. Fifteen of these 21 participants died from intercurrent disease, six in the laparoscopy group and nine in the laparotomy group. Six participants died from endometrial cancer: three in each group.
Two studies reported perioperative mortality (Mourits 2010; Walker 2012). Walker 2012 found no statistically significant differences in perioperative death rate within 30 days between women in the laparoscopy and laparotomy groups (RR 0.68, 95% CI 0.27 to 1.71; Figure 4). Mourits 2010 found no statistically significant differences in perioperative death rate within six weeks between women in the laparoscopy and laparotomy groups (RR 1.54, 95% CI 0.16 to 14.61). Four studies observed no women in either group died within 30 days or six weeks of their surgery (Janda 2017; Malzoni 2009; Tozzi 2005; Zullo 2009). From these results, we suggested that for apparent early stage endometrial cancer, laparoscopy is associated with similar outcomes in terms of survival and recurrence rate.
Meta‐analysis of three RCTs, assessing 313 participants, found that laparoscopy was associated with a large and statistically significant reduction in blood loss compared with laparotomy (MD –106.82, 95% CI –141.59 to –72.06) (Malzoni 2009; Tozzi 2005; Zullo 2009). In addition, laparoscopy was associated with a significantly shorter hospital stay and reduced postoperative complications.
Three studies reported QoL data using validated scales (Janda 2017; Mourits 2010; Zullo 2009). During the first three years from surgery in Zullo 2009, the SF‐36 scores were significantly higher in the laparoscopic group than in the laparotomy group (P < 0.05). At the four‐year follow‐up visit and at the next visits, there was no significant difference between groups. Janda 2017 reported early (up to four weeks) and late (up to six months) postoperative recovery, these time periods were adequate in allowing a satisfactory assessment of QoL over a longer period of time. Similarly, Mourits 2010 assessed QoL after six weeks and three months postsurgery. Participants who had laparoscopy scored significantly higher on the physical functioning sub scale of the SF‐36 at six weeks, and on the role‐physical sub scale at three months after the procedure. Participants who had laparotomy scored significantly higher on the vitality sub scale of the mental dimension three months after surgery. There were no differences between groups for the other subscales.
Overall completeness and applicability of evidence
We identified nine RCTs that compared laparoscopy with laparotomy in women with early stage endometrial cancer. All trials included at least 70% of women with early stage disease.
Overall, the certainty of the evidence was moderate (GRADE Working Group). Although there were nine included trials, only six reported on survival data that could be pooled in a meta‐analysis using an HR.
While the review found no evidence of a difference between the two types of surgery in terms of OS and RFS and QoL was incompletely reported, it does suggest that laparoscopy may be associated with significantly fewer postoperative complications with less estimated blood loss and a lower rate of severe postoperative complications.
Walker 2012 assessed severe postoperative adverse events in 2591 women of which there were 596 events (the meta‐analysis, which included Janda 2017 and Walker 2012, assessed 3344 women and noted 675 events).
There was no statistically significant difference in any of the adverse event categories.
Quality of the evidence
Including the available evidence of the large RCT (Walker 2012), allows robust conclusions for the comparison in terms of efficacy, despite inconsistency in the survival point estimates in the smaller trials that addressed these outcomes. There is evidence to suggest that laparoscopy is a safer method of surgery than laparotomy for women with early stage endometrial cancer.
The reporting of the methodological quality of the trials showed that most trials were at moderate risk of bias while three trials were at high risk of bias as they did not satisfy any of the criteria used to assess risk of bias (Fram 2002; Tozzi 2005; Zorlu 2005).
In the six trials that reported survival, an HR was either reported explicitly or it was possible to deduce one using the methods of Parmar 1998. An HR is the best statistic to summarise the difference in risk in two treatment groups over the duration of a trial, when there is 'censoring' (i.e. the time to death (or disease recurrence) is unknown for some women as they were still alive (or disease free) at the end of the trial).
Three trials reported QoL data using a validated scale (Janda 2017; Mourits 2010; Zullo 2009), but only Zullo 2009 used an adequate follow‐up period of four years. QoL should be assessed at different time intervals and, to allow a satisfactory assessment, should continue for a reasonably long period of time.
Overall, the certainty of evidence for OS and RFS was moderate and was downgraded for unclear risk of bias profiles and imprecision in effect estimates. However, most trials used adequate methods of sequence generation and concealment of allocation so trials were not prone to selection bias. Adverse event outcomes were downgraded for the same reasons and additionally for low event rates and low power, thus these outcomes provided low certainty evidence.
Potential biases in the review process
We attempted to reduce bias in the review process by performing a comprehensive search, including a thorough search of the grey literature and ensuring that all studies were sifted and data extracted independently by two review authors. We also restricted the included studies to RCTs as they provide the strongest level of evidence available.
The greatest threat to the validity of the review was likely to be the possibility of publication bias (i.e. studies that did found the treatment ineffective may not have been published). We were unable to assess this possibility as all the treatment comparisons were restricted to meta‐analyses of up to seven trials.
Agreements and disagreements with other studies or reviews
To the best of our knowledge there have been several reviews and four meta‐analysis comparing laparoscopy and laparotomy in the management of early endometrial cancer (de la Orden 2008; He 2013; Imesch 2009; Palomba 2009). All four meta‐analyses suggested that the laparoscopic approach is an effective procedure for treating women with endometrial cancer.
de la Orden 2008: a systematic review and meta‐analysis of four randomised trials showing laparoscopy offers advantages with respect to postoperative recovery, including reduced bleeding and reduced need for analgesics. In addition, intraoperative and postoperative complications were fewer among women who underwent laparoscopic hysterectomy in all the studies. The mean hospital stay of women who underwent laparoscopy was three to four days shorter, and they returned to normal activity sooner. The number of lymph glands resected was the same with both techniques. Laparoscopy was associated with a better QoL after surgery. With respect to long term results, there were no significant differences in relation to OS, DFS or cause‐specific survival, according to one study.
He 2013: a systematic review and meta‐analysis of nine eligible RCTs (1361 in the laparotomy group and 2255 in the laparoscopy group). They found no significant difference between laparoscopy and laparotomy approaches to endometrial cancer in three‐year OS. They reported the benefits of laparoscopic surgery versus laparotomy, which were shorter length of hospital stay. Disadvantages were higher rates of intraoperative complications and longer duration of surgical procedures.
Imesch 2009: a systematic review suggesting that randomised trials demonstrate the safety, feasibility and effectiveness of laparoscopy. In addition, the impact on survival and DFS is equivalent to that of laparotomy.
Palomba 2009: a systematic review and meta‐analysis of four RCTs suggested that the laparoscopic approach is an effective procedure for treating endometrial cancer, even if limited to early stages. Notwithstanding the longer operative time, advantages of the laparoscopy over laparotomy were reduced intraoperative blood loss and postoperative complications.
Authors' conclusions
Implications for practice.
For early stage endometrioid adenocarcinoma of the endometrium laparoscopy is associated with similar overall and disease free survival.
Laparoscopy is effective and is associated with reduced operative morbidity and hospital stay.
There is no significant difference in the quality of life between treatment with laparoscopy and laparotomy.
Implications for research.
Randomised controlled trials are recommended to determine the benefits and harms of laparoscopy in the management of uterine sarcomas/carcinosarcoma.
Studies on economic evaluation and cost effectiveness of laparoscopy versus laparotomy for endometrial cancer would improve clinical decisions.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2018 | Amended | Alberto Lopes contact details amended. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 22 October 2018 | New search has been performed | Search updated 14 June 2018. |
| 22 October 2018 | New citation required but conclusions have not changed | One new study identified. Author citation revised. |
| 27 March 2014 | Amended | Contact details updated. |
Acknowledgements
We thank Jo Morrison for clinical advice and support. We thank Jane Hayes and Jo Platt for designing the search strategies and Gail Quinn and Clare Jess for their contribution to the editorial process. We also acknowledge and thank A D Fisher, M Al‐Khaduri and Fiona Kew for their contributions to earlier versions of this review.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health, UK.
Appendices
Appendix 1. 1989 FIGO Staging
Stage I
Stage I endometrial cancer is carcinoma confined to the corpus uteri.
Stage IA: tumour limited to endometrium.
Stage IB: invasion to less than 50% of the myometrium.
Stage IC: invasion to greater than 50% of the myometrium.
Stage II
Stage II endometrial cancer involves the corpus and the cervix but has not extended outside the uterus.
Stage IIA: endocervical glandular involvement only.
Stage IIB: cervical stromal invasion.
Stage III
Stage III endometrial cancer extends outside of the uterus but is confined to the true pelvis.
Stage IIIA: tumour invades serosa or adnexa or positive peritoneal cytology, or a combination of these.
Stage IIIB: vaginal metastases.
Stage IIIC: metastases to pelvic or para‐aortic lymph nodes, or both.
Stage IV
Stage IV endometrial cancer involves the bladder or bowel mucosa or has metastasised to distant sites.
Stage IVA: tumour invasion of bladder or bowel mucosa, or both.
Stage IVB: distant metastases, including intra‐abdominal or inguinal lymph nodes, or both.
Appendix 2. 2009 FIGO Staging
Stage I
Tumour confined to the corpus uteri.
Stage IA: no or less than half myometrial invasion.
Stage IB: invasion equal to or more than half of the myometrium.
Stage II
Tumour invades cervical stroma, but does not extend beyond the uterus.
Stage III
Local or regional (or both) spread of the tumour.
Stage IIIA: tumour invades the serosa of the corpus uteri or adnexae, or both.
Stage IIIB: vaginal or parametrial involvement, or both.
Stage IIIC: metastases to pelvic or para‐aortic lymph nodes, or both.
Stage IIIC1: positive pelvic nodes.
Stage IIIC2: positive para‐aortic lymph nodes with or without positive pelvic lymph node.
Stage IV
Tumour invades bladder or bowel mucosa or distant metastases, or a combination of these.
Stage IVA: tumour invasion of bladder or bowel mucosa, or both.
Stage IVB: distant metastases, including intra‐abdominal metastases or inguinal lymph nodes, or both.
Appendix 3. CENTRAL search strategy
CENTRAL 2018, Issue 5.
MeSH descriptor Endometrial Neoplasms explode all trees
(endometri* or uter*) near/5 (cancer* or carcinoma* or tumor* or tumour* or malignan* or neoplas*)
(#1 OR #2)
MeSH descriptor Laparoscopy explode all trees
laparoscop*
MeSH descriptor Laparotomy explode all trees
laparotom*
TAH or LAVH or TLH
MeSH descriptor Hysterectomy explode all trees
hysterectom*
(#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
(#3 AND #11)
Appendix 4. MEDLINE search strategy
MEDLINE (Ovid) to April 2012 to June week 2 2018.
exp Endometrial Neoplasms/
((endometri* or uter*) adj5 (cancer* or carcinoma* or tumor* or tumour* or malignan* or neoplas*)).mp.
1 or 2
Laparoscopy/
laparoscop*.mp.
Laparotomy/
laparotom*.mp.
(TAH or LAVH or TLH).mp.
exp Hysterectomy/
hysterectom*.mp.
4 or 5 or 6 or 7 or 8 or 9 or 10
3 and 11
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
13 or 14 or 15 or 16 or 17 or 18 or 19
12 and 20
key: mp=mp=title, original title, abstract, name of substance word, subject heading word, unique identifier, pt=publication type, ab=abstract, sh=subject heading, ab=abstract
Appendix 5. Embase search strategy
Embase (Ovid) April 2012 to 2018 week 24.
exp endometrium tumor/
((endometri* or uter*) adj5 (cancer* or carcinoma* or tumor* or tumour* or malignan* or neoplas*)).mp.
1 or 2
laparoscopy/
laparoscop*.mp.
laparotomy/
laparotom*.mp.
(TAH or LAVH or TLH).mp.
exp hysterectomy/
hysterectom*.mp.
4 or 5 or 6 or 7 or 8 or 9 or 10
3 and 11
crossover procedure/
double blind procedure/
randomized controlled trial/
single blind procedure/
random*.mp.
factorial*.mp.
cross over*.mp.
cross‐over*.mp.
placebo*.mp.
(doubl* adj blind*).mp.
(singl* adj blind*).mp.
assign*.mp.
allocat*.mp.
volunteer*.mp.
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
12 and 27
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 6 | 3993 | Hazard Ratio (Random, 95% CI) | 1.04 [0.86, 1.25] |
| 2 Recurrence free survival | 5 | 3710 | Hazard Ratio (Random, 95% CI) | 1.14 [0.90, 1.43] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative death | 5 | 3233 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.32, 1.79] |
| 1.1 Within 30 days | 4 | 2950 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.27, 1.71] |
| 1.2 Within 6 weeks | 1 | 283 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.16, 14.61] |
| 2 Bladder injury | 7 | 3342 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.54, 2.32] |
| 3 Urethral injury | 6 | 3463 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.60, 3.28] |
| 4 Vascular injury | 5 | 3401 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.23, 2.94] |
| 5 Bowel injury | 4 | 3070 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.75, 2.18] |
| 6 Severe postoperative adverse events | 2 | 3344 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.44, 1.38] |
| 7 Estimated blood loss (mL) | 3 | 313 | Mean Difference (IV, Random, 95% CI) | ‐106.82 [‐141.59, ‐72.06] |
| 8 Blood transfusion required | 8 | 3894 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.22, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fram 2002.
| Methods | Randomised controlled trial Study dates: July 1996 to July 1998 (24 months) |
|
| Participants | 61 women randomised 32 in laparotomy group, mean age 60.6 years, BMI 26.2, stages not documented, histology not documented (SD not documented). 29 in laparoscopy group, mean age 61.2 years, BMI 25.7, stages not documented, histology not documented (SD not documented). Inclusion criteria: stage I EC Exclusion criteria: not documented |
|
| Interventions |
Intervention: LAVH, bilateral salpingo‐oophorectomy, peritoneal washings, ± pelvic lymph node dissection (node dissection described in detail). Control: laparotomy, TAH, bilateral salpingo‐oophorectomy, peritoneal washings, ± pelvic lymph node dissection. Procedure described as "traditional approach", no further details given. Number of surgeons who performed procedures/level of experience: not documented. |
|
| Outcomes | Days of hospitalisation Duration of procedure Intraoperative bleeding Complications Conversion from laparoscopy to laparotomy |
|
| Notes | Follow‐up: period of follow‐up and loss to follow‐up rate not documented. Analysis: no comment on study power, no comment on ITT analysis. Final stage and histopathological characteristics: not documented. There was a significant difference (P < 0.05) in days of hospitalisation between the laparoscopic (group A) and laparotomy (group B) groups (2.3 vs 5.5 days). Laparoscopy was associated with longer operating time (136.2 min vs 101.9 min) (P < 0.05). There was no significant difference in the number of lymph nodes obtained in both groups of participants who required pelvic lymphadenectomy, neither on each side alone nor in total (21.3 in laparoscopy group vs. 21.9 in laparotomy group), and it was noted that the number of lymph nodes obtained from the right sides of participants were more than those obtained from the left sides in both groups (11.9 in laparoscopy group vs 12.2 in laparotomy group on the right side, and 9.4 in laparoscopy group vs 9.7 in laparotomy group on the left side). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Concerns regarding this study being a randomised controlled trial, despite the use of the phrase "the patients were randomly allocated into two groups", as there was little documentation regarding method of randomisation and study methodology. The phrase "there were 32 patients in group B who were matched as a control group" implies a case controlled study. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No documentation regarding completeness of data. No information on attrition. No comment on cases included for analysis. |
| Selective reporting (reporting bias) | Unclear risk | insufficient information to permit judgement. |
| Other bias | Unclear risk | insufficient information to assess whether additional form of bias may have been present. |
Janda 2017.
| Methods | Multicentre randomised controlled trial with 2:1 intervention:control Study dates: December 2006 to January 2009 |
|
| Participants | 332 participants included in QoL component. 142 in TAH group, mean age 62.7 years (SD 9.7), 49 (34.5%) had BMI ≥ 35, all had PS 0–1. 190 in TLH group, mean age 62.8 years (SD 10), 72 (38%) had BMI ≥ 35, all had PS 0–1. Inclusion criteria: histologically confirmed primary endometrioid adenocarcinoma of the endometrium, clinical stage I disease. Women aged ≥ 18 years with PS ECOG 0/1. Surgeons: qualified gynaecological oncologists, with evidence of a minimal number of 20 supervised and documented TLHs performed as the main surgeon. "TLHs will also be video recorded with regular random audits conducted by the trial safety committee". Exclusion criteria: histological cell type other than endometrioid; history of previous or concomitant cancer; clinically advanced disease (stages II/IV); uterine size > 10 weeks' gestation or uterine size > 8 cm transverse diameter on ultrasound; estimated life expectancy < 6 months; enlarged aortic lymph nodes; unfit for surgery due to serious concomitant systemic disorders incompatible with study; unable to complete QoL measures; live too far from a treatment centre to allow adequate follow‐up. |
|
| Interventions |
Intervention: TLH, bilateral salpingo‐oophorectomy, peritoneal washings, ± pelvic lymph node dissection ± para‐aortic lymph node dissection. 190 women. Control: laparotomy, vertical midline skin incision, TAH, bilateral salpingo‐oophorectomy, peritoneal washings, ± pelvic lymph node dissection ± para‐aortic lymph node dissection. 142 women. |
|
| Outcomes | DFS Hospital stay Postoperative pain QoL Treatment related morbidity: "as evaluated by intraoperative complications; perioperative complications, pulmonary, renal and cerebrovascular morbidity; wound and vault complications (infection, breakdown, and dehiscence); septicaemia and thromboembolic complications (DVT, PE); lymphocyst or abscess formation; early postoperative (< 4 weeks) complications; long‐term (4 weeks to 6 months after surgery) morbidity (lymphoedema, incisional hernia formation); estimated blood loss (haemoglobin change from baseline); postoperative pain and analgesic consumption. Other outcomes were also assessed, such as participant perception of body image; costs and cost‐effectiveness of treatments; patterns of recurrence and overall survival". |
|
| Notes | Protocols for pelvic or par‐aortic lymphadenectomy, or both: per clinical practice guidelines for each institution, to document preoperatively if women considered unfit for pelvic and para‐aortic lymph node dissection. Protocols for adjuvant therapy: per institutional guidelines. Follow‐up: on‐going to determine disease specific outcomes. Analysis on ITT principles. TLH associated with equivalent or improved QoL at 6 months when compared to TAH. In early recovery (up to 4 weeks after surgery), participants in TLH group had significantly greater improvements in most QoL measurements than participants in TAH group. Greatest differences noted in FWB (13% greater improvement for participants in TLH group), PWB (11%), EnWB (6%) and the overall FACT‐G summary score (7%; P = 0.001 for all comparisons). Participants in TLH group reported a 5% (P = 0.001) greater improvement in body image and 7.5% (P = 0.001) greater improvement in overall QoL (EuroQoL‐VAS) than participants in TAH group. During early recovery, neither EWB (P = 0.39) nor SWB (P = 0.59) differed significantly between participants assigned to TAH or TLH. During late postoperative recovery phase (3–6 months after surgery), participants in TLH group recovered significantly more in their physical (P = 0.008), functional (P = 0.009), EC specific (P = 0.003) and overall well‐being (FACT‐G; P = 0.03), and also had better QoL recovery with regard to body image (P = 0.001). Significantly higher proportion of participants in TAH group stayed in hospital for > 2 days (139/142 in TAH group vs 72/190 in TLH group; P < 0.0001). In addition, participants in TLH group had less postoperative pain and reduced analgesic consumption compared with participants in TAH group. TLH was associated with equivalent DFS when compared with TAH for women with stage I EC. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via computer generated, web based system using stratified permuted blocks of 3 and 6 participants. |
| Allocation concealment (selection bias) | Low risk | Randomisation centrally and independent from other study procedures via a computer generated, web based system (providing concealment of the next assigned treatment). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 332/332 (100%) participants analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias was present. |
Lu 2013.
| Methods | Prospective randomised trial comparing laparoscopy with laparotomy Study dates: January 2000 to December 2010 All records were kept prospectively in a computerised database. Randomisation performed using a central managed random number table. All participants entered into study had their initial pathological diagnosis confirmed. |
|
| Participants | 318 consecutive women referred to Beijing Chao‐Yang Hospital affiliated Capital Medical University for treatment for histologically confirmed EC. 272 women met the inclusion criteria. 151 women in laparoscopy group, median age 56.6 years (range 27–82). 121 women in laparotomy group, median age 57.2 (range 29–79). Exclusion criteria: ovarian lesions, contraindications for general anaesthesia, systemic infections, bulky uterus C12 weeks' size and documented significant cardiopulmonary disease. |
|
| Interventions |
Intervention: laparoscopy Control: laparotomy All participants received pelvic lymphadenectomy and para‐aortic lymph nodes sampling. Para‐aortic lymphadenectomy up to the level of the inferior mesenteric artery was performed in participants with positive pelvic lymph node discovered at frozen section evaluation and non‐endometrioid carcinomas. |
|
| Outcomes | Long term recurrence rate OS: calculated from date of EC diagnosis to death from any cause. Recurrences were classified by site of first recurrence. |
|
| Notes | All participants who underwent laparoscopic approach were informed that conversion from laparoscopy to laparotomy was expected in case of intraoperative complications or other difficulties. All participants and their spouses were comprehensively counselled about the benefits and potential risk of each approach. Staging of participants done according to FIGO 1988 staging system. The median operating time was 211 min (range 100–460) in the laparoscopy group and 261 min (range 90–570) in the laparotomy group (P < 0.01). Median blood loss 86 mL in laparoscopy group and 419 mL in laparotomy group (P < 0.05). Median length of hospital stay 3 days in laparoscopy group and 6 days in laparotomy group (P < 0.05). Pelvic lymphadenectomy performed in all participants and para‐aortic lymphadenectomy in 15% of laparoscopy group and 31.4% of laparotomy group (P < 0.05). 7 (4.6%) participants in laparoscopy group had recurrence vs 6 (5.0%) participants in laparotomy group. Overall, 21 (7.7%) participants died, 9 (6%) in laparoscopy group and 12 (9.9%) in laparotomy group. 15/ 21 participants died from intercurrent disease, 6 in laparoscopy group and 9 in laparotomy group. 6 participants died from EC: 3 in each group. TLH: OS 94%, 5‐year survival rate 96%; TAH: OS 90.1%, TAH 91% (P > 0.05). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by central managed random number table. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias was present. |
Malzoni 2009.
| Methods | Randomised controlled trial, computer generated lists Study dates: November 2001 to January 2006 (50 months) |
|
| Participants | 159 women randomised 78 women in laparotomy group, mean age 63 years (SD 14; 95% CI 43 to 84), mean BMI 29 (SD 7.3; 95% CI 17 to 39), stage I/IIA disease 68/78 (87%). 81 women in laparoscopy group, mean age 60 years (SD 11) (95% CI 39 to 81), mean BMI 28 (SD 6.9; 95% CI 19 to 37), stage I/IIA disease 75/81 (92.5%). Inclusion criteria: clinical stage I EC Exclusion criteria: ovarian lesions; metastases beyond uterus; contraindication for general anaesthesia; systemic infections; abnormal Papanicolau smear; bulky uterus ≥ 12 weeks' size; where vaginal removal of uterus may require morcellation; significant cardiopulmonary disease which would be a contraindication for prolonged Trendelenburg position; severe hip disease precluding use of dorsolithotomy position; inadequate bone marrow, renal and hepatic function; BMI ≥ 40; aged ≥ 80 years; treatment with prior pelvic radiotherapy or chemotherapy (or both); no available follow‐up information. |
|
| Interventions |
Intervention: total LPS, bilateral salpingo‐oophorectomy, peritoneal washings, + pelvic lymph node dissection, ± para‐aortic lymph node dissection. Control: total LPT, bilateral salpingo‐oophorectomy, peritoneal washings, + pelvic lymph node dissection, ± para‐aortic lymph node dissection. For all cases of papillary serous or clear cell tumours and adenosquamous carcinomas, infracolic omentectomy performed. |
|
| Outcomes |
Primary outcomes: OS DFS Secondary outcomes: Operative time Blood loss Hospital stay |
|
| Notes | Follow‐up duration: 38.5 months (2–81 months). Protocol: visits monthly for first 3 months, every 3 months for 2 years, 6 monthly for 3 years and yearly thereafter. 61% of participants received adjuvant therapy. Total specified but no breakdown between 2 groups, reported as a difference. Adjuvant therapy: 98/159 (61%); brachytherapy only: 45/159 (28%); radiotherapy + brachytherapy: 41/159 (21%); chemotherapy + radiotherapy: 12/159 (7%). Randomisation: computer generated list. Analysis: no comment on study power, no comment on ITT analysis. 12 (7.5%) participants died, 5 (6.1%) in LPS group and 7 (8.9%) in LPT group. 3/10 participants died from intercurrent disease, 1 in LPS group and 2 in LPT group. 9 (4.4%) participants died from EC: 4 (4.9%) in LPS group and 5 (6.4%) in LPT group. 91.4% participants free of disease in LPS group vs 88.5% in LPT group. No significant difference in recurrence rate (P > 0.05). Mean operative time: 136 min (SD 31; 95% CI 118 to 181) in LPS group and 123 min (SD 29; 95% CI 111 to 198) in LPT group (P < 0.01). Mean blood loss: 50 mL (SD 12; 95% CI 20 to 90) in LPS group and 145 mL (SD 35; 95% CI 60 to 255) in LPT group (P < 0.01). Mean length of hospital stay: 5.1 (SD 1.2; 95% CI 1 to 7) in LPT group and 2.1 (SD 0.5; 95% CI 1 to 5) in LPS group (P < 0.01). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Yes, computer generated randomisation list, drawn up by a statistician. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 159/159 (100%) participants analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias was present. |
Mourits 2010.
| Methods | Multicentre randomised controlled trial with 2:1 intervention:control randomisation Study dates: 1 February 2007 to 15 January 2009 |
|
| Participants | 283 women assessed for eligibility, 4 excluded as did not fulfil inclusion criteria: 2 in each group. 94 in laparotomy group, mean age 63 (range 39–86), mean BMI 28 (range 19–48), stage I disease 75/185 (87.2%). 185 in laparoscopy group, mean age 62 (range 40–89), mean BMI 29 (range 17–55), stage I disease 130/185 (87.2%). Inclusion criteria: participants: histologically confirmed grade I–II EC or complex atypical hyperplasia, clinically confined to the uterine corpus (i.e. clinical stage I), aged > 18 years, prior to study entry "an endocervical curettage is performed to make sure that the participant has clinical stage I disease and the cervix is not involved". Exclusion criteria: non‐endometrioid adenocarcinoma histological types, uterine size larger than that expected at 12 weeks' pregnancy (original protocol stated uterine size > 10 weeks' gestation), cardiopulmonary contraindications for laparoscopy or laparotomy, stage II–IV disease. |
|
| Interventions |
Intervention: TLH, bilateral salpingo‐oophorectomy, peritoneal washings. All TLH procedures were done by 24 certified surgeons who achieved sufficient laparoscopic skills for performing a TLH. Control: laparotomy, TAH, bilateral salpingo‐oophorectomy, peritoneal washings. TAH procedures were done by fully trained, established, gynaecological surgeons; any 1 of the 24 certified surgeons or a colleague. |
|
| Outcomes |
Primary outcome: Major complication rate Secondary outcomes: Minor complications Treatment‐related outcomes QoL: SF‐36, SAQ, Body Image Scale and VAS at baseline, 6 weeks, 3 and 6 months postsurgery |
|
| Notes | Questionnaire regarding: QoL SF‐36. The SAQ "was completed only by patients that had been sexually active in the month before receiving the questionnaire". Follow‐up: outcomes assessed "Pre‐operatively, after 6 weeks, and after 3 and 6 months". No lymphadenectomy performed. Adjuvant radiotherapy: 38 (20.0%) in laparoscopy group, 25 (26.6%) in laparotomy group. 2 centres did not comply with randomisation procedure and were banned from participation shortly after start of study. These centres assumed an advantage for laparoscopy and intended to first offer laparoscopy and subsequently randomise women who had no preference. 1 of these centres had not yet randomised women. The other centre randomised 4 women before beginning the selective randomisation; therefore, these women were not excluded from the study. According to protocol, uterine size > 10 weeks' gestation was a contraindication, but trial included uterine size > 12 weeks' gestation. Conversion to laparotomy occurred in 20/185 (10.8%) of laparoscopic procedures. TLH was associated with significantly less blood loss (P < 0.0001), less use of pain medication (P < 0.0001), shorter hospital stay (P < 0.0001) and faster recovery (P = 0.002), but procedure took longer than TAH (P < 0.0001). Participants who had laparoscopy scored significantly higher on the physical functioning sub scale of the SF‐36 at 6 weeks, and on the role‐physical sub scale at 3 months after the procedure. Participants who had laparotomy scored significantly higher on the vitality sub scale of the mental dimension 3 months after surgery. No differences for the other subscales. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by sequential number generation done centrally in alternate blocks of 6 and 3 participants, with stratification by trial centre. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 283/283 (100%) participants analysed Loss to follow‐up: documented. "All adverse events are followed until they have abated, or until a stable situation has been reached". Missing data: documented and addressed. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement, although according to protocol, secondary outcome measures were costs and cost‐effectiveness and included additional home care, but these were not reported. |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias was present. |
Tozzi 2005.
| Methods | Randomised controlled trial Study dates: July 1995 to December 2002 |
|
| Participants | 122 women randomised 63 women in laparoscopy group, mean age 67 years (range 35–88), mean BMI 31.3 (range 20.2–43.6), stage I disease "similar in 2 groups" (initial numbers quoted for 31/37 (83.7%) women in 2000 paper), endometrioid histology 52 (82.5%). 59 women in laparotomy group, mean age 66 (range 36–89), mean BMI 32.1 (range 20–51.3), stage I disease "similar in 2 groups" (initial numbers quoted for 28/33 (84.8%) women in 2000 paper), endometrioid histology 52 (88.1%). Inclusion criteria: histologically confirmed EC. Exclusion criteria: uterine size > 8 cm transverse diameter on sonography. |
|
| Interventions |
Intervention: LAVH, bilateral salpingo‐oophorectomy, peritoneal washings, ± pelvic lymph node dissection, ± para‐aortic lymph node dissection. Control: laparotomy, TAH, bilateral salpingo‐oophorectomy, peritoneal washings, ± pelvic lymph node dissection, ± para‐aortic lymph node dissection. Decision for lymph node dissection based on intraoperative frozen section of the uterus: FIGO > 1 or G3, FIGO IBG2 or IC: proceed to frozen section ± pelvic lymph node dissection, and if frozen section of pelvic nodes positive, proceed to para‐aortic lymph node dissection. Stage III and IV disease: debulking by laparotomy. Histological involvement of cervix at D&C ("occult stage II"): type II radical hysterectomy. |
|
| Outcomes |
Primary outcomes: DFS (87.4% in laparoscopy group vs 91.6% in laparotomy group). OS (82.7% in laparoscopy group vs 86.5% in laparotomy group). Cause‐specific survival (90.5% in laparoscopy group vs 94.9% in laparotomy group). Overall complication rates, blood loss (mean blood loss, difference haemoglobin, number transfusions), duration of hospital stay, postoperative infusion, return of bowel activity. |
|
| Notes | Median follow‐up 44 months (5–96 months), total loss to follow‐up rate documented; however, distribution of cases unclear. Equal distribution of final stage and histopathological characteristics in both groups. Bilateral tubal coagulation at start of procedure, no uterine manipulator used. DFS: 87.4% in laparoscopy group vs 91.6% in laparotomy group. OS: 82.7% in laparoscopy group vs 86.5% in laparotomy group. CSS: 90.5% in laparoscopy group vs 94.9% in laparotomy group. Overall complications: 12/122 (9.8%) participants experienced intraoperative, 43/122 (35.2%) participants experienced early postoperative and 25/122 (20.4%) participants experienced late postoperative complications. Intraoperative complication rate: overall: 3/63 (4.7%) in laparoscopy group vs 9/59 (15.2%) in laparotomy group; P = 0.082. Early postoperative complication rate: 15/63 (23.8%) in laparoscopy group vs 28/59 (47.4%) in laparotomy group; P = 0.011. Late postoperative complication rate: 5/63 (7.9%) in laparoscopy group vs 21/59 (35.5%) in laparotomy group; P = 0.001. 3 publications on the same study population. Malur 2001 detailed immediate operative and postoperative data on initial 70 women (37 in laparoscopy group vs 33 in laparotomy group) with shorter follow‐up. 2Tozzi 2005 publications detailed long term follow‐up of final study population of 122 women (63 in laparoscopy group vs 59 in laparotomy group). Both 2005 papers reported outcomes with ranges, and Malur 2001 reported data with SDs. Data with SDs have been used where available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central managed random number table. |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | Definitions varied between 3 papers of the same trial. Immediate postoperative complications were variously described as "until day 7" or "<15 days", with "longer‐term" duration not defined or late postoperative defined as "15 days for 6 months". "Major complication" not clearly defined: one 2005 paper (Journal Minimally Invasive Gynecology) cited "one major complication occurred in each group", while the other 2005 paper (Gynaecological Oncology) cited "intraoperative complications as 1 ureteric, 1 bowel, 2 nerve and 1 vaginal injuries with 9 blood losses documented as >1500ml". Number of women receiving adjuvant treatment reported as "seventy‐eight (63.9%) of 122 patients and "seventy‐six out of 122 patients (61.4%)". |
| Other bias | Unclear risk | Insufficient information to assess any whether additional risk of bias was present. |
Walker 2012.
| Methods | Multicentre randomised controlled trial with 2:1 intervention:control randomisation Study dates: May 1996 to September 2005 (112 months) |
|
| Participants | 2616 women randomised, 1696 in laparoscopy group, 920 in laparotomy group. 1630 women included in analysis in laparoscopy group: median age 63 (IQR 55–‐72), median BMI 28 (IQR 24–34), stage I/IIA disease 1287/1630 (76.5%). 886 women included in analysis in laparotomy group: median age 63 (IQR 55–71), median BMI 29 (IQR 24–34), stage I/IIA disease 700/886 (77%). Inclusion criteria: stage I to IIA uterine cancer (FIGO 1988); adenocarcinoma or uterine sarcoma; aged > 18 years; no contraindication to laparoscopy; no clinical or chest X‐ray evidence of metastases beyond the uterus or macroscopic involvement of cervix; PS GOG 0–3; white cell count > 3000/mm³; platelet count > 100 000/mm³; bilirubin ≤ 1.5 times normal; SGOT ≤ 3 times normal; creatinine ≤ 2 mg/dL; prior malignancy allowed if no current evidence of disease. Exclusion criteria: prior pelvic or abdominal radiotherapy; no prior retroperitoneal surgery; pregnancy. |
|
| Interventions |
Intervention: LPS included laparoscopic assisted techniques, total laparoscopic approaches, and rarely robotics. Washings, extrafascial hysterectomy and bilateral salpingo‐oophorectomy, + pelvic lymph node sampling + para‐aortic lymph node sampling. Control: laparotomy, washings, extrafascial hysterectomy and bilateral salpingo‐oophorectomy, + pelvic lymph node sampling + para‐aortic lymph node sampling. |
|
| Outcomes | OS and recurrence free survival Severe postoperative adverse events Intraoperative complications Operative time Hospitalisation > 2 days |
|
| Notes | Conversion from laparoscopy to laparotomy was secondary to poor visibility in 246 (14.6%) participants, metastatic cancer in 69 (4.1%) participants, bleeding in 49 (2.9%) participants and other cause in 70 (4.2%) participants. Original protocol Primary outcomes: conversion to laparotomy rate, operative time, duration of hospital stay, blood transfusion, death in 6 weeks, readmission, reoperation rate, postoperative complications for 6 weeks postoperative, pathological stage by nodal status, pathological stage by pelvic washing, node positivity rate, first site of recurrence after 5 years, progression free survival every 3 months for 2 years, and every 6 months for 5 years. Secondary outcomes: QoL assessed by FACT‐G; body image; sexual function; SF‐36; BPI: personal appearance; return to work before surgery; and 1 week, 6 weeks and 1 year. QoL measures: FACT‐G; additional treatment related symptoms (AP); resumption of normal activities; BPI; fear of recurrence; body image; return to work at baseline, 1 week, 3 weeks, 6 weeks and 6 months postsurgery. Amended protocol Primary outcomes: recurrence free survival Protocol amendment: trial was originally designed to accrue 800 participants over 3‐year period to evaluate surgical complications, adverse events, length of hospital stay and improving QoL. In 2001, protocol was amended, and sample size increased to 2550 to assess whether laparoscopy could be considered not inferior to open laparotomy with regard to recurrence free survival. Secondary outcomes: perioperative adverse events, laparoscopy conversion to laparotomy, length of hospital stay after surgery, operative time, QoL, sites of recurrence, survival. Reported to date: 2009 6‐week morbidity and mortality, hospital length of stay, conversion from laparoscopy to laparotomy, recurrence free survival, site of recurrence and participant reported QoL outcomes. Follow‐up: protocol: 6 weeks, every 3 months for 2 years, every 6 months for 3 years Drop‐out rate: not documented Final stage and histopathological characteristics: "Final FIGO staging results were the same by the randomisation arm". Not yet reported: survival and long‐term outcome Laparoscopy had fewer moderate to severe postoperative adverse events than laparotomy (14% in laparoscopy group vs 21% in laparotomy group; P < 0.0001) but similar rates of intraoperative complications, despite having a significantly longer operative time (median: 204 min in laparoscopy group vs 130 min in laparotomy group; P < 0.001). Hospitalisation > 2 days was significantly lower with laparoscopy (52% in laparoscopy group vs 94% in laparotomy group; P < 0.0001). Pelvic and para‐aortic nodes were not removed in 8% of participants in laparoscopy group and 4% of participants in laparotomy group (P < 0.0001). No difference in overall detection of advanced stage (stage IIIA, IIIC or IVB) (17% in laparoscopy group vs 17% in laparotomy group; P < 0.841). Median follow‐up time of 59.3 months for both groups, 350 participants (13.5%) died (229 (13.6%) in laparoscopy group vs 121 (13.3%) in laparotomy group), of which 224 deaths resulted from disease (152 in laparoscopy group vs 72 in laparotomy group). 309 (11.9%) participants had recurrent disease (210 (12.5%) in laparoscopy group vs 99 (10.9%) in laparotomy group). Hazard ratio for recurrence free survival for laparoscopy relative to laparotomy: 1.14. Sites of recurrence were similar between groups (P = 0.470). Postoperative adjuvant therapy was similar between groups (P = 0.607). Estimated 5‐year OS: 89.9% in laparoscopy group vs 89.9% in laparotomy group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment using permuted block design such that approximately twice as many registered participants underwent laparoscopy compared with laparotomy. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded (surgery refused or contraindicated): 11 in laparoscopy group, 14 in laparotomy group. Used for surgical analysis: 1682 in laparoscopy group, 909 in laparotomy group. Underwent assigned procedure: 1682 in laparoscopy group, 901 in laparotomy group. Excluded following pathology review: 52 in laparoscopy group, 23 in laparotomy group. Participants included in analysis of pathology: 1630 in laparoscopy group, 886 in laparotomy group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. Results of long‐term follow‐up awaited. |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias was present. |
Zorlu 2005.
| Methods | Randomised controlled trial Study dates: 1998–2002 |
|
| Participants | 52 women with early stage EC. 26 women in laparoscopy group: mean age 56.6 years (range 40 to 72), BMI 24.4. 26 women in laparotomy group: mean age 54.9 years (range 36 to77), BMI 26.2. Exclusion criteria: clinically advanced disease |
|
| Interventions |
Intervention: laparoscopy Control: laparotomy |
|
| Outcomes | Length of hospital stay Operative time Wound complications Hospital stay 8 units of red blood cell suspension were transfused to participants in laparotomy group and 6 units to participants in laparoscopy group. |
|
| Notes | 11 (42.3%) participants in laparoscopy group and 10 (38.5%) participants in laparotomy group were later scheduled for adjuvant radiotherapy. Length of hospitalisation: significantly shorter in laparoscopy group (4.1 days in laparoscopy group vs 8.2 days in laparotomy group; z = 1.96; P < 0.05). Operative time: similar between groups (155 min in laparoscopy group vs 144 min in laparotomy group; P = 0.05). Wound complications: occurred in 5 in participants in laparotomy group, of which 1 was evisceration and needed reoperation for closure. 8 units of red blood cell suspension transfused to participants in laparotomy group vs 6 units to participants in laparoscopy group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated that participants were randomly assigned to surgical approach. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No documentation |
| Selective reporting (reporting bias) | Unclear risk | No documentation |
| Other bias | Unclear risk | No documentation |
Zullo 2009.
| Methods | Multicentre randomised controlled trial Study dates: March 2001 to December 2003 (33 months) |
|
| Participants | 84 women randomised 42 in laparoscopy group, mean age 62.1 years (SD 14.5), BMI 29.9 (SD 7.5). 42 in laparotomy group, mean age 61.5 years (SD 13.3), BMI31.8 (SD 8.5). Inclusion criteria: early stage EC. Exclusion criteria: advanced stage EC; other pre malignancies or malignancies; major medical conditions; psychiatric disorders; current or history of acute or chronic physical illness; premenstrual syndrome; current or past (within 6 months from study enrolment) use of drugs influencing cognition. |
|
| Interventions |
Intervention: LAVH, bilateral salpingo‐oophorectomy, peritoneal washings, + pelvic lymph node dissection, ± para‐aortic lymph node dissection, "uterus cannulated with minimal manipulation". Control: laparotomy, vertical midline skin incision, TAH, bilateral salpingo‐oophorectomy, peritoneal washings, pelvic lymph node dissection, ± para‐aortic lymph node dissection. Decision for para‐aortic lymph node dissection based on intraoperative frozen section of pelvic nodes and if frozen section positive, proceed to para‐aortic lymph node dissection. For serous papillary carcinoma, proceeded to omentectomy and appendicectomy. Surgeons: 1 surgeon performed all procedures. |
|
| Outcomes |
Primary outcomes: OS DFS Secondary outcomes: Moderate and major adverse events including intraoperative blood loss Postoperative pain Hospital stay QoL measures: SF‐36 and Kupperman Index assessed at baseline; 1, 3 and 6 months postsurgery SF‐36 scores annually for 7 years Follow‐up: every 3 months for 2 years, every 6 months for 3 years, yearly thereafter; vaginal and abdominal examination, ultrasound, bloods for haematology, CA125 if initially elevated, assessed QoL and climacteric symptoms with Italian Short‐Form Health Survey SF‐36 and Kupperman Index at each visit, vaginal smears every 6 months for first 2 years and yearly thereafter, chest X‐ray yearly. |
|
| Notes | Duration of follow‐up: 78 months (range 19–84), loss to follow‐up documented for entire study for intervention vs control: non‐attenders after 3 months (2 in laparoscopy group vs 4 in laparotomy group), non‐compliance with protocol following surgery documented (3 in laparoscopy group vs 2 in laparotomy group). Definition of disease failure: locoregional (vaginal or pelvic recurrence, or both) or distant (para‐aortic node metastases, abdominal, liver, lung, bone and diffuse metastatic disease). Follow‐up data: 7 years available. Women who had not participated in the first follow‐up visit after randomisation were excluded from the final analysis. Postoperative complications: within 30 days from surgery. Adjuvant therapy: "Patients with advanced stage surgical cancer (stage I) were treated according Network 2008 guidelines. Combined chemotherapy/brachytherapy regimen was prescribed for patients with aggressive non endometrioid cancers (i.e., uterine papillary serous carcinoma and uterine clear cell carcinoma)". Pelvic radiotherapy: 46 Gy, with 2 Gy daily fractions for 5 days per week. Brachytherapy: 22 Gy, with 5.5 Gy, twice weekly, 4 doses. Chemotherapy: 6 cycles of paclitaxel and cisplatin/carboplatin or doxorubicin. Adjuvant treatment received: 23 (57.5%) in laparoscopy group,19 (50%) in laparotomy group; P = 0.507. No differences in percentage of moderate/major adverse events were recorded throughout the study (4 (10.0%) participants in laparoscopy group vs 6 (15.7%) participants in laparotomy group; P = 0.445). Number of lymph nodules were similar between groups (pelvic: 11.5 (SD 4.6) in laparoscopy group vs 10.7 (SD 5.5) in laparotomy group: para‐aortic: 5.8 (SD 4.2) in laparoscopy group vs 4.9 (SD 3.9) in laparotomy group). Postoperative pain: no difference between groups during first 48 hours from surgery, whereas at hospital discharge VAS score was significantly lower in laparoscopic group (P < 0.05). Mean hospital stay and length time needed to return to full activity or work (or both) were significantly (P < 0.05) lower in laparoscopy group (hospital stay: 3.0 (SD 1.4) in laparoscopy group vs 6.9 (SD 2.6) in laparotomy group); time needed to return to full activity or work: 28.2 (SD 12.8) in laparoscopy group vs 47.8 (SD 24.7) in laparotomy group). Missing data: women who missed first follow‐up excluded from final analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Single blocks, computer generated lists. |
| Allocation concealment (selection bias) | Low risk | Yes. All eligible participants were assigned randomly in single blocks in 2 surgical treatment groups with the use of a computer generated randomisation list. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 78/84 (93%) participants analysed Participants who had not participated in the first follow‐up visit after randomisation were excluded from final analysis. |
| Selective reporting (reporting bias) | Low risk | All data analysed by ITT method on basis of treatment assignment and not on treatment receipt; only those participants who had not participated in the first follow‐up visit after randomisation were excluded from final analysis. |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias was present. |
AP: BMI: body mass index; BPI: Brief Pain Inventory; D&C: dilation and curettage; DFS: disease free survival; DVT: deep vein thrombosis; EC: endometrial cancer; ECOG: Eastern Cooperative Oncology Group; EnWB; EWB: ; FACT‐G: Functional Assessment of Cancer Therapy scale – General; FIGO: International Federation of Gynaecology and Obstetrics; FWB: ; GOG: Gynecologic Oncology Group; IQR: interquartile range; ITT: intention to treat; LAVH: laparoscopically assisted vaginal hysterectomy; LPS: laparoscopic hysterectomy; LPT: abdominal hysterectomy; min: minute; OS: overall survival; PE: pulmonary embolism; PS: performance status; PWB: ; QoL: quality of life; SAQ: Sexual Activity Questionnaire; SD: standard deviation; SF‐36: 36‐item Short‐Form Healthy Survey; SGOT: serum glutamic oxaloacetic transaminase (enzyme); SWB: ; TAH: total abdominal hysterectomy; TLH: total laparoscopic hysterectomy; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| de la Orden 2008 | Not a randomised trial. |
| Ghezzi 2006 | Laparoscopic assisted vaginal hysterectomy vs total laparoscopic hysterectomy. No laparotomy comparison arm, compared 2 laparoscopic procedures. |
| Ghezzi 2010 | Not a randomised controlled trial. |
| Imesch 2009 | A systematic review article, not a randomised controlled trial. |
| Ju 2009 | Not a randomised controlled trial. |
| Lin 2007 | Not a randomised controlled trial. |
| Liu 2009 | Not a randomised controlled trial. |
| Palomba 2009 | A systematic review article, not a randomised controlled trial. An updated version of this review was also identified and subsequently excluded. |
Contributions of authors
KG: is the main author. She developed the protocol, reviewed the papers and wrote the review. HD: sifted the top up search results, and drafted the updated results section of the review. AB: drafted the methodological and statistical sections of the review. AL: provided clinical expertise and designed the protocol and drafted the clinical sections of the review.
Sources of support
Internal sources
Northern Gynaecology Oncology Centre, Queen Elizabeth Hospital, Gateshead, UK.
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Declarations of interest
KG: none known. HD: none known. AB: none known. AL: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Fram 2002 {published data only}
- Fram KM. Laparoscopically assisted vaginal hysterectomy versus abdominal hysterectomy in stage I endometrial cancer. International Journal of Gynecological Cancer 2002;12(1):57‐61. [MEDLINE: ; 1048‐891X] [DOI] [PubMed] [Google Scholar]
Janda 2017 {published data only}
- Janda M, Gebski V, Brand A, Hogg R, Jobling TW, Land R, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncology 2010;11(8):772‐80. [DOI: 10.1016/S1470-2045(10)70145-5] [DOI] [PubMed] [Google Scholar]
- Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease‐free survival among women with stage I endometrial cancer: a randomized clinical trial. JAMA 2017;317(12):1224‐33. [DOI] [PubMed] [Google Scholar]
- Janda M, Gebski V, Forder P, Jackson D, Williams G, Obermair A, LACE Trial Committee. Total laparoscopic versus open surgery for stage 1 endometrial cancer: the LACE randomized controlled trial. Contemporary Clinical Trials 2006;27(4):353‐63. [Clinical Trials identifier: NCT00096408; DOI: 10.1016/j.cct.2006.03.004; PUBMED: 16678497] [DOI] [PubMed] [Google Scholar]
- Kondalsamy‐Chennakesavan S, Janda M, Gebski V, Baker J, Brand A, Hogg R, et al. Risk factors to predict the incidence of surgical adverse events following open or laparoscopic surgery for apparent early stage endometrial cancer: results from a randomised controlled trial. European Journal of Cancer 2012;48(14):2155‐62. [DOI] [PubMed] [Google Scholar]
- Obermair A, Janda M, Baker J, Kondalsamy‐Chennakesavan S, Brand A, Hogg R, et al. Improved surgical safety after laparoscopic compared to open surgery for apparent early stage endometrial cancer: results from a randomised controlled trial. European Journal of Cancer 2012;48(8):1147‐53. [DOI] [PubMed] [Google Scholar]
Lu 2013 {published data only}
- Lu Q, Liu H, Liu C, Wang S, Li S, Guo S, et al. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: a prospective randomized study with 11‐year experience. Journal of Cancer Research and Clinical Oncology 2013;139:1853‐9. [DOI] [PubMed] [Google Scholar]
Malzoni 2009 {published data only}
- Malzoni M, Tinelli R, Consentino F, Perone C, Rasile M, Iuzzolino D, et al. Total laparoscopic hysterectomy vs. abdominal hysterectomy with lymphadenectomy for early stage endometrial cancer: a prospective randomized study. Gynecologic Oncology 2009;112:126‐33. [DOI] [PubMed] [Google Scholar]
Mourits 2010 {published data only}
- Bijen CB, Briët JM, Bock GH, Arts HJ, Bergsma‐Kadijk JA, Mourits MJ. Total laparoscopic hysterectomy versus abdominal hysterectomy in the treatment of patients with early stage endometrial cancer: a randomized multi center study. BMC Cancer 2009;9(1):23. [Dutch Trial Registry: NTR821] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourits MJ, Bijen CB, Arts HJ, Ter Brugge HG, Sidje R, Paulsen L, et al. Safety of laparoscopy versus laparotomy in early‐stage endometrial cancer: a randomised trial. Lancet Oncology 2010;11(8):763‐71. [DOI: 10.1016/S1470-2045(10)70143-1; Dutch Trial Registry: NTR821] [DOI] [PubMed] [Google Scholar]
Tozzi 2005 {published data only}
- Malur S, Possover M, Michels W, Schneider A. Laparoscopic‐assisted vaginal versus abdominal surgery in patients with endometrial cancer‐a prospective randomized trial. Gynecologic Oncology 2001;80(2):239‐44. [DOI] [PubMed] [Google Scholar]
- Tozzi R, Malur S, Koehler C, Schneider A. Analysis of morbidity in patients with endometrial cancer: is there a commitment to offer laparoscopy?. Gynecologic Oncology 2005;97(1):4‐9. [DOI] [PubMed] [Google Scholar]
- Tozzi R, Malur S, Koehler C, Schneider A. Laparoscopy versus laparotomy in endometrial cancer: first analysis of survival of a randomized prospective study. Journal of Minimally Invasive Gynecology 2005;12(2):130‐6. [DOI] [PubMed] [Google Scholar]
Walker 2012 {published data only}
- Kornblith AB, Huang HQ, Walker JL, Spirtos NM, Rotmensch J, Cella D. Quality of life of patients with endometrial cancer undergoing laparoscopic International Federation of Gynecology and Obstetrics staging compared with laparotomy: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2009;27(32):5337‐42. [DOI: 10.1200/JCO.2009.22.3529] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Piedmonte M, Spirtos N, Eisenkop S, Schlaerth J, Mannel RS, et al. Surgical staging of uterine cancer: randomized phase III trial of laparoscopy vs laparotomy – a Gyneclogic Oncology Group Study (GOG): preliminary results. Journal of Clinical Oncology 2006;24(18S):5010. [Google Scholar]
- Walker JL, Piedmonte M, Spirtos N, Eisenkop S, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. Journal of Clinical Oncology 2009;27:5331‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Barakat R, Pearl ML, Sharma SK. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. Journal of Clinical Oncology Mar 1, 2012;30(7):695‐700. [DOI: 10.1200/JCO.2011.38.8645.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. Journal of Clinical Oncology 2012;30(7):695‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zorlu 2005 {published data only}
- Zorlu CG, Simsek T, Seker Ari E. Laparoscopy or laparotomy for the management of endometrial cancer. Journal of the Society of Laparoendoscopic Surgeons 2005;9:442‐6. [PMC free article] [PubMed] [Google Scholar]
Zullo 2009 {published data only}
- Zullo F, Palomba S, Falbo A, Russo T, Mocciaro R, Tartaglia E, et al. Laparoscopic surgery vs laparotomy for early stage endometrial cancer: long‐term data of a randomized controlled trial. American Journal of Obstetrics and Gynecology 2009; Vol. 200, issue 3:e1‐9. [0002‐9378; PUBMED: 19167698 ] [DOI] [PubMed]
- Zullo F, Palomba S, Russo T, Falbo A, Costantino M, Tolino A, et al. A prospective randomized comparison between laparoscopic and laparotomic approaches in women with early stage endometrial cancer: a focus on the quality of life. American Journal of Obstetrics and Gynecology 2005;1934:1344‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
de la Orden 2008 {published data only}
- Orden SG, Reza MM, Blasco JA, Andradas E, Callejo D, Pérez T. Laparoscopic hysterectomy in the treatment of endometrial cancer: a systematic review. Journal of Minimally Invasive Gynecology 2008;15(4):395‐401. [DOI] [PubMed] [Google Scholar]
Ghezzi 2006 {published data only}
- Ghezzi F, Cromi A, Bergamini V, Uccella S, Beretta P, Franchi M, et al. Laparoscopic‐assisted vaginal hysterectomy versus total laparoscopic hysterectomy for the management of endometrial cancer: a randomized clinical trial. Journal of Minimally Invasive Gynecology 2006;13(2):114‐20. [MEDLINE: ; 1553‐4650] [DOI] [PubMed] [Google Scholar]
Ghezzi 2010 {published data only}
- Ghezzi F, Cromi A, Uccella S, Siesto G, Giudici S, Serati M, et al. Laparoscopic versus open surgery for endometrial cancer: a minimum 3‐year follow‐up study. Annals of Surgical Oncology 2010;17:271‐8. [DOI: 10.1245/s10434-009-0720-1] [DOI] [PubMed] [Google Scholar]
Imesch 2009 {published data only}
- Imesch P, Dedes K, Fink D. Laparoscopic approach in endometrial cancer: current data and evidence [Laparoskopische Therapie des Korpuskarzinoms: Datenlage und Evidenz]. Gynakologisch‐geburtshilfliche Rundschau 2009;49(3):111‐6. [DOI] [PubMed] [Google Scholar]
Ju 2009 {published data only}
- Ju W, Myung SK, Kim Y, Choi HJ, Kim SC. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: a meta‐analysis. International Journal of Gynecological Cancer 2009;19(3):400‐6. [DOI: 10.1111/IGC.0b013e3181a1caf8] [DOI] [PubMed] [Google Scholar]
Lin 2007 {published data only}
- Lin F, Zhang QJ, Zheng FY, Zhao HQ, Zeng QQ, Zeng MH, et al. Laparoscopically assisted versus open surgery for endometrial cancer? A meta‐analysis of randomized controlled trials. International Journal of Gynecological Cancer 2007;18(6):1315‐25. [DOI: 10.1111/j.1525-1438.2007.01180.x] [DOI] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu J, Zhou X, Liang Y. Laparoscopic and abdominal hysterectomy: systematic evaluation and meta‐analysis of randomized controlled trials. Journal of Practical Obstetrics and Gynecology 2009. [DOI: ] [Google Scholar]
Palomba 2009 {published data only}
- Palomba S, Falbo A, Mocciaro R, Russo T, Zullo F. Laparoscopic treatment for endometrial cancer: a meta‐analysis of randomized controlled trials (RCTs). Gynecologic Oncology 2009;112(2):415‐21. [DOI: 10.1016/j.ygyno.2008.09.014] [DOI] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Russo T, Zullo F. Updating of a recent meta‐analysis of randomized controlled trials to assess the safety and the efficacy of the laparoscopic surgery for treating early stage endometrial cancer. Gynecologic Oncology 2009;114(1):135‐6. [PUBMED: 19375153] [DOI] [PubMed] [Google Scholar]
Additional references
Berek 2010
- Berek JS, Hacker NF. Berek and Hacker's Gynecologic Oncology. 5th Edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2010. [Google Scholar]
Creasman 2006
- Creasman WT, Odicino F, Mausinneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. International Journal of Gynaecology and Obstetrics 2006;95(Suppl 1):S105‐43. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- Common Terminology Criteria for Adverse Events v3.0 (CTCAE). ctep.cancer.gov/forms/CTCAEv3.pdf 9 August 2006.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Egger M, Davey Smith G, Altman DG, editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context, 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Eltabbakh 2000
- Eltabbakh GH, Shamonki MI, Moody JM, Garafano LL. Hysterectomy for obese women with endometrial cancer: laparoscopy or laparotomy?. Gynecologic Oncology 2000;78(3):329‐35. [DOI] [PubMed] [Google Scholar]
Eltabbakh 2002
- Eltabbakh GH. Analysis of survival after laparoscopy in women with endometrial carcinoma. Cancer 2002;95(9):1894‐901. [DOI] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI: 10.1002/ijc.29210] [DOI] [PubMed] [Google Scholar]
FIGO Staging 2009
- FIGO Committee on Gyneacologic Oncology. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International Journal of Gynecology and Obstetrics 2009;105:103‐4. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, cancer incidence and mortality worldwide, 2010. globocan.iarc.fr. Lyon, France, (accessed prior to 12 October 2018).
GRADE Working Group
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauspy 2010
- Hauspy J, Jiménez W, Rosen B, Gotlieb WH, Fung‐Kee‐Fung M, Plante M. Laparoscopic surgery for endometrial cancer: a review. Journal of Obstetrics and Gynaecology Canada 2010;32(6):570‐9. [DOI] [PubMed] [Google Scholar]
He 2013
- He H, Zeng D, Ou H, Tang Y, Li J, Zhong H. Laparoscopic treatment of endometrial cancer: systematic review. Journal of Minimally Invasive Gynecology 2013;20(4):413‐23. [DOI: 10.1016/j.jmig.2013.01.005] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Chichester: John Wiley & Sons, 2011.
Holub 2000
- Holub Z, Bartös P, Jabor A, Eim J, Fischlová D, Kliment L. Laparoscopic surgery in obese women with endometrial cancer. Journal of the American Association of Gynecologic Laparoscopists 2000;7(1):83‐8. [MEDLINE: ; 1074‐3804] [DOI] [PubMed] [Google Scholar]
Marana 1999
- Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multicenter study. American Journal of Obstetrics & Gynecology 1999;180:270‐5. [DOI] [PubMed] [Google Scholar]
Muntz 1999
- Muntz HG, Goff BA, Madsen BL, Yon JL. Port‐site recurrence after laparoscopic surgery for endometrial carcinoma. Obstetrics and Gynecology 1999;93(5):807‐9. [DOI] [PubMed] [Google Scholar]
Obermair 2004
- Obermair A, Manolitsas TP, Leung Y, Hammond IG, McCartney AJ. Total laparoscopic hysterectomy for endometrial cancer: patterns of recurrence and survival. Gynecologic Oncology 2004;92(3):789‐93. [DOI] [PubMed] [Google Scholar]
Obermair 2005
- Obermair A, Manolitsas TP, Leung Y, Hammond IG, McCartney AJ. Total laparoscopic hysterectomy versus total abdominal hysterectomy for obese women with endometrial cancer. International Journal of Gynecological Cancer 2005;15:319‐24. [MEDLINE: ; 1048‐891X] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanjuan 2005
- Sanjuan A, Hernandez S, Pahisa J, Ayuso JR, Torne A, Martinez Roman S, et al. Port‐site metastasis after laparoscopic surgery for endometrial carcinoma: two case reports. Gynecologic Oncology 2005;96(2):539‐54. [DOI] [PubMed] [Google Scholar]
Scribner 2001
- Scribner J, Dennis R, Walker JL, Johnson GA, McMeekin SD, Gold MA, et al. Surgical management of early‐stage endometrial cancer in the elderly: is laparoscopy feasible?. Gynecologic Oncology 2001;83(3):563‐8. [DOI] [PubMed] [Google Scholar]
Seracchioli 2005
- Seracchioli R, Venturoli S, Ceccarin M, Cantarelli M, Ceccaroni M, Pignotti E, et al. Is total laparoscopic surgery for endometrial carcinoma at risk of local recurrence? A long‐term survival. Anticancer Research 2005;25:2423‐8. [PubMed] [Google Scholar]
Shepherd 1989
- Shepherd JH. Revised FIGO staging for gynaecological cancer. British Journal of Obstetrics and Gynaecology 1989;96(8):889‐92. [DOI] [PubMed] [Google Scholar]
Smits 2013
- Smits A, Lopes A, Das N, Bekkers R, Galaal K. The impact of BMI on quality of life in obese endometrial cancer survivors: does size matter?. Gynecologic Oncology 2013;132(1):137‐41. [DOI: 10.1016/j.ygyno.2013.11.018] [DOI] [PubMed] [Google Scholar]
Tjalma 2003
- Tjalma WA. Laparoscopic surgery and port‐site metastases: routine measurements to reduce the risk. European Journal of Gynaecological Oncology 2003;24:3‐4. [PubMed] [Google Scholar]


