Abstract
Background
Despite efforts to preserve the neurovascular bundles with nerve‐sparing surgery, erectile dysfunction remains common following radical prostatectomy. Postoperative penile rehabilitation seeks to restore erectile function but results have been conflicting.
Objectives
To evaluate the effects of penile rehabilitation strategies in restoring erectile function following radical prostatectomy for prostate cancer.
Search methods
We performed a comprehensive search of multiple databases (CENTRAL, MEDLINE, Embase), the Cochrane Library, Web of Science, clinical trial registries (ClinicalTrials.gov, International Clinical Trials Registry Platform) and a grey literature repository (Grey Literature Report) from their inception through to 3 January 2018. We also searched the reference lists of other relevant publications and abstract proceedings. We applied no language restrictions.
Selection criteria
We included randomised or quasi‐randomised trials with a parallel or cross‐over design.
Data collection and analysis
We used standard Cochrane methodological procedures. Two review authors independently screened the literature, extracted data, assessed risk of bias and rated quality of evidence according to GRADE on a per‐outcome basis. Primary outcomes were self‐reported potency, erectile function measured by validated questionnaires (with potency defined as an International Index of Erectile Function (IIEF‐EF) score of 19 or greater and or an IIEF‐5 of score of 17 or greater) and serious adverse events. For all quality of life assessments on a continuous scale, higher values indicated better quality of life.
Main results
We included eight randomised controlled trials with 1699 participants across three comparisons. This abstract focuses on the primary outcomes of this review only.
Scheduled phosphodiesterase type 5 inhibitors (PDE5I) versus placebo or no treatment
Scheduled PDE5I may have little or no effect on short‐term (up to 12 months) self‐reported potency (risk ratio (RR) 1.13, 95% confidence interval (CI) 0.91 to1.41; very low quality evidence), which corresponds to 47 more men with self‐reported potency per 1000 (95% CI 33 fewer to 149 more) and short‐term erectile function as assessed by a validated instrument (RR 1.11, 95% CI 0.80 to 1.55; very low quality evidence), which corresponds to 28 more men per 1000 (95% CI 50 fewer to 138 more), but we are very uncertain of both of these findings. Scheduled PDE5I may result in fewer serious adverse events compared to placebo (RR 0.32, 95% CI 0.11 to 0.94; low quality evidence), though this does not appear biologically plausible and may represent a chance finding. We are also very uncertain of this finding. We found no long‐term (longer than 12 months) data for any of the three primary outcomes.
Scheduled PDE5I versus on‐demand PDE5I
Daily PDE5I appears to result in little to no difference in both short‐term and long‐term (greater than 12 months) self‐reported potency (short term: RR 0.97, 95% CI 0.62 to 1.53; long term: RR 1.00, 95% CI 0.60 to 1.67; both very low quality evidence); this corresponds to nine fewer men with self‐reported short‐term potency per 1000 (95% CI 119 fewer to 166 more) and zero fewer men with self‐reported long‐term potency per 1000 (95% CI 153 fewer to 257 more). We are very uncertain of these findings. Daily PDE5I appears to result in little to no difference in short‐term and long‐term erectile function (short term: RR 1.00, 95% CI 0.65 to 1.55; long term; RR 0.74, 95% CI 0.48 to 1.14; both very‐low quality evidence), which corresponds to zero men with short‐term erectile dysfunction per 1000 (95% CI 80 fewer to 125 more) and 119 fewer men with long‐term erectile dysfunction per 1000 (95% CI 239 fewer to 64 more). We are very uncertain of these findings. Scheduled PDE5I may result in little or no effects on short‐term adverse events (RR 0.69 95% CI 0.12 to 4.04; very low quality evidence), which corresponds to seven fewer men with short‐term serious adverse events (95% CI 18 fewer to 64 more), but we are very uncertain of these findings. We found no long‐term data for serious adverse events.
Scheduled PDE5I versus scheduled intraurethral prostaglandin E1
At short‐term follow‐up, daily PDE5I may result in little or no effect on self‐reported potency (RR 1.10, 95% CI 0.79, to 1.52; very low quality evidence), which corresponds to 46 more men per 1000 (95% CI 97 fewer to 241 more). Daily PDE5I may result in a small improvement of erectile function (RR 1.64, 95% CI 0.84 to 3.20; very low quality evidence), which corresponds to 92 more men per 1000 (95% CI 23 fewer to 318 more) but we are very uncertain of both these findings. We found no long‐term (longer than 12 months) data for any of the three primary outcomes.
We found no evidence for any other comparisons and were unable to perform any of the preplanned subgroup analyses based on nerve‐sparing approach, age or baseline erectile function.
Authors' conclusions
Based on mostly very‐low and some low‐quality evidence, penile rehabilitation strategies consisting of scheduled PDE5I use following radical prostatectomy may not promote self‐reported potency and erectile function any more than on demand use.
Plain language summary
Penile rehabilitation for post prostatectomy erectile dysfunction
Review question
How well do treatments work to restore men's ability to have erections after surgery for prostate cancer?
Background
Many men have problems with erections after having their prostate removed for prostate cancer. Studies suggest that taking certain medicines or using devices to help with erection may help men's erections recover faster and better when used on a regular, scheduled basis (like daily or twice a week) rather than as needed. However, it is unclear how well these treatments actually work.
Study characteristics
We included eight randomised studies (clinical studies where people are randomly put into one of two or more treatment groups) with 1699 participants. Five trials compared the scheduled use of phosphodiesterase inhibitors (a type of medicine) to either no treatment or a placebo (a pretend drug with no effect). Two studies compared the use of phosphodiesterase inhibitors either as a daily prescription or as needed. One study compared the daily use of either a phosphodiesterase inhibitor or a medicine called prostaglandin E1 that is placed into the tip of the penis like a suppository. The main outcomes of this review that we felt were most important to men were how good they thought their erections were (self‐reported potency), how good their erections were based on a specialised erection questionnaire (quality of erections) and any whether there were any major unwanted side effects.
Key results
We found that the men who used these medicines on a scheduled basis may have had similar self‐reported erections and quality of erections (based on questionnaires they filled out) as men who took no medication regularly or use it as needed. They also had similar rates of serious unwanted side effects and similar rates of stopping the drug before the end of the treatment duration. However, we are very uncertain of these findings. We were unable to research whether these results would be different in different groups of men based on whether the surgeon tried to preserve the nerves that help with erections or not, based on men's age and how good their erections were beforehand because we found no studies.
Reliability of evidence
The quality of evidence was very low for most main outcomes. That means we are very uncertain of the results of this review. Further research will likely change these findings.
Summary of findings
Background
Prostate cancer is the most common non‐skin cancer in men. In 2014 in the UK alone, there were 46,700 new cases of prostate cancer diagnosed accounting for about 13% of all new cancer diagnoses. Prostate cancer in 2016 resulted in about 11,500 deaths in the UK making it the second most common cancer related cause of death in men (Cancer Research UK). In the USA, prostate cancer accounted for 172,258 new cancer diagnoses and caused 28,343 deaths in 2014 (U.S. Cancer Statistics Working Group). For organ‐confined prostate cancer (pT2), treatment options with curative intent include mainly radical prostatectomy (RP) and radiotherapy. RP can be undertaken as an open procedure typically through a retropubic approach (RRP), laparoscopic (LRP) or robotic‐assisted (RARP). Radiotherapeutic options for prostate cancer include external beam radiotherapy (EBRT) typically delivered as 2 Gy fractions over seven weeks to a total of 70 Gy with or without concomitant hormone treatment. Other therapeutic options that involve radiotherapy include intensity‐modulated radiotherapy and brachytherapy. Active surveillance of prostate cancer also falls into the category of treatments with curative intent. This treatment approach consists of an active decision not to treat the prostate cancer at the time of diagnosis but rather to monitor the man closely to enable the proper timing of curative treatment, taking into account the man's life expectancy. It is advocated by European Heidenreich 2014 and American Sanda 2017 urological guidelines in men with low‐risk organ‐confined prostate cancer.
RP has the potential to completely remove the tumour and remains a preferred and effective treatment modality utilised as a first option in approximately 33% of prostate cancer cases with organ‐confined disease and in 52% of cases in men aged over 62 years of age (Lalong‐Muh 2012; American Cancer Society 2014). In 2010 in the US alone, there were 11,290 prostatectomies, two‐thirds of which were RARP. These figures compared to the data from 2004, when there were 6188 prostatectomies, of which only 8% were RARP, suggests that RP rates have risen exponentially since the introduction of RARP (Lowrance 2012).
The common adverse effects of RP include erectile dysfunction (ED) and urinary incontinence. Many factors influence the incidence and severity of postoperative ED, including man's age, tumour stage, preoperative potency, length of surgical intervention and experience of the surgeon (Wang 2014). Despite meticulous dissection in an attempt to preserve the neurovascular bundles with nerve‐sparing surgery, ED remains common. Even with nerve‐sparing surgery, there is a period of neuropraxia during which the man has no spontaneous erections. This can lead to penile hypoxia and long‐lasting damage to the erectile tissue (Burnett 2005; Raina 2010). The length of time that neuropraxia and consequent ED will last is difficult to predict, with some studies suggesting many men require more than two years to recover erectile function satisfactorily (Rabbani 2010).
The introduction of the robot‐assisted technology has refined nerve‐sparing procedures mainly through three‐dimensional magnification and movement calibration that could result in reduced postprostatectomy ED rates. One systematic review evaluated the prevalence and the potential risk factors of ED after RARP. Their findings suggested that the prevalence of ED ranged from 54% to 90% at 12 months and 63% to 94% at 24 months (Ficarra 2012). RARP had a significant advantage over RRP with an ED prevalence of 24.2% with RARP versus 47.8% with RRP at 12 months (Ficarra 2012). However, despite these technological advances, ED is still significant in this patient population. This has led to the development of penile rehabilitation programmes that aim to promote male sexual function before and after any insult to the penile erectile physiological axis. Penile rehabilitation has now become an integral part of patient management after RP and most urologists advocate that this should be commenced as soon as possible following surgery.
Description of the condition
Male sexual dysfunction related to prostate cancer treatment can be divided into three broad categories: ED and changes in penile size; ejaculatory and orgasmic dysfunction; and psychosexual impairment with changes in sexual desire, intimacy and mental health (Chung 2013). ED is defined as the inability of a man to achieve and maintain an erection of sufficient strength for satisfactory sexual activity (NIH Consensus Conference 1993). It's incidence reported in the literature after RP varies dramatically from 20% to 90% (Fowler 1993; Rabbani 2000; Stanford 2000; Kundu 2004; Rozet 2005; Penson 2008; Alemozaffar 2011). The discrepancy in the reported rates of erectile function after RP is due to many factors. These include variations in study population demographics, methods of data acquisition, variability in questionnaire use, duration of postoperative follow‐up, variations in baseline erectile function status, inconsistency in defining adequate erectile function, surgical technique, and the definition of quality and consistency of erection (Mulhall 2009). ED can have a major impact on the man's self‐esteem, quality of life (QoL), confidence and life satisfaction, causing depression in certain cases (Kubin 2003). Quantifying accurately the prevalence of ED after RP is of utmost importance in evaluating the burden of this treatment‐related adverse effect, in order to set appropriate expectations and facilitate medical decision making. One analysis identified 24 studies that originated from major cancer centres and reported ED recovery outcomes after RP, in large participant cohorts (Mulhall 2009). In these studies, the mean overall rates of erectile function recovery were 48% (standard deviation (SD) 25%; range 12% to 96%). When nerve sparing was accounted for, as it was in 14 (58%) of the 24 articles reviewed, mean erectile function recovery rates were 50% (SD 24%) for bilateral and 34% (SD 16%) for unilateral nerve‐sparing surgery.
The starting point for analysing data on penile rehabilitation is objectively defining ED and reaching a consensus as to the definition of return to potency following RARP. Unfortunately, there remains significant heterogeneity in the literature in terms of definitions of ED after RP, and a significant number of studies do not clearly state their definitions of ED or return to sexual function. Scoring systems such as Sexual Health Inventory For Men (SHIM) scores, International Index of Erectile Function (IIEF‐5), sexual questionnaires, and patient and partner reporting are all prone to inaccuracies. Therefore, evaluating return of potency following RARP in the absence of a consensus definition was a challenge for this review. For the purposes of this study as outlined in more detail in the 'methodology' section, we included men with erectile function sufficient for intercourse. According to the IIEF‐5 and IIEF questionnaires, we defined 'sufficient for intercourse' as men with mild (IIEF‐5 greater than 17) or no (IIEF greater than 19) ED. Therefore, we defined return to sexual function as return to baseline IIEF‐5/IIEF scores.
Description of the intervention
Penile rehabilitation following RP revolves around the use of medications (alone or in combination) or devices to preserve erectile tissue health and maximise erectile function recovery or both medications and devices (Mulhall 2010). The treatment options include: phosphodiesterase‐5 inhibitors (PDE5I) (sildenafil citrate; tadalafil; vardenafil) scheduled or daily dosing; alprostadil preparations (prostaglandin E1, such as Viridal Duo or Caverject as injectables, or Medicated Urethral System for Erections (MUSE) as urethral pellets), and vacuum erection or vacuum constriction devices (VED/VCD) (Steggall 2011; Weyne 2015). These interventions have been used singly or in combination, either presurgery or following successful trial without catheter following surgery, and at different strengths, dosing frequencies and combinations, to attempt to identify the most suitable option to prevent or limit neuropraxia, recover erections and restore sexual activity.
How the intervention might work
The main pathophysiological mechanism which underlies the development of ED after RP is damage to the cavernosal nerves and vascular injury. Damage to these nerves occurs either due to their complete transection during non‐nerve‐sparing procedures or due to neuropraxia which commonly occurs during nerve‐sparing RP. Neuropraxia is defined by the transient block of nerve transmission despite an anatomically intact nerve, caused in this case by direct trauma, stretching, heating due to electrocautery, ischaemia and local inflammation (Fode 2013). Vascular injury primarily involves damage to the accessory pudendal arteries. This, together with the direct effect of loss of cavernosal nerve function results in a reduction in the oxygenation of penile tissues due to structural changes in vascular smooth muscle and endothelium. This ultimately causes loss of smooth muscle due to apoptosis (Kendirci 2006), impaired veno‐occlusive function, collagen accumulation and penile fibrosis (Hatzimouratidis 2009; Kacker 2013). Collectively these physiological changes result in ED and penile shortening.
Surgical intervention is known to induce hypoxia in a time‐dependent manner, such that the potential for recovery of erectile function decreases with time. The goal of early intervention with penile rehabilitation strategies is to improve the oxygenation of cavernosal tissue during the period of neuropraxia, to prevent uninhibited deterioration of penile tissues and to minimise (if not abrogate) the adverse structural and physiological changes that occur in the penis following RP. Penile rehabilitation also ensures that the man is well‐placed to regain presurgery erectile function and not remain dependent on erectile aids following surgery (Burnett 2013; Segal 2013). Oral PDE5I by virtue of their ease of use, are often considered as the mainstay of ED management. They are generally well‐tolerated, have proved to be relatively safe and are the preferred treatment after RP in some centres. Nevertheless, there are a number of men with postsurgery ED, who do not respond to PDE5I, or who become less responsive and less satisfied as treatment progresses. In some men, PDE5I are contraindicated by virtue of the use of nitrate medication and the risk of consequent hypotension. Apart from the oral PDE5I, the other options for management of postprostatectomy ED (including MUSE and intracavernosal injections (ICIs)) are invasive, uncomfortable, unappealing and sometimes ineffective for some men. While PDE5I may be appealing as they appear 'easy' to use, there are limited data examining whether PDE5I aid penile rehabilitation in a time‐dependent manner, which is critical as men often prefer to manage their incontinence before their erections, and if treatment is not introduced early, there is a risk of penile atrophy that will make the recovery of erections more problematic.
Why it is important to do this review
ED is a common adverse event of RP and it significantly affects QoL. The aforementioned new insights into the pathophysiology of post‐RP ED have led to the development of a multitude of different penile rehabilitation strategies which aim to improve the oxygenation of penile tissues during the period of neuropraxia that inevitably follows RP in the hope to reduce the rate of postprostatectomy ED and restore sexual activity without the use of erectogenic aids. Several randomised controlled trials (RCTs) have been published which address the question of whether these treatment modalities (alone/in combination and at different dosages or dosing schedules) are of any benefit in reducing the incidence of ED after RP and hasten the return to unassisted sexual function. Currently there is still controversy regarding the effectiveness of rehabilitation programmes. The purpose of this review is to systematically evaluate these treatment options and combinations to identify whether any of these can recover erections and restore sexual activity in addition to evaluating other important clinical outcomes such as adverse events, treatment acceptability by patients, treatment discontinuation rates and QoL. Our further aim is to compare, where evidence exists, different treatment modalities between them and determine which, if any, of these treatments may be most beneficial to restoring unassisted erectile function in men with postprostatectomy ED.
Objectives
To evaluate the effects of penile rehabilitation strategies in restoring erectile function following radical prostatectomy for prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs with a parallel or cross‐over design, and quasi‐randomised controlled trials (where participants were allocated to different arms of the trial using a method of allocation that was not truly random). Due to the nature of the review question, we did not consider cluster‐RCTs.
Types of participants
Men (aged 18 years or over), who received radical surgical intervention for clinically organ‐confined prostate cancer (cT1 or T2, N0 and M0) irrespective of disease risk status. We also considered men with T3 disease who were treated by RP alone and received no other form of adjuvant or neoadjuvant therapy. We considered all surgical approaches of RP such as RRP, radical perineal prostatectomy, laparoscopic prostatectomy and robot‐assisted laparoscopic prostatectomy, irrespective of the nerve‐sparing status. We excluded men who had received RP as a salvage procedure following failed primary therapy with another treatment modality. We also excluded men who were administered androgen deprivation therapy (ADT) or salvage RT due to biochemical recurrence following RP. We only included men who had erectile function sufficient for intercourse prior to surgery, as documented by an IIEF score. We defined these men as those who had IIEF or IIEF‐5 scores within the mild or no ED range (mild: IIEF 19 or greater; none: IIEF‐5 17 or greater). We chose these baseline IIEF scores as they included men with mild and no erectile function which we consider as having erectile function sufficient for intercourse. Men also needed to have a heterosexual partner and be sexually active. We focused on men in heterosexual relationships since it has been reported that anal intercourse requires 33% greater penile rigidity (Gebert 2014).
Types of interventions
To allow a fair and accurate comparison of efficacy of these agents in improving the recovery of erectile function, participants within experimental or placebo groups needed to, at the time of outcome assessment, be receiving no treatment for erectile function or be receiving the same treatment (e.g. the same type and dosage of PDE5I). We excluded any RCTs that did not provide this fair comparison. We included studies of psychological interventions only if these were offered in combination with pharmacological interventions, or were received by participants in both the intervention and control groups.
We planned to investigate the following experimental versus comparison interventions.
Experimental interventions
PDE5I scheduled (e.g. daily or twice per week).
Prostaglandin E1 (alprostadil) scheduled administered as ICIs.
Prostaglandin E1 (alprostadil) scheduled administered intraurethrally (MUSE and Vitaros (alprostadil topical cream)).
Scheduled use of VEDs or VCDs
Scheduled use of combination treatments (e.g. PDE5I and VEDs).
Comparator interventions
Placebo or no intervention/observation.
On demand intervention
Different types of active interventions listed under the experimental interventions above but administered on demand
Comparisons
Experimental intervention versus comparator intervention.
Types of outcome measures
We considered trials with a minimum follow‐up of six weeks.
Primary outcomes
Self‐reported potency.
Erectile function.
Serious adverse events.
Secondary outcomes
Sexual QoL.
Treatment discontinuation.
IIEF‐5 or IIEF‐EF.
Acceptability of the intervention.
Method and timing of outcome measurement
-
Self‐reported potency
Number or percentage of participants achieving self‐reported potency after RP defined as an erection firm enough and of sufficient duration to have sexual intercourse.
-
Erectile function
Number or percentage of participants achieving potency after RP according to IIEF‐EF and IIEF‐5 scores (Rosen 1997). We defined achieving potency as IIEF‐EF of 19 or greater (mild ED) and IIEF‐5 of 17 or greater (no ED).
-
Serious adverse events
Rate of participants who experienced at least one serious adverse events using an erectile aid (using the NCI Common Terminology Criteria for Adverse Events (CTCAE) reporting; grades 3 to 5) (National Cancer Institute). If the study authors of eligible studies did not use the CTCAE system, we judged the adverse events by severity using the available information described in the studies.
-
Sexual QoL
Mean change assessed with validated questionnaires such as sexual domain of Expanded Prostate Cancer Index Composite (EPIC) (Wei 2000; Szymanski 2010; Chang 2011).
-
Treatment discontinuation
Defined as treatment discontinuation from any cause at any time after participants were randomised to intervention/comparator groups.
-
International Index of Erectile Function (IIEF) or IIEF‐5
Mean change or final value, measured as EF domain of IIEF or total score of IIEF‐5 questionnaire (Rosen 1997).
-
Acceptability of the intervention
Evaluated by Treatment Acceptability Questionnaires (TAQ) (Hunsley 1992).
We considered clinically important difference for the review outcomes to rate quality of the evidence for imprecision in the 'Summary of findings' tables (Jaeschke 1989; Johnston 2013). There was no reported threshold in self‐reported potency, erectile function, serious adverse events, treatment discontinuation, and TAQ. We considered the clinically important difference for self‐reported potency, erectile function, serious adverse events, treatment discontinuation and TAQ for acceptability of the intervention as relative risk reduction of at least 25% (Guyatt 2011a). We used the minimal clinically important difference (MCID) of sexual domain of EPIC of 10 points (Skolarus 2015). We considered the MCID in the erectile function domain score of IIEF of four (Rosen 2011). We also considered IIEF‐5 of over five points as MCID (Spaliviero 2010).
We planned to assess the outcomes as short‐term and long‐term outcomes.
Up to and including 12 months postintervention (short‐term).
More than 12 months postintervention (long‐term).
'Summary of findings' tables
We presented 'Summary of findings' tables reporting the following outcomes listed according to priority.
Self‐reported potency.
Erectile function.
Serious adverse events.
Sexual QoL.
Treatment discontinuation.
Search methods for identification of studies
Electronic searches
We initially searched the following sources from inception of each database to 3 January 2018 (see Appendix 1).
-
The Cochrane Library (via Wiley; for the search strategy)
Cochrane Database of Systematic Reviews.
Cochrane Central Register of Controlled Trials.
Database of Abstracts of Reviews of Effects.
Health Technology Assessment Database.
MEDLINE (via Ovid).
Embase (via Ovid).
CINAHL.
PsycINFO.
Searching other resources
We examined the reference lists of relevant obtained articles, systematic reviews and clinical practice guidelines to check for additional related published and unpublished studies.
We searched the Conference Proceedings Citation Index (available through the Web of Science database). Additionally, we searched specific conference proceedings for the British Association of Urological Surgeons (BAUS); European Association of Urology (EAU) and American Urological Association (AUA) (from 2008 to June 2017). We selected 2008 as a cut‐off as most conference proceedings were available on international urological associations' websites from 2008 onwards.
We searched consensus papers and proceedings from specialist meetings (e.g. Sexual Function Health Council of the American Foundation for Urologic Disease).
We planned to contact experts in the field to enquire about any relevant clinical trials or journal articles that were not listed in other sources.
We attempted to contact drug manufacturers, to enquire about any relevant trials or journal articles that were not listed in other sources.
Additionally, we searched the following central registers of clinical trials on 6 June 2018 to identify any unpublished, ongoing or proposed new trials:
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch/);
Current Controlled Trials (www.controlled‐trials.com/);
UK Clinical Research Network Portfolio Database (public.ukcrn.org.uk/search/);
UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk/default.aspx);
ClinicalTrials.gov register (www.clinicaltrials.gov/);
Current Controlled Trials (ISRCTN Register) (www.controlled‐trials.com/mrct/);
ClinicalStudyResults.org (www.clinicalstudyresults.org).
Data collection and analysis
Selection of studies
We used Covidence to identify and remove potential duplicate records. Two review authors (YP, MS) independently scanned the abstract, title, or both, of remaining records retrieved, to determine which records should be assessed further. Two review authors (YP, MS) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification or ongoing studies, in accordance with the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any discrepancies through consensus or recourse to a third review author (PD). If resolution of a disagreement was not possible, we designated the study as 'awaiting classification' and we contacted study authors for clarification. We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in the Characteristics of excluded studies table. Studies were included regardless of whether outcomes were reported in a useable way. Any studies whereby intervention and comparator groups were not compared at time of study end‐point and outcome assessment in a fair manner were excluded. For study inclusion, both intervention and comparator arms had to, at the time of outcome assessment, be receiving no intervention (i.e. no treatment) or receiving the same intervention. If this was a PDE5I, the same dose and dosing schedule should have been used. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
Two review authors (YP, JJH) independently extracted data using a form based on the standardised Cochrane data extraction form. We performed a pilot test run of the data abstraction form in advance to confirm its usability.
For studies that fulfilled the inclusion criteria, two review authors (YP, JJH) independently abstracted the following information, which we provided in the Characteristics of included studies table:
study design;
study dates (or report if these were not made available);
participant details and baseline demographics;
inclusion and exclusion criteria;
number of participants by study/study arm;
details of the intervention such as timing and dosage;
definitions of outcomes, details of outcomes and how/when they were measured, as well as any relevant subgroups;
study funding sources;
declarations of interest by the investigators.
We resolved any disagreements regarding study characteristics or outcome measures by discussion, or if required, by consultation with a third review author (PD).
Assessment of risk of bias in included studies
We used the Cochrane tool for assessing risk of bias to objectively assess the included studies (Jüni 2001; Higgins 2011a). Two review authors (YP, JH) independently assessed the risk of bias of each included study. We resolved disagreements by consensus, or by consultation with a third review author (PD). We judged the risk of bias on an outcome‐specific basis as 'low risk,' high risk' or 'unclear risk' for each of the following individual items:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessors (detection bias);
incomplete outcome reporting (attrition bias);
selective outcome reporting;
other biases.
We judged risk of bias domains and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented a 'Risk of bias' summary figure to illustrate these findings (Figure 1; Figure 2).
1.
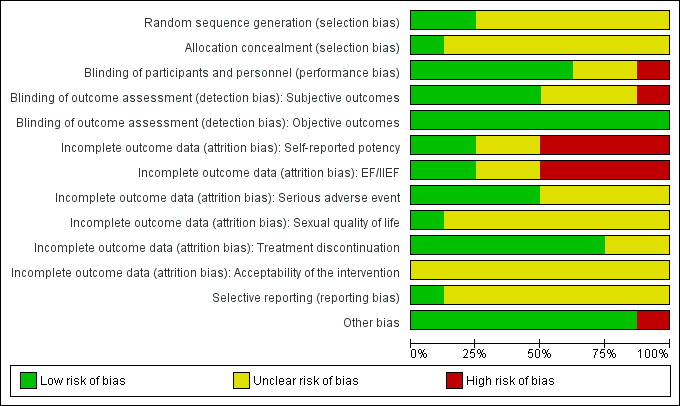
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
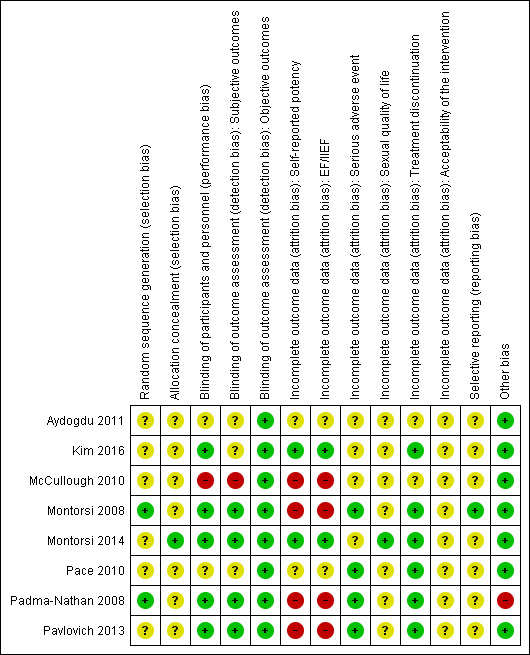
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For selection bias (random sequence generation and allocation concealment), we evaluated risk of bias at a trial level.
For performance bias (blinding of participants and personnel), we considered all outcomes to be susceptible to performance bias and assessed this individually per outcome.
For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective) outcomes.
We defined the following outcomes as subjective outcomes:
self‐reported potency;
erectile function;
serious adverse events;
sexual QoL;
IIEF;
acceptability of the intervention.
We defined the following outcomes as objective outcomes:
treatment discontinuation.
We initially assessed attrition bias (incomplete outcome data) on a per‐outcome basis but created groups of outcomes based on similar reporting characteristics.
For reporting bias (selective reporting), we evaluated risk of bias on a trial level.
We further summarised the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome, in accordance with the approach for summary assessments of the risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs unless different studies used different measures to assess the same outcome, in which case we used standardised mean differences (SMDs).
Unit of analysis issues
The unit of analysis was the individual participant. For cross‐over trials or trials with more than two intervention groups, we planned to incorporate these study designs in meta‐analyses in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We attempted to obtain missing data from study authors and performed intention‐to‐treat analyses if data were available; we otherwise performed available‐case analyses. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpreted the I2 statistic as follows:
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we attempted to determine possible reasons for this by examining individual study and subgroup characteristics. In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report study results as the pooled effect estimate in a meta‐analysis but provided a narrative description of the results of each study.
Assessment of reporting biases
If we included 10 studies or more investigating a particular outcome, we planned to use funnel plots to assess small‐study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias (Egger 1997; Sterne 2011). However, all comparisons in this review include fewer than 10 RCTs.
Data synthesis
We combined data from trials that were sufficiently similar and of sufficient quality to provide pooled effect estimates.
We summarised data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method. We used Review Manager 5 to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following variables to be potential sources of heterogeneity and, therefore, planned to perform the following subgroup analyses to determine potential qualitative or quantitative interactions of the following subgroups with the effect estimate.
Nerve‐sparing approach (none versus unilateral or bilateral, partial or complete nerve‐sparing) since it may affect the potential for recovery.
Age of participants (under 65 years versus 65 years or older); older men may have diminished recovery potential.
Baseline erectile function scores (IIEF‐5: 17 to 21 versus 22 to 25 or IIEF: 19 to 24 versus 25 to 30); men with diminished baseline erectile function may have diminished recovery potential.
Subgroup analyses of the nerve‐sparing approach are important to determine whether differences exist in effect estimate, if any, of penile rehabilitation strategies on erectile function recovery following RP between the subgroups. Age and baseline erectile function scores are important covariates that can affect the degree of erectile function recovery offered by the penile rehabilitation strategies under investigation, and, therefore, it is important to evaluate these in separate subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes.
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' of bias.
Restricting the analysis by taking into account washout effect, by excluding studies without washout at outcome assessment.
'Summary of findings' tables
We presented the overall quality of evidence (QoE) for each outcome according to the GRADE approach, which takes into account five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (directness of results) (Guyatt 2011b). Two review authors (YP, JH) independently rated the QoE for each outcome as 'high,' 'moderate,' 'low' or 'very low;' we resolved discrepancies by consensus, or, if needed, by arbitration by a third review author (PD). We presented a summary of the evidence for the main outcomes in 'Summary of findings' tables, which provide key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Schünemann 2011). This was performed in GRADEpro GDT.
Results
Description of studies
Results of the search
We identified the 3542 records through searching electronic databases, trial registries and handsearching abstract proceedings of relevant meetings since their inception. We identified three other relevant records (Cavallini 2005; Nehra 2005; Seo 2014) by searching the reference lists of CADTH guideline (CADTH 2017). After removal of 2047 duplicates, we screened the titles and abstracts of 1498 records, and excluded 1452 records as evidently irrelevant. We screened 46 studies for full‐text eligibility, and excluded 24 studies that did not meet the inclusion criteria or were not relevant to the question under trial. We included eight studies (22 records) in the review. We identified no studies as awaiting classification or part of an ongoing trial. The flow of literature through the assessment process is shown in the PRISMA flowchart (Figure 3).
3.
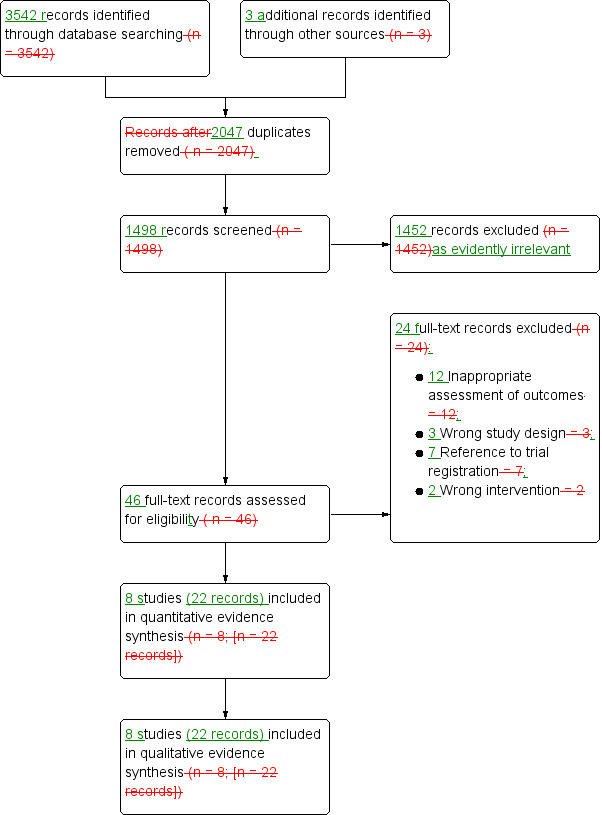
Study flow diagram.
Included studies
Details of included studies are presented elsewhere (see Characteristics of included studies; Table 6; Table 7).
1. Baseline characteristics.
| Study | Trial period | Setting/country | Description of participants | Intervention(s) and comparator(s) | Duration of intervention, washout and total follow‐upa |
Mean age (SD) |
Mean PSA (SD) |
Pathological Gleason score n (%) |
Pathological tumour stage n (%) |
| Aydogdu 2011 | 2006–2008 | Single institution/Turkey | Men aged < 65 years, preoperative full potency (IIEF‐EF scores > 25 and answered SEP questions 2–3 'yes'), no history of penile plaques or previous penile surgery, clinical stage T1c or lower, PSA < 10 ng/mL and a biopsy Gleason score < 8. No exclusion criteria reported |
Tadalafil 20 mg/day, 3 days/week | Intervention: 6 months Washout: 5 months and 10–14 days Follow‐up: 12 months |
56.2 | 6.3 | NR | NR |
| No treatment | 58.1 | 5.8 | NR | NR | |||||
| Kim 2016 | 2006–2012 | Single institution/USA | Men with localised prostate cancer who elected to go surgical treatment. These men had normal preoperative EF, defined as Sexual Health Index for Men score ≥ 21 and at > 1 erectile event with tip penile rigidity > 60% and lasting > 10 minutes in duration documented by Rigiscan. Men with known risk factors for ED and men with health conditions which are potential contraindications for PDE5I therapy were excluded from study. Men taking potent cytochrome P450 inhibitors or alpha‐adrenergic blocking agents (which could interact with sildenafil), or with known hypersensitivity to sildenafil or other ingredients of Viagra were also excluded. |
Nightly sildenafil 50 mg + 6 tablets of sildenafil 100 mg per month for on‐demand use | Intervention: 12 months Washout: 1 month Follow‐up: 13 months |
54.3 (7.1) | 5.1 (2.9) | 3+3: 35 (74.5) 3+4: 6 (12.8) 4+3: 3 (6.4) 4+4: 3 (6.4) |
T1c: 34 (72.3) T2a‐T2c: 12 (25.5) T3:1 (2.1) |
| Matched placebo + 6 tablets of sildenafil 100 mg per month for on‐demand use | 53.7 (7.1) | 4.2 (2.8) | 3+3: 39 (83.0) 3+4: 5 (10.6) 4+3: 0 (0.0) 4+4: 3 (6.4) |
T1c: 30 (63.8) T2a‐T2c: 16 (34.0) T3: 1 (2.1) |
|||||
| McCullough 2010 | NA | Multicentre/USA | Men aged < 70 years, sexually active in a stable relationship, with normal EF as determined by IIEF‐EF questionnaire (IIEF‐EF score ≥ 26) and scheduled to undergo bilateral nsRP were included. Those men with Gleason Score > 7, PSA > 20 ng/mL and postoperative RT or ADT were excluded. |
Nightly sildenafil 50 mg | Intervention: 9 months Washout: 1 month Follow‐up: 11 months |
55.6 (5.9) | NR | NR | NR |
| Nightly intraurethral alprostadil | 56.8 (6.4) | NR | NR | NR | |||||
| Montorsi 2008 | 2004–2007 | 87 centres across Europe, the US, Canada, and South Africa | Men, aged 18–64 years, in a heterosexual relationship, and scheduled to undergo bilateral NSRP within approximately 1mo of screening; an interest in resuming sexual activity as soon as possible after surgery; normal preoperative EF (IIIEF ≥ 26 at screening without the use of therapy or devices for the improvement of erections and no previous use of therapy or devices for ED; historical total PSA <10 ng/ml; Gleason tumour score ≤ 7 on biopsy; no tumour perforation of the prostate capsule were included. Men who had residual prostate cancer or requirement for RT or adjuvant therapy; need for further surgery due to haemorrhage; and urethral catheter expected to be in place for ≥ 3 weeks due to anastomotic fistula; had contraindication of PDE5I were excluded. |
Nightly vardenafil 10 mg (which could be decreased to 5 mg if required) plus on‐demand placebo | Intervention: 9 months Washout: 2 months Follow‐up: 13.5 months |
57.4 | NR | NR | NR |
| Flexible‐dose (starting at 10 mg with the option to titrate to 5 mg or 20 mg), on‐demand vardenafil plus nightly placebo | 56.8 | NR | NR | NR | |||||
| Nightly placebo, on‐demand placebo | 57.1 | NR | NR | NR | |||||
| Montorsi 2014 | 2009–2011 | Multicentre/Europe & Canada | Men aged < 68 years with normal EF who underwent nsRP for organ‐confined, non‐metastatic prostate cancer (Gleason Score ≤ 7, PSA ≤ 10 ng/mL). Postsurgical inclusion criteria included the development of ED as measured by a participant‐reported Residual Erection Function Score of ≤ 3 (= "penis is hard enough for penetration but not completely hard") were included. Men 1) with history of ED 2) who received prior PDE5I treatments 3) who received neoadjuvant RT or a ADT or were due to receive adjuvant RT or ADT, 4) with history of prostatic surgery or prostatic physical treatments, 5) with history of diabetes mellitus, 6) with history of galactose intolerance, lapp lactase deficiency, or glucose‐galactose malabsorption 7) who have clinically significant renal insufficiency were excluded. |
Tadalafil 5 mg once daily | Intervention: 9 months Washout: 6 weeks Follow‐up: 13.5 months |
58.6 (5.07) | NR | NR | NR |
| Tadalafil 20 mg on‐demand | 57.5 (5.91) | NR | NR | NR | |||||
| Placebo | 57.6 (5.69) | NR | NR | NR | |||||
| Pace 2010 | 2005–2009 | Single centre/Italy | Men with total PSA level < 10 ng/mL, Gleason score ≤ 7 on biopsy, no capsular involvement, a normal preoperative EF assessed by an IIEF score ≥ 26, without the use of any therapy for improving erection | Sildenafil 50 mg or 100 mg at night for 8 weeks | Intervention: 8 weeks Washout: 14 weeks Follow‐up: 24 months |
NR | 5.5 (range 1.2–9.9) | 6: 18 (90.0%) 7: 2 (10.0%) |
T1b and T1c: 10 (50.0%), T2a: 9 (45.0%), T2b: 1 (5.0%) |
| No treatment | NR | 6 (range 1.8–8.9) | 6: 18 (90.0%) 7: 2 (10.0%) |
T1b and T1c: 9 (45.0%); T2a: 9 (45.0%); T2b: 2 (10.0%) | |||||
| Padma‐Nathan 2008 | 1999–2001 | 16 sites in North America, France, Belgium and Australia screened participants, and 11 sites in North America and France | Men aged 18–70 years, weighing 50–125 kg who had to had normal preoperative EF (combined score ≥ 8 on questions 3 and 4 of the IIEF questionnaire) and wish to return to sexual activity after surgery and be in a stable, heterosexual relationship for the past 6 months | Sildenafil 100 mg once nightly | Intervention: 36 weeks Washout: 8 weeks Follow‐up: 48 weeks |
55 ± 6/ | NR | NR | NR |
| Sildenafil 50 mg once nightly | 55 ± 6 | NR | NR | NR | |||||
| Placebo once nightly | 57 ± 7 | NR | NR | NR | |||||
| Pavlovich 2013 | 2006– 2007 | Single institution/USA | Men who chose to undergo nsRP who satisfied the following criteria: aged < 65 years, untreated prostate cancer < cT2b, biopsy Gleason score < 8, baseline IIEF‐EF score ≥ 25/30, no PDE5I use, and presence of a steady sexual partner | Nightly sildenafil 50 mg with on‐demand placebo | Intervention: 12 months Washout: 4 weeks Follow‐up: 13 months |
54.3 (range 2–63) | 4.7 (range 0.6–14) | 6: 41 (82.0%) 7: 9 (18.0%) | T1c: 37 (74.0%), T2a: 13 (26.0%) |
| On‐demand sildenafil 50 mg (maximum 6 tablets/month) with nightly placebo | 53.6 (range 40–64) | 5.1 (range 0.8–9.0) | 6: 42 (84.0%)/7: 8 (16.0%) | T1c: 40 (80.0%); T2a: 10 (20.0%) |
ADT: androgen deprivation therapy; ED: erectile dysfunction; EF: erectile function; IIEF‐EF: International Index of Erectile Function – Erectile Function domain; n: number of participants; NA: not applicable; NR: not reported; nsRP: nerve‐sparing radical prostatectomy; PDE5I: phosphodiesterase Inhibitor 5 inhibitor; PSA: prostate‐specific antigen; RT: radiotherapy; SD: standard deviation; SEP: Sexual Encounter Profile.
aIntervention started within 1 month after surgery in all included studies except Montorsi 2008 (14 days after surgery) and Montorsi 2014 (starting date of intervention: not defined).
2. Participants disposition of included studies.
| Study | Intervention (s) and comparator (s) | Screened/eligible (n) | randomised (n) | Analysed (efficacy; n)a | Analysed (safety; n) | Finishing trial (n (%))b |
| Aydogdu 2011 | Tadalafil 20 mg/day, 3 days/week | 85/74 | NR | 32 | NR | 32 |
| No treatment | NR | 33 | NR | 33 | ||
| Total | 74 | 65 | — | 65 | ||
| Kim 2016 | Daily sildenafil 50 mg | 100/97 | 49 | 47 | 47 | 37 |
| On‐demand sildenafil 100 mg | 48 | 47 | 47 | 37 | ||
| Total | 97 | 94 | 94 | 74 | ||
| McCullough 2010 | Daily sildenafil citrate 50 mg | 227/212 | 73 | 59 | NR | 59 |
| Daily intraurethral alprostadil 125 μg (dose titration 250 μg) | 139 | 97 | NR | 97 | ||
| Total | 212 | 156 | — | 156 | ||
| Montorsi 2008 | Daily vardenafil 5–10 mg | 997/628 | 210 | 143 | 207 | 137 |
| On‐demand vardenafil 5–20 mg | 208 | 149 | 204 | 141 | ||
| Placebo | 210 | 153 | 206 | 145 | ||
| Total | 628 | 445 | 617 | 423 | ||
| Montorsi 2014 | Daily tadalafil 5 mg | 583/423 | 139 | 139 | 139 | 98 |
| On‐demand tadalafil 20 mg | 143 | 142 | 143 | 112 | ||
| Placebo | 141 | 141 | 141 | 105 | ||
| Total | 423 | 422 | 423 | 315 | ||
| Pace 2010 | Daily sildenafil 50 mg or 100 mg | NR/40 | 20 | 20 | NR | 20 |
| No treatment | 20 | 20 | NR | 20 | ||
| Total | 40 | 40 | — | 40 | ||
| Padma‐Nathan 2008 | Daily sildenafil 100 mg | 238/125 | 41 | 28 | 41 | 28 |
| Daily sildenafil 50 mg | 41 | 23 | 40 | 23 | ||
| Placebo | 43 | 25 | 42 | 25 | ||
| Total | 125 | 76 | 123 | 76 | ||
| Pavlovich 2013 | Daily sildenafil 50 mg with on‐demand placebo | 102/100 | 50 | 36 | 50 | 36 |
| On‐demand sildenafil 50 mg (maximum 6 tablets/month) with daily placebo | 50 | 38 | 50 | 38 | ||
| Total | 100 | 74 | 100 | 74 | ||
| Grand Total | 1699 | 1307 | 1357 | 1223 | ||
n: number of participants; NR: not reported.
aThe number of participants in erectile function outcome.
bThe number of participants finishing the trial at the end of the washout period or open‐label treatment.
Source of data
We included eight published studies. All studies were identified through our electronic database search and were published in English.
Study design and settings
All included studies were parallel randomised‐controlled trials. Five of seven studies were reported as "double‐blinded" (Montorsi 2008; Padma‐Nathan 2008; Pavlovich 2013; Montorsi 2014; Kim 2016). The participants and investigators were blinded in one study (Montorsi 2014) and participants and personnel were blinded in one study (Kim 2016). Montorsi 2008 blinded participant, personnel, and investigator. Two studies were reported to be "double‐blinded" but it was not clear who was blinded (Padma‐Nathan 2008; Pavlovich 2013). One study was an open label trial (McCullough 2010). The remaining trials had no information regarding blinding (Pace 2010; Aydogdu 2011). All included trials had a washout period (treatment discontinuation for all randomised participants) before the assessment of outcomes.
All studies were likely conducted in an outpatient clinic setting. Most of the included studies were performed in the US and Europe (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Pace 2010; Aydogdu 2011; Pavlovich 2013; Montorsi 2014), except one study which was performed in Asia (Kim 2016). Four trials were multicentre trials (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Montorsi 2014). The studies were performed from year 1999 to 2012.
Participants
We included 1699 randomised participants, of which 1223 participants finished the trial. The mean age was 56.7 years and mean prostate‐specific antigen (PSA) was 4.98 ng/mL. Only three studies reported pathological Gleason score and tumour stage (Pace 2010; Pavlovich 2013; Kim 2016). Pathological Gleason score ranged from six to eight. Kim 2016 included one participant with T3 disease. The remaining studies included T1 and T2 disease.
Bilateral nerve‐sparing RP was performed in most of the included studies (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Pace 2010; Aydogdu 2011; Montorsi 2014). Kim 2016 performed bilateral nerve‐sparing procedure except in one participant who underwent unilateral nerve sparing. Pavlovich 2013 used a nerve‐sparing procedure but did not describe whether it was unilateral or bilateral. RARP or LRP was performed in three of eight trials (McCullough 2010; Pavlovich 2013; Kim 2016).
Major exclusion criteria included known risk factors for ED such as diabetes mellitus and cardiovascular disease, prior treatment with experimental interventions, neoadjuvant or adjuvant treatment with other prostate cancer therapies such as radiotherapy or hormone therapy and the presence of ED at baseline. Padma‐Nathan 2008 additionally described sleep disorder as an exclusion criterion. One study did not describe exclusion criteria (Aydogdu 2011).
Interventions and comparators
Sildenafil was used in all studies except three studies (vardenafil: Montorsi 2008; tadalafil: Aydogdu 2011; Montorsi 2014). Daily sildenafil was administrated as an oral dose of 50 mg (McCullough 2010; Pavlovich 2013; Kim 2016) or 100 mg (Padma‐Nathan 2008). One study did not specify the exact dose of sildenafil (Pace 2010; both 50 mg or 100 mg). Daily vardenafil was administrated as an oral dose of 10 mg but decreased to 5 mg, if required (Montorsi 2008). Daily tadalafil was administrated as an oral dose of 5 mg (Montorsi 2014). On‐demand sildenafil was also administrated as an oral dose of 50 mg (Padma‐Nathan 2008; Pavlovich 2013) or 100 mg (Kim 2016). On‐demand vardenafil was used as a flexible‐dose (starting at 10 mg with the option to titrate to 5 mg or 20 mg) (Montorsi 2008). Tadalafil was administrated as an oral dose of 20 mg three times a week Aydogdu 2011 or on‐demand (Montorsi 2014).
Daily prostaglandin E1 was administrated intraurethrally (McCullough 2010). The drug dose was titrated (125 μg followed by 250 μg) during study period.
Placebo was used as comparators in three studies (Montorsi 2008; Padma‐Nathan 2008; Montorsi 2014). Two studies used 'no treatment' as a comparator (Pace 2010; Aydogdu 2011).
The duration of intervention ranged from eight weeks to 12 months. All interventions were administrated within one month after surgery in all included studies. The duration of washout ranged from four weeks to eight weeks in six of the included studies (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Pavlovich 2013; Montorsi 2014; Kim 2016). Aydogdu 2011 administrated the intervention for six months and assessed the outcomes at 12 months after surgery. One study administered the intervention for eight weeks and assessed the outcomes at 24 weeks after surgery (Pace 2010).
Comparisons
We included three comparisons in this review, which were informed by eight studies. Two studies were three‐armed trials and contributed to two comparisons each.
Five studies compared scheduled (daily or twice a week) PDE5I use to placebo or no treatment (Aydogdu 2011; Montorsi 2008; Montorsi 2014; Pace 2010; Padma‐Nathan 2008).
Four studies compared daily PDE5I to on‐demand PDE5I (Montorsi 2008; Pavlovich 2013; Montorsi 2014; Kim 2016), and
One study compared daily PDE5I to daily intraurethral prostaglandin E1 (McCullough 2010).
Tables 1 and 2 (Table 6; Table 7) provide further details about the specifics of the comparison.
Outcomes
We identified the primary outcomes in each of the included studies for all comparisons of PDE5I (i.e. versus placebo/no treatment, on‐demand use and intraurethral prostaglandin E1). For self‐reported potency, we used different questionnaires or definitions for outcome measurement such as Rigiscan (Kim 2016); participant‐reported intercourse success rate (McCullough 2010); Sexual Encounter Profile (SEP) question (Montorsi 2008; Aydogdu 2011; Montorsi 2014); and potency rate (not defined) (Pace 2010). Return to normal erectile function was defined as IIEF‐EF greater than 22 (Montorsi 2008; Pavlovich 2013; Montorsi 2014; Kim 2016); IIEF‐EF greater than 26 and greater than 17 (McCullough 2010); IIEF‐EF 26 or greater (Aydogdu 2011); IIEF question 3 and 4 of 8 or greater (Padma‐Nathan 2008); IIEF‐EF (not defined) (Pace 2010). Given that none of included studies reported serious adverse events using CTCAE, we used the available information described in the studies. No trial reported on our predefined secondary outcomes of acceptability of the intervention. Other secondary outcomes were reported in at least one of the included studies.
We used the outcomes that were assessed after washout period to make a fair comparison. For long‐term follow‐up, we used the sexual QoL and treatment discontinuation outcomes that were assessed after the open‐label period (with all participants taking on‐demand PDE5I for that time period).
Funding sources and conflicts of interest
Four studies were supported by pharmaceutical companies (Montorsi 2008; Padma‐Nathan 2008; Montorsi 2014; Kim 2016), and one study explicitly reported no funding source (Pavlovich 2013). Three studies did not disclose funding sources (McCullough 2010; Pace 2010; Aydogdu 2011). Four studies reported having relationships with pharmaceutical companies (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Montorsi 2014), and three studies reported no conflicts of interests (Pace 2010; Aydogdu 2011; Pavlovich 2013). One study did not disclose whether conflicts of interest were present (Kim 2016).
Excluded studies
We assessed 46 full‐text records and excluded 24 studies (see Characteristics of excluded studies table).
Studies awaiting classification
We found no studies awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
Allocation
Random sequence generation
Two studies were at low risk of bias (Montorsi 2008; Padma‐Nathan 2008). The remaining studies were at unclear risk of bias.
Allocation concealment
Only one study was at low risk of bias (Montorsi 2008), and the remaining studies were at unclear risk of bias.
Blinding
Blinding of participants and personnel
We rated five studies at low risk of bias (Montorsi 2008; Padma‐Nathan 2008; Pavlovich 2013; Montorsi 2014; Kim 2016). We judged one study at high risk of bias (McCullough 2010), and the remaining studies at unclear risk of bias.
Blinding of outcome assessment
Subjective outcomes: self‐reported potency, erectile function, serious adverse events, sexual quality of life, IIEF and acceptability of the intervention
We rated four studies at low risk of bias (Montorsi 2008; Padma‐Nathan 2008; Pavlovich 2013; Montorsi 2014). We judged one study at high risk of bias (McCullough 2010), and the remaining studies at unclear risk of bias.
Objective outcomes: treatment discontinuation
We rated all studies at low risk of bias as objective outcomes are unlikely to be affected by lack of blinding.
Incomplete outcome data
Self‐reported potency
We rated two studies at low risk of bias (Montorsi 2014; Kim 2016). We judged four studies at high risk of bias (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Pavlovich 2013), and the remaining studies at unclear risk of bias.
Erectile function/International Index of Erectile Function
We rated two studies at low risk of bias (Montorsi 2014; Kim 2016). We judged four studies at high risk of bias (Montorsi 2008; Padma‐Nathan 2008; McCullough 2010; Pavlovich 2013), and the remaining studies at unclear risk of bias.
Serious adverse events
We rated four studies at low risk of bias (Montorsi 2008; Padma‐Nathan 2008; Pace 2010; Pavlovich 2013), and the remaining studies at unclear risk of bias.
Sexual quality of life
We rated one study at low risk of bias (Montorsi 2014), and the remaining studies at unclear risk of bias.
Treatment discontinuation
We rated six studies at low risk of bias (Montorsi 2008; Padma‐Nathan 2008; Pace 2010; Pavlovich 2013; Montorsi 2014; Kim 2016), and the two remaining studies at unclear risk of bias (Aydogdu 2011McCullough 2010).
Acceptability of the intervention
None of the included studies reported acceptability of the intervention; therefore, we rated all studies at unclear risk of bias.
Selective reporting
We rated one study at low risk of bias (Montorsi 2008), and the remaining studies at unclear risk of bias.
Other potential sources of bias
We rated seven studies at low risk of bias (Montorsi 2008; McCullough 2010; Pace 2010; Aydogdu 2011; Pavlovich 2013; Montorsi 2014; Kim 2016). We judged one study at high risk of bias due to premature termination as a result of lack of efficacy of the intervention (Padma‐Nathan 2008).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Scheduled PDE5I compared to on demand placebo or no treatment for post‐prostatectomy erectile dysfunction (short‐term).
| Scheduled PDE5I compared to on demand placebo or no treatment for post‐prostatectomy erectile dysfunction (short‐term) | |||||
| Patient or population: post‐prostatectomy erectile dysfunction (short‐term) Setting: outpatient clinic Intervention: scheduled PDE5I Comparison: on demand placebo or no treatment | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with on demand placebo or no treatment | Risk difference with scheduled PDE5I | ||||
| Self‐reported potency assessed with: Sexual Encounter Profile diary question 3 or self report follow up: range 24 weeks to 46 weeks | 628 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 1.13 (0.91 to 1.41) | Study population | |
| 364 per 1,000 | 47 more per 1,000 (33 fewer to 149 more) | ||||
| Erectile function assessed with: International Index of Erectile Function‐Erectile Function domain follow up: range 24 weeks to 48 weeks | 757 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 1.11 (0.80 to 1.55) | Study population | |
| 250 per 1,000 | 28 more per 1,000 (50 fewer to 138 more) | ||||
| Serious adverse events follow up: range 24 weeks to 48 weeks | 443 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | RR 0.32 (0.11 to 0.94) | Study population | |
| 64 per 1,000 | 44 fewer per 1,000 (57 fewer to 4 fewer) | ||||
| Sexual quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Treatment discontinuation follow up: range 24 weeks to 48 weeks | 443 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 0.98 (0.72 to 1.34) | Study population | |
| 246 per 1,000 | 5 fewer per 1,000 (69 fewer to 84 more) | ||||
| International Index of Erectile Function assessed with: International Index of Erectile Function‐Erectile Function domain Scale from: 1 (worst: severe ED) to 30 (best: no ED) follow up: mean 48 weeks | 356 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 4 5 | ‐ | The mean international Index of Erectile Function ranged from 8.8 to 12.4 | MD 2.09 higher (1.85 lower to 6.03 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level for study limitations: unclear or high risk of bias in one or more domains.
2 Downgraded by one level for indirectness: difference in outcome measure.
3 Downgraded by two levels for imprecision: wide confidence interval crosses assumed threshold of clinically important difference.
4 Downgraded by one level for imprecision: confidence interval crosses assumed threshold of clinically important difference.
5 Not downgraded for inconsistency despite substantial heterogeneity given that likely not clinically meaningful.
Summary of findings 2. Scheduled PDE5I compared to on demand placebo or no treatment for post‐prostatectomy erectile dysfunction (long‐term).
| Scheduled PDE5I compared to on demand placebo or no treatment for post‐prostatectomy erectile dysfunction (long‐term) | |||||
| Patient or population: post‐prostatectomy erectile dysfunction (long‐term) Setting: outpatient clinic Intervention: scheduled PDE5I Comparison: on demand placebo or no treatment | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with on demand placebo or no treatment | Risk difference with scheduled PDE5I | ||||
| Self‐reported potency ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Erectile function ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Serious adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual quality of life assessed with: Expanded Prostate Cancer Index Composite (sexual domain) Scale from: 0 (worst) to 100 (best) follow up: mean 54 weeks | 280 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ‐ | The mean quality of life was 33.4 | MD 3.2 higher (5.91 lower to 12.31 higher) |
| Treatment discontinuation follow up: mean 54 weeks | 420 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | RR 1.12 (0.85 to 1.48) | Study population | |
| 310 per 1,000 | 37 more per 1,000 (46 fewer to 149 more) | ||||
| International Index of Erectile Function ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level for imprecision: confidence interval crosses assumed threshold of clinically important difference.
Summary of findings 3. Scheduled PDE5I compared to on demand PDE5I for post‐prostatectomy erectile dysfunction (short‐term).
| Scheduled PDE5I compared to on demand PDE5I for post‐prostatectomy erectile dysfunction (short‐term) | |||||
| Patient or population: post‐prostatectomy erectile dysfunction (short‐term) Setting: outpatient clinic Intervention: scheduled PDE5I Comparison: on demand PDE5I | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with on demand PDE5I | Risk difference with scheduled PDE5I | ||||
| Self‐reported potency assessed with: Sexual Encounter Profile diary question 3 follow up: range 42 weeks to 46 weeks | 532 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 0.97 (0.62 to 1.53) | Study population | |
| 314 per 1,000 | 9 fewer per 1,000 (119 fewer to 166 more) | ||||
| Erectile function assessed with: International Index of Erectile Function‐Erectile Function domain follow up: range 42 weeks to 46 weeks | 573 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 1.00 (0.65 to 1.55) | Study population | |
| 227 per 1,000 | 0 fewer per 1,000 (79 fewer to 125 more) | ||||
| Serious adverse events follow up: mean 42 weeks | 282 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 0.69 (0.12 to 4.04) | Study population | |
| 21 per 1,000 | 7 fewer per 1,000 (18 fewer to 64 more) | ||||
| Sexual quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Treatment discontinuation follow up: mean 42 weeks | 282 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | RR 1.35 (0.85 to 2.12) | Study population | |
| 182 per 1,000 | 64 more per 1,000 (27 fewer to 204 more) | ||||
| International Index of Erectile Function assessed with: International Index of Erectile Function‐Erectile Function domain Scale from: 1 (worst: severe ED) to 30 (best: no ED) follow up: mean 42 weeks | 281 (1 RCT) | ⊕⊕⊕⊕ HIGH | ‐ | The mean international Index of Erectile Function ranged from 2.38 | MD 0.16 higher (0.15 lower to 0.47 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level for study limitations: unclear or high risk of bias in one or more domains.
2 Not downgraded for inconsistency despite moderate or substantial heterogeneity given that likely not clinically meaningful.
3 Downgraded by two levels for imprecision: wide confidence interval crosses assumed threshold of clinically important difference.
4 Downgraded by two levels for imprecision: very rare event resulting in wide confidence interval.
Summary of findings 4. Scheduled PDE5I compared to on demand PDE5I for post‐prostatectomy erectile dysfunction (long‐term).
| Scheduled PDE5I compared to on demand PDE5I for post‐prostatectomy erectile dysfunction (long‐term) | |||||
| Patient or population: post‐prostatectomy erectile dysfunction (long‐term) Setting: outpatient clinic Intervention: scheduled PDE5I Comparison: on demand PDE5I | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with on demand PDE5I | Risk difference with scheduled PDE5I | ||||
| Self‐reported potency assessed with: Rigi scan follow up: mean 13 months | 94 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 1.00 (0.60 to 1.67) | Study population | |
| 383 per 1,000 | 0 fewer per 1,000 (153 fewer to 257 more) | ||||
| Erectile function assessed with: International Index of Erectile Function‐Erectile Function domain follow up: mean 13 months | 168 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.74 (0.48 to 1.14) | Study population | |
| 459 per 1,000 | 119 fewer per 1,000 (239 fewer to 64 more) | ||||
| Serious adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual quality of life assessed with: Expanded Prostate Cancer Index Composite (sexual domain) Scale from: 0 (worst) to 100 (best) follow up: mean 54 weeks | 281 (1 RCT) | ⊕⊕⊕⊝ MODERATE 4 | ‐ | The mean sexual quality of life was 32.6 | MD 4 higher (4.84 lower to 12.84 higher) |
| Treatment discontinuation follow up: range 52 weeks to 54 weeks | 612 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 5 | RR 1.09 (0.86 to 1.38) | Study population | |
| 295 per 1,000 | 27 more per 1,000 (41 fewer to 112 more) | ||||
| International Index of Erectile Function ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level for study limitations: unclear or high risk of bias in one or more domains.
2 Downgraded by two levels for imprecision: wide confidence interval crosses assumed threshold of clinically important difference.
3 Downgraded by two levels for imprecision: very rare event resulting in wide confidence interval.
4 Downgraded by one level for imprecision: confidence interval crosses assumed threshold of clinically important difference.
5 Downgraded by one level for indirectness: difference in intervention at the time of outcome assessment (no treatment versus on demand PDE5I).
Summary of findings 5. Scheduled PDE5I compared to scheduled intraurethral prostaglandin E1 for post‐prostatectomy erectile dysfunction (short term).
| Scheduled PDE5I compared to scheduled intraurethral prostaglandin E1 for post‐prostatectomy erectile dysfunction (short term) | |||||
| Patient or population: post‐prostatectomy erectile dysfunction (short term) Setting: outpatient clinic Intervention: scheduled PDE5I Comparison: scheduled intraurethral prostaglandin E1 | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with scheduled intraurethral prostaglandin E1 | Risk difference with scheduled PDE5I | ||||
| Self‐reported potency assessed with: Intercourse success rate follow up: mean 11 months | 156 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 1.10 (0.79 to 1.52) | Study population | |
| 464 per 1,000 | 46 more per 1,000 (97 fewer to 241 more) | ||||
| Erectile function assessed with: International Index of Erectile Function‐Erectile Function domain > 26 follow up: mean 11 months | 156 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 1.64 (0.84 to 3.20) | Study population | |
| 144 per 1,000 | 92 more per 1,000 (23 fewer to 318 more) | ||||
| Erectile function assessed with: International Index of Erectile Function‐Erectile Function domain > 17 follow up: mean 11 months | 156 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 1.20 (0.79 to 1.81) | Study population | |
| 340 per 1,000 | 68 more per 1,000 (71 fewer to 276 more) | ||||
| Serious adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sexual quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Treatment discontinuation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| International Index of Erectile Function ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level for study limitations: unclear or high risk of bias in almost all domains.
2 Downgraded by two levels for imprecision: wide confidence interval crosses assumed threshold of clinically important difference.
1. Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment
We included five studies comparing scheduled PDE5I versus placebo or no treatment short‐term (Montorsi 2008; Padma‐Nathan 2008; Pace 2010; Aydogdu 2011; Montorsi 2014). We included two studies comparing daily PDE5I versus placebo long‐term (Montorsi 2008; Montorsi 2014).
1.1. Self‐reported potency
We included four RCTs with 628 participants in the short‐term analysis (scheduled PDE5I 307, placebo or no treatment 321) (Montorsi 2008; Pace 2010; Montorsi 2014). Three studies used SEP diary question 3 (change from baseline in 'Yes' answers to questions) (Montorsi 2008; Aydogdu 2011; Montorsi 2014), and one study used self‐reported potency rate that was not further defined in the methods section of the study (Pace 2010).
Scheduled PDE5I may result in little to no difference in self‐reported potency (RR 1.13, 95% CI 0.91 to 1.41; I2 = 33%; Analysis 1.1), which corresponds to 47 more men with self‐reported potency per 1000 (95% CI 33 fewer to 149 more), but we are very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations, indirectness and imprecision.
1.1. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 1 Self‐reported potency (short term).
We found no studies that reported long‐term data for self‐reported potency.
1.2. Erectile function
We included five RCTs with 757 participants in the short‐term analysis (scheduled PDE5I 385, placebo or no treatment 372) (Montorsi 2008; Padma‐Nathan 2008; Pace 2010; Aydogdu 2011; Montorsi 2014). One study reported the proportion of participants with IIEF‐EF greater than 25 (no dysfunction) (Aydogdu 2011). Two studies reported the proportion of participants with IIEF‐EF greater than 21 (mild to no dysfunction) (Montorsi 2008; Montorsi 2014), and one study reported the proportion of participants who scored 8 or greater on Qquestion 3 and question 4 of the IIEF and also answered 'yes' to the question "Over the past 4 weeks, have your erections been good enough for satisfactory sexual activity?" after treatment (Padma‐Nathan 2008).
Scheduled PDE5I may result in little to no difference in erectile function (RR 1.11, 95% CI 0.80 to 1.55; I2 = 37%; Analysis 1.2), which corresponds to 28 more men per 1000 (95% CI 50 fewer to 138 more) but we are very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations, indirectness and imprecision.
1.2. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 2 Erectile function (short term).
We found no studies that reported long‐term data for erectile function.
1.3. Serious adverse events
We included three RCTs with 443 participants in the analysis (scheduled PDE5I 240, placebo or no treatment 203) (Padma‐Nathan 2008; Pace 2010; Montorsi 2014).
Scheduled PDE5I may result in less serious adverse events (RR 0.32, 95% CI 0.11 to 0.94; I2 = 0%; Analysis 1.3), which corresponds to 44 fewer men per 1000 (95% CI 57 fewer to 4 fewer), but we considered this to have low biological plausibility and to likely represent a chance finding. We rated the QoE as low, downgrading for study limitations and imprecision.
1.3. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 3 Serious adverse event (short term).
We found no studies that reported long‐term data for serious adverse events.
1.4. Sexual quality of life
We found no studies that reported short‐term data for sexual QoL.
We included one RCT with 280 participants in the analysis (daily PDE5I 139, placebo 141) that reported long‐term data (Montorsi 2014).
Scheduled PDE5I likely results in little to no difference in sexual QoL long term (MD 3.20 points, 95% CI –5.91 to 12.31; Analysis 1.6). We rated the QoE as moderate, downgrading for imprecision.
1.6. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 6 Sexual quality of life (long term).
1.5. Treatment discontinuation
We included three RCTs with 443 participants in the short‐term analysis (scheduled PDE5I 240, placebo or no treatment 203) (Padma‐Nathan 2008; Pace 2010; Montorsi 2014).
Scheduled PDE5I appears to result in little to no difference on treatment discontinuation (RR 0.98, 95% CI 0.72 to 1.34; I2 = 0%; Analysis 1.4),which corresponds to 5 fewer men per 1000 (95%CI 69 fewer to 84 more), but we are very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
1.4. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 4 Treatment discontinuation (short term).
We included one RCT with 420 participants in the long‐term analysis (daily PDE5I 210, placebo 210) (Montorsi 2008).
Scheduled PDE5I likely results in little to no difference in rates of treatment discontinuation (RR 1.12, 95% CI 0.85 to 1.48; Analysis 1.7), which corresponds to 37 more men per 1000 who discontinued treatment (95%CI 46 fewer to 149 more). We rated the QoE as moderate, downgrading for imprecision.
1.7. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 7 Treatment discontinuation (long term).
1.6. International Index of Erectile Function
We included two RCTs with 356 participants in the short‐term analysis (scheduled PDE5I 190, placebo or no treatment 166) (Padma‐Nathan 2008; Montorsi 2014). We used the change from baseline in Montorsi 2014 and final value in Padma‐Nathan 2008.
Scheduled PDE5I may result in little to no difference in IIEF‐EF domain score (RR 2.09, 95% CI –1.85 to 6.03; I2 = 64%; Analysis 1.5). We rated the QoE as low, downgrading for study limitations and imprecision.
1.5. Analysis.
Comparison 1 Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment, Outcome 5 International Index of Erectile Function – Erectile Function domain (IIEF‐EF) (short term).
We found no studies that reported long‐term data for IIEF.
1.7. Acceptability of the intervention
We found no studies that reported acceptability of the intervention either short‐term or long‐term.
Subgroup analysis
We were unable to perform any subgroup analyses due to no relevant data in the included studies.
Sensitivity analysis
As we rated only one study at low risk of bias (Montorsi 2014), and all included studies had a washout period (Montorsi 2008; Padma‐Nathan 2008; Montorsi 2014) or no treatment period (Pace 2010), we were unable to perform any meaningful sensitivity analyses.
2. Scheduled phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor
We included two studies comparing daily PDE5I versus on‐demand PDE5I short‐term (Montorsi 2008; Montorsi 2014).
We included four studies comparing daily PDE5I versus on‐demand PDE5I long‐term (Montorsi 2008; Pavlovich 2013; Montorsi 2014; Kim 2016).
2.1. Self‐reported potency
We included two RCTs with 532 participants in the short‐term analysis (daily PDE5I 255, on‐demand PDE5I 277) (Montorsi 2008; Montorsi 2014). We used the results of SEP diary question 3 to evaluate self‐reported potency.
Daily PDE5I may result in little to no difference in on self‐reported potency short‐term (RR 0.97, 95% CI 0.62 to 1.53; I2 = 67%; Analysis 2.1), which corresponds to nine fewer men with self‐reported short‐term potency per 1000 (95% CI 119 fewer to 166 more), but we were very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
2.1. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 1 Self‐reported potency (short term).
We included one RCT with 94 participants in the long‐term analysis (daily PDE5I 47, on‐demand PDE5I 47) (Kim 2016). While Kim 2016 reported Rigiscan as objective assessment of erectile function; we used these data as self‐reported potency.
Daily PDE5I may result in little to no difference in self‐reported potency long term (RR 1.00, 95% CI 0.60 to 1.67; Analysis 2.6), which corresponds to zero fewer men with self‐reported long‐term potency per 1000 (95% CI 153 fewer to 257 more) but we are very uncertain of this finding. We rated the quality of evidence as very low, downgrading for study limitations and imprecision.
2.6. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 6 Self‐reported potency (long term).
2.2. Erectile function
We included two RCTs with 573 participants in the short‐term analysis (daily PDE5I 282, on‐demand PDE5I 291) (Montorsi 2008; Montorsi 2014). All included studies reported the proportion of participants with IIEF‐EF greater than 21 (mild to no dysfunction) after treatment.
Daily PDE5I may result in little to no difference in short term erectile function (RR 1.00, 95% CI 0.65 to 1.55; I2 = 49%; Analysis 2.2), which corresponds to zero fewer men with short‐term erectile dysfunction per 1000 (95% CI 79 fewer to 125 more), but we were very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
2.2. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 2 Erectile function (short term).
We included two RCTs with 168 participants in the long‐term analysis (daily PDE5I 83, on‐demand PDE5I 85) (Pavlovich 2013; Kim 2016). All included studies reported the proportion of participants with IIEF‐EF greater than 21 (mild to no dysfunction).
Daily PDE5I appears to result in little to no difference in erectile function long term (RR 0.74, 95% CI 0.48 to 1.14; I2 = 0%; Analysis 2.7), which corresponds to 119 fewer men with long‐term erectile dysfunction per 1000 (95% CI 239 fewer to 64 more), but we were very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
2.7. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 7 Erectile function (long term).
2.3. Serious adverse events
We included one RCT with 282 participants in the short‐term analysis (daily PDE5I 139, on‐demand PDE5I 143) (Montorsi 2014).
Daily PDE5I appeared to result in little to no difference in serious adverse events short term (RR 0.69, 95% CI 0.12 to 4.04; Analysis 2.3), which corresponds to seven fewer men with short‐term serious adverse events (95% CI 18 fewer to 64 more) but we are very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
2.3. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 3 Serious adverse event (short term).
2.4. Sexual quality of life
We found no studies that reported short‐term outcomes for sexual QoL.
We included one RCT with 281 participants in the long‐term analysis (daily PDE5I 139, on‐demand PDE5I 142) (Montorsi 2014).
Daily PDE5I likely results in little or no difference in sexual QoL long‐term (MD 4.00 points, 95% CI –4.84 to 12.84; Analysis 2.9). We rated the QoE as moderate, downgrading for imprecision.
2.9. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 9 Sexual quality of life (long term).
2.5. Treatment discontinuation
We included one RCT with 282 participants in the short‐term analysis (daily PDE5I 139, on‐demand PDE5I 143) (Montorsi 2014).
Daily PDE5I may result in little to no difference in treatment discontinuation short‐term (RR 1.35, 95% CI 0.85 to 2.12; Analysis 2.4), which corresponds to 63 more men who discontinued treatment (95% CI 36 fewer to 203 more). We rated the QoE as low, downgrading for study limitations and imprecision.
2.4. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 4 Treatment discontinuation (short term).
We included three RCTs with 612 participants in the long‐term analysis (daily PDE5I 307, on‐demand PDE5I 305) (Pavlovich 2013; Montorsi 2014; Kim 2016).
Daily PDE5I may result in little to no difference in treatment discontinuation long‐term (RR 1.09, 95% CI 0.86 to 1.38; Analysis 2.10), which corresponds to 27 more men who discontinued treatment (95% CI 41 fewer to 112 more), but we are very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations, indirectness and imprecision.
2.10. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 10 Treatment discontinuation (long term).
2.6. International Index of Erectile Function
We included one RCT with 281 participants in the short‐term analysis (daily PDE5I 139, placebo 142) (Montorsi 2014).
Daily PDE5I results in little or no difference in IIEF‐EF domain score short‐term (MD 0.16, 95% CI –0.15 to 0.47; Analysis 2.5). We rated the QoE as high.
2.5. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 5 International Index of Erectile Function – Erectile Function domain (IIEF‐EF) (short term).
We found no studies that reported long‐term outcomes.
2.7. Acceptability of the intervention
We found no studies that reported short‐term or long‐term outcomes.
Subgroup analysis
We were unable to perform any subgroup analyses due to no relevant data in the included studies.
Sensitivity analysis
As we rated only one study at low risk of bias (Montorsi 2014), and all included studies had washout periods, we were unable to perform sensitivity analyses.
3. Daily phosphodiesterase 5 inhibitor versus daily intraurethral prostaglandin E1 (short‐term)
We included one study with 156 participants (on‐demand PDE5I 59, placebo 97) comparing daily PDE5I versus intraurethral prostaglandin E1 with short‐term follow‐up (McCullough 2010). We found no studies with long‐term follow‐up.
3.1. Self‐reported potency
McCullough 2010 reported the proportion of participants who had successful sexual intercourse short‐term.
Daily PDE5I appears to result in little to no difference in self‐reported potency (RR 1.10, 95% CI 0.79, to 1.52; Analysis 3.1), which corresponds to 46 more men per 1000 (95% CI 97 fewer to 241 more) but we were very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
3.1. Analysis.
Comparison 3 Daily phosphodiesterase 5 inhibitor (PDE5I) versus daily intraurethral prostaglandin (IUP) E1 (short term), Outcome 1 Self‐reported potency.
3.2. Erectile function
McCullough 2010 reported erectile function based on IIEF‐EF greater than 26 (no dysfunction) and IIEF‐EF greater than 17 (moderate to no dysfunction).
Daily PDE5I may result in a small improvement in erectile function (IIEF‐EF greater than 26: RR 1.64, 95% CI 0.84 to 3.20; Analysis 3.2; IIEF‐EF greater than 17: RR 1.20, 95% CI 0.79 to 1.81; Analysis 3.3), which corresponds to 92 more men per 1000 (95% CI 23 fewer to 318 more), but we are very uncertain of this finding. We rated the QoE as very low, downgrading for study limitations and imprecision.
3.2. Analysis.
Comparison 3 Daily phosphodiesterase 5 inhibitor (PDE5I) versus daily intraurethral prostaglandin (IUP) E1 (short term), Outcome 2 Erectile function (International Index of Erectile Function – Erectile Function domain (IIEF‐EF) > 26).
3.3. Analysis.
Comparison 3 Daily phosphodiesterase 5 inhibitor (PDE5I) versus daily intraurethral prostaglandin (IUP) E1 (short term), Outcome 3 Erectile function (IIEF‐EF > 17).
3.3. Serious adverse events
We found no studies that reported serious adverse events.
3.4. Sexual quality of life
We found no studies that reported sexual QoL.
3.5. Treatment discontinuation
We found no studies that reported treatment discontinuation.
3.6. International Index of Erectile Function
We found no studies that reported IIEF.
3.7. Acceptability of the intervention
We found no studies that reported acceptability of the intervention.
Subgroup analysis
We were unable to perform any subgroup analyses due to no relevant data in the included studies.
Sensitivity analysis
We were unable to perform any subgroup analyses due to paucity of included studies.
Discussion
Summary of main results
This review included eight studies with 1699 randomised participants across three comparisons. The mean participant age was 56.7 years and the preoperative PSA level was approximately 5 ng/mL.
We only included the trials with participants who received no treatment or the same treatment (e.g. the same type and dosage of PDE5I) for both intervention and control group at the time of outcome assessment to allow a fair comparison of efficacy of these agents. This is a critical methodological aspect of this review.
We found trial evidence for two comparisons that assessed scheduled medication versus placebo or no treatment or on‐demand use only. Although we were very uncertain of the result due to very low QoE, neither suggested a benefit of a rehabilitation regimen that relied on scheduled medication dosage over placebo/no treatment or on‐demand use only.
We found trial evidence for one comparison of different rehabilitation regimens, namely daily PDE5I versus daily intraurethral prostaglandin E1. Similarly, based on very low quality evidence, efficacy was comparable.
We found limited evidence on serious adverse events for the three available comparisons. We found no eligible trials for any other comparisons, such as those comparing scheduled intracavernosal injections versus on‐demand use.
We were unable to conduct any of the preplanned subgroup analyses, namely based on nerve‐sparing status, age and baseline erectile function due to lack of relevant data.
Overall completeness and applicability of evidence
Several issues impact the completeness and applicability of the summarised evidence:
Participant population
Almost all studies were conducted in western countries. Given the increasing prevalence rate of prostate cancer in Asia further studies performed in Asian countries would be valuable in validating these findings.
Although all studies included participants who had normal or mild erectile function at screening and developed ED as a result of prostatectomy, the definitions of baseline ED were different between studies.
We were unable to conduct any of the preplanned subgroup analyses, namely based on nerve‐sparing status, age and baseline erectile function.
Interventions and comparators
Included studies used different PDE5I. Given that tadalafil 20 mg has a long half‐life, we combined the results with scheduled tadalafil (twice a week; Aydogdu 2011) and daily dosing PDE5I. These differences in the interventions may have affected the review results.
There were different treatment durations and follow‐up periods among included studies that contributed clinical heterogeneity and may have affected the results of this review.
There were no trials on several comparisons of interest, for example scheduled versus on‐demand intracavernosal injections.
Outcomes
Even though the primary outcome of erectile function was determined with validated questionnaires, there was clinical and/or methodological heterogeneity among studies in how they measured and reported these outcomes. Also studies used different definitions of recovery of ED for outcome measurement.
Most included trials focused on short‐term outcomes. Given the long‐lasting impact of RP on ED, such short‐term outcomes appear insufficient to provide assurance of long‐term effectiveness.
Quality of the evidence
We downgraded the QoE to very low for nearly all primary outcomes. Issues that lowered our confidence in the estimates of the effect were study limitations, specifically selection bias (unclear allocation), performance and detection bias (lack of blinding for subjective outcomes), and other biases such as attrition bias and baseline imbalances in study characteristics.
We also downgraded for indirectness and imprecision due to differences in methods of outcome measurements (e.g. different definitions of self‐reported potency and recovery of erectile function according to IIEF/IIEF‐EF scores) and wide CIs.
Potential biases in the review process
Although we conducted this systematic review with a comprehensive search strategy consistent with current Cochrane standards, there was the possibility of bias.
Despite a comprehensive search of published and unpublished studies without language restrictions, which included contacting the principal investigators of the existing studies and experts in the field, we may have missed relevant studies. This may be because they were published in non‐indexed journals or were unpublished.
Most studies were industry‐sponsored and may have been particularly susceptible to publication bias. Given the paucity of studies we encountered, statistical tests for publication bias were not meaningful.
Agreements and disagreements with other studies or reviews
Our systematic review stands out for its high methodological standards as documented by the a priori published protocol (Philippou 2016), its comprehensive search strategy with a focus on RCTs and its use of GRADE to rate the QoE on a per‐outcome basis.
Several systematic reviews have been published on this topic. These include Tian 2017 (eight RCTs), Qiu 2016 (14 RCTs), Cui 2016 (six RCTs), Li 2014 (seven RCTs) and Wang 2014 (eight RCTs). Universally all of these studies concluded that PDE5I were safe, well‐tolerated and reported significant improvements in the IIEF‐EF scores as compared with placebo or no treatment (Tian 2017: short‐term: MD 2.26, 95% CI 1.45 to 3.08; P < 0.00001; long‐term: MD 4.5, 95% CI 3.6 to 5.4; P < 0.00001; Qiu 2016: MD 4.89, 95% CI 4.25 to 5.53; P < 0.001; Cui 2016: MD 4.04, 95% CI 2.87 to 5.22; P < 0.00001; Li 2014: MD 4.35, 95% CI 3.42 to 5.29; P < 0.00001; Wang 2014: MD 5.63, 95% CI 4.26 to 6.99; P < 0.00001 in favour of the PDE5I arm.
Our review differed in two important ways.
We focused on patient‐important outcomes and rated the QoE using GRADE on a per‐outcome basis. Therefore, this review is the first to describe the degree of certainty we can place in these results. For the most part, this confidence was very low indicating major uncertainty.
Many existing reviews choose to include all existing trials for a given comparison irrespective of whether they provided a fair comparison when it came to outcome assessment. For example, Tian 2017 included four, Qiu 2016 included nine, Cui 2016 included two, Li 2014 included three and Wang 2014 included five such studies, which we excluded on the basis of inappropriate assessment of outcomes. This probably explained the difference in our results compared to the other systematic reviews.
Despite these results from the other reviews which concluded that PDE5I were efficacious in restoring erectile function, there appears awareness of these methodological issues mentioned above by the International Society of Sexual Medicine. In the guideline document from the Fourth International Consultation for Sexual Medicine, the authors indicated that data were conflicting as to whether penile rehabilitation with PDE5Is improved recovery of spontaneous erections (Salonia 2017). Our review underscores the concern that scheduled use of PDE5I has no advantage over on demand use, but likely increase the costs of treatment.
Authors' conclusions
Implications for practice.
The evidence summarised in this systematic review does not provide support for penile rehabilitation after radical prostatectomy (RP) as a means to restore erectile function to its unassisted preoperative state compared to no treatment or placebo or on‐demand treatment alone. However, it should be noted that the evidence provided in this review includes studies that evaluated only phosphodiesterase‐5 inhibitors (PDE5I) and intraurethral prostaglandin as monotherapy from the armoury of current penile rehabilitation methods.
Implications for research.
The very low quality evidence indicates major methodological limitations of this body of evidence. These relate to standard methodological issues such as allocation concealment, blinding and completeness of follow‐up. In addition, there were concerns about inconsistency owing to clinical and methodological heterogeneity as well as imprecision, due to inadequately powered studies. Therefore, both for these comparisons and other rehabilitation strategies there is a need for better quality research focused on patient‐important outcomes. Rather than continued use in clinical practice for which this review demonstrates no apparent benefit, the focus might be placed on patient enrolment in meaningfully designed clinical trials.
Notes
We have based parts of the 'Methods' section of this protocol on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by Cochrane Urology.
Acknowledgements
We are grateful to the editorial team of the Cochrane Urology Review Group for their help and support. We are very grateful to Gareth Brown, Frank Chinegwundoh, Suks Minhas, and David Ralph for having served as peer reviewers. We thank Cochrane Urology and our contact editor Mike Risk for the support we received.
Appendices
Appendix 1. MEDLINE (via OvidSP)
1. Alprostadil/
2. "Prostaglandin E1".tw
3. alprostadil.tw
4. sildenafil.tw
5. viagra.tw
6. tadalafil.tw
7. cialis.tw
8. vardenafil.tw
9. levitra.tw
10. "penile rehabilitation".tw
11. "erect$ rehabilitation".tw
12. "vacuum therapy".tw
13. "vacuum erection device$".tw
14. VED.tw
15. "vacuum constriction device$".tw
16. VCD.tw
17. exp Phosphodiesterase 5 Inhibitors/
18. (Phosphodiesterase adj1 "5 Inhibit$").tw
19. (Phosphodiesterase adj1 "V Inhibit$").tw
20. (PDE5 OR PDE‐5 OR "PDE 5") adj1 inhibit$.tw
21. PDE5‐I.tw
22. Muse$.tw
23. ICI.tw
24. "intracavernosal injection$".tw
25. OR/1‐24
26. exp Erectile Dysfunction/
27. "erectile dysfunction".tw
28. "erectile function".tw
29. ED.tw
30. impoten$.tw
31. poten$.tw
32. ((sex or sexual$) adj3 (function$ or dysfunc$ or satisf$ or problem$ or symptom$ or arous$ or activ$ or rehabilitation OR "quality of life")).tw
33. OR/26‐32
34. exp Prostatectomy/
35. prostatectom$.tw
36. RP.tw
37. OR/34‐36
38. exp Prostatic Neoplasms/
39. "prostat$ cancer".tw
40. "prostat$ neoplasm$".tw
41. CaP.tw
42. OR/38‐41
43. 37 AND 42
44. 25 AND 33 AND 43
Appendix 2. MEDLINE via Embase
1. prostaglandin E1/
2. "Prostaglandin E1".tw
3. alprostadil.tw
4. sildenafil.tw
5. viagra.tw
6. tadalafil.tw
7. cialis.tw
8. vardenafil.tw
9. levitra.tw
10. "penile rehabilitation".tw
11. "erect$ rehabilitation".tw
12. "vacuum therapy".tw
13. "vacuum erection device$".tw
14. VED.tw
15. "vacuum constriction device$".tw
16. VCD.tw
17. exp Phosphodiesterase V Inhibitors/
18. (Phosphodiesterase adj1 "5 Inhibit$").tw
19. (Phosphodiesterase adj1 "V Inhibit$").tw
20. ((PDE5 OR PDE‐5 OR "PDE 5") adj1 inhibit$).tw
21. PDE5‐I.tw
22. Muse$.tw
23. ICI.tw
24. "intracavernosal injection$".tw
25. OR/1‐24
26. exp Erectile Dysfunction/
27. "erectile dysfunction".tw
28. "erectile function".tw
29. ED.tw
30. Impotence/
31. impoten$.tw
32. poten$.tw
33. ((sex or sexual$) adj3 (function$ or dysfunc$ or satisf$ or problem$ or symptom$ or arous$ or activ$ or rehabilitation OR "quality of life")).tw
34. OR/26‐33
35. exp Prostatectomy/
36. Prostatectom$.tw
37. RP.tw
38. OR/35‐37
39. exp prostate tumor/
40. "prostat$ cancer".tw
41. "prostat$ neoplasm$".tw
42. CaP.tw
43. OR/39‐42
44. 38 AND 43
45. 25 AND 34 AND 44
Appendix 3. The Cochrane Library via Wiley
1. MeSH descriptor: [Alprostadil] explode all trees
2. “Prostaglandin E1":ti,ab,kw
3. alprostadil:ti,ab,kw
4. sildenafil:ti,ab,kw
5. viagra:ti,ab,kw
6. tadalafil:ti,ab,kw
7. cialis:ti,ab,kw
8. vardenafil:ti,ab,kw
9. levitra:ti,ab,kw
10. "penile rehab*":ti,ab,kw
11. "erect* rehab*":ti,ab,kw
12. "vacuum therapy":ti,ab,kw
13. "vacuum erection device*":ti,ab,kw
14. VED:ti,ab,kw
15. "vacuum constriction device*":ti,ab,kw
16. VCD:ti,ab,kw
17. MeSH descriptor: [Phosphodiesterase 5 Inhibitors] explode all trees
18. (Phosphodiesterase next "5 Inhibit*"):ti,ab,kw
19. (Phosphodiesterase next "v Inhibit*"):ti,ab,kw
20. ((PDE5 or PDE‐5 or "PDE 5") next inhibit*):ti,ab,kw
21. (PDE5‐I):ti,ab,kw
22. muse*:ti,ab,kw
23. ICI:ti,ab,kw
24. "intracavernosal injection*":ti,ab,kw
25. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
26. MeSH descriptor: [Erectile Dysfunction] explode all trees
27. (erect* near/3 (dysfunction or function* or capacity or failure)):ti,ab,kw
28. ED:ti,ab,kw
29. impoten*:ti,ab,kw
30. poten*:ti,ab,kw
31. ((sex or sexual*) near/3 (function* or dysfunc* or satisf* or problem* or symptom* or arous* or activ* or rehabilitation or "quality of life")):ti,ab,kw
32. #26 or #27 or #28 or #29 or #30 or #31
33. MeSH descriptor: [Prostatectomy] explode all trees
34. Prostatectom*:ti,ab,kw
35. rp:ti,ab,kw
36. #33 or #34 or #35
37. MeSH descriptor: [Prostatic Neoplasms] explode all trees
38. "prostat* cancer":ti,ab,kw
39. "prostat* neoplasm*":ti,ab,kw
40. CaP:ti,ab,kw
41. #37 or #38 or #39 or #40
42. #36 and #41
43. #25 and #32 and #42
Data and analyses
Comparison 1. Scheduled phosphodiesterase 5 inhibitor versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐reported potency (short term) | 4 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.91, 1.41] |
| 2 Erectile function (short term) | 5 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.55] |
| 3 Serious adverse event (short term) | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.94] |
| 4 Treatment discontinuation (short term) | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.72, 1.34] |
| 5 International Index of Erectile Function – Erectile Function domain (IIEF‐EF) (short term) | 2 | 356 | Mean Difference (IV, Random, 95% CI) | 2.09 [‐1.85, 6.03] |
| 6 Sexual quality of life (long term) | 1 | 280 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐5.91, 12.31] |
| 7 Treatment discontinuation (long term) | 1 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.85, 1.48] |
Comparison 2. Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐reported potency (short term) | 2 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.62, 1.53] |
| 2 Erectile function (short term) | 2 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.65, 1.55] |
| 3 Serious adverse event (short term) | 1 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 4.04] |
| 4 Treatment discontinuation (short term) | 1 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.85, 2.12] |
| 5 International Index of Erectile Function – Erectile Function domain (IIEF‐EF) (short term) | 1 | 281 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.15, 0.47] |
| 6 Self‐reported potency (long term) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.60, 1.67] |
| 7 Erectile function (long term) | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.14] |
| 8 Serious adverse event (long term) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.92] |
| 9 Sexual quality of life (long term) | 1 | 281 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐4.84, 12.84] |
| 10 Treatment discontinuation (long term) | 3 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.38] |
2.8. Analysis.
Comparison 2 Daily phosphodiesterase 5 inhibitor versus on‐demand phosphodiesterase 5 inhibitor, Outcome 8 Serious adverse event (long term).
Comparison 3. Daily phosphodiesterase 5 inhibitor (PDE5I) versus daily intraurethral prostaglandin (IUP) E1 (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐reported potency | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.79, 1.52] |
| 2 Erectile function (International Index of Erectile Function – Erectile Function domain (IIEF‐EF) > 26) | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.84, 3.20] |
| 3 Erectile function (IIEF‐EF > 17) | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aydogdu 2011.
| Methods |
Study design: parallel RCT Setting/country: single centre, Turkey Dates when study was conducted: 2006–2008 |
|
| Participants |
Inclusion criteria: aged < 65 years, preoperative full potency (IIEF‐EF scores > 25 and answered SEP question 2–3 'yes'), no history of penile plaques or previous penile surgery, clinical stage T1c or lower, PSA < 10 ng/mL and biopsy Gleason score < 8 Exclusion criteria: NR Total number of participants randomly assigned: 74 Group A
Group B
|
|
| Interventions |
Group A: tadalafil 20 mg/day, 3 days/week Group B: no treatment Surgery or cointervention: BNSRRP Interval between surgery and intervention: 14–20 days Intervention duration: 6 months Washout period before outcome assessment: 5 months and 10–14 days Total follow‐up period: 12 months |
|
| Outcomes |
Primary outcomes
How measured: IIEF‐EF Questionnaire, SEP question 2 and question 3, penile length and circumference at both flaccid and at maximum erection Time points measured: baseline, 3, 6 and 12 months Time points reported: 3, 6 and 12 months Secondary outcomes: NR Safety outcomes: NR Subgroup: none |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | Unclear risk | 9/74 (12.1%) randomised participants not included in analysis; group allocation unclear. |
| Incomplete outcome data (attrition bias) EF/IIEF | Unclear risk | 9/74 (12.1%) randomised participants not included in analysis; group allocation unclear. |
| Incomplete outcome data (attrition bias) Serious adverse event | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes well described but protocol not available |
| Other bias | Low risk | Not detected |
Kim 2016.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Setting/country: USA Dates when study was conducted: 2006–2012 |
|
| Participants |
Inclusion criteria: IIEF ≥ 21, nsRP Exclusion criteria: known risk factors for ED, poor surgical candidates, health conditions that are potential contraindications for PDE5I therapy, prior treatment with PDE5I, and taking potent cytochrome P450 inhibitors or alpha‐adrenergic blocking agents (which could interact with sildenafil), or with known hypersensitivity to sildenafil or other ingredients of Viagra Total number of participants randomly assigned: 97 Group A
Group B
|
|
| Interventions |
Group A: nightly PDE5I (sildenafil 50 mg) Group B: placebo with 6 tablets of SC (100 mg) every 30 days for on‐demand use Surgery or cointervention: nsRP (RRP or RARP) Interval between surgery and intervention: 1 day Intervention duration: 12 months Washout period before outcome assessment: 1 month Total follow‐up period: 13 months |
|
| Outcomes |
Primary outcomes
How measured: Rigiscan device; IIEF‐EF Questionnaire Time points measured: 2 weeks, and then at 3, 6, 9 and 12 months Time points reported: 2 weeks, and then at 3, 6, 9 and 12 months Secondary outcomes: NR Safety outcomes: NR Subgroup: none |
|
| Funding sources | Pfizer Inc. | |
| Declarations of interest | None reported | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatment arm was concealed from patients and clinical personnel until all interventions and assessments were complete." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote: "Double‐blind" Judgement: not described who was blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcomes were unlikely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | Low risk | 2/49 (4.0%) participants in experimental group and 1/48 (2.0%) participant in control group were not included in analysis. |
| Incomplete outcome data (attrition bias) EF/IIEF | Low risk | 2/49 (4.0%) participants in experimental group and 1/48 (2.0%) participant in control group were not included in analysis. |
| Incomplete outcome data (attrition bias) Serious adverse event | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Low risk | All participants included in analysis |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes well described but protocol not available. |
| Other bias | Low risk | Not detected |
McCullough 2010.
| Methods |
Study design: parallel, open‐label, randomised‐controlled trial Setting/country: USA Dates when study was conducted: NR |
|
| Participants |
Inclusion criteria: men < 70 years, sexually active in a stable relationship, with normal EF as determined by the IIEF‐EF domain score (IIEF‐EF score ≥ 26 was required to be eligible for study) and scheduled to undergo BNSRP. Exclusion criteria: men with Gleason score > 7, PSA > 20 ng/mL and postoperative radiation therapy or androgen ablation Total number of participants randomly assigned: 212 Group A
Group B
|
|
| Interventions |
Group A: nightly SC 50 mg Group B: intraurethral alprostadil 125 μg daily/at 2 month after surgery dose titration 250 μg Surgery or cointervention: BNSRP Interval between surgery and intervention: 1 month Intervention duration: 8 months Washout period before outcome assessment: 1 month Total follow‐up period: 11 months |
|
| Outcomes |
Primary outcomes
How measured: EDITS; IIEF‐EF; GAQ/SPL (SPL): measured from pubic bone to coronal sulcus with a rigid ruler, adverse events as reported in study Time points measured: EDITS: 11 months; other outcomes: 1, 9 and 11 months Time points reported: EDITS: 11 months; other outcomes: 1, 9 and 11 months Secondary outcome: none reported Safety outcomes: adverse events How measured: NR Time point measured: postoperative 1, 9 and 11 months Time point reported: postoperative 1, 9 and 11 months Subgroup: none |
|
| Funding sources | Vivus, Pfizer, Med Reviews, American Medical Systems, Auxilium, Coloplast, Cook, GlaxoSmithKline/Schering Plough, Indevus, Johnson & Johnson, Medtronic, National Institute of Health, Plethora, Sanofi‐Aventis, Solvay, Theralogix, Timm Medical, Augusta Medical, Watson, Aeterna‐Zentaris, Steba‐Pharma, Serenity and USOHIFU | |
| Declarations of interest | None reported | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label study" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "open label study" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | High risk | 42/139 (30.2%) participants in experimental group and 14/73 (19.1%) participants in control group not included in the analysis. |
| Incomplete outcome data (attrition bias) EF/IIEF | High risk | 42/139 (30.2%) participants in experimental group and 14/73 (19.1%) participants in control group not included in the analysis. |
| Incomplete outcome data (attrition bias) Serious adverse event | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes well described but protocol not available. |
| Other bias | Low risk | Not detected |
Montorsi 2008.
| Methods |
Study design: randomised, double‐blind, double‐dummy, multicentre, parallel group trial Setting/country: 87 centres across Europe, US, Canada and South Africa Dates when study was conducted: December 2004 to September 2007 |
|
| Participants |
Inclusion criteria: men, aged 18–64 years, in heterosexual relationship and scheduled to undergo BNSRP surgery within approximately 1 month of screening; interest in resuming sexual activity as soon as possible after surgery; normal preoperative EF (IIEF‐EF domain score ≥ 26 at screening without use of therapy or devices for improvement of erections and no previous use of therapy or devices for ED; historical total PSA < 10 ng/mL; Gleason tumour score ≤ 7 on biopsy; no tumour perforation of the prostate capsule Exclusion criteria: men with residual prostate cancer or requirement for radiotherapy or adjuvant therapy; need for further surgery due to haemorrhage; and urethral catheter expected to be in place for ≥ 3 weeks due to anastomotic fistula; had contraindication of PDE5I. Total number of participants randomly assigned: 628 Group A
Group B
Group C
|
|
| Interventions |
Group A: daily PDE5I: 10 mg nightly vardenafil (which could be decreased to 5 mg if required) plus on‐demand placebo) Group B: on‐demand PDE5i: flexible‐dose (starting at 10 mg with the option to titrate to 5 mg or 20 mg), on‐demand vardenafil plus nightly placebo Group C: nightly placebo, on‐demand placebo Surgery or cointervention: BNSRRP Interval between surgery and intervention: 14 days Intervention duration: 9 months Washout period before outcome assessment: 2 months Total follow‐up period: 13.5 months |
|
| Outcomes |
Primary outcomes
How measured: IIEF‐EF Questionnaire Time points measured: at study endpoint after the 2‐month washout period Time points reported: at study endpoint after the 2‐month washout period Secondary outcomes
How measured: IIEF‐EF Questionnaire/SEP Questionnaire Time points measured: at the end of the DBT period and at the end of the open‐label period Time points reported: at the end of the DBT period and at the end of the open‐label period Safety outcomes How measured: adverse events Time points measured: NR Time points reported: NR Subgroup: none |
|
| Funding sources | Bayer Schering Pharma AG | |
| Declarations of interest | Professor Montorsi, Dr Brock, Dr Lee, and Professor Stief have acted as paid consultants or investigators for Bayer Schering Pharma AG. | |
| Notes |
Protocol: NCT00492635 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation codes were computer generated by Bayer Schering Pharma, Germany." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Triple (Participant, Care Provider, Investigator)" in the protocol. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Triple (Participant, Care Provider, Investigator)" in the protocol. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome not likely affected by lack of blinding, |
| Incomplete outcome data (attrition bias) Self‐reported potency | High risk | 94/210 (44.7%) participants in daily vardenafil group, 73/208 (35.0%) participants in on‐demand vardenafil group and 4/210 (39.5%) participants in placebo group were not included in the analysis. |
| Incomplete outcome data (attrition bias) EF/IIEF | High risk | 67/210 (31.9%) participants in daily vardenafil group, 59/208 (28.3%) participants in on‐demand vardenafil group and 57/210 (27.1%) participants in placebo group were not included in the analysis. |
| Incomplete outcome data (attrition bias) Serious adverse event | Low risk | 3/210 (1.4%) participants in daily vardenafil group, 4/208 (1.9%) participants in on‐demand vardenafil group and 4/210 (1.9%) participants in placebo group were not included in the analysis. |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Low risk | Protocol was published and prespecified study outcomes were analysed as planned. |
| Other bias | Low risk | Not detected |
Montorsi 2014.
| Methods |
Study design: randomised double‐blind placebo‐controlled trial Setting/country: 50 centres from 9 European countries and Canada Dates when study was conducted: November 2009 to August 2011 |
|
| Participants |
Inclusion criteria: men aged < 68 years at the time of nsRP with normal preoperative EF who underwent nsRP for organ‐confined, non‐metastatic prostate cancer (Gleason score ≤ 7, PSA < 10 ng/mL). Postsurgical inclusion criteria included the development of ED, as measured by a participant‐reported Residual Erection Function score of ≤ 3 ("penis is hard enough for penetration but not completely hard"). Exclusion criteria: men with no history of ED, who had received previous or current treatment with tadalafil or any other PDE5I; had undergone, or planned to undergo, radiation or hormonal therapy for prostate cancer; history of prostatic surgery or prostatic physical treatments; history of diabetes mellitus; history of galactose intolerance, lapp lactase deficiency or glucose‐galactose malabsorption; clinically significant renal insufficiency as determined by the investigator Total number of participants randomly assigned: 423 Group A
Group B
Group C
|
|
| Interventions |
Group A: tadalafil 5 mg once daily Group B: on‐demand tadalafil 20 mg Group C: placebo Surgery or cointervention: bilateral nerve‐sparing surgery during screening period Interval between surgery and intervention: NR Intervention duration: 9 months Washout period before outcome assessment: 6 weeks (washout) followed by 3 months' open label Total follow‐up period: 13.5 months (after 3 months' open label) |
|
| Outcomes |
Primary outcomes
How measured: proportion of men achieving an IIEF‐EF score ≥ 22 Time points measured: at end of DFW Time points reported: at end of DFW (primary outcome) Secondary outcomes
How measured: Montorsi 2014: IIEF and SEP Questionnaire: measurements were taken before administration of any sedatives or anaesthetics. Moncada 2015: defined as the time from baseline to reach an IIEF‐EF ≥ 22 during DBT IIEF‐EF scores were categorised into the following ED severity categories: severe (0–10), moderate (11–16), mild (17–25) and normal (26–30). ED severity was assessed at baseline, end of DBT, and end of DFW. Improvement was defined as an IIEF‐EF score of ≥ 1 category higher than baseline (or maintaining normal EF) at the end of DBT. Maintenance of treatment response, assessed for participants who improved ≥ 1 category after DBT, was defined as either maintaining this improved category until the end of DFW or declining after DBT but still maintaining a higher category at the end of DFW than at baseline. Time points measured: Montorsi 2014 (at 9, 10.5 and 13 months, before RP and 9 months (after DBT))/Moncada 2015 (time to event: at baseline, end of DBT and end of DFW; at the end of DBT; at baseline, DBT and DFW) Time points reported: Montorsi 2014 (at 9, 10.5 and 13 months, before RP and 9 months (after DBT))/Moncada 2015 (time to event: at baseline, end of DBT and end of DFW; at the end of DBT; at baseline, DBT and DFW) Safety outcomes How measured: adverse events Time points measured: after DBT Time points reported: after DBT Subgroup: none |
|
| Funding sources | Eli Lilly | |
| Declarations of interest | I Moncada has been a consultant for, and received speaker honoraria and travel expenses from, Eli Lilly. C Henneges, C Turbi and H Buettner are employees of Eli Lilly and Company and own Eli Lilly stock. FR de Bethencourt, E Lledó‐García, JI Martinez‐Salamanca and J Romero‐Otero have no conflicts of interest to disclose. | |
| Notes |
Protocol: NCT01026818 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "interactive voice response system and stratified by age group and country." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Matching placebo tablets identical to the 5‐mg and 20‐mg tadalafil tablets were used to ensure that the blinded regimen was identical." Judgement: "Double‐blind (Participants and investigator)" in protocol and "placebo controlled" in article. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Matching placebo tablets identical to the 5‐mg and 20‐mg tadalafil tablets were used to ensure that the blinded regimen was identical." Judgement: "Double‐blind (Participants and investigator)" in protocol and "placebo controlled" in article. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | Low risk | All participants were included in the analysis except 1 participant in tadalafil on‐demand group. |
| Incomplete outcome data (attrition bias) EF/IIEF | Low risk | All participants were included in the analysis except 1 participant in tadalafil on‐demand group. |
| Incomplete outcome data (attrition bias) Serious adverse event | Unclear risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Sexual quality of life | Low risk | All participants were included in the analysis except 1 participant in tadalafil on‐demand group. |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | While protocol was published, a few predefined outcomes in protocol were not reported in article. |
| Other bias | Low risk | Not detected |
Pace 2010.
| Methods |
Study design: RCT Setting/country: Italy Dates when study was conducted: 2005–2009 |
|
| Participants |
Inclusion criteria: men with total PSA 510 ng/mL, Gleason score ≤ 7 on biopsy, no capsular involvement, normal preoperative EF assessed by an IIEF score ≥ 26, without the use of any therapy for improving erection Exclusion criteria: men with cardiovascular diseases and previous pharmacological treatments which did not allow the contemporary use of PDE5I Total number of participants randomly assigned: 40 Group A
Group B
|
|
| Interventions |
Group A: sildenafil 50 mg or 100 mg at night Group B: no treatment Surgery or cointervention: BNSRRP Interval between surgery and intervention: 2 weeks Intervention duration: 8 weeks Washout period before outcome assessment: 14 weeks Total follow‐up period: 24 weeks |
|
| Outcomes |
Primary outcomes
How measured: NR (maybe IIEF‐EF); NR; NR; IIEF‐EF; NR Time points measured: before surgery and then at 3, 6, 12 and 24 weeks after NSRP; NR; NR; before surgery and then at 3, 6, 12 and 24 weeks after NSRP; NR Time points reported: before surgery and then at 3, 6, 12 and 24 weeks after NSRP; before and at 24 weeks; before and at 24 weeks; before and at 24 weeks; before and at 24 weeks Secondary outcomes
How measured: NR Time points measured: 8 weeks Time points reported: 8 weeks Safety outcomes How measured: adverse events Time points measured: NR Time points reported: NR Subgroup: none |
|
| Funding sources | NR | |
| Declarations of interest | NR | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | Unclear risk | Not available |
| Incomplete outcome data (attrition bias) EF/IIEF | Unclear risk | Not available |
| Incomplete outcome data (attrition bias) Serious adverse event | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes well described but protocol was not available. |
| Other bias | Low risk | Not detected |
Padma‐Nathan 2008.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Setting/country: 16 sites in North America, France, Belgium and Australia screened participants and 11 sites in North America and France Dates when study was conducted: April 1999 to October 2001 |
|
| Participants |
Inclusion criteria: men aged 18–70 years; weighing 50–125 kg with normal preoperative EF (combined score of at least 8 on question 3 and question 4 of IIEF); wish to return to sexual activity after surgery and be in a stable, heterosexual relationship for the past 6 months Exclusion criteria: pathological stage > pT2; tumour Gleason score ≥ 8 on preoperative biopsy; PSA ≥ 20 mg/L; positive lymph nodes or required postoperative radiation or androgen ablation therapy; had a sleep disorder; were taking sedative/hypnotics as sleep aids or receiving nitrates or any treatment for ED Total number of participants randomly assigned: 125 Group A
Group B
Group C
|
|
| Interventions |
Group A: sildenafil 100 mg once daily night‐time Group B: sildenafil 50 mg once daily night‐time Group C: placebo once daily night‐time Surgery or cointervention: BNSRRP by experienced surgeons Interval between surgery and intervention: 4 weeks Intervention duration: 36 weeks' double‐blind study period Washout period before outcome assessment: 8 weeks Total follow‐up period: 48 weeks |
|
| Outcomes |
Primary outcomes
How measured: stringent responder definition was established a priori as those participants who, at the end of phase 3, had a combined score of ≥ 8 for question 3 and question 4 of the IIEF, and also answered 'yes' to the question, "Over the past 4 weeks, have your erections been good enough for satisfactory sexual activity?" Time points measured: at 44 weeks of treatment (36 weeks' double blind + 8 weeks washout; 48 weeks after surgery) Time points reported: at 44 weeks of treatment (36 weeks' double blind + 8 weeks washout; 48 weeks after surgery) Secondary outcomes
How measured: IIEF‐EF Questionnaire; plethysmography Time points measured: baseline, 12, 24 and 36 weeks after treatment (double blinded; for 40 weeks after surgery), 44 weeks after treatment (additional 8 weeks' washout; 48 weeks after surgery) Time points reported: before and at 44 weeks of treatment (36 weeks double blinded+ 8 weeks washout; 48 weeks after surgery) Safety outcomes How measured: adverse events Time points measured: NR Time points reported: NR Subgroup: none |
|
| Funding sources | Pfizer Inc | |
| Declarations of interest | R Siegel was an employee of Pfizer at the time of this research. Gerald Brock: consultant, investigator for clinical research, and speakers bureau member for Pfizer Inc, Lilly‐ICOS, Bayer, GSK, Johnson & Johnson, Coloplast and AMS. Francois Giuliano: investigator for clinical and preclinical research, meeting lecturer and member of advisory board for Pfizer Inc, Bayer‐GSK, Lilly‐ICOS, Johnson & Johnson; preclinical research for Solvay Pharmaceuticals, Roche; investigator for preclinical research and meeting lecturer for Sanofi‐Aventis. Larry Levine: Consultant for Pfizer Inc, Lilly‐ICOS, Auxillium, Johnson & Johnson; investigator for clinical research for Pfizer Inc, Bayer‐GSK, Auxillium; lecturer for Pfizer Inc, Lilly‐ICOS, Schering‐Plough. Larry Lipshultz: Consultant for Pfizer Inc, Lilly‐ICOS, Auxillium, and Solvay Pharmaceuticals; investigator for clinical research for Pfizer Inc and Lilly‐ICOS. Andrew McCullough: Consultant for Pfizer Inc, Lilly‐ICOS, Auxillium, Johnson & Johnson; investigator for clinical research for Pfizer Inc, Lilly‐ICOS, Bayer‐GSK, Guilford Pharmaceuticals, Ion Channel, Johnson & Johnson and Schering Plough; advisory board and lecturer for Pfizer Inc, Lilly‐ICOS and Auxillium. Francesco Montorsi: Consultant for American Medical System, Bayer‐GSK, Johnson & Johnson, Lilly ICOS, Pfizer Inc and Takeda. Harin Padma‐Nathan: Consultant, received grant support (for this and other clinical trials) from, and participated in CME educational program for Pfizer Inc, Lilly‐ICOS, Bayer‐GSK, NexMed and Palatin Technologies. |
|
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation was in a 1:1:1 ratio using the method of random permuted blocks and a pseudo‐random number generator." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind, placebo controlled" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Double‐blind, placebo controlled" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcome are not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | High risk | 13/41 (31.7%) participants in sildenafil 100 mg group, 17/40 (42.5%) participants in sildenafil 50 mg group and 17/42 (40.4%) participants in placebo group were not included in the analysis. |
| Incomplete outcome data (attrition bias) EF/IIEF | High risk | 13/41 (31.7%) participants in sildenafil 100 mg group, 17/40 (42.5%) participants in sildenafil 50 mg group and 17/42 (40.4%) participants in placebo group were not included in the analysis. |
| Incomplete outcome data (attrition bias) Serious adverse event | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes were well described but protocol was not available. |
| Other bias | High risk | Premature termination due to lack of efficacy of intervention. Statistical method change during study. |
Pavlovich 2013.
| Methods |
Study design: randomised double‐blind, placebo‐controlled trial Setting/country: single institution in USA Dates when study was conducted: 2006–2007 |
|
| Participants |
Inclusion criteria: men choosing to undergo nsRP who satisfied the following criteria: aged < 65 years, untreated prostate cancer < cT2b, biopsy Gleason score < 8, baseline IIEF‐EF score ≥ 25/30, no PDE5I use and presence of a steady sexual partner Exclusion criteria: NR Total number of participants randomly assigned: 100 Group A
Group B
|
|
| Interventions |
Group A: nightly sildenafil 50 mg with on‐demand placebo Group B: on‐demand sildenafil 50 mg (maximum 6 tablets/month) with nightly placebo Surgery or cointervention: nerve‐sparing minimally invasive RP (either laparoscopic or RARP). Interval between surgery and intervention: 1 day Intervention duration: 1 year double‐blind study period Washout period before outcome assessment: 4 weeks Total follow‐up period: 13 months |
|
| Outcomes |
Primary outcomes
How measured: IIEF‐EF Questionnaire (not defined) Time points measured: baseline, at 1, 3, 6, 9, 12 and 13 months Time points reported: baseline, at 1, 3, 6, 9, 12 and 13 months Secondary outcomes
How measured: EPIC Questionnaire and IIEF Time points measured: baseline, at 1, 3, 6, 9, 12 and 13 months Time points reported: baseline, at 1, 3, 6, 9, 12 and 13 months Safety outcomes How measured: adverse events Time points measured: NR Time points reported: NR Subgroup: none |
|
| Funding sources | None (acknowledgement: this trial was supported with an independent investigator‐initiated grant from Pfizer Pharmaceuticals) | |
| Declarations of interest | None | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind, placebo controlled" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Double‐blind, placebo controlled" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Self‐reported potency | High risk | Short term: 16/50 (32.0%) participants in daily group and 20/50 (40.0%) participants in on‐demand group were not included in the analysis. Long term: 14/50 (28.0%) participants in daily group and 12/50 (24.0%) participants in on‐demand group were not included in the analysis. |
| Incomplete outcome data (attrition bias) EF/IIEF | High risk | Short term: 16/50 (32.0%) participants in daily group and 20/50 (40.0%) participants in on‐demand group were not included in the analysis. Long term: 14/50 (28.0%) participants in daily group and 12/50 (24.0%) participants in on‐demand group were not included in the analysis. |
| Incomplete outcome data (attrition bias) Serious adverse event | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Sexual quality of life | Unclear risk | Quote: "None differed significantly between treatment groups" Judgement: no available data |
| Incomplete outcome data (attrition bias) Treatment discontinuation | Low risk | All participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acceptability of the intervention | Unclear risk | No information given |
| Selective reporting (reporting bias) | Unclear risk | Predefined outcomes are well described but protocol was not available. |
| Other bias | Low risk | Not detected |
BNSRP: bilateral nerve‐sparing radical retropubic prostatectomy; BNSRRP: bilateral nerve‐sparing radical retropubic prostatectomy; DBT: double‐blind treatment; DFW: drug‐free washout; ED: erectile dysfunction; EDITS: Erectile Dysfunction Inventory of Treatment Satisfaction; EF: erectile function; GAQ: Global Assessment Question; IIEF: International Index of Erectile Function; IIEF‐EF: International Index of Erectile Function – Erectile Function domain; LOCF: last observation carried forward; PSA: prostate‐specific antigen; NA: not applicable; NR: not reported; nsRP: nerve‐sparing radical prostatectomy; PDE5I: phosphodiesterase 5 inhibitor; RARP: robot‐assisted radical prostatectomy; RCT: randomised controlled trial; RP: retropubic prostatectomy; RRP: radical retropubic prostatectomy; SC: sildenafil citrate; SD: standard deviation; SEP: Sexual Encounter Profile; SPL: stretched penile length.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bannowsky 2008 | Inappropriate assessment of outcome (not fair comparison at the time of outcome assessment) |
| Bannowsky 2010 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Bannowsky 2012 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Brock 2003 | Inappropriate assessment of outcome (not fair comparison at the time of outcome assessment) |
| Canat 2015 | Inappropriate assessment of outcome (not fair comparison at the time of outcome assessment) |
| Cavallini 2005 | Inappropriate assessment of outcome (not fair comparison at the time of outcome assessment) |
| Chambers 2015 | Wrong intervention |
| Engel 2011 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Fode 2014 | Wrong intervention |
| Kim 2017 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Kohler 2007 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Kosev 2013 | Wrong study design |
| McCullough 2008 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Montorsi 1997 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Montorsi 2004 | Inappropriate assessment of outcome (not fair comparison at the time of outcome assessment) |
| Mulhall 2013 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Naccarato 2016 | Inappropriate assessment of outcomes (not fair comparison at the time of outcome assessment) |
| Nehra 2005 | Inappropriate assessment of outcome (not fair comparison at the time of outcome assessment) |
| Raina 2007 | Wrong study design |
| Seo 2014 | Wrong study design (retrospective study) |
| Yassin 2010 | Incorrect reference |
Differences between protocol and review
This review was based on a published protocol with differences as described below (Philippou 2016).
We removed the comparison of on‐demand PDE5 versus placebo/no treatment as on‐demand dosing is not an established method of penile rehabilitation.
We divided time points into short‐term and long‐term instead of the time points outlined in the protocol of six, 12 and 24 months. Short‐term time points were 12 months or less and long‐term time point were greater than 12 months.
Contributions of authors
YP: drafted the protocol, undertook data collection, analysis and drafted the final review.
JHJ: undertook data collection, analysis and drafted the final review.
MS: conceptualised the review topic and drafted the protocol.
SO: developed and ran the search strategy.
CB: developed and ran the search strategy.
JB: undertook data collection,
PD: conceptualised the review topic, drafted the protocol and provided oversight.
Sources of support
Internal sources
-
Minneapolis VAMC and UMN Department of Urology, USA.
Protected research time
External sources
No sources of support supplied
Declarations of interest
YP: none.
JHJ: none.
MS: none.
SO: none.
CB: none.
JB: none
PD: none.
New
References
References to studies included in this review
Aydogdu 2011 {published data only}
- Aydogdu O, Gokce MI, Burgu B, Baltacı S, Yaman O. Tadalafil rehabilitation therapy preserves penile size after bilateral nerve sparing radical retropubic prostatectomy. International Brazilian Journal of Urology 2011;37(3):336‐44. [DOI] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Hawksworth DJ, Kim DJ, Travis JA, Cullen J, Hurwitz L, Rosner IL, et al. Evaluation of nocturnal tumescence and its response to nightly sildenafil citrate during acute recovery following radical prostatectomy: a randomized, double blind, placebo‐controlled study. Journal of Sexual Medicine 2014;11:189‐90. [Google Scholar]
- Kim DJ, Hawksworth DJ, Hurwitz LM, Cullen J, Rosner IL, Lue TF, et al. A prospective, randomized, placebo‐controlled trial of on‐demand vs. nightly sildenafil citrate as assessed by Rigiscan and the International Index of Erectile Function. Andrology 2016;4(1):27‐32. [DOI] [PubMed] [Google Scholar]
McCullough 2010 {published data only}
- McCullough AR, Hellstrom WG, Wang R, Lepor H, Wagner KR, Engel JD. Recovery of erectile function after nerve sparing radical prostatectomy and penile rehabilitation with nightly intraurethral alprostadil versus sildenafil citrate. Journal of Urology 2010;183(6):2451‐6. [DOI] [PubMed] [Google Scholar]
Montorsi 2008 {published data only}
- Montorsi F, Brock G, Lee J, Shapiro J, Poppel H, Graefen M, et al. Effect of nightly versus on‐demand vardenafil on recovery of erectile function in men following bilateral nerve‐sparing radical prostatectomy. European Urology 2008;54(4):924‐31. [DOI] [PubMed] [Google Scholar]
Montorsi 2014 {published data only}
- Brock G, Montorsi F, Costa P, Shah N, Jabaloyas JM, Hammerer P, et al. Tadalafil once a day significantly reduces penile length‐loss in patients post bilateral nerve‐sparing radical prostatectomy (NSRP) – results from a randomized controlled trial. Journal of Urology 2014;191(4 Suppl):e530‐1. [Google Scholar]
- Brock G, Montorsi F, Costa P, Shah N, Martinez‐Jabaloyas JM, Hammerer P, et al. Effect of tadalafil once daily on penile length loss and morning erections in patients after bilateral nerve‐sparing radical prostatectomy: results from a randomized controlled trial. Urology 2015;85(5):1090‐6. [DOI] [PubMed] [Google Scholar]
- Moncada I, Bethencourt F, Turbi C, Buettner H, Henneges C, Salamanca J. Effects of tadalafil once daily (OaD) or on‐demand (PRN) versus placebo on time to recovery of erectile function (EF) in patients post bilateral nerve‐sparing radical prostatectomy (NSRP). Journal of Sexual Medicine 2014;11:19‐20. [Google Scholar]
- Moncada I, Bethencourt FR, Turbi C, Buttner H, Henneges C, Martinez Salamanca JI. Effects of tadalafil once daily (OaD) or on‐demand (PRN) versus placebo on time to recovery of erectile function (EF) in patients post bilateral nerve‐sparing radical prostatectomy (nsRP). European Urology, Supplements 2014;13(1):e598‐a. [Google Scholar]
- Moncada I, Bethencourt FR, Lledó‐García E, Romero‐Otero J, Turbi C, Büttner H, et al. Effects of tadalafil once daily or on demand versus placebo on time to recovery of erectile function in patients after bilateral nerve‐sparing radical prostatectomy. World Journal of Urology 2015;33(7):1031‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montorsi F, Brock G, Stolzenburg JU, Mulhall J, Moncada I, Patel HR, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve‐sparing radical prostatectomy: a randomised placebo‐controlled study (REACTT). European Urology 2014;65(3):587‐96. [DOI] [PubMed] [Google Scholar]
- Mulhall JP, Brock G, Oelke M, Fode M, Probst KA, Henneges C, et al. Effects of tadalafil once‐daily or on‐demand vs placebo on return to baseline erectile function after bilateral nerve‐sparing radical prostatectomy – results from a randomized controlled trial (REACTT). Journal of Sexual Medicine 2016;13(4):679‐83. [DOI] [PubMed] [Google Scholar]
- Patel H, Shah N, Lundmark J, Cooper‐Jones J, Buttner H, Henneges C, et al. Effects of tadalafil treatment post bilateral nerve‐sparing radical prostatectomy: quality of life, psychosocial outcomes and treatment satisfaction. Urology 2014;84(4 Suppl):s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HR, Ilo D, Shah N, Cuzin B, Chadwick D, Andrianne R, et al. Effects of tadalafil treatment after bilateral nerve‐sparing radical prostatectomy: quality of life, psychosocial outcomes, and treatment satisfaction results from a randomized, placebo‐controlled phase IV study. BMC Urology 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HR, Shah N, Lundmark J, Cooper Jones J, Buttner H, Henneges C, et al. Effects of tadalafil treatment post bilateral nerve‐sparing radical prostatectomy (NSRP): quality of life (QOL), psychosocial outcomes and treatment satisfaction. Journal of Urology 2014;191(Suppl 4):e532. [Google Scholar]
- Patel HR, Shah N, Lundmark J, Cooper Jones J, Büttner H, Henneges C, et al. Effects of tadalafil treatment postbilateral nerve‐sparing radical prostatectomy (NSRP): quality of life (QOL), psychosocial outcomes and treatment satisfaction. Journal of Sexual Medicine 2014;11(Suppl 1):19. [Google Scholar]
- Schostak M, Graefen M, Kriegel C, Michl U, Morales AM, Pommerville PJ, et al. Impact of surgical approach on erectile function recovery following bilateral nerve sparing‐radical prostatectomy: results from a randomized controlled trial of tadalafil versus placebo (REACTT). Journal of Urology 2015;193(4 Suppl 1):e510‐1. [Google Scholar]
- Stolzenburg JU, Graefen M, Kriegel C, Michl U, Martin Morales A, Pommerville PJ, et al. Effect of surgical approach on erectile function recovery following bilateral nerve‐sparing radical prostatectomy: an evaluation utilising data from a randomised, double‐blind, double‐dummy multicentre trial of tadalafil vs placebo. BJU International 2015;116(2):241‐51. [DOI] [PubMed] [Google Scholar]
- Stolzenburg JU, Graefen M, Kriegel C, Michl U, Martin Morales A, Pommerville PJ, et al. Impact of surgical approach on recovery of erectile function following bilateral nerve‐sparing radical prostatectomy: results from a randomized controlled trial of tadalafil versus placebo (REACTT). European Urology, Supplements 2015;14(2):e622‐a. [Google Scholar]
Pace 2010 {published data only}
- Pace G, Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disability and Rehabilitation 2010;32(14):1204‐8. [DOI] [PubMed] [Google Scholar]
Padma‐Nathan 2008 {published data only}
- Padma‐Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, Montorsi F, et al. Randomized, double‐blind, placebo‐controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve‐sparing radical prostatectomy. International Journal of Impotence Research 2008;20(5):479‐86. [DOI] [PubMed] [Google Scholar]
Pavlovich 2013 {published data only}
- Pavlovich CP, Levinson AW, Su LM, Mettee LZ, Feng Z, Bivalacqua TJ, et al. Nightly vs on‐demand sildenafil for penile rehabilitation after minimally invasive nerve‐sparing radical prostatectomy: results of a randomized double‐blind trial with placebo. BJU International 2013;112(6):844‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bannowsky 2008 {published data only}
- Bannowsky A, Schulze H, Horst C, Hautmann S, Junemann KP. Recovery of erectile function after nerve‐sparing radical prostatectomy: improvement with nightly low‐dose sildenafil. BJU International 2008;101(10):1279‐83. [DOI] [PubMed] [Google Scholar]
Bannowsky 2010 {published data only}
- Bannowsky A, Schulze H, Junemann KP. Rehabilitative therapy for erectile function after nerve‐sparing radical prostatectomy. Journal of Men's Health December 2010;7(4):390‐395. [Google Scholar]
- Bannowsky A, Schulze H, Uckert S, Junemann KP. Rehabilitation of erectile function two years after nerve‐sparing radical prostatectomy: Is there a real significant effect with nightly low‐dose sildenafil (25 mg)?. [German]. Journal fur Urologie und Urogynakologie 2014;21(3):16‐21. [Google Scholar]
- Bannowsky A, Schulze H, Horst C, Junemann KP. Nerve‐sparing radical prostatectomy with nightly low‐dose sildenafil. Rehabilitation of erectile function. Urologe 2010;49(12):1516‐21. [DOI] [PubMed] [Google Scholar]
Bannowsky 2012 {published data only}
- Bannowsky A, Ahlen H, Loch, T. Increasing the dose of vardenafil on a daily basis does not improve erectile function after unilateral nerve‐sparing radical prostatectomy. Journal of Sexual Medicine 2012;9(5):1448‐53. [DOI] [PubMed] [Google Scholar]
Brock 2003 {published data only}
- Brock G, Nehra A, Lipshultz LI, Karlin GS, Gleave M, Seger M, et al. Safety and efficacy of vardenafil for the treatment of men with erectile dysfunction after radical retropubic prostatectomy. Journal of Urology 2003;170(4 Pt 1):1278‐83. [DOI] [PubMed] [Google Scholar]
Canat 2015 {published data only}
- Canat L, Guner B, Gurbuz C, Atis G, Caskurlu T. Effects of three‐times‐per‐week versus on‐demand tadalafil treatment on erectile function and continence recovery following bilateral nerve sparing radical prostatectomy: results of a prospective, randomized, and single‐center study. Kaohsiung Journal of Medical Sciences 2015;31(2):90‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavallini 2005 {published data only}
- Cavallini G, Modenini F, Vitali G, Koverech A. Acetyl‐L‐carnitine plus propionyl‐L‐carnitine improve efficacy of sildenafil in treatment of erectile dysfunction after bilateral nerve‐sparing radical retropubic prostatectomy. Urology 2005;66(5):1080‐5. [DOI] [PubMed] [Google Scholar]
Chambers 2015 {published data only}
- Chambers SK, Occhipinti S, Schover L, Nielsen L, Zajdlewicz L, Clutton S, et al. A randomised controlled trial of a couples‐based sexuality intervention for men with localised prostate cancer and their female partners. Psycho‐Oncology 2015;24(7):748‐56. [DOI] [PubMed] [Google Scholar]
Engel 2011 {published data only}
- Engel JD. Effect on sexual function of a vacuum erection device post‐prostatectomy. Canadian Journal of Urology 2011;18(3):5721‐5. [PubMed] [Google Scholar]
Fode 2014 {published data only}
- Fode M, Borre M, Ohl DA, Lichtbach J, Sonksen J. Penile vibratory stimulation in the recovery of urinary continence and erectile function after nerve‐sparing radical prostatectomy: a randomized, controlled trial. BJU International 2014;114(1):111‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim KS, Kim TH, Noh JH, Bae JH, Oh CY, Cho JS, et al. Comparison of efficacy and safety for erectile dysfunction of mirodenalfil 50mg once daily and 100mg on‐demand in patients with radical prostatectomy: multicenter, randomized trial. Journal of Urology 2017;197(4 Suppl 1):e1345. [Google Scholar]
Kohler 2007 {published data only}
- Kohler TS, Pedro R, Hendlin K, Utz W, Ugarte R, Reddy P, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU International 2007;100(4):858‐62. [DOI] [PubMed] [Google Scholar]
Kosev 2013 {published data only}
- Kosev P, Anakievski D, Chankov P, Hinev A. Sexual rehabilitation after radical prostatectomy. European Urology, Supplements 2013;12(4):e1117. [Google Scholar]
McCullough 2008 {published data only}
- McCullough AR, LevineLA, Padma‐Nathan H. Return of nocturnal erections and erectile function after bilateral nerve‐sparing radical prostatectomy in men treated nightly with sildenafil citrate: subanalysis of a longitudinal randomized double‐blind placebo‐controlled trial. Journal of Sexual Medicine Feb 2008;5(2):476‐84. [DOI] [PubMed] [Google Scholar]
Montorsi 1997 {published data only}
- Montorsi F, Guazzoni G, Strambi LF, Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve‐sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. Journal of Urology 1997;158(4):1408‐10. [PubMed] [Google Scholar]
Montorsi 2004 {published data only}
- Montorsi F, Nathan HP, McCullough A, Brock GB, Broderick G, Ahuja S, et al. Tadalafil in the treatment of erectile dysfunction following bilateral nerve sparing radical retropubic prostatectomy: a randomized, double‐blind, placebo controlled trial. Journal of Urology 2004;172(3):1036‐41. [DOI] [PubMed] [Google Scholar]
Mulhall 2013 {published data only}
- Mulhall JP, Burnett AL, Wang R, McVary KT, Moul JW, Bowden CH, et al. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. Journal of Urology 2013;189(6):2229‐36. [DOI] [PubMed] [Google Scholar]
- Mulhall JP, Moul JW, Wang R, Shin D, Engel JD, Day WW, et al. A randomized, double‐blind, placebo controlled, parallel group, multicenter study of the safety and efficacy of avanafil in the treatment of erectile dysfunction following bilateral, nerve‐sparing radical prostatectomy. Journal of Sexual Medicine 2013;10:147. [Google Scholar]
Naccarato 2016 {published data only}
- Naccarato AM, Reis LO, Ferreira U, Denardi F. Psychotherapy and phosphodiesterase‐5 inhibitor in early rehabilitation after radical prostatectomy: a prospective randomised controlled trial. Andrologia 2016;48(10):1183‐7. [DOI] [PubMed] [Google Scholar]
Nehra 2005 {published data only}
- Nehra A, Grantmyre J, Nadel A, Thibonnier M, Brock G. Vardenafil improved patient satisfaction with erectile hardness, orgasmic function and sexual experience in men with erectile dysfunction following nerve sparing radical prostatectomy. Journal of Urology 2005;173(6):2067‐71. [DOI] [PubMed] [Google Scholar]
Raina 2007 {published data only}
- Raina R, Pahlajani G, Agarwal A, Zippe CD. The early use of transurethral alprostadil after radical prostatectomy potentially facilitates an earlier return of erectile function and successful sexual activity. BJU International 2007;100(6):1317‐21. [DOI] [PubMed] [Google Scholar]
Seo 2014 {published data only}
- Seo YE, Kim SD, Kim TH, Sung GT. The efficacy and safety of tadalafil 5 mg once daily in the treatment of erectile dysfunction after robot‐assisted laparoscopic radical prostatectomy: 1‐year follow‐up. Korean Journal of Urology 2014;55(2):112‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yassin 2010 {published data only}
- Yassin AA, Haider A, Bernhard Mohr. Combination of vacuum device and PDE‐5 inhibitors for penis rehabilitation in patients after nerve‐sparing radical prostatectomy. A randomised prospective study. Sexologies 2010;19:259. [Google Scholar]
Additional references
Alemozaffar 2011
- Alemozaffar M, Regan MM, Cooperberg MR, Wei JT, Michalski JM, Sandler HM, et al. Prediction of erectile function following treatment for prostate cancer. JAMA 2011;306(11):1205‐14. [PUBMED: 21934053] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Cancer Society 2014
- American Cancer Society. Cancer treatment and survivorship facts and figures 2014‐2015. www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/cancer‐treatment‐and‐survivorship‐facts‐and‐figures/cancer‐treatment‐and‐survivorship‐facts‐and‐figures‐2014‐2015.pdf (accessed prior to 26 August 2018).
Burnett 2005
- Burnett AL. Erectile dysfunction following radical prostatectomy. JAMA 2005;293(21):2648‐53. [DOI] [PubMed] [Google Scholar]
Burnett 2013
- Burnett AL. Current rehabilitation strategy: clinical evidence for erection recovery after radical prostatectomy. Translational Andrology and Urology 2013;2(1):24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
CADTH 2017
- CADTH. Phosphodiesterase type 5 inhibitors for penile rehabilitation post radical prostatectomy: a review of clinical effectiveness and guidelines. www.cadth.ca/phosphodiesterase‐type‐5‐inhibitors‐penile‐rehabilitation‐post‐radical‐prostatectomy‐review‐clinical (accessed 7 November 2017). [PubMed]
Cancer Research UK
- Cancer Research UK. Prostate cancer statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/prostate‐cancer#heading‐One (accessed 8 December 2017).
Chang 2011
- Chang P, Szymanski KM, Dunn RL, Chipman JJ, Litwin MS, Nguyen PL, et al. Expanded Prostate cancer Index Composite for clinical practice: development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. Journal of Urology 2011;186(3):865‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2013
- Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of the art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. Journal of Sexual Medicine 2013;10(Suppl 1):102‐11. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2013.
Cui 2016
- Cui Y, Liu X, Shi L, Gao Z. Efficacy and safety of phosphodiesterase type 5 (PDE 5) inhibitors in treating erectile dysfunction after bilateral nerve‐sparing radical prostatectomy. Andrologia 2016;48(1):20‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ficarra 2012
- Ficarra V, Novora G, Alhering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta‐analysis of studies reporting potency rates after robot‐assisted radical prostatectomy. European Urology 2012;62:418‐30. [DOI] [PubMed] [Google Scholar]
Fode 2013
- Fode M, Ohl DA, Ralph D, Sonksen J. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU international 2013;112(7):998‐1008. [PUBMED: 23826962] [DOI] [PubMed] [Google Scholar]
Fowler 1993
- Fowler FJ Jr, Barry MJ, Lu‐Yao G, Roman A, Wasson J, Wennberg JE. Patient‐reported complications and follow‐up treatment after radical prostatectomy. The National Medicare Experience: 1988‐1990 (updated June 1993). Urology 1993;42(6):622‐9. [PUBMED: 8256394] [DOI] [PubMed] [Google Scholar]
Gebert 2014
- Gebert S. Are penile prostheses a viable option to recommend for gay men?. International Journal of Urological Nursing 2014;8(3):111‐3. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 23 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Hatzimouratidis 2009
- Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. European Urology 2009;55:334‐47. [DOI] [PubMed] [Google Scholar]
Heidenreich 2014
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Kwast T, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent‐update 2013. European Urology 2014;65(1):124‐37. [PUBMED: 24207135] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunsley 1992
- Hunsley J. Development of the Treatment Acceptability Questionnaire. Journal of Psychopathology and Behavioral Assessment 1992;14(1):55‐64. [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407‐15. [DOI] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient‐reported outcomes in meta‐analyses – Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kacker 2013
- Kacker R, O'Leary MP. Penile rehabilitation after radical prostatectomy. Trends in Urology & Men's Health 2013;September/October:12‐17. [Google Scholar]
Kendirci 2006
- Kendirci M, Bejma J, Hellstrom WJG. Update on erectile dysfunction in prostate cancer patients. Current Opinion in Urology 2006;16(3):186‐95. [DOI] [PubMed] [Google Scholar]
Kubin 2003
- Kubin M, Wagner G, Fugl‐Meyer AR. Epidemiology of erectile dysfunction. International Journal of Impotence Research 2003;15(1):63‐71. [DOI] [PubMed] [Google Scholar]
Kundu 2004
- Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. Journal of Urology 2004;172(6 Pt 1):2227‐31. [PUBMED: 15538237] [DOI] [PubMed] [Google Scholar]
Lalong‐Muh 2012
- Lalong‐Muh J, Treacy C, Steggall M. Erectile dysfunction following retropubic prostatectomy. British Journal of Nursing 2012;22(4):S4‐S9. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li J, Shi Q, Pu C, Tang Y, Bai Y, Yuan H, et al. Phosphodiesterase type 5 inhibitors for the treatment of post‐nerve sparing radical prostatectomy erectile dysfunction in men. Scientific Reports 2014;4:5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowrance 2012
- Lowrance WT, Eastham JA, Savage C, Maschino AC, Laudone VP, Dechet CB, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. Journal of Urology 2012;187(6):2087‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulhall 2009
- Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. Journal of Urology 2009;181(2):462‐71. [PUBMED: 19084865] [DOI] [PubMed] [Google Scholar]
Mulhall 2010
- Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. Journal of Sexual Medicine 2010;7(4 (Pt 2)):1687‐98. [DOI] [PubMed] [Google Scholar]
National Cancer Institute
- National Cancer Institute. Common toxicity criteria. ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed 9 November 2017).
NIH Consensus Conference 1993
- NIH Consensus Conference. Impotence – Consensus Development Panel on Impotence. Journal of the American Medical Association 1993;270:83‐90. [Google Scholar]
Penson 2008
- Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5‐year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. Journal of Urology 2008;179(5 Suppl):S40‐4. [PUBMED: 18405749] [DOI] [PubMed] [Google Scholar]
Philippou 2016
- Philippou YA, Steggall MJ, Treacy CL, Hirani S, O'Driscoll ST, Bakker CJ, et al. Penile rehabilitation for post‐prostatectomy erectile dysfunction. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012414] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2016
- Qiu S, Tang Z, Deng L, Liu L, Han P, Yang L, et al. Comparisons of regular and on‐demand regimen of PED5‐Is in the treatment of ED after nerve‐sparing radical prostatectomy for prostate cancer. Scientific Reports 2016;6:32853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabbani 2000
- Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. Journal of Urology 2000;164(6):1929‐34. [PUBMED: 11061884] [PubMed] [Google Scholar]
Rabbani 2010
- Rabbani F, Schiff J, Peicuch M, Yunis LH, Eastham JA, Scardino PT, et al. Time course of recovery of erectile function after radical retropubic prostatectomy: does anyone recovery after 2 years?. Journal of Sexual Medicine 2010;7:3984‐90. [DOI] [PubMed] [Google Scholar]
Raina 2010
- Raina R, Pahlajani G, Agarwal A, Jones S, Zippe C. Long‐term potency after early use of a vacuum erection device following radical prostatectomy. British Journal of Urology International 2010;106(11):1719‐22. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822‐30. [DOI] [PubMed] [Google Scholar]
Rosen 2011
- Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. European Urology 2011;60(5):1010‐6. [DOI] [PubMed] [Google Scholar]
Rozet 2005
- Rozet F, Galiano M, Cathelineau X, Barret E, Cathala N, Vallancien G. Extraperitoneal laparoscopic radical prostatectomy: a prospective evaluation of 600 cases. Journal of Urology 2005;174(3):908‐11. [PUBMED: 16093985] [DOI] [PubMed] [Google Scholar]
Salonia 2017
- Salonia A, Adaikan G, Buvat J, Carrier S, El‐Meliegy A, Hatzimouratidis K, et al. Sexual rehabilitation after treatment for prostate cancer—Part 2: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). Journal of Sexual Medicine 2017;14:297‐315. [DOI] [PubMed] [Google Scholar]
Sanda 2017
- Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM Taplin ME, Treadwell JR. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options.. J Urol. 2017 Dec 15;199(3):683–690. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Segal 2013
- Segal RL, Bivalacqua TJ, Burnett AL. Current penile‐rehabilitation strategies: clinical evidence. Arab Journal of Urology 2013;11:230‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skolarus 2015
- Skolarus TA, Dunn RL, Sanda MG, Chang P, Greenfield TK, Litwin MS, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology 2015;85(1):101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaliviero 2010
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C. Does Greenlight HPS (™) laser photoselective vaporization prostatectomy affect sexual function?. Journal of Endourology 2010;24(12):2051‐7. [DOI] [PubMed] [Google Scholar]
Stanford 2000
- Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283(3):354‐60. [PUBMED: 10647798] [DOI] [PubMed] [Google Scholar]
Steggall 2011
- Steggall MJ. Clinical management of erectile dysfunction. International Journal of Urological Nursing 2011;5(2):52‐8. [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Szymanski 2010
- Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the Expanded Prostate cancer Index Composite instrument for measuring health‐related quality of life among prostate cancer survivors. Urology 2010;76(5):1245‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tian 2017
- Tian D, Wang XY, Zong HT, Zhang Y. Efficacy and safety of short‐and long‐term, regular and on‐demand regimens of phosphodiesterase type 5 inhibitors in treating erectile dysfunction after nerve‐sparing radical prostatectomy: a systematic review and meta‐analysis.. Clinical Interventions in Aging 2017;12:405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
U.S. Cancer Statistics Working Group
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999‐2014 Incidence and Mortality Web‐based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2017. www.cdc.gov/uscs (accessed 8 December 2017).
Wang 2014
- Wang X, Wang X, Liu T, He Q, Wang Y, Zhang X. Systematic review and meta‐analysis of the use of phosphodiesterase type 5 inhibitors for treatment of erectile dysfunction following bilateral nerve‐sparing radical prostatectomy. PloS One 2014;9(3):e91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2000
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate cancer Index Composite (EPIC) for comprehensive assessment of health‐related quality of life in men with prostate cancer. Urology 2000;56(6):899‐905. [DOI] [PubMed] [Google Scholar]
Weyne 2015
- Weyne E, Castiglione F, Aa F, Bivalacqua TJ, Albersen M. Landmarks in erectile function recovery after radical prostatectomy. Nature Reviews. Urology 2015;12(5):289‐97. [PUBMED: 25868558] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI: 10.1136/bmj.39465.451748.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]


