Abstract
Background
Central venous catheters (CVCs) can help with diagnosis and treatment of the critically ill. The catheter may be placed in a large vein in the neck (internal jugular vein), upper chest (subclavian vein) or groin (femoral vein). Whilst this is beneficial overall, inserting the catheter risks arterial puncture and other complications and should be performed with as few attempts as possible. Traditionally, anatomical ‘landmarks’ on the body surface were used to find the correct place in which to insert catheters, but ultrasound imaging is now available. A Doppler mode is sometimes used to supplement plain ‘two‐dimensional’ ultrasound.
Objectives
The primary objective of this review was to evaluate the effectiveness and safety of two‐dimensional (imaging ultrasound (US) or ultrasound Doppler (USD)) guided puncture techniques for insertion of central venous catheters via the internal jugular vein in adults and children. We assessed whether there was a difference in complication rates between traditional landmark‐guided and any ultrasound‐guided central vein puncture.
Our secondary objectives were to assess whether the effect differs between US and USD; whether the effect differs between ultrasound used throughout the puncture ('direct') and ultrasound used only to identify and mark the vein before the start of the puncture procedure (indirect'); and whether the effect differs between different groups of patients or between different levels of experience among those inserting the catheters.
Search methods
We searched the Central Register of Controlled Trials (CENTRAL) (2013, Issue 1), MEDLINE (1966 to 15 January 2013), EMBASE (1966 to 15 January 2013), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 15 January 2013 ), reference lists of articles, 'grey literature' and dissertations. An additional handsearch focused on intensive care and anaesthesia journals and abstracts and proceedings of scientific meetings. We attempted to identify unpublished or ongoing studies by contacting companies and experts in the field, and we searched trial registers. We reran the search in August 2014. We will deal with identified studies of interest when we update the review.
Selection criteria
We included randomized and quasi‐randomized controlled trials comparing two‐dimensional ultrasound or Doppler ultrasound with an anatomical 'landmark' technique during insertion of internal jugular venous catheters in both adults and children.
Data collection and analysis
Three review authors independently extracted data on methodological quality, participants, interventions and outcomes of interest using a standardized form. A priori, we aimed to perform subgroup analyses, when possible, for adults and children, and for experienced operators and inexperienced operators.
Main results
Of 735 identified citations, 35 studies enrolling 5108 participants fulfilled the inclusion criteria. The quality of evidence was very low for most of the outcomes and was moderate at best for four of the outcomes. Most trials had an unclear risk of bias across the six domains, and heterogeneity among the studies was significant.
Use of two‐dimensional ultrasound reduced the rate of total complications overall by 71% (14 trials, 2406 participants, risk ratio (RR) 0.29, 95% confidence interval (CI) 0.17 to 0.52; P value < 0.0001, I² = 57%), and the number of participants with an inadvertent arterial puncture by 72% (22 trials, 4388 participants, RR 0.28, 95% CI 0.18 to 0.44; P value < 0.00001, I² = 35%). Overall success rates were modestly increased in all groups combined at 12% (23 trials, 4340 participants, RR 1.12, 95% CI 1.08 to 1.17; P value < 0.00001, I² = 85%), and similar benefit was noted across all subgroups. The number of attempts needed for successful cannulation was decreased overall (16 trials, 3302 participants, mean difference (MD) ‐1.19 attempts, 95% CI ‐1.45 to ‐0.92; P value < 0.00001, I² = 96%) and in all subgroups. Use of two‐dimensional ultrasound increased the chance of success at the first attempt by 57% (18 trials, 2681 participants, RR 1.57, 95% CI 1.36 to 1.82; P value < 0.00001, I² = 82%) and reduced the chance of haematoma formation (overall reduction 73%, 13 trials, 3233 participants, RR 0.27, 95% CI 0.13 to 0.55; P value 0.0004, I² = 54%). Use of two‐dimensional ultrasound decreased the time to successful cannulation by 30.52 seconds (MD ‐30.52 seconds, 95% CI ‐55.21 to ‐5.82; P value 0.02, I² = 97%). Additional data are available to support use of ultrasound during, not simply before, line insertion.
Use of Doppler ultrasound increased the chance of success at the first attempt by 58% (four trials, 199 participants, RR 1.58, 95% CI 1.02 to 2.43; P value 0.04, I² = 57%). No evidence showed a difference for the total numbers of perioperative and postoperative complications/adverse events (three trials, 93 participants, RR 0.52, 95% CI 0.16 to 1.71; P value 0.28), the overall success rate (seven trials, 289 participants, RR 1.09, 95% CI 0.95 to 1.25; P value 0.20), the total number of attempts until success (two trials, 69 participants, MD ‐0.63, 95% CI ‐1.92 to 0.66; P value 0.34), the overall number of participants with an arterial puncture (six trials, 213 participants, RR 0.61, 95% CI 0.21 to 1.73; P value 0.35) and time to successful cannulation (five trials, 214 participants, each using a different definition for this outcome; MD 62.04 seconds, 95% CI ‐13.47 to 137.55; P value 0.11) when Doppler ultrasound was used. It was not possible to perform analyses for the other outcomes because they were reported in only one trial.
Authors' conclusions
Based on available data, we conclude that two‐dimensional ultrasound offers gains in safety and quality when compared with an anatomical landmark technique. Because of missing data, we did not compare effects with experienced versus inexperienced operators for all outcomes (arterial puncture, haematoma formation, other complications, success with attempt number one), and so the relative utility of ultrasound in these groups remains unclear and no data are available on use of this technique in patients at high risk of complications. The results for Doppler ultrasound techniques versus anatomical landmark techniques are also uncertain.
Plain language summary
Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization
People who are critically ill sometimes need a catheter in a central vein to help with diagnosis and treatment. The catheter may be placed in a large vein in the neck (internal jugular vein), upper chest (subclavian vein) or groin (femoral vein). However, this procedure carries risks such as arterial puncture (puncturing an artery instead of the vein might result in a haematoma, which can become infected or can lead to compression of the carotid artery) and other complications (thrombosis, embolism, pneumothorax, nerve injury) and should be performed with as few attempts as possible.
Puncture‐related complications can result from patient‐specific features such as an abnormal weight‐to‐height ratio, variations in anatomical structure (the probability of which is given in the literature as up to 29%), thrombosis‐related changes in wall structure (Caridi 1998; Denys 1991; Ferral 1998; McIntyre 1992), an existing hypovolaemia or a coagulopathy (Bernard 1971). In addition, the experience of the practitioner (Bernard 1971), the environment in which the insertion is effected (Bo‐Linn 1982), the position and the risk inherent in the particular puncture procedure contribute to the occurrence of complications.
In the past, ‘landmarks’ on the body surface were used to find the correct place to insert catheters, but ultrasound imaging is now available.
This Cochrane systematic review compared landmark techniques versus ultrasound to guide the insertion of a catheter into the large vein in the neck (the internal jugular vein). In 2013 we included in the review 35 studies enrolling 5108 participants (adults and children). These studies were varied, and their quality was moderate at best. We reran the search in August 2014. We will deal with any studies of interest when we update the review.
Nevertheless, ultrasound offered some benefits. Using ultrasound reduced the rate of complications (‐71%), including severe bruising (‐73%) and accidental puncturing of an artery instead of the vein (72%). It also increased success rates, including success rates at the first attempt (+57%) and reduced the time taken to perform the procedure. None of the included studies reported on death or patient‐reported outcomes (patient discomfort).
Based on available data, we conclude that two‐dimensional ultrasound offers improved safety and quality when compared with an anatomical landmark technique, but these findings do not necessarily hold for all users or for patients at high risk of complications. The relative utility of ultrasound when operators are experienced or inexperienced in central line insertion, however, remains unclear for some outcomes. The results for Doppler ultrasound techniques versus an anatomical landmark technique are also uncertain.
Summary of findings
Background
Description of the condition
Puncture of vessels with the insertion of catheters for diagnostic or therapeutic purposes is often a vital component of perioperative or intensive care management. Approximately six million central venous catheterizations are performed each year in Europe and the USA (Calvert 2003; FDA Drug Bull 1989).
The benefits of these central venous catheters (CVCs) lie in their ability to allow the recording of central venous pressure or other haemodynamic parameters (Rajaram 2013) and the infusion of agents that are too potent (e.g. catecholamines) or too irritating (e.g. chemotherapeutical substances, parenteral nutrition solutions (Joffe 2009)) to be applied via peripheral veins; they also can be used to carry out dialysis therapy in cases of acute renal failure.
Puncture of vessels that are suitable for bringing in CVCs traditionally takes place by the landmark puncture technique (LM). The orientation of the insertion is governed by the basic anatomical structures, and during puncture of the internal jugular vein (IJV) by palpation of the carotid artery (the arterial counterpart to the IJV). This method however remains unsuccessful in up to 35% of cases (Bernard 1971; Defalque 1974; Sznajder 1986), and the total rate of complications is given in the literature as up to 19% (Merrer 2001). Nine per cent of patients have abnormalities of the anatomy of the central veins that make the puncture or the following catheterization difficult, dangerous or impossible (Denys 1991a). A multitude of puncture‐ and catheter‐related complications of all degrees of severity have previously been described in the literature (Bodenham 2011; Cook 2011; Domino 2004; Pikwer 2012; van Miert 2012). The US Food and Drug Administration (FDA) described a total puncture‐related rate of 5% to 20% (FDA Drug Bull 1989), Johnson a rate of arterial puncture of up to 37.8% (Johnson 1994) and Polderman a rate of catheter‐related infection (CRI) of 1% to 40% (Polderman 2002). Different sites of insertion carry different rates of risk. For instance, catheters in the femoral vein or the internal jugular vein are more likely to be associated with thrombotic or infectious complications (catheter colonization, catheter‐related bloodstream infection (CRBSI)) than those in the subclavian vein; fewer mechanical complications have occurred in femoral catheters (Ge 2012).
Puncture‐related complications can result from patient‐specific features such as an abnormal weight‐to‐height ratio (obesity, cachexia), variations in anatomical structure (a probability of which is given in the literature as up to 29%), thrombosis‐related changes in wall structure (Caridi 1998; Ferral 1998; McIntyre 1992), an existing hypovolaemia or a coagulopathy (Bernard 1971). In addition, the experience of the practitioner (Bernard 1971), the environment in which the insertion is effected (Bo‐Linn 1982), the position of the patient and the risk inherent in the particular puncture procedure contribute to the occurrence of complications.
Many attempts have been made to reduce the number of complications associated with central venous catheterizations. These attempts have involved the development of ever newer types of access and puncture techniques and materials, as well as utilization of various ultrasound procedures (imaging ultrasound (US) or ultrasound Doppler (USD), direct or indirect, with or without needle guide).
Description of the intervention
In 1982 Peters et al reported for the first time the use of an ultrasound Doppler sonographic device to facilitate locating the subclavian vein (Peters 1982). In 1984 Legler and Nugent reported for the first time use of an ultrasound Doppler sonographic device to facilitate locating the internal jugular vein before inserting central venous catheters (Legler 1984). Since that time, ultrasound imaging procedures have also been tried, first for locating the internal jugular vein (Yonei 1986), then for locating the subclavian vein (Yonei 1988). These procedures, at first, made use of ultrasound scanners that were already used by the respective departments for diagnostic purposes. Later, scanners were developed especially for the purpose of vessel location, such as the SmartNeedle system® (SN) and the SiteRite scanner® (SR). Sonographic techniques (ultrasound Doppler (USD) and imaging ultrasound (US)) are referred to as direct (D; ultrasound during puncture; real‐time ultrasound) or indirect (ID; looking for the vessel by means of ultrasound and marking the puncture site on the skin; following puncture performed without sonographic guidance). Real‐time ultrasound guidance of CVC insertion provides the operator the benefit of visualizing the target vein and surrounding anatomical structures before and during the procedure. Several accessories have been developed to provide assistance during the procedure. Sterile sheaths prevent contamination by the ultrasound probe and can be filled with sterile ultrasonic transmitting gel. A needle guide—a piece of plastic that angles the needle so it will intersect the center of the vessel—can be attached to the probe to ensure optimal positioning of the needle during vessel puncture. Passage of the introducer needle into the vein can be performed using a transverse (short axis) view or a longitudinal (long axis) view. Benefits of the transverse view are that it is generally associated with a shorter learning curve and can make it easier to visualize small vessels. The primary advantage of the longitudinal view is that it allows better visualization of the advancing needle tip, which may reduce perforation of the posterior vessel wall (Atkinson 2005). For this reason, the American College of Emergency Physicians has recommended the longitudinal view (American College of Emergency Physicians 2007).
The last paper related to USD guidance was published in 2000 (Verghese 2000). This study was published first as a congress poster in 1995 (Verghese 1995). Reduced interest in this technique may be related to its lower effectiveness in comparison with US techniques and increasing distribution of ultrasonic apparatus, as well as the various possibilities for use of US devices (e.g. evaluation of vessel diameter, control of the position of the catheter tip, peripheral venous and arterial cannulation, performing regional anaesthesia with the help of ultrasound). Some of the studies evaluated by review authors for this review permit the conclusion that Doppler ultrasound for vascular access is associated with a longer learning curve, longer insertion times and higher costs than are reported for B‐mode ultrasound (Bold 1998; Gilbert 1995; Legler 1984). Other studies found it "easy to learn, and efficient ..." (Branger 1995), or that “Finally, training did not influence the course of the study....This suggests that training had no influence on Doppler guidance procedure and that it could be learned easily and quickly” (Lefrant 1998).
How the intervention might work
Use of sonographic techniques (ultrasound Doppler (USD) or imaging ultrasound (US), direct (D; ultrasound during puncture or indirect (ID; looking for the vessel by means of ultrasound and marking the puncture site on the skin; following puncture performed without sonographic guidance)) for better locating vessels for insertion of CVCs will help make the procedure safer, faster, freer of complications and more often successful. One explanation for these benefits is that real‐time ultrasonography clarifies the relative position of the needle and the vein and structures surrounding the vein. The image offered by two‐dimensional ultrasonography allows the user to predict variant vascular anatomy (e.g. transposition of the vein and the artery, overlap of the artery and the vein) or abnormal patient anatomy (e.g. morbid obesity, cachexia, local scarring) and to assess the patency of a target vein (thrombosis, small diameter) before and during the procedure. Examination of the vessel in different positioning maneuvers (e.g. turning the head; patient down, flat, up; arching the shoulders or not; leg straight or abducted) allows the operator to determine optimal storage for the puncture. Because of the risk of catheter‐related thrombosis along with other factors affected by the relationship between the diameter of the catheter and that of the vessel, the external diameter of the catheter should not exceed one‐third the internal diameter of the vein (Debordeau 2009; Lamperti 2012). If catheter diameter is excessive, the possibly taller vessel of the opposite side or another vessel should be punctured and catheterized. For these reasons, supporters of ultrasound‐guided puncture propagate primary use in all patients. Abnormalities can be recognized and the puncture made easier or safer by selection of another access route or with the help of improved storage.
Why it is important to do this review
Growing numbers of publications and meta‐analyses (Calvert 2003; Hind 2003; Keenan 2002; Randolph 1996; Rothschild 2001) have compared the effectiveness of ultrasound guidance versus the traditional landmark technique for central vein catheterization. However, these reviews are 10 years old, and sonographic devices and their uses have changed.
The meta‐analysis from Wu (Wu 2013) was conducted to compare the use of anatomical landmark techniques for central venous cannulation versus real‐time, two‐dimensional ultrasound guidance to determine whether ultrasound techniques decreased risks of cannulation failure, arterial puncture, haematoma and haemothorax in adults and children. USD techniques and indirect (ID) proceedings were not taken into account.
Many RCTs and six meta‐analyses have suggested that the use of ultrasound may be associated with reduced complication rates and improved first‐pass and overall success rates when catheters are placed via the internal jugular vein. Furthermore, a multitude of publications from all sorts of institutions have strongly recommended the use of ultrasound to assist vessel puncture for CVC catheterization (Alderson 1993; Calvert 2003; Rothschild 2001). Although a variety of scientific proofs and recommendations have covered the use of these procedures, great resistance against their incorporation into clinical practice continues (Howard 2007).
Therefore, we systematically reviewed the literature to assess both efficacy and safety outcomes of the use of sonographic techniques for internal jugular vein puncture during CVC instillation to see whether this approach makes the procedure safer, faster, freer of complications and more often successful. This review is one of a pair of Cochrane reviews on this topic. The other Cochrane review focuses on evidence on the use of ultrasound in catheterization of the subclavian and femoral veins (Brass 2013b).
Objectives
Primary objective
The primary objective of this review was to evaluate the effectiveness and safety of two‐dimensional (imaging ultrasound (US) or ultrasound Doppler (USD)) guided puncture techniques for insertion of central venous catheters via the internal jugular vein in adults and children. We assessed whether there was a difference in complication rates between traditional landmark‐guided and any ultrasound‐guided central vein puncture.
Secondary objectives
Our secondary objectives were to assess whether the effect differs between US and USD; whether the effect differs between ultrasound used throughout the puncture ('direct') and ultrasound used only to identify and mark the vein before the start of the puncture procedure (indirect'); and whether the effect differs between different groups of patients or between different levels of experience among those inserting the catheters.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized controlled trials (RCTs) in all languages eligible for inclusion in the review, with an RCT defined as a study in which participants were allocated to treatment groups on the basis of a random or quasi‐random method (e.g. using random number tables, hospital number, date of birth). We also included controlled clinical trials (CCTs).
Types of participants
We included all patients (children and adults) who required insertion of a central venous catheter via the internal jugular vein.
We applied no restrictions with respect to specific population characteristics (e.g. age; gender; race; presence of a particular condition, for example, risk factors), study settings (intensive care unit (ICU); operation room; participant awake or anaesthetized/with anaesthesia) or practitioners' experience.
Types of interventions
We included all studies in which conventional techniques oriented to anatomical landmarks (LMs) for puncture of the internal jugular vein (control intervention) were compared with techniques by which punctures were performed with the help of imaging (US) or Doppler (USD) ultrasonographic devices (experimental intervention). We included all studies, irrespective of whether the puncture was performed directly (using sonographic control) or indirectly (looking for the vessel by means of ultrasound and marking the puncture site on the skin; following puncture performed without sonographic guidance).
Types of outcome measures
Outcome measures did not constitute criteria for including studies.
Primary outcomes
The primary outcome measured was the total number of perioperative and postoperative complications/adverse events ((*) absolute numbers (n/N) and expressed as percentages (%)).
Secondary outcomes
Secondary outcomes included the following.
Overall success rate (*).
Number of attempts until success (*).
Number of participants with an arterial puncture (*).
Number of participants with significant haematoma formation (*).
Numbers of participants with other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (*).
Time needed for success (*).
Success with attempt number 1, 2, 3 (*).
Participant discomfort (*).
Mortality (*).
All outcomes were defined as stated by the study authors. We differentiated between intraoperative, postoperative and long‐term complications. We included studies irrespective of whether all of this information was available.
Search methods for identification of studies
We employed the standard methods of the Cochrane Anaesthesia Review Group.
Two review authors (PB, LK) independently assessed the titles and abstracts (when available) of all reports identified by electronic searching, manual searching, snowballing and making contact with experts and industry.
We assessed the reports as follows.
Patrick Brass (PB) assessed all reports.
Laurentius Kolodziej (LK) assessed all reports.
We retrieved and evaluated potentially relevant studies, chosen by at least one review author, in full‐text versions. We masked all selected studies by obscuring study authors' names and institutions, location of study, reference list, journal of publication and any other potential identifiers.
Electronic searches
One review author (PB) and the CARG TSC (KH) searched the following databases for relevant trials:
the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 1; see Appendix 1 for detailed search strategy); Ovid MEDLINE (1966 to 15 January 2013; see Appendix 2); Ovid EMBASE (1980 to 15 January 2013; see Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1982 to 15 January 2013; see Appendix 4); MedPilot (1980 to 15 January 2013; see Appendix 5); and registers of clinical trials. We developed a specific strategy for each database.
We reran the search in August 2014. We will deal with any studies of interest when we update the review.
We did not limit the search by language or publication status.
We used the optimally sensitive strategies of The Cochrane Collaboration to identify RCTs for MEDLINE and EMBASE searches (Dickersin 1994; Lefebvre 2001; Robinson 2002).
We combined the MEDLINE search strategy with the Cochrane highly sensitive search strategy phases one and two, as contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted our MEDLINE search strategy for searching the other databases.
We attempted to identify unpublished or ongoing studies by searching the following two trial registries (searched on 20 March 2014) for all years available in all possible fields using the basic search function (using separately the following keyword terms: "ultrasound", "central vein catheterization", "central vein catheter").
Current Controlled Trials: www.controlled‐trials.com.
ClinicalTrials.gov: www.clinicaltrials.gov.
Searching other resources
We performed an additional handsearch focused on intensive care and anaesthesia journals, abstracts and proceedings of scientific meetings (e.g. proceedings of the Annual Congress of the European Society of Intensive Care Medicine (ESICM), the Annual Congress of the German Society of Anaesthesia (DAK), the Annual Congress of the European Society of Anaesthesia (ESA)) (2003 to 2013; last search 20 January 2013); references lists; 'grey literature' (System for Information on Grey Literature in Europe (SIGLE and Zetoc); the Index to Scientific and Technical Proceedings (from the Institute for Scientific Information); and dissertations.
We attempted to identify unpublished or ongoing studies by contacting the companies medilab GmbH (SiteRite®, Dymax Corporation), Medimex (P.D. Access®/SmartNeedle®) and SonoSite.
We contacted experts in the field to identify unpublished studies and studies presented in abstract form at major international meetings.
We (PB, LK) checked the bibliographies of all identified studies. We repeated this approach until no further studies could be identified.
Data collection and analysis
Selection of studies
Two review authors (PB, LK) independently screened the titles and abstracts of reports identified by electronic searching, manual searching, snowballing and making contact with experts and industry for relevance. At this stage, we excluded only citations that were clearly irrelevant. We obtained full copies of all potentially relevant papers.
Two review authors (PB, LK) independently screened the full papers, identified relevant studies and assessed eligibility of studies for inclusion. We selected trials that met the inclusion criteria, using a checklist designed in advance for that purpose. We resolved disagreements on the eligibility of studies through discussion. When resolution was not possible, we consulted a third review author (GS).
We assessed the quality of all studies meeting the inclusion criteria and extracted data from them. We excluded all irrelevant records and recorded details of the studies and reasons for exclusion.
Data extraction and management
Two review authors (PB, LK) independently extracted the data using a specially designed data extraction form. We resolved disagreements by discussion; when necessary, we consulted a third review author (GS). Once we had resolved disagreements, we recorded extracted data on the final data extraction form.
We contacted study authors to ask for clarification or to request missing information. We excluded data until further clarification was provided if we could not reach agreement.
One review author (PB) transcribed the data into RevMan 5.2 (RevMan 5.2), and another review author (LK) checked the data entered to look for discrepancies.
In addition to details related to the risk of bias of included studies, we extracted two sets of data.
Study characteristics: place of publication; date of publication; population characteristics; setting; detailed nature of intervention; detailed nature of comparator; and detailed nature of outcomes. A key purpose of these data was to define unexpected clinical heterogeneity in included studies independently from the analysis of results.
Results of included studies with respect to each of the main outcomes indicated in the review question. We carefully recorded reasons why an included study did not contribute data on a particular outcome and considered the possibility of selective reporting of results on particular outcomes.
We recorded for each trial the following data.
Authors.
Year of publication.
Study design.
Population.
Inclusion procedure: (‐) means non‐consecutive/unknown; (+) means consecutive.
Setting: university/other/unknown.
Participant characteristics (age, gender, height, weight, body mass index (BMI)) recorded as stated in the study.
Punctured vessel/punctured side.
Intervention (US or USD, puncture occurred directly (DUS or DUSD) or indirectly (IDUS or IDUSD) (puncture method: USA: information on applied ultrasound procedure and on position in which the puncture was performed; LM: information on position in which the puncture was performed. Puncture method: +: standardized; ‐: not standardized).
Study design: P: prospective; R: randomized; C: controlled; Cr.‐o.: cross‐over; information on randomization method; exclusion of participants after randomization: +: yes; ‐: no; intention‐to‐treat evaluation plan: +: yes; ‐: no.
Number and experience of practitioners.
Numbers of punctures and participants.
LM/US: number of conventional/sonographic punctures.
Details of the outcome (all studies included, irrespective of whether they provided complete information on overall success rate;total number of attempts needed until success;number of punctures that were successful at first, second, third, etc., attempt;overall complication rate or number of individual complications; and time required until success, or whether some of this information was lacking).
Conclusions of study authors.
Assessment of risk of bias in included studies
Two review authors (PB, LK) independently assessed the methodological quality of each included study using a simple form and following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains as having low, unclear or high risk of bias.
Random sequence generation.
Allocation concealment.
Participant blinding.
Provider/physician blinding.
Outcome assessor blinding.
Incomplete outcome data addressed.
Selective outcome reporting.
Other source of bias.
We reviewed the assessments and discussed inconsistencies between review authors in interpretation of inclusion criteria and their significance to selected studies. We resolved disagreements through discussion with a third review author.
We did not automatically exclude any study as the result of a rating of ’unclear risk of bias’ or ’high risk of bias.’ We presented our evaluation of the Risk of bias in included studies in tabular form in the Results section of the review.
A summary of bias was given for each study, and the results were summarized in the 'Risk of bias' table in the Results section of the review. We predicted that, given the nature of the intervention, blinding of the practitioner would not be possible. We noted measures of clinical performance. For instance, when given, we recorded the experience and number of practitioners performing the procedures in a trial.
Second, we assessed the quality of evidence at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Measures of treatment effect
We analysed extracted data using Review Manager (RevMan 5.2).
For dichotomous data, we described results both as a relative measure (risk ratio (RR)) with 95% confidence intervals (CIs) and as an absolute measure (number needed to treat for an additional beneficial outcome and risk difference). Relative measures can be used to combine studies, but absolute measures can be more informative than relative measures because they reflect the baseline risk as well as the change in risk noted with the intervention.
For continuous outcomes, we used the mean difference (MD) and the standard deviation (SD) to summarize the data for each group. This provides the advantage of summarizing results in natural units that are easily understood.
Unit of analysis issues
We included cross‐over studies in this review, but we did not analyse the endpoint success rate after cross‐over.
The unit of analysis was the individual participant.
Dealing with missing data
No simple solution is known for the problem of missing data. We handled this problem by contacting the investigators, when possible, to clarify some methodological issues and to request additional data. In addition, the assumption of whatever method was used to cope with missing data was made explicit. We included studies irrespective of whether all of the outcome information was available. However, to date, we have not received data beyond those presented in the primary reports. If we subsequently receive additional information, we plan to incorporate these data into the next update of this review.
Assessment of heterogeneity
We assessed heterogeneity between trials by visually inspecting forest plots, and we quantified statistical heterogeneity by calculating the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2003). We regarded heterogeneity as low if I2 was less than 25%, as moderate if I2 was between 25% and 50% and as substantial if I2 was greater than 50%. If evidence of substantial heterogeneity was found, we investigated and reported possible reasons for this.
The predetermined significance level of heterogeneity was the P value of .05. Both the typical effect size and the effect size relative to specific study characteristics will be interpreted cautiously if heterogeneity is significant.
Assessment of reporting biases
We made a great effort to identify unpublished studies and to minimize the impact of possible publication bias by using a comprehensive research strategy.
Publication bias occurs when published studies are not representative of all studies that have been done, usually because positive results tend to be submitted and published more often than negative results. Because detecting publication bias is difficult, we tried to minimize it by performing comprehensive literature searches, using study registries and contacting the manufacturers of ultrasound devices (Glasziou 2001).
We assessed reporting bias also by trying to identify whether the study was included in a trial registry, whether a protocol was available and whether the Methods section provided a list of outcomes. We compared outcomes listed in those sources versus outcomes reported in the published paper.
We used a graphical display (funnel plot) of the size of the treatment effect against the precision of the trial (one/standard error) to investigate publication bias by examining for signs of asymmetry. Publication bias is associated with asymmetry (Light 1984). In the absence of publication bias, a plot of study sample size (or study weight) versus outcome (i.e. log relative risk) should have a bell or inverted funnel shape, with the apex near the summary effect estimate (funnel plot). If asymmetry was found, we also searched for reasons other than publication bias, such as poor methodological quality of smaller studies, true heterogeneity, artefactual reasons or chance (Egger 1997).
We did not use funnel plots to assess publication bias when we found fewer than 10 trials for an endpoint, as asymmetry is difficult to detect when a small number of studies are examined.
Data synthesis
We reviewed the data from included studies qualitatively and then, if possible, combined the data quantitatively by population, intervention and outcome, using the statistical software of The Cochrane Collaboration, Review Manager (RevMan 5.2).
We performed a meta‐analysis when studies of similar comparisons reported the same outcome measures. We used models with random effects (i.e. the Mantel‐Haenszel (MH) method for dichotomous data (using risk ratio as effect measure) and the inverse variance (IV) method for continuous data (using standardized mean difference (SMD) as effect measure) when between‐study heterogeneity was apparent, as assessed by Q and I2 statistics. Confidence intervals were calculated at the 95% level, and corresponding P values equal to or less than 5% (two‐sided alpha) were considered statistically significant.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis of different sonographic techniques ((D)/(ID)/US/USD), puncture sites, groups of participants (adults, children) and practitioners (experienced, not experienced).
The experience of practitioners and their faculties in both ultrasound techniques and control techniques involved varied across trials from medical student (Turker 2009) to "10 years of experience in IJV (LM) catheter placement....at least 5 years of experience in performing this method (US)" (Karakitsos 2006). In 19 trials the level of experience in performing the procedures was stated (not stated in nine (Chuan 2005; Hayashi 1998; Johnson 1994; Ovezov 2010; Scherhag 1989; Soyer 1993; Troianos 1990; Troianos 1991; Verghese 1995)). In some studies the level of experience in performing the procedures was stated only for the landmark group. Information given ranged from "experienced cardiac anaesthetist" (Alderson 1992) or "familiar with both cannulation techniques" (Hayashi 2002) to very firm descriptions of experience (Böck 1999; Karakitsos 2006; Palepu 2009). The definitions of an experienced operator and of an inexperienced operator varied across a large range.
According to the Cochrane Handbook for Systematic Reviews of Interventions, Section 9.6.3, we should like to compare the magnitude of effects only informally. The limitation of this approach (i.e. differences may be explained by chance alone) is acknowledged. In a future version of this review, we will apply the Borenstein approach as well.
Sensitivity analysis
A priori, we planned sensitivity analyses to test how sensitive the results would be to reasonable changes in assumptions made during the review process and in the protocol for combining data (Lau 1998).
We planned to perform sensitivity analyses regarding 'randomized versus quasi randomized' and eventually 'good quality studies versus poor quality studies.' We defined a good quality study as one that includes all of the following domains: adequate allocation concealment, blinding of outcome assessment and data analysis performed according to the intention‐to‐treat principle. A poor quality study, for the purposes of the proposed sensitivity analysis, was defined as one that lacks one or more of these key domains.
We have not performed a sensitivity analysis, as almost all of the included studies have high risk of bias. For example, in no study was the outcome assessor blinded, and in only four studies was adequate sequence generation or adequate allocation concealment reported. Inclusion and exclusion criteria were clearly defined in only 10 studies (Agarwal 2009; Böck 1999; Chuan 2005; Hayashi 2002; Hrics 1998; Karakitsos 2006; Leung 2006; Milling 2005; Scherhag 1989; Turker 2009), and treatment and control groups were adequately described at entry in only eight studies (Böck 1999; Hayashi 2002; Karakitsos 2006; Leung 2006; Lin 1998; Scherhag 1989; Sulek 2000; Turker 2009).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The January 2013 search strategy and our previous search identified a total of 704 citations.
A search of other sources yielded a total of 31 citations: 10 from an additional handsearch focused on intensive care and anaesthesia journals and abstracts and proceedings of scientific meetings (e.g. proceedings of the Annual Congress of the European Society of Intensive Care Medicine (ESICM) or of the Annual Congress of the European Society of Anaesthesia (ESA)), four from reference lists and 17 from companies that we contacted for references. After reviewing the titles and abstracts, we identified and retrieved for review 11 articles in full text (see Figure 1).
1.

Study flow diagram.
Altogether, 735 citations, including 439 duplicates, were identified. After title and abstract screening of the 296 unique citations, 243 citations were excluded. A total of 53 full texts were screened, of which 13 reports were excluded (for reasons for exclusion, see Excluded studies section below).
We reran the search in August 2014. We found eight new citations, of which five are studies of interest (Airapetian 2013; Bikash 2014; Cajozzo 2004; Gok 2013; Shrestha 2011) (see Characteristics of studies awaiting classification). We will deal with studies of interest when we update the review.
We identified no ongoing studies.
Altogether, we included 35 studies in the quantitative synthesis.
Included studies
In this review we included 35 studies from 1989 to the date of the search, with 5108 participants, as described in the Characteristics of included studies. The individual studies involved sample sizes of 21 (Branger 1994) to 900 participants (Karakitsos 2006). The studies took place in different hospital settings all over the world. Of the 35 studies, 29 were RCTs and four were QRCTs (Armstrong 1993; Denys 1993; Grebenik 2004; Lin 1998); it is unclear whether two studies are RCTs or CCTs (Branger 1994; Branger 1995).
Study authors used two‐dimensional ultrasound to scan the insertion site before, but not during, puncture ('indirect puncture') in five studies (Alderson 1992; Armstrong 1993; Chuan 2005; Hayashi 1998; Hayashi 2002), and during insertion ('direct puncture') in 19 studies (Agarwal 2009; Bansal 2005; Böck 1999; Denys 1993; Grebenik 2004; Johnson 1994; Karakitsos 2006; Leung 2006; Lin 1998; Mallory 1990; Ovezov 2010; Palepu 2009; Scherhag 1989; Soyer 1993; Sulek 2000; Teichgräber 1997; Troianos 1990; Troianos 1991; Turker 2009). It was unclear whether direct or indirect puncture had been used in three studies (Heatly 1995; Verghese 1995; Verghese 1996); two studies (Hrics 1998; Milling 2005) used both.
In eight studies Doppler ultrasound was used; one study used indirect puncture (Legler 1983), and seven used direct puncture (Branger 1994; Branger 1995; Gilbert 1995; Gratz 1994; Scherhag 1989; Verghese 1995; Vucevic 1994). Two studies (Scherhag 1989; Verghese 1995) used both two‐dimensional and Doppler modes. In two studies Doppler ultrasound machines without a needle guide were used (Legler 1983; Scherhag 1989), and in four SmartNeedle®, a Doppler‐guided needle device, was used (Gilbert 1995; Gratz 1994; Verghese 1995; Vucevic 1994). Branger et al (Branger 1994; Branger 1995) used a pulsed Doppler probe, which had been developed by the study authors.
The ultrasound probe was wrapped in a sterile glove in five studies (Böck 1999; Leung 2006; Mallory 1990; Scherhag 1989; Sulek 2000), in a sterile sheath in seven studies (Agarwal 2009; Grebenik 2004; Karakitsos 2006; Milling 2005; Palepu 2009; Troianos 1990; Troianos 1991) and in a sterile plastic bag in three studies (Denys 1993; Hrics 1998; Lin 1998). The probe was sterilized with povidone‐iodine in one study (Soyer 1993)and with ethylenoxide gas in two studies (Branger 1994; Branger 1995); it was disinfected in one study (Bansal 2005), and nothing was reported in eight studies (Heatly 1995; Johnson 1994; Legler 1983; Ovezov 2010; Teichgräber 1997; Turker 2009; Verghese 1995; Verghese 1996). In four studies (Gilbert 1995; Gratz 1994; Verghese 2000; Vucevic 1994), the sterile needle from SmartNeedle® was used.
Whilst most studies used only the internal jugular vein, three used both the internal jugular vein and the subclavian vein (Branger 1994; Branger 1995; Palepu 2009), and in three studies in which the internal jugular vein was used, investigators examined the use of US and USD (Scherhag 1989; Verghese 1995; Verghese 2000).
Only 20 studies provided information about the puncture side. In 14 studies only the right side was used; in six studies both sides were used. In 14 studies no details were given, and in one study (Scherhag 1989) the side of insertion was specified only when Doppler ultrasound was used.
In six (Armstrong 1993; Denys 1993; Hrics 1998; Lin 1998; Verghese 1995; Verghese 1996) of 10 studies (Alderson 1992; Armstrong 1993; Denys 1993; Grebenik 2004; Hrics 1998; Lin 1998; Troianos 1990; Troianos 1991; Verghese 1995; Verghese 1996) in which the SiteRite® ultrasound device was used for ultrasound‐guided internal jugular vein cannulation, the study authors claimed that they had used the needle holder/guide. In these studies, it can be assumed that passage of the introducer needle into the vein was performed in the transverse (short axis) view. In addition, representation of the vein in the short axis was used in the following studies: Agarwal 2009; Bansal 2005; Böck 1999; Hayashi 2002; Leung 2006; Mallory 1990; Palepu 2009; Scherhag 1989; Soyer 1993; Teichgräber 1997. Passage of the introducer needle into the vein was performed in the longitudinal (long axis) view only in the study conducted by Karakitsos (Karakitsos 2006).
Participants were adults of both sexes in 23 studies (USD N = 5, US N = 18) (Agarwal 2009; Bansal 2005; Böck 1999; Denys 1993; Hayashi 1998; Hayashi 2002; Karakitsos 2006; Leung 2006; Lin 1998; Mallory 1990; Milling 2005; Palepu 2009; Scherhag 1989; Soyer 1993; Sulek 2000; Troianos 1990; Turker 2009; Troianos 1991) and were children in six studies (Alderson 1992; Chuan 2005; Grebenik 2004; Ovezov 2010; Verghese 1995; Verghese 1996); no such details were given in seven studies (Armstrong 1993; Branger 1994; Gratz 1994; Heatly 1995; Hrics 1998; Johnson 1994; Teichgräber 1997).
Procedures were carried out when participants were awake in eight studies, all including adults (Bansal 2005; Denys 1993; Lin 1998; Scherhag 1989; Soyer 1993; Troianos 1990; Troianos 1991; Turker 2009); were anaesthetized in eight studies, four including adults (Hayashi 1998; Hayashi 2002; Sulek 2000; Vucevic 1994) and four including children (Chuan 2005; Grebenik 2004; Verghese 1995; Verghese 1996). Timing was not specified in one study (Armstrong 1993), and various combinations were reported in others: one anaesthetized/sedated (Karakitsos 2006); and three anaesthetized or awake (Branger 1994; Branger 1995; Gilbert 1995). No details of this were provided in 14 studies.
In 24 of the studies, no details on the number of operators who carried out the procedure were provided (19 two‐dimensional ultrasound: Agarwal 2009; Alderson 1992; Armstrong 1993; Bansal 2005; Chuan 2005; Hayashi 1998; Johnson 1994; Karakitsos 2006; Lin 1998; Mallory 1990; Ovezov 2010; Palepu 2009; Scherhag 1989; Sulek 2000; Teichgräber 1997; Troianos 1990; Troianos 1991; Verghese 1995; Verghese 1996; five Doppler: Gilbert 1995; Gratz 1994; Legler 1983; Scherhag 1989; Verghese 1995).
In 13 of the studies, details on the number of operators who carried out the procedure were provided (Böck 1999; Branger 1994; Branger 1995; Denys 1993; Grebenik 2004; Hayashi 2002; Heatly 1995; Hrics 1998; Leung 2006; Milling 2005; Soyer 1993; Turker 2009; Vucevic 1994).
In only 25 of the studies were details of the experience of the operators who carried out the procedure provided. These procedures were carried out by senior fellows (Mallory 1990), experienced operators (Alderson 1992; Bansal 2005; Denys 1993; Lin 1998; Sulek 2000; Teichgräber 1997), operators with ample experience (Heatly 1995), registrars (Armstrong 1993), fellows and attendings (Verghese 1996), residents and attendings (Hayashi 2002; Hrics 1998), attendings (Karakitsos 2006), experienced anaesthetists (Böck 1999; Gratz 1994; Vucevic 1994), consultant paediatric cardiac anaesthetists (Grebenik 2004), a medical student (Turker 2009), registrars and consultants (Palepu 2009), senior residents and consultants (Agarwal 2009), junior residents or seniors (Branger 1994; Branger 1995), emergency physicians or registrars working in the ED (Leung 2006), internal medicine and surgery residents with varying levels of experience (Milling 2005) and inexperienced juniors (Gilbert 1995).
In addition, no study describes the learning curve of the operators within the study. However, the operator experience plays an important role, for both US‐guided and traditional landmark techniques can introduce significant bias in either direction.
In none of the studies was the outcome assessor blinded.
Grebenick`s study was criticized for the high rates of dropout and the statistical analysis used (Grau 2005).
Inclusion and exclusion criteria were clearly defined in 10 studies (Agarwal 2009; Böck 1999; Chuan 2005; Hayashi 2002; Hrics 1998; Karakitsos 2006; Leung 2006; Milling 2005; Scherhag 1989; Turker 2009), and treatment and control groups were adequately described at entry in only nine studies (Böck 1999; Hayashi 2002; Karakitsos 2006; Leung 2006; Lin 1998; Milling 2005; Scherhag 1989; Sulek 2000; Turker 2009).
Of the 35 included studies, 14 evaluated the primary outcome of total complication rate (Agarwal 2009; Bansal 2005; Böck 1999; Denys 1993; Grebenik 2004; Heatly 1995; Leung 2006; Lin 1998; Milling 2005; Palepu 2009; Soyer 1993; Turker 2009; Verghese 1995; Verghese 1996); 21 did not (Alderson 1992; Armstrong 1993; Branger 1994; Branger 1995; Chuan 2005; Gilbert 1995; Gratz 1994; Hayashi 1998; Hayashi 2002; Hrics 1998; Johnson 1994; Karakitsos 2006; Legler 1983; Mallory 1990; Ovezov 2010; Scherhag 1989; Sulek 2000; Teichgräber 1997; Troianos 1990; Troianos 1991; Vucevic 1994). Of the included studies, 23 studies evaluated the overall success rate (Alderson 1992; Armstrong 1993; Bansal 2005; Chuan 2005; Denys 1993; Grebenik 2004; Hayashi 2002; Heatly 1995; Hrics 1998; Johnson 1994; Karakitsos 2006; Leung 2006; Lin 1998; Mallory 1990; Milling 2005; Ovezov 2010; Palepu 2009; Scherhag 1989; Soyer 1993; Troianos 1990; Troianos 1991; Turker 2009; Verghese 1996); 12 did not (Agarwal 2009; Böck 1999; Branger 1994; Branger 1995; Gilbert 1995; Gratz 1994; Hayashi 1998; Legler 1983; Sulek 2000; Teichgräber 1997; Verghese 1995; Vucevic 1994). In all, 16 studies evaluated the number of attempts needed for success, 20 the time to successful cannulation and 18 the numbers of successes on the first to fifth attempts.
Excluded studies
We excluded 13 studies from the review for the following reasons.
Five were not randomized trials: Denys 1990; Denys 1991 (prospective study, not randomized, used only ultrasound); Gallieni 1995 (observational study, LM used first for 10 participants, then US for an additional 31 participants); Koski 1992 (observational study, used ultrasound‐guided technique during first half of the study and the conventional method during second half of the study); and Serafimidis 2009 (no details on whether the study is prospective and randomized). In one study, no report of ethical approval or participant consent was provided and randomization was balanced for procedures performed by interns or residents (Slama 1997). Four studies were published twice: first as a congress poster (Alderson 1992; Legler 1983; Verghese 1995; Verghese 1996), then as an article (Alderson 1993; Legler 1984; Verghese 1999; Verghese 2000).
In one of the studies, study authors made no statements about the punctured vessels (Woody 2001); in two studies, study authors used different vessels, but the results were stated together (Froehlich 2009; Miller 2002).
See the Characteristics of excluded studies table.
Awaiting classification
Five studies are awaiting classification (Airapetian 2013; Bikash 2014; Cajozzo 2004; Gok 2013; Shrestha 2011). See the Characteristics of studies awaiting classification table.
Risk of bias in included studies
We used the domain‐based evaluation table of The Cochrane Collaboration provided in RevMan 5.2 to assess the validity and the quality of included trials.
We have detailed in the Characteristics of included studies table methods of randomization, outcome assessment details and exclusion criteria. A summary of our assessment of methodological quality of included studies is given in Figure 2 and Figure 3.
2.
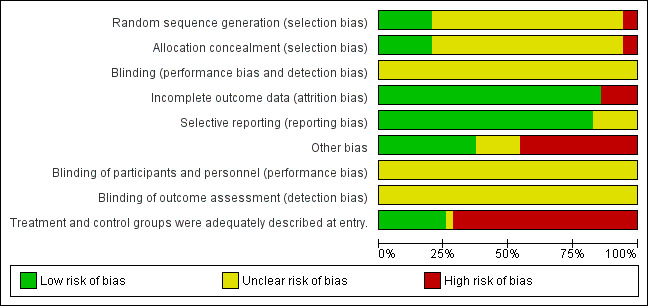
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
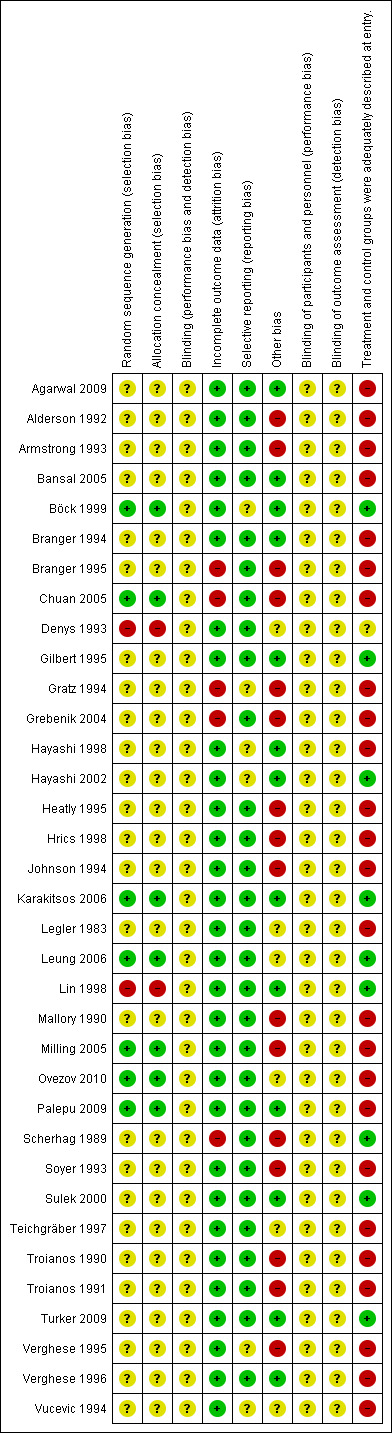
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The quality of evidence was very low or low for most of the outcomes, and was moderate at best for four of the outcomes. Most of the trials had unclear risk of bias across the six domains.
We believe that the inability to blind the practitioner performing the puncture, especially when the same person was performing all punctures, was a potential source of performance bias. One further source of potential bias lies in the fact that in none of the studies was the outcome assessor blinded. For this reason, all included trials should be considered as having at least moderate risk of bias. Because of the nature of the intervention, blinding of the practitioner was never going to be possible, and this is an unavoidable source of bias. We are aware that these studies are at potential risk of bias and have taken this into account when assessing their results.
Allocation
Allocation concealment was inadequate in two studies (Denys 1993; Lin 1998), adequate in seven studies (Böck 1999; Chuan 2005; Karakitsos 2006; Leung 2006; Milling 2005; Ovezov 2010; Palepu 2009) and unclear in 26 studies (20 two‐dimensional ultrasound: Agarwal 2009; Alderson 1992; Armstrong 1993; Bansal 2005; Grebenik 2004; Hayashi 1998; Hayashi 2002; Heatly 1995; Hrics 1998; Johnson 1994; Mallory 1990; Scherhag 1989; Soyer 1993; Sulek 2000; Teichgräber 1997; Troianos 1990; Troianos 1991; Turker 2009; Verghese 1995; Verghese 1996; and nine Doppler: Branger 1994; Branger 1995; Gilbert 1995; Gratz 1994; Legler 1983; Scherhag 1989; Verghese 1995; Verghese 1996; Vucevic 1994. Sequence generation was inadequate in two studies (Denys 1993; Lin 1998), adequate in eight studies (Böck 1999; Chuan 2005; Karakitsos 2006; Leung 2006; Lin 1998; Milling 2005; Ovezov 2010; Palepu 2009) and unclear in 26 studies (21 two‐dimensional ultrasound, five Doppler). We are aware that these studies are at potential risk of bias and have taken this into account when assessing their results.
The four studies that were published twice had the following unusual features: In Alderson 1992 and Alderson 1993, as well as in Legler 1983 and Legler 1984, allocation concealment was unclear. In Verghese 1995 and Verghese 1996, allocation concealment was unclear in the congress poster and adequate in the articles (Verghese 1999 and Verghese 2000).
Blinding
None of the studies was free from other problems that could put it at risk of bias. Given the nature of the intervention, blinding to the intervention was not always (participants) or was never (personnel) feasible; however, we assessed the risk of bias depending on whether or not outcome assessors were independent from those involved in participant care management decisions. In none of the 32 trials was it stated that the outcome assessor was blinded. We have described above whether cannulation was performed with participants awake, sedated or anaesthetized. However, in no trial was any attempt made to blind participants to the technique being used. This may be a potential source of detection bias, as several of the assessed outcomes may be subjective (e.g. complication rate, participant satisfaction), although in fact no trial studied participant‐reported outcome measures.
Incomplete outcome data
Completeness of data on main outcomes
Incomplete outcome data were addressed in 30 studies (US N = 24, USD N = 4 (Branger 1994; Gilbert 1995; Legler 1983; Vucevic 1994), US and USD N = 2 (Verghese 1995; Verghese 1996)) with low risk of attrition bias and in five studies (US N = 2 (Chuan 2005; Grebenik 2004), USD N = 2 (Branger 1995; Gratz 1994), US and USD N = 1 (Scherhag 1989)) with high risk of attrition bias. In these five trials, incomplete outcome data were not adequately addressed. (Outcomes of participants who withdrew or were excluded after allocation were neither detailed separately nor included in an intention‐to‐treat analysis, or the text stated that no withdrawals occurred (Branger 1995; Chuan 2005; Gratz 1994, Grebenik 2004; Scherhag 1989)). We believe that the potential for attrition bias is therefore high in these studies.
A comparison of outcomes mentioned in the publication versus endpoints planned in the study protocol was not possible for any of the studies because not a single protocol was published.
In 25 studies, included participants were selected (US N = 19 (Agarwal 2009; Alderson 1992; Armstrong 1993; Bansal 2005; Böck 1999; Chuan 2005; Grebenik 2004; Hayashi 2002; Hrics 1998; Leung 2006; Lin 1998; Mallory 1990; Milling 2005; Palepu 2009; Soyer 1993; Sulek 2000; Troianos 1990; Troianos 1991; Turker 2009), USD N = 3 (Branger 1994; Branger 1995; Gilbert 1995), US and USD N = 3 (Scherhag 1989; Verghese 1995; Verghese 1996)), in four they were not selected (US N = 4 (Denys 1993; Hayashi 1998; Karakitsos 2006; Teichgräber 1997)) and in six selection was unclear (Gratz 1994; Heatly 1995; Johnson 1994; Legler 1983; Ovezov 2010; Vucevic 1994). However we believe that the potential for selection bias is low in these studies.
In 19 studies (US N = 16 (Alderson 1992; Armstrong 1993; Böck 1999; Chuan 2005; Denys 1993; Hayashi 1998; Hayashi 2002; Heatly 1995; Johnson 1994; Lin 1998; Mallory 1990; Ovezov 2010; Soyer 1993; Teichgräber 1997; Troianos 1990; Troianos 1991), USD N = 1 (Legler 1983), US and USD N = 2 ( Verghese 1995; Verghese 1996)), it remains unclear whether there were withdrawals. In 15 studies no withdrawals were reported, and in one study, withdrawals were described (Hrics 1998).
In seven studies (US N = four (Chuan 2005; Grebenik 2004; Hrics 1998; Palepu 2009), USD N = 2 (Branger 1995; Gratz 1994), US and USD N = 1 (Scherhag 1989)) participants were excluded after randomization, in 23 studies no postrandomization exclusion occurred and in five studies this remains unclear (US N = four (Alderson 1992; Heatly 1995; Johnson 1994; Ovezov 2010), USD N = 1 (Verghese 1995)).
No intention‐to‐treat (ITT) analyses were performed in nine studies (US N = 5 (Chuan 2005; Grebenik 2004; Hrics 1998; Johnson 1994; Palepu 2009), USD N = 3 (Branger 1994; Branger 1995; Gratz 1994), US and USD N = 1 (Scherhag 1989)). In 17 studies ITT analyses were performed (Alderson 1992; Bansal 2005; Böck 1999; Denys 1993; Gilbert 1995; Karakitsos 2006; Legler 1983; Leung 2006; Mallory 1990; Milling 2005; Soyer 1993; Sulek 2000; Teichgräber 1997; Troianos 1990; Troianos 1991; Turker 2009; Vucevic 1994), and in nine studies it is unclear whether ITT analyses were performed.
In none of the studies did we find an excessive dropout rate.
Selective reporting
In no study can selective reporting (selective availability of data; selective reporting of outcomes, time points, subgroups or analyses) be excluded because none of the studies had a published protocol.
Two of the studies were not free from the suggestion of selective outcome reporting but had low risk of bias (LM group complication rate indicated, US group complication rate not indicated (Hayashi 2002; Scherhag 1989)).
We believe that all other studies were free from the suggestion of selective outcome reporting. Outcomes listed in the Methods section (if a Methods section was provided) were reported in the Results section in all studies.
Other potential sources of bias
A priori sample size calculations were conducted in none of the studies. None of the studies was stopped early, for example, by the data monitoring committee. Conflicts of interest were not reported in any of the studies.
Effects of interventions
Summary of findings for the main comparison. Ultrasound guidance compared with anatomical landmarks for internal jugular vein cannulation for central vein catheterization.
| Ultrasound guidance compared with anatomical landmarks for internal jugular vein cannulation for central vein catheterization | ||||||
| Patient or population: patients with internal jugular vein cannulation for central vein catheterization Settings: Intervention: ultrasound guidance Comparison: anatomical landmark | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anatomical landmark | Ultrasound guidance | |||||
| Complication rate total | Study population | RR 0.29 (0.17 to 0.52) | 2406 (14 studies) | ⊕⊝⊝⊝ Very lowa,b,c,d | ||
| 135 per 1000 | 39 per 1000 (23 to 70) | |||||
| Moderate | ||||||
| 136 per 1000 | 39 per 1000 (23 to 71) | |||||
| Overall success rate | Study population | RR 1.12 (1.08 to 1.17) | 4340 (23 studies) | ⊕⊝⊝⊝ Very lowc,e,f,g | ||
| 876 per 1000 | 982 per 1000 (946 to 1000) | |||||
| Moderate | ||||||
| 850 per 1000 | 952 per 1000 (918 to 994) | |||||
| Number of attempts until success | Mean number of attempts until success in the intervention groups was 1.19 lower (1.45 to 0.92 lower) | 3302 (16 studies) | ⊕⊝⊝⊝ Very lowc,g,h,i | |||
| Arterial puncture | Study population | RR 0.28 (0.18 to 0.44) | 4388 (22 studies) | ⊕⊕⊝⊝ Lowc,j,k,l | ||
| 94 per 1000 | 26 per 1000 (17 to 41) | |||||
| Moderate | ||||||
| 84 per 1000 | 24 per 1000 (15 to 37) | |||||
| Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) | Study population | RR 0.34 (0.15 to 0.76) | 3042 (11 studies) | ⊕⊕⊕⊝ Moderatec,m,n,o | ||
| 30 per 1000 | 10 per 1000 (4 to 23) | |||||
| Moderate | ||||||
| 23 per 1000 | 8 per 1000 (3 to 17) | |||||
| Time to successful cannulation | Mean time to successful cannulation in the intervention groups was 30.52 lower (55.21 to 5.82 lower) | 3451 (20 studies) | ⊕⊝⊝⊝ Very lowl,p,q,r | |||
| Success with attempt number 1 | Study population | RR 1.57 (1.36 to 1.82) | 2681 (18 studies) | ⊕⊕⊕⊝ Moderatec,s,t | ||
| 501 per 1000 | 787 per 1000 (682 to 912) | |||||
| Moderate | ||||||
| 545 per 1000 | 856 per 1000 (741 to 992) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aLack of allocation concealment: unclear in 8 of 14 studies, inadequate in 1 study. Incomplete outcome data addressed in 5 studies. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 7 from 14 studies, unclear in 2 studies. Treatment and control groups were adequately described at entry in 4 of 14 studies. bUnexplained substantial heterogeneity: P value 0.005; I² = 57%. cA precise result of appreciable benefit. dFunnel plot shows remarkable heterogeneity at the top and asymmetry at the bottom of the funnel. e Lack of allocation concealment: unclear in 15 of 23 studies, inadequate in 1 study. Incomplete outcome data addressed in 3 studies. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 7 from 23 studies, unclear in 3 studies. Treatment and control groups were adequately described at entry in 6 of 23 studies. fUnexplained substantial heterogeneity: P value < 0.00001, I² = 84%. gFunnel plot shows heterogeneity at the top and asymmetry at the bottom of the funnel. hLack of allocation concealment: unclear in 11 of 16 studies, inadequate in 1 study. Incomplete outcome data addressed in 1 study. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 6 from 16 studies, unclear in 2 studies. Treatment and control groups were adequately described at entry in 4 of 16 studies. iUnexplained substantial heterogeneity: P value < 0.00001, I² = 96%. jLack of allocation concealment: unclear in 14 of 22 studies, inadequate in 1 study. Incomplete outcome data addressed in 2 studies. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 11 from 22 studies, unclear in 4 studies. Treatment and control groups were adequately described at entry in 7 of 22 studies. kNo heterogeneity: P value 0.05, I² = 35%. lFunnel plot shows remarkable heterogeneity and asymmetry of the funnel. mLack of allocation concealment: unclear in 6 of 11 studies, inadequate in 1 study. Incomplete outcome data addressed in 1 study. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 6 from 11 studies, unclear in 3 studies. Treatment and control groups were adequately described at entry in 4 of 11 studies. nNo heterogeneity: P value 0.3, I² = 17%. oFewer than 10 trials for this endpoint. pLack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 7 from 20 studies, unclear in 2 studies. Treatment and control groups were adequately described at entry in 6 of 20 studies. qSubstantial heterogeneity: P value < 0.00001, I² = 97%. rAn imprecise result of appreciable or no appreciable effect. sLack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 6 from 18 studies, unclear in 4 studies. Treatment and control groups were adequately described at entry in 4 of 18 studies. tUnexplained substantial heterogeneity: P value < 0.00001, I² = 82%.
Summary of findings 2. Doppler guidance compared with anatomical landmarks for internal jugular vein cannulation for central vein catheterization.
| Doppler guidance compared with anatomical landmark for internal jugular vein cannulation for central vein catheterization | ||||||
| Patient or population: patients with internal jugular vein cannulation for central vein catheterization Settings: Intervention: Doppler guidance Comparison: Anatomical landmark | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anatomical landmark | Doppler guidance | |||||
| Complication rate total | Study population | RR 0.52 (0.16 to 1.71) | 93 (3 studies) | ⊕⊕⊝⊝ Lowa,b,c | ||
| 149 per 1000 | 77 per 1000 (24 to 255) | |||||
| Moderate | ||||||
| 188 per 1000 | 98 per 1000 (30 to 321) | |||||
| Overall success rate | Study population | RR 1.09 (0.95 to 1.25) | 289 (7 studies) | ⊕⊝⊝⊝ Very lowc,d,e,f | ||
| 800 per 1000 | 872 per 1000 (760 to 1000) | |||||
| Moderate | ||||||
| 800 per 1000 | 872 per 1000 (760 to 1000) | |||||
| Number of attempts until success | Mean number of attempts until success in the intervention groups was 0.63 lower (1.92 lower to 0.66 higher) | 69 (2 studies) | ⊕⊝⊝⊝ Very lowc,f,g,h | |||
| Arterial puncture | Study population | RR 0.61 (0.21 to 1.73) | 213 (6 studies) | ⊕⊕⊝⊝ Lowb,c,i,j | ||
| 75 per 1000 | 46 per 1000 (16 to 129) | |||||
| Moderate | ||||||
| 50 per 1000 | 31 per 1000 (10 to 87) | |||||
| Time to successful cannulation | Mean time to successful cannulation in the intervention groups was 62.04 higher (13.47 lower to 137.55 higher) | 214 (5 studies) | ⊕⊕⊕⊝ Moderateb,c,k | |||
| Success with attempt number 1 | Study population | RR 1.58 (1.02 to 2.43) | 199 (4 studies) | ⊕⊕⊝⊝ Lowc,l | ||
| 390 per 1000 | 617 per 1000 (398 to 949) | |||||
| Moderate | ||||||
| 423 per 1000 | 668 per 1000 (431 to 1000) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aNo heterogeneity: P value 0.72; I² = 0%. bAn imprecise result including appreciable benefit or harm. Total number of events is less than 300. cFewer than 10 trials for this endpoint, dLack of allocation concealment: unclear in all 7 studies. Incomplete outcome data addressed in 3 studies. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 2 from 7 studies, unclear in 2 studies. Treatment and control groups were adequately described at study entry in 2 of 7 studies. eUnexplained substantial heterogeneity: P value 0.001; I² = 72%. fAn imprecise result of appreciable or no appreciable effect. Total number of events is less than 300. gLack of allocation concealment: unclear in 2 of 2 studies. Incomplete outcome data addressed in 2 studies. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in neither of the 2 studies. Treatment and control groups were adequately described at study entry in none of the studies. hUnexplained substantial heterogeneity: P value 0.05; I² = 75%. iLack of allocation concealment: unclear in 6 of 6 studies. Incomplete outcome data addressed in 2 studies. Lack of blinding: Participants, operators and outcome assessors are aware of the arm to which participants are allocated in none of the studies. Free of other bias in 1 from 6 studies, unclear in 2 studies. Treatment and control groups were adequately described at entry in 1 of 6 studies. jNo heterogeneity: P value 0.96; I² = 0%. kNo heterogeneity: P value 0.09; I² = 50%. lUnexplained substantial heterogeneity: P value 0.07; I² = 57%.
Almost all of the included studies had high risk of bias, and heterogeneity was substantial. Our results therefore must be interpreted with caution. Further, our planned sensitivity analyses were not feasible, as these trials could not be separated into 'high quality' and 'poor quality' studies.
The results are presented in two sections.
A. Anatomical landmark versus two‐dimensional ultrasound.
B. Anatomical landmark versus Doppler ultrasound.
For each outcome, differential effects between studies in which ultrasound was used for puncture, or indirectly to locate the vein before puncture, or for which the method was not reported, when available, can be found in the tables within the ‘Data and analyses’ section later in the review. None of the studies assessed participant discomfort during the procedure, and none assessed mortality.
Section A. Landmark versus two‐dimensional ultrasound
Heterogeneity was substantial for all comparisons except the adult subgroup analysis for the risk of arterial puncture. A random‐effects model was used for all analyses.
1. Total number of perioperative and postoperative complications/adverse events
All participants
This outcome was reported in 14 trials, including 2406 participants (Agarwal 2009; Bansal 2005; Böck 1999; Denys 1993; Grebenik 2004; Heatly 1995; Leung 2006; Lin 1998; Milling 2005; Palepu 2009; Soyer 1993; Turker 2009; Verghese 1995; Verghese 1996) (see Figure 4 and Figure 5). Use of two‐dimensional ultrasound decreased the total number of perioperative and postoperative complications by 71% (risk ratio (RR) 0.29, 95% confidence interval (CI) 0.17 to 0.52; P value < 0.0001, I² = 57%) (see Analysis 1.1). The quality of evidence was very low (Table 1). The inverted funnel plot for the primary outcome of the total number of perioperative and postoperative complications/adverse events did suggest publication bias, but trials were relatively few to permit an accurate assessment (Figure 5).
4.
Forest plot of comparison: 1 Traditional landmark versus ultrasound guidance for internal jugular vein cannulation for central vein catheterization, outcome: 1.1 Complication rate total.
5.

Funnel plot of comparison: 1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization, outcome: 1.1 Complication rate total.
1.1. Analysis.
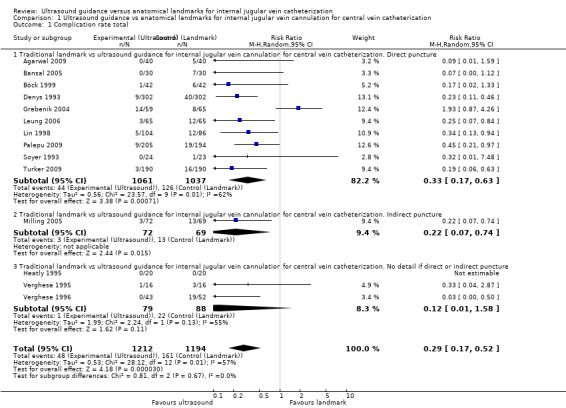
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 1 Complication rate total.
The funnel plot including all studies of traditional landmark guidance versus ultrasound guidance for internal jugular vein cannulation for central vein catheterization shows marked heterogeneity at the top and asymmetry at the bottom of the funnel. The small studies by Verghese 1996 (RR 0.03, 95% CI 0.00 to 0.50; 43 vs 52 participants) and Grebenik 2004 (RR 1.93, 95% CI 0.87 to 4.26; 59 vs 65 participants) may be considered outliers. They may indicate risk for publication bias (i.e. small studies with null effect are less likely to get published) or very poor implementation of the experimental intervention, respectively. However, inclusion of both outlying studies in the analysis seems to result in a conservative estimate of treatment effect in favour of the experimental intervention.
Adults
This outcome was analysable in 10 studies (Agarwal 2009; Bansal 2005; Böck 1999; Denys 1993; Heatly 1995; Leung 2006; Lin 1998; Palepu 2009; Soyer 1993; Turker 2009) including 2014 adults. Use of two‐dimensional ultrasound decreased the total number of perioperative and postoperative complications and reduced the complication rate by 73% (RR 0.27, 95% CI 0.18 to 0.40; P value < 0.00001, I² = 0%) (see Analysis 3.1). The inverted funnel plot for this outcome did not suggest publication bias, but trials were relatively few to permit an accurate assessment.
3.1. Analysis.
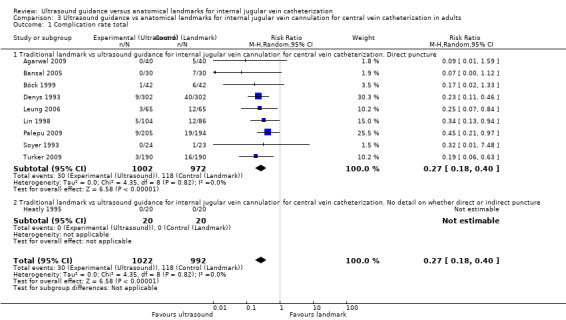
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 1 Complication rate total.
Children
This outcome was studied in four trials including 291 children (Alderson 1992; Grebenik 2004; Verghese 1995; Verghese 1996). No evidence was found of a reduction in complications with the use of ultrasound (RR 0.37, 95% CI 0.09 to 1.46; P value 0.16, I² = 77%) (see Analysis 4.1).
4.1. Analysis.
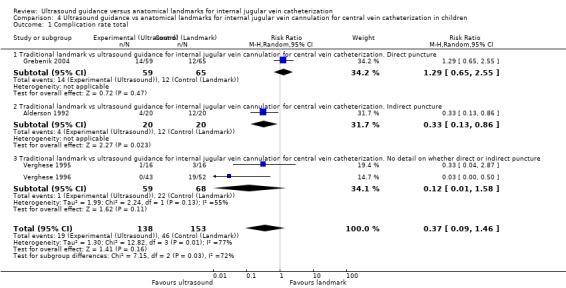
Comparison 4 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children, Outcome 1 Complication rate total.
Inexperienced operators
Data for this subgroup were presented in five studies including 643 participants (Bansal 2005; Grebenik 2004; Soyer 1993; Turker 2009; Verghese 1995). No evidence was found of a reduction in complications for inexperienced operators (RR 0.35, 95% CI 0.10 to 1.28; P value 0.11, I² = 67%) (see Analysis 5.1).
5.1. Analysis.

Comparison 5 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and inexperienced operators, Outcome 1 Complication rate total.
Experienced operators
Data for this subgroup were presented in eight studies including 1532 participants. Use of two‐dimensional ultrasound decreased the total number of perioperative and postoperative complications by 71% (RR 0.29, 95% CI 0.19 to 0.43; P value < 0.00001, I² = 0%) (see Analysis 6.1).
6.1. Analysis.

Comparison 6 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators, Outcome 1 Complication rate total.
2. Overall success rate
All participants
This outcome was reported in 23 trials including 4340 participants (Alderson 1992; Armstrong 1993; Bansal 2005; Chuan 2005; Denys 1993; Grebenik 2004; Hayashi 2002; Heatly 1995; Hrics 1998; Johnson 1994; Karakitsos 2006; Leung 2006; Lin 1998; Mallory 1990; Milling 2005; Ovezov 2010; Palepu 2009; Scherhag 1989; Soyer 1993; Troianos 1990; Troianos 1991; Turker 2009; Verghese 1996). Use of two‐dimensional ultrasound increased the overall success rate by 12% (RR 1.12, 95% CI 1.08 to 1.17; P value < 0.00001, I² = 85%) (see Analysis 1.2). The quality of the evidence was very low (Table 1).
1.2. Analysis.
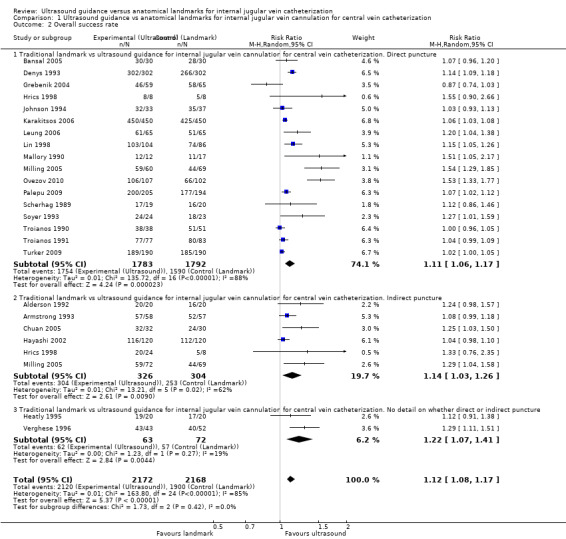
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 2 Overall success rate.
Adults
This outcome was presented in 18 trials including 3669 participants (Armstrong 1993; Bansal 2005; Denys 1993; Hayashi 2002; Heatly 1995; Hrics 1998; Johnson 1994; Karakitsos 2006; Leung 2006; Lin 1998; Mallory 1990; Milling 2005; Palepu 2009; Scherhag 1989; Soyer 1993; Troianos 1990; Troianos 1991; Turker 2009). Use of two‐dimensional ultrasound increased the overall success rate by 9% (RR 1.09, 95% CI 1.05 to 1.13; P value < 0.00001, I² = 80%) (see Analysis 3.2).
3.2. Analysis.
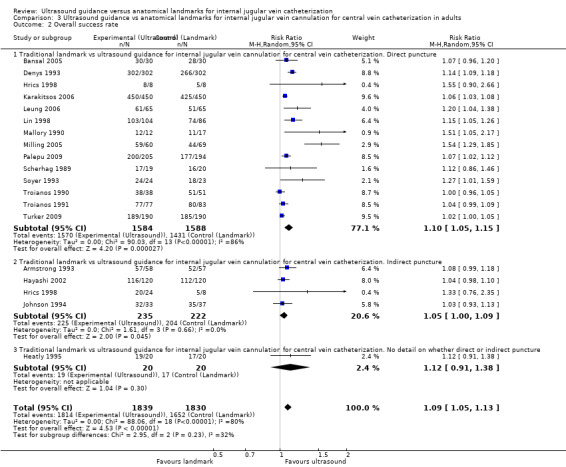
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 2 Overall success rate.
Children
This outcome was reported in five studies, including 530 children (Alderson 1992; Chuan 2005; Grebenik 2004; Ovezov 2010; Verghese 1996). Use of two‐dimensional ultrasound increased the overall success rate by 22% (RR 1.22, 95% CI 1.00 to 1.49; P value 0.05, I² = 85%) (see Analysis 4.2).
4.2. Analysis.

Comparison 4 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children, Outcome 2 Overall success rate.
Inexperienced operators
This outcome was reported in 13 studies including 1427 participants (Armstrong 1993; Bansal 2005; Chuan 2005; Grebenik 2004; Heatly 1995; Hrics 1998; Johnson 1994; Ovezov 2010; Scherhag 1989; Soyer 1993; Troianos 1990; Troianos 1991; Turker 2009). Use of two‐dimensional ultrasound increased the overall success rate by 9% (RR 1.09, 95% CI 1.02 to 1.16; P value 0.01, I² = 86%) (see Analysis 5.2).
5.2. Analysis.
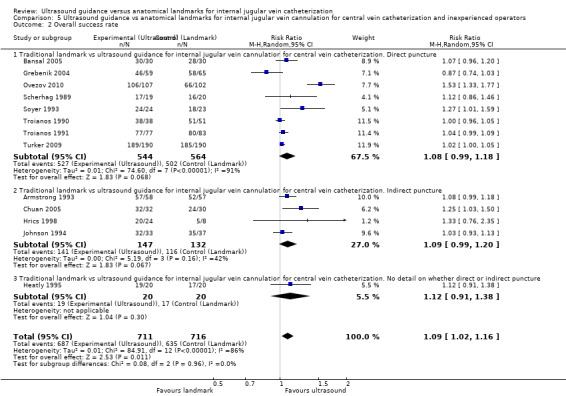
Comparison 5 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and inexperienced operators, Outcome 2 Overall success rate.
Experienced operators
This outcome was reported in nine studies including 2513 participants (Alderson 1992; Denys 1993; Hayashi 2002; Hrics 1998; Karakitsos 2006; Lin 1998; Mallory 1990; Palepu 2009; Verghese 1996). Use of two‐dimensional ultrasound increased the overall success rate by 11% (RR 1.11, 95% CI 1.06 to 1.16; P value < 0.00001, I² = 72%) (see Analysis 6.2).
6.2. Analysis.

Comparison 6 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators, Outcome 2 Overall success rate.
3. Number of attempts until success
All participants
This outcome was reported in 16 trials including 3302 participants (Agarwal 2009; Alderson 1992; Armstrong 1993; Chuan 2005; Denys 1993; Johnson 1994; Karakitsos 2006; Lin 1998; Milling 2005; Ovezov 2010; Soyer 1993; Sulek 2000; Troianos 1990; Troianos 1991; Turker 2009; Verghese 1996). Use of two‐dimensional ultrasound decreased the number of attempts needed to succeed (mean difference (MD) ‐1.19 attempts, 95% CI ‐1.45 to ‐0.92; P value < 0.00001, I² = 96%) (see Analysis 1.3). The quality of the evidence was very low (Table 1).
1.3. Analysis.

Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 3 Number of attempts until success.
Adults
This outcome was reported in 12 studies including 2896 participants (Agarwal 2009; Armstrong 1993; Denys 1993; Johnson 1994; Karakitsos 2006; Lin 1998; Milling 2005; Soyer 1993; Sulek 2000; Troianos 1990; Troianos 1991; Turker 2009). Use of two‐dimensional ultrasound decreased the number of attempts needed to succeed (MD ‐1.18 attempts, 95% CI ‐1.50 to ‐0.85; P value < 0.00001, I² = 93%) (see Analysis 3.3).
3.3. Analysis.

Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 3 Number of attempts until success.
Children
This outcome was reported in four studies including 406 children. If one looks at these studies, which exclusively included children (Alderson 1992; Chuan 2005; Ovezov 2010; Verghese 1996), use of two‐dimensional ultrasound decreased the number of attempts needed to succeed (MD ‐1.24 attempts, 95% CI ‐1.72 to ‐0.77; P value < 0.00001, I² = 75%) (see Analysis 4.3).
4.3. Analysis.

Comparison 4 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children, Outcome 3 Number of attempts until success.
Inexperienced operators
Data were presented for this outcome in eight studies including 1132 participants. If one looks at the eight studies, which exclusively included inexperienced operators (D: Ovezov 2010; Soyer 1993; Troianos 1990; Troianos 1991; Turker 2009; ID: Armstrong 1993; Chuan 2005; Johnson 1994), use of two‐dimensional ultrasound decreased the number of attempts needed to succeed (MD ‐1.21 attempts, 95% CI ‐1.59 to ‐0.83; P value < 0.00001, I² = 97%) (see Analysis 5.3).
5.3. Analysis.

Comparison 5 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and inexperienced operators, Outcome 3 Number of attempts until success.
Experienced operators
Data were presented for this outcome in seven studies including 2029 participants (Agarwal 2009; Alderson 1992; Denys 1993; Karakitsos 2006; Lin 1998; Sulek 2000; Verghese 1996). Use of two‐dimensional ultrasound decreased the number of attempts needed to succeed (MD ‐1.09, 95% CI ‐1.52 to ‐0.66; P value < 0.00001, I² = 88%) (see Analysis 6.3).
6.3. Analysis.

Comparison 6 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators, Outcome 3 Number of attempts until success.
4. Number of participants with an arterial puncture
All participants
In 22 studies including 4388 participants, the overall number of participants with an arterial puncture was reported (Agarwal 2009; Alderson 1992; Armstrong 1993; Bansal 2005; Böck 1999; Chuan 2005; Denys 1993; Grebenik 2004; Hayashi 1998; Hayashi 2002; Karakitsos 2006; Leung 2006; Lin 1998; Ovezov 2010; Palepu 2009; Soyer 1993; Sulek 2000; Teichgräber 1997; Troianos 1990; Troianos 1991; Turker 2009; Verghese 1996). Use of two‐dimensional ultrasound decreased the number of participants with an arterial puncture by 72% (RR 0.28, 95% CI 0.18 to 0.44; P value < 0.00001, I² = 35%) (see Analysis 1.4). The quality of the evidence was low (Table 1).
1.4. Analysis.
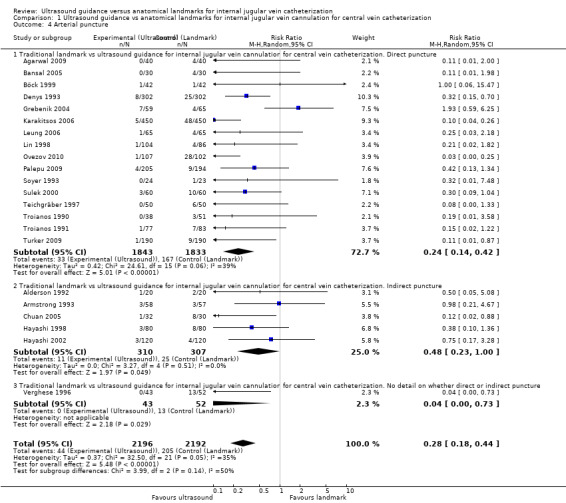
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 4 Arterial puncture.
Adults
This outcome was reported in 18 studies including 3920 adults. Use of two‐dimensional ultrasound decreased the number of participants with an arterial puncture by 74% (RR 0.26, 95% CI 0.18 to 0.37; P value < 0.00001, I² = 0%) (see Analysis 3.4).
3.4. Analysis.
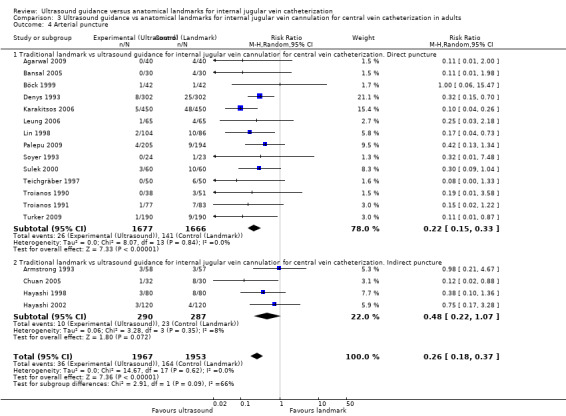
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 4 Arterial puncture.
Children
This outcome was reported in five studies, including 530 children. No evidence of a difference was found when two‐dimensional ultrasound was used (RR 0.20, 95% CI 0.03 to 1.35; P value 0.10, I² = 79%) (see Analysis 4.4).
4.4. Analysis.
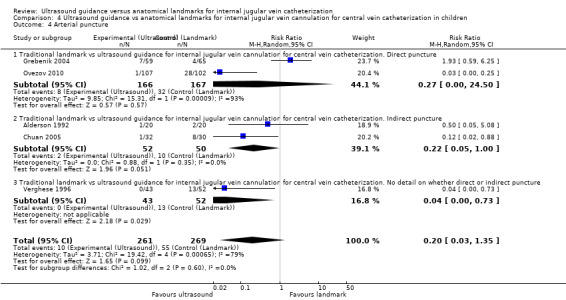
Comparison 4 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children, Outcome 4 Arterial puncture.
Experienced operators
Data were presented for this outcome in 10 studies including 2632 participants (Agarwal 2009; Alderson 1992; Armstrong 1993; Böck 1999; Denys 1993; Karakitsos 2006; Lin 1998; Palepu 2009; Sulek 2000; Teichgräber 1997). Use of two‐dimensional ultrasound decreased the number of participants with an arterial puncture by 73% (RR 0.27, 95% CI 0.17 to 0.44; P value < 0.00001, I² = 16%) (see Analysis 6.4).
6.4. Analysis.

Comparison 6 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators, Outcome 4 Arterial puncture.
5. Number of participants with significant haematoma formation
All participants
The number of participants with significant haematoma formation was reported in 13 trials including 3233 participants (Agarwal 2009; Bansal 2005; Böck 1999; Chuan 2005; Denys 1993; Grebenik 2004; Karakitsos 2006; Leung 2006; Lin 1998; Palepu 2009; Sulek 2000; Teichgräber 1997; Turker 2009). Use of two‐dimensional ultrasound decreased the number of participants with significant haematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P value 0.0004, I² = 54%) (see Analysis 1.5). The quality of the evidence was very low.
1.5. Analysis.
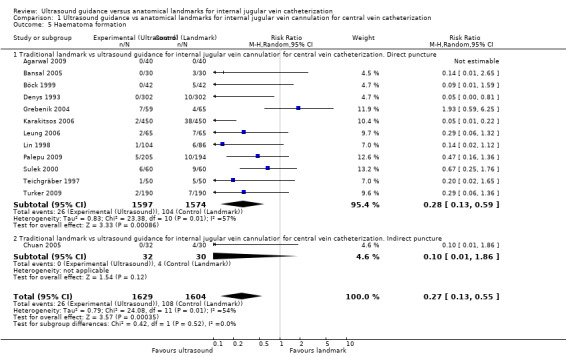
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 5 Haematoma formation.
Adults
This outcome was reported in 11 studies including 3047 participants (Agarwal 2009; Bansal 2005; Böck 1999; Denys 1993; Karakitsos 2006; Leung 2006; Lin 1998; Palepu 2009; Sulek 2000; Teichgräber 1997; Turker 2009). Use of two‐dimensional ultrasound decreased the number of participants with significant haematoma formation by 77% (RR 0.23, 95% CI 0.12 to 0.44; P value < 0.00001, I² = 35%) (see Analysis 3.5).
3.5. Analysis.
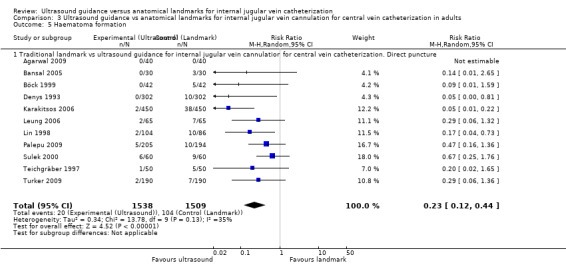
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 5 Haematoma formation.
6. Number of participants with other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury)
All participants
This outcome was reported in 11 trials including 3042 participants (Agarwal 2009; Alderson 1992; Denys 1993; Grebenik 2004; Karakitsos 2006; Leung 2006; Lin 1998; Palepu 2009; Teichgräber 1997; Turker 2009; Verghese 1996). Use of two‐dimensional ultrasound decreased the number of participants with other complications by 66% (RR 0.34, 95% CI 0.15 to 0.76; P value 0.009, I² = 17%) (see Analysis 1.6). The quality of the evidence was moderate (Table 1).
1.6. Analysis.
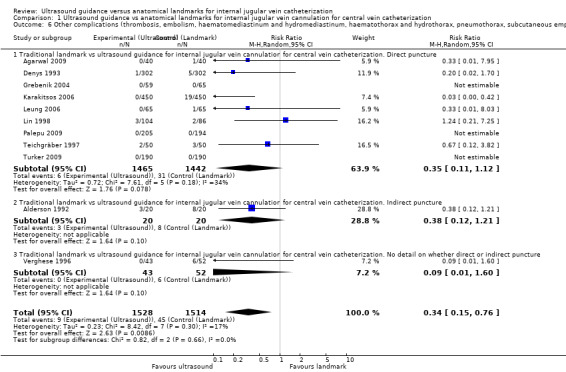
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 6 Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury).
Adults
In adults (nine trials, 2830 adults) (Agarwal 2009; Denys 1993; Karakitsos 2006; Leung 2006; Lin 1998; Palepu 2009; Soyer 1993; Teichgräber 1997; Turker 2009), no evidence of a difference was found (RR 0.35, 95% CI 0.11 to 1.12; P value 0.08, I² = 34%) (see Analysis 3.6).
3.6. Analysis.
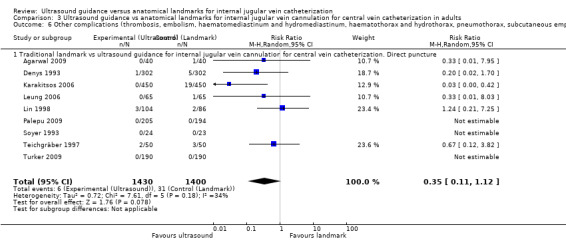
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 6 Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury).
Children
In children (three trials, 259 children), use of two‐dimensional ultrasound decreased the number of participants with other complications by 73% (RR 0.27, 95% CI 0.10 to 0.76; P value 0.01, I² = 0%) (see Analysis 4.5).
4.5. Analysis.

Comparison 4 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children, Outcome 5 Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury).
7. Time to successful cannulation
Overall, 14 different definitions of time taken for cannulation were reported in 20 trials including 3451 participants. Overall, use of two‐dimensional ultrasound decreased the time to successful cannulation by 30.52 seconds (MD ‐30.52 seconds, 95% CI ‐55.21 to ‐5.82; P value 0.02, I² = 97%) (see Analysis 1.7). The quality of the evidence was very low (Table 1).
1.7. Analysis.
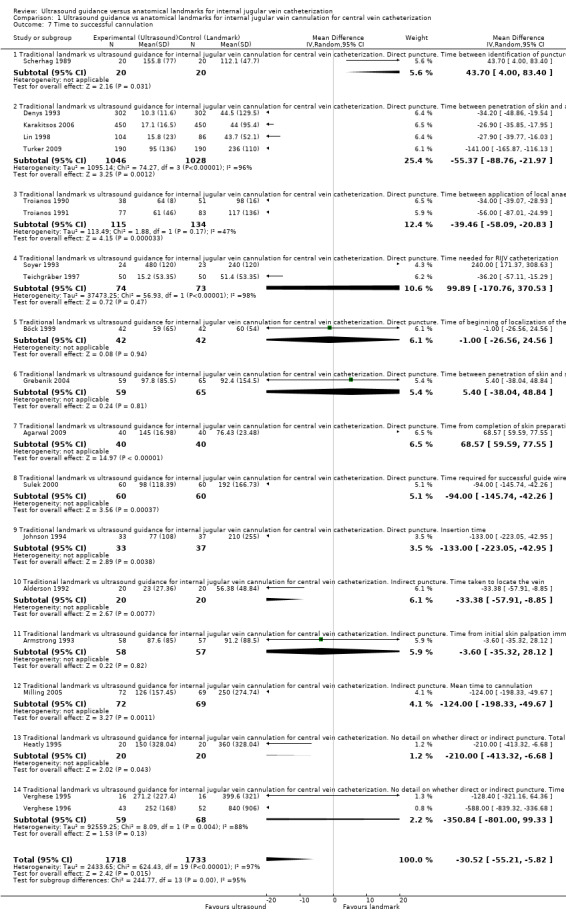
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 7 Time to successful cannulation.
This finding was not repeated in the subgroups examined: adults (11 different definitions, 16 trials, 3160 participants) (MD ‐13.07 seconds, 95% CI ‐40.57 to 14.44; P value 0.35, I² = 98%) (see Analysis 3.7); children (three different definitions, four trials, 291 children) (MD ‐90.70 seconds, 95% CI ‐184.74 to 3.35; P value 0.06, I² = 87%) (see Analysis 4.6); inexperienced operators (eight different definitions, nine trials, 1057 participants) (MD 5.6 seconds, 95% CI ‐50.51 to 61.71; P value 0.84, I² = 97%) (see Analysis 5.4); and experienced operators (seven trials, 2073 participants) (MD ‐31.9 seconds, 95% CI ‐76.07 to 12.28; P value 0.16, I² = 98%) (see Analysis 6.6). We made no further differentiation regarding the different times, as a variety of definitions of time to successful cannulation were involved.
3.7. Analysis.
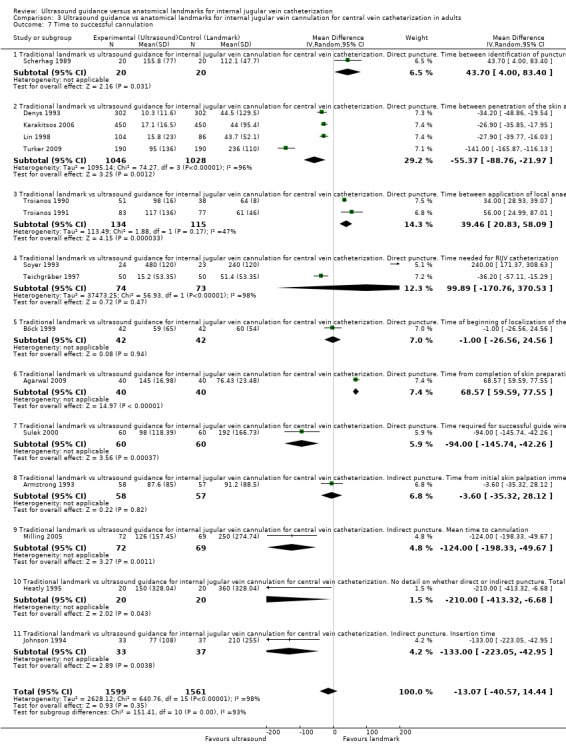
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 7 Time to successful cannulation.
4.6. Analysis.

Comparison 4 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children, Outcome 6 Time to successful cannulation.
5.4. Analysis.

Comparison 5 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and inexperienced operators, Outcome 4 Time to successful cannulation.
6.6. Analysis.

Comparison 6 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators, Outcome 6 Time to successful cannulation.
8. Success on the first attempt
Overall, success at the first attempt was reported in 18 trials including 2681 participants (Agarwal 2009; Armstrong 1993; Bansal 2005; Böck 1999; Denys 1993; Hayashi 1998; Hrics 1998; Johnson 1994; Leung 2006; Lin 1998; Mallory 1990; Milling 2005; Ovezov 2010; Palepu 2009; Scherhag 1989; Teichgräber 1997; Troianos 1990; Troianos 1991). Use of two‐dimensional ultrasound increased the chance of success at the first attempt by 57% (RR 1.57, 95% CI 1.36 to 1.82; P value < 0.00001, I² = 82%) (see Analysis 1.8). The quality of the evidence was moderate (Table 1). In adults—the only subgroup for which data were available (15 studies, 2291 adults)—use of two‐dimensional ultrasound increased the chance of success at the first attempt by 51% (RR 1.51, 95% CI 1.30 to 1.75; P value < 0.00001, I² = 82%) (see Analysis 3.8).
1.8. Analysis.
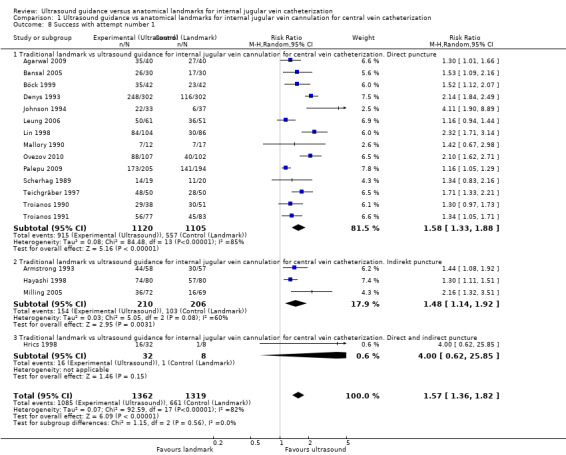
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 8 Success with attempt number 1 .
3.8. Analysis.
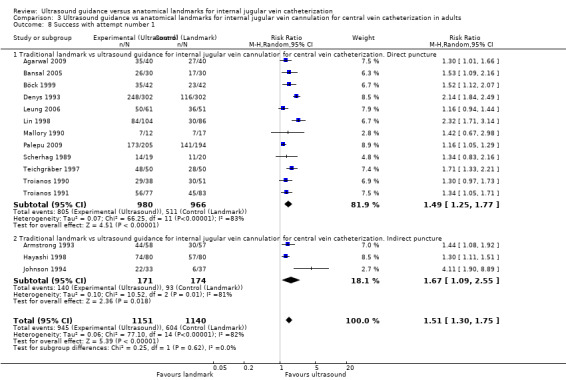
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 8 Success with attempt number 1 .
9. Success on the second attempt
Success on the second attempt was reported in six trials including 1156 adults (Böck 1999; Denys 1993; Hayashi 2002; Lin 1998; Mallory 1990; Troianos 1990). Use of two‐dimensional ultrasound increased the chance of success at the second attempt by 19% (RR 1.19, 95% CI 1.07 to 1.32; P value 0.001, I² = 78%) (see Analysis 1.9). The quality of the evidence was low.
1.9. Analysis.
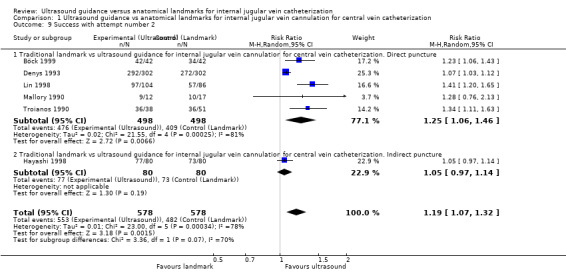
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 9 Success with attempt number 2.
10. Success on the third attempt
Success on the third attempt was reported in two trials including 189 adults (Hayashi 2002; Mallory 1990). No evidence of a difference was found when ultrasound was used (RR 1.22, 95% CI 0.66 to 2.28; P value 0.52, I² = 88%) (see Analysis 1.10).
1.10. Analysis.
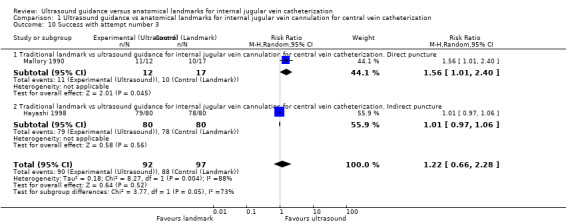
Comparison 1 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 10 Success with attempt number 3.
Section B. Landmark versus Doppler ultrasound
For many of the analyses, it was not possible to perform subgroup analyses because the relevant groups of participants had not been studied. Heteregeneity was largely low, except for time to successful cannulation, for which it was moderate. We used a random‐effects model throughout.
1. Total number of perioperative and postoperative complications/adverse events
The total number of perioperative and postoperative complications/adverse events was reported in three trials including 93 participants (Branger 1994; Legler 1983; Verghese 1995). No evidence was found of a difference when Doppler ultrasound was used (RR 0.52, 95% CI 0.16 to 1.71; P value 0.28, I² = 0%) (see Analysis 2.1). The quality of the evidence was low (Table 2).
2.1. Analysis.

Comparison 2 Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 1 Complication rate total.
2. Overall success rate
The overall success rate was reported in seven trials including 289 participants (Branger 1994; Branger 1995; Gilbert 1995; Gratz 1994; Legler 1983; Scherhag 1989; Vucevic 1994). No evidence of a difference in this outcome was found (RR 1.09, 95% CI 0.95 to 1.25; P value 0.20, I² = 72%) (see Analysis 2.2). The quality of the evidence was very low (Table 2).
2.2. Analysis.
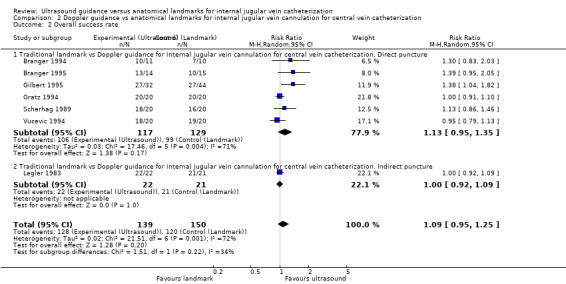
Comparison 2 Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 2 Overall success rate.
3. Number of attempts until success
The total number of attempts until success was reported in two trials including 69 participants (Branger 1995; Gratz 1994). No evidence of a difference in this outcome was found (MD ‐0.63, 95% CI ‐1.92 to 0.66; P value 0.34, I² = 75%) (see Analysis 2.3). The quality of the evidence was very low (Table 2).
2.3. Analysis.
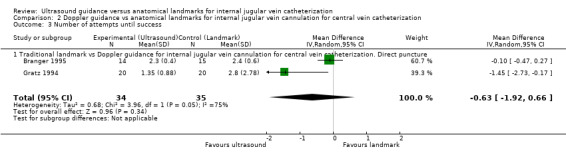
Comparison 2 Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 3 Number of attempts until success.
4. Number of participants with an arterial puncture
The overall number of participants with an arterial puncture was reported in six trials including 213 participants (Branger 1994; Gratz 1994; Legler 1983; Scherhag 1989; Verghese 1995; Vucevic 1994). No evidence of a difference for this outcome was found (RR 0.61, 95% CI 0.21 to 1.73; P value 0.35, I² = 0%) (see Analysis 2.4). The quality of the evidence was low (Table 2).
2.4. Analysis.
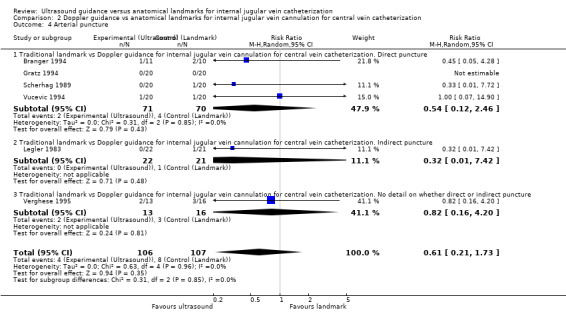
Comparison 2 Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 4 Arterial puncture.
5. Number of participants with significant haematoma formation
This outcome was reported in only one trial.
6. Number of participants with other complications
None of the trial authors reported this outcome.
7. Time to successful cannulation
We included five trials (214 participants), each using a different definition for this outcome (Branger 1994; Gilbert 1995; Gratz 1994; Scherhag 1989; Verghese 1995). No evidence of a difference in this outcome was found (MD 62.04 seconds, 95% CI ‐13.47 to 137.55; P value 0.11, I² = 50%). We made no further differentiation regarding the different times, as such a variety of definitions were involved (see Analysis 2.5). The quality of the evidence was moderate (Table 2).
2.5. Analysis.

Comparison 2 Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 5 Time to successful cannulation.
8. Success on the first attempt
This outcome was reported in four trials including 199 participants (Gilbert 1995; Gratz 1994; Legler 1983; Scherhag 1989). Overall, use of Doppler ultrasound increased the chance of success at the first attempt by 58% (RR 1.58, 95% CI 1.02 to 2.43; P value 0.04, I² = 57%) (see Analysis 2.6). The quality of the evidence was low (Table 2).
2.6. Analysis.
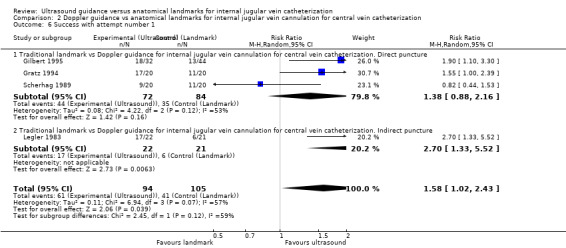
Comparison 2 Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization, Outcome 6 Success with attempt number 1.
9. Success on the second attempt
Success with attempt number two was reported in only one trial (Scherhag 1989).
10. Success on the third attempt
Success with attempt number three was reported in only one trial (Scherhag 1989).
Discussion
Summary of main results
Our analyses of available data suggest that two‐dimensional ultrasound improves many, but not all, aspects of the effectiveness and safety of venous catheter insertion into the internal jugular vein.
The methodological quality of the studies varied from very low to moderate (see Table 1 and Table 2). Based on available evidence, use of two‐dimensional ultrasound reduced the rate of total complications (all participants, adults, experienced operators), the number of participants with an inadvertent arterial puncture (all participants, adults, experienced operators) and the time taken for successful cannulation (all participants). It also increased overall success rates (all participants, adults, children, inexperienced operators, experienced operators) and decreased the number of attempts needed for successful cannulation (all groups). It increased the chance of success at the first attempt (all participants, adults) whilst reducing the chance of haematoma formation (all participants, adults, experienced operators). Further, more data are available to support the use of ultrasound during, not simply before, line insertion. Because of missing data, we did not compare the effects in experienced versus inexperienced operators for all outcomes (arterial puncture, haematoma formation, other complications, success with attempt number one), and so the relative utility of ultrasound in these groups remains unclear, and no data are available on use of this technique in patients at high risk for complications.
Use of Doppler ultrasound increased the chance of success at the first attempt. No evidence was found of differences in the total number of perioperative and postoperative complications/adverse events, the overall success rate, the total number of attempts until success, the overall number of participants with an arterial puncture and the time to successful cannulation when Doppler ultrasound was used. It was not possible to perform analyses for the other outcomes because they were reported in only one trial.
None of the studies addressed the impact of ultrasound guidance on mortality, length of hospital stay or patient‐reported outcomes (pain, discomfort). Finally, whether infection rates are increased by the use of ultrasonic apparatus because the transducer is brought into the puncture field, which may possibly lead to local infection, or if the number of required puncture attempts is reduced, was investigated by none of the reviewed studies and remains unanswered, as does the question of whether shorter puncture duration and smaller numbers of punctures of the arteria carotis and haematomas lead to a reduction in the infection rate.
Our review was not able to provide a complete answer to the question of whether ultrasound helps inexperienced practitioners more (or indeed less) than it helps experienced staff. Using ultrasound safely requires consideration of the following points. Use of US for vascular access requires training (Feller‐Kopman 2007; Lamperti 2010; Resnick 2008). The operator should learn the physical fundamentals of the procedure and its limitations, and should learn to deal with the equipment used (image optimization, probe manipulation, imaging techniques) and simultaneous handling of the transducer and the needle both inside and outside of the plane (French 2008). The operator should then practise under experienced supervision (Feller‐Kopman 2007), as with adequate training in US‐guided vascular access, complications are reduced (Seto 2010; Schoenfeld 2011), but this approach may be harmful if training is inadequate (Weiner 2012). Whether the infection rate is increased by the use of ultrasonic apparatus because the transducer is brought into the puncture field, may lead to additional local infection, or if the number of required puncture attempts is reduced, was investigated by none of the reviewed studies and remains unanswered, as does the question of whether shorter puncture duration and smaller numbers of punctures of the arteria carotis and haematomas lead to a reduction in the infection rate.Aseptic procedures should be performed to avoid infection. Current guidelines of the Centers for Disease Control and Prevention (CDC) suggest that sterile US cover shields should be used to reduce the risk of central line–associated bloodstream infection (CLABSI).
The results of our analyses must be interpreted with caution for several reasons. The methodological quality of the evidence was very low or low for most of the outcomes and was moderate at best for four of the outcomes. Most of the included trials had unclear risk of bias across the six domains and heterogeneity among the studies was significant. Possible reasons for this are the various access approaches, patient positions and techniques of both puncture and cannulation that were used. Another major problem in evaluating these studies was that exact details on the training experience of the operators for each method were absent or inaccurate, and that the experience that the operators had with each method was very unevenly distributed in most of the studies. It must be pointed out that in many studies included in this review, operators with limited experience in US‐guided vascular access techniques were included; however, these techniques require training and experience for optimization of the risk‐benefit ratio. Experience with the landmark technique and limited practice with US‐guided vascular access will lead to an underestimation of the potential beneficial effects of the US‐guided technique. In addition, only one study describes the 'learning curve' of the operators within the study, and this only for those performing the US technique. These factors could have introduced significant bias in either direction. Additional limitations included the unblinded design (operator bias, outcome assessor bias) and failure to clearly define the outcomes measured. It is not clear whether the results mentioned above and the conclusions derived from them are also valid for emergency procedures. Unfortunately none of the studies evaluated for this review contains a cost‐benefit analysis for ultrasound guidance. In addition, more than half of the studies reviewed are older than 15 years. So they were performed at a time when the technology of the equipment and experience in dealing with it were still significantly limited.
In general, it will become more difficult in the future to justify catheterization of the internal jugular vein without ultrasound. In time, use of ultrasound for invasive procedures is likely to become as fundamental a part of anaesthetic practice as preoperative fasting (Smith 1997). However, evidence is lacking for patients at higher risk of complications—for instance, in the presence of anatomical variation or difficult veins (obese patients, patients with oedema or haematomas, those with weak or missing arterial pulsations, children) or coagulation disorders. Ultrasound in itself will help screen for vessel patency and vascular abnormalities and variants. No evidence suggests whether it should be used from the outset, or whether it should be a 'fall back' technique when the landmark approach has failed, and opinions vary (Atkinson 2005; Calvert 2003; Muhm 2002; Scott 2004; Watters 2002). Formal guidance advocating the use of ultrasound‐guided catheterization is available from the US Agency for Healthcare Research and Quality in the United States (Shojania 2001), the UK National Institute of Clinical Excellence (NICE) (NICE 2002), the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists (Troianos 2012) and the American Society of Anesthesiologists (Rupp 2012).
The review authors' personal view is that ultrasound guidance should not be required in all patients. We think that it should be used at any rate in patients with anatomical variation or difficult veins (obese patients, patients with oedema or haematoma, those with weak or missing arterial pulsations, paediatric patients) or coagulative disorders. Also ultrasound is helpful in screening for vessel patency and vascular abnormalities and variants. Some experts believe that it is indefensible to not use ultrasound (Bodenham 2006). However, we believe it is vital to maintain skills with the landmark technique for use when ultrasound is not available (Brass 2001; NICE 2002), and to remind practitioners that it is not always necessary to slavishly follow guidance in cases where it is not indicated, although some are wary of medicolegal consequences if they do not (Augoustides 2009; Hessel 2009).
Likewise, we do not accept economic arguments against the widespread introduction of ultrasound‐guided methods; although none of our review data allow us to comment further on this, others have explored this aspect in greater detail (Calvert 2003; Calvert 2004; Kinsella 2009).
Applying guidelines to real‐life clinical practice can be difficult because their effectiveness is dependent upon many factors including clinician acceptance of them, workload, availability of equipment, frequency of assessments and continuing assessment and feedback to ensure compliance with them (Girard 2005; Tovey 2007). Also, data on patient‐relevant outcomes such as mortality or patient discomfort are sparse (small number of events for mortality) or are not available for any study (end‐organ damage) for adequate evaluation of the efficacy of using ultrasound techniques. Because our systematic review shows the benefit of using two‐dimensional ultrasound for real‐time sonographic cannulation of the internal jugular vein in most subgroups and groups of operators, it will become more difficult to justify use of the landmark technique in the future.
Overall completeness and applicability of evidence
The included 35 studies recruited 5108 patients with a variety of underlying diseases in a variety of settings and a variety of operators (different disciplines and experience), which should increase the applicability of the results.
Our systematic approach to the search, study selection and data extraction should have minimized the likelihood of missing relevant studies.
Because of our comprehensive search strategy, the additional handsearch and contact with different companies and experts in the field, we are confident that we have identified all randomized trials comparing ultrasound techniques for internal jugular vein puncture during central venous catheter instillation in adults and children with landmark‐guided puncture techniques.
With respect to the reports of Hayashi (Hayashi 1998; Hayashi 2002) and Troianos (Troianos 1990; Troianos 1991), we assumed that the two publications from each study author reported two separate studies. Regarding the study of Ovezov (Ovezov 2010), data are also available on the Internet; we wrote to the study author to ask for clarification and to request additional information related to study methods and data, but our enquiry remains unanswered. We included the study with conservative results.
Quality of the evidence
The quality of the evidence was very low for most of the outcomes (N = 5) and moderate at best for three of the outcomes for using US. For using USD the quality of the evidence was low (N = 3) or very low (N = 2) for most of the outcomes and moderate at best for one of the outcomes. Most of the trials had unclear risk of bias across the six domains and heterogeneity among the studies was significant.
We originally planned to undertake exploratory subgroup analysis to find out if contextual factors (type of operator, setting) or intervention factors (type of protocol or approach) were the cause of the heterogeneity. However, because of the wide variety of procedures, operators and circumstances under which cannulations took place, we performed subgroup analyses only on the impact of types of participants (adults, children) and experience of the operators.
It is not easy to isolate the reasons for heterogeneity because puncture of vessels with insertion of catheters is a complex process. It is plausible that the discordance in results among studies may be due to contextual factors (differences in participant populations and practice) or intervention factors.
In relation to intervention factors, many methodological differences among studies may have contributed to heterogeneity. In relation to risk of bias within studies, methodological quality ranged from very low to moderate. The intervention could not be blinded to personnel, which is understandable. It is plausible therefore that the unblinded nature of the intervention may have prompted a change in behaviour, and this may have affected results.
The methodological quality of the trials was moderate at best. Allocation concealment was described adequately in seven of 35 trials. In all studies outcome assessment was not blinded, or it was unclear. Clearly blinding of the operator is not possible in this type of work; however no trial except the one in which participants were sedated or anaesthetized attempted to blind the participant. Clinical heterogeneity was considerable in terms of the range of patients and operators studied, the approaches used and the ultrasound machines and probes involved. Further, different studies used different methods and time periods for puncturing the vein and placing the catheter.
Performance of central venous catheterization is clearly dependent on the expertise of the operator for the landmark and for the ultrasound method and technique used. Advances in medicine do not come simply from the availability of new technology but depend on how the technology is actually applied (Guimares 2009). The experience of practitioners and their faculties in both ultrasound techniques and control techniques and the number of practitioners involved varied across trials. In 10 of the studies no details on the experience of the operators who carried out the procedure were provided. In 25 of the studies details on the experience of the operators who carried out the procedure were provided. Procedures were carried out by medical students (Turker 2009) to experienced anaesthetists (Böck 1999; Gratz 1994; Vucevic 1994). Furthermore, whatever the experience of the operator, certain 'tacit' factors involved in performing practical procedures are not (and indeed cannot be) recorded in the report of a clinical trial but nevertheless influence the effectiveness and safety of the procedure (Goodwin 2005; Mort 2009). Some of these include non‐technical skills and, although less obvious, are an essential part of expert performance (Smith 2009; Smith 2010; Smith 2011). It may be that some of our findings (e.g. the apparent lack of benefit for experienced operators in number of attempts needed for success) are a result of the fact that these practitioners are already highly skilled. It is also possible that use of ultrasound may have differential effects on quality as opposed to safety, and even experienced operators can become safer even when their success rates do not improve.
The included studies cover a period of 21 years, during which considerable change has occurred in the technology of ultrasound devices and the availability of ultrasound in anaesthetic practice.
Potential biases in the review process
Our systematic approach to searching, selecting studies and extracting data should have minimized the likelihood of missing relevant studies. A very comprehensive search strategy was applied to identify all potential studies and their reports. However, although 35 studies were identified, information on several relevant outcome data prespecified in our protocol was not always or was never reported (patient discomfort). Several of these outcome measures are important in making an informed and balanced decision regarding which technique should be used in which situation. Some most likely were not ascertained during the trial; others could have been collected but not reported. Unfortunately, even after contacting the primary investigators, we have not been able to obtain additional data to date. We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (e.g. extracting data independently in duplicate to minimize error and reduce bias in the process).
One particular outcome deserves mention here: The definition of 'time to cannulation' varied considerably between studies. We made the decision to pool data for this outcome, but given high heterogeneity, the results should be interpreted with caution.
Given the lag time between the date of the search (January 2013) and publication of the review, it is possible that studies of interest were not considered. We reran the search in August 2014 and found five eligible studies (Airapetian 2013; Bikash 2014; Cajozzo 2004; Gok 2013; Shrestha 2011), which now are awaiting classification. We will deal with them when we update the review.
Agreements and disagreements with other studies or reviews
Seven meta‐analyses (Calvert 2003; Hind 2003; Keenan 2002; Randolph 1996; Rothschild 2001; Sigaut 2009; Wu 2013) have compared the effectiveness of ultrasound guidance versus the traditional landmark technique for central vein catheterization.
Calvert and Hind et al conducted a meta‐analysis to assess the evidence for clinical effectiveness of ultrasound‐guided central venous cannulation (Calvert 2003; Hind 2003). That meta‐analysis included only studies in which investigators used real‐time two‐dimensional ultrasonography or Doppler needles and probes and compared this method with the anatomical landmark method of cannulation, and in which the study authors used a different statistic and did not report any subgroup analysis. Their systematic reviews show clear benefit from two‐dimensional ultrasound guidance for central venous access compared with the landmark method. This was manifest in a lower technical failure rate (overall and on first attempt), a reduction in complications and faster access. The study authors wrote that one explanation for these benefits is that ultrasonography clarifies the relative position of the needle and the vein and its surrounding structures, and that the image offered by two‐dimensional ultrasonography allows the user to predict variant anatomy and to assess the patency of a target vein. The study authors concluded that "catheterization under two dimensional ultrasound guidance is quicker and safer than the landmark method in both adults and children. Two dimensional ultrasound guidance is more effective than Doppler ultrasound guidance for more difficult procedures."
Randolph et al conducted a meta‐analysis to evaluate the effect of real‐time ultrasound guidance using a regular or Doppler ultrasound technique for placement of central venous catheters (Randolph 1996). The results are similar to those of the previous meta‐analysis: however, this study inappropriately pooled the results from trials of both Doppler ultrasound guidance and two‐dimensional ultrasound guidance. Evidence presented in that analysis favours the use of two‐dimensional ultrasound guidance for cannulation of the subclavian vein, with Doppler ultrasound guidance less successful and more time consuming than even the landmark method. This method also proved more successful than Doppler ultrasound guidance or the landmark method when the internal jugular vein of infants was cannulated, with the image aiding the navigation of diminutive anatomy, although this evidence was derived from only one study. Ultrasound guidance therefore is likely to confer benefit to patients through a reduction in the risks of the procedure, and patients are less likely to undergo a prolonged, sometimes uncomfortable and possibly fruitless attempt at central venous cannulation. The study authors concluded that "when used for vessel location and catheter placement, real‐time ultrasound guidance or Doppler ultrasound guidance improves success rates and decreases the complications associated with internal jugular and subclavian venous catheter placement."
Keenan et al (Keenan 2002) found in their review that "adoption of real‐time ultrasound to guide CVC placement has the potential to improve successful line placement and minimized complications. It can improve patient safety. However, there are significant cost concerns and the reported adverse events are generally minor and easy to treat. Before creating study protocols to increase usage of this technology, both current usage and cost effectiveness should be determined."
Sigaut et al (Sigaut 2009) conducted a systematic review to address the question of whether ultrasound prelocation and/or guidance (UPG) of the internal jugular vein (IJV) offers advantages over the anatomical landmarks (AL) method during IJV access in children and infants. The authors concluded that "they do not found the utility of ultrasound during IJV access in children and infants in increasing the success rate and in decreasing complications."
The meta‐analysis from Wu (Wu 2013) was conducted to compare the use of anatomical landmark techniques for central venous cannulation versus real‐time two‐dimensional ultrasound guidance to determine whether ultrasound techniques decreased risks of cannulation failure, arterial puncture, haematoma and haemothorax in adults and children. USD techniques or indirect (ID) proceedings were not taken into account. These review authors came to the conclusion that use of real‐time two‐dimensional ultrasound‐guided techniques (RTUS) in adults receiving CVC was associated with decreased risks of cannulation failure, arterial puncture, haematoma and haemothorax. However, RTUS did not lead to a reduction in the risks of cannulation failure, arterial puncture, haematoma, pneumothorax and haemothorax in children or in infants when the limited data were analysed, and additional data from randomized studies are needed for evaluation of these outcomes in paediatric patients. Their results correspond to ours. In addition, we could demonstrate that the use of two‐dimensional ultrasound decreased the number of attempts needed to succeed.
Authors' conclusions
Implications for practice.
Several important implications for practice can be seen in our systematic review and meta‐analysis.
Our systematic review shows the benefit of using two‐dimensional ultrasound techniques for cannulation of the internal jugular vein in terms of complication rates, the overall success rate, the number of attempts made, success at first attempt, time to successful cannulation and risk of severe bruising and accidental arterial puncture. These benefits are seen in most subgroups and are consistent across experienced and inexperienced operators (when data were available on complication rate total, overall success rate and number of attempts until success). Results comparing Doppler ultrasound for cannulation versus traditional landmark techniques were more uncertain. Use of Doppler ultrasound increased the chance of success at the first attempt. No evidence showed differences for the other outcomes. More data are available to support use of ultrasound during ('direct'), not simply before ('indirect'), line insertion. However, no data on mortality, patient‐reported outcomes (e.g. pain, discomfort, length of stay in hospital/on ICU) or rate of catheter‐related bloodstream infection were provided. The quality of the evidence was very low for most outcomes and heterogeneity among the studies was significant; therefore the results must be interpreted with caution.
Implications for research.
For many studies, many important items were not described in sufficient detail including the nature of the landmarks used, the experience of the person inserting the catheter and some of the outcomes. Furthermore, important outcomes, such as patient‐reported outcomes, infection (at the site of insertion or in the bloodstream) and bleeding and haematoma formation in patients with coagulopathy, have not been addressed. Likewise, it would be possible to compare 'in‐plane' and 'out‐of‐plane' approaches.
However, two of our key questions—whether ultrasound improves safety and effectiveness of insertion in patients at higher risk of complications, and whether it helps inexperienced practitioners more (or indeed less) than experienced staff—remain unanswered. Whether the infection rate is increased by the use of ultrasonic apparatus because the transducer is brought into the puncture field, which may lead to local infection, or if the number of required puncture attempts is reduced was investigated by only one of the reviewed studies (Karakitsos 2006) and therefore would remain unanswered, as was the question of whether the shorter puncture duration and the smaller numbers of punctures of the arteria carotis and haematomas lead to a reduction in the infection rate.
Opinions are divided over whether further trials are necessary. Some argue that current evidence is sufficient to support the use of ultrasound (Bodenham 2006; Scott 2004). However, given that the studies that we have identified are not of optimum quality and do not address all unanswered questions about the technique, we believe that this view is premature and somewhat nihilistic. Future trials should be designed with a focus on the methodological issues highlighted in this review and the gaps in knowledge that need to be filled. A broader, mixed‐methods approach might be better suited to some aspects of this complex intervention, incorporating process evaluation to understand how context influences outcome and to provide insights to aid implementation in other settings. In addition, an economic evaluation taking into consideration the cost‐effectiveness of the method, not only from the payer's perspective but also from that of service users and society as a whole, would be useful for decision makers.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 28 May 2010 | Amended | Contact details updated |
| 4 November 2008 | Amended | Change to list of review authors |
Acknowledgements
We would like to thank Harald Herkner (content editor); Cathal Walsh (statistical editor); Massimo Lamperti, Cliff L Shelton and Bernard Coronel (peer reviewers); and Robert Wyllie (consumer) for their help and editorial advice during preparation of this systematic review. Many special thanks to Jane Cracknell for her great patience, help and editorial advice during preparation of the protocol and the systematic review.
We would like to thank Harald Herkner, Daniel Hind, Bernard Coronel, Janet Wale and Mark Edward for their help and editorial advice during preparation of this protocol, and Karen Hovhannisyan (Cochrane Anaesthesis Review Group (CARG) Trial's Search Co‐ordinator) for his help in preparing the search strategies.
We would like to acknowledge Prof. Dr. med. Ulf Börner's contribution to the protocol (Brass 2008). Prof. Dr. med. Ulf Börner was listed as an author of the protocol for this systematic review before the time of his death in 2008.
Appendices
Appendix 1. Search strategy for CENTRAL (Wiley Interscience)
#1 MeSH descriptor Catheterization, Central Venous explode all trees #2 MeSH descriptor Central Venous Pressure explode all trees #3 central venous line* #4 central venous pressure:TI,AB #5 (venous or vein*) near (cannualation or access or catheter*) #6 pulmonary art* flotation* #7 central line* insertion* #8 hickman near line* #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Ultrasonics explode all trees #11 MeSH descriptor Ultrasonography explode all trees #12 (imag* near guid*) #13 (ultrasound* or ultrasonic* or doppler) #14 (#10 OR #11 OR #12 OR #13) #15 (#9 AND #14)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. (zentralveno?s* kathet* or (venostrom* or venenkathe*) or hickman line* or central line* insertion* or pulmonary arter* flotation* or ((venous or vein*) adj4 (cannulation or access or catheter* puncture)) or central venous line* or central venous pressure).mp. or exp Venous Cutdown/ or Central Venous Pressure/ or exp Catheterization Central Venous/ 2. (ultrasound* or ultrasonic* or Doppler or echography or ultrasonograpgh*).mp. or exp Ultrasonography Doppler Color/ or exp Echocardiography Doppler/ or exp Ultrasonography/ or exp Ultrasonics/ 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. central venous catheterization/ or central venous pressure/ or zentralveno?s* kathet*.mp. or (venostrom* or venenkathe*).mp. or hickman line*.mp. or central line* insertion*.mp. or pulmonary arter* flotation*.mp. or ((venous or vein*) adj4 (cannulation or access or catheter* puncture)).mp. or central venous line*.mp. or central venous pressure.mp. 2. ultrasound/ or explode echography/ or (ultrasound* or ultrasonic* or Doppler or echography or ultrasonograpgh*).mp. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 ( (MH "Catheterization, Peripheral Central Venous") OR (MH "Central Venous Pressure") OR (MH "Venous Cutdown") ) OR ( (zentralveno?s* kathet* or (venostrom* or venenkathe*) or hickman line* or central line* insertion* or pulmonary arter* flotation* or ((venous or vein*) and (cannulation or access or catheter* puncture)) or central venous line* or central venous pressure) ) S2 ( (MH "Ultrasonography, Doppler, Color") OR (MH "Echocardiography, Doppler") OR (MH "Ultrasonography") OR (MH "Ultrasonics") ) OR AB ( ultrasound* or ultrasonic* or Doppler or echography or ultrasonograpgh* ) S3 S1 and S2
Appendix 5. Search strategy for GRIPS‐WEB search (DIMDI)
1 KL97; SM78; SPPP; SP97; CA66; CL63; MEOO; ME66; MEOA; ME6O; T165; MK77; GE79; EU93; PX97; PY81; HN69; CB85; SU88; SV88; AZ72; EM74; EM83; EM9O; PT85; TV01 2 ct d ultrasonics 3 ft=(ultrasound; ultrasonic) 4 ct d ultrasonography 5 cc d A##lus 6 cc d A1/us 7 cc d A2/us 8 cc d A3/us 9 cc d A4/us 10 cc d A5/us 11 cc d A6/us 12 cc d A7/us 13cc d A8/us 14 cc d A9/us 15 cc d A14/us 16 cc d c1/us 17 cc d c2/us 18 cc d c3/us 19 cc d c4/us 20 cc d c5/us 21 cc d c6/us 22 cc d c7/us 23 cc d c8/us 24 cc d c9/us 25 cc d c10us 26 cc d c11/us 27 cc d c12/us 28 cc d c13/us 29 cc d c14/us 30 cc d e15/us 31 cc d c16/us 32 co d e17/us 33 cc d c18/us 34 cc d c19/us 35 cc d c20/us 36 co d c21/us 37 cc d c23/us 38 cc d f3/us 39 ct d catheterization 40 ct=venous cutdown 41 ft=(vein cutdown; venostom?; venenkathe?) 42 ft=(central venous cathe?; zentralveno#s?kath?) 43 (cathether AND venous) /same sent 44 (Kathe? AND ven?) /same sent 45 (cathet? AND ven?) /same sent 46 S=45 OR S=44 OR S=43 OR S=42 OR S=41 OR S=40 OR S=39 47 S=46 OR S=38 OR S=37 OR S=36 OR S=35 OR S=34 OR S=33 OR S=32 OR S=31 OR S=30 OR S=29 OR S=28 OR S=27 OR S=26 OR S=25 OR S=24 OR S=23 OR S=22 OR S=21 OR S=20 OR S=19 OR S=18 OR S=17 OR S=16 OR S=15 OR S=14 OR S=13 OR S=12 OR S=11 OR S=10 OR S=9 OR S=8 OR S=7 OR S=6 OR S=5 OR S=4 OR S=3 OR S=2 48 S=47 AND S=46 49 48 AND (study; studie#) 50 49 AND (zufall?; random?) 51 50 and prospe#tiv? 52 CT="RANDOMIZED CONTROLLED TRIAL" 53 CT="CLINICAL TRIAL" 54 CT="CENTRAL VENOUS CATHETER" 55 CT=' PROSPECTIVE STUDIES" 56 CT="CATHETERIZATION" 57 CT="CATHETERIZATION, CENTRAL VENOUS" 58 CT="PROSPECTIVE STUDY" 59 S=58 OR S=57 OR S=56 OR S=55 OR S=54 OR S=53 OR S=52 60 S=59 AND S=51 61 check duplicates: unique in s=60 62 doppler/(ti; ct; ab) 63 vein puncture 64 venous puncture 65 cannulation 66 zentralveno#ese punktion 67 S=66 OR S=65 OR S=64 OR S=63 68 ultras? 69 S=68 OR S=62 70 67 AND 69 71 70 NOT 61 72 71 AND (studie#; study) 73 check duplicates: unique in s=72 74 73 AND Prospe#tiv?
Data and analyses
Comparison 1. Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complication rate total | 14 | 2406 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.52] |
| 1.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 10 | 2098 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.17, 0.63] |
| 1.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.74] |
| 1.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail if direct or indirect puncture | 3 | 167 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.58] |
| 2 Overall success rate | 23 | 4340 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.08, 1.17] |
| 2.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 17 | 3575 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.06, 1.17] |
| 2.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 6 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.03, 1.26] |
| 2.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.41] |
| 3 Number of attempts until success | 16 | 3302 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.45, ‐0.92] |
| 3.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 11 | 2849 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.50, ‐0.88] |
| 3.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 4 | 358 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.42, ‐0.45] |
| 3.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.78, ‐1.22] |
| 4 Arterial puncture | 22 | 4388 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.18, 0.44] |
| 4.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 16 | 3676 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.14, 0.42] |
| 4.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 5 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.23, 1.00] |
| 4.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.73] |
| 5 Haematoma formation | 13 | 3233 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.55] |
| 5.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 12 | 3171 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.59] |
| 5.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.86] |
| 6 Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) | 11 | 3042 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.76] |
| 6.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 9 | 2907 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.11, 1.12] |
| 6.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.12, 1.21] |
| 6.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.60] |
| 7 Time to successful cannulation | 20 | 3451 | Mean Difference (IV, Random, 95% CI) | ‐30.52 [‐55.21, ‐5.82] |
| 7.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between identification of puncture site and final catheter placement | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 43.70 [4.00, 83.40] |
| 7.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of skin and aspiration of venous blood into the syringe | 4 | 2074 | Mean Difference (IV, Random, 95% CI) | ‐55.37 [‐88.76, ‐21.97] |
| 7.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between application of local anaesthetic and RJI puncture | 2 | 249 | Mean Difference (IV, Random, 95% CI) | ‐39.46 [‐58.09, ‐20.83] |
| 7.4 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time needed for RIJV catheterization | 2 | 147 | Mean Difference (IV, Random, 95% CI) | 99.89 [‐170.76, 370.53] |
| 7.5 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time of beginning of localization of the vessel up to aspiration of venous blood | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐26.56, 24.56] |
| 7.6 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of skin and successful placement of guide wire within the internal jugular vein | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐38.04, 48.84] |
| 7.7 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time from completion of skin preparation and draping to successful aspiration of venous blood into the syringe | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 68.57 [59.59, 77.55] |
| 7.8 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time required for successful guide wire insertion | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐92.00 [‐145.74, ‐42.26] |
| 7.9 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Insertion time | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐133.0 [‐223.05, ‐42.95] |
| 7.10 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Time taken to locate the vein | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐33.38 [‐57.91, ‐8.85] |
| 7.11 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Time from initial skin palpation immediately before initial needle insertion to removal of 18‐gauge cannula from the guide wire | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐35.32, 28.12] |
| 7.12 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Mean time to cannulation | 1 | 141 | Mean Difference (IV, Random, 95% CI) | ‐124.0 [‐198.33, ‐49.67] |
| 7.13 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Total time | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐210.0 [‐413.32, ‐6.68] |
| 7.14 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Time between insertion of needle into the skin until free flow of blood from the catheter | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐350.84 [‐801.00, 99.33] |
| 8 Success with attempt number 1 | 18 | 2681 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.36, 1.82] |
| 8.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 14 | 2225 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.33, 1.88] |
| 8.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirekt puncture | 3 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.14, 1.92] |
| 8.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct and indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.62, 25.85] |
| 9 Success with attempt number 2 | 6 | 1156 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.07, 1.32] |
| 9.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 5 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.06, 1.46] |
| 9.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.14] |
| 10 Success with attempt number 3 | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.66, 2.28] |
| 10.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.01, 2.40] |
| 10.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.97, 1.06] |
Comparison 2. Doppler guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complication rate total | 3 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.71] |
| 1.1 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.16, 2.04] |
| 1.2 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.42] |
| 2 Overall success rate | 7 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.95, 1.25] |
| 2.1 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 6 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.95, 1.35] |
| 2.2 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 3 Number of attempts until success | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.92, 0.66] |
| 3.1 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.92, 0.66] |
| 4 Arterial puncture | 6 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.73] |
| 4.1 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 4 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.12, 2.46] |
| 4.2 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.42] |
| 4.3 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.16, 4.20] |
| 5 Time to successful cannulation | 5 | 214 | Mean Difference (IV, Random, 95% CI) | 62.04 [‐13.47, 137.55] |
| 5.1 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between identification of puncture site and final catheter placement | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 54.90 [16.46, 93.34] |
| 5.2 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between injection of local anaesthetic and insertion of cannula into the IJV | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐117.00 [‐274.74, 40.74] |
| 5.3 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Total duration of venous catheterization | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 214.0 [11.55, 416.45] |
| 5.4 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Mean times required to achieve successful cannulation | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 95.0 [‐2.40, 192.40] |
| 5.5 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Time between insertion of needle into the skin until free flow of blood from the catheter | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 135.60 [‐117.76, 388.96] |
| 6 Success with attempt number 1 | 4 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.02, 2.43] |
| 6.1 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 3 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.88, 2.16] |
| 6.2 Traditional landmark vs Doppler guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [1.33, 5.52] |
Comparison 3. Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complication rate total | 10 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.18, 0.40] |
| 1.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 9 | 1974 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.18, 0.40] |
| 1.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Overall success rate | 18 | 3669 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.05, 1.13] |
| 2.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 14 | 3172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.05, 1.15] |
| 2.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 4 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.00, 1.09] |
| 2.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.38] |
| 3 Number of attempts until success | 12 | 2896 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.50, ‐0.85] |
| 3.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 9 | 2570 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.50, ‐0.77] |
| 3.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 3 | 326 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.31, ‐0.50] |
| 4 Arterial puncture | 18 | 3920 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.18, 0.37] |
| 4.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 14 | 3343 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.15, 0.33] |
| 4.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 4 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.07] |
| 5 Haematoma formation | 11 | 3047 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.44] |
| 5.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 11 | 3047 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.44] |
| 6 Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) | 9 | 2830 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.11, 1.12] |
| 6.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 9 | 2830 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.11, 1.12] |
| 7 Time to successful cannulation | 16 | 3160 | Mean Difference (IV, Random, 95% CI) | ‐13.07 [‐40.57, 14.44] |
| 7.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between identification of puncture site and final catheter placement | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 43.70 [4.00, 83.40] |
| 7.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of the skin and aspiration of venous blood into the syringe | 4 | 2074 | Mean Difference (IV, Random, 95% CI) | ‐55.37 [‐88.76, ‐21.97] |
| 7.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between application of local anaesthetic and RJI puncture | 2 | 249 | Mean Difference (IV, Random, 95% CI) | 39.46 [20.83, 58.09] |
| 7.4 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time needed for RIJV catheterization | 2 | 147 | Mean Difference (IV, Random, 95% CI) | 99.89 [‐170.76, 370.53] |
| 7.5 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time of beginning of localization of the vessel up to aspiration of venous blood | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐26.56, 24.56] |
| 7.6 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time from completion of skin preparation and draping to successful aspiration of venous blood into the syringe | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 68.57 [59.59, 77.55] |
| 7.7 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time required for successful guide wire insertion | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐92.00 [‐145.74, ‐42.26] |
| 7.8 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Time from initial skin palpation immediately before initial needle insertion to removal of 18gauge cannula from the guide wire | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐35.32, 28.12] |
| 7.9 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Mean time to cannulation | 1 | 141 | Mean Difference (IV, Random, 95% CI) | ‐124.0 [‐198.33, ‐49.67] |
| 7.10 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Total time | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐210.0 [‐413.32, ‐6.68] |
| 7.11 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Insertion time | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐133.0 [‐223.05, ‐42.95] |
| 8 Success with attempt number 1 | 15 | 2291 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.30, 1.75] |
| 8.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 12 | 1946 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.25, 1.77] |
| 8.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.09, 2.55] |
| 9 Success with attempt number 2 | 7 | 1196 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.07, 1.30] |
| 9.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 6 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.06, 1.41] |
| 9.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.14] |
| 10 Success with attempt number 3 | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.85, 1.51] |
| 10.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.85, 1.81] |
| 10.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.97, 1.06] |
3.9. Analysis.
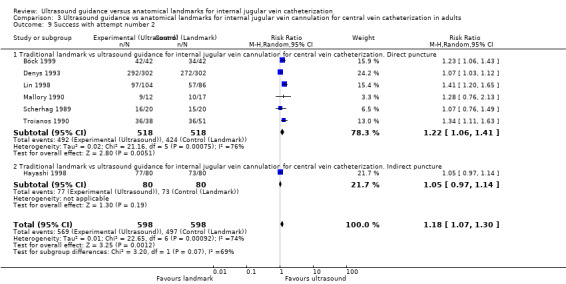
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 9 Success with attempt number 2.
3.10. Analysis.
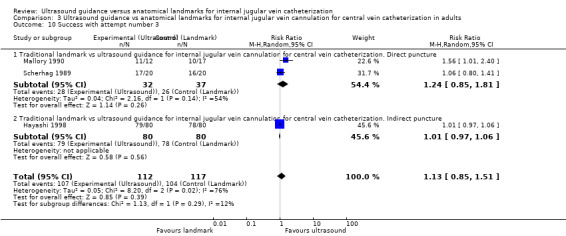
Comparison 3 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in adults, Outcome 10 Success with attempt number 3.
Comparison 4. Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complication rate total | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.09, 1.46] |
| 1.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.65, 2.55] |
| 1.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.86] |
| 1.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.58] |
| 2 Overall success rate | 5 | 530 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.00, 1.49] |
| 2.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.66, 2.02] |
| 2.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.08, 1.44] |
| 2.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.11, 1.51] |
| 3 Number of attempts until success | 4 | 406 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐1.72, ‐0.77] |
| 3.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 1 | 209 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.46, ‐1.38] |
| 3.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.18, ‐0.34] |
| 3.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.78, ‐1.22] |
| 4 Arterial puncture | 5 | 530 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.35] |
| 4.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.00, 24.50] |
| 4.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.00] |
| 4.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.73] |
| 5 Other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) | 3 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.76] |
| 5.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.22] |
| 5.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.12, 1.21] |
| 5.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.60] |
| 6 Time to successful cannulation | 4 | 291 | Mean Difference (IV, Random, 95% CI) | ‐90.70 [‐184.74, 3.35] |
| 6.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of skin and successful placement of guide wire within the internal jugular vein | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐38.04, 48.84] |
| 6.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Time taken to locate the vein | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐33.38 [‐57.91, ‐8.85] |
| 6.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Time between insertion of needle into the skin until free flow of blood from the catheter | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐350.84 [‐801.00, 99.33] |
Comparison 5. Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and inexperienced operators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complication rate total | 5 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.28] |
| 1.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 4 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.07, 1.63] |
| 1.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.87] |
| 2 Overall success rate | 13 | 1427 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.16] |
| 2.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 8 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.99, 1.18] |
| 2.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 4 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.99, 1.20] |
| 2.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.38] |
| 3 Number of attempts until success | 8 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.59, ‐0.83] |
| 3.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 5 | 885 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.75, ‐0.82] |
| 3.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 3 | 247 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.53, ‐0.51] |
| 4 Time to successful cannulation | 9 | 1057 | Mean Difference (IV, Random, 95% CI) | 5.60 [‐50.51, 61.71] |
| 4.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between identification of puncture site and final catheter placement | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 43.70 [4.00, 83.40] |
| 4.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of skin and aspiration of venous blood into the syringe | 1 | 380 | Mean Difference (IV, Random, 95% CI) | ‐141.0 [‐165.87, ‐116.13] |
| 4.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of skin and successful placement of guide wire within the internal jugular vein | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐38.04, 48.84] |
| 4.4 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between application of local anaesthetic and RJI puncture | 2 | 249 | Mean Difference (IV, Random, 95% CI) | 39.46 [20.83, 58.09] |
| 4.5 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time needed for RIJV catheterization | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 240.0 [171.37, 308.63] |
| 4.6 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Time from initial skin palpation immediately before initial needle insertion to removal of 18‐gauge cannula from the guide wire | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐35.32, 28.12] |
| 4.7 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Time between insertion of needle into skin until free flow of blood from catheter | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐128.40 [‐321.16, 64.36] |
| 4.8 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture. Insertion time | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐133.0 [‐223.05, ‐42.95] |
Comparison 6. Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complication rate total | 8 | 1532 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.19, 0.43] |
| 1.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 5 | 1357 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.19, 0.46] |
| 1.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.86] |
| 1.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.50] |
| 2 Overall success rate | 9 | 2513 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.06, 1.16] |
| 2.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 6 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.05, 1.16] |
| 2.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.92, 1.31] |
| 2.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.11, 1.51] |
| 3 Number of attempts until success | 7 | 2029 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.52, ‐0.66] |
| 3.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 5 | 1894 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.54, ‐0.54] |
| 3.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.17, ‐0.13] |
| 3.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture | 1 | 95 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.78, ‐1.22] |
| 4 Arterial puncture | 10 | 2632 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.44] |
| 4.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 8 | 2477 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.15, 0.36] |
| 4.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Indirect puncture | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.22, 2.90] |
| 5 Haematoma formation | 8 | 2477 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.50] |
| 5.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture | 8 | 2477 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.50] |
| 6 Time to successful cannulation | 7 | 2073 | Mean Difference (IV, Random, 95% CI) | ‐31.90 [‐76.07, 12.28] |
| 6.1 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time between penetration of skin and aspiration of venous blood into the syringe | 3 | 1694 | Mean Difference (IV, Random, 95% CI) | ‐28.59 [‐35.01, ‐22.17] |
| 6.2 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time of beginning of localization of the vessel up to aspiration of venous blood | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐26.56, 24.56] |
| 6.3 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time from completion of skin preparation and draping to successful aspiration of venous blood into the syringe | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 68.57 [59.59, 77.55] |
| 6.4 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. Direct puncture. Time required for successful guide wire insertion | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐92.00 [‐145.74, ‐42.26] |
| 6.5 Traditional landmark vs ultrasound guidance for internal jugular vein cannulation for central vein catheterization. No detail on whether direct or indirect puncture. Time between insertion of needle into the skin until free flow of blood from the catheter | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐588.0 [‐839.32, ‐336.68] |
6.5. Analysis.
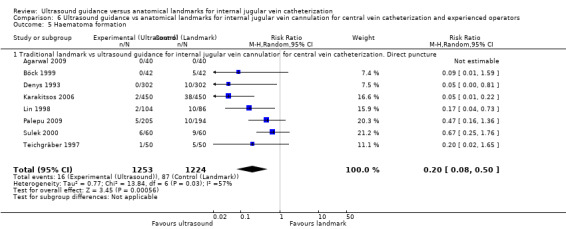
Comparison 6 Ultrasound guidance vs anatomical landmarks for internal jugular vein cannulation for central vein catheterization and experienced operators, Outcome 5 Haematoma formation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2009.
| Methods | Randomized controlled trial (RCT) Randomization method: no details in the text (B) |
|
| Participants | Medical and surgical patients requiring CVCs for difficult peripheral venous access, need for invasive haemodynamic monitoring and delivery of inotropic medications or antibiotics in a medical and surgical intensive care unit (ICU) Exclusion criteria for the study: patients with previous CVC within 15 days, anatomical deformity (e.g. neck surgery, malignancy, burns at the site of insertion), emergency conditions not permitting time to arrange equipment for the study, bleeding disorders, age younger than 18 years and refusal to give consent for inclusion in the study Inclusion and exclusion criteria clearly defined in the text Treatment and control groups not adequately described at study entry No admission details described No information on whether participants were anaesthetized or sedated or awake Operators: number: no details Experience: senior residents or consultants. All had undergone training in US‐guided cannulation techniques and had been performing the procedure for at least 1 year |
|
| Interventions | Technique: Landmark (LM): no details vs Ultrasound (US): SonoSite Micromaxx® with a 7.5‐MHz ultrasound probe covered with a sterile sheath ((short axis) see typical image and description in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side US Direct puncture Technique standardized: unclear Head flat, head rotation: no details Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Number of attempts until success (absolute numbers (n/N) and standard deviation (SD)): attempted entry of needle into the skin and its removal from the skin Complication rates: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (absolute numbers (n/N) and expressed as percentages (%)) Time to successful cannulation (seconds) Success with attempt number 1 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intension‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Alderson 1992.
| Methods | Congress poster Prospectively randomized: randomization method: no details in the text |
|
| Participants | 40 patients younger than 2 years of age undergoing cardiac surgery Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry Two admission details described (age, weight) No information on whether participants were anaesthetized or sedated or awake Operators: number: no details Experience: experienced cardiac anaesthesiologists |
|
| Interventions | Technique: LM: no details vs US: ultrasound with 7.5‐MHz resolution (SiteRite scanner without needle guide) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV no details US Indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (n, %) Time to successful cannulation (seconds): time taken to locate the vein |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture Congress poster |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: Unclear _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Armstrong 1993.
| Methods | Prospectively quasi‐randomized trial All internal jugular vein cannulations performed over a period of 6 weeks were assessed. The ‘SiteRite’ was used exclusively in one operating theatre. and cannulations in the other were performed in a standard manner using anatomical landmarks alone |
|
| Participants | Patients before operations Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 3 admission details described (sex, weight, height) Admission details not described, only “equal demographic data”: __X__ Participants anaesthetized Operators: number: no details Experience: anaesthetists of registrar grade or above |
|
| Interventions | Technique: LM: finder needle used vs US: ultrasound with 7.5‐MHz resolution (SiteRite scanner) without needle guide, finder needle used. (After skin cleaning and draping, the internal jugular vein was located with a 21 G needle. After the internal jugular vein was located, an 18‐gauge cannula was inserted with the initial needle acting as a guide. A guide wire was then inserted through the cannula) ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side US Indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Overall success rate (N, %) in 100 seconds Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (n, %) Time to successful cannulation (seconds) (time from initial skin palpation immediately before initial needle insertion to removal of the 18‐gauge cannula from the guide wire). In cases for which the internal jugular vein was not located, cannulation times were disregarded Success with attempt number 1 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture 5 insertions into the right internal jugular vein were abandoned in the control group. In 3 individuals, the vein was not located, and later use of the ‘SiteRite’ demonstrated very small veins adjacent to the carotid artery. In one case, a cannula had been inserted but was shown to be outside the vein when examined using the ‘SiteRite’; in the fifth case, the carotid artery was punctured by the seeking needle and the procedure abandoned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Bansal 2005.
| Methods | Prospectively randomized controlled trial Randomization method: no details Methods of concealment: unclear Randomized study conducted to compare the procedure success rate and periprocedural complications in participants undergoing ultrasound‐guided vs non–ultrasound‐guided IJVC insertion for temporary haemodialysis access |
|
| Participants | All patients subjected to insertion of an IJVC for temporary haemodialysis access Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 4 admission details described (sex, age, underlying disorders, anatomical distinctiveness) Participants awake, local anaesthesia Operators: number: no details Experience: All procedures were performed by nephrologists without involvement of a radiologist. All nephrologists of our unit who had done at least 25 cases by either method were eligible to perform the procedure in the study population |
|
| Interventions | Blind (group A) or ultrasound‐guided (group B) procedure Technique: LM: no details vs US: Portable ordinary ultrasound machine with a 3.5‐MHz curved probe without a needle guide or any colour Doppler facility was used. Ultrasound probe disinfected (short axis) LM Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side IJV, right US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg): down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side IJV, right |
|
| Outcomes | Number of attempts until success (N, SD) Primary outcome: Each push of the needle was counted as an attempt, and change in direction of the needle, even without coming out of the skin puncture, was counted as a separate attempt Failure rate (N, %): More than 3 attempts or inability to cannulate was counted as a failed procedure Complication rate (N, %): Complications such as carotid artery puncture and haematoma formation and any others were recorded Occurrence of adverse outcomes (failed procedure, carotid puncture, haematoma), blood loss mL (mean ± SD) |
|
| Notes | No cross‐over No sample size estimation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Branger 1994.
| Methods | Controlled clinical trial (CCT) Randomization method: predetermined list; no other details in the text |
|
| Participants | Consecutive patients requiring central venous catheter for haemodialysis, apheresis or parenteral nutrition; patients with known risk factors were excluded Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission details described Participants awake Operators: number: no details Experience: junior residents, senior staff members (LM 6J 4S, US 5J 6S) |
|
| Interventions | Technique: LM: no details vs US: 5 MHz with needle guide, developed by study authors LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV and SV no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV and SV side: no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %): failure defined in the text, see text Complication rates: total, arterial puncture, haematoma formation (N, %) Success rate after cross‐over (N, %) |
|
| Notes | Cross‐over landmark‐guided puncture and ultrasound‐guided puncture LM: Cross‐over after failure of initial technique after 30 minutes 3 LM → 2 (66.7%) success with US US: cross‐over after failure of initial technique after 30 minutes 1 Do → (100%) success with senior |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: predetermined list; no other details in the text |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: predetermined list; no other details in the text (C) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Branger 1995.
| Methods | Controlled clinical trial (CCT) Randomization method: 100 consecutive patients with subclavian vein catheterization and 30 patients with IJV catheterization were included in the study. Choices of vessel, puncture site and catheter were made according to patient`s history and clinical status before non‐Doppler or Doppler technique was selected from random tables (with separated tables for subclavian and for IJV catheterization) |
|
| Participants | Consecutive patients requiring central venous catheter for haemodialysis, apheresis or parenteral nutrition; patients with known risk factors such as thoracic abnormality, respiratory distress, major obesity or restlessness were excluded Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details described (sex, age) Participants awake and anaesthetized Operators: number: 22 Experience: 14 junior residents (postgraduate students < 5 years of clinical experience), 8 senior staff members (> 5 years of clinical experience), members of the nephrology, emergency and intensive care departments. They were taught the Doppler technique over a 2‐week period by the 2 senior members, who were previously involved in animal experimental study; participants had to achieve at least 1 venous catheterization with the non‐Doppler and with the Doppler technique before entering the study. The operator for each venous catheterization was chosen according to a random table (LM 10J 5S, US 6J 8S) |
|
| Interventions | Technique: LM: no details vs US: hand‐held pulsed Doppler probe for co‐axial guidance of the puncture needle and a dedicated 4‐MHz pulsed Doppler, probe sterilized, developed by study authors LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV and SV: no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV and SV side: no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %): failure defined as inability to obtain venous blood after longer than 30 minutes. After onset of local anaesthesia or after more than 4 attempts at venous puncture Number of attempts until success (N, SD) Time to successful cannulation (seconds) Success rate after cross‐over (N, %) |
|
| Notes | Cross‐over: landmark‐guided puncture and ultrasound‐guided puncture LM: cross‐over after failure of initial technique In case of failure of the initial attempt at catheterization by the non‐Doppler technique, the operator was allowed to use the Doppler technique 1 J LM → 1 (100%) success with Doppler 4 S LM → 2 (50%) successes with Doppler US: cross‐over after failure of the initial technique In case of failure of the Doppler technique used by a junior staff member, a senior staff member was asked to perform Doppler venous catheterization 1 J Do→ 1 (100%) success with senior |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Controlled clinical trial (CCT) Randomization method: random tables (C) |
| Allocation concealment (selection bias) | Unclear risk | Controlled clinical trial (CCT) Randomization method: random tables (C) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Böck 1999.
| Methods | Prospectively randomized controlled trial | |
| Participants | Patients who needed CVC for thoracic or cardiac surgery. Number enrolled in study: 77 (7 patients had 2 IJV punctures) > 84 punctures Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at study entry 5 admission details described (sex, weight, height, age, anatomical distinctiveness) No information on whether participants were anaesthetized or sedated or awake Operators: number: 7 Experience: experienced anaesthetists (5 to 10 years clinically active, approximately 350 to 800 LM of CVC placements), US technology demonstrated and was assisted once by an expert before beginning of the studies |
|
| Interventions | Technique: LM: standard approach described by English, with seeking puncture vs US: 7.5‐MHz ultrasound covered with a sterile glove, technique described by Denys et al without seeking puncture ((short axis) see typical image in the article, described in the text) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time of beginning of localization of the vessel up to aspiration of venous blood Success with attempt number 1, 2, 3 (N, %) Outcomes measures defined: unsuccessful first puncture, unsuccessful puncture, arterial puncture, haematoma formation, pneumothorax, infection, nerve injury |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization method adequate |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate (e.g. numbered, sealed opaque envelopes drawn) Non‐consecutively (A) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention to treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Chuan 2005.
| Methods | Randomized controlled trial (RCT) (A) Randomization method: random table |
|
| Participants | 62 infants (body weight < 12 kg) undergoing elective surgery for congenital heart disease Inclusion and exclusion criteria clearly defined in the text Treatment and control groups not adequately described at study entry 3 admission details described (weight, age, underlying disorders) Participants anaesthetized Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: approach described by Verghese vs US: intraoperative probe attached to the TEE machine (HP SONOS 4500 TEE 15.0 to 6.0 MHz) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique, catheter over needle: no details Vessel and side: IJV right side US Indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique, catheter over needle: no details Vessel and side: IJV right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %): failures: more than 7 attempts in the same position regardless of the occurrence of artery puncture; duration of cannulation longer than 45 minutes; haematoma formation or haemopneumothorax caused by unintentional arterial puncture and need for catheterization via an alternative route or method. If arterial puncture did not cause haematoma, cannulation may be attempted at the same site Number of attempts until success (N, SD) Arterial puncture (N, %) |
|
| Notes | 1 case (in the LM group) had several failures at multiple sites and had to be catheterized via surgical cut‐down of the femoral vein. Number of attempts (> 20) was not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table |
| Allocation concealment (selection bias) | Low risk | Random table |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of participants who withdrew or were excluded after allocation were NEITHER detailed separately NOR included in an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ (see comment on treatment) Withdrawals: Unclear _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Denys 1993.
| Methods | "… sequential protocol was used in this study. Since we have a similar number of procedures each week, the ultrasound device was used one week and the landmark technique was used the next week. This was continued until we had 302 patients in each group. Thereafter, the ultrasound technique was used exclusively in an additional 626 patients. There was no provision for crossover in this study design. Because many patients had more than one procedure, it was possible that the same patient was cannulated using a different technique on separate occasions" | |
| Participants | "… evaluated an ultrasound‐guided method in 302 patients undergoing internal jugular venous cannulation and compared the results with 302 patients in whom an external landmark‐guided technique was used. Ultrasound was used exclusively in an additional 626 patients. Patients undergoing internal jugular venous cannulation as part of a cardiac catheterization or placement of a central venous line (N =1,230) were studied" Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details described (sex, age) Participants awake Operators: number: 29. 15 operators performed fewer than 20 procedures (range, 1 to 19), and 14 operators performed more than 20 (range, 20 to 288) Experience: All cannulations were performed by operators with extensive experience in landmark‐guided internal jugular vein access, including attending cardiologists and cardiology fellows |
|
| Interventions | Technique: LM: no details, finder needle used vs US: ultrasound with 7.5‐MHz resolution (SiteRite scanner) with needle guide probe wrapped in a sterile plastic bag ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV, RIJV 96.4% (N = 894), LIJV 3.6% (N = 34) because IJV absent or very small |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time between penetration of the skin and aspiration of venous blood into the syringe. When multiple sticks were required, only the time when the needle was on the skin or was advanced was taken into account Success with attempt number 1, 2 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequential protocol was used in this study |
| Allocation concealment (selection bias) | High risk | Sequential protocol was used in this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | Participant selection: No _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Unclear risk | No |
Gilbert 1995.
| Methods | Prospectively randomized controlled trial (RCT) Randomization method: no details in the text |
|
| Participants | 76 consecutive, consenting adult patients with preexisting obesity or coagulopathy requiring central venous access. Obesity was defined as weight greater than 130% of ideal body weight for height and body mass index greater than 28. Coagulopathy was defined as a platelet count less than 50,000 thrombocytes/mm3 or an increase of greater than 30% above maximum laboratory control value for 1 or more of the following variables: prothrombin time; partial thromboplastin time; activated clotting time; or template bleeding time Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at entry. A minimum of 4 admission details were described. 6 admission details was described (sex, weight, height, BMI, age, anatomical distinctiveness) Participants awake and anaesthetized Operators: number: no details Experience: junior house staff, who were relatively inexperienced in using either technique, performed all cannulations under the direct supervision of attending faculty. They were instructed in ultrasound device use by listening to a prepared 5‐minute audiotape depicting arterial and venous signals. Years of postgraduate training and experience in control or ultrasonic techniques were similar among junior house staff for both groups of participants. The average operator was in the third postgraduate year and had greater familiarity with use of the control technique than the ultrasound technique |
|
| Interventions | Technique: LM: high/central approach, initially performing venipuncture with a 22‐gauge finder needle vs US: SmartNeedle LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details; only positioning was similar for all participants Seldinger technique: catheter over needle, no details Vessel and side: IJV no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details; only positioning was similar in all participants Seldinger technique Vessel and side: IJV no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, haematoma formation (N, %) Carotid artery puncture was defined as inadvertent placement of any size needle or catheter into a neck vessel that yielded bright red or pulsatile blood Haematoma formation was defined as the appearance of visible neck swelling at the site of cannulation (or attempted cannulation) and distortion of existing anatomical landmarks within 1 hour of study Time to successful cannulation (seconds): Time for cannulation was recorded with a finder (control) or cannulation (ultrasound) needle, beginning with the initial skin puncture and ending with successful placement of a Seldinger wire, or until a given technique failed Success with attempt number 1 (N, %) Success rate after cross‐over (N, %) |
|
| Notes | Cross‐over landmark‐guided puncture and ultrasound‐guided puncture 3 cannulation attempts were allowed with the initial randomized technique before cross‐over to 3 attempts with the alternative technique. The study was discontinued if more than 6 total attempts were required LM: cross‐over after failure of the initial technique 17 LM → 12 (70.6%) success with Doppler US: cross‐over after failure of the initial technique 5 Do → 2 (40%) successes with LM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Gratz 1994.
| Methods | Randomized controlled trial (RCT) Randomization method: no details in the text (B) |
|
| Participants | Patients scheduled for cardiothoracic or major vascular operations who required IJV cannulation 1 participant in the Doppler group was dropped from the study because of a user error in connecting the Doppler needle to the transducer. This participant was not included in the statistical analysis Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 1 admission detail was described (sex) Admission details not described, only “equal demographic data” Participants awake Operators: number: no details Experience: experienced anaesthesiologists |
|
| Interventions | Technique: LM: no details vs US: 14.3‐MHz SmartNeedle LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), Head rotation no details Catheter over needle Vessel and side: IJV side no detail US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Catheter over needle Vessel and side: IJV side no detail |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Arterial puncture (n, %) Time to successful cannulation (seconds): time interval between injection of local anaesthetic and insertion of the cannula into the IJV Success with attempt number 1 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of participants who withdrew or were excluded after allocation were NEITHER detailed separately NOR included in an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Yes |
| Other bias | High risk | Participant selection: Unclear _X_ Withdrawals: No _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Grebenik 2004.
| Methods | Quasi‐randomized controlled trial (Q‐RCT) (D) Randomization method: Block randomization was performed by the anaesthetic assistant immediately before anaesthesia; the anaesthetist was then informed of the technique to be used |
|
| Participants | 124 infants and children presenting for cardiac surgery were prospectively examined; ultrasound guidance was used for central venous catheterization in children undergoing heart surgery On 10 occasions, the ultrasound probe was not available or the batteries were uncharged. These 10 cases were therefore excluded from further analysis, so that a total of 59 patients were included in the ultrasound group Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details described (weight, age) Participants anaesthetized Operators: number: 1 of 3 Experience: All procedures were undertaken by 1 of 3 consultant paediatric cardiac anaesthetists, all of whom had some experience in using the ultrasound probe. Extent of previous experience varied, but the least experienced operator had performed 5 cannulations with the ultrasound probe before the start of the study |
|
| Interventions | Technique: LM: no details vs US: 7.5‐MHz ultrasound (SiteRite scanner) with needle guide ((short axis) no details in the article), wrapped in a sterile sheath LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg) and hepatic compression Seldinger technique Vessel and side: IJV right side US Direct puncture Technique standardized: yes Head down (Trendelenburg) and hepatic compression Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Outcomes Overall success rate (N, %) Failure rate (N, %): No time limit was set, but the procedure was recorded as a failure if right internal jugular cannulation was abandoned and an alternative site was used for central venous cannulation Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): Time from the moment of needle insertion through the skin to the time at which the guide wire was successfully placed within the internal jugular vein was measured |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture On 10 occasions, the ultrasound probe was not available or the batteries were uncharged. These 10 cases therefore were excluded from further analysis, so that a total of 59 participants were included in the ultrasound group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization was performed by the anaesthetic assistant immediately before anaesthesia; the anaesthetist was then informed of the technique to be used |
| Allocation concealment (selection bias) | Unclear risk | Block randomization was performed by the anaesthetic assistant immediately before anaesthesia; the anaesthetist was then informed of the technique to be used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of participants who withdrew or were excluded after allocation were NEITHER detailed separately NOR included in an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | Participant selection: Yes _X_ (see comment on treatment) Withdrawals: No _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Hayashi 1998.
| Methods | Congress poster Prospectively randomized; randomization method no details in the text |
|
| Participants | "… 160 adult patients aged 27 to 89 undergoing general anaesthesia and RIJV cannulation …" Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 1 admission detail described (age) Participants anaesthetized Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: with seeking puncture vs US: 7.5‐MHz or 3.75‐MHz ultrasound with seeking puncture (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique, catheter over needle, no details Vessel and side: IJV right side US Indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique, catheter over needle, no details Vessel and side: IJV right side |
|
| Outcomes | Arterial puncture (n, %) Success with attempt number 1, 2, 3, 4, 5 (n, %) |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture Congress poster |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment unclear (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Yes |
| Other bias | Low risk | Participant selection: No _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Hayashi 2002.
| Methods | Prospectively randomized controlled trial Randomization method: no details in the text |
|
| Participants | 240 randomly selected adult patients requiring RIJV catheter placement under general endotracheal anaesthesia for elective surgery ... patients with a history of previous neck surgery or RIJV cannulation were not included in the study Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at study entry 5 admission details were described (sex, weight, height, BMI, age) Participants anaesthetized Operators: number: 6 Each of these anaesthesiologists performed RIJV cannulation for 40 participants, who were assigned randomly to the landmark group or the ultrasound group (n = 20 each) Experience: 2 residents and 4 attending physicians. All anaesthesiologists were familiar with both cannulation techniques using landmark and ultrasound at the beginning of the study |
|
| Interventions | Technique: LM: RIJV puncture was attempted using respiratory jugular venodilation as the primary landmark for locating the RIJV When not observed, approach described by Bazaral and Harlan was used, with seeking puncture vs US: 7.5 (N = 60)‐ or 3.75 (N = 60)‐MHz ultrasound without seeking puncture LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side US Indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Arterial puncture was identified by forceful pulsatile return of brightly coloured blood from a needle |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment unclear (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Heatly 1995.
| Methods | Congress poster Prospectively randomized; randomization method: no details in the text |
|
| Participants | Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission details described No information on whether participants anaesthetized or sedated or awake Operators: number: 1 individual Experience: ample |
|
| Interventions | Technique: LM: no details vs US: no details (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details US Unclear whether direct or indirect puncture Technique standardized: yes Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate total (N, %) Time to successful cannulation (seconds) Success rate after cross‐over (N, %) Outcome measures not defined |
|
| Notes | LM: cross‐over after 5 attempts (N = 5 → US 5/5 successes) US: cross‐ over after 5 attempts (N = 1 → US 1/1 success) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | Participant selection: Unclear _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: Unclear _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Hrics 1998.
| Methods | Prospectively randomized; randomization method: no details in the text | |
| Participants | All patients needing urgent CVC placement were considered for the study. Urgent placement was defined as needing venous access for intravenous fluids, blood products, medications, dialysis or cardiac pacing within 1 hour of arrival to the ED. Only patients having internal jugular lines were included in the study. The site of line placement was determined by the examining physician and was not dictated by the study. Patients requiring emergent CVC for cardiac or traumatic arrest were excluded Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission detail described No information on whether participants were anaesthetized or sedated or awake Operators: number: 16 Experience: 9 residents and 7 attending emergency physicians who participated in a 2‐hour in‐service demonstrating the use of ultrasound in CVC placement. 2 of the primary investigators (PH, SW) responsible for the training of all operators in the use of ultrasound for CVC placement were available for consultation 24 hours a day |
|
| Interventions | Technique: LM: Standard approach described by Defalque 8 and Advanced Cardiac Life Support texts vs US: 7.5‐MHz SiteRite ultrasound with needle guide sterile sleeve, technique described by Denys et al ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side US N = 32 (24 indirect punctures/8 direct punctures) Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (n, %) Success with attempt number 1 (n, %) Success rate after cross‐over (n, %) Outcome measures not defined |
|
| Notes | Cross‐over landmark‐guided puncture or ultrasound‐guided puncture LM: cross‐over 2 LM proc not successful; → 2/2 (100%) success with US US: no cross‐over 8 participants without landmarks→ 7/7 (100%) success with US, 0/1 (0%) success with LM US N = 32 (24 indirect punctures/8 direct punctures). Outcomes of overall success rate and failure rate were shown separately; other outcomes were shown together |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment unclear (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Yes _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Johnson 1994.
| Methods | Congress poster Prospectively randomized; randomization method: no details in the text |
|
| Participants | 70 critically ill patients Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry Admission details not described, only “equal demographic data” No admission detail described No information on whether participants were anaesthetized or sedated or awake Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: no details vs US: no details (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details US Indirect puncture Technique standardized: yes Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds) Success with attempt number 1 (N, %) Outcomes measures not defined |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | Participant selection: Unclear _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: Unclear _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Karakitsos 2006.
| Methods | Randomized controlled trial (RCT) (A) Randomization method: Participants were randomly assigned in a 1‐to‐1 ratio. Randomization was performed by means of a computer‐generated random‐numbers table, and participants were stratified with regard to age, gender and BMI. Block randomization was used to ensure equal numbers of participants in the above groups |
|
| Participants | 900 mechanically ventilated critical care patients Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at study entry 5 admission details described (sex, BMI, age, coagulation status, anatomical distinctiveness) Participants anaesthetized or sedated Operators: number: no details Experience: ".. well‐trained attending cardiologists, intensivists, and surgeons with similar experience (10 years of experience in IJV catheter placements) to minimise the ... physicians who performed the ultrasound‐guided method were well trained and had at least 5 years of experience in performing this method" |
|
| Interventions | Technique: LM: with seeking puncture vs US: 7.5‐MHz ultrasound wrapped in a sterile plastic sheath, without seeking puncture ((long axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Flat, head rotation, no details Seldinger technique Vessel and side: IJV right side 232, left 218 US Unclear whether direct or indirect puncture Technique standardized: unclear Flat, head rotation, no details Seldinger technique Vessel and side: IJV right side 228, left 222 |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD): average number of attempts before successful placement (defined as separate skin punctures) Arterial puncture (N, %): Carotid artery puncture was noted by forceful pulsatile expulsion of bright red blood from the needle, haematoma formation, other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time between penetration of skin and aspiration of venous blood into the syringe |
|
| Notes | LM: CVC BSI 16% US: CVC BSI 10.4% No cross‐over landmark‐guided puncture or ultrasound‐guided puncture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1‐to‐1 ratio. Randomization was performed by means of a computer‐generated random‐numbers table, and participants were stratified with regard to age, gender and BMI. Block randomization was used to ensure equal numbers of participants in the above groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned in a 1‐to‐1 ratio. Randomization was performed by means of a computer‐generated random‐numbers table, and participants were stratified with regard to age, gender and BMI. Block randomization was used to ensure equal numbers of participants in the above groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: No _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Legler 1983.
| Methods | Congress poster Prospectively randomized trial (RCT); randomization method: no details in the text |
|
| Participants | Patients scheduled for major vascular or cardiac surgery Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission detail described No information on whether participants anaesthetized or sedated or awake Operators: number: no details Experience: no details (... under the supervision of a staff anaesthesiologist) |
|
| Interventions | Technique: LM: no details vs US: 10‐MHz Doppler LM indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat, head rotation no details Seldinger technique Vessel and side: IJV right side US Indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Arterial puncture (N, %) Success with attempt number 1 (N, %) Outcome measures not defined |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | Participant selection: Unclear _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Leung 2006.
| Methods | Prospectively randomized controlled trial Randomization method: computer‐generated block randomization. Allocation assignments were concealed in serially numbered opaque sealed envelopes. The operator and the participant became aware of the insertion technique only after enrolment, not consecutively |
|
| Participants | Patients presenting to an ED who required central venous access as part of their treatment Indications for central venous access in the ED included difficult peripheral venous access, need for invasive haemodynamic monitoring, delivery of inotropic medications or antibiotics, delivery of fluids and blood when no other access was available and temporary internal pacing. All patients were older than 18 years Inclusion and exclusion (exclusion criteria were trauma patients in whom the cervical spine could not be cleared clinically or radiologically before line insertion and patients with severe coagulopathy (consistent history and active bleeding) that could not be corrected with platelets, fresh frozen plasma or other blood products) criteria not clearly defined in the text Treatment and control groups adequately described at study entry 5 admission details described (sex, age, coagulation status, anatomical distinctiveness, underlying disorders) No information on whether participants were anaesthetized or sedated or awake Operators: number: 13 Experience: 5 experienced and 8 inexperienced emergency physicians or registrars (trainees of the Australasian College for Emergency Medicine, postgraduate year 3 or above) working in the ED Experienced operators were defined as those who had successfully performed more than 25 traditional landmark internal jugular vein catheterizations without supervision, and inexperienced operators as those who had performed fewer than 25 traditional landmark internal jugular vein catheterizations. There were 13 operators; 5 were experienced and 8 were inexperienced. Before commencement of the study, operators participated in a minimum 2‐hour education programme outlining the landmark technique, use of the ultrasonographic machine in locating the internal jugular vein and subsequent insertion of the catheter under real‐time ultrasonographic guidance |
|
| Interventions | Technique: LM: central, anterior or posterior approach, depending on operator experience and preference vs US: SonoSite18010‐5 MHz 38‐mm linear array Transducer covered with a sterile glove without a needle guide, ((short axis) see typical image in the article and description in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV no details US Direct puncture Technique standardized: unclear Head down (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique Vessel and side: IJV no details |
|
| Outcomes | Overall success rate (N, %): success: IJV was cannulated, which resulted in successful aspiration of blood Failure rate (N, %): failure: Operator was unable to perform cannulation of the IJV after 3 attempts. Failure was due to inability to locate or puncture the internal jugular vein or inability to feed the guide wire or catheter. An attempt was defined as entry of the introducer needle into the skin followed by its removal from the skin Time to successful cannulation (seconds): For each technique, 2 access times were recorded: time to initial flash of blood (start to flash time) and time to successful insertion of the central venous catheter (start to line working time). Time needed to set up the ultrasonographic machine and prepare the probe was not included |
|
| Notes | Cross‐over Provision was made in the study for cross‐over to the other technique on the ipsilateral side of the neck, depending on complications and participant cooperation If the initial method was unsuccessful after a maximum of 3 attempts, provision was made in the study for cross‐over to the other technique. Again, 3 attempts could be made with the second technique. If both methods were unsuccessful, or if cross‐over did not occur, alternative access was obtained and documented. Alternative access sites included the contralateral internal jugular vein, subclavian vein, or femoral vein LM: cross‐over 12/14; 11/12 successes with US cross‐over were not attempted in 2 of 14 failed landmark cases because the guide wire could not be fed through the vein US: cross‐over 0/4. Cross‐over was not attempted in the 4 failed ultrasonographic cases because the internal jugular vein was poorly visualized or the guide wire could not be fed through the vein |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Lin 1998.
| Methods | Q‐RCT The ultrasound device and the landmark‐guided technique were used during alternating weeks throughout the 6‐month study period |
|
| Participants | "… 190 patients undergoing jugular venous cannulation for haemodialysis …" Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups adequately described at entry. A minimum of 4 admission details were described (sex, age, underlying disorders, coagulation status) Participants awake Operators: number: no details Experience: All operators were fellow nephrologists experienced in landmark‐guided jugular venous cannulation for haemodialysis catheter |
|
| Interventions | Technique: LM: This detecting needle penetrated the skin at the top of the triangle between the sternal and the clavicular head of the sternocleidomastoid muscle with a 45° angle and was aimed at the ipsilateral nipple... vs US: 7.5‐MHz (SiteRite scanner) ultrasound with needle guide covered in a sterile plastic bag ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Flat, head rotation Seldinger technique Vessel and side IJV right side N = 54 (62.8%), left side N = 32 (37.2%) US Direct puncture Technique standardized: unclear Flat, head rotation Seldinger technique Vessel and side IJV right side N = 69 (66.3%), left side N = 35 (33.7%) |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time between the first skin puncture and aspiration of venous blood into the syringe; time required for searching the actual venous location with a detecting needle was not included in recorded access time. Time between puncture attempts was neglected when multiple punctures were needed Success with attempt number 1, 2, > 3 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture and ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Ultrasound device and landmark‐guided technique were used during alternating weeks throughout the 6‐month study period |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed (D) Ultrasound device and landmark‐guided technique were used during alternating weeks throughout the 6‐month study period |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Mallory 1990.
| Methods | Prospectively randomized controlled trial Randomization method: no details in the text |
|
| Participants | Patients who required urgent or urgent‐elective IJV cannulation in the medical/surgical ICU over a 3‐month period Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission detail described No information on whether participants were anaesthetized or sedated or awake Operators: number: no details Experience: senior ICU staff or critical care fellows with at least 6 months of clinical experience in the ICU. Operator experience was similar for each randomization group Postgraduate training years 6.67 ± 1.95 (SD) vs 6.23 ± 2.01 years |
|
| Interventions | Technique: LM: no details vs US: 2‐dimensional ultrasound with 5‐MHz resolution wrapped in a sterile glove (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg): down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side IJV, no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side IJV, no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Success with attempt number 1, 2, 3, 4 (N, %) Success rate after cross‐over (N, %) Outcome measures not defined |
|
| Notes | Cross‐over landmark‐guided puncture: Participants who could not be cannulated during the initial 5 needle passes were then crossed over to receive the alternate technique for the next 5 passes No cross‐over ultrasound‐guided puncture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Milling 2005.
| Methods | Prospectively randomized controlled trial Randomization method: random numbers table Enrolment forms were sealed in coded opaque envelopes During the 6‐month trial period, 235 patients underwent central cannula placement and were eligible for enrolment. A total of 34 patients were not enrolled because of the unavailability of an investigator (10) and were not called (24). No patients refused enrolment. 201 patients were enrolled and randomly assigned |
|
| Participants | Patients undergoing internal jugular vein central venous cannulation The study population was enrolled when 1 of 7 study investigators was available. Most participants were from the emergency department and the medical intensive care unit Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at study entry 3 admission details described (sex, age, anatomical distinctiveness) No information on whether participants were anaesthetized or sedated or awake Operators: 22 Experience: 14 internal medicine and surgery residents (postgraduate years 2 and 3) with varying levels of experience; the lead author performed just over half of the procedures in the study Study investigators were emergency medicine residents and attending physicians who had received a 1‐hour bedside teaching session on identifying the carotid artery and the internal jugular vein with an iLook25 SonoSite ultrasound machine (SonoSite, Bothell, WA) with a 7.5‐MHz linear array probe; the same equipment was used on all study participants. Subsequently, they had to demonstrate proficiency at dynamic ultrasound‐guided central venous cannulation by performing the procedure a minimum of 10 times. Study investigators performed or assisted in all dynamic procedures. The least experienced investigator had placed 30 cannulas at the study’s outset. The most experienced had placed 100. Any doctor credentialed by the hospital for central cannula placement, including study investigators, performed procedures in the S and LM groups. The non‐ultrasound central cannulization credentialing process requires 5 supervised procedures per anatomical location (internal jugular, femoral, subclavian) and subjective assessment of proficiency in the procedure by a supervising physician |
|
| Interventions | Technique: LM: no details vs US: dynamic ultrasound (D): iLook25 SonoSite with a 7.5‐MHz linear array probe, covered with sterile sheath (axis no details) US: static ultrasound (ID): iLook25 SonoSite with a 7.5‐MHz linear array probe (axis no details) LM Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg)/flat; head rotation no details Seldinger technique: catheter over needle, no details Vessel and side IJV, both sides US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Seldinger technique: catheter over needle, no details Vessel and side IJV, both sides US Indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Seldinger technique: catheter over needle, no details Vessel and side IJV: both sides |
|
| Outcomes | Overall success rate (N, %) Primary outcome: successful cannulation: Cannulation was successful if the J‐wire was placed without resistance Failure rate (N, %) Number of attempts until success (N, SD) Cannulation attempt. An attempt was a single pass of the 18‐gauge locator needle with no degree of withdrawal or redirection and with subsequent forward movement, whether or not a new skin puncture was made. Each successive withdrawal or redirection with subsequent forward movement was considered another attempt Complication rate (N, %) arterial puncture. Arterial puncture involved aspiration of pulsatile arterial blood into an 18‐gauge locator needle syringe Time to cannulation (seconds): Cannulation time, i.e. from “needle to skin to J‐wire in,” was measured in seconds. Time includes only the time taken while attempting central cannulation by the technique to which it was randomly assigned. For failures, it includes only the time until the technique was abandoned (after either 5 sticks or 5 minutes). It does not include rescue time Success with attempt number 1 (N, %): secondary outcomes: first‐attempt cannulation success: Cannulation was considered successful at the first attempt if it was achieved with the first needle pass Success rate after cross‐over (N, %) Rescue: After 5 attempts or 5 minutes of attempting cannulation, the participant was rescued by the dynamic technique |
|
| Notes | Cross‐over landmark‐guided puncture Cross‐over ultrasound‐guided puncture Sample size estimate We estimated that, given 70 participants in each group (S, D, LM), or 210 total, we would have 80% power to detect a 25% difference in success rates at a test level of 0.05 Presentation of results for the primary endpoint is done according to the original allocation of participants into 3 groups (N = 60 dynamic ultrasound, N = 72 static ultrasound, N = 69 landmarks technique) Presentation of results of the other endpoints is done in the way that rescue experiments (N = 13 static ultrasonic, N = 27 landmarks technique) are presented together with those of the group "dynamic ultrasound" (then N = 100) So only the primary endpoint could be used for the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Enrolment forms were sealed in coded opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Ovezov 2010.
| Methods | Randomized controlled trial (RCT) Randomization method: computer‐generated randomization table |
|
| Participants | Median age of participants undergoing catheterization procedure in the main group: 53 months; in the control group: 52 months; median weight in the main group: 15 kg; in the control group: 16.4 kg Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details were described (weight, age) No information on participants were anaesthetized or sedated or awake Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: no details vs US: 10‐MHz ultrasound probe (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Seldinger technique Vessel and side: IJV, no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Seldinger technique Vessel and side: IJV, no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (n, %) Time to successful cannulation (seconds): median time spent on the implementation of catheterization Success with attempt number 1 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture Congress poster and presentation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization method: computer‐generated randomization table (A) |
| Allocation concealment (selection bias) | Low risk | Randomization method: computer‐generated randomization table (A) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | Patient selection: Unclear _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: Unclear _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Palepu 2009.
| Methods | Randomized controlled trial (RCT) Randomization method: computer‐generated randomization table All patients admitted to the ICU between April 2007 and September 2008 and requiring central venous access as part of their management were enrolled in the study. Patients younger than 18 years and those refusing to give consent for inclusion in the study were excluded. As the number of femoral vein catheters was small in both groups, they were not included in the analysis |
|
| Participants | "...patients requiring CVC for difficult peripheral venous access, need for invasive haemodynamic monitoring and delivery of inotropic medications or antibiotics in a medical and surgical ICU" Inclusion and exclusion criteria clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details described (sex, age) Admission details not described, only “equal demographic data” Participants awake "...after giving local anesthesia..." Operators: number: no details Experience: registrars with < 6 years of experience, consultants with > 6 years of experience in the field of anesthesia and critical care |
|
| Interventions | Technique: LM: technique (see picture in the article), without finder needle Cannulation using the landmark technique performed as per standard guidelines vs US: 6‐ to 13‐MHz ultrasound probe covered with sterile sheath, without finder needle ((short axis) see typical image in the article and description in the article) LM Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Seldinger technique Vessel and side: Right internal jugular vein (IJV) was the first choice for cannulation. Other sites such as left IJV, left or right subclavian vein (SCV) or femoral veins were cannulated only if the right IJV was not available for cannulation because of the presence of a previously inserted CVC or dialysis catheter IJV 194 (86.2%); right side 178 (91.8%) SCV 28 (12.4%); right side 23 (82.1%) Femoral vein 3 (1.3%) US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Seldinger technique Vessel and side: IJV 205 (91.1%); right side 182 (88.8%) SCV 17 (7.6%); right side 16 (94.1%) Fem v 3 (1.3%) |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %): failure: Operator was unable to cannulate the vein within 3 attempts Number of attempts until success (N, SD): Attempt needle`s entry into the skin and its removal from the skin Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Success with attempt number 1 (N, %) Success rate after cross‐over (N, %) |
|
| Notes | Cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture If the initial method was unsuccessful after a maximum of 3 attempts, an alternative method was used for example, USG was used if the insertion was being done by the ALT technique, help was taken from a more experienced operator or an alternative site was chosen LM: 10/10 success with US and 7/7 success on the same side by a more experienced operator |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomization table |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | Yes |
Scherhag 1989.
| Methods | Prospectively randomized controlled trial Randomization plan, but no details in the text |
|
| Participants | Patients who required a CVC and in whom CVC placement was possible in the right IJV. Other patients were excluded Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at study entry. Minimum of 4 admission details described. 4 admission details described (sex, weight, height, age) Participants awake Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: technique described by Bazaral and Harlan vs Do: 4‐MHz Doppler, wrapped in a sterile glove, technique described by Scherhag vs US: 5‐MHz US, wrapped in a sterile glove, technique described by Scherhag ((short axis) see typical image in the article and description elsewhere) LM Unclear whether direct or indirect puncture Technique standardized: yes Flat head rotation Seldinger technique Vessel and side: IJV right side Do Direct puncture Technique standardized: yes Flat head rotation Seldinger technique Vessel and side IJV: right side US Direct puncture Technique standardized: yes Flat head rotation Seldinger technique Vessel and side IJV: right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %): failure: Operator was unable to cannulate the vein within 3 attempts. Change in direction without a new puncture/without reinsertion of the cannula was also included as an attempt Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): Cannulation time was defined as the time needed for identification of the puncture site and final catheter placement Success with attempt number 1, 2, 3 (N, %) Success rate after cross‐over (N, %) |
|
| Notes | Cross‐over landmark‐guided puncture: Participants who could not be cannulated during the initial 3 needle passes were crossed over to receive the alternate technique for the next 5 passes Cross‐over landmark‐guided puncture and Doppler‐guided puncture No cross‐over ultrasound‐guided puncture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization plan, but no details in the text |
| Allocation concealment (selection bias) | Unclear risk | Randomization plan, but no details in the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes LM group complication rate indicated, US group complication rate not indicated |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: Yes _X_ Intention‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Soyer 1993.
| Methods | Prospectively randomized controlled trial Randomization method: no details in the text Patients were prospectively and randomly selected into 2 groups |
|
| Participants | 47 patients with liver dysfunction underwent transjugular liver biopsy in our department Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details were described (sex, age) Participants awake Operators: number: 2 Experience: performed randomly by 2 different operators with the same experience in transjugular liver biopsy |
|
| Interventions | Technique: LM: participants awake vs US: participants awake, ultrasound with 7.5‐MHz resolution, probe sterilized with povidone‐iodine ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Catheter over needle Vessel and side: IJV right side US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Catheter over needle Vessel and side IJV: right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (n, %) Time to successful cannulation (seconds); time needed for RIJV catheterization Success rate after cross‐over (N, %): cross‐over after 6 attempts |
|
| Notes | Participants who could not be cannulated during the initial 6 needle passes were then crossed over Cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Sulek 2000.
| Methods | Randomized controlled trial (RCT) Randomization method: no details in the text (B) |
|
| Participants | 120 adult patients without previous IJV catheter placement scheduled for elective abdominal, vascular or cardiothoracic
procedures with general anaesthesia and mechanical ventilation Exclusion criteria for the study included the following: Patients were excluded from randomization if they had a history of radical neck dissection, carotid endarterectomy, carotid artery stenosis, contraindications to the Trendelenburg position or refusal to participate Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at entry 4 admission details were described (age, sex, weight, height) Participants anaesthetized Operators: number: no details Experience: All cannulation attempts were performed by operators experienced in IJV cannulation (at least 60 IJV catheter placements) with known expertise in use of the ultrasound‐guided IJV technique |
|
| Interventions | Technique: LM: technique well described in the article vs US: ultrasound with 5‐MHz resolution covered with a sterile glove ((long axis) see description in the article) LM Unclear whether direct or indirect puncture Technique standardized: yes Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV both sides US Direct puncture Technique standardized: yes Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV both sides |
|
| Outcomes | Number of attempts until success (N, SD) Complication rate: arterial puncture, haematoma formation (N, %) Time to successful cannulation (seconds): time required for successful guide wire insertion |
|
| Notes | No cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Yes__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Yes__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Teichgräber 1997.
| Methods | Prospectively randomized; randomization method: no details in the text | |
| Participants | 100 patients undergoing routine catheterization of the IJV Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission detail described No information on whether participants were anaesthetized or sedated or awake Operators: number: no details. 2 operators were necessary for this technique Experience: mean number of years of postgraduate clinical training LM group (6.9 ± 3.2 postgraduate); US group (3.8 ± 3.1 postgraduate) |
|
| Interventions | Technique: LM: no details vs US: 5‐MHz ultrasound ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head up (anti‐Trendelenburg) down (Trendelenburg); flat head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details US Direct puncture Technique standardized: unclear Head up (anti‐Trendelenburg); down (Trendelenburg); flat head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details |
|
| Outcomes | Complication rate: arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): Time to IJ access was measured Success with attempt number 1 (N, %) |
|
| Notes | No cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | Participant selection: No _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Troianos 1990.
| Methods | Prospectively randomized controlled trial Randomization method: no details in the text |
|
| Participants | 89 cardiothoracic surgical patients undergoing RIJ cannulation Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry No admission detail described Participants awake Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: participants awake vs US: participants awake, ultrasound with 7.5‐MHz resolution (SiteRite scanner without needle guide) covered by a sterile sheath. External landmarks were used to identify the site for injection of local anaesthetic (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Catheter over needle Vessel and side IJV: right side US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Catheter over needle Vessel and side IJV: right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time between application of local anaesthetic and RJI puncture Success with attempt number 1, 2 (N, %) |
|
| Notes | Participants who could not be cannulated during the initial 5 needle passes were crossed over to receive the alternate technique for the next 5 passes. But no Cross‐over landmark‐guided puncture or ultrasound‐guided puncture Congress poster |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Troianos 1991.
| Methods | Prospectively randomized controlled trial Randomization method: no details in the text The 2 groups were similar with respect to age, height, weight, presence of good anatomical landmarks and clinical experience |
|
| Participants | 160 cardiothoracic surgical patients undergoing RIJ cannulation Level of clinical experience of the person performing the cannulation was recorded, as was the presence or absence of good anatomical landmarks. Good landmarks included palpable division of the sternocleidomastoid muscle and a palpable carotid artery pulse Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry Admission details not described, only “equal demographic data” No admission detail described. Participants awake Operators: number: no details Experience: similar with respect to clinical experience |
|
| Interventions | Technique: LM: participants awake vs US: participants awake, ultrasound with 5‐ or 7.5‐MHz resolution (SonoSite 500 or SiteRite scanner without needle guide) covered by a sterile sheath; external landmarks were used to identify the site for injection of local anaesthetic ((short axis) see typical image in the article) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Catheter over needle Vessel and side IJV: right side US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Catheter over needle Vessel and side IJV: right side |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time between application of local anaesthetic and RJI puncture Success with attempt number 1 (N, %) Success rate after cross‐over (N, %) |
|
| Notes | Participants who could not be cannulated during the initial 3 needle passes were crossed over to receive the alternate technique for the next 5 passes Cross‐over landmark‐guided puncture; no cross‐over ultrasound‐guided puncture |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text The 2 groups were similar with respect to age, height, weight, presence of good anatomical landmarks and clinical experience |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text The 2 groups were similar with respect to age, height, weight, presence of good anatomical landmarks and clinical experience |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Turker 2009.
| Methods | Randomized controlled trial (RCT) Randomization method: no details in the text |
|
| Participants | "... spontaneously breathing patients ... who required internal jugular vein cannulation. All catheters were inserted to give total parenteral nutrition solution and chemotherapeutics or to measure the central venous pressure for i. v. fluid management" "... patients were enrolled in between April and November, 2008. Patients with local or systemic infection, known vascular abnormalities, untreated coagulopathy (international normalization ratio > 1.5 and platelets < 50000/mm3) were excluded" Inclusion and exclusion criteria clearly defined in the text Treatment and control groups adequately described at study entry 5 admission details described (sex, BMI, age, coagulation status, anatomical distinctiveness) Participants awake Operators: number: 1 Experience: senior medical student in final year |
|
| Interventions | Technique: LM: with finder needle vs US: 7.5‐MHz ultrasound probe, without finder needle (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side 94.73%, left 5.27% US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side IJV: right side 90.52%, left 9.48% |
|
| Outcomes | Overall success rate (N, %): Successful placement was defined as observation of the catheters in the proper position by X‐ray and functional determinants (i.e. no difficulty in the infusion or aspiration of venous blood). Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): access time between first skin puncture and aspiration of venous blood into the syringe |
|
| Notes | No cross‐over landmark‐guided puncture or ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | Low risk | Yes |
Verghese 1995.
| Methods | Congress poster Prospectively randomized: randomization method: no details in the text |
|
| Participants | 45 infants ASA status III or IV: Infants were randomly assigned to 1 of 3 groups (SmartNeedle (internal Doppler ultrasound), landmark‐guided, SiteRite) Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at entry 2 admission details described (weight, age) Other admission details not described, only “equal demographic data” Participants anaesthetized Operators: number: no details Experience: no details |
|
| Interventions | Technique: LM: no details vs US: ultrasound with 7.5‐MHz resolution (SiteRite scanner) needle guide no details (axis no details) vs Doppler: no details LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique: catheter over needle, no details Vessel and side: IJV no details US Unclear whether direct or indirect puncture Technique standardized: yes Head down (Trendelenburg), head rotation Seldinger technique: catheter over needle, no details Vessel and side: IJV no details Doppler Unclear whether direct or indirect puncture Technique standardized: yes Head down (Trendelenburg), head rotation Seldinger technique: catheter over needle, no details Vessel and side: IJV no details |
|
| Outcomes | Number of attempts until success (N, SD) Complication rate total (N, %) Time to successful cannulation (seconds): time between insertion of needle into the skin until free flow of blood from the catheter |
|
| Notes | No cross‐over landmark‐guided puncture; no ultrasound‐guided puncture Congress poster |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Yes |
| Other bias | High risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: Unclear _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Verghese 1996.
| Methods | Congress poster Prospectively randomized; randomization method: no details in the text |
|
| Participants | 95 infants (1 to 12 months of age) ASA status III or IV, scheduled to undergo IJ cannulation Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry 2 admission details described (weight, age) Other admission details not described, only “equal demographic data” Participants anaesthetized Operators: number: no details Experience: paediatric anesthesia fellows or attendings |
|
| Interventions | Technique: LM: no details vs US: 7.5‐MHz resolution SiteRite scanner, needle guide no details (axis no details) LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details US Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation no details Seldinger technique: catheter over needle, no details Vessel and side: IJV no details |
|
| Outcomes | Overall success rate (N, %) Failure rate (N, %) Number of attempts until success (N, SD) Complication rate: total, arterial puncture, local bleeding, haematoma formation, cardiac complications, malpositioned catheter tips, rate of catheter‐related infection, mortality, rate of other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) (N, %) Time to successful cannulation (seconds): time between insertion of the needle into the skin until free flow of blood from the catheter |
|
| Notes | No cross‐over landmark‐guided puncture; no ultrasound‐guided puncture Congress poster |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Participant selection: Yes _X_ Withdrawals: Unclear _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
Vucevic 1994.
| Methods | Randomized controlled trial (RCT) Randomization method: no details in the text (B) |
|
| Participants | Adult patients requiring central venous cannulation for cardiac surgery or in the ICU 40 patients, randomly allocated into 4 groups of 10. In group A (control), no problems were anticipated in cannulation. In group B, the SMART needle was used, and again no problems were anticipated as regards cannulation. In groups C (control) and D (SMART), potential problems were anticipated because of obesity, previous cannulations or previous unsuccessful attempts. Groups A and B are designated as ‘easy’ groups, and groups C and D as ‘difficult’ Inclusion and exclusion criteria not clearly defined in the text Treatment and control groups not adequately described at study entry Admission details not described, only “equal demographic data” No admission detail described Participants anaesthetized Operators: number: 2 Experience: 1 of 2 consultant anaesthetists, both with extensive experience in jugular venous access using the standard Seldinger technique. As neither anaesthetist had previously used the SMART needle, both performed 10 SMART needle cannulations before the start of the study to familiarize themselves with this new technique |
|
| Interventions | Technique: LM: no details vs US: 14.3‐MHz SmartNeedle LM Unclear whether direct or indirect puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side US Direct puncture Technique standardized: unclear Head down (Trendelenburg), head rotation Seldinger technique Vessel and side: IJV right side |
|
| Outcomes | Overall success rate (n, %) Failure rate (n, %) Number of attempts until success (n): number of attempts at cannulation: A single pass was defined as aspiration of blood on the way in or on withdrawal. Redirection of the needle counted as a further attempt Arterial puncture (n, %) Time to successful cannulation (seconds): time to successful insertion of the Seldinger wire Success with attempt number 1, 2, 3, 4, 5 (n, %) Success rate after cross‐over (n, %) |
|
| Notes | Cross‐over landmark‐guided puncture and ultrasound‐guided puncture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Allocation concealment (selection bias) | Unclear risk | Randomization method: no details in the text (B) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of participants who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Yes |
| Other bias | Unclear risk | Participant selection: Unclear _X_ Withdrawals: No _X_ Postrandomization exclusion: No _X_ Intention‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
| Treatment and control groups were adequately described at entry. | High risk | No |
ASA = American Society of Anesthesiologists.
BSI = blood stream infection.
CCT = controlled clinical trial.
CVC = central venous catheter.
Do = Doppler.
ED = emergency department.
ICU = intensive care unit.
IJV = internal jugular vein.
IJVC = internal jugular vein cannulation.
LIJV = left internal jugular vein.
LM = landmark puncture technique.
Q‐RCT = quasi‐randomized controlled trial.
RCT = randomized controlled trial.
RIJV = right internal jugular vein.
SD = standard deviation.
SV = subclavian vein.
TEE = transesophageal echocardiography.
US = ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alderson 1993 | Published twice (Congress poster → article) (see Alderson 1992) |
| Denys 1990 | Prospective study, not randomized; only ultrasound used; published twice (see Denys 1991) |
| Denys 1991 | Prospective study, not randomized; only ultrasound used; published twice (see Denys 1990) |
| Froehlich 2009 | Different vessels were punctured and were statistically analysed together |
| Gallieni 1995 | Observational study; LM used first for 10 patients, then US for additional 31 patients |
| Koski 1992 | Observational study; ultrasound‐guided technique was used during first half of the study and conventional method during second half of the study |
| Legler 1984 | Published twice (Congress poster → article) (see Legler 1983) |
| Miller 2002 | Prospectively randomized (C) controlled trial with different vessels punctured and statistically analysed together |
| Serafimidis 2009 | No details on whether the study is prospective and randomized |
| Slama 1997 | No report of ethical approval; nor did study authors ask for patients’ consent. Randomization was balanced for procedures performed by interns or residents |
| Verghese 1999 | Published twice (Congress poster → article) (see Verghese 1996) |
| Verghese 2000 | Published twice (Congress poster → article) (see Verghese 1995) |
| Woody 2001 | Prospectively randomized study. No details on punctured vessels were given; no usable data |
Characteristics of studies awaiting assessment [ordered by study ID]
Airapetian 2013.
| Methods | Prospective randomized single‐centre controlled trial |
| Participants | A total of 118 patients requiring jugular or femoral central cannula placement were randomly assigned to 3 groups |
| Interventions | Quick‐look ultrasound with a skin mark (UM) has been used frequently for central vein cannulation. The aim of this study was to compare this method with landmark (LM) and ultrasound‐guided (UG) cannulation of jugular and femoral veins by inexperienced operators |
| Outcomes | Primary outcome was success rate; secondary outcomes were placement time, number of attempts, mechanical complication rate and catheter colonization rate |
| Notes |
Bikash 2014.
| Methods | Prospective randomized observational study |
| Participants | 120 patients scheduled for elective or emergency surgery or who during their stay in the ICU required IJV catheterization were included in this study |
| Interventions | This study compares the ultrasound‐guided technique (real‐time image during cannulation, relocation of the IJV before cannulation) versus the classical anatomical landmark technique (central approach) for right IJV cannulation |
| Outcomes | Number of attempts, success rate, venous access time, catheterization time and complications |
| Notes |
Cajozzo 2004.
| Methods | Prospective randomized study |
| Participants | 196 patients: 105 received US‐guided CVC, and 91 received CVC without US guide |
| Interventions | US‐guided CVC and CVC without US guide |
| Outcomes | Time to perform CVC, success, major complications |
| Notes |
Gok 2013.
| Methods | Prospective randomized single‐centre study |
| Participants | Critical care patients suffering cardiac arrest, congestive cardiac failure, acute pulmonary embolism, ARDS, postoperative respiratory failure, trauma, neuromuscular disease, cerebrovascular accident, metabolic disease, organophosphorus poisoning and catheterization |
| Interventions | 97 real‐time USG‐guided internal vein catheterizations compared with the landmark technique used in 97 critical care patients |
| Outcomes | Incidence of catheter‐related bloodstream infection, average access time, time for insertion, attempts required, mechanical complications |
| Notes |
Shrestha 2011.
| Methods | Prospective randomized comparative study |
| Participants | 120 patients in an intensive care unit requiring central venous cannulation |
| Interventions | Ultrasound technique for cannulation of the right internal jugular vein vs conventional landmark technique |
| Outcomes | Success, number of attempts, time and first attempt success rate |
| Notes |
ARDS = acute respiratory distress syndrome.
CVCs = central venous catheters.
ICU = intensive care unit.
LM = landmark.
UG = ultrasound‐guided.
US = ultrasound.
Differences between protocol and review
Five differences between the published protocol (Brass 2008) and the review should be noted.
We used the new domain‐based evaluation of The Cochrane Collaboration to assess the validity and quality of included studies because this tool was released after publication of the protocol.
We planned to perform sensitivity analysis regarding 'randomized versus quasi‐randomized' and eventually 'good quality studies versus poor quality studies' to test how sensitive the results are to reasonable changes in assumptions made and in the protocol for combining the data. We have not performed the sensitivity analysis, as almost all studies included in this review have unclear risk of bias across the six domains.
The original protocol (Brass 2008) proposed a single review including all anatomical sites for central venous catheterization. In view of the numbers of eligible studies and comparisons, we have split the material into two reviews: This review will focus on the internal jugular vein, and the other review on the subclavian and femoral veins (Brass 2013b).
We planned to consider the following additional outcomes: number of participants with significant local bleeding, number of participants with significant cardiac complications, rate of malpositioned catheter tips, number of participants with a significant pneumothorax, rate of catheter‐related infection and success rate after cross‐over. During our evaluation, we have determined that it is more useful to look at the number of participants with other complications (thrombosis, embolism, haematomediastinum and hydromediastinum, haematothorax and hydrothorax, pneumothorax, subcutaneous emphysema, nerve injury) together. We planned to examine the costs connected with application of the new method and whether the additional financial expenditure is in reasonable proportion to the possible ensurance of improvement/advantages. We have not undertaken these analyses, as none of the studies assessed costs.
We planned to use a fixed‐effect model when between‐studies heterogeneity was negligible; otherwise we planned to use a random‐effects model, which takes into account between‐study variability as well as within‐study variability. We have used a random‐effects model for all analyses regardless of heterogeneity, as in most comparisons, the heterogeneity that cannot be readily explained is > 25%. This is the more conservative approach.
Contributions of authors
Patrick Brass (PB), Martin Hellmich (HM), Laurentius Kolodziej (LK), Guido Schick (GS), Andrew F Smith (AFS).
Conceiving of the review: PB. Designing the review: PB. Co‐ordinating the review: PB. Undertaking manual searches: PB. Undertaking electronic searches: Karen Hovhannisyan, PB. Screening search results: PB, LK. Organizing retrieval of papers: PB, LK. Screening retrieved papers against inclusion criteria: PB, LK, GS. Appraising quality of papers: PB, LK, GS. Abstracting data from papers: PB, LK, GS. Writing to authors of papers to ask for additional information: PB. Obtaining additional data about papers: PB. Obtaining and screening data on unpublished studies: PB, LK. Managing data for the review: MH. Entering data into Review Manager (RevMan 5.2): PB, LK, GS. Analysing data: PB, GS. Interpreting data: PB, GS, MH, AFS. Writing the review: PB, AFS. Performing previous work that served as the foundation of the present study: PB. Serving as guarantor for the review (one review author): PB. Performing statistical analysis: PB, MH.
Sources of support
Internal sources
New source of support, Other.
External sources
-
National Institute for Health Research, UK.
Salary support for Andrew Smith
Declarations of interest
Patrick Brass: none known.
Martin Hellmich: none known.
Laurentius Kolodziej: none known.
Guido Schick: none known.
Andrew F Smith: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Agarwal 2009 {published data only}
- Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian Journal of Critical Care Medicine 2009;13(4):213‐6. [DOI: 10.4103/0972-5229.60174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alderson 1992 {published data only}
- Alderson PJ, Burrows FA, Holtby HM. The use of ultrasound to facilitate central venous cannulation in young children. Anesthesiology 1992;77(3A):A1196. [Google Scholar]
Armstrong 1993 {published data only}
- Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine—an aid to internal jugular vein cannulation. Anaesthesia 1993;48(4):319‐23. [DOI] [PubMed] [Google Scholar]
Bansal 2005 {published data only}
- Bansal R, Agarwal SK, Tiwari SC, Dash SC. A prospective randomized study to compare ultrasound‐guided with nonultrasound‐guided double lumen internal jugular catheter insertion as a temporary hemodialysis access. Renal Failure 2005;27(5):561‐4. [PUBMED: 16152994] [DOI] [PubMed] [Google Scholar]
Böck 1999 {published data only}
- Böck U, Möllhoff T, Förster R. Ultrasonography guided versus anatomically oriented puncture of the internal jugular vein for central venous catheterization [Ultraschall gesteuerte versus anatomisch orientierte Punktion der Vena jugularis interna zur zentralvenösen Katheterisierung]. Ultraschall in der Medizin 1999;20(3):98‐103. [DOI] [PubMed] [Google Scholar]
Branger 1994 {published data only}
- Branger B, Zabadani B, Vecina F, Juan JM, Dauzat M. Continuous guidance for venous punctures using a new pulsed Doppler probe: efficiency, safety [Guidage continu des ponctions veineuses par une nouvelle sonde Doppler pulsee: efficacite, securite]. Nephrologie 1994;15(2):137‐40. [PubMed] [Google Scholar]
Branger 1995 {published data only}
- Branger B, Dauzat M, Zabadani B, Vecina F, Lefranc JY. Pulsed Doppler sonography for the guidance of vein puncture: a prospective study. Artificial Organs 1995;19(9):933‐8. [DOI] [PubMed] [Google Scholar]
Chuan 2005 {published data only}
- Chuan Wx, Wei W, Yu L. A randomized controlled study of ultrasound prelocation vs anatomical landmark‐guided cannulation of the internal jugular vein in infants and children. Pediatric Anesthesia 2005;15(9):733‐8. [DOI] [PubMed] [Google Scholar]
Denys 1993 {published data only}
- Denys BG, Uretsky BF, Reddy PS. Ultrasound‐assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark‐guided technique. Circulation 1993;87(5):1557‐62. [DOI] [PubMed] [Google Scholar]
Gilbert 1995 {published data only}
- Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio‐guided Doppler ultrasound vascular access device: results from a prospective, dual‐center, randomized, crossover clinical study. Critical Care Medicine 1995;23(1):60‐5. [DOI] [PubMed] [Google Scholar]
Gratz 1994 {published data only}
- Gratz I, Afshar M, Kidwell P, Weiman DS, Shariff HM. Doppler‐guided cannulation of the internal jugular vein: a prospective, randomized trial. Journal of Clinical Monitoring 1994;10(3):185‐8. [DOI] [PubMed] [Google Scholar]
Grebenik 2004 {published data only}
- Grebenik CR, Boyce A, Sinclair ME, Evans RD, Mason DG, Martin B. NICE guidelines for central venous catheterization in children. Is the evidence base sufficient?. British Journal of Anaesthesia 2004;92(6):827‐30. [DOI] [PubMed] [Google Scholar]
Hayashi 1998 {published data only}
- Hayashi H, Tsuzuku M, Amano M. Simplfied echo‐guided internal jugular vein puncture: a comparison to the landmark‐guided technique. Anesthesia and Analgesia. 1998; Vol. 86:SCA89.
Hayashi 2002 {published data only}
- Hayashi H, Amano M. Does ultrasound imaging before puncture facilitate internal jugular vein cannulation? Prospective randomized comparison with landmark‐guided puncture in ventilated patients. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(5):572‐5. [DOI] [PubMed] [Google Scholar]
Heatly 1995 {published data only}
- Heatly T, Berger R. Comparison of the conventional landmark technique and an ultrasound‐guided approach for the placement of central venous catheters. American Journal of Respiratory and Critical Care Medicine 1995;151(S):A333. [Google Scholar]
Hrics 1998 {published data only}
- Hrics P, Wilber S, Blanda MP, Gallo U. Ultrasound‐assisted internal jugular vein catheterization in the ED. The American Journal of Emergency Medicine 1998;16(4):401‐3. [DOI] [PubMed] [Google Scholar]
Johnson 1994 {published data only}
- Johnson R, ODonnell J, Fielder K. Ultrasound guidance for cannulation of the internal jugular vein (IJV) in the critically ill. A randomized prospective study. Critical Care Medicine 1994;22(1):A28. [Google Scholar]
Karakitsos 2006 {published data only}
- Karakitsos D, Labropoulos N, Groot E, Patrianakos AP, Kouraklis G, Poularas J, et al. Real‐time ultrasound‐guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Critical Care 2006;10(6):R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legler 1983 {published data only}
- Legler D, Nugent M. Doppler localization of the internal jugular vein facilitates its cannulation. Anesthesiology 1983;59:A179. [DOI] [PubMed] [Google Scholar]
Leung 2006 {published data only}
- Leung J, Duffy M, Finckh A. Real‐time ultrasonographically‐guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: a randomized, prospective study. Annals of Emergency Medicine 2006;48(5):540‐7. [DOI: 10.1016/j.annemergmed.2006.01.011; PUBMED: 17052555] [DOI] [PubMed] [Google Scholar]
Lin 1998 {published data only}
- Lin BS, Huang TP, Tang GJ, Tarng DC, Kong CW. Ultrasound‐guided cannulation of the internal jugular vein for dialysis vascular access in uremic patients. Nephron 1998;78(4):423‐8. [DOI] [PubMed] [Google Scholar]
Mallory 1990 {published data only}
- Mallory DL, McGee WT, Shawker TH, Brenner M, Bailey KR, Evans RG, et al. Ultrasound guidance improves the success rate of internal jugular vein cannulation. A prospective, randomized trial. Chest 1990;98:157‐60. [DOI] [PubMed] [Google Scholar]
Milling 2005 {published data only}
- Milling TJ Jr, Rose J, Briggs WM, Birkhahn R, Gaeta TJ, Bove JJ, et al. Randomized, controlled clinical trial of point‐of‐care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP‐3) Trial. Critical Care Medicine 2005;33(8):1764‐9. [PUBMED: 16096454] [DOI] [PubMed] [Google Scholar]
Ovezov 2010 {published data only}
- Ovezov A, Zakirov I, Vishnyakova M. Effectiveness and safety of the internal jugular vein catheterization in pediatrics: ultrasound navigation vs anatomical landmarks (a prospective, randomized, double‐blind study). Electronic poster. Barcelona, ESICM, 2010.
Palepu 2009 {published data only}
- Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian Journal of Radiology and Imaging 2009;19(3):191‐8. [DOI: 10.4103/0971-3026.54877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherhag 1989 {published data only}
- Scherhag A, Klein A, Jantzen JP. Cannulation of the internal jugular vein using 2 ultrasonic technics. A comparative controlled study. [Die Vena jugularis interna‐Kanülierung mit Hilfe zweier Ultraschallverfahren. Eine vergleichende, kontrollierte Untersuchung]. Anaesthesist 1989;38(11):633‐8. [PubMed] [Google Scholar]
Soyer 1993 {published data only}
- Soyer P, Lacheheb D, Levesque M. High‐resolution sonographic guidance for transjugular liver biopsy. Abdominal Imaging 1993;18(4):360‐2. [DOI] [PubMed] [Google Scholar]
Sulek 2000 {published data only}
- Sulek CA, Blas ML, Lobato EB. A randomized study of left versus right internal jugular vein cannulation in adults. Journal of Clinical Anesthesia 2000;12(2):142‐5. [PUBMED: 10818329] [DOI] [PubMed] [Google Scholar]
Teichgräber 1997 {published data only}
- Teichgräber UK, Benter T, Gebel M, Manns MP. A sonographically guided technique for central venous access. American Journal of Roentgenology 1997;169(3):731‐3. [DOI] [PubMed] [Google Scholar]
Troianos 1990 {published data only}
- Troianos CA, Jobes DR, Ellison N. Ultrasound guided cannulation of the internal jugular vein. Anesthesiology 1990;73, No 3A:A451. [DOI] [PubMed] [Google Scholar]
Troianos 1991 {published data only}
- Troianos CA, Jobes DR, Ellison N. Ultrasound‐guided cannulation of the internal jugular vein. A prospective, randomized study. Anesthesia and Analgesia 1991;72(6):823‐6. [DOI] [PubMed] [Google Scholar]
Turker 2009 {published data only}
- Turker G, Kaya FN, Gurbet A, Aksu H, Erdogan C, Atlas A. Internal jugular vein cannulation: an ultrasound‐guided technique versus a landmark‐guided technique. Clinics 2009;64(10):989‐92. [DOI: 10.1590/S1807-59322009001000009; PUBMED: 19841706] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verghese 1995 {published data only}
- Verghese ST, McGill WA, Patel RI, Sell JE, Midgley FM, Ruttimann UE. Approaches to internal jugular vein cannulation in infants: seeing, hearing vs. feeling. Anesthesia and Analgesia 1995;80:S525. [Google Scholar]
Verghese 1996 {published data only}
- Verghese S, McGill WA, Patel R, Norden J, Ruttiman U. Internal jugular vein cannulation in infants: palpation vs. imaging. Anesthesiology 1996;85, 3A:1078. [Google Scholar]
Vucevic 1994 {published data only}
- Vucevic M, Tehan B, Gamlin F, Berridge JC, Boylan M. The SMART needle. A new Doppler ultrasound‐guided vascular access needle. Anaesthesia 1994;49(10):889‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alderson 1993 {published data only}
- Alderson PJ, Burrows FA, Stemp LI, Holtby HM. Use of ultrasound to evaluate internal jugular vein anatomy and to facilitate central venous cannulation in paediatric patients. British Journal of Anaesthesia 1993;70(2):145‐8. [DOI] [PubMed] [Google Scholar]
Denys 1990 {published data only}
- Denys BG, Uretsky BF, Ruffner RJ, Sandhu JS, Reddy PS. The use of ultrasound to access the internal jugular vein: a prospective study in 300 patients. Circulation 1990;82(S III):67. [Google Scholar]
Denys 1991 {published data only}
- Denys BG, Uretsky BF, Reddy PS, Ruffner RJ, Sandhu JS, Breishlatt WM. An ultrasound method for safe and rapid central venous access. The New England Journal of Medicine 1991;324(8):566. [PUBMED: 1992315] [DOI] [PubMed] [Google Scholar]
Froehlich 2009 {published data only}
- Froehlich CD, Rigby MR, Rosenberg ES, Li R, Roerig PL, Easley KA, et al. Ultrasound‐guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Critical Care Medicine 2009;37(3):1090‐6. [DOI: 10.1097/CCM.0b013e31819b570e] [DOI] [PubMed] [Google Scholar]
Gallieni 1995 {published data only}
- Gallieni S, Cozzolino M. Uncomplicated central vein catheterization of high risk patients with real time ultrasound guidance. The International Journal of Artificial Organs 1995;18(3):117‐21. [PubMed] [Google Scholar]
Koski 1992 {published data only}
- Koski EM, Suhonen M, Mattila MA. Ultrasound‐facilitated central venous cannulation. Critical Care Medicine 1992;20(3):424‐6. [PMID: 1541105] [DOI] [PubMed] [Google Scholar]
Legler 1984 {published data only}
- Legler D, Nugent M. Doppler localization of the internal jugular vein facilitates its cannulation. Anesthesiology 1984;60(5):481‐2. [DOI] [PubMed] [Google Scholar]
Miller 2002 {published data only}
- Miller AH, Roth BA, Mills TJ, Woody JR, Longmoor CE, Foster B. Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department. Academic Emergency Medicine 2002;9(8):800‐5. [DOI] [PubMed] [Google Scholar]
Serafimidis 2009 {published data only}
- Serafimidis K, Sakorafas GH, Konstantoudakis G, Petropoulou K, Giannopoulos GP, Danias N, et al. Ultrasound‐guided catheterization of the internal jugular vein in oncologic patients; comparison with the classical anatomic landmark technique: a prospective study. International Journal of Surgery 2009;7(6):526‐8. [DOI: 10.1016/j.ijsu.2009.08.011] [DOI] [PubMed] [Google Scholar]
Slama 1997 {published data only}
- Slama M, Novara A, Safavian A, Ossart M, Safar M, Fagon JY. Improvement of internal jugular vein cannulation using an ultrasound‐guided technique. Intensive Care Medicine 1997;23(8):916‐9. [DOI] [PubMed] [Google Scholar]
Verghese 1999 {published data only}
- Verghese ST, McGill WA, Patel RI, Sell JE, Midgley FM, Ruttimann UE. Ultrasound‐guided internal jugular venous cannulation in infants: a prospective comparison with the traditional palpation method. Anesthesiology 1999;91(1):71‐7. [DOI] [PubMed] [Google Scholar]
Verghese 2000 {published data only}
- Verghese ST, McGill WA, Patel RI, Sell JE, Midgley FM, Ruttimann UE. Comparison of three techniques for internal jugular vein cannulation in infants. Paediatric Anaesthesia 2000;10(5):505‐11. [DOI] [PubMed] [Google Scholar]
Woody 2001 {published data only}
- Woody JR, Miller AH, Mills TJ, Roth BA. Using ultrasound for central venous line placement (CVP) in the emergency department. Academic Emergency Medicine 2001;8:580. [DOI: 10.1111/j.1553-2712.2001.tb00169.x] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Airapetian 2013 {published data only}
- Airapetian N, Maizel J, Langelle F, Modeliar SS, Karakitsos D, Dupont H, et al. Ultrasound‐guided central venous cannulation is superior to quick‐look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Medicine 2013;39(11):1938‐44. [DOI: 10.1007/s00134-013-3072-z; PUBMED: 24026296] [DOI] [PubMed] [Google Scholar]
Bikash 2014 {published data only}
- Bikash RR, Virender KM, Lokesh K, Dilip S, Vanlal MD, Ravindra KP. Internal jugular vein cannulation: a comparison of three techniques. Journal of Anaesthesiology Clinical Pharmacology 2014;29(3):367‐71. [DOI: 10.4103/0970-9185.117115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cajozzo 2004 {published data only}
- Cajozzo M, Quintini G, Cocchiera G, Greco G, Vaglica R, Pezzano G, et al. Comparison of central venous catheterization with and without ultrasound guide. Transfusion and Apheresis Science 2004;31(3):199‐202. [PUBMED: 15556467] [DOI] [PubMed] [Google Scholar]
Gok 2013 {published data only}
- Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter‐associated bloodstream infection in critical care patients. Acta Medica Mediterranea 2013;29:677‐82. [Google Scholar]
Shrestha 2011 {published data only}
- Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. Journal of the Nepal Medical Association 2011;51(182):56‐61. [PUBMED: 22916513] [PubMed] [Google Scholar]
Additional references
American College of Emergency Physicians 2007
Atkinson 2005
- Atkinson P, Boyle A, Robinson S, Campbell‐Hewson G. Should ultrasound guidance be used for central venous catheterisation in the emergency department ?. Emergency Medicine Journal 2005;22:158‐64. [DOI: 10.1136/emj.2003.011288] [DOI] [PMC free article] [PubMed] [Google Scholar]
Augoustides 2009
- Augoustides JG, Cheung AT. Pro: ultrasound should be the standard of care for central catheter insertion. Journal of Cardiothoracic and Vascular Anesthesia 2009;23(5):720‐4. [DOI: 10.1053/j.jvca.2009.06.012; PUBMED: 19686963] [DOI] [PubMed] [Google Scholar]
Bernard 1971
- Bernard RW, Stahl WM. Subclavian vein catheterization: a prospective study. I. Non‐infectious complications. Annals of Surgery 1971;173(2):184‐90. [PUBMED: 5100094] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bo‐Linn 1982
- Bo‐Linn GW, Anderson DJ, Anderson KC, McGoon MD. Percutaneous central venous catheterization performed by medical house officers: a prospective study. Catheterization and Cardiovascular Diagnosis 1982;8(1):23‐9. [PUBMED: 7060113] [DOI] [PubMed] [Google Scholar]
Bodenham 2006
- Bodenham AR. Commentary. Can you justify not using ultrasound guidance for central venous access?. Critical Care 2006;10(6):175. [DOI: 10.1186/cc5079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bodenham 2011
- Bodenham A. Reducing major procedural complications from central venous catheterisation. Anaesthesia 2011;66:6‐9. [DOI: 10.1111/j.1365-2044.2010.06583.x; PUBMED: 21198502] [DOI] [PubMed] [Google Scholar]
Bold 1998
- Bold RJ, Winchester DJ, Madary AR, Gergurich MA, Mansfield PF. Prospective, randomized trial of Doppler‐assisted subclavian vein catheterization. Archives of Surgery 1998;133(10):1089‐93. [DOI] [PubMed] [Google Scholar]
Brass 2001
- Brass P, Volk O, Leben J, Schregel W. Central venous cannulation—always with ultrasound support? [Zentralvenöse Punktion Nur Noch mit Ultraschall?]. Anästesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2001;36(10):619‐27. [DOI] [PubMed] [Google Scholar]
Brass 2013b
- Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Traditional landmark versus ultrasound guidance for internal jugular vein catheterization. Cochrane Database of Systematic Reviews In process, issue In process. [DOI] [PMC free article] [PubMed]
Calvert 2003
- Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A. The effectiveness and cost‐effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technology Assessment 2003;7(12):1‐84. [PUBMED: 12709290] [DOI] [PubMed] [Google Scholar]
Calvert 2004
- Calvert N, Hind D, McWilliams R, Davidson A, Beverley C, Thomas SM. Ultrasound for central venous cannulation: economic evaluation of cost‐effectiveness. Anaesthesia 2004;59(11):1116‐20. [PUBMED: 15479322] [DOI] [PubMed] [Google Scholar]
Caridi 1998
- Caridi JG, Hawkins IF Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. American Journal of Roentgenology 1998;171(5):1259‐63. [PUBMED: 9798857] [DOI] [PubMed] [Google Scholar]
Cook 2011
- Cook TM. Litigation related to central venous access by anaesthetists: an analysis of claims against the NHS in England 1995‐2009. Anaesthesia 2011;66(1):56‐7. [DOI: 10.1111/j.1365-2044.2010.06569.x; PUBMED: 21198504] [DOI] [PubMed] [Google Scholar]
Debordeau 2009
- Debourdeau P, Kassab Chahmi D, Gal G, Kriegel I, Desruennes E, Douard MC, et al. Working group of the SOR, French National Feberation of Cancer Centers. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Annals of Oncology 2009;20(9):1459–71. [DOI: 10.1093/annonc/mdp052; PUBMED: 19525362] [DOI] [PubMed] [Google Scholar]
Defalque 1974
- Defalque RJ. Percutaneous catheterization of the internal jugular vein. Anesthesia and Analgesia 1974;53(1):116‐21. [PUBMED: 4589503] [PubMed] [Google Scholar]
Denys 1991a
- Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Critical Care Medicine 1991;19(12):1516‐9. [PUBMED: 1959371] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [PUBMED: 7718048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Domino 2004
- Domino KB, Bowdle TA, Posner KL, Spitellie PH, Lee LA, Cheney FW. Injuries and liability related to central vascular catheters: a closed claims analysis. Anesthesiology 2004;100(6):1411‐18. [PUBMED: 15166560] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [PUBMED: 9432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA Drug Bull 1989
- Food, Drug Administration. Precautions necessary with central venous catheters. FDA Drug Bulletin 1989;July:15‐6. [Google Scholar]
Feller‐Kopman 2007
- Feller‐Kopman D. Ultrasound‐guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest 2007;132(1):302‐9. [PUBMED: 17625091] [DOI] [PubMed] [Google Scholar]
Ferral 1998
- Ferral H. US‐guided puncture of the internal jugular vein: an unexpected anatomic relationship. Journal of Vascular and Interventional Radiology 1998;9(5):854‐5. [PUBMED: 9756083] [DOI] [PubMed] [Google Scholar]
French 2008
- French J, Raine‐Fenning N, Hardmann J. Pitfalls of ultrasound guided vascular access: the use of three four dimensional ultrasound. Anaesthesia 2008;63:806–13. [DOI] [PubMed] [Google Scholar]
Ge 2012
- Ge X, Cavallazzi R, Li C, Pan SM, Wang YW, Wang FL. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004084.pub3; PUBMED: 22419292] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girard 2005
- Girard TD, Schectman JM. Ultrasound guidance during central venous catheterization: a survey of use by house staff physicians. Journal of Critical Care 2005;20(3):224‐9. [PUBMED: 16253790] [DOI] [PubMed] [Google Scholar]
Glasziou 2001
- Glasziou P, Irwig L, Bain C, Colditz G. Systematic Reviews in Health Care: A Practical Guide. 1st Edition. Cambridge, UK: Cambridge University Press, 2001. [NLM ID: 101133142] [Google Scholar]
Goodwin 2005
- Goodwin D, Pope C, Mort M, Smith AF. Access, boundaries and their effects: legitimate participation in anaesthesia. Sociology of Health and Ilness 2005;27:855‐71. [PUBMED: 16283902] [DOI] [PubMed] [Google Scholar]
Grau 2005
- Grau T, Kessler J, Mansmann U. Re: central venous catheterization in infants. British Journal of Anaesthesia 2005;94(1):135. [PUBMED: 15637785] [PubMed] [Google Scholar]
Guimares 2009
- Guimaraes MM, Dib R, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006058.pub2] [DOI] [PubMed] [Google Scholar]
Hessel 2009
- Hessel EA 2nd. Con: we should not enforce the use of ultrasound as a standard of care for obtaining central venous access. Journal of Cardiothoracic and Vascular Anesthesia. 2009;23(5):725‐8. [DOI: 10.1053/j.jvca.2009.06.020; PUBMED: 19789059] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG (editors) editor(s). In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons, Ltd, 2011. [Google Scholar]
Hind 2003
- Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C, et al. Ultrasonic locating devices for central venous cannulation: meta‐analysis. BMJ 2003;327(7411):361. [PUBMED: 12919984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2007
- Howard S. A survey measuring the impact of NICE guidance 49: the use of ultrasound locating devices for placing central venous catheters. http://www.nice.org.uk/pdf/Final_CVC_placement_survey_report.pdf (accessed 4 June 2007).
Joffe 2009
- Joffe A, Anton N, Lequier L, Vandermeer B, Tjosvold L, Larsen B, et al. Nutritional support for critically ill children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005144.pub2] [DOI] [PubMed] [Google Scholar]
Keenan 2002
- Keenan SP. Use of ultrasound to place central lines. Journal of Critical Care 2002;17(2):126‐37. [PUBMED: 12096376] [DOI] [PubMed] [Google Scholar]
Kinsella 2009
- Kinsella S, Young N. Ultrasound‐guided central line placement as compared with standard landmark technique: some unpleasant arithmetic for the economics of medical innovation. Value Health 2009;12(1):98‐100. [DOI: 10.1111/j.1524-4733.2008.00427.x; PUBMED: 18647249] [DOI] [PubMed] [Google Scholar]
Lamperti 2010
- Lamperti M, Cortellazzi P, Caldiroli D. Ultrasound‐guided cannulation of IJV in pediatric patients: are meta‐analyses sufficient?. Paediatric Anaesthesia 2010;20(4):373‐4. [DOI: 10.1111/j.1460-9592.2010.03276.x.; PUBMED: 20470347] [DOI] [PubMed] [Google Scholar]
Lamperti 2012
- Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Medicine 2012;38(7):1105‐17. [DOI: 10.1007/s00134-012-2597-x; PUBMED: 22614241] [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. In: Mulrow C, Cook D editor(s). Systematic Reviews: Synthesis of Best Evidence for Healthcare Decisions. 1st Edition. Philadelphia: American College of Physicians, 1998:91‐101. [Google Scholar]
Lefebvre 2001
- Lefebvre C, Clarke M. Identifying randomized trials. In: Egger M, Davey Smith G, Altman D editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001:69‐86. [NLM ID: 101093083] [Google Scholar]
Lefrant 1998
- Lefrant JY, Cuvillon P, Bénézet JF, Dauzat M, Peray P, Saïssi G, et al. Pulsed Doppler ultrasonography guidance for catheterization of the subclavian vein. Anesthesiology 1998;88(5):1195‐201. [PUBMED: 9605678] [DOI] [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing Up: The Science of Reviewing Research. Cambridge, Massachusetts: Harvard University Press, 1984. [NLM ID: 8505340] [Google Scholar]
McIntyre 1992
- McIntyre AS, Levison RA, Wood S, Phillips RK, Lennard‐Jones JE. Duplex Doppler ultrasound identifies veins suitable for insertion of central feeding catheters. Journal of Parenteral and Enteral Nutrition 1992;16(3):264‐7. [PUBMED: 1501358] [DOI] [PubMed] [Google Scholar]
Merrer 2001
- Merrer J, Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286(6):700‐7. [PUBMED: 11495620] [DOI] [PubMed] [Google Scholar]
Mort 2009
- Mort M, Smith AF. Beyond information: intimate relations in sociotechnical practice. Sociology 2009;43:215‐31. [doi: 10.1177/0038038508101162] [Google Scholar]
Muhm 2002
- Muhm M. Ultrasound guided central venous access. BMJ 2002;325(7377):1373‐4. [PUBMED: 12480829] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2002
- National Institute for Health and Clinical Excellence Appraisal Committee Members. Guidance on the use of ultrasound locating devices for placing central venous catheters. Technology Appraisal No. 49 September 2002;49:1‐24. [Google Scholar]
Peters 1982
- Peters JL, Belsham PA, Garrett CP, Kurzer M. Doppler ultrasound technique for safer percutaneous catheterization of the infraclavicular subclavian vein. American Journal of Surgery 1982;143(3):391‐3. [PUBMED: 7065360] [DOI] [PubMed] [Google Scholar]
Pikwer 2012
- Pikwer A, Åkeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia 2012;67(1):65‐71. [DOI: 10.1111/j.1365-2044.2011.06911.x; PUBMED: 19370617] [DOI] [PubMed] [Google Scholar]
Polderman 2002
- Polderman KH, Girbes AR. Central venous catheter use. Part 1: mechanical complications. Intensive Care Medicine 2002;28(1):1‐17. [PUBMED: 11818994] [DOI] [PubMed] [Google Scholar]
Rajaram 2013
- Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD003408.pub3; PUBMED: 23450539] [DOI] [PMC free article] [PubMed] [Google Scholar]
Randolph 1996
- Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature. Critical Care Medicine 1996;24(12):2053‐8. [PUBMED: 8968276] [DOI] [PubMed] [Google Scholar]
Resnick 2008
- Resnick JR, Cydulka RK, Donato J. Success of ultrasound‐guided peripheral intravenous access with skin marking. Academic Emergency Medicine 2008;15(8):723‐30. [PUBMED: 18637084] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, November 8 2012.
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150‐3. [PUBMED: 11914311] [DOI] [PubMed] [Google Scholar]
Rothschild 2001
- Rothschild JM. Ultrasound guidance of central vein catheterization. In: Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ editor(s). Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Vol. 43, Rockville, MD: Agency for Healthcare Research and Quality, 2001:244‐52. [AHRQ Publication No. 01‐E058] [Google Scholar]
Rupp 2012
- American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, Blitt C, Caplan RA, Connis RT, Domino KB, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology 2012;116(3):539‐73. [DOI: 10.1097/ALN.0b013e31823c9569; PUBMED: 22307320] [DOI] [PubMed] [Google Scholar]
Schoenfeld 2011
- Schoenfeld E, Shokoohi H, Boniface K. Ultrasound‐guided peripheral intravenous access in the emergency department: patient‐centered survey. The Western Journal of Emergency Medicine 2011;12(4):475‐7. [DOI: 10.5811/westjem.2011.3.1920; PUBMED: 22224141] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2004
- Scott DHT. Editorial II: the king of the blind extends his frontiers. British Journal of Anaesthesia 2004;93(2):175‐7. [DOI: 10.1093/bja/aeh183] [DOI] [PubMed] [Google Scholar]
Seto 2010
- Seto AH, Abu‐Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real‐time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). Cardiovascular interventions. Journal of the American College of Cardiology Cardiovascular Intervention 2010;3(7):751‐8. [DOI: 10.1016/j.jcin.2010.04.015] [DOI] [PubMed] [Google Scholar]
Shojania 2001
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment 2001;43(i‐x):1‐668. [PUBMED: 11510252] [PMC free article] [PubMed] [Google Scholar]
Sigaut 2009
- Sigaut S, Skhiri A, Stany I, Golmar J, Nivoche Y, Constant I, et al. Ultrasound guided internal jugular vein access in children and infant: a meta‐analysis of published studies. Paediatric Anaesthesia 2009;19(12):1199‐206. [DOI: 10.1111/j.1460-9592.2009.03171.x; PMID: 19863734] [DOI] [PubMed] [Google Scholar]
Smith 1997
- Smith AF, Vallance H, Slater RM. Shorter fluid fasts reduce postoperative emesis. BMJ 1997;314:1486. [PUBMED: 9167597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009
- Smith AF. In search of excellence in anesthesiology. Anesthesiology 2009;110(1):4‐5. [DOI: 10.1097/ALN.0b013e318190b263] [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith AF, Greaves JD. Beyond competence: defining and promoting excellence in anaesthesia. Anaesthesia 2010;65:184‐91. [PUBMED: 20003114] [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith AF, Glavin R, Greaves JD. Defining excellence in anaesthesia: the role of personal qualities and practice environment. British Journal of Anaesthesia 2011;106(1):38‐43. [DOI: 10.1093/bja/aeq308; PUBMED: 21118845] [DOI] [PubMed] [Google Scholar]
Sznajder 1986
- Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Archives of Internal Medicine 1986;146(2):259‐61. [PUBMED: 3947185] [DOI] [PubMed] [Google Scholar]
Tovey 2007
- Tovey G, Stokes M. A survey of the use of 2D ultrasound guidance for insertion of central venous catheters by UK consultant paediatric anaesthetists. European Journal of Anaesthesiology 2007;24(1):71‐5. [PUBMED: 16895614] [DOI] [PubMed] [Google Scholar]
Troianos 2012
- Troianos CA, Hartman GS, Glas KE, Skubas NJ, Eberhardt RT, Walker JD, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesthesia and Analgesia 2012;114(1):46‐72. [DOI: 10.1213/ANE.0b013e3182407cd8; PUBMED: 22127816] [DOI] [PubMed] [Google Scholar]
van Miert 2012
- Miert C, Hill R, Jones L. Interventions for restoring patency of occluded central venous catheter lumens. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD007119.pub2; PUBMED: 22513946] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watters 2002
- Watters MP. Where is the harm in using ultrasound guidance?. BMJ; http://www.bmj.com/rapid‐response/2011/10/29/where‐harm‐using‐ultrasound‐guidance; accessed Nov 2013 2002.
Weiner 2012
- Weiner MM, Geldard P, Mittnacht AJ. Ultrasound‐guided vascular access: a comprehensive review. Journal of Cardiothoracic Anesthesia 2013;27(2):345‐60. [doi: 10.1053/j.jvca.2012.07.007; PUBMED: 22995457] [DOI] [PubMed] [Google Scholar]
Wu 2013
- Wu SY, Ling Q, Cao LH, Wang J, Xu MX, Zeng WA. Real‐time two‐dimensional ultrasound guidance for central venous cannulation: a meta‐analysis. Anesthesiology 2013;118(2):361‐75. [DOI: 10.1097/ALN.0b013e31827bd172; PUBMED: 23249991] [DOI] [PubMed] [Google Scholar]
Yonei 1986
- Yonei A, Nonoue T, Sari A. Real‐time ultrasonic guidance for percutaneous puncture of the internal jugular vein. Anesthesiology 1986;64(6):830‐1. [PUBMED: 3717653] [DOI] [PubMed] [Google Scholar]
Yonei 1988
- Yonei A, Yokota K, Yamashita S, Sari A. Ultrasound‐guided catheterization of the subclavian vein. Journal of Clinical Ultrasound 1988;16(7):499‐501. [PUBMED: 3152446] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brass 2008
- Brass P, Hellmich M, Kolodziej L, Kullmer B, Schick G, Schregel W. Traditional landmark versus ultrasound guidance for central vein catheterization. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006962] [DOI] [Google Scholar]



