Abstract
Background
This is an update of the original review published in 2007.
Carcinoma of the rectum is a common malignancy, especially in high income countries. Local recurrence may occur after surgery alone. Preoperative radiotherapy (PRT) has the potential to reduce the risk of local recurrence and improve outcomes in rectal cancer.
Objectives
To determine the effect of preoperative radiotherapy for people with localised resectable rectal cancer compared to surgery alone.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library; Issue 5, 2018) (4 June 2018), MEDLINE (Ovid) (1950 to 4 June 2018), and Embase (Ovid) (1974 to 4 June 2018). We also searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for relevant ongoing trials (4 June 2018).
Selection criteria
We included randomised controlled trials comparing PRT and surgery with surgery alone for people with localised advanced rectal cancer planned for radical surgery. We excluded trials that did not use contemporary radiotherapy techniques (with more than two fields to the pelvis).
Data collection and analysis
Two review authors independently assessed the 'Risk of bias' domains for each included trial, and extracted data. For time‐to‐event data, we calculated the Peto odds ratio (Peto OR) and variances, and for dichotomous data we calculated risk ratios (RR) using the random‐effects method. Potential sources of heterogeneity hypothesised a priori included study quality, staging, and the use of total mesorectal excision (TME) surgery.
Main results
We included four trials with a total of 4663 participants. All four trials reported short PRT courses, with three trials using 25 Gy in five fractions, and one trial using 20 Gy in four fractions. Only one study specifically required TME surgery for inclusion, whereas in another study 90% of participants received TME surgery.
Preoperative radiotherapy probably reduces overall mortality at 4 to 12 years' follow‐up (4 trials, 4663 participants; Peto OR 0.90, 95% CI 0.83 to 0.98; moderate‐quality evidence). For every 1000 people who undergo surgery alone, 454 would die compared with 45 fewer (the true effect may lie between 77 fewer to 9 fewer) in the PRT group. There was some evidence from subgroup analyses that in trials using TME no or little effect of PRT on survival (P = 0.03 for the difference between subgroups).
Preoperative radiotherapy may have little or no effect in reducing cause‐specific mortality for rectal cancer (2 trials, 2145 participants; Peto OR 0.89, 95% CI 0.77 to 1.03; low‐quality evidence).
We found moderate‐quality evidence that PRT reduces local recurrence (4 trials, 4663 participants; Peto OR 0.48, 95% CI 0.40 to 0.57). In absolute terms, 161 out of 1000 patients receiving surgery alone would experience local recurrence compared with 83 fewer with PRT. The results were consistent in TME and non‐TME studies.
There may be little or no difference in curative resection (4 trials, 4673 participants; RR 1.00, 95% CI 0.97 to 1.02; low‐quality evidence) or in the need for sphincter‐sparing surgery (3 trials, 4379 participants; RR 0.99, 95% CI 0.94 to 1.04; I2 = 0%; low‐quality evidence) between PRT and surgery alone.
Low‐quality evidence suggests that PRT may increase the risk of sepsis from 13% to 16% (2 trials, 2698 participants; RR 1.25, 95% CI 1.04 to 1.52) and surgical complications from 25% to 30% (2 trials, 2698 participants; RR 1.20, 95% CI 1.01 to 1.42) compared to surgery alone.
Two trials evaluated quality of life using different scales. Both studies concluded that sexual dysfunction occurred more in the PRT group. Mixed results were found for faecal incontinence, and irradiated participants tended to resume work later than non‐irradiated participants between 6 and 12 months, but this effect had attenuated after 18 months (low‐quality evidence).
Authors' conclusions
We found moderate‐quality evidence that PRT reduces overall mortality. Subgroup analysis did not confirm this effect in people undergoing TME surgery. We found consistent evidence that PRT reduces local recurrence. Risk of sepsis and postsurgical complications may be higher with PRT.
The main limitation of the findings of the present review concerns their applicability. The included trials only assessed short‐course radiotherapy and did not use chemotherapy, which is widely used in the contemporary management of rectal cancer disease. The differences between the trials regarding the criteria used to define rectal cancer, staging, radiotherapy delivered, the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy did not appear to influence the size of effect across the studies.
Future trials should focus on identifying participants that are most likely to benefit from PRT especially in terms of improving local control, sphincter preservation, and overall survival while reducing acute and late toxicities (especially rectal and sexual function), as well as determining the effect of radiotherapy when chemotherapy is used and the optimal timing of surgery following radiotherapy.
Plain language summary
Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma
Background
Rectal cancer is one of the most common causes of cancer deaths in the western world. Individuals diagnosed with rectal cancer are mainly treated with surgery. However, the risk remains that rectal cancer will recur after surgical treatment. A course of radiotherapy before surgery might reduce the risk of local recurrence because radiotherapy can destroy smaller residual tumours and enhance the effects of surgery.
Study characteristics
We searched medical databases on 4 June 2018 for randomised trials (experimental studies where people are randomly allocated to one of two or more treatment groups) to determine whether there is any benefit to radiotherapy before surgical treatment for people with rectal cancer in terms of reducing the risk of dying from any cause, the risk of dying from cancer, and the risk of cancer recurring in the pelvis. We considered high‐dose regimen of radiotherapy followed by any type of surgical treatment to remove cancer of the rectum.
Results
We found four trials involving 4663 people with operable rectal cancer. Our results suggest that administering short‐course radiotherapy before surgery probably reduces mortality. However, when our analysis was limited to a contemporary type of surgery (total mesorectal excision), there was no evidence of a difference between the group receiving radiotherapy before surgery and the group receiving surgery alone. There may be little or no difference between groups in cancer‐related death when short‐course radiotherapy is used.
We found moderate quality evidence that using preoperative radiotherapy compared to surgery alone may provide substantial benefit in terms of reduction of local recurrence of the cancer.
There was little or no effect of preoperative radiotherapy on curative resection and sphincter‐sparing surgery.
We found higher rates of sepsis, surgical complications, and sexual complications in participants treated with radiotherapy compared to those who received only surgery.
Quality of the evidence
Overall the studies were well‐designed. We judged the quality of the evidence as moderate for cancer recurrence and overall mortality, as there were serious concerns regarding the applicability of the findings to the contemporary management of rectal cancer.
We further downgraded the quality of the evidence for the remaining outcomes due to imprecise results and/or variations between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, the type of surgery performed, the radiation dose and fractioning, the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy.
Summary of findings
Summary of findings for the main comparison. Preoperative radiotherapy compared to surgery alone for the management of localised rectal carcinoma.
| Preoperative radiotherapy compared to surgery alone for the management of localised rectal carcinoma | ||||||
|
Patient or population: People with localised rectal carcinoma
Intervention: Preoperative radiotherapy Control: Surgery alone Settings: Hospital | ||||||
| Outcomes | № of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | Comment | |
| Risk with surgery alone | Risk difference with preoperative radiotherapy | |||||
|
Overall mortality (follow‐up 4 to 12 years) |
4663 (4 studies) | Peto OR 0.90 (0.83 to 0.98) | 454 per 1000 | 45 fewer per 1000 (77 fewer to 9 fewer) | ⊕⊕⊕⊝ moderate1,2,3,4 | |
|
Overall mortality ‐ only total mesorectal excision (follow‐up 4 to 12 years) |
3211 (2 studies) | Peto OR 0.97 (0.87 to 1.08) |
410 per 1000 | 9 fewer per 1000 (42 fewer to 24 more) | ⊕⊕⊝⊝ low3,5 | |
|
Cause‐specific mortality (follow‐up 4 to 12 years) |
2145 (2 studies) | Peto OR 0.89 (0.77 to 1.03) | 355 per 1000 | 39 fewer per 1000 (82 fewer to 11 more) | ⊕⊕⊝⊝ low3,5 | |
|
Local recurrence (follow‐up 4 to 12 years) |
4663 (4 studies) | Peto OR 0.48 (0.40 to 0.57) | 161 per 1000 | 83 fewer per 1000 (96 fewer to 69 fewer) | ⊕⊕⊕⊝ moderate1,3,6 | |
|
Curative resection (follow‐up 4 to 12 years) |
4673 (4 studies) | RR 1.00 (0.97 to 1.02) | 809 per 1000 | 0 fewer per 1000 (24 fewer to 16 more) | ⊕⊕⊝⊝ low1,3,5 | |
|
Sphincter preservation (follow‐up 4 to 12 years) |
4379 (3 studies) | RR 0.99 (0.94 to 1.04) | 588 per 1000 | 6 fewer per 1000 (35 fewer to 24 more) | ⊕⊕⊝⊝ low3,5,7 | |
| Postoperative morbidity ‐ sepsis (within 30 days after surgery) | 2698 (2 studies) | RR 1.25 (1.04 to 1.52) | 128 per 1000 | 32 more per 1000 (5 more to 67 more) | ⊕⊕⊝⊝ low3, 7 | |
| Postoperative morbidity ‐ surgical complications (within 30 days after surgery) | 2698 (2 studies) | RR 1.20 (1.01 to 1.42) | 248 per 1000 | 50 more per 1000 (2 more to 104 more) | ⊕⊕⊝⊝ low3,7 | |
|
Quality of life (follow‐up range 6 to 18 months) |
3211 (2 studies) |
See comment | ⊕⊕⊝⊝ low3,7 | 2 studies evaluated quality of life using different scales (Sebag‐Montefiore 2009; van Gijn 2011). Both studies concluded that sexual dysfunction occurred more in the preoperative radiotherapy group; results for faecal incontinence were mixed; and irradiated participants tended to resume work later than non‐irradiated participants between 6 to 12 months, but with no difference after 18 months. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Three out of four studies reported an adequate method of allocation concealment; any potential performance bias or detection bias was not taken into account given the outcome under consideration was an objective outcome. We did not downgrade for risk of bias. 2Heterogeneity was moderate (I2 = 42%) and could be explained by differences between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed, radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy. However, we did not downgrade the evidence, as we judged heterogeneity not serious because the confidence intervals showed substantial overlap, and the statistical test for heterogeneity was low (P = 0.16). 3We downgraded for indirectness: the patient population treated in these trials might differ from the population treated in the present day, with more accurate methods of preoperative imaging, accurate staging for distant metastatic disease, use of TME, and use of chemotherapy. 4We did not downgrade for imprecision: the optimal information size criterion was met, and the 95% CI excludes no effect. 5We downgraded for imprecision: the optimal information size criterion was met, but the 95% CI comprises no effect. 6Heterogeneity was moderate (I2 = 51%) and could be explained by differences between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed, radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy. However, we judged heterogeneity not serious because the confidence intervals showed substantial overlap, and the statistical test for heterogeneity was P = 0.10. In addition, the exclusion of the older trial, Marsh 1994, reduced the I2 to 23% (P = 0.23). 7It was unclear whether the outcome assessor was blinded. We considered the outcome to be subjective and downgraded the evidence because of risk of bias.
Background
Description of the condition
Colorectal cancer is the third most common cancer worldwide (746,000 cases in men and 614,000 cases in women). Almost 55% of cases occur in high income countries, with rectal cancer accounting for ˜30% of cases (Ferlay 2015). Incidence is low in people aged 50 years or less, but strongly increases with age. The median age at diagnosis is around 70 years in high income countries (Siegel 2012). Colorectal cancer is the third most common cause of cancer death in men, and the fourth in women (Ferlay 2015).
Anatomically the rectum extends from the anal verge for about 12 cm to 15 cm. Since rectal cancer symptoms generally include rectal bleeding or changes in bowel habits that may be misdiagnosed as benign disease, cancer diagnosis is often delayed. Consequently, at diagnosis some patients may have evidence of locally advanced (i.e. when the tumour infiltrates beyond the muscular wall into adjacent tissues or into regional lymph nodes) or metastatic disease (i.e. the tumour has spread to another part of the body).
Complete visualisation of the colon (either with colonoscopy or computed tomographic (CT) colonography) is needed to identify synchronous neoplastic lesions, which are found in about 2% to 4% of patients with colorectal cancer(Park 2012).
Accurate staging to define the extent of disease is essential to guide optimal treatment. Diagnostic imaging has significantly improved over time. Magnetic resonance imaging (MRI) using a phased array coil is now recommended (Beets‐Tan 2003; Beets‐Tan 2005; Puli 2009; van de Velde 2013), although endoscopic ultrasound can be used for the earliest‐stage tumours. Accuracy is improved when MRI and ultrasound are combined (Swartling 2013). Nodal staging is performed with MRI, although accuracy is low (Fernandez‐Esparrach 2011). Abdominal and chest computed tomography (CT) scans are recommended to detect distant metastases.
The Tumour Node Metastases (TNM) classification staging system of the American Joint Committee on Cancer/Union for International Cancer Control is the preferred staging system for colorectal cancer (Table 2) (Sobin 2010), and has replaced the older Dukes classification (Table 3) (Dukes 1932). Table 4 shows colorectal cancer staging based on anatomic and prognostic factors.
1. Classification of colorectal cancers according to TNM (T stage: local invasion depth; N stage: lymph node involvement; and M stage: presence of distant metastases).
| Definition | |
| T stage | |
| Tx | No information about local tumour infiltration available |
| Tis | Tumour restricted to mucosa, no infiltration of lamina muscularis mucosae. |
| T1 | Infiltration through lamina muscularis mucosae into submucosa, no infiltration of lamina muscularis propria |
| T2 | Infiltration into, but not beyond, lamina muscularis propria |
| T3 | Infiltration into subserosa or non‐peritonealised pericolic or perirectal tissue, or both; no infiltration of serosa or neighbouring organs |
| T4a | Infiltration of the serosa |
| T4b | Infiltration of neighbouring tissues or organs |
| N stage | |
| Nx | No information about lymph node involvement available |
| N0 | No lymph node involvement |
| N1a | Cancer cells detectable in 1 regional lymph node |
| N1b | Cancer cells detectable in 2 to 3 regional lymph nodes |
| N1c | Tumour satellites in subserosa or pericolic or perirectal fat tissue, regional lymph nodes not involved |
| N2a | Cancer cells detectable in 4 to 6 regional lymph nodes |
| N2b | Cancer cells detectable in 7 or greater regional lymph nodes |
| M stage | |
| Mx | No information about distant metastases available |
| M0 | No distant metastases detectable |
| M1a | Metastasis to 1 distant organ or distant lymph nodes |
| M1b | Metastasis to more than 1 distant organ or set of distant lymph nodes or peritoneal metastasis |
2. Classification of colorectal cancers according to Dukes.
| Stage | Description |
| A | Limited to muscularis propria; nodes not involved |
| B | Extending beyond muscularis propria; nodes not involved |
| C | Lymph nodes involved |
| D | Distant metastatic spread |
3. Anatomic stage/prognostic groups.
| Stage | T | N | M | Dukes |
| 0 | Tis | N0 | M0 | ‐‐ |
| I | T1 | N0 | M0 | A |
| T2 | N0 | M0 | A | |
| IIA | T3 | N0 | M0 | B |
| IIB | T4a | N0 | M0 | B |
| IIC | T4b | N0 | M0 | B |
| IIIA | T1‐T2 | N1/N1c | M0 | C |
| T1 | N2a | M0 | C | |
| IIIB | T3‐T4a | N1/N1c | M0 | C |
| T2‐T3 | N2a | M0 | C | |
| T1‐T2 | N2b | M0 | C | |
| IIIC | T4a | N2a | M0 | C |
| T3‐T4a | N2b | M0 | C | |
| T4b | N1‐N2 | M0 | C | |
| IVA | Any T | Any N | M1a | ‐‐ |
| IVB | Any T | Any N | M1b | ‐‐ |
Surgical intervention is the mainstay of rectal cancer treatment. For all but very early tumours, radical excision is required, either with an abdominoperineal resection, or a low anterior resection. The type of procedure depends on the stage, size, and site of disease. Abdominoperineal resection is the removal of the anus, rectum, and part of the sigmoid colon along with the regional lymph nodes, through incisions made in the abdomen and perineum resulting in a permanent colostomy (Mauvais 2011; Miles 1908; Perry 2007). Abdominoperineal resection is preferred for low‐lying rectal cancers where there is concern about achieving clear distal resection margins, or concern about postsurgical sphincter function. An alternative, 'sphincter‐sparing' surgical approach for tumours of the mid‐ to upper rectum is low anterior resection, which involves removal of the sigmoid colon and rectum to a level where the distal margin is free of cancer. Low anterior resection preserves the anal sphincter but carries a risk of anastomotic leakage (Lipska 2006; Matthiessen 2004; Pakkastie 1994).
Despite radical surgery, disease can recur either locally in the pelvis or distantly. Because of the proximity of the rectum to important pelvic structures and the difficulty in achieving clear surgical margins, local relapse is a much greater concern than with colon cancer, and local relapse rates ranging from 20% to 70% have been reported after surgery alone in older trials (Eu 1998; McCall 1995). The risk of local recurrence is increased with disease that extends beyond the muscularis propria of the rectal wall or to regional lymph nodes (Gilbert 1978; Mendenhall 1983; Walz 1981). Local relapses often cause severe morbidity including pain, bowel dysfunction, or bleeding, are difficult to treat, and are associated with a poor prognosis (Cai 2014; Caricato 2006; Holm 1994; Tanis 2013; Wong 1998).
A number of strategies have been investigated to reduce the risk of local recurrence, including the use of adjuvant or neoadjuvant radiotherapy, the use of chemotherapy, and improvements in surgical technique.
A significant advance in surgical technique has occurred with the widespread adoption of total mesorectal excision (TME), first described by Heald in 1982 (Heald 1982). It is the removal of the rectum and surrounding mesorectum enveloped within the visceral pelvic fascia to the level of the levators using sharp dissection (Enker 1997). One of the main prognostic factors for rectal cancer recurrence is a positive circumferential resection margin (Caricato 2006; Nagtegaal 2008), which is defined as a distance of 1 mm or less between the tumour border and resection margin. Clinicopathologic studies reported that most recurrences occurred when tumour spread to the radial excision margins, suggesting that recurrence was related to the persistence of tumour foci within the mesorectum which may be distal to the primary tumour (Quirke 1986). Total mesorectal excision improves the chance of achieving clear circumferential resection margins, and has significantly reduced the local relapse rate to below 10% (Enker 1999; Heald 1986), although the risk of anastomotic leaks is increased (Goldberg 1998; Wiig 1998).
Description of the intervention
Radiotherapy, a local treatment, aims at delivering a precise dose of ionising radiation to a well‐defined target volume with minimal damage to healthy surrounding organs. It is commonly administered using an external‐beam technique that delivers several beams of high‐energy photons generated outside the patient to the target volume. Photons produced by linear accelerators (x rays) are most often used today to deliver the external‐beam treatment, although in the past 60Cobalt units producing lower‐energy γ rays were used. Radiotherapy delivery has evolved significantly over the years, with changes in target volume, definition of target volume, and number of fields used. In earlier trials of radiotherapy, the target volume included the tumour, its containing mesorectum, regional pelvic nodes, and para‐aortic nodes. Although this resulted in reduced local recurrence, it increased the risk of perioperative morbidity and mortality (Cedermark 1995). Contemporary radiotherapy usually limits radiotherapy to the tumour, its containing mesorectum, and regional lymph nodes in the pelvis only, covering the posterior pelvis. Earlier radiotherapy treatments used two fields (anterior and posterior fields) to treat the target volume, whereas modern radiotherapy uses three or more radiotherapy fields to reduce the amount of normal tissue in the field (especially small bowel). Earlier two‐dimensional techniques used bones as markers to define the treatment field. Newer radiotherapy techniques such as three‐dimensional conformal radiotherapy (3DCRT) use CT to define the target volume and normal tissues or organs at risk. Multileaf collimators in the treatment head can provide shielding of fields to limit the dose to normal tissues. Magnetic resonance imaging (MRI) and positron emission tomography (PET) can be used to better define the tumour. Contemporary treatment planning systems provide a more accurate estimate of dose distribution. More recently, highly conformal radiotherapy techniques such as intensity modulated and volumetric radiotherapy use inverse planning and multileaf collimators to provide even greater conformality.
Another type of radiation therapy is brachytherapy, which utilizes radioactive seeds or sources placed inside the patient's body within cavities or tissues. It is not the focus of this review.
How the intervention might work
People with stage I disease may not need any additional treatment after surgery if the risk of recurrence is very low. People with stage II or III disease have a higher risk of local recurrence after surgery, which is thought to be due to microscopic residual disease.
Pelvic radiotherapy has the capacity to treat microscopic residual disease beyond or at the edge of the surgical field and reduce the risk of local recurrence. Radiotherapy has been used either postoperatively or preoperatively. Early randomised trials demonstrated that radiotherapy given postoperatively for locally advanced disease (stage II and III) with the aim of destroying microscopic residual disease reduced the risk of local recurrence (Fisher 1988; Gastrointestinal Tumor Study Group 1985). The addition of chemotherapy to postoperative radiotherapy improved survival and further reduced local recurrence compared with surgery and radiotherapy alone (Gastrointestinal Tumor Study Group 1985; Krook 1991). Based on these findings, in 1990 the National Institutes of Health Consensus Conference recommended that postoperative chemotherapy and radiotherapy be given concurrently as standard therapy for people with stage II and III rectal cancer (NIH consensus conference 1990).
An alternative approach that has been investigated is the use of preoperative radiotherapy (PRT), with or without chemotherapy. The theoretical advantages of preoperative compared with postoperative radiotherapy include the potential for tumour down‐staging with better chances of complete resection with clear margins and less risk of tumour seeding. It is possible that cytoreduction may enable sphincter preservation in lower rectal cancers that would otherwise require an abdominoperineal resection. Postoperatively there may be alterations in vasculature that result in hypoxia which may reduce the sensitivity to radiotherapy of residual tumour cells (Perez 1992). A preoperative approach also has the potential to reduce toxicity by avoiding treatment of the anastomosis (if a low anterior resection is performed) and reducing the amount of small bowel in the radiotherapy field. The preoperative approach has been compared with postoperative radiotherapy in randomised trials and has been shown to result in a lower risk of local recurrence and less toxicity compared with a postoperative approach (Sauer 2004;Sebag‐Montefiore 2009). In addition, Adam 1994 demonstrated that involvement of this margin (defined as microscopic tumour present 1 mm or less from the radial margin) was associated with a high risk of local recurrence. This approach permitted the identification of the few patients at high risk of failure who might benefit from selective postoperative chemoradiotherapy (Sebag‐Montefiore 2009).
Two differing radiotherapy dose/fractionation schemes for preoperative radiotherapy have emerged in common use:
long‐course radiotherapy employs standard fractionation of 1.8 Gy to 2 Gy per day for five days a week to a total dose of 45 Gy to 50 Gy in 25 to 28 fractions, which may be given with chemotherapy. Surgery is usually delayed for at least six weeks after completion to allow maximal cytoreduction. This regimen is thought to be preferable in disease which is fixed, unresectable, or borderline resectable at presentation;
short‐course radiotherapy utilises hypofractionated schemes (e.g. 5 Gy a day for five consecutive days for a total dose of 25 Gy), and surgery usually occurs within seven days following completion.
Why it is important to do this review
Many people with resectable locally advanced rectal cancer recur after surgery alone. Preoperative radiotherapy has the potential to reduce the risk of local recurrence. However, there are potential disadvantages with PRT: it can be logistically difficult requiring multiple treatments; it results in a delay to definitive surgery; and it may be associated with perioperative morbidity and acute and late toxicity. Improvements in surgical technique and the widespread adoption of TME have lowered the local recurrence of rectal cancer, and the effect of PRT when TME is used is unclear. A systematic review was essential to determine the effect of preoperative radiotherapy, in terms of efficacy and toxicity. This review is an update of an earlier Cochrane Review that assessed the effect of preoperative radiotherapy with surgery alone, as well as the effect of other preoperative therapy, including the addition of chemotherapy to preoperative radiotherapy (Wong 2007). These questions have now been separated into two reviews. A Cochrane Review assessing the effect of the addition of chemotherapy to preoperative radiotherapy was recently published (De Caluwe 2013), and this review therefore only addressed the question of the effect of preoperative radiotherapy followed by surgery compared to surgery. There have been significant advances in surgery and radiotherapy for rectal cancer which justify an updated review, with exclusion of randomised trials using surgery or radiotherapy considered unacceptable by current standards.
Objectives
To determine the effect of preoperative radiotherapy for people with resectable rectal cancer compared to surgery alone.
Methods
Criteria for considering studies for this review
Types of studies
Eligible studies were randomised controlled trials (RCTs) that compared PRT and surgery versus surgery alone in people diagnosed with localised resectable rectal cancer. Cluster RCTs were eligible. We excluded studies including both colon and rectal cancer with no subgroup results for participants with rectal cancer. We included studies irrespective of their publication status and language of publication.
Types of participants
We included trials on adults (aged 18 or above) diagnosed with a locally advanced carcinoma of the rectum, with no evidence of distant metastasis.
Types of interventions
Surgery
We included trials that considered any radical surgical intervention (e.g. Hartmann procedure, anterior resection, or abdominal perineal resection). Total mesorectal excision was not mandated.
Radiotherapy
Active group
Preoperative radiotherapy: we considered trials that assessed pelvic radiotherapy that was delivered with mega‐voltage external‐beam radiation with a biological effective dose (BED) of at least 30 Gy (assuming an alpha/beta ratio of 10 Gy) for inclusion. The prior publication of this review revealed no improvement in local control in subset analysis of studies with BED less than 30 Gy, and such low doses do not reflect contemporary radiotherapy of interest in this review (Wong 2007). We excluded trials using only two fields, or very large fields that included elective treatment of the para‐aortic nodes.
We excluded trials that used brachytherapy.
Control group
The control group did not receive preoperative radiotherapy. Chemotherapy was permitted, provided it was given in both arms.
Types of outcome measures
Primary outcomes
Overall mortality.
Secondary outcomes
Cause‐specific mortality (rectal cancer‐related mortality).
Local recurrence (defined as an intrapelvic recurrence following a primary rectal cancer resection, with or without distal metastasis).
Distant metastasis (in any organ documented).
Any recurrence (distant or local).
Curative resection (resection is defined as curative if all the macroscopic disease could be removed at the end of surgery with negative histological margin) and overall resectability (Law 2004).
Sphincter preservation.
Postoperative morbidity (including overall complication within 30 days after surgery).
Postoperative mortality (defined as death within 30 days after surgery).
Acute radiotherapy toxicity (within six months).
Late toxicity (after six months).
Quality of life (using validated scales, as reported by study authors).
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restriction. We searched the following electronic databases to identify potential studies:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library; Issue 5, 2018) (4 June 2018) (Appendix 1);
Ovid MEDLINE (1950 to 4 June 2018) (Appendix 2);
Ovid Embase (1974 to 4 June 2018) (Appendix 3).
Searching other resources
We searched the following trial registers on 4 June 2018:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
We searched relevant websites and checked reference lists of all included studies for additional eligible studies.
Data collection and analysis
Selection of studies
Two review authors (IA, RC) independently scanned the titles and abstracts of all records identified by the electronic searches. For records with insufficient data to make a clear decision or abstracts appearing to meet the inclusion criteria, we obtained the full text of the study. Pairs of review authors (RC, IP, ML, RDF) independently selected articles of interest. Disagreements were resolved through discussion or by the involvement of a third review author (IA).
Data extraction and management
Pairs of review authors (IA IP, ML, RDF) independently extracted data from all included trials. Disagreements were resolved through discussion by the involvement of a third review author when necessary. We attempted to contact authors for clarification whenever necessary.
We recorded the following data for each trial: year of publication, the number and details of participants including demographic characteristics (e.g. location of cancer, resectability, staging work‐up, stage distribution, definition used to define rectal cancer), details of radiotherapy (dose, fractionation, and volume), type of surgery, type of outcome, outcome measure, and duration of follow‐up.
Assessment of risk of bias in included studies
Two review authors (IA, RC) independently assessed the risk of bias as specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), according to the outlined criteria for judgement (Appendix 4). We tabulated risk of bias for each included study along with a judgement of low, high, or unclear risk of bias for each domain.
We addressed the following domains: sequence generation; allocation sequence concealment (Savovic 2012; Wood 2008); blinding of participants, surgeons, and assessors (Savovic 2012; Schulz 1996; Wood 2008); incomplete outcome data (Abraha 2015; Abraha 2017); and selective outcome reporting (Chan 2004; Macura 2010). We based blinding of participants, surgeons, and assessors on whether we judged the outcome to be subjective or objective. Except for quality of life, we considered all outcomes to be objective.
Any disagreements were resolved by consensus or with the assistance of a third review author when necessary.
Measures of treatment effect
We compared the outcomes of overall mortality, cause‐specific mortality, any recurrence, and local recurrence using reported or estimated Peto odds ratio (OR) and its variance (Parmar 1998).
For other outcomes, risk ratios (RR) (with 95% confidence interval (CI)), pooled using the random‐effects model (DerSimonian 1996), were used for the analyses.
For relevant outcomes, in addition to Peto OR or RR we calculated absolute effect using risk difference (RD) and relative CI.
Unit of analysis issues
Randomisation took place on an individual basis for each participant receiving the intervention. We did not identify any cluster RCTs. The unit of analysis was thus the individual participant.
Dealing with missing data
We attempted to contact trial investigators to obtain information on unpublished missing data, without success.
Assessment of heterogeneity
We assessed heterogeneity of study characteristics and statistical heterogeneity. We evaluated the former by examining the corresponding table of characteristics of the included population, the type of interventions, and the type of outcome measures.
We assessed statistical heterogeneity for each meta‐analysis through a visual assessment of the forest plot, in addition to evaluating the Chi2 test and I2 statistic. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we considered a Chi2 test with a P value of 0.10 to be significant, and we interpreted the I2statistic as: 0% to 40% unimportant heterogeneity; 30% to 60% moderate heterogeneity; 50% to 90% substantial heterogeneity; and 75% to 100% considerable heterogeneity.
Assessment of reporting biases
We did our best to include data from all trials on all prespecified outcomes, obtained from secondary publications. We planned a funnel plot of effect estimates against their standard errors to assess possible between‐study reporting bias. Given the limited number of included studies, we did not assess funnel plot asymmetry for reported outcomes as recommended and described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
We performed meta‐analysis of outcomes in which we had comparable effect measures for more than one study, and when measures of clinical and methodological heterogeneity indicated that pooling was appropriate. Pooled data were presented with the number of included studies, the number of participants, the summary statistic with 95% CI followed by an assessment of the test for homogeneity.
If the difference was statistically significant, for hazard ratios, an estimate of the effect of the event rates for selected time points (e.g. 1, 5, 10 years) was calculated to provide estimates of the magnitude of effect.
We used the hazard ratio and variance corresponding to the published survival data. Where this was not directly available from the paper, it was estimated using log rank P value, number randomised, events, or survival curves where available. An Excel (MS Excel 2010) spreadsheet developed by the Meta‐analysis Group of the Medical Research Council Clinical Trials Unit, London was used to facilitate the calculation (Tierney 2007). We used the individual participant data outcome in Review Manager 5 (RevMan 2014) to handle the hazard ratio. The number of events (n) entered into the MetaView tables was the number surviving at the end of the follow‐up period as published. The hazard ratios appear under 'Peto OR', the default label applied by the Review Manager 5 analysis software (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out subgroup analyses with investigation of interactions for overall mortality and local recurrence according to:
risk of bias (selection bias): inadequate/unclear allocation concealment or adequate allocation concealment;
stage: TNM I/II or Duke A/B or TNM III or Duke C;
surgery: TME or not TME;
-
distance of the tumour from the anal verge (local recurrence only):
less or equal to 5;
from 6 to 10;
higher than 10.
We used the test for subgroup differences in Review Manager 5 to compare subgroup analyses.
Sensitivity analysis
We assessed the robustness of our findings by performing the following sensitivity analyses when data were sufficient. We performed sensitivity analysis:
restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' for selection bias;
restricting the analysis to studies that used TME surgery.
Quality of the evidence
We used the GRADE approach to assess the quality of the evidence for all outcomes (Schünemann 2011a; Schünemann 2011b). The quality of evidence can be downgraded by one (serious concern) or two (very serious concern) levels for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes), imprecision (wide confidence interval, small sample size), and risk of publication bias (Balshem 2011). Key findings of the review including summary of the amount of data, the magnitude of the effect size, and the overall quality of the evidence for the most important outcomes are presented in the Table 1. Ratings for all outcomes are given in Table 5.
4. GRADE ratings for all outcomes.
| Outcomes | № of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | Comment | |
| Risk with surgery alone | Risk difference with preoperative radiotherapy | |||||
|
Overall mortality (follow‐up 4 to 12 years) |
4663 (4 studies) | Peto OR 0.90 (0.83 to 0.98) | 454 per 1000 | 45 fewer per 1000 (77 fewer to 9 fewer) | ⊕⊕⊕⊝ moderate1,2,3,4 | |
|
Overall mortality ‐ TME only (follow‐up 4 to 12 years) |
3211 (2 studies) | Peto OR 0.97 (0.87 to 1.08) |
410 per 1000 | 9 fewer per 1000 (from 42 fewer to 24 more) | ⊕⊕⊝⊝ low3,5 | |
|
Cause‐specific mortality (follow‐up 4 to 12 years) |
2145 (2 studies) | Peto OR 0.89 (0.77 to 1.03) | 355 per 1000 | 39 fewer per 1000 (82 fewer to 11 more) | ⊕⊕⊝⊝ low3,5 | |
|
Local recurrence (follow‐up 4 to 12 years) |
4663 (4 studies) | Peto OR 0.48 (0.40 to 0.57) | 161 per 1000 | 83 fewer per 1000 (96 fewer to 69 fewer) | ⊕⊕⊕⊝ moderate1,3,6 | |
|
Local recurrence ‐ TME only (follow‐up 4 to 12 years) |
3211 (2 studies) | HR 0.46 (0.35 to 0.59) | 108 per 1000 | 57 fewer per 1000 (from 43 fewer to 69 fewer) | ⊕⊕⊕⊝ moderate3 | |
|
Distant metastases (follow‐up 4 to 12 years) |
4485 (4 studies) | RR 0.96 (0.85 to 1.08) | 207 per 1000 | 8 fewer per 1000 (31 fewer to 17 more) | ⊕⊕⊝⊝ low1,3,5 | |
|
Any recurrence (median follow‐up 10 years) |
1861 (1 study) | Peto OR 0.82 (0.68 to 0.99) | 270 per 1000 | 49 fewer per 1000 (86 fewer to 3 fewer) | ⊕⊕⊝⊝ low3,7 | |
|
Curative resection (follow‐up 4 to 12 years) |
4673 (4 studies) | RR 1.00 (0.97 to 1.02) | 809 per 1000 | 0 fewer per 1000 (24 fewer to 16 more) | ⊕⊕⊝⊝ low1,3,5 | |
|
Overall resectability (follow‐up 5 to 8 years) |
2802 (3 studies) | RR 0.99 (0.95 to 1.04) | 897 per 1000 | 9 fewer per 1000 (45 fewer to 36 more) | ⊕⊝⊝⊝ very low3,5,8 | |
|
Sphincter preservation (follow‐up 4 to 12 years) |
4379 (3 studies) | RR 0.99 (0.94 to 1.04) | 588 per 1000 | 6 fewer per 1000 (35 fewer to 24 more) | ⊕⊕⊝⊝ low3,5,9 | |
| Postoperative morbidity ‐ sepsis (within 30 days after surgery) | 2698 (2 studies) | RR 1.25 (1.04 to 1.52) | 128 per 1000 | 32 more per 1000 (5 more to 67 more) | ⊕⊕⊝⊝ low3,9 | |
| Postoperative morbidity ‐ surgical complications (within 30 days after surgery) | 2698 (2 studies) | RR 1.20 (1.01 to 1.42) | 248 per 1000 | 50 more per 1000 (2 more to 104 more) | ⊕⊕⊝⊝ low3,9 | |
| Postoperative mortality (within 30 days after surgery) | 1960 (2 studies) | RR 0.75 (0.46 to 1.22) | 37 per 1000 | 9 fewer per 1000 (20 fewer to 8 more) | ⊕⊕⊝⊝ low3,5 | |
| Acute radiotherapy toxicity (within 6 months) | 1530 (1 study) |
See comment | ⊕⊕⊝⊝ low3, 9 | Only 1 study reported acute radiotherapy toxicity (van Gijn 2011): grade 1 toxicity occurred in 19% (145/761) of participants, grade 2 and 3 occurred in 7% (53/761), whereas none of the participants developed grade 4 or 5 side effects. | ||
| Late toxicities (after 6 months) | See comment | ⊕⊕⊝⊝ low3,9 | No study evaluated late toxicity. However, 1 trial provided data on long‐term rectal function based on subgroup of participants (Swedish RCT 1997). Compared to open surgery alone, after PRT there were more participants with increased stool frequency (20% (17/84) vs 8% (7/87); RR 2.52 (95% CI 1.1 to 5.75)) and continence problems (50% (42/84) vs 24% (21/87); RR 2.07 (95% CI 1.35 to 3.18)). The rates of tenesmus were similar between the 2 groups (27% (23/84) vs 33% (29/97); RR 0.82 (95% CI 0.52 to 1.30)). | |||
|
Quality of life (follow‐up range 6 to 18 months) |
3211 (2 studies) |
See comment | ⊕⊕⊝⊝ low3,9 | 2 studies evaluated quality of life using different scales (Sebag‐Montefiore 2009; van Gijn 2011). Both studies concluded that sexual dysfunction occurred more in the PRT group; results for faecal incontinence were mixed; and irradiated participants tended to resume work later than non‐irradiated participants between 6 to 12 months, but with no difference after 18 months. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; OR: odds ratio; PRT: preoperative radiotherapy; RR: risk ratio; TME: total mesorectal excision | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Three out of four studies reported an adequate method of allocation concealment; any potential performance bias or detection bias was not taken into account given the outcome under consideration was an objective outcome. We did not downgrade for risk of bias. 2Heterogeneity was moderate (I2 = 42%) and could be explained by differences between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed, radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy. However, we did not downgrade the evidence, as we judged heterogeneity not serious because the confidence intervals showed substantial overlap, and the statistical test for heterogeneity was low (P = 0.16). 3We downgraded for indirectness: the patient population treated in these trials might differ from the population treated in the present day, with more accurate methods of preoperative imaging, accurate staging for distant metastatic disease, use of TME, and use of chemotherapy. 4We did not downgrade for imprecision: the optimal information size criterion was met, and the 95% CI excludes no effect. 5We downgraded for imprecision: the optimal information size criterion was met, but the 95% CI comprises no effect. 6Heterogeneity was moderate (I2 = 51%) and could be explained by differences between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed, radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy. However, we judged heterogeneity not serious because the confidence intervals showed substantial overlap, and the statistical test for heterogeneity was P = 0.10. In addition, the exclusion of the older trial, Marsh 1994, reduced the I2 to 23% (P = 0.23). 7We downgraded by one level for imprecision: large confidence interval. 8We downgraded by one level for inconsistency: unexplained moderate heterogeneity. 9It was unclear whether the outcome assessor was blinded. We considered the outcome to be subjective and downgraded the evidence because of risk of bias.
Results
Description of studies
Results of the search
We conducted a literature search on 4 June 2018 and identified a total of 4231 citations. Of these, we screened the titles and abstracts of 2944 records after removing 1287 duplicates. We excluded 2830 records after title and abstract screening and assessed 114 full‐text records for eligibility. We excluded 48 trials (constituting 76 records), with reasons provided in the Characteristics of excluded studies table.
We identified 5 trials (constituting 39 records) addressing preoperative radiotherapy versus surgery alone that were eligible for inclusion (Marsh 1994; Sebag‐Montefiore 2009; Stockholm 1996; Swedish RCT 1997; van Gijn 2011). However, as the Swedish RCT 1997 included part of the population of the Stockholm 1996, we used data only from the Swedish trial to avoid double counting (Swedish RCT 1997).
The study screening process is presented in Figure 1.
1.
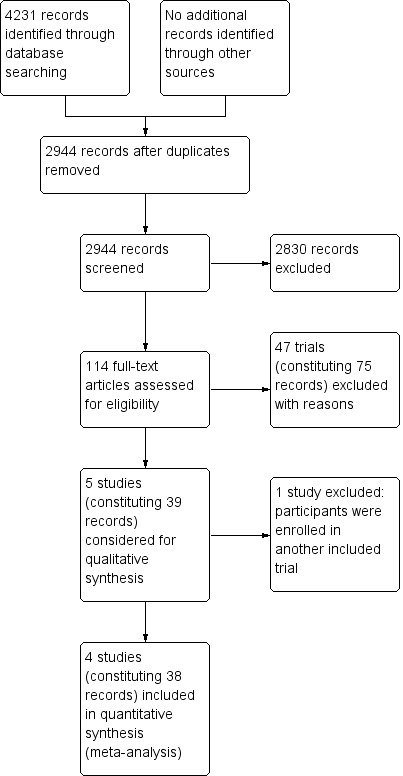
Study flow diagram.
Included studies
Analysis and interpretation are based on four trials (Marsh 1994; Sebag‐Montefiore 2009; Swedish RCT 1997; van Gijn 2011).
The four included studies were published between 1994 and 2011. The number of included participants was 4663 across all the trials. Follow‐up ranged between 4 to 12 years: the minimum follow‐up was 4 years for Sebag‐Montefiore 2009, 5 years for Swedish RCT 1997, 8 years for Marsh 1994, and 12 years for van Gijn 2011.
The trials differed in a number of factors including the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed (and in particular the requirement for TME), radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, the use of adjuvant or postoperative therapy, and the outcomes reported. Details are given in the Characteristics of included studies table.
Criteria used to define rectal cancer
The four included studies used different criteria to define rectal cancer: one study defined rectal cancer below the sacral promontory (Swedish RCT 1997), while the others used a defined distance from the anal verge: 13 cm in Marsh 1994 and 14 cm in van Gijn 2011 and Sebag‐Montefiore 2009.
Stage of participants and staging work‐up
Marsh 1994 included participants with "locally advanced" disease defined by primary tumour being fixed or tethered but operable on examination under anaesthetic. No information was given about the use of preoperative imaging to stage for metastatic disease. Swedish RCT 1997, van Gijn 2011, and Sebag‐Montefiore 2009 included participants with clinically resectable stage I to III rectal cancer. van Gijn 2011 commented specifically about excluding fixed tumours. Swedish RCT 1997 and van Gijn 2011 provided no details about what imaging was used to identify metastatic disease. In Sebag‐Montefiore 2009, liver ultrasound or CT and chest X‐ray were used to identify people with metastatic disease for exclusion.
Radiotherapy and radiotherapy‐to‐surgery interval
Marsh 1994 used a rotational three‐field wedged technique using 4MeV linear accelerator to treat the posterior pelvis giving 20 Gy in four daily fractions. Surgery was performed within a week of completion of radiotherapy. In Swedish RCT 1997, van Gijn 2011, and Sebag‐Montefiore 2009, three or four fields were used to treat to a dose of 25 Gy in five daily fractions. Surgery was to be performed within one week of completion of radiotherapy in Swedish RCT 1997 and van Gijn 2011, and within 10 days in Sebag‐Montefiore 2009.
Surgery
Only one study specifically required TME (van Gijn 2011). In Sebag‐Montefiore 2009, TME was not mandated in the trial protocol, however surgeons were encouraged to use it, and as a consequence, 92% (n = 1143) of the resections were recorded as TME.
Postoperative therapy
No postoperative therapy was described in Marsh 1994 or Swedish RCT 1997.
In van Gijn 2011, participants with positive margins were to receive postoperative radiotherapy to a dose of 50.4 Gy in 28 daily fractions.
In Sebag‐Montefiore 2009, participants with positive margins were to receive postoperative radiotherapy to a dose of 45 Gy in 25 fractions with concurrent chemotherapy with infusional or bolus 5‐fluorouracil and leucovorin. Adjuvant chemotherapy using 5‐fluorouracil and leucovorin either monthly or weekly was permitted. Participating centres were required to state their local policy for the use of chemotherapy according to either positive margins or lymph node involvement, and were required to apply this to both treatment groups. If postoperative chemoradiotherapy was required for positive margins, it was given first. Seventy‐seven of 676 (12%) participants in the surgery‐alone arm had positive margins. Fifty‐five of these received chemoradiotherapy, and seven received radiotherapy alone. Given that the number of participants receiving postoperative chemoradiotherapy (n = 77) was very small compared to the overall sample size of the study (n = 1350), we have included this study in our meta‐analysis. Forty per cent of participants in the PRT and 45% in the control arm received adjuvant chemotherapy.
Stage distribution
One study provided stage distribution according to Dukes' classification (Table 3) (Swedish RCT 1997); approximately 33% of participants were Dukes' A. Two studies provided stage distribution according to TNM classification (Table 2) (Sebag‐Montefiore 2009; van Gijn 2011); 26% and 31% of participants were TNM I in Sebag‐Montefiore 2009 and van Gijn 2011, respectively. Marsh 1994 did not report stage but included by protocol participants with "locally advanced", "fixed" disease. In the absence of contemporary imaging, 9% of the participants were found to be inoperable at laparotomy because of either extensive local or metastatic disease. Only approximately one‐half of the participants in each arm underwent curative surgery, and approximately one‐third received palliative operations.
Three studies provided subgroup analysis of outcomes based on pathological stage. Three trials analysed local recurrence according to pathological stage (Sebag‐Montefiore 2009; Swedish RCT 1997; van Gijn 2011). Two trials analysed overall mortality according to stage (Swedish RCT 1997; van Gijn 2011).
Excluded studies
We excluded 48 studies (constituting 76 records). We excluded 13 trials because both allocated groups received radiation therapy (Atif 2012; Bujko 2013; Dubois 2011; Francois 2014; Frykholm 2001; Gerard 2011Gérard 2012; Guckenberger 2012; Latkauskas 2012; Ngan 2012; Pettersson 2015; Rouanet 2006; Valentini 2008); six studies because the radiotherapy beam was extended beyond the pelvis (Cedermark 1995; Gerard 1988; Kligerman 1972; MRC 1996; Reis Neto 1989; You 1993); six studies because the BED was less than 30 Gy10 (Dahl 1990; Goldberg 1994; Higgins 1986; MRC 1984; Petersen 1998; Rider 1977); six studies were reviews or meta‐analyses (Camma 2000; CCCG 2001; Ceelen 2005; Figueredo 2003; Gunderson 2003; Zehra 2015); one study used postoperative radiotherapy in both arms (Kim 2011); and one study was a secondary analysis of a randomised trial of two types of surgical procedures, where preoperative radiotherapy was given at the surgeons' discretion (Parc 2009). Furthermore, four potentially eligible studies that were included in an individual patient data meta‐analysis, CCCG 2001, were not included in the present analysis either because they were not provided with sufficient data (Cummings 1985; Niebel 1988; Sause(RTOG81‐15)1994), or because during enrolment participants had evidence of distant metastases (Higgins 1975). We excluded the remaining 11 studies for various reasons, as stated in the Characteristics of excluded studies table (Bosset 2004; Boulis‐Wassif 1982; Boulis‐Wassif 1984; Bujko 2004; Erlandsson 2017; Frykholm 1993; Gerard 2004; Glehen 2003; Illenyi 1994; Kimura 1989; Stockholm 1996).
Risk of bias in included studies
The description of our 'Risk of bias' assessment for each study follows. Details can be found in the 'Risk of bias' tables in (Characteristics of included studies. Figure 2 displays our 'Risk of bias' judgements about each 'Risk of bias' item for each included study.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials reported details of the random sequence generation and were considered as at low risk of selection bias (Sebag‐Montefiore 2009; van Gijn 2011). The remaining trials did not report the method of randomisation and were judged as at unclear risk of bias (Marsh 1994; Swedish RCT 1997).
Three trials reported adequate allocation concealment (Sebag‐Montefiore 2009; Swedish RCT 1997; van Gijn 2011), and one trial did not clearly report the methods used to conceal allocation (Marsh 1994).
Blinding
Given the nature of the intervention, it was not possible to blind participants and personnel, thus we considered all trials to be at high risk of performance bias independent from the information provided. The outcome assessor was blinded in only two trials (Swedish RCT 1997; van Gijn 2011). The remaining two trials did not report information regarding blinding of the outcome assessor (Marsh 1994; Sebag‐Montefiore 2009). However, for objective outcomes such as mortality, the absence of blinding was not considered as a source of bias in the development of the 'Summary of findings' table (Table 1).
Incomplete outcome data
We considered all of the included trials to be at low risk of attrition bias.
Selective reporting
We identified fewer than 10 RCTs, which hindered the possibility of evaluating publication bias. In future updates we plan to use visual asymmetry on a funnel plot to explore reporting bias.
Effects of interventions
See: Table 1
1. Primary outcome
1.1 Overall mortality
See: Table 1
The proportion of mortality was 42.5% (987/2324) in the PRT group and 45.4% (1063/2339) in the control group. Moderate‐quality evidence suggests that PRT was associated with a reduced overall mortality hazard rate (Analysis 1.1: studies = 4; participants = 4663; Peto odds ratio (OR) 0.90, 95% confidence interval (CI) 0.83 to 0.98; P = 0.02; I2= 42%, P = 0.16; Figure 3). In absolute terms, this means that for every 1000 patients receiving radiotherapy, 45 fewer per 1000 more would die, but the true effect may lie between 77 fewer and 9 fewer.
1.1. Analysis.
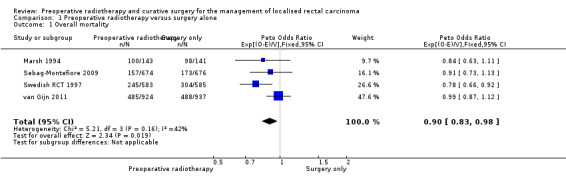
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 1 Overall mortality.
3.
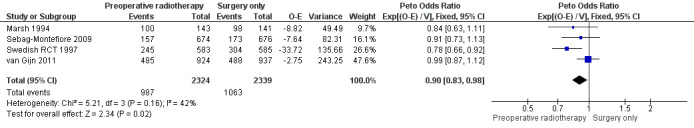
Forest plot of comparison: 1 Preoperative radiotherapy versus surgery alone, outcome: 1.1 Overall mortality.
We downgraded the evidence only for indirectness since the patient population treated in these trials might differ from the population treated at the present time, regarding more accurate methods of preoperative imaging, accurate staging for distant metastatic disease, use of TME, and use of chemotherapy.
Three of four trials reported an adequate method of allocation concealment, and as the outcome under consideration was objective, we did not take into account any potential performance bias or detection bias and therefore did not downgrade the evidence due to risk of bias. We found moderate heterogeneity (I2 = 42%), which could be explained by differences between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed, radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy. However, we judged heterogeneity as not serious because the confidence intervals showed substantial overlap, and the statistical test for heterogeneity was low (P = 0.16).
2. Secondary outcomes
See: Table 1
2.1 Cause‐specific mortality
Two trials reported cause‐specific mortality (Marsh 1994; van Gijn 2011). The proportion of mortality was 32.6% (348/1067) for the PRT group and 31.9% (383/1078) for the control group. Analysis revealed no evidence of a difference between the two interventions (Analysis 1.2: studies = 2, participants = 2145; Peto OR 0.89, 95% CI 0.77 to 1.03; I2 = 10%; low‐quality evidence; Figure 4). In absolute terms, for every 1000 patients receiving radiotherapy, 39 fewer per 1000 would die, but the true effect may lie between 82 fewer and 11 more.
1.2. Analysis.
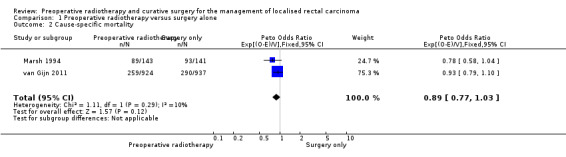
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 2 Cause‐specific mortality.
4.
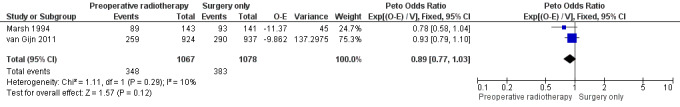
Forest plot of comparison: 1 Preoperative radiotherapy versus surgery alone, outcome: 1.2 Cause‐specific mortality.
2.2 Local recurrence
All trials reported local recurrence. The proportion of local recurrence was 6.7% (153/2294) in the PRT group and 16.1% (371/2311) in the control group.
Moderate‐quality evidence shows that PRT may be associated with reduced local recurrence compared to surgery alone (Analysis 1.3: studies = 4; participants = 4663; Peto OR 0.48, 95% CI 0.40 to 0.57; I2 = 51%, P = 0.10; Figure 5). In absolute terms, for every 1000 patients receiving radiotherapy, 83 fewer per 1000 more would have local recurrence, but the true effect may lie between 96 fewer and 69 fewer.
1.3. Analysis.
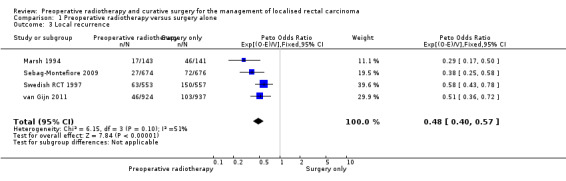
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 3 Local recurrence.
5.
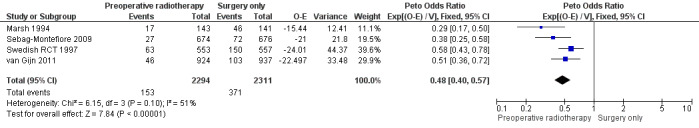
Forest plot of comparison: 1 Preoperative radiotherapy versus surgery alone, outcome: 1.3 Local recurrence.
2.3 Distant metastases
All trials reported distant metastases. The proportion of events was similar between the two groups: 19.6% (438/2235) in the control group and 20.7% (465/2250) in the PRT group (Analysis 1.4: studies = 4; participants = 4485; risk ratio (RR) 0.96, 95% CI 0.85 to 1.08; I2 = 8%; low‐quality evidence; Figure 6).
1.4. Analysis.
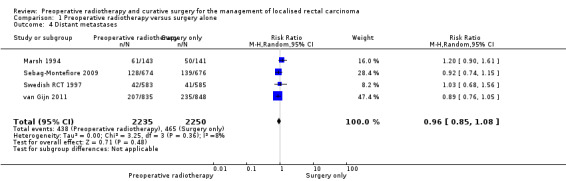
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 4 Distant metastases.
6.
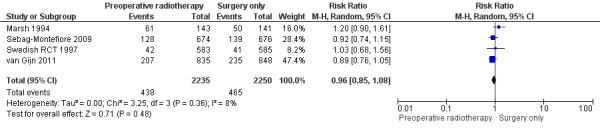
Forest plot of comparison: 1 Preoperative radiotherapy versus surgery alone, outcome: 1.4 Distant metastases.
2.4 Any recurrence
Only one trial reported the outcome any recurrence (van Gijn 2011). The proportions of the events were 20% (185/924) in the PRT group and 27% (253/937) in the control group. Low evidence suggests that compared to surgery alone, PRT reduces any recurrence (Analysis 1.5: studies = 1; participants = 1861; Peto OR 0.82, 95% CI 0.68 to 0.99).
1.5. Analysis.
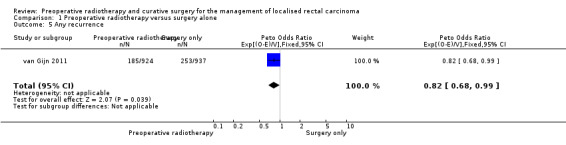
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 5 Any recurrence.
2.5 Curative resection and overall resectability
The proportion of curative resection was similar between the groups: 80.9% (1888/2335) in the PRT group and 80.9% (1891/2338) in the control group (Analysis 1.6: studies = 4; participants = 4673; RR 1.00, 95% CI 0.97 to 1.02; I2 = 0%; low‐quality evidence).
1.6. Analysis.
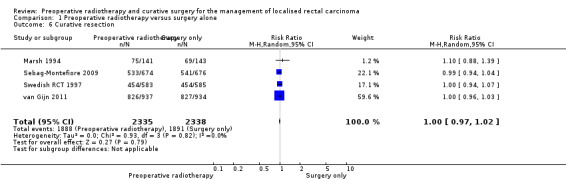
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 6 Curative resection.
Three trials reported on overall resectability (Marsh 1994; Sebag‐Montefiore 2009; Swedish RCT 1997). The proportion of the events was similar between the two groups: 88.9% (1243/1398) in the PRT group and 1260/1404 (89.7%) in the control group with no evidence of difference (Analysis 1.7: studies = 3; participants = 2802; RR 0.99, 95% CI 0.95 to 1.04; I2 = 59%; very low‐quality evidence).
1.7. Analysis.
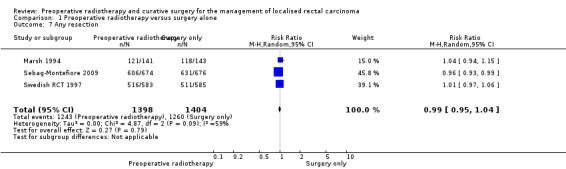
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 7 Any resection.
2.6 Sphincter preservation
Three trials reported sphincter‐sparing surgery (Sebag‐Montefiore 2009; Swedish RCT 1997; van Gijn 2011). The proportion of events was similar between the two groups, with no evidence of difference (Analysis 1.8: studies = 3; participants = 4379; RR 0.99, 95% CI 0.94 to 1.04; I2 = 0%; low‐quality evidence).
1.8. Analysis.
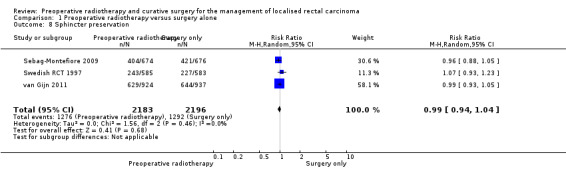
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 8 Sphincter preservation.
2.7 Postoperative morbidity
We did not grade postoperative morbidity, as each study presented data differently. We were able to group the available data by event, such as sepsis or infection and surgical complication.
Sepsis
Two studies reported infection‐related events (Swedish RCT 1997; van Gijn 2011). Low‐quality evidence suggests that infection or sepsis can be associated with preoperative radiotherapy (Analysis 1.9: studies = 2; participants = 2698; RR 1.25, 95% CI 1.04 to 1.52; I2 = 5%). In absolute terms, for every 1000 patients receiving radiotherapy, 32 more sepsis will occur with a true effect that may lie between 5 more and 67 more.
1.9. Analysis.
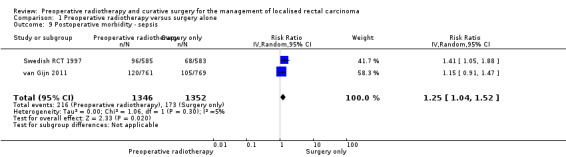
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 9 Postoperative morbidity ‐ sepsis.
Surgical complications within 30 days after surgery
Two studies reported surgical complications and were combinable in a meta‐analysis.
van Gijn 2011 provided data about postoperative complications, which included perineal wound healing, perforation, intestinal necrosis, fistula, stoma, bleeding, ileus, abdominal dehiscence, abdominoperineal resection or low anterior resection. The overall complication rate was higher in the PRT group (48%) than in the control group (41%); according to the authors, the difference was mainly attributable to the difference in perineal wound healing.
Swedish RCT 1997 provided data about surgical complications including anastomotic dehiscence, wound rupture, ileus, and others.
After excluding infection or sepsis, we attempted to pool the data regarding postoperative surgical complications. Low‐quality evidence showed that PRT can be associated with surgical complication (Analysis 1.10: studies = 2; participants = 2698; RR 1.20, 95% CI 1.01 to 1.42; I2 = 46%). In absolute terms, for every 1000 patients receiving radiotherapy, 50 more surgical complications will occur with a true effect that may lie between 2 more and 104 more.
1.10. Analysis.
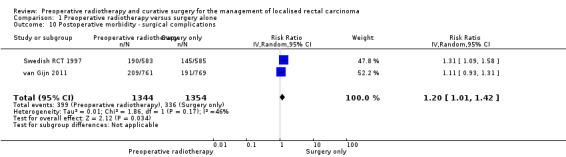
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 10 Postoperative morbidity ‐ surgical complications.
A third study reported subgroup analysis on surgical complication (Sebag‐Montefiore 2009). The trials reported that in participants who had an anterior resection, the clinical anastomotic leak rates at one month were similar in both groups (preoperative radiotherapy 9% (32/338); selective postoperative chemoradiotherapy 7% (26/370)), whereas in those who had an abdominoperineal excision, participants in the preoperative group had higher rates of a non‐healing perineum than those in the control group (70/202 (35%) versus 44/202 (22%), respectively). At 12 and 24 months' follow‐up, in participants with an abdominoperineal excision, the authors reported that rates of small bowel obstruction, perineal wound failure to heal, and lumbar or sacral neuropathy did not differ between the two treatment groups. However, data were not reported.
2.8 Postoperative mortality
Two studies reported postoperative mortality (Sebag‐Montefiore 2009; Swedish RCT 1997), and there was no evidence in favour of one of the interventions (Analysis 1.11: studies = 2; participants = 1960; RR 0.75, 95% CI 0.46 to 1.22; low‐quality evidence).
1.11. Analysis.
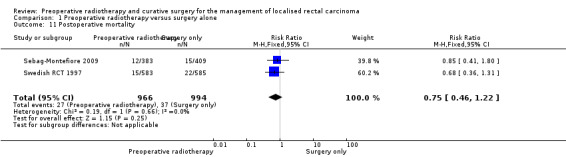
Comparison 1 Preoperative radiotherapy versus surgery alone, Outcome 11 Postoperative mortality.
2.9 Acute radiotherapy toxicity
Only one study reported acute radiotherapy side effects (van Gijn 2011): grade 1 toxicity occurred in 19% (145/761) of participants, grade 2 and 3 occurred in 7% (53/761), whereas no participants developed grade 4 or 5 side effects. The most commonly reported side effect was diarrhoea (n = 256) followed by dermatitis (n = 59), neurological symptoms (n = 35), cystitis (n = 27), and thromboembolic events (n = 2).
2.10 Late toxicities
No study evaluated late toxicity. However, Swedish RCT 1997 provided data on long‐term rectal function based on subgroup of participants. A questionnaire was sent to 220 treated participants, and a response was obtained from 92% (n=203) of participants who were alive after a minimum of five years. Thirty‐two participants were excluded, mainly because of postoperative stomas and dementia, which left 171 for analysis.
Compared to open surgery alone, after PRT there were more participants with increased stool frequency (20% (17/84) versus 8% (7/87); RR 2.52, 95% CI 1.1 to 5.75) and continence problems (50% (42/84) versus 24% (21/87); RR 2.07, 95% CI 1.35 to 3.18). The rates of tenesmus were similar between the two groups (27% (23/84) versus 33% (29/97); RR 0.82, 95% CI 0.52 to 1.30).
2.11 Quality of life
Two studies reported on quality of life (Sebag‐Montefiore 2009; van Gijn 2011).
Sebag‐Montefiore 2009 administered to all 1350 enrolled participants the Medical Outcomes Study Short‐Form 36‐item (MOS SF‐36) and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Colorectal 38‐item (EORTC QLQ‐CR38) questionnaires at baseline (before random assignment), every 3 months for 1 year, and then every 6 months until 3 years from random assignment (Stephens 2010). At six months' follow‐up, male sexual dysfunction was significantly increased following surgery in the group that received PRT (P < 0.001). No major changes between treatments or time points in terms of general health or bowel function were observed, but exploratory analysis indicated a significant increase in the level of faecal incontinence with PRT (53.2% versus 37.3%; P = 0.007 at 2 years) (Stephens 2010).
The van Gijn 2011 trial compared health‐related quality of life and sexual function between the treatment arms (Marijnen 2005). Analysis was based on 990 eligible participants. Health‐related quality of life (as measured by the Rotterdam Symptom Checklist) improved over time but did not differ significantly between the treatment arms except for on the activity scale. Similarly, there was no treatment effect in the defecation scale. However, sexual function was significantly worse for both males and females. The economic impact of rectal cancer and the effect of preoperative radiotherapy were reported for the same study (van den Brink 2005). Of the 292 eligible participants who had paid labour before treatment (total study sample 1530), only 61% resumed work at 24 months. Irradiated participants tended to resume work later than non‐irradiated participants between 6 and 12 months, although there was no difference after 18 months (van den Brink 2005).
In a subsequent evaluation, van Gijn 2011 assessed bowel function 14 years after PRT and TME. A questionnaire was sent to the surviving participants (n = 583) in 2012, and 242 non‐stoma participants were included in the analysis. The questionnaires included the Low Anterior Resection Syndrome Score (LARS score), European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core (EORTC QLQ‐C30) and Colorectal Module (EORTC QLQ‐CR29). The LARS score range was divided into "no LARS", "minor LARS", and "major LARS" categories in ascending severity of bowel dysfunction (Chen 2015). Major bowel dysfunction was reported by 56% of the participants allocated to the PRT + TME group compared to 35% of the participants that received TME alone (P = 0.01).
3. Subgroup analyses
3.1 Overall mortality according to risk of bias (adequate versus unclear/inadequate allocation concealment)
When we considered the only study with unclear allocation concealment, there was no evidence of difference (Peto OR 0.84, 95% CI 0.63 to 1.11) compared to the pooled analysis of the studies at low risk of selection bias (Analysis 2.1.1: studies = 3; participants = 4379; Peto OR 0.91, 95% CI 0.83 to 1.00). No change in heterogeneity was observed (I2 = 59%) within the studies with adequate allocation concealment, and the test for subgroup difference was not statistically significant (P = 0.58).
2.1. Analysis.
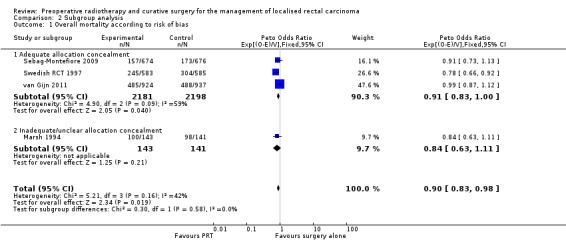
Comparison 2 Subgroup analysis, Outcome 1 Overall mortality according to risk of bias.
3.2 Overall mortality according to stage
We performed subgroup analysis for overall mortality according to stage. Hence we attempted to pool the data by combining Dukes A/B stage with TNM I/II stages and Dukes C with TNM stage III, without success.
Swedish RCT 1997 reported no difference between PRT and surgery alone in terms of overall survival at five years across all the stage groups (Analysis 2.2). In a subsequent publication (Folkesson 2005), an analysis limited to curatively treated participants (908/1168) at a median follow‐up of 13 years showed no survival benefit was observed across all the stages.
2.2. Analysis.
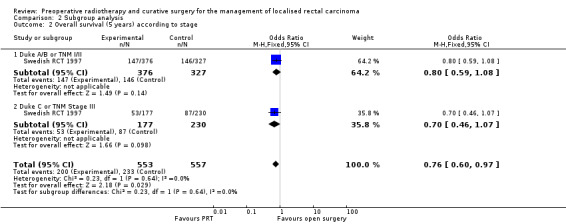
Comparison 2 Subgroup analysis, Outcome 2 Overall survival (5 years) according to stage.
van Gijn 2011 reported 10 years' follow‐up data for TNM I to III for all eligible participants and for participants with negative circumferential margin. Irrespective of the status of circumferential resection margin, radiotherapy was not associated with an increase in overall survival (Analysis 2.3). When analysis was restricted to participants with negative circumferential resection margin, 10‐year overall survival was better in the radiotherapy group than in the controls within the TNM III subgroup (45% (101/210) in the PRT group and 37% (84/225) in the surgery‐alone group; Peto OR 0.76, 95% CI 0.59 to 0.98; Analysis 2.6).
2.3. Analysis.
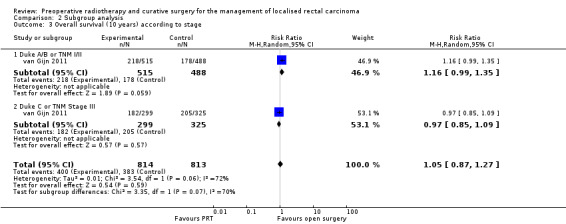
Comparison 2 Subgroup analysis, Outcome 3 Overall survival (10 years) according to stage.
2.6. Analysis.
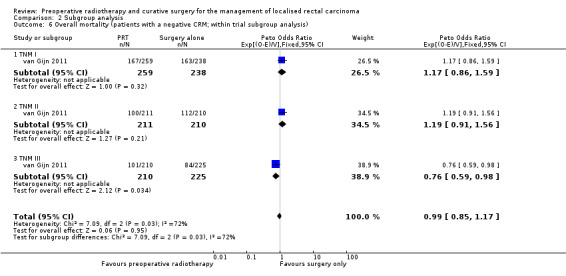
Comparison 2 Subgroup analysis, Outcome 6 Overall mortality (patients with a negative CRM; within trial subgroup analysis).
3.3 Overall mortality according to TME
Two trials were conducted before the TME era, and therefore the majority of patients would not have undergone TME (Marsh 1994; Swedish RCT 1997). All participants were to undergo TME in van Gijn 2011 according to protocol. Although TME was not mandated in Sebag‐Montefiore 2009, due to its widespread adoption at the time, 92% of the participants had TME, and for that reason we considered this trial as a TME trial for the subgroup analysis.
In the trials where TME was not performed, overall survival was significantly reduced with PRT (Analysis 2.4.1: studies = 2; participants = 1452; Peto OR 0.79, 95% CI 0.69 to 0.92). Conversely, in the trials where participants underwent TME, there was no effect in favour of one the two treatment groups under investigation (Analysis 2.4.2: studies = 2; participants = 3211; Peto OR 0.97, 95% CI 0.87 to 1.08). The test for subgroup difference was statistically significant (P = 0.03) (Analysis 2.4).
2.4. Analysis.
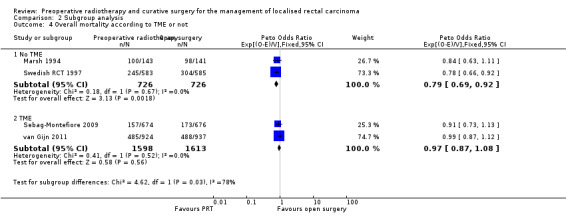
Comparison 2 Subgroup analysis, Outcome 4 Overall mortality according to TME or not.
3.4 Local recurrence according to risk of bias (adequate versus unclear/inadequate allocation concealment)
There was no evidence of subgroup difference in the treatment effect between the trial where the allocation concealment was unclear (studies = 1; participants = 284; Peto OR 0.29, 95% CI 0.17 to 0.50) and the trials with adequate allocation concealment (studies = 3; participants = 4321; Peto OR 0.51, 95% CI 0.42 to 0.62) (Analysis 2.5).
2.5. Analysis.
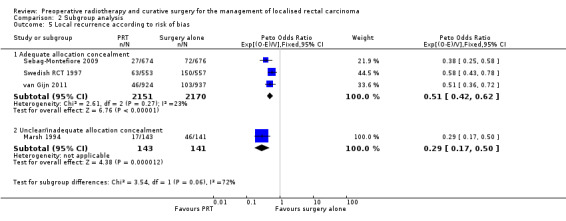
Comparison 2 Subgroup analysis, Outcome 5 Local recurrence according to risk of bias.
3.5 Local recurrence according to stage
Based on stage, we attempted to pool the data by combining Dukes A/B stage with TNM I/II stages and Dukes C with TNM stage III.
Swedish RCT 1997 calculated local recurrence according to stage. At five‐year follow‐up, local recurrence was lower in the PRT group both at higher (studies = 1; participants = 407; RR 0.49, 95% CI 0.35 to 0.68) or lower stages (studies = 1; participants = 1003; RR 1.16, 95% CI 0.99 to 1.35). The test for subgroup difference was statistically significant (P < 0.001) (Analysis 2.7). These favourable results remained constant at 10 years' follow‐up for lower stages (studies = 2; participants = 1710; RR 0.46, 95% CI 0.27 to 0.76) and higher stages (studies = 2; participants = 1132; RR 0.48, 95% CI 0.35 to 0.67; Analysis 2.8).
2.7. Analysis.
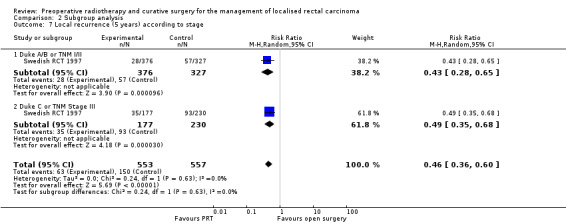
Comparison 2 Subgroup analysis, Outcome 7 Local recurrence (5 years) according to stage.
2.8. Analysis.
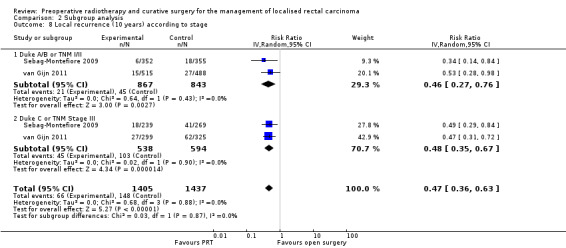
Comparison 2 Subgroup analysis, Outcome 8 Local recurrence (10 years) according to stage.
3.6 Local recurrence according to distance of the tumour from the anal verge
Three studies evaluated the relationship between the distance of the tumour from the anal verge and the effect of radiotherapy on local recurrence (Sebag‐Montefiore 2009; Swedish RCT 1997; van Gijn 2011). Data were presented in different ways, therefore it was not possible to perform meta‐analyses. In a secondary analysis of participants that received curative surgery (based on the absence of distant metastases and R0 surgery) (Folkesson 2005), Swedish RCT 1997 reported a lower recurrence rate at < = 5 cm and 6 to 10 cm from anal verge in favour of PRT, but not for tumours originating from greater than 10 from the anal verge (Analysis 2.9).
2.9. Analysis.
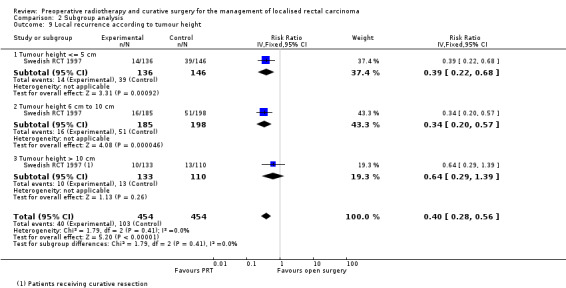
Comparison 2 Subgroup analysis, Outcome 9 Local recurrence according to tumour height.
In Sebag‐Montefiore 2009, three‐year local recurrence rate was significantly lower in the PRT group across all the tumour height groups, and the effect of radiotherapy became stronger as the distance from the anal verge increased (<= 5 cm Peto OR 0.45 (95% CI 0.23 to 0.88); 6 cm to 10 cm, Peto OR 0.50 (95% CI 0.28 to 0.90); > 10 cm, Peto OR 0.19 (95% CI 0.07 to 0.47)).
Similarly, in van Gijn 2011 the benefit of radiotherapy was significant as the distance from the anal verge increased with a significant distance‐by‐treatment interaction. However, when participants with a positive circumferential resection margin were excluded from the analyses, the relationship between distance from the anal verge and the effect of radiotherapy disappeared. Details were not provided in the article (van Gijn 2011).
4. Sensitivity analyses
4.1 Restricting analysis to studies with adequate allocation concealment
When we restricted the analysis to the studies with adequate allocation concealment (Sebag‐Montefiore 2009; Swedish RCT 1997; van Gijn 2011), the results for overall mortality showed a slight change in the effect estimate as the extreme value of the confidence interval null value of 1.00, but the direction or magnitude of the effect estimate remained substantially the same compared with the original analysis (Analysis 2.1: studies = 3; participants = 4379; Peto OR 0.91, 95% CI 0.83 to 1.00).
For the outcome local recurrence, exclusion of the study with unclear allocation concealment, Marsh 1994, did not affect the results, as no substantial change in the direction or magnitude of the effect estimate compared with the original analysis was observed. (Analysis 2.5).
See Table 6 for comparison.
5. Sensitivity analyses.
| Sensitivity analyses for overall mortality and local recurrence | Original analysis (effect estimate (95% CI)) | Sensitivity analysis (effect estimate (95% CI)) |
| Restricting analysis to studies with adequate allocation concealment (outcome: overall mortality) | Peto OR 0.90 (0.83 to 0.98) | Peto OR 0.91 (0.83 to 1.00) |
| Restricting analysis to studies with adequate allocation concealment (outcome: local recurrence) | Peto OR 0.48 (0.40 to 0.57) | Peto OR 0.51 (0.42 to 0.62) |
| Restrcting analysis to studies that used TME surgery (outcome: overall mortality) | Peto OR 0.90 (0.83 to 0.98) | Peto OR 0.97 (0.87 to 1.08) |
| Restricting analysis to studies that used TME surgery (outcome: local recurrence) | Peto OR 0.48 (0.40 to 0.57) | Peto OR 0.46 (0.35 to 0.59) |
CI: confidence interval; OR: odds ratio; TME: total mesorectal excision
4.2 Restricting analysis to studies that used TME surgery
When we restricted the analysis to the studies that used TME surgery (Sebag‐Montefiore 2009; van Gijn 2011), there was no evidence of a difference between PRT and open surgery alone in terms of overall mortality (Analysis 2.4: participants = 3211; Peto OR 0.97, 95% CI 0.87 to 1.08). However, for the outcome local recurrence, exclusion of the no‐TME studies did not affect the results, as no substantial change in the direction or magnitude of the effect estimate compared with the original analysis was observed.
See Table 6 for comparison.
Discussion
Although the protocol for this review, Wong 2000, intended to consider only preoperative radiotherapy, the previous version of this review, Wong 2007, considered the inclusion of trials that dealt with adjuvant or neoadjuvant strategies in the control group. Regarding the comparison between PRT and surgery alone, the review included 19 trials and concluded that overall mortality was marginally improved (Peto OR 0.93, 95% CI to 0.87 to 1) and local recurrence was improved, but the magnitude of benefit was heterogeneous across trials. The review also noted that sensitivity analysis showed benefits of PRT in participants treated with BED > 30 Gy10 and multiple‐field radiotherapy techniques.
In the current version of the review we only considered trials that evaluated PRT versus surgery alone, as was stated in the original protocol. However, the protocol was published 16 years ago, and the characteristic of radiotherapy has changed during this period. We therefore did not consider the trials with mega‐voltage of at least 30 Gy and two‐fields techniques in the present update. There remained a total of four trials for inclusion, and with respect to the previous version of the review, regarding PRT and surgery alone, the present review includes the final results of the MRC CR07 and NCIC‐CTG C016 trial (Sebag‐Montefiore 2009), which enrolled 1350 participants, and the updated results of the Dutch trial on 1861 participants that reported results at a follow‐up of 11.6 years (van Gijn 2011). In conclusion, the evidence from the present review concerns solely short‐course radiotherapy, limiting its applicability to people with resectable disease. Individuals with borderline unresectable or unresectable disease may benefit from long‐course radiotherapy, and this review is unable to contribute any evidence to support or refute that.
Summary of main results
The overall evidence was of moderate quality and sufficient to conclude that modern radiotherapy reduces local recurrence in people with locally advanced rectal cancer. Subgroup analysis indicated that this improvement persisted in participants that received TME. Whereas there was an improvement in overall survival in favour of PRT treatment, this advantage disappeared when analysis was limited to the trials that used TME. Mortality and local recurrence were not completely reported according to stage, and no conclusion can be formulated based on stage.
Overall completeness and applicability of evidence
Despite there having been significant advances in tumour staging, radiotherapy delivery, and surgical techniques as well as trial design (van de Velde 2013; van de Velde 2014), none of the included studies used contemporary staging with CT chest/abdomen MRI pelvis for staging, limiting the applicability of the evidence to current practice. In particular in the Marsh 1994 trial, 50% of participants did not undergo curative resection because of understaging without contemporary imaging. In addition, since the Marsh 1994 trial was also designed to evaluate PRT in participants with locally advanced carcinomas of the rectum located within 13 cm of the anal verge, short‐course radiation treatment was probably inadequate.
It is generally accepted that patients with stage I disease should not be given any treatment in addition to surgery because the local recurrence rate is low and the benefit from neoadjuvant treatment very small. In addition, most would accept that patients with locally advanced disease benefit from additional treatment, whereas the benefit for patients with stage II disease is less clear (Brenner 2014). We were unable to obtain sufficient data from the trials to perform subgroup analysis and are unable to conclude which patient would benefit from neoadjuvant PRT treatment.
We did not compare the more commonly used fractionation regimens in current practice, short‐course radiotherapy (SCRT) versus long‐course chemoradiotherapy (LCRT + CT), as our review was limited to studies using radiotherapy alone. Two randomised trials have compared SCRT with LCRT, both finding no significant difference in outcomes except for pathologic complete response and down‐staging rates, which were significantly higher in the LCRT + CT group (Bujko 2004; Bujko 2006; Ngan 2012). Moreover, Bujko and colleagues found that LCRT + CT was associated with higher rates of acute toxicity. An ongoing German study, Siegel 2009, is designed to compare the same schedules, and expects to enrol 760 participants with T2, node‐positive, or T3 disease. The Stockholm III trial is comparing LCRT + CT with delayed surgery eight weeks later, SCRT with immediate surgery, and SCRT followed by surgery eight weeks later. Early analysis showed that SCRT with immediate surgery, but 10 days or more after the start of radiotherapy, was significantly associated with higher postoperative complication rates, while SCRT followed by delayed surgery was feasible and had a down‐staging effect (Pettersson 2015). Other efficacy outcomes will be reported with longer follow‐up.
The present update shows also that grade 1 toxicity occurred in 19% and grade 2 and 3 occurred in 7% (53/761) of participants who received PRT. In addition, PRT may be associated with an increased risk of sepsis or postoperative perineal/pelvic infection, surgical complications, as well as sexual dysfunction. However, not all the studies reported side effects uniformly, and improvements in the quality and consistency of reporting of acute and late toxicity and quality of life are needed.
The issue of the type of surgery in relation to the applicability of the evidence from the present review is important. Despite concerns regarding functional outcomes such as sexual and urinary dysfunction, TME has significantly improved oncologic outcomes in terms of local recurrence and cancer‐specific survival (Heald 1982), and consequently has become a standard surgical approach for colorectal cancer removal worldwide (Stewart 2007). In our review, 3076 participants received TME surgery (Sebag‐Montefiore 2009; van Gijn 2011), amounting to 66% of the entire population included in the four trials.
The present review excluded by protocol trials that assessed the efficacy of adding chemotherapy to PRT compared to radiotherapy alone Chemotherapy, usually consisting of the cytotoxic agent 5‐fluorouracil, may accomplish more tumour cell killing than PRT alone, thereby improving resectability, reducing local recurrence, and improving sphincter preservation. In addition, it may act systemically, thereby reducing the risk of distant metastases and improving overall survival. Another Cochrane Review addressed the issue of preoperative chemo‐radiation for people with colorectal cancer (De Caluwe 2013). Several trials have demonstrated that chemoradiotherapy provides better local control than the same radiotherapy alone (Bosset 2006; Braendengen 2008; Gerard 2006; Glimelius 2008). The European Society for Medical Oncology (ESMO) Clinical Practice Guidelines recommend preoperative chemotherapy if judged necessary after pathological evaluation (Glimelius 2013). The National Comprehensive Cancer Network (NCCN) Guidelines recommend combined modality with addition of chemotherapy for most patients with stage II or III rectal cancer (Benson 2015). In conclusion, the applicability of the current review to modern practice is limited further because chemotherapy was not routinely given in all trials except in Sebag‐Montefiore 2009.
Quality of the evidence
We evaluated the quality of the evidence using the GRADE approach, which also considers the 'Risk of bias' tool. In terms of risk of selection bias, only one trial was at unclear risk of bias for allocation concealment. Overall, this trial represented 6% of the entire population included in the review, and potential selection bias was not taken into account. As blinding of participants and personnel was impossible, we considered all of the included studies as at high risk of performance bias, but took no further action to downgrade the evidence, in particular for the objective outcomes (mortality, local recurrence, distant metastases, any recurrence, curative resection). In addition, three of the four trials did not report whether the outcome assessor was blinded, but we did not take any action to downgrade the evidence because of detection bias for objective outcomes.
Heterogeneity for overall mortality and local recurrence was moderate, and this could be explained by differences between the trials regarding the criteria used to define rectal cancer, the stage of participants, preoperative imaging used for assessing stage, surgery performed, radiotherapy delivered (including dose and fractionation), the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy. However, we judged heterogeneity as not serious because the confidence intervals showed substantial overlap, and the statistical test for heterogeneity was not significant for both analyses. We therefore decided not to downgrade the evidence because of inconsistency.
We found serious concerns regarding applicability since the patient population treated in these trials might differ from the population treated in the present day, as the latter may receive more accurate methods of preoperative imaging, accurate staging for distant metastatic disease, use of TME, and use of chemotherapy. Hence, we downgraded the evidence due to indirectness.
Consequently, we judged the evidence for overall mortality and local recurrence to be of moderate quality, and further downgraded the quality of the evidence for the remaining outcomes to low either because of imprecision, indirectness, or risk of bias (Table 5).
Potential biases in the review process
The review was comprehensive in terms of the search strategies adopted and the electronic databases searched. In addition, we screened the reference lists of reviews and contacted trial authors for clarification. Two review authors independently carried out screening of titles and abstracts, full‐text assessment of potentially relevant studies, and data extraction. One review author performed analyses, which a second review author checked. We were unable to obtain the full text for three studies, Boulis‐Wassif 1979; Kimura 1989; Illenyi 1994, that were evaluated in the previous version of the review (Wong 2007), however it should be acknowledged that all of these studies used older radiotherapy techniques, and their absence should not affect the applicability of the review.
Agreements and disagreements with other studies or reviews
We identified several systematic reviews on this topic in the medical literature (Camma 2000; CCCG 2001; Glimelius 2003; Figueredo 2003; Ooi 1999; Twomey 1989; Viani 2011). The studies included in these reviews varied with regard to focus, prespecified inclusion and exclusion criteria, search strategy, and the time frame the search was conducted.
Two reviews evaluated PRT versus surgery (CCCG 2001; Viani 2011).
The first review was from The Colorectal Cancer Collaborative Group, who were able to perform an individual patient data meta‐analysis using 22 randomised comparisons between preoperative radiotherapy and no radiotherapy for rectal cancer (6350 participants in 14 trials) (CCCG 2001). The authors of this review were able to obtain hazard ratios and variance from the primary investigators for this individual patient data meta‐analysis, and included studies that we excluded from our assessment with reasons (Cummings 1982; Niebel 1988; Sause(RTOG81‐15)1994). The magnitude and the direction of the effect for the outcome overall survival was similar with that of the present review.
Viani 2011 identified 20 primary studies including the trials we identified except for Sebag‐Montefiore 2009. The authors performed a meta‐regression analysis and concluded that PRT with a BED of > 30 Gy10 is more efficient in reducing local recurrence and mortality rates than a BED of less than 30 Gy10, independent of the schedule of fractionation used. Their results were similar to our conclusion.
Preoperative radiotherapy in current clinical guidelines
In order to define the extent of surgery and the requirement for neoadjuvant therapy, clinical guidelines classify rectal cancer into four groups: very early (some cT1), early (cT1‐2, some cT3), intermediate (most cT3, some cT4), and locally advanced (some cT3, most cT4) (Glimelius 2013). For therapeutic decision, the guidelines take into account other factors such tumour height, closeness to the mesorectal fascia, as well as nodal (cN) stage and vascular and nerve invasion.
In very early rectal cancer cases, guidelines consider the transanal endoscopic microsurgery technique to be sufficient. In early‐stage cases (cT1‐2, some cT3), TME technique‐based surgery is considered appropriate, while the addition of PRT is not suggested, as it is considered to be overtreatment (Valentini 2009). However, in very low‐located tumours (especially located anterior), PRT may be indicated, since the distance to the mesorectal fascia is very small.
In intermediate cases, PRT is recommended for most cT3 (cT3(b)c+ without threatened or involved mesorectal fascia (mrf‐) according to MRI. Chemotherapy with 5‐fluorouracil (bolus, continuous infusion, or oral) added to PRT to 46 to 50.4 Gy, 1.8 to 2.0 Gy/fraction is considered an alternative or is suggested in low‐located rectal cancers.
In locally advanced cases (cT3 mrf+, cT4 with overgrowth to other organs (cT4b)), preoperative chemoradiotherapy, 50.4 Gy, 1.8 Gy/fraction with concomitant 5‐fluorouracil‐based therapy, is recommended followed by radical surgery six to eight weeks later (Glimelius 2013).
Authors' conclusions
Implications for practice.
We found moderate‐quality evidence that preoperative radiotherapy (PRT) reduces overall mortality. Subgroup analysis did not confirm this effect in people undergoing TME surgery. We found consistent evidence that PRT reduces local recurrence. Risk of sepsis and postsurgical complications may be higher with PRT. We downgraded the level of evidence for overall mortality and local recurrence due to indirectness. We further downgraded cause‐specific mortality, curative resection, and sphincter‐sparing surgery due to wide confidence intervals. We downgraded postoperative morbidity due to risk of bias and indirectness.
The differences between the trials regarding the criteria used to define rectal cancer, staging, radiotherapy delivered, the time between radiotherapy and surgery, and the use of adjuvant or postoperative therapy did not appear to influence the size of effect across the studies.
Implications for research.
Future trials should take into account the following issues.
Which patients benefit from PRT: Generally guidelines do not recommend neoadjuvant therapy for patients with stage I disease, given that the rate of local recurrence is low and the benefit of adjuvant chemoradiation therapy is very small. While the benefit of neoadjuvant therapy is very clear for stage III disease, its benefit for stage II patients is less clear, and further investigation is needed.
Short‐ versus long‐course radiotherapy: The question of whether PRT is best given as a short‐course (5 Gy × 5) schedule or as long‐course conventionally fractionated radiotherapy (1.8 to 2.0 Gy × 25 to 28) remains. Two trials that evaluated preoperative short‐course radiotherapy with preoperative conventionally fractionated chemoradiation therapy did not find any differences in local recurrence, disease‐free survival, and overall survival (Bujko 2013; Ngan 2012). A German study designed to compare the same schedules and expecting to enrol 760 participants with T2, node‐positive, or T3 disease is still ongoing (Siegel 2009). The Stockholm III trial randomised 845 participants to either short‐course with immediate surgery, short‐course with delayed (four to eight weeks) surgery, and 2 Gy × 25 with delayed surgery. Interim analyses based on 462 participants showed that participants randomised to short‐course radiotherapy with delayed surgery had a higher rate of pathological complete responses (Pettersson 2015). These results need further confirmation.
Additional chemotherapy or intensified chemotherapy: Although chemoradiotherapy treatment improves local control, it should be recognised that the advantage from the addition of chemotherapy is obtained at a price of increased acute toxicity (Fiorica 2010). 5‐fluorouracil is the drug most utilised to sensitise radiation treatment. Combinations of 5‐fluorouracil with oxaliplatin, irinotecan, or other targeted drugs have been extensively experimented. Several large randomised trials have failed to show any benefit from the addition of oxaliplatin (Aschele 2011; Gerard 2006). The addition of cetuximab to capecitabine‐based chemotherapy in a phase II study did not improve complete response rate, the primary endpoint, but an improvement in overall survival in the KRAS wild‐type population was observed. These results need to be confirmed.
The best timing between radiotherapy and surgery: A trial showed that a longer interval between neoadjuvant radiation and surgery was associated with improved tumour clinical response and pathologic down‐staging, however without determining a precise timing after radiotherapy (Francois 1999). A recent observational study based on an observational National Cancer Data Base ‐ which collects information on approximately 70% of newly diagnosed cancer cases in the United States and Puerto Rico from more than 1,500 cancer centers.‐ reported that eight weeks may be the optimum time for surgery after chemoradiotherapy for best pathologic down‐staging and successful surgical outcome as determined from margin positivity (Sun 2016). However, these results need to be confirmed based on randomised trials on relevant outcomes such as local recurrence and mortality.
Benefit of adjuvant chemotherapy: The issue of the benefit of adjuvant chemotherapy has been partly addressed by a Cochrane Review (Petersen 2012). The review included 21 trials with 9221 participants and concluded that a significant advantage was observed in favour of adjuvant chemotherapy concerning disease‐free and overall survival in people with rectal cancer operated for cure. However, the review was not able to define which patients benefit most based on the Tumour Node Metastases (TNM) stage. In addition, it must be emphasised that the included participants were treated over several decades, during which time both surgery and the use of additional (chemo)radiotherapy have evolved considerably (Glimelius 2013). Hence, the advantages of adjuvant chemotherapy within the multimodal treatment of rectal cancer need to be clarified.
Relevant outcomes for future trials should include anastomotic leak rate, quality of life, and radiotherapy toxicity.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2018 | New search has been performed | New and up‐to‐date version of the updated review |
| 4 June 2018 | New citation required and conclusions have changed | Since the original review was published in 2007, advances has been made regarding the techniques used to deliver radiotherapy. When the original review was performed, many trials used old techniques that are not justified in contemporary clinical practice. In this update we have modified the inclusion criteria and excluded trials that used low‐energy radiotherapy, two‐field approaches with AP‐PA fields, and very large fields (pelvic plus para‐aortic). |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 3 April 2015 | Amended | Updated |
| 22 April 2014 | New search has been performed | New searches performed. One new trial included in meta‐analysis, and data from one trial updated in the meta‐analyses. |
| 29 December 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the Editors and the Cochrane Colorectal Cancer Group editorial office for their patience and assistance in the development of this complex review. We would like to thank Dr Tiffany Daly for her helpful comments and suggestions.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
The Cochrane Library (Issue 1, January 2017)
#1 MeSH descriptor Radiotherapy explode all trees
#2 (radiotherap*):ti,ab,kw
#3 (#1 OR #2)
#4 MeSH descriptor Colorectal Surgery explode all trees
#5 (surger*):ti,ab,kw
#6 MeSH descriptor Neoadjuvant Therapy explode all trees
#7 MeSH descriptor Combined Modality Therapy explode all trees
#8 MeSH descriptor Preoperative Care explode all trees
#9 (neoadjuvant* or adjuvant*) near3 (therap*):ti,ab,kw
#10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Rectal Neoplasms explode all trees
#12 ((rect* or anal* or anus*) near3 (carcinom* or neoplas* or adenocarcinom* or cancer* tumor*
or tumour* or sarcom*)):ti,ab,kw
#13 (#11 OR #12)
#14 (#3 AND #10 AND #13)
Appendix 2. MEDLINE search strategy
MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present)
1. exp Radiotherapy/
2. radiotherap*.mp.
3. 1 or 2
4. exp Colorectal Surgery/
5. surger*.mp.
6. exp Neoadjuvant Therapy/
7. exp Combined Modality Therapy/
8. exp Preoperative Care/
10. 4 or 5 or 6 or 7 or 8 or 9
11. exp Rectal Neoplasms/
12. ((rect* or anal* or anus*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*)).mp.
13. 11 or 12
14. 3 and 10 and 13
15. randomized controlled trial.pt.
16. controlled clinical trial.pt.
17. randomized.ab.
18. placebo.ab.
19. clinical trials as topic.sh.
20. randomly.ab.
21. trial.ti.
22. or/15‐21
23. exp animals/ not humans.sh.
24. 22 not 23
25. 14 and 24
Appendix 3. Embase search strategy
Embase Ovid (1974 to 2017 Week 03)
1. exp preoperative radiotherapy/
2. radiotherap*.mp.
3. 1 or 2
4. exp colorectal surgery/
5. surger*.mp.
6. exp cancer adjuvant therapy/
7. exp multimodality cancer therapy/
8. exp preoperative care/
9. ((neoadjuvant* or adjuvant*) adj3 therap*).mp.
10. 4 or 5 or 6 or 7 or 8 or 9
11. exp rectum tumor/
12. ((rect* or anal* or anus*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom*)).mp.
13. 11 or 12
14. 3 and 10 and 13
15. CROSSOVER PROCEDURE.sh.
16. DOUBLE‐BLIND PROCEDURE.sh.
17. SINGLE‐BLIND PROCEDURE.sh.
18. (crossover* or cross over*).ti,ab.
19. placebo*.ti,ab.
20. (doubl* adj blind*).ti,ab.
21. allocat*.ti,ab.
22. trial.ti.
23. RANDOMIZED CONTROLLED TRIAL.sh.
24. random*.ti,ab.
25. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
27. 25 not 26
28. (prostate* or head* or neck* or breast* or endometrial* or hepato* or ovarian* or pelvic* or cervix* or cervical* or liver* or bone* or hodgkin* or lung* or brain* or pancreatic* or nasopharyn*).m_titl.
29. 27 not 28
30. 14 and 29
Appendix 4. 'Risk of bias' assessment
Risk of bias in randomised trials
Extracted from the Cochrane Handbook for Systematic Reviews of Interventions (handbook.cochrane.org)
Table 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘low risk’ of bias | The investigators describe a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘high risk’ of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of low or high risk |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | |
| Criteria for a judgement of ‘low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘high risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, such as if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature, or handling of incomplete outcome data | |
| Criteria for a judgement of ‘low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Any one of the following:
|
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘low risk’ of bias | Any of the following:
|
| Criteria for the judgement of ‘high risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘unclear risk’ of bias | Insufficient information to permit judgement of low or high risk. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table | |
| Criteria for a judgement of ‘low risk’ of bias | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘high risk’ of bias | There is at least one important risk of bias. For example, the study:
|
| Criteria for the judgement of ‘unclear risk’ of bias | There may be a risk of bias, but there is either:
|
Data and analyses
Comparison 1. Preoperative radiotherapy versus surgery alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality | 4 | 4663 | Peto Odds Ratio (95% CI) | 0.90 [0.83, 0.98] |
| 2 Cause‐specific mortality | 2 | 2145 | Peto Odds Ratio (95% CI) | 0.89 [0.77, 1.03] |
| 3 Local recurrence | 4 | 4605 | Peto Odds Ratio (95% CI) | 0.48 [0.40, 0.57] |
| 4 Distant metastases | 4 | 4485 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 5 Any recurrence | 1 | 1861 | Peto Odds Ratio (95% CI) | 0.82 [0.68, 0.99] |
| 6 Curative resection | 4 | 4673 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 7 Any resection | 3 | 2802 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.95, 1.04] |
| 8 Sphincter preservation | 3 | 4379 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.94, 1.04] |
| 9 Postoperative morbidity ‐ sepsis | 2 | 2698 | Risk Ratio (IV, Random, 95% CI) | 1.25 [1.04, 1.52] |
| 10 Postoperative morbidity ‐ surgical complications | 2 | 2698 | Risk Ratio (IV, Random, 95% CI) | 1.20 [1.01, 1.42] |
| 11 Postoperative mortality | 2 | 1960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.22] |
Comparison 2. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality according to risk of bias | 4 | 4663 | Peto Odds Ratio (95% CI) | 0.90 [0.83, 0.98] |
| 1.1 Adequate allocation concealment | 3 | 4379 | Peto Odds Ratio (95% CI) | 0.91 [0.83, 1.00] |
| 1.2 Inadequate/unclear allocation concealment | 1 | 284 | Peto Odds Ratio (95% CI) | 0.84 [0.63, 1.11] |
| 2 Overall survival (5 years) according to stage | 1 | 1110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.97] |
| 2.1 Duke A/B or TNM I/II | 1 | 703 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.08] |
| 2.2 Duke C or TNM Stage III | 1 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.07] |
| 3 Overall survival (10 years) according to stage | 1 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.27] |
| 3.1 Duke A/B or TNM I/II | 1 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.99, 1.35] |
| 3.2 Duke C or TNM Stage III | 1 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.09] |
| 4 Overall mortality according to TME or not | 4 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 4.1 No TME | 2 | 1452 | Peto Odds Ratio (95% CI) | 0.79 [0.69, 0.92] |
| 4.2 TME | 2 | 3211 | Peto Odds Ratio (95% CI) | 0.97 [0.87, 1.08] |
| 5 Local recurrence according to risk of bias | 4 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 5.1 Adequate allocation concealment | 3 | 4321 | Peto Odds Ratio (95% CI) | 0.51 [0.42, 0.62] |
| 5.2 Unclear/inadequate allocation concealment | 1 | 284 | Peto Odds Ratio (95% CI) | 0.29 [0.17, 0.50] |
| 6 Overall mortality (patients with a negative CRM; within trial subgroup analysis) | 1 | 1353 | Peto Odds Ratio (95% CI) | 0.99 [0.85, 1.17] |
| 6.1 TNM I | 1 | 497 | Peto Odds Ratio (95% CI) | 1.17 [0.86, 1.59] |
| 6.2 TNM II | 1 | 421 | Peto Odds Ratio (95% CI) | 1.19 [0.91, 1.56] |
| 6.3 TNM III | 1 | 435 | Peto Odds Ratio (95% CI) | 0.76 [0.59, 0.98] |
| 7 Local recurrence (5 years) according to stage | 1 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.36, 0.60] |
| 7.1 Duke A/B or TNM I/II | 1 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.65] |
| 7.2 Duke C or TNM Stage III | 1 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.35, 0.68] |
| 8 Local recurrence (10 years) according to stage | 2 | 2842 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.36, 0.63] |
| 8.1 Duke A/B or TNM I/II | 2 | 1710 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.27, 0.76] |
| 8.2 Duke C or TNM Stage III | 2 | 1132 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.35, 0.67] |
| 9 Local recurrence according to tumour height | 1 | 908 | Risk Ratio (IV, Fixed, 95% CI) | 0.40 [0.28, 0.56] |
| 9.1 Tumour height <= 5 cm | 1 | 282 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.22, 0.68] |
| 9.2 Tumour height 6 cm to 10 cm | 1 | 383 | Risk Ratio (IV, Fixed, 95% CI) | 0.34 [0.20, 0.57] |
| 9.3 Tumour height > 10 cm | 1 | 243 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.29, 1.39] |
| 10 Local recurrence according to TME or not | 4 | 4605 | Peto Odds Ratio (95% CI) | 0.48 [0.40, 0.57] |
| 10.1 No TME | 2 | 1394 | Peto Odds Ratio (95% CI) | 0.50 [0.38, 0.65] |
| 10.2 TME | 2 | 3211 | Peto Odds Ratio (95% CI) | 0.46 [0.35, 0.59] |
2.10. Analysis.
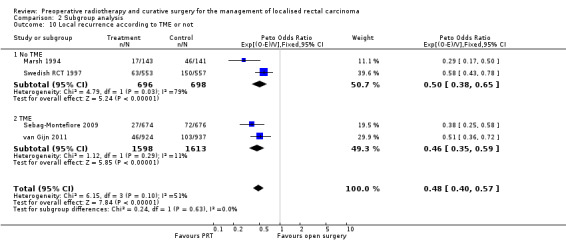
Comparison 2 Subgroup analysis, Outcome 10 Local recurrence according to TME or not.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Marsh 1994.
| Methods | Randomisation method not available. Enrolment period: 1982 to 1986 Abdominal imaging: not stated Chest imaging: not stated Study arm: 143 randomised, 0 excluded; median time from randomisation to surgery 27 days (IQR 21 to 33) Control arm: 141 randomised , 0 excluded; median time from randomisation to surgery 19 days (IQR 12 to 26) | |
| Participants | Rectal cancer Location: </= 13 cm Resectability: locally advanced (tethered or fixed) but operable (within 13 cm of the anal verge) | |
| Interventions | Surgery: not stated RT: 2000 in 4 fr BED: 31.8 Gy10 RT volume: 10x10x10 cm posterior pelvis RT‐S: </= 1 week Technique: rotational field Co‐intervention: none | |
| Outcomes |
|
|
| Notes | Definition for:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was unclear how the method of randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear how allocation of participants was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible with the type of intervention. |
| Blinding the outcome assessor (detection bias; objective outcomes) Outcomes: mortality; local recurrence; distant metastases; curative resection Outcomes: mortality; local recurrence; distant metastases; any recurrence; curative resection | Low risk | Mortality: no information was provided on the blinding of the outcome evaluator. Recurrence: no information was provided on the blinding of the outcome evaluator. Metastases: no information was provided on the blinding of the outcome evaluator. Curative resection: It was unclear whether the operating surgeon or the pathologist was blinded. Quote: "The operating surgeon recorded a 'curative' resection if the carcinoma was removed with neither spillage nor perforation, and there was no macroscopic evidence of residua I local disease or distant metastases. The degree of local invasion present at operation was also noted. Pathologic information on the resected tumour was recorded prospectively by pathologists from the referral hospital on a standard form for each of the 284 patients. Lymph nodes were sampled and assessed in the normal way, as was the presence of venous invasion." Since the outcomes were objective, we considered the study to be at low risk of detection bias for the listed outcomes. |
| Blinding the outcome assessor (detection bias): subjective outcomes: Postoperative morbidity; sphincter preservation; acute and late toxicities; quality of life Outcomes: Postoperative morbidity; sphyncter preservation | Unclear risk | Postoperative morbidity was not assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although intention‐to‐treat was not stated, it appears that all participants were analysed according to their initial allocation. No apparent significant loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Relevant clinical outcomes were considered. |
Sebag‐Montefiore 2009.
| Methods | Randomisation method: “minimisation procedure”. Enrolment period: March 1998 to August 2005
Abdominal imaging: liver ultrasound or CT scan
Chest imaging: CXR
2‐arm study: short‐course preoperative radiotherapy (25 Gy in 5 fractions) (n = 674) vs initial surgery with selective postoperative chemoradiotherapy (45 Gy in 25 fractions with concurrent 5‐fluorouracil) (n = 676) Total randomised: 1350 participants |
|
| Participants | Rectal cancer Location: within 15 cm from anal verge Resectability: locally resectable WHO PS 0 to 3 Age: </= 87 years | |
| Interventions | Short‐course preoperative radiotherapy (25 Gy in 5 fractions) (n = 674) vs initial surgery with selective postoperative chemoradiotherapy (45 Gy in 25 fractions with concurrent 5‐fluorouracil) restricted to patients with involvement of the circumferential resection margin (n = 676) RT target volume: sacral promontory superiorly, 3 to 5 cm below the inferior tumour extent, 2 to 3 cm anterior to the sacral promontory, 1 cm posterior to the anterior sacrum, and 1 cm lateral to the most lateral aspect of the bony true pelvis |
|
| Outcomes | Primary outcome: local recurrence Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible consenting patients were randomly assigned to treatment groups by the MRC Clinical Trials Unit by a minimisation procedure, with stratification for surgeon, distance of distal tumour extent from the anal verge, and WHO performance status." |
| Allocation concealment (selection bias) | Low risk | "Eligible consenting patients were randomly assigned to treatment groups by the MRC Clinical Trials Unit by a minimisation procedure, with stratification for surgeon, distance of distal tumour extent from the anal verge, and WHO performance status." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible with the type of intervention. |
| Blinding the outcome assessor (detection bias; objective outcomes) Outcomes: mortality; local recurrence; distant metastases; curative resection Outcomes: mortality; local recurrence; distant metastases; any recurrence; curative resection | Unclear risk | Mortality: no information provided on the blinding of the outcome evaluator. Recurrence: no information provided on the blinding of the outcome evaluator. Quote: "Confirmed local recurrence was defined as intraluminal tumour confirmed by a biopsy sample, positive imaging, or equivocal pelvic imaging with a raised serum carcino‐embryonic antigen without distant metastases" Metastases: no information provided on the blinding of the outcome evaluator. Curative resection: no information was provided as to whether the surgeon was blinded. Quote: "a simple grading system of the resected macroscopic surgical specimen was prospectively assessed as part of the trial." Since the outcomes were objective, we considered the study to be at low risk of detection bias for the listed outcomes. |
| Blinding the outcome assessor (detection bias): subjective outcomes: Postoperative morbidity; sphincter preservation; acute and late toxicities; quality of life Outcomes: Postoperative morbidity; sphyncter preservation | Unclear risk | No information about the blinding of the outcome assessor regarding postoperative morbidity was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed on an intention‐to‐treat basis. No relevant missing data |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes were reported. |
Swedish RCT 1997.
| Methods | Randomisation method: telephone. Enrolment period: March 1987 to February 1990 Abdominal imaging: not stated Chest imaging: not stated Study arm: 585 randomised, 10 excluded Control arm: 583 randomised, 11 excluded | |
| Participants | Rectal cancer Location: below sacral promontory by barium enema Resectability: locally resectable | |
| Interventions | Surgery: AP/anterior resection RT : 25.00 Gy in 5 fr BED: 38.7 Gy10 RT volume: L5 to obturator. To include anal canal, tumour, mesorectum, presacral nodes, internal iliac nodes RT‐S: within 1 week 3‐ or 4‐field Co‐intervention: none | |
| Outcomes |
|
|
| Notes | Definitions for:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description was provided |
| Allocation concealment (selection bias) | Low risk | Allocation was central. Quote: "Patients were randomly assigned to treatment groups, with stratification according to hospital, by telephone contact with the trial center in one of the six Swedish health care regions" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of staff and personnel is not possible with the type of intervention. |
| Blinding the outcome assessor (detection bias; objective outcomes) Outcomes: mortality; local recurrence; distant metastases; curative resection Outcomes: mortality; local recurrence; distant metastases; any recurrence; curative resection | Unclear risk | Mortality: outcome assessor was adequately blinded. Quote: "All case‐record forms were checked by an independent observer against the clinical records during an audit in 1995. The causes of death of all patients who died were checked against the National Causes of Death Registry by computerized linkage" Recurrence: outcome evaluator was blinded. Quote: "clinical evaluation twice a year during the first five years after surgery was stipulated in the protocol. Any clinically detectable tumour, whether morphologically verified or not, within the dorsal parts of the pelvis, including the urinary bladder, was considered a local recurrence. Laboratory tests, imaging, and biochemical tests were performed only if a local or distant recurrence was suspected. All case‐record forms were checked by an independent observer against the clinical records during an audit in 1995" Metastases: no information was provided on the blinding of the outcome evaluator. Curative resection: no information was provided as to whether the surgeon or the pathologist were blinded. Quote: "Surgery was considered locally curative if both the surgeon and the histopathologist considered the margins of the resected tissue to be free of tumour, even if the bowel was perforated during surgery. The locally curative nature of surgery was defined as uncertain when either the surgeon or the pathologist reported a questionable margin." Since the outcomes were objective, we considered the study to be at low risk of detection bias for the listed outcomes. |
| Blinding the outcome assessor (detection bias): subjective outcomes: Postoperative morbidity; sphincter preservation; acute and late toxicities; quality of life Outcomes: Postoperative morbidity; sphyncter preservation | Unclear risk | No information about the blinding of the outcome assessor regarding postoperative morbidity was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was performed according to the intention‐to‐treat principle. No apparent relevant missing data or attrition |
| Selective reporting (reporting bias) | Unclear risk | Results on toxicity were not given. |
van Gijn 2011.
| Methods | Randomisation method: computer generated and based on permuted blocks of 6 with stratification according to centre and the expected type of surgery. Enrolment period: January 1996 to December 1999 Abdominal imaging: none Chest imaging: not stated 2‐arm study: arm 1 preoperative radiotherapy + TME (897 allocated to treatment) and arm 2 TME alone (908 allocated to treatment) (ratio 1:1) Total randomised 1861 with 56 excluded (allocated to treatment: 1805).
|
|
| Participants | Adenocarcinoma of the rectum without evidence of distant disease Location: below the level of S1/S2 with an inferior tumour margin located 15 cm or less from the anal verge Resectability: clinically defined No upper age limit was given. 64% male and 36% female TNM stage:
|
|
| Interventions | Surgery: AP/anterior resection/HP with TME technique RT : 25.00 Gy in 5 fr BED: 38.7 Gy10 RT volume: primary tumour, mesentery with vascular supply, perirectal, presacral, internal iliac nodes up to S1‐2 RT‐S: within 10 days Multiple fields Co‐intervention: postoperative radiotherapy was used for participants who had positive margins (< 1 mm) and did not receive preoperative XRT. |
|
| Outcomes |
|
|
| Notes | Overall recurrence analyses were done on the basis of the number of eligible participants who had a macroscopically complete local resection without distant metastases at the time of surgery. As specified in the trial protocol, secondary analyses were done on participants with a negative circumferential resection margin (> 1 mm) and no signs of distant tumour spread. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomsation was computer generated. Quote: "Randomisation was computer‐generated and based on permuted blocks of six with stratification according to centre and the expected type of surgery. Randomisation was managed centrally at the data centre of the Department of Surgery of Leiden University Medical Centre, Netherlands. For every stratification group and participating centre, a list was printed by the Department of Medical Statistics. Patients were assigned to a treatment by these lists, which were only available in the central data centre. Local investigators enrolling patients had no knowledge of the next assignment in the sequence." |
| Allocation concealment (selection bias) | Low risk | The allocation was adequate and clearly reported. Quote: "Randomisation was computer‐generated and based on permuted blocks of six with stratification according to centre and the expected type of surgery. Randomisation was managed centrally at the data centre of the Department of Surgery of Leiden University Medical Centre, Netherlands. For every stratification group and participating centre, a list was printed by the Department of Medical Statistics. Patients were assigned to a treatment by these lists, which were only available in the central data centre. Local investigators enrolling patients had no knowledge of the next assignment in the sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible with the type of intervention. |
| Blinding the outcome assessor (detection bias; objective outcomes) Outcomes: mortality; local recurrence; distant metastases; curative resection Outcomes: mortality; local recurrence; distant metastases; any recurrence; curative resection | Unclear risk | Mortality: no information was provided on the blinding of the outcome evaluator. Recurrence: it was unclear whether the outcome evaluator was blinded. Quote: "Investigators reviewing primary endpoints [i.e. local recurrence] were not aware of the allocated treatment and those analysing data were unmasked" Metastases: no information was provided on the blinding of the outcome evaluator. Quote: "Distant recurrence analyses were done on all eligible patients who did not have distant metastases at the time of surgery." Curative resection: no clear information was provided. Quote: "Local recurrence analyses were done on all eligible patients who underwent a macroscopically complete local resection" Since the outcomes were objective, we considered the study to be at low risk of detection bias for the listed outcomes. |
| Blinding the outcome assessor (detection bias): subjective outcomes: Postoperative morbidity; sphincter preservation; acute and late toxicities; quality of life Outcomes: Postoperative morbidity; sphyncter preservation | Unclear risk | No information about the blinding of the outcome assessor regarding postoperative morbidity was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Local recurrence: due to macroscopically incomplete resection, 24 (2.7%) in the preoperative radiotherapy group and 33 (3.6%) in the surgery‐alone group were excluded from local recurrence analysis. We concluded that the proportion of exclusions was not relevant to introduce bias in the results. Survival analysis: no exclusions, missing data, or loss to follow‐up. All participants were included in analysis. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes were considered. |
AP: abdominal perineal; BED: biological equivalent dose; CRM: circumferential resection margin; CT: computed tomography; CXR: chest X‐ray; fr: fraction; FU: follow‐up; IQR: interquartile range; Mets @ lap: metastatic identified at the time of laparotomy; HP: Hartmann procedure; RT: radiotherapy; RTS: radiotherapy + surgery; RT‐S: time between radiotherapy and surgery; S: surgery; TME: total mesorectal excision; Tox post RT: toxicity postradiotherapy; WHO PS: World Health Organization Performance Status; XRT: Chermoradiation therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atif 2012 | Randomised trial of preoperative radiotherapy versus postoperative radiotherapy. Both arms received radiotherapy. |
| Bosset 2004 | Randomised trial of preoperative chemoradiotherapy versus preoperative radiotherapy. Both arms received radiotherapy. |
| Boulis‐Wassif 1982 | Large fields including para‐aortics and only 2 fields |
| Boulis‐Wassif 1984 | Randomised trial of preoperative administration of radiotherapy, with or without 5‐fluorouracil before radical surgery |
| Bujko 2004 | Randomised trial of short‐term radiotherapy vs conventionally fractionated radiochemotherapy |
| Bujko 2013 | Randomised those with early rectal cancer to preoperative short‐course radiotherapy (25 Gy in 5 fractions + 4 Gy boost) or long‐course chemoradiotherapy (55.8 Gy in 31 fractions with concurrent 5‐fluorouracil/leucovorin) followed by local excision. Radical surgery only for poor responders. Excluded given that both arms received radiotherapy, and radical surgery not done for all participants |
| Camma 2000 | Meta‐analysis, preoperative radiotherapy for rectal cancer |
| CCCG 2001 | Systematic review |
| Cedermark 1995 | Large‐field RT with elective para‐aortic node irradiation |
| Ceelen 2005 | Systematic review on preoperative chemoradiotherapy for locally advanced rectal cancer |
| Cummings 1985 | [trial ‐ primary reference]. No data regarding the study. Included in CCCG 2001 review by obtaining individual patient data (no published data available) |
| Dahl 1990 | Large fields with superior border at the top of L1 and only 2 fields |
| Dubois 2011 | Preoperative RT was performed in all participants before randomisation to either surgical resection alone or surgical resection and intraoperative radiation therapy. |
| Erlandsson 2017 | No surgery‐alone arm |
| Figueredo 2003 | Meta‐analysis and practice guideline for Cancer Care Ontario |
| Francois 2014 | ACCORD12/0405 PRODIGE: both arms used preoperative chemoradiotherapy. The experimental arm used additional oxaliplatin. |
| Frykholm 1993 | Randomised trial of preoperative radiotherapy vs postoperative radiotherapy. Both arms received radiotherapy. |
| Frykholm 2001 | Compared chemoradiotherapy vs radiotherapy preoperatively for unresectable rectal cancer. Both arms received radiotherapy. |
| Gerard 1988 | The trial used only 2 fields and large‐field RT with superior border at top of second lumbar vertebra. |
| Gerard 2004 | Randomised trial of preoperative external‐beam radiotherapy (39 Gy in 13 fractions over 17 days) vs the same external‐beam radiotherapy with boost (85 Gy in 3 fractions) using endocavitary contact X‐ray |
| Gerard 2011 | Both arms received radiotherapy. |
| Glehen 2003 | Randomised trial of short‐interval (2 weeks) preoperative radiotherapy vs long‐interval (4 to 6 weeks) |
| Goldberg 1994 | The trial used low RT dose (15 Gy in 3 fractions) and only 2 fields. |
| Guckenberger 2012 | Both arms received radiation therapy. |
| Gunderson 2003 | Review article. |
| Gérard 2012 | Both arms received radiation therapy. |
| Higgins 1975 | Patients at enrolment were with evidence of distant metastases. |
| Higgins 1986 | The trial used low RT energy, only 2 fields, and large fields with the superior border at the top of the second lumbar vertebra. |
| Illenyi 1994 | The trial used only 2 fields. |
| Kim 2011 | Radiotherapy was performed postoperatively in all participants. The study compared early (started on the first day of the first chemotherapy cycle) and late RT (started on the first day of the third chemotherapy cycle). |
| Kimura 1989 | No information available regarding fractionation, fields, or field arrangement |
| Kligerman 1972 | The trial used large‐field RT. |
| Latkauskas 2012 | Both arms received radiation therapy. |
| MRC 1984 | The trial used low RT dose (20 Gy in 10 fractions or 5 Gy single fraction) and only 2 fields. |
| MRC 1996 | The trial used only 2 fields. |
| Ngan 2012 | Both arms received radiation therapy. This is a randomised trial comparing short‐course radiotherapy with long‐course chemoradiotherapy. |
| Niebel 1988 | A randomised 3‐arm study: (1) preoperative radiotherapy (25 Gy in 2.5 weeks) with a postoperative boost (25 Gy) for participants with pT3 and pT4 stages; (2) postoperative radiotherapy; and (3) surgery. The authors reported low compliance to postoperative boost without providing numbers: "many patients with pT3/pT4‐stage disease postoperatively refused the intended radiation therapy in spite of having given informed consent or were not radiated for various reasons which reflect the doctor's or the patient's bias". In addition, neither the number of allocated participants in the groups nor the results for the evaluated outcomes were adequately reported. |
| Parc 2009 | Secondary analysis of a randomised trial of 2 different surgical procedures: coloplasty versus J‐pouch. The use of preoperative RT was not randomised, and was left to surgeons’ discretion. |
| Petersen 1998 | The trial used low RT dose (16.5 Gy in 5 fractions). |
| Pettersson 2015 | No surgery‐alone arm |
| Reis Neto 1989 | The trial used large‐field RT. |
| Rider 1977 | The trial used low RT dose (5 Gy). |
| Rouanet 2006 | Both arms received RT. Randomisation between preoperative RT alone (45 + 18 Gy) and preoperative chemoradiotherapy (45 Gy + infusional 5‐fluorouracil) |
| Sause(RTOG81‐15)1994 | Low dose (5 Gy) |
| Stockholm 1996 | 316 participants from this trial were included in Swedish RCT 1997, therefore we have excluded this trial to avoid double counting. |
| Valentini 2008 | The study compared 2 different chemoradiotherapy schemes (both arms received radiotherapy). |
| You 1993 | The trial used large‐field RT. |
| Zehra 2015 | This was an abstract of a review about rectal dysfunction and quality of life following curative treatment for rectal cancer. |
RT: radiotherapy
Differences between protocol and review
Since the publication of the protocol for this review in 2000 (Wong 2000), advances have been made with respect to the techniques used to deliver radiotherapy. The original review included 19 studies (Wong 2007). However, given that many trials used very old techniques that are not justified in contemporary clinical practice, we modified the inclusion criteria in the present update, and hence excluded trials that used low‐energy radiotherapy, two‐field approaches with AP‐PA fields, and very large fields (pelvic + para‐aortic).
Consequently, there remained four studies that constituted the base of the evidence in this updated review.
Contributions of authors
Co‐ordinating the review: Iosief Abraha, Cynthia Aristei
Data collection for the review: Iosief Abraha, Isabella Palumbo, Marco Lupattelli, Rita De Florio, Roberto Cirocchi, and Stefano Trastulli
Data management for the review: Iosief Abraha
Analysis of data: Iosief Abraha, Cynthia Aristei, Vincenzo Valentini
Interpretation of data: Cynthia Aristei, Vincenzo Valentini, Iosief Abraha, Isabella Palumbo, Marco Lupattelli, Rita De Florio, Roberto Cirocchi, and Stefano Trastulli
Writing the review: Cynthia Aristei, Vincenzo Valentini, Iosief Abraha, Isabella Palumbo, Marco Lupattelli, Rita De Florio, Roberto Cirocchi, and Stefano Trastulli
Providing general advice on the review: Cynthia Aristei, Vincenzo Valentini
Sources of support
Internal sources
None, Other.
External sources
No sources of support supplied
Declarations of interest
Iosief Abraha: no conflict of interest
Cynthia Aristei: no conflict of interest
Isabella Palumbo: no conflict of interest
Marco Lupattelli: no conflict of interest
Stefano Trastulli: no conflict of interest
Roberto Cirocchi: no conflict of interest
Rita De Florio: no conflict of interest
Vincenzo Valentini: no conflict of interest
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Marsh 1994 {published data only}
- Jones DJ, Zaloudik J, James RD, Haboubi N, Moore M, Schofield PF. Predicting local recurrence of carcinoma of the rectum after preoperative radiotherapy and surgery. British Journal of Surgery 1989;76:1172‐5. [DOI] [PubMed] [Google Scholar]
- Marsh PJ, James RD, Schofield PF. Adjuvant preoperative radiotherapy for locally advanced rectal carcinoma: results of a prospective randomized trial. Diseases of the Colon & Rectum 1994;37:1205‐14. [DOI] [PubMed] [Google Scholar]
- Marsh PJ, James RD, Schofield PF. Definition of local recurrence after surgery for rectal carcinoma. British Journal of Surgery 1995;82(4):465‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sebag‐Montefiore 2009 {published data only}
- Quirke P, Sebag‐Montefiore D, Steele R, Khanna S, Monson J, Holliday A, et al. Local recurrence after rectal cancer resection is strongly related to the plane of surgical dissection and is further reduced by pre‐operative short course radiotherapy. Preliminary results of the Medical Research Council (MRC) CR07 trial. Journal of Clinical Oncology 2006;24(18 suppl):3512. [Google Scholar]
- Sebag‐Montefiore D, Steele R, Monson J, Couture J, Metz C, Pugh C, et al. OC‐0219 THE MRC CR07 TRIAL NCIC CO16 TRIAL AFTER A MEDIAN FOLLOW UP OF 8 YEARS. OC‐0219 THE MRC CR07 TRIAL NCIC CO16 TRIAL AFTER A MEDIAN FOLLOW UP OF 8 YEARS. 103 2012.
- Sebag‐Montefiore D, Steele R, Quirke P, Grieve R, Khanna S, Monson J, et al. Routine short course pre‐op radiotherapy or selective post‐op chemoradiotherapy for resectable rectal cancer? Preliminary results of the MRC CR07 randomised trial. Journal of Clinical Oncology 2006, 2006;24(18_suppl):3511. [Google Scholar]
- Sebag‐Montefiore D, Steele R, Quirke P, Grieve R, Khanna S, Monson J, et al. Routine short course pre‐op radiotherapy or selective post‐op chemoradiotherapy for resectable rectal cancer? Preliminary results of the MRC CR07 randomised trial. Journal of Clinical Oncology 2006;24(suppl 18):3511. [Google Scholar]
- Sebag‐Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC‐CTG C016): a multicentre, randomised trial. Lancet 2009;373(9666):811‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RJ, Thompson LC, Quirke P, Steele R, Grieve R, Couture J, et al. Impact of short‐course preoperative radiotherapy for rectal cancer on patients' quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. Journal of Clinical Oncology 2010;28(27):4233‐9. [DOI] [PubMed] [Google Scholar]
Swedish RCT 1997 {published data only}
- Adell G, Sun Xiao‐Feng, Stal O, Klintenberg C, Sjödahl R. p53 status: an indicator for the effect of preoperative radiotherapy of rectal cancer. Radiotherapy and Oncology 1999;51:169‐74. [DOI] [PubMed] [Google Scholar]
- Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Adverse effects of preoperative radiation therapy for rectal cancer: long term follow‐up of the Swedish Rectal Cancer Trial. Journal of Clinical Oncology 2005;23(34):8697‐9705. [DOI] [PubMed] [Google Scholar]
- Dahlberg M, Glimelius B, Graf W, Påhlman L. Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Diseases of the Colon & Rectum 1998;41(5):543‐9. [DOI] [PubMed] [Google Scholar]
- Dahlberg M, Glimelius B, Påhlman L. Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish Rectal Cancer Trial. Annals of Surgery 1999;229:493‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg M, Stenborg A, Påhlman L, Glimelius B. Cost‐effectiveness of preoperative radiotherapy in rectal cancer: results from the Swedish Rectal Cancer Trial. International Journal of Radiation Oncology * Biology * Physics 2002;54(3):654‐60. [DOI] [PubMed] [Google Scholar]
- Folkesson J, Birgisson H, Påhlman L, Cedermark B, Glimeliux B. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. Journal of Clinical Oncology 2005;23(24):5644‐50. [DOI] [PubMed] [Google Scholar]
- Graf W, Dahlberg M, Osman MM, Holmberg L, Påhlman L, Glimelius B. Short‐term preoperative radiotherapy results in down‐staging of rectal cancer: a study of 1316 patients. Radiotherapy & Oncology 1997;43(2):133‐7. [DOI] [PubMed] [Google Scholar]
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. New England Journal of Medicine 1997;336:980‐7. [DOI] [PubMed] [Google Scholar]
- Swedish Rectal Cancer Trial. Increased 5‐years survival after preoperative irradiation in rectal cancer. International Journal of Colorectal Disease 1996;11:128. [Google Scholar]
- Swedish Rectal Cancer Trial. Initial report from a Swedish multicenter study examining the role of preoperative irradiation in the treatment of patients with resectable rectal carcinoma. British Journal of Surgery 1993;80:1333‐6. [DOI] [PubMed] [Google Scholar]
- Swedish Rectal Cancer Trial. Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. European Journal of Surgery 1996;162:397‐402. [PubMed] [Google Scholar]
van Gijn 2011 {published data only}
- Chen TYT, Wiltink LM, Nout RA, Meershoek‐Klein Kranenbarg E, Laurberg SO, Marijnen CAM, et al. Bowel function 14 years after preoperative short‐course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clinical Colorectal Cancer 2015;14(2):106‐14. [DOI] [PubMed] [Google Scholar]
- Kapiteijn E, Kranenbarg EK, Steup WH, Taat CW, Rutten HJ, Wiggers T, et al. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch Rectal Cancer Group. European Journal of Surgery 1999;165(5):410‐20. [DOI] [PubMed] [Google Scholar]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New England Journal of Medicine 2001;345(9):690‐2. [DOI] [PubMed] [Google Scholar]
- Kusters M, Marijnen CA, Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. European Journal of Surgical Oncology 2010;36(5):470‐6. [PUBMED: 20096534] [DOI] [PubMed] [Google Scholar]
- Lange MM, Dulk M, Bossema ER, Maas CP, Peeters KC, Rutten HJ, et al. Risk factors for faecal incontinence after rectal cancer treatment. British Journal of Surgery 2007;94(10):1278‐84. [PUBMED: 17579345] [DOI] [PubMed] [Google Scholar]
- Marijnen CA, Kapiteijn E, Nagtegaal ID, Mulder‐Stapel AA, Velde CJH, Schrier P, et al. p53 expression in human rectal tissue after radiotherapy: up regulation in normal mucosa versus functional loss in rectal carcinomas. International Journal of Radiation Oncology * Biology * Physics 2002;52(3):720‐8. [DOI] [PubMed] [Google Scholar]
- Marijnen CA, Kapiteijn E, Velde CJ, Marrtijn H, Steup WH, Wiggers T, et al. Acute side effects and complications after short‐term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. Journal of Clinical Oncology 2002;20(3):817‐25. [DOI] [PubMed] [Google Scholar]
- Marijnen CA, Nagtegaal ID, Kapiteijn E, Kranenbarg EK, Noordijk EM, Velde CJH, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. International Journal of Radiation Oncology * Biology * Physics 2003;55(5):1311‐20. [DOI] [PubMed] [Google Scholar]
- Marijnen CA, Nagtegaal ID, Klein KE, Hermans J, velde CJ, Leer JW, et al. No downstaging after short‐term preoperative radiotherapy in rectal cancer patients. Journal of Clinical Oncology 2001;19(7):1976‐84. [DOI] [PubMed] [Google Scholar]
- Marijnen CA, Velde CJH, Putter H, Brink M, Maas CP, Martijn H, et al. Impact of short‐term preoperative radiotherapy on health related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. Journal of Clinical Oncology 2005;23(9):1847‐58. [DOI] [PubMed] [Google Scholar]
- Nagtegaal I, Velde CJH, Marijnen CA, Krieken JHJM. Low rectal cancer: a call for a change of approach in abdominoperineal resection. Journal of Clinical Oncology 2005;23(36):9257‐64. [DOI] [PubMed] [Google Scholar]
- Nagtegaal ID, Velde CJH, Warp E, Kapiteijn E, Quirke P, Krieken HJM, the Pathology Review Committee for the Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macrosopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. Journal of Clinical Oncology 2002;20(7):1729‐34. [DOI] [PubMed] [Google Scholar]
- Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow‐up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Annals of Surgery 2007;246(5):693‐701. [PUBMED: 17968156] [DOI] [PubMed] [Google Scholar]
- Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM‐K, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12‐year follow‐up of the multicentre, randomised controlled TME trial. Lancet Oncology 2011;12(6):575‐82. [DOI] [PubMed] [Google Scholar]
- Brink M, Stiggelbout AM, Hout WB, Kievit J, Kranenbarg EK, Marijnen CA, et al. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. Journal of Clinical Oncology 2004;22(19):3958‐64. [DOI] [PubMed] [Google Scholar]
- Brink M, can den Hout WB, Kievit J, Marijnen AM, Putter H, Velde CJH, et al. The impact of diagnosis and treatment of rectal cancer on paid and unpaid labor. Diseases of the Colon & Rectum 2005;48(10):1875‐82. [DOI] [PubMed] [Google Scholar]
- Brink M, Hout WB, Stiggelbout AM, Kranenbarg EK, Marijnen CAM, Velde CJH, et al. Cost‐utility analysis of preoperative radiotherapy in patients with rectal cancer undergoing total mesorectal excision: a study of the Dutch Colorectal Cancer Group. Journal of Clinical Oncology 2004;22(2):244‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atif 2012 {published data only}
- Atif E, Sakr H, Teama S, Zayed D. Effect of radical surgery combined with pre‐ or postoperative radiotherapy in treatment of resectable rectal cancer. Chinese‐German Journal of Clinical Oncology 2012;11(7):384‐90. [Google Scholar]
- Gerard J, Romestaing P, Chapet O, Ortholan C. Clinical Tumor Response after Neoadjuvant Radiotherapy in Rectal Cancer and Conservative Treatment: Ten Years Results of the Lyon R96‐02 Randomized Trial. International Journal of Radiation Oncology • Biology • Physics 2011;81(2):S95‐6. [DOI] [PubMed] [Google Scholar]
Bosset 2004 {published data only}
- Bosset JF, Calais G, Daban A, Berger C, Radosevic‐Jelic L, Maingon P, et al. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. European Journal of Cancer (Oxford, England: 1990) 2004;40(2):219‐24. [PUBMED: 14728936] [DOI] [PubMed] [Google Scholar]
Boulis‐Wassif 1982 {published data only}
- Boulis‐Wassif S. The role of pre‐operative adjuvant therapy in the management of borderline operability rectal cancer. Clinical Radiology 1982;33(3):353‐8. [DOI] [PubMed] [Google Scholar]
Boulis‐Wassif 1984 {published data only}
- Boulis‐Wassif S, Gerard A, Loygue J, Camelot D, Buyse M, Duez N. Final results of a randomized trial on the treatment of rectal cancer with preoperative radiotherapy alone or in combination with 5‐fluorouracil, followed by radical surgery. Trial of the European Organization on Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. Cancer 1984;53(9):1811‐8. [PUBMED: 6423263] [DOI] [PubMed] [Google Scholar]
Bujko 2004 {published data only}
- Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, Michalski W, Bebenek M, Pudelko M, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short‐term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiotherapy and Oncology: journal of the European Society for Therapeutic Radiology and Oncology 2004;72(1):15‐24. [PUBMED: 15236870] [DOI] [PubMed] [Google Scholar]
Bujko 2013 {published data only}
- Bujko K, Richter P, Smith FM, Polkowski W, Szczepkowski M, Rutkowski A, et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re‐operation for poor responders: a prospective multicentre study. Radiotherapy and Oncology: journal of the European Society for Therapeutic Radiology and Oncology 2013;106(2):198‐205. [PUBMED: 23333016] [DOI] [PubMed] [Google Scholar]
Camma 2000 {published data only}
- Camma C, Giunta M, Fiorica F, Pagliaro L, Craxi A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: a metaanalysis. JAMA 2000;284(8):1008‐15. [DOI] [PubMed] [Google Scholar]
CCCG 2001 {published data only}
- CCCG, Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic review of 8507 patients from 22 randomized trials. Lancet 2001;358(9290):1291‐304. [DOI] [PubMed] [Google Scholar]
Cedermark 1995 {published data only}
- Cedermark B, Johansson H, Rutqvist LE, Wilking N for the Stockholm Colorectal Cancer Study Group. The Stockholm I Trial of preoperative short term radiotherapy in operable rectal carcinoma. Cancer 1995;75:2269‐75. [DOI] [PubMed] [Google Scholar]
- Cedermark B, Theve NO, Rieger A. Preoperative short‐term radiotherapy in rectal carcinoma: a preliminary report of a prospective randomized study. Cancer 1985;55:1182‐5. [DOI] [PubMed] [Google Scholar]
- Holm T, Cedermark B, Rutqvist LE. Local recurrence of rectal adenocarcinoma after "curative" surgery with and without preoperative radiotherapy. British Journal of Surgery 1994;81:452‐5. [DOI] [PubMed] [Google Scholar]
- Holm T, Johansson H, Cedermark B, Ekelund G, Rutqvist LE. Influence of hospital and surgeon related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. British Journal of Surgery 1997;84(5):657‐63. [PubMed] [Google Scholar]
- Holm T, Rutqvist LE, Johansson H, Cedermark B. Abdominoperineal resection and anterior resection in the treatment of rectal cancer. British Journal of Surgery 1995;82:1213‐6. [DOI] [PubMed] [Google Scholar]
- Holm T, Rutqvist LE, Johansson H, Cedermark B. Postoperative mortality in rectal cancer treated with or without preoperative radiotherapy: causes and risk factors. British Journal of Surgery 1996;83:964‐8. [DOI] [PubMed] [Google Scholar]
- Pollack J, Holm T, Cedermark B, Holmström B, Mellgren A. Long‐term effect of preoperative radiation therapy on anorectal function. Diseases of the Colon & Rectum 2006;49(3):345‐52. [DOI] [PubMed] [Google Scholar]
- Stockholm Rectal Cancer Study Group. Preoperative short‐term radiation therapy in operable rectal carcinoma. Cancer 1990;66:49‐55. [DOI] [PubMed] [Google Scholar]
- Stockholm Rectal Cancer Study Group. Short ‐term preoperative radiotherapy for adenocarcinoma of the rectum: an interim analysis of a randomized muticenter trial. American Journal of Clinical Oncology 1987;10(5):369‐75. [DOI] [PubMed] [Google Scholar]
Ceelen 2005 {published data only}
- Ceelen W, Pattyn P, Boterberg T, Peeters M. Preoperative combined modality therapy in the management of locally advanced rectal cancer. European Journal of Surgical Oncology 2005;32:259‐68. [DOI] [PubMed] [Google Scholar]
Cummings 1985 {unpublished data only}
- Cummings BJ. Radiation therapy and rectal carcinoma: The Princess Margaret Hospital experience. British Journal of Surgery 1985;72 Suppl:S64‐6. [DOI] [PubMed] [Google Scholar]
Dahl 1990 {published data only}
- Dahl O, Horn A, Mella O. Do acute side‐effects during radiotherapy predict tumour response in rectal carcinoma?. Acta Oncologica 1994;33(4):409‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dahl O, Horn A, Morild I, Halvorsen JF, Odland G, Reinertsen S, et al. Low‐dose preoperative radiation postpones recurrences in operable rectal cancer: results of a randomized multicenter trial in western Norway. Cancer 1990;66:2286‐94. [DOI] [PubMed] [Google Scholar]
- Horn A, Halvorsen JF, Dahl O. Preoperative radiotherapy in operable rectal cancer. Diseases of the Colon & Rectum 1990;33:823‐8. [DOI] [PubMed] [Google Scholar]
- Horn A, Morild I, Dahl O. Tumor shrinkage and down staging after preoperative radiation of rectal adenocarcinomas. Radiotherapy & Oncology 1990;18:19‐28. [DOI] [PubMed] [Google Scholar]
Dubois 2011 {published data only}
- Dubois JB, Bussieres E, Richaud P, Rouanet P, Becouarn Y, Mathoulin‐Pelissier S, et al. Intra‐operative radiotherapy of rectal cancer: results of the French multi‐institutional randomized study. Radiotherapy and Oncology: journal of the European Society for Therapeutic Radiology and Oncology 2011;98(3):298‐303. [PUBMED: 21339010] [DOI] [PubMed] [Google Scholar]
Erlandsson 2017 {published data only}
- Erlandsson J, Holm T, Pettersson D, Berglund A, Cedermark B, Radu C, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non‐blinded, phase 3, non‐inferiority trial. Lancet Oncology 2017;18(3):336‐46. [PUBMED: 28190762] [DOI] [PubMed] [Google Scholar]
Figueredo 2003 {published data only}
- Figueredo A, Zuraw L, Wong RKS, Agboola O, Rumble B, Tandan V, members of the CCO's Program in Evidence Based Care's Gastrointestinal Cancer Disease Site Group. The use of preoperative radiotherapy in the management of patients with clinically resectable rectal cancer: a practice guideline. BioMed Central 2003;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francois 2014 {published data only}
- Francois E, Azria D, Gourgou‐Bourgade S, Jarlier M, Martel‐Laffay I, Hennequin C, et al. Results in the elderly with locally advanced rectal cancer from the ACCOR12/PRODIGE 2 phase III trial: tolerance and efficacy. Radiotherapy & Oncology 2014;110(1):144‐9. [DOI] [PubMed] [Google Scholar]
Frykholm 1993 {published data only}
- Frykholm GJ, Glimelius B, Påhlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Diseases of the Colon & Rectum 1993;36(6):564‐72. [PUBMED: 8500374] [DOI] [PubMed] [Google Scholar]
Frykholm 2001 {published data only}
- Frykholm GJ, Påhlman L, Glimelius B. Combined chemo and radiotherapy vs radiotherapy alone in the treatment of primary, non resectable adenocarcinoma of the rectum. International Journal of Radiation Oncology * Biology * Physics 2001;50(2):427‐34. [DOI] [PubMed] [Google Scholar]
Gerard 1988 {published data only}
- Gerard A, Berrod J‐L, Pene F, Loygue J, Laugier A, Bruckner R, et al. Interim analysis of a phase III study on preoperative radiation therapy in resectable rectal carcinoma. Cancer 1985;55:2373‐9. [DOI] [PubMed] [Google Scholar]
- Gerard A, Berrod JL, Pene F, Loygue J, Laugier A, Bruckner R. Preoperative radiotherapy and radical surgery as combined treatment in rectal cancer. Recent Results in Cancer Research 1988;110:130‐3. [DOI] [PubMed] [Google Scholar]
- Gerard A, Buyse M, Nordlinger B, Loygue J, Pene F, Kempf P, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer: final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Annals of Surgery 1988;208(5):606‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzner J, Bruckner R, Kempf P. Preoperative radiotherapy in rectal cancer. Strahlentherapie 1984;160:236‐8. [PubMed] [Google Scholar]
- Metzger U, Magdeburg W, Largiader F. Preliminary radiation for rectal carcinoma. Results of a randomised European multicenter study. Helvetica Chimica Acta 1985;52:707‐11. [PubMed] [Google Scholar]
Gerard 2004 {published data only}
- Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R, et al. Improved sphincter preservation in low rectal cancer with high‐dose preoperative radiotherapy: the Lyon R96‐02 randomized trial. Journal of Clinical Oncology 2004;22(12):2404‐9. [PUBMED: 15197202] [DOI] [PubMed] [Google Scholar]
Gerard 2011 {published data only}
- Gerard J, Romestaing P, Chapet O, Ortholan C. Clinical tumor response after neoadjuvant radiotherapy in rectal cancer and conservative treatment: ten years results of the Lyon R96‐02 Randomized Trial. International Journal of Radiation Oncology * Biology* Physics 2011;81(2):S95‐6. [DOI] [PubMed] [Google Scholar]
Gérard 2012 {published and unpublished data}
- Gérard J‐P, Ortholan C, Chapet O, Romestaing P. Ten‐year results of the Lyon R96‐2 randomized trial in distal rectal cancer: radiation dose escalation to increase organ preservation. Journal of Clinical Oncology 2012;30(Suppl 4):Abstract 556. [Google Scholar]
Glehen 2003 {published data only}
- Glehen O, Chapet O, Adham M, Nemoz JC, Gerard J‐P. Long‐term results of the Lyons R90‐01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter‐saving surgery in rectal cancer. British Journal of Surgery 2003;90(8):996‐8. [PUBMED: 12905554] [DOI] [PubMed] [Google Scholar]
Goldberg 1994 {published data only}
- Goldberg PA, Nicholls RJ. Prediction of local recurrence and survival of carcinoma of the rectum by surgical and histopathological assessment of local clearance. British Journal of Surgery 1995;82(8):1054‐6. [DOI] [PubMed] [Google Scholar]
- Goldberg PA, Nicholls RJ, Porter NH, Love S, Grimsey JE. Long term results of a randomised trial of short course low‐dose adjuvant preoperative radiotherapy for rectal cancer: reduction in local treatment failure. European Journal of Cancer 1994;30A(11):1602‐6. [DOI] [PubMed] [Google Scholar]
- Porter NH, Nicholls RJ. Pre‐operative radiotherapy in operable rectal cancer: interim report of a trial carried out by the Rectal Cancer Group. British Journal of Surgery 1985;72 Suppl:62‐4. [DOI] [PubMed] [Google Scholar]
Guckenberger 2012 {published data only}
- Guckenberger M, Saur G, Wehner D, Sweeney RA, Thalheimer A, Germer CT, et al. Comparison of preoperative short‐course radiotherapy and long‐course radiochemotherapy for locally advanced rectal cancer. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft ... [et al] 2012;188(7):551‐7. [PUBMED: 22638934] [DOI] [PubMed] [Google Scholar]
Gunderson 2003 {published data only}
- Gunderson LL, Haddock MG, Schild SE. Preoperative versus postoperative irradiation as a component of adjuvant treatment. Seminars in Radiation Oncology 2003;13(4):419‐32. [DOI] [PubMed] [Google Scholar]
Higgins 1975 {published data only}
- Higgins GA, Conn JH, Jordan PH, Humphrey EW, Roswit B, Keehn RJ. Preoperative radiotherapy for colorectal cancer. Annals of Surgery 1975;181(5):624‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 1986 {published data only}
- Higgins GA, Humphrey EW, Dwight RW, Roswit B, Lee LE, Keehn RJ. Preoperative radiation and surgery for cancer of the rectum: Veterans Administration Surgical Oncology Group Trial II. Cancer 1986;58:352‐9. [DOI] [PubMed] [Google Scholar]
Illenyi 1994 {published data only}
- Illenyi L, Grexa E, Gecser G, Kott I. Local recurrence of rectal cancer following preoperative irradiation. Acta Chirurgica Hungarica 1994;34(3‐4):333‐47. [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim TW, Lee JH, Lee JH, Ahn JH, Kang YK, Lee KH, et al. Randomized trial of postoperative adjuvant therapy in Stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: 10‐year follow‐up. International Journal of Radiation Oncology * Biology * Physics 2011;81(4):1025‐31. [PUBMED: 20932669] [DOI] [PubMed] [Google Scholar]
Kimura 1989 {published data only}
- Kimura K, Tuchiya S, Yasutomi M, Ohkawa T, Hirose T, Tani C, et al. Comparison of surgical therapy and combined irradiation in rectal cancer. First report, Effect of irradiation on the tumor. Japanese Journal of Cancer and Chemotherapy 1989;16(9):3161‐72. [PubMed] [Google Scholar]
Kligerman 1972 {published data only}
- Kligerman MM, Urdaneta N, Knowlton A, Vidone R, Hartman PV, Vera R. Preoperative irradiation of rectosigmoid carcinoma including its regional lymph nodes. American Journal of Roentgenology 1972;114:498‐503. [DOI] [PubMed] [Google Scholar]
Latkauskas 2012 {published data only}
- Latkauskas T, Pauzas H, Gineikiene I, Janciauskiene R, Juozaityte E, Saladzinskas Z, et al. Initial results of a randomized controlled trial comparing clinical and pathological downstaging of rectal cancer after preoperative short‐course radiotherapy or long‐term chemoradiotherapy, both with delayed surgery. Colorectal Disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 2012;14(3):294‐8. [PUBMED: 21899712] [DOI] [PubMed] [Google Scholar]
MRC 1984 {published data only}
- MRC Working Party. A trial of preoperative radiotherapy in the management of operable rectal cancer: first report of an MRC Working Party. British Journal of Surgery 1982;69:513‐9. [DOI] [PubMed] [Google Scholar]
- MRC Working Party. Clinico‐pathological features of prognostic significance in operable rectal cancer in 17 centers in the U.K.: third report of an MRC Working Party. British Journal of Cancer 1984;50:435‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC Working Party. The evaluation of low dose pre‐operative X‐ray therapy in the management of operable rectal cancer; results of a randomly controlled trial: second report of an MRC Working Party. British Journal of Surgery 1984;71:21‐5. [DOI] [PubMed] [Google Scholar]
MRC 1996 {published data only}
- Medical Research Council Rectal Cancer Working Party. Randomized trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 1996;348:1605‐10. [PubMed] [Google Scholar]
Ngan 2012 {published data only}
- Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short‐course radiotherapy versus long‐course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans‐Tasman Radiation Oncology Group trial 01.04. Journal of Clinical Oncology 2012;30(31):3827‐33. [PUBMED: 23008301] [DOI] [PubMed] [Google Scholar]
Niebel 1988 {published data only}
- Niebel W, Schulz U, Ried M, Erhard J, Beersiek F, Blocher G, et al. Five year results of a prospective and randomized study: experience with combined radiotherapy and surgery of primary rectal carcinoma. Recent Results in Cancer Research 1988;110:111‐3. [DOI] [PubMed] [Google Scholar]
Parc 2009 {published data only}
- Parc Y, Zutshi M, Zalinski S, Ruppert R, Furst A, Fazio VW. Preoperative radiotherapy is associated with worse functional results after coloanal anastomosis for rectal cancer. Diseases of the Colon & Rectum 2009;52(12):2004‐14. [DOI] [PubMed] [Google Scholar]
Petersen 1998 {published data only}
- Herrmann T, Petersen S, Hellmich G, Baumann M, Ludwig K. Delayed toxicity of brief preoperative irradiation and risk‐adjusted postoperative radiotherapy of operative rectal carcinoma. Results of a randomized prospective study. Strahlentherapie und Onkologie 1999;175(9):430‐6. [DOI] [PubMed] [Google Scholar]
- Petersen S, Baumann M, Hellmich G, Herrmann Th, Ludwig K. Preoperative short‐term radiotherapy of rectal cancer: results of a prospective randomised trial. The European Society of Therapeutic Radiology and Oncology. 1998; Vol. 48:S1‐240.
- Petersen S, Hellmich G, Baumann M, Herrmann T, Henke G, Ludwig K. Brief preoperative radiotherapy in surgical therapy of rectal carcinoma. Chirurg 1998;69(7):759‐65. [DOI] [PubMed] [Google Scholar]
Pettersson 2015 {published data only}
- Pettersson D, Lorinc E, Holm T, Iversen H, Cedermark B, Glimelius B, et al. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. British Journal of Surgery 2015;102(8):972‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reis Neto 1989 {published data only}
- Reis Neto JA, Quilici FA, Reis JA Jr. A comparison of nonoperative vs preoperative radiotherapy in rectal carcinoma: a 10 year randomized trial. Diseases of the Colon & Rectum 1989;32:702‐10. [DOI] [PubMed] [Google Scholar]
Rider 1977 {published data only}
- Rider WD, Palmer JA, Mahoney LJ, Robertson CT. Preoperative irradiation in operable cancer of the rectum: report of the Toronto Trial. Canadian Journal of Surgery 1977;20(4):335‐8. [PubMed] [Google Scholar]
Rouanet 2006 {published data only}
- Rouanet P, Rivoire M, Lelong B, Rullier E, Dravet F, Mineur L, et al. Sphincter preserving surgery after preoperative treatment for ultra‐low rectal carcinoma. A French multicenter prospective trial: GRECCAR 1. Journal of Clinical Oncology 2006;24(suppl 18):3527. [Google Scholar]
Sause(RTOG81‐15)1994 {published data only}
- Sause WT, Pajak TF, Noyes RD, Dobelbower R, Fischbach J, Doggett S, et al. Evaluation of preoperative radiation therapy in operable colorectal cancer. Annals of Surgery 1994;220(5):668‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockholm 1996 {published data only}
- Cedermark B. The Stockholm II trial on preoperative short term radiotherapy in operable rectal carcinoma: a prospective randomised trial. Proceedings of the American Society for Clinical Oncology 1994;14:198. [Google Scholar]
- Holm T, Johannson H, Rutqvist LE, Cedermark B. Tumor location and the effects of preoperative radiotherapy in the treatment of rectal cancer. British Journal of Surgery 2001;88:839‐43. [DOI] [PubMed] [Google Scholar]
- Holm T, Rutqvist LE, Johansson H, Cedermark B. Abdominoperineal resection and anterior resection in the treatment of rectal cancer. British Journal of Surgery 1995;82:1213‐6. [DOI] [PubMed] [Google Scholar]
- Holm T, Rutqvist LE, Johansson H, Cedermark B. Postoperative mortality in rectal cancer treated with or without preoperative radiotherapy: causes and risk factors. British Journal of Surgery 1996;83:964‐8. [DOI] [PubMed] [Google Scholar]
- Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark B, the Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma ‐ long term follow up of a population based study. Cancer 2001;92:896‐902. [DOI] [PubMed] [Google Scholar]
- Stockholm Colorectal Cancer Study Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Annals of Surgical Oncology 1996;3(5):423‐30. [DOI] [PubMed] [Google Scholar]
- Stockholm Rectal Cancer Study Group. Preoperative short‐term radiation therapy in operable rectal carcinoma. Cancer 1990;66:49‐55. [DOI] [PubMed] [Google Scholar]
Valentini 2008 {published data only}
- Valentini V, Coco C, Minsky BD, Gambacorta MA, Cosimelli M, Bellavita R, et al. Randomized, multicenter, phase IIb study of preoperative chemoradiotherapy in T3 mid‐distal rectal cancer: raltitrexed + oxaliplatin + radiotherapy versus cisplatin + 5‐fluorouracil + radiotherapy. International Journal of Radiation Oncology * Biology * Physics 2008;70(2):403‐12. [PUBMED: 17919844] [DOI] [PubMed] [Google Scholar]
You 1993 {published data only}
- You QS, Wang RZ, Suen GQ, Yan FC, Gao YJ, Cui SR, et al. Combination preoperative radiation and endocavitary hyperthermia for rectal cancer: long term results of 44 patients. International Journal of Hyperthermia 1993;9:19‐24. [DOI] [PubMed] [Google Scholar]
Zehra 2015 {published data only}
- Zehra F, Siddiqui MRS, Andrews KG, Faiz O, Jalaludin B, Warusavitarne J. Rectal dysfunction and QOL in patients after curative treatment for rectal cancer. Colorectal Disease 2015;17:45. [Google Scholar]
Additional references
Abraha 2015
- Abraha I, Cherubini A, Cozzolino F, Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta‐epidemiological study. BMJ (Clinical research ed.) 2015;350:h2445. [PUBMED: 26016488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abraha 2017
- Abraha I, Cozzolino F, Orso M, Marchesi M, Germani A, Lombardo G, et al. A systematic review found that deviations from intention‐to‐treat are common in randomized trials and systematic reviews. Journal of Clinical Epidemiology 2017;84:37‐46. [PUBMED: 28088592] [DOI] [PubMed] [Google Scholar]
Adam 1994
- Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994;344(8924):707‐11. [PUBMED: 7915774] [DOI] [PubMed] [Google Scholar]
Aschele 2011
- Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR‐01 randomized phase III trial. Journal of Clinical Oncology 2011;29(20):2773‐80. [PUBMED: 21606427] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Beets‐Tan 2003
- Beets‐Tan RG. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Disease 2003;5(5):392‐5. [PUBMED: 12925068] [DOI] [PubMed] [Google Scholar]
Beets‐Tan 2005
- Beets‐Tan RG, Lettinga T, Beets GL. Pre‐operative imaging of rectal cancer and its impact on surgical performance and treatment outcome. European Journal of Surgical Oncology 2005;31(6):681‐8. [PUBMED: 16023947] [DOI] [PubMed] [Google Scholar]
Benson 2015
- Benson AB 3rd, Venook AP, Bekaii‐Saab T, Chan E, Chen YJ, Cooper HS, et al. Rectal Cancer, Version 2.2015. Journal of the National Comprehensive Cancer Network 2015;13(6):719‐28; quiz 728. [PUBMED: 26085388] [DOI] [PubMed] [Google Scholar]
Bosset 2006
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic‐Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. New England Journal of Medicine 2006;355(11):1114‐23. [PUBMED: 16971718] [DOI] [PubMed] [Google Scholar]
Boulis‐Wassif 1979
- Boulis‐Wassif S, Langenhorst BL, Hop WCJ. The contribution of preoperative radiotherapy in the management of borderline rectal cancer. Adjuvant Therapy of Cancer. Vol. II, Grune & Stratton, 1979:613‐20. [Google Scholar]
Braendengen 2008
- Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Pahlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. Journal of Clinical Oncology 2008;26(22):3687‐94. [PUBMED: 18669453] [DOI] [PubMed] [Google Scholar]
Brenner 2014
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383(9927):1490‐502. [PUBMED: 24225001] [DOI] [PubMed] [Google Scholar]
Bujko 2006
- Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, Michalski W, Bebenek M, Kryj M. Long‐term results of a randomized trial comparing preoperative short‐course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. British Journal of Surgery 2006;93(10):1215‐23. [PUBMED: 16983741] [DOI] [PubMed] [Google Scholar]
Cai 2014
- Cai Y, Li Z, Gu X, Fang Y, Xiang J, Chen Z. Prognostic factors associated with locally recurrent rectal cancer following primary surgery (Review). Oncology Letters 2014;7(1):10‐6. [PUBMED: 24348812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caricato 2006
- Caricato M, Borzomati D, Ausania F, Valeri S, Rosignoli A, Coppola R. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. European Journal of Surgical Oncology 2006;32(2):126‐32. [PUBMED: 16377120] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hrobjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen TYT, Wiltink LM, Nout RA, Meershoek‐Klein Kranenbarg E, Laurberg S, Marijnen CA, et al. Bowel function 14 years after preoperative short‐course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clinical Colorectal Cancer 2015;14(2):106‐14. [DOI] [PubMed] [Google Scholar]
Cummings 1982
- Cummings B, Rider W, Harwood A, Keane T, Thomas G. Radical external beam radiation therapy for adenocarcinoma of the rectum. Diseases of the Colon & Rectum 1982;26:30‐6. [DOI] [PubMed] [Google Scholar]
De Caluwe 2013
- Caluwe L, Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD006041.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1996
Dukes 1932
- Dukes CE. The classification of cancer of the rectum. Journal of Pathological Bacteriology 1932;35:323. [Google Scholar]
Enker 1997
- Enker WE. Total Mesorectal Excision ‐ The new golden standard of surgery for rectal cancer. Annals of Medicine 1997;29:127‐33. [DOI] [PubMed] [Google Scholar]
Enker 1999
- Enker WE, Merchant N, Cohen AM, Lanouette NM, Swallow C, Guillem J, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Annals of Surgery 1999;230(4):544‐52; discussion 552‐4. [PUBMED: 10522724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eu 1998
- Eu KW, Seow‐Choen F, Ho JM, Ho YH, Leong AF. Local recurrence following rectal resection for cancer. Journal of the Royal College of Surgeons of Edinburgh 1998;43(6):393‐6. [PUBMED: 9990786] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [PUBMED: 25220842] [DOI] [PubMed] [Google Scholar]
Fernandez‐Esparrach 2011
- Fernandez‐Esparrach G, Ayuso‐Colella JR, Sendino O, Pages M, Cuatrecasas M, Pellise M, et al. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointestinal Endoscopy 2011;74(2):347‐54. [PUBMED: 21802588] [DOI] [PubMed] [Google Scholar]
Fiorica 2010
- Fiorica F, Cartei F, Licata A, Enea M, Ursino S, Colosimo C, et al. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta‐analysis of literature data. Cancer Treatment Reviews 2010;36(7):539‐49. [PUBMED: 20334979] [DOI] [PubMed] [Google Scholar]
Fisher 1988
- Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R‐01. Journal of the National Cancer Institute 1988;80(1):21‐9. [PUBMED: 3276900] [DOI] [PubMed] [Google Scholar]
Folkesson 2005
- Folkesson J, Birgisson H, Påhlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. Journal of Clinical Oncology 2005;23(24):5644‐50. [PUBMED: 16110023] [DOI] [PubMed] [Google Scholar]
Francois 1999
- Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter‐sparing surgery for rectal cancer: the Lyon R90‐01 randomized trial. Journal of Clinical Oncology 1999;17(8):2396. [PUBMED: 10561302] [DOI] [PubMed] [Google Scholar]
Gastrointestinal Tumor Study Group 1985
- Gastrointestinal Tumor Study Group. Prolongation of the disease‐free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. New England Journal of Medicine 1985;312(23):1465‐72. [PUBMED: 2859523] [DOI] [PubMed] [Google Scholar]
Gerard 2006
- Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon‐Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203. Journal of Clinical Oncology 2006;24(28):4620‐5. [PUBMED: 17008704] [DOI] [PubMed] [Google Scholar]
Gilbert 1978
- Gilbert SG. Symptomatic local tumor failure following abdomino‐perineal resection. International Journal of Radiation Oncology * Biology * Physics 1978;4(9‐10):801‐7. [PUBMED: 711549] [DOI] [PubMed] [Google Scholar]
Glimelius 2003
- Glimelius B, Grönberg H, Jarhult J, Wallgren A, Cavallin‐Stahl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncologica 2003;42(5‐6):476‐92. [DOI] [PubMed] [Google Scholar]
Glimelius 2008
- Glimelius B, Holm T, Blomqvist L. Chemotherapy in addition to preoperative radiotherapy in locally advanced rectal cancer ‐ a systematic overview. Reviews on Recent Clinical Trials 2008;3(3):204‐11. [PUBMED: 18782078] [DOI] [PubMed] [Google Scholar]
Glimelius 2013
- Glimelius B, Tiret E, Cervantes A, Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;24 Suppl 6:vi81‐8. [PUBMED: 24078665] [DOI] [PubMed] [Google Scholar]
Goldberg 1998
- Goldberg S, Klas JV. Total mesorectal excision in the treatment of rectal cancer: a view from the USA. Seminars in Surgical Oncology 1998;15:87‐90. [DOI] [PubMed] [Google Scholar]
Heald 1982
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery ‐ the clue to pelvic recurrence?. British Journal of Surgery 1982;69(10):613‐6. [PUBMED: 6751457] [DOI] [PubMed] [Google Scholar]
Heald 1986
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1(8496):1479‐82. [PUBMED: 2425199] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Chapter 8: Assessing risk of bias in included studies. In Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org 2011.
Holm 1994
- Holm T, Cedermark B, Rutqvist LE. Local recurrence of rectal adenocarcinoma after 'curative' surgery with and without preoperative radiotherapy. British Journal of Surgery 1994;81:452‐5. [DOI] [PubMed] [Google Scholar]
Krook 1991
- Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high‐risk rectal carcinoma. New England Journal of Medicine 1991;324(11):709‐15. [PUBMED: 1997835] [DOI] [PubMed] [Google Scholar]
Law 2004
- Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Annals of Surgery 2004;240(2):260‐8. [PUBMED: 15273550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipska 2006
- Lipska MA, Bissett IP, Parry BR, Merrie AE. Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ journal of surgery 2006;76(7):579‐85. [PUBMED: 16813622] [DOI] [PubMed] [Google Scholar]
Macura 2010
- Macura A, Abraha I, Kirkham J, Gensini GF, Moja L, Iorio A. Selective outcome reporting: telling and detecting true lies. The state of the science. Internal and Emergency Medicine 2010;5(2):151‐5. [PUBMED: 20300879] [DOI] [PubMed] [Google Scholar]
Marijnen 2005
- Marijnen CA, Velde CJ, Putter H, Brink M, Maas CP, Martijn H, et al. Impact of short‐term preoperative radiotherapy on health‐related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. Journal of Clinical Oncology 2005;23(9):1847‐58. [PUBMED: 15774778] [DOI] [PubMed] [Google Scholar]
Matthiessen 2004
- Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjödahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 2004;6(6):462‐9. [PUBMED: 15521937] [DOI] [PubMed] [Google Scholar]
Mauvais 2011
- Mauvais F, Sabbagh C, Brehant O, Viart L, Benhaim T, Fuks D, et al. The current abdominoperineal resection: oncological problems and surgical modifications for low rectal cancer. Journal of Visceral Surgery 2011;148(2):e85‐93. [PUBMED: 21481666] [DOI] [PubMed] [Google Scholar]
McCall 1995
- McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. International Journal of Colorectal Disease 1995;10(3):126‐32. [PUBMED: 7561427] [DOI] [PubMed] [Google Scholar]
Mendenhall 1983
- Mendenhall WM, Million RR, Pfaff WW. Patterns of recurrence in adenocarcinoma of the rectum and rectosigmoid treated with surgery alone: implications in treatment planning with adjuvant radiation therapy. International Journal of Radiation Oncology * Biology * Physics 1983;9(7):977‐85. [PUBMED: 6863077] [DOI] [PubMed] [Google Scholar]
Miles 1908
- Miles WE. A method of performing abdominoperineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon. Lancet 1908;2:1812–3. [Google Scholar]
MS Excel 2010 [Computer program]
- Microsoft Corporation. Microsoft Excel. Microsoft Corporation, 2010.
Nagtegaal 2008
- Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer?. Journal of Clinical Oncology 2008;26(2):303‐12. [PUBMED: 18182672] [DOI] [PubMed] [Google Scholar]
NIH consensus conference 1990
- NIH consensus conference 1990. NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264(11):1444‐50. [PUBMED: 2202842] [PubMed] [Google Scholar]
Ooi 1999
- Ooi BS, Tjandra JJ, Green M. Morbidities of adjuvant chemotherapy and radiotherapy for resectable rectal cancer. An overview. Diseases of the Colon & Rectum 1999;42:403‐18. [DOI] [PubMed] [Google Scholar]
Pakkastie 1994
- Pakkastie TE, Luukkonen PE, Jarvinen HJ. Anastomotic leakage after anterior resection of the rectum. European Journal of Surgery (Acta Chirurgica) 1994;160(5):293‐7; discussion 299‐300. [PUBMED: 8075199] [PubMed] [Google Scholar]
Park 2012
- Park SH, Lee JH, Lee SS, Kim JC, Yu CS, Kim HC, et al. CT colonography for detection and characterisation of synchronous proximal colonic lesions in patients with stenosing colorectal cancer. Gut 2012;61(12):1716‐22. [PUBMED: 22115824] [DOI] [PubMed] [Google Scholar]
Parmar 1998
Perez 1992
- Perez CA, Brady LW. Chapter 1. Principles and Practice of Radiation Oncology. Lippincott Williams & Wilkins, 1992. [Google Scholar]
Perry 2007
- Perry BW, Connaughton CJ. Abdominoperineal resection: how is it done and what are the results?. Clinics in Colon and Rectal Surgery 2007;20(3):213‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2012
- Petersen SH, Harling H, Kirkeby LT, Wille‐Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004078.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puli 2009
- Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta‐analysis and systematic review. Annals of Surgical Oncology 2009;16(2):254‐65. [PUBMED: 19018597] [DOI] [PubMed] [Google Scholar]
Quirke 1986
- Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2(8514):996‐9. [PUBMED: 2430152] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sauer 2004
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New England Journal of Medicine 2004;351(17):1731‐40. [PUBMED: 15496622] [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Schulz 1996
- Schulz KF, Grimes DA, Altman DG, Hayes RJ. Blinding and exclusions after allocation in randomised controlled trials: survey of published parallel group trials in obstetrics and gynaecology. BMJ (Clinical research ed.) 1996;312(7033):742‐4. [PUBMED: 8605459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11. Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Siegel 2009
- Siegel R, Burock S, Wernecke KD, Kretzschmar A, Dietel M, Loy V, et al. Preoperative short‐course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi‐centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer 2009;9:50. [PUBMED: 19200365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2012
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA: A Cancer Journal for Clinicians 2012;62(4):220‐41. [PUBMED: 22700443] [DOI] [PubMed] [Google Scholar]
Sobin 2010
- Sobin LH, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th Edition. New York: Wiley‐Blackwell, 2010. [Google Scholar]
Stephens 2010
- Stephens RJ, Thompson LC, Quirke P, Steele R, Grieve R, Couture J, et al. Impact of short‐course preoperative radiotherapy for rectal cancer on patients' quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. Journal of Clinical Oncology 2010;28(27):4233‐9. [PUBMED: 20585099] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stewart 2007
- Stewart DB, Dietz DW. Total mesorectal excision: what are we doing?. Clinics in Colon and Rectal Surgery 2007;20(3):190‐202. [PUBMED: 20011200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2016
- Sun Z, Adam MA, Kim J, Shenoi M, Migaly J, Mantyh CR. Optimal timing to surgery after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Journal of the American College of Surgeons 2016;222(4):367‐74. [PUBMED: 26897480] [DOI] [PubMed] [Google Scholar]
Swartling 2013
- Swartling T, Kalebo P, Derwinger K, Gustavsson B, Kurlberg G. Stage and size using magnetic resonance imaging and endosonography in neoadjuvantly‐treated rectal cancer. World Journal of Gastroenterology 2013;19(21):3263‐71. [PUBMED: 23745028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanis 2013
- Tanis PJ, Doeksen A, Lanschot JJB. Intentionally curative treatment of locally recurrent rectal cancer: a systematic review. Canadian Journal of Surgery 2013;56(2):135‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Twomey 1989
- Twomey P, Burchell M, Strawn D, Guernsey J. Local control in rectal cancer. A clinical review and meta‐analysis. Archives of Surgery 1989;124:1174‐9. [DOI] [PubMed] [Google Scholar]
Valentini 2009
- Valentini V, Aristei C, Glimelius B, Minsky BD, Beets‐Tan R, Borras JM, et al. Multidisciplinary Rectal Cancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA‐CC2). Radiotherapy and Oncology 2009;92(2):148‐63. [PUBMED: 19595467] [DOI] [PubMed] [Google Scholar]
van de Velde 2013
- Velde CJ, Aristei C, Boelens PG, Beets‐Tan RG, Blomqvist L, Borras JM, et al. EURECCA colorectal: multidisciplinary mission statement on better care for patients with colon and rectal cancer in Europe. European Journal of Cancer 2013;49(13):2784‐90. [PUBMED: 23769991] [DOI] [PubMed] [Google Scholar]
van de Velde 2014
- Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: Multidisciplinary management: European consensus conference colon & rectum. European Journal of Cancer 2014;50(1):1.e1‐34. [PUBMED: 24183379] [DOI] [PubMed] [Google Scholar]
van den Brink 2005
- Brink M, Hout WB, Kievit J, Marijnen CA, Putter H, Velde CJ, et al. The impact of diagnosis and treatment of rectal cancer on paid and unpaid labor. Diseases of the Colon & Rectum 2005;48(10):1875‐82. [PUBMED: 16175329] [DOI] [PubMed] [Google Scholar]
Viani 2011
- Viani GA, Stefano EJ, Soares FV, Afonso SL. Evaluation of biologic effective dose and schedule of fractionation for preoperative radiotherapy for rectal cancer: meta‐analyses and meta‐regression. International Journal of Radiation Oncology * Biology * Physics 2011;80(4):985‐91. [PUBMED: 20615619] [DOI] [PubMed] [Google Scholar]
Walz 1981
- Walz BJ, Green MR, Lindstrom ER, Butcher HR Jr. Anatomical prognostic factors after abdominal perineal resection. International Journal of Radiation Oncology * Biology * Physics 1981;7(4):477‐84. [PUBMED: 7251417] [DOI] [PubMed] [Google Scholar]
Wiig 1998
- Wiig JN, Søreide O. Mesorectal excision for rectal cancer: a view from Europe. Seminars in Surgical Oncology 1998;15(2):78‐86. [DOI] [PubMed] [Google Scholar]
Wong 1998
- Wong CS, Cummings BJ, Brierley MB, Catton CN, McLean M, Catton P, et al. Treatment of locally recurrent rectal carcinoma ‐ results and prognostic factors. International Journal of Radiation Oncology * Biology * Physics 1998;40(2):427‐35. [DOI] [PubMed] [Google Scholar]
Wong 2000
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wong 2007
- Wong RK, Tandan V, Silva S, Figueredo A. Pre‐operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002102.pub2] [DOI] [PubMed] [Google Scholar]


